User login
Reflectance Confocal Microscopy as a Diagnostic Aid in Allergic Contact Dermatitis to Mango Sap
The mango tree (Mangifera indica) produces nutrient-dense fruit—known colloquially as the “king of fruits”—that is widely consumed across the world. Native to southern Asia, the mango tree is a member of the Anacardiaceae family, a large family of flowering, fruit-bearing plants.1 Many members of the Anacardiaceae family, which includes poison ivy and poison oak, are known to produce urushiol, a skin irritant associated with allergic contact dermatitis (ACD).2 Interestingly, despite its widespread consumption and categorization in the Anacardiaceae family, allergic reactions to mango are comparatively rare; they occur as either immediate type I hypersensitivity reactions manifesting with rapid-onset symptoms such as urticaria, wheezing, and angioedema, or delayed type IV hypersensitivity reactions manifesting as ACD.3 Although exposure to components of the mango tree has been most characteristically linked to type IV hypersensitivity reactions, there remain fewer than 40 reported cases of mango-induced ACD since it was first described in 1939.4
Evaluation of ACD most commonly includes a thorough clinical assessment with diagnostic support from patch testing and histopathologic review following skin biopsy. In recent years, reflectance confocal microscopy (RCM) has shown promising potential to join the repertoire of diagnostic tools for ACD by enabling dynamic and high-resolution imaging of contact dermatitis in vivo.5-10 Reflectance confocal microscopy is a noninvasive optical imaging technique that uses a low-energy diode laser to penetrate the layers of the skin. The resulting reflected light generates images that facilitate visualization of cutaneous structures to the depth of the papillary dermis.11 While it is most commonly used in skin cancer diagnostics, preliminary studies also have shown an emerging role for RCM in the evaluation of eczematous and inflammatory skin disease, including contact dermatitis.5-10 Herein, we present a unique case of mango sap–induced ACD imaged and diagnosed in real time via RCM.
Case Report
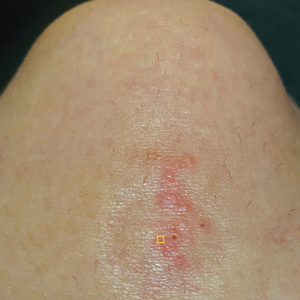
A 39-year-old woman presented to our clinic with a pruritic vesicular eruption on the right leg of 2 weeks’ duration that initially had developed within 7 days of exposure to mango tree sap (Figure 1). The patient reported having experienced similar pruritic eruptions in the past following contact with mango sap while eating mangos but denied any history of reactions from ingestion of the fruit. She also reported a history of robust reactions to poison ivy; however, a timeline specifying the order of first exposure to these irritants was unknown. She denied any personal or family history of atopic conditions.
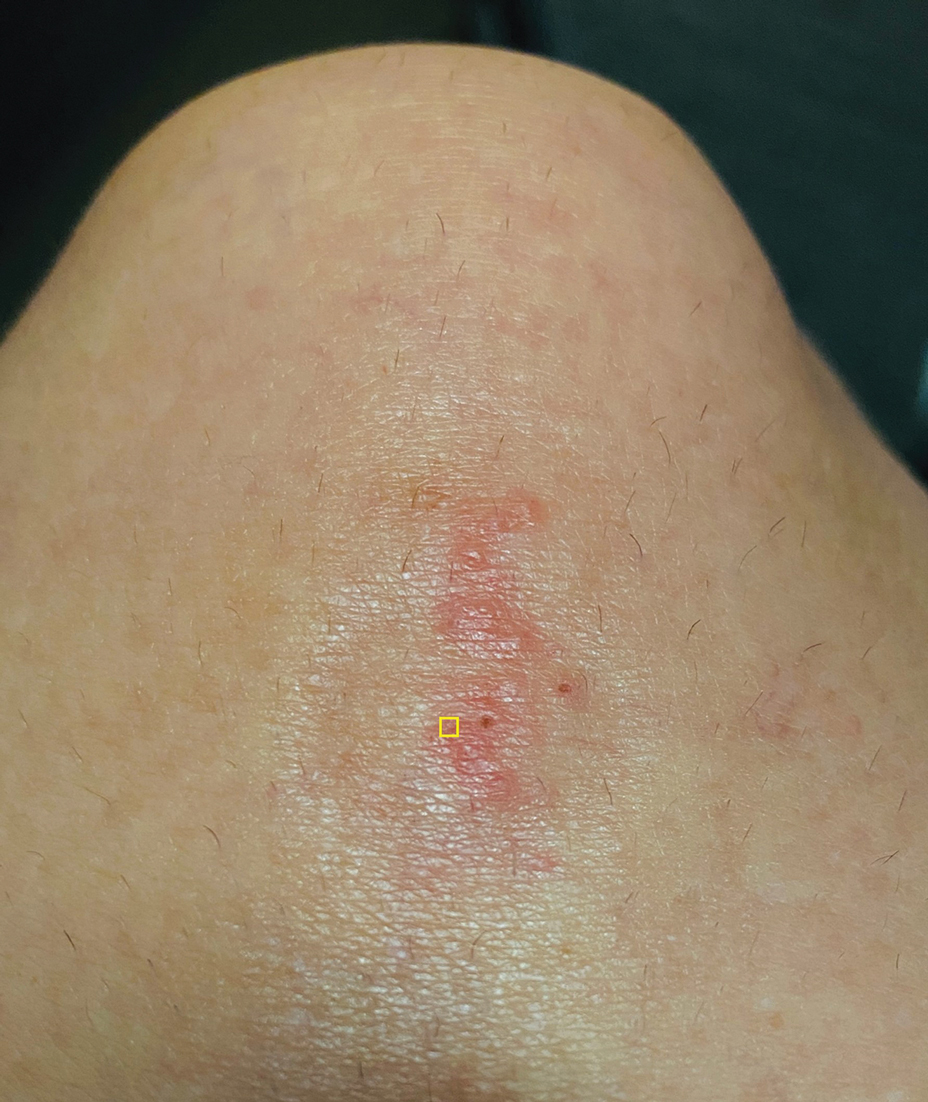
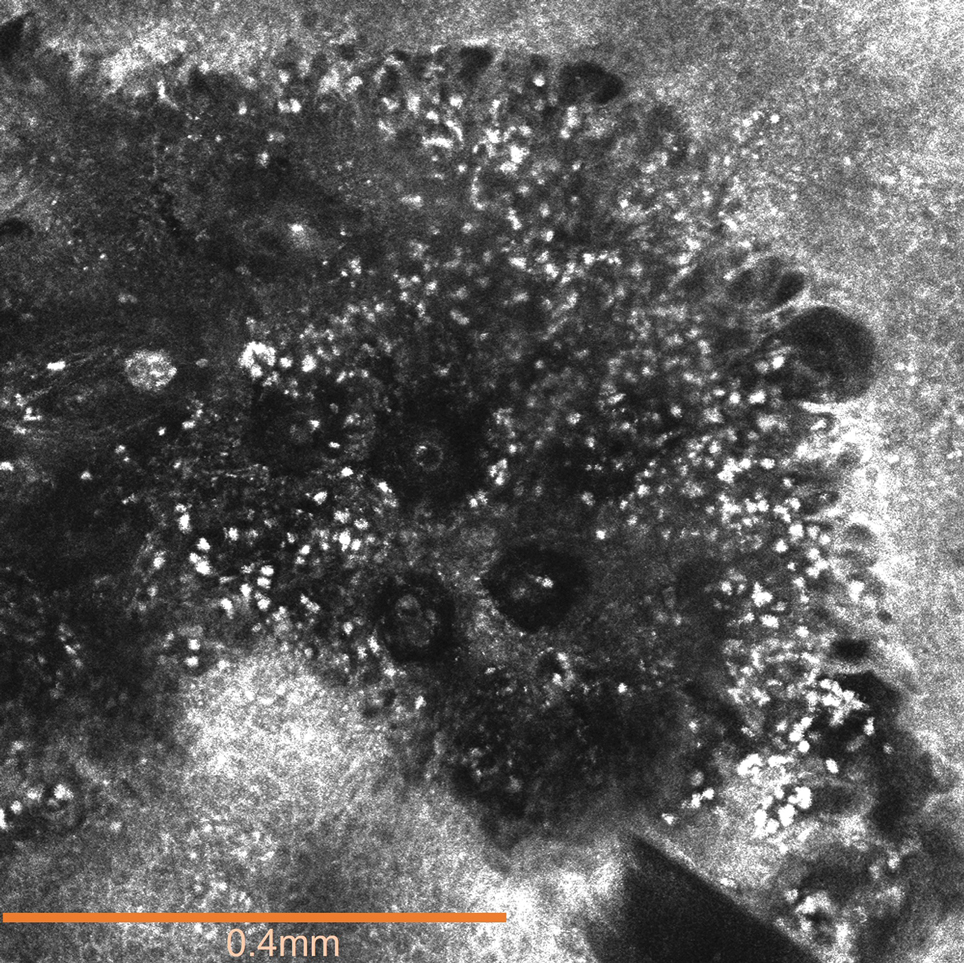
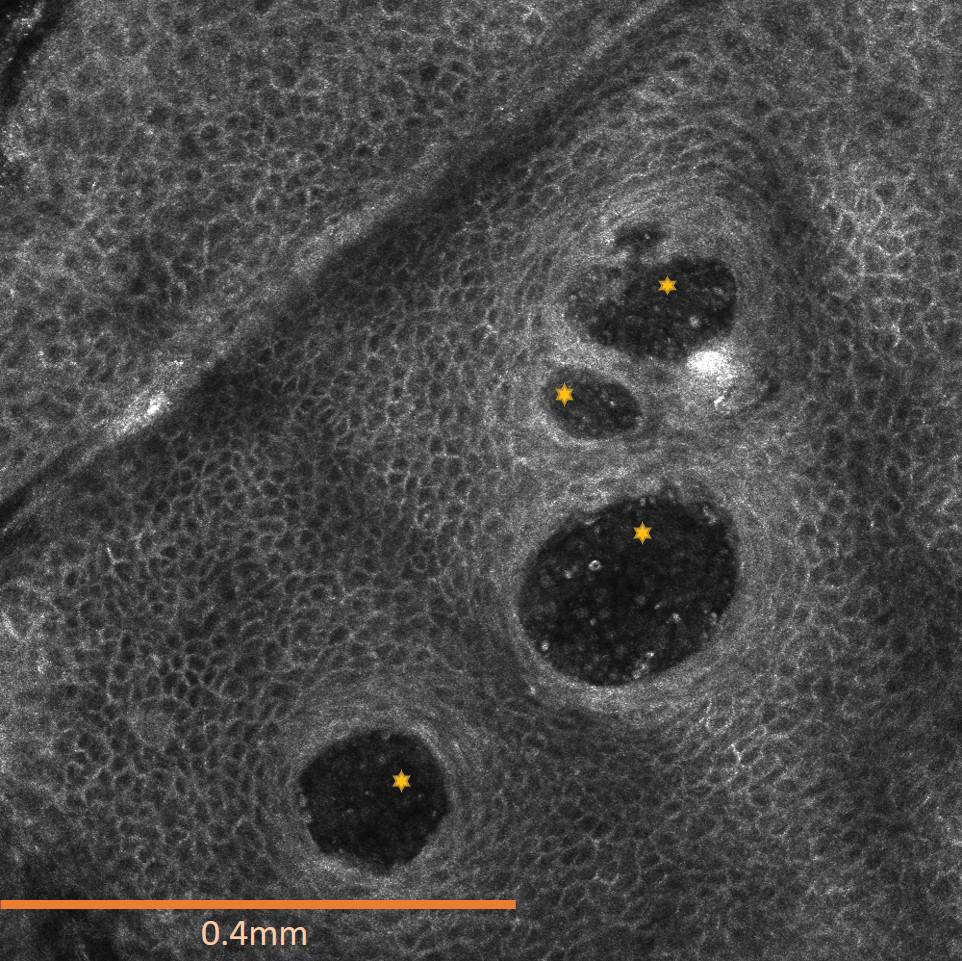
The affected skin was imaged in real time during clinic using RCM, which showed an inflammatory infiltrate represented by dark spongiotic vesicles containing bright cells (Figure 2). Additional RCM imaging at the level of the stratum spinosum showed dark spongiotic areas with bright inflammatory cells infiltrating the vesicles, which were surrounded by normal skin showing a typical epidermal honeycomb pattern (Figure 3). These findings were diagnostic of ACD secondary to exposure to mango sap. The patient was advised to apply clobetasol cream 0.05% to the affected area. Notable improvement of the rash was noted within 10 days of treatment.
Comment
Exposure to the mango tree and its fruit is a rare cause of ACD, with few reported cases in the literature. The majority of known instances have occurred in non–mango-cultivating countries, largely the United States, although cases also have been reported in Canada, Australia, France, Japan, and Thailand.3,12 Mango-induced contact allergy follows a roughly equal distribution between males and females and most often occurs in young adults during the third and fourth decades of life.4,12-21 Importantly, delayed-type hypersensitivity reactions to mango can manifest as either localized or systemic ACD. Localized ACD can be induced via direct contact with the mango tree and its components or ingestion of the fruit.3,12,22 Conversely, systemic ACD is primarily stimulated by ingestion of the fruit. In our case, the patient had no history of allergy following mango ingestion, and her ACD was prompted by isolated contact with mango sap. The time from exposure to symptom onset of known instances of mango ACD varies widely, ranging from less than 24 hours to as long as 9 days.3,12 Diagnosis of mango-induced ACD largely is guided by clinical findings. Presenting symptoms often include an eczematous, vesicular, pruritic rash on affected areas of the skin, frequently the head, neck, and extremities. Patients also commonly present with linear papulovesicular lesions and periorbital or perioral edema.
The suspected allergens responsible for mango-induced ACD are derived from resorcinol—specifically heptadecadienyl resorcinol, heptadecenyl resorcinol, and pentadecyl resorcinol, which are collectively known as mango allergens.23 These allergens can be found within the pulp and skin of the mango fruit as well as in the bark and leaves of the mango tree, which may explain observed allergic reactions to components of both the mango fruit and tree.12 Similar to these resorcinol derivatives, the urushiol resin found in poison ivy and poison oak is a catechol derivative.2 Importantly, both resorcinols and catechols are isomers of the same aromatic phenol—dihydroxybenzene. Because of these similarities, it is thought that the allergens in mangos may cross-react with urushiol in poison ivy or poison oak.23 Alongside their shared categorization in the Anacardiaceae family, it is hypothesized that this cross-reactivity underlies the sensitization that has been noted between mango and poison ivy or poison oak exposure.12,23,24 Thus, ACD often can occur on initial contact with the mango tree or its components, as a prior exposure to poison ivy or poison oak may serve as the inciting factor for hypersensitization. The majority of reported cases in the literature also occurred in countries where exposure to poison ivy and poison oak are common, further supporting the notion that these compounds may provide a sensitizing trigger for a future mango contact allergy.12
A detailed clinical history combined with adjunctive diagnostic support from patch testing and histopathology of biopsied skin lesions classically are used in the diagnosis of mango-induced ACD. Due to its ability to provide quick and noninvasive in vivo imaging of cutaneous lesions, RCM's applications have expanded to include evaluation of inflammatory skin diseases such as contact dermatitis. Many features of contact dermatitis identified via RCM are common between ACD and irritant contact dermatitis (ICD) and include disruption of the stratum corneum, parakeratosis, vesiculation, spongiosis, and exocytosis.6,10,25 Studies also have described features shown via RCM that are unique to ACD, including vasodilation and intercellular edema, compared to more distinct targetoid keratinocytes and detached corneocytes seen in ICD.6,10,25 Studies by Astner et al5,6 demonstrated a wide range of sensitivity from 52% to 96% and a high specificity of RCM greater than 95% for many of the aforementioned features of contact dermatitis, including disruption of the stratum corneum, parakeratosis, spongiosis, and exocytosis. Additional studies have further strengthened these findings, demonstrating sensitivity and specificity values of 83% and 92% for contact dermatitis under RCM, respectively.26 Importantly, given the similarities and potentially large overlap of features between ACD and ICD identified via RCM as well as findings seen on physical examination and histopathology, an emphasis on clinical correlation is essential when differentiating between these 2 variants of contact dermatitis. Thus, taken in consideration with clinical contexts, RCM has shown potent diagnostic accuracy and great potential to support the evaluation of ACD alongside patch testing and histopathology.
Final Thoughts
Contact allergy to the mango tree and its components is uncommon. We report a unique case of mango sap–induced ACD evaluated and diagnosed via dynamic visualization under RCM. As a noninvasive and reproducible imaging technique with resolutions comparable to histopathologic analysis, RCM is a promising tool that can be used to support the diagnostic evaluation of ACD.
- Shah KA, Patel MB, Patel RJ, et al. Mangifera indica (mango). Pharmacogn Rev. 2010;4:42-48.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed September 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557866
- Sareen R, Shah A. Hypersensitivity manifestations to the fruit mango. Asia Pac Allergy. 2011;1:43-49.
- Zakon SJ. Contact dermatitis due to mango. JAMA. 1939;113:1808.
- Astner S, Gonzalez E, Cheung A, et al. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986-992.
- Astner S, Gonzalez S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182-191.
- Csuka EA, Ward SC, Ekelem C, et al. Reflectance confocal microscopy, optical coherence tomography, and multiphoton microscopy in inflammatory skin disease diagnosis. Lasers Surg Med. 2021;53:776-797.
- Guichard A, Fanian F, Girardin P, et al. Allergic patch test and contact dermatitis by in vivo reflectance confocal microscopy [in French]. Ann Dermatol Venereol. 2014;141:805-807.
- Sakanashi EN, Matsumura M, Kikuchi K, et al. A comparative study of allergic contact dermatitis by patch test versus reflectance confocal laser microscopy, with nickel and cobalt. Eur J Dermatol. 2010;20:705-711.
- Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
- Shahriari N, Grant-Kels JM, Rabinovitz H, et al. Reflectance confocal microscopy: principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84:1-14.
- Berghea EC, Craiu M, Ali S, et al. Contact allergy induced by mango (Mangifera indica): a relevant topic? Medicina (Kaunas). 2021;57:1240.
- O’Hern K, Zhang F, Zug KA, et al. “Mango slice” dermatitis: pediatric allergic contact dermatitis to mango pulp and skin. Dermatitis. 2022;33:E46-E47.
- Raison-Peyron N, Aljaber F, Al Ali OA, et al. Mango dermatitis: an unusual cause of eyelid dermatitis in France. Contact Dermatitis. 2021;85:599-600.
- Alipour Tehrany Y, Coulombe J. Mango allergic contact dermatitis. Contact Dermatitis. 2021;85:241-242.
- Yoo MJ, Carius BM. Mango dermatitis after urushiol sensitization. Clin Pract Cases Emerg Med. 2019;3:361-363.
- Miyazawa H, Nishie W, Hata H, et al. A severe case of mango dermatitis. J Eur Acad Dermatol Venereol. 2018;32:E160-E161.
- Trehan I, Meuli GJ. Mango contact allergy. J Travel Med. 2010;17:284.
- Wiwanitkit V. Mango dermatitis. Indian J Dermatol. 2008;53:158.
- Weinstein S, Bassiri-Tehrani S, Cohen DE. Allergic contact dermatitis to mango flesh. Int J Dermatol. 2004;43:195-196.
- Calvert ML, Robertson I, Samaratunga H. Mango dermatitis: allergic contact dermatitis to Mangifera indica. Australas J Dermatol. 1996;37:59-60.
- Thoo CH, Freeman S. Hypersensitivity reaction to the ingestion of mango flesh. Australas J Dermatol. 2008;49:116-119.
- Oka K, Saito F, Yasuhara T, et al. A study of cross-reactions between mango contact allergens and urushiol. Contact Dermatitis. 2004;51:292-296.
- Keil H, Wasserman D, Dawson CR. Mango dermatitis and its relationship to poison ivy hypersensitivity. Ann Allergy. 1946;4: 268-281.
- Maarouf M, Costello CM, Gonzalez S, et al. In vivo reflectance confocal microscopy: emerging role in noninvasive diagnosis and monitoring of eczematous dermatoses. Actas Dermosifiliogr (Engl Ed). 2019;110:626-636.
- Koller S, Gerger A, Ahlgrimm-Siess V, et al. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp Dermatol. 2009;18:536-540.
The mango tree (Mangifera indica) produces nutrient-dense fruit—known colloquially as the “king of fruits”—that is widely consumed across the world. Native to southern Asia, the mango tree is a member of the Anacardiaceae family, a large family of flowering, fruit-bearing plants.1 Many members of the Anacardiaceae family, which includes poison ivy and poison oak, are known to produce urushiol, a skin irritant associated with allergic contact dermatitis (ACD).2 Interestingly, despite its widespread consumption and categorization in the Anacardiaceae family, allergic reactions to mango are comparatively rare; they occur as either immediate type I hypersensitivity reactions manifesting with rapid-onset symptoms such as urticaria, wheezing, and angioedema, or delayed type IV hypersensitivity reactions manifesting as ACD.3 Although exposure to components of the mango tree has been most characteristically linked to type IV hypersensitivity reactions, there remain fewer than 40 reported cases of mango-induced ACD since it was first described in 1939.4
Evaluation of ACD most commonly includes a thorough clinical assessment with diagnostic support from patch testing and histopathologic review following skin biopsy. In recent years, reflectance confocal microscopy (RCM) has shown promising potential to join the repertoire of diagnostic tools for ACD by enabling dynamic and high-resolution imaging of contact dermatitis in vivo.5-10 Reflectance confocal microscopy is a noninvasive optical imaging technique that uses a low-energy diode laser to penetrate the layers of the skin. The resulting reflected light generates images that facilitate visualization of cutaneous structures to the depth of the papillary dermis.11 While it is most commonly used in skin cancer diagnostics, preliminary studies also have shown an emerging role for RCM in the evaluation of eczematous and inflammatory skin disease, including contact dermatitis.5-10 Herein, we present a unique case of mango sap–induced ACD imaged and diagnosed in real time via RCM.
Case Report
A 39-year-old woman presented to our clinic with a pruritic vesicular eruption on the right leg of 2 weeks’ duration that initially had developed within 7 days of exposure to mango tree sap (Figure 1). The patient reported having experienced similar pruritic eruptions in the past following contact with mango sap while eating mangos but denied any history of reactions from ingestion of the fruit. She also reported a history of robust reactions to poison ivy; however, a timeline specifying the order of first exposure to these irritants was unknown. She denied any personal or family history of atopic conditions.

The affected skin was imaged in real time during clinic using RCM, which showed an inflammatory infiltrate represented by dark spongiotic vesicles containing bright cells (Figure 2). Additional RCM imaging at the level of the stratum spinosum showed dark spongiotic areas with bright inflammatory cells infiltrating the vesicles, which were surrounded by normal skin showing a typical epidermal honeycomb pattern (Figure 3). These findings were diagnostic of ACD secondary to exposure to mango sap. The patient was advised to apply clobetasol cream 0.05% to the affected area. Notable improvement of the rash was noted within 10 days of treatment.


Comment
Exposure to the mango tree and its fruit is a rare cause of ACD, with few reported cases in the literature. The majority of known instances have occurred in non–mango-cultivating countries, largely the United States, although cases also have been reported in Canada, Australia, France, Japan, and Thailand.3,12 Mango-induced contact allergy follows a roughly equal distribution between males and females and most often occurs in young adults during the third and fourth decades of life.4,12-21 Importantly, delayed-type hypersensitivity reactions to mango can manifest as either localized or systemic ACD. Localized ACD can be induced via direct contact with the mango tree and its components or ingestion of the fruit.3,12,22 Conversely, systemic ACD is primarily stimulated by ingestion of the fruit. In our case, the patient had no history of allergy following mango ingestion, and her ACD was prompted by isolated contact with mango sap. The time from exposure to symptom onset of known instances of mango ACD varies widely, ranging from less than 24 hours to as long as 9 days.3,12 Diagnosis of mango-induced ACD largely is guided by clinical findings. Presenting symptoms often include an eczematous, vesicular, pruritic rash on affected areas of the skin, frequently the head, neck, and extremities. Patients also commonly present with linear papulovesicular lesions and periorbital or perioral edema.
The suspected allergens responsible for mango-induced ACD are derived from resorcinol—specifically heptadecadienyl resorcinol, heptadecenyl resorcinol, and pentadecyl resorcinol, which are collectively known as mango allergens.23 These allergens can be found within the pulp and skin of the mango fruit as well as in the bark and leaves of the mango tree, which may explain observed allergic reactions to components of both the mango fruit and tree.12 Similar to these resorcinol derivatives, the urushiol resin found in poison ivy and poison oak is a catechol derivative.2 Importantly, both resorcinols and catechols are isomers of the same aromatic phenol—dihydroxybenzene. Because of these similarities, it is thought that the allergens in mangos may cross-react with urushiol in poison ivy or poison oak.23 Alongside their shared categorization in the Anacardiaceae family, it is hypothesized that this cross-reactivity underlies the sensitization that has been noted between mango and poison ivy or poison oak exposure.12,23,24 Thus, ACD often can occur on initial contact with the mango tree or its components, as a prior exposure to poison ivy or poison oak may serve as the inciting factor for hypersensitization. The majority of reported cases in the literature also occurred in countries where exposure to poison ivy and poison oak are common, further supporting the notion that these compounds may provide a sensitizing trigger for a future mango contact allergy.12
A detailed clinical history combined with adjunctive diagnostic support from patch testing and histopathology of biopsied skin lesions classically are used in the diagnosis of mango-induced ACD. Due to its ability to provide quick and noninvasive in vivo imaging of cutaneous lesions, RCM's applications have expanded to include evaluation of inflammatory skin diseases such as contact dermatitis. Many features of contact dermatitis identified via RCM are common between ACD and irritant contact dermatitis (ICD) and include disruption of the stratum corneum, parakeratosis, vesiculation, spongiosis, and exocytosis.6,10,25 Studies also have described features shown via RCM that are unique to ACD, including vasodilation and intercellular edema, compared to more distinct targetoid keratinocytes and detached corneocytes seen in ICD.6,10,25 Studies by Astner et al5,6 demonstrated a wide range of sensitivity from 52% to 96% and a high specificity of RCM greater than 95% for many of the aforementioned features of contact dermatitis, including disruption of the stratum corneum, parakeratosis, spongiosis, and exocytosis. Additional studies have further strengthened these findings, demonstrating sensitivity and specificity values of 83% and 92% for contact dermatitis under RCM, respectively.26 Importantly, given the similarities and potentially large overlap of features between ACD and ICD identified via RCM as well as findings seen on physical examination and histopathology, an emphasis on clinical correlation is essential when differentiating between these 2 variants of contact dermatitis. Thus, taken in consideration with clinical contexts, RCM has shown potent diagnostic accuracy and great potential to support the evaluation of ACD alongside patch testing and histopathology.
Final Thoughts
Contact allergy to the mango tree and its components is uncommon. We report a unique case of mango sap–induced ACD evaluated and diagnosed via dynamic visualization under RCM. As a noninvasive and reproducible imaging technique with resolutions comparable to histopathologic analysis, RCM is a promising tool that can be used to support the diagnostic evaluation of ACD.
The mango tree (Mangifera indica) produces nutrient-dense fruit—known colloquially as the “king of fruits”—that is widely consumed across the world. Native to southern Asia, the mango tree is a member of the Anacardiaceae family, a large family of flowering, fruit-bearing plants.1 Many members of the Anacardiaceae family, which includes poison ivy and poison oak, are known to produce urushiol, a skin irritant associated with allergic contact dermatitis (ACD).2 Interestingly, despite its widespread consumption and categorization in the Anacardiaceae family, allergic reactions to mango are comparatively rare; they occur as either immediate type I hypersensitivity reactions manifesting with rapid-onset symptoms such as urticaria, wheezing, and angioedema, or delayed type IV hypersensitivity reactions manifesting as ACD.3 Although exposure to components of the mango tree has been most characteristically linked to type IV hypersensitivity reactions, there remain fewer than 40 reported cases of mango-induced ACD since it was first described in 1939.4
Evaluation of ACD most commonly includes a thorough clinical assessment with diagnostic support from patch testing and histopathologic review following skin biopsy. In recent years, reflectance confocal microscopy (RCM) has shown promising potential to join the repertoire of diagnostic tools for ACD by enabling dynamic and high-resolution imaging of contact dermatitis in vivo.5-10 Reflectance confocal microscopy is a noninvasive optical imaging technique that uses a low-energy diode laser to penetrate the layers of the skin. The resulting reflected light generates images that facilitate visualization of cutaneous structures to the depth of the papillary dermis.11 While it is most commonly used in skin cancer diagnostics, preliminary studies also have shown an emerging role for RCM in the evaluation of eczematous and inflammatory skin disease, including contact dermatitis.5-10 Herein, we present a unique case of mango sap–induced ACD imaged and diagnosed in real time via RCM.
Case Report
A 39-year-old woman presented to our clinic with a pruritic vesicular eruption on the right leg of 2 weeks’ duration that initially had developed within 7 days of exposure to mango tree sap (Figure 1). The patient reported having experienced similar pruritic eruptions in the past following contact with mango sap while eating mangos but denied any history of reactions from ingestion of the fruit. She also reported a history of robust reactions to poison ivy; however, a timeline specifying the order of first exposure to these irritants was unknown. She denied any personal or family history of atopic conditions.

The affected skin was imaged in real time during clinic using RCM, which showed an inflammatory infiltrate represented by dark spongiotic vesicles containing bright cells (Figure 2). Additional RCM imaging at the level of the stratum spinosum showed dark spongiotic areas with bright inflammatory cells infiltrating the vesicles, which were surrounded by normal skin showing a typical epidermal honeycomb pattern (Figure 3). These findings were diagnostic of ACD secondary to exposure to mango sap. The patient was advised to apply clobetasol cream 0.05% to the affected area. Notable improvement of the rash was noted within 10 days of treatment.


Comment
Exposure to the mango tree and its fruit is a rare cause of ACD, with few reported cases in the literature. The majority of known instances have occurred in non–mango-cultivating countries, largely the United States, although cases also have been reported in Canada, Australia, France, Japan, and Thailand.3,12 Mango-induced contact allergy follows a roughly equal distribution between males and females and most often occurs in young adults during the third and fourth decades of life.4,12-21 Importantly, delayed-type hypersensitivity reactions to mango can manifest as either localized or systemic ACD. Localized ACD can be induced via direct contact with the mango tree and its components or ingestion of the fruit.3,12,22 Conversely, systemic ACD is primarily stimulated by ingestion of the fruit. In our case, the patient had no history of allergy following mango ingestion, and her ACD was prompted by isolated contact with mango sap. The time from exposure to symptom onset of known instances of mango ACD varies widely, ranging from less than 24 hours to as long as 9 days.3,12 Diagnosis of mango-induced ACD largely is guided by clinical findings. Presenting symptoms often include an eczematous, vesicular, pruritic rash on affected areas of the skin, frequently the head, neck, and extremities. Patients also commonly present with linear papulovesicular lesions and periorbital or perioral edema.
The suspected allergens responsible for mango-induced ACD are derived from resorcinol—specifically heptadecadienyl resorcinol, heptadecenyl resorcinol, and pentadecyl resorcinol, which are collectively known as mango allergens.23 These allergens can be found within the pulp and skin of the mango fruit as well as in the bark and leaves of the mango tree, which may explain observed allergic reactions to components of both the mango fruit and tree.12 Similar to these resorcinol derivatives, the urushiol resin found in poison ivy and poison oak is a catechol derivative.2 Importantly, both resorcinols and catechols are isomers of the same aromatic phenol—dihydroxybenzene. Because of these similarities, it is thought that the allergens in mangos may cross-react with urushiol in poison ivy or poison oak.23 Alongside their shared categorization in the Anacardiaceae family, it is hypothesized that this cross-reactivity underlies the sensitization that has been noted between mango and poison ivy or poison oak exposure.12,23,24 Thus, ACD often can occur on initial contact with the mango tree or its components, as a prior exposure to poison ivy or poison oak may serve as the inciting factor for hypersensitization. The majority of reported cases in the literature also occurred in countries where exposure to poison ivy and poison oak are common, further supporting the notion that these compounds may provide a sensitizing trigger for a future mango contact allergy.12
A detailed clinical history combined with adjunctive diagnostic support from patch testing and histopathology of biopsied skin lesions classically are used in the diagnosis of mango-induced ACD. Due to its ability to provide quick and noninvasive in vivo imaging of cutaneous lesions, RCM's applications have expanded to include evaluation of inflammatory skin diseases such as contact dermatitis. Many features of contact dermatitis identified via RCM are common between ACD and irritant contact dermatitis (ICD) and include disruption of the stratum corneum, parakeratosis, vesiculation, spongiosis, and exocytosis.6,10,25 Studies also have described features shown via RCM that are unique to ACD, including vasodilation and intercellular edema, compared to more distinct targetoid keratinocytes and detached corneocytes seen in ICD.6,10,25 Studies by Astner et al5,6 demonstrated a wide range of sensitivity from 52% to 96% and a high specificity of RCM greater than 95% for many of the aforementioned features of contact dermatitis, including disruption of the stratum corneum, parakeratosis, spongiosis, and exocytosis. Additional studies have further strengthened these findings, demonstrating sensitivity and specificity values of 83% and 92% for contact dermatitis under RCM, respectively.26 Importantly, given the similarities and potentially large overlap of features between ACD and ICD identified via RCM as well as findings seen on physical examination and histopathology, an emphasis on clinical correlation is essential when differentiating between these 2 variants of contact dermatitis. Thus, taken in consideration with clinical contexts, RCM has shown potent diagnostic accuracy and great potential to support the evaluation of ACD alongside patch testing and histopathology.
Final Thoughts
Contact allergy to the mango tree and its components is uncommon. We report a unique case of mango sap–induced ACD evaluated and diagnosed via dynamic visualization under RCM. As a noninvasive and reproducible imaging technique with resolutions comparable to histopathologic analysis, RCM is a promising tool that can be used to support the diagnostic evaluation of ACD.
- Shah KA, Patel MB, Patel RJ, et al. Mangifera indica (mango). Pharmacogn Rev. 2010;4:42-48.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed September 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557866
- Sareen R, Shah A. Hypersensitivity manifestations to the fruit mango. Asia Pac Allergy. 2011;1:43-49.
- Zakon SJ. Contact dermatitis due to mango. JAMA. 1939;113:1808.
- Astner S, Gonzalez E, Cheung A, et al. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986-992.
- Astner S, Gonzalez S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182-191.
- Csuka EA, Ward SC, Ekelem C, et al. Reflectance confocal microscopy, optical coherence tomography, and multiphoton microscopy in inflammatory skin disease diagnosis. Lasers Surg Med. 2021;53:776-797.
- Guichard A, Fanian F, Girardin P, et al. Allergic patch test and contact dermatitis by in vivo reflectance confocal microscopy [in French]. Ann Dermatol Venereol. 2014;141:805-807.
- Sakanashi EN, Matsumura M, Kikuchi K, et al. A comparative study of allergic contact dermatitis by patch test versus reflectance confocal laser microscopy, with nickel and cobalt. Eur J Dermatol. 2010;20:705-711.
- Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
- Shahriari N, Grant-Kels JM, Rabinovitz H, et al. Reflectance confocal microscopy: principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84:1-14.
- Berghea EC, Craiu M, Ali S, et al. Contact allergy induced by mango (Mangifera indica): a relevant topic? Medicina (Kaunas). 2021;57:1240.
- O’Hern K, Zhang F, Zug KA, et al. “Mango slice” dermatitis: pediatric allergic contact dermatitis to mango pulp and skin. Dermatitis. 2022;33:E46-E47.
- Raison-Peyron N, Aljaber F, Al Ali OA, et al. Mango dermatitis: an unusual cause of eyelid dermatitis in France. Contact Dermatitis. 2021;85:599-600.
- Alipour Tehrany Y, Coulombe J. Mango allergic contact dermatitis. Contact Dermatitis. 2021;85:241-242.
- Yoo MJ, Carius BM. Mango dermatitis after urushiol sensitization. Clin Pract Cases Emerg Med. 2019;3:361-363.
- Miyazawa H, Nishie W, Hata H, et al. A severe case of mango dermatitis. J Eur Acad Dermatol Venereol. 2018;32:E160-E161.
- Trehan I, Meuli GJ. Mango contact allergy. J Travel Med. 2010;17:284.
- Wiwanitkit V. Mango dermatitis. Indian J Dermatol. 2008;53:158.
- Weinstein S, Bassiri-Tehrani S, Cohen DE. Allergic contact dermatitis to mango flesh. Int J Dermatol. 2004;43:195-196.
- Calvert ML, Robertson I, Samaratunga H. Mango dermatitis: allergic contact dermatitis to Mangifera indica. Australas J Dermatol. 1996;37:59-60.
- Thoo CH, Freeman S. Hypersensitivity reaction to the ingestion of mango flesh. Australas J Dermatol. 2008;49:116-119.
- Oka K, Saito F, Yasuhara T, et al. A study of cross-reactions between mango contact allergens and urushiol. Contact Dermatitis. 2004;51:292-296.
- Keil H, Wasserman D, Dawson CR. Mango dermatitis and its relationship to poison ivy hypersensitivity. Ann Allergy. 1946;4: 268-281.
- Maarouf M, Costello CM, Gonzalez S, et al. In vivo reflectance confocal microscopy: emerging role in noninvasive diagnosis and monitoring of eczematous dermatoses. Actas Dermosifiliogr (Engl Ed). 2019;110:626-636.
- Koller S, Gerger A, Ahlgrimm-Siess V, et al. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp Dermatol. 2009;18:536-540.
- Shah KA, Patel MB, Patel RJ, et al. Mangifera indica (mango). Pharmacogn Rev. 2010;4:42-48.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed September 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557866
- Sareen R, Shah A. Hypersensitivity manifestations to the fruit mango. Asia Pac Allergy. 2011;1:43-49.
- Zakon SJ. Contact dermatitis due to mango. JAMA. 1939;113:1808.
- Astner S, Gonzalez E, Cheung A, et al. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986-992.
- Astner S, Gonzalez S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182-191.
- Csuka EA, Ward SC, Ekelem C, et al. Reflectance confocal microscopy, optical coherence tomography, and multiphoton microscopy in inflammatory skin disease diagnosis. Lasers Surg Med. 2021;53:776-797.
- Guichard A, Fanian F, Girardin P, et al. Allergic patch test and contact dermatitis by in vivo reflectance confocal microscopy [in French]. Ann Dermatol Venereol. 2014;141:805-807.
- Sakanashi EN, Matsumura M, Kikuchi K, et al. A comparative study of allergic contact dermatitis by patch test versus reflectance confocal laser microscopy, with nickel and cobalt. Eur J Dermatol. 2010;20:705-711.
- Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
- Shahriari N, Grant-Kels JM, Rabinovitz H, et al. Reflectance confocal microscopy: principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84:1-14.
- Berghea EC, Craiu M, Ali S, et al. Contact allergy induced by mango (Mangifera indica): a relevant topic? Medicina (Kaunas). 2021;57:1240.
- O’Hern K, Zhang F, Zug KA, et al. “Mango slice” dermatitis: pediatric allergic contact dermatitis to mango pulp and skin. Dermatitis. 2022;33:E46-E47.
- Raison-Peyron N, Aljaber F, Al Ali OA, et al. Mango dermatitis: an unusual cause of eyelid dermatitis in France. Contact Dermatitis. 2021;85:599-600.
- Alipour Tehrany Y, Coulombe J. Mango allergic contact dermatitis. Contact Dermatitis. 2021;85:241-242.
- Yoo MJ, Carius BM. Mango dermatitis after urushiol sensitization. Clin Pract Cases Emerg Med. 2019;3:361-363.
- Miyazawa H, Nishie W, Hata H, et al. A severe case of mango dermatitis. J Eur Acad Dermatol Venereol. 2018;32:E160-E161.
- Trehan I, Meuli GJ. Mango contact allergy. J Travel Med. 2010;17:284.
- Wiwanitkit V. Mango dermatitis. Indian J Dermatol. 2008;53:158.
- Weinstein S, Bassiri-Tehrani S, Cohen DE. Allergic contact dermatitis to mango flesh. Int J Dermatol. 2004;43:195-196.
- Calvert ML, Robertson I, Samaratunga H. Mango dermatitis: allergic contact dermatitis to Mangifera indica. Australas J Dermatol. 1996;37:59-60.
- Thoo CH, Freeman S. Hypersensitivity reaction to the ingestion of mango flesh. Australas J Dermatol. 2008;49:116-119.
- Oka K, Saito F, Yasuhara T, et al. A study of cross-reactions between mango contact allergens and urushiol. Contact Dermatitis. 2004;51:292-296.
- Keil H, Wasserman D, Dawson CR. Mango dermatitis and its relationship to poison ivy hypersensitivity. Ann Allergy. 1946;4: 268-281.
- Maarouf M, Costello CM, Gonzalez S, et al. In vivo reflectance confocal microscopy: emerging role in noninvasive diagnosis and monitoring of eczematous dermatoses. Actas Dermosifiliogr (Engl Ed). 2019;110:626-636.
- Koller S, Gerger A, Ahlgrimm-Siess V, et al. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp Dermatol. 2009;18:536-540.
Practice Points
- Contact with mango tree sap can induce allergic contact dermatitis.
- Reflectance confocal microscopy (RCM) is a noninvasive imaging technique that can provide real-time in vivo visualization of affected skin in contact dermatitis.
- Predominant findings of contact dermatitis under RCM include disruption of the stratum corneum; parakeratosis; vesiculation; spongiosis; and exocytosis, vasodilation, and intercellular edema more specific to the allergic subtype.
Does allergic conjunctivitis always require prescription eyedrops?
No, not all patients with allergic conjunctivitis need prescription eyedrops.
For mild symptoms, basic nonpharmacologic eye care often suffices. Advise the patient to avoid rubbing the eyes, to use artificial tears as needed, to apply cold compresses, to limit or temporarily discontinue contact lens wear, and to avoid exposure to known allergens.
Topical therapy with an over-the-counter eyedrop that combines an antihistamine and a mast cell stabilizer is another first-line measure.
Prescription eyedrops are usually reserved for patients who have persistent bothersome symptoms despite use of over-the-counter eyedrops. Also, some patients have difficulty with the regimens for over-the-counter eyedrops, since most must be applied two to four times per day. In addition, patients with concomitant allergic rhinitis may benefit from an intranasal corticosteroid.
ALLERGIC CONJUNCTIVITIS: A BRIEF OVERVIEW
Allergic conjunctivitis, caused by exposure of the eye to airborne allergens, affects up to 40% of the US population, predominantly young adults.1 Bilateral pruritus is the chief symptom. The absence of pruritus should prompt consideration of a more serious eye condition.
Other common symptoms of allergic conjunctivitis include redness, tearing (a clear, watery discharge), eyelid edema, burning, and mild photophobia. Some patients may have infraorbital edema and darkening around the eye, dubbed an “allergic shiner.”1
Allergic conjunctivitis can be acute, with sudden onset of symptoms upon exposure to an isolated allergen. It can be seasonal, from exposure to pollen and with a more gradual onset. It can also be perennial, from year-round exposure to indoor allergens such as animal dander, dust mites, and mold.
Allergic conjunctivitis often occurs together with allergic rhinitis, which is also caused by exposure to aeroallergens and is characterized by nasal congestion, pruritus, rhinorrhea (anterior and posterior), and sneezing.2
Pollen is more commonly associated with rhinoconjunctivitis, whereas dust mite allergy is more likely to cause rhinitis alone.
An immunoglobulin E-mediated reaction
Allergic conjunctivitis is a type I immunoglobulin E-mediated immediate hypersensitivity reaction. In the early phase, ie, within minutes of allergen exposure, previously sensitized mast cells are exposed to an allergen, causing degranulation and release of inflammatory mediators, primarily histamine. The late phase, ie, 6 to 10 hours after the initial exposure, involves an influx of inflammatory cells such as eosinophils, basophils, and neutrophils.3
Differential diagnosis
The differential diagnosis of allergic conjunctivitis includes infectious conjunctivitis, chronic dry eye, preservative toxicity, giant papillary conjunctivitis, atopic keratoconjunctivitis, and vernal keratoconjunctivitis.3 Giant papillary conjunctivitis is an inflammatory reaction to a foreign substance, such as a contact lens. Atopic keratoconjunctivitis and vernal keratoconjunctivitis can be vision-threatening and require referral to an ophthalmologist. Atopic keratoconjunctivitis is associated with eczematous lesions of the lids and skin, and vernal keratoconjunctivitis involves chronic inflammation of the palpebral conjunctivae. Warning signs include photophobia, pain, abnormal findings on pupillary examination, blurred vision (unrelated to excessively watery eyes), unilateral eye complaints, and ciliary flush.2
Bacterial conjunctivitis is highly contagious and usually presents with hyperemia, “stuck eye” upon awakening, and thick, purulent discharge. It is usually unilateral. Symptoms include burning, foreign-body sensation, and discomfort rather than pruritus. Patients with allergic conjunctivitis may have concomitant bacterial conjunctivitis and so require a topical antibiotic as well as treatment for allergic conjunctivitis.
Viral conjunctivitis usually affects one eye, is self-limited, and typically presents with other symptoms of a viral syndrome.
MANAGEMENT OPTIONS
Management of allergic conjunctivitis consists of basic eye care, avoidance of allergy triggers, and over-the-counter and prescription topical and systemic therapies, as well as allergen immunotherapy.3
Avoidance
Triggers for the allergic reaction, such as pollen, can be identified with aeroallergen skin testing by an allergist. But simple avoidance measures are helpful, such as closing windows, using air conditioning, limiting exposure to the outdoors when pollen counts are high, wearing sunglasses, showering before bedtime, avoiding exposure to animal dander, and using zippered casings for bedding to minimize exposure to dust mites.3
Patients who wear contact lenses should reduce or discontinue their use, as allergens adhere to contact lens surfaces.
Topical therapies
If avoidance is not feasible or if symptoms persist despite avoidance measures, patients should be started on eyedrops.
Eyedrops for allergic conjunctivitis are classified by mechanism of action: topical antihistamines, mast-cell stabilizers, and combination preparations of antihistamine and mast-cell stabilizer (Table 1). Algorithms for managing allergic conjunctivitis exist2 but are based on expert consensus, since there are no randomized clinical trials with head-to-head comparisons of topical agents for allergic conjunctivitis.
In our practice, we use a three-step approach to treat allergic conjunctivitis (Table 2). Combination antihistamine and mast-cell stabilizer eyedrops are the first line, used as needed, daily, seasonally, or year-round, based on the patient’s symptoms and allergen profile. Antihistamine or combination eyedrops are preferred as they have a faster onset of action than mast-cell stabilizers alone,3 which have an onset of action of 3 to 5 days. The combination drops provide an effect on the late-phase response and a longer duration of action.
Currently, the only over-the-counter eyedrops for allergic conjunctivitis are cromolyn (a mast-cell stabilizer) and ketotifen 0.025% (a combination antihistamine and mast-cell stabilizer). Most drops for allergic conjunctivitis are taken two to four times a day. Two once-daily eyedrop formulations for allergic conjunctivitis—available only by prescription—are olopatadine 0.2% and alcaftadine. However, these are very expensive (Table 1) and so may not be an appropriate choice for some patients. On the other hand, a study from the United Kingdom4 found that patients using olopatadine made fewer visits to their general practitioner than patients using cromolyn, resulting in lower overall cost of healthcare. Results of studies of patient preferences and efficacy of olopatadine 0.1% (twice-daily preparation) vs ketotifen 0.025% are mixed,5–8 and no study has compared olopatadine 0.2% (once-daily preparation) with over-the-counter ketotifen.
Adverse effects of eyedrops
Common adverse effects include stinging and burning immediately after use; this effect may be reduced by keeping the eyedrops in the refrigerator. Patients who wear contact lenses should remove them before using eyedrops for allergic conjunctivitis, and wait at least 10 minutes to replace them if the eye is no longer red.2 Antihistamine drops are contraindicated in patients at risk for angle-closure glaucoma.
Whenever possible, patients with seasonal allergic conjunctivitis should begin treatment 2 to 4 weeks before the relevant pollen season, as guided by the patient’s experience in past seasons or by the results of aeroallergen skin testing. This modifies the “priming” effect, in which the amount of allergen required to induce an immediate allergic response decreases with repeated exposure to the allergen.
OTHER TREATMENT OPTIONS
Vasoconstrictor or decongestant eyedrops are indicated to relieve eye redness but have little or no effect on pruritus, and prolonged use may lead to rebound hyperemia. Thus, they are not generally recommended for long-term treatment of allergic conjunctivitis.3 Also, patients with glaucoma should be advised against long-term use of over-the-counter vasoconstrictor eyedrops.
Corticosteroid eyedrops are reserved for refractory and severe cases. Their use requires close follow-up with an ophthalmologist to monitor for complications such as increased intraocular pressure, infection, and cataracts.2
Patients presenting with an acute severe episode of allergic conjunctivitis that has not responded to oral antihistamines or combination eyedrops may be treated with a short course of an oral corticosteroid, if the benefit outweighs the risk in that patient.
Oral antihistamines are generally less effective than topical ophthalmic agents in relieving ocular allergy symptoms and have a slower onset of action.2 They are useful in patients who have an aversion to instilling eyedrops on a regular basis or who wear contact lenses.
For patients who have associated allergic rhinitis—ie, most patients with allergic conjunctivitis—intranasal corticosteroids and intranasal antihistamines are the most effective treatments for rhinitis and are also effective for allergic conjunctivitis. Monotherapy with an intranasal medication may provide sufficient relief of conjunctivitis symptoms or allow ocular medications to be used on a less frequent basis.
Allergen immunotherapy
Referral to an allergist for consideration of allergen immunotherapy is an option when avoidance measures are ineffective or unfeasible, when first-line treatments are ineffective, and when the patient does not wish to use medications.
Allergen immunotherapy is the only disease-modifying therapy available for allergic conjunctivitis. Two forms are available: traditional subcutaneous immunotherapy, and sublingual tablet immunotherapy, recently approved by the US Food and Drug Administration.9 Subcutaneous immunotherapy targets specific aeroallergens for patients allergic to multiple allergens. The new sublingual immunotherapy tablets target only grass pollen and ragweed pollen.9 Most patients in the United States are polysensitized.10 Both forms of immunotherapy can result in sustained effectiveness following discontinuation. Sublingual therapy may be administered year-round, before allergy season, or during allergy season (depending on the type of allergy).
TAILORING TREATMENT
We recommend a case-by-case approach to the management of patients with allergic conjunctivitis, tailoring treatment to the patient’s symptoms, allergen profile, and personal preferences.
For example, if adherence is a challenge we recommend a once-daily combination eyedrop (olopatadine 0.2%, or alcaftadine). If cost is a barrier, we recommend the combination over-the-counter drop (ketotifen).
Medications may be used during allergy season or year-round depending on the patient’s symptom and allergen profile. Patients whose symptoms are not relieved with these measures should be referred to an allergist for further evaluation and management, or to an ophthalmologist to monitor for complications of topical steroid use and other warning signs, as discussed earlier, or to weigh in on the differential diagnosis.
- Bielory L, Friedlaender MH. Allergic conjunctivitis. Immunol Allergy Clin North Am 2008; 28:43–58.
- Bielory L, Meltzer EO, Nichols KK, Melton R, Thomas RK, Bartlett JD. An algorithm for the management of allergic conjunctivitis. Allergy Asthma Proc 2013; 34:408–420.
- Wallace DV, Dykewicz MS, Bernstein DI, et al; Joint Task Force on Practice; American Academy of Allergy; Asthma & Immunology; American College of Allergy; Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol 2008; 122(suppl 2):S1–S84.
- Guest JF, Clegg JP, Smith AF. Health economic impact of olopatadine compared to branded and generic sodium cromoglycate in the treatment of seasonal allergic conjunctivitis in the UK. Curr Med Res Opin 2006; 22:1777–1785.
- Leonardi A, Zafirakis P. Efficacy and comfort of olopatadine versus ketotifen ophthalmic solutions: a double-masked, environmental study of patient preference. Curr Med Res Opin 2004; 20:1167–1173.
- Ganz M, Koll E, Gausche J, Detjen P, Orfan N. Ketotifen fumarate and olopatadine hydrochloride in the treatment of allergic conjunctivitis: a real-world comparison of efficacy and ocular comfort. Adv Ther 2003; 20:79–91.
- Aguilar AJ. Comparative study of clinical efficacy and tolerance in seasonal allergic conjunctivitis management with 0.1% olopatadine hydrochloride versus 0.05% ketotifen fumarate. Acta Ophthalmol Scand Suppl 2000; 230:52–55.
- Artal MN, Luna JD, Discepola M. A forced choice comfort study of olopatadine hydrochloride 0.1% versus ketotifen fumarate 0.05%. Acta Ophthalmol Scand Suppl 2000; 230:64–65.
- Cox L. Sublingual immunotherapy for aeroallergens: status in the United States. Allergy Asthma Proc 2014; 35:34–42.
- Salo PM, Arbes SJ Jr, Jaramillo R, et al. Prevalence of allergic sensitization in the United States: results from the National Health and Nutrition Examination Survey (NHANES) 2005-2006. J Allergy Clin Immunol 2014; 134:350–359.
No, not all patients with allergic conjunctivitis need prescription eyedrops.
For mild symptoms, basic nonpharmacologic eye care often suffices. Advise the patient to avoid rubbing the eyes, to use artificial tears as needed, to apply cold compresses, to limit or temporarily discontinue contact lens wear, and to avoid exposure to known allergens.
Topical therapy with an over-the-counter eyedrop that combines an antihistamine and a mast cell stabilizer is another first-line measure.
Prescription eyedrops are usually reserved for patients who have persistent bothersome symptoms despite use of over-the-counter eyedrops. Also, some patients have difficulty with the regimens for over-the-counter eyedrops, since most must be applied two to four times per day. In addition, patients with concomitant allergic rhinitis may benefit from an intranasal corticosteroid.
ALLERGIC CONJUNCTIVITIS: A BRIEF OVERVIEW
Allergic conjunctivitis, caused by exposure of the eye to airborne allergens, affects up to 40% of the US population, predominantly young adults.1 Bilateral pruritus is the chief symptom. The absence of pruritus should prompt consideration of a more serious eye condition.
Other common symptoms of allergic conjunctivitis include redness, tearing (a clear, watery discharge), eyelid edema, burning, and mild photophobia. Some patients may have infraorbital edema and darkening around the eye, dubbed an “allergic shiner.”1
Allergic conjunctivitis can be acute, with sudden onset of symptoms upon exposure to an isolated allergen. It can be seasonal, from exposure to pollen and with a more gradual onset. It can also be perennial, from year-round exposure to indoor allergens such as animal dander, dust mites, and mold.
Allergic conjunctivitis often occurs together with allergic rhinitis, which is also caused by exposure to aeroallergens and is characterized by nasal congestion, pruritus, rhinorrhea (anterior and posterior), and sneezing.2
Pollen is more commonly associated with rhinoconjunctivitis, whereas dust mite allergy is more likely to cause rhinitis alone.
An immunoglobulin E-mediated reaction
Allergic conjunctivitis is a type I immunoglobulin E-mediated immediate hypersensitivity reaction. In the early phase, ie, within minutes of allergen exposure, previously sensitized mast cells are exposed to an allergen, causing degranulation and release of inflammatory mediators, primarily histamine. The late phase, ie, 6 to 10 hours after the initial exposure, involves an influx of inflammatory cells such as eosinophils, basophils, and neutrophils.3
Differential diagnosis
The differential diagnosis of allergic conjunctivitis includes infectious conjunctivitis, chronic dry eye, preservative toxicity, giant papillary conjunctivitis, atopic keratoconjunctivitis, and vernal keratoconjunctivitis.3 Giant papillary conjunctivitis is an inflammatory reaction to a foreign substance, such as a contact lens. Atopic keratoconjunctivitis and vernal keratoconjunctivitis can be vision-threatening and require referral to an ophthalmologist. Atopic keratoconjunctivitis is associated with eczematous lesions of the lids and skin, and vernal keratoconjunctivitis involves chronic inflammation of the palpebral conjunctivae. Warning signs include photophobia, pain, abnormal findings on pupillary examination, blurred vision (unrelated to excessively watery eyes), unilateral eye complaints, and ciliary flush.2
Bacterial conjunctivitis is highly contagious and usually presents with hyperemia, “stuck eye” upon awakening, and thick, purulent discharge. It is usually unilateral. Symptoms include burning, foreign-body sensation, and discomfort rather than pruritus. Patients with allergic conjunctivitis may have concomitant bacterial conjunctivitis and so require a topical antibiotic as well as treatment for allergic conjunctivitis.
Viral conjunctivitis usually affects one eye, is self-limited, and typically presents with other symptoms of a viral syndrome.
MANAGEMENT OPTIONS
Management of allergic conjunctivitis consists of basic eye care, avoidance of allergy triggers, and over-the-counter and prescription topical and systemic therapies, as well as allergen immunotherapy.3
Avoidance
Triggers for the allergic reaction, such as pollen, can be identified with aeroallergen skin testing by an allergist. But simple avoidance measures are helpful, such as closing windows, using air conditioning, limiting exposure to the outdoors when pollen counts are high, wearing sunglasses, showering before bedtime, avoiding exposure to animal dander, and using zippered casings for bedding to minimize exposure to dust mites.3
Patients who wear contact lenses should reduce or discontinue their use, as allergens adhere to contact lens surfaces.
Topical therapies
If avoidance is not feasible or if symptoms persist despite avoidance measures, patients should be started on eyedrops.
Eyedrops for allergic conjunctivitis are classified by mechanism of action: topical antihistamines, mast-cell stabilizers, and combination preparations of antihistamine and mast-cell stabilizer (Table 1). Algorithms for managing allergic conjunctivitis exist2 but are based on expert consensus, since there are no randomized clinical trials with head-to-head comparisons of topical agents for allergic conjunctivitis.
In our practice, we use a three-step approach to treat allergic conjunctivitis (Table 2). Combination antihistamine and mast-cell stabilizer eyedrops are the first line, used as needed, daily, seasonally, or year-round, based on the patient’s symptoms and allergen profile. Antihistamine or combination eyedrops are preferred as they have a faster onset of action than mast-cell stabilizers alone,3 which have an onset of action of 3 to 5 days. The combination drops provide an effect on the late-phase response and a longer duration of action.
Currently, the only over-the-counter eyedrops for allergic conjunctivitis are cromolyn (a mast-cell stabilizer) and ketotifen 0.025% (a combination antihistamine and mast-cell stabilizer). Most drops for allergic conjunctivitis are taken two to four times a day. Two once-daily eyedrop formulations for allergic conjunctivitis—available only by prescription—are olopatadine 0.2% and alcaftadine. However, these are very expensive (Table 1) and so may not be an appropriate choice for some patients. On the other hand, a study from the United Kingdom4 found that patients using olopatadine made fewer visits to their general practitioner than patients using cromolyn, resulting in lower overall cost of healthcare. Results of studies of patient preferences and efficacy of olopatadine 0.1% (twice-daily preparation) vs ketotifen 0.025% are mixed,5–8 and no study has compared olopatadine 0.2% (once-daily preparation) with over-the-counter ketotifen.
Adverse effects of eyedrops
Common adverse effects include stinging and burning immediately after use; this effect may be reduced by keeping the eyedrops in the refrigerator. Patients who wear contact lenses should remove them before using eyedrops for allergic conjunctivitis, and wait at least 10 minutes to replace them if the eye is no longer red.2 Antihistamine drops are contraindicated in patients at risk for angle-closure glaucoma.
Whenever possible, patients with seasonal allergic conjunctivitis should begin treatment 2 to 4 weeks before the relevant pollen season, as guided by the patient’s experience in past seasons or by the results of aeroallergen skin testing. This modifies the “priming” effect, in which the amount of allergen required to induce an immediate allergic response decreases with repeated exposure to the allergen.
OTHER TREATMENT OPTIONS
Vasoconstrictor or decongestant eyedrops are indicated to relieve eye redness but have little or no effect on pruritus, and prolonged use may lead to rebound hyperemia. Thus, they are not generally recommended for long-term treatment of allergic conjunctivitis.3 Also, patients with glaucoma should be advised against long-term use of over-the-counter vasoconstrictor eyedrops.
Corticosteroid eyedrops are reserved for refractory and severe cases. Their use requires close follow-up with an ophthalmologist to monitor for complications such as increased intraocular pressure, infection, and cataracts.2
Patients presenting with an acute severe episode of allergic conjunctivitis that has not responded to oral antihistamines or combination eyedrops may be treated with a short course of an oral corticosteroid, if the benefit outweighs the risk in that patient.
Oral antihistamines are generally less effective than topical ophthalmic agents in relieving ocular allergy symptoms and have a slower onset of action.2 They are useful in patients who have an aversion to instilling eyedrops on a regular basis or who wear contact lenses.
For patients who have associated allergic rhinitis—ie, most patients with allergic conjunctivitis—intranasal corticosteroids and intranasal antihistamines are the most effective treatments for rhinitis and are also effective for allergic conjunctivitis. Monotherapy with an intranasal medication may provide sufficient relief of conjunctivitis symptoms or allow ocular medications to be used on a less frequent basis.
Allergen immunotherapy
Referral to an allergist for consideration of allergen immunotherapy is an option when avoidance measures are ineffective or unfeasible, when first-line treatments are ineffective, and when the patient does not wish to use medications.
Allergen immunotherapy is the only disease-modifying therapy available for allergic conjunctivitis. Two forms are available: traditional subcutaneous immunotherapy, and sublingual tablet immunotherapy, recently approved by the US Food and Drug Administration.9 Subcutaneous immunotherapy targets specific aeroallergens for patients allergic to multiple allergens. The new sublingual immunotherapy tablets target only grass pollen and ragweed pollen.9 Most patients in the United States are polysensitized.10 Both forms of immunotherapy can result in sustained effectiveness following discontinuation. Sublingual therapy may be administered year-round, before allergy season, or during allergy season (depending on the type of allergy).
TAILORING TREATMENT
We recommend a case-by-case approach to the management of patients with allergic conjunctivitis, tailoring treatment to the patient’s symptoms, allergen profile, and personal preferences.
For example, if adherence is a challenge we recommend a once-daily combination eyedrop (olopatadine 0.2%, or alcaftadine). If cost is a barrier, we recommend the combination over-the-counter drop (ketotifen).
Medications may be used during allergy season or year-round depending on the patient’s symptom and allergen profile. Patients whose symptoms are not relieved with these measures should be referred to an allergist for further evaluation and management, or to an ophthalmologist to monitor for complications of topical steroid use and other warning signs, as discussed earlier, or to weigh in on the differential diagnosis.
No, not all patients with allergic conjunctivitis need prescription eyedrops.
For mild symptoms, basic nonpharmacologic eye care often suffices. Advise the patient to avoid rubbing the eyes, to use artificial tears as needed, to apply cold compresses, to limit or temporarily discontinue contact lens wear, and to avoid exposure to known allergens.
Topical therapy with an over-the-counter eyedrop that combines an antihistamine and a mast cell stabilizer is another first-line measure.
Prescription eyedrops are usually reserved for patients who have persistent bothersome symptoms despite use of over-the-counter eyedrops. Also, some patients have difficulty with the regimens for over-the-counter eyedrops, since most must be applied two to four times per day. In addition, patients with concomitant allergic rhinitis may benefit from an intranasal corticosteroid.
ALLERGIC CONJUNCTIVITIS: A BRIEF OVERVIEW
Allergic conjunctivitis, caused by exposure of the eye to airborne allergens, affects up to 40% of the US population, predominantly young adults.1 Bilateral pruritus is the chief symptom. The absence of pruritus should prompt consideration of a more serious eye condition.
Other common symptoms of allergic conjunctivitis include redness, tearing (a clear, watery discharge), eyelid edema, burning, and mild photophobia. Some patients may have infraorbital edema and darkening around the eye, dubbed an “allergic shiner.”1
Allergic conjunctivitis can be acute, with sudden onset of symptoms upon exposure to an isolated allergen. It can be seasonal, from exposure to pollen and with a more gradual onset. It can also be perennial, from year-round exposure to indoor allergens such as animal dander, dust mites, and mold.
Allergic conjunctivitis often occurs together with allergic rhinitis, which is also caused by exposure to aeroallergens and is characterized by nasal congestion, pruritus, rhinorrhea (anterior and posterior), and sneezing.2
Pollen is more commonly associated with rhinoconjunctivitis, whereas dust mite allergy is more likely to cause rhinitis alone.
An immunoglobulin E-mediated reaction
Allergic conjunctivitis is a type I immunoglobulin E-mediated immediate hypersensitivity reaction. In the early phase, ie, within minutes of allergen exposure, previously sensitized mast cells are exposed to an allergen, causing degranulation and release of inflammatory mediators, primarily histamine. The late phase, ie, 6 to 10 hours after the initial exposure, involves an influx of inflammatory cells such as eosinophils, basophils, and neutrophils.3
Differential diagnosis
The differential diagnosis of allergic conjunctivitis includes infectious conjunctivitis, chronic dry eye, preservative toxicity, giant papillary conjunctivitis, atopic keratoconjunctivitis, and vernal keratoconjunctivitis.3 Giant papillary conjunctivitis is an inflammatory reaction to a foreign substance, such as a contact lens. Atopic keratoconjunctivitis and vernal keratoconjunctivitis can be vision-threatening and require referral to an ophthalmologist. Atopic keratoconjunctivitis is associated with eczematous lesions of the lids and skin, and vernal keratoconjunctivitis involves chronic inflammation of the palpebral conjunctivae. Warning signs include photophobia, pain, abnormal findings on pupillary examination, blurred vision (unrelated to excessively watery eyes), unilateral eye complaints, and ciliary flush.2
Bacterial conjunctivitis is highly contagious and usually presents with hyperemia, “stuck eye” upon awakening, and thick, purulent discharge. It is usually unilateral. Symptoms include burning, foreign-body sensation, and discomfort rather than pruritus. Patients with allergic conjunctivitis may have concomitant bacterial conjunctivitis and so require a topical antibiotic as well as treatment for allergic conjunctivitis.
Viral conjunctivitis usually affects one eye, is self-limited, and typically presents with other symptoms of a viral syndrome.
MANAGEMENT OPTIONS
Management of allergic conjunctivitis consists of basic eye care, avoidance of allergy triggers, and over-the-counter and prescription topical and systemic therapies, as well as allergen immunotherapy.3
Avoidance
Triggers for the allergic reaction, such as pollen, can be identified with aeroallergen skin testing by an allergist. But simple avoidance measures are helpful, such as closing windows, using air conditioning, limiting exposure to the outdoors when pollen counts are high, wearing sunglasses, showering before bedtime, avoiding exposure to animal dander, and using zippered casings for bedding to minimize exposure to dust mites.3
Patients who wear contact lenses should reduce or discontinue their use, as allergens adhere to contact lens surfaces.
Topical therapies
If avoidance is not feasible or if symptoms persist despite avoidance measures, patients should be started on eyedrops.
Eyedrops for allergic conjunctivitis are classified by mechanism of action: topical antihistamines, mast-cell stabilizers, and combination preparations of antihistamine and mast-cell stabilizer (Table 1). Algorithms for managing allergic conjunctivitis exist2 but are based on expert consensus, since there are no randomized clinical trials with head-to-head comparisons of topical agents for allergic conjunctivitis.
In our practice, we use a three-step approach to treat allergic conjunctivitis (Table 2). Combination antihistamine and mast-cell stabilizer eyedrops are the first line, used as needed, daily, seasonally, or year-round, based on the patient’s symptoms and allergen profile. Antihistamine or combination eyedrops are preferred as they have a faster onset of action than mast-cell stabilizers alone,3 which have an onset of action of 3 to 5 days. The combination drops provide an effect on the late-phase response and a longer duration of action.
Currently, the only over-the-counter eyedrops for allergic conjunctivitis are cromolyn (a mast-cell stabilizer) and ketotifen 0.025% (a combination antihistamine and mast-cell stabilizer). Most drops for allergic conjunctivitis are taken two to four times a day. Two once-daily eyedrop formulations for allergic conjunctivitis—available only by prescription—are olopatadine 0.2% and alcaftadine. However, these are very expensive (Table 1) and so may not be an appropriate choice for some patients. On the other hand, a study from the United Kingdom4 found that patients using olopatadine made fewer visits to their general practitioner than patients using cromolyn, resulting in lower overall cost of healthcare. Results of studies of patient preferences and efficacy of olopatadine 0.1% (twice-daily preparation) vs ketotifen 0.025% are mixed,5–8 and no study has compared olopatadine 0.2% (once-daily preparation) with over-the-counter ketotifen.
Adverse effects of eyedrops
Common adverse effects include stinging and burning immediately after use; this effect may be reduced by keeping the eyedrops in the refrigerator. Patients who wear contact lenses should remove them before using eyedrops for allergic conjunctivitis, and wait at least 10 minutes to replace them if the eye is no longer red.2 Antihistamine drops are contraindicated in patients at risk for angle-closure glaucoma.
Whenever possible, patients with seasonal allergic conjunctivitis should begin treatment 2 to 4 weeks before the relevant pollen season, as guided by the patient’s experience in past seasons or by the results of aeroallergen skin testing. This modifies the “priming” effect, in which the amount of allergen required to induce an immediate allergic response decreases with repeated exposure to the allergen.
OTHER TREATMENT OPTIONS
Vasoconstrictor or decongestant eyedrops are indicated to relieve eye redness but have little or no effect on pruritus, and prolonged use may lead to rebound hyperemia. Thus, they are not generally recommended for long-term treatment of allergic conjunctivitis.3 Also, patients with glaucoma should be advised against long-term use of over-the-counter vasoconstrictor eyedrops.
Corticosteroid eyedrops are reserved for refractory and severe cases. Their use requires close follow-up with an ophthalmologist to monitor for complications such as increased intraocular pressure, infection, and cataracts.2
Patients presenting with an acute severe episode of allergic conjunctivitis that has not responded to oral antihistamines or combination eyedrops may be treated with a short course of an oral corticosteroid, if the benefit outweighs the risk in that patient.
Oral antihistamines are generally less effective than topical ophthalmic agents in relieving ocular allergy symptoms and have a slower onset of action.2 They are useful in patients who have an aversion to instilling eyedrops on a regular basis or who wear contact lenses.
For patients who have associated allergic rhinitis—ie, most patients with allergic conjunctivitis—intranasal corticosteroids and intranasal antihistamines are the most effective treatments for rhinitis and are also effective for allergic conjunctivitis. Monotherapy with an intranasal medication may provide sufficient relief of conjunctivitis symptoms or allow ocular medications to be used on a less frequent basis.
Allergen immunotherapy
Referral to an allergist for consideration of allergen immunotherapy is an option when avoidance measures are ineffective or unfeasible, when first-line treatments are ineffective, and when the patient does not wish to use medications.
Allergen immunotherapy is the only disease-modifying therapy available for allergic conjunctivitis. Two forms are available: traditional subcutaneous immunotherapy, and sublingual tablet immunotherapy, recently approved by the US Food and Drug Administration.9 Subcutaneous immunotherapy targets specific aeroallergens for patients allergic to multiple allergens. The new sublingual immunotherapy tablets target only grass pollen and ragweed pollen.9 Most patients in the United States are polysensitized.10 Both forms of immunotherapy can result in sustained effectiveness following discontinuation. Sublingual therapy may be administered year-round, before allergy season, or during allergy season (depending on the type of allergy).
TAILORING TREATMENT
We recommend a case-by-case approach to the management of patients with allergic conjunctivitis, tailoring treatment to the patient’s symptoms, allergen profile, and personal preferences.
For example, if adherence is a challenge we recommend a once-daily combination eyedrop (olopatadine 0.2%, or alcaftadine). If cost is a barrier, we recommend the combination over-the-counter drop (ketotifen).
Medications may be used during allergy season or year-round depending on the patient’s symptom and allergen profile. Patients whose symptoms are not relieved with these measures should be referred to an allergist for further evaluation and management, or to an ophthalmologist to monitor for complications of topical steroid use and other warning signs, as discussed earlier, or to weigh in on the differential diagnosis.
- Bielory L, Friedlaender MH. Allergic conjunctivitis. Immunol Allergy Clin North Am 2008; 28:43–58.
- Bielory L, Meltzer EO, Nichols KK, Melton R, Thomas RK, Bartlett JD. An algorithm for the management of allergic conjunctivitis. Allergy Asthma Proc 2013; 34:408–420.
- Wallace DV, Dykewicz MS, Bernstein DI, et al; Joint Task Force on Practice; American Academy of Allergy; Asthma & Immunology; American College of Allergy; Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol 2008; 122(suppl 2):S1–S84.
- Guest JF, Clegg JP, Smith AF. Health economic impact of olopatadine compared to branded and generic sodium cromoglycate in the treatment of seasonal allergic conjunctivitis in the UK. Curr Med Res Opin 2006; 22:1777–1785.
- Leonardi A, Zafirakis P. Efficacy and comfort of olopatadine versus ketotifen ophthalmic solutions: a double-masked, environmental study of patient preference. Curr Med Res Opin 2004; 20:1167–1173.
- Ganz M, Koll E, Gausche J, Detjen P, Orfan N. Ketotifen fumarate and olopatadine hydrochloride in the treatment of allergic conjunctivitis: a real-world comparison of efficacy and ocular comfort. Adv Ther 2003; 20:79–91.
- Aguilar AJ. Comparative study of clinical efficacy and tolerance in seasonal allergic conjunctivitis management with 0.1% olopatadine hydrochloride versus 0.05% ketotifen fumarate. Acta Ophthalmol Scand Suppl 2000; 230:52–55.
- Artal MN, Luna JD, Discepola M. A forced choice comfort study of olopatadine hydrochloride 0.1% versus ketotifen fumarate 0.05%. Acta Ophthalmol Scand Suppl 2000; 230:64–65.
- Cox L. Sublingual immunotherapy for aeroallergens: status in the United States. Allergy Asthma Proc 2014; 35:34–42.
- Salo PM, Arbes SJ Jr, Jaramillo R, et al. Prevalence of allergic sensitization in the United States: results from the National Health and Nutrition Examination Survey (NHANES) 2005-2006. J Allergy Clin Immunol 2014; 134:350–359.
- Bielory L, Friedlaender MH. Allergic conjunctivitis. Immunol Allergy Clin North Am 2008; 28:43–58.
- Bielory L, Meltzer EO, Nichols KK, Melton R, Thomas RK, Bartlett JD. An algorithm for the management of allergic conjunctivitis. Allergy Asthma Proc 2013; 34:408–420.
- Wallace DV, Dykewicz MS, Bernstein DI, et al; Joint Task Force on Practice; American Academy of Allergy; Asthma & Immunology; American College of Allergy; Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol 2008; 122(suppl 2):S1–S84.
- Guest JF, Clegg JP, Smith AF. Health economic impact of olopatadine compared to branded and generic sodium cromoglycate in the treatment of seasonal allergic conjunctivitis in the UK. Curr Med Res Opin 2006; 22:1777–1785.
- Leonardi A, Zafirakis P. Efficacy and comfort of olopatadine versus ketotifen ophthalmic solutions: a double-masked, environmental study of patient preference. Curr Med Res Opin 2004; 20:1167–1173.
- Ganz M, Koll E, Gausche J, Detjen P, Orfan N. Ketotifen fumarate and olopatadine hydrochloride in the treatment of allergic conjunctivitis: a real-world comparison of efficacy and ocular comfort. Adv Ther 2003; 20:79–91.
- Aguilar AJ. Comparative study of clinical efficacy and tolerance in seasonal allergic conjunctivitis management with 0.1% olopatadine hydrochloride versus 0.05% ketotifen fumarate. Acta Ophthalmol Scand Suppl 2000; 230:52–55.
- Artal MN, Luna JD, Discepola M. A forced choice comfort study of olopatadine hydrochloride 0.1% versus ketotifen fumarate 0.05%. Acta Ophthalmol Scand Suppl 2000; 230:64–65.
- Cox L. Sublingual immunotherapy for aeroallergens: status in the United States. Allergy Asthma Proc 2014; 35:34–42.
- Salo PM, Arbes SJ Jr, Jaramillo R, et al. Prevalence of allergic sensitization in the United States: results from the National Health and Nutrition Examination Survey (NHANES) 2005-2006. J Allergy Clin Immunol 2014; 134:350–359.
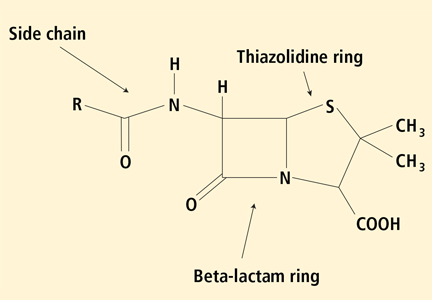
Penicillin allergy: A practical guide for clinicians
Most patients who report that they are allergic to penicillin can ultimately receive penicillin or a penicillin-type antibiotic again after an appropriate evaluation and, possibly, treatment. This course of action decreases the need for broad-spectrum antibiotics,1–4 reduces health care costs, and prevents the development of multidrug-resistant pathogens.5
About 10% of the general population say that they are allergic to penicillin.1,6,7 Although the prevalence of life-threatening anaphylactic reactions to penicillin has been estimated to be between 0.02% and 0.04%,6 the most common reaction is a cutaneous eruption. Since anaphylactic reactions are mediated by immunoglobulin E (IgE), evaluation of patients with a history of penicillin allergy by penicillin skin testing is recommended to rule out IgE-mediated reactions.
This review outlines a practical approach to evaluating a suspected IgE-mediated reaction to penicillin, with key points in the history and diagnostic testing. We also review subsequent management and cross-reactivity with other beta-lactam-containing antibiotics.
EVALUATING ALLERGIC PATIENTS
Evaluation of patients with a history of penicillin allergy can be improved with an understanding of the classification of drug reactions, risk factors for allergy, and the pathophysiology of penicillin allergy.
Classification of drug reactions
Adverse drug reactions include all unintended pharmacologic effects of a drug and can be classified as predictable (type A) or unpredictable (type B). Predictable reactions are dose-dependent, are related to the known pharmacologic actions of the medication, and occur in otherwise healthy individuals. Unpredictable reactions are further classified into drug intolerance, drug idiosyncrasy, drug allergy, and pseudoallergic reactions.8,9
Penicillin allergy can manifest as any hypersensitivity reaction of the Gell and Coombs classification (Table 1).9 Type I (immediate) and type IV (delayed) reactions are the most common types of reactions that occur with antibiotics and should be classified based on the onset of symptoms as immediate (within 1 hour) or delayed (days or weeks).8
Risk factors for IgE-mediated reaction
Risk factors for a hypersensitivity reaction include frequent or repetitive courses of penicillin10 and high-dose parenteral (rather than oral) administration.
Age and atopy are not risk factors for penicillin allergy.7 However, atopy increases the risk of a more severe anaphylactic reaction to penicillin, and anaphylactic reactions are most commonly reported between the ages of 20 and 49.6
Pathophysiology of penicillin allergy
All penicillins share a common core ring structure (beta-lactam and thiazolidine rings) but differ in their side chains (R group) (Figure 1).
Under physiologic conditions, the core ring structure is metabolized into major (penicilloyl) and minor (penicillin itself, penicilloate and penilloate) antigenic determinants that may trigger an immediate IgE-dependent response.9 In the United States, commercial forms of antigenic determinates for skin testing exist in the form of penicillin G (minor determinant) and penicilloyl-polylysine, better known as Prepen (major determinant).
Immediate-type reactions to similar antibiotics such as aminopenicillins and cephalosporins may be caused by IgE antibodies against the R-group side chain rather than the core penicillin major and minor determinants.11
Questions to ask patients who have a history of penicillin allergy
Patients should be questioned closely about previous and current reactions to penicillin and should undergo skin-prick and intradermal testing, followed by graded-dose challenge or drug tolerance desensitization (Figure 2).
Questions to ask patients who have a history of penicillin allergy (Table 2)9,12 include the following:
Do you remember the details of the reaction? These include the route of administration, the time between the dose of penicillin and the appearance of symptoms, and how the reaction was managed.
Immediate reactions (ie, IgE-mediated, or Gell and Coombs type I) usually occur within the first hour after the first dose of the antibiotic, although they occasionally take up to 2 hours to occur, especially if the medication is taken orally and is taken with food. Symptoms consistent with IgE-mediated reactions include urticaria (most common), pruritus, angioedema, laryngeal edema, wheezing, shortness of breath, presyncope or syncope, hypotension, and cardiorespiratory collapse.
In contrast, symptoms of a non–IgE-mediated reaction are delayed in onset, occurring after days of treatment. They include nonpruritic maculopapular eruptions, hemolytic anemia, serum sickness, Stevens-Johnson syndrome, drug rash with eosinophilia and systemic symptoms, acute interstitial nephritis, and toxic epidermal necrolysis.9
If the patient has had severe non–IgE-mediated reactions to penicillin (eg, Stevens-Johnson syndrome, toxic epidermal necrolysis, acute interstitial nephritis, hemolytic anemia, or serum sickness) in the past, skin testing, graded-dose challenge, and desensitization are contraindicated.
How many years ago did the reaction occur? Most patients lose their sensitivity to penicillin over time.7,13–15 Nearly 50% of patients with IgE-mediated penicillin allergy lose their sensitivity within 5 years of the reaction,15 increasing to 80% or more by 10 years.13
How was the reaction managed? What was the outcome? Use of and positive response to epinephrine and histamine 1 receptor antagonists (antihistamines) with resolution or significant improvement of symptoms within a few hours may indicate an IgE-mediated reaction.
What was the indication for penicillin? Many cutaneous reactions are a result of an underlying viral or bacterial infection. For example, up to 90% of patients with Epstein-Barr virus infection develop a maculopapular rash when given penicillin.16
Have you tolerated other forms of penicillin since the reaction? Sometimes the patient has already tolerated other beta-lactams such as aminopenicillins, cephalosporins, and semisynthetic penicillins (piperacillin-tazobactam). Patients who tolerate other beta-lactams without adverse reactions are not allergic to beta-lactams.
Diagnostic tests
Skin testing. The only validated test for diagnosing IgE-mediated reactions caused by penicillin is the immediate hypersensitivity skin test,9 which should be performed by a board-certified allergist. The test consists of skin-prick and intradermal testing with the major determinant (penicilloyl-polylysine), the minor determinant (penicillin G), a negative control (normal saline), and a positive control (histamine). Minor-determinant mix is not commercially available in the United States.
Results of skin-prick testing are read 15 minutes after application. A positive response is a wheal at least 3 mm larger in diameter (with equivalent erythema) than the negative control done simultaneously. Intradermal testing is only done after a negative skin-prick test. If the allergic reaction was severe (ie, anaphylaxis), skin testing should be done at least 4 to 6 weeks after the reaction.
A history of severe non–IgE-mediated reaction to penicillin is a contraindication to skin-prick testing for penicillin allergy. The positive predictive value of penicillin skin testing is 50%, and the negative predictive value is 97%.3,7,9,13
Commercial in vitro testing (serum-specific IgE assays) for IgE-mediated hypersensitivity to penicillin is inferior to skin testing in terms of the negative predictive value and is not a suitable substitute for penicillin skin testing.
MANAGING PENICILLIN ALLERGY
If skin testing is positive, use another antibiotic, or refer for desensitization
If penicillin skin testing is positive (Figure 2), use another antibiotic that is equally efficacious. Patients who absolutely need a beta-lactam may undergo drug desensitization, performed by a board-certified allergist.
During desensitization, patients receive progressively higher doses of the drug every 15 to 20 minutes subcutaneously or intravenously, or every 20 to 30 minutes orally, until a full therapeutic dose is tolerated. Most protocols begin with a dose ranging from 1/10,000 to 1/1,000 of the final dose, depending on the severity of the allergic reaction.9,17
Using modern protocols, the success rate for tolerance induction is extremely high (75% to 100% in patients with cystic fibrosis, a group with a high rate of drug allergy18–20).
Drug desensitization is contraindicated in patients with non–IgE-mediated reactions.
If skin testing is negative, refer for graded-dose challenge
If skin testing is negative (Figure 2), graded-dose challenge is recommended. This procedure must be done by a board-certified allergist. If the original reaction was life-threatening, graded-dose challenge may entail giving 1/100 of the therapeutic dose. Then, if no reaction occurs during a brief observation period (usually 30 minutes), a full dose is given. However, many patients can start with 1/10 or even a full dose of the drug, especially if the original reaction was limited to the skin and the penicillin skin test is negative.
Graded-dose challenge is contraindicated if the original reaction was a severe non–IgE-mediated reaction.
UNDERSTANDING CROSS-REACTIVITY OF PENICILLIN
Penicillin is the only antibiotic for which skin testing is reliable and validated. If a drug that cross-reacts with penicillin is needed, it is important to know the rate of cross-reactivity (Table 3). The rate of cross-reactivity between penicillin and aminopenicillins (amoxicillin and ampicillin) is less than 1.3% in the United States.10,21 However, the cross-reactivity rate among aminopenicillins and cephalosporins is between 10% to 40%. For that reason, patients with prior reactions to aminopenicillins should avoid cephalosporins that share identical R-chain side groups with aminopenicillins.9,22
The rate of cross-reactivity between penicillin and cephalosporins was reported as 10% 40 years ago.23,24 But this was with early, first-generation cephalosporins that may have been contaminated with penicillin. The cross-reactivity rate with cephalosporins today is 3%.25 In general, first- and second-generation cephalosporins cause more allergic reactions than third- and fourth-generation cephalosporins.26
Patients with a history of penicillin allergy who require a cephalosporin should still undergo penicillin skin testing. Skin testing with cephalosporins has not been validated. However, skin testing with nonirritating concentrations of cephalosporins9 may be done to elucidate IgE reactions.
In a study by Romano et al,27 110 patients who had positive results on penicillin skin testing completed graded-dose challenge with the carbapenem antibiotic imipenem. The rate of cross-reactivity between penicillin and imipenem was less than 1%.
Monobactam antibiotics do not cross-react with other beta-lactams, except ceftazidime with aztreonam. This is probably because of similarities in their chemical structure.
- Park M, Markus P, Matesic D, Li JT. Safety and effectiveness of a preoperative allergy clinic in decreasing vancomycin use in patients with a history of penicillin allergy. Ann Allergy Asthma Immunol 2006; 97:681–687.
- Arroliga ME, Radojicic C, Gordon SM, et al. A prospective observational study of the effect of penicillin skin testing on antibiotic use in the intensive care unit. Infect Control Hosp Epidemiol 2003; 24:347–350.
- del Real GA, Rose ME, Ramirez-Atamoros MT, et al. Penicillin skin testing in patients with a history of beta-lactam allergy. Ann Allergy Asthma Immunol 2007; 98:355–359.
- Nadarajah K, Green GR, Naglak M. Clinical outcomes of penicillin skin testing. Ann Allergy Asthma Immunol 2005; 95:541–545.
- Harris AD, Sauberman L, Kabbash L, Greineder DK, Samore MH. Penicillin skin testing: a way to optimize antibiotic utilization. Am J Med 1999; 107:166–168.
- Idsoe O, Guthe T, Willcox RR, de Weck AL. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ 1968; 38:159–188.
- Gadde J, Spence M, Wheeler B, Adkinson NF Jr. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA 1993; 270:2456–2463.
- Johansson SG, Bieber T, Dahl R, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol 2004; 113:832–836.
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol 2010; 105:259–273.
- Park MA, Matesic D, Markus PJ, Li JT. Female sex as a risk factor for penicillin allergy. Ann Allergy Asthma Immunol 2007; 99:54–58.
- Moreno E, Macias E, Davila I, Laffond E, Ruiz A, Lorente F. Hypersensitivity reactions to cephalosporins. Expert Opin Drug Saf 2008; 7:295–304.
- Salkind AR, Cuddy PG, Foxworth JW. The rational clinical examination. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA 2001; 285:2498–2505.
- Sullivan TJ, Wedner HJ, Shatz GS, Yecies LD, Parker CW. Skin testing to detect penicillin allergy. J Allergy Clin Immunol 1981; 68:171–180.
- Macy E, Schatz M, Lin C, Poon KY. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J 2009; 13:12–18.
- Blanca M, Torres MJ, Garcia JJ, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol 1999; 103:918–924.
- Patel BM. Skin rash with infectious mononucleosis and ampicillin. Pediatrics 1967; 40:910–911.
- Liu A, Fanning L, Chong H, et al. Desensitization regimens for drug allergy: state of the art in the 21st century. Clin Exp Allergy 2011; 41:1679–1688.
- Burrows JA, Toon M, Bell SC. Antibiotic desensitization in adults with cystic fibrosis. Respirology 2003; 8:359–364.
- Turvey SE, Cronin B, Arnold AD, Dioun AF. Antibiotic desensitization for the allergic patient: 5 years of experience and practice. Ann Allergy Asthma Immunol 2004; 92:426–432.
- Legere HJ 3rd, Palis RI, Rodriguez Bouza T, Uluer AZ, Castells MC. A safe protocol for rapid desensitization in patients with cystic fibrosis and antibiotic hypersensitivity. J Cyst Fibros 2009; 8:418–424.
- Lin E, Saxon A, Riedl M. Penicillin allergy: value of including amoxicillin as a determinant in penicillin skin testing. Int Arch Allergy Immunol 2010; 152:313–318.
- Dickson SD, Salazar KC. Diagnosis and management of immediate hypersensitivity reactions to cephalosporins. Clin Rev Allergy Immunol 2013; 45:131–142.
- Dash CH. Penicillin allergy and the cephalosporins. J Antimicrob Chemother 1975; 1(suppl 3):107–118.
- Petz LD. Immunologic cross-reactivity between penicillins and cephalosporins: a review. J Infect Dis 1978; 137(suppl):S74–S79.
- American Academy of Allergy Asthma & Immunology. Cephalosporin administration to patients with a history of penicillin allergy. www.aaaai.org/Aaaai/media/MediaLibrary/PDF%20Documents/Practice%20and%20Parameters/Cephalosporin-administration-2009.pdf. Accessed April 2, 2015.
- Fonacier L, Hirschberg R, Gerson S. Adverse drug reactions to cephalosporins in hospitalized patients with a history of penicillin allergy. Allergy Asthma Proc 2005; 26:135–141.
- Romano A, Viola M, Gueant-Rodriguez RM, Gaeta F, Pettinato R, Gueant JL. Imipenem in patients with immediate hypersensitivity to penicillins. N Engl J Med 2006; 354:2835–2837.
Most patients who report that they are allergic to penicillin can ultimately receive penicillin or a penicillin-type antibiotic again after an appropriate evaluation and, possibly, treatment. This course of action decreases the need for broad-spectrum antibiotics,1–4 reduces health care costs, and prevents the development of multidrug-resistant pathogens.5
About 10% of the general population say that they are allergic to penicillin.1,6,7 Although the prevalence of life-threatening anaphylactic reactions to penicillin has been estimated to be between 0.02% and 0.04%,6 the most common reaction is a cutaneous eruption. Since anaphylactic reactions are mediated by immunoglobulin E (IgE), evaluation of patients with a history of penicillin allergy by penicillin skin testing is recommended to rule out IgE-mediated reactions.
This review outlines a practical approach to evaluating a suspected IgE-mediated reaction to penicillin, with key points in the history and diagnostic testing. We also review subsequent management and cross-reactivity with other beta-lactam-containing antibiotics.
EVALUATING ALLERGIC PATIENTS
Evaluation of patients with a history of penicillin allergy can be improved with an understanding of the classification of drug reactions, risk factors for allergy, and the pathophysiology of penicillin allergy.
Classification of drug reactions
Adverse drug reactions include all unintended pharmacologic effects of a drug and can be classified as predictable (type A) or unpredictable (type B). Predictable reactions are dose-dependent, are related to the known pharmacologic actions of the medication, and occur in otherwise healthy individuals. Unpredictable reactions are further classified into drug intolerance, drug idiosyncrasy, drug allergy, and pseudoallergic reactions.8,9
Penicillin allergy can manifest as any hypersensitivity reaction of the Gell and Coombs classification (Table 1).9 Type I (immediate) and type IV (delayed) reactions are the most common types of reactions that occur with antibiotics and should be classified based on the onset of symptoms as immediate (within 1 hour) or delayed (days or weeks).8
Risk factors for IgE-mediated reaction
Risk factors for a hypersensitivity reaction include frequent or repetitive courses of penicillin10 and high-dose parenteral (rather than oral) administration.
Age and atopy are not risk factors for penicillin allergy.7 However, atopy increases the risk of a more severe anaphylactic reaction to penicillin, and anaphylactic reactions are most commonly reported between the ages of 20 and 49.6
Pathophysiology of penicillin allergy
All penicillins share a common core ring structure (beta-lactam and thiazolidine rings) but differ in their side chains (R group) (Figure 1).
Under physiologic conditions, the core ring structure is metabolized into major (penicilloyl) and minor (penicillin itself, penicilloate and penilloate) antigenic determinants that may trigger an immediate IgE-dependent response.9 In the United States, commercial forms of antigenic determinates for skin testing exist in the form of penicillin G (minor determinant) and penicilloyl-polylysine, better known as Prepen (major determinant).
Immediate-type reactions to similar antibiotics such as aminopenicillins and cephalosporins may be caused by IgE antibodies against the R-group side chain rather than the core penicillin major and minor determinants.11
Questions to ask patients who have a history of penicillin allergy
Patients should be questioned closely about previous and current reactions to penicillin and should undergo skin-prick and intradermal testing, followed by graded-dose challenge or drug tolerance desensitization (Figure 2).
Questions to ask patients who have a history of penicillin allergy (Table 2)9,12 include the following:
Do you remember the details of the reaction? These include the route of administration, the time between the dose of penicillin and the appearance of symptoms, and how the reaction was managed.
Immediate reactions (ie, IgE-mediated, or Gell and Coombs type I) usually occur within the first hour after the first dose of the antibiotic, although they occasionally take up to 2 hours to occur, especially if the medication is taken orally and is taken with food. Symptoms consistent with IgE-mediated reactions include urticaria (most common), pruritus, angioedema, laryngeal edema, wheezing, shortness of breath, presyncope or syncope, hypotension, and cardiorespiratory collapse.
In contrast, symptoms of a non–IgE-mediated reaction are delayed in onset, occurring after days of treatment. They include nonpruritic maculopapular eruptions, hemolytic anemia, serum sickness, Stevens-Johnson syndrome, drug rash with eosinophilia and systemic symptoms, acute interstitial nephritis, and toxic epidermal necrolysis.9
If the patient has had severe non–IgE-mediated reactions to penicillin (eg, Stevens-Johnson syndrome, toxic epidermal necrolysis, acute interstitial nephritis, hemolytic anemia, or serum sickness) in the past, skin testing, graded-dose challenge, and desensitization are contraindicated.
How many years ago did the reaction occur? Most patients lose their sensitivity to penicillin over time.7,13–15 Nearly 50% of patients with IgE-mediated penicillin allergy lose their sensitivity within 5 years of the reaction,15 increasing to 80% or more by 10 years.13
How was the reaction managed? What was the outcome? Use of and positive response to epinephrine and histamine 1 receptor antagonists (antihistamines) with resolution or significant improvement of symptoms within a few hours may indicate an IgE-mediated reaction.
What was the indication for penicillin? Many cutaneous reactions are a result of an underlying viral or bacterial infection. For example, up to 90% of patients with Epstein-Barr virus infection develop a maculopapular rash when given penicillin.16
Have you tolerated other forms of penicillin since the reaction? Sometimes the patient has already tolerated other beta-lactams such as aminopenicillins, cephalosporins, and semisynthetic penicillins (piperacillin-tazobactam). Patients who tolerate other beta-lactams without adverse reactions are not allergic to beta-lactams.
Diagnostic tests
Skin testing. The only validated test for diagnosing IgE-mediated reactions caused by penicillin is the immediate hypersensitivity skin test,9 which should be performed by a board-certified allergist. The test consists of skin-prick and intradermal testing with the major determinant (penicilloyl-polylysine), the minor determinant (penicillin G), a negative control (normal saline), and a positive control (histamine). Minor-determinant mix is not commercially available in the United States.
Results of skin-prick testing are read 15 minutes after application. A positive response is a wheal at least 3 mm larger in diameter (with equivalent erythema) than the negative control done simultaneously. Intradermal testing is only done after a negative skin-prick test. If the allergic reaction was severe (ie, anaphylaxis), skin testing should be done at least 4 to 6 weeks after the reaction.
A history of severe non–IgE-mediated reaction to penicillin is a contraindication to skin-prick testing for penicillin allergy. The positive predictive value of penicillin skin testing is 50%, and the negative predictive value is 97%.3,7,9,13
Commercial in vitro testing (serum-specific IgE assays) for IgE-mediated hypersensitivity to penicillin is inferior to skin testing in terms of the negative predictive value and is not a suitable substitute for penicillin skin testing.
MANAGING PENICILLIN ALLERGY
If skin testing is positive, use another antibiotic, or refer for desensitization
If penicillin skin testing is positive (Figure 2), use another antibiotic that is equally efficacious. Patients who absolutely need a beta-lactam may undergo drug desensitization, performed by a board-certified allergist.
During desensitization, patients receive progressively higher doses of the drug every 15 to 20 minutes subcutaneously or intravenously, or every 20 to 30 minutes orally, until a full therapeutic dose is tolerated. Most protocols begin with a dose ranging from 1/10,000 to 1/1,000 of the final dose, depending on the severity of the allergic reaction.9,17
Using modern protocols, the success rate for tolerance induction is extremely high (75% to 100% in patients with cystic fibrosis, a group with a high rate of drug allergy18–20).
Drug desensitization is contraindicated in patients with non–IgE-mediated reactions.
If skin testing is negative, refer for graded-dose challenge
If skin testing is negative (Figure 2), graded-dose challenge is recommended. This procedure must be done by a board-certified allergist. If the original reaction was life-threatening, graded-dose challenge may entail giving 1/100 of the therapeutic dose. Then, if no reaction occurs during a brief observation period (usually 30 minutes), a full dose is given. However, many patients can start with 1/10 or even a full dose of the drug, especially if the original reaction was limited to the skin and the penicillin skin test is negative.
Graded-dose challenge is contraindicated if the original reaction was a severe non–IgE-mediated reaction.
UNDERSTANDING CROSS-REACTIVITY OF PENICILLIN
Penicillin is the only antibiotic for which skin testing is reliable and validated. If a drug that cross-reacts with penicillin is needed, it is important to know the rate of cross-reactivity (Table 3). The rate of cross-reactivity between penicillin and aminopenicillins (amoxicillin and ampicillin) is less than 1.3% in the United States.10,21 However, the cross-reactivity rate among aminopenicillins and cephalosporins is between 10% to 40%. For that reason, patients with prior reactions to aminopenicillins should avoid cephalosporins that share identical R-chain side groups with aminopenicillins.9,22
The rate of cross-reactivity between penicillin and cephalosporins was reported as 10% 40 years ago.23,24 But this was with early, first-generation cephalosporins that may have been contaminated with penicillin. The cross-reactivity rate with cephalosporins today is 3%.25 In general, first- and second-generation cephalosporins cause more allergic reactions than third- and fourth-generation cephalosporins.26
Patients with a history of penicillin allergy who require a cephalosporin should still undergo penicillin skin testing. Skin testing with cephalosporins has not been validated. However, skin testing with nonirritating concentrations of cephalosporins9 may be done to elucidate IgE reactions.
In a study by Romano et al,27 110 patients who had positive results on penicillin skin testing completed graded-dose challenge with the carbapenem antibiotic imipenem. The rate of cross-reactivity between penicillin and imipenem was less than 1%.
Monobactam antibiotics do not cross-react with other beta-lactams, except ceftazidime with aztreonam. This is probably because of similarities in their chemical structure.
Most patients who report that they are allergic to penicillin can ultimately receive penicillin or a penicillin-type antibiotic again after an appropriate evaluation and, possibly, treatment. This course of action decreases the need for broad-spectrum antibiotics,1–4 reduces health care costs, and prevents the development of multidrug-resistant pathogens.5
About 10% of the general population say that they are allergic to penicillin.1,6,7 Although the prevalence of life-threatening anaphylactic reactions to penicillin has been estimated to be between 0.02% and 0.04%,6 the most common reaction is a cutaneous eruption. Since anaphylactic reactions are mediated by immunoglobulin E (IgE), evaluation of patients with a history of penicillin allergy by penicillin skin testing is recommended to rule out IgE-mediated reactions.
This review outlines a practical approach to evaluating a suspected IgE-mediated reaction to penicillin, with key points in the history and diagnostic testing. We also review subsequent management and cross-reactivity with other beta-lactam-containing antibiotics.
EVALUATING ALLERGIC PATIENTS
Evaluation of patients with a history of penicillin allergy can be improved with an understanding of the classification of drug reactions, risk factors for allergy, and the pathophysiology of penicillin allergy.
Classification of drug reactions
Adverse drug reactions include all unintended pharmacologic effects of a drug and can be classified as predictable (type A) or unpredictable (type B). Predictable reactions are dose-dependent, are related to the known pharmacologic actions of the medication, and occur in otherwise healthy individuals. Unpredictable reactions are further classified into drug intolerance, drug idiosyncrasy, drug allergy, and pseudoallergic reactions.8,9
Penicillin allergy can manifest as any hypersensitivity reaction of the Gell and Coombs classification (Table 1).9 Type I (immediate) and type IV (delayed) reactions are the most common types of reactions that occur with antibiotics and should be classified based on the onset of symptoms as immediate (within 1 hour) or delayed (days or weeks).8
Risk factors for IgE-mediated reaction
Risk factors for a hypersensitivity reaction include frequent or repetitive courses of penicillin10 and high-dose parenteral (rather than oral) administration.
Age and atopy are not risk factors for penicillin allergy.7 However, atopy increases the risk of a more severe anaphylactic reaction to penicillin, and anaphylactic reactions are most commonly reported between the ages of 20 and 49.6
Pathophysiology of penicillin allergy
All penicillins share a common core ring structure (beta-lactam and thiazolidine rings) but differ in their side chains (R group) (Figure 1).
Under physiologic conditions, the core ring structure is metabolized into major (penicilloyl) and minor (penicillin itself, penicilloate and penilloate) antigenic determinants that may trigger an immediate IgE-dependent response.9 In the United States, commercial forms of antigenic determinates for skin testing exist in the form of penicillin G (minor determinant) and penicilloyl-polylysine, better known as Prepen (major determinant).
Immediate-type reactions to similar antibiotics such as aminopenicillins and cephalosporins may be caused by IgE antibodies against the R-group side chain rather than the core penicillin major and minor determinants.11
Questions to ask patients who have a history of penicillin allergy
Patients should be questioned closely about previous and current reactions to penicillin and should undergo skin-prick and intradermal testing, followed by graded-dose challenge or drug tolerance desensitization (Figure 2).
Questions to ask patients who have a history of penicillin allergy (Table 2)9,12 include the following:
Do you remember the details of the reaction? These include the route of administration, the time between the dose of penicillin and the appearance of symptoms, and how the reaction was managed.
Immediate reactions (ie, IgE-mediated, or Gell and Coombs type I) usually occur within the first hour after the first dose of the antibiotic, although they occasionally take up to 2 hours to occur, especially if the medication is taken orally and is taken with food. Symptoms consistent with IgE-mediated reactions include urticaria (most common), pruritus, angioedema, laryngeal edema, wheezing, shortness of breath, presyncope or syncope, hypotension, and cardiorespiratory collapse.
In contrast, symptoms of a non–IgE-mediated reaction are delayed in onset, occurring after days of treatment. They include nonpruritic maculopapular eruptions, hemolytic anemia, serum sickness, Stevens-Johnson syndrome, drug rash with eosinophilia and systemic symptoms, acute interstitial nephritis, and toxic epidermal necrolysis.9
If the patient has had severe non–IgE-mediated reactions to penicillin (eg, Stevens-Johnson syndrome, toxic epidermal necrolysis, acute interstitial nephritis, hemolytic anemia, or serum sickness) in the past, skin testing, graded-dose challenge, and desensitization are contraindicated.
How many years ago did the reaction occur? Most patients lose their sensitivity to penicillin over time.7,13–15 Nearly 50% of patients with IgE-mediated penicillin allergy lose their sensitivity within 5 years of the reaction,15 increasing to 80% or more by 10 years.13
How was the reaction managed? What was the outcome? Use of and positive response to epinephrine and histamine 1 receptor antagonists (antihistamines) with resolution or significant improvement of symptoms within a few hours may indicate an IgE-mediated reaction.
What was the indication for penicillin? Many cutaneous reactions are a result of an underlying viral or bacterial infection. For example, up to 90% of patients with Epstein-Barr virus infection develop a maculopapular rash when given penicillin.16
Have you tolerated other forms of penicillin since the reaction? Sometimes the patient has already tolerated other beta-lactams such as aminopenicillins, cephalosporins, and semisynthetic penicillins (piperacillin-tazobactam). Patients who tolerate other beta-lactams without adverse reactions are not allergic to beta-lactams.
Diagnostic tests
Skin testing. The only validated test for diagnosing IgE-mediated reactions caused by penicillin is the immediate hypersensitivity skin test,9 which should be performed by a board-certified allergist. The test consists of skin-prick and intradermal testing with the major determinant (penicilloyl-polylysine), the minor determinant (penicillin G), a negative control (normal saline), and a positive control (histamine). Minor-determinant mix is not commercially available in the United States.
Results of skin-prick testing are read 15 minutes after application. A positive response is a wheal at least 3 mm larger in diameter (with equivalent erythema) than the negative control done simultaneously. Intradermal testing is only done after a negative skin-prick test. If the allergic reaction was severe (ie, anaphylaxis), skin testing should be done at least 4 to 6 weeks after the reaction.
A history of severe non–IgE-mediated reaction to penicillin is a contraindication to skin-prick testing for penicillin allergy. The positive predictive value of penicillin skin testing is 50%, and the negative predictive value is 97%.3,7,9,13
Commercial in vitro testing (serum-specific IgE assays) for IgE-mediated hypersensitivity to penicillin is inferior to skin testing in terms of the negative predictive value and is not a suitable substitute for penicillin skin testing.
MANAGING PENICILLIN ALLERGY
If skin testing is positive, use another antibiotic, or refer for desensitization
If penicillin skin testing is positive (Figure 2), use another antibiotic that is equally efficacious. Patients who absolutely need a beta-lactam may undergo drug desensitization, performed by a board-certified allergist.
During desensitization, patients receive progressively higher doses of the drug every 15 to 20 minutes subcutaneously or intravenously, or every 20 to 30 minutes orally, until a full therapeutic dose is tolerated. Most protocols begin with a dose ranging from 1/10,000 to 1/1,000 of the final dose, depending on the severity of the allergic reaction.9,17
Using modern protocols, the success rate for tolerance induction is extremely high (75% to 100% in patients with cystic fibrosis, a group with a high rate of drug allergy18–20).
Drug desensitization is contraindicated in patients with non–IgE-mediated reactions.
If skin testing is negative, refer for graded-dose challenge
If skin testing is negative (Figure 2), graded-dose challenge is recommended. This procedure must be done by a board-certified allergist. If the original reaction was life-threatening, graded-dose challenge may entail giving 1/100 of the therapeutic dose. Then, if no reaction occurs during a brief observation period (usually 30 minutes), a full dose is given. However, many patients can start with 1/10 or even a full dose of the drug, especially if the original reaction was limited to the skin and the penicillin skin test is negative.
Graded-dose challenge is contraindicated if the original reaction was a severe non–IgE-mediated reaction.
UNDERSTANDING CROSS-REACTIVITY OF PENICILLIN
Penicillin is the only antibiotic for which skin testing is reliable and validated. If a drug that cross-reacts with penicillin is needed, it is important to know the rate of cross-reactivity (Table 3). The rate of cross-reactivity between penicillin and aminopenicillins (amoxicillin and ampicillin) is less than 1.3% in the United States.10,21 However, the cross-reactivity rate among aminopenicillins and cephalosporins is between 10% to 40%. For that reason, patients with prior reactions to aminopenicillins should avoid cephalosporins that share identical R-chain side groups with aminopenicillins.9,22
The rate of cross-reactivity between penicillin and cephalosporins was reported as 10% 40 years ago.23,24 But this was with early, first-generation cephalosporins that may have been contaminated with penicillin. The cross-reactivity rate with cephalosporins today is 3%.25 In general, first- and second-generation cephalosporins cause more allergic reactions than third- and fourth-generation cephalosporins.26
Patients with a history of penicillin allergy who require a cephalosporin should still undergo penicillin skin testing. Skin testing with cephalosporins has not been validated. However, skin testing with nonirritating concentrations of cephalosporins9 may be done to elucidate IgE reactions.
In a study by Romano et al,27 110 patients who had positive results on penicillin skin testing completed graded-dose challenge with the carbapenem antibiotic imipenem. The rate of cross-reactivity between penicillin and imipenem was less than 1%.
Monobactam antibiotics do not cross-react with other beta-lactams, except ceftazidime with aztreonam. This is probably because of similarities in their chemical structure.
- Park M, Markus P, Matesic D, Li JT. Safety and effectiveness of a preoperative allergy clinic in decreasing vancomycin use in patients with a history of penicillin allergy. Ann Allergy Asthma Immunol 2006; 97:681–687.
- Arroliga ME, Radojicic C, Gordon SM, et al. A prospective observational study of the effect of penicillin skin testing on antibiotic use in the intensive care unit. Infect Control Hosp Epidemiol 2003; 24:347–350.
- del Real GA, Rose ME, Ramirez-Atamoros MT, et al. Penicillin skin testing in patients with a history of beta-lactam allergy. Ann Allergy Asthma Immunol 2007; 98:355–359.
- Nadarajah K, Green GR, Naglak M. Clinical outcomes of penicillin skin testing. Ann Allergy Asthma Immunol 2005; 95:541–545.
- Harris AD, Sauberman L, Kabbash L, Greineder DK, Samore MH. Penicillin skin testing: a way to optimize antibiotic utilization. Am J Med 1999; 107:166–168.
- Idsoe O, Guthe T, Willcox RR, de Weck AL. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ 1968; 38:159–188.
- Gadde J, Spence M, Wheeler B, Adkinson NF Jr. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA 1993; 270:2456–2463.
- Johansson SG, Bieber T, Dahl R, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol 2004; 113:832–836.
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol 2010; 105:259–273.
- Park MA, Matesic D, Markus PJ, Li JT. Female sex as a risk factor for penicillin allergy. Ann Allergy Asthma Immunol 2007; 99:54–58.
- Moreno E, Macias E, Davila I, Laffond E, Ruiz A, Lorente F. Hypersensitivity reactions to cephalosporins. Expert Opin Drug Saf 2008; 7:295–304.
- Salkind AR, Cuddy PG, Foxworth JW. The rational clinical examination. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA 2001; 285:2498–2505.
- Sullivan TJ, Wedner HJ, Shatz GS, Yecies LD, Parker CW. Skin testing to detect penicillin allergy. J Allergy Clin Immunol 1981; 68:171–180.
- Macy E, Schatz M, Lin C, Poon KY. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J 2009; 13:12–18.
- Blanca M, Torres MJ, Garcia JJ, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol 1999; 103:918–924.
- Patel BM. Skin rash with infectious mononucleosis and ampicillin. Pediatrics 1967; 40:910–911.
- Liu A, Fanning L, Chong H, et al. Desensitization regimens for drug allergy: state of the art in the 21st century. Clin Exp Allergy 2011; 41:1679–1688.
- Burrows JA, Toon M, Bell SC. Antibiotic desensitization in adults with cystic fibrosis. Respirology 2003; 8:359–364.
- Turvey SE, Cronin B, Arnold AD, Dioun AF. Antibiotic desensitization for the allergic patient: 5 years of experience and practice. Ann Allergy Asthma Immunol 2004; 92:426–432.
- Legere HJ 3rd, Palis RI, Rodriguez Bouza T, Uluer AZ, Castells MC. A safe protocol for rapid desensitization in patients with cystic fibrosis and antibiotic hypersensitivity. J Cyst Fibros 2009; 8:418–424.
- Lin E, Saxon A, Riedl M. Penicillin allergy: value of including amoxicillin as a determinant in penicillin skin testing. Int Arch Allergy Immunol 2010; 152:313–318.
- Dickson SD, Salazar KC. Diagnosis and management of immediate hypersensitivity reactions to cephalosporins. Clin Rev Allergy Immunol 2013; 45:131–142.
- Dash CH. Penicillin allergy and the cephalosporins. J Antimicrob Chemother 1975; 1(suppl 3):107–118.
- Petz LD. Immunologic cross-reactivity between penicillins and cephalosporins: a review. J Infect Dis 1978; 137(suppl):S74–S79.
- American Academy of Allergy Asthma & Immunology. Cephalosporin administration to patients with a history of penicillin allergy. www.aaaai.org/Aaaai/media/MediaLibrary/PDF%20Documents/Practice%20and%20Parameters/Cephalosporin-administration-2009.pdf. Accessed April 2, 2015.
- Fonacier L, Hirschberg R, Gerson S. Adverse drug reactions to cephalosporins in hospitalized patients with a history of penicillin allergy. Allergy Asthma Proc 2005; 26:135–141.
- Romano A, Viola M, Gueant-Rodriguez RM, Gaeta F, Pettinato R, Gueant JL. Imipenem in patients with immediate hypersensitivity to penicillins. N Engl J Med 2006; 354:2835–2837.
- Park M, Markus P, Matesic D, Li JT. Safety and effectiveness of a preoperative allergy clinic in decreasing vancomycin use in patients with a history of penicillin allergy. Ann Allergy Asthma Immunol 2006; 97:681–687.
- Arroliga ME, Radojicic C, Gordon SM, et al. A prospective observational study of the effect of penicillin skin testing on antibiotic use in the intensive care unit. Infect Control Hosp Epidemiol 2003; 24:347–350.
- del Real GA, Rose ME, Ramirez-Atamoros MT, et al. Penicillin skin testing in patients with a history of beta-lactam allergy. Ann Allergy Asthma Immunol 2007; 98:355–359.
- Nadarajah K, Green GR, Naglak M. Clinical outcomes of penicillin skin testing. Ann Allergy Asthma Immunol 2005; 95:541–545.
- Harris AD, Sauberman L, Kabbash L, Greineder DK, Samore MH. Penicillin skin testing: a way to optimize antibiotic utilization. Am J Med 1999; 107:166–168.
- Idsoe O, Guthe T, Willcox RR, de Weck AL. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ 1968; 38:159–188.
- Gadde J, Spence M, Wheeler B, Adkinson NF Jr. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA 1993; 270:2456–2463.
- Johansson SG, Bieber T, Dahl R, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol 2004; 113:832–836.
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol 2010; 105:259–273.
- Park MA, Matesic D, Markus PJ, Li JT. Female sex as a risk factor for penicillin allergy. Ann Allergy Asthma Immunol 2007; 99:54–58.
- Moreno E, Macias E, Davila I, Laffond E, Ruiz A, Lorente F. Hypersensitivity reactions to cephalosporins. Expert Opin Drug Saf 2008; 7:295–304.
- Salkind AR, Cuddy PG, Foxworth JW. The rational clinical examination. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA 2001; 285:2498–2505.
- Sullivan TJ, Wedner HJ, Shatz GS, Yecies LD, Parker CW. Skin testing to detect penicillin allergy. J Allergy Clin Immunol 1981; 68:171–180.
- Macy E, Schatz M, Lin C, Poon KY. The falling rate of positive penicillin skin tests from 1995 to 2007. Perm J 2009; 13:12–18.
- Blanca M, Torres MJ, Garcia JJ, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol 1999; 103:918–924.
- Patel BM. Skin rash with infectious mononucleosis and ampicillin. Pediatrics 1967; 40:910–911.
- Liu A, Fanning L, Chong H, et al. Desensitization regimens for drug allergy: state of the art in the 21st century. Clin Exp Allergy 2011; 41:1679–1688.
- Burrows JA, Toon M, Bell SC. Antibiotic desensitization in adults with cystic fibrosis. Respirology 2003; 8:359–364.
- Turvey SE, Cronin B, Arnold AD, Dioun AF. Antibiotic desensitization for the allergic patient: 5 years of experience and practice. Ann Allergy Asthma Immunol 2004; 92:426–432.
- Legere HJ 3rd, Palis RI, Rodriguez Bouza T, Uluer AZ, Castells MC. A safe protocol for rapid desensitization in patients with cystic fibrosis and antibiotic hypersensitivity. J Cyst Fibros 2009; 8:418–424.
- Lin E, Saxon A, Riedl M. Penicillin allergy: value of including amoxicillin as a determinant in penicillin skin testing. Int Arch Allergy Immunol 2010; 152:313–318.
- Dickson SD, Salazar KC. Diagnosis and management of immediate hypersensitivity reactions to cephalosporins. Clin Rev Allergy Immunol 2013; 45:131–142.
- Dash CH. Penicillin allergy and the cephalosporins. J Antimicrob Chemother 1975; 1(suppl 3):107–118.
- Petz LD. Immunologic cross-reactivity between penicillins and cephalosporins: a review. J Infect Dis 1978; 137(suppl):S74–S79.
- American Academy of Allergy Asthma & Immunology. Cephalosporin administration to patients with a history of penicillin allergy. www.aaaai.org/Aaaai/media/MediaLibrary/PDF%20Documents/Practice%20and%20Parameters/Cephalosporin-administration-2009.pdf. Accessed April 2, 2015.
- Fonacier L, Hirschberg R, Gerson S. Adverse drug reactions to cephalosporins in hospitalized patients with a history of penicillin allergy. Allergy Asthma Proc 2005; 26:135–141.
- Romano A, Viola M, Gueant-Rodriguez RM, Gaeta F, Pettinato R, Gueant JL. Imipenem in patients with immediate hypersensitivity to penicillins. N Engl J Med 2006; 354:2835–2837.
KEY POINTS
- The prevalence of reported penicillin allergy is 10% in the general population. However, more than 90% of these patients are found not to be allergic to penicillin after skin testing.
- In patients found to have penicillin allergy, the frequency of positive results on skin testing decreases by 10% per year of avoidance. Therefore, 80% to 100% of patients are expected to test negative for penicillin allergy by 10 years after their reaction.
- Skin testing for penicillin allergy is only useful for type 1 IgE-mediated reactions. However, in properly selected patients, the negative predictive value of penicillin skin testing is nearly 97%.
- The rate of cross-reactivity between penicillin and cephalosporins is approximately 3%.