User login
Cutaneous Metastasis of an Undiagnosed Prostatic Adenocarcinoma
Cutaneous Metastasis of an Undiagnosed Prostatic Adenocarcinoma
To the Editor:
Cutaneous metastasis of prostate cancer is rare and portends a bleak prognosis. Diagnosis of the primary cancer can be challenging, as skin metastasis can mimic a variety of conditions. We report a case of metastatic prostatic adenocarcinoma confirmed via biopsy of a new skin lesion.
A 97-year-old man presented to the dermatology clinic for routine follow-up of psoriasis. During the visit, a family member mentioned a new bleeding lesion on the left shoulder. It was not known how long the lesion had been present. Four months prior, the patient had a prostate-specific antigen (PSA) level of 582 ng/mL (reference range, 0-6.5 ng/mL), and computed tomography of the chest had shown innumerable pulmonary nodules in addition to lymphadenopathy of the left axilla, clavicle, and mediastinum. The imaging was ordered by the patient’s urologist as part of routine workup, as he had a history of obstructive renal failure and was being monitored for an indwelling catheter. Two months later, a bone scan ordered by the urologist due to high PSA levels showed extensive osteoblastic metastatic disease throughout the axial and proximal appendicular skeleton. The elevated PSA levels and findings of pulmonary and osteoblastic metastasis suggested a diagnosis of metastatic prostatic adenocarcinoma, but no confirmatory biopsy was performed following the imaging because the patient’s family declined additional workup or intervention.
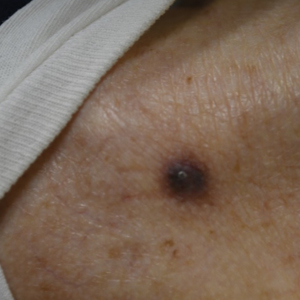
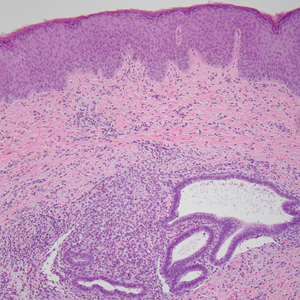
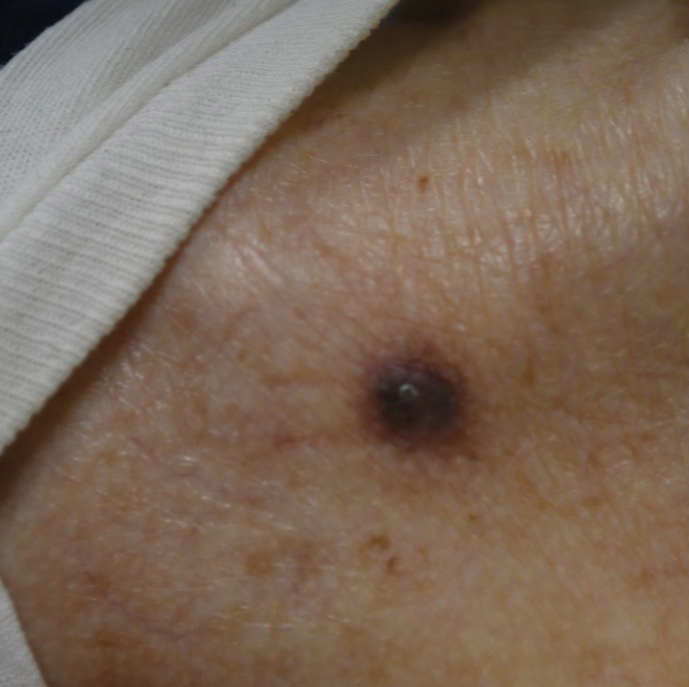
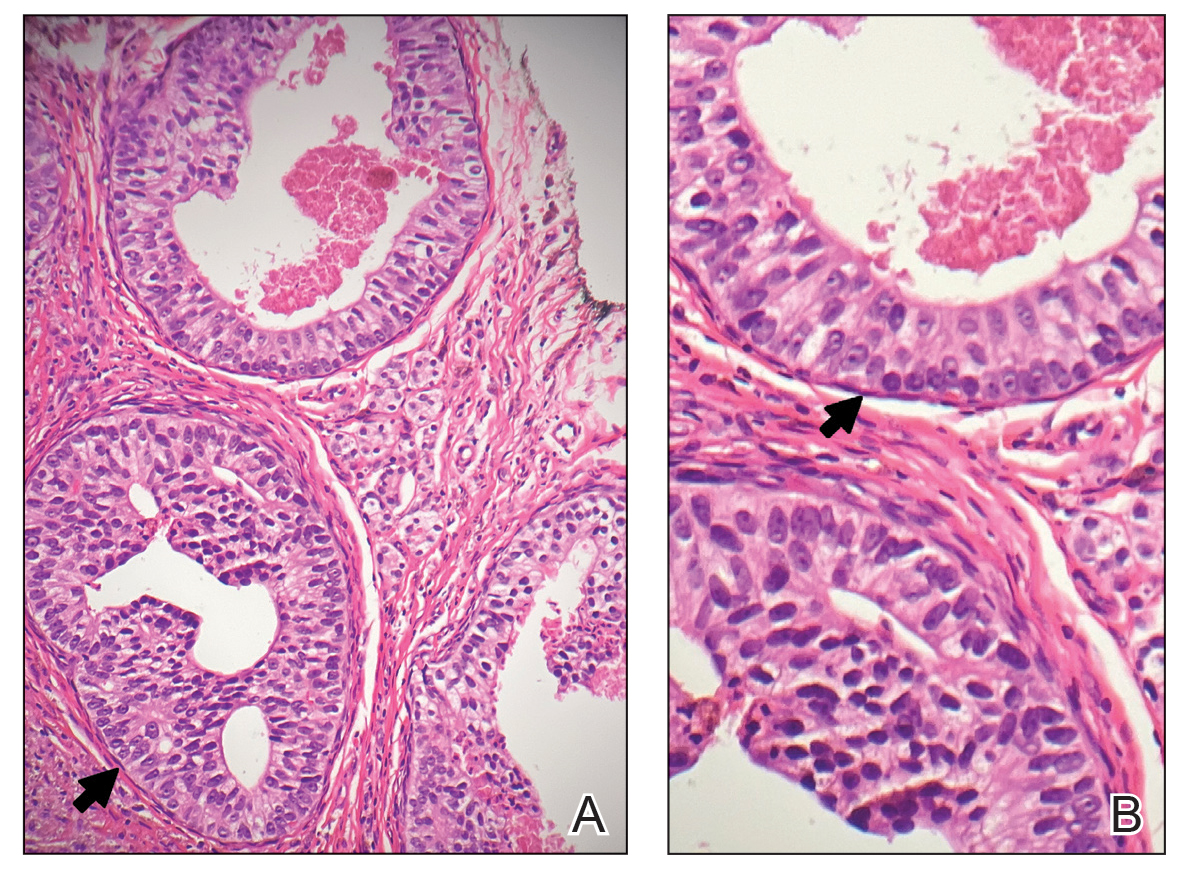
Physical examination at the current presentation revealed an 8-mm brown papule with an overlying blue-white veil (Figure 1). There were no other skin findings. Primary differential diagnoses included metastatic prostate cancer, nodular melanoma, and traumatized seborrheic keratosis. A shave biopsy of the lesion showed multiple glandular structures infiltrating the dermis lined by monomorphic epithelial cells with prominent eosinophilic nucleoli (Figures 2 and 3). Focal cribriform architecture of the glands was present as well as dermal hemorrhage and a lymphohistiocytic infiltrate (Figure 2A). Interestingly, in-transit vascular metastases were confirmed with the support of ERG, CD34, and CD31 immunohistochemical staining of the vessels.
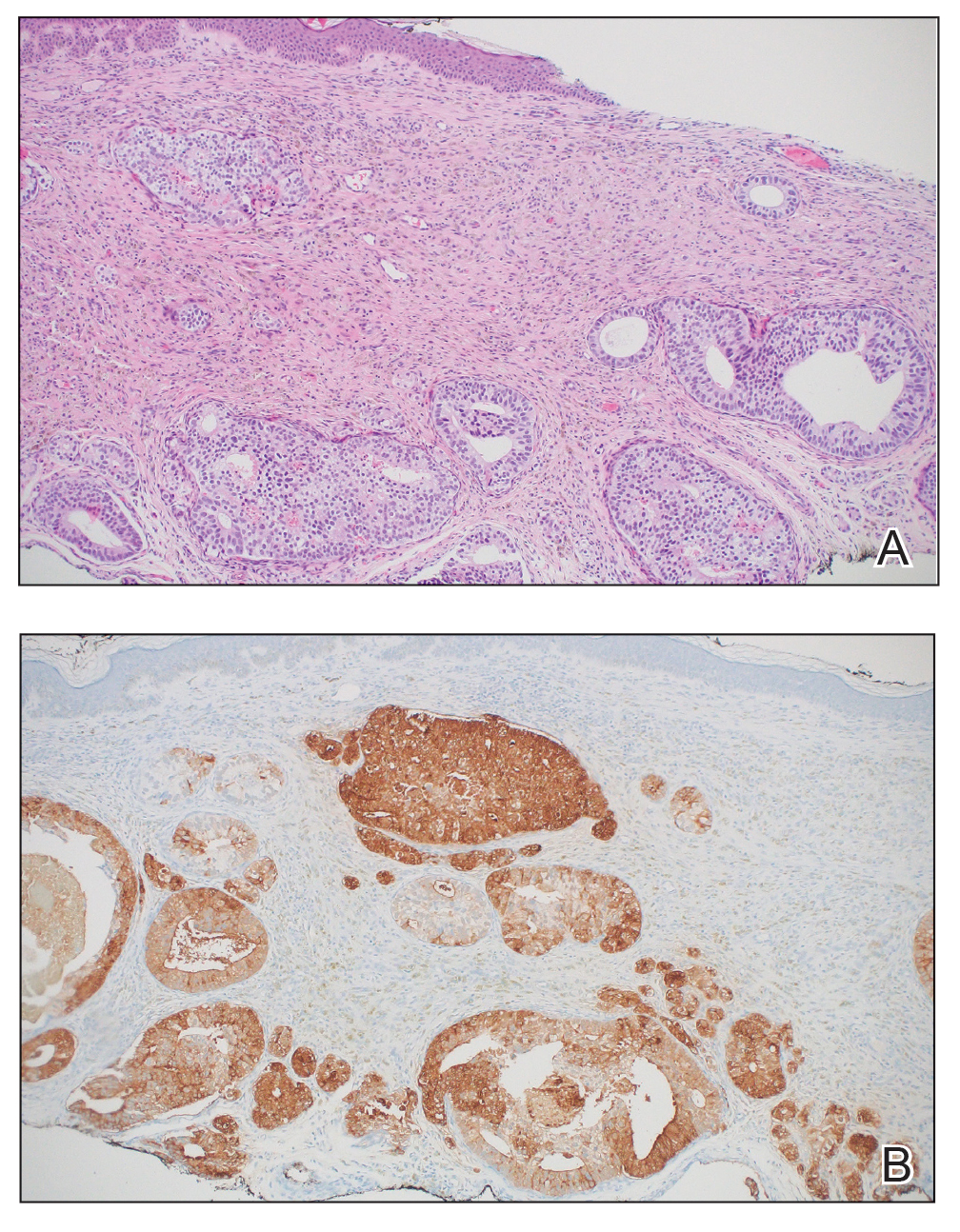
Immunohistochemical staining was positive for PSA (Figure 2B), NKX 3.1, and ERG in the invasive glandular structures, which also displayed patchy weak staining with AMACR. Staining was negative for prostein, cytokeratin (CK) 7, CK20, CK5/6, p63, p40, CDX2, and thyroid transcription factor 1. These findings were consistent with a diagnosis of cutaneous metastatic prostatic adenocarcinoma. Next-generation sequencing showed trans-membrane protease serine 2:v-ets erythroblastosis virus E26 oncogene homolog (TMPRSS2-ERG) fusion compatible with the positive ERG immunohistochemical staining. The patient and family declined any treatment due to his age, comorbidities, and rapid decline. He died 2 months after diagnosis of the skin metastasis.
Aside from nonmelanoma skin cancer, prostate cancer is the most common cancer and the second leading cause of cancer-related deaths among men in the United States.1 It most commonly metastasizes to the bones, nonregional lymph nodes, liver, and thorax.2 Metastasis to the skin is very rare, with only a 0.36% incidence.3 When prostate cancer does metastasize to the skin, the prognosis is poor, with an estimated mean survival of 7 months after diagnosis of cutaneous metastasis.4 Our patient’s survival time was even shorter—only 2 months after diagnosis of cutaneous metastasis, likely the result of his late diagnosis.
Clinically, cutaneous metastasis of prostate cancer can manifest as a wide variety of lesions; in one report of 78 cases, 56 (72%) were hard nodules, 11 (14%) were single nodules, 5 (7%) were edema or lymphedema, and 5 (7%) were an unspecific rash.4 Diagnosis of cutaneous metastasis of prostate cancer can be challenging, as it often is mistaken for other skin conditions including herpes zoster, basal cell carcinoma, angiosarcoma, cellulitis, mammary Paget disease, telangiectasia, pyoderma, morphea, and trichoepithelioma.5 In our patient, the clinical appearance of the lesion resembled a nodular melanoma. Thus, in patients with a history of prostate cancer, it is important to keep cutaneous metastasis in the differential when examining the skin because of the prognostic implications. Cutaneous metastasis of prostate cancer often indicates a poor prognosis.
In a report of 78 patients, the most common sites of skin metastasis for prostate cancer were the inguinal area and penis (28% [22/78]), abdomen (23% [18/78]), head and neck (16% [12/78]), and chest (14% [11/78]); the extremities and back were less frequently involved (10% [8/78] and 9% [7/78], respectively).4 Generally, cutaneous metastasis of internal malignancies involves the deep dermis and the subcutaneous tissue. It is common for cutaneous metastases to show histologic features of the primary tumor, as we saw in our patient. In a case series with 45 histologic diagnoses of cutaneous metastases from internal malignancies, 75.5% (34/45) of cases showed morphologic features of the primary tumor.6 However, this is not always the case, and the histologic appearance may vary. Metastatic prostate cancer may manifest as sheets, nests, or cords and often may have nuclear pleomorphism with prominent nucleoli.7
Immunohistochemical staining can help make a definitive diagnosis and differentiate the source of the tumor. Prostate cancer metastases often will stain positive for NKX3.1, PSA, AMACR, ERG, PSMA, and prosaposin, with PSA being the most specific marker.7,8 In our patient, no prostate biopsy had been performed, thus the skin biopsy was the diagnostic tissue for the prostatic adenocarcinoma.
Next-generation sequencing showed a TMPRSS2- ERG fusion, which commonly is seen in prostate cancer.9 A search of Google Scholar using the terms next-generation sequencing, cutaneous metastasis, and prostate adenocarcinoma yielded 3 additional cases of cutaneous metastasis of prostate cancer in which next-generation sequencing was performed.10-12 One case showed mutations of the tumor protein 53 (TP53) and phosphatase and tensin homolog (PTEN) genes; one showed just a TP53 mutation; and one showed inactivation of the breast cancer predisposition gene 2 (BRCA2) and amplification of MYC proto-oncogene, BHLH transcription factor (MYC) and fibroblast growth factor receptor 1 (FGFR1).10,11,12 While limited by a small number of reported cases, there does not appear to be a repeating mutation to suggest a genetic mechanism of skin metastasis.
The route of cutaneous metastasis of prostate cancer still is unclear, but hypothesized mechanisms include hematogenous or lymphatic spread, direct infiltration, or implantation from a surgical scar.11 When cutaneous involvement occurs in an area far from the primary tumor, it is thought to be the result of hematogenous spread, which would be consistent with our patient’s findings.13 Given the role of Batson venous plexus as a conduit from the prostate to the vertebral column for metastatic spread and considering the location of the lesion on our patient’s back, we hypothesized that the mechanism of metastasis to the skin was from vascular extension of the metastatic foci involving the vertebrae.
Our case highlights the importance of considering cutaneous involvement of prostatic adenocarcinoma in patients with new skin lesions, particularly in the setting of a known or suspected prostate malignancy. Skin metastasis can have a range of manifestations and provides prognostic information that can help determine the course of treatment.
- US Cancer Statistics Working Group. US cancer statistics data visualizations tool, based on 2022 submission data (1999-2020). US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. November 2023. Accessed November 11, 2024. https://www.cdc.gov/cancer/dataviz
- Gandaglia G, Abdollah F, Schiffmann J, et al. Distribution of metastatic sites in patients with prostate cancer: a population-based analysis. Prostate. 2014;74:210-216. doi:10.1002/pros.22742
- Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026. doi:10.1016/j.urology.2004.01.014
- Wang SQ, Mecca PS, Myskowski PL, et al. Scrotal and penile papules and plaques as the initial manifestation of a cutaneous metastasis of adenocarcinoma of the prostate: case report and review of the literature. J Cutan Pathol. 2008;35:681-684. doi:10.1111/j.1600-0560.2007.00873.x
- Reddy S, Bang RH, Contreras ME. Telangiectatic cutaneous metastasis from carcinoma of the prostate. Br J Dermatol. 2007;156:598-600. doi:10.1111/j.1365-2133.2006.07696.x
- Guanziroli E, Coggi A, Venegoni L, et al. Cutaneous metastases of internal malignancies: an experience from a single institution. Eur J Dermatol. 2017;27:609-614. doi:10.1684/ejd.2017.3142
- Onalaja-Underwood AA, Sokumbi O. Eruptive papules as a cutaneous manifestation of metastatic prostate adenocarcinoma. Am J Dermatopathol. 2023;45:828-830. doi:10.1097/DAD.0000000000002559
- Oesterling JE. Prostate specific antigen: a critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. J Urol. 1991;145:907-923. doi:10.1016/s0022-5347(17)38491-4
- Wang Z, Wang Y, Zhang J, et al. Significance of the TMPRSS2:ERG gene fusion in prostate cancer. Mol Med Rep. 2017;16:5450-5458. doi:10.3892/mmr.2017.7281
- Sharma H, Franklin M, Braunberger R, et al. Cutaneous metastasis from prostate cancer: a case report with literature review. Curr Probl Cancer Case Rep. 2022;7:100175. doi:10.1016/j.cpccr.2022.100175
- Dills A, Obi O, Bustos K, et al. Cutaneous manifestation of prostate adenocarcinoma: a rare presentation of a common disease. J Investig Med High Impact Case Rep. 2021;9:2324709621990769. doi:10.1177/2324709621990769
- Fadel CA, Kallab AM. Cutaneous scrotal metastasis secondary to primary prostate adenocarcinoma responding to immunotherapy. Ann Intern Med: Clinical Cases. 2022;1. doi:10.7326/aimcc.2022.0682
- Powell FC, Venencie PY, Winkelmann RK. Metastatic prostate carcinoma manifesting as penile nodules. Arch Dermatol. 1984;120:1604- 1606. doi:10.1001/archderm.1984.01650480066022
To the Editor:
Cutaneous metastasis of prostate cancer is rare and portends a bleak prognosis. Diagnosis of the primary cancer can be challenging, as skin metastasis can mimic a variety of conditions. We report a case of metastatic prostatic adenocarcinoma confirmed via biopsy of a new skin lesion.
A 97-year-old man presented to the dermatology clinic for routine follow-up of psoriasis. During the visit, a family member mentioned a new bleeding lesion on the left shoulder. It was not known how long the lesion had been present. Four months prior, the patient had a prostate-specific antigen (PSA) level of 582 ng/mL (reference range, 0-6.5 ng/mL), and computed tomography of the chest had shown innumerable pulmonary nodules in addition to lymphadenopathy of the left axilla, clavicle, and mediastinum. The imaging was ordered by the patient’s urologist as part of routine workup, as he had a history of obstructive renal failure and was being monitored for an indwelling catheter. Two months later, a bone scan ordered by the urologist due to high PSA levels showed extensive osteoblastic metastatic disease throughout the axial and proximal appendicular skeleton. The elevated PSA levels and findings of pulmonary and osteoblastic metastasis suggested a diagnosis of metastatic prostatic adenocarcinoma, but no confirmatory biopsy was performed following the imaging because the patient’s family declined additional workup or intervention.
Physical examination at the current presentation revealed an 8-mm brown papule with an overlying blue-white veil (Figure 1). There were no other skin findings. Primary differential diagnoses included metastatic prostate cancer, nodular melanoma, and traumatized seborrheic keratosis. A shave biopsy of the lesion showed multiple glandular structures infiltrating the dermis lined by monomorphic epithelial cells with prominent eosinophilic nucleoli (Figures 2 and 3). Focal cribriform architecture of the glands was present as well as dermal hemorrhage and a lymphohistiocytic infiltrate (Figure 2A). Interestingly, in-transit vascular metastases were confirmed with the support of ERG, CD34, and CD31 immunohistochemical staining of the vessels.



Immunohistochemical staining was positive for PSA (Figure 2B), NKX 3.1, and ERG in the invasive glandular structures, which also displayed patchy weak staining with AMACR. Staining was negative for prostein, cytokeratin (CK) 7, CK20, CK5/6, p63, p40, CDX2, and thyroid transcription factor 1. These findings were consistent with a diagnosis of cutaneous metastatic prostatic adenocarcinoma. Next-generation sequencing showed trans-membrane protease serine 2:v-ets erythroblastosis virus E26 oncogene homolog (TMPRSS2-ERG) fusion compatible with the positive ERG immunohistochemical staining. The patient and family declined any treatment due to his age, comorbidities, and rapid decline. He died 2 months after diagnosis of the skin metastasis.
Aside from nonmelanoma skin cancer, prostate cancer is the most common cancer and the second leading cause of cancer-related deaths among men in the United States.1 It most commonly metastasizes to the bones, nonregional lymph nodes, liver, and thorax.2 Metastasis to the skin is very rare, with only a 0.36% incidence.3 When prostate cancer does metastasize to the skin, the prognosis is poor, with an estimated mean survival of 7 months after diagnosis of cutaneous metastasis.4 Our patient’s survival time was even shorter—only 2 months after diagnosis of cutaneous metastasis, likely the result of his late diagnosis.
Clinically, cutaneous metastasis of prostate cancer can manifest as a wide variety of lesions; in one report of 78 cases, 56 (72%) were hard nodules, 11 (14%) were single nodules, 5 (7%) were edema or lymphedema, and 5 (7%) were an unspecific rash.4 Diagnosis of cutaneous metastasis of prostate cancer can be challenging, as it often is mistaken for other skin conditions including herpes zoster, basal cell carcinoma, angiosarcoma, cellulitis, mammary Paget disease, telangiectasia, pyoderma, morphea, and trichoepithelioma.5 In our patient, the clinical appearance of the lesion resembled a nodular melanoma. Thus, in patients with a history of prostate cancer, it is important to keep cutaneous metastasis in the differential when examining the skin because of the prognostic implications. Cutaneous metastasis of prostate cancer often indicates a poor prognosis.
In a report of 78 patients, the most common sites of skin metastasis for prostate cancer were the inguinal area and penis (28% [22/78]), abdomen (23% [18/78]), head and neck (16% [12/78]), and chest (14% [11/78]); the extremities and back were less frequently involved (10% [8/78] and 9% [7/78], respectively).4 Generally, cutaneous metastasis of internal malignancies involves the deep dermis and the subcutaneous tissue. It is common for cutaneous metastases to show histologic features of the primary tumor, as we saw in our patient. In a case series with 45 histologic diagnoses of cutaneous metastases from internal malignancies, 75.5% (34/45) of cases showed morphologic features of the primary tumor.6 However, this is not always the case, and the histologic appearance may vary. Metastatic prostate cancer may manifest as sheets, nests, or cords and often may have nuclear pleomorphism with prominent nucleoli.7
Immunohistochemical staining can help make a definitive diagnosis and differentiate the source of the tumor. Prostate cancer metastases often will stain positive for NKX3.1, PSA, AMACR, ERG, PSMA, and prosaposin, with PSA being the most specific marker.7,8 In our patient, no prostate biopsy had been performed, thus the skin biopsy was the diagnostic tissue for the prostatic adenocarcinoma.
Next-generation sequencing showed a TMPRSS2- ERG fusion, which commonly is seen in prostate cancer.9 A search of Google Scholar using the terms next-generation sequencing, cutaneous metastasis, and prostate adenocarcinoma yielded 3 additional cases of cutaneous metastasis of prostate cancer in which next-generation sequencing was performed.10-12 One case showed mutations of the tumor protein 53 (TP53) and phosphatase and tensin homolog (PTEN) genes; one showed just a TP53 mutation; and one showed inactivation of the breast cancer predisposition gene 2 (BRCA2) and amplification of MYC proto-oncogene, BHLH transcription factor (MYC) and fibroblast growth factor receptor 1 (FGFR1).10,11,12 While limited by a small number of reported cases, there does not appear to be a repeating mutation to suggest a genetic mechanism of skin metastasis.
The route of cutaneous metastasis of prostate cancer still is unclear, but hypothesized mechanisms include hematogenous or lymphatic spread, direct infiltration, or implantation from a surgical scar.11 When cutaneous involvement occurs in an area far from the primary tumor, it is thought to be the result of hematogenous spread, which would be consistent with our patient’s findings.13 Given the role of Batson venous plexus as a conduit from the prostate to the vertebral column for metastatic spread and considering the location of the lesion on our patient’s back, we hypothesized that the mechanism of metastasis to the skin was from vascular extension of the metastatic foci involving the vertebrae.
Our case highlights the importance of considering cutaneous involvement of prostatic adenocarcinoma in patients with new skin lesions, particularly in the setting of a known or suspected prostate malignancy. Skin metastasis can have a range of manifestations and provides prognostic information that can help determine the course of treatment.
To the Editor:
Cutaneous metastasis of prostate cancer is rare and portends a bleak prognosis. Diagnosis of the primary cancer can be challenging, as skin metastasis can mimic a variety of conditions. We report a case of metastatic prostatic adenocarcinoma confirmed via biopsy of a new skin lesion.
A 97-year-old man presented to the dermatology clinic for routine follow-up of psoriasis. During the visit, a family member mentioned a new bleeding lesion on the left shoulder. It was not known how long the lesion had been present. Four months prior, the patient had a prostate-specific antigen (PSA) level of 582 ng/mL (reference range, 0-6.5 ng/mL), and computed tomography of the chest had shown innumerable pulmonary nodules in addition to lymphadenopathy of the left axilla, clavicle, and mediastinum. The imaging was ordered by the patient’s urologist as part of routine workup, as he had a history of obstructive renal failure and was being monitored for an indwelling catheter. Two months later, a bone scan ordered by the urologist due to high PSA levels showed extensive osteoblastic metastatic disease throughout the axial and proximal appendicular skeleton. The elevated PSA levels and findings of pulmonary and osteoblastic metastasis suggested a diagnosis of metastatic prostatic adenocarcinoma, but no confirmatory biopsy was performed following the imaging because the patient’s family declined additional workup or intervention.
Physical examination at the current presentation revealed an 8-mm brown papule with an overlying blue-white veil (Figure 1). There were no other skin findings. Primary differential diagnoses included metastatic prostate cancer, nodular melanoma, and traumatized seborrheic keratosis. A shave biopsy of the lesion showed multiple glandular structures infiltrating the dermis lined by monomorphic epithelial cells with prominent eosinophilic nucleoli (Figures 2 and 3). Focal cribriform architecture of the glands was present as well as dermal hemorrhage and a lymphohistiocytic infiltrate (Figure 2A). Interestingly, in-transit vascular metastases were confirmed with the support of ERG, CD34, and CD31 immunohistochemical staining of the vessels.



Immunohistochemical staining was positive for PSA (Figure 2B), NKX 3.1, and ERG in the invasive glandular structures, which also displayed patchy weak staining with AMACR. Staining was negative for prostein, cytokeratin (CK) 7, CK20, CK5/6, p63, p40, CDX2, and thyroid transcription factor 1. These findings were consistent with a diagnosis of cutaneous metastatic prostatic adenocarcinoma. Next-generation sequencing showed trans-membrane protease serine 2:v-ets erythroblastosis virus E26 oncogene homolog (TMPRSS2-ERG) fusion compatible with the positive ERG immunohistochemical staining. The patient and family declined any treatment due to his age, comorbidities, and rapid decline. He died 2 months after diagnosis of the skin metastasis.
Aside from nonmelanoma skin cancer, prostate cancer is the most common cancer and the second leading cause of cancer-related deaths among men in the United States.1 It most commonly metastasizes to the bones, nonregional lymph nodes, liver, and thorax.2 Metastasis to the skin is very rare, with only a 0.36% incidence.3 When prostate cancer does metastasize to the skin, the prognosis is poor, with an estimated mean survival of 7 months after diagnosis of cutaneous metastasis.4 Our patient’s survival time was even shorter—only 2 months after diagnosis of cutaneous metastasis, likely the result of his late diagnosis.
Clinically, cutaneous metastasis of prostate cancer can manifest as a wide variety of lesions; in one report of 78 cases, 56 (72%) were hard nodules, 11 (14%) were single nodules, 5 (7%) were edema or lymphedema, and 5 (7%) were an unspecific rash.4 Diagnosis of cutaneous metastasis of prostate cancer can be challenging, as it often is mistaken for other skin conditions including herpes zoster, basal cell carcinoma, angiosarcoma, cellulitis, mammary Paget disease, telangiectasia, pyoderma, morphea, and trichoepithelioma.5 In our patient, the clinical appearance of the lesion resembled a nodular melanoma. Thus, in patients with a history of prostate cancer, it is important to keep cutaneous metastasis in the differential when examining the skin because of the prognostic implications. Cutaneous metastasis of prostate cancer often indicates a poor prognosis.
In a report of 78 patients, the most common sites of skin metastasis for prostate cancer were the inguinal area and penis (28% [22/78]), abdomen (23% [18/78]), head and neck (16% [12/78]), and chest (14% [11/78]); the extremities and back were less frequently involved (10% [8/78] and 9% [7/78], respectively).4 Generally, cutaneous metastasis of internal malignancies involves the deep dermis and the subcutaneous tissue. It is common for cutaneous metastases to show histologic features of the primary tumor, as we saw in our patient. In a case series with 45 histologic diagnoses of cutaneous metastases from internal malignancies, 75.5% (34/45) of cases showed morphologic features of the primary tumor.6 However, this is not always the case, and the histologic appearance may vary. Metastatic prostate cancer may manifest as sheets, nests, or cords and often may have nuclear pleomorphism with prominent nucleoli.7
Immunohistochemical staining can help make a definitive diagnosis and differentiate the source of the tumor. Prostate cancer metastases often will stain positive for NKX3.1, PSA, AMACR, ERG, PSMA, and prosaposin, with PSA being the most specific marker.7,8 In our patient, no prostate biopsy had been performed, thus the skin biopsy was the diagnostic tissue for the prostatic adenocarcinoma.
Next-generation sequencing showed a TMPRSS2- ERG fusion, which commonly is seen in prostate cancer.9 A search of Google Scholar using the terms next-generation sequencing, cutaneous metastasis, and prostate adenocarcinoma yielded 3 additional cases of cutaneous metastasis of prostate cancer in which next-generation sequencing was performed.10-12 One case showed mutations of the tumor protein 53 (TP53) and phosphatase and tensin homolog (PTEN) genes; one showed just a TP53 mutation; and one showed inactivation of the breast cancer predisposition gene 2 (BRCA2) and amplification of MYC proto-oncogene, BHLH transcription factor (MYC) and fibroblast growth factor receptor 1 (FGFR1).10,11,12 While limited by a small number of reported cases, there does not appear to be a repeating mutation to suggest a genetic mechanism of skin metastasis.
The route of cutaneous metastasis of prostate cancer still is unclear, but hypothesized mechanisms include hematogenous or lymphatic spread, direct infiltration, or implantation from a surgical scar.11 When cutaneous involvement occurs in an area far from the primary tumor, it is thought to be the result of hematogenous spread, which would be consistent with our patient’s findings.13 Given the role of Batson venous plexus as a conduit from the prostate to the vertebral column for metastatic spread and considering the location of the lesion on our patient’s back, we hypothesized that the mechanism of metastasis to the skin was from vascular extension of the metastatic foci involving the vertebrae.
Our case highlights the importance of considering cutaneous involvement of prostatic adenocarcinoma in patients with new skin lesions, particularly in the setting of a known or suspected prostate malignancy. Skin metastasis can have a range of manifestations and provides prognostic information that can help determine the course of treatment.
- US Cancer Statistics Working Group. US cancer statistics data visualizations tool, based on 2022 submission data (1999-2020). US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. November 2023. Accessed November 11, 2024. https://www.cdc.gov/cancer/dataviz
- Gandaglia G, Abdollah F, Schiffmann J, et al. Distribution of metastatic sites in patients with prostate cancer: a population-based analysis. Prostate. 2014;74:210-216. doi:10.1002/pros.22742
- Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026. doi:10.1016/j.urology.2004.01.014
- Wang SQ, Mecca PS, Myskowski PL, et al. Scrotal and penile papules and plaques as the initial manifestation of a cutaneous metastasis of adenocarcinoma of the prostate: case report and review of the literature. J Cutan Pathol. 2008;35:681-684. doi:10.1111/j.1600-0560.2007.00873.x
- Reddy S, Bang RH, Contreras ME. Telangiectatic cutaneous metastasis from carcinoma of the prostate. Br J Dermatol. 2007;156:598-600. doi:10.1111/j.1365-2133.2006.07696.x
- Guanziroli E, Coggi A, Venegoni L, et al. Cutaneous metastases of internal malignancies: an experience from a single institution. Eur J Dermatol. 2017;27:609-614. doi:10.1684/ejd.2017.3142
- Onalaja-Underwood AA, Sokumbi O. Eruptive papules as a cutaneous manifestation of metastatic prostate adenocarcinoma. Am J Dermatopathol. 2023;45:828-830. doi:10.1097/DAD.0000000000002559
- Oesterling JE. Prostate specific antigen: a critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. J Urol. 1991;145:907-923. doi:10.1016/s0022-5347(17)38491-4
- Wang Z, Wang Y, Zhang J, et al. Significance of the TMPRSS2:ERG gene fusion in prostate cancer. Mol Med Rep. 2017;16:5450-5458. doi:10.3892/mmr.2017.7281
- Sharma H, Franklin M, Braunberger R, et al. Cutaneous metastasis from prostate cancer: a case report with literature review. Curr Probl Cancer Case Rep. 2022;7:100175. doi:10.1016/j.cpccr.2022.100175
- Dills A, Obi O, Bustos K, et al. Cutaneous manifestation of prostate adenocarcinoma: a rare presentation of a common disease. J Investig Med High Impact Case Rep. 2021;9:2324709621990769. doi:10.1177/2324709621990769
- Fadel CA, Kallab AM. Cutaneous scrotal metastasis secondary to primary prostate adenocarcinoma responding to immunotherapy. Ann Intern Med: Clinical Cases. 2022;1. doi:10.7326/aimcc.2022.0682
- Powell FC, Venencie PY, Winkelmann RK. Metastatic prostate carcinoma manifesting as penile nodules. Arch Dermatol. 1984;120:1604- 1606. doi:10.1001/archderm.1984.01650480066022
- US Cancer Statistics Working Group. US cancer statistics data visualizations tool, based on 2022 submission data (1999-2020). US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. November 2023. Accessed November 11, 2024. https://www.cdc.gov/cancer/dataviz
- Gandaglia G, Abdollah F, Schiffmann J, et al. Distribution of metastatic sites in patients with prostate cancer: a population-based analysis. Prostate. 2014;74:210-216. doi:10.1002/pros.22742
- Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026. doi:10.1016/j.urology.2004.01.014
- Wang SQ, Mecca PS, Myskowski PL, et al. Scrotal and penile papules and plaques as the initial manifestation of a cutaneous metastasis of adenocarcinoma of the prostate: case report and review of the literature. J Cutan Pathol. 2008;35:681-684. doi:10.1111/j.1600-0560.2007.00873.x
- Reddy S, Bang RH, Contreras ME. Telangiectatic cutaneous metastasis from carcinoma of the prostate. Br J Dermatol. 2007;156:598-600. doi:10.1111/j.1365-2133.2006.07696.x
- Guanziroli E, Coggi A, Venegoni L, et al. Cutaneous metastases of internal malignancies: an experience from a single institution. Eur J Dermatol. 2017;27:609-614. doi:10.1684/ejd.2017.3142
- Onalaja-Underwood AA, Sokumbi O. Eruptive papules as a cutaneous manifestation of metastatic prostate adenocarcinoma. Am J Dermatopathol. 2023;45:828-830. doi:10.1097/DAD.0000000000002559
- Oesterling JE. Prostate specific antigen: a critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. J Urol. 1991;145:907-923. doi:10.1016/s0022-5347(17)38491-4
- Wang Z, Wang Y, Zhang J, et al. Significance of the TMPRSS2:ERG gene fusion in prostate cancer. Mol Med Rep. 2017;16:5450-5458. doi:10.3892/mmr.2017.7281
- Sharma H, Franklin M, Braunberger R, et al. Cutaneous metastasis from prostate cancer: a case report with literature review. Curr Probl Cancer Case Rep. 2022;7:100175. doi:10.1016/j.cpccr.2022.100175
- Dills A, Obi O, Bustos K, et al. Cutaneous manifestation of prostate adenocarcinoma: a rare presentation of a common disease. J Investig Med High Impact Case Rep. 2021;9:2324709621990769. doi:10.1177/2324709621990769
- Fadel CA, Kallab AM. Cutaneous scrotal metastasis secondary to primary prostate adenocarcinoma responding to immunotherapy. Ann Intern Med: Clinical Cases. 2022;1. doi:10.7326/aimcc.2022.0682
- Powell FC, Venencie PY, Winkelmann RK. Metastatic prostate carcinoma manifesting as penile nodules. Arch Dermatol. 1984;120:1604- 1606. doi:10.1001/archderm.1984.01650480066022
Cutaneous Metastasis of an Undiagnosed Prostatic Adenocarcinoma
Cutaneous Metastasis of an Undiagnosed Prostatic Adenocarcinoma
PRACTICE POINTS
- Cutaneous metastasis of prostate cancer can have various manifestations and portends a poor prognosis.
- New skin lesions that develop in patients with a high clinical suspicion for prostate cancer warrant consideration of cutaneous metastasis.
Break the Itch-Scratch Cycle to Treat Prurigo Nodularis
Break the Itch-Scratch Cycle to Treat Prurigo Nodularis
Prurigo nodularis (PN) is a chronic inflammatory skin condition characterized by firm hyperkeratotic nodules that develops when patients persistently scratch or rub intensely itchy areas of the skin. This potent itch-scratch cycle can be traced back to a dysfunctional interplay between cutaneous nerve fibers and the local immune environment.1-3 Pruritis lasting at least 6 weeks is a hallmark symptom of PN and can be accompanied by pain and/or a burning sensation.4 The lesions are symmetrically distributed in areas that are easy to scratch (eg, arms, legs, trunk), typically sparing the face, palms, and soles; however, facial lesions have been reported in pediatric patients with PN, who also are more likely to have back, hand, and foot involvement.5,6
PN can greatly affect patients’ quality of life, leading to increased rates of depression and anxiety.7-9 Patients with severe symptoms also report increased sleep disturbance, distraction from work, selfconsciousness leading to social isolation, and missed days of work/school.9 In one study, patients with PN reported missing at least 1 day of work, school, training, or learning; giving up a leisure activity or sport; or refusing an invitation to dinner or a party in the past 3 months due to the disease.10
Epidemiology
PN has a prevalence of 72 per 100,000 individuals in the United States, most commonly affecting adults aged 51 to 65 years and disproportionately affecting African American and female patients.11-13 Most patients with PN experience a 2-year delay in diagnosis after initial onset of symptoms. 10 Adults with PN have an increased likelihood of having other dermatologic conditions, including atopic dermatitis (AD) and psoriasis.11 Nearly two-thirds of pediatric patients with PN present with AD, and those with AD showed more resistance to first-line treatment options.5
Key Clinical Features
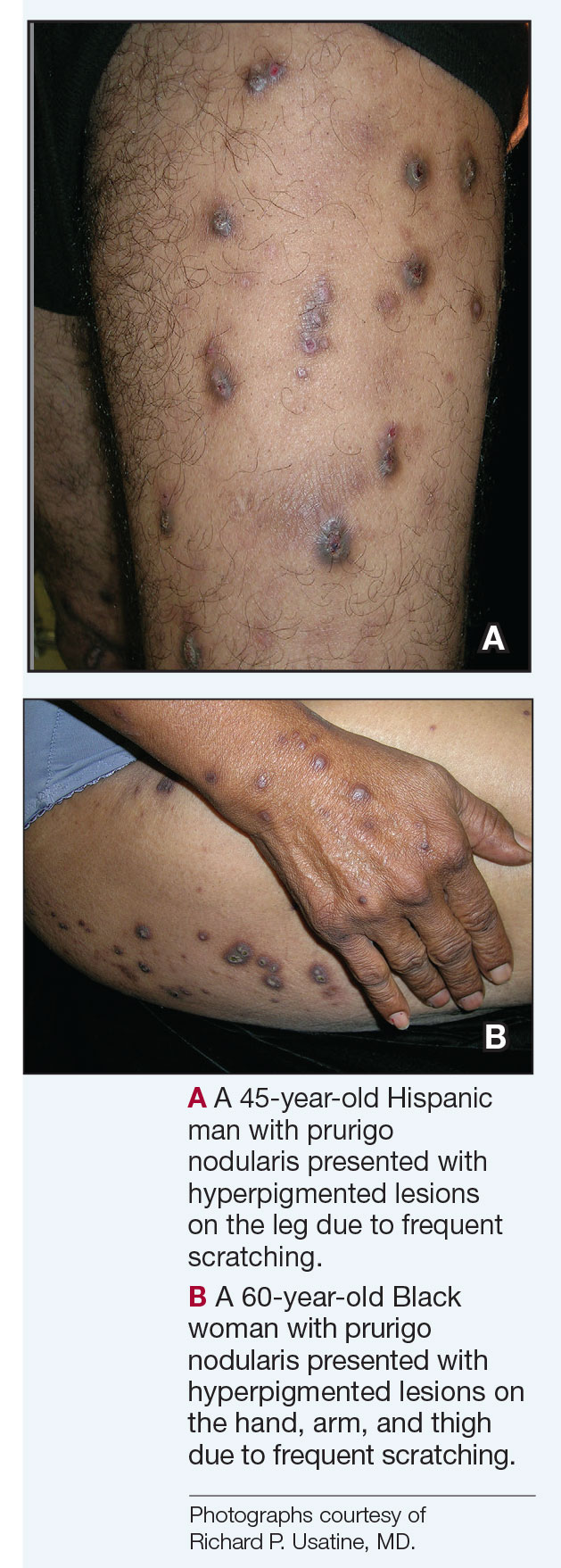
Compared to White patients, who typically present with lesions that appear erythematous or pink, patients with darker skin tones may present with hyperpigmented nodules that are larger and darker.12 The pruritic nodules often show signs of scratching or picking (eg, excoriations, lichenification, and angulated erosions).4
Worth Noting
Diagnosis of PN is made clinically, but skin biopsy may be helpful to rule out alternative diseases. Histologically, the hairy palm sign may be present in addition to other histologic features commonly associated with excessive scratching or rubbing of the skin.
Patients with PN have a high risk for HIV, which is not surprising considering HIV is a known systemic cause of generalized chronic pruritus. Other associations include type 2 diabetes mellitus and thyroid, kidney, and liver disease. 11,13 Workup for patients with PN should include a complete blood count with differential; liver and renal function testing; and testing for C-reactive protein, thyroid-stimulating hormone, and lactate dehydrogenase.4,14 Hemoglobin A1c and HIV testing as well as a hepatitis panel should be considered when appropriate. Because generalized pruritus may be a sign of malignancy, chest radiography and lymph node and abdominal ultrasonography should be performed in patients who have experienced itch for less than 1 year along with B symptoms (fever, night sweats, ≥ 10% weight loss over 6 months, fatigue).14 Frequent scratching can disrupt the skin barrier, contributing to the increased risk for skin infections.13 All patients with a suspected PN diagnosis also should undergo screening for depression and anxiety, as patients with PN are at an increased risk for these conditions.4
Treatment of PN starts with breaking the itch-scratch cycle by addressing the underlying cause of the pruritus. Therapies are focused on addressing the immunologic and neural components of the disease. Topical treatments include moderate to strong corticosteroids, calcineurin inhibitors (tacrolimus or pimecrolimus), capsaicin, and antipruritic emollients. Systemic agents include phototherapy (narrow-band UVB or excimer laser), gabapentin, pregabalin, paroxetine, and amitriptyline to address the neural component of itch. Methotrexate or cyclosporine can be used to address the immunologic component of PN and diminish the itch. That said, methotrexate and cyclosporine often are inadequate to control pruritus. 10 Of note, sedating antihistamines are not effective in treating itch in PN but can be used as an adjuvant therapy for sleep disturbances in these patients.15
The only drugs currently approved by the US Food and Drug Administration to treat PN are the biologics dupilumab (targeting the IL-4 receptor) approved in 2022 and nemolizumab (targeting the IL-31 receptor) approved in 2024.16-18 The evidence that these injectable biologics work is heartening in a condition that has historically been very challenging to treat.16,18 It should be noted that the high cost of these 2 medications can restrict access to care for patients who are uninsured or underinsured.
Resolution of a prurigo nodule may result in a hyperpigmented macule taking months to years to fade.
Health Disparity Highlight
Patients with PN have a considerable comorbidity burden, negative impact on quality of life, and increased health care utilization rates.12 PN is 3.4 times more common in Black patients than White patients.13 Black patients with PN have increased mortality, higher health care utilization rates, and increased systemic inflammation compared to White patients.12,19,20
- Cevikbas F, Wang X, Akiyama T, et al. A sensory neuron– expressed IL-31 receptor mediates T helper cell–dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448-460.
- Lou H, Lu J, Choi EB, et al. Expression of IL-22 in the skin causes Th2-biased immunity, epidermal barrier dysfunction, and pruritus via stimulating epithelial Th2 cytokines and the GRP pathway. J Immunol. 2017;198:2543-2555.
- Sutaria N, Adawi W, Goldberg R, et al. Itch: pathogenesis and treatment. J Am Acad Dermatol. 2022;86:17-34.
- Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84:747-760.
- Kyvayko R, Fachler-Sharp T, Greenberger S, et al. Characterization of paediatric prurigo nodularis: a multicentre retrospective, observational study. Acta Derm Venereol. 2024;104:adv15771.
- Aggarwal P, Choi J, Sutaria N, et al. Clinical characteristics and disease burden in prurigo nodularis. Clin Exp Dermatol. 2021;46:1277-1284.
- Whang KA, Le TK, Khanna R, et al. Health-related quality of life and economic burden of prurigo nodularis. J Am Acad Dermatol. 2022;86:573-580.
- Jørgensen KM, Egeberg A, Gislason GH, et al. Anxiety, depression and suicide in patients with prurigo nodularis. J Eur Acad Dermatol Venereol. 2017;31:E106-E107.
- Rodriguez D, Kwatra SG, Dias-Barbosa C, et al. Patient perspectives on living with severe prurigo nodularis. JAMA Dermatol. 2023;159:1205-1212.
- Misery L, Patras de Campaigno C, Taieb C, et al. Impact of chronic prurigo nodularis on daily life and stigmatization. J Eur Acad Dermatol Venereol. 2023;37:E908-E909.
- Huang AH, Canner JK, Khanna R, et al. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Invest Dermatol. 2020;140:480-483.e4.
- Sutaria N, Adawi W, Brown I, et al. Racial disparities in mortality among patients with prurigo nodularis: a multicenter cohort study. J Am Acad Dermatol. 2022;82:487- 490.
- Boozalis E, Tang O, Patel S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol. 2018;79:714-719.e3.
- Müller S, Zeidler C, Ständer S. Chronic prurigo including prurigo nodularis: new insights and treatments. Am J Clin Dermatol. 2024;25:15-33.
- Williams KA, Roh YS, Brown I, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14:67-77.
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589.
- Beck KM, Yang EJ, Sekhon S, et al. Dupilumab treatment for generalized prurigo nodularis. JAMA Dermatol. 2019;155:118-120.
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double- blind, placebo- controlled phase 3 trials. Nat Med. 2023;29:1180-1190.
- Wongvibulsin S, Sutaria N, Williams KA, et al. A nationwide study of prurigo nodularis: disease burden and healthcare utilization in the United States. J Invest Dermatol. 2021;141:2530-2533.e1.
- Sutaria N, Alphonse MP, Marani M, et al. Cluster analysis of circulating plasma biomarkers in prurigo nodularis reveals a distinct systemic inflammatory signature in African Americans. J Invest Dermatol. 2022;142:1300-1308.e3.
Prurigo nodularis (PN) is a chronic inflammatory skin condition characterized by firm hyperkeratotic nodules that develops when patients persistently scratch or rub intensely itchy areas of the skin. This potent itch-scratch cycle can be traced back to a dysfunctional interplay between cutaneous nerve fibers and the local immune environment.1-3 Pruritis lasting at least 6 weeks is a hallmark symptom of PN and can be accompanied by pain and/or a burning sensation.4 The lesions are symmetrically distributed in areas that are easy to scratch (eg, arms, legs, trunk), typically sparing the face, palms, and soles; however, facial lesions have been reported in pediatric patients with PN, who also are more likely to have back, hand, and foot involvement.5,6
PN can greatly affect patients’ quality of life, leading to increased rates of depression and anxiety.7-9 Patients with severe symptoms also report increased sleep disturbance, distraction from work, selfconsciousness leading to social isolation, and missed days of work/school.9 In one study, patients with PN reported missing at least 1 day of work, school, training, or learning; giving up a leisure activity or sport; or refusing an invitation to dinner or a party in the past 3 months due to the disease.10

Epidemiology
PN has a prevalence of 72 per 100,000 individuals in the United States, most commonly affecting adults aged 51 to 65 years and disproportionately affecting African American and female patients.11-13 Most patients with PN experience a 2-year delay in diagnosis after initial onset of symptoms. 10 Adults with PN have an increased likelihood of having other dermatologic conditions, including atopic dermatitis (AD) and psoriasis.11 Nearly two-thirds of pediatric patients with PN present with AD, and those with AD showed more resistance to first-line treatment options.5
Key Clinical Features
Compared to White patients, who typically present with lesions that appear erythematous or pink, patients with darker skin tones may present with hyperpigmented nodules that are larger and darker.12 The pruritic nodules often show signs of scratching or picking (eg, excoriations, lichenification, and angulated erosions).4
Worth Noting
Diagnosis of PN is made clinically, but skin biopsy may be helpful to rule out alternative diseases. Histologically, the hairy palm sign may be present in addition to other histologic features commonly associated with excessive scratching or rubbing of the skin.
Patients with PN have a high risk for HIV, which is not surprising considering HIV is a known systemic cause of generalized chronic pruritus. Other associations include type 2 diabetes mellitus and thyroid, kidney, and liver disease. 11,13 Workup for patients with PN should include a complete blood count with differential; liver and renal function testing; and testing for C-reactive protein, thyroid-stimulating hormone, and lactate dehydrogenase.4,14 Hemoglobin A1c and HIV testing as well as a hepatitis panel should be considered when appropriate. Because generalized pruritus may be a sign of malignancy, chest radiography and lymph node and abdominal ultrasonography should be performed in patients who have experienced itch for less than 1 year along with B symptoms (fever, night sweats, ≥ 10% weight loss over 6 months, fatigue).14 Frequent scratching can disrupt the skin barrier, contributing to the increased risk for skin infections.13 All patients with a suspected PN diagnosis also should undergo screening for depression and anxiety, as patients with PN are at an increased risk for these conditions.4
Treatment of PN starts with breaking the itch-scratch cycle by addressing the underlying cause of the pruritus. Therapies are focused on addressing the immunologic and neural components of the disease. Topical treatments include moderate to strong corticosteroids, calcineurin inhibitors (tacrolimus or pimecrolimus), capsaicin, and antipruritic emollients. Systemic agents include phototherapy (narrow-band UVB or excimer laser), gabapentin, pregabalin, paroxetine, and amitriptyline to address the neural component of itch. Methotrexate or cyclosporine can be used to address the immunologic component of PN and diminish the itch. That said, methotrexate and cyclosporine often are inadequate to control pruritus. 10 Of note, sedating antihistamines are not effective in treating itch in PN but can be used as an adjuvant therapy for sleep disturbances in these patients.15
The only drugs currently approved by the US Food and Drug Administration to treat PN are the biologics dupilumab (targeting the IL-4 receptor) approved in 2022 and nemolizumab (targeting the IL-31 receptor) approved in 2024.16-18 The evidence that these injectable biologics work is heartening in a condition that has historically been very challenging to treat.16,18 It should be noted that the high cost of these 2 medications can restrict access to care for patients who are uninsured or underinsured.
Resolution of a prurigo nodule may result in a hyperpigmented macule taking months to years to fade.
Health Disparity Highlight
Patients with PN have a considerable comorbidity burden, negative impact on quality of life, and increased health care utilization rates.12 PN is 3.4 times more common in Black patients than White patients.13 Black patients with PN have increased mortality, higher health care utilization rates, and increased systemic inflammation compared to White patients.12,19,20
Prurigo nodularis (PN) is a chronic inflammatory skin condition characterized by firm hyperkeratotic nodules that develops when patients persistently scratch or rub intensely itchy areas of the skin. This potent itch-scratch cycle can be traced back to a dysfunctional interplay between cutaneous nerve fibers and the local immune environment.1-3 Pruritis lasting at least 6 weeks is a hallmark symptom of PN and can be accompanied by pain and/or a burning sensation.4 The lesions are symmetrically distributed in areas that are easy to scratch (eg, arms, legs, trunk), typically sparing the face, palms, and soles; however, facial lesions have been reported in pediatric patients with PN, who also are more likely to have back, hand, and foot involvement.5,6
PN can greatly affect patients’ quality of life, leading to increased rates of depression and anxiety.7-9 Patients with severe symptoms also report increased sleep disturbance, distraction from work, selfconsciousness leading to social isolation, and missed days of work/school.9 In one study, patients with PN reported missing at least 1 day of work, school, training, or learning; giving up a leisure activity or sport; or refusing an invitation to dinner or a party in the past 3 months due to the disease.10

Epidemiology
PN has a prevalence of 72 per 100,000 individuals in the United States, most commonly affecting adults aged 51 to 65 years and disproportionately affecting African American and female patients.11-13 Most patients with PN experience a 2-year delay in diagnosis after initial onset of symptoms. 10 Adults with PN have an increased likelihood of having other dermatologic conditions, including atopic dermatitis (AD) and psoriasis.11 Nearly two-thirds of pediatric patients with PN present with AD, and those with AD showed more resistance to first-line treatment options.5
Key Clinical Features
Compared to White patients, who typically present with lesions that appear erythematous or pink, patients with darker skin tones may present with hyperpigmented nodules that are larger and darker.12 The pruritic nodules often show signs of scratching or picking (eg, excoriations, lichenification, and angulated erosions).4
Worth Noting
Diagnosis of PN is made clinically, but skin biopsy may be helpful to rule out alternative diseases. Histologically, the hairy palm sign may be present in addition to other histologic features commonly associated with excessive scratching or rubbing of the skin.
Patients with PN have a high risk for HIV, which is not surprising considering HIV is a known systemic cause of generalized chronic pruritus. Other associations include type 2 diabetes mellitus and thyroid, kidney, and liver disease. 11,13 Workup for patients with PN should include a complete blood count with differential; liver and renal function testing; and testing for C-reactive protein, thyroid-stimulating hormone, and lactate dehydrogenase.4,14 Hemoglobin A1c and HIV testing as well as a hepatitis panel should be considered when appropriate. Because generalized pruritus may be a sign of malignancy, chest radiography and lymph node and abdominal ultrasonography should be performed in patients who have experienced itch for less than 1 year along with B symptoms (fever, night sweats, ≥ 10% weight loss over 6 months, fatigue).14 Frequent scratching can disrupt the skin barrier, contributing to the increased risk for skin infections.13 All patients with a suspected PN diagnosis also should undergo screening for depression and anxiety, as patients with PN are at an increased risk for these conditions.4
Treatment of PN starts with breaking the itch-scratch cycle by addressing the underlying cause of the pruritus. Therapies are focused on addressing the immunologic and neural components of the disease. Topical treatments include moderate to strong corticosteroids, calcineurin inhibitors (tacrolimus or pimecrolimus), capsaicin, and antipruritic emollients. Systemic agents include phototherapy (narrow-band UVB or excimer laser), gabapentin, pregabalin, paroxetine, and amitriptyline to address the neural component of itch. Methotrexate or cyclosporine can be used to address the immunologic component of PN and diminish the itch. That said, methotrexate and cyclosporine often are inadequate to control pruritus. 10 Of note, sedating antihistamines are not effective in treating itch in PN but can be used as an adjuvant therapy for sleep disturbances in these patients.15
The only drugs currently approved by the US Food and Drug Administration to treat PN are the biologics dupilumab (targeting the IL-4 receptor) approved in 2022 and nemolizumab (targeting the IL-31 receptor) approved in 2024.16-18 The evidence that these injectable biologics work is heartening in a condition that has historically been very challenging to treat.16,18 It should be noted that the high cost of these 2 medications can restrict access to care for patients who are uninsured or underinsured.
Resolution of a prurigo nodule may result in a hyperpigmented macule taking months to years to fade.
Health Disparity Highlight
Patients with PN have a considerable comorbidity burden, negative impact on quality of life, and increased health care utilization rates.12 PN is 3.4 times more common in Black patients than White patients.13 Black patients with PN have increased mortality, higher health care utilization rates, and increased systemic inflammation compared to White patients.12,19,20
- Cevikbas F, Wang X, Akiyama T, et al. A sensory neuron– expressed IL-31 receptor mediates T helper cell–dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448-460.
- Lou H, Lu J, Choi EB, et al. Expression of IL-22 in the skin causes Th2-biased immunity, epidermal barrier dysfunction, and pruritus via stimulating epithelial Th2 cytokines and the GRP pathway. J Immunol. 2017;198:2543-2555.
- Sutaria N, Adawi W, Goldberg R, et al. Itch: pathogenesis and treatment. J Am Acad Dermatol. 2022;86:17-34.
- Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84:747-760.
- Kyvayko R, Fachler-Sharp T, Greenberger S, et al. Characterization of paediatric prurigo nodularis: a multicentre retrospective, observational study. Acta Derm Venereol. 2024;104:adv15771.
- Aggarwal P, Choi J, Sutaria N, et al. Clinical characteristics and disease burden in prurigo nodularis. Clin Exp Dermatol. 2021;46:1277-1284.
- Whang KA, Le TK, Khanna R, et al. Health-related quality of life and economic burden of prurigo nodularis. J Am Acad Dermatol. 2022;86:573-580.
- Jørgensen KM, Egeberg A, Gislason GH, et al. Anxiety, depression and suicide in patients with prurigo nodularis. J Eur Acad Dermatol Venereol. 2017;31:E106-E107.
- Rodriguez D, Kwatra SG, Dias-Barbosa C, et al. Patient perspectives on living with severe prurigo nodularis. JAMA Dermatol. 2023;159:1205-1212.
- Misery L, Patras de Campaigno C, Taieb C, et al. Impact of chronic prurigo nodularis on daily life and stigmatization. J Eur Acad Dermatol Venereol. 2023;37:E908-E909.
- Huang AH, Canner JK, Khanna R, et al. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Invest Dermatol. 2020;140:480-483.e4.
- Sutaria N, Adawi W, Brown I, et al. Racial disparities in mortality among patients with prurigo nodularis: a multicenter cohort study. J Am Acad Dermatol. 2022;82:487- 490.
- Boozalis E, Tang O, Patel S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol. 2018;79:714-719.e3.
- Müller S, Zeidler C, Ständer S. Chronic prurigo including prurigo nodularis: new insights and treatments. Am J Clin Dermatol. 2024;25:15-33.
- Williams KA, Roh YS, Brown I, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14:67-77.
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589.
- Beck KM, Yang EJ, Sekhon S, et al. Dupilumab treatment for generalized prurigo nodularis. JAMA Dermatol. 2019;155:118-120.
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double- blind, placebo- controlled phase 3 trials. Nat Med. 2023;29:1180-1190.
- Wongvibulsin S, Sutaria N, Williams KA, et al. A nationwide study of prurigo nodularis: disease burden and healthcare utilization in the United States. J Invest Dermatol. 2021;141:2530-2533.e1.
- Sutaria N, Alphonse MP, Marani M, et al. Cluster analysis of circulating plasma biomarkers in prurigo nodularis reveals a distinct systemic inflammatory signature in African Americans. J Invest Dermatol. 2022;142:1300-1308.e3.
- Cevikbas F, Wang X, Akiyama T, et al. A sensory neuron– expressed IL-31 receptor mediates T helper cell–dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448-460.
- Lou H, Lu J, Choi EB, et al. Expression of IL-22 in the skin causes Th2-biased immunity, epidermal barrier dysfunction, and pruritus via stimulating epithelial Th2 cytokines and the GRP pathway. J Immunol. 2017;198:2543-2555.
- Sutaria N, Adawi W, Goldberg R, et al. Itch: pathogenesis and treatment. J Am Acad Dermatol. 2022;86:17-34.
- Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84:747-760.
- Kyvayko R, Fachler-Sharp T, Greenberger S, et al. Characterization of paediatric prurigo nodularis: a multicentre retrospective, observational study. Acta Derm Venereol. 2024;104:adv15771.
- Aggarwal P, Choi J, Sutaria N, et al. Clinical characteristics and disease burden in prurigo nodularis. Clin Exp Dermatol. 2021;46:1277-1284.
- Whang KA, Le TK, Khanna R, et al. Health-related quality of life and economic burden of prurigo nodularis. J Am Acad Dermatol. 2022;86:573-580.
- Jørgensen KM, Egeberg A, Gislason GH, et al. Anxiety, depression and suicide in patients with prurigo nodularis. J Eur Acad Dermatol Venereol. 2017;31:E106-E107.
- Rodriguez D, Kwatra SG, Dias-Barbosa C, et al. Patient perspectives on living with severe prurigo nodularis. JAMA Dermatol. 2023;159:1205-1212.
- Misery L, Patras de Campaigno C, Taieb C, et al. Impact of chronic prurigo nodularis on daily life and stigmatization. J Eur Acad Dermatol Venereol. 2023;37:E908-E909.
- Huang AH, Canner JK, Khanna R, et al. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Invest Dermatol. 2020;140:480-483.e4.
- Sutaria N, Adawi W, Brown I, et al. Racial disparities in mortality among patients with prurigo nodularis: a multicenter cohort study. J Am Acad Dermatol. 2022;82:487- 490.
- Boozalis E, Tang O, Patel S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol. 2018;79:714-719.e3.
- Müller S, Zeidler C, Ständer S. Chronic prurigo including prurigo nodularis: new insights and treatments. Am J Clin Dermatol. 2024;25:15-33.
- Williams KA, Roh YS, Brown I, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14:67-77.
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589.
- Beck KM, Yang EJ, Sekhon S, et al. Dupilumab treatment for generalized prurigo nodularis. JAMA Dermatol. 2019;155:118-120.
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double- blind, placebo- controlled phase 3 trials. Nat Med. 2023;29:1180-1190.
- Wongvibulsin S, Sutaria N, Williams KA, et al. A nationwide study of prurigo nodularis: disease burden and healthcare utilization in the United States. J Invest Dermatol. 2021;141:2530-2533.e1.
- Sutaria N, Alphonse MP, Marani M, et al. Cluster analysis of circulating plasma biomarkers in prurigo nodularis reveals a distinct systemic inflammatory signature in African Americans. J Invest Dermatol. 2022;142:1300-1308.e3.
Break the Itch-Scratch Cycle to Treat Prurigo Nodularis
Break the Itch-Scratch Cycle to Treat Prurigo Nodularis
Improving Interprofessional Neurology Training Using Tele-Education
Improving Interprofessional Neurology Training Using Tele-Education
Neurologic disorders are major causes of death and disability. Globally, the burden of neurologic disorders continues to increase. The prevalence of disabling neurologic disorders significantly increases with age. As people live longer, health care systems will face increasing demands for treatment, rehabilitation, and support services for neurologic disorders. The scarcity of established modifiable risks for most of the neurologic burden demonstrates how new knowledge is required to develop effective prevention and treatment strategies.1
A single-center study for chronic headache at a rural institution found that, when combined with public education, clinician education not only can increase access to care but also reduce specialist overuse, hospitalizations, polypharmacy, and emergency department visits.2 A predicted shortage of neurologists has sparked increased interest in the field and individual neurology educators are helping fuel its popularity.3-5
TELE-EDUCATION
Educating the next generation of health professionals is 1 of 4 statutory missions of the US Department of Veterans Affairs (VA).6 Tele-education (also known as telelearning and distance learning) deviates from traditional in-person classroom settings, in which the lecture has been a core pedagogic method.7 Audio, video, and online technologies provide health education and can overcome geographic barriers for rural and remote clinicians.8 Recent technological improvements have allowed for inexpensive and efficient dissemination of educational materials, including video lectures, podcasts, online modules, assessment materials, and even entire curricula.9
There has been an increase in the awareness of the parallel curriculum involving self-directed and asynchronous learning opportunities. 10 Several studies report knowledge gained via tele-education is comparable to conventional classroom learning.11-13 A systematic review of e-learning perceptions among health care students suggested benefits (eg, learning flexibility, pedagogical design, online interactions, basic computer skills, and access to technology) and drawbacks (eg, limited acquisition of clinical skills, internet connection problems, and issues with using educational platforms).1
The COVID-19 pandemic forced an abrupt cessation of traditional in-person education, forcing educational institutions and medical organizations to transition to telelearning. Solutions in the education field appeared during the pandemic, such as videoconferencing, social media, and telemedicine, that effectively addressed the sudden cessation of in-person medical education.15
Graduate medical education in neurology residency programs served as an experimental set up for tele-education during the pandemic. Residents from neurology training programs outlined the benefits of a volunteer lecturer-based online didactic program that was established to meet this need, which included exposure to subspeciality topics, access to subspecialist experts not available within the department, exposure to different pedagogic methods, interaction with members of other educational institutions and training programs, career development opportunities, and the potential for forming a community of learning.16
Not all recent educational developments are technology-based. For example, instruction focused on specific patient experiences, and learning processes that emphasize problem solving and personal responsibility over specific knowledge have been successful in neurology.17,18 Departments and institutions must be creative in finding ways to fund continuing education, especially when budgets are limited.19
ANNUAL NEUROLOGY SEMINAR
An annual Veterans Health Administration (VHA) neurology seminar began in 2019 as a 1-day in-person event. Neurologists at the Michael E. DeBakey VA Medical Center in Houston presented in 50-minute sessions. Nonspecialist clinical personnel and neurology clinicians attended the event. Attendees requested making the presentations widely available and regularly repeating the seminar.
The second neurology seminar took place during the COVID-19 pandemic. It was conducted online and advertised across the Veterans Integrated Services Network (VISN) 16. The 1-day program had 204 participants who were primarily nurses (59%) and physicians (21%); 94% agreed with the program objectives (Table 1). Participants could earn CME credits for the 7 presentations primarily by VHA experts.
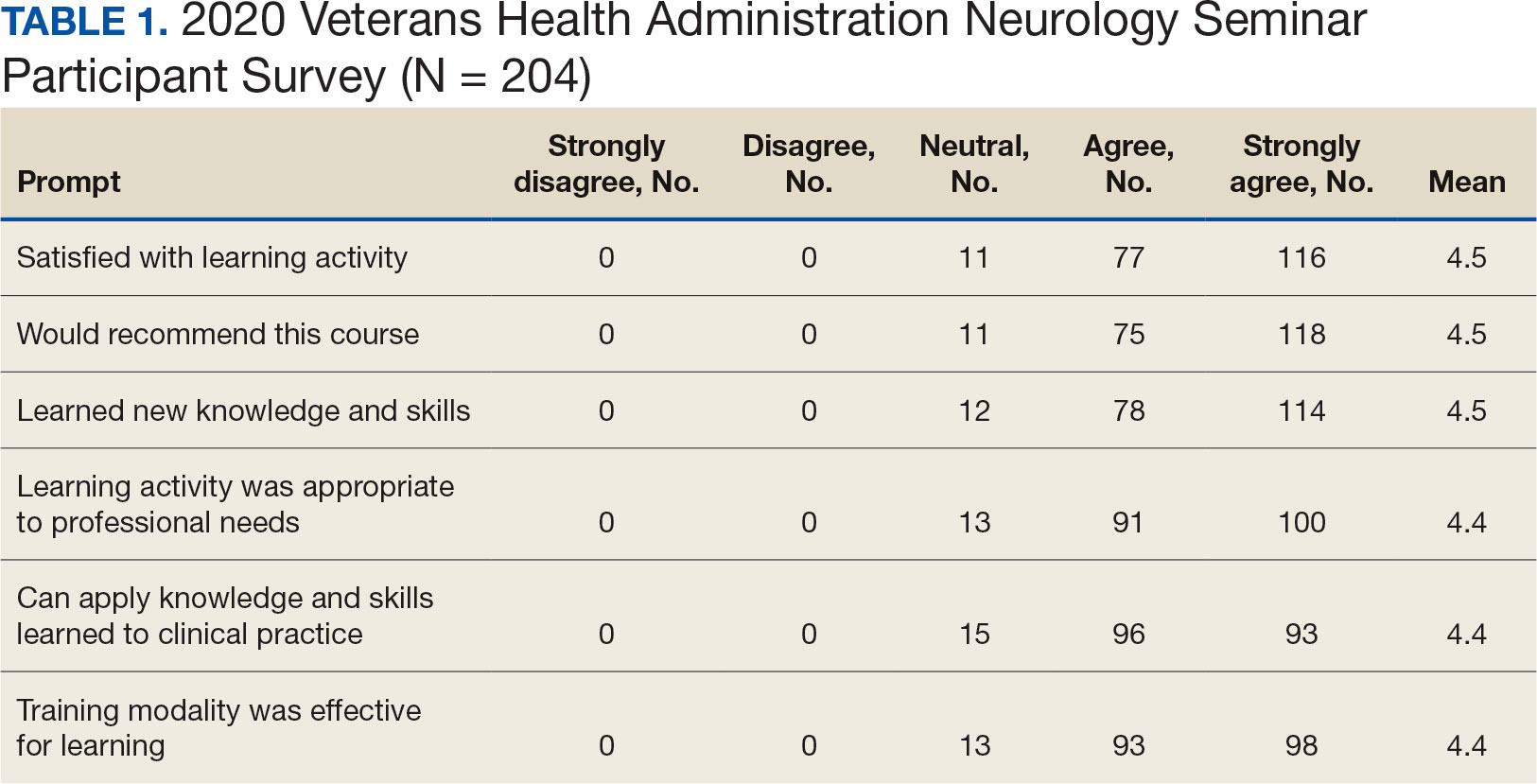
Based on feedback and a needs assessment, the program expanded in 2021 and 2022. With support from the national VHA neurology office and VHA Employee Education System (EES), the Institute for Learning, Education, and Development (ILEAD), the feedback identified topics that resonate with VHA clinicians. Neurological disorders in the fields of stroke, dementia, and headache were included since veterans with these disorders regularly visit primary care, geriatrics, mental health, and other clinical offices. Updates provided in the diagnosis and treatment of common neurological disorders were well received. Almost all speakers were VHA clinicians, which allowed them to focus on topics relevant to clinical practice at the VHA.
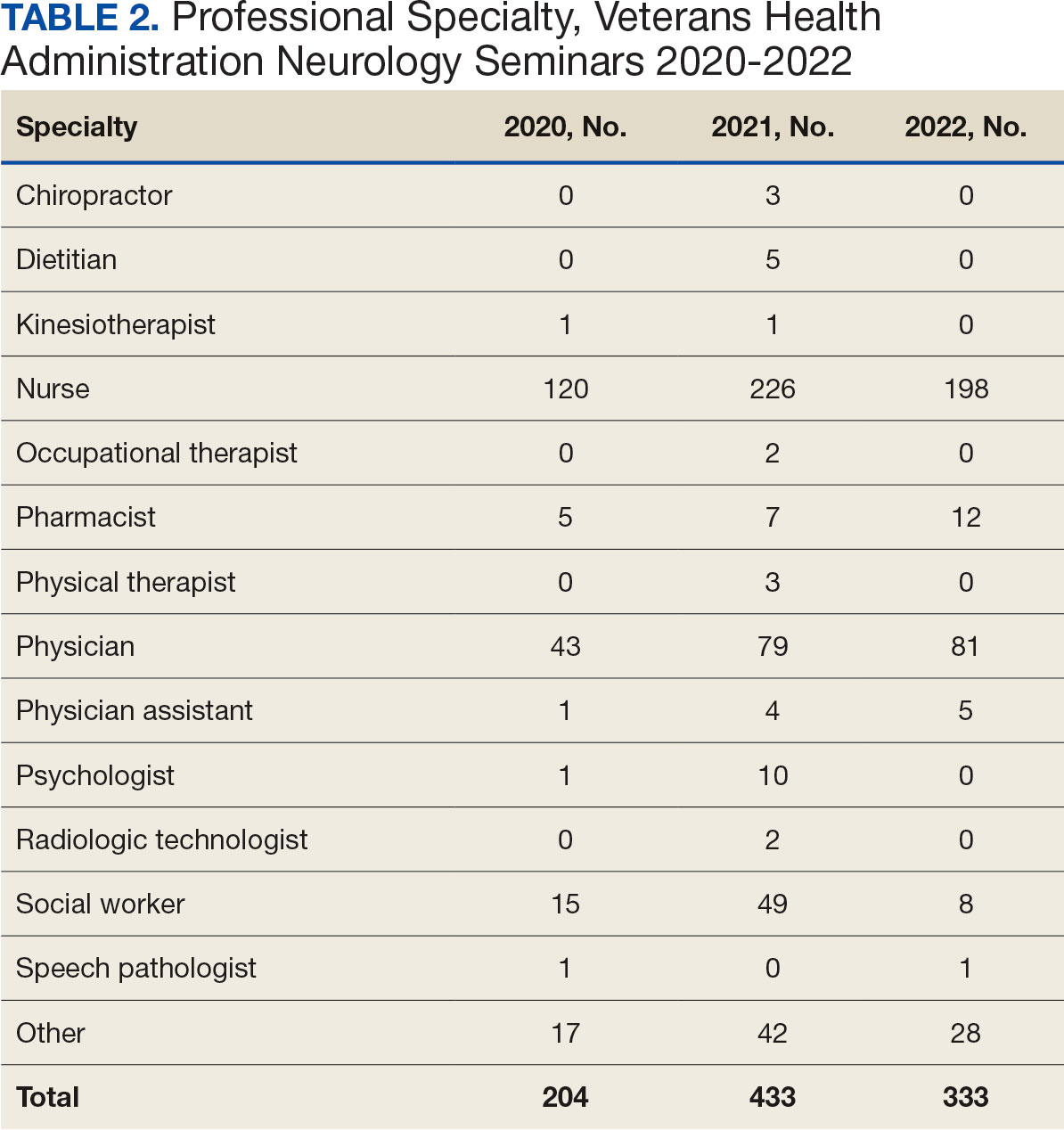
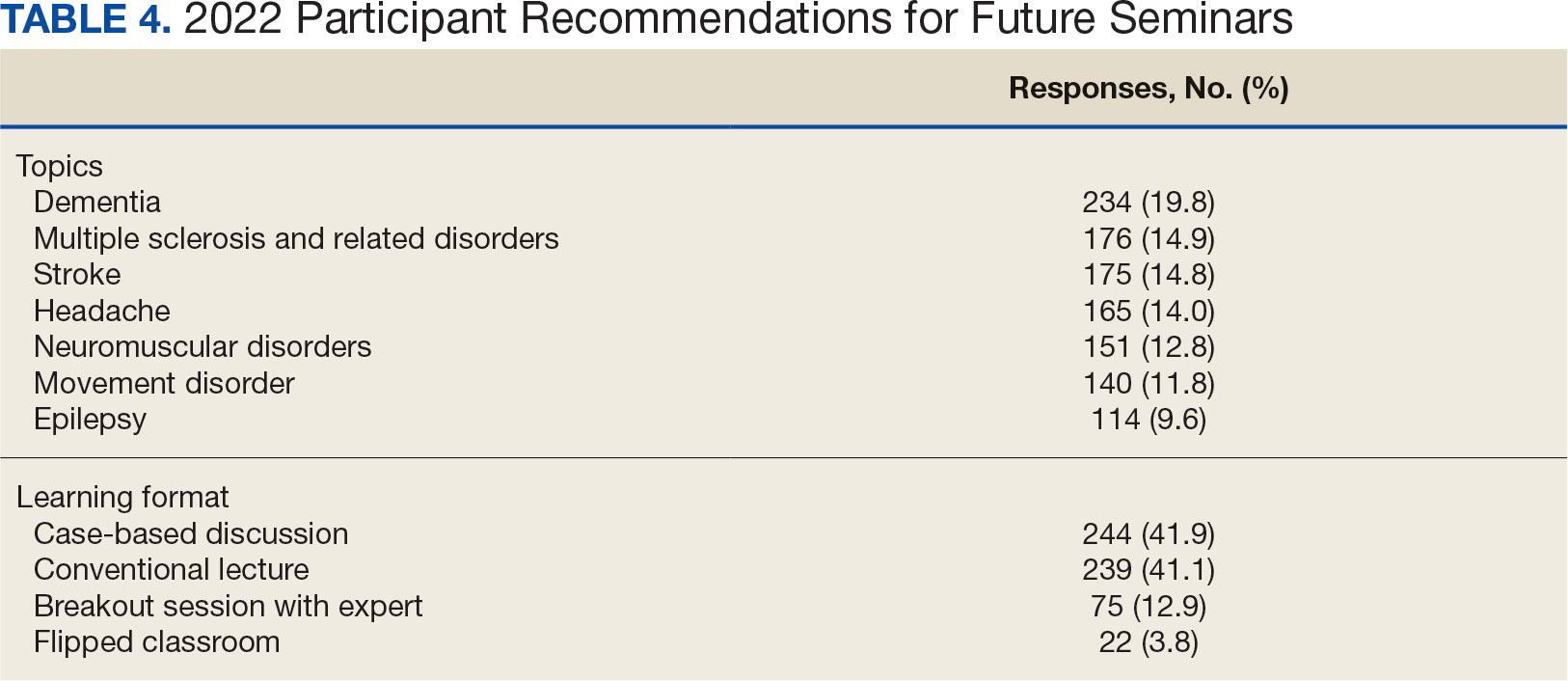
Attendance has increased annually. In 2021, 550 clinicians registered (52% nurses) and 433 completed the postseminar survey (Table 2). In 2022, 635 participants registered and 342 completed evaluations, including attendees from other federal agencies who were invited to participate via EES TRAIN (Training Finder Real-time Affiliate Integrated Network). Forty-seven participants from other federal agencies, including the US Department of Defense, National Institute of Health, and Centers for Disease Control and Prevention, completed the feedback evaluation via TRAIN (Table 3). Participants report high levels of satisfaction each year (mean of 4.5 on a 5-point scale). Respondents preferred conventional lecture presentation and case-based discussions for the teaching format and dementia was the most requested topic for future seminars (Table 4).

The content of each seminar was designed to include . 1 topic relevant to current clinical practice. The 2020 seminar covered topics of cerebrovascular complications of COVID- 19 and living well with neurodegenerative disease in the COVID-19 era. In 2021, the seminar included COVID-19 and neurologic manifestations. In 2022, topics included trends in stroke rehabilitation. In addition, ≥ 1 session addressed neurologic issues within the VHA. In 2020, the VA Deputy National Director of Neurology presented on the VHA stroke systems of care. In 2021, there was a presentation on traumatic brain injury (TBI) in the military. In 2022, sessions covered long term neurologic consequences of TBI and use of telemedicine for neurologic disorders. Feedback on the sessions were positive (eAppendix, available at doi:10.12788/fp.0545).

At the request of the participants, individual presentations were shared via email by the course director and speakers. In collaboration with the EES, each session was recorded and the 2022 seminar was made available to registrants in TMS and EES TRAIN and via the VHA Neurology SharePoint.
DISCUSSION
The annual VHA neurology seminar is a 1-day neurology conference that provides education to general neurologists and other clinicians caring for patients with neurologic disorders. It is the first of its kind neurology education program in the VHA covering most subspecialties in neurology and aims at improving neurologic patient care and access through education. Sessions have covered stroke, epilepsy, sleep, amyotrophic lateral sclerosis, neuropathy, dementia, movement disorders and Parkinson disease, headaches, multiple sclerosis, neurorehabilitation, and telehealth.
The seminar has transitioned from an inperson meeting to a virtual format, making neurology education more convenient and accessible. The virtual format provides the means to increase educational collaborations and share lecture platforms with other federal agencies. The program offers CME credits at no cost to government employees. Recorded lectures can also be asynchronously viewed from the Neurology SharePoint without the ability to earn CME credits. These recordings may be used to educate trainees as well.
The seminar aims to educate all health care professionals caring for patients with neurologic disorders. It aims to eliminate neurophobia, the fear of neural sciences and clinical neurology, and help general practitioners, especially in rural areas, take care of patients with neurologic disorders. The seminars introduce general practitioners to VHA neurology experts; the epilepsy, headache multiple sclerosis, and Parkinson disease centers of excellence; and the national programs for telestroke and teleneurology.
Education Support in the VHA
The EES/ILEAD provides a wide variety of learning opportunities to VHA employees on a broad range of topics, making it one of the largest medical education programs in the country. Pharmacists, social workers, psychologists, therapists, nurses, physician assistants, and physicians have access to certified training opportunities to gain knowledge and skills needed to provide high-quality, veteran-centered care.
A review of geriatrics learning activities through the EES found > 15,000 lectures from 1999 to 2009 for > 300,000 attendees.20 To our knowledge, a review of neurology-related learning activities offered by the EES/ILEAD has not been completed, but the study on geriatrics shows that a similar review would be feasible, given the integrated education system, and helpful in identifying what topics are covered, formats are used, and participants are engaged in neurology education at the VHA. This is a future project planned by the neurology education workgroup.
The EES/ILEAD arranged CME credit for the VHA Neurology Seminar and assisted in organizing an online event with > 500 attendees. Technology support and tools provided by EES during the virtual seminar, such as polling and chat features, kept the audience engaged. Other specialties may similarly value a virtual, all-day seminar format that is efficient and can encourage increased participation from practitioners, nurses, and clinicians.
Future Growth
We plan to increase future participation in the annual neurology seminar with primary care, geriatrics, neurology, and other specialties by instituting an improved and earlier marketing strategy. This includes working with the VHA neurology office to inform neurology practitioners as well as other program offices in the VHA. We intend to host the seminar the same day every year to make it easy for attendees to plan accordingly. In the future we may consider hybrid in-person and virtual modalities if feasible. We plan to focus on reaching out to other government agencies through platforms like TRAIN and the American Academy of Neurology government sections. Securing funding, administrative staff, and protected time in the future may help expand the program further.
Limitations
While a virtual format offers several advantages, using it removes the feel of an in-person meeting, which could be viewed by some attendees as a limitation. The other challenges and drawbacks of transitioning to the virtual platform for a national meeting are similar to those reported in the literature: time zone differences, internet issues, and participants having difficulty using certain online platforms. Attendance could also be limited by scheduling conflicts.16 Despite a large audience attending the seminar, many clinicians do not get protected time from their institutions. Institutional and leadership support at national and local levels will likely improve participation and help participants earn CME credits. While we are still doing a preliminary needs assessment, a formal needs assessment across federal governmental organizations will be helpful.
CONCLUSIONS
The annual VHA neurology seminar promotes interprofessional education, introduces neurology subspecialty centers of excellence, improves access to renowned neurology experts, and provides neurology-related updates through a VHA lens. The program not only provides educational updates to neurology clinicians, but also increases the confidence of non-neurology clinicians called to care for veterans with neurological disorders in their respective clinics.
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990- 2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459-480. doi:10.1016/S1474-4422(18)30499-X
- Baker V, Hack N. Improving access to care for patients with migraine in a remote Pacific population. Neurol Clin Pract. 2020;10(5):444-448. doi:10.1212/CPJ.0000000000000774
- Gutmann L, Cahill C, Jordan JT, et al. Characteristics of graduating US allopathic medical students pursuing a career in neurology. Neurology. 2019;92(17):e2051-e2063. doi:10.1212/WNL.0000000000007369
- Jordan JT, Cahill C, Ostendorf T, et al. Attracting neurology’s next generation: a qualitative study of specialty choice and perceptions. Neurology. 2020;95(8):e1080- e1090. doi:10.1212/WNL.0000000000009461
- Minen MT, Kaplan K, Akter S, et al. Understanding how to strengthen the neurology pipeline with insights from undergraduate neuroscience students. Neurology 2022;98(8):314-323. doi:10.1212/WNL.0000000000013259
- US Department of Veterans Affairs, Office of Academic Affiliations. To Educate for VA and the Nation. Updated August 1, 2024. Accessed August 15, 2024. https://www.va.gov/oaa/
- Schaefer SM, Dominguez M, Moeller JJ. The future of the lecture in neurology education. Semin Neurol. 2018;38(4):418-427. doi:10.1055/s-0038-1667042
- Curran VR. Tele-education. J Telemed Telecare. 2006;12(2):57-63. doi:10.1258/135763306776084400
- Lau KHV, Lakhan SE, Achike F. New media, technology and neurology education. Semin Neurol. 2018;38(4):457- 464. doi:10.1055/s-0038-1666985
- Quirk M, Chumley H. The adaptive medical curriculum: a model for continuous improvement. Med Teach. 2018;40(8):786-790. doi:10.1080/0142159X.2018.1484896
- Brockfeld T, Müller B, de Laffolie J. Video versus live lecture courses: a comparative evaluation of lecture types and results. Med Educ Online. 2018;23(1):1555434. doi:10.1080/10872981.2018.1555434
- Davis J, Crabb S, Rogers E, Zamora J, Khan K. Computer-based teaching is as good as face to face lecture-based teaching of evidence based medicine: a randomized controlled trial. Med Teach. 2008;30(3):302-307. doi:10.1080/01421590701784349
- Markova T, Roth LM, Monsur J. Synchronous distance learning as an effective and feasible method for delivering residency didactics. Fam Med. 2005;37(8):570-575.
- Naciri A, Radid M, Kharbach A, Chemsi G. E-learning in health professions education during the COVID-19 pandemic: a systematic review. J Educ Eval Health Prof. 2021;18:27. doi:10.3352/jeehp.2021.18.27
- Dedeilia A, Sotiropoulos MG, Hanrahan JG, Janga D, Dedeilias P, Sideris M. Medical and surgical education challenges and innovations in the COVID-19 era: a systematic review. In Vivo. 2020;34(3 Suppl):1603-1611. doi:10.21873/invivo.11950
- Weber DJ, Albert DVF, Aravamuthan BR, Bernson-Leung ME, Bhatti D, Milligan TA. Training in neurology: rapid implementation of cross-institutional neurology resident education in the time of COVID-19. Neurology. 2020;95(19):883-886. doi:10.1212/WNL.0000000000010753
- Frey J, Neeley B, Umer A, et al. Training in neurology: neuro day: an innovative curriculum connecting medical students with patients. Neurology. 2021;96(10):e1482- e1486. doi:10.1212/WNL.0000000000010859
- Schwartzstein RM, Dienstag JL, King RW, et al. The Harvard Medical School Pathways Curriculum: reimagining developmentally appropriate medical education for contemporary learners. Acad Med. 2020;95(11):1687-1695. doi:10.1097/ACM.0000000000003270
- Greer DM, Moeller J, Torres DR, et al. Funding the educational mission in neurology. Neurology. 2021;96(12):574- 582. doi:10.1212/WNL.0000000000011635
- Thielke S, Tumosa N, Lindenfeld R, Shay K. Geriatric focused educational offerings in the Department of Veterans Affairs from 1999 to 2009. Gerontol Geriatr Educ. 2011;32(1):38-53. doi:10.1080/02701960.2011.550214
Neurologic disorders are major causes of death and disability. Globally, the burden of neurologic disorders continues to increase. The prevalence of disabling neurologic disorders significantly increases with age. As people live longer, health care systems will face increasing demands for treatment, rehabilitation, and support services for neurologic disorders. The scarcity of established modifiable risks for most of the neurologic burden demonstrates how new knowledge is required to develop effective prevention and treatment strategies.1
A single-center study for chronic headache at a rural institution found that, when combined with public education, clinician education not only can increase access to care but also reduce specialist overuse, hospitalizations, polypharmacy, and emergency department visits.2 A predicted shortage of neurologists has sparked increased interest in the field and individual neurology educators are helping fuel its popularity.3-5
TELE-EDUCATION
Educating the next generation of health professionals is 1 of 4 statutory missions of the US Department of Veterans Affairs (VA).6 Tele-education (also known as telelearning and distance learning) deviates from traditional in-person classroom settings, in which the lecture has been a core pedagogic method.7 Audio, video, and online technologies provide health education and can overcome geographic barriers for rural and remote clinicians.8 Recent technological improvements have allowed for inexpensive and efficient dissemination of educational materials, including video lectures, podcasts, online modules, assessment materials, and even entire curricula.9
There has been an increase in the awareness of the parallel curriculum involving self-directed and asynchronous learning opportunities. 10 Several studies report knowledge gained via tele-education is comparable to conventional classroom learning.11-13 A systematic review of e-learning perceptions among health care students suggested benefits (eg, learning flexibility, pedagogical design, online interactions, basic computer skills, and access to technology) and drawbacks (eg, limited acquisition of clinical skills, internet connection problems, and issues with using educational platforms).1
The COVID-19 pandemic forced an abrupt cessation of traditional in-person education, forcing educational institutions and medical organizations to transition to telelearning. Solutions in the education field appeared during the pandemic, such as videoconferencing, social media, and telemedicine, that effectively addressed the sudden cessation of in-person medical education.15
Graduate medical education in neurology residency programs served as an experimental set up for tele-education during the pandemic. Residents from neurology training programs outlined the benefits of a volunteer lecturer-based online didactic program that was established to meet this need, which included exposure to subspeciality topics, access to subspecialist experts not available within the department, exposure to different pedagogic methods, interaction with members of other educational institutions and training programs, career development opportunities, and the potential for forming a community of learning.16
Not all recent educational developments are technology-based. For example, instruction focused on specific patient experiences, and learning processes that emphasize problem solving and personal responsibility over specific knowledge have been successful in neurology.17,18 Departments and institutions must be creative in finding ways to fund continuing education, especially when budgets are limited.19
ANNUAL NEUROLOGY SEMINAR
An annual Veterans Health Administration (VHA) neurology seminar began in 2019 as a 1-day in-person event. Neurologists at the Michael E. DeBakey VA Medical Center in Houston presented in 50-minute sessions. Nonspecialist clinical personnel and neurology clinicians attended the event. Attendees requested making the presentations widely available and regularly repeating the seminar.
The second neurology seminar took place during the COVID-19 pandemic. It was conducted online and advertised across the Veterans Integrated Services Network (VISN) 16. The 1-day program had 204 participants who were primarily nurses (59%) and physicians (21%); 94% agreed with the program objectives (Table 1). Participants could earn CME credits for the 7 presentations primarily by VHA experts.

Based on feedback and a needs assessment, the program expanded in 2021 and 2022. With support from the national VHA neurology office and VHA Employee Education System (EES), the Institute for Learning, Education, and Development (ILEAD), the feedback identified topics that resonate with VHA clinicians. Neurological disorders in the fields of stroke, dementia, and headache were included since veterans with these disorders regularly visit primary care, geriatrics, mental health, and other clinical offices. Updates provided in the diagnosis and treatment of common neurological disorders were well received. Almost all speakers were VHA clinicians, which allowed them to focus on topics relevant to clinical practice at the VHA.
Attendance has increased annually. In 2021, 550 clinicians registered (52% nurses) and 433 completed the postseminar survey (Table 2). In 2022, 635 participants registered and 342 completed evaluations, including attendees from other federal agencies who were invited to participate via EES TRAIN (Training Finder Real-time Affiliate Integrated Network). Forty-seven participants from other federal agencies, including the US Department of Defense, National Institute of Health, and Centers for Disease Control and Prevention, completed the feedback evaluation via TRAIN (Table 3). Participants report high levels of satisfaction each year (mean of 4.5 on a 5-point scale). Respondents preferred conventional lecture presentation and case-based discussions for the teaching format and dementia was the most requested topic for future seminars (Table 4).



The content of each seminar was designed to include . 1 topic relevant to current clinical practice. The 2020 seminar covered topics of cerebrovascular complications of COVID- 19 and living well with neurodegenerative disease in the COVID-19 era. In 2021, the seminar included COVID-19 and neurologic manifestations. In 2022, topics included trends in stroke rehabilitation. In addition, ≥ 1 session addressed neurologic issues within the VHA. In 2020, the VA Deputy National Director of Neurology presented on the VHA stroke systems of care. In 2021, there was a presentation on traumatic brain injury (TBI) in the military. In 2022, sessions covered long term neurologic consequences of TBI and use of telemedicine for neurologic disorders. Feedback on the sessions were positive (eAppendix, available at doi:10.12788/fp.0545).

At the request of the participants, individual presentations were shared via email by the course director and speakers. In collaboration with the EES, each session was recorded and the 2022 seminar was made available to registrants in TMS and EES TRAIN and via the VHA Neurology SharePoint.
DISCUSSION
The annual VHA neurology seminar is a 1-day neurology conference that provides education to general neurologists and other clinicians caring for patients with neurologic disorders. It is the first of its kind neurology education program in the VHA covering most subspecialties in neurology and aims at improving neurologic patient care and access through education. Sessions have covered stroke, epilepsy, sleep, amyotrophic lateral sclerosis, neuropathy, dementia, movement disorders and Parkinson disease, headaches, multiple sclerosis, neurorehabilitation, and telehealth.
The seminar has transitioned from an inperson meeting to a virtual format, making neurology education more convenient and accessible. The virtual format provides the means to increase educational collaborations and share lecture platforms with other federal agencies. The program offers CME credits at no cost to government employees. Recorded lectures can also be asynchronously viewed from the Neurology SharePoint without the ability to earn CME credits. These recordings may be used to educate trainees as well.
The seminar aims to educate all health care professionals caring for patients with neurologic disorders. It aims to eliminate neurophobia, the fear of neural sciences and clinical neurology, and help general practitioners, especially in rural areas, take care of patients with neurologic disorders. The seminars introduce general practitioners to VHA neurology experts; the epilepsy, headache multiple sclerosis, and Parkinson disease centers of excellence; and the national programs for telestroke and teleneurology.
Education Support in the VHA
The EES/ILEAD provides a wide variety of learning opportunities to VHA employees on a broad range of topics, making it one of the largest medical education programs in the country. Pharmacists, social workers, psychologists, therapists, nurses, physician assistants, and physicians have access to certified training opportunities to gain knowledge and skills needed to provide high-quality, veteran-centered care.
A review of geriatrics learning activities through the EES found > 15,000 lectures from 1999 to 2009 for > 300,000 attendees.20 To our knowledge, a review of neurology-related learning activities offered by the EES/ILEAD has not been completed, but the study on geriatrics shows that a similar review would be feasible, given the integrated education system, and helpful in identifying what topics are covered, formats are used, and participants are engaged in neurology education at the VHA. This is a future project planned by the neurology education workgroup.
The EES/ILEAD arranged CME credit for the VHA Neurology Seminar and assisted in organizing an online event with > 500 attendees. Technology support and tools provided by EES during the virtual seminar, such as polling and chat features, kept the audience engaged. Other specialties may similarly value a virtual, all-day seminar format that is efficient and can encourage increased participation from practitioners, nurses, and clinicians.
Future Growth
We plan to increase future participation in the annual neurology seminar with primary care, geriatrics, neurology, and other specialties by instituting an improved and earlier marketing strategy. This includes working with the VHA neurology office to inform neurology practitioners as well as other program offices in the VHA. We intend to host the seminar the same day every year to make it easy for attendees to plan accordingly. In the future we may consider hybrid in-person and virtual modalities if feasible. We plan to focus on reaching out to other government agencies through platforms like TRAIN and the American Academy of Neurology government sections. Securing funding, administrative staff, and protected time in the future may help expand the program further.
Limitations
While a virtual format offers several advantages, using it removes the feel of an in-person meeting, which could be viewed by some attendees as a limitation. The other challenges and drawbacks of transitioning to the virtual platform for a national meeting are similar to those reported in the literature: time zone differences, internet issues, and participants having difficulty using certain online platforms. Attendance could also be limited by scheduling conflicts.16 Despite a large audience attending the seminar, many clinicians do not get protected time from their institutions. Institutional and leadership support at national and local levels will likely improve participation and help participants earn CME credits. While we are still doing a preliminary needs assessment, a formal needs assessment across federal governmental organizations will be helpful.
CONCLUSIONS
The annual VHA neurology seminar promotes interprofessional education, introduces neurology subspecialty centers of excellence, improves access to renowned neurology experts, and provides neurology-related updates through a VHA lens. The program not only provides educational updates to neurology clinicians, but also increases the confidence of non-neurology clinicians called to care for veterans with neurological disorders in their respective clinics.
Neurologic disorders are major causes of death and disability. Globally, the burden of neurologic disorders continues to increase. The prevalence of disabling neurologic disorders significantly increases with age. As people live longer, health care systems will face increasing demands for treatment, rehabilitation, and support services for neurologic disorders. The scarcity of established modifiable risks for most of the neurologic burden demonstrates how new knowledge is required to develop effective prevention and treatment strategies.1
A single-center study for chronic headache at a rural institution found that, when combined with public education, clinician education not only can increase access to care but also reduce specialist overuse, hospitalizations, polypharmacy, and emergency department visits.2 A predicted shortage of neurologists has sparked increased interest in the field and individual neurology educators are helping fuel its popularity.3-5
TELE-EDUCATION
Educating the next generation of health professionals is 1 of 4 statutory missions of the US Department of Veterans Affairs (VA).6 Tele-education (also known as telelearning and distance learning) deviates from traditional in-person classroom settings, in which the lecture has been a core pedagogic method.7 Audio, video, and online technologies provide health education and can overcome geographic barriers for rural and remote clinicians.8 Recent technological improvements have allowed for inexpensive and efficient dissemination of educational materials, including video lectures, podcasts, online modules, assessment materials, and even entire curricula.9
There has been an increase in the awareness of the parallel curriculum involving self-directed and asynchronous learning opportunities. 10 Several studies report knowledge gained via tele-education is comparable to conventional classroom learning.11-13 A systematic review of e-learning perceptions among health care students suggested benefits (eg, learning flexibility, pedagogical design, online interactions, basic computer skills, and access to technology) and drawbacks (eg, limited acquisition of clinical skills, internet connection problems, and issues with using educational platforms).1
The COVID-19 pandemic forced an abrupt cessation of traditional in-person education, forcing educational institutions and medical organizations to transition to telelearning. Solutions in the education field appeared during the pandemic, such as videoconferencing, social media, and telemedicine, that effectively addressed the sudden cessation of in-person medical education.15
Graduate medical education in neurology residency programs served as an experimental set up for tele-education during the pandemic. Residents from neurology training programs outlined the benefits of a volunteer lecturer-based online didactic program that was established to meet this need, which included exposure to subspeciality topics, access to subspecialist experts not available within the department, exposure to different pedagogic methods, interaction with members of other educational institutions and training programs, career development opportunities, and the potential for forming a community of learning.16
Not all recent educational developments are technology-based. For example, instruction focused on specific patient experiences, and learning processes that emphasize problem solving and personal responsibility over specific knowledge have been successful in neurology.17,18 Departments and institutions must be creative in finding ways to fund continuing education, especially when budgets are limited.19
ANNUAL NEUROLOGY SEMINAR
An annual Veterans Health Administration (VHA) neurology seminar began in 2019 as a 1-day in-person event. Neurologists at the Michael E. DeBakey VA Medical Center in Houston presented in 50-minute sessions. Nonspecialist clinical personnel and neurology clinicians attended the event. Attendees requested making the presentations widely available and regularly repeating the seminar.
The second neurology seminar took place during the COVID-19 pandemic. It was conducted online and advertised across the Veterans Integrated Services Network (VISN) 16. The 1-day program had 204 participants who were primarily nurses (59%) and physicians (21%); 94% agreed with the program objectives (Table 1). Participants could earn CME credits for the 7 presentations primarily by VHA experts.

Based on feedback and a needs assessment, the program expanded in 2021 and 2022. With support from the national VHA neurology office and VHA Employee Education System (EES), the Institute for Learning, Education, and Development (ILEAD), the feedback identified topics that resonate with VHA clinicians. Neurological disorders in the fields of stroke, dementia, and headache were included since veterans with these disorders regularly visit primary care, geriatrics, mental health, and other clinical offices. Updates provided in the diagnosis and treatment of common neurological disorders were well received. Almost all speakers were VHA clinicians, which allowed them to focus on topics relevant to clinical practice at the VHA.
Attendance has increased annually. In 2021, 550 clinicians registered (52% nurses) and 433 completed the postseminar survey (Table 2). In 2022, 635 participants registered and 342 completed evaluations, including attendees from other federal agencies who were invited to participate via EES TRAIN (Training Finder Real-time Affiliate Integrated Network). Forty-seven participants from other federal agencies, including the US Department of Defense, National Institute of Health, and Centers for Disease Control and Prevention, completed the feedback evaluation via TRAIN (Table 3). Participants report high levels of satisfaction each year (mean of 4.5 on a 5-point scale). Respondents preferred conventional lecture presentation and case-based discussions for the teaching format and dementia was the most requested topic for future seminars (Table 4).



The content of each seminar was designed to include . 1 topic relevant to current clinical practice. The 2020 seminar covered topics of cerebrovascular complications of COVID- 19 and living well with neurodegenerative disease in the COVID-19 era. In 2021, the seminar included COVID-19 and neurologic manifestations. In 2022, topics included trends in stroke rehabilitation. In addition, ≥ 1 session addressed neurologic issues within the VHA. In 2020, the VA Deputy National Director of Neurology presented on the VHA stroke systems of care. In 2021, there was a presentation on traumatic brain injury (TBI) in the military. In 2022, sessions covered long term neurologic consequences of TBI and use of telemedicine for neurologic disorders. Feedback on the sessions were positive (eAppendix, available at doi:10.12788/fp.0545).

At the request of the participants, individual presentations were shared via email by the course director and speakers. In collaboration with the EES, each session was recorded and the 2022 seminar was made available to registrants in TMS and EES TRAIN and via the VHA Neurology SharePoint.
DISCUSSION
The annual VHA neurology seminar is a 1-day neurology conference that provides education to general neurologists and other clinicians caring for patients with neurologic disorders. It is the first of its kind neurology education program in the VHA covering most subspecialties in neurology and aims at improving neurologic patient care and access through education. Sessions have covered stroke, epilepsy, sleep, amyotrophic lateral sclerosis, neuropathy, dementia, movement disorders and Parkinson disease, headaches, multiple sclerosis, neurorehabilitation, and telehealth.
The seminar has transitioned from an inperson meeting to a virtual format, making neurology education more convenient and accessible. The virtual format provides the means to increase educational collaborations and share lecture platforms with other federal agencies. The program offers CME credits at no cost to government employees. Recorded lectures can also be asynchronously viewed from the Neurology SharePoint without the ability to earn CME credits. These recordings may be used to educate trainees as well.
The seminar aims to educate all health care professionals caring for patients with neurologic disorders. It aims to eliminate neurophobia, the fear of neural sciences and clinical neurology, and help general practitioners, especially in rural areas, take care of patients with neurologic disorders. The seminars introduce general practitioners to VHA neurology experts; the epilepsy, headache multiple sclerosis, and Parkinson disease centers of excellence; and the national programs for telestroke and teleneurology.
Education Support in the VHA
The EES/ILEAD provides a wide variety of learning opportunities to VHA employees on a broad range of topics, making it one of the largest medical education programs in the country. Pharmacists, social workers, psychologists, therapists, nurses, physician assistants, and physicians have access to certified training opportunities to gain knowledge and skills needed to provide high-quality, veteran-centered care.
A review of geriatrics learning activities through the EES found > 15,000 lectures from 1999 to 2009 for > 300,000 attendees.20 To our knowledge, a review of neurology-related learning activities offered by the EES/ILEAD has not been completed, but the study on geriatrics shows that a similar review would be feasible, given the integrated education system, and helpful in identifying what topics are covered, formats are used, and participants are engaged in neurology education at the VHA. This is a future project planned by the neurology education workgroup.
The EES/ILEAD arranged CME credit for the VHA Neurology Seminar and assisted in organizing an online event with > 500 attendees. Technology support and tools provided by EES during the virtual seminar, such as polling and chat features, kept the audience engaged. Other specialties may similarly value a virtual, all-day seminar format that is efficient and can encourage increased participation from practitioners, nurses, and clinicians.
Future Growth
We plan to increase future participation in the annual neurology seminar with primary care, geriatrics, neurology, and other specialties by instituting an improved and earlier marketing strategy. This includes working with the VHA neurology office to inform neurology practitioners as well as other program offices in the VHA. We intend to host the seminar the same day every year to make it easy for attendees to plan accordingly. In the future we may consider hybrid in-person and virtual modalities if feasible. We plan to focus on reaching out to other government agencies through platforms like TRAIN and the American Academy of Neurology government sections. Securing funding, administrative staff, and protected time in the future may help expand the program further.
Limitations
While a virtual format offers several advantages, using it removes the feel of an in-person meeting, which could be viewed by some attendees as a limitation. The other challenges and drawbacks of transitioning to the virtual platform for a national meeting are similar to those reported in the literature: time zone differences, internet issues, and participants having difficulty using certain online platforms. Attendance could also be limited by scheduling conflicts.16 Despite a large audience attending the seminar, many clinicians do not get protected time from their institutions. Institutional and leadership support at national and local levels will likely improve participation and help participants earn CME credits. While we are still doing a preliminary needs assessment, a formal needs assessment across federal governmental organizations will be helpful.
CONCLUSIONS
The annual VHA neurology seminar promotes interprofessional education, introduces neurology subspecialty centers of excellence, improves access to renowned neurology experts, and provides neurology-related updates through a VHA lens. The program not only provides educational updates to neurology clinicians, but also increases the confidence of non-neurology clinicians called to care for veterans with neurological disorders in their respective clinics.
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990- 2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459-480. doi:10.1016/S1474-4422(18)30499-X
- Baker V, Hack N. Improving access to care for patients with migraine in a remote Pacific population. Neurol Clin Pract. 2020;10(5):444-448. doi:10.1212/CPJ.0000000000000774
- Gutmann L, Cahill C, Jordan JT, et al. Characteristics of graduating US allopathic medical students pursuing a career in neurology. Neurology. 2019;92(17):e2051-e2063. doi:10.1212/WNL.0000000000007369
- Jordan JT, Cahill C, Ostendorf T, et al. Attracting neurology’s next generation: a qualitative study of specialty choice and perceptions. Neurology. 2020;95(8):e1080- e1090. doi:10.1212/WNL.0000000000009461
- Minen MT, Kaplan K, Akter S, et al. Understanding how to strengthen the neurology pipeline with insights from undergraduate neuroscience students. Neurology 2022;98(8):314-323. doi:10.1212/WNL.0000000000013259
- US Department of Veterans Affairs, Office of Academic Affiliations. To Educate for VA and the Nation. Updated August 1, 2024. Accessed August 15, 2024. https://www.va.gov/oaa/
- Schaefer SM, Dominguez M, Moeller JJ. The future of the lecture in neurology education. Semin Neurol. 2018;38(4):418-427. doi:10.1055/s-0038-1667042
- Curran VR. Tele-education. J Telemed Telecare. 2006;12(2):57-63. doi:10.1258/135763306776084400
- Lau KHV, Lakhan SE, Achike F. New media, technology and neurology education. Semin Neurol. 2018;38(4):457- 464. doi:10.1055/s-0038-1666985
- Quirk M, Chumley H. The adaptive medical curriculum: a model for continuous improvement. Med Teach. 2018;40(8):786-790. doi:10.1080/0142159X.2018.1484896
- Brockfeld T, Müller B, de Laffolie J. Video versus live lecture courses: a comparative evaluation of lecture types and results. Med Educ Online. 2018;23(1):1555434. doi:10.1080/10872981.2018.1555434
- Davis J, Crabb S, Rogers E, Zamora J, Khan K. Computer-based teaching is as good as face to face lecture-based teaching of evidence based medicine: a randomized controlled trial. Med Teach. 2008;30(3):302-307. doi:10.1080/01421590701784349
- Markova T, Roth LM, Monsur J. Synchronous distance learning as an effective and feasible method for delivering residency didactics. Fam Med. 2005;37(8):570-575.
- Naciri A, Radid M, Kharbach A, Chemsi G. E-learning in health professions education during the COVID-19 pandemic: a systematic review. J Educ Eval Health Prof. 2021;18:27. doi:10.3352/jeehp.2021.18.27
- Dedeilia A, Sotiropoulos MG, Hanrahan JG, Janga D, Dedeilias P, Sideris M. Medical and surgical education challenges and innovations in the COVID-19 era: a systematic review. In Vivo. 2020;34(3 Suppl):1603-1611. doi:10.21873/invivo.11950
- Weber DJ, Albert DVF, Aravamuthan BR, Bernson-Leung ME, Bhatti D, Milligan TA. Training in neurology: rapid implementation of cross-institutional neurology resident education in the time of COVID-19. Neurology. 2020;95(19):883-886. doi:10.1212/WNL.0000000000010753
- Frey J, Neeley B, Umer A, et al. Training in neurology: neuro day: an innovative curriculum connecting medical students with patients. Neurology. 2021;96(10):e1482- e1486. doi:10.1212/WNL.0000000000010859
- Schwartzstein RM, Dienstag JL, King RW, et al. The Harvard Medical School Pathways Curriculum: reimagining developmentally appropriate medical education for contemporary learners. Acad Med. 2020;95(11):1687-1695. doi:10.1097/ACM.0000000000003270
- Greer DM, Moeller J, Torres DR, et al. Funding the educational mission in neurology. Neurology. 2021;96(12):574- 582. doi:10.1212/WNL.0000000000011635
- Thielke S, Tumosa N, Lindenfeld R, Shay K. Geriatric focused educational offerings in the Department of Veterans Affairs from 1999 to 2009. Gerontol Geriatr Educ. 2011;32(1):38-53. doi:10.1080/02701960.2011.550214
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990- 2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459-480. doi:10.1016/S1474-4422(18)30499-X
- Baker V, Hack N. Improving access to care for patients with migraine in a remote Pacific population. Neurol Clin Pract. 2020;10(5):444-448. doi:10.1212/CPJ.0000000000000774
- Gutmann L, Cahill C, Jordan JT, et al. Characteristics of graduating US allopathic medical students pursuing a career in neurology. Neurology. 2019;92(17):e2051-e2063. doi:10.1212/WNL.0000000000007369
- Jordan JT, Cahill C, Ostendorf T, et al. Attracting neurology’s next generation: a qualitative study of specialty choice and perceptions. Neurology. 2020;95(8):e1080- e1090. doi:10.1212/WNL.0000000000009461
- Minen MT, Kaplan K, Akter S, et al. Understanding how to strengthen the neurology pipeline with insights from undergraduate neuroscience students. Neurology 2022;98(8):314-323. doi:10.1212/WNL.0000000000013259
- US Department of Veterans Affairs, Office of Academic Affiliations. To Educate for VA and the Nation. Updated August 1, 2024. Accessed August 15, 2024. https://www.va.gov/oaa/
- Schaefer SM, Dominguez M, Moeller JJ. The future of the lecture in neurology education. Semin Neurol. 2018;38(4):418-427. doi:10.1055/s-0038-1667042
- Curran VR. Tele-education. J Telemed Telecare. 2006;12(2):57-63. doi:10.1258/135763306776084400
- Lau KHV, Lakhan SE, Achike F. New media, technology and neurology education. Semin Neurol. 2018;38(4):457- 464. doi:10.1055/s-0038-1666985
- Quirk M, Chumley H. The adaptive medical curriculum: a model for continuous improvement. Med Teach. 2018;40(8):786-790. doi:10.1080/0142159X.2018.1484896
- Brockfeld T, Müller B, de Laffolie J. Video versus live lecture courses: a comparative evaluation of lecture types and results. Med Educ Online. 2018;23(1):1555434. doi:10.1080/10872981.2018.1555434
- Davis J, Crabb S, Rogers E, Zamora J, Khan K. Computer-based teaching is as good as face to face lecture-based teaching of evidence based medicine: a randomized controlled trial. Med Teach. 2008;30(3):302-307. doi:10.1080/01421590701784349
- Markova T, Roth LM, Monsur J. Synchronous distance learning as an effective and feasible method for delivering residency didactics. Fam Med. 2005;37(8):570-575.
- Naciri A, Radid M, Kharbach A, Chemsi G. E-learning in health professions education during the COVID-19 pandemic: a systematic review. J Educ Eval Health Prof. 2021;18:27. doi:10.3352/jeehp.2021.18.27
- Dedeilia A, Sotiropoulos MG, Hanrahan JG, Janga D, Dedeilias P, Sideris M. Medical and surgical education challenges and innovations in the COVID-19 era: a systematic review. In Vivo. 2020;34(3 Suppl):1603-1611. doi:10.21873/invivo.11950
- Weber DJ, Albert DVF, Aravamuthan BR, Bernson-Leung ME, Bhatti D, Milligan TA. Training in neurology: rapid implementation of cross-institutional neurology resident education in the time of COVID-19. Neurology. 2020;95(19):883-886. doi:10.1212/WNL.0000000000010753
- Frey J, Neeley B, Umer A, et al. Training in neurology: neuro day: an innovative curriculum connecting medical students with patients. Neurology. 2021;96(10):e1482- e1486. doi:10.1212/WNL.0000000000010859
- Schwartzstein RM, Dienstag JL, King RW, et al. The Harvard Medical School Pathways Curriculum: reimagining developmentally appropriate medical education for contemporary learners. Acad Med. 2020;95(11):1687-1695. doi:10.1097/ACM.0000000000003270
- Greer DM, Moeller J, Torres DR, et al. Funding the educational mission in neurology. Neurology. 2021;96(12):574- 582. doi:10.1212/WNL.0000000000011635
- Thielke S, Tumosa N, Lindenfeld R, Shay K. Geriatric focused educational offerings in the Department of Veterans Affairs from 1999 to 2009. Gerontol Geriatr Educ. 2011;32(1):38-53. doi:10.1080/02701960.2011.550214
Improving Interprofessional Neurology Training Using Tele-Education
Improving Interprofessional Neurology Training Using Tele-Education
Development of an Integrative Medicine Rotation for Family Medicine and Preventive Medicine Residency
Development of an Integrative Medicine Rotation for Family Medicine and Preventive Medicine Residency
Integrative medicine or complementary alternative medicine (IM/CAM) is increasingly being recognized as an integral part of optimal health and healing. IM/CAM “reaffirms the importance of the relationship between practitioner and patient, focuses on the whole person, is informed by evidence, and makes use of all appropriate therapeutic approaches, healthcare professionals and disciplines.”1 IM/CAM encompasses a wide range of therapies, conceptual frameworks, and health care-related professions, such as acupuncture, massage, dietary supplements, mindfulness, yoga, meditation and guided imagery.1 Research has found that 30% to 98% of patients with chronic conditions seek IM/CAM therapies.1-3
Despite the high prevalence of patients utilizing IM/CAM therapies and the National Institutes of Health grants for IM/CAM education, implementation of IM/CAM instruction in graduate medical education programs remains inconsistent.1 Barriers cited by programs include a lack of IM/CAM experts in the program, faculty training, competing financial resources, and an already full resident education schedule.4 As a result, many physicians have limited or no training in IM/CAM.1,5
The US Department of Veterans Affairs (VA) offers IM/CAM health programs to veterans and caregivers as part of its whole health care initiative.6 Several VA health care systems have adopted whole health and IM/CAM through programs for mental health integration into primary care; women’s health; integrative pain care; geriatrics, through adoption of Age-Friendly Health Systems standards; and nutrition and physical activity.7-13 The VA provides training to more medical students than any other health system: > 95% of US medical schools are affiliated with a VA medical center (VAMC).14 As part of the training mission, VA seeks to encourage students of diverse professions to consider careers in the VA.14
Residency is a time for newly licensed physicians to acquire additional experience and training to translate knowledge and skills acquired during medical school directly to patient care.15 However, residency curricula have limited time to incorporate IM/CAM training. Residency training is also physically and psychosocially demanding, often resulting in inadequate self-care, poor work-life balance, and disrupted sleep.16-18 Resident wellness is at a historic low, resulting in high rates of burnout during training.4,15
Residency programs are required to provide wellness education; however, most programs include minimal content.19 Despite high rates of burnout, formal curricula on the topic have not been established. 20 IM/CAM education also can provide a path for residents to learn about and engage in mindfulness-based training or cognitive stress reduction for self-care.
INTEGRATIVE WHOLE HEALTH ROTATION
In 2017, the Baltimore Geriatric Research Education and Clinical Center (GRECC) established an IM/whole health residency rotation and created a structured curriculum incorporating self-assessment, active reflection, and self-care to complement training in specific IM/CAM modalities for residents in family medicine. The curriculum evaluated how this training improved residents’ perceptions of IM/CAM and how it personally and professionally impacted the practice of self-care as a strategy to decrease burnout. We hypothesized that this structured experience would increase IM/CAM knowledge among clinicians while promoting the importance and practice of self-care to reduce burnout.
The 2-week IM/CAM curriculum was developed by University of Maryland School of Medicine faculty in partnership with the Baltimore GRECC and staff at the VA Maryland Health Care System. The curriculum was designed to expose residents to the 8 components of the whole health Circle of Health (moving the body; surroundings; personal development; food and drink; recharge; family, friends, and coworkers; spirit and soul; and power of the mind) in addition to IM/ CAM modalities the VA is mandated to offer to veterans (acupuncture, chiropractic, meditation, massage therapy, biofeedback, clinical hypnosis, guided imagery, yoga, and tai chi).21 Twelve residents (1 preventive medicine and 11 third-year family medicine residents) rotated individually throughout the year as part of their behavioral health block rotation. All residents completed the 2-week curriculum as their schedules allowed. The curriculum consisted of didactics sessions and activities at the Baltimore, Loch Raven, and Perry Point VAMCs. Residents completed evaluations before and after the rotation. The experience described in this article by the residents and the survey data were collected from the 2018/2019 training year. A rotation syllabus, competencies adapted from Locke and colleagues and skills residents obtain during this rotation that support these competencies, as well as a resident sample schedule were developed (eAppendix is available at doi:10.12788/fp.0544).1

Rotation Overview
for each resident were built around instructional opportunities, which included 1-on-1 didactics, direct observation of treatment modalities, and personal reflection of the residents’ self-care practices. While each resident’s rotation schedule varied slightly due to their schedules, the foundational instruction elements were the same. Didactic session themes included an overview of IM/CAM, nutrition, narrative medicine, pain psychology, music therapy, chaplain services, motor-cognitive training, and exercise guidelines. Assigned readings, including peer-reviewed literature on IM/CAM therapies, complemented all sessions. Residents created an evidence-supported integrative treatment plan for a patient with a condition of interest to them.
Residents observed clinician-led veteran group sessions on IM/CAM treatment modalities, including guided meditation, mindfulness and relaxation, self-awareness, living well with chronic pain, tai chi, drumming for health and balance, anger management, recovery group, acceptance and commitment therapy, and Gerofit exercise. The group classes allowed residents to actively participate in the activity or discussion. Residents also shadowed VA clinicians in sleep, pain, nutrition, acupuncture, and mental health clinics.
Residents were encouraged to practice self-care during the 2-week rotation. The rotation schedule built in free time, including a 1-hour daily lunch period, for residents to consider their own health habits, complete a personal health inventory, and try self-care activities outlined on the syllabus with links to resources. These resources also served as educational materials that residents could share with patients. All materials, including didactic lectures, journal articles and self-care resources, were provided to each resident through a free online course to ensure residents had access throughout and following completion of the rotation. This content, including the rotation evaluation metrics, is available upon request from the corresponding author.
Evaluations
Residents completed a survey before and after the rotation to measure IM/CAM knowledge and application and self-care/ burnout perceptions. Residents were asked to evaluate rotation sessions and comment on whether this rotation benefited them personally and professionally (Table 1). Descriptive statistics were analyzed using Microsoft Excel. Given the small sample size and lack of statistical power, only mean survey results are reported in this article. Because this opportunity is specific to the University of Maryland School of Medicine and the proposed project was part of ordinary educational practice, the study was deemed not human subject research by the University of Maryland Institutional Review Board (HP-00089256).

Perceptions and attitudes toward IM/CAM were assessed using a survey designed by the University of Minnesota Academic Health Center. It included 18 items scored on a 5-point semantic rating scale (1, strongly disagree; 5, strongly agree).22 Residents rated their level of agreement with statements reflecting both positive (eg, clinical care should integrate the best of conventional and CAM practices) and negative (eg, CAM is a threat to public health) views. Three questions adapted from the NHIS Adult Complementary Health Questionnaire and UC Irvine Survey of Health Care Use and Practice assessed the use of IM/CAM resources.23,24
Resident knowledge and application of IM/CAM were measured using a case study designed by the course faculty. The case listed a chief complaint of nerve pain, with a history of chronic pain, neuropathic pain, anxiety, chronic fatigue, depression, insomnia, posttraumatic stress disorder, history of present illness, past surgical history, medication list, review of symptoms, laboratory values, and physical examination. The residents completed an assessment before and after the rotation. Residents rated their confidence in the diagnosis and treatment of 8 medical conditions using a 5-point semantic rating scale (Table 2). Self-care importance and selfcare frequency were measured by a variety of means, including 3 survey questions, the Five Facet Mindfulness Questionnaire, 2 prompts on a 7-point semantic scale, and a slightly modified version of the validated Perceived Stress Scale.25-28
Survey Results
Residents gave the rotation positive feedback with a mean score of 8.5 out of 10. They reported the beneficial impact of seeing the nontraditional and nonpharmacological practices in treating patients, chronic pain management team approaches, and enjoyed being able to participate in group classes with patients. Many residents expressed a desire for a longer rotation to have more time to experience the behavioral health-focused sessions. Residents also requested additional information on nutritional supplements/natural medicines, battlefield acupuncture training and osteopathic manipulative therapy practices. All residents reported the rotation personally and/or professionally benefited them (Appendix).
Given the sample of 12 residents, values are presented as prerotation to postrotation comparisons without statistical analysis. There was a trend towards an increase in the reported use and recommendation of 26 modalities of nonconventional therapies following the rotation. There was also a slight increase in resource knowledge and use of these resources, and residents reported accessing more types of resources. Mean scores of the case study to gauge knowledge and application of IM increased from 7.5 at baseline to 11.0 after the rotation. Resident confidence in diagnosis increased for all 8 conditions, but confidence in treatment only increased for 4 conditions.
Results of self-care importance, self-care frequency and mindfulness were consistent baseline to postrotation. The mean time residents spent regularly practicing self-care during a work week increased slightly while feelings of burnout decreased. The perceived stress scale average score decreased from 13.4 at baseline to 10.5 after rotation.
DISCUSSION
The implementation of an IM residency rotation that incorporates whole health and interprofessional practices demonstrated improved perception and increased use of IM/CAM resources and knowledge among a small sample of third-year residents. Residents reported they had a positive experience participating in the rotation and gained knowledge, resources, and skills they felt confident discussing with their patients.
Many studies reported favorable attitudes and perceptions of IM/CAM use among physicians, but few have assessed these measures while implementing a training curriculum.3,4,22 Gardiner and colleagues reported on the perception and use of IM resources among family medicine residents.4 The study found that while 58% of all residents reported IM/CAM as an important part of their training, only 60% reported they received it or had specific learning objectives in their curriculum. 4 The program outlined in this study and previous research illustrate that physicians recognize the importance of IM/CAM education in training programs, but most were unaware of the resources available or did not feel comfortable counseling patients about most IM/CAM applications.
Residents in this program slightly increased their use of IM/CAM to diagnose and treat medical conditions after the rotation. A study by Wahner-Roedler and colleagues assessed physician knowledge regarding common IM/CAM therapies.3 On average, physicians only felt knowledgeable and comfortable counseling patients for 3 of 13 listed treatments/techniques and few natural herbal treatments. The study also found that most physicians had difficulty accessing IM/CAM information at their institution despite having free access to electronic databases. However, this study only assessed physician attitudes of IM/CAM and did not include an educational component to increase their knowledge of the modalities.3 This evaluation supports the need for interventions like the program described in this article that provide physicians with access to evidence-based resources combined with the applied experiences to increase their comfort within this growing field.
Though the sample size in this study was small, its results support existing research indicating that clinicians view selfcare as important. Many residents were already using a self-care plan at baseline, but there was slight increase in the practice of self-care during the rotation and a slight decrease in burnout. Previous research reflects high rates of burnout and relatively poor quality of life among primary care physicians.15 Burnout is associated with lower quality of care, lower patient satisfaction and contributes to medical errors. Studies suggest as many as 60% of primary care physicians report symptoms of burnout, which negatively affected the quality of patient care they provide.15
Despite the profound effects burnout has on physicians and patient care, a standardized wellness education or self-care tool kit is not currently available. The University of Massachusetts recently introduced a pilot program to promote resident wellness that demonstrated favorable results.15 A meta-analysis of physicians and medical trainees found decreases in anxiety and symptoms of anxiety as well as a decrease in burnout among participants in cognitive, behavioral and mindfulness interventions.29 However, unlike our program, these programs focused solely on the well-being of medical trainees, residents, and physicians and didn’t focus on the patient-clinician interactions. Given the impact on patient care, there is a need to develop and implement additional programs like our residency rotation that promote health and wellness among physicians while also evaluating how physicians may translate these skills to patient education.
While this program st i l l exists for third-year residents at Baltimore GRECC, it has significantly changed since the COVID-19 pandemic. For about the first 6 months of the pandemic, when physical distancing requirements were in place, family medicine trainees were not able to rotate. Upon return to the facility, many group classes were cancelled and some clinicians no longer offered the sessions. The rotation has evolved to a hybrid format, where many group classes for veteran patients are offered virtually, and residents observe a mix of virtual and in-person shadowing opportunities. Our formal evaluation included administering the survey and occurred from July 2018 to July 2019 but wasn’t implemented upon return to post-COVID activities due to the inconsistent experiences offered to residents over the past few years. Future research should evaluate the impact of this hybrid program on the clinicians and explore dissemination to other VAMCs and their academic affiliates.
Limitations
Project recruitment was limited to 11 family medicine and 1 preventive medicine resident. Perceptions, use of IM/CAM, and knowledge about IM/CAM could be considerably different in different departments with varying schedules, hours worked, and patient volumes. Secondly, the survey was conducted 2 weeks apart. Indications of self-care and burnout may not reflect long-term effects, adoption, or maintenance. Future research should include longer follow up to examine how this type of educational activity may impact burnout rates of physicians following the completion of residency, as well as changes in perspectives of IM/CAM while practicing as a physician. Trainees were exposed to a wide range of health care professions, but additional research is needed regarding medical resident perceptions of the roles of specific professions in a collaborative health care team.30,31
CONCLUSIONS
The residency rotation program illustrates the benefits of establishing a standardized IM/CAM rotation that includes self-care resources in family medicine programs to adequately train clinicians to practice wellness and promote it to their patients. The results of this project suggest this type of training will help residents assess the literature to better counsel patients on IM/CAM options while also providing strategies for maintaining optimal health and well-being for health care professionals. Broadening and shifting the scope of medicine from treatment to prevention, personal wellness, and optimal healing should be a top priority.
- Locke AB, Gordon A, Guerrera MP, Gardiner P, Lebensohn P. Recommended integrative medicine competencies for family medicine residents. Explore (NY). 2013;9(5):308-313. doi:10.1016/j.explore.2013.06.005
- Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA. 1998;280(18):1569-1575. doi:10.1001/jama.280.18.1569
- Wahner-Roedler DL, Vincent A, Elkin PL, Loehrer LL, Cha SS, Bauer BA. Physicians’ attitudes toward complementary and alternative medicine and their knowledge of specific therapies: a survey at an academic medical center. Evid Based Complement Alternat Med. 2006;3(4):495-501. doi:10.1093/ecam/nel036
- Gardiner P, Filippelli AC, Lebensohn P, Bonakdar R. Family medicine residency program directors attitudes and knowledge of family medicine CAM competencies. Explore (NY). 2013;9(5):299-307. doi:10.1016/j.explore.2013.06.002
- Sierpina V, Levine R, Astin J, Tan A. Use of mind-body therapies in psychiatry and family medicine faculty and residents: attitudes, barriers, and gender differences. Explore (NY). 2007;3(2):129-135. doi:10.1016/j.explore.2006.12.001
- Krist AH, South-Paul J, Meisnere M, eds. Achieving Whole Health: A New Approach for Veterans and the Nation. The National Academies Press; 2023.
- Bokhour BG, DeFaccio R, Gaj L, et al. Changes in patientreported outcomes associated with receiving whole health in the Veteran Health Administration (VHA)’s National Demonstration Project. J Gen Intern Med. 2024;39(1):84-94. doi:10.1007/s11606-023-08376-0
- Courtney RE, Schadegg MJ, Bolton R, Smith S, Harden SM. Using a whole health approach to build biopsychosocial- spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Gabrielian S, Jones AL, Hoge AE, et al. Enhancing primary care experiences for homeless patients with serious mental illness: results from a national survey. J Prim Care Community Health. 2021;12:2150132721993654. doi:10.1177/2150132721993654
- Matthieu MM, Church KA, Taylor LD, et al. Integrating the age-friendly health systems movement in Veterans Health Administration: national advance care planning via group visits and the 4Ms framework. Health Soc Work. 2023;48(4):277-280. doi:10.1093/hsw/hlad022
- Meisler AW, Gianoli MO, Na PJ, Pietrzak RH. Functional disability in US military veterans: the importance of integrated whole health initiatives. Prim Care Companion CNS Disord. 2023;25(4):22m03461. doi:10.4088/PCC.22m03461
- Ortmeyer HK, Giffuni J, Etchberger D, Katzel L. The role of companion dogs in the VA Maryland Health Care System Whole Health(y) GeroFit Program. Animals (Basel). 2023;13(19):3047. doi:10.3390/ani13193047
- Sullivan MB, Hill K, Ballengee LA, et al. Remotely delivered psychologically informed mindful movement physical therapy for pain care: a framework for operationalization. Glob Adv Integr Med Health. 2023;12:27536130231209751. doi:10.1177/27536130231209751
- (OAA) OoAA. 75th Anniversary: Passion to learn. Power to heal. Washington DC.: US Department of Veterans Affairs; 2021. https://content.yudu.com/web/448fx/0A448g9/75thAnniversary2021/html/index.html?page=24&origin=reader
- Runyan C, Savageau JA, Potts S, Weinreb L. Impact of a family medicine resident wellness curriculum: a feasibility study. Med Educ Online. 2016;21:30648. doi:10.3402/meo.v21.30648
- Lafreniere JP, Rios R, Packer H, Ghazarian S, Wright SM, Levine RB. Burned out at the bedside: patient perceptions of physician burnout in an internal medicine resident continuity clinic. J Gen Intern Med. 2016;31(2):203-208. doi:10.1007/s11606-015-3503-3
- Freedy JR, Staley C, Mims LD, et al. Social, individual, and environmental characteristics of family medicine resident burnout: a CERA study. Fam Med. 2022;54(4):270-276. doi:10.22454/FamMed.2022.526799
- Alrishan MA, Alshammari SA. Prevalence of sleep deprivation and its effect on the performance of family medicine residents in Riyadh, Saudi Arabia. J Family Community Med. 2020;27(2):125-130. doi:10.4103/jfcm.JFCM_9_20
- ACGME. ACGME Program Requirements for Graduate Medical Education in Family Medicine. https://www.acgme.org/globalassets/pfassets/programrequirements/120_familymedicine_2024.pdf
- Nene Y, Tadi P. Resident Burnout. In: StatPearls; 2023.
- Bokhour BG, Haun JN, Hyde J, Charns M, Kligler B. Transforming the veterans affairs to a whole health system of care: time for action and research. Med Care. 2020;58(4):295-300. doi:10.1097/MLF.0000000000001316
- Kreitzer MJ, Mitten D, Harris I, Shandeling J. Attitudes toward CAM among medical, nursing, and pharmacy faculty and students: a comparative analysis. Altern Ther Health Med. 2002;8(6):44-53.
- Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015(79):1-16.
- Nguyen J, Liu MA, Patel RJ, Tahara K, Nguyen AL. Use and interest in complementary and alternative medicine among college students seeking healthcare at a university campus student health center. Complement Ther Clin Pract. 2016;24:103-108. doi:10.1016/j.ctcp.2016.06.001
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13(1):27-45. doi:10.1177/1073191105283504
- Baer RA, Smith GT, Lykins E, et al. Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples. Assessment. 2008;15(3):329-342. doi:10.1177/1073191107313003
- West CP, Dyrbye LN, Sloan JA, Shanafelt TD. Single item measures of emotional exhaustion and depersonalization are useful for assessing burnout in medical professionals. J Gen Intern Med. 2009;24(12):1318- 1321. doi:10.1007/s11606-009-1129-z
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396.
- Regehr C, Glancy D, Pitts A, LeBlanc VR. Interventions to reduce the consequences of stress in physicians: a review and meta-analysis. J Nerv Ment Dis. 2014;202(5):353-359. doi:10.1097/NMD.0000000000000130
- Visser CLF, Ket JCF, Croiset G, Kusurkar RA. Perceptions of residents, medical and nursing students about interprofessional education: a systematic review of the quantitative and qualitative literature. BMC Med Educ. 2017;17(1):77. doi:10.1186/s12909-017-0909-0
- Lingard L, Espin S, Evans C, Hawryluck L. The rules of the game: interprofessional collaboration on the intensive care unit team. Crit Care. 2004;8(6):R403-408. doi:10.1186/cc2958
Integrative medicine or complementary alternative medicine (IM/CAM) is increasingly being recognized as an integral part of optimal health and healing. IM/CAM “reaffirms the importance of the relationship between practitioner and patient, focuses on the whole person, is informed by evidence, and makes use of all appropriate therapeutic approaches, healthcare professionals and disciplines.”1 IM/CAM encompasses a wide range of therapies, conceptual frameworks, and health care-related professions, such as acupuncture, massage, dietary supplements, mindfulness, yoga, meditation and guided imagery.1 Research has found that 30% to 98% of patients with chronic conditions seek IM/CAM therapies.1-3
Despite the high prevalence of patients utilizing IM/CAM therapies and the National Institutes of Health grants for IM/CAM education, implementation of IM/CAM instruction in graduate medical education programs remains inconsistent.1 Barriers cited by programs include a lack of IM/CAM experts in the program, faculty training, competing financial resources, and an already full resident education schedule.4 As a result, many physicians have limited or no training in IM/CAM.1,5
The US Department of Veterans Affairs (VA) offers IM/CAM health programs to veterans and caregivers as part of its whole health care initiative.6 Several VA health care systems have adopted whole health and IM/CAM through programs for mental health integration into primary care; women’s health; integrative pain care; geriatrics, through adoption of Age-Friendly Health Systems standards; and nutrition and physical activity.7-13 The VA provides training to more medical students than any other health system: > 95% of US medical schools are affiliated with a VA medical center (VAMC).14 As part of the training mission, VA seeks to encourage students of diverse professions to consider careers in the VA.14
Residency is a time for newly licensed physicians to acquire additional experience and training to translate knowledge and skills acquired during medical school directly to patient care.15 However, residency curricula have limited time to incorporate IM/CAM training. Residency training is also physically and psychosocially demanding, often resulting in inadequate self-care, poor work-life balance, and disrupted sleep.16-18 Resident wellness is at a historic low, resulting in high rates of burnout during training.4,15
Residency programs are required to provide wellness education; however, most programs include minimal content.19 Despite high rates of burnout, formal curricula on the topic have not been established. 20 IM/CAM education also can provide a path for residents to learn about and engage in mindfulness-based training or cognitive stress reduction for self-care.
INTEGRATIVE WHOLE HEALTH ROTATION
In 2017, the Baltimore Geriatric Research Education and Clinical Center (GRECC) established an IM/whole health residency rotation and created a structured curriculum incorporating self-assessment, active reflection, and self-care to complement training in specific IM/CAM modalities for residents in family medicine. The curriculum evaluated how this training improved residents’ perceptions of IM/CAM and how it personally and professionally impacted the practice of self-care as a strategy to decrease burnout. We hypothesized that this structured experience would increase IM/CAM knowledge among clinicians while promoting the importance and practice of self-care to reduce burnout.
The 2-week IM/CAM curriculum was developed by University of Maryland School of Medicine faculty in partnership with the Baltimore GRECC and staff at the VA Maryland Health Care System. The curriculum was designed to expose residents to the 8 components of the whole health Circle of Health (moving the body; surroundings; personal development; food and drink; recharge; family, friends, and coworkers; spirit and soul; and power of the mind) in addition to IM/ CAM modalities the VA is mandated to offer to veterans (acupuncture, chiropractic, meditation, massage therapy, biofeedback, clinical hypnosis, guided imagery, yoga, and tai chi).21 Twelve residents (1 preventive medicine and 11 third-year family medicine residents) rotated individually throughout the year as part of their behavioral health block rotation. All residents completed the 2-week curriculum as their schedules allowed. The curriculum consisted of didactics sessions and activities at the Baltimore, Loch Raven, and Perry Point VAMCs. Residents completed evaluations before and after the rotation. The experience described in this article by the residents and the survey data were collected from the 2018/2019 training year. A rotation syllabus, competencies adapted from Locke and colleagues and skills residents obtain during this rotation that support these competencies, as well as a resident sample schedule were developed (eAppendix is available at doi:10.12788/fp.0544).1

Rotation Overview
for each resident were built around instructional opportunities, which included 1-on-1 didactics, direct observation of treatment modalities, and personal reflection of the residents’ self-care practices. While each resident’s rotation schedule varied slightly due to their schedules, the foundational instruction elements were the same. Didactic session themes included an overview of IM/CAM, nutrition, narrative medicine, pain psychology, music therapy, chaplain services, motor-cognitive training, and exercise guidelines. Assigned readings, including peer-reviewed literature on IM/CAM therapies, complemented all sessions. Residents created an evidence-supported integrative treatment plan for a patient with a condition of interest to them.
Residents observed clinician-led veteran group sessions on IM/CAM treatment modalities, including guided meditation, mindfulness and relaxation, self-awareness, living well with chronic pain, tai chi, drumming for health and balance, anger management, recovery group, acceptance and commitment therapy, and Gerofit exercise. The group classes allowed residents to actively participate in the activity or discussion. Residents also shadowed VA clinicians in sleep, pain, nutrition, acupuncture, and mental health clinics.
Residents were encouraged to practice self-care during the 2-week rotation. The rotation schedule built in free time, including a 1-hour daily lunch period, for residents to consider their own health habits, complete a personal health inventory, and try self-care activities outlined on the syllabus with links to resources. These resources also served as educational materials that residents could share with patients. All materials, including didactic lectures, journal articles and self-care resources, were provided to each resident through a free online course to ensure residents had access throughout and following completion of the rotation. This content, including the rotation evaluation metrics, is available upon request from the corresponding author.
Evaluations
Residents completed a survey before and after the rotation to measure IM/CAM knowledge and application and self-care/ burnout perceptions. Residents were asked to evaluate rotation sessions and comment on whether this rotation benefited them personally and professionally (Table 1). Descriptive statistics were analyzed using Microsoft Excel. Given the small sample size and lack of statistical power, only mean survey results are reported in this article. Because this opportunity is specific to the University of Maryland School of Medicine and the proposed project was part of ordinary educational practice, the study was deemed not human subject research by the University of Maryland Institutional Review Board (HP-00089256).

Perceptions and attitudes toward IM/CAM were assessed using a survey designed by the University of Minnesota Academic Health Center. It included 18 items scored on a 5-point semantic rating scale (1, strongly disagree; 5, strongly agree).22 Residents rated their level of agreement with statements reflecting both positive (eg, clinical care should integrate the best of conventional and CAM practices) and negative (eg, CAM is a threat to public health) views. Three questions adapted from the NHIS Adult Complementary Health Questionnaire and UC Irvine Survey of Health Care Use and Practice assessed the use of IM/CAM resources.23,24
Resident knowledge and application of IM/CAM were measured using a case study designed by the course faculty. The case listed a chief complaint of nerve pain, with a history of chronic pain, neuropathic pain, anxiety, chronic fatigue, depression, insomnia, posttraumatic stress disorder, history of present illness, past surgical history, medication list, review of symptoms, laboratory values, and physical examination. The residents completed an assessment before and after the rotation. Residents rated their confidence in the diagnosis and treatment of 8 medical conditions using a 5-point semantic rating scale (Table 2). Self-care importance and selfcare frequency were measured by a variety of means, including 3 survey questions, the Five Facet Mindfulness Questionnaire, 2 prompts on a 7-point semantic scale, and a slightly modified version of the validated Perceived Stress Scale.25-28

Survey Results
Residents gave the rotation positive feedback with a mean score of 8.5 out of 10. They reported the beneficial impact of seeing the nontraditional and nonpharmacological practices in treating patients, chronic pain management team approaches, and enjoyed being able to participate in group classes with patients. Many residents expressed a desire for a longer rotation to have more time to experience the behavioral health-focused sessions. Residents also requested additional information on nutritional supplements/natural medicines, battlefield acupuncture training and osteopathic manipulative therapy practices. All residents reported the rotation personally and/or professionally benefited them (Appendix).
Given the sample of 12 residents, values are presented as prerotation to postrotation comparisons without statistical analysis. There was a trend towards an increase in the reported use and recommendation of 26 modalities of nonconventional therapies following the rotation. There was also a slight increase in resource knowledge and use of these resources, and residents reported accessing more types of resources. Mean scores of the case study to gauge knowledge and application of IM increased from 7.5 at baseline to 11.0 after the rotation. Resident confidence in diagnosis increased for all 8 conditions, but confidence in treatment only increased for 4 conditions.
Results of self-care importance, self-care frequency and mindfulness were consistent baseline to postrotation. The mean time residents spent regularly practicing self-care during a work week increased slightly while feelings of burnout decreased. The perceived stress scale average score decreased from 13.4 at baseline to 10.5 after rotation.
DISCUSSION
The implementation of an IM residency rotation that incorporates whole health and interprofessional practices demonstrated improved perception and increased use of IM/CAM resources and knowledge among a small sample of third-year residents. Residents reported they had a positive experience participating in the rotation and gained knowledge, resources, and skills they felt confident discussing with their patients.
Many studies reported favorable attitudes and perceptions of IM/CAM use among physicians, but few have assessed these measures while implementing a training curriculum.3,4,22 Gardiner and colleagues reported on the perception and use of IM resources among family medicine residents.4 The study found that while 58% of all residents reported IM/CAM as an important part of their training, only 60% reported they received it or had specific learning objectives in their curriculum. 4 The program outlined in this study and previous research illustrate that physicians recognize the importance of IM/CAM education in training programs, but most were unaware of the resources available or did not feel comfortable counseling patients about most IM/CAM applications.
Residents in this program slightly increased their use of IM/CAM to diagnose and treat medical conditions after the rotation. A study by Wahner-Roedler and colleagues assessed physician knowledge regarding common IM/CAM therapies.3 On average, physicians only felt knowledgeable and comfortable counseling patients for 3 of 13 listed treatments/techniques and few natural herbal treatments. The study also found that most physicians had difficulty accessing IM/CAM information at their institution despite having free access to electronic databases. However, this study only assessed physician attitudes of IM/CAM and did not include an educational component to increase their knowledge of the modalities.3 This evaluation supports the need for interventions like the program described in this article that provide physicians with access to evidence-based resources combined with the applied experiences to increase their comfort within this growing field.
Though the sample size in this study was small, its results support existing research indicating that clinicians view selfcare as important. Many residents were already using a self-care plan at baseline, but there was slight increase in the practice of self-care during the rotation and a slight decrease in burnout. Previous research reflects high rates of burnout and relatively poor quality of life among primary care physicians.15 Burnout is associated with lower quality of care, lower patient satisfaction and contributes to medical errors. Studies suggest as many as 60% of primary care physicians report symptoms of burnout, which negatively affected the quality of patient care they provide.15
Despite the profound effects burnout has on physicians and patient care, a standardized wellness education or self-care tool kit is not currently available. The University of Massachusetts recently introduced a pilot program to promote resident wellness that demonstrated favorable results.15 A meta-analysis of physicians and medical trainees found decreases in anxiety and symptoms of anxiety as well as a decrease in burnout among participants in cognitive, behavioral and mindfulness interventions.29 However, unlike our program, these programs focused solely on the well-being of medical trainees, residents, and physicians and didn’t focus on the patient-clinician interactions. Given the impact on patient care, there is a need to develop and implement additional programs like our residency rotation that promote health and wellness among physicians while also evaluating how physicians may translate these skills to patient education.
While this program st i l l exists for third-year residents at Baltimore GRECC, it has significantly changed since the COVID-19 pandemic. For about the first 6 months of the pandemic, when physical distancing requirements were in place, family medicine trainees were not able to rotate. Upon return to the facility, many group classes were cancelled and some clinicians no longer offered the sessions. The rotation has evolved to a hybrid format, where many group classes for veteran patients are offered virtually, and residents observe a mix of virtual and in-person shadowing opportunities. Our formal evaluation included administering the survey and occurred from July 2018 to July 2019 but wasn’t implemented upon return to post-COVID activities due to the inconsistent experiences offered to residents over the past few years. Future research should evaluate the impact of this hybrid program on the clinicians and explore dissemination to other VAMCs and their academic affiliates.
Limitations
Project recruitment was limited to 11 family medicine and 1 preventive medicine resident. Perceptions, use of IM/CAM, and knowledge about IM/CAM could be considerably different in different departments with varying schedules, hours worked, and patient volumes. Secondly, the survey was conducted 2 weeks apart. Indications of self-care and burnout may not reflect long-term effects, adoption, or maintenance. Future research should include longer follow up to examine how this type of educational activity may impact burnout rates of physicians following the completion of residency, as well as changes in perspectives of IM/CAM while practicing as a physician. Trainees were exposed to a wide range of health care professions, but additional research is needed regarding medical resident perceptions of the roles of specific professions in a collaborative health care team.30,31
CONCLUSIONS
The residency rotation program illustrates the benefits of establishing a standardized IM/CAM rotation that includes self-care resources in family medicine programs to adequately train clinicians to practice wellness and promote it to their patients. The results of this project suggest this type of training will help residents assess the literature to better counsel patients on IM/CAM options while also providing strategies for maintaining optimal health and well-being for health care professionals. Broadening and shifting the scope of medicine from treatment to prevention, personal wellness, and optimal healing should be a top priority.
Integrative medicine or complementary alternative medicine (IM/CAM) is increasingly being recognized as an integral part of optimal health and healing. IM/CAM “reaffirms the importance of the relationship between practitioner and patient, focuses on the whole person, is informed by evidence, and makes use of all appropriate therapeutic approaches, healthcare professionals and disciplines.”1 IM/CAM encompasses a wide range of therapies, conceptual frameworks, and health care-related professions, such as acupuncture, massage, dietary supplements, mindfulness, yoga, meditation and guided imagery.1 Research has found that 30% to 98% of patients with chronic conditions seek IM/CAM therapies.1-3
Despite the high prevalence of patients utilizing IM/CAM therapies and the National Institutes of Health grants for IM/CAM education, implementation of IM/CAM instruction in graduate medical education programs remains inconsistent.1 Barriers cited by programs include a lack of IM/CAM experts in the program, faculty training, competing financial resources, and an already full resident education schedule.4 As a result, many physicians have limited or no training in IM/CAM.1,5
The US Department of Veterans Affairs (VA) offers IM/CAM health programs to veterans and caregivers as part of its whole health care initiative.6 Several VA health care systems have adopted whole health and IM/CAM through programs for mental health integration into primary care; women’s health; integrative pain care; geriatrics, through adoption of Age-Friendly Health Systems standards; and nutrition and physical activity.7-13 The VA provides training to more medical students than any other health system: > 95% of US medical schools are affiliated with a VA medical center (VAMC).14 As part of the training mission, VA seeks to encourage students of diverse professions to consider careers in the VA.14
Residency is a time for newly licensed physicians to acquire additional experience and training to translate knowledge and skills acquired during medical school directly to patient care.15 However, residency curricula have limited time to incorporate IM/CAM training. Residency training is also physically and psychosocially demanding, often resulting in inadequate self-care, poor work-life balance, and disrupted sleep.16-18 Resident wellness is at a historic low, resulting in high rates of burnout during training.4,15
Residency programs are required to provide wellness education; however, most programs include minimal content.19 Despite high rates of burnout, formal curricula on the topic have not been established. 20 IM/CAM education also can provide a path for residents to learn about and engage in mindfulness-based training or cognitive stress reduction for self-care.
INTEGRATIVE WHOLE HEALTH ROTATION
In 2017, the Baltimore Geriatric Research Education and Clinical Center (GRECC) established an IM/whole health residency rotation and created a structured curriculum incorporating self-assessment, active reflection, and self-care to complement training in specific IM/CAM modalities for residents in family medicine. The curriculum evaluated how this training improved residents’ perceptions of IM/CAM and how it personally and professionally impacted the practice of self-care as a strategy to decrease burnout. We hypothesized that this structured experience would increase IM/CAM knowledge among clinicians while promoting the importance and practice of self-care to reduce burnout.
The 2-week IM/CAM curriculum was developed by University of Maryland School of Medicine faculty in partnership with the Baltimore GRECC and staff at the VA Maryland Health Care System. The curriculum was designed to expose residents to the 8 components of the whole health Circle of Health (moving the body; surroundings; personal development; food and drink; recharge; family, friends, and coworkers; spirit and soul; and power of the mind) in addition to IM/ CAM modalities the VA is mandated to offer to veterans (acupuncture, chiropractic, meditation, massage therapy, biofeedback, clinical hypnosis, guided imagery, yoga, and tai chi).21 Twelve residents (1 preventive medicine and 11 third-year family medicine residents) rotated individually throughout the year as part of their behavioral health block rotation. All residents completed the 2-week curriculum as their schedules allowed. The curriculum consisted of didactics sessions and activities at the Baltimore, Loch Raven, and Perry Point VAMCs. Residents completed evaluations before and after the rotation. The experience described in this article by the residents and the survey data were collected from the 2018/2019 training year. A rotation syllabus, competencies adapted from Locke and colleagues and skills residents obtain during this rotation that support these competencies, as well as a resident sample schedule were developed (eAppendix is available at doi:10.12788/fp.0544).1

Rotation Overview
for each resident were built around instructional opportunities, which included 1-on-1 didactics, direct observation of treatment modalities, and personal reflection of the residents’ self-care practices. While each resident’s rotation schedule varied slightly due to their schedules, the foundational instruction elements were the same. Didactic session themes included an overview of IM/CAM, nutrition, narrative medicine, pain psychology, music therapy, chaplain services, motor-cognitive training, and exercise guidelines. Assigned readings, including peer-reviewed literature on IM/CAM therapies, complemented all sessions. Residents created an evidence-supported integrative treatment plan for a patient with a condition of interest to them.
Residents observed clinician-led veteran group sessions on IM/CAM treatment modalities, including guided meditation, mindfulness and relaxation, self-awareness, living well with chronic pain, tai chi, drumming for health and balance, anger management, recovery group, acceptance and commitment therapy, and Gerofit exercise. The group classes allowed residents to actively participate in the activity or discussion. Residents also shadowed VA clinicians in sleep, pain, nutrition, acupuncture, and mental health clinics.
Residents were encouraged to practice self-care during the 2-week rotation. The rotation schedule built in free time, including a 1-hour daily lunch period, for residents to consider their own health habits, complete a personal health inventory, and try self-care activities outlined on the syllabus with links to resources. These resources also served as educational materials that residents could share with patients. All materials, including didactic lectures, journal articles and self-care resources, were provided to each resident through a free online course to ensure residents had access throughout and following completion of the rotation. This content, including the rotation evaluation metrics, is available upon request from the corresponding author.
Evaluations
Residents completed a survey before and after the rotation to measure IM/CAM knowledge and application and self-care/ burnout perceptions. Residents were asked to evaluate rotation sessions and comment on whether this rotation benefited them personally and professionally (Table 1). Descriptive statistics were analyzed using Microsoft Excel. Given the small sample size and lack of statistical power, only mean survey results are reported in this article. Because this opportunity is specific to the University of Maryland School of Medicine and the proposed project was part of ordinary educational practice, the study was deemed not human subject research by the University of Maryland Institutional Review Board (HP-00089256).

Perceptions and attitudes toward IM/CAM were assessed using a survey designed by the University of Minnesota Academic Health Center. It included 18 items scored on a 5-point semantic rating scale (1, strongly disagree; 5, strongly agree).22 Residents rated their level of agreement with statements reflecting both positive (eg, clinical care should integrate the best of conventional and CAM practices) and negative (eg, CAM is a threat to public health) views. Three questions adapted from the NHIS Adult Complementary Health Questionnaire and UC Irvine Survey of Health Care Use and Practice assessed the use of IM/CAM resources.23,24
Resident knowledge and application of IM/CAM were measured using a case study designed by the course faculty. The case listed a chief complaint of nerve pain, with a history of chronic pain, neuropathic pain, anxiety, chronic fatigue, depression, insomnia, posttraumatic stress disorder, history of present illness, past surgical history, medication list, review of symptoms, laboratory values, and physical examination. The residents completed an assessment before and after the rotation. Residents rated their confidence in the diagnosis and treatment of 8 medical conditions using a 5-point semantic rating scale (Table 2). Self-care importance and selfcare frequency were measured by a variety of means, including 3 survey questions, the Five Facet Mindfulness Questionnaire, 2 prompts on a 7-point semantic scale, and a slightly modified version of the validated Perceived Stress Scale.25-28

Survey Results
Residents gave the rotation positive feedback with a mean score of 8.5 out of 10. They reported the beneficial impact of seeing the nontraditional and nonpharmacological practices in treating patients, chronic pain management team approaches, and enjoyed being able to participate in group classes with patients. Many residents expressed a desire for a longer rotation to have more time to experience the behavioral health-focused sessions. Residents also requested additional information on nutritional supplements/natural medicines, battlefield acupuncture training and osteopathic manipulative therapy practices. All residents reported the rotation personally and/or professionally benefited them (Appendix).
Given the sample of 12 residents, values are presented as prerotation to postrotation comparisons without statistical analysis. There was a trend towards an increase in the reported use and recommendation of 26 modalities of nonconventional therapies following the rotation. There was also a slight increase in resource knowledge and use of these resources, and residents reported accessing more types of resources. Mean scores of the case study to gauge knowledge and application of IM increased from 7.5 at baseline to 11.0 after the rotation. Resident confidence in diagnosis increased for all 8 conditions, but confidence in treatment only increased for 4 conditions.
Results of self-care importance, self-care frequency and mindfulness were consistent baseline to postrotation. The mean time residents spent regularly practicing self-care during a work week increased slightly while feelings of burnout decreased. The perceived stress scale average score decreased from 13.4 at baseline to 10.5 after rotation.
DISCUSSION
The implementation of an IM residency rotation that incorporates whole health and interprofessional practices demonstrated improved perception and increased use of IM/CAM resources and knowledge among a small sample of third-year residents. Residents reported they had a positive experience participating in the rotation and gained knowledge, resources, and skills they felt confident discussing with their patients.
Many studies reported favorable attitudes and perceptions of IM/CAM use among physicians, but few have assessed these measures while implementing a training curriculum.3,4,22 Gardiner and colleagues reported on the perception and use of IM resources among family medicine residents.4 The study found that while 58% of all residents reported IM/CAM as an important part of their training, only 60% reported they received it or had specific learning objectives in their curriculum. 4 The program outlined in this study and previous research illustrate that physicians recognize the importance of IM/CAM education in training programs, but most were unaware of the resources available or did not feel comfortable counseling patients about most IM/CAM applications.
Residents in this program slightly increased their use of IM/CAM to diagnose and treat medical conditions after the rotation. A study by Wahner-Roedler and colleagues assessed physician knowledge regarding common IM/CAM therapies.3 On average, physicians only felt knowledgeable and comfortable counseling patients for 3 of 13 listed treatments/techniques and few natural herbal treatments. The study also found that most physicians had difficulty accessing IM/CAM information at their institution despite having free access to electronic databases. However, this study only assessed physician attitudes of IM/CAM and did not include an educational component to increase their knowledge of the modalities.3 This evaluation supports the need for interventions like the program described in this article that provide physicians with access to evidence-based resources combined with the applied experiences to increase their comfort within this growing field.
Though the sample size in this study was small, its results support existing research indicating that clinicians view selfcare as important. Many residents were already using a self-care plan at baseline, but there was slight increase in the practice of self-care during the rotation and a slight decrease in burnout. Previous research reflects high rates of burnout and relatively poor quality of life among primary care physicians.15 Burnout is associated with lower quality of care, lower patient satisfaction and contributes to medical errors. Studies suggest as many as 60% of primary care physicians report symptoms of burnout, which negatively affected the quality of patient care they provide.15
Despite the profound effects burnout has on physicians and patient care, a standardized wellness education or self-care tool kit is not currently available. The University of Massachusetts recently introduced a pilot program to promote resident wellness that demonstrated favorable results.15 A meta-analysis of physicians and medical trainees found decreases in anxiety and symptoms of anxiety as well as a decrease in burnout among participants in cognitive, behavioral and mindfulness interventions.29 However, unlike our program, these programs focused solely on the well-being of medical trainees, residents, and physicians and didn’t focus on the patient-clinician interactions. Given the impact on patient care, there is a need to develop and implement additional programs like our residency rotation that promote health and wellness among physicians while also evaluating how physicians may translate these skills to patient education.
While this program st i l l exists for third-year residents at Baltimore GRECC, it has significantly changed since the COVID-19 pandemic. For about the first 6 months of the pandemic, when physical distancing requirements were in place, family medicine trainees were not able to rotate. Upon return to the facility, many group classes were cancelled and some clinicians no longer offered the sessions. The rotation has evolved to a hybrid format, where many group classes for veteran patients are offered virtually, and residents observe a mix of virtual and in-person shadowing opportunities. Our formal evaluation included administering the survey and occurred from July 2018 to July 2019 but wasn’t implemented upon return to post-COVID activities due to the inconsistent experiences offered to residents over the past few years. Future research should evaluate the impact of this hybrid program on the clinicians and explore dissemination to other VAMCs and their academic affiliates.
Limitations
Project recruitment was limited to 11 family medicine and 1 preventive medicine resident. Perceptions, use of IM/CAM, and knowledge about IM/CAM could be considerably different in different departments with varying schedules, hours worked, and patient volumes. Secondly, the survey was conducted 2 weeks apart. Indications of self-care and burnout may not reflect long-term effects, adoption, or maintenance. Future research should include longer follow up to examine how this type of educational activity may impact burnout rates of physicians following the completion of residency, as well as changes in perspectives of IM/CAM while practicing as a physician. Trainees were exposed to a wide range of health care professions, but additional research is needed regarding medical resident perceptions of the roles of specific professions in a collaborative health care team.30,31
CONCLUSIONS
The residency rotation program illustrates the benefits of establishing a standardized IM/CAM rotation that includes self-care resources in family medicine programs to adequately train clinicians to practice wellness and promote it to their patients. The results of this project suggest this type of training will help residents assess the literature to better counsel patients on IM/CAM options while also providing strategies for maintaining optimal health and well-being for health care professionals. Broadening and shifting the scope of medicine from treatment to prevention, personal wellness, and optimal healing should be a top priority.
- Locke AB, Gordon A, Guerrera MP, Gardiner P, Lebensohn P. Recommended integrative medicine competencies for family medicine residents. Explore (NY). 2013;9(5):308-313. doi:10.1016/j.explore.2013.06.005
- Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA. 1998;280(18):1569-1575. doi:10.1001/jama.280.18.1569
- Wahner-Roedler DL, Vincent A, Elkin PL, Loehrer LL, Cha SS, Bauer BA. Physicians’ attitudes toward complementary and alternative medicine and their knowledge of specific therapies: a survey at an academic medical center. Evid Based Complement Alternat Med. 2006;3(4):495-501. doi:10.1093/ecam/nel036
- Gardiner P, Filippelli AC, Lebensohn P, Bonakdar R. Family medicine residency program directors attitudes and knowledge of family medicine CAM competencies. Explore (NY). 2013;9(5):299-307. doi:10.1016/j.explore.2013.06.002
- Sierpina V, Levine R, Astin J, Tan A. Use of mind-body therapies in psychiatry and family medicine faculty and residents: attitudes, barriers, and gender differences. Explore (NY). 2007;3(2):129-135. doi:10.1016/j.explore.2006.12.001
- Krist AH, South-Paul J, Meisnere M, eds. Achieving Whole Health: A New Approach for Veterans and the Nation. The National Academies Press; 2023.
- Bokhour BG, DeFaccio R, Gaj L, et al. Changes in patientreported outcomes associated with receiving whole health in the Veteran Health Administration (VHA)’s National Demonstration Project. J Gen Intern Med. 2024;39(1):84-94. doi:10.1007/s11606-023-08376-0
- Courtney RE, Schadegg MJ, Bolton R, Smith S, Harden SM. Using a whole health approach to build biopsychosocial- spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Gabrielian S, Jones AL, Hoge AE, et al. Enhancing primary care experiences for homeless patients with serious mental illness: results from a national survey. J Prim Care Community Health. 2021;12:2150132721993654. doi:10.1177/2150132721993654
- Matthieu MM, Church KA, Taylor LD, et al. Integrating the age-friendly health systems movement in Veterans Health Administration: national advance care planning via group visits and the 4Ms framework. Health Soc Work. 2023;48(4):277-280. doi:10.1093/hsw/hlad022
- Meisler AW, Gianoli MO, Na PJ, Pietrzak RH. Functional disability in US military veterans: the importance of integrated whole health initiatives. Prim Care Companion CNS Disord. 2023;25(4):22m03461. doi:10.4088/PCC.22m03461
- Ortmeyer HK, Giffuni J, Etchberger D, Katzel L. The role of companion dogs in the VA Maryland Health Care System Whole Health(y) GeroFit Program. Animals (Basel). 2023;13(19):3047. doi:10.3390/ani13193047
- Sullivan MB, Hill K, Ballengee LA, et al. Remotely delivered psychologically informed mindful movement physical therapy for pain care: a framework for operationalization. Glob Adv Integr Med Health. 2023;12:27536130231209751. doi:10.1177/27536130231209751
- (OAA) OoAA. 75th Anniversary: Passion to learn. Power to heal. Washington DC.: US Department of Veterans Affairs; 2021. https://content.yudu.com/web/448fx/0A448g9/75thAnniversary2021/html/index.html?page=24&origin=reader
- Runyan C, Savageau JA, Potts S, Weinreb L. Impact of a family medicine resident wellness curriculum: a feasibility study. Med Educ Online. 2016;21:30648. doi:10.3402/meo.v21.30648
- Lafreniere JP, Rios R, Packer H, Ghazarian S, Wright SM, Levine RB. Burned out at the bedside: patient perceptions of physician burnout in an internal medicine resident continuity clinic. J Gen Intern Med. 2016;31(2):203-208. doi:10.1007/s11606-015-3503-3
- Freedy JR, Staley C, Mims LD, et al. Social, individual, and environmental characteristics of family medicine resident burnout: a CERA study. Fam Med. 2022;54(4):270-276. doi:10.22454/FamMed.2022.526799
- Alrishan MA, Alshammari SA. Prevalence of sleep deprivation and its effect on the performance of family medicine residents in Riyadh, Saudi Arabia. J Family Community Med. 2020;27(2):125-130. doi:10.4103/jfcm.JFCM_9_20
- ACGME. ACGME Program Requirements for Graduate Medical Education in Family Medicine. https://www.acgme.org/globalassets/pfassets/programrequirements/120_familymedicine_2024.pdf
- Nene Y, Tadi P. Resident Burnout. In: StatPearls; 2023.
- Bokhour BG, Haun JN, Hyde J, Charns M, Kligler B. Transforming the veterans affairs to a whole health system of care: time for action and research. Med Care. 2020;58(4):295-300. doi:10.1097/MLF.0000000000001316
- Kreitzer MJ, Mitten D, Harris I, Shandeling J. Attitudes toward CAM among medical, nursing, and pharmacy faculty and students: a comparative analysis. Altern Ther Health Med. 2002;8(6):44-53.
- Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015(79):1-16.
- Nguyen J, Liu MA, Patel RJ, Tahara K, Nguyen AL. Use and interest in complementary and alternative medicine among college students seeking healthcare at a university campus student health center. Complement Ther Clin Pract. 2016;24:103-108. doi:10.1016/j.ctcp.2016.06.001
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13(1):27-45. doi:10.1177/1073191105283504
- Baer RA, Smith GT, Lykins E, et al. Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples. Assessment. 2008;15(3):329-342. doi:10.1177/1073191107313003
- West CP, Dyrbye LN, Sloan JA, Shanafelt TD. Single item measures of emotional exhaustion and depersonalization are useful for assessing burnout in medical professionals. J Gen Intern Med. 2009;24(12):1318- 1321. doi:10.1007/s11606-009-1129-z
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396.
- Regehr C, Glancy D, Pitts A, LeBlanc VR. Interventions to reduce the consequences of stress in physicians: a review and meta-analysis. J Nerv Ment Dis. 2014;202(5):353-359. doi:10.1097/NMD.0000000000000130
- Visser CLF, Ket JCF, Croiset G, Kusurkar RA. Perceptions of residents, medical and nursing students about interprofessional education: a systematic review of the quantitative and qualitative literature. BMC Med Educ. 2017;17(1):77. doi:10.1186/s12909-017-0909-0
- Lingard L, Espin S, Evans C, Hawryluck L. The rules of the game: interprofessional collaboration on the intensive care unit team. Crit Care. 2004;8(6):R403-408. doi:10.1186/cc2958
- Locke AB, Gordon A, Guerrera MP, Gardiner P, Lebensohn P. Recommended integrative medicine competencies for family medicine residents. Explore (NY). 2013;9(5):308-313. doi:10.1016/j.explore.2013.06.005
- Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA. 1998;280(18):1569-1575. doi:10.1001/jama.280.18.1569
- Wahner-Roedler DL, Vincent A, Elkin PL, Loehrer LL, Cha SS, Bauer BA. Physicians’ attitudes toward complementary and alternative medicine and their knowledge of specific therapies: a survey at an academic medical center. Evid Based Complement Alternat Med. 2006;3(4):495-501. doi:10.1093/ecam/nel036
- Gardiner P, Filippelli AC, Lebensohn P, Bonakdar R. Family medicine residency program directors attitudes and knowledge of family medicine CAM competencies. Explore (NY). 2013;9(5):299-307. doi:10.1016/j.explore.2013.06.002
- Sierpina V, Levine R, Astin J, Tan A. Use of mind-body therapies in psychiatry and family medicine faculty and residents: attitudes, barriers, and gender differences. Explore (NY). 2007;3(2):129-135. doi:10.1016/j.explore.2006.12.001
- Krist AH, South-Paul J, Meisnere M, eds. Achieving Whole Health: A New Approach for Veterans and the Nation. The National Academies Press; 2023.
- Bokhour BG, DeFaccio R, Gaj L, et al. Changes in patientreported outcomes associated with receiving whole health in the Veteran Health Administration (VHA)’s National Demonstration Project. J Gen Intern Med. 2024;39(1):84-94. doi:10.1007/s11606-023-08376-0
- Courtney RE, Schadegg MJ, Bolton R, Smith S, Harden SM. Using a whole health approach to build biopsychosocial- spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Gabrielian S, Jones AL, Hoge AE, et al. Enhancing primary care experiences for homeless patients with serious mental illness: results from a national survey. J Prim Care Community Health. 2021;12:2150132721993654. doi:10.1177/2150132721993654
- Matthieu MM, Church KA, Taylor LD, et al. Integrating the age-friendly health systems movement in Veterans Health Administration: national advance care planning via group visits and the 4Ms framework. Health Soc Work. 2023;48(4):277-280. doi:10.1093/hsw/hlad022
- Meisler AW, Gianoli MO, Na PJ, Pietrzak RH. Functional disability in US military veterans: the importance of integrated whole health initiatives. Prim Care Companion CNS Disord. 2023;25(4):22m03461. doi:10.4088/PCC.22m03461
- Ortmeyer HK, Giffuni J, Etchberger D, Katzel L. The role of companion dogs in the VA Maryland Health Care System Whole Health(y) GeroFit Program. Animals (Basel). 2023;13(19):3047. doi:10.3390/ani13193047
- Sullivan MB, Hill K, Ballengee LA, et al. Remotely delivered psychologically informed mindful movement physical therapy for pain care: a framework for operationalization. Glob Adv Integr Med Health. 2023;12:27536130231209751. doi:10.1177/27536130231209751
- (OAA) OoAA. 75th Anniversary: Passion to learn. Power to heal. Washington DC.: US Department of Veterans Affairs; 2021. https://content.yudu.com/web/448fx/0A448g9/75thAnniversary2021/html/index.html?page=24&origin=reader
- Runyan C, Savageau JA, Potts S, Weinreb L. Impact of a family medicine resident wellness curriculum: a feasibility study. Med Educ Online. 2016;21:30648. doi:10.3402/meo.v21.30648
- Lafreniere JP, Rios R, Packer H, Ghazarian S, Wright SM, Levine RB. Burned out at the bedside: patient perceptions of physician burnout in an internal medicine resident continuity clinic. J Gen Intern Med. 2016;31(2):203-208. doi:10.1007/s11606-015-3503-3
- Freedy JR, Staley C, Mims LD, et al. Social, individual, and environmental characteristics of family medicine resident burnout: a CERA study. Fam Med. 2022;54(4):270-276. doi:10.22454/FamMed.2022.526799
- Alrishan MA, Alshammari SA. Prevalence of sleep deprivation and its effect on the performance of family medicine residents in Riyadh, Saudi Arabia. J Family Community Med. 2020;27(2):125-130. doi:10.4103/jfcm.JFCM_9_20
- ACGME. ACGME Program Requirements for Graduate Medical Education in Family Medicine. https://www.acgme.org/globalassets/pfassets/programrequirements/120_familymedicine_2024.pdf
- Nene Y, Tadi P. Resident Burnout. In: StatPearls; 2023.
- Bokhour BG, Haun JN, Hyde J, Charns M, Kligler B. Transforming the veterans affairs to a whole health system of care: time for action and research. Med Care. 2020;58(4):295-300. doi:10.1097/MLF.0000000000001316
- Kreitzer MJ, Mitten D, Harris I, Shandeling J. Attitudes toward CAM among medical, nursing, and pharmacy faculty and students: a comparative analysis. Altern Ther Health Med. 2002;8(6):44-53.
- Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015(79):1-16.
- Nguyen J, Liu MA, Patel RJ, Tahara K, Nguyen AL. Use and interest in complementary and alternative medicine among college students seeking healthcare at a university campus student health center. Complement Ther Clin Pract. 2016;24:103-108. doi:10.1016/j.ctcp.2016.06.001
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13(1):27-45. doi:10.1177/1073191105283504
- Baer RA, Smith GT, Lykins E, et al. Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples. Assessment. 2008;15(3):329-342. doi:10.1177/1073191107313003
- West CP, Dyrbye LN, Sloan JA, Shanafelt TD. Single item measures of emotional exhaustion and depersonalization are useful for assessing burnout in medical professionals. J Gen Intern Med. 2009;24(12):1318- 1321. doi:10.1007/s11606-009-1129-z
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396.
- Regehr C, Glancy D, Pitts A, LeBlanc VR. Interventions to reduce the consequences of stress in physicians: a review and meta-analysis. J Nerv Ment Dis. 2014;202(5):353-359. doi:10.1097/NMD.0000000000000130
- Visser CLF, Ket JCF, Croiset G, Kusurkar RA. Perceptions of residents, medical and nursing students about interprofessional education: a systematic review of the quantitative and qualitative literature. BMC Med Educ. 2017;17(1):77. doi:10.1186/s12909-017-0909-0
- Lingard L, Espin S, Evans C, Hawryluck L. The rules of the game: interprofessional collaboration on the intensive care unit team. Crit Care. 2004;8(6):R403-408. doi:10.1186/cc2958
Development of an Integrative Medicine Rotation for Family Medicine and Preventive Medicine Residency
Development of an Integrative Medicine Rotation for Family Medicine and Preventive Medicine Residency
Pharmacist-Led Deprescribing of Aspirin for Primary Prevention of Cardiovascular Disease Among Geriatric Veterans
Pharmacist-Led Deprescribing of Aspirin for Primary Prevention of Cardiovascular Disease Among Geriatric Veterans
Low-dose aspirin commonly is used for the prevention of cardiovascular disease (CVD) but is associated with an increased risk of major bleeding.1 The use of aspirin for primary prevention is largely extrapolated from clinical trials showing benefit in the secondary prevention of myocardial infarction and ischemic stroke. However, results from the Aspirin in Reducing Events in the Elderly (ASPREE) trial challenged this practice.2 The ASPREE trial, conducted in the United States and Australia from 2010 to 2014, sought to determine whether daily 100 mg aspirin, was superior to placebo in promoting disability-free survival among older adults. Participants were aged ≥ 70 years (≥ 65 years for Hispanic and Black US participants), living in the community, and were free from preexisting CVD, cerebrovascular disease, or any chronic condition likely to limit survival to < 5 years. The study found no significant difference in the primary endpoints of death, dementia, or persistent physical disability, but there was a significantly higher risk of major hemorrhage in the aspirin group (3.8% vs 2.8%; hazard ratio, 1.38; 95% CI, 1.18-1.62; P < .001).
Several medical societies have updated their guideline recommendations for aspirin for primary prevention of CVD. The 2022 United States Public Service Task Force (USPSTF) provides a grade C recommendation (at least moderate certainty that the net benefit is small) to consider low-dose aspirin for the primary prevention of CVD on an individual patient basis for adults aged 40 to 59 years who have a ≥ 10% 10-year CVD risk. For adults aged ≥ 60 years, the USPSTF recommendation is grade D (moderate or high certainty that the practice has no net benefit or that harms outweigh the benefits) for low-dose aspirin use.1,3 The American College of Cardiology and American Heart Association (ACC/AHA) recommend considering low-dose aspirin for primary prevention of atherosclerotic cardiovascular disease (ASCVD) among select adults aged 40 to 70 years at higher CVD risk but not at increased risk of bleeding.4 The American Diabetes Association (ADA) recommends low-dose aspirin for primary prevention of CVD in patients with diabetes and additional risk factors such as family history of premature ASCVD, hypertension, dyslipidemia, smoking, or chronic kidney disease, and who are not at higher risk of bleeding.5 The ADA standards also caution against the use of aspirin as primary prevention in patients aged > 70 years. Low-dose aspirin use is not recommended for the primary prevention of CVD in older adults or adults of any age who are at increased risk of bleeding.
Recent literature using the US Department of Veterans Affairs (VA) Corporate Data Warehouse database confirms 86,555 of 1.8 million veterans aged > 70 years (5%) were taking low-dose aspirin for primary prevention of ASCVD despite guideline recommendations.6 Higher risk of gastrointestinal and other major bleeding from low-dose aspirin has been reported in the literature.1 Major bleeds represent a significant burden to the health care system with an estimated mean $13,093 cost for gastrointestinal bleed hospitalization.7
Considering the large scale aspirin use without appropriate indication within the veteran population, the risk of adverse effects, and the significant cost to patients and the health care system, it is imperative to determine the best approach to efficiently deprescribe aspirin for primary prevention among geriatric patients. Deprescribing refers to the systematic and supervised process of dose reduction or drug discontinuation with the goal of improving health and/or reducing the risk of adverse effects.8 During patient visits, primary care practitioners (PCPs) have opportunities to discontinue aspirin, but these encounters are time-limited and deprescribing might be secondary to more acute primary care needs. The shortage of PCPs is expected to worsen in coming years, which could further reduce their availability to assess inappropriate aspirin use.9
VA clinical pharmacist practitioners (CPPs) serve as medication experts and work autonomously under a broad scope of practice as part of the patient aligned care team.10-12 CPPs can free up time for PCPs and facilitate deprescribing efforts, especially for older adults. One retrospective cohort study conducted at a VA medical center found that CPPs deprescribed more potentially inappropriate medications among individuals aged ≥ 80 years compared with usual care with PCPs (26.8% vs 16.1%; P < .001).12,13 An aspirin deprescribing protocol conducted in 2022 resulted in nearly half of veterans aged ≥ 70 years contacted by phone agreeing to stop aspirin. Although this study supports the role pharmacists can play in reducing aspirin use in accordance with guidelines, the authors acknowledge that their interventions had a mean time of 12 minutes per patient and would require workflow changes.14 The purpose of this study is to evaluate the efficiency of aspirin deprescribing through 2 approaches: direct deprescribing by pharmacists using populationlevel review compared with clinicians following a pharmacist-led education.
Methods
This was a single-center quality improvement cohort study at the Durham VA Health Care System (DVAHCS) in North Carolina. Patients included were aged ≥ 70 years without known ASCVD who received care at any of 3 DVAHCS community-based outpatient clinics and prescribed aspirin. Patient data was obtained using the VIONE (Deprescribing Dashboard called Vital, Important, Optional, Not indicated, and Every medication has a specific indication or diagnosis) dashboard.15 VIONE was developed to identify potentially inappropriate medications (PIMs) that are eligible to deprescribe based on Beers Criteria, Screening Tool of Older Personsf Prescriptions criteria, and common clinical scenarios when clinicians determine the risk outweighs the benefit to continue a specific medication. 16,17 VIONE is used to reduce polypharmacy and improve patient safety, comfort, and medication adherence. Aspirin for patients aged ≥ 70 years without a history of ASCVD is a PIM identified by VIONE. Patients aged ≥ 70 years were chosen as an inclusion criteria in this study to match the ASPREE trial inclusion criteria and age inclusion criteria in the VIONE dashboard for aspirin deprescribing.2 Patient lists were generated for these potentially inappropriate aspirin prescriptions for 3 months before clinician staff education presentations, the day of the presentations, and 3 months after.
The primary endpoint was the number of veterans with aspirin deprescribed directly by 2 pharmacists over 12 weeks, divided by total patient care time spent, compared with the change in number of veterans with aspirin deprescribed by any DVAHCS physician, nurse practitioner, physician assistant, or CPP over 12 weeks, divided by the total pharmacist time spent on PCP education. Secondary endpoints were the number of aspirin orders discontinued by pharmacists and CPPs, the number of aspirin orders discontinued 12 weeks before pharmacist-led education compared with the number of aspirin orders discontinued 12 weeks after CPP-led education, average and median pharmacist time spent per patient encounter, and time of direct patient encounters vs time spent on PCP education.
Pharmacists reviewed each patient who met the inclusion criteria from the list generated by VIONE on December 1, 2022, for aspirin appropriateness according to the ACC/AHA and USPSTF guidelines, with the goal to discontinue aspirin for primary prevention of ASCVD and no other indications.1,4 Pharmacists documented their visits using VIONE methodology in the Computerized Patient Record System (CPRS) using a polypharmacy review note. CPPs contacted patients who were taking aspirin for primary prevention by unscheduled telephone call to assess for aspirin adherence, undocumented history of ASCVD, cardiovascular risk factors, and history of bleeding. Aspirin was discontinued if patients met guideline criteria recommendations and agreed to discontinuation. Risk-benefit discussions were completed when patients without known ASCVD were considered high risk because the ACC/AHA guidelines mention there is insufficient evidence of safety and efficacy of aspirin for primary prevention for patients with other known ASCVD risk factors (eg, strong family history of premature myocardial infarction, inability to achieve lipid, blood pressure, or glucose targets, or significant elevation in coronary artery calcium score).
High risk was defined as family history of premature ASCVD (in a male first-degree relative aged < 55 years or a female first-degree relative aged < 65 years), most recent blood pressure or 2 blood pressure results in the last 12 months > 160/100 mm Hg, recent hemoglobin A1c > 9%, and/or low-density lipoprotein > 190 mg/dL or not prescribed an indicated statin.3 Aspirin was continued or discontinued according to patient preference after the personalized risk-benefit discussion.
For patients with a clinical indication for aspirin use other than ASCVD (eg, atrial fibrillation not on anticoagulation, venous thromboembolism prophylaxis, carotid artery disease), CPPs documented their assessment and when appropriate deferred to the PCP for consideration of stopping aspirin. For patients with undocumented ASCVD, CPPs added their ASCVD history to their problem list and aspirin was continued. PCPs were notified by alert when aspirin was discontinued and when patients could not be reached by telephone.
presented a review of recent guideline updates and supporting literature at 2 online staff meetings. The education sessions lasted about 10 minutes and were presented to PCPs across 3 community-based outpatient clinics. An estimated 40 minutes were spent creating the PowerPoint education materials, seeking feedback, making edits, and answering questions or emails from PCPs after the presentation. During the presentation, pharmacists encouraged PCPs to discontinue aspirin (active VA prescriptions and reported over-the-counter use) for primary prevention of ASCVD in patients aged ≥ 70 years during their upcoming appointments and consider risk factors recommended by the ACC/AHA guidelines when applicable. PCPs were notified that CPPs planned to start a population review for discontinuing active VA aspirin prescriptions on December 1, 2022. The primary endpoint and secondary endpoints were analyzed using descriptive statistics. All data were analyzed using Microsoft Excel.
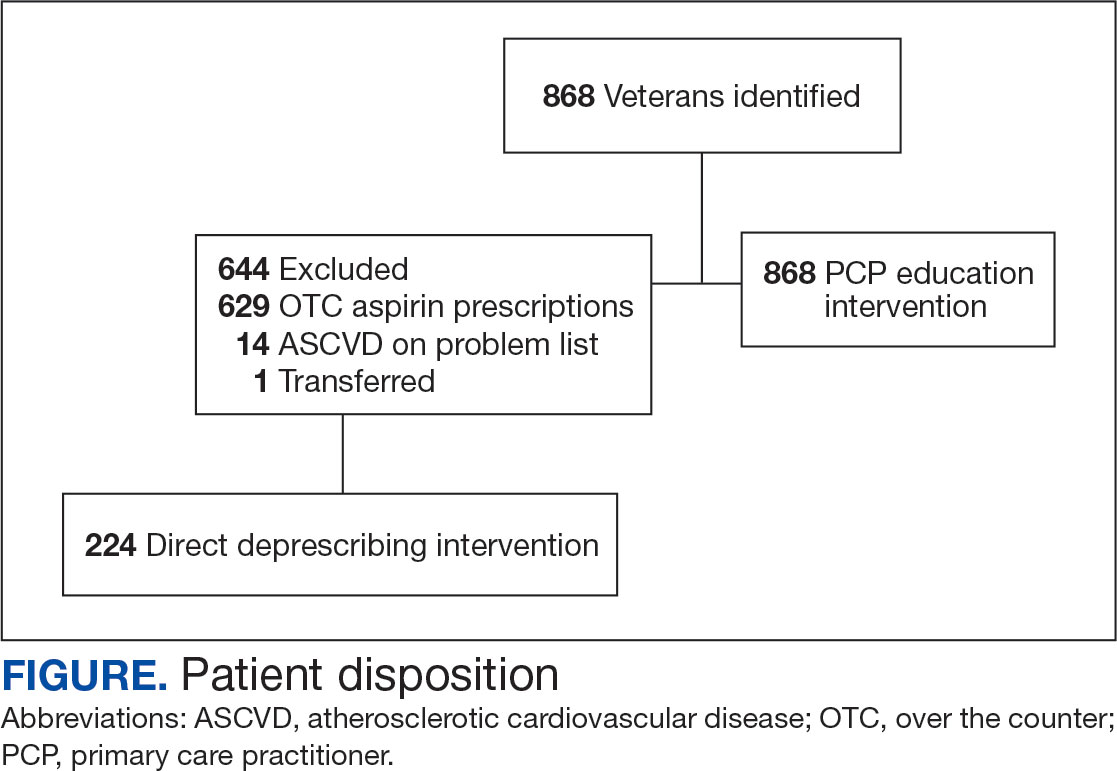
Results
A total of 868 patients aged ≥ 70 years with active prescriptions for aspirin were identified on December 1, 2022. After applying inclusion and exclusion criteria for the pharmacist population review, 224 patients were included for cohort final analysis (Figure). All 868 patients were eligible for the CPP intervention. Primary reasons for exclusion from the CPP population included over-thecounter aspirin and a history of ASCVD in the patient’s problem list. All patients were male, with a mean (SD) age of 75 (4.4) years (Table 1). Most patients were prescribed aspirin, 81 mg daily (n = 220; 98%).
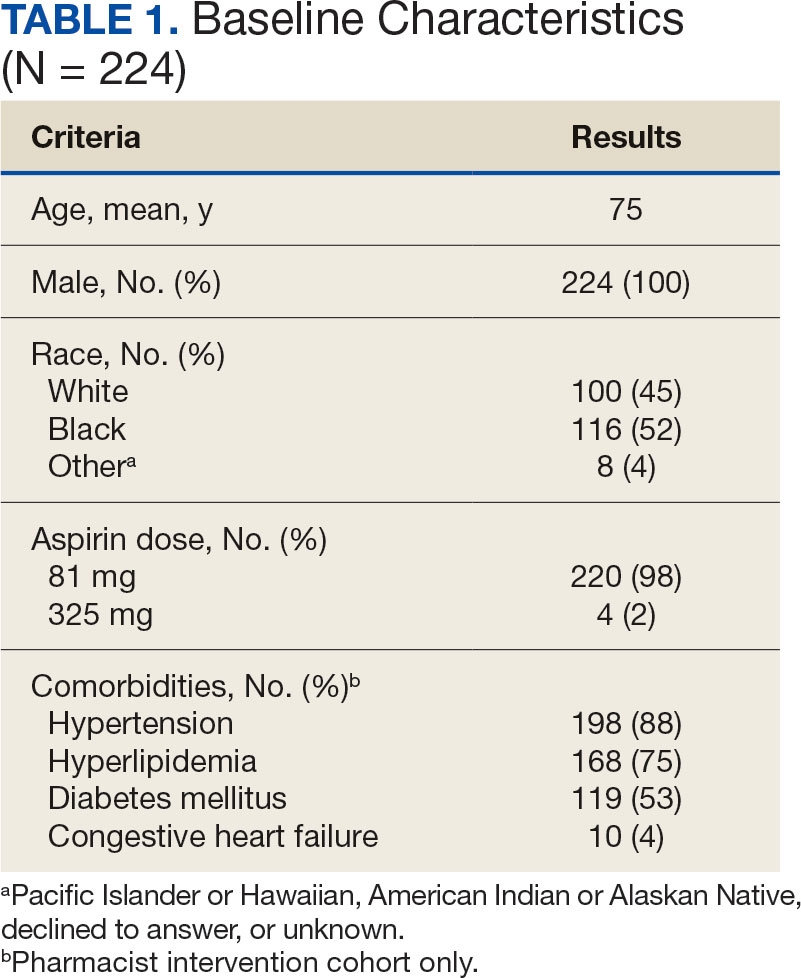
The direct CPP deprescribing intervention resulted in 2 aspirin prescriptions discontinued per hour of pharmacist time and 67 aspirin prescriptions discontinued per hour of pharmacist time via the PCP education intervention. CPPs discontinued 66 aspirin orders in the 12 weeks before the PCP education sessions. A total of 230 aspirin prescriptions were discontinued in the 12 weeks following the PCP education sessions, with 97 discontinued directly by CPPs and 133 discontinued by PCPs. The PCP education session yielded an additional 67 discontinued aspirin orders compared with the 12 weeks before the education sessions (Table 2).
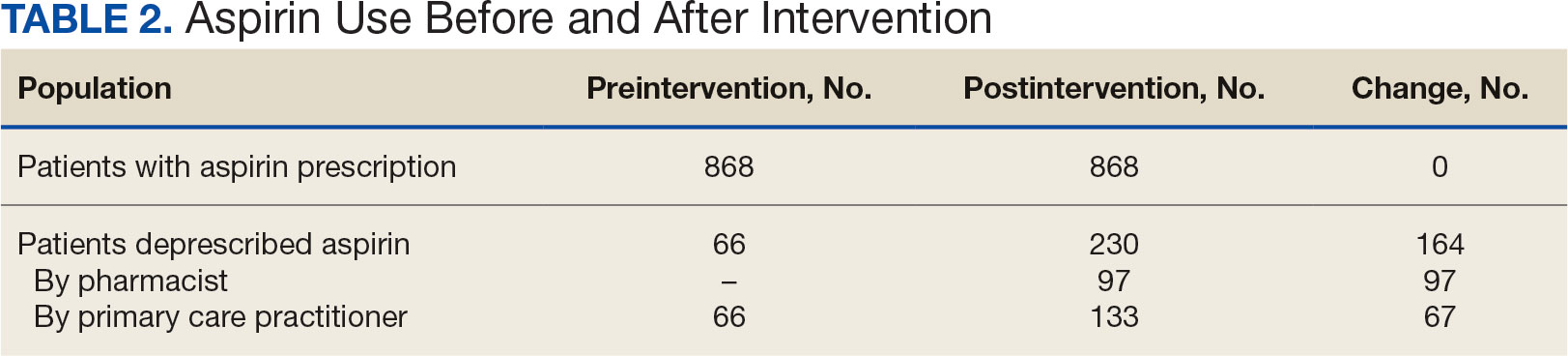
The CPP direct deprescribing intervention took about 48.3 hours, accounting for health record review and time interacting with patients. The PCP education intervention took about 60 minutes, which included time for preparing and delivering education materials (Table 3). CPP deprescribing encounter types, interventions, and related subcategories, and other identified indications to continue aspirin are listed in Table 4.

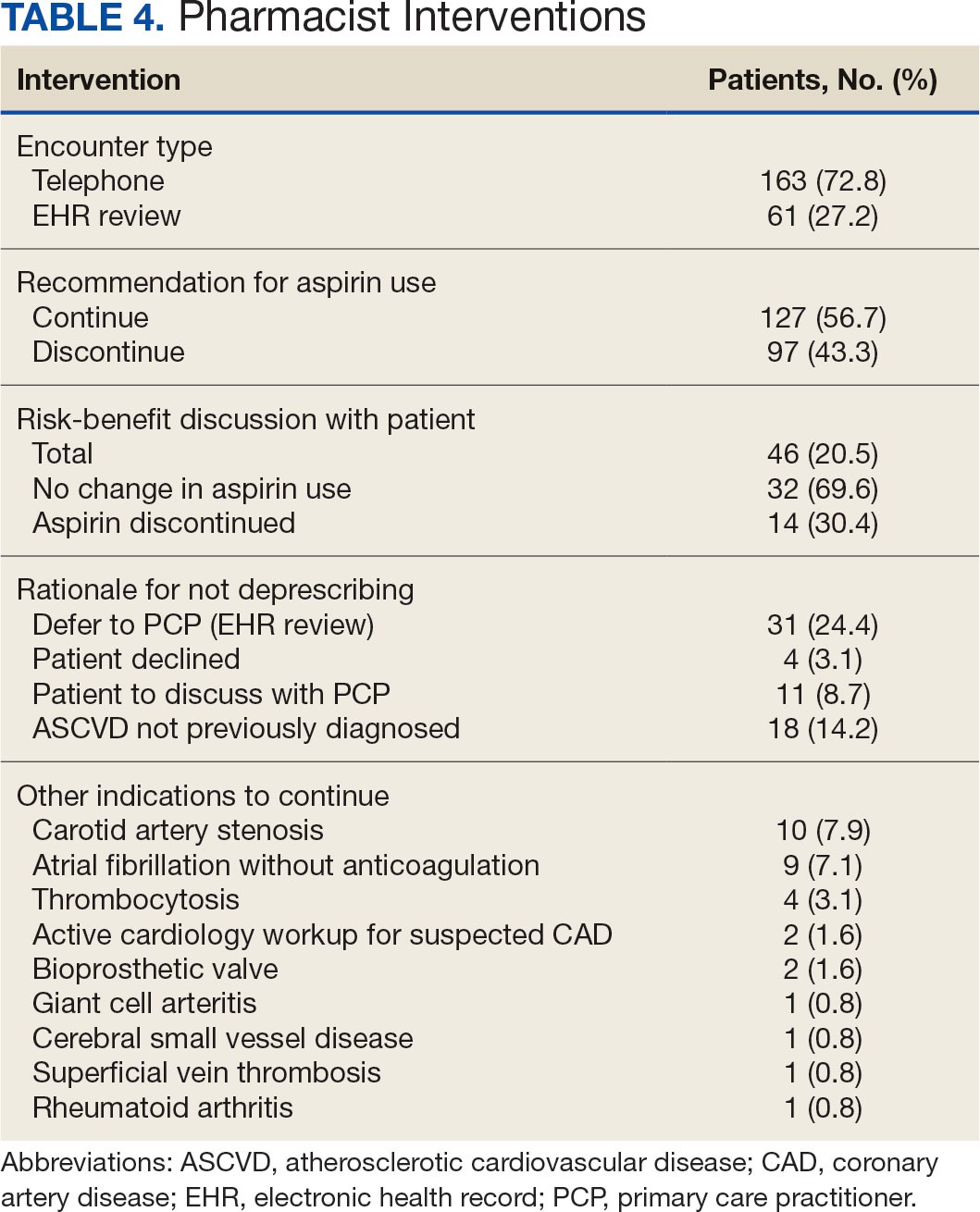
Discussion
Compared with direct deprescribing by pharmacists, the PCP education intervention was more efficient based on number of aspirin orders discontinued by pharmacist time. PCPs discontinued twice as many aspirin prescriptions in the 12 weeks after pharmacist-led education compared with the 12 weeks before.
Patients were primarily contacted by telephone (73%) for deprescribing. Among the 163 patients reached by phone and encouraged to discontinue aspirin, 97 patients (60%) accepted the recommendation, which was similar to the acceptance rates found in the literature (48% to 55%).14,18 Although many veterans continued taking aspirin (78%), most had indications for its continued use, such as a history of ASCVD, atrial fibrillation without anticoagulation, and carotid artery stenosis, and complex comorbidities that required further discussion with their PCP. Less common uses for aspirin were identified through CPRS review or patient reports included cerebral small vessel disease without history of ASCVD, subclavian artery stenosis, thrombocytosis, bioprosthetic valve replacement, giant cell arteritis, rheumatoid arthritis, and prevention of second eye involvement of ischemic optic neuropathy.
to describe the benefit of clinical pharmacy services for deprescribing aspirin for primary prevention of ASCVD through PCP education. Previously published literature has assessed alternative ways to identify or discontinue PIMs—including aspirin—among geriatric patients. One study evaluated the use of marking inappropriate aspirin prescriptions in the electronic health database, leading to a significant reduction in incidence of inappropriate aspirin prescribing; however, it did not assess changes in discontinuation rates of existing aspirin prescriptions.19 The previous VA pharmacist aspirin deprescribing protocol demonstrated pharmacists’ aptitude at discontinuing aspirin for primary prevention but only used direct patient contact and did not compare efficiency with other methods, including PCP education.14
This quality improvement project contributes new data to the existing literature to support the use of clinical pharmacists to discontinue aspirin for primary prevention and suggests a strong role for pharmacists as educators on clinical guidelines, in addition to their roles directly deprescribing PIMs in clinical practice. This study is further strengthened by its use of VIONE, which previously has demonstrated effectiveness in deprescribing a variety of PIMs in primary care settings.20
Despite using VIONE for generating a list of patients eligible for deprescription, our CPRS review found that this list was frequently inaccurate. For example, a small portion of patients were on the VIONE generated list indicating they had no ASCVD history, but had transient ischemic attack listed in their problem lists. Patient problem lists often were missing documented ASCVD history that was revealed by patient interview or CPRS review. It is possible that patients interviewed might have omitted relevant ASCVD history because of low health literacy, conditions affecting memory, or use of health care services outside the VA system.
There were several instances of aspirin used for other non-ASCVD indications, such as primary stroke prevention in atrial fibrillation. The ACC/AHA atrial fibrillation guidelines previously provided a Class IIb recommendation (benefit is greater than risk but additional studies are needed) for considering no antithrombic therapy or treatment with oral anticoagulant or aspirin for nonvalvular atrial fibrillation with CHA2DS2-VASc (Congestive heart failure, Hypertension, Age [> 65 y, 1 point; > 75 y, 2 points], Diabetes, previous Stroke/transient ischemic attack [2 points]) score of 1.21 The ACC/ AHA guidelines were updated in 2023 to recommend against antiplatelet therapy as an alternative to anticoagulation for reducing cardioembolic stroke risk among patients with atrial fibrillation with no indication for antiplatelet therapy because of risk of harm.22 If a patient has no risk factors for stroke, aspirin is not recommended to prevent thromboembolic events because of a lack of benefit. Interventions from this quality improvement study were completed before the 2023 atrial fibrillation guideline was published and therefore in this study aspirin was not discontinued when used for atrial fibrillation. Aspirin use for atrial fibrillation might benefit from similar discontinuation efforts analyzed within this study. Beyond atrial fibrillation, major guidelines do not comment on the use of aspirin for any other indications in the absence of clinical ASCVD.
Limitations
This study is limited by the lack of clinical consensus for complex patients and demonstrates the importance of individualized patient assessment when considering discontinuing aspirin. Because of the project’s relatively short intervention period, aspirin deprescribing rates could decrease over time and repeated education efforts might be necessary to see lasting impact. Health care professionals from services outside of primary care also might have discontinued aspirin during the study period unrelated to the education and these discontinued aspirin prescriptions could contribute to the higher rate observed among PCPs. This study included a specific population cohort of male, US veterans and might not reflect other populations where these interventions could be implemented.
The measurement of time spent by pharmacists and PCPs is an additional limitation. Although it is expected that PCPs attempt to discontinue aspirin during their existing patient care appointments, the time spent during visits was not measured or documented. Direct deprescribing by pharmacist CPRS review required a significant amount of time and could be a barrier to successful intervention by CPPs in patient aligned care teams.
To reduce the time pharmacists spent completing CPRS reviews, an aspirin deprescribing clinical reminder tool could be used to assess use and appropriate indication quickly during any primary care visit led by a PCP or CPP. In addition, it is recommended that pharmacists regularly educate health care professionals on guideline recommendations for aspirin use among geriatric patients. Future studies of the incidence of major cardiovascular events after aspirin deprescribing among geriatric patients and a longitudinal cost/benefit analysis could support these initiatives.
Conclusions
In this study, pharmacists successfully deprescribed inappropriate medications, such as aspirin. However, pharmacist-led PCP education is more efficient compared with direct deprescribing using a population-level review. PCP education requires less time and could allow ambulatory care pharmacists to spend more time on other direct patient care interventions to improve quality and access to care in primary care clinics. This study’s results further support the role of pharmacists in deprescribing PIMs for older adults and the use of a deprescribing tool, such as VIONE, in a primary care setting.
- US Preventive Services Task Force; Davidson KW, Barry MJ, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force recommendation statement. JAMA. 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983
- McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379(16):1519-1528. doi:10.1056/NEJMoa1803955
- Barry MJ, Wolff TA, Pbert L, et al. Putting evidence into practice: an update on the US Preventive Services Task Force methods for developing recommendations for preventive services. Ann Fam Med. 2023;21(2):165-171. doi:10.1370/afm.2946
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/ AHA Guideline on the Primary Prevention of Cardiovascular Disease: A report of the American College of Cardiology/ American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: Standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S179-S218. doi:10.2337/dc24-S010
- Ong SY, Chui P, Bhargava A, Justice A, Hauser RG. Estimating aspirin overuse for primary prevention of atherosclerotic cardiovascular disease (from a nationwide healthcare system). Am J Cardiol. 2020;137:25-30. doi:10.1016/j.amjcard.2020.09.042
- Weiss AJ, Jiang HJ. Overview of clinical conditions with frequent and costly hospital readmissions by payer, 2018. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); July 20, 2021.
- Krishnaswami A, Steinman MA, Goyal P, et al. Deprescribing in older adults with cardiovascular disease. J Am Coll Cardiol. 2019;73(20):2584-2595. doi:10.1016/j.jacc.2019.03.467
- Association of American Medical Colleges. The complexities of physician supply and demand: projections from 2019 to 2034. Accessed March 17, 2024. https://www.aamc.org/media/54681/download
- US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1108.07(1): General pharmacy service requirements. November 28, 2022. Accessed March 17, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=10045
- US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1108.11(3): Clinical pharmacy services. July 1, 2015. Accessed March 17, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3120
- US Department of Veterans Affairs. Clinical pharmacist practitioner (CPP) to improve access to and quality of care August 2021. August 2021. Accessed May 19, 2023. https://www.pbm.va.gov/PBM/CPPO/Documents/ExternalFactSheet_OptimizingtheCPPToImproveAccess_508.pdf
- Ammerman CA, Simpkins BA, Warman N, Downs TN. Potentially inappropriate medications in older adults: Deprescribing with a clinical pharmacist. J Am Geriatr Soc. 2019;67(1):115-118. doi:10.1111/jgs.15623
- Rothbauer K, Siodlak M, Dreischmeier E, Ranola TS, Welch L. Evaluation of a pharmacist-driven ambulatory aspirin deprescribing protocol. Fed Pract. 2022;39(suppl 5):S37- S41a. doi:10.12788/fp.0294
- US Department of Veterans Affairs. VIONE changes the way VA handles prescriptions. January 25, 2020. Accessed May 21, 2023. https://news.va.gov/70709/vione-changes-way-va-handles-prescriptions/
- 2023 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052- 2081. doi:10.1111/jgs.18372
- O’Mahony D, Cherubini A, Guiteras AR, et al. STOPP/ START criteria for potentially inappropriate prescribing in older people: version 3. Eur Geriatr Med. 2023;14(4):625- 632. doi:10.1007/s41999-023-00777-y
- Draeger C, Lodhi F, Geissinger N, Larson T, Griesbach S. Interdisciplinary deprescribing of aspirin through prescriber education and provision of patient-specific recommendations. WMJ. 2022;121(3):220-225
- de Lusignan S, Hinton W, Seidu S, et al. Dashboards to reduce inappropriate prescribing of metformin and aspirin: A quality assurance programme in a primary care sentinel network. Prim Care Diabetes. 2021;15(6):1075-1079. doi:10.1016/j.pcd.2021.06.003
- Nelson MW, Downs TN, Puglisi GM, Simpkins BA, Collier AS. Use of a deprescribing tool in an interdisciplinary primary-care patient-aligned care team. Sr Care Pharm. 2022;37(1):34-43. doi:10.4140/TCP.n.2022.34
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199-e267. doi:10.1161/CIR.0000000000000041
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/ AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines Circulation. 2024;149(1):e1- e156. doi:10.1161/CIR.0000000000001193
Low-dose aspirin commonly is used for the prevention of cardiovascular disease (CVD) but is associated with an increased risk of major bleeding.1 The use of aspirin for primary prevention is largely extrapolated from clinical trials showing benefit in the secondary prevention of myocardial infarction and ischemic stroke. However, results from the Aspirin in Reducing Events in the Elderly (ASPREE) trial challenged this practice.2 The ASPREE trial, conducted in the United States and Australia from 2010 to 2014, sought to determine whether daily 100 mg aspirin, was superior to placebo in promoting disability-free survival among older adults. Participants were aged ≥ 70 years (≥ 65 years for Hispanic and Black US participants), living in the community, and were free from preexisting CVD, cerebrovascular disease, or any chronic condition likely to limit survival to < 5 years. The study found no significant difference in the primary endpoints of death, dementia, or persistent physical disability, but there was a significantly higher risk of major hemorrhage in the aspirin group (3.8% vs 2.8%; hazard ratio, 1.38; 95% CI, 1.18-1.62; P < .001).
Several medical societies have updated their guideline recommendations for aspirin for primary prevention of CVD. The 2022 United States Public Service Task Force (USPSTF) provides a grade C recommendation (at least moderate certainty that the net benefit is small) to consider low-dose aspirin for the primary prevention of CVD on an individual patient basis for adults aged 40 to 59 years who have a ≥ 10% 10-year CVD risk. For adults aged ≥ 60 years, the USPSTF recommendation is grade D (moderate or high certainty that the practice has no net benefit or that harms outweigh the benefits) for low-dose aspirin use.1,3 The American College of Cardiology and American Heart Association (ACC/AHA) recommend considering low-dose aspirin for primary prevention of atherosclerotic cardiovascular disease (ASCVD) among select adults aged 40 to 70 years at higher CVD risk but not at increased risk of bleeding.4 The American Diabetes Association (ADA) recommends low-dose aspirin for primary prevention of CVD in patients with diabetes and additional risk factors such as family history of premature ASCVD, hypertension, dyslipidemia, smoking, or chronic kidney disease, and who are not at higher risk of bleeding.5 The ADA standards also caution against the use of aspirin as primary prevention in patients aged > 70 years. Low-dose aspirin use is not recommended for the primary prevention of CVD in older adults or adults of any age who are at increased risk of bleeding.
Recent literature using the US Department of Veterans Affairs (VA) Corporate Data Warehouse database confirms 86,555 of 1.8 million veterans aged > 70 years (5%) were taking low-dose aspirin for primary prevention of ASCVD despite guideline recommendations.6 Higher risk of gastrointestinal and other major bleeding from low-dose aspirin has been reported in the literature.1 Major bleeds represent a significant burden to the health care system with an estimated mean $13,093 cost for gastrointestinal bleed hospitalization.7
Considering the large scale aspirin use without appropriate indication within the veteran population, the risk of adverse effects, and the significant cost to patients and the health care system, it is imperative to determine the best approach to efficiently deprescribe aspirin for primary prevention among geriatric patients. Deprescribing refers to the systematic and supervised process of dose reduction or drug discontinuation with the goal of improving health and/or reducing the risk of adverse effects.8 During patient visits, primary care practitioners (PCPs) have opportunities to discontinue aspirin, but these encounters are time-limited and deprescribing might be secondary to more acute primary care needs. The shortage of PCPs is expected to worsen in coming years, which could further reduce their availability to assess inappropriate aspirin use.9
VA clinical pharmacist practitioners (CPPs) serve as medication experts and work autonomously under a broad scope of practice as part of the patient aligned care team.10-12 CPPs can free up time for PCPs and facilitate deprescribing efforts, especially for older adults. One retrospective cohort study conducted at a VA medical center found that CPPs deprescribed more potentially inappropriate medications among individuals aged ≥ 80 years compared with usual care with PCPs (26.8% vs 16.1%; P < .001).12,13 An aspirin deprescribing protocol conducted in 2022 resulted in nearly half of veterans aged ≥ 70 years contacted by phone agreeing to stop aspirin. Although this study supports the role pharmacists can play in reducing aspirin use in accordance with guidelines, the authors acknowledge that their interventions had a mean time of 12 minutes per patient and would require workflow changes.14 The purpose of this study is to evaluate the efficiency of aspirin deprescribing through 2 approaches: direct deprescribing by pharmacists using populationlevel review compared with clinicians following a pharmacist-led education.
Methods
This was a single-center quality improvement cohort study at the Durham VA Health Care System (DVAHCS) in North Carolina. Patients included were aged ≥ 70 years without known ASCVD who received care at any of 3 DVAHCS community-based outpatient clinics and prescribed aspirin. Patient data was obtained using the VIONE (Deprescribing Dashboard called Vital, Important, Optional, Not indicated, and Every medication has a specific indication or diagnosis) dashboard.15 VIONE was developed to identify potentially inappropriate medications (PIMs) that are eligible to deprescribe based on Beers Criteria, Screening Tool of Older Personsf Prescriptions criteria, and common clinical scenarios when clinicians determine the risk outweighs the benefit to continue a specific medication. 16,17 VIONE is used to reduce polypharmacy and improve patient safety, comfort, and medication adherence. Aspirin for patients aged ≥ 70 years without a history of ASCVD is a PIM identified by VIONE. Patients aged ≥ 70 years were chosen as an inclusion criteria in this study to match the ASPREE trial inclusion criteria and age inclusion criteria in the VIONE dashboard for aspirin deprescribing.2 Patient lists were generated for these potentially inappropriate aspirin prescriptions for 3 months before clinician staff education presentations, the day of the presentations, and 3 months after.
The primary endpoint was the number of veterans with aspirin deprescribed directly by 2 pharmacists over 12 weeks, divided by total patient care time spent, compared with the change in number of veterans with aspirin deprescribed by any DVAHCS physician, nurse practitioner, physician assistant, or CPP over 12 weeks, divided by the total pharmacist time spent on PCP education. Secondary endpoints were the number of aspirin orders discontinued by pharmacists and CPPs, the number of aspirin orders discontinued 12 weeks before pharmacist-led education compared with the number of aspirin orders discontinued 12 weeks after CPP-led education, average and median pharmacist time spent per patient encounter, and time of direct patient encounters vs time spent on PCP education.
Pharmacists reviewed each patient who met the inclusion criteria from the list generated by VIONE on December 1, 2022, for aspirin appropriateness according to the ACC/AHA and USPSTF guidelines, with the goal to discontinue aspirin for primary prevention of ASCVD and no other indications.1,4 Pharmacists documented their visits using VIONE methodology in the Computerized Patient Record System (CPRS) using a polypharmacy review note. CPPs contacted patients who were taking aspirin for primary prevention by unscheduled telephone call to assess for aspirin adherence, undocumented history of ASCVD, cardiovascular risk factors, and history of bleeding. Aspirin was discontinued if patients met guideline criteria recommendations and agreed to discontinuation. Risk-benefit discussions were completed when patients without known ASCVD were considered high risk because the ACC/AHA guidelines mention there is insufficient evidence of safety and efficacy of aspirin for primary prevention for patients with other known ASCVD risk factors (eg, strong family history of premature myocardial infarction, inability to achieve lipid, blood pressure, or glucose targets, or significant elevation in coronary artery calcium score).
High risk was defined as family history of premature ASCVD (in a male first-degree relative aged < 55 years or a female first-degree relative aged < 65 years), most recent blood pressure or 2 blood pressure results in the last 12 months > 160/100 mm Hg, recent hemoglobin A1c > 9%, and/or low-density lipoprotein > 190 mg/dL or not prescribed an indicated statin.3 Aspirin was continued or discontinued according to patient preference after the personalized risk-benefit discussion.
For patients with a clinical indication for aspirin use other than ASCVD (eg, atrial fibrillation not on anticoagulation, venous thromboembolism prophylaxis, carotid artery disease), CPPs documented their assessment and when appropriate deferred to the PCP for consideration of stopping aspirin. For patients with undocumented ASCVD, CPPs added their ASCVD history to their problem list and aspirin was continued. PCPs were notified by alert when aspirin was discontinued and when patients could not be reached by telephone.
presented a review of recent guideline updates and supporting literature at 2 online staff meetings. The education sessions lasted about 10 minutes and were presented to PCPs across 3 community-based outpatient clinics. An estimated 40 minutes were spent creating the PowerPoint education materials, seeking feedback, making edits, and answering questions or emails from PCPs after the presentation. During the presentation, pharmacists encouraged PCPs to discontinue aspirin (active VA prescriptions and reported over-the-counter use) for primary prevention of ASCVD in patients aged ≥ 70 years during their upcoming appointments and consider risk factors recommended by the ACC/AHA guidelines when applicable. PCPs were notified that CPPs planned to start a population review for discontinuing active VA aspirin prescriptions on December 1, 2022. The primary endpoint and secondary endpoints were analyzed using descriptive statistics. All data were analyzed using Microsoft Excel.

Results
A total of 868 patients aged ≥ 70 years with active prescriptions for aspirin were identified on December 1, 2022. After applying inclusion and exclusion criteria for the pharmacist population review, 224 patients were included for cohort final analysis (Figure). All 868 patients were eligible for the CPP intervention. Primary reasons for exclusion from the CPP population included over-thecounter aspirin and a history of ASCVD in the patient’s problem list. All patients were male, with a mean (SD) age of 75 (4.4) years (Table 1). Most patients were prescribed aspirin, 81 mg daily (n = 220; 98%).

The direct CPP deprescribing intervention resulted in 2 aspirin prescriptions discontinued per hour of pharmacist time and 67 aspirin prescriptions discontinued per hour of pharmacist time via the PCP education intervention. CPPs discontinued 66 aspirin orders in the 12 weeks before the PCP education sessions. A total of 230 aspirin prescriptions were discontinued in the 12 weeks following the PCP education sessions, with 97 discontinued directly by CPPs and 133 discontinued by PCPs. The PCP education session yielded an additional 67 discontinued aspirin orders compared with the 12 weeks before the education sessions (Table 2).

The CPP direct deprescribing intervention took about 48.3 hours, accounting for health record review and time interacting with patients. The PCP education intervention took about 60 minutes, which included time for preparing and delivering education materials (Table 3). CPP deprescribing encounter types, interventions, and related subcategories, and other identified indications to continue aspirin are listed in Table 4.


Discussion
Compared with direct deprescribing by pharmacists, the PCP education intervention was more efficient based on number of aspirin orders discontinued by pharmacist time. PCPs discontinued twice as many aspirin prescriptions in the 12 weeks after pharmacist-led education compared with the 12 weeks before.
Patients were primarily contacted by telephone (73%) for deprescribing. Among the 163 patients reached by phone and encouraged to discontinue aspirin, 97 patients (60%) accepted the recommendation, which was similar to the acceptance rates found in the literature (48% to 55%).14,18 Although many veterans continued taking aspirin (78%), most had indications for its continued use, such as a history of ASCVD, atrial fibrillation without anticoagulation, and carotid artery stenosis, and complex comorbidities that required further discussion with their PCP. Less common uses for aspirin were identified through CPRS review or patient reports included cerebral small vessel disease without history of ASCVD, subclavian artery stenosis, thrombocytosis, bioprosthetic valve replacement, giant cell arteritis, rheumatoid arthritis, and prevention of second eye involvement of ischemic optic neuropathy.
to describe the benefit of clinical pharmacy services for deprescribing aspirin for primary prevention of ASCVD through PCP education. Previously published literature has assessed alternative ways to identify or discontinue PIMs—including aspirin—among geriatric patients. One study evaluated the use of marking inappropriate aspirin prescriptions in the electronic health database, leading to a significant reduction in incidence of inappropriate aspirin prescribing; however, it did not assess changes in discontinuation rates of existing aspirin prescriptions.19 The previous VA pharmacist aspirin deprescribing protocol demonstrated pharmacists’ aptitude at discontinuing aspirin for primary prevention but only used direct patient contact and did not compare efficiency with other methods, including PCP education.14
This quality improvement project contributes new data to the existing literature to support the use of clinical pharmacists to discontinue aspirin for primary prevention and suggests a strong role for pharmacists as educators on clinical guidelines, in addition to their roles directly deprescribing PIMs in clinical practice. This study is further strengthened by its use of VIONE, which previously has demonstrated effectiveness in deprescribing a variety of PIMs in primary care settings.20
Despite using VIONE for generating a list of patients eligible for deprescription, our CPRS review found that this list was frequently inaccurate. For example, a small portion of patients were on the VIONE generated list indicating they had no ASCVD history, but had transient ischemic attack listed in their problem lists. Patient problem lists often were missing documented ASCVD history that was revealed by patient interview or CPRS review. It is possible that patients interviewed might have omitted relevant ASCVD history because of low health literacy, conditions affecting memory, or use of health care services outside the VA system.
There were several instances of aspirin used for other non-ASCVD indications, such as primary stroke prevention in atrial fibrillation. The ACC/AHA atrial fibrillation guidelines previously provided a Class IIb recommendation (benefit is greater than risk but additional studies are needed) for considering no antithrombic therapy or treatment with oral anticoagulant or aspirin for nonvalvular atrial fibrillation with CHA2DS2-VASc (Congestive heart failure, Hypertension, Age [> 65 y, 1 point; > 75 y, 2 points], Diabetes, previous Stroke/transient ischemic attack [2 points]) score of 1.21 The ACC/ AHA guidelines were updated in 2023 to recommend against antiplatelet therapy as an alternative to anticoagulation for reducing cardioembolic stroke risk among patients with atrial fibrillation with no indication for antiplatelet therapy because of risk of harm.22 If a patient has no risk factors for stroke, aspirin is not recommended to prevent thromboembolic events because of a lack of benefit. Interventions from this quality improvement study were completed before the 2023 atrial fibrillation guideline was published and therefore in this study aspirin was not discontinued when used for atrial fibrillation. Aspirin use for atrial fibrillation might benefit from similar discontinuation efforts analyzed within this study. Beyond atrial fibrillation, major guidelines do not comment on the use of aspirin for any other indications in the absence of clinical ASCVD.
Limitations
This study is limited by the lack of clinical consensus for complex patients and demonstrates the importance of individualized patient assessment when considering discontinuing aspirin. Because of the project’s relatively short intervention period, aspirin deprescribing rates could decrease over time and repeated education efforts might be necessary to see lasting impact. Health care professionals from services outside of primary care also might have discontinued aspirin during the study period unrelated to the education and these discontinued aspirin prescriptions could contribute to the higher rate observed among PCPs. This study included a specific population cohort of male, US veterans and might not reflect other populations where these interventions could be implemented.
The measurement of time spent by pharmacists and PCPs is an additional limitation. Although it is expected that PCPs attempt to discontinue aspirin during their existing patient care appointments, the time spent during visits was not measured or documented. Direct deprescribing by pharmacist CPRS review required a significant amount of time and could be a barrier to successful intervention by CPPs in patient aligned care teams.
To reduce the time pharmacists spent completing CPRS reviews, an aspirin deprescribing clinical reminder tool could be used to assess use and appropriate indication quickly during any primary care visit led by a PCP or CPP. In addition, it is recommended that pharmacists regularly educate health care professionals on guideline recommendations for aspirin use among geriatric patients. Future studies of the incidence of major cardiovascular events after aspirin deprescribing among geriatric patients and a longitudinal cost/benefit analysis could support these initiatives.
Conclusions
In this study, pharmacists successfully deprescribed inappropriate medications, such as aspirin. However, pharmacist-led PCP education is more efficient compared with direct deprescribing using a population-level review. PCP education requires less time and could allow ambulatory care pharmacists to spend more time on other direct patient care interventions to improve quality and access to care in primary care clinics. This study’s results further support the role of pharmacists in deprescribing PIMs for older adults and the use of a deprescribing tool, such as VIONE, in a primary care setting.
Low-dose aspirin commonly is used for the prevention of cardiovascular disease (CVD) but is associated with an increased risk of major bleeding.1 The use of aspirin for primary prevention is largely extrapolated from clinical trials showing benefit in the secondary prevention of myocardial infarction and ischemic stroke. However, results from the Aspirin in Reducing Events in the Elderly (ASPREE) trial challenged this practice.2 The ASPREE trial, conducted in the United States and Australia from 2010 to 2014, sought to determine whether daily 100 mg aspirin, was superior to placebo in promoting disability-free survival among older adults. Participants were aged ≥ 70 years (≥ 65 years for Hispanic and Black US participants), living in the community, and were free from preexisting CVD, cerebrovascular disease, or any chronic condition likely to limit survival to < 5 years. The study found no significant difference in the primary endpoints of death, dementia, or persistent physical disability, but there was a significantly higher risk of major hemorrhage in the aspirin group (3.8% vs 2.8%; hazard ratio, 1.38; 95% CI, 1.18-1.62; P < .001).
Several medical societies have updated their guideline recommendations for aspirin for primary prevention of CVD. The 2022 United States Public Service Task Force (USPSTF) provides a grade C recommendation (at least moderate certainty that the net benefit is small) to consider low-dose aspirin for the primary prevention of CVD on an individual patient basis for adults aged 40 to 59 years who have a ≥ 10% 10-year CVD risk. For adults aged ≥ 60 years, the USPSTF recommendation is grade D (moderate or high certainty that the practice has no net benefit or that harms outweigh the benefits) for low-dose aspirin use.1,3 The American College of Cardiology and American Heart Association (ACC/AHA) recommend considering low-dose aspirin for primary prevention of atherosclerotic cardiovascular disease (ASCVD) among select adults aged 40 to 70 years at higher CVD risk but not at increased risk of bleeding.4 The American Diabetes Association (ADA) recommends low-dose aspirin for primary prevention of CVD in patients with diabetes and additional risk factors such as family history of premature ASCVD, hypertension, dyslipidemia, smoking, or chronic kidney disease, and who are not at higher risk of bleeding.5 The ADA standards also caution against the use of aspirin as primary prevention in patients aged > 70 years. Low-dose aspirin use is not recommended for the primary prevention of CVD in older adults or adults of any age who are at increased risk of bleeding.
Recent literature using the US Department of Veterans Affairs (VA) Corporate Data Warehouse database confirms 86,555 of 1.8 million veterans aged > 70 years (5%) were taking low-dose aspirin for primary prevention of ASCVD despite guideline recommendations.6 Higher risk of gastrointestinal and other major bleeding from low-dose aspirin has been reported in the literature.1 Major bleeds represent a significant burden to the health care system with an estimated mean $13,093 cost for gastrointestinal bleed hospitalization.7
Considering the large scale aspirin use without appropriate indication within the veteran population, the risk of adverse effects, and the significant cost to patients and the health care system, it is imperative to determine the best approach to efficiently deprescribe aspirin for primary prevention among geriatric patients. Deprescribing refers to the systematic and supervised process of dose reduction or drug discontinuation with the goal of improving health and/or reducing the risk of adverse effects.8 During patient visits, primary care practitioners (PCPs) have opportunities to discontinue aspirin, but these encounters are time-limited and deprescribing might be secondary to more acute primary care needs. The shortage of PCPs is expected to worsen in coming years, which could further reduce their availability to assess inappropriate aspirin use.9
VA clinical pharmacist practitioners (CPPs) serve as medication experts and work autonomously under a broad scope of practice as part of the patient aligned care team.10-12 CPPs can free up time for PCPs and facilitate deprescribing efforts, especially for older adults. One retrospective cohort study conducted at a VA medical center found that CPPs deprescribed more potentially inappropriate medications among individuals aged ≥ 80 years compared with usual care with PCPs (26.8% vs 16.1%; P < .001).12,13 An aspirin deprescribing protocol conducted in 2022 resulted in nearly half of veterans aged ≥ 70 years contacted by phone agreeing to stop aspirin. Although this study supports the role pharmacists can play in reducing aspirin use in accordance with guidelines, the authors acknowledge that their interventions had a mean time of 12 minutes per patient and would require workflow changes.14 The purpose of this study is to evaluate the efficiency of aspirin deprescribing through 2 approaches: direct deprescribing by pharmacists using populationlevel review compared with clinicians following a pharmacist-led education.
Methods
This was a single-center quality improvement cohort study at the Durham VA Health Care System (DVAHCS) in North Carolina. Patients included were aged ≥ 70 years without known ASCVD who received care at any of 3 DVAHCS community-based outpatient clinics and prescribed aspirin. Patient data was obtained using the VIONE (Deprescribing Dashboard called Vital, Important, Optional, Not indicated, and Every medication has a specific indication or diagnosis) dashboard.15 VIONE was developed to identify potentially inappropriate medications (PIMs) that are eligible to deprescribe based on Beers Criteria, Screening Tool of Older Personsf Prescriptions criteria, and common clinical scenarios when clinicians determine the risk outweighs the benefit to continue a specific medication. 16,17 VIONE is used to reduce polypharmacy and improve patient safety, comfort, and medication adherence. Aspirin for patients aged ≥ 70 years without a history of ASCVD is a PIM identified by VIONE. Patients aged ≥ 70 years were chosen as an inclusion criteria in this study to match the ASPREE trial inclusion criteria and age inclusion criteria in the VIONE dashboard for aspirin deprescribing.2 Patient lists were generated for these potentially inappropriate aspirin prescriptions for 3 months before clinician staff education presentations, the day of the presentations, and 3 months after.
The primary endpoint was the number of veterans with aspirin deprescribed directly by 2 pharmacists over 12 weeks, divided by total patient care time spent, compared with the change in number of veterans with aspirin deprescribed by any DVAHCS physician, nurse practitioner, physician assistant, or CPP over 12 weeks, divided by the total pharmacist time spent on PCP education. Secondary endpoints were the number of aspirin orders discontinued by pharmacists and CPPs, the number of aspirin orders discontinued 12 weeks before pharmacist-led education compared with the number of aspirin orders discontinued 12 weeks after CPP-led education, average and median pharmacist time spent per patient encounter, and time of direct patient encounters vs time spent on PCP education.
Pharmacists reviewed each patient who met the inclusion criteria from the list generated by VIONE on December 1, 2022, for aspirin appropriateness according to the ACC/AHA and USPSTF guidelines, with the goal to discontinue aspirin for primary prevention of ASCVD and no other indications.1,4 Pharmacists documented their visits using VIONE methodology in the Computerized Patient Record System (CPRS) using a polypharmacy review note. CPPs contacted patients who were taking aspirin for primary prevention by unscheduled telephone call to assess for aspirin adherence, undocumented history of ASCVD, cardiovascular risk factors, and history of bleeding. Aspirin was discontinued if patients met guideline criteria recommendations and agreed to discontinuation. Risk-benefit discussions were completed when patients without known ASCVD were considered high risk because the ACC/AHA guidelines mention there is insufficient evidence of safety and efficacy of aspirin for primary prevention for patients with other known ASCVD risk factors (eg, strong family history of premature myocardial infarction, inability to achieve lipid, blood pressure, or glucose targets, or significant elevation in coronary artery calcium score).
High risk was defined as family history of premature ASCVD (in a male first-degree relative aged < 55 years or a female first-degree relative aged < 65 years), most recent blood pressure or 2 blood pressure results in the last 12 months > 160/100 mm Hg, recent hemoglobin A1c > 9%, and/or low-density lipoprotein > 190 mg/dL or not prescribed an indicated statin.3 Aspirin was continued or discontinued according to patient preference after the personalized risk-benefit discussion.
For patients with a clinical indication for aspirin use other than ASCVD (eg, atrial fibrillation not on anticoagulation, venous thromboembolism prophylaxis, carotid artery disease), CPPs documented their assessment and when appropriate deferred to the PCP for consideration of stopping aspirin. For patients with undocumented ASCVD, CPPs added their ASCVD history to their problem list and aspirin was continued. PCPs were notified by alert when aspirin was discontinued and when patients could not be reached by telephone.
presented a review of recent guideline updates and supporting literature at 2 online staff meetings. The education sessions lasted about 10 minutes and were presented to PCPs across 3 community-based outpatient clinics. An estimated 40 minutes were spent creating the PowerPoint education materials, seeking feedback, making edits, and answering questions or emails from PCPs after the presentation. During the presentation, pharmacists encouraged PCPs to discontinue aspirin (active VA prescriptions and reported over-the-counter use) for primary prevention of ASCVD in patients aged ≥ 70 years during their upcoming appointments and consider risk factors recommended by the ACC/AHA guidelines when applicable. PCPs were notified that CPPs planned to start a population review for discontinuing active VA aspirin prescriptions on December 1, 2022. The primary endpoint and secondary endpoints were analyzed using descriptive statistics. All data were analyzed using Microsoft Excel.

Results
A total of 868 patients aged ≥ 70 years with active prescriptions for aspirin were identified on December 1, 2022. After applying inclusion and exclusion criteria for the pharmacist population review, 224 patients were included for cohort final analysis (Figure). All 868 patients were eligible for the CPP intervention. Primary reasons for exclusion from the CPP population included over-thecounter aspirin and a history of ASCVD in the patient’s problem list. All patients were male, with a mean (SD) age of 75 (4.4) years (Table 1). Most patients were prescribed aspirin, 81 mg daily (n = 220; 98%).

The direct CPP deprescribing intervention resulted in 2 aspirin prescriptions discontinued per hour of pharmacist time and 67 aspirin prescriptions discontinued per hour of pharmacist time via the PCP education intervention. CPPs discontinued 66 aspirin orders in the 12 weeks before the PCP education sessions. A total of 230 aspirin prescriptions were discontinued in the 12 weeks following the PCP education sessions, with 97 discontinued directly by CPPs and 133 discontinued by PCPs. The PCP education session yielded an additional 67 discontinued aspirin orders compared with the 12 weeks before the education sessions (Table 2).

The CPP direct deprescribing intervention took about 48.3 hours, accounting for health record review and time interacting with patients. The PCP education intervention took about 60 minutes, which included time for preparing and delivering education materials (Table 3). CPP deprescribing encounter types, interventions, and related subcategories, and other identified indications to continue aspirin are listed in Table 4.


Discussion
Compared with direct deprescribing by pharmacists, the PCP education intervention was more efficient based on number of aspirin orders discontinued by pharmacist time. PCPs discontinued twice as many aspirin prescriptions in the 12 weeks after pharmacist-led education compared with the 12 weeks before.
Patients were primarily contacted by telephone (73%) for deprescribing. Among the 163 patients reached by phone and encouraged to discontinue aspirin, 97 patients (60%) accepted the recommendation, which was similar to the acceptance rates found in the literature (48% to 55%).14,18 Although many veterans continued taking aspirin (78%), most had indications for its continued use, such as a history of ASCVD, atrial fibrillation without anticoagulation, and carotid artery stenosis, and complex comorbidities that required further discussion with their PCP. Less common uses for aspirin were identified through CPRS review or patient reports included cerebral small vessel disease without history of ASCVD, subclavian artery stenosis, thrombocytosis, bioprosthetic valve replacement, giant cell arteritis, rheumatoid arthritis, and prevention of second eye involvement of ischemic optic neuropathy.
to describe the benefit of clinical pharmacy services for deprescribing aspirin for primary prevention of ASCVD through PCP education. Previously published literature has assessed alternative ways to identify or discontinue PIMs—including aspirin—among geriatric patients. One study evaluated the use of marking inappropriate aspirin prescriptions in the electronic health database, leading to a significant reduction in incidence of inappropriate aspirin prescribing; however, it did not assess changes in discontinuation rates of existing aspirin prescriptions.19 The previous VA pharmacist aspirin deprescribing protocol demonstrated pharmacists’ aptitude at discontinuing aspirin for primary prevention but only used direct patient contact and did not compare efficiency with other methods, including PCP education.14
This quality improvement project contributes new data to the existing literature to support the use of clinical pharmacists to discontinue aspirin for primary prevention and suggests a strong role for pharmacists as educators on clinical guidelines, in addition to their roles directly deprescribing PIMs in clinical practice. This study is further strengthened by its use of VIONE, which previously has demonstrated effectiveness in deprescribing a variety of PIMs in primary care settings.20
Despite using VIONE for generating a list of patients eligible for deprescription, our CPRS review found that this list was frequently inaccurate. For example, a small portion of patients were on the VIONE generated list indicating they had no ASCVD history, but had transient ischemic attack listed in their problem lists. Patient problem lists often were missing documented ASCVD history that was revealed by patient interview or CPRS review. It is possible that patients interviewed might have omitted relevant ASCVD history because of low health literacy, conditions affecting memory, or use of health care services outside the VA system.
There were several instances of aspirin used for other non-ASCVD indications, such as primary stroke prevention in atrial fibrillation. The ACC/AHA atrial fibrillation guidelines previously provided a Class IIb recommendation (benefit is greater than risk but additional studies are needed) for considering no antithrombic therapy or treatment with oral anticoagulant or aspirin for nonvalvular atrial fibrillation with CHA2DS2-VASc (Congestive heart failure, Hypertension, Age [> 65 y, 1 point; > 75 y, 2 points], Diabetes, previous Stroke/transient ischemic attack [2 points]) score of 1.21 The ACC/ AHA guidelines were updated in 2023 to recommend against antiplatelet therapy as an alternative to anticoagulation for reducing cardioembolic stroke risk among patients with atrial fibrillation with no indication for antiplatelet therapy because of risk of harm.22 If a patient has no risk factors for stroke, aspirin is not recommended to prevent thromboembolic events because of a lack of benefit. Interventions from this quality improvement study were completed before the 2023 atrial fibrillation guideline was published and therefore in this study aspirin was not discontinued when used for atrial fibrillation. Aspirin use for atrial fibrillation might benefit from similar discontinuation efforts analyzed within this study. Beyond atrial fibrillation, major guidelines do not comment on the use of aspirin for any other indications in the absence of clinical ASCVD.
Limitations
This study is limited by the lack of clinical consensus for complex patients and demonstrates the importance of individualized patient assessment when considering discontinuing aspirin. Because of the project’s relatively short intervention period, aspirin deprescribing rates could decrease over time and repeated education efforts might be necessary to see lasting impact. Health care professionals from services outside of primary care also might have discontinued aspirin during the study period unrelated to the education and these discontinued aspirin prescriptions could contribute to the higher rate observed among PCPs. This study included a specific population cohort of male, US veterans and might not reflect other populations where these interventions could be implemented.
The measurement of time spent by pharmacists and PCPs is an additional limitation. Although it is expected that PCPs attempt to discontinue aspirin during their existing patient care appointments, the time spent during visits was not measured or documented. Direct deprescribing by pharmacist CPRS review required a significant amount of time and could be a barrier to successful intervention by CPPs in patient aligned care teams.
To reduce the time pharmacists spent completing CPRS reviews, an aspirin deprescribing clinical reminder tool could be used to assess use and appropriate indication quickly during any primary care visit led by a PCP or CPP. In addition, it is recommended that pharmacists regularly educate health care professionals on guideline recommendations for aspirin use among geriatric patients. Future studies of the incidence of major cardiovascular events after aspirin deprescribing among geriatric patients and a longitudinal cost/benefit analysis could support these initiatives.
Conclusions
In this study, pharmacists successfully deprescribed inappropriate medications, such as aspirin. However, pharmacist-led PCP education is more efficient compared with direct deprescribing using a population-level review. PCP education requires less time and could allow ambulatory care pharmacists to spend more time on other direct patient care interventions to improve quality and access to care in primary care clinics. This study’s results further support the role of pharmacists in deprescribing PIMs for older adults and the use of a deprescribing tool, such as VIONE, in a primary care setting.
- US Preventive Services Task Force; Davidson KW, Barry MJ, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force recommendation statement. JAMA. 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983
- McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379(16):1519-1528. doi:10.1056/NEJMoa1803955
- Barry MJ, Wolff TA, Pbert L, et al. Putting evidence into practice: an update on the US Preventive Services Task Force methods for developing recommendations for preventive services. Ann Fam Med. 2023;21(2):165-171. doi:10.1370/afm.2946
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/ AHA Guideline on the Primary Prevention of Cardiovascular Disease: A report of the American College of Cardiology/ American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: Standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S179-S218. doi:10.2337/dc24-S010
- Ong SY, Chui P, Bhargava A, Justice A, Hauser RG. Estimating aspirin overuse for primary prevention of atherosclerotic cardiovascular disease (from a nationwide healthcare system). Am J Cardiol. 2020;137:25-30. doi:10.1016/j.amjcard.2020.09.042
- Weiss AJ, Jiang HJ. Overview of clinical conditions with frequent and costly hospital readmissions by payer, 2018. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); July 20, 2021.
- Krishnaswami A, Steinman MA, Goyal P, et al. Deprescribing in older adults with cardiovascular disease. J Am Coll Cardiol. 2019;73(20):2584-2595. doi:10.1016/j.jacc.2019.03.467
- Association of American Medical Colleges. The complexities of physician supply and demand: projections from 2019 to 2034. Accessed March 17, 2024. https://www.aamc.org/media/54681/download
- US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1108.07(1): General pharmacy service requirements. November 28, 2022. Accessed March 17, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=10045
- US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1108.11(3): Clinical pharmacy services. July 1, 2015. Accessed March 17, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3120
- US Department of Veterans Affairs. Clinical pharmacist practitioner (CPP) to improve access to and quality of care August 2021. August 2021. Accessed May 19, 2023. https://www.pbm.va.gov/PBM/CPPO/Documents/ExternalFactSheet_OptimizingtheCPPToImproveAccess_508.pdf
- Ammerman CA, Simpkins BA, Warman N, Downs TN. Potentially inappropriate medications in older adults: Deprescribing with a clinical pharmacist. J Am Geriatr Soc. 2019;67(1):115-118. doi:10.1111/jgs.15623
- Rothbauer K, Siodlak M, Dreischmeier E, Ranola TS, Welch L. Evaluation of a pharmacist-driven ambulatory aspirin deprescribing protocol. Fed Pract. 2022;39(suppl 5):S37- S41a. doi:10.12788/fp.0294
- US Department of Veterans Affairs. VIONE changes the way VA handles prescriptions. January 25, 2020. Accessed May 21, 2023. https://news.va.gov/70709/vione-changes-way-va-handles-prescriptions/
- 2023 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052- 2081. doi:10.1111/jgs.18372
- O’Mahony D, Cherubini A, Guiteras AR, et al. STOPP/ START criteria for potentially inappropriate prescribing in older people: version 3. Eur Geriatr Med. 2023;14(4):625- 632. doi:10.1007/s41999-023-00777-y
- Draeger C, Lodhi F, Geissinger N, Larson T, Griesbach S. Interdisciplinary deprescribing of aspirin through prescriber education and provision of patient-specific recommendations. WMJ. 2022;121(3):220-225
- de Lusignan S, Hinton W, Seidu S, et al. Dashboards to reduce inappropriate prescribing of metformin and aspirin: A quality assurance programme in a primary care sentinel network. Prim Care Diabetes. 2021;15(6):1075-1079. doi:10.1016/j.pcd.2021.06.003
- Nelson MW, Downs TN, Puglisi GM, Simpkins BA, Collier AS. Use of a deprescribing tool in an interdisciplinary primary-care patient-aligned care team. Sr Care Pharm. 2022;37(1):34-43. doi:10.4140/TCP.n.2022.34
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199-e267. doi:10.1161/CIR.0000000000000041
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/ AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines Circulation. 2024;149(1):e1- e156. doi:10.1161/CIR.0000000000001193
- US Preventive Services Task Force; Davidson KW, Barry MJ, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force recommendation statement. JAMA. 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983
- McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379(16):1519-1528. doi:10.1056/NEJMoa1803955
- Barry MJ, Wolff TA, Pbert L, et al. Putting evidence into practice: an update on the US Preventive Services Task Force methods for developing recommendations for preventive services. Ann Fam Med. 2023;21(2):165-171. doi:10.1370/afm.2946
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/ AHA Guideline on the Primary Prevention of Cardiovascular Disease: A report of the American College of Cardiology/ American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: Standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S179-S218. doi:10.2337/dc24-S010
- Ong SY, Chui P, Bhargava A, Justice A, Hauser RG. Estimating aspirin overuse for primary prevention of atherosclerotic cardiovascular disease (from a nationwide healthcare system). Am J Cardiol. 2020;137:25-30. doi:10.1016/j.amjcard.2020.09.042
- Weiss AJ, Jiang HJ. Overview of clinical conditions with frequent and costly hospital readmissions by payer, 2018. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); July 20, 2021.
- Krishnaswami A, Steinman MA, Goyal P, et al. Deprescribing in older adults with cardiovascular disease. J Am Coll Cardiol. 2019;73(20):2584-2595. doi:10.1016/j.jacc.2019.03.467
- Association of American Medical Colleges. The complexities of physician supply and demand: projections from 2019 to 2034. Accessed March 17, 2024. https://www.aamc.org/media/54681/download
- US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1108.07(1): General pharmacy service requirements. November 28, 2022. Accessed March 17, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=10045
- US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1108.11(3): Clinical pharmacy services. July 1, 2015. Accessed March 17, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3120
- US Department of Veterans Affairs. Clinical pharmacist practitioner (CPP) to improve access to and quality of care August 2021. August 2021. Accessed May 19, 2023. https://www.pbm.va.gov/PBM/CPPO/Documents/ExternalFactSheet_OptimizingtheCPPToImproveAccess_508.pdf
- Ammerman CA, Simpkins BA, Warman N, Downs TN. Potentially inappropriate medications in older adults: Deprescribing with a clinical pharmacist. J Am Geriatr Soc. 2019;67(1):115-118. doi:10.1111/jgs.15623
- Rothbauer K, Siodlak M, Dreischmeier E, Ranola TS, Welch L. Evaluation of a pharmacist-driven ambulatory aspirin deprescribing protocol. Fed Pract. 2022;39(suppl 5):S37- S41a. doi:10.12788/fp.0294
- US Department of Veterans Affairs. VIONE changes the way VA handles prescriptions. January 25, 2020. Accessed May 21, 2023. https://news.va.gov/70709/vione-changes-way-va-handles-prescriptions/
- 2023 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052- 2081. doi:10.1111/jgs.18372
- O’Mahony D, Cherubini A, Guiteras AR, et al. STOPP/ START criteria for potentially inappropriate prescribing in older people: version 3. Eur Geriatr Med. 2023;14(4):625- 632. doi:10.1007/s41999-023-00777-y
- Draeger C, Lodhi F, Geissinger N, Larson T, Griesbach S. Interdisciplinary deprescribing of aspirin through prescriber education and provision of patient-specific recommendations. WMJ. 2022;121(3):220-225
- de Lusignan S, Hinton W, Seidu S, et al. Dashboards to reduce inappropriate prescribing of metformin and aspirin: A quality assurance programme in a primary care sentinel network. Prim Care Diabetes. 2021;15(6):1075-1079. doi:10.1016/j.pcd.2021.06.003
- Nelson MW, Downs TN, Puglisi GM, Simpkins BA, Collier AS. Use of a deprescribing tool in an interdisciplinary primary-care patient-aligned care team. Sr Care Pharm. 2022;37(1):34-43. doi:10.4140/TCP.n.2022.34
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199-e267. doi:10.1161/CIR.0000000000000041
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/ AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines Circulation. 2024;149(1):e1- e156. doi:10.1161/CIR.0000000000001193
Pharmacist-Led Deprescribing of Aspirin for Primary Prevention of Cardiovascular Disease Among Geriatric Veterans
Pharmacist-Led Deprescribing of Aspirin for Primary Prevention of Cardiovascular Disease Among Geriatric Veterans
Indeterminate Cell Histiocytosis and a Review of Current Treatment
Indeterminate Cell Histiocytosis and a Review of Current Treatment
To the Editor:
Indeterminate cell histiocytosis (ICH) is a rare neoplastic dendritic cell disorder with a poorly understood histogenesis and pathogenesis.1 The clinical manifestation of ICH is broad and can include isolated or multiple papules or nodules on the face, neck, trunk, arms, or legs. Our case demonstrates a rare occurrence of ICH that initially was misdiagnosed and highlights the use of cobimetinib, a MEK inhibitor, as a potential new therapeutic option for ICH.
A 74-year-old man with a history of type 2 diabetes mellitus presented for evaluation of a progressive pruritic rash of approximately 5 years’ duration. The eruption previously had been diagnosed as Langerhans cell histiocytosis. It started on the chest and spread to the face, neck, trunk, and arms. The patient denied systemic symptoms and had no known history of malignancy.
Physical examination revealed pink to orange smooth papules, nodules, and small plaques on the ears, cheeks, trunk, neck, and arms (Figure 1). Baseline laboratory results showed a normal complete blood count and comprehensive metabolic panel, elevated lactate dehydrogenase and erythrocyte sedimentation rate, and hyperlipidemia. Serology for hepatitis B and C was negative. Bone marrow biopsy was normal, and positron emission tomography/ computed tomography demonstrated no evidence of extracutaneous disease. A punch biopsy of a lesion on the left forearm revealed epithelioid histiocytic proliferation in the dermis extending into the subcutis with a background infiltrate of small lymphocytes. Immunohistochemistry was positive for CD1a and CD56 and was variably positive for CD4 but negative for CD163, CD68, S100, Langerin, cyclin D1, myeloperoxidase, CD21, and CD23. No mutation was detected in BRAF codon 600. Given the negative Langerin stain, these findings were compatible with a diagnosis of ICH. After considering the lack of standard treatment options as well as the recent approval of cobimetinib for histiocytic disorders, we initiated treatment with cobimetinib at the standard dose of 60 mg daily for 21 days followed by a 7-day break.

One month into treatment, the patient’s lesions were less erythematous, and he reported improvement in pruritus. Two months into treatment, there was continued improvement in cutaneous symptoms with flattening of the lesions on the chest and back. At this time, the patient developed edema of the face and ears (Figure 2) and reported weakness, blurred vision, and decreased appetite. He was advised to take an additional 7-day treatment break before resuming cobimetinib at a decreased dose of 40 mg daily. The patient returned to the clinic 1 month later with improved systemic symptoms and continued flattening of the lesions. Five months into treatment, the lesions had continued to improve with complete resolution of the facial plaques (Figure 3).


Indeterminate cell histiocytosis is a rarely diagnosed condition characterized by the proliferation of indeterminate histiocytes that morphologically and immunophenotypically resemble Langerhans cells but lack their characteristic Birbeck granules.2 There is no standard treatment for ICH, but previous reports have described improvement with a variety of treatment options including methotrexate,3,4 UVB phototherapy,5 and topical delgocitinib 0.5%.6
Because histiocytic disorders are characterized by mutations in the mitogen-activated protein kinase pathway, it is possible that they would be responsive to MEK inhibition. Cobimetinib, a MEK inhibitor initially approved to treat metastatic melanoma, was approved by the US Food and Drug Administration to treat histiocytic disorders in October 2022.7 The approval followed the release of data from a phase 2 trial of cobimetinib in 18 adults with various histiocytic disorders, which demonstrated an 89% (16/18) overall response rate with 94% (17/18) of patients remaining progression free at 1 year.8 While cobimetinib has not specifically been studied in ICH, given the high response rate in histiocytic disorders and the lack of standard treatment options for ICH, the decision was made to initiate treatment with cobimetinib in our patient. Based on the observed improvement in our patient, we propose cobimetinib as a treatment option for patients with cutaneous ICH and recommend additional studies to confirm its safety and efficacy in patients with this disorder.
- Bakry OA, Samaka RM, Kandil MA, et al. Indeterminate cell histiocytosis with naïve cells. Rare Tumors. 2013;5:e13. doi:10.4081 /rt.2013.e13
- Manente L, Cotellessa C, Schmitt I, et al. Indeterminate cell histiocytosis: a rare histiocytic disorder. Am J Dermatopathol. 1997; 19:276-283. doi:10.1097/00000372-199706000-00014
- Lie E, Jedrych J, Sweren R, et al. Generalized indeterminate cell histiocytosis successfully treated with methotrexate. JAAD Case Rep. 2022;25:93-96. doi:10.1016/j.jdcr.2022.05.027
- Fournier J, Ingraffea A, Pedvis-Leftick A. Successful treatment of indeterminate cell histiocytosis with low-dose methotrexate. J Dermatol. 2011;38:937-939. doi:10.1111/j.1346-8138.2010.01148.x
- Logemann N, Thomas B, Yetto T. Indeterminate cell histiocytosis successfully treated with narrowband UVB. Dermatol Online J. 2013;19:20031. doi:10.5070/D31910020031
- Fujimoto RFT, Miura H, Takata M, et al. Indeterminate cell histiocytosis treated with 0.5% delgocitinib ointment. Br J Dermatol. 2023;188:E39. doi:10.1093/bjd/ljad029
- Diamond EL, Durham B, Dogan A, et al. Phase 2 trial of single-agent cobimetinib for adults with histiocytic neoplasms. Blood. 2023;142:1812. doi:10.1182/blood-2023-187508
- Diamond EL, Durham BH, Ulaner GA, et al. Efficacy of MEK inhibition in patients with histiocytic neoplasms. Nature. 2019;567:521-524. doi:10.1038/s41586-019-1012-y
To the Editor:
Indeterminate cell histiocytosis (ICH) is a rare neoplastic dendritic cell disorder with a poorly understood histogenesis and pathogenesis.1 The clinical manifestation of ICH is broad and can include isolated or multiple papules or nodules on the face, neck, trunk, arms, or legs. Our case demonstrates a rare occurrence of ICH that initially was misdiagnosed and highlights the use of cobimetinib, a MEK inhibitor, as a potential new therapeutic option for ICH.
A 74-year-old man with a history of type 2 diabetes mellitus presented for evaluation of a progressive pruritic rash of approximately 5 years’ duration. The eruption previously had been diagnosed as Langerhans cell histiocytosis. It started on the chest and spread to the face, neck, trunk, and arms. The patient denied systemic symptoms and had no known history of malignancy.
Physical examination revealed pink to orange smooth papules, nodules, and small plaques on the ears, cheeks, trunk, neck, and arms (Figure 1). Baseline laboratory results showed a normal complete blood count and comprehensive metabolic panel, elevated lactate dehydrogenase and erythrocyte sedimentation rate, and hyperlipidemia. Serology for hepatitis B and C was negative. Bone marrow biopsy was normal, and positron emission tomography/ computed tomography demonstrated no evidence of extracutaneous disease. A punch biopsy of a lesion on the left forearm revealed epithelioid histiocytic proliferation in the dermis extending into the subcutis with a background infiltrate of small lymphocytes. Immunohistochemistry was positive for CD1a and CD56 and was variably positive for CD4 but negative for CD163, CD68, S100, Langerin, cyclin D1, myeloperoxidase, CD21, and CD23. No mutation was detected in BRAF codon 600. Given the negative Langerin stain, these findings were compatible with a diagnosis of ICH. After considering the lack of standard treatment options as well as the recent approval of cobimetinib for histiocytic disorders, we initiated treatment with cobimetinib at the standard dose of 60 mg daily for 21 days followed by a 7-day break.

One month into treatment, the patient’s lesions were less erythematous, and he reported improvement in pruritus. Two months into treatment, there was continued improvement in cutaneous symptoms with flattening of the lesions on the chest and back. At this time, the patient developed edema of the face and ears (Figure 2) and reported weakness, blurred vision, and decreased appetite. He was advised to take an additional 7-day treatment break before resuming cobimetinib at a decreased dose of 40 mg daily. The patient returned to the clinic 1 month later with improved systemic symptoms and continued flattening of the lesions. Five months into treatment, the lesions had continued to improve with complete resolution of the facial plaques (Figure 3).


Indeterminate cell histiocytosis is a rarely diagnosed condition characterized by the proliferation of indeterminate histiocytes that morphologically and immunophenotypically resemble Langerhans cells but lack their characteristic Birbeck granules.2 There is no standard treatment for ICH, but previous reports have described improvement with a variety of treatment options including methotrexate,3,4 UVB phototherapy,5 and topical delgocitinib 0.5%.6
Because histiocytic disorders are characterized by mutations in the mitogen-activated protein kinase pathway, it is possible that they would be responsive to MEK inhibition. Cobimetinib, a MEK inhibitor initially approved to treat metastatic melanoma, was approved by the US Food and Drug Administration to treat histiocytic disorders in October 2022.7 The approval followed the release of data from a phase 2 trial of cobimetinib in 18 adults with various histiocytic disorders, which demonstrated an 89% (16/18) overall response rate with 94% (17/18) of patients remaining progression free at 1 year.8 While cobimetinib has not specifically been studied in ICH, given the high response rate in histiocytic disorders and the lack of standard treatment options for ICH, the decision was made to initiate treatment with cobimetinib in our patient. Based on the observed improvement in our patient, we propose cobimetinib as a treatment option for patients with cutaneous ICH and recommend additional studies to confirm its safety and efficacy in patients with this disorder.
To the Editor:
Indeterminate cell histiocytosis (ICH) is a rare neoplastic dendritic cell disorder with a poorly understood histogenesis and pathogenesis.1 The clinical manifestation of ICH is broad and can include isolated or multiple papules or nodules on the face, neck, trunk, arms, or legs. Our case demonstrates a rare occurrence of ICH that initially was misdiagnosed and highlights the use of cobimetinib, a MEK inhibitor, as a potential new therapeutic option for ICH.
A 74-year-old man with a history of type 2 diabetes mellitus presented for evaluation of a progressive pruritic rash of approximately 5 years’ duration. The eruption previously had been diagnosed as Langerhans cell histiocytosis. It started on the chest and spread to the face, neck, trunk, and arms. The patient denied systemic symptoms and had no known history of malignancy.
Physical examination revealed pink to orange smooth papules, nodules, and small plaques on the ears, cheeks, trunk, neck, and arms (Figure 1). Baseline laboratory results showed a normal complete blood count and comprehensive metabolic panel, elevated lactate dehydrogenase and erythrocyte sedimentation rate, and hyperlipidemia. Serology for hepatitis B and C was negative. Bone marrow biopsy was normal, and positron emission tomography/ computed tomography demonstrated no evidence of extracutaneous disease. A punch biopsy of a lesion on the left forearm revealed epithelioid histiocytic proliferation in the dermis extending into the subcutis with a background infiltrate of small lymphocytes. Immunohistochemistry was positive for CD1a and CD56 and was variably positive for CD4 but negative for CD163, CD68, S100, Langerin, cyclin D1, myeloperoxidase, CD21, and CD23. No mutation was detected in BRAF codon 600. Given the negative Langerin stain, these findings were compatible with a diagnosis of ICH. After considering the lack of standard treatment options as well as the recent approval of cobimetinib for histiocytic disorders, we initiated treatment with cobimetinib at the standard dose of 60 mg daily for 21 days followed by a 7-day break.

One month into treatment, the patient’s lesions were less erythematous, and he reported improvement in pruritus. Two months into treatment, there was continued improvement in cutaneous symptoms with flattening of the lesions on the chest and back. At this time, the patient developed edema of the face and ears (Figure 2) and reported weakness, blurred vision, and decreased appetite. He was advised to take an additional 7-day treatment break before resuming cobimetinib at a decreased dose of 40 mg daily. The patient returned to the clinic 1 month later with improved systemic symptoms and continued flattening of the lesions. Five months into treatment, the lesions had continued to improve with complete resolution of the facial plaques (Figure 3).


Indeterminate cell histiocytosis is a rarely diagnosed condition characterized by the proliferation of indeterminate histiocytes that morphologically and immunophenotypically resemble Langerhans cells but lack their characteristic Birbeck granules.2 There is no standard treatment for ICH, but previous reports have described improvement with a variety of treatment options including methotrexate,3,4 UVB phototherapy,5 and topical delgocitinib 0.5%.6
Because histiocytic disorders are characterized by mutations in the mitogen-activated protein kinase pathway, it is possible that they would be responsive to MEK inhibition. Cobimetinib, a MEK inhibitor initially approved to treat metastatic melanoma, was approved by the US Food and Drug Administration to treat histiocytic disorders in October 2022.7 The approval followed the release of data from a phase 2 trial of cobimetinib in 18 adults with various histiocytic disorders, which demonstrated an 89% (16/18) overall response rate with 94% (17/18) of patients remaining progression free at 1 year.8 While cobimetinib has not specifically been studied in ICH, given the high response rate in histiocytic disorders and the lack of standard treatment options for ICH, the decision was made to initiate treatment with cobimetinib in our patient. Based on the observed improvement in our patient, we propose cobimetinib as a treatment option for patients with cutaneous ICH and recommend additional studies to confirm its safety and efficacy in patients with this disorder.
- Bakry OA, Samaka RM, Kandil MA, et al. Indeterminate cell histiocytosis with naïve cells. Rare Tumors. 2013;5:e13. doi:10.4081 /rt.2013.e13
- Manente L, Cotellessa C, Schmitt I, et al. Indeterminate cell histiocytosis: a rare histiocytic disorder. Am J Dermatopathol. 1997; 19:276-283. doi:10.1097/00000372-199706000-00014
- Lie E, Jedrych J, Sweren R, et al. Generalized indeterminate cell histiocytosis successfully treated with methotrexate. JAAD Case Rep. 2022;25:93-96. doi:10.1016/j.jdcr.2022.05.027
- Fournier J, Ingraffea A, Pedvis-Leftick A. Successful treatment of indeterminate cell histiocytosis with low-dose methotrexate. J Dermatol. 2011;38:937-939. doi:10.1111/j.1346-8138.2010.01148.x
- Logemann N, Thomas B, Yetto T. Indeterminate cell histiocytosis successfully treated with narrowband UVB. Dermatol Online J. 2013;19:20031. doi:10.5070/D31910020031
- Fujimoto RFT, Miura H, Takata M, et al. Indeterminate cell histiocytosis treated with 0.5% delgocitinib ointment. Br J Dermatol. 2023;188:E39. doi:10.1093/bjd/ljad029
- Diamond EL, Durham B, Dogan A, et al. Phase 2 trial of single-agent cobimetinib for adults with histiocytic neoplasms. Blood. 2023;142:1812. doi:10.1182/blood-2023-187508
- Diamond EL, Durham BH, Ulaner GA, et al. Efficacy of MEK inhibition in patients with histiocytic neoplasms. Nature. 2019;567:521-524. doi:10.1038/s41586-019-1012-y
- Bakry OA, Samaka RM, Kandil MA, et al. Indeterminate cell histiocytosis with naïve cells. Rare Tumors. 2013;5:e13. doi:10.4081 /rt.2013.e13
- Manente L, Cotellessa C, Schmitt I, et al. Indeterminate cell histiocytosis: a rare histiocytic disorder. Am J Dermatopathol. 1997; 19:276-283. doi:10.1097/00000372-199706000-00014
- Lie E, Jedrych J, Sweren R, et al. Generalized indeterminate cell histiocytosis successfully treated with methotrexate. JAAD Case Rep. 2022;25:93-96. doi:10.1016/j.jdcr.2022.05.027
- Fournier J, Ingraffea A, Pedvis-Leftick A. Successful treatment of indeterminate cell histiocytosis with low-dose methotrexate. J Dermatol. 2011;38:937-939. doi:10.1111/j.1346-8138.2010.01148.x
- Logemann N, Thomas B, Yetto T. Indeterminate cell histiocytosis successfully treated with narrowband UVB. Dermatol Online J. 2013;19:20031. doi:10.5070/D31910020031
- Fujimoto RFT, Miura H, Takata M, et al. Indeterminate cell histiocytosis treated with 0.5% delgocitinib ointment. Br J Dermatol. 2023;188:E39. doi:10.1093/bjd/ljad029
- Diamond EL, Durham B, Dogan A, et al. Phase 2 trial of single-agent cobimetinib for adults with histiocytic neoplasms. Blood. 2023;142:1812. doi:10.1182/blood-2023-187508
- Diamond EL, Durham BH, Ulaner GA, et al. Efficacy of MEK inhibition in patients with histiocytic neoplasms. Nature. 2019;567:521-524. doi:10.1038/s41586-019-1012-y
Indeterminate Cell Histiocytosis and a Review of Current Treatment
Indeterminate Cell Histiocytosis and a Review of Current Treatment
PRACTICE POINTS
- Indeterminate cell histiocytosis (ICH) is a rare neoplastic dendritic cell disorder that can manifest as isolated or multiple papules or nodules on the face, neck, trunk, arms, or legs.
- Although there is no standard treatment for ICH, histiocytic disorders are characterized by mutations in the mitogen-activated protein kinase pathway and may be responsive to MEK inhibition.
- Cobimetinib, a MEK inhibitor initially approved to treat metastatic melanoma, was approved by the US Food and Drug Administration to treat histiocytic disorders in October 2022.
Bimekizumab for Hidradenitis Suppurativa: Pathophysiology and Promising Interventions
Bimekizumab for Hidradenitis Suppurativa: Pathophysiology and Promising Interventions
Hidradenitis suppurativa (HS) is a debilitating dermatologic condition characterized by recurrent episodes of neutrophilic inflammation affecting the apocrine and pilosebaceous units that most commonly affects individuals aged 20 to 40 years. Originating from the hair follicles, inflammation initiates the formation of painful nodules and abscesses that can progress to sinus tracts or fistulas accompanied by the development of extensive scarring, exquisite pain, and malodorous drainage.1 The lesions most commonly occur in intertriginous zones as well as areas rich in apocrine glands. The distinctive and sometimes irreversible clinical features of HS profoundly influence patients’ well-being and have lasting social, personal, and emotional impacts on their lives.2
Bimekizumab is a monoclonal antibody that specifically targets IL-17A and IL-17F, aiming to inhibit the downstream effects responsible for the chronic inflammation and tissue damage characteristic of HS.3 In HS lesions, IL-17 cytokines produced by T helper 17 (Th17) cells stimulate the production of chemokines (such as CC motif chemokine ligand 20) and neutrophil-attracting chemokines (including C-X-C motif chemokine ligands 1 and 8), cytokines (such as granulocyte colony-stimulating factor and IL-19), and epidermal antimicrobial proteins.1,2 This cascade results in the chemotaxis of monocytes and neutrophils in the skin, recruiting additional Th17 and myeloid cells and further amplifying IL-17 production.1
Bimekizumab’s mechanism of action strategically disrupts this feed-forward inflammatory loop, decreasing the transcription of neutrophil-attracting chemokines, IL-19, and epidermal antimicrobial proteins (Figure).1,2 This leads to diminished recruitment of Th17 cells and inhibits the chemotaxis of monocytes and neutrophils in the skin, effectively addressing the chronic inflammation and tissue damage characteristic of HS.
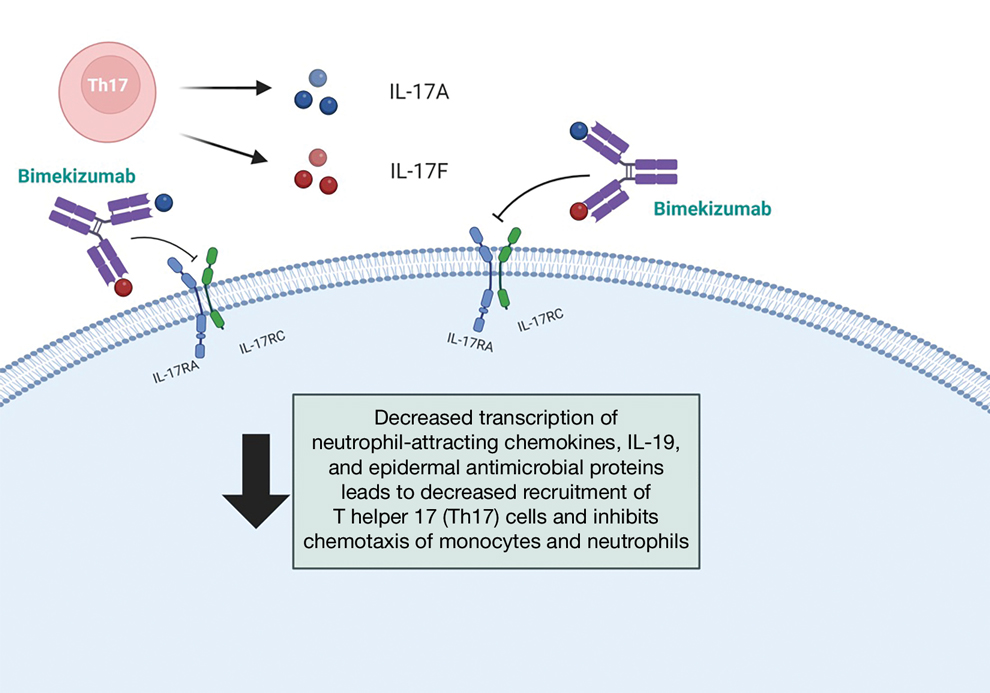
We present a comprehensive review of the current standards of care, the underlying molecular pathophysiology of HS, and evaluation of the efficacy and safety of bimekizumab.
Evaluating HS Severity
The Hurley staging system provides a valuable framework for evaluating the severity of HS based on lesion characteristics. Stage I is characterized by abscess formation without tracts or scars. Stage II is characterized by recurrent abscesses with sinus tracts and scarring. Stage III is characterized by diffuse involvement, multiple interconnected sinus tracts, and abscesses across an entire area, leaving little to no uninvolved skin.4
Treatment strategies for HS vary based on Hurley staging (eTable).5-11 For mild cases (stage I), topical and intralesional therapies are common, while moderate to severe cases (stages II and III) may require extensive surgical approaches or systemic drugs such as antibiotics, hormonal therapies, retinoids, or immunosuppressive/biologic agents.2
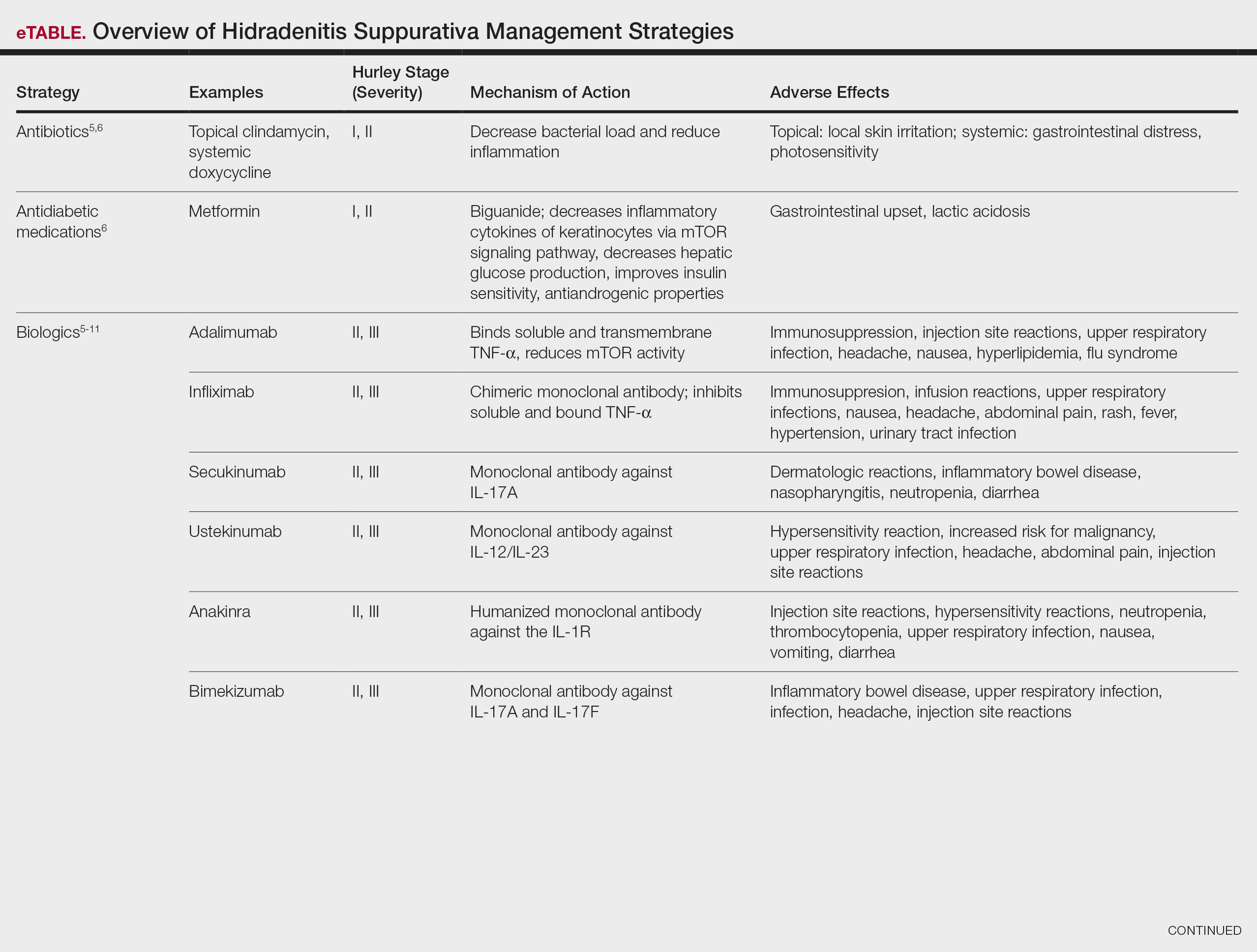
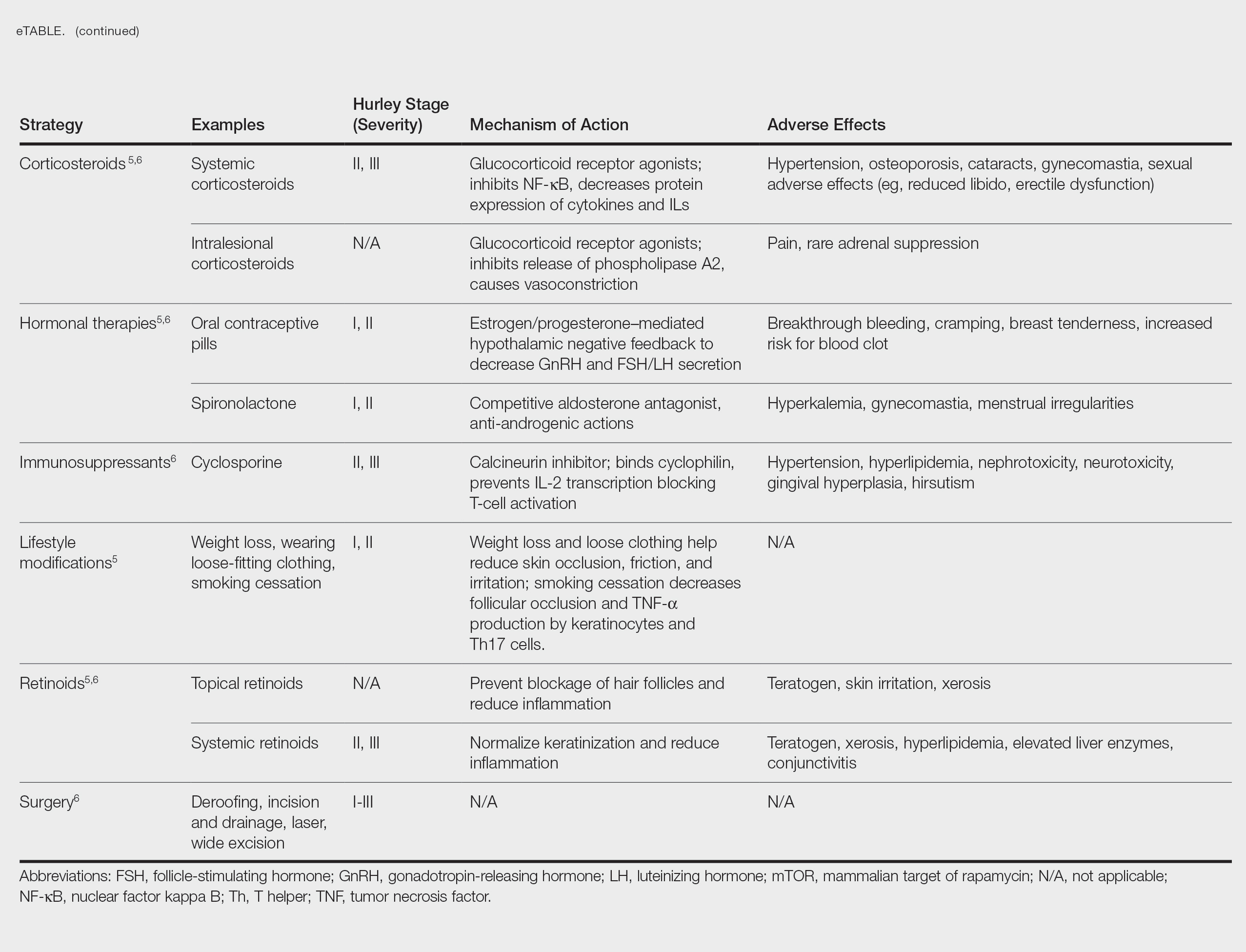
Adalimumab, an anti–tumor necrosis factor (TNF) α monoclonal antibody, was the first US Food and Drug Administration (FDA)–approved biologic for HS. Secukinumab, a monoclonal antibody against IL-17A, subsequently was approved by the FDA for moderate to severe HS.12 Off-label use of biologics including infliximab and ustekinumab expands the available treatment options for HS. In one Phase II randomized clinical trial (RCT), infliximab showed efficacy in reducing Hidradenitis Suppurativa Severity Index scores, with 26.7% (4/15) of patients achieving a 50% or greater reduction compared to placebo, although this was not statistically significant. Similarly, ustekinumab demonstrated promising results, with 47.1% (8/17) of patients achieving Hidradenitis Suppurativa Clinical Response (HiSCR) at week 40.2 This multifaceted approach aims to address the varying degrees of severity and optimize outcomes for individuals with HS.
Molecular Pathophysiology of HS
The pathogenesis of HS is multifactorial, involving a complex interplay of genetic, environmental, and behavioral factors.2 Approximately 33% to 40% of patients with HS worldwide report a first-degree relative with the condition, indicating a hereditary element with an autosomal-dominant transmission pattern and highlighting the global relevance of genetic factors in HS.4 Hidradenitis suppurativa is highly prevalent in individuals with obesity, likely due to increased intertriginous surface area, skin friction, sweat production, and hormonal changes in these patients. Smoking also commonly is associated with HS, with nicotine potentially contributing to increased follicular plugging.1 Hormonal influences also play a role, as evidenced by a greater prevalence of HS in females, disease onset typically occurring between puberty and menopause, and symptomatic fluctuations correlating with menstrual cycles and exogenous hormones.4
Altered infundibular keratinization with subsequent hyperkeratosis/occlusion and innate immune pathway activation are key events leading to development of HS.1 These events are mediated by release of pathogen- and danger-associated molecular patterns, leading to inflammasome-mediated IL-1α release, followed by downstream cytokine release.2 Elevated levels of TNF-α, IL-1Β, IL-10, IL-17, and particularly IL-17A have been detected in HS lesional skin. The IL-17 family comprises multiple members, namely IL-17A, IL-17C, IL-17E, and IL17F. IL-17A and IL-17F often are co-expressed and secreted predominantly by a subset of CD4+ T helper cells, namely Th17 cells.2 IL-17 cytokines exert pro-inflammatory effects, influencing immune cell activity and contributing to skin inflammation, particularly in HS.
Given the pivotal role of IL-17 in the pathogenesis of HS, the exploration of IL-17–targeted agents has become a focal point in clinical research. Bimekizumab, a novel IL-17 inhibitor, has emerged as a promising candidate, offering a potential breakthrough in the treatment landscape for individuals affected by HS.
Bimekizumab for HS Management
A phase II, double-blind, placebo-controlled RCT included 90 patients with moderate to severe HS (age range, 18-70 years) who were randomly assigned in a 2:1:1 ratio to receive either bimekizumab 320 mg every 2 weeks (with a 640-mg loading dose at baseline)(n=46), placebo (n=21), or adalimumab 40 mg once weekly from week 4 onward (following an initial 160-mg loading dose at baseline and 80-mg dose at week 2)(n=21). The study included a 12-week treatment period followed by a 20-week safety follow-up period. The primary endpoint was the achievement of HiSCR50—defined as a reduction of at least 50% nodules, coupled with no increase in the number of abscesses or draining fistulas relative to baseline—at week 12. Additionally, the study assessed the number of patients who achieved a modified HiSCR with 75% reduction (HiSCR75) of combined abscess and inflammatory nodule count or a modified HiSCR with 90% reduction (HiSCR90). At week 12, the modeled response rates were estimated using a Bayesian logistic regression model. For HiSCR50, the modeled rate for bimekizumab was 57.3%, with an observed rate of 62.5% (25/40), compared to a modeled rate of 26.1% for placebo (observed rate, 27.8% [5/18]). The posterior probability of superiority for bimekizumab over placebo was 0.998. By week 12, bimekizumab-treated patients achieved modeled HiSCR75 and HiSCR90 rates of 46.0% and 32.0%, respectively, with observed rates of 50.0% (20/40) for HiSCR75 and 35.0% (14/40) for HiSCR90. In comparison, placebo-treated patients achieved modeled HiSCR75 and HiSCR90 rates of 10.0% and 0%, respectively, with observed rates of 11.1% (2/18) for HiSCR75 and 0% (0/18) for HiSCR90. Adalimumab-treated participants demonstrated intermediate results, achieving modeled HiSCR75 and HiSCR90 rates of 35.0% and 15.0%, respectively, with observed rates of 38.88% (7/18) for HiSCR75 and 16.66% (3/18) for HiSCR90.7
Bimekizumab was effective in the treatment of moderate to severe HS with comparable results to adalimumab.7 The incidence of treatment-emergent adverse events was similar across treatment arms (bimekizumab, 69.6% [32/46]; placebo, 61.9% [13/21]; adalimumab, 71.4% [15/21]). The most common treatment-emergent adverse events in the biologic treatment arms were infections (43.5% [20/46] in the bimekizumab group and 42.9% [9/21] in the adalimumab group), skin and subcutaneous tissue disorders (28.3% [13/46] in the bimekizumab group and 42.9% [9/21] in the adalimumab group), and general disorders/administration site conditions (21.7% [10/46] in the bimekizumab group and 23.8% [5/21] in the adalimumab group). Serious adverse events occurred in 4.3% (2/46) of patients in the bimekizumab group, 9.5% (2/21) of patients in the placebo group, and 4.8% (1/21) of patients in the adalimumab group. Serious adverse events that required hospitalization were due to anemia and empyema in the bimekizumab group; worsening HS in the adalimumab group; and myocardial infarction, hypoesthesia, headache, and dizziness in the placebo group. No deaths occurred in this study. Overall, bimekizumab was well tolerated, and discontinuation rates were low across all arms. The primary reason for discontinuation was withdrawal of consent (not due to an adverse event) or loss to follow-up.7
Two completed 48-week phase III RCTs, BE HEARD I and BE HEARD II, evaluated the efficacy and safety of bimekizumab in patients with moderate to severe HS.13 In both trials, 2 bimekizumab dosing regimens (320 mg every 2 weeks and 320 mg every 4 weeks) were compared with placebo during the 16-week initial and 32-week maintenance treatment periods. The primary endpoint of week 16 was achieved by 47.8% (138/289) and 51.9% (151/291) of patients receiving bimekizumab every 2 weeks in BE HEARD I (n=505) and BE HEARD II (n=509), respectively, compared with 29.2% (21/72) and 32.4% (24/74) of the placebo group. The bimekizumab 320 mg every 4 weeks dosing regimen met the primary endpoint only in BE HEARD II, with 53.5% (77/144) of patients achieving HiSCR50 compared to 32.4% (24/74) with placebo (P=0.0038).13 Both trials met the key secondary endpoint of HiSCR75 at week 16 for bimekizumab 320 mg every 2 weeks vs placebo. In BE HEARD I, 33.6% (97/289) of patients receiving bimekizumab achieved HiSCR75 versus 18.1% (13/72) taking placebo. In BE HEARD II, 35.7% (104/291) of patients receiving bimekizumab achieved HiSCR75 vs 16.2% (12/74) taking placebo. Responses were maintained or increased through week 48 in both trials. The most common treatment-emergent adverse events through week 48 were worsening HS, COVID-19 infection, diarrhea, oral candidiasis, and headache.13
A smaller scale case series investigated the use of bimekizumab in 4 female patients aged 20 to 62 years with moderate to severe HS and concomitant plaque or inverse psoriasis.8 A monthly loading dose of 320 mg was given during weeks 0 to 12 followed by a maintenance dose of 320 mg administered every 8 weeks. The International Hidradenitis Suppurativa Score System, visual analogue scale, and Dermatology Life Quality Index were used to assess the effectiveness of therapy by comparing scores before and after 4 and 16 weeks of treatment. A reduction of pain and improvement of HS lesions was observed in 3 (75.0%) patients after the first dosage of bimekizumab, with completed remission of HS by week 16. The fourth patient (25.0%) experienced substantial improvement in all measures, although not complete remission. All 4 patients remained on bimekizumab, and no adverse effects were reported.8
A meta-analysis evaluated 16 RCTs of 9 biologics and 3 small-molecule inhibitors in 2076 patients with HS.10 Secukinumab was not included in this meta-analysis. Only adalimumab (risk ratio, 1.77; 95% CI, 1.44-2.17) and bimekizumab (risk ratio, 2.25; 95% CI, 1.03-4.92) were superior to placebo in achieving HiSCR response at weeks 12 to 16 in 5 RCTs and 1 RCT, respectively; however, no statistically significant differences were noted between adalimumab and bimekizumab (P=.56). This analysis concluded that adalimumab and bimekizumab are the only 2 biologics efficacious in reaching HiSCR and consistently improved both disease severity and quality of life in patients with HS with an acceptable safety profile.10 Furthermore, these biologics had no increase in serious adverse events when compared to placebo.10
A network meta-analysis of 10 clinical trials involving more than 900 total participants evaluated nonsurgical therapies for HS. The analysis used Surface Under the Cumulative Ranking curve (SUCRA) values to estimate the efficacy of treatments in achieving clinical response according to HiSCR criteria. These values range from 0% to 100%, with 100% representing the best possible ranking for efficacy. Bimekizumab showed the highest estimated efficacy with a SUCRA value of 67%, followed by adalimumab (64%), anakinra (49%), and placebo (19%). These SUCRA values indicate the relative ranking of treatments, with higher values suggesting greater likelihood of achieving clinical response, rather than representing the actual percentage of patients achieving HiSCR. Bimekizumab was found to be more efficacious than placebo (P<.05).14
Building on the initial evidence of bimekizumab’s efficacy, BE HEARD I and BE HEARD II addressed some limitations of prior studies, including small sample sizes and insufficient stratification.13 Notably, stratification by baseline Hurley stage severity (ie, the most severe stage of disease assigned at baseline) and baseline systemic antibiotic use helped mitigate bias and ensured a more robust assessment of treatment efficacy; however, certain limitations persist. While the trials demonstrated rapid and clinically meaningful responses maintained up to 48 weeks, longer-term data beyond this period are limited, leaving gaps in understanding the durability of treatment effects over years. Additionally, despite appropriate stratification, the generalizability of the findings to broader patient populations remains unclear, as trial participants may not fully represent the diversity of patients seen in clinical practice.13
Future research is needed to address these limitations. The use of validated HS biomarkers as endpoints could enhance the ability to evaluate biologic efficacy and identify predictors of response. Comparative studies with other biologics also are warranted to establish the relative efficacy of bimekizumab within the growing therapeutic landscape for HS. Finally, real-world evidence from larger and more diverse populations will be critical to confirm the trial findings and assess long-term safety and effectiveness in routine clinical practice.13
Conclusion
The existing literature and recent phase III RCTs, BE HEARD I and BE HEARD II, demonstrate that bimekizumab is an effective treatment for moderate to severe HS, with robust efficacy according to HiSCR scores and sustained responses through 48 weeks. These trials addressed some prior limitations, including small sample sizes and insufficient stratification, providing a more comprehensive evaluation of bimekizumab’s clinical impact. The safety profile of bimekizumab remains favorable, with low discontinuation rates and manageable adverse events, such as infection, gastrointestinal upset, headache, and injection-site reactions. Long-term efficacy and safety data beyond 48 weeks still are needed to fully establish its durability and impact in diverse populations. The recent FDA approval of bimekizumab for moderate to severe HS provides patients with a new treatment option, offering a more positive clinical outlook.
- Malvaso D, Calabrese L, Chiricozzi A, et al. IL-17 inhibition: a valid therapeutic strategy in the management of hidradenitis suppurativa. Pharmaceutics. 2023;15:2450. doi:10.3390 /pharmaceutics15102450
- Markota C¡agalj A, Marinovic´ B, Bukvic´ Mokos Z. New and emerging targeted therapies for hidradenitis suppurativa. Int J Mol Sci. 2022;23:3753. doi:10.3390/ijms23073753
- Zouboulis CC, Frew JW, Giamarellos-Bourboulis EJ, et al. Target molecules for future hidradenitis suppurativa treatment. Exp Dermatol. 2021;30 suppl 1:8-17. doi:10.1111/exd.14338
- Ballard K, Shuman VL. Hidradenitis suppurativa. StatPearls [Internet]. Updated May 6, 2024. Accessed December 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK534867/
- Rathod U, Prasad PN, Patel BM, et al. Hidradenitis suppurativa: a literature review comparing current therapeutic modalities. Cureus. 2023;15:E43695. doi:10.7759/cureus.43695
- Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: current and emerging treatments. J Am Acad Dermatol. 2020;82:1061-1082. doi:10.1016/j.jaad.2019.08.089
- Glatt S, Jemec GBE, Forman S, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, doubleblind, placebo-controlled randomized clinical trial. JAMA Dermatol. 2021;157:1279-1288. doi:10.1001/jamadermatol.2021.2905
- Molinelli E, Gambini D, Maurizi A, et al. Bimekizumab in hidradenitis suppurativa: a valid and effective emerging treatment. Clin Exp Dermatol. 2023;48:1272-1274. doi:10.1093/ced/llad229
- Martora F, Megna M, Battista T, et al. Adalimumab, ustekinumab, and secukinumab in the management of hidradenitis suppurativa: a review of the real-life experience. Clin Cosmet Investig Dermatol. 2023;16:135-148. doi:10.2147/CCID.S391356
- Huang CH, Huang IH, Tai CC, et al. Biologics and small molecule inhibitors for treating hidradenitis suppurativa: a systematic review and meta-analysis. Biomedicines. 2022;10:1303. doi:10.3390 /biomedicines10061303
- Ojeda Gómez A, Madero Velázquez L, Buendía Sanchez L, et al. Inflammatory bowel disease new-onset during secukinumab therapy: real-world data from a tertiary center. Rev Esp Enferm Dig. 2021;113: 858-859. doi:10.17235/reed.2021.8397/2021
- Martora F, Marasca C, Cacciapuoti S, et al. Secukinumab in hidradenitis suppurativa patients who failed adalimumab: a 52-week real-life study. Clin Cosmet Investig Dermatol. 2024;17:159-166. doi:10.2147 /CCID.S449367
- Kimball AB, Jemec GBE, Sayed CJ, et al. Efficacy and safety of bimekizumab in patients with moderate-to-severe hidradenitis suppurativa (BE HEARD I and BE HEARD II): two 48-week, randomised, double-blind, placebo-controlled, multicentre phase 3 trials. Lancet. 2024;403:2504-2519. doi:10.1016 /S0140-6736(24)00101-6
- Gupta AK, Shear NH, Piguet V, et al. Efficacy of non-surgical monotherapies for hidradenitis suppurativa: a systematic review and network meta-analyses of randomized trials. J Dermatolog Treat. 2022;33:2149-2160. doi:10.1080/09546634.2021.1927949
Hidradenitis suppurativa (HS) is a debilitating dermatologic condition characterized by recurrent episodes of neutrophilic inflammation affecting the apocrine and pilosebaceous units that most commonly affects individuals aged 20 to 40 years. Originating from the hair follicles, inflammation initiates the formation of painful nodules and abscesses that can progress to sinus tracts or fistulas accompanied by the development of extensive scarring, exquisite pain, and malodorous drainage.1 The lesions most commonly occur in intertriginous zones as well as areas rich in apocrine glands. The distinctive and sometimes irreversible clinical features of HS profoundly influence patients’ well-being and have lasting social, personal, and emotional impacts on their lives.2
Bimekizumab is a monoclonal antibody that specifically targets IL-17A and IL-17F, aiming to inhibit the downstream effects responsible for the chronic inflammation and tissue damage characteristic of HS.3 In HS lesions, IL-17 cytokines produced by T helper 17 (Th17) cells stimulate the production of chemokines (such as CC motif chemokine ligand 20) and neutrophil-attracting chemokines (including C-X-C motif chemokine ligands 1 and 8), cytokines (such as granulocyte colony-stimulating factor and IL-19), and epidermal antimicrobial proteins.1,2 This cascade results in the chemotaxis of monocytes and neutrophils in the skin, recruiting additional Th17 and myeloid cells and further amplifying IL-17 production.1
Bimekizumab’s mechanism of action strategically disrupts this feed-forward inflammatory loop, decreasing the transcription of neutrophil-attracting chemokines, IL-19, and epidermal antimicrobial proteins (Figure).1,2 This leads to diminished recruitment of Th17 cells and inhibits the chemotaxis of monocytes and neutrophils in the skin, effectively addressing the chronic inflammation and tissue damage characteristic of HS.

We present a comprehensive review of the current standards of care, the underlying molecular pathophysiology of HS, and evaluation of the efficacy and safety of bimekizumab.
Evaluating HS Severity
The Hurley staging system provides a valuable framework for evaluating the severity of HS based on lesion characteristics. Stage I is characterized by abscess formation without tracts or scars. Stage II is characterized by recurrent abscesses with sinus tracts and scarring. Stage III is characterized by diffuse involvement, multiple interconnected sinus tracts, and abscesses across an entire area, leaving little to no uninvolved skin.4
Treatment strategies for HS vary based on Hurley staging (eTable).5-11 For mild cases (stage I), topical and intralesional therapies are common, while moderate to severe cases (stages II and III) may require extensive surgical approaches or systemic drugs such as antibiotics, hormonal therapies, retinoids, or immunosuppressive/biologic agents.2


Adalimumab, an anti–tumor necrosis factor (TNF) α monoclonal antibody, was the first US Food and Drug Administration (FDA)–approved biologic for HS. Secukinumab, a monoclonal antibody against IL-17A, subsequently was approved by the FDA for moderate to severe HS.12 Off-label use of biologics including infliximab and ustekinumab expands the available treatment options for HS. In one Phase II randomized clinical trial (RCT), infliximab showed efficacy in reducing Hidradenitis Suppurativa Severity Index scores, with 26.7% (4/15) of patients achieving a 50% or greater reduction compared to placebo, although this was not statistically significant. Similarly, ustekinumab demonstrated promising results, with 47.1% (8/17) of patients achieving Hidradenitis Suppurativa Clinical Response (HiSCR) at week 40.2 This multifaceted approach aims to address the varying degrees of severity and optimize outcomes for individuals with HS.
Molecular Pathophysiology of HS
The pathogenesis of HS is multifactorial, involving a complex interplay of genetic, environmental, and behavioral factors.2 Approximately 33% to 40% of patients with HS worldwide report a first-degree relative with the condition, indicating a hereditary element with an autosomal-dominant transmission pattern and highlighting the global relevance of genetic factors in HS.4 Hidradenitis suppurativa is highly prevalent in individuals with obesity, likely due to increased intertriginous surface area, skin friction, sweat production, and hormonal changes in these patients. Smoking also commonly is associated with HS, with nicotine potentially contributing to increased follicular plugging.1 Hormonal influences also play a role, as evidenced by a greater prevalence of HS in females, disease onset typically occurring between puberty and menopause, and symptomatic fluctuations correlating with menstrual cycles and exogenous hormones.4
Altered infundibular keratinization with subsequent hyperkeratosis/occlusion and innate immune pathway activation are key events leading to development of HS.1 These events are mediated by release of pathogen- and danger-associated molecular patterns, leading to inflammasome-mediated IL-1α release, followed by downstream cytokine release.2 Elevated levels of TNF-α, IL-1Β, IL-10, IL-17, and particularly IL-17A have been detected in HS lesional skin. The IL-17 family comprises multiple members, namely IL-17A, IL-17C, IL-17E, and IL17F. IL-17A and IL-17F often are co-expressed and secreted predominantly by a subset of CD4+ T helper cells, namely Th17 cells.2 IL-17 cytokines exert pro-inflammatory effects, influencing immune cell activity and contributing to skin inflammation, particularly in HS.
Given the pivotal role of IL-17 in the pathogenesis of HS, the exploration of IL-17–targeted agents has become a focal point in clinical research. Bimekizumab, a novel IL-17 inhibitor, has emerged as a promising candidate, offering a potential breakthrough in the treatment landscape for individuals affected by HS.
Bimekizumab for HS Management
A phase II, double-blind, placebo-controlled RCT included 90 patients with moderate to severe HS (age range, 18-70 years) who were randomly assigned in a 2:1:1 ratio to receive either bimekizumab 320 mg every 2 weeks (with a 640-mg loading dose at baseline)(n=46), placebo (n=21), or adalimumab 40 mg once weekly from week 4 onward (following an initial 160-mg loading dose at baseline and 80-mg dose at week 2)(n=21). The study included a 12-week treatment period followed by a 20-week safety follow-up period. The primary endpoint was the achievement of HiSCR50—defined as a reduction of at least 50% nodules, coupled with no increase in the number of abscesses or draining fistulas relative to baseline—at week 12. Additionally, the study assessed the number of patients who achieved a modified HiSCR with 75% reduction (HiSCR75) of combined abscess and inflammatory nodule count or a modified HiSCR with 90% reduction (HiSCR90). At week 12, the modeled response rates were estimated using a Bayesian logistic regression model. For HiSCR50, the modeled rate for bimekizumab was 57.3%, with an observed rate of 62.5% (25/40), compared to a modeled rate of 26.1% for placebo (observed rate, 27.8% [5/18]). The posterior probability of superiority for bimekizumab over placebo was 0.998. By week 12, bimekizumab-treated patients achieved modeled HiSCR75 and HiSCR90 rates of 46.0% and 32.0%, respectively, with observed rates of 50.0% (20/40) for HiSCR75 and 35.0% (14/40) for HiSCR90. In comparison, placebo-treated patients achieved modeled HiSCR75 and HiSCR90 rates of 10.0% and 0%, respectively, with observed rates of 11.1% (2/18) for HiSCR75 and 0% (0/18) for HiSCR90. Adalimumab-treated participants demonstrated intermediate results, achieving modeled HiSCR75 and HiSCR90 rates of 35.0% and 15.0%, respectively, with observed rates of 38.88% (7/18) for HiSCR75 and 16.66% (3/18) for HiSCR90.7
Bimekizumab was effective in the treatment of moderate to severe HS with comparable results to adalimumab.7 The incidence of treatment-emergent adverse events was similar across treatment arms (bimekizumab, 69.6% [32/46]; placebo, 61.9% [13/21]; adalimumab, 71.4% [15/21]). The most common treatment-emergent adverse events in the biologic treatment arms were infections (43.5% [20/46] in the bimekizumab group and 42.9% [9/21] in the adalimumab group), skin and subcutaneous tissue disorders (28.3% [13/46] in the bimekizumab group and 42.9% [9/21] in the adalimumab group), and general disorders/administration site conditions (21.7% [10/46] in the bimekizumab group and 23.8% [5/21] in the adalimumab group). Serious adverse events occurred in 4.3% (2/46) of patients in the bimekizumab group, 9.5% (2/21) of patients in the placebo group, and 4.8% (1/21) of patients in the adalimumab group. Serious adverse events that required hospitalization were due to anemia and empyema in the bimekizumab group; worsening HS in the adalimumab group; and myocardial infarction, hypoesthesia, headache, and dizziness in the placebo group. No deaths occurred in this study. Overall, bimekizumab was well tolerated, and discontinuation rates were low across all arms. The primary reason for discontinuation was withdrawal of consent (not due to an adverse event) or loss to follow-up.7
Two completed 48-week phase III RCTs, BE HEARD I and BE HEARD II, evaluated the efficacy and safety of bimekizumab in patients with moderate to severe HS.13 In both trials, 2 bimekizumab dosing regimens (320 mg every 2 weeks and 320 mg every 4 weeks) were compared with placebo during the 16-week initial and 32-week maintenance treatment periods. The primary endpoint of week 16 was achieved by 47.8% (138/289) and 51.9% (151/291) of patients receiving bimekizumab every 2 weeks in BE HEARD I (n=505) and BE HEARD II (n=509), respectively, compared with 29.2% (21/72) and 32.4% (24/74) of the placebo group. The bimekizumab 320 mg every 4 weeks dosing regimen met the primary endpoint only in BE HEARD II, with 53.5% (77/144) of patients achieving HiSCR50 compared to 32.4% (24/74) with placebo (P=0.0038).13 Both trials met the key secondary endpoint of HiSCR75 at week 16 for bimekizumab 320 mg every 2 weeks vs placebo. In BE HEARD I, 33.6% (97/289) of patients receiving bimekizumab achieved HiSCR75 versus 18.1% (13/72) taking placebo. In BE HEARD II, 35.7% (104/291) of patients receiving bimekizumab achieved HiSCR75 vs 16.2% (12/74) taking placebo. Responses were maintained or increased through week 48 in both trials. The most common treatment-emergent adverse events through week 48 were worsening HS, COVID-19 infection, diarrhea, oral candidiasis, and headache.13
A smaller scale case series investigated the use of bimekizumab in 4 female patients aged 20 to 62 years with moderate to severe HS and concomitant plaque or inverse psoriasis.8 A monthly loading dose of 320 mg was given during weeks 0 to 12 followed by a maintenance dose of 320 mg administered every 8 weeks. The International Hidradenitis Suppurativa Score System, visual analogue scale, and Dermatology Life Quality Index were used to assess the effectiveness of therapy by comparing scores before and after 4 and 16 weeks of treatment. A reduction of pain and improvement of HS lesions was observed in 3 (75.0%) patients after the first dosage of bimekizumab, with completed remission of HS by week 16. The fourth patient (25.0%) experienced substantial improvement in all measures, although not complete remission. All 4 patients remained on bimekizumab, and no adverse effects were reported.8
A meta-analysis evaluated 16 RCTs of 9 biologics and 3 small-molecule inhibitors in 2076 patients with HS.10 Secukinumab was not included in this meta-analysis. Only adalimumab (risk ratio, 1.77; 95% CI, 1.44-2.17) and bimekizumab (risk ratio, 2.25; 95% CI, 1.03-4.92) were superior to placebo in achieving HiSCR response at weeks 12 to 16 in 5 RCTs and 1 RCT, respectively; however, no statistically significant differences were noted between adalimumab and bimekizumab (P=.56). This analysis concluded that adalimumab and bimekizumab are the only 2 biologics efficacious in reaching HiSCR and consistently improved both disease severity and quality of life in patients with HS with an acceptable safety profile.10 Furthermore, these biologics had no increase in serious adverse events when compared to placebo.10
A network meta-analysis of 10 clinical trials involving more than 900 total participants evaluated nonsurgical therapies for HS. The analysis used Surface Under the Cumulative Ranking curve (SUCRA) values to estimate the efficacy of treatments in achieving clinical response according to HiSCR criteria. These values range from 0% to 100%, with 100% representing the best possible ranking for efficacy. Bimekizumab showed the highest estimated efficacy with a SUCRA value of 67%, followed by adalimumab (64%), anakinra (49%), and placebo (19%). These SUCRA values indicate the relative ranking of treatments, with higher values suggesting greater likelihood of achieving clinical response, rather than representing the actual percentage of patients achieving HiSCR. Bimekizumab was found to be more efficacious than placebo (P<.05).14
Building on the initial evidence of bimekizumab’s efficacy, BE HEARD I and BE HEARD II addressed some limitations of prior studies, including small sample sizes and insufficient stratification.13 Notably, stratification by baseline Hurley stage severity (ie, the most severe stage of disease assigned at baseline) and baseline systemic antibiotic use helped mitigate bias and ensured a more robust assessment of treatment efficacy; however, certain limitations persist. While the trials demonstrated rapid and clinically meaningful responses maintained up to 48 weeks, longer-term data beyond this period are limited, leaving gaps in understanding the durability of treatment effects over years. Additionally, despite appropriate stratification, the generalizability of the findings to broader patient populations remains unclear, as trial participants may not fully represent the diversity of patients seen in clinical practice.13
Future research is needed to address these limitations. The use of validated HS biomarkers as endpoints could enhance the ability to evaluate biologic efficacy and identify predictors of response. Comparative studies with other biologics also are warranted to establish the relative efficacy of bimekizumab within the growing therapeutic landscape for HS. Finally, real-world evidence from larger and more diverse populations will be critical to confirm the trial findings and assess long-term safety and effectiveness in routine clinical practice.13
Conclusion
The existing literature and recent phase III RCTs, BE HEARD I and BE HEARD II, demonstrate that bimekizumab is an effective treatment for moderate to severe HS, with robust efficacy according to HiSCR scores and sustained responses through 48 weeks. These trials addressed some prior limitations, including small sample sizes and insufficient stratification, providing a more comprehensive evaluation of bimekizumab’s clinical impact. The safety profile of bimekizumab remains favorable, with low discontinuation rates and manageable adverse events, such as infection, gastrointestinal upset, headache, and injection-site reactions. Long-term efficacy and safety data beyond 48 weeks still are needed to fully establish its durability and impact in diverse populations. The recent FDA approval of bimekizumab for moderate to severe HS provides patients with a new treatment option, offering a more positive clinical outlook.
Hidradenitis suppurativa (HS) is a debilitating dermatologic condition characterized by recurrent episodes of neutrophilic inflammation affecting the apocrine and pilosebaceous units that most commonly affects individuals aged 20 to 40 years. Originating from the hair follicles, inflammation initiates the formation of painful nodules and abscesses that can progress to sinus tracts or fistulas accompanied by the development of extensive scarring, exquisite pain, and malodorous drainage.1 The lesions most commonly occur in intertriginous zones as well as areas rich in apocrine glands. The distinctive and sometimes irreversible clinical features of HS profoundly influence patients’ well-being and have lasting social, personal, and emotional impacts on their lives.2
Bimekizumab is a monoclonal antibody that specifically targets IL-17A and IL-17F, aiming to inhibit the downstream effects responsible for the chronic inflammation and tissue damage characteristic of HS.3 In HS lesions, IL-17 cytokines produced by T helper 17 (Th17) cells stimulate the production of chemokines (such as CC motif chemokine ligand 20) and neutrophil-attracting chemokines (including C-X-C motif chemokine ligands 1 and 8), cytokines (such as granulocyte colony-stimulating factor and IL-19), and epidermal antimicrobial proteins.1,2 This cascade results in the chemotaxis of monocytes and neutrophils in the skin, recruiting additional Th17 and myeloid cells and further amplifying IL-17 production.1
Bimekizumab’s mechanism of action strategically disrupts this feed-forward inflammatory loop, decreasing the transcription of neutrophil-attracting chemokines, IL-19, and epidermal antimicrobial proteins (Figure).1,2 This leads to diminished recruitment of Th17 cells and inhibits the chemotaxis of monocytes and neutrophils in the skin, effectively addressing the chronic inflammation and tissue damage characteristic of HS.

We present a comprehensive review of the current standards of care, the underlying molecular pathophysiology of HS, and evaluation of the efficacy and safety of bimekizumab.
Evaluating HS Severity
The Hurley staging system provides a valuable framework for evaluating the severity of HS based on lesion characteristics. Stage I is characterized by abscess formation without tracts or scars. Stage II is characterized by recurrent abscesses with sinus tracts and scarring. Stage III is characterized by diffuse involvement, multiple interconnected sinus tracts, and abscesses across an entire area, leaving little to no uninvolved skin.4
Treatment strategies for HS vary based on Hurley staging (eTable).5-11 For mild cases (stage I), topical and intralesional therapies are common, while moderate to severe cases (stages II and III) may require extensive surgical approaches or systemic drugs such as antibiotics, hormonal therapies, retinoids, or immunosuppressive/biologic agents.2


Adalimumab, an anti–tumor necrosis factor (TNF) α monoclonal antibody, was the first US Food and Drug Administration (FDA)–approved biologic for HS. Secukinumab, a monoclonal antibody against IL-17A, subsequently was approved by the FDA for moderate to severe HS.12 Off-label use of biologics including infliximab and ustekinumab expands the available treatment options for HS. In one Phase II randomized clinical trial (RCT), infliximab showed efficacy in reducing Hidradenitis Suppurativa Severity Index scores, with 26.7% (4/15) of patients achieving a 50% or greater reduction compared to placebo, although this was not statistically significant. Similarly, ustekinumab demonstrated promising results, with 47.1% (8/17) of patients achieving Hidradenitis Suppurativa Clinical Response (HiSCR) at week 40.2 This multifaceted approach aims to address the varying degrees of severity and optimize outcomes for individuals with HS.
Molecular Pathophysiology of HS
The pathogenesis of HS is multifactorial, involving a complex interplay of genetic, environmental, and behavioral factors.2 Approximately 33% to 40% of patients with HS worldwide report a first-degree relative with the condition, indicating a hereditary element with an autosomal-dominant transmission pattern and highlighting the global relevance of genetic factors in HS.4 Hidradenitis suppurativa is highly prevalent in individuals with obesity, likely due to increased intertriginous surface area, skin friction, sweat production, and hormonal changes in these patients. Smoking also commonly is associated with HS, with nicotine potentially contributing to increased follicular plugging.1 Hormonal influences also play a role, as evidenced by a greater prevalence of HS in females, disease onset typically occurring between puberty and menopause, and symptomatic fluctuations correlating with menstrual cycles and exogenous hormones.4
Altered infundibular keratinization with subsequent hyperkeratosis/occlusion and innate immune pathway activation are key events leading to development of HS.1 These events are mediated by release of pathogen- and danger-associated molecular patterns, leading to inflammasome-mediated IL-1α release, followed by downstream cytokine release.2 Elevated levels of TNF-α, IL-1Β, IL-10, IL-17, and particularly IL-17A have been detected in HS lesional skin. The IL-17 family comprises multiple members, namely IL-17A, IL-17C, IL-17E, and IL17F. IL-17A and IL-17F often are co-expressed and secreted predominantly by a subset of CD4+ T helper cells, namely Th17 cells.2 IL-17 cytokines exert pro-inflammatory effects, influencing immune cell activity and contributing to skin inflammation, particularly in HS.
Given the pivotal role of IL-17 in the pathogenesis of HS, the exploration of IL-17–targeted agents has become a focal point in clinical research. Bimekizumab, a novel IL-17 inhibitor, has emerged as a promising candidate, offering a potential breakthrough in the treatment landscape for individuals affected by HS.
Bimekizumab for HS Management
A phase II, double-blind, placebo-controlled RCT included 90 patients with moderate to severe HS (age range, 18-70 years) who were randomly assigned in a 2:1:1 ratio to receive either bimekizumab 320 mg every 2 weeks (with a 640-mg loading dose at baseline)(n=46), placebo (n=21), or adalimumab 40 mg once weekly from week 4 onward (following an initial 160-mg loading dose at baseline and 80-mg dose at week 2)(n=21). The study included a 12-week treatment period followed by a 20-week safety follow-up period. The primary endpoint was the achievement of HiSCR50—defined as a reduction of at least 50% nodules, coupled with no increase in the number of abscesses or draining fistulas relative to baseline—at week 12. Additionally, the study assessed the number of patients who achieved a modified HiSCR with 75% reduction (HiSCR75) of combined abscess and inflammatory nodule count or a modified HiSCR with 90% reduction (HiSCR90). At week 12, the modeled response rates were estimated using a Bayesian logistic regression model. For HiSCR50, the modeled rate for bimekizumab was 57.3%, with an observed rate of 62.5% (25/40), compared to a modeled rate of 26.1% for placebo (observed rate, 27.8% [5/18]). The posterior probability of superiority for bimekizumab over placebo was 0.998. By week 12, bimekizumab-treated patients achieved modeled HiSCR75 and HiSCR90 rates of 46.0% and 32.0%, respectively, with observed rates of 50.0% (20/40) for HiSCR75 and 35.0% (14/40) for HiSCR90. In comparison, placebo-treated patients achieved modeled HiSCR75 and HiSCR90 rates of 10.0% and 0%, respectively, with observed rates of 11.1% (2/18) for HiSCR75 and 0% (0/18) for HiSCR90. Adalimumab-treated participants demonstrated intermediate results, achieving modeled HiSCR75 and HiSCR90 rates of 35.0% and 15.0%, respectively, with observed rates of 38.88% (7/18) for HiSCR75 and 16.66% (3/18) for HiSCR90.7
Bimekizumab was effective in the treatment of moderate to severe HS with comparable results to adalimumab.7 The incidence of treatment-emergent adverse events was similar across treatment arms (bimekizumab, 69.6% [32/46]; placebo, 61.9% [13/21]; adalimumab, 71.4% [15/21]). The most common treatment-emergent adverse events in the biologic treatment arms were infections (43.5% [20/46] in the bimekizumab group and 42.9% [9/21] in the adalimumab group), skin and subcutaneous tissue disorders (28.3% [13/46] in the bimekizumab group and 42.9% [9/21] in the adalimumab group), and general disorders/administration site conditions (21.7% [10/46] in the bimekizumab group and 23.8% [5/21] in the adalimumab group). Serious adverse events occurred in 4.3% (2/46) of patients in the bimekizumab group, 9.5% (2/21) of patients in the placebo group, and 4.8% (1/21) of patients in the adalimumab group. Serious adverse events that required hospitalization were due to anemia and empyema in the bimekizumab group; worsening HS in the adalimumab group; and myocardial infarction, hypoesthesia, headache, and dizziness in the placebo group. No deaths occurred in this study. Overall, bimekizumab was well tolerated, and discontinuation rates were low across all arms. The primary reason for discontinuation was withdrawal of consent (not due to an adverse event) or loss to follow-up.7
Two completed 48-week phase III RCTs, BE HEARD I and BE HEARD II, evaluated the efficacy and safety of bimekizumab in patients with moderate to severe HS.13 In both trials, 2 bimekizumab dosing regimens (320 mg every 2 weeks and 320 mg every 4 weeks) were compared with placebo during the 16-week initial and 32-week maintenance treatment periods. The primary endpoint of week 16 was achieved by 47.8% (138/289) and 51.9% (151/291) of patients receiving bimekizumab every 2 weeks in BE HEARD I (n=505) and BE HEARD II (n=509), respectively, compared with 29.2% (21/72) and 32.4% (24/74) of the placebo group. The bimekizumab 320 mg every 4 weeks dosing regimen met the primary endpoint only in BE HEARD II, with 53.5% (77/144) of patients achieving HiSCR50 compared to 32.4% (24/74) with placebo (P=0.0038).13 Both trials met the key secondary endpoint of HiSCR75 at week 16 for bimekizumab 320 mg every 2 weeks vs placebo. In BE HEARD I, 33.6% (97/289) of patients receiving bimekizumab achieved HiSCR75 versus 18.1% (13/72) taking placebo. In BE HEARD II, 35.7% (104/291) of patients receiving bimekizumab achieved HiSCR75 vs 16.2% (12/74) taking placebo. Responses were maintained or increased through week 48 in both trials. The most common treatment-emergent adverse events through week 48 were worsening HS, COVID-19 infection, diarrhea, oral candidiasis, and headache.13
A smaller scale case series investigated the use of bimekizumab in 4 female patients aged 20 to 62 years with moderate to severe HS and concomitant plaque or inverse psoriasis.8 A monthly loading dose of 320 mg was given during weeks 0 to 12 followed by a maintenance dose of 320 mg administered every 8 weeks. The International Hidradenitis Suppurativa Score System, visual analogue scale, and Dermatology Life Quality Index were used to assess the effectiveness of therapy by comparing scores before and after 4 and 16 weeks of treatment. A reduction of pain and improvement of HS lesions was observed in 3 (75.0%) patients after the first dosage of bimekizumab, with completed remission of HS by week 16. The fourth patient (25.0%) experienced substantial improvement in all measures, although not complete remission. All 4 patients remained on bimekizumab, and no adverse effects were reported.8
A meta-analysis evaluated 16 RCTs of 9 biologics and 3 small-molecule inhibitors in 2076 patients with HS.10 Secukinumab was not included in this meta-analysis. Only adalimumab (risk ratio, 1.77; 95% CI, 1.44-2.17) and bimekizumab (risk ratio, 2.25; 95% CI, 1.03-4.92) were superior to placebo in achieving HiSCR response at weeks 12 to 16 in 5 RCTs and 1 RCT, respectively; however, no statistically significant differences were noted between adalimumab and bimekizumab (P=.56). This analysis concluded that adalimumab and bimekizumab are the only 2 biologics efficacious in reaching HiSCR and consistently improved both disease severity and quality of life in patients with HS with an acceptable safety profile.10 Furthermore, these biologics had no increase in serious adverse events when compared to placebo.10
A network meta-analysis of 10 clinical trials involving more than 900 total participants evaluated nonsurgical therapies for HS. The analysis used Surface Under the Cumulative Ranking curve (SUCRA) values to estimate the efficacy of treatments in achieving clinical response according to HiSCR criteria. These values range from 0% to 100%, with 100% representing the best possible ranking for efficacy. Bimekizumab showed the highest estimated efficacy with a SUCRA value of 67%, followed by adalimumab (64%), anakinra (49%), and placebo (19%). These SUCRA values indicate the relative ranking of treatments, with higher values suggesting greater likelihood of achieving clinical response, rather than representing the actual percentage of patients achieving HiSCR. Bimekizumab was found to be more efficacious than placebo (P<.05).14
Building on the initial evidence of bimekizumab’s efficacy, BE HEARD I and BE HEARD II addressed some limitations of prior studies, including small sample sizes and insufficient stratification.13 Notably, stratification by baseline Hurley stage severity (ie, the most severe stage of disease assigned at baseline) and baseline systemic antibiotic use helped mitigate bias and ensured a more robust assessment of treatment efficacy; however, certain limitations persist. While the trials demonstrated rapid and clinically meaningful responses maintained up to 48 weeks, longer-term data beyond this period are limited, leaving gaps in understanding the durability of treatment effects over years. Additionally, despite appropriate stratification, the generalizability of the findings to broader patient populations remains unclear, as trial participants may not fully represent the diversity of patients seen in clinical practice.13
Future research is needed to address these limitations. The use of validated HS biomarkers as endpoints could enhance the ability to evaluate biologic efficacy and identify predictors of response. Comparative studies with other biologics also are warranted to establish the relative efficacy of bimekizumab within the growing therapeutic landscape for HS. Finally, real-world evidence from larger and more diverse populations will be critical to confirm the trial findings and assess long-term safety and effectiveness in routine clinical practice.13
Conclusion
The existing literature and recent phase III RCTs, BE HEARD I and BE HEARD II, demonstrate that bimekizumab is an effective treatment for moderate to severe HS, with robust efficacy according to HiSCR scores and sustained responses through 48 weeks. These trials addressed some prior limitations, including small sample sizes and insufficient stratification, providing a more comprehensive evaluation of bimekizumab’s clinical impact. The safety profile of bimekizumab remains favorable, with low discontinuation rates and manageable adverse events, such as infection, gastrointestinal upset, headache, and injection-site reactions. Long-term efficacy and safety data beyond 48 weeks still are needed to fully establish its durability and impact in diverse populations. The recent FDA approval of bimekizumab for moderate to severe HS provides patients with a new treatment option, offering a more positive clinical outlook.
- Malvaso D, Calabrese L, Chiricozzi A, et al. IL-17 inhibition: a valid therapeutic strategy in the management of hidradenitis suppurativa. Pharmaceutics. 2023;15:2450. doi:10.3390 /pharmaceutics15102450
- Markota C¡agalj A, Marinovic´ B, Bukvic´ Mokos Z. New and emerging targeted therapies for hidradenitis suppurativa. Int J Mol Sci. 2022;23:3753. doi:10.3390/ijms23073753
- Zouboulis CC, Frew JW, Giamarellos-Bourboulis EJ, et al. Target molecules for future hidradenitis suppurativa treatment. Exp Dermatol. 2021;30 suppl 1:8-17. doi:10.1111/exd.14338
- Ballard K, Shuman VL. Hidradenitis suppurativa. StatPearls [Internet]. Updated May 6, 2024. Accessed December 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK534867/
- Rathod U, Prasad PN, Patel BM, et al. Hidradenitis suppurativa: a literature review comparing current therapeutic modalities. Cureus. 2023;15:E43695. doi:10.7759/cureus.43695
- Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: current and emerging treatments. J Am Acad Dermatol. 2020;82:1061-1082. doi:10.1016/j.jaad.2019.08.089
- Glatt S, Jemec GBE, Forman S, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, doubleblind, placebo-controlled randomized clinical trial. JAMA Dermatol. 2021;157:1279-1288. doi:10.1001/jamadermatol.2021.2905
- Molinelli E, Gambini D, Maurizi A, et al. Bimekizumab in hidradenitis suppurativa: a valid and effective emerging treatment. Clin Exp Dermatol. 2023;48:1272-1274. doi:10.1093/ced/llad229
- Martora F, Megna M, Battista T, et al. Adalimumab, ustekinumab, and secukinumab in the management of hidradenitis suppurativa: a review of the real-life experience. Clin Cosmet Investig Dermatol. 2023;16:135-148. doi:10.2147/CCID.S391356
- Huang CH, Huang IH, Tai CC, et al. Biologics and small molecule inhibitors for treating hidradenitis suppurativa: a systematic review and meta-analysis. Biomedicines. 2022;10:1303. doi:10.3390 /biomedicines10061303
- Ojeda Gómez A, Madero Velázquez L, Buendía Sanchez L, et al. Inflammatory bowel disease new-onset during secukinumab therapy: real-world data from a tertiary center. Rev Esp Enferm Dig. 2021;113: 858-859. doi:10.17235/reed.2021.8397/2021
- Martora F, Marasca C, Cacciapuoti S, et al. Secukinumab in hidradenitis suppurativa patients who failed adalimumab: a 52-week real-life study. Clin Cosmet Investig Dermatol. 2024;17:159-166. doi:10.2147 /CCID.S449367
- Kimball AB, Jemec GBE, Sayed CJ, et al. Efficacy and safety of bimekizumab in patients with moderate-to-severe hidradenitis suppurativa (BE HEARD I and BE HEARD II): two 48-week, randomised, double-blind, placebo-controlled, multicentre phase 3 trials. Lancet. 2024;403:2504-2519. doi:10.1016 /S0140-6736(24)00101-6
- Gupta AK, Shear NH, Piguet V, et al. Efficacy of non-surgical monotherapies for hidradenitis suppurativa: a systematic review and network meta-analyses of randomized trials. J Dermatolog Treat. 2022;33:2149-2160. doi:10.1080/09546634.2021.1927949
- Malvaso D, Calabrese L, Chiricozzi A, et al. IL-17 inhibition: a valid therapeutic strategy in the management of hidradenitis suppurativa. Pharmaceutics. 2023;15:2450. doi:10.3390 /pharmaceutics15102450
- Markota C¡agalj A, Marinovic´ B, Bukvic´ Mokos Z. New and emerging targeted therapies for hidradenitis suppurativa. Int J Mol Sci. 2022;23:3753. doi:10.3390/ijms23073753
- Zouboulis CC, Frew JW, Giamarellos-Bourboulis EJ, et al. Target molecules for future hidradenitis suppurativa treatment. Exp Dermatol. 2021;30 suppl 1:8-17. doi:10.1111/exd.14338
- Ballard K, Shuman VL. Hidradenitis suppurativa. StatPearls [Internet]. Updated May 6, 2024. Accessed December 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK534867/
- Rathod U, Prasad PN, Patel BM, et al. Hidradenitis suppurativa: a literature review comparing current therapeutic modalities. Cureus. 2023;15:E43695. doi:10.7759/cureus.43695
- Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: current and emerging treatments. J Am Acad Dermatol. 2020;82:1061-1082. doi:10.1016/j.jaad.2019.08.089
- Glatt S, Jemec GBE, Forman S, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, doubleblind, placebo-controlled randomized clinical trial. JAMA Dermatol. 2021;157:1279-1288. doi:10.1001/jamadermatol.2021.2905
- Molinelli E, Gambini D, Maurizi A, et al. Bimekizumab in hidradenitis suppurativa: a valid and effective emerging treatment. Clin Exp Dermatol. 2023;48:1272-1274. doi:10.1093/ced/llad229
- Martora F, Megna M, Battista T, et al. Adalimumab, ustekinumab, and secukinumab in the management of hidradenitis suppurativa: a review of the real-life experience. Clin Cosmet Investig Dermatol. 2023;16:135-148. doi:10.2147/CCID.S391356
- Huang CH, Huang IH, Tai CC, et al. Biologics and small molecule inhibitors for treating hidradenitis suppurativa: a systematic review and meta-analysis. Biomedicines. 2022;10:1303. doi:10.3390 /biomedicines10061303
- Ojeda Gómez A, Madero Velázquez L, Buendía Sanchez L, et al. Inflammatory bowel disease new-onset during secukinumab therapy: real-world data from a tertiary center. Rev Esp Enferm Dig. 2021;113: 858-859. doi:10.17235/reed.2021.8397/2021
- Martora F, Marasca C, Cacciapuoti S, et al. Secukinumab in hidradenitis suppurativa patients who failed adalimumab: a 52-week real-life study. Clin Cosmet Investig Dermatol. 2024;17:159-166. doi:10.2147 /CCID.S449367
- Kimball AB, Jemec GBE, Sayed CJ, et al. Efficacy and safety of bimekizumab in patients with moderate-to-severe hidradenitis suppurativa (BE HEARD I and BE HEARD II): two 48-week, randomised, double-blind, placebo-controlled, multicentre phase 3 trials. Lancet. 2024;403:2504-2519. doi:10.1016 /S0140-6736(24)00101-6
- Gupta AK, Shear NH, Piguet V, et al. Efficacy of non-surgical monotherapies for hidradenitis suppurativa: a systematic review and network meta-analyses of randomized trials. J Dermatolog Treat. 2022;33:2149-2160. doi:10.1080/09546634.2021.1927949
Bimekizumab for Hidradenitis Suppurativa: Pathophysiology and Promising Interventions
Bimekizumab for Hidradenitis Suppurativa: Pathophysiology and Promising Interventions
PRACTICE POINTS
- Management of hidradenitis suppurativa (HS) includes lifestyle modifications as well as topical and systemic antibiotics, intralesional and systemic corticosteroids, retinoids, hormonal therapies, immunosuppressants, biologic agents, and minor to invasive surgical procedures.
- Adalimumab, secukinumab, and more recently bimekizumab are biologics that are approved by the US Food and Drug Administration for the treatment of moderate to severe HS.
- Bimekizumab is a monoclonal antibody targeting IL-17A and IL-17F that has demonstrated strong clinical efficacy in generating a sustained clinical response in moderate to severe HS-related clinical features.
Solitary Lesion on the Umbilicus
Solitary Lesion on the Umbilicus
THE DIAGNOSIS: Cutaneous Endometriosis
Endometriosis is the ectopic presence of endometrial tissue and occurs in approximately 13% of women of childbearing age.1 This non-neoplastic lesion can manifest on the skin in less than 5.5% of endometriosis cases worldwide. Historically, secondary cutaneous endometriosis (CE) most frequently has been associated with prior gynecologic surgery (often cesarean section)2; however, an increased incidence of primary CE in patients without prior surgical history recently has been documented in the literature.3 While secondary CE usually manifests at the site of a surgical scar, primary CE has a predilection for the umbilicus (Villar nodule). In both primary and secondary CE, patients present clinically with a solitary nodule and abdominal pain that may be exacerbated during menstruation. Bleeding without associated pain may be more common in primary CE, while bleeding with pain may be more common in secondary CE. Cutaneous endometriosis often is overlooked given its low incidence, leading to delayed diagnosis. Primary CE often is misdiagnosed clinically as a pyogenic granuloma, Sister Mary Joseph nodule, or keloid, while secondary CE may be mistaken for a fibroma, incisional hernia, or granuloma.2
Primary and secondary CE have identical histopathologic features. Glands of variable size consisting of a single epithelial layer of columnar cells are present in the reticular dermis or subcutis (quiz image).4 The accompanying periglandular stroma often is uniform, consisting of spindle-shaped basophilic cells with abundant vascular structures. The stroma may contain moderate numbers of mitotic figures, a chronic inflammatory infiltrate, and extravasated red blood cells. The ectopic tissue may be inactive or display morphologic changes resembling those of the endometrium in the normal menstrual cycle.4 As the ectopic tissue progresses through the stages of menstruation, the glandular morphology also transforms. The proliferative stage demonstrates increased epithelial mitotic figures, the secretory stage exhibits intraluminal secretion, and during menstruation there are degenerative epithelial cells and evidence of vascular congestion. A mixture of glandular stages may be seen in biopsy results. Robust immunohistochemical expression of CD10 in the endometrial stroma can aid in diagnosis (Figure 1). Estrogen and progesterone receptor immunostaining also shows strong nuclear positivity, except in decidualized tissue.4 Unlike intestinal glands, endometrial glands do not express CDX2 or CK20.5 Complete surgical excision of CE usually is curative; however, recurrence has been documented in 10% (3/30) of cases.2
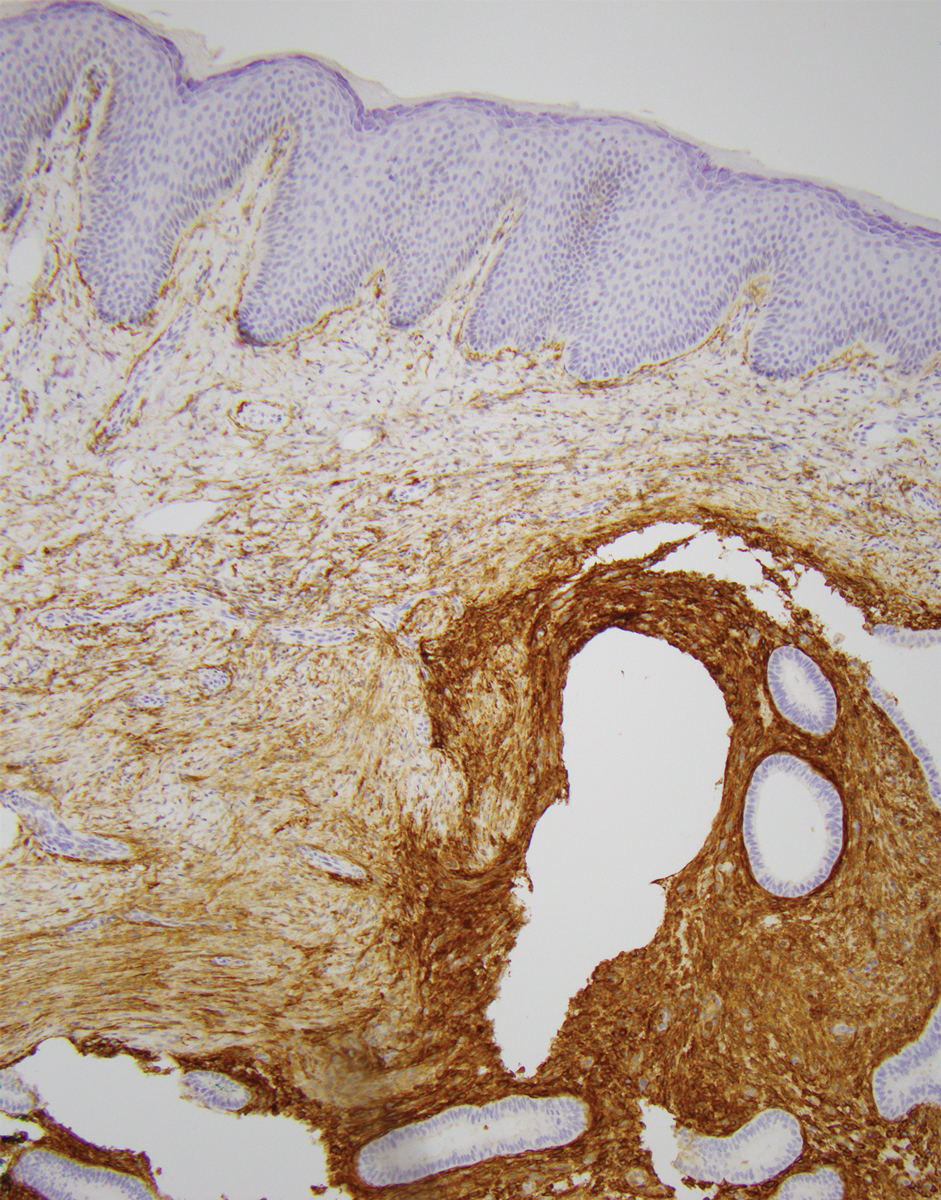
Breast carcinoma is the most common internal malignancy associated with cutaneous metastasis and may develop prior to visceral diagnosis. It is possible that tumor cells travel through the communicating networks of the cutaneous lymphatic ducts and the mammary lymphatic plexus; however, cutaneous manifestation often is located on the ipsilateral breast, and therefore tumor expansion rather than true metastasis cannot always be ruled out. On histopathology, findings of breast adenocarcinoma include tumor cells that tend to show either interstitial, nodular, mixed, or intravascular growth patterns (Figure 2). Tumor cells may invade the stroma in clusters or as individual cells. Sites of distant metastasis may show an increased likelihood of vascular and lymphatic invasion.6
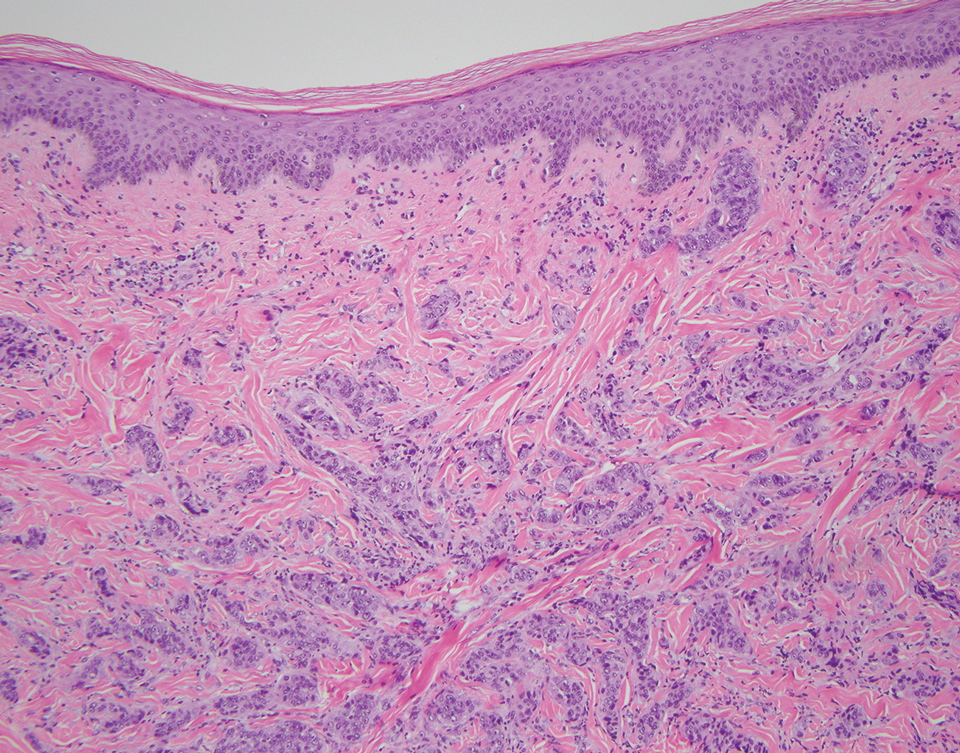
Nodular hidradenoma often manifests as a solitary nodule in the head or neck region, predominantly in women.7 Pathology shows well-demarcated intradermal aggregates of tumor cells within a hyalinized stroma; connection to the epidermis is not a feature of nodular hidradenoma. The epithelial component consists of polygonal cells with eosinophilic to amphophilic cytoplasm as well as large glycogenated cells with pale to clear cytoplasm (leading to the alternative term clear cell hidradenoma)(Figure 3). The cystic portion represents deterioration of tumor cells. Surgical excision usually is curative, although lesions may recur. Malignant transformation is rare.7
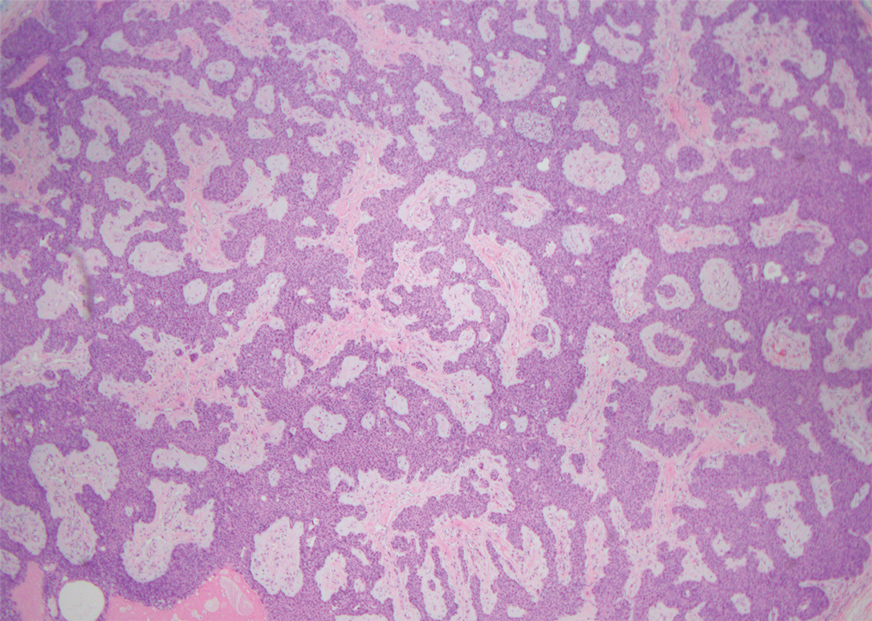
Sister Mary Joseph nodule is a cutaneous involvement of the umbilicus by a metastatic malignancy, often from an intra-abdominal primary malignancy (most commonly ovarian carcinoma in women and colonic carcinoma in men). Clinically, patients present with a solitary firm nodule or plaque within the umbilicus.8,9 Histopathology recapitulates the primary tumor (Figure 4).9 Sister Mary Joseph nodule portends a poor prognosis, with a survival rate of less than 8 months from the time of diagnosis.10
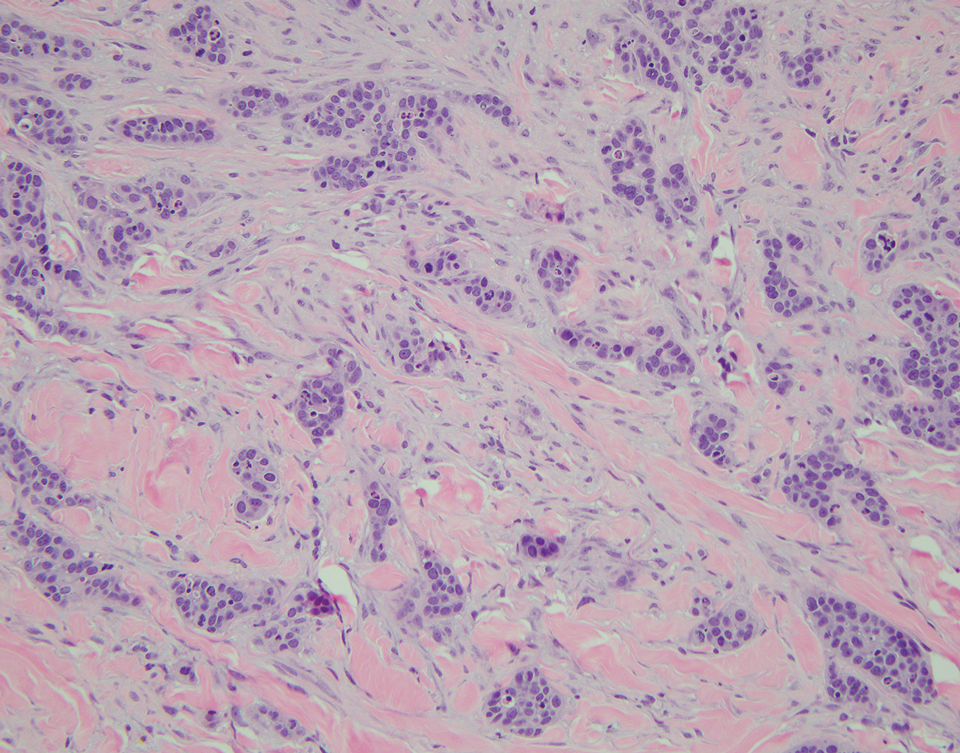
Urachal duct cyst develops from a remnant of the urachus that closed appropriately at the umbilicus and bladder but did not completely regress. It may manifest as an extraperitoneal mass at the umbilicus. Clinically, urachal duct cysts may be asymptomatic until an inciting event (eg, inflammation, deposition of calculus, or malignancy) occurs.11 Histopathology shows cystically dilated structures lined with a transitional epithelium (Figure 5).12 Urachal duct cysts usually are diagnosed in children or young adults and subsequently are excised.11
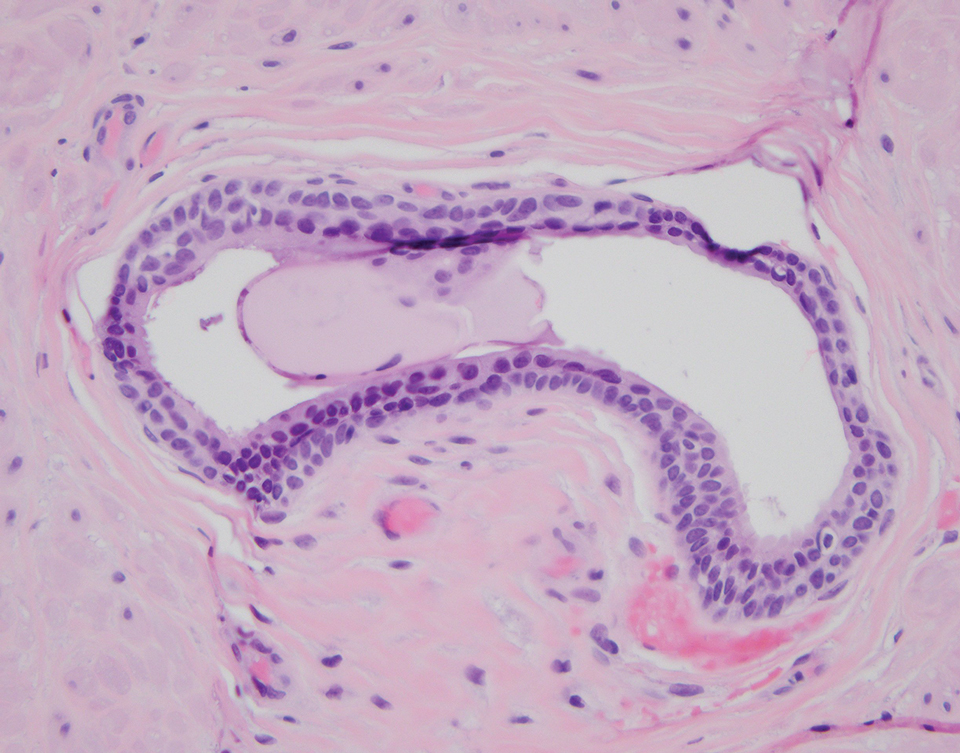
- Harder C, Velho RV, Brandes I, et al. Assessing the true prevalence of endometriosis: a narrative review of literature data. Int J Gynaecol Obstet. 2024;167:883-900. doi:10.1002/ijgo.15756
- Lopez-Soto A, Sanchez-Zapata MI, Martinez-Cendan JP, et al. Cutaneous endometriosis: presentation of 33 cases and literature review. Eur J Obstet Gynecol Reprod Biol. Feb 2018;221:58-63. doi:10.1016 /j.ejogrb.2017.11.024
- Dridi D, Chiaffarino F, Parazzini F, et al. Umbilical endometriosis: a systematic literature review and pathogenic theory proposal. J Clin Med. 2022;11:995. doi:10.3390/jcm11040995
- Farooq U, Laureano AC, Miteva M, Elgart GW. Cutaneous endometriosis: diagnostic immunohistochemistry and clinicopathologic correlation. J Cutan Pathol. 2011;38:525-528. doi:10.1111/j.1600-0560.2011.01681.x
- Gadducci A, Zannoni GF. Endometriosis-associated extraovarian malignancies: a challenging question for the clinician and the pathologist. Anticancer Res. 2020;40:2429-2438. doi:10.21873/anticanres.14212
- Ronen S, Suster D, Chen WS, et al. Histologic patterns of cutaneous metastases of breast carcinoma: a clinicopathologic study of 232 cases. Am J Dermatopathol. 2021;43:401-411. doi:10.1097 /DAD.0000000000001841
- Nandeesh BN, Rajalakshmi T. A study of histopathologic spectrum of nodular hidradenoma. Am J Dermatopathol. 2012;34:461-470. doi:10.1097/DAD.0b013e31821a4d33
- Abu-Hilal M, Newman JS. Sister Mary Joseph and her nodule: historical and clinical perspective. Am J Med Sci. 2009;337:271-273. doi:10.1097/MAJ.0b013e3181954187
- Powell FC, Cooper AJ, Massa MC, et al. Sister Mary Joseph’s nodule: a clinical and histologic study. J Am Acad Dermatol. 1984;10:610-615. doi:10.1016/s0190-9622(84)80265-0
- Hugen N, Kanne H, Simmer F, et al. Umbilical metastases: real-world data shows abysmal outcome. Int J Cancer. 2021;149: 1266-1273. doi:10.1002/ijc.33684
- Al-Salem A. An Illustrated Guide to Pediatric Urology. 1st ed. Springer Cham; 2016.
- Schubert GE, Pavkovic MB, Bethke-Bedürftig BA. Tubular urachal remnants in adult bladders. J Urol. 1982;127:40-42. doi:10.1016/s0022- 5347(17)53595-8
THE DIAGNOSIS: Cutaneous Endometriosis
Endometriosis is the ectopic presence of endometrial tissue and occurs in approximately 13% of women of childbearing age.1 This non-neoplastic lesion can manifest on the skin in less than 5.5% of endometriosis cases worldwide. Historically, secondary cutaneous endometriosis (CE) most frequently has been associated with prior gynecologic surgery (often cesarean section)2; however, an increased incidence of primary CE in patients without prior surgical history recently has been documented in the literature.3 While secondary CE usually manifests at the site of a surgical scar, primary CE has a predilection for the umbilicus (Villar nodule). In both primary and secondary CE, patients present clinically with a solitary nodule and abdominal pain that may be exacerbated during menstruation. Bleeding without associated pain may be more common in primary CE, while bleeding with pain may be more common in secondary CE. Cutaneous endometriosis often is overlooked given its low incidence, leading to delayed diagnosis. Primary CE often is misdiagnosed clinically as a pyogenic granuloma, Sister Mary Joseph nodule, or keloid, while secondary CE may be mistaken for a fibroma, incisional hernia, or granuloma.2
Primary and secondary CE have identical histopathologic features. Glands of variable size consisting of a single epithelial layer of columnar cells are present in the reticular dermis or subcutis (quiz image).4 The accompanying periglandular stroma often is uniform, consisting of spindle-shaped basophilic cells with abundant vascular structures. The stroma may contain moderate numbers of mitotic figures, a chronic inflammatory infiltrate, and extravasated red blood cells. The ectopic tissue may be inactive or display morphologic changes resembling those of the endometrium in the normal menstrual cycle.4 As the ectopic tissue progresses through the stages of menstruation, the glandular morphology also transforms. The proliferative stage demonstrates increased epithelial mitotic figures, the secretory stage exhibits intraluminal secretion, and during menstruation there are degenerative epithelial cells and evidence of vascular congestion. A mixture of glandular stages may be seen in biopsy results. Robust immunohistochemical expression of CD10 in the endometrial stroma can aid in diagnosis (Figure 1). Estrogen and progesterone receptor immunostaining also shows strong nuclear positivity, except in decidualized tissue.4 Unlike intestinal glands, endometrial glands do not express CDX2 or CK20.5 Complete surgical excision of CE usually is curative; however, recurrence has been documented in 10% (3/30) of cases.2

Breast carcinoma is the most common internal malignancy associated with cutaneous metastasis and may develop prior to visceral diagnosis. It is possible that tumor cells travel through the communicating networks of the cutaneous lymphatic ducts and the mammary lymphatic plexus; however, cutaneous manifestation often is located on the ipsilateral breast, and therefore tumor expansion rather than true metastasis cannot always be ruled out. On histopathology, findings of breast adenocarcinoma include tumor cells that tend to show either interstitial, nodular, mixed, or intravascular growth patterns (Figure 2). Tumor cells may invade the stroma in clusters or as individual cells. Sites of distant metastasis may show an increased likelihood of vascular and lymphatic invasion.6

Nodular hidradenoma often manifests as a solitary nodule in the head or neck region, predominantly in women.7 Pathology shows well-demarcated intradermal aggregates of tumor cells within a hyalinized stroma; connection to the epidermis is not a feature of nodular hidradenoma. The epithelial component consists of polygonal cells with eosinophilic to amphophilic cytoplasm as well as large glycogenated cells with pale to clear cytoplasm (leading to the alternative term clear cell hidradenoma)(Figure 3). The cystic portion represents deterioration of tumor cells. Surgical excision usually is curative, although lesions may recur. Malignant transformation is rare.7

Sister Mary Joseph nodule is a cutaneous involvement of the umbilicus by a metastatic malignancy, often from an intra-abdominal primary malignancy (most commonly ovarian carcinoma in women and colonic carcinoma in men). Clinically, patients present with a solitary firm nodule or plaque within the umbilicus.8,9 Histopathology recapitulates the primary tumor (Figure 4).9 Sister Mary Joseph nodule portends a poor prognosis, with a survival rate of less than 8 months from the time of diagnosis.10

Urachal duct cyst develops from a remnant of the urachus that closed appropriately at the umbilicus and bladder but did not completely regress. It may manifest as an extraperitoneal mass at the umbilicus. Clinically, urachal duct cysts may be asymptomatic until an inciting event (eg, inflammation, deposition of calculus, or malignancy) occurs.11 Histopathology shows cystically dilated structures lined with a transitional epithelium (Figure 5).12 Urachal duct cysts usually are diagnosed in children or young adults and subsequently are excised.11

THE DIAGNOSIS: Cutaneous Endometriosis
Endometriosis is the ectopic presence of endometrial tissue and occurs in approximately 13% of women of childbearing age.1 This non-neoplastic lesion can manifest on the skin in less than 5.5% of endometriosis cases worldwide. Historically, secondary cutaneous endometriosis (CE) most frequently has been associated with prior gynecologic surgery (often cesarean section)2; however, an increased incidence of primary CE in patients without prior surgical history recently has been documented in the literature.3 While secondary CE usually manifests at the site of a surgical scar, primary CE has a predilection for the umbilicus (Villar nodule). In both primary and secondary CE, patients present clinically with a solitary nodule and abdominal pain that may be exacerbated during menstruation. Bleeding without associated pain may be more common in primary CE, while bleeding with pain may be more common in secondary CE. Cutaneous endometriosis often is overlooked given its low incidence, leading to delayed diagnosis. Primary CE often is misdiagnosed clinically as a pyogenic granuloma, Sister Mary Joseph nodule, or keloid, while secondary CE may be mistaken for a fibroma, incisional hernia, or granuloma.2
Primary and secondary CE have identical histopathologic features. Glands of variable size consisting of a single epithelial layer of columnar cells are present in the reticular dermis or subcutis (quiz image).4 The accompanying periglandular stroma often is uniform, consisting of spindle-shaped basophilic cells with abundant vascular structures. The stroma may contain moderate numbers of mitotic figures, a chronic inflammatory infiltrate, and extravasated red blood cells. The ectopic tissue may be inactive or display morphologic changes resembling those of the endometrium in the normal menstrual cycle.4 As the ectopic tissue progresses through the stages of menstruation, the glandular morphology also transforms. The proliferative stage demonstrates increased epithelial mitotic figures, the secretory stage exhibits intraluminal secretion, and during menstruation there are degenerative epithelial cells and evidence of vascular congestion. A mixture of glandular stages may be seen in biopsy results. Robust immunohistochemical expression of CD10 in the endometrial stroma can aid in diagnosis (Figure 1). Estrogen and progesterone receptor immunostaining also shows strong nuclear positivity, except in decidualized tissue.4 Unlike intestinal glands, endometrial glands do not express CDX2 or CK20.5 Complete surgical excision of CE usually is curative; however, recurrence has been documented in 10% (3/30) of cases.2

Breast carcinoma is the most common internal malignancy associated with cutaneous metastasis and may develop prior to visceral diagnosis. It is possible that tumor cells travel through the communicating networks of the cutaneous lymphatic ducts and the mammary lymphatic plexus; however, cutaneous manifestation often is located on the ipsilateral breast, and therefore tumor expansion rather than true metastasis cannot always be ruled out. On histopathology, findings of breast adenocarcinoma include tumor cells that tend to show either interstitial, nodular, mixed, or intravascular growth patterns (Figure 2). Tumor cells may invade the stroma in clusters or as individual cells. Sites of distant metastasis may show an increased likelihood of vascular and lymphatic invasion.6

Nodular hidradenoma often manifests as a solitary nodule in the head or neck region, predominantly in women.7 Pathology shows well-demarcated intradermal aggregates of tumor cells within a hyalinized stroma; connection to the epidermis is not a feature of nodular hidradenoma. The epithelial component consists of polygonal cells with eosinophilic to amphophilic cytoplasm as well as large glycogenated cells with pale to clear cytoplasm (leading to the alternative term clear cell hidradenoma)(Figure 3). The cystic portion represents deterioration of tumor cells. Surgical excision usually is curative, although lesions may recur. Malignant transformation is rare.7

Sister Mary Joseph nodule is a cutaneous involvement of the umbilicus by a metastatic malignancy, often from an intra-abdominal primary malignancy (most commonly ovarian carcinoma in women and colonic carcinoma in men). Clinically, patients present with a solitary firm nodule or plaque within the umbilicus.8,9 Histopathology recapitulates the primary tumor (Figure 4).9 Sister Mary Joseph nodule portends a poor prognosis, with a survival rate of less than 8 months from the time of diagnosis.10

Urachal duct cyst develops from a remnant of the urachus that closed appropriately at the umbilicus and bladder but did not completely regress. It may manifest as an extraperitoneal mass at the umbilicus. Clinically, urachal duct cysts may be asymptomatic until an inciting event (eg, inflammation, deposition of calculus, or malignancy) occurs.11 Histopathology shows cystically dilated structures lined with a transitional epithelium (Figure 5).12 Urachal duct cysts usually are diagnosed in children or young adults and subsequently are excised.11

- Harder C, Velho RV, Brandes I, et al. Assessing the true prevalence of endometriosis: a narrative review of literature data. Int J Gynaecol Obstet. 2024;167:883-900. doi:10.1002/ijgo.15756
- Lopez-Soto A, Sanchez-Zapata MI, Martinez-Cendan JP, et al. Cutaneous endometriosis: presentation of 33 cases and literature review. Eur J Obstet Gynecol Reprod Biol. Feb 2018;221:58-63. doi:10.1016 /j.ejogrb.2017.11.024
- Dridi D, Chiaffarino F, Parazzini F, et al. Umbilical endometriosis: a systematic literature review and pathogenic theory proposal. J Clin Med. 2022;11:995. doi:10.3390/jcm11040995
- Farooq U, Laureano AC, Miteva M, Elgart GW. Cutaneous endometriosis: diagnostic immunohistochemistry and clinicopathologic correlation. J Cutan Pathol. 2011;38:525-528. doi:10.1111/j.1600-0560.2011.01681.x
- Gadducci A, Zannoni GF. Endometriosis-associated extraovarian malignancies: a challenging question for the clinician and the pathologist. Anticancer Res. 2020;40:2429-2438. doi:10.21873/anticanres.14212
- Ronen S, Suster D, Chen WS, et al. Histologic patterns of cutaneous metastases of breast carcinoma: a clinicopathologic study of 232 cases. Am J Dermatopathol. 2021;43:401-411. doi:10.1097 /DAD.0000000000001841
- Nandeesh BN, Rajalakshmi T. A study of histopathologic spectrum of nodular hidradenoma. Am J Dermatopathol. 2012;34:461-470. doi:10.1097/DAD.0b013e31821a4d33
- Abu-Hilal M, Newman JS. Sister Mary Joseph and her nodule: historical and clinical perspective. Am J Med Sci. 2009;337:271-273. doi:10.1097/MAJ.0b013e3181954187
- Powell FC, Cooper AJ, Massa MC, et al. Sister Mary Joseph’s nodule: a clinical and histologic study. J Am Acad Dermatol. 1984;10:610-615. doi:10.1016/s0190-9622(84)80265-0
- Hugen N, Kanne H, Simmer F, et al. Umbilical metastases: real-world data shows abysmal outcome. Int J Cancer. 2021;149: 1266-1273. doi:10.1002/ijc.33684
- Al-Salem A. An Illustrated Guide to Pediatric Urology. 1st ed. Springer Cham; 2016.
- Schubert GE, Pavkovic MB, Bethke-Bedürftig BA. Tubular urachal remnants in adult bladders. J Urol. 1982;127:40-42. doi:10.1016/s0022- 5347(17)53595-8
- Harder C, Velho RV, Brandes I, et al. Assessing the true prevalence of endometriosis: a narrative review of literature data. Int J Gynaecol Obstet. 2024;167:883-900. doi:10.1002/ijgo.15756
- Lopez-Soto A, Sanchez-Zapata MI, Martinez-Cendan JP, et al. Cutaneous endometriosis: presentation of 33 cases and literature review. Eur J Obstet Gynecol Reprod Biol. Feb 2018;221:58-63. doi:10.1016 /j.ejogrb.2017.11.024
- Dridi D, Chiaffarino F, Parazzini F, et al. Umbilical endometriosis: a systematic literature review and pathogenic theory proposal. J Clin Med. 2022;11:995. doi:10.3390/jcm11040995
- Farooq U, Laureano AC, Miteva M, Elgart GW. Cutaneous endometriosis: diagnostic immunohistochemistry and clinicopathologic correlation. J Cutan Pathol. 2011;38:525-528. doi:10.1111/j.1600-0560.2011.01681.x
- Gadducci A, Zannoni GF. Endometriosis-associated extraovarian malignancies: a challenging question for the clinician and the pathologist. Anticancer Res. 2020;40:2429-2438. doi:10.21873/anticanres.14212
- Ronen S, Suster D, Chen WS, et al. Histologic patterns of cutaneous metastases of breast carcinoma: a clinicopathologic study of 232 cases. Am J Dermatopathol. 2021;43:401-411. doi:10.1097 /DAD.0000000000001841
- Nandeesh BN, Rajalakshmi T. A study of histopathologic spectrum of nodular hidradenoma. Am J Dermatopathol. 2012;34:461-470. doi:10.1097/DAD.0b013e31821a4d33
- Abu-Hilal M, Newman JS. Sister Mary Joseph and her nodule: historical and clinical perspective. Am J Med Sci. 2009;337:271-273. doi:10.1097/MAJ.0b013e3181954187
- Powell FC, Cooper AJ, Massa MC, et al. Sister Mary Joseph’s nodule: a clinical and histologic study. J Am Acad Dermatol. 1984;10:610-615. doi:10.1016/s0190-9622(84)80265-0
- Hugen N, Kanne H, Simmer F, et al. Umbilical metastases: real-world data shows abysmal outcome. Int J Cancer. 2021;149: 1266-1273. doi:10.1002/ijc.33684
- Al-Salem A. An Illustrated Guide to Pediatric Urology. 1st ed. Springer Cham; 2016.
- Schubert GE, Pavkovic MB, Bethke-Bedürftig BA. Tubular urachal remnants in adult bladders. J Urol. 1982;127:40-42. doi:10.1016/s0022- 5347(17)53595-8
Solitary Lesion on the Umbilicus
Solitary Lesion on the Umbilicus
A 33-year-old woman with no notable medical or surgical history presented to our clinic with a solitary indurated nodule on the umbilicus that had been progressively enlarging for 1 year. The patient reported that she had undergone piercing of the umbilicus more than 5 years prior. She noted that the lesion was uncomfortable and pruritic and occasionally bled spontaneously. Physical examination revealed no other mucosal or cutaneous findings. A shave biopsy of the nodule was performed.
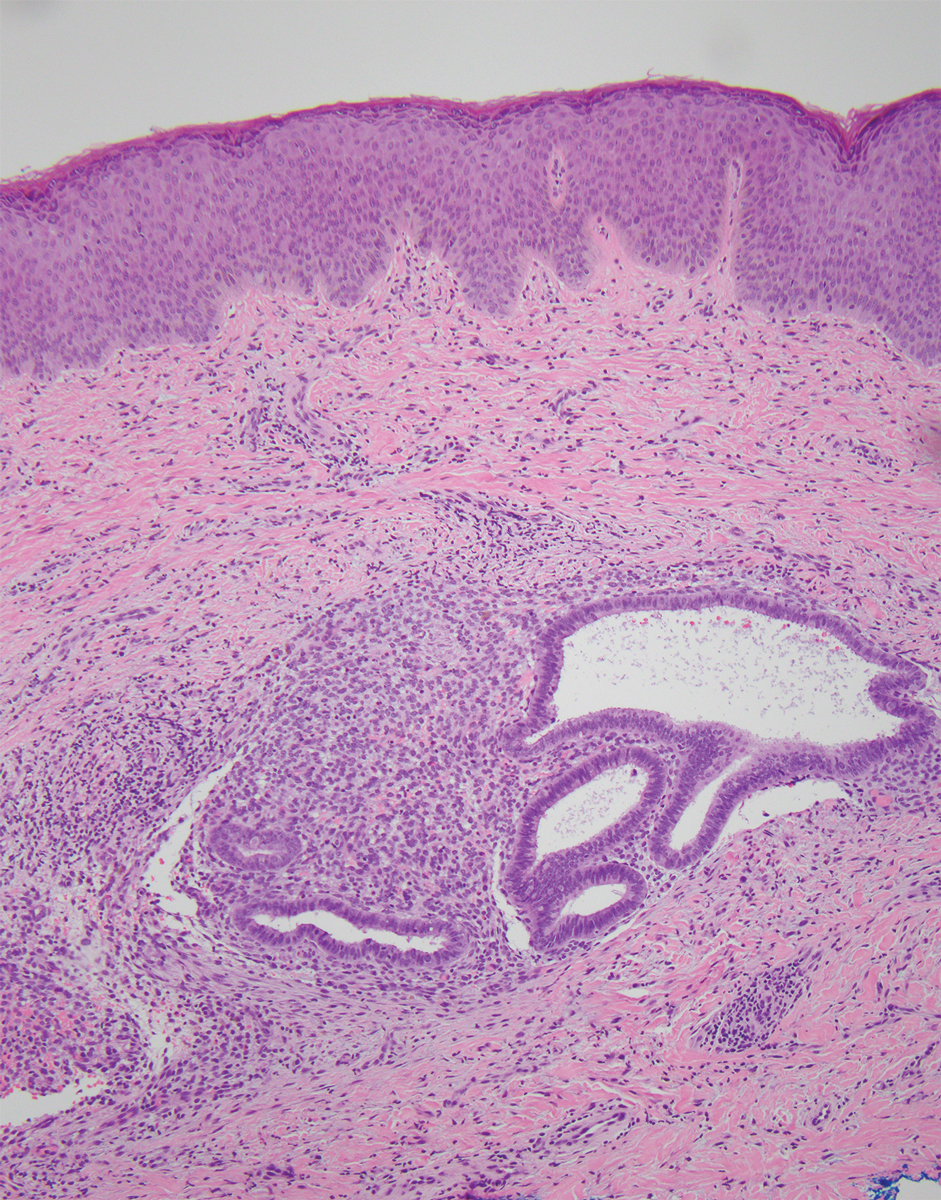
Treatment of Seborrheic Dermatitis in Black Patients
Treatment of Seborrheic Dermatitis in Black Patients
Seborrheic dermatitis (SD) is a common chronic inflammatory skin condition that predominantly affects areas with high concentrations of sebaceous glands such as the scalp and face. Up to 5% of the worldwide population is affected by SD each year, causing a major burden of disease for patients and the health care system.1 In 2023, the cost of medical treatment for SD in the United States was $300 million, with outpatient office visits alone costing $58 million and prescription drugs costing $109 million. Indirect costs of disease (eg, lost workdays) account for another $51 million.1 Since SD frequently manifests on the face, it tends to have negative effects on the patient’s quality of life, resulting in psychological distress and low self-esteem.2
Patients with SD may describe symptoms of excessive dandruff and itching along with hyperpigmentation or hypopigmentation of the skin; Black patients tend to present with the classic manifestations: a combination of scaling, flaking, and erythematous patches on the scalp, ears, and face, particularly around the eyebrows, eyelids, and nose. With SD being the second most common diagnosis in Black patients who seek care from a dermatologist, it is important to have effective treatment approaches for SD in this patient population.3
In this study, we aimed to evaluate medical and nonmedical treatment options for SD in Black patients by identifying common practices and products mentioned on consumer websites and in the medical literature.
Methods
A Google search was conducted during 2 time periods (September 2022—October 2022 and March 2023—April 2023) using the terms products for itchy scalp in Black patients, products for dandruff in Black patients, itchy scalp in Black women, itchy scalp in Black men, treatment for scalp itch in Black patients, and dry scalp in Black hair. Products that were recommended by at least 1 website on the first page of search results were included in our list of products, and the ingredients were reviewed by the authors. We excluded individual retailer websites as well as those that did not provide specific recommendations on products or ingredients to use when treating SD. To ensure reliability and standardization, we did not review products that were suggested by ads in the shopping section on the first page of search results.
We also evaluated medical treatments used for SD in dermatology literature. A PubMed search of articles indexed for MEDLINE using the terms seborrheic dermatitis treatment for Black patients, treatment for dandruff for Black patients, and seborrheic dermatitis and skin of color was conducted. We excluded articles that did not address treatment options for SD, were specific to treating SD in patient populations with specific comorbidities being studied, discussed SD in animals, or were published prior to 1990.
Results
We identified 16 unique consumer websites with product or ingredient recommendations for SD in Black patients, none of which were provided by authors with a medical or scientific background; however, 4 (25%) websites included insights from board-certified dermatologists. A total of 16 ingredients were recommended, 15 (94%) of which were mentioned at least twice in our search results (eTable 1).
Overall, we noticed that ingredients labeled as natural or organic were common in over-the-counter (OTC) products, and ingredients such as sulfates and parabens were avoided. Common OTC ingredients for antidandruff and anti-itch shampoos and conditioners include zinc pyrithione, selenium sulfide, coal tar, salicylic acid, and citric acid. Additionally, coconut oil, tea tree oil, apple cider vinegar, and charcoal are common natural alternatives used to address SD symptoms.
Our review of the literature yielded limited recommendations tailored specifically to Black patients with SD. Of 108 abstracts, articles, or textbook chapters providing treatment recommendations for SD, 6 (6%) specifically discussed treatments for Black patients. All articles were written by authors with medical or scientific backgrounds. Of the treatment options discussed, topical antifungals generally were considered first-line for SD in all patients, with ketoconazole shampoo being a common first choice.4,5
Comment
Our study indicated that many consumer websites recommend unstudied nonmedical treatments for SD. Zinc pyrithione was one of the most commonly mentioned ingredients in OTC products to treat SD targeted toward Black patients, as its properties have contributed to ease of hair combing and less frizz.6 Zinc pyrithione has antifungal properties that reduce the proliferation of Malassezia furfur as well as anti-inflammatory properties that reduce irritation, pruritus, and erythema in areas affected by SD.7 Tea tree and peppermint oils also were commonly mentioned; the theory is that these oils mitigate SD by reducing yeast growth and soothing inflammation through antioxidant activity.8,9 Coal tar also is used due to its keratoplastic properties, which slow the growth of skin cells and ultimately reduce scaling and dryness.10 Yeast thrives in basic pH conditions; apple cider vinegar is used as an ingredient in OTC products for SD because its acidic pH creates a less favorable environment for yeast to grow.11 Although many of the ingredients found in OTC products we identified have not yet been studied, they have properties that theoretically would be helpful in treating SD.
Our review of the medical literature revealed that while there are treatments that are effective for SD, the recommended use may not consider the cultural differences that exist for Black patients. For instance, reports in the literature regarding ketoconazole shampoo revealed that ketoconazole increases the risk for hair shaft dryness, damage, and subsequent breakage, especially in Black women who also may be using heat styling or chemical relaxers.5 As a result, ketoconazole should be used with caution in Black women, with an emphasis on direct application to the scalp rather than the hair shafts.12 Additional options reported for Black patients include ciclopirox olamine and zinc pyrithione, which may have fewer risks.13
When prescribing medicated shampoos, traditional instructions regarding frequency of use to control symptoms of SD range from 2 to 3 times weekly to daily for a specified period of time determined by the dermatologist.14 However, frequency of hair washing varies greatly among Black patients, sometimes occurring only once monthly. The frequency also may change based on styling techniques (eg, braids, weaves, and wigs).15 Based on previous research underscoring the tendency for Black patients to use medicated shampoos less frequently than White patients, it is important for clinicians to understand that these cultural practices can undermine the effectiveness when medicated shampoos are prescribed for SD.16
Additionally, topical corticosteroids often are used in conjunction with antifungals to help decrease inflammation of the scalp.17 An option reported for Black patients is topical fluocinolone 0.01%; however, package instructions state to apply topically to the scalp nightly and wash the hair thoroughly each morning, which may not be feasible for Black patients based on previously mentioned differences in hair-washing techniques. An alternative option may be to apply the medication 3 to 4 times per week, washing the hair weekly rather than daily.18 Fluocinolone can be used as an ointment, solution, oil, or cream.19,20 When comparing treatment vehicles for SD, a study conducted by Chappell et al21 found that Black patients preferred using ointment or oil vehicles; White patients preferred foams and sprays, which may not be suitable for Afro hair patterns. As such, using less-drying modalities may increase compliance and treatment success in Black patients. For patients who may have involvement on the hairline, face, or ears along with hypopigmentation (which is a common skin concern associated with SD), calcineurin inhibitors can be used until resolution occurs.5,22 High et al15 found that twice-daily use of pimecrolimus rapidly normalized skin pigmentation during the first 2 weeks of use. Overall, personalization of treatment may not only avoid adverse effects but also ensure patient compliance, with the overall goal of treating to reduce yeast activity, pruritus, and dyschromia.22
Interestingly, after the website searches were completed for this study, the US Food and Drug Administration approved topical roflumilast foam for SD. In a phase III trial of 457 total patients, 36 Black patients were included.23 It was determined that 79.5% of patients overall throughout the trial achieved Investigator Global Assessment success (score of 0 [clear] or 1 [almost clear]) plus ≥2-point improvement from baseline (on a scale of 0 [clear] to 4 [severe]) at weeks 2, 4, and 8. Although there currently are no long-term studies, roflumilast may be a promising option for Black patients with SD.23
Aside from developing an individualized treatment approach for Black patients with SD, it is important to ask targeted questions during the clinical encounter to identify factors that may be exacerbating symptoms, especially due to the wide range of hair care practices used by the Black community (eTable 2). Asking targeted questions is especially important, as prior studies have shown that extensions, hair relaxers, and particular hair products can irritate the scalp and increase the likelihood of developing SD.21,24 Rucker Wright et al25 evaluated different hair care practices among young Black females and their association with the development of SD. The authors found that using hair extensions (either braided, cornrowed, or ponytails), chemical relaxers, and hair oils every 2 weeks was associated with SD. The study also found that SD rates were roughly 20% higher among Black girls with extensions compared to Black girls without extensions, regardless of how frequently hair was washed.25

Many Black patients grease the scalp with oils that are beneficial for lubrication and reduction of abrasive damage caused by grooming; however, they also may increase incidence of SD.26 Tight curls worn by Black patients also can impede sebum from traveling down the hair shaft, leading to oil buildup on the scalp. This is the ideal environment for increased Malassezia density and higher risk for SD development.27 To balance the beneficial effects of hair oils with the increased susceptibility for SD, providers should emphasize applying these oils only to distal hair shafts, which are more likely to be damaged, and avoiding application to the scalp.19
Conclusion
Given its long-term relapsing and remitting nature, SD can be distressing for Black patients, many of whom may seek additional treatment options aside from those recommended by health care professionals. In order to better educate patients, it is important for dermatologists to know not only the common ingredients that may be present in OTC products but also the thought process behind why patients use them. Additionally, prescription treatments for Black patients with SD may require nuanced alterations to the product instructions that may prevent health disparities and provide culturally sensitive care. Overall, the literature regarding treatment for Black patients with SD is limited, and more high-quality studies are needed.
- Tucker D, Masood S. Seborrheic dermatitis. StatPearls [Internet]. Updated March 1, 2024. Accessed December 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK551707/
- Borda LJ, Wikramanayake TC. Seborrheic dermatitis and dandruff: a comprehensive review. J Clin Investig Dermatol. 2015;3:10.13188 /2373-1044.1000019.
- American Academy of Dermatology. Seborrheic dermatitis by the numbers. American Academy of Dermatology Skin Disease Briefs. Updated May 5, 2018. Accessed November 22, 2024. https://www.aad.org/asset/49w949DPcF8RSJYIRHfDon
- Davis SA, Naarahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Borda LJ, Perper M, Keri JE. Treatment of seborrheic dermatitis: a comprehensive review. J Dermatolog Treat. 2019;30:158-169.
- Draelos ZD, Kenneally DC, Hodges LT, et al. A comparison of hair quality and cosmetic acceptance following the use of two anti-dandruff shampoos. J Investig Dermatol Symp Proc. 2005;10:201-214.
- Barak-Shinar D, Green LJ. Scalp seborrheic dermatitis and dandruff therapy using a herbal and zinc pyrithione-based therapy of shampoo and scalp lotion. J Clin Aesthet Dermatol. 2018;11:26-31.
- Satchell AC, Saurajen A, Bell C, et al. Treatment of dandruff with 5% tea tree oil shampoo. J Am Acad Dermatol. 2002;47:852-855.
- Herro E, Jacob SE. Mentha piperita (peppermint). Dermatitis. 2010;21:327-329.
- Sanfilippo A, English JC. An overview of medicated shampoos used in dandruff treatment. Pharm Ther. 2006;31:396-400.
- Arun PVPS, Vineetha Y, Waheed M, et al. Quantification of the minimum amount of lemon juice and apple cider vinegar required for the growth inhibition of dandruff causing fungi Malassezia furfur. Int J Sci Res in Biological Sciences. 2019;6:144-147.
- Gao HY, Li Wan Po A. Topical formulations of fluocinolone acetonide. Are creams, gels and ointments bioequivalent and does dilution affect activity? Eur J Clin Pharmacol. 1994;46:71-75.
- Pauporte M, Maibach H, Lowe N, et al. Fluocinolone acetonide topical oil for scalp psoriasis. J Dermatolog Treat. 2004;15:360-364.
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- High WA, Pandya AG. Pilot trial of 1% pimecrolimus cream in the treatment of seborrheic dermatitis in African American adults with associated hypopigmentation. J Am Acad Dermatol. 2006;54:1083-1088.
- Hollins LC, Butt M, Hong J, et al. Research in brief: survey of hair care practices in various ethnic and racial pediatric populations. Pediatr Dermatol. 2022;39:494-496.
- Halder RM, Roberts CI, Nootheti PK. Cutaneous diseases in the black races. Dermatol Clin. 2003;21:679-687, ix.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Friedmann DP, Mishra V, Batty T. Progressive facial papules in an African- American patient: an atypical presentation of seborrheic dermatitis. J Clin Aesthet Dermatol. 2018;11:44-45.
- Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
- Chappell J, Mattox A, Simonetta C, et al. Seborrheic dermatitis of the scalp in populations practicing less frequent hair washing: ketoconazole 2% foam versus ketoconazole 2% shampoo. three-year data. J Am Acad Dermatol. 2014;70:AB54.
- Dadzie OE, Salam A. The hair grooming practices of women of African descent in London, United Kingdom: findings of a cross-sectional study. J Eur Acad Dermatol Venereol. 2016;30:1021-1024.
- Blauvelt A, Draelos ZD, Stein Gold L, et al. Roflumilast foam 0.3% for adolescent and adult patients with seborrheic dermatitis: a randomized, double-blinded, vehicle-controlled, phase 3 trial. J Am Acad Dermatol. 2024;90:986-993.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262.
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319.
- Mayo T, Dinkins J, Elewski B. Hair oils may worsen seborrheic dermatitis in Black patients. Skin Appendage Disord. 2023;9:151-152.
Seborrheic dermatitis (SD) is a common chronic inflammatory skin condition that predominantly affects areas with high concentrations of sebaceous glands such as the scalp and face. Up to 5% of the worldwide population is affected by SD each year, causing a major burden of disease for patients and the health care system.1 In 2023, the cost of medical treatment for SD in the United States was $300 million, with outpatient office visits alone costing $58 million and prescription drugs costing $109 million. Indirect costs of disease (eg, lost workdays) account for another $51 million.1 Since SD frequently manifests on the face, it tends to have negative effects on the patient’s quality of life, resulting in psychological distress and low self-esteem.2
Patients with SD may describe symptoms of excessive dandruff and itching along with hyperpigmentation or hypopigmentation of the skin; Black patients tend to present with the classic manifestations: a combination of scaling, flaking, and erythematous patches on the scalp, ears, and face, particularly around the eyebrows, eyelids, and nose. With SD being the second most common diagnosis in Black patients who seek care from a dermatologist, it is important to have effective treatment approaches for SD in this patient population.3
In this study, we aimed to evaluate medical and nonmedical treatment options for SD in Black patients by identifying common practices and products mentioned on consumer websites and in the medical literature.
Methods
A Google search was conducted during 2 time periods (September 2022—October 2022 and March 2023—April 2023) using the terms products for itchy scalp in Black patients, products for dandruff in Black patients, itchy scalp in Black women, itchy scalp in Black men, treatment for scalp itch in Black patients, and dry scalp in Black hair. Products that were recommended by at least 1 website on the first page of search results were included in our list of products, and the ingredients were reviewed by the authors. We excluded individual retailer websites as well as those that did not provide specific recommendations on products or ingredients to use when treating SD. To ensure reliability and standardization, we did not review products that were suggested by ads in the shopping section on the first page of search results.
We also evaluated medical treatments used for SD in dermatology literature. A PubMed search of articles indexed for MEDLINE using the terms seborrheic dermatitis treatment for Black patients, treatment for dandruff for Black patients, and seborrheic dermatitis and skin of color was conducted. We excluded articles that did not address treatment options for SD, were specific to treating SD in patient populations with specific comorbidities being studied, discussed SD in animals, or were published prior to 1990.
Results
We identified 16 unique consumer websites with product or ingredient recommendations for SD in Black patients, none of which were provided by authors with a medical or scientific background; however, 4 (25%) websites included insights from board-certified dermatologists. A total of 16 ingredients were recommended, 15 (94%) of which were mentioned at least twice in our search results (eTable 1).

Overall, we noticed that ingredients labeled as natural or organic were common in over-the-counter (OTC) products, and ingredients such as sulfates and parabens were avoided. Common OTC ingredients for antidandruff and anti-itch shampoos and conditioners include zinc pyrithione, selenium sulfide, coal tar, salicylic acid, and citric acid. Additionally, coconut oil, tea tree oil, apple cider vinegar, and charcoal are common natural alternatives used to address SD symptoms.
Our review of the literature yielded limited recommendations tailored specifically to Black patients with SD. Of 108 abstracts, articles, or textbook chapters providing treatment recommendations for SD, 6 (6%) specifically discussed treatments for Black patients. All articles were written by authors with medical or scientific backgrounds. Of the treatment options discussed, topical antifungals generally were considered first-line for SD in all patients, with ketoconazole shampoo being a common first choice.4,5
Comment
Our study indicated that many consumer websites recommend unstudied nonmedical treatments for SD. Zinc pyrithione was one of the most commonly mentioned ingredients in OTC products to treat SD targeted toward Black patients, as its properties have contributed to ease of hair combing and less frizz.6 Zinc pyrithione has antifungal properties that reduce the proliferation of Malassezia furfur as well as anti-inflammatory properties that reduce irritation, pruritus, and erythema in areas affected by SD.7 Tea tree and peppermint oils also were commonly mentioned; the theory is that these oils mitigate SD by reducing yeast growth and soothing inflammation through antioxidant activity.8,9 Coal tar also is used due to its keratoplastic properties, which slow the growth of skin cells and ultimately reduce scaling and dryness.10 Yeast thrives in basic pH conditions; apple cider vinegar is used as an ingredient in OTC products for SD because its acidic pH creates a less favorable environment for yeast to grow.11 Although many of the ingredients found in OTC products we identified have not yet been studied, they have properties that theoretically would be helpful in treating SD.
Our review of the medical literature revealed that while there are treatments that are effective for SD, the recommended use may not consider the cultural differences that exist for Black patients. For instance, reports in the literature regarding ketoconazole shampoo revealed that ketoconazole increases the risk for hair shaft dryness, damage, and subsequent breakage, especially in Black women who also may be using heat styling or chemical relaxers.5 As a result, ketoconazole should be used with caution in Black women, with an emphasis on direct application to the scalp rather than the hair shafts.12 Additional options reported for Black patients include ciclopirox olamine and zinc pyrithione, which may have fewer risks.13
When prescribing medicated shampoos, traditional instructions regarding frequency of use to control symptoms of SD range from 2 to 3 times weekly to daily for a specified period of time determined by the dermatologist.14 However, frequency of hair washing varies greatly among Black patients, sometimes occurring only once monthly. The frequency also may change based on styling techniques (eg, braids, weaves, and wigs).15 Based on previous research underscoring the tendency for Black patients to use medicated shampoos less frequently than White patients, it is important for clinicians to understand that these cultural practices can undermine the effectiveness when medicated shampoos are prescribed for SD.16
Additionally, topical corticosteroids often are used in conjunction with antifungals to help decrease inflammation of the scalp.17 An option reported for Black patients is topical fluocinolone 0.01%; however, package instructions state to apply topically to the scalp nightly and wash the hair thoroughly each morning, which may not be feasible for Black patients based on previously mentioned differences in hair-washing techniques. An alternative option may be to apply the medication 3 to 4 times per week, washing the hair weekly rather than daily.18 Fluocinolone can be used as an ointment, solution, oil, or cream.19,20 When comparing treatment vehicles for SD, a study conducted by Chappell et al21 found that Black patients preferred using ointment or oil vehicles; White patients preferred foams and sprays, which may not be suitable for Afro hair patterns. As such, using less-drying modalities may increase compliance and treatment success in Black patients. For patients who may have involvement on the hairline, face, or ears along with hypopigmentation (which is a common skin concern associated with SD), calcineurin inhibitors can be used until resolution occurs.5,22 High et al15 found that twice-daily use of pimecrolimus rapidly normalized skin pigmentation during the first 2 weeks of use. Overall, personalization of treatment may not only avoid adverse effects but also ensure patient compliance, with the overall goal of treating to reduce yeast activity, pruritus, and dyschromia.22
Interestingly, after the website searches were completed for this study, the US Food and Drug Administration approved topical roflumilast foam for SD. In a phase III trial of 457 total patients, 36 Black patients were included.23 It was determined that 79.5% of patients overall throughout the trial achieved Investigator Global Assessment success (score of 0 [clear] or 1 [almost clear]) plus ≥2-point improvement from baseline (on a scale of 0 [clear] to 4 [severe]) at weeks 2, 4, and 8. Although there currently are no long-term studies, roflumilast may be a promising option for Black patients with SD.23
Aside from developing an individualized treatment approach for Black patients with SD, it is important to ask targeted questions during the clinical encounter to identify factors that may be exacerbating symptoms, especially due to the wide range of hair care practices used by the Black community (eTable 2). Asking targeted questions is especially important, as prior studies have shown that extensions, hair relaxers, and particular hair products can irritate the scalp and increase the likelihood of developing SD.21,24 Rucker Wright et al25 evaluated different hair care practices among young Black females and their association with the development of SD. The authors found that using hair extensions (either braided, cornrowed, or ponytails), chemical relaxers, and hair oils every 2 weeks was associated with SD. The study also found that SD rates were roughly 20% higher among Black girls with extensions compared to Black girls without extensions, regardless of how frequently hair was washed.25

Many Black patients grease the scalp with oils that are beneficial for lubrication and reduction of abrasive damage caused by grooming; however, they also may increase incidence of SD.26 Tight curls worn by Black patients also can impede sebum from traveling down the hair shaft, leading to oil buildup on the scalp. This is the ideal environment for increased Malassezia density and higher risk for SD development.27 To balance the beneficial effects of hair oils with the increased susceptibility for SD, providers should emphasize applying these oils only to distal hair shafts, which are more likely to be damaged, and avoiding application to the scalp.19
Conclusion
Given its long-term relapsing and remitting nature, SD can be distressing for Black patients, many of whom may seek additional treatment options aside from those recommended by health care professionals. In order to better educate patients, it is important for dermatologists to know not only the common ingredients that may be present in OTC products but also the thought process behind why patients use them. Additionally, prescription treatments for Black patients with SD may require nuanced alterations to the product instructions that may prevent health disparities and provide culturally sensitive care. Overall, the literature regarding treatment for Black patients with SD is limited, and more high-quality studies are needed.
Seborrheic dermatitis (SD) is a common chronic inflammatory skin condition that predominantly affects areas with high concentrations of sebaceous glands such as the scalp and face. Up to 5% of the worldwide population is affected by SD each year, causing a major burden of disease for patients and the health care system.1 In 2023, the cost of medical treatment for SD in the United States was $300 million, with outpatient office visits alone costing $58 million and prescription drugs costing $109 million. Indirect costs of disease (eg, lost workdays) account for another $51 million.1 Since SD frequently manifests on the face, it tends to have negative effects on the patient’s quality of life, resulting in psychological distress and low self-esteem.2
Patients with SD may describe symptoms of excessive dandruff and itching along with hyperpigmentation or hypopigmentation of the skin; Black patients tend to present with the classic manifestations: a combination of scaling, flaking, and erythematous patches on the scalp, ears, and face, particularly around the eyebrows, eyelids, and nose. With SD being the second most common diagnosis in Black patients who seek care from a dermatologist, it is important to have effective treatment approaches for SD in this patient population.3
In this study, we aimed to evaluate medical and nonmedical treatment options for SD in Black patients by identifying common practices and products mentioned on consumer websites and in the medical literature.
Methods
A Google search was conducted during 2 time periods (September 2022—October 2022 and March 2023—April 2023) using the terms products for itchy scalp in Black patients, products for dandruff in Black patients, itchy scalp in Black women, itchy scalp in Black men, treatment for scalp itch in Black patients, and dry scalp in Black hair. Products that were recommended by at least 1 website on the first page of search results were included in our list of products, and the ingredients were reviewed by the authors. We excluded individual retailer websites as well as those that did not provide specific recommendations on products or ingredients to use when treating SD. To ensure reliability and standardization, we did not review products that were suggested by ads in the shopping section on the first page of search results.
We also evaluated medical treatments used for SD in dermatology literature. A PubMed search of articles indexed for MEDLINE using the terms seborrheic dermatitis treatment for Black patients, treatment for dandruff for Black patients, and seborrheic dermatitis and skin of color was conducted. We excluded articles that did not address treatment options for SD, were specific to treating SD in patient populations with specific comorbidities being studied, discussed SD in animals, or were published prior to 1990.
Results
We identified 16 unique consumer websites with product or ingredient recommendations for SD in Black patients, none of which were provided by authors with a medical or scientific background; however, 4 (25%) websites included insights from board-certified dermatologists. A total of 16 ingredients were recommended, 15 (94%) of which were mentioned at least twice in our search results (eTable 1).

Overall, we noticed that ingredients labeled as natural or organic were common in over-the-counter (OTC) products, and ingredients such as sulfates and parabens were avoided. Common OTC ingredients for antidandruff and anti-itch shampoos and conditioners include zinc pyrithione, selenium sulfide, coal tar, salicylic acid, and citric acid. Additionally, coconut oil, tea tree oil, apple cider vinegar, and charcoal are common natural alternatives used to address SD symptoms.
Our review of the literature yielded limited recommendations tailored specifically to Black patients with SD. Of 108 abstracts, articles, or textbook chapters providing treatment recommendations for SD, 6 (6%) specifically discussed treatments for Black patients. All articles were written by authors with medical or scientific backgrounds. Of the treatment options discussed, topical antifungals generally were considered first-line for SD in all patients, with ketoconazole shampoo being a common first choice.4,5
Comment
Our study indicated that many consumer websites recommend unstudied nonmedical treatments for SD. Zinc pyrithione was one of the most commonly mentioned ingredients in OTC products to treat SD targeted toward Black patients, as its properties have contributed to ease of hair combing and less frizz.6 Zinc pyrithione has antifungal properties that reduce the proliferation of Malassezia furfur as well as anti-inflammatory properties that reduce irritation, pruritus, and erythema in areas affected by SD.7 Tea tree and peppermint oils also were commonly mentioned; the theory is that these oils mitigate SD by reducing yeast growth and soothing inflammation through antioxidant activity.8,9 Coal tar also is used due to its keratoplastic properties, which slow the growth of skin cells and ultimately reduce scaling and dryness.10 Yeast thrives in basic pH conditions; apple cider vinegar is used as an ingredient in OTC products for SD because its acidic pH creates a less favorable environment for yeast to grow.11 Although many of the ingredients found in OTC products we identified have not yet been studied, they have properties that theoretically would be helpful in treating SD.
Our review of the medical literature revealed that while there are treatments that are effective for SD, the recommended use may not consider the cultural differences that exist for Black patients. For instance, reports in the literature regarding ketoconazole shampoo revealed that ketoconazole increases the risk for hair shaft dryness, damage, and subsequent breakage, especially in Black women who also may be using heat styling or chemical relaxers.5 As a result, ketoconazole should be used with caution in Black women, with an emphasis on direct application to the scalp rather than the hair shafts.12 Additional options reported for Black patients include ciclopirox olamine and zinc pyrithione, which may have fewer risks.13
When prescribing medicated shampoos, traditional instructions regarding frequency of use to control symptoms of SD range from 2 to 3 times weekly to daily for a specified period of time determined by the dermatologist.14 However, frequency of hair washing varies greatly among Black patients, sometimes occurring only once monthly. The frequency also may change based on styling techniques (eg, braids, weaves, and wigs).15 Based on previous research underscoring the tendency for Black patients to use medicated shampoos less frequently than White patients, it is important for clinicians to understand that these cultural practices can undermine the effectiveness when medicated shampoos are prescribed for SD.16
Additionally, topical corticosteroids often are used in conjunction with antifungals to help decrease inflammation of the scalp.17 An option reported for Black patients is topical fluocinolone 0.01%; however, package instructions state to apply topically to the scalp nightly and wash the hair thoroughly each morning, which may not be feasible for Black patients based on previously mentioned differences in hair-washing techniques. An alternative option may be to apply the medication 3 to 4 times per week, washing the hair weekly rather than daily.18 Fluocinolone can be used as an ointment, solution, oil, or cream.19,20 When comparing treatment vehicles for SD, a study conducted by Chappell et al21 found that Black patients preferred using ointment or oil vehicles; White patients preferred foams and sprays, which may not be suitable for Afro hair patterns. As such, using less-drying modalities may increase compliance and treatment success in Black patients. For patients who may have involvement on the hairline, face, or ears along with hypopigmentation (which is a common skin concern associated with SD), calcineurin inhibitors can be used until resolution occurs.5,22 High et al15 found that twice-daily use of pimecrolimus rapidly normalized skin pigmentation during the first 2 weeks of use. Overall, personalization of treatment may not only avoid adverse effects but also ensure patient compliance, with the overall goal of treating to reduce yeast activity, pruritus, and dyschromia.22
Interestingly, after the website searches were completed for this study, the US Food and Drug Administration approved topical roflumilast foam for SD. In a phase III trial of 457 total patients, 36 Black patients were included.23 It was determined that 79.5% of patients overall throughout the trial achieved Investigator Global Assessment success (score of 0 [clear] or 1 [almost clear]) plus ≥2-point improvement from baseline (on a scale of 0 [clear] to 4 [severe]) at weeks 2, 4, and 8. Although there currently are no long-term studies, roflumilast may be a promising option for Black patients with SD.23
Aside from developing an individualized treatment approach for Black patients with SD, it is important to ask targeted questions during the clinical encounter to identify factors that may be exacerbating symptoms, especially due to the wide range of hair care practices used by the Black community (eTable 2). Asking targeted questions is especially important, as prior studies have shown that extensions, hair relaxers, and particular hair products can irritate the scalp and increase the likelihood of developing SD.21,24 Rucker Wright et al25 evaluated different hair care practices among young Black females and their association with the development of SD. The authors found that using hair extensions (either braided, cornrowed, or ponytails), chemical relaxers, and hair oils every 2 weeks was associated with SD. The study also found that SD rates were roughly 20% higher among Black girls with extensions compared to Black girls without extensions, regardless of how frequently hair was washed.25

Many Black patients grease the scalp with oils that are beneficial for lubrication and reduction of abrasive damage caused by grooming; however, they also may increase incidence of SD.26 Tight curls worn by Black patients also can impede sebum from traveling down the hair shaft, leading to oil buildup on the scalp. This is the ideal environment for increased Malassezia density and higher risk for SD development.27 To balance the beneficial effects of hair oils with the increased susceptibility for SD, providers should emphasize applying these oils only to distal hair shafts, which are more likely to be damaged, and avoiding application to the scalp.19
Conclusion
Given its long-term relapsing and remitting nature, SD can be distressing for Black patients, many of whom may seek additional treatment options aside from those recommended by health care professionals. In order to better educate patients, it is important for dermatologists to know not only the common ingredients that may be present in OTC products but also the thought process behind why patients use them. Additionally, prescription treatments for Black patients with SD may require nuanced alterations to the product instructions that may prevent health disparities and provide culturally sensitive care. Overall, the literature regarding treatment for Black patients with SD is limited, and more high-quality studies are needed.
- Tucker D, Masood S. Seborrheic dermatitis. StatPearls [Internet]. Updated March 1, 2024. Accessed December 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK551707/
- Borda LJ, Wikramanayake TC. Seborrheic dermatitis and dandruff: a comprehensive review. J Clin Investig Dermatol. 2015;3:10.13188 /2373-1044.1000019.
- American Academy of Dermatology. Seborrheic dermatitis by the numbers. American Academy of Dermatology Skin Disease Briefs. Updated May 5, 2018. Accessed November 22, 2024. https://www.aad.org/asset/49w949DPcF8RSJYIRHfDon
- Davis SA, Naarahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Borda LJ, Perper M, Keri JE. Treatment of seborrheic dermatitis: a comprehensive review. J Dermatolog Treat. 2019;30:158-169.
- Draelos ZD, Kenneally DC, Hodges LT, et al. A comparison of hair quality and cosmetic acceptance following the use of two anti-dandruff shampoos. J Investig Dermatol Symp Proc. 2005;10:201-214.
- Barak-Shinar D, Green LJ. Scalp seborrheic dermatitis and dandruff therapy using a herbal and zinc pyrithione-based therapy of shampoo and scalp lotion. J Clin Aesthet Dermatol. 2018;11:26-31.
- Satchell AC, Saurajen A, Bell C, et al. Treatment of dandruff with 5% tea tree oil shampoo. J Am Acad Dermatol. 2002;47:852-855.
- Herro E, Jacob SE. Mentha piperita (peppermint). Dermatitis. 2010;21:327-329.
- Sanfilippo A, English JC. An overview of medicated shampoos used in dandruff treatment. Pharm Ther. 2006;31:396-400.
- Arun PVPS, Vineetha Y, Waheed M, et al. Quantification of the minimum amount of lemon juice and apple cider vinegar required for the growth inhibition of dandruff causing fungi Malassezia furfur. Int J Sci Res in Biological Sciences. 2019;6:144-147.
- Gao HY, Li Wan Po A. Topical formulations of fluocinolone acetonide. Are creams, gels and ointments bioequivalent and does dilution affect activity? Eur J Clin Pharmacol. 1994;46:71-75.
- Pauporte M, Maibach H, Lowe N, et al. Fluocinolone acetonide topical oil for scalp psoriasis. J Dermatolog Treat. 2004;15:360-364.
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- High WA, Pandya AG. Pilot trial of 1% pimecrolimus cream in the treatment of seborrheic dermatitis in African American adults with associated hypopigmentation. J Am Acad Dermatol. 2006;54:1083-1088.
- Hollins LC, Butt M, Hong J, et al. Research in brief: survey of hair care practices in various ethnic and racial pediatric populations. Pediatr Dermatol. 2022;39:494-496.
- Halder RM, Roberts CI, Nootheti PK. Cutaneous diseases in the black races. Dermatol Clin. 2003;21:679-687, ix.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Friedmann DP, Mishra V, Batty T. Progressive facial papules in an African- American patient: an atypical presentation of seborrheic dermatitis. J Clin Aesthet Dermatol. 2018;11:44-45.
- Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
- Chappell J, Mattox A, Simonetta C, et al. Seborrheic dermatitis of the scalp in populations practicing less frequent hair washing: ketoconazole 2% foam versus ketoconazole 2% shampoo. three-year data. J Am Acad Dermatol. 2014;70:AB54.
- Dadzie OE, Salam A. The hair grooming practices of women of African descent in London, United Kingdom: findings of a cross-sectional study. J Eur Acad Dermatol Venereol. 2016;30:1021-1024.
- Blauvelt A, Draelos ZD, Stein Gold L, et al. Roflumilast foam 0.3% for adolescent and adult patients with seborrheic dermatitis: a randomized, double-blinded, vehicle-controlled, phase 3 trial. J Am Acad Dermatol. 2024;90:986-993.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262.
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319.
- Mayo T, Dinkins J, Elewski B. Hair oils may worsen seborrheic dermatitis in Black patients. Skin Appendage Disord. 2023;9:151-152.
- Tucker D, Masood S. Seborrheic dermatitis. StatPearls [Internet]. Updated March 1, 2024. Accessed December 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK551707/
- Borda LJ, Wikramanayake TC. Seborrheic dermatitis and dandruff: a comprehensive review. J Clin Investig Dermatol. 2015;3:10.13188 /2373-1044.1000019.
- American Academy of Dermatology. Seborrheic dermatitis by the numbers. American Academy of Dermatology Skin Disease Briefs. Updated May 5, 2018. Accessed November 22, 2024. https://www.aad.org/asset/49w949DPcF8RSJYIRHfDon
- Davis SA, Naarahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Borda LJ, Perper M, Keri JE. Treatment of seborrheic dermatitis: a comprehensive review. J Dermatolog Treat. 2019;30:158-169.
- Draelos ZD, Kenneally DC, Hodges LT, et al. A comparison of hair quality and cosmetic acceptance following the use of two anti-dandruff shampoos. J Investig Dermatol Symp Proc. 2005;10:201-214.
- Barak-Shinar D, Green LJ. Scalp seborrheic dermatitis and dandruff therapy using a herbal and zinc pyrithione-based therapy of shampoo and scalp lotion. J Clin Aesthet Dermatol. 2018;11:26-31.
- Satchell AC, Saurajen A, Bell C, et al. Treatment of dandruff with 5% tea tree oil shampoo. J Am Acad Dermatol. 2002;47:852-855.
- Herro E, Jacob SE. Mentha piperita (peppermint). Dermatitis. 2010;21:327-329.
- Sanfilippo A, English JC. An overview of medicated shampoos used in dandruff treatment. Pharm Ther. 2006;31:396-400.
- Arun PVPS, Vineetha Y, Waheed M, et al. Quantification of the minimum amount of lemon juice and apple cider vinegar required for the growth inhibition of dandruff causing fungi Malassezia furfur. Int J Sci Res in Biological Sciences. 2019;6:144-147.
- Gao HY, Li Wan Po A. Topical formulations of fluocinolone acetonide. Are creams, gels and ointments bioequivalent and does dilution affect activity? Eur J Clin Pharmacol. 1994;46:71-75.
- Pauporte M, Maibach H, Lowe N, et al. Fluocinolone acetonide topical oil for scalp psoriasis. J Dermatolog Treat. 2004;15:360-364.
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- High WA, Pandya AG. Pilot trial of 1% pimecrolimus cream in the treatment of seborrheic dermatitis in African American adults with associated hypopigmentation. J Am Acad Dermatol. 2006;54:1083-1088.
- Hollins LC, Butt M, Hong J, et al. Research in brief: survey of hair care practices in various ethnic and racial pediatric populations. Pediatr Dermatol. 2022;39:494-496.
- Halder RM, Roberts CI, Nootheti PK. Cutaneous diseases in the black races. Dermatol Clin. 2003;21:679-687, ix.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Friedmann DP, Mishra V, Batty T. Progressive facial papules in an African- American patient: an atypical presentation of seborrheic dermatitis. J Clin Aesthet Dermatol. 2018;11:44-45.
- Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
- Chappell J, Mattox A, Simonetta C, et al. Seborrheic dermatitis of the scalp in populations practicing less frequent hair washing: ketoconazole 2% foam versus ketoconazole 2% shampoo. three-year data. J Am Acad Dermatol. 2014;70:AB54.
- Dadzie OE, Salam A. The hair grooming practices of women of African descent in London, United Kingdom: findings of a cross-sectional study. J Eur Acad Dermatol Venereol. 2016;30:1021-1024.
- Blauvelt A, Draelos ZD, Stein Gold L, et al. Roflumilast foam 0.3% for adolescent and adult patients with seborrheic dermatitis: a randomized, double-blinded, vehicle-controlled, phase 3 trial. J Am Acad Dermatol. 2024;90:986-993.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262.
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319.
- Mayo T, Dinkins J, Elewski B. Hair oils may worsen seborrheic dermatitis in Black patients. Skin Appendage Disord. 2023;9:151-152.
Treatment of Seborrheic Dermatitis in Black Patients
Treatment of Seborrheic Dermatitis in Black Patients
PRACTICE POINTS
- Cultural awareness when treating Black patients with seborrheic dermatitis is vital to providing appropriate care, as hair care practices may impact treatment options and regimen.
- Knowledge about over-the-counter products that are targeted toward Black patients and the ingredients they contain can assist in providing better counseling to patients and improve shared decision-making.
Best Practices for Capturing Clinical and Dermoscopic Images With Smartphone Photography
Best Practices for Capturing Clinical and Dermoscopic Images With Smartphone Photography
PRACTICE GAP
Photography is an essential tool in modern dermatologic practice, aiding in the evaluation, documentation, and monitoring of nevi, skin cancers, and other cutaneous pathologies.1 With the rapid technologic advancement of smartphone cameras, high-quality clinical and dermoscopic images have become increasingly easy to attain; however, best practices for optimizing smartphone photography are limited in the medical literature. We have collated a series of recommendations to help fill this knowledge gap.
A search of PubMed articles indexed for MEDLINE was conducted using the terms clinical imaging AND smartphone, clinical photography AND smartphone, dermatology AND photography, dermatology AND imaging, dermoscopy AND photography, and dermoscopy AND imaging. We also consulted with Elizabeth Seiverling, MD (Annville, Pennsylvania) and Jennifer Stein, MD (New York, New York)—both renowned experts in the fields of dermatology, dermoscopy, and medical photography—via email and video meetings conducted during the period from June 1, 2022, through August 20, 2022. Our goal in creating this guide is to facilitate standardized yet simple ways to integrate smartphone photography into current dermatologic practice.
THE TECHNIQUE
Clinical Photography
Clinical images should be captured in a space with ample indirect natural light, such as a patient examination room with frosted or draped windows, ensuring patient privacy is maintained.1,2 The smartphone’s flash can be used if natural lighting is insufficient, but caution should be exercised when photographing patients with darker skin types, as the flash may create an undesired glare. To combat this, consider using a small clip-on light-emitting diode ring light positioned at a 45° angle for more uniform lighting and reduced glare (eFigures 1 and 2).2 This additional light source helps to distribute light evenly across the patient’s skin, enhancing detail visibility, minimizing harsh shadows, and ensuring a more accurate representation of skin pigmentation.2

When a magnified image is required (eg, to capture suspicious lesions with unique and detailed findings such as irregular borders or atypical pigmentation), use the smartphone’s digital zoom function rather than physically moving the camera lens closer to the subject. Moving the camera too close can cause proximity distortion, artificially enlarging objects close to the lens and degrading the quality of the image.1,2 Unnecessary camera features such as portrait mode, live focus, and filters should be turned off to maintain image accuracy. It also is important to avoid excessive manual adjustments to exposure and brightness settings.1,2 The tap-to-focus feature that is integrated into many smartphone cameras can be utilized to ensure the capture of sharp, focused images. After verifying the image preview on the smartphone display, take the photograph. Immediately review the captured image to ensure it is clear and well lit and accurately depicts the area of interest, including its color, texture, and any relevant details, without glare or distortion. If the image does not meet these criteria, promptly reattempt to achieve the desired quality.
Dermoscopic Photography
Dermoscopy, which enables magnified examination of skin lesions, is increasingly being utilized in dermatology. While traditional dermoscopic photography requires specialized equipment, such as large single-lens reflex cameras with dedicated dermoscopic lens attachments, smartphone cameras now can be used to obtain dermoscopic images of reasonable quality.3,4 Adhering to specific practices can help to optimize the quality of dermoscopic images obtained via this technique.
Before capturing an image, it is essential to prepare both the lesion and the surrounding skin. Ensure the area is cleaned thoroughly and trim any hairs that may obscure the image. Apply an interface fluid such as rubbing alcohol or ultrasonography gel to improve image clarity by reducing surface tension and reflections, minimizing glare, and ensuring even light transmission throughout the lesion.5 As recommended for clinical photography, images should be captured in a space with ample indirect light. For best results, we recommend utilizing the primary photo capture option instead of portrait or panoramic mode or additional settings. It is crucial to disable features such as live focus, filters, night mode, and flash, as they may alter image accuracy; however, use of the tap-to-focus feature or manual settings adjustment is encouraged to ensure a high-resolution photograph.
Once these smartphone settings have been verified, position the dermatoscope directly over the lesion of interest. Next, place the smartphone camera lens directly against the eyepiece of the dermatoscope (Figure). Center the lesion in the field of view on the screen. Most smartphones enable adjustment to the image magnification on the photo capture screen. A single tap on the screen should populate the zoom options (eg, ×0.5, ×1, ×3) and allow for adjustment. For the majority of dermoscopic photographs, we recommend standard ×1 magnification, as it typically provides a clear and accurate representation of the lesion without introducing the possibility of image distortion. To obtain a close-up image, use the smartphone’s digital zoom function prior to taking the photograph rather than zooming in on the image after it has been captured; however, to minimize proximity distortion and maintain optimal image quality, avoid exceeding the halfway point on the camera’s zoom dial. After verifying the image preview on the smartphone display, capture the photograph. Immediate review is recommended to allow for prompt reattempt at capturing the image if needed.
PRACTICE IMPLICATIONS
The inherent convenience and accessibility offered by smartphone photography further solidifies its status as a valuable tool in modern dermatologic practice. By adhering to the best practices outlined in this guide, dermatologists can utilize smartphones to capture high-quality clinical and dermoscopic images that support accurate diagnosis and enhance patient care. This approach helps streamline workflows, enhance consistency in image quality, and standardize image capture across different settings and providers.
Additionally, smartphone photography can enhance both education and telemedicine by enabling physicians to easily share high-quality images with colleagues for virtual consultations, second opinions, and collaborative diagnoses. This sharing of images fosters learning opportunities, supports knowledge exchange, and allows for real-time feedback—all of which can improve clinical decision-making. Moreover, it broadens access to dermatologic expertise, strengthens communication between health care providers, and facilitates timely decision-making. As a result, patients benefit from more efficient, accurate, and collaborative care.
- Muraco L. Improved medical photography: key tips for creating images of lasting value. JAMA Dermatol. 2020;156:121-123. doi:10.1001 /jamadermatol.2019.3849
- Alvarado SM, Flessland P, Grant-Kels JM, et al. Practical strategies for improving clinical photography of dark skin. J Am Acad Dermatol. 2022;86:E21-E23. doi:10.1016/j.jaad.2021.09.001
- Pagliarello C, Feliciani C, Fantini C, et al. Use of the dermoscope as a smartphone close-up lens and LED annular macro ring flash. J Am Acad Dermatol. 2016;75:E27–E28. doi:10.1016/j.jaad .2015.12.04
- Zuo KJ, Guo D, Rao J. Mobile teledermatology: a promising future in clinical practice. J Cutan Med Surg. 2013;17:387-391. doi:10.2310/7750.2013.13030
- Gewirtzman AJ, Saurat J-H, Braun RP. An evaluation of dermscopy fluids and application techniques. Br J Dermatol. 2003;149:59-63. doi:10.1046/j.1365-2133.2003.05366.x
PRACTICE GAP
Photography is an essential tool in modern dermatologic practice, aiding in the evaluation, documentation, and monitoring of nevi, skin cancers, and other cutaneous pathologies.1 With the rapid technologic advancement of smartphone cameras, high-quality clinical and dermoscopic images have become increasingly easy to attain; however, best practices for optimizing smartphone photography are limited in the medical literature. We have collated a series of recommendations to help fill this knowledge gap.
A search of PubMed articles indexed for MEDLINE was conducted using the terms clinical imaging AND smartphone, clinical photography AND smartphone, dermatology AND photography, dermatology AND imaging, dermoscopy AND photography, and dermoscopy AND imaging. We also consulted with Elizabeth Seiverling, MD (Annville, Pennsylvania) and Jennifer Stein, MD (New York, New York)—both renowned experts in the fields of dermatology, dermoscopy, and medical photography—via email and video meetings conducted during the period from June 1, 2022, through August 20, 2022. Our goal in creating this guide is to facilitate standardized yet simple ways to integrate smartphone photography into current dermatologic practice.
THE TECHNIQUE
Clinical Photography
Clinical images should be captured in a space with ample indirect natural light, such as a patient examination room with frosted or draped windows, ensuring patient privacy is maintained.1,2 The smartphone’s flash can be used if natural lighting is insufficient, but caution should be exercised when photographing patients with darker skin types, as the flash may create an undesired glare. To combat this, consider using a small clip-on light-emitting diode ring light positioned at a 45° angle for more uniform lighting and reduced glare (eFigures 1 and 2).2 This additional light source helps to distribute light evenly across the patient’s skin, enhancing detail visibility, minimizing harsh shadows, and ensuring a more accurate representation of skin pigmentation.2


When a magnified image is required (eg, to capture suspicious lesions with unique and detailed findings such as irregular borders or atypical pigmentation), use the smartphone’s digital zoom function rather than physically moving the camera lens closer to the subject. Moving the camera too close can cause proximity distortion, artificially enlarging objects close to the lens and degrading the quality of the image.1,2 Unnecessary camera features such as portrait mode, live focus, and filters should be turned off to maintain image accuracy. It also is important to avoid excessive manual adjustments to exposure and brightness settings.1,2 The tap-to-focus feature that is integrated into many smartphone cameras can be utilized to ensure the capture of sharp, focused images. After verifying the image preview on the smartphone display, take the photograph. Immediately review the captured image to ensure it is clear and well lit and accurately depicts the area of interest, including its color, texture, and any relevant details, without glare or distortion. If the image does not meet these criteria, promptly reattempt to achieve the desired quality.
Dermoscopic Photography
Dermoscopy, which enables magnified examination of skin lesions, is increasingly being utilized in dermatology. While traditional dermoscopic photography requires specialized equipment, such as large single-lens reflex cameras with dedicated dermoscopic lens attachments, smartphone cameras now can be used to obtain dermoscopic images of reasonable quality.3,4 Adhering to specific practices can help to optimize the quality of dermoscopic images obtained via this technique.
Before capturing an image, it is essential to prepare both the lesion and the surrounding skin. Ensure the area is cleaned thoroughly and trim any hairs that may obscure the image. Apply an interface fluid such as rubbing alcohol or ultrasonography gel to improve image clarity by reducing surface tension and reflections, minimizing glare, and ensuring even light transmission throughout the lesion.5 As recommended for clinical photography, images should be captured in a space with ample indirect light. For best results, we recommend utilizing the primary photo capture option instead of portrait or panoramic mode or additional settings. It is crucial to disable features such as live focus, filters, night mode, and flash, as they may alter image accuracy; however, use of the tap-to-focus feature or manual settings adjustment is encouraged to ensure a high-resolution photograph.
Once these smartphone settings have been verified, position the dermatoscope directly over the lesion of interest. Next, place the smartphone camera lens directly against the eyepiece of the dermatoscope (Figure). Center the lesion in the field of view on the screen. Most smartphones enable adjustment to the image magnification on the photo capture screen. A single tap on the screen should populate the zoom options (eg, ×0.5, ×1, ×3) and allow for adjustment. For the majority of dermoscopic photographs, we recommend standard ×1 magnification, as it typically provides a clear and accurate representation of the lesion without introducing the possibility of image distortion. To obtain a close-up image, use the smartphone’s digital zoom function prior to taking the photograph rather than zooming in on the image after it has been captured; however, to minimize proximity distortion and maintain optimal image quality, avoid exceeding the halfway point on the camera’s zoom dial. After verifying the image preview on the smartphone display, capture the photograph. Immediate review is recommended to allow for prompt reattempt at capturing the image if needed.

PRACTICE IMPLICATIONS
The inherent convenience and accessibility offered by smartphone photography further solidifies its status as a valuable tool in modern dermatologic practice. By adhering to the best practices outlined in this guide, dermatologists can utilize smartphones to capture high-quality clinical and dermoscopic images that support accurate diagnosis and enhance patient care. This approach helps streamline workflows, enhance consistency in image quality, and standardize image capture across different settings and providers.
Additionally, smartphone photography can enhance both education and telemedicine by enabling physicians to easily share high-quality images with colleagues for virtual consultations, second opinions, and collaborative diagnoses. This sharing of images fosters learning opportunities, supports knowledge exchange, and allows for real-time feedback—all of which can improve clinical decision-making. Moreover, it broadens access to dermatologic expertise, strengthens communication between health care providers, and facilitates timely decision-making. As a result, patients benefit from more efficient, accurate, and collaborative care.
PRACTICE GAP
Photography is an essential tool in modern dermatologic practice, aiding in the evaluation, documentation, and monitoring of nevi, skin cancers, and other cutaneous pathologies.1 With the rapid technologic advancement of smartphone cameras, high-quality clinical and dermoscopic images have become increasingly easy to attain; however, best practices for optimizing smartphone photography are limited in the medical literature. We have collated a series of recommendations to help fill this knowledge gap.
A search of PubMed articles indexed for MEDLINE was conducted using the terms clinical imaging AND smartphone, clinical photography AND smartphone, dermatology AND photography, dermatology AND imaging, dermoscopy AND photography, and dermoscopy AND imaging. We also consulted with Elizabeth Seiverling, MD (Annville, Pennsylvania) and Jennifer Stein, MD (New York, New York)—both renowned experts in the fields of dermatology, dermoscopy, and medical photography—via email and video meetings conducted during the period from June 1, 2022, through August 20, 2022. Our goal in creating this guide is to facilitate standardized yet simple ways to integrate smartphone photography into current dermatologic practice.
THE TECHNIQUE
Clinical Photography
Clinical images should be captured in a space with ample indirect natural light, such as a patient examination room with frosted or draped windows, ensuring patient privacy is maintained.1,2 The smartphone’s flash can be used if natural lighting is insufficient, but caution should be exercised when photographing patients with darker skin types, as the flash may create an undesired glare. To combat this, consider using a small clip-on light-emitting diode ring light positioned at a 45° angle for more uniform lighting and reduced glare (eFigures 1 and 2).2 This additional light source helps to distribute light evenly across the patient’s skin, enhancing detail visibility, minimizing harsh shadows, and ensuring a more accurate representation of skin pigmentation.2


When a magnified image is required (eg, to capture suspicious lesions with unique and detailed findings such as irregular borders or atypical pigmentation), use the smartphone’s digital zoom function rather than physically moving the camera lens closer to the subject. Moving the camera too close can cause proximity distortion, artificially enlarging objects close to the lens and degrading the quality of the image.1,2 Unnecessary camera features such as portrait mode, live focus, and filters should be turned off to maintain image accuracy. It also is important to avoid excessive manual adjustments to exposure and brightness settings.1,2 The tap-to-focus feature that is integrated into many smartphone cameras can be utilized to ensure the capture of sharp, focused images. After verifying the image preview on the smartphone display, take the photograph. Immediately review the captured image to ensure it is clear and well lit and accurately depicts the area of interest, including its color, texture, and any relevant details, without glare or distortion. If the image does not meet these criteria, promptly reattempt to achieve the desired quality.
Dermoscopic Photography
Dermoscopy, which enables magnified examination of skin lesions, is increasingly being utilized in dermatology. While traditional dermoscopic photography requires specialized equipment, such as large single-lens reflex cameras with dedicated dermoscopic lens attachments, smartphone cameras now can be used to obtain dermoscopic images of reasonable quality.3,4 Adhering to specific practices can help to optimize the quality of dermoscopic images obtained via this technique.
Before capturing an image, it is essential to prepare both the lesion and the surrounding skin. Ensure the area is cleaned thoroughly and trim any hairs that may obscure the image. Apply an interface fluid such as rubbing alcohol or ultrasonography gel to improve image clarity by reducing surface tension and reflections, minimizing glare, and ensuring even light transmission throughout the lesion.5 As recommended for clinical photography, images should be captured in a space with ample indirect light. For best results, we recommend utilizing the primary photo capture option instead of portrait or panoramic mode or additional settings. It is crucial to disable features such as live focus, filters, night mode, and flash, as they may alter image accuracy; however, use of the tap-to-focus feature or manual settings adjustment is encouraged to ensure a high-resolution photograph.
Once these smartphone settings have been verified, position the dermatoscope directly over the lesion of interest. Next, place the smartphone camera lens directly against the eyepiece of the dermatoscope (Figure). Center the lesion in the field of view on the screen. Most smartphones enable adjustment to the image magnification on the photo capture screen. A single tap on the screen should populate the zoom options (eg, ×0.5, ×1, ×3) and allow for adjustment. For the majority of dermoscopic photographs, we recommend standard ×1 magnification, as it typically provides a clear and accurate representation of the lesion without introducing the possibility of image distortion. To obtain a close-up image, use the smartphone’s digital zoom function prior to taking the photograph rather than zooming in on the image after it has been captured; however, to minimize proximity distortion and maintain optimal image quality, avoid exceeding the halfway point on the camera’s zoom dial. After verifying the image preview on the smartphone display, capture the photograph. Immediate review is recommended to allow for prompt reattempt at capturing the image if needed.

PRACTICE IMPLICATIONS
The inherent convenience and accessibility offered by smartphone photography further solidifies its status as a valuable tool in modern dermatologic practice. By adhering to the best practices outlined in this guide, dermatologists can utilize smartphones to capture high-quality clinical and dermoscopic images that support accurate diagnosis and enhance patient care. This approach helps streamline workflows, enhance consistency in image quality, and standardize image capture across different settings and providers.
Additionally, smartphone photography can enhance both education and telemedicine by enabling physicians to easily share high-quality images with colleagues for virtual consultations, second opinions, and collaborative diagnoses. This sharing of images fosters learning opportunities, supports knowledge exchange, and allows for real-time feedback—all of which can improve clinical decision-making. Moreover, it broadens access to dermatologic expertise, strengthens communication between health care providers, and facilitates timely decision-making. As a result, patients benefit from more efficient, accurate, and collaborative care.
- Muraco L. Improved medical photography: key tips for creating images of lasting value. JAMA Dermatol. 2020;156:121-123. doi:10.1001 /jamadermatol.2019.3849
- Alvarado SM, Flessland P, Grant-Kels JM, et al. Practical strategies for improving clinical photography of dark skin. J Am Acad Dermatol. 2022;86:E21-E23. doi:10.1016/j.jaad.2021.09.001
- Pagliarello C, Feliciani C, Fantini C, et al. Use of the dermoscope as a smartphone close-up lens and LED annular macro ring flash. J Am Acad Dermatol. 2016;75:E27–E28. doi:10.1016/j.jaad .2015.12.04
- Zuo KJ, Guo D, Rao J. Mobile teledermatology: a promising future in clinical practice. J Cutan Med Surg. 2013;17:387-391. doi:10.2310/7750.2013.13030
- Gewirtzman AJ, Saurat J-H, Braun RP. An evaluation of dermscopy fluids and application techniques. Br J Dermatol. 2003;149:59-63. doi:10.1046/j.1365-2133.2003.05366.x
- Muraco L. Improved medical photography: key tips for creating images of lasting value. JAMA Dermatol. 2020;156:121-123. doi:10.1001 /jamadermatol.2019.3849
- Alvarado SM, Flessland P, Grant-Kels JM, et al. Practical strategies for improving clinical photography of dark skin. J Am Acad Dermatol. 2022;86:E21-E23. doi:10.1016/j.jaad.2021.09.001
- Pagliarello C, Feliciani C, Fantini C, et al. Use of the dermoscope as a smartphone close-up lens and LED annular macro ring flash. J Am Acad Dermatol. 2016;75:E27–E28. doi:10.1016/j.jaad .2015.12.04
- Zuo KJ, Guo D, Rao J. Mobile teledermatology: a promising future in clinical practice. J Cutan Med Surg. 2013;17:387-391. doi:10.2310/7750.2013.13030
- Gewirtzman AJ, Saurat J-H, Braun RP. An evaluation of dermscopy fluids and application techniques. Br J Dermatol. 2003;149:59-63. doi:10.1046/j.1365-2133.2003.05366.x
Best Practices for Capturing Clinical and Dermoscopic Images With Smartphone Photography
Best Practices for Capturing Clinical and Dermoscopic Images With Smartphone Photography