User login
Weighted Blankets May Help Reduce Preoperative Anxiety During Mohs Micrographic Surgery
Weighted Blankets May Help Reduce Preoperative Anxiety During Mohs Micrographic Surgery
To the Editor:
Patients with nonmelanoma skin cancers exhibit high quality-of-life satisfaction after treatment with Mohs micrographic surgery (MMS) or excision.1,2 However, perioperative anxiety in patients undergoing MMS is common, especially during the immediate preoperative period.3 Anxiety activates the sympathetic nervous system, resulting in physiologic changes such as tachycardia and hypertension.4,5 These sequelae may not only increase patient distress but also increase intraoperative bleeding, complication rates, and recovery times.4,5 Thus, the preoperative period represents a critical window for interventions aimed at reducing anxiety. Anxiety peaks during the perioperative period for a myriad of reasons, including anticipation of pain or potential complications. Enhancing patient comfort and well-being during the procedure may help reduce negative emotional sequelae, alleviate fear during procedures, and increase patient satisfaction.3
Weighted blankets (WBs) frequently are utilized in occupational and physical therapy as a deep pressure stimulation tool to alleviate anxiety by mimicking the experience of being massaged or swaddled.6 Deep pressure tools increase parasympathetic tone, help reduce anxiety, and provide a calming effect.7,8 Nonhospitalized individuals were more relaxed during mental health evaluations when using a WB, and deep pressure tools have frequently been used to calm individuals with autism spectrum disorders or attention-deficit/hyperactivity disorders.6 Furthermore, WBs have successfully been used to reduce anxiety in mental health care settings, as well as during chemotherapy infusions.6,9 The literature is sparse regarding the use of WB in the perioperative setting. Potential benefit has been demonstrated in the setting of dental cleanings and wisdom teeth extractions.7,8 In the current study, we investigated whether use of a WB could reduce preoperative anxiety in the setting of MMS.
Institutional review board approval was obtained from the University of Virginia (Charlottesville, Virginia), and adult patients undergoing MMS to the head or neck were recruited to participate in a single-blind randomized controlled trial in the spring of 2023. Patients undergoing MMS on other areas of the body were excluded because the placement of the WB could interfere with the procedure. Other exclusion criteria included pregnancy, dementia, or current treatment with an anxiolytic medication.
Twenty-seven patients were included in the study, and informed consent was obtained. Patients were randomized to use a WB or standard hospital towel (control). The medical-grade WBs weighed 8.5 pounds, while the cotton hospital towels weighed less than 1 pound. The WBs were cleaned in between patients with standard germicidal disposable wipes.
Patient data were collected from electronic medical records including age, sex, weight, history of prior MMS, and current use of antihypertensives and/or beta-blockers. Data also were collected on the presence of anxiety disorders, major depression, fibromyalgia, tobacco and alcohol use, hyperthyroidism, hyperhidrosis, cardiac arrhythmias (including atrial fibrillation), chronic obstructive pulmonary disease, asthma, coronary artery disease, diabetes mellitus, peripheral neuropathy, and menopausal symptoms.
During the procedure, anxiety was monitored using the State-Trait Anxiety Inventory (STAI) Form Y-1, the visual analogue scale for anxiety (VAS-A), and vital signs including heart rate, blood pressure, and respiratory rate. Vital signs were evaluated by nursing staff with the patient sitting up and the WB or hospital towel removed. Using these assessments, anxiety was measured at 3 different timepoints: upon arrival to the clinic (timepoint A), after the patient rested in a reclined beach-chair position with the WB or hospital towel placed over them for 10 minutes before administration of local anesthetic and starting the procedure (timepoint B), and after the first MMS stage was taken (timepoint C).
A power analysis was not completed due to a lack of previous studies on the use of WBs during MMS. Group means were analyzed using two-tailed t-tests and one-way analysis of variance. A P value of .05 indicated statistical significance.
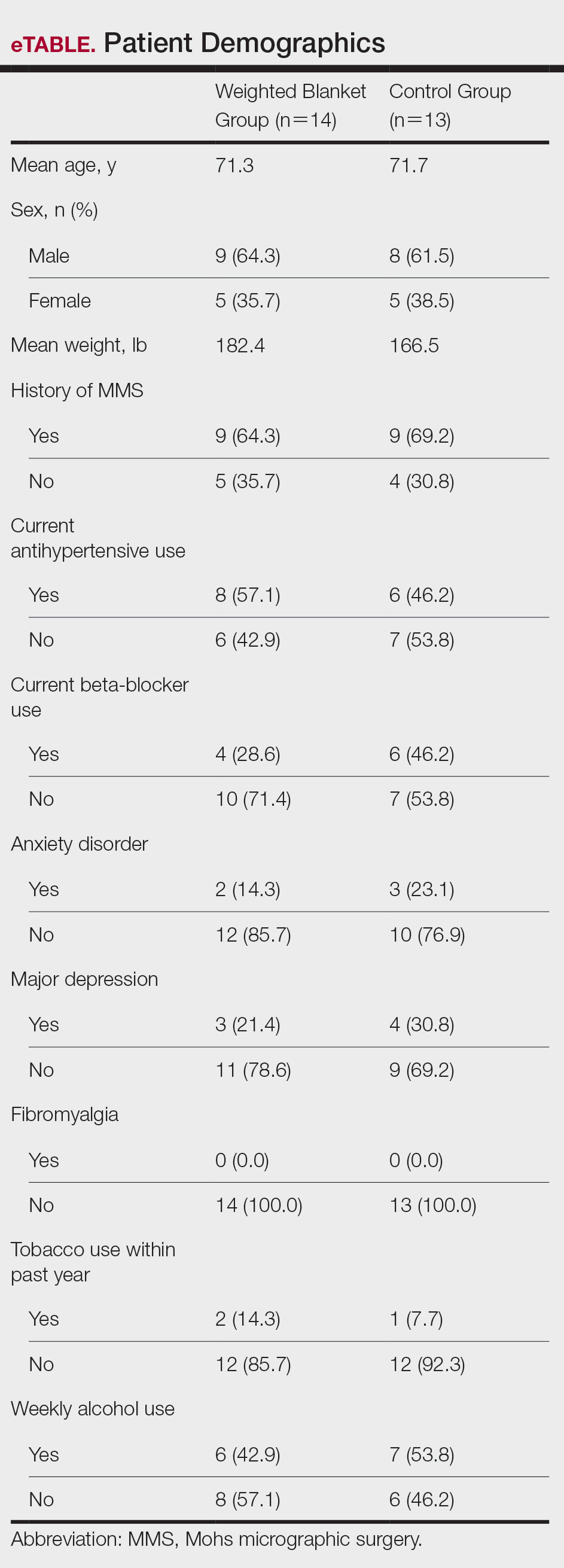
Fourteen patients were randomized to the WB group and 13 were randomized to the control group. Patient demographics are outlined in the eTable. In the WB group, mean STAI scores progressively decreased at each timepoint (A: 15.3, B: 13.6, C: 12.7) and mean VAS-A scores followed a similar trend (A: 24.2, B: 19.3, C: 10.5). In the control group, the mean STAI scores remained stable at timepoints A and B (17.7) and then decreased at timepoint C (14.8). The mean VAS-A scores in the control group followed a similar pattern, remaining stable at timepoints A (22.9) and B (22.8) and then decreasing at timepoint C (14.4). These changes were not statistically significant.
Mean vital signs for both the WB and control groups were relatively stable across all timepoints, although they tended to decrease by timepoint C. In the WB group, mean heart rates were 69, 69, and 67 beats per minute at timepoints A, B, and C, respectively. Mean systolic blood pressures were 137 mm Hg, 138 mm Hg, and 136 mm Hg and mean diastolic pressures were 71 mm Hg, 68 mm Hg, and 66 mm Hg at timepoints A, B, and C, respectively. Mean respiratory rates were 20, 19, and 18 breaths per minute at timepoints A, B, and C, respectively. In the control group, mean heart rates were 70, 69, and 68 beats per minute across timepoints A, B, and C, respectively. Mean systolic blood pressures were 137 mm Hg, 138 mm Hg, and 133 mm Hg and mean diastolic pressures were 71 mm Hg, 74 mm Hg, and 68 mm Hg at timepoints A, B, and C, respectively. Mean respiratory rates were 19, 18, and 18 breaths per minute at timepoints A, B, and C, respectively. These changes were not statistically significant.
Our pilot study examined the effects of using a WB to alleviate preoperative anxiety during MMS. Our results suggest that WBs may modestly improve subjective anxiety immediately prior to undergoing MMS. Mean STAI and VAS-A scores decreased from timepoint A to timepoint B in the WB group vs the control group in which these scores remained stable. Although our study was not powered to determine statistical differences and significance was not reached, our results suggest a favorable trend in decreased anxiety scores. Our analysis was limited by a small sample size; therefore, additional larger-scale studies will be needed to confirm this trend.
Our results are broadly consistent with earlier studies that found improvement in physiologic proxies of anxiety with the use of WBs during chemotherapy infusions, dental procedures, and acute inpatient mental health hospitalizations.7-10 During periods of high anxiety, use of WBs shifts the autonomic nervous system from a sympathetic to a parasympathetic state, as demonstrated by increased high-frequency heart rate variability, a marker of parasympathetic activity.6,11 While the exact mechanism of how WBs and other deep pressure stimulation tools affect high-frequency heart rate variability is unclear, one study showed that patients undergoing dental extractions were better equipped when using deep pressure stimulation tools to utilize calming techniques and regulate stress.12 The use of WBs and other deep pressure stimulation tools may extend beyond the perioperative setting and also may be an effective tool for clinicians in other settings (eg, clinic visits, physical examinations).
In our study, all participants demonstrated the greatest reduction in anxiety at timepoint C after the first MMS stage, likely related to patients relaxing more after knowing what to expect from the surgery; this also may have been reflected somewhat in the slight downward trend noted in vital signs across both study groups. One concern regarding WB use in surgical settings is whether the added pressure could trigger unfavorable circulatory effects, such as elevated blood pressure. In our study, with the exception of diastolic blood pressure, vital signs appeared unaffected by the type of blanket used and remained relatively stable from timepoint A to timepoint B and decreased at timepoint C. Diastolic blood pressure in the WB group decreased from timepoint A to timepoint B, then decreased further from timepoint B to timepoint C. This mirrored the decreasing STAI score trend, compared to the control group who increased from timepoint A to timepoint B and reached a nadir at timepoint C. Consistent with prior WB studies, there were no adverse effects from WBs, including adverse impacts on vital signs.6,9
The original recruitment goal was not met due to staffing issues related to the COVID-19 pandemic, and subgroup analyses were deferred as a result of sample size limitations. It is possible that the WB intervention may have a larger impact on subpopulations more prone to perioperative anxiety (eg, patients undergoing MMS for the first time). However, the results of our pilot study suggest a beneficial effect from the use of WBs. While these preliminary data are promising, additional studies in the perioperative setting are needed to more accurately determine the clinical utility of WBs during MMS and other procedures.
- Eberle FC, Schippert W, Trilling B, et al. Cosmetic results of histographically controlled excision of non-melanoma skin cancer in the head and neck region. J Dtsch Dermatol Ges. 2005;3:109-112. doi:10.1111/j.1610-0378.2005.04738.x
- Chren MM, Sahay AP, Bertenthal DS, et al. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127:1351-1357. doi:10.1038/sj.jid.5700740
- Kossintseva I, Zloty D. Determinants and timeline of perioperative anxiety in Mohs surgery. Dermatol Surg. 2017;43:1029-1035. doi:10.1097 /DSS.0000000000001152
- Pritchard MJ. Identifying and assessing anxiety in pre-operative patients. Nurs Stand. 2009;23:35-40. doi:10.7748/ns2009.08.23.51.35.c7222.
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:E20306. doi:10.1371/journal.pone.0020306
- Mullen B, Champagne T, Krishnamurty S, et al. Exploring the safety and therapeutic effects of deep pressure stimulation using a weighted blanket. Occup Ther Ment Health. 2008;24:65-89. doi:10.1300/ J004v24n01_05
- Chen HY, Yang H, Chi HJ, et al. Physiological effects of deep touch pressure on anxiety alleviation: the weighted blanket approach. J Med Biol Eng. 2013;33:463-470. doi:10.5405/jmbe.1043
- Chen HY, Yang H, Meng LF, et al. Effect of deep pressure input on parasympathetic system in patients with wisdom tooth surgery. J Formos Med Assoc. 2016;115:853-859. doi:10.1016 /j.jfma.2016.07.008
- Vinson J, Powers J, Mosesso K. Weighted blankets: anxiety reduction in adult patients receiving chemotherapy. Clin J Oncol Nurs. 2020; 24:360-368. doi:10.1188/20.CJON.360-368
- Champagne T, Mullen B, Dickson D, et al. Evaluating the safety and effectiveness of the weighted blanket with adults during an inpatient mental health hospitalization. Occup Ther Ment Health. 2015;31:211-233. doi:10.1080/0164212X.2015.1066220
- Lane RD, McRae K, Reiman EM, et al. Neural correlates of heart rate variability during emotion. Neuroimage. 2009;44:213-222. doi: 10.1016/j.neuroimage.2008.07.056
- Moyer CA, Rounds J, Hannum JW. A meta-analysis of massage therapy research. Psychol Bull. 2004;130:3-18. doi: 10.1037 /0033-2909.130.1.3
To the Editor:
Patients with nonmelanoma skin cancers exhibit high quality-of-life satisfaction after treatment with Mohs micrographic surgery (MMS) or excision.1,2 However, perioperative anxiety in patients undergoing MMS is common, especially during the immediate preoperative period.3 Anxiety activates the sympathetic nervous system, resulting in physiologic changes such as tachycardia and hypertension.4,5 These sequelae may not only increase patient distress but also increase intraoperative bleeding, complication rates, and recovery times.4,5 Thus, the preoperative period represents a critical window for interventions aimed at reducing anxiety. Anxiety peaks during the perioperative period for a myriad of reasons, including anticipation of pain or potential complications. Enhancing patient comfort and well-being during the procedure may help reduce negative emotional sequelae, alleviate fear during procedures, and increase patient satisfaction.3
Weighted blankets (WBs) frequently are utilized in occupational and physical therapy as a deep pressure stimulation tool to alleviate anxiety by mimicking the experience of being massaged or swaddled.6 Deep pressure tools increase parasympathetic tone, help reduce anxiety, and provide a calming effect.7,8 Nonhospitalized individuals were more relaxed during mental health evaluations when using a WB, and deep pressure tools have frequently been used to calm individuals with autism spectrum disorders or attention-deficit/hyperactivity disorders.6 Furthermore, WBs have successfully been used to reduce anxiety in mental health care settings, as well as during chemotherapy infusions.6,9 The literature is sparse regarding the use of WB in the perioperative setting. Potential benefit has been demonstrated in the setting of dental cleanings and wisdom teeth extractions.7,8 In the current study, we investigated whether use of a WB could reduce preoperative anxiety in the setting of MMS.
Institutional review board approval was obtained from the University of Virginia (Charlottesville, Virginia), and adult patients undergoing MMS to the head or neck were recruited to participate in a single-blind randomized controlled trial in the spring of 2023. Patients undergoing MMS on other areas of the body were excluded because the placement of the WB could interfere with the procedure. Other exclusion criteria included pregnancy, dementia, or current treatment with an anxiolytic medication.
Twenty-seven patients were included in the study, and informed consent was obtained. Patients were randomized to use a WB or standard hospital towel (control). The medical-grade WBs weighed 8.5 pounds, while the cotton hospital towels weighed less than 1 pound. The WBs were cleaned in between patients with standard germicidal disposable wipes.
Patient data were collected from electronic medical records including age, sex, weight, history of prior MMS, and current use of antihypertensives and/or beta-blockers. Data also were collected on the presence of anxiety disorders, major depression, fibromyalgia, tobacco and alcohol use, hyperthyroidism, hyperhidrosis, cardiac arrhythmias (including atrial fibrillation), chronic obstructive pulmonary disease, asthma, coronary artery disease, diabetes mellitus, peripheral neuropathy, and menopausal symptoms.
During the procedure, anxiety was monitored using the State-Trait Anxiety Inventory (STAI) Form Y-1, the visual analogue scale for anxiety (VAS-A), and vital signs including heart rate, blood pressure, and respiratory rate. Vital signs were evaluated by nursing staff with the patient sitting up and the WB or hospital towel removed. Using these assessments, anxiety was measured at 3 different timepoints: upon arrival to the clinic (timepoint A), after the patient rested in a reclined beach-chair position with the WB or hospital towel placed over them for 10 minutes before administration of local anesthetic and starting the procedure (timepoint B), and after the first MMS stage was taken (timepoint C).
A power analysis was not completed due to a lack of previous studies on the use of WBs during MMS. Group means were analyzed using two-tailed t-tests and one-way analysis of variance. A P value of .05 indicated statistical significance.
Fourteen patients were randomized to the WB group and 13 were randomized to the control group. Patient demographics are outlined in the eTable. In the WB group, mean STAI scores progressively decreased at each timepoint (A: 15.3, B: 13.6, C: 12.7) and mean VAS-A scores followed a similar trend (A: 24.2, B: 19.3, C: 10.5). In the control group, the mean STAI scores remained stable at timepoints A and B (17.7) and then decreased at timepoint C (14.8). The mean VAS-A scores in the control group followed a similar pattern, remaining stable at timepoints A (22.9) and B (22.8) and then decreasing at timepoint C (14.4). These changes were not statistically significant.

Mean vital signs for both the WB and control groups were relatively stable across all timepoints, although they tended to decrease by timepoint C. In the WB group, mean heart rates were 69, 69, and 67 beats per minute at timepoints A, B, and C, respectively. Mean systolic blood pressures were 137 mm Hg, 138 mm Hg, and 136 mm Hg and mean diastolic pressures were 71 mm Hg, 68 mm Hg, and 66 mm Hg at timepoints A, B, and C, respectively. Mean respiratory rates were 20, 19, and 18 breaths per minute at timepoints A, B, and C, respectively. In the control group, mean heart rates were 70, 69, and 68 beats per minute across timepoints A, B, and C, respectively. Mean systolic blood pressures were 137 mm Hg, 138 mm Hg, and 133 mm Hg and mean diastolic pressures were 71 mm Hg, 74 mm Hg, and 68 mm Hg at timepoints A, B, and C, respectively. Mean respiratory rates were 19, 18, and 18 breaths per minute at timepoints A, B, and C, respectively. These changes were not statistically significant.
Our pilot study examined the effects of using a WB to alleviate preoperative anxiety during MMS. Our results suggest that WBs may modestly improve subjective anxiety immediately prior to undergoing MMS. Mean STAI and VAS-A scores decreased from timepoint A to timepoint B in the WB group vs the control group in which these scores remained stable. Although our study was not powered to determine statistical differences and significance was not reached, our results suggest a favorable trend in decreased anxiety scores. Our analysis was limited by a small sample size; therefore, additional larger-scale studies will be needed to confirm this trend.
Our results are broadly consistent with earlier studies that found improvement in physiologic proxies of anxiety with the use of WBs during chemotherapy infusions, dental procedures, and acute inpatient mental health hospitalizations.7-10 During periods of high anxiety, use of WBs shifts the autonomic nervous system from a sympathetic to a parasympathetic state, as demonstrated by increased high-frequency heart rate variability, a marker of parasympathetic activity.6,11 While the exact mechanism of how WBs and other deep pressure stimulation tools affect high-frequency heart rate variability is unclear, one study showed that patients undergoing dental extractions were better equipped when using deep pressure stimulation tools to utilize calming techniques and regulate stress.12 The use of WBs and other deep pressure stimulation tools may extend beyond the perioperative setting and also may be an effective tool for clinicians in other settings (eg, clinic visits, physical examinations).
In our study, all participants demonstrated the greatest reduction in anxiety at timepoint C after the first MMS stage, likely related to patients relaxing more after knowing what to expect from the surgery; this also may have been reflected somewhat in the slight downward trend noted in vital signs across both study groups. One concern regarding WB use in surgical settings is whether the added pressure could trigger unfavorable circulatory effects, such as elevated blood pressure. In our study, with the exception of diastolic blood pressure, vital signs appeared unaffected by the type of blanket used and remained relatively stable from timepoint A to timepoint B and decreased at timepoint C. Diastolic blood pressure in the WB group decreased from timepoint A to timepoint B, then decreased further from timepoint B to timepoint C. This mirrored the decreasing STAI score trend, compared to the control group who increased from timepoint A to timepoint B and reached a nadir at timepoint C. Consistent with prior WB studies, there were no adverse effects from WBs, including adverse impacts on vital signs.6,9
The original recruitment goal was not met due to staffing issues related to the COVID-19 pandemic, and subgroup analyses were deferred as a result of sample size limitations. It is possible that the WB intervention may have a larger impact on subpopulations more prone to perioperative anxiety (eg, patients undergoing MMS for the first time). However, the results of our pilot study suggest a beneficial effect from the use of WBs. While these preliminary data are promising, additional studies in the perioperative setting are needed to more accurately determine the clinical utility of WBs during MMS and other procedures.
To the Editor:
Patients with nonmelanoma skin cancers exhibit high quality-of-life satisfaction after treatment with Mohs micrographic surgery (MMS) or excision.1,2 However, perioperative anxiety in patients undergoing MMS is common, especially during the immediate preoperative period.3 Anxiety activates the sympathetic nervous system, resulting in physiologic changes such as tachycardia and hypertension.4,5 These sequelae may not only increase patient distress but also increase intraoperative bleeding, complication rates, and recovery times.4,5 Thus, the preoperative period represents a critical window for interventions aimed at reducing anxiety. Anxiety peaks during the perioperative period for a myriad of reasons, including anticipation of pain or potential complications. Enhancing patient comfort and well-being during the procedure may help reduce negative emotional sequelae, alleviate fear during procedures, and increase patient satisfaction.3
Weighted blankets (WBs) frequently are utilized in occupational and physical therapy as a deep pressure stimulation tool to alleviate anxiety by mimicking the experience of being massaged or swaddled.6 Deep pressure tools increase parasympathetic tone, help reduce anxiety, and provide a calming effect.7,8 Nonhospitalized individuals were more relaxed during mental health evaluations when using a WB, and deep pressure tools have frequently been used to calm individuals with autism spectrum disorders or attention-deficit/hyperactivity disorders.6 Furthermore, WBs have successfully been used to reduce anxiety in mental health care settings, as well as during chemotherapy infusions.6,9 The literature is sparse regarding the use of WB in the perioperative setting. Potential benefit has been demonstrated in the setting of dental cleanings and wisdom teeth extractions.7,8 In the current study, we investigated whether use of a WB could reduce preoperative anxiety in the setting of MMS.
Institutional review board approval was obtained from the University of Virginia (Charlottesville, Virginia), and adult patients undergoing MMS to the head or neck were recruited to participate in a single-blind randomized controlled trial in the spring of 2023. Patients undergoing MMS on other areas of the body were excluded because the placement of the WB could interfere with the procedure. Other exclusion criteria included pregnancy, dementia, or current treatment with an anxiolytic medication.
Twenty-seven patients were included in the study, and informed consent was obtained. Patients were randomized to use a WB or standard hospital towel (control). The medical-grade WBs weighed 8.5 pounds, while the cotton hospital towels weighed less than 1 pound. The WBs were cleaned in between patients with standard germicidal disposable wipes.
Patient data were collected from electronic medical records including age, sex, weight, history of prior MMS, and current use of antihypertensives and/or beta-blockers. Data also were collected on the presence of anxiety disorders, major depression, fibromyalgia, tobacco and alcohol use, hyperthyroidism, hyperhidrosis, cardiac arrhythmias (including atrial fibrillation), chronic obstructive pulmonary disease, asthma, coronary artery disease, diabetes mellitus, peripheral neuropathy, and menopausal symptoms.
During the procedure, anxiety was monitored using the State-Trait Anxiety Inventory (STAI) Form Y-1, the visual analogue scale for anxiety (VAS-A), and vital signs including heart rate, blood pressure, and respiratory rate. Vital signs were evaluated by nursing staff with the patient sitting up and the WB or hospital towel removed. Using these assessments, anxiety was measured at 3 different timepoints: upon arrival to the clinic (timepoint A), after the patient rested in a reclined beach-chair position with the WB or hospital towel placed over them for 10 minutes before administration of local anesthetic and starting the procedure (timepoint B), and after the first MMS stage was taken (timepoint C).
A power analysis was not completed due to a lack of previous studies on the use of WBs during MMS. Group means were analyzed using two-tailed t-tests and one-way analysis of variance. A P value of .05 indicated statistical significance.
Fourteen patients were randomized to the WB group and 13 were randomized to the control group. Patient demographics are outlined in the eTable. In the WB group, mean STAI scores progressively decreased at each timepoint (A: 15.3, B: 13.6, C: 12.7) and mean VAS-A scores followed a similar trend (A: 24.2, B: 19.3, C: 10.5). In the control group, the mean STAI scores remained stable at timepoints A and B (17.7) and then decreased at timepoint C (14.8). The mean VAS-A scores in the control group followed a similar pattern, remaining stable at timepoints A (22.9) and B (22.8) and then decreasing at timepoint C (14.4). These changes were not statistically significant.

Mean vital signs for both the WB and control groups were relatively stable across all timepoints, although they tended to decrease by timepoint C. In the WB group, mean heart rates were 69, 69, and 67 beats per minute at timepoints A, B, and C, respectively. Mean systolic blood pressures were 137 mm Hg, 138 mm Hg, and 136 mm Hg and mean diastolic pressures were 71 mm Hg, 68 mm Hg, and 66 mm Hg at timepoints A, B, and C, respectively. Mean respiratory rates were 20, 19, and 18 breaths per minute at timepoints A, B, and C, respectively. In the control group, mean heart rates were 70, 69, and 68 beats per minute across timepoints A, B, and C, respectively. Mean systolic blood pressures were 137 mm Hg, 138 mm Hg, and 133 mm Hg and mean diastolic pressures were 71 mm Hg, 74 mm Hg, and 68 mm Hg at timepoints A, B, and C, respectively. Mean respiratory rates were 19, 18, and 18 breaths per minute at timepoints A, B, and C, respectively. These changes were not statistically significant.
Our pilot study examined the effects of using a WB to alleviate preoperative anxiety during MMS. Our results suggest that WBs may modestly improve subjective anxiety immediately prior to undergoing MMS. Mean STAI and VAS-A scores decreased from timepoint A to timepoint B in the WB group vs the control group in which these scores remained stable. Although our study was not powered to determine statistical differences and significance was not reached, our results suggest a favorable trend in decreased anxiety scores. Our analysis was limited by a small sample size; therefore, additional larger-scale studies will be needed to confirm this trend.
Our results are broadly consistent with earlier studies that found improvement in physiologic proxies of anxiety with the use of WBs during chemotherapy infusions, dental procedures, and acute inpatient mental health hospitalizations.7-10 During periods of high anxiety, use of WBs shifts the autonomic nervous system from a sympathetic to a parasympathetic state, as demonstrated by increased high-frequency heart rate variability, a marker of parasympathetic activity.6,11 While the exact mechanism of how WBs and other deep pressure stimulation tools affect high-frequency heart rate variability is unclear, one study showed that patients undergoing dental extractions were better equipped when using deep pressure stimulation tools to utilize calming techniques and regulate stress.12 The use of WBs and other deep pressure stimulation tools may extend beyond the perioperative setting and also may be an effective tool for clinicians in other settings (eg, clinic visits, physical examinations).
In our study, all participants demonstrated the greatest reduction in anxiety at timepoint C after the first MMS stage, likely related to patients relaxing more after knowing what to expect from the surgery; this also may have been reflected somewhat in the slight downward trend noted in vital signs across both study groups. One concern regarding WB use in surgical settings is whether the added pressure could trigger unfavorable circulatory effects, such as elevated blood pressure. In our study, with the exception of diastolic blood pressure, vital signs appeared unaffected by the type of blanket used and remained relatively stable from timepoint A to timepoint B and decreased at timepoint C. Diastolic blood pressure in the WB group decreased from timepoint A to timepoint B, then decreased further from timepoint B to timepoint C. This mirrored the decreasing STAI score trend, compared to the control group who increased from timepoint A to timepoint B and reached a nadir at timepoint C. Consistent with prior WB studies, there were no adverse effects from WBs, including adverse impacts on vital signs.6,9
The original recruitment goal was not met due to staffing issues related to the COVID-19 pandemic, and subgroup analyses were deferred as a result of sample size limitations. It is possible that the WB intervention may have a larger impact on subpopulations more prone to perioperative anxiety (eg, patients undergoing MMS for the first time). However, the results of our pilot study suggest a beneficial effect from the use of WBs. While these preliminary data are promising, additional studies in the perioperative setting are needed to more accurately determine the clinical utility of WBs during MMS and other procedures.
- Eberle FC, Schippert W, Trilling B, et al. Cosmetic results of histographically controlled excision of non-melanoma skin cancer in the head and neck region. J Dtsch Dermatol Ges. 2005;3:109-112. doi:10.1111/j.1610-0378.2005.04738.x
- Chren MM, Sahay AP, Bertenthal DS, et al. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127:1351-1357. doi:10.1038/sj.jid.5700740
- Kossintseva I, Zloty D. Determinants and timeline of perioperative anxiety in Mohs surgery. Dermatol Surg. 2017;43:1029-1035. doi:10.1097 /DSS.0000000000001152
- Pritchard MJ. Identifying and assessing anxiety in pre-operative patients. Nurs Stand. 2009;23:35-40. doi:10.7748/ns2009.08.23.51.35.c7222.
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:E20306. doi:10.1371/journal.pone.0020306
- Mullen B, Champagne T, Krishnamurty S, et al. Exploring the safety and therapeutic effects of deep pressure stimulation using a weighted blanket. Occup Ther Ment Health. 2008;24:65-89. doi:10.1300/ J004v24n01_05
- Chen HY, Yang H, Chi HJ, et al. Physiological effects of deep touch pressure on anxiety alleviation: the weighted blanket approach. J Med Biol Eng. 2013;33:463-470. doi:10.5405/jmbe.1043
- Chen HY, Yang H, Meng LF, et al. Effect of deep pressure input on parasympathetic system in patients with wisdom tooth surgery. J Formos Med Assoc. 2016;115:853-859. doi:10.1016 /j.jfma.2016.07.008
- Vinson J, Powers J, Mosesso K. Weighted blankets: anxiety reduction in adult patients receiving chemotherapy. Clin J Oncol Nurs. 2020; 24:360-368. doi:10.1188/20.CJON.360-368
- Champagne T, Mullen B, Dickson D, et al. Evaluating the safety and effectiveness of the weighted blanket with adults during an inpatient mental health hospitalization. Occup Ther Ment Health. 2015;31:211-233. doi:10.1080/0164212X.2015.1066220
- Lane RD, McRae K, Reiman EM, et al. Neural correlates of heart rate variability during emotion. Neuroimage. 2009;44:213-222. doi: 10.1016/j.neuroimage.2008.07.056
- Moyer CA, Rounds J, Hannum JW. A meta-analysis of massage therapy research. Psychol Bull. 2004;130:3-18. doi: 10.1037 /0033-2909.130.1.3
- Eberle FC, Schippert W, Trilling B, et al. Cosmetic results of histographically controlled excision of non-melanoma skin cancer in the head and neck region. J Dtsch Dermatol Ges. 2005;3:109-112. doi:10.1111/j.1610-0378.2005.04738.x
- Chren MM, Sahay AP, Bertenthal DS, et al. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127:1351-1357. doi:10.1038/sj.jid.5700740
- Kossintseva I, Zloty D. Determinants and timeline of perioperative anxiety in Mohs surgery. Dermatol Surg. 2017;43:1029-1035. doi:10.1097 /DSS.0000000000001152
- Pritchard MJ. Identifying and assessing anxiety in pre-operative patients. Nurs Stand. 2009;23:35-40. doi:10.7748/ns2009.08.23.51.35.c7222.
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:E20306. doi:10.1371/journal.pone.0020306
- Mullen B, Champagne T, Krishnamurty S, et al. Exploring the safety and therapeutic effects of deep pressure stimulation using a weighted blanket. Occup Ther Ment Health. 2008;24:65-89. doi:10.1300/ J004v24n01_05
- Chen HY, Yang H, Chi HJ, et al. Physiological effects of deep touch pressure on anxiety alleviation: the weighted blanket approach. J Med Biol Eng. 2013;33:463-470. doi:10.5405/jmbe.1043
- Chen HY, Yang H, Meng LF, et al. Effect of deep pressure input on parasympathetic system in patients with wisdom tooth surgery. J Formos Med Assoc. 2016;115:853-859. doi:10.1016 /j.jfma.2016.07.008
- Vinson J, Powers J, Mosesso K. Weighted blankets: anxiety reduction in adult patients receiving chemotherapy. Clin J Oncol Nurs. 2020; 24:360-368. doi:10.1188/20.CJON.360-368
- Champagne T, Mullen B, Dickson D, et al. Evaluating the safety and effectiveness of the weighted blanket with adults during an inpatient mental health hospitalization. Occup Ther Ment Health. 2015;31:211-233. doi:10.1080/0164212X.2015.1066220
- Lane RD, McRae K, Reiman EM, et al. Neural correlates of heart rate variability during emotion. Neuroimage. 2009;44:213-222. doi: 10.1016/j.neuroimage.2008.07.056
- Moyer CA, Rounds J, Hannum JW. A meta-analysis of massage therapy research. Psychol Bull. 2004;130:3-18. doi: 10.1037 /0033-2909.130.1.3
Weighted Blankets May Help Reduce Preoperative Anxiety During Mohs Micrographic Surgery
Weighted Blankets May Help Reduce Preoperative Anxiety During Mohs Micrographic Surgery
PRACTICE POINTS
- Preoperative anxiety in patients during Mohs micrographic surgery (MMS) may increase intraoperative bleeding, complication rates, and recovery times.
- Using weighted blankets may reduce anxiety in patients undergoing MMS of the head and neck.
Dermatologic Implications of Glycemic Control Medications for Patients with Type 2 Diabetes Mellitus
Dermatologic Implications of Glycemic Control Medications for Patients with Type 2 Diabetes Mellitus
Type 2 diabetes mellitus (T2DM) is a chronic disease characterized by uncontrolled hyperglycemia. Over the past few decades, its prevalence has steadily increased, now affecting approximately 10% of adults worldwide and ranking among the top 10 leading causes of death globally.1 The pathophysiology of T2DM involves persistent hyperglycemia that drives insulin resistance and a progressive decline in insulin production from the pancreas.2 Medical management of this condition aims to reduce blood glucose levels or enhance insulin production and sensitivity. Aside from lifestyle modifications, metformin is considered the first-line treatment for glycemic control according to the 2023 American Association of Clinical Endocrinology’s T2DM management algorithm.3 These updated guidelines stratify adjunct treatments by individualized glycemic targets and patient needs. For patients who are overweight or obese, glucagonlike peptide 1 (GLP-1) and dual GLP-1/ gastric inhibitory polypeptide (GIP) agonists are the preferred adjunct or second-line treatments.3
In this review, we highlight the dermatologic adverse effects and potential therapeutic benefits of metformin as well as GLP-1 and GLP-1/GIP agonists.
METFORMIN
Metformin is a biguanide agent used as a first-line treatment for T2DM because of its ability to reduce hepatic glucose production and increase peripheral tissue glucose uptake.4 In addition to its effects on glucose, metformin has been shown to have anti-inflammatory properties via inhibition of the nuclear factor κB and mammalian target of rapamycin (mTOR) pathways, leading to decreased production of cytokines associated with T helper (Th) 1 and Th17 cell responses, such as IL-17, interferon gamma (IFN-γ), and tumor necrosis factor α (TNF-α).5-7 These findings have spurred interest among clinicians in the potential use of metformin for inflammatory conditions, including dermatologic diseases such as psoriasis and hidradenitis suppurativa (HS).8
Adverse Effects
Metformin is administered orally and generally is well tolerated. The most common adverse effects include gastrointestinal symptoms such as diarrhea, nausea, vomiting, and abdominal pain.9 While cutaneous adverse effects are rare, multiple dermatologic adverse reactions to metformin have been reported,10,11 including leukocytoclastic vasculitis,11-13 fixed drug eruptions,14-17 drug rash with eosinophilia and systemic symptoms (DRESS) syndrome,18 and photosensitivity reactions.19 Leukocytoclastic vasculitis and DRESS syndrome typically develop within the first month following metformin initiation, while fixed drug eruption and photosensitivity reactions have more variable timing, occurring weeks to years after treatment initiation.12-19
Dermatologic Implications
Acanthosis Nigricans—Acanthosis nigricans (AN) is characterized by hyperpigmentation and velvety skin thickening, typically in intertriginous areas such as the back of the neck, axillae, and groin.20 It commonly is associated with insulin resistance and obesity.21-23 Treatments for AN primarily center around insulin sensitivity and weight loss,24,25 with some benefit observed from the use of keratolytic agents.26,27 Metformin may have utility in treating AN through its effects on insulin sensitivity and glycemic control. Multiple case reports have noted marked improvements in AN in patients with and without obesity with the addition of metformin to their existing treatment regimens in doses ranging from 500 mg to 1700 mg daily.28-30 However, an unblinded randomized controlled trial (RCT) comparing the efficacy of metformin (500 mg 3 times daily) with rosiglitazone (4 mg/d), another T2DM medication, on AN neck lesions in patients who were overweight and obese found no significant effects in lesion severity and only modest improvements in skin texture in both groups at 12 weeks following treatment initiation.31 Another RCT comparing metformin (500 mg twice daily) with a twice-daily capsule containing α-lipoic acid, biotin, chromium polynicotinate, and zinc sulfate, showed significant (P<.001) improvements in AN neck lesions in both groups after 12 weeks.32 According to Sung et al,8 longer duration of therapy (>6 months), higher doses (1700–2000 mg), and lower baseline weight were associated with higher efficacy of metformin for treatment of AN. Overall, the use of metformin as an adjunct treatment for AN, particularly in patients with underlying hyperglycemia, is supported in the literature, but further studies are needed to clarify dosing, duration of therapy, and patient populations that will benefit most from adding metformin to their treatment regimens.
Hirsutism—Hirsutism, which is characterized by excessive hair growth in androgen-dependent areas, can be challenging to treat. Metformin has been shown to reduce circulating insulin, luteinizing hormone, androstenedione, and testosterone, thus improving underlying hyperandrogenism, particularly in patients with polycystic ovary syndrome (PCOS).33-35 Although single studies evaluating the efficacy of metformin for treatment of hirsutism in patients with PCOS have shown potential benefits,36-38 meta-analyses showed no significant effects of metformin compared to placebo or oral contraceptives and decreased benefits compared to spironolactone and flutamide.39 Given these findings showing that metformin was no more effective than placebo or other treatments, the current Endocrine Society guidelines recommend against the use of metformin for hirsutism.39,40 There may be a role for metformin as an adjuvant therapy in certain populations (eg, patients with comorbid T2DM), although further studies stratifying risk factors such as body mass index and age are needed.41
Hidradenitis Suppurativa—Hidradenitis suppurativa is a follicular occlusive disease characterized by recurrent inflamed nodules leading to chronic dermal abscesses, fibrosis, and sinus tract formation primarily in intertriginous areas such as the axillae and groin.42 Medical management depends on disease severity but usually involves antibiotic treatment with adjunct therapies such as oral contraceptives, antiandrogenic medications (eg, spironolactone), biologic medications, and metformin.42 Preclinical and clinical data suggest that metformin can impact HS through metabolic and immunomodulatory mechanisms.5,42 Like many chronic inflammatory disorders, HS is associated with metabolic syndrome.43,44 A study evaluating insulin secretion after oral glucose tolerance testing showed increased insulin levels in patients with HS compared to controls (P=.02), with 60% (6/10) of patients with HS meeting criteria for insulin resistance. In addition, serum insulin levels in insulin-resistant patients with HS correlated with increased lesional skin mTOR gene expression at 30 (r=.80) and 60 (r=1.00) minutes, and mTOR was found to be upregulated in lesional and extralesional skin in patients with HS compared to healthy controls (P<.01).45 Insulin activates mTOR signaling, which mediates cell growth and survival, among other processes.46 Thus, metformin’s ability to increase insulin sensitivity and inhibit mTOR signaling could be beneficial in the setting of HS. Additionally, insulin and insulinlike growth factor 1 (IGF-1) increase androgen signaling, a process that has been implicated in HS.47
Metformin also may impact HS through its effects on testosterone and other hormones.48 A study evaluating peripheral blood mononuclear cells in patients with HS showed reduced IL-17, IFN-γ, TNF-α, and IL-6 levels in patients who were taking metformin (dose not reported) for longer than 6 months compared to patients who were not on metformin. Further analysis of ex vivo HS lesions cultured with metformin showed decreased IL-17, IFN-γ, TNF-α, and IL-8 expression in tissue, suggesting an antiinflammatory role of metformin in HS.5
Although there are no known RCTs assessing the efficacy of metformin in HS, existing clinical data are supportive of the use of metformin for refractory HS.49 Following a case report describing a patient with T2DM and stable HS while on metformin,50 several cohort studies have assessed the efficacy of metformin for the treatment of HS. A prospective study evaluating the efficacy of metformin monotherapy (starting dose of 500 mg/d, titrated to 500 mg 3 times daily) in patients with and without T2DM with HS refractory to other therapies found clinical improvement in 72% (18/25) of patients using the Sartorius Hidradenitis Suppurativa Score, improving from a mean (SD) score of 34.40 (12.46) to 26.76 (11.22) at 12 weeks (P=.0055,) and 22.39 (11.30) at 24 weeks (P=.0001). Additionally, 64% (16/25) of patients showed improved quality of life as evaluated by the Dermatology Life Quality Index (DLQI), which decreased from a mean (SD) score of 15.00 (4.96) to 10.08 (5.96)(P=.0017) at 12 weeks and 7.65 (7.12)(P=.000009) at 24 weeks on treatment.48 In a retrospective study of 53 patients with HS taking metformin started at 500 mg daily and increased to 500 mg twice daily after 2 weeks (when tolerated), 68% (36/53) showed some clinical response, with 19% (7/36) of those patients having achieved complete response to metformin monotherapy (defined as no active HS).51 Similarly, a retrospective study of pediatric patients with HS evaluating metformin (doses ranging from 500-2000 mg daily) as an adjunct therapy described a subset of patients with decreased frequency of HS flares with metformin.52 These studies emphasize the safety profile of metformin and support its current use as an adjunctive therapy for HS.
Acne Vulgaris—Acne vulgaris (AV) is a chronic inflammatory disorder affecting the pilosebaceous follicles.11 Similar to HS, AV has metabolic and hormonal influences that can be targeted by metformin.53 In AV, androgens lead to increased sebum production by binding to androgen receptors on sebocytes, which in turn attracts Cutibacterium acnes and promotes hyperkeratinization, inducing inflammation.54 Thus, the antiandrogenic effects of metformin may be beneficial for treatment of AV. Additionally, sebocytes express receptors for insulin and IGF-1, which can increase the size and number of sebocytes, as well as promote lipogenesis and inflammatory response, influencing sebum production.54 Serum levels for IGF-1 have been observed to be increased in patients with AV55 and reduced by metformin.56 A recent meta-analysis assessing the efficacy of metformin on AV indicated that 87% (13/15) of studies noted disease improvement on metformin, with 47% (7/15) of studies showing statistically significant (P<0.05) decreases in acne severity.57 Although most studies showed improvement, 47% (7/15) did not find significant differences between metformin and other interventions, indicating the availability of comparable treatment options. Overall, there has been a positive association between metformin use and acne improvement.57 However, it is important to note that most studies have focused on females with PCOS,57 and the main benefits of metformin in acne might be seen when managing comorbid conditions, particularly those associated with metabolic dysregulation and insulin resistance. Further studies are needed to determine the generalizability of prior results.
Psoriasis—Psoriasis is a chronic autoinflammatory disease characterized by epidermal hyperplasia with multiple cutaneous manifestations and potential for multiorgan involvement. Comorbid conditions include psoriatic arthritis, metabolic syndrome, and cardiovascular disease.58 Current treatment options depend on several factors (eg, disease severity, location of cutaneous lesions, comorbidities) and include topical, systemic, and phototherapy options, many of which target the immune system.58,59 A meta-analysis of 3 RCTs showed that metformin (500 mg/d or 1000 mg/d) was associated with significantly improved Psoriasis Area and Severity Index (PASI) 75% reductions (odds ratio [OR], 22.02; 95% CI, 2.12-228.49; P=.01) and 75% reductions in erythema, scaling, and induration (OR, 9.12; 95% CI, 2.13-39.02; P=.003) compared to placebo.60 In addition, an RCT evaluating the efficacy of metformin (1000 mg/d) or pioglitazone (30 mg/d) for 12 weeks in patients with psoriasis with metabolic syndrome found significant improvements in PASI75 (P=.001) and erythema, scaling, and induration (P=.016) scores as well as in Physician Global Assessment scores (P=.012) compared to placebo and no differences compared to pioglitazone.61 While current psoriasis management guidelines do not include metformin, its use may be worth consideration as an adjunct therapy in patients with psoriasis and comorbidities such as T2DM and metabolic syndrome.59 Metformin’s potential benefits in psoriasis may lie outside its metabolic influences and occur secondary to its immunomodulatory effects, including targeting of the Th17 axis or cytokine-specific pathways such as TNF-α, which are known to be involved in psoriasis pathogenesis.58
Central Centrifugal Cicatricial Alopecia—Central centrifugal cicatricial alopecia (CCCA) is a form of scarring alopecia characterized by chronic inflammation leading to permanent loss of hair follicles on the crown of the scalp.62 Current treatments include topical and intralesional corticosteroids, as well as oral antibiotics. In addition, therapies including the antimalarial hydroxychloroquine and immunosuppressants mycophenolate and cyclosporine are used in refractory disease.63,64 A case report described 2 patients with hair regrowth after 4 and 6 months of treatment with topical metformin 10% compounded in a proprietary transdermal vehicle.65 The authors speculated that metformin’s effects on CCCA could be attributed to its known agonistic effects on the adenosine monophosphate-activated protein kinase (AMPK) pathway with subsequent reduction in inflammation-induced fibrosis.65,66 Microarray67 and proteomic68 analysis have shown that AMPK is known to be downregulated in CCCA , making it an interesting therapeutic target in this disease. A recent retrospective case series demonstrated that 67% (8/12) of patients with refractory CCCA had symptomatic improvement, and 50% (6/12) showed hair regrowth after 6 months of low-dose (500 mg/d) oral metformin treatment.62 In addition, metformin therapy showed antifibrotic and anti-inflammatory effects when comparing scalp biopsies before and after treatment. Results showed decreased expression of fibrosisrelated genes (matrix metalloproteinase 7, collagen type IV á 1 chain), and gene set variation analysis showing reduced Th17 (P=.04) and increased AMPK signaling (P=.02) gene set expression.62 These findings are consistent with previous studies describing the upregulation of AMPK66 and downregulation of Th176 following metformin treatment. The immunomodulatory effects of metformin could be attributed to AMPK-mediated mTOR and NF-κB downregulation,62 although more studies are needed to understand these mechanisms and further explore the use of metformin in CCCA.
Skin Cancer—Metformin also has been evaluated in the setting of skin malignancies, including melanoma, squamous cell carcinoma, and basal cell carcinoma. Preclinical data suggest that metformin decreases cell viability in tumors through interactions with pathways involved in proinflammatory and prosurvival mechanisms such as NF-κB and mTOR.69,70 Additionally, given metformin’s inhibitory effects on oxidative phosphorylation, it has been postulated that it could be used to overcome treatment resistance driven by metabolic reprogramming.71,72 Most studies related to metformin and skin malignancies are still in preclinical stages; however, a meta-analysis of RCTs and cohort studies did not find significant associations between metformin use and skin cancer risk, although data trended toward a modest reduction in skin cancer among metformin users.73 A retrospective cohort study of melanoma in patients with T2DM taking metformin (250-2000 mg/d) found that the 5-year incidence of recurrence was lower in the metformin cohort compared to nonusers (43.8% vs 58.2%, respectively)(P=.002), and overall survival rates trended upward in the higher body mass index (>30) and melanoma stages 1 and 2 groups but did not reach statistical significance.74 In addition, a whole population casecontrol study in Iceland reported that metformin use at least 2 years before first-time basal cell carcinoma diagnosis was associated with a lower risk for disease (adjusted OR, 0.71; 95% CI, 0.61-0.83) with no significant dose-dependent differences; there were no notable effects on squamous cell carcinoma risk.75 Further preclinical and clinical data are needed to elucidate metformin’s effects on skin malignancies.
GLP-1 AND DUAL GLP-1/GIP AGONISTS
Glucagonlike peptide 1 and dual GLP-1/GIP agonists are emerging classes of medications currently approved as adjunct and second-line therapies for T2DM, particularly in patients who are overweight or obese as well as in those who are at risk for hypoglycemia.3 Currently approved GLP-1 agonists for T2DM include semaglutide, dulaglutide, exenatide, liraglutide, and lixisenatide, while tirzepatide is the only approved dual GLP-1/GIP agonist. Activating GLP-1 and GIP receptors stimulates insulin secretion and decreases glucagon production by the pancreas, thereby reducing blood glucose levels. Additionally, some of these medications are approved for obesity given their effects in delayed gastric emptying and increased satiety, among other factors.
Over the past few years, multiple case reports have described the associations between GLP-1 agonist use and improvement of dermatologic conditions, particularly those associated with T2DM and obesity, including HS and psoriasis.76,77 The mechanisms through which this occurs are not fully elucidated, although basic science and clinical studies have shown that GLP-1 agonists have immunomodulatory effects by reducing proinflammatory cytokines and altering immune cell populations.77-80 The numerous ongoing clinical trials and research studies will help further elucidate their benefits in other disease settings.81
Adverse Reactions
Most GLP-1 and GLP-1/GIP agonists are administered subcutaneously, and the most commonly reported cutaneous adverse effects are injection site reactions.82 Anaphylactic reactions to these medications also have been reported, although it is unclear if these were specific to the active ingredients or to injection excipients.83,84 A review of 33 cases of cutaneous reactions to GLP-1 agonists reported 11 (33%) dermal hypersensitivity reactions occurring as early as 4 weeks and as late as 3 years after treatment initiation. It also described 10 (30%) cases of eosinophilic panniculitis that developed within 3 weeks to 5 months of GLP-1 treatment, 3 (9%) cases of bullous pemphigoid that occurred within the first 2 months, 2 (6%) morbilliform drug eruptions that occurred within 5 weeks, 2 (6%) cases of angioedema that occurred 15 minutes to 2 weeks after treatment initiation, and 7 (21%) other isolated cutaneous reactions. Extended-release exenatide had the most reported reactions followed by liraglutide and subcutaneous semaglutide.85
In a different study, semaglutide use was most commonly associated with injection site reactions followed by alopecia, especially with oral administration. Unique cases of angioedema (2 days after injection), cutaneous hypersensitivity (within 10 months on treatment), bullous pemphigoid (within 2 months on treatment), eosinophilic fasciitis (within 2 weeks on treatment), and leukocytoclastic vasculitis (unclear timing), most of which resolved after discontinuation, also were reported.86 A recent case report linked semaglutide (0.5 mg/wk) to a case of drug-induced systemic lupus erythematosus that developed within 3 months of treatment initiation and described systemic lupus erythematosus–like symptoms in a subset of patients using this medication, namely females older than 60 years, within the first month of treatment.87 Hyperhidrosis was listed as a common adverse event in exenatide clinical trials, and various cases of panniculitis with exenatide use have been reported.82,88 Alopecia, mainly attributed to accelerated telogen effluvium secondary to rapid weight loss, also has been reported, although hair loss is not officially listed as an adverse effect of GLP-1 agonists, and reports are highly variable.89 Also secondary to weight loss, facial changes including sunken eyes, development of wrinkles, sagging jowls around the neck and jaw, and a hollowed appearance, among others, are recognized as undesirable adverse effects.90 Mansour et al90 described the potential challenges and considerations to these rising concerns associated with GLP1-agonist use.
Dermatologic Implications
Hidradenitis Suppurativa—Weight loss commonly is recommended as a lifestyle modification in the management of HS. Multiple reports have described clinical improvement of HS following weight loss with other medical interventions, such as dietary measures and bariatric surgery.91-94 Thus, it has been postulated that medically supported weight loss with GLP-1 agonists can help improve HS95; however, the data on the effectiveness of GLP-1 agonists on HS are still scarce and mostly have been reported in individual patients. One case report described a patient with improvements in their recalcitrant HS and DLQI score following weight loss on liraglutide (initial dose of 0.6 mg/d, titrated to 1.8 mg/d).76 In addition, a recent case report described improvements in HS and DLQI score following concomitant tirzepatide (initial dose of 2.5 mg/0.5 mL weekly, titrated to 7.5 mg/0.5 mL weekly) and infliximab treatment.96 The off-label use of these medications for HS is debated, and further studies regarding the benefits of GLP-1 agonists on HS still are needed.
Psoriasis—Similarly, several case reports have commented on the effects of GLP-1 agonists on psoriasis.97,98 An early study found GLP-1 receptors were expressed in psoriasis plaques but not in healthy skin and discussed that this could be due to immune infiltration in the plaques, providing a potential rationale for using anti-inflammatory GLP-1 agonists for psoriasis.99 Two prospective cohort studies observed improvements in PASI and DLQI scores in patients with psoriasis and T2DM after liraglutide treatment and noted important changes in immune cell populations.80,100 A recent RCT also found improvements in DLQI and PASI scores (P<.05) in patients with T2DM following liraglutide (1.8 mg/d) treatment, along with overall decreases in inflammatory cytokines, such as IL-23, IL-17, and TNF-α.77 However, another RCT in patients with obesity did not observe significant improvements in PASI and DLQI scores compared to placebo after 8 weeks of liraglutide (initial dose of 0.6 mg/d, titrated to 1.8 mg/d) treatment. 99 Although these results could have been influenced by the short length of treatment compared to other studies, which observed participants for more than 10 weeks, they highlight the need for tailored studies considering the different comorbidities to identify patients who could benefit the most from these therapies.
Alopecia—Although some studies have reported increased rates of alopecia following GLP-1 agonist treatment, others have speculated about the potential role of these medications in treating hair loss through improved insulin sensitivity and scalp blood flow.86,89 For example, a case report described a patient with improvement in androgenetic alopecia within 6 months of tirzepatide monotherapy at 2.5 mg weekly for the first 3 months followed by an increased dose of 5 mg weekly.101 The authors described the role of insulin in increasing dihydrotestosterone levels, which leads to miniaturization of the dermal papilla of hair follicles and argued that improvement of insulin resistance could benefit hair loss. Further studies can help elucidate the role of these medications on alopecia.
FINAL THOUGHTS
Standard T2DM treatments including metformin and GLP-1 and GLP-1/GIP agonists exhibit metabolic, immunologic, and hormonal effects that should be explored in other disease contexts. We reviewed the current data on T2DM medications in dermatologic conditions to highlight the need for additional studies to better understand the role that these medications play across diverse patient populations. Type 2 diabetes mellitus is a common comorbidity in dermatology patients, and understanding the multifactorial effects of these medications can help optimize treatment strategies, especially in patients with coexisting dermatologic and metabolic diseases.
- Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. doi:10.1038/nrendo.2017.151
- Ahmad E, Lim S, Lamptey R, et al. Type 2 diabetes. Lancet. 2022;400: 1803-1820. doi:10.1016/s0140-6736(22)01655-5
- Samson SL, Vellanki P, Blonde L, et al. American Association of Clinical Endocrinology Consensus Statement: comprehensive type 2 diabetes management algorithm—2023 update. Endocr Pract. 2023;29:305-340. doi:10.1016/j.eprac.2023.02.001
- LaMoia TE, Shulman GI. Cellular and molecular mechanisms of metformin action. Endocr Rev. 2021;42:77-96. doi:10.1210/endrev/bnaa023
- Petrasca A, Hambly R, Kearney N, et al. Metformin has antiinflammatory effects and induces immunometabolic reprogramming via multiple mechanisms in hidradenitis suppurativa. Br J Dermatol. 2023;189:730-740. doi:10.1093/bjd/ljad305
- Duan W, Ding Y, Yu X, et al. Metformin mitigates autoimmune insulitis by inhibiting Th1 and Th17 responses while promoting Treg production. Am J Transl Res. 2019;11:2393-2402.
- Bharath LP, Nikolajczyk BS. The intersection of metformin and inflammation. Am J Physiol Cell Physiol. 2021;320:C873-C879. doi:10.1152 /ajpcell.00604.2020
- Sung CT, Chao T, Lee A, et al. Oral metformin for treating dermatological diseases: a systematic review. J Drugs Dermatol. 2020;19:713-720. doi:10.36849/jdd.2020.4874
- Feng J, Wang X, Ye X, et al. Mitochondria as an important target of metformin: the mechanism of action, toxic and side effects, and new therapeutic applications. Pharmacol Res. 2022;177:106114. doi:10.1016/j.phrs.2022.106114
- Klapholz L, Leitersdorf E, Weinrauch L. Leucocytoclastic vasculitis and pneumonitis induced by metformin. Br Med J (Clin Res Ed). 1986;293:483. doi:10.1136/bmj.293.6545.483
- Badr D, Kurban M, Abbas O. Metformin in dermatology: an overview. J Eur Acad Dermatol Venereol. 2013;27:1329-1335. doi:10.1111/jdv.12116
- Czarnowicki T, Ramot Y, Ingber A, et al. Metformin-induced leukocytoclastic vasculitis: a case report. Am J Clin Dermatol. 2012;13:61-63. doi:10.2165/11593230-000000000-00000
- Ben Salem C, Hmouda H, Slim R, et al. Rare case of metformininduced leukocytoclastic vasculitis. Ann Pharmacother. 2006;40:1685-1687. doi:10.1345/aph.1H155
- Abtahi-Naeini B, Momen T, Amiri R, et al. Metformin-induced generalized bullous fixed-drug eruption with a positive dechallengerechallenge test: a case report and literature review. Case Rep Dermatol Med. 2023;2023:6353919. doi:10.1155/2023/6353919
- Al Masri D, Fleifel M, Hirbli K. Fixed drug eruption secondary to four anti-diabetic medications: an unusual case of polysensitivity. Cureus. 2021;13:E18599. doi:10.7759/cureus.18599
- Ramírez-Bellver JL, Lopez J, Macias E, et al. Metformin-induced generalized fixed drug eruption with cutaneous hemophagocytosis. Am J Dermatopathol. 2017;39:471-475. doi:10.1097/dad.0000000000000800
- Steber CJ, Perkins SL, Harris KB. Metformin-induced fixed-drug eruption confirmed by multiple exposures. Am J Case Rep. 2016;17:231-234. doi:10.12659/ajcr.896424
- Voore P, Odigwe C, Mirrakhimov AE, et al. DRESS syndrome following metformin administration: a case report and review of the literature. Am J Ther. 2016;23:E1970-E1973. doi:10.1097/mjt.0000000000000292
- Kastalli S, El Aïdli S, Chaabane A, et al. Photosensitivity induced by metformin: a report of 3 cases. Article in French. Tunis Med. 2009;87:703-705.
- Karadağ AS, You Y, Danarti R, et al. Acanthosis nigricans and the metabolic syndrome. Clin Dermatol. 2018;36:48-53. doi:10.1016/j.clindermatol.2017.09.008
- Kong AS, Williams RL, Smith M, et al. Acanthosis nigricans and diabetes risk factors: prevalence in young persons seen in southwestern US primary care practices. Ann Fam Med. 2007;5:202-208. doi:10.1370/afm.678
- Stuart CA, Gilkison CR, Smith MM, et al. Acanthosis nigricans as a risk factor for non-insulin dependent diabetes mellitus. Clin Pediatr (Phila). 1998;37:73-79. doi:10.1177/000992289803700203
- Hud JA Jr, Cohen JB, Wagner JM, et al. Prevalence and significance of acanthosis nigricans in an adult obese population. Arch Dermatol. 1992;128:941-944.
- Novotny R, Davis J, Butel J, et al. Effect of the Children’s Healthy Living Program on young child overweight, obesity, and acanthosis nigricans in the US-affiliated Pacific region: a randomized clinical trial. JAMA Netw Open. 2018;1:E183896. doi:10.1001/jamanetworkopen.2018.3896
- Romo A, Benavides S. Treatment options in insulin resistance obesityrelated acanthosis nigricans. Ann Pharmacother. 2008;42:1090-1094. doi:10.1345/aph.1K446
- Treesirichod A, Chaithirayanon S, Chaikul T, et al. The randomized trials of 10% urea cream and 0.025% tretinoin cream in the treatment of acanthosis nigricans. J Dermatolog Treat. 2021;32:837-842. doi:10.108 0/09546634.2019.1708855
- Treesirichod A, Chaithirayanon S, Wongjitrat N. Comparison of the efficacy and safety of 0.1% adapalene gel and 0.025% tretinoin cream in the treatment of childhood acanthosis nigricans. Pediatr Dermatol. 2019;36:330-334. doi:10.1111/pde.13799
- Hermanns-Lê T, Hermanns JF, Piérard GE. Juvenile acanthosis nigricans and insulin resistance. Pediatr Dermatol. 2002;19:12-14. doi:10.1046 /j.1525-1470.2002.00013.x
- Walling HW, Messingham M, Myers LM, et al. Improvement of acanthosis nigricans on isotretinoin and metformin. J Drugs Dermatol. 2003;2:677-681.
- Giri D, Alsaffar H, Ramakrishnan R. Acanthosis nigricans and its response to metformin. Pediatr Dermatol. 2017;34:e281-e282. doi:10.1111/pde.13206
- Bellot-Rojas P, Posadas-Sanchez R, Caracas-Portilla N, et al. Comparison of metformin versus rosiglitazone in patients with acanthosis nigricans: a pilot study. J Drugs Dermatol. 2006;5:884-889.
- Sett A, Pradhan S, Sancheti K, et al. Effectiveness and safety of metformin versus Canthex™ in patients with acanthosis nigricans: a randomized, double-blind controlled trial. Indian J Dermatol. 2019;64:115-121. doi:10.4103/ijd.IJD_417_17
- Genazzani AD, Battaglia C, Malavasi B, et al. Metformin administration modulates and restores luteinizing hormone spontaneous episodic secretion and ovarian function in nonobese patients with polycystic ovary syndrome. Fertil Steril. 2004;81:114-119. doi:10.1016 /j.fertnstert.2003.05.020
- Kazerooni T, Dehghan-Kooshkghazi M. Effects of metformin therapy on hyperandrogenism in women with polycystic ovarian syndrome. Gynecol Endocrinol. 2003;17:51-56.
- Kolodziejczyk B, Duleba AJ, Spaczynski RZ, et al. Metformin therapy decreases hyperandrogenism and hyperinsulinemia in women with polycystic ovary syndrome. Fertil Steril. 2000;73:1149-1154. doi:10.1016 /s0015-0282(00)00501-x
- Kelly CJ, Gordon D. The effect of metformin on hirsutism in polycystic ovary syndrome. Eur J Endocrinol. 2002;147:217-221. doi:10.1530/eje.0.1470217
- Harborne L, Fleming R, Lyall H, et al. Metformin or antiandrogen in the treatment of hirsutism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:4116-4123. doi:10.1210/jc.2003-030424
- Rezvanian H, Adibi N, Siavash M, et al. Increased insulin sensitivity by metformin enhances intense-pulsed-light-assisted hair removal in patients with polycystic ovary syndrome. Dermatology. 2009;218: 231-236. doi:10.1159/000187718
- Cosma M, Swiglo BA, Flynn DN, et al. Clinical review: insulin sensitizers for the treatment of hirsutism: a systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab. 2008;93:1135-1142. doi:10.1210/jc.2007-2429
- Martin KA, Anderson RR, Chang RJ, et al. Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1233-1257.
- Fraison E, Kostova E, Moran LJ, et al. Metformin versus the combined oral contraceptive pill for hirsutism, acne, and menstrual pattern in polycystic ovary syndrome. Cochrane Database Syst Rev. 2020;8:CD005552. doi:10.1002/14651858.CD005552.pub3
- Hambly R, Kearney N, Hughes R, et al. Metformin treatment of hidradenitis suppurativa: effect on metabolic parameters, inflammation, cardiovascular risk biomarkers, and immune mediators. Int J Mol Sci. 2023;24:6969. doi:10.3390/ijms24086969
- Gold DA, Reeder VJ, Mahan MG, et al. The prevalence of metabolic syndrome in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2014;70:699-703. doi:10.1016/j.jaad.2013.11.014
- Miller IM, Ellervik C, Vinding GR, et al. Association of metabolic syndrome and hidradenitis suppurativa. JAMA Dermatol. 2014;150: 1273-1280. doi:10.1001/jamadermatol.2014.1165
- Monfrecola G, Balato A, Caiazzo G, et al. Mammalian target of rapamycin, insulin resistance and hidradenitis suppurativa: a possible metabolic loop. J Eur Acad Dermatol Venereol. 2016;30:1631-1633. doi:10.1111/jdv.13233
- Yoon MS. The role of mammalian target of rapamycin (mTOR) in insulin signaling. Nutrients. 2017;9:1176. doi:10.3390/nu9111176
- Abu Rached N, Gambichler T, Dietrich JW, et al. The role of hormones in hidradenitis suppurativa: a systematic review. Int J Mol Sci. 2022;23:15250. doi:10.3390/ijms232315250
- Verdolini R, Clayton N, Smith A, et al. Metformin for the treatment of hidradenitis suppurativa: a little help along the way. J Eur Acad Dermatol Venereol. 2013;27:1101-1108. doi:10.1111/j.1468-3083.2012.04668.x
- Tsentemeidou A, Vakirlis E, Papadimitriou I, et al. Metformin in hidradenitis suppurativa: is it worth pursuing further? Skin Appendage Disord. 2023;9:187-190. doi:10.1159/000529359
- Arun B, Loffeld A. Long-standing hidradenitis suppurativa treated effectively with metformin. Clin Exp Dermatol. 2009;34:920-921. doi:10.1111/j.1365-2230.2008.03121.x
- Jennings L, Hambly R, Hughes R, et al. Metformin use in hidradenitis suppurativa. J Dermatolog Treat. 2020;31:261-263. doi:10.1080/09546634 .2019.1592100
- Moussa C, Wadowski L, Price H, et al. Metformin as adjunctive therapy for pediatric patients with hidradenitis suppurativa. J Drugs Dermatol. 2020;19:1231-1234. doi:10.36849/jdd.2020.5447
- Cho M, Woo YR, Cho SH, et al. Metformin: a potential treatment for acne, hidradenitis suppurativa and rosacea. Acta Derm Venereol. 2023;103:adv18392. doi:10.2340/actadv.v103.18392
- Del Rosso JQ, Kircik L. The cutaneous effects of androgens and androgen-mediated sebum production and their pathophysiologic and therapeutic importance in acne vulgaris. J Dermatolog Treat. 2024;35:2298878. doi:10.1080/09546634.2023.2298878
- El-Tahlawi S, Ezzat Mohammad N, Mohamed El-Amir A, et al. Survivin and insulin-like growth factor-I: potential role in the pathogenesis of acne and post-acne scar. Scars Burn Heal. 2019;5:2059513118818031. doi:10.1177/2059513118818031
- Albalat W, Darwish H, Abd-Elaal WH, et al. The potential role of insulin-like growth factor 1 in acne vulgaris and its correlation with the clinical response before and after treatment with metformin. J Cosmet Dermatol. 2022;21:6209-6214. doi:10.1111/jocd.15210
- Nguyen S, Nguyen ML, Roberts WS, et al. The efficacy of metformin as a therapeutic agent in the treatment of acne vulgaris: a systematic review. Cureus. 2024;16:E56246. doi:10.7759/cureus.56246
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983-994. doi:10.1016 /s0140-6736(14)61909-7
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113. doi:10.1016/j.jaad.2018.11.058
- Huang Z, Li J, Chen H, et al. The efficacy of metformin for the treatment of psoriasis: a meta-analysis study. Postepy Dermatol Alergol. 2023;40:606-610. doi:10.5114/ada.2023.130524
- Singh S, Bhansali A. Randomized placebo control study of insulin sensitizers (metformin and pioglitazone) in psoriasis patients with metabolic syndrome (topical treatment cohort). BMC Dermatol. 2016;16:12. doi:10.1186 /s12895-016-0049-y
- Bao A, Qadri A, Gadre A, et al. Low-dose metformin and profibrotic signature in central centrifugal cicatricial alopecia. JAMA Dermatol. 2024;E243062. doi:10.1001/jamadermatol.2024.3062
- Lawson CN, Bakayoko A, Callender VD. Central centrifugal cicatricial alopecia: challenges and treatments. Dermatol Clin. 2021;39:389-405. doi:10.1016/j.det.2021.03.004
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668. doi:10.1016/j .jaad.2008.09.066
- Araoye EF, Thomas JAL, Aguh CU. Hair regrowth in 2 patients with recalcitrant central centrifugal cicatricial alopecia after use of topical metformin. JAAD Case Rep. 2020;6:106-108. doi:10.1016/j.jdcr.2019.12.008
- Foretz M, Guigas B, Bertrand L, et al. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20:953-966. doi:10.1016 /j.cmet.2014.09.018
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904-912.e1. doi:10.1016/j.jaad.2018.05.1257
- Gadre A, Dyson T, Jedrych J, et al. Proteomic profiling of central centrifugal cicatricial alopecia reveals role of humoral immune response pathway and metabolic dysregulation. JID Innov. 2024;4:100263. doi:10.1016/j.xjidi.2024.100263
- Chaudhary SC, Kurundkar D, Elmets CA, et al. Metformin, an antidiabetic agent reduces growth of cutaneous squamous cell carcinoma by targeting mTOR signaling pathway. Photochem Photobiol. 2012;88:1149-1156. doi:10.1111/j.1751-1097.2012.01165.x
- Tomic T, Botton T, Cerezo M, et al. Metformin inhibits melanoma development through autophagy and apoptosis mechanisms. Cell Death Dis. 2011;2:e199. doi:10.1038/cddis.2011.86
- Mascaraque-Checa M, Gallego-Rentero M, Nicolás-Morala J, et al. Metformin overcomes metabolic reprogramming-induced resistance of skin squamous cell carcinoma to photodynamic therapy. Mol Metab. 2022;60:101496. doi:10.1016/j.molmet.2022.101496
- Mascaraque M, Delgado-Wicke P, Nuevo-Tapioles C, et al. Metformin as an adjuvant to photodynamic therapy in resistant basal cell carcinoma cells. Cancers (Basel). 2020;12:668. doi:10.3390/cancers12030668
- Chang MS, Hartman RI, Xue J, et al. Risk of skin cancer associated with metformin use: a meta-analysis of randomized controlled trials and observational studies. Cancer Prev Res (Phila). 2021;14:77-84. doi:10.1158/1940-6207.Capr-20-0376
- Augustin RC, Huang Z, Ding F, et al. Metformin is associated with improved clinical outcomes in patients with melanoma: a retrospective, multi-institutional study. Front Oncol. 2023;13:1075823. doi:10.3389 /fonc.2023.1075823
- Adalsteinsson JA, Muzumdar S, Waldman R, et al. Metformin is associated with decreased risk of basal cell carcinoma: a whole-population casecontrol study from Iceland. J Am Acad Dermatol. 2021;85:56-61. doi:10.1016/j.jaad.2021.02.042
- Jennings L, Nestor L, Molloy O, et al. The treatment of hidradenitis suppurativa with the glucagon-like peptide-1 agonist liraglutide. Br J Dermatol. 2017;177:858-859. doi:10.1111/bjd.15233
- Lin L, Xu X, Yu Y, et al. Glucagon-like peptide-1 receptor agonist liraglutide therapy for psoriasis patients with type 2 diabetes: a randomized-controlled trial. J Dermatolog Treat. 2022;33: 1428-1434. doi:10.1080/09546634.2020.1826392
- Karacabeyli D, Lacaille D. Glucagon-like peptide 1 receptor agonists in patients with inflammatory arthritis or psoriasis: a scoping review. J Clin Rheumatol. 2024;30:26-31. doi:10.1097/rhu.0000000000001949
- Yang J, Wang Z, Zhang X. GLP-1 receptor agonist impairs keratinocytes inflammatory signals by activating AMPK. Exp Mol Pathol. 2019;107: 124-128. doi:10.1016/j.yexmp.2019.01.014
- Buysschaert M, Baeck M, Preumont V, et al. Improvement of psoriasis during glucagon-like peptide-1 analogue therapy in type 2 diabetes is associated with decreasing dermal Υϛ T-cell number: a prospective case-series study. Br J Dermatol. 2014;171:155-161. doi:10.1111/bjd.12886
- Wilbon SS, Kolonin MG. GLP1 receptor agonists-effects beyond obesity and diabetes. Cells. 2023;13:65. doi:10.3390/cells13010065
- Filippatos TD, Panagiotopoulou TV, Elisaf MS. Adverse effects of GLP-1 receptor agonists. Rev Diabet Stud. 2014;11:202-230. doi:10.1900 /rds.2014.11.202
- He Z, Tabe AN, Rana S, et al. Tirzepatide-induced biphasic anaphylactic reaction: a case report. Cureus. 2023;15:e50112. doi:10.7759/cureus.50112
- Anthony MS, Aroda VR, Parlett LE, et al. Risk of anaphylaxis among new users of glp-1 receptor agonists: a cohort study. Diabetes Care. 2024;47:712-719. doi:10.2337/dc23-1911
- Salazar CE, Patil MK, Aihie O, et al. Rare cutaneous adverse reactions associated with GLP-1 agonists: a review of the published literature. Arch Dermatol Res. 2024;316:248. doi:10.1007/s00403-024-02969-3
- Tran MM, Mirza FN, Lee AC, et al. Dermatologic findings associated with semaglutide use: a scoping review. J Am Acad Dermatol. 2024;91:166-168. doi:10.1016/j.jaad.2024.03.021
- Castellanos V, Workneh H, Malik A, et al. Semaglutide-induced lupus erythematosus with multiorgan involvement. Cureus. 2024;16:E55324. doi:10.7759/cureus.55324
- Boccardi A, Shubrook JH. Cutaneous reactions to antidiabetic agents: a narrative review. Diabetology. 2022;3:97-107.
- Desai DD, Sikora M, Nohria A, et al. GLP-1 agonists and hair loss: a call for further investigation. Int J Dermatol. 2024;63:1128-1130. doi:10.1111 /ijd.17246
- Mansour MR, Hannawa OM, Yaldo MM, et al. The rise of “Ozempic face”: analyzing trends and treatment challenges associated with rapid facial weight loss induced by GLP-1 agonists. J Plast Reconstr Aesthet Surg. 2024;96:225-227. doi:10.1016/j.bjps.2024.07.051
- Sivanand A, Gulliver WP, Josan CK, et al. Weight loss and dietary interventions for hidradenitis suppurativa: a systematic review. J Cutan Med Surg. 2020;24:64-72. doi:10.1177/1203475419874412
- Boer J. Resolution of hidradenitis suppurativa after weight loss by dietary measures, especially on frictional locations. J Eur Acad Dermatol Venereol. 2016;30:895-896. doi:10.1111/jdv.13059
- Thomas CL, Gordon KD, Mortimer PS. Rapid resolution of hidradenitis suppurativa after bariatric surgical intervention. Clin Exp Dermatol. 2014;39:315-7; quiz 317-8. doi:10.1111/ced.12269
- Mandour MO, Al-Musawi S, Idowu E, et al. Metabolic endoscopy and a simplified low-carbohydrate-high-dietary fiber template as novel treatments for hidradenitis suppurativa—a case series. JAAD Case Rep. 2023;34:23-26. doi:10.1016/j.jdcr.2023.01.035
- Henry T, Cahn B, Haber R, et al. Therapeutic potential of GLP-1 agonists for hidradenitis suppurativa. Int J Dermatol. 2023;62:1543-1544. doi:10.1111/ijd.16892
- Chan LJ, Kaur M, Kaffenberger BH. A case of recalcitrant hidradenitis suppurativa concomitantly treated with tirzepatide. JAAD Case Rep. 2024;52:101-102. doi:10.1016/j.jdcr.2024.02.023
- Costanzo G, Curatolo S, Busà B, et al. Two birds one stone: semaglutide is highly effective against severe psoriasis in a type 2 diabetic patient. Endocrinol Diabetes Metab Case Rep. 2021;2021:21-00007. doi:10.1530 /edm-21-0007
- Buysschaert M, Tennstedt D, Preumont V. Improvement of psoriasis during exenatide treatment in a patient with diabetes. Diabetes Metab. 2012;38:86-88. doi:10.1016/j.diabet.2011.11.004
- Faurschou A, Gyldenløve M, Rohde U, et al. Lack of effect of the glucagonlike peptide-1 receptor agonist liraglutide on psoriasis in glucose-tolerant patients--a randomized placebo-controlled trial. J Eur Acad Dermatol Venereol. 2015;29:555-559. doi:10.1111/jdv.12629
- Ahern T, Tobin AM, Corrigan M, et al. Glucagon-like peptide-1 analogue therapy for psoriasis patients with obesity and type 2 diabetes: a prospective cohort study. J Eur Acad Dermatol Venereol. 2013;27:1440-1443. doi:10.1111/j.1468-3083.2012.04609.x
- Gordon ER, Musleh S, Bordone LA. Treatment of insulin resistance with tirzepatide leading to improvement of hair loss. JAAD Case Rep. 2024;50:123-125. doi:10.1016/j.jdcr.2024.06.001
Type 2 diabetes mellitus (T2DM) is a chronic disease characterized by uncontrolled hyperglycemia. Over the past few decades, its prevalence has steadily increased, now affecting approximately 10% of adults worldwide and ranking among the top 10 leading causes of death globally.1 The pathophysiology of T2DM involves persistent hyperglycemia that drives insulin resistance and a progressive decline in insulin production from the pancreas.2 Medical management of this condition aims to reduce blood glucose levels or enhance insulin production and sensitivity. Aside from lifestyle modifications, metformin is considered the first-line treatment for glycemic control according to the 2023 American Association of Clinical Endocrinology’s T2DM management algorithm.3 These updated guidelines stratify adjunct treatments by individualized glycemic targets and patient needs. For patients who are overweight or obese, glucagonlike peptide 1 (GLP-1) and dual GLP-1/ gastric inhibitory polypeptide (GIP) agonists are the preferred adjunct or second-line treatments.3
In this review, we highlight the dermatologic adverse effects and potential therapeutic benefits of metformin as well as GLP-1 and GLP-1/GIP agonists.
METFORMIN
Metformin is a biguanide agent used as a first-line treatment for T2DM because of its ability to reduce hepatic glucose production and increase peripheral tissue glucose uptake.4 In addition to its effects on glucose, metformin has been shown to have anti-inflammatory properties via inhibition of the nuclear factor κB and mammalian target of rapamycin (mTOR) pathways, leading to decreased production of cytokines associated with T helper (Th) 1 and Th17 cell responses, such as IL-17, interferon gamma (IFN-γ), and tumor necrosis factor α (TNF-α).5-7 These findings have spurred interest among clinicians in the potential use of metformin for inflammatory conditions, including dermatologic diseases such as psoriasis and hidradenitis suppurativa (HS).8
Adverse Effects
Metformin is administered orally and generally is well tolerated. The most common adverse effects include gastrointestinal symptoms such as diarrhea, nausea, vomiting, and abdominal pain.9 While cutaneous adverse effects are rare, multiple dermatologic adverse reactions to metformin have been reported,10,11 including leukocytoclastic vasculitis,11-13 fixed drug eruptions,14-17 drug rash with eosinophilia and systemic symptoms (DRESS) syndrome,18 and photosensitivity reactions.19 Leukocytoclastic vasculitis and DRESS syndrome typically develop within the first month following metformin initiation, while fixed drug eruption and photosensitivity reactions have more variable timing, occurring weeks to years after treatment initiation.12-19
Dermatologic Implications
Acanthosis Nigricans—Acanthosis nigricans (AN) is characterized by hyperpigmentation and velvety skin thickening, typically in intertriginous areas such as the back of the neck, axillae, and groin.20 It commonly is associated with insulin resistance and obesity.21-23 Treatments for AN primarily center around insulin sensitivity and weight loss,24,25 with some benefit observed from the use of keratolytic agents.26,27 Metformin may have utility in treating AN through its effects on insulin sensitivity and glycemic control. Multiple case reports have noted marked improvements in AN in patients with and without obesity with the addition of metformin to their existing treatment regimens in doses ranging from 500 mg to 1700 mg daily.28-30 However, an unblinded randomized controlled trial (RCT) comparing the efficacy of metformin (500 mg 3 times daily) with rosiglitazone (4 mg/d), another T2DM medication, on AN neck lesions in patients who were overweight and obese found no significant effects in lesion severity and only modest improvements in skin texture in both groups at 12 weeks following treatment initiation.31 Another RCT comparing metformin (500 mg twice daily) with a twice-daily capsule containing α-lipoic acid, biotin, chromium polynicotinate, and zinc sulfate, showed significant (P<.001) improvements in AN neck lesions in both groups after 12 weeks.32 According to Sung et al,8 longer duration of therapy (>6 months), higher doses (1700–2000 mg), and lower baseline weight were associated with higher efficacy of metformin for treatment of AN. Overall, the use of metformin as an adjunct treatment for AN, particularly in patients with underlying hyperglycemia, is supported in the literature, but further studies are needed to clarify dosing, duration of therapy, and patient populations that will benefit most from adding metformin to their treatment regimens.
Hirsutism—Hirsutism, which is characterized by excessive hair growth in androgen-dependent areas, can be challenging to treat. Metformin has been shown to reduce circulating insulin, luteinizing hormone, androstenedione, and testosterone, thus improving underlying hyperandrogenism, particularly in patients with polycystic ovary syndrome (PCOS).33-35 Although single studies evaluating the efficacy of metformin for treatment of hirsutism in patients with PCOS have shown potential benefits,36-38 meta-analyses showed no significant effects of metformin compared to placebo or oral contraceptives and decreased benefits compared to spironolactone and flutamide.39 Given these findings showing that metformin was no more effective than placebo or other treatments, the current Endocrine Society guidelines recommend against the use of metformin for hirsutism.39,40 There may be a role for metformin as an adjuvant therapy in certain populations (eg, patients with comorbid T2DM), although further studies stratifying risk factors such as body mass index and age are needed.41
Hidradenitis Suppurativa—Hidradenitis suppurativa is a follicular occlusive disease characterized by recurrent inflamed nodules leading to chronic dermal abscesses, fibrosis, and sinus tract formation primarily in intertriginous areas such as the axillae and groin.42 Medical management depends on disease severity but usually involves antibiotic treatment with adjunct therapies such as oral contraceptives, antiandrogenic medications (eg, spironolactone), biologic medications, and metformin.42 Preclinical and clinical data suggest that metformin can impact HS through metabolic and immunomodulatory mechanisms.5,42 Like many chronic inflammatory disorders, HS is associated with metabolic syndrome.43,44 A study evaluating insulin secretion after oral glucose tolerance testing showed increased insulin levels in patients with HS compared to controls (P=.02), with 60% (6/10) of patients with HS meeting criteria for insulin resistance. In addition, serum insulin levels in insulin-resistant patients with HS correlated with increased lesional skin mTOR gene expression at 30 (r=.80) and 60 (r=1.00) minutes, and mTOR was found to be upregulated in lesional and extralesional skin in patients with HS compared to healthy controls (P<.01).45 Insulin activates mTOR signaling, which mediates cell growth and survival, among other processes.46 Thus, metformin’s ability to increase insulin sensitivity and inhibit mTOR signaling could be beneficial in the setting of HS. Additionally, insulin and insulinlike growth factor 1 (IGF-1) increase androgen signaling, a process that has been implicated in HS.47
Metformin also may impact HS through its effects on testosterone and other hormones.48 A study evaluating peripheral blood mononuclear cells in patients with HS showed reduced IL-17, IFN-γ, TNF-α, and IL-6 levels in patients who were taking metformin (dose not reported) for longer than 6 months compared to patients who were not on metformin. Further analysis of ex vivo HS lesions cultured with metformin showed decreased IL-17, IFN-γ, TNF-α, and IL-8 expression in tissue, suggesting an antiinflammatory role of metformin in HS.5
Although there are no known RCTs assessing the efficacy of metformin in HS, existing clinical data are supportive of the use of metformin for refractory HS.49 Following a case report describing a patient with T2DM and stable HS while on metformin,50 several cohort studies have assessed the efficacy of metformin for the treatment of HS. A prospective study evaluating the efficacy of metformin monotherapy (starting dose of 500 mg/d, titrated to 500 mg 3 times daily) in patients with and without T2DM with HS refractory to other therapies found clinical improvement in 72% (18/25) of patients using the Sartorius Hidradenitis Suppurativa Score, improving from a mean (SD) score of 34.40 (12.46) to 26.76 (11.22) at 12 weeks (P=.0055,) and 22.39 (11.30) at 24 weeks (P=.0001). Additionally, 64% (16/25) of patients showed improved quality of life as evaluated by the Dermatology Life Quality Index (DLQI), which decreased from a mean (SD) score of 15.00 (4.96) to 10.08 (5.96)(P=.0017) at 12 weeks and 7.65 (7.12)(P=.000009) at 24 weeks on treatment.48 In a retrospective study of 53 patients with HS taking metformin started at 500 mg daily and increased to 500 mg twice daily after 2 weeks (when tolerated), 68% (36/53) showed some clinical response, with 19% (7/36) of those patients having achieved complete response to metformin monotherapy (defined as no active HS).51 Similarly, a retrospective study of pediatric patients with HS evaluating metformin (doses ranging from 500-2000 mg daily) as an adjunct therapy described a subset of patients with decreased frequency of HS flares with metformin.52 These studies emphasize the safety profile of metformin and support its current use as an adjunctive therapy for HS.
Acne Vulgaris—Acne vulgaris (AV) is a chronic inflammatory disorder affecting the pilosebaceous follicles.11 Similar to HS, AV has metabolic and hormonal influences that can be targeted by metformin.53 In AV, androgens lead to increased sebum production by binding to androgen receptors on sebocytes, which in turn attracts Cutibacterium acnes and promotes hyperkeratinization, inducing inflammation.54 Thus, the antiandrogenic effects of metformin may be beneficial for treatment of AV. Additionally, sebocytes express receptors for insulin and IGF-1, which can increase the size and number of sebocytes, as well as promote lipogenesis and inflammatory response, influencing sebum production.54 Serum levels for IGF-1 have been observed to be increased in patients with AV55 and reduced by metformin.56 A recent meta-analysis assessing the efficacy of metformin on AV indicated that 87% (13/15) of studies noted disease improvement on metformin, with 47% (7/15) of studies showing statistically significant (P<0.05) decreases in acne severity.57 Although most studies showed improvement, 47% (7/15) did not find significant differences between metformin and other interventions, indicating the availability of comparable treatment options. Overall, there has been a positive association between metformin use and acne improvement.57 However, it is important to note that most studies have focused on females with PCOS,57 and the main benefits of metformin in acne might be seen when managing comorbid conditions, particularly those associated with metabolic dysregulation and insulin resistance. Further studies are needed to determine the generalizability of prior results.
Psoriasis—Psoriasis is a chronic autoinflammatory disease characterized by epidermal hyperplasia with multiple cutaneous manifestations and potential for multiorgan involvement. Comorbid conditions include psoriatic arthritis, metabolic syndrome, and cardiovascular disease.58 Current treatment options depend on several factors (eg, disease severity, location of cutaneous lesions, comorbidities) and include topical, systemic, and phototherapy options, many of which target the immune system.58,59 A meta-analysis of 3 RCTs showed that metformin (500 mg/d or 1000 mg/d) was associated with significantly improved Psoriasis Area and Severity Index (PASI) 75% reductions (odds ratio [OR], 22.02; 95% CI, 2.12-228.49; P=.01) and 75% reductions in erythema, scaling, and induration (OR, 9.12; 95% CI, 2.13-39.02; P=.003) compared to placebo.60 In addition, an RCT evaluating the efficacy of metformin (1000 mg/d) or pioglitazone (30 mg/d) for 12 weeks in patients with psoriasis with metabolic syndrome found significant improvements in PASI75 (P=.001) and erythema, scaling, and induration (P=.016) scores as well as in Physician Global Assessment scores (P=.012) compared to placebo and no differences compared to pioglitazone.61 While current psoriasis management guidelines do not include metformin, its use may be worth consideration as an adjunct therapy in patients with psoriasis and comorbidities such as T2DM and metabolic syndrome.59 Metformin’s potential benefits in psoriasis may lie outside its metabolic influences and occur secondary to its immunomodulatory effects, including targeting of the Th17 axis or cytokine-specific pathways such as TNF-α, which are known to be involved in psoriasis pathogenesis.58
Central Centrifugal Cicatricial Alopecia—Central centrifugal cicatricial alopecia (CCCA) is a form of scarring alopecia characterized by chronic inflammation leading to permanent loss of hair follicles on the crown of the scalp.62 Current treatments include topical and intralesional corticosteroids, as well as oral antibiotics. In addition, therapies including the antimalarial hydroxychloroquine and immunosuppressants mycophenolate and cyclosporine are used in refractory disease.63,64 A case report described 2 patients with hair regrowth after 4 and 6 months of treatment with topical metformin 10% compounded in a proprietary transdermal vehicle.65 The authors speculated that metformin’s effects on CCCA could be attributed to its known agonistic effects on the adenosine monophosphate-activated protein kinase (AMPK) pathway with subsequent reduction in inflammation-induced fibrosis.65,66 Microarray67 and proteomic68 analysis have shown that AMPK is known to be downregulated in CCCA , making it an interesting therapeutic target in this disease. A recent retrospective case series demonstrated that 67% (8/12) of patients with refractory CCCA had symptomatic improvement, and 50% (6/12) showed hair regrowth after 6 months of low-dose (500 mg/d) oral metformin treatment.62 In addition, metformin therapy showed antifibrotic and anti-inflammatory effects when comparing scalp biopsies before and after treatment. Results showed decreased expression of fibrosisrelated genes (matrix metalloproteinase 7, collagen type IV á 1 chain), and gene set variation analysis showing reduced Th17 (P=.04) and increased AMPK signaling (P=.02) gene set expression.62 These findings are consistent with previous studies describing the upregulation of AMPK66 and downregulation of Th176 following metformin treatment. The immunomodulatory effects of metformin could be attributed to AMPK-mediated mTOR and NF-κB downregulation,62 although more studies are needed to understand these mechanisms and further explore the use of metformin in CCCA.
Skin Cancer—Metformin also has been evaluated in the setting of skin malignancies, including melanoma, squamous cell carcinoma, and basal cell carcinoma. Preclinical data suggest that metformin decreases cell viability in tumors through interactions with pathways involved in proinflammatory and prosurvival mechanisms such as NF-κB and mTOR.69,70 Additionally, given metformin’s inhibitory effects on oxidative phosphorylation, it has been postulated that it could be used to overcome treatment resistance driven by metabolic reprogramming.71,72 Most studies related to metformin and skin malignancies are still in preclinical stages; however, a meta-analysis of RCTs and cohort studies did not find significant associations between metformin use and skin cancer risk, although data trended toward a modest reduction in skin cancer among metformin users.73 A retrospective cohort study of melanoma in patients with T2DM taking metformin (250-2000 mg/d) found that the 5-year incidence of recurrence was lower in the metformin cohort compared to nonusers (43.8% vs 58.2%, respectively)(P=.002), and overall survival rates trended upward in the higher body mass index (>30) and melanoma stages 1 and 2 groups but did not reach statistical significance.74 In addition, a whole population casecontrol study in Iceland reported that metformin use at least 2 years before first-time basal cell carcinoma diagnosis was associated with a lower risk for disease (adjusted OR, 0.71; 95% CI, 0.61-0.83) with no significant dose-dependent differences; there were no notable effects on squamous cell carcinoma risk.75 Further preclinical and clinical data are needed to elucidate metformin’s effects on skin malignancies.
GLP-1 AND DUAL GLP-1/GIP AGONISTS
Glucagonlike peptide 1 and dual GLP-1/GIP agonists are emerging classes of medications currently approved as adjunct and second-line therapies for T2DM, particularly in patients who are overweight or obese as well as in those who are at risk for hypoglycemia.3 Currently approved GLP-1 agonists for T2DM include semaglutide, dulaglutide, exenatide, liraglutide, and lixisenatide, while tirzepatide is the only approved dual GLP-1/GIP agonist. Activating GLP-1 and GIP receptors stimulates insulin secretion and decreases glucagon production by the pancreas, thereby reducing blood glucose levels. Additionally, some of these medications are approved for obesity given their effects in delayed gastric emptying and increased satiety, among other factors.
Over the past few years, multiple case reports have described the associations between GLP-1 agonist use and improvement of dermatologic conditions, particularly those associated with T2DM and obesity, including HS and psoriasis.76,77 The mechanisms through which this occurs are not fully elucidated, although basic science and clinical studies have shown that GLP-1 agonists have immunomodulatory effects by reducing proinflammatory cytokines and altering immune cell populations.77-80 The numerous ongoing clinical trials and research studies will help further elucidate their benefits in other disease settings.81
Adverse Reactions
Most GLP-1 and GLP-1/GIP agonists are administered subcutaneously, and the most commonly reported cutaneous adverse effects are injection site reactions.82 Anaphylactic reactions to these medications also have been reported, although it is unclear if these were specific to the active ingredients or to injection excipients.83,84 A review of 33 cases of cutaneous reactions to GLP-1 agonists reported 11 (33%) dermal hypersensitivity reactions occurring as early as 4 weeks and as late as 3 years after treatment initiation. It also described 10 (30%) cases of eosinophilic panniculitis that developed within 3 weeks to 5 months of GLP-1 treatment, 3 (9%) cases of bullous pemphigoid that occurred within the first 2 months, 2 (6%) morbilliform drug eruptions that occurred within 5 weeks, 2 (6%) cases of angioedema that occurred 15 minutes to 2 weeks after treatment initiation, and 7 (21%) other isolated cutaneous reactions. Extended-release exenatide had the most reported reactions followed by liraglutide and subcutaneous semaglutide.85
In a different study, semaglutide use was most commonly associated with injection site reactions followed by alopecia, especially with oral administration. Unique cases of angioedema (2 days after injection), cutaneous hypersensitivity (within 10 months on treatment), bullous pemphigoid (within 2 months on treatment), eosinophilic fasciitis (within 2 weeks on treatment), and leukocytoclastic vasculitis (unclear timing), most of which resolved after discontinuation, also were reported.86 A recent case report linked semaglutide (0.5 mg/wk) to a case of drug-induced systemic lupus erythematosus that developed within 3 months of treatment initiation and described systemic lupus erythematosus–like symptoms in a subset of patients using this medication, namely females older than 60 years, within the first month of treatment.87 Hyperhidrosis was listed as a common adverse event in exenatide clinical trials, and various cases of panniculitis with exenatide use have been reported.82,88 Alopecia, mainly attributed to accelerated telogen effluvium secondary to rapid weight loss, also has been reported, although hair loss is not officially listed as an adverse effect of GLP-1 agonists, and reports are highly variable.89 Also secondary to weight loss, facial changes including sunken eyes, development of wrinkles, sagging jowls around the neck and jaw, and a hollowed appearance, among others, are recognized as undesirable adverse effects.90 Mansour et al90 described the potential challenges and considerations to these rising concerns associated with GLP1-agonist use.
Dermatologic Implications
Hidradenitis Suppurativa—Weight loss commonly is recommended as a lifestyle modification in the management of HS. Multiple reports have described clinical improvement of HS following weight loss with other medical interventions, such as dietary measures and bariatric surgery.91-94 Thus, it has been postulated that medically supported weight loss with GLP-1 agonists can help improve HS95; however, the data on the effectiveness of GLP-1 agonists on HS are still scarce and mostly have been reported in individual patients. One case report described a patient with improvements in their recalcitrant HS and DLQI score following weight loss on liraglutide (initial dose of 0.6 mg/d, titrated to 1.8 mg/d).76 In addition, a recent case report described improvements in HS and DLQI score following concomitant tirzepatide (initial dose of 2.5 mg/0.5 mL weekly, titrated to 7.5 mg/0.5 mL weekly) and infliximab treatment.96 The off-label use of these medications for HS is debated, and further studies regarding the benefits of GLP-1 agonists on HS still are needed.
Psoriasis—Similarly, several case reports have commented on the effects of GLP-1 agonists on psoriasis.97,98 An early study found GLP-1 receptors were expressed in psoriasis plaques but not in healthy skin and discussed that this could be due to immune infiltration in the plaques, providing a potential rationale for using anti-inflammatory GLP-1 agonists for psoriasis.99 Two prospective cohort studies observed improvements in PASI and DLQI scores in patients with psoriasis and T2DM after liraglutide treatment and noted important changes in immune cell populations.80,100 A recent RCT also found improvements in DLQI and PASI scores (P<.05) in patients with T2DM following liraglutide (1.8 mg/d) treatment, along with overall decreases in inflammatory cytokines, such as IL-23, IL-17, and TNF-α.77 However, another RCT in patients with obesity did not observe significant improvements in PASI and DLQI scores compared to placebo after 8 weeks of liraglutide (initial dose of 0.6 mg/d, titrated to 1.8 mg/d) treatment. 99 Although these results could have been influenced by the short length of treatment compared to other studies, which observed participants for more than 10 weeks, they highlight the need for tailored studies considering the different comorbidities to identify patients who could benefit the most from these therapies.
Alopecia—Although some studies have reported increased rates of alopecia following GLP-1 agonist treatment, others have speculated about the potential role of these medications in treating hair loss through improved insulin sensitivity and scalp blood flow.86,89 For example, a case report described a patient with improvement in androgenetic alopecia within 6 months of tirzepatide monotherapy at 2.5 mg weekly for the first 3 months followed by an increased dose of 5 mg weekly.101 The authors described the role of insulin in increasing dihydrotestosterone levels, which leads to miniaturization of the dermal papilla of hair follicles and argued that improvement of insulin resistance could benefit hair loss. Further studies can help elucidate the role of these medications on alopecia.
FINAL THOUGHTS
Standard T2DM treatments including metformin and GLP-1 and GLP-1/GIP agonists exhibit metabolic, immunologic, and hormonal effects that should be explored in other disease contexts. We reviewed the current data on T2DM medications in dermatologic conditions to highlight the need for additional studies to better understand the role that these medications play across diverse patient populations. Type 2 diabetes mellitus is a common comorbidity in dermatology patients, and understanding the multifactorial effects of these medications can help optimize treatment strategies, especially in patients with coexisting dermatologic and metabolic diseases.
Type 2 diabetes mellitus (T2DM) is a chronic disease characterized by uncontrolled hyperglycemia. Over the past few decades, its prevalence has steadily increased, now affecting approximately 10% of adults worldwide and ranking among the top 10 leading causes of death globally.1 The pathophysiology of T2DM involves persistent hyperglycemia that drives insulin resistance and a progressive decline in insulin production from the pancreas.2 Medical management of this condition aims to reduce blood glucose levels or enhance insulin production and sensitivity. Aside from lifestyle modifications, metformin is considered the first-line treatment for glycemic control according to the 2023 American Association of Clinical Endocrinology’s T2DM management algorithm.3 These updated guidelines stratify adjunct treatments by individualized glycemic targets and patient needs. For patients who are overweight or obese, glucagonlike peptide 1 (GLP-1) and dual GLP-1/ gastric inhibitory polypeptide (GIP) agonists are the preferred adjunct or second-line treatments.3
In this review, we highlight the dermatologic adverse effects and potential therapeutic benefits of metformin as well as GLP-1 and GLP-1/GIP agonists.
METFORMIN
Metformin is a biguanide agent used as a first-line treatment for T2DM because of its ability to reduce hepatic glucose production and increase peripheral tissue glucose uptake.4 In addition to its effects on glucose, metformin has been shown to have anti-inflammatory properties via inhibition of the nuclear factor κB and mammalian target of rapamycin (mTOR) pathways, leading to decreased production of cytokines associated with T helper (Th) 1 and Th17 cell responses, such as IL-17, interferon gamma (IFN-γ), and tumor necrosis factor α (TNF-α).5-7 These findings have spurred interest among clinicians in the potential use of metformin for inflammatory conditions, including dermatologic diseases such as psoriasis and hidradenitis suppurativa (HS).8
Adverse Effects
Metformin is administered orally and generally is well tolerated. The most common adverse effects include gastrointestinal symptoms such as diarrhea, nausea, vomiting, and abdominal pain.9 While cutaneous adverse effects are rare, multiple dermatologic adverse reactions to metformin have been reported,10,11 including leukocytoclastic vasculitis,11-13 fixed drug eruptions,14-17 drug rash with eosinophilia and systemic symptoms (DRESS) syndrome,18 and photosensitivity reactions.19 Leukocytoclastic vasculitis and DRESS syndrome typically develop within the first month following metformin initiation, while fixed drug eruption and photosensitivity reactions have more variable timing, occurring weeks to years after treatment initiation.12-19
Dermatologic Implications
Acanthosis Nigricans—Acanthosis nigricans (AN) is characterized by hyperpigmentation and velvety skin thickening, typically in intertriginous areas such as the back of the neck, axillae, and groin.20 It commonly is associated with insulin resistance and obesity.21-23 Treatments for AN primarily center around insulin sensitivity and weight loss,24,25 with some benefit observed from the use of keratolytic agents.26,27 Metformin may have utility in treating AN through its effects on insulin sensitivity and glycemic control. Multiple case reports have noted marked improvements in AN in patients with and without obesity with the addition of metformin to their existing treatment regimens in doses ranging from 500 mg to 1700 mg daily.28-30 However, an unblinded randomized controlled trial (RCT) comparing the efficacy of metformin (500 mg 3 times daily) with rosiglitazone (4 mg/d), another T2DM medication, on AN neck lesions in patients who were overweight and obese found no significant effects in lesion severity and only modest improvements in skin texture in both groups at 12 weeks following treatment initiation.31 Another RCT comparing metformin (500 mg twice daily) with a twice-daily capsule containing α-lipoic acid, biotin, chromium polynicotinate, and zinc sulfate, showed significant (P<.001) improvements in AN neck lesions in both groups after 12 weeks.32 According to Sung et al,8 longer duration of therapy (>6 months), higher doses (1700–2000 mg), and lower baseline weight were associated with higher efficacy of metformin for treatment of AN. Overall, the use of metformin as an adjunct treatment for AN, particularly in patients with underlying hyperglycemia, is supported in the literature, but further studies are needed to clarify dosing, duration of therapy, and patient populations that will benefit most from adding metformin to their treatment regimens.
Hirsutism—Hirsutism, which is characterized by excessive hair growth in androgen-dependent areas, can be challenging to treat. Metformin has been shown to reduce circulating insulin, luteinizing hormone, androstenedione, and testosterone, thus improving underlying hyperandrogenism, particularly in patients with polycystic ovary syndrome (PCOS).33-35 Although single studies evaluating the efficacy of metformin for treatment of hirsutism in patients with PCOS have shown potential benefits,36-38 meta-analyses showed no significant effects of metformin compared to placebo or oral contraceptives and decreased benefits compared to spironolactone and flutamide.39 Given these findings showing that metformin was no more effective than placebo or other treatments, the current Endocrine Society guidelines recommend against the use of metformin for hirsutism.39,40 There may be a role for metformin as an adjuvant therapy in certain populations (eg, patients with comorbid T2DM), although further studies stratifying risk factors such as body mass index and age are needed.41
Hidradenitis Suppurativa—Hidradenitis suppurativa is a follicular occlusive disease characterized by recurrent inflamed nodules leading to chronic dermal abscesses, fibrosis, and sinus tract formation primarily in intertriginous areas such as the axillae and groin.42 Medical management depends on disease severity but usually involves antibiotic treatment with adjunct therapies such as oral contraceptives, antiandrogenic medications (eg, spironolactone), biologic medications, and metformin.42 Preclinical and clinical data suggest that metformin can impact HS through metabolic and immunomodulatory mechanisms.5,42 Like many chronic inflammatory disorders, HS is associated with metabolic syndrome.43,44 A study evaluating insulin secretion after oral glucose tolerance testing showed increased insulin levels in patients with HS compared to controls (P=.02), with 60% (6/10) of patients with HS meeting criteria for insulin resistance. In addition, serum insulin levels in insulin-resistant patients with HS correlated with increased lesional skin mTOR gene expression at 30 (r=.80) and 60 (r=1.00) minutes, and mTOR was found to be upregulated in lesional and extralesional skin in patients with HS compared to healthy controls (P<.01).45 Insulin activates mTOR signaling, which mediates cell growth and survival, among other processes.46 Thus, metformin’s ability to increase insulin sensitivity and inhibit mTOR signaling could be beneficial in the setting of HS. Additionally, insulin and insulinlike growth factor 1 (IGF-1) increase androgen signaling, a process that has been implicated in HS.47
Metformin also may impact HS through its effects on testosterone and other hormones.48 A study evaluating peripheral blood mononuclear cells in patients with HS showed reduced IL-17, IFN-γ, TNF-α, and IL-6 levels in patients who were taking metformin (dose not reported) for longer than 6 months compared to patients who were not on metformin. Further analysis of ex vivo HS lesions cultured with metformin showed decreased IL-17, IFN-γ, TNF-α, and IL-8 expression in tissue, suggesting an antiinflammatory role of metformin in HS.5
Although there are no known RCTs assessing the efficacy of metformin in HS, existing clinical data are supportive of the use of metformin for refractory HS.49 Following a case report describing a patient with T2DM and stable HS while on metformin,50 several cohort studies have assessed the efficacy of metformin for the treatment of HS. A prospective study evaluating the efficacy of metformin monotherapy (starting dose of 500 mg/d, titrated to 500 mg 3 times daily) in patients with and without T2DM with HS refractory to other therapies found clinical improvement in 72% (18/25) of patients using the Sartorius Hidradenitis Suppurativa Score, improving from a mean (SD) score of 34.40 (12.46) to 26.76 (11.22) at 12 weeks (P=.0055,) and 22.39 (11.30) at 24 weeks (P=.0001). Additionally, 64% (16/25) of patients showed improved quality of life as evaluated by the Dermatology Life Quality Index (DLQI), which decreased from a mean (SD) score of 15.00 (4.96) to 10.08 (5.96)(P=.0017) at 12 weeks and 7.65 (7.12)(P=.000009) at 24 weeks on treatment.48 In a retrospective study of 53 patients with HS taking metformin started at 500 mg daily and increased to 500 mg twice daily after 2 weeks (when tolerated), 68% (36/53) showed some clinical response, with 19% (7/36) of those patients having achieved complete response to metformin monotherapy (defined as no active HS).51 Similarly, a retrospective study of pediatric patients with HS evaluating metformin (doses ranging from 500-2000 mg daily) as an adjunct therapy described a subset of patients with decreased frequency of HS flares with metformin.52 These studies emphasize the safety profile of metformin and support its current use as an adjunctive therapy for HS.
Acne Vulgaris—Acne vulgaris (AV) is a chronic inflammatory disorder affecting the pilosebaceous follicles.11 Similar to HS, AV has metabolic and hormonal influences that can be targeted by metformin.53 In AV, androgens lead to increased sebum production by binding to androgen receptors on sebocytes, which in turn attracts Cutibacterium acnes and promotes hyperkeratinization, inducing inflammation.54 Thus, the antiandrogenic effects of metformin may be beneficial for treatment of AV. Additionally, sebocytes express receptors for insulin and IGF-1, which can increase the size and number of sebocytes, as well as promote lipogenesis and inflammatory response, influencing sebum production.54 Serum levels for IGF-1 have been observed to be increased in patients with AV55 and reduced by metformin.56 A recent meta-analysis assessing the efficacy of metformin on AV indicated that 87% (13/15) of studies noted disease improvement on metformin, with 47% (7/15) of studies showing statistically significant (P<0.05) decreases in acne severity.57 Although most studies showed improvement, 47% (7/15) did not find significant differences between metformin and other interventions, indicating the availability of comparable treatment options. Overall, there has been a positive association between metformin use and acne improvement.57 However, it is important to note that most studies have focused on females with PCOS,57 and the main benefits of metformin in acne might be seen when managing comorbid conditions, particularly those associated with metabolic dysregulation and insulin resistance. Further studies are needed to determine the generalizability of prior results.
Psoriasis—Psoriasis is a chronic autoinflammatory disease characterized by epidermal hyperplasia with multiple cutaneous manifestations and potential for multiorgan involvement. Comorbid conditions include psoriatic arthritis, metabolic syndrome, and cardiovascular disease.58 Current treatment options depend on several factors (eg, disease severity, location of cutaneous lesions, comorbidities) and include topical, systemic, and phototherapy options, many of which target the immune system.58,59 A meta-analysis of 3 RCTs showed that metformin (500 mg/d or 1000 mg/d) was associated with significantly improved Psoriasis Area and Severity Index (PASI) 75% reductions (odds ratio [OR], 22.02; 95% CI, 2.12-228.49; P=.01) and 75% reductions in erythema, scaling, and induration (OR, 9.12; 95% CI, 2.13-39.02; P=.003) compared to placebo.60 In addition, an RCT evaluating the efficacy of metformin (1000 mg/d) or pioglitazone (30 mg/d) for 12 weeks in patients with psoriasis with metabolic syndrome found significant improvements in PASI75 (P=.001) and erythema, scaling, and induration (P=.016) scores as well as in Physician Global Assessment scores (P=.012) compared to placebo and no differences compared to pioglitazone.61 While current psoriasis management guidelines do not include metformin, its use may be worth consideration as an adjunct therapy in patients with psoriasis and comorbidities such as T2DM and metabolic syndrome.59 Metformin’s potential benefits in psoriasis may lie outside its metabolic influences and occur secondary to its immunomodulatory effects, including targeting of the Th17 axis or cytokine-specific pathways such as TNF-α, which are known to be involved in psoriasis pathogenesis.58
Central Centrifugal Cicatricial Alopecia—Central centrifugal cicatricial alopecia (CCCA) is a form of scarring alopecia characterized by chronic inflammation leading to permanent loss of hair follicles on the crown of the scalp.62 Current treatments include topical and intralesional corticosteroids, as well as oral antibiotics. In addition, therapies including the antimalarial hydroxychloroquine and immunosuppressants mycophenolate and cyclosporine are used in refractory disease.63,64 A case report described 2 patients with hair regrowth after 4 and 6 months of treatment with topical metformin 10% compounded in a proprietary transdermal vehicle.65 The authors speculated that metformin’s effects on CCCA could be attributed to its known agonistic effects on the adenosine monophosphate-activated protein kinase (AMPK) pathway with subsequent reduction in inflammation-induced fibrosis.65,66 Microarray67 and proteomic68 analysis have shown that AMPK is known to be downregulated in CCCA , making it an interesting therapeutic target in this disease. A recent retrospective case series demonstrated that 67% (8/12) of patients with refractory CCCA had symptomatic improvement, and 50% (6/12) showed hair regrowth after 6 months of low-dose (500 mg/d) oral metformin treatment.62 In addition, metformin therapy showed antifibrotic and anti-inflammatory effects when comparing scalp biopsies before and after treatment. Results showed decreased expression of fibrosisrelated genes (matrix metalloproteinase 7, collagen type IV á 1 chain), and gene set variation analysis showing reduced Th17 (P=.04) and increased AMPK signaling (P=.02) gene set expression.62 These findings are consistent with previous studies describing the upregulation of AMPK66 and downregulation of Th176 following metformin treatment. The immunomodulatory effects of metformin could be attributed to AMPK-mediated mTOR and NF-κB downregulation,62 although more studies are needed to understand these mechanisms and further explore the use of metformin in CCCA.
Skin Cancer—Metformin also has been evaluated in the setting of skin malignancies, including melanoma, squamous cell carcinoma, and basal cell carcinoma. Preclinical data suggest that metformin decreases cell viability in tumors through interactions with pathways involved in proinflammatory and prosurvival mechanisms such as NF-κB and mTOR.69,70 Additionally, given metformin’s inhibitory effects on oxidative phosphorylation, it has been postulated that it could be used to overcome treatment resistance driven by metabolic reprogramming.71,72 Most studies related to metformin and skin malignancies are still in preclinical stages; however, a meta-analysis of RCTs and cohort studies did not find significant associations between metformin use and skin cancer risk, although data trended toward a modest reduction in skin cancer among metformin users.73 A retrospective cohort study of melanoma in patients with T2DM taking metformin (250-2000 mg/d) found that the 5-year incidence of recurrence was lower in the metformin cohort compared to nonusers (43.8% vs 58.2%, respectively)(P=.002), and overall survival rates trended upward in the higher body mass index (>30) and melanoma stages 1 and 2 groups but did not reach statistical significance.74 In addition, a whole population casecontrol study in Iceland reported that metformin use at least 2 years before first-time basal cell carcinoma diagnosis was associated with a lower risk for disease (adjusted OR, 0.71; 95% CI, 0.61-0.83) with no significant dose-dependent differences; there were no notable effects on squamous cell carcinoma risk.75 Further preclinical and clinical data are needed to elucidate metformin’s effects on skin malignancies.
GLP-1 AND DUAL GLP-1/GIP AGONISTS
Glucagonlike peptide 1 and dual GLP-1/GIP agonists are emerging classes of medications currently approved as adjunct and second-line therapies for T2DM, particularly in patients who are overweight or obese as well as in those who are at risk for hypoglycemia.3 Currently approved GLP-1 agonists for T2DM include semaglutide, dulaglutide, exenatide, liraglutide, and lixisenatide, while tirzepatide is the only approved dual GLP-1/GIP agonist. Activating GLP-1 and GIP receptors stimulates insulin secretion and decreases glucagon production by the pancreas, thereby reducing blood glucose levels. Additionally, some of these medications are approved for obesity given their effects in delayed gastric emptying and increased satiety, among other factors.
Over the past few years, multiple case reports have described the associations between GLP-1 agonist use and improvement of dermatologic conditions, particularly those associated with T2DM and obesity, including HS and psoriasis.76,77 The mechanisms through which this occurs are not fully elucidated, although basic science and clinical studies have shown that GLP-1 agonists have immunomodulatory effects by reducing proinflammatory cytokines and altering immune cell populations.77-80 The numerous ongoing clinical trials and research studies will help further elucidate their benefits in other disease settings.81
Adverse Reactions
Most GLP-1 and GLP-1/GIP agonists are administered subcutaneously, and the most commonly reported cutaneous adverse effects are injection site reactions.82 Anaphylactic reactions to these medications also have been reported, although it is unclear if these were specific to the active ingredients or to injection excipients.83,84 A review of 33 cases of cutaneous reactions to GLP-1 agonists reported 11 (33%) dermal hypersensitivity reactions occurring as early as 4 weeks and as late as 3 years after treatment initiation. It also described 10 (30%) cases of eosinophilic panniculitis that developed within 3 weeks to 5 months of GLP-1 treatment, 3 (9%) cases of bullous pemphigoid that occurred within the first 2 months, 2 (6%) morbilliform drug eruptions that occurred within 5 weeks, 2 (6%) cases of angioedema that occurred 15 minutes to 2 weeks after treatment initiation, and 7 (21%) other isolated cutaneous reactions. Extended-release exenatide had the most reported reactions followed by liraglutide and subcutaneous semaglutide.85
In a different study, semaglutide use was most commonly associated with injection site reactions followed by alopecia, especially with oral administration. Unique cases of angioedema (2 days after injection), cutaneous hypersensitivity (within 10 months on treatment), bullous pemphigoid (within 2 months on treatment), eosinophilic fasciitis (within 2 weeks on treatment), and leukocytoclastic vasculitis (unclear timing), most of which resolved after discontinuation, also were reported.86 A recent case report linked semaglutide (0.5 mg/wk) to a case of drug-induced systemic lupus erythematosus that developed within 3 months of treatment initiation and described systemic lupus erythematosus–like symptoms in a subset of patients using this medication, namely females older than 60 years, within the first month of treatment.87 Hyperhidrosis was listed as a common adverse event in exenatide clinical trials, and various cases of panniculitis with exenatide use have been reported.82,88 Alopecia, mainly attributed to accelerated telogen effluvium secondary to rapid weight loss, also has been reported, although hair loss is not officially listed as an adverse effect of GLP-1 agonists, and reports are highly variable.89 Also secondary to weight loss, facial changes including sunken eyes, development of wrinkles, sagging jowls around the neck and jaw, and a hollowed appearance, among others, are recognized as undesirable adverse effects.90 Mansour et al90 described the potential challenges and considerations to these rising concerns associated with GLP1-agonist use.
Dermatologic Implications
Hidradenitis Suppurativa—Weight loss commonly is recommended as a lifestyle modification in the management of HS. Multiple reports have described clinical improvement of HS following weight loss with other medical interventions, such as dietary measures and bariatric surgery.91-94 Thus, it has been postulated that medically supported weight loss with GLP-1 agonists can help improve HS95; however, the data on the effectiveness of GLP-1 agonists on HS are still scarce and mostly have been reported in individual patients. One case report described a patient with improvements in their recalcitrant HS and DLQI score following weight loss on liraglutide (initial dose of 0.6 mg/d, titrated to 1.8 mg/d).76 In addition, a recent case report described improvements in HS and DLQI score following concomitant tirzepatide (initial dose of 2.5 mg/0.5 mL weekly, titrated to 7.5 mg/0.5 mL weekly) and infliximab treatment.96 The off-label use of these medications for HS is debated, and further studies regarding the benefits of GLP-1 agonists on HS still are needed.
Psoriasis—Similarly, several case reports have commented on the effects of GLP-1 agonists on psoriasis.97,98 An early study found GLP-1 receptors were expressed in psoriasis plaques but not in healthy skin and discussed that this could be due to immune infiltration in the plaques, providing a potential rationale for using anti-inflammatory GLP-1 agonists for psoriasis.99 Two prospective cohort studies observed improvements in PASI and DLQI scores in patients with psoriasis and T2DM after liraglutide treatment and noted important changes in immune cell populations.80,100 A recent RCT also found improvements in DLQI and PASI scores (P<.05) in patients with T2DM following liraglutide (1.8 mg/d) treatment, along with overall decreases in inflammatory cytokines, such as IL-23, IL-17, and TNF-α.77 However, another RCT in patients with obesity did not observe significant improvements in PASI and DLQI scores compared to placebo after 8 weeks of liraglutide (initial dose of 0.6 mg/d, titrated to 1.8 mg/d) treatment. 99 Although these results could have been influenced by the short length of treatment compared to other studies, which observed participants for more than 10 weeks, they highlight the need for tailored studies considering the different comorbidities to identify patients who could benefit the most from these therapies.
Alopecia—Although some studies have reported increased rates of alopecia following GLP-1 agonist treatment, others have speculated about the potential role of these medications in treating hair loss through improved insulin sensitivity and scalp blood flow.86,89 For example, a case report described a patient with improvement in androgenetic alopecia within 6 months of tirzepatide monotherapy at 2.5 mg weekly for the first 3 months followed by an increased dose of 5 mg weekly.101 The authors described the role of insulin in increasing dihydrotestosterone levels, which leads to miniaturization of the dermal papilla of hair follicles and argued that improvement of insulin resistance could benefit hair loss. Further studies can help elucidate the role of these medications on alopecia.
FINAL THOUGHTS
Standard T2DM treatments including metformin and GLP-1 and GLP-1/GIP agonists exhibit metabolic, immunologic, and hormonal effects that should be explored in other disease contexts. We reviewed the current data on T2DM medications in dermatologic conditions to highlight the need for additional studies to better understand the role that these medications play across diverse patient populations. Type 2 diabetes mellitus is a common comorbidity in dermatology patients, and understanding the multifactorial effects of these medications can help optimize treatment strategies, especially in patients with coexisting dermatologic and metabolic diseases.
- Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. doi:10.1038/nrendo.2017.151
- Ahmad E, Lim S, Lamptey R, et al. Type 2 diabetes. Lancet. 2022;400: 1803-1820. doi:10.1016/s0140-6736(22)01655-5
- Samson SL, Vellanki P, Blonde L, et al. American Association of Clinical Endocrinology Consensus Statement: comprehensive type 2 diabetes management algorithm—2023 update. Endocr Pract. 2023;29:305-340. doi:10.1016/j.eprac.2023.02.001
- LaMoia TE, Shulman GI. Cellular and molecular mechanisms of metformin action. Endocr Rev. 2021;42:77-96. doi:10.1210/endrev/bnaa023
- Petrasca A, Hambly R, Kearney N, et al. Metformin has antiinflammatory effects and induces immunometabolic reprogramming via multiple mechanisms in hidradenitis suppurativa. Br J Dermatol. 2023;189:730-740. doi:10.1093/bjd/ljad305
- Duan W, Ding Y, Yu X, et al. Metformin mitigates autoimmune insulitis by inhibiting Th1 and Th17 responses while promoting Treg production. Am J Transl Res. 2019;11:2393-2402.
- Bharath LP, Nikolajczyk BS. The intersection of metformin and inflammation. Am J Physiol Cell Physiol. 2021;320:C873-C879. doi:10.1152 /ajpcell.00604.2020
- Sung CT, Chao T, Lee A, et al. Oral metformin for treating dermatological diseases: a systematic review. J Drugs Dermatol. 2020;19:713-720. doi:10.36849/jdd.2020.4874
- Feng J, Wang X, Ye X, et al. Mitochondria as an important target of metformin: the mechanism of action, toxic and side effects, and new therapeutic applications. Pharmacol Res. 2022;177:106114. doi:10.1016/j.phrs.2022.106114
- Klapholz L, Leitersdorf E, Weinrauch L. Leucocytoclastic vasculitis and pneumonitis induced by metformin. Br Med J (Clin Res Ed). 1986;293:483. doi:10.1136/bmj.293.6545.483
- Badr D, Kurban M, Abbas O. Metformin in dermatology: an overview. J Eur Acad Dermatol Venereol. 2013;27:1329-1335. doi:10.1111/jdv.12116
- Czarnowicki T, Ramot Y, Ingber A, et al. Metformin-induced leukocytoclastic vasculitis: a case report. Am J Clin Dermatol. 2012;13:61-63. doi:10.2165/11593230-000000000-00000
- Ben Salem C, Hmouda H, Slim R, et al. Rare case of metformininduced leukocytoclastic vasculitis. Ann Pharmacother. 2006;40:1685-1687. doi:10.1345/aph.1H155
- Abtahi-Naeini B, Momen T, Amiri R, et al. Metformin-induced generalized bullous fixed-drug eruption with a positive dechallengerechallenge test: a case report and literature review. Case Rep Dermatol Med. 2023;2023:6353919. doi:10.1155/2023/6353919
- Al Masri D, Fleifel M, Hirbli K. Fixed drug eruption secondary to four anti-diabetic medications: an unusual case of polysensitivity. Cureus. 2021;13:E18599. doi:10.7759/cureus.18599
- Ramírez-Bellver JL, Lopez J, Macias E, et al. Metformin-induced generalized fixed drug eruption with cutaneous hemophagocytosis. Am J Dermatopathol. 2017;39:471-475. doi:10.1097/dad.0000000000000800
- Steber CJ, Perkins SL, Harris KB. Metformin-induced fixed-drug eruption confirmed by multiple exposures. Am J Case Rep. 2016;17:231-234. doi:10.12659/ajcr.896424
- Voore P, Odigwe C, Mirrakhimov AE, et al. DRESS syndrome following metformin administration: a case report and review of the literature. Am J Ther. 2016;23:E1970-E1973. doi:10.1097/mjt.0000000000000292
- Kastalli S, El Aïdli S, Chaabane A, et al. Photosensitivity induced by metformin: a report of 3 cases. Article in French. Tunis Med. 2009;87:703-705.
- Karadağ AS, You Y, Danarti R, et al. Acanthosis nigricans and the metabolic syndrome. Clin Dermatol. 2018;36:48-53. doi:10.1016/j.clindermatol.2017.09.008
- Kong AS, Williams RL, Smith M, et al. Acanthosis nigricans and diabetes risk factors: prevalence in young persons seen in southwestern US primary care practices. Ann Fam Med. 2007;5:202-208. doi:10.1370/afm.678
- Stuart CA, Gilkison CR, Smith MM, et al. Acanthosis nigricans as a risk factor for non-insulin dependent diabetes mellitus. Clin Pediatr (Phila). 1998;37:73-79. doi:10.1177/000992289803700203
- Hud JA Jr, Cohen JB, Wagner JM, et al. Prevalence and significance of acanthosis nigricans in an adult obese population. Arch Dermatol. 1992;128:941-944.
- Novotny R, Davis J, Butel J, et al. Effect of the Children’s Healthy Living Program on young child overweight, obesity, and acanthosis nigricans in the US-affiliated Pacific region: a randomized clinical trial. JAMA Netw Open. 2018;1:E183896. doi:10.1001/jamanetworkopen.2018.3896
- Romo A, Benavides S. Treatment options in insulin resistance obesityrelated acanthosis nigricans. Ann Pharmacother. 2008;42:1090-1094. doi:10.1345/aph.1K446
- Treesirichod A, Chaithirayanon S, Chaikul T, et al. The randomized trials of 10% urea cream and 0.025% tretinoin cream in the treatment of acanthosis nigricans. J Dermatolog Treat. 2021;32:837-842. doi:10.108 0/09546634.2019.1708855
- Treesirichod A, Chaithirayanon S, Wongjitrat N. Comparison of the efficacy and safety of 0.1% adapalene gel and 0.025% tretinoin cream in the treatment of childhood acanthosis nigricans. Pediatr Dermatol. 2019;36:330-334. doi:10.1111/pde.13799
- Hermanns-Lê T, Hermanns JF, Piérard GE. Juvenile acanthosis nigricans and insulin resistance. Pediatr Dermatol. 2002;19:12-14. doi:10.1046 /j.1525-1470.2002.00013.x
- Walling HW, Messingham M, Myers LM, et al. Improvement of acanthosis nigricans on isotretinoin and metformin. J Drugs Dermatol. 2003;2:677-681.
- Giri D, Alsaffar H, Ramakrishnan R. Acanthosis nigricans and its response to metformin. Pediatr Dermatol. 2017;34:e281-e282. doi:10.1111/pde.13206
- Bellot-Rojas P, Posadas-Sanchez R, Caracas-Portilla N, et al. Comparison of metformin versus rosiglitazone in patients with acanthosis nigricans: a pilot study. J Drugs Dermatol. 2006;5:884-889.
- Sett A, Pradhan S, Sancheti K, et al. Effectiveness and safety of metformin versus Canthex™ in patients with acanthosis nigricans: a randomized, double-blind controlled trial. Indian J Dermatol. 2019;64:115-121. doi:10.4103/ijd.IJD_417_17
- Genazzani AD, Battaglia C, Malavasi B, et al. Metformin administration modulates and restores luteinizing hormone spontaneous episodic secretion and ovarian function in nonobese patients with polycystic ovary syndrome. Fertil Steril. 2004;81:114-119. doi:10.1016 /j.fertnstert.2003.05.020
- Kazerooni T, Dehghan-Kooshkghazi M. Effects of metformin therapy on hyperandrogenism in women with polycystic ovarian syndrome. Gynecol Endocrinol. 2003;17:51-56.
- Kolodziejczyk B, Duleba AJ, Spaczynski RZ, et al. Metformin therapy decreases hyperandrogenism and hyperinsulinemia in women with polycystic ovary syndrome. Fertil Steril. 2000;73:1149-1154. doi:10.1016 /s0015-0282(00)00501-x
- Kelly CJ, Gordon D. The effect of metformin on hirsutism in polycystic ovary syndrome. Eur J Endocrinol. 2002;147:217-221. doi:10.1530/eje.0.1470217
- Harborne L, Fleming R, Lyall H, et al. Metformin or antiandrogen in the treatment of hirsutism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:4116-4123. doi:10.1210/jc.2003-030424
- Rezvanian H, Adibi N, Siavash M, et al. Increased insulin sensitivity by metformin enhances intense-pulsed-light-assisted hair removal in patients with polycystic ovary syndrome. Dermatology. 2009;218: 231-236. doi:10.1159/000187718
- Cosma M, Swiglo BA, Flynn DN, et al. Clinical review: insulin sensitizers for the treatment of hirsutism: a systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab. 2008;93:1135-1142. doi:10.1210/jc.2007-2429
- Martin KA, Anderson RR, Chang RJ, et al. Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1233-1257.
- Fraison E, Kostova E, Moran LJ, et al. Metformin versus the combined oral contraceptive pill for hirsutism, acne, and menstrual pattern in polycystic ovary syndrome. Cochrane Database Syst Rev. 2020;8:CD005552. doi:10.1002/14651858.CD005552.pub3
- Hambly R, Kearney N, Hughes R, et al. Metformin treatment of hidradenitis suppurativa: effect on metabolic parameters, inflammation, cardiovascular risk biomarkers, and immune mediators. Int J Mol Sci. 2023;24:6969. doi:10.3390/ijms24086969
- Gold DA, Reeder VJ, Mahan MG, et al. The prevalence of metabolic syndrome in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2014;70:699-703. doi:10.1016/j.jaad.2013.11.014
- Miller IM, Ellervik C, Vinding GR, et al. Association of metabolic syndrome and hidradenitis suppurativa. JAMA Dermatol. 2014;150: 1273-1280. doi:10.1001/jamadermatol.2014.1165
- Monfrecola G, Balato A, Caiazzo G, et al. Mammalian target of rapamycin, insulin resistance and hidradenitis suppurativa: a possible metabolic loop. J Eur Acad Dermatol Venereol. 2016;30:1631-1633. doi:10.1111/jdv.13233
- Yoon MS. The role of mammalian target of rapamycin (mTOR) in insulin signaling. Nutrients. 2017;9:1176. doi:10.3390/nu9111176
- Abu Rached N, Gambichler T, Dietrich JW, et al. The role of hormones in hidradenitis suppurativa: a systematic review. Int J Mol Sci. 2022;23:15250. doi:10.3390/ijms232315250
- Verdolini R, Clayton N, Smith A, et al. Metformin for the treatment of hidradenitis suppurativa: a little help along the way. J Eur Acad Dermatol Venereol. 2013;27:1101-1108. doi:10.1111/j.1468-3083.2012.04668.x
- Tsentemeidou A, Vakirlis E, Papadimitriou I, et al. Metformin in hidradenitis suppurativa: is it worth pursuing further? Skin Appendage Disord. 2023;9:187-190. doi:10.1159/000529359
- Arun B, Loffeld A. Long-standing hidradenitis suppurativa treated effectively with metformin. Clin Exp Dermatol. 2009;34:920-921. doi:10.1111/j.1365-2230.2008.03121.x
- Jennings L, Hambly R, Hughes R, et al. Metformin use in hidradenitis suppurativa. J Dermatolog Treat. 2020;31:261-263. doi:10.1080/09546634 .2019.1592100
- Moussa C, Wadowski L, Price H, et al. Metformin as adjunctive therapy for pediatric patients with hidradenitis suppurativa. J Drugs Dermatol. 2020;19:1231-1234. doi:10.36849/jdd.2020.5447
- Cho M, Woo YR, Cho SH, et al. Metformin: a potential treatment for acne, hidradenitis suppurativa and rosacea. Acta Derm Venereol. 2023;103:adv18392. doi:10.2340/actadv.v103.18392
- Del Rosso JQ, Kircik L. The cutaneous effects of androgens and androgen-mediated sebum production and their pathophysiologic and therapeutic importance in acne vulgaris. J Dermatolog Treat. 2024;35:2298878. doi:10.1080/09546634.2023.2298878
- El-Tahlawi S, Ezzat Mohammad N, Mohamed El-Amir A, et al. Survivin and insulin-like growth factor-I: potential role in the pathogenesis of acne and post-acne scar. Scars Burn Heal. 2019;5:2059513118818031. doi:10.1177/2059513118818031
- Albalat W, Darwish H, Abd-Elaal WH, et al. The potential role of insulin-like growth factor 1 in acne vulgaris and its correlation with the clinical response before and after treatment with metformin. J Cosmet Dermatol. 2022;21:6209-6214. doi:10.1111/jocd.15210
- Nguyen S, Nguyen ML, Roberts WS, et al. The efficacy of metformin as a therapeutic agent in the treatment of acne vulgaris: a systematic review. Cureus. 2024;16:E56246. doi:10.7759/cureus.56246
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983-994. doi:10.1016 /s0140-6736(14)61909-7
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113. doi:10.1016/j.jaad.2018.11.058
- Huang Z, Li J, Chen H, et al. The efficacy of metformin for the treatment of psoriasis: a meta-analysis study. Postepy Dermatol Alergol. 2023;40:606-610. doi:10.5114/ada.2023.130524
- Singh S, Bhansali A. Randomized placebo control study of insulin sensitizers (metformin and pioglitazone) in psoriasis patients with metabolic syndrome (topical treatment cohort). BMC Dermatol. 2016;16:12. doi:10.1186 /s12895-016-0049-y
- Bao A, Qadri A, Gadre A, et al. Low-dose metformin and profibrotic signature in central centrifugal cicatricial alopecia. JAMA Dermatol. 2024;E243062. doi:10.1001/jamadermatol.2024.3062
- Lawson CN, Bakayoko A, Callender VD. Central centrifugal cicatricial alopecia: challenges and treatments. Dermatol Clin. 2021;39:389-405. doi:10.1016/j.det.2021.03.004
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668. doi:10.1016/j .jaad.2008.09.066
- Araoye EF, Thomas JAL, Aguh CU. Hair regrowth in 2 patients with recalcitrant central centrifugal cicatricial alopecia after use of topical metformin. JAAD Case Rep. 2020;6:106-108. doi:10.1016/j.jdcr.2019.12.008
- Foretz M, Guigas B, Bertrand L, et al. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20:953-966. doi:10.1016 /j.cmet.2014.09.018
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904-912.e1. doi:10.1016/j.jaad.2018.05.1257
- Gadre A, Dyson T, Jedrych J, et al. Proteomic profiling of central centrifugal cicatricial alopecia reveals role of humoral immune response pathway and metabolic dysregulation. JID Innov. 2024;4:100263. doi:10.1016/j.xjidi.2024.100263
- Chaudhary SC, Kurundkar D, Elmets CA, et al. Metformin, an antidiabetic agent reduces growth of cutaneous squamous cell carcinoma by targeting mTOR signaling pathway. Photochem Photobiol. 2012;88:1149-1156. doi:10.1111/j.1751-1097.2012.01165.x
- Tomic T, Botton T, Cerezo M, et al. Metformin inhibits melanoma development through autophagy and apoptosis mechanisms. Cell Death Dis. 2011;2:e199. doi:10.1038/cddis.2011.86
- Mascaraque-Checa M, Gallego-Rentero M, Nicolás-Morala J, et al. Metformin overcomes metabolic reprogramming-induced resistance of skin squamous cell carcinoma to photodynamic therapy. Mol Metab. 2022;60:101496. doi:10.1016/j.molmet.2022.101496
- Mascaraque M, Delgado-Wicke P, Nuevo-Tapioles C, et al. Metformin as an adjuvant to photodynamic therapy in resistant basal cell carcinoma cells. Cancers (Basel). 2020;12:668. doi:10.3390/cancers12030668
- Chang MS, Hartman RI, Xue J, et al. Risk of skin cancer associated with metformin use: a meta-analysis of randomized controlled trials and observational studies. Cancer Prev Res (Phila). 2021;14:77-84. doi:10.1158/1940-6207.Capr-20-0376
- Augustin RC, Huang Z, Ding F, et al. Metformin is associated with improved clinical outcomes in patients with melanoma: a retrospective, multi-institutional study. Front Oncol. 2023;13:1075823. doi:10.3389 /fonc.2023.1075823
- Adalsteinsson JA, Muzumdar S, Waldman R, et al. Metformin is associated with decreased risk of basal cell carcinoma: a whole-population casecontrol study from Iceland. J Am Acad Dermatol. 2021;85:56-61. doi:10.1016/j.jaad.2021.02.042
- Jennings L, Nestor L, Molloy O, et al. The treatment of hidradenitis suppurativa with the glucagon-like peptide-1 agonist liraglutide. Br J Dermatol. 2017;177:858-859. doi:10.1111/bjd.15233
- Lin L, Xu X, Yu Y, et al. Glucagon-like peptide-1 receptor agonist liraglutide therapy for psoriasis patients with type 2 diabetes: a randomized-controlled trial. J Dermatolog Treat. 2022;33: 1428-1434. doi:10.1080/09546634.2020.1826392
- Karacabeyli D, Lacaille D. Glucagon-like peptide 1 receptor agonists in patients with inflammatory arthritis or psoriasis: a scoping review. J Clin Rheumatol. 2024;30:26-31. doi:10.1097/rhu.0000000000001949
- Yang J, Wang Z, Zhang X. GLP-1 receptor agonist impairs keratinocytes inflammatory signals by activating AMPK. Exp Mol Pathol. 2019;107: 124-128. doi:10.1016/j.yexmp.2019.01.014
- Buysschaert M, Baeck M, Preumont V, et al. Improvement of psoriasis during glucagon-like peptide-1 analogue therapy in type 2 diabetes is associated with decreasing dermal Υϛ T-cell number: a prospective case-series study. Br J Dermatol. 2014;171:155-161. doi:10.1111/bjd.12886
- Wilbon SS, Kolonin MG. GLP1 receptor agonists-effects beyond obesity and diabetes. Cells. 2023;13:65. doi:10.3390/cells13010065
- Filippatos TD, Panagiotopoulou TV, Elisaf MS. Adverse effects of GLP-1 receptor agonists. Rev Diabet Stud. 2014;11:202-230. doi:10.1900 /rds.2014.11.202
- He Z, Tabe AN, Rana S, et al. Tirzepatide-induced biphasic anaphylactic reaction: a case report. Cureus. 2023;15:e50112. doi:10.7759/cureus.50112
- Anthony MS, Aroda VR, Parlett LE, et al. Risk of anaphylaxis among new users of glp-1 receptor agonists: a cohort study. Diabetes Care. 2024;47:712-719. doi:10.2337/dc23-1911
- Salazar CE, Patil MK, Aihie O, et al. Rare cutaneous adverse reactions associated with GLP-1 agonists: a review of the published literature. Arch Dermatol Res. 2024;316:248. doi:10.1007/s00403-024-02969-3
- Tran MM, Mirza FN, Lee AC, et al. Dermatologic findings associated with semaglutide use: a scoping review. J Am Acad Dermatol. 2024;91:166-168. doi:10.1016/j.jaad.2024.03.021
- Castellanos V, Workneh H, Malik A, et al. Semaglutide-induced lupus erythematosus with multiorgan involvement. Cureus. 2024;16:E55324. doi:10.7759/cureus.55324
- Boccardi A, Shubrook JH. Cutaneous reactions to antidiabetic agents: a narrative review. Diabetology. 2022;3:97-107.
- Desai DD, Sikora M, Nohria A, et al. GLP-1 agonists and hair loss: a call for further investigation. Int J Dermatol. 2024;63:1128-1130. doi:10.1111 /ijd.17246
- Mansour MR, Hannawa OM, Yaldo MM, et al. The rise of “Ozempic face”: analyzing trends and treatment challenges associated with rapid facial weight loss induced by GLP-1 agonists. J Plast Reconstr Aesthet Surg. 2024;96:225-227. doi:10.1016/j.bjps.2024.07.051
- Sivanand A, Gulliver WP, Josan CK, et al. Weight loss and dietary interventions for hidradenitis suppurativa: a systematic review. J Cutan Med Surg. 2020;24:64-72. doi:10.1177/1203475419874412
- Boer J. Resolution of hidradenitis suppurativa after weight loss by dietary measures, especially on frictional locations. J Eur Acad Dermatol Venereol. 2016;30:895-896. doi:10.1111/jdv.13059
- Thomas CL, Gordon KD, Mortimer PS. Rapid resolution of hidradenitis suppurativa after bariatric surgical intervention. Clin Exp Dermatol. 2014;39:315-7; quiz 317-8. doi:10.1111/ced.12269
- Mandour MO, Al-Musawi S, Idowu E, et al. Metabolic endoscopy and a simplified low-carbohydrate-high-dietary fiber template as novel treatments for hidradenitis suppurativa—a case series. JAAD Case Rep. 2023;34:23-26. doi:10.1016/j.jdcr.2023.01.035
- Henry T, Cahn B, Haber R, et al. Therapeutic potential of GLP-1 agonists for hidradenitis suppurativa. Int J Dermatol. 2023;62:1543-1544. doi:10.1111/ijd.16892
- Chan LJ, Kaur M, Kaffenberger BH. A case of recalcitrant hidradenitis suppurativa concomitantly treated with tirzepatide. JAAD Case Rep. 2024;52:101-102. doi:10.1016/j.jdcr.2024.02.023
- Costanzo G, Curatolo S, Busà B, et al. Two birds one stone: semaglutide is highly effective against severe psoriasis in a type 2 diabetic patient. Endocrinol Diabetes Metab Case Rep. 2021;2021:21-00007. doi:10.1530 /edm-21-0007
- Buysschaert M, Tennstedt D, Preumont V. Improvement of psoriasis during exenatide treatment in a patient with diabetes. Diabetes Metab. 2012;38:86-88. doi:10.1016/j.diabet.2011.11.004
- Faurschou A, Gyldenløve M, Rohde U, et al. Lack of effect of the glucagonlike peptide-1 receptor agonist liraglutide on psoriasis in glucose-tolerant patients--a randomized placebo-controlled trial. J Eur Acad Dermatol Venereol. 2015;29:555-559. doi:10.1111/jdv.12629
- Ahern T, Tobin AM, Corrigan M, et al. Glucagon-like peptide-1 analogue therapy for psoriasis patients with obesity and type 2 diabetes: a prospective cohort study. J Eur Acad Dermatol Venereol. 2013;27:1440-1443. doi:10.1111/j.1468-3083.2012.04609.x
- Gordon ER, Musleh S, Bordone LA. Treatment of insulin resistance with tirzepatide leading to improvement of hair loss. JAAD Case Rep. 2024;50:123-125. doi:10.1016/j.jdcr.2024.06.001
- Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. doi:10.1038/nrendo.2017.151
- Ahmad E, Lim S, Lamptey R, et al. Type 2 diabetes. Lancet. 2022;400: 1803-1820. doi:10.1016/s0140-6736(22)01655-5
- Samson SL, Vellanki P, Blonde L, et al. American Association of Clinical Endocrinology Consensus Statement: comprehensive type 2 diabetes management algorithm—2023 update. Endocr Pract. 2023;29:305-340. doi:10.1016/j.eprac.2023.02.001
- LaMoia TE, Shulman GI. Cellular and molecular mechanisms of metformin action. Endocr Rev. 2021;42:77-96. doi:10.1210/endrev/bnaa023
- Petrasca A, Hambly R, Kearney N, et al. Metformin has antiinflammatory effects and induces immunometabolic reprogramming via multiple mechanisms in hidradenitis suppurativa. Br J Dermatol. 2023;189:730-740. doi:10.1093/bjd/ljad305
- Duan W, Ding Y, Yu X, et al. Metformin mitigates autoimmune insulitis by inhibiting Th1 and Th17 responses while promoting Treg production. Am J Transl Res. 2019;11:2393-2402.
- Bharath LP, Nikolajczyk BS. The intersection of metformin and inflammation. Am J Physiol Cell Physiol. 2021;320:C873-C879. doi:10.1152 /ajpcell.00604.2020
- Sung CT, Chao T, Lee A, et al. Oral metformin for treating dermatological diseases: a systematic review. J Drugs Dermatol. 2020;19:713-720. doi:10.36849/jdd.2020.4874
- Feng J, Wang X, Ye X, et al. Mitochondria as an important target of metformin: the mechanism of action, toxic and side effects, and new therapeutic applications. Pharmacol Res. 2022;177:106114. doi:10.1016/j.phrs.2022.106114
- Klapholz L, Leitersdorf E, Weinrauch L. Leucocytoclastic vasculitis and pneumonitis induced by metformin. Br Med J (Clin Res Ed). 1986;293:483. doi:10.1136/bmj.293.6545.483
- Badr D, Kurban M, Abbas O. Metformin in dermatology: an overview. J Eur Acad Dermatol Venereol. 2013;27:1329-1335. doi:10.1111/jdv.12116
- Czarnowicki T, Ramot Y, Ingber A, et al. Metformin-induced leukocytoclastic vasculitis: a case report. Am J Clin Dermatol. 2012;13:61-63. doi:10.2165/11593230-000000000-00000
- Ben Salem C, Hmouda H, Slim R, et al. Rare case of metformininduced leukocytoclastic vasculitis. Ann Pharmacother. 2006;40:1685-1687. doi:10.1345/aph.1H155
- Abtahi-Naeini B, Momen T, Amiri R, et al. Metformin-induced generalized bullous fixed-drug eruption with a positive dechallengerechallenge test: a case report and literature review. Case Rep Dermatol Med. 2023;2023:6353919. doi:10.1155/2023/6353919
- Al Masri D, Fleifel M, Hirbli K. Fixed drug eruption secondary to four anti-diabetic medications: an unusual case of polysensitivity. Cureus. 2021;13:E18599. doi:10.7759/cureus.18599
- Ramírez-Bellver JL, Lopez J, Macias E, et al. Metformin-induced generalized fixed drug eruption with cutaneous hemophagocytosis. Am J Dermatopathol. 2017;39:471-475. doi:10.1097/dad.0000000000000800
- Steber CJ, Perkins SL, Harris KB. Metformin-induced fixed-drug eruption confirmed by multiple exposures. Am J Case Rep. 2016;17:231-234. doi:10.12659/ajcr.896424
- Voore P, Odigwe C, Mirrakhimov AE, et al. DRESS syndrome following metformin administration: a case report and review of the literature. Am J Ther. 2016;23:E1970-E1973. doi:10.1097/mjt.0000000000000292
- Kastalli S, El Aïdli S, Chaabane A, et al. Photosensitivity induced by metformin: a report of 3 cases. Article in French. Tunis Med. 2009;87:703-705.
- Karadağ AS, You Y, Danarti R, et al. Acanthosis nigricans and the metabolic syndrome. Clin Dermatol. 2018;36:48-53. doi:10.1016/j.clindermatol.2017.09.008
- Kong AS, Williams RL, Smith M, et al. Acanthosis nigricans and diabetes risk factors: prevalence in young persons seen in southwestern US primary care practices. Ann Fam Med. 2007;5:202-208. doi:10.1370/afm.678
- Stuart CA, Gilkison CR, Smith MM, et al. Acanthosis nigricans as a risk factor for non-insulin dependent diabetes mellitus. Clin Pediatr (Phila). 1998;37:73-79. doi:10.1177/000992289803700203
- Hud JA Jr, Cohen JB, Wagner JM, et al. Prevalence and significance of acanthosis nigricans in an adult obese population. Arch Dermatol. 1992;128:941-944.
- Novotny R, Davis J, Butel J, et al. Effect of the Children’s Healthy Living Program on young child overweight, obesity, and acanthosis nigricans in the US-affiliated Pacific region: a randomized clinical trial. JAMA Netw Open. 2018;1:E183896. doi:10.1001/jamanetworkopen.2018.3896
- Romo A, Benavides S. Treatment options in insulin resistance obesityrelated acanthosis nigricans. Ann Pharmacother. 2008;42:1090-1094. doi:10.1345/aph.1K446
- Treesirichod A, Chaithirayanon S, Chaikul T, et al. The randomized trials of 10% urea cream and 0.025% tretinoin cream in the treatment of acanthosis nigricans. J Dermatolog Treat. 2021;32:837-842. doi:10.108 0/09546634.2019.1708855
- Treesirichod A, Chaithirayanon S, Wongjitrat N. Comparison of the efficacy and safety of 0.1% adapalene gel and 0.025% tretinoin cream in the treatment of childhood acanthosis nigricans. Pediatr Dermatol. 2019;36:330-334. doi:10.1111/pde.13799
- Hermanns-Lê T, Hermanns JF, Piérard GE. Juvenile acanthosis nigricans and insulin resistance. Pediatr Dermatol. 2002;19:12-14. doi:10.1046 /j.1525-1470.2002.00013.x
- Walling HW, Messingham M, Myers LM, et al. Improvement of acanthosis nigricans on isotretinoin and metformin. J Drugs Dermatol. 2003;2:677-681.
- Giri D, Alsaffar H, Ramakrishnan R. Acanthosis nigricans and its response to metformin. Pediatr Dermatol. 2017;34:e281-e282. doi:10.1111/pde.13206
- Bellot-Rojas P, Posadas-Sanchez R, Caracas-Portilla N, et al. Comparison of metformin versus rosiglitazone in patients with acanthosis nigricans: a pilot study. J Drugs Dermatol. 2006;5:884-889.
- Sett A, Pradhan S, Sancheti K, et al. Effectiveness and safety of metformin versus Canthex™ in patients with acanthosis nigricans: a randomized, double-blind controlled trial. Indian J Dermatol. 2019;64:115-121. doi:10.4103/ijd.IJD_417_17
- Genazzani AD, Battaglia C, Malavasi B, et al. Metformin administration modulates and restores luteinizing hormone spontaneous episodic secretion and ovarian function in nonobese patients with polycystic ovary syndrome. Fertil Steril. 2004;81:114-119. doi:10.1016 /j.fertnstert.2003.05.020
- Kazerooni T, Dehghan-Kooshkghazi M. Effects of metformin therapy on hyperandrogenism in women with polycystic ovarian syndrome. Gynecol Endocrinol. 2003;17:51-56.
- Kolodziejczyk B, Duleba AJ, Spaczynski RZ, et al. Metformin therapy decreases hyperandrogenism and hyperinsulinemia in women with polycystic ovary syndrome. Fertil Steril. 2000;73:1149-1154. doi:10.1016 /s0015-0282(00)00501-x
- Kelly CJ, Gordon D. The effect of metformin on hirsutism in polycystic ovary syndrome. Eur J Endocrinol. 2002;147:217-221. doi:10.1530/eje.0.1470217
- Harborne L, Fleming R, Lyall H, et al. Metformin or antiandrogen in the treatment of hirsutism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:4116-4123. doi:10.1210/jc.2003-030424
- Rezvanian H, Adibi N, Siavash M, et al. Increased insulin sensitivity by metformin enhances intense-pulsed-light-assisted hair removal in patients with polycystic ovary syndrome. Dermatology. 2009;218: 231-236. doi:10.1159/000187718
- Cosma M, Swiglo BA, Flynn DN, et al. Clinical review: insulin sensitizers for the treatment of hirsutism: a systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab. 2008;93:1135-1142. doi:10.1210/jc.2007-2429
- Martin KA, Anderson RR, Chang RJ, et al. Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1233-1257.
- Fraison E, Kostova E, Moran LJ, et al. Metformin versus the combined oral contraceptive pill for hirsutism, acne, and menstrual pattern in polycystic ovary syndrome. Cochrane Database Syst Rev. 2020;8:CD005552. doi:10.1002/14651858.CD005552.pub3
- Hambly R, Kearney N, Hughes R, et al. Metformin treatment of hidradenitis suppurativa: effect on metabolic parameters, inflammation, cardiovascular risk biomarkers, and immune mediators. Int J Mol Sci. 2023;24:6969. doi:10.3390/ijms24086969
- Gold DA, Reeder VJ, Mahan MG, et al. The prevalence of metabolic syndrome in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2014;70:699-703. doi:10.1016/j.jaad.2013.11.014
- Miller IM, Ellervik C, Vinding GR, et al. Association of metabolic syndrome and hidradenitis suppurativa. JAMA Dermatol. 2014;150: 1273-1280. doi:10.1001/jamadermatol.2014.1165
- Monfrecola G, Balato A, Caiazzo G, et al. Mammalian target of rapamycin, insulin resistance and hidradenitis suppurativa: a possible metabolic loop. J Eur Acad Dermatol Venereol. 2016;30:1631-1633. doi:10.1111/jdv.13233
- Yoon MS. The role of mammalian target of rapamycin (mTOR) in insulin signaling. Nutrients. 2017;9:1176. doi:10.3390/nu9111176
- Abu Rached N, Gambichler T, Dietrich JW, et al. The role of hormones in hidradenitis suppurativa: a systematic review. Int J Mol Sci. 2022;23:15250. doi:10.3390/ijms232315250
- Verdolini R, Clayton N, Smith A, et al. Metformin for the treatment of hidradenitis suppurativa: a little help along the way. J Eur Acad Dermatol Venereol. 2013;27:1101-1108. doi:10.1111/j.1468-3083.2012.04668.x
- Tsentemeidou A, Vakirlis E, Papadimitriou I, et al. Metformin in hidradenitis suppurativa: is it worth pursuing further? Skin Appendage Disord. 2023;9:187-190. doi:10.1159/000529359
- Arun B, Loffeld A. Long-standing hidradenitis suppurativa treated effectively with metformin. Clin Exp Dermatol. 2009;34:920-921. doi:10.1111/j.1365-2230.2008.03121.x
- Jennings L, Hambly R, Hughes R, et al. Metformin use in hidradenitis suppurativa. J Dermatolog Treat. 2020;31:261-263. doi:10.1080/09546634 .2019.1592100
- Moussa C, Wadowski L, Price H, et al. Metformin as adjunctive therapy for pediatric patients with hidradenitis suppurativa. J Drugs Dermatol. 2020;19:1231-1234. doi:10.36849/jdd.2020.5447
- Cho M, Woo YR, Cho SH, et al. Metformin: a potential treatment for acne, hidradenitis suppurativa and rosacea. Acta Derm Venereol. 2023;103:adv18392. doi:10.2340/actadv.v103.18392
- Del Rosso JQ, Kircik L. The cutaneous effects of androgens and androgen-mediated sebum production and their pathophysiologic and therapeutic importance in acne vulgaris. J Dermatolog Treat. 2024;35:2298878. doi:10.1080/09546634.2023.2298878
- El-Tahlawi S, Ezzat Mohammad N, Mohamed El-Amir A, et al. Survivin and insulin-like growth factor-I: potential role in the pathogenesis of acne and post-acne scar. Scars Burn Heal. 2019;5:2059513118818031. doi:10.1177/2059513118818031
- Albalat W, Darwish H, Abd-Elaal WH, et al. The potential role of insulin-like growth factor 1 in acne vulgaris and its correlation with the clinical response before and after treatment with metformin. J Cosmet Dermatol. 2022;21:6209-6214. doi:10.1111/jocd.15210
- Nguyen S, Nguyen ML, Roberts WS, et al. The efficacy of metformin as a therapeutic agent in the treatment of acne vulgaris: a systematic review. Cureus. 2024;16:E56246. doi:10.7759/cureus.56246
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983-994. doi:10.1016 /s0140-6736(14)61909-7
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113. doi:10.1016/j.jaad.2018.11.058
- Huang Z, Li J, Chen H, et al. The efficacy of metformin for the treatment of psoriasis: a meta-analysis study. Postepy Dermatol Alergol. 2023;40:606-610. doi:10.5114/ada.2023.130524
- Singh S, Bhansali A. Randomized placebo control study of insulin sensitizers (metformin and pioglitazone) in psoriasis patients with metabolic syndrome (topical treatment cohort). BMC Dermatol. 2016;16:12. doi:10.1186 /s12895-016-0049-y
- Bao A, Qadri A, Gadre A, et al. Low-dose metformin and profibrotic signature in central centrifugal cicatricial alopecia. JAMA Dermatol. 2024;E243062. doi:10.1001/jamadermatol.2024.3062
- Lawson CN, Bakayoko A, Callender VD. Central centrifugal cicatricial alopecia: challenges and treatments. Dermatol Clin. 2021;39:389-405. doi:10.1016/j.det.2021.03.004
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668. doi:10.1016/j .jaad.2008.09.066
- Araoye EF, Thomas JAL, Aguh CU. Hair regrowth in 2 patients with recalcitrant central centrifugal cicatricial alopecia after use of topical metformin. JAAD Case Rep. 2020;6:106-108. doi:10.1016/j.jdcr.2019.12.008
- Foretz M, Guigas B, Bertrand L, et al. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20:953-966. doi:10.1016 /j.cmet.2014.09.018
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904-912.e1. doi:10.1016/j.jaad.2018.05.1257
- Gadre A, Dyson T, Jedrych J, et al. Proteomic profiling of central centrifugal cicatricial alopecia reveals role of humoral immune response pathway and metabolic dysregulation. JID Innov. 2024;4:100263. doi:10.1016/j.xjidi.2024.100263
- Chaudhary SC, Kurundkar D, Elmets CA, et al. Metformin, an antidiabetic agent reduces growth of cutaneous squamous cell carcinoma by targeting mTOR signaling pathway. Photochem Photobiol. 2012;88:1149-1156. doi:10.1111/j.1751-1097.2012.01165.x
- Tomic T, Botton T, Cerezo M, et al. Metformin inhibits melanoma development through autophagy and apoptosis mechanisms. Cell Death Dis. 2011;2:e199. doi:10.1038/cddis.2011.86
- Mascaraque-Checa M, Gallego-Rentero M, Nicolás-Morala J, et al. Metformin overcomes metabolic reprogramming-induced resistance of skin squamous cell carcinoma to photodynamic therapy. Mol Metab. 2022;60:101496. doi:10.1016/j.molmet.2022.101496
- Mascaraque M, Delgado-Wicke P, Nuevo-Tapioles C, et al. Metformin as an adjuvant to photodynamic therapy in resistant basal cell carcinoma cells. Cancers (Basel). 2020;12:668. doi:10.3390/cancers12030668
- Chang MS, Hartman RI, Xue J, et al. Risk of skin cancer associated with metformin use: a meta-analysis of randomized controlled trials and observational studies. Cancer Prev Res (Phila). 2021;14:77-84. doi:10.1158/1940-6207.Capr-20-0376
- Augustin RC, Huang Z, Ding F, et al. Metformin is associated with improved clinical outcomes in patients with melanoma: a retrospective, multi-institutional study. Front Oncol. 2023;13:1075823. doi:10.3389 /fonc.2023.1075823
- Adalsteinsson JA, Muzumdar S, Waldman R, et al. Metformin is associated with decreased risk of basal cell carcinoma: a whole-population casecontrol study from Iceland. J Am Acad Dermatol. 2021;85:56-61. doi:10.1016/j.jaad.2021.02.042
- Jennings L, Nestor L, Molloy O, et al. The treatment of hidradenitis suppurativa with the glucagon-like peptide-1 agonist liraglutide. Br J Dermatol. 2017;177:858-859. doi:10.1111/bjd.15233
- Lin L, Xu X, Yu Y, et al. Glucagon-like peptide-1 receptor agonist liraglutide therapy for psoriasis patients with type 2 diabetes: a randomized-controlled trial. J Dermatolog Treat. 2022;33: 1428-1434. doi:10.1080/09546634.2020.1826392
- Karacabeyli D, Lacaille D. Glucagon-like peptide 1 receptor agonists in patients with inflammatory arthritis or psoriasis: a scoping review. J Clin Rheumatol. 2024;30:26-31. doi:10.1097/rhu.0000000000001949
- Yang J, Wang Z, Zhang X. GLP-1 receptor agonist impairs keratinocytes inflammatory signals by activating AMPK. Exp Mol Pathol. 2019;107: 124-128. doi:10.1016/j.yexmp.2019.01.014
- Buysschaert M, Baeck M, Preumont V, et al. Improvement of psoriasis during glucagon-like peptide-1 analogue therapy in type 2 diabetes is associated with decreasing dermal Υϛ T-cell number: a prospective case-series study. Br J Dermatol. 2014;171:155-161. doi:10.1111/bjd.12886
- Wilbon SS, Kolonin MG. GLP1 receptor agonists-effects beyond obesity and diabetes. Cells. 2023;13:65. doi:10.3390/cells13010065
- Filippatos TD, Panagiotopoulou TV, Elisaf MS. Adverse effects of GLP-1 receptor agonists. Rev Diabet Stud. 2014;11:202-230. doi:10.1900 /rds.2014.11.202
- He Z, Tabe AN, Rana S, et al. Tirzepatide-induced biphasic anaphylactic reaction: a case report. Cureus. 2023;15:e50112. doi:10.7759/cureus.50112
- Anthony MS, Aroda VR, Parlett LE, et al. Risk of anaphylaxis among new users of glp-1 receptor agonists: a cohort study. Diabetes Care. 2024;47:712-719. doi:10.2337/dc23-1911
- Salazar CE, Patil MK, Aihie O, et al. Rare cutaneous adverse reactions associated with GLP-1 agonists: a review of the published literature. Arch Dermatol Res. 2024;316:248. doi:10.1007/s00403-024-02969-3
- Tran MM, Mirza FN, Lee AC, et al. Dermatologic findings associated with semaglutide use: a scoping review. J Am Acad Dermatol. 2024;91:166-168. doi:10.1016/j.jaad.2024.03.021
- Castellanos V, Workneh H, Malik A, et al. Semaglutide-induced lupus erythematosus with multiorgan involvement. Cureus. 2024;16:E55324. doi:10.7759/cureus.55324
- Boccardi A, Shubrook JH. Cutaneous reactions to antidiabetic agents: a narrative review. Diabetology. 2022;3:97-107.
- Desai DD, Sikora M, Nohria A, et al. GLP-1 agonists and hair loss: a call for further investigation. Int J Dermatol. 2024;63:1128-1130. doi:10.1111 /ijd.17246
- Mansour MR, Hannawa OM, Yaldo MM, et al. The rise of “Ozempic face”: analyzing trends and treatment challenges associated with rapid facial weight loss induced by GLP-1 agonists. J Plast Reconstr Aesthet Surg. 2024;96:225-227. doi:10.1016/j.bjps.2024.07.051
- Sivanand A, Gulliver WP, Josan CK, et al. Weight loss and dietary interventions for hidradenitis suppurativa: a systematic review. J Cutan Med Surg. 2020;24:64-72. doi:10.1177/1203475419874412
- Boer J. Resolution of hidradenitis suppurativa after weight loss by dietary measures, especially on frictional locations. J Eur Acad Dermatol Venereol. 2016;30:895-896. doi:10.1111/jdv.13059
- Thomas CL, Gordon KD, Mortimer PS. Rapid resolution of hidradenitis suppurativa after bariatric surgical intervention. Clin Exp Dermatol. 2014;39:315-7; quiz 317-8. doi:10.1111/ced.12269
- Mandour MO, Al-Musawi S, Idowu E, et al. Metabolic endoscopy and a simplified low-carbohydrate-high-dietary fiber template as novel treatments for hidradenitis suppurativa—a case series. JAAD Case Rep. 2023;34:23-26. doi:10.1016/j.jdcr.2023.01.035
- Henry T, Cahn B, Haber R, et al. Therapeutic potential of GLP-1 agonists for hidradenitis suppurativa. Int J Dermatol. 2023;62:1543-1544. doi:10.1111/ijd.16892
- Chan LJ, Kaur M, Kaffenberger BH. A case of recalcitrant hidradenitis suppurativa concomitantly treated with tirzepatide. JAAD Case Rep. 2024;52:101-102. doi:10.1016/j.jdcr.2024.02.023
- Costanzo G, Curatolo S, Busà B, et al. Two birds one stone: semaglutide is highly effective against severe psoriasis in a type 2 diabetic patient. Endocrinol Diabetes Metab Case Rep. 2021;2021:21-00007. doi:10.1530 /edm-21-0007
- Buysschaert M, Tennstedt D, Preumont V. Improvement of psoriasis during exenatide treatment in a patient with diabetes. Diabetes Metab. 2012;38:86-88. doi:10.1016/j.diabet.2011.11.004
- Faurschou A, Gyldenløve M, Rohde U, et al. Lack of effect of the glucagonlike peptide-1 receptor agonist liraglutide on psoriasis in glucose-tolerant patients--a randomized placebo-controlled trial. J Eur Acad Dermatol Venereol. 2015;29:555-559. doi:10.1111/jdv.12629
- Ahern T, Tobin AM, Corrigan M, et al. Glucagon-like peptide-1 analogue therapy for psoriasis patients with obesity and type 2 diabetes: a prospective cohort study. J Eur Acad Dermatol Venereol. 2013;27:1440-1443. doi:10.1111/j.1468-3083.2012.04609.x
- Gordon ER, Musleh S, Bordone LA. Treatment of insulin resistance with tirzepatide leading to improvement of hair loss. JAAD Case Rep. 2024;50:123-125. doi:10.1016/j.jdcr.2024.06.001
Dermatologic Implications of Glycemic Control Medications for Patients with Type 2 Diabetes Mellitus
Dermatologic Implications of Glycemic Control Medications for Patients with Type 2 Diabetes Mellitus
PRACTICE POINTS
- Type 2 diabetes mellitus (T2DM) is highly prevalent in patients with various dermatologic conditions; therefore, it is important for dermatologists to understand the adverse effects of T2DM medications to optimize treatment strategies.
- In addition to glycemic control and management, the hormonal and immunologic effects of T2DM medications can be leveraged to treat dermatologic conditions, particularly those associated with metabolic dysregulation.
Dome-Shaped White Papules on the Earlobe
Dome-Shaped White Papules on the Earlobe
THE DIAGNOSIS: Trichodiscoma
Histologic evaluation revealed an unremarkable epidermal surface and a subjacent well-demarcated superficial dermal nodule showing a proliferation, sometimes fascicular, of wavy and spindled fibroblasts with some stellate forms within a variably loose fibrous stroma. Some angioplasia and vascular ectasia also were seen (Figure). A diagnosis of trichodiscoma was made based on these histologic findings.
While the patient’s personal and family history of pneumothorax originally had been attributed to other causes, the diagnosis of trichodiscoma raised suspicion for Birt-Hogg-Dubé syndrome due to the classic association of skin lesions (often trichodiscomas), renal cell carcinoma, and spontaneous pneumothorax in this condition. The patient was sent for genetic testing for the associated folliculin (FLCN) gene, which was positive and thereby confirmed the diagnosis of Birt-Hogg-Dubé syndrome. At the most recent follow-up almost 2 years after initial presentation, the lesions on the earlobe were stable. The patient has since undergone screening for abdominal and renal neoplasia with negative results, and he has had no other occurrences of pneumothorax.
Our case highlights the association between trichodiscomas and Birt-Hogg-Dubé syndrome, which necessitates screening for renal cell carcinoma, pneumothorax, and lung cysts.1 Birt-Hogg-Dubé syndrome is an autosomal- dominant disorder of the skin and lungs that is characterized by a predisposition for renal carcinoma, pneumothorax, and colon polyps as well as cutaneous markers that include fibrofolliculomas, acrochordons, and trichodiscomas; the trichodiscomas tend to manifest as numerous smooth, flesh-colored or grayish-white papules on the face, ears, neck, and/or upper trunk.1
Trichodiscomas are benign lesions and do not require treatment2; however, if they are cosmetically bothersome to the patient, surgical excision is an option for single lesions. For more widespread cutaneous disease, combination therapy with a CO2 laser and erbium-doped yttrium aluminum garnet laser may be utilized.3 The differential diagnosis for trichodiscoma includes basal cell carcinoma, fibrous papule, dermal nevus, and trichofolliculoma.
Basal cell carcinoma is the most common type of skin cancer.4 Clinically, it typically manifests as pink or flesh-colored papules on the head or neck, often with overlying ulceration or telangiectasia. Due to its association with chronic sun exposure, the median age of diagnosis for basal cell carcinoma is 68 years. Histopathologically, basal cell carcinoma is characterized by islands or nests of atypical basaloid cells with palisading cells at the periphery.4 Treatment depends on the location and size of the lesion, but Mohs micrographic surgery is the most common intervention on the face and ears.5
In contrast, fibrous papules are benign lesions that manifest clinically as small, firm, flesh-colored papules that most commonly are found on the nose.6,7 On dermatopathology, classic findings include fibrovascular proliferation and scattered multinucleated triangular or stellate cells in the upper dermis.7 Due to the benign nature of the lesion, treatment is not required6; however, shave excision, electrodessication, and laser therapies can be attempted if the patient chooses to pursue treatment.8
Dermal nevus is a type of benign acquired melanocytic nevus that manifests clinically as a light-brown to flesh-colored, dome-shaped or papillomatous papule.9 It typically develops in areas that are exposed to the sun, including the face.10 There also have been cases of dermal nevi on the ear.11 Histopathology shows melanocytic nevus cells that have completely detached from the epidermis and are located entirely in the dermis.12 While dermal nevi are benign and treatment is not necessary, surgical excision is an option for patients who request removal.13
Trichofolliculoma is a benign tumor of the adnexa that shows follicular differentiation on histopathology.14 On physical examination, it manifests as an isolated flesh-colored papule or nodule with a central pore from which tufted hairs protrude. These lesions usually appear on the face or scalp and occur more commonly in women than in men. While these may be clinically indistinguishable from trichodiscomas, the absence of protruding hair in our patient’s case makes trichofolliculoma less likely. When biopsied, histopathology classically shows a cystically dilated hair follicle with keratinous material and several mature and immature branched follicular structures. Preferred treatment for trichofolliculomas is surgical excision, and recurrence is rare.14
- Toro JR, Glenn G, Duray P, et al. Birt-Hogg-Dubé syndrome: a novel marker of kidney neoplasia. Arch Dermatol. 1999;135:1195-202. doi:10.1001/archderm.135.10.1195
- Tong Y, Coda AB, Schneider JA, et al. Familial multiple trichodiscomas: case report and concise review. Cureus. 2017;9:E1596. doi:10.7759/cureus.1596
- Riley J, Athalye L, Tran D, et al. Concomitant fibrofolliculoma and trichodiscoma on the abdomen. Cutis. 2018;102:E30-E32.
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. StatPearls [Internet]. Updated March 13, 2024. Accessed December 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482439/
- Bittner GC, Kubo EM, Fantini BC, et al. Auricular reconstruction after Mohs micrographic surgery: analysis of 101 cases. An Bras Dermatol. 2021;96:408-415. doi:10.1016/j.abd.2020.12.008
- Damman J, Biswas A. Fibrous papule: a histopathologic review. Am J Dermatopathol. 2018;40:551-560. doi:10.1097/DAD.0000000000001083
- Jacyk WK, Rütten A, Requena L. Fibrous papule of the face with granular cells. Dermatology. 2008;216:56-59. doi:10.1159/000109359
- Macri A, Kwan E, Tanner LS. Cutaneous angiofibroma. StatPearls [Internet]. Updated July 19, 2024. Accessed December 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482470/
- Sardana K, Chakravarty P, Goel K. Optimal management of common acquired melanocytic nevi (moles): current perspectives. Clin Cosmet Investig Dermatol. 2014;7:89-103. doi:10.2147/CCID.S57782
- Conforti C, Giuffrida R, Agozzino M, et al. Basal cell carcinoma and dermal nevi of the face: comparison of localization and dermatoscopic features. Int J Dermatol. 2021;60:996-1002. doi:10.1111/ijd.15554
- Alves RV, Brandão FH, Aquino JE, et al. Intradermal melanocytic nevus of the external auditory canal. Braz J Otorhinolaryngol. 2005;71:104-106. doi: 10.1016/s1808-8694(15)31295-7
- Muradia I, Khunger N, Yadav AK. A clinical, dermoscopic, and histopathological analysis of common acquired melanocytic nevi in skin of color. J Clin Aesthet Dermatol. 2022;15:41-51.
- Sardana K, Chakravarty P, Goel K. Optimal management of common acquired melanocytic nevi (moles): current perspectives. Clin Cosmet Investig Dermatol. 2014;7:89-103. doi:10.2147/CCID.S57782
- Massara B, Sellami K, Graja S, et al. Trichofolliculoma: a case series. J Clin Aesthet Dermatol. 2023;16:41-43.
THE DIAGNOSIS: Trichodiscoma
Histologic evaluation revealed an unremarkable epidermal surface and a subjacent well-demarcated superficial dermal nodule showing a proliferation, sometimes fascicular, of wavy and spindled fibroblasts with some stellate forms within a variably loose fibrous stroma. Some angioplasia and vascular ectasia also were seen (Figure). A diagnosis of trichodiscoma was made based on these histologic findings.

While the patient’s personal and family history of pneumothorax originally had been attributed to other causes, the diagnosis of trichodiscoma raised suspicion for Birt-Hogg-Dubé syndrome due to the classic association of skin lesions (often trichodiscomas), renal cell carcinoma, and spontaneous pneumothorax in this condition. The patient was sent for genetic testing for the associated folliculin (FLCN) gene, which was positive and thereby confirmed the diagnosis of Birt-Hogg-Dubé syndrome. At the most recent follow-up almost 2 years after initial presentation, the lesions on the earlobe were stable. The patient has since undergone screening for abdominal and renal neoplasia with negative results, and he has had no other occurrences of pneumothorax.
Our case highlights the association between trichodiscomas and Birt-Hogg-Dubé syndrome, which necessitates screening for renal cell carcinoma, pneumothorax, and lung cysts.1 Birt-Hogg-Dubé syndrome is an autosomal- dominant disorder of the skin and lungs that is characterized by a predisposition for renal carcinoma, pneumothorax, and colon polyps as well as cutaneous markers that include fibrofolliculomas, acrochordons, and trichodiscomas; the trichodiscomas tend to manifest as numerous smooth, flesh-colored or grayish-white papules on the face, ears, neck, and/or upper trunk.1
Trichodiscomas are benign lesions and do not require treatment2; however, if they are cosmetically bothersome to the patient, surgical excision is an option for single lesions. For more widespread cutaneous disease, combination therapy with a CO2 laser and erbium-doped yttrium aluminum garnet laser may be utilized.3 The differential diagnosis for trichodiscoma includes basal cell carcinoma, fibrous papule, dermal nevus, and trichofolliculoma.
Basal cell carcinoma is the most common type of skin cancer.4 Clinically, it typically manifests as pink or flesh-colored papules on the head or neck, often with overlying ulceration or telangiectasia. Due to its association with chronic sun exposure, the median age of diagnosis for basal cell carcinoma is 68 years. Histopathologically, basal cell carcinoma is characterized by islands or nests of atypical basaloid cells with palisading cells at the periphery.4 Treatment depends on the location and size of the lesion, but Mohs micrographic surgery is the most common intervention on the face and ears.5
In contrast, fibrous papules are benign lesions that manifest clinically as small, firm, flesh-colored papules that most commonly are found on the nose.6,7 On dermatopathology, classic findings include fibrovascular proliferation and scattered multinucleated triangular or stellate cells in the upper dermis.7 Due to the benign nature of the lesion, treatment is not required6; however, shave excision, electrodessication, and laser therapies can be attempted if the patient chooses to pursue treatment.8
Dermal nevus is a type of benign acquired melanocytic nevus that manifests clinically as a light-brown to flesh-colored, dome-shaped or papillomatous papule.9 It typically develops in areas that are exposed to the sun, including the face.10 There also have been cases of dermal nevi on the ear.11 Histopathology shows melanocytic nevus cells that have completely detached from the epidermis and are located entirely in the dermis.12 While dermal nevi are benign and treatment is not necessary, surgical excision is an option for patients who request removal.13
Trichofolliculoma is a benign tumor of the adnexa that shows follicular differentiation on histopathology.14 On physical examination, it manifests as an isolated flesh-colored papule or nodule with a central pore from which tufted hairs protrude. These lesions usually appear on the face or scalp and occur more commonly in women than in men. While these may be clinically indistinguishable from trichodiscomas, the absence of protruding hair in our patient’s case makes trichofolliculoma less likely. When biopsied, histopathology classically shows a cystically dilated hair follicle with keratinous material and several mature and immature branched follicular structures. Preferred treatment for trichofolliculomas is surgical excision, and recurrence is rare.14
THE DIAGNOSIS: Trichodiscoma
Histologic evaluation revealed an unremarkable epidermal surface and a subjacent well-demarcated superficial dermal nodule showing a proliferation, sometimes fascicular, of wavy and spindled fibroblasts with some stellate forms within a variably loose fibrous stroma. Some angioplasia and vascular ectasia also were seen (Figure). A diagnosis of trichodiscoma was made based on these histologic findings.

While the patient’s personal and family history of pneumothorax originally had been attributed to other causes, the diagnosis of trichodiscoma raised suspicion for Birt-Hogg-Dubé syndrome due to the classic association of skin lesions (often trichodiscomas), renal cell carcinoma, and spontaneous pneumothorax in this condition. The patient was sent for genetic testing for the associated folliculin (FLCN) gene, which was positive and thereby confirmed the diagnosis of Birt-Hogg-Dubé syndrome. At the most recent follow-up almost 2 years after initial presentation, the lesions on the earlobe were stable. The patient has since undergone screening for abdominal and renal neoplasia with negative results, and he has had no other occurrences of pneumothorax.
Our case highlights the association between trichodiscomas and Birt-Hogg-Dubé syndrome, which necessitates screening for renal cell carcinoma, pneumothorax, and lung cysts.1 Birt-Hogg-Dubé syndrome is an autosomal- dominant disorder of the skin and lungs that is characterized by a predisposition for renal carcinoma, pneumothorax, and colon polyps as well as cutaneous markers that include fibrofolliculomas, acrochordons, and trichodiscomas; the trichodiscomas tend to manifest as numerous smooth, flesh-colored or grayish-white papules on the face, ears, neck, and/or upper trunk.1
Trichodiscomas are benign lesions and do not require treatment2; however, if they are cosmetically bothersome to the patient, surgical excision is an option for single lesions. For more widespread cutaneous disease, combination therapy with a CO2 laser and erbium-doped yttrium aluminum garnet laser may be utilized.3 The differential diagnosis for trichodiscoma includes basal cell carcinoma, fibrous papule, dermal nevus, and trichofolliculoma.
Basal cell carcinoma is the most common type of skin cancer.4 Clinically, it typically manifests as pink or flesh-colored papules on the head or neck, often with overlying ulceration or telangiectasia. Due to its association with chronic sun exposure, the median age of diagnosis for basal cell carcinoma is 68 years. Histopathologically, basal cell carcinoma is characterized by islands or nests of atypical basaloid cells with palisading cells at the periphery.4 Treatment depends on the location and size of the lesion, but Mohs micrographic surgery is the most common intervention on the face and ears.5
In contrast, fibrous papules are benign lesions that manifest clinically as small, firm, flesh-colored papules that most commonly are found on the nose.6,7 On dermatopathology, classic findings include fibrovascular proliferation and scattered multinucleated triangular or stellate cells in the upper dermis.7 Due to the benign nature of the lesion, treatment is not required6; however, shave excision, electrodessication, and laser therapies can be attempted if the patient chooses to pursue treatment.8
Dermal nevus is a type of benign acquired melanocytic nevus that manifests clinically as a light-brown to flesh-colored, dome-shaped or papillomatous papule.9 It typically develops in areas that are exposed to the sun, including the face.10 There also have been cases of dermal nevi on the ear.11 Histopathology shows melanocytic nevus cells that have completely detached from the epidermis and are located entirely in the dermis.12 While dermal nevi are benign and treatment is not necessary, surgical excision is an option for patients who request removal.13
Trichofolliculoma is a benign tumor of the adnexa that shows follicular differentiation on histopathology.14 On physical examination, it manifests as an isolated flesh-colored papule or nodule with a central pore from which tufted hairs protrude. These lesions usually appear on the face or scalp and occur more commonly in women than in men. While these may be clinically indistinguishable from trichodiscomas, the absence of protruding hair in our patient’s case makes trichofolliculoma less likely. When biopsied, histopathology classically shows a cystically dilated hair follicle with keratinous material and several mature and immature branched follicular structures. Preferred treatment for trichofolliculomas is surgical excision, and recurrence is rare.14
- Toro JR, Glenn G, Duray P, et al. Birt-Hogg-Dubé syndrome: a novel marker of kidney neoplasia. Arch Dermatol. 1999;135:1195-202. doi:10.1001/archderm.135.10.1195
- Tong Y, Coda AB, Schneider JA, et al. Familial multiple trichodiscomas: case report and concise review. Cureus. 2017;9:E1596. doi:10.7759/cureus.1596
- Riley J, Athalye L, Tran D, et al. Concomitant fibrofolliculoma and trichodiscoma on the abdomen. Cutis. 2018;102:E30-E32.
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. StatPearls [Internet]. Updated March 13, 2024. Accessed December 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482439/
- Bittner GC, Kubo EM, Fantini BC, et al. Auricular reconstruction after Mohs micrographic surgery: analysis of 101 cases. An Bras Dermatol. 2021;96:408-415. doi:10.1016/j.abd.2020.12.008
- Damman J, Biswas A. Fibrous papule: a histopathologic review. Am J Dermatopathol. 2018;40:551-560. doi:10.1097/DAD.0000000000001083
- Jacyk WK, Rütten A, Requena L. Fibrous papule of the face with granular cells. Dermatology. 2008;216:56-59. doi:10.1159/000109359
- Macri A, Kwan E, Tanner LS. Cutaneous angiofibroma. StatPearls [Internet]. Updated July 19, 2024. Accessed December 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482470/
- Sardana K, Chakravarty P, Goel K. Optimal management of common acquired melanocytic nevi (moles): current perspectives. Clin Cosmet Investig Dermatol. 2014;7:89-103. doi:10.2147/CCID.S57782
- Conforti C, Giuffrida R, Agozzino M, et al. Basal cell carcinoma and dermal nevi of the face: comparison of localization and dermatoscopic features. Int J Dermatol. 2021;60:996-1002. doi:10.1111/ijd.15554
- Alves RV, Brandão FH, Aquino JE, et al. Intradermal melanocytic nevus of the external auditory canal. Braz J Otorhinolaryngol. 2005;71:104-106. doi: 10.1016/s1808-8694(15)31295-7
- Muradia I, Khunger N, Yadav AK. A clinical, dermoscopic, and histopathological analysis of common acquired melanocytic nevi in skin of color. J Clin Aesthet Dermatol. 2022;15:41-51.
- Sardana K, Chakravarty P, Goel K. Optimal management of common acquired melanocytic nevi (moles): current perspectives. Clin Cosmet Investig Dermatol. 2014;7:89-103. doi:10.2147/CCID.S57782
- Massara B, Sellami K, Graja S, et al. Trichofolliculoma: a case series. J Clin Aesthet Dermatol. 2023;16:41-43.
- Toro JR, Glenn G, Duray P, et al. Birt-Hogg-Dubé syndrome: a novel marker of kidney neoplasia. Arch Dermatol. 1999;135:1195-202. doi:10.1001/archderm.135.10.1195
- Tong Y, Coda AB, Schneider JA, et al. Familial multiple trichodiscomas: case report and concise review. Cureus. 2017;9:E1596. doi:10.7759/cureus.1596
- Riley J, Athalye L, Tran D, et al. Concomitant fibrofolliculoma and trichodiscoma on the abdomen. Cutis. 2018;102:E30-E32.
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. StatPearls [Internet]. Updated March 13, 2024. Accessed December 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482439/
- Bittner GC, Kubo EM, Fantini BC, et al. Auricular reconstruction after Mohs micrographic surgery: analysis of 101 cases. An Bras Dermatol. 2021;96:408-415. doi:10.1016/j.abd.2020.12.008
- Damman J, Biswas A. Fibrous papule: a histopathologic review. Am J Dermatopathol. 2018;40:551-560. doi:10.1097/DAD.0000000000001083
- Jacyk WK, Rütten A, Requena L. Fibrous papule of the face with granular cells. Dermatology. 2008;216:56-59. doi:10.1159/000109359
- Macri A, Kwan E, Tanner LS. Cutaneous angiofibroma. StatPearls [Internet]. Updated July 19, 2024. Accessed December 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482470/
- Sardana K, Chakravarty P, Goel K. Optimal management of common acquired melanocytic nevi (moles): current perspectives. Clin Cosmet Investig Dermatol. 2014;7:89-103. doi:10.2147/CCID.S57782
- Conforti C, Giuffrida R, Agozzino M, et al. Basal cell carcinoma and dermal nevi of the face: comparison of localization and dermatoscopic features. Int J Dermatol. 2021;60:996-1002. doi:10.1111/ijd.15554
- Alves RV, Brandão FH, Aquino JE, et al. Intradermal melanocytic nevus of the external auditory canal. Braz J Otorhinolaryngol. 2005;71:104-106. doi: 10.1016/s1808-8694(15)31295-7
- Muradia I, Khunger N, Yadav AK. A clinical, dermoscopic, and histopathological analysis of common acquired melanocytic nevi in skin of color. J Clin Aesthet Dermatol. 2022;15:41-51.
- Sardana K, Chakravarty P, Goel K. Optimal management of common acquired melanocytic nevi (moles): current perspectives. Clin Cosmet Investig Dermatol. 2014;7:89-103. doi:10.2147/CCID.S57782
- Massara B, Sellami K, Graja S, et al. Trichofolliculoma: a case series. J Clin Aesthet Dermatol. 2023;16:41-43.
Dome-Shaped White Papules on the Earlobe
Dome-Shaped White Papules on the Earlobe
A 70-year-old man presented to the dermatology clinic for a routine full-body skin examination that revealed multiple asymptomatic, dome-shaped, white papules on the left posterior earlobe. The patient had a personal and family history of spontaneous pneumothorax and no history of cancer. A shave biopsy of one of the papules was performed.
Debunking Dermatology Myths to Enhance Patient Care
Debunking Dermatology Myths to Enhance Patient Care
The advent of social media has revolutionized the way patients access and consume health information. While this increased access has its merits, it also has given rise to the proliferation of medical myths, which have considerable effects on patient-physician interactions.1 Myths are prevalent across all fields of health care, ranging from misconceptions about disease etiology and prevention to the efficacy and safety of treatments. This influx of misinformation can derail the clinical encounter, shifting the focus from evidence-based medicine to myth-busting.2 The COVID-19 pandemic exacerbated this issue, as widespread lockdowns and social distancing measures limited access to in-person medical consultations, prompting patients to increasingly turn to online sources for health information that often were unreliable, thereby bypassing professional medical advice.3 Herein, we highlight the challenges and implications of common dermatology myths and provide strategies for effectively debunking these myths to enhance patient care.
Common Dermatology Myths
In dermatology, where visible and often distressing conditions such as acne and hair loss are common, the impact of myths on patient perceptions and treatment outcomes can be particularly profound. Patients often arrive for consultations with preconceived notions that are not grounded in scientific evidence. Common dermatologic myths include eczema and the efficacy of topical corticosteroids, the causes and treatment of hair loss, and risk factors associated with skin cancer.
Eczema and Topical Corticosteroids—Topical corticosteroids for eczema are safe and effective, but nonadherence due to phobias stemming from misinformation online can impede treatment.4 Myths such as red skin syndrome and topical corticosteroid addiction are prevalent. Red skin syndrome refers to claims that prolonged use of topical corticosteroids causes severe redness and burning of the skin and worsening eczema symptoms upon withdrawal. Topical corticosteroid addiction suggests that patients become dependent on corticosteroids, requiring higher doses over time to maintain efficacy. These misconceptions contribute to fear and avoidance of prescribed treatments.
Eczema myths often divert focus from its true etiology as a genetic inflammatory skin disease, suggesting instead that it is caused by leaky gut or food intolerances.4 Risks such as skin thinning and stunted growth often are exaggerated on social media and other nonmedical platforms, though these adverse effects rarely are seen when topical corticosteroids are used appropriately under medical supervision. Misinformation often is linked to companies promoting unregulated consultations, tests, or supposedly natural treatments, including herbal remedies that may surreptitiously contain corticosteroids without clear labeling. This fosters distrust of US Food and Drug Administration– approved and dermatologist-prescribed treatments, as patients may cite concerns based on experiences with or claims about unapproved products.4
Sunscreen and Skin Cancer—In 2018, the American Academy of Dermatology prioritized skin cancer prevention due to suboptimal public adoption of photoprotection measures.5 However, the proliferation of misinformation regarding sunscreen and its potential to cause skin cancer is a more pressing issue. Myths range from claims that sunscreen is ineffective to warnings that it is dangerous, with some social media influencers even suggesting that sunscreen causes skin cancer due to toxic ingredients.6 Oxybenzone, typically found in chemical sunscreens, has been criticized by some advocacy groups and social media influencers as a potential hormone disruptor (ie, a chemical that could interfere with hormone production).7 However, no conclusive evidence has shown that oxybenzone is harmful to humans. Consumer concerns often are based on animal studies in which rats are fed oxybenzone, but mathematical modeling has indicated it would take 277 years of sunscreen use by humans to match the doses used in these studies.8 The false association between sunscreen use and skin cancer is based on flawed studies that found higher rates of skin cancer—including melanoma—in sunscreen users compared to those who did not use sunscreen. However, those using sunscreen also were more likely to travel to sunnier climates and engage in sunbathing, and it may have been this increased sun exposure that elevated their risk for skin cancer.7 It is imperative that the dermatology community counteract this type of misinformation with evidence-based advice.
Hair Loss—Some patients believe that hair loss is caused by wearing hats, frequent shampooing, or even stress in a way that oversimplifies complex physiological processes. Biotin, which commonly is added to supplements for hair, skin, and nails, has been linked to potential risks, such as interference with laboratory testing and false-positive or false-negative results in critical medical tests, which can lead to misdiagnosis or inappropriate treatment.9 Biotin interference can result in falsely low troponin readings, which are critical in diagnosing acute myocardial infarction. Tests for other hormones such as cortisol and parathyroid hormone also are affected, potentially impacting the evaluation and management of endocrine disorders. The US Food and Drug Administration has issued warnings for patients on this topic, emphasizing the importance of informing health care providers about any biotin supplementation prior to laboratory testing. Despite its popularity, there is no substantial scientific evidence to suggest that biotin supplementation promotes hair growth in anyone other than those with deficiency, which is quite rare.9
Myths and the Patient-Physician Relationship
The proliferation of medical myths and misinformation affects the dynamic between patients and dermatologists in several ways. Research across various medical fields has demonstrated that misinformation can substantially impact patient behavior and treatment adherence. Like many other specialists, dermatologists often spend considerable time during consultations with patients debunking myths and correcting misconceptions, which can detract from discussing more critical aspects of the patient’s condition and treatment plan and lead to frustration and anxiety among patients. It also can be challenging for physicians to have these conversations without alienating patients, who may distrust medical recommendations and believe that natural or alternative treatments are superior. This can lead to noncompliance with prescribed treatments, and patients may instead opt to try unproven remedies they encounter online, ultimately resulting in poorer health outcomes.
Strategies to Debunk Myths
By implementing the following strategies, dermatologists can combat the spread of myths, foster trust among patients, and promote adherence to evidence-based treatments:
- Provide educational outreach. Preemptively address myths by giving patients accurate and accessible resources. Including a dedicated section on your clinic’s website with articles, frequently asked questions, videos, and links to reputable sources can be effective. Sharing patient testimonials and before-and-after photographs to demonstrate the success of evidence-based treatments also is recommended, as real-life stories can be powerful tools in dispelling myths.
- Practice effective communication. Involve patients in the decision-making process by discussing their treatment goals, preferences, and concerns. It is important to present all options clearly, including the potential benefits and adverse effects. Discuss the expected outcomes and timelines, and be transparent about the limitations of certain treatment—honesty helps build trust and sets realistic expectations.
- Conduct structured consultations. Ensure that consultations with patients follow a structured format—history, physical examination, and discussion—to help keep the focus on evidence-based practice.
- Leverage technology. Guide patients toward reliable digital patient education tools to empower them with accurate information. Hosting live sessions on social media platforms during which patients can ask questions and receive evidence-based answers also can be beneficial.
Final Thoughts
In summary, the rise of medical myths poses a considerable challenge to dermatologic practice. By understanding the sources and impacts of these myths and employing strategies to dispel them, dermatologists can better navigate the complexities of modern patient interactions and ensure that care remains grounded in scientific evidence.
- Kessler SH, Bachmann E. Debunking health myths on the internet: the persuasive effect of (visual) online communication. Z Gesundheitswissenschaften J Public Health. 2022;30:1823-1835.
- Fridman I, Johnson S, Elston Lafata J. Health information and misinformation: a framework to guide research and practice. JMIR Med Educ. 2023;9:E38687.
- Di Novi C, Kovacic M, Orso CE. Online health information seeking behavior, healthcare access, and health status during exceptional times. J Econ Behav Organ. 2024;220:675-690.
- Finnegan P, Murphy M, O’Connor C. #corticophobia: a review on online misinformation related to topical steroids. Clin Exp Dermatol. 2023;48:112-115.
- Yang EJ, Beck KM, Maarouf M, et al. Truths and myths in sunscreen labeling. J Cosmet Dermatol. 2018;17:1288-1292.
- Hopkins C. What Gen Z gets wrong about sunscreen. New York Times. Published May 27, 2024. Accessed December 16, 2024. https://www.nytimes.com/2024/05/27/well/live/sunscreen-skin-cancer-gen-z.html
- Harvard Health Publishing. The science of sunscreen. Published February 15, 2021. Accessed December 9, 2024. https://www.health.harvard.edu/staying-healthy/the-science-of-sunscreen
- Lim HW, Arellano-Mendoza MI, Stengel F. Current challenges in photoprotection. J Am Acad Dermatol. 2017;76:S91-S99.
- Li D, Ferguson A, Cervinski MA, et al. AACC guidance document on biotin interference in laboratory tests. J Appl Lab Med. 2020; 5:575-587.
The advent of social media has revolutionized the way patients access and consume health information. While this increased access has its merits, it also has given rise to the proliferation of medical myths, which have considerable effects on patient-physician interactions.1 Myths are prevalent across all fields of health care, ranging from misconceptions about disease etiology and prevention to the efficacy and safety of treatments. This influx of misinformation can derail the clinical encounter, shifting the focus from evidence-based medicine to myth-busting.2 The COVID-19 pandemic exacerbated this issue, as widespread lockdowns and social distancing measures limited access to in-person medical consultations, prompting patients to increasingly turn to online sources for health information that often were unreliable, thereby bypassing professional medical advice.3 Herein, we highlight the challenges and implications of common dermatology myths and provide strategies for effectively debunking these myths to enhance patient care.
Common Dermatology Myths
In dermatology, where visible and often distressing conditions such as acne and hair loss are common, the impact of myths on patient perceptions and treatment outcomes can be particularly profound. Patients often arrive for consultations with preconceived notions that are not grounded in scientific evidence. Common dermatologic myths include eczema and the efficacy of topical corticosteroids, the causes and treatment of hair loss, and risk factors associated with skin cancer.
Eczema and Topical Corticosteroids—Topical corticosteroids for eczema are safe and effective, but nonadherence due to phobias stemming from misinformation online can impede treatment.4 Myths such as red skin syndrome and topical corticosteroid addiction are prevalent. Red skin syndrome refers to claims that prolonged use of topical corticosteroids causes severe redness and burning of the skin and worsening eczema symptoms upon withdrawal. Topical corticosteroid addiction suggests that patients become dependent on corticosteroids, requiring higher doses over time to maintain efficacy. These misconceptions contribute to fear and avoidance of prescribed treatments.
Eczema myths often divert focus from its true etiology as a genetic inflammatory skin disease, suggesting instead that it is caused by leaky gut or food intolerances.4 Risks such as skin thinning and stunted growth often are exaggerated on social media and other nonmedical platforms, though these adverse effects rarely are seen when topical corticosteroids are used appropriately under medical supervision. Misinformation often is linked to companies promoting unregulated consultations, tests, or supposedly natural treatments, including herbal remedies that may surreptitiously contain corticosteroids without clear labeling. This fosters distrust of US Food and Drug Administration– approved and dermatologist-prescribed treatments, as patients may cite concerns based on experiences with or claims about unapproved products.4
Sunscreen and Skin Cancer—In 2018, the American Academy of Dermatology prioritized skin cancer prevention due to suboptimal public adoption of photoprotection measures.5 However, the proliferation of misinformation regarding sunscreen and its potential to cause skin cancer is a more pressing issue. Myths range from claims that sunscreen is ineffective to warnings that it is dangerous, with some social media influencers even suggesting that sunscreen causes skin cancer due to toxic ingredients.6 Oxybenzone, typically found in chemical sunscreens, has been criticized by some advocacy groups and social media influencers as a potential hormone disruptor (ie, a chemical that could interfere with hormone production).7 However, no conclusive evidence has shown that oxybenzone is harmful to humans. Consumer concerns often are based on animal studies in which rats are fed oxybenzone, but mathematical modeling has indicated it would take 277 years of sunscreen use by humans to match the doses used in these studies.8 The false association between sunscreen use and skin cancer is based on flawed studies that found higher rates of skin cancer—including melanoma—in sunscreen users compared to those who did not use sunscreen. However, those using sunscreen also were more likely to travel to sunnier climates and engage in sunbathing, and it may have been this increased sun exposure that elevated their risk for skin cancer.7 It is imperative that the dermatology community counteract this type of misinformation with evidence-based advice.
Hair Loss—Some patients believe that hair loss is caused by wearing hats, frequent shampooing, or even stress in a way that oversimplifies complex physiological processes. Biotin, which commonly is added to supplements for hair, skin, and nails, has been linked to potential risks, such as interference with laboratory testing and false-positive or false-negative results in critical medical tests, which can lead to misdiagnosis or inappropriate treatment.9 Biotin interference can result in falsely low troponin readings, which are critical in diagnosing acute myocardial infarction. Tests for other hormones such as cortisol and parathyroid hormone also are affected, potentially impacting the evaluation and management of endocrine disorders. The US Food and Drug Administration has issued warnings for patients on this topic, emphasizing the importance of informing health care providers about any biotin supplementation prior to laboratory testing. Despite its popularity, there is no substantial scientific evidence to suggest that biotin supplementation promotes hair growth in anyone other than those with deficiency, which is quite rare.9
Myths and the Patient-Physician Relationship
The proliferation of medical myths and misinformation affects the dynamic between patients and dermatologists in several ways. Research across various medical fields has demonstrated that misinformation can substantially impact patient behavior and treatment adherence. Like many other specialists, dermatologists often spend considerable time during consultations with patients debunking myths and correcting misconceptions, which can detract from discussing more critical aspects of the patient’s condition and treatment plan and lead to frustration and anxiety among patients. It also can be challenging for physicians to have these conversations without alienating patients, who may distrust medical recommendations and believe that natural or alternative treatments are superior. This can lead to noncompliance with prescribed treatments, and patients may instead opt to try unproven remedies they encounter online, ultimately resulting in poorer health outcomes.
Strategies to Debunk Myths
By implementing the following strategies, dermatologists can combat the spread of myths, foster trust among patients, and promote adherence to evidence-based treatments:
- Provide educational outreach. Preemptively address myths by giving patients accurate and accessible resources. Including a dedicated section on your clinic’s website with articles, frequently asked questions, videos, and links to reputable sources can be effective. Sharing patient testimonials and before-and-after photographs to demonstrate the success of evidence-based treatments also is recommended, as real-life stories can be powerful tools in dispelling myths.
- Practice effective communication. Involve patients in the decision-making process by discussing their treatment goals, preferences, and concerns. It is important to present all options clearly, including the potential benefits and adverse effects. Discuss the expected outcomes and timelines, and be transparent about the limitations of certain treatment—honesty helps build trust and sets realistic expectations.
- Conduct structured consultations. Ensure that consultations with patients follow a structured format—history, physical examination, and discussion—to help keep the focus on evidence-based practice.
- Leverage technology. Guide patients toward reliable digital patient education tools to empower them with accurate information. Hosting live sessions on social media platforms during which patients can ask questions and receive evidence-based answers also can be beneficial.
Final Thoughts
In summary, the rise of medical myths poses a considerable challenge to dermatologic practice. By understanding the sources and impacts of these myths and employing strategies to dispel them, dermatologists can better navigate the complexities of modern patient interactions and ensure that care remains grounded in scientific evidence.
The advent of social media has revolutionized the way patients access and consume health information. While this increased access has its merits, it also has given rise to the proliferation of medical myths, which have considerable effects on patient-physician interactions.1 Myths are prevalent across all fields of health care, ranging from misconceptions about disease etiology and prevention to the efficacy and safety of treatments. This influx of misinformation can derail the clinical encounter, shifting the focus from evidence-based medicine to myth-busting.2 The COVID-19 pandemic exacerbated this issue, as widespread lockdowns and social distancing measures limited access to in-person medical consultations, prompting patients to increasingly turn to online sources for health information that often were unreliable, thereby bypassing professional medical advice.3 Herein, we highlight the challenges and implications of common dermatology myths and provide strategies for effectively debunking these myths to enhance patient care.
Common Dermatology Myths
In dermatology, where visible and often distressing conditions such as acne and hair loss are common, the impact of myths on patient perceptions and treatment outcomes can be particularly profound. Patients often arrive for consultations with preconceived notions that are not grounded in scientific evidence. Common dermatologic myths include eczema and the efficacy of topical corticosteroids, the causes and treatment of hair loss, and risk factors associated with skin cancer.
Eczema and Topical Corticosteroids—Topical corticosteroids for eczema are safe and effective, but nonadherence due to phobias stemming from misinformation online can impede treatment.4 Myths such as red skin syndrome and topical corticosteroid addiction are prevalent. Red skin syndrome refers to claims that prolonged use of topical corticosteroids causes severe redness and burning of the skin and worsening eczema symptoms upon withdrawal. Topical corticosteroid addiction suggests that patients become dependent on corticosteroids, requiring higher doses over time to maintain efficacy. These misconceptions contribute to fear and avoidance of prescribed treatments.
Eczema myths often divert focus from its true etiology as a genetic inflammatory skin disease, suggesting instead that it is caused by leaky gut or food intolerances.4 Risks such as skin thinning and stunted growth often are exaggerated on social media and other nonmedical platforms, though these adverse effects rarely are seen when topical corticosteroids are used appropriately under medical supervision. Misinformation often is linked to companies promoting unregulated consultations, tests, or supposedly natural treatments, including herbal remedies that may surreptitiously contain corticosteroids without clear labeling. This fosters distrust of US Food and Drug Administration– approved and dermatologist-prescribed treatments, as patients may cite concerns based on experiences with or claims about unapproved products.4
Sunscreen and Skin Cancer—In 2018, the American Academy of Dermatology prioritized skin cancer prevention due to suboptimal public adoption of photoprotection measures.5 However, the proliferation of misinformation regarding sunscreen and its potential to cause skin cancer is a more pressing issue. Myths range from claims that sunscreen is ineffective to warnings that it is dangerous, with some social media influencers even suggesting that sunscreen causes skin cancer due to toxic ingredients.6 Oxybenzone, typically found in chemical sunscreens, has been criticized by some advocacy groups and social media influencers as a potential hormone disruptor (ie, a chemical that could interfere with hormone production).7 However, no conclusive evidence has shown that oxybenzone is harmful to humans. Consumer concerns often are based on animal studies in which rats are fed oxybenzone, but mathematical modeling has indicated it would take 277 years of sunscreen use by humans to match the doses used in these studies.8 The false association between sunscreen use and skin cancer is based on flawed studies that found higher rates of skin cancer—including melanoma—in sunscreen users compared to those who did not use sunscreen. However, those using sunscreen also were more likely to travel to sunnier climates and engage in sunbathing, and it may have been this increased sun exposure that elevated their risk for skin cancer.7 It is imperative that the dermatology community counteract this type of misinformation with evidence-based advice.
Hair Loss—Some patients believe that hair loss is caused by wearing hats, frequent shampooing, or even stress in a way that oversimplifies complex physiological processes. Biotin, which commonly is added to supplements for hair, skin, and nails, has been linked to potential risks, such as interference with laboratory testing and false-positive or false-negative results in critical medical tests, which can lead to misdiagnosis or inappropriate treatment.9 Biotin interference can result in falsely low troponin readings, which are critical in diagnosing acute myocardial infarction. Tests for other hormones such as cortisol and parathyroid hormone also are affected, potentially impacting the evaluation and management of endocrine disorders. The US Food and Drug Administration has issued warnings for patients on this topic, emphasizing the importance of informing health care providers about any biotin supplementation prior to laboratory testing. Despite its popularity, there is no substantial scientific evidence to suggest that biotin supplementation promotes hair growth in anyone other than those with deficiency, which is quite rare.9
Myths and the Patient-Physician Relationship
The proliferation of medical myths and misinformation affects the dynamic between patients and dermatologists in several ways. Research across various medical fields has demonstrated that misinformation can substantially impact patient behavior and treatment adherence. Like many other specialists, dermatologists often spend considerable time during consultations with patients debunking myths and correcting misconceptions, which can detract from discussing more critical aspects of the patient’s condition and treatment plan and lead to frustration and anxiety among patients. It also can be challenging for physicians to have these conversations without alienating patients, who may distrust medical recommendations and believe that natural or alternative treatments are superior. This can lead to noncompliance with prescribed treatments, and patients may instead opt to try unproven remedies they encounter online, ultimately resulting in poorer health outcomes.
Strategies to Debunk Myths
By implementing the following strategies, dermatologists can combat the spread of myths, foster trust among patients, and promote adherence to evidence-based treatments:
- Provide educational outreach. Preemptively address myths by giving patients accurate and accessible resources. Including a dedicated section on your clinic’s website with articles, frequently asked questions, videos, and links to reputable sources can be effective. Sharing patient testimonials and before-and-after photographs to demonstrate the success of evidence-based treatments also is recommended, as real-life stories can be powerful tools in dispelling myths.
- Practice effective communication. Involve patients in the decision-making process by discussing their treatment goals, preferences, and concerns. It is important to present all options clearly, including the potential benefits and adverse effects. Discuss the expected outcomes and timelines, and be transparent about the limitations of certain treatment—honesty helps build trust and sets realistic expectations.
- Conduct structured consultations. Ensure that consultations with patients follow a structured format—history, physical examination, and discussion—to help keep the focus on evidence-based practice.
- Leverage technology. Guide patients toward reliable digital patient education tools to empower them with accurate information. Hosting live sessions on social media platforms during which patients can ask questions and receive evidence-based answers also can be beneficial.
Final Thoughts
In summary, the rise of medical myths poses a considerable challenge to dermatologic practice. By understanding the sources and impacts of these myths and employing strategies to dispel them, dermatologists can better navigate the complexities of modern patient interactions and ensure that care remains grounded in scientific evidence.
- Kessler SH, Bachmann E. Debunking health myths on the internet: the persuasive effect of (visual) online communication. Z Gesundheitswissenschaften J Public Health. 2022;30:1823-1835.
- Fridman I, Johnson S, Elston Lafata J. Health information and misinformation: a framework to guide research and practice. JMIR Med Educ. 2023;9:E38687.
- Di Novi C, Kovacic M, Orso CE. Online health information seeking behavior, healthcare access, and health status during exceptional times. J Econ Behav Organ. 2024;220:675-690.
- Finnegan P, Murphy M, O’Connor C. #corticophobia: a review on online misinformation related to topical steroids. Clin Exp Dermatol. 2023;48:112-115.
- Yang EJ, Beck KM, Maarouf M, et al. Truths and myths in sunscreen labeling. J Cosmet Dermatol. 2018;17:1288-1292.
- Hopkins C. What Gen Z gets wrong about sunscreen. New York Times. Published May 27, 2024. Accessed December 16, 2024. https://www.nytimes.com/2024/05/27/well/live/sunscreen-skin-cancer-gen-z.html
- Harvard Health Publishing. The science of sunscreen. Published February 15, 2021. Accessed December 9, 2024. https://www.health.harvard.edu/staying-healthy/the-science-of-sunscreen
- Lim HW, Arellano-Mendoza MI, Stengel F. Current challenges in photoprotection. J Am Acad Dermatol. 2017;76:S91-S99.
- Li D, Ferguson A, Cervinski MA, et al. AACC guidance document on biotin interference in laboratory tests. J Appl Lab Med. 2020; 5:575-587.
- Kessler SH, Bachmann E. Debunking health myths on the internet: the persuasive effect of (visual) online communication. Z Gesundheitswissenschaften J Public Health. 2022;30:1823-1835.
- Fridman I, Johnson S, Elston Lafata J. Health information and misinformation: a framework to guide research and practice. JMIR Med Educ. 2023;9:E38687.
- Di Novi C, Kovacic M, Orso CE. Online health information seeking behavior, healthcare access, and health status during exceptional times. J Econ Behav Organ. 2024;220:675-690.
- Finnegan P, Murphy M, O’Connor C. #corticophobia: a review on online misinformation related to topical steroids. Clin Exp Dermatol. 2023;48:112-115.
- Yang EJ, Beck KM, Maarouf M, et al. Truths and myths in sunscreen labeling. J Cosmet Dermatol. 2018;17:1288-1292.
- Hopkins C. What Gen Z gets wrong about sunscreen. New York Times. Published May 27, 2024. Accessed December 16, 2024. https://www.nytimes.com/2024/05/27/well/live/sunscreen-skin-cancer-gen-z.html
- Harvard Health Publishing. The science of sunscreen. Published February 15, 2021. Accessed December 9, 2024. https://www.health.harvard.edu/staying-healthy/the-science-of-sunscreen
- Lim HW, Arellano-Mendoza MI, Stengel F. Current challenges in photoprotection. J Am Acad Dermatol. 2017;76:S91-S99.
- Li D, Ferguson A, Cervinski MA, et al. AACC guidance document on biotin interference in laboratory tests. J Appl Lab Med. 2020; 5:575-587.
Debunking Dermatology Myths to Enhance Patient Care
Debunking Dermatology Myths to Enhance Patient Care
Mentorship in Residency
Mentorship in Residency
The year was 2023, and I was on my way to the American Academy of Dermatology meeting in New Orleans, Louisiana. “Geaux Tigers!” I exclaimed to a stranger as she walked by in her purple and gold shoes and scrubs. We chatted for a minute or two about Louisiana State University (LSU) football, then went our separate ways. Later that day, in the hands-on wound closures workshop, I was surprised to see my new acquaintance step up to the podium to lecture, then make rounds across the room to instruct residents. I didn’t know it at the time, but those purple and gold shoes sparked a conversation with a fellowship program director who would become one of my most valued mentors.
I didn’t set out to find a mentor that day—I simply was excited to connect with a fellow Tigers fan. But mentorship often finds us unexpectedly, and that encounter serves as a reminder that mentorship doesn’t always start in a formal setting. Sometimes it begins with a quick conversation in the right place at the right time. This story is one of many experiences that taught me valuable lessons about mentorship—its importance, how it can grow naturally, and the impact it can have.
Residency is a pivotal time in a physician’s life, filled with rapid learning, complex challenges, and new professional relationships. Amidst the long hours and heavy responsibilities, mentorship stands out as a support system for guiding residents toward professional and personal growth. Herein, I share more about my experiences with mentorship in residency, the lessons I have learned, and how they can serve as guidance for residents.
The Value of Mentorship
Mentorship in residency has been shown to have a major impact on career satisfaction, clinical confidence, and professional development.1 A good mentor offers more than just advice—he or she can provide a model of professionalism and skills that resonates with the mentee’s own aspirations. Mentorship can help residents refine their clinical skills, navigate the complexities of patient care, engage in research, and connect with professionals in their field.2
Mentorship can be sought intentionally or arise naturally from shared interests and connections. Some residents reach out to potential mentors directly through emails, set up one-on-one meetings, or shadow them to gain firsthand experience. Others find mentorship simply by putting themselves in situations that foster these connections, such as attending conferences or lectures. Both approaches can lead to impactful relationships that shape a resident’s career and personal growth.
For residents involved in research, an effective faculty research mentor is particularly impactful. Studies show that residents who work with knowledgeable research mentors are more likely to experience success and productivity in their research efforts.3 Research mentors can provide essential guidance—from helping formulate research questions to navigating the complexities of publishing—which makes them invaluable in a resident’s academic development.
If you have interests in specific areas not heavily emphasized within your residency program (eg, transplantation dermatology, hair restoration, cutaneous lymphoma), consider checking within your broader medical community for specialists. Many dermatologists and other specialists welcome the opportunity to mentor residents who express a sincere interest in learning. By reaching out to these professionals, you not only expand your clinical knowledge but also gain access to niche areas of dermatology that can shape and refine your future practice. Often, these experiences lead to invaluable mentorships that may otherwise be unavailable within your immediate training environment.
Networking Through Professional Society Rotational and Mentorship Programs
The Women’s Dermatologic Society (https://www.womensderm.org/), the American Society for Dermatologic Surgery (https://www.asds.net/), and the American Society for Laser Medicine and Surgery (https://www.aslms.org/) all provide excellent formalized mentorship or preceptorship programs. Check their websites for application requirements and timelines. Participating in these programs is a great way to network with experts in dermatology, providing a structured way to interact with physicians who share your interests. Whether you are interested in medical dermatology, surgery, pediatrics, dermatopathology, or cosmetics, there are many mentors who greatly enjoy sharing their knowledge and experience with residents. Oftentimes, these programs include stipends to assist with costs that are awarded as accolades that can enhance your curriculum vitae. Engaging in these recognized preceptorship programs often builds lasting connections and ensures that both mentor and mentee have a vested interest in the relationship’s success.
Making Connections at Conferences and Maximizing Hands-on Learning
Professional conferences offer valuable opportunities to connect with mentors, whether you are proactively seeking mentorship or simply allowing connections to happen naturally. Conferences such as those of the American Academy of Dermatology and American Society for Dermatologic Surgery publish educational booklets and schedules online prior to the event, giving you a chance to explore both topics and speaker names ahead of time. This can be an excellent opportunity to create a day-by-day game plan, identifying sessions and lectures of interest as well as specific authors or experts you might like to meet. Planning in advance makes it easier to engage with leaders in the field, introduce yourself, and make meaningful connections.
Oftentimes, these society meetings offer hands-on courses, which are a great way to meet mentors and learn from direct instruction. Instructors for these courses often are leaders in dermatology who are passionate about teaching. With small group sizes, hands-on courses offer both technical skill-building opportunities and a chance to connect personally with instructors. Take a moment to introduce yourself and engage in a quick conversation, and if you feel it is appropriate, follow up with an email after the conference. This helps keep the connection alive beyond the event and may open doors for future mentorship opportunities.
Away Rotations
For residents looking to build specialized skills and connect with mentors outside their own program—especially those considering fellowship—away rotations can be a great tool. Though it may require using vacation time, an away rotation offers immersive learning in a particular area while providing opportunities to observe new mentors and establish relationships within a desired subspecialty or program. By simply reaching out and expressing interest, residents can connect with physicians who may become lasting mentors and advocates.
Building a Mentor-Mentee Relationship
A meaningful mentor-mentee relationship requires time, effort, and effective communication, with clear expectations around mentorship goals, time commitments, and how both parties envision the relationship evolving.4 Ideally, mentees should feel comfortable sharing their goals with mentors and asking for feedback. In the right context, a simple and effective practice is to send your mentor a brief update on your progress every few months. This could be a quick email sharing your latest projects, ideas, and/or achievements. By regularly checking in, you show your mentor that you are committed to growing from their guidance and respect their time.
The Lasting Impact of Mentorship
The effects of mentorship in residency extend well beyond the training years, as mentors often become lifelong guides and professional advocates for their mentees.5 Residency often is the last time a resident trains under the direct supervision of an attending physician, making it a unique and formative period. After graduation, many new physicians find the transition to independent practice challenging, and the “real world” can be a shock. Having a mentor during this time, or maintaining connections with mentors from residency, can be invaluable. Mentors can offer advice, act as sounding boards, and remind new graduates of the importance of being lifelong learners. These relationships help ease the transition into practice, instilling a commitment to continuous improvement and professional growth. For me, a conversation about LSU football at the AAD meeting in New Orleans exemplifies how mentorship can begin in the most unexpected ways. That casual exchange led to an away rotation, a fellowship interview, connections at national meetings, and the start of what I hope will be a lifelong friendship.
- Ramanan RA, Taylor WC, Davis RB, et al. Mentoring matters. mentoring and career preparation in internal medicine residency training. J Gen Intern Med. 2006;21:340-345.
- Sambunjak D, Straus SE, Marusic´ A. Mentoring in academic medicine: a systematic review. JAMA. 2006;296:1103-1115.
- Ercan-Fang NG, Mahmoud MA, Cottrell C, et al. Best practices in resident research-a national survey of high functioning internal medicine residency programs in resident research in USA. Am J Med Sci. 2021;361:23-29.
- Allen TD, Eby LT, Poteet ML, et al. Career benefits associated with mentoring for protégeé: a meta-analysis. J Appl Psychol. 2004;89:127-136.
- Kashiwagi DT, Varkey P, Cook DA. Mentoring programs for physicians in academic medicine: a systematic review. Acad Med. 2013;88:1029-1037.
The year was 2023, and I was on my way to the American Academy of Dermatology meeting in New Orleans, Louisiana. “Geaux Tigers!” I exclaimed to a stranger as she walked by in her purple and gold shoes and scrubs. We chatted for a minute or two about Louisiana State University (LSU) football, then went our separate ways. Later that day, in the hands-on wound closures workshop, I was surprised to see my new acquaintance step up to the podium to lecture, then make rounds across the room to instruct residents. I didn’t know it at the time, but those purple and gold shoes sparked a conversation with a fellowship program director who would become one of my most valued mentors.
I didn’t set out to find a mentor that day—I simply was excited to connect with a fellow Tigers fan. But mentorship often finds us unexpectedly, and that encounter serves as a reminder that mentorship doesn’t always start in a formal setting. Sometimes it begins with a quick conversation in the right place at the right time. This story is one of many experiences that taught me valuable lessons about mentorship—its importance, how it can grow naturally, and the impact it can have.
Residency is a pivotal time in a physician’s life, filled with rapid learning, complex challenges, and new professional relationships. Amidst the long hours and heavy responsibilities, mentorship stands out as a support system for guiding residents toward professional and personal growth. Herein, I share more about my experiences with mentorship in residency, the lessons I have learned, and how they can serve as guidance for residents.
The Value of Mentorship
Mentorship in residency has been shown to have a major impact on career satisfaction, clinical confidence, and professional development.1 A good mentor offers more than just advice—he or she can provide a model of professionalism and skills that resonates with the mentee’s own aspirations. Mentorship can help residents refine their clinical skills, navigate the complexities of patient care, engage in research, and connect with professionals in their field.2
Mentorship can be sought intentionally or arise naturally from shared interests and connections. Some residents reach out to potential mentors directly through emails, set up one-on-one meetings, or shadow them to gain firsthand experience. Others find mentorship simply by putting themselves in situations that foster these connections, such as attending conferences or lectures. Both approaches can lead to impactful relationships that shape a resident’s career and personal growth.
For residents involved in research, an effective faculty research mentor is particularly impactful. Studies show that residents who work with knowledgeable research mentors are more likely to experience success and productivity in their research efforts.3 Research mentors can provide essential guidance—from helping formulate research questions to navigating the complexities of publishing—which makes them invaluable in a resident’s academic development.
If you have interests in specific areas not heavily emphasized within your residency program (eg, transplantation dermatology, hair restoration, cutaneous lymphoma), consider checking within your broader medical community for specialists. Many dermatologists and other specialists welcome the opportunity to mentor residents who express a sincere interest in learning. By reaching out to these professionals, you not only expand your clinical knowledge but also gain access to niche areas of dermatology that can shape and refine your future practice. Often, these experiences lead to invaluable mentorships that may otherwise be unavailable within your immediate training environment.
Networking Through Professional Society Rotational and Mentorship Programs
The Women’s Dermatologic Society (https://www.womensderm.org/), the American Society for Dermatologic Surgery (https://www.asds.net/), and the American Society for Laser Medicine and Surgery (https://www.aslms.org/) all provide excellent formalized mentorship or preceptorship programs. Check their websites for application requirements and timelines. Participating in these programs is a great way to network with experts in dermatology, providing a structured way to interact with physicians who share your interests. Whether you are interested in medical dermatology, surgery, pediatrics, dermatopathology, or cosmetics, there are many mentors who greatly enjoy sharing their knowledge and experience with residents. Oftentimes, these programs include stipends to assist with costs that are awarded as accolades that can enhance your curriculum vitae. Engaging in these recognized preceptorship programs often builds lasting connections and ensures that both mentor and mentee have a vested interest in the relationship’s success.
Making Connections at Conferences and Maximizing Hands-on Learning
Professional conferences offer valuable opportunities to connect with mentors, whether you are proactively seeking mentorship or simply allowing connections to happen naturally. Conferences such as those of the American Academy of Dermatology and American Society for Dermatologic Surgery publish educational booklets and schedules online prior to the event, giving you a chance to explore both topics and speaker names ahead of time. This can be an excellent opportunity to create a day-by-day game plan, identifying sessions and lectures of interest as well as specific authors or experts you might like to meet. Planning in advance makes it easier to engage with leaders in the field, introduce yourself, and make meaningful connections.
Oftentimes, these society meetings offer hands-on courses, which are a great way to meet mentors and learn from direct instruction. Instructors for these courses often are leaders in dermatology who are passionate about teaching. With small group sizes, hands-on courses offer both technical skill-building opportunities and a chance to connect personally with instructors. Take a moment to introduce yourself and engage in a quick conversation, and if you feel it is appropriate, follow up with an email after the conference. This helps keep the connection alive beyond the event and may open doors for future mentorship opportunities.
Away Rotations
For residents looking to build specialized skills and connect with mentors outside their own program—especially those considering fellowship—away rotations can be a great tool. Though it may require using vacation time, an away rotation offers immersive learning in a particular area while providing opportunities to observe new mentors and establish relationships within a desired subspecialty or program. By simply reaching out and expressing interest, residents can connect with physicians who may become lasting mentors and advocates.
Building a Mentor-Mentee Relationship
A meaningful mentor-mentee relationship requires time, effort, and effective communication, with clear expectations around mentorship goals, time commitments, and how both parties envision the relationship evolving.4 Ideally, mentees should feel comfortable sharing their goals with mentors and asking for feedback. In the right context, a simple and effective practice is to send your mentor a brief update on your progress every few months. This could be a quick email sharing your latest projects, ideas, and/or achievements. By regularly checking in, you show your mentor that you are committed to growing from their guidance and respect their time.
The Lasting Impact of Mentorship
The effects of mentorship in residency extend well beyond the training years, as mentors often become lifelong guides and professional advocates for their mentees.5 Residency often is the last time a resident trains under the direct supervision of an attending physician, making it a unique and formative period. After graduation, many new physicians find the transition to independent practice challenging, and the “real world” can be a shock. Having a mentor during this time, or maintaining connections with mentors from residency, can be invaluable. Mentors can offer advice, act as sounding boards, and remind new graduates of the importance of being lifelong learners. These relationships help ease the transition into practice, instilling a commitment to continuous improvement and professional growth. For me, a conversation about LSU football at the AAD meeting in New Orleans exemplifies how mentorship can begin in the most unexpected ways. That casual exchange led to an away rotation, a fellowship interview, connections at national meetings, and the start of what I hope will be a lifelong friendship.
The year was 2023, and I was on my way to the American Academy of Dermatology meeting in New Orleans, Louisiana. “Geaux Tigers!” I exclaimed to a stranger as she walked by in her purple and gold shoes and scrubs. We chatted for a minute or two about Louisiana State University (LSU) football, then went our separate ways. Later that day, in the hands-on wound closures workshop, I was surprised to see my new acquaintance step up to the podium to lecture, then make rounds across the room to instruct residents. I didn’t know it at the time, but those purple and gold shoes sparked a conversation with a fellowship program director who would become one of my most valued mentors.
I didn’t set out to find a mentor that day—I simply was excited to connect with a fellow Tigers fan. But mentorship often finds us unexpectedly, and that encounter serves as a reminder that mentorship doesn’t always start in a formal setting. Sometimes it begins with a quick conversation in the right place at the right time. This story is one of many experiences that taught me valuable lessons about mentorship—its importance, how it can grow naturally, and the impact it can have.
Residency is a pivotal time in a physician’s life, filled with rapid learning, complex challenges, and new professional relationships. Amidst the long hours and heavy responsibilities, mentorship stands out as a support system for guiding residents toward professional and personal growth. Herein, I share more about my experiences with mentorship in residency, the lessons I have learned, and how they can serve as guidance for residents.
The Value of Mentorship
Mentorship in residency has been shown to have a major impact on career satisfaction, clinical confidence, and professional development.1 A good mentor offers more than just advice—he or she can provide a model of professionalism and skills that resonates with the mentee’s own aspirations. Mentorship can help residents refine their clinical skills, navigate the complexities of patient care, engage in research, and connect with professionals in their field.2
Mentorship can be sought intentionally or arise naturally from shared interests and connections. Some residents reach out to potential mentors directly through emails, set up one-on-one meetings, or shadow them to gain firsthand experience. Others find mentorship simply by putting themselves in situations that foster these connections, such as attending conferences or lectures. Both approaches can lead to impactful relationships that shape a resident’s career and personal growth.
For residents involved in research, an effective faculty research mentor is particularly impactful. Studies show that residents who work with knowledgeable research mentors are more likely to experience success and productivity in their research efforts.3 Research mentors can provide essential guidance—from helping formulate research questions to navigating the complexities of publishing—which makes them invaluable in a resident’s academic development.
If you have interests in specific areas not heavily emphasized within your residency program (eg, transplantation dermatology, hair restoration, cutaneous lymphoma), consider checking within your broader medical community for specialists. Many dermatologists and other specialists welcome the opportunity to mentor residents who express a sincere interest in learning. By reaching out to these professionals, you not only expand your clinical knowledge but also gain access to niche areas of dermatology that can shape and refine your future practice. Often, these experiences lead to invaluable mentorships that may otherwise be unavailable within your immediate training environment.
Networking Through Professional Society Rotational and Mentorship Programs
The Women’s Dermatologic Society (https://www.womensderm.org/), the American Society for Dermatologic Surgery (https://www.asds.net/), and the American Society for Laser Medicine and Surgery (https://www.aslms.org/) all provide excellent formalized mentorship or preceptorship programs. Check their websites for application requirements and timelines. Participating in these programs is a great way to network with experts in dermatology, providing a structured way to interact with physicians who share your interests. Whether you are interested in medical dermatology, surgery, pediatrics, dermatopathology, or cosmetics, there are many mentors who greatly enjoy sharing their knowledge and experience with residents. Oftentimes, these programs include stipends to assist with costs that are awarded as accolades that can enhance your curriculum vitae. Engaging in these recognized preceptorship programs often builds lasting connections and ensures that both mentor and mentee have a vested interest in the relationship’s success.
Making Connections at Conferences and Maximizing Hands-on Learning
Professional conferences offer valuable opportunities to connect with mentors, whether you are proactively seeking mentorship or simply allowing connections to happen naturally. Conferences such as those of the American Academy of Dermatology and American Society for Dermatologic Surgery publish educational booklets and schedules online prior to the event, giving you a chance to explore both topics and speaker names ahead of time. This can be an excellent opportunity to create a day-by-day game plan, identifying sessions and lectures of interest as well as specific authors or experts you might like to meet. Planning in advance makes it easier to engage with leaders in the field, introduce yourself, and make meaningful connections.
Oftentimes, these society meetings offer hands-on courses, which are a great way to meet mentors and learn from direct instruction. Instructors for these courses often are leaders in dermatology who are passionate about teaching. With small group sizes, hands-on courses offer both technical skill-building opportunities and a chance to connect personally with instructors. Take a moment to introduce yourself and engage in a quick conversation, and if you feel it is appropriate, follow up with an email after the conference. This helps keep the connection alive beyond the event and may open doors for future mentorship opportunities.
Away Rotations
For residents looking to build specialized skills and connect with mentors outside their own program—especially those considering fellowship—away rotations can be a great tool. Though it may require using vacation time, an away rotation offers immersive learning in a particular area while providing opportunities to observe new mentors and establish relationships within a desired subspecialty or program. By simply reaching out and expressing interest, residents can connect with physicians who may become lasting mentors and advocates.
Building a Mentor-Mentee Relationship
A meaningful mentor-mentee relationship requires time, effort, and effective communication, with clear expectations around mentorship goals, time commitments, and how both parties envision the relationship evolving.4 Ideally, mentees should feel comfortable sharing their goals with mentors and asking for feedback. In the right context, a simple and effective practice is to send your mentor a brief update on your progress every few months. This could be a quick email sharing your latest projects, ideas, and/or achievements. By regularly checking in, you show your mentor that you are committed to growing from their guidance and respect their time.
The Lasting Impact of Mentorship
The effects of mentorship in residency extend well beyond the training years, as mentors often become lifelong guides and professional advocates for their mentees.5 Residency often is the last time a resident trains under the direct supervision of an attending physician, making it a unique and formative period. After graduation, many new physicians find the transition to independent practice challenging, and the “real world” can be a shock. Having a mentor during this time, or maintaining connections with mentors from residency, can be invaluable. Mentors can offer advice, act as sounding boards, and remind new graduates of the importance of being lifelong learners. These relationships help ease the transition into practice, instilling a commitment to continuous improvement and professional growth. For me, a conversation about LSU football at the AAD meeting in New Orleans exemplifies how mentorship can begin in the most unexpected ways. That casual exchange led to an away rotation, a fellowship interview, connections at national meetings, and the start of what I hope will be a lifelong friendship.
- Ramanan RA, Taylor WC, Davis RB, et al. Mentoring matters. mentoring and career preparation in internal medicine residency training. J Gen Intern Med. 2006;21:340-345.
- Sambunjak D, Straus SE, Marusic´ A. Mentoring in academic medicine: a systematic review. JAMA. 2006;296:1103-1115.
- Ercan-Fang NG, Mahmoud MA, Cottrell C, et al. Best practices in resident research-a national survey of high functioning internal medicine residency programs in resident research in USA. Am J Med Sci. 2021;361:23-29.
- Allen TD, Eby LT, Poteet ML, et al. Career benefits associated with mentoring for protégeé: a meta-analysis. J Appl Psychol. 2004;89:127-136.
- Kashiwagi DT, Varkey P, Cook DA. Mentoring programs for physicians in academic medicine: a systematic review. Acad Med. 2013;88:1029-1037.
- Ramanan RA, Taylor WC, Davis RB, et al. Mentoring matters. mentoring and career preparation in internal medicine residency training. J Gen Intern Med. 2006;21:340-345.
- Sambunjak D, Straus SE, Marusic´ A. Mentoring in academic medicine: a systematic review. JAMA. 2006;296:1103-1115.
- Ercan-Fang NG, Mahmoud MA, Cottrell C, et al. Best practices in resident research-a national survey of high functioning internal medicine residency programs in resident research in USA. Am J Med Sci. 2021;361:23-29.
- Allen TD, Eby LT, Poteet ML, et al. Career benefits associated with mentoring for protégeé: a meta-analysis. J Appl Psychol. 2004;89:127-136.
- Kashiwagi DT, Varkey P, Cook DA. Mentoring programs for physicians in academic medicine: a systematic review. Acad Med. 2013;88:1029-1037.
Mentorship in Residency
Mentorship in Residency
RESIDENT PEARLS
- Mentorship can help residents refine their clinical skills, navigate the complexities of patient care, engage in research, and connect with professionals in their field.
- The effects of mentorship in residency extend well beyond the training years, as mentors often become lifelong guides and professional advocates for their mentees.
Recurrent Nodule on the First Toe
Recurrent Nodule on the First Toe
THE DIAGNOSIS: Hidradenocarcinoma
Both the original and recurrent lesions were interpreted as a chondroid syringoma, a benign adnexal tumor; however, the third biopsy of the lesion revealed a low-grade adnexal neoplasm with irregular nests of variably sized epithelial cells demonstrating mild nuclear atypia and low mitotic activity. Given the multiple recurrences, accelerated growth, and more aggressive histologic findings, the patient was referred to our clinic for surgical management.
We elected to perform modified Mohs micrographic surgery (MMS) with permanent tissue sections to enable the application of immunohistochemical stains to fully characterize the tumor. Histopathology showed a poorly circumscribed infiltrative dermal neoplasm composed of basaloid cells with a solid and cystic growth pattern in a background of hyalinized, fibrotic stroma (Figure, A and B). There were focal clear cell and squamous features as well as focal ductal differentiation (Figure, C and D). No obvious papillary structures were noted. The tumor cells were positive for D2-40, and staining for CD31 failed to reveal lymphovascular invasion. Based on the infiltrative features in conjunction with the findings from the prior biopsies, a diagnosis of hidradenocarcinoma (HAC) was made. Deep and peripheral margins were cleared after 2 stages of MMS.
Initially described in 1954, HAC is an exceedingly rare adnexal tumor of apocrine and eccrine derivation.1 Historically, nomenclature for this entity has varied in the literature, including synonyms such as malignant nodular hidradenoma, malignant acrospiroma, solid-cystic adenocarcinoma, and malignant clear cell myoepithelioma.2,3 Approximately 6% of all malignant eccrine tumors worldwide are HACs, which account for only 1 in 13,000 dermatopathology specimens.1 These tumors may transform from clear cell hidradenomas (their benign counterparts) but more commonly arise de novo. Compared to benign hidradenomas, HACs are poorly circumscribed with infiltrative growth patterns on histopathology and may exhibit nuclear pleomorphism, prominent mitotic activity, necrosis, and perineural or vascular invasion.2
Clinically, HAC manifests as a 1- to 5-cm, solitary, firm, intradermal pink or violaceous nodule with possible ulceration.2,4 The nodule often is asymptomatic but may be tender, as in our patient. There seems to be no clear anatomic site of predilection, with approximately 42% of HACs localized to the head and neck and the remainder occurring on the trunk, arms, and legs.3,5-7 Females and males are affected equally, and lesions tend to arise in the seventh decade of life.7
Reports in the literature suggest that HAC is a very aggressive tumor with a generally poor prognosis.1 Several studies have found that up to half of tumors locally recur despite aggressive surgical management, and metastasis occurs in 20% to 60% of patients.3,8 However, a large study of US Surveillance, Epidemiology, and End Results data investigating the clinicopathologic characteristics of 289 patients with HAC revealed a more favorable prognosis.7 Mean overall survival and cancer-specific survival were greater than 13 years, and 10-year overall survival and cancer-specific survival rates were 60.2% and 90.5%, respectively.
Traditionally used to treat keratinocyte carcinomas, including basal cell carcinoma and squamous cell carcinoma, complete margin assessment with MMS is increasingly being utilized in the management of other cutaneous malignancies, including adnexal tumors.8 Due to its rarity, there remains no standard optimal treatment approach for HAC. One small retrospective study of 10 patients with HAC treated with MMS demonstrated favorable outcomes with no cases of recurrence, metastasis, or diseaserelated mortality in a mean 7-year follow-up period.9
Whole-body positron emission tomography/computed tomography performed in our patient approximately 1 month after MMS revealed mildly hypermetabolic left inguinal lymph nodes, which were thought to be reactive, and a question of small hypermetabolic foci in the liver. Follow-up computed tomography of the abdomen subsequently was performed and was negative for hepatic metastases. The patient will be monitored closely for local recurrence; however, the clearance of the tumor with MMS, which allowed complete margin assessment, is encouraging and supports MMS as superior to traditional surgical excision in the treatment of HAC. At his most recent examination 17 months after Mohs surgery, the patient remained tumor free.
Aggressive digital papillary adenocarcinoma (ADPA) is a rare malignant tumor originating in the sweat glands that can occur on the first toe but most commonly arises on the fingers. While both HAC and ADPA can manifest with an infiltrative growth pattern and cytologic atypia, ADPA classically reveals a well-circumscribed multinodular tumor in the dermis comprised of solid and cystic proliferation as well as papillary projections. In addition, ADPA has been described as having back-to-back glandular and ductal structures.10 Giant cell tumor of the tendon sheath is a benign fibrohistiocytic tumor that also typically manifests on the fingers but rarely can occur on the foot, including the first toe.11,12 This tumor is more common in women and most frequently affects individuals aged 30 to 50 years.12 Microscopically, giant cell tumor of the tendon sheath is characterized by a proliferation of osteoclastlike giant cells, epithelioid histiocytelike cells, mononuclear cells, and xanthomatous cells among collagenous bands.11
Osteosarcoma is an uncommon tumor of osteoidproducing cells that usually arises in the metaphysis of long bones and manifests as a tender subcutaneous mass. It has a bimodal age distribution, peaking in adolescents and adults older than 65 years.13 While very rare, osteosarcoma has been reported to occur in the bones of the feet, including the phalanges.14 Given the recurrent nature of our patient’s tumor, metastasis should always be considered; however, in his case, full-body imaging was negative for additional malignancy.
- Gauerke S, Driscoll JJ. Hidradenocarcinomas: a brief review and future directions. Arch Pathol Lab Med. 2010;134:781-785. doi:10.5858/134.5.781
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/J.HOC.2018.09.002
- Ohta M, Hiramoto M, Fujii M, et al. Nodular hidradenocarcinoma on the scalp of a young woman: case report and review of literature. Dermatol Surg. 2004;30:1265-1268. doi:10.1111/J.1524-4725.2004.30390.X
- Souvatzidis P, Sbano P, Mandato F, et al. Malignant nodular hidradenoma of the skin: report of seven cases. J Eur Acad Dermatol Venereol. 2008;22:549-554. doi:10.1111/J.1468-3083.2007.02504.X
- Yavel R, Hinshaw M, Rao V, et al. Hidradenomas and a hidradenocarcinoma of the scalp managed using Mohs micrographic surgery and a multidisciplinary approach: case reports and review of the literature. Dermatolog Surg. 2009;35:273-281. doi:10.1111/j.1524-4725.2008.34424.x
- Kazakov DV, Ivan D, Kutzner H, et al. Cutaneous hidradenocarcinoma: a clinicopathological, immunohistochemical, and molecular biologic study of 14 cases, including Her2/neu gene expression/ amplification, TP53 gene mutation analysis, and t(11;19) translocation. Am J Dermatopathol. 2009;31:236-247. doi:10.1097/DAD.0B013E3181984F10
- Gao T, Pan S, Li M, et al. Prognostic analysis of hidradenocarcinoma: a SEER-based observational study. Ann Med. 2022;54:454-463. doi:10 .1080/07853890.2022.2032313
- Tolkachjov SN. Adnexal carcinomas treated with Mohs micrographic surgery: a comprehensive review. Dermatol Surg. 2017;43:1199-1207. doi:10.1097/DSS.0000000000001167
- Tolkachjov SN, Hocker TL, Hochwalt PC, et al. Mohs micrographic surgery for the treatment of hidradenocarcinoma: the mayo clinic experience from 1993 to 2013. Dermatolog Surg. 2015;41:226-231. doi:10.1097/DSS.0000000000000242
- Weingertner N, Gressel A, Battistella M, et al. Aggressive digital papillary adenocarcinoma: a clinicopathological study of 19 cases. J Am Acad Dermatol. 2017;77:549-558.e1. doi:10.1016/J.JAAD.2017.02.028
- Paral KM, Petronic-Rosic V. Acral manifestations of soft tissue tumors. Clin Dermatol. 2017;35:85-98. doi:10.1016/J.CLINDER MATOL.2016.09.012
- Kondo RN, Crespigio J, Pavezzi PD, et al. Giant cell tumors of the tendon sheath in the left hallux. An Bras Dermatol. 2016;91:704-705. doi:10.1590/ABD1806-4841.20165769
- Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3-13. doi:10.1007/978-1-4419-0284-9_1
- Anninga JK, Picci P, Fiocco M, et al. Osteosarcoma of the hands and feet: a distinct clinico-pathological subgroup. Virchows Arch. 2013;462:109- 120. doi:10.1007/S00428-012-1339-3
THE DIAGNOSIS: Hidradenocarcinoma
Both the original and recurrent lesions were interpreted as a chondroid syringoma, a benign adnexal tumor; however, the third biopsy of the lesion revealed a low-grade adnexal neoplasm with irregular nests of variably sized epithelial cells demonstrating mild nuclear atypia and low mitotic activity. Given the multiple recurrences, accelerated growth, and more aggressive histologic findings, the patient was referred to our clinic for surgical management.
We elected to perform modified Mohs micrographic surgery (MMS) with permanent tissue sections to enable the application of immunohistochemical stains to fully characterize the tumor. Histopathology showed a poorly circumscribed infiltrative dermal neoplasm composed of basaloid cells with a solid and cystic growth pattern in a background of hyalinized, fibrotic stroma (Figure, A and B). There were focal clear cell and squamous features as well as focal ductal differentiation (Figure, C and D). No obvious papillary structures were noted. The tumor cells were positive for D2-40, and staining for CD31 failed to reveal lymphovascular invasion. Based on the infiltrative features in conjunction with the findings from the prior biopsies, a diagnosis of hidradenocarcinoma (HAC) was made. Deep and peripheral margins were cleared after 2 stages of MMS.

Initially described in 1954, HAC is an exceedingly rare adnexal tumor of apocrine and eccrine derivation.1 Historically, nomenclature for this entity has varied in the literature, including synonyms such as malignant nodular hidradenoma, malignant acrospiroma, solid-cystic adenocarcinoma, and malignant clear cell myoepithelioma.2,3 Approximately 6% of all malignant eccrine tumors worldwide are HACs, which account for only 1 in 13,000 dermatopathology specimens.1 These tumors may transform from clear cell hidradenomas (their benign counterparts) but more commonly arise de novo. Compared to benign hidradenomas, HACs are poorly circumscribed with infiltrative growth patterns on histopathology and may exhibit nuclear pleomorphism, prominent mitotic activity, necrosis, and perineural or vascular invasion.2
Clinically, HAC manifests as a 1- to 5-cm, solitary, firm, intradermal pink or violaceous nodule with possible ulceration.2,4 The nodule often is asymptomatic but may be tender, as in our patient. There seems to be no clear anatomic site of predilection, with approximately 42% of HACs localized to the head and neck and the remainder occurring on the trunk, arms, and legs.3,5-7 Females and males are affected equally, and lesions tend to arise in the seventh decade of life.7
Reports in the literature suggest that HAC is a very aggressive tumor with a generally poor prognosis.1 Several studies have found that up to half of tumors locally recur despite aggressive surgical management, and metastasis occurs in 20% to 60% of patients.3,8 However, a large study of US Surveillance, Epidemiology, and End Results data investigating the clinicopathologic characteristics of 289 patients with HAC revealed a more favorable prognosis.7 Mean overall survival and cancer-specific survival were greater than 13 years, and 10-year overall survival and cancer-specific survival rates were 60.2% and 90.5%, respectively.
Traditionally used to treat keratinocyte carcinomas, including basal cell carcinoma and squamous cell carcinoma, complete margin assessment with MMS is increasingly being utilized in the management of other cutaneous malignancies, including adnexal tumors.8 Due to its rarity, there remains no standard optimal treatment approach for HAC. One small retrospective study of 10 patients with HAC treated with MMS demonstrated favorable outcomes with no cases of recurrence, metastasis, or diseaserelated mortality in a mean 7-year follow-up period.9
Whole-body positron emission tomography/computed tomography performed in our patient approximately 1 month after MMS revealed mildly hypermetabolic left inguinal lymph nodes, which were thought to be reactive, and a question of small hypermetabolic foci in the liver. Follow-up computed tomography of the abdomen subsequently was performed and was negative for hepatic metastases. The patient will be monitored closely for local recurrence; however, the clearance of the tumor with MMS, which allowed complete margin assessment, is encouraging and supports MMS as superior to traditional surgical excision in the treatment of HAC. At his most recent examination 17 months after Mohs surgery, the patient remained tumor free.
Aggressive digital papillary adenocarcinoma (ADPA) is a rare malignant tumor originating in the sweat glands that can occur on the first toe but most commonly arises on the fingers. While both HAC and ADPA can manifest with an infiltrative growth pattern and cytologic atypia, ADPA classically reveals a well-circumscribed multinodular tumor in the dermis comprised of solid and cystic proliferation as well as papillary projections. In addition, ADPA has been described as having back-to-back glandular and ductal structures.10 Giant cell tumor of the tendon sheath is a benign fibrohistiocytic tumor that also typically manifests on the fingers but rarely can occur on the foot, including the first toe.11,12 This tumor is more common in women and most frequently affects individuals aged 30 to 50 years.12 Microscopically, giant cell tumor of the tendon sheath is characterized by a proliferation of osteoclastlike giant cells, epithelioid histiocytelike cells, mononuclear cells, and xanthomatous cells among collagenous bands.11
Osteosarcoma is an uncommon tumor of osteoidproducing cells that usually arises in the metaphysis of long bones and manifests as a tender subcutaneous mass. It has a bimodal age distribution, peaking in adolescents and adults older than 65 years.13 While very rare, osteosarcoma has been reported to occur in the bones of the feet, including the phalanges.14 Given the recurrent nature of our patient’s tumor, metastasis should always be considered; however, in his case, full-body imaging was negative for additional malignancy.
THE DIAGNOSIS: Hidradenocarcinoma
Both the original and recurrent lesions were interpreted as a chondroid syringoma, a benign adnexal tumor; however, the third biopsy of the lesion revealed a low-grade adnexal neoplasm with irregular nests of variably sized epithelial cells demonstrating mild nuclear atypia and low mitotic activity. Given the multiple recurrences, accelerated growth, and more aggressive histologic findings, the patient was referred to our clinic for surgical management.
We elected to perform modified Mohs micrographic surgery (MMS) with permanent tissue sections to enable the application of immunohistochemical stains to fully characterize the tumor. Histopathology showed a poorly circumscribed infiltrative dermal neoplasm composed of basaloid cells with a solid and cystic growth pattern in a background of hyalinized, fibrotic stroma (Figure, A and B). There were focal clear cell and squamous features as well as focal ductal differentiation (Figure, C and D). No obvious papillary structures were noted. The tumor cells were positive for D2-40, and staining for CD31 failed to reveal lymphovascular invasion. Based on the infiltrative features in conjunction with the findings from the prior biopsies, a diagnosis of hidradenocarcinoma (HAC) was made. Deep and peripheral margins were cleared after 2 stages of MMS.

Initially described in 1954, HAC is an exceedingly rare adnexal tumor of apocrine and eccrine derivation.1 Historically, nomenclature for this entity has varied in the literature, including synonyms such as malignant nodular hidradenoma, malignant acrospiroma, solid-cystic adenocarcinoma, and malignant clear cell myoepithelioma.2,3 Approximately 6% of all malignant eccrine tumors worldwide are HACs, which account for only 1 in 13,000 dermatopathology specimens.1 These tumors may transform from clear cell hidradenomas (their benign counterparts) but more commonly arise de novo. Compared to benign hidradenomas, HACs are poorly circumscribed with infiltrative growth patterns on histopathology and may exhibit nuclear pleomorphism, prominent mitotic activity, necrosis, and perineural or vascular invasion.2
Clinically, HAC manifests as a 1- to 5-cm, solitary, firm, intradermal pink or violaceous nodule with possible ulceration.2,4 The nodule often is asymptomatic but may be tender, as in our patient. There seems to be no clear anatomic site of predilection, with approximately 42% of HACs localized to the head and neck and the remainder occurring on the trunk, arms, and legs.3,5-7 Females and males are affected equally, and lesions tend to arise in the seventh decade of life.7
Reports in the literature suggest that HAC is a very aggressive tumor with a generally poor prognosis.1 Several studies have found that up to half of tumors locally recur despite aggressive surgical management, and metastasis occurs in 20% to 60% of patients.3,8 However, a large study of US Surveillance, Epidemiology, and End Results data investigating the clinicopathologic characteristics of 289 patients with HAC revealed a more favorable prognosis.7 Mean overall survival and cancer-specific survival were greater than 13 years, and 10-year overall survival and cancer-specific survival rates were 60.2% and 90.5%, respectively.
Traditionally used to treat keratinocyte carcinomas, including basal cell carcinoma and squamous cell carcinoma, complete margin assessment with MMS is increasingly being utilized in the management of other cutaneous malignancies, including adnexal tumors.8 Due to its rarity, there remains no standard optimal treatment approach for HAC. One small retrospective study of 10 patients with HAC treated with MMS demonstrated favorable outcomes with no cases of recurrence, metastasis, or diseaserelated mortality in a mean 7-year follow-up period.9
Whole-body positron emission tomography/computed tomography performed in our patient approximately 1 month after MMS revealed mildly hypermetabolic left inguinal lymph nodes, which were thought to be reactive, and a question of small hypermetabolic foci in the liver. Follow-up computed tomography of the abdomen subsequently was performed and was negative for hepatic metastases. The patient will be monitored closely for local recurrence; however, the clearance of the tumor with MMS, which allowed complete margin assessment, is encouraging and supports MMS as superior to traditional surgical excision in the treatment of HAC. At his most recent examination 17 months after Mohs surgery, the patient remained tumor free.
Aggressive digital papillary adenocarcinoma (ADPA) is a rare malignant tumor originating in the sweat glands that can occur on the first toe but most commonly arises on the fingers. While both HAC and ADPA can manifest with an infiltrative growth pattern and cytologic atypia, ADPA classically reveals a well-circumscribed multinodular tumor in the dermis comprised of solid and cystic proliferation as well as papillary projections. In addition, ADPA has been described as having back-to-back glandular and ductal structures.10 Giant cell tumor of the tendon sheath is a benign fibrohistiocytic tumor that also typically manifests on the fingers but rarely can occur on the foot, including the first toe.11,12 This tumor is more common in women and most frequently affects individuals aged 30 to 50 years.12 Microscopically, giant cell tumor of the tendon sheath is characterized by a proliferation of osteoclastlike giant cells, epithelioid histiocytelike cells, mononuclear cells, and xanthomatous cells among collagenous bands.11
Osteosarcoma is an uncommon tumor of osteoidproducing cells that usually arises in the metaphysis of long bones and manifests as a tender subcutaneous mass. It has a bimodal age distribution, peaking in adolescents and adults older than 65 years.13 While very rare, osteosarcoma has been reported to occur in the bones of the feet, including the phalanges.14 Given the recurrent nature of our patient’s tumor, metastasis should always be considered; however, in his case, full-body imaging was negative for additional malignancy.
- Gauerke S, Driscoll JJ. Hidradenocarcinomas: a brief review and future directions. Arch Pathol Lab Med. 2010;134:781-785. doi:10.5858/134.5.781
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/J.HOC.2018.09.002
- Ohta M, Hiramoto M, Fujii M, et al. Nodular hidradenocarcinoma on the scalp of a young woman: case report and review of literature. Dermatol Surg. 2004;30:1265-1268. doi:10.1111/J.1524-4725.2004.30390.X
- Souvatzidis P, Sbano P, Mandato F, et al. Malignant nodular hidradenoma of the skin: report of seven cases. J Eur Acad Dermatol Venereol. 2008;22:549-554. doi:10.1111/J.1468-3083.2007.02504.X
- Yavel R, Hinshaw M, Rao V, et al. Hidradenomas and a hidradenocarcinoma of the scalp managed using Mohs micrographic surgery and a multidisciplinary approach: case reports and review of the literature. Dermatolog Surg. 2009;35:273-281. doi:10.1111/j.1524-4725.2008.34424.x
- Kazakov DV, Ivan D, Kutzner H, et al. Cutaneous hidradenocarcinoma: a clinicopathological, immunohistochemical, and molecular biologic study of 14 cases, including Her2/neu gene expression/ amplification, TP53 gene mutation analysis, and t(11;19) translocation. Am J Dermatopathol. 2009;31:236-247. doi:10.1097/DAD.0B013E3181984F10
- Gao T, Pan S, Li M, et al. Prognostic analysis of hidradenocarcinoma: a SEER-based observational study. Ann Med. 2022;54:454-463. doi:10 .1080/07853890.2022.2032313
- Tolkachjov SN. Adnexal carcinomas treated with Mohs micrographic surgery: a comprehensive review. Dermatol Surg. 2017;43:1199-1207. doi:10.1097/DSS.0000000000001167
- Tolkachjov SN, Hocker TL, Hochwalt PC, et al. Mohs micrographic surgery for the treatment of hidradenocarcinoma: the mayo clinic experience from 1993 to 2013. Dermatolog Surg. 2015;41:226-231. doi:10.1097/DSS.0000000000000242
- Weingertner N, Gressel A, Battistella M, et al. Aggressive digital papillary adenocarcinoma: a clinicopathological study of 19 cases. J Am Acad Dermatol. 2017;77:549-558.e1. doi:10.1016/J.JAAD.2017.02.028
- Paral KM, Petronic-Rosic V. Acral manifestations of soft tissue tumors. Clin Dermatol. 2017;35:85-98. doi:10.1016/J.CLINDER MATOL.2016.09.012
- Kondo RN, Crespigio J, Pavezzi PD, et al. Giant cell tumors of the tendon sheath in the left hallux. An Bras Dermatol. 2016;91:704-705. doi:10.1590/ABD1806-4841.20165769
- Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3-13. doi:10.1007/978-1-4419-0284-9_1
- Anninga JK, Picci P, Fiocco M, et al. Osteosarcoma of the hands and feet: a distinct clinico-pathological subgroup. Virchows Arch. 2013;462:109- 120. doi:10.1007/S00428-012-1339-3
- Gauerke S, Driscoll JJ. Hidradenocarcinomas: a brief review and future directions. Arch Pathol Lab Med. 2010;134:781-785. doi:10.5858/134.5.781
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/J.HOC.2018.09.002
- Ohta M, Hiramoto M, Fujii M, et al. Nodular hidradenocarcinoma on the scalp of a young woman: case report and review of literature. Dermatol Surg. 2004;30:1265-1268. doi:10.1111/J.1524-4725.2004.30390.X
- Souvatzidis P, Sbano P, Mandato F, et al. Malignant nodular hidradenoma of the skin: report of seven cases. J Eur Acad Dermatol Venereol. 2008;22:549-554. doi:10.1111/J.1468-3083.2007.02504.X
- Yavel R, Hinshaw M, Rao V, et al. Hidradenomas and a hidradenocarcinoma of the scalp managed using Mohs micrographic surgery and a multidisciplinary approach: case reports and review of the literature. Dermatolog Surg. 2009;35:273-281. doi:10.1111/j.1524-4725.2008.34424.x
- Kazakov DV, Ivan D, Kutzner H, et al. Cutaneous hidradenocarcinoma: a clinicopathological, immunohistochemical, and molecular biologic study of 14 cases, including Her2/neu gene expression/ amplification, TP53 gene mutation analysis, and t(11;19) translocation. Am J Dermatopathol. 2009;31:236-247. doi:10.1097/DAD.0B013E3181984F10
- Gao T, Pan S, Li M, et al. Prognostic analysis of hidradenocarcinoma: a SEER-based observational study. Ann Med. 2022;54:454-463. doi:10 .1080/07853890.2022.2032313
- Tolkachjov SN. Adnexal carcinomas treated with Mohs micrographic surgery: a comprehensive review. Dermatol Surg. 2017;43:1199-1207. doi:10.1097/DSS.0000000000001167
- Tolkachjov SN, Hocker TL, Hochwalt PC, et al. Mohs micrographic surgery for the treatment of hidradenocarcinoma: the mayo clinic experience from 1993 to 2013. Dermatolog Surg. 2015;41:226-231. doi:10.1097/DSS.0000000000000242
- Weingertner N, Gressel A, Battistella M, et al. Aggressive digital papillary adenocarcinoma: a clinicopathological study of 19 cases. J Am Acad Dermatol. 2017;77:549-558.e1. doi:10.1016/J.JAAD.2017.02.028
- Paral KM, Petronic-Rosic V. Acral manifestations of soft tissue tumors. Clin Dermatol. 2017;35:85-98. doi:10.1016/J.CLINDER MATOL.2016.09.012
- Kondo RN, Crespigio J, Pavezzi PD, et al. Giant cell tumors of the tendon sheath in the left hallux. An Bras Dermatol. 2016;91:704-705. doi:10.1590/ABD1806-4841.20165769
- Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3-13. doi:10.1007/978-1-4419-0284-9_1
- Anninga JK, Picci P, Fiocco M, et al. Osteosarcoma of the hands and feet: a distinct clinico-pathological subgroup. Virchows Arch. 2013;462:109- 120. doi:10.1007/S00428-012-1339-3
Recurrent Nodule on the First Toe
Recurrent Nodule on the First Toe
A 56-year-old man was referred to the dermatology clinic for treatment of a recurrent nodule on the left first toe. The lesion first appeared 12 years prior and was resected by an outside dermatologist, who diagnosed the lesion as benign based on biopsy results. Approximately 10 years later, the lesion began to grow back with a similar appearance to the original nodule; it again was diagnosed as benign based on another biopsy and excised by the outside dermatologist. Two years later, the patient had a second recurrence of the lesion, which was excised by his dermatologist. The biopsy report at that time identified the lesion as a low-grade adnexal neoplasm. The patient had a rapid recurrence of the tumor after 6 months and was referred to our clinic for Mohs micrographic surgery. Physical examination revealed a tender, 2.5×1.8-cm, firm, exophytic, subcutaneous nodule on the left first toe with no associated lymphadenopathy.
Impact of NSAID Use on Bleeding Rates for Patients Taking Rivaroxaban or Apixaban
Impact of NSAID Use on Bleeding Rates for Patients Taking Rivaroxaban or Apixaban
Clinical practice has shifted from vitamin K antagonists to direct oral anticoagulants (DOACs) for atrial fibrillation treatment due to their more favorable risk-benefit profile and less lifestyle modification required.1,2 However, the advantage of a lower bleeding risk with DOACs could be compromised by potentially problematic pharmacokinetic interactions like those conferred by antiplatelets or nonsteroidal anti-inflammatory drugs (NSAIDs).3,4 Treating a patient needing anticoagulation with a DOAC who has comorbidities may introduce unavoidable drug-drug interactions. This particularly happens with over-the-counter and prescription NSAIDs used for the management of pain and inflammatory conditions.5
NSAIDs primarily affect 2 cyclooxygenase (COX) enzyme isomers, COX-1 and COX-2.6 COX-1 helps maintain gastrointestinal (GI) mucosa integrity and platelet aggregation processes, whereas COX-2 is engaged in pain signaling and inflammation mediation. COX-1 inhibition is associated with more bleeding-related adverse events (AEs), especially in the GI tract. COX-2 inhibition is thought to provide analgesia and anti-inflammatory properties without elevating bleeding risk. This premise is responsible for the preferential use of celecoxib, a COX-2 selective NSAID, which should confer a lower bleeding risk compared to nonselective NSAIDs such as ibuprofen and naproxen.7 NSAIDs have been documented as independent risk factors for bleeding. NSAID users are about 3 times as likely to develop GI AEs compared to nonNSAID users.8
Many clinicians aim to further mitigate NSAID-associated bleeding risk by coprescribing a proton pump inhibitor (PPI). PPIs provide gastroprotection against NSAID-induced mucosal injury and sequential complication of GI bleeding. In a multicenter randomized control trial, patients who received concomitant PPI therapy while undergoing chronic NSAID therapy—including nonselective and COX-2 selective NSAIDs—had a significantly lower risk of GI ulcer development (placebo, 17.0%; 20 mg esomeprazole, 5.2%; 40 mg esomeprazole, 4.6%).9 Current clinical guidelines for preventing NSAIDassociated bleeding complications recommend using a COX-2 selective NSAID in combination with PPI therapy for patients at high risk for GI-related bleeding, including the concomitant use of anticoagulants.10
There is evidence suggesting an increased bleeding risk with NSAIDs when used in combination with vitamin K antagonists such as warfarin.11,12 A systematic review of warfarin and concomitant NSAID use found an increased risk of overall bleeding with NSAID use in combination with warfarin (odds ratio 1.58; 95% CI, 1.18-2.12), compared to warfarin alone.12
Posthoc analyses of randomized clinical trials have also demonstrated an increased bleeding risk with oral anticoagulation and concomitant NSAID use.13,14 In the RE-LY trial, NSAID users on warfarin or dabigatran had a statistically significant increased risk of major bleeding compared to non-NSAID users (hazard ratio [HR] 1.68; 95% CI, 1.40- 2.02; P < .001).13 In the ARISTOTLE trial, patients on warfarin or apixaban who were incident NSAID users were found to have an increased risk of major bleeding (HR 1.61; 95% CI, 1.11-2.33) and clinically relevant nonmajor bleeding (HR 1.70; 95% CI, 1.16- 2.48).14 These trials found a statistically significant increased bleeding risk associated with NSAID use, though the populations evaluated included patients taking warfarin and patients taking DOACs. These trials did not evaluate the bleeding risk of concomitant NSAID use among DOACs alone.
Evidence on NSAID-associated bleeding risk with DOACs is lacking in settings where the patient population, prescribing practices, and monitoring levels are variable. Within the Veterans Health Administration, clinical pharmacist practitioners (CPPs) in anticoagulation clinics oversee DOAC therapy management. CPPs monitor safety and efficacy of DOAC therapies through a population health management tool, the DOAC Dashboard.15 The DOAC Dashboard creates alerts for patients who may require an intervention based on certain clinical parameters, such as drug-drug interactions.16 Whenever a patient on a DOAC is prescribed an NSAID, an alert is generated on the DOAC Dashboard to flag the CPPs for the potential need for an intervention. If NSAID therapy remains clinically indicated, CPPs may recommend risk reduction strategies such as a COX-2 selective NSAID or coprescribing a PPI.10
The DOAC Dashboard provides an ideal setting for investigating the effects of NSAID use, NSAID selectivity, and PPI coprescribing on DOAC bleeding rates. With an increasing population of patients receiving anticoagulation therapy with a DOAC, more guidance regarding the bleeding risk of concomitant NSAID use with DOACs is needed. Studies evaluating the bleeding risk with concomitant NSAID use in patients on a DOAC alone are limited. This is the first study to date to compare bleeding risk with concomitant NSAID use between DOACs. This study provides information on bleeding risk with NSAID use among commonly prescribed DOACs, rivaroxaban and apixaban, and the potential impacts of current risk reduction strategies.
METHODS
This single-center retrospective cohort review was performed using the electronic health records (EHRs) of patients enrolled in the US Department of Veterans Affairs (VA) Mountain Home Healthcare System who received rivaroxaban or apixaban from December 2020 to December 2022. This study received approval from the East Tennessee State University/VA Institutional Review Board committee.
Patients were identified through the DOAC Dashboard, aged 21 to 100 years, and received rivaroxaban or apixaban at a therapeutic dose: rivaroxaban 10 to 20 mg daily or apixaban 2.5 to 5 mg twice daily. Patients were excluded if they were prescribed dual antiplatelet therapy, received rivaroxaban at dosing indicated for peripheral vascular disease, were undergoing dialysis, had evidence of moderate to severe hepatic impairment or any hepatic disease with coagulopathy, were undergoing chemotherapy or radiation, or had hematological conditions with predisposed bleeding risk. These patients were excluded to mitigate the potential confounding impact from nontherapeutic DOAC dosing strategies and conditions associated with an increased bleeding risk.
Eligible patients were stratified based on NSAID use. NSAID users were defined as patients prescribed an oral NSAID, including both acute and chronic courses, at any point during the study time frame while actively on a DOAC. Bleeding events were reviewed to evaluate rates between rivaroxaban and apixaban among NSAID and nonNSAID users. Identified NSAID users were further assessed for NSAID selectivity and PPI coprescribing as a subgroup analysis for the secondary assessment.
Data Collection
Baseline data were collected, including age, body mass index, anticoagulation indication, DOAC agent, DOAC dose, and DOAC total daily dose. Baseline serum creatinine levels, liver function tests, hemoglobin levels, and platelet counts were collected from the most recent data available immediately prior to the bleeding event, if applicable.
The DOAC Dashboard was reviewed for active and dismissed drug interaction alerts to identify patients taking rivaroxaban or apixaban who were prescribed an NSAID. Patients were categorized in the NSAID group if an interacting drug alert with an NSAID was reported during the study time frame. Data available through the interacting drug alerts on NSAID use were limited to the interacting drug name and date of the reported flag. Manual EHR review was required to confirm dates of NSAID therapy initiation and NSAID discontinuation, if applicable.
Data regarding concomitant antiplatelet use were obtained through review of the active and dismissed drug interaction alerts on the DOAC Dashboard. Concomitant antiplatelet use was defined as the prescribing of a single antiplatelet agent at any point while receiving DOAC therapy. Data on concomitant antiplatelets were collected regardless of NSAID status.
Data on coprescribed PPI therapy were obtained through manual EHR review of identified NSAID users. Coprescribed PPI therapy was defined as the prescribing of a PPI at any point during NSAID therapy. Data regarding PPI use among non-NSAID users were not collected because the secondary endpoint was designed to assess PPI use only among patients coprescribed a DOAC and NSAID.
Outcomes
Bleeding events were identified through an outcomes report generated by the DOAC Dashboard based on International Classification of Diseases, Tenth Revision diagnosis codes associated with a bleeding event. The outcomes report captures diagnoses from the outpatient and inpatient care settings. Reported bleeding events were limited to patients who received a DOAC at any point in the 6 months prior to the event and excluded patients with recent DOAC initiation within 7 days of the event, as these patients are not captured on the DOAC Dashboard.
All reported bleeding events were manually reviewed in the EHR and categorized as a major or clinically relevant nonmajor bleed, according to International Society of Thrombosis and Haemostasis criteria. Validated bleeding events were then crossreferenced with the interacting drug alerts report to identify events with potentially overlapping NSAID therapy at the time of the event. Overlapping NSAID therapy was defined as the prescribing of an NSAID at any point in the 6 months prior to the event. All events with potential overlapping NSAID therapies were manually reviewed for confirmation of NSAID status at the time of the event.
The primary endpoint was a composite of any bleeding event per International Society of Thrombosis and Haemostasis criteria. The secondary endpoint evaluated the potential impact of NSAID selectivity or PPI coprescribing on the bleeding rate among the NSAID user groups.
Statistical Analysis
Analyses were performed consistent with the methods used in the ARISTOTLE and RE-LY trials. It was determined that a sample size of 504 patients, with ≥ 168 patients in each group, would provide 80% power using a 2-sided a of 0.05. HRs with 95% CIs and respective P values were calculated using a SPSS-adapted online calculator.
RESULTS
The DOAC Dashboard identified 681 patients on rivaroxaban and 3225 patients on apixaban; 72 patients on rivaroxaban (10.6%) and 300 patients on apixaban (9.3%) were NSAID users. The mean age of NSAID users was 66.9 years in the rivaroxaban group and 72.4 years in the apixaban group. The mean age of non-NSAID users was 71.5 years in the rivaroxaban group and 75.6 years in the apixaban group. No appreciable differences were observed among subgroups in body mass index, renal function, hepatic function, hemoglobin, or platelet counts, and no statistically significant differences were identified (Table 1). Antiplatelet agents identified included aspirin, clopidogrel, prasugrel, and ticagrelor. Fifteen patients (20.3%) in the rivaroxaban group and 87 patients (28.7%) in the apixaban group had concomitant antiplatelet and NSAID use. Forty-five patients on rivaroxaban (60.8%) and 170 (55.9%) on apixaban were prescribed concomitant PPI and NSAID at baseline. Among non-NSAID users, there was concomitant antiplatelet use for 265 patients (43.6%) in the rivaroxaban group and 1401 patients (47.9%) in the apixaban group. Concomitant PPI use was identified among 63 patients (60.0%) taking selective NSAIDs and 182 (57.2%) taking nonselective NSAIDs.
A total of 423 courses of NSAIDs were identified: 85 NSAID courses in the rivaroxaban group and 338 NSAID courses in the apixaban group. Most NSAID courses involved a nonselective NSAID in the rivaroxaban and apixaban NSAID user groups: 75.2% (n = 318) aggregately compared to 71.8% (n = 61) and 76.0% (n = 257) in the rivaroxaban and apixaban groups, respectively. The most frequent NSAID courses identified were meloxicam (26.7%; n = 113), celecoxib (24.8%; n = 105), ibuprofen (19.1%; n = 81), and naproxen (13.5%; n = 57). Data regarding NSAID therapy initiation and discontinuation dates were not readily available. As a result, the duration of NSAID courses was not captured.
There was no statistically significant difference in bleeding rates between rivaroxaban and apixaban among NSAID users (HR 1.04; 95% CI, 0.98-1.12) or non-NSAID users (HR 1.15; 95% CI, 0.80-1.66) (Table 2). Apixaban non-NSAID users had a higher rate of major bleeds (HR 0.32; 95% CI, 0.17-0.61) while rivaroxaban non-NSAID users had a higher rate of clinically relevant nonmajor bleeds (HR 1.63; 95% CI, 1.10-2.54).
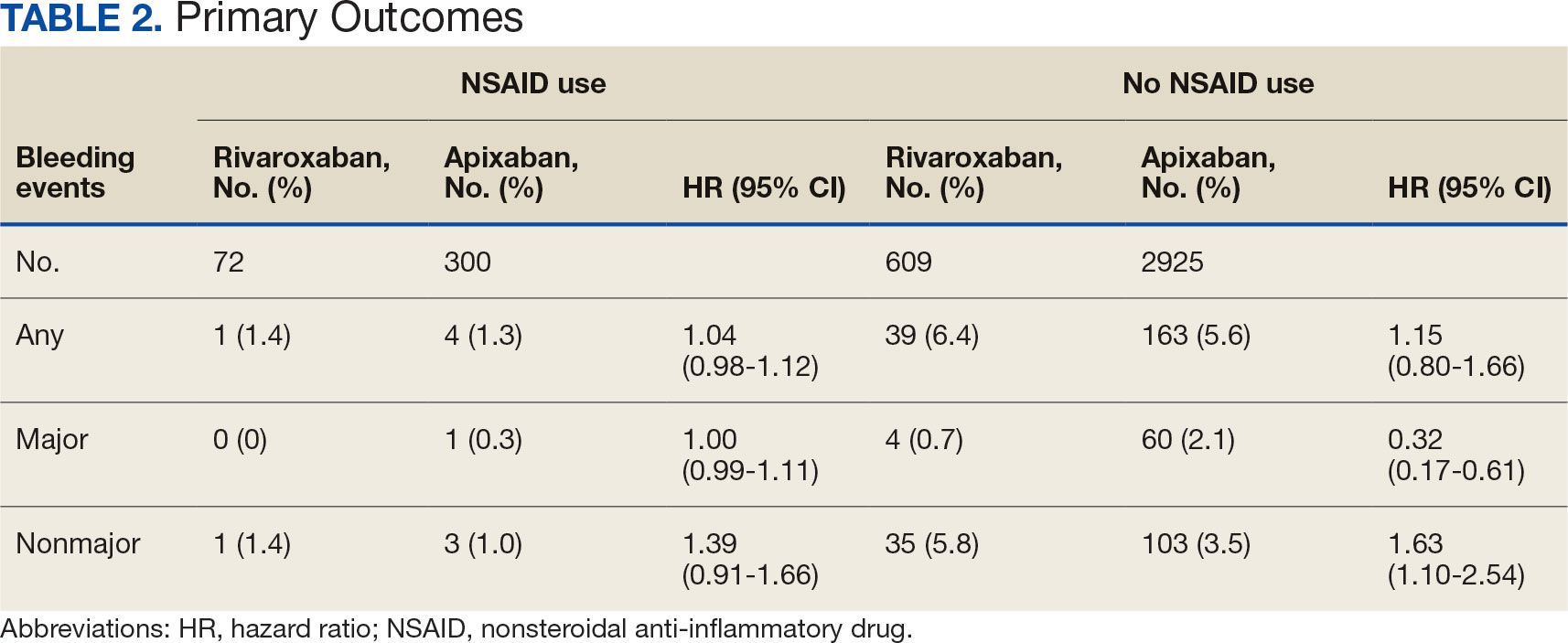
The sample size for the secondary endpoint consisted of bleeding events that were confirmed to have had an overlapping NSAID prescribed at the time of the event. For this secondary assessment, there was 1 rivaroxaban NSAID user bleeding event and 4 apixaban NSAID user bleeding events. For the rivaroxaban NSAID user bleeding event, the NSAID was nonselective and a PPI was not coprescribed. For the apixaban NSAID user bleeding events, 2 NSAIDs were nonselective and 2 were selective. All patients with apixaban and NSAID bleeding events had a coprescribed PPI. There was no clinically significant difference in the bleeding rates observed for NSAID selectivity or PPI coprescribing among the NSAID user subgroups.
DISCUSSION
This study found that there was no statistically significant difference for bleeding rates of major and nonmajor bleeding events between rivaroxaban and apixaban among NSAID users and non-NSAID users. This study did not identify a clinically significant impact on bleeding rates from NSAID selectivity or PPI coprescribing among the NSAID users.
There were notable but not statistically significant differences in baseline characteristics observed between the NSAID and non-NSAID user groups. On average, the rivaroxaban and apixaban NSAID users were younger compared with those not taking NSAIDs. NSAIDs, specifically nonselective NSAIDs, are recognized as potentially inappropriate medications for older adults given that this population is at an increased risk for GI ulcer development and/or GI bleeding.17 The non-NSAID user group likely consisted of older patients compared to the NSAID user group as clinicians may avoid prescribing NSAIDs to older adults regardless of concomitant DOAC therapy.
In addition to having an older patient population, non-NSAID users were more frequently prescribed a concomitant antiplatelet when compared with NSAID users. This prescribing pattern may be due to clinicians avoiding the use of NSAIDs in patients receiving DOAC therapy in combination with antiplatelet therapy, as these patients have been found to have an increased bleeding rate compared to DOAC therapy alone.18
Non-NSAID users had an overall higher bleeding rate for both major and nonmajor bleeding events. Based on this observation, it could be hypothesized that antiplatelet agents have a higher risk of bleeding in comparison to NSAIDs. In a subanalysis of the EXPAND study evaluating risk factors of major bleeding in patients receiving rivaroxaban, concomitant use of antiplatelet agents demonstrated a statistically significant increased risk of bleeding (HR 1.6; 95% CI, 1.2-2.3; P = .003) while concomitant use of NSAIDs did not (HR 0.8; 95% CI, 0.3-2.2; P = .67).19
In assessing PPI status at baseline, a majority of both rivaroxaban and apixaban NSAID users were coprescribed a PPI. This trend aligns with current clinical guideline recommendations for the prescribing of PPI therapy for GI protection in high-risk patients, such as those on DOAC therapy and concomitant NSAID therapy.10 Given the high proportion of NSAID users coprescribed a PPI at baseline, it may be possible that the true incidence of NSAID-associated bleeding events was higher than what this study found. This observation may reflect the impact from timely implementation of risk mitigation strategies by CPPs in the anticoagulation clinic. However, this study was not constructed to assess the efficacy of PPI use in this manner.
It is important to note the patients included in this study were followed by a pharmacist in an anticoagulation clinic using the DOAC Dashboard.15 This population management tool allows CPPs to make proactive interventions when a patient taking a DOAC receives an NSAID prescription, such as recommending the coprescribing of a PPI or use of a selective NSAID.10,16 These standards of care may have contributed to an overall reduced bleeding rate among the NSAID user group and may not be reflective of private practice.
The planned analysis of this study was modeled after the posthoc analysis of the RE-LY and ARISTOTLE trials. Both trials demonstrated an increased risk of bleeding with oral anticoagulation, including DOAC and warfarin, in combination with NSAID use. However, both trials found that NSAID use in patients treated with a DOAC was not independently associated with increased bleeding events compared with warfarin.13,14 The results of this study are comparable to the RE-LY and ARISTOTLE findings that NSAID use among patients treated with rivaroxaban or apixaban did not demonstrate a statistically significant increased bleeding risk.
Studies of NSAID use in combination with DOAC therapy have been limited to patient populations consisting of both DOAC and warfarin. Evidence from these trials outlines the increased bleeding risk associated with NSAID use in combination with oral anticoagulation; however, these patient populations include those on a DOAC and warfarin.13,14,19,20 Given the limited evidence on NSAID use among DOACs alone, it is assumed NSAID use in combination with DOACs has a similar risk of bleeding as warfarin use. This may cause clinicians to automatically exclude NSAID therapy as a treatment option for patients on a DOAC who are otherwise clinically appropriate candidates, such as those with underlying inflammatory conditions. Avoiding NSAID therapy in this patient population may lead to suboptimal pain management and increase the risk of patient harm from methods such as inappropriate opioid therapy prescribing.
DOAC therapy should not be a universal limitation to the use of NSAIDs. Although the risk of bleeding with NSAID therapy is always present, deliberate NSAID prescribing in addition to the timely implementation of risk mitigation strategies may provide an avenue for safe NSAID prescribing in patients receiving a DOAC. A population health-based approach to DOAC management, such as the DOAC Dashboard, appears to be effective at preventing patient harm when NSAIDs are prescribed in conjunction with DOACs.
Limitations
The DOAC Dashboard has been shown to be effective and efficient at monitoring DOAC therapy from a population-based approach.16 Reports generated through the DOAC Dashboard provide convenient access to patient data which allows for timely interventions; however, there are limits to its use for data collection. All the data elements necessary to properly assess bleeding risk with validated tools, such as HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/ alcohol concomitantly), are not available on DOAC Dashboard reports. Due to this constraint, bleeding risk assessments were not conducted at baseline and this study was unable to include risk modeling. Additionally, data elements like initiation and discontinuation dates and duration of therapies were not readily available. As a result, this study was unable to incorporate time as a data point.
This was a retrospective study that relied on manual review of chart documentation to verify bleeding events, but data obtained through the DOAC Dashboard were transferred directly from the EHR.15 Bleeding events available for evaluation were restricted to those that occurred at a VA facility. Additionally, the sample size within the rivaroxaban NSAID user group did not reach the predefined sample size required to reach power and may have been too small to detect a difference if one did exist. The secondary assessment had a low sample size of NSAID user bleeding events, making it difficult to fully assess its impact on NSAID selectivity and PPI coprescribing on bleeding rates. All courses of NSAIDs were equally valued regardless of the dose or therapy duration; however, this is consistent with how NSAID use was defined in the RE-LY and ARISTOTLE trials.
CONCLUSIONS
This retrospective cohort review found no statistically significant difference in the composite bleeding rates between rivaroxaban and apixaban among NSAID users and non-NSAID users. Moreover, there was no clinically significant impact observed for bleeding rates in regard to NSAID selectivity and PPI coprescribing among NSAID users. However, coprescribing of PPI therapy to patients on a DOAC who are clinically indicated for an NSAID may reduce the risk of bleeding. Population health management tools, such as the DOAC Dashboard, may also allow clinicians to safely prescribe NSAIDs to patients on a DOAC. Further large-scale observational studies are needed to quantify the real-world risk of bleeding with concomitant NSAID use among DOACs alone and to evaluate the impact from NSAID selectivity or PPI coprescribing.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e44S-e88S. doi:10.1378/chest.11-2292
- Eikelboom J, Merli G. Bleeding with direct oral anticoagulants vs warfarin: clinical experience. Am J Med. 2016;129(11S):S33-S40. doi:10.1016/j.amjmed.2016.06.003
- Vranckx P, Valgimigli M, Heidbuchel H. The significance of drug-drug and drug-food interactions of oral anticoagulation. Arrhythm Electrophysiol Rev. 2018;7(1):55-61. doi:10.15420/aer.2017.50.1
- Davis JS, Lee HY, Kim J, et al. Use of non-steroidal antiinflammatory drugs in US adults: changes over time and by demographic. Open Heart. 2017;4(1):e000550. doi:10.1136/openhrt-2016-000550
- Schafer AI. Effects of nonsteroidal antiinflammatory drugs on platelet function and systemic hemostasis. J Clin Pharmacol. 1995;35(3):209-219. doi:10.1002/j.1552-4604.1995.tb04050.x
- Al-Saeed A. Gastrointestinal and cardiovascular risk of nonsteroidal anti-inflammatory drugs. Oman Med J. 2011;26(6):385-391. doi:10.5001/omj.2011.101
- Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991;115(10):787-796. doi:10.7326/0003-4819-115-10-787
- Scheiman JM, Yeomans ND, Talley NJ, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006;101(4):701-710. doi:10.1111/j.1572-0241.2006.00499.x
- Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152(4):706-715. doi:10.1053/j.gastro.2017.01.031
- Lamberts M, Lip GYH, Hansen ML, et al. Relation of nonsteroidal anti-inflammatory drugs to serious bleeding and thromboembolism risk in patients with atrial fibrillation receiving antithrombotic therapy: a nationwide cohort study. Ann Intern Med. 2014;161(10):690-698. doi:10.7326/M13-1581
- Villa Zapata L, Hansten PD, Panic J, et al. Risk of bleeding with exposure to warfarin and nonsteroidal anti-inflammatory drugs: a systematic review and metaanalysis. Thromb Haemost. 2020;120(7):1066-1074. doi:10.1055/s-0040-1710592
- Kent AP, Brueckmann M, Fraessdorf M, et al. Concomitant oral anticoagulant and nonsteroidal anti-inflammatory drug therapy in patients with atrial fibrillation. J Am Coll Cardiol. 2018;72(3):255-267. doi:10.1016/j.jacc.2018.04.063
- Dalgaard F, Mulder H, Wojdyla DM, et al. Patients with atrial fibrillation taking nonsteroidal antiinflammatory drugs and oral anticoagulants in the ARISTOTLE Trial. Circulation. 2020;141(1):10-20. doi:10.1161/CIRCULATIONAHA.119.041296
- Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Asssoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
- . Valencia D, Spoutz P, Stoppi J, et al. Impact of a direct oral anticoagulant population management tool on anticoagulation therapy monitoring in clinical practice. Ann Pharmacother. 2019;53(8):806-811. doi:10.1177/1060028019835843
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Kumar S, Danik SB, Altman RK, et al. Non-vitamin K antagonist oral anticoagulants and antiplatelet therapy for stroke prevention in patients with atrial fibrillation. Cardiol Rev. 2016;24(5):218-223. doi:10.1097/CRD.0000000000000088
- Sakuma I, Uchiyama S, Atarashi H, et al. Clinical risk factors of stroke and major bleeding in patients with nonvalvular atrial fibrillation under rivaroxaban: the EXPAND study sub-analysis. Heart Vessels. 2019;34(11):1839-1851. doi:10.1007/s00380-019-01425-x
- Davidson BL, Verheijen S, Lensing AWA, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med. 2014;174(6):947-953. doi:10.1001/jamainternmed.2014.946
Clinical practice has shifted from vitamin K antagonists to direct oral anticoagulants (DOACs) for atrial fibrillation treatment due to their more favorable risk-benefit profile and less lifestyle modification required.1,2 However, the advantage of a lower bleeding risk with DOACs could be compromised by potentially problematic pharmacokinetic interactions like those conferred by antiplatelets or nonsteroidal anti-inflammatory drugs (NSAIDs).3,4 Treating a patient needing anticoagulation with a DOAC who has comorbidities may introduce unavoidable drug-drug interactions. This particularly happens with over-the-counter and prescription NSAIDs used for the management of pain and inflammatory conditions.5
NSAIDs primarily affect 2 cyclooxygenase (COX) enzyme isomers, COX-1 and COX-2.6 COX-1 helps maintain gastrointestinal (GI) mucosa integrity and platelet aggregation processes, whereas COX-2 is engaged in pain signaling and inflammation mediation. COX-1 inhibition is associated with more bleeding-related adverse events (AEs), especially in the GI tract. COX-2 inhibition is thought to provide analgesia and anti-inflammatory properties without elevating bleeding risk. This premise is responsible for the preferential use of celecoxib, a COX-2 selective NSAID, which should confer a lower bleeding risk compared to nonselective NSAIDs such as ibuprofen and naproxen.7 NSAIDs have been documented as independent risk factors for bleeding. NSAID users are about 3 times as likely to develop GI AEs compared to nonNSAID users.8
Many clinicians aim to further mitigate NSAID-associated bleeding risk by coprescribing a proton pump inhibitor (PPI). PPIs provide gastroprotection against NSAID-induced mucosal injury and sequential complication of GI bleeding. In a multicenter randomized control trial, patients who received concomitant PPI therapy while undergoing chronic NSAID therapy—including nonselective and COX-2 selective NSAIDs—had a significantly lower risk of GI ulcer development (placebo, 17.0%; 20 mg esomeprazole, 5.2%; 40 mg esomeprazole, 4.6%).9 Current clinical guidelines for preventing NSAIDassociated bleeding complications recommend using a COX-2 selective NSAID in combination with PPI therapy for patients at high risk for GI-related bleeding, including the concomitant use of anticoagulants.10
There is evidence suggesting an increased bleeding risk with NSAIDs when used in combination with vitamin K antagonists such as warfarin.11,12 A systematic review of warfarin and concomitant NSAID use found an increased risk of overall bleeding with NSAID use in combination with warfarin (odds ratio 1.58; 95% CI, 1.18-2.12), compared to warfarin alone.12
Posthoc analyses of randomized clinical trials have also demonstrated an increased bleeding risk with oral anticoagulation and concomitant NSAID use.13,14 In the RE-LY trial, NSAID users on warfarin or dabigatran had a statistically significant increased risk of major bleeding compared to non-NSAID users (hazard ratio [HR] 1.68; 95% CI, 1.40- 2.02; P < .001).13 In the ARISTOTLE trial, patients on warfarin or apixaban who were incident NSAID users were found to have an increased risk of major bleeding (HR 1.61; 95% CI, 1.11-2.33) and clinically relevant nonmajor bleeding (HR 1.70; 95% CI, 1.16- 2.48).14 These trials found a statistically significant increased bleeding risk associated with NSAID use, though the populations evaluated included patients taking warfarin and patients taking DOACs. These trials did not evaluate the bleeding risk of concomitant NSAID use among DOACs alone.
Evidence on NSAID-associated bleeding risk with DOACs is lacking in settings where the patient population, prescribing practices, and monitoring levels are variable. Within the Veterans Health Administration, clinical pharmacist practitioners (CPPs) in anticoagulation clinics oversee DOAC therapy management. CPPs monitor safety and efficacy of DOAC therapies through a population health management tool, the DOAC Dashboard.15 The DOAC Dashboard creates alerts for patients who may require an intervention based on certain clinical parameters, such as drug-drug interactions.16 Whenever a patient on a DOAC is prescribed an NSAID, an alert is generated on the DOAC Dashboard to flag the CPPs for the potential need for an intervention. If NSAID therapy remains clinically indicated, CPPs may recommend risk reduction strategies such as a COX-2 selective NSAID or coprescribing a PPI.10
The DOAC Dashboard provides an ideal setting for investigating the effects of NSAID use, NSAID selectivity, and PPI coprescribing on DOAC bleeding rates. With an increasing population of patients receiving anticoagulation therapy with a DOAC, more guidance regarding the bleeding risk of concomitant NSAID use with DOACs is needed. Studies evaluating the bleeding risk with concomitant NSAID use in patients on a DOAC alone are limited. This is the first study to date to compare bleeding risk with concomitant NSAID use between DOACs. This study provides information on bleeding risk with NSAID use among commonly prescribed DOACs, rivaroxaban and apixaban, and the potential impacts of current risk reduction strategies.
METHODS
This single-center retrospective cohort review was performed using the electronic health records (EHRs) of patients enrolled in the US Department of Veterans Affairs (VA) Mountain Home Healthcare System who received rivaroxaban or apixaban from December 2020 to December 2022. This study received approval from the East Tennessee State University/VA Institutional Review Board committee.
Patients were identified through the DOAC Dashboard, aged 21 to 100 years, and received rivaroxaban or apixaban at a therapeutic dose: rivaroxaban 10 to 20 mg daily or apixaban 2.5 to 5 mg twice daily. Patients were excluded if they were prescribed dual antiplatelet therapy, received rivaroxaban at dosing indicated for peripheral vascular disease, were undergoing dialysis, had evidence of moderate to severe hepatic impairment or any hepatic disease with coagulopathy, were undergoing chemotherapy or radiation, or had hematological conditions with predisposed bleeding risk. These patients were excluded to mitigate the potential confounding impact from nontherapeutic DOAC dosing strategies and conditions associated with an increased bleeding risk.
Eligible patients were stratified based on NSAID use. NSAID users were defined as patients prescribed an oral NSAID, including both acute and chronic courses, at any point during the study time frame while actively on a DOAC. Bleeding events were reviewed to evaluate rates between rivaroxaban and apixaban among NSAID and nonNSAID users. Identified NSAID users were further assessed for NSAID selectivity and PPI coprescribing as a subgroup analysis for the secondary assessment.
Data Collection
Baseline data were collected, including age, body mass index, anticoagulation indication, DOAC agent, DOAC dose, and DOAC total daily dose. Baseline serum creatinine levels, liver function tests, hemoglobin levels, and platelet counts were collected from the most recent data available immediately prior to the bleeding event, if applicable.
The DOAC Dashboard was reviewed for active and dismissed drug interaction alerts to identify patients taking rivaroxaban or apixaban who were prescribed an NSAID. Patients were categorized in the NSAID group if an interacting drug alert with an NSAID was reported during the study time frame. Data available through the interacting drug alerts on NSAID use were limited to the interacting drug name and date of the reported flag. Manual EHR review was required to confirm dates of NSAID therapy initiation and NSAID discontinuation, if applicable.
Data regarding concomitant antiplatelet use were obtained through review of the active and dismissed drug interaction alerts on the DOAC Dashboard. Concomitant antiplatelet use was defined as the prescribing of a single antiplatelet agent at any point while receiving DOAC therapy. Data on concomitant antiplatelets were collected regardless of NSAID status.
Data on coprescribed PPI therapy were obtained through manual EHR review of identified NSAID users. Coprescribed PPI therapy was defined as the prescribing of a PPI at any point during NSAID therapy. Data regarding PPI use among non-NSAID users were not collected because the secondary endpoint was designed to assess PPI use only among patients coprescribed a DOAC and NSAID.
Outcomes
Bleeding events were identified through an outcomes report generated by the DOAC Dashboard based on International Classification of Diseases, Tenth Revision diagnosis codes associated with a bleeding event. The outcomes report captures diagnoses from the outpatient and inpatient care settings. Reported bleeding events were limited to patients who received a DOAC at any point in the 6 months prior to the event and excluded patients with recent DOAC initiation within 7 days of the event, as these patients are not captured on the DOAC Dashboard.
All reported bleeding events were manually reviewed in the EHR and categorized as a major or clinically relevant nonmajor bleed, according to International Society of Thrombosis and Haemostasis criteria. Validated bleeding events were then crossreferenced with the interacting drug alerts report to identify events with potentially overlapping NSAID therapy at the time of the event. Overlapping NSAID therapy was defined as the prescribing of an NSAID at any point in the 6 months prior to the event. All events with potential overlapping NSAID therapies were manually reviewed for confirmation of NSAID status at the time of the event.
The primary endpoint was a composite of any bleeding event per International Society of Thrombosis and Haemostasis criteria. The secondary endpoint evaluated the potential impact of NSAID selectivity or PPI coprescribing on the bleeding rate among the NSAID user groups.
Statistical Analysis
Analyses were performed consistent with the methods used in the ARISTOTLE and RE-LY trials. It was determined that a sample size of 504 patients, with ≥ 168 patients in each group, would provide 80% power using a 2-sided a of 0.05. HRs with 95% CIs and respective P values were calculated using a SPSS-adapted online calculator.
RESULTS
The DOAC Dashboard identified 681 patients on rivaroxaban and 3225 patients on apixaban; 72 patients on rivaroxaban (10.6%) and 300 patients on apixaban (9.3%) were NSAID users. The mean age of NSAID users was 66.9 years in the rivaroxaban group and 72.4 years in the apixaban group. The mean age of non-NSAID users was 71.5 years in the rivaroxaban group and 75.6 years in the apixaban group. No appreciable differences were observed among subgroups in body mass index, renal function, hepatic function, hemoglobin, or platelet counts, and no statistically significant differences were identified (Table 1). Antiplatelet agents identified included aspirin, clopidogrel, prasugrel, and ticagrelor. Fifteen patients (20.3%) in the rivaroxaban group and 87 patients (28.7%) in the apixaban group had concomitant antiplatelet and NSAID use. Forty-five patients on rivaroxaban (60.8%) and 170 (55.9%) on apixaban were prescribed concomitant PPI and NSAID at baseline. Among non-NSAID users, there was concomitant antiplatelet use for 265 patients (43.6%) in the rivaroxaban group and 1401 patients (47.9%) in the apixaban group. Concomitant PPI use was identified among 63 patients (60.0%) taking selective NSAIDs and 182 (57.2%) taking nonselective NSAIDs.

A total of 423 courses of NSAIDs were identified: 85 NSAID courses in the rivaroxaban group and 338 NSAID courses in the apixaban group. Most NSAID courses involved a nonselective NSAID in the rivaroxaban and apixaban NSAID user groups: 75.2% (n = 318) aggregately compared to 71.8% (n = 61) and 76.0% (n = 257) in the rivaroxaban and apixaban groups, respectively. The most frequent NSAID courses identified were meloxicam (26.7%; n = 113), celecoxib (24.8%; n = 105), ibuprofen (19.1%; n = 81), and naproxen (13.5%; n = 57). Data regarding NSAID therapy initiation and discontinuation dates were not readily available. As a result, the duration of NSAID courses was not captured.
There was no statistically significant difference in bleeding rates between rivaroxaban and apixaban among NSAID users (HR 1.04; 95% CI, 0.98-1.12) or non-NSAID users (HR 1.15; 95% CI, 0.80-1.66) (Table 2). Apixaban non-NSAID users had a higher rate of major bleeds (HR 0.32; 95% CI, 0.17-0.61) while rivaroxaban non-NSAID users had a higher rate of clinically relevant nonmajor bleeds (HR 1.63; 95% CI, 1.10-2.54).

The sample size for the secondary endpoint consisted of bleeding events that were confirmed to have had an overlapping NSAID prescribed at the time of the event. For this secondary assessment, there was 1 rivaroxaban NSAID user bleeding event and 4 apixaban NSAID user bleeding events. For the rivaroxaban NSAID user bleeding event, the NSAID was nonselective and a PPI was not coprescribed. For the apixaban NSAID user bleeding events, 2 NSAIDs were nonselective and 2 were selective. All patients with apixaban and NSAID bleeding events had a coprescribed PPI. There was no clinically significant difference in the bleeding rates observed for NSAID selectivity or PPI coprescribing among the NSAID user subgroups.
DISCUSSION
This study found that there was no statistically significant difference for bleeding rates of major and nonmajor bleeding events between rivaroxaban and apixaban among NSAID users and non-NSAID users. This study did not identify a clinically significant impact on bleeding rates from NSAID selectivity or PPI coprescribing among the NSAID users.
There were notable but not statistically significant differences in baseline characteristics observed between the NSAID and non-NSAID user groups. On average, the rivaroxaban and apixaban NSAID users were younger compared with those not taking NSAIDs. NSAIDs, specifically nonselective NSAIDs, are recognized as potentially inappropriate medications for older adults given that this population is at an increased risk for GI ulcer development and/or GI bleeding.17 The non-NSAID user group likely consisted of older patients compared to the NSAID user group as clinicians may avoid prescribing NSAIDs to older adults regardless of concomitant DOAC therapy.
In addition to having an older patient population, non-NSAID users were more frequently prescribed a concomitant antiplatelet when compared with NSAID users. This prescribing pattern may be due to clinicians avoiding the use of NSAIDs in patients receiving DOAC therapy in combination with antiplatelet therapy, as these patients have been found to have an increased bleeding rate compared to DOAC therapy alone.18
Non-NSAID users had an overall higher bleeding rate for both major and nonmajor bleeding events. Based on this observation, it could be hypothesized that antiplatelet agents have a higher risk of bleeding in comparison to NSAIDs. In a subanalysis of the EXPAND study evaluating risk factors of major bleeding in patients receiving rivaroxaban, concomitant use of antiplatelet agents demonstrated a statistically significant increased risk of bleeding (HR 1.6; 95% CI, 1.2-2.3; P = .003) while concomitant use of NSAIDs did not (HR 0.8; 95% CI, 0.3-2.2; P = .67).19
In assessing PPI status at baseline, a majority of both rivaroxaban and apixaban NSAID users were coprescribed a PPI. This trend aligns with current clinical guideline recommendations for the prescribing of PPI therapy for GI protection in high-risk patients, such as those on DOAC therapy and concomitant NSAID therapy.10 Given the high proportion of NSAID users coprescribed a PPI at baseline, it may be possible that the true incidence of NSAID-associated bleeding events was higher than what this study found. This observation may reflect the impact from timely implementation of risk mitigation strategies by CPPs in the anticoagulation clinic. However, this study was not constructed to assess the efficacy of PPI use in this manner.
It is important to note the patients included in this study were followed by a pharmacist in an anticoagulation clinic using the DOAC Dashboard.15 This population management tool allows CPPs to make proactive interventions when a patient taking a DOAC receives an NSAID prescription, such as recommending the coprescribing of a PPI or use of a selective NSAID.10,16 These standards of care may have contributed to an overall reduced bleeding rate among the NSAID user group and may not be reflective of private practice.
The planned analysis of this study was modeled after the posthoc analysis of the RE-LY and ARISTOTLE trials. Both trials demonstrated an increased risk of bleeding with oral anticoagulation, including DOAC and warfarin, in combination with NSAID use. However, both trials found that NSAID use in patients treated with a DOAC was not independently associated with increased bleeding events compared with warfarin.13,14 The results of this study are comparable to the RE-LY and ARISTOTLE findings that NSAID use among patients treated with rivaroxaban or apixaban did not demonstrate a statistically significant increased bleeding risk.
Studies of NSAID use in combination with DOAC therapy have been limited to patient populations consisting of both DOAC and warfarin. Evidence from these trials outlines the increased bleeding risk associated with NSAID use in combination with oral anticoagulation; however, these patient populations include those on a DOAC and warfarin.13,14,19,20 Given the limited evidence on NSAID use among DOACs alone, it is assumed NSAID use in combination with DOACs has a similar risk of bleeding as warfarin use. This may cause clinicians to automatically exclude NSAID therapy as a treatment option for patients on a DOAC who are otherwise clinically appropriate candidates, such as those with underlying inflammatory conditions. Avoiding NSAID therapy in this patient population may lead to suboptimal pain management and increase the risk of patient harm from methods such as inappropriate opioid therapy prescribing.
DOAC therapy should not be a universal limitation to the use of NSAIDs. Although the risk of bleeding with NSAID therapy is always present, deliberate NSAID prescribing in addition to the timely implementation of risk mitigation strategies may provide an avenue for safe NSAID prescribing in patients receiving a DOAC. A population health-based approach to DOAC management, such as the DOAC Dashboard, appears to be effective at preventing patient harm when NSAIDs are prescribed in conjunction with DOACs.
Limitations
The DOAC Dashboard has been shown to be effective and efficient at monitoring DOAC therapy from a population-based approach.16 Reports generated through the DOAC Dashboard provide convenient access to patient data which allows for timely interventions; however, there are limits to its use for data collection. All the data elements necessary to properly assess bleeding risk with validated tools, such as HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/ alcohol concomitantly), are not available on DOAC Dashboard reports. Due to this constraint, bleeding risk assessments were not conducted at baseline and this study was unable to include risk modeling. Additionally, data elements like initiation and discontinuation dates and duration of therapies were not readily available. As a result, this study was unable to incorporate time as a data point.
This was a retrospective study that relied on manual review of chart documentation to verify bleeding events, but data obtained through the DOAC Dashboard were transferred directly from the EHR.15 Bleeding events available for evaluation were restricted to those that occurred at a VA facility. Additionally, the sample size within the rivaroxaban NSAID user group did not reach the predefined sample size required to reach power and may have been too small to detect a difference if one did exist. The secondary assessment had a low sample size of NSAID user bleeding events, making it difficult to fully assess its impact on NSAID selectivity and PPI coprescribing on bleeding rates. All courses of NSAIDs were equally valued regardless of the dose or therapy duration; however, this is consistent with how NSAID use was defined in the RE-LY and ARISTOTLE trials.
CONCLUSIONS
This retrospective cohort review found no statistically significant difference in the composite bleeding rates between rivaroxaban and apixaban among NSAID users and non-NSAID users. Moreover, there was no clinically significant impact observed for bleeding rates in regard to NSAID selectivity and PPI coprescribing among NSAID users. However, coprescribing of PPI therapy to patients on a DOAC who are clinically indicated for an NSAID may reduce the risk of bleeding. Population health management tools, such as the DOAC Dashboard, may also allow clinicians to safely prescribe NSAIDs to patients on a DOAC. Further large-scale observational studies are needed to quantify the real-world risk of bleeding with concomitant NSAID use among DOACs alone and to evaluate the impact from NSAID selectivity or PPI coprescribing.
Clinical practice has shifted from vitamin K antagonists to direct oral anticoagulants (DOACs) for atrial fibrillation treatment due to their more favorable risk-benefit profile and less lifestyle modification required.1,2 However, the advantage of a lower bleeding risk with DOACs could be compromised by potentially problematic pharmacokinetic interactions like those conferred by antiplatelets or nonsteroidal anti-inflammatory drugs (NSAIDs).3,4 Treating a patient needing anticoagulation with a DOAC who has comorbidities may introduce unavoidable drug-drug interactions. This particularly happens with over-the-counter and prescription NSAIDs used for the management of pain and inflammatory conditions.5
NSAIDs primarily affect 2 cyclooxygenase (COX) enzyme isomers, COX-1 and COX-2.6 COX-1 helps maintain gastrointestinal (GI) mucosa integrity and platelet aggregation processes, whereas COX-2 is engaged in pain signaling and inflammation mediation. COX-1 inhibition is associated with more bleeding-related adverse events (AEs), especially in the GI tract. COX-2 inhibition is thought to provide analgesia and anti-inflammatory properties without elevating bleeding risk. This premise is responsible for the preferential use of celecoxib, a COX-2 selective NSAID, which should confer a lower bleeding risk compared to nonselective NSAIDs such as ibuprofen and naproxen.7 NSAIDs have been documented as independent risk factors for bleeding. NSAID users are about 3 times as likely to develop GI AEs compared to nonNSAID users.8
Many clinicians aim to further mitigate NSAID-associated bleeding risk by coprescribing a proton pump inhibitor (PPI). PPIs provide gastroprotection against NSAID-induced mucosal injury and sequential complication of GI bleeding. In a multicenter randomized control trial, patients who received concomitant PPI therapy while undergoing chronic NSAID therapy—including nonselective and COX-2 selective NSAIDs—had a significantly lower risk of GI ulcer development (placebo, 17.0%; 20 mg esomeprazole, 5.2%; 40 mg esomeprazole, 4.6%).9 Current clinical guidelines for preventing NSAIDassociated bleeding complications recommend using a COX-2 selective NSAID in combination with PPI therapy for patients at high risk for GI-related bleeding, including the concomitant use of anticoagulants.10
There is evidence suggesting an increased bleeding risk with NSAIDs when used in combination with vitamin K antagonists such as warfarin.11,12 A systematic review of warfarin and concomitant NSAID use found an increased risk of overall bleeding with NSAID use in combination with warfarin (odds ratio 1.58; 95% CI, 1.18-2.12), compared to warfarin alone.12
Posthoc analyses of randomized clinical trials have also demonstrated an increased bleeding risk with oral anticoagulation and concomitant NSAID use.13,14 In the RE-LY trial, NSAID users on warfarin or dabigatran had a statistically significant increased risk of major bleeding compared to non-NSAID users (hazard ratio [HR] 1.68; 95% CI, 1.40- 2.02; P < .001).13 In the ARISTOTLE trial, patients on warfarin or apixaban who were incident NSAID users were found to have an increased risk of major bleeding (HR 1.61; 95% CI, 1.11-2.33) and clinically relevant nonmajor bleeding (HR 1.70; 95% CI, 1.16- 2.48).14 These trials found a statistically significant increased bleeding risk associated with NSAID use, though the populations evaluated included patients taking warfarin and patients taking DOACs. These trials did not evaluate the bleeding risk of concomitant NSAID use among DOACs alone.
Evidence on NSAID-associated bleeding risk with DOACs is lacking in settings where the patient population, prescribing practices, and monitoring levels are variable. Within the Veterans Health Administration, clinical pharmacist practitioners (CPPs) in anticoagulation clinics oversee DOAC therapy management. CPPs monitor safety and efficacy of DOAC therapies through a population health management tool, the DOAC Dashboard.15 The DOAC Dashboard creates alerts for patients who may require an intervention based on certain clinical parameters, such as drug-drug interactions.16 Whenever a patient on a DOAC is prescribed an NSAID, an alert is generated on the DOAC Dashboard to flag the CPPs for the potential need for an intervention. If NSAID therapy remains clinically indicated, CPPs may recommend risk reduction strategies such as a COX-2 selective NSAID or coprescribing a PPI.10
The DOAC Dashboard provides an ideal setting for investigating the effects of NSAID use, NSAID selectivity, and PPI coprescribing on DOAC bleeding rates. With an increasing population of patients receiving anticoagulation therapy with a DOAC, more guidance regarding the bleeding risk of concomitant NSAID use with DOACs is needed. Studies evaluating the bleeding risk with concomitant NSAID use in patients on a DOAC alone are limited. This is the first study to date to compare bleeding risk with concomitant NSAID use between DOACs. This study provides information on bleeding risk with NSAID use among commonly prescribed DOACs, rivaroxaban and apixaban, and the potential impacts of current risk reduction strategies.
METHODS
This single-center retrospective cohort review was performed using the electronic health records (EHRs) of patients enrolled in the US Department of Veterans Affairs (VA) Mountain Home Healthcare System who received rivaroxaban or apixaban from December 2020 to December 2022. This study received approval from the East Tennessee State University/VA Institutional Review Board committee.
Patients were identified through the DOAC Dashboard, aged 21 to 100 years, and received rivaroxaban or apixaban at a therapeutic dose: rivaroxaban 10 to 20 mg daily or apixaban 2.5 to 5 mg twice daily. Patients were excluded if they were prescribed dual antiplatelet therapy, received rivaroxaban at dosing indicated for peripheral vascular disease, were undergoing dialysis, had evidence of moderate to severe hepatic impairment or any hepatic disease with coagulopathy, were undergoing chemotherapy or radiation, or had hematological conditions with predisposed bleeding risk. These patients were excluded to mitigate the potential confounding impact from nontherapeutic DOAC dosing strategies and conditions associated with an increased bleeding risk.
Eligible patients were stratified based on NSAID use. NSAID users were defined as patients prescribed an oral NSAID, including both acute and chronic courses, at any point during the study time frame while actively on a DOAC. Bleeding events were reviewed to evaluate rates between rivaroxaban and apixaban among NSAID and nonNSAID users. Identified NSAID users were further assessed for NSAID selectivity and PPI coprescribing as a subgroup analysis for the secondary assessment.
Data Collection
Baseline data were collected, including age, body mass index, anticoagulation indication, DOAC agent, DOAC dose, and DOAC total daily dose. Baseline serum creatinine levels, liver function tests, hemoglobin levels, and platelet counts were collected from the most recent data available immediately prior to the bleeding event, if applicable.
The DOAC Dashboard was reviewed for active and dismissed drug interaction alerts to identify patients taking rivaroxaban or apixaban who were prescribed an NSAID. Patients were categorized in the NSAID group if an interacting drug alert with an NSAID was reported during the study time frame. Data available through the interacting drug alerts on NSAID use were limited to the interacting drug name and date of the reported flag. Manual EHR review was required to confirm dates of NSAID therapy initiation and NSAID discontinuation, if applicable.
Data regarding concomitant antiplatelet use were obtained through review of the active and dismissed drug interaction alerts on the DOAC Dashboard. Concomitant antiplatelet use was defined as the prescribing of a single antiplatelet agent at any point while receiving DOAC therapy. Data on concomitant antiplatelets were collected regardless of NSAID status.
Data on coprescribed PPI therapy were obtained through manual EHR review of identified NSAID users. Coprescribed PPI therapy was defined as the prescribing of a PPI at any point during NSAID therapy. Data regarding PPI use among non-NSAID users were not collected because the secondary endpoint was designed to assess PPI use only among patients coprescribed a DOAC and NSAID.
Outcomes
Bleeding events were identified through an outcomes report generated by the DOAC Dashboard based on International Classification of Diseases, Tenth Revision diagnosis codes associated with a bleeding event. The outcomes report captures diagnoses from the outpatient and inpatient care settings. Reported bleeding events were limited to patients who received a DOAC at any point in the 6 months prior to the event and excluded patients with recent DOAC initiation within 7 days of the event, as these patients are not captured on the DOAC Dashboard.
All reported bleeding events were manually reviewed in the EHR and categorized as a major or clinically relevant nonmajor bleed, according to International Society of Thrombosis and Haemostasis criteria. Validated bleeding events were then crossreferenced with the interacting drug alerts report to identify events with potentially overlapping NSAID therapy at the time of the event. Overlapping NSAID therapy was defined as the prescribing of an NSAID at any point in the 6 months prior to the event. All events with potential overlapping NSAID therapies were manually reviewed for confirmation of NSAID status at the time of the event.
The primary endpoint was a composite of any bleeding event per International Society of Thrombosis and Haemostasis criteria. The secondary endpoint evaluated the potential impact of NSAID selectivity or PPI coprescribing on the bleeding rate among the NSAID user groups.
Statistical Analysis
Analyses were performed consistent with the methods used in the ARISTOTLE and RE-LY trials. It was determined that a sample size of 504 patients, with ≥ 168 patients in each group, would provide 80% power using a 2-sided a of 0.05. HRs with 95% CIs and respective P values were calculated using a SPSS-adapted online calculator.
RESULTS
The DOAC Dashboard identified 681 patients on rivaroxaban and 3225 patients on apixaban; 72 patients on rivaroxaban (10.6%) and 300 patients on apixaban (9.3%) were NSAID users. The mean age of NSAID users was 66.9 years in the rivaroxaban group and 72.4 years in the apixaban group. The mean age of non-NSAID users was 71.5 years in the rivaroxaban group and 75.6 years in the apixaban group. No appreciable differences were observed among subgroups in body mass index, renal function, hepatic function, hemoglobin, or platelet counts, and no statistically significant differences were identified (Table 1). Antiplatelet agents identified included aspirin, clopidogrel, prasugrel, and ticagrelor. Fifteen patients (20.3%) in the rivaroxaban group and 87 patients (28.7%) in the apixaban group had concomitant antiplatelet and NSAID use. Forty-five patients on rivaroxaban (60.8%) and 170 (55.9%) on apixaban were prescribed concomitant PPI and NSAID at baseline. Among non-NSAID users, there was concomitant antiplatelet use for 265 patients (43.6%) in the rivaroxaban group and 1401 patients (47.9%) in the apixaban group. Concomitant PPI use was identified among 63 patients (60.0%) taking selective NSAIDs and 182 (57.2%) taking nonselective NSAIDs.

A total of 423 courses of NSAIDs were identified: 85 NSAID courses in the rivaroxaban group and 338 NSAID courses in the apixaban group. Most NSAID courses involved a nonselective NSAID in the rivaroxaban and apixaban NSAID user groups: 75.2% (n = 318) aggregately compared to 71.8% (n = 61) and 76.0% (n = 257) in the rivaroxaban and apixaban groups, respectively. The most frequent NSAID courses identified were meloxicam (26.7%; n = 113), celecoxib (24.8%; n = 105), ibuprofen (19.1%; n = 81), and naproxen (13.5%; n = 57). Data regarding NSAID therapy initiation and discontinuation dates were not readily available. As a result, the duration of NSAID courses was not captured.
There was no statistically significant difference in bleeding rates between rivaroxaban and apixaban among NSAID users (HR 1.04; 95% CI, 0.98-1.12) or non-NSAID users (HR 1.15; 95% CI, 0.80-1.66) (Table 2). Apixaban non-NSAID users had a higher rate of major bleeds (HR 0.32; 95% CI, 0.17-0.61) while rivaroxaban non-NSAID users had a higher rate of clinically relevant nonmajor bleeds (HR 1.63; 95% CI, 1.10-2.54).

The sample size for the secondary endpoint consisted of bleeding events that were confirmed to have had an overlapping NSAID prescribed at the time of the event. For this secondary assessment, there was 1 rivaroxaban NSAID user bleeding event and 4 apixaban NSAID user bleeding events. For the rivaroxaban NSAID user bleeding event, the NSAID was nonselective and a PPI was not coprescribed. For the apixaban NSAID user bleeding events, 2 NSAIDs were nonselective and 2 were selective. All patients with apixaban and NSAID bleeding events had a coprescribed PPI. There was no clinically significant difference in the bleeding rates observed for NSAID selectivity or PPI coprescribing among the NSAID user subgroups.
DISCUSSION
This study found that there was no statistically significant difference for bleeding rates of major and nonmajor bleeding events between rivaroxaban and apixaban among NSAID users and non-NSAID users. This study did not identify a clinically significant impact on bleeding rates from NSAID selectivity or PPI coprescribing among the NSAID users.
There were notable but not statistically significant differences in baseline characteristics observed between the NSAID and non-NSAID user groups. On average, the rivaroxaban and apixaban NSAID users were younger compared with those not taking NSAIDs. NSAIDs, specifically nonselective NSAIDs, are recognized as potentially inappropriate medications for older adults given that this population is at an increased risk for GI ulcer development and/or GI bleeding.17 The non-NSAID user group likely consisted of older patients compared to the NSAID user group as clinicians may avoid prescribing NSAIDs to older adults regardless of concomitant DOAC therapy.
In addition to having an older patient population, non-NSAID users were more frequently prescribed a concomitant antiplatelet when compared with NSAID users. This prescribing pattern may be due to clinicians avoiding the use of NSAIDs in patients receiving DOAC therapy in combination with antiplatelet therapy, as these patients have been found to have an increased bleeding rate compared to DOAC therapy alone.18
Non-NSAID users had an overall higher bleeding rate for both major and nonmajor bleeding events. Based on this observation, it could be hypothesized that antiplatelet agents have a higher risk of bleeding in comparison to NSAIDs. In a subanalysis of the EXPAND study evaluating risk factors of major bleeding in patients receiving rivaroxaban, concomitant use of antiplatelet agents demonstrated a statistically significant increased risk of bleeding (HR 1.6; 95% CI, 1.2-2.3; P = .003) while concomitant use of NSAIDs did not (HR 0.8; 95% CI, 0.3-2.2; P = .67).19
In assessing PPI status at baseline, a majority of both rivaroxaban and apixaban NSAID users were coprescribed a PPI. This trend aligns with current clinical guideline recommendations for the prescribing of PPI therapy for GI protection in high-risk patients, such as those on DOAC therapy and concomitant NSAID therapy.10 Given the high proportion of NSAID users coprescribed a PPI at baseline, it may be possible that the true incidence of NSAID-associated bleeding events was higher than what this study found. This observation may reflect the impact from timely implementation of risk mitigation strategies by CPPs in the anticoagulation clinic. However, this study was not constructed to assess the efficacy of PPI use in this manner.
It is important to note the patients included in this study were followed by a pharmacist in an anticoagulation clinic using the DOAC Dashboard.15 This population management tool allows CPPs to make proactive interventions when a patient taking a DOAC receives an NSAID prescription, such as recommending the coprescribing of a PPI or use of a selective NSAID.10,16 These standards of care may have contributed to an overall reduced bleeding rate among the NSAID user group and may not be reflective of private practice.
The planned analysis of this study was modeled after the posthoc analysis of the RE-LY and ARISTOTLE trials. Both trials demonstrated an increased risk of bleeding with oral anticoagulation, including DOAC and warfarin, in combination with NSAID use. However, both trials found that NSAID use in patients treated with a DOAC was not independently associated with increased bleeding events compared with warfarin.13,14 The results of this study are comparable to the RE-LY and ARISTOTLE findings that NSAID use among patients treated with rivaroxaban or apixaban did not demonstrate a statistically significant increased bleeding risk.
Studies of NSAID use in combination with DOAC therapy have been limited to patient populations consisting of both DOAC and warfarin. Evidence from these trials outlines the increased bleeding risk associated with NSAID use in combination with oral anticoagulation; however, these patient populations include those on a DOAC and warfarin.13,14,19,20 Given the limited evidence on NSAID use among DOACs alone, it is assumed NSAID use in combination with DOACs has a similar risk of bleeding as warfarin use. This may cause clinicians to automatically exclude NSAID therapy as a treatment option for patients on a DOAC who are otherwise clinically appropriate candidates, such as those with underlying inflammatory conditions. Avoiding NSAID therapy in this patient population may lead to suboptimal pain management and increase the risk of patient harm from methods such as inappropriate opioid therapy prescribing.
DOAC therapy should not be a universal limitation to the use of NSAIDs. Although the risk of bleeding with NSAID therapy is always present, deliberate NSAID prescribing in addition to the timely implementation of risk mitigation strategies may provide an avenue for safe NSAID prescribing in patients receiving a DOAC. A population health-based approach to DOAC management, such as the DOAC Dashboard, appears to be effective at preventing patient harm when NSAIDs are prescribed in conjunction with DOACs.
Limitations
The DOAC Dashboard has been shown to be effective and efficient at monitoring DOAC therapy from a population-based approach.16 Reports generated through the DOAC Dashboard provide convenient access to patient data which allows for timely interventions; however, there are limits to its use for data collection. All the data elements necessary to properly assess bleeding risk with validated tools, such as HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/ alcohol concomitantly), are not available on DOAC Dashboard reports. Due to this constraint, bleeding risk assessments were not conducted at baseline and this study was unable to include risk modeling. Additionally, data elements like initiation and discontinuation dates and duration of therapies were not readily available. As a result, this study was unable to incorporate time as a data point.
This was a retrospective study that relied on manual review of chart documentation to verify bleeding events, but data obtained through the DOAC Dashboard were transferred directly from the EHR.15 Bleeding events available for evaluation were restricted to those that occurred at a VA facility. Additionally, the sample size within the rivaroxaban NSAID user group did not reach the predefined sample size required to reach power and may have been too small to detect a difference if one did exist. The secondary assessment had a low sample size of NSAID user bleeding events, making it difficult to fully assess its impact on NSAID selectivity and PPI coprescribing on bleeding rates. All courses of NSAIDs were equally valued regardless of the dose or therapy duration; however, this is consistent with how NSAID use was defined in the RE-LY and ARISTOTLE trials.
CONCLUSIONS
This retrospective cohort review found no statistically significant difference in the composite bleeding rates between rivaroxaban and apixaban among NSAID users and non-NSAID users. Moreover, there was no clinically significant impact observed for bleeding rates in regard to NSAID selectivity and PPI coprescribing among NSAID users. However, coprescribing of PPI therapy to patients on a DOAC who are clinically indicated for an NSAID may reduce the risk of bleeding. Population health management tools, such as the DOAC Dashboard, may also allow clinicians to safely prescribe NSAIDs to patients on a DOAC. Further large-scale observational studies are needed to quantify the real-world risk of bleeding with concomitant NSAID use among DOACs alone and to evaluate the impact from NSAID selectivity or PPI coprescribing.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e44S-e88S. doi:10.1378/chest.11-2292
- Eikelboom J, Merli G. Bleeding with direct oral anticoagulants vs warfarin: clinical experience. Am J Med. 2016;129(11S):S33-S40. doi:10.1016/j.amjmed.2016.06.003
- Vranckx P, Valgimigli M, Heidbuchel H. The significance of drug-drug and drug-food interactions of oral anticoagulation. Arrhythm Electrophysiol Rev. 2018;7(1):55-61. doi:10.15420/aer.2017.50.1
- Davis JS, Lee HY, Kim J, et al. Use of non-steroidal antiinflammatory drugs in US adults: changes over time and by demographic. Open Heart. 2017;4(1):e000550. doi:10.1136/openhrt-2016-000550
- Schafer AI. Effects of nonsteroidal antiinflammatory drugs on platelet function and systemic hemostasis. J Clin Pharmacol. 1995;35(3):209-219. doi:10.1002/j.1552-4604.1995.tb04050.x
- Al-Saeed A. Gastrointestinal and cardiovascular risk of nonsteroidal anti-inflammatory drugs. Oman Med J. 2011;26(6):385-391. doi:10.5001/omj.2011.101
- Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991;115(10):787-796. doi:10.7326/0003-4819-115-10-787
- Scheiman JM, Yeomans ND, Talley NJ, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006;101(4):701-710. doi:10.1111/j.1572-0241.2006.00499.x
- Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152(4):706-715. doi:10.1053/j.gastro.2017.01.031
- Lamberts M, Lip GYH, Hansen ML, et al. Relation of nonsteroidal anti-inflammatory drugs to serious bleeding and thromboembolism risk in patients with atrial fibrillation receiving antithrombotic therapy: a nationwide cohort study. Ann Intern Med. 2014;161(10):690-698. doi:10.7326/M13-1581
- Villa Zapata L, Hansten PD, Panic J, et al. Risk of bleeding with exposure to warfarin and nonsteroidal anti-inflammatory drugs: a systematic review and metaanalysis. Thromb Haemost. 2020;120(7):1066-1074. doi:10.1055/s-0040-1710592
- Kent AP, Brueckmann M, Fraessdorf M, et al. Concomitant oral anticoagulant and nonsteroidal anti-inflammatory drug therapy in patients with atrial fibrillation. J Am Coll Cardiol. 2018;72(3):255-267. doi:10.1016/j.jacc.2018.04.063
- Dalgaard F, Mulder H, Wojdyla DM, et al. Patients with atrial fibrillation taking nonsteroidal antiinflammatory drugs and oral anticoagulants in the ARISTOTLE Trial. Circulation. 2020;141(1):10-20. doi:10.1161/CIRCULATIONAHA.119.041296
- Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Asssoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
- . Valencia D, Spoutz P, Stoppi J, et al. Impact of a direct oral anticoagulant population management tool on anticoagulation therapy monitoring in clinical practice. Ann Pharmacother. 2019;53(8):806-811. doi:10.1177/1060028019835843
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Kumar S, Danik SB, Altman RK, et al. Non-vitamin K antagonist oral anticoagulants and antiplatelet therapy for stroke prevention in patients with atrial fibrillation. Cardiol Rev. 2016;24(5):218-223. doi:10.1097/CRD.0000000000000088
- Sakuma I, Uchiyama S, Atarashi H, et al. Clinical risk factors of stroke and major bleeding in patients with nonvalvular atrial fibrillation under rivaroxaban: the EXPAND study sub-analysis. Heart Vessels. 2019;34(11):1839-1851. doi:10.1007/s00380-019-01425-x
- Davidson BL, Verheijen S, Lensing AWA, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med. 2014;174(6):947-953. doi:10.1001/jamainternmed.2014.946
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e44S-e88S. doi:10.1378/chest.11-2292
- Eikelboom J, Merli G. Bleeding with direct oral anticoagulants vs warfarin: clinical experience. Am J Med. 2016;129(11S):S33-S40. doi:10.1016/j.amjmed.2016.06.003
- Vranckx P, Valgimigli M, Heidbuchel H. The significance of drug-drug and drug-food interactions of oral anticoagulation. Arrhythm Electrophysiol Rev. 2018;7(1):55-61. doi:10.15420/aer.2017.50.1
- Davis JS, Lee HY, Kim J, et al. Use of non-steroidal antiinflammatory drugs in US adults: changes over time and by demographic. Open Heart. 2017;4(1):e000550. doi:10.1136/openhrt-2016-000550
- Schafer AI. Effects of nonsteroidal antiinflammatory drugs on platelet function and systemic hemostasis. J Clin Pharmacol. 1995;35(3):209-219. doi:10.1002/j.1552-4604.1995.tb04050.x
- Al-Saeed A. Gastrointestinal and cardiovascular risk of nonsteroidal anti-inflammatory drugs. Oman Med J. 2011;26(6):385-391. doi:10.5001/omj.2011.101
- Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991;115(10):787-796. doi:10.7326/0003-4819-115-10-787
- Scheiman JM, Yeomans ND, Talley NJ, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006;101(4):701-710. doi:10.1111/j.1572-0241.2006.00499.x
- Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152(4):706-715. doi:10.1053/j.gastro.2017.01.031
- Lamberts M, Lip GYH, Hansen ML, et al. Relation of nonsteroidal anti-inflammatory drugs to serious bleeding and thromboembolism risk in patients with atrial fibrillation receiving antithrombotic therapy: a nationwide cohort study. Ann Intern Med. 2014;161(10):690-698. doi:10.7326/M13-1581
- Villa Zapata L, Hansten PD, Panic J, et al. Risk of bleeding with exposure to warfarin and nonsteroidal anti-inflammatory drugs: a systematic review and metaanalysis. Thromb Haemost. 2020;120(7):1066-1074. doi:10.1055/s-0040-1710592
- Kent AP, Brueckmann M, Fraessdorf M, et al. Concomitant oral anticoagulant and nonsteroidal anti-inflammatory drug therapy in patients with atrial fibrillation. J Am Coll Cardiol. 2018;72(3):255-267. doi:10.1016/j.jacc.2018.04.063
- Dalgaard F, Mulder H, Wojdyla DM, et al. Patients with atrial fibrillation taking nonsteroidal antiinflammatory drugs and oral anticoagulants in the ARISTOTLE Trial. Circulation. 2020;141(1):10-20. doi:10.1161/CIRCULATIONAHA.119.041296
- Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Asssoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
- . Valencia D, Spoutz P, Stoppi J, et al. Impact of a direct oral anticoagulant population management tool on anticoagulation therapy monitoring in clinical practice. Ann Pharmacother. 2019;53(8):806-811. doi:10.1177/1060028019835843
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Kumar S, Danik SB, Altman RK, et al. Non-vitamin K antagonist oral anticoagulants and antiplatelet therapy for stroke prevention in patients with atrial fibrillation. Cardiol Rev. 2016;24(5):218-223. doi:10.1097/CRD.0000000000000088
- Sakuma I, Uchiyama S, Atarashi H, et al. Clinical risk factors of stroke and major bleeding in patients with nonvalvular atrial fibrillation under rivaroxaban: the EXPAND study sub-analysis. Heart Vessels. 2019;34(11):1839-1851. doi:10.1007/s00380-019-01425-x
- Davidson BL, Verheijen S, Lensing AWA, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med. 2014;174(6):947-953. doi:10.1001/jamainternmed.2014.946
Impact of NSAID Use on Bleeding Rates for Patients Taking Rivaroxaban or Apixaban
Impact of NSAID Use on Bleeding Rates for Patients Taking Rivaroxaban or Apixaban
Physician Attitudes About Veterans Affairs Video Connect Encounters
Physician Attitudes About Veterans Affairs Video Connect Encounters
Prior to the COVID-19 pandemic, health care systems had been increasingly focused on expanding care delivery through clinical video telehealth (CVT) services.1-3 These modalities offer clinicians and patients opportunities to interact without needing face-to-face visits. CVT services offer significant advantages to patients who encounter challenges accessing traditional face-to-face services, including those living in rural or underserved areas, individuals with mobility limitations, and those with difficulty attending appointments due to work or caregiving commitments.4 The COVID-19 pandemic accelerated the expansion of CVT services to mitigate the spread of the virus.1
Despite its evident advantages, widespread adoption of CVT has encountered resistance.2 Physicians have frequently expressed concerns about the reliability and functionality of CVT platforms for scheduled encounters and frustration with inadequate training.4-6 Additionally, there is a lack trust in the technology, as physicians are unfamiliar with reimbursement or workload capture associated with CVT. Physicians have concerns that telecommunication may diminish the intangible aspects of the “art of medicine.”4 As a result, the implementation of telehealth services has been inconsistent, with successful adoption limited to specific medical and surgical specialties.4 Only recently have entire departments within major health care systems expressed interest in providing comprehensive CVT services in response to the challenges posed by the COVID-19 pandemic.4
The Veterans Health Administration (VHA) of the US Department of Veterans Affairs (VA) provides an appropriate setting for assessing clinician perceptions of telehealth services. Since 2003, the VHA has significantly expanded CVT services to eligible veterans and has used the VA Video Connect (VVC) platform since 2018.7-10 Through VVC, VA staff and clinicians may schedule video visits with patients, meet with patients through virtual face-to-face interaction, and share relevant laboratory results and imaging through screen sharing. Prior research has shown increased accessibility to care through VVC. For example, a single-site study demonstrated that VVC implementation for delivering psychotherapies significantly increased CVT encounters from 15% to 85% among veterans with anxiety and/or depression.11
The VA New Mexico Healthcare System (VANMHCS) serves a high volume of veterans living in remote and rural regions and significantly increased its use of CVT during the COVID-19 pandemic to reduce in-person visits. Expectedly, this was met with a variety of challenges. Herein, we sought to assess physician perspectives, concerns, and attitudes toward VVC via semistructured interviews. Our hypothesis was that VA physicians may feel uncomfortable with video encounters but recognize the growing importance of such practices providing specialty care to veterans in rural areas.
METHODS
A semistructured interview protocol was created following discussions with physicians from the VANMHCS Medicine Service. Questions were constructed to assess the following domains: overarching views of video telehealth, perceptions of various applications for conducting VVC encounters, and barriers to the broad implementation of video telehealth. A qualitative investigation specialist aided with question development. Two pilot interviews were conducted prior to performing the interviews with the recruited participants to evaluate the quality and delivery of questions.
All VANMHCS physicians who provided outpatient care within the Department of Medicine and had completed ≥ 1 VVC encounter were eligible to participate. Invitations were disseminated via email, and follow-up emails to encourage participation were sent periodically for 2 months following the initial request. Union approval was obtained to interview employees for a research study. In total, 64 physicians were invited and 13 (20%) chose to participate. As the study did not involve assessing medical interventions among patients, a waiver of informed consent was granted by the VANMHCS Institutional Review Board. Physicians who participated in this study were informed that their responses would be used for reporting purposes and could be rescinded at any time.
Data Analysis
Semistructured interviews were conducted by a single interviewer and recorded using Microsoft Teams. The interviews took place between February 2021 and December 2021 and lasted 5 to 15 minutes, with a mean duration of 9 minutes. Verbal informed consent was obtained from all participants before the interviews. Interviewees were encouraged to expand on their responses to structured questions by recounting past experiences with VVC. Recorded audio was additionally transcribed via Microsoft Teams, and the research team reviewed the transcriptions to ensure accuracy.
The tracking and coding of responses to interview questions were conducted using Microsoft Excel. Initially, 5 transcripts were reviewed and responses were assessed by 2 study team members through open coding. All team members examined the 5 coded transcripts to identify differences and reach a consensus for any discrepancies. Based on recommendations from all team members regarding nuanced excerpts of transcripts, 1 study team member coded the remaining interviews. Thematic analysis was subsequently conducted according to the method described by Braun and Clarke.12 Themes were developed both deductively and inductively by reviewing the direct responses to interview questions and identifying emerging patterns of data, respectively. Indicative quotes representing each theme were carefully chosen for reporting.
RESULTS
Thirteen interviews were conducted and 9 participants (69%) were female. Participating physicians included 3 internal medicine/primary care physicians (23%), 2 nephrologists (15%), and 1 (8%) from cardiology, endocrinology, hematology, infectious diseases, palliative care, critical care, pulmonology, and sleep medicine. Years of post training experience among physicians ranged from 1 to 9 years (n = 5, 38%), 10 to 19 years (n = 3, 23%), and . 20 years (n = 5, 38%). Seven participants (54%) had conducted ≥ 5 VVC visits, with 1 physician completing > 50 video visits (Table).
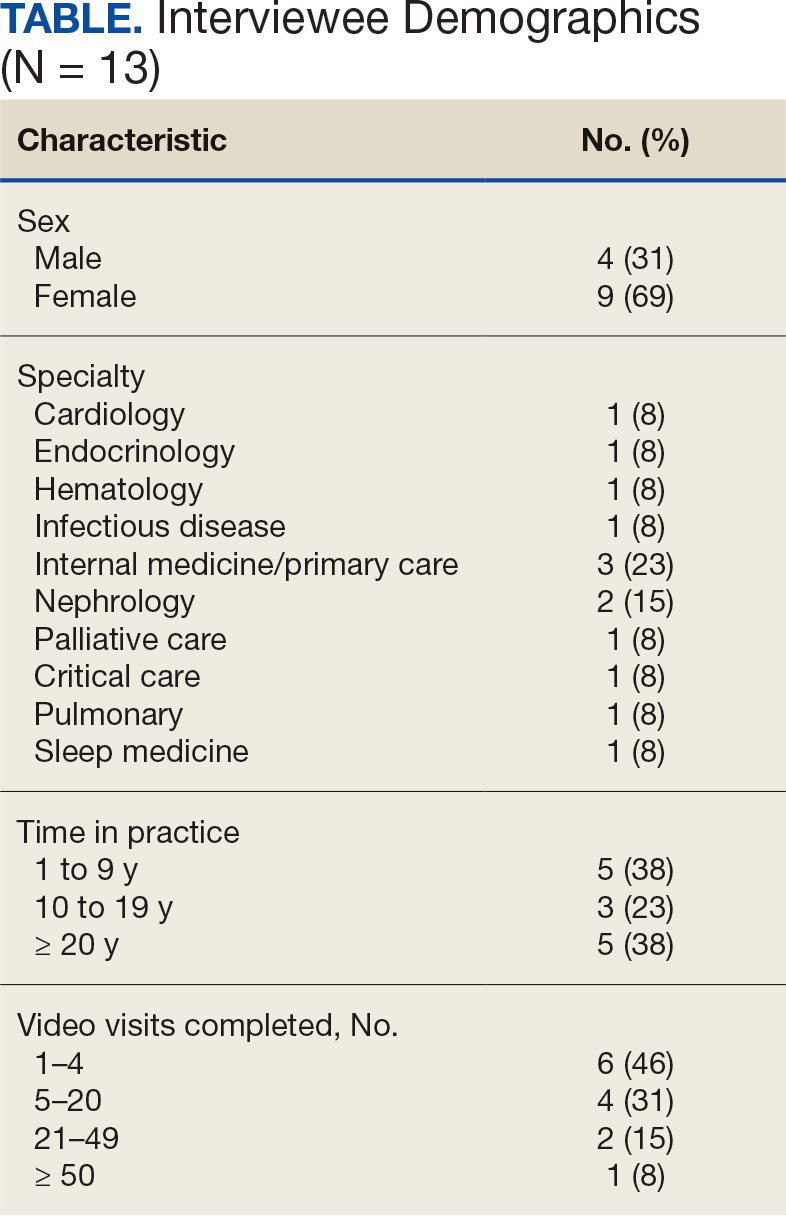
Using open coding and a deductive approach to thematic analysis, 5 themes were identified: (1) VVC software and internet connection issues affected implementation; (2) patient technological literacy affected veteran and physician comfort with VVC; (3) integration of supportive measures was desired; (4) CVT services may increasingly be used to enhance access to care; and (5) in-person encounters afforded unique advantages over CVT. Illustrative quotes from physicians that reflect these themes can be found in the Appendix.
Theme 1: VVC software and internet connection issues affected its implementation. Most participants expressed concern about the technical challenges with VVC. Interviewees cited inconsistencies for both patients and physicians receiving emails with links to join VVC visits, which should be generated when appointments are scheduled. Some physicians were unaware of scheduled VVC visits until the day of the appointment and only received the link via email. Such issues appeared to occur regardless whether the physicians or support staff scheduled the encounter. Poor video and audio quality was also cited as significant barriers to successful VVC visits and were often not resolvable through troubleshooting efforts by physicians, patients, or support personnel. Given the limited time allotted to each patient encounter, such issues could significantly impact the physician’s ability to remain on schedule. Moreover, connectivity problems led to significant lapses, delays in audio and video transmission, and complete disconnections from the VVC encounter. This was a significant concern for participants, given the rural nature of New Mexico and the large geographical gaps in internet service throughout the state.
Theme 2: Patient technological literacy affected veteran and physician comfort with VVC. Successful VVC appointments require high-speed Internet and compatible hardware. Physicians indicated that some patients reported difficulties with critical steps in the process, such as logging into the VVC platform or ensuring their microphones and cameras were active. Physicians also expressed concern about older veterans’ ability to utilize electronic devices, noting they may generally be less technology savvy. Additionally, physicians reported that despite offering the option of a virtual visit, many veterans preferred in-person visits, regardless of the drive time required. This appeared related to a fear of using the technology, which led veterans to believe that virtual visits do not provide the same quality of care as in-person visits.
Theme 3: Integration of supportive measures is desired. Interviewees felt that integrated VVC technical assistance and technology literacy education were imperative. First, training the patient or the patient’s caregiver on how to complete a VVC encounter using the preferred device and the VVC platform would be beneficial. Second, education to inform physicians about common troubleshooting issues could help streamline VVC encounters. Third, managing a VVC encounter similarly to standard in-person visits could allow for better patient and physician experience. For example, physicians suggested that a medical assistant or a nurse triage the patient, take vital signs, and set them up in a room, potentially at a regional VA community based outpatient clinic. Such efforts would also allow patients to receive specialty care in remote areas where only primary care is generally offered. Support staff could assist with technological issues, such as setting up the VVC encounter and addressing potential problems before the physician joins the encounter, thereby preventing delays in patient care. Finally, physicians felt that designating a day solely for CVT visits would help prevent disruption in care with in-person visits.
Theme 4: CVT services may increasingly be used to enhance access to care. Physicians felt that VVC would help patients encountering obstacles in accessing conventional in person services, including patients in rural and underserved areas, with disabilities, or with scheduling challenges.4 Patients with chronic conditions might drive the use of virtual visits, as many of these patients are already accustomed to remote medical monitoring. Data from devices such as scales and continuous glucose monitors can be easily reviewed during VVC visits. Second, video encounters facilitate closer monitoring that some patients might otherwise skip due to significant travel barriers, especially in a rural state like New Mexico. Lastly, VVC may be more efficient than in person visits as they eliminate the need for lengthy parking, checking in, and checking out processes. Thus, if technological issues are resolved, a typical physician’s day in the clinic may be more efficient with virtual visits.
Theme 5: In-person encounters afforded unique advantages over CVT. Some physicians felt in-person visits still offer unique advantages. They opined that the selection of appropriate candidates for CVT is critical. Patients requiring a physical examination should be scheduled for in person visits. For example, patients with advanced chronic kidney disease who require accurate volume status assessment or patients who have recently undergone surgery and need detailed wound inspection should be seen in the clinic. In-person visits may also be preferable for patients with recurrent admissions, or those whose condition is difficult to assess; accurate assessments of such patients may help prevent readmissions. Finally, many patients are more comfortable and satisfied with in-person visits, which are perceived as a more standard or traditional process. Respondents noted that some patients felt physicians may not focus as much attention during a VVC visit as they do during in-person visits. There were also concerns that some patients feel more motivation to come to in-person visits, as they see the VA as a place to interact with other veterans and staff with whom they are familiar and comfortable.
DISCUSSION
VANMHCS physicians, which serves veterans across an expansive territory ranging from Southern Colorado to West Texas. About 4.6 million veterans reside in rural regions, constituting roughly 25% of the total veteran population, a pattern mirrored in New Mexico.13 Medicine Service physicians agreed on a number of themes: VVC user-interface issues may affect its use and effectiveness, technological literacy was important for both patients and health care staff, technical support staff roles before and during VVC visits should be standardized, CVT is likely to increase health care delivery, and in-person encounters are preferred for many patients.
This is the first study to qualitatively evaluate a diverse group of physicians at a VA medical center incorporating CVT services across specialties. A few related qualitative studies have been conducted external to VHA, generally evaluating clinicians within a single specialty. Kalicki and colleagues surveyed 16 physicians working at a large home-based primary care program in New York City between April and June 2020 to identify and explore barriers to telehealth among homebound older adults. Similarly to our study, physicians noted that many patients required assistance (family members or caregivers) with the visit, either due to technological literacy issues or medical conditions like dementia.14
Heyer and colleagues surveyed 29 oncologists at an urban academic center prior to the COVID-19 pandemic. Similar to our observations, the oncologists said telemedicine helped eliminate travel as a barrier to health care. Heyer and colleagues noted difficulty for oncologists in performing virtual physical examinations, despite training. This group did note the benefits when being selective as to which clinical issues they would handle virtually vs in person.15
Budhwani and colleagues reported that mental health professionals in an academic setting cited difficulty establishing therapeutic relationships via telehealth and felt that this affected quality of care.16 While this was not a topic during our interviews, it is reasonable to question how potentially missed nonverbal cues may impact patient assessments.
Notably, technological issues were common among all reviewed studies. These ranged from internet connectivity issues to necessary electronic devices. As mentioned, these barriers are more prevalent in rural states like New Mexico.
Limitations
All participants in this study were Medicine Service physicians of a single VA health care system, which may limit generalizability. Many of our respondents were female (69%), compared with 39.2% of active internal medicine physicians and therefore may not be representative.17 Nearly one-half of our participants only completed 1 to 4 VVC encounters, which may have contributed to the emergence of a common theme regarding technological issues. Physicians with more experience with CVT services may be more skilled at troubleshooting technological issues that arise during visits.
CONCLUSIONS
Our study, conducted with VANMHCS physicians, illuminated 5 key themes influencing the use and implementation of video encounters: technological issues, technological literacy, a desire for integrated support measures, perceived future growth of video telehealth, and the unique advantages of in-person visits. Addressing technological barriers and providing more extensive training may streamline CVT use. However, it is vital to recognize the unique benefits of in-person visits and consider the benefits of each modality along with patient preferences when selecting the best care venue. As health care evolves, better understanding and acting upon these themes will optimize telehealth services, particularly in rural areas. Future research should involve patients and other health care team members to further explore strategies for effective CVT service integration.
Appendix
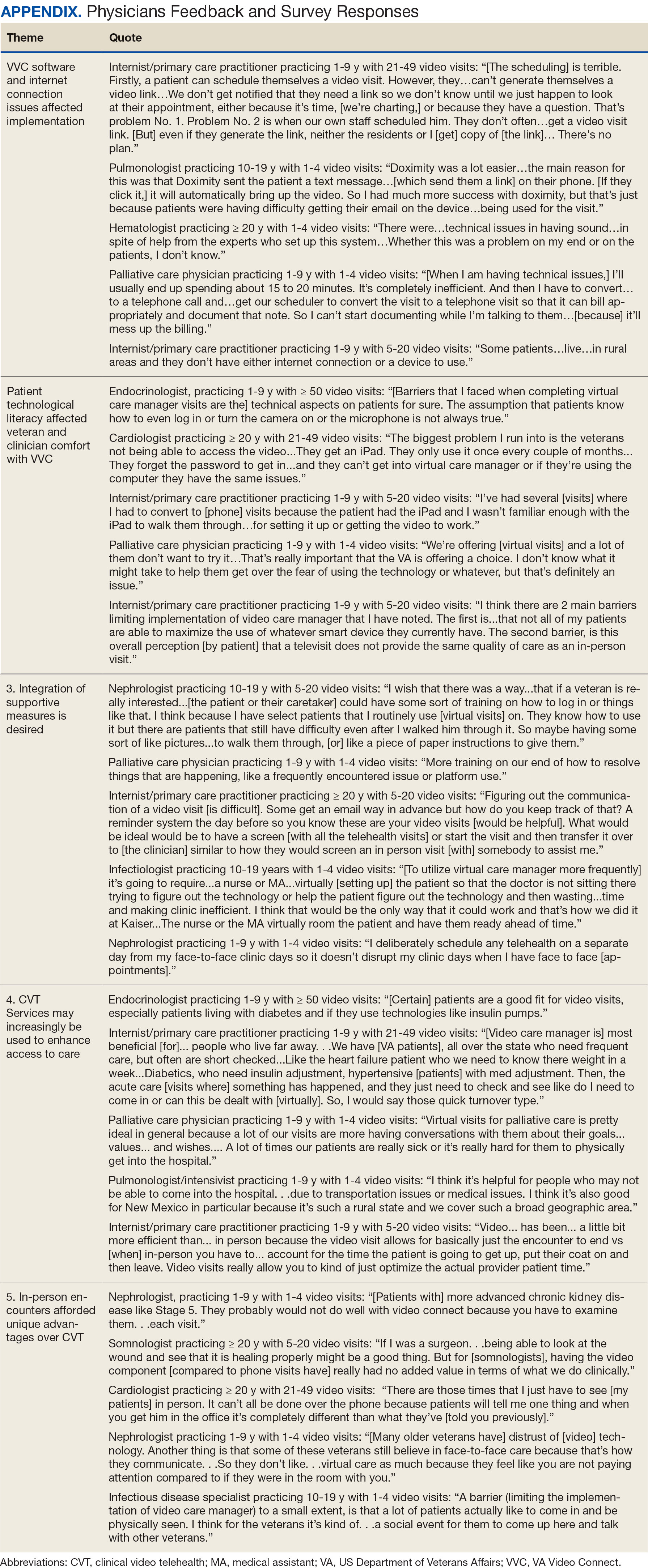
- Monaghesh E, Hajizadeh A. The role of telehealth during covid-19 outbreak: a systematic review based on current evidence. BMC Public Health. 2020;20(1):1193. doi:10.1186/s12889-020-09301-4
- Scott Kruse C, Karem P, Shifflett K, Vegi L, Ravi K, Brooks M. Evaluating barriers to adopting telemedicine worldwide: a systematic review. J Telemed Telecare. 2018;24(1):4-12. doi:10.1177/1357633X16674087
- Bashshur RL, Howell JD, Krupinski EA, Harms KM, Bashshur N, Doarn CR. The empirical foundations of telemedicine interventions in primary care. Telemed J E Health. 2016;22(5):342-375. doi:10.1089/tmj.2016.0045
- Yellowlees P, Nakagawa K, Pakyurek M, Hanson A, Elder J, Kales HC. Rapid conversion of an outpatient psychiatric clinic to a 100% virtual telepsychiatry clinic in response to covid-19. Pyschiatr Serv. 2020;71(7):749-752. doi:10.1176/appi.ps.202000230
- Hailey D, Ohinmaa A, Roine R. Study quality and evidence of benefit in recent assessments of telemedicine. J Telemed Telecare. 2004;10(6):318-324. doi:10.1258/1357633042602053
- Osuji TA, Macias M, McMullen C, et al. Clinician perspectives on implementing video visits in home-based palliative care. Palliat Med Rep. 2020;1(1):221-226. doi:10.1089/pmr.2020.0074
- Darkins A. The growth of telehealth services in the Veterans Health Administration between 1994 and 2014: a study in the diffusion of innovation. Telemed J E Health. 2014;20(9):761-768. doi:10.1089/tmj.2014.0143
- Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375(2):154-161. doi:10.1056/nejmra1601705
- Alexander NB, Phillips K, Wagner-Felkey J, et al. Team VA video connect (VVC) to optimize mobility and physical activity in post-hospital discharge older veterans: Baseline assessment. BMC Geriatr. 2021;21(1):502. doi:10.1186/s12877-021-02454-w
- Padala KP, Wilson KB, Gauss CH, Stovall JD, Padala PR. VA video connect for clinical care in older adults in a rural state during the covid-19 pandemic: cross-sectional study. J Med Internet Res. 2020;22(9)e21561. doi:10.2196/21561
- Myers US, Coulon S, Knies K, et al. Lessons learned in implementing VA video connect for evidence-based psychotherapies for anxiety and depression in the veterans healthcare administration. J Technol Behav Sci. 2020;6(2):320-326. doi:10.1007/s41347-020-00161-8
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77-101. doi:10.1191/1478088706qp063oa
- US Department of Veterans Affairs, National Center for Feterans Analysis and Statistics. Accessed September 18, 2024. www.va.gov/vetdata/report.asp
- Kalicki AV, Moody KA, Franzosa E, Gliatto PM, Ornstein KA. Barriers to telehealth access among homebound older adults. J Am Geriatr Soc. 2021;69(9):2404-2411. doi:10.1111/jgs.17163
- Heyer A, Granberg RE, Rising KL, Binder AF, Gentsch AT, Handley NR. Medical oncology professionals’ perceptions of telehealth video visits. JAMA Netw Open. 2021;4(1) e2033967. doi:10.1001/jamanetworkopen.2020.33967
- Budhwani S, Fujioka JK, Chu C, et al. Delivering mental health care virtually during the COVID-19 pandemic: qualitative evaluation of provider experiences in a scaled context. JMIR Form Res. 2021;5(9)e30280. doi:10.2196/30280
- Association of American Medical Colleges. Active physicians by sex and specialty, 2021. AAMC. Accessed September 18, 2024. https://www.aamc.org/data-reports/workforce/data/active-physicians-sex-specialty-2021
Prior to the COVID-19 pandemic, health care systems had been increasingly focused on expanding care delivery through clinical video telehealth (CVT) services.1-3 These modalities offer clinicians and patients opportunities to interact without needing face-to-face visits. CVT services offer significant advantages to patients who encounter challenges accessing traditional face-to-face services, including those living in rural or underserved areas, individuals with mobility limitations, and those with difficulty attending appointments due to work or caregiving commitments.4 The COVID-19 pandemic accelerated the expansion of CVT services to mitigate the spread of the virus.1
Despite its evident advantages, widespread adoption of CVT has encountered resistance.2 Physicians have frequently expressed concerns about the reliability and functionality of CVT platforms for scheduled encounters and frustration with inadequate training.4-6 Additionally, there is a lack trust in the technology, as physicians are unfamiliar with reimbursement or workload capture associated with CVT. Physicians have concerns that telecommunication may diminish the intangible aspects of the “art of medicine.”4 As a result, the implementation of telehealth services has been inconsistent, with successful adoption limited to specific medical and surgical specialties.4 Only recently have entire departments within major health care systems expressed interest in providing comprehensive CVT services in response to the challenges posed by the COVID-19 pandemic.4
The Veterans Health Administration (VHA) of the US Department of Veterans Affairs (VA) provides an appropriate setting for assessing clinician perceptions of telehealth services. Since 2003, the VHA has significantly expanded CVT services to eligible veterans and has used the VA Video Connect (VVC) platform since 2018.7-10 Through VVC, VA staff and clinicians may schedule video visits with patients, meet with patients through virtual face-to-face interaction, and share relevant laboratory results and imaging through screen sharing. Prior research has shown increased accessibility to care through VVC. For example, a single-site study demonstrated that VVC implementation for delivering psychotherapies significantly increased CVT encounters from 15% to 85% among veterans with anxiety and/or depression.11
The VA New Mexico Healthcare System (VANMHCS) serves a high volume of veterans living in remote and rural regions and significantly increased its use of CVT during the COVID-19 pandemic to reduce in-person visits. Expectedly, this was met with a variety of challenges. Herein, we sought to assess physician perspectives, concerns, and attitudes toward VVC via semistructured interviews. Our hypothesis was that VA physicians may feel uncomfortable with video encounters but recognize the growing importance of such practices providing specialty care to veterans in rural areas.
METHODS
A semistructured interview protocol was created following discussions with physicians from the VANMHCS Medicine Service. Questions were constructed to assess the following domains: overarching views of video telehealth, perceptions of various applications for conducting VVC encounters, and barriers to the broad implementation of video telehealth. A qualitative investigation specialist aided with question development. Two pilot interviews were conducted prior to performing the interviews with the recruited participants to evaluate the quality and delivery of questions.
All VANMHCS physicians who provided outpatient care within the Department of Medicine and had completed ≥ 1 VVC encounter were eligible to participate. Invitations were disseminated via email, and follow-up emails to encourage participation were sent periodically for 2 months following the initial request. Union approval was obtained to interview employees for a research study. In total, 64 physicians were invited and 13 (20%) chose to participate. As the study did not involve assessing medical interventions among patients, a waiver of informed consent was granted by the VANMHCS Institutional Review Board. Physicians who participated in this study were informed that their responses would be used for reporting purposes and could be rescinded at any time.
Data Analysis
Semistructured interviews were conducted by a single interviewer and recorded using Microsoft Teams. The interviews took place between February 2021 and December 2021 and lasted 5 to 15 minutes, with a mean duration of 9 minutes. Verbal informed consent was obtained from all participants before the interviews. Interviewees were encouraged to expand on their responses to structured questions by recounting past experiences with VVC. Recorded audio was additionally transcribed via Microsoft Teams, and the research team reviewed the transcriptions to ensure accuracy.
The tracking and coding of responses to interview questions were conducted using Microsoft Excel. Initially, 5 transcripts were reviewed and responses were assessed by 2 study team members through open coding. All team members examined the 5 coded transcripts to identify differences and reach a consensus for any discrepancies. Based on recommendations from all team members regarding nuanced excerpts of transcripts, 1 study team member coded the remaining interviews. Thematic analysis was subsequently conducted according to the method described by Braun and Clarke.12 Themes were developed both deductively and inductively by reviewing the direct responses to interview questions and identifying emerging patterns of data, respectively. Indicative quotes representing each theme were carefully chosen for reporting.
RESULTS
Thirteen interviews were conducted and 9 participants (69%) were female. Participating physicians included 3 internal medicine/primary care physicians (23%), 2 nephrologists (15%), and 1 (8%) from cardiology, endocrinology, hematology, infectious diseases, palliative care, critical care, pulmonology, and sleep medicine. Years of post training experience among physicians ranged from 1 to 9 years (n = 5, 38%), 10 to 19 years (n = 3, 23%), and . 20 years (n = 5, 38%). Seven participants (54%) had conducted ≥ 5 VVC visits, with 1 physician completing > 50 video visits (Table).

Using open coding and a deductive approach to thematic analysis, 5 themes were identified: (1) VVC software and internet connection issues affected implementation; (2) patient technological literacy affected veteran and physician comfort with VVC; (3) integration of supportive measures was desired; (4) CVT services may increasingly be used to enhance access to care; and (5) in-person encounters afforded unique advantages over CVT. Illustrative quotes from physicians that reflect these themes can be found in the Appendix.
Theme 1: VVC software and internet connection issues affected its implementation. Most participants expressed concern about the technical challenges with VVC. Interviewees cited inconsistencies for both patients and physicians receiving emails with links to join VVC visits, which should be generated when appointments are scheduled. Some physicians were unaware of scheduled VVC visits until the day of the appointment and only received the link via email. Such issues appeared to occur regardless whether the physicians or support staff scheduled the encounter. Poor video and audio quality was also cited as significant barriers to successful VVC visits and were often not resolvable through troubleshooting efforts by physicians, patients, or support personnel. Given the limited time allotted to each patient encounter, such issues could significantly impact the physician’s ability to remain on schedule. Moreover, connectivity problems led to significant lapses, delays in audio and video transmission, and complete disconnections from the VVC encounter. This was a significant concern for participants, given the rural nature of New Mexico and the large geographical gaps in internet service throughout the state.
Theme 2: Patient technological literacy affected veteran and physician comfort with VVC. Successful VVC appointments require high-speed Internet and compatible hardware. Physicians indicated that some patients reported difficulties with critical steps in the process, such as logging into the VVC platform or ensuring their microphones and cameras were active. Physicians also expressed concern about older veterans’ ability to utilize electronic devices, noting they may generally be less technology savvy. Additionally, physicians reported that despite offering the option of a virtual visit, many veterans preferred in-person visits, regardless of the drive time required. This appeared related to a fear of using the technology, which led veterans to believe that virtual visits do not provide the same quality of care as in-person visits.
Theme 3: Integration of supportive measures is desired. Interviewees felt that integrated VVC technical assistance and technology literacy education were imperative. First, training the patient or the patient’s caregiver on how to complete a VVC encounter using the preferred device and the VVC platform would be beneficial. Second, education to inform physicians about common troubleshooting issues could help streamline VVC encounters. Third, managing a VVC encounter similarly to standard in-person visits could allow for better patient and physician experience. For example, physicians suggested that a medical assistant or a nurse triage the patient, take vital signs, and set them up in a room, potentially at a regional VA community based outpatient clinic. Such efforts would also allow patients to receive specialty care in remote areas where only primary care is generally offered. Support staff could assist with technological issues, such as setting up the VVC encounter and addressing potential problems before the physician joins the encounter, thereby preventing delays in patient care. Finally, physicians felt that designating a day solely for CVT visits would help prevent disruption in care with in-person visits.
Theme 4: CVT services may increasingly be used to enhance access to care. Physicians felt that VVC would help patients encountering obstacles in accessing conventional in person services, including patients in rural and underserved areas, with disabilities, or with scheduling challenges.4 Patients with chronic conditions might drive the use of virtual visits, as many of these patients are already accustomed to remote medical monitoring. Data from devices such as scales and continuous glucose monitors can be easily reviewed during VVC visits. Second, video encounters facilitate closer monitoring that some patients might otherwise skip due to significant travel barriers, especially in a rural state like New Mexico. Lastly, VVC may be more efficient than in person visits as they eliminate the need for lengthy parking, checking in, and checking out processes. Thus, if technological issues are resolved, a typical physician’s day in the clinic may be more efficient with virtual visits.
Theme 5: In-person encounters afforded unique advantages over CVT. Some physicians felt in-person visits still offer unique advantages. They opined that the selection of appropriate candidates for CVT is critical. Patients requiring a physical examination should be scheduled for in person visits. For example, patients with advanced chronic kidney disease who require accurate volume status assessment or patients who have recently undergone surgery and need detailed wound inspection should be seen in the clinic. In-person visits may also be preferable for patients with recurrent admissions, or those whose condition is difficult to assess; accurate assessments of such patients may help prevent readmissions. Finally, many patients are more comfortable and satisfied with in-person visits, which are perceived as a more standard or traditional process. Respondents noted that some patients felt physicians may not focus as much attention during a VVC visit as they do during in-person visits. There were also concerns that some patients feel more motivation to come to in-person visits, as they see the VA as a place to interact with other veterans and staff with whom they are familiar and comfortable.
DISCUSSION
VANMHCS physicians, which serves veterans across an expansive territory ranging from Southern Colorado to West Texas. About 4.6 million veterans reside in rural regions, constituting roughly 25% of the total veteran population, a pattern mirrored in New Mexico.13 Medicine Service physicians agreed on a number of themes: VVC user-interface issues may affect its use and effectiveness, technological literacy was important for both patients and health care staff, technical support staff roles before and during VVC visits should be standardized, CVT is likely to increase health care delivery, and in-person encounters are preferred for many patients.
This is the first study to qualitatively evaluate a diverse group of physicians at a VA medical center incorporating CVT services across specialties. A few related qualitative studies have been conducted external to VHA, generally evaluating clinicians within a single specialty. Kalicki and colleagues surveyed 16 physicians working at a large home-based primary care program in New York City between April and June 2020 to identify and explore barriers to telehealth among homebound older adults. Similarly to our study, physicians noted that many patients required assistance (family members or caregivers) with the visit, either due to technological literacy issues or medical conditions like dementia.14
Heyer and colleagues surveyed 29 oncologists at an urban academic center prior to the COVID-19 pandemic. Similar to our observations, the oncologists said telemedicine helped eliminate travel as a barrier to health care. Heyer and colleagues noted difficulty for oncologists in performing virtual physical examinations, despite training. This group did note the benefits when being selective as to which clinical issues they would handle virtually vs in person.15
Budhwani and colleagues reported that mental health professionals in an academic setting cited difficulty establishing therapeutic relationships via telehealth and felt that this affected quality of care.16 While this was not a topic during our interviews, it is reasonable to question how potentially missed nonverbal cues may impact patient assessments.
Notably, technological issues were common among all reviewed studies. These ranged from internet connectivity issues to necessary electronic devices. As mentioned, these barriers are more prevalent in rural states like New Mexico.
Limitations
All participants in this study were Medicine Service physicians of a single VA health care system, which may limit generalizability. Many of our respondents were female (69%), compared with 39.2% of active internal medicine physicians and therefore may not be representative.17 Nearly one-half of our participants only completed 1 to 4 VVC encounters, which may have contributed to the emergence of a common theme regarding technological issues. Physicians with more experience with CVT services may be more skilled at troubleshooting technological issues that arise during visits.
CONCLUSIONS
Our study, conducted with VANMHCS physicians, illuminated 5 key themes influencing the use and implementation of video encounters: technological issues, technological literacy, a desire for integrated support measures, perceived future growth of video telehealth, and the unique advantages of in-person visits. Addressing technological barriers and providing more extensive training may streamline CVT use. However, it is vital to recognize the unique benefits of in-person visits and consider the benefits of each modality along with patient preferences when selecting the best care venue. As health care evolves, better understanding and acting upon these themes will optimize telehealth services, particularly in rural areas. Future research should involve patients and other health care team members to further explore strategies for effective CVT service integration.
Appendix

Prior to the COVID-19 pandemic, health care systems had been increasingly focused on expanding care delivery through clinical video telehealth (CVT) services.1-3 These modalities offer clinicians and patients opportunities to interact without needing face-to-face visits. CVT services offer significant advantages to patients who encounter challenges accessing traditional face-to-face services, including those living in rural or underserved areas, individuals with mobility limitations, and those with difficulty attending appointments due to work or caregiving commitments.4 The COVID-19 pandemic accelerated the expansion of CVT services to mitigate the spread of the virus.1
Despite its evident advantages, widespread adoption of CVT has encountered resistance.2 Physicians have frequently expressed concerns about the reliability and functionality of CVT platforms for scheduled encounters and frustration with inadequate training.4-6 Additionally, there is a lack trust in the technology, as physicians are unfamiliar with reimbursement or workload capture associated with CVT. Physicians have concerns that telecommunication may diminish the intangible aspects of the “art of medicine.”4 As a result, the implementation of telehealth services has been inconsistent, with successful adoption limited to specific medical and surgical specialties.4 Only recently have entire departments within major health care systems expressed interest in providing comprehensive CVT services in response to the challenges posed by the COVID-19 pandemic.4
The Veterans Health Administration (VHA) of the US Department of Veterans Affairs (VA) provides an appropriate setting for assessing clinician perceptions of telehealth services. Since 2003, the VHA has significantly expanded CVT services to eligible veterans and has used the VA Video Connect (VVC) platform since 2018.7-10 Through VVC, VA staff and clinicians may schedule video visits with patients, meet with patients through virtual face-to-face interaction, and share relevant laboratory results and imaging through screen sharing. Prior research has shown increased accessibility to care through VVC. For example, a single-site study demonstrated that VVC implementation for delivering psychotherapies significantly increased CVT encounters from 15% to 85% among veterans with anxiety and/or depression.11
The VA New Mexico Healthcare System (VANMHCS) serves a high volume of veterans living in remote and rural regions and significantly increased its use of CVT during the COVID-19 pandemic to reduce in-person visits. Expectedly, this was met with a variety of challenges. Herein, we sought to assess physician perspectives, concerns, and attitudes toward VVC via semistructured interviews. Our hypothesis was that VA physicians may feel uncomfortable with video encounters but recognize the growing importance of such practices providing specialty care to veterans in rural areas.
METHODS
A semistructured interview protocol was created following discussions with physicians from the VANMHCS Medicine Service. Questions were constructed to assess the following domains: overarching views of video telehealth, perceptions of various applications for conducting VVC encounters, and barriers to the broad implementation of video telehealth. A qualitative investigation specialist aided with question development. Two pilot interviews were conducted prior to performing the interviews with the recruited participants to evaluate the quality and delivery of questions.
All VANMHCS physicians who provided outpatient care within the Department of Medicine and had completed ≥ 1 VVC encounter were eligible to participate. Invitations were disseminated via email, and follow-up emails to encourage participation were sent periodically for 2 months following the initial request. Union approval was obtained to interview employees for a research study. In total, 64 physicians were invited and 13 (20%) chose to participate. As the study did not involve assessing medical interventions among patients, a waiver of informed consent was granted by the VANMHCS Institutional Review Board. Physicians who participated in this study were informed that their responses would be used for reporting purposes and could be rescinded at any time.
Data Analysis
Semistructured interviews were conducted by a single interviewer and recorded using Microsoft Teams. The interviews took place between February 2021 and December 2021 and lasted 5 to 15 minutes, with a mean duration of 9 minutes. Verbal informed consent was obtained from all participants before the interviews. Interviewees were encouraged to expand on their responses to structured questions by recounting past experiences with VVC. Recorded audio was additionally transcribed via Microsoft Teams, and the research team reviewed the transcriptions to ensure accuracy.
The tracking and coding of responses to interview questions were conducted using Microsoft Excel. Initially, 5 transcripts were reviewed and responses were assessed by 2 study team members through open coding. All team members examined the 5 coded transcripts to identify differences and reach a consensus for any discrepancies. Based on recommendations from all team members regarding nuanced excerpts of transcripts, 1 study team member coded the remaining interviews. Thematic analysis was subsequently conducted according to the method described by Braun and Clarke.12 Themes were developed both deductively and inductively by reviewing the direct responses to interview questions and identifying emerging patterns of data, respectively. Indicative quotes representing each theme were carefully chosen for reporting.
RESULTS
Thirteen interviews were conducted and 9 participants (69%) were female. Participating physicians included 3 internal medicine/primary care physicians (23%), 2 nephrologists (15%), and 1 (8%) from cardiology, endocrinology, hematology, infectious diseases, palliative care, critical care, pulmonology, and sleep medicine. Years of post training experience among physicians ranged from 1 to 9 years (n = 5, 38%), 10 to 19 years (n = 3, 23%), and . 20 years (n = 5, 38%). Seven participants (54%) had conducted ≥ 5 VVC visits, with 1 physician completing > 50 video visits (Table).

Using open coding and a deductive approach to thematic analysis, 5 themes were identified: (1) VVC software and internet connection issues affected implementation; (2) patient technological literacy affected veteran and physician comfort with VVC; (3) integration of supportive measures was desired; (4) CVT services may increasingly be used to enhance access to care; and (5) in-person encounters afforded unique advantages over CVT. Illustrative quotes from physicians that reflect these themes can be found in the Appendix.
Theme 1: VVC software and internet connection issues affected its implementation. Most participants expressed concern about the technical challenges with VVC. Interviewees cited inconsistencies for both patients and physicians receiving emails with links to join VVC visits, which should be generated when appointments are scheduled. Some physicians were unaware of scheduled VVC visits until the day of the appointment and only received the link via email. Such issues appeared to occur regardless whether the physicians or support staff scheduled the encounter. Poor video and audio quality was also cited as significant barriers to successful VVC visits and were often not resolvable through troubleshooting efforts by physicians, patients, or support personnel. Given the limited time allotted to each patient encounter, such issues could significantly impact the physician’s ability to remain on schedule. Moreover, connectivity problems led to significant lapses, delays in audio and video transmission, and complete disconnections from the VVC encounter. This was a significant concern for participants, given the rural nature of New Mexico and the large geographical gaps in internet service throughout the state.
Theme 2: Patient technological literacy affected veteran and physician comfort with VVC. Successful VVC appointments require high-speed Internet and compatible hardware. Physicians indicated that some patients reported difficulties with critical steps in the process, such as logging into the VVC platform or ensuring their microphones and cameras were active. Physicians also expressed concern about older veterans’ ability to utilize electronic devices, noting they may generally be less technology savvy. Additionally, physicians reported that despite offering the option of a virtual visit, many veterans preferred in-person visits, regardless of the drive time required. This appeared related to a fear of using the technology, which led veterans to believe that virtual visits do not provide the same quality of care as in-person visits.
Theme 3: Integration of supportive measures is desired. Interviewees felt that integrated VVC technical assistance and technology literacy education were imperative. First, training the patient or the patient’s caregiver on how to complete a VVC encounter using the preferred device and the VVC platform would be beneficial. Second, education to inform physicians about common troubleshooting issues could help streamline VVC encounters. Third, managing a VVC encounter similarly to standard in-person visits could allow for better patient and physician experience. For example, physicians suggested that a medical assistant or a nurse triage the patient, take vital signs, and set them up in a room, potentially at a regional VA community based outpatient clinic. Such efforts would also allow patients to receive specialty care in remote areas where only primary care is generally offered. Support staff could assist with technological issues, such as setting up the VVC encounter and addressing potential problems before the physician joins the encounter, thereby preventing delays in patient care. Finally, physicians felt that designating a day solely for CVT visits would help prevent disruption in care with in-person visits.
Theme 4: CVT services may increasingly be used to enhance access to care. Physicians felt that VVC would help patients encountering obstacles in accessing conventional in person services, including patients in rural and underserved areas, with disabilities, or with scheduling challenges.4 Patients with chronic conditions might drive the use of virtual visits, as many of these patients are already accustomed to remote medical monitoring. Data from devices such as scales and continuous glucose monitors can be easily reviewed during VVC visits. Second, video encounters facilitate closer monitoring that some patients might otherwise skip due to significant travel barriers, especially in a rural state like New Mexico. Lastly, VVC may be more efficient than in person visits as they eliminate the need for lengthy parking, checking in, and checking out processes. Thus, if technological issues are resolved, a typical physician’s day in the clinic may be more efficient with virtual visits.
Theme 5: In-person encounters afforded unique advantages over CVT. Some physicians felt in-person visits still offer unique advantages. They opined that the selection of appropriate candidates for CVT is critical. Patients requiring a physical examination should be scheduled for in person visits. For example, patients with advanced chronic kidney disease who require accurate volume status assessment or patients who have recently undergone surgery and need detailed wound inspection should be seen in the clinic. In-person visits may also be preferable for patients with recurrent admissions, or those whose condition is difficult to assess; accurate assessments of such patients may help prevent readmissions. Finally, many patients are more comfortable and satisfied with in-person visits, which are perceived as a more standard or traditional process. Respondents noted that some patients felt physicians may not focus as much attention during a VVC visit as they do during in-person visits. There were also concerns that some patients feel more motivation to come to in-person visits, as they see the VA as a place to interact with other veterans and staff with whom they are familiar and comfortable.
DISCUSSION
VANMHCS physicians, which serves veterans across an expansive territory ranging from Southern Colorado to West Texas. About 4.6 million veterans reside in rural regions, constituting roughly 25% of the total veteran population, a pattern mirrored in New Mexico.13 Medicine Service physicians agreed on a number of themes: VVC user-interface issues may affect its use and effectiveness, technological literacy was important for both patients and health care staff, technical support staff roles before and during VVC visits should be standardized, CVT is likely to increase health care delivery, and in-person encounters are preferred for many patients.
This is the first study to qualitatively evaluate a diverse group of physicians at a VA medical center incorporating CVT services across specialties. A few related qualitative studies have been conducted external to VHA, generally evaluating clinicians within a single specialty. Kalicki and colleagues surveyed 16 physicians working at a large home-based primary care program in New York City between April and June 2020 to identify and explore barriers to telehealth among homebound older adults. Similarly to our study, physicians noted that many patients required assistance (family members or caregivers) with the visit, either due to technological literacy issues or medical conditions like dementia.14
Heyer and colleagues surveyed 29 oncologists at an urban academic center prior to the COVID-19 pandemic. Similar to our observations, the oncologists said telemedicine helped eliminate travel as a barrier to health care. Heyer and colleagues noted difficulty for oncologists in performing virtual physical examinations, despite training. This group did note the benefits when being selective as to which clinical issues they would handle virtually vs in person.15
Budhwani and colleagues reported that mental health professionals in an academic setting cited difficulty establishing therapeutic relationships via telehealth and felt that this affected quality of care.16 While this was not a topic during our interviews, it is reasonable to question how potentially missed nonverbal cues may impact patient assessments.
Notably, technological issues were common among all reviewed studies. These ranged from internet connectivity issues to necessary electronic devices. As mentioned, these barriers are more prevalent in rural states like New Mexico.
Limitations
All participants in this study were Medicine Service physicians of a single VA health care system, which may limit generalizability. Many of our respondents were female (69%), compared with 39.2% of active internal medicine physicians and therefore may not be representative.17 Nearly one-half of our participants only completed 1 to 4 VVC encounters, which may have contributed to the emergence of a common theme regarding technological issues. Physicians with more experience with CVT services may be more skilled at troubleshooting technological issues that arise during visits.
CONCLUSIONS
Our study, conducted with VANMHCS physicians, illuminated 5 key themes influencing the use and implementation of video encounters: technological issues, technological literacy, a desire for integrated support measures, perceived future growth of video telehealth, and the unique advantages of in-person visits. Addressing technological barriers and providing more extensive training may streamline CVT use. However, it is vital to recognize the unique benefits of in-person visits and consider the benefits of each modality along with patient preferences when selecting the best care venue. As health care evolves, better understanding and acting upon these themes will optimize telehealth services, particularly in rural areas. Future research should involve patients and other health care team members to further explore strategies for effective CVT service integration.
Appendix

- Monaghesh E, Hajizadeh A. The role of telehealth during covid-19 outbreak: a systematic review based on current evidence. BMC Public Health. 2020;20(1):1193. doi:10.1186/s12889-020-09301-4
- Scott Kruse C, Karem P, Shifflett K, Vegi L, Ravi K, Brooks M. Evaluating barriers to adopting telemedicine worldwide: a systematic review. J Telemed Telecare. 2018;24(1):4-12. doi:10.1177/1357633X16674087
- Bashshur RL, Howell JD, Krupinski EA, Harms KM, Bashshur N, Doarn CR. The empirical foundations of telemedicine interventions in primary care. Telemed J E Health. 2016;22(5):342-375. doi:10.1089/tmj.2016.0045
- Yellowlees P, Nakagawa K, Pakyurek M, Hanson A, Elder J, Kales HC. Rapid conversion of an outpatient psychiatric clinic to a 100% virtual telepsychiatry clinic in response to covid-19. Pyschiatr Serv. 2020;71(7):749-752. doi:10.1176/appi.ps.202000230
- Hailey D, Ohinmaa A, Roine R. Study quality and evidence of benefit in recent assessments of telemedicine. J Telemed Telecare. 2004;10(6):318-324. doi:10.1258/1357633042602053
- Osuji TA, Macias M, McMullen C, et al. Clinician perspectives on implementing video visits in home-based palliative care. Palliat Med Rep. 2020;1(1):221-226. doi:10.1089/pmr.2020.0074
- Darkins A. The growth of telehealth services in the Veterans Health Administration between 1994 and 2014: a study in the diffusion of innovation. Telemed J E Health. 2014;20(9):761-768. doi:10.1089/tmj.2014.0143
- Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375(2):154-161. doi:10.1056/nejmra1601705
- Alexander NB, Phillips K, Wagner-Felkey J, et al. Team VA video connect (VVC) to optimize mobility and physical activity in post-hospital discharge older veterans: Baseline assessment. BMC Geriatr. 2021;21(1):502. doi:10.1186/s12877-021-02454-w
- Padala KP, Wilson KB, Gauss CH, Stovall JD, Padala PR. VA video connect for clinical care in older adults in a rural state during the covid-19 pandemic: cross-sectional study. J Med Internet Res. 2020;22(9)e21561. doi:10.2196/21561
- Myers US, Coulon S, Knies K, et al. Lessons learned in implementing VA video connect for evidence-based psychotherapies for anxiety and depression in the veterans healthcare administration. J Technol Behav Sci. 2020;6(2):320-326. doi:10.1007/s41347-020-00161-8
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77-101. doi:10.1191/1478088706qp063oa
- US Department of Veterans Affairs, National Center for Feterans Analysis and Statistics. Accessed September 18, 2024. www.va.gov/vetdata/report.asp
- Kalicki AV, Moody KA, Franzosa E, Gliatto PM, Ornstein KA. Barriers to telehealth access among homebound older adults. J Am Geriatr Soc. 2021;69(9):2404-2411. doi:10.1111/jgs.17163
- Heyer A, Granberg RE, Rising KL, Binder AF, Gentsch AT, Handley NR. Medical oncology professionals’ perceptions of telehealth video visits. JAMA Netw Open. 2021;4(1) e2033967. doi:10.1001/jamanetworkopen.2020.33967
- Budhwani S, Fujioka JK, Chu C, et al. Delivering mental health care virtually during the COVID-19 pandemic: qualitative evaluation of provider experiences in a scaled context. JMIR Form Res. 2021;5(9)e30280. doi:10.2196/30280
- Association of American Medical Colleges. Active physicians by sex and specialty, 2021. AAMC. Accessed September 18, 2024. https://www.aamc.org/data-reports/workforce/data/active-physicians-sex-specialty-2021
- Monaghesh E, Hajizadeh A. The role of telehealth during covid-19 outbreak: a systematic review based on current evidence. BMC Public Health. 2020;20(1):1193. doi:10.1186/s12889-020-09301-4
- Scott Kruse C, Karem P, Shifflett K, Vegi L, Ravi K, Brooks M. Evaluating barriers to adopting telemedicine worldwide: a systematic review. J Telemed Telecare. 2018;24(1):4-12. doi:10.1177/1357633X16674087
- Bashshur RL, Howell JD, Krupinski EA, Harms KM, Bashshur N, Doarn CR. The empirical foundations of telemedicine interventions in primary care. Telemed J E Health. 2016;22(5):342-375. doi:10.1089/tmj.2016.0045
- Yellowlees P, Nakagawa K, Pakyurek M, Hanson A, Elder J, Kales HC. Rapid conversion of an outpatient psychiatric clinic to a 100% virtual telepsychiatry clinic in response to covid-19. Pyschiatr Serv. 2020;71(7):749-752. doi:10.1176/appi.ps.202000230
- Hailey D, Ohinmaa A, Roine R. Study quality and evidence of benefit in recent assessments of telemedicine. J Telemed Telecare. 2004;10(6):318-324. doi:10.1258/1357633042602053
- Osuji TA, Macias M, McMullen C, et al. Clinician perspectives on implementing video visits in home-based palliative care. Palliat Med Rep. 2020;1(1):221-226. doi:10.1089/pmr.2020.0074
- Darkins A. The growth of telehealth services in the Veterans Health Administration between 1994 and 2014: a study in the diffusion of innovation. Telemed J E Health. 2014;20(9):761-768. doi:10.1089/tmj.2014.0143
- Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375(2):154-161. doi:10.1056/nejmra1601705
- Alexander NB, Phillips K, Wagner-Felkey J, et al. Team VA video connect (VVC) to optimize mobility and physical activity in post-hospital discharge older veterans: Baseline assessment. BMC Geriatr. 2021;21(1):502. doi:10.1186/s12877-021-02454-w
- Padala KP, Wilson KB, Gauss CH, Stovall JD, Padala PR. VA video connect for clinical care in older adults in a rural state during the covid-19 pandemic: cross-sectional study. J Med Internet Res. 2020;22(9)e21561. doi:10.2196/21561
- Myers US, Coulon S, Knies K, et al. Lessons learned in implementing VA video connect for evidence-based psychotherapies for anxiety and depression in the veterans healthcare administration. J Technol Behav Sci. 2020;6(2):320-326. doi:10.1007/s41347-020-00161-8
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77-101. doi:10.1191/1478088706qp063oa
- US Department of Veterans Affairs, National Center for Feterans Analysis and Statistics. Accessed September 18, 2024. www.va.gov/vetdata/report.asp
- Kalicki AV, Moody KA, Franzosa E, Gliatto PM, Ornstein KA. Barriers to telehealth access among homebound older adults. J Am Geriatr Soc. 2021;69(9):2404-2411. doi:10.1111/jgs.17163
- Heyer A, Granberg RE, Rising KL, Binder AF, Gentsch AT, Handley NR. Medical oncology professionals’ perceptions of telehealth video visits. JAMA Netw Open. 2021;4(1) e2033967. doi:10.1001/jamanetworkopen.2020.33967
- Budhwani S, Fujioka JK, Chu C, et al. Delivering mental health care virtually during the COVID-19 pandemic: qualitative evaluation of provider experiences in a scaled context. JMIR Form Res. 2021;5(9)e30280. doi:10.2196/30280
- Association of American Medical Colleges. Active physicians by sex and specialty, 2021. AAMC. Accessed September 18, 2024. https://www.aamc.org/data-reports/workforce/data/active-physicians-sex-specialty-2021
Physician Attitudes About Veterans Affairs Video Connect Encounters
Physician Attitudes About Veterans Affairs Video Connect Encounters
Painful Oral, Groin, and Scalp Lesions in a Young Man
Painful Oral, Groin, and Scalp Lesions in a Young Man
THE DIAGNOSIS: Pemphigus Vegetans
Histopathologic examination of the biopsies from the scalp and left anterior thigh revealed suprabasal clefting with acantholytic cells extending into the follicular infundibulum with eosinophilic pustules within the epidermis. The dermis contained perivascular lymphohistiocytic and eosinophilic inflammatory infiltrates without viral cytopathic effects (Figure 1). Direct immunofluorescence revealed strong IgG and moderate IgA pericellular deposition around keratinocyte cytoplasms (Figure 2). Serologic evaluation demonstrated anti–desmoglein 3 antibodies. Based on the clinical presentation and histopathologic correlation, a diagnosis of pemphigus vegetans was made.
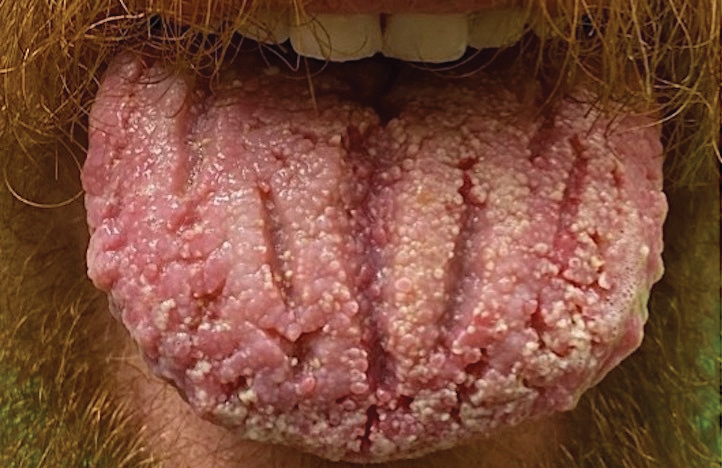
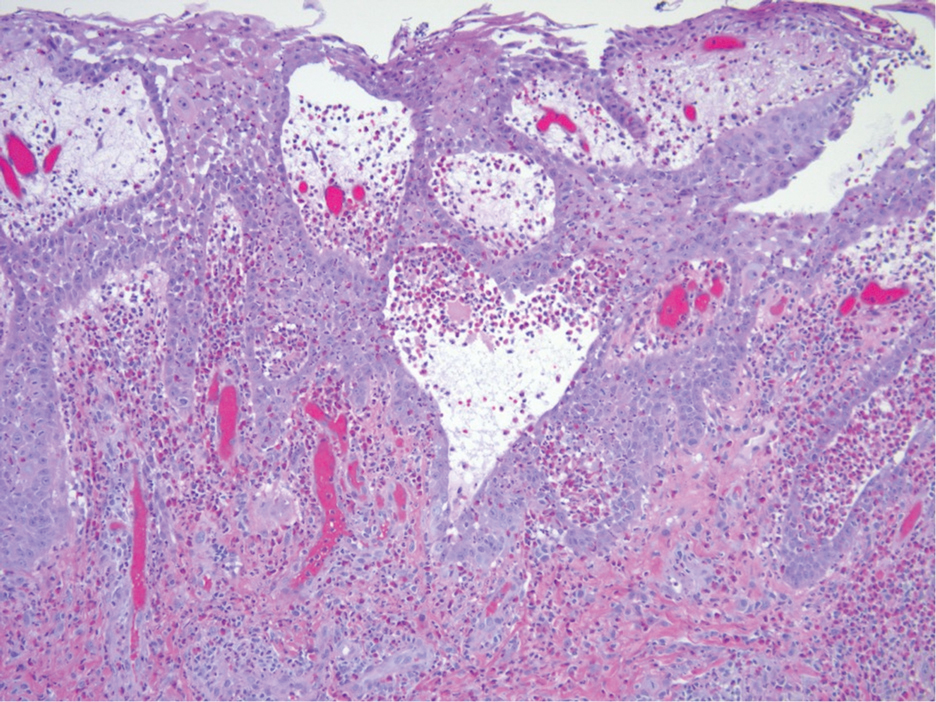
Pemphigus vegetans is a vesiculobullous autoimmune disease that is similar to pemphigus vulgaris but is characterized by the formation of vegetative plaques along the intertriginous areas and on the oral mucosa.1 It is the rarest variant of all pemphigus subtypes and was first described by Neumann in 1876.2 There are 2 subtypes of this variant: Hallopeau and Neumann, each with unique characteristics and physical manifestations. The Hallopeau type initially manifests with pustular lesions that rupture and evolve into erosions that commonly become infected. Gradually they merge and multiply to become more painful and vegetative.3 It has a more indolent course and typically responds well to treatment, and prolonged remission can be reached.4 The Neumann type is more severe and manifests with large vesiculobullous and erosive lesions that rupture and ulcerate, forming verrucous crusted vegetative plaques over the erosions.5 The erosions along the edge of the lesions induce new vegetation, becoming dry, hyperkeratotic, and fissured.3 The Neumann type often requires higher-dose steroids and typically is resistant to treatment.4 Patients can present with oral stomatitis and occasionally can develop a fissured or cerebriform appearance of the tongue, as seen in our patient (Figure 3).1,2 Nail changes include onychorrhexis, onychomadesis, subungual pustules, and ultimately nail atrophy.5
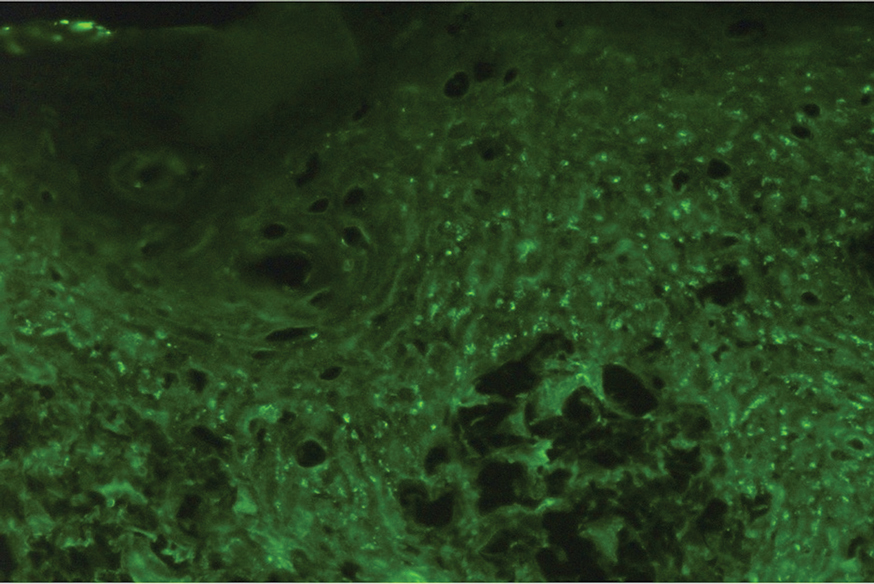
Pemphigus diseases are characterized by IgG autoantibodies against desmoglein 3 and/or desmoglein 1. These are components of desmosomes that are responsible for keratinocyte adhesion, disruption of which results in the blister formation seen in pemphigus subtypes. The unique physical manifestation of pemphigus vegetans is thought to be due not only to autoantibodies against desmogleins 1 and 3 but also to autoantibodies against desmocollin 1 and 2.1
Histopathologic examination reveals hyperkeratosis and pseudoepitheliomatous hyperplasia with acantholysis that creates a suprabasal cleft. Basal cells remain intact to the basement membrane by hemidesmosomes, resulting in a tombstone appearance. The Hallopeau type typically manifests with a large eosinophilic inflammatory response, leading to eosinophilic spongiosis and intraepidermal microabscesses. The Neumann type manifests with more of a neutrophilic and lymphocytic infiltrate, accompanied by the eosinophilic response.1 For evaluation, obtain histopathology as well as direct immunofluorescence or enzyme-linked immunosorbent assay to look for intracellular deposition of desmoglein autoantibodies.
First-line treatment for pemphigus vulgaris and its variants is rituximab, an anti-CD20 monoclonal antibody. It has also been shown to have therapeutic benefit with combination of corticosteroids and rituximab. Corticosteroids should be given at a dose of 1 mg/kg daily for 2 to 4 weeks. Other immunosuppressive agents (steroid sparing) include azathioprine, dapsone, mycophenolate mofetil, methotrexate, cyclophosphamide, cyclosporine, and intravenous immunoglobulin. Pulse therapy with intermittent intravenous corticosteroids and immunosuppressants is another second-line therapeutic option. Topical therapeutic options include steroids, tacrolimus, and nicotinamide with oral tetracycline at onset and relapse. The goal of therapy is to maintain remission for 1 year then slowly taper treatment over another year.1
Our patient initially was treated with prednisone, and subsequent courses of azathioprine and mycophenolate mofetil failed. He then was treated with 2 infusions of rituximab that were given 2 weeks apart. He was able to taper off the prednisone 1 month after the last infusion with complete remission of disease. He has been disease free for more than 9 months postinfusion.
Differential diagnoses for pemphigus vegetans can include bullous pemphigoid, bullous systemic lupus erythematosus, dermatitis herpetiformis, and pemphigus vulgaris. Lesion characteristics are key to differentiating pemphigus vegetans from other autoimmune blistering disorders. Bullous pemphigoid will manifest with tense blisters where pemphigus vulgaris will be flaccid; this is due to the difference in autoantibody targets between the conditions. Diagnosis depends on clinical presentation and histopathologic findings.
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls [Internet]. Updated June 26, 2023. Accessed December 16, 2024. https://www.ncbi.nlm.nih.gov/books/NBK545229/
- Rebello MS, Ramesh BM, Sukumar D, et al. Cerebriform cutaneous lesions in pemphigus vegetans. Indian J Dermatol. 2016;61:206-208.
- Ruocco V, Ruocco E, Caccavale S, et al. Pemphigus vegetans of the folds (intertriginous areas). Clin Dermatol. 2015;33:471-476.
- Ajbani AA, Mehta KS, Marfatia YS. Verrucous lesions over external genitalia as a presenting feature of pemphigus vegetans. Indian J Sex Transm Dis AIDS. 2019;40:176-179.
- Vinay K, De D, Handa S, et al. Pemphigus vegetans presenting as a verrucous plaque on the finger. Clin Exp Dermatol. 2016;41:316-317.
THE DIAGNOSIS: Pemphigus Vegetans
Histopathologic examination of the biopsies from the scalp and left anterior thigh revealed suprabasal clefting with acantholytic cells extending into the follicular infundibulum with eosinophilic pustules within the epidermis. The dermis contained perivascular lymphohistiocytic and eosinophilic inflammatory infiltrates without viral cytopathic effects (Figure 1). Direct immunofluorescence revealed strong IgG and moderate IgA pericellular deposition around keratinocyte cytoplasms (Figure 2). Serologic evaluation demonstrated anti–desmoglein 3 antibodies. Based on the clinical presentation and histopathologic correlation, a diagnosis of pemphigus vegetans was made.


Pemphigus vegetans is a vesiculobullous autoimmune disease that is similar to pemphigus vulgaris but is characterized by the formation of vegetative plaques along the intertriginous areas and on the oral mucosa.1 It is the rarest variant of all pemphigus subtypes and was first described by Neumann in 1876.2 There are 2 subtypes of this variant: Hallopeau and Neumann, each with unique characteristics and physical manifestations. The Hallopeau type initially manifests with pustular lesions that rupture and evolve into erosions that commonly become infected. Gradually they merge and multiply to become more painful and vegetative.3 It has a more indolent course and typically responds well to treatment, and prolonged remission can be reached.4 The Neumann type is more severe and manifests with large vesiculobullous and erosive lesions that rupture and ulcerate, forming verrucous crusted vegetative plaques over the erosions.5 The erosions along the edge of the lesions induce new vegetation, becoming dry, hyperkeratotic, and fissured.3 The Neumann type often requires higher-dose steroids and typically is resistant to treatment.4 Patients can present with oral stomatitis and occasionally can develop a fissured or cerebriform appearance of the tongue, as seen in our patient (Figure 3).1,2 Nail changes include onychorrhexis, onychomadesis, subungual pustules, and ultimately nail atrophy.5

Pemphigus diseases are characterized by IgG autoantibodies against desmoglein 3 and/or desmoglein 1. These are components of desmosomes that are responsible for keratinocyte adhesion, disruption of which results in the blister formation seen in pemphigus subtypes. The unique physical manifestation of pemphigus vegetans is thought to be due not only to autoantibodies against desmogleins 1 and 3 but also to autoantibodies against desmocollin 1 and 2.1
Histopathologic examination reveals hyperkeratosis and pseudoepitheliomatous hyperplasia with acantholysis that creates a suprabasal cleft. Basal cells remain intact to the basement membrane by hemidesmosomes, resulting in a tombstone appearance. The Hallopeau type typically manifests with a large eosinophilic inflammatory response, leading to eosinophilic spongiosis and intraepidermal microabscesses. The Neumann type manifests with more of a neutrophilic and lymphocytic infiltrate, accompanied by the eosinophilic response.1 For evaluation, obtain histopathology as well as direct immunofluorescence or enzyme-linked immunosorbent assay to look for intracellular deposition of desmoglein autoantibodies.
First-line treatment for pemphigus vulgaris and its variants is rituximab, an anti-CD20 monoclonal antibody. It has also been shown to have therapeutic benefit with combination of corticosteroids and rituximab. Corticosteroids should be given at a dose of 1 mg/kg daily for 2 to 4 weeks. Other immunosuppressive agents (steroid sparing) include azathioprine, dapsone, mycophenolate mofetil, methotrexate, cyclophosphamide, cyclosporine, and intravenous immunoglobulin. Pulse therapy with intermittent intravenous corticosteroids and immunosuppressants is another second-line therapeutic option. Topical therapeutic options include steroids, tacrolimus, and nicotinamide with oral tetracycline at onset and relapse. The goal of therapy is to maintain remission for 1 year then slowly taper treatment over another year.1
Our patient initially was treated with prednisone, and subsequent courses of azathioprine and mycophenolate mofetil failed. He then was treated with 2 infusions of rituximab that were given 2 weeks apart. He was able to taper off the prednisone 1 month after the last infusion with complete remission of disease. He has been disease free for more than 9 months postinfusion.
Differential diagnoses for pemphigus vegetans can include bullous pemphigoid, bullous systemic lupus erythematosus, dermatitis herpetiformis, and pemphigus vulgaris. Lesion characteristics are key to differentiating pemphigus vegetans from other autoimmune blistering disorders. Bullous pemphigoid will manifest with tense blisters where pemphigus vulgaris will be flaccid; this is due to the difference in autoantibody targets between the conditions. Diagnosis depends on clinical presentation and histopathologic findings.
THE DIAGNOSIS: Pemphigus Vegetans
Histopathologic examination of the biopsies from the scalp and left anterior thigh revealed suprabasal clefting with acantholytic cells extending into the follicular infundibulum with eosinophilic pustules within the epidermis. The dermis contained perivascular lymphohistiocytic and eosinophilic inflammatory infiltrates without viral cytopathic effects (Figure 1). Direct immunofluorescence revealed strong IgG and moderate IgA pericellular deposition around keratinocyte cytoplasms (Figure 2). Serologic evaluation demonstrated anti–desmoglein 3 antibodies. Based on the clinical presentation and histopathologic correlation, a diagnosis of pemphigus vegetans was made.


Pemphigus vegetans is a vesiculobullous autoimmune disease that is similar to pemphigus vulgaris but is characterized by the formation of vegetative plaques along the intertriginous areas and on the oral mucosa.1 It is the rarest variant of all pemphigus subtypes and was first described by Neumann in 1876.2 There are 2 subtypes of this variant: Hallopeau and Neumann, each with unique characteristics and physical manifestations. The Hallopeau type initially manifests with pustular lesions that rupture and evolve into erosions that commonly become infected. Gradually they merge and multiply to become more painful and vegetative.3 It has a more indolent course and typically responds well to treatment, and prolonged remission can be reached.4 The Neumann type is more severe and manifests with large vesiculobullous and erosive lesions that rupture and ulcerate, forming verrucous crusted vegetative plaques over the erosions.5 The erosions along the edge of the lesions induce new vegetation, becoming dry, hyperkeratotic, and fissured.3 The Neumann type often requires higher-dose steroids and typically is resistant to treatment.4 Patients can present with oral stomatitis and occasionally can develop a fissured or cerebriform appearance of the tongue, as seen in our patient (Figure 3).1,2 Nail changes include onychorrhexis, onychomadesis, subungual pustules, and ultimately nail atrophy.5

Pemphigus diseases are characterized by IgG autoantibodies against desmoglein 3 and/or desmoglein 1. These are components of desmosomes that are responsible for keratinocyte adhesion, disruption of which results in the blister formation seen in pemphigus subtypes. The unique physical manifestation of pemphigus vegetans is thought to be due not only to autoantibodies against desmogleins 1 and 3 but also to autoantibodies against desmocollin 1 and 2.1
Histopathologic examination reveals hyperkeratosis and pseudoepitheliomatous hyperplasia with acantholysis that creates a suprabasal cleft. Basal cells remain intact to the basement membrane by hemidesmosomes, resulting in a tombstone appearance. The Hallopeau type typically manifests with a large eosinophilic inflammatory response, leading to eosinophilic spongiosis and intraepidermal microabscesses. The Neumann type manifests with more of a neutrophilic and lymphocytic infiltrate, accompanied by the eosinophilic response.1 For evaluation, obtain histopathology as well as direct immunofluorescence or enzyme-linked immunosorbent assay to look for intracellular deposition of desmoglein autoantibodies.
First-line treatment for pemphigus vulgaris and its variants is rituximab, an anti-CD20 monoclonal antibody. It has also been shown to have therapeutic benefit with combination of corticosteroids and rituximab. Corticosteroids should be given at a dose of 1 mg/kg daily for 2 to 4 weeks. Other immunosuppressive agents (steroid sparing) include azathioprine, dapsone, mycophenolate mofetil, methotrexate, cyclophosphamide, cyclosporine, and intravenous immunoglobulin. Pulse therapy with intermittent intravenous corticosteroids and immunosuppressants is another second-line therapeutic option. Topical therapeutic options include steroids, tacrolimus, and nicotinamide with oral tetracycline at onset and relapse. The goal of therapy is to maintain remission for 1 year then slowly taper treatment over another year.1
Our patient initially was treated with prednisone, and subsequent courses of azathioprine and mycophenolate mofetil failed. He then was treated with 2 infusions of rituximab that were given 2 weeks apart. He was able to taper off the prednisone 1 month after the last infusion with complete remission of disease. He has been disease free for more than 9 months postinfusion.
Differential diagnoses for pemphigus vegetans can include bullous pemphigoid, bullous systemic lupus erythematosus, dermatitis herpetiformis, and pemphigus vulgaris. Lesion characteristics are key to differentiating pemphigus vegetans from other autoimmune blistering disorders. Bullous pemphigoid will manifest with tense blisters where pemphigus vulgaris will be flaccid; this is due to the difference in autoantibody targets between the conditions. Diagnosis depends on clinical presentation and histopathologic findings.
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls [Internet]. Updated June 26, 2023. Accessed December 16, 2024. https://www.ncbi.nlm.nih.gov/books/NBK545229/
- Rebello MS, Ramesh BM, Sukumar D, et al. Cerebriform cutaneous lesions in pemphigus vegetans. Indian J Dermatol. 2016;61:206-208.
- Ruocco V, Ruocco E, Caccavale S, et al. Pemphigus vegetans of the folds (intertriginous areas). Clin Dermatol. 2015;33:471-476.
- Ajbani AA, Mehta KS, Marfatia YS. Verrucous lesions over external genitalia as a presenting feature of pemphigus vegetans. Indian J Sex Transm Dis AIDS. 2019;40:176-179.
- Vinay K, De D, Handa S, et al. Pemphigus vegetans presenting as a verrucous plaque on the finger. Clin Exp Dermatol. 2016;41:316-317.
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls [Internet]. Updated June 26, 2023. Accessed December 16, 2024. https://www.ncbi.nlm.nih.gov/books/NBK545229/
- Rebello MS, Ramesh BM, Sukumar D, et al. Cerebriform cutaneous lesions in pemphigus vegetans. Indian J Dermatol. 2016;61:206-208.
- Ruocco V, Ruocco E, Caccavale S, et al. Pemphigus vegetans of the folds (intertriginous areas). Clin Dermatol. 2015;33:471-476.
- Ajbani AA, Mehta KS, Marfatia YS. Verrucous lesions over external genitalia as a presenting feature of pemphigus vegetans. Indian J Sex Transm Dis AIDS. 2019;40:176-179.
- Vinay K, De D, Handa S, et al. Pemphigus vegetans presenting as a verrucous plaque on the finger. Clin Exp Dermatol. 2016;41:316-317.
Painful Oral, Groin, and Scalp Lesions in a Young Man
Painful Oral, Groin, and Scalp Lesions in a Young Man
A 27-year-old man presented to the dermatology department with painful oral and groin lesions of 2 years’ duration as well as lip ulceration that had been present for 1 month. The patient also reported moderately tender scalp and face lesions that had been present for several weeks. The lip ulceration was previously treated by his primary care provider with valacyclovir (1 g daily for 2 weeks) without improvement. Six months prior to the current presentation, we treated the groin lesions as condyloma involving the perineum and genital region at our clinic with no response to cryotherapy, topical imiquimod, or extensive surgical excision with skin grafting. Pathology at the time showed condyloma but was negative for human papillomavirus. Physical examination at the current presentation revealed superficial erosions along the vermilion border. The oral mucosa exhibited cobblestoning, and fissures were present on the tongue. Eroded pink plaques studded with vesicles were present on the vertex scalp and left chin. The bilateral inguinal regions extending to anterior-lateral upper thighs and posterior buttocks revealed erythematous, arcuate, and annular erosive plaques with verrucous hyperkeratotic borders and fissuring on the leading edge. Pink erosive and verrucous erythematous plaques were noted on the penile shaft, scrotum, and perineum. Punch biopsies of the scalp and left anterior thigh as well as direct immunofluorescence were performed.
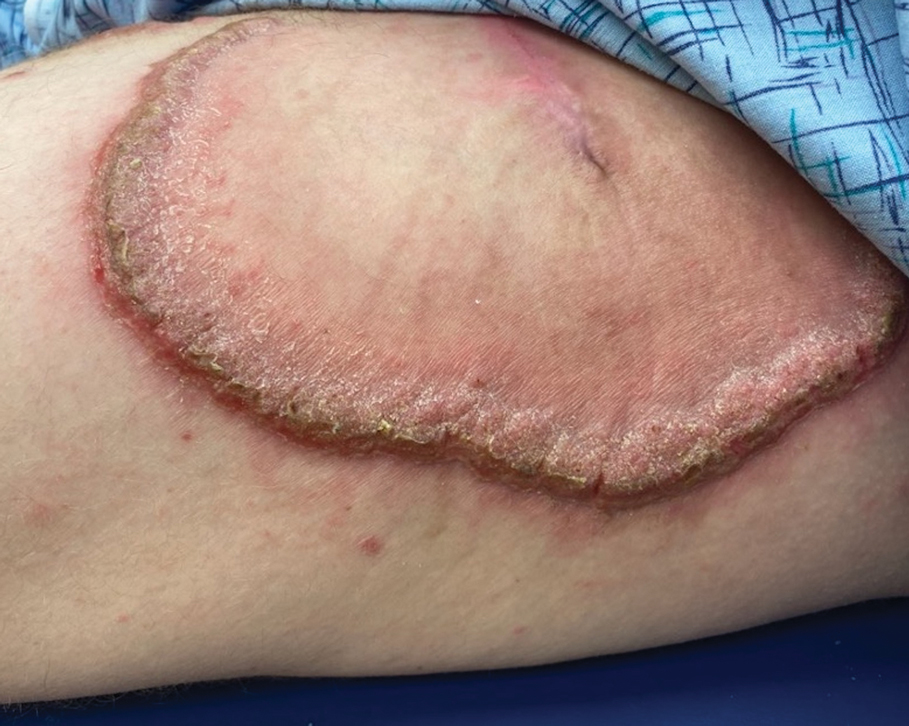
Optimal Exercise Levels for Dermatology Patients With Psoriasis
Optimal Exercise Levels for Dermatology Patients With Psoriasis
There is a direct link between psoriasis and metabolic conditions such as diabetes mellitus and obesity.1 Exercise of varied intensity in patients with chronic inflammatory and metabolic conditions can help improve quality of life and severity of disease; however, there has not been a clear consensus on the recommended duration and types of exercise that are most advantageous.1-5 We reviewed the literature to identify physical and mental health impacts of exercise on patients with psoriasis, and we present the recommended duration and types of exercise that are most impactful for these patients.
One indicator of the link between psoriasis and exercise is the level of peroxisome proliferator activated receptor gamma coactivator-1 α (PGC-1α) in muscle cells.2 This marker reduces inflammation. When levels are low in muscle cells, an induction occurs that leads to systemic or local inflammation; however, skeletal muscle PGC-1α levels increase following exercise, indicating reduced inflammation.2 The level of PGC-1α is measured through muscle biopsy and polymerase chain reaction.6 Another indicator of the correlation between exercise and inflammation is lipoprotein-associated phospholipase A2, which is produced by inflammatory cells and has a correlation with cardiovascular disease. Exercise reduces lipoprotein-associated phospholipase A2 levels, and a sedentary lifestyle correlates with increased levels of this marker.3 Lipoprotein-associated phospholipase A2 is measured through an enzyme-linked immunosorbent assay of the blood, with levels around 200 ng/mL considered high.7 Patients with psoriasis are 30% less likely to participate in physical activity compared to patients without psoriasis, which can be attributed to psychosocial impairment and other factors. Sedentary lifestyle is associated with new or worsening metabolic disease and prevalence of psoriatic lesions.1
A metabolic equivalent task score is a classification system that measures the rate of the body’s oxygen uptake for any given activity.4 A score of 20.9 or more metabolic equivalent task hours of vigorous exercise per week—equal to 105 minutes of running or 180 minutes of swimming or playing tennis—is linked with a 25% to 30% risk reduction of psoriasis in women.4 Therefore, we recommend 30 minutes of exercise 4 to 5 times per week for women. These periods of exercise should consist mainly of activities that will not cause psoriasis flares due to excessive sweating, skin trauma, or prolonged sun exposure.5 Walking, yoga, and bike riding all could be good exercise options for those with psoriasis. The National Psoriasis Foundation offers guidance on physical activity in patients with psoriasis or psoriatic arthritis.8 Psoriasis has apparent physical and psychosocial impacts on patients that can be prevented and improved through the exercise recommendations presented in this article. Dermatologists should use these recommendations to address psoriasis in their everyday practice.
- Do YK, Lakhani N, Malhotra R, et al. Association between psoriasis and leisure-time physical activity: findings from the National Health and Nutrition Examination Survey. J Dermatol. 2015;42:148-153. doi:10.1111/1346-8138.12721
- Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454: 463-469. doi:10.1038/nature07206
- Clark K, Sharp S, Womack CJ, et al. Increased sedentary time and decreased physical activity increases lipoprotein associated phospholipase A2 in obese individuals. Nutr Metab Cardiovasc Dis. 2022;32:1703-1710. doi:10.1016/j.numecd.2022.04.023
- Yeh C, Flatley E, Elkattawy O, et al. Exercise in dermatology: exercise’s influence on skin aging, skin cancer, psoriasis, venous ulcers, and androgenetic alopecia. J Am Acad Dermatol. 2022;87:183-184. doi:10.1016/j.jaad.2021.07.023
- Sheppard R, Gan WK, Onambele-Pearson GL, et al. Developing an aerobic exercise intervention for patients with psoriasis to support lifestyle behaviour change and improve health outcomes. Clin Exp Dermatol. 2023;48:5-11. doi:10.1093/ced/llac008
- Lin J, Wu H, Tarr P, et al. Transcriptional co-activator PGC-1a drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797-801. doi:10.1038/nature00904
- Lin J, Wu H, Tarr P, et al. Transcriptional co-activator PGC-1a drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797-801. doi:10.1038/nature00904.023
- National Psoriasis Foundation. Active and mindful lifestyles. https://www.psoriasis.org/active-and-mindful-lifestyles/
There is a direct link between psoriasis and metabolic conditions such as diabetes mellitus and obesity.1 Exercise of varied intensity in patients with chronic inflammatory and metabolic conditions can help improve quality of life and severity of disease; however, there has not been a clear consensus on the recommended duration and types of exercise that are most advantageous.1-5 We reviewed the literature to identify physical and mental health impacts of exercise on patients with psoriasis, and we present the recommended duration and types of exercise that are most impactful for these patients.
One indicator of the link between psoriasis and exercise is the level of peroxisome proliferator activated receptor gamma coactivator-1 α (PGC-1α) in muscle cells.2 This marker reduces inflammation. When levels are low in muscle cells, an induction occurs that leads to systemic or local inflammation; however, skeletal muscle PGC-1α levels increase following exercise, indicating reduced inflammation.2 The level of PGC-1α is measured through muscle biopsy and polymerase chain reaction.6 Another indicator of the correlation between exercise and inflammation is lipoprotein-associated phospholipase A2, which is produced by inflammatory cells and has a correlation with cardiovascular disease. Exercise reduces lipoprotein-associated phospholipase A2 levels, and a sedentary lifestyle correlates with increased levels of this marker.3 Lipoprotein-associated phospholipase A2 is measured through an enzyme-linked immunosorbent assay of the blood, with levels around 200 ng/mL considered high.7 Patients with psoriasis are 30% less likely to participate in physical activity compared to patients without psoriasis, which can be attributed to psychosocial impairment and other factors. Sedentary lifestyle is associated with new or worsening metabolic disease and prevalence of psoriatic lesions.1
A metabolic equivalent task score is a classification system that measures the rate of the body’s oxygen uptake for any given activity.4 A score of 20.9 or more metabolic equivalent task hours of vigorous exercise per week—equal to 105 minutes of running or 180 minutes of swimming or playing tennis—is linked with a 25% to 30% risk reduction of psoriasis in women.4 Therefore, we recommend 30 minutes of exercise 4 to 5 times per week for women. These periods of exercise should consist mainly of activities that will not cause psoriasis flares due to excessive sweating, skin trauma, or prolonged sun exposure.5 Walking, yoga, and bike riding all could be good exercise options for those with psoriasis. The National Psoriasis Foundation offers guidance on physical activity in patients with psoriasis or psoriatic arthritis.8 Psoriasis has apparent physical and psychosocial impacts on patients that can be prevented and improved through the exercise recommendations presented in this article. Dermatologists should use these recommendations to address psoriasis in their everyday practice.
There is a direct link between psoriasis and metabolic conditions such as diabetes mellitus and obesity.1 Exercise of varied intensity in patients with chronic inflammatory and metabolic conditions can help improve quality of life and severity of disease; however, there has not been a clear consensus on the recommended duration and types of exercise that are most advantageous.1-5 We reviewed the literature to identify physical and mental health impacts of exercise on patients with psoriasis, and we present the recommended duration and types of exercise that are most impactful for these patients.
One indicator of the link between psoriasis and exercise is the level of peroxisome proliferator activated receptor gamma coactivator-1 α (PGC-1α) in muscle cells.2 This marker reduces inflammation. When levels are low in muscle cells, an induction occurs that leads to systemic or local inflammation; however, skeletal muscle PGC-1α levels increase following exercise, indicating reduced inflammation.2 The level of PGC-1α is measured through muscle biopsy and polymerase chain reaction.6 Another indicator of the correlation between exercise and inflammation is lipoprotein-associated phospholipase A2, which is produced by inflammatory cells and has a correlation with cardiovascular disease. Exercise reduces lipoprotein-associated phospholipase A2 levels, and a sedentary lifestyle correlates with increased levels of this marker.3 Lipoprotein-associated phospholipase A2 is measured through an enzyme-linked immunosorbent assay of the blood, with levels around 200 ng/mL considered high.7 Patients with psoriasis are 30% less likely to participate in physical activity compared to patients without psoriasis, which can be attributed to psychosocial impairment and other factors. Sedentary lifestyle is associated with new or worsening metabolic disease and prevalence of psoriatic lesions.1
A metabolic equivalent task score is a classification system that measures the rate of the body’s oxygen uptake for any given activity.4 A score of 20.9 or more metabolic equivalent task hours of vigorous exercise per week—equal to 105 minutes of running or 180 minutes of swimming or playing tennis—is linked with a 25% to 30% risk reduction of psoriasis in women.4 Therefore, we recommend 30 minutes of exercise 4 to 5 times per week for women. These periods of exercise should consist mainly of activities that will not cause psoriasis flares due to excessive sweating, skin trauma, or prolonged sun exposure.5 Walking, yoga, and bike riding all could be good exercise options for those with psoriasis. The National Psoriasis Foundation offers guidance on physical activity in patients with psoriasis or psoriatic arthritis.8 Psoriasis has apparent physical and psychosocial impacts on patients that can be prevented and improved through the exercise recommendations presented in this article. Dermatologists should use these recommendations to address psoriasis in their everyday practice.
- Do YK, Lakhani N, Malhotra R, et al. Association between psoriasis and leisure-time physical activity: findings from the National Health and Nutrition Examination Survey. J Dermatol. 2015;42:148-153. doi:10.1111/1346-8138.12721
- Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454: 463-469. doi:10.1038/nature07206
- Clark K, Sharp S, Womack CJ, et al. Increased sedentary time and decreased physical activity increases lipoprotein associated phospholipase A2 in obese individuals. Nutr Metab Cardiovasc Dis. 2022;32:1703-1710. doi:10.1016/j.numecd.2022.04.023
- Yeh C, Flatley E, Elkattawy O, et al. Exercise in dermatology: exercise’s influence on skin aging, skin cancer, psoriasis, venous ulcers, and androgenetic alopecia. J Am Acad Dermatol. 2022;87:183-184. doi:10.1016/j.jaad.2021.07.023
- Sheppard R, Gan WK, Onambele-Pearson GL, et al. Developing an aerobic exercise intervention for patients with psoriasis to support lifestyle behaviour change and improve health outcomes. Clin Exp Dermatol. 2023;48:5-11. doi:10.1093/ced/llac008
- Lin J, Wu H, Tarr P, et al. Transcriptional co-activator PGC-1a drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797-801. doi:10.1038/nature00904
- Lin J, Wu H, Tarr P, et al. Transcriptional co-activator PGC-1a drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797-801. doi:10.1038/nature00904.023
- National Psoriasis Foundation. Active and mindful lifestyles. https://www.psoriasis.org/active-and-mindful-lifestyles/
- Do YK, Lakhani N, Malhotra R, et al. Association between psoriasis and leisure-time physical activity: findings from the National Health and Nutrition Examination Survey. J Dermatol. 2015;42:148-153. doi:10.1111/1346-8138.12721
- Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454: 463-469. doi:10.1038/nature07206
- Clark K, Sharp S, Womack CJ, et al. Increased sedentary time and decreased physical activity increases lipoprotein associated phospholipase A2 in obese individuals. Nutr Metab Cardiovasc Dis. 2022;32:1703-1710. doi:10.1016/j.numecd.2022.04.023
- Yeh C, Flatley E, Elkattawy O, et al. Exercise in dermatology: exercise’s influence on skin aging, skin cancer, psoriasis, venous ulcers, and androgenetic alopecia. J Am Acad Dermatol. 2022;87:183-184. doi:10.1016/j.jaad.2021.07.023
- Sheppard R, Gan WK, Onambele-Pearson GL, et al. Developing an aerobic exercise intervention for patients with psoriasis to support lifestyle behaviour change and improve health outcomes. Clin Exp Dermatol. 2023;48:5-11. doi:10.1093/ced/llac008
- Lin J, Wu H, Tarr P, et al. Transcriptional co-activator PGC-1a drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797-801. doi:10.1038/nature00904
- Lin J, Wu H, Tarr P, et al. Transcriptional co-activator PGC-1a drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797-801. doi:10.1038/nature00904.023
- National Psoriasis Foundation. Active and mindful lifestyles. https://www.psoriasis.org/active-and-mindful-lifestyles/
Optimal Exercise Levels for Dermatology Patients With Psoriasis
Optimal Exercise Levels for Dermatology Patients With Psoriasis
PRACTICE POINTS
- Patients with psoriasis should exercise for less time (~30 min) more frequently (4–5 times per week).
- Exercise that involves excessive sweating should be avoided; recommended types of exercise for patients with psoriasis include walking, yoga, and bike riding.
- Physicians should educate patients on the processes behind psoriasis and direct them to the National Psoriasis Foundation’s website when needed.