User login
Primary Cutaneous Plasmacytoma
To the Editor:
Primary extramedullary plasmacytoma is a rare plasma cell neoplasm that occurs as an isolated finding without detectable underlying multiple myeloma. Uncommonly, it has been reported to occur in the skin as a primary cutaneous plasmacytoma (PCP). We describe a case of an asymptomatic plaque on the back that was found to contain a monoclonal proliferation of plasma cells without evidence of systemic disease despite thorough evaluation. We also include a brief discussion of PCPs, including diagnosis and treatment.
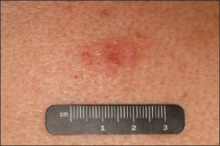
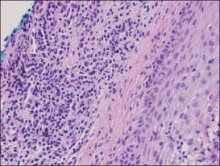
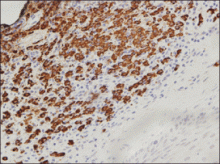
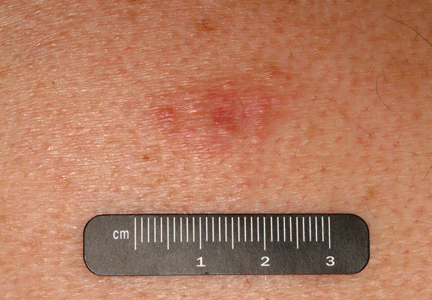
An indurated plaque was discovered on the upper back of a 57-year-old man during a routine follow-up visit for acne treatment. The patient reported the lesion had been present for approximately 2 years; it was asymptomatic and had not increased in size. A review of systems did not reveal any abnormalities. His medical history included hypercholesterolemia, hypertension, osteoarthritis, acne, type 2 diabetes mellitus, well-controlled herpes labialis, and gastroesophageal reflux disease. Physical examination revealed a nontender 2-cm erythematous plaque on the right side of the upper back (Figure 1). No other lesions or lymphadenopathy were noted. A biopsy was performed to rule out a primary neoplasm or metastatic lesion. Histopathologic examination of 3 separate punch biopsies revealed a perivascular infiltrate of lymphocytes and plasma cells in the papillary and superficial reticular dermis (Figure 2). On immunohistochemical staining, the plasma cells essentially were all κ positive, with rare λ-positive plasma cells (Figure 3).
The patient underwent complete evaluation for myeloma. A bone marrow biopsy was negative with no evidence of plasma cell dyscrasia. A skeletal survey showed no lytic lesions, and serum and urine protein electrophoreses were negative for M proteins. Urine immunofixation was negative for Bence Jones proteins. Computed tomography of the chest, abdomen, and pelvis showed no evidence of disease. Serum free k light chains were elevated at 32 mg/L (reference range, <19.4 mg/L), and IgA and b2-microglobulin also were elevated at 435 mg/dL (reference range, <400 mg/dL) and 2.8 mg/L (reference range, <1.8 mg/L), respectively. The k:λ ratio was slightly elevated at 2.2 (reference range, 0.26–1.65).
Local radiotherapy was considered but was rejected by the patient. A single 5-mg/cc intralesional injection of triamcinolone acetonide (1 cc total) was administered, which resulted in modest improvement but did not yield complete resolution. A repeat biopsy showed a monoclonal proliferation of plasma cells with areas of follicular mucinosis. Immunohistochemical studies also demonstrated CD3+ lymphocytes with more CD4+ cells than CD8+ cells and scattered CD20+ cells. Repeat serum and urine protein electrophoreses as well as bone marrow biopsy were negative or myeloma.
Because of the localized and recalcitrant nature of the neoplasm, the patient underwent wide local excision. Positron emission tomography performed 1 year following surgery demonstrated no residual disease. The patient has had no clinical recurrence but has demonstrated persistently elevated free k light chains. Seven years following the excision, there was no evidence of systemic disease.
Primary extramedullary plasmacytoma is a rare plasma cell neoplasm that occurs without detectable underlying multiple myeloma.1 Originally classified as a discrete entity, PCP currently is considered to be a variant of primary cutaneous marginal zone B-cell lymphoma.2 Cases of localization to the skin are thought to represent 2% to 4% of all primary extramedullary plasmacytomas.3 Very few cases of PCPs have been reported in the literature thus far.1 Primary cutaneous plasmacytomas are most commonly reported in Asian men, with an age range of 22 to 88 years; lesions typically present as slow-growing, reddish brown macules or plaques on the face, trunk, or extremities.4 They may be single or multiple, though solitary lesions are thought to portend a better prognosis. Associated features include lymphadenopathy, hepatosplenomegaly, hypergammaglobulinemia, and an elevated erythrocyte sedimentation rate.5 Multiple myeloma has been estimated to develop in one-third of patients.6 Primary cutaneous plasmacytoma also has been linked to organ transplantation and Epstein-Barr virus–associated proteins7 as well as recurrent herpes simplex virus infections.8
Diagnosis is based on a proliferation of a clonal k or λ light chain with the exclusion of multiple myeloma.9 Typical histologic findings include a diffuse or nodular dermal and subcuticular infiltrate of plasma cells, often with a grenz zone. Less commonly, an interstitial pattern predominates, mimicking a benign inflammatory process.4 The plasma cells may display varying degrees of maturation and atypia but no epidermotropism.10 On immunohistochemical staining, plasma cells stain positive for CD79a, CD34, and CD138, and also may stain positively for epithelial membrane antigen and vimentin.4
The differential diagnosis includes cutaneous plasmacytoma occurring in the setting of systemic disease, and reactive polyclonal processes occurring in the setting of chronic infection or autoimmune disease. Possible treatment options include intralesional steroids, psoralen plus UVA, topical immunomodulators,10 local radiotherapy,11 and excision. Prognosis is varied, with reported cases showing complete remission, local recurrence, and progression to systemic disease, with the development of multiple myeloma in one-third of cases.4 More aggressive cases are associated with multiple lesions, larger size, and IgA secretion by neoplastic cells.
Our patient with a solitary dermal infiltrate of monoclonal plasma cells did not have bone marrow involvement and had an unexplained elevated level of free k light chains in the serum. This unexplained relationship underscores the importance of continued surveillance in any cutaneous lymphoma.
1. Rongioletti F, Patterson JW, Rebora A. The histological and pathogenetic spectrum of cutaneous disease in monoclonal gammopathies. J Cutan Pathol. 2008;35:705-721.
2. Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
3. Muscardin LM, Pulsoni A, Cerroni L. Primary cutaneous plasmacytoma: report of a case with review of the literature. J Am Acad Dermatol. 2000;43(5, pt 2):962-965.
4. Kazakov DV, Belousova IE, Müller B, et al. Primary cutaneous plasmacytoma: a clinicopathological study of two cases with a long-term follow-up and review of the literature. J Cutan Pathol. 2002;29:244-248.
5. Aricò M, Bongiorno MR. Primary cutaneous plasmacytosis in a child. is this a new entity? J Eur Acad Dermatol. 2002;16:164-167.
6. Willoughby V, Werlang-Perurena A, Kelly A, et al. Primary cutaneous plasmacytoma (posttransplant lymphoproliferative disorder, plasmacytoma-like) in a heart transplant patient. Am J Dermatopathol. 2006;28:442-445.
7. Buffet M, Dupin N, Carlotti A, et al. Epstein-Barr virus–associated cutaneous plasmacytoma post–solid organ transplantation [in French]. Ann Dermatol Venereol. 2004;131:1085-1091.
8. Zendri E, Venturi C, Ricci R, et al. Primary cutaneous plasmacytoma: a role for a triggering stimulus? Clin Exp Dermatol. 2005;30:229-231.
9. Tüting T, Bork K. Primary plasmacytoma of the skin. J Am Acad Dermatol. 1996;34(2, pt 2):386-390.
10. Miura H, Itami S, Yoshikawa K. Treatment of facial lesion of cutaneous plasmacytosis with tacrolimus ointment. J Am Acad Dermatol. 2003;49:1195-1196.
11. Tessari G, Fabbian F, Colato C, et al. Primary cutaneous plasmacytoma after rejection of a transplanted kidney: case report and review of the literature. Int J Hematol. 2004;80:361-364.
To the Editor:
Primary extramedullary plasmacytoma is a rare plasma cell neoplasm that occurs as an isolated finding without detectable underlying multiple myeloma. Uncommonly, it has been reported to occur in the skin as a primary cutaneous plasmacytoma (PCP). We describe a case of an asymptomatic plaque on the back that was found to contain a monoclonal proliferation of plasma cells without evidence of systemic disease despite thorough evaluation. We also include a brief discussion of PCPs, including diagnosis and treatment.
An indurated plaque was discovered on the upper back of a 57-year-old man during a routine follow-up visit for acne treatment. The patient reported the lesion had been present for approximately 2 years; it was asymptomatic and had not increased in size. A review of systems did not reveal any abnormalities. His medical history included hypercholesterolemia, hypertension, osteoarthritis, acne, type 2 diabetes mellitus, well-controlled herpes labialis, and gastroesophageal reflux disease. Physical examination revealed a nontender 2-cm erythematous plaque on the right side of the upper back (Figure 1). No other lesions or lymphadenopathy were noted. A biopsy was performed to rule out a primary neoplasm or metastatic lesion. Histopathologic examination of 3 separate punch biopsies revealed a perivascular infiltrate of lymphocytes and plasma cells in the papillary and superficial reticular dermis (Figure 2). On immunohistochemical staining, the plasma cells essentially were all κ positive, with rare λ-positive plasma cells (Figure 3).
The patient underwent complete evaluation for myeloma. A bone marrow biopsy was negative with no evidence of plasma cell dyscrasia. A skeletal survey showed no lytic lesions, and serum and urine protein electrophoreses were negative for M proteins. Urine immunofixation was negative for Bence Jones proteins. Computed tomography of the chest, abdomen, and pelvis showed no evidence of disease. Serum free k light chains were elevated at 32 mg/L (reference range, <19.4 mg/L), and IgA and b2-microglobulin also were elevated at 435 mg/dL (reference range, <400 mg/dL) and 2.8 mg/L (reference range, <1.8 mg/L), respectively. The k:λ ratio was slightly elevated at 2.2 (reference range, 0.26–1.65).
Local radiotherapy was considered but was rejected by the patient. A single 5-mg/cc intralesional injection of triamcinolone acetonide (1 cc total) was administered, which resulted in modest improvement but did not yield complete resolution. A repeat biopsy showed a monoclonal proliferation of plasma cells with areas of follicular mucinosis. Immunohistochemical studies also demonstrated CD3+ lymphocytes with more CD4+ cells than CD8+ cells and scattered CD20+ cells. Repeat serum and urine protein electrophoreses as well as bone marrow biopsy were negative or myeloma.
Because of the localized and recalcitrant nature of the neoplasm, the patient underwent wide local excision. Positron emission tomography performed 1 year following surgery demonstrated no residual disease. The patient has had no clinical recurrence but has demonstrated persistently elevated free k light chains. Seven years following the excision, there was no evidence of systemic disease.
Primary extramedullary plasmacytoma is a rare plasma cell neoplasm that occurs without detectable underlying multiple myeloma.1 Originally classified as a discrete entity, PCP currently is considered to be a variant of primary cutaneous marginal zone B-cell lymphoma.2 Cases of localization to the skin are thought to represent 2% to 4% of all primary extramedullary plasmacytomas.3 Very few cases of PCPs have been reported in the literature thus far.1 Primary cutaneous plasmacytomas are most commonly reported in Asian men, with an age range of 22 to 88 years; lesions typically present as slow-growing, reddish brown macules or plaques on the face, trunk, or extremities.4 They may be single or multiple, though solitary lesions are thought to portend a better prognosis. Associated features include lymphadenopathy, hepatosplenomegaly, hypergammaglobulinemia, and an elevated erythrocyte sedimentation rate.5 Multiple myeloma has been estimated to develop in one-third of patients.6 Primary cutaneous plasmacytoma also has been linked to organ transplantation and Epstein-Barr virus–associated proteins7 as well as recurrent herpes simplex virus infections.8
Diagnosis is based on a proliferation of a clonal k or λ light chain with the exclusion of multiple myeloma.9 Typical histologic findings include a diffuse or nodular dermal and subcuticular infiltrate of plasma cells, often with a grenz zone. Less commonly, an interstitial pattern predominates, mimicking a benign inflammatory process.4 The plasma cells may display varying degrees of maturation and atypia but no epidermotropism.10 On immunohistochemical staining, plasma cells stain positive for CD79a, CD34, and CD138, and also may stain positively for epithelial membrane antigen and vimentin.4
The differential diagnosis includes cutaneous plasmacytoma occurring in the setting of systemic disease, and reactive polyclonal processes occurring in the setting of chronic infection or autoimmune disease. Possible treatment options include intralesional steroids, psoralen plus UVA, topical immunomodulators,10 local radiotherapy,11 and excision. Prognosis is varied, with reported cases showing complete remission, local recurrence, and progression to systemic disease, with the development of multiple myeloma in one-third of cases.4 More aggressive cases are associated with multiple lesions, larger size, and IgA secretion by neoplastic cells.
Our patient with a solitary dermal infiltrate of monoclonal plasma cells did not have bone marrow involvement and had an unexplained elevated level of free k light chains in the serum. This unexplained relationship underscores the importance of continued surveillance in any cutaneous lymphoma.
To the Editor:
Primary extramedullary plasmacytoma is a rare plasma cell neoplasm that occurs as an isolated finding without detectable underlying multiple myeloma. Uncommonly, it has been reported to occur in the skin as a primary cutaneous plasmacytoma (PCP). We describe a case of an asymptomatic plaque on the back that was found to contain a monoclonal proliferation of plasma cells without evidence of systemic disease despite thorough evaluation. We also include a brief discussion of PCPs, including diagnosis and treatment.
An indurated plaque was discovered on the upper back of a 57-year-old man during a routine follow-up visit for acne treatment. The patient reported the lesion had been present for approximately 2 years; it was asymptomatic and had not increased in size. A review of systems did not reveal any abnormalities. His medical history included hypercholesterolemia, hypertension, osteoarthritis, acne, type 2 diabetes mellitus, well-controlled herpes labialis, and gastroesophageal reflux disease. Physical examination revealed a nontender 2-cm erythematous plaque on the right side of the upper back (Figure 1). No other lesions or lymphadenopathy were noted. A biopsy was performed to rule out a primary neoplasm or metastatic lesion. Histopathologic examination of 3 separate punch biopsies revealed a perivascular infiltrate of lymphocytes and plasma cells in the papillary and superficial reticular dermis (Figure 2). On immunohistochemical staining, the plasma cells essentially were all κ positive, with rare λ-positive plasma cells (Figure 3).
The patient underwent complete evaluation for myeloma. A bone marrow biopsy was negative with no evidence of plasma cell dyscrasia. A skeletal survey showed no lytic lesions, and serum and urine protein electrophoreses were negative for M proteins. Urine immunofixation was negative for Bence Jones proteins. Computed tomography of the chest, abdomen, and pelvis showed no evidence of disease. Serum free k light chains were elevated at 32 mg/L (reference range, <19.4 mg/L), and IgA and b2-microglobulin also were elevated at 435 mg/dL (reference range, <400 mg/dL) and 2.8 mg/L (reference range, <1.8 mg/L), respectively. The k:λ ratio was slightly elevated at 2.2 (reference range, 0.26–1.65).
Local radiotherapy was considered but was rejected by the patient. A single 5-mg/cc intralesional injection of triamcinolone acetonide (1 cc total) was administered, which resulted in modest improvement but did not yield complete resolution. A repeat biopsy showed a monoclonal proliferation of plasma cells with areas of follicular mucinosis. Immunohistochemical studies also demonstrated CD3+ lymphocytes with more CD4+ cells than CD8+ cells and scattered CD20+ cells. Repeat serum and urine protein electrophoreses as well as bone marrow biopsy were negative or myeloma.
Because of the localized and recalcitrant nature of the neoplasm, the patient underwent wide local excision. Positron emission tomography performed 1 year following surgery demonstrated no residual disease. The patient has had no clinical recurrence but has demonstrated persistently elevated free k light chains. Seven years following the excision, there was no evidence of systemic disease.
Primary extramedullary plasmacytoma is a rare plasma cell neoplasm that occurs without detectable underlying multiple myeloma.1 Originally classified as a discrete entity, PCP currently is considered to be a variant of primary cutaneous marginal zone B-cell lymphoma.2 Cases of localization to the skin are thought to represent 2% to 4% of all primary extramedullary plasmacytomas.3 Very few cases of PCPs have been reported in the literature thus far.1 Primary cutaneous plasmacytomas are most commonly reported in Asian men, with an age range of 22 to 88 years; lesions typically present as slow-growing, reddish brown macules or plaques on the face, trunk, or extremities.4 They may be single or multiple, though solitary lesions are thought to portend a better prognosis. Associated features include lymphadenopathy, hepatosplenomegaly, hypergammaglobulinemia, and an elevated erythrocyte sedimentation rate.5 Multiple myeloma has been estimated to develop in one-third of patients.6 Primary cutaneous plasmacytoma also has been linked to organ transplantation and Epstein-Barr virus–associated proteins7 as well as recurrent herpes simplex virus infections.8
Diagnosis is based on a proliferation of a clonal k or λ light chain with the exclusion of multiple myeloma.9 Typical histologic findings include a diffuse or nodular dermal and subcuticular infiltrate of plasma cells, often with a grenz zone. Less commonly, an interstitial pattern predominates, mimicking a benign inflammatory process.4 The plasma cells may display varying degrees of maturation and atypia but no epidermotropism.10 On immunohistochemical staining, plasma cells stain positive for CD79a, CD34, and CD138, and also may stain positively for epithelial membrane antigen and vimentin.4
The differential diagnosis includes cutaneous plasmacytoma occurring in the setting of systemic disease, and reactive polyclonal processes occurring in the setting of chronic infection or autoimmune disease. Possible treatment options include intralesional steroids, psoralen plus UVA, topical immunomodulators,10 local radiotherapy,11 and excision. Prognosis is varied, with reported cases showing complete remission, local recurrence, and progression to systemic disease, with the development of multiple myeloma in one-third of cases.4 More aggressive cases are associated with multiple lesions, larger size, and IgA secretion by neoplastic cells.
Our patient with a solitary dermal infiltrate of monoclonal plasma cells did not have bone marrow involvement and had an unexplained elevated level of free k light chains in the serum. This unexplained relationship underscores the importance of continued surveillance in any cutaneous lymphoma.
1. Rongioletti F, Patterson JW, Rebora A. The histological and pathogenetic spectrum of cutaneous disease in monoclonal gammopathies. J Cutan Pathol. 2008;35:705-721.
2. Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
3. Muscardin LM, Pulsoni A, Cerroni L. Primary cutaneous plasmacytoma: report of a case with review of the literature. J Am Acad Dermatol. 2000;43(5, pt 2):962-965.
4. Kazakov DV, Belousova IE, Müller B, et al. Primary cutaneous plasmacytoma: a clinicopathological study of two cases with a long-term follow-up and review of the literature. J Cutan Pathol. 2002;29:244-248.
5. Aricò M, Bongiorno MR. Primary cutaneous plasmacytosis in a child. is this a new entity? J Eur Acad Dermatol. 2002;16:164-167.
6. Willoughby V, Werlang-Perurena A, Kelly A, et al. Primary cutaneous plasmacytoma (posttransplant lymphoproliferative disorder, plasmacytoma-like) in a heart transplant patient. Am J Dermatopathol. 2006;28:442-445.
7. Buffet M, Dupin N, Carlotti A, et al. Epstein-Barr virus–associated cutaneous plasmacytoma post–solid organ transplantation [in French]. Ann Dermatol Venereol. 2004;131:1085-1091.
8. Zendri E, Venturi C, Ricci R, et al. Primary cutaneous plasmacytoma: a role for a triggering stimulus? Clin Exp Dermatol. 2005;30:229-231.
9. Tüting T, Bork K. Primary plasmacytoma of the skin. J Am Acad Dermatol. 1996;34(2, pt 2):386-390.
10. Miura H, Itami S, Yoshikawa K. Treatment of facial lesion of cutaneous plasmacytosis with tacrolimus ointment. J Am Acad Dermatol. 2003;49:1195-1196.
11. Tessari G, Fabbian F, Colato C, et al. Primary cutaneous plasmacytoma after rejection of a transplanted kidney: case report and review of the literature. Int J Hematol. 2004;80:361-364.
1. Rongioletti F, Patterson JW, Rebora A. The histological and pathogenetic spectrum of cutaneous disease in monoclonal gammopathies. J Cutan Pathol. 2008;35:705-721.
2. Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
3. Muscardin LM, Pulsoni A, Cerroni L. Primary cutaneous plasmacytoma: report of a case with review of the literature. J Am Acad Dermatol. 2000;43(5, pt 2):962-965.
4. Kazakov DV, Belousova IE, Müller B, et al. Primary cutaneous plasmacytoma: a clinicopathological study of two cases with a long-term follow-up and review of the literature. J Cutan Pathol. 2002;29:244-248.
5. Aricò M, Bongiorno MR. Primary cutaneous plasmacytosis in a child. is this a new entity? J Eur Acad Dermatol. 2002;16:164-167.
6. Willoughby V, Werlang-Perurena A, Kelly A, et al. Primary cutaneous plasmacytoma (posttransplant lymphoproliferative disorder, plasmacytoma-like) in a heart transplant patient. Am J Dermatopathol. 2006;28:442-445.
7. Buffet M, Dupin N, Carlotti A, et al. Epstein-Barr virus–associated cutaneous plasmacytoma post–solid organ transplantation [in French]. Ann Dermatol Venereol. 2004;131:1085-1091.
8. Zendri E, Venturi C, Ricci R, et al. Primary cutaneous plasmacytoma: a role for a triggering stimulus? Clin Exp Dermatol. 2005;30:229-231.
9. Tüting T, Bork K. Primary plasmacytoma of the skin. J Am Acad Dermatol. 1996;34(2, pt 2):386-390.
10. Miura H, Itami S, Yoshikawa K. Treatment of facial lesion of cutaneous plasmacytosis with tacrolimus ointment. J Am Acad Dermatol. 2003;49:1195-1196.
11. Tessari G, Fabbian F, Colato C, et al. Primary cutaneous plasmacytoma after rejection of a transplanted kidney: case report and review of the literature. Int J Hematol. 2004;80:361-364.