User login
Assessment steps and treatment tips for ankle arthritis
CASE › A 57-year-old man had been experiencing intermittent pain in his left ankle for the past 2.5 years. About 6 weeks before coming to our clinic, his symptoms became significantly worse after playing a pickup game of basketball. At the clinic visit, he reported no other recent injury or trauma to the leg. However, 15 years earlier he had fractured his left ankle and was treated conservatively with a short period in a cast followed by a course of physical therapy. After completing the physical therapy, he noted significant improvement, although he continued to have minor episodes of pain. He felt no instability or mechanical locking but did note a decreased ability to move the ankle. And it felt much stiffer than his right ankle.
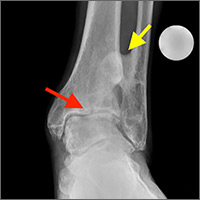
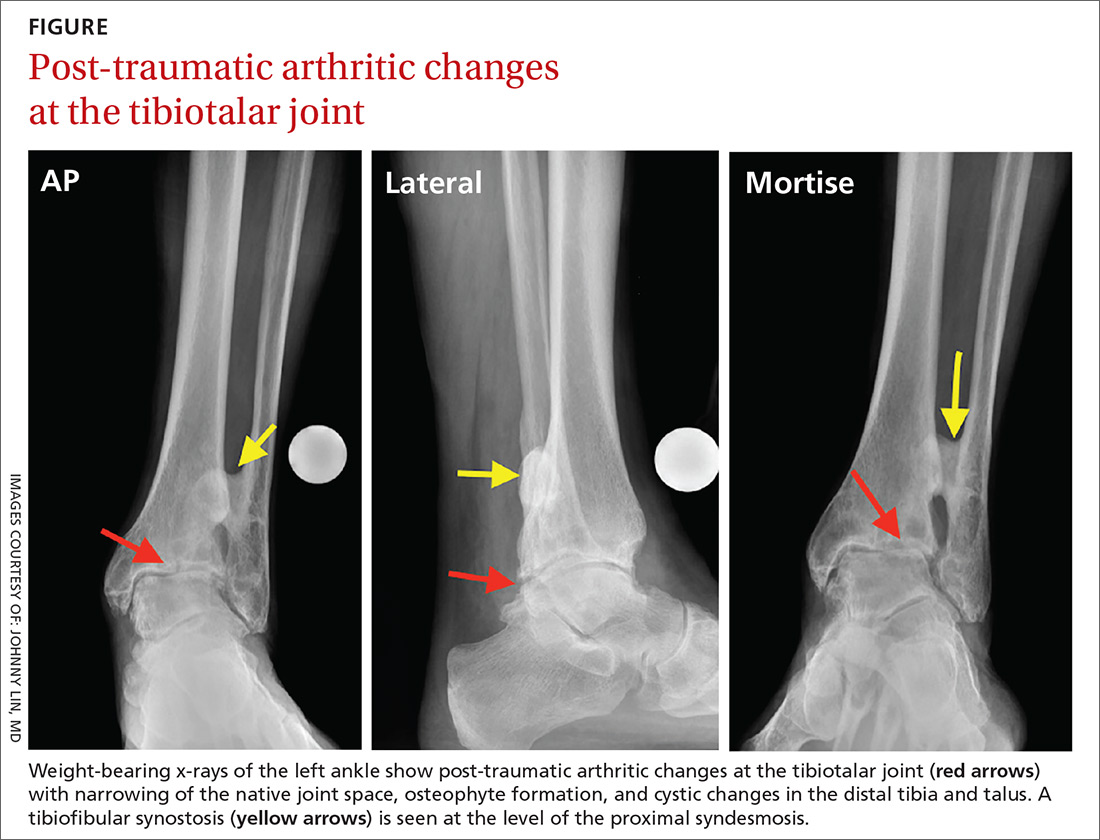
Examination of his left ankle revealed tenderness over the anterior aspect at the tibiotalar joint. He also exhibited decreased dorsiflexion and was unable to perform a toe raise. There was no tenderness over the major ligaments, and results of anterior drawer and talar tilt tests were normal. X-rays revealed tibiotalar joint arthritis (FIGURE).
How would you proceed if this were your patient?
Arthritis of the tibiotalar joint, which has an estimated prevalence of approximately 1%, occurs much less frequently than arthritis of the knee or hip joints.1 This low prevalence is primarily due to the ankle joint’s unique biomechanics and the features of the cartilage within the joint, including its thickness.2
Specifically, the hip and knee joints have greater degrees of freedom than the tibiotalar articulation, which is significantly constrained. The bony congruity between the talus, tibia, and fibula provides inherent stability to the ankle joint, thus protecting against primary osteoarthritis (OA).
Additionally, the large number of ligamentous structures and overall strength of the ligaments provide significant supplemental stability to the ankle joint articulation. Articular cartilage within the ankle joint is thicker than that of the knee and hip (1-1.7 mm). This cartilage also tends to retain its tensile strength with age, unlike cartilage in the hip; the ankle is therefore more resistant to age-related degeneration.3
Metabolic factors also protect against arthritis. Chondrocytes in the ankle are less responsive to inflammatory mediators, including interleukin-1 (IL-1), and therefore produce fewer matrix metalloproteinases.1,2,4 There are also fewer IL-1 receptors on ankle chondrocytes.
The role of trauma in ankle OA. Given the ankle joint’s inherent stability, the most common cause of ankle OA is trauma,4 mainly ankle fracture and, less commonly, ligamentous injury.5,6 Other rarer causes of ankle arthritis include primary OA, crystalline arthropathy, inflammatory disease, septic arthritis, neuroarthropathy, hemochromatosis, and ochronosis.
The ankle’s characteristics that protect it against primary OA may facilitate the pathogenesis of post-traumatic OA through 2 main mechanisms. First, direct trauma to the chondral surfaces can hasten the onset of progressive degeneration. Second, articular incongruity from a fracture can lead to insidious deterioration. The stiffer cartilage layer may be less adaptable to malalignment, and incongruity may cause secondary instability and chronic overloading. Ultimately, the joint breaks down with associated cartilage wear.6,7
The importance of the normal ankle’s congruity and stability became clear in the landmark study by Ramsey and colleagues,8 showing that the contact area between the talus and the tibia decreases as talar displacement increases laterally. This innate stability explains why the contact area of the ankle joint can bear loads similar to those of the hip and knee, yet does not experience primary OA nearly as often.
A stepwise diagnostic appraisal
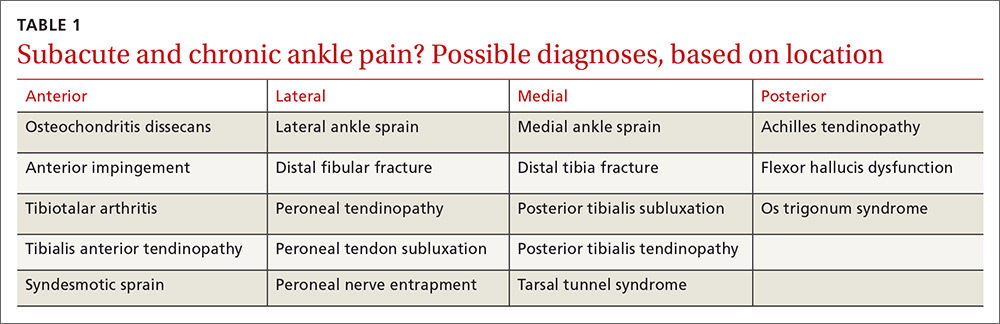
Ask these questions. Since most ankle pain results from trauma, ask about any recent or remote injury to the affected ankle. Knowing the type of injury that occurred and the exact treatment, if received, may shed light on the relationship between the injury and current symptoms. Acute traumatic events can cause fractures or injury to various soft-tissue structures traversing the ankle joint. Ankle ligament sprains or tendon strains may result after abnormal rotation of the foot. Alternatively, chronic overuse injuries may lead to tendinopathy in any of the tendons that control motion throughout the foot and ankle or degenerative changes within the tibiotalar joint. Knowing the exact location of pain may also help identify the pathology (TABLE 1).
The patient in our case had not suffered a recent injury, so it was important to learn as much as possible about his prior fracture. Was the injury treated conservatively or surgically? If management was conservative, the type and duration of treatment could offer clues to the mechanism underlying symptoms. If a patient has undergone surgery, knowledge of the exact procedure could suggest specific problems. For example, surgical fixation would likely indicate there was ankle instability, thus altering the normal biomechanics in the injured tibiotalar joint.
Other key questions to ask. Most patients with ankle pain also complain of limitations in their usual activities. Ask about the duration and type of pain and other symptoms. Also ask about the position of the foot and ankle when the pain is at its greatest, which will provide insight into likely areas of pathology. For example, if pain arises when the patient navigates uneven ground, subtalar pathology is highly likely. If the patient complains of pain while walking down stairs, suspect injury to the posterior (plantar flexed) ankle; pain while walking up stairs more likely indicates anterior (dorsiflexed) pathology.
Finally, ask about nonorthopedic medical problems and all medications being taken. Systemic conditions, too, can lead to ankle pain—eg, inflammatory arthropathies, infections, and crystalline arthropathy.
Physical examination. Observe the patient’s gait to assess any functional or range-of-motion limitations or abnormal loading throughout the foot and ankle.9 With the patient standing, evaluate any malalignment from the foot through the knees and to the hips. Evaluate the skin for any lesions, wounds, or evidence of trauma or surgery. Next, with the patient seated, examine carefully for neuropathy or vascular abnormalities. Evaluate the ankle’s range of motion and assess for any mechanical locking, clicking, or crepitus. Palpate all bony and ligamentous landmarks to reveal areas of tenderness or swelling. Perform anterior drawer and varus tilt tests to determine overall ligamentous stability of the ankle, and compare your findings with test results of the opposite, uninjured ankle.
Diagnostic imaging. Order weight-bearing radiographs of the foot and ankle. Including the foot allows you to identify additional potential concerns such as malalignment, deformity, or adjacent joint arthritis. Look particularly for joint space narrowing, malalignment, post-traumatic changes, or implanted hardware. Advanced imaging studies—computerized tomography, magnetic resonance imaging, bone scan—are reserved for cases that necessitate ruling out alternative diagnoses, or for preoperative evaluation by an orthopedic surgeon.
Management: Make use of multiple modalities
Conservative management options for ankle OA are limited, and high-quality evidence of efficacy is lacking. Surgical alternatives, however, are invasive and yield modest outcomes. Therefore, unless specific indications for surgery are present, exhaust conservative options (TABLE 2) before considering referral.
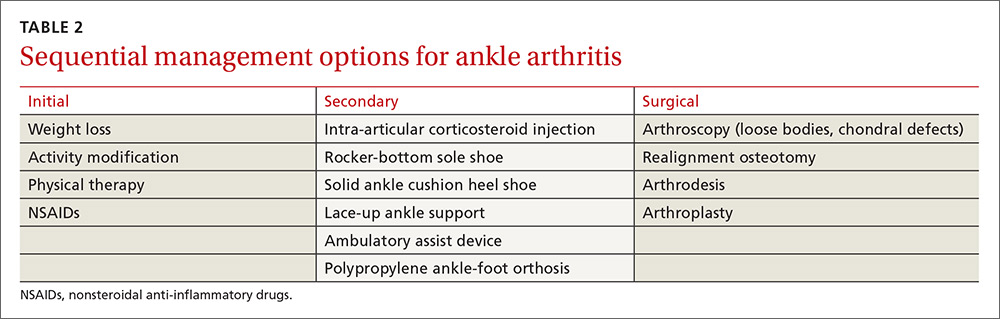
Weight loss is important for those who are overweight—as with knee OA management—to decrease the reactive forces within the ankle joint and to decrease pain. Weight loss will also enhance the outcomes of other treatment modalities and improve overall health.10,11
Activity modification is usually required, even though this may make weight loss more difficult. Avoiding vigorous activities, restricting work-related movements that place high-impact stress on the ankle, and decreasing overall walking time often reduce the severity of symptoms and improve functioning in other activities. Use of assistive-devices, such as a cane, can decrease the weight-bearing load on the affected joint.10,11
Physical therapy has not been shown to alleviate pain in ankle arthritis, although stretching, joint mobilization, and gait training may help prevent further progression of arthritis and improve function.11 The strength of dorsiflexion and plantar-flexion muscles is often decreased in individuals with ankle arthritis. Strengthening exercises may be indicated in individuals exhibiting deficits.
Prescriptive conservative management. Begin with a combination, as needed, of anti-inflammatory medications, orthotic devices, and footwear modifications.
Nonsteroidal anti-inflammatory agents are generally safe, but long-term use requires monitoring. Intra-articular steroid injections have some supporting evidence of effectiveness, but any benefit is short-lived.12 Glucosamine and chondroitin, although unlikely to cause harm, are not supported by the evidence for use in ankle arthritis. Intra-articular viscosupplementation is controversial, and evidence is limited regarding its efficacy.1
Adding a rocker-bottom sole and a solid ankle cushion heel to a shoe helps decrease heel strike impact in individuals with decreased ankle motion, and they aid in the transition from the heel strike to the push-off during level walking.11 If the arthritic joint is unstable, a lace-up ankle support may help with proprioception and stability. A polypropylene ankle-foot orthosis, custom leather ankle corset, or a double-upright brace with a patellar-tendon-bearing support are options to restrict ankle motion and decrease weight-bearing forces.10,11
Immobilization is not recommended except for short-term use during an arthritic flare. Limiting ankle motion reduces pain, but the downside tradeoff is acquired stiffness and weakness that accompanies prolonged periods of immobilization. A controlled ankle motion walking boot or walking plaster cast are both reasonable options for the short term.
Consider surgical referral for specific indications such as osteophytes, loose bodies, and chondral defects, which may be treated with arthroscopy. Patients with large areas of exposed chondral bone or rapid onset of degeneration have poorer outcomes with conservative management and should also be referred to a surgeon earlier. Otherwise, consider surgical referral only after a full trial of conservative management.11
Surgical options vary in scope and effectiveness and include osteotomy, arthrodesis, and arthroplasty. Osteotomies can be performed in early OA to correct bony alignment deformities. Arthrodesis in neutral dorsiflexion with roughly 5 degrees of external rotation is reserved for end-stage ankle OA to allow for near normal gait and pain relief. Total ankle arthroplasty is an emerging option for severe ankle OA, resulting in improved pain relief, gait, and patient satisfaction, but potentially has a higher reoperation rate when compared with arthrodesis.1,2
CASE › We prescribed short-term immobilization with a controlled ankle motion boot and administered an intra-articular corticosteroid injection. At the patient’s follow-up visit 6 weeks later, he reported only moderate improvement in pain. We then advised physical therapy at a specialty ankle rehabilitation program to focus on mobilization, strengthening, and gait training. Nearly one year after his initial visit to our clinic, he is doing well. He understands, however, that the nature of his ankle arthrosis may necessitate surgical intervention in the future.
CORRESPONDENCE
Adam Bitterman, DO, Department of Orthopedic Surgery, Hofstra Northwell School of Medicine at Huntington Hospital, 155 East Main Street, Huntington, NY 11743; [email protected].
1. Valderrabano V, Horisberger M, Russell I, et al. Etiology of ankle osteoarthritis. Clin Orthop Relat Res. 2009;467:1800-1806.
2. Huch K, Kuettner KE, Dieppe P. Osteoarthritis in ankle and knee joints. Semin Arthritis Rheum. 1997;26:667-674.
3. Kempson GE. Age-related changes in the tensile properties of human articular cartilage: a comparative study between the femoral head of the hip joint and the talus of the ankle joint. Biochim Biophys Acta. 1991;1075:223-230.
4. Saltzman CL, Salamon ML, Blanchard GM, et al. Epidemiology of ankle arthritis: Report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44-46.
5. Brown T, Johnston R, Saltzman C, et al. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20:739-744.
6. Barg A, Pagenstert G, Hügle T, et al. Ankle osteoarthritis etiology, diagnostics and classification. Foot Ankle Clin. 2013;18:411-426.
7. Schenker M, Mauck R, Ahn J, et al. Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J Am Acad Orthop Surg. 2014;22:20-28.
8. Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58:356-357.
9. Hayes BJ, Gonzalez T, Smith JT, et al. Ankle arthritis: you can’t always replace it. J Am Acad Orthop Surg. 2016;24:e29-e38.
10. Thomas R, Daniels T. Current concepts review ankle arthritis. J Bone Joint Surg Am. 2003;85A:923-936.
11. Martin RL, Stewart GW, Conti SF. Posttraumatic ankle arthritis: an update on conservative and surgical management. J Orthop Sports Phys Ther. 2007:37:253-259.
12. Pekarek B, Osher L, Buck S, et al. Intra-articular corticosteroid injections: a critical literature review with up-to-date findings. Foot. 2011;21:66-70.
13. Abate M, Schiavone C, Salini V. Hyaluronic acid in ankle arthritis: why evidence of efficacy is still lacking? Clin Exp Rheumatol. 2012;30:277-281.
14. Witteveen AG, Hofstad CJ, Kerkhoffs GM. Hyaluronic acid and other conservative treatment options for osteoarthritis of the ankle. Cochrane Database Syst Rev. 2015;(10):CD010643.
15. Rao S, Ellis SJ, Deland JT, et al. Nonmedicinal therapy in the management of ankle arthritis. Curr Opin Rheumatol. 2010;22:223-228.
CASE › A 57-year-old man had been experiencing intermittent pain in his left ankle for the past 2.5 years. About 6 weeks before coming to our clinic, his symptoms became significantly worse after playing a pickup game of basketball. At the clinic visit, he reported no other recent injury or trauma to the leg. However, 15 years earlier he had fractured his left ankle and was treated conservatively with a short period in a cast followed by a course of physical therapy. After completing the physical therapy, he noted significant improvement, although he continued to have minor episodes of pain. He felt no instability or mechanical locking but did note a decreased ability to move the ankle. And it felt much stiffer than his right ankle.
Examination of his left ankle revealed tenderness over the anterior aspect at the tibiotalar joint. He also exhibited decreased dorsiflexion and was unable to perform a toe raise. There was no tenderness over the major ligaments, and results of anterior drawer and talar tilt tests were normal. X-rays revealed tibiotalar joint arthritis (FIGURE).

How would you proceed if this were your patient?
Arthritis of the tibiotalar joint, which has an estimated prevalence of approximately 1%, occurs much less frequently than arthritis of the knee or hip joints.1 This low prevalence is primarily due to the ankle joint’s unique biomechanics and the features of the cartilage within the joint, including its thickness.2
Specifically, the hip and knee joints have greater degrees of freedom than the tibiotalar articulation, which is significantly constrained. The bony congruity between the talus, tibia, and fibula provides inherent stability to the ankle joint, thus protecting against primary osteoarthritis (OA).
Additionally, the large number of ligamentous structures and overall strength of the ligaments provide significant supplemental stability to the ankle joint articulation. Articular cartilage within the ankle joint is thicker than that of the knee and hip (1-1.7 mm). This cartilage also tends to retain its tensile strength with age, unlike cartilage in the hip; the ankle is therefore more resistant to age-related degeneration.3
Metabolic factors also protect against arthritis. Chondrocytes in the ankle are less responsive to inflammatory mediators, including interleukin-1 (IL-1), and therefore produce fewer matrix metalloproteinases.1,2,4 There are also fewer IL-1 receptors on ankle chondrocytes.
The role of trauma in ankle OA. Given the ankle joint’s inherent stability, the most common cause of ankle OA is trauma,4 mainly ankle fracture and, less commonly, ligamentous injury.5,6 Other rarer causes of ankle arthritis include primary OA, crystalline arthropathy, inflammatory disease, septic arthritis, neuroarthropathy, hemochromatosis, and ochronosis.
The ankle’s characteristics that protect it against primary OA may facilitate the pathogenesis of post-traumatic OA through 2 main mechanisms. First, direct trauma to the chondral surfaces can hasten the onset of progressive degeneration. Second, articular incongruity from a fracture can lead to insidious deterioration. The stiffer cartilage layer may be less adaptable to malalignment, and incongruity may cause secondary instability and chronic overloading. Ultimately, the joint breaks down with associated cartilage wear.6,7
The importance of the normal ankle’s congruity and stability became clear in the landmark study by Ramsey and colleagues,8 showing that the contact area between the talus and the tibia decreases as talar displacement increases laterally. This innate stability explains why the contact area of the ankle joint can bear loads similar to those of the hip and knee, yet does not experience primary OA nearly as often.
A stepwise diagnostic appraisal
Ask these questions. Since most ankle pain results from trauma, ask about any recent or remote injury to the affected ankle. Knowing the type of injury that occurred and the exact treatment, if received, may shed light on the relationship between the injury and current symptoms. Acute traumatic events can cause fractures or injury to various soft-tissue structures traversing the ankle joint. Ankle ligament sprains or tendon strains may result after abnormal rotation of the foot. Alternatively, chronic overuse injuries may lead to tendinopathy in any of the tendons that control motion throughout the foot and ankle or degenerative changes within the tibiotalar joint. Knowing the exact location of pain may also help identify the pathology (TABLE 1).

The patient in our case had not suffered a recent injury, so it was important to learn as much as possible about his prior fracture. Was the injury treated conservatively or surgically? If management was conservative, the type and duration of treatment could offer clues to the mechanism underlying symptoms. If a patient has undergone surgery, knowledge of the exact procedure could suggest specific problems. For example, surgical fixation would likely indicate there was ankle instability, thus altering the normal biomechanics in the injured tibiotalar joint.
Other key questions to ask. Most patients with ankle pain also complain of limitations in their usual activities. Ask about the duration and type of pain and other symptoms. Also ask about the position of the foot and ankle when the pain is at its greatest, which will provide insight into likely areas of pathology. For example, if pain arises when the patient navigates uneven ground, subtalar pathology is highly likely. If the patient complains of pain while walking down stairs, suspect injury to the posterior (plantar flexed) ankle; pain while walking up stairs more likely indicates anterior (dorsiflexed) pathology.
Finally, ask about nonorthopedic medical problems and all medications being taken. Systemic conditions, too, can lead to ankle pain—eg, inflammatory arthropathies, infections, and crystalline arthropathy.
Physical examination. Observe the patient’s gait to assess any functional or range-of-motion limitations or abnormal loading throughout the foot and ankle.9 With the patient standing, evaluate any malalignment from the foot through the knees and to the hips. Evaluate the skin for any lesions, wounds, or evidence of trauma or surgery. Next, with the patient seated, examine carefully for neuropathy or vascular abnormalities. Evaluate the ankle’s range of motion and assess for any mechanical locking, clicking, or crepitus. Palpate all bony and ligamentous landmarks to reveal areas of tenderness or swelling. Perform anterior drawer and varus tilt tests to determine overall ligamentous stability of the ankle, and compare your findings with test results of the opposite, uninjured ankle.
Diagnostic imaging. Order weight-bearing radiographs of the foot and ankle. Including the foot allows you to identify additional potential concerns such as malalignment, deformity, or adjacent joint arthritis. Look particularly for joint space narrowing, malalignment, post-traumatic changes, or implanted hardware. Advanced imaging studies—computerized tomography, magnetic resonance imaging, bone scan—are reserved for cases that necessitate ruling out alternative diagnoses, or for preoperative evaluation by an orthopedic surgeon.
Management: Make use of multiple modalities
Conservative management options for ankle OA are limited, and high-quality evidence of efficacy is lacking. Surgical alternatives, however, are invasive and yield modest outcomes. Therefore, unless specific indications for surgery are present, exhaust conservative options (TABLE 2) before considering referral.

Weight loss is important for those who are overweight—as with knee OA management—to decrease the reactive forces within the ankle joint and to decrease pain. Weight loss will also enhance the outcomes of other treatment modalities and improve overall health.10,11
Activity modification is usually required, even though this may make weight loss more difficult. Avoiding vigorous activities, restricting work-related movements that place high-impact stress on the ankle, and decreasing overall walking time often reduce the severity of symptoms and improve functioning in other activities. Use of assistive-devices, such as a cane, can decrease the weight-bearing load on the affected joint.10,11
Physical therapy has not been shown to alleviate pain in ankle arthritis, although stretching, joint mobilization, and gait training may help prevent further progression of arthritis and improve function.11 The strength of dorsiflexion and plantar-flexion muscles is often decreased in individuals with ankle arthritis. Strengthening exercises may be indicated in individuals exhibiting deficits.
Prescriptive conservative management. Begin with a combination, as needed, of anti-inflammatory medications, orthotic devices, and footwear modifications.
Nonsteroidal anti-inflammatory agents are generally safe, but long-term use requires monitoring. Intra-articular steroid injections have some supporting evidence of effectiveness, but any benefit is short-lived.12 Glucosamine and chondroitin, although unlikely to cause harm, are not supported by the evidence for use in ankle arthritis. Intra-articular viscosupplementation is controversial, and evidence is limited regarding its efficacy.1
Adding a rocker-bottom sole and a solid ankle cushion heel to a shoe helps decrease heel strike impact in individuals with decreased ankle motion, and they aid in the transition from the heel strike to the push-off during level walking.11 If the arthritic joint is unstable, a lace-up ankle support may help with proprioception and stability. A polypropylene ankle-foot orthosis, custom leather ankle corset, or a double-upright brace with a patellar-tendon-bearing support are options to restrict ankle motion and decrease weight-bearing forces.10,11
Immobilization is not recommended except for short-term use during an arthritic flare. Limiting ankle motion reduces pain, but the downside tradeoff is acquired stiffness and weakness that accompanies prolonged periods of immobilization. A controlled ankle motion walking boot or walking plaster cast are both reasonable options for the short term.
Consider surgical referral for specific indications such as osteophytes, loose bodies, and chondral defects, which may be treated with arthroscopy. Patients with large areas of exposed chondral bone or rapid onset of degeneration have poorer outcomes with conservative management and should also be referred to a surgeon earlier. Otherwise, consider surgical referral only after a full trial of conservative management.11
Surgical options vary in scope and effectiveness and include osteotomy, arthrodesis, and arthroplasty. Osteotomies can be performed in early OA to correct bony alignment deformities. Arthrodesis in neutral dorsiflexion with roughly 5 degrees of external rotation is reserved for end-stage ankle OA to allow for near normal gait and pain relief. Total ankle arthroplasty is an emerging option for severe ankle OA, resulting in improved pain relief, gait, and patient satisfaction, but potentially has a higher reoperation rate when compared with arthrodesis.1,2
CASE › We prescribed short-term immobilization with a controlled ankle motion boot and administered an intra-articular corticosteroid injection. At the patient’s follow-up visit 6 weeks later, he reported only moderate improvement in pain. We then advised physical therapy at a specialty ankle rehabilitation program to focus on mobilization, strengthening, and gait training. Nearly one year after his initial visit to our clinic, he is doing well. He understands, however, that the nature of his ankle arthrosis may necessitate surgical intervention in the future.
CORRESPONDENCE
Adam Bitterman, DO, Department of Orthopedic Surgery, Hofstra Northwell School of Medicine at Huntington Hospital, 155 East Main Street, Huntington, NY 11743; [email protected].
CASE › A 57-year-old man had been experiencing intermittent pain in his left ankle for the past 2.5 years. About 6 weeks before coming to our clinic, his symptoms became significantly worse after playing a pickup game of basketball. At the clinic visit, he reported no other recent injury or trauma to the leg. However, 15 years earlier he had fractured his left ankle and was treated conservatively with a short period in a cast followed by a course of physical therapy. After completing the physical therapy, he noted significant improvement, although he continued to have minor episodes of pain. He felt no instability or mechanical locking but did note a decreased ability to move the ankle. And it felt much stiffer than his right ankle.
Examination of his left ankle revealed tenderness over the anterior aspect at the tibiotalar joint. He also exhibited decreased dorsiflexion and was unable to perform a toe raise. There was no tenderness over the major ligaments, and results of anterior drawer and talar tilt tests were normal. X-rays revealed tibiotalar joint arthritis (FIGURE).

How would you proceed if this were your patient?
Arthritis of the tibiotalar joint, which has an estimated prevalence of approximately 1%, occurs much less frequently than arthritis of the knee or hip joints.1 This low prevalence is primarily due to the ankle joint’s unique biomechanics and the features of the cartilage within the joint, including its thickness.2
Specifically, the hip and knee joints have greater degrees of freedom than the tibiotalar articulation, which is significantly constrained. The bony congruity between the talus, tibia, and fibula provides inherent stability to the ankle joint, thus protecting against primary osteoarthritis (OA).
Additionally, the large number of ligamentous structures and overall strength of the ligaments provide significant supplemental stability to the ankle joint articulation. Articular cartilage within the ankle joint is thicker than that of the knee and hip (1-1.7 mm). This cartilage also tends to retain its tensile strength with age, unlike cartilage in the hip; the ankle is therefore more resistant to age-related degeneration.3
Metabolic factors also protect against arthritis. Chondrocytes in the ankle are less responsive to inflammatory mediators, including interleukin-1 (IL-1), and therefore produce fewer matrix metalloproteinases.1,2,4 There are also fewer IL-1 receptors on ankle chondrocytes.
The role of trauma in ankle OA. Given the ankle joint’s inherent stability, the most common cause of ankle OA is trauma,4 mainly ankle fracture and, less commonly, ligamentous injury.5,6 Other rarer causes of ankle arthritis include primary OA, crystalline arthropathy, inflammatory disease, septic arthritis, neuroarthropathy, hemochromatosis, and ochronosis.
The ankle’s characteristics that protect it against primary OA may facilitate the pathogenesis of post-traumatic OA through 2 main mechanisms. First, direct trauma to the chondral surfaces can hasten the onset of progressive degeneration. Second, articular incongruity from a fracture can lead to insidious deterioration. The stiffer cartilage layer may be less adaptable to malalignment, and incongruity may cause secondary instability and chronic overloading. Ultimately, the joint breaks down with associated cartilage wear.6,7
The importance of the normal ankle’s congruity and stability became clear in the landmark study by Ramsey and colleagues,8 showing that the contact area between the talus and the tibia decreases as talar displacement increases laterally. This innate stability explains why the contact area of the ankle joint can bear loads similar to those of the hip and knee, yet does not experience primary OA nearly as often.
A stepwise diagnostic appraisal
Ask these questions. Since most ankle pain results from trauma, ask about any recent or remote injury to the affected ankle. Knowing the type of injury that occurred and the exact treatment, if received, may shed light on the relationship between the injury and current symptoms. Acute traumatic events can cause fractures or injury to various soft-tissue structures traversing the ankle joint. Ankle ligament sprains or tendon strains may result after abnormal rotation of the foot. Alternatively, chronic overuse injuries may lead to tendinopathy in any of the tendons that control motion throughout the foot and ankle or degenerative changes within the tibiotalar joint. Knowing the exact location of pain may also help identify the pathology (TABLE 1).

The patient in our case had not suffered a recent injury, so it was important to learn as much as possible about his prior fracture. Was the injury treated conservatively or surgically? If management was conservative, the type and duration of treatment could offer clues to the mechanism underlying symptoms. If a patient has undergone surgery, knowledge of the exact procedure could suggest specific problems. For example, surgical fixation would likely indicate there was ankle instability, thus altering the normal biomechanics in the injured tibiotalar joint.
Other key questions to ask. Most patients with ankle pain also complain of limitations in their usual activities. Ask about the duration and type of pain and other symptoms. Also ask about the position of the foot and ankle when the pain is at its greatest, which will provide insight into likely areas of pathology. For example, if pain arises when the patient navigates uneven ground, subtalar pathology is highly likely. If the patient complains of pain while walking down stairs, suspect injury to the posterior (plantar flexed) ankle; pain while walking up stairs more likely indicates anterior (dorsiflexed) pathology.
Finally, ask about nonorthopedic medical problems and all medications being taken. Systemic conditions, too, can lead to ankle pain—eg, inflammatory arthropathies, infections, and crystalline arthropathy.
Physical examination. Observe the patient’s gait to assess any functional or range-of-motion limitations or abnormal loading throughout the foot and ankle.9 With the patient standing, evaluate any malalignment from the foot through the knees and to the hips. Evaluate the skin for any lesions, wounds, or evidence of trauma or surgery. Next, with the patient seated, examine carefully for neuropathy or vascular abnormalities. Evaluate the ankle’s range of motion and assess for any mechanical locking, clicking, or crepitus. Palpate all bony and ligamentous landmarks to reveal areas of tenderness or swelling. Perform anterior drawer and varus tilt tests to determine overall ligamentous stability of the ankle, and compare your findings with test results of the opposite, uninjured ankle.
Diagnostic imaging. Order weight-bearing radiographs of the foot and ankle. Including the foot allows you to identify additional potential concerns such as malalignment, deformity, or adjacent joint arthritis. Look particularly for joint space narrowing, malalignment, post-traumatic changes, or implanted hardware. Advanced imaging studies—computerized tomography, magnetic resonance imaging, bone scan—are reserved for cases that necessitate ruling out alternative diagnoses, or for preoperative evaluation by an orthopedic surgeon.
Management: Make use of multiple modalities
Conservative management options for ankle OA are limited, and high-quality evidence of efficacy is lacking. Surgical alternatives, however, are invasive and yield modest outcomes. Therefore, unless specific indications for surgery are present, exhaust conservative options (TABLE 2) before considering referral.

Weight loss is important for those who are overweight—as with knee OA management—to decrease the reactive forces within the ankle joint and to decrease pain. Weight loss will also enhance the outcomes of other treatment modalities and improve overall health.10,11
Activity modification is usually required, even though this may make weight loss more difficult. Avoiding vigorous activities, restricting work-related movements that place high-impact stress on the ankle, and decreasing overall walking time often reduce the severity of symptoms and improve functioning in other activities. Use of assistive-devices, such as a cane, can decrease the weight-bearing load on the affected joint.10,11
Physical therapy has not been shown to alleviate pain in ankle arthritis, although stretching, joint mobilization, and gait training may help prevent further progression of arthritis and improve function.11 The strength of dorsiflexion and plantar-flexion muscles is often decreased in individuals with ankle arthritis. Strengthening exercises may be indicated in individuals exhibiting deficits.
Prescriptive conservative management. Begin with a combination, as needed, of anti-inflammatory medications, orthotic devices, and footwear modifications.
Nonsteroidal anti-inflammatory agents are generally safe, but long-term use requires monitoring. Intra-articular steroid injections have some supporting evidence of effectiveness, but any benefit is short-lived.12 Glucosamine and chondroitin, although unlikely to cause harm, are not supported by the evidence for use in ankle arthritis. Intra-articular viscosupplementation is controversial, and evidence is limited regarding its efficacy.1
Adding a rocker-bottom sole and a solid ankle cushion heel to a shoe helps decrease heel strike impact in individuals with decreased ankle motion, and they aid in the transition from the heel strike to the push-off during level walking.11 If the arthritic joint is unstable, a lace-up ankle support may help with proprioception and stability. A polypropylene ankle-foot orthosis, custom leather ankle corset, or a double-upright brace with a patellar-tendon-bearing support are options to restrict ankle motion and decrease weight-bearing forces.10,11
Immobilization is not recommended except for short-term use during an arthritic flare. Limiting ankle motion reduces pain, but the downside tradeoff is acquired stiffness and weakness that accompanies prolonged periods of immobilization. A controlled ankle motion walking boot or walking plaster cast are both reasonable options for the short term.
Consider surgical referral for specific indications such as osteophytes, loose bodies, and chondral defects, which may be treated with arthroscopy. Patients with large areas of exposed chondral bone or rapid onset of degeneration have poorer outcomes with conservative management and should also be referred to a surgeon earlier. Otherwise, consider surgical referral only after a full trial of conservative management.11
Surgical options vary in scope and effectiveness and include osteotomy, arthrodesis, and arthroplasty. Osteotomies can be performed in early OA to correct bony alignment deformities. Arthrodesis in neutral dorsiflexion with roughly 5 degrees of external rotation is reserved for end-stage ankle OA to allow for near normal gait and pain relief. Total ankle arthroplasty is an emerging option for severe ankle OA, resulting in improved pain relief, gait, and patient satisfaction, but potentially has a higher reoperation rate when compared with arthrodesis.1,2
CASE › We prescribed short-term immobilization with a controlled ankle motion boot and administered an intra-articular corticosteroid injection. At the patient’s follow-up visit 6 weeks later, he reported only moderate improvement in pain. We then advised physical therapy at a specialty ankle rehabilitation program to focus on mobilization, strengthening, and gait training. Nearly one year after his initial visit to our clinic, he is doing well. He understands, however, that the nature of his ankle arthrosis may necessitate surgical intervention in the future.
CORRESPONDENCE
Adam Bitterman, DO, Department of Orthopedic Surgery, Hofstra Northwell School of Medicine at Huntington Hospital, 155 East Main Street, Huntington, NY 11743; [email protected].
1. Valderrabano V, Horisberger M, Russell I, et al. Etiology of ankle osteoarthritis. Clin Orthop Relat Res. 2009;467:1800-1806.
2. Huch K, Kuettner KE, Dieppe P. Osteoarthritis in ankle and knee joints. Semin Arthritis Rheum. 1997;26:667-674.
3. Kempson GE. Age-related changes in the tensile properties of human articular cartilage: a comparative study between the femoral head of the hip joint and the talus of the ankle joint. Biochim Biophys Acta. 1991;1075:223-230.
4. Saltzman CL, Salamon ML, Blanchard GM, et al. Epidemiology of ankle arthritis: Report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44-46.
5. Brown T, Johnston R, Saltzman C, et al. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20:739-744.
6. Barg A, Pagenstert G, Hügle T, et al. Ankle osteoarthritis etiology, diagnostics and classification. Foot Ankle Clin. 2013;18:411-426.
7. Schenker M, Mauck R, Ahn J, et al. Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J Am Acad Orthop Surg. 2014;22:20-28.
8. Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58:356-357.
9. Hayes BJ, Gonzalez T, Smith JT, et al. Ankle arthritis: you can’t always replace it. J Am Acad Orthop Surg. 2016;24:e29-e38.
10. Thomas R, Daniels T. Current concepts review ankle arthritis. J Bone Joint Surg Am. 2003;85A:923-936.
11. Martin RL, Stewart GW, Conti SF. Posttraumatic ankle arthritis: an update on conservative and surgical management. J Orthop Sports Phys Ther. 2007:37:253-259.
12. Pekarek B, Osher L, Buck S, et al. Intra-articular corticosteroid injections: a critical literature review with up-to-date findings. Foot. 2011;21:66-70.
13. Abate M, Schiavone C, Salini V. Hyaluronic acid in ankle arthritis: why evidence of efficacy is still lacking? Clin Exp Rheumatol. 2012;30:277-281.
14. Witteveen AG, Hofstad CJ, Kerkhoffs GM. Hyaluronic acid and other conservative treatment options for osteoarthritis of the ankle. Cochrane Database Syst Rev. 2015;(10):CD010643.
15. Rao S, Ellis SJ, Deland JT, et al. Nonmedicinal therapy in the management of ankle arthritis. Curr Opin Rheumatol. 2010;22:223-228.
1. Valderrabano V, Horisberger M, Russell I, et al. Etiology of ankle osteoarthritis. Clin Orthop Relat Res. 2009;467:1800-1806.
2. Huch K, Kuettner KE, Dieppe P. Osteoarthritis in ankle and knee joints. Semin Arthritis Rheum. 1997;26:667-674.
3. Kempson GE. Age-related changes in the tensile properties of human articular cartilage: a comparative study between the femoral head of the hip joint and the talus of the ankle joint. Biochim Biophys Acta. 1991;1075:223-230.
4. Saltzman CL, Salamon ML, Blanchard GM, et al. Epidemiology of ankle arthritis: Report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44-46.
5. Brown T, Johnston R, Saltzman C, et al. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20:739-744.
6. Barg A, Pagenstert G, Hügle T, et al. Ankle osteoarthritis etiology, diagnostics and classification. Foot Ankle Clin. 2013;18:411-426.
7. Schenker M, Mauck R, Ahn J, et al. Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J Am Acad Orthop Surg. 2014;22:20-28.
8. Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58:356-357.
9. Hayes BJ, Gonzalez T, Smith JT, et al. Ankle arthritis: you can’t always replace it. J Am Acad Orthop Surg. 2016;24:e29-e38.
10. Thomas R, Daniels T. Current concepts review ankle arthritis. J Bone Joint Surg Am. 2003;85A:923-936.
11. Martin RL, Stewart GW, Conti SF. Posttraumatic ankle arthritis: an update on conservative and surgical management. J Orthop Sports Phys Ther. 2007:37:253-259.
12. Pekarek B, Osher L, Buck S, et al. Intra-articular corticosteroid injections: a critical literature review with up-to-date findings. Foot. 2011;21:66-70.
13. Abate M, Schiavone C, Salini V. Hyaluronic acid in ankle arthritis: why evidence of efficacy is still lacking? Clin Exp Rheumatol. 2012;30:277-281.
14. Witteveen AG, Hofstad CJ, Kerkhoffs GM. Hyaluronic acid and other conservative treatment options for osteoarthritis of the ankle. Cochrane Database Syst Rev. 2015;(10):CD010643.
15. Rao S, Ellis SJ, Deland JT, et al. Nonmedicinal therapy in the management of ankle arthritis. Curr Opin Rheumatol. 2010;22:223-228.
PRACTICE RECOMMENDATIONS
› Always ask that the foot be included in ankle x-rays to aid in identifying malalignment, deformity, or joint arthritis. C
› Use anti-inflammatory medications, orthotic devices, and footwear modifications, as needed, for ankle osteoarthritis. C
› Avoid ankle immobilization except, perhaps, during arthritic flare. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Superior Mesenteric Artery Syndrome as a Complication of Scoliosis Surgery
Take-Home Points
- Adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome following pediatric spine surgery.
- Must recognize nonspecific symptoms such as abdominal pain, tenderness, distention, bilious or projectile vomiting, hypoactive bowel sounds, and anorexia postoperatively.
- Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, or cardiovascular collapse and death.
Superior mesenteric artery (SMA) syndrome resulting from surgical treatment of scoliosis has been recognized in the medical literature since 1752.1 Throughout the literature, SMA syndrome variably has been referred to as cast syndrome, Wilkie syndrome, arteriomesenteric duodenal obstruction, and chronic duodenal ileus.2 We now recognize numerous etiologies of SMA syndrome, as several sources can externally compress the duodenum. Classic acute symptoms of bowel obstruction include bilious vomiting, nausea, and epigastric pain. Chronic manifestations of SMA syndrome may include weight loss and decreased appetite. Our literature review revealed that adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome after pediatric spine surgery.
We report the case of a 14-year-old boy who developed SMA syndrome after undergoing scoliosis surgery. The patient and his mother provided written informed consent for print and electronic publication of this case report.
Case Report
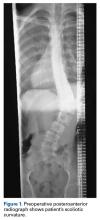
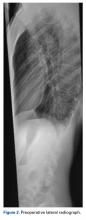
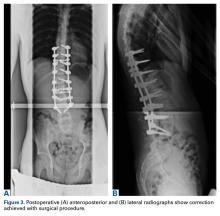
A 14-year-old boy with a history of idiopathic scoliosis presented to Cohen Children’s Hospital (Long Island Jewish Medical Center) with bilious vomiting that had persisted for 7 days after posterior T9–L4 fusion with instrumentation.
Discussion
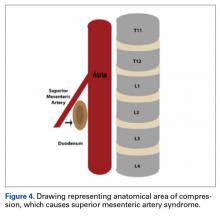
SMA syndrome is attributed to the anatomical orientation of the third part of the duodenum, which passes between the aorta and the SMA (Figure 4).
Adolescents are particularly vulnerable to this condition. Faster adolescent bone growth relative to visceral growth is accompanied by a decrease in SMA angle.3 Occasionally, body casts are used after surgery to immobilize the vertebrae and augment healing. Cast syndrome occurs when pressure from a body cast causes a bowel obstruction secondary to spinal hyperextension and amplified spinal lordosis.2 This finding, dating to the 19th century, was reported by Willet4 when a patient died 48 hours after application of a body cast. In 1950, the term cast syndrome was coined after a motorcyclist’s injuries were treated with a hip spica cast and the patient died of cardiovascular collapse secondary to persistent vomiting.5
Table 1 summarizes various evaluation, diagnosis, and treatment algorithms designed to optimize nutrition and weight in patients developing signs and symptoms of SMA syndrome after posterior spinal instrumentation and fusion for adolescent idiopathic scoliosis (AIS).
The third unique feature in this case is electrocardiogram findings. Although some cases briefly discussed electrolyte abnormalities, none presented evidence that these abnormalities caused cardiac changes.6,16,18 The overall clinical significance of the QT prolongation in our patient’s case is unknown, as this finding was improved with correction of the electrolyte abnormalities and appropriate fluid replenishment.
Early recognition of nonspecific symptoms (eg, abdominal pain, tenderness, distension, bilious or projectile vomiting, hypoactive bowel sounds, anorexia) plays a key role in preventing severe morbidity and mortality from SMA syndrome after scoliosis surgery. Although many patients present in the semiclassic obstructed pattern, notable reasons for diagnostic delay include normal appetite and bowel sounds.3 For example, SMA syndrome may be misdiagnosed as stomach flu because of unfamiliarity with disease diagnosis and management.20 Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, and cardiovascular collapse and death.
Am J Orthop. 2017;46(2):E124-E130. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Evarts CM, Winter RB, Hall JE. Vascular compression of the duodenum associated with the treatment of scoliosis. Review of the literature and report of eighteen cases. J Bone Joint Surg Am. 1971;53(3):431-444.
2. Zhu ZZ, Qiu Y. Superior mesenteric artery syndrome following scoliosis surgery: its risk indicators and treatment strategy. World J Gastroenterol. 2005;11(21):3307-3310.
3. Hutchinson DT, Bassett GS. Superior mesenteric artery syndrome in pediatric orthopedic patients. Clin Orthop Relat Res. 1990;(250):250-257.
4. Willet A. Fatal vomiting following application of plaster-of-Paris bandage in case of spinal curvature. St Barth Hosp Rep. 1878;14:333-335.
5. Dorph MH. The cast syndrome; review of the literature and report of a case. N Engl J Med. 1950;243(12):440-442.
6. Lam DJ, Lee JZ, Chua JH, Lee YT, Lim KB. Superior mesenteric artery syndrome following surgery for adolescent idiopathic scoliosis: a case series, review of the literature, and an algorithm for management. J Pediatr Orthop B. 2014;23(4):312-318.
7. Tsirikos AI, Anakwe RE, Baker AD. Late presentation of superior mesenteric artery syndrome following scoliosis surgery: a case report. J Med Case Rep. 2008;2:9.
8. Akin JT Jr, Skandalakis JE, Gray SW. The anatomic basis of vascular compression of the duodenum. Surg Clin North Am. 1974;54(6):1361-1370.
9. Amy BW, Priebe CJ Jr, King A. Superior mesenteric artery syndrome associated with scoliosis treated by a modified Ladd procedure. J Pediatr Orthop. 1985;5(3):361-363.
10. Richardson WS, Surowiec WJ. Laparoscopic repair of superior mesenteric artery syndrome. Am J Surg. 2001;181(4):377-378.
11. Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83(8):1169-1181.
12. Braun SV, Hedden DM, Howard AW. Superior mesenteric artery syndrome following spinal deformity correction. J Bone Joint Surg Am. 2006;88(10):2252-2257.
13. Smith BG, Hakim-Zargar M, Thomson JD. Low body mass index: a risk factor for superior mesenteric artery syndrome in adolescents undergoing spinal fusion for scoliosis. J Spinal Disord Tech. 2009;22(2):144-148.
14. Pan CH, Tzeng ST, Chen CS, Chen PQ. Superior mesenteric artery syndrome complicating staged corrective surgery for scoliosis. J Formos Med Assoc. 2007;106(2 suppl):S37-S45.
15. Kennedy RH, Cooper MJ. An unusually severe case of the cast syndrome. Postgrad Med J. 1983;59(694):539-540.
16. Keskin M, Akgül T, Bayraktar A, Dikici F, Balik E. Superior mesenteric artery syndrome: an infrequent complication of scoliosis surgery. Case Rep Surg. 2014;2014:263431.
17. Amarawickrama H, Harikrishnan A, Krijgsman B. Superior mesenteric artery syndrome in a young girl following spinal surgery for scoliosis. Br J Hosp Med. 2005;66(12):700-701.
18. Crowther MA, Webb PJ, Eyre-Brook IA. Superior mesenteric artery syndrome following surgery for scoliosis. Spine. 2002;27(24):E528-E533.
19. Moskovich R, Cheong-Leen P. Vascular compression of the duodenum. J R Soc Med. 1986;79(8):465-467.
20. Shah MA, Albright MB, Vogt MT, Moreland MS. Superior mesenteric artery syndrome in scoliosis surgery: weight percentile for height as an indicator of risk. J Pediatr Orthop. 2003;23(5):665-668.
Take-Home Points
- Adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome following pediatric spine surgery.
- Must recognize nonspecific symptoms such as abdominal pain, tenderness, distention, bilious or projectile vomiting, hypoactive bowel sounds, and anorexia postoperatively.
- Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, or cardiovascular collapse and death.
Superior mesenteric artery (SMA) syndrome resulting from surgical treatment of scoliosis has been recognized in the medical literature since 1752.1 Throughout the literature, SMA syndrome variably has been referred to as cast syndrome, Wilkie syndrome, arteriomesenteric duodenal obstruction, and chronic duodenal ileus.2 We now recognize numerous etiologies of SMA syndrome, as several sources can externally compress the duodenum. Classic acute symptoms of bowel obstruction include bilious vomiting, nausea, and epigastric pain. Chronic manifestations of SMA syndrome may include weight loss and decreased appetite. Our literature review revealed that adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome after pediatric spine surgery.
We report the case of a 14-year-old boy who developed SMA syndrome after undergoing scoliosis surgery. The patient and his mother provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old boy with a history of idiopathic scoliosis presented to Cohen Children’s Hospital (Long Island Jewish Medical Center) with bilious vomiting that had persisted for 7 days after posterior T9–L4 fusion with instrumentation.
Discussion
SMA syndrome is attributed to the anatomical orientation of the third part of the duodenum, which passes between the aorta and the SMA (Figure 4).
Adolescents are particularly vulnerable to this condition. Faster adolescent bone growth relative to visceral growth is accompanied by a decrease in SMA angle.3 Occasionally, body casts are used after surgery to immobilize the vertebrae and augment healing. Cast syndrome occurs when pressure from a body cast causes a bowel obstruction secondary to spinal hyperextension and amplified spinal lordosis.2 This finding, dating to the 19th century, was reported by Willet4 when a patient died 48 hours after application of a body cast. In 1950, the term cast syndrome was coined after a motorcyclist’s injuries were treated with a hip spica cast and the patient died of cardiovascular collapse secondary to persistent vomiting.5
Table 1 summarizes various evaluation, diagnosis, and treatment algorithms designed to optimize nutrition and weight in patients developing signs and symptoms of SMA syndrome after posterior spinal instrumentation and fusion for adolescent idiopathic scoliosis (AIS).
The third unique feature in this case is electrocardiogram findings. Although some cases briefly discussed electrolyte abnormalities, none presented evidence that these abnormalities caused cardiac changes.6,16,18 The overall clinical significance of the QT prolongation in our patient’s case is unknown, as this finding was improved with correction of the electrolyte abnormalities and appropriate fluid replenishment.
Early recognition of nonspecific symptoms (eg, abdominal pain, tenderness, distension, bilious or projectile vomiting, hypoactive bowel sounds, anorexia) plays a key role in preventing severe morbidity and mortality from SMA syndrome after scoliosis surgery. Although many patients present in the semiclassic obstructed pattern, notable reasons for diagnostic delay include normal appetite and bowel sounds.3 For example, SMA syndrome may be misdiagnosed as stomach flu because of unfamiliarity with disease diagnosis and management.20 Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, and cardiovascular collapse and death.
Am J Orthop. 2017;46(2):E124-E130. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome following pediatric spine surgery.
- Must recognize nonspecific symptoms such as abdominal pain, tenderness, distention, bilious or projectile vomiting, hypoactive bowel sounds, and anorexia postoperatively.
- Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, or cardiovascular collapse and death.
Superior mesenteric artery (SMA) syndrome resulting from surgical treatment of scoliosis has been recognized in the medical literature since 1752.1 Throughout the literature, SMA syndrome variably has been referred to as cast syndrome, Wilkie syndrome, arteriomesenteric duodenal obstruction, and chronic duodenal ileus.2 We now recognize numerous etiologies of SMA syndrome, as several sources can externally compress the duodenum. Classic acute symptoms of bowel obstruction include bilious vomiting, nausea, and epigastric pain. Chronic manifestations of SMA syndrome may include weight loss and decreased appetite. Our literature review revealed that adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome after pediatric spine surgery.
We report the case of a 14-year-old boy who developed SMA syndrome after undergoing scoliosis surgery. The patient and his mother provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old boy with a history of idiopathic scoliosis presented to Cohen Children’s Hospital (Long Island Jewish Medical Center) with bilious vomiting that had persisted for 7 days after posterior T9–L4 fusion with instrumentation.
Discussion
SMA syndrome is attributed to the anatomical orientation of the third part of the duodenum, which passes between the aorta and the SMA (Figure 4).
Adolescents are particularly vulnerable to this condition. Faster adolescent bone growth relative to visceral growth is accompanied by a decrease in SMA angle.3 Occasionally, body casts are used after surgery to immobilize the vertebrae and augment healing. Cast syndrome occurs when pressure from a body cast causes a bowel obstruction secondary to spinal hyperextension and amplified spinal lordosis.2 This finding, dating to the 19th century, was reported by Willet4 when a patient died 48 hours after application of a body cast. In 1950, the term cast syndrome was coined after a motorcyclist’s injuries were treated with a hip spica cast and the patient died of cardiovascular collapse secondary to persistent vomiting.5
Table 1 summarizes various evaluation, diagnosis, and treatment algorithms designed to optimize nutrition and weight in patients developing signs and symptoms of SMA syndrome after posterior spinal instrumentation and fusion for adolescent idiopathic scoliosis (AIS).
The third unique feature in this case is electrocardiogram findings. Although some cases briefly discussed electrolyte abnormalities, none presented evidence that these abnormalities caused cardiac changes.6,16,18 The overall clinical significance of the QT prolongation in our patient’s case is unknown, as this finding was improved with correction of the electrolyte abnormalities and appropriate fluid replenishment.
Early recognition of nonspecific symptoms (eg, abdominal pain, tenderness, distension, bilious or projectile vomiting, hypoactive bowel sounds, anorexia) plays a key role in preventing severe morbidity and mortality from SMA syndrome after scoliosis surgery. Although many patients present in the semiclassic obstructed pattern, notable reasons for diagnostic delay include normal appetite and bowel sounds.3 For example, SMA syndrome may be misdiagnosed as stomach flu because of unfamiliarity with disease diagnosis and management.20 Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, and cardiovascular collapse and death.
Am J Orthop. 2017;46(2):E124-E130. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Evarts CM, Winter RB, Hall JE. Vascular compression of the duodenum associated with the treatment of scoliosis. Review of the literature and report of eighteen cases. J Bone Joint Surg Am. 1971;53(3):431-444.
2. Zhu ZZ, Qiu Y. Superior mesenteric artery syndrome following scoliosis surgery: its risk indicators and treatment strategy. World J Gastroenterol. 2005;11(21):3307-3310.
3. Hutchinson DT, Bassett GS. Superior mesenteric artery syndrome in pediatric orthopedic patients. Clin Orthop Relat Res. 1990;(250):250-257.
4. Willet A. Fatal vomiting following application of plaster-of-Paris bandage in case of spinal curvature. St Barth Hosp Rep. 1878;14:333-335.
5. Dorph MH. The cast syndrome; review of the literature and report of a case. N Engl J Med. 1950;243(12):440-442.
6. Lam DJ, Lee JZ, Chua JH, Lee YT, Lim KB. Superior mesenteric artery syndrome following surgery for adolescent idiopathic scoliosis: a case series, review of the literature, and an algorithm for management. J Pediatr Orthop B. 2014;23(4):312-318.
7. Tsirikos AI, Anakwe RE, Baker AD. Late presentation of superior mesenteric artery syndrome following scoliosis surgery: a case report. J Med Case Rep. 2008;2:9.
8. Akin JT Jr, Skandalakis JE, Gray SW. The anatomic basis of vascular compression of the duodenum. Surg Clin North Am. 1974;54(6):1361-1370.
9. Amy BW, Priebe CJ Jr, King A. Superior mesenteric artery syndrome associated with scoliosis treated by a modified Ladd procedure. J Pediatr Orthop. 1985;5(3):361-363.
10. Richardson WS, Surowiec WJ. Laparoscopic repair of superior mesenteric artery syndrome. Am J Surg. 2001;181(4):377-378.
11. Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83(8):1169-1181.
12. Braun SV, Hedden DM, Howard AW. Superior mesenteric artery syndrome following spinal deformity correction. J Bone Joint Surg Am. 2006;88(10):2252-2257.
13. Smith BG, Hakim-Zargar M, Thomson JD. Low body mass index: a risk factor for superior mesenteric artery syndrome in adolescents undergoing spinal fusion for scoliosis. J Spinal Disord Tech. 2009;22(2):144-148.
14. Pan CH, Tzeng ST, Chen CS, Chen PQ. Superior mesenteric artery syndrome complicating staged corrective surgery for scoliosis. J Formos Med Assoc. 2007;106(2 suppl):S37-S45.
15. Kennedy RH, Cooper MJ. An unusually severe case of the cast syndrome. Postgrad Med J. 1983;59(694):539-540.
16. Keskin M, Akgül T, Bayraktar A, Dikici F, Balik E. Superior mesenteric artery syndrome: an infrequent complication of scoliosis surgery. Case Rep Surg. 2014;2014:263431.
17. Amarawickrama H, Harikrishnan A, Krijgsman B. Superior mesenteric artery syndrome in a young girl following spinal surgery for scoliosis. Br J Hosp Med. 2005;66(12):700-701.
18. Crowther MA, Webb PJ, Eyre-Brook IA. Superior mesenteric artery syndrome following surgery for scoliosis. Spine. 2002;27(24):E528-E533.
19. Moskovich R, Cheong-Leen P. Vascular compression of the duodenum. J R Soc Med. 1986;79(8):465-467.
20. Shah MA, Albright MB, Vogt MT, Moreland MS. Superior mesenteric artery syndrome in scoliosis surgery: weight percentile for height as an indicator of risk. J Pediatr Orthop. 2003;23(5):665-668.
1. Evarts CM, Winter RB, Hall JE. Vascular compression of the duodenum associated with the treatment of scoliosis. Review of the literature and report of eighteen cases. J Bone Joint Surg Am. 1971;53(3):431-444.
2. Zhu ZZ, Qiu Y. Superior mesenteric artery syndrome following scoliosis surgery: its risk indicators and treatment strategy. World J Gastroenterol. 2005;11(21):3307-3310.
3. Hutchinson DT, Bassett GS. Superior mesenteric artery syndrome in pediatric orthopedic patients. Clin Orthop Relat Res. 1990;(250):250-257.
4. Willet A. Fatal vomiting following application of plaster-of-Paris bandage in case of spinal curvature. St Barth Hosp Rep. 1878;14:333-335.
5. Dorph MH. The cast syndrome; review of the literature and report of a case. N Engl J Med. 1950;243(12):440-442.
6. Lam DJ, Lee JZ, Chua JH, Lee YT, Lim KB. Superior mesenteric artery syndrome following surgery for adolescent idiopathic scoliosis: a case series, review of the literature, and an algorithm for management. J Pediatr Orthop B. 2014;23(4):312-318.
7. Tsirikos AI, Anakwe RE, Baker AD. Late presentation of superior mesenteric artery syndrome following scoliosis surgery: a case report. J Med Case Rep. 2008;2:9.
8. Akin JT Jr, Skandalakis JE, Gray SW. The anatomic basis of vascular compression of the duodenum. Surg Clin North Am. 1974;54(6):1361-1370.
9. Amy BW, Priebe CJ Jr, King A. Superior mesenteric artery syndrome associated with scoliosis treated by a modified Ladd procedure. J Pediatr Orthop. 1985;5(3):361-363.
10. Richardson WS, Surowiec WJ. Laparoscopic repair of superior mesenteric artery syndrome. Am J Surg. 2001;181(4):377-378.
11. Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83(8):1169-1181.
12. Braun SV, Hedden DM, Howard AW. Superior mesenteric artery syndrome following spinal deformity correction. J Bone Joint Surg Am. 2006;88(10):2252-2257.
13. Smith BG, Hakim-Zargar M, Thomson JD. Low body mass index: a risk factor for superior mesenteric artery syndrome in adolescents undergoing spinal fusion for scoliosis. J Spinal Disord Tech. 2009;22(2):144-148.
14. Pan CH, Tzeng ST, Chen CS, Chen PQ. Superior mesenteric artery syndrome complicating staged corrective surgery for scoliosis. J Formos Med Assoc. 2007;106(2 suppl):S37-S45.
15. Kennedy RH, Cooper MJ. An unusually severe case of the cast syndrome. Postgrad Med J. 1983;59(694):539-540.
16. Keskin M, Akgül T, Bayraktar A, Dikici F, Balik E. Superior mesenteric artery syndrome: an infrequent complication of scoliosis surgery. Case Rep Surg. 2014;2014:263431.
17. Amarawickrama H, Harikrishnan A, Krijgsman B. Superior mesenteric artery syndrome in a young girl following spinal surgery for scoliosis. Br J Hosp Med. 2005;66(12):700-701.
18. Crowther MA, Webb PJ, Eyre-Brook IA. Superior mesenteric artery syndrome following surgery for scoliosis. Spine. 2002;27(24):E528-E533.
19. Moskovich R, Cheong-Leen P. Vascular compression of the duodenum. J R Soc Med. 1986;79(8):465-467.
20. Shah MA, Albright MB, Vogt MT, Moreland MS. Superior mesenteric artery syndrome in scoliosis surgery: weight percentile for height as an indicator of risk. J Pediatr Orthop. 2003;23(5):665-668.