User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Trends in Utilization of Total Hip Arthroplasty for Femoral Neck Fractures in the United States
ABSTRACT
The ideal mode of fixation for patients with femoral neck fractures is not well defined in the current literature. This study describes the recent trends in surgical management of femoral neck fractures with an analysis on perioperative outcomes.
The National Hospital Discharge Survey was used to identify femoral neck fractures in the United States between 1990 and 2007 (n = 1,155,960) treated with open reduction and internal fixation (ORIF), total hip arthroplasty (THA), or hemiarthroplasty (HA). Trends were examined over the following 3 time periods: 1990 to 1995 (group 1), 1996 to 2001 (group 2), and 2002 to 2007 (group 3). Elixhauser Comorbidity Index and perioperative complications were calculated.
Use of HA increased (74.4% to 84.6%), whereas that of THA (7.3% to 4.9%) and ORIF (18.3% to 10.6%) decreased, from group 1 to group 3 in the age group of >80 years. The use of ORIF increased (63.9% to 81.4%), whereas the use of both HA and THA decreased, from group 1 to group 3 in the age group of <50 years. The rate of adverse events increased across all fixation types but was greatest among THA (32.2% to 48.3%).
The femoral neck patient population is now older and has more medical comorbidities. We observed a trend toward performing HA in older patients and ORIF in younger patients. Despite superior functional outcomes reported in THA, this study found a decreased utilization of THA in all age groups along with an increase in adverse events and nonroutine discharges for patients with femoral neck fractures treated with THA.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Continue to: Femoral neck fractures...
Femoral neck fractures are a common occurrence in the United States. A recent study estimated an incidence of >63 per 100,000 population.1-8 Although the incidence appears to have decreased over recent decades, there is a projected exponential increase in the incidence of hip fractures over the next 30 years in the baby boomer population.8,9 Given that these fractures have a significant impact on patient morbidity, mortality, and quality of life, research efforts have been directed toward optimizing the treatment of affected patients and improving the outcomes.4,9-24
The treatment of choice for femoral neck fractures and the use of total hip arthroplasty (THA)11 have been a topic of debate.4,9,10,15-17,22,25 Total hip arthroplasty has been advocated for younger, more active patients, whereas hemiarthroplasty (HA) has been reserved for patients who are older and less active. Although several studies have demonstrated that arthroplasty outperforms open reduction and internal fixation (ORIF) in the elderly population with displaced femoral neck fractures, ORIF is still commonly performed in the United States for nondisplaced fractures and in patients aged <50 years.26-29
In an attempt to quantify the use of THA in the treatment of femoral neck fractures and demonstrate the national trends, Miller and colleagues5 pooled the American Board of Orthopaedic Surgery (ABOS) database and analyzed the treatment trends of surgeons taking part II of the ABOS examination from 1999 to 2011. The authors found an increased utilization of THA by recently graduated orthopedic surgeons. In contrast, Jain and colleagues30 found different national trends when they analyzed data from the National Inpatient Sample containing data between 1990 and 2001 and further found decreased utilization of THA procedures by orthopedic surgeons of all levels of training nationwide. However, neither of these studies reported about the trends in demographics, comorbidities, risk factors, or outcomes in this patient population following surgery.
The purpose of this study was to help clarify the findings of these authors using the largest dataset to date and also report on the perioperative complications associated with each mode of fixation in patients who undergo operative treatment for femoral neck fractures in the United States. Our hypotheses were that the femoral neck fracture patient population has become older and has more medical comorbidities. We also hypothesized that there has been a trend toward performing fewer THA procedures in the United States and that THA is associated with increased perioperative complications compared to those with HA and ORIF.
MATERIALS AND METHODS
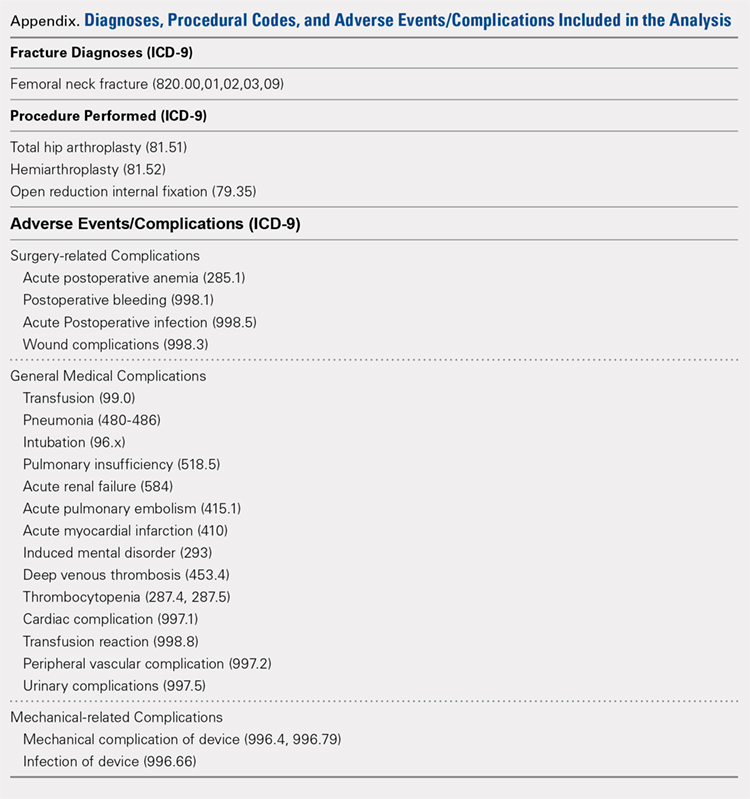
We conducted a retrospective epidemiological study using the National Hospital Discharge Survey (NHDS) on surgical trends in the management of femoral neck fractures. The NHDS is a publicly available survey that is conducted annually to provide data of nonfederal, short-stay hospitals to the public. The sample data are weighted to provide nationwide estimates of annual inpatient care. The NHDS includes up to 7 medical diagnoses and 4 procedural codes per case, which are categorized using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes, that were collected along with patient demographic information, length of stay (LOS), and discharge disposition. The diagnostic and procedural codes used for this study are presented in the Appendix. The year 2007 was chosen as the endpoint of this study due to the fact that the relative standard error of the NHDS doubled in 2008 as a result of a decrease in its survey size. As this is a publicly available database, our study was exempt from institutional review board approval.
Continue to: All pateints admitted...
All patients admitted with a primary diagnosis of closed transcervical fracture of the femoral neck (ICD-9-CM 820.0x) were selected. This resulted in 1,674,160 fractures. All patients with fractures with a concurrent primary procedural code of ORIF (79.35), HA (81.52), or THA (81.51) were identified, resulting in a total sample size of 1,155,960 surgical fractures. Analysis of the fractures based on additional specificity,ie subcapital versus midcervical versus basicervical, was not carried out because >90% of femoral neck fractures in the database were coded as “unspecified” or “other” (ICD9 CM 820.00 and 820.09, respectively).
Comorbidity burden was quantified using Elixhauser coding algorithms as previously described.31 The Elixhauser comorbidity measure is a model consisting of 31 conditions and has recently been identified as a better predictor of mortality in patients undergoing orthopedic procedures when compared with the Charlson Comorbidity Index.31 Dichotomous variables for each Elixhauser comorbidity were created, and χ2 tests were utilized to assess the association between each comorbidity and mortality. The weighted Elixhauser score for each statistically significant comorbidity was calculated as described by van Walraven and colleagues.32 The Elixhauser comorbidity score was then calculated for each patient by summing the individual weights of all comorbidities. Postoperative adverse events were determined using the complication-screening-package as previously described.33
All adverse events were categorized into 3 categories, including general medical complications, mechanical complications, and surgical complications. All adverse events recorded in the NHDS database are events that occurred during a single hospitalization. Therefore, it does not take into account adverse events that occurred after discharge, and, for example, mortality refers to postoperative mortality that occurs prior to discharge. The study period comprised data captured from 1990 to 2007, and 3 groups were generated from this time period to better characterize patients throughout the large study time frame. Group 1 comprised patients who underwent surgical management of femoral neck fractures from 1990 to 1995, group 2 consisted of patients treated from 1996 to 2001, and group 3 included patients treated from 2002 to 2007.
Categorical data were analyzed using the χ2 test, and continuous data were analyzed by the independent-samples t test and ANOVA. Multivariable binary logistic regression analyses were performed to assess the contributions of individual comorbidities to mortality, adverse events, and nonroutine discharge. Elixhauser comorbidities with a P value of < .10 in the bivariate analysis and presenting in at least 0.2% of the population were included in the logistic regression.31 Odds ratios and confidence intervals were calculated to assess the association between comorbidities and our dichotomous variables. A P value of < .001 defined statistical significance.33 Statistical analysis was conducted using SPSS version 21 (IBM).
RESULTS
Patient Demographics
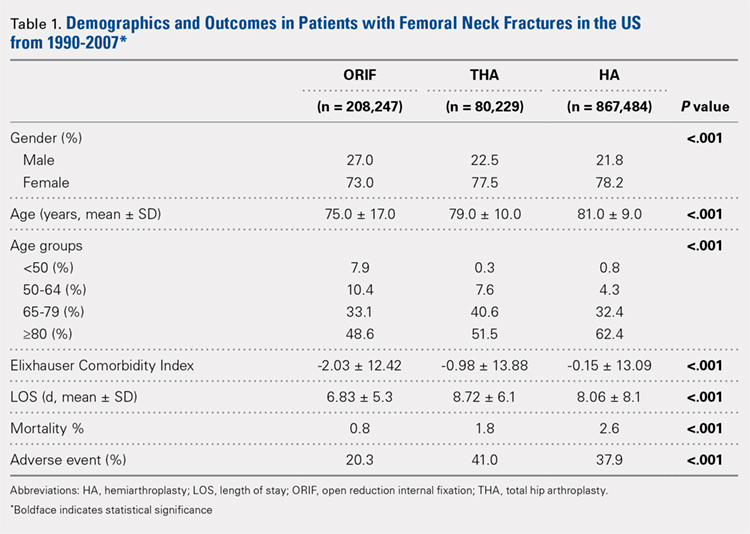
Our query demonstrated a total of 1,155,960 patients who underwent surgical fixation of femoral neck fractures (Table 1). The most commonly used treatment modality was HA (75%), followed by ORIF (18%) and later by THA (7%). The majority of patients were females in each treatment group. Patients’ age varied according to treatment group, with patients undergoing HA having a mean age of 81.0 ± 9.0 years, patients undergoing ORIF having a mean age of 75.0 ± 17.0 years, and those undergoing THA having a mean age of 79.0 ± 10.0 years (P < .001). The majority of patients were ≥80 years in all treatment groups, but the ORIF group had the greatest proportion of patients <65 years (P < .001). Among patients undergoing HA, 62.4% were ≥80 years, while the ORIF and HA groups consisted of 48.6% and 51.5% of patients in that same age group, respectively.
Continue to: TRENDS ANALYSIS
TRENDS ANALYSIS
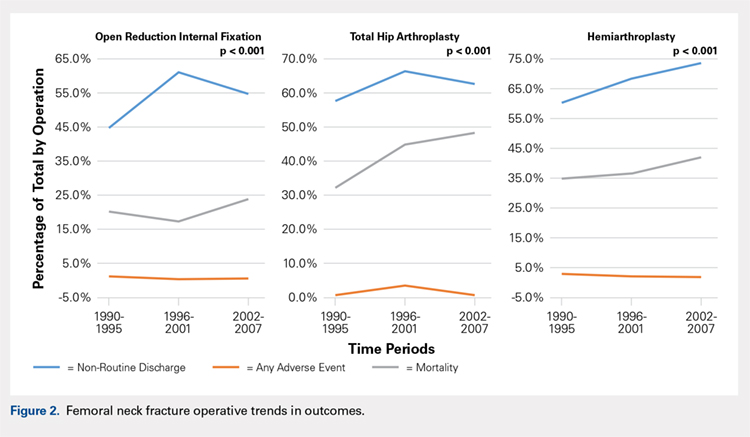
There was a significant change in the distributions of the procedures performed according to age group over time. Patients >80 years continued to undergo primarily HA, with an increase from 74.4% during 1990 to 1995 up to 84.6% during the 2002 to 2007 period and a concomitant decrease in ORIF from 18.3% to 10.6% during the same time period in this age group. Surgical trends in patients 65 to 79 years demonstrated a significant decrease in management with ORIF from 19.1% in 1990 to 1995 to 16.8% in the 2002 to 2007 cohort (P < .001 for all, Table 2). There was an increase in the use of HA from 71.9% during the 1990 to 1995 period to 75.5% during the final study period (Table 2, Figure 1). The use of THA for all age groups decreased between 1990 and 2007, except for the 50- to 64-year-old group where THA utilization remained constant.
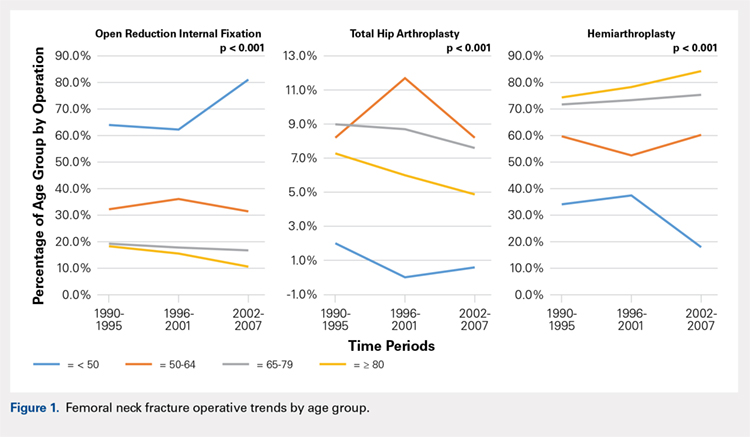
Management patterns in patients 50 to 64 years varied throughout the analysis and demonstrated the following trend: treatment with HA remained the most common technique used but varied slightly from 59.7% during 1990 to 1995 to 60.3% during 2002 to2007 (P < .001, Table 2). The second most common treatment used was ORIF, which decreased from 32.2% to 31.5% (P < .001, Table 2). The use of THA varied significantly from 8.2% among those managed during 1990 to 1995 to 11.7% during 1996 to 2001 but later declined to the initial 8.2% (P < .001, Table 2).
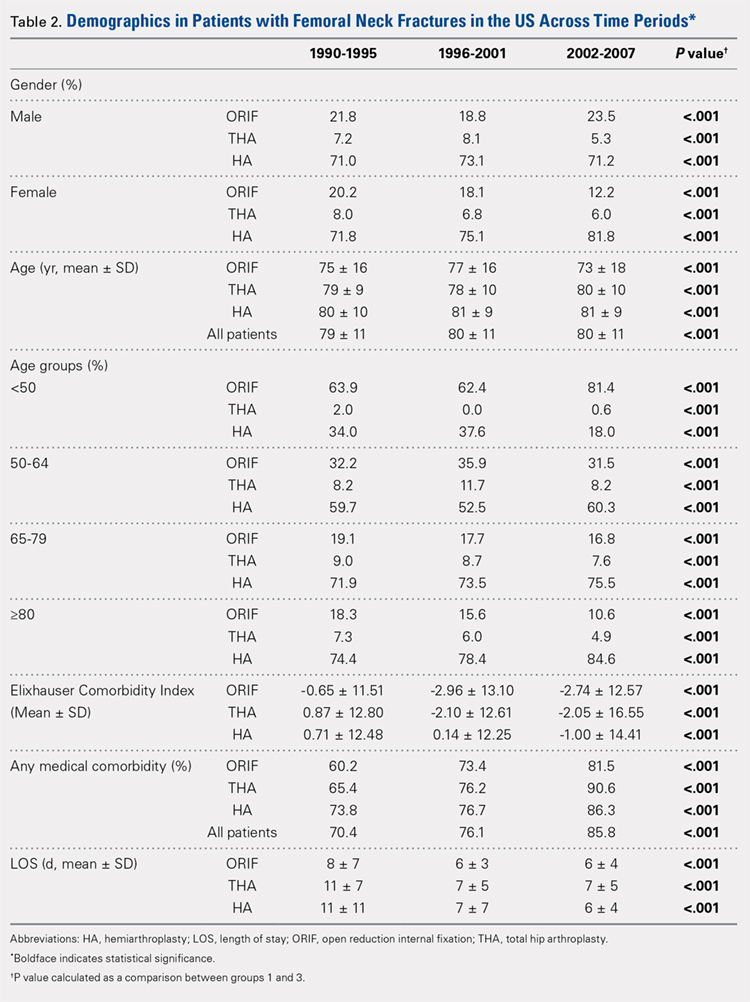
Analysis of patients ≤49 years demonstrated that ORIF was the preferred technique, which experienced a growth from 63.9% during 1990 to 1995 to 81.4% during the 2002 to 2007 period (P < .001, Table 2). A decreased use in THA was observed from 2.0% in the initial period to 0.6% in the final period (P < .001, Table 2). Use of HA decreased from 34.0% in 1990 to 1995 to 18.0% in 2002 to 2007 (P < .001, Table 2).
LENGTH OF STAY
Mean number of in-hospital days decreased throughout the study period for all treatment techniques. During the 1990 to 1995 study period, patients who underwent ORIF had a mean LOS of 8 ± 7 days, which decreased (P < .001, Table 2) to 6 ± 3 days in 1996 to 2001 and remained constant during 2002 to 2007 (mean 6 ± 4 days). This decrease in LOS was also observed in patients who underwent THA (P < .001, Table 2), who initially had a mean LOS of 11 ± 7 days during 1990 to 1995, which later decreased to 7 ± 5 days for the remainder of the study. The LOS for patients who underwent HA also decreased (P < .001, Table 2), which initially was reported to be 11 ± 11 days during 1990 to 1995, decreasing to 7 ±7 days in 1996–2001 and later to 6 ± 4 days in 2002 to 2007.
COMORBIDITIY ANALYSIS
The Elixhauser Comorbidity Index varied significantly among groups over time (P < .001, Table 2). Overall mean Elixhauser Comorbidity Index score per procedure type is provided in Table 1, with HA patients having the highest score (-0.15 ± 13.09, p<.001).
Continue to: Analysis of the preoperative comorbidities...
Analysis of the preoperative comorbidities demonstrated significant differences among each surgical treatment group (P < .001 for all, Table 3). The most common comorbidities in patients who underwent HA were uncomplicated hypertension (33.2%), fluid/electrolyte disorders (17.4%), chronic pulmonary disease (14.9%), and congestive heart failure (13.7%). The most common comorbidities in the ORIF group were uncomplicated hypertension (30.8%), fluid/electrolyte disorders (14.5%), chronic pulmonary disease (14.0%), and uncomplicated diabetes (10.9%). Patients treated with THA had most commonly uncomplicated hypertension (30.1%), fluid/electrolyte disorders (17.2%), uncomplicated diabetes (15.5%), and chronic pulmonary disease (14.4%). The prevalence of comorbidities is displayed in Table 3.
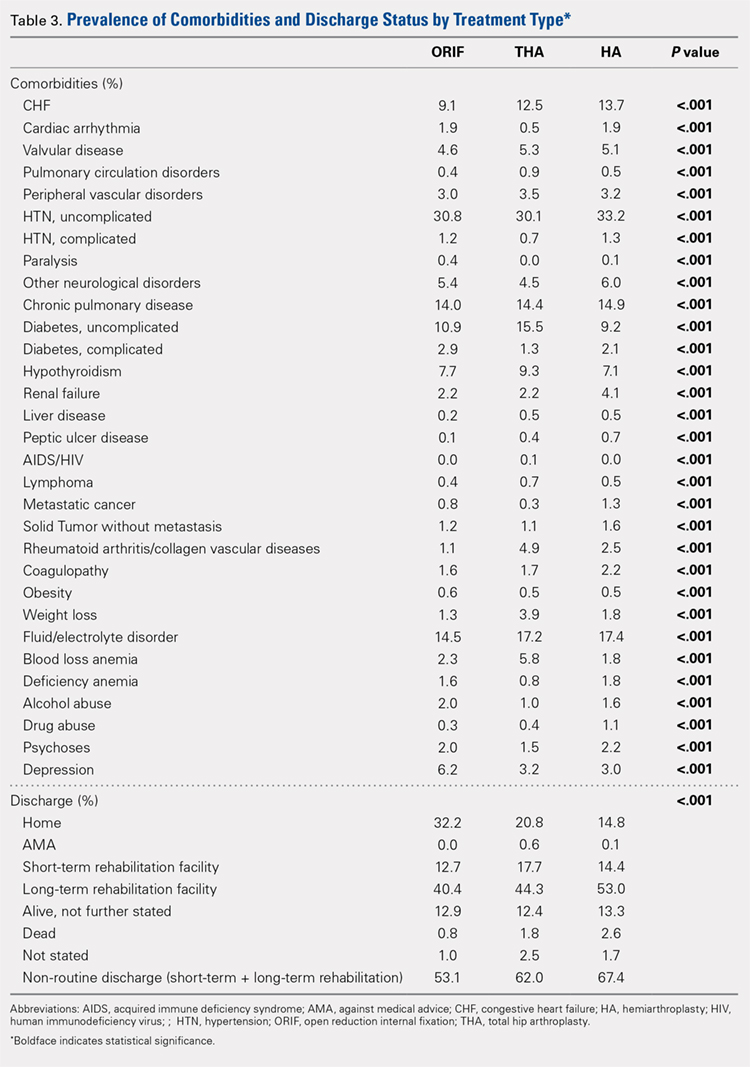
DISCHARGE STATUS
Mortality varied significantly, being lowest in those who underwent ORIF (0.8%), followed those who underwent THA (1.8%), and HA (2.6%) (P < .001, Table 1).
The majority of patients in each group were discharged to long-term rehabilitation facilities, including 53.0% of those treated with HA, 40.4% of those treated with ORIF, and 44.3% of patients treated with THA. The second most common discharge location was home, which included 14.8% of patients who underwent HA, 32.2% of patients treated with ORIF, and 20.8% of those who underwent THA. Table 3 demonstrates the details of the discharge settings.
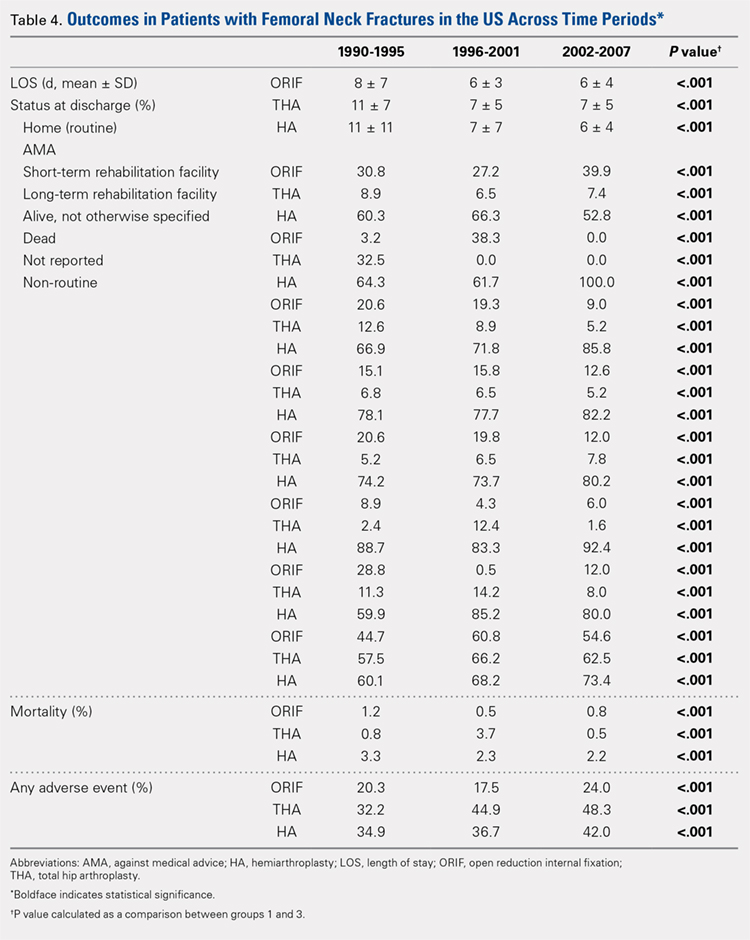
Mortality analysis over time demonstrated a significant decrease in each treatment group (P < .001). Mortality in the ORIF group decreased from 1.2% during 1990 to 1995 to 0.8% in 2002 to 2007. Mortality in the THA group also decreased significantly from 0.8% during 1990 to 1995 to 0.5% during the 2002 to 2007 time period. Patients who underwent HA also exhibited a decrease in mortality rate from 3.3% during 1990 to 1995 to 2.2% during 2002 to 2007 (P < .001, Table 4, Figure 2).
GENERAL ADVERSE EVENTS
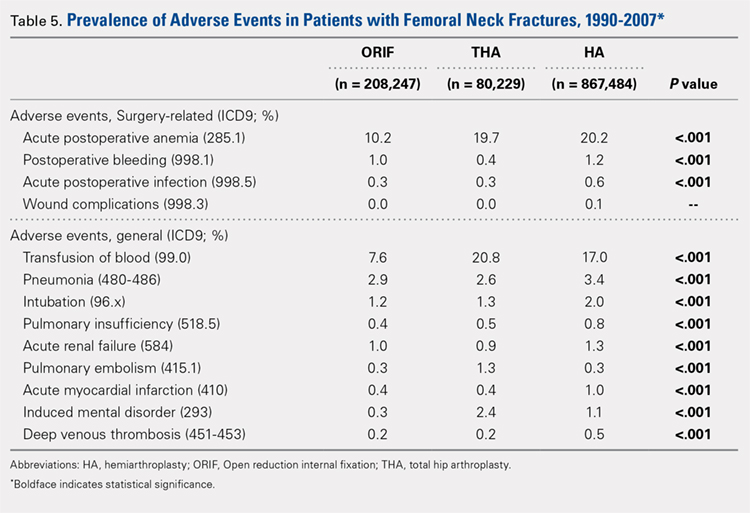
There was a significant difference (P < .001) in the percentage of adverse events experienced, the maximum being observed in the THA group (41.0%), followed by the HA group (37.9%) and trailed by the ORIF group (20.3%, (P < .001, Table 1). The prevalence of adverse events is detailed in Table 5.
Continue to: Patients who underwent THA...
Patients who underwent THA had the highest rate of any adverse event, LOS, and transfusion rate (Table 1 and Table 5).
The prevalence of postoperative pneumonia was highest in the HA group (3.4%), followed by the ORIF group (2.9%), and the THA group (2.6%) (P < .001, Table 5). There was also a significant difference in rates of intubation, pulmonary insufficiency, acute renal failure, pulmonary embolism, acute myocardial infarction, induced mental disorder, and deep venous thrombosis (P < .001 for all, Table 5).
SURGERY-RELATED ADVERSE EVENTS
Surgery-related outcomes over the entire study period were significantly different according to the type of procedure performed (P < .001, Table 5). Patients who underwent HA had the highest rate of acute postoperative anemia (20.2%), followed by those who underwent THA (19.7%), and ORIF (10.2%). Postoperative bleeding rates also varied significantly, with 1.2% in the HA group, followed by 1.0% in the ORIF group and 0.4% in the THA group (P < .001, Table 5). Acute postoperative infection rates also varied significantly, with the highest rate being observed in the HA group (0.6%) compared to that in the THA and ORIF groups (both 0.3%) (P < .001, Table 5).
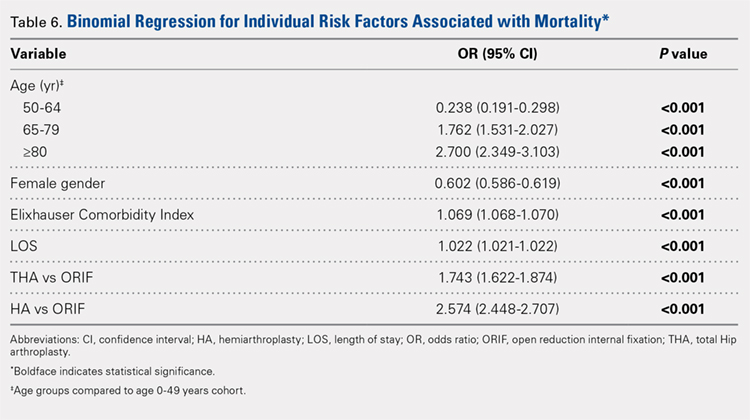
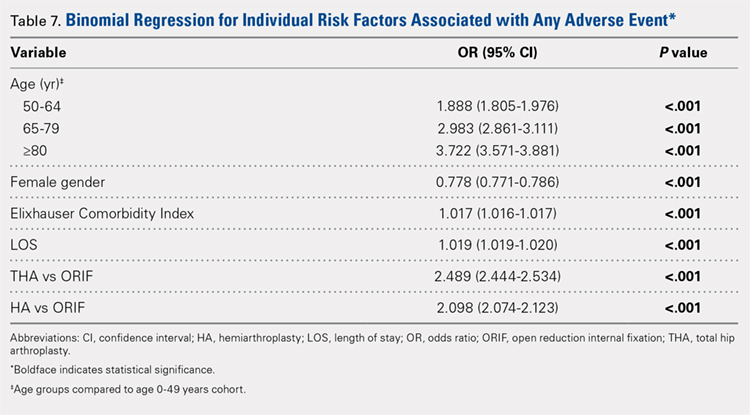
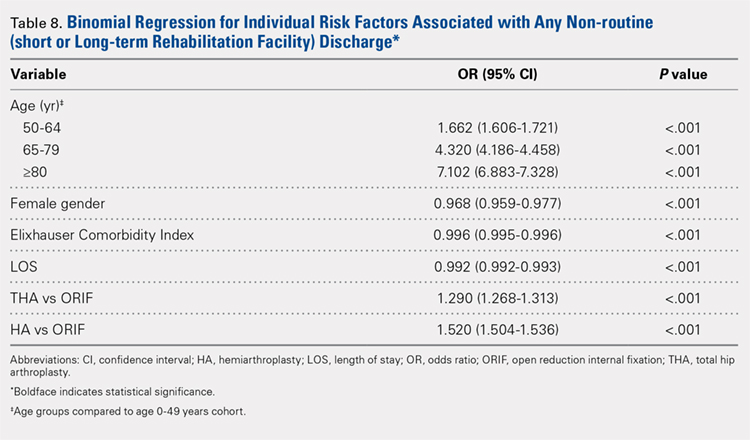
Table 6, Table 7, and Table 8 detail the results of regression analyses in patients with femoral neck fractures for individual risk factors associated with mortality, any adverse event, and nonroutine discharge to a short- or long-term rehabilitation facility, respectively. Increasing age (50–64 years, OR: 0.238; 65–79 years, OR: 1.762; and ≥80 years, OR: 2.700), THA (OR: 1.743), and HA (OR: 2.574) were found to be independent risk factors for mortality in the perioperative period (P < .001 for each, Table 6). Increasing age (50–64 years, OR: 1.888; 65–79 years, OR: 2.983; and ≥80 years, OR: 3.722), THA (OR: 2.489), and HA (OR: 2.098) were also found to be independent risk factors for any adverse event in the perioperative period (P < .001, Table 7). Age (50–64 years, OR: 1.662; 65–79 years, OR: 4.320; and ≥80 years, OR: 7.102) was the best predictor for nonroutine discharge to a short- or long-term rehabilitation facility (P < .001, Table 8).
DISCUSSION
Femoral neck fractures in the elderly population present a significant financial burden to the healthcare system.1-3,24,25 Consistent with previous epidemiological studies, our results show that the femoral neck fracture population has become older and has more medical comorbidities over the last 3 decades.27,28. Similarly, we also found that the rate of medical, surgical, and mechanical perioperative complications has increased in the same time period. Interestingly, the mortality rate has remained relatively similar.
Continue to: Although patients undergoing HA...
Although patients undergoing HA for femoral neck fractures are older and have more medical comorbidities, we found that the rate of adverse events in the perioperative period for patients undergoing THA was higher than that in the HA group. Consistent with prior studies, patients who underwent THA had higher rates of blood transfusion, pulmonary embolism, and induced mental disorders.34 Multivariable regression analysis demonstrated that after controlling for age, medical comorbidity, and type of surgery performed, THA emerged as an independent risk factor for any adverse event in the perioperative period. Increased anesthesia time, reaming of the acetabulum, and increased complexity of surgery probably account for these changes.
Our study results are consistent with those of Jain and colleagues,30 which showed a decrease in utilization of THA for femoral neck fractures between 1990 and 2001. Since THA is generally indicated for younger, more active patients in relatively good health, this would explain why changes in baseline health in this cohort over the last 20 years would lead to fewer THA procedures being performed. Surgeons in the US may be finding there are fewer patients who are candidates for THA. Miller and colleagues5 reported conflicting results and showed an increase in THA utilization in this patient population. However, their study evaluated treatment trends based on data from the ABOS part II of recently graduated orthopedic surgeons and may not be an accurate representation of national practice trends in the US. The trend toward increased subspecialization may explain their findings. As the authors noted, although they found an increase in the use of THA for femoral neck fractures by new adult reconstruction surgeons, the percentage of new surgeons treating femoral neck fractures has declined.5
Our analysis showed very concrete trends in treatment management at the extremes of the age ranges. There were substantial increases in the use of ORIF for patients <50 years (from 63.9% in 1990–1995 to 81.4% in 2002–2007, P < .001) and in the use of HA for patients >80 years (from 74.4% in 1990–1995 to 84.6% in 2002–2007, P < .001). This trend parallels recent studies that purport better outcomes for young patients undergoing ORIF and elderly patients undergoing HA.30 Our analysis did not demonstrate a large shift in surgeon preference for treatment of patients between 50 and 80 years, although there was a statistically significant decrease in ORIF and THA usage and a reflective increase in HA usage in this population as well. The fact that there has not been as substantial a shift in treatment trends for this large age group is potentially due to the wide variations in comorbid conditions and the functionality that abounds in this age group.1
The limitations of the current study are those inherent with a retrospective database analysis. The reliance on accurate coding brings up a potential for error; however, it is unlikely that comorbidities and outcomes are undercoded as hospitals are incentivized to input values that increase the acuity and thus reimbursement for each hospital stay.35 The database also relies on the ICD-9 procedural and diagnostic codes, which are not as specific as the currently adopted ICD-10 codes; hence, we are unable to distinguish between different forms of internal fixation, for example intramedullary nailing versus dynamic hip screw. This also precludes us from including other critical data such as degree of fracture displacement, cemented versus uncemented implantation, surgical approach for arthroplasty, and functional outcomes of individual patients. Moreover, the database used, although the largest inpatient sample available for analysis, represents only approximately 20% of hospitals nationwide. In addition, as patients cannot be tracked over time within the database, we are limited to outcomes in the perioperative period captured in a single hospital stay and cannot identify readmissions. Finally, our analysis is limited to the years 1990 to 2007 because of an increase in the relative standard error of the database in more recent years. Although this results in data that are not the most current, we believe that this study provides valuable insight regarding the trends in surgical treatment and acute postoperative outcomes of these injuries that have hitherto not been reported. To limit the inherent biases and the limitations within this study, prospective, randomized studies with long-term follow-up comparing outcomes across modes of treatment are needed to definitively determine the optimum form of treatment for this fracture type.
CONCLUSION
This is the largest study to date reporting on national trends in the surgical treatment and outcomes of the femoral neck fracture population. Orthopedic surgeons performing THA should be aware that the femoral neck fracture population is changing and at higher risk for perioperative complications. The advent of bisphosphonate therapy has been suggested as a possible reason for the decrease in fragility fractures and why a larger proportion of the femoral neck fracture population is now >80 years.36,37 With an aging population at a higher risk for perioperative complications, clinicians must take special care in choosing the appropriate surgical intervention that will give their patients the best functional outcome while minimizing the risk of surgical complications. Orthopedic surgeons should weigh the added risk associated with THA in this population.
1. Bishop J, Yang A, Githens M, Sox AH. Evaluation of contemporary trends in femoral neck fracture management reveals discrepancies in treatment. Geriatr Orthop Surg Rehabil. 2016;7(3):135. doi:10.1177/2151458516658328.
2. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res Off J Am Soc Bone Miner Res. 2007;22(3):465. doi:10.1359/jbmr.061113.
3. Kannus P, Parkkari J, Sievanen H, Heinonen A, Vuori I, Jarvinen M. Epidemiology of hip fractures. Bone. 1996;18(1 Suppl.):57s. doi:10.1016/8756-3282(95)00381-9.
4. Koval KJ, Zuckerman JD. Hip fractures: I. Overview and evaluation and treatment of femoral-neck fractures. J Am Acad Orthop Surg. 1994;2(3):141. doi:10.5435/00124635-199405000-00002.
5. Miller BJ, Callaghan JJ, Cram P, Karam M, Marsh JL, Noiseux NO. Changing trends in the treatment of femoral neck fractures: a review of the American Board of Orthopaedic Surgery database. J Bone Joint Surg. (American) 2014;96(17):e149. doi:10.2106/JBJS.M.01122.
6. Miller BJ, Lu X, Cram P. The trends in treatment of femoral neck fractures in the Medicare population from 1991 to 2008. J Bone Joint Surg. (American) 2013;95(18):e132. doi:10.2106/JBJS.L.01163.
7. Nwachukwu BU, McCormick F, Provencher MT, Roche M, Rubash HE. A comprehensive analysis of Medicare trends in utilization and hospital economics for total knee and hip arthroplasty from 2005 to 2011. J Arthroplast. 2015;30(1):15. doi:10.1016/j.arth.2014.08.025.
8. Su EP, Su SL. Femoral neck fractures: a changing paradigm. Bone Joint J. 2014;96-b(11) Supple A):43. doi:10.1302/0301-620X.96B11.34334.
9. Ahn J, Man LX, Park S, Sodl JF, Esterhai JL. Systematic review of cemented and uncemented hemiarthroplasty outcomes for femoral neck fractures. Clin Orthop Relat Res. 2008;466(10):2513. doi:10.1007/s11999-008-0368-3.
10. Alolabi B, Bajammal S, Shirali J, Karanicolas PJ, Gafni A, Bhandari M. Treatment of displaced femoral neck fractures in the elderly: a cost-benefit analysis. J Orthop Trauma. 2009;23(6):442. doi:10.1097/BOT.0b013e31817614dd.
11. Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290. doi:10.1093/aje/kwp266.
12. Brox WT, Chan PH, Cafri G, Inacio MC. Similar mortality with general or regional anesthesia in elderly hip fracture patients. Acta Orthop. 2016;87(2):152. doi:10.3109/17453674.2015.1128781.
13. Catal B, Sener M. Treatment and displacement affect the reoperation rate for femoral neck fracture. Clin Orthop Relat Res. 2013;471(12):4096. doi:10.1007/s11999-013-3295-x.
14. Dailiana Z, Papakostidou I, Varitimidis S, Michalitsis S, Veloni A, Malizos K. Surgical treatment of hip fractures: factors influencing mortality. Hippokratia. 2013;17(3):252.
15. Deangelis JP, Ademi A, Staff I, Lewis CG. Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures: a prospective randomized trial with early follow-up. J Orthop Trauma. 2012;26(3):135. doi:10.1097/BOT.0b013e318238b7a5.
16. Hedbeck CJ, Inngul C, Blomfeldt R, Ponzer S, Tornkvist H, Enocson A. Internal fixation versus cemented hemiarthroplasty for displaced femoral neck fractures in patients with severe cognitive dysfunction: a randomized controlled trial. J Orthop Trauma. 2013;27(12):690. doi:10.1097/BOT.0b013e318291f544.
17. Jia Z, Ding F, Wu Y, et al. Unipolar versus bipolar hemiarthroplasty for displaced femoral neck fractures: a systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2015;10:8. doi:10.1186/s13018-015-0165-0.
18. Lapidus LJ, Charalampidis A, Rundgren J, Enocson A. Internal fixation of garden I and II femoral neck fractures: posterior tilt did not influence the reoperation rate in 382 consecutive hips followed for a minimum of 5 years. J Orthop Trauma. 2013;27(7):386. doi:10.1097/BOT.0b013e318281da6e.
19. Mariconda M, Costa GG, Cerbasi S, et al. Factors predicting mobility and the change in Activities of Daily Living After hip fracture: A 1-year prospective cohort study. J Orthop Trauma. 2016;30(2):71. doi:10.1097/BOT.0000000000000448.
20. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality After proximal femoral fracture: A retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg. (American) 2015;97(16):1333. doi:10.2106/JBJS.O.00029.
21. Samuel AM, Russo GS, Lukasiewicz AM, et al. Surgical treatment of femoral neck fractures after 24 hours in patients between the ages of 18 and 49 is associated with poor inpatient outcomes: an analysis of 1361 patients in the National Trauma Data Bank. J Orthop Trauma. 2016;30(2):89. doi:10.1097/BOT.0000000000000456.
22. Yu L, Wang Y, Chen J. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures: meta-analysis of randomized trials. Clin Orthop Relat Res. 2012;470(8):2235. doi:10.1007/s11999-012-2293-8.
23. Zi-Sheng A, You-Shui G, Zhi-Zhen J, Ting Y, Chang-Qing Z. Hemiarthroplasty vs primary total hip arthroplasty for displaced fractures of the femoral neck in the elderly: a meta-analysis. J Arthroplast. 2012;27(4):583. doi:10.1016/j.arth.2011.07.009.
24. Zielinski SM, Keijsers NL, Praet SF, et al. Functional outcome after successful internal fixation versus salvage arthroplasty of patients with a femoral neck fracture. J Orthop Trauma. 2014;28(12):e273. doi:10.1097/BOT.0000000000000123.
25. Gu Q, Koenig L, Mather RC, 3rd, Tongue J. Surgery for hip fracture yields societal benefits that exceed the direct medical costs. Clin Orthop Relat Res. 2014;472(11):3536. doi:10.1007/s11999-014-3820-6.
26. Forsh DA, Ferguson TA. Contemporary management of femoral neck fractures: the young and the old. Curr Rev Musculoskelet Med. 2012;5(3):214. doi:10.1007/s12178-012-9127-x.
27. Macaulay W, Pagnotto MR, Iorio R, Mont MA, Saleh KJ. Displaced femoral neck fractures in the elderly: hemiarthroplasty versus total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(5):287. doi:10.5435/00124635-200605000-00004.
28. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: Femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596. doi:10.5435/00124635-200810000-00005.
29. Probe R, Ward R. Internal fixation of femoral neck fractures. J Am Acad Orthop Surg. 2006;14(9):565. doi:10.5435/00124635-200609000-00006.
30. Jain NB, Losina E, Ward DM, Harris MB, Katz JN. Trends in surgical management of femoral neck fractures in the United States. Clin Orthop Relat Res. 2008;466(12):3116. doi:10.1007/s11999-008-0392-3.
31. Menendez ME, Neuhaus V, van Dijk CN, Ring D. The Elixhauser comorbidity method outperforms the Charlson index in predicting inpatient death after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(9):2878. doi:10.1007/s11999-014-3686-7.
32. Van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser Comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633.
33. Best MJ, Buller LT, Falakassa J, Vecchione D. Risk factors for nonroutine discharge in patients undergoing spinal fusion for intervertebral disc disorders. Iowa Orthop J. 2015;35:147.
34. Schairer WW, Lane JM, Halsey DA, Iorio R, Padgett DE, McLawhorn AS. The Frank Stinchfield award: total hip arthroplasty for femoral neck fracture is not a typical DRG 470: A propensity-matched cohort study. Clin Orthop Relat Res. 2017;475(2):353-360. doi:10.1007/s11999-016-4868-2.
35. Nikkel LE, Fox EJ, Black KP, Davis C, Andersen L, Hollenbeak CS. Impact of comorbidities on hospitalization costs following hip fracture. J Bone Joint Surg Am. 2012;94(1):9. doi:10.2106/JBJS.J.01077.
36. Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009;122(2 Suppl.):S14. doi:10.1016/j.amjmed.2008.12.003.
37. Siris ES, Pasquale MK, Wang Y, Watts NB. Estimating bisphosphonate use and fracture reduction among US women aged 45 years and older, 2001-2008. J Bone Miner Res Off J Am Soc Bone Miner Res. 2011;26(1):3. doi:10.1002/jbmr.189.
ABSTRACT
The ideal mode of fixation for patients with femoral neck fractures is not well defined in the current literature. This study describes the recent trends in surgical management of femoral neck fractures with an analysis on perioperative outcomes.
The National Hospital Discharge Survey was used to identify femoral neck fractures in the United States between 1990 and 2007 (n = 1,155,960) treated with open reduction and internal fixation (ORIF), total hip arthroplasty (THA), or hemiarthroplasty (HA). Trends were examined over the following 3 time periods: 1990 to 1995 (group 1), 1996 to 2001 (group 2), and 2002 to 2007 (group 3). Elixhauser Comorbidity Index and perioperative complications were calculated.
Use of HA increased (74.4% to 84.6%), whereas that of THA (7.3% to 4.9%) and ORIF (18.3% to 10.6%) decreased, from group 1 to group 3 in the age group of >80 years. The use of ORIF increased (63.9% to 81.4%), whereas the use of both HA and THA decreased, from group 1 to group 3 in the age group of <50 years. The rate of adverse events increased across all fixation types but was greatest among THA (32.2% to 48.3%).
The femoral neck patient population is now older and has more medical comorbidities. We observed a trend toward performing HA in older patients and ORIF in younger patients. Despite superior functional outcomes reported in THA, this study found a decreased utilization of THA in all age groups along with an increase in adverse events and nonroutine discharges for patients with femoral neck fractures treated with THA.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Continue to: Femoral neck fractures...
Femoral neck fractures are a common occurrence in the United States. A recent study estimated an incidence of >63 per 100,000 population.1-8 Although the incidence appears to have decreased over recent decades, there is a projected exponential increase in the incidence of hip fractures over the next 30 years in the baby boomer population.8,9 Given that these fractures have a significant impact on patient morbidity, mortality, and quality of life, research efforts have been directed toward optimizing the treatment of affected patients and improving the outcomes.4,9-24
The treatment of choice for femoral neck fractures and the use of total hip arthroplasty (THA)11 have been a topic of debate.4,9,10,15-17,22,25 Total hip arthroplasty has been advocated for younger, more active patients, whereas hemiarthroplasty (HA) has been reserved for patients who are older and less active. Although several studies have demonstrated that arthroplasty outperforms open reduction and internal fixation (ORIF) in the elderly population with displaced femoral neck fractures, ORIF is still commonly performed in the United States for nondisplaced fractures and in patients aged <50 years.26-29
In an attempt to quantify the use of THA in the treatment of femoral neck fractures and demonstrate the national trends, Miller and colleagues5 pooled the American Board of Orthopaedic Surgery (ABOS) database and analyzed the treatment trends of surgeons taking part II of the ABOS examination from 1999 to 2011. The authors found an increased utilization of THA by recently graduated orthopedic surgeons. In contrast, Jain and colleagues30 found different national trends when they analyzed data from the National Inpatient Sample containing data between 1990 and 2001 and further found decreased utilization of THA procedures by orthopedic surgeons of all levels of training nationwide. However, neither of these studies reported about the trends in demographics, comorbidities, risk factors, or outcomes in this patient population following surgery.
The purpose of this study was to help clarify the findings of these authors using the largest dataset to date and also report on the perioperative complications associated with each mode of fixation in patients who undergo operative treatment for femoral neck fractures in the United States. Our hypotheses were that the femoral neck fracture patient population has become older and has more medical comorbidities. We also hypothesized that there has been a trend toward performing fewer THA procedures in the United States and that THA is associated with increased perioperative complications compared to those with HA and ORIF.
MATERIALS AND METHODS
We conducted a retrospective epidemiological study using the National Hospital Discharge Survey (NHDS) on surgical trends in the management of femoral neck fractures. The NHDS is a publicly available survey that is conducted annually to provide data of nonfederal, short-stay hospitals to the public. The sample data are weighted to provide nationwide estimates of annual inpatient care. The NHDS includes up to 7 medical diagnoses and 4 procedural codes per case, which are categorized using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes, that were collected along with patient demographic information, length of stay (LOS), and discharge disposition. The diagnostic and procedural codes used for this study are presented in the Appendix. The year 2007 was chosen as the endpoint of this study due to the fact that the relative standard error of the NHDS doubled in 2008 as a result of a decrease in its survey size. As this is a publicly available database, our study was exempt from institutional review board approval.

Continue to: All pateints admitted...
All patients admitted with a primary diagnosis of closed transcervical fracture of the femoral neck (ICD-9-CM 820.0x) were selected. This resulted in 1,674,160 fractures. All patients with fractures with a concurrent primary procedural code of ORIF (79.35), HA (81.52), or THA (81.51) were identified, resulting in a total sample size of 1,155,960 surgical fractures. Analysis of the fractures based on additional specificity,ie subcapital versus midcervical versus basicervical, was not carried out because >90% of femoral neck fractures in the database were coded as “unspecified” or “other” (ICD9 CM 820.00 and 820.09, respectively).
Comorbidity burden was quantified using Elixhauser coding algorithms as previously described.31 The Elixhauser comorbidity measure is a model consisting of 31 conditions and has recently been identified as a better predictor of mortality in patients undergoing orthopedic procedures when compared with the Charlson Comorbidity Index.31 Dichotomous variables for each Elixhauser comorbidity were created, and χ2 tests were utilized to assess the association between each comorbidity and mortality. The weighted Elixhauser score for each statistically significant comorbidity was calculated as described by van Walraven and colleagues.32 The Elixhauser comorbidity score was then calculated for each patient by summing the individual weights of all comorbidities. Postoperative adverse events were determined using the complication-screening-package as previously described.33
All adverse events were categorized into 3 categories, including general medical complications, mechanical complications, and surgical complications. All adverse events recorded in the NHDS database are events that occurred during a single hospitalization. Therefore, it does not take into account adverse events that occurred after discharge, and, for example, mortality refers to postoperative mortality that occurs prior to discharge. The study period comprised data captured from 1990 to 2007, and 3 groups were generated from this time period to better characterize patients throughout the large study time frame. Group 1 comprised patients who underwent surgical management of femoral neck fractures from 1990 to 1995, group 2 consisted of patients treated from 1996 to 2001, and group 3 included patients treated from 2002 to 2007.
Categorical data were analyzed using the χ2 test, and continuous data were analyzed by the independent-samples t test and ANOVA. Multivariable binary logistic regression analyses were performed to assess the contributions of individual comorbidities to mortality, adverse events, and nonroutine discharge. Elixhauser comorbidities with a P value of < .10 in the bivariate analysis and presenting in at least 0.2% of the population were included in the logistic regression.31 Odds ratios and confidence intervals were calculated to assess the association between comorbidities and our dichotomous variables. A P value of < .001 defined statistical significance.33 Statistical analysis was conducted using SPSS version 21 (IBM).
RESULTS
Patient Demographics
Our query demonstrated a total of 1,155,960 patients who underwent surgical fixation of femoral neck fractures (Table 1). The most commonly used treatment modality was HA (75%), followed by ORIF (18%) and later by THA (7%). The majority of patients were females in each treatment group. Patients’ age varied according to treatment group, with patients undergoing HA having a mean age of 81.0 ± 9.0 years, patients undergoing ORIF having a mean age of 75.0 ± 17.0 years, and those undergoing THA having a mean age of 79.0 ± 10.0 years (P < .001). The majority of patients were ≥80 years in all treatment groups, but the ORIF group had the greatest proportion of patients <65 years (P < .001). Among patients undergoing HA, 62.4% were ≥80 years, while the ORIF and HA groups consisted of 48.6% and 51.5% of patients in that same age group, respectively.

Continue to: TRENDS ANALYSIS
TRENDS ANALYSIS
There was a significant change in the distributions of the procedures performed according to age group over time. Patients >80 years continued to undergo primarily HA, with an increase from 74.4% during 1990 to 1995 up to 84.6% during the 2002 to 2007 period and a concomitant decrease in ORIF from 18.3% to 10.6% during the same time period in this age group. Surgical trends in patients 65 to 79 years demonstrated a significant decrease in management with ORIF from 19.1% in 1990 to 1995 to 16.8% in the 2002 to 2007 cohort (P < .001 for all, Table 2). There was an increase in the use of HA from 71.9% during the 1990 to 1995 period to 75.5% during the final study period (Table 2, Figure 1). The use of THA for all age groups decreased between 1990 and 2007, except for the 50- to 64-year-old group where THA utilization remained constant.

Management patterns in patients 50 to 64 years varied throughout the analysis and demonstrated the following trend: treatment with HA remained the most common technique used but varied slightly from 59.7% during 1990 to 1995 to 60.3% during 2002 to2007 (P < .001, Table 2). The second most common treatment used was ORIF, which decreased from 32.2% to 31.5% (P < .001, Table 2). The use of THA varied significantly from 8.2% among those managed during 1990 to 1995 to 11.7% during 1996 to 2001 but later declined to the initial 8.2% (P < .001, Table 2).

Analysis of patients ≤49 years demonstrated that ORIF was the preferred technique, which experienced a growth from 63.9% during 1990 to 1995 to 81.4% during the 2002 to 2007 period (P < .001, Table 2). A decreased use in THA was observed from 2.0% in the initial period to 0.6% in the final period (P < .001, Table 2). Use of HA decreased from 34.0% in 1990 to 1995 to 18.0% in 2002 to 2007 (P < .001, Table 2).
LENGTH OF STAY
Mean number of in-hospital days decreased throughout the study period for all treatment techniques. During the 1990 to 1995 study period, patients who underwent ORIF had a mean LOS of 8 ± 7 days, which decreased (P < .001, Table 2) to 6 ± 3 days in 1996 to 2001 and remained constant during 2002 to 2007 (mean 6 ± 4 days). This decrease in LOS was also observed in patients who underwent THA (P < .001, Table 2), who initially had a mean LOS of 11 ± 7 days during 1990 to 1995, which later decreased to 7 ± 5 days for the remainder of the study. The LOS for patients who underwent HA also decreased (P < .001, Table 2), which initially was reported to be 11 ± 11 days during 1990 to 1995, decreasing to 7 ±7 days in 1996–2001 and later to 6 ± 4 days in 2002 to 2007.
COMORBIDITIY ANALYSIS
The Elixhauser Comorbidity Index varied significantly among groups over time (P < .001, Table 2). Overall mean Elixhauser Comorbidity Index score per procedure type is provided in Table 1, with HA patients having the highest score (-0.15 ± 13.09, p<.001).
Continue to: Analysis of the preoperative comorbidities...
Analysis of the preoperative comorbidities demonstrated significant differences among each surgical treatment group (P < .001 for all, Table 3). The most common comorbidities in patients who underwent HA were uncomplicated hypertension (33.2%), fluid/electrolyte disorders (17.4%), chronic pulmonary disease (14.9%), and congestive heart failure (13.7%). The most common comorbidities in the ORIF group were uncomplicated hypertension (30.8%), fluid/electrolyte disorders (14.5%), chronic pulmonary disease (14.0%), and uncomplicated diabetes (10.9%). Patients treated with THA had most commonly uncomplicated hypertension (30.1%), fluid/electrolyte disorders (17.2%), uncomplicated diabetes (15.5%), and chronic pulmonary disease (14.4%). The prevalence of comorbidities is displayed in Table 3.

DISCHARGE STATUS
Mortality varied significantly, being lowest in those who underwent ORIF (0.8%), followed those who underwent THA (1.8%), and HA (2.6%) (P < .001, Table 1).
The majority of patients in each group were discharged to long-term rehabilitation facilities, including 53.0% of those treated with HA, 40.4% of those treated with ORIF, and 44.3% of patients treated with THA. The second most common discharge location was home, which included 14.8% of patients who underwent HA, 32.2% of patients treated with ORIF, and 20.8% of those who underwent THA. Table 3 demonstrates the details of the discharge settings.

Mortality analysis over time demonstrated a significant decrease in each treatment group (P < .001). Mortality in the ORIF group decreased from 1.2% during 1990 to 1995 to 0.8% in 2002 to 2007. Mortality in the THA group also decreased significantly from 0.8% during 1990 to 1995 to 0.5% during the 2002 to 2007 time period. Patients who underwent HA also exhibited a decrease in mortality rate from 3.3% during 1990 to 1995 to 2.2% during 2002 to 2007 (P < .001, Table 4, Figure 2).

GENERAL ADVERSE EVENTS
There was a significant difference (P < .001) in the percentage of adverse events experienced, the maximum being observed in the THA group (41.0%), followed by the HA group (37.9%) and trailed by the ORIF group (20.3%, (P < .001, Table 1). The prevalence of adverse events is detailed in Table 5.

Continue to: Patients who underwent THA...
Patients who underwent THA had the highest rate of any adverse event, LOS, and transfusion rate (Table 1 and Table 5).
The prevalence of postoperative pneumonia was highest in the HA group (3.4%), followed by the ORIF group (2.9%), and the THA group (2.6%) (P < .001, Table 5). There was also a significant difference in rates of intubation, pulmonary insufficiency, acute renal failure, pulmonary embolism, acute myocardial infarction, induced mental disorder, and deep venous thrombosis (P < .001 for all, Table 5).
SURGERY-RELATED ADVERSE EVENTS
Surgery-related outcomes over the entire study period were significantly different according to the type of procedure performed (P < .001, Table 5). Patients who underwent HA had the highest rate of acute postoperative anemia (20.2%), followed by those who underwent THA (19.7%), and ORIF (10.2%). Postoperative bleeding rates also varied significantly, with 1.2% in the HA group, followed by 1.0% in the ORIF group and 0.4% in the THA group (P < .001, Table 5). Acute postoperative infection rates also varied significantly, with the highest rate being observed in the HA group (0.6%) compared to that in the THA and ORIF groups (both 0.3%) (P < .001, Table 5).

Table 6, Table 7, and Table 8 detail the results of regression analyses in patients with femoral neck fractures for individual risk factors associated with mortality, any adverse event, and nonroutine discharge to a short- or long-term rehabilitation facility, respectively. Increasing age (50–64 years, OR: 0.238; 65–79 years, OR: 1.762; and ≥80 years, OR: 2.700), THA (OR: 1.743), and HA (OR: 2.574) were found to be independent risk factors for mortality in the perioperative period (P < .001 for each, Table 6). Increasing age (50–64 years, OR: 1.888; 65–79 years, OR: 2.983; and ≥80 years, OR: 3.722), THA (OR: 2.489), and HA (OR: 2.098) were also found to be independent risk factors for any adverse event in the perioperative period (P < .001, Table 7). Age (50–64 years, OR: 1.662; 65–79 years, OR: 4.320; and ≥80 years, OR: 7.102) was the best predictor for nonroutine discharge to a short- or long-term rehabilitation facility (P < .001, Table 8).

DISCUSSION
Femoral neck fractures in the elderly population present a significant financial burden to the healthcare system.1-3,24,25 Consistent with previous epidemiological studies, our results show that the femoral neck fracture population has become older and has more medical comorbidities over the last 3 decades.27,28. Similarly, we also found that the rate of medical, surgical, and mechanical perioperative complications has increased in the same time period. Interestingly, the mortality rate has remained relatively similar.

Continue to: Although patients undergoing HA...
Although patients undergoing HA for femoral neck fractures are older and have more medical comorbidities, we found that the rate of adverse events in the perioperative period for patients undergoing THA was higher than that in the HA group. Consistent with prior studies, patients who underwent THA had higher rates of blood transfusion, pulmonary embolism, and induced mental disorders.34 Multivariable regression analysis demonstrated that after controlling for age, medical comorbidity, and type of surgery performed, THA emerged as an independent risk factor for any adverse event in the perioperative period. Increased anesthesia time, reaming of the acetabulum, and increased complexity of surgery probably account for these changes.
Our study results are consistent with those of Jain and colleagues,30 which showed a decrease in utilization of THA for femoral neck fractures between 1990 and 2001. Since THA is generally indicated for younger, more active patients in relatively good health, this would explain why changes in baseline health in this cohort over the last 20 years would lead to fewer THA procedures being performed. Surgeons in the US may be finding there are fewer patients who are candidates for THA. Miller and colleagues5 reported conflicting results and showed an increase in THA utilization in this patient population. However, their study evaluated treatment trends based on data from the ABOS part II of recently graduated orthopedic surgeons and may not be an accurate representation of national practice trends in the US. The trend toward increased subspecialization may explain their findings. As the authors noted, although they found an increase in the use of THA for femoral neck fractures by new adult reconstruction surgeons, the percentage of new surgeons treating femoral neck fractures has declined.5
Our analysis showed very concrete trends in treatment management at the extremes of the age ranges. There were substantial increases in the use of ORIF for patients <50 years (from 63.9% in 1990–1995 to 81.4% in 2002–2007, P < .001) and in the use of HA for patients >80 years (from 74.4% in 1990–1995 to 84.6% in 2002–2007, P < .001). This trend parallels recent studies that purport better outcomes for young patients undergoing ORIF and elderly patients undergoing HA.30 Our analysis did not demonstrate a large shift in surgeon preference for treatment of patients between 50 and 80 years, although there was a statistically significant decrease in ORIF and THA usage and a reflective increase in HA usage in this population as well. The fact that there has not been as substantial a shift in treatment trends for this large age group is potentially due to the wide variations in comorbid conditions and the functionality that abounds in this age group.1
The limitations of the current study are those inherent with a retrospective database analysis. The reliance on accurate coding brings up a potential for error; however, it is unlikely that comorbidities and outcomes are undercoded as hospitals are incentivized to input values that increase the acuity and thus reimbursement for each hospital stay.35 The database also relies on the ICD-9 procedural and diagnostic codes, which are not as specific as the currently adopted ICD-10 codes; hence, we are unable to distinguish between different forms of internal fixation, for example intramedullary nailing versus dynamic hip screw. This also precludes us from including other critical data such as degree of fracture displacement, cemented versus uncemented implantation, surgical approach for arthroplasty, and functional outcomes of individual patients. Moreover, the database used, although the largest inpatient sample available for analysis, represents only approximately 20% of hospitals nationwide. In addition, as patients cannot be tracked over time within the database, we are limited to outcomes in the perioperative period captured in a single hospital stay and cannot identify readmissions. Finally, our analysis is limited to the years 1990 to 2007 because of an increase in the relative standard error of the database in more recent years. Although this results in data that are not the most current, we believe that this study provides valuable insight regarding the trends in surgical treatment and acute postoperative outcomes of these injuries that have hitherto not been reported. To limit the inherent biases and the limitations within this study, prospective, randomized studies with long-term follow-up comparing outcomes across modes of treatment are needed to definitively determine the optimum form of treatment for this fracture type.
CONCLUSION
This is the largest study to date reporting on national trends in the surgical treatment and outcomes of the femoral neck fracture population. Orthopedic surgeons performing THA should be aware that the femoral neck fracture population is changing and at higher risk for perioperative complications. The advent of bisphosphonate therapy has been suggested as a possible reason for the decrease in fragility fractures and why a larger proportion of the femoral neck fracture population is now >80 years.36,37 With an aging population at a higher risk for perioperative complications, clinicians must take special care in choosing the appropriate surgical intervention that will give their patients the best functional outcome while minimizing the risk of surgical complications. Orthopedic surgeons should weigh the added risk associated with THA in this population.
ABSTRACT
The ideal mode of fixation for patients with femoral neck fractures is not well defined in the current literature. This study describes the recent trends in surgical management of femoral neck fractures with an analysis on perioperative outcomes.
The National Hospital Discharge Survey was used to identify femoral neck fractures in the United States between 1990 and 2007 (n = 1,155,960) treated with open reduction and internal fixation (ORIF), total hip arthroplasty (THA), or hemiarthroplasty (HA). Trends were examined over the following 3 time periods: 1990 to 1995 (group 1), 1996 to 2001 (group 2), and 2002 to 2007 (group 3). Elixhauser Comorbidity Index and perioperative complications were calculated.
Use of HA increased (74.4% to 84.6%), whereas that of THA (7.3% to 4.9%) and ORIF (18.3% to 10.6%) decreased, from group 1 to group 3 in the age group of >80 years. The use of ORIF increased (63.9% to 81.4%), whereas the use of both HA and THA decreased, from group 1 to group 3 in the age group of <50 years. The rate of adverse events increased across all fixation types but was greatest among THA (32.2% to 48.3%).
The femoral neck patient population is now older and has more medical comorbidities. We observed a trend toward performing HA in older patients and ORIF in younger patients. Despite superior functional outcomes reported in THA, this study found a decreased utilization of THA in all age groups along with an increase in adverse events and nonroutine discharges for patients with femoral neck fractures treated with THA.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Continue to: Femoral neck fractures...
Femoral neck fractures are a common occurrence in the United States. A recent study estimated an incidence of >63 per 100,000 population.1-8 Although the incidence appears to have decreased over recent decades, there is a projected exponential increase in the incidence of hip fractures over the next 30 years in the baby boomer population.8,9 Given that these fractures have a significant impact on patient morbidity, mortality, and quality of life, research efforts have been directed toward optimizing the treatment of affected patients and improving the outcomes.4,9-24
The treatment of choice for femoral neck fractures and the use of total hip arthroplasty (THA)11 have been a topic of debate.4,9,10,15-17,22,25 Total hip arthroplasty has been advocated for younger, more active patients, whereas hemiarthroplasty (HA) has been reserved for patients who are older and less active. Although several studies have demonstrated that arthroplasty outperforms open reduction and internal fixation (ORIF) in the elderly population with displaced femoral neck fractures, ORIF is still commonly performed in the United States for nondisplaced fractures and in patients aged <50 years.26-29
In an attempt to quantify the use of THA in the treatment of femoral neck fractures and demonstrate the national trends, Miller and colleagues5 pooled the American Board of Orthopaedic Surgery (ABOS) database and analyzed the treatment trends of surgeons taking part II of the ABOS examination from 1999 to 2011. The authors found an increased utilization of THA by recently graduated orthopedic surgeons. In contrast, Jain and colleagues30 found different national trends when they analyzed data from the National Inpatient Sample containing data between 1990 and 2001 and further found decreased utilization of THA procedures by orthopedic surgeons of all levels of training nationwide. However, neither of these studies reported about the trends in demographics, comorbidities, risk factors, or outcomes in this patient population following surgery.
The purpose of this study was to help clarify the findings of these authors using the largest dataset to date and also report on the perioperative complications associated with each mode of fixation in patients who undergo operative treatment for femoral neck fractures in the United States. Our hypotheses were that the femoral neck fracture patient population has become older and has more medical comorbidities. We also hypothesized that there has been a trend toward performing fewer THA procedures in the United States and that THA is associated with increased perioperative complications compared to those with HA and ORIF.
MATERIALS AND METHODS
We conducted a retrospective epidemiological study using the National Hospital Discharge Survey (NHDS) on surgical trends in the management of femoral neck fractures. The NHDS is a publicly available survey that is conducted annually to provide data of nonfederal, short-stay hospitals to the public. The sample data are weighted to provide nationwide estimates of annual inpatient care. The NHDS includes up to 7 medical diagnoses and 4 procedural codes per case, which are categorized using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes, that were collected along with patient demographic information, length of stay (LOS), and discharge disposition. The diagnostic and procedural codes used for this study are presented in the Appendix. The year 2007 was chosen as the endpoint of this study due to the fact that the relative standard error of the NHDS doubled in 2008 as a result of a decrease in its survey size. As this is a publicly available database, our study was exempt from institutional review board approval.

Continue to: All pateints admitted...
All patients admitted with a primary diagnosis of closed transcervical fracture of the femoral neck (ICD-9-CM 820.0x) were selected. This resulted in 1,674,160 fractures. All patients with fractures with a concurrent primary procedural code of ORIF (79.35), HA (81.52), or THA (81.51) were identified, resulting in a total sample size of 1,155,960 surgical fractures. Analysis of the fractures based on additional specificity,ie subcapital versus midcervical versus basicervical, was not carried out because >90% of femoral neck fractures in the database were coded as “unspecified” or “other” (ICD9 CM 820.00 and 820.09, respectively).
Comorbidity burden was quantified using Elixhauser coding algorithms as previously described.31 The Elixhauser comorbidity measure is a model consisting of 31 conditions and has recently been identified as a better predictor of mortality in patients undergoing orthopedic procedures when compared with the Charlson Comorbidity Index.31 Dichotomous variables for each Elixhauser comorbidity were created, and χ2 tests were utilized to assess the association between each comorbidity and mortality. The weighted Elixhauser score for each statistically significant comorbidity was calculated as described by van Walraven and colleagues.32 The Elixhauser comorbidity score was then calculated for each patient by summing the individual weights of all comorbidities. Postoperative adverse events were determined using the complication-screening-package as previously described.33
All adverse events were categorized into 3 categories, including general medical complications, mechanical complications, and surgical complications. All adverse events recorded in the NHDS database are events that occurred during a single hospitalization. Therefore, it does not take into account adverse events that occurred after discharge, and, for example, mortality refers to postoperative mortality that occurs prior to discharge. The study period comprised data captured from 1990 to 2007, and 3 groups were generated from this time period to better characterize patients throughout the large study time frame. Group 1 comprised patients who underwent surgical management of femoral neck fractures from 1990 to 1995, group 2 consisted of patients treated from 1996 to 2001, and group 3 included patients treated from 2002 to 2007.
Categorical data were analyzed using the χ2 test, and continuous data were analyzed by the independent-samples t test and ANOVA. Multivariable binary logistic regression analyses were performed to assess the contributions of individual comorbidities to mortality, adverse events, and nonroutine discharge. Elixhauser comorbidities with a P value of < .10 in the bivariate analysis and presenting in at least 0.2% of the population were included in the logistic regression.31 Odds ratios and confidence intervals were calculated to assess the association between comorbidities and our dichotomous variables. A P value of < .001 defined statistical significance.33 Statistical analysis was conducted using SPSS version 21 (IBM).
RESULTS
Patient Demographics
Our query demonstrated a total of 1,155,960 patients who underwent surgical fixation of femoral neck fractures (Table 1). The most commonly used treatment modality was HA (75%), followed by ORIF (18%) and later by THA (7%). The majority of patients were females in each treatment group. Patients’ age varied according to treatment group, with patients undergoing HA having a mean age of 81.0 ± 9.0 years, patients undergoing ORIF having a mean age of 75.0 ± 17.0 years, and those undergoing THA having a mean age of 79.0 ± 10.0 years (P < .001). The majority of patients were ≥80 years in all treatment groups, but the ORIF group had the greatest proportion of patients <65 years (P < .001). Among patients undergoing HA, 62.4% were ≥80 years, while the ORIF and HA groups consisted of 48.6% and 51.5% of patients in that same age group, respectively.

Continue to: TRENDS ANALYSIS
TRENDS ANALYSIS
There was a significant change in the distributions of the procedures performed according to age group over time. Patients >80 years continued to undergo primarily HA, with an increase from 74.4% during 1990 to 1995 up to 84.6% during the 2002 to 2007 period and a concomitant decrease in ORIF from 18.3% to 10.6% during the same time period in this age group. Surgical trends in patients 65 to 79 years demonstrated a significant decrease in management with ORIF from 19.1% in 1990 to 1995 to 16.8% in the 2002 to 2007 cohort (P < .001 for all, Table 2). There was an increase in the use of HA from 71.9% during the 1990 to 1995 period to 75.5% during the final study period (Table 2, Figure 1). The use of THA for all age groups decreased between 1990 and 2007, except for the 50- to 64-year-old group where THA utilization remained constant.

Management patterns in patients 50 to 64 years varied throughout the analysis and demonstrated the following trend: treatment with HA remained the most common technique used but varied slightly from 59.7% during 1990 to 1995 to 60.3% during 2002 to2007 (P < .001, Table 2). The second most common treatment used was ORIF, which decreased from 32.2% to 31.5% (P < .001, Table 2). The use of THA varied significantly from 8.2% among those managed during 1990 to 1995 to 11.7% during 1996 to 2001 but later declined to the initial 8.2% (P < .001, Table 2).

Analysis of patients ≤49 years demonstrated that ORIF was the preferred technique, which experienced a growth from 63.9% during 1990 to 1995 to 81.4% during the 2002 to 2007 period (P < .001, Table 2). A decreased use in THA was observed from 2.0% in the initial period to 0.6% in the final period (P < .001, Table 2). Use of HA decreased from 34.0% in 1990 to 1995 to 18.0% in 2002 to 2007 (P < .001, Table 2).
LENGTH OF STAY
Mean number of in-hospital days decreased throughout the study period for all treatment techniques. During the 1990 to 1995 study period, patients who underwent ORIF had a mean LOS of 8 ± 7 days, which decreased (P < .001, Table 2) to 6 ± 3 days in 1996 to 2001 and remained constant during 2002 to 2007 (mean 6 ± 4 days). This decrease in LOS was also observed in patients who underwent THA (P < .001, Table 2), who initially had a mean LOS of 11 ± 7 days during 1990 to 1995, which later decreased to 7 ± 5 days for the remainder of the study. The LOS for patients who underwent HA also decreased (P < .001, Table 2), which initially was reported to be 11 ± 11 days during 1990 to 1995, decreasing to 7 ±7 days in 1996–2001 and later to 6 ± 4 days in 2002 to 2007.
COMORBIDITIY ANALYSIS
The Elixhauser Comorbidity Index varied significantly among groups over time (P < .001, Table 2). Overall mean Elixhauser Comorbidity Index score per procedure type is provided in Table 1, with HA patients having the highest score (-0.15 ± 13.09, p<.001).
Continue to: Analysis of the preoperative comorbidities...
Analysis of the preoperative comorbidities demonstrated significant differences among each surgical treatment group (P < .001 for all, Table 3). The most common comorbidities in patients who underwent HA were uncomplicated hypertension (33.2%), fluid/electrolyte disorders (17.4%), chronic pulmonary disease (14.9%), and congestive heart failure (13.7%). The most common comorbidities in the ORIF group were uncomplicated hypertension (30.8%), fluid/electrolyte disorders (14.5%), chronic pulmonary disease (14.0%), and uncomplicated diabetes (10.9%). Patients treated with THA had most commonly uncomplicated hypertension (30.1%), fluid/electrolyte disorders (17.2%), uncomplicated diabetes (15.5%), and chronic pulmonary disease (14.4%). The prevalence of comorbidities is displayed in Table 3.

DISCHARGE STATUS
Mortality varied significantly, being lowest in those who underwent ORIF (0.8%), followed those who underwent THA (1.8%), and HA (2.6%) (P < .001, Table 1).
The majority of patients in each group were discharged to long-term rehabilitation facilities, including 53.0% of those treated with HA, 40.4% of those treated with ORIF, and 44.3% of patients treated with THA. The second most common discharge location was home, which included 14.8% of patients who underwent HA, 32.2% of patients treated with ORIF, and 20.8% of those who underwent THA. Table 3 demonstrates the details of the discharge settings.

Mortality analysis over time demonstrated a significant decrease in each treatment group (P < .001). Mortality in the ORIF group decreased from 1.2% during 1990 to 1995 to 0.8% in 2002 to 2007. Mortality in the THA group also decreased significantly from 0.8% during 1990 to 1995 to 0.5% during the 2002 to 2007 time period. Patients who underwent HA also exhibited a decrease in mortality rate from 3.3% during 1990 to 1995 to 2.2% during 2002 to 2007 (P < .001, Table 4, Figure 2).

GENERAL ADVERSE EVENTS
There was a significant difference (P < .001) in the percentage of adverse events experienced, the maximum being observed in the THA group (41.0%), followed by the HA group (37.9%) and trailed by the ORIF group (20.3%, (P < .001, Table 1). The prevalence of adverse events is detailed in Table 5.

Continue to: Patients who underwent THA...
Patients who underwent THA had the highest rate of any adverse event, LOS, and transfusion rate (Table 1 and Table 5).
The prevalence of postoperative pneumonia was highest in the HA group (3.4%), followed by the ORIF group (2.9%), and the THA group (2.6%) (P < .001, Table 5). There was also a significant difference in rates of intubation, pulmonary insufficiency, acute renal failure, pulmonary embolism, acute myocardial infarction, induced mental disorder, and deep venous thrombosis (P < .001 for all, Table 5).
SURGERY-RELATED ADVERSE EVENTS
Surgery-related outcomes over the entire study period were significantly different according to the type of procedure performed (P < .001, Table 5). Patients who underwent HA had the highest rate of acute postoperative anemia (20.2%), followed by those who underwent THA (19.7%), and ORIF (10.2%). Postoperative bleeding rates also varied significantly, with 1.2% in the HA group, followed by 1.0% in the ORIF group and 0.4% in the THA group (P < .001, Table 5). Acute postoperative infection rates also varied significantly, with the highest rate being observed in the HA group (0.6%) compared to that in the THA and ORIF groups (both 0.3%) (P < .001, Table 5).

Table 6, Table 7, and Table 8 detail the results of regression analyses in patients with femoral neck fractures for individual risk factors associated with mortality, any adverse event, and nonroutine discharge to a short- or long-term rehabilitation facility, respectively. Increasing age (50–64 years, OR: 0.238; 65–79 years, OR: 1.762; and ≥80 years, OR: 2.700), THA (OR: 1.743), and HA (OR: 2.574) were found to be independent risk factors for mortality in the perioperative period (P < .001 for each, Table 6). Increasing age (50–64 years, OR: 1.888; 65–79 years, OR: 2.983; and ≥80 years, OR: 3.722), THA (OR: 2.489), and HA (OR: 2.098) were also found to be independent risk factors for any adverse event in the perioperative period (P < .001, Table 7). Age (50–64 years, OR: 1.662; 65–79 years, OR: 4.320; and ≥80 years, OR: 7.102) was the best predictor for nonroutine discharge to a short- or long-term rehabilitation facility (P < .001, Table 8).

DISCUSSION
Femoral neck fractures in the elderly population present a significant financial burden to the healthcare system.1-3,24,25 Consistent with previous epidemiological studies, our results show that the femoral neck fracture population has become older and has more medical comorbidities over the last 3 decades.27,28. Similarly, we also found that the rate of medical, surgical, and mechanical perioperative complications has increased in the same time period. Interestingly, the mortality rate has remained relatively similar.

Continue to: Although patients undergoing HA...
Although patients undergoing HA for femoral neck fractures are older and have more medical comorbidities, we found that the rate of adverse events in the perioperative period for patients undergoing THA was higher than that in the HA group. Consistent with prior studies, patients who underwent THA had higher rates of blood transfusion, pulmonary embolism, and induced mental disorders.34 Multivariable regression analysis demonstrated that after controlling for age, medical comorbidity, and type of surgery performed, THA emerged as an independent risk factor for any adverse event in the perioperative period. Increased anesthesia time, reaming of the acetabulum, and increased complexity of surgery probably account for these changes.
Our study results are consistent with those of Jain and colleagues,30 which showed a decrease in utilization of THA for femoral neck fractures between 1990 and 2001. Since THA is generally indicated for younger, more active patients in relatively good health, this would explain why changes in baseline health in this cohort over the last 20 years would lead to fewer THA procedures being performed. Surgeons in the US may be finding there are fewer patients who are candidates for THA. Miller and colleagues5 reported conflicting results and showed an increase in THA utilization in this patient population. However, their study evaluated treatment trends based on data from the ABOS part II of recently graduated orthopedic surgeons and may not be an accurate representation of national practice trends in the US. The trend toward increased subspecialization may explain their findings. As the authors noted, although they found an increase in the use of THA for femoral neck fractures by new adult reconstruction surgeons, the percentage of new surgeons treating femoral neck fractures has declined.5
Our analysis showed very concrete trends in treatment management at the extremes of the age ranges. There were substantial increases in the use of ORIF for patients <50 years (from 63.9% in 1990–1995 to 81.4% in 2002–2007, P < .001) and in the use of HA for patients >80 years (from 74.4% in 1990–1995 to 84.6% in 2002–2007, P < .001). This trend parallels recent studies that purport better outcomes for young patients undergoing ORIF and elderly patients undergoing HA.30 Our analysis did not demonstrate a large shift in surgeon preference for treatment of patients between 50 and 80 years, although there was a statistically significant decrease in ORIF and THA usage and a reflective increase in HA usage in this population as well. The fact that there has not been as substantial a shift in treatment trends for this large age group is potentially due to the wide variations in comorbid conditions and the functionality that abounds in this age group.1
The limitations of the current study are those inherent with a retrospective database analysis. The reliance on accurate coding brings up a potential for error; however, it is unlikely that comorbidities and outcomes are undercoded as hospitals are incentivized to input values that increase the acuity and thus reimbursement for each hospital stay.35 The database also relies on the ICD-9 procedural and diagnostic codes, which are not as specific as the currently adopted ICD-10 codes; hence, we are unable to distinguish between different forms of internal fixation, for example intramedullary nailing versus dynamic hip screw. This also precludes us from including other critical data such as degree of fracture displacement, cemented versus uncemented implantation, surgical approach for arthroplasty, and functional outcomes of individual patients. Moreover, the database used, although the largest inpatient sample available for analysis, represents only approximately 20% of hospitals nationwide. In addition, as patients cannot be tracked over time within the database, we are limited to outcomes in the perioperative period captured in a single hospital stay and cannot identify readmissions. Finally, our analysis is limited to the years 1990 to 2007 because of an increase in the relative standard error of the database in more recent years. Although this results in data that are not the most current, we believe that this study provides valuable insight regarding the trends in surgical treatment and acute postoperative outcomes of these injuries that have hitherto not been reported. To limit the inherent biases and the limitations within this study, prospective, randomized studies with long-term follow-up comparing outcomes across modes of treatment are needed to definitively determine the optimum form of treatment for this fracture type.
CONCLUSION
This is the largest study to date reporting on national trends in the surgical treatment and outcomes of the femoral neck fracture population. Orthopedic surgeons performing THA should be aware that the femoral neck fracture population is changing and at higher risk for perioperative complications. The advent of bisphosphonate therapy has been suggested as a possible reason for the decrease in fragility fractures and why a larger proportion of the femoral neck fracture population is now >80 years.36,37 With an aging population at a higher risk for perioperative complications, clinicians must take special care in choosing the appropriate surgical intervention that will give their patients the best functional outcome while minimizing the risk of surgical complications. Orthopedic surgeons should weigh the added risk associated with THA in this population.
1. Bishop J, Yang A, Githens M, Sox AH. Evaluation of contemporary trends in femoral neck fracture management reveals discrepancies in treatment. Geriatr Orthop Surg Rehabil. 2016;7(3):135. doi:10.1177/2151458516658328.
2. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res Off J Am Soc Bone Miner Res. 2007;22(3):465. doi:10.1359/jbmr.061113.
3. Kannus P, Parkkari J, Sievanen H, Heinonen A, Vuori I, Jarvinen M. Epidemiology of hip fractures. Bone. 1996;18(1 Suppl.):57s. doi:10.1016/8756-3282(95)00381-9.
4. Koval KJ, Zuckerman JD. Hip fractures: I. Overview and evaluation and treatment of femoral-neck fractures. J Am Acad Orthop Surg. 1994;2(3):141. doi:10.5435/00124635-199405000-00002.
5. Miller BJ, Callaghan JJ, Cram P, Karam M, Marsh JL, Noiseux NO. Changing trends in the treatment of femoral neck fractures: a review of the American Board of Orthopaedic Surgery database. J Bone Joint Surg. (American) 2014;96(17):e149. doi:10.2106/JBJS.M.01122.
6. Miller BJ, Lu X, Cram P. The trends in treatment of femoral neck fractures in the Medicare population from 1991 to 2008. J Bone Joint Surg. (American) 2013;95(18):e132. doi:10.2106/JBJS.L.01163.
7. Nwachukwu BU, McCormick F, Provencher MT, Roche M, Rubash HE. A comprehensive analysis of Medicare trends in utilization and hospital economics for total knee and hip arthroplasty from 2005 to 2011. J Arthroplast. 2015;30(1):15. doi:10.1016/j.arth.2014.08.025.
8. Su EP, Su SL. Femoral neck fractures: a changing paradigm. Bone Joint J. 2014;96-b(11) Supple A):43. doi:10.1302/0301-620X.96B11.34334.
9. Ahn J, Man LX, Park S, Sodl JF, Esterhai JL. Systematic review of cemented and uncemented hemiarthroplasty outcomes for femoral neck fractures. Clin Orthop Relat Res. 2008;466(10):2513. doi:10.1007/s11999-008-0368-3.
10. Alolabi B, Bajammal S, Shirali J, Karanicolas PJ, Gafni A, Bhandari M. Treatment of displaced femoral neck fractures in the elderly: a cost-benefit analysis. J Orthop Trauma. 2009;23(6):442. doi:10.1097/BOT.0b013e31817614dd.
11. Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290. doi:10.1093/aje/kwp266.
12. Brox WT, Chan PH, Cafri G, Inacio MC. Similar mortality with general or regional anesthesia in elderly hip fracture patients. Acta Orthop. 2016;87(2):152. doi:10.3109/17453674.2015.1128781.
13. Catal B, Sener M. Treatment and displacement affect the reoperation rate for femoral neck fracture. Clin Orthop Relat Res. 2013;471(12):4096. doi:10.1007/s11999-013-3295-x.
14. Dailiana Z, Papakostidou I, Varitimidis S, Michalitsis S, Veloni A, Malizos K. Surgical treatment of hip fractures: factors influencing mortality. Hippokratia. 2013;17(3):252.
15. Deangelis JP, Ademi A, Staff I, Lewis CG. Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures: a prospective randomized trial with early follow-up. J Orthop Trauma. 2012;26(3):135. doi:10.1097/BOT.0b013e318238b7a5.
16. Hedbeck CJ, Inngul C, Blomfeldt R, Ponzer S, Tornkvist H, Enocson A. Internal fixation versus cemented hemiarthroplasty for displaced femoral neck fractures in patients with severe cognitive dysfunction: a randomized controlled trial. J Orthop Trauma. 2013;27(12):690. doi:10.1097/BOT.0b013e318291f544.
17. Jia Z, Ding F, Wu Y, et al. Unipolar versus bipolar hemiarthroplasty for displaced femoral neck fractures: a systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2015;10:8. doi:10.1186/s13018-015-0165-0.
18. Lapidus LJ, Charalampidis A, Rundgren J, Enocson A. Internal fixation of garden I and II femoral neck fractures: posterior tilt did not influence the reoperation rate in 382 consecutive hips followed for a minimum of 5 years. J Orthop Trauma. 2013;27(7):386. doi:10.1097/BOT.0b013e318281da6e.
19. Mariconda M, Costa GG, Cerbasi S, et al. Factors predicting mobility and the change in Activities of Daily Living After hip fracture: A 1-year prospective cohort study. J Orthop Trauma. 2016;30(2):71. doi:10.1097/BOT.0000000000000448.
20. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality After proximal femoral fracture: A retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg. (American) 2015;97(16):1333. doi:10.2106/JBJS.O.00029.
21. Samuel AM, Russo GS, Lukasiewicz AM, et al. Surgical treatment of femoral neck fractures after 24 hours in patients between the ages of 18 and 49 is associated with poor inpatient outcomes: an analysis of 1361 patients in the National Trauma Data Bank. J Orthop Trauma. 2016;30(2):89. doi:10.1097/BOT.0000000000000456.
22. Yu L, Wang Y, Chen J. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures: meta-analysis of randomized trials. Clin Orthop Relat Res. 2012;470(8):2235. doi:10.1007/s11999-012-2293-8.
23. Zi-Sheng A, You-Shui G, Zhi-Zhen J, Ting Y, Chang-Qing Z. Hemiarthroplasty vs primary total hip arthroplasty for displaced fractures of the femoral neck in the elderly: a meta-analysis. J Arthroplast. 2012;27(4):583. doi:10.1016/j.arth.2011.07.009.
24. Zielinski SM, Keijsers NL, Praet SF, et al. Functional outcome after successful internal fixation versus salvage arthroplasty of patients with a femoral neck fracture. J Orthop Trauma. 2014;28(12):e273. doi:10.1097/BOT.0000000000000123.
25. Gu Q, Koenig L, Mather RC, 3rd, Tongue J. Surgery for hip fracture yields societal benefits that exceed the direct medical costs. Clin Orthop Relat Res. 2014;472(11):3536. doi:10.1007/s11999-014-3820-6.
26. Forsh DA, Ferguson TA. Contemporary management of femoral neck fractures: the young and the old. Curr Rev Musculoskelet Med. 2012;5(3):214. doi:10.1007/s12178-012-9127-x.
27. Macaulay W, Pagnotto MR, Iorio R, Mont MA, Saleh KJ. Displaced femoral neck fractures in the elderly: hemiarthroplasty versus total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(5):287. doi:10.5435/00124635-200605000-00004.
28. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: Femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596. doi:10.5435/00124635-200810000-00005.
29. Probe R, Ward R. Internal fixation of femoral neck fractures. J Am Acad Orthop Surg. 2006;14(9):565. doi:10.5435/00124635-200609000-00006.
30. Jain NB, Losina E, Ward DM, Harris MB, Katz JN. Trends in surgical management of femoral neck fractures in the United States. Clin Orthop Relat Res. 2008;466(12):3116. doi:10.1007/s11999-008-0392-3.
31. Menendez ME, Neuhaus V, van Dijk CN, Ring D. The Elixhauser comorbidity method outperforms the Charlson index in predicting inpatient death after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(9):2878. doi:10.1007/s11999-014-3686-7.
32. Van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser Comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633.
33. Best MJ, Buller LT, Falakassa J, Vecchione D. Risk factors for nonroutine discharge in patients undergoing spinal fusion for intervertebral disc disorders. Iowa Orthop J. 2015;35:147.
34. Schairer WW, Lane JM, Halsey DA, Iorio R, Padgett DE, McLawhorn AS. The Frank Stinchfield award: total hip arthroplasty for femoral neck fracture is not a typical DRG 470: A propensity-matched cohort study. Clin Orthop Relat Res. 2017;475(2):353-360. doi:10.1007/s11999-016-4868-2.
35. Nikkel LE, Fox EJ, Black KP, Davis C, Andersen L, Hollenbeak CS. Impact of comorbidities on hospitalization costs following hip fracture. J Bone Joint Surg Am. 2012;94(1):9. doi:10.2106/JBJS.J.01077.
36. Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009;122(2 Suppl.):S14. doi:10.1016/j.amjmed.2008.12.003.
37. Siris ES, Pasquale MK, Wang Y, Watts NB. Estimating bisphosphonate use and fracture reduction among US women aged 45 years and older, 2001-2008. J Bone Miner Res Off J Am Soc Bone Miner Res. 2011;26(1):3. doi:10.1002/jbmr.189.
1. Bishop J, Yang A, Githens M, Sox AH. Evaluation of contemporary trends in femoral neck fracture management reveals discrepancies in treatment. Geriatr Orthop Surg Rehabil. 2016;7(3):135. doi:10.1177/2151458516658328.
2. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res Off J Am Soc Bone Miner Res. 2007;22(3):465. doi:10.1359/jbmr.061113.
3. Kannus P, Parkkari J, Sievanen H, Heinonen A, Vuori I, Jarvinen M. Epidemiology of hip fractures. Bone. 1996;18(1 Suppl.):57s. doi:10.1016/8756-3282(95)00381-9.
4. Koval KJ, Zuckerman JD. Hip fractures: I. Overview and evaluation and treatment of femoral-neck fractures. J Am Acad Orthop Surg. 1994;2(3):141. doi:10.5435/00124635-199405000-00002.
5. Miller BJ, Callaghan JJ, Cram P, Karam M, Marsh JL, Noiseux NO. Changing trends in the treatment of femoral neck fractures: a review of the American Board of Orthopaedic Surgery database. J Bone Joint Surg. (American) 2014;96(17):e149. doi:10.2106/JBJS.M.01122.
6. Miller BJ, Lu X, Cram P. The trends in treatment of femoral neck fractures in the Medicare population from 1991 to 2008. J Bone Joint Surg. (American) 2013;95(18):e132. doi:10.2106/JBJS.L.01163.
7. Nwachukwu BU, McCormick F, Provencher MT, Roche M, Rubash HE. A comprehensive analysis of Medicare trends in utilization and hospital economics for total knee and hip arthroplasty from 2005 to 2011. J Arthroplast. 2015;30(1):15. doi:10.1016/j.arth.2014.08.025.
8. Su EP, Su SL. Femoral neck fractures: a changing paradigm. Bone Joint J. 2014;96-b(11) Supple A):43. doi:10.1302/0301-620X.96B11.34334.
9. Ahn J, Man LX, Park S, Sodl JF, Esterhai JL. Systematic review of cemented and uncemented hemiarthroplasty outcomes for femoral neck fractures. Clin Orthop Relat Res. 2008;466(10):2513. doi:10.1007/s11999-008-0368-3.
10. Alolabi B, Bajammal S, Shirali J, Karanicolas PJ, Gafni A, Bhandari M. Treatment of displaced femoral neck fractures in the elderly: a cost-benefit analysis. J Orthop Trauma. 2009;23(6):442. doi:10.1097/BOT.0b013e31817614dd.
11. Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290. doi:10.1093/aje/kwp266.
12. Brox WT, Chan PH, Cafri G, Inacio MC. Similar mortality with general or regional anesthesia in elderly hip fracture patients. Acta Orthop. 2016;87(2):152. doi:10.3109/17453674.2015.1128781.
13. Catal B, Sener M. Treatment and displacement affect the reoperation rate for femoral neck fracture. Clin Orthop Relat Res. 2013;471(12):4096. doi:10.1007/s11999-013-3295-x.
14. Dailiana Z, Papakostidou I, Varitimidis S, Michalitsis S, Veloni A, Malizos K. Surgical treatment of hip fractures: factors influencing mortality. Hippokratia. 2013;17(3):252.
15. Deangelis JP, Ademi A, Staff I, Lewis CG. Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures: a prospective randomized trial with early follow-up. J Orthop Trauma. 2012;26(3):135. doi:10.1097/BOT.0b013e318238b7a5.
16. Hedbeck CJ, Inngul C, Blomfeldt R, Ponzer S, Tornkvist H, Enocson A. Internal fixation versus cemented hemiarthroplasty for displaced femoral neck fractures in patients with severe cognitive dysfunction: a randomized controlled trial. J Orthop Trauma. 2013;27(12):690. doi:10.1097/BOT.0b013e318291f544.
17. Jia Z, Ding F, Wu Y, et al. Unipolar versus bipolar hemiarthroplasty for displaced femoral neck fractures: a systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2015;10:8. doi:10.1186/s13018-015-0165-0.
18. Lapidus LJ, Charalampidis A, Rundgren J, Enocson A. Internal fixation of garden I and II femoral neck fractures: posterior tilt did not influence the reoperation rate in 382 consecutive hips followed for a minimum of 5 years. J Orthop Trauma. 2013;27(7):386. doi:10.1097/BOT.0b013e318281da6e.
19. Mariconda M, Costa GG, Cerbasi S, et al. Factors predicting mobility and the change in Activities of Daily Living After hip fracture: A 1-year prospective cohort study. J Orthop Trauma. 2016;30(2):71. doi:10.1097/BOT.0000000000000448.
20. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality After proximal femoral fracture: A retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg. (American) 2015;97(16):1333. doi:10.2106/JBJS.O.00029.
21. Samuel AM, Russo GS, Lukasiewicz AM, et al. Surgical treatment of femoral neck fractures after 24 hours in patients between the ages of 18 and 49 is associated with poor inpatient outcomes: an analysis of 1361 patients in the National Trauma Data Bank. J Orthop Trauma. 2016;30(2):89. doi:10.1097/BOT.0000000000000456.
22. Yu L, Wang Y, Chen J. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures: meta-analysis of randomized trials. Clin Orthop Relat Res. 2012;470(8):2235. doi:10.1007/s11999-012-2293-8.
23. Zi-Sheng A, You-Shui G, Zhi-Zhen J, Ting Y, Chang-Qing Z. Hemiarthroplasty vs primary total hip arthroplasty for displaced fractures of the femoral neck in the elderly: a meta-analysis. J Arthroplast. 2012;27(4):583. doi:10.1016/j.arth.2011.07.009.
24. Zielinski SM, Keijsers NL, Praet SF, et al. Functional outcome after successful internal fixation versus salvage arthroplasty of patients with a femoral neck fracture. J Orthop Trauma. 2014;28(12):e273. doi:10.1097/BOT.0000000000000123.
25. Gu Q, Koenig L, Mather RC, 3rd, Tongue J. Surgery for hip fracture yields societal benefits that exceed the direct medical costs. Clin Orthop Relat Res. 2014;472(11):3536. doi:10.1007/s11999-014-3820-6.
26. Forsh DA, Ferguson TA. Contemporary management of femoral neck fractures: the young and the old. Curr Rev Musculoskelet Med. 2012;5(3):214. doi:10.1007/s12178-012-9127-x.
27. Macaulay W, Pagnotto MR, Iorio R, Mont MA, Saleh KJ. Displaced femoral neck fractures in the elderly: hemiarthroplasty versus total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(5):287. doi:10.5435/00124635-200605000-00004.
28. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: Femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596. doi:10.5435/00124635-200810000-00005.
29. Probe R, Ward R. Internal fixation of femoral neck fractures. J Am Acad Orthop Surg. 2006;14(9):565. doi:10.5435/00124635-200609000-00006.
30. Jain NB, Losina E, Ward DM, Harris MB, Katz JN. Trends in surgical management of femoral neck fractures in the United States. Clin Orthop Relat Res. 2008;466(12):3116. doi:10.1007/s11999-008-0392-3.
31. Menendez ME, Neuhaus V, van Dijk CN, Ring D. The Elixhauser comorbidity method outperforms the Charlson index in predicting inpatient death after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(9):2878. doi:10.1007/s11999-014-3686-7.
32. Van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser Comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633.
33. Best MJ, Buller LT, Falakassa J, Vecchione D. Risk factors for nonroutine discharge in patients undergoing spinal fusion for intervertebral disc disorders. Iowa Orthop J. 2015;35:147.
34. Schairer WW, Lane JM, Halsey DA, Iorio R, Padgett DE, McLawhorn AS. The Frank Stinchfield award: total hip arthroplasty for femoral neck fracture is not a typical DRG 470: A propensity-matched cohort study. Clin Orthop Relat Res. 2017;475(2):353-360. doi:10.1007/s11999-016-4868-2.
35. Nikkel LE, Fox EJ, Black KP, Davis C, Andersen L, Hollenbeak CS. Impact of comorbidities on hospitalization costs following hip fracture. J Bone Joint Surg Am. 2012;94(1):9. doi:10.2106/JBJS.J.01077.
36. Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009;122(2 Suppl.):S14. doi:10.1016/j.amjmed.2008.12.003.
37. Siris ES, Pasquale MK, Wang Y, Watts NB. Estimating bisphosphonate use and fracture reduction among US women aged 45 years and older, 2001-2008. J Bone Miner Res Off J Am Soc Bone Miner Res. 2011;26(1):3. doi:10.1002/jbmr.189.
TAKE-HOME POINTS
- The femoral neck patient population is older and has more medical comorbidities.
- Hemiarthroplasty (HA) is being performed more commonly in patients > 50 years old for femoral neck fractures.
- Open reduction and internal fixation is being performed more commonly in patients > 80 years old for femoral neck fractures.
- The rate of adverse events following femoral neck fracture is higher in the total hip arthroplasty (THA) group than in the HA group.
- THA is an independent risk factor for adverse events following femoral neck fracture.
Incidental Asymptomatic Fibular Stress Fractures Presenting as Varus Knee Osteoarthritis: A Case Report
ABSTRACT
Stress fractures are often missed, especially in unusual clinical settings. We report on 2 patients who presented to our orthopedic surgery clinic with incidental findings of asymptomatic proximal fibular tension side stress fractures in severe longstanding varus osteoarthritic knees. Initial plain films demonstrated an expansile deformity of the proximal fibular shaft, and differential diagnosis included a healed or healing fracture versus possible neoplasm. Magnetic resonance imaging with and without gadolinium was utilized to rule out the latter prior to planned total knee arthroplasty.
Continue to: The proximal fibula...
The proximal fibula is a rare site for stress fractures, with most of these fractures occurring in military recruits.1 To the authors’ knowledge, there has been only 1 documented case of a proximal fibular stress fracture in patients with severe osteoarthritis (OA) and fixed varus deformity, which mimicked L5 radiculopathy.2 We are not aware of any reports of asymptomatic tension-side fibular stress fractures in varus knees. In our 2 cases, the patients were indicated for total knee arthroplasty (TKA) for varus degenerative joint disease after failing nonoperative treatment; however, further work-up was justified to rule out neoplasm after plain films revealed expansile deformities of the proximal fibular shaft. Each patient subsequently underwent magnetic resonance imaging (MRI) with and without gadolinium contrast, which demonstrated a healed and healing proximal fibular stress fracture. Magnetic resonance imaging is rarely indicated in the evaluation of degenerative joint disease, and stress fractures about a varus knee generally occur on the compression side of the tibia and are symptomatic.3-7 The patients provided informed written consent for print and electronic publication of this case report.
CASE REPORT
The first patient was a 77-year-old male who presented with longstanding knee pain, left greater than right, exacerbated by weight-bearing activities. The patient had no improvement with physical therapy or anti-inflammatory medication. He denied any history of trauma, weakness, paresthesias, or a recent increase in activity. The patient also denied any fevers, chills, night sweats, or other constitutional symptoms. On physical examination, the patient had an antalgic gait and limited range of motion bilaterally. Examination of his right lower extremity demonstrated a fixed 5° varus deformity. No distinct point tenderness was noted.
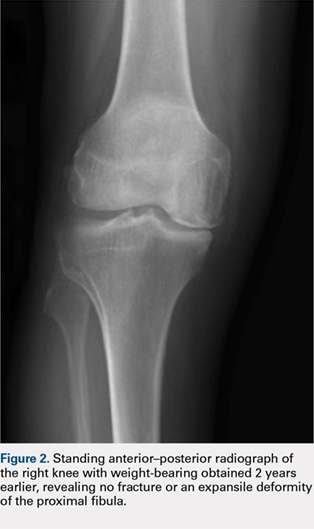
Radiographs of the right knee demonstrated varus deformity and tricompartmental degenerative changes with severe medial joint space narrowing. An expansile deformity of the proximal right fibular shaft was also noted (Figure 1), which was not present on the films 2 years earlier (Figure 2). The absence of this deformity on previous imaging raised the suspicion of a tumor. An MRI with and without gadolinium, which was obtained to rule out a neoplastic process, showed an old, healed proximal fibular shaft fracture with chronic periosteal reaction (Figure 3). There was no marrow edema to suggest acute injury and no neoplastic lesion. He was reassured regarding the benign findings and was scheduled for a left TKA, as his pain was more severe on the left knee. The patient’s stress fracture healed without complications, and he underwent a successful left TKA. He returned approximately 6 months after his procedure with worsening right knee pain and underwent a successful TKA on the right knee as well.
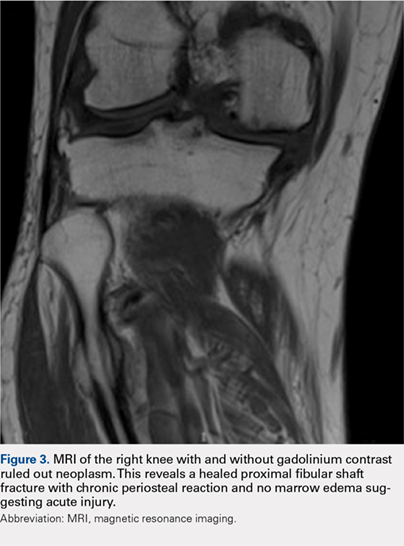
The second patient was a 67-year-old male with longstanding bilateral knee pain, right greater than left, with no antecedent trauma. He denied a history of increased activity, or weakness or paresthesias. He denied any fevers, chills, night sweats, or other constitutional symptoms. One year prior to presentation at our clinic, he had received corticosteroid injections and hyaluronic acid, without relief. The patient also had a history with another surgeon of arthroscopy 1 year earlier and subchondroplasty 3 years before presentation to our clinic. On physical examination, the patient’s right knee displayed a fixed 7° varus deformity with decreased range of motion, effusion, and diffuse crepitus. Further examination revealed tenderness to palpation of the proximal fibula.
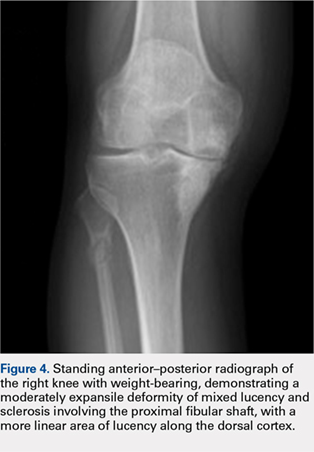
Radiographs of the right knee showed degenerative joint disease with varus deformity and medial compartment joint space narrowing. They also demonstrated an expansile deformity of mixed lucency and sclerosis involving the proximal right fibular shaft (Figure 4). Although these findings appeared to be consistent with a stress fracture, their appearance was also suspicious for a neoplasm. To rule out malignancy, an MRI with and without gadolinium was obtained that revealed a healing stress fracture of the proximal fibula (Figure 5). The patient was reassured, and plans were made to proceed with a TKA. The patient’s stress fracture healed without complications, and he underwent successful right TKA. Radiographs from the patient’s 8-week follow-up showed a healed fibular stress fracture (Figure 6).
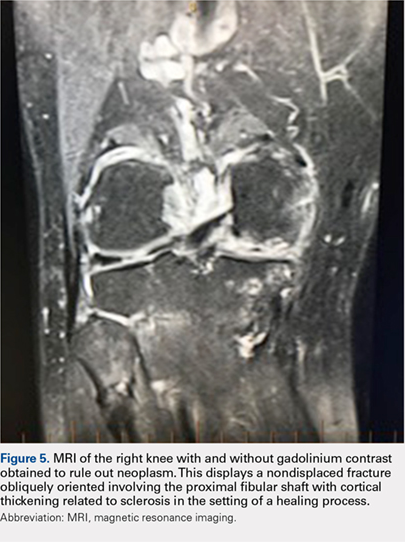

Continue to: DISCUSSION
DISCUSSION
To our knowledge, this is the first report of incidental tension-side stress fractures in varus osteoarthritic knees. Stress fractures have been classified into 2 groups, fatigue fractures and insufficiency fractures. Fatigue fractures occur when abnormal stress is applied to normal bones, and insufficiency fractures result when normal stress is applied to abnormal bones.8 Stress fractures can also be classified into risk categories based on which bone is involved and the loading of the bone.9 Sites loaded in tension have increased risk of nonunion, progression to complete fracture, and reoccurrence compared with sites loaded in compression.9 Stress fractures of the fibula occur rarely, and when present, they are more commonly observed in the distal fibula in athletes and military recruits.1 Stress fractures occur rarely in patients with primary OA, and when present in this setting, obesity and malalignment are the contributing factors.3 Neither patient was obese in our case (body mass index of 27 and 28, respectively), but significant varus deformity was present in both patients. Stress fractures occurring near the knee in the setting of a varus deformity generally occur on the compression side of the tibia and are symptomatic.3-7
Regarding malalignment, Cheung and colleagues10 reported about a case of an elderly female with OA of the knee with valgus deformity that initially developed a proximal fibular stress fracture followed by a proximal tibial stress fracture. However, both of our patients had varus deformities. Mullaji and Shetty3 documented stress fractures in 34 patients with OA, a majority with varus deformities, but did not report any isolated proximal fibular stress fractures. Manish and colleagues2 reported the only documented case of an isolated proximal fibular stress fracture in a patient with osteoarthritic varus deformity. The patient presented initially with pain and paresthesias of the lower thigh and leg consistent with an L5 radiculopathy. They believed that the varus deformity and the repetitive contraction of the lateral knee muscles put increased shear forces on the fibula leading to the stress fracture. Our patients did not present with any radicular symptoms, a history of acute worsening pain, or an increased activity concerning for a stress fracture. Instead, our patients presented with progressively worsening knee pain typical of severe OA and incidental findings on imaging of tension-side fibular stress fractures. An MRI with and without gadolinium confirmed the diagnosis of a healed fracture in our first patient and a healing fracture in our second patient.
CONCLUSION
Although exceedingly rare in osteoarthritic varus knees, we presented 2 cases of MRI-confirmed proximal fibular stress fractures in this report. As demonstrated, patients may present with symptoms of OA or radicular symptoms as described by Manish and colleagues.2 Presentation may also include an expansile lesion on imaging, prompting a differential diagnosis that includes a neoplasm. If present in the setting of an osteoarthritic varus knee, stress fractures of the proximal fibula should heal with conservative treatment and not affect the plan or outcome of TKA.
- Devas MB, Sweetnam R. Stress fractures of the fibula; a review of fifty cases in athletes. J Bone Joint Surg Br. 1956;38-B(4):818-829.
- Manish KK, Agnivesh T, Pramod PS, Samir SD. Isolated proximal fibular stress fracture in osteoarthritis knee presenting as L5 radiculopathy. J Orthop Case Reports. 2015;5(3):75-77. doi:10.13107/jocr.2250-0685.315.
- Mullaji A, Shetty G. Total knee arthroplasty for arthritic knees with tibiofibular stress fractures: classification and treatment guidelines. J Arthroplasty. 2010;25(2):295-301. doi:10.1016/j.arth.2008.11.012.
- Sourlas I, Papachristou G, Pilichou A, Giannoudis PV, Efstathopoulos N, Nikolaou VS. Proximal tibial stress fractures associated with primary degenerative knee osteoarthritis. Am J Orthop (Belle Mead NJ). 2009;38(3):120-124
- Demir B, Gursu S, Oke R, Ozturk K, Sahin V. Proximal tibia stress fracture caused by severe arthrosis of the knee with varus deformity. Am J Orthop (Belle Mead NJ). 2009;38(9):457-459.
- Satku K, Kumar VP, Pho RW. Stress fractures of the tibia in osteoarthritis of the knee. J Bone Joint Surg Br. 1987;69(2):309-311. doi:10.1302/0301-620X.69B2.3818767.
- Martin LM, Bourne RB, Rorabeck CH. Stress fractures associated with osteoarthritis of the knee. A report of three cases. J Bone Joint Surg Am. 1988;70(5):771-774.
- Hong SH, Chu IT. Stress fracture of the proximal fibula in military recruits. Clin Orthop Surg. 2009;1(3):161-164. doi:10.4055/cios.2009.1.3.161
- Knapik JJ, Reynolds K, Hoedebecke KL. Stress fractures: Etiology, epidemiology, diagnosis, treatment, and prevention. J Spec Oper Med. 17(2):120-130.
- Cheung MHS, Lee M-F, Lui TH. Insufficiency fracture of the proximal fibula and then tibia: A case report. J Orthop Surg. 2013;21(1):103-105. doi:10.1177/230949901302100126
ABSTRACT
Stress fractures are often missed, especially in unusual clinical settings. We report on 2 patients who presented to our orthopedic surgery clinic with incidental findings of asymptomatic proximal fibular tension side stress fractures in severe longstanding varus osteoarthritic knees. Initial plain films demonstrated an expansile deformity of the proximal fibular shaft, and differential diagnosis included a healed or healing fracture versus possible neoplasm. Magnetic resonance imaging with and without gadolinium was utilized to rule out the latter prior to planned total knee arthroplasty.
Continue to: The proximal fibula...
The proximal fibula is a rare site for stress fractures, with most of these fractures occurring in military recruits.1 To the authors’ knowledge, there has been only 1 documented case of a proximal fibular stress fracture in patients with severe osteoarthritis (OA) and fixed varus deformity, which mimicked L5 radiculopathy.2 We are not aware of any reports of asymptomatic tension-side fibular stress fractures in varus knees. In our 2 cases, the patients were indicated for total knee arthroplasty (TKA) for varus degenerative joint disease after failing nonoperative treatment; however, further work-up was justified to rule out neoplasm after plain films revealed expansile deformities of the proximal fibular shaft. Each patient subsequently underwent magnetic resonance imaging (MRI) with and without gadolinium contrast, which demonstrated a healed and healing proximal fibular stress fracture. Magnetic resonance imaging is rarely indicated in the evaluation of degenerative joint disease, and stress fractures about a varus knee generally occur on the compression side of the tibia and are symptomatic.3-7 The patients provided informed written consent for print and electronic publication of this case report.

CASE REPORT
The first patient was a 77-year-old male who presented with longstanding knee pain, left greater than right, exacerbated by weight-bearing activities. The patient had no improvement with physical therapy or anti-inflammatory medication. He denied any history of trauma, weakness, paresthesias, or a recent increase in activity. The patient also denied any fevers, chills, night sweats, or other constitutional symptoms. On physical examination, the patient had an antalgic gait and limited range of motion bilaterally. Examination of his right lower extremity demonstrated a fixed 5° varus deformity. No distinct point tenderness was noted.

Radiographs of the right knee demonstrated varus deformity and tricompartmental degenerative changes with severe medial joint space narrowing. An expansile deformity of the proximal right fibular shaft was also noted (Figure 1), which was not present on the films 2 years earlier (Figure 2). The absence of this deformity on previous imaging raised the suspicion of a tumor. An MRI with and without gadolinium, which was obtained to rule out a neoplastic process, showed an old, healed proximal fibular shaft fracture with chronic periosteal reaction (Figure 3). There was no marrow edema to suggest acute injury and no neoplastic lesion. He was reassured regarding the benign findings and was scheduled for a left TKA, as his pain was more severe on the left knee. The patient’s stress fracture healed without complications, and he underwent a successful left TKA. He returned approximately 6 months after his procedure with worsening right knee pain and underwent a successful TKA on the right knee as well.

The second patient was a 67-year-old male with longstanding bilateral knee pain, right greater than left, with no antecedent trauma. He denied a history of increased activity, or weakness or paresthesias. He denied any fevers, chills, night sweats, or other constitutional symptoms. One year prior to presentation at our clinic, he had received corticosteroid injections and hyaluronic acid, without relief. The patient also had a history with another surgeon of arthroscopy 1 year earlier and subchondroplasty 3 years before presentation to our clinic. On physical examination, the patient’s right knee displayed a fixed 7° varus deformity with decreased range of motion, effusion, and diffuse crepitus. Further examination revealed tenderness to palpation of the proximal fibula.

Radiographs of the right knee showed degenerative joint disease with varus deformity and medial compartment joint space narrowing. They also demonstrated an expansile deformity of mixed lucency and sclerosis involving the proximal right fibular shaft (Figure 4). Although these findings appeared to be consistent with a stress fracture, their appearance was also suspicious for a neoplasm. To rule out malignancy, an MRI with and without gadolinium was obtained that revealed a healing stress fracture of the proximal fibula (Figure 5). The patient was reassured, and plans were made to proceed with a TKA. The patient’s stress fracture healed without complications, and he underwent successful right TKA. Radiographs from the patient’s 8-week follow-up showed a healed fibular stress fracture (Figure 6).


Continue to: DISCUSSION
DISCUSSION
To our knowledge, this is the first report of incidental tension-side stress fractures in varus osteoarthritic knees. Stress fractures have been classified into 2 groups, fatigue fractures and insufficiency fractures. Fatigue fractures occur when abnormal stress is applied to normal bones, and insufficiency fractures result when normal stress is applied to abnormal bones.8 Stress fractures can also be classified into risk categories based on which bone is involved and the loading of the bone.9 Sites loaded in tension have increased risk of nonunion, progression to complete fracture, and reoccurrence compared with sites loaded in compression.9 Stress fractures of the fibula occur rarely, and when present, they are more commonly observed in the distal fibula in athletes and military recruits.1 Stress fractures occur rarely in patients with primary OA, and when present in this setting, obesity and malalignment are the contributing factors.3 Neither patient was obese in our case (body mass index of 27 and 28, respectively), but significant varus deformity was present in both patients. Stress fractures occurring near the knee in the setting of a varus deformity generally occur on the compression side of the tibia and are symptomatic.3-7
Regarding malalignment, Cheung and colleagues10 reported about a case of an elderly female with OA of the knee with valgus deformity that initially developed a proximal fibular stress fracture followed by a proximal tibial stress fracture. However, both of our patients had varus deformities. Mullaji and Shetty3 documented stress fractures in 34 patients with OA, a majority with varus deformities, but did not report any isolated proximal fibular stress fractures. Manish and colleagues2 reported the only documented case of an isolated proximal fibular stress fracture in a patient with osteoarthritic varus deformity. The patient presented initially with pain and paresthesias of the lower thigh and leg consistent with an L5 radiculopathy. They believed that the varus deformity and the repetitive contraction of the lateral knee muscles put increased shear forces on the fibula leading to the stress fracture. Our patients did not present with any radicular symptoms, a history of acute worsening pain, or an increased activity concerning for a stress fracture. Instead, our patients presented with progressively worsening knee pain typical of severe OA and incidental findings on imaging of tension-side fibular stress fractures. An MRI with and without gadolinium confirmed the diagnosis of a healed fracture in our first patient and a healing fracture in our second patient.
CONCLUSION
Although exceedingly rare in osteoarthritic varus knees, we presented 2 cases of MRI-confirmed proximal fibular stress fractures in this report. As demonstrated, patients may present with symptoms of OA or radicular symptoms as described by Manish and colleagues.2 Presentation may also include an expansile lesion on imaging, prompting a differential diagnosis that includes a neoplasm. If present in the setting of an osteoarthritic varus knee, stress fractures of the proximal fibula should heal with conservative treatment and not affect the plan or outcome of TKA.
ABSTRACT
Stress fractures are often missed, especially in unusual clinical settings. We report on 2 patients who presented to our orthopedic surgery clinic with incidental findings of asymptomatic proximal fibular tension side stress fractures in severe longstanding varus osteoarthritic knees. Initial plain films demonstrated an expansile deformity of the proximal fibular shaft, and differential diagnosis included a healed or healing fracture versus possible neoplasm. Magnetic resonance imaging with and without gadolinium was utilized to rule out the latter prior to planned total knee arthroplasty.
Continue to: The proximal fibula...
The proximal fibula is a rare site for stress fractures, with most of these fractures occurring in military recruits.1 To the authors’ knowledge, there has been only 1 documented case of a proximal fibular stress fracture in patients with severe osteoarthritis (OA) and fixed varus deformity, which mimicked L5 radiculopathy.2 We are not aware of any reports of asymptomatic tension-side fibular stress fractures in varus knees. In our 2 cases, the patients were indicated for total knee arthroplasty (TKA) for varus degenerative joint disease after failing nonoperative treatment; however, further work-up was justified to rule out neoplasm after plain films revealed expansile deformities of the proximal fibular shaft. Each patient subsequently underwent magnetic resonance imaging (MRI) with and without gadolinium contrast, which demonstrated a healed and healing proximal fibular stress fracture. Magnetic resonance imaging is rarely indicated in the evaluation of degenerative joint disease, and stress fractures about a varus knee generally occur on the compression side of the tibia and are symptomatic.3-7 The patients provided informed written consent for print and electronic publication of this case report.

CASE REPORT
The first patient was a 77-year-old male who presented with longstanding knee pain, left greater than right, exacerbated by weight-bearing activities. The patient had no improvement with physical therapy or anti-inflammatory medication. He denied any history of trauma, weakness, paresthesias, or a recent increase in activity. The patient also denied any fevers, chills, night sweats, or other constitutional symptoms. On physical examination, the patient had an antalgic gait and limited range of motion bilaterally. Examination of his right lower extremity demonstrated a fixed 5° varus deformity. No distinct point tenderness was noted.

Radiographs of the right knee demonstrated varus deformity and tricompartmental degenerative changes with severe medial joint space narrowing. An expansile deformity of the proximal right fibular shaft was also noted (Figure 1), which was not present on the films 2 years earlier (Figure 2). The absence of this deformity on previous imaging raised the suspicion of a tumor. An MRI with and without gadolinium, which was obtained to rule out a neoplastic process, showed an old, healed proximal fibular shaft fracture with chronic periosteal reaction (Figure 3). There was no marrow edema to suggest acute injury and no neoplastic lesion. He was reassured regarding the benign findings and was scheduled for a left TKA, as his pain was more severe on the left knee. The patient’s stress fracture healed without complications, and he underwent a successful left TKA. He returned approximately 6 months after his procedure with worsening right knee pain and underwent a successful TKA on the right knee as well.

The second patient was a 67-year-old male with longstanding bilateral knee pain, right greater than left, with no antecedent trauma. He denied a history of increased activity, or weakness or paresthesias. He denied any fevers, chills, night sweats, or other constitutional symptoms. One year prior to presentation at our clinic, he had received corticosteroid injections and hyaluronic acid, without relief. The patient also had a history with another surgeon of arthroscopy 1 year earlier and subchondroplasty 3 years before presentation to our clinic. On physical examination, the patient’s right knee displayed a fixed 7° varus deformity with decreased range of motion, effusion, and diffuse crepitus. Further examination revealed tenderness to palpation of the proximal fibula.

Radiographs of the right knee showed degenerative joint disease with varus deformity and medial compartment joint space narrowing. They also demonstrated an expansile deformity of mixed lucency and sclerosis involving the proximal right fibular shaft (Figure 4). Although these findings appeared to be consistent with a stress fracture, their appearance was also suspicious for a neoplasm. To rule out malignancy, an MRI with and without gadolinium was obtained that revealed a healing stress fracture of the proximal fibula (Figure 5). The patient was reassured, and plans were made to proceed with a TKA. The patient’s stress fracture healed without complications, and he underwent successful right TKA. Radiographs from the patient’s 8-week follow-up showed a healed fibular stress fracture (Figure 6).


Continue to: DISCUSSION
DISCUSSION
To our knowledge, this is the first report of incidental tension-side stress fractures in varus osteoarthritic knees. Stress fractures have been classified into 2 groups, fatigue fractures and insufficiency fractures. Fatigue fractures occur when abnormal stress is applied to normal bones, and insufficiency fractures result when normal stress is applied to abnormal bones.8 Stress fractures can also be classified into risk categories based on which bone is involved and the loading of the bone.9 Sites loaded in tension have increased risk of nonunion, progression to complete fracture, and reoccurrence compared with sites loaded in compression.9 Stress fractures of the fibula occur rarely, and when present, they are more commonly observed in the distal fibula in athletes and military recruits.1 Stress fractures occur rarely in patients with primary OA, and when present in this setting, obesity and malalignment are the contributing factors.3 Neither patient was obese in our case (body mass index of 27 and 28, respectively), but significant varus deformity was present in both patients. Stress fractures occurring near the knee in the setting of a varus deformity generally occur on the compression side of the tibia and are symptomatic.3-7
Regarding malalignment, Cheung and colleagues10 reported about a case of an elderly female with OA of the knee with valgus deformity that initially developed a proximal fibular stress fracture followed by a proximal tibial stress fracture. However, both of our patients had varus deformities. Mullaji and Shetty3 documented stress fractures in 34 patients with OA, a majority with varus deformities, but did not report any isolated proximal fibular stress fractures. Manish and colleagues2 reported the only documented case of an isolated proximal fibular stress fracture in a patient with osteoarthritic varus deformity. The patient presented initially with pain and paresthesias of the lower thigh and leg consistent with an L5 radiculopathy. They believed that the varus deformity and the repetitive contraction of the lateral knee muscles put increased shear forces on the fibula leading to the stress fracture. Our patients did not present with any radicular symptoms, a history of acute worsening pain, or an increased activity concerning for a stress fracture. Instead, our patients presented with progressively worsening knee pain typical of severe OA and incidental findings on imaging of tension-side fibular stress fractures. An MRI with and without gadolinium confirmed the diagnosis of a healed fracture in our first patient and a healing fracture in our second patient.
CONCLUSION
Although exceedingly rare in osteoarthritic varus knees, we presented 2 cases of MRI-confirmed proximal fibular stress fractures in this report. As demonstrated, patients may present with symptoms of OA or radicular symptoms as described by Manish and colleagues.2 Presentation may also include an expansile lesion on imaging, prompting a differential diagnosis that includes a neoplasm. If present in the setting of an osteoarthritic varus knee, stress fractures of the proximal fibula should heal with conservative treatment and not affect the plan or outcome of TKA.
- Devas MB, Sweetnam R. Stress fractures of the fibula; a review of fifty cases in athletes. J Bone Joint Surg Br. 1956;38-B(4):818-829.
- Manish KK, Agnivesh T, Pramod PS, Samir SD. Isolated proximal fibular stress fracture in osteoarthritis knee presenting as L5 radiculopathy. J Orthop Case Reports. 2015;5(3):75-77. doi:10.13107/jocr.2250-0685.315.
- Mullaji A, Shetty G. Total knee arthroplasty for arthritic knees with tibiofibular stress fractures: classification and treatment guidelines. J Arthroplasty. 2010;25(2):295-301. doi:10.1016/j.arth.2008.11.012.
- Sourlas I, Papachristou G, Pilichou A, Giannoudis PV, Efstathopoulos N, Nikolaou VS. Proximal tibial stress fractures associated with primary degenerative knee osteoarthritis. Am J Orthop (Belle Mead NJ). 2009;38(3):120-124
- Demir B, Gursu S, Oke R, Ozturk K, Sahin V. Proximal tibia stress fracture caused by severe arthrosis of the knee with varus deformity. Am J Orthop (Belle Mead NJ). 2009;38(9):457-459.
- Satku K, Kumar VP, Pho RW. Stress fractures of the tibia in osteoarthritis of the knee. J Bone Joint Surg Br. 1987;69(2):309-311. doi:10.1302/0301-620X.69B2.3818767.
- Martin LM, Bourne RB, Rorabeck CH. Stress fractures associated with osteoarthritis of the knee. A report of three cases. J Bone Joint Surg Am. 1988;70(5):771-774.
- Hong SH, Chu IT. Stress fracture of the proximal fibula in military recruits. Clin Orthop Surg. 2009;1(3):161-164. doi:10.4055/cios.2009.1.3.161
- Knapik JJ, Reynolds K, Hoedebecke KL. Stress fractures: Etiology, epidemiology, diagnosis, treatment, and prevention. J Spec Oper Med. 17(2):120-130.
- Cheung MHS, Lee M-F, Lui TH. Insufficiency fracture of the proximal fibula and then tibia: A case report. J Orthop Surg. 2013;21(1):103-105. doi:10.1177/230949901302100126
- Devas MB, Sweetnam R. Stress fractures of the fibula; a review of fifty cases in athletes. J Bone Joint Surg Br. 1956;38-B(4):818-829.
- Manish KK, Agnivesh T, Pramod PS, Samir SD. Isolated proximal fibular stress fracture in osteoarthritis knee presenting as L5 radiculopathy. J Orthop Case Reports. 2015;5(3):75-77. doi:10.13107/jocr.2250-0685.315.
- Mullaji A, Shetty G. Total knee arthroplasty for arthritic knees with tibiofibular stress fractures: classification and treatment guidelines. J Arthroplasty. 2010;25(2):295-301. doi:10.1016/j.arth.2008.11.012.
- Sourlas I, Papachristou G, Pilichou A, Giannoudis PV, Efstathopoulos N, Nikolaou VS. Proximal tibial stress fractures associated with primary degenerative knee osteoarthritis. Am J Orthop (Belle Mead NJ). 2009;38(3):120-124
- Demir B, Gursu S, Oke R, Ozturk K, Sahin V. Proximal tibia stress fracture caused by severe arthrosis of the knee with varus deformity. Am J Orthop (Belle Mead NJ). 2009;38(9):457-459.
- Satku K, Kumar VP, Pho RW. Stress fractures of the tibia in osteoarthritis of the knee. J Bone Joint Surg Br. 1987;69(2):309-311. doi:10.1302/0301-620X.69B2.3818767.
- Martin LM, Bourne RB, Rorabeck CH. Stress fractures associated with osteoarthritis of the knee. A report of three cases. J Bone Joint Surg Am. 1988;70(5):771-774.
- Hong SH, Chu IT. Stress fracture of the proximal fibula in military recruits. Clin Orthop Surg. 2009;1(3):161-164. doi:10.4055/cios.2009.1.3.161
- Knapik JJ, Reynolds K, Hoedebecke KL. Stress fractures: Etiology, epidemiology, diagnosis, treatment, and prevention. J Spec Oper Med. 17(2):120-130.
- Cheung MHS, Lee M-F, Lui TH. Insufficiency fracture of the proximal fibula and then tibia: A case report. J Orthop Surg. 2013;21(1):103-105. doi:10.1177/230949901302100126
TAKE-HOME POINTS
- Proximal fibular stress fractures in patients with primary osteoarthritis and fixed varus deformity have rarely been reported.
- Stress fractures occurring near the knee in the setting of a varus deformity generally occur on the compression side of the tibia and are symptomatic.
- Proximal fibular stress fractures may present as an incidental finding of an expansile deformity on plain films in patients with varus osteoarthritic knees.
- Magnetic resonance imaging is rarely indicated in the evaluation of degenerative joint disease; however, it was justified in our case to rule out neoplasm.
- When present in the setting of an osteoarthritic varus knee, stress fractures of the proximal fibula should heal with conservative treatment and should not affect the plan or outcome of TKA.
Arthroscopic SLAP IIb Repair Using Knot-Tying Versus Knotless Suture Anchors: Is There a Difference?
ABSTRACT
The use of knotless suture anchors has increased in popularity; however, there is a paucity of literature examining the difference in clinical outcomes with traditional knotted fixation. It was hypothesized that knotless fixation would provide superior clinical outcomes, improved return to play (RTP), and lower revision rates as compared with traditional knotted fixation in the repair of SLAP IIb tears. Seventy-four athletes who underwent arthroscopic SLAP IIb repair with traditional (n = 42) and knotless anchors (n = 32) by a single surgeon were evaluated after a minimum 2-year follow. Demographic and surgical data, RTP, Kerlan-Jobe Orthopaedic Clinic (KJOC) score, American Shoulder and Elbow Surgeons (ASES) score, stability, strength, and pain scores were compared. Knotless anchors had slightly higher RTP (93.5% vs 90.2%, P = .94) and RTP at the same level (58.1% vs 53.7% P = .81) compared with knotted fixation, but the difference did not reach statistical significance. Knotless anchors were less likely to require revision surgery than traditional anchors (9% vs 17%, P = .50), but the difference was not statistically significant. When comparing knotless and traditional knotted suture anchor repair of type llb SLAP tears, knotless fixation required less revision surgery and had higher RTP, ASES, and KJOC scores; however, statistical significance was not achieved in this relatively small cohort.
Continue to: Injury of the anterosuperior...
Injury of the anterosuperior labrum near the biceps origin was first described by Andrews and colleagues in 1985 in overhead athletes.1 The term SLAP, or a tear in the superior labrum anterior to posterior, was coined a few years later by Snyder and colleagues.2 They described an injury to the superior labrum beginning posteriorly and extending anteriorly, including the “anchor” of the biceps tendon to the labrum. Snyder further delineated SLAP lesions into 4 subtypes, the most common being type II, which he described as “degenerative fraying of the labrum with additional detachment of the superior labrum and biceps from the glenoid resulting in an unstable labral anchor.”2,3 Type II tears are of particular importance as they are the most common SLAP lesions, with an incidence of 55%, and comprise nearly 75% of SLAP repairs performed.2,4
Morgan and colleagues further delineated type II SLAP tears into IIa (anterior), IIb (posterior), and IIc (combined). Their group found that SLAP IIb tears were the most common type in overhead throwers, accounting for 47% of overhead athletes with type II tears.5 Further, type IIb tears can have a significant impact in throwers, in part due to greater shoulder instability as well as anterior pseudolaxity.5 SLAP injuries typically have been difficult to successfully treat nonoperatively in overhead athletes.6 A study by Edwards and colleagues6 examined 39 patients with all types of SLAP tears. Although, in their study, nonoperative management failed in 20 patients and they required surgery, 10 of the 15 overhead athletes in whom nonoperative treatment did not fail initially returned to sport at a level equal to or better than their pre-injury level, indicating that nonoperative treatment may play a role in some patients’ recovery.6
Surgical outcomes of SLAP IIb repairs have traditionally been less predictable than those of other shoulder injuries. Some believe that traditional knotted anchors may be partially to blame by abrading the rotator cuff, possibly leading to rotator cuff tears and pain. Further, knotted anchors are typically bulkier and require more experience with tying and tensioning and, therefore, may lead to less consistent results.7 The purpose of this study was to investigate if knotless anchors result in more favorable outcomes in repair of type IIb SLAP lesions when compared with traditional knotted anchors. It was hypothesized that knotless fixation will provide superior clinical outcomes, improved return to play (RTP), and lower revision rates as compared with traditional knotted fixation in the repair of SLAP IIb tears.
METHODS
PATIENT SELECTION
The authors retrospectively reviewed SLAP tears repaired by the senior author from June 2000 to September 2015. The inclusion criteria consisted of all athletes at any level who were diagnosed intraoperatively with a type IIb SLAP tear as defined by Morgan and colleagues5 with a minimum 2-year follow-up. The exclusion criteria were any patients with a previous shoulder surgery and the presence of any labral pathology aside from a SLAP IIb tear. Patients with rotator cuff or biceps pathologies were included. In all included patients, an initial course of preoperative physical therapy, including strengthening and stabilization of the scapulothoracic joint, had failed. Patient-directed surveys evaluated RTP, as well as the Kerlan-Jobe Orthopaedic Clinic (KJOC) score, American Shoulder and Elbow Surgeons (ASES) score, stability, range of motion (ROM), strength, and pain scores, as previously described.8-10 Institutional Review Board and informed consent approval were acquired prior to initiation of the study.
PATIENT EVALUATION
An appropriate preoperative history was taken, and physical examinations were performed, including evaluation of the scapulothoracic joint, as well as tests to evaluate the presence of a SLAP tear, anterior instability, posterior instability, multi-directional instability, and rotator cuff tears, as previously described.11 Patients with a history and physical examination concerning SLAP pathology underwent an magnetic resonance imaging (MRI) arthrogram, which was used in conjunction with intraoperative findings to diagnose type IIb SLAP tears.
Continue to: SURGICAL TECHNIQUE
SURGICAL TECHNIQUE
All surgeries were performed arthroscopically with the patient in the lateral decubitus position. The SLAP lesions were subsequently repaired using a technique similar to that described by Burkhart and colleagues.12 The traditional knotted fixation incorporated the use of 3.0 Bio-FASTak (Arthrex) with #2 FiberWire (Arthrex). Knotless anchor fixation was performed using 2.9 mm × 12.5 mm or 2.4 mm × 11.3 mm BioComposite PushLock (Arthrex) suture anchors, based on the size of the glenoid, with LabralTape or SutureTape (Arthrex). Patients who had surgery before January 1, 2013 underwent fixation with traditional knotted fixation; after that date, patients underwent fixation with knotless anchors.
POSTOPERATIVE REHABILITATION
Patients underwent a strict postoperative protocol in which they were kept in a sling with an abduction pillow for the first 6 weeks and performed pendulum exercises and passive motion only. A formal physical therapy regimen started at 4 weeks with passive ROM, passive posterior capsular and internal rotation stretching, scapulothoracic mobility, and biceps, rotator cuff, and capsular stabilizer strengthening. At 10 weeks, patients began biceps, rotator cuff, and scapular stabilizer resistance exercises, and at 16 weeks, throwing athletes began an interval throwing program. Patients were first eligible to return to sport without limitation at 9 months.
STATISTICAL ANALYSIS
Return to play, KJOC, ASES, stability, ROM, strength, and pain scores were analyzed and compared using Fisher exact test, the Kruskal-Wallis test, and the Wilcoxon rank sum test, where appropriate. The level of statistical significance was α = 0.05.
RESULTS
Table 1. Patient Demographics | |
Athletes (N) | 74 |
Age (yr) | 30.1 (14-64) |
Knotless anchors | 32 (43.2%) |
Knotted anchors | 42 (56.8%) |
Overhead athletes | 53 (72%) |
Throwing athletes | 29 (39%) |
Follow-up (yr) | 6.5 (2-12) |
Of the 74 athletes who met inclusion criteria, 28 were female (37.8%) and 46 (62.2%) were male. The average follow-up was 6.5 years with a minimum of 2 years and a maximum of 12 years. Forty-two (56.8%) patients underwent traditional knotted suture anchor fixation and 32 (43.2%) underwent knotless anchor fixation. The average age was 30.1 +/– 13.6 years, with a range of 14 to 64 years. The majority of athletes were right hand dominant (79.9%). Fifty-three (72%) were overhead athletes and 29 (39%) were throwing athletes (Table 1). The average age in the knotted group was 33.3 years: 29 of 42 (69%) were overhead athletes and 20 (47.6%) were throwing athletes. In the knotless group, the average age was 25.8 years: 24 of 32 (75.0%) were overhead athletes and 9 (28.1%) were throwing athletes. Primary sports at the time of injury are listed in Table 2. The average number of anchors used was 3.1, with 17 patients (23.0%) requiring ≤2 anchors, 39 (52.7%) requiring 3 anchors, and 18 (24.3%) requiring ≥4 anchors for repair. The number of anchors used was determined intraoperatively by the surgeon on the basis of the size and extent of the tear. Of the entire group of 74 patients, 91.9% returned to sport, 56.8% returned to the same level, 35.1% returned at a lower capacity, and 8.1% were unable to return to sport. Knotless anchors had a slightly higher overall RTP compared with traditional anchors (93.5% vs 90.2%, P = .94), as well as a higher RTP at the same level (58.1% vs 53.7%, P = .81). These differences were, however, not statistically significant (Table 3).
Table 2. Primary Sport at Time of SLAP IIb Injury | |
Primary Sport | n (%) |
Baseball | 14 (19.7%) |
Softball | 8 (11.3%) |
Volleyball | 6 (8.5%) |
Basketball | 5 (7.0%) |
Golf | 5 (7.0%) |
Other Sport | 33 (46.5%) |
No Primary Sport | 3 (4.1%) |
Abbreviation: SLAP, superior labrum anterior to posterior.
Knotless anchors were less likely to require revision surgery than traditional anchors (9% vs 17%, P = .50), but this difference was not statistically significant (Table 3). In the knotted group, 5 patients had revision surgery for rotator cuff tears, and 2 patients had recurrent SLAP tears. In the knotless group, 2 patients had revision surgeries for a torn rotator cuff, and 1 patient had a snapping scapula. A power analysis found that it would take over 300 athletes in each group to detect a significant difference in the revision rate between knotless and traditional anchors.
Table 3. Comparison of Anchor Type in Surgical Fixation of SLAP IIb Tears | |||||
| RTP | RTP Same Level | ASES | KJOC | Revision Rate |
Knotless anchors (n = 32) | 93.5% | 58.1% | 86.3 + 10.5 | 66.1 + 29.6 | 9% |
Traditional anchors (n = 42) | 90.2% | 53.7% | 85.3 + 15.6 | 65.6 + 27.2 | 17% |
P-value | .94 | .81 | .79 | .61 | .50 |
Abbreviations: ASES, American Shoulder and Elbow Surgeons; KJOC, Kerlan-Jobe Orthopaedic Clinic; RTP: return to play. | |||||
Continue to: Although KJOC...
Although KJOC (66.1 vs 65.6 P = .61) and ASES (86.3 vs 85.3 P = .79) scores were also superior with knotless anchors, these differences in scores were not statistically significant (Table 3). Pain was the only variable that was linked to decreased RTP, as patients who rated higher on a pain scale of 0 to 10 were less likely to return to their sport (P < .0001). There was no correlation in outcome measures or RTP with gender, age, number of anchors, or sport type (P > .05). There was no statistically significant difference in RTP, KJOC, or ASES scores between non-overhead and overhead athletes (Table 4). Overall return to sport in throwers was 85.7% (24/28), while 39.3% (11/28) returned at the same level, 46.4% (13/28) at a lower level, and 14.3% (4/28) did not return to sport.
Table 4. Overhead vs Non-Overhead Athletes After Surgical Fixation of SLAP IIb Tears | |||||
| RTP | RTP Same Level | ASES | ASES Good-Excellent | KJOC |
Overhead | 90.6% | 52.3% | 91.7 + 14.1 | 98.1% | 64.6 + 25.7 |
Non-Overhead | 95.5% | 72.7% | 86.7 + 12.7 | 100% | 88.5 + 29.6 |
P value | 0.1 | 0.29 | 0.76 | 0.50 | 0.49 |
Abbreviations: ASES, American Shoulder and Elbow Surgeons; KJOC, Kerlan-Jobe Orthopaedic Clinic; RTP: return to play. | |||||
DISCUSSION
There was no significant difference between knotted and knotless fixation in clinical outcomes or return to sport in the repair of SLAP IIb tears; however, there was a trend toward knotless anchors requiring less revision surgery and having higher RTP, ASES, and KJOC scores than knotted fixation. Despite the inclusion of 74 patients, this study was significantly underpowered, as a power analysis calculated that over 300 athletes would be required in each group to detect a difference in the revision rate.
SLAP tears, traditionally treated with knotted suture anchors, have yielded varying results in the literature, with good to excellent results being reported in 65% to 94% of patients.13-17 The success of SLAP repairs in athletes, especially overhead athletes, remains a difficult problem, as they are common injuries, and RTP is less predictable. Studies differ with regard to the percentage of overhead athletes who are able to return to their previous level of sport, with ranges being reported from 22% to 92%.16,18,19 In a systematic review of 198 patients, Sayde and colleagues16 found that 63% of overhead athletes treated with anchor fixation, tacks, or staples were able to return to their previous level of play. Morgan and colleagues5 found a higher return to sport when compared with other studies, reporting that 83% of patients undergoing SLAP repairs using traditional suture anchors had excellent results, and 87% of the 53 overhead athletes had excellent results based on UCLA shoulder scores. Further, 37 of the 44 pitchers examined (84%) were able to return to their pre-injury levels.5 This is in contrast to Friel and colleagues20 who found that in 48 patients with type II SLAP tears treated with traditional anchors, 23% reported excellent and 56% reported good results in regards to UCLA shoulder scores. Friel and colleagues also found that 62% of all athletes and 59% of overhead athletes were able to return to their previous levels of sport, which is similar to the current study.20 The large discrepancy in RTP at the pre-injury level between this study and that of Morgan and colleagues5 may be due to the shorter minimum follow-up of 1 year as well as the inclusion of all subtypes of SLAP II tears in the latter. The current study had a minimum 2-year follow-up period, with an average of 6.5 years, and was limited to SLAP IIb tears. With a longer follow-up period, patient outcomes and RTP, particularly in overhead sports, may deteriorate; therefore, the current study likely shows a more complete and accurate result.
Knotless anchors were originally introduced as a less time consuming, lower profile, and simpler device to learn and use for arthroscopic procedures.21 Kocaoglu and colleagues22 found that in Bankart repairs, the mean time per anchor placement for knotted anchors was 380 seconds, whereas placement of knotless anchors took on average 225 seconds. A learning curve also exists for proper and efficient knot tying.7 There is also variation in knot tying between surgeons, as evidenced by a wide range in both load to failure and knot height.7 A study performed by Hanypsiak and colleagues7 found that the surgical knot was the weakest portion of the suture-anchor construct, as the knot’s load to failure was less than the pullout strength of the anchor.
There is also concern for the added height associated with traditional knotted fixation, which has been supported by case reports of knot-induced glenoid erosion after arthroscopic fixation of a SLAP tear.23 Hanypsiak and colleagues7 also found that the average knot height occupied 50% to 95% of the space between the humeral head and the acromion when the shoulder is in a neutral position, indicating that the higher profile knotted anchors may contact the undersurface of the acromion, which could affect the labral repair as well as cause rotator cuff injury. Abrasion of the rotator cuff by a prominent knot may cause pain, tearing, and disability. A recent study by Park and colleagues24 reported on 11 patients with knot-induced pain after type II SLAP repair. All complained of sharp pain, with 64% also complaining of clicking. Knot location did not seem to matter, as there was no difference in preoperative symptoms, with 5 of the 11 patients having knots on the glenoid side of the repair on repeat arthroscopy. Patients with knots on the labral side did, however, have humeral head cartilage damage. The knots appeared to be the cause of pain and clicking, as after arthroscopic knot removal, dramatic pain relief was seen, with Constant and UCLA scores significantly improving in all 11 patients. All patients also had positive preoperative compression-rotation testing, and at 6 weeks after surgical knot removal, all were negative.24
Continue to: Further, as shown by Dines and colleagues...
Further, as shown by Dines and colleagues25, knotless anchors may help to better restore the meniscoid anatomy of the superior labrum better than knotted suture anchors. With regards to fixation strength, Uggen and colleagues26, using a cadaveric model, found no difference in initial fixation strength of knotless and traditional suture anchor repair of SLAP II tears, and both restored glenohumeral rotation without over-constraining the shoulder.
Despite the shorter operative time, lower profile, and more consistent tensioning with knotless anchors, the literature is limited with regard to evaluating patient outcomes. In a study by Yung and colleagues13 14 of the 16 patients with type II SLAP tears were treated with knotless anchors, and the authors found that 31.3% of patients had an excellent UCLA score while 43.8% had a good score. This is similar to the outcomes illustrated in studies by both Friel and colleagues20 and Sayde and colleagues.16 In a more recent study, Yang and colleagues27 did find some benefit in regard to ROM with knotless fixation. Their study consisted of 21 patients who underwent surgery with traditional knotted anchor fixation and 20 who underwent knotless horizontal mattress fixation. They found an average UCLA score of 37.6 and ASES score of 91.5 in patients undergoing knotless fixation, and the knotless fixation group had 5% greater total ROM, 15.6% more internal rotation at abduction, and 11.4% more external rotation at the side as compared with patients undergoing the traditional knotted technique. When compared with the current study, this study also had a significantly shorter follow-up period of 3 years.27 In a 2017 study, Bents and colleagues28 compared 44 patients who underwent knotless and 119 who underwent knotted fixation of SLAP tears. They found no statistically significant difference between knotless and knotted fixation in the ASES score, Visual Analog Scale (VAS), ASES, or Veterans RAND 12-Item Health Survey (VR-12) at 1 year postoperatively. Their outcomes were similar to those of the current study, but as in other mentioned literature, the study by Bents and colleagues28 included multiple surgeons with different postoperative protocols, was not limited to SLAP IIb tears, and also had a shorter follow-up of 1 year. Like Kocaoglu and colleagues22, Bents and colleagues did find knotless anchors to be more efficient, as operative time was reduced by 5.3 minutes per anchor. This likely would have a significant impact on surgical cost and surgeon productivity.28
One limitation of the current study was that despite the inclusion of >70 patients, the study was still significantly underpowered. It was determined that >300 patients in each group would be required to detect a significant difference in the revision rate between the 2 anchor types. Also, due to the retrospective nature of this study, no preoperative scores were collected. The inclusion of objective clinical measurements and follow-up imaging evaluating the rotator cuff and other anatomy would also be of interest.
Although statistical significance was not achieved, there was a trend toward knotless fixation requiring less revision surgery and having higher RTP, ASES, and KJOC scores when compared with traditional knotted fixation at 6.5-year follow-up. Larger studies with longer follow-up periods are necessary to determine the effects of knotted and knotless anchors on rotator cuff tears, patient reported outcomes, and RTP. These complications have been shown in the literature, mostly in case reports, and typically develop over a longer period.23 Despite this, other advantages of knotless fixation, such as its lower profile, the ability to better provide consistent tensioning, and decreased surgical time are important to consider.
1. Andrews JR, Carson WG, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341. doi:10.1177/036354658501300508.
2. Snyder SJ, Karzel RP, Pizzo WD, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthrosc J Arthrosc Relat Surg. 1990;6(4):274-279. doi:10.1016/0749-8063(90)90056-J.
3. Ahsan ZS, Hsu JE, Gee AO. The Snyder classification of superior labrum anterior and posterior (SLAP) lesions. Clin Orthop. 2016;474(9):2075-2078. doi:10.1007/s11999-016-4826-z.
4. Erickson BJ, Jain A, Abrams GD, et al. SLAP Lesions: Trends in treatment. Arthrosc J Arthrosc Relat Surg. 2016;32(6):976-981. doi:10.1016/j.arthro.2015.11.044.
5. Morgan C, Burkhart S, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthrosc J Arthrosc Relat Surg. 1998;14(6):553-565. doi:10.1016/S0749-8063(98)70049-0.
6. Edwards SL, Lee JA, Bell J-E, et al. nonoperative treatment of superior labrum anterior posterior tears: Improvements in pain, function, and quality of life. Am J Sports Med. 2010;38(7):1456-1461. doi:10.1177/0363546510370937.
7. Hanypsiak BT, DeLong JM, Simmons L, Lowe W, Burkhart S. Knot strength varies widely among expert arthroscopists. Am J Sports Med. 2014;42(8):1978-1984. doi:10.1177/0363546514535554.
8. Alberta FG, ElAttrache NS, Bissell S, et al. The development and validation of a functional assessment tool for the upper extremity in the overhead athlete. Am J Sports Med. 2010;38(5):903-911. doi:10.1177/0363546509355642.
9. Bradley JP, McClincy MP, Arner JW, Tejwani SG. Arthroscopic capsulolabral reconstruction for posterior instability of the shoulder: a prospective study of 200 shoulders. Am J Sports Med. 2013;41(9):2005-2014. doi:10.1177/0363546513493599.
10. Michener LA, McClure PW, Sennett BJ. American shoulder and elbow surgeons standardized shoulder assessment form, patient self-report section: Reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594. doi:10.1067/mse.2002.127096.
11. Cook C, Hegedus EJ. Orthopedic Physical Examination Tests: An Evidence-Based Approach. Upper Saddle River, NJ: PearsonPrentice Hall; 2008.
12. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: Spectrum of pathology part I: Pathoanatomy and biomechanics. Arthrosc J Arthrosc Relat Surg. 2003;19(4):404-420. doi:10.1053/jars.2003.50128.
13. Yung PS-H, Fong DT-P, Kong M-F, et al. Arthroscopic repair of isolated type II superior labrum anterior–posterior lesion. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1151-1157. doi:10.1007/s00167-008-0629-4.
14. Brockmeier SF, Voos JE, Williams RJ, Altchek DW, Cordasco FA, Allen AA. Outcomes After Arthroscopic Repair of Type-II SLAP Lesions: J Bone Jt Surg-Am Vol. 2009;91(7):1595-1603. doi:10.2106/JBJS.H.00205.
15. Galano GJ, Ahmad CS, Bigliani L, Levine W. Percutaneous SLAP lesion repair technique is an effective alternative to portal of Wilmington. Orthopedics. 2010;33(11). doi:10.3928/01477447-20100924-15.
16. Sayde WM, Cohen SB, Ciccotti MG, Dodson CC. Return to play after type II superior labral anterior-posterior lesion repairs in athletes: A systematic review. Clin Orthop Relat Res. 2012;470(6):1595-1600. doi:10.1007/s11999-012-2295-6.
17. Kim K-H, Bin S-I, Kim J-M. The correlation between posterior tibial slope and maximal angle of flexion after total knee arthroplasty. Knee Surg Relat Res. 2012;24(3):158-163. doi:10.5792/ksrr.2012.24.3.158.
18. Kim S-H, Ha K-I, Kim S-H, Choi H-J. Results of arthroscopic treatment of superior labral lesions. J Bone Joint Surg Am. 2002;84-A(6):981-985.
19. Pagnani MJ, Speer KP, Altchek DW, Warren RF, Dines DM. Arthroscopic fixation of superior labral lesions using a biodegradable implant: a preliminary report. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc. 1995;11(2):194-198.
20. Friel NA, Karas V, Slabaugh MA, Cole BJ. Outcomes of type II superior labrum, anterior to posterior (SLAP) repair: Prospective evaluation at a minimum two-year follow-up. J Shoulder Elbow Surg. 2010;19(6):859-867. doi:10.1016/j.jse.2010.03.004.
21. Thal R. A knotless suture anchor. Arthrosc J Arthrosc Relat Surg. 2001;17(2):213-218. doi:10.1053/jars.2001.20666.
22. Kocaoglu B, Guven O, Nalbantoglu U, Aydin N, Haklar U. No difference between knotless sutures and suture anchors in arthroscopic repair of Bankart lesions in collision athletes. Knee Surg Sports Traumatol Arthrosc. 2009;17(7):844-849. doi:10.1007/s00167-009-0811-3.
23. Rhee YG, Ha JH. Knot-induced glenoid erosion after arthroscopic fixation for unstable superior labrum anterior-posterior lesion: Case report. J Shoulder Elbow Surg. 2006;15(3):391-393. doi:10.1016/j.jse.2005.03.010.
24. Park JG, Cho NS, Kim JY, Song JH, Hong SJ, Rhee YG. Arthroscopic Knot Removal for Failed Superior Labrum Anterior-Posterior Repair Secondary to Knot-Induced Pain. Am J Sports Med. 2017;45(11):2563-2568. doi:10.1177/0363546517713662.
25. Dines JS, ElAttrache NS. Horizontal Mattress With a Knotless Anchor to Better Recreate the Normal Superior Labrum Anatomy. Arthrosc J Arthrosc Relat Surg. 2008;24(12):1422-1425. doi:10.1016/j.arthro.2008.06.012.
26. Uggen C, Wei A, Glousman RE, et al. Biomechanical Comparison of Knotless Anchor Repair Versus Simple Suture Repair for Type II SLAP Lesions. Arthrosc J Arthrosc Relat Surg. 2009;25(10):1085-1092. doi:10.1016/j.arthro.2009.03.022.
27. Yang HJ, Yoon K, Jin H, Song HS. Clinical outcome of arthroscopic SLAP repair: conventional vertical knot versus knotless horizontal mattress sutures. Knee Surg Sports Traumatol Arthrosc. 2016;24(2):464-469. doi:10.1007/s00167-014-3449-8.
28. Bents EJ, Brady PC, Adams CR, Tokish JM, Higgins LD, Denard PJ. Patient-reported outcomes of knotted and knotless glenohumeral labral repairs are equivalent. Am J Orthop. 2017;46(6):279-283.
ABSTRACT
The use of knotless suture anchors has increased in popularity; however, there is a paucity of literature examining the difference in clinical outcomes with traditional knotted fixation. It was hypothesized that knotless fixation would provide superior clinical outcomes, improved return to play (RTP), and lower revision rates as compared with traditional knotted fixation in the repair of SLAP IIb tears. Seventy-four athletes who underwent arthroscopic SLAP IIb repair with traditional (n = 42) and knotless anchors (n = 32) by a single surgeon were evaluated after a minimum 2-year follow. Demographic and surgical data, RTP, Kerlan-Jobe Orthopaedic Clinic (KJOC) score, American Shoulder and Elbow Surgeons (ASES) score, stability, strength, and pain scores were compared. Knotless anchors had slightly higher RTP (93.5% vs 90.2%, P = .94) and RTP at the same level (58.1% vs 53.7% P = .81) compared with knotted fixation, but the difference did not reach statistical significance. Knotless anchors were less likely to require revision surgery than traditional anchors (9% vs 17%, P = .50), but the difference was not statistically significant. When comparing knotless and traditional knotted suture anchor repair of type llb SLAP tears, knotless fixation required less revision surgery and had higher RTP, ASES, and KJOC scores; however, statistical significance was not achieved in this relatively small cohort.
Continue to: Injury of the anterosuperior...
Injury of the anterosuperior labrum near the biceps origin was first described by Andrews and colleagues in 1985 in overhead athletes.1 The term SLAP, or a tear in the superior labrum anterior to posterior, was coined a few years later by Snyder and colleagues.2 They described an injury to the superior labrum beginning posteriorly and extending anteriorly, including the “anchor” of the biceps tendon to the labrum. Snyder further delineated SLAP lesions into 4 subtypes, the most common being type II, which he described as “degenerative fraying of the labrum with additional detachment of the superior labrum and biceps from the glenoid resulting in an unstable labral anchor.”2,3 Type II tears are of particular importance as they are the most common SLAP lesions, with an incidence of 55%, and comprise nearly 75% of SLAP repairs performed.2,4
Morgan and colleagues further delineated type II SLAP tears into IIa (anterior), IIb (posterior), and IIc (combined). Their group found that SLAP IIb tears were the most common type in overhead throwers, accounting for 47% of overhead athletes with type II tears.5 Further, type IIb tears can have a significant impact in throwers, in part due to greater shoulder instability as well as anterior pseudolaxity.5 SLAP injuries typically have been difficult to successfully treat nonoperatively in overhead athletes.6 A study by Edwards and colleagues6 examined 39 patients with all types of SLAP tears. Although, in their study, nonoperative management failed in 20 patients and they required surgery, 10 of the 15 overhead athletes in whom nonoperative treatment did not fail initially returned to sport at a level equal to or better than their pre-injury level, indicating that nonoperative treatment may play a role in some patients’ recovery.6
Surgical outcomes of SLAP IIb repairs have traditionally been less predictable than those of other shoulder injuries. Some believe that traditional knotted anchors may be partially to blame by abrading the rotator cuff, possibly leading to rotator cuff tears and pain. Further, knotted anchors are typically bulkier and require more experience with tying and tensioning and, therefore, may lead to less consistent results.7 The purpose of this study was to investigate if knotless anchors result in more favorable outcomes in repair of type IIb SLAP lesions when compared with traditional knotted anchors. It was hypothesized that knotless fixation will provide superior clinical outcomes, improved return to play (RTP), and lower revision rates as compared with traditional knotted fixation in the repair of SLAP IIb tears.
METHODS
PATIENT SELECTION
The authors retrospectively reviewed SLAP tears repaired by the senior author from June 2000 to September 2015. The inclusion criteria consisted of all athletes at any level who were diagnosed intraoperatively with a type IIb SLAP tear as defined by Morgan and colleagues5 with a minimum 2-year follow-up. The exclusion criteria were any patients with a previous shoulder surgery and the presence of any labral pathology aside from a SLAP IIb tear. Patients with rotator cuff or biceps pathologies were included. In all included patients, an initial course of preoperative physical therapy, including strengthening and stabilization of the scapulothoracic joint, had failed. Patient-directed surveys evaluated RTP, as well as the Kerlan-Jobe Orthopaedic Clinic (KJOC) score, American Shoulder and Elbow Surgeons (ASES) score, stability, range of motion (ROM), strength, and pain scores, as previously described.8-10 Institutional Review Board and informed consent approval were acquired prior to initiation of the study.
PATIENT EVALUATION
An appropriate preoperative history was taken, and physical examinations were performed, including evaluation of the scapulothoracic joint, as well as tests to evaluate the presence of a SLAP tear, anterior instability, posterior instability, multi-directional instability, and rotator cuff tears, as previously described.11 Patients with a history and physical examination concerning SLAP pathology underwent an magnetic resonance imaging (MRI) arthrogram, which was used in conjunction with intraoperative findings to diagnose type IIb SLAP tears.
Continue to: SURGICAL TECHNIQUE
SURGICAL TECHNIQUE
All surgeries were performed arthroscopically with the patient in the lateral decubitus position. The SLAP lesions were subsequently repaired using a technique similar to that described by Burkhart and colleagues.12 The traditional knotted fixation incorporated the use of 3.0 Bio-FASTak (Arthrex) with #2 FiberWire (Arthrex). Knotless anchor fixation was performed using 2.9 mm × 12.5 mm or 2.4 mm × 11.3 mm BioComposite PushLock (Arthrex) suture anchors, based on the size of the glenoid, with LabralTape or SutureTape (Arthrex). Patients who had surgery before January 1, 2013 underwent fixation with traditional knotted fixation; after that date, patients underwent fixation with knotless anchors.
POSTOPERATIVE REHABILITATION
Patients underwent a strict postoperative protocol in which they were kept in a sling with an abduction pillow for the first 6 weeks and performed pendulum exercises and passive motion only. A formal physical therapy regimen started at 4 weeks with passive ROM, passive posterior capsular and internal rotation stretching, scapulothoracic mobility, and biceps, rotator cuff, and capsular stabilizer strengthening. At 10 weeks, patients began biceps, rotator cuff, and scapular stabilizer resistance exercises, and at 16 weeks, throwing athletes began an interval throwing program. Patients were first eligible to return to sport without limitation at 9 months.
STATISTICAL ANALYSIS
Return to play, KJOC, ASES, stability, ROM, strength, and pain scores were analyzed and compared using Fisher exact test, the Kruskal-Wallis test, and the Wilcoxon rank sum test, where appropriate. The level of statistical significance was α = 0.05.
RESULTS
Table 1. Patient Demographics | |
Athletes (N) | 74 |
Age (yr) | 30.1 (14-64) |
Knotless anchors | 32 (43.2%) |
Knotted anchors | 42 (56.8%) |
Overhead athletes | 53 (72%) |
Throwing athletes | 29 (39%) |
Follow-up (yr) | 6.5 (2-12) |
Of the 74 athletes who met inclusion criteria, 28 were female (37.8%) and 46 (62.2%) were male. The average follow-up was 6.5 years with a minimum of 2 years and a maximum of 12 years. Forty-two (56.8%) patients underwent traditional knotted suture anchor fixation and 32 (43.2%) underwent knotless anchor fixation. The average age was 30.1 +/– 13.6 years, with a range of 14 to 64 years. The majority of athletes were right hand dominant (79.9%). Fifty-three (72%) were overhead athletes and 29 (39%) were throwing athletes (Table 1). The average age in the knotted group was 33.3 years: 29 of 42 (69%) were overhead athletes and 20 (47.6%) were throwing athletes. In the knotless group, the average age was 25.8 years: 24 of 32 (75.0%) were overhead athletes and 9 (28.1%) were throwing athletes. Primary sports at the time of injury are listed in Table 2. The average number of anchors used was 3.1, with 17 patients (23.0%) requiring ≤2 anchors, 39 (52.7%) requiring 3 anchors, and 18 (24.3%) requiring ≥4 anchors for repair. The number of anchors used was determined intraoperatively by the surgeon on the basis of the size and extent of the tear. Of the entire group of 74 patients, 91.9% returned to sport, 56.8% returned to the same level, 35.1% returned at a lower capacity, and 8.1% were unable to return to sport. Knotless anchors had a slightly higher overall RTP compared with traditional anchors (93.5% vs 90.2%, P = .94), as well as a higher RTP at the same level (58.1% vs 53.7%, P = .81). These differences were, however, not statistically significant (Table 3).
Table 2. Primary Sport at Time of SLAP IIb Injury | |
Primary Sport | n (%) |
Baseball | 14 (19.7%) |
Softball | 8 (11.3%) |
Volleyball | 6 (8.5%) |
Basketball | 5 (7.0%) |
Golf | 5 (7.0%) |
Other Sport | 33 (46.5%) |
No Primary Sport | 3 (4.1%) |
Abbreviation: SLAP, superior labrum anterior to posterior.
Knotless anchors were less likely to require revision surgery than traditional anchors (9% vs 17%, P = .50), but this difference was not statistically significant (Table 3). In the knotted group, 5 patients had revision surgery for rotator cuff tears, and 2 patients had recurrent SLAP tears. In the knotless group, 2 patients had revision surgeries for a torn rotator cuff, and 1 patient had a snapping scapula. A power analysis found that it would take over 300 athletes in each group to detect a significant difference in the revision rate between knotless and traditional anchors.
Table 3. Comparison of Anchor Type in Surgical Fixation of SLAP IIb Tears | |||||
| RTP | RTP Same Level | ASES | KJOC | Revision Rate |
Knotless anchors (n = 32) | 93.5% | 58.1% | 86.3 + 10.5 | 66.1 + 29.6 | 9% |
Traditional anchors (n = 42) | 90.2% | 53.7% | 85.3 + 15.6 | 65.6 + 27.2 | 17% |
P-value | .94 | .81 | .79 | .61 | .50 |
Abbreviations: ASES, American Shoulder and Elbow Surgeons; KJOC, Kerlan-Jobe Orthopaedic Clinic; RTP: return to play. | |||||
Continue to: Although KJOC...
Although KJOC (66.1 vs 65.6 P = .61) and ASES (86.3 vs 85.3 P = .79) scores were also superior with knotless anchors, these differences in scores were not statistically significant (Table 3). Pain was the only variable that was linked to decreased RTP, as patients who rated higher on a pain scale of 0 to 10 were less likely to return to their sport (P < .0001). There was no correlation in outcome measures or RTP with gender, age, number of anchors, or sport type (P > .05). There was no statistically significant difference in RTP, KJOC, or ASES scores between non-overhead and overhead athletes (Table 4). Overall return to sport in throwers was 85.7% (24/28), while 39.3% (11/28) returned at the same level, 46.4% (13/28) at a lower level, and 14.3% (4/28) did not return to sport.
Table 4. Overhead vs Non-Overhead Athletes After Surgical Fixation of SLAP IIb Tears | |||||
| RTP | RTP Same Level | ASES | ASES Good-Excellent | KJOC |
Overhead | 90.6% | 52.3% | 91.7 + 14.1 | 98.1% | 64.6 + 25.7 |
Non-Overhead | 95.5% | 72.7% | 86.7 + 12.7 | 100% | 88.5 + 29.6 |
P value | 0.1 | 0.29 | 0.76 | 0.50 | 0.49 |
Abbreviations: ASES, American Shoulder and Elbow Surgeons; KJOC, Kerlan-Jobe Orthopaedic Clinic; RTP: return to play. | |||||
DISCUSSION
There was no significant difference between knotted and knotless fixation in clinical outcomes or return to sport in the repair of SLAP IIb tears; however, there was a trend toward knotless anchors requiring less revision surgery and having higher RTP, ASES, and KJOC scores than knotted fixation. Despite the inclusion of 74 patients, this study was significantly underpowered, as a power analysis calculated that over 300 athletes would be required in each group to detect a difference in the revision rate.
SLAP tears, traditionally treated with knotted suture anchors, have yielded varying results in the literature, with good to excellent results being reported in 65% to 94% of patients.13-17 The success of SLAP repairs in athletes, especially overhead athletes, remains a difficult problem, as they are common injuries, and RTP is less predictable. Studies differ with regard to the percentage of overhead athletes who are able to return to their previous level of sport, with ranges being reported from 22% to 92%.16,18,19 In a systematic review of 198 patients, Sayde and colleagues16 found that 63% of overhead athletes treated with anchor fixation, tacks, or staples were able to return to their previous level of play. Morgan and colleagues5 found a higher return to sport when compared with other studies, reporting that 83% of patients undergoing SLAP repairs using traditional suture anchors had excellent results, and 87% of the 53 overhead athletes had excellent results based on UCLA shoulder scores. Further, 37 of the 44 pitchers examined (84%) were able to return to their pre-injury levels.5 This is in contrast to Friel and colleagues20 who found that in 48 patients with type II SLAP tears treated with traditional anchors, 23% reported excellent and 56% reported good results in regards to UCLA shoulder scores. Friel and colleagues also found that 62% of all athletes and 59% of overhead athletes were able to return to their previous levels of sport, which is similar to the current study.20 The large discrepancy in RTP at the pre-injury level between this study and that of Morgan and colleagues5 may be due to the shorter minimum follow-up of 1 year as well as the inclusion of all subtypes of SLAP II tears in the latter. The current study had a minimum 2-year follow-up period, with an average of 6.5 years, and was limited to SLAP IIb tears. With a longer follow-up period, patient outcomes and RTP, particularly in overhead sports, may deteriorate; therefore, the current study likely shows a more complete and accurate result.
Knotless anchors were originally introduced as a less time consuming, lower profile, and simpler device to learn and use for arthroscopic procedures.21 Kocaoglu and colleagues22 found that in Bankart repairs, the mean time per anchor placement for knotted anchors was 380 seconds, whereas placement of knotless anchors took on average 225 seconds. A learning curve also exists for proper and efficient knot tying.7 There is also variation in knot tying between surgeons, as evidenced by a wide range in both load to failure and knot height.7 A study performed by Hanypsiak and colleagues7 found that the surgical knot was the weakest portion of the suture-anchor construct, as the knot’s load to failure was less than the pullout strength of the anchor.
There is also concern for the added height associated with traditional knotted fixation, which has been supported by case reports of knot-induced glenoid erosion after arthroscopic fixation of a SLAP tear.23 Hanypsiak and colleagues7 also found that the average knot height occupied 50% to 95% of the space between the humeral head and the acromion when the shoulder is in a neutral position, indicating that the higher profile knotted anchors may contact the undersurface of the acromion, which could affect the labral repair as well as cause rotator cuff injury. Abrasion of the rotator cuff by a prominent knot may cause pain, tearing, and disability. A recent study by Park and colleagues24 reported on 11 patients with knot-induced pain after type II SLAP repair. All complained of sharp pain, with 64% also complaining of clicking. Knot location did not seem to matter, as there was no difference in preoperative symptoms, with 5 of the 11 patients having knots on the glenoid side of the repair on repeat arthroscopy. Patients with knots on the labral side did, however, have humeral head cartilage damage. The knots appeared to be the cause of pain and clicking, as after arthroscopic knot removal, dramatic pain relief was seen, with Constant and UCLA scores significantly improving in all 11 patients. All patients also had positive preoperative compression-rotation testing, and at 6 weeks after surgical knot removal, all were negative.24
Continue to: Further, as shown by Dines and colleagues...
Further, as shown by Dines and colleagues25, knotless anchors may help to better restore the meniscoid anatomy of the superior labrum better than knotted suture anchors. With regards to fixation strength, Uggen and colleagues26, using a cadaveric model, found no difference in initial fixation strength of knotless and traditional suture anchor repair of SLAP II tears, and both restored glenohumeral rotation without over-constraining the shoulder.
Despite the shorter operative time, lower profile, and more consistent tensioning with knotless anchors, the literature is limited with regard to evaluating patient outcomes. In a study by Yung and colleagues13 14 of the 16 patients with type II SLAP tears were treated with knotless anchors, and the authors found that 31.3% of patients had an excellent UCLA score while 43.8% had a good score. This is similar to the outcomes illustrated in studies by both Friel and colleagues20 and Sayde and colleagues.16 In a more recent study, Yang and colleagues27 did find some benefit in regard to ROM with knotless fixation. Their study consisted of 21 patients who underwent surgery with traditional knotted anchor fixation and 20 who underwent knotless horizontal mattress fixation. They found an average UCLA score of 37.6 and ASES score of 91.5 in patients undergoing knotless fixation, and the knotless fixation group had 5% greater total ROM, 15.6% more internal rotation at abduction, and 11.4% more external rotation at the side as compared with patients undergoing the traditional knotted technique. When compared with the current study, this study also had a significantly shorter follow-up period of 3 years.27 In a 2017 study, Bents and colleagues28 compared 44 patients who underwent knotless and 119 who underwent knotted fixation of SLAP tears. They found no statistically significant difference between knotless and knotted fixation in the ASES score, Visual Analog Scale (VAS), ASES, or Veterans RAND 12-Item Health Survey (VR-12) at 1 year postoperatively. Their outcomes were similar to those of the current study, but as in other mentioned literature, the study by Bents and colleagues28 included multiple surgeons with different postoperative protocols, was not limited to SLAP IIb tears, and also had a shorter follow-up of 1 year. Like Kocaoglu and colleagues22, Bents and colleagues did find knotless anchors to be more efficient, as operative time was reduced by 5.3 minutes per anchor. This likely would have a significant impact on surgical cost and surgeon productivity.28
One limitation of the current study was that despite the inclusion of >70 patients, the study was still significantly underpowered. It was determined that >300 patients in each group would be required to detect a significant difference in the revision rate between the 2 anchor types. Also, due to the retrospective nature of this study, no preoperative scores were collected. The inclusion of objective clinical measurements and follow-up imaging evaluating the rotator cuff and other anatomy would also be of interest.
Although statistical significance was not achieved, there was a trend toward knotless fixation requiring less revision surgery and having higher RTP, ASES, and KJOC scores when compared with traditional knotted fixation at 6.5-year follow-up. Larger studies with longer follow-up periods are necessary to determine the effects of knotted and knotless anchors on rotator cuff tears, patient reported outcomes, and RTP. These complications have been shown in the literature, mostly in case reports, and typically develop over a longer period.23 Despite this, other advantages of knotless fixation, such as its lower profile, the ability to better provide consistent tensioning, and decreased surgical time are important to consider.
ABSTRACT
The use of knotless suture anchors has increased in popularity; however, there is a paucity of literature examining the difference in clinical outcomes with traditional knotted fixation. It was hypothesized that knotless fixation would provide superior clinical outcomes, improved return to play (RTP), and lower revision rates as compared with traditional knotted fixation in the repair of SLAP IIb tears. Seventy-four athletes who underwent arthroscopic SLAP IIb repair with traditional (n = 42) and knotless anchors (n = 32) by a single surgeon were evaluated after a minimum 2-year follow. Demographic and surgical data, RTP, Kerlan-Jobe Orthopaedic Clinic (KJOC) score, American Shoulder and Elbow Surgeons (ASES) score, stability, strength, and pain scores were compared. Knotless anchors had slightly higher RTP (93.5% vs 90.2%, P = .94) and RTP at the same level (58.1% vs 53.7% P = .81) compared with knotted fixation, but the difference did not reach statistical significance. Knotless anchors were less likely to require revision surgery than traditional anchors (9% vs 17%, P = .50), but the difference was not statistically significant. When comparing knotless and traditional knotted suture anchor repair of type llb SLAP tears, knotless fixation required less revision surgery and had higher RTP, ASES, and KJOC scores; however, statistical significance was not achieved in this relatively small cohort.
Continue to: Injury of the anterosuperior...
Injury of the anterosuperior labrum near the biceps origin was first described by Andrews and colleagues in 1985 in overhead athletes.1 The term SLAP, or a tear in the superior labrum anterior to posterior, was coined a few years later by Snyder and colleagues.2 They described an injury to the superior labrum beginning posteriorly and extending anteriorly, including the “anchor” of the biceps tendon to the labrum. Snyder further delineated SLAP lesions into 4 subtypes, the most common being type II, which he described as “degenerative fraying of the labrum with additional detachment of the superior labrum and biceps from the glenoid resulting in an unstable labral anchor.”2,3 Type II tears are of particular importance as they are the most common SLAP lesions, with an incidence of 55%, and comprise nearly 75% of SLAP repairs performed.2,4
Morgan and colleagues further delineated type II SLAP tears into IIa (anterior), IIb (posterior), and IIc (combined). Their group found that SLAP IIb tears were the most common type in overhead throwers, accounting for 47% of overhead athletes with type II tears.5 Further, type IIb tears can have a significant impact in throwers, in part due to greater shoulder instability as well as anterior pseudolaxity.5 SLAP injuries typically have been difficult to successfully treat nonoperatively in overhead athletes.6 A study by Edwards and colleagues6 examined 39 patients with all types of SLAP tears. Although, in their study, nonoperative management failed in 20 patients and they required surgery, 10 of the 15 overhead athletes in whom nonoperative treatment did not fail initially returned to sport at a level equal to or better than their pre-injury level, indicating that nonoperative treatment may play a role in some patients’ recovery.6
Surgical outcomes of SLAP IIb repairs have traditionally been less predictable than those of other shoulder injuries. Some believe that traditional knotted anchors may be partially to blame by abrading the rotator cuff, possibly leading to rotator cuff tears and pain. Further, knotted anchors are typically bulkier and require more experience with tying and tensioning and, therefore, may lead to less consistent results.7 The purpose of this study was to investigate if knotless anchors result in more favorable outcomes in repair of type IIb SLAP lesions when compared with traditional knotted anchors. It was hypothesized that knotless fixation will provide superior clinical outcomes, improved return to play (RTP), and lower revision rates as compared with traditional knotted fixation in the repair of SLAP IIb tears.
METHODS
PATIENT SELECTION
The authors retrospectively reviewed SLAP tears repaired by the senior author from June 2000 to September 2015. The inclusion criteria consisted of all athletes at any level who were diagnosed intraoperatively with a type IIb SLAP tear as defined by Morgan and colleagues5 with a minimum 2-year follow-up. The exclusion criteria were any patients with a previous shoulder surgery and the presence of any labral pathology aside from a SLAP IIb tear. Patients with rotator cuff or biceps pathologies were included. In all included patients, an initial course of preoperative physical therapy, including strengthening and stabilization of the scapulothoracic joint, had failed. Patient-directed surveys evaluated RTP, as well as the Kerlan-Jobe Orthopaedic Clinic (KJOC) score, American Shoulder and Elbow Surgeons (ASES) score, stability, range of motion (ROM), strength, and pain scores, as previously described.8-10 Institutional Review Board and informed consent approval were acquired prior to initiation of the study.
PATIENT EVALUATION
An appropriate preoperative history was taken, and physical examinations were performed, including evaluation of the scapulothoracic joint, as well as tests to evaluate the presence of a SLAP tear, anterior instability, posterior instability, multi-directional instability, and rotator cuff tears, as previously described.11 Patients with a history and physical examination concerning SLAP pathology underwent an magnetic resonance imaging (MRI) arthrogram, which was used in conjunction with intraoperative findings to diagnose type IIb SLAP tears.
Continue to: SURGICAL TECHNIQUE
SURGICAL TECHNIQUE
All surgeries were performed arthroscopically with the patient in the lateral decubitus position. The SLAP lesions were subsequently repaired using a technique similar to that described by Burkhart and colleagues.12 The traditional knotted fixation incorporated the use of 3.0 Bio-FASTak (Arthrex) with #2 FiberWire (Arthrex). Knotless anchor fixation was performed using 2.9 mm × 12.5 mm or 2.4 mm × 11.3 mm BioComposite PushLock (Arthrex) suture anchors, based on the size of the glenoid, with LabralTape or SutureTape (Arthrex). Patients who had surgery before January 1, 2013 underwent fixation with traditional knotted fixation; after that date, patients underwent fixation with knotless anchors.
POSTOPERATIVE REHABILITATION
Patients underwent a strict postoperative protocol in which they were kept in a sling with an abduction pillow for the first 6 weeks and performed pendulum exercises and passive motion only. A formal physical therapy regimen started at 4 weeks with passive ROM, passive posterior capsular and internal rotation stretching, scapulothoracic mobility, and biceps, rotator cuff, and capsular stabilizer strengthening. At 10 weeks, patients began biceps, rotator cuff, and scapular stabilizer resistance exercises, and at 16 weeks, throwing athletes began an interval throwing program. Patients were first eligible to return to sport without limitation at 9 months.
STATISTICAL ANALYSIS
Return to play, KJOC, ASES, stability, ROM, strength, and pain scores were analyzed and compared using Fisher exact test, the Kruskal-Wallis test, and the Wilcoxon rank sum test, where appropriate. The level of statistical significance was α = 0.05.
RESULTS
Table 1. Patient Demographics | |
Athletes (N) | 74 |
Age (yr) | 30.1 (14-64) |
Knotless anchors | 32 (43.2%) |
Knotted anchors | 42 (56.8%) |
Overhead athletes | 53 (72%) |
Throwing athletes | 29 (39%) |
Follow-up (yr) | 6.5 (2-12) |
Of the 74 athletes who met inclusion criteria, 28 were female (37.8%) and 46 (62.2%) were male. The average follow-up was 6.5 years with a minimum of 2 years and a maximum of 12 years. Forty-two (56.8%) patients underwent traditional knotted suture anchor fixation and 32 (43.2%) underwent knotless anchor fixation. The average age was 30.1 +/– 13.6 years, with a range of 14 to 64 years. The majority of athletes were right hand dominant (79.9%). Fifty-three (72%) were overhead athletes and 29 (39%) were throwing athletes (Table 1). The average age in the knotted group was 33.3 years: 29 of 42 (69%) were overhead athletes and 20 (47.6%) were throwing athletes. In the knotless group, the average age was 25.8 years: 24 of 32 (75.0%) were overhead athletes and 9 (28.1%) were throwing athletes. Primary sports at the time of injury are listed in Table 2. The average number of anchors used was 3.1, with 17 patients (23.0%) requiring ≤2 anchors, 39 (52.7%) requiring 3 anchors, and 18 (24.3%) requiring ≥4 anchors for repair. The number of anchors used was determined intraoperatively by the surgeon on the basis of the size and extent of the tear. Of the entire group of 74 patients, 91.9% returned to sport, 56.8% returned to the same level, 35.1% returned at a lower capacity, and 8.1% were unable to return to sport. Knotless anchors had a slightly higher overall RTP compared with traditional anchors (93.5% vs 90.2%, P = .94), as well as a higher RTP at the same level (58.1% vs 53.7%, P = .81). These differences were, however, not statistically significant (Table 3).
Table 2. Primary Sport at Time of SLAP IIb Injury | |
Primary Sport | n (%) |
Baseball | 14 (19.7%) |
Softball | 8 (11.3%) |
Volleyball | 6 (8.5%) |
Basketball | 5 (7.0%) |
Golf | 5 (7.0%) |
Other Sport | 33 (46.5%) |
No Primary Sport | 3 (4.1%) |
Abbreviation: SLAP, superior labrum anterior to posterior.
Knotless anchors were less likely to require revision surgery than traditional anchors (9% vs 17%, P = .50), but this difference was not statistically significant (Table 3). In the knotted group, 5 patients had revision surgery for rotator cuff tears, and 2 patients had recurrent SLAP tears. In the knotless group, 2 patients had revision surgeries for a torn rotator cuff, and 1 patient had a snapping scapula. A power analysis found that it would take over 300 athletes in each group to detect a significant difference in the revision rate between knotless and traditional anchors.
Table 3. Comparison of Anchor Type in Surgical Fixation of SLAP IIb Tears | |||||
| RTP | RTP Same Level | ASES | KJOC | Revision Rate |
Knotless anchors (n = 32) | 93.5% | 58.1% | 86.3 + 10.5 | 66.1 + 29.6 | 9% |
Traditional anchors (n = 42) | 90.2% | 53.7% | 85.3 + 15.6 | 65.6 + 27.2 | 17% |
P-value | .94 | .81 | .79 | .61 | .50 |
Abbreviations: ASES, American Shoulder and Elbow Surgeons; KJOC, Kerlan-Jobe Orthopaedic Clinic; RTP: return to play. | |||||
Continue to: Although KJOC...
Although KJOC (66.1 vs 65.6 P = .61) and ASES (86.3 vs 85.3 P = .79) scores were also superior with knotless anchors, these differences in scores were not statistically significant (Table 3). Pain was the only variable that was linked to decreased RTP, as patients who rated higher on a pain scale of 0 to 10 were less likely to return to their sport (P < .0001). There was no correlation in outcome measures or RTP with gender, age, number of anchors, or sport type (P > .05). There was no statistically significant difference in RTP, KJOC, or ASES scores between non-overhead and overhead athletes (Table 4). Overall return to sport in throwers was 85.7% (24/28), while 39.3% (11/28) returned at the same level, 46.4% (13/28) at a lower level, and 14.3% (4/28) did not return to sport.
Table 4. Overhead vs Non-Overhead Athletes After Surgical Fixation of SLAP IIb Tears | |||||
| RTP | RTP Same Level | ASES | ASES Good-Excellent | KJOC |
Overhead | 90.6% | 52.3% | 91.7 + 14.1 | 98.1% | 64.6 + 25.7 |
Non-Overhead | 95.5% | 72.7% | 86.7 + 12.7 | 100% | 88.5 + 29.6 |
P value | 0.1 | 0.29 | 0.76 | 0.50 | 0.49 |
Abbreviations: ASES, American Shoulder and Elbow Surgeons; KJOC, Kerlan-Jobe Orthopaedic Clinic; RTP: return to play. | |||||
DISCUSSION
There was no significant difference between knotted and knotless fixation in clinical outcomes or return to sport in the repair of SLAP IIb tears; however, there was a trend toward knotless anchors requiring less revision surgery and having higher RTP, ASES, and KJOC scores than knotted fixation. Despite the inclusion of 74 patients, this study was significantly underpowered, as a power analysis calculated that over 300 athletes would be required in each group to detect a difference in the revision rate.
SLAP tears, traditionally treated with knotted suture anchors, have yielded varying results in the literature, with good to excellent results being reported in 65% to 94% of patients.13-17 The success of SLAP repairs in athletes, especially overhead athletes, remains a difficult problem, as they are common injuries, and RTP is less predictable. Studies differ with regard to the percentage of overhead athletes who are able to return to their previous level of sport, with ranges being reported from 22% to 92%.16,18,19 In a systematic review of 198 patients, Sayde and colleagues16 found that 63% of overhead athletes treated with anchor fixation, tacks, or staples were able to return to their previous level of play. Morgan and colleagues5 found a higher return to sport when compared with other studies, reporting that 83% of patients undergoing SLAP repairs using traditional suture anchors had excellent results, and 87% of the 53 overhead athletes had excellent results based on UCLA shoulder scores. Further, 37 of the 44 pitchers examined (84%) were able to return to their pre-injury levels.5 This is in contrast to Friel and colleagues20 who found that in 48 patients with type II SLAP tears treated with traditional anchors, 23% reported excellent and 56% reported good results in regards to UCLA shoulder scores. Friel and colleagues also found that 62% of all athletes and 59% of overhead athletes were able to return to their previous levels of sport, which is similar to the current study.20 The large discrepancy in RTP at the pre-injury level between this study and that of Morgan and colleagues5 may be due to the shorter minimum follow-up of 1 year as well as the inclusion of all subtypes of SLAP II tears in the latter. The current study had a minimum 2-year follow-up period, with an average of 6.5 years, and was limited to SLAP IIb tears. With a longer follow-up period, patient outcomes and RTP, particularly in overhead sports, may deteriorate; therefore, the current study likely shows a more complete and accurate result.
Knotless anchors were originally introduced as a less time consuming, lower profile, and simpler device to learn and use for arthroscopic procedures.21 Kocaoglu and colleagues22 found that in Bankart repairs, the mean time per anchor placement for knotted anchors was 380 seconds, whereas placement of knotless anchors took on average 225 seconds. A learning curve also exists for proper and efficient knot tying.7 There is also variation in knot tying between surgeons, as evidenced by a wide range in both load to failure and knot height.7 A study performed by Hanypsiak and colleagues7 found that the surgical knot was the weakest portion of the suture-anchor construct, as the knot’s load to failure was less than the pullout strength of the anchor.
There is also concern for the added height associated with traditional knotted fixation, which has been supported by case reports of knot-induced glenoid erosion after arthroscopic fixation of a SLAP tear.23 Hanypsiak and colleagues7 also found that the average knot height occupied 50% to 95% of the space between the humeral head and the acromion when the shoulder is in a neutral position, indicating that the higher profile knotted anchors may contact the undersurface of the acromion, which could affect the labral repair as well as cause rotator cuff injury. Abrasion of the rotator cuff by a prominent knot may cause pain, tearing, and disability. A recent study by Park and colleagues24 reported on 11 patients with knot-induced pain after type II SLAP repair. All complained of sharp pain, with 64% also complaining of clicking. Knot location did not seem to matter, as there was no difference in preoperative symptoms, with 5 of the 11 patients having knots on the glenoid side of the repair on repeat arthroscopy. Patients with knots on the labral side did, however, have humeral head cartilage damage. The knots appeared to be the cause of pain and clicking, as after arthroscopic knot removal, dramatic pain relief was seen, with Constant and UCLA scores significantly improving in all 11 patients. All patients also had positive preoperative compression-rotation testing, and at 6 weeks after surgical knot removal, all were negative.24
Continue to: Further, as shown by Dines and colleagues...
Further, as shown by Dines and colleagues25, knotless anchors may help to better restore the meniscoid anatomy of the superior labrum better than knotted suture anchors. With regards to fixation strength, Uggen and colleagues26, using a cadaveric model, found no difference in initial fixation strength of knotless and traditional suture anchor repair of SLAP II tears, and both restored glenohumeral rotation without over-constraining the shoulder.
Despite the shorter operative time, lower profile, and more consistent tensioning with knotless anchors, the literature is limited with regard to evaluating patient outcomes. In a study by Yung and colleagues13 14 of the 16 patients with type II SLAP tears were treated with knotless anchors, and the authors found that 31.3% of patients had an excellent UCLA score while 43.8% had a good score. This is similar to the outcomes illustrated in studies by both Friel and colleagues20 and Sayde and colleagues.16 In a more recent study, Yang and colleagues27 did find some benefit in regard to ROM with knotless fixation. Their study consisted of 21 patients who underwent surgery with traditional knotted anchor fixation and 20 who underwent knotless horizontal mattress fixation. They found an average UCLA score of 37.6 and ASES score of 91.5 in patients undergoing knotless fixation, and the knotless fixation group had 5% greater total ROM, 15.6% more internal rotation at abduction, and 11.4% more external rotation at the side as compared with patients undergoing the traditional knotted technique. When compared with the current study, this study also had a significantly shorter follow-up period of 3 years.27 In a 2017 study, Bents and colleagues28 compared 44 patients who underwent knotless and 119 who underwent knotted fixation of SLAP tears. They found no statistically significant difference between knotless and knotted fixation in the ASES score, Visual Analog Scale (VAS), ASES, or Veterans RAND 12-Item Health Survey (VR-12) at 1 year postoperatively. Their outcomes were similar to those of the current study, but as in other mentioned literature, the study by Bents and colleagues28 included multiple surgeons with different postoperative protocols, was not limited to SLAP IIb tears, and also had a shorter follow-up of 1 year. Like Kocaoglu and colleagues22, Bents and colleagues did find knotless anchors to be more efficient, as operative time was reduced by 5.3 minutes per anchor. This likely would have a significant impact on surgical cost and surgeon productivity.28
One limitation of the current study was that despite the inclusion of >70 patients, the study was still significantly underpowered. It was determined that >300 patients in each group would be required to detect a significant difference in the revision rate between the 2 anchor types. Also, due to the retrospective nature of this study, no preoperative scores were collected. The inclusion of objective clinical measurements and follow-up imaging evaluating the rotator cuff and other anatomy would also be of interest.
Although statistical significance was not achieved, there was a trend toward knotless fixation requiring less revision surgery and having higher RTP, ASES, and KJOC scores when compared with traditional knotted fixation at 6.5-year follow-up. Larger studies with longer follow-up periods are necessary to determine the effects of knotted and knotless anchors on rotator cuff tears, patient reported outcomes, and RTP. These complications have been shown in the literature, mostly in case reports, and typically develop over a longer period.23 Despite this, other advantages of knotless fixation, such as its lower profile, the ability to better provide consistent tensioning, and decreased surgical time are important to consider.
1. Andrews JR, Carson WG, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341. doi:10.1177/036354658501300508.
2. Snyder SJ, Karzel RP, Pizzo WD, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthrosc J Arthrosc Relat Surg. 1990;6(4):274-279. doi:10.1016/0749-8063(90)90056-J.
3. Ahsan ZS, Hsu JE, Gee AO. The Snyder classification of superior labrum anterior and posterior (SLAP) lesions. Clin Orthop. 2016;474(9):2075-2078. doi:10.1007/s11999-016-4826-z.
4. Erickson BJ, Jain A, Abrams GD, et al. SLAP Lesions: Trends in treatment. Arthrosc J Arthrosc Relat Surg. 2016;32(6):976-981. doi:10.1016/j.arthro.2015.11.044.
5. Morgan C, Burkhart S, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthrosc J Arthrosc Relat Surg. 1998;14(6):553-565. doi:10.1016/S0749-8063(98)70049-0.
6. Edwards SL, Lee JA, Bell J-E, et al. nonoperative treatment of superior labrum anterior posterior tears: Improvements in pain, function, and quality of life. Am J Sports Med. 2010;38(7):1456-1461. doi:10.1177/0363546510370937.
7. Hanypsiak BT, DeLong JM, Simmons L, Lowe W, Burkhart S. Knot strength varies widely among expert arthroscopists. Am J Sports Med. 2014;42(8):1978-1984. doi:10.1177/0363546514535554.
8. Alberta FG, ElAttrache NS, Bissell S, et al. The development and validation of a functional assessment tool for the upper extremity in the overhead athlete. Am J Sports Med. 2010;38(5):903-911. doi:10.1177/0363546509355642.
9. Bradley JP, McClincy MP, Arner JW, Tejwani SG. Arthroscopic capsulolabral reconstruction for posterior instability of the shoulder: a prospective study of 200 shoulders. Am J Sports Med. 2013;41(9):2005-2014. doi:10.1177/0363546513493599.
10. Michener LA, McClure PW, Sennett BJ. American shoulder and elbow surgeons standardized shoulder assessment form, patient self-report section: Reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594. doi:10.1067/mse.2002.127096.
11. Cook C, Hegedus EJ. Orthopedic Physical Examination Tests: An Evidence-Based Approach. Upper Saddle River, NJ: PearsonPrentice Hall; 2008.
12. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: Spectrum of pathology part I: Pathoanatomy and biomechanics. Arthrosc J Arthrosc Relat Surg. 2003;19(4):404-420. doi:10.1053/jars.2003.50128.
13. Yung PS-H, Fong DT-P, Kong M-F, et al. Arthroscopic repair of isolated type II superior labrum anterior–posterior lesion. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1151-1157. doi:10.1007/s00167-008-0629-4.
14. Brockmeier SF, Voos JE, Williams RJ, Altchek DW, Cordasco FA, Allen AA. Outcomes After Arthroscopic Repair of Type-II SLAP Lesions: J Bone Jt Surg-Am Vol. 2009;91(7):1595-1603. doi:10.2106/JBJS.H.00205.
15. Galano GJ, Ahmad CS, Bigliani L, Levine W. Percutaneous SLAP lesion repair technique is an effective alternative to portal of Wilmington. Orthopedics. 2010;33(11). doi:10.3928/01477447-20100924-15.
16. Sayde WM, Cohen SB, Ciccotti MG, Dodson CC. Return to play after type II superior labral anterior-posterior lesion repairs in athletes: A systematic review. Clin Orthop Relat Res. 2012;470(6):1595-1600. doi:10.1007/s11999-012-2295-6.
17. Kim K-H, Bin S-I, Kim J-M. The correlation between posterior tibial slope and maximal angle of flexion after total knee arthroplasty. Knee Surg Relat Res. 2012;24(3):158-163. doi:10.5792/ksrr.2012.24.3.158.
18. Kim S-H, Ha K-I, Kim S-H, Choi H-J. Results of arthroscopic treatment of superior labral lesions. J Bone Joint Surg Am. 2002;84-A(6):981-985.
19. Pagnani MJ, Speer KP, Altchek DW, Warren RF, Dines DM. Arthroscopic fixation of superior labral lesions using a biodegradable implant: a preliminary report. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc. 1995;11(2):194-198.
20. Friel NA, Karas V, Slabaugh MA, Cole BJ. Outcomes of type II superior labrum, anterior to posterior (SLAP) repair: Prospective evaluation at a minimum two-year follow-up. J Shoulder Elbow Surg. 2010;19(6):859-867. doi:10.1016/j.jse.2010.03.004.
21. Thal R. A knotless suture anchor. Arthrosc J Arthrosc Relat Surg. 2001;17(2):213-218. doi:10.1053/jars.2001.20666.
22. Kocaoglu B, Guven O, Nalbantoglu U, Aydin N, Haklar U. No difference between knotless sutures and suture anchors in arthroscopic repair of Bankart lesions in collision athletes. Knee Surg Sports Traumatol Arthrosc. 2009;17(7):844-849. doi:10.1007/s00167-009-0811-3.
23. Rhee YG, Ha JH. Knot-induced glenoid erosion after arthroscopic fixation for unstable superior labrum anterior-posterior lesion: Case report. J Shoulder Elbow Surg. 2006;15(3):391-393. doi:10.1016/j.jse.2005.03.010.
24. Park JG, Cho NS, Kim JY, Song JH, Hong SJ, Rhee YG. Arthroscopic Knot Removal for Failed Superior Labrum Anterior-Posterior Repair Secondary to Knot-Induced Pain. Am J Sports Med. 2017;45(11):2563-2568. doi:10.1177/0363546517713662.
25. Dines JS, ElAttrache NS. Horizontal Mattress With a Knotless Anchor to Better Recreate the Normal Superior Labrum Anatomy. Arthrosc J Arthrosc Relat Surg. 2008;24(12):1422-1425. doi:10.1016/j.arthro.2008.06.012.
26. Uggen C, Wei A, Glousman RE, et al. Biomechanical Comparison of Knotless Anchor Repair Versus Simple Suture Repair for Type II SLAP Lesions. Arthrosc J Arthrosc Relat Surg. 2009;25(10):1085-1092. doi:10.1016/j.arthro.2009.03.022.
27. Yang HJ, Yoon K, Jin H, Song HS. Clinical outcome of arthroscopic SLAP repair: conventional vertical knot versus knotless horizontal mattress sutures. Knee Surg Sports Traumatol Arthrosc. 2016;24(2):464-469. doi:10.1007/s00167-014-3449-8.
28. Bents EJ, Brady PC, Adams CR, Tokish JM, Higgins LD, Denard PJ. Patient-reported outcomes of knotted and knotless glenohumeral labral repairs are equivalent. Am J Orthop. 2017;46(6):279-283.
1. Andrews JR, Carson WG, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341. doi:10.1177/036354658501300508.
2. Snyder SJ, Karzel RP, Pizzo WD, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthrosc J Arthrosc Relat Surg. 1990;6(4):274-279. doi:10.1016/0749-8063(90)90056-J.
3. Ahsan ZS, Hsu JE, Gee AO. The Snyder classification of superior labrum anterior and posterior (SLAP) lesions. Clin Orthop. 2016;474(9):2075-2078. doi:10.1007/s11999-016-4826-z.
4. Erickson BJ, Jain A, Abrams GD, et al. SLAP Lesions: Trends in treatment. Arthrosc J Arthrosc Relat Surg. 2016;32(6):976-981. doi:10.1016/j.arthro.2015.11.044.
5. Morgan C, Burkhart S, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthrosc J Arthrosc Relat Surg. 1998;14(6):553-565. doi:10.1016/S0749-8063(98)70049-0.
6. Edwards SL, Lee JA, Bell J-E, et al. nonoperative treatment of superior labrum anterior posterior tears: Improvements in pain, function, and quality of life. Am J Sports Med. 2010;38(7):1456-1461. doi:10.1177/0363546510370937.
7. Hanypsiak BT, DeLong JM, Simmons L, Lowe W, Burkhart S. Knot strength varies widely among expert arthroscopists. Am J Sports Med. 2014;42(8):1978-1984. doi:10.1177/0363546514535554.
8. Alberta FG, ElAttrache NS, Bissell S, et al. The development and validation of a functional assessment tool for the upper extremity in the overhead athlete. Am J Sports Med. 2010;38(5):903-911. doi:10.1177/0363546509355642.
9. Bradley JP, McClincy MP, Arner JW, Tejwani SG. Arthroscopic capsulolabral reconstruction for posterior instability of the shoulder: a prospective study of 200 shoulders. Am J Sports Med. 2013;41(9):2005-2014. doi:10.1177/0363546513493599.
10. Michener LA, McClure PW, Sennett BJ. American shoulder and elbow surgeons standardized shoulder assessment form, patient self-report section: Reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594. doi:10.1067/mse.2002.127096.
11. Cook C, Hegedus EJ. Orthopedic Physical Examination Tests: An Evidence-Based Approach. Upper Saddle River, NJ: PearsonPrentice Hall; 2008.
12. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: Spectrum of pathology part I: Pathoanatomy and biomechanics. Arthrosc J Arthrosc Relat Surg. 2003;19(4):404-420. doi:10.1053/jars.2003.50128.
13. Yung PS-H, Fong DT-P, Kong M-F, et al. Arthroscopic repair of isolated type II superior labrum anterior–posterior lesion. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1151-1157. doi:10.1007/s00167-008-0629-4.
14. Brockmeier SF, Voos JE, Williams RJ, Altchek DW, Cordasco FA, Allen AA. Outcomes After Arthroscopic Repair of Type-II SLAP Lesions: J Bone Jt Surg-Am Vol. 2009;91(7):1595-1603. doi:10.2106/JBJS.H.00205.
15. Galano GJ, Ahmad CS, Bigliani L, Levine W. Percutaneous SLAP lesion repair technique is an effective alternative to portal of Wilmington. Orthopedics. 2010;33(11). doi:10.3928/01477447-20100924-15.
16. Sayde WM, Cohen SB, Ciccotti MG, Dodson CC. Return to play after type II superior labral anterior-posterior lesion repairs in athletes: A systematic review. Clin Orthop Relat Res. 2012;470(6):1595-1600. doi:10.1007/s11999-012-2295-6.
17. Kim K-H, Bin S-I, Kim J-M. The correlation between posterior tibial slope and maximal angle of flexion after total knee arthroplasty. Knee Surg Relat Res. 2012;24(3):158-163. doi:10.5792/ksrr.2012.24.3.158.
18. Kim S-H, Ha K-I, Kim S-H, Choi H-J. Results of arthroscopic treatment of superior labral lesions. J Bone Joint Surg Am. 2002;84-A(6):981-985.
19. Pagnani MJ, Speer KP, Altchek DW, Warren RF, Dines DM. Arthroscopic fixation of superior labral lesions using a biodegradable implant: a preliminary report. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc. 1995;11(2):194-198.
20. Friel NA, Karas V, Slabaugh MA, Cole BJ. Outcomes of type II superior labrum, anterior to posterior (SLAP) repair: Prospective evaluation at a minimum two-year follow-up. J Shoulder Elbow Surg. 2010;19(6):859-867. doi:10.1016/j.jse.2010.03.004.
21. Thal R. A knotless suture anchor. Arthrosc J Arthrosc Relat Surg. 2001;17(2):213-218. doi:10.1053/jars.2001.20666.
22. Kocaoglu B, Guven O, Nalbantoglu U, Aydin N, Haklar U. No difference between knotless sutures and suture anchors in arthroscopic repair of Bankart lesions in collision athletes. Knee Surg Sports Traumatol Arthrosc. 2009;17(7):844-849. doi:10.1007/s00167-009-0811-3.
23. Rhee YG, Ha JH. Knot-induced glenoid erosion after arthroscopic fixation for unstable superior labrum anterior-posterior lesion: Case report. J Shoulder Elbow Surg. 2006;15(3):391-393. doi:10.1016/j.jse.2005.03.010.
24. Park JG, Cho NS, Kim JY, Song JH, Hong SJ, Rhee YG. Arthroscopic Knot Removal for Failed Superior Labrum Anterior-Posterior Repair Secondary to Knot-Induced Pain. Am J Sports Med. 2017;45(11):2563-2568. doi:10.1177/0363546517713662.
25. Dines JS, ElAttrache NS. Horizontal Mattress With a Knotless Anchor to Better Recreate the Normal Superior Labrum Anatomy. Arthrosc J Arthrosc Relat Surg. 2008;24(12):1422-1425. doi:10.1016/j.arthro.2008.06.012.
26. Uggen C, Wei A, Glousman RE, et al. Biomechanical Comparison of Knotless Anchor Repair Versus Simple Suture Repair for Type II SLAP Lesions. Arthrosc J Arthrosc Relat Surg. 2009;25(10):1085-1092. doi:10.1016/j.arthro.2009.03.022.
27. Yang HJ, Yoon K, Jin H, Song HS. Clinical outcome of arthroscopic SLAP repair: conventional vertical knot versus knotless horizontal mattress sutures. Knee Surg Sports Traumatol Arthrosc. 2016;24(2):464-469. doi:10.1007/s00167-014-3449-8.
28. Bents EJ, Brady PC, Adams CR, Tokish JM, Higgins LD, Denard PJ. Patient-reported outcomes of knotted and knotless glenohumeral labral repairs are equivalent. Am J Orthop. 2017;46(6):279-283.
TAKE-HOME POINTS
- SLAP IIb tears are common injuries in overhead athletes, yet surgical outcomes are variable, with throwers commonly having difficulty with return to play at the same level.
- In this study, 92% of athletes returned to play post-operatively, yet only around 55% returned at the same level.
- In overhead athletes, overall return to play was 85.7%, yet only 39.3% returned at the same level.
- Knotless fixation required less revision surgery and had higher outcome scores and return to play when compared to knotted fixation; however, this did not reach statistical significance.
- Knotless fixation should be considered in SLAP IIb repairs given their lower profile leading to less rotator cuff irritation, the ability to better provide more consistent tensioning, and decreased surgical time.
Nutrition-Related Considerations in Soccer: A Review
Soccer is the world’s most popular sport. As the sport has grown, so have the physical demands and the search for ways to edge out the competition with the use of sports science and nutrition. The demands, which include intense training, ≥90 minutes matches, congested fixtures, and travel, lead to increased energy and nutrient requirements, stress on the body, and risk of impaired sleep cycles. Identifying key areas to enhance a player’s performance is an ongoing effort because of individual differences. Moreover, new information is being discovered via research, and advancing technology to measure performance is always evolving. This article focuses on the core nutrition principles known to lay the foundation for a better soccer player. These principles are obvious for some; however, nutrition and hydration are often undervalued, leaving the individual player with the responsibility to eat right. This review addresses the most applicable nutrition-related recommendations for soccer players.
Technical, tactical, and physical skills are key factors in a soccer player’s performance. However, energy demands of matches and training sessions require adequate fuel and hydration to maximize those key factors. Athletes may need to manage carbohydrates, protein, and fat separately to achieve optimal body size and body composition, and to maximize performance.
Nutrition plays a vital role in keeping the player healthy, reducing risk of injuries, speeding up recovery, and enhancing training adaptations. Research has shown what we eat and when we eat can significantly impact skeletal muscle adaptation, inflammation, immune response, and energy metabolism. These are all essential nutrition considerations for soccer players.
ENERGY METABOLISM IN SOCCER
Understanding energy demands will help determine energy requirements: type, amount, and timing of macronutrients and micronutrients. Soccer utilizes both aerobic and anaerobic energy systems. Soccer is an intermittent team-based sport; thus, it contains various high-intensity movements, such as sprinting, jumping, dribbling, and frequent changing of direction performed in between numerous low-intensity slow movements. The high intense movements collectively account for about 30% of match play, whereas 70% is walking, jogging, and standing. Although sprinting and jumping are not a large part of the 90 minutes of match play, they have a huge impact on the outcome of the match. Distance covered in the last 15 minutes of match play decreases by 14% to 45% compared with the first 15 minutes of play.1 Krustrup and colleagues2 found muscles in the quadriceps to be empty or nearly empty of glycogen (stored carbohydrates) after match play. This phenomenon can help explain a significant decrease in sprinting, jumping, and intermittent movements toward the end of a match—energy demands that rely on glycogen as the primary fuel source. Being well-fueled and hydrated and having the ability to delay fatigue can place a team at a performance advantage.
ENERGY EXPENDITURE
Beyond training load or match intensity, a soccer player’s body composition, gender, age, and position can affect energy needs. Position differences in elite soccer players show that the greatest total distance covered is by central midfielders and wide midfielders (~12 km –13 km), whereas central defenders cover the least area of the field players (≤~10 km).3,4 The environment can also play a role in energy expenditure. To further understand calorie needs, total daily energy expenditure in soccer players has been measured using doubly labeled water and estimated using heart rate, global positioning system, video match analysis, and activity records.5,6 One study estimated that energy expended during a training day for elite male soccer players is between 3442 kcal and 3824 kcal.6 Another study using doubly labeled water concluded that mean energy expenditure of elite male soccer players is 3566 kcal over a 7-day period, which included 5 training days and 2 matches.7 In terms of energy expenditure for elite female soccer players, the mean values for match day, training days, and rest days were 2914, 2783, and 2213 calories, respectively.8
Continue to: FUELING THE SOCCER PLAYER
FUELING THE SOCCER PLAYER
Depending on the match fixture, proper fueling can be a challenge due to the number of matches, travel time, and limited recovery time. Macronutrients will provide the mainstay of fuel for a player, specifically carbohydrates and fats. Carbohydrates are the preferred source of fuel for the majority of the calories consumed. Using body weight (kg) is a more current and accurate method of recommending the amount of each macronutrient an individual player should eat as compared to using a percentage of total daily calories.
- Carbohydrates: 5–10 g/kg/day
- Protein: 1.2–2.0 g/kg/day
- Fat: 0.8–1.5 g/kg/day
CARBOHYDRATE AND SOCCER PERFORMANCE
Carbohydrates are a limited supply of fuel compared with fat stores. They are an important fuel source for soccer players, as muscle glycogen is vital to performance during high intense training and match play (Table 1). Yet current research shows that a high carbohydrate intake is not required to be followed every day due to varied energy demands.9 This newer strategy is referred to as “training low,” allowing the athlete to train at a low-moderate intensity in a low glycogen state. The glycogen status of the muscle can alter the training adaptations through cellular changes in the mitochondria. Therefore, carbohydrate needs should reflect the work required or demand for optimal performance. However, on high-training load days or 24 hours pre-match, carbohydrate intake should be increased to maximize muscle glycogen stores. Soccer players need to consume up to 8-10 g/kg body weight during the 24 hours before a match.10 On low or rest days, carbohydrate intake should be reduced to reflect the decreased training load. For example, recent research has demonstrated potential training adaptations when muscle glycogen stores are not consistently high11 or intentionally kept low depending on the training load. Adjusting carbohydrate intake to the physical demands of an athlete is a strategy called nutrition periodization.
Table 1. Carbohydrates | ||
Timing | Amount | Application |
| Daily | 5–7 g/kg/day | Low–moderate training load. Match amount to training session intensity. |
Pre-Training/Match | 1–4 gm/kg | Adjust to players’ tolerance, preferences and training load. |
| During Training | 0–30 g/h | Light training session |
| Recovery/After Training | Balance meal 1.0–1.2 g/kg/h, ASAP. | Light training: < 2 h Heavy training/2 sessions/day |
| Match day -1, match day, match day +1 | 7–10 g/kg/d | Adjust to players’ tolerance, preferences. |
| During/half time | 30–60 g/h | High glycemic carbohydrates |
| Recovery/after match | 1.0–1.2 g/kg/h | High glycemic carbohydrates |
However, if glycogen stores are not well supplied before a match >90 minutes, then the muscles and the brain will become fatigued and lead to poor performance. Glycogen depletion contributes to fatigue toward the end of a match.10 In the early 1970s, Saltin and colleagues12 showed that players with high muscle glycogen stores (~400 mmol/kg dry wt) achieve higher movement intensities and cover more total distance than those players who start the match with low glycogen stores (~200 mmol/kg dry wt). Another study examined pre-match diets of male soccer players (65% vs 30% daily carbohydrate intake) to determine the effect on performance outcomes and glycogen concentrations. Results showed high-muscle glycogen concentrations in the 65% carbohydrate diet and a significantly higher amount of intense exercise bouts. More acutely, studies have shown a meal containing 200 to 300 grams of carbohydrates 2 to 4 hours before exercise prolongs endurance.13-15 Ideally, consuming fast-digesting carbohydrate sources during or at half time will help maintain blood glucose concentrations and spare muscle glycogen reserves. The majority of literature shows a 6% to 8% solution of combined fast-digesting carbohydrates (ie, glucose, fructose, sucrose, or maltodextrin) at a rate of 30 to 60 g/h enhances at least 1 aspect of performance in soccer.16-18 These performance benefits include increased running time, improved time to fatigue, and enhanced technical skills. Regarding recovery, soccer players should begin consuming carbohydrate-rich foods and beverages immediately after exhaustive training or a match to optimize glycogen reloading. Ingesting post-exercise carbohydrates stimulates muscle and liver glycogen synthesis up to tenfold compared with post-intake of no carbohydrates.19 This recovery period becomes vital when there are <8 hours between training sessions or another match, such as in youth tournaments. The form of carbohydrate, solid or liquid, can be based on preference and tolerance, as long as the source provides a large glycemic and insulin response.
An easy way to adjust daily carbohydrate intake is to schedule carbohydrate-rich foods at meals or snacks around important training sessions or before/during/after on match day. Anderson and colleagues10 looked at training loads for 1, 2, and 3 matches per week, recommending high carbohydrate intake match day minus 1, on match day, and match day plus 1 for 1 and 2 matches per week and lower carbohydrate intake on the other days. During a 3-match week, lowering carbohydrates any day of that week is not recommended. More research is needed to determine the best strategy for performance regarding carbohydrate periodization in soccer.
PROTEIN AND SOCCER PERFORMANCE
Protein is important to soccer players for muscle tissue repair, strength, bone health, and the immune system (Table 2). The American College of Sports Medicine, the Academy of Nutrition and Dietetics, and the Dietitians of Canada recommend 1.2 to 2.0 g/kg/day.20 Most soccer players meet the daily protein requirements; however, the key to optimizing the total daily amount is focusing on the source/amino acid profile, timing, and amount per feeding. Consuming divided doses of protein (20 g to 40 g) every 3 to 4 hours gives the body a continuous flow of amino acids to support muscle synthesis and recovery. In terms of body size, the recommendation is 0.25 to 0.4 g/kg every 3 to 4 hours, which includes pre-training/match and post-training/match. Protein/amino acids consumed around strength training and high-intensity sessions can promote muscle adaptations, minimize tissue breakdown, and speed recovery. Soccer matches lead to significant muscle damage21 especially at 2 sessions/day or multiple matches in a week. Protein is not a priority during training or matches, as its role is not to provide energy, and the primary goal during soccer activities is energy production. Research supports an intake of 30 to 40 g of casein, which is a slow digesting protein, at night before bed when a strength-training session has been performed that day.22,23
Table 2. Protein | ||
Timing | Amount | Application |
| Daily | 1.2–2.0 g/kg | High quality sources; chicken, lean meats, fish, seafood, eggs, dairy, beans, soy |
Pre-training/match; | 20–40 g or 0.25–0.40 g/kg | Meal/snack |
| During training/match | None needed | If training session <3 h |
| Recovery/after training Night-time feeding | 20–40 g | <30–60 min, whey, casein/whey, pea, soy protein Casein (slow-absorbing protein), strength training days |
Continue to: FAT AND SOCCER PERFORMANCE
FAT AND SOCCER PERFORMANCE
Fat is the primary source of energy at rest and at low-training intensities, such as walking or jogging for soccer players (Table 3). Besides providing slow, long-lasting energy, fat helps absorb vitamins A, D, E, and K; produce hormones; protect organs; and support the cell membrane structure. The dietary recommendations of total fat intake for athletes are similar to or slightly greater than those recommended for non-athletes. The total amount required depends on the training demands and the players’ goals. The recommended amount of dietary fat is between 20% and 35% of total daily energy intake.
Table 3. Fat | ||
Timing | Amount | Application |
Daily | 0.8–1.5 g/kg | Include well balanced meals, primarily polyunsaturated and monounsaturated fats. |
Pre-Training/Match; | ~10–30 g/meal | Limit amount. Avoid digestion and gastrointestinal issues. |
During Training/Match | None needed | Risk of gastrointestinal intolerances. |
Recovery/After Training | ~10–30 g | Include well-balanced meals, primarily polyunsaturated and monounsaturated fats. |
The key to gaining performance benefits from dietary fat depends on the type of fat selected. Some fats in excess, such as omega-6 fatty acids and saturated fats, may promote inflammation, hinder recovery, and affect brain health. Other types can help reduce inflammation, enhance muscle recovery, and improve brain health. These types include polyunsaturated omega-3 fatty acids, which are essential for the health of the athlete, allowing for a balanced fatty acid profile.23 Specific omega-3 fatty acids (EPA and DHA) have shown an improvement in the function of the mitochondria, enhancing energy cell metabolism. They also have potential to be highly anti-inflammatory, benefit rehabilitation during soft-tissue injury, and help decrease secondary damage from a concussion.
In addition, research shows that omega-3 may enhance the energy production of the mitochondria, resulting in less oxidative damage to the muscle cell.25 More research is needed on the effects of performance on soccer players. Given the slow digestion and absorption of fats, fat intake must be limited leading up to or during training sessions or matches, which may risk gastrointestinal issues and displacement of carbohydrates. Low to moderate monounsaturated and polyunsaturated fats in a recovery meal have not been shown to inhibit muscle glycogen reloading or muscle protein synthesis.26,27 In regard to fat intake post-match, fat is not a key nutrient of concern for muscle recovery, as it can be included in the next balanced meal.
MICRONUTRIENTS, VITAMINS, AND MINERALS
Exercise stresses many of the metabolic pathways where vitamins and minerals are required. High-level training demands may also increase the turnover rate of vitamins and minerals. As a result, greater dietary intakes of vitamins and minerals may be warranted. Soccer players at the greatest risk for poor vitamin and mineral levels are those who skip meals, who eliminate ≥1 of the food groups from their diet (such as vegans), or who consume unbalanced and highly processed foods. In soccer players, the micronutrients of concern include iron and vitamin D. In young female soccer players, calcium intake must be assessed along with adequate energy intake for optimal bone density. Vegetarians, vegans, and/or athletes who do not consume meat, eggs, and/or dairy in their diet are at risk for vitamin B12 deficiency. The key to obtaining all the vitamins and minerals an athlete will need is to eat a wide variety of nutrient-dense foods.
IRON
Iron deficiency, with or without anemia, may impair muscle function and limit exercise capacity. Adequate iron intake in athletes with iron deficiencies and/or anemia can improve exercise capacity. Iron depletion is 1 of the most common nutrient deficiencies observed among endurance athletes. Foot strike hemolysis can destroy red blood cells during activities such as running. Research has shown that 30% of professional male soccer players have ferritin levels <30 mcg/L at the end of a soccer season.28 Thus, fatigue and poor recovery time place soccer players at risk of an iron imbalance.29,30
Continue to: Landahl and colleagues...
Landahl and colleagues31 found that iron deficiency and iron deficiency anemia are common in female soccer players at the elite level. In their study of 28 female national soccer players, 57% had iron deficiency and 29% presented with iron deficiency anemia 6 months before the FIFA Women's World Cup. Testing hemoglobin alone is insufficient to detect relative anemia. Regular monitoring of hemoglobin and ferritin concentrations may be necessary to determine appropriate iron needs.
VITAMIN D
Vitamin D is required for optimal bone health, as it helps regulate calcium and phosphorus. Further research shows a link between vitamin D and non–bone-related functions, such as muscle health, immune support, and anti-inflammatory roles, which may be linked to performance. Soccer players with low levels of vitamin D (<30 ng/mL) may be more at risk for musculoskeletal injuries and stress fractures.34 In other sports, vitamin D may enhance muscle strength; however, no association between vitamin D and muscle strength has been found in soccer players.34,35 The geographic location of an athlete seems to be irrelevant to serum levels, as insufficient levels can be found at various latitudes.34,36-38
Evidence has shown that vitamin D may improve athletic performance in vitamin D-depleted athletes, thereby improving vertical jumps, lowering risks of muscle injury/strains and stress fractures, and reducing risk of colds/flu. In 2013, researchers showed for the first time a link between vitamin D and muscle aerobic metabolism by studying the energy efficiency of the mitochondria.32 Athletes with low vitamin D levels increased their ATP production within the muscle with vitamin D supplementation over 10 weeks to 12 weeks.33
CALCIUM
Soccer players present with stronger and denser bones than non-athletes due to running and jumping in their sport. Weight-bearing sites such as lumbar spine, hip, femoral neck, trochanter, intertrochanteric region, and both legs are sensitive to the impact of soccer movements.39 Calcium and vitamin D are also important for muscle contraction.
Given the variation in genetics, sports, and gender, optimal performance requires a healthy eating plan tailored to the individual athlete. A healthy eating plan allows an athlete to train longer and harder, delay the onset of fatigue, and speed recovery. Nutrition supports optimal performance through real food, proper hydration, nutrient timing, and supplementation.
Continue to: FLUID REQUIREMENTS FOR SOCCER PLAYERS
FLUID REQUIREMENTS FOR SOCCER PLAYERS
Many athletes overlook the importance of hydration on performance, either assuming they are hydrated or they miscalculate fluid and electrolyte needs to actual sweat losses. Numerous factors play a part in optimal hydration such as sweat rate, environment, training intensity, duration, body size, and body composition. Soccer players have fewer breaks to consume fluids during a match compared with basketball, baseball, or American football players. These breaks include a 15-minute half between coming off the pitch to the locker room and back, as well as time spent with coaches reviewing strategies; this short window of time must be maximized to rehydrate. Fluids with a carbohydrate concentration of 4% to 8% at 5 to 10 ounces and breaks every 15 to 20 minutes are optimal to maximize uptake while avoiding gastric intolerance.
Studies have shown that most players do not drink sufficiently during a match to optimize hydration, replacing only ~40% to 45% of their sweat losses.40, 41 Maughan and colleagues measured high levels of urine osmolality in some soccer players, thereby indicating that the players started their training session dehydrated.41 Soccer players must begin training or a match well hydrated due to the limited opportunities after kick-off. The athlete should drink at least 4 hours prior to exercise; if no urine is produced or urine is dark in color, then the athlete should drink again 2 hours prior.
Table 4. Sweat Rate Calculation Steps | ||
|
Changes in body mass, urine color, and thirst offer clues to the need for rehydration. Advanced hydration measurement includes testing urine specific gravity (USG) values. For example, testing pre-training or pre-match can be conducted to determine hydration status and trending changes from day to day. A USG value >1.020 is considered dehydrated in accordance with the NATA position statement.42 Calculating a sweat rate is a practical approach to determining individual hydration needs (see Table 4). Sweat rates will vary between soccer players based on their position and intensity of play, along with total match time.39 Soccer players will lose ~1.5 to 4.5 liters during match play.43-46 In general, athletes, including soccer players, should limit body weight loss to ≤2% to 3% to maintain performance. Studies have shown that >2% body mass loss can hinder soccer-specific performance, such as dribbling skills and intermittent high intensity sprinting.49-51) Table 5 outlines the detrimental effects dehydration has on performance. Urine-specific gravity values between 1.021 and 1.030 may reflect 3% to 5% change in body weight.
Table 5. Performance Outcomes at Various Dehydration Levels | ||
|
ELECTROLYTES
Sodium is the primary electrolyte lost in sweat. Other electrolytes (potassium, magnesium, and calcium) are lost at much lower levels and typically replaced through diet. Soccer players can lose large amounts of sodium; between 700 and 1500 mg of sodium/L of sweat has been reported in several studies.42-44 Studies of professional male soccer players have shown potassium losses in the range of 165 mg/L to 234 mg/L.42,51,52 Sodium in a sports drink or in food aids with water uptake from the intestines and enhances the thirst mechanism in the brain, resulting in additional fluid being retained in the body.
REHYDRATION AFTER TRAINING OR COMPETITION
Within 2 hours after training or competition, the rehydration strategy should provide water to restore body fluid status, carbohydrates to replenish glycogen (fuel) stores, and electrolytes to speed rehydration (Table 6). The volume of fluids and type of fluids over the next 24 hours dictate the hydration status prior to the next day’s training session. It is a continuous cycle. Over time, an athlete increases the risk of being in a chronic dehydrated state, resulting in lack of motivation, risk of injury, and illness, fatigue, and poor performance. The current recommendation is to drink ~50% more in volume than the amount of weight lost, such as 22 to 24 ounces/pound lost.52
Table 6. Hydration | ||
Timing | Amount | Application |
Daily | 3.7 L adult males | Monitor urine color. |
Pre-training/match; | 16 oz or 5–7 mL/kg | Monitor urine production and color |
During training/match | 13–28 oz/h (400- | Every 15–20 min. *Dependent on sweat rate. |
Recovery/after training | 22–24 oz/1 lb body weight lost | Water + food (carbohydrates/electrolytes) |
- Mohr M, Krustrup P, Bangsbo J. Match performance of high-standard soccer players with special reference to development of fatigue. J Sports Sci. 2003;21:519-528.
- Krustrup P, Mohr M, Steensberg A, Bencke J, Kjaer M, Bangsbo J. Muscle and blood metabolites during a soccer game: implications for sprint performance. Med Sci Sports Exerc. 2006;38:1165-1174.
- Di Salvo V, Gregson W, Atkinson G, Tordoff P, Drust B. Analysis of high intensity activity in Premier League soccer. Int J Sports Med. 2009;30:205-212.
- Di Salvo V, Baron R, Tschan H, Calderon Montero FJ, Bachl N, Pigozzi F. Performance characteristics according to playing position in elite soccer. Int J Sports Med. 2007;28:222-227.
- Reilly T, Thomas V. Estimated daily energy expenditures of professional association footballers. Ergonomics. 1979;22:541-548.
- Osgnach C, Poser S, Bernardini R, Rinaldo R, di Prampero P.E. Energy cost and metabolic power in elite soccer: A new match analysis approach. Med Sci Sports Exerc. 2010;42:170-178.
- Anderson L, Orme P, Naughton RJ, Close, GL, Milsom J, Rydings D, et al. Energy intake and expenditure of professional soccer players of the English Premier League: evidence of carbohydrate periodization. Int J Sport Nutr Exerc Metab. 2017;1-25.
- Mara JK, Thompson KG, Pumpa KL. Assessing the energy expenditure of elite female soccer layers: a preliminary study. J Strength Cond Res. 2015;2780-2786.
- Bartlett JD, Hawley JA, Morton JP. Eur J Sport Sci. 2015;15(1):1, 3-12.
- Anderson L, Orme P, Di Michele R, Close GL, Morgans R, Drust B, Morton JP. Quantification of training load during one-, two- and three-game week schedules in professional soccer players from the English Premier League: implications for carbohydrate periodisation. J Sports Sci. 2016;34;1250-1259.
- Hawley JA, Morton JP. Ramping up the signal: promoting endurance training adaptation in skeletal muscle by nutritional manipulation. Clin Exp Pharmacol Physiol. 2014;41:608-613.
- Saltin B. Metabolic fundamentals in exercise. 1973;:137-146.
- Balsom PD, Wood K, Olsson P, Ekblom B. Carbohydrate intake and multiple sprint sports: With special reference to football (soccer). Int J Sports Med. 1999;20:48-52.
- Neufer PD, Costill DL, Flynn MG, Kirwan JP, Mitchell JB, Houmard J. Improvements in exercise performance: Effects of carbohydrate feedings and diet. J Appl Physiol. 1987;62:983-988.
- Sherman WM, Brodowicz G, Wright DA, Allen WK, Simonsen J, Dernbach A. Effects of 4 h preexercise carbohydrate feedings on cycling performance. Med Sci Sports Exerc. 1989;21:598-604.
- Baker LB, Rollo I, Stein KW, Jeukendrup AE. Acute effects of carbohydrate supplementation on intermittent sports performance. Nutrients. 2015;7:5733-5763.
- Goedecke JH, White NJ, Chicktay W, Mahomed H, Durandt J, Lambert MI. The effect of carbohydrate ingestion on performance during a simulated soccer match. Nutrients. 2013;5:5193-5204.
- Nicholas CW, Williams C, Lakomy HK, Phillips G, Nowitz A. Influence of ingesting a carbohydrate-electrolyte solution on endurance capacity during intermittent, high-intensity shuttle running. J Sports Sci. 1995;13:283-290.
- Burke LM, van Loon LJC, Hawley JA. Post-exercise muscle glycogen resynthesis in humans. J Appl Physiol. 2016;122:1055-1067.
- Rodriquez NR, DiMarco NM, Langley S. Position of the American Dietetic Association, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. J Am Diet Assoc. 2009;109(3):509-527.
- Romagnoli M, Sanchis-Gomar F, Alis R, Risso-Ballester J, Bosio A, Graziani RL, Rampinini E. Changes in muscle damage, inflammation, and fatigue-related parameters in young elite soccer players after a match. J. Sports Med Phys Fit. 2016;56:1198-1205.
- Res PT, Groen B, Pennings B, Beelen M, Wallis GA, Gijsen AP, et al.Protein ingestion before sleep improves postexercise overnight recovery. Med Sci Sports Exerc. 2012;44:1560-1569.
- Snijders T, Res PT, Smeets JSJ, Van Vliet S, Van Kranenburg J, Maase K, et al.Protein ingestion before sleep increases muscle mass and strength gains during prolonged resistance-type exercise training in healthy young men. J Nutr. 2015;145:1178-1184.
- Simopoulos AP. Omega-3 fatty acids and athletics. Curr Sports Med Rep. 2007;6230-236.
- Peoples GE, McLennan PL, Howe P, Groeller H. Fish oil reduces apparent myocardial oxygen consumption in trained cyclists but does not change time to fatigue. Presented at the Fourth International Conference on Nutrition and Fitness; May 25-29, 2000; Ancient Olympia, Greece.
- Burke LM, Collier GR, Beasley S.K, Davis PG, Fricker PA, Heeley P, et al. Effect of coingestion of fat and protein with carbohydrate feedings on muscle glycogen storage. J Appl Physiol. 1995;78:2187-2192.
- Roy BD, Tarnopolsky MA. Influence of differing macronutrient intakes on muscle glycogen resynthesis after resistance exercise. J Appl Physiol. 1998;84:890-896.
- Reinke S, Taylor W.R, Duda GN, von Haehling S, Reinke P, Volk H-D et al. Absolute and functional iron deficiency in professional athletes during training and recovery. Int J Cardiol. 2012;156:186-191.
- Escanero JF, Villanueva J, Rojo A, Herrera A, del Diego C, Guerra M. Iron stores in professional athletes throughout the sports season. Physiol Behav. 1997;62:811-814.
- Heisterberg MF, Fahrenkrug J, Krustrup P, Storskov A, Kjær, M, Andersen JL. Extensive monitoring
- Landahl G, Adolfsson P, Borjesson M, Mannheimer C, Rodjer S. Iron deficiency and anemia: a common problem in female elite soccer players. Int J Sport Nutr Exerc Metab. 2005;15(6):689-694.
- Sinha A, Hollingsworth K, Ball S, Cheetham T. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. Endocrine Abstracts, 2013;31.OC1.6
- Shuler FD, Wingate MK, Moore GH, Giangarra C. Sports health benefits of vitamin D. Sports Health. 2012;4:496-501.
- Hamilton B, Whiteley R, Farooq A, Chalabi H. Vitamin D concentration in 342 professional football players and association with lower limb isokinetic function. J Sci. Med Sport. 2014;17:139-143.
- Ksiażek A, Zagrodna A, Dziubek W, Pietraszewski B, Ochmann B, Słowińska-Lisowska M,25(OH)D3 levels relative to muscle strength and maximum oxygen uptake in athletes. J Hum Kinet. 2016;50:71-77.
- Kopeć A, Solarz K, Majda F, Słowińska-Lisowska M, Medraś M. An evaluation of the levels of vitamin D and bone turnover markers after the summer and winter periods in Polish professional soccer players. J Hum Kinet. 2013;38:135-140.
- Vander Slagmolen G, van Hellemondt FJ, Wielders JPM. Do professional soccer players have a vitamin D status supporting optimal performance in winter time? J Sports Med Doping Stud. 2014,4:2.
- Morton JP, Iqbal Z, Drust B, Burgess D, Close GL, Brukner PD. Seasonal variation in vitamin D status in professional soccer players of the English Premier League. Appl Physiol Nutr Metab. 2012;37:798-802.
- Lozano-Berges G, Matute-Llorente A, Gonzalez-Aguero A, Gomez-Bruton A, Gomez-Cabelloa A, Vincente-Rodriguez G, Casajus JA. Soccer helps build strong bones during growth: a systematic review and meta-analysis. Eur J Pediatr. 2018;177(3):295-310.
- Burke LM. Fluid balance during team sports. J Sports Sci. 1997;15:287-295.
- Maughan RJ, Merson SJ, Broad NP, Shirreffs SM. Fluid and electrolyte intake and loss in elite soccer players during training. Int J Sport Nutr Exerc Metab. 2004;14:333-346.
- Brendon P, McDermott, P, Anderson SA, Armstrong LE, Casa DJ, Cheuvront SN, et al. National Athletic Trainers’ Association Position Statement: Fluid Replacement for the Physically Active. J Athl Train. 2017;52(9):877-895.
- Shirreffs SM, Aragon-Vargas LF, Chamorro M, Maughan RJ, Serratosa L, Zachwieja JJ. The sweating response of elite professional soccer players to training in the heat. Int J Sports Med. 2005;26: 90-95.
- Maughan RJ, Watson P, Evans GH, Broad N, Shirreffs SM. Water balance and salt losses in competitive football. Int J Sport Nutr Exerc Metab. 2007;17:583-594.
- Aragón-Vargas LF, Moncada-Jiménez J, Hernández-Elizondo J, Barrenechea A,Monge-Alvarado M. Evaluation of pre-game hydration status, heat stress, and fluid balance during professional soccer competition in the heat. Eur J Sport Sci. 2009;9:269-276.
- Maughan RJ, Shirreffs SM, Merson SJ, Horswill CA. Fluid and electrolyte balance in elite male football (soccer) players training in a cool environment. J Sports Sci. 2005;23:73-79.
- Duffield R, McCall A, Coutts AJ, Peiffer JJ. Hydration, sweat and thermoregulatory responses to professional football training in the heat. J Sports Sci. 2012;30:957-965.
- Shirreffs SM, Aragon-Vargas LF, Chamorro M, Maughan RJ, Serratosa L, Zachwieja JJ. The sweating response of elite professional soccer players to training in the heat. Int J Sports Med. 2005;26:90-95.
- Edwards AM, Mann ME, Marfell-Jones MJ, Rankin DM, Noakes TD, Shillington DP. Influence of moderate dehydration on soccer performance: Physiological responses to 45 min of outdoor match-play and the immediate subsequent performance of sport-specific and mental concentration tests. Br J Sports Med. 2007;41:385-391.
- McGregor SJ, Nicholas CW, Lakomy HK, Williams C. The influence of intermittent high-intensity shuttle running and fluid ingestion on the performance of a soccer skill. J Sports Sci. 1999;17:895-903.
- Maughan RJ, Merson SJ, Broad NP, Shirreffs SM. Fluid and electrolyte intake and loss in elite soccer players during training. Int J Sport Nutr Exerc Metab. 2004;14:333-346.
- Shirreffs SM, Sawka MN, Stone M. Water and electrolyte needs for football training and match-play. J Sports Sci. 2006;24:699-707.
Soccer is the world’s most popular sport. As the sport has grown, so have the physical demands and the search for ways to edge out the competition with the use of sports science and nutrition. The demands, which include intense training, ≥90 minutes matches, congested fixtures, and travel, lead to increased energy and nutrient requirements, stress on the body, and risk of impaired sleep cycles. Identifying key areas to enhance a player’s performance is an ongoing effort because of individual differences. Moreover, new information is being discovered via research, and advancing technology to measure performance is always evolving. This article focuses on the core nutrition principles known to lay the foundation for a better soccer player. These principles are obvious for some; however, nutrition and hydration are often undervalued, leaving the individual player with the responsibility to eat right. This review addresses the most applicable nutrition-related recommendations for soccer players.
Technical, tactical, and physical skills are key factors in a soccer player’s performance. However, energy demands of matches and training sessions require adequate fuel and hydration to maximize those key factors. Athletes may need to manage carbohydrates, protein, and fat separately to achieve optimal body size and body composition, and to maximize performance.
Nutrition plays a vital role in keeping the player healthy, reducing risk of injuries, speeding up recovery, and enhancing training adaptations. Research has shown what we eat and when we eat can significantly impact skeletal muscle adaptation, inflammation, immune response, and energy metabolism. These are all essential nutrition considerations for soccer players.
ENERGY METABOLISM IN SOCCER
Understanding energy demands will help determine energy requirements: type, amount, and timing of macronutrients and micronutrients. Soccer utilizes both aerobic and anaerobic energy systems. Soccer is an intermittent team-based sport; thus, it contains various high-intensity movements, such as sprinting, jumping, dribbling, and frequent changing of direction performed in between numerous low-intensity slow movements. The high intense movements collectively account for about 30% of match play, whereas 70% is walking, jogging, and standing. Although sprinting and jumping are not a large part of the 90 minutes of match play, they have a huge impact on the outcome of the match. Distance covered in the last 15 minutes of match play decreases by 14% to 45% compared with the first 15 minutes of play.1 Krustrup and colleagues2 found muscles in the quadriceps to be empty or nearly empty of glycogen (stored carbohydrates) after match play. This phenomenon can help explain a significant decrease in sprinting, jumping, and intermittent movements toward the end of a match—energy demands that rely on glycogen as the primary fuel source. Being well-fueled and hydrated and having the ability to delay fatigue can place a team at a performance advantage.
ENERGY EXPENDITURE
Beyond training load or match intensity, a soccer player’s body composition, gender, age, and position can affect energy needs. Position differences in elite soccer players show that the greatest total distance covered is by central midfielders and wide midfielders (~12 km –13 km), whereas central defenders cover the least area of the field players (≤~10 km).3,4 The environment can also play a role in energy expenditure. To further understand calorie needs, total daily energy expenditure in soccer players has been measured using doubly labeled water and estimated using heart rate, global positioning system, video match analysis, and activity records.5,6 One study estimated that energy expended during a training day for elite male soccer players is between 3442 kcal and 3824 kcal.6 Another study using doubly labeled water concluded that mean energy expenditure of elite male soccer players is 3566 kcal over a 7-day period, which included 5 training days and 2 matches.7 In terms of energy expenditure for elite female soccer players, the mean values for match day, training days, and rest days were 2914, 2783, and 2213 calories, respectively.8
Continue to: FUELING THE SOCCER PLAYER
FUELING THE SOCCER PLAYER
Depending on the match fixture, proper fueling can be a challenge due to the number of matches, travel time, and limited recovery time. Macronutrients will provide the mainstay of fuel for a player, specifically carbohydrates and fats. Carbohydrates are the preferred source of fuel for the majority of the calories consumed. Using body weight (kg) is a more current and accurate method of recommending the amount of each macronutrient an individual player should eat as compared to using a percentage of total daily calories.
- Carbohydrates: 5–10 g/kg/day
- Protein: 1.2–2.0 g/kg/day
- Fat: 0.8–1.5 g/kg/day
CARBOHYDRATE AND SOCCER PERFORMANCE
Carbohydrates are a limited supply of fuel compared with fat stores. They are an important fuel source for soccer players, as muscle glycogen is vital to performance during high intense training and match play (Table 1). Yet current research shows that a high carbohydrate intake is not required to be followed every day due to varied energy demands.9 This newer strategy is referred to as “training low,” allowing the athlete to train at a low-moderate intensity in a low glycogen state. The glycogen status of the muscle can alter the training adaptations through cellular changes in the mitochondria. Therefore, carbohydrate needs should reflect the work required or demand for optimal performance. However, on high-training load days or 24 hours pre-match, carbohydrate intake should be increased to maximize muscle glycogen stores. Soccer players need to consume up to 8-10 g/kg body weight during the 24 hours before a match.10 On low or rest days, carbohydrate intake should be reduced to reflect the decreased training load. For example, recent research has demonstrated potential training adaptations when muscle glycogen stores are not consistently high11 or intentionally kept low depending on the training load. Adjusting carbohydrate intake to the physical demands of an athlete is a strategy called nutrition periodization.
Table 1. Carbohydrates | ||
Timing | Amount | Application |
| Daily | 5–7 g/kg/day | Low–moderate training load. Match amount to training session intensity. |
Pre-Training/Match | 1–4 gm/kg | Adjust to players’ tolerance, preferences and training load. |
| During Training | 0–30 g/h | Light training session |
| Recovery/After Training | Balance meal 1.0–1.2 g/kg/h, ASAP. | Light training: < 2 h Heavy training/2 sessions/day |
| Match day -1, match day, match day +1 | 7–10 g/kg/d | Adjust to players’ tolerance, preferences. |
| During/half time | 30–60 g/h | High glycemic carbohydrates |
| Recovery/after match | 1.0–1.2 g/kg/h | High glycemic carbohydrates |
However, if glycogen stores are not well supplied before a match >90 minutes, then the muscles and the brain will become fatigued and lead to poor performance. Glycogen depletion contributes to fatigue toward the end of a match.10 In the early 1970s, Saltin and colleagues12 showed that players with high muscle glycogen stores (~400 mmol/kg dry wt) achieve higher movement intensities and cover more total distance than those players who start the match with low glycogen stores (~200 mmol/kg dry wt). Another study examined pre-match diets of male soccer players (65% vs 30% daily carbohydrate intake) to determine the effect on performance outcomes and glycogen concentrations. Results showed high-muscle glycogen concentrations in the 65% carbohydrate diet and a significantly higher amount of intense exercise bouts. More acutely, studies have shown a meal containing 200 to 300 grams of carbohydrates 2 to 4 hours before exercise prolongs endurance.13-15 Ideally, consuming fast-digesting carbohydrate sources during or at half time will help maintain blood glucose concentrations and spare muscle glycogen reserves. The majority of literature shows a 6% to 8% solution of combined fast-digesting carbohydrates (ie, glucose, fructose, sucrose, or maltodextrin) at a rate of 30 to 60 g/h enhances at least 1 aspect of performance in soccer.16-18 These performance benefits include increased running time, improved time to fatigue, and enhanced technical skills. Regarding recovery, soccer players should begin consuming carbohydrate-rich foods and beverages immediately after exhaustive training or a match to optimize glycogen reloading. Ingesting post-exercise carbohydrates stimulates muscle and liver glycogen synthesis up to tenfold compared with post-intake of no carbohydrates.19 This recovery period becomes vital when there are <8 hours between training sessions or another match, such as in youth tournaments. The form of carbohydrate, solid or liquid, can be based on preference and tolerance, as long as the source provides a large glycemic and insulin response.
An easy way to adjust daily carbohydrate intake is to schedule carbohydrate-rich foods at meals or snacks around important training sessions or before/during/after on match day. Anderson and colleagues10 looked at training loads for 1, 2, and 3 matches per week, recommending high carbohydrate intake match day minus 1, on match day, and match day plus 1 for 1 and 2 matches per week and lower carbohydrate intake on the other days. During a 3-match week, lowering carbohydrates any day of that week is not recommended. More research is needed to determine the best strategy for performance regarding carbohydrate periodization in soccer.
PROTEIN AND SOCCER PERFORMANCE
Protein is important to soccer players for muscle tissue repair, strength, bone health, and the immune system (Table 2). The American College of Sports Medicine, the Academy of Nutrition and Dietetics, and the Dietitians of Canada recommend 1.2 to 2.0 g/kg/day.20 Most soccer players meet the daily protein requirements; however, the key to optimizing the total daily amount is focusing on the source/amino acid profile, timing, and amount per feeding. Consuming divided doses of protein (20 g to 40 g) every 3 to 4 hours gives the body a continuous flow of amino acids to support muscle synthesis and recovery. In terms of body size, the recommendation is 0.25 to 0.4 g/kg every 3 to 4 hours, which includes pre-training/match and post-training/match. Protein/amino acids consumed around strength training and high-intensity sessions can promote muscle adaptations, minimize tissue breakdown, and speed recovery. Soccer matches lead to significant muscle damage21 especially at 2 sessions/day or multiple matches in a week. Protein is not a priority during training or matches, as its role is not to provide energy, and the primary goal during soccer activities is energy production. Research supports an intake of 30 to 40 g of casein, which is a slow digesting protein, at night before bed when a strength-training session has been performed that day.22,23
Table 2. Protein | ||
Timing | Amount | Application |
| Daily | 1.2–2.0 g/kg | High quality sources; chicken, lean meats, fish, seafood, eggs, dairy, beans, soy |
Pre-training/match; | 20–40 g or 0.25–0.40 g/kg | Meal/snack |
| During training/match | None needed | If training session <3 h |
| Recovery/after training Night-time feeding | 20–40 g | <30–60 min, whey, casein/whey, pea, soy protein Casein (slow-absorbing protein), strength training days |
Continue to: FAT AND SOCCER PERFORMANCE
FAT AND SOCCER PERFORMANCE
Fat is the primary source of energy at rest and at low-training intensities, such as walking or jogging for soccer players (Table 3). Besides providing slow, long-lasting energy, fat helps absorb vitamins A, D, E, and K; produce hormones; protect organs; and support the cell membrane structure. The dietary recommendations of total fat intake for athletes are similar to or slightly greater than those recommended for non-athletes. The total amount required depends on the training demands and the players’ goals. The recommended amount of dietary fat is between 20% and 35% of total daily energy intake.
Table 3. Fat | ||
Timing | Amount | Application |
Daily | 0.8–1.5 g/kg | Include well balanced meals, primarily polyunsaturated and monounsaturated fats. |
Pre-Training/Match; | ~10–30 g/meal | Limit amount. Avoid digestion and gastrointestinal issues. |
During Training/Match | None needed | Risk of gastrointestinal intolerances. |
Recovery/After Training | ~10–30 g | Include well-balanced meals, primarily polyunsaturated and monounsaturated fats. |
The key to gaining performance benefits from dietary fat depends on the type of fat selected. Some fats in excess, such as omega-6 fatty acids and saturated fats, may promote inflammation, hinder recovery, and affect brain health. Other types can help reduce inflammation, enhance muscle recovery, and improve brain health. These types include polyunsaturated omega-3 fatty acids, which are essential for the health of the athlete, allowing for a balanced fatty acid profile.23 Specific omega-3 fatty acids (EPA and DHA) have shown an improvement in the function of the mitochondria, enhancing energy cell metabolism. They also have potential to be highly anti-inflammatory, benefit rehabilitation during soft-tissue injury, and help decrease secondary damage from a concussion.
In addition, research shows that omega-3 may enhance the energy production of the mitochondria, resulting in less oxidative damage to the muscle cell.25 More research is needed on the effects of performance on soccer players. Given the slow digestion and absorption of fats, fat intake must be limited leading up to or during training sessions or matches, which may risk gastrointestinal issues and displacement of carbohydrates. Low to moderate monounsaturated and polyunsaturated fats in a recovery meal have not been shown to inhibit muscle glycogen reloading or muscle protein synthesis.26,27 In regard to fat intake post-match, fat is not a key nutrient of concern for muscle recovery, as it can be included in the next balanced meal.
MICRONUTRIENTS, VITAMINS, AND MINERALS
Exercise stresses many of the metabolic pathways where vitamins and minerals are required. High-level training demands may also increase the turnover rate of vitamins and minerals. As a result, greater dietary intakes of vitamins and minerals may be warranted. Soccer players at the greatest risk for poor vitamin and mineral levels are those who skip meals, who eliminate ≥1 of the food groups from their diet (such as vegans), or who consume unbalanced and highly processed foods. In soccer players, the micronutrients of concern include iron and vitamin D. In young female soccer players, calcium intake must be assessed along with adequate energy intake for optimal bone density. Vegetarians, vegans, and/or athletes who do not consume meat, eggs, and/or dairy in their diet are at risk for vitamin B12 deficiency. The key to obtaining all the vitamins and minerals an athlete will need is to eat a wide variety of nutrient-dense foods.
IRON
Iron deficiency, with or without anemia, may impair muscle function and limit exercise capacity. Adequate iron intake in athletes with iron deficiencies and/or anemia can improve exercise capacity. Iron depletion is 1 of the most common nutrient deficiencies observed among endurance athletes. Foot strike hemolysis can destroy red blood cells during activities such as running. Research has shown that 30% of professional male soccer players have ferritin levels <30 mcg/L at the end of a soccer season.28 Thus, fatigue and poor recovery time place soccer players at risk of an iron imbalance.29,30
Continue to: Landahl and colleagues...
Landahl and colleagues31 found that iron deficiency and iron deficiency anemia are common in female soccer players at the elite level. In their study of 28 female national soccer players, 57% had iron deficiency and 29% presented with iron deficiency anemia 6 months before the FIFA Women's World Cup. Testing hemoglobin alone is insufficient to detect relative anemia. Regular monitoring of hemoglobin and ferritin concentrations may be necessary to determine appropriate iron needs.
VITAMIN D
Vitamin D is required for optimal bone health, as it helps regulate calcium and phosphorus. Further research shows a link between vitamin D and non–bone-related functions, such as muscle health, immune support, and anti-inflammatory roles, which may be linked to performance. Soccer players with low levels of vitamin D (<30 ng/mL) may be more at risk for musculoskeletal injuries and stress fractures.34 In other sports, vitamin D may enhance muscle strength; however, no association between vitamin D and muscle strength has been found in soccer players.34,35 The geographic location of an athlete seems to be irrelevant to serum levels, as insufficient levels can be found at various latitudes.34,36-38
Evidence has shown that vitamin D may improve athletic performance in vitamin D-depleted athletes, thereby improving vertical jumps, lowering risks of muscle injury/strains and stress fractures, and reducing risk of colds/flu. In 2013, researchers showed for the first time a link between vitamin D and muscle aerobic metabolism by studying the energy efficiency of the mitochondria.32 Athletes with low vitamin D levels increased their ATP production within the muscle with vitamin D supplementation over 10 weeks to 12 weeks.33
CALCIUM
Soccer players present with stronger and denser bones than non-athletes due to running and jumping in their sport. Weight-bearing sites such as lumbar spine, hip, femoral neck, trochanter, intertrochanteric region, and both legs are sensitive to the impact of soccer movements.39 Calcium and vitamin D are also important for muscle contraction.
Given the variation in genetics, sports, and gender, optimal performance requires a healthy eating plan tailored to the individual athlete. A healthy eating plan allows an athlete to train longer and harder, delay the onset of fatigue, and speed recovery. Nutrition supports optimal performance through real food, proper hydration, nutrient timing, and supplementation.
Continue to: FLUID REQUIREMENTS FOR SOCCER PLAYERS
FLUID REQUIREMENTS FOR SOCCER PLAYERS
Many athletes overlook the importance of hydration on performance, either assuming they are hydrated or they miscalculate fluid and electrolyte needs to actual sweat losses. Numerous factors play a part in optimal hydration such as sweat rate, environment, training intensity, duration, body size, and body composition. Soccer players have fewer breaks to consume fluids during a match compared with basketball, baseball, or American football players. These breaks include a 15-minute half between coming off the pitch to the locker room and back, as well as time spent with coaches reviewing strategies; this short window of time must be maximized to rehydrate. Fluids with a carbohydrate concentration of 4% to 8% at 5 to 10 ounces and breaks every 15 to 20 minutes are optimal to maximize uptake while avoiding gastric intolerance.
Studies have shown that most players do not drink sufficiently during a match to optimize hydration, replacing only ~40% to 45% of their sweat losses.40, 41 Maughan and colleagues measured high levels of urine osmolality in some soccer players, thereby indicating that the players started their training session dehydrated.41 Soccer players must begin training or a match well hydrated due to the limited opportunities after kick-off. The athlete should drink at least 4 hours prior to exercise; if no urine is produced or urine is dark in color, then the athlete should drink again 2 hours prior.
Table 4. Sweat Rate Calculation Steps | ||
|
Changes in body mass, urine color, and thirst offer clues to the need for rehydration. Advanced hydration measurement includes testing urine specific gravity (USG) values. For example, testing pre-training or pre-match can be conducted to determine hydration status and trending changes from day to day. A USG value >1.020 is considered dehydrated in accordance with the NATA position statement.42 Calculating a sweat rate is a practical approach to determining individual hydration needs (see Table 4). Sweat rates will vary between soccer players based on their position and intensity of play, along with total match time.39 Soccer players will lose ~1.5 to 4.5 liters during match play.43-46 In general, athletes, including soccer players, should limit body weight loss to ≤2% to 3% to maintain performance. Studies have shown that >2% body mass loss can hinder soccer-specific performance, such as dribbling skills and intermittent high intensity sprinting.49-51) Table 5 outlines the detrimental effects dehydration has on performance. Urine-specific gravity values between 1.021 and 1.030 may reflect 3% to 5% change in body weight.
Table 5. Performance Outcomes at Various Dehydration Levels | ||
|
ELECTROLYTES
Sodium is the primary electrolyte lost in sweat. Other electrolytes (potassium, magnesium, and calcium) are lost at much lower levels and typically replaced through diet. Soccer players can lose large amounts of sodium; between 700 and 1500 mg of sodium/L of sweat has been reported in several studies.42-44 Studies of professional male soccer players have shown potassium losses in the range of 165 mg/L to 234 mg/L.42,51,52 Sodium in a sports drink or in food aids with water uptake from the intestines and enhances the thirst mechanism in the brain, resulting in additional fluid being retained in the body.
REHYDRATION AFTER TRAINING OR COMPETITION
Within 2 hours after training or competition, the rehydration strategy should provide water to restore body fluid status, carbohydrates to replenish glycogen (fuel) stores, and electrolytes to speed rehydration (Table 6). The volume of fluids and type of fluids over the next 24 hours dictate the hydration status prior to the next day’s training session. It is a continuous cycle. Over time, an athlete increases the risk of being in a chronic dehydrated state, resulting in lack of motivation, risk of injury, and illness, fatigue, and poor performance. The current recommendation is to drink ~50% more in volume than the amount of weight lost, such as 22 to 24 ounces/pound lost.52
Table 6. Hydration | ||
Timing | Amount | Application |
Daily | 3.7 L adult males | Monitor urine color. |
Pre-training/match; | 16 oz or 5–7 mL/kg | Monitor urine production and color |
During training/match | 13–28 oz/h (400- | Every 15–20 min. *Dependent on sweat rate. |
Recovery/after training | 22–24 oz/1 lb body weight lost | Water + food (carbohydrates/electrolytes) |
Soccer is the world’s most popular sport. As the sport has grown, so have the physical demands and the search for ways to edge out the competition with the use of sports science and nutrition. The demands, which include intense training, ≥90 minutes matches, congested fixtures, and travel, lead to increased energy and nutrient requirements, stress on the body, and risk of impaired sleep cycles. Identifying key areas to enhance a player’s performance is an ongoing effort because of individual differences. Moreover, new information is being discovered via research, and advancing technology to measure performance is always evolving. This article focuses on the core nutrition principles known to lay the foundation for a better soccer player. These principles are obvious for some; however, nutrition and hydration are often undervalued, leaving the individual player with the responsibility to eat right. This review addresses the most applicable nutrition-related recommendations for soccer players.
Technical, tactical, and physical skills are key factors in a soccer player’s performance. However, energy demands of matches and training sessions require adequate fuel and hydration to maximize those key factors. Athletes may need to manage carbohydrates, protein, and fat separately to achieve optimal body size and body composition, and to maximize performance.
Nutrition plays a vital role in keeping the player healthy, reducing risk of injuries, speeding up recovery, and enhancing training adaptations. Research has shown what we eat and when we eat can significantly impact skeletal muscle adaptation, inflammation, immune response, and energy metabolism. These are all essential nutrition considerations for soccer players.
ENERGY METABOLISM IN SOCCER
Understanding energy demands will help determine energy requirements: type, amount, and timing of macronutrients and micronutrients. Soccer utilizes both aerobic and anaerobic energy systems. Soccer is an intermittent team-based sport; thus, it contains various high-intensity movements, such as sprinting, jumping, dribbling, and frequent changing of direction performed in between numerous low-intensity slow movements. The high intense movements collectively account for about 30% of match play, whereas 70% is walking, jogging, and standing. Although sprinting and jumping are not a large part of the 90 minutes of match play, they have a huge impact on the outcome of the match. Distance covered in the last 15 minutes of match play decreases by 14% to 45% compared with the first 15 minutes of play.1 Krustrup and colleagues2 found muscles in the quadriceps to be empty or nearly empty of glycogen (stored carbohydrates) after match play. This phenomenon can help explain a significant decrease in sprinting, jumping, and intermittent movements toward the end of a match—energy demands that rely on glycogen as the primary fuel source. Being well-fueled and hydrated and having the ability to delay fatigue can place a team at a performance advantage.
ENERGY EXPENDITURE
Beyond training load or match intensity, a soccer player’s body composition, gender, age, and position can affect energy needs. Position differences in elite soccer players show that the greatest total distance covered is by central midfielders and wide midfielders (~12 km –13 km), whereas central defenders cover the least area of the field players (≤~10 km).3,4 The environment can also play a role in energy expenditure. To further understand calorie needs, total daily energy expenditure in soccer players has been measured using doubly labeled water and estimated using heart rate, global positioning system, video match analysis, and activity records.5,6 One study estimated that energy expended during a training day for elite male soccer players is between 3442 kcal and 3824 kcal.6 Another study using doubly labeled water concluded that mean energy expenditure of elite male soccer players is 3566 kcal over a 7-day period, which included 5 training days and 2 matches.7 In terms of energy expenditure for elite female soccer players, the mean values for match day, training days, and rest days were 2914, 2783, and 2213 calories, respectively.8
Continue to: FUELING THE SOCCER PLAYER
FUELING THE SOCCER PLAYER
Depending on the match fixture, proper fueling can be a challenge due to the number of matches, travel time, and limited recovery time. Macronutrients will provide the mainstay of fuel for a player, specifically carbohydrates and fats. Carbohydrates are the preferred source of fuel for the majority of the calories consumed. Using body weight (kg) is a more current and accurate method of recommending the amount of each macronutrient an individual player should eat as compared to using a percentage of total daily calories.
- Carbohydrates: 5–10 g/kg/day
- Protein: 1.2–2.0 g/kg/day
- Fat: 0.8–1.5 g/kg/day
CARBOHYDRATE AND SOCCER PERFORMANCE
Carbohydrates are a limited supply of fuel compared with fat stores. They are an important fuel source for soccer players, as muscle glycogen is vital to performance during high intense training and match play (Table 1). Yet current research shows that a high carbohydrate intake is not required to be followed every day due to varied energy demands.9 This newer strategy is referred to as “training low,” allowing the athlete to train at a low-moderate intensity in a low glycogen state. The glycogen status of the muscle can alter the training adaptations through cellular changes in the mitochondria. Therefore, carbohydrate needs should reflect the work required or demand for optimal performance. However, on high-training load days or 24 hours pre-match, carbohydrate intake should be increased to maximize muscle glycogen stores. Soccer players need to consume up to 8-10 g/kg body weight during the 24 hours before a match.10 On low or rest days, carbohydrate intake should be reduced to reflect the decreased training load. For example, recent research has demonstrated potential training adaptations when muscle glycogen stores are not consistently high11 or intentionally kept low depending on the training load. Adjusting carbohydrate intake to the physical demands of an athlete is a strategy called nutrition periodization.
Table 1. Carbohydrates | ||
Timing | Amount | Application |
| Daily | 5–7 g/kg/day | Low–moderate training load. Match amount to training session intensity. |
Pre-Training/Match | 1–4 gm/kg | Adjust to players’ tolerance, preferences and training load. |
| During Training | 0–30 g/h | Light training session |
| Recovery/After Training | Balance meal 1.0–1.2 g/kg/h, ASAP. | Light training: < 2 h Heavy training/2 sessions/day |
| Match day -1, match day, match day +1 | 7–10 g/kg/d | Adjust to players’ tolerance, preferences. |
| During/half time | 30–60 g/h | High glycemic carbohydrates |
| Recovery/after match | 1.0–1.2 g/kg/h | High glycemic carbohydrates |
However, if glycogen stores are not well supplied before a match >90 minutes, then the muscles and the brain will become fatigued and lead to poor performance. Glycogen depletion contributes to fatigue toward the end of a match.10 In the early 1970s, Saltin and colleagues12 showed that players with high muscle glycogen stores (~400 mmol/kg dry wt) achieve higher movement intensities and cover more total distance than those players who start the match with low glycogen stores (~200 mmol/kg dry wt). Another study examined pre-match diets of male soccer players (65% vs 30% daily carbohydrate intake) to determine the effect on performance outcomes and glycogen concentrations. Results showed high-muscle glycogen concentrations in the 65% carbohydrate diet and a significantly higher amount of intense exercise bouts. More acutely, studies have shown a meal containing 200 to 300 grams of carbohydrates 2 to 4 hours before exercise prolongs endurance.13-15 Ideally, consuming fast-digesting carbohydrate sources during or at half time will help maintain blood glucose concentrations and spare muscle glycogen reserves. The majority of literature shows a 6% to 8% solution of combined fast-digesting carbohydrates (ie, glucose, fructose, sucrose, or maltodextrin) at a rate of 30 to 60 g/h enhances at least 1 aspect of performance in soccer.16-18 These performance benefits include increased running time, improved time to fatigue, and enhanced technical skills. Regarding recovery, soccer players should begin consuming carbohydrate-rich foods and beverages immediately after exhaustive training or a match to optimize glycogen reloading. Ingesting post-exercise carbohydrates stimulates muscle and liver glycogen synthesis up to tenfold compared with post-intake of no carbohydrates.19 This recovery period becomes vital when there are <8 hours between training sessions or another match, such as in youth tournaments. The form of carbohydrate, solid or liquid, can be based on preference and tolerance, as long as the source provides a large glycemic and insulin response.
An easy way to adjust daily carbohydrate intake is to schedule carbohydrate-rich foods at meals or snacks around important training sessions or before/during/after on match day. Anderson and colleagues10 looked at training loads for 1, 2, and 3 matches per week, recommending high carbohydrate intake match day minus 1, on match day, and match day plus 1 for 1 and 2 matches per week and lower carbohydrate intake on the other days. During a 3-match week, lowering carbohydrates any day of that week is not recommended. More research is needed to determine the best strategy for performance regarding carbohydrate periodization in soccer.
PROTEIN AND SOCCER PERFORMANCE
Protein is important to soccer players for muscle tissue repair, strength, bone health, and the immune system (Table 2). The American College of Sports Medicine, the Academy of Nutrition and Dietetics, and the Dietitians of Canada recommend 1.2 to 2.0 g/kg/day.20 Most soccer players meet the daily protein requirements; however, the key to optimizing the total daily amount is focusing on the source/amino acid profile, timing, and amount per feeding. Consuming divided doses of protein (20 g to 40 g) every 3 to 4 hours gives the body a continuous flow of amino acids to support muscle synthesis and recovery. In terms of body size, the recommendation is 0.25 to 0.4 g/kg every 3 to 4 hours, which includes pre-training/match and post-training/match. Protein/amino acids consumed around strength training and high-intensity sessions can promote muscle adaptations, minimize tissue breakdown, and speed recovery. Soccer matches lead to significant muscle damage21 especially at 2 sessions/day or multiple matches in a week. Protein is not a priority during training or matches, as its role is not to provide energy, and the primary goal during soccer activities is energy production. Research supports an intake of 30 to 40 g of casein, which is a slow digesting protein, at night before bed when a strength-training session has been performed that day.22,23
Table 2. Protein | ||
Timing | Amount | Application |
| Daily | 1.2–2.0 g/kg | High quality sources; chicken, lean meats, fish, seafood, eggs, dairy, beans, soy |
Pre-training/match; | 20–40 g or 0.25–0.40 g/kg | Meal/snack |
| During training/match | None needed | If training session <3 h |
| Recovery/after training Night-time feeding | 20–40 g | <30–60 min, whey, casein/whey, pea, soy protein Casein (slow-absorbing protein), strength training days |
Continue to: FAT AND SOCCER PERFORMANCE
FAT AND SOCCER PERFORMANCE
Fat is the primary source of energy at rest and at low-training intensities, such as walking or jogging for soccer players (Table 3). Besides providing slow, long-lasting energy, fat helps absorb vitamins A, D, E, and K; produce hormones; protect organs; and support the cell membrane structure. The dietary recommendations of total fat intake for athletes are similar to or slightly greater than those recommended for non-athletes. The total amount required depends on the training demands and the players’ goals. The recommended amount of dietary fat is between 20% and 35% of total daily energy intake.
Table 3. Fat | ||
Timing | Amount | Application |
Daily | 0.8–1.5 g/kg | Include well balanced meals, primarily polyunsaturated and monounsaturated fats. |
Pre-Training/Match; | ~10–30 g/meal | Limit amount. Avoid digestion and gastrointestinal issues. |
During Training/Match | None needed | Risk of gastrointestinal intolerances. |
Recovery/After Training | ~10–30 g | Include well-balanced meals, primarily polyunsaturated and monounsaturated fats. |
The key to gaining performance benefits from dietary fat depends on the type of fat selected. Some fats in excess, such as omega-6 fatty acids and saturated fats, may promote inflammation, hinder recovery, and affect brain health. Other types can help reduce inflammation, enhance muscle recovery, and improve brain health. These types include polyunsaturated omega-3 fatty acids, which are essential for the health of the athlete, allowing for a balanced fatty acid profile.23 Specific omega-3 fatty acids (EPA and DHA) have shown an improvement in the function of the mitochondria, enhancing energy cell metabolism. They also have potential to be highly anti-inflammatory, benefit rehabilitation during soft-tissue injury, and help decrease secondary damage from a concussion.
In addition, research shows that omega-3 may enhance the energy production of the mitochondria, resulting in less oxidative damage to the muscle cell.25 More research is needed on the effects of performance on soccer players. Given the slow digestion and absorption of fats, fat intake must be limited leading up to or during training sessions or matches, which may risk gastrointestinal issues and displacement of carbohydrates. Low to moderate monounsaturated and polyunsaturated fats in a recovery meal have not been shown to inhibit muscle glycogen reloading or muscle protein synthesis.26,27 In regard to fat intake post-match, fat is not a key nutrient of concern for muscle recovery, as it can be included in the next balanced meal.
MICRONUTRIENTS, VITAMINS, AND MINERALS
Exercise stresses many of the metabolic pathways where vitamins and minerals are required. High-level training demands may also increase the turnover rate of vitamins and minerals. As a result, greater dietary intakes of vitamins and minerals may be warranted. Soccer players at the greatest risk for poor vitamin and mineral levels are those who skip meals, who eliminate ≥1 of the food groups from their diet (such as vegans), or who consume unbalanced and highly processed foods. In soccer players, the micronutrients of concern include iron and vitamin D. In young female soccer players, calcium intake must be assessed along with adequate energy intake for optimal bone density. Vegetarians, vegans, and/or athletes who do not consume meat, eggs, and/or dairy in their diet are at risk for vitamin B12 deficiency. The key to obtaining all the vitamins and minerals an athlete will need is to eat a wide variety of nutrient-dense foods.
IRON
Iron deficiency, with or without anemia, may impair muscle function and limit exercise capacity. Adequate iron intake in athletes with iron deficiencies and/or anemia can improve exercise capacity. Iron depletion is 1 of the most common nutrient deficiencies observed among endurance athletes. Foot strike hemolysis can destroy red blood cells during activities such as running. Research has shown that 30% of professional male soccer players have ferritin levels <30 mcg/L at the end of a soccer season.28 Thus, fatigue and poor recovery time place soccer players at risk of an iron imbalance.29,30
Continue to: Landahl and colleagues...
Landahl and colleagues31 found that iron deficiency and iron deficiency anemia are common in female soccer players at the elite level. In their study of 28 female national soccer players, 57% had iron deficiency and 29% presented with iron deficiency anemia 6 months before the FIFA Women's World Cup. Testing hemoglobin alone is insufficient to detect relative anemia. Regular monitoring of hemoglobin and ferritin concentrations may be necessary to determine appropriate iron needs.
VITAMIN D
Vitamin D is required for optimal bone health, as it helps regulate calcium and phosphorus. Further research shows a link between vitamin D and non–bone-related functions, such as muscle health, immune support, and anti-inflammatory roles, which may be linked to performance. Soccer players with low levels of vitamin D (<30 ng/mL) may be more at risk for musculoskeletal injuries and stress fractures.34 In other sports, vitamin D may enhance muscle strength; however, no association between vitamin D and muscle strength has been found in soccer players.34,35 The geographic location of an athlete seems to be irrelevant to serum levels, as insufficient levels can be found at various latitudes.34,36-38
Evidence has shown that vitamin D may improve athletic performance in vitamin D-depleted athletes, thereby improving vertical jumps, lowering risks of muscle injury/strains and stress fractures, and reducing risk of colds/flu. In 2013, researchers showed for the first time a link between vitamin D and muscle aerobic metabolism by studying the energy efficiency of the mitochondria.32 Athletes with low vitamin D levels increased their ATP production within the muscle with vitamin D supplementation over 10 weeks to 12 weeks.33
CALCIUM
Soccer players present with stronger and denser bones than non-athletes due to running and jumping in their sport. Weight-bearing sites such as lumbar spine, hip, femoral neck, trochanter, intertrochanteric region, and both legs are sensitive to the impact of soccer movements.39 Calcium and vitamin D are also important for muscle contraction.
Given the variation in genetics, sports, and gender, optimal performance requires a healthy eating plan tailored to the individual athlete. A healthy eating plan allows an athlete to train longer and harder, delay the onset of fatigue, and speed recovery. Nutrition supports optimal performance through real food, proper hydration, nutrient timing, and supplementation.
Continue to: FLUID REQUIREMENTS FOR SOCCER PLAYERS
FLUID REQUIREMENTS FOR SOCCER PLAYERS
Many athletes overlook the importance of hydration on performance, either assuming they are hydrated or they miscalculate fluid and electrolyte needs to actual sweat losses. Numerous factors play a part in optimal hydration such as sweat rate, environment, training intensity, duration, body size, and body composition. Soccer players have fewer breaks to consume fluids during a match compared with basketball, baseball, or American football players. These breaks include a 15-minute half between coming off the pitch to the locker room and back, as well as time spent with coaches reviewing strategies; this short window of time must be maximized to rehydrate. Fluids with a carbohydrate concentration of 4% to 8% at 5 to 10 ounces and breaks every 15 to 20 minutes are optimal to maximize uptake while avoiding gastric intolerance.
Studies have shown that most players do not drink sufficiently during a match to optimize hydration, replacing only ~40% to 45% of their sweat losses.40, 41 Maughan and colleagues measured high levels of urine osmolality in some soccer players, thereby indicating that the players started their training session dehydrated.41 Soccer players must begin training or a match well hydrated due to the limited opportunities after kick-off. The athlete should drink at least 4 hours prior to exercise; if no urine is produced or urine is dark in color, then the athlete should drink again 2 hours prior.
Table 4. Sweat Rate Calculation Steps | ||
|
Changes in body mass, urine color, and thirst offer clues to the need for rehydration. Advanced hydration measurement includes testing urine specific gravity (USG) values. For example, testing pre-training or pre-match can be conducted to determine hydration status and trending changes from day to day. A USG value >1.020 is considered dehydrated in accordance with the NATA position statement.42 Calculating a sweat rate is a practical approach to determining individual hydration needs (see Table 4). Sweat rates will vary between soccer players based on their position and intensity of play, along with total match time.39 Soccer players will lose ~1.5 to 4.5 liters during match play.43-46 In general, athletes, including soccer players, should limit body weight loss to ≤2% to 3% to maintain performance. Studies have shown that >2% body mass loss can hinder soccer-specific performance, such as dribbling skills and intermittent high intensity sprinting.49-51) Table 5 outlines the detrimental effects dehydration has on performance. Urine-specific gravity values between 1.021 and 1.030 may reflect 3% to 5% change in body weight.
Table 5. Performance Outcomes at Various Dehydration Levels | ||
|
ELECTROLYTES
Sodium is the primary electrolyte lost in sweat. Other electrolytes (potassium, magnesium, and calcium) are lost at much lower levels and typically replaced through diet. Soccer players can lose large amounts of sodium; between 700 and 1500 mg of sodium/L of sweat has been reported in several studies.42-44 Studies of professional male soccer players have shown potassium losses in the range of 165 mg/L to 234 mg/L.42,51,52 Sodium in a sports drink or in food aids with water uptake from the intestines and enhances the thirst mechanism in the brain, resulting in additional fluid being retained in the body.
REHYDRATION AFTER TRAINING OR COMPETITION
Within 2 hours after training or competition, the rehydration strategy should provide water to restore body fluid status, carbohydrates to replenish glycogen (fuel) stores, and electrolytes to speed rehydration (Table 6). The volume of fluids and type of fluids over the next 24 hours dictate the hydration status prior to the next day’s training session. It is a continuous cycle. Over time, an athlete increases the risk of being in a chronic dehydrated state, resulting in lack of motivation, risk of injury, and illness, fatigue, and poor performance. The current recommendation is to drink ~50% more in volume than the amount of weight lost, such as 22 to 24 ounces/pound lost.52
Table 6. Hydration | ||
Timing | Amount | Application |
Daily | 3.7 L adult males | Monitor urine color. |
Pre-training/match; | 16 oz or 5–7 mL/kg | Monitor urine production and color |
During training/match | 13–28 oz/h (400- | Every 15–20 min. *Dependent on sweat rate. |
Recovery/after training | 22–24 oz/1 lb body weight lost | Water + food (carbohydrates/electrolytes) |
- Mohr M, Krustrup P, Bangsbo J. Match performance of high-standard soccer players with special reference to development of fatigue. J Sports Sci. 2003;21:519-528.
- Krustrup P, Mohr M, Steensberg A, Bencke J, Kjaer M, Bangsbo J. Muscle and blood metabolites during a soccer game: implications for sprint performance. Med Sci Sports Exerc. 2006;38:1165-1174.
- Di Salvo V, Gregson W, Atkinson G, Tordoff P, Drust B. Analysis of high intensity activity in Premier League soccer. Int J Sports Med. 2009;30:205-212.
- Di Salvo V, Baron R, Tschan H, Calderon Montero FJ, Bachl N, Pigozzi F. Performance characteristics according to playing position in elite soccer. Int J Sports Med. 2007;28:222-227.
- Reilly T, Thomas V. Estimated daily energy expenditures of professional association footballers. Ergonomics. 1979;22:541-548.
- Osgnach C, Poser S, Bernardini R, Rinaldo R, di Prampero P.E. Energy cost and metabolic power in elite soccer: A new match analysis approach. Med Sci Sports Exerc. 2010;42:170-178.
- Anderson L, Orme P, Naughton RJ, Close, GL, Milsom J, Rydings D, et al. Energy intake and expenditure of professional soccer players of the English Premier League: evidence of carbohydrate periodization. Int J Sport Nutr Exerc Metab. 2017;1-25.
- Mara JK, Thompson KG, Pumpa KL. Assessing the energy expenditure of elite female soccer layers: a preliminary study. J Strength Cond Res. 2015;2780-2786.
- Bartlett JD, Hawley JA, Morton JP. Eur J Sport Sci. 2015;15(1):1, 3-12.
- Anderson L, Orme P, Di Michele R, Close GL, Morgans R, Drust B, Morton JP. Quantification of training load during one-, two- and three-game week schedules in professional soccer players from the English Premier League: implications for carbohydrate periodisation. J Sports Sci. 2016;34;1250-1259.
- Hawley JA, Morton JP. Ramping up the signal: promoting endurance training adaptation in skeletal muscle by nutritional manipulation. Clin Exp Pharmacol Physiol. 2014;41:608-613.
- Saltin B. Metabolic fundamentals in exercise. 1973;:137-146.
- Balsom PD, Wood K, Olsson P, Ekblom B. Carbohydrate intake and multiple sprint sports: With special reference to football (soccer). Int J Sports Med. 1999;20:48-52.
- Neufer PD, Costill DL, Flynn MG, Kirwan JP, Mitchell JB, Houmard J. Improvements in exercise performance: Effects of carbohydrate feedings and diet. J Appl Physiol. 1987;62:983-988.
- Sherman WM, Brodowicz G, Wright DA, Allen WK, Simonsen J, Dernbach A. Effects of 4 h preexercise carbohydrate feedings on cycling performance. Med Sci Sports Exerc. 1989;21:598-604.
- Baker LB, Rollo I, Stein KW, Jeukendrup AE. Acute effects of carbohydrate supplementation on intermittent sports performance. Nutrients. 2015;7:5733-5763.
- Goedecke JH, White NJ, Chicktay W, Mahomed H, Durandt J, Lambert MI. The effect of carbohydrate ingestion on performance during a simulated soccer match. Nutrients. 2013;5:5193-5204.
- Nicholas CW, Williams C, Lakomy HK, Phillips G, Nowitz A. Influence of ingesting a carbohydrate-electrolyte solution on endurance capacity during intermittent, high-intensity shuttle running. J Sports Sci. 1995;13:283-290.
- Burke LM, van Loon LJC, Hawley JA. Post-exercise muscle glycogen resynthesis in humans. J Appl Physiol. 2016;122:1055-1067.
- Rodriquez NR, DiMarco NM, Langley S. Position of the American Dietetic Association, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. J Am Diet Assoc. 2009;109(3):509-527.
- Romagnoli M, Sanchis-Gomar F, Alis R, Risso-Ballester J, Bosio A, Graziani RL, Rampinini E. Changes in muscle damage, inflammation, and fatigue-related parameters in young elite soccer players after a match. J. Sports Med Phys Fit. 2016;56:1198-1205.
- Res PT, Groen B, Pennings B, Beelen M, Wallis GA, Gijsen AP, et al.Protein ingestion before sleep improves postexercise overnight recovery. Med Sci Sports Exerc. 2012;44:1560-1569.
- Snijders T, Res PT, Smeets JSJ, Van Vliet S, Van Kranenburg J, Maase K, et al.Protein ingestion before sleep increases muscle mass and strength gains during prolonged resistance-type exercise training in healthy young men. J Nutr. 2015;145:1178-1184.
- Simopoulos AP. Omega-3 fatty acids and athletics. Curr Sports Med Rep. 2007;6230-236.
- Peoples GE, McLennan PL, Howe P, Groeller H. Fish oil reduces apparent myocardial oxygen consumption in trained cyclists but does not change time to fatigue. Presented at the Fourth International Conference on Nutrition and Fitness; May 25-29, 2000; Ancient Olympia, Greece.
- Burke LM, Collier GR, Beasley S.K, Davis PG, Fricker PA, Heeley P, et al. Effect of coingestion of fat and protein with carbohydrate feedings on muscle glycogen storage. J Appl Physiol. 1995;78:2187-2192.
- Roy BD, Tarnopolsky MA. Influence of differing macronutrient intakes on muscle glycogen resynthesis after resistance exercise. J Appl Physiol. 1998;84:890-896.
- Reinke S, Taylor W.R, Duda GN, von Haehling S, Reinke P, Volk H-D et al. Absolute and functional iron deficiency in professional athletes during training and recovery. Int J Cardiol. 2012;156:186-191.
- Escanero JF, Villanueva J, Rojo A, Herrera A, del Diego C, Guerra M. Iron stores in professional athletes throughout the sports season. Physiol Behav. 1997;62:811-814.
- Heisterberg MF, Fahrenkrug J, Krustrup P, Storskov A, Kjær, M, Andersen JL. Extensive monitoring
- Landahl G, Adolfsson P, Borjesson M, Mannheimer C, Rodjer S. Iron deficiency and anemia: a common problem in female elite soccer players. Int J Sport Nutr Exerc Metab. 2005;15(6):689-694.
- Sinha A, Hollingsworth K, Ball S, Cheetham T. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. Endocrine Abstracts, 2013;31.OC1.6
- Shuler FD, Wingate MK, Moore GH, Giangarra C. Sports health benefits of vitamin D. Sports Health. 2012;4:496-501.
- Hamilton B, Whiteley R, Farooq A, Chalabi H. Vitamin D concentration in 342 professional football players and association with lower limb isokinetic function. J Sci. Med Sport. 2014;17:139-143.
- Ksiażek A, Zagrodna A, Dziubek W, Pietraszewski B, Ochmann B, Słowińska-Lisowska M,25(OH)D3 levels relative to muscle strength and maximum oxygen uptake in athletes. J Hum Kinet. 2016;50:71-77.
- Kopeć A, Solarz K, Majda F, Słowińska-Lisowska M, Medraś M. An evaluation of the levels of vitamin D and bone turnover markers after the summer and winter periods in Polish professional soccer players. J Hum Kinet. 2013;38:135-140.
- Vander Slagmolen G, van Hellemondt FJ, Wielders JPM. Do professional soccer players have a vitamin D status supporting optimal performance in winter time? J Sports Med Doping Stud. 2014,4:2.
- Morton JP, Iqbal Z, Drust B, Burgess D, Close GL, Brukner PD. Seasonal variation in vitamin D status in professional soccer players of the English Premier League. Appl Physiol Nutr Metab. 2012;37:798-802.
- Lozano-Berges G, Matute-Llorente A, Gonzalez-Aguero A, Gomez-Bruton A, Gomez-Cabelloa A, Vincente-Rodriguez G, Casajus JA. Soccer helps build strong bones during growth: a systematic review and meta-analysis. Eur J Pediatr. 2018;177(3):295-310.
- Burke LM. Fluid balance during team sports. J Sports Sci. 1997;15:287-295.
- Maughan RJ, Merson SJ, Broad NP, Shirreffs SM. Fluid and electrolyte intake and loss in elite soccer players during training. Int J Sport Nutr Exerc Metab. 2004;14:333-346.
- Brendon P, McDermott, P, Anderson SA, Armstrong LE, Casa DJ, Cheuvront SN, et al. National Athletic Trainers’ Association Position Statement: Fluid Replacement for the Physically Active. J Athl Train. 2017;52(9):877-895.
- Shirreffs SM, Aragon-Vargas LF, Chamorro M, Maughan RJ, Serratosa L, Zachwieja JJ. The sweating response of elite professional soccer players to training in the heat. Int J Sports Med. 2005;26: 90-95.
- Maughan RJ, Watson P, Evans GH, Broad N, Shirreffs SM. Water balance and salt losses in competitive football. Int J Sport Nutr Exerc Metab. 2007;17:583-594.
- Aragón-Vargas LF, Moncada-Jiménez J, Hernández-Elizondo J, Barrenechea A,Monge-Alvarado M. Evaluation of pre-game hydration status, heat stress, and fluid balance during professional soccer competition in the heat. Eur J Sport Sci. 2009;9:269-276.
- Maughan RJ, Shirreffs SM, Merson SJ, Horswill CA. Fluid and electrolyte balance in elite male football (soccer) players training in a cool environment. J Sports Sci. 2005;23:73-79.
- Duffield R, McCall A, Coutts AJ, Peiffer JJ. Hydration, sweat and thermoregulatory responses to professional football training in the heat. J Sports Sci. 2012;30:957-965.
- Shirreffs SM, Aragon-Vargas LF, Chamorro M, Maughan RJ, Serratosa L, Zachwieja JJ. The sweating response of elite professional soccer players to training in the heat. Int J Sports Med. 2005;26:90-95.
- Edwards AM, Mann ME, Marfell-Jones MJ, Rankin DM, Noakes TD, Shillington DP. Influence of moderate dehydration on soccer performance: Physiological responses to 45 min of outdoor match-play and the immediate subsequent performance of sport-specific and mental concentration tests. Br J Sports Med. 2007;41:385-391.
- McGregor SJ, Nicholas CW, Lakomy HK, Williams C. The influence of intermittent high-intensity shuttle running and fluid ingestion on the performance of a soccer skill. J Sports Sci. 1999;17:895-903.
- Maughan RJ, Merson SJ, Broad NP, Shirreffs SM. Fluid and electrolyte intake and loss in elite soccer players during training. Int J Sport Nutr Exerc Metab. 2004;14:333-346.
- Shirreffs SM, Sawka MN, Stone M. Water and electrolyte needs for football training and match-play. J Sports Sci. 2006;24:699-707.
- Mohr M, Krustrup P, Bangsbo J. Match performance of high-standard soccer players with special reference to development of fatigue. J Sports Sci. 2003;21:519-528.
- Krustrup P, Mohr M, Steensberg A, Bencke J, Kjaer M, Bangsbo J. Muscle and blood metabolites during a soccer game: implications for sprint performance. Med Sci Sports Exerc. 2006;38:1165-1174.
- Di Salvo V, Gregson W, Atkinson G, Tordoff P, Drust B. Analysis of high intensity activity in Premier League soccer. Int J Sports Med. 2009;30:205-212.
- Di Salvo V, Baron R, Tschan H, Calderon Montero FJ, Bachl N, Pigozzi F. Performance characteristics according to playing position in elite soccer. Int J Sports Med. 2007;28:222-227.
- Reilly T, Thomas V. Estimated daily energy expenditures of professional association footballers. Ergonomics. 1979;22:541-548.
- Osgnach C, Poser S, Bernardini R, Rinaldo R, di Prampero P.E. Energy cost and metabolic power in elite soccer: A new match analysis approach. Med Sci Sports Exerc. 2010;42:170-178.
- Anderson L, Orme P, Naughton RJ, Close, GL, Milsom J, Rydings D, et al. Energy intake and expenditure of professional soccer players of the English Premier League: evidence of carbohydrate periodization. Int J Sport Nutr Exerc Metab. 2017;1-25.
- Mara JK, Thompson KG, Pumpa KL. Assessing the energy expenditure of elite female soccer layers: a preliminary study. J Strength Cond Res. 2015;2780-2786.
- Bartlett JD, Hawley JA, Morton JP. Eur J Sport Sci. 2015;15(1):1, 3-12.
- Anderson L, Orme P, Di Michele R, Close GL, Morgans R, Drust B, Morton JP. Quantification of training load during one-, two- and three-game week schedules in professional soccer players from the English Premier League: implications for carbohydrate periodisation. J Sports Sci. 2016;34;1250-1259.
- Hawley JA, Morton JP. Ramping up the signal: promoting endurance training adaptation in skeletal muscle by nutritional manipulation. Clin Exp Pharmacol Physiol. 2014;41:608-613.
- Saltin B. Metabolic fundamentals in exercise. 1973;:137-146.
- Balsom PD, Wood K, Olsson P, Ekblom B. Carbohydrate intake and multiple sprint sports: With special reference to football (soccer). Int J Sports Med. 1999;20:48-52.
- Neufer PD, Costill DL, Flynn MG, Kirwan JP, Mitchell JB, Houmard J. Improvements in exercise performance: Effects of carbohydrate feedings and diet. J Appl Physiol. 1987;62:983-988.
- Sherman WM, Brodowicz G, Wright DA, Allen WK, Simonsen J, Dernbach A. Effects of 4 h preexercise carbohydrate feedings on cycling performance. Med Sci Sports Exerc. 1989;21:598-604.
- Baker LB, Rollo I, Stein KW, Jeukendrup AE. Acute effects of carbohydrate supplementation on intermittent sports performance. Nutrients. 2015;7:5733-5763.
- Goedecke JH, White NJ, Chicktay W, Mahomed H, Durandt J, Lambert MI. The effect of carbohydrate ingestion on performance during a simulated soccer match. Nutrients. 2013;5:5193-5204.
- Nicholas CW, Williams C, Lakomy HK, Phillips G, Nowitz A. Influence of ingesting a carbohydrate-electrolyte solution on endurance capacity during intermittent, high-intensity shuttle running. J Sports Sci. 1995;13:283-290.
- Burke LM, van Loon LJC, Hawley JA. Post-exercise muscle glycogen resynthesis in humans. J Appl Physiol. 2016;122:1055-1067.
- Rodriquez NR, DiMarco NM, Langley S. Position of the American Dietetic Association, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. J Am Diet Assoc. 2009;109(3):509-527.
- Romagnoli M, Sanchis-Gomar F, Alis R, Risso-Ballester J, Bosio A, Graziani RL, Rampinini E. Changes in muscle damage, inflammation, and fatigue-related parameters in young elite soccer players after a match. J. Sports Med Phys Fit. 2016;56:1198-1205.
- Res PT, Groen B, Pennings B, Beelen M, Wallis GA, Gijsen AP, et al.Protein ingestion before sleep improves postexercise overnight recovery. Med Sci Sports Exerc. 2012;44:1560-1569.
- Snijders T, Res PT, Smeets JSJ, Van Vliet S, Van Kranenburg J, Maase K, et al.Protein ingestion before sleep increases muscle mass and strength gains during prolonged resistance-type exercise training in healthy young men. J Nutr. 2015;145:1178-1184.
- Simopoulos AP. Omega-3 fatty acids and athletics. Curr Sports Med Rep. 2007;6230-236.
- Peoples GE, McLennan PL, Howe P, Groeller H. Fish oil reduces apparent myocardial oxygen consumption in trained cyclists but does not change time to fatigue. Presented at the Fourth International Conference on Nutrition and Fitness; May 25-29, 2000; Ancient Olympia, Greece.
- Burke LM, Collier GR, Beasley S.K, Davis PG, Fricker PA, Heeley P, et al. Effect of coingestion of fat and protein with carbohydrate feedings on muscle glycogen storage. J Appl Physiol. 1995;78:2187-2192.
- Roy BD, Tarnopolsky MA. Influence of differing macronutrient intakes on muscle glycogen resynthesis after resistance exercise. J Appl Physiol. 1998;84:890-896.
- Reinke S, Taylor W.R, Duda GN, von Haehling S, Reinke P, Volk H-D et al. Absolute and functional iron deficiency in professional athletes during training and recovery. Int J Cardiol. 2012;156:186-191.
- Escanero JF, Villanueva J, Rojo A, Herrera A, del Diego C, Guerra M. Iron stores in professional athletes throughout the sports season. Physiol Behav. 1997;62:811-814.
- Heisterberg MF, Fahrenkrug J, Krustrup P, Storskov A, Kjær, M, Andersen JL. Extensive monitoring
- Landahl G, Adolfsson P, Borjesson M, Mannheimer C, Rodjer S. Iron deficiency and anemia: a common problem in female elite soccer players. Int J Sport Nutr Exerc Metab. 2005;15(6):689-694.
- Sinha A, Hollingsworth K, Ball S, Cheetham T. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. Endocrine Abstracts, 2013;31.OC1.6
- Shuler FD, Wingate MK, Moore GH, Giangarra C. Sports health benefits of vitamin D. Sports Health. 2012;4:496-501.
- Hamilton B, Whiteley R, Farooq A, Chalabi H. Vitamin D concentration in 342 professional football players and association with lower limb isokinetic function. J Sci. Med Sport. 2014;17:139-143.
- Ksiażek A, Zagrodna A, Dziubek W, Pietraszewski B, Ochmann B, Słowińska-Lisowska M,25(OH)D3 levels relative to muscle strength and maximum oxygen uptake in athletes. J Hum Kinet. 2016;50:71-77.
- Kopeć A, Solarz K, Majda F, Słowińska-Lisowska M, Medraś M. An evaluation of the levels of vitamin D and bone turnover markers after the summer and winter periods in Polish professional soccer players. J Hum Kinet. 2013;38:135-140.
- Vander Slagmolen G, van Hellemondt FJ, Wielders JPM. Do professional soccer players have a vitamin D status supporting optimal performance in winter time? J Sports Med Doping Stud. 2014,4:2.
- Morton JP, Iqbal Z, Drust B, Burgess D, Close GL, Brukner PD. Seasonal variation in vitamin D status in professional soccer players of the English Premier League. Appl Physiol Nutr Metab. 2012;37:798-802.
- Lozano-Berges G, Matute-Llorente A, Gonzalez-Aguero A, Gomez-Bruton A, Gomez-Cabelloa A, Vincente-Rodriguez G, Casajus JA. Soccer helps build strong bones during growth: a systematic review and meta-analysis. Eur J Pediatr. 2018;177(3):295-310.
- Burke LM. Fluid balance during team sports. J Sports Sci. 1997;15:287-295.
- Maughan RJ, Merson SJ, Broad NP, Shirreffs SM. Fluid and electrolyte intake and loss in elite soccer players during training. Int J Sport Nutr Exerc Metab. 2004;14:333-346.
- Brendon P, McDermott, P, Anderson SA, Armstrong LE, Casa DJ, Cheuvront SN, et al. National Athletic Trainers’ Association Position Statement: Fluid Replacement for the Physically Active. J Athl Train. 2017;52(9):877-895.
- Shirreffs SM, Aragon-Vargas LF, Chamorro M, Maughan RJ, Serratosa L, Zachwieja JJ. The sweating response of elite professional soccer players to training in the heat. Int J Sports Med. 2005;26: 90-95.
- Maughan RJ, Watson P, Evans GH, Broad N, Shirreffs SM. Water balance and salt losses in competitive football. Int J Sport Nutr Exerc Metab. 2007;17:583-594.
- Aragón-Vargas LF, Moncada-Jiménez J, Hernández-Elizondo J, Barrenechea A,Monge-Alvarado M. Evaluation of pre-game hydration status, heat stress, and fluid balance during professional soccer competition in the heat. Eur J Sport Sci. 2009;9:269-276.
- Maughan RJ, Shirreffs SM, Merson SJ, Horswill CA. Fluid and electrolyte balance in elite male football (soccer) players training in a cool environment. J Sports Sci. 2005;23:73-79.
- Duffield R, McCall A, Coutts AJ, Peiffer JJ. Hydration, sweat and thermoregulatory responses to professional football training in the heat. J Sports Sci. 2012;30:957-965.
- Shirreffs SM, Aragon-Vargas LF, Chamorro M, Maughan RJ, Serratosa L, Zachwieja JJ. The sweating response of elite professional soccer players to training in the heat. Int J Sports Med. 2005;26:90-95.
- Edwards AM, Mann ME, Marfell-Jones MJ, Rankin DM, Noakes TD, Shillington DP. Influence of moderate dehydration on soccer performance: Physiological responses to 45 min of outdoor match-play and the immediate subsequent performance of sport-specific and mental concentration tests. Br J Sports Med. 2007;41:385-391.
- McGregor SJ, Nicholas CW, Lakomy HK, Williams C. The influence of intermittent high-intensity shuttle running and fluid ingestion on the performance of a soccer skill. J Sports Sci. 1999;17:895-903.
- Maughan RJ, Merson SJ, Broad NP, Shirreffs SM. Fluid and electrolyte intake and loss in elite soccer players during training. Int J Sport Nutr Exerc Metab. 2004;14:333-346.
- Shirreffs SM, Sawka MN, Stone M. Water and electrolyte needs for football training and match-play. J Sports Sci. 2006;24:699-707.
TAKE-HOME POINTS:
- Nutrition plays a vital role in keeping the player healthy, reducing risk for injury, speeding up recovery, and enhancing training adaptations.
- Average energy expenditure during a training day is ~3500-3600 kcal for elite male soccer players and ~2700-2800 kcal for elite female soccer players.
- Carbohydrate needs should reflect the work required/demand to produce optimal performance.
- Vitamin D and iron are two common nutrients of concern for soccer players.
- Studies have shown that most players do not drink sufficiently during a match to optimize hydration, replacing only ~40% to 45% of their sweat losses. Soccer players can also lose large amounts of sodium: between 700 and 1500 mg of sodium/L of sweat.
Lower Extremity Injuries in Ice Hockey: Current Concepts
ABSTRACT
Ice hockey is a fast-paced, collision sport requiring tremendous skill and finesse, yet ice hockey can be a harsh and violent game. It has one of the highest musculoskeletal injury rates in all of competitive sports. Razor sharp skates, aluminum sticks and boards made from high density polyethylene (HDPE), all contribute to the intrinsic hazards of the game. The objective of this article is to review evaluation, management, and return-to-the-rink guidelines after common lower extremity ice hockey injuries.
“Hockey is a fast body-contact game played by men with clubs in their hands and knives laced to their feet, since the skates are razor sharp, and before the evening is over it is almost a certainty that someone will be hurt and will fleck the ice with a generous contribution of gore before he is led away to be hemstitched together again.” —Paul Gallico in Farewell to Sport (1938)
Ice hockey is a collision sport with player speeds in excess of 30 miles/hour, on a sheet of ice surrounded by unforgiving boards, with a vulcanized rubber puck moving at speeds approaching 100 miles/hour.1-3 Understanding injuries specific to this fast-paced sport is an essential part of being a team physician at any level of competitive ice hockey. We are continuing to improve our ability to correctly identify and treat injuries in ice hockey players.2,4 On the prevention side, rule changes in hockey have been implemented, such as raising the age to allow checking and penalties for deliberate hits to the head and checking from behind, to make the game safer to play.3 Additionally, advancements in biomechanical research and 3D modeling are providing new insights into the pathoanatomy of the hip joint, which can be utilized for surgical planning in hockey players and goalies suffering from symptomatic femoroacetabular impingement (FAI) of the hip.5
During the 2010 Winter Olympics, more than 30% of ice hockey players were injured, which was the highest percentage amongst all competing sports.6 They also tallied the highest percentage of player-to-player injuries during the Olympics of any sport. Consequently, the team physician covering ice hockey should be prepared to manage upper and lower extremity musculoskeletal injuries, but also concussions, cervical spine injuries, and ocular and dental trauma.2
One of the earliest epidemiological studies of ice hockey injuries looked at elite Danish hockey players over 2 seasons and found that head trauma accounted for 28% of all injuries, followed by lower extremity injuries at 27% with upper extremity injuries accounting for 19%.7 More recent epidemiological studies have shown similar rates based on body region while further defining individual diagnoses and their incidence. This should help clinicians and researchers develop prevention strategies, as well as improve treatments to optimize player outcomes and return to sport.8,9 Our group recently reviewed the evaluation and management of common head, neck, and shoulder injuries at all competitive levels of ice hockey, and this article serves to complement the former by focusing on lower extremity injuries (Table).2
Continue to: Hip and groin...
EVALUATION AND MANAGEMENT OF COMMON LOWER EXTREMITY HOCKEY INJURIES
HIP INJURIES
Hip and groin injuries are very common amongst this group of athletes and account for approximately 9% of all ice hockey injuries.1 Unfortunately, they are also known for their high recurrence rates, which may be in part due to delayed diagnosis, inadequate rest and rehabilitation, as well as the extreme loads that are placed on the hip during competition.10,11 In hockey, the most commonly reported hip injuries include goaltender’s hip, FAI, sports hernia/hockey groin syndrome, adductor strains, hip pointer, and quadriceps contusions. Dalton and colleagues12 performed the largest epidemiological study to date on hip and groin injuries amongst National Collegiate Athletic Association ice hockey players and reported that the most common injury mechanism was noncontact in nature. Contact injuries accounted for 13% (55 of 421) in men’s ice hockey players while less than 4% (4 of 114) injuries in female ice hockey players, which is likely attributed to a no checking rule in the women’s division. Some of these hip and groin injuries are difficult to diagnose so it is important for the team physician to perform a thorough history and physical examination. Advanced imaging (magnetic resonance imaging [MRI] or a computed tomography (CT) scan with 3D reconstructions) may be necessary to make the correct diagnosis. This is important for providing proper treatment as well as setting player expectations for return to sport.12
Table 1. Return-to-Play Guidelines for Common Lower Extremity Ice Hockey Injuries | ||
Lower Extremity Injury | Treatment Options | Return-to-the-Rink Goal |
FAI | In-season: injection, physical therapy program, NSAIDS. Off-season or unable to play: requires arthroscopic surgery | Nonoperative can take up to 6 weeks. Surgical depends on what is fixed but goal is 4 months to return to ice24,26
|
Sports hernia/athletic pubalgia
| In-season: physical therapy program, NSAIDS Off-season or unable to play requiring surgery. Essential to make sure no other pathology (eg, FAI, osteitis pubis, adductor strain) to maximize success
| Nonoperative 6-8 wk trial of physical therapy Operative: depends if concomitant FAI but in isolation goal is 3-4 mo33,54
|
Adductor strains | Ice, NSAIDS, physical therapy, use of Hypervolt Hyperice | Depends on position (goalie vs skater) and severity; can take up to 4-8 wk to return to ice. Want 70% strength and painless ROM to skate successfully;55 in chronic cases, may take up to 6 mo35
|
Quadriceps contusion
| Hinged knee brace to maintain 120° of flexion, ice, compression wrap.
| When player regains motion and strength, return to ice can be as fast a couple of days or as long as 3 wk8,46
|
MCL | Hinged knee brace, shin pad modification, ice, NSAIDs | Depends on Grade; if Grade I, 1-2 wk; Grade II, 2-4 wk; Grade III, 4-6 wk8
|
ACL | Surgery autograft BTB autograft soft tissue
| 9-10 mo41 |
Meniscus tear | Depends on type of tear and seasonal timing (in-season or off-season) | If surgical, 3-4 mo; if repair, 4-6 wk if partial menisectomy
|
High ankle sprain
| Cam boot, NSAIDS, ice and physical therapy
| 6 wk49 |
Boot top laceration | Repair of cut structures, depends on depth and what is injured; best treatment is prevention with Kevlar socks | If laceration is deep and severs any medial tendons/vascular structures, return to ice can be ≥6 mo
|
Lace bite
| Bunga pad, ice, diclofenac gel
| Couple of days to up to 2 wk in recalcitrant cases3 |
Abbreviations: ACL, anterior cruciate ligament; BTB, bone-patellar tendon-bone; Cam, controlled ankle motion boot; MCL, medial collateral ligament; FAI, femoroacetabular impingement; NSAIDS, nonsteroidal anti-inflammatory drugs; ROM, range of motion.
Throughout the hockey community, FAI is being examined as a possible source of symptomatic hip pain amongst players at all levels. A recent study, which utilized the National Hockey League (NHL) injury surveillance database, reported that FAI accounted for 5.3% of all hip and groin injuries.13 The etiology of FAI is thought to arise from a combination of genetic predisposition coupled with repetitive axial loading/hip flexion. This causes a bony overgrowth of the proximal femoral physes resulting in a cam deformity (Figure 1).5,14 The abnormal bony anatomy allows for impingement between the acetabulum and proximal femur, which can injure the labrum and articular cartilage of the hip joint.
In the recent study by Ross and colleagues,15 the authors focused on symptomatic hip impingement in ice hockey goalies.15 Goaltender’s hip may be the result of the “butterfly style,” which is a technique of goaltending that emphasizes guarding the lower part of the goal. The goalie drops to his/her knees and internally rotates the hips to allow the leg pads to be parallel to the ice. This style acquired the name butterfly because of the resemblance of the spread goalie pads to a butterfly’s wings. Bedi and associates16 have evaluated hip biomechanics using 3D-generated bone models and showed in their study that arthroscopic treatment can improve hip kinematics and range of motion.
Plain radiographs showed that 90% (61 of 68) of hockey goalies had an elevated alpha angle signifying a femoral cam-type deformity.15 Goalies had a significantly lower mean lateral center-edge angle (27.3° vs 29.6°; P = .03) and 13.2% of them were found to have acetabular dysplasia (lateral center-edge angle<20°) compared to only 3% of positional players. The CT scan measurements demonstrated that hockey goalies have a unique cam-type deformity that is located more lateral (1:00 o’clock vs 1:45 o’clock; P < .0001) along the proximal femur, an elevated maximum alpha angle (80.9° vs 68.6°; P < .0001) and loss of offset, when compared to positional players. These findings provide an anatomical basis in support of reports that goaltenders are more likely to experience intra-articular hip injuries compared to other positional players.13
Regardless of position, symptomatic FAI in a hockey player is generally a problem that slowly builds and is made worse with activity.17 On examination, the player may have limited hip flexion and internal rotation, as well as weakness compared to the contralateral side when testing hip flexion and abduction.18,19 Plain radiographs plus MRI or CT allow for proper characterization and diagnosis (to include underlying chondrolabral pathology).20,21
In the young athlete, initial management includes physical therapy, which focuses on core strengthening. Emphasis is placed on hip flexion and extension, as well as abduction and external rotation with the goal of reducing symptoms and avoiding injuries.22 A similar approach may be applied to the elite athlete, but failure of nonoperative management may necessitate surgical intervention. Hip arthroscopy continues to grow in popularity over open surgical dislocation with low complication rate and high return-to-play rate.23-25
For the in-season athlete, attempts to continue to play can be assisted with the role of an intra-articular corticosteroid injection, which can help calm inflammation within the hip joint and mitigate pain, while rehabilitation focuses on core stabilization, postural retraining and focusing on any muscle imbalances that might be present. For positional players, ice time and shift duration can be adjusted to give the player’s hip a period of rest; meanwhile, for goaltenders, shot volumes in practice can be decreased.
Continue to: For athletes who...
For athletes who fail nonoperative care, surgical treatment varies depending on underlying hip pathology and may include femoroplasty, acetabuloplasty, and microfracture as well as labral repair or debridement. Though data are limited, Philippon and colleagues26 have published promising results in a case series of 28 NHL players after surgical intervention for FAI. All players returned to sport at an average of 3.8 months and players who had surgery within 1 year of injury returned on average 1.1 months sooner than those who waited more than 1 year. Rehabilitation protocol varies between goaltenders compared to defensemen and offensive players due to the different demands required for blocking shots on goal.27
One of the most challenging injuries to correctly identify in the hip area is athletic pubalgia (also referred to as sports hernia or core muscle injury) because pain in the groin may be referred from the lumbar spine, hip joint, urologic, or perineal etiologies.28 Sports hernias involve dilatation of the external ring of the inguinal canal and thinning of the posterior wall. Players may report to the athletic trainer or team physician with a complaint of groin pain that is worse when pushing off with their skate or taking a slap shot.29 On exam, pain can be reproduced by hip extension, contralateral torso rotation, or with a resisted sit-up with palpation of the inferolateral edge of the distal rectus abdominus.30 An MRI with specific sequences centered over the pubic symphysis is usually warranted to aid in the workup of sports hernia. An MRI in these cases may also demonstrate avulsions of the rectus abdominus.31
Most of these injuries are managed conservatively but can warrant surgical intervention if the symptoms persist. In the study by Jakoi and colleagues,32 they identified 43 ice hockey players over an 8-year period (2001-2008) who had repairs of their sports hernias and assessed the statistics during the 2 years prior and 2 years after surgery. The authors found that 80% of these players were able to return to the ice for 2 or more full seasons. The return-to-sport rate was comparable to other sports after sports hernia repair, but players who had played in ≥7 seasons demonstrated a greater decrease in number of games played, goals, assists and time on ice compared to those who had played in ≤6 seasons prior to the time of injury. Between 1989 and 2000, 22 NHL players who failed to respond to nonoperative management of their groin injuries underwent surgical exploration.29 At the time of surgical exploration, their hockey groin syndrome, consisted of small tears in the external oblique aponeurosis through which branches of the ilioinguinal or iliohypogastric could be identified. These surgical procedures were all through a standard inguinal approach and the perforating neurovascular structures were excised, while the main trunk of the ilioinguinal nerve was ablated and the external oblique aponeurosis was repaired and reinforced with Goretex (W.L. Gore & Associates Inc, Flagstaff, AZ). At follow-up, 18 of the 22 players (82%) had no pain and 19 (86%) were able to resume their careers in the NHL.29 Ice hockey players with sports hernias or hockey groin syndrome often return to the sport, but it is important to identify these problems early so that surgical options can be discussed if the player fails conservative management. It is also critical to make sure that all pathology is identified, because in players with mixed sports hernia and FAI, return-to-play results improve when both issues are addressed. In a study of athletes (some of whom were ice hockey players), who had both FAI and sports hernia, and only hernia/pubalgia surgery was performed, 25% of these athletes returned to sport. If only FAI was addressed, 50% of the athletes returned to sport; however, when hernia and FAI were treated, 89% returned to play.33
Adductor strains includes injury to the adductor muscles, pectineus, obturator externus and gracilis, and are prevalent in ice hockey players. A study of elite Swedish ice hockey players published in 1988 reported that adductor strains accounted for 10% (10 of 95) of all injuries.34 Given the prevalence of these injuries, considerable research has been dedicated to understanding their mechanism and prevention.35 Adductor strains within the ice hockey population have been attributed to the eccentric forces on the adductors when players attempt to decelerate the leg during a stride.36 A study of NHL players revealed that a ratio <80% of adductor-to-abductor muscle is the best predictor of a groin strain.37
These injuries are also well known for their recurrence rates, as was the case in an NHL study where 4 of the 9 adductor strains (44%) were recurrent injuries.37 The authors attributed the recurrence to an incomplete rehabilitation program and an accelerated return to sport. This was followed by an NHL prevention program that spanned 2 seasons and analyzed 58 players whose adductor-to-abductor ratio was <80% and placed them into a 6-week intervention program during the preseason.37 Only 3 players sustained an adductor strain in the 2 subsequent seasons after the intervention, compared to 11 strains in the previous 2 seasons. Thus, early identification of muscle strength imbalance coupled with an appropriate intervention program has proven to be an effective means of reducing adductor strains in this at-risk population.
Continue to: Contact injuries may...
Contact injuries may vary with checking into the boards being unique to men’s ice hockey. Hip pointers occur as a result of a direct compression injury to the iliac crest, which causes trauma to the bone but also to the overlying hip abductor musculature, and represent roughly 2.4% of ice hockey injuries.23 The resulting contusion may cause a local hematoma formation. Early identification of the injury plus treatment with RICE (rest, ice, compression, elevation) coupled with crutches to limit weight-bearing status may minimize soft tissue trauma and swelling, and ultimately aid in pain control and return to sport.38 Hip abductor strengthening, added padding over the injured area, as well as a compressive hip spica wrapping, have all been suggested to expedite return to play and help prevent recurrence of the hip pointer.8
KNEE INJURIES
Injury to the medial collateral ligament (MCL) is the most commonly reported knee injury (Figure 2) and second only to concussion amongst all injuries in National Collegiate Athletic Association ice hockey players.8,39 The mechanism of injury typically involves a valgus force on the knee, which is often caused by collision into another player.39 Valgus stress testing with the knee in 30° of flexion is used to grade the severity of injury (Grade I: 0-5 mm of medial opening; Grade II: 5-10 mm of medial opening; Grade III: >10 mm of medial opening).39 One study that followed a single college hockey team for 8 seasons reported that 77% of injuries (10 of 13) occurred during player-to-player collision,39 with 5 being Grade 1 injuries, 6 Grade 2 injuries, 1 Grade 3; information was missing for 1 player. Nonoperative management of incomplete injuries, grade 1 and 2 sprains, with RICE and early physical therapy intervention to work on knee range of motion and quadriceps strengthening typically helps the player return to sport within days for grade 1 and 2 injuries to 3 weeks for grade 2 injuries. Complete tears have been managed both operatively and nonoperatively with evidence to suggest better outcomes after surgical intervention if there is a concomitant ACL injury requiring reconstruction.8,9
Anterior cruciate ligament (ACL) tears occur less frequently in hockey players compared to the players in other sports such as football and basketball.38,40 Between 2006 and 2010, 47 players were identified by the NHL Injury Surveillance System as having sustained an ACL injury, which equates to an incidence of 9.4 ACL injuries per NHL season over this time span.41 The mechanism of ACL tears in ice hockey players appears to be different from other sports players based on a recent MRI study that evaluated players for concomitant injuries following ACL tear and noted significantly fewer bone bruises on the lateral femoral condyle compared to players in other sports.42 Early evaluation after injury with Lachman and/or pivot shift tests aids the diagnosis. Data from the NHL study identified 32 players (68%) with concomitant meniscal injuries and 32 (68%) had MCL injuries in conjunction with their ACL tears.41 Average length in the league prior to injury was 5.65 seasons. Twenty-nine of the injured players (61.7%) underwent reconstruction with a patellar tendon autograft, 13 (27.7%) had a hamstring autograft, and 5 (10.6%) had either a patellar tendon or hamstring allograft.41 Meniscus and ACL injuries were associated with a decreased length of career compared to age-matched controls and, notably, players >30 years at the time of injury had only a 67% rate of return to sport whereas those <30 years had a return-to-sport rate of 80%. Players who were able to return did so at an average of 9.8 months (range, 6-21 months) and had a significant reduction in total number of goals, assists, and points scored compared to controls. Decline in performance was typically associated with forwards and wings, while defensemen did not demonstrate the same decrease in performance following return to ice hockey.41
Meniscal tears are a well-documented concomitant injury with ruptures of the ACL, and the combination is a known pattern associated with shorter careers compared to isolated ACL tears in ice hockey players.41 The lateral meniscus is known for increased mobility compared to the medial meniscus and is more commonly injured (39% vs 8.5%) in ACL tears that occur in contact sports and downhill skiing.42 Ice hockey presents a scenario that is different from other contact sports because of the near frictionless interaction between the player’s ice skates and playing surface. This likely equates to a different injury mechanism and dissipation of energy after contact as well as non-contact injuries.38 A recent study reviewed knee MRI findings associated with ACL tears in collegiate ice hockey players and compared to other sports known for their high rates of concomitant meniscal pathology. The authors reported a statistically significant decrease in lateral meniscus tears and bone-bruising patterns in ice hockey players with ACL injuries compared to athletes with ACL tears in other sports.43 In contrast, an NHL study of ACL tears in professional ice hockey players found that 68% of players had concomitant meniscal tears (32 out of 47 players).41
Continue to: The presence of...
The presence of a meniscal tear on MRI is typically a surgical problem, especially if it occurred with an ACL injury. Meniscal repair is preferable, if possible, because there is a known association of increased cartilage contact pressures associated with meniscal debridement. Return to sport following meniscus injury hinges upon whether it is an isolated injury and how it is treated. If the meniscus injury occurs in isolation and can be treated with a debridement and partial resection alone, there is obviously a quicker return to sport as the player can be weight-bearing immediately following surgery. Return to skating after meniscal debridement and partial resection is usually 4 to 6 weeks, whereas meniscal repair protocols vary depending on surgeon; players may need 3 months to 4 months to return to the ice.
Quadriceps contusions are contact injuries that are not unique to ice hockey (Figure 3). They may result from player collision but also from direct blows from a hockey puck. A high velocity puck is known to cause immense trauma to the quadriceps muscles, which may result in localized bleeding and hematoma formation. If the player is able to anticipate the event, active contraction of the quadriceps muscle has been shown to absorb some of the energy and result in a less traumatic injury, but in a fast paced ice hockey game, the player’s anticipation is less likely than in other sports such as baseball.44Interestingly, the degree of knee flexion after injury is predictive of injury severity with milder injuries associated with angles >90 and more severe injuries resulting in knee flexion angles <45° and typically an antalgic gait.45 It is important to treat these injuries during the first 24 hours with the knee maintained in 120°of flexion, plus ice and compression, which can be achieved using a locked knee brace or elastic compression wrap. Quadriceps stretching and isometric strengthening should immediately follow the period of immobilization. The addition of NSAIDs may help prevent the formation of myositis ossificans. A study from West Point suggests that the average return to sport or activity ranges from 13 days (mild contusion) to 21 days (severe contusions), while others8 have indicated that if the injury is treated acutely and a player is able to regain motion and strength, return to ice hockey within a few days is possible.
FOOT AND ANKLE
Ice hockey has some unique injuries that can be attributed to the use of ice skates for play. One such injury is boot-top lacerations, which are fortunately rare as they can be a career-ending injury.47 The spectrum of injury ranges from superficial abrasions to more severe soft tissue disruption, including the extensor tendons and neurovascular structures. The actual mechanism of injury involves an opponent’s skate blade cutting across the anterior ankle. One early case report described a protective method of having players place their skate tongues deep to their protective shin pads, instead of turning the tongues down.47 Kevlar socks have also been shown to help prevent or minimize the damage from a skate blade.48
Injury to the lateral ankle ligaments, anterior talofibular ligament or calcaneofibular ligament, are usually more common than the higher ankle sprains involving the syndesmosis. However, this is not the case in ice hockey. The rigidity of the ice skate at the level of the lateral ligaments seems to impart a protective mechanism to the lower ligaments, but this results in a higher incidence of syndesmotic injuries. These high ankle injuries are unfortunately more debilitating and often require a longer recovery period. In a study of these injuries in NHL players, syndesmotic sprains made up 74% of all ankle sprains, whereas only 18.4% of ankle sprains involved the syndesmosis in American football players..49,50 The average number of days between injury and return to play is 45 days, and some authors believe that defensemen may have a harder time recovering because of the demands on their ankles by having to switch continuously between forward and backward skating.49
Most patients are treated conservatively when their ankle plain radiographs show a congruent mortise and no evidence of syndesmotic widening. If the player expresses pain when squeezing the syndesmosis, it is helpful to obtain stress radiographs to further evaluate for syndesmotic injury. Nonoperative management includes RICE, immobilization in a rigid boot with crutches to protect weight-bearing with gradual advancements and eventually physical therapy to address any ankle stiffness, followed by dynamic functional activities. Treatment options for syndesmotic widening and failed conservative management includes both screw and plate options as well as suture buttons.49,51,52
Ankle and foot fractures were historically a rare injury in ice hockey players based on radiograph evaluation; however, the recent study by Baker and colleagues4 demonstrated that MRI can be helpful in detecting subradiographic fractures. Most of the injuries detected after MRI were from being hit by a hockey puck; this was a novel mechanism that had not been previously reported in the literature.4 Of the injuries that resulted from a direct blow, 14 of 17 occurred on the medial aspect of the foot and ankle, which is believed to result another word? from a defender skating towards an offensive player and attempting to block shots on goal. In this study, all occult fractures involving the medial malleolus were eventually treated with open reduction and internal fixation and underwent routine healing.4 The navicular bone and base of the first metatarsal accounted for the remaining medial-sided fractures. In a recent analysis of risk factors for reoperation following operative fixation of foot fractures across the National Basketball Association, the National Football Leagues, Major League Baseball, and the National Hockey League only a total of 3 fractures involving the foot (1 navicular and 2 first metatarsal) were identified in NHL players over a 30-year period.53 The study acknowledged a major limitation being a public source for identifying players with fractures.
Lace bite is another common ice hockey injury. It typically occurs at the beginning of a season or whenever a player is breaking in a new pair of skates. The cause of the lace bite is the rigid tongue in the skate that rubs against the anterior ankle. Skating causes inflammation in the area of the tibialis anterior tendon, and the player will complain of significant anterior ankle pain. First line treatment for lace bite is ice (Figure 4A), NSAID gel (eg, diclofenac 1%), and a Bunga lace-bite pad (Absolute Athletics). (Figure 4B).
SUMMARY
Lower extremity injuries are common in ice hockey players, and a covering physician should be comfortable managing these injuries from breezers to skate. Proper evaluation and work-up is critical for early diagnosis and identification of pathology, which can minimize the impact of the injury and expedite a treatment plan to return the player safely to the ice and in the game.
1. Flik K, Lyman S, Marx RG. American collegiate men's ice hockey: an analysis of injuries. Am J Sports Med. 2005;33(2):183-187.
2. Popkin CA, Nelson BJ, Park CN, et al. Head, neck, and shoulder injuries in ice hockey: current concepts. Am J Orthop (Belle Mead NJ). 2017;46(3):123-134.
3. Popkin CA, Schulz BM, Park CN, Bottiglieri TS, Lynch TS. Evaluation, management and prevention of lower extremity youth ice hockey injuries. Open Access J Sports Med. 534 2016;7:167-176.
4. Baker JC, Hoover EG, Hillen TJ, Smith MV, Wright RW, Rubin DA. Subradiographic foot and ankle fractures and bone contusions detected by MRI in elite ice hockey players. Am J Sports Med. 2016;44(5):1317-1323.
5. Philippon MJ, Ho CP, Briggs KK, Stull J, LaPrade RF. Prevalence of increased alpha angles as a measure of cam-type femoroacetabular impingement in youth ice hockey players. Am J Sports Med. 2013;41(6):1357-1362.
6. Engebretsen L, Steffen K, Alonso JM, et al. Sports injuries and illnesses during the Winter Olympic Games 2010. Br J Sports Med. 2010;44(11):772-780.
7. Jorgensen U, Schmidt-Olsen S. The epidemiology of ice hockey injuries. Br J Sports Med. 1986;20(1):7-9.
8. Laprade RF, Surowiec RK, Sochanska AN, et al. Epidemiology, identification, treatment and return to play of musculoskeletal-based ice hockey injuries. BrJ Sports Med. 2014;48(1):4-10.
9. Mosenthal W, Kim M, Holzshu R, Hanypsiak B, Athiviraham A. Common ice hockey injuries and treatment: a current concepts review. Curr Sports Med Rep. 2017;16(5):357-362.
10. Tyler TF, Silvers HJ, Gerhardt MB, Nicholas SJ. Groin injuries in sports medicine. Sports Health. 2010;2(3):231-236.
11. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533.
12. Dalton SL, Zupon AB, Gardner EC, Djoko A, Dompier TP, Kerr ZY. The epidemiology of hip/groin injuries in National Collegiate Athletic Association men's and women's ice hockey: 2009-2010 through 2014-2015 academic years. Orthop J Sports Med. 2016;4(3):2325967116632692.
13. Epstein DM, McHugh M, Yorio M, Neri B. Intra-articular hip injuries in national hockey league players: a descriptive epidemiological study. Am J Sports Med. 2013;41(2):343-348.
14. Nepple JJ, Vigdorchik JM, Clohisy JC. What is the association between sports participation and the development of proximal femoral cam deformity? A systematic review and meta-analysis. Am J Sports Med. 2015;43(11):2833-2840.
15. Ross JR, Bedi A, Stone RM, Sibilsky Enselman E, Kelly BT, Larson CM. Characterization of symptomatic hip impingement in butterfly ice hockey goalies. Arthroscopy. 2015;31(4):635-642.
16. Bedi A, Dolan M, Hetsroni I, et al. Surgical treatment of femoroacetabular impingement improves hip kinematics: a computer-assisted model. Am J Sports Med. 2011;39(Suppl):43S-49S.
17. Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467(3):638-644.
18. Nepple JJ, Goljan P, Briggs KK, Garvey SE, Ryan M, Philippon MJ. Hip strength deficits in patients with symptomatic femoroacetabular impingement and labral ears. Arthroscopy. 2015;31(11):2106-2111.
19. Audenaert EA, Peeters I, Vigneron L, Baelde N, Pattyn C. Hip morphological characteristics and range of internal rotation in femoroacetabular impingement. Am J Sports Med. 2012;40(6):1329-1336.
20. Notzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84(4):556-560.
21. Kuhn AW, Ross JR, Bedi A. Three-dimensional imaging and computer navigation in planning for hip preservation surgery. Sports Med Arthrosc Rev. 2015;23(4):e31-e38.
22. Wall PD, Fernandez M, Griffin DR, Foster NE. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM R. 2013;5(5):418-426.
23. Kuhn AW, Noonan BC, Kelly BT, Larson CM, Bedi A. The hip in ice hockey: a current concepts review. Arthroscopy. 2016;32(9):1928-1938.
24. O'Connor M, Minkara AA, Westermann RW, Rosneck J, Lynch TS. Return to play after hip arthroscopy: a systematic review and meta-analysis. Am J Sports Med. 2018:46(11):2780-2788.
25. Minkara AA, Westermann RW, Rosneck J, Lynch TS. Systematic review and meta-analysis of outcomes after hip arthroscopy in femoroacetabular impingement. Am J Sports Med. 2018:363546517749475.
26. Philippon MJ, Weiss DR, Kuppersmith DA, Briggs KK, Hay CJ. Arthroscopic labral repair and treatment of femoroacetabular impingement in professional hockey players. Am J Sports Med. 2010;38(1):99-104.
27. Pierce CM, Laprade RF, Wahoff M, O'Brien L, Philippon MJ. Ice hockey goaltender rehabilitation, including on-ice progression, after arthroscopic hip surgery for femoroacetabular impingement. J Orthop Sports Phys Ther. 2013;43(3):129-141.
28. MacLeod DA, Gibbon WW. The sportsman's groin. Br J Surg. 1999;86(7):849-850.
29. Irshad K, Feldman LS, Lavoie C, Lacroix VJ, Mulder DS, Brown RA. Operative management of "hockey groin syndrome": 12 years of experience in National Hockey League players. Surgery. 2001;130(4):759-764; discussion 764-756.
30. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
31. Zoga AC, Kavanagh EC, Omar IM, et al. Athletic pubalgia and the "sports hernia": MR imaging findings. Radiology. 2008;247(3):797-807.
32. Jakoi A, O'Neill C, Damsgaard C, Fehring K, Tom J. Sports hernia in National Hockey League players: does surgery affect performance? Am J Sports Med. 2013;41(1):107-110.
33. Larson CM, Pierce BR, Giveans MR. Treatment of athletes with symptomatic intra-articular hip pathology and athletic pubalgia/sports hernia: a case series. Arthroscopy.2011;27(6):768-775.
34. Lorentzon R, Wedren H, Pietila T. Incidence, nature, and causes of ice hockey injuries. A three-year prospective study of a Swedish elite ice hockey team. Am J Sports Med. 1988;16(4):392-396.
35. Holmich P, Uhrskou P, Ulnits L, et al. Effectiveness of active physical training as treatment for long-standing adductor-related groin pain in athletes: randomised trial. Lancet. 1999;353(9151):439-443.
36. Sim FH, Chao EY. Injury potential in modern ice hockey. Am J Sports Med. 1978;6(6):378-384.
37. Tyler TF, Nicholas SJ, Campbell RJ, McHugh MP. The association of hip strength and flexibility with the incidence of adductor muscle strains in professional ice hockey players. Am J Sports Med. 2001;29(2):124-128.
38. LaPrade RF, Wijdicks CA, Griffith CJ. Division I intercollegiate ice hockey team coverage. BrJ Sports Med. 2009;43(13):1000-1005.
39. Grant JA, Bedi A, Kurz J, Bancroft R, Miller BS. Incidence and injury characteristics of medial collateral ligament injuries in male collegiate ice hockey players. Sports Health. 2013;5(3):270-272.
40. Erickson BJ, Harris JD, Cole BJ, et al. Performance and return to sport after anterior cruciate ligament reconstruction in National Hockey League players. Orthop J Sports Med. 2014;2(9):2325967114548831.
41. Sikka R, Kurtenbach C, Steubs JT, Boyd JL, Nelson BJ. Anterior Cruciate Ligament Injuries in Professional Hockey Players. Am J Sports Med. 2016;44(2):378-383.
42. Friden T, Erlandsson T, Zatterstrom R, Lindstrand A, Moritz U. Compression or distraction of the anterior cruciate injured knee: variations in injury pattern in contact sports and downhill skiing. Knee Surg Sports Traumatol Arthrosc. 1995;3(3):144-147.
43. Kluczynski MA, Kang JV, Marzo JM, Bisson LJ. Magnetic resonance imaging and intra-articular findings after anterior cruciate ligament injuries in ice hockey versus other sports. Orthop J Sports Med. 2016;4(5):2325967116646534. 44. Beiner JM, Jokl P. Muscle contusion injuries: current treatment options. J Am Acad Orthop Surg. 2001;9(4):227-237.
45. Jackson DW, Feagin JA. Quadriceps contusions in young athletes. Relation of severity of injury to treatment and prognosis. J Bone Joint Surg Am. 1973;55(1):95-105.
46. Ryan JB, Wheeler JH, Hopkinson WJ, Arciero RA, Kolakowski KR. Quadriceps contusions. West Point update. Am J Sports Med. 1991;19(3):299-304.
47. Johnson PN, Mark; Green, Eric. Boot-top lacerations in ice hockey players: a new injury. Clin J Sports Med. 1991:205-208.
48. Nauth A, Aziz M, Tsuji M, Whalen DB, Theodoropoulos JS, Zdero R. The protective effect of Kevlar socks against hockey skate blade injuries: a biomechanical study. Orthop J Sports Med. 2014;2(Suppl 2):7.
49. Wright RW, Barile RJ, Surprenant DA, Matava MJ. Ankle syndesmosis sprains in national hockey league players. Am J Sports Med. 2004;32(8):1941-1945.
50. Boytim MJ, Fischer DA, Neumann L. Syndesmotic ankle sprains. Am J Sports Med. 1991;19(3):294-298.
51. Marymont JV, Lynch MA, Henning CE. Acute ligamentous diastasis of the ankle without fracture. Evaluation by radionuclide imaging. Am J Sports Med. 1986;14(5):407-409.
52. Miller CD, Shelton WR, Barrett GR, Savoie FH, Dukes AD. Deltoid and syndesmosis ligament injury of the ankle without fracture. Am J Sports Med. 1995;23(6):746-750.
53. Singh SK, Larkin KE, Kadakia AR, Hsu WK. Risk factors for reoperation and performance-based outcomes after operative fixation of foot fractures in the professional athlete: a cross-sport analysis. Sports Health. 2018;10(1):70-74.
54. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144.
55. Elattar O, Choi HR, Dills VD, Busconi B. Groin injuries (athletic pubalgia) and return to play. Sports Health. 2016;8(4):313-323.
ABSTRACT
Ice hockey is a fast-paced, collision sport requiring tremendous skill and finesse, yet ice hockey can be a harsh and violent game. It has one of the highest musculoskeletal injury rates in all of competitive sports. Razor sharp skates, aluminum sticks and boards made from high density polyethylene (HDPE), all contribute to the intrinsic hazards of the game. The objective of this article is to review evaluation, management, and return-to-the-rink guidelines after common lower extremity ice hockey injuries.
“Hockey is a fast body-contact game played by men with clubs in their hands and knives laced to their feet, since the skates are razor sharp, and before the evening is over it is almost a certainty that someone will be hurt and will fleck the ice with a generous contribution of gore before he is led away to be hemstitched together again.” —Paul Gallico in Farewell to Sport (1938)
Ice hockey is a collision sport with player speeds in excess of 30 miles/hour, on a sheet of ice surrounded by unforgiving boards, with a vulcanized rubber puck moving at speeds approaching 100 miles/hour.1-3 Understanding injuries specific to this fast-paced sport is an essential part of being a team physician at any level of competitive ice hockey. We are continuing to improve our ability to correctly identify and treat injuries in ice hockey players.2,4 On the prevention side, rule changes in hockey have been implemented, such as raising the age to allow checking and penalties for deliberate hits to the head and checking from behind, to make the game safer to play.3 Additionally, advancements in biomechanical research and 3D modeling are providing new insights into the pathoanatomy of the hip joint, which can be utilized for surgical planning in hockey players and goalies suffering from symptomatic femoroacetabular impingement (FAI) of the hip.5
During the 2010 Winter Olympics, more than 30% of ice hockey players were injured, which was the highest percentage amongst all competing sports.6 They also tallied the highest percentage of player-to-player injuries during the Olympics of any sport. Consequently, the team physician covering ice hockey should be prepared to manage upper and lower extremity musculoskeletal injuries, but also concussions, cervical spine injuries, and ocular and dental trauma.2
One of the earliest epidemiological studies of ice hockey injuries looked at elite Danish hockey players over 2 seasons and found that head trauma accounted for 28% of all injuries, followed by lower extremity injuries at 27% with upper extremity injuries accounting for 19%.7 More recent epidemiological studies have shown similar rates based on body region while further defining individual diagnoses and their incidence. This should help clinicians and researchers develop prevention strategies, as well as improve treatments to optimize player outcomes and return to sport.8,9 Our group recently reviewed the evaluation and management of common head, neck, and shoulder injuries at all competitive levels of ice hockey, and this article serves to complement the former by focusing on lower extremity injuries (Table).2
Continue to: Hip and groin...
EVALUATION AND MANAGEMENT OF COMMON LOWER EXTREMITY HOCKEY INJURIES
HIP INJURIES
Hip and groin injuries are very common amongst this group of athletes and account for approximately 9% of all ice hockey injuries.1 Unfortunately, they are also known for their high recurrence rates, which may be in part due to delayed diagnosis, inadequate rest and rehabilitation, as well as the extreme loads that are placed on the hip during competition.10,11 In hockey, the most commonly reported hip injuries include goaltender’s hip, FAI, sports hernia/hockey groin syndrome, adductor strains, hip pointer, and quadriceps contusions. Dalton and colleagues12 performed the largest epidemiological study to date on hip and groin injuries amongst National Collegiate Athletic Association ice hockey players and reported that the most common injury mechanism was noncontact in nature. Contact injuries accounted for 13% (55 of 421) in men’s ice hockey players while less than 4% (4 of 114) injuries in female ice hockey players, which is likely attributed to a no checking rule in the women’s division. Some of these hip and groin injuries are difficult to diagnose so it is important for the team physician to perform a thorough history and physical examination. Advanced imaging (magnetic resonance imaging [MRI] or a computed tomography (CT) scan with 3D reconstructions) may be necessary to make the correct diagnosis. This is important for providing proper treatment as well as setting player expectations for return to sport.12
Table 1. Return-to-Play Guidelines for Common Lower Extremity Ice Hockey Injuries | ||
Lower Extremity Injury | Treatment Options | Return-to-the-Rink Goal |
FAI | In-season: injection, physical therapy program, NSAIDS. Off-season or unable to play: requires arthroscopic surgery | Nonoperative can take up to 6 weeks. Surgical depends on what is fixed but goal is 4 months to return to ice24,26
|
Sports hernia/athletic pubalgia
| In-season: physical therapy program, NSAIDS Off-season or unable to play requiring surgery. Essential to make sure no other pathology (eg, FAI, osteitis pubis, adductor strain) to maximize success
| Nonoperative 6-8 wk trial of physical therapy Operative: depends if concomitant FAI but in isolation goal is 3-4 mo33,54
|
Adductor strains | Ice, NSAIDS, physical therapy, use of Hypervolt Hyperice | Depends on position (goalie vs skater) and severity; can take up to 4-8 wk to return to ice. Want 70% strength and painless ROM to skate successfully;55 in chronic cases, may take up to 6 mo35
|
Quadriceps contusion
| Hinged knee brace to maintain 120° of flexion, ice, compression wrap.
| When player regains motion and strength, return to ice can be as fast a couple of days or as long as 3 wk8,46
|
MCL | Hinged knee brace, shin pad modification, ice, NSAIDs | Depends on Grade; if Grade I, 1-2 wk; Grade II, 2-4 wk; Grade III, 4-6 wk8
|
ACL | Surgery autograft BTB autograft soft tissue
| 9-10 mo41 |
Meniscus tear | Depends on type of tear and seasonal timing (in-season or off-season) | If surgical, 3-4 mo; if repair, 4-6 wk if partial menisectomy
|
High ankle sprain
| Cam boot, NSAIDS, ice and physical therapy
| 6 wk49 |
Boot top laceration | Repair of cut structures, depends on depth and what is injured; best treatment is prevention with Kevlar socks | If laceration is deep and severs any medial tendons/vascular structures, return to ice can be ≥6 mo
|
Lace bite
| Bunga pad, ice, diclofenac gel
| Couple of days to up to 2 wk in recalcitrant cases3 |
Abbreviations: ACL, anterior cruciate ligament; BTB, bone-patellar tendon-bone; Cam, controlled ankle motion boot; MCL, medial collateral ligament; FAI, femoroacetabular impingement; NSAIDS, nonsteroidal anti-inflammatory drugs; ROM, range of motion.
Throughout the hockey community, FAI is being examined as a possible source of symptomatic hip pain amongst players at all levels. A recent study, which utilized the National Hockey League (NHL) injury surveillance database, reported that FAI accounted for 5.3% of all hip and groin injuries.13 The etiology of FAI is thought to arise from a combination of genetic predisposition coupled with repetitive axial loading/hip flexion. This causes a bony overgrowth of the proximal femoral physes resulting in a cam deformity (Figure 1).5,14 The abnormal bony anatomy allows for impingement between the acetabulum and proximal femur, which can injure the labrum and articular cartilage of the hip joint.
In the recent study by Ross and colleagues,15 the authors focused on symptomatic hip impingement in ice hockey goalies.15 Goaltender’s hip may be the result of the “butterfly style,” which is a technique of goaltending that emphasizes guarding the lower part of the goal. The goalie drops to his/her knees and internally rotates the hips to allow the leg pads to be parallel to the ice. This style acquired the name butterfly because of the resemblance of the spread goalie pads to a butterfly’s wings. Bedi and associates16 have evaluated hip biomechanics using 3D-generated bone models and showed in their study that arthroscopic treatment can improve hip kinematics and range of motion.
Plain radiographs showed that 90% (61 of 68) of hockey goalies had an elevated alpha angle signifying a femoral cam-type deformity.15 Goalies had a significantly lower mean lateral center-edge angle (27.3° vs 29.6°; P = .03) and 13.2% of them were found to have acetabular dysplasia (lateral center-edge angle<20°) compared to only 3% of positional players. The CT scan measurements demonstrated that hockey goalies have a unique cam-type deformity that is located more lateral (1:00 o’clock vs 1:45 o’clock; P < .0001) along the proximal femur, an elevated maximum alpha angle (80.9° vs 68.6°; P < .0001) and loss of offset, when compared to positional players. These findings provide an anatomical basis in support of reports that goaltenders are more likely to experience intra-articular hip injuries compared to other positional players.13
Regardless of position, symptomatic FAI in a hockey player is generally a problem that slowly builds and is made worse with activity.17 On examination, the player may have limited hip flexion and internal rotation, as well as weakness compared to the contralateral side when testing hip flexion and abduction.18,19 Plain radiographs plus MRI or CT allow for proper characterization and diagnosis (to include underlying chondrolabral pathology).20,21
In the young athlete, initial management includes physical therapy, which focuses on core strengthening. Emphasis is placed on hip flexion and extension, as well as abduction and external rotation with the goal of reducing symptoms and avoiding injuries.22 A similar approach may be applied to the elite athlete, but failure of nonoperative management may necessitate surgical intervention. Hip arthroscopy continues to grow in popularity over open surgical dislocation with low complication rate and high return-to-play rate.23-25
For the in-season athlete, attempts to continue to play can be assisted with the role of an intra-articular corticosteroid injection, which can help calm inflammation within the hip joint and mitigate pain, while rehabilitation focuses on core stabilization, postural retraining and focusing on any muscle imbalances that might be present. For positional players, ice time and shift duration can be adjusted to give the player’s hip a period of rest; meanwhile, for goaltenders, shot volumes in practice can be decreased.
Continue to: For athletes who...
For athletes who fail nonoperative care, surgical treatment varies depending on underlying hip pathology and may include femoroplasty, acetabuloplasty, and microfracture as well as labral repair or debridement. Though data are limited, Philippon and colleagues26 have published promising results in a case series of 28 NHL players after surgical intervention for FAI. All players returned to sport at an average of 3.8 months and players who had surgery within 1 year of injury returned on average 1.1 months sooner than those who waited more than 1 year. Rehabilitation protocol varies between goaltenders compared to defensemen and offensive players due to the different demands required for blocking shots on goal.27
One of the most challenging injuries to correctly identify in the hip area is athletic pubalgia (also referred to as sports hernia or core muscle injury) because pain in the groin may be referred from the lumbar spine, hip joint, urologic, or perineal etiologies.28 Sports hernias involve dilatation of the external ring of the inguinal canal and thinning of the posterior wall. Players may report to the athletic trainer or team physician with a complaint of groin pain that is worse when pushing off with their skate or taking a slap shot.29 On exam, pain can be reproduced by hip extension, contralateral torso rotation, or with a resisted sit-up with palpation of the inferolateral edge of the distal rectus abdominus.30 An MRI with specific sequences centered over the pubic symphysis is usually warranted to aid in the workup of sports hernia. An MRI in these cases may also demonstrate avulsions of the rectus abdominus.31
Most of these injuries are managed conservatively but can warrant surgical intervention if the symptoms persist. In the study by Jakoi and colleagues,32 they identified 43 ice hockey players over an 8-year period (2001-2008) who had repairs of their sports hernias and assessed the statistics during the 2 years prior and 2 years after surgery. The authors found that 80% of these players were able to return to the ice for 2 or more full seasons. The return-to-sport rate was comparable to other sports after sports hernia repair, but players who had played in ≥7 seasons demonstrated a greater decrease in number of games played, goals, assists and time on ice compared to those who had played in ≤6 seasons prior to the time of injury. Between 1989 and 2000, 22 NHL players who failed to respond to nonoperative management of their groin injuries underwent surgical exploration.29 At the time of surgical exploration, their hockey groin syndrome, consisted of small tears in the external oblique aponeurosis through which branches of the ilioinguinal or iliohypogastric could be identified. These surgical procedures were all through a standard inguinal approach and the perforating neurovascular structures were excised, while the main trunk of the ilioinguinal nerve was ablated and the external oblique aponeurosis was repaired and reinforced with Goretex (W.L. Gore & Associates Inc, Flagstaff, AZ). At follow-up, 18 of the 22 players (82%) had no pain and 19 (86%) were able to resume their careers in the NHL.29 Ice hockey players with sports hernias or hockey groin syndrome often return to the sport, but it is important to identify these problems early so that surgical options can be discussed if the player fails conservative management. It is also critical to make sure that all pathology is identified, because in players with mixed sports hernia and FAI, return-to-play results improve when both issues are addressed. In a study of athletes (some of whom were ice hockey players), who had both FAI and sports hernia, and only hernia/pubalgia surgery was performed, 25% of these athletes returned to sport. If only FAI was addressed, 50% of the athletes returned to sport; however, when hernia and FAI were treated, 89% returned to play.33
Adductor strains includes injury to the adductor muscles, pectineus, obturator externus and gracilis, and are prevalent in ice hockey players. A study of elite Swedish ice hockey players published in 1988 reported that adductor strains accounted for 10% (10 of 95) of all injuries.34 Given the prevalence of these injuries, considerable research has been dedicated to understanding their mechanism and prevention.35 Adductor strains within the ice hockey population have been attributed to the eccentric forces on the adductors when players attempt to decelerate the leg during a stride.36 A study of NHL players revealed that a ratio <80% of adductor-to-abductor muscle is the best predictor of a groin strain.37
These injuries are also well known for their recurrence rates, as was the case in an NHL study where 4 of the 9 adductor strains (44%) were recurrent injuries.37 The authors attributed the recurrence to an incomplete rehabilitation program and an accelerated return to sport. This was followed by an NHL prevention program that spanned 2 seasons and analyzed 58 players whose adductor-to-abductor ratio was <80% and placed them into a 6-week intervention program during the preseason.37 Only 3 players sustained an adductor strain in the 2 subsequent seasons after the intervention, compared to 11 strains in the previous 2 seasons. Thus, early identification of muscle strength imbalance coupled with an appropriate intervention program has proven to be an effective means of reducing adductor strains in this at-risk population.
Continue to: Contact injuries may...
Contact injuries may vary with checking into the boards being unique to men’s ice hockey. Hip pointers occur as a result of a direct compression injury to the iliac crest, which causes trauma to the bone but also to the overlying hip abductor musculature, and represent roughly 2.4% of ice hockey injuries.23 The resulting contusion may cause a local hematoma formation. Early identification of the injury plus treatment with RICE (rest, ice, compression, elevation) coupled with crutches to limit weight-bearing status may minimize soft tissue trauma and swelling, and ultimately aid in pain control and return to sport.38 Hip abductor strengthening, added padding over the injured area, as well as a compressive hip spica wrapping, have all been suggested to expedite return to play and help prevent recurrence of the hip pointer.8
KNEE INJURIES
Injury to the medial collateral ligament (MCL) is the most commonly reported knee injury (Figure 2) and second only to concussion amongst all injuries in National Collegiate Athletic Association ice hockey players.8,39 The mechanism of injury typically involves a valgus force on the knee, which is often caused by collision into another player.39 Valgus stress testing with the knee in 30° of flexion is used to grade the severity of injury (Grade I: 0-5 mm of medial opening; Grade II: 5-10 mm of medial opening; Grade III: >10 mm of medial opening).39 One study that followed a single college hockey team for 8 seasons reported that 77% of injuries (10 of 13) occurred during player-to-player collision,39 with 5 being Grade 1 injuries, 6 Grade 2 injuries, 1 Grade 3; information was missing for 1 player. Nonoperative management of incomplete injuries, grade 1 and 2 sprains, with RICE and early physical therapy intervention to work on knee range of motion and quadriceps strengthening typically helps the player return to sport within days for grade 1 and 2 injuries to 3 weeks for grade 2 injuries. Complete tears have been managed both operatively and nonoperatively with evidence to suggest better outcomes after surgical intervention if there is a concomitant ACL injury requiring reconstruction.8,9
Anterior cruciate ligament (ACL) tears occur less frequently in hockey players compared to the players in other sports such as football and basketball.38,40 Between 2006 and 2010, 47 players were identified by the NHL Injury Surveillance System as having sustained an ACL injury, which equates to an incidence of 9.4 ACL injuries per NHL season over this time span.41 The mechanism of ACL tears in ice hockey players appears to be different from other sports players based on a recent MRI study that evaluated players for concomitant injuries following ACL tear and noted significantly fewer bone bruises on the lateral femoral condyle compared to players in other sports.42 Early evaluation after injury with Lachman and/or pivot shift tests aids the diagnosis. Data from the NHL study identified 32 players (68%) with concomitant meniscal injuries and 32 (68%) had MCL injuries in conjunction with their ACL tears.41 Average length in the league prior to injury was 5.65 seasons. Twenty-nine of the injured players (61.7%) underwent reconstruction with a patellar tendon autograft, 13 (27.7%) had a hamstring autograft, and 5 (10.6%) had either a patellar tendon or hamstring allograft.41 Meniscus and ACL injuries were associated with a decreased length of career compared to age-matched controls and, notably, players >30 years at the time of injury had only a 67% rate of return to sport whereas those <30 years had a return-to-sport rate of 80%. Players who were able to return did so at an average of 9.8 months (range, 6-21 months) and had a significant reduction in total number of goals, assists, and points scored compared to controls. Decline in performance was typically associated with forwards and wings, while defensemen did not demonstrate the same decrease in performance following return to ice hockey.41
Meniscal tears are a well-documented concomitant injury with ruptures of the ACL, and the combination is a known pattern associated with shorter careers compared to isolated ACL tears in ice hockey players.41 The lateral meniscus is known for increased mobility compared to the medial meniscus and is more commonly injured (39% vs 8.5%) in ACL tears that occur in contact sports and downhill skiing.42 Ice hockey presents a scenario that is different from other contact sports because of the near frictionless interaction between the player’s ice skates and playing surface. This likely equates to a different injury mechanism and dissipation of energy after contact as well as non-contact injuries.38 A recent study reviewed knee MRI findings associated with ACL tears in collegiate ice hockey players and compared to other sports known for their high rates of concomitant meniscal pathology. The authors reported a statistically significant decrease in lateral meniscus tears and bone-bruising patterns in ice hockey players with ACL injuries compared to athletes with ACL tears in other sports.43 In contrast, an NHL study of ACL tears in professional ice hockey players found that 68% of players had concomitant meniscal tears (32 out of 47 players).41
Continue to: The presence of...
The presence of a meniscal tear on MRI is typically a surgical problem, especially if it occurred with an ACL injury. Meniscal repair is preferable, if possible, because there is a known association of increased cartilage contact pressures associated with meniscal debridement. Return to sport following meniscus injury hinges upon whether it is an isolated injury and how it is treated. If the meniscus injury occurs in isolation and can be treated with a debridement and partial resection alone, there is obviously a quicker return to sport as the player can be weight-bearing immediately following surgery. Return to skating after meniscal debridement and partial resection is usually 4 to 6 weeks, whereas meniscal repair protocols vary depending on surgeon; players may need 3 months to 4 months to return to the ice.
Quadriceps contusions are contact injuries that are not unique to ice hockey (Figure 3). They may result from player collision but also from direct blows from a hockey puck. A high velocity puck is known to cause immense trauma to the quadriceps muscles, which may result in localized bleeding and hematoma formation. If the player is able to anticipate the event, active contraction of the quadriceps muscle has been shown to absorb some of the energy and result in a less traumatic injury, but in a fast paced ice hockey game, the player’s anticipation is less likely than in other sports such as baseball.44Interestingly, the degree of knee flexion after injury is predictive of injury severity with milder injuries associated with angles >90 and more severe injuries resulting in knee flexion angles <45° and typically an antalgic gait.45 It is important to treat these injuries during the first 24 hours with the knee maintained in 120°of flexion, plus ice and compression, which can be achieved using a locked knee brace or elastic compression wrap. Quadriceps stretching and isometric strengthening should immediately follow the period of immobilization. The addition of NSAIDs may help prevent the formation of myositis ossificans. A study from West Point suggests that the average return to sport or activity ranges from 13 days (mild contusion) to 21 days (severe contusions), while others8 have indicated that if the injury is treated acutely and a player is able to regain motion and strength, return to ice hockey within a few days is possible.
FOOT AND ANKLE
Ice hockey has some unique injuries that can be attributed to the use of ice skates for play. One such injury is boot-top lacerations, which are fortunately rare as they can be a career-ending injury.47 The spectrum of injury ranges from superficial abrasions to more severe soft tissue disruption, including the extensor tendons and neurovascular structures. The actual mechanism of injury involves an opponent’s skate blade cutting across the anterior ankle. One early case report described a protective method of having players place their skate tongues deep to their protective shin pads, instead of turning the tongues down.47 Kevlar socks have also been shown to help prevent or minimize the damage from a skate blade.48
Injury to the lateral ankle ligaments, anterior talofibular ligament or calcaneofibular ligament, are usually more common than the higher ankle sprains involving the syndesmosis. However, this is not the case in ice hockey. The rigidity of the ice skate at the level of the lateral ligaments seems to impart a protective mechanism to the lower ligaments, but this results in a higher incidence of syndesmotic injuries. These high ankle injuries are unfortunately more debilitating and often require a longer recovery period. In a study of these injuries in NHL players, syndesmotic sprains made up 74% of all ankle sprains, whereas only 18.4% of ankle sprains involved the syndesmosis in American football players..49,50 The average number of days between injury and return to play is 45 days, and some authors believe that defensemen may have a harder time recovering because of the demands on their ankles by having to switch continuously between forward and backward skating.49
Most patients are treated conservatively when their ankle plain radiographs show a congruent mortise and no evidence of syndesmotic widening. If the player expresses pain when squeezing the syndesmosis, it is helpful to obtain stress radiographs to further evaluate for syndesmotic injury. Nonoperative management includes RICE, immobilization in a rigid boot with crutches to protect weight-bearing with gradual advancements and eventually physical therapy to address any ankle stiffness, followed by dynamic functional activities. Treatment options for syndesmotic widening and failed conservative management includes both screw and plate options as well as suture buttons.49,51,52
Ankle and foot fractures were historically a rare injury in ice hockey players based on radiograph evaluation; however, the recent study by Baker and colleagues4 demonstrated that MRI can be helpful in detecting subradiographic fractures. Most of the injuries detected after MRI were from being hit by a hockey puck; this was a novel mechanism that had not been previously reported in the literature.4 Of the injuries that resulted from a direct blow, 14 of 17 occurred on the medial aspect of the foot and ankle, which is believed to result another word? from a defender skating towards an offensive player and attempting to block shots on goal. In this study, all occult fractures involving the medial malleolus were eventually treated with open reduction and internal fixation and underwent routine healing.4 The navicular bone and base of the first metatarsal accounted for the remaining medial-sided fractures. In a recent analysis of risk factors for reoperation following operative fixation of foot fractures across the National Basketball Association, the National Football Leagues, Major League Baseball, and the National Hockey League only a total of 3 fractures involving the foot (1 navicular and 2 first metatarsal) were identified in NHL players over a 30-year period.53 The study acknowledged a major limitation being a public source for identifying players with fractures.
Lace bite is another common ice hockey injury. It typically occurs at the beginning of a season or whenever a player is breaking in a new pair of skates. The cause of the lace bite is the rigid tongue in the skate that rubs against the anterior ankle. Skating causes inflammation in the area of the tibialis anterior tendon, and the player will complain of significant anterior ankle pain. First line treatment for lace bite is ice (Figure 4A), NSAID gel (eg, diclofenac 1%), and a Bunga lace-bite pad (Absolute Athletics). (Figure 4B).
SUMMARY
Lower extremity injuries are common in ice hockey players, and a covering physician should be comfortable managing these injuries from breezers to skate. Proper evaluation and work-up is critical for early diagnosis and identification of pathology, which can minimize the impact of the injury and expedite a treatment plan to return the player safely to the ice and in the game.
ABSTRACT
Ice hockey is a fast-paced, collision sport requiring tremendous skill and finesse, yet ice hockey can be a harsh and violent game. It has one of the highest musculoskeletal injury rates in all of competitive sports. Razor sharp skates, aluminum sticks and boards made from high density polyethylene (HDPE), all contribute to the intrinsic hazards of the game. The objective of this article is to review evaluation, management, and return-to-the-rink guidelines after common lower extremity ice hockey injuries.
“Hockey is a fast body-contact game played by men with clubs in their hands and knives laced to their feet, since the skates are razor sharp, and before the evening is over it is almost a certainty that someone will be hurt and will fleck the ice with a generous contribution of gore before he is led away to be hemstitched together again.” —Paul Gallico in Farewell to Sport (1938)
Ice hockey is a collision sport with player speeds in excess of 30 miles/hour, on a sheet of ice surrounded by unforgiving boards, with a vulcanized rubber puck moving at speeds approaching 100 miles/hour.1-3 Understanding injuries specific to this fast-paced sport is an essential part of being a team physician at any level of competitive ice hockey. We are continuing to improve our ability to correctly identify and treat injuries in ice hockey players.2,4 On the prevention side, rule changes in hockey have been implemented, such as raising the age to allow checking and penalties for deliberate hits to the head and checking from behind, to make the game safer to play.3 Additionally, advancements in biomechanical research and 3D modeling are providing new insights into the pathoanatomy of the hip joint, which can be utilized for surgical planning in hockey players and goalies suffering from symptomatic femoroacetabular impingement (FAI) of the hip.5
During the 2010 Winter Olympics, more than 30% of ice hockey players were injured, which was the highest percentage amongst all competing sports.6 They also tallied the highest percentage of player-to-player injuries during the Olympics of any sport. Consequently, the team physician covering ice hockey should be prepared to manage upper and lower extremity musculoskeletal injuries, but also concussions, cervical spine injuries, and ocular and dental trauma.2
One of the earliest epidemiological studies of ice hockey injuries looked at elite Danish hockey players over 2 seasons and found that head trauma accounted for 28% of all injuries, followed by lower extremity injuries at 27% with upper extremity injuries accounting for 19%.7 More recent epidemiological studies have shown similar rates based on body region while further defining individual diagnoses and their incidence. This should help clinicians and researchers develop prevention strategies, as well as improve treatments to optimize player outcomes and return to sport.8,9 Our group recently reviewed the evaluation and management of common head, neck, and shoulder injuries at all competitive levels of ice hockey, and this article serves to complement the former by focusing on lower extremity injuries (Table).2
Continue to: Hip and groin...
EVALUATION AND MANAGEMENT OF COMMON LOWER EXTREMITY HOCKEY INJURIES
HIP INJURIES
Hip and groin injuries are very common amongst this group of athletes and account for approximately 9% of all ice hockey injuries.1 Unfortunately, they are also known for their high recurrence rates, which may be in part due to delayed diagnosis, inadequate rest and rehabilitation, as well as the extreme loads that are placed on the hip during competition.10,11 In hockey, the most commonly reported hip injuries include goaltender’s hip, FAI, sports hernia/hockey groin syndrome, adductor strains, hip pointer, and quadriceps contusions. Dalton and colleagues12 performed the largest epidemiological study to date on hip and groin injuries amongst National Collegiate Athletic Association ice hockey players and reported that the most common injury mechanism was noncontact in nature. Contact injuries accounted for 13% (55 of 421) in men’s ice hockey players while less than 4% (4 of 114) injuries in female ice hockey players, which is likely attributed to a no checking rule in the women’s division. Some of these hip and groin injuries are difficult to diagnose so it is important for the team physician to perform a thorough history and physical examination. Advanced imaging (magnetic resonance imaging [MRI] or a computed tomography (CT) scan with 3D reconstructions) may be necessary to make the correct diagnosis. This is important for providing proper treatment as well as setting player expectations for return to sport.12
Table 1. Return-to-Play Guidelines for Common Lower Extremity Ice Hockey Injuries | ||
Lower Extremity Injury | Treatment Options | Return-to-the-Rink Goal |
FAI | In-season: injection, physical therapy program, NSAIDS. Off-season or unable to play: requires arthroscopic surgery | Nonoperative can take up to 6 weeks. Surgical depends on what is fixed but goal is 4 months to return to ice24,26
|
Sports hernia/athletic pubalgia
| In-season: physical therapy program, NSAIDS Off-season or unable to play requiring surgery. Essential to make sure no other pathology (eg, FAI, osteitis pubis, adductor strain) to maximize success
| Nonoperative 6-8 wk trial of physical therapy Operative: depends if concomitant FAI but in isolation goal is 3-4 mo33,54
|
Adductor strains | Ice, NSAIDS, physical therapy, use of Hypervolt Hyperice | Depends on position (goalie vs skater) and severity; can take up to 4-8 wk to return to ice. Want 70% strength and painless ROM to skate successfully;55 in chronic cases, may take up to 6 mo35
|
Quadriceps contusion
| Hinged knee brace to maintain 120° of flexion, ice, compression wrap.
| When player regains motion and strength, return to ice can be as fast a couple of days or as long as 3 wk8,46
|
MCL | Hinged knee brace, shin pad modification, ice, NSAIDs | Depends on Grade; if Grade I, 1-2 wk; Grade II, 2-4 wk; Grade III, 4-6 wk8
|
ACL | Surgery autograft BTB autograft soft tissue
| 9-10 mo41 |
Meniscus tear | Depends on type of tear and seasonal timing (in-season or off-season) | If surgical, 3-4 mo; if repair, 4-6 wk if partial menisectomy
|
High ankle sprain
| Cam boot, NSAIDS, ice and physical therapy
| 6 wk49 |
Boot top laceration | Repair of cut structures, depends on depth and what is injured; best treatment is prevention with Kevlar socks | If laceration is deep and severs any medial tendons/vascular structures, return to ice can be ≥6 mo
|
Lace bite
| Bunga pad, ice, diclofenac gel
| Couple of days to up to 2 wk in recalcitrant cases3 |
Abbreviations: ACL, anterior cruciate ligament; BTB, bone-patellar tendon-bone; Cam, controlled ankle motion boot; MCL, medial collateral ligament; FAI, femoroacetabular impingement; NSAIDS, nonsteroidal anti-inflammatory drugs; ROM, range of motion.
Throughout the hockey community, FAI is being examined as a possible source of symptomatic hip pain amongst players at all levels. A recent study, which utilized the National Hockey League (NHL) injury surveillance database, reported that FAI accounted for 5.3% of all hip and groin injuries.13 The etiology of FAI is thought to arise from a combination of genetic predisposition coupled with repetitive axial loading/hip flexion. This causes a bony overgrowth of the proximal femoral physes resulting in a cam deformity (Figure 1).5,14 The abnormal bony anatomy allows for impingement between the acetabulum and proximal femur, which can injure the labrum and articular cartilage of the hip joint.
In the recent study by Ross and colleagues,15 the authors focused on symptomatic hip impingement in ice hockey goalies.15 Goaltender’s hip may be the result of the “butterfly style,” which is a technique of goaltending that emphasizes guarding the lower part of the goal. The goalie drops to his/her knees and internally rotates the hips to allow the leg pads to be parallel to the ice. This style acquired the name butterfly because of the resemblance of the spread goalie pads to a butterfly’s wings. Bedi and associates16 have evaluated hip biomechanics using 3D-generated bone models and showed in their study that arthroscopic treatment can improve hip kinematics and range of motion.
Plain radiographs showed that 90% (61 of 68) of hockey goalies had an elevated alpha angle signifying a femoral cam-type deformity.15 Goalies had a significantly lower mean lateral center-edge angle (27.3° vs 29.6°; P = .03) and 13.2% of them were found to have acetabular dysplasia (lateral center-edge angle<20°) compared to only 3% of positional players. The CT scan measurements demonstrated that hockey goalies have a unique cam-type deformity that is located more lateral (1:00 o’clock vs 1:45 o’clock; P < .0001) along the proximal femur, an elevated maximum alpha angle (80.9° vs 68.6°; P < .0001) and loss of offset, when compared to positional players. These findings provide an anatomical basis in support of reports that goaltenders are more likely to experience intra-articular hip injuries compared to other positional players.13
Regardless of position, symptomatic FAI in a hockey player is generally a problem that slowly builds and is made worse with activity.17 On examination, the player may have limited hip flexion and internal rotation, as well as weakness compared to the contralateral side when testing hip flexion and abduction.18,19 Plain radiographs plus MRI or CT allow for proper characterization and diagnosis (to include underlying chondrolabral pathology).20,21
In the young athlete, initial management includes physical therapy, which focuses on core strengthening. Emphasis is placed on hip flexion and extension, as well as abduction and external rotation with the goal of reducing symptoms and avoiding injuries.22 A similar approach may be applied to the elite athlete, but failure of nonoperative management may necessitate surgical intervention. Hip arthroscopy continues to grow in popularity over open surgical dislocation with low complication rate and high return-to-play rate.23-25
For the in-season athlete, attempts to continue to play can be assisted with the role of an intra-articular corticosteroid injection, which can help calm inflammation within the hip joint and mitigate pain, while rehabilitation focuses on core stabilization, postural retraining and focusing on any muscle imbalances that might be present. For positional players, ice time and shift duration can be adjusted to give the player’s hip a period of rest; meanwhile, for goaltenders, shot volumes in practice can be decreased.
Continue to: For athletes who...
For athletes who fail nonoperative care, surgical treatment varies depending on underlying hip pathology and may include femoroplasty, acetabuloplasty, and microfracture as well as labral repair or debridement. Though data are limited, Philippon and colleagues26 have published promising results in a case series of 28 NHL players after surgical intervention for FAI. All players returned to sport at an average of 3.8 months and players who had surgery within 1 year of injury returned on average 1.1 months sooner than those who waited more than 1 year. Rehabilitation protocol varies between goaltenders compared to defensemen and offensive players due to the different demands required for blocking shots on goal.27
One of the most challenging injuries to correctly identify in the hip area is athletic pubalgia (also referred to as sports hernia or core muscle injury) because pain in the groin may be referred from the lumbar spine, hip joint, urologic, or perineal etiologies.28 Sports hernias involve dilatation of the external ring of the inguinal canal and thinning of the posterior wall. Players may report to the athletic trainer or team physician with a complaint of groin pain that is worse when pushing off with their skate or taking a slap shot.29 On exam, pain can be reproduced by hip extension, contralateral torso rotation, or with a resisted sit-up with palpation of the inferolateral edge of the distal rectus abdominus.30 An MRI with specific sequences centered over the pubic symphysis is usually warranted to aid in the workup of sports hernia. An MRI in these cases may also demonstrate avulsions of the rectus abdominus.31
Most of these injuries are managed conservatively but can warrant surgical intervention if the symptoms persist. In the study by Jakoi and colleagues,32 they identified 43 ice hockey players over an 8-year period (2001-2008) who had repairs of their sports hernias and assessed the statistics during the 2 years prior and 2 years after surgery. The authors found that 80% of these players were able to return to the ice for 2 or more full seasons. The return-to-sport rate was comparable to other sports after sports hernia repair, but players who had played in ≥7 seasons demonstrated a greater decrease in number of games played, goals, assists and time on ice compared to those who had played in ≤6 seasons prior to the time of injury. Between 1989 and 2000, 22 NHL players who failed to respond to nonoperative management of their groin injuries underwent surgical exploration.29 At the time of surgical exploration, their hockey groin syndrome, consisted of small tears in the external oblique aponeurosis through which branches of the ilioinguinal or iliohypogastric could be identified. These surgical procedures were all through a standard inguinal approach and the perforating neurovascular structures were excised, while the main trunk of the ilioinguinal nerve was ablated and the external oblique aponeurosis was repaired and reinforced with Goretex (W.L. Gore & Associates Inc, Flagstaff, AZ). At follow-up, 18 of the 22 players (82%) had no pain and 19 (86%) were able to resume their careers in the NHL.29 Ice hockey players with sports hernias or hockey groin syndrome often return to the sport, but it is important to identify these problems early so that surgical options can be discussed if the player fails conservative management. It is also critical to make sure that all pathology is identified, because in players with mixed sports hernia and FAI, return-to-play results improve when both issues are addressed. In a study of athletes (some of whom were ice hockey players), who had both FAI and sports hernia, and only hernia/pubalgia surgery was performed, 25% of these athletes returned to sport. If only FAI was addressed, 50% of the athletes returned to sport; however, when hernia and FAI were treated, 89% returned to play.33
Adductor strains includes injury to the adductor muscles, pectineus, obturator externus and gracilis, and are prevalent in ice hockey players. A study of elite Swedish ice hockey players published in 1988 reported that adductor strains accounted for 10% (10 of 95) of all injuries.34 Given the prevalence of these injuries, considerable research has been dedicated to understanding their mechanism and prevention.35 Adductor strains within the ice hockey population have been attributed to the eccentric forces on the adductors when players attempt to decelerate the leg during a stride.36 A study of NHL players revealed that a ratio <80% of adductor-to-abductor muscle is the best predictor of a groin strain.37
These injuries are also well known for their recurrence rates, as was the case in an NHL study where 4 of the 9 adductor strains (44%) were recurrent injuries.37 The authors attributed the recurrence to an incomplete rehabilitation program and an accelerated return to sport. This was followed by an NHL prevention program that spanned 2 seasons and analyzed 58 players whose adductor-to-abductor ratio was <80% and placed them into a 6-week intervention program during the preseason.37 Only 3 players sustained an adductor strain in the 2 subsequent seasons after the intervention, compared to 11 strains in the previous 2 seasons. Thus, early identification of muscle strength imbalance coupled with an appropriate intervention program has proven to be an effective means of reducing adductor strains in this at-risk population.
Continue to: Contact injuries may...
Contact injuries may vary with checking into the boards being unique to men’s ice hockey. Hip pointers occur as a result of a direct compression injury to the iliac crest, which causes trauma to the bone but also to the overlying hip abductor musculature, and represent roughly 2.4% of ice hockey injuries.23 The resulting contusion may cause a local hematoma formation. Early identification of the injury plus treatment with RICE (rest, ice, compression, elevation) coupled with crutches to limit weight-bearing status may minimize soft tissue trauma and swelling, and ultimately aid in pain control and return to sport.38 Hip abductor strengthening, added padding over the injured area, as well as a compressive hip spica wrapping, have all been suggested to expedite return to play and help prevent recurrence of the hip pointer.8
KNEE INJURIES
Injury to the medial collateral ligament (MCL) is the most commonly reported knee injury (Figure 2) and second only to concussion amongst all injuries in National Collegiate Athletic Association ice hockey players.8,39 The mechanism of injury typically involves a valgus force on the knee, which is often caused by collision into another player.39 Valgus stress testing with the knee in 30° of flexion is used to grade the severity of injury (Grade I: 0-5 mm of medial opening; Grade II: 5-10 mm of medial opening; Grade III: >10 mm of medial opening).39 One study that followed a single college hockey team for 8 seasons reported that 77% of injuries (10 of 13) occurred during player-to-player collision,39 with 5 being Grade 1 injuries, 6 Grade 2 injuries, 1 Grade 3; information was missing for 1 player. Nonoperative management of incomplete injuries, grade 1 and 2 sprains, with RICE and early physical therapy intervention to work on knee range of motion and quadriceps strengthening typically helps the player return to sport within days for grade 1 and 2 injuries to 3 weeks for grade 2 injuries. Complete tears have been managed both operatively and nonoperatively with evidence to suggest better outcomes after surgical intervention if there is a concomitant ACL injury requiring reconstruction.8,9
Anterior cruciate ligament (ACL) tears occur less frequently in hockey players compared to the players in other sports such as football and basketball.38,40 Between 2006 and 2010, 47 players were identified by the NHL Injury Surveillance System as having sustained an ACL injury, which equates to an incidence of 9.4 ACL injuries per NHL season over this time span.41 The mechanism of ACL tears in ice hockey players appears to be different from other sports players based on a recent MRI study that evaluated players for concomitant injuries following ACL tear and noted significantly fewer bone bruises on the lateral femoral condyle compared to players in other sports.42 Early evaluation after injury with Lachman and/or pivot shift tests aids the diagnosis. Data from the NHL study identified 32 players (68%) with concomitant meniscal injuries and 32 (68%) had MCL injuries in conjunction with their ACL tears.41 Average length in the league prior to injury was 5.65 seasons. Twenty-nine of the injured players (61.7%) underwent reconstruction with a patellar tendon autograft, 13 (27.7%) had a hamstring autograft, and 5 (10.6%) had either a patellar tendon or hamstring allograft.41 Meniscus and ACL injuries were associated with a decreased length of career compared to age-matched controls and, notably, players >30 years at the time of injury had only a 67% rate of return to sport whereas those <30 years had a return-to-sport rate of 80%. Players who were able to return did so at an average of 9.8 months (range, 6-21 months) and had a significant reduction in total number of goals, assists, and points scored compared to controls. Decline in performance was typically associated with forwards and wings, while defensemen did not demonstrate the same decrease in performance following return to ice hockey.41
Meniscal tears are a well-documented concomitant injury with ruptures of the ACL, and the combination is a known pattern associated with shorter careers compared to isolated ACL tears in ice hockey players.41 The lateral meniscus is known for increased mobility compared to the medial meniscus and is more commonly injured (39% vs 8.5%) in ACL tears that occur in contact sports and downhill skiing.42 Ice hockey presents a scenario that is different from other contact sports because of the near frictionless interaction between the player’s ice skates and playing surface. This likely equates to a different injury mechanism and dissipation of energy after contact as well as non-contact injuries.38 A recent study reviewed knee MRI findings associated with ACL tears in collegiate ice hockey players and compared to other sports known for their high rates of concomitant meniscal pathology. The authors reported a statistically significant decrease in lateral meniscus tears and bone-bruising patterns in ice hockey players with ACL injuries compared to athletes with ACL tears in other sports.43 In contrast, an NHL study of ACL tears in professional ice hockey players found that 68% of players had concomitant meniscal tears (32 out of 47 players).41
Continue to: The presence of...
The presence of a meniscal tear on MRI is typically a surgical problem, especially if it occurred with an ACL injury. Meniscal repair is preferable, if possible, because there is a known association of increased cartilage contact pressures associated with meniscal debridement. Return to sport following meniscus injury hinges upon whether it is an isolated injury and how it is treated. If the meniscus injury occurs in isolation and can be treated with a debridement and partial resection alone, there is obviously a quicker return to sport as the player can be weight-bearing immediately following surgery. Return to skating after meniscal debridement and partial resection is usually 4 to 6 weeks, whereas meniscal repair protocols vary depending on surgeon; players may need 3 months to 4 months to return to the ice.
Quadriceps contusions are contact injuries that are not unique to ice hockey (Figure 3). They may result from player collision but also from direct blows from a hockey puck. A high velocity puck is known to cause immense trauma to the quadriceps muscles, which may result in localized bleeding and hematoma formation. If the player is able to anticipate the event, active contraction of the quadriceps muscle has been shown to absorb some of the energy and result in a less traumatic injury, but in a fast paced ice hockey game, the player’s anticipation is less likely than in other sports such as baseball.44Interestingly, the degree of knee flexion after injury is predictive of injury severity with milder injuries associated with angles >90 and more severe injuries resulting in knee flexion angles <45° and typically an antalgic gait.45 It is important to treat these injuries during the first 24 hours with the knee maintained in 120°of flexion, plus ice and compression, which can be achieved using a locked knee brace or elastic compression wrap. Quadriceps stretching and isometric strengthening should immediately follow the period of immobilization. The addition of NSAIDs may help prevent the formation of myositis ossificans. A study from West Point suggests that the average return to sport or activity ranges from 13 days (mild contusion) to 21 days (severe contusions), while others8 have indicated that if the injury is treated acutely and a player is able to regain motion and strength, return to ice hockey within a few days is possible.
FOOT AND ANKLE
Ice hockey has some unique injuries that can be attributed to the use of ice skates for play. One such injury is boot-top lacerations, which are fortunately rare as they can be a career-ending injury.47 The spectrum of injury ranges from superficial abrasions to more severe soft tissue disruption, including the extensor tendons and neurovascular structures. The actual mechanism of injury involves an opponent’s skate blade cutting across the anterior ankle. One early case report described a protective method of having players place their skate tongues deep to their protective shin pads, instead of turning the tongues down.47 Kevlar socks have also been shown to help prevent or minimize the damage from a skate blade.48
Injury to the lateral ankle ligaments, anterior talofibular ligament or calcaneofibular ligament, are usually more common than the higher ankle sprains involving the syndesmosis. However, this is not the case in ice hockey. The rigidity of the ice skate at the level of the lateral ligaments seems to impart a protective mechanism to the lower ligaments, but this results in a higher incidence of syndesmotic injuries. These high ankle injuries are unfortunately more debilitating and often require a longer recovery period. In a study of these injuries in NHL players, syndesmotic sprains made up 74% of all ankle sprains, whereas only 18.4% of ankle sprains involved the syndesmosis in American football players..49,50 The average number of days between injury and return to play is 45 days, and some authors believe that defensemen may have a harder time recovering because of the demands on their ankles by having to switch continuously between forward and backward skating.49
Most patients are treated conservatively when their ankle plain radiographs show a congruent mortise and no evidence of syndesmotic widening. If the player expresses pain when squeezing the syndesmosis, it is helpful to obtain stress radiographs to further evaluate for syndesmotic injury. Nonoperative management includes RICE, immobilization in a rigid boot with crutches to protect weight-bearing with gradual advancements and eventually physical therapy to address any ankle stiffness, followed by dynamic functional activities. Treatment options for syndesmotic widening and failed conservative management includes both screw and plate options as well as suture buttons.49,51,52
Ankle and foot fractures were historically a rare injury in ice hockey players based on radiograph evaluation; however, the recent study by Baker and colleagues4 demonstrated that MRI can be helpful in detecting subradiographic fractures. Most of the injuries detected after MRI were from being hit by a hockey puck; this was a novel mechanism that had not been previously reported in the literature.4 Of the injuries that resulted from a direct blow, 14 of 17 occurred on the medial aspect of the foot and ankle, which is believed to result another word? from a defender skating towards an offensive player and attempting to block shots on goal. In this study, all occult fractures involving the medial malleolus were eventually treated with open reduction and internal fixation and underwent routine healing.4 The navicular bone and base of the first metatarsal accounted for the remaining medial-sided fractures. In a recent analysis of risk factors for reoperation following operative fixation of foot fractures across the National Basketball Association, the National Football Leagues, Major League Baseball, and the National Hockey League only a total of 3 fractures involving the foot (1 navicular and 2 first metatarsal) were identified in NHL players over a 30-year period.53 The study acknowledged a major limitation being a public source for identifying players with fractures.
Lace bite is another common ice hockey injury. It typically occurs at the beginning of a season or whenever a player is breaking in a new pair of skates. The cause of the lace bite is the rigid tongue in the skate that rubs against the anterior ankle. Skating causes inflammation in the area of the tibialis anterior tendon, and the player will complain of significant anterior ankle pain. First line treatment for lace bite is ice (Figure 4A), NSAID gel (eg, diclofenac 1%), and a Bunga lace-bite pad (Absolute Athletics). (Figure 4B).
SUMMARY
Lower extremity injuries are common in ice hockey players, and a covering physician should be comfortable managing these injuries from breezers to skate. Proper evaluation and work-up is critical for early diagnosis and identification of pathology, which can minimize the impact of the injury and expedite a treatment plan to return the player safely to the ice and in the game.
1. Flik K, Lyman S, Marx RG. American collegiate men's ice hockey: an analysis of injuries. Am J Sports Med. 2005;33(2):183-187.
2. Popkin CA, Nelson BJ, Park CN, et al. Head, neck, and shoulder injuries in ice hockey: current concepts. Am J Orthop (Belle Mead NJ). 2017;46(3):123-134.
3. Popkin CA, Schulz BM, Park CN, Bottiglieri TS, Lynch TS. Evaluation, management and prevention of lower extremity youth ice hockey injuries. Open Access J Sports Med. 534 2016;7:167-176.
4. Baker JC, Hoover EG, Hillen TJ, Smith MV, Wright RW, Rubin DA. Subradiographic foot and ankle fractures and bone contusions detected by MRI in elite ice hockey players. Am J Sports Med. 2016;44(5):1317-1323.
5. Philippon MJ, Ho CP, Briggs KK, Stull J, LaPrade RF. Prevalence of increased alpha angles as a measure of cam-type femoroacetabular impingement in youth ice hockey players. Am J Sports Med. 2013;41(6):1357-1362.
6. Engebretsen L, Steffen K, Alonso JM, et al. Sports injuries and illnesses during the Winter Olympic Games 2010. Br J Sports Med. 2010;44(11):772-780.
7. Jorgensen U, Schmidt-Olsen S. The epidemiology of ice hockey injuries. Br J Sports Med. 1986;20(1):7-9.
8. Laprade RF, Surowiec RK, Sochanska AN, et al. Epidemiology, identification, treatment and return to play of musculoskeletal-based ice hockey injuries. BrJ Sports Med. 2014;48(1):4-10.
9. Mosenthal W, Kim M, Holzshu R, Hanypsiak B, Athiviraham A. Common ice hockey injuries and treatment: a current concepts review. Curr Sports Med Rep. 2017;16(5):357-362.
10. Tyler TF, Silvers HJ, Gerhardt MB, Nicholas SJ. Groin injuries in sports medicine. Sports Health. 2010;2(3):231-236.
11. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533.
12. Dalton SL, Zupon AB, Gardner EC, Djoko A, Dompier TP, Kerr ZY. The epidemiology of hip/groin injuries in National Collegiate Athletic Association men's and women's ice hockey: 2009-2010 through 2014-2015 academic years. Orthop J Sports Med. 2016;4(3):2325967116632692.
13. Epstein DM, McHugh M, Yorio M, Neri B. Intra-articular hip injuries in national hockey league players: a descriptive epidemiological study. Am J Sports Med. 2013;41(2):343-348.
14. Nepple JJ, Vigdorchik JM, Clohisy JC. What is the association between sports participation and the development of proximal femoral cam deformity? A systematic review and meta-analysis. Am J Sports Med. 2015;43(11):2833-2840.
15. Ross JR, Bedi A, Stone RM, Sibilsky Enselman E, Kelly BT, Larson CM. Characterization of symptomatic hip impingement in butterfly ice hockey goalies. Arthroscopy. 2015;31(4):635-642.
16. Bedi A, Dolan M, Hetsroni I, et al. Surgical treatment of femoroacetabular impingement improves hip kinematics: a computer-assisted model. Am J Sports Med. 2011;39(Suppl):43S-49S.
17. Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467(3):638-644.
18. Nepple JJ, Goljan P, Briggs KK, Garvey SE, Ryan M, Philippon MJ. Hip strength deficits in patients with symptomatic femoroacetabular impingement and labral ears. Arthroscopy. 2015;31(11):2106-2111.
19. Audenaert EA, Peeters I, Vigneron L, Baelde N, Pattyn C. Hip morphological characteristics and range of internal rotation in femoroacetabular impingement. Am J Sports Med. 2012;40(6):1329-1336.
20. Notzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84(4):556-560.
21. Kuhn AW, Ross JR, Bedi A. Three-dimensional imaging and computer navigation in planning for hip preservation surgery. Sports Med Arthrosc Rev. 2015;23(4):e31-e38.
22. Wall PD, Fernandez M, Griffin DR, Foster NE. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM R. 2013;5(5):418-426.
23. Kuhn AW, Noonan BC, Kelly BT, Larson CM, Bedi A. The hip in ice hockey: a current concepts review. Arthroscopy. 2016;32(9):1928-1938.
24. O'Connor M, Minkara AA, Westermann RW, Rosneck J, Lynch TS. Return to play after hip arthroscopy: a systematic review and meta-analysis. Am J Sports Med. 2018:46(11):2780-2788.
25. Minkara AA, Westermann RW, Rosneck J, Lynch TS. Systematic review and meta-analysis of outcomes after hip arthroscopy in femoroacetabular impingement. Am J Sports Med. 2018:363546517749475.
26. Philippon MJ, Weiss DR, Kuppersmith DA, Briggs KK, Hay CJ. Arthroscopic labral repair and treatment of femoroacetabular impingement in professional hockey players. Am J Sports Med. 2010;38(1):99-104.
27. Pierce CM, Laprade RF, Wahoff M, O'Brien L, Philippon MJ. Ice hockey goaltender rehabilitation, including on-ice progression, after arthroscopic hip surgery for femoroacetabular impingement. J Orthop Sports Phys Ther. 2013;43(3):129-141.
28. MacLeod DA, Gibbon WW. The sportsman's groin. Br J Surg. 1999;86(7):849-850.
29. Irshad K, Feldman LS, Lavoie C, Lacroix VJ, Mulder DS, Brown RA. Operative management of "hockey groin syndrome": 12 years of experience in National Hockey League players. Surgery. 2001;130(4):759-764; discussion 764-756.
30. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
31. Zoga AC, Kavanagh EC, Omar IM, et al. Athletic pubalgia and the "sports hernia": MR imaging findings. Radiology. 2008;247(3):797-807.
32. Jakoi A, O'Neill C, Damsgaard C, Fehring K, Tom J. Sports hernia in National Hockey League players: does surgery affect performance? Am J Sports Med. 2013;41(1):107-110.
33. Larson CM, Pierce BR, Giveans MR. Treatment of athletes with symptomatic intra-articular hip pathology and athletic pubalgia/sports hernia: a case series. Arthroscopy.2011;27(6):768-775.
34. Lorentzon R, Wedren H, Pietila T. Incidence, nature, and causes of ice hockey injuries. A three-year prospective study of a Swedish elite ice hockey team. Am J Sports Med. 1988;16(4):392-396.
35. Holmich P, Uhrskou P, Ulnits L, et al. Effectiveness of active physical training as treatment for long-standing adductor-related groin pain in athletes: randomised trial. Lancet. 1999;353(9151):439-443.
36. Sim FH, Chao EY. Injury potential in modern ice hockey. Am J Sports Med. 1978;6(6):378-384.
37. Tyler TF, Nicholas SJ, Campbell RJ, McHugh MP. The association of hip strength and flexibility with the incidence of adductor muscle strains in professional ice hockey players. Am J Sports Med. 2001;29(2):124-128.
38. LaPrade RF, Wijdicks CA, Griffith CJ. Division I intercollegiate ice hockey team coverage. BrJ Sports Med. 2009;43(13):1000-1005.
39. Grant JA, Bedi A, Kurz J, Bancroft R, Miller BS. Incidence and injury characteristics of medial collateral ligament injuries in male collegiate ice hockey players. Sports Health. 2013;5(3):270-272.
40. Erickson BJ, Harris JD, Cole BJ, et al. Performance and return to sport after anterior cruciate ligament reconstruction in National Hockey League players. Orthop J Sports Med. 2014;2(9):2325967114548831.
41. Sikka R, Kurtenbach C, Steubs JT, Boyd JL, Nelson BJ. Anterior Cruciate Ligament Injuries in Professional Hockey Players. Am J Sports Med. 2016;44(2):378-383.
42. Friden T, Erlandsson T, Zatterstrom R, Lindstrand A, Moritz U. Compression or distraction of the anterior cruciate injured knee: variations in injury pattern in contact sports and downhill skiing. Knee Surg Sports Traumatol Arthrosc. 1995;3(3):144-147.
43. Kluczynski MA, Kang JV, Marzo JM, Bisson LJ. Magnetic resonance imaging and intra-articular findings after anterior cruciate ligament injuries in ice hockey versus other sports. Orthop J Sports Med. 2016;4(5):2325967116646534. 44. Beiner JM, Jokl P. Muscle contusion injuries: current treatment options. J Am Acad Orthop Surg. 2001;9(4):227-237.
45. Jackson DW, Feagin JA. Quadriceps contusions in young athletes. Relation of severity of injury to treatment and prognosis. J Bone Joint Surg Am. 1973;55(1):95-105.
46. Ryan JB, Wheeler JH, Hopkinson WJ, Arciero RA, Kolakowski KR. Quadriceps contusions. West Point update. Am J Sports Med. 1991;19(3):299-304.
47. Johnson PN, Mark; Green, Eric. Boot-top lacerations in ice hockey players: a new injury. Clin J Sports Med. 1991:205-208.
48. Nauth A, Aziz M, Tsuji M, Whalen DB, Theodoropoulos JS, Zdero R. The protective effect of Kevlar socks against hockey skate blade injuries: a biomechanical study. Orthop J Sports Med. 2014;2(Suppl 2):7.
49. Wright RW, Barile RJ, Surprenant DA, Matava MJ. Ankle syndesmosis sprains in national hockey league players. Am J Sports Med. 2004;32(8):1941-1945.
50. Boytim MJ, Fischer DA, Neumann L. Syndesmotic ankle sprains. Am J Sports Med. 1991;19(3):294-298.
51. Marymont JV, Lynch MA, Henning CE. Acute ligamentous diastasis of the ankle without fracture. Evaluation by radionuclide imaging. Am J Sports Med. 1986;14(5):407-409.
52. Miller CD, Shelton WR, Barrett GR, Savoie FH, Dukes AD. Deltoid and syndesmosis ligament injury of the ankle without fracture. Am J Sports Med. 1995;23(6):746-750.
53. Singh SK, Larkin KE, Kadakia AR, Hsu WK. Risk factors for reoperation and performance-based outcomes after operative fixation of foot fractures in the professional athlete: a cross-sport analysis. Sports Health. 2018;10(1):70-74.
54. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144.
55. Elattar O, Choi HR, Dills VD, Busconi B. Groin injuries (athletic pubalgia) and return to play. Sports Health. 2016;8(4):313-323.
1. Flik K, Lyman S, Marx RG. American collegiate men's ice hockey: an analysis of injuries. Am J Sports Med. 2005;33(2):183-187.
2. Popkin CA, Nelson BJ, Park CN, et al. Head, neck, and shoulder injuries in ice hockey: current concepts. Am J Orthop (Belle Mead NJ). 2017;46(3):123-134.
3. Popkin CA, Schulz BM, Park CN, Bottiglieri TS, Lynch TS. Evaluation, management and prevention of lower extremity youth ice hockey injuries. Open Access J Sports Med. 534 2016;7:167-176.
4. Baker JC, Hoover EG, Hillen TJ, Smith MV, Wright RW, Rubin DA. Subradiographic foot and ankle fractures and bone contusions detected by MRI in elite ice hockey players. Am J Sports Med. 2016;44(5):1317-1323.
5. Philippon MJ, Ho CP, Briggs KK, Stull J, LaPrade RF. Prevalence of increased alpha angles as a measure of cam-type femoroacetabular impingement in youth ice hockey players. Am J Sports Med. 2013;41(6):1357-1362.
6. Engebretsen L, Steffen K, Alonso JM, et al. Sports injuries and illnesses during the Winter Olympic Games 2010. Br J Sports Med. 2010;44(11):772-780.
7. Jorgensen U, Schmidt-Olsen S. The epidemiology of ice hockey injuries. Br J Sports Med. 1986;20(1):7-9.
8. Laprade RF, Surowiec RK, Sochanska AN, et al. Epidemiology, identification, treatment and return to play of musculoskeletal-based ice hockey injuries. BrJ Sports Med. 2014;48(1):4-10.
9. Mosenthal W, Kim M, Holzshu R, Hanypsiak B, Athiviraham A. Common ice hockey injuries and treatment: a current concepts review. Curr Sports Med Rep. 2017;16(5):357-362.
10. Tyler TF, Silvers HJ, Gerhardt MB, Nicholas SJ. Groin injuries in sports medicine. Sports Health. 2010;2(3):231-236.
11. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533.
12. Dalton SL, Zupon AB, Gardner EC, Djoko A, Dompier TP, Kerr ZY. The epidemiology of hip/groin injuries in National Collegiate Athletic Association men's and women's ice hockey: 2009-2010 through 2014-2015 academic years. Orthop J Sports Med. 2016;4(3):2325967116632692.
13. Epstein DM, McHugh M, Yorio M, Neri B. Intra-articular hip injuries in national hockey league players: a descriptive epidemiological study. Am J Sports Med. 2013;41(2):343-348.
14. Nepple JJ, Vigdorchik JM, Clohisy JC. What is the association between sports participation and the development of proximal femoral cam deformity? A systematic review and meta-analysis. Am J Sports Med. 2015;43(11):2833-2840.
15. Ross JR, Bedi A, Stone RM, Sibilsky Enselman E, Kelly BT, Larson CM. Characterization of symptomatic hip impingement in butterfly ice hockey goalies. Arthroscopy. 2015;31(4):635-642.
16. Bedi A, Dolan M, Hetsroni I, et al. Surgical treatment of femoroacetabular impingement improves hip kinematics: a computer-assisted model. Am J Sports Med. 2011;39(Suppl):43S-49S.
17. Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467(3):638-644.
18. Nepple JJ, Goljan P, Briggs KK, Garvey SE, Ryan M, Philippon MJ. Hip strength deficits in patients with symptomatic femoroacetabular impingement and labral ears. Arthroscopy. 2015;31(11):2106-2111.
19. Audenaert EA, Peeters I, Vigneron L, Baelde N, Pattyn C. Hip morphological characteristics and range of internal rotation in femoroacetabular impingement. Am J Sports Med. 2012;40(6):1329-1336.
20. Notzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84(4):556-560.
21. Kuhn AW, Ross JR, Bedi A. Three-dimensional imaging and computer navigation in planning for hip preservation surgery. Sports Med Arthrosc Rev. 2015;23(4):e31-e38.
22. Wall PD, Fernandez M, Griffin DR, Foster NE. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM R. 2013;5(5):418-426.
23. Kuhn AW, Noonan BC, Kelly BT, Larson CM, Bedi A. The hip in ice hockey: a current concepts review. Arthroscopy. 2016;32(9):1928-1938.
24. O'Connor M, Minkara AA, Westermann RW, Rosneck J, Lynch TS. Return to play after hip arthroscopy: a systematic review and meta-analysis. Am J Sports Med. 2018:46(11):2780-2788.
25. Minkara AA, Westermann RW, Rosneck J, Lynch TS. Systematic review and meta-analysis of outcomes after hip arthroscopy in femoroacetabular impingement. Am J Sports Med. 2018:363546517749475.
26. Philippon MJ, Weiss DR, Kuppersmith DA, Briggs KK, Hay CJ. Arthroscopic labral repair and treatment of femoroacetabular impingement in professional hockey players. Am J Sports Med. 2010;38(1):99-104.
27. Pierce CM, Laprade RF, Wahoff M, O'Brien L, Philippon MJ. Ice hockey goaltender rehabilitation, including on-ice progression, after arthroscopic hip surgery for femoroacetabular impingement. J Orthop Sports Phys Ther. 2013;43(3):129-141.
28. MacLeod DA, Gibbon WW. The sportsman's groin. Br J Surg. 1999;86(7):849-850.
29. Irshad K, Feldman LS, Lavoie C, Lacroix VJ, Mulder DS, Brown RA. Operative management of "hockey groin syndrome": 12 years of experience in National Hockey League players. Surgery. 2001;130(4):759-764; discussion 764-756.
30. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
31. Zoga AC, Kavanagh EC, Omar IM, et al. Athletic pubalgia and the "sports hernia": MR imaging findings. Radiology. 2008;247(3):797-807.
32. Jakoi A, O'Neill C, Damsgaard C, Fehring K, Tom J. Sports hernia in National Hockey League players: does surgery affect performance? Am J Sports Med. 2013;41(1):107-110.
33. Larson CM, Pierce BR, Giveans MR. Treatment of athletes with symptomatic intra-articular hip pathology and athletic pubalgia/sports hernia: a case series. Arthroscopy.2011;27(6):768-775.
34. Lorentzon R, Wedren H, Pietila T. Incidence, nature, and causes of ice hockey injuries. A three-year prospective study of a Swedish elite ice hockey team. Am J Sports Med. 1988;16(4):392-396.
35. Holmich P, Uhrskou P, Ulnits L, et al. Effectiveness of active physical training as treatment for long-standing adductor-related groin pain in athletes: randomised trial. Lancet. 1999;353(9151):439-443.
36. Sim FH, Chao EY. Injury potential in modern ice hockey. Am J Sports Med. 1978;6(6):378-384.
37. Tyler TF, Nicholas SJ, Campbell RJ, McHugh MP. The association of hip strength and flexibility with the incidence of adductor muscle strains in professional ice hockey players. Am J Sports Med. 2001;29(2):124-128.
38. LaPrade RF, Wijdicks CA, Griffith CJ. Division I intercollegiate ice hockey team coverage. BrJ Sports Med. 2009;43(13):1000-1005.
39. Grant JA, Bedi A, Kurz J, Bancroft R, Miller BS. Incidence and injury characteristics of medial collateral ligament injuries in male collegiate ice hockey players. Sports Health. 2013;5(3):270-272.
40. Erickson BJ, Harris JD, Cole BJ, et al. Performance and return to sport after anterior cruciate ligament reconstruction in National Hockey League players. Orthop J Sports Med. 2014;2(9):2325967114548831.
41. Sikka R, Kurtenbach C, Steubs JT, Boyd JL, Nelson BJ. Anterior Cruciate Ligament Injuries in Professional Hockey Players. Am J Sports Med. 2016;44(2):378-383.
42. Friden T, Erlandsson T, Zatterstrom R, Lindstrand A, Moritz U. Compression or distraction of the anterior cruciate injured knee: variations in injury pattern in contact sports and downhill skiing. Knee Surg Sports Traumatol Arthrosc. 1995;3(3):144-147.
43. Kluczynski MA, Kang JV, Marzo JM, Bisson LJ. Magnetic resonance imaging and intra-articular findings after anterior cruciate ligament injuries in ice hockey versus other sports. Orthop J Sports Med. 2016;4(5):2325967116646534. 44. Beiner JM, Jokl P. Muscle contusion injuries: current treatment options. J Am Acad Orthop Surg. 2001;9(4):227-237.
45. Jackson DW, Feagin JA. Quadriceps contusions in young athletes. Relation of severity of injury to treatment and prognosis. J Bone Joint Surg Am. 1973;55(1):95-105.
46. Ryan JB, Wheeler JH, Hopkinson WJ, Arciero RA, Kolakowski KR. Quadriceps contusions. West Point update. Am J Sports Med. 1991;19(3):299-304.
47. Johnson PN, Mark; Green, Eric. Boot-top lacerations in ice hockey players: a new injury. Clin J Sports Med. 1991:205-208.
48. Nauth A, Aziz M, Tsuji M, Whalen DB, Theodoropoulos JS, Zdero R. The protective effect of Kevlar socks against hockey skate blade injuries: a biomechanical study. Orthop J Sports Med. 2014;2(Suppl 2):7.
49. Wright RW, Barile RJ, Surprenant DA, Matava MJ. Ankle syndesmosis sprains in national hockey league players. Am J Sports Med. 2004;32(8):1941-1945.
50. Boytim MJ, Fischer DA, Neumann L. Syndesmotic ankle sprains. Am J Sports Med. 1991;19(3):294-298.
51. Marymont JV, Lynch MA, Henning CE. Acute ligamentous diastasis of the ankle without fracture. Evaluation by radionuclide imaging. Am J Sports Med. 1986;14(5):407-409.
52. Miller CD, Shelton WR, Barrett GR, Savoie FH, Dukes AD. Deltoid and syndesmosis ligament injury of the ankle without fracture. Am J Sports Med. 1995;23(6):746-750.
53. Singh SK, Larkin KE, Kadakia AR, Hsu WK. Risk factors for reoperation and performance-based outcomes after operative fixation of foot fractures in the professional athlete: a cross-sport analysis. Sports Health. 2018;10(1):70-74.
54. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144.
55. Elattar O, Choi HR, Dills VD, Busconi B. Groin injuries (athletic pubalgia) and return to play. Sports Health. 2016;8(4):313-323.
TAKE-HOME POINTS:
Ice hockey is a high-speed, collision sport with one of the highest injury rates in all of sports.
Femoroacetabular impingement is a cause of hip pain at all levels of ice hockey; studies indicate goaltenders are at high risk—particularly those who utilize the butterfly, as opposed to hybrid or stand-up, goaltending style.
Medial collateral ligament (MCL) tears are common in ice hockey and are usually the result of a collision with another player.
Use of Kevlar socks and placement of skate tongues deep to the shin pads can help reduce the chance of a boot-top laceration.
High-ankle sprains are more prevalent in ice hockey because of the rigidity of hockey skates and can be a cause of significant loss of time away from the rink.
Safety and Efficacy of Percutaneous Injection of Lipogems Micro-Fractured Adipose Tissue for Osteoarthritic Knees
ABSTRACT
The aim of this study was to evaluate the safety and efficacy of using autologous, micro-fractured, minimally manipulated adipose tissue in patients with refractory knee osteoarthritis (OA). A total of 17 subjects (26 knees) with a median age of 72 years (range: 54-78 years) and a history of knee OA (Kellgren–Lawrence, grade of 3 or 4) underwent treatment with ultrasound-guided injection of micro-fractured adipose tissue. Micro-fractured fat was obtained using a minimal manipulation technique in a closed system (Lipogems), without the addition of enzymes or any other additives. The study subjects were clinically evaluated using the numerical pain rating scale (NPRS), the 100-point Knee Society Score (KSS) with its functional component (FXN), and the lower extremity activity scale (LEAS) at 6 weeks, 6 months, and 12 months following this procedure.
When compared with baseline, significant improvements were noted in the mean values of NPRS, FXN, and LEAS at 6 weeks, 6 months, and 12 months. The mean KSS significantly improved at 6 weeks and 12 months. In particular, the average KSS score improved from 74 to 82, the FXN score improved from 65 to 76, and the LEAS score improved from 36 to 47. These values were significantly greater than the previously published minimal clinically important difference described for KSS and FXN in patients who underwent total knee arthroplasty for primary OA. No serious adverse events were reported. The injection of autologous, micro-fractured, minimally manipulated adipose tissue appears to be a safe and effective treatment option for patients with refractory, severe (grade 3 or 4) knee OA.
This study demonstrated significant improvements in pain, quality of life, and function for at least 12 months in this study population. This intervention may represent a nonsurgical treatment option to avoid knee joint replacement in this population; however, further investigation is needed.
Continue to: Knee OA is...
Knee OA is a chronic disease that affects all races, genders, and ages, but it is most prevalent in obese and elderly people. Worldwide, arthritis is considered to be the fourth leading cause of disability.1 In developing and developed countries, knee OA may cause a significant decline in the quality of life for individuals >65 years due to joint pain and disability.1 Nonoperative treatment can be successful in patients with mild to moderate arthritis with pain.
Current treatment options for knee OA, including physical therapy and anti-inflammatory drugs, aim to remedy the symptoms, but they do little to treat the underlying causes of knee OA pain. When a patient presents with advanced arthritis of the knee as confirmed by radiographic findings (classified as Kellgren–Lawrence grade of 3 or 4), the standard approach has been a total knee arthroplasty (TKA) after the patient has failed conservative treatment. The annual rate of total knee replacement in the United States has doubled since 2000, especially in those 45 – 65 years.2 The total number of procedures performed each year now exceeds 640,000, at a total annual cost of about $10.2 billion.2 Multiple studies show that TKA has favorable outcomes in pain relief and functional improvement in patients >60 years when evaluated at a follow-up of 10 years after surgery.2
However, some patients are hesitant to proceed with surgery due to fear of surgical pain and procedural complications. The known complications include deep vein thrombosis, pulmonary embolism, nerve injury, and infection. In addition, up to 20% of patients continue to complain of pain following a total knee replacement.3 Finally, in the young population (<50 years), there are concerns related to the potential need of revision knee surgery in the future.3
Alternative treatments for knee OA have recently emerged, including the use of platelet-rich plasma (PRP). A recent meta-analysis that included 10 randomized controlled trials with a total of 1069 patients demonstrated that, compared with hyaluronic acid and saline, intra-articular PRP injection may have more benefits in pain relief and functional improvement in patients with symptomatic knee OA at 1-year post-injection.4 Another smaller study examined patients who had experienced mild knee OA (Kellgren–Lawrence grade <3) for an average of 14 months. Each patient underwent magnetic resonance imaging for the evaluation of joint damage and then received a single PRP injection. The patients were assessed at regular intervals, with improvement in pain lasting up to 12 months.5
Additional orthobiologic options include the use of bone marrow and adipose-derived stem cell (ASC) injections for a variety of knee conditions, including knee OA. Mesenchymal stem cells (MSCs) are multipotent cells that have been used for the treatment of OA in clinical trials because of their regeneration potential and anti-inflammatory effects.6 Bone marrow stem cells (BMSCs) were first used to repair cartilage damage in humans in 1998. However, BMSCs had particular challenges, including low stem cell yield, pain, and possible morbidities during bone marrow aspiration. An alternative is ASCs, which may be more suitable clinically because of the high stem cell yield from lipoaspirates, faster cell proliferation, and less discomfort and morbidities during the harvesting procedure.7 In addition, these adult stem cells can contribute to the chondrogenic, osteogenic, adipogenic, myogenic, and neurogenic lineages.8 One study demonstrated that the contents of cartilage glycosaminoglycans significantly increased in specific areas of a knee joint treated with ASCs.9,10 This increased glycosaminoglycan content in hyaline cartilage may explain the observed visual analog score (VAS) improvement and clinical results. Other studies suggest that the chondrogenic action of ASCs may depend more on regenerative signaling by activated perivascular cells and signaling of trophic and paracrine mediators, such as vascular endothelial growth factor.9,10 Finally, the mechanism of action may include providing volume, support, cushioning, and filling of soft tissue defects.11
The Lipogems method and device, approved by the U.S. Food and Drug Administration, is used to harvest ASCs, cleanse, and micro-fracture adipose tissue while maintaining the perivascular niche that contains pericytes. The purpose of this study was to evaluate the safety and efficacy of using autologous, micro-fractured, minimally manipulated adipose tissue in patients with severe refractory knee OA.
Continue to: This report details...
STUDY PRESENTATION
This report details the outcome of an IRB-approved study of 17 subjects with 26 symptomatic knees with a history of knee OA (Kellgren–Lawrence grade of 3 or 4) diagnosed by a radiograph. Patient demographics are described in the Table.
TABLE. Patient Demographics | |
Male n (%) | 10 (58.8) |
Age, mean ± SD (range) | 68.27 ± 7.43 |
BMI, mean ± SD (range) | 28.98 ± 4.50 |
Kellgren–Lawrence grade 3 (n) | 7 |
Kellgren–Lawrence grade 4 (n) | 19 |
Abbreviation: BMI, body mass index.
The study patients were evaluated by an orthopedic surgeon, Mitchell Sheinkop, who commonly performs total joint replacement in his practice and considers potential patients as candidates for TKA. These patients presented with a Kellgren-Lawrence grade of 3 or 4 knee OA, and all had significant pain that was refractory to conservative treatment, which included medications, physical therapy, and injections. The study patients were offered the Lipogems procedure as an alternative to TKA. Following this procedure, the study subjects were clinically evaluated using the numerical pain rating scale (NPRS), the 100-point Knee Society Score (KSS) with its functional component (FXN), and the lower extremity activity scale (LEAS) at 6 weeks, 6 months, and 12 months. The 1989 KSS12 was used for this study. Adverse reactions were also monitored throughout the study period.
METHODS
After obtaining informed consent, the subjects were taken into the operating room, moved to the procedure table, and placed in the prone position for aspiration. After scrubbing with Betadine and draping, 1 mL of lidocaine was used to anesthetize the skin, and a pre-prepared preparation of lidocaine, epinephrine, and sterile saline was infused into the subcutaneous tissue. The micro-fragmented adipose tissue was obtained with minimal manipulation using Lipogems, a closed system using mild mechanical forces and reduction filters. The system processes the lipoaspirate without the addition of enzymes or any other additives. The final product consists of adipose tissue clusters with preserved vascular stromal niche of approximately 500 microns. The lipoaspirate was processed in the same room via a closed system. During the processing, the subject’s puncture wounds were dressed. The knee injection site was prepped with a Betadine swab and DuraPrep. Then, Lipogems was injected intra-articularly under ultrasound guidance.
After the completion of the injection, manual range of motion was administered to the treated joint. The subject was then transferred to the recovery room where vital signs were monitored. Post-procedure instructions were reviewed with the patient by the study staff. The subject was instructed to use an assistive device and avoid weight-bearing for 48 hours and maintain the activities of daily living to a minimum on the day of the procedure. Non-weight-bearing for 48 hours was recommended for reducing discomfort to avoid the use of opioids. Nonsteroidal anti-inflammatory drugs, alcohol, and marijuana must be avoided for 4 weeks after the procedure. Pretreatment and post-treatment outcomes were collected using the NPRS, the 100-point KSS with its FXN, and the LEAS at 6 weeks, 6 months, and 12 months after this procedure. The 1989 KSS12 was used for this study since the same scale was used for previous TKA procedures by our authors, allowing for future comparisons of results.
STATISTICAL ANALYSIS
Mean and standard deviation were used to estimate central tendency and variability. Outcome measures were analyzed using the t test, with the pairwise t test was used for paired and subsequent measurements of the same patient or a knee. All analyses were performed with significance set at P <.05. The minimal clinically important difference (MCID) in patients who underwent TKA for primary OA was between 5.3 and 5.9 for KSS, while the MCID for FXN was between 6.1 and 6.4.13 These values were referenced for our analysis.
Continue to: No significant adverse...
RESULTS
No significant adverse events were reported in the subjects of this study. Common minor adverse events included pain and swelling, which generally resolved in 48 to 72 hours after the procedure.
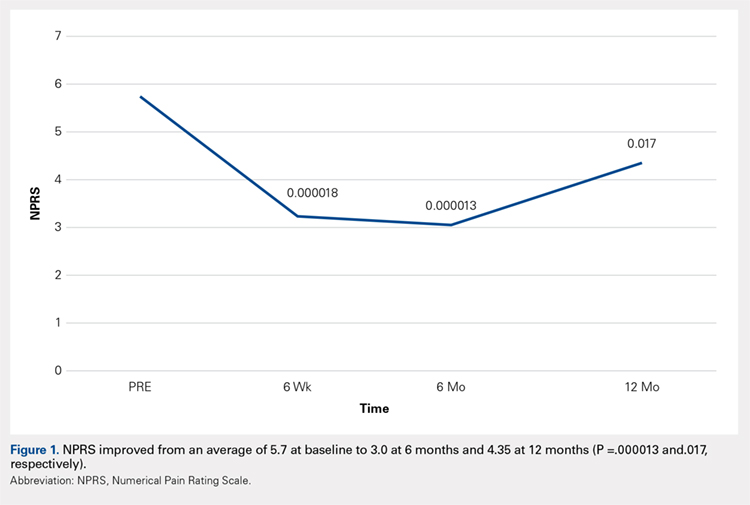
Compared with baseline, significant improvements were noted in the mean values of NPRS (Figure 1) at 6 weeks, 6 months, and 12 months. The mean KSS significantly improved from baseline at 6 weeks and 12 months (Figure 2). Significant improvements were also noted in the mean values of FXN (Figure 3) and the mean LEAS significantly improved from baseline at 6 weeks and 6 months (Figure 4).
DISCUSSION
Knee OA is a disabling condition that affects a substantial proportion of the aging population. The current treatment methods do little to address the degenerative environment of the joint, which includes cytokines such as IL-1 and IL-2. Orthobiologic agents have been used recently to address these issues, which include PRP and MSCs from various sources, including bone marrow and adipose tissue.
A recent meta-analysis conducted by Cui and colleagues14 evaluated 18 studies of MSC treatment for knee OA with a total of 565 participants (226 males and 339 females). The duration from the onset of knee pain to registration in each study ranged from 3 months to ≥7 years. The follow-up period was 3 months -24 months. The majority of studies recruited patients with knee OA with a severity grade of 1-4 on the K-L scale; K-L grades 1 and 2 and grades 3 and 4 were defined as early OA and advanced OA, respectively. The results suggested that MSC treatment significantly improved pain and functional status, relative to the baseline evaluations in knee OA, and the beneficial effect was maintained for 2 years after treatment. Furthermore, the treatment effectiveness was not reduced over time.14
Included in the abovementioned meta-analysis were 2 papers by Koh and colleagues in 2012 and 2013 on the use of AMSCs for the treatment of OA. 15,16 The first study included 18 patients whose adipose tissue was harvested from the inner side of the infrapatellar fat pad via a skin incision after arthroscopic debridement. The cells were centrifuged and injected into the patient’s knee the same day. The results showed a significant reduction of pain and an increased quality of life for all patients, and a positive correlation was found between the number of cells injected and pain improvements. The authors concluded that AMSCs were a valid cell source for treating cartilage damage.15
In their second study, Koh and colleagues reported their results of treating 30 elderly patients with OA (≥65 years), who had failed conventional treatment, using intra-articular injections of AMSCs.16 This patient population is important since OA most commonly occurs in the elderly population. Patients underwent arthroscopic lavage and cartilage evaluation before receiving an injection of AMSCs delivered in PRP. The authors demonstrated that AMSC therapy for elderly patients with mild to moderate OA was an effective treatment resulting in reduction of pain and regeneration of cartilage.16
In another study, Adriani and colleagues17 performed autologous percutaneous fat injection from January 2012 to March 2015 for the treatment of knee OA. Their 30 patients (12 males and 18 females; mean age of 63.3 years; mean body mass index of 25.1) had stable or progressive knee OA for at least 12 months, no other injection treatments during the previous 12 months, and no prior knee surgeries. The patients were evaluated at baseline and 1 week and at 1, 3, 6, and 12 months after treatment using the NPRS and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) as outcome measures. The average VAS was 7.7 at baseline and improved to 4.3 at 3-month follow-up; however, a slight deterioration (VAS 5.0) was noted at 1 year. Total WOMAC score was 89.9 at baseline, 68.6 at 3 months, and 73.2 at 12-month follow-up.17
Continue to: The results of...
The results of this study demonstrated significant improvements in pain, quality of life, and function at 12 months after ultrasound-guided injection of ASCs in patients with severe knee OA. Significant improvement that was noted at 6 weeks was maintained through 12 months after the treatment. Improvement was noted in all scales, including the NPRS, the KSS, and the FXN beginning at 3 months and continuing through 12 months. The LEAS was statistically significant through 6 months after the treatment but not significant at 12 months. No serious adverse events were recorded.
In a study by Lee and colleagues,13 the MCID was described for KSS and FXN in patients who underwent TKA for primary OA. This is the minimal change in a scoring measure that is perceived by the patient to be beneficial or harmful. The MCID for KSS was noted to be between 5.3 and 5.9, while the MCID for FXN was between 6.1 and 6.4.13 In our study, the KSS score improved from an average of 74.0 at baseline to 79.6 at 6 months and 81.6 at 12 months (a difference of 5.6 and 7.6; P = .18 and.014, respectively). The FXN improved from an average of 65.4 at baseline to 75.2 at 6 months and 76.4 at 12 months (a difference of 9.9 and 11; P = .041 and.014, respectively). Therefore, a clinically important difference of KSS and FXN scores was noted at both 6 and 12 months.
The technique used in this study provides autologous, minimally manipulated, fat graft performed in a short time (60-90 minutes), without expansion and/or enzymatic treatment. In addition, the harvesting and the injection of stem cells on the same day is a simple, office-based procedure, and compliant with the U. S. Food and Drug Administration regulations.18 The cost of the procedure averages $3500.
A study limitation is that it is a case series with relatively small numbers and not a randomized controlled study. Therefore, a placebo effect may play a role in our results. Further study with a larger number of patients and randomized controlled studies would be beneficial to support the findings of this study.
CONCLUSION
The injection of autologous, micro-fractured, minimally manipulated adipose tissue appears to be a safe and effective treatment option in patients with refractory severe (grade 3 or 4) knee OA. This study showed significant improvements in pain, quality of life, and function for at least 12 months in this study population. This intervention may represent a nonsurgical treatment option to avoid knee joint replacement in this population; however, further investigation is needed.
- Yubo M, Yanyan L, Li L, Tao S, Bo L, Lin C. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: A meta-analysis. PLoS One. 2017;12(4):e0175449.
- Jauregui JJ, Cherian JJ, Pierce TP, Beaver WB, Issa K, Mont MA. Long-Term Survivorship and Clinical Outcomes Following Total Knee Arthroplasty. J Arthroplasty. 2015;30(12):2164-2166.
- Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
- Dai W-L, Zhou A-G, Zhang H, Zhang J. Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Meta-analysis of Randomized Controlled Trials. Arthroscopy.33(3):659-670.e651.
- Halpern B CS, Rodeo SA, Hayter C, Bogner E, Potter HG, Nguyen J. Clinical and MRI outcomes after platelet-rich plasma treatment for knee osteoarthritis. Clin J Sport Med. 2013 May;23.
- Mamidi MK, Das AK, Zakaria Z, Bhonde R. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthritis Cartilage. 2016;24(8):1307-1316.
- Tang Y, Pan ZY, Zou Y, et al. A comparative assessment of adipose-derived stem cells from subcutaneous and visceral fat as a potential cell source for knee osteoarthritis treatment. J Cell Mol Med. 2017.
- Izadpanah R, Trygg C, Patel B, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. Journal of cellular biochemistry. 2006;99(5):1285-1297.
- Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med. 2010;16(5):203-209.
- Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. A J Physiol Renal Physiol. 2007;292(5):F1626-1635.
- Mestak O, Sukop A, Hsueh YS, et al. Centrifugation versus PureGraft for fatgrafting to the breast after breast-conserving therapy. World J Surg Oncol. 2014;12:178.
- Insall JN DL, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989 Nov;(248):13-4.
- Lee WC, Kwan YH, Chong HC, Yeo SJ. The minimal clinically important difference for Knee Society Clinical Rating System after total knee arthroplasty for primary osteoarthritis. Knee Surgery, Sports Traumatology, Arthroscopy. 2016.
- Cui GH, Wang YY, Li CJ, Shi CH, Wang WS. Efficacy of mesenchymal stem cells in treating patients with osteoarthritis of the knee: A meta-analysis. Exp Ther Med. 2016;12(5):3390-3400.
- Koh Y-GC, Yun-Jin. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19(6):902-907.
- Koh Y-GC, Yun-Jin. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29(4):748-755.
- Adriani E. MM, et al. Percutaneous Fat Transfer to Treat Knee Osteoarthritis Symptoms: Preliminary Results. Joints. 2017.
- Bianchi F, Maioli M, Leonardi E, et al. A New Nonenzymatic Method and Device to Obtain a Fat Tissue Derivative Highly Enriched in Pericyte-Like Elements by Mild Mechanical Forces From Human Lipoaspirates. Cell Transplantation. 2013;22(11):2063-2077
ABSTRACT
The aim of this study was to evaluate the safety and efficacy of using autologous, micro-fractured, minimally manipulated adipose tissue in patients with refractory knee osteoarthritis (OA). A total of 17 subjects (26 knees) with a median age of 72 years (range: 54-78 years) and a history of knee OA (Kellgren–Lawrence, grade of 3 or 4) underwent treatment with ultrasound-guided injection of micro-fractured adipose tissue. Micro-fractured fat was obtained using a minimal manipulation technique in a closed system (Lipogems), without the addition of enzymes or any other additives. The study subjects were clinically evaluated using the numerical pain rating scale (NPRS), the 100-point Knee Society Score (KSS) with its functional component (FXN), and the lower extremity activity scale (LEAS) at 6 weeks, 6 months, and 12 months following this procedure.
When compared with baseline, significant improvements were noted in the mean values of NPRS, FXN, and LEAS at 6 weeks, 6 months, and 12 months. The mean KSS significantly improved at 6 weeks and 12 months. In particular, the average KSS score improved from 74 to 82, the FXN score improved from 65 to 76, and the LEAS score improved from 36 to 47. These values were significantly greater than the previously published minimal clinically important difference described for KSS and FXN in patients who underwent total knee arthroplasty for primary OA. No serious adverse events were reported. The injection of autologous, micro-fractured, minimally manipulated adipose tissue appears to be a safe and effective treatment option for patients with refractory, severe (grade 3 or 4) knee OA.
This study demonstrated significant improvements in pain, quality of life, and function for at least 12 months in this study population. This intervention may represent a nonsurgical treatment option to avoid knee joint replacement in this population; however, further investigation is needed.
Continue to: Knee OA is...
Knee OA is a chronic disease that affects all races, genders, and ages, but it is most prevalent in obese and elderly people. Worldwide, arthritis is considered to be the fourth leading cause of disability.1 In developing and developed countries, knee OA may cause a significant decline in the quality of life for individuals >65 years due to joint pain and disability.1 Nonoperative treatment can be successful in patients with mild to moderate arthritis with pain.
Current treatment options for knee OA, including physical therapy and anti-inflammatory drugs, aim to remedy the symptoms, but they do little to treat the underlying causes of knee OA pain. When a patient presents with advanced arthritis of the knee as confirmed by radiographic findings (classified as Kellgren–Lawrence grade of 3 or 4), the standard approach has been a total knee arthroplasty (TKA) after the patient has failed conservative treatment. The annual rate of total knee replacement in the United States has doubled since 2000, especially in those 45 – 65 years.2 The total number of procedures performed each year now exceeds 640,000, at a total annual cost of about $10.2 billion.2 Multiple studies show that TKA has favorable outcomes in pain relief and functional improvement in patients >60 years when evaluated at a follow-up of 10 years after surgery.2
However, some patients are hesitant to proceed with surgery due to fear of surgical pain and procedural complications. The known complications include deep vein thrombosis, pulmonary embolism, nerve injury, and infection. In addition, up to 20% of patients continue to complain of pain following a total knee replacement.3 Finally, in the young population (<50 years), there are concerns related to the potential need of revision knee surgery in the future.3
Alternative treatments for knee OA have recently emerged, including the use of platelet-rich plasma (PRP). A recent meta-analysis that included 10 randomized controlled trials with a total of 1069 patients demonstrated that, compared with hyaluronic acid and saline, intra-articular PRP injection may have more benefits in pain relief and functional improvement in patients with symptomatic knee OA at 1-year post-injection.4 Another smaller study examined patients who had experienced mild knee OA (Kellgren–Lawrence grade <3) for an average of 14 months. Each patient underwent magnetic resonance imaging for the evaluation of joint damage and then received a single PRP injection. The patients were assessed at regular intervals, with improvement in pain lasting up to 12 months.5
Additional orthobiologic options include the use of bone marrow and adipose-derived stem cell (ASC) injections for a variety of knee conditions, including knee OA. Mesenchymal stem cells (MSCs) are multipotent cells that have been used for the treatment of OA in clinical trials because of their regeneration potential and anti-inflammatory effects.6 Bone marrow stem cells (BMSCs) were first used to repair cartilage damage in humans in 1998. However, BMSCs had particular challenges, including low stem cell yield, pain, and possible morbidities during bone marrow aspiration. An alternative is ASCs, which may be more suitable clinically because of the high stem cell yield from lipoaspirates, faster cell proliferation, and less discomfort and morbidities during the harvesting procedure.7 In addition, these adult stem cells can contribute to the chondrogenic, osteogenic, adipogenic, myogenic, and neurogenic lineages.8 One study demonstrated that the contents of cartilage glycosaminoglycans significantly increased in specific areas of a knee joint treated with ASCs.9,10 This increased glycosaminoglycan content in hyaline cartilage may explain the observed visual analog score (VAS) improvement and clinical results. Other studies suggest that the chondrogenic action of ASCs may depend more on regenerative signaling by activated perivascular cells and signaling of trophic and paracrine mediators, such as vascular endothelial growth factor.9,10 Finally, the mechanism of action may include providing volume, support, cushioning, and filling of soft tissue defects.11
The Lipogems method and device, approved by the U.S. Food and Drug Administration, is used to harvest ASCs, cleanse, and micro-fracture adipose tissue while maintaining the perivascular niche that contains pericytes. The purpose of this study was to evaluate the safety and efficacy of using autologous, micro-fractured, minimally manipulated adipose tissue in patients with severe refractory knee OA.
Continue to: This report details...
STUDY PRESENTATION
This report details the outcome of an IRB-approved study of 17 subjects with 26 symptomatic knees with a history of knee OA (Kellgren–Lawrence grade of 3 or 4) diagnosed by a radiograph. Patient demographics are described in the Table.
TABLE. Patient Demographics | |
Male n (%) | 10 (58.8) |
Age, mean ± SD (range) | 68.27 ± 7.43 |
BMI, mean ± SD (range) | 28.98 ± 4.50 |
Kellgren–Lawrence grade 3 (n) | 7 |
Kellgren–Lawrence grade 4 (n) | 19 |
Abbreviation: BMI, body mass index.
The study patients were evaluated by an orthopedic surgeon, Mitchell Sheinkop, who commonly performs total joint replacement in his practice and considers potential patients as candidates for TKA. These patients presented with a Kellgren-Lawrence grade of 3 or 4 knee OA, and all had significant pain that was refractory to conservative treatment, which included medications, physical therapy, and injections. The study patients were offered the Lipogems procedure as an alternative to TKA. Following this procedure, the study subjects were clinically evaluated using the numerical pain rating scale (NPRS), the 100-point Knee Society Score (KSS) with its functional component (FXN), and the lower extremity activity scale (LEAS) at 6 weeks, 6 months, and 12 months. The 1989 KSS12 was used for this study. Adverse reactions were also monitored throughout the study period.
METHODS
After obtaining informed consent, the subjects were taken into the operating room, moved to the procedure table, and placed in the prone position for aspiration. After scrubbing with Betadine and draping, 1 mL of lidocaine was used to anesthetize the skin, and a pre-prepared preparation of lidocaine, epinephrine, and sterile saline was infused into the subcutaneous tissue. The micro-fragmented adipose tissue was obtained with minimal manipulation using Lipogems, a closed system using mild mechanical forces and reduction filters. The system processes the lipoaspirate without the addition of enzymes or any other additives. The final product consists of adipose tissue clusters with preserved vascular stromal niche of approximately 500 microns. The lipoaspirate was processed in the same room via a closed system. During the processing, the subject’s puncture wounds were dressed. The knee injection site was prepped with a Betadine swab and DuraPrep. Then, Lipogems was injected intra-articularly under ultrasound guidance.
After the completion of the injection, manual range of motion was administered to the treated joint. The subject was then transferred to the recovery room where vital signs were monitored. Post-procedure instructions were reviewed with the patient by the study staff. The subject was instructed to use an assistive device and avoid weight-bearing for 48 hours and maintain the activities of daily living to a minimum on the day of the procedure. Non-weight-bearing for 48 hours was recommended for reducing discomfort to avoid the use of opioids. Nonsteroidal anti-inflammatory drugs, alcohol, and marijuana must be avoided for 4 weeks after the procedure. Pretreatment and post-treatment outcomes were collected using the NPRS, the 100-point KSS with its FXN, and the LEAS at 6 weeks, 6 months, and 12 months after this procedure. The 1989 KSS12 was used for this study since the same scale was used for previous TKA procedures by our authors, allowing for future comparisons of results.
STATISTICAL ANALYSIS
Mean and standard deviation were used to estimate central tendency and variability. Outcome measures were analyzed using the t test, with the pairwise t test was used for paired and subsequent measurements of the same patient or a knee. All analyses were performed with significance set at P <.05. The minimal clinically important difference (MCID) in patients who underwent TKA for primary OA was between 5.3 and 5.9 for KSS, while the MCID for FXN was between 6.1 and 6.4.13 These values were referenced for our analysis.
Continue to: No significant adverse...
RESULTS
No significant adverse events were reported in the subjects of this study. Common minor adverse events included pain and swelling, which generally resolved in 48 to 72 hours after the procedure.

Compared with baseline, significant improvements were noted in the mean values of NPRS (Figure 1) at 6 weeks, 6 months, and 12 months. The mean KSS significantly improved from baseline at 6 weeks and 12 months (Figure 2). Significant improvements were also noted in the mean values of FXN (Figure 3) and the mean LEAS significantly improved from baseline at 6 weeks and 6 months (Figure 4).

DISCUSSION
Knee OA is a disabling condition that affects a substantial proportion of the aging population. The current treatment methods do little to address the degenerative environment of the joint, which includes cytokines such as IL-1 and IL-2. Orthobiologic agents have been used recently to address these issues, which include PRP and MSCs from various sources, including bone marrow and adipose tissue.

A recent meta-analysis conducted by Cui and colleagues14 evaluated 18 studies of MSC treatment for knee OA with a total of 565 participants (226 males and 339 females). The duration from the onset of knee pain to registration in each study ranged from 3 months to ≥7 years. The follow-up period was 3 months -24 months. The majority of studies recruited patients with knee OA with a severity grade of 1-4 on the K-L scale; K-L grades 1 and 2 and grades 3 and 4 were defined as early OA and advanced OA, respectively. The results suggested that MSC treatment significantly improved pain and functional status, relative to the baseline evaluations in knee OA, and the beneficial effect was maintained for 2 years after treatment. Furthermore, the treatment effectiveness was not reduced over time.14

Included in the abovementioned meta-analysis were 2 papers by Koh and colleagues in 2012 and 2013 on the use of AMSCs for the treatment of OA. 15,16 The first study included 18 patients whose adipose tissue was harvested from the inner side of the infrapatellar fat pad via a skin incision after arthroscopic debridement. The cells were centrifuged and injected into the patient’s knee the same day. The results showed a significant reduction of pain and an increased quality of life for all patients, and a positive correlation was found between the number of cells injected and pain improvements. The authors concluded that AMSCs were a valid cell source for treating cartilage damage.15
In their second study, Koh and colleagues reported their results of treating 30 elderly patients with OA (≥65 years), who had failed conventional treatment, using intra-articular injections of AMSCs.16 This patient population is important since OA most commonly occurs in the elderly population. Patients underwent arthroscopic lavage and cartilage evaluation before receiving an injection of AMSCs delivered in PRP. The authors demonstrated that AMSC therapy for elderly patients with mild to moderate OA was an effective treatment resulting in reduction of pain and regeneration of cartilage.16
In another study, Adriani and colleagues17 performed autologous percutaneous fat injection from January 2012 to March 2015 for the treatment of knee OA. Their 30 patients (12 males and 18 females; mean age of 63.3 years; mean body mass index of 25.1) had stable or progressive knee OA for at least 12 months, no other injection treatments during the previous 12 months, and no prior knee surgeries. The patients were evaluated at baseline and 1 week and at 1, 3, 6, and 12 months after treatment using the NPRS and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) as outcome measures. The average VAS was 7.7 at baseline and improved to 4.3 at 3-month follow-up; however, a slight deterioration (VAS 5.0) was noted at 1 year. Total WOMAC score was 89.9 at baseline, 68.6 at 3 months, and 73.2 at 12-month follow-up.17
Continue to: The results of...
The results of this study demonstrated significant improvements in pain, quality of life, and function at 12 months after ultrasound-guided injection of ASCs in patients with severe knee OA. Significant improvement that was noted at 6 weeks was maintained through 12 months after the treatment. Improvement was noted in all scales, including the NPRS, the KSS, and the FXN beginning at 3 months and continuing through 12 months. The LEAS was statistically significant through 6 months after the treatment but not significant at 12 months. No serious adverse events were recorded.
In a study by Lee and colleagues,13 the MCID was described for KSS and FXN in patients who underwent TKA for primary OA. This is the minimal change in a scoring measure that is perceived by the patient to be beneficial or harmful. The MCID for KSS was noted to be between 5.3 and 5.9, while the MCID for FXN was between 6.1 and 6.4.13 In our study, the KSS score improved from an average of 74.0 at baseline to 79.6 at 6 months and 81.6 at 12 months (a difference of 5.6 and 7.6; P = .18 and.014, respectively). The FXN improved from an average of 65.4 at baseline to 75.2 at 6 months and 76.4 at 12 months (a difference of 9.9 and 11; P = .041 and.014, respectively). Therefore, a clinically important difference of KSS and FXN scores was noted at both 6 and 12 months.
The technique used in this study provides autologous, minimally manipulated, fat graft performed in a short time (60-90 minutes), without expansion and/or enzymatic treatment. In addition, the harvesting and the injection of stem cells on the same day is a simple, office-based procedure, and compliant with the U. S. Food and Drug Administration regulations.18 The cost of the procedure averages $3500.
A study limitation is that it is a case series with relatively small numbers and not a randomized controlled study. Therefore, a placebo effect may play a role in our results. Further study with a larger number of patients and randomized controlled studies would be beneficial to support the findings of this study.
CONCLUSION
The injection of autologous, micro-fractured, minimally manipulated adipose tissue appears to be a safe and effective treatment option in patients with refractory severe (grade 3 or 4) knee OA. This study showed significant improvements in pain, quality of life, and function for at least 12 months in this study population. This intervention may represent a nonsurgical treatment option to avoid knee joint replacement in this population; however, further investigation is needed.
ABSTRACT
The aim of this study was to evaluate the safety and efficacy of using autologous, micro-fractured, minimally manipulated adipose tissue in patients with refractory knee osteoarthritis (OA). A total of 17 subjects (26 knees) with a median age of 72 years (range: 54-78 years) and a history of knee OA (Kellgren–Lawrence, grade of 3 or 4) underwent treatment with ultrasound-guided injection of micro-fractured adipose tissue. Micro-fractured fat was obtained using a minimal manipulation technique in a closed system (Lipogems), without the addition of enzymes or any other additives. The study subjects were clinically evaluated using the numerical pain rating scale (NPRS), the 100-point Knee Society Score (KSS) with its functional component (FXN), and the lower extremity activity scale (LEAS) at 6 weeks, 6 months, and 12 months following this procedure.
When compared with baseline, significant improvements were noted in the mean values of NPRS, FXN, and LEAS at 6 weeks, 6 months, and 12 months. The mean KSS significantly improved at 6 weeks and 12 months. In particular, the average KSS score improved from 74 to 82, the FXN score improved from 65 to 76, and the LEAS score improved from 36 to 47. These values were significantly greater than the previously published minimal clinically important difference described for KSS and FXN in patients who underwent total knee arthroplasty for primary OA. No serious adverse events were reported. The injection of autologous, micro-fractured, minimally manipulated adipose tissue appears to be a safe and effective treatment option for patients with refractory, severe (grade 3 or 4) knee OA.
This study demonstrated significant improvements in pain, quality of life, and function for at least 12 months in this study population. This intervention may represent a nonsurgical treatment option to avoid knee joint replacement in this population; however, further investigation is needed.
Continue to: Knee OA is...
Knee OA is a chronic disease that affects all races, genders, and ages, but it is most prevalent in obese and elderly people. Worldwide, arthritis is considered to be the fourth leading cause of disability.1 In developing and developed countries, knee OA may cause a significant decline in the quality of life for individuals >65 years due to joint pain and disability.1 Nonoperative treatment can be successful in patients with mild to moderate arthritis with pain.
Current treatment options for knee OA, including physical therapy and anti-inflammatory drugs, aim to remedy the symptoms, but they do little to treat the underlying causes of knee OA pain. When a patient presents with advanced arthritis of the knee as confirmed by radiographic findings (classified as Kellgren–Lawrence grade of 3 or 4), the standard approach has been a total knee arthroplasty (TKA) after the patient has failed conservative treatment. The annual rate of total knee replacement in the United States has doubled since 2000, especially in those 45 – 65 years.2 The total number of procedures performed each year now exceeds 640,000, at a total annual cost of about $10.2 billion.2 Multiple studies show that TKA has favorable outcomes in pain relief and functional improvement in patients >60 years when evaluated at a follow-up of 10 years after surgery.2
However, some patients are hesitant to proceed with surgery due to fear of surgical pain and procedural complications. The known complications include deep vein thrombosis, pulmonary embolism, nerve injury, and infection. In addition, up to 20% of patients continue to complain of pain following a total knee replacement.3 Finally, in the young population (<50 years), there are concerns related to the potential need of revision knee surgery in the future.3
Alternative treatments for knee OA have recently emerged, including the use of platelet-rich plasma (PRP). A recent meta-analysis that included 10 randomized controlled trials with a total of 1069 patients demonstrated that, compared with hyaluronic acid and saline, intra-articular PRP injection may have more benefits in pain relief and functional improvement in patients with symptomatic knee OA at 1-year post-injection.4 Another smaller study examined patients who had experienced mild knee OA (Kellgren–Lawrence grade <3) for an average of 14 months. Each patient underwent magnetic resonance imaging for the evaluation of joint damage and then received a single PRP injection. The patients were assessed at regular intervals, with improvement in pain lasting up to 12 months.5
Additional orthobiologic options include the use of bone marrow and adipose-derived stem cell (ASC) injections for a variety of knee conditions, including knee OA. Mesenchymal stem cells (MSCs) are multipotent cells that have been used for the treatment of OA in clinical trials because of their regeneration potential and anti-inflammatory effects.6 Bone marrow stem cells (BMSCs) were first used to repair cartilage damage in humans in 1998. However, BMSCs had particular challenges, including low stem cell yield, pain, and possible morbidities during bone marrow aspiration. An alternative is ASCs, which may be more suitable clinically because of the high stem cell yield from lipoaspirates, faster cell proliferation, and less discomfort and morbidities during the harvesting procedure.7 In addition, these adult stem cells can contribute to the chondrogenic, osteogenic, adipogenic, myogenic, and neurogenic lineages.8 One study demonstrated that the contents of cartilage glycosaminoglycans significantly increased in specific areas of a knee joint treated with ASCs.9,10 This increased glycosaminoglycan content in hyaline cartilage may explain the observed visual analog score (VAS) improvement and clinical results. Other studies suggest that the chondrogenic action of ASCs may depend more on regenerative signaling by activated perivascular cells and signaling of trophic and paracrine mediators, such as vascular endothelial growth factor.9,10 Finally, the mechanism of action may include providing volume, support, cushioning, and filling of soft tissue defects.11
The Lipogems method and device, approved by the U.S. Food and Drug Administration, is used to harvest ASCs, cleanse, and micro-fracture adipose tissue while maintaining the perivascular niche that contains pericytes. The purpose of this study was to evaluate the safety and efficacy of using autologous, micro-fractured, minimally manipulated adipose tissue in patients with severe refractory knee OA.
Continue to: This report details...
STUDY PRESENTATION
This report details the outcome of an IRB-approved study of 17 subjects with 26 symptomatic knees with a history of knee OA (Kellgren–Lawrence grade of 3 or 4) diagnosed by a radiograph. Patient demographics are described in the Table.
TABLE. Patient Demographics | |
Male n (%) | 10 (58.8) |
Age, mean ± SD (range) | 68.27 ± 7.43 |
BMI, mean ± SD (range) | 28.98 ± 4.50 |
Kellgren–Lawrence grade 3 (n) | 7 |
Kellgren–Lawrence grade 4 (n) | 19 |
Abbreviation: BMI, body mass index.
The study patients were evaluated by an orthopedic surgeon, Mitchell Sheinkop, who commonly performs total joint replacement in his practice and considers potential patients as candidates for TKA. These patients presented with a Kellgren-Lawrence grade of 3 or 4 knee OA, and all had significant pain that was refractory to conservative treatment, which included medications, physical therapy, and injections. The study patients were offered the Lipogems procedure as an alternative to TKA. Following this procedure, the study subjects were clinically evaluated using the numerical pain rating scale (NPRS), the 100-point Knee Society Score (KSS) with its functional component (FXN), and the lower extremity activity scale (LEAS) at 6 weeks, 6 months, and 12 months. The 1989 KSS12 was used for this study. Adverse reactions were also monitored throughout the study period.
METHODS
After obtaining informed consent, the subjects were taken into the operating room, moved to the procedure table, and placed in the prone position for aspiration. After scrubbing with Betadine and draping, 1 mL of lidocaine was used to anesthetize the skin, and a pre-prepared preparation of lidocaine, epinephrine, and sterile saline was infused into the subcutaneous tissue. The micro-fragmented adipose tissue was obtained with minimal manipulation using Lipogems, a closed system using mild mechanical forces and reduction filters. The system processes the lipoaspirate without the addition of enzymes or any other additives. The final product consists of adipose tissue clusters with preserved vascular stromal niche of approximately 500 microns. The lipoaspirate was processed in the same room via a closed system. During the processing, the subject’s puncture wounds were dressed. The knee injection site was prepped with a Betadine swab and DuraPrep. Then, Lipogems was injected intra-articularly under ultrasound guidance.
After the completion of the injection, manual range of motion was administered to the treated joint. The subject was then transferred to the recovery room where vital signs were monitored. Post-procedure instructions were reviewed with the patient by the study staff. The subject was instructed to use an assistive device and avoid weight-bearing for 48 hours and maintain the activities of daily living to a minimum on the day of the procedure. Non-weight-bearing for 48 hours was recommended for reducing discomfort to avoid the use of opioids. Nonsteroidal anti-inflammatory drugs, alcohol, and marijuana must be avoided for 4 weeks after the procedure. Pretreatment and post-treatment outcomes were collected using the NPRS, the 100-point KSS with its FXN, and the LEAS at 6 weeks, 6 months, and 12 months after this procedure. The 1989 KSS12 was used for this study since the same scale was used for previous TKA procedures by our authors, allowing for future comparisons of results.
STATISTICAL ANALYSIS
Mean and standard deviation were used to estimate central tendency and variability. Outcome measures were analyzed using the t test, with the pairwise t test was used for paired and subsequent measurements of the same patient or a knee. All analyses were performed with significance set at P <.05. The minimal clinically important difference (MCID) in patients who underwent TKA for primary OA was between 5.3 and 5.9 for KSS, while the MCID for FXN was between 6.1 and 6.4.13 These values were referenced for our analysis.
Continue to: No significant adverse...
RESULTS
No significant adverse events were reported in the subjects of this study. Common minor adverse events included pain and swelling, which generally resolved in 48 to 72 hours after the procedure.

Compared with baseline, significant improvements were noted in the mean values of NPRS (Figure 1) at 6 weeks, 6 months, and 12 months. The mean KSS significantly improved from baseline at 6 weeks and 12 months (Figure 2). Significant improvements were also noted in the mean values of FXN (Figure 3) and the mean LEAS significantly improved from baseline at 6 weeks and 6 months (Figure 4).

DISCUSSION
Knee OA is a disabling condition that affects a substantial proportion of the aging population. The current treatment methods do little to address the degenerative environment of the joint, which includes cytokines such as IL-1 and IL-2. Orthobiologic agents have been used recently to address these issues, which include PRP and MSCs from various sources, including bone marrow and adipose tissue.

A recent meta-analysis conducted by Cui and colleagues14 evaluated 18 studies of MSC treatment for knee OA with a total of 565 participants (226 males and 339 females). The duration from the onset of knee pain to registration in each study ranged from 3 months to ≥7 years. The follow-up period was 3 months -24 months. The majority of studies recruited patients with knee OA with a severity grade of 1-4 on the K-L scale; K-L grades 1 and 2 and grades 3 and 4 were defined as early OA and advanced OA, respectively. The results suggested that MSC treatment significantly improved pain and functional status, relative to the baseline evaluations in knee OA, and the beneficial effect was maintained for 2 years after treatment. Furthermore, the treatment effectiveness was not reduced over time.14

Included in the abovementioned meta-analysis were 2 papers by Koh and colleagues in 2012 and 2013 on the use of AMSCs for the treatment of OA. 15,16 The first study included 18 patients whose adipose tissue was harvested from the inner side of the infrapatellar fat pad via a skin incision after arthroscopic debridement. The cells were centrifuged and injected into the patient’s knee the same day. The results showed a significant reduction of pain and an increased quality of life for all patients, and a positive correlation was found between the number of cells injected and pain improvements. The authors concluded that AMSCs were a valid cell source for treating cartilage damage.15
In their second study, Koh and colleagues reported their results of treating 30 elderly patients with OA (≥65 years), who had failed conventional treatment, using intra-articular injections of AMSCs.16 This patient population is important since OA most commonly occurs in the elderly population. Patients underwent arthroscopic lavage and cartilage evaluation before receiving an injection of AMSCs delivered in PRP. The authors demonstrated that AMSC therapy for elderly patients with mild to moderate OA was an effective treatment resulting in reduction of pain and regeneration of cartilage.16
In another study, Adriani and colleagues17 performed autologous percutaneous fat injection from January 2012 to March 2015 for the treatment of knee OA. Their 30 patients (12 males and 18 females; mean age of 63.3 years; mean body mass index of 25.1) had stable or progressive knee OA for at least 12 months, no other injection treatments during the previous 12 months, and no prior knee surgeries. The patients were evaluated at baseline and 1 week and at 1, 3, 6, and 12 months after treatment using the NPRS and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) as outcome measures. The average VAS was 7.7 at baseline and improved to 4.3 at 3-month follow-up; however, a slight deterioration (VAS 5.0) was noted at 1 year. Total WOMAC score was 89.9 at baseline, 68.6 at 3 months, and 73.2 at 12-month follow-up.17
Continue to: The results of...
The results of this study demonstrated significant improvements in pain, quality of life, and function at 12 months after ultrasound-guided injection of ASCs in patients with severe knee OA. Significant improvement that was noted at 6 weeks was maintained through 12 months after the treatment. Improvement was noted in all scales, including the NPRS, the KSS, and the FXN beginning at 3 months and continuing through 12 months. The LEAS was statistically significant through 6 months after the treatment but not significant at 12 months. No serious adverse events were recorded.
In a study by Lee and colleagues,13 the MCID was described for KSS and FXN in patients who underwent TKA for primary OA. This is the minimal change in a scoring measure that is perceived by the patient to be beneficial or harmful. The MCID for KSS was noted to be between 5.3 and 5.9, while the MCID for FXN was between 6.1 and 6.4.13 In our study, the KSS score improved from an average of 74.0 at baseline to 79.6 at 6 months and 81.6 at 12 months (a difference of 5.6 and 7.6; P = .18 and.014, respectively). The FXN improved from an average of 65.4 at baseline to 75.2 at 6 months and 76.4 at 12 months (a difference of 9.9 and 11; P = .041 and.014, respectively). Therefore, a clinically important difference of KSS and FXN scores was noted at both 6 and 12 months.
The technique used in this study provides autologous, minimally manipulated, fat graft performed in a short time (60-90 minutes), without expansion and/or enzymatic treatment. In addition, the harvesting and the injection of stem cells on the same day is a simple, office-based procedure, and compliant with the U. S. Food and Drug Administration regulations.18 The cost of the procedure averages $3500.
A study limitation is that it is a case series with relatively small numbers and not a randomized controlled study. Therefore, a placebo effect may play a role in our results. Further study with a larger number of patients and randomized controlled studies would be beneficial to support the findings of this study.
CONCLUSION
The injection of autologous, micro-fractured, minimally manipulated adipose tissue appears to be a safe and effective treatment option in patients with refractory severe (grade 3 or 4) knee OA. This study showed significant improvements in pain, quality of life, and function for at least 12 months in this study population. This intervention may represent a nonsurgical treatment option to avoid knee joint replacement in this population; however, further investigation is needed.
- Yubo M, Yanyan L, Li L, Tao S, Bo L, Lin C. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: A meta-analysis. PLoS One. 2017;12(4):e0175449.
- Jauregui JJ, Cherian JJ, Pierce TP, Beaver WB, Issa K, Mont MA. Long-Term Survivorship and Clinical Outcomes Following Total Knee Arthroplasty. J Arthroplasty. 2015;30(12):2164-2166.
- Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
- Dai W-L, Zhou A-G, Zhang H, Zhang J. Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Meta-analysis of Randomized Controlled Trials. Arthroscopy.33(3):659-670.e651.
- Halpern B CS, Rodeo SA, Hayter C, Bogner E, Potter HG, Nguyen J. Clinical and MRI outcomes after platelet-rich plasma treatment for knee osteoarthritis. Clin J Sport Med. 2013 May;23.
- Mamidi MK, Das AK, Zakaria Z, Bhonde R. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthritis Cartilage. 2016;24(8):1307-1316.
- Tang Y, Pan ZY, Zou Y, et al. A comparative assessment of adipose-derived stem cells from subcutaneous and visceral fat as a potential cell source for knee osteoarthritis treatment. J Cell Mol Med. 2017.
- Izadpanah R, Trygg C, Patel B, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. Journal of cellular biochemistry. 2006;99(5):1285-1297.
- Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med. 2010;16(5):203-209.
- Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. A J Physiol Renal Physiol. 2007;292(5):F1626-1635.
- Mestak O, Sukop A, Hsueh YS, et al. Centrifugation versus PureGraft for fatgrafting to the breast after breast-conserving therapy. World J Surg Oncol. 2014;12:178.
- Insall JN DL, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989 Nov;(248):13-4.
- Lee WC, Kwan YH, Chong HC, Yeo SJ. The minimal clinically important difference for Knee Society Clinical Rating System after total knee arthroplasty for primary osteoarthritis. Knee Surgery, Sports Traumatology, Arthroscopy. 2016.
- Cui GH, Wang YY, Li CJ, Shi CH, Wang WS. Efficacy of mesenchymal stem cells in treating patients with osteoarthritis of the knee: A meta-analysis. Exp Ther Med. 2016;12(5):3390-3400.
- Koh Y-GC, Yun-Jin. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19(6):902-907.
- Koh Y-GC, Yun-Jin. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29(4):748-755.
- Adriani E. MM, et al. Percutaneous Fat Transfer to Treat Knee Osteoarthritis Symptoms: Preliminary Results. Joints. 2017.
- Bianchi F, Maioli M, Leonardi E, et al. A New Nonenzymatic Method and Device to Obtain a Fat Tissue Derivative Highly Enriched in Pericyte-Like Elements by Mild Mechanical Forces From Human Lipoaspirates. Cell Transplantation. 2013;22(11):2063-2077
- Yubo M, Yanyan L, Li L, Tao S, Bo L, Lin C. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: A meta-analysis. PLoS One. 2017;12(4):e0175449.
- Jauregui JJ, Cherian JJ, Pierce TP, Beaver WB, Issa K, Mont MA. Long-Term Survivorship and Clinical Outcomes Following Total Knee Arthroplasty. J Arthroplasty. 2015;30(12):2164-2166.
- Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
- Dai W-L, Zhou A-G, Zhang H, Zhang J. Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Meta-analysis of Randomized Controlled Trials. Arthroscopy.33(3):659-670.e651.
- Halpern B CS, Rodeo SA, Hayter C, Bogner E, Potter HG, Nguyen J. Clinical and MRI outcomes after platelet-rich plasma treatment for knee osteoarthritis. Clin J Sport Med. 2013 May;23.
- Mamidi MK, Das AK, Zakaria Z, Bhonde R. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthritis Cartilage. 2016;24(8):1307-1316.
- Tang Y, Pan ZY, Zou Y, et al. A comparative assessment of adipose-derived stem cells from subcutaneous and visceral fat as a potential cell source for knee osteoarthritis treatment. J Cell Mol Med. 2017.
- Izadpanah R, Trygg C, Patel B, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. Journal of cellular biochemistry. 2006;99(5):1285-1297.
- Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med. 2010;16(5):203-209.
- Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. A J Physiol Renal Physiol. 2007;292(5):F1626-1635.
- Mestak O, Sukop A, Hsueh YS, et al. Centrifugation versus PureGraft for fatgrafting to the breast after breast-conserving therapy. World J Surg Oncol. 2014;12:178.
- Insall JN DL, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989 Nov;(248):13-4.
- Lee WC, Kwan YH, Chong HC, Yeo SJ. The minimal clinically important difference for Knee Society Clinical Rating System after total knee arthroplasty for primary osteoarthritis. Knee Surgery, Sports Traumatology, Arthroscopy. 2016.
- Cui GH, Wang YY, Li CJ, Shi CH, Wang WS. Efficacy of mesenchymal stem cells in treating patients with osteoarthritis of the knee: A meta-analysis. Exp Ther Med. 2016;12(5):3390-3400.
- Koh Y-GC, Yun-Jin. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19(6):902-907.
- Koh Y-GC, Yun-Jin. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29(4):748-755.
- Adriani E. MM, et al. Percutaneous Fat Transfer to Treat Knee Osteoarthritis Symptoms: Preliminary Results. Joints. 2017.
- Bianchi F, Maioli M, Leonardi E, et al. A New Nonenzymatic Method and Device to Obtain a Fat Tissue Derivative Highly Enriched in Pericyte-Like Elements by Mild Mechanical Forces From Human Lipoaspirates. Cell Transplantation. 2013;22(11):2063-2077
TAKE-HOME POINTS
- Severe knee osteoarthritis causes pain and limits functions in a substantial proportion of the aging population.
- Total knee arthroplasty is often recommended in this group of patients when conservative management has failed.
- Many patients in this group continue to seek a nonsurgical option for this process.
- Autologous, micro-fractured, minimally manipulated adipose tissue is easy to harvest, and injection into a knee joint resulted in significant improvement in pain and function for at least 12 months in this study population.
- This intervention may represent a nonsurgical treatment option to avoid knee joint replacement in this population.
Geniculate Artery Injury During Primary Total Knee Arthroplasty
ABSTRACT
Major arterial injury associated with total knee arthroplasty (TKA) is a rare and potentially devastating complication. However, the rate of injury to smaller periarticular vessels and the clinical significance of such an injury have not been well investigated. The purpose of this study is to describe the rate and outcomes of geniculate artery (GA) injury, the time at which injury occurs, and any associations with tourniquet use.
From November 2015 to February 2016, 3 surgeons at a single institution performed 100 consecutive primary TKAs and documented the presence or absence and the timing of GA injury. The data were then retrospectively reviewed. All TKAs had no prior surgery on the operative extremity. Other variables collected included tourniquet use, tranexamic acid (TXA) administration, intraoperative blood loss, postoperative drain output, and blood transfusion.
The overall rate of GA injury was 38%, with lateral inferior and middle GA injury in 31% and 15% of TKAs, respectively. Most of the injuries were visualized during bone cuts or meniscectomy. The rate of overall or isolated GA injury was not significantly different (P > .05) with either use of intravenous (84 patients) or topical (14 patients) TXA administration. Comparing selective tourniquet use (only during cementation) vs routine use showed no differences in GA injury rate (P = .37), blood loss (P = .07), or drain output (P = .46).
There is a relatively high rate of GA injury, with injury to the lateral GA occurring more often than the middle GA. Routine or selective tourniquet use does not affect the rate of injury.
Continue to: Major arterial injury...
Major arterial injury associated with total knee arthroplasty (TKA) is a rare and potentially devastating complication. The majority of literature in this context consists of case reports, small case series, and large retrospective studies that have examined the type, location, and mechanism of injury present in these cases.1-13 Reported arterial injuries include occlusion, laceration, aneurysm, pseudoaneurysm, and arteriovenous fistula formation in the femoral (believed to be due to the tourniquet around the proximal thigh) and popliteal arteries causing combinations of ischemia and hemorrhage necessitating treatment ranging from endovascular arterial intervention to amputation.4,5,9-11,13-17 In addition, several studies have asserted that the risk of major arterial injury may be increased with tourniquet use, suggesting that tourniquet use should be minimized for routine primary TKAs.3,6
There are very few cases in the literature specifically addressing injury to the more commonly encountered geniculate arteries (GAs). The medial GAs are typically visualized and coagulated during the standard medial parapatellar approach. In addition, if performed, a lateral release can damage the lateral superior and inferior GAs and the middle GA can be cut with posterior cruciate ligament resection. However, the middle and lateral inferior GAs are anecdotally the most difficult to detect and treat intraoperatively, especially after implantation of TKA and deflation of the tourniquet. The potential lack of recognition of such GA injury can result in harmful sequelae, including ischemia of the patella, hemorrhage, and painful pseudoaneurysms.2,18-29 Currently, there are only 2 case reports of lateral inferior GA injury, 2 cases of medial inferior GA injury, and no reports of middle GA injury.2,23,24,29
The rate, the timing within surgery, the risk factors, including tourniquet use, and the clinical effects of GA injury are largely unknown. If these factors were better understood, prophylactic measures and/or awareness could be better applied to prevent adverse outcomes, especially in cases of the middle and lateral inferior GAs. The aims of this study are to elucidate the rate and timing of middle and lateral inferior GA injury during primary TKA; determine the factors related to injury, including intraoperative blood loss, postoperative drain output, and tranexamic (TXA) acid use; and investigate any differences in the rate of injury with and without the use of a tourniquet.
MATERIALS AND METHODS
PATIENT DEMOGRAPHICS AND SURGICAL TECHNIQUE
From November 2015 to February 2016, 3 surgeons (MJT, TMM, and RTT) at a single institution performed 100 consecutive unilateral primary TKAs and documented the presence or absence and the timing of GA injury. After obtaining approval from our Institutional Review Board, a retrospective study was performed to investigate the prospectively recorded rate of middle and lateral inferior GA injuries occurring during primary TKAs. Patients with a diagnosis of isolated osteoarthritis were included, and those with any previous surgery on the operative knee were excluded. The average age of patients at the time of surgery was 67 years (range, 25-91 years), the average body mass index was 33 kg/m2 (range, 18-54 kg/m2), and there were 63 (63%) female patients.
All TKAs were performed through a medial parapatellar approach with a posterior-stabilized, cemented design, and each patient received a postoperative surgical drain. One of the 3 lead surgeons (TMM) in this study used a tourniquet from the time of incision until the completion of cementation, and the other 2 (MJT and RTT) predominantly used the tourniquet only during cementation. To elucidate any differences in GA injury between these 2 methods of tourniquet use, the patients were categorized into 2 groups base d on tourniquet use. Group 1 included patients in whom a tourniquet was used to maintain a bloodless surgical field from the time of incision until the completion of cementation, and Group 2 included patients in whom tourniquet use was more selective (ie, applied only during cementation). Group 1 comprised 31% (31/100) of patients, while Group 2 comprised 67% (67/100) of patients; no tourniquet was used in 2% (2/100) of cases. In addition, TXA was used in 98% (98/100) of patients: 84 patients received intravenous (IV) and 14 received topical TXA administration.
Continue to: ANALYSIS OF GENICULATE ARTERY INJURY
ANALYSIS OF GENICULATE ARTERY INJURY
The senior authors critically evaluated the GA during the primary TKAs and documented the presence or absence of injury in the operative reports. GA injury was reported if there was intraoperative visualization of pulsatile bleeding or visualization of arterial lumen in the anatomic areas of the middle and lateral inferior GAs. At 3 separate occasions during the operation, the surgeon looked specifically for pulsatile bleeding or arterial lumen in the areas of the middle and lateral inferior GAs, including after all the femoral and tibial bone cuts were completed, immediately before preparing to cement (before the tourniquet was inflated if there was not one inflated from the start of the procedure), and immediately after the tourniquet was deflated (Figure 1). All bleeding GAs that were visualized were effectively coagulated by cautery. Details regarding the use of TXA (topical or IV), intraoperative blood loss, postsurgical drain output for 24 hours after surgery, and blood transfusion were collected from the patients’ medical records (Table 1).

| Table 1. Operative Variables | |
Variable | Value |
Total number | 100 (100%) |
Intraoperative blood loss (mL) | 160 (25-500) |
Drain output 1st 24 hours (mL) | 488 (75-1980) |
Total output (mL) | 618 (75-2130) |
Use of TXA | 98 (98%) |
Topical TXA | 84 (84%) |
IV TXA | 14 (14%) |
Tourniquet entire procedure | 31 (31%) |
Operative variables other than geniculate artery injury. Data presented as mean (range) or n (%). TXA = tranexamic acid.
STATISTICAL METHODS
Statistical analysis was performed using the JMP software version 10.0.0 (SAS Institute, Inc). The overall rate of GA injury was determined, including the rates of GA injury based on location, time point, and method of diagnosis (pulsatile bleeding or arterial lumen visualization). If >1 GA injury occurred in the same knee, only 1 GA injury was calculated for the overall rate; however, each injury was specified separately when calculating the injury rate for the specific GA. Intraoperative blood loss, postoperative drain output, and the use of TXA were compared between cases in which a GA injury was detected and those in which it was not detected. Before conducting the retrospective review, a power analysis determined that we would require 100 patients to detect a difference in GA injury between Groups 1 and 2 (33 in Group 1 and 67 in Group 2), assuming a 30% rate in Group 1 and a 5% rate of GA injury in Group 2 using Fisher’s exact test. The Fisher’s exact test was used to compare categorical variables, and the Wilcoxon rank sum test was used to compare continuous variables. An alpha value of .05 was considered as statistically significant.
RESULTS
RATE OF GENICULATE ARTERY INJURY
The overall rate of any GA injury was 38% (38/100). Lateral inferior GA injury was more frequently detected than middle GA injury (31% vs 15% of TKAs, respectively; Table 2). Among the 31 lateral inferior GA injuries, 14 were identified as pulsatile bleeding, 7 as lumen visualizations, and 6 as both pulsatile bleeding and lumen visualization; 4 were detected by methods not recorded in the operative report. Of the lateral inferior GA injuries, 11 were identified after the bone cuts, 7 during meniscus removal, 3 during exposure, 1 after tourniquet deflation, and 9 at a time not recorded in the operative report. Of the 15 middle GA injuries, 9 were identified as pulsatile bleeding, 2 as lumen visualizations, and 4 as both pulsatile bleeding and lumen visualization. In addition, 7 of these GA injuries were identified after the bone cuts, 3 during cruciate removal, 1 after meniscus removal, 1 during exposure, and 3 at a time not recorded in the operative report (Table 3).
| Table 2. Rates of Geniculate Artery Injury Based on Location and Method | ||||
Location | Pulsatile Bleeding | Arterial Lumen | Both | Overall Rate |
Lateral inferior GA | 14 (14%) | 7 (7%) | 6 (6%) | 31 (31%) |
Middle GA | 9 (9%) | 2 (2%) | 4 (4%) | 15 (15%) |
Rates of geniculate artery injury based on location and method of diagnosis. Data presented as n (%). There were 4 additional lateral inferior and 9 middle GA injuries identified by a method not specified in the operative report. GA = geniculate artery.
Table 3. Rates of Geniculate Artery Injury Based on Time Point | ||
Time | Lateral Inferior GA | Middle GA |
After bone cuts | 11 (11%) | 7 (7%) |
During meniscus removal | 7 (7%) | 1 (1%) |
During exposure | 3 (3%) | 1 (1%) |
After tourniquet deflation | 1 (1%) | 0 (0%) |
During cruciate removal | 0 (0%) | 3 (3%) |
Not reported | 9 (9%) | 3 (3%) |
Rates of geniculate artery injury based on time point and method of diagnosis. GA = geniculate artery. Data presented as n (%).
FACTORS ASSOCIATED WITH GENICULATE ARTERY INJURY
Mean intraoperative estimated blood loss was 186 mL (standard deviation [SD], 111; range 50–500 mL) in those with a GA injury versus 147 mL (range, 82.25–400 mL) in those without injury (P = .14). Postoperative drain output in the 24 hours after surgery was 467 mL (SD 253, range 100–1105 mL) versus 502 mL (SD 378, range 75–1980 mL) in TKAs with and without GA injury, respectively (P = .82). Total estimated blood loss (combined intraoperative blood loss and 24-hour postoperative drain output) was 613 mL (SD 252, range 150–1105 mL) in TKAs with GA injury versus 620 mL (SD 393, range 75–2130 mL) without injury (P = .44) (Table 4). Overall, there was no statistical difference in blood loss, drain output, or combined output when analyzed according to lateral inferior or middle GA injury (P = .24–.82) (Table 5 and Table 6). No patients required blood transfusion postoperatively after TKA.
| Table 4. Factors Associated with GA Injury | |||
Outcome | GA Injury | No GA Injury | P Value |
Blood loss (mL) | 186 (50-500) | 147 (25-400) | .1366 |
24-Hour drain output (mL) | 467 (100-1105) | 502 (75-1980) | .8240 |
Total output (mL) | 613 (150-1105) | 620 (75-2130) | .4368 |
Differences in outcomes based on presence or absence of GA injury. Note that there were no significant differences. Values are reported as average (range). GA = geniculate artery.
| Table 5. Factors Associated with LIGA Injury | |||
Outcome | LIGA Injury | No LIGA Injury | P Value |
Blood loss (mL) | 178 (50-400) | 153 (25-500) | .2401 |
24-Hour drain output (mL) | 461 (100-890) | 501 (75-1980) | .8187 |
Total output (mL) | 610 (150-1080) | 621 (75-2130) | .4165 |
Differences in outcomes based on presence or absence of LIGA injury. Note that there were no significant differences. Values are reported as average (range). LIGA = lateral inferior geniculate artery.
| Table 6. Factors Associated with MGA Injury | |||
Outcome | MGA Injury | No MGA Injury | P Value |
Blood loss (mL) | 190 (75-500) | 156 (25-400) | .6225 |
24-Hour drain output (mL) | 455 (125-1105) | 494 (75-1980) | .6428 |
Total output (mL) | 582 (200-1105) | 624 (75-2130) | .6535 |
Differences in outcomes based on presence or absence of MGA injury. Note that there were no significant differences. Values are reported as average (range). MGA = middle geniculate artery.
IV administration of TXA was associated with a 37% (31/84) rate of GA injury, whereas topical TXA administration was associated with a 43% (6/14) rate of GA injury (P = .77). The rate of overall or isolated GA injury was not significantly different (P = .35–1.0) between IV and topical TXA administration (Table 7). In addition, total combined output was not significantly different (P = .1032) when comparing GA injury and noninjury in the subgroup analysis based on TXA use (IV or topical); however, topical administration was associated with lower intraoperative blood loss than IV administration (P = .0489), whereas IV administration was associated with lower 24-hour postoperative drain output than topical administration (P = .0169). There was no difference in blood loss, 24-hour drain output, or total output between those who did and did not sustain a GA injury in the group of patients who received IV TXA administration (Table 8, P = .2118–.7091). The same was true for those receiving topical TXA administration (Table 9, P = .0912–.9485).
Table 7. Factors Associated with TXA Injury | |||
Outcome | IV TXA (n = 84) | Topical TXA (n = 14) | P Value |
Any GA injury | 31 (37%) | 6 (43%) | .7683 |
LIGA injury | 24 (29%) | 6 (43%) | .3498 |
MGA injury | 13 (15%) | 2 (14%) | 1.0 |
Blood loss (mL) | 170 (25-500) | 113 (40-240) | .0489* |
24-Hour drain output (mL) | 454 (75-1980) | 662 (75-1800) | .0169* |
Total output (mL) | 592 (75-2130) | 751 (75-2130) | .1032 |
Differences in outcomes based on presence or absence of MGA injury. Note that there were no significant differences. Values are reported as n (%) or average (range). TXA = tranexamic acid, GA = geniculate artery, LIGA = lateral inferior geniculate artery, MGA = middle geniculate artery. *denotes statistical significance (P < .05).
| Table 8. Factors Associated with GA Injury Given IV TXA Use | ||||
Outcome | GA Injury | No GA Injury | Difference | P Value |
Blood loss (mL) | 195 (50-500) | 157 (25-400) | 38 | .2118 |
24-Hour drain output (mL) | 436 (100-1105) | 464 (75-1980) | 28 | .7091 |
Total output (mL) | 594 (150-1105) | 592 (75-2130) | 2 | .6982 |
Differences in outcomes of those patients who received IV TXA based on presence or absence of GA injury. Note that there were no significant differences. Values are reported as average (range). GA = geniculate artery, TXA = tranexamic acid.
| Table 9. Factors Associated with GA Injury Given Topical TXA Use | ||||
Outcome | GA Injury | No GA Injury | Difference | P Value |
Blood loss (mL) | 163 (100-250) | 84 (40-150) | 79 | .0912 |
24-Hour drain output (mL) | 610 (205-890) | 701 (415-1800) | 91 | .9485 |
Total output (mL) | 719 (405-960) | 775 (455-1900) | 56 | .6982 |
Differences in outcomes based on presence or absence of GA injury. Note that there were no significant differences. Values are reported as average (range). GA = geniculate artery.
Continue to: TOURNIQUET USE
TOURNIQUET USE
Comparison between Groups 1 (tourniquet use) and 2 (selective tourniquet use) revealed similar rates of overall and specific GA injury, intraoperative blood loss, and 24-hour postoperative drain output (Table 10). Group 1 demonstrated a 29% (9/31) rate of any GA injury versus 40% (27/67) in Group 2 (P = .37). For the specific lateral inferior GA injury, there was an equivalent rate of injury at 29% (9/31 in Group 1, 20/67 in Group 2; P = 1.0). Similarly, Group 1 patients had a 10% (3/31) rate of middle GA injury compared to 16% (11/67) in Group 2 patients (P = .53). Intraoperative estimated blood loss was lower in Group 1 (140 mL; range 25–400 mL) than in Group 2 (171 mL; range 40–500 mL) (P = .07), whereas the average 24-hour postoperative drain output was similar for Groups 1 (484 mL; range 75–1800 mL) and 2 (488 mL; range 100–1980 mL) (P = .46). Total estimated output was slightly less for Group 1 (593 mL; range 75–1900 mL) than for Group 2 (626 mL; range 125–2130 mL) (P = .38). A post hoc power analysis showed that with these rates of GA injury in Groups 1 and 2 and given a 2:1 ratio of the number of patients in Group 2 versus Group 1, a total of 185 patients in Group 1 and 370 patients in Group 2 would be needed to detect a statistically significant difference (P < .05) with a power of 80%.
| Table 10. Factors Associated with Tourniquet Use | ||||
Injury | Group 1 (n = 31) | Group 2 (n = 67) | Difference | P Value |
Overall GA injury | 9 (29%) | 27 (40%) | 11% | .3687 |
Lateral inferior GA | 9 (29%) | 20 (29%) | 0% | 1.0 |
Middle GA | 3 (10%) | 11 (16%) | 6% | .5382 |
Blood loss (mL) | 140 (25-400) | 171 (40-500) | 31 | .0661 |
24-Hour drain output (mL) | 484 (75-1800) | 488 (100-1980) | 4 | .4580 |
Total output (mL) | 593 (75-1900) | 626 (125-2130) | 33 | .3776 |
Differences in outcomes separated based on use of a tourniquet for the entire case (Group 1) vs use of a tourniquet only during cementation (Group 2). Note that there were no significant differences. Values are reported as n (%) or average (range). GA = geniculate artery.
DISCUSSION
Major arterial injury associated with TKA is a well-known, rare, and potentially devastating complication.1-13 However, the rate of injury to smaller periarticular vessels and the clinical significance of such injury have not been studied. The present study found a high rate of GA injury but no clinically significant difference in intraoperative blood loss or postoperative drain output between patients with GA injury (which was identified and managed with cautery) and those without GA injury. In addition, tourniquet use did not affect the rate of injury or the associated blood loss. To our knowledge, this is the first study that has critically evaluated the rate of GA injury occurring during TKA.
The overall rate of GA injury occurring during primary TKA was 38% with a higher predominance of lateral inferior than middle GA injury (31% vs 15%). Anatomically, it would follow that the lateral GA could be injured at a higher rate as it courses on top of the lateral meniscus, thus being susceptible to injury during cutting of the tibial plateau and meniscectomy. In addition, because the meniscectomy is performed longitudinally along the course of the artery, it may also be potentially lacerated in multiple locations and lengthwise. In theory, there should be a 100% rate of middle GA injury during posterior-stabilized TKA as this artery runs through the cruciate ligaments, which are resected during these cases. However, vessel injury was defined in this study as the visualization of pulsatile bleeding or vessel lumen. It is probable that in the cases in which injury to the middle GA was not visualized, it was cut but simultaneously cauterized. Thus, a lower rate (15%) of injury was detected. Nonetheless, these results still suggest that these periarticular arteries are injured at a higher rate; therefore, it is important for surgeons to specifically identify these injuries intraoperatively and adequately cauterize these vessels. As long as these arteries are cauterized, additional blood loss and potential vascular pseudoaneurysms should be prevented.
The effect of GA injury on intraoperative blood loss, 24-hour postoperative drain output, and total estimated blood loss showed no significant clinical findings in the present study cohort. In addition, examining the injury rate and blood loss based on TXA use also revealed no detrimental clinical associations. Although GA injury could inherently be associated with higher levels of blood loss and drain output, it is important to note that all GA injuries were also effectively coagulated, thus explaining the indifferent results. Accordingly, it should be recommended to surgeons performing primary TKAs to carefully evaluate for GA injury to prevent excessive blood loss or painful pseudoaneurysms. However, there is also a potential for beta error in this study in which a true difference did exist but no statistical difference was found due to the study being underpowered.
Full or selective tourniquet use during TKA did not appear to have any effect on the rate of GA injury, intraoperative blood loss, or 24-hour postoperative drain output. The similarity between GA injury rates perhaps further indicates an equivalent ability to detect these injuries between these two methods because of operative inspection for such injuries. With regard to intraoperative blood loss and drain output, the present findings are similar to previous studies demonstrating equivocal results despite variable tourniquet utilization in TKA.15,30 However, these results differ from those of Harvey and colleagues31, who demonstrated that blood loss inversely correlated with intraoperative tourniquet time. There are risks and benefits related to the use of both full and selective tourniquet methods, but either method does not appear to be advantageous in decreasing the rate of GA injury.
Continue to: Although this is the first study...
Although this is the first study to investigate the rates of GA injury and the potential clinical effects, there are limitations to this research. First, the study was retrospective in nature despite the fact that the data were collected prospectively. Only acute perioperative follow-up was performed, and thus, we were unable to evaluate longer term effects of GA injury on TKA outcomes. Furthermore, this study is potentially prone to beta error. As discussed above, 185 patients in Group 1 and 370 patients in Group 2 would be needed to detect a statistical difference in the rate of GA injury based on the rates found in this study. This study could also have been underpowered to identify differences in other aspects, such as differences in blood loss and drain. Furthermore, the data collected regarding intraoperative blood loss are estimated data and can be variable. Finally, visualization of vessel lumen and pulsatile bleeding is not a validated method to diagnose GA injuries, and potential injuries may have been missed. Despite such disadvantages, the strengths of this study include the concise results in consecutive patients, the generalizability of the data as multiple surgeons participated, and its first report of nonmajor periarticular artery injury.
CONCLUSIONS
There is a relatively high rate of GA injury, with injury to the lateral GA being visualized more often than injury to the middle GA. The majority of GA injuries occur around the time of bone cuts and meniscectomy, and tourniquet use does not affect the rate of injury. To reduce intraoperative blood loss and postoperative drain output, surgeons should identify and coagulate GA injuries routinely during primary TKA.
1. Calligaro KD, Dougherty MJ, Ryan S, Booth RE. Acute arterial complications associated with total hip and knee arthroplasty. J Vasc Surg. 2003;38(6):1170-1177. doi: 10.1016/S0741-5214(03)00918-2.
2. Dennis DA, Neumann RD, Toma P, Rosenberg G, Mallory TH. Arteriovenous fistula with false aneurysm of the inferior medial geniculate artery. A complication of total knee arthroplasty. Clin Orthop Relat Res. 1987(222):255-260.
3. Hagan PF, Kaufman EE. Vascular complication of knee arthroplasty under tourniquet. A case report. Clin Orthop Relat Res. 1990(257):159-161.
4. Holmberg A, Milbrink J, Bergqvist D. Arterial complications after knee arthroplasty: 4 cases and a review of the literature. Acta Orthop Scand. 1996;67(1):75-78. doi: 10.3109/17453679608995616.
5. Hozack WJ, Cole PA, Gardner R, Corces A. Popliteal aneurysm after total knee arthroplasty. Case reports and review of the literature. J Arthroplasty. 1990;5(4):301-305. doi: 10.1016/S0883-5403(08)80087-3.
6. Jeyaseelan S, Stevenson TM, Pfitzner J. Tourniquet failure and arterial calcification. Case report and theoretical dangers. Anaesthesia. 1981;36(1):48-50. doi: 10.1111/j.1365-2044.1981.tb08599.x
7. Mureebe L, Gahtan V, Kahn MB, Kerstein MD, Roberts AB. Popliteal artery injury after total knee arthroplasty. Am Surg. 1996;62(5):366-368.
8. O'Connor JV, Stocks G, Crabtree JD, Jr., Galasso P, Wallsh E. Popliteal pseudoaneurysm following total knee arthroplasty. J Arthroplasty. 1998;13(7):830-832. doi: 10.1016/S0883-5403(98)90039-0.
9. Ohira T, Fujimoto T, Taniwaki K. Acute popliteal artery occlusion after total knee arthroplasty. Arch Orthop Trauma Surg. 1997;116(6-7):429-430. doi: 10.1007/BF00434007.
10. Parfenchuck TA, Young TR. Intraoperative arterial occlusion in total joint arthroplasty. J Arthroplasty. 1994;9(2):217-220. doi: 10.1016/0883-5403(94)90071-X.
11. Rush JH, Vidovich JD, Johnson MA. Arterial complications of total knee replacement. The Australian experience. J Bone Joint Surg Br. 1987;69(3):400-402. doi: 10.1302/0301-620X.69B3.3584193.
12. Smith DE, McGraw RW, Taylor DC, Masri BA. Arterial complications and total knee arthroplasty. J Am Acad Orthop Surg. 2001;9(4):253-257.
13. Zahrani HA, Cuschieri RJ. Vascular complications after total knee replacement. J Cardiovasc Surg (Torino). 1989;30(6):951-952.
14. Isiklar ZU, Landon GC, Tullos HS. Amputation after failed total knee arthroplasty. Clin Orthop Relat Res. 1994(299):173-178.
15. Wakankar HM, Nicholl JE, Koka R, D'Arcy JC. The tourniquet in total knee arthroplasty. A prospective, randomised study. J Bone Joint Surg Br. 1999;81(1):30-33. doi: 10.1302/0301-620X.81B1.0810030.
16. Kumar SN, Chapman JA, Rawlins I. Vascular injuries in total knee arthroplasty. A review of the problem with special reference to the possible effects of the tourniquet. J Arthroplasty. 1998;13(2):211-216. doi: 10.1016/S0883-5403(98)90102-4.
17. DeLaurentis DA, Levitsky KA, Booth RE, et al. Arterial and ischemic aspects of total knee arthroplasty. Am J Surg. 1992;164(3):237-240. doi: 10.1016/S0002-9610(05)81078-5.
18. Langkamer VG. Local vascular complications after knee replacement: a review with illustrative case reports. Knee. 2001;8(4):259-264. doi: 10.1016/S0968-0160(01)00103-X.
19. Moran M, Hodgkinson J, Tait W. False aneurysm of the superior lateral geniculate artery following Total Knee Replacement. Knee. 2002;9(4):349-351. doi: 10.1016/S0968-0160(02)00061-3.
20. Pritsch T, Parnes N, Menachem A. A bleeding pseudoaneurysm of the lateral genicular artery after total knee arthroplasty--a case report. Acta Orthop. 2005;76(1):138-140. doi: 10.1080/00016470510030463.
21. Gaheer RS, Chirputkar K, Sarungi M. Spontaneous resolution of superior medial geniculate artery pseudoaneurysm following total knee arthroplasty. Knee. 2014;21(2):586-588. doi: 10.1016/j.knee.2012.10.021.
22. Law KY, Cheung KW, Chiu KH, Antonio GE. Pseudoaneurysm of the geniculate artery following total knee arthroplasty: a report of two cases. J Orthop Surg (Hong Kong). 2007;15(3):386-389. /doi: 10.1177/230949900701500331.
23. Noorpuri BS, Maxwell-Armstrong CA, Lamerton AJ. Pseudo-aneurysm of a geniculate collateral artery complicating total knee replacement. Eur J Vasc Endovasc Surg. 1999;18(6):534-535.
24. Pai VS. Traumatic aneurysm of the inferior lateral geniculate artery after total knee replacement. J Arthroplasty. 1999;14(5):633-634. doi: 10.1016/S0883-5403(99)90089-X.
25. Julien TP, Gravereaux E, Martin S. Superior medial geniculate artery pseudoaneurysm after primary total knee arthroplasty. J Arthroplasty. 2012;27(2):323 e313-326. doi: 10.1016/j.arth.2011.02.009.
26. Kalsi PS, Carrington RJ, Skinner JS. Therapeutic embolization for the treatment of recurrent hemarthrosis after total knee arthroplasty due to an arteriovenous fistula. J Arthroplasty. 2007;22(8):1223-1225. /doi: 10.1016/j.arth.2006.11.012.
27. Ritter MA, Herbst SA, Keating EM, Faris PM, Meding JB. Patellofemoral complications following total knee arthroplasty. Effect of a lateral release and sacrifice of the superior lateral geniculate artery. J Arthroplasty. 1996;11(4):368-372. doi: 10.1016/S0883-5403(96)80024-6.
28. Aldrich D, Anschuetz R, LoPresti C, Fumich M, Pitluk H, O'Brien W. Pseudoaneurysm complicating knee arthroscopy. Arthroscopy. 1995;11(2):229-230. doi: 10.1016/0749-8063(95)90073-X.
29. Sharma H, Singh GK, Cavanagh SP, Kay D. Pseudoaneurysm of the inferior medial geniculate artery following primary total knee arthroplasty: delayed presentation with recurrent haemorrhagic episodes. Knee Surg Sports Traumatol Arthrosc. 2006;14(2):153-155. doi: 10.1007/s00167-005-0639-4.
30. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253. doi: 10.1302/0301-620X.77B2.7706340.
31. Harvey EJ, Leclerc J, Brooks CE, Burke DL. Effect of tourniquet use on blood loss and incidence of deep vein thrombosis in total knee arthroplasty. J Arthroplasty. 1997;12(3):291-296. doi: 10.1016/S0883-5403(97)90025-5.
ABSTRACT
Major arterial injury associated with total knee arthroplasty (TKA) is a rare and potentially devastating complication. However, the rate of injury to smaller periarticular vessels and the clinical significance of such an injury have not been well investigated. The purpose of this study is to describe the rate and outcomes of geniculate artery (GA) injury, the time at which injury occurs, and any associations with tourniquet use.
From November 2015 to February 2016, 3 surgeons at a single institution performed 100 consecutive primary TKAs and documented the presence or absence and the timing of GA injury. The data were then retrospectively reviewed. All TKAs had no prior surgery on the operative extremity. Other variables collected included tourniquet use, tranexamic acid (TXA) administration, intraoperative blood loss, postoperative drain output, and blood transfusion.
The overall rate of GA injury was 38%, with lateral inferior and middle GA injury in 31% and 15% of TKAs, respectively. Most of the injuries were visualized during bone cuts or meniscectomy. The rate of overall or isolated GA injury was not significantly different (P > .05) with either use of intravenous (84 patients) or topical (14 patients) TXA administration. Comparing selective tourniquet use (only during cementation) vs routine use showed no differences in GA injury rate (P = .37), blood loss (P = .07), or drain output (P = .46).
There is a relatively high rate of GA injury, with injury to the lateral GA occurring more often than the middle GA. Routine or selective tourniquet use does not affect the rate of injury.
Continue to: Major arterial injury...
Major arterial injury associated with total knee arthroplasty (TKA) is a rare and potentially devastating complication. The majority of literature in this context consists of case reports, small case series, and large retrospective studies that have examined the type, location, and mechanism of injury present in these cases.1-13 Reported arterial injuries include occlusion, laceration, aneurysm, pseudoaneurysm, and arteriovenous fistula formation in the femoral (believed to be due to the tourniquet around the proximal thigh) and popliteal arteries causing combinations of ischemia and hemorrhage necessitating treatment ranging from endovascular arterial intervention to amputation.4,5,9-11,13-17 In addition, several studies have asserted that the risk of major arterial injury may be increased with tourniquet use, suggesting that tourniquet use should be minimized for routine primary TKAs.3,6
There are very few cases in the literature specifically addressing injury to the more commonly encountered geniculate arteries (GAs). The medial GAs are typically visualized and coagulated during the standard medial parapatellar approach. In addition, if performed, a lateral release can damage the lateral superior and inferior GAs and the middle GA can be cut with posterior cruciate ligament resection. However, the middle and lateral inferior GAs are anecdotally the most difficult to detect and treat intraoperatively, especially after implantation of TKA and deflation of the tourniquet. The potential lack of recognition of such GA injury can result in harmful sequelae, including ischemia of the patella, hemorrhage, and painful pseudoaneurysms.2,18-29 Currently, there are only 2 case reports of lateral inferior GA injury, 2 cases of medial inferior GA injury, and no reports of middle GA injury.2,23,24,29
The rate, the timing within surgery, the risk factors, including tourniquet use, and the clinical effects of GA injury are largely unknown. If these factors were better understood, prophylactic measures and/or awareness could be better applied to prevent adverse outcomes, especially in cases of the middle and lateral inferior GAs. The aims of this study are to elucidate the rate and timing of middle and lateral inferior GA injury during primary TKA; determine the factors related to injury, including intraoperative blood loss, postoperative drain output, and tranexamic (TXA) acid use; and investigate any differences in the rate of injury with and without the use of a tourniquet.
MATERIALS AND METHODS
PATIENT DEMOGRAPHICS AND SURGICAL TECHNIQUE
From November 2015 to February 2016, 3 surgeons (MJT, TMM, and RTT) at a single institution performed 100 consecutive unilateral primary TKAs and documented the presence or absence and the timing of GA injury. After obtaining approval from our Institutional Review Board, a retrospective study was performed to investigate the prospectively recorded rate of middle and lateral inferior GA injuries occurring during primary TKAs. Patients with a diagnosis of isolated osteoarthritis were included, and those with any previous surgery on the operative knee were excluded. The average age of patients at the time of surgery was 67 years (range, 25-91 years), the average body mass index was 33 kg/m2 (range, 18-54 kg/m2), and there were 63 (63%) female patients.
All TKAs were performed through a medial parapatellar approach with a posterior-stabilized, cemented design, and each patient received a postoperative surgical drain. One of the 3 lead surgeons (TMM) in this study used a tourniquet from the time of incision until the completion of cementation, and the other 2 (MJT and RTT) predominantly used the tourniquet only during cementation. To elucidate any differences in GA injury between these 2 methods of tourniquet use, the patients were categorized into 2 groups base d on tourniquet use. Group 1 included patients in whom a tourniquet was used to maintain a bloodless surgical field from the time of incision until the completion of cementation, and Group 2 included patients in whom tourniquet use was more selective (ie, applied only during cementation). Group 1 comprised 31% (31/100) of patients, while Group 2 comprised 67% (67/100) of patients; no tourniquet was used in 2% (2/100) of cases. In addition, TXA was used in 98% (98/100) of patients: 84 patients received intravenous (IV) and 14 received topical TXA administration.
Continue to: ANALYSIS OF GENICULATE ARTERY INJURY
ANALYSIS OF GENICULATE ARTERY INJURY
The senior authors critically evaluated the GA during the primary TKAs and documented the presence or absence of injury in the operative reports. GA injury was reported if there was intraoperative visualization of pulsatile bleeding or visualization of arterial lumen in the anatomic areas of the middle and lateral inferior GAs. At 3 separate occasions during the operation, the surgeon looked specifically for pulsatile bleeding or arterial lumen in the areas of the middle and lateral inferior GAs, including after all the femoral and tibial bone cuts were completed, immediately before preparing to cement (before the tourniquet was inflated if there was not one inflated from the start of the procedure), and immediately after the tourniquet was deflated (Figure 1). All bleeding GAs that were visualized were effectively coagulated by cautery. Details regarding the use of TXA (topical or IV), intraoperative blood loss, postsurgical drain output for 24 hours after surgery, and blood transfusion were collected from the patients’ medical records (Table 1).

| Table 1. Operative Variables | |
Variable | Value |
Total number | 100 (100%) |
Intraoperative blood loss (mL) | 160 (25-500) |
Drain output 1st 24 hours (mL) | 488 (75-1980) |
Total output (mL) | 618 (75-2130) |
Use of TXA | 98 (98%) |
Topical TXA | 84 (84%) |
IV TXA | 14 (14%) |
Tourniquet entire procedure | 31 (31%) |
Operative variables other than geniculate artery injury. Data presented as mean (range) or n (%). TXA = tranexamic acid.
STATISTICAL METHODS
Statistical analysis was performed using the JMP software version 10.0.0 (SAS Institute, Inc). The overall rate of GA injury was determined, including the rates of GA injury based on location, time point, and method of diagnosis (pulsatile bleeding or arterial lumen visualization). If >1 GA injury occurred in the same knee, only 1 GA injury was calculated for the overall rate; however, each injury was specified separately when calculating the injury rate for the specific GA. Intraoperative blood loss, postoperative drain output, and the use of TXA were compared between cases in which a GA injury was detected and those in which it was not detected. Before conducting the retrospective review, a power analysis determined that we would require 100 patients to detect a difference in GA injury between Groups 1 and 2 (33 in Group 1 and 67 in Group 2), assuming a 30% rate in Group 1 and a 5% rate of GA injury in Group 2 using Fisher’s exact test. The Fisher’s exact test was used to compare categorical variables, and the Wilcoxon rank sum test was used to compare continuous variables. An alpha value of .05 was considered as statistically significant.
RESULTS
RATE OF GENICULATE ARTERY INJURY
The overall rate of any GA injury was 38% (38/100). Lateral inferior GA injury was more frequently detected than middle GA injury (31% vs 15% of TKAs, respectively; Table 2). Among the 31 lateral inferior GA injuries, 14 were identified as pulsatile bleeding, 7 as lumen visualizations, and 6 as both pulsatile bleeding and lumen visualization; 4 were detected by methods not recorded in the operative report. Of the lateral inferior GA injuries, 11 were identified after the bone cuts, 7 during meniscus removal, 3 during exposure, 1 after tourniquet deflation, and 9 at a time not recorded in the operative report. Of the 15 middle GA injuries, 9 were identified as pulsatile bleeding, 2 as lumen visualizations, and 4 as both pulsatile bleeding and lumen visualization. In addition, 7 of these GA injuries were identified after the bone cuts, 3 during cruciate removal, 1 after meniscus removal, 1 during exposure, and 3 at a time not recorded in the operative report (Table 3).
| Table 2. Rates of Geniculate Artery Injury Based on Location and Method | ||||
Location | Pulsatile Bleeding | Arterial Lumen | Both | Overall Rate |
Lateral inferior GA | 14 (14%) | 7 (7%) | 6 (6%) | 31 (31%) |
Middle GA | 9 (9%) | 2 (2%) | 4 (4%) | 15 (15%) |
Rates of geniculate artery injury based on location and method of diagnosis. Data presented as n (%). There were 4 additional lateral inferior and 9 middle GA injuries identified by a method not specified in the operative report. GA = geniculate artery.
Table 3. Rates of Geniculate Artery Injury Based on Time Point | ||
Time | Lateral Inferior GA | Middle GA |
After bone cuts | 11 (11%) | 7 (7%) |
During meniscus removal | 7 (7%) | 1 (1%) |
During exposure | 3 (3%) | 1 (1%) |
After tourniquet deflation | 1 (1%) | 0 (0%) |
During cruciate removal | 0 (0%) | 3 (3%) |
Not reported | 9 (9%) | 3 (3%) |
Rates of geniculate artery injury based on time point and method of diagnosis. GA = geniculate artery. Data presented as n (%).
FACTORS ASSOCIATED WITH GENICULATE ARTERY INJURY
Mean intraoperative estimated blood loss was 186 mL (standard deviation [SD], 111; range 50–500 mL) in those with a GA injury versus 147 mL (range, 82.25–400 mL) in those without injury (P = .14). Postoperative drain output in the 24 hours after surgery was 467 mL (SD 253, range 100–1105 mL) versus 502 mL (SD 378, range 75–1980 mL) in TKAs with and without GA injury, respectively (P = .82). Total estimated blood loss (combined intraoperative blood loss and 24-hour postoperative drain output) was 613 mL (SD 252, range 150–1105 mL) in TKAs with GA injury versus 620 mL (SD 393, range 75–2130 mL) without injury (P = .44) (Table 4). Overall, there was no statistical difference in blood loss, drain output, or combined output when analyzed according to lateral inferior or middle GA injury (P = .24–.82) (Table 5 and Table 6). No patients required blood transfusion postoperatively after TKA.
| Table 4. Factors Associated with GA Injury | |||
Outcome | GA Injury | No GA Injury | P Value |
Blood loss (mL) | 186 (50-500) | 147 (25-400) | .1366 |
24-Hour drain output (mL) | 467 (100-1105) | 502 (75-1980) | .8240 |
Total output (mL) | 613 (150-1105) | 620 (75-2130) | .4368 |
Differences in outcomes based on presence or absence of GA injury. Note that there were no significant differences. Values are reported as average (range). GA = geniculate artery.
| Table 5. Factors Associated with LIGA Injury | |||
Outcome | LIGA Injury | No LIGA Injury | P Value |
Blood loss (mL) | 178 (50-400) | 153 (25-500) | .2401 |
24-Hour drain output (mL) | 461 (100-890) | 501 (75-1980) | .8187 |
Total output (mL) | 610 (150-1080) | 621 (75-2130) | .4165 |
Differences in outcomes based on presence or absence of LIGA injury. Note that there were no significant differences. Values are reported as average (range). LIGA = lateral inferior geniculate artery.
| Table 6. Factors Associated with MGA Injury | |||
Outcome | MGA Injury | No MGA Injury | P Value |
Blood loss (mL) | 190 (75-500) | 156 (25-400) | .6225 |
24-Hour drain output (mL) | 455 (125-1105) | 494 (75-1980) | .6428 |
Total output (mL) | 582 (200-1105) | 624 (75-2130) | .6535 |
Differences in outcomes based on presence or absence of MGA injury. Note that there were no significant differences. Values are reported as average (range). MGA = middle geniculate artery.
IV administration of TXA was associated with a 37% (31/84) rate of GA injury, whereas topical TXA administration was associated with a 43% (6/14) rate of GA injury (P = .77). The rate of overall or isolated GA injury was not significantly different (P = .35–1.0) between IV and topical TXA administration (Table 7). In addition, total combined output was not significantly different (P = .1032) when comparing GA injury and noninjury in the subgroup analysis based on TXA use (IV or topical); however, topical administration was associated with lower intraoperative blood loss than IV administration (P = .0489), whereas IV administration was associated with lower 24-hour postoperative drain output than topical administration (P = .0169). There was no difference in blood loss, 24-hour drain output, or total output between those who did and did not sustain a GA injury in the group of patients who received IV TXA administration (Table 8, P = .2118–.7091). The same was true for those receiving topical TXA administration (Table 9, P = .0912–.9485).
Table 7. Factors Associated with TXA Injury | |||
Outcome | IV TXA (n = 84) | Topical TXA (n = 14) | P Value |
Any GA injury | 31 (37%) | 6 (43%) | .7683 |
LIGA injury | 24 (29%) | 6 (43%) | .3498 |
MGA injury | 13 (15%) | 2 (14%) | 1.0 |
Blood loss (mL) | 170 (25-500) | 113 (40-240) | .0489* |
24-Hour drain output (mL) | 454 (75-1980) | 662 (75-1800) | .0169* |
Total output (mL) | 592 (75-2130) | 751 (75-2130) | .1032 |
Differences in outcomes based on presence or absence of MGA injury. Note that there were no significant differences. Values are reported as n (%) or average (range). TXA = tranexamic acid, GA = geniculate artery, LIGA = lateral inferior geniculate artery, MGA = middle geniculate artery. *denotes statistical significance (P < .05).
| Table 8. Factors Associated with GA Injury Given IV TXA Use | ||||
Outcome | GA Injury | No GA Injury | Difference | P Value |
Blood loss (mL) | 195 (50-500) | 157 (25-400) | 38 | .2118 |
24-Hour drain output (mL) | 436 (100-1105) | 464 (75-1980) | 28 | .7091 |
Total output (mL) | 594 (150-1105) | 592 (75-2130) | 2 | .6982 |
Differences in outcomes of those patients who received IV TXA based on presence or absence of GA injury. Note that there were no significant differences. Values are reported as average (range). GA = geniculate artery, TXA = tranexamic acid.
| Table 9. Factors Associated with GA Injury Given Topical TXA Use | ||||
Outcome | GA Injury | No GA Injury | Difference | P Value |
Blood loss (mL) | 163 (100-250) | 84 (40-150) | 79 | .0912 |
24-Hour drain output (mL) | 610 (205-890) | 701 (415-1800) | 91 | .9485 |
Total output (mL) | 719 (405-960) | 775 (455-1900) | 56 | .6982 |
Differences in outcomes based on presence or absence of GA injury. Note that there were no significant differences. Values are reported as average (range). GA = geniculate artery.
Continue to: TOURNIQUET USE
TOURNIQUET USE
Comparison between Groups 1 (tourniquet use) and 2 (selective tourniquet use) revealed similar rates of overall and specific GA injury, intraoperative blood loss, and 24-hour postoperative drain output (Table 10). Group 1 demonstrated a 29% (9/31) rate of any GA injury versus 40% (27/67) in Group 2 (P = .37). For the specific lateral inferior GA injury, there was an equivalent rate of injury at 29% (9/31 in Group 1, 20/67 in Group 2; P = 1.0). Similarly, Group 1 patients had a 10% (3/31) rate of middle GA injury compared to 16% (11/67) in Group 2 patients (P = .53). Intraoperative estimated blood loss was lower in Group 1 (140 mL; range 25–400 mL) than in Group 2 (171 mL; range 40–500 mL) (P = .07), whereas the average 24-hour postoperative drain output was similar for Groups 1 (484 mL; range 75–1800 mL) and 2 (488 mL; range 100–1980 mL) (P = .46). Total estimated output was slightly less for Group 1 (593 mL; range 75–1900 mL) than for Group 2 (626 mL; range 125–2130 mL) (P = .38). A post hoc power analysis showed that with these rates of GA injury in Groups 1 and 2 and given a 2:1 ratio of the number of patients in Group 2 versus Group 1, a total of 185 patients in Group 1 and 370 patients in Group 2 would be needed to detect a statistically significant difference (P < .05) with a power of 80%.
| Table 10. Factors Associated with Tourniquet Use | ||||
Injury | Group 1 (n = 31) | Group 2 (n = 67) | Difference | P Value |
Overall GA injury | 9 (29%) | 27 (40%) | 11% | .3687 |
Lateral inferior GA | 9 (29%) | 20 (29%) | 0% | 1.0 |
Middle GA | 3 (10%) | 11 (16%) | 6% | .5382 |
Blood loss (mL) | 140 (25-400) | 171 (40-500) | 31 | .0661 |
24-Hour drain output (mL) | 484 (75-1800) | 488 (100-1980) | 4 | .4580 |
Total output (mL) | 593 (75-1900) | 626 (125-2130) | 33 | .3776 |
Differences in outcomes separated based on use of a tourniquet for the entire case (Group 1) vs use of a tourniquet only during cementation (Group 2). Note that there were no significant differences. Values are reported as n (%) or average (range). GA = geniculate artery.
DISCUSSION
Major arterial injury associated with TKA is a well-known, rare, and potentially devastating complication.1-13 However, the rate of injury to smaller periarticular vessels and the clinical significance of such injury have not been studied. The present study found a high rate of GA injury but no clinically significant difference in intraoperative blood loss or postoperative drain output between patients with GA injury (which was identified and managed with cautery) and those without GA injury. In addition, tourniquet use did not affect the rate of injury or the associated blood loss. To our knowledge, this is the first study that has critically evaluated the rate of GA injury occurring during TKA.
The overall rate of GA injury occurring during primary TKA was 38% with a higher predominance of lateral inferior than middle GA injury (31% vs 15%). Anatomically, it would follow that the lateral GA could be injured at a higher rate as it courses on top of the lateral meniscus, thus being susceptible to injury during cutting of the tibial plateau and meniscectomy. In addition, because the meniscectomy is performed longitudinally along the course of the artery, it may also be potentially lacerated in multiple locations and lengthwise. In theory, there should be a 100% rate of middle GA injury during posterior-stabilized TKA as this artery runs through the cruciate ligaments, which are resected during these cases. However, vessel injury was defined in this study as the visualization of pulsatile bleeding or vessel lumen. It is probable that in the cases in which injury to the middle GA was not visualized, it was cut but simultaneously cauterized. Thus, a lower rate (15%) of injury was detected. Nonetheless, these results still suggest that these periarticular arteries are injured at a higher rate; therefore, it is important for surgeons to specifically identify these injuries intraoperatively and adequately cauterize these vessels. As long as these arteries are cauterized, additional blood loss and potential vascular pseudoaneurysms should be prevented.
The effect of GA injury on intraoperative blood loss, 24-hour postoperative drain output, and total estimated blood loss showed no significant clinical findings in the present study cohort. In addition, examining the injury rate and blood loss based on TXA use also revealed no detrimental clinical associations. Although GA injury could inherently be associated with higher levels of blood loss and drain output, it is important to note that all GA injuries were also effectively coagulated, thus explaining the indifferent results. Accordingly, it should be recommended to surgeons performing primary TKAs to carefully evaluate for GA injury to prevent excessive blood loss or painful pseudoaneurysms. However, there is also a potential for beta error in this study in which a true difference did exist but no statistical difference was found due to the study being underpowered.
Full or selective tourniquet use during TKA did not appear to have any effect on the rate of GA injury, intraoperative blood loss, or 24-hour postoperative drain output. The similarity between GA injury rates perhaps further indicates an equivalent ability to detect these injuries between these two methods because of operative inspection for such injuries. With regard to intraoperative blood loss and drain output, the present findings are similar to previous studies demonstrating equivocal results despite variable tourniquet utilization in TKA.15,30 However, these results differ from those of Harvey and colleagues31, who demonstrated that blood loss inversely correlated with intraoperative tourniquet time. There are risks and benefits related to the use of both full and selective tourniquet methods, but either method does not appear to be advantageous in decreasing the rate of GA injury.
Continue to: Although this is the first study...
Although this is the first study to investigate the rates of GA injury and the potential clinical effects, there are limitations to this research. First, the study was retrospective in nature despite the fact that the data were collected prospectively. Only acute perioperative follow-up was performed, and thus, we were unable to evaluate longer term effects of GA injury on TKA outcomes. Furthermore, this study is potentially prone to beta error. As discussed above, 185 patients in Group 1 and 370 patients in Group 2 would be needed to detect a statistical difference in the rate of GA injury based on the rates found in this study. This study could also have been underpowered to identify differences in other aspects, such as differences in blood loss and drain. Furthermore, the data collected regarding intraoperative blood loss are estimated data and can be variable. Finally, visualization of vessel lumen and pulsatile bleeding is not a validated method to diagnose GA injuries, and potential injuries may have been missed. Despite such disadvantages, the strengths of this study include the concise results in consecutive patients, the generalizability of the data as multiple surgeons participated, and its first report of nonmajor periarticular artery injury.
CONCLUSIONS
There is a relatively high rate of GA injury, with injury to the lateral GA being visualized more often than injury to the middle GA. The majority of GA injuries occur around the time of bone cuts and meniscectomy, and tourniquet use does not affect the rate of injury. To reduce intraoperative blood loss and postoperative drain output, surgeons should identify and coagulate GA injuries routinely during primary TKA.
ABSTRACT
Major arterial injury associated with total knee arthroplasty (TKA) is a rare and potentially devastating complication. However, the rate of injury to smaller periarticular vessels and the clinical significance of such an injury have not been well investigated. The purpose of this study is to describe the rate and outcomes of geniculate artery (GA) injury, the time at which injury occurs, and any associations with tourniquet use.
From November 2015 to February 2016, 3 surgeons at a single institution performed 100 consecutive primary TKAs and documented the presence or absence and the timing of GA injury. The data were then retrospectively reviewed. All TKAs had no prior surgery on the operative extremity. Other variables collected included tourniquet use, tranexamic acid (TXA) administration, intraoperative blood loss, postoperative drain output, and blood transfusion.
The overall rate of GA injury was 38%, with lateral inferior and middle GA injury in 31% and 15% of TKAs, respectively. Most of the injuries were visualized during bone cuts or meniscectomy. The rate of overall or isolated GA injury was not significantly different (P > .05) with either use of intravenous (84 patients) or topical (14 patients) TXA administration. Comparing selective tourniquet use (only during cementation) vs routine use showed no differences in GA injury rate (P = .37), blood loss (P = .07), or drain output (P = .46).
There is a relatively high rate of GA injury, with injury to the lateral GA occurring more often than the middle GA. Routine or selective tourniquet use does not affect the rate of injury.
Continue to: Major arterial injury...
Major arterial injury associated with total knee arthroplasty (TKA) is a rare and potentially devastating complication. The majority of literature in this context consists of case reports, small case series, and large retrospective studies that have examined the type, location, and mechanism of injury present in these cases.1-13 Reported arterial injuries include occlusion, laceration, aneurysm, pseudoaneurysm, and arteriovenous fistula formation in the femoral (believed to be due to the tourniquet around the proximal thigh) and popliteal arteries causing combinations of ischemia and hemorrhage necessitating treatment ranging from endovascular arterial intervention to amputation.4,5,9-11,13-17 In addition, several studies have asserted that the risk of major arterial injury may be increased with tourniquet use, suggesting that tourniquet use should be minimized for routine primary TKAs.3,6
There are very few cases in the literature specifically addressing injury to the more commonly encountered geniculate arteries (GAs). The medial GAs are typically visualized and coagulated during the standard medial parapatellar approach. In addition, if performed, a lateral release can damage the lateral superior and inferior GAs and the middle GA can be cut with posterior cruciate ligament resection. However, the middle and lateral inferior GAs are anecdotally the most difficult to detect and treat intraoperatively, especially after implantation of TKA and deflation of the tourniquet. The potential lack of recognition of such GA injury can result in harmful sequelae, including ischemia of the patella, hemorrhage, and painful pseudoaneurysms.2,18-29 Currently, there are only 2 case reports of lateral inferior GA injury, 2 cases of medial inferior GA injury, and no reports of middle GA injury.2,23,24,29
The rate, the timing within surgery, the risk factors, including tourniquet use, and the clinical effects of GA injury are largely unknown. If these factors were better understood, prophylactic measures and/or awareness could be better applied to prevent adverse outcomes, especially in cases of the middle and lateral inferior GAs. The aims of this study are to elucidate the rate and timing of middle and lateral inferior GA injury during primary TKA; determine the factors related to injury, including intraoperative blood loss, postoperative drain output, and tranexamic (TXA) acid use; and investigate any differences in the rate of injury with and without the use of a tourniquet.
MATERIALS AND METHODS
PATIENT DEMOGRAPHICS AND SURGICAL TECHNIQUE
From November 2015 to February 2016, 3 surgeons (MJT, TMM, and RTT) at a single institution performed 100 consecutive unilateral primary TKAs and documented the presence or absence and the timing of GA injury. After obtaining approval from our Institutional Review Board, a retrospective study was performed to investigate the prospectively recorded rate of middle and lateral inferior GA injuries occurring during primary TKAs. Patients with a diagnosis of isolated osteoarthritis were included, and those with any previous surgery on the operative knee were excluded. The average age of patients at the time of surgery was 67 years (range, 25-91 years), the average body mass index was 33 kg/m2 (range, 18-54 kg/m2), and there were 63 (63%) female patients.
All TKAs were performed through a medial parapatellar approach with a posterior-stabilized, cemented design, and each patient received a postoperative surgical drain. One of the 3 lead surgeons (TMM) in this study used a tourniquet from the time of incision until the completion of cementation, and the other 2 (MJT and RTT) predominantly used the tourniquet only during cementation. To elucidate any differences in GA injury between these 2 methods of tourniquet use, the patients were categorized into 2 groups base d on tourniquet use. Group 1 included patients in whom a tourniquet was used to maintain a bloodless surgical field from the time of incision until the completion of cementation, and Group 2 included patients in whom tourniquet use was more selective (ie, applied only during cementation). Group 1 comprised 31% (31/100) of patients, while Group 2 comprised 67% (67/100) of patients; no tourniquet was used in 2% (2/100) of cases. In addition, TXA was used in 98% (98/100) of patients: 84 patients received intravenous (IV) and 14 received topical TXA administration.
Continue to: ANALYSIS OF GENICULATE ARTERY INJURY
ANALYSIS OF GENICULATE ARTERY INJURY
The senior authors critically evaluated the GA during the primary TKAs and documented the presence or absence of injury in the operative reports. GA injury was reported if there was intraoperative visualization of pulsatile bleeding or visualization of arterial lumen in the anatomic areas of the middle and lateral inferior GAs. At 3 separate occasions during the operation, the surgeon looked specifically for pulsatile bleeding or arterial lumen in the areas of the middle and lateral inferior GAs, including after all the femoral and tibial bone cuts were completed, immediately before preparing to cement (before the tourniquet was inflated if there was not one inflated from the start of the procedure), and immediately after the tourniquet was deflated (Figure 1). All bleeding GAs that were visualized were effectively coagulated by cautery. Details regarding the use of TXA (topical or IV), intraoperative blood loss, postsurgical drain output for 24 hours after surgery, and blood transfusion were collected from the patients’ medical records (Table 1).

| Table 1. Operative Variables | |
Variable | Value |
Total number | 100 (100%) |
Intraoperative blood loss (mL) | 160 (25-500) |
Drain output 1st 24 hours (mL) | 488 (75-1980) |
Total output (mL) | 618 (75-2130) |
Use of TXA | 98 (98%) |
Topical TXA | 84 (84%) |
IV TXA | 14 (14%) |
Tourniquet entire procedure | 31 (31%) |
Operative variables other than geniculate artery injury. Data presented as mean (range) or n (%). TXA = tranexamic acid.
STATISTICAL METHODS
Statistical analysis was performed using the JMP software version 10.0.0 (SAS Institute, Inc). The overall rate of GA injury was determined, including the rates of GA injury based on location, time point, and method of diagnosis (pulsatile bleeding or arterial lumen visualization). If >1 GA injury occurred in the same knee, only 1 GA injury was calculated for the overall rate; however, each injury was specified separately when calculating the injury rate for the specific GA. Intraoperative blood loss, postoperative drain output, and the use of TXA were compared between cases in which a GA injury was detected and those in which it was not detected. Before conducting the retrospective review, a power analysis determined that we would require 100 patients to detect a difference in GA injury between Groups 1 and 2 (33 in Group 1 and 67 in Group 2), assuming a 30% rate in Group 1 and a 5% rate of GA injury in Group 2 using Fisher’s exact test. The Fisher’s exact test was used to compare categorical variables, and the Wilcoxon rank sum test was used to compare continuous variables. An alpha value of .05 was considered as statistically significant.
RESULTS
RATE OF GENICULATE ARTERY INJURY
The overall rate of any GA injury was 38% (38/100). Lateral inferior GA injury was more frequently detected than middle GA injury (31% vs 15% of TKAs, respectively; Table 2). Among the 31 lateral inferior GA injuries, 14 were identified as pulsatile bleeding, 7 as lumen visualizations, and 6 as both pulsatile bleeding and lumen visualization; 4 were detected by methods not recorded in the operative report. Of the lateral inferior GA injuries, 11 were identified after the bone cuts, 7 during meniscus removal, 3 during exposure, 1 after tourniquet deflation, and 9 at a time not recorded in the operative report. Of the 15 middle GA injuries, 9 were identified as pulsatile bleeding, 2 as lumen visualizations, and 4 as both pulsatile bleeding and lumen visualization. In addition, 7 of these GA injuries were identified after the bone cuts, 3 during cruciate removal, 1 after meniscus removal, 1 during exposure, and 3 at a time not recorded in the operative report (Table 3).
| Table 2. Rates of Geniculate Artery Injury Based on Location and Method | ||||
Location | Pulsatile Bleeding | Arterial Lumen | Both | Overall Rate |
Lateral inferior GA | 14 (14%) | 7 (7%) | 6 (6%) | 31 (31%) |
Middle GA | 9 (9%) | 2 (2%) | 4 (4%) | 15 (15%) |
Rates of geniculate artery injury based on location and method of diagnosis. Data presented as n (%). There were 4 additional lateral inferior and 9 middle GA injuries identified by a method not specified in the operative report. GA = geniculate artery.
Table 3. Rates of Geniculate Artery Injury Based on Time Point | ||
Time | Lateral Inferior GA | Middle GA |
After bone cuts | 11 (11%) | 7 (7%) |
During meniscus removal | 7 (7%) | 1 (1%) |
During exposure | 3 (3%) | 1 (1%) |
After tourniquet deflation | 1 (1%) | 0 (0%) |
During cruciate removal | 0 (0%) | 3 (3%) |
Not reported | 9 (9%) | 3 (3%) |
Rates of geniculate artery injury based on time point and method of diagnosis. GA = geniculate artery. Data presented as n (%).
FACTORS ASSOCIATED WITH GENICULATE ARTERY INJURY
Mean intraoperative estimated blood loss was 186 mL (standard deviation [SD], 111; range 50–500 mL) in those with a GA injury versus 147 mL (range, 82.25–400 mL) in those without injury (P = .14). Postoperative drain output in the 24 hours after surgery was 467 mL (SD 253, range 100–1105 mL) versus 502 mL (SD 378, range 75–1980 mL) in TKAs with and without GA injury, respectively (P = .82). Total estimated blood loss (combined intraoperative blood loss and 24-hour postoperative drain output) was 613 mL (SD 252, range 150–1105 mL) in TKAs with GA injury versus 620 mL (SD 393, range 75–2130 mL) without injury (P = .44) (Table 4). Overall, there was no statistical difference in blood loss, drain output, or combined output when analyzed according to lateral inferior or middle GA injury (P = .24–.82) (Table 5 and Table 6). No patients required blood transfusion postoperatively after TKA.
| Table 4. Factors Associated with GA Injury | |||
Outcome | GA Injury | No GA Injury | P Value |
Blood loss (mL) | 186 (50-500) | 147 (25-400) | .1366 |
24-Hour drain output (mL) | 467 (100-1105) | 502 (75-1980) | .8240 |
Total output (mL) | 613 (150-1105) | 620 (75-2130) | .4368 |
Differences in outcomes based on presence or absence of GA injury. Note that there were no significant differences. Values are reported as average (range). GA = geniculate artery.
| Table 5. Factors Associated with LIGA Injury | |||
Outcome | LIGA Injury | No LIGA Injury | P Value |
Blood loss (mL) | 178 (50-400) | 153 (25-500) | .2401 |
24-Hour drain output (mL) | 461 (100-890) | 501 (75-1980) | .8187 |
Total output (mL) | 610 (150-1080) | 621 (75-2130) | .4165 |
Differences in outcomes based on presence or absence of LIGA injury. Note that there were no significant differences. Values are reported as average (range). LIGA = lateral inferior geniculate artery.
| Table 6. Factors Associated with MGA Injury | |||
Outcome | MGA Injury | No MGA Injury | P Value |
Blood loss (mL) | 190 (75-500) | 156 (25-400) | .6225 |
24-Hour drain output (mL) | 455 (125-1105) | 494 (75-1980) | .6428 |
Total output (mL) | 582 (200-1105) | 624 (75-2130) | .6535 |
Differences in outcomes based on presence or absence of MGA injury. Note that there were no significant differences. Values are reported as average (range). MGA = middle geniculate artery.
IV administration of TXA was associated with a 37% (31/84) rate of GA injury, whereas topical TXA administration was associated with a 43% (6/14) rate of GA injury (P = .77). The rate of overall or isolated GA injury was not significantly different (P = .35–1.0) between IV and topical TXA administration (Table 7). In addition, total combined output was not significantly different (P = .1032) when comparing GA injury and noninjury in the subgroup analysis based on TXA use (IV or topical); however, topical administration was associated with lower intraoperative blood loss than IV administration (P = .0489), whereas IV administration was associated with lower 24-hour postoperative drain output than topical administration (P = .0169). There was no difference in blood loss, 24-hour drain output, or total output between those who did and did not sustain a GA injury in the group of patients who received IV TXA administration (Table 8, P = .2118–.7091). The same was true for those receiving topical TXA administration (Table 9, P = .0912–.9485).
Table 7. Factors Associated with TXA Injury | |||
Outcome | IV TXA (n = 84) | Topical TXA (n = 14) | P Value |
Any GA injury | 31 (37%) | 6 (43%) | .7683 |
LIGA injury | 24 (29%) | 6 (43%) | .3498 |
MGA injury | 13 (15%) | 2 (14%) | 1.0 |
Blood loss (mL) | 170 (25-500) | 113 (40-240) | .0489* |
24-Hour drain output (mL) | 454 (75-1980) | 662 (75-1800) | .0169* |
Total output (mL) | 592 (75-2130) | 751 (75-2130) | .1032 |
Differences in outcomes based on presence or absence of MGA injury. Note that there were no significant differences. Values are reported as n (%) or average (range). TXA = tranexamic acid, GA = geniculate artery, LIGA = lateral inferior geniculate artery, MGA = middle geniculate artery. *denotes statistical significance (P < .05).
| Table 8. Factors Associated with GA Injury Given IV TXA Use | ||||
Outcome | GA Injury | No GA Injury | Difference | P Value |
Blood loss (mL) | 195 (50-500) | 157 (25-400) | 38 | .2118 |
24-Hour drain output (mL) | 436 (100-1105) | 464 (75-1980) | 28 | .7091 |
Total output (mL) | 594 (150-1105) | 592 (75-2130) | 2 | .6982 |
Differences in outcomes of those patients who received IV TXA based on presence or absence of GA injury. Note that there were no significant differences. Values are reported as average (range). GA = geniculate artery, TXA = tranexamic acid.
| Table 9. Factors Associated with GA Injury Given Topical TXA Use | ||||
Outcome | GA Injury | No GA Injury | Difference | P Value |
Blood loss (mL) | 163 (100-250) | 84 (40-150) | 79 | .0912 |
24-Hour drain output (mL) | 610 (205-890) | 701 (415-1800) | 91 | .9485 |
Total output (mL) | 719 (405-960) | 775 (455-1900) | 56 | .6982 |
Differences in outcomes based on presence or absence of GA injury. Note that there were no significant differences. Values are reported as average (range). GA = geniculate artery.
Continue to: TOURNIQUET USE
TOURNIQUET USE
Comparison between Groups 1 (tourniquet use) and 2 (selective tourniquet use) revealed similar rates of overall and specific GA injury, intraoperative blood loss, and 24-hour postoperative drain output (Table 10). Group 1 demonstrated a 29% (9/31) rate of any GA injury versus 40% (27/67) in Group 2 (P = .37). For the specific lateral inferior GA injury, there was an equivalent rate of injury at 29% (9/31 in Group 1, 20/67 in Group 2; P = 1.0). Similarly, Group 1 patients had a 10% (3/31) rate of middle GA injury compared to 16% (11/67) in Group 2 patients (P = .53). Intraoperative estimated blood loss was lower in Group 1 (140 mL; range 25–400 mL) than in Group 2 (171 mL; range 40–500 mL) (P = .07), whereas the average 24-hour postoperative drain output was similar for Groups 1 (484 mL; range 75–1800 mL) and 2 (488 mL; range 100–1980 mL) (P = .46). Total estimated output was slightly less for Group 1 (593 mL; range 75–1900 mL) than for Group 2 (626 mL; range 125–2130 mL) (P = .38). A post hoc power analysis showed that with these rates of GA injury in Groups 1 and 2 and given a 2:1 ratio of the number of patients in Group 2 versus Group 1, a total of 185 patients in Group 1 and 370 patients in Group 2 would be needed to detect a statistically significant difference (P < .05) with a power of 80%.
| Table 10. Factors Associated with Tourniquet Use | ||||
Injury | Group 1 (n = 31) | Group 2 (n = 67) | Difference | P Value |
Overall GA injury | 9 (29%) | 27 (40%) | 11% | .3687 |
Lateral inferior GA | 9 (29%) | 20 (29%) | 0% | 1.0 |
Middle GA | 3 (10%) | 11 (16%) | 6% | .5382 |
Blood loss (mL) | 140 (25-400) | 171 (40-500) | 31 | .0661 |
24-Hour drain output (mL) | 484 (75-1800) | 488 (100-1980) | 4 | .4580 |
Total output (mL) | 593 (75-1900) | 626 (125-2130) | 33 | .3776 |
Differences in outcomes separated based on use of a tourniquet for the entire case (Group 1) vs use of a tourniquet only during cementation (Group 2). Note that there were no significant differences. Values are reported as n (%) or average (range). GA = geniculate artery.
DISCUSSION
Major arterial injury associated with TKA is a well-known, rare, and potentially devastating complication.1-13 However, the rate of injury to smaller periarticular vessels and the clinical significance of such injury have not been studied. The present study found a high rate of GA injury but no clinically significant difference in intraoperative blood loss or postoperative drain output between patients with GA injury (which was identified and managed with cautery) and those without GA injury. In addition, tourniquet use did not affect the rate of injury or the associated blood loss. To our knowledge, this is the first study that has critically evaluated the rate of GA injury occurring during TKA.
The overall rate of GA injury occurring during primary TKA was 38% with a higher predominance of lateral inferior than middle GA injury (31% vs 15%). Anatomically, it would follow that the lateral GA could be injured at a higher rate as it courses on top of the lateral meniscus, thus being susceptible to injury during cutting of the tibial plateau and meniscectomy. In addition, because the meniscectomy is performed longitudinally along the course of the artery, it may also be potentially lacerated in multiple locations and lengthwise. In theory, there should be a 100% rate of middle GA injury during posterior-stabilized TKA as this artery runs through the cruciate ligaments, which are resected during these cases. However, vessel injury was defined in this study as the visualization of pulsatile bleeding or vessel lumen. It is probable that in the cases in which injury to the middle GA was not visualized, it was cut but simultaneously cauterized. Thus, a lower rate (15%) of injury was detected. Nonetheless, these results still suggest that these periarticular arteries are injured at a higher rate; therefore, it is important for surgeons to specifically identify these injuries intraoperatively and adequately cauterize these vessels. As long as these arteries are cauterized, additional blood loss and potential vascular pseudoaneurysms should be prevented.
The effect of GA injury on intraoperative blood loss, 24-hour postoperative drain output, and total estimated blood loss showed no significant clinical findings in the present study cohort. In addition, examining the injury rate and blood loss based on TXA use also revealed no detrimental clinical associations. Although GA injury could inherently be associated with higher levels of blood loss and drain output, it is important to note that all GA injuries were also effectively coagulated, thus explaining the indifferent results. Accordingly, it should be recommended to surgeons performing primary TKAs to carefully evaluate for GA injury to prevent excessive blood loss or painful pseudoaneurysms. However, there is also a potential for beta error in this study in which a true difference did exist but no statistical difference was found due to the study being underpowered.
Full or selective tourniquet use during TKA did not appear to have any effect on the rate of GA injury, intraoperative blood loss, or 24-hour postoperative drain output. The similarity between GA injury rates perhaps further indicates an equivalent ability to detect these injuries between these two methods because of operative inspection for such injuries. With regard to intraoperative blood loss and drain output, the present findings are similar to previous studies demonstrating equivocal results despite variable tourniquet utilization in TKA.15,30 However, these results differ from those of Harvey and colleagues31, who demonstrated that blood loss inversely correlated with intraoperative tourniquet time. There are risks and benefits related to the use of both full and selective tourniquet methods, but either method does not appear to be advantageous in decreasing the rate of GA injury.
Continue to: Although this is the first study...
Although this is the first study to investigate the rates of GA injury and the potential clinical effects, there are limitations to this research. First, the study was retrospective in nature despite the fact that the data were collected prospectively. Only acute perioperative follow-up was performed, and thus, we were unable to evaluate longer term effects of GA injury on TKA outcomes. Furthermore, this study is potentially prone to beta error. As discussed above, 185 patients in Group 1 and 370 patients in Group 2 would be needed to detect a statistical difference in the rate of GA injury based on the rates found in this study. This study could also have been underpowered to identify differences in other aspects, such as differences in blood loss and drain. Furthermore, the data collected regarding intraoperative blood loss are estimated data and can be variable. Finally, visualization of vessel lumen and pulsatile bleeding is not a validated method to diagnose GA injuries, and potential injuries may have been missed. Despite such disadvantages, the strengths of this study include the concise results in consecutive patients, the generalizability of the data as multiple surgeons participated, and its first report of nonmajor periarticular artery injury.
CONCLUSIONS
There is a relatively high rate of GA injury, with injury to the lateral GA being visualized more often than injury to the middle GA. The majority of GA injuries occur around the time of bone cuts and meniscectomy, and tourniquet use does not affect the rate of injury. To reduce intraoperative blood loss and postoperative drain output, surgeons should identify and coagulate GA injuries routinely during primary TKA.
1. Calligaro KD, Dougherty MJ, Ryan S, Booth RE. Acute arterial complications associated with total hip and knee arthroplasty. J Vasc Surg. 2003;38(6):1170-1177. doi: 10.1016/S0741-5214(03)00918-2.
2. Dennis DA, Neumann RD, Toma P, Rosenberg G, Mallory TH. Arteriovenous fistula with false aneurysm of the inferior medial geniculate artery. A complication of total knee arthroplasty. Clin Orthop Relat Res. 1987(222):255-260.
3. Hagan PF, Kaufman EE. Vascular complication of knee arthroplasty under tourniquet. A case report. Clin Orthop Relat Res. 1990(257):159-161.
4. Holmberg A, Milbrink J, Bergqvist D. Arterial complications after knee arthroplasty: 4 cases and a review of the literature. Acta Orthop Scand. 1996;67(1):75-78. doi: 10.3109/17453679608995616.
5. Hozack WJ, Cole PA, Gardner R, Corces A. Popliteal aneurysm after total knee arthroplasty. Case reports and review of the literature. J Arthroplasty. 1990;5(4):301-305. doi: 10.1016/S0883-5403(08)80087-3.
6. Jeyaseelan S, Stevenson TM, Pfitzner J. Tourniquet failure and arterial calcification. Case report and theoretical dangers. Anaesthesia. 1981;36(1):48-50. doi: 10.1111/j.1365-2044.1981.tb08599.x
7. Mureebe L, Gahtan V, Kahn MB, Kerstein MD, Roberts AB. Popliteal artery injury after total knee arthroplasty. Am Surg. 1996;62(5):366-368.
8. O'Connor JV, Stocks G, Crabtree JD, Jr., Galasso P, Wallsh E. Popliteal pseudoaneurysm following total knee arthroplasty. J Arthroplasty. 1998;13(7):830-832. doi: 10.1016/S0883-5403(98)90039-0.
9. Ohira T, Fujimoto T, Taniwaki K. Acute popliteal artery occlusion after total knee arthroplasty. Arch Orthop Trauma Surg. 1997;116(6-7):429-430. doi: 10.1007/BF00434007.
10. Parfenchuck TA, Young TR. Intraoperative arterial occlusion in total joint arthroplasty. J Arthroplasty. 1994;9(2):217-220. doi: 10.1016/0883-5403(94)90071-X.
11. Rush JH, Vidovich JD, Johnson MA. Arterial complications of total knee replacement. The Australian experience. J Bone Joint Surg Br. 1987;69(3):400-402. doi: 10.1302/0301-620X.69B3.3584193.
12. Smith DE, McGraw RW, Taylor DC, Masri BA. Arterial complications and total knee arthroplasty. J Am Acad Orthop Surg. 2001;9(4):253-257.
13. Zahrani HA, Cuschieri RJ. Vascular complications after total knee replacement. J Cardiovasc Surg (Torino). 1989;30(6):951-952.
14. Isiklar ZU, Landon GC, Tullos HS. Amputation after failed total knee arthroplasty. Clin Orthop Relat Res. 1994(299):173-178.
15. Wakankar HM, Nicholl JE, Koka R, D'Arcy JC. The tourniquet in total knee arthroplasty. A prospective, randomised study. J Bone Joint Surg Br. 1999;81(1):30-33. doi: 10.1302/0301-620X.81B1.0810030.
16. Kumar SN, Chapman JA, Rawlins I. Vascular injuries in total knee arthroplasty. A review of the problem with special reference to the possible effects of the tourniquet. J Arthroplasty. 1998;13(2):211-216. doi: 10.1016/S0883-5403(98)90102-4.
17. DeLaurentis DA, Levitsky KA, Booth RE, et al. Arterial and ischemic aspects of total knee arthroplasty. Am J Surg. 1992;164(3):237-240. doi: 10.1016/S0002-9610(05)81078-5.
18. Langkamer VG. Local vascular complications after knee replacement: a review with illustrative case reports. Knee. 2001;8(4):259-264. doi: 10.1016/S0968-0160(01)00103-X.
19. Moran M, Hodgkinson J, Tait W. False aneurysm of the superior lateral geniculate artery following Total Knee Replacement. Knee. 2002;9(4):349-351. doi: 10.1016/S0968-0160(02)00061-3.
20. Pritsch T, Parnes N, Menachem A. A bleeding pseudoaneurysm of the lateral genicular artery after total knee arthroplasty--a case report. Acta Orthop. 2005;76(1):138-140. doi: 10.1080/00016470510030463.
21. Gaheer RS, Chirputkar K, Sarungi M. Spontaneous resolution of superior medial geniculate artery pseudoaneurysm following total knee arthroplasty. Knee. 2014;21(2):586-588. doi: 10.1016/j.knee.2012.10.021.
22. Law KY, Cheung KW, Chiu KH, Antonio GE. Pseudoaneurysm of the geniculate artery following total knee arthroplasty: a report of two cases. J Orthop Surg (Hong Kong). 2007;15(3):386-389. /doi: 10.1177/230949900701500331.
23. Noorpuri BS, Maxwell-Armstrong CA, Lamerton AJ. Pseudo-aneurysm of a geniculate collateral artery complicating total knee replacement. Eur J Vasc Endovasc Surg. 1999;18(6):534-535.
24. Pai VS. Traumatic aneurysm of the inferior lateral geniculate artery after total knee replacement. J Arthroplasty. 1999;14(5):633-634. doi: 10.1016/S0883-5403(99)90089-X.
25. Julien TP, Gravereaux E, Martin S. Superior medial geniculate artery pseudoaneurysm after primary total knee arthroplasty. J Arthroplasty. 2012;27(2):323 e313-326. doi: 10.1016/j.arth.2011.02.009.
26. Kalsi PS, Carrington RJ, Skinner JS. Therapeutic embolization for the treatment of recurrent hemarthrosis after total knee arthroplasty due to an arteriovenous fistula. J Arthroplasty. 2007;22(8):1223-1225. /doi: 10.1016/j.arth.2006.11.012.
27. Ritter MA, Herbst SA, Keating EM, Faris PM, Meding JB. Patellofemoral complications following total knee arthroplasty. Effect of a lateral release and sacrifice of the superior lateral geniculate artery. J Arthroplasty. 1996;11(4):368-372. doi: 10.1016/S0883-5403(96)80024-6.
28. Aldrich D, Anschuetz R, LoPresti C, Fumich M, Pitluk H, O'Brien W. Pseudoaneurysm complicating knee arthroscopy. Arthroscopy. 1995;11(2):229-230. doi: 10.1016/0749-8063(95)90073-X.
29. Sharma H, Singh GK, Cavanagh SP, Kay D. Pseudoaneurysm of the inferior medial geniculate artery following primary total knee arthroplasty: delayed presentation with recurrent haemorrhagic episodes. Knee Surg Sports Traumatol Arthrosc. 2006;14(2):153-155. doi: 10.1007/s00167-005-0639-4.
30. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253. doi: 10.1302/0301-620X.77B2.7706340.
31. Harvey EJ, Leclerc J, Brooks CE, Burke DL. Effect of tourniquet use on blood loss and incidence of deep vein thrombosis in total knee arthroplasty. J Arthroplasty. 1997;12(3):291-296. doi: 10.1016/S0883-5403(97)90025-5.
1. Calligaro KD, Dougherty MJ, Ryan S, Booth RE. Acute arterial complications associated with total hip and knee arthroplasty. J Vasc Surg. 2003;38(6):1170-1177. doi: 10.1016/S0741-5214(03)00918-2.
2. Dennis DA, Neumann RD, Toma P, Rosenberg G, Mallory TH. Arteriovenous fistula with false aneurysm of the inferior medial geniculate artery. A complication of total knee arthroplasty. Clin Orthop Relat Res. 1987(222):255-260.
3. Hagan PF, Kaufman EE. Vascular complication of knee arthroplasty under tourniquet. A case report. Clin Orthop Relat Res. 1990(257):159-161.
4. Holmberg A, Milbrink J, Bergqvist D. Arterial complications after knee arthroplasty: 4 cases and a review of the literature. Acta Orthop Scand. 1996;67(1):75-78. doi: 10.3109/17453679608995616.
5. Hozack WJ, Cole PA, Gardner R, Corces A. Popliteal aneurysm after total knee arthroplasty. Case reports and review of the literature. J Arthroplasty. 1990;5(4):301-305. doi: 10.1016/S0883-5403(08)80087-3.
6. Jeyaseelan S, Stevenson TM, Pfitzner J. Tourniquet failure and arterial calcification. Case report and theoretical dangers. Anaesthesia. 1981;36(1):48-50. doi: 10.1111/j.1365-2044.1981.tb08599.x
7. Mureebe L, Gahtan V, Kahn MB, Kerstein MD, Roberts AB. Popliteal artery injury after total knee arthroplasty. Am Surg. 1996;62(5):366-368.
8. O'Connor JV, Stocks G, Crabtree JD, Jr., Galasso P, Wallsh E. Popliteal pseudoaneurysm following total knee arthroplasty. J Arthroplasty. 1998;13(7):830-832. doi: 10.1016/S0883-5403(98)90039-0.
9. Ohira T, Fujimoto T, Taniwaki K. Acute popliteal artery occlusion after total knee arthroplasty. Arch Orthop Trauma Surg. 1997;116(6-7):429-430. doi: 10.1007/BF00434007.
10. Parfenchuck TA, Young TR. Intraoperative arterial occlusion in total joint arthroplasty. J Arthroplasty. 1994;9(2):217-220. doi: 10.1016/0883-5403(94)90071-X.
11. Rush JH, Vidovich JD, Johnson MA. Arterial complications of total knee replacement. The Australian experience. J Bone Joint Surg Br. 1987;69(3):400-402. doi: 10.1302/0301-620X.69B3.3584193.
12. Smith DE, McGraw RW, Taylor DC, Masri BA. Arterial complications and total knee arthroplasty. J Am Acad Orthop Surg. 2001;9(4):253-257.
13. Zahrani HA, Cuschieri RJ. Vascular complications after total knee replacement. J Cardiovasc Surg (Torino). 1989;30(6):951-952.
14. Isiklar ZU, Landon GC, Tullos HS. Amputation after failed total knee arthroplasty. Clin Orthop Relat Res. 1994(299):173-178.
15. Wakankar HM, Nicholl JE, Koka R, D'Arcy JC. The tourniquet in total knee arthroplasty. A prospective, randomised study. J Bone Joint Surg Br. 1999;81(1):30-33. doi: 10.1302/0301-620X.81B1.0810030.
16. Kumar SN, Chapman JA, Rawlins I. Vascular injuries in total knee arthroplasty. A review of the problem with special reference to the possible effects of the tourniquet. J Arthroplasty. 1998;13(2):211-216. doi: 10.1016/S0883-5403(98)90102-4.
17. DeLaurentis DA, Levitsky KA, Booth RE, et al. Arterial and ischemic aspects of total knee arthroplasty. Am J Surg. 1992;164(3):237-240. doi: 10.1016/S0002-9610(05)81078-5.
18. Langkamer VG. Local vascular complications after knee replacement: a review with illustrative case reports. Knee. 2001;8(4):259-264. doi: 10.1016/S0968-0160(01)00103-X.
19. Moran M, Hodgkinson J, Tait W. False aneurysm of the superior lateral geniculate artery following Total Knee Replacement. Knee. 2002;9(4):349-351. doi: 10.1016/S0968-0160(02)00061-3.
20. Pritsch T, Parnes N, Menachem A. A bleeding pseudoaneurysm of the lateral genicular artery after total knee arthroplasty--a case report. Acta Orthop. 2005;76(1):138-140. doi: 10.1080/00016470510030463.
21. Gaheer RS, Chirputkar K, Sarungi M. Spontaneous resolution of superior medial geniculate artery pseudoaneurysm following total knee arthroplasty. Knee. 2014;21(2):586-588. doi: 10.1016/j.knee.2012.10.021.
22. Law KY, Cheung KW, Chiu KH, Antonio GE. Pseudoaneurysm of the geniculate artery following total knee arthroplasty: a report of two cases. J Orthop Surg (Hong Kong). 2007;15(3):386-389. /doi: 10.1177/230949900701500331.
23. Noorpuri BS, Maxwell-Armstrong CA, Lamerton AJ. Pseudo-aneurysm of a geniculate collateral artery complicating total knee replacement. Eur J Vasc Endovasc Surg. 1999;18(6):534-535.
24. Pai VS. Traumatic aneurysm of the inferior lateral geniculate artery after total knee replacement. J Arthroplasty. 1999;14(5):633-634. doi: 10.1016/S0883-5403(99)90089-X.
25. Julien TP, Gravereaux E, Martin S. Superior medial geniculate artery pseudoaneurysm after primary total knee arthroplasty. J Arthroplasty. 2012;27(2):323 e313-326. doi: 10.1016/j.arth.2011.02.009.
26. Kalsi PS, Carrington RJ, Skinner JS. Therapeutic embolization for the treatment of recurrent hemarthrosis after total knee arthroplasty due to an arteriovenous fistula. J Arthroplasty. 2007;22(8):1223-1225. /doi: 10.1016/j.arth.2006.11.012.
27. Ritter MA, Herbst SA, Keating EM, Faris PM, Meding JB. Patellofemoral complications following total knee arthroplasty. Effect of a lateral release and sacrifice of the superior lateral geniculate artery. J Arthroplasty. 1996;11(4):368-372. doi: 10.1016/S0883-5403(96)80024-6.
28. Aldrich D, Anschuetz R, LoPresti C, Fumich M, Pitluk H, O'Brien W. Pseudoaneurysm complicating knee arthroscopy. Arthroscopy. 1995;11(2):229-230. doi: 10.1016/0749-8063(95)90073-X.
29. Sharma H, Singh GK, Cavanagh SP, Kay D. Pseudoaneurysm of the inferior medial geniculate artery following primary total knee arthroplasty: delayed presentation with recurrent haemorrhagic episodes. Knee Surg Sports Traumatol Arthrosc. 2006;14(2):153-155. doi: 10.1007/s00167-005-0639-4.
30. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253. doi: 10.1302/0301-620X.77B2.7706340.
31. Harvey EJ, Leclerc J, Brooks CE, Burke DL. Effect of tourniquet use on blood loss and incidence of deep vein thrombosis in total knee arthroplasty. J Arthroplasty. 1997;12(3):291-296. doi: 10.1016/S0883-5403(97)90025-5.
TAKE-HOME POINTS
- During total knee arthroscopy (TKA), 38% of patients will have an injury of a geniculate artery.
- The lateral inferior geniculate artery is most commonly injured, with a rate of injury of 31%.
- The middle geniculate artery is injured 15% of the time.
- The most common time of geniculate artery injury is during bone cutting or removal of the meniscus.
- There is no difference in rate of geniculate artery injury identification with or without the use of a tourniquet.
Foot and Ankle Injuries in Soccer
ABSTRACT
The ankle is one of the most commonly injured joints in soccer and represents a significant cost to the healthcare system. The ligaments that stabilize the ankle joint determine its biomechanics—alterations of which result from various soccer-related injuries. Acute sprains are among the most common injury in soccer players and are generally treated conservatively, with emphasis placed on secondary prevention to reduce the risk for future sprains and progression to chronic ankle instability. Repetitive ankle injuries in soccer players may cause chronic ankle instability, which includes both mechanical ligamentous laxity and functional changes. Chronic ankle pathology often requires surgery to repair ligamentous damage and remove soft-tissue or osseous impingement. Proper initial treatment, rehabilitation, and secondary prevention of ankle injuries can limit the amount of time lost from play and avoid negative long-term sequelae (eg, osteochondral lesions, arthritis). On the other hand, high ankle sprains portend a poorer prognosis and a longer recovery. These injuries will typically require surgical stabilization. Impingement-like syndromes of the ankle can undergo an initial trial of conservative treatment; when this fails, however, soccer players respond favorably to arthroscopic debridement of the lesions causing impingement. Finally, other pathologies (eg, stress fractures) are highly encouraged to be treated with surgical stabilization in elite soccer players.
Continue to: EPIDEMIOLOGY
EPIDEMIOLOGY
With roughly 200,000 professional and around 240 million amateur soccer players, soccer has been recognized as the most popular sport worldwide. Nevertheless, given its rising popularity in society, one must also consider the increasing incidence of injuries as a result. Elite soccer players sustain between 10 and 35 injuries per 1000 competitive playing hours.1 Approximately 80% are traumatic, and 20% are overuse injuries.2 Soccer injuries are more frequent with increasing age of the participants, whereas the incidence of injuries in preadolescent players is low. The incidence of injuries has been found to be higher during competition when compared with practice/training sessions, with some studies showing that 59% of injuries occurred during games.2 Amateur or recreational soccer players sustain fewer injuries than professional soccer players, as one would expect, given both the higher intensity of training and match schedule in professionals.
The ankle is one of the most commonly injured joints in soccer, with some studies suggesting it comprises one-fifth of all injuries sustained during soccer, which is only second to those of the knee.2 Ankle sprains specifically are quite a common occurrence in soccer.3-9 A recent study of an English premier league club showed that over a 4-season period, 20% of injuries were of the foot and ankle with a mean return to sport time of 54 days.10 Of all foot and ankle related injuries, ankle sprains are the most common, followed by bruises/contusions, and tendon lesions. Fractures are very rare (1%) in soccer, but when they do occur they impart a much more extended recovery. During the 2010 Fédération Internationale de Football Association (FIFA) World Cup, ankle sprains were among the most common injuries and approximately half lead to players missing training or competitive matches.5
ANATOMY
Knowledge of the biomechanics of both the foot and ankle joints is essential to understand soccer injuries. The ankle joint (talocrural articulation) consists of the distal ends of the tibia and fibula, which form the mortise, and the superior aspect of the talar dome.11 As a hinge joint, the ankle provides 20° of dorsiflexion and 50° of plantar flexion,12 with stability provided by the lateral, medial, and superior ligamentous complexes. The superior articular surface of the talus is narrower posteriorly, which creates a looser fit within the mortise during plantar flexion.11 This decreased stability could help explain why the most common injury in soccer involves a plantar flexion mechanism.13,14 Inferiorly, the talus articulates with the calcaneus to form the subtalar joint. It is at this site that the majority of both foot inversion and eversion occurs. The transverse tarsal joints (Chopart’s joints) separate the hindfoot from the midfoot. Movement of this joint depends on the relative alignment of its 2 articulations: the talonavicular and calcaneocuboid joints. During foot eversion, these 2 joints are parallel to each other allowing supple motion and aiding in shock absorption during the heel strike phase of the gait cycle. With foot inversion, the joints become nonparallel and thus lock the transverse tarsal joints providing a rigid lever needed for push-off.11,12
LATERAL LIGAMENTS
The ankle joint is stabilized laterally by a ligament complex consisting of 3 individual ligaments, all originating from the lateral malleolus: the anterior talofibular ligament (ATFL), the posterior talofibular ligament (PTFL), and the calcaneofibular ligament (CFL) (Figure 1).11,12,15 The ATFL is the primary restraint to inversion in plantar flexion, and it helps resist anterolateral translation of the talus in the mortise. However, it is the weakest and therefore the most frequently injured of the lateral ligaments. The PTFL plays only a supplementary role in ankle stability when the lateral ligament complex is intact. It is under the greatest strain in ankle dorsiflexion and acts to limit posterior talar displacement within the mortise as well as talar external rotation.13,16 The CFL is the primary restraint to inversion in the neutral or dorsiflexed position. It restrains subtalar inversion, thereby limiting talar tilt within the mortise.

DELTOID LIGAMENT
The deltoid ligament complex consists of 6 continuous adjacent superficial and deep ligaments that function synergistically to resist valgus and pronation forces, as well as external rotation of the talus in the mortise.11-13,17 The superficial layer crosses both ankle and subtalar joints. It originates from the anterior colliculus and fans out to insert into the navicular, neck of the talus, sustentaculum tali, and posteromedial talar tubercle. The tibiocalcaneal (sustentaculum tali) portion is the strongest component in the superficial layer and resists calcaneal eversion. The deep layer crosses the ankle joint only. It functions as the primary stabilizer of the medial ankle and prevents both lateral displacement and external rotation of the talus. It originates from the inferior and posterior aspects of the medial malleolus and inserts on the medial and posteromedial aspects of the talus.12,17,18
Continue to: SYNDESMOSIS
SYNDESMOSIS
The ankle syndesmosis, or inferior tibiofibular joint, is the distal articulation between the tibia and fibula. The syndesmosis contributes to ankle mortise integrity through its firm fixation of the lateral malleolus against the lateral surface of the talus. Ligaments comprising the ankle syndesmosis include the anterior inferior tibiofibular ligament (AITFL), the posterior inferior tibiofibular ligament (PITFL), the inferior transverse ligament, and the interosseous ligament (IOL).12
ANKLE SPRAINS
Ankle sprains are the most common pathology encountered amongst soccer players, representing from one-half to two-thirds of all ankle related injuries. Most sprains occur outside of player contact.
LATERAL ANKLE SPRAINS AND INSTABILITY
Injury to the lateral ligaments of the ankle represents 77% to 91% of all ankle sprains in soccer.6,19 The greatest risk factor for an ankle sprain in a soccer player is a history of prior sprain.20 Other risk factors include increasing age, player-to-player contact, condition of the pitch, weight-bearing status of the injured limb at the time of injury, and joint instability or laxity.21,22
The evaluation of an ankle sprain to determine its severity is best done after the acute phase, approximately 4 to 7 days after the initial injury when both pain and swelling have subsided.23 The anterior drawer (ATFL instability) and talar tilt (CFL instability) tests are useful in evaluating ankle instability in the delayed or chronic setting; however, both have been shown to have limited sensitivity and significant variability amongst different examiners.24
Clinical examination will direct further diagnostic tests including X-rays, magnetic resonance imaging (MRI), and computed tomography (CT). The Ottawa ankle rules are generally helpful in determining whether plain X-rays are indicated in the acute setting.25,26 (Figure 2) According to these rules, ankle radiographs should be obtained if ankle pain is reported near the malleoli and 1 or more of the following is seen during examination: inability to bear weight immediately after injury and for 4 steps in the emergency department, and bony tenderness at the posterior edge or tip of the malleolus. Stress X-rays are generally not indicated in acute injuries but may be useful in chronic ankle instability cases.23

Continue to: Ankle sprains cover...
Ankle sprains cover a broad spectrum of injuries; therefore, a grading system was devised to aid in guiding treatment. Grade I (mild) sprains are those with minimal swelling and tenderness but have the ligaments still intact. Grade II (moderate) sprains occur when there are partial ligament tears associated with moderate pain, swelling, and tenderness. Finally, Grade III (severe) sprains are complete ligament tears with marked swelling, hemorrhage, tenderness, loss of function, and abnormal joint motion and instability.23, 24
Initial treatment for all ankle sprains is nonoperative and involves the RICE (rest, ice, compression, elevation) protocol with the use of nonsteroidal anti-inflammatory drugs (NSAIDs) during the acute phase (first 4-5 days) with a short period (no >2 weeks) of immobilization.27 Most authors agree that early mobilization followed by phased rehabilitation is warranted to minimize time away from sports.28-31 Prolonged immobilization (>2 weeks) has detrimental effects and may lead to a longer return to play.28-31 The rehabilitation protocol is divided into stages: (1) pain and edema control, (2) range of motion (ROM) and strengthening exercises, (3) soccer specific functional training, and (4) prophylactic intervention with balance and proprioception exercises. Surgical intervention is rarely indicated for acute ankle sprains. There are exceptions, however, such as when ankle sprains are associated with other injuries that require acute intervention (eg, fracture, osteochondral lesion). Surgery is indicated in the setting of chronic, recurrent mechanical instability. Anatomical repairs (modified Brostrom) seem to produce better outcomes than non-anatomical reconstructions (eg, Chrisman-Snook). Surgical outcomes are good, and most athletes are able to return to their pre-injury level of function.32
In athletes, prevention of recurrent sprains is key. Braces may help prevent ankle sprains and bracing has been shown to be superior to taping, as tape loses its restrictive properties within 20 to 30 minutes of initiating activity.33,34 Application of an orthosis (lace-up ankle orthosis) has been shown to reduce the incidence of ankle re-injury in soccer players with previous ankle sprains. Several studies have found minimal, if any, effect of orthoses on athletic performance.20,35,36 Low-profile braces for soccer have been developed which allow for minimal disruption of the player’s boot and space proximally to insert the shin guard. Another essential component of prevention is prophylactic intervention with balance and proprioceptive exercises. A study looking at first division men’s league football (soccer) players in Iran showed a significant decrease in re-injury rates with proprioceptive training.37 In 2003, FIFA introduced a comprehensive warm-up program (FIFA 11+), which has since been shown in several studies to decrease the risk of injury in amateur soccer players.38-40
MEDIAL ANKLE SPRAINS AND INSTABILITY
Soccer places an unusually high demand on both the medial foot and ankle structures when compared with other sports. For instance, striking the ball requires the player to abduct and externally rotate the foot, which preloads medial structures.9 Hintermann18 looked at 54 cases of medial ankle instability and found that injury commonly occurred during landing on an uneven surface, which applies to soccer players when landing after heading the ball or jumping over a tackle. Pronation with eversion and extreme rotational injuries are well known to cause deltoid ligament injury. However, complete rupture of the deltoid ligament is rare and is more often associated with ankle fractures.41 Due to its close proximity and similarly shared function in medial plantar arch stabilization with the tibiospring and spring ligaments, posterior tibialis tendon dysfunction is also frequently seen in medial ankle instability.17 After an acute injury, patients can present with a medial ankle hematoma and pain along the deltoid ligament. Although chronic insufficiency is diagnosed based on the feeling of “giving way,” pain in the medial gutter of the ankle and a valgus and pronation deformity of the foot can be corrected by activating the peroneus tertius muscle. Arthroscopy is the most specific way to confirm clinically suspected instability of the medial ankle; however, MRI can demonstrate loss of organized medial fibers (Figures 3A, 3B).18 Primary surgical repair of deltoid ligament tears yield good to excellent results and should be considered in the soccer player to prevent problems associated with chronic non-repaired tears such as instability, osteoarthritis, and impingement syndromes.18 After surgical repair, players will undergo extensive physical therapy that progresses to sport-specific exercises with the ultimate goal of returning to competitive play around 4-6 months post-operatively.

HIGH ANKLE SPRAINS (SYNDESMOSIS)
High ankle sprains are much less common than low ankle sprains; however, when they do occur they portend a lengthier rehabilitation and a poorer prognosis, especially if undiagnosed. Lubberts and colleagues42 studied the epidemiology of isolated syndesmotic injuries in professional football players. They pooled data from 15 consecutive seasons of European professional football between 2001 and 2016. They examined a total of 3677 players from 61 teams across 17 countries. There were 1320 ankle ligament injuries registered during 15 seasons, of which 94 (7%) were isolated syndesmotic injuries. The incidence of these injuries increased annually between 2001 and 2016. Injuries were 74% contact-related, and isolated syndesmotic injuries were followed by a mean of a 39-day absence.42 Moreover, football players may have an increased risk of syndesmotic sprains due to foot planting and cutting action.41
Continue to: These injuriesa are typically...
These injuries are typically identified with pain over the AITFL and interosseous membrane. Physical examination tests that help identify syndesmotic injuries include the squeeze test, external rotation test, and crossed-leg test.41 The diagnosis can be made on plain X-ray when there is clear diastasis between the distal tibia and fibula. Two critical measurements on plain films are made 1 cm above the tibial plafond and are used to evaluate the integrity of the syndesmosis: tibiofibular clear space >6 mm, and tibiofibular overlap <1 mm, which indicate disruption of the syndesmosis.43 More subtle injuries can be diagnosed with better sensitivity and specificity using MRI, which can also reveal secondary findings such as bone bruises, ATFL injury, osteochondral lesions, and tibiofibular incongruity.44,45 Arthroscopy is an invaluable diagnostic tool for syndesmotic injuries with a characteristic triad finding of PITFL scarring, disrupted interosseous ligament, and posterolateral tibial plafond chondral damage.46
Classification of the ligaments involved can aid in the selection of appropriate treatment. Grade I injuries involve AITFL tears. Grade IIa injuries involve AITFL and IOL tears. Grade IIb injuries include AITFL, PITFL, and IOL tears. Grade III injuries involve injury to all 3 ligaments, as well as a fibular fracture. Conservative treatment is recommended for Grades I and IIa, while surgical intervention is necessary for Grades IIb and III (Figures 4A, 4B). Compared with other ankle sprains, syndesmotic injuries typically require a more prolonged recovery/rehabilitation. Some studies suggest that these injuries require twice as long to heal.47 Hopkinson and colleagues48 reported a mean recovery time of 55 days following syndesmotic injuries in cadets at the United States Military Academy at West Point. Some surgeons advocate surgical intervention in professional athletes with mild sprains to expedite return to play.49
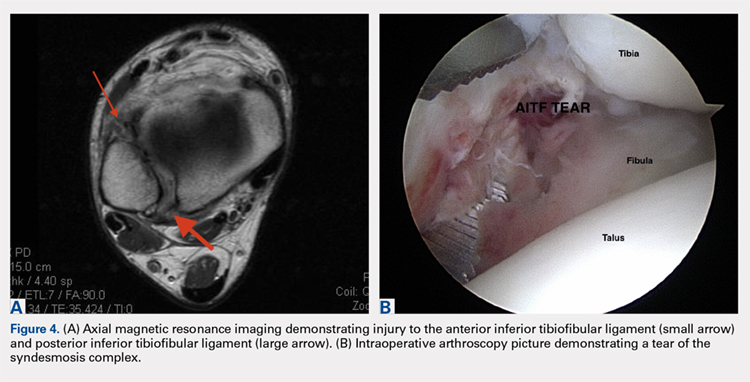
Surgery has been well established as necessary in more severe injuries where there is clear diastasis or instability of the syndesmosis. Traditionally, screws were used for surgical fixation; however, they often required a second surgery for subsequent removal. There is no general consensus on the optimal screw size, level of placement, or timing of removal.50,51 More recently, non-absorbable suture button fixation (eg, TightRope; Arthrex) has become more popular and provides certain advantages over screw fixation, such as avoiding the need for hardware removal. TightRope has been shown to provide more accurate stabilization of the syndesmosis as compared with screw fixation.52 Since malreduction is the most important indicator of poor long-term functional outcome, suture button fixation should be considered in the treatment of the football player.53 Finally, Colcuc and colleagues54 reported a lower complication rate and earlier return to sports in patients treated with knotless suture button devices compared with screw fixation.
OSTEOCHONDRAL LESIONS
Osteochondral lesions (OCLs) are cartilage-bone defects that are usually located in the talus. They can be caused by an acute traumatic event or repetitive microtrauma with no apparent history of trauma (eg, ankle instability). Acute OCLs can occur in soccer secondary to an ankle sprain or ankle fracture. Symptoms of OCLs include pain, swelling, and mechanical symptoms such as catching or locking, and on physical examination, one might see an effusion. The initial imaging modality of choice is radiographing; however, in ankle sprains with continued pain and swelling MRI may be indicated to rule out an underlying OCL. Missed acute lesions have a tendency not to heal and become chronic lesions, which can cause pain and playing disability. It is well established that chronic ankle instability is an important etiologic factor for OCLs. With the normal hydrostatic pressure within the ankle joint, synovial fluid gets pushed into cartilage/bone fissures, which can then lead to cystic degeneration of the subchondral bone.55-57
Surgical repair of an OCL is dependent on both the size and location of the lesion. Acute lesions can be managed by arthroscopic débridement, microfracture, or fixation of the lesion if enough bone remains attached to the chondral lesion. Return to play is based on development and maturation of fibrocartilage over the lesion (debridement/microfracture) or healing and incorporation of the new graft (chondral repair procedures). Meanwhile, chronic lesions can be managed in 1-stage (microfracture, osteochondral autograft transfer or 2-stage (autologous chondrocyte implantation [ACI]) procedures.56-57 Additional biologic healing augmentation with platelet-rich plasma has been described as well.58 Newer techniques in treating chronic talus OCLs, including ones that have failed to respond to bone marrow stimulation techniques, have been developed more recently such as the use of particulated juvenile articular cartilage allograft (DeNovo NT Natural Tissue Graft®; Zimmer Biomet).59 These newer techniques avoid the need for a 2-stage procedure, as is the case with ACI.
Continue to: Further studies are needed...
Further studies are needed to both investigate long-term outcomes and determine the superiority of the arthroscopic juvenile cartilage procedure compared with microfracture and other cartilage resurfacing procedures. When surgically treating OCLs, one must also restore normal ankle joint biomechanics for the lesion to heal. For instance, in the presence of ankle instability, ligament reconstruction must be performed. Also, one should also consider addressing any hindfoot malalignment with an osteotomy (calcaneus, supramalleolar). In a recent retrospective study, van Eekeren and colleagues60 showed that approximately 76% of patients were able to return to sports at long-term follow-up after arthroscopic débridement and bone marrow stimulation of talar OCLs. However, the activity level decreased at long-term follow-up and never attained the pre-injury level.60
ANKLE IMPINGEMENT
ANTERIOR ANKLE IMPINGEMENT (FOOTBALLER'S ANKLE)
Anterior ankle impingement is caused by anterior osteophytes on both the distal tibia and talar neck. It is thought to be related to repetitive microtrauma to the anteromedial aspect of the ankle from recurrent ball impact.61 It is very common amongst soccer players with some studies suggesting that 60% of soccer players have this syndrome. Ankle impingement is characterized by anterior pain with ankle dorsiflexion, decreased dorsiflexion, and swelling. It is primarily diagnosed with lateral ankle X-rays, which will show the osteophytes. An oblique anteromedial X-ray may increase detection of osteophytes (Figure 5). The early stages of anterior impingement can be treated successfully with injections and heel lifts. Treatment of lesions that fail to respond to conservative management involves arthroscopic or open excision of osteophytes. Most patients with no preexisting osteoarthritis treated arthroscopically will experience pain relief and return to full activity, though recurrent osteophyte formation has been noted at long-term follow-up.62
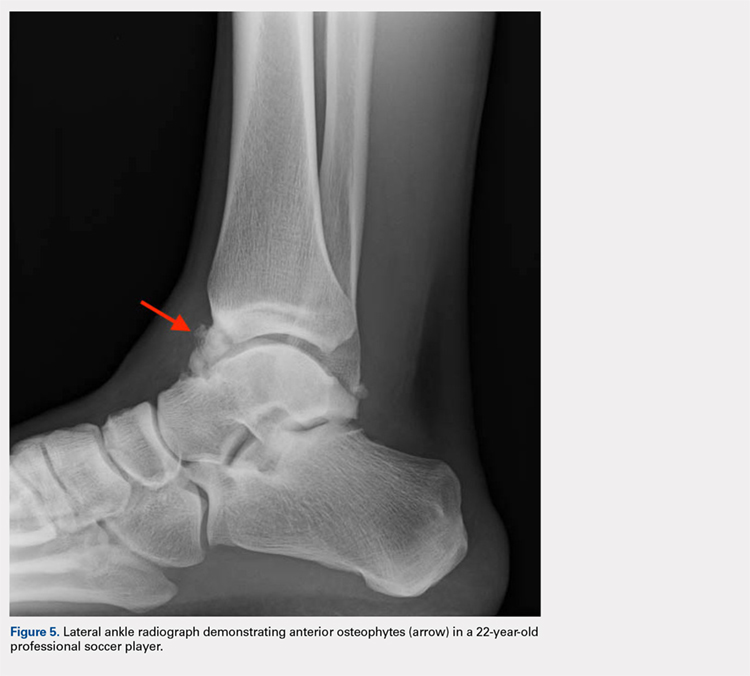
Anterior ankle impingement is most often caused by acute ankle sprains with an inversion type of mechanism.62 The subsequent reactive inflammation can cause fibrosis leading to distal fascicle enlargement of the AITFL. Impingement in the anterolateral gutter of this enlarged fascicle can also cause both chronic reactive synovitis and chondromalacia of the lateral talar dome.63 MRI can identify abnormal areas of pathology; however, 50% of cases are diagnosed based on clinical examination alone.63 Patients generally present with a history of anterolateral ankle pain and swelling with an occasional popping or snapping sensation.
Soccer players commonly develop anterior bony impingement due to repetitive loading of the anterior ankle from striking the ball. This repetition can lead to osteophyte formation of the anterior distal tibia and talar neck. After the osteophytes form, decreased dorsiflexion can occur due to a mechanical stop and inflammation of the interposed capsule.
The patient will exhibit tenderness to palpation along the anterolateral aspect of the ankle, with pain elicited at extreme passive dorsiflexion.62 Initially, an injection with local anesthetic and corticosteroid can serve both a diagnostic and therapeutic purpose; however, patients who fail conservative treatment can be treated with arthroscopy and resection of the involved scar tissue and osteophytes. The best results are seen in those patients with no concurrent intra-articular lesions or ankle osteoarthritis (Figure 5).62 When treated non-operatively, a player may return to play when pain resolves; however, if treated surgically with arthroscopic debridement/resection, a player must wait until his surgical scars have healed prior to attempting return to play.
Continue to: ANTEROMEDIAL ANKLE IMPINGEMENT
ANTEROMEDIAL ANKLE IMPINGEMENT
Anteromedial ankle impingement is a less common ankle impingement syndrome. It is associated with eversion injuries or following medial malleolar or talar fractures.64,65 Previous injury to the anterior tibiotalar fascicle of the deltoid complex leads to ligament thickening and subsequent impingement in the anteromedial corner of the talus. Adjacent fibrosis and synovitis are common consequences of impingement; however, osteophyte formation and chondral stripping along the anteromedial talus can also be seen. Patients typically complain of pain along the anteromedial joint line that is worse with activity, clicking or popping sensations, and painful, limited dorsiflexion. On examination, impingement can be detected through palpation over the anterior tibiotalar fascicle of the deltoid ligament and eversion or extreme passive dorsiflexion of the foot, all of which will elicit medial ankle tenderness.17,62 Initial treatment consists of rest, physical therapy, and NSAIDs. Refractory cases may be amenable to arthroscopic or open resection of the anterior tibiotalar fascicle with débridement of any adjacent synovitis and scar tissue.62
POSTERIOR ANKLE IMPINGEMENT
Posterior ankle impingement is often referred to as “os trigonum syndrome” since the posterior impingement is frequently associated with a prominent os trigonum. An os trigonum is an accessory ossicle representing the separated posterolateral tubercle of the talus. It is usually asymptomatic. However, in soccer players, pain can occur from impaction between the posterior tibial plafond and the os trigonum, or because of soft tissue compression between the 2 opposing osseous structures. The pain is due to repetitive microtrauma (ankle plantarflexion) or acute forced plantarflexion, which can present as an acute fracture of the os trigonum. Because soccer is a sport requiring both repetitive and extreme plantarflexion, it may predispose players to posterior ankle impingement (Figures 6A, 6B).62,66
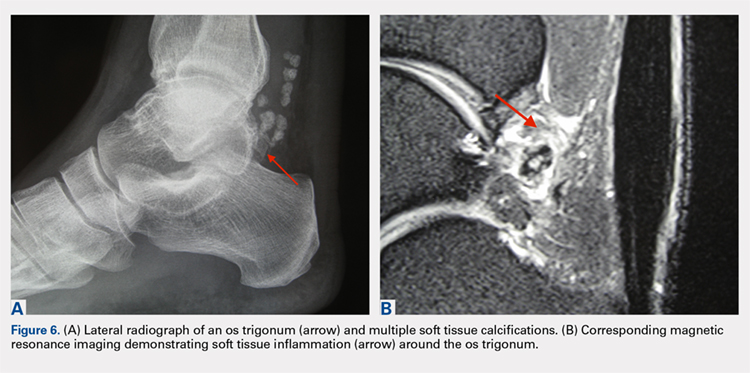
Clinically, it can be very difficult to detect and diagnose because the affected structures lie deep and it can coexist with other disease processes (eg, peroneal tendinopathy, Achilles tendinopathy).62,66 Patients will complain of chronic deep posterior ankle pain that is worse with push-off activities (eg, jumping). On examination, patients will exhibit pain with palpation over the posterolateral process and with the crunch test. Lateral radiograph with the foot in plantar flexion will show the os trigonum impinged between the posterior tibial malleolus and the calcaneal tuberosity. An MRI will demonstrate the os trigonum as well as any associated inflammation and edema, while it can also demonstrate coexisting pathologies.
Initial treatment consists of rest, NSAIDs, and taping to prevent plantar flexion. Ultrasound-guided cortisone injection of the capsule and posterior bursa can be both therapeutic and diagnostic. A posterior injection can be used to temporize the symptoms so that the soccer player can make it through the season.
Surgical excision is saved for refractory cases, and this can be done either through an open posterolateral approach or arthroscopic techniques. Recently, Georgiannos and Bisbinas67 showed in an athletic population that endoscopic excision had both a lower complication rate and a quicker return to sports compared with the traditional open approach. Carreira and colleagues68 conducted a retrospective case series of 20 patients (mostly competitive athletes). They found that posterior ankle arthroscopy to address posterior impingement allowed for the maintenance or restoration of anatomic ROM of the ankle and hindfoot, ability to return to at least the previous level of activity, and improvement in objective assessment of pain relief and a higher level of function parameters.68
Continue to: TENDON PATHOLOGY
TENDON PATHOLOGY
SUPERIOR PERONEAL RETINACULUM INJURY
The superior peroneal retinaculum (SPR) forms the roof of the superior peroneal tunnel. The tunnel contains the peroneus brevis and longus tendons and is bordered by the retromalleolar groove of the fibula and the lower aspect of the posterior intramuscular septum of the leg.69,70 The SPR originates from the posterolateral ridge of the fibula and inserts onto the lateral calcaneus, and it is the primary restraint of the peroneal tendons within the retromalleolar sulcus.
Injury to the retinaculum results from both ankle dorsiflexion and inversion, and forceful reflex contraction of the peroneal muscles, which causes subluxation or dislocation of the contained tendons.69 A high level of suspicion is required regarding these injuries since the mechanism of injury is similar to that of a simple lateral ankle sprain. In the setting of retrofibular pain, snapping or popping sensations around the lateral malleolus, or chronic ankle instability that worsens on uneven surfaces, one must consider an injury to the SPR.69 Radiographs are not always diagnostic; however, occasionally on an internal rotation view, one may see a cortical avulsion off the distal tip of the lateral malleolus (“fleck sign”) indicating a rim fracture from an SPR injury (Figure 7). MRI is the best imaging modality to assess the peroneal tendons, as well as an SPR injury. Recently, ultrasound has grown in popularity and may be more useful, since it allows for dynamic evaluation of subluxating/dislocating tendons.69

Conservative management is often associated with poor outcomes, and surgery is indicated for all acute and chronic dislocations in athletes.71 Anatomic reconstruction of the SPR is the preferred surgical method.72 Peroneus brevis debulking and fibular groove deepening may also augment the retinaculum repair.73 van Dijk and colleagues in their systematic review showed that patients treated with both groove deepening and SPR repair have higher rates of return to the sport than patients treated with SPR repair alone.74
STRESS FRACTURES
FIFTH METATARSAL
Fifth metatarsal stress fractures usually occur secondary to lateral overload or avulsion of the peroneus brevis. The fifth metatarsal base can be susceptible to injury in a cavovarus foot. Non-operative treatment typically requires a longer period of immobilization (boot or cast) and necessitates a longer period of non–weight-bearing (anywhere between 6-12 weeks). Therefore, surgery is typically recommended in athletes or in the setting of a recurrent base of the fifth metatarsal fracture to expedite healing and return to play. Return to play is still not recommended until there is evidence of radiographic healing of the fracture. There are certain distinctions with fifth metatarsal stress fractures regarding location and healing rates that need to be taken into account.75,76 In particular, zone 2 injuries (Jones fractures) represent a vascular watershed area, making these fractures prone to nonunion with nonunion rates as high as 15% to 30%. Occasionally, the cavovarus deformity will require correction as well as a reduction in the risk of recurrence or nonunion. Surgical fixation most commonly consists of a single screw placed in an antegrade fashion.77 One must pay attention to screw size since smaller diameter screws (<4.5 mm) are associated with delayed union or nonunion. Moreover, screws that are too long will straighten the curved metatarsal shaft and can lead to fracture distraction or malreduction (Figures 8A, 8B).77
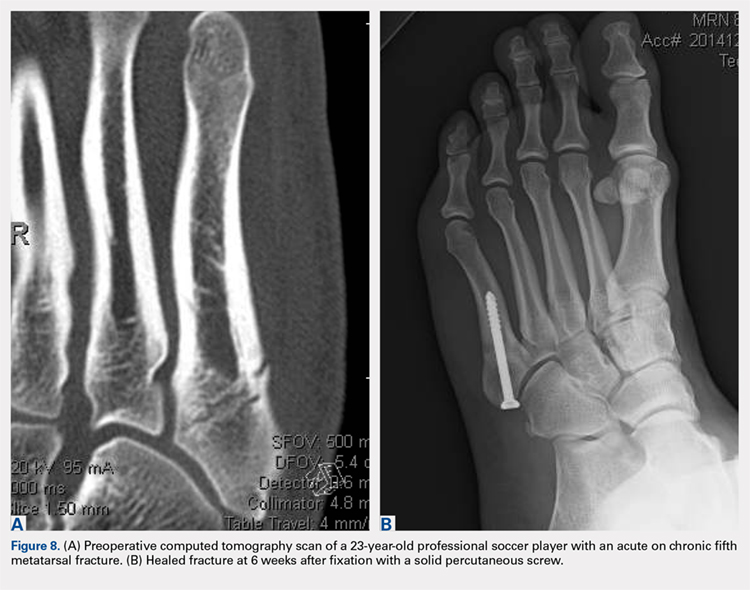
Patients have returned to competitive sports within 6 weeks; however, it should be noted that causes of failure were linked to early return and return to sports before a radiographic union can lead to failure of fixation. Ekstrand and van Dijk78 studied a large group of professional soccer players and found that out of 13,754 injuries, 0.5% (67) were fifth metatarsal fractures. Of note, they found that 45% of players had prodromal symptoms. Furthermore, after surgical treatment the fractures healed faster, compared with conservative treatment (75% vs 33%); however, there was no significant difference in lay-off days between both groups (80 vs 74 days).78 Matsuda and colleagues79 looked at 335 male collegiate soccer players, 29 of whom had a history of a fifth metatarsal stress fracture. They found that playing the midfield position and having an everted rearfoot and inverted forefoot alignment were associated with fifth metatarsal stress fractures.79 Saita and colleagues80 found that restricted hip internal rotation was associated with an increased risk of developing a Jones fracture in 162 professional football players. Finally, Fujitaka and colleagues81 looked at 273 male soccer players between 2005 and 2013. They found an association between weak toe-grip strength and fifth metatarsal fractures, suggesting that weak toe-grip may lead to an increase in the load applied onto the lateral side of the foot, resulting in a stress fracture.81
Continue to: NAVICULAR
NAVICULAR
Another common tarsal bone that sustains stress fractures is the navicular. It is not as common as calcaneal stress fractures in military recruits but can occur in the same type of population, as well as explosive athletics such as sprinters and soccer players. It commonly presents with an indistinct vague achy pain with activity that improves with rest, and pain at the dorsum of the midfoot or along the medial longitudinal arch with activity. It can easily go undiagnosed for quite some time given the difficulty in visualizing the navicular with plain radiographs. Clinically, it is difficult to make the diagnosis, and therefore advanced imaging is necessary when the injury is suspected. Both MRI and CT scans can be used to understand the extent of the injury (Figures 9A-9C). In non-displaced stress fractures, conservative non-operative treatment is the appropriate treatment modality with a brief period of immobilization and non–weight-bearing;82 however, operative treatment is also considered in elite athletes. In either case, return to play is discouraged until there is evidence of radiographic healing. When a displacement is noted, or there is a delay in diagnosis, then operative treatment is recommended.
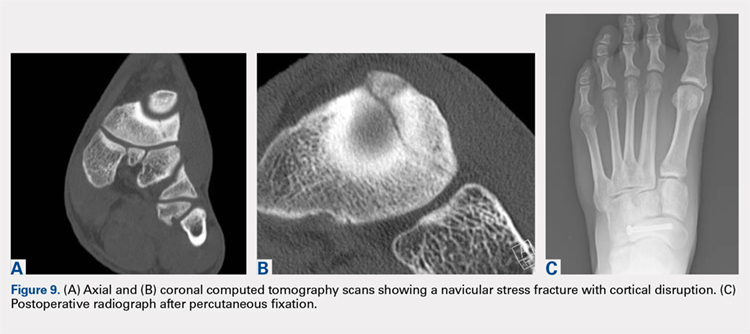
CONCLUSION
Ankle injuries are very common in soccer and can result in decreased performance or significant loss of playing time. Treatment of acute injury generally follows a conservative route, with surgical intervention reserved for severe ruptures or osteochondral fracture of the ankle joint. Chronic ankle pathology resulting in mechanical or functional instability generally requires surgery to repair ligamentous damage and restore normal ankle kinematics. It is critical for the soccer player to receive appropriate rehabilitation prior to returning to play in order to reduce the risk for reinjury and further chronic instability. Prevention and early intervention of ankle injuries is key in preventing the long-term sequelae of ankle injuries, such as arthritis, in former soccer players.
1. Dvorak J, Junge A. Football injuries and physical symptoms. A review of the literature. Am J Sports Med. 2000;28(5 Suppl):S3-S9.
2. Chomiak J, Junge A, Peterson L, Dvorak J. Severe injuries in football players. Am J Sports Med. 2000;28(5 Suppl):S58-S68.
3. Cloke DJ, Ansell P, Avery P, Deehan D. Ankle injuries in football academies: a three-centre prospective study. Br J Sports Med. 2011;45(9):702-708. doi:10.1136/bjsm.2009.067900.
4. Cloke DJ, Spencer S, Hodson A, Deehan D. The epidemiology of ankle injuries occurring in English Football Association academies. Br J Sports Med. 2009;43(14):1119-1125. doi:10.1136/bjsm.2008.052050.
5. Dvorak J, Junge A, Derman W, Schwellnus M. Injuries and illnesses of football players during the 2010 FIFA World Cup. Br J Sports Med. 2011;45(8):626-630. doi:10.1136/bjsm.2010.079905.
6. Ekstrand J, Gillquist J. Soccer injuries and their mechanisms: a prospective study. Med Sci Sports Exerc. 1983;15(3):267-270.
7. Fousekis K, Tsepis E, Vagenas G. Intrinsic risk factors of noncontact ankle sprains in soccer: a prospective study on 100 professional players. Am J Sports Med. 2012;40(8):1842-1850. doi:10.1177/0363546512449602.
8. Gaulrapp H, Becker A, Walther M, Hess H. Injuries in women’s soccer: a 1-year all players prospective field study of the women’s Bundesliga (German premiere league). Clin J Sports Med. 2010;20(4):264-271. doi:10.1097/JSM.0b013e3181e78e33.
9. Morgan BE, Oberlander MA. An examination of injuries in major league soccer. The inaugural season. Am J Sports Med. 2001;29(4):426-430. doi:10.1177/03635465010290040701.
10. Jain N, Murray D, Kemp S, Calder J. Frequency and trends in foot and ankle injuries within an English Premier League Football Club using a new impact factor of injury to identify a focus for injury prevention. Foot Ankle Surg. 2014;20(4):237-240. doi:10.1016/j.fas.2014.05.004.
11. Moore KL, Dalley AF, Agur AMR. Clinically Oriented Anatomy. 6th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins, 2010:xxix, 1134.
12. Thompson JC, Netter FH. Netter’s Concise Orthopaedic Anatomy. 2nd ed. Philadelphia, PA: Saunders Elsevier, 2010:x, 404.
13. Giza E, Mandelbaum B. Chronic footballer’s ankle. In: Football Traumatology. Springer Milan, 2006:333-351.
14. Garrick JG. The frequency of injury, mechanism of injury, and epidemiology of ankle sprains. Am J Sports Med. 1977:5(6):241-242. doi:10.1177/036354657700500606.
15. Agur AMR, Grant JCB. Grant’s Atlas of Anatomy. 13th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins. 2011.
16. Renstrom PA, Konradsen L. Ankle ligament injuries. Br J Sports Med. 1997;31(1):11-20.
17. Chhabra A, Subhawong TK, Carrino JA. MR imaging of deltoid ligament pathologic findings and associated impingement syndromes. Radiographics. 2010;30(3):751-761. doi:10.1148/rg.303095756.
18. Hintermann B. Medial ankle instability. Foot Ankle Clin. 2003;8(4):723-738.
19. Woods C, Hawkins R, Hulse M, Hodson A. The Football Association Medical Research Programme: an audit of injuries in professional football: an analysis of ankle sprains. Br J Sports Med. 2003;37(3):233-238.
20. Thacker SB, Stroup DF, Branche CM, Gilchrist J, Goodman RA, Weitman EA. The prevention of ankle sprains in sports. A systematic review of the literature. Am J Sports Med. 1999;27(6):753-760. doi:10.1177/03635465990270061201.
21. Giza E, Fuller C, Junge A, Dvorak J. Mechanisms of foot and ankle injuries in soccer. Am J Sports Med. 2003;31(4):550-554. doi:10.1177/03635465030310041201.
22. Tucker AM. Common soccer injuries. Diagnosis, treatment and rehabilitation. Sports Med. 1997;23(1):21-32.
23. Lynch SA, Renstrom PA. Treatment of acute lateral ankle ligament rupture in the athlete. Conservative versus surgical treatment. Sports Med. 1999;27(1):61-71.
24. Chan KW, Ding BC, Mroczek KJ. Acute and chronic lateral ankle instability in the athlete. Bull NYU Hosp Jt Dis. 2011;69(1):17-26.
25. Stiell IG, Greenberg GH, McKnight RD, Nair RC, McDowell I, Worthington JR. A study to develop clinical decision rules for the use of radiography in acute ankle injuries. Ann Emerg Med. 1992;21(4):384-390.
26. Bachmann LM, Kolb E, Koller MT, Steurer J, ter Riet G. Accuracy of Ottawa ankle rules to exclude fractures of the ankle and mid-foot: systematic review. BMJ. 2003;326(7386):417. doi:10.1136/bmj.326.7386.417.
27. Balduini FC, Vegso JJ, Torg JS, Torg E. Management and rehabilitation of ligamentous injuries to the ankle. Sports Med. 1987;4(5):364-380.
28. Kerkhoffs GM, Rowe BH, Assendelft WJ, Kelly KD, Struijs PA, van Dijk CN. Immobilisation for acute ankle sprain. A systematic review. Arch Orthop Trauma Surg. 2001;121(8):462-471.
29. Konradsen L, Holmer P, Sondergaard L. Early mobilizing treatment for grade III ankle ligament injuries. Foot Ankle. 1991;12(2):69-73.
30. Eiff MP, Smith AT, Smith GE. Early mobilization versus immobilization in the treatment of lateral ankle sprains. Am J Sports Med. 1994;22(1):83-88. doi:10.1177/036354659402200115.
31. Shrier I. Treatment of lateral collateral ligament sprains of the ankle: a critical appraisal of the literature. Clin J Sport Med. 1995;5(3):187-195.
32. DiGiovanni BF, Partal G, Baumhauer JF. Acute ankle injury and chronic lateral instability in the athlete. Clin Sports Med. 2004;23(1):1-19, v. doi:10.1016/S0278-5919(03)00095-4.
33. Alt W, Lohrer H, Gollhofer A. Functional properties of adhesive ankle taping: neuromuscular and mechanical effects before and after exercise. Foot Ankle Int. 1999;20(4):238-245. doi:10.1177/107110079902000406.
34. Garrick JG, Requa RK. Role of external support in the prevention of ankle sprains. Med Sci Sports. 1973;5(3):200-203.
35. Sharpe SR, Knapik J, Jones B. Ankle braces effectively reduce recurrence of ankle sprains in female soccer players. J Athl Train. 1997;32(1):21-24.
36. Surve I, Schwellnus MP, Noakes T, Lombard C. A fivefold reduction in the incidence of recurrent ankle sprains in soccer players using the Sport-Stirrup orthosis. Am J Sports Med. 1994;22(5):601-606. doi:10.1177/036354659402200506.
37. Mohammadi F. Comparison of 3 preventive methods to reduce the recurrence of ankle inversion sprains in male soccer players. Am J Sports Med. 2007;35(6):922-926. doi:10.1177/0363546507299259.
38. Steffen K, Meeuwisse WH, Romiti M, et al. Evaluation of how different implementation strategies of an injury prevention programme (FIFA 11+) impact team adherence and injury risk in Canadian female youth football players: a cluster-randomised trial. Br J Sports Med. 2013;47(8):480-487. doi:10.1136/bjsports-2012-091887.
39. Steffen K, Emery CA, Romiti M, et al. High adherence to a neuromuscular injury prevention programme (FIFA 11+) improves functional balance and reduces injury risk in Canadian youth female football players: a cluster randomised trial. Br J Sports Med. 2013;47(12):794-802. doi: 10.1136/bjsports-2012-091886.
40. Junge A, Lamprecht M, Stamm H, et al. Countrywide campaign to prevent soccer injuries in Swiss amateur players. Am J Sports Med. 2011;39(1):57-63. doi:10.1177/0363546510377424.
41. Lin CF, Gross ML, Weinhold P. Ankle syndesmosis injuries: anatomy, biomechanics, mechanism of injury, and clinical guidelines for diagnosis and intervention. J Orthop Sports Phys Ther. 2006;36(6):372-384. doi:10.2519/jospt.2006.2195.
42. Lubberts B, D’Hooghe P, Bengtsson H, DiGiovanni CW, Calder J, Ekstrand J. Epidemiology and return to play following isolated syndesmotic injuries of the ankle: a prospective cohort study of 3677 male professional football players in the UEFA Elite Club Injury Study. Br J Sports Med. 2017. doi:10.1136/bjsports-2017-097710.
43. Harper MC, Keller TS. A radiographic evaluation of the tibiofibular syndesmosis. Foot Ankle. 1989;10(3):156-160.
44. Vogl TJ, Hochmuth K, Diebold T, et al. Magnetic resonance imaging in the diagnosis of acute injured distal tibiofibular syndesmosis. Invest Radiol. 1997;32(7):401-409.
45. Brown KW, Morrison WB, Schweitzer ME, Parellada JA, Nothnagel H. MRI findings associated with distal tibiofibular syndesmosis injury. AJR Am J Roentgenol. 2004;182(1):131-136. doi:10.2214/ajr.182.1.1820131.
46. Ogilvie-Harris DJ, Reed SC, Hedman TP. Disruption of the ankle syndesmosis: biomechanical study of the ligamentous restraints. Arthroscopy. 1994;10(5):558-560.
47. Boytim MJ, Fischer DA, Neumann L. Syndesmotic ankle sprains. Am J Sports Med. 1991;19(3):294-298. doi:10.1177/036354659101900315.
48. Hopkinson WJ, St Pierre P, Ryan JB, Wheeler JH. Syndesmosis sprains of the ankle. Foot Ankle. 1990;10(6):325-330. doi:10.1177/107110079001000607.
49. Del Buono A, Florio A, Boccanera MS, Maffulli N. Syndesmosis injuries of the ankle. Curr Rev Musculoskelet Med. 2013;6(4):313-319. doi:10.1007/s12178-013-9183-x.
50. Dattani R, Patnaik S, Kantak A, Srikanth B, Selvan TP. Injuries to the tibiofibular syndesmosis. J Bone Joint Surg Br. 2008;90(4):405-410. doi:10.1302/0301-620X.90B4.19750.
51. Schepers T. To retain or remove the syndesmotic screw: a review of literature. Arch Orthop Trauma Surg. 2011;131(7):879-883. doi:10.1007/s00402-010-1225-x.
52. Naqvi GA, Cunningham P, Lynch B, Galvin R, Awan N. Fixation of ankle syndesmotic injuries: comparison of tightrope fixation and syndesmotic screw fixation for accuracy of syndesmotic reduction. Am J Sports Med. 2012;40(12):2828-2835. doi:10.1177/0363546512461480.
53. Weening B, Bhandari M. Predictors of functional outcome following transsyndesmotic screw fixation of ankle fractures. J Orthop Trauma. 2005;19(2):102-108.
54. Colcuc C, Blank M, Stein T, et al. Lower complication rate and faster return to sports in patients with acute syndesmotic rupture treated with a new knotless suture button device. Knee Surg Sports Traumatol Arthrosc. 2017. doi:10.1007/s00167-017-4820-4823.
55. Savage-Elliott I, Ross KA, Smyth NA, Murawski CD, Kennedy JG. Osteochondral lesions of the talus: a current concepts review and evidence-based treatment paradigm. Foot Ankle Spec. 2014;7(5):414-422. doi:10.1177/1938640014543362.
56. Talusan PG, Milewski MD, Toy JO, Wall EJ. Osteochondritis dissecans of the talus: diagnosis and treatment in athletes. Clin Sports Med. 2014;33(2):267-284. doi:10.1016/j.csm.2014.01.003.
57. Murawski CD, Kennedy JG. Operative treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 2013;95(11):1045-1054. doi:10.2106/JBJS.L.00773.
58. Guney A, Akar M, Karaman I, Oner M, Guney B. Clinical outcomes of platelet rich plasma (PRP) as an adjunct to microfracture surgery in osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2384-2389. doi:10.1007/s00167-013-2784-5.
59. Hatic SO, Berlet GC. Particulated juvenile articular cartilage graft (DeNovo NT Graft) for treatment of osteochondral lesions of the talus. Foot Ankle Spec. 2010;3(6):361-364. doi:10.1177/1938640010388602.
60. van Eekeren IC, van Bergen CJ, Sierevelt IN, Reilingh ML, van Dijk CN. Return to sports after arthroscopic debridement and bone marrow stimulation of osteochondral talar defects: a 5- to 24-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1311-1315. doi:10.1007/s00167-016-3992-6.
61. Tol JL, Slim E, van Soest AJ, van Dijk CN. The relationship of the kicking action in soccer and anterior ankle impingement syndrome. A biomechanical analysis. Am J Sports Med. 2002;30(1):45-50. doi:10.1177/03635465020300012101.
62. Sanders TG, Rathur SK. Impingement syndromes of the ankle. Magn Reson Imaging Clin N Am. 2008;16(1):29-38. doi:10.1016/j.mric.2008.02.005.
63. Ogilvie-Harris DJ, Gilbart MK, Chorney K. Chronic pain following ankle sprains in athletes: the role of arthroscopic surgery. Arthroscopy. 1997;13(5):564-574.
64. Robinson P, White LM, Salonen D, Ogilvie-Harris D. Anteromedial impingement of the ankle: using MR arthrography to assess the anteromedial recess. AJR Am J Roentgenol. 2002;178(3):601-604. doi:10.2214/ajr.178.3.1780601.
65. Mosier-La Clair SM, Monroe MT, Manoli A. Medial impingement syndrome of the anterior tibiotalar fascicle of the deltoid ligament on the talus. Foot Ankle Int. 2000;21(5):385-391.
66. Maquirriain J. Posterior ankle impingement syndrome. J Am Acad Orthop Surg. 2005;13(6):365-371.
67. Georgiannos D, Bisbinas I. Endoscopic versus open excision of os trigonum for the treatment of posterior ankle impingement syndrome in an athletic population: a randomized controlled study with 5-year follow-up. Am J Sports Med. 2017;45(6):1388-1394. doi:10.1177/0363546516682498.
68. Carreira DS, Vora AM, Hearne KL, Kozy J. Outcome of arthroscopic treatment of posterior impingement of the ankle. Foot Ankle Int. 2016;37(4):394-400. doi:10.1177/1071100715620857.
69. Roth JA, Taylor WC, Whalen J. Peroneal tendon subluxation: the other lateral ankle injury. Br J Sports Med. 2010;44(14):1047-1053. doi:10.1136/bjsm.2008.057182.
70. Athavale SA, Swathi, Vangara SV. Anatomy of the superior peroneal tunnel. J Bone Joint Surg Am. 2011;93(6):564-571. doi:10.2106/JBJS.17.00836.
71. Porter D, McCarroll J, Knapp E, Torma J. Peroneal tendon subluxation in athletes: fibular groove deepening and retinacular reconstruction. Foot Ankle Int. 2005;26(6):436-441.
72. Ferran NA, Oliva F, Maffulli N. Recurrent subluxation of the peroneal tendons. Sports Med. 2006;36(10):839-846. doi:10.1053/j.jfas.2010.02.007.
73. Saxena A, Ewen B. Peroneal subluxation: surgical results in 31 athletic patients. J Foot Ankle Surg. 2010;49(3):238-241.
74. van Dijk PA, Gianakos AL, Kerkhoffs GM, Kennedy JG. Return to sports and clinical outcomes in patients treated for peroneal tendon dislocation: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1155-1164. doi:10.1007/s00167-015-3833-z.
75. Lee KT, Park YU, Young KW, Kim JS, Kim JB. The plantar gap: another prognostic factor for fifth metatarsal stress fracture. Am J Sports Med. 2011;39(10):2206-2211. doi:10.1177/0363546511414856.
76. Torg JS. Fractures of the base of the fifth metatarsal distal to the tuberosity. Orthopedics. 1990;13:731-737.
77. Smith TO, Clark A, Hing CB. Interventions for treating proximal fifth metatarsal fractures in adults: a meta-analysis of the current evidence-base. Foot Ankle Surg. 2011;17(4):300-307. doi:10.1016/j.fas.2010.12.005.
78. Ekstrand J, van Dijk CN. Fifth metatarsal fractures among male professional footballers: a potential career-ending disease. Br J Sports Med. 2013;47(12):754-758.
79. Matsuda S, Fukubayashi T, Hirose N. Characteristics of the foot static alignment and the plantar pressure associated with fifth metatarsal stress fracture history in male soccer players: a case-control study. Sports Med Open. 2017;3(1):27.
80. Saita Y, Nagao M, Kawasaki T, et al. Range limitation in hip internal rotation and fifth metatarsal stress fractures (Jones fracture) in professional football players. Knee Surg Sports Traumatol Arthrosc. 2018;26(7):1943-1949. doi:10.1007/s00167-017-4552-4.
81. Fujitaka K, Taniguchi A, Isomoto S, et al. Pathogenesis of fifth metatarsal fractures in college soccer players. Orthop J Sports Med. 2015;18;3(9):2325967115603654.
82. Torg J, Moyer J, Gaughan J, Boden B. Management of tarsal navicular stress fractures: conservative versus surgical treatment: a meta-analysis. Am J Sports Med. 2010;38(5):1048-1053.
83. Haytmanek CT, Williams BT, James EW, et al. Radiographic identification of the primary lateral ankle structures. Am J Sports Med. 2015;43(1):79-87. doi:10.1177/0363546514553778.
ABSTRACT
The ankle is one of the most commonly injured joints in soccer and represents a significant cost to the healthcare system. The ligaments that stabilize the ankle joint determine its biomechanics—alterations of which result from various soccer-related injuries. Acute sprains are among the most common injury in soccer players and are generally treated conservatively, with emphasis placed on secondary prevention to reduce the risk for future sprains and progression to chronic ankle instability. Repetitive ankle injuries in soccer players may cause chronic ankle instability, which includes both mechanical ligamentous laxity and functional changes. Chronic ankle pathology often requires surgery to repair ligamentous damage and remove soft-tissue or osseous impingement. Proper initial treatment, rehabilitation, and secondary prevention of ankle injuries can limit the amount of time lost from play and avoid negative long-term sequelae (eg, osteochondral lesions, arthritis). On the other hand, high ankle sprains portend a poorer prognosis and a longer recovery. These injuries will typically require surgical stabilization. Impingement-like syndromes of the ankle can undergo an initial trial of conservative treatment; when this fails, however, soccer players respond favorably to arthroscopic debridement of the lesions causing impingement. Finally, other pathologies (eg, stress fractures) are highly encouraged to be treated with surgical stabilization in elite soccer players.
Continue to: EPIDEMIOLOGY
EPIDEMIOLOGY
With roughly 200,000 professional and around 240 million amateur soccer players, soccer has been recognized as the most popular sport worldwide. Nevertheless, given its rising popularity in society, one must also consider the increasing incidence of injuries as a result. Elite soccer players sustain between 10 and 35 injuries per 1000 competitive playing hours.1 Approximately 80% are traumatic, and 20% are overuse injuries.2 Soccer injuries are more frequent with increasing age of the participants, whereas the incidence of injuries in preadolescent players is low. The incidence of injuries has been found to be higher during competition when compared with practice/training sessions, with some studies showing that 59% of injuries occurred during games.2 Amateur or recreational soccer players sustain fewer injuries than professional soccer players, as one would expect, given both the higher intensity of training and match schedule in professionals.
The ankle is one of the most commonly injured joints in soccer, with some studies suggesting it comprises one-fifth of all injuries sustained during soccer, which is only second to those of the knee.2 Ankle sprains specifically are quite a common occurrence in soccer.3-9 A recent study of an English premier league club showed that over a 4-season period, 20% of injuries were of the foot and ankle with a mean return to sport time of 54 days.10 Of all foot and ankle related injuries, ankle sprains are the most common, followed by bruises/contusions, and tendon lesions. Fractures are very rare (1%) in soccer, but when they do occur they impart a much more extended recovery. During the 2010 Fédération Internationale de Football Association (FIFA) World Cup, ankle sprains were among the most common injuries and approximately half lead to players missing training or competitive matches.5
ANATOMY
Knowledge of the biomechanics of both the foot and ankle joints is essential to understand soccer injuries. The ankle joint (talocrural articulation) consists of the distal ends of the tibia and fibula, which form the mortise, and the superior aspect of the talar dome.11 As a hinge joint, the ankle provides 20° of dorsiflexion and 50° of plantar flexion,12 with stability provided by the lateral, medial, and superior ligamentous complexes. The superior articular surface of the talus is narrower posteriorly, which creates a looser fit within the mortise during plantar flexion.11 This decreased stability could help explain why the most common injury in soccer involves a plantar flexion mechanism.13,14 Inferiorly, the talus articulates with the calcaneus to form the subtalar joint. It is at this site that the majority of both foot inversion and eversion occurs. The transverse tarsal joints (Chopart’s joints) separate the hindfoot from the midfoot. Movement of this joint depends on the relative alignment of its 2 articulations: the talonavicular and calcaneocuboid joints. During foot eversion, these 2 joints are parallel to each other allowing supple motion and aiding in shock absorption during the heel strike phase of the gait cycle. With foot inversion, the joints become nonparallel and thus lock the transverse tarsal joints providing a rigid lever needed for push-off.11,12
LATERAL LIGAMENTS
The ankle joint is stabilized laterally by a ligament complex consisting of 3 individual ligaments, all originating from the lateral malleolus: the anterior talofibular ligament (ATFL), the posterior talofibular ligament (PTFL), and the calcaneofibular ligament (CFL) (Figure 1).11,12,15 The ATFL is the primary restraint to inversion in plantar flexion, and it helps resist anterolateral translation of the talus in the mortise. However, it is the weakest and therefore the most frequently injured of the lateral ligaments. The PTFL plays only a supplementary role in ankle stability when the lateral ligament complex is intact. It is under the greatest strain in ankle dorsiflexion and acts to limit posterior talar displacement within the mortise as well as talar external rotation.13,16 The CFL is the primary restraint to inversion in the neutral or dorsiflexed position. It restrains subtalar inversion, thereby limiting talar tilt within the mortise.

DELTOID LIGAMENT
The deltoid ligament complex consists of 6 continuous adjacent superficial and deep ligaments that function synergistically to resist valgus and pronation forces, as well as external rotation of the talus in the mortise.11-13,17 The superficial layer crosses both ankle and subtalar joints. It originates from the anterior colliculus and fans out to insert into the navicular, neck of the talus, sustentaculum tali, and posteromedial talar tubercle. The tibiocalcaneal (sustentaculum tali) portion is the strongest component in the superficial layer and resists calcaneal eversion. The deep layer crosses the ankle joint only. It functions as the primary stabilizer of the medial ankle and prevents both lateral displacement and external rotation of the talus. It originates from the inferior and posterior aspects of the medial malleolus and inserts on the medial and posteromedial aspects of the talus.12,17,18
Continue to: SYNDESMOSIS
SYNDESMOSIS
The ankle syndesmosis, or inferior tibiofibular joint, is the distal articulation between the tibia and fibula. The syndesmosis contributes to ankle mortise integrity through its firm fixation of the lateral malleolus against the lateral surface of the talus. Ligaments comprising the ankle syndesmosis include the anterior inferior tibiofibular ligament (AITFL), the posterior inferior tibiofibular ligament (PITFL), the inferior transverse ligament, and the interosseous ligament (IOL).12
ANKLE SPRAINS
Ankle sprains are the most common pathology encountered amongst soccer players, representing from one-half to two-thirds of all ankle related injuries. Most sprains occur outside of player contact.
LATERAL ANKLE SPRAINS AND INSTABILITY
Injury to the lateral ligaments of the ankle represents 77% to 91% of all ankle sprains in soccer.6,19 The greatest risk factor for an ankle sprain in a soccer player is a history of prior sprain.20 Other risk factors include increasing age, player-to-player contact, condition of the pitch, weight-bearing status of the injured limb at the time of injury, and joint instability or laxity.21,22
The evaluation of an ankle sprain to determine its severity is best done after the acute phase, approximately 4 to 7 days after the initial injury when both pain and swelling have subsided.23 The anterior drawer (ATFL instability) and talar tilt (CFL instability) tests are useful in evaluating ankle instability in the delayed or chronic setting; however, both have been shown to have limited sensitivity and significant variability amongst different examiners.24
Clinical examination will direct further diagnostic tests including X-rays, magnetic resonance imaging (MRI), and computed tomography (CT). The Ottawa ankle rules are generally helpful in determining whether plain X-rays are indicated in the acute setting.25,26 (Figure 2) According to these rules, ankle radiographs should be obtained if ankle pain is reported near the malleoli and 1 or more of the following is seen during examination: inability to bear weight immediately after injury and for 4 steps in the emergency department, and bony tenderness at the posterior edge or tip of the malleolus. Stress X-rays are generally not indicated in acute injuries but may be useful in chronic ankle instability cases.23

Continue to: Ankle sprains cover...
Ankle sprains cover a broad spectrum of injuries; therefore, a grading system was devised to aid in guiding treatment. Grade I (mild) sprains are those with minimal swelling and tenderness but have the ligaments still intact. Grade II (moderate) sprains occur when there are partial ligament tears associated with moderate pain, swelling, and tenderness. Finally, Grade III (severe) sprains are complete ligament tears with marked swelling, hemorrhage, tenderness, loss of function, and abnormal joint motion and instability.23, 24
Initial treatment for all ankle sprains is nonoperative and involves the RICE (rest, ice, compression, elevation) protocol with the use of nonsteroidal anti-inflammatory drugs (NSAIDs) during the acute phase (first 4-5 days) with a short period (no >2 weeks) of immobilization.27 Most authors agree that early mobilization followed by phased rehabilitation is warranted to minimize time away from sports.28-31 Prolonged immobilization (>2 weeks) has detrimental effects and may lead to a longer return to play.28-31 The rehabilitation protocol is divided into stages: (1) pain and edema control, (2) range of motion (ROM) and strengthening exercises, (3) soccer specific functional training, and (4) prophylactic intervention with balance and proprioception exercises. Surgical intervention is rarely indicated for acute ankle sprains. There are exceptions, however, such as when ankle sprains are associated with other injuries that require acute intervention (eg, fracture, osteochondral lesion). Surgery is indicated in the setting of chronic, recurrent mechanical instability. Anatomical repairs (modified Brostrom) seem to produce better outcomes than non-anatomical reconstructions (eg, Chrisman-Snook). Surgical outcomes are good, and most athletes are able to return to their pre-injury level of function.32
In athletes, prevention of recurrent sprains is key. Braces may help prevent ankle sprains and bracing has been shown to be superior to taping, as tape loses its restrictive properties within 20 to 30 minutes of initiating activity.33,34 Application of an orthosis (lace-up ankle orthosis) has been shown to reduce the incidence of ankle re-injury in soccer players with previous ankle sprains. Several studies have found minimal, if any, effect of orthoses on athletic performance.20,35,36 Low-profile braces for soccer have been developed which allow for minimal disruption of the player’s boot and space proximally to insert the shin guard. Another essential component of prevention is prophylactic intervention with balance and proprioceptive exercises. A study looking at first division men’s league football (soccer) players in Iran showed a significant decrease in re-injury rates with proprioceptive training.37 In 2003, FIFA introduced a comprehensive warm-up program (FIFA 11+), which has since been shown in several studies to decrease the risk of injury in amateur soccer players.38-40
MEDIAL ANKLE SPRAINS AND INSTABILITY
Soccer places an unusually high demand on both the medial foot and ankle structures when compared with other sports. For instance, striking the ball requires the player to abduct and externally rotate the foot, which preloads medial structures.9 Hintermann18 looked at 54 cases of medial ankle instability and found that injury commonly occurred during landing on an uneven surface, which applies to soccer players when landing after heading the ball or jumping over a tackle. Pronation with eversion and extreme rotational injuries are well known to cause deltoid ligament injury. However, complete rupture of the deltoid ligament is rare and is more often associated with ankle fractures.41 Due to its close proximity and similarly shared function in medial plantar arch stabilization with the tibiospring and spring ligaments, posterior tibialis tendon dysfunction is also frequently seen in medial ankle instability.17 After an acute injury, patients can present with a medial ankle hematoma and pain along the deltoid ligament. Although chronic insufficiency is diagnosed based on the feeling of “giving way,” pain in the medial gutter of the ankle and a valgus and pronation deformity of the foot can be corrected by activating the peroneus tertius muscle. Arthroscopy is the most specific way to confirm clinically suspected instability of the medial ankle; however, MRI can demonstrate loss of organized medial fibers (Figures 3A, 3B).18 Primary surgical repair of deltoid ligament tears yield good to excellent results and should be considered in the soccer player to prevent problems associated with chronic non-repaired tears such as instability, osteoarthritis, and impingement syndromes.18 After surgical repair, players will undergo extensive physical therapy that progresses to sport-specific exercises with the ultimate goal of returning to competitive play around 4-6 months post-operatively.

HIGH ANKLE SPRAINS (SYNDESMOSIS)
High ankle sprains are much less common than low ankle sprains; however, when they do occur they portend a lengthier rehabilitation and a poorer prognosis, especially if undiagnosed. Lubberts and colleagues42 studied the epidemiology of isolated syndesmotic injuries in professional football players. They pooled data from 15 consecutive seasons of European professional football between 2001 and 2016. They examined a total of 3677 players from 61 teams across 17 countries. There were 1320 ankle ligament injuries registered during 15 seasons, of which 94 (7%) were isolated syndesmotic injuries. The incidence of these injuries increased annually between 2001 and 2016. Injuries were 74% contact-related, and isolated syndesmotic injuries were followed by a mean of a 39-day absence.42 Moreover, football players may have an increased risk of syndesmotic sprains due to foot planting and cutting action.41
Continue to: These injuriesa are typically...
These injuries are typically identified with pain over the AITFL and interosseous membrane. Physical examination tests that help identify syndesmotic injuries include the squeeze test, external rotation test, and crossed-leg test.41 The diagnosis can be made on plain X-ray when there is clear diastasis between the distal tibia and fibula. Two critical measurements on plain films are made 1 cm above the tibial plafond and are used to evaluate the integrity of the syndesmosis: tibiofibular clear space >6 mm, and tibiofibular overlap <1 mm, which indicate disruption of the syndesmosis.43 More subtle injuries can be diagnosed with better sensitivity and specificity using MRI, which can also reveal secondary findings such as bone bruises, ATFL injury, osteochondral lesions, and tibiofibular incongruity.44,45 Arthroscopy is an invaluable diagnostic tool for syndesmotic injuries with a characteristic triad finding of PITFL scarring, disrupted interosseous ligament, and posterolateral tibial plafond chondral damage.46
Classification of the ligaments involved can aid in the selection of appropriate treatment. Grade I injuries involve AITFL tears. Grade IIa injuries involve AITFL and IOL tears. Grade IIb injuries include AITFL, PITFL, and IOL tears. Grade III injuries involve injury to all 3 ligaments, as well as a fibular fracture. Conservative treatment is recommended for Grades I and IIa, while surgical intervention is necessary for Grades IIb and III (Figures 4A, 4B). Compared with other ankle sprains, syndesmotic injuries typically require a more prolonged recovery/rehabilitation. Some studies suggest that these injuries require twice as long to heal.47 Hopkinson and colleagues48 reported a mean recovery time of 55 days following syndesmotic injuries in cadets at the United States Military Academy at West Point. Some surgeons advocate surgical intervention in professional athletes with mild sprains to expedite return to play.49

Surgery has been well established as necessary in more severe injuries where there is clear diastasis or instability of the syndesmosis. Traditionally, screws were used for surgical fixation; however, they often required a second surgery for subsequent removal. There is no general consensus on the optimal screw size, level of placement, or timing of removal.50,51 More recently, non-absorbable suture button fixation (eg, TightRope; Arthrex) has become more popular and provides certain advantages over screw fixation, such as avoiding the need for hardware removal. TightRope has been shown to provide more accurate stabilization of the syndesmosis as compared with screw fixation.52 Since malreduction is the most important indicator of poor long-term functional outcome, suture button fixation should be considered in the treatment of the football player.53 Finally, Colcuc and colleagues54 reported a lower complication rate and earlier return to sports in patients treated with knotless suture button devices compared with screw fixation.
OSTEOCHONDRAL LESIONS
Osteochondral lesions (OCLs) are cartilage-bone defects that are usually located in the talus. They can be caused by an acute traumatic event or repetitive microtrauma with no apparent history of trauma (eg, ankle instability). Acute OCLs can occur in soccer secondary to an ankle sprain or ankle fracture. Symptoms of OCLs include pain, swelling, and mechanical symptoms such as catching or locking, and on physical examination, one might see an effusion. The initial imaging modality of choice is radiographing; however, in ankle sprains with continued pain and swelling MRI may be indicated to rule out an underlying OCL. Missed acute lesions have a tendency not to heal and become chronic lesions, which can cause pain and playing disability. It is well established that chronic ankle instability is an important etiologic factor for OCLs. With the normal hydrostatic pressure within the ankle joint, synovial fluid gets pushed into cartilage/bone fissures, which can then lead to cystic degeneration of the subchondral bone.55-57
Surgical repair of an OCL is dependent on both the size and location of the lesion. Acute lesions can be managed by arthroscopic débridement, microfracture, or fixation of the lesion if enough bone remains attached to the chondral lesion. Return to play is based on development and maturation of fibrocartilage over the lesion (debridement/microfracture) or healing and incorporation of the new graft (chondral repair procedures). Meanwhile, chronic lesions can be managed in 1-stage (microfracture, osteochondral autograft transfer or 2-stage (autologous chondrocyte implantation [ACI]) procedures.56-57 Additional biologic healing augmentation with platelet-rich plasma has been described as well.58 Newer techniques in treating chronic talus OCLs, including ones that have failed to respond to bone marrow stimulation techniques, have been developed more recently such as the use of particulated juvenile articular cartilage allograft (DeNovo NT Natural Tissue Graft®; Zimmer Biomet).59 These newer techniques avoid the need for a 2-stage procedure, as is the case with ACI.
Continue to: Further studies are needed...
Further studies are needed to both investigate long-term outcomes and determine the superiority of the arthroscopic juvenile cartilage procedure compared with microfracture and other cartilage resurfacing procedures. When surgically treating OCLs, one must also restore normal ankle joint biomechanics for the lesion to heal. For instance, in the presence of ankle instability, ligament reconstruction must be performed. Also, one should also consider addressing any hindfoot malalignment with an osteotomy (calcaneus, supramalleolar). In a recent retrospective study, van Eekeren and colleagues60 showed that approximately 76% of patients were able to return to sports at long-term follow-up after arthroscopic débridement and bone marrow stimulation of talar OCLs. However, the activity level decreased at long-term follow-up and never attained the pre-injury level.60
ANKLE IMPINGEMENT
ANTERIOR ANKLE IMPINGEMENT (FOOTBALLER'S ANKLE)
Anterior ankle impingement is caused by anterior osteophytes on both the distal tibia and talar neck. It is thought to be related to repetitive microtrauma to the anteromedial aspect of the ankle from recurrent ball impact.61 It is very common amongst soccer players with some studies suggesting that 60% of soccer players have this syndrome. Ankle impingement is characterized by anterior pain with ankle dorsiflexion, decreased dorsiflexion, and swelling. It is primarily diagnosed with lateral ankle X-rays, which will show the osteophytes. An oblique anteromedial X-ray may increase detection of osteophytes (Figure 5). The early stages of anterior impingement can be treated successfully with injections and heel lifts. Treatment of lesions that fail to respond to conservative management involves arthroscopic or open excision of osteophytes. Most patients with no preexisting osteoarthritis treated arthroscopically will experience pain relief and return to full activity, though recurrent osteophyte formation has been noted at long-term follow-up.62

Anterior ankle impingement is most often caused by acute ankle sprains with an inversion type of mechanism.62 The subsequent reactive inflammation can cause fibrosis leading to distal fascicle enlargement of the AITFL. Impingement in the anterolateral gutter of this enlarged fascicle can also cause both chronic reactive synovitis and chondromalacia of the lateral talar dome.63 MRI can identify abnormal areas of pathology; however, 50% of cases are diagnosed based on clinical examination alone.63 Patients generally present with a history of anterolateral ankle pain and swelling with an occasional popping or snapping sensation.
Soccer players commonly develop anterior bony impingement due to repetitive loading of the anterior ankle from striking the ball. This repetition can lead to osteophyte formation of the anterior distal tibia and talar neck. After the osteophytes form, decreased dorsiflexion can occur due to a mechanical stop and inflammation of the interposed capsule.
The patient will exhibit tenderness to palpation along the anterolateral aspect of the ankle, with pain elicited at extreme passive dorsiflexion.62 Initially, an injection with local anesthetic and corticosteroid can serve both a diagnostic and therapeutic purpose; however, patients who fail conservative treatment can be treated with arthroscopy and resection of the involved scar tissue and osteophytes. The best results are seen in those patients with no concurrent intra-articular lesions or ankle osteoarthritis (Figure 5).62 When treated non-operatively, a player may return to play when pain resolves; however, if treated surgically with arthroscopic debridement/resection, a player must wait until his surgical scars have healed prior to attempting return to play.
Continue to: ANTEROMEDIAL ANKLE IMPINGEMENT
ANTEROMEDIAL ANKLE IMPINGEMENT
Anteromedial ankle impingement is a less common ankle impingement syndrome. It is associated with eversion injuries or following medial malleolar or talar fractures.64,65 Previous injury to the anterior tibiotalar fascicle of the deltoid complex leads to ligament thickening and subsequent impingement in the anteromedial corner of the talus. Adjacent fibrosis and synovitis are common consequences of impingement; however, osteophyte formation and chondral stripping along the anteromedial talus can also be seen. Patients typically complain of pain along the anteromedial joint line that is worse with activity, clicking or popping sensations, and painful, limited dorsiflexion. On examination, impingement can be detected through palpation over the anterior tibiotalar fascicle of the deltoid ligament and eversion or extreme passive dorsiflexion of the foot, all of which will elicit medial ankle tenderness.17,62 Initial treatment consists of rest, physical therapy, and NSAIDs. Refractory cases may be amenable to arthroscopic or open resection of the anterior tibiotalar fascicle with débridement of any adjacent synovitis and scar tissue.62
POSTERIOR ANKLE IMPINGEMENT
Posterior ankle impingement is often referred to as “os trigonum syndrome” since the posterior impingement is frequently associated with a prominent os trigonum. An os trigonum is an accessory ossicle representing the separated posterolateral tubercle of the talus. It is usually asymptomatic. However, in soccer players, pain can occur from impaction between the posterior tibial plafond and the os trigonum, or because of soft tissue compression between the 2 opposing osseous structures. The pain is due to repetitive microtrauma (ankle plantarflexion) or acute forced plantarflexion, which can present as an acute fracture of the os trigonum. Because soccer is a sport requiring both repetitive and extreme plantarflexion, it may predispose players to posterior ankle impingement (Figures 6A, 6B).62,66

Clinically, it can be very difficult to detect and diagnose because the affected structures lie deep and it can coexist with other disease processes (eg, peroneal tendinopathy, Achilles tendinopathy).62,66 Patients will complain of chronic deep posterior ankle pain that is worse with push-off activities (eg, jumping). On examination, patients will exhibit pain with palpation over the posterolateral process and with the crunch test. Lateral radiograph with the foot in plantar flexion will show the os trigonum impinged between the posterior tibial malleolus and the calcaneal tuberosity. An MRI will demonstrate the os trigonum as well as any associated inflammation and edema, while it can also demonstrate coexisting pathologies.
Initial treatment consists of rest, NSAIDs, and taping to prevent plantar flexion. Ultrasound-guided cortisone injection of the capsule and posterior bursa can be both therapeutic and diagnostic. A posterior injection can be used to temporize the symptoms so that the soccer player can make it through the season.
Surgical excision is saved for refractory cases, and this can be done either through an open posterolateral approach or arthroscopic techniques. Recently, Georgiannos and Bisbinas67 showed in an athletic population that endoscopic excision had both a lower complication rate and a quicker return to sports compared with the traditional open approach. Carreira and colleagues68 conducted a retrospective case series of 20 patients (mostly competitive athletes). They found that posterior ankle arthroscopy to address posterior impingement allowed for the maintenance or restoration of anatomic ROM of the ankle and hindfoot, ability to return to at least the previous level of activity, and improvement in objective assessment of pain relief and a higher level of function parameters.68
Continue to: TENDON PATHOLOGY
TENDON PATHOLOGY
SUPERIOR PERONEAL RETINACULUM INJURY
The superior peroneal retinaculum (SPR) forms the roof of the superior peroneal tunnel. The tunnel contains the peroneus brevis and longus tendons and is bordered by the retromalleolar groove of the fibula and the lower aspect of the posterior intramuscular septum of the leg.69,70 The SPR originates from the posterolateral ridge of the fibula and inserts onto the lateral calcaneus, and it is the primary restraint of the peroneal tendons within the retromalleolar sulcus.
Injury to the retinaculum results from both ankle dorsiflexion and inversion, and forceful reflex contraction of the peroneal muscles, which causes subluxation or dislocation of the contained tendons.69 A high level of suspicion is required regarding these injuries since the mechanism of injury is similar to that of a simple lateral ankle sprain. In the setting of retrofibular pain, snapping or popping sensations around the lateral malleolus, or chronic ankle instability that worsens on uneven surfaces, one must consider an injury to the SPR.69 Radiographs are not always diagnostic; however, occasionally on an internal rotation view, one may see a cortical avulsion off the distal tip of the lateral malleolus (“fleck sign”) indicating a rim fracture from an SPR injury (Figure 7). MRI is the best imaging modality to assess the peroneal tendons, as well as an SPR injury. Recently, ultrasound has grown in popularity and may be more useful, since it allows for dynamic evaluation of subluxating/dislocating tendons.69

Conservative management is often associated with poor outcomes, and surgery is indicated for all acute and chronic dislocations in athletes.71 Anatomic reconstruction of the SPR is the preferred surgical method.72 Peroneus brevis debulking and fibular groove deepening may also augment the retinaculum repair.73 van Dijk and colleagues in their systematic review showed that patients treated with both groove deepening and SPR repair have higher rates of return to the sport than patients treated with SPR repair alone.74
STRESS FRACTURES
FIFTH METATARSAL
Fifth metatarsal stress fractures usually occur secondary to lateral overload or avulsion of the peroneus brevis. The fifth metatarsal base can be susceptible to injury in a cavovarus foot. Non-operative treatment typically requires a longer period of immobilization (boot or cast) and necessitates a longer period of non–weight-bearing (anywhere between 6-12 weeks). Therefore, surgery is typically recommended in athletes or in the setting of a recurrent base of the fifth metatarsal fracture to expedite healing and return to play. Return to play is still not recommended until there is evidence of radiographic healing of the fracture. There are certain distinctions with fifth metatarsal stress fractures regarding location and healing rates that need to be taken into account.75,76 In particular, zone 2 injuries (Jones fractures) represent a vascular watershed area, making these fractures prone to nonunion with nonunion rates as high as 15% to 30%. Occasionally, the cavovarus deformity will require correction as well as a reduction in the risk of recurrence or nonunion. Surgical fixation most commonly consists of a single screw placed in an antegrade fashion.77 One must pay attention to screw size since smaller diameter screws (<4.5 mm) are associated with delayed union or nonunion. Moreover, screws that are too long will straighten the curved metatarsal shaft and can lead to fracture distraction or malreduction (Figures 8A, 8B).77

Patients have returned to competitive sports within 6 weeks; however, it should be noted that causes of failure were linked to early return and return to sports before a radiographic union can lead to failure of fixation. Ekstrand and van Dijk78 studied a large group of professional soccer players and found that out of 13,754 injuries, 0.5% (67) were fifth metatarsal fractures. Of note, they found that 45% of players had prodromal symptoms. Furthermore, after surgical treatment the fractures healed faster, compared with conservative treatment (75% vs 33%); however, there was no significant difference in lay-off days between both groups (80 vs 74 days).78 Matsuda and colleagues79 looked at 335 male collegiate soccer players, 29 of whom had a history of a fifth metatarsal stress fracture. They found that playing the midfield position and having an everted rearfoot and inverted forefoot alignment were associated with fifth metatarsal stress fractures.79 Saita and colleagues80 found that restricted hip internal rotation was associated with an increased risk of developing a Jones fracture in 162 professional football players. Finally, Fujitaka and colleagues81 looked at 273 male soccer players between 2005 and 2013. They found an association between weak toe-grip strength and fifth metatarsal fractures, suggesting that weak toe-grip may lead to an increase in the load applied onto the lateral side of the foot, resulting in a stress fracture.81
Continue to: NAVICULAR
NAVICULAR
Another common tarsal bone that sustains stress fractures is the navicular. It is not as common as calcaneal stress fractures in military recruits but can occur in the same type of population, as well as explosive athletics such as sprinters and soccer players. It commonly presents with an indistinct vague achy pain with activity that improves with rest, and pain at the dorsum of the midfoot or along the medial longitudinal arch with activity. It can easily go undiagnosed for quite some time given the difficulty in visualizing the navicular with plain radiographs. Clinically, it is difficult to make the diagnosis, and therefore advanced imaging is necessary when the injury is suspected. Both MRI and CT scans can be used to understand the extent of the injury (Figures 9A-9C). In non-displaced stress fractures, conservative non-operative treatment is the appropriate treatment modality with a brief period of immobilization and non–weight-bearing;82 however, operative treatment is also considered in elite athletes. In either case, return to play is discouraged until there is evidence of radiographic healing. When a displacement is noted, or there is a delay in diagnosis, then operative treatment is recommended.

CONCLUSION
Ankle injuries are very common in soccer and can result in decreased performance or significant loss of playing time. Treatment of acute injury generally follows a conservative route, with surgical intervention reserved for severe ruptures or osteochondral fracture of the ankle joint. Chronic ankle pathology resulting in mechanical or functional instability generally requires surgery to repair ligamentous damage and restore normal ankle kinematics. It is critical for the soccer player to receive appropriate rehabilitation prior to returning to play in order to reduce the risk for reinjury and further chronic instability. Prevention and early intervention of ankle injuries is key in preventing the long-term sequelae of ankle injuries, such as arthritis, in former soccer players.
ABSTRACT
The ankle is one of the most commonly injured joints in soccer and represents a significant cost to the healthcare system. The ligaments that stabilize the ankle joint determine its biomechanics—alterations of which result from various soccer-related injuries. Acute sprains are among the most common injury in soccer players and are generally treated conservatively, with emphasis placed on secondary prevention to reduce the risk for future sprains and progression to chronic ankle instability. Repetitive ankle injuries in soccer players may cause chronic ankle instability, which includes both mechanical ligamentous laxity and functional changes. Chronic ankle pathology often requires surgery to repair ligamentous damage and remove soft-tissue or osseous impingement. Proper initial treatment, rehabilitation, and secondary prevention of ankle injuries can limit the amount of time lost from play and avoid negative long-term sequelae (eg, osteochondral lesions, arthritis). On the other hand, high ankle sprains portend a poorer prognosis and a longer recovery. These injuries will typically require surgical stabilization. Impingement-like syndromes of the ankle can undergo an initial trial of conservative treatment; when this fails, however, soccer players respond favorably to arthroscopic debridement of the lesions causing impingement. Finally, other pathologies (eg, stress fractures) are highly encouraged to be treated with surgical stabilization in elite soccer players.
Continue to: EPIDEMIOLOGY
EPIDEMIOLOGY
With roughly 200,000 professional and around 240 million amateur soccer players, soccer has been recognized as the most popular sport worldwide. Nevertheless, given its rising popularity in society, one must also consider the increasing incidence of injuries as a result. Elite soccer players sustain between 10 and 35 injuries per 1000 competitive playing hours.1 Approximately 80% are traumatic, and 20% are overuse injuries.2 Soccer injuries are more frequent with increasing age of the participants, whereas the incidence of injuries in preadolescent players is low. The incidence of injuries has been found to be higher during competition when compared with practice/training sessions, with some studies showing that 59% of injuries occurred during games.2 Amateur or recreational soccer players sustain fewer injuries than professional soccer players, as one would expect, given both the higher intensity of training and match schedule in professionals.
The ankle is one of the most commonly injured joints in soccer, with some studies suggesting it comprises one-fifth of all injuries sustained during soccer, which is only second to those of the knee.2 Ankle sprains specifically are quite a common occurrence in soccer.3-9 A recent study of an English premier league club showed that over a 4-season period, 20% of injuries were of the foot and ankle with a mean return to sport time of 54 days.10 Of all foot and ankle related injuries, ankle sprains are the most common, followed by bruises/contusions, and tendon lesions. Fractures are very rare (1%) in soccer, but when they do occur they impart a much more extended recovery. During the 2010 Fédération Internationale de Football Association (FIFA) World Cup, ankle sprains were among the most common injuries and approximately half lead to players missing training or competitive matches.5
ANATOMY
Knowledge of the biomechanics of both the foot and ankle joints is essential to understand soccer injuries. The ankle joint (talocrural articulation) consists of the distal ends of the tibia and fibula, which form the mortise, and the superior aspect of the talar dome.11 As a hinge joint, the ankle provides 20° of dorsiflexion and 50° of plantar flexion,12 with stability provided by the lateral, medial, and superior ligamentous complexes. The superior articular surface of the talus is narrower posteriorly, which creates a looser fit within the mortise during plantar flexion.11 This decreased stability could help explain why the most common injury in soccer involves a plantar flexion mechanism.13,14 Inferiorly, the talus articulates with the calcaneus to form the subtalar joint. It is at this site that the majority of both foot inversion and eversion occurs. The transverse tarsal joints (Chopart’s joints) separate the hindfoot from the midfoot. Movement of this joint depends on the relative alignment of its 2 articulations: the talonavicular and calcaneocuboid joints. During foot eversion, these 2 joints are parallel to each other allowing supple motion and aiding in shock absorption during the heel strike phase of the gait cycle. With foot inversion, the joints become nonparallel and thus lock the transverse tarsal joints providing a rigid lever needed for push-off.11,12
LATERAL LIGAMENTS
The ankle joint is stabilized laterally by a ligament complex consisting of 3 individual ligaments, all originating from the lateral malleolus: the anterior talofibular ligament (ATFL), the posterior talofibular ligament (PTFL), and the calcaneofibular ligament (CFL) (Figure 1).11,12,15 The ATFL is the primary restraint to inversion in plantar flexion, and it helps resist anterolateral translation of the talus in the mortise. However, it is the weakest and therefore the most frequently injured of the lateral ligaments. The PTFL plays only a supplementary role in ankle stability when the lateral ligament complex is intact. It is under the greatest strain in ankle dorsiflexion and acts to limit posterior talar displacement within the mortise as well as talar external rotation.13,16 The CFL is the primary restraint to inversion in the neutral or dorsiflexed position. It restrains subtalar inversion, thereby limiting talar tilt within the mortise.

DELTOID LIGAMENT
The deltoid ligament complex consists of 6 continuous adjacent superficial and deep ligaments that function synergistically to resist valgus and pronation forces, as well as external rotation of the talus in the mortise.11-13,17 The superficial layer crosses both ankle and subtalar joints. It originates from the anterior colliculus and fans out to insert into the navicular, neck of the talus, sustentaculum tali, and posteromedial talar tubercle. The tibiocalcaneal (sustentaculum tali) portion is the strongest component in the superficial layer and resists calcaneal eversion. The deep layer crosses the ankle joint only. It functions as the primary stabilizer of the medial ankle and prevents both lateral displacement and external rotation of the talus. It originates from the inferior and posterior aspects of the medial malleolus and inserts on the medial and posteromedial aspects of the talus.12,17,18
Continue to: SYNDESMOSIS
SYNDESMOSIS
The ankle syndesmosis, or inferior tibiofibular joint, is the distal articulation between the tibia and fibula. The syndesmosis contributes to ankle mortise integrity through its firm fixation of the lateral malleolus against the lateral surface of the talus. Ligaments comprising the ankle syndesmosis include the anterior inferior tibiofibular ligament (AITFL), the posterior inferior tibiofibular ligament (PITFL), the inferior transverse ligament, and the interosseous ligament (IOL).12
ANKLE SPRAINS
Ankle sprains are the most common pathology encountered amongst soccer players, representing from one-half to two-thirds of all ankle related injuries. Most sprains occur outside of player contact.
LATERAL ANKLE SPRAINS AND INSTABILITY
Injury to the lateral ligaments of the ankle represents 77% to 91% of all ankle sprains in soccer.6,19 The greatest risk factor for an ankle sprain in a soccer player is a history of prior sprain.20 Other risk factors include increasing age, player-to-player contact, condition of the pitch, weight-bearing status of the injured limb at the time of injury, and joint instability or laxity.21,22
The evaluation of an ankle sprain to determine its severity is best done after the acute phase, approximately 4 to 7 days after the initial injury when both pain and swelling have subsided.23 The anterior drawer (ATFL instability) and talar tilt (CFL instability) tests are useful in evaluating ankle instability in the delayed or chronic setting; however, both have been shown to have limited sensitivity and significant variability amongst different examiners.24
Clinical examination will direct further diagnostic tests including X-rays, magnetic resonance imaging (MRI), and computed tomography (CT). The Ottawa ankle rules are generally helpful in determining whether plain X-rays are indicated in the acute setting.25,26 (Figure 2) According to these rules, ankle radiographs should be obtained if ankle pain is reported near the malleoli and 1 or more of the following is seen during examination: inability to bear weight immediately after injury and for 4 steps in the emergency department, and bony tenderness at the posterior edge or tip of the malleolus. Stress X-rays are generally not indicated in acute injuries but may be useful in chronic ankle instability cases.23

Continue to: Ankle sprains cover...
Ankle sprains cover a broad spectrum of injuries; therefore, a grading system was devised to aid in guiding treatment. Grade I (mild) sprains are those with minimal swelling and tenderness but have the ligaments still intact. Grade II (moderate) sprains occur when there are partial ligament tears associated with moderate pain, swelling, and tenderness. Finally, Grade III (severe) sprains are complete ligament tears with marked swelling, hemorrhage, tenderness, loss of function, and abnormal joint motion and instability.23, 24
Initial treatment for all ankle sprains is nonoperative and involves the RICE (rest, ice, compression, elevation) protocol with the use of nonsteroidal anti-inflammatory drugs (NSAIDs) during the acute phase (first 4-5 days) with a short period (no >2 weeks) of immobilization.27 Most authors agree that early mobilization followed by phased rehabilitation is warranted to minimize time away from sports.28-31 Prolonged immobilization (>2 weeks) has detrimental effects and may lead to a longer return to play.28-31 The rehabilitation protocol is divided into stages: (1) pain and edema control, (2) range of motion (ROM) and strengthening exercises, (3) soccer specific functional training, and (4) prophylactic intervention with balance and proprioception exercises. Surgical intervention is rarely indicated for acute ankle sprains. There are exceptions, however, such as when ankle sprains are associated with other injuries that require acute intervention (eg, fracture, osteochondral lesion). Surgery is indicated in the setting of chronic, recurrent mechanical instability. Anatomical repairs (modified Brostrom) seem to produce better outcomes than non-anatomical reconstructions (eg, Chrisman-Snook). Surgical outcomes are good, and most athletes are able to return to their pre-injury level of function.32
In athletes, prevention of recurrent sprains is key. Braces may help prevent ankle sprains and bracing has been shown to be superior to taping, as tape loses its restrictive properties within 20 to 30 minutes of initiating activity.33,34 Application of an orthosis (lace-up ankle orthosis) has been shown to reduce the incidence of ankle re-injury in soccer players with previous ankle sprains. Several studies have found minimal, if any, effect of orthoses on athletic performance.20,35,36 Low-profile braces for soccer have been developed which allow for minimal disruption of the player’s boot and space proximally to insert the shin guard. Another essential component of prevention is prophylactic intervention with balance and proprioceptive exercises. A study looking at first division men’s league football (soccer) players in Iran showed a significant decrease in re-injury rates with proprioceptive training.37 In 2003, FIFA introduced a comprehensive warm-up program (FIFA 11+), which has since been shown in several studies to decrease the risk of injury in amateur soccer players.38-40
MEDIAL ANKLE SPRAINS AND INSTABILITY
Soccer places an unusually high demand on both the medial foot and ankle structures when compared with other sports. For instance, striking the ball requires the player to abduct and externally rotate the foot, which preloads medial structures.9 Hintermann18 looked at 54 cases of medial ankle instability and found that injury commonly occurred during landing on an uneven surface, which applies to soccer players when landing after heading the ball or jumping over a tackle. Pronation with eversion and extreme rotational injuries are well known to cause deltoid ligament injury. However, complete rupture of the deltoid ligament is rare and is more often associated with ankle fractures.41 Due to its close proximity and similarly shared function in medial plantar arch stabilization with the tibiospring and spring ligaments, posterior tibialis tendon dysfunction is also frequently seen in medial ankle instability.17 After an acute injury, patients can present with a medial ankle hematoma and pain along the deltoid ligament. Although chronic insufficiency is diagnosed based on the feeling of “giving way,” pain in the medial gutter of the ankle and a valgus and pronation deformity of the foot can be corrected by activating the peroneus tertius muscle. Arthroscopy is the most specific way to confirm clinically suspected instability of the medial ankle; however, MRI can demonstrate loss of organized medial fibers (Figures 3A, 3B).18 Primary surgical repair of deltoid ligament tears yield good to excellent results and should be considered in the soccer player to prevent problems associated with chronic non-repaired tears such as instability, osteoarthritis, and impingement syndromes.18 After surgical repair, players will undergo extensive physical therapy that progresses to sport-specific exercises with the ultimate goal of returning to competitive play around 4-6 months post-operatively.

HIGH ANKLE SPRAINS (SYNDESMOSIS)
High ankle sprains are much less common than low ankle sprains; however, when they do occur they portend a lengthier rehabilitation and a poorer prognosis, especially if undiagnosed. Lubberts and colleagues42 studied the epidemiology of isolated syndesmotic injuries in professional football players. They pooled data from 15 consecutive seasons of European professional football between 2001 and 2016. They examined a total of 3677 players from 61 teams across 17 countries. There were 1320 ankle ligament injuries registered during 15 seasons, of which 94 (7%) were isolated syndesmotic injuries. The incidence of these injuries increased annually between 2001 and 2016. Injuries were 74% contact-related, and isolated syndesmotic injuries were followed by a mean of a 39-day absence.42 Moreover, football players may have an increased risk of syndesmotic sprains due to foot planting and cutting action.41
Continue to: These injuriesa are typically...
These injuries are typically identified with pain over the AITFL and interosseous membrane. Physical examination tests that help identify syndesmotic injuries include the squeeze test, external rotation test, and crossed-leg test.41 The diagnosis can be made on plain X-ray when there is clear diastasis between the distal tibia and fibula. Two critical measurements on plain films are made 1 cm above the tibial plafond and are used to evaluate the integrity of the syndesmosis: tibiofibular clear space >6 mm, and tibiofibular overlap <1 mm, which indicate disruption of the syndesmosis.43 More subtle injuries can be diagnosed with better sensitivity and specificity using MRI, which can also reveal secondary findings such as bone bruises, ATFL injury, osteochondral lesions, and tibiofibular incongruity.44,45 Arthroscopy is an invaluable diagnostic tool for syndesmotic injuries with a characteristic triad finding of PITFL scarring, disrupted interosseous ligament, and posterolateral tibial plafond chondral damage.46
Classification of the ligaments involved can aid in the selection of appropriate treatment. Grade I injuries involve AITFL tears. Grade IIa injuries involve AITFL and IOL tears. Grade IIb injuries include AITFL, PITFL, and IOL tears. Grade III injuries involve injury to all 3 ligaments, as well as a fibular fracture. Conservative treatment is recommended for Grades I and IIa, while surgical intervention is necessary for Grades IIb and III (Figures 4A, 4B). Compared with other ankle sprains, syndesmotic injuries typically require a more prolonged recovery/rehabilitation. Some studies suggest that these injuries require twice as long to heal.47 Hopkinson and colleagues48 reported a mean recovery time of 55 days following syndesmotic injuries in cadets at the United States Military Academy at West Point. Some surgeons advocate surgical intervention in professional athletes with mild sprains to expedite return to play.49

Surgery has been well established as necessary in more severe injuries where there is clear diastasis or instability of the syndesmosis. Traditionally, screws were used for surgical fixation; however, they often required a second surgery for subsequent removal. There is no general consensus on the optimal screw size, level of placement, or timing of removal.50,51 More recently, non-absorbable suture button fixation (eg, TightRope; Arthrex) has become more popular and provides certain advantages over screw fixation, such as avoiding the need for hardware removal. TightRope has been shown to provide more accurate stabilization of the syndesmosis as compared with screw fixation.52 Since malreduction is the most important indicator of poor long-term functional outcome, suture button fixation should be considered in the treatment of the football player.53 Finally, Colcuc and colleagues54 reported a lower complication rate and earlier return to sports in patients treated with knotless suture button devices compared with screw fixation.
OSTEOCHONDRAL LESIONS
Osteochondral lesions (OCLs) are cartilage-bone defects that are usually located in the talus. They can be caused by an acute traumatic event or repetitive microtrauma with no apparent history of trauma (eg, ankle instability). Acute OCLs can occur in soccer secondary to an ankle sprain or ankle fracture. Symptoms of OCLs include pain, swelling, and mechanical symptoms such as catching or locking, and on physical examination, one might see an effusion. The initial imaging modality of choice is radiographing; however, in ankle sprains with continued pain and swelling MRI may be indicated to rule out an underlying OCL. Missed acute lesions have a tendency not to heal and become chronic lesions, which can cause pain and playing disability. It is well established that chronic ankle instability is an important etiologic factor for OCLs. With the normal hydrostatic pressure within the ankle joint, synovial fluid gets pushed into cartilage/bone fissures, which can then lead to cystic degeneration of the subchondral bone.55-57
Surgical repair of an OCL is dependent on both the size and location of the lesion. Acute lesions can be managed by arthroscopic débridement, microfracture, or fixation of the lesion if enough bone remains attached to the chondral lesion. Return to play is based on development and maturation of fibrocartilage over the lesion (debridement/microfracture) or healing and incorporation of the new graft (chondral repair procedures). Meanwhile, chronic lesions can be managed in 1-stage (microfracture, osteochondral autograft transfer or 2-stage (autologous chondrocyte implantation [ACI]) procedures.56-57 Additional biologic healing augmentation with platelet-rich plasma has been described as well.58 Newer techniques in treating chronic talus OCLs, including ones that have failed to respond to bone marrow stimulation techniques, have been developed more recently such as the use of particulated juvenile articular cartilage allograft (DeNovo NT Natural Tissue Graft®; Zimmer Biomet).59 These newer techniques avoid the need for a 2-stage procedure, as is the case with ACI.
Continue to: Further studies are needed...
Further studies are needed to both investigate long-term outcomes and determine the superiority of the arthroscopic juvenile cartilage procedure compared with microfracture and other cartilage resurfacing procedures. When surgically treating OCLs, one must also restore normal ankle joint biomechanics for the lesion to heal. For instance, in the presence of ankle instability, ligament reconstruction must be performed. Also, one should also consider addressing any hindfoot malalignment with an osteotomy (calcaneus, supramalleolar). In a recent retrospective study, van Eekeren and colleagues60 showed that approximately 76% of patients were able to return to sports at long-term follow-up after arthroscopic débridement and bone marrow stimulation of talar OCLs. However, the activity level decreased at long-term follow-up and never attained the pre-injury level.60
ANKLE IMPINGEMENT
ANTERIOR ANKLE IMPINGEMENT (FOOTBALLER'S ANKLE)
Anterior ankle impingement is caused by anterior osteophytes on both the distal tibia and talar neck. It is thought to be related to repetitive microtrauma to the anteromedial aspect of the ankle from recurrent ball impact.61 It is very common amongst soccer players with some studies suggesting that 60% of soccer players have this syndrome. Ankle impingement is characterized by anterior pain with ankle dorsiflexion, decreased dorsiflexion, and swelling. It is primarily diagnosed with lateral ankle X-rays, which will show the osteophytes. An oblique anteromedial X-ray may increase detection of osteophytes (Figure 5). The early stages of anterior impingement can be treated successfully with injections and heel lifts. Treatment of lesions that fail to respond to conservative management involves arthroscopic or open excision of osteophytes. Most patients with no preexisting osteoarthritis treated arthroscopically will experience pain relief and return to full activity, though recurrent osteophyte formation has been noted at long-term follow-up.62

Anterior ankle impingement is most often caused by acute ankle sprains with an inversion type of mechanism.62 The subsequent reactive inflammation can cause fibrosis leading to distal fascicle enlargement of the AITFL. Impingement in the anterolateral gutter of this enlarged fascicle can also cause both chronic reactive synovitis and chondromalacia of the lateral talar dome.63 MRI can identify abnormal areas of pathology; however, 50% of cases are diagnosed based on clinical examination alone.63 Patients generally present with a history of anterolateral ankle pain and swelling with an occasional popping or snapping sensation.
Soccer players commonly develop anterior bony impingement due to repetitive loading of the anterior ankle from striking the ball. This repetition can lead to osteophyte formation of the anterior distal tibia and talar neck. After the osteophytes form, decreased dorsiflexion can occur due to a mechanical stop and inflammation of the interposed capsule.
The patient will exhibit tenderness to palpation along the anterolateral aspect of the ankle, with pain elicited at extreme passive dorsiflexion.62 Initially, an injection with local anesthetic and corticosteroid can serve both a diagnostic and therapeutic purpose; however, patients who fail conservative treatment can be treated with arthroscopy and resection of the involved scar tissue and osteophytes. The best results are seen in those patients with no concurrent intra-articular lesions or ankle osteoarthritis (Figure 5).62 When treated non-operatively, a player may return to play when pain resolves; however, if treated surgically with arthroscopic debridement/resection, a player must wait until his surgical scars have healed prior to attempting return to play.
Continue to: ANTEROMEDIAL ANKLE IMPINGEMENT
ANTEROMEDIAL ANKLE IMPINGEMENT
Anteromedial ankle impingement is a less common ankle impingement syndrome. It is associated with eversion injuries or following medial malleolar or talar fractures.64,65 Previous injury to the anterior tibiotalar fascicle of the deltoid complex leads to ligament thickening and subsequent impingement in the anteromedial corner of the talus. Adjacent fibrosis and synovitis are common consequences of impingement; however, osteophyte formation and chondral stripping along the anteromedial talus can also be seen. Patients typically complain of pain along the anteromedial joint line that is worse with activity, clicking or popping sensations, and painful, limited dorsiflexion. On examination, impingement can be detected through palpation over the anterior tibiotalar fascicle of the deltoid ligament and eversion or extreme passive dorsiflexion of the foot, all of which will elicit medial ankle tenderness.17,62 Initial treatment consists of rest, physical therapy, and NSAIDs. Refractory cases may be amenable to arthroscopic or open resection of the anterior tibiotalar fascicle with débridement of any adjacent synovitis and scar tissue.62
POSTERIOR ANKLE IMPINGEMENT
Posterior ankle impingement is often referred to as “os trigonum syndrome” since the posterior impingement is frequently associated with a prominent os trigonum. An os trigonum is an accessory ossicle representing the separated posterolateral tubercle of the talus. It is usually asymptomatic. However, in soccer players, pain can occur from impaction between the posterior tibial plafond and the os trigonum, or because of soft tissue compression between the 2 opposing osseous structures. The pain is due to repetitive microtrauma (ankle plantarflexion) or acute forced plantarflexion, which can present as an acute fracture of the os trigonum. Because soccer is a sport requiring both repetitive and extreme plantarflexion, it may predispose players to posterior ankle impingement (Figures 6A, 6B).62,66

Clinically, it can be very difficult to detect and diagnose because the affected structures lie deep and it can coexist with other disease processes (eg, peroneal tendinopathy, Achilles tendinopathy).62,66 Patients will complain of chronic deep posterior ankle pain that is worse with push-off activities (eg, jumping). On examination, patients will exhibit pain with palpation over the posterolateral process and with the crunch test. Lateral radiograph with the foot in plantar flexion will show the os trigonum impinged between the posterior tibial malleolus and the calcaneal tuberosity. An MRI will demonstrate the os trigonum as well as any associated inflammation and edema, while it can also demonstrate coexisting pathologies.
Initial treatment consists of rest, NSAIDs, and taping to prevent plantar flexion. Ultrasound-guided cortisone injection of the capsule and posterior bursa can be both therapeutic and diagnostic. A posterior injection can be used to temporize the symptoms so that the soccer player can make it through the season.
Surgical excision is saved for refractory cases, and this can be done either through an open posterolateral approach or arthroscopic techniques. Recently, Georgiannos and Bisbinas67 showed in an athletic population that endoscopic excision had both a lower complication rate and a quicker return to sports compared with the traditional open approach. Carreira and colleagues68 conducted a retrospective case series of 20 patients (mostly competitive athletes). They found that posterior ankle arthroscopy to address posterior impingement allowed for the maintenance or restoration of anatomic ROM of the ankle and hindfoot, ability to return to at least the previous level of activity, and improvement in objective assessment of pain relief and a higher level of function parameters.68
Continue to: TENDON PATHOLOGY
TENDON PATHOLOGY
SUPERIOR PERONEAL RETINACULUM INJURY
The superior peroneal retinaculum (SPR) forms the roof of the superior peroneal tunnel. The tunnel contains the peroneus brevis and longus tendons and is bordered by the retromalleolar groove of the fibula and the lower aspect of the posterior intramuscular septum of the leg.69,70 The SPR originates from the posterolateral ridge of the fibula and inserts onto the lateral calcaneus, and it is the primary restraint of the peroneal tendons within the retromalleolar sulcus.
Injury to the retinaculum results from both ankle dorsiflexion and inversion, and forceful reflex contraction of the peroneal muscles, which causes subluxation or dislocation of the contained tendons.69 A high level of suspicion is required regarding these injuries since the mechanism of injury is similar to that of a simple lateral ankle sprain. In the setting of retrofibular pain, snapping or popping sensations around the lateral malleolus, or chronic ankle instability that worsens on uneven surfaces, one must consider an injury to the SPR.69 Radiographs are not always diagnostic; however, occasionally on an internal rotation view, one may see a cortical avulsion off the distal tip of the lateral malleolus (“fleck sign”) indicating a rim fracture from an SPR injury (Figure 7). MRI is the best imaging modality to assess the peroneal tendons, as well as an SPR injury. Recently, ultrasound has grown in popularity and may be more useful, since it allows for dynamic evaluation of subluxating/dislocating tendons.69

Conservative management is often associated with poor outcomes, and surgery is indicated for all acute and chronic dislocations in athletes.71 Anatomic reconstruction of the SPR is the preferred surgical method.72 Peroneus brevis debulking and fibular groove deepening may also augment the retinaculum repair.73 van Dijk and colleagues in their systematic review showed that patients treated with both groove deepening and SPR repair have higher rates of return to the sport than patients treated with SPR repair alone.74
STRESS FRACTURES
FIFTH METATARSAL
Fifth metatarsal stress fractures usually occur secondary to lateral overload or avulsion of the peroneus brevis. The fifth metatarsal base can be susceptible to injury in a cavovarus foot. Non-operative treatment typically requires a longer period of immobilization (boot or cast) and necessitates a longer period of non–weight-bearing (anywhere between 6-12 weeks). Therefore, surgery is typically recommended in athletes or in the setting of a recurrent base of the fifth metatarsal fracture to expedite healing and return to play. Return to play is still not recommended until there is evidence of radiographic healing of the fracture. There are certain distinctions with fifth metatarsal stress fractures regarding location and healing rates that need to be taken into account.75,76 In particular, zone 2 injuries (Jones fractures) represent a vascular watershed area, making these fractures prone to nonunion with nonunion rates as high as 15% to 30%. Occasionally, the cavovarus deformity will require correction as well as a reduction in the risk of recurrence or nonunion. Surgical fixation most commonly consists of a single screw placed in an antegrade fashion.77 One must pay attention to screw size since smaller diameter screws (<4.5 mm) are associated with delayed union or nonunion. Moreover, screws that are too long will straighten the curved metatarsal shaft and can lead to fracture distraction or malreduction (Figures 8A, 8B).77

Patients have returned to competitive sports within 6 weeks; however, it should be noted that causes of failure were linked to early return and return to sports before a radiographic union can lead to failure of fixation. Ekstrand and van Dijk78 studied a large group of professional soccer players and found that out of 13,754 injuries, 0.5% (67) were fifth metatarsal fractures. Of note, they found that 45% of players had prodromal symptoms. Furthermore, after surgical treatment the fractures healed faster, compared with conservative treatment (75% vs 33%); however, there was no significant difference in lay-off days between both groups (80 vs 74 days).78 Matsuda and colleagues79 looked at 335 male collegiate soccer players, 29 of whom had a history of a fifth metatarsal stress fracture. They found that playing the midfield position and having an everted rearfoot and inverted forefoot alignment were associated with fifth metatarsal stress fractures.79 Saita and colleagues80 found that restricted hip internal rotation was associated with an increased risk of developing a Jones fracture in 162 professional football players. Finally, Fujitaka and colleagues81 looked at 273 male soccer players between 2005 and 2013. They found an association between weak toe-grip strength and fifth metatarsal fractures, suggesting that weak toe-grip may lead to an increase in the load applied onto the lateral side of the foot, resulting in a stress fracture.81
Continue to: NAVICULAR
NAVICULAR
Another common tarsal bone that sustains stress fractures is the navicular. It is not as common as calcaneal stress fractures in military recruits but can occur in the same type of population, as well as explosive athletics such as sprinters and soccer players. It commonly presents with an indistinct vague achy pain with activity that improves with rest, and pain at the dorsum of the midfoot or along the medial longitudinal arch with activity. It can easily go undiagnosed for quite some time given the difficulty in visualizing the navicular with plain radiographs. Clinically, it is difficult to make the diagnosis, and therefore advanced imaging is necessary when the injury is suspected. Both MRI and CT scans can be used to understand the extent of the injury (Figures 9A-9C). In non-displaced stress fractures, conservative non-operative treatment is the appropriate treatment modality with a brief period of immobilization and non–weight-bearing;82 however, operative treatment is also considered in elite athletes. In either case, return to play is discouraged until there is evidence of radiographic healing. When a displacement is noted, or there is a delay in diagnosis, then operative treatment is recommended.

CONCLUSION
Ankle injuries are very common in soccer and can result in decreased performance or significant loss of playing time. Treatment of acute injury generally follows a conservative route, with surgical intervention reserved for severe ruptures or osteochondral fracture of the ankle joint. Chronic ankle pathology resulting in mechanical or functional instability generally requires surgery to repair ligamentous damage and restore normal ankle kinematics. It is critical for the soccer player to receive appropriate rehabilitation prior to returning to play in order to reduce the risk for reinjury and further chronic instability. Prevention and early intervention of ankle injuries is key in preventing the long-term sequelae of ankle injuries, such as arthritis, in former soccer players.
1. Dvorak J, Junge A. Football injuries and physical symptoms. A review of the literature. Am J Sports Med. 2000;28(5 Suppl):S3-S9.
2. Chomiak J, Junge A, Peterson L, Dvorak J. Severe injuries in football players. Am J Sports Med. 2000;28(5 Suppl):S58-S68.
3. Cloke DJ, Ansell P, Avery P, Deehan D. Ankle injuries in football academies: a three-centre prospective study. Br J Sports Med. 2011;45(9):702-708. doi:10.1136/bjsm.2009.067900.
4. Cloke DJ, Spencer S, Hodson A, Deehan D. The epidemiology of ankle injuries occurring in English Football Association academies. Br J Sports Med. 2009;43(14):1119-1125. doi:10.1136/bjsm.2008.052050.
5. Dvorak J, Junge A, Derman W, Schwellnus M. Injuries and illnesses of football players during the 2010 FIFA World Cup. Br J Sports Med. 2011;45(8):626-630. doi:10.1136/bjsm.2010.079905.
6. Ekstrand J, Gillquist J. Soccer injuries and their mechanisms: a prospective study. Med Sci Sports Exerc. 1983;15(3):267-270.
7. Fousekis K, Tsepis E, Vagenas G. Intrinsic risk factors of noncontact ankle sprains in soccer: a prospective study on 100 professional players. Am J Sports Med. 2012;40(8):1842-1850. doi:10.1177/0363546512449602.
8. Gaulrapp H, Becker A, Walther M, Hess H. Injuries in women’s soccer: a 1-year all players prospective field study of the women’s Bundesliga (German premiere league). Clin J Sports Med. 2010;20(4):264-271. doi:10.1097/JSM.0b013e3181e78e33.
9. Morgan BE, Oberlander MA. An examination of injuries in major league soccer. The inaugural season. Am J Sports Med. 2001;29(4):426-430. doi:10.1177/03635465010290040701.
10. Jain N, Murray D, Kemp S, Calder J. Frequency and trends in foot and ankle injuries within an English Premier League Football Club using a new impact factor of injury to identify a focus for injury prevention. Foot Ankle Surg. 2014;20(4):237-240. doi:10.1016/j.fas.2014.05.004.
11. Moore KL, Dalley AF, Agur AMR. Clinically Oriented Anatomy. 6th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins, 2010:xxix, 1134.
12. Thompson JC, Netter FH. Netter’s Concise Orthopaedic Anatomy. 2nd ed. Philadelphia, PA: Saunders Elsevier, 2010:x, 404.
13. Giza E, Mandelbaum B. Chronic footballer’s ankle. In: Football Traumatology. Springer Milan, 2006:333-351.
14. Garrick JG. The frequency of injury, mechanism of injury, and epidemiology of ankle sprains. Am J Sports Med. 1977:5(6):241-242. doi:10.1177/036354657700500606.
15. Agur AMR, Grant JCB. Grant’s Atlas of Anatomy. 13th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins. 2011.
16. Renstrom PA, Konradsen L. Ankle ligament injuries. Br J Sports Med. 1997;31(1):11-20.
17. Chhabra A, Subhawong TK, Carrino JA. MR imaging of deltoid ligament pathologic findings and associated impingement syndromes. Radiographics. 2010;30(3):751-761. doi:10.1148/rg.303095756.
18. Hintermann B. Medial ankle instability. Foot Ankle Clin. 2003;8(4):723-738.
19. Woods C, Hawkins R, Hulse M, Hodson A. The Football Association Medical Research Programme: an audit of injuries in professional football: an analysis of ankle sprains. Br J Sports Med. 2003;37(3):233-238.
20. Thacker SB, Stroup DF, Branche CM, Gilchrist J, Goodman RA, Weitman EA. The prevention of ankle sprains in sports. A systematic review of the literature. Am J Sports Med. 1999;27(6):753-760. doi:10.1177/03635465990270061201.
21. Giza E, Fuller C, Junge A, Dvorak J. Mechanisms of foot and ankle injuries in soccer. Am J Sports Med. 2003;31(4):550-554. doi:10.1177/03635465030310041201.
22. Tucker AM. Common soccer injuries. Diagnosis, treatment and rehabilitation. Sports Med. 1997;23(1):21-32.
23. Lynch SA, Renstrom PA. Treatment of acute lateral ankle ligament rupture in the athlete. Conservative versus surgical treatment. Sports Med. 1999;27(1):61-71.
24. Chan KW, Ding BC, Mroczek KJ. Acute and chronic lateral ankle instability in the athlete. Bull NYU Hosp Jt Dis. 2011;69(1):17-26.
25. Stiell IG, Greenberg GH, McKnight RD, Nair RC, McDowell I, Worthington JR. A study to develop clinical decision rules for the use of radiography in acute ankle injuries. Ann Emerg Med. 1992;21(4):384-390.
26. Bachmann LM, Kolb E, Koller MT, Steurer J, ter Riet G. Accuracy of Ottawa ankle rules to exclude fractures of the ankle and mid-foot: systematic review. BMJ. 2003;326(7386):417. doi:10.1136/bmj.326.7386.417.
27. Balduini FC, Vegso JJ, Torg JS, Torg E. Management and rehabilitation of ligamentous injuries to the ankle. Sports Med. 1987;4(5):364-380.
28. Kerkhoffs GM, Rowe BH, Assendelft WJ, Kelly KD, Struijs PA, van Dijk CN. Immobilisation for acute ankle sprain. A systematic review. Arch Orthop Trauma Surg. 2001;121(8):462-471.
29. Konradsen L, Holmer P, Sondergaard L. Early mobilizing treatment for grade III ankle ligament injuries. Foot Ankle. 1991;12(2):69-73.
30. Eiff MP, Smith AT, Smith GE. Early mobilization versus immobilization in the treatment of lateral ankle sprains. Am J Sports Med. 1994;22(1):83-88. doi:10.1177/036354659402200115.
31. Shrier I. Treatment of lateral collateral ligament sprains of the ankle: a critical appraisal of the literature. Clin J Sport Med. 1995;5(3):187-195.
32. DiGiovanni BF, Partal G, Baumhauer JF. Acute ankle injury and chronic lateral instability in the athlete. Clin Sports Med. 2004;23(1):1-19, v. doi:10.1016/S0278-5919(03)00095-4.
33. Alt W, Lohrer H, Gollhofer A. Functional properties of adhesive ankle taping: neuromuscular and mechanical effects before and after exercise. Foot Ankle Int. 1999;20(4):238-245. doi:10.1177/107110079902000406.
34. Garrick JG, Requa RK. Role of external support in the prevention of ankle sprains. Med Sci Sports. 1973;5(3):200-203.
35. Sharpe SR, Knapik J, Jones B. Ankle braces effectively reduce recurrence of ankle sprains in female soccer players. J Athl Train. 1997;32(1):21-24.
36. Surve I, Schwellnus MP, Noakes T, Lombard C. A fivefold reduction in the incidence of recurrent ankle sprains in soccer players using the Sport-Stirrup orthosis. Am J Sports Med. 1994;22(5):601-606. doi:10.1177/036354659402200506.
37. Mohammadi F. Comparison of 3 preventive methods to reduce the recurrence of ankle inversion sprains in male soccer players. Am J Sports Med. 2007;35(6):922-926. doi:10.1177/0363546507299259.
38. Steffen K, Meeuwisse WH, Romiti M, et al. Evaluation of how different implementation strategies of an injury prevention programme (FIFA 11+) impact team adherence and injury risk in Canadian female youth football players: a cluster-randomised trial. Br J Sports Med. 2013;47(8):480-487. doi:10.1136/bjsports-2012-091887.
39. Steffen K, Emery CA, Romiti M, et al. High adherence to a neuromuscular injury prevention programme (FIFA 11+) improves functional balance and reduces injury risk in Canadian youth female football players: a cluster randomised trial. Br J Sports Med. 2013;47(12):794-802. doi: 10.1136/bjsports-2012-091886.
40. Junge A, Lamprecht M, Stamm H, et al. Countrywide campaign to prevent soccer injuries in Swiss amateur players. Am J Sports Med. 2011;39(1):57-63. doi:10.1177/0363546510377424.
41. Lin CF, Gross ML, Weinhold P. Ankle syndesmosis injuries: anatomy, biomechanics, mechanism of injury, and clinical guidelines for diagnosis and intervention. J Orthop Sports Phys Ther. 2006;36(6):372-384. doi:10.2519/jospt.2006.2195.
42. Lubberts B, D’Hooghe P, Bengtsson H, DiGiovanni CW, Calder J, Ekstrand J. Epidemiology and return to play following isolated syndesmotic injuries of the ankle: a prospective cohort study of 3677 male professional football players in the UEFA Elite Club Injury Study. Br J Sports Med. 2017. doi:10.1136/bjsports-2017-097710.
43. Harper MC, Keller TS. A radiographic evaluation of the tibiofibular syndesmosis. Foot Ankle. 1989;10(3):156-160.
44. Vogl TJ, Hochmuth K, Diebold T, et al. Magnetic resonance imaging in the diagnosis of acute injured distal tibiofibular syndesmosis. Invest Radiol. 1997;32(7):401-409.
45. Brown KW, Morrison WB, Schweitzer ME, Parellada JA, Nothnagel H. MRI findings associated with distal tibiofibular syndesmosis injury. AJR Am J Roentgenol. 2004;182(1):131-136. doi:10.2214/ajr.182.1.1820131.
46. Ogilvie-Harris DJ, Reed SC, Hedman TP. Disruption of the ankle syndesmosis: biomechanical study of the ligamentous restraints. Arthroscopy. 1994;10(5):558-560.
47. Boytim MJ, Fischer DA, Neumann L. Syndesmotic ankle sprains. Am J Sports Med. 1991;19(3):294-298. doi:10.1177/036354659101900315.
48. Hopkinson WJ, St Pierre P, Ryan JB, Wheeler JH. Syndesmosis sprains of the ankle. Foot Ankle. 1990;10(6):325-330. doi:10.1177/107110079001000607.
49. Del Buono A, Florio A, Boccanera MS, Maffulli N. Syndesmosis injuries of the ankle. Curr Rev Musculoskelet Med. 2013;6(4):313-319. doi:10.1007/s12178-013-9183-x.
50. Dattani R, Patnaik S, Kantak A, Srikanth B, Selvan TP. Injuries to the tibiofibular syndesmosis. J Bone Joint Surg Br. 2008;90(4):405-410. doi:10.1302/0301-620X.90B4.19750.
51. Schepers T. To retain or remove the syndesmotic screw: a review of literature. Arch Orthop Trauma Surg. 2011;131(7):879-883. doi:10.1007/s00402-010-1225-x.
52. Naqvi GA, Cunningham P, Lynch B, Galvin R, Awan N. Fixation of ankle syndesmotic injuries: comparison of tightrope fixation and syndesmotic screw fixation for accuracy of syndesmotic reduction. Am J Sports Med. 2012;40(12):2828-2835. doi:10.1177/0363546512461480.
53. Weening B, Bhandari M. Predictors of functional outcome following transsyndesmotic screw fixation of ankle fractures. J Orthop Trauma. 2005;19(2):102-108.
54. Colcuc C, Blank M, Stein T, et al. Lower complication rate and faster return to sports in patients with acute syndesmotic rupture treated with a new knotless suture button device. Knee Surg Sports Traumatol Arthrosc. 2017. doi:10.1007/s00167-017-4820-4823.
55. Savage-Elliott I, Ross KA, Smyth NA, Murawski CD, Kennedy JG. Osteochondral lesions of the talus: a current concepts review and evidence-based treatment paradigm. Foot Ankle Spec. 2014;7(5):414-422. doi:10.1177/1938640014543362.
56. Talusan PG, Milewski MD, Toy JO, Wall EJ. Osteochondritis dissecans of the talus: diagnosis and treatment in athletes. Clin Sports Med. 2014;33(2):267-284. doi:10.1016/j.csm.2014.01.003.
57. Murawski CD, Kennedy JG. Operative treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 2013;95(11):1045-1054. doi:10.2106/JBJS.L.00773.
58. Guney A, Akar M, Karaman I, Oner M, Guney B. Clinical outcomes of platelet rich plasma (PRP) as an adjunct to microfracture surgery in osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2384-2389. doi:10.1007/s00167-013-2784-5.
59. Hatic SO, Berlet GC. Particulated juvenile articular cartilage graft (DeNovo NT Graft) for treatment of osteochondral lesions of the talus. Foot Ankle Spec. 2010;3(6):361-364. doi:10.1177/1938640010388602.
60. van Eekeren IC, van Bergen CJ, Sierevelt IN, Reilingh ML, van Dijk CN. Return to sports after arthroscopic debridement and bone marrow stimulation of osteochondral talar defects: a 5- to 24-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1311-1315. doi:10.1007/s00167-016-3992-6.
61. Tol JL, Slim E, van Soest AJ, van Dijk CN. The relationship of the kicking action in soccer and anterior ankle impingement syndrome. A biomechanical analysis. Am J Sports Med. 2002;30(1):45-50. doi:10.1177/03635465020300012101.
62. Sanders TG, Rathur SK. Impingement syndromes of the ankle. Magn Reson Imaging Clin N Am. 2008;16(1):29-38. doi:10.1016/j.mric.2008.02.005.
63. Ogilvie-Harris DJ, Gilbart MK, Chorney K. Chronic pain following ankle sprains in athletes: the role of arthroscopic surgery. Arthroscopy. 1997;13(5):564-574.
64. Robinson P, White LM, Salonen D, Ogilvie-Harris D. Anteromedial impingement of the ankle: using MR arthrography to assess the anteromedial recess. AJR Am J Roentgenol. 2002;178(3):601-604. doi:10.2214/ajr.178.3.1780601.
65. Mosier-La Clair SM, Monroe MT, Manoli A. Medial impingement syndrome of the anterior tibiotalar fascicle of the deltoid ligament on the talus. Foot Ankle Int. 2000;21(5):385-391.
66. Maquirriain J. Posterior ankle impingement syndrome. J Am Acad Orthop Surg. 2005;13(6):365-371.
67. Georgiannos D, Bisbinas I. Endoscopic versus open excision of os trigonum for the treatment of posterior ankle impingement syndrome in an athletic population: a randomized controlled study with 5-year follow-up. Am J Sports Med. 2017;45(6):1388-1394. doi:10.1177/0363546516682498.
68. Carreira DS, Vora AM, Hearne KL, Kozy J. Outcome of arthroscopic treatment of posterior impingement of the ankle. Foot Ankle Int. 2016;37(4):394-400. doi:10.1177/1071100715620857.
69. Roth JA, Taylor WC, Whalen J. Peroneal tendon subluxation: the other lateral ankle injury. Br J Sports Med. 2010;44(14):1047-1053. doi:10.1136/bjsm.2008.057182.
70. Athavale SA, Swathi, Vangara SV. Anatomy of the superior peroneal tunnel. J Bone Joint Surg Am. 2011;93(6):564-571. doi:10.2106/JBJS.17.00836.
71. Porter D, McCarroll J, Knapp E, Torma J. Peroneal tendon subluxation in athletes: fibular groove deepening and retinacular reconstruction. Foot Ankle Int. 2005;26(6):436-441.
72. Ferran NA, Oliva F, Maffulli N. Recurrent subluxation of the peroneal tendons. Sports Med. 2006;36(10):839-846. doi:10.1053/j.jfas.2010.02.007.
73. Saxena A, Ewen B. Peroneal subluxation: surgical results in 31 athletic patients. J Foot Ankle Surg. 2010;49(3):238-241.
74. van Dijk PA, Gianakos AL, Kerkhoffs GM, Kennedy JG. Return to sports and clinical outcomes in patients treated for peroneal tendon dislocation: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1155-1164. doi:10.1007/s00167-015-3833-z.
75. Lee KT, Park YU, Young KW, Kim JS, Kim JB. The plantar gap: another prognostic factor for fifth metatarsal stress fracture. Am J Sports Med. 2011;39(10):2206-2211. doi:10.1177/0363546511414856.
76. Torg JS. Fractures of the base of the fifth metatarsal distal to the tuberosity. Orthopedics. 1990;13:731-737.
77. Smith TO, Clark A, Hing CB. Interventions for treating proximal fifth metatarsal fractures in adults: a meta-analysis of the current evidence-base. Foot Ankle Surg. 2011;17(4):300-307. doi:10.1016/j.fas.2010.12.005.
78. Ekstrand J, van Dijk CN. Fifth metatarsal fractures among male professional footballers: a potential career-ending disease. Br J Sports Med. 2013;47(12):754-758.
79. Matsuda S, Fukubayashi T, Hirose N. Characteristics of the foot static alignment and the plantar pressure associated with fifth metatarsal stress fracture history in male soccer players: a case-control study. Sports Med Open. 2017;3(1):27.
80. Saita Y, Nagao M, Kawasaki T, et al. Range limitation in hip internal rotation and fifth metatarsal stress fractures (Jones fracture) in professional football players. Knee Surg Sports Traumatol Arthrosc. 2018;26(7):1943-1949. doi:10.1007/s00167-017-4552-4.
81. Fujitaka K, Taniguchi A, Isomoto S, et al. Pathogenesis of fifth metatarsal fractures in college soccer players. Orthop J Sports Med. 2015;18;3(9):2325967115603654.
82. Torg J, Moyer J, Gaughan J, Boden B. Management of tarsal navicular stress fractures: conservative versus surgical treatment: a meta-analysis. Am J Sports Med. 2010;38(5):1048-1053.
83. Haytmanek CT, Williams BT, James EW, et al. Radiographic identification of the primary lateral ankle structures. Am J Sports Med. 2015;43(1):79-87. doi:10.1177/0363546514553778.
1. Dvorak J, Junge A. Football injuries and physical symptoms. A review of the literature. Am J Sports Med. 2000;28(5 Suppl):S3-S9.
2. Chomiak J, Junge A, Peterson L, Dvorak J. Severe injuries in football players. Am J Sports Med. 2000;28(5 Suppl):S58-S68.
3. Cloke DJ, Ansell P, Avery P, Deehan D. Ankle injuries in football academies: a three-centre prospective study. Br J Sports Med. 2011;45(9):702-708. doi:10.1136/bjsm.2009.067900.
4. Cloke DJ, Spencer S, Hodson A, Deehan D. The epidemiology of ankle injuries occurring in English Football Association academies. Br J Sports Med. 2009;43(14):1119-1125. doi:10.1136/bjsm.2008.052050.
5. Dvorak J, Junge A, Derman W, Schwellnus M. Injuries and illnesses of football players during the 2010 FIFA World Cup. Br J Sports Med. 2011;45(8):626-630. doi:10.1136/bjsm.2010.079905.
6. Ekstrand J, Gillquist J. Soccer injuries and their mechanisms: a prospective study. Med Sci Sports Exerc. 1983;15(3):267-270.
7. Fousekis K, Tsepis E, Vagenas G. Intrinsic risk factors of noncontact ankle sprains in soccer: a prospective study on 100 professional players. Am J Sports Med. 2012;40(8):1842-1850. doi:10.1177/0363546512449602.
8. Gaulrapp H, Becker A, Walther M, Hess H. Injuries in women’s soccer: a 1-year all players prospective field study of the women’s Bundesliga (German premiere league). Clin J Sports Med. 2010;20(4):264-271. doi:10.1097/JSM.0b013e3181e78e33.
9. Morgan BE, Oberlander MA. An examination of injuries in major league soccer. The inaugural season. Am J Sports Med. 2001;29(4):426-430. doi:10.1177/03635465010290040701.
10. Jain N, Murray D, Kemp S, Calder J. Frequency and trends in foot and ankle injuries within an English Premier League Football Club using a new impact factor of injury to identify a focus for injury prevention. Foot Ankle Surg. 2014;20(4):237-240. doi:10.1016/j.fas.2014.05.004.
11. Moore KL, Dalley AF, Agur AMR. Clinically Oriented Anatomy. 6th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins, 2010:xxix, 1134.
12. Thompson JC, Netter FH. Netter’s Concise Orthopaedic Anatomy. 2nd ed. Philadelphia, PA: Saunders Elsevier, 2010:x, 404.
13. Giza E, Mandelbaum B. Chronic footballer’s ankle. In: Football Traumatology. Springer Milan, 2006:333-351.
14. Garrick JG. The frequency of injury, mechanism of injury, and epidemiology of ankle sprains. Am J Sports Med. 1977:5(6):241-242. doi:10.1177/036354657700500606.
15. Agur AMR, Grant JCB. Grant’s Atlas of Anatomy. 13th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins. 2011.
16. Renstrom PA, Konradsen L. Ankle ligament injuries. Br J Sports Med. 1997;31(1):11-20.
17. Chhabra A, Subhawong TK, Carrino JA. MR imaging of deltoid ligament pathologic findings and associated impingement syndromes. Radiographics. 2010;30(3):751-761. doi:10.1148/rg.303095756.
18. Hintermann B. Medial ankle instability. Foot Ankle Clin. 2003;8(4):723-738.
19. Woods C, Hawkins R, Hulse M, Hodson A. The Football Association Medical Research Programme: an audit of injuries in professional football: an analysis of ankle sprains. Br J Sports Med. 2003;37(3):233-238.
20. Thacker SB, Stroup DF, Branche CM, Gilchrist J, Goodman RA, Weitman EA. The prevention of ankle sprains in sports. A systematic review of the literature. Am J Sports Med. 1999;27(6):753-760. doi:10.1177/03635465990270061201.
21. Giza E, Fuller C, Junge A, Dvorak J. Mechanisms of foot and ankle injuries in soccer. Am J Sports Med. 2003;31(4):550-554. doi:10.1177/03635465030310041201.
22. Tucker AM. Common soccer injuries. Diagnosis, treatment and rehabilitation. Sports Med. 1997;23(1):21-32.
23. Lynch SA, Renstrom PA. Treatment of acute lateral ankle ligament rupture in the athlete. Conservative versus surgical treatment. Sports Med. 1999;27(1):61-71.
24. Chan KW, Ding BC, Mroczek KJ. Acute and chronic lateral ankle instability in the athlete. Bull NYU Hosp Jt Dis. 2011;69(1):17-26.
25. Stiell IG, Greenberg GH, McKnight RD, Nair RC, McDowell I, Worthington JR. A study to develop clinical decision rules for the use of radiography in acute ankle injuries. Ann Emerg Med. 1992;21(4):384-390.
26. Bachmann LM, Kolb E, Koller MT, Steurer J, ter Riet G. Accuracy of Ottawa ankle rules to exclude fractures of the ankle and mid-foot: systematic review. BMJ. 2003;326(7386):417. doi:10.1136/bmj.326.7386.417.
27. Balduini FC, Vegso JJ, Torg JS, Torg E. Management and rehabilitation of ligamentous injuries to the ankle. Sports Med. 1987;4(5):364-380.
28. Kerkhoffs GM, Rowe BH, Assendelft WJ, Kelly KD, Struijs PA, van Dijk CN. Immobilisation for acute ankle sprain. A systematic review. Arch Orthop Trauma Surg. 2001;121(8):462-471.
29. Konradsen L, Holmer P, Sondergaard L. Early mobilizing treatment for grade III ankle ligament injuries. Foot Ankle. 1991;12(2):69-73.
30. Eiff MP, Smith AT, Smith GE. Early mobilization versus immobilization in the treatment of lateral ankle sprains. Am J Sports Med. 1994;22(1):83-88. doi:10.1177/036354659402200115.
31. Shrier I. Treatment of lateral collateral ligament sprains of the ankle: a critical appraisal of the literature. Clin J Sport Med. 1995;5(3):187-195.
32. DiGiovanni BF, Partal G, Baumhauer JF. Acute ankle injury and chronic lateral instability in the athlete. Clin Sports Med. 2004;23(1):1-19, v. doi:10.1016/S0278-5919(03)00095-4.
33. Alt W, Lohrer H, Gollhofer A. Functional properties of adhesive ankle taping: neuromuscular and mechanical effects before and after exercise. Foot Ankle Int. 1999;20(4):238-245. doi:10.1177/107110079902000406.
34. Garrick JG, Requa RK. Role of external support in the prevention of ankle sprains. Med Sci Sports. 1973;5(3):200-203.
35. Sharpe SR, Knapik J, Jones B. Ankle braces effectively reduce recurrence of ankle sprains in female soccer players. J Athl Train. 1997;32(1):21-24.
36. Surve I, Schwellnus MP, Noakes T, Lombard C. A fivefold reduction in the incidence of recurrent ankle sprains in soccer players using the Sport-Stirrup orthosis. Am J Sports Med. 1994;22(5):601-606. doi:10.1177/036354659402200506.
37. Mohammadi F. Comparison of 3 preventive methods to reduce the recurrence of ankle inversion sprains in male soccer players. Am J Sports Med. 2007;35(6):922-926. doi:10.1177/0363546507299259.
38. Steffen K, Meeuwisse WH, Romiti M, et al. Evaluation of how different implementation strategies of an injury prevention programme (FIFA 11+) impact team adherence and injury risk in Canadian female youth football players: a cluster-randomised trial. Br J Sports Med. 2013;47(8):480-487. doi:10.1136/bjsports-2012-091887.
39. Steffen K, Emery CA, Romiti M, et al. High adherence to a neuromuscular injury prevention programme (FIFA 11+) improves functional balance and reduces injury risk in Canadian youth female football players: a cluster randomised trial. Br J Sports Med. 2013;47(12):794-802. doi: 10.1136/bjsports-2012-091886.
40. Junge A, Lamprecht M, Stamm H, et al. Countrywide campaign to prevent soccer injuries in Swiss amateur players. Am J Sports Med. 2011;39(1):57-63. doi:10.1177/0363546510377424.
41. Lin CF, Gross ML, Weinhold P. Ankle syndesmosis injuries: anatomy, biomechanics, mechanism of injury, and clinical guidelines for diagnosis and intervention. J Orthop Sports Phys Ther. 2006;36(6):372-384. doi:10.2519/jospt.2006.2195.
42. Lubberts B, D’Hooghe P, Bengtsson H, DiGiovanni CW, Calder J, Ekstrand J. Epidemiology and return to play following isolated syndesmotic injuries of the ankle: a prospective cohort study of 3677 male professional football players in the UEFA Elite Club Injury Study. Br J Sports Med. 2017. doi:10.1136/bjsports-2017-097710.
43. Harper MC, Keller TS. A radiographic evaluation of the tibiofibular syndesmosis. Foot Ankle. 1989;10(3):156-160.
44. Vogl TJ, Hochmuth K, Diebold T, et al. Magnetic resonance imaging in the diagnosis of acute injured distal tibiofibular syndesmosis. Invest Radiol. 1997;32(7):401-409.
45. Brown KW, Morrison WB, Schweitzer ME, Parellada JA, Nothnagel H. MRI findings associated with distal tibiofibular syndesmosis injury. AJR Am J Roentgenol. 2004;182(1):131-136. doi:10.2214/ajr.182.1.1820131.
46. Ogilvie-Harris DJ, Reed SC, Hedman TP. Disruption of the ankle syndesmosis: biomechanical study of the ligamentous restraints. Arthroscopy. 1994;10(5):558-560.
47. Boytim MJ, Fischer DA, Neumann L. Syndesmotic ankle sprains. Am J Sports Med. 1991;19(3):294-298. doi:10.1177/036354659101900315.
48. Hopkinson WJ, St Pierre P, Ryan JB, Wheeler JH. Syndesmosis sprains of the ankle. Foot Ankle. 1990;10(6):325-330. doi:10.1177/107110079001000607.
49. Del Buono A, Florio A, Boccanera MS, Maffulli N. Syndesmosis injuries of the ankle. Curr Rev Musculoskelet Med. 2013;6(4):313-319. doi:10.1007/s12178-013-9183-x.
50. Dattani R, Patnaik S, Kantak A, Srikanth B, Selvan TP. Injuries to the tibiofibular syndesmosis. J Bone Joint Surg Br. 2008;90(4):405-410. doi:10.1302/0301-620X.90B4.19750.
51. Schepers T. To retain or remove the syndesmotic screw: a review of literature. Arch Orthop Trauma Surg. 2011;131(7):879-883. doi:10.1007/s00402-010-1225-x.
52. Naqvi GA, Cunningham P, Lynch B, Galvin R, Awan N. Fixation of ankle syndesmotic injuries: comparison of tightrope fixation and syndesmotic screw fixation for accuracy of syndesmotic reduction. Am J Sports Med. 2012;40(12):2828-2835. doi:10.1177/0363546512461480.
53. Weening B, Bhandari M. Predictors of functional outcome following transsyndesmotic screw fixation of ankle fractures. J Orthop Trauma. 2005;19(2):102-108.
54. Colcuc C, Blank M, Stein T, et al. Lower complication rate and faster return to sports in patients with acute syndesmotic rupture treated with a new knotless suture button device. Knee Surg Sports Traumatol Arthrosc. 2017. doi:10.1007/s00167-017-4820-4823.
55. Savage-Elliott I, Ross KA, Smyth NA, Murawski CD, Kennedy JG. Osteochondral lesions of the talus: a current concepts review and evidence-based treatment paradigm. Foot Ankle Spec. 2014;7(5):414-422. doi:10.1177/1938640014543362.
56. Talusan PG, Milewski MD, Toy JO, Wall EJ. Osteochondritis dissecans of the talus: diagnosis and treatment in athletes. Clin Sports Med. 2014;33(2):267-284. doi:10.1016/j.csm.2014.01.003.
57. Murawski CD, Kennedy JG. Operative treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 2013;95(11):1045-1054. doi:10.2106/JBJS.L.00773.
58. Guney A, Akar M, Karaman I, Oner M, Guney B. Clinical outcomes of platelet rich plasma (PRP) as an adjunct to microfracture surgery in osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2384-2389. doi:10.1007/s00167-013-2784-5.
59. Hatic SO, Berlet GC. Particulated juvenile articular cartilage graft (DeNovo NT Graft) for treatment of osteochondral lesions of the talus. Foot Ankle Spec. 2010;3(6):361-364. doi:10.1177/1938640010388602.
60. van Eekeren IC, van Bergen CJ, Sierevelt IN, Reilingh ML, van Dijk CN. Return to sports after arthroscopic debridement and bone marrow stimulation of osteochondral talar defects: a 5- to 24-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1311-1315. doi:10.1007/s00167-016-3992-6.
61. Tol JL, Slim E, van Soest AJ, van Dijk CN. The relationship of the kicking action in soccer and anterior ankle impingement syndrome. A biomechanical analysis. Am J Sports Med. 2002;30(1):45-50. doi:10.1177/03635465020300012101.
62. Sanders TG, Rathur SK. Impingement syndromes of the ankle. Magn Reson Imaging Clin N Am. 2008;16(1):29-38. doi:10.1016/j.mric.2008.02.005.
63. Ogilvie-Harris DJ, Gilbart MK, Chorney K. Chronic pain following ankle sprains in athletes: the role of arthroscopic surgery. Arthroscopy. 1997;13(5):564-574.
64. Robinson P, White LM, Salonen D, Ogilvie-Harris D. Anteromedial impingement of the ankle: using MR arthrography to assess the anteromedial recess. AJR Am J Roentgenol. 2002;178(3):601-604. doi:10.2214/ajr.178.3.1780601.
65. Mosier-La Clair SM, Monroe MT, Manoli A. Medial impingement syndrome of the anterior tibiotalar fascicle of the deltoid ligament on the talus. Foot Ankle Int. 2000;21(5):385-391.
66. Maquirriain J. Posterior ankle impingement syndrome. J Am Acad Orthop Surg. 2005;13(6):365-371.
67. Georgiannos D, Bisbinas I. Endoscopic versus open excision of os trigonum for the treatment of posterior ankle impingement syndrome in an athletic population: a randomized controlled study with 5-year follow-up. Am J Sports Med. 2017;45(6):1388-1394. doi:10.1177/0363546516682498.
68. Carreira DS, Vora AM, Hearne KL, Kozy J. Outcome of arthroscopic treatment of posterior impingement of the ankle. Foot Ankle Int. 2016;37(4):394-400. doi:10.1177/1071100715620857.
69. Roth JA, Taylor WC, Whalen J. Peroneal tendon subluxation: the other lateral ankle injury. Br J Sports Med. 2010;44(14):1047-1053. doi:10.1136/bjsm.2008.057182.
70. Athavale SA, Swathi, Vangara SV. Anatomy of the superior peroneal tunnel. J Bone Joint Surg Am. 2011;93(6):564-571. doi:10.2106/JBJS.17.00836.
71. Porter D, McCarroll J, Knapp E, Torma J. Peroneal tendon subluxation in athletes: fibular groove deepening and retinacular reconstruction. Foot Ankle Int. 2005;26(6):436-441.
72. Ferran NA, Oliva F, Maffulli N. Recurrent subluxation of the peroneal tendons. Sports Med. 2006;36(10):839-846. doi:10.1053/j.jfas.2010.02.007.
73. Saxena A, Ewen B. Peroneal subluxation: surgical results in 31 athletic patients. J Foot Ankle Surg. 2010;49(3):238-241.
74. van Dijk PA, Gianakos AL, Kerkhoffs GM, Kennedy JG. Return to sports and clinical outcomes in patients treated for peroneal tendon dislocation: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1155-1164. doi:10.1007/s00167-015-3833-z.
75. Lee KT, Park YU, Young KW, Kim JS, Kim JB. The plantar gap: another prognostic factor for fifth metatarsal stress fracture. Am J Sports Med. 2011;39(10):2206-2211. doi:10.1177/0363546511414856.
76. Torg JS. Fractures of the base of the fifth metatarsal distal to the tuberosity. Orthopedics. 1990;13:731-737.
77. Smith TO, Clark A, Hing CB. Interventions for treating proximal fifth metatarsal fractures in adults: a meta-analysis of the current evidence-base. Foot Ankle Surg. 2011;17(4):300-307. doi:10.1016/j.fas.2010.12.005.
78. Ekstrand J, van Dijk CN. Fifth metatarsal fractures among male professional footballers: a potential career-ending disease. Br J Sports Med. 2013;47(12):754-758.
79. Matsuda S, Fukubayashi T, Hirose N. Characteristics of the foot static alignment and the plantar pressure associated with fifth metatarsal stress fracture history in male soccer players: a case-control study. Sports Med Open. 2017;3(1):27.
80. Saita Y, Nagao M, Kawasaki T, et al. Range limitation in hip internal rotation and fifth metatarsal stress fractures (Jones fracture) in professional football players. Knee Surg Sports Traumatol Arthrosc. 2018;26(7):1943-1949. doi:10.1007/s00167-017-4552-4.
81. Fujitaka K, Taniguchi A, Isomoto S, et al. Pathogenesis of fifth metatarsal fractures in college soccer players. Orthop J Sports Med. 2015;18;3(9):2325967115603654.
82. Torg J, Moyer J, Gaughan J, Boden B. Management of tarsal navicular stress fractures: conservative versus surgical treatment: a meta-analysis. Am J Sports Med. 2010;38(5):1048-1053.
83. Haytmanek CT, Williams BT, James EW, et al. Radiographic identification of the primary lateral ankle structures. Am J Sports Med. 2015;43(1):79-87. doi:10.1177/0363546514553778.
TAKE-HOME POINTS
- Soccer injuries of the foot and ankle are becoming more prevalent due to the ever-growing popularity of the sport.
- Low ankle sprains represent the majority of foot and ankle–related injuries due to soccer and most can be treated non-operatively, with an early mobilization protocol followed by a phased rehabilitation.
- High ankle sprains are less common than low ankle sprains; however, they require a lengthier rehabilitation and most of the time are treated surgically.
- Impingement-like syndromes are common among soccer players and can be due to repetitive microtrauma from recurrent ball impact. Most of these syndromes respond favorably to non-operative modalities.
- Stress fractures of the foot, although less common, often require surgical stabilization in soccer players.
Use of Musculoskeletal Ultrasound and Regenerative Therapies in Soccer
ABSTRACT
Improvements in ultrasound technology have increased the popularity and use of ultrasound as a diagnostic and therapeutic modality for many soccer-related musculoskeletal (MSK) injuries. As a dynamic imaging modality, ultrasound offers increased accuracy and efficacy with minimally invasive procedures, such as guided injections, percutaneous tenotomy, and regenerative therapies, in the clinical setting. Emerging evidence indicates that regenerative therapies, such as platelet-rich-plasma (PRP), mesenchymal stem cells, and amniotic products, are a promising treatment for many MSK injuries and are gaining popularity among professional athletes. PRP is a safe treatment for a number of MSK conditions and has been included in the standard of care. However, conflicting evidence on return-to-play timeframes and efficacy in certain MSK conditions have led to inconsistent recommendations on indications for use, dose, and timing of treatment. Mesenchymal stem cell therapy, while promising, lacks high-level evidence of efficacy despite its increasing use among athletes. Currently, no data are available regarding the outcome of the use of amniotic products for the treatment of injuries in athletes. Furthermore, preparation of many regenerative therapies eclipses the concept of minimal manipulation and is subject to US Food and Drug Administration phase I to III trials. High-level research on regenerative medicine therapies should be continuously conducted to establish their clinical efficacy and safety data.
ULTRASOUND
Ultrasound (US) was first introduced for musculoskeletal (MSK) evaluation in 1957.1 Since then, US has gained increasing attention due to its ease of utilization in the clinical setting, repeatability, noninvasiveness, capability for contralateral comparison, lack of radiation exposure, and capability to provide real-time dynamic tissue assessment.1 Compared with magnetic resonance imaging or computed tomography, US presents limitations, including decreased resolution of certain tissues, limited field of view, limited penetration beyond osseous structures, incomplete evaluation of a joint or structure, and operator experience. However, advancements in technology, image resolution, and portability have improved the visualization of multiple anatomic structures and the accuracy of minimally invasive ultrasound-guided procedures at the point of care. The use of US for guided hip injections possibly decreases the cost relative to fluoroscopic guidance.2 Other studies have reported that US, as a result of its safety profile, has replaced fluoroscopy for certain procedures, such as barbotage of calcific tendinosis.3 US has been used for diagnostic purposes and guidance for therapeutic interventions, such as needle aspiration, diagnostic or therapeutic injection, needle tenotomy, tissue release, hydro-dissection, and biopsy.3 Given its expanding application, US has been increasingly used in the clinical setting, athletic training room, and sidelines of athletic events.
DIAGNOSTIC ULTRASOUND
An epidemiologic review of the National Collegiate Athletic Association (NCAA) men’s and women’s soccer injuries from 1988 to 2003 reported over 24,000 combined injuries. Over 70% of these injuries are MSK in nature and often affect the lower extremities.4,5 Ekstrand and colleagues6 also conducted an epidemiological review of muscle injuries among professional soccer players from 2001 to 2009. They found that 92% of all muscle injuries involved the lower extremities. The portability of US allows it to serve as an ideal modality for diagnostic evaluation of acute MSK injuries. Klauser and colleagues7 developed consensus based on the recommendations of the European Society of Musculoskeletal Radiology (ESSR) for the clinical indication of diagnostic ultrasound. A grading system was developed to describe the clinical utility of diagnostic US evaluation of MSK structures:
• Grade 0: Ultrasound is not indicated;
• Grade 1: Ultrasound is indicated if other imaging techniques are not appropriate;
• Grade 2: Ultrasound indication is equivalent to other imaging modalities;
• Grade 3: Ultrasound is the first-choice technique.
Henderson and colleagues8 conducted a review of 95 studies (12 systemic reviews and 83 diagnostic studies) that investigated the accuracy of diagnostic US imaging on soft tissue MSK injuries of the upper and lower extremities. They reported the sensitivity and specificity of the method for detection of over 40 hip, knee, ankle, and foot injuries and assigned corresponding grades based on diagnostic accuracy by using the same system developed by Klauser and colleagues.7,8 Common MSK injuries of the lower extremity and their corresponding ESSR grades are listed in the Table. This study demonstrated that diagnostic US is highly accurate for a number of soft tissue MSK injuries of the lower extremity and consistently matches the recommendation grades issued by Klauser and colleagues.7 In the hands of a skilled operator, US has become an increasingly popular and cost-effective modality for diagnosis and monitoring of acute muscle injuries and chronic tendinopathies among soccer athletes.
Table. Clinical Indication Grades for Diagnostic Ultrasound Evaluation of Common Lower Extremity Injuries7,8
Hip | Knee | Foot/Ankle |
Synovitis/Effusion: 3 | Quadricep tendinosis/tear: 3 | Anterior talofibular ligament injury: 3 |
Snapping hip (extra-articular): 3 | Patella tendinopathy: 3 | Calcaneofibular ligament injury: 3 |
Gluteal tendon tear: 3 | Pes anserine bursitis: 3 | Peroneal tendon tear/subluxation: 3 |
Meralgia paresthetica: 3 | Periarticular bursitis & ganglion: 3 | Posterior tibial tendinopathy: 3 |
Lateral femoral cutaneous nerve injury: 3 | Osgood-Schlatter & Sinding-Larsen: 3 | Plantaris tendon tear: 3 |
Femoral nerve injury: 3 | Synovitis/Effusion: 3 | Plantar fasciitis: 3 |
Sports hernia: 3 | Baker’s Cyst: 2-3 | Calcific tendonitis: 3 |
Morel-Lavallée lesions: 3 | MCL injury: 2 | Retrocalcaneal bursitis: 3 |
Muscle injury (high grade): 3 | IT band friction: 2 | Joint effusion: 3 |
Trochanteric bursitis: 2 | Medial patella plica syndrome: 2 | Ganglion cyst: 3 |
Proximal hamstring injury: 2 | Meniscal cyst: 2 | Retinacula pathology: 3 |
Sciatica: 1-2 | Common perineal neuropathy: 2 | Achilles tendinopathy: 2 |
Muscle injury (low grade): 1 | Distal hamstring tendon injury: 1-2 | Haglund disease: 2 |
Psoas tendon pathology: 1 | Intra-articular ganglion: 1 | Deltoid ligament injury: 2 |
Osteoarthritis: 0 | Hoffa’s fat pad syndrome: 1 | Plantar plate tear: 2 |
Labral tear: 0 | Loose bodies: 1 | Syndesmotic injury: 2 |
| LCL injury: 0-1 | Morton’s neuroma: 2 |
| Popliteal injury: 0-1 | Deltoid ligament injury: 1 |
| Plica syndrome: 0 | Spring ligament injury: 1 |
| Full/partial ACL tear: 0 | Anterolateral ankle impingement: 0 |
| PCL tear: 0 | Posterior talofibular ligament injury: 0 |
| Medial/lateral meniscus tear: 0 |
|
| Osteochondritis dissecans: 0 |
|
Abbreviations: ACL, anterior cruciate ligament; IT, iliotibial; LCL, lateral collateral ligament; MCL, medial collateral ligament; PCL, posterior cruciate ligament.
ULTRASOUND-GUIDED THERAPEUTIC PROCEDURES
The use of US at the point of care for needle guidance has led to its widespread application for therapeutic procedures, including injections and multiple regenerative therapies. Intra-articular US-guided injection and aspiration are common therapeutic interventions performed in the clinical setting. In a position statement of the American Medical Society for Sports Medicine, US-guided injections were found to be more accurate (SORT A evidence), effective (SORT B evidence), and cost effective (SORT B evidence) than landmark-guided injections.3 A recent meta-analysis conducted by Daniels and colleagues1 demonstrated the improved accuracy and efficacy of US-guided injections at the knee, ankle, and foot. Injections may serve a diagnostic purpose when anesthetics, such as lidocaine, are used in isolation, a therapeutic purpose, or both.
Continue to: Percutaneous tenotomy involve...
REGENERATIVE THERAPIES FOR MUSCULOSKELETAL CONDITIONS
PERCUTANEOUS TENOTOMY
Percutaneous tenotomy involves the introduction of a needle into damaged soft tissues, most often tendons (“needling”), in an effort to stimulate a healing response and resect the diseased tendon tissue. Although tenotomy was initially performed as an open or arthroscopic surgical technique, advances in US technology have led to improved sensitivity and specificity identifying areas of tendinous injury (hypervascularity, hypoechogenicity, and calcification); as such, the combination of these techniques has been used in the outpatient setting. New commercial models incorporate ultrasound guidance with needles or micro-resection probes for real-time débridement of damaged tissues. Percutaneous tenotomy has been described in the management of tendinopathy involving the rotator cuff, medial and lateral epicondyles, patellar and Achilles tendons, and plantar fascia.
Housner and colleagues9 evaluated the safety and short-term efficacy of US-guided needle tenotomy in 13 patients with chronic tendinosis of the patella, Achilles tendon, gluteus medius, iliotibial tract, hamstring, and rectus femoris. They reported no procedural complications and a significant decrease in pain scores at 4 and 12 weeks of follow-up.
Koh and colleagues10 conducted a prospective case series to evaluate the safety and efficacy of office-based, US-guided percutaneous tenotomy (using a commercial model) on 20 patients with chronic lateral epicondylitis. The authors reported no wound complications and significant improvement in pain scores at each follow-up period up to 1 year. Subsequent post-procedural US evaluation of injured tissues revealed evidence of healing (decreased tendon thickness, vascularity, and hypoechogenicity) in over half the cohort after 6 months compared with the baseline.11
Lee and colleagues12 evaluated the efficacy of US-guided needle tenotomy combined with platelet-rich plasma (PRP) injection on chronic recalcitrant gluteus medius tendinopathy. In this case series, 21 patients underwent PRP and “needling” through the hypoechoic regions of the injured tendon under direct US guidance. After a period of rest, all patients completed the structured rehabilitation protocol. After an average follow-up of 10 months, all patients displayed significant improvements in all outcome questionnaires and did not report any significant adverse events. The authors concluded that tenotomy combined with PRP is a safe and effective method for treatment for recalcitrant gluteus medius tendinopathy.
These studies indicate that US-guided percutaneous tenotomy, alone or in combination with regenerative therapies, such as PRP, is a safe and effective treatment option for various tendinopathies. However, while tenotomy appears safe with promising results and no reported major adverse events, the level of evidence remains low.
ORTHOBIOLOGICS
Orthobiologics are substances composed of biological materials that can be used to aid or even hasten the healing of bones, muscles, tendons, and ligaments. Orthobiologics may contain growth factors, which initiate or stimulate the body’s reparative process; matrix proteins, which serve as scaffolding for healing tissues; or stem cells, specifically adult stem cells, which are multipotent and can differentiate into several cell lines. Adult stem cells are categorized as hematopoietic, neural, epithelial, skin, and mesenchymal types. Mesenchymal stem cells (MSCs) are of particular interest in sports medicine applications because they secrete growth factors and cytokines with trophic, chemotactic, and immunosuppressive properties.13 MSCs are also multipotent and can differentiate into bones, muscles, cartilages, and tendons.14-17MSCs are readily isolated from many sources, including bone marrow, adipose tissues, synovial tissues, peripheral blood, skeletal muscles, umbilical cord blood, and placenta.13,14Several types of regenerative therapies used in orthopedic and sports medicine practice include PRP, stem cell therapy, and amniotic membrane/fluid preparations. While each therapy possesses the potential for promising results, the paucity of research and discrepancies among studies regarding the description of stem cell lines used limit the available evidence on the true clinical benefits of these regenerative therapies.
[HEAD 3] PLATELET-RICH PLASMA
PRP is an autologous product that has been used to stimulate biological factors and promote healing since the 1970s. Through the activation of platelets, PRP improves localized recruitment, proliferation, and differentiation of cells involved in tissue repair. Platelets, which are non-nucleated bodies located in peripheral blood, contain and release 3 groups of bioactive factors that enhance the healing process. Growth factors and cytokines released from alpha-granules play a role in cell proliferation, chemotaxis, cell differentiation, and angiogenesis. Bioactive factors, such as serotonin and histamine, released from dense granules, increase capillary permeability and improve cell recruitment and migration. Adhesion molecules also assist in cell migration and creation of an extracellular matrix, which acts as a scaffold for wound healing.18 Platelets are activated by mechanical trauma or contact with multiple activators, including Von Willebrand factor, collagen, thrombin, or calcium chloride. When activated, platelets release growth factors and cytokines, which create a pro-inflammatory environment that mediates the tissue repair process. After the procedure, the pro-inflammatory environment may result in patient discomfort, which can be managed with ice and acetaminophen. Use of nonsteroidal anti-inflammatory drugs may theoretically inhibit the inflammatory cascade induced by PRP, and they are avoided before and after the procedure, although evidence regarding necessary time frames is lacking.
Continue to: PRP consists of...
PRP consists of the fractionated liquid component of autologous whole blood, which contains increased concentrations of platelets and cytokines. Different methods and commercial preparations are available for collecting and preparing PRP. Variations in the amount of blood drawn, use of anticoagulants, presence or absence of an activating agent, number of centrifuge spins, and overall platelet and white blood cell concentrations lead to difficulty in evaluating and interpreting the available evidence regarding PRP therapy.
In vitro and animal studies demonstrated promising and safe results regarding the healing effect of PRP on injured soft tissues, such as tendons, ligaments, and muscles. In this regard, a number of studies have evaluated the effect of PRP on human MSK injuries. However, in addition to the above-mentioned variabilities in PRP, many of such studies lack standardization and randomization techniques and include a small number of patients only, thereby limiting the overall comparison and clinical application.
A landmark study conducted by Mishra and Pavelko19 concluded that PRP significantly reduced pain in patients with chronic elbow tendinosis. Similar findings were reported in high-level overhead athletes with ulnar collateral ligament insufficiency, which did not improve with conservative management.20 Fitzpatrick and colleagues21 found improvements in pain with the use of single PRP injection as treatment for chronic gluteal tendinopathy. PRP can effectively improve pain and recovery in chronic ligament and tendon injuries, such as lateral epicondylitis, patellar tendinopathy, and plantar fasciitis, when patients are unresponsive to traditional conservative management. The application of PRP to treat acute MSK injuries has produced mixed results. Hamid and colleagues22 conducted a level II randomized controlled trial to evaluate the effect of PRP combined with a rehabilitation program for treatment of grade 2 hamstring injuries on return-to-play compared with rehabilitation alone. Fourteen athletes were randomized into the study and control groups. Hamid and colleagues22 reported improved return-to-play in the study group compared with that in the control (26.7 and 42.5 days, respectively). This study also reported lower pain scores in the PRP group over time, but the difference was not statistically significant. Zanon and colleagues23 conducted a prospective study to evaluate return-to-play in professional soccer players with acute hamstring strains treated with PRP and a rehabilitation program. This study determined that athletes treated with PRP were “match fit,” meaning they would be available for match selection in an average of 36.8 days. However, Zanon and colleagues23 did not include a control group for comparison. Other studies reported that PRP treatment of acutely injured muscles and medial collateral ligaments of soccer and basketball players decreased their return-to-play interval.18 Reviews by Hamilton and colleagues24 and Pas and colleagues25 concluded that PRP treatment of acutely injured tissues with good blood supply (eg, hamstring muscles) did not improve pain or return-to-play compared with standardized rehabilitation protocols. Similarly, in a double-blinded placebo controlled trial, Reurink and colleagues26 evaluated return-to-play in 80 athletes with acute hamstring injuries treated with a rehabilitation program and either PRP or placebo. Reurink and colleagues26 found no difference in return-to-play (42 days for both groups), but the difference was not statistically significant. PRP has also been used intraoperatively and shows promising results in total knee arthroplasty, anterior cruciate ligament reconstruction, acute Achilles tendon repair, rotator cuff repair, and cartilage repair. However, many of these intraoperative studies are limited to animal models.
In 2009, the World Anti-Doping Agency (WADA) prohibited the use of PRP because it contains autologous growth factors and IGF-1, which could produce an anabolic effect. Recent studies have failed to demonstrate any athletic advantages of using PRP. WADA has since removed PRP from its prohibited list. PRP is also not prohibited by the US Anti-Doping Agency (USADA) and many major professional sporting leagues in the United States. However, care must be taken in reviewing the components of PRP because many commercially available products differ in PRP formulation. Since 2010, many team physicians have increasingly used PRP to treat a wide range of athletic injuries. A recent anonymous survey conducted by a team of physicians on PRP use in elite athletes revealed minimal complications but significant variability among physicians with regard to timing, belief in evidence, and formulation and dosing of PRP treatments. Many physicians did implicate athlete desire as the main indication for treatment.27
As an autologous treatment, PRP injection has no serious adverse effects beyond mild discomfort as a result of the procedure and pro-inflammatory state in the days following injection. Recent concerns regarding the potential of PRP treatment for heterotopic ossification have been reported, but published information is limited to case reports. PRP can improve pain and function in patients with chronic MSK injury. PRP appears to be a safe and effective alternative to surgery for patients with injury to poorly perfused tissue, which has not improved with conservative measures, such as rest, physical therapy, and anti-inflammatory medications. Care should be taken when treating athletes with PRP to establish regulations on doping by individual governing bodies.
Continue to: Use of stem...
STEM CELL THERAPY
Use of stem cell therapy is based on the properties of the proliferation and differentiation of multipoint MSC lines. These stem cells can theoretically regenerate injured tissues and influence repair through immunomodulation; paracrine activity through the release of bioactive agents, such as cytokines, trophic, and chemotactic molecules; and cell differentiation into various cell lineages.15,16,13,17 Orthopedic surgeons have used microfracture to recruit MSCs during cartilage repair procedures for over 20 years. This procedure draws multipotent MSCs to the injured site to induce chondrogenic proliferation and fibrocartilage repair.28
Adult MSCs provide a readily accessible autologous source of stem cells for regenerative therapies. MSCs can be isolated from a variety of tissues, including bone marrow, adipose tissues, synovia, human umbilical cord blood, and peripheral blood. The majority of stem cell therapies in the United States for sports medicine purposes are conducted using bone marrow aspirate concentrate (BMAC) and adipose tissues. The US Food and Drug Administration (FDA) allows the use of minimally manipulated autologous stem cells to be injected into the same patient on the same day. However, some studies reported that culturing stem cells or introducing products, such as collagenase to stem cells, can increase the stem cell concentration prior to injection. These processes constitute more than “minimal manipulation” and therefore would require drug trials prior to use in the United States.
Although MSCs can be readily obtained from a variety of tissue sources, the makeup of the cell concentrate differs. Bone marrow and adipose tissues are readily available sources of homogenous MSCs. Harvesting stem cells from adipose tissues provides a less invasive route of collection than from BMAC. Harvested BMAC and adipose tissues consist of heterogeneous cell populations that are composed of precursor and accessory cells, such as pericytes, endothelial cells, smooth muscle cells, fibroblasts, and macrophages in addition to MSCs.
Animal studies reported promising results when evaluating soft tissue lesions in small and large animal models.14,15 Although clinical and human evidence remains limited, the potential of MSCs for regenerative repair has led to a recent increase in the number of related clinical studies. Multiple systematic reviews have concluded that MSC therapy is safe for the treatment of osteoarthritis, cartilage lesions, and tendinopathies. Limited evidence is available regarding the safety of intramuscular use, and a theoretical concern arises on the development of heterotopic bone formation as a result of treatment.13,16 The efficacy of MSC therapy is difficult to determine due to the lack of standardization in stem cell populations, adjuvants (eg, PRP, hyaluronic acid, and scaffolding preparations), and delivery methods used.13,17
Similar to PRP, the increased use of MSC therapy among high-profile athletes has led to the promotion of these therapies as safe and effective despite limited evidence.29 Although MSC therapy is a promising and safe treatment option for patients with soft tissue injuries, the paucity in data and human studies limit its clinical use. Moreover, data of MSC efficacy is complicated because of the disparity between clinical studies regarding MSC collection method (many of which eclipse the “minimal manipulation” standard), description of isolated cell concentrates, dosage, method of delivery, use of adjuvants, and lack of randomization. Further studies using [standardized] methods are needed before establishing a true consensus on the safety and efficacy of MSC therapy.
AMNIOTIC MEMBRANE
The placenta is a source of MSCs, a collagen-rich extracellular matrix, and bioactive growth and regulatory factors. The capacity of the placenta to modulate biological activities and tissue formation is thought to provide a means of tissue repair and healing. The placenta consists of amniotic fluid, amniotic membrane (AM), chorionic membrane, and umbilical cord blood and tissues. Although MSCs have been isolated from each component of placental tissues, amniotic and chorionic membranes and umbilical cord tissues yield the highest concentration.
The majority of regenerative studies involving the placenta used AM alone or in combination with other placental tissues. AM is a metabolically active tissue that consists of an epithelial layer, a basement membrane, and a mesenchymal tissue layer. In addition to being a source of stem cells, AM synthesizes many growth factors, vasoactive peptides, and cytokines, which are capable of tissue regeneration. AM was initially used as a biological scaffold for the treatment of skin burns and wounds. Other intrinsic properties of AM include the provision of a matrix for cellular migration and proliferation, enhanced wound healing with reduced scar formation, antibacterial activity, and lastly, non-immunogenic and immunosuppressive properties. These inherent characteristics have spurred studies on the potential use of AM in sports medicine as a minimally invasive means to treat osteoarthritis and injuries of tendons, ligaments, muscles, fascia, and cartilages.
Continue to: Animal studies reported...
Animal studies reported positive results with the use of AM to treat osteoarthritis, cartilage defects, and tendon and ligament injuries. Few studies involving human participants also revealed favorable results with regard to the use of AM for the treatment of plantar fasciitis and osteoarthritis; however, these studies are industry-sponsored and employed small sample sizes. The unique mixture of a collagen-rich extracellular matrix, bioactive growth factors, and pluripotent stem cells may allow AM to become an effective treatment for MSK injuries. Although initial animal and human studies show promising results, variabilities regarding models (animal and human), pathologies, placental tissues, and methods of preparation, preservation, and delivery used limit the ability for comparison, analysis, and drawing of definitive conclusions. Thus far, no studies have evaluated the use of currently available AM products for the treatment of injuries sustained by soccer players.
Despite the current popularity of AM as regenerative therapy in academic research and potential use in clinical treatment in sports medicine, physicians should remain aware of the limited evidence available. Other barriers to research and use AM as a regenerative therapy include regulatory classifications based on the concept of “minimal manipulation” in biologic therapies. Minimally manipulated placental allografts are less regulated, less costly to study, and more easily commercialized. These products are not required to undergo FDA phase I to III trials prior to premarket approval. In 2000, the FDA position on all AM products falls into 2 categories. The first position states that AM that contains allogenic stem cells mixed with another drug that is micronized and/or cryopreserved is more than “minimally manipulated” and therefore categorized as “biologic” and would be subject to phase I to III trials. Dehydrated and decellularized AM, however, may meet the concept of minimal manipulation and is only approved by the FDA as a wound covering. Thus, any application of AM for the treatment of sports medicine pathology is not currently FDA-approved, considered off-label, not covered by insurance, and subject to out-of-pocket pay.30,31
CONCLUSION
With improvements in technology and portability, US has become an effective imaging modality for point-of-care evaluation, diagnosis, and continuous monitoring of many MSK injuries. Additionally, as a dynamic imaging modality, US allows for increased accuracy and efficacy when combined with minimally invasive procedures, such as diagnostic and therapeutic guided injections and percutaneous tenotomy, in the clinical setting; thereby decreasing the overall healthcare costs. PRP is proven to be a safe treatment for several MSK conditions, such as lateral epicondylitis, patellar tendonitis, and plantar fasciitis. Although PRP has been included in the standard of care in some areas, this technique may be predominantly athlete driven. Conflicting evidence with regard to return-to-play timeframes following PRP treatment for muscular injuries and poor evidence in conditions, such as Achilles tendonitis, have led to inconsistent indications for use, dose, and timing of treatment. Although early evidence of MSC therapy is promising, high-level evidence for MSC therapy is insufficient, despite its increased use among athletes. Thus far, no data are available regarding the outcomes of the use of amniotic products for the treatment of injuries among athletes. Furthermore, the preparation of amniotic products has many regulatory concerns. The authors advocate for continuous high-level research on regenerative medicine therapies to establish clinical efficacy and safety data.
1. Daniels E, Cole D, Jacobs B, Phillips S. Existing Evidence on ultrasound-guided injections in sports medicine. Orthop J Sports Med. 2018;6(2):2325967118756576. doi:10.1177/2325967118756576.
2. Henne M, Centurion A, Rosas S, Youmans H, Osbahr D. Trends in utilization of image-guided hip joint injections. Unpublished. 2018.
3. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine position statement: Interventional musculoskeletal ultrasound in sports medicine. Clin J Sport Med. 2015;25:6-22. doi:10.1097/JSM.0000000000000175.
4. Agel J, Evans TA, Dick R, Putukian M, Marshal S. Descriptive epidemiology of collegiate men’s soccer injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2002-2003. J Athl Train. 2007;42(2):270-277.
5. Dick R, Putukian M, Agel J, Evans T, Marshall S. Descriptive epidemiology of collegiate women’s soccer injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2002-2003. J Athl Train. 2007;42(2):278-285.
6. Ekstrand J, Hagglund M, Walden M. Epidemiology of muscle injuries in professional football (soccer). Am J Sports Med. 2011;39(6):1226-1232. doi:10.1177/0363546510395879.
7. Klauser A, Tagliafico A, Allen G, et al. Clinical indications for musculoskeletal ultrasound: A Delphi-based consensus paper of the European society of musculoskeletal radiology. Eur Radiol. 2012;22(5):1140-1148. doi:10.1007/s00330-011-2356-3.
8. Henderson R, Walker B, Young K. The accuracy of diagnostic ultrasound imaging for musculoskeletal soft tissue pathology of the extremities: a comprehensive review of the literature. Chiropr Man Therap. 2015;23(1):31. doi:10.1186/s12998-015-0076-5.
9. Housner JA, Jacobson JA, Misko R. Sonographically guided percutaneous needle tenotomy for the treatment of chronic tendinosis. J Ultrasound Med. 2009;28(9):1187-1192. doi:10.7863/jum.2009.28.9.1187.
10. Koh J, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644. doi:10.1177/0363546512470625.
11. Seng C, Mohan PC, Koh J, et al. Ultrasonic percutaneous tenotomy for recalcitrant lateral elbow tendinopathy: sustainability and sonographic progression at 3 years. Am J Sports Med. 2015;44(2):504-510. doi:10.1177/0363546515612758.
12. Lee J, Harrison J, Boachie-Adjei K, Vargas E, Moley P. Platelet-rich plasma injections with needle tenotomy for gluteus medius tendinopathy: A registry study with prospective follow-up. Orthop J Sports Med. 2016;4(11):2325967116671692. doi:10.1177/2325967116671692.
13. Osborne H, Anderson L, Burt P, Young M, Gerrard D. Australasian College of Sports Physicians-Position statement: the place of mesenchymal stem/stromal cell therapies in sport and exercise medicine. Br J Sports Med. 2016;50:1237-1244. doi:10.1136/bjsports-2015-095711.
14. Anderson J, Little D, Toth A, et al. Stem cell therapies for knee cartilage repair. The current status of preclinical and clinical studies. Am J Sports Med. 2013;42(9)2253-2261. doi:10.1177/0363546513508744.
15. Lee S, Kwon B, Lee Kyoungbun, Son Y, Chung S. Therapeutic mechanisms of human adipose-derived mesenchymal stem cells in a rat tendon injury model. Am J Sports Med. 2017;45(6):1429-1439. doi:10.1177/0363546517689874.
16. McIntyre J, Jones I, Han B, Vangsness C. Intra-articular mesenchymal stem cell therapy for the human joint. A systematic review. Am J Sports Med. 2017;0363546517735844. doi:10.1177/0363546517735844.
17. Pas HIMFL, Moen M, Haisma J, Winters M. No evidence for the use of stem cell therapy for tendon disorders: a systematic review. Br J Sports Med. 2017;51:996-1002. doi:10.1136/bjsports-2016-096794.
18. Foster T, Puskas B, Mandelbaum B, Gerhardt M, Rodeo S. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37(11):2259-2272. doi:10.1177/0363546509349921.
19. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778. doi:10.1177/0363546506288850.
20. Dines J, Williams P, ElAttrache N, et al. Platelet-rich plasma can be used to successfully treat elbow ulnar collateral ligament insufficiency in high-level throwers. Am J Orthop. 2016;45(4):296-300.
21. Fitzpatrick J, Bulsara M, O’Donnel J, McCrory P, Zheng M. The effectiveness of platelet-rich plasma injections in gluteal tendinopathy. A randomized, double-blind controlled trial comparing a single platelet-rich plasma injection with a single corticosteroid injection. Am J Sports Med. 2018;46(4)933-939. doi:10.1177/0363546517745525.
22. Hamid M, Ali M, Yusof A, George J, Lee L. Platelet-rich plasma injections for the treatment of hamstring injuries: A randomized controlled trial. Am J Sports Med. 2014;42(10):2410-2418. doi:10.1177/0363546514541540.
23. Zanon G, Combi F, Combi A, Perticarini L, Sammarchi L, Benazzo F. Platelet-rich plasma in the treatment of acute hamstring injuries in professional football players. Joints. 2016;4(1):17-23. doi:10.11138/jts/2016.4.1.017.
24. Hamilton B, Tol JL, Almusa E, et al. Platelet-rich plasma does not enhance return to play in hamstring injuries: a randomized controlled trial. Br J Sports Med. 2015;49:943-950. doi:10.1136/bjsports-2015-094603.
25. Pas HIMFL, Reurink G, Tol JL, Wier A, Winters M, Moen M. Efficacy of rehabilitation (lengthening) exercises, platelet-rich plasma injections, and other conservative interventions in acute hamstring injuries: an updated systematic review and meta-analysis. Br J Sports Med. 2015;49:1197-1205. doi:10.1136/bjsports-2015-094879.
26. Reurink G, Goudswaard G, Moen M, et al. Platelet-rich plasma injections in acute muscle injury. N Engl J Med. 2014;370:2546-2547. doi:10.1056/NEJMc1402340.
27. Kantrowitz D, Padaki A, Ahmad C, Lynch T. Defining platelet-rich plasma usage by team physicians in elite athletes. Orthop J Sports Med. 2018;6(4):2325967118767077. doi:10.1177/2325967118767077.
28. Mithoefer K, Peterson L, Zenobi-Wong M, Mandelbaum B. Cartilage issues in football-today’s problems and tomorrow’s solutions. Br J Sports Med. 2015;49(9):590-596. doi:1136/bjsports-2015-094772.
29. Matthews K, Cuchiara M. Regional regulatory insights: U.S. National Football League Athletes seeking unproven stem cell treatments. Stem Cells Dev. 2014;23(S1):60-64. doi:10.1089/scd.2014.0358.
30. McIntyre J, Jones I, Danilkovich A, Vangsness T. The placenta: applications in orthopaedic sports medicine. Am J Sports Med. 2018;46(1):234-247. doi:10.1177/0363546517697682.
31. Riboh J, Saltzman B, Yankee A, Cole BJ. Human amniotic membrane-derived products in sports medicine: Basic science, early results, and potential clinical applications. Am J Sports Med. 2015;44(9)2425-2434. doi:10.1177/0363546515612750.
ABSTRACT
Improvements in ultrasound technology have increased the popularity and use of ultrasound as a diagnostic and therapeutic modality for many soccer-related musculoskeletal (MSK) injuries. As a dynamic imaging modality, ultrasound offers increased accuracy and efficacy with minimally invasive procedures, such as guided injections, percutaneous tenotomy, and regenerative therapies, in the clinical setting. Emerging evidence indicates that regenerative therapies, such as platelet-rich-plasma (PRP), mesenchymal stem cells, and amniotic products, are a promising treatment for many MSK injuries and are gaining popularity among professional athletes. PRP is a safe treatment for a number of MSK conditions and has been included in the standard of care. However, conflicting evidence on return-to-play timeframes and efficacy in certain MSK conditions have led to inconsistent recommendations on indications for use, dose, and timing of treatment. Mesenchymal stem cell therapy, while promising, lacks high-level evidence of efficacy despite its increasing use among athletes. Currently, no data are available regarding the outcome of the use of amniotic products for the treatment of injuries in athletes. Furthermore, preparation of many regenerative therapies eclipses the concept of minimal manipulation and is subject to US Food and Drug Administration phase I to III trials. High-level research on regenerative medicine therapies should be continuously conducted to establish their clinical efficacy and safety data.
ULTRASOUND
Ultrasound (US) was first introduced for musculoskeletal (MSK) evaluation in 1957.1 Since then, US has gained increasing attention due to its ease of utilization in the clinical setting, repeatability, noninvasiveness, capability for contralateral comparison, lack of radiation exposure, and capability to provide real-time dynamic tissue assessment.1 Compared with magnetic resonance imaging or computed tomography, US presents limitations, including decreased resolution of certain tissues, limited field of view, limited penetration beyond osseous structures, incomplete evaluation of a joint or structure, and operator experience. However, advancements in technology, image resolution, and portability have improved the visualization of multiple anatomic structures and the accuracy of minimally invasive ultrasound-guided procedures at the point of care. The use of US for guided hip injections possibly decreases the cost relative to fluoroscopic guidance.2 Other studies have reported that US, as a result of its safety profile, has replaced fluoroscopy for certain procedures, such as barbotage of calcific tendinosis.3 US has been used for diagnostic purposes and guidance for therapeutic interventions, such as needle aspiration, diagnostic or therapeutic injection, needle tenotomy, tissue release, hydro-dissection, and biopsy.3 Given its expanding application, US has been increasingly used in the clinical setting, athletic training room, and sidelines of athletic events.
DIAGNOSTIC ULTRASOUND
An epidemiologic review of the National Collegiate Athletic Association (NCAA) men’s and women’s soccer injuries from 1988 to 2003 reported over 24,000 combined injuries. Over 70% of these injuries are MSK in nature and often affect the lower extremities.4,5 Ekstrand and colleagues6 also conducted an epidemiological review of muscle injuries among professional soccer players from 2001 to 2009. They found that 92% of all muscle injuries involved the lower extremities. The portability of US allows it to serve as an ideal modality for diagnostic evaluation of acute MSK injuries. Klauser and colleagues7 developed consensus based on the recommendations of the European Society of Musculoskeletal Radiology (ESSR) for the clinical indication of diagnostic ultrasound. A grading system was developed to describe the clinical utility of diagnostic US evaluation of MSK structures:
• Grade 0: Ultrasound is not indicated;
• Grade 1: Ultrasound is indicated if other imaging techniques are not appropriate;
• Grade 2: Ultrasound indication is equivalent to other imaging modalities;
• Grade 3: Ultrasound is the first-choice technique.
Henderson and colleagues8 conducted a review of 95 studies (12 systemic reviews and 83 diagnostic studies) that investigated the accuracy of diagnostic US imaging on soft tissue MSK injuries of the upper and lower extremities. They reported the sensitivity and specificity of the method for detection of over 40 hip, knee, ankle, and foot injuries and assigned corresponding grades based on diagnostic accuracy by using the same system developed by Klauser and colleagues.7,8 Common MSK injuries of the lower extremity and their corresponding ESSR grades are listed in the Table. This study demonstrated that diagnostic US is highly accurate for a number of soft tissue MSK injuries of the lower extremity and consistently matches the recommendation grades issued by Klauser and colleagues.7 In the hands of a skilled operator, US has become an increasingly popular and cost-effective modality for diagnosis and monitoring of acute muscle injuries and chronic tendinopathies among soccer athletes.
Table. Clinical Indication Grades for Diagnostic Ultrasound Evaluation of Common Lower Extremity Injuries7,8
Hip | Knee | Foot/Ankle |
Synovitis/Effusion: 3 | Quadricep tendinosis/tear: 3 | Anterior talofibular ligament injury: 3 |
Snapping hip (extra-articular): 3 | Patella tendinopathy: 3 | Calcaneofibular ligament injury: 3 |
Gluteal tendon tear: 3 | Pes anserine bursitis: 3 | Peroneal tendon tear/subluxation: 3 |
Meralgia paresthetica: 3 | Periarticular bursitis & ganglion: 3 | Posterior tibial tendinopathy: 3 |
Lateral femoral cutaneous nerve injury: 3 | Osgood-Schlatter & Sinding-Larsen: 3 | Plantaris tendon tear: 3 |
Femoral nerve injury: 3 | Synovitis/Effusion: 3 | Plantar fasciitis: 3 |
Sports hernia: 3 | Baker’s Cyst: 2-3 | Calcific tendonitis: 3 |
Morel-Lavallée lesions: 3 | MCL injury: 2 | Retrocalcaneal bursitis: 3 |
Muscle injury (high grade): 3 | IT band friction: 2 | Joint effusion: 3 |
Trochanteric bursitis: 2 | Medial patella plica syndrome: 2 | Ganglion cyst: 3 |
Proximal hamstring injury: 2 | Meniscal cyst: 2 | Retinacula pathology: 3 |
Sciatica: 1-2 | Common perineal neuropathy: 2 | Achilles tendinopathy: 2 |
Muscle injury (low grade): 1 | Distal hamstring tendon injury: 1-2 | Haglund disease: 2 |
Psoas tendon pathology: 1 | Intra-articular ganglion: 1 | Deltoid ligament injury: 2 |
Osteoarthritis: 0 | Hoffa’s fat pad syndrome: 1 | Plantar plate tear: 2 |
Labral tear: 0 | Loose bodies: 1 | Syndesmotic injury: 2 |
| LCL injury: 0-1 | Morton’s neuroma: 2 |
| Popliteal injury: 0-1 | Deltoid ligament injury: 1 |
| Plica syndrome: 0 | Spring ligament injury: 1 |
| Full/partial ACL tear: 0 | Anterolateral ankle impingement: 0 |
| PCL tear: 0 | Posterior talofibular ligament injury: 0 |
| Medial/lateral meniscus tear: 0 |
|
| Osteochondritis dissecans: 0 |
|
Abbreviations: ACL, anterior cruciate ligament; IT, iliotibial; LCL, lateral collateral ligament; MCL, medial collateral ligament; PCL, posterior cruciate ligament.
ULTRASOUND-GUIDED THERAPEUTIC PROCEDURES
The use of US at the point of care for needle guidance has led to its widespread application for therapeutic procedures, including injections and multiple regenerative therapies. Intra-articular US-guided injection and aspiration are common therapeutic interventions performed in the clinical setting. In a position statement of the American Medical Society for Sports Medicine, US-guided injections were found to be more accurate (SORT A evidence), effective (SORT B evidence), and cost effective (SORT B evidence) than landmark-guided injections.3 A recent meta-analysis conducted by Daniels and colleagues1 demonstrated the improved accuracy and efficacy of US-guided injections at the knee, ankle, and foot. Injections may serve a diagnostic purpose when anesthetics, such as lidocaine, are used in isolation, a therapeutic purpose, or both.
Continue to: Percutaneous tenotomy involve...
REGENERATIVE THERAPIES FOR MUSCULOSKELETAL CONDITIONS
PERCUTANEOUS TENOTOMY
Percutaneous tenotomy involves the introduction of a needle into damaged soft tissues, most often tendons (“needling”), in an effort to stimulate a healing response and resect the diseased tendon tissue. Although tenotomy was initially performed as an open or arthroscopic surgical technique, advances in US technology have led to improved sensitivity and specificity identifying areas of tendinous injury (hypervascularity, hypoechogenicity, and calcification); as such, the combination of these techniques has been used in the outpatient setting. New commercial models incorporate ultrasound guidance with needles or micro-resection probes for real-time débridement of damaged tissues. Percutaneous tenotomy has been described in the management of tendinopathy involving the rotator cuff, medial and lateral epicondyles, patellar and Achilles tendons, and plantar fascia.
Housner and colleagues9 evaluated the safety and short-term efficacy of US-guided needle tenotomy in 13 patients with chronic tendinosis of the patella, Achilles tendon, gluteus medius, iliotibial tract, hamstring, and rectus femoris. They reported no procedural complications and a significant decrease in pain scores at 4 and 12 weeks of follow-up.
Koh and colleagues10 conducted a prospective case series to evaluate the safety and efficacy of office-based, US-guided percutaneous tenotomy (using a commercial model) on 20 patients with chronic lateral epicondylitis. The authors reported no wound complications and significant improvement in pain scores at each follow-up period up to 1 year. Subsequent post-procedural US evaluation of injured tissues revealed evidence of healing (decreased tendon thickness, vascularity, and hypoechogenicity) in over half the cohort after 6 months compared with the baseline.11
Lee and colleagues12 evaluated the efficacy of US-guided needle tenotomy combined with platelet-rich plasma (PRP) injection on chronic recalcitrant gluteus medius tendinopathy. In this case series, 21 patients underwent PRP and “needling” through the hypoechoic regions of the injured tendon under direct US guidance. After a period of rest, all patients completed the structured rehabilitation protocol. After an average follow-up of 10 months, all patients displayed significant improvements in all outcome questionnaires and did not report any significant adverse events. The authors concluded that tenotomy combined with PRP is a safe and effective method for treatment for recalcitrant gluteus medius tendinopathy.
These studies indicate that US-guided percutaneous tenotomy, alone or in combination with regenerative therapies, such as PRP, is a safe and effective treatment option for various tendinopathies. However, while tenotomy appears safe with promising results and no reported major adverse events, the level of evidence remains low.
ORTHOBIOLOGICS
Orthobiologics are substances composed of biological materials that can be used to aid or even hasten the healing of bones, muscles, tendons, and ligaments. Orthobiologics may contain growth factors, which initiate or stimulate the body’s reparative process; matrix proteins, which serve as scaffolding for healing tissues; or stem cells, specifically adult stem cells, which are multipotent and can differentiate into several cell lines. Adult stem cells are categorized as hematopoietic, neural, epithelial, skin, and mesenchymal types. Mesenchymal stem cells (MSCs) are of particular interest in sports medicine applications because they secrete growth factors and cytokines with trophic, chemotactic, and immunosuppressive properties.13 MSCs are also multipotent and can differentiate into bones, muscles, cartilages, and tendons.14-17MSCs are readily isolated from many sources, including bone marrow, adipose tissues, synovial tissues, peripheral blood, skeletal muscles, umbilical cord blood, and placenta.13,14Several types of regenerative therapies used in orthopedic and sports medicine practice include PRP, stem cell therapy, and amniotic membrane/fluid preparations. While each therapy possesses the potential for promising results, the paucity of research and discrepancies among studies regarding the description of stem cell lines used limit the available evidence on the true clinical benefits of these regenerative therapies.
[HEAD 3] PLATELET-RICH PLASMA
PRP is an autologous product that has been used to stimulate biological factors and promote healing since the 1970s. Through the activation of platelets, PRP improves localized recruitment, proliferation, and differentiation of cells involved in tissue repair. Platelets, which are non-nucleated bodies located in peripheral blood, contain and release 3 groups of bioactive factors that enhance the healing process. Growth factors and cytokines released from alpha-granules play a role in cell proliferation, chemotaxis, cell differentiation, and angiogenesis. Bioactive factors, such as serotonin and histamine, released from dense granules, increase capillary permeability and improve cell recruitment and migration. Adhesion molecules also assist in cell migration and creation of an extracellular matrix, which acts as a scaffold for wound healing.18 Platelets are activated by mechanical trauma or contact with multiple activators, including Von Willebrand factor, collagen, thrombin, or calcium chloride. When activated, platelets release growth factors and cytokines, which create a pro-inflammatory environment that mediates the tissue repair process. After the procedure, the pro-inflammatory environment may result in patient discomfort, which can be managed with ice and acetaminophen. Use of nonsteroidal anti-inflammatory drugs may theoretically inhibit the inflammatory cascade induced by PRP, and they are avoided before and after the procedure, although evidence regarding necessary time frames is lacking.
Continue to: PRP consists of...
PRP consists of the fractionated liquid component of autologous whole blood, which contains increased concentrations of platelets and cytokines. Different methods and commercial preparations are available for collecting and preparing PRP. Variations in the amount of blood drawn, use of anticoagulants, presence or absence of an activating agent, number of centrifuge spins, and overall platelet and white blood cell concentrations lead to difficulty in evaluating and interpreting the available evidence regarding PRP therapy.
In vitro and animal studies demonstrated promising and safe results regarding the healing effect of PRP on injured soft tissues, such as tendons, ligaments, and muscles. In this regard, a number of studies have evaluated the effect of PRP on human MSK injuries. However, in addition to the above-mentioned variabilities in PRP, many of such studies lack standardization and randomization techniques and include a small number of patients only, thereby limiting the overall comparison and clinical application.
A landmark study conducted by Mishra and Pavelko19 concluded that PRP significantly reduced pain in patients with chronic elbow tendinosis. Similar findings were reported in high-level overhead athletes with ulnar collateral ligament insufficiency, which did not improve with conservative management.20 Fitzpatrick and colleagues21 found improvements in pain with the use of single PRP injection as treatment for chronic gluteal tendinopathy. PRP can effectively improve pain and recovery in chronic ligament and tendon injuries, such as lateral epicondylitis, patellar tendinopathy, and plantar fasciitis, when patients are unresponsive to traditional conservative management. The application of PRP to treat acute MSK injuries has produced mixed results. Hamid and colleagues22 conducted a level II randomized controlled trial to evaluate the effect of PRP combined with a rehabilitation program for treatment of grade 2 hamstring injuries on return-to-play compared with rehabilitation alone. Fourteen athletes were randomized into the study and control groups. Hamid and colleagues22 reported improved return-to-play in the study group compared with that in the control (26.7 and 42.5 days, respectively). This study also reported lower pain scores in the PRP group over time, but the difference was not statistically significant. Zanon and colleagues23 conducted a prospective study to evaluate return-to-play in professional soccer players with acute hamstring strains treated with PRP and a rehabilitation program. This study determined that athletes treated with PRP were “match fit,” meaning they would be available for match selection in an average of 36.8 days. However, Zanon and colleagues23 did not include a control group for comparison. Other studies reported that PRP treatment of acutely injured muscles and medial collateral ligaments of soccer and basketball players decreased their return-to-play interval.18 Reviews by Hamilton and colleagues24 and Pas and colleagues25 concluded that PRP treatment of acutely injured tissues with good blood supply (eg, hamstring muscles) did not improve pain or return-to-play compared with standardized rehabilitation protocols. Similarly, in a double-blinded placebo controlled trial, Reurink and colleagues26 evaluated return-to-play in 80 athletes with acute hamstring injuries treated with a rehabilitation program and either PRP or placebo. Reurink and colleagues26 found no difference in return-to-play (42 days for both groups), but the difference was not statistically significant. PRP has also been used intraoperatively and shows promising results in total knee arthroplasty, anterior cruciate ligament reconstruction, acute Achilles tendon repair, rotator cuff repair, and cartilage repair. However, many of these intraoperative studies are limited to animal models.
In 2009, the World Anti-Doping Agency (WADA) prohibited the use of PRP because it contains autologous growth factors and IGF-1, which could produce an anabolic effect. Recent studies have failed to demonstrate any athletic advantages of using PRP. WADA has since removed PRP from its prohibited list. PRP is also not prohibited by the US Anti-Doping Agency (USADA) and many major professional sporting leagues in the United States. However, care must be taken in reviewing the components of PRP because many commercially available products differ in PRP formulation. Since 2010, many team physicians have increasingly used PRP to treat a wide range of athletic injuries. A recent anonymous survey conducted by a team of physicians on PRP use in elite athletes revealed minimal complications but significant variability among physicians with regard to timing, belief in evidence, and formulation and dosing of PRP treatments. Many physicians did implicate athlete desire as the main indication for treatment.27
As an autologous treatment, PRP injection has no serious adverse effects beyond mild discomfort as a result of the procedure and pro-inflammatory state in the days following injection. Recent concerns regarding the potential of PRP treatment for heterotopic ossification have been reported, but published information is limited to case reports. PRP can improve pain and function in patients with chronic MSK injury. PRP appears to be a safe and effective alternative to surgery for patients with injury to poorly perfused tissue, which has not improved with conservative measures, such as rest, physical therapy, and anti-inflammatory medications. Care should be taken when treating athletes with PRP to establish regulations on doping by individual governing bodies.
Continue to: Use of stem...
STEM CELL THERAPY
Use of stem cell therapy is based on the properties of the proliferation and differentiation of multipoint MSC lines. These stem cells can theoretically regenerate injured tissues and influence repair through immunomodulation; paracrine activity through the release of bioactive agents, such as cytokines, trophic, and chemotactic molecules; and cell differentiation into various cell lineages.15,16,13,17 Orthopedic surgeons have used microfracture to recruit MSCs during cartilage repair procedures for over 20 years. This procedure draws multipotent MSCs to the injured site to induce chondrogenic proliferation and fibrocartilage repair.28
Adult MSCs provide a readily accessible autologous source of stem cells for regenerative therapies. MSCs can be isolated from a variety of tissues, including bone marrow, adipose tissues, synovia, human umbilical cord blood, and peripheral blood. The majority of stem cell therapies in the United States for sports medicine purposes are conducted using bone marrow aspirate concentrate (BMAC) and adipose tissues. The US Food and Drug Administration (FDA) allows the use of minimally manipulated autologous stem cells to be injected into the same patient on the same day. However, some studies reported that culturing stem cells or introducing products, such as collagenase to stem cells, can increase the stem cell concentration prior to injection. These processes constitute more than “minimal manipulation” and therefore would require drug trials prior to use in the United States.
Although MSCs can be readily obtained from a variety of tissue sources, the makeup of the cell concentrate differs. Bone marrow and adipose tissues are readily available sources of homogenous MSCs. Harvesting stem cells from adipose tissues provides a less invasive route of collection than from BMAC. Harvested BMAC and adipose tissues consist of heterogeneous cell populations that are composed of precursor and accessory cells, such as pericytes, endothelial cells, smooth muscle cells, fibroblasts, and macrophages in addition to MSCs.
Animal studies reported promising results when evaluating soft tissue lesions in small and large animal models.14,15 Although clinical and human evidence remains limited, the potential of MSCs for regenerative repair has led to a recent increase in the number of related clinical studies. Multiple systematic reviews have concluded that MSC therapy is safe for the treatment of osteoarthritis, cartilage lesions, and tendinopathies. Limited evidence is available regarding the safety of intramuscular use, and a theoretical concern arises on the development of heterotopic bone formation as a result of treatment.13,16 The efficacy of MSC therapy is difficult to determine due to the lack of standardization in stem cell populations, adjuvants (eg, PRP, hyaluronic acid, and scaffolding preparations), and delivery methods used.13,17
Similar to PRP, the increased use of MSC therapy among high-profile athletes has led to the promotion of these therapies as safe and effective despite limited evidence.29 Although MSC therapy is a promising and safe treatment option for patients with soft tissue injuries, the paucity in data and human studies limit its clinical use. Moreover, data of MSC efficacy is complicated because of the disparity between clinical studies regarding MSC collection method (many of which eclipse the “minimal manipulation” standard), description of isolated cell concentrates, dosage, method of delivery, use of adjuvants, and lack of randomization. Further studies using [standardized] methods are needed before establishing a true consensus on the safety and efficacy of MSC therapy.
AMNIOTIC MEMBRANE
The placenta is a source of MSCs, a collagen-rich extracellular matrix, and bioactive growth and regulatory factors. The capacity of the placenta to modulate biological activities and tissue formation is thought to provide a means of tissue repair and healing. The placenta consists of amniotic fluid, amniotic membrane (AM), chorionic membrane, and umbilical cord blood and tissues. Although MSCs have been isolated from each component of placental tissues, amniotic and chorionic membranes and umbilical cord tissues yield the highest concentration.
The majority of regenerative studies involving the placenta used AM alone or in combination with other placental tissues. AM is a metabolically active tissue that consists of an epithelial layer, a basement membrane, and a mesenchymal tissue layer. In addition to being a source of stem cells, AM synthesizes many growth factors, vasoactive peptides, and cytokines, which are capable of tissue regeneration. AM was initially used as a biological scaffold for the treatment of skin burns and wounds. Other intrinsic properties of AM include the provision of a matrix for cellular migration and proliferation, enhanced wound healing with reduced scar formation, antibacterial activity, and lastly, non-immunogenic and immunosuppressive properties. These inherent characteristics have spurred studies on the potential use of AM in sports medicine as a minimally invasive means to treat osteoarthritis and injuries of tendons, ligaments, muscles, fascia, and cartilages.
Continue to: Animal studies reported...
Animal studies reported positive results with the use of AM to treat osteoarthritis, cartilage defects, and tendon and ligament injuries. Few studies involving human participants also revealed favorable results with regard to the use of AM for the treatment of plantar fasciitis and osteoarthritis; however, these studies are industry-sponsored and employed small sample sizes. The unique mixture of a collagen-rich extracellular matrix, bioactive growth factors, and pluripotent stem cells may allow AM to become an effective treatment for MSK injuries. Although initial animal and human studies show promising results, variabilities regarding models (animal and human), pathologies, placental tissues, and methods of preparation, preservation, and delivery used limit the ability for comparison, analysis, and drawing of definitive conclusions. Thus far, no studies have evaluated the use of currently available AM products for the treatment of injuries sustained by soccer players.
Despite the current popularity of AM as regenerative therapy in academic research and potential use in clinical treatment in sports medicine, physicians should remain aware of the limited evidence available. Other barriers to research and use AM as a regenerative therapy include regulatory classifications based on the concept of “minimal manipulation” in biologic therapies. Minimally manipulated placental allografts are less regulated, less costly to study, and more easily commercialized. These products are not required to undergo FDA phase I to III trials prior to premarket approval. In 2000, the FDA position on all AM products falls into 2 categories. The first position states that AM that contains allogenic stem cells mixed with another drug that is micronized and/or cryopreserved is more than “minimally manipulated” and therefore categorized as “biologic” and would be subject to phase I to III trials. Dehydrated and decellularized AM, however, may meet the concept of minimal manipulation and is only approved by the FDA as a wound covering. Thus, any application of AM for the treatment of sports medicine pathology is not currently FDA-approved, considered off-label, not covered by insurance, and subject to out-of-pocket pay.30,31
CONCLUSION
With improvements in technology and portability, US has become an effective imaging modality for point-of-care evaluation, diagnosis, and continuous monitoring of many MSK injuries. Additionally, as a dynamic imaging modality, US allows for increased accuracy and efficacy when combined with minimally invasive procedures, such as diagnostic and therapeutic guided injections and percutaneous tenotomy, in the clinical setting; thereby decreasing the overall healthcare costs. PRP is proven to be a safe treatment for several MSK conditions, such as lateral epicondylitis, patellar tendonitis, and plantar fasciitis. Although PRP has been included in the standard of care in some areas, this technique may be predominantly athlete driven. Conflicting evidence with regard to return-to-play timeframes following PRP treatment for muscular injuries and poor evidence in conditions, such as Achilles tendonitis, have led to inconsistent indications for use, dose, and timing of treatment. Although early evidence of MSC therapy is promising, high-level evidence for MSC therapy is insufficient, despite its increased use among athletes. Thus far, no data are available regarding the outcomes of the use of amniotic products for the treatment of injuries among athletes. Furthermore, the preparation of amniotic products has many regulatory concerns. The authors advocate for continuous high-level research on regenerative medicine therapies to establish clinical efficacy and safety data.
ABSTRACT
Improvements in ultrasound technology have increased the popularity and use of ultrasound as a diagnostic and therapeutic modality for many soccer-related musculoskeletal (MSK) injuries. As a dynamic imaging modality, ultrasound offers increased accuracy and efficacy with minimally invasive procedures, such as guided injections, percutaneous tenotomy, and regenerative therapies, in the clinical setting. Emerging evidence indicates that regenerative therapies, such as platelet-rich-plasma (PRP), mesenchymal stem cells, and amniotic products, are a promising treatment for many MSK injuries and are gaining popularity among professional athletes. PRP is a safe treatment for a number of MSK conditions and has been included in the standard of care. However, conflicting evidence on return-to-play timeframes and efficacy in certain MSK conditions have led to inconsistent recommendations on indications for use, dose, and timing of treatment. Mesenchymal stem cell therapy, while promising, lacks high-level evidence of efficacy despite its increasing use among athletes. Currently, no data are available regarding the outcome of the use of amniotic products for the treatment of injuries in athletes. Furthermore, preparation of many regenerative therapies eclipses the concept of minimal manipulation and is subject to US Food and Drug Administration phase I to III trials. High-level research on regenerative medicine therapies should be continuously conducted to establish their clinical efficacy and safety data.
ULTRASOUND
Ultrasound (US) was first introduced for musculoskeletal (MSK) evaluation in 1957.1 Since then, US has gained increasing attention due to its ease of utilization in the clinical setting, repeatability, noninvasiveness, capability for contralateral comparison, lack of radiation exposure, and capability to provide real-time dynamic tissue assessment.1 Compared with magnetic resonance imaging or computed tomography, US presents limitations, including decreased resolution of certain tissues, limited field of view, limited penetration beyond osseous structures, incomplete evaluation of a joint or structure, and operator experience. However, advancements in technology, image resolution, and portability have improved the visualization of multiple anatomic structures and the accuracy of minimally invasive ultrasound-guided procedures at the point of care. The use of US for guided hip injections possibly decreases the cost relative to fluoroscopic guidance.2 Other studies have reported that US, as a result of its safety profile, has replaced fluoroscopy for certain procedures, such as barbotage of calcific tendinosis.3 US has been used for diagnostic purposes and guidance for therapeutic interventions, such as needle aspiration, diagnostic or therapeutic injection, needle tenotomy, tissue release, hydro-dissection, and biopsy.3 Given its expanding application, US has been increasingly used in the clinical setting, athletic training room, and sidelines of athletic events.
DIAGNOSTIC ULTRASOUND
An epidemiologic review of the National Collegiate Athletic Association (NCAA) men’s and women’s soccer injuries from 1988 to 2003 reported over 24,000 combined injuries. Over 70% of these injuries are MSK in nature and often affect the lower extremities.4,5 Ekstrand and colleagues6 also conducted an epidemiological review of muscle injuries among professional soccer players from 2001 to 2009. They found that 92% of all muscle injuries involved the lower extremities. The portability of US allows it to serve as an ideal modality for diagnostic evaluation of acute MSK injuries. Klauser and colleagues7 developed consensus based on the recommendations of the European Society of Musculoskeletal Radiology (ESSR) for the clinical indication of diagnostic ultrasound. A grading system was developed to describe the clinical utility of diagnostic US evaluation of MSK structures:
• Grade 0: Ultrasound is not indicated;
• Grade 1: Ultrasound is indicated if other imaging techniques are not appropriate;
• Grade 2: Ultrasound indication is equivalent to other imaging modalities;
• Grade 3: Ultrasound is the first-choice technique.
Henderson and colleagues8 conducted a review of 95 studies (12 systemic reviews and 83 diagnostic studies) that investigated the accuracy of diagnostic US imaging on soft tissue MSK injuries of the upper and lower extremities. They reported the sensitivity and specificity of the method for detection of over 40 hip, knee, ankle, and foot injuries and assigned corresponding grades based on diagnostic accuracy by using the same system developed by Klauser and colleagues.7,8 Common MSK injuries of the lower extremity and their corresponding ESSR grades are listed in the Table. This study demonstrated that diagnostic US is highly accurate for a number of soft tissue MSK injuries of the lower extremity and consistently matches the recommendation grades issued by Klauser and colleagues.7 In the hands of a skilled operator, US has become an increasingly popular and cost-effective modality for diagnosis and monitoring of acute muscle injuries and chronic tendinopathies among soccer athletes.
Table. Clinical Indication Grades for Diagnostic Ultrasound Evaluation of Common Lower Extremity Injuries7,8
Hip | Knee | Foot/Ankle |
Synovitis/Effusion: 3 | Quadricep tendinosis/tear: 3 | Anterior talofibular ligament injury: 3 |
Snapping hip (extra-articular): 3 | Patella tendinopathy: 3 | Calcaneofibular ligament injury: 3 |
Gluteal tendon tear: 3 | Pes anserine bursitis: 3 | Peroneal tendon tear/subluxation: 3 |
Meralgia paresthetica: 3 | Periarticular bursitis & ganglion: 3 | Posterior tibial tendinopathy: 3 |
Lateral femoral cutaneous nerve injury: 3 | Osgood-Schlatter & Sinding-Larsen: 3 | Plantaris tendon tear: 3 |
Femoral nerve injury: 3 | Synovitis/Effusion: 3 | Plantar fasciitis: 3 |
Sports hernia: 3 | Baker’s Cyst: 2-3 | Calcific tendonitis: 3 |
Morel-Lavallée lesions: 3 | MCL injury: 2 | Retrocalcaneal bursitis: 3 |
Muscle injury (high grade): 3 | IT band friction: 2 | Joint effusion: 3 |
Trochanteric bursitis: 2 | Medial patella plica syndrome: 2 | Ganglion cyst: 3 |
Proximal hamstring injury: 2 | Meniscal cyst: 2 | Retinacula pathology: 3 |
Sciatica: 1-2 | Common perineal neuropathy: 2 | Achilles tendinopathy: 2 |
Muscle injury (low grade): 1 | Distal hamstring tendon injury: 1-2 | Haglund disease: 2 |
Psoas tendon pathology: 1 | Intra-articular ganglion: 1 | Deltoid ligament injury: 2 |
Osteoarthritis: 0 | Hoffa’s fat pad syndrome: 1 | Plantar plate tear: 2 |
Labral tear: 0 | Loose bodies: 1 | Syndesmotic injury: 2 |
| LCL injury: 0-1 | Morton’s neuroma: 2 |
| Popliteal injury: 0-1 | Deltoid ligament injury: 1 |
| Plica syndrome: 0 | Spring ligament injury: 1 |
| Full/partial ACL tear: 0 | Anterolateral ankle impingement: 0 |
| PCL tear: 0 | Posterior talofibular ligament injury: 0 |
| Medial/lateral meniscus tear: 0 |
|
| Osteochondritis dissecans: 0 |
|
Abbreviations: ACL, anterior cruciate ligament; IT, iliotibial; LCL, lateral collateral ligament; MCL, medial collateral ligament; PCL, posterior cruciate ligament.
ULTRASOUND-GUIDED THERAPEUTIC PROCEDURES
The use of US at the point of care for needle guidance has led to its widespread application for therapeutic procedures, including injections and multiple regenerative therapies. Intra-articular US-guided injection and aspiration are common therapeutic interventions performed in the clinical setting. In a position statement of the American Medical Society for Sports Medicine, US-guided injections were found to be more accurate (SORT A evidence), effective (SORT B evidence), and cost effective (SORT B evidence) than landmark-guided injections.3 A recent meta-analysis conducted by Daniels and colleagues1 demonstrated the improved accuracy and efficacy of US-guided injections at the knee, ankle, and foot. Injections may serve a diagnostic purpose when anesthetics, such as lidocaine, are used in isolation, a therapeutic purpose, or both.
Continue to: Percutaneous tenotomy involve...
REGENERATIVE THERAPIES FOR MUSCULOSKELETAL CONDITIONS
PERCUTANEOUS TENOTOMY
Percutaneous tenotomy involves the introduction of a needle into damaged soft tissues, most often tendons (“needling”), in an effort to stimulate a healing response and resect the diseased tendon tissue. Although tenotomy was initially performed as an open or arthroscopic surgical technique, advances in US technology have led to improved sensitivity and specificity identifying areas of tendinous injury (hypervascularity, hypoechogenicity, and calcification); as such, the combination of these techniques has been used in the outpatient setting. New commercial models incorporate ultrasound guidance with needles or micro-resection probes for real-time débridement of damaged tissues. Percutaneous tenotomy has been described in the management of tendinopathy involving the rotator cuff, medial and lateral epicondyles, patellar and Achilles tendons, and plantar fascia.
Housner and colleagues9 evaluated the safety and short-term efficacy of US-guided needle tenotomy in 13 patients with chronic tendinosis of the patella, Achilles tendon, gluteus medius, iliotibial tract, hamstring, and rectus femoris. They reported no procedural complications and a significant decrease in pain scores at 4 and 12 weeks of follow-up.
Koh and colleagues10 conducted a prospective case series to evaluate the safety and efficacy of office-based, US-guided percutaneous tenotomy (using a commercial model) on 20 patients with chronic lateral epicondylitis. The authors reported no wound complications and significant improvement in pain scores at each follow-up period up to 1 year. Subsequent post-procedural US evaluation of injured tissues revealed evidence of healing (decreased tendon thickness, vascularity, and hypoechogenicity) in over half the cohort after 6 months compared with the baseline.11
Lee and colleagues12 evaluated the efficacy of US-guided needle tenotomy combined with platelet-rich plasma (PRP) injection on chronic recalcitrant gluteus medius tendinopathy. In this case series, 21 patients underwent PRP and “needling” through the hypoechoic regions of the injured tendon under direct US guidance. After a period of rest, all patients completed the structured rehabilitation protocol. After an average follow-up of 10 months, all patients displayed significant improvements in all outcome questionnaires and did not report any significant adverse events. The authors concluded that tenotomy combined with PRP is a safe and effective method for treatment for recalcitrant gluteus medius tendinopathy.
These studies indicate that US-guided percutaneous tenotomy, alone or in combination with regenerative therapies, such as PRP, is a safe and effective treatment option for various tendinopathies. However, while tenotomy appears safe with promising results and no reported major adverse events, the level of evidence remains low.
ORTHOBIOLOGICS
Orthobiologics are substances composed of biological materials that can be used to aid or even hasten the healing of bones, muscles, tendons, and ligaments. Orthobiologics may contain growth factors, which initiate or stimulate the body’s reparative process; matrix proteins, which serve as scaffolding for healing tissues; or stem cells, specifically adult stem cells, which are multipotent and can differentiate into several cell lines. Adult stem cells are categorized as hematopoietic, neural, epithelial, skin, and mesenchymal types. Mesenchymal stem cells (MSCs) are of particular interest in sports medicine applications because they secrete growth factors and cytokines with trophic, chemotactic, and immunosuppressive properties.13 MSCs are also multipotent and can differentiate into bones, muscles, cartilages, and tendons.14-17MSCs are readily isolated from many sources, including bone marrow, adipose tissues, synovial tissues, peripheral blood, skeletal muscles, umbilical cord blood, and placenta.13,14Several types of regenerative therapies used in orthopedic and sports medicine practice include PRP, stem cell therapy, and amniotic membrane/fluid preparations. While each therapy possesses the potential for promising results, the paucity of research and discrepancies among studies regarding the description of stem cell lines used limit the available evidence on the true clinical benefits of these regenerative therapies.
[HEAD 3] PLATELET-RICH PLASMA
PRP is an autologous product that has been used to stimulate biological factors and promote healing since the 1970s. Through the activation of platelets, PRP improves localized recruitment, proliferation, and differentiation of cells involved in tissue repair. Platelets, which are non-nucleated bodies located in peripheral blood, contain and release 3 groups of bioactive factors that enhance the healing process. Growth factors and cytokines released from alpha-granules play a role in cell proliferation, chemotaxis, cell differentiation, and angiogenesis. Bioactive factors, such as serotonin and histamine, released from dense granules, increase capillary permeability and improve cell recruitment and migration. Adhesion molecules also assist in cell migration and creation of an extracellular matrix, which acts as a scaffold for wound healing.18 Platelets are activated by mechanical trauma or contact with multiple activators, including Von Willebrand factor, collagen, thrombin, or calcium chloride. When activated, platelets release growth factors and cytokines, which create a pro-inflammatory environment that mediates the tissue repair process. After the procedure, the pro-inflammatory environment may result in patient discomfort, which can be managed with ice and acetaminophen. Use of nonsteroidal anti-inflammatory drugs may theoretically inhibit the inflammatory cascade induced by PRP, and they are avoided before and after the procedure, although evidence regarding necessary time frames is lacking.
Continue to: PRP consists of...
PRP consists of the fractionated liquid component of autologous whole blood, which contains increased concentrations of platelets and cytokines. Different methods and commercial preparations are available for collecting and preparing PRP. Variations in the amount of blood drawn, use of anticoagulants, presence or absence of an activating agent, number of centrifuge spins, and overall platelet and white blood cell concentrations lead to difficulty in evaluating and interpreting the available evidence regarding PRP therapy.
In vitro and animal studies demonstrated promising and safe results regarding the healing effect of PRP on injured soft tissues, such as tendons, ligaments, and muscles. In this regard, a number of studies have evaluated the effect of PRP on human MSK injuries. However, in addition to the above-mentioned variabilities in PRP, many of such studies lack standardization and randomization techniques and include a small number of patients only, thereby limiting the overall comparison and clinical application.
A landmark study conducted by Mishra and Pavelko19 concluded that PRP significantly reduced pain in patients with chronic elbow tendinosis. Similar findings were reported in high-level overhead athletes with ulnar collateral ligament insufficiency, which did not improve with conservative management.20 Fitzpatrick and colleagues21 found improvements in pain with the use of single PRP injection as treatment for chronic gluteal tendinopathy. PRP can effectively improve pain and recovery in chronic ligament and tendon injuries, such as lateral epicondylitis, patellar tendinopathy, and plantar fasciitis, when patients are unresponsive to traditional conservative management. The application of PRP to treat acute MSK injuries has produced mixed results. Hamid and colleagues22 conducted a level II randomized controlled trial to evaluate the effect of PRP combined with a rehabilitation program for treatment of grade 2 hamstring injuries on return-to-play compared with rehabilitation alone. Fourteen athletes were randomized into the study and control groups. Hamid and colleagues22 reported improved return-to-play in the study group compared with that in the control (26.7 and 42.5 days, respectively). This study also reported lower pain scores in the PRP group over time, but the difference was not statistically significant. Zanon and colleagues23 conducted a prospective study to evaluate return-to-play in professional soccer players with acute hamstring strains treated with PRP and a rehabilitation program. This study determined that athletes treated with PRP were “match fit,” meaning they would be available for match selection in an average of 36.8 days. However, Zanon and colleagues23 did not include a control group for comparison. Other studies reported that PRP treatment of acutely injured muscles and medial collateral ligaments of soccer and basketball players decreased their return-to-play interval.18 Reviews by Hamilton and colleagues24 and Pas and colleagues25 concluded that PRP treatment of acutely injured tissues with good blood supply (eg, hamstring muscles) did not improve pain or return-to-play compared with standardized rehabilitation protocols. Similarly, in a double-blinded placebo controlled trial, Reurink and colleagues26 evaluated return-to-play in 80 athletes with acute hamstring injuries treated with a rehabilitation program and either PRP or placebo. Reurink and colleagues26 found no difference in return-to-play (42 days for both groups), but the difference was not statistically significant. PRP has also been used intraoperatively and shows promising results in total knee arthroplasty, anterior cruciate ligament reconstruction, acute Achilles tendon repair, rotator cuff repair, and cartilage repair. However, many of these intraoperative studies are limited to animal models.
In 2009, the World Anti-Doping Agency (WADA) prohibited the use of PRP because it contains autologous growth factors and IGF-1, which could produce an anabolic effect. Recent studies have failed to demonstrate any athletic advantages of using PRP. WADA has since removed PRP from its prohibited list. PRP is also not prohibited by the US Anti-Doping Agency (USADA) and many major professional sporting leagues in the United States. However, care must be taken in reviewing the components of PRP because many commercially available products differ in PRP formulation. Since 2010, many team physicians have increasingly used PRP to treat a wide range of athletic injuries. A recent anonymous survey conducted by a team of physicians on PRP use in elite athletes revealed minimal complications but significant variability among physicians with regard to timing, belief in evidence, and formulation and dosing of PRP treatments. Many physicians did implicate athlete desire as the main indication for treatment.27
As an autologous treatment, PRP injection has no serious adverse effects beyond mild discomfort as a result of the procedure and pro-inflammatory state in the days following injection. Recent concerns regarding the potential of PRP treatment for heterotopic ossification have been reported, but published information is limited to case reports. PRP can improve pain and function in patients with chronic MSK injury. PRP appears to be a safe and effective alternative to surgery for patients with injury to poorly perfused tissue, which has not improved with conservative measures, such as rest, physical therapy, and anti-inflammatory medications. Care should be taken when treating athletes with PRP to establish regulations on doping by individual governing bodies.
Continue to: Use of stem...
STEM CELL THERAPY
Use of stem cell therapy is based on the properties of the proliferation and differentiation of multipoint MSC lines. These stem cells can theoretically regenerate injured tissues and influence repair through immunomodulation; paracrine activity through the release of bioactive agents, such as cytokines, trophic, and chemotactic molecules; and cell differentiation into various cell lineages.15,16,13,17 Orthopedic surgeons have used microfracture to recruit MSCs during cartilage repair procedures for over 20 years. This procedure draws multipotent MSCs to the injured site to induce chondrogenic proliferation and fibrocartilage repair.28
Adult MSCs provide a readily accessible autologous source of stem cells for regenerative therapies. MSCs can be isolated from a variety of tissues, including bone marrow, adipose tissues, synovia, human umbilical cord blood, and peripheral blood. The majority of stem cell therapies in the United States for sports medicine purposes are conducted using bone marrow aspirate concentrate (BMAC) and adipose tissues. The US Food and Drug Administration (FDA) allows the use of minimally manipulated autologous stem cells to be injected into the same patient on the same day. However, some studies reported that culturing stem cells or introducing products, such as collagenase to stem cells, can increase the stem cell concentration prior to injection. These processes constitute more than “minimal manipulation” and therefore would require drug trials prior to use in the United States.
Although MSCs can be readily obtained from a variety of tissue sources, the makeup of the cell concentrate differs. Bone marrow and adipose tissues are readily available sources of homogenous MSCs. Harvesting stem cells from adipose tissues provides a less invasive route of collection than from BMAC. Harvested BMAC and adipose tissues consist of heterogeneous cell populations that are composed of precursor and accessory cells, such as pericytes, endothelial cells, smooth muscle cells, fibroblasts, and macrophages in addition to MSCs.
Animal studies reported promising results when evaluating soft tissue lesions in small and large animal models.14,15 Although clinical and human evidence remains limited, the potential of MSCs for regenerative repair has led to a recent increase in the number of related clinical studies. Multiple systematic reviews have concluded that MSC therapy is safe for the treatment of osteoarthritis, cartilage lesions, and tendinopathies. Limited evidence is available regarding the safety of intramuscular use, and a theoretical concern arises on the development of heterotopic bone formation as a result of treatment.13,16 The efficacy of MSC therapy is difficult to determine due to the lack of standardization in stem cell populations, adjuvants (eg, PRP, hyaluronic acid, and scaffolding preparations), and delivery methods used.13,17
Similar to PRP, the increased use of MSC therapy among high-profile athletes has led to the promotion of these therapies as safe and effective despite limited evidence.29 Although MSC therapy is a promising and safe treatment option for patients with soft tissue injuries, the paucity in data and human studies limit its clinical use. Moreover, data of MSC efficacy is complicated because of the disparity between clinical studies regarding MSC collection method (many of which eclipse the “minimal manipulation” standard), description of isolated cell concentrates, dosage, method of delivery, use of adjuvants, and lack of randomization. Further studies using [standardized] methods are needed before establishing a true consensus on the safety and efficacy of MSC therapy.
AMNIOTIC MEMBRANE
The placenta is a source of MSCs, a collagen-rich extracellular matrix, and bioactive growth and regulatory factors. The capacity of the placenta to modulate biological activities and tissue formation is thought to provide a means of tissue repair and healing. The placenta consists of amniotic fluid, amniotic membrane (AM), chorionic membrane, and umbilical cord blood and tissues. Although MSCs have been isolated from each component of placental tissues, amniotic and chorionic membranes and umbilical cord tissues yield the highest concentration.
The majority of regenerative studies involving the placenta used AM alone or in combination with other placental tissues. AM is a metabolically active tissue that consists of an epithelial layer, a basement membrane, and a mesenchymal tissue layer. In addition to being a source of stem cells, AM synthesizes many growth factors, vasoactive peptides, and cytokines, which are capable of tissue regeneration. AM was initially used as a biological scaffold for the treatment of skin burns and wounds. Other intrinsic properties of AM include the provision of a matrix for cellular migration and proliferation, enhanced wound healing with reduced scar formation, antibacterial activity, and lastly, non-immunogenic and immunosuppressive properties. These inherent characteristics have spurred studies on the potential use of AM in sports medicine as a minimally invasive means to treat osteoarthritis and injuries of tendons, ligaments, muscles, fascia, and cartilages.
Continue to: Animal studies reported...
Animal studies reported positive results with the use of AM to treat osteoarthritis, cartilage defects, and tendon and ligament injuries. Few studies involving human participants also revealed favorable results with regard to the use of AM for the treatment of plantar fasciitis and osteoarthritis; however, these studies are industry-sponsored and employed small sample sizes. The unique mixture of a collagen-rich extracellular matrix, bioactive growth factors, and pluripotent stem cells may allow AM to become an effective treatment for MSK injuries. Although initial animal and human studies show promising results, variabilities regarding models (animal and human), pathologies, placental tissues, and methods of preparation, preservation, and delivery used limit the ability for comparison, analysis, and drawing of definitive conclusions. Thus far, no studies have evaluated the use of currently available AM products for the treatment of injuries sustained by soccer players.
Despite the current popularity of AM as regenerative therapy in academic research and potential use in clinical treatment in sports medicine, physicians should remain aware of the limited evidence available. Other barriers to research and use AM as a regenerative therapy include regulatory classifications based on the concept of “minimal manipulation” in biologic therapies. Minimally manipulated placental allografts are less regulated, less costly to study, and more easily commercialized. These products are not required to undergo FDA phase I to III trials prior to premarket approval. In 2000, the FDA position on all AM products falls into 2 categories. The first position states that AM that contains allogenic stem cells mixed with another drug that is micronized and/or cryopreserved is more than “minimally manipulated” and therefore categorized as “biologic” and would be subject to phase I to III trials. Dehydrated and decellularized AM, however, may meet the concept of minimal manipulation and is only approved by the FDA as a wound covering. Thus, any application of AM for the treatment of sports medicine pathology is not currently FDA-approved, considered off-label, not covered by insurance, and subject to out-of-pocket pay.30,31
CONCLUSION
With improvements in technology and portability, US has become an effective imaging modality for point-of-care evaluation, diagnosis, and continuous monitoring of many MSK injuries. Additionally, as a dynamic imaging modality, US allows for increased accuracy and efficacy when combined with minimally invasive procedures, such as diagnostic and therapeutic guided injections and percutaneous tenotomy, in the clinical setting; thereby decreasing the overall healthcare costs. PRP is proven to be a safe treatment for several MSK conditions, such as lateral epicondylitis, patellar tendonitis, and plantar fasciitis. Although PRP has been included in the standard of care in some areas, this technique may be predominantly athlete driven. Conflicting evidence with regard to return-to-play timeframes following PRP treatment for muscular injuries and poor evidence in conditions, such as Achilles tendonitis, have led to inconsistent indications for use, dose, and timing of treatment. Although early evidence of MSC therapy is promising, high-level evidence for MSC therapy is insufficient, despite its increased use among athletes. Thus far, no data are available regarding the outcomes of the use of amniotic products for the treatment of injuries among athletes. Furthermore, the preparation of amniotic products has many regulatory concerns. The authors advocate for continuous high-level research on regenerative medicine therapies to establish clinical efficacy and safety data.
1. Daniels E, Cole D, Jacobs B, Phillips S. Existing Evidence on ultrasound-guided injections in sports medicine. Orthop J Sports Med. 2018;6(2):2325967118756576. doi:10.1177/2325967118756576.
2. Henne M, Centurion A, Rosas S, Youmans H, Osbahr D. Trends in utilization of image-guided hip joint injections. Unpublished. 2018.
3. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine position statement: Interventional musculoskeletal ultrasound in sports medicine. Clin J Sport Med. 2015;25:6-22. doi:10.1097/JSM.0000000000000175.
4. Agel J, Evans TA, Dick R, Putukian M, Marshal S. Descriptive epidemiology of collegiate men’s soccer injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2002-2003. J Athl Train. 2007;42(2):270-277.
5. Dick R, Putukian M, Agel J, Evans T, Marshall S. Descriptive epidemiology of collegiate women’s soccer injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2002-2003. J Athl Train. 2007;42(2):278-285.
6. Ekstrand J, Hagglund M, Walden M. Epidemiology of muscle injuries in professional football (soccer). Am J Sports Med. 2011;39(6):1226-1232. doi:10.1177/0363546510395879.
7. Klauser A, Tagliafico A, Allen G, et al. Clinical indications for musculoskeletal ultrasound: A Delphi-based consensus paper of the European society of musculoskeletal radiology. Eur Radiol. 2012;22(5):1140-1148. doi:10.1007/s00330-011-2356-3.
8. Henderson R, Walker B, Young K. The accuracy of diagnostic ultrasound imaging for musculoskeletal soft tissue pathology of the extremities: a comprehensive review of the literature. Chiropr Man Therap. 2015;23(1):31. doi:10.1186/s12998-015-0076-5.
9. Housner JA, Jacobson JA, Misko R. Sonographically guided percutaneous needle tenotomy for the treatment of chronic tendinosis. J Ultrasound Med. 2009;28(9):1187-1192. doi:10.7863/jum.2009.28.9.1187.
10. Koh J, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644. doi:10.1177/0363546512470625.
11. Seng C, Mohan PC, Koh J, et al. Ultrasonic percutaneous tenotomy for recalcitrant lateral elbow tendinopathy: sustainability and sonographic progression at 3 years. Am J Sports Med. 2015;44(2):504-510. doi:10.1177/0363546515612758.
12. Lee J, Harrison J, Boachie-Adjei K, Vargas E, Moley P. Platelet-rich plasma injections with needle tenotomy for gluteus medius tendinopathy: A registry study with prospective follow-up. Orthop J Sports Med. 2016;4(11):2325967116671692. doi:10.1177/2325967116671692.
13. Osborne H, Anderson L, Burt P, Young M, Gerrard D. Australasian College of Sports Physicians-Position statement: the place of mesenchymal stem/stromal cell therapies in sport and exercise medicine. Br J Sports Med. 2016;50:1237-1244. doi:10.1136/bjsports-2015-095711.
14. Anderson J, Little D, Toth A, et al. Stem cell therapies for knee cartilage repair. The current status of preclinical and clinical studies. Am J Sports Med. 2013;42(9)2253-2261. doi:10.1177/0363546513508744.
15. Lee S, Kwon B, Lee Kyoungbun, Son Y, Chung S. Therapeutic mechanisms of human adipose-derived mesenchymal stem cells in a rat tendon injury model. Am J Sports Med. 2017;45(6):1429-1439. doi:10.1177/0363546517689874.
16. McIntyre J, Jones I, Han B, Vangsness C. Intra-articular mesenchymal stem cell therapy for the human joint. A systematic review. Am J Sports Med. 2017;0363546517735844. doi:10.1177/0363546517735844.
17. Pas HIMFL, Moen M, Haisma J, Winters M. No evidence for the use of stem cell therapy for tendon disorders: a systematic review. Br J Sports Med. 2017;51:996-1002. doi:10.1136/bjsports-2016-096794.
18. Foster T, Puskas B, Mandelbaum B, Gerhardt M, Rodeo S. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37(11):2259-2272. doi:10.1177/0363546509349921.
19. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778. doi:10.1177/0363546506288850.
20. Dines J, Williams P, ElAttrache N, et al. Platelet-rich plasma can be used to successfully treat elbow ulnar collateral ligament insufficiency in high-level throwers. Am J Orthop. 2016;45(4):296-300.
21. Fitzpatrick J, Bulsara M, O’Donnel J, McCrory P, Zheng M. The effectiveness of platelet-rich plasma injections in gluteal tendinopathy. A randomized, double-blind controlled trial comparing a single platelet-rich plasma injection with a single corticosteroid injection. Am J Sports Med. 2018;46(4)933-939. doi:10.1177/0363546517745525.
22. Hamid M, Ali M, Yusof A, George J, Lee L. Platelet-rich plasma injections for the treatment of hamstring injuries: A randomized controlled trial. Am J Sports Med. 2014;42(10):2410-2418. doi:10.1177/0363546514541540.
23. Zanon G, Combi F, Combi A, Perticarini L, Sammarchi L, Benazzo F. Platelet-rich plasma in the treatment of acute hamstring injuries in professional football players. Joints. 2016;4(1):17-23. doi:10.11138/jts/2016.4.1.017.
24. Hamilton B, Tol JL, Almusa E, et al. Platelet-rich plasma does not enhance return to play in hamstring injuries: a randomized controlled trial. Br J Sports Med. 2015;49:943-950. doi:10.1136/bjsports-2015-094603.
25. Pas HIMFL, Reurink G, Tol JL, Wier A, Winters M, Moen M. Efficacy of rehabilitation (lengthening) exercises, platelet-rich plasma injections, and other conservative interventions in acute hamstring injuries: an updated systematic review and meta-analysis. Br J Sports Med. 2015;49:1197-1205. doi:10.1136/bjsports-2015-094879.
26. Reurink G, Goudswaard G, Moen M, et al. Platelet-rich plasma injections in acute muscle injury. N Engl J Med. 2014;370:2546-2547. doi:10.1056/NEJMc1402340.
27. Kantrowitz D, Padaki A, Ahmad C, Lynch T. Defining platelet-rich plasma usage by team physicians in elite athletes. Orthop J Sports Med. 2018;6(4):2325967118767077. doi:10.1177/2325967118767077.
28. Mithoefer K, Peterson L, Zenobi-Wong M, Mandelbaum B. Cartilage issues in football-today’s problems and tomorrow’s solutions. Br J Sports Med. 2015;49(9):590-596. doi:1136/bjsports-2015-094772.
29. Matthews K, Cuchiara M. Regional regulatory insights: U.S. National Football League Athletes seeking unproven stem cell treatments. Stem Cells Dev. 2014;23(S1):60-64. doi:10.1089/scd.2014.0358.
30. McIntyre J, Jones I, Danilkovich A, Vangsness T. The placenta: applications in orthopaedic sports medicine. Am J Sports Med. 2018;46(1):234-247. doi:10.1177/0363546517697682.
31. Riboh J, Saltzman B, Yankee A, Cole BJ. Human amniotic membrane-derived products in sports medicine: Basic science, early results, and potential clinical applications. Am J Sports Med. 2015;44(9)2425-2434. doi:10.1177/0363546515612750.
1. Daniels E, Cole D, Jacobs B, Phillips S. Existing Evidence on ultrasound-guided injections in sports medicine. Orthop J Sports Med. 2018;6(2):2325967118756576. doi:10.1177/2325967118756576.
2. Henne M, Centurion A, Rosas S, Youmans H, Osbahr D. Trends in utilization of image-guided hip joint injections. Unpublished. 2018.
3. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine position statement: Interventional musculoskeletal ultrasound in sports medicine. Clin J Sport Med. 2015;25:6-22. doi:10.1097/JSM.0000000000000175.
4. Agel J, Evans TA, Dick R, Putukian M, Marshal S. Descriptive epidemiology of collegiate men’s soccer injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2002-2003. J Athl Train. 2007;42(2):270-277.
5. Dick R, Putukian M, Agel J, Evans T, Marshall S. Descriptive epidemiology of collegiate women’s soccer injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2002-2003. J Athl Train. 2007;42(2):278-285.
6. Ekstrand J, Hagglund M, Walden M. Epidemiology of muscle injuries in professional football (soccer). Am J Sports Med. 2011;39(6):1226-1232. doi:10.1177/0363546510395879.
7. Klauser A, Tagliafico A, Allen G, et al. Clinical indications for musculoskeletal ultrasound: A Delphi-based consensus paper of the European society of musculoskeletal radiology. Eur Radiol. 2012;22(5):1140-1148. doi:10.1007/s00330-011-2356-3.
8. Henderson R, Walker B, Young K. The accuracy of diagnostic ultrasound imaging for musculoskeletal soft tissue pathology of the extremities: a comprehensive review of the literature. Chiropr Man Therap. 2015;23(1):31. doi:10.1186/s12998-015-0076-5.
9. Housner JA, Jacobson JA, Misko R. Sonographically guided percutaneous needle tenotomy for the treatment of chronic tendinosis. J Ultrasound Med. 2009;28(9):1187-1192. doi:10.7863/jum.2009.28.9.1187.
10. Koh J, Mohan PC, Howe TS, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644. doi:10.1177/0363546512470625.
11. Seng C, Mohan PC, Koh J, et al. Ultrasonic percutaneous tenotomy for recalcitrant lateral elbow tendinopathy: sustainability and sonographic progression at 3 years. Am J Sports Med. 2015;44(2):504-510. doi:10.1177/0363546515612758.
12. Lee J, Harrison J, Boachie-Adjei K, Vargas E, Moley P. Platelet-rich plasma injections with needle tenotomy for gluteus medius tendinopathy: A registry study with prospective follow-up. Orthop J Sports Med. 2016;4(11):2325967116671692. doi:10.1177/2325967116671692.
13. Osborne H, Anderson L, Burt P, Young M, Gerrard D. Australasian College of Sports Physicians-Position statement: the place of mesenchymal stem/stromal cell therapies in sport and exercise medicine. Br J Sports Med. 2016;50:1237-1244. doi:10.1136/bjsports-2015-095711.
14. Anderson J, Little D, Toth A, et al. Stem cell therapies for knee cartilage repair. The current status of preclinical and clinical studies. Am J Sports Med. 2013;42(9)2253-2261. doi:10.1177/0363546513508744.
15. Lee S, Kwon B, Lee Kyoungbun, Son Y, Chung S. Therapeutic mechanisms of human adipose-derived mesenchymal stem cells in a rat tendon injury model. Am J Sports Med. 2017;45(6):1429-1439. doi:10.1177/0363546517689874.
16. McIntyre J, Jones I, Han B, Vangsness C. Intra-articular mesenchymal stem cell therapy for the human joint. A systematic review. Am J Sports Med. 2017;0363546517735844. doi:10.1177/0363546517735844.
17. Pas HIMFL, Moen M, Haisma J, Winters M. No evidence for the use of stem cell therapy for tendon disorders: a systematic review. Br J Sports Med. 2017;51:996-1002. doi:10.1136/bjsports-2016-096794.
18. Foster T, Puskas B, Mandelbaum B, Gerhardt M, Rodeo S. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37(11):2259-2272. doi:10.1177/0363546509349921.
19. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778. doi:10.1177/0363546506288850.
20. Dines J, Williams P, ElAttrache N, et al. Platelet-rich plasma can be used to successfully treat elbow ulnar collateral ligament insufficiency in high-level throwers. Am J Orthop. 2016;45(4):296-300.
21. Fitzpatrick J, Bulsara M, O’Donnel J, McCrory P, Zheng M. The effectiveness of platelet-rich plasma injections in gluteal tendinopathy. A randomized, double-blind controlled trial comparing a single platelet-rich plasma injection with a single corticosteroid injection. Am J Sports Med. 2018;46(4)933-939. doi:10.1177/0363546517745525.
22. Hamid M, Ali M, Yusof A, George J, Lee L. Platelet-rich plasma injections for the treatment of hamstring injuries: A randomized controlled trial. Am J Sports Med. 2014;42(10):2410-2418. doi:10.1177/0363546514541540.
23. Zanon G, Combi F, Combi A, Perticarini L, Sammarchi L, Benazzo F. Platelet-rich plasma in the treatment of acute hamstring injuries in professional football players. Joints. 2016;4(1):17-23. doi:10.11138/jts/2016.4.1.017.
24. Hamilton B, Tol JL, Almusa E, et al. Platelet-rich plasma does not enhance return to play in hamstring injuries: a randomized controlled trial. Br J Sports Med. 2015;49:943-950. doi:10.1136/bjsports-2015-094603.
25. Pas HIMFL, Reurink G, Tol JL, Wier A, Winters M, Moen M. Efficacy of rehabilitation (lengthening) exercises, platelet-rich plasma injections, and other conservative interventions in acute hamstring injuries: an updated systematic review and meta-analysis. Br J Sports Med. 2015;49:1197-1205. doi:10.1136/bjsports-2015-094879.
26. Reurink G, Goudswaard G, Moen M, et al. Platelet-rich plasma injections in acute muscle injury. N Engl J Med. 2014;370:2546-2547. doi:10.1056/NEJMc1402340.
27. Kantrowitz D, Padaki A, Ahmad C, Lynch T. Defining platelet-rich plasma usage by team physicians in elite athletes. Orthop J Sports Med. 2018;6(4):2325967118767077. doi:10.1177/2325967118767077.
28. Mithoefer K, Peterson L, Zenobi-Wong M, Mandelbaum B. Cartilage issues in football-today’s problems and tomorrow’s solutions. Br J Sports Med. 2015;49(9):590-596. doi:1136/bjsports-2015-094772.
29. Matthews K, Cuchiara M. Regional regulatory insights: U.S. National Football League Athletes seeking unproven stem cell treatments. Stem Cells Dev. 2014;23(S1):60-64. doi:10.1089/scd.2014.0358.
30. McIntyre J, Jones I, Danilkovich A, Vangsness T. The placenta: applications in orthopaedic sports medicine. Am J Sports Med. 2018;46(1):234-247. doi:10.1177/0363546517697682.
31. Riboh J, Saltzman B, Yankee A, Cole BJ. Human amniotic membrane-derived products in sports medicine: Basic science, early results, and potential clinical applications. Am J Sports Med. 2015;44(9)2425-2434. doi:10.1177/0363546515612750.
TAKE-HOME POINTS
- Improvements in ultrasound technology have increased its use as a therapeutic and diagnostic modality.
- Ultrasound offers increased accuracy and efficacy with minimally invasive procedures.
- PRP is a safe and effective treatment for many musculoskeletal injuries, however return-to-play time frames limit its efficacy.
- While stem cell and amniotic products offer promising results, the paucity in data limits overall use.
- Care should be taken when discussing regenerative therapy as many products eclipse the concept of “minimal manipulation” and therefore require USFDA trials to establish safety data.
The PASTA Bridge – A Repair Technique for Partial Articular-Sided Rotator Cuff Tears: A Biomechanical Evaluation of Construct Strength
ABSTRACT
Partial articular-sided supraspinatus tendon avulsion (PASTA) tears are a common clinical problem that can require surgical intervention to reduce patient symptoms. Currently, no consensus has been reached regarding the optimal repair technique. The PASTA Bridge technique was developed by the senior author to address these types of lesions. A controlled laboratory study was performed comparing the PASTA Bridge with a standard transtendon rotator cuff repair to confirm its biomechanical efficacy. A 50% articular-sided partial tear of the supraspinatus tendon was created on 6 matched pairs of fresh-frozen cadaveric shoulders. For each matched pair, 1 humerus received a PASTA Bridge repair, whereas the contralateral side received a repair using a single suture anchor with a horizontal mattress suture. The ultimate load, yield load, and stiffness were determined from the load-displacement results for each sample. Video tracking software was used to determine the cyclic displacement of each sample at the articular margin and the repair site. Strain at the margin and repair site was then calculated using this collected data. There were no significant differences between the 2 repairs in ultimate load (P = .577), strain at the repair site (P = .355), or strain at the margin (P = .801). No instance of failure was due to the PASTA Bridge construct itself. The results of this study have established that the PASTA Bridge is biomechanically equivalent to the transtendon repair technique. The PASTA Bridge is technically easy, percutaneous, reproducible, and is associated with fewer risks.
Continue to: Rotator cuff tests...
Rotator cuff tears can be classified as full-thickness or partial-thickness; the latter being further divided into the bursal surface, articular-sided, or intratendinous tears. A study analyzing the anatomical distribution of partial tears found that approximately 50% of those at the rotator cuff footprint were articular-sided and predominantly involved the supraspinatus tendon.1 These partial-thickness articular-sided supraspinatus tendon avulsion tears have been coined “PASTA lesions.” Current treatment recommendations suggest that a debridement, a transtendon technique, or a “takedown” method of completing a partial tear and performing a full-thickness repair be utilized for partial-thickness rotator cuff repairs.
The primary goal of a partial cuff repair is to reestablish the tendon footprint at the humeral head. It has been argued that the “takedown” method alters the normal footprint and presents tension complications that can result in poor outcomes.2-5 Also, if the full-thickness repair fails, the patient is left with a full-thickness tear that could be more disabling. The trans-tendon technique has proven to be superior in this sense, demonstrating an improvement in both footprint contact and healing potential.3-5 This article aims to evaluate the biomechanical effectiveness of a new PASTA lesion repair technique, the PASTA Bridge,6 when compared with a traditional transtendon suture anchor repair.
MATERIALS AND METHODS
BIOMECHANICAL OPERATIVE TECHNIQUE: PASTA BRIDGE REPAIR
A 17-gauge spinal needle was used to create a puncture in the supraspinatus tendon approximately 7.5 mm anterior to the centerline of the footprint and just medial to the simulated tear line. A 1.1-mm blunt Nitinol wire (Arthrex) was placed over the top of the spinal needle, and the spinal needle was removed. A 2.4-mm portal dilation instrument (Arthrex) was placed over the top of the 1.1 blunt wire (Arthrex) followed by the drill spear for the 2.4-mm BioComposite SutureTak (Arthrex). A pilot hole was created just medial to the simulated tear using the spear and a 1.8-mm drill followed by insertion of a 2.4-mm BioComposite SutureTak (Arthrex). This process was repeated approximately 5 mm posterior to the centerline of the footprint. A strand of suture from each anchor was tied in a manner similar to the “double pulley” method described by Lo and Burkhart.3 The opposing 2 limbs were tensioned to pull the knot taut over the repair site and fixed laterally with a 4.75-mm BioComposite SwiveLock (Arthrex) placed approximately 1 cm lateral to the greater tuberosity.
BIOMECHANICAL OPERATIVE TECHNIQUE: CONTROL (4.5-MM CORKSCREW FT GROUP)
A No. 11 scalpel was used to create a puncture in the tendon for a transtendon approach. A 4.5-mm titanium Corkscrew FT (Arthrex) was placed just medial to the beginning of the simulated tear. The No. 2 FiberWire (Arthrex) was passed anterior and posterior to the hole made for the transtendon approach. A horizontal mattress stitch was tied using a standard 2-handed knot technique.
BIOMECHANICAL ANALYSIS
The proximal humeri with intact supraspinatus tendons were removed from 6 matched pairs of fresh-frozen cadaver shoulders (3 males, 3 females; average age, 49 ± 12 years). The shaft of the humerus was potted in fiberglass resin. For each sample, a partial tear of the supraspinatus tendon was replicated by using a sharp blade to transect 50% of the medial side of the supraspinatus from the tuberosity.2,5 From each matched pair, 1 humerus was selected to receive a PASTA Bridge repair,6 and the contralateral repair was performed using one 4.5-mm titanium Corkscrew FT. Half of the samples of each repair were performed on the right humerus to avoid a mechanical bias. Each repair was performed by the same orthopedic surgeon.
Continue to: Biomechanical testing was...
Biomechanical testing was conducted using an INSTRON 8871 Axial Table Top Servo-hydraulic Testing System (INSTRON), with a 5 kN load cell attached to the crosshead. The system was calibrated using FastTrack software (AEC Software), and both the load and position controls were run through WaveMaker software (WaveMaker). Each sample was positioned on a fixed angle fixture and secured to the testing surface so that the direction of pull would be performed 45° to the humeral shaft. A custom fixture with inter-digitated brass clamps was attached to the crosshead, and dry ice was used to freeze the tendon to the clamp. The test setup can be seen in Figures 1A, 1B.
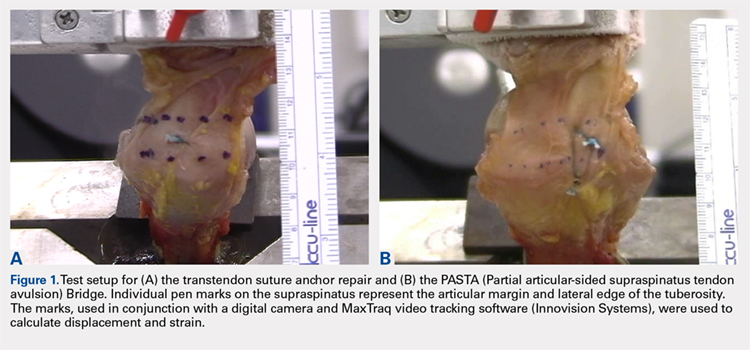
Each sample was pre-loaded to 10 N to remove slack from the system. Pre-loading was followed by cyclic loading between 10 N and 100 N,7-11 at 1 Hz, for 100 cycles. One-hundred cycles were chosen based on literature stating that the majority of the cyclic displacement occurs in the first 100 cycles.7-10 Post cycling, the samples were loaded to failure at a rate of 33 mm/sec.7-12 Load and position data were recorded at 500 Hz, and the mode of failure was noted for each sample.
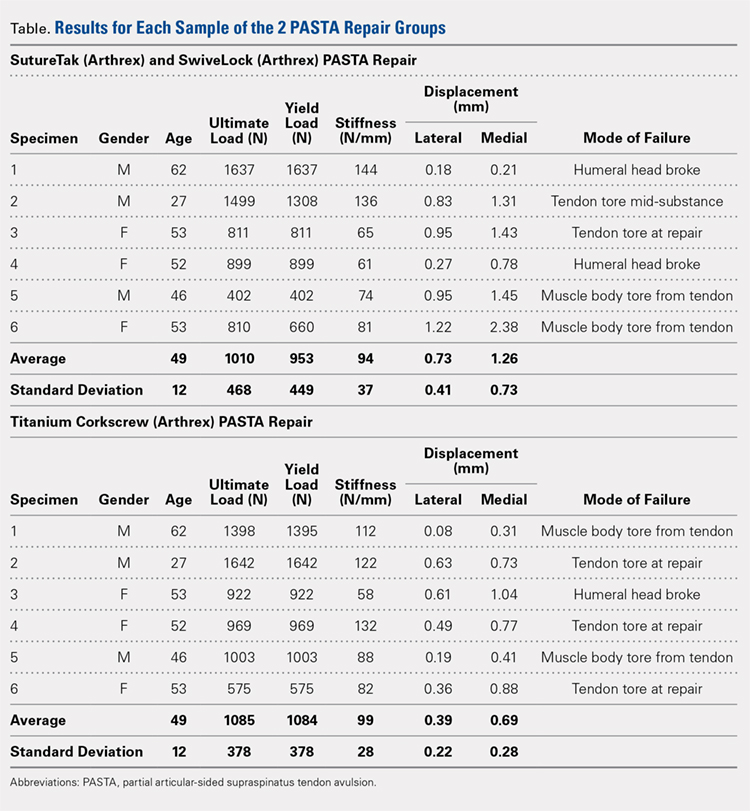
Before loading, a soft-tissue marker was used to create individual marks on the supraspinatus in-line with the articular margin and lateral edge of the tuberosity (Figures 1A, 1B). The individual marks, a digital camera, and MaxTraq video tracking software (Innovision Systems) were used to calculate displacement and strain.
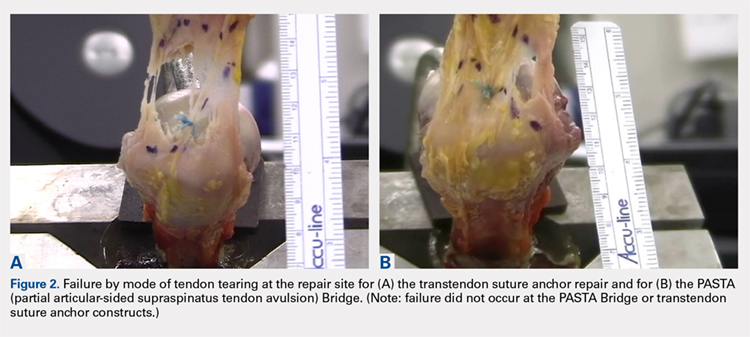
For each sample, the ultimate load, yield load, and stiffness were determined from the load-displacement results. Video tracking software was used to determine the cyclic displacement of each sample at both the articular margin (medial dots) and at the repair site. The strain at these 2 locations was calculated by dividing the cyclic displacement of the respective site by the distance between the site of interest and the lateral edge of the tuberosity (lateral marks) (ΔL/L). Paired t tests (α = 0.05) were used to determine if differences in ultimate load or strain between the 2 repairs were significant.
RESULTS
BIOMECHANICAL ANALYSIS
The results of the biomechanical testing are provided in the Table. There were no significant differences between the 2 repairs in ultimate load (P = .577), strain at the repair site (P = .355), or strain at the margin (P = .801). A post-hoc power analysis revealed that a sample size of at least 20 matched pairs would be needed to establish a significant difference for strain at the repair site. The modes of failure were mid-substance tendon tearing, the humeral head breaking, tearing at the musculotendinous junction, or the tendon tearing at the repair site. All 4 modes of failure occurred in at least 1 sample from both repair groups (Figures 2-4). Visual inspection of the samples post-testing revealed no damage to the anchors or sutures. A representative picture of the tendon tearing at the repair site can be seen in Figures 2A, 2B.
Continue to: The purpose of...
DISCUSSION
The purpose of this study was to evaluate the biomechanical strength of a new technique for PASTA repairs—the PASTA Bridge.6 After creation of a partial-thickness tear on a cadaveric model, we compared the PASTA Bridge technique6 with a standard transtendon suture anchor repair. We hypothesized that the PASTA Bridge would yield equivalent or better biomechanical properties including the ultimate load to failure and the degree of strain at different locations in the repair. Our results supported this hypothesis. The PASTA Bridge was biomechanically equivalent to transtendon repair.
For repairs of partial-thickness rotator cuff tears, 2 traditional techniques are transtendon repairs and the “takedown” method of completing a partial tear into a full tear with a subsequent repair.13 While clinical outcomes of the 2 methods suggest no superiority over the other,13 studies have demonstrated a biomechanical advantage with transtendon repairs. Repairs of PASTA lesions exhibit both lower strain and displacement of the repaired tendon compared with a full-thickness repair.2-5 Failure of the “takedown” method results in a full-thickness rotator cuff tear as opposed to a partial tear. This outcome can prove to be more debilitating for the patient. Furthermore, Mazzocca and colleagues5 illustrated that for partial tears >25% thickness, the cuff strain returned to the intact state once repaired.
Our data suggest that biomechanically the transtendon and the PASTA Bridge6 techniques were equivalent. While the ultimate load and strain at repair sites are comparable, the PASTA Bridge is percutaneous and presents significantly less risk of complications. The PASTA Bridge6 uses a medial row horizontal mattress with a lateral row fixation to recreate the rotator cuff footprint. It has been postulated that reestablishing a higher percentage of the footprint can aide in tendon-bone healing, having valuable implications for both biological and clinical outcomes of the patient.3,4,14 Greater contact at the tendon-bone interface may allow more fibers to participate in the healing process.14 In their analysis of rotator cuff repair, Apreleva and colleagues14 asserted that more laterally placed suture anchors may increase the repair-site area. The lateral anchors of the PASTA Bridge help not only to increase the footprint and thereby the healing potential of the repair but also assist in taking pressure off the medial row anchors.
In their report on double-row rotator cuff repair, Lo and Burkhart3 suggest that double-row fixation is superior to single-row repairs for a variety of reasons. Primarily, double-row techniques increase the number of points of fixation, which will secondarily reduce both the stress and load at each suture point.3 This effect improves the overall strength of the repair construct. Use of the lateral anchor of the PASTA Bridge6 allows the medial anchors to act as pivot points. Placing the stress laterally, the configuration allows for movement and strain distribution without sacrificing the integrity of the repair. In our analysis, failure occurred by the tendon tearing mid-substance, humeral head breaking, tendon tearing at the repair site, and tearing at the musculotendinous junction (Figures 2-4). There was no instance of failure due to the construct itself indicating that the 2.4-mm medial anchors are more than adequate for the PASTA Bridge.6 When visually inspecting the samples after failure, there was no damage to the anchors or sutures. This observation indicates that the PASTA Bridge construct is remarkably strong and capable of withstanding excessive forces.
There were some potential limitations of this study. The small sample size modified the potential for identifying significant differences between the groups. A post-hoc power analysis revealed that a sample size of at least 20 matched pairs would be required to determine a significant difference between the 2 repair groups in strain at the repair site. We did not test this many pairs because the data was so similar after 6 matched pairs that it did not warrant continuing further. Additional research should be done with larger sample populations to evaluate the biomechanical efficacy of this technique further.
CONCLUSION
The PASTA Bridge6 creates a strong construct for repair of articular-sided partial-thickness tears of the supraspinatus. The data suggest the PASTA Bridge6 is biomechanically equivalent to the gold standard transtendon suture anchor repair. The PASTA Bridge6 is technically sound, percutaneous, and presents less risk of complications. It does not require arthroscopic knot tying and carries only minimal risk of damage to residual tissues. In our analysis, there were no failures of the actual construct, asserting that the PASTA Bridge6 is a strong, durable repair. The PASTA Bridge6 should be strongly considered by surgeons treating PASTA lesions.
1. Schaeffeler C, Mueller D, Kirchhoff C, Wolf P, Rummeny EJ, Woertler K. Tears at the rotator cuff footprint: prevalence and imaging characteristics in 305 MR arthrograms of the shoulder. Eur Radiol. 2011;21:1477-1484. doi:10.1007/s00330-011-2066-x.
2. Gonzalez-Lomas G, Kippe MA, Brown GD, et al. In situ transtendon repair outperforms tear completion and repair for partial articular-sided supraspinatus tendon tears. J Shoulder Elbow Surg. 2008;17(5):722-728.
3. Lo IKY, Burkhart SS. Transtendon arthroscopic repair of partial-thickness, articular surface tears of the rotator cuff. Arthroscopy. 2004; 20(2):214-220. doi:10.1016/j.arthro.2003.11.042.
4. Mazzocca AD, Millett PJ, Guanche CA, Santangelo SA, Arciero RA. Arthroscopic single-row versus double-row suture anchor rotator cuff repair. Am J Sports Med. 2005;33(12):1861-1868.
5. Mazzocca AD, Rincon LM, O’Connor RW, et al. Intra-articular partial-thickness rotator cuff tears: analysis of injured and repaired strain behavior. Am J Sports Med. 2008;36(1):110-116. doi:10.1177/0363546507307502.
6. Hirahara AM, Andersen WJ. The PASTA bridge: a technique for the arthroscopic repair of PASTA lesions. Arthrosc Tech. In Press. Epub 2017 Sept 18.
7. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing and ultimate failure strength of biodegradable glenoid anchors. Arthroscopy. 2008; 24(2):224-228. doi:10.1016/j.arthro.2007.08.011.
8. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007; 23(4):355-360. doi:10.1016/j.arthro.2006.12.009.
9. Barber FA, Feder SM, Burkhart SS, Ahrens J. The relationship of suture anchor failure and bone density to proximal humerus location: a cadaveric study. Arthroscopy. 1997;13(3):340-345. doi:10.1016/j.jbiomech.2009.12.007.
10. Barber FA, Herbert MA, Richards DP. Sutures and suture anchors: update 2003. Arthroscopy. 2003;19(9):985-990.
11. Burkhart SS, Johnson TC, Wirth MA, Athanasiou KA. Cyclic loading of transosseous rotator cuff repairs: tension overload as a possible cause of failure. Arthroscopy. 1997;13(2):172-176. doi:10.1016/S0749-8063(97)90151-1.
12. Hecker AT, Shea M, Hayhurst JO, Myers ER, Meeks LW, Hayes WC. Pull-out strength of suture anchors for rotator cuff and bankart lesion repairs. Am J Sports Med. 1993; 21(6):874-879.
13. Strauss EJ, Salata MJ, Kercher J, et al. The arthroscopic management of partial-thickness rotator cuff tears: a systematic review of the literature. Arthroscopy. 2011;27(4):568-580. doi:10.1016/j.arthro.2010.09.019.
14. Apreleva M, Özbaydar M, Fitzgibbons PG, Warner JJP. Rotator cuff tears: the effect of the reconstruction method on three-dimensional repair-site area. Arthroscopy. 2002;18(5):519-526. doi:10.1053/jars.2002.32930.
ABSTRACT
Partial articular-sided supraspinatus tendon avulsion (PASTA) tears are a common clinical problem that can require surgical intervention to reduce patient symptoms. Currently, no consensus has been reached regarding the optimal repair technique. The PASTA Bridge technique was developed by the senior author to address these types of lesions. A controlled laboratory study was performed comparing the PASTA Bridge with a standard transtendon rotator cuff repair to confirm its biomechanical efficacy. A 50% articular-sided partial tear of the supraspinatus tendon was created on 6 matched pairs of fresh-frozen cadaveric shoulders. For each matched pair, 1 humerus received a PASTA Bridge repair, whereas the contralateral side received a repair using a single suture anchor with a horizontal mattress suture. The ultimate load, yield load, and stiffness were determined from the load-displacement results for each sample. Video tracking software was used to determine the cyclic displacement of each sample at the articular margin and the repair site. Strain at the margin and repair site was then calculated using this collected data. There were no significant differences between the 2 repairs in ultimate load (P = .577), strain at the repair site (P = .355), or strain at the margin (P = .801). No instance of failure was due to the PASTA Bridge construct itself. The results of this study have established that the PASTA Bridge is biomechanically equivalent to the transtendon repair technique. The PASTA Bridge is technically easy, percutaneous, reproducible, and is associated with fewer risks.
Continue to: Rotator cuff tests...
Rotator cuff tears can be classified as full-thickness or partial-thickness; the latter being further divided into the bursal surface, articular-sided, or intratendinous tears. A study analyzing the anatomical distribution of partial tears found that approximately 50% of those at the rotator cuff footprint were articular-sided and predominantly involved the supraspinatus tendon.1 These partial-thickness articular-sided supraspinatus tendon avulsion tears have been coined “PASTA lesions.” Current treatment recommendations suggest that a debridement, a transtendon technique, or a “takedown” method of completing a partial tear and performing a full-thickness repair be utilized for partial-thickness rotator cuff repairs.
The primary goal of a partial cuff repair is to reestablish the tendon footprint at the humeral head. It has been argued that the “takedown” method alters the normal footprint and presents tension complications that can result in poor outcomes.2-5 Also, if the full-thickness repair fails, the patient is left with a full-thickness tear that could be more disabling. The trans-tendon technique has proven to be superior in this sense, demonstrating an improvement in both footprint contact and healing potential.3-5 This article aims to evaluate the biomechanical effectiveness of a new PASTA lesion repair technique, the PASTA Bridge,6 when compared with a traditional transtendon suture anchor repair.
MATERIALS AND METHODS
BIOMECHANICAL OPERATIVE TECHNIQUE: PASTA BRIDGE REPAIR
A 17-gauge spinal needle was used to create a puncture in the supraspinatus tendon approximately 7.5 mm anterior to the centerline of the footprint and just medial to the simulated tear line. A 1.1-mm blunt Nitinol wire (Arthrex) was placed over the top of the spinal needle, and the spinal needle was removed. A 2.4-mm portal dilation instrument (Arthrex) was placed over the top of the 1.1 blunt wire (Arthrex) followed by the drill spear for the 2.4-mm BioComposite SutureTak (Arthrex). A pilot hole was created just medial to the simulated tear using the spear and a 1.8-mm drill followed by insertion of a 2.4-mm BioComposite SutureTak (Arthrex). This process was repeated approximately 5 mm posterior to the centerline of the footprint. A strand of suture from each anchor was tied in a manner similar to the “double pulley” method described by Lo and Burkhart.3 The opposing 2 limbs were tensioned to pull the knot taut over the repair site and fixed laterally with a 4.75-mm BioComposite SwiveLock (Arthrex) placed approximately 1 cm lateral to the greater tuberosity.
BIOMECHANICAL OPERATIVE TECHNIQUE: CONTROL (4.5-MM CORKSCREW FT GROUP)
A No. 11 scalpel was used to create a puncture in the tendon for a transtendon approach. A 4.5-mm titanium Corkscrew FT (Arthrex) was placed just medial to the beginning of the simulated tear. The No. 2 FiberWire (Arthrex) was passed anterior and posterior to the hole made for the transtendon approach. A horizontal mattress stitch was tied using a standard 2-handed knot technique.
BIOMECHANICAL ANALYSIS
The proximal humeri with intact supraspinatus tendons were removed from 6 matched pairs of fresh-frozen cadaver shoulders (3 males, 3 females; average age, 49 ± 12 years). The shaft of the humerus was potted in fiberglass resin. For each sample, a partial tear of the supraspinatus tendon was replicated by using a sharp blade to transect 50% of the medial side of the supraspinatus from the tuberosity.2,5 From each matched pair, 1 humerus was selected to receive a PASTA Bridge repair,6 and the contralateral repair was performed using one 4.5-mm titanium Corkscrew FT. Half of the samples of each repair were performed on the right humerus to avoid a mechanical bias. Each repair was performed by the same orthopedic surgeon.
Continue to: Biomechanical testing was...
Biomechanical testing was conducted using an INSTRON 8871 Axial Table Top Servo-hydraulic Testing System (INSTRON), with a 5 kN load cell attached to the crosshead. The system was calibrated using FastTrack software (AEC Software), and both the load and position controls were run through WaveMaker software (WaveMaker). Each sample was positioned on a fixed angle fixture and secured to the testing surface so that the direction of pull would be performed 45° to the humeral shaft. A custom fixture with inter-digitated brass clamps was attached to the crosshead, and dry ice was used to freeze the tendon to the clamp. The test setup can be seen in Figures 1A, 1B.

Each sample was pre-loaded to 10 N to remove slack from the system. Pre-loading was followed by cyclic loading between 10 N and 100 N,7-11 at 1 Hz, for 100 cycles. One-hundred cycles were chosen based on literature stating that the majority of the cyclic displacement occurs in the first 100 cycles.7-10 Post cycling, the samples were loaded to failure at a rate of 33 mm/sec.7-12 Load and position data were recorded at 500 Hz, and the mode of failure was noted for each sample.

Before loading, a soft-tissue marker was used to create individual marks on the supraspinatus in-line with the articular margin and lateral edge of the tuberosity (Figures 1A, 1B). The individual marks, a digital camera, and MaxTraq video tracking software (Innovision Systems) were used to calculate displacement and strain.

For each sample, the ultimate load, yield load, and stiffness were determined from the load-displacement results. Video tracking software was used to determine the cyclic displacement of each sample at both the articular margin (medial dots) and at the repair site. The strain at these 2 locations was calculated by dividing the cyclic displacement of the respective site by the distance between the site of interest and the lateral edge of the tuberosity (lateral marks) (ΔL/L). Paired t tests (α = 0.05) were used to determine if differences in ultimate load or strain between the 2 repairs were significant.

RESULTS
BIOMECHANICAL ANALYSIS
The results of the biomechanical testing are provided in the Table. There were no significant differences between the 2 repairs in ultimate load (P = .577), strain at the repair site (P = .355), or strain at the margin (P = .801). A post-hoc power analysis revealed that a sample size of at least 20 matched pairs would be needed to establish a significant difference for strain at the repair site. The modes of failure were mid-substance tendon tearing, the humeral head breaking, tearing at the musculotendinous junction, or the tendon tearing at the repair site. All 4 modes of failure occurred in at least 1 sample from both repair groups (Figures 2-4). Visual inspection of the samples post-testing revealed no damage to the anchors or sutures. A representative picture of the tendon tearing at the repair site can be seen in Figures 2A, 2B.

Continue to: The purpose of...
DISCUSSION
The purpose of this study was to evaluate the biomechanical strength of a new technique for PASTA repairs—the PASTA Bridge.6 After creation of a partial-thickness tear on a cadaveric model, we compared the PASTA Bridge technique6 with a standard transtendon suture anchor repair. We hypothesized that the PASTA Bridge would yield equivalent or better biomechanical properties including the ultimate load to failure and the degree of strain at different locations in the repair. Our results supported this hypothesis. The PASTA Bridge was biomechanically equivalent to transtendon repair.
For repairs of partial-thickness rotator cuff tears, 2 traditional techniques are transtendon repairs and the “takedown” method of completing a partial tear into a full tear with a subsequent repair.13 While clinical outcomes of the 2 methods suggest no superiority over the other,13 studies have demonstrated a biomechanical advantage with transtendon repairs. Repairs of PASTA lesions exhibit both lower strain and displacement of the repaired tendon compared with a full-thickness repair.2-5 Failure of the “takedown” method results in a full-thickness rotator cuff tear as opposed to a partial tear. This outcome can prove to be more debilitating for the patient. Furthermore, Mazzocca and colleagues5 illustrated that for partial tears >25% thickness, the cuff strain returned to the intact state once repaired.
Our data suggest that biomechanically the transtendon and the PASTA Bridge6 techniques were equivalent. While the ultimate load and strain at repair sites are comparable, the PASTA Bridge is percutaneous and presents significantly less risk of complications. The PASTA Bridge6 uses a medial row horizontal mattress with a lateral row fixation to recreate the rotator cuff footprint. It has been postulated that reestablishing a higher percentage of the footprint can aide in tendon-bone healing, having valuable implications for both biological and clinical outcomes of the patient.3,4,14 Greater contact at the tendon-bone interface may allow more fibers to participate in the healing process.14 In their analysis of rotator cuff repair, Apreleva and colleagues14 asserted that more laterally placed suture anchors may increase the repair-site area. The lateral anchors of the PASTA Bridge help not only to increase the footprint and thereby the healing potential of the repair but also assist in taking pressure off the medial row anchors.
In their report on double-row rotator cuff repair, Lo and Burkhart3 suggest that double-row fixation is superior to single-row repairs for a variety of reasons. Primarily, double-row techniques increase the number of points of fixation, which will secondarily reduce both the stress and load at each suture point.3 This effect improves the overall strength of the repair construct. Use of the lateral anchor of the PASTA Bridge6 allows the medial anchors to act as pivot points. Placing the stress laterally, the configuration allows for movement and strain distribution without sacrificing the integrity of the repair. In our analysis, failure occurred by the tendon tearing mid-substance, humeral head breaking, tendon tearing at the repair site, and tearing at the musculotendinous junction (Figures 2-4). There was no instance of failure due to the construct itself indicating that the 2.4-mm medial anchors are more than adequate for the PASTA Bridge.6 When visually inspecting the samples after failure, there was no damage to the anchors or sutures. This observation indicates that the PASTA Bridge construct is remarkably strong and capable of withstanding excessive forces.
There were some potential limitations of this study. The small sample size modified the potential for identifying significant differences between the groups. A post-hoc power analysis revealed that a sample size of at least 20 matched pairs would be required to determine a significant difference between the 2 repair groups in strain at the repair site. We did not test this many pairs because the data was so similar after 6 matched pairs that it did not warrant continuing further. Additional research should be done with larger sample populations to evaluate the biomechanical efficacy of this technique further.
CONCLUSION
The PASTA Bridge6 creates a strong construct for repair of articular-sided partial-thickness tears of the supraspinatus. The data suggest the PASTA Bridge6 is biomechanically equivalent to the gold standard transtendon suture anchor repair. The PASTA Bridge6 is technically sound, percutaneous, and presents less risk of complications. It does not require arthroscopic knot tying and carries only minimal risk of damage to residual tissues. In our analysis, there were no failures of the actual construct, asserting that the PASTA Bridge6 is a strong, durable repair. The PASTA Bridge6 should be strongly considered by surgeons treating PASTA lesions.
ABSTRACT
Partial articular-sided supraspinatus tendon avulsion (PASTA) tears are a common clinical problem that can require surgical intervention to reduce patient symptoms. Currently, no consensus has been reached regarding the optimal repair technique. The PASTA Bridge technique was developed by the senior author to address these types of lesions. A controlled laboratory study was performed comparing the PASTA Bridge with a standard transtendon rotator cuff repair to confirm its biomechanical efficacy. A 50% articular-sided partial tear of the supraspinatus tendon was created on 6 matched pairs of fresh-frozen cadaveric shoulders. For each matched pair, 1 humerus received a PASTA Bridge repair, whereas the contralateral side received a repair using a single suture anchor with a horizontal mattress suture. The ultimate load, yield load, and stiffness were determined from the load-displacement results for each sample. Video tracking software was used to determine the cyclic displacement of each sample at the articular margin and the repair site. Strain at the margin and repair site was then calculated using this collected data. There were no significant differences between the 2 repairs in ultimate load (P = .577), strain at the repair site (P = .355), or strain at the margin (P = .801). No instance of failure was due to the PASTA Bridge construct itself. The results of this study have established that the PASTA Bridge is biomechanically equivalent to the transtendon repair technique. The PASTA Bridge is technically easy, percutaneous, reproducible, and is associated with fewer risks.
Continue to: Rotator cuff tests...
Rotator cuff tears can be classified as full-thickness or partial-thickness; the latter being further divided into the bursal surface, articular-sided, or intratendinous tears. A study analyzing the anatomical distribution of partial tears found that approximately 50% of those at the rotator cuff footprint were articular-sided and predominantly involved the supraspinatus tendon.1 These partial-thickness articular-sided supraspinatus tendon avulsion tears have been coined “PASTA lesions.” Current treatment recommendations suggest that a debridement, a transtendon technique, or a “takedown” method of completing a partial tear and performing a full-thickness repair be utilized for partial-thickness rotator cuff repairs.
The primary goal of a partial cuff repair is to reestablish the tendon footprint at the humeral head. It has been argued that the “takedown” method alters the normal footprint and presents tension complications that can result in poor outcomes.2-5 Also, if the full-thickness repair fails, the patient is left with a full-thickness tear that could be more disabling. The trans-tendon technique has proven to be superior in this sense, demonstrating an improvement in both footprint contact and healing potential.3-5 This article aims to evaluate the biomechanical effectiveness of a new PASTA lesion repair technique, the PASTA Bridge,6 when compared with a traditional transtendon suture anchor repair.
MATERIALS AND METHODS
BIOMECHANICAL OPERATIVE TECHNIQUE: PASTA BRIDGE REPAIR
A 17-gauge spinal needle was used to create a puncture in the supraspinatus tendon approximately 7.5 mm anterior to the centerline of the footprint and just medial to the simulated tear line. A 1.1-mm blunt Nitinol wire (Arthrex) was placed over the top of the spinal needle, and the spinal needle was removed. A 2.4-mm portal dilation instrument (Arthrex) was placed over the top of the 1.1 blunt wire (Arthrex) followed by the drill spear for the 2.4-mm BioComposite SutureTak (Arthrex). A pilot hole was created just medial to the simulated tear using the spear and a 1.8-mm drill followed by insertion of a 2.4-mm BioComposite SutureTak (Arthrex). This process was repeated approximately 5 mm posterior to the centerline of the footprint. A strand of suture from each anchor was tied in a manner similar to the “double pulley” method described by Lo and Burkhart.3 The opposing 2 limbs were tensioned to pull the knot taut over the repair site and fixed laterally with a 4.75-mm BioComposite SwiveLock (Arthrex) placed approximately 1 cm lateral to the greater tuberosity.
BIOMECHANICAL OPERATIVE TECHNIQUE: CONTROL (4.5-MM CORKSCREW FT GROUP)
A No. 11 scalpel was used to create a puncture in the tendon for a transtendon approach. A 4.5-mm titanium Corkscrew FT (Arthrex) was placed just medial to the beginning of the simulated tear. The No. 2 FiberWire (Arthrex) was passed anterior and posterior to the hole made for the transtendon approach. A horizontal mattress stitch was tied using a standard 2-handed knot technique.
BIOMECHANICAL ANALYSIS
The proximal humeri with intact supraspinatus tendons were removed from 6 matched pairs of fresh-frozen cadaver shoulders (3 males, 3 females; average age, 49 ± 12 years). The shaft of the humerus was potted in fiberglass resin. For each sample, a partial tear of the supraspinatus tendon was replicated by using a sharp blade to transect 50% of the medial side of the supraspinatus from the tuberosity.2,5 From each matched pair, 1 humerus was selected to receive a PASTA Bridge repair,6 and the contralateral repair was performed using one 4.5-mm titanium Corkscrew FT. Half of the samples of each repair were performed on the right humerus to avoid a mechanical bias. Each repair was performed by the same orthopedic surgeon.
Continue to: Biomechanical testing was...
Biomechanical testing was conducted using an INSTRON 8871 Axial Table Top Servo-hydraulic Testing System (INSTRON), with a 5 kN load cell attached to the crosshead. The system was calibrated using FastTrack software (AEC Software), and both the load and position controls were run through WaveMaker software (WaveMaker). Each sample was positioned on a fixed angle fixture and secured to the testing surface so that the direction of pull would be performed 45° to the humeral shaft. A custom fixture with inter-digitated brass clamps was attached to the crosshead, and dry ice was used to freeze the tendon to the clamp. The test setup can be seen in Figures 1A, 1B.

Each sample was pre-loaded to 10 N to remove slack from the system. Pre-loading was followed by cyclic loading between 10 N and 100 N,7-11 at 1 Hz, for 100 cycles. One-hundred cycles were chosen based on literature stating that the majority of the cyclic displacement occurs in the first 100 cycles.7-10 Post cycling, the samples were loaded to failure at a rate of 33 mm/sec.7-12 Load and position data were recorded at 500 Hz, and the mode of failure was noted for each sample.

Before loading, a soft-tissue marker was used to create individual marks on the supraspinatus in-line with the articular margin and lateral edge of the tuberosity (Figures 1A, 1B). The individual marks, a digital camera, and MaxTraq video tracking software (Innovision Systems) were used to calculate displacement and strain.

For each sample, the ultimate load, yield load, and stiffness were determined from the load-displacement results. Video tracking software was used to determine the cyclic displacement of each sample at both the articular margin (medial dots) and at the repair site. The strain at these 2 locations was calculated by dividing the cyclic displacement of the respective site by the distance between the site of interest and the lateral edge of the tuberosity (lateral marks) (ΔL/L). Paired t tests (α = 0.05) were used to determine if differences in ultimate load or strain between the 2 repairs were significant.

RESULTS
BIOMECHANICAL ANALYSIS
The results of the biomechanical testing are provided in the Table. There were no significant differences between the 2 repairs in ultimate load (P = .577), strain at the repair site (P = .355), or strain at the margin (P = .801). A post-hoc power analysis revealed that a sample size of at least 20 matched pairs would be needed to establish a significant difference for strain at the repair site. The modes of failure were mid-substance tendon tearing, the humeral head breaking, tearing at the musculotendinous junction, or the tendon tearing at the repair site. All 4 modes of failure occurred in at least 1 sample from both repair groups (Figures 2-4). Visual inspection of the samples post-testing revealed no damage to the anchors or sutures. A representative picture of the tendon tearing at the repair site can be seen in Figures 2A, 2B.

Continue to: The purpose of...
DISCUSSION
The purpose of this study was to evaluate the biomechanical strength of a new technique for PASTA repairs—the PASTA Bridge.6 After creation of a partial-thickness tear on a cadaveric model, we compared the PASTA Bridge technique6 with a standard transtendon suture anchor repair. We hypothesized that the PASTA Bridge would yield equivalent or better biomechanical properties including the ultimate load to failure and the degree of strain at different locations in the repair. Our results supported this hypothesis. The PASTA Bridge was biomechanically equivalent to transtendon repair.
For repairs of partial-thickness rotator cuff tears, 2 traditional techniques are transtendon repairs and the “takedown” method of completing a partial tear into a full tear with a subsequent repair.13 While clinical outcomes of the 2 methods suggest no superiority over the other,13 studies have demonstrated a biomechanical advantage with transtendon repairs. Repairs of PASTA lesions exhibit both lower strain and displacement of the repaired tendon compared with a full-thickness repair.2-5 Failure of the “takedown” method results in a full-thickness rotator cuff tear as opposed to a partial tear. This outcome can prove to be more debilitating for the patient. Furthermore, Mazzocca and colleagues5 illustrated that for partial tears >25% thickness, the cuff strain returned to the intact state once repaired.
Our data suggest that biomechanically the transtendon and the PASTA Bridge6 techniques were equivalent. While the ultimate load and strain at repair sites are comparable, the PASTA Bridge is percutaneous and presents significantly less risk of complications. The PASTA Bridge6 uses a medial row horizontal mattress with a lateral row fixation to recreate the rotator cuff footprint. It has been postulated that reestablishing a higher percentage of the footprint can aide in tendon-bone healing, having valuable implications for both biological and clinical outcomes of the patient.3,4,14 Greater contact at the tendon-bone interface may allow more fibers to participate in the healing process.14 In their analysis of rotator cuff repair, Apreleva and colleagues14 asserted that more laterally placed suture anchors may increase the repair-site area. The lateral anchors of the PASTA Bridge help not only to increase the footprint and thereby the healing potential of the repair but also assist in taking pressure off the medial row anchors.
In their report on double-row rotator cuff repair, Lo and Burkhart3 suggest that double-row fixation is superior to single-row repairs for a variety of reasons. Primarily, double-row techniques increase the number of points of fixation, which will secondarily reduce both the stress and load at each suture point.3 This effect improves the overall strength of the repair construct. Use of the lateral anchor of the PASTA Bridge6 allows the medial anchors to act as pivot points. Placing the stress laterally, the configuration allows for movement and strain distribution without sacrificing the integrity of the repair. In our analysis, failure occurred by the tendon tearing mid-substance, humeral head breaking, tendon tearing at the repair site, and tearing at the musculotendinous junction (Figures 2-4). There was no instance of failure due to the construct itself indicating that the 2.4-mm medial anchors are more than adequate for the PASTA Bridge.6 When visually inspecting the samples after failure, there was no damage to the anchors or sutures. This observation indicates that the PASTA Bridge construct is remarkably strong and capable of withstanding excessive forces.
There were some potential limitations of this study. The small sample size modified the potential for identifying significant differences between the groups. A post-hoc power analysis revealed that a sample size of at least 20 matched pairs would be required to determine a significant difference between the 2 repair groups in strain at the repair site. We did not test this many pairs because the data was so similar after 6 matched pairs that it did not warrant continuing further. Additional research should be done with larger sample populations to evaluate the biomechanical efficacy of this technique further.
CONCLUSION
The PASTA Bridge6 creates a strong construct for repair of articular-sided partial-thickness tears of the supraspinatus. The data suggest the PASTA Bridge6 is biomechanically equivalent to the gold standard transtendon suture anchor repair. The PASTA Bridge6 is technically sound, percutaneous, and presents less risk of complications. It does not require arthroscopic knot tying and carries only minimal risk of damage to residual tissues. In our analysis, there were no failures of the actual construct, asserting that the PASTA Bridge6 is a strong, durable repair. The PASTA Bridge6 should be strongly considered by surgeons treating PASTA lesions.
1. Schaeffeler C, Mueller D, Kirchhoff C, Wolf P, Rummeny EJ, Woertler K. Tears at the rotator cuff footprint: prevalence and imaging characteristics in 305 MR arthrograms of the shoulder. Eur Radiol. 2011;21:1477-1484. doi:10.1007/s00330-011-2066-x.
2. Gonzalez-Lomas G, Kippe MA, Brown GD, et al. In situ transtendon repair outperforms tear completion and repair for partial articular-sided supraspinatus tendon tears. J Shoulder Elbow Surg. 2008;17(5):722-728.
3. Lo IKY, Burkhart SS. Transtendon arthroscopic repair of partial-thickness, articular surface tears of the rotator cuff. Arthroscopy. 2004; 20(2):214-220. doi:10.1016/j.arthro.2003.11.042.
4. Mazzocca AD, Millett PJ, Guanche CA, Santangelo SA, Arciero RA. Arthroscopic single-row versus double-row suture anchor rotator cuff repair. Am J Sports Med. 2005;33(12):1861-1868.
5. Mazzocca AD, Rincon LM, O’Connor RW, et al. Intra-articular partial-thickness rotator cuff tears: analysis of injured and repaired strain behavior. Am J Sports Med. 2008;36(1):110-116. doi:10.1177/0363546507307502.
6. Hirahara AM, Andersen WJ. The PASTA bridge: a technique for the arthroscopic repair of PASTA lesions. Arthrosc Tech. In Press. Epub 2017 Sept 18.
7. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing and ultimate failure strength of biodegradable glenoid anchors. Arthroscopy. 2008; 24(2):224-228. doi:10.1016/j.arthro.2007.08.011.
8. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007; 23(4):355-360. doi:10.1016/j.arthro.2006.12.009.
9. Barber FA, Feder SM, Burkhart SS, Ahrens J. The relationship of suture anchor failure and bone density to proximal humerus location: a cadaveric study. Arthroscopy. 1997;13(3):340-345. doi:10.1016/j.jbiomech.2009.12.007.
10. Barber FA, Herbert MA, Richards DP. Sutures and suture anchors: update 2003. Arthroscopy. 2003;19(9):985-990.
11. Burkhart SS, Johnson TC, Wirth MA, Athanasiou KA. Cyclic loading of transosseous rotator cuff repairs: tension overload as a possible cause of failure. Arthroscopy. 1997;13(2):172-176. doi:10.1016/S0749-8063(97)90151-1.
12. Hecker AT, Shea M, Hayhurst JO, Myers ER, Meeks LW, Hayes WC. Pull-out strength of suture anchors for rotator cuff and bankart lesion repairs. Am J Sports Med. 1993; 21(6):874-879.
13. Strauss EJ, Salata MJ, Kercher J, et al. The arthroscopic management of partial-thickness rotator cuff tears: a systematic review of the literature. Arthroscopy. 2011;27(4):568-580. doi:10.1016/j.arthro.2010.09.019.
14. Apreleva M, Özbaydar M, Fitzgibbons PG, Warner JJP. Rotator cuff tears: the effect of the reconstruction method on three-dimensional repair-site area. Arthroscopy. 2002;18(5):519-526. doi:10.1053/jars.2002.32930.
1. Schaeffeler C, Mueller D, Kirchhoff C, Wolf P, Rummeny EJ, Woertler K. Tears at the rotator cuff footprint: prevalence and imaging characteristics in 305 MR arthrograms of the shoulder. Eur Radiol. 2011;21:1477-1484. doi:10.1007/s00330-011-2066-x.
2. Gonzalez-Lomas G, Kippe MA, Brown GD, et al. In situ transtendon repair outperforms tear completion and repair for partial articular-sided supraspinatus tendon tears. J Shoulder Elbow Surg. 2008;17(5):722-728.
3. Lo IKY, Burkhart SS. Transtendon arthroscopic repair of partial-thickness, articular surface tears of the rotator cuff. Arthroscopy. 2004; 20(2):214-220. doi:10.1016/j.arthro.2003.11.042.
4. Mazzocca AD, Millett PJ, Guanche CA, Santangelo SA, Arciero RA. Arthroscopic single-row versus double-row suture anchor rotator cuff repair. Am J Sports Med. 2005;33(12):1861-1868.
5. Mazzocca AD, Rincon LM, O’Connor RW, et al. Intra-articular partial-thickness rotator cuff tears: analysis of injured and repaired strain behavior. Am J Sports Med. 2008;36(1):110-116. doi:10.1177/0363546507307502.
6. Hirahara AM, Andersen WJ. The PASTA bridge: a technique for the arthroscopic repair of PASTA lesions. Arthrosc Tech. In Press. Epub 2017 Sept 18.
7. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing and ultimate failure strength of biodegradable glenoid anchors. Arthroscopy. 2008; 24(2):224-228. doi:10.1016/j.arthro.2007.08.011.
8. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007; 23(4):355-360. doi:10.1016/j.arthro.2006.12.009.
9. Barber FA, Feder SM, Burkhart SS, Ahrens J. The relationship of suture anchor failure and bone density to proximal humerus location: a cadaveric study. Arthroscopy. 1997;13(3):340-345. doi:10.1016/j.jbiomech.2009.12.007.
10. Barber FA, Herbert MA, Richards DP. Sutures and suture anchors: update 2003. Arthroscopy. 2003;19(9):985-990.
11. Burkhart SS, Johnson TC, Wirth MA, Athanasiou KA. Cyclic loading of transosseous rotator cuff repairs: tension overload as a possible cause of failure. Arthroscopy. 1997;13(2):172-176. doi:10.1016/S0749-8063(97)90151-1.
12. Hecker AT, Shea M, Hayhurst JO, Myers ER, Meeks LW, Hayes WC. Pull-out strength of suture anchors for rotator cuff and bankart lesion repairs. Am J Sports Med. 1993; 21(6):874-879.
13. Strauss EJ, Salata MJ, Kercher J, et al. The arthroscopic management of partial-thickness rotator cuff tears: a systematic review of the literature. Arthroscopy. 2011;27(4):568-580. doi:10.1016/j.arthro.2010.09.019.
14. Apreleva M, Özbaydar M, Fitzgibbons PG, Warner JJP. Rotator cuff tears: the effect of the reconstruction method on three-dimensional repair-site area. Arthroscopy. 2002;18(5):519-526. doi:10.1053/jars.2002.32930.
TAKE-HOME POINTS
- The PASTA Bridge is biomechanically equivalent to the gold-standard transtendon repair technique.
- The configuration is a double-row repair, increasing the number of fixation points.
- The lateral anchor of the PASTA Bridge assumes the stress of the repair, allowing the medial anchors to act as pivot points.
- The PASTA Bridge is strong and capable of withstanding excessive forces.
- The PASTA Bridge poses less risk of complication.