User login
House bill would allow corrective action plan for DEA violators
A new bill aims to clarify the rules of the Controlled Substances Act to ensure that legitimate operators stay in business and patients get needed medication, according to congressional backers of the bill, which was approved by the House of Representatives on July 29.
The bill still has to be taken up by the Senate, where there is no companion legislation.
The Ensuring Patient Access and Effective Drug Enforcement Act of 2014 (H.R. 4709) would ensure that restrictions on distribution of controlled substances are not so onerous as to inhibit access for patients, would require the U.S. Attorney General to give DEA registrant pharmacies and physicians who violate the rules an opportunity to submit a corrective action plan that might defer suspension of their registration, and would establish a working group to make recommendations to Congress on federal policies to reduce prescription drug diversion and abuse.
These measures are among the major policy goals of the Alliance to Prevent the Abuse of Medicines. The Washington, D.C.–based group includes among its members the American Medical Association, Cardinal Health, CVS Caremark, the Health Industry Distributors Association, and Teva.
The National Association of Chain Drug Stores "and chain pharmacy are committed to partnering with federal and state agencies, law enforcement personnel, policymakers, and other stakeholders to work on viable strategies to simultaneously advance patient health and prevent prescription drug abuse," NACDS President and CEO Steven C. Anderson, said in a statement regarding the bill.
Rep. Marsha Blackburn (R-Tenn.), a cosponsor of the bill, said in a statement that simply acknowledging the epidemic of prescription drug abuse isn’t enough. "Congress has a responsibility to make sure the law is crystal clear for both the DEA and legitimate businesses who want to understand what the rules are so they can do the right thing."
In House testimony last April, DEA Deputy Assistant Administrator Joseph Rannazzisi said the agency’s job is getting tougher. The number of registrants that the DEA regulates has mushroomed from 480,000 in 1973 to 1.5 million today, he said. At the same time, diversion and abuse have risen steeply, with opioids selling on the black market for 5-10 times their retail value.
In the last 3 years, the DEA’s Tactical Diversion Squads have increased from 37 to 66, and the agency has been applying its stiffest penalty – an immediate suspension order – in a judicious manner, according to Mr. Rannazzisi. From October 2013 through March 2014, 20 suspensions were ordered.
On Twitter @aliciaault
A new bill aims to clarify the rules of the Controlled Substances Act to ensure that legitimate operators stay in business and patients get needed medication, according to congressional backers of the bill, which was approved by the House of Representatives on July 29.
The bill still has to be taken up by the Senate, where there is no companion legislation.
The Ensuring Patient Access and Effective Drug Enforcement Act of 2014 (H.R. 4709) would ensure that restrictions on distribution of controlled substances are not so onerous as to inhibit access for patients, would require the U.S. Attorney General to give DEA registrant pharmacies and physicians who violate the rules an opportunity to submit a corrective action plan that might defer suspension of their registration, and would establish a working group to make recommendations to Congress on federal policies to reduce prescription drug diversion and abuse.
These measures are among the major policy goals of the Alliance to Prevent the Abuse of Medicines. The Washington, D.C.–based group includes among its members the American Medical Association, Cardinal Health, CVS Caremark, the Health Industry Distributors Association, and Teva.
The National Association of Chain Drug Stores "and chain pharmacy are committed to partnering with federal and state agencies, law enforcement personnel, policymakers, and other stakeholders to work on viable strategies to simultaneously advance patient health and prevent prescription drug abuse," NACDS President and CEO Steven C. Anderson, said in a statement regarding the bill.
Rep. Marsha Blackburn (R-Tenn.), a cosponsor of the bill, said in a statement that simply acknowledging the epidemic of prescription drug abuse isn’t enough. "Congress has a responsibility to make sure the law is crystal clear for both the DEA and legitimate businesses who want to understand what the rules are so they can do the right thing."
In House testimony last April, DEA Deputy Assistant Administrator Joseph Rannazzisi said the agency’s job is getting tougher. The number of registrants that the DEA regulates has mushroomed from 480,000 in 1973 to 1.5 million today, he said. At the same time, diversion and abuse have risen steeply, with opioids selling on the black market for 5-10 times their retail value.
In the last 3 years, the DEA’s Tactical Diversion Squads have increased from 37 to 66, and the agency has been applying its stiffest penalty – an immediate suspension order – in a judicious manner, according to Mr. Rannazzisi. From October 2013 through March 2014, 20 suspensions were ordered.
On Twitter @aliciaault
A new bill aims to clarify the rules of the Controlled Substances Act to ensure that legitimate operators stay in business and patients get needed medication, according to congressional backers of the bill, which was approved by the House of Representatives on July 29.
The bill still has to be taken up by the Senate, where there is no companion legislation.
The Ensuring Patient Access and Effective Drug Enforcement Act of 2014 (H.R. 4709) would ensure that restrictions on distribution of controlled substances are not so onerous as to inhibit access for patients, would require the U.S. Attorney General to give DEA registrant pharmacies and physicians who violate the rules an opportunity to submit a corrective action plan that might defer suspension of their registration, and would establish a working group to make recommendations to Congress on federal policies to reduce prescription drug diversion and abuse.
These measures are among the major policy goals of the Alliance to Prevent the Abuse of Medicines. The Washington, D.C.–based group includes among its members the American Medical Association, Cardinal Health, CVS Caremark, the Health Industry Distributors Association, and Teva.
The National Association of Chain Drug Stores "and chain pharmacy are committed to partnering with federal and state agencies, law enforcement personnel, policymakers, and other stakeholders to work on viable strategies to simultaneously advance patient health and prevent prescription drug abuse," NACDS President and CEO Steven C. Anderson, said in a statement regarding the bill.
Rep. Marsha Blackburn (R-Tenn.), a cosponsor of the bill, said in a statement that simply acknowledging the epidemic of prescription drug abuse isn’t enough. "Congress has a responsibility to make sure the law is crystal clear for both the DEA and legitimate businesses who want to understand what the rules are so they can do the right thing."
In House testimony last April, DEA Deputy Assistant Administrator Joseph Rannazzisi said the agency’s job is getting tougher. The number of registrants that the DEA regulates has mushroomed from 480,000 in 1973 to 1.5 million today, he said. At the same time, diversion and abuse have risen steeply, with opioids selling on the black market for 5-10 times their retail value.
In the last 3 years, the DEA’s Tactical Diversion Squads have increased from 37 to 66, and the agency has been applying its stiffest penalty – an immediate suspension order – in a judicious manner, according to Mr. Rannazzisi. From October 2013 through March 2014, 20 suspensions were ordered.
On Twitter @aliciaault
House panel looks into rocky rollout of healthcare.gov
WASHINGTON – Despite a scathing report on past and ongoing failures with healthcare.gov, the Affordable Care Act’s website will be ready to go when enrollment begins again in November, Andy Slavitt, principal deputy administrator of the Centers for Medicare & Medicaid Services testified to a House subcommittee July 31.
Mr. Slavitt, who took his post 3 weeks ago, was called to Capitol Hill to respond to a Government Accountability Office report that is critical of both the rollout of healthcare.gov and the ongoing management of the portal. The GAO is a nonpartisan agency that serves as the investigative arm of Congress.
Mr. Slavitt acknowledged problems listed by the GAO. "Fortunately, or unfortunately, the GAO report wasn’t news to the people at CMS," he testified before the House Energy & Commerce Committee Subcommittee on Oversight and Investigations.
But many have been addressed, he said, adding that there is more accountability, better communication with contractors, and clearly defined performance goals.
"This coming year will be one of continuous and visible improvement, but not perfection," Mr. Slavitt testified.
The GAO report chronicled mismanagement, limited oversight, and a chaotic planning process within CMS during the lead-up to the launch of healthcare.gov in October 2013. Officials knew that the site was only 65% ready that spring, but put off a review of performance until September, GAO official William T. Woods testified at the hearing.
Constantly changing requirements led to huge cost increases – which appear to have continued. As of March 2014, the federal government had spent $840 million on the website. The CMS did not properly review or approve work by the contractors and "took only limited steps to hold the contractor accountable," Mr. Woods said in written testimony.
The GAO report notes that the cost of working with Accenture Federal Services – the contractor hired to replace the site’s original builder – had ballooned from $91 million to more than $175 million in the first half of 2014, because of new requirements and other changes ordered by CMS.
Despite rising costs, key parts of the website are still not functional, including the financial management module, which handles financial interactions with insurers. That contract continues, "so costs on that particular contract are almost certainly higher today than they were when we completed our audit work," Mr. Woods said.
Rep. Tim Murphy (R-Pa.), chairman of the oversight subcommittee said, "We still don’t know if the Administration has a system in place capable of handling inconsistencies, inaccurate subsidies, or whether CMS will ever put in place a functioning payments system."
Rep. Murphy and his Republican colleagues also questioned whether top CMS officials, such as Administrator Marilyn Tavenner, were aware that the site was so far behind in its performance goals and deadlines. At one point, Rep. Murphy suggested that, if they had known, they might have perjured themselves in appearances before the Energy & Commerce Committee in 2013.
Democrats on the panel said that it was important to acknowledge the failures of the site’s launch, but only to inform future solutions. "We will stipulate the rollout of the ACA was an unmitigated disaster," said Rep. Diana DeGette (D-Colo.). But she and her colleagues said it was time to move forward.
"My point is not to excuse the healthcare.gov problems but to put them in context," said Rep. Henry Waxman (D-Calif.). He said the problems were fixed, though not quick enough for his liking. But, Rep. Waxman added, "I want to learn what went wrong so CMS can do a better job for the next time."
On Twitter @aliciaault
WASHINGTON – Despite a scathing report on past and ongoing failures with healthcare.gov, the Affordable Care Act’s website will be ready to go when enrollment begins again in November, Andy Slavitt, principal deputy administrator of the Centers for Medicare & Medicaid Services testified to a House subcommittee July 31.
Mr. Slavitt, who took his post 3 weeks ago, was called to Capitol Hill to respond to a Government Accountability Office report that is critical of both the rollout of healthcare.gov and the ongoing management of the portal. The GAO is a nonpartisan agency that serves as the investigative arm of Congress.
Mr. Slavitt acknowledged problems listed by the GAO. "Fortunately, or unfortunately, the GAO report wasn’t news to the people at CMS," he testified before the House Energy & Commerce Committee Subcommittee on Oversight and Investigations.
But many have been addressed, he said, adding that there is more accountability, better communication with contractors, and clearly defined performance goals.
"This coming year will be one of continuous and visible improvement, but not perfection," Mr. Slavitt testified.
The GAO report chronicled mismanagement, limited oversight, and a chaotic planning process within CMS during the lead-up to the launch of healthcare.gov in October 2013. Officials knew that the site was only 65% ready that spring, but put off a review of performance until September, GAO official William T. Woods testified at the hearing.
Constantly changing requirements led to huge cost increases – which appear to have continued. As of March 2014, the federal government had spent $840 million on the website. The CMS did not properly review or approve work by the contractors and "took only limited steps to hold the contractor accountable," Mr. Woods said in written testimony.
The GAO report notes that the cost of working with Accenture Federal Services – the contractor hired to replace the site’s original builder – had ballooned from $91 million to more than $175 million in the first half of 2014, because of new requirements and other changes ordered by CMS.
Despite rising costs, key parts of the website are still not functional, including the financial management module, which handles financial interactions with insurers. That contract continues, "so costs on that particular contract are almost certainly higher today than they were when we completed our audit work," Mr. Woods said.
Rep. Tim Murphy (R-Pa.), chairman of the oversight subcommittee said, "We still don’t know if the Administration has a system in place capable of handling inconsistencies, inaccurate subsidies, or whether CMS will ever put in place a functioning payments system."
Rep. Murphy and his Republican colleagues also questioned whether top CMS officials, such as Administrator Marilyn Tavenner, were aware that the site was so far behind in its performance goals and deadlines. At one point, Rep. Murphy suggested that, if they had known, they might have perjured themselves in appearances before the Energy & Commerce Committee in 2013.
Democrats on the panel said that it was important to acknowledge the failures of the site’s launch, but only to inform future solutions. "We will stipulate the rollout of the ACA was an unmitigated disaster," said Rep. Diana DeGette (D-Colo.). But she and her colleagues said it was time to move forward.
"My point is not to excuse the healthcare.gov problems but to put them in context," said Rep. Henry Waxman (D-Calif.). He said the problems were fixed, though not quick enough for his liking. But, Rep. Waxman added, "I want to learn what went wrong so CMS can do a better job for the next time."
On Twitter @aliciaault
WASHINGTON – Despite a scathing report on past and ongoing failures with healthcare.gov, the Affordable Care Act’s website will be ready to go when enrollment begins again in November, Andy Slavitt, principal deputy administrator of the Centers for Medicare & Medicaid Services testified to a House subcommittee July 31.
Mr. Slavitt, who took his post 3 weeks ago, was called to Capitol Hill to respond to a Government Accountability Office report that is critical of both the rollout of healthcare.gov and the ongoing management of the portal. The GAO is a nonpartisan agency that serves as the investigative arm of Congress.
Mr. Slavitt acknowledged problems listed by the GAO. "Fortunately, or unfortunately, the GAO report wasn’t news to the people at CMS," he testified before the House Energy & Commerce Committee Subcommittee on Oversight and Investigations.
But many have been addressed, he said, adding that there is more accountability, better communication with contractors, and clearly defined performance goals.
"This coming year will be one of continuous and visible improvement, but not perfection," Mr. Slavitt testified.
The GAO report chronicled mismanagement, limited oversight, and a chaotic planning process within CMS during the lead-up to the launch of healthcare.gov in October 2013. Officials knew that the site was only 65% ready that spring, but put off a review of performance until September, GAO official William T. Woods testified at the hearing.
Constantly changing requirements led to huge cost increases – which appear to have continued. As of March 2014, the federal government had spent $840 million on the website. The CMS did not properly review or approve work by the contractors and "took only limited steps to hold the contractor accountable," Mr. Woods said in written testimony.
The GAO report notes that the cost of working with Accenture Federal Services – the contractor hired to replace the site’s original builder – had ballooned from $91 million to more than $175 million in the first half of 2014, because of new requirements and other changes ordered by CMS.
Despite rising costs, key parts of the website are still not functional, including the financial management module, which handles financial interactions with insurers. That contract continues, "so costs on that particular contract are almost certainly higher today than they were when we completed our audit work," Mr. Woods said.
Rep. Tim Murphy (R-Pa.), chairman of the oversight subcommittee said, "We still don’t know if the Administration has a system in place capable of handling inconsistencies, inaccurate subsidies, or whether CMS will ever put in place a functioning payments system."
Rep. Murphy and his Republican colleagues also questioned whether top CMS officials, such as Administrator Marilyn Tavenner, were aware that the site was so far behind in its performance goals and deadlines. At one point, Rep. Murphy suggested that, if they had known, they might have perjured themselves in appearances before the Energy & Commerce Committee in 2013.
Democrats on the panel said that it was important to acknowledge the failures of the site’s launch, but only to inform future solutions. "We will stipulate the rollout of the ACA was an unmitigated disaster," said Rep. Diana DeGette (D-Colo.). But she and her colleagues said it was time to move forward.
"My point is not to excuse the healthcare.gov problems but to put them in context," said Rep. Henry Waxman (D-Calif.). He said the problems were fixed, though not quick enough for his liking. But, Rep. Waxman added, "I want to learn what went wrong so CMS can do a better job for the next time."
On Twitter @aliciaault
FROM A HOUSE ENERGY & COMMERCE COMMITTEE HEARING
House votes to speed sunscreen approvals
The U.S. House of Representatives passed legislation to speed up Food and Drug Administration approvals of new sunscreen ingredients and to eliminate a backlog.
The Sunscreen Innovation Act (H.R. 4250) was passed by a voice vote and now awaits action by the Senate. The companion bill in the Senate, S. 2141, was introduced by Jack Reed (D-R.I.) and has been referred to the Committee on Health, Education, Labor, and Pensions but has not received a hearing yet.
Backers of the legislation are encouraging the Senate to move quickly.
"Even though the Food and Drug Administration has listed action on sunscreen ingredient applications as a priority since 2008, no new sunscreen ingredients have been approved by the FDA in 15 years," Rep. Ed Whitfield (R-Ky.), one of the lead sponsors of the House bill, said in a statement after the approval.
"The framework outlined in this legislation strikes an appropriate balance between consumer safety and access to new sunscreen products," he said, adding, "I am pleased to see my legislation pass the House of Representatives, and call on the Senate to act."
The bill requires the FDA to make final decisions within a year on the backlog of ingredients under review, and within a year and a half on new applications. It also sets up more Congressional oversight of the process and makes "sunscreens that have been marketed for five continuous years in the United States or other countries and in sufficient quantity eligible for review under this Act."
Currently, according to the American Cancer Society Cancer Action Network (ACS CAN), only three of the seven UVA-blocking ingredients sold in Europe are approved in the United States.
The Sunscreen Innovation Act has many supporters in the health care field and among consumer advocates and manufacturers, many of whom belong to the PASS (Public Access to SunScreens) Coalition. In a statement issued by the coalition, the American College of Mohs Surgery said, "This important legislation will go a long way in helping prevent skin cancers by improving the availability of more effective sunscreen products, and we are committed to its passage."
The Environmental Working Group, a frequent critic of the FDA’s regulation of sunscreens and of manufacturers, also applauded House passage of the bill.
The American Cancer Society was pleased as well. "The House took a critical step today to fix a broken process at FDA for the review of new sunscreen ingredients that could potentially help more Americans prevent skin cancer," Chris Hansen, president of the ACS CAN, said in a statement.
"American consumers should have access to the broadest choice of sunscreens – including those in use for years in other countries – once they are shown to be safe and effective," he noted.
On Twitter @aliciaault
The U.S. House of Representatives passed legislation to speed up Food and Drug Administration approvals of new sunscreen ingredients and to eliminate a backlog.
The Sunscreen Innovation Act (H.R. 4250) was passed by a voice vote and now awaits action by the Senate. The companion bill in the Senate, S. 2141, was introduced by Jack Reed (D-R.I.) and has been referred to the Committee on Health, Education, Labor, and Pensions but has not received a hearing yet.
Backers of the legislation are encouraging the Senate to move quickly.
"Even though the Food and Drug Administration has listed action on sunscreen ingredient applications as a priority since 2008, no new sunscreen ingredients have been approved by the FDA in 15 years," Rep. Ed Whitfield (R-Ky.), one of the lead sponsors of the House bill, said in a statement after the approval.
"The framework outlined in this legislation strikes an appropriate balance between consumer safety and access to new sunscreen products," he said, adding, "I am pleased to see my legislation pass the House of Representatives, and call on the Senate to act."
The bill requires the FDA to make final decisions within a year on the backlog of ingredients under review, and within a year and a half on new applications. It also sets up more Congressional oversight of the process and makes "sunscreens that have been marketed for five continuous years in the United States or other countries and in sufficient quantity eligible for review under this Act."
Currently, according to the American Cancer Society Cancer Action Network (ACS CAN), only three of the seven UVA-blocking ingredients sold in Europe are approved in the United States.
The Sunscreen Innovation Act has many supporters in the health care field and among consumer advocates and manufacturers, many of whom belong to the PASS (Public Access to SunScreens) Coalition. In a statement issued by the coalition, the American College of Mohs Surgery said, "This important legislation will go a long way in helping prevent skin cancers by improving the availability of more effective sunscreen products, and we are committed to its passage."
The Environmental Working Group, a frequent critic of the FDA’s regulation of sunscreens and of manufacturers, also applauded House passage of the bill.
The American Cancer Society was pleased as well. "The House took a critical step today to fix a broken process at FDA for the review of new sunscreen ingredients that could potentially help more Americans prevent skin cancer," Chris Hansen, president of the ACS CAN, said in a statement.
"American consumers should have access to the broadest choice of sunscreens – including those in use for years in other countries – once they are shown to be safe and effective," he noted.
On Twitter @aliciaault
The U.S. House of Representatives passed legislation to speed up Food and Drug Administration approvals of new sunscreen ingredients and to eliminate a backlog.
The Sunscreen Innovation Act (H.R. 4250) was passed by a voice vote and now awaits action by the Senate. The companion bill in the Senate, S. 2141, was introduced by Jack Reed (D-R.I.) and has been referred to the Committee on Health, Education, Labor, and Pensions but has not received a hearing yet.
Backers of the legislation are encouraging the Senate to move quickly.
"Even though the Food and Drug Administration has listed action on sunscreen ingredient applications as a priority since 2008, no new sunscreen ingredients have been approved by the FDA in 15 years," Rep. Ed Whitfield (R-Ky.), one of the lead sponsors of the House bill, said in a statement after the approval.
"The framework outlined in this legislation strikes an appropriate balance between consumer safety and access to new sunscreen products," he said, adding, "I am pleased to see my legislation pass the House of Representatives, and call on the Senate to act."
The bill requires the FDA to make final decisions within a year on the backlog of ingredients under review, and within a year and a half on new applications. It also sets up more Congressional oversight of the process and makes "sunscreens that have been marketed for five continuous years in the United States or other countries and in sufficient quantity eligible for review under this Act."
Currently, according to the American Cancer Society Cancer Action Network (ACS CAN), only three of the seven UVA-blocking ingredients sold in Europe are approved in the United States.
The Sunscreen Innovation Act has many supporters in the health care field and among consumer advocates and manufacturers, many of whom belong to the PASS (Public Access to SunScreens) Coalition. In a statement issued by the coalition, the American College of Mohs Surgery said, "This important legislation will go a long way in helping prevent skin cancers by improving the availability of more effective sunscreen products, and we are committed to its passage."
The Environmental Working Group, a frequent critic of the FDA’s regulation of sunscreens and of manufacturers, also applauded House passage of the bill.
The American Cancer Society was pleased as well. "The House took a critical step today to fix a broken process at FDA for the review of new sunscreen ingredients that could potentially help more Americans prevent skin cancer," Chris Hansen, president of the ACS CAN, said in a statement.
"American consumers should have access to the broadest choice of sunscreens – including those in use for years in other countries – once they are shown to be safe and effective," he noted.
On Twitter @aliciaault
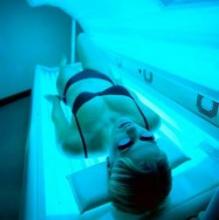
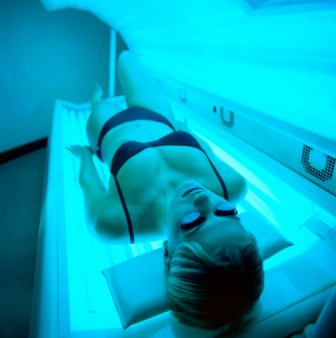
Surgeon General: Tanning, indoor tanning must stop
The U.S. Surgeon General’s office is calling on Americans to do more to help prevent skin cancer, saying that it is a growing public health problem.
The five-point call to action singled out ultraviolet light exposure – from indoor and outdoor tanning – as a major culprit in the growing incidence of all cancers, and melanoma, in particular.
"Tanned skin is damaged skin," Acting Surgeon General, Boris D. Lushniak, a dermatologist, said at a briefing to release the report.
"When people tan or get sunburned, they increase their risk of getting skin cancer later in life," he said, adding that the nation needs to change its cultural acceptance of tanning as a sign of health.
The report noted that about 5 million Americans are treated for skin cancer each year, at a cost of $8 billion. Melanoma is of the greatest concern; its incidence has tripled over the past 3 decades, with 63,000 new cases per year. Some 9,000 Americans die from melanoma each year, and death rates are on the rise in men, Dr. Howard Koh, assistant secretary for health at the U.S. Department of Health & Human Services, said at the briefing.
Both Dr. Koh and Dr. Lushniak singled out indoor tanning as particularly dangerous. The Surgeon General’s report cited research showing that indoor tanning may be associated with more than 400,000 cases of skin cancer, including 6,000 melanomas, per year.
Forty-four states and the District of Columbia have some type of indoor tanning laws or regulations. Eleven states – California, Delaware, Hawaii, Illinois, Louisiana, Minnesota, Nevada, Oregon, Texas, Vermont and Washington – prohibit indoor tanning by individuals under age 18. But indoor tanning remains popular: One in three white women aged 16-25 years engages in indoor tanning each year, according to the report.
In May, the Food and Drug Administration issued stricter rules for sunlamps used with indoor tanning, requiring a boxed warning that they should not be used on anyone younger than 18 years. The agency also required that more information on risks and contraindications be provided to sunlamp users, FDA Commissioner Margaret Hamburg noted in a statement on the Surgeon General’s report.
The five main points in the Surgeon General’s call to action are:
• Increase opportunities for sun protection in outdoor settings, such as increasing shade in recreational areas, and providing more protection for school children and outdoor workers.
• Give individuals information to make informed, healthy choices about UV exposure, including providing materials in workplaces and schools, and partnering with physicians and health systems.
• Promote policies that advance the goal of preventing skin cancer, such as encouraging electronic reporting of reportable skin cancers.
• Reduce harms from indoor tanning by enforcing existing laws and considering additional restrictions.
• Strengthen research, surveillance, monitoring, and evaluation.
The American Academy of Dermatology Association applauded the report.
"The American public needs to be aware that the dangers of ultraviolet radiation exposure are real," Dr. Brett M. Coldiron, president of the AADA, said in a statement. "The AADA is particularly pleased that the HHS and the Office of the Surgeon General have highlighted methods for the public to prevent skin cancer in this white paper, that include seeking shade, wearing protective clothing, applying sunscreen, and avoiding dangerous indoor tanning devices."
John Seffrin, Ph.D., chief executive officer of the American Cancer Society Cancer Action Network, noted that this is the first time there has been a national action plan regarding skin cancer.
"By bringing national attention to this growing public health crisis, the Surgeon General is calling on all of us to reinvigorate the fight against skin cancer," he said. "The Surgeon General’s Call to Action outlines achievable goals and strategies to support more Americans in making healthy choices about protecting their skin," Dr. Seffrin said.
On Twitter @aliciaault
The U.S. Surgeon General’s office is calling on Americans to do more to help prevent skin cancer, saying that it is a growing public health problem.
The five-point call to action singled out ultraviolet light exposure – from indoor and outdoor tanning – as a major culprit in the growing incidence of all cancers, and melanoma, in particular.
"Tanned skin is damaged skin," Acting Surgeon General, Boris D. Lushniak, a dermatologist, said at a briefing to release the report.
"When people tan or get sunburned, they increase their risk of getting skin cancer later in life," he said, adding that the nation needs to change its cultural acceptance of tanning as a sign of health.
The report noted that about 5 million Americans are treated for skin cancer each year, at a cost of $8 billion. Melanoma is of the greatest concern; its incidence has tripled over the past 3 decades, with 63,000 new cases per year. Some 9,000 Americans die from melanoma each year, and death rates are on the rise in men, Dr. Howard Koh, assistant secretary for health at the U.S. Department of Health & Human Services, said at the briefing.
Both Dr. Koh and Dr. Lushniak singled out indoor tanning as particularly dangerous. The Surgeon General’s report cited research showing that indoor tanning may be associated with more than 400,000 cases of skin cancer, including 6,000 melanomas, per year.
Forty-four states and the District of Columbia have some type of indoor tanning laws or regulations. Eleven states – California, Delaware, Hawaii, Illinois, Louisiana, Minnesota, Nevada, Oregon, Texas, Vermont and Washington – prohibit indoor tanning by individuals under age 18. But indoor tanning remains popular: One in three white women aged 16-25 years engages in indoor tanning each year, according to the report.
In May, the Food and Drug Administration issued stricter rules for sunlamps used with indoor tanning, requiring a boxed warning that they should not be used on anyone younger than 18 years. The agency also required that more information on risks and contraindications be provided to sunlamp users, FDA Commissioner Margaret Hamburg noted in a statement on the Surgeon General’s report.
The five main points in the Surgeon General’s call to action are:
• Increase opportunities for sun protection in outdoor settings, such as increasing shade in recreational areas, and providing more protection for school children and outdoor workers.
• Give individuals information to make informed, healthy choices about UV exposure, including providing materials in workplaces and schools, and partnering with physicians and health systems.
• Promote policies that advance the goal of preventing skin cancer, such as encouraging electronic reporting of reportable skin cancers.
• Reduce harms from indoor tanning by enforcing existing laws and considering additional restrictions.
• Strengthen research, surveillance, monitoring, and evaluation.
The American Academy of Dermatology Association applauded the report.
"The American public needs to be aware that the dangers of ultraviolet radiation exposure are real," Dr. Brett M. Coldiron, president of the AADA, said in a statement. "The AADA is particularly pleased that the HHS and the Office of the Surgeon General have highlighted methods for the public to prevent skin cancer in this white paper, that include seeking shade, wearing protective clothing, applying sunscreen, and avoiding dangerous indoor tanning devices."
John Seffrin, Ph.D., chief executive officer of the American Cancer Society Cancer Action Network, noted that this is the first time there has been a national action plan regarding skin cancer.
"By bringing national attention to this growing public health crisis, the Surgeon General is calling on all of us to reinvigorate the fight against skin cancer," he said. "The Surgeon General’s Call to Action outlines achievable goals and strategies to support more Americans in making healthy choices about protecting their skin," Dr. Seffrin said.
On Twitter @aliciaault
The U.S. Surgeon General’s office is calling on Americans to do more to help prevent skin cancer, saying that it is a growing public health problem.
The five-point call to action singled out ultraviolet light exposure – from indoor and outdoor tanning – as a major culprit in the growing incidence of all cancers, and melanoma, in particular.
"Tanned skin is damaged skin," Acting Surgeon General, Boris D. Lushniak, a dermatologist, said at a briefing to release the report.
"When people tan or get sunburned, they increase their risk of getting skin cancer later in life," he said, adding that the nation needs to change its cultural acceptance of tanning as a sign of health.
The report noted that about 5 million Americans are treated for skin cancer each year, at a cost of $8 billion. Melanoma is of the greatest concern; its incidence has tripled over the past 3 decades, with 63,000 new cases per year. Some 9,000 Americans die from melanoma each year, and death rates are on the rise in men, Dr. Howard Koh, assistant secretary for health at the U.S. Department of Health & Human Services, said at the briefing.
Both Dr. Koh and Dr. Lushniak singled out indoor tanning as particularly dangerous. The Surgeon General’s report cited research showing that indoor tanning may be associated with more than 400,000 cases of skin cancer, including 6,000 melanomas, per year.
Forty-four states and the District of Columbia have some type of indoor tanning laws or regulations. Eleven states – California, Delaware, Hawaii, Illinois, Louisiana, Minnesota, Nevada, Oregon, Texas, Vermont and Washington – prohibit indoor tanning by individuals under age 18. But indoor tanning remains popular: One in three white women aged 16-25 years engages in indoor tanning each year, according to the report.
In May, the Food and Drug Administration issued stricter rules for sunlamps used with indoor tanning, requiring a boxed warning that they should not be used on anyone younger than 18 years. The agency also required that more information on risks and contraindications be provided to sunlamp users, FDA Commissioner Margaret Hamburg noted in a statement on the Surgeon General’s report.
The five main points in the Surgeon General’s call to action are:
• Increase opportunities for sun protection in outdoor settings, such as increasing shade in recreational areas, and providing more protection for school children and outdoor workers.
• Give individuals information to make informed, healthy choices about UV exposure, including providing materials in workplaces and schools, and partnering with physicians and health systems.
• Promote policies that advance the goal of preventing skin cancer, such as encouraging electronic reporting of reportable skin cancers.
• Reduce harms from indoor tanning by enforcing existing laws and considering additional restrictions.
• Strengthen research, surveillance, monitoring, and evaluation.
The American Academy of Dermatology Association applauded the report.
"The American public needs to be aware that the dangers of ultraviolet radiation exposure are real," Dr. Brett M. Coldiron, president of the AADA, said in a statement. "The AADA is particularly pleased that the HHS and the Office of the Surgeon General have highlighted methods for the public to prevent skin cancer in this white paper, that include seeking shade, wearing protective clothing, applying sunscreen, and avoiding dangerous indoor tanning devices."
John Seffrin, Ph.D., chief executive officer of the American Cancer Society Cancer Action Network, noted that this is the first time there has been a national action plan regarding skin cancer.
"By bringing national attention to this growing public health crisis, the Surgeon General is calling on all of us to reinvigorate the fight against skin cancer," he said. "The Surgeon General’s Call to Action outlines achievable goals and strategies to support more Americans in making healthy choices about protecting their skin," Dr. Seffrin said.
On Twitter @aliciaault
FDA gives full approval to ibrutinib for CLL
The Food and Drug Administration has given full approval for ibrutinib (Imbruvica) for patients with chronic lymphocytic leukemia who have received at least one prior therapy and for those who have a deletion in chromosome 17 (17p deletion) and may or may not have received previous treatment.
The drug received conditional, accelerated approval from the FDA in February for CLL. The agency said that new trial results that looked at overall survival and progression-free survival confirmed the drug’s benefit. Ibrutinib’s label will be updated to show that it has a confirmed survival benefit.
The FDA also designated ibrutinib as a breakthrough therapy for CLL with 17p deletion.
"Imbruvica is the fourth drug approved to treat CLL that received a breakthrough therapy designation, reflecting the promise of the breakthrough therapy designation program and demonstrating the FDA’s commitment to working cooperatively with companies to expedite the development, review, and approval of these important new drugs," said Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, in a statement.
"This FDA approval for Imbruvica is a major step toward chemo-free treatment in CLL," said Dr. John Byrd, director of the hematology division at the Ohio State University Comprehensive Cancer Center, Columbus, in a statement issued by Pharmacyclics, the Sunnyvale, Calif.–based company that is comarketing the drug with Janssen Biotech.
"I continue to be awed by the duration of my patients’ responses to Imbruvica and am grateful Imbruvica now is available to a broader group of CLL patients," said Dr. Byrd, who was a lead investigator for the main trial evaluating ibrutinib, RESONATE.
Three other drugs for CLL have also received breakthrough designations: obinutuzumab (Gazyva), ofatumumab (Arzerra), and most recently, idelalisib (Zydelig), earlier in July.
CLL is a non-Hodgkin’s lymphoma that primarily affects older individuals. The National Cancer Institute estimates that 15,720 Americans will be diagnosed and 4,600 will die from CLL in 2014, the FDA said.
In RESONATE, 391 previously treated patients received either ibrutinib or ofatumumab. Of those, 127 patients had a 17p deletion. The trial was stopped early because overall, patients receiving ibrutinib had a 78% reduction in the risk of disease progression or death, and a 57% reduction in risk of death. For the patients with 17p, there was a 75% reduction in the risk of disease progression or death.
This is the third FDA approval for ibrutinib. In addition to the accelerated approval for CLL in February, the drug was approved for mantle cell lymphoma in November 2013.
Dr. Byrd has served as an unpaid adviser to both Pharmacyclics and Janssen. He reported no financial interest in either company.
On Twitter @aliciaault
The Food and Drug Administration has given full approval for ibrutinib (Imbruvica) for patients with chronic lymphocytic leukemia who have received at least one prior therapy and for those who have a deletion in chromosome 17 (17p deletion) and may or may not have received previous treatment.
The drug received conditional, accelerated approval from the FDA in February for CLL. The agency said that new trial results that looked at overall survival and progression-free survival confirmed the drug’s benefit. Ibrutinib’s label will be updated to show that it has a confirmed survival benefit.
The FDA also designated ibrutinib as a breakthrough therapy for CLL with 17p deletion.
"Imbruvica is the fourth drug approved to treat CLL that received a breakthrough therapy designation, reflecting the promise of the breakthrough therapy designation program and demonstrating the FDA’s commitment to working cooperatively with companies to expedite the development, review, and approval of these important new drugs," said Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, in a statement.
"This FDA approval for Imbruvica is a major step toward chemo-free treatment in CLL," said Dr. John Byrd, director of the hematology division at the Ohio State University Comprehensive Cancer Center, Columbus, in a statement issued by Pharmacyclics, the Sunnyvale, Calif.–based company that is comarketing the drug with Janssen Biotech.
"I continue to be awed by the duration of my patients’ responses to Imbruvica and am grateful Imbruvica now is available to a broader group of CLL patients," said Dr. Byrd, who was a lead investigator for the main trial evaluating ibrutinib, RESONATE.
Three other drugs for CLL have also received breakthrough designations: obinutuzumab (Gazyva), ofatumumab (Arzerra), and most recently, idelalisib (Zydelig), earlier in July.
CLL is a non-Hodgkin’s lymphoma that primarily affects older individuals. The National Cancer Institute estimates that 15,720 Americans will be diagnosed and 4,600 will die from CLL in 2014, the FDA said.
In RESONATE, 391 previously treated patients received either ibrutinib or ofatumumab. Of those, 127 patients had a 17p deletion. The trial was stopped early because overall, patients receiving ibrutinib had a 78% reduction in the risk of disease progression or death, and a 57% reduction in risk of death. For the patients with 17p, there was a 75% reduction in the risk of disease progression or death.
This is the third FDA approval for ibrutinib. In addition to the accelerated approval for CLL in February, the drug was approved for mantle cell lymphoma in November 2013.
Dr. Byrd has served as an unpaid adviser to both Pharmacyclics and Janssen. He reported no financial interest in either company.
On Twitter @aliciaault
The Food and Drug Administration has given full approval for ibrutinib (Imbruvica) for patients with chronic lymphocytic leukemia who have received at least one prior therapy and for those who have a deletion in chromosome 17 (17p deletion) and may or may not have received previous treatment.
The drug received conditional, accelerated approval from the FDA in February for CLL. The agency said that new trial results that looked at overall survival and progression-free survival confirmed the drug’s benefit. Ibrutinib’s label will be updated to show that it has a confirmed survival benefit.
The FDA also designated ibrutinib as a breakthrough therapy for CLL with 17p deletion.
"Imbruvica is the fourth drug approved to treat CLL that received a breakthrough therapy designation, reflecting the promise of the breakthrough therapy designation program and demonstrating the FDA’s commitment to working cooperatively with companies to expedite the development, review, and approval of these important new drugs," said Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, in a statement.
"This FDA approval for Imbruvica is a major step toward chemo-free treatment in CLL," said Dr. John Byrd, director of the hematology division at the Ohio State University Comprehensive Cancer Center, Columbus, in a statement issued by Pharmacyclics, the Sunnyvale, Calif.–based company that is comarketing the drug with Janssen Biotech.
"I continue to be awed by the duration of my patients’ responses to Imbruvica and am grateful Imbruvica now is available to a broader group of CLL patients," said Dr. Byrd, who was a lead investigator for the main trial evaluating ibrutinib, RESONATE.
Three other drugs for CLL have also received breakthrough designations: obinutuzumab (Gazyva), ofatumumab (Arzerra), and most recently, idelalisib (Zydelig), earlier in July.
CLL is a non-Hodgkin’s lymphoma that primarily affects older individuals. The National Cancer Institute estimates that 15,720 Americans will be diagnosed and 4,600 will die from CLL in 2014, the FDA said.
In RESONATE, 391 previously treated patients received either ibrutinib or ofatumumab. Of those, 127 patients had a 17p deletion. The trial was stopped early because overall, patients receiving ibrutinib had a 78% reduction in the risk of disease progression or death, and a 57% reduction in risk of death. For the patients with 17p, there was a 75% reduction in the risk of disease progression or death.
This is the third FDA approval for ibrutinib. In addition to the accelerated approval for CLL in February, the drug was approved for mantle cell lymphoma in November 2013.
Dr. Byrd has served as an unpaid adviser to both Pharmacyclics and Janssen. He reported no financial interest in either company.
On Twitter @aliciaault
Conflicting rulings raise questions about legality of ACA premium subsidies
Two conflicting appeals court decisions issued July 22 seem to put the tax subsidies offered by the Affordable Care Act – and potentially, the entire law itself – on either shaky or firm legal footing, depending on who’s doing the analysis.
Both cases were originally brought by plaintiffs who contended that the Obama Administration did not have the legal authority to issue subsidies to low-income individuals who buy insurance on the federal marketplace. They said that the ACA explicitly said that credits were only available to "state-established exchanges." When lower courts ruled against them, they appealed.
The District of Columbia Circuit of the U.S. Court of Appeals sided with the plaintiffs in Halbig v. Burwell, 2-1. The 4th Circuit of the U.S. Court of Appeals, on the other hand, sided unanimously with the government in King v. Burwell.
An estimated 5 million people have received subsidies from the 36 federal marketplaces, putting them at risk for losing those tax credits. For the time being, however, people who have been deemed eligible for subsidies will continue to receive them. And those who sign up for insurance in the next open enrollment period beginning Nov. 15 will also likely get subsidies.
"This ruling does not have any practical impact on Americans’ ability to receive tax credits right now," White House spokesman Josh Earnest said about the ruling in Halbig v. Burwell. In a briefing after both decisions were handed down, Mr. Earnest also said that the administration would essentially appeal that ruling by seeking a decision by the full panel of 11 judges who sit on the D.C. Circuit.
"You don’t need a fancy legal degree to understand that Congress intended for every eligible American to have access to tax credits that would lower their health care costs," whether state or federal officials were running the marketplace, Mr. Earnest said.
The lead plaintiff in the case heard by the D.C. Circuit was brought by Jacqueline Halbig, a senior policy adviser in the Department of Health & Human Services under President George W. Bush. The two judges ruling for her and her coplaintiffs said that their reading of the ACA "plainly makes subsidies available only on exchanges established by the states." The "legislative record provides little indication one way or the other of congressional intent, but the statutory text does," they said, adding that the language in the law is "conclusive evidence of Congress’ intent."
They said they reached their conclusion "with reluctance," because "our ruling will likely have significant consequences both for the millions of individuals receiving tax credits through federal exchanges and for health insurance markets more broadly."
Judge Harry Edwards dissented. "This case is about appellants’ not-so-veiled attempt to gut the Patient Protection and Affordable Care Act." The argument that Congress only intended to pay subsidies on state exchanges as a way to encourage states to run their own exchanges "is nonsense, made up out of whole cloth."
Judge Edwards added, "There is no credible evidence in the record that Congress intended to condition subsidies on whether a state, as opposed to HHS, established the exchange. Nor is there credible evidence that any state even considered the possibility that its taxpayers would be denied subsidies if the state opted to allow HHS to establish an exchange on its behalf."
Those who support premium subsidies said they were dismayed by the D.C. Circuit’s ruling, but that it would not likely stand.
"Today’s decision represents the high-water mark for Affordable Care Act opponents, but the water will recede very quickly," said Ron Pollack, Executive Director of Families USA, in a statement.
The American Cancer Society, American Cancer Society Cancer Action Network, American Diabetes Association, and American Heart Association filed a joint statement saying that they believed the Halbig decision could be disastrous. "On behalf of the tens of millions of people nationwide who have experienced cancer, diabetes, heart disease, and stroke, we are deeply disappointed with the decision of the U.S. Court of Appeals for the D.C. Circuit, which denies premium tax credits that make health coverage more affordable to people who buy a plan in the federally facilitated marketplace," they said. But, they added that it would not likely be upheld.
Others who opposed the ACA and the subsidy scheme applauded the Halbig decision.
"The president has been spending money illegally, the court has ruled," said Michael Cannon, director of Health Policy Studies at the Washington, D.C.-based Cato Institute, in a briefing. An article by Mr. Cannon and economist Jonathan Adler led to the Halbig filing.
In a statement, Sen. Ted Cruz (R-Tex.), who filed a friend of the court brief in King v. Burwell, said that the D.C. Circuit’s decision "is a repudiation of Obamacare and all the lawlessness that has come with it."
The plaintiffs in King v. Burwell, however, were handed a setback by the 4th Circuit. The four individual plaintiffs, all of whom live in Virginia, which does not have a state-run exchange, did not want to be forced to buy health insurance, but said that if they didn’t, they’d be penalized. Since the ACA will give them a subsidy to buy coverage, they would be forced either to buy insurance or pay a penalty for not having coverage, they said.
They added that Congress said that only state-run marketplaces could receive federal subsidies.
The judges ruled against them, saying "we are not persuaded by the plaintiffs’ ‘coercion’ argument." They did say, however, that there was some confusion in the law. "We cannot discern whether Congress intended one way or another to make the tax credits available on HHS-facilitated exchanges," they wrote, adding that "the relevant statutory sections appear to conflict with one another, yielding different possible interpretations."
But in the end, the judges concluded that it was "clear that widely available tax credits are essential to fulfilling the Act’s primary goals and that Congress was aware of their importance when drafting the bill." Therefore, the Internal Revenue Service’s rule authorizing tax credits was "a permissible exercise of the agency’s discretion."
Mr. Cannon of the Cato Institute said that he views the 4th Circuit ruling as a kind of loss for the Obama administration because the judges said that the language in the ACA was ambiguous on the subsidies.
Families USA’s Ron Pollack, however, said that he thought the administration would ultimately prevail.
"As of today, eight judges – two federal district court judges and six appellate judges – have ruled on these challenges. Altogether, six judges have ruled that the cases should be dismissed, and only two have upheld plaintiffs’ claims," he said in a statement.
There are still more legal proceedings to be decided before there is a definitive answer on whether the subsidies – and the ACA itself – are legal. The full D.C. Circuit has to choose whether to hear the Halbig case, and the plaintiffs in the 4th Circuit could also ask for a hearing by the full panel of judges.
There are two additional major cases questioning the legality of the subsidies pending at the appeals court level.
One or all of the cases could be taken to the U.S. Supreme Court.
On Twitter @aliciaault
Two conflicting appeals court decisions issued July 22 seem to put the tax subsidies offered by the Affordable Care Act – and potentially, the entire law itself – on either shaky or firm legal footing, depending on who’s doing the analysis.
Both cases were originally brought by plaintiffs who contended that the Obama Administration did not have the legal authority to issue subsidies to low-income individuals who buy insurance on the federal marketplace. They said that the ACA explicitly said that credits were only available to "state-established exchanges." When lower courts ruled against them, they appealed.
The District of Columbia Circuit of the U.S. Court of Appeals sided with the plaintiffs in Halbig v. Burwell, 2-1. The 4th Circuit of the U.S. Court of Appeals, on the other hand, sided unanimously with the government in King v. Burwell.
An estimated 5 million people have received subsidies from the 36 federal marketplaces, putting them at risk for losing those tax credits. For the time being, however, people who have been deemed eligible for subsidies will continue to receive them. And those who sign up for insurance in the next open enrollment period beginning Nov. 15 will also likely get subsidies.
"This ruling does not have any practical impact on Americans’ ability to receive tax credits right now," White House spokesman Josh Earnest said about the ruling in Halbig v. Burwell. In a briefing after both decisions were handed down, Mr. Earnest also said that the administration would essentially appeal that ruling by seeking a decision by the full panel of 11 judges who sit on the D.C. Circuit.
"You don’t need a fancy legal degree to understand that Congress intended for every eligible American to have access to tax credits that would lower their health care costs," whether state or federal officials were running the marketplace, Mr. Earnest said.
The lead plaintiff in the case heard by the D.C. Circuit was brought by Jacqueline Halbig, a senior policy adviser in the Department of Health & Human Services under President George W. Bush. The two judges ruling for her and her coplaintiffs said that their reading of the ACA "plainly makes subsidies available only on exchanges established by the states." The "legislative record provides little indication one way or the other of congressional intent, but the statutory text does," they said, adding that the language in the law is "conclusive evidence of Congress’ intent."
They said they reached their conclusion "with reluctance," because "our ruling will likely have significant consequences both for the millions of individuals receiving tax credits through federal exchanges and for health insurance markets more broadly."
Judge Harry Edwards dissented. "This case is about appellants’ not-so-veiled attempt to gut the Patient Protection and Affordable Care Act." The argument that Congress only intended to pay subsidies on state exchanges as a way to encourage states to run their own exchanges "is nonsense, made up out of whole cloth."
Judge Edwards added, "There is no credible evidence in the record that Congress intended to condition subsidies on whether a state, as opposed to HHS, established the exchange. Nor is there credible evidence that any state even considered the possibility that its taxpayers would be denied subsidies if the state opted to allow HHS to establish an exchange on its behalf."
Those who support premium subsidies said they were dismayed by the D.C. Circuit’s ruling, but that it would not likely stand.
"Today’s decision represents the high-water mark for Affordable Care Act opponents, but the water will recede very quickly," said Ron Pollack, Executive Director of Families USA, in a statement.
The American Cancer Society, American Cancer Society Cancer Action Network, American Diabetes Association, and American Heart Association filed a joint statement saying that they believed the Halbig decision could be disastrous. "On behalf of the tens of millions of people nationwide who have experienced cancer, diabetes, heart disease, and stroke, we are deeply disappointed with the decision of the U.S. Court of Appeals for the D.C. Circuit, which denies premium tax credits that make health coverage more affordable to people who buy a plan in the federally facilitated marketplace," they said. But, they added that it would not likely be upheld.
Others who opposed the ACA and the subsidy scheme applauded the Halbig decision.
"The president has been spending money illegally, the court has ruled," said Michael Cannon, director of Health Policy Studies at the Washington, D.C.-based Cato Institute, in a briefing. An article by Mr. Cannon and economist Jonathan Adler led to the Halbig filing.
In a statement, Sen. Ted Cruz (R-Tex.), who filed a friend of the court brief in King v. Burwell, said that the D.C. Circuit’s decision "is a repudiation of Obamacare and all the lawlessness that has come with it."
The plaintiffs in King v. Burwell, however, were handed a setback by the 4th Circuit. The four individual plaintiffs, all of whom live in Virginia, which does not have a state-run exchange, did not want to be forced to buy health insurance, but said that if they didn’t, they’d be penalized. Since the ACA will give them a subsidy to buy coverage, they would be forced either to buy insurance or pay a penalty for not having coverage, they said.
They added that Congress said that only state-run marketplaces could receive federal subsidies.
The judges ruled against them, saying "we are not persuaded by the plaintiffs’ ‘coercion’ argument." They did say, however, that there was some confusion in the law. "We cannot discern whether Congress intended one way or another to make the tax credits available on HHS-facilitated exchanges," they wrote, adding that "the relevant statutory sections appear to conflict with one another, yielding different possible interpretations."
But in the end, the judges concluded that it was "clear that widely available tax credits are essential to fulfilling the Act’s primary goals and that Congress was aware of their importance when drafting the bill." Therefore, the Internal Revenue Service’s rule authorizing tax credits was "a permissible exercise of the agency’s discretion."
Mr. Cannon of the Cato Institute said that he views the 4th Circuit ruling as a kind of loss for the Obama administration because the judges said that the language in the ACA was ambiguous on the subsidies.
Families USA’s Ron Pollack, however, said that he thought the administration would ultimately prevail.
"As of today, eight judges – two federal district court judges and six appellate judges – have ruled on these challenges. Altogether, six judges have ruled that the cases should be dismissed, and only two have upheld plaintiffs’ claims," he said in a statement.
There are still more legal proceedings to be decided before there is a definitive answer on whether the subsidies – and the ACA itself – are legal. The full D.C. Circuit has to choose whether to hear the Halbig case, and the plaintiffs in the 4th Circuit could also ask for a hearing by the full panel of judges.
There are two additional major cases questioning the legality of the subsidies pending at the appeals court level.
One or all of the cases could be taken to the U.S. Supreme Court.
On Twitter @aliciaault
Two conflicting appeals court decisions issued July 22 seem to put the tax subsidies offered by the Affordable Care Act – and potentially, the entire law itself – on either shaky or firm legal footing, depending on who’s doing the analysis.
Both cases were originally brought by plaintiffs who contended that the Obama Administration did not have the legal authority to issue subsidies to low-income individuals who buy insurance on the federal marketplace. They said that the ACA explicitly said that credits were only available to "state-established exchanges." When lower courts ruled against them, they appealed.
The District of Columbia Circuit of the U.S. Court of Appeals sided with the plaintiffs in Halbig v. Burwell, 2-1. The 4th Circuit of the U.S. Court of Appeals, on the other hand, sided unanimously with the government in King v. Burwell.
An estimated 5 million people have received subsidies from the 36 federal marketplaces, putting them at risk for losing those tax credits. For the time being, however, people who have been deemed eligible for subsidies will continue to receive them. And those who sign up for insurance in the next open enrollment period beginning Nov. 15 will also likely get subsidies.
"This ruling does not have any practical impact on Americans’ ability to receive tax credits right now," White House spokesman Josh Earnest said about the ruling in Halbig v. Burwell. In a briefing after both decisions were handed down, Mr. Earnest also said that the administration would essentially appeal that ruling by seeking a decision by the full panel of 11 judges who sit on the D.C. Circuit.
"You don’t need a fancy legal degree to understand that Congress intended for every eligible American to have access to tax credits that would lower their health care costs," whether state or federal officials were running the marketplace, Mr. Earnest said.
The lead plaintiff in the case heard by the D.C. Circuit was brought by Jacqueline Halbig, a senior policy adviser in the Department of Health & Human Services under President George W. Bush. The two judges ruling for her and her coplaintiffs said that their reading of the ACA "plainly makes subsidies available only on exchanges established by the states." The "legislative record provides little indication one way or the other of congressional intent, but the statutory text does," they said, adding that the language in the law is "conclusive evidence of Congress’ intent."
They said they reached their conclusion "with reluctance," because "our ruling will likely have significant consequences both for the millions of individuals receiving tax credits through federal exchanges and for health insurance markets more broadly."
Judge Harry Edwards dissented. "This case is about appellants’ not-so-veiled attempt to gut the Patient Protection and Affordable Care Act." The argument that Congress only intended to pay subsidies on state exchanges as a way to encourage states to run their own exchanges "is nonsense, made up out of whole cloth."
Judge Edwards added, "There is no credible evidence in the record that Congress intended to condition subsidies on whether a state, as opposed to HHS, established the exchange. Nor is there credible evidence that any state even considered the possibility that its taxpayers would be denied subsidies if the state opted to allow HHS to establish an exchange on its behalf."
Those who support premium subsidies said they were dismayed by the D.C. Circuit’s ruling, but that it would not likely stand.
"Today’s decision represents the high-water mark for Affordable Care Act opponents, but the water will recede very quickly," said Ron Pollack, Executive Director of Families USA, in a statement.
The American Cancer Society, American Cancer Society Cancer Action Network, American Diabetes Association, and American Heart Association filed a joint statement saying that they believed the Halbig decision could be disastrous. "On behalf of the tens of millions of people nationwide who have experienced cancer, diabetes, heart disease, and stroke, we are deeply disappointed with the decision of the U.S. Court of Appeals for the D.C. Circuit, which denies premium tax credits that make health coverage more affordable to people who buy a plan in the federally facilitated marketplace," they said. But, they added that it would not likely be upheld.
Others who opposed the ACA and the subsidy scheme applauded the Halbig decision.
"The president has been spending money illegally, the court has ruled," said Michael Cannon, director of Health Policy Studies at the Washington, D.C.-based Cato Institute, in a briefing. An article by Mr. Cannon and economist Jonathan Adler led to the Halbig filing.
In a statement, Sen. Ted Cruz (R-Tex.), who filed a friend of the court brief in King v. Burwell, said that the D.C. Circuit’s decision "is a repudiation of Obamacare and all the lawlessness that has come with it."
The plaintiffs in King v. Burwell, however, were handed a setback by the 4th Circuit. The four individual plaintiffs, all of whom live in Virginia, which does not have a state-run exchange, did not want to be forced to buy health insurance, but said that if they didn’t, they’d be penalized. Since the ACA will give them a subsidy to buy coverage, they would be forced either to buy insurance or pay a penalty for not having coverage, they said.
They added that Congress said that only state-run marketplaces could receive federal subsidies.
The judges ruled against them, saying "we are not persuaded by the plaintiffs’ ‘coercion’ argument." They did say, however, that there was some confusion in the law. "We cannot discern whether Congress intended one way or another to make the tax credits available on HHS-facilitated exchanges," they wrote, adding that "the relevant statutory sections appear to conflict with one another, yielding different possible interpretations."
But in the end, the judges concluded that it was "clear that widely available tax credits are essential to fulfilling the Act’s primary goals and that Congress was aware of their importance when drafting the bill." Therefore, the Internal Revenue Service’s rule authorizing tax credits was "a permissible exercise of the agency’s discretion."
Mr. Cannon of the Cato Institute said that he views the 4th Circuit ruling as a kind of loss for the Obama administration because the judges said that the language in the ACA was ambiguous on the subsidies.
Families USA’s Ron Pollack, however, said that he thought the administration would ultimately prevail.
"As of today, eight judges – two federal district court judges and six appellate judges – have ruled on these challenges. Altogether, six judges have ruled that the cases should be dismissed, and only two have upheld plaintiffs’ claims," he said in a statement.
There are still more legal proceedings to be decided before there is a definitive answer on whether the subsidies – and the ACA itself – are legal. The full D.C. Circuit has to choose whether to hear the Halbig case, and the plaintiffs in the 4th Circuit could also ask for a hearing by the full panel of judges.
There are two additional major cases questioning the legality of the subsidies pending at the appeals court level.
One or all of the cases could be taken to the U.S. Supreme Court.
On Twitter @aliciaault
Delegates direct AMA to study effect of Maintenance of Certification
CHICAGO – The American Medical Association should continue to work with the American Board of Medical Specialties to address physicians’ concerns about Maintenance of Certification – that was the consensus at the annual meeting of the AMA House of Delegates.
The AMA’s delegates defeated a resolution that asked the organization to put a moratorium on MOC until it was proven to improve the quality of care and patient outcomes. However, they did agree to a new policy that directs the AMA to:
• Explore with independent entities the feasibility of conducting a study to evaluate the effect MOC requirements and Maintenance of Licensure principles have on workforce, practice costs, patient outcomes, patient safety, and patient access.
• Work with the American Board of Medical Specialties and its 24 member boards to collect data on why physicians choose to maintain or discontinue their board certification.
• Work with the ABMS and the Federation of State Medical Boards to study whether MOC and the principles of Maintenance of Licensure are important factors to physicians when deciding whether to retire and whether they have a direct effect on workforce.
• Oppose making MOC mandatory as a condition of medical licensure, and encourage physicians to strive constantly to improve their care of patients by the means they find most effective.
The new policy applies to both the ABMS MOC process and the Osteopathic Continuous Certification (OCC) process.
Physicians have increasingly voiced their concerns about MOC. Dr. Paul Teirstein, chief of cardiology and director of interventional cardiology for Scripps Clinic in La Jolla, Calif., launched a petition drive to overhaul the American Board of Internal Medicine’s MOC process. The petition has more than 17,000 signatures.
The ABIM says that it is listening to physicians and is making changes in the process, but also recently said that more than 150,000 physicians had participated in its MOC process – making the May 1 deadline to be listed on the ABIM website as having met the MOC criteria.
But anger is still bubbling up, and was expressed at the AMA’s meeting.
"Practicing physicians on the front lines are increasingly burdened, hassled, and confused by the onerous and expensive process of Maintenance of Certification and Maintenance of Licensure," said Dr. James A. Goodyear, a delegate from Pennsylvania.
Dr. Goodyear introduced the resolution to seek a moratorium on the MOC.
But Dr. Darlyne Menscer, a member of the AMA Council on Medical Education, told the delegates that such a moratorium would put a wedge in the close working relationship the AMA has had with the ABMS. "This is more prescriptive than we can commit to as a council, although we definitely do hear the concerns of the House," added Dr. Menscer.
The AMA has been discussing the concerns about MOC with the ABMS, most recently holding a meeting in Chicago in early June.
Dr. Joshua Cohen, a delegate from the American Academy of Neurology, and a member of the AMA Foundation’s Board of Directors, who attended that meeting, also argued against a moratorium. "It would make it impossible for the AMA to improve the process going forward," said Dr. Cohen.
Dr. Chuck Wilson, a pediatrician and delegate from the North Carolina delegation, also opposed any major change in direction for the AMA. He noted that if the AMA was seen as opposed to MOC, it might not be viewed well. "We all want it to be less onerous," said Dr. Wilson. But, he noted, "the Council on Medical Education is working in that direction. Let’s give them a chance to be successful."
In a statement after the HOD meeting, the AMA said that it "continues to ensure the MOC process does not disrupt physician practice or reduce the capacity of the overall physician workforce." Concerns about MOC "center around the need for relevance to the daily practice of physicians and the better integration into physician practices to optimally support learning and improvement."
On Twitter @aliciaault
CHICAGO – The American Medical Association should continue to work with the American Board of Medical Specialties to address physicians’ concerns about Maintenance of Certification – that was the consensus at the annual meeting of the AMA House of Delegates.
The AMA’s delegates defeated a resolution that asked the organization to put a moratorium on MOC until it was proven to improve the quality of care and patient outcomes. However, they did agree to a new policy that directs the AMA to:
• Explore with independent entities the feasibility of conducting a study to evaluate the effect MOC requirements and Maintenance of Licensure principles have on workforce, practice costs, patient outcomes, patient safety, and patient access.
• Work with the American Board of Medical Specialties and its 24 member boards to collect data on why physicians choose to maintain or discontinue their board certification.
• Work with the ABMS and the Federation of State Medical Boards to study whether MOC and the principles of Maintenance of Licensure are important factors to physicians when deciding whether to retire and whether they have a direct effect on workforce.
• Oppose making MOC mandatory as a condition of medical licensure, and encourage physicians to strive constantly to improve their care of patients by the means they find most effective.
The new policy applies to both the ABMS MOC process and the Osteopathic Continuous Certification (OCC) process.
Physicians have increasingly voiced their concerns about MOC. Dr. Paul Teirstein, chief of cardiology and director of interventional cardiology for Scripps Clinic in La Jolla, Calif., launched a petition drive to overhaul the American Board of Internal Medicine’s MOC process. The petition has more than 17,000 signatures.
The ABIM says that it is listening to physicians and is making changes in the process, but also recently said that more than 150,000 physicians had participated in its MOC process – making the May 1 deadline to be listed on the ABIM website as having met the MOC criteria.
But anger is still bubbling up, and was expressed at the AMA’s meeting.
"Practicing physicians on the front lines are increasingly burdened, hassled, and confused by the onerous and expensive process of Maintenance of Certification and Maintenance of Licensure," said Dr. James A. Goodyear, a delegate from Pennsylvania.
Dr. Goodyear introduced the resolution to seek a moratorium on the MOC.
But Dr. Darlyne Menscer, a member of the AMA Council on Medical Education, told the delegates that such a moratorium would put a wedge in the close working relationship the AMA has had with the ABMS. "This is more prescriptive than we can commit to as a council, although we definitely do hear the concerns of the House," added Dr. Menscer.
The AMA has been discussing the concerns about MOC with the ABMS, most recently holding a meeting in Chicago in early June.
Dr. Joshua Cohen, a delegate from the American Academy of Neurology, and a member of the AMA Foundation’s Board of Directors, who attended that meeting, also argued against a moratorium. "It would make it impossible for the AMA to improve the process going forward," said Dr. Cohen.
Dr. Chuck Wilson, a pediatrician and delegate from the North Carolina delegation, also opposed any major change in direction for the AMA. He noted that if the AMA was seen as opposed to MOC, it might not be viewed well. "We all want it to be less onerous," said Dr. Wilson. But, he noted, "the Council on Medical Education is working in that direction. Let’s give them a chance to be successful."
In a statement after the HOD meeting, the AMA said that it "continues to ensure the MOC process does not disrupt physician practice or reduce the capacity of the overall physician workforce." Concerns about MOC "center around the need for relevance to the daily practice of physicians and the better integration into physician practices to optimally support learning and improvement."
On Twitter @aliciaault
CHICAGO – The American Medical Association should continue to work with the American Board of Medical Specialties to address physicians’ concerns about Maintenance of Certification – that was the consensus at the annual meeting of the AMA House of Delegates.
The AMA’s delegates defeated a resolution that asked the organization to put a moratorium on MOC until it was proven to improve the quality of care and patient outcomes. However, they did agree to a new policy that directs the AMA to:
• Explore with independent entities the feasibility of conducting a study to evaluate the effect MOC requirements and Maintenance of Licensure principles have on workforce, practice costs, patient outcomes, patient safety, and patient access.
• Work with the American Board of Medical Specialties and its 24 member boards to collect data on why physicians choose to maintain or discontinue their board certification.
• Work with the ABMS and the Federation of State Medical Boards to study whether MOC and the principles of Maintenance of Licensure are important factors to physicians when deciding whether to retire and whether they have a direct effect on workforce.
• Oppose making MOC mandatory as a condition of medical licensure, and encourage physicians to strive constantly to improve their care of patients by the means they find most effective.
The new policy applies to both the ABMS MOC process and the Osteopathic Continuous Certification (OCC) process.
Physicians have increasingly voiced their concerns about MOC. Dr. Paul Teirstein, chief of cardiology and director of interventional cardiology for Scripps Clinic in La Jolla, Calif., launched a petition drive to overhaul the American Board of Internal Medicine’s MOC process. The petition has more than 17,000 signatures.
The ABIM says that it is listening to physicians and is making changes in the process, but also recently said that more than 150,000 physicians had participated in its MOC process – making the May 1 deadline to be listed on the ABIM website as having met the MOC criteria.
But anger is still bubbling up, and was expressed at the AMA’s meeting.
"Practicing physicians on the front lines are increasingly burdened, hassled, and confused by the onerous and expensive process of Maintenance of Certification and Maintenance of Licensure," said Dr. James A. Goodyear, a delegate from Pennsylvania.
Dr. Goodyear introduced the resolution to seek a moratorium on the MOC.
But Dr. Darlyne Menscer, a member of the AMA Council on Medical Education, told the delegates that such a moratorium would put a wedge in the close working relationship the AMA has had with the ABMS. "This is more prescriptive than we can commit to as a council, although we definitely do hear the concerns of the House," added Dr. Menscer.
The AMA has been discussing the concerns about MOC with the ABMS, most recently holding a meeting in Chicago in early June.
Dr. Joshua Cohen, a delegate from the American Academy of Neurology, and a member of the AMA Foundation’s Board of Directors, who attended that meeting, also argued against a moratorium. "It would make it impossible for the AMA to improve the process going forward," said Dr. Cohen.
Dr. Chuck Wilson, a pediatrician and delegate from the North Carolina delegation, also opposed any major change in direction for the AMA. He noted that if the AMA was seen as opposed to MOC, it might not be viewed well. "We all want it to be less onerous," said Dr. Wilson. But, he noted, "the Council on Medical Education is working in that direction. Let’s give them a chance to be successful."
In a statement after the HOD meeting, the AMA said that it "continues to ensure the MOC process does not disrupt physician practice or reduce the capacity of the overall physician workforce." Concerns about MOC "center around the need for relevance to the daily practice of physicians and the better integration into physician practices to optimally support learning and improvement."
On Twitter @aliciaault
AT THE AMA HOD MEETING
Teens Who Sleep Less at Risk for Greater Insulin Resistance
MINNEAPOLIS – Teens who do not sleep enough may be at risk for gaining weight and increased insulin resistance.
That’s the conclusion of a small pilot study conducted by Dr. Dorit Koren and her colleagues at the University of Chicago.
There is already considerable epidemiologic data that lack of sleep is a risk factor for obesity in children and young adults, said Dr. Koren who is with the departments of pediatrics and medicine in the pediatric endocrinology department at the University of Chicago.
There have been studies examining the risk of type 2 diabetes with sleep deprivation in adults, but there has been no population-based data in children examining the risk of type 2 diabetes in children and adolescents – and that’s important because they are not just small adults, she said.
Adolescents tend to be more insulin resistant because of the pubertal growth spurt, and they have a different sleep architecture than do adults, as they tend to be late to bed and late to rise, said Dr. Koren.
Previous studies looking at glucose homeostasis in adolescents have mostly looked at fasting rather than dynamic measures of glucose homeostasis and that is a limitation because fasting measures reflect primarily hepatic insulin sensitivity, she said. Most studies also were conducted in a sleep lab, which is not a natural environment.
She and her colleagues wanted to study adolescents at home and also gauge postprandial glucose metabolism. They enrolled 10 adolescents, aged 13-18 years. A total of 70% were black and 30% were non-Hispanic white. Just under half were male. They were mostly overweight, as measured by body mass index, although some were very lean, and some were very obese, said Dr. Koren.
The patients were first given an overnight polysomnogram, and then told to measure sleep at home through an actigraphy device, and sleep diaries. The actigraphy helped back up the diaries, which are known to be "remarkably inaccurate" among adolescents, said Dr. Koren. They kept track of their sleep for 2 weeks.
The teens then returned for a second visit to the clinic. The researchers analyzed the average bedtime and waking time, and then asked them to restrict their sleep by going to bed an hour later. After returning again, the new measures after sleep restriction were compared with the earlier measures.
There was a strong correlation between weight and sleep duration, with longer sleep associated with less weight. They also saw a trend toward a greater waist circumference in adolescents who slept less.
There was a significant negative association between sleep duration and the 90-minute oral glucose tolerance test, with a P = .036. Restricted sleep also led to greater insulin resistance as measured by the homeostasis model assessment of insulin resistance (P = .091), and the whole-body insulin sensitivity index (P = .091).
Dr. Koren and her colleagues also performed linear regression analyses, controlling for either waist circumference or weight. Sleep deprivation was still the most significant factor as measured on the 90-minute glucose tolerance test and by the whole-body insulin sensitivity index.
"The model suggests that these relationships between home sleep deprivation and insulin resistance or hyperglycemia are independent of obesity, generalized or central," said Dr. Koren.
She cited the example of a 15-year-old female subject, who was lean. Her sleep went from 8.7 hours at baseline to 7.9 hours with the restriction. Her glucose values did not change significantly between baseline and restriction, but her insulin levels were noticeably higher in the sleep-restricted state, said Dr. Koren. Those levels rose an hour into the 90-minute tolerance test, which suggests that she was insulin resistant and needed to secrete more insulin to maintain glycemia.
Dr. Koren and her colleagues hope to replicate the study in a larger cohort.
Dr. Koren reported no relevant financial conflicts.
On Twitter @aliciaault
MINNEAPOLIS – Teens who do not sleep enough may be at risk for gaining weight and increased insulin resistance.
That’s the conclusion of a small pilot study conducted by Dr. Dorit Koren and her colleagues at the University of Chicago.
There is already considerable epidemiologic data that lack of sleep is a risk factor for obesity in children and young adults, said Dr. Koren who is with the departments of pediatrics and medicine in the pediatric endocrinology department at the University of Chicago.
There have been studies examining the risk of type 2 diabetes with sleep deprivation in adults, but there has been no population-based data in children examining the risk of type 2 diabetes in children and adolescents – and that’s important because they are not just small adults, she said.
Adolescents tend to be more insulin resistant because of the pubertal growth spurt, and they have a different sleep architecture than do adults, as they tend to be late to bed and late to rise, said Dr. Koren.
Previous studies looking at glucose homeostasis in adolescents have mostly looked at fasting rather than dynamic measures of glucose homeostasis and that is a limitation because fasting measures reflect primarily hepatic insulin sensitivity, she said. Most studies also were conducted in a sleep lab, which is not a natural environment.
She and her colleagues wanted to study adolescents at home and also gauge postprandial glucose metabolism. They enrolled 10 adolescents, aged 13-18 years. A total of 70% were black and 30% were non-Hispanic white. Just under half were male. They were mostly overweight, as measured by body mass index, although some were very lean, and some were very obese, said Dr. Koren.
The patients were first given an overnight polysomnogram, and then told to measure sleep at home through an actigraphy device, and sleep diaries. The actigraphy helped back up the diaries, which are known to be "remarkably inaccurate" among adolescents, said Dr. Koren. They kept track of their sleep for 2 weeks.
The teens then returned for a second visit to the clinic. The researchers analyzed the average bedtime and waking time, and then asked them to restrict their sleep by going to bed an hour later. After returning again, the new measures after sleep restriction were compared with the earlier measures.
There was a strong correlation between weight and sleep duration, with longer sleep associated with less weight. They also saw a trend toward a greater waist circumference in adolescents who slept less.
There was a significant negative association between sleep duration and the 90-minute oral glucose tolerance test, with a P = .036. Restricted sleep also led to greater insulin resistance as measured by the homeostasis model assessment of insulin resistance (P = .091), and the whole-body insulin sensitivity index (P = .091).
Dr. Koren and her colleagues also performed linear regression analyses, controlling for either waist circumference or weight. Sleep deprivation was still the most significant factor as measured on the 90-minute glucose tolerance test and by the whole-body insulin sensitivity index.
"The model suggests that these relationships between home sleep deprivation and insulin resistance or hyperglycemia are independent of obesity, generalized or central," said Dr. Koren.
She cited the example of a 15-year-old female subject, who was lean. Her sleep went from 8.7 hours at baseline to 7.9 hours with the restriction. Her glucose values did not change significantly between baseline and restriction, but her insulin levels were noticeably higher in the sleep-restricted state, said Dr. Koren. Those levels rose an hour into the 90-minute tolerance test, which suggests that she was insulin resistant and needed to secrete more insulin to maintain glycemia.
Dr. Koren and her colleagues hope to replicate the study in a larger cohort.
Dr. Koren reported no relevant financial conflicts.
On Twitter @aliciaault
MINNEAPOLIS – Teens who do not sleep enough may be at risk for gaining weight and increased insulin resistance.
That’s the conclusion of a small pilot study conducted by Dr. Dorit Koren and her colleagues at the University of Chicago.
There is already considerable epidemiologic data that lack of sleep is a risk factor for obesity in children and young adults, said Dr. Koren who is with the departments of pediatrics and medicine in the pediatric endocrinology department at the University of Chicago.
There have been studies examining the risk of type 2 diabetes with sleep deprivation in adults, but there has been no population-based data in children examining the risk of type 2 diabetes in children and adolescents – and that’s important because they are not just small adults, she said.
Adolescents tend to be more insulin resistant because of the pubertal growth spurt, and they have a different sleep architecture than do adults, as they tend to be late to bed and late to rise, said Dr. Koren.
Previous studies looking at glucose homeostasis in adolescents have mostly looked at fasting rather than dynamic measures of glucose homeostasis and that is a limitation because fasting measures reflect primarily hepatic insulin sensitivity, she said. Most studies also were conducted in a sleep lab, which is not a natural environment.
She and her colleagues wanted to study adolescents at home and also gauge postprandial glucose metabolism. They enrolled 10 adolescents, aged 13-18 years. A total of 70% were black and 30% were non-Hispanic white. Just under half were male. They were mostly overweight, as measured by body mass index, although some were very lean, and some were very obese, said Dr. Koren.
The patients were first given an overnight polysomnogram, and then told to measure sleep at home through an actigraphy device, and sleep diaries. The actigraphy helped back up the diaries, which are known to be "remarkably inaccurate" among adolescents, said Dr. Koren. They kept track of their sleep for 2 weeks.
The teens then returned for a second visit to the clinic. The researchers analyzed the average bedtime and waking time, and then asked them to restrict their sleep by going to bed an hour later. After returning again, the new measures after sleep restriction were compared with the earlier measures.
There was a strong correlation between weight and sleep duration, with longer sleep associated with less weight. They also saw a trend toward a greater waist circumference in adolescents who slept less.
There was a significant negative association between sleep duration and the 90-minute oral glucose tolerance test, with a P = .036. Restricted sleep also led to greater insulin resistance as measured by the homeostasis model assessment of insulin resistance (P = .091), and the whole-body insulin sensitivity index (P = .091).
Dr. Koren and her colleagues also performed linear regression analyses, controlling for either waist circumference or weight. Sleep deprivation was still the most significant factor as measured on the 90-minute glucose tolerance test and by the whole-body insulin sensitivity index.
"The model suggests that these relationships between home sleep deprivation and insulin resistance or hyperglycemia are independent of obesity, generalized or central," said Dr. Koren.
She cited the example of a 15-year-old female subject, who was lean. Her sleep went from 8.7 hours at baseline to 7.9 hours with the restriction. Her glucose values did not change significantly between baseline and restriction, but her insulin levels were noticeably higher in the sleep-restricted state, said Dr. Koren. Those levels rose an hour into the 90-minute tolerance test, which suggests that she was insulin resistant and needed to secrete more insulin to maintain glycemia.
Dr. Koren and her colleagues hope to replicate the study in a larger cohort.
Dr. Koren reported no relevant financial conflicts.
On Twitter @aliciaault
FROM SLEEP 2014
Sleep Society: Screen for Apnea at First Medicare Visit
MINNEAPOLIS – The American Academy of Sleep Medicine is pushing to have a simple sleep apnea questionnaire included in the initial Welcome to Medicare preventive care visit.
Including such a screening tool would help identify obstructive sleep apnea (OSA) when patients first join the Medicare program and thus improve the odds of diagnosing and treating the condition, said Dr. Timothy Morgenthaler, president of the AASM. Getting a handle on OSA could also reduce the potential that the beneficiary will develop related chronic conditions, and that will help Medicare curb expenditures, he said.
An estimated 20% of current Medicare beneficiaries have OSA. That number is expected to grow with the rising obesity rates, he said. Untreated OSA can increase the risk of hypertension, heart disease, type 2 diabetes, and stroke, said Dr. Morgenthaler, who is professor of medicine at the Mayo Clinic in Rochester, Minn.
The AASM has been lobbying Congress to include a validated OSA screen in the initial Medicare visit and found sponsors in Rep. Michael Burgess (R-Tex.) and Rep. Bobby Rush (D-Ill.). The two congressmen introduced a bill (H.R. 4695) that would do just that on May 21.
"This important legislation addresses the barriers that prevent new Medicare beneficiaries from receiving what we know to be required sleep apnea services," Dr. Morgenthaler said at the annual meeting of the Associated Professional Sleep Societies.
Rep. Erik Paulsen (R-Minn.), who recently signed on to the bill as a cosponsor, told AASM attendees that adding an OSA screen to the initial Medicare visit would help increase detection of disease, raise patient awareness, and "improve health care quality and reduce costs to the Medicare program," over the long term.
The AASM is asking its members to back the legislation and educate local lawmakers and patients through the group’s Seniors Sleep Campaign.
The association also wants to make it easier for board-certified sleep medicine specialists to care for Medicare patients from start to finish. Currently, antikickback laws prevent sleep specialists and sleep centers from directly providing therapeutic durable medical equipment to Medicare patients, said Dr. Morgenthaler.
The AASM has developed model language for an exception to that statute, which it hopes legislators or regulators will approve, he said. It would allow board-certified specialists to provide the continuum of care from start to finish, including durable medical equipment such as continuous positive airway pressure devices.
Eliminating the current fragmented system of care would eliminate waste, simplify the work flow, and improve the quality of care and reduce costs, said Dr. Morgenthaler.
On Twitter @aliciaault
MINNEAPOLIS – The American Academy of Sleep Medicine is pushing to have a simple sleep apnea questionnaire included in the initial Welcome to Medicare preventive care visit.
Including such a screening tool would help identify obstructive sleep apnea (OSA) when patients first join the Medicare program and thus improve the odds of diagnosing and treating the condition, said Dr. Timothy Morgenthaler, president of the AASM. Getting a handle on OSA could also reduce the potential that the beneficiary will develop related chronic conditions, and that will help Medicare curb expenditures, he said.
An estimated 20% of current Medicare beneficiaries have OSA. That number is expected to grow with the rising obesity rates, he said. Untreated OSA can increase the risk of hypertension, heart disease, type 2 diabetes, and stroke, said Dr. Morgenthaler, who is professor of medicine at the Mayo Clinic in Rochester, Minn.
The AASM has been lobbying Congress to include a validated OSA screen in the initial Medicare visit and found sponsors in Rep. Michael Burgess (R-Tex.) and Rep. Bobby Rush (D-Ill.). The two congressmen introduced a bill (H.R. 4695) that would do just that on May 21.
"This important legislation addresses the barriers that prevent new Medicare beneficiaries from receiving what we know to be required sleep apnea services," Dr. Morgenthaler said at the annual meeting of the Associated Professional Sleep Societies.
Rep. Erik Paulsen (R-Minn.), who recently signed on to the bill as a cosponsor, told AASM attendees that adding an OSA screen to the initial Medicare visit would help increase detection of disease, raise patient awareness, and "improve health care quality and reduce costs to the Medicare program," over the long term.
The AASM is asking its members to back the legislation and educate local lawmakers and patients through the group’s Seniors Sleep Campaign.
The association also wants to make it easier for board-certified sleep medicine specialists to care for Medicare patients from start to finish. Currently, antikickback laws prevent sleep specialists and sleep centers from directly providing therapeutic durable medical equipment to Medicare patients, said Dr. Morgenthaler.
The AASM has developed model language for an exception to that statute, which it hopes legislators or regulators will approve, he said. It would allow board-certified specialists to provide the continuum of care from start to finish, including durable medical equipment such as continuous positive airway pressure devices.
Eliminating the current fragmented system of care would eliminate waste, simplify the work flow, and improve the quality of care and reduce costs, said Dr. Morgenthaler.
On Twitter @aliciaault
MINNEAPOLIS – The American Academy of Sleep Medicine is pushing to have a simple sleep apnea questionnaire included in the initial Welcome to Medicare preventive care visit.
Including such a screening tool would help identify obstructive sleep apnea (OSA) when patients first join the Medicare program and thus improve the odds of diagnosing and treating the condition, said Dr. Timothy Morgenthaler, president of the AASM. Getting a handle on OSA could also reduce the potential that the beneficiary will develop related chronic conditions, and that will help Medicare curb expenditures, he said.
An estimated 20% of current Medicare beneficiaries have OSA. That number is expected to grow with the rising obesity rates, he said. Untreated OSA can increase the risk of hypertension, heart disease, type 2 diabetes, and stroke, said Dr. Morgenthaler, who is professor of medicine at the Mayo Clinic in Rochester, Minn.
The AASM has been lobbying Congress to include a validated OSA screen in the initial Medicare visit and found sponsors in Rep. Michael Burgess (R-Tex.) and Rep. Bobby Rush (D-Ill.). The two congressmen introduced a bill (H.R. 4695) that would do just that on May 21.
"This important legislation addresses the barriers that prevent new Medicare beneficiaries from receiving what we know to be required sleep apnea services," Dr. Morgenthaler said at the annual meeting of the Associated Professional Sleep Societies.
Rep. Erik Paulsen (R-Minn.), who recently signed on to the bill as a cosponsor, told AASM attendees that adding an OSA screen to the initial Medicare visit would help increase detection of disease, raise patient awareness, and "improve health care quality and reduce costs to the Medicare program," over the long term.
The AASM is asking its members to back the legislation and educate local lawmakers and patients through the group’s Seniors Sleep Campaign.
The association also wants to make it easier for board-certified sleep medicine specialists to care for Medicare patients from start to finish. Currently, antikickback laws prevent sleep specialists and sleep centers from directly providing therapeutic durable medical equipment to Medicare patients, said Dr. Morgenthaler.
The AASM has developed model language for an exception to that statute, which it hopes legislators or regulators will approve, he said. It would allow board-certified specialists to provide the continuum of care from start to finish, including durable medical equipment such as continuous positive airway pressure devices.
Eliminating the current fragmented system of care would eliminate waste, simplify the work flow, and improve the quality of care and reduce costs, said Dr. Morgenthaler.
On Twitter @aliciaault
AT SLEEP 2014
ACA: How will the recent Supreme Court decision impact contraception?
It may be months, even years before it’s clear whether access to and payment for contraception will be limited in the wake of the U.S. Supreme Court’s decision that for-profit companies can forego health insurance coverage of birth control methods that violate their religious belief.
At this point, only the two plaintiffs in the case – Hobby Lobby and Conestoga Wood Specialties – can change their health insurance coverage of contraception, based on the June 30 decision by the U.S. Supreme Court.
In the meantime, there is a lot of confusion among patients – and uncertainty among doctors -- about what the ruling means.
"We’re all scratching our heads and saying, ‘Now what?’ " said Dr. Anne Davis, medical director of Physicians for Reproductive Health. "All of us are very used to hurdles with coverage, but this is a new one."
Now, ob.gyns and other physicians may have to figure out if a patient works for an employer whose insurance will not cover certain types of contraception, or perhaps all birth control, said Dr. Davis of the department of clinical obstetrics and gynecology at Columbia University, New York.
The Supreme Court ruling is "a huge step backward," said Dr. Eve Espey, chair of obstetrics and gynecology at the University of New Mexico, Albuquerque. For physicians, a key concern is whether they will be paid for inserting an IUD or providing another service; for patients, it’s whether they can get the contraception and if they can afford it, she said.
When concerns mount, "those services tend to not happen," Dr. Espey said.
Ob.gyns. also expressed concern that the ruling had opened the door to interference in the physician-patient relationship.
Clinicians need the freedom to select the birth control method that makes the most sense for the patient, said Dr. Davis. "It’s going to be really unfortunate if you and the patient agree, and it’s the right thing and you find out that her employer has decided it’s not the right kind of birth control because of their beliefs," she said.
Dr. Jeanne Conry, immediate past president of the American Congress of Obstetricians and Gynecologists (ACOG), said that if she has decided that an IUD is best for a 34-year-old diabetic patient, but her employer says it won’t pay for that method, "that’s not in her best interest."
"They’ve just interfered with my best treatment for the patient," said Dr. Conry, an ob.gyn with Kaiser Permanente in Roseville, Calif.
Dr. Mitchell Creinin, chair of the obstetrics and gynecology department at University of California, Davis, said that the ruling could return contraception to a means-tested environment, in which only those who have the means will get the most effective methods, like the IUD. Instead, he said, it should be a benefit for all. Preventing pregnancy "is a public health issue and we’ve got to look at it that way," Dr. Creinin said.
Just after the ruling, ACOG President John Jennings agreed, expressing dismay that the Court was overlooking what had been firmly established. "The value of family planning – including contraception – has been clearly demonstrated," he said, in a statement.
But pro-life groups, including Americans United for Life and the Association of Pro-Life Obstetricians and Gynecologists, applauded the Hobby Lobby decision, saying that it helps preserve the right to practice according to conscience.
"Respect for the constitutionally guaranteed rights of conscience – whether held by business owners or medical professionals – must and should be protected," said Dr. Monique Chireau, AUL board member, with the department of ob.gyn at Duke University, Durham, N.C.
Dr. Gene Rudd, senior vice president for the Christian Medical and Dental Associations (CMDA), said that he did not think the decision would have any direct impact on ob.gyns.’ practices. However, he said, "Had the Justices allowed the government to force business owners to act against their conscience, that could establish a legal precedent by which the government might one day force physicians to act against their consciences."
The CMDA’s membership view the ACA’s mandate that women receive contraceptives free of charge as a potential infringement on their right to practice according to their beliefs, said Dr. Rudd, an ob.gyn.
In a CMDA survey, "95% of our members said they’d leave medicine before being required to violate their conscience," he said. They want "women free to pursue getting the contraceptive of their choice," but not to force either individual physicians or religiously affiliated organizations or small private employers to do things that are against their beliefs.
Who might be affected?
For now, there is no definitive answer on how many women could potentially lose coverage of contraception as a result of the Supreme Court decision. Hobby Lobby has 500 stores and 13,000 employees. Conestoga Wood Specialties has 950 employees. There are 90-100 cases challenging the contraception requirement, with about half them from nonprofit organizations and half from for-profit companies. And there’s motivation to sue: Companies that do not comply with the ACA requirement face fines of $100 per day per enrollee.
According to the Dept. of Health & Human Services, about 48 million women are eligible for preventive services because they are in "non-grandfathered" health plans.
Millions of women receive family planning services outside of employer-sponsored insurance, and thus may not be directly affected. A Guttmacher Institute report shows that 19 million women need publicly funded services and supplies because they are low income or are assumed to have a low income because they are under age 20 years. Family planning services provided through federal and state government funding helped women avoid 2.2 million unintended pregnancies in 2010, according to Guttmacher.
Reversing the decision
A handful of Democrats in the House and Senate introduced legislation July 9 to reverse the Supreme Court’s decision. The Protect Women's Health From Corporate Interference Act, introduced by Sen. Patty Murray (D-Wash.), would eliminate the loophole given by the court to companies that are not religiously affiliated.
ACOG praised the effort and applauded the legislators "for recognizing that contraceptives and other essential health care services should not be treated differently than other forms of health care.
"We look forward to working with them and other Congressional leaders to ensure that the ability of physicians to provide care for women is no longer subject to outside interference," Dr. Jennings said in a statement.
The White House said in an official Statement of Administration Policy that it strongly supported the legislation, saying that it "would prevent owners of for-profit companies from asserting their personal religious views to deny their employees federally-required health benefits."
Senate Democrats attempted to bring the bill to the floor on July 16, but failed to secure enough votes for debate. Republican Senators in the meantime introduced their own legislation to guarantee that employers could not bar "access" to contraceptives. The Preserving Religious Freedom and a Woman’s Access to Contraception Act (S. 2605) was introduced by Sen. Kelly Ayotte (R-N.H.).
Neither of the bills are expected to gain much traction in either the Senate or the House.
ACOG has asked its members to report coverage problems to their regional chapters, Dr. Conry said.
The federal government also could pursue several methods of reinstating the coverage. It could give for-profit employers the same accommodation that it has given nonprofit religiously affiliated employers like schools or charities. If these employers self-attest that paying for birth control violates their beliefs, then the employer’s insurer pays for the contraception. For self-insured employers, the exemption is not as easily navigated, because they would have to pay for the coverage.
That accommodation is being challenged by at least 45 legal cases that argue filling out the form violates plaintiffs’ religious beliefs because it means they are facilitating access to birth control.
The government could also pay directly for contraception for women whose employers receive an exemption. Supreme Court Justice Samuel Alito suggested such a possibility in his majority opinion in the Hobby Lobby case.
Dr. Davis said that, ideally, gynecologists would be able to give patients the contraception of choice on the same day of a visit and that the physician would be reimbursed for the cost of the device plus a fee for placing it. And, physicians should be able to counsel patients without thought of potential oversight from an employer or anyone else.
But, she said, "We are very far away from that right now."
On Twitter @aliciaault
It may be months, even years before it’s clear whether access to and payment for contraception will be limited in the wake of the U.S. Supreme Court’s decision that for-profit companies can forego health insurance coverage of birth control methods that violate their religious belief.
At this point, only the two plaintiffs in the case – Hobby Lobby and Conestoga Wood Specialties – can change their health insurance coverage of contraception, based on the June 30 decision by the U.S. Supreme Court.
In the meantime, there is a lot of confusion among patients – and uncertainty among doctors -- about what the ruling means.
"We’re all scratching our heads and saying, ‘Now what?’ " said Dr. Anne Davis, medical director of Physicians for Reproductive Health. "All of us are very used to hurdles with coverage, but this is a new one."
Now, ob.gyns and other physicians may have to figure out if a patient works for an employer whose insurance will not cover certain types of contraception, or perhaps all birth control, said Dr. Davis of the department of clinical obstetrics and gynecology at Columbia University, New York.
The Supreme Court ruling is "a huge step backward," said Dr. Eve Espey, chair of obstetrics and gynecology at the University of New Mexico, Albuquerque. For physicians, a key concern is whether they will be paid for inserting an IUD or providing another service; for patients, it’s whether they can get the contraception and if they can afford it, she said.
When concerns mount, "those services tend to not happen," Dr. Espey said.
Ob.gyns. also expressed concern that the ruling had opened the door to interference in the physician-patient relationship.
Clinicians need the freedom to select the birth control method that makes the most sense for the patient, said Dr. Davis. "It’s going to be really unfortunate if you and the patient agree, and it’s the right thing and you find out that her employer has decided it’s not the right kind of birth control because of their beliefs," she said.
Dr. Jeanne Conry, immediate past president of the American Congress of Obstetricians and Gynecologists (ACOG), said that if she has decided that an IUD is best for a 34-year-old diabetic patient, but her employer says it won’t pay for that method, "that’s not in her best interest."
"They’ve just interfered with my best treatment for the patient," said Dr. Conry, an ob.gyn with Kaiser Permanente in Roseville, Calif.
Dr. Mitchell Creinin, chair of the obstetrics and gynecology department at University of California, Davis, said that the ruling could return contraception to a means-tested environment, in which only those who have the means will get the most effective methods, like the IUD. Instead, he said, it should be a benefit for all. Preventing pregnancy "is a public health issue and we’ve got to look at it that way," Dr. Creinin said.
Just after the ruling, ACOG President John Jennings agreed, expressing dismay that the Court was overlooking what had been firmly established. "The value of family planning – including contraception – has been clearly demonstrated," he said, in a statement.
But pro-life groups, including Americans United for Life and the Association of Pro-Life Obstetricians and Gynecologists, applauded the Hobby Lobby decision, saying that it helps preserve the right to practice according to conscience.
"Respect for the constitutionally guaranteed rights of conscience – whether held by business owners or medical professionals – must and should be protected," said Dr. Monique Chireau, AUL board member, with the department of ob.gyn at Duke University, Durham, N.C.
Dr. Gene Rudd, senior vice president for the Christian Medical and Dental Associations (CMDA), said that he did not think the decision would have any direct impact on ob.gyns.’ practices. However, he said, "Had the Justices allowed the government to force business owners to act against their conscience, that could establish a legal precedent by which the government might one day force physicians to act against their consciences."
The CMDA’s membership view the ACA’s mandate that women receive contraceptives free of charge as a potential infringement on their right to practice according to their beliefs, said Dr. Rudd, an ob.gyn.
In a CMDA survey, "95% of our members said they’d leave medicine before being required to violate their conscience," he said. They want "women free to pursue getting the contraceptive of their choice," but not to force either individual physicians or religiously affiliated organizations or small private employers to do things that are against their beliefs.
Who might be affected?
For now, there is no definitive answer on how many women could potentially lose coverage of contraception as a result of the Supreme Court decision. Hobby Lobby has 500 stores and 13,000 employees. Conestoga Wood Specialties has 950 employees. There are 90-100 cases challenging the contraception requirement, with about half them from nonprofit organizations and half from for-profit companies. And there’s motivation to sue: Companies that do not comply with the ACA requirement face fines of $100 per day per enrollee.
According to the Dept. of Health & Human Services, about 48 million women are eligible for preventive services because they are in "non-grandfathered" health plans.
Millions of women receive family planning services outside of employer-sponsored insurance, and thus may not be directly affected. A Guttmacher Institute report shows that 19 million women need publicly funded services and supplies because they are low income or are assumed to have a low income because they are under age 20 years. Family planning services provided through federal and state government funding helped women avoid 2.2 million unintended pregnancies in 2010, according to Guttmacher.
Reversing the decision
A handful of Democrats in the House and Senate introduced legislation July 9 to reverse the Supreme Court’s decision. The Protect Women's Health From Corporate Interference Act, introduced by Sen. Patty Murray (D-Wash.), would eliminate the loophole given by the court to companies that are not religiously affiliated.
ACOG praised the effort and applauded the legislators "for recognizing that contraceptives and other essential health care services should not be treated differently than other forms of health care.
"We look forward to working with them and other Congressional leaders to ensure that the ability of physicians to provide care for women is no longer subject to outside interference," Dr. Jennings said in a statement.
The White House said in an official Statement of Administration Policy that it strongly supported the legislation, saying that it "would prevent owners of for-profit companies from asserting their personal religious views to deny their employees federally-required health benefits."
Senate Democrats attempted to bring the bill to the floor on July 16, but failed to secure enough votes for debate. Republican Senators in the meantime introduced their own legislation to guarantee that employers could not bar "access" to contraceptives. The Preserving Religious Freedom and a Woman’s Access to Contraception Act (S. 2605) was introduced by Sen. Kelly Ayotte (R-N.H.).
Neither of the bills are expected to gain much traction in either the Senate or the House.
ACOG has asked its members to report coverage problems to their regional chapters, Dr. Conry said.
The federal government also could pursue several methods of reinstating the coverage. It could give for-profit employers the same accommodation that it has given nonprofit religiously affiliated employers like schools or charities. If these employers self-attest that paying for birth control violates their beliefs, then the employer’s insurer pays for the contraception. For self-insured employers, the exemption is not as easily navigated, because they would have to pay for the coverage.
That accommodation is being challenged by at least 45 legal cases that argue filling out the form violates plaintiffs’ religious beliefs because it means they are facilitating access to birth control.
The government could also pay directly for contraception for women whose employers receive an exemption. Supreme Court Justice Samuel Alito suggested such a possibility in his majority opinion in the Hobby Lobby case.
Dr. Davis said that, ideally, gynecologists would be able to give patients the contraception of choice on the same day of a visit and that the physician would be reimbursed for the cost of the device plus a fee for placing it. And, physicians should be able to counsel patients without thought of potential oversight from an employer or anyone else.
But, she said, "We are very far away from that right now."
On Twitter @aliciaault
It may be months, even years before it’s clear whether access to and payment for contraception will be limited in the wake of the U.S. Supreme Court’s decision that for-profit companies can forego health insurance coverage of birth control methods that violate their religious belief.
At this point, only the two plaintiffs in the case – Hobby Lobby and Conestoga Wood Specialties – can change their health insurance coverage of contraception, based on the June 30 decision by the U.S. Supreme Court.
In the meantime, there is a lot of confusion among patients – and uncertainty among doctors -- about what the ruling means.
"We’re all scratching our heads and saying, ‘Now what?’ " said Dr. Anne Davis, medical director of Physicians for Reproductive Health. "All of us are very used to hurdles with coverage, but this is a new one."
Now, ob.gyns and other physicians may have to figure out if a patient works for an employer whose insurance will not cover certain types of contraception, or perhaps all birth control, said Dr. Davis of the department of clinical obstetrics and gynecology at Columbia University, New York.
The Supreme Court ruling is "a huge step backward," said Dr. Eve Espey, chair of obstetrics and gynecology at the University of New Mexico, Albuquerque. For physicians, a key concern is whether they will be paid for inserting an IUD or providing another service; for patients, it’s whether they can get the contraception and if they can afford it, she said.
When concerns mount, "those services tend to not happen," Dr. Espey said.
Ob.gyns. also expressed concern that the ruling had opened the door to interference in the physician-patient relationship.
Clinicians need the freedom to select the birth control method that makes the most sense for the patient, said Dr. Davis. "It’s going to be really unfortunate if you and the patient agree, and it’s the right thing and you find out that her employer has decided it’s not the right kind of birth control because of their beliefs," she said.
Dr. Jeanne Conry, immediate past president of the American Congress of Obstetricians and Gynecologists (ACOG), said that if she has decided that an IUD is best for a 34-year-old diabetic patient, but her employer says it won’t pay for that method, "that’s not in her best interest."
"They’ve just interfered with my best treatment for the patient," said Dr. Conry, an ob.gyn with Kaiser Permanente in Roseville, Calif.
Dr. Mitchell Creinin, chair of the obstetrics and gynecology department at University of California, Davis, said that the ruling could return contraception to a means-tested environment, in which only those who have the means will get the most effective methods, like the IUD. Instead, he said, it should be a benefit for all. Preventing pregnancy "is a public health issue and we’ve got to look at it that way," Dr. Creinin said.
Just after the ruling, ACOG President John Jennings agreed, expressing dismay that the Court was overlooking what had been firmly established. "The value of family planning – including contraception – has been clearly demonstrated," he said, in a statement.
But pro-life groups, including Americans United for Life and the Association of Pro-Life Obstetricians and Gynecologists, applauded the Hobby Lobby decision, saying that it helps preserve the right to practice according to conscience.
"Respect for the constitutionally guaranteed rights of conscience – whether held by business owners or medical professionals – must and should be protected," said Dr. Monique Chireau, AUL board member, with the department of ob.gyn at Duke University, Durham, N.C.
Dr. Gene Rudd, senior vice president for the Christian Medical and Dental Associations (CMDA), said that he did not think the decision would have any direct impact on ob.gyns.’ practices. However, he said, "Had the Justices allowed the government to force business owners to act against their conscience, that could establish a legal precedent by which the government might one day force physicians to act against their consciences."
The CMDA’s membership view the ACA’s mandate that women receive contraceptives free of charge as a potential infringement on their right to practice according to their beliefs, said Dr. Rudd, an ob.gyn.
In a CMDA survey, "95% of our members said they’d leave medicine before being required to violate their conscience," he said. They want "women free to pursue getting the contraceptive of their choice," but not to force either individual physicians or religiously affiliated organizations or small private employers to do things that are against their beliefs.
Who might be affected?
For now, there is no definitive answer on how many women could potentially lose coverage of contraception as a result of the Supreme Court decision. Hobby Lobby has 500 stores and 13,000 employees. Conestoga Wood Specialties has 950 employees. There are 90-100 cases challenging the contraception requirement, with about half them from nonprofit organizations and half from for-profit companies. And there’s motivation to sue: Companies that do not comply with the ACA requirement face fines of $100 per day per enrollee.
According to the Dept. of Health & Human Services, about 48 million women are eligible for preventive services because they are in "non-grandfathered" health plans.
Millions of women receive family planning services outside of employer-sponsored insurance, and thus may not be directly affected. A Guttmacher Institute report shows that 19 million women need publicly funded services and supplies because they are low income or are assumed to have a low income because they are under age 20 years. Family planning services provided through federal and state government funding helped women avoid 2.2 million unintended pregnancies in 2010, according to Guttmacher.
Reversing the decision
A handful of Democrats in the House and Senate introduced legislation July 9 to reverse the Supreme Court’s decision. The Protect Women's Health From Corporate Interference Act, introduced by Sen. Patty Murray (D-Wash.), would eliminate the loophole given by the court to companies that are not religiously affiliated.
ACOG praised the effort and applauded the legislators "for recognizing that contraceptives and other essential health care services should not be treated differently than other forms of health care.
"We look forward to working with them and other Congressional leaders to ensure that the ability of physicians to provide care for women is no longer subject to outside interference," Dr. Jennings said in a statement.
The White House said in an official Statement of Administration Policy that it strongly supported the legislation, saying that it "would prevent owners of for-profit companies from asserting their personal religious views to deny their employees federally-required health benefits."
Senate Democrats attempted to bring the bill to the floor on July 16, but failed to secure enough votes for debate. Republican Senators in the meantime introduced their own legislation to guarantee that employers could not bar "access" to contraceptives. The Preserving Religious Freedom and a Woman’s Access to Contraception Act (S. 2605) was introduced by Sen. Kelly Ayotte (R-N.H.).
Neither of the bills are expected to gain much traction in either the Senate or the House.
ACOG has asked its members to report coverage problems to their regional chapters, Dr. Conry said.
The federal government also could pursue several methods of reinstating the coverage. It could give for-profit employers the same accommodation that it has given nonprofit religiously affiliated employers like schools or charities. If these employers self-attest that paying for birth control violates their beliefs, then the employer’s insurer pays for the contraception. For self-insured employers, the exemption is not as easily navigated, because they would have to pay for the coverage.
That accommodation is being challenged by at least 45 legal cases that argue filling out the form violates plaintiffs’ religious beliefs because it means they are facilitating access to birth control.
The government could also pay directly for contraception for women whose employers receive an exemption. Supreme Court Justice Samuel Alito suggested such a possibility in his majority opinion in the Hobby Lobby case.
Dr. Davis said that, ideally, gynecologists would be able to give patients the contraception of choice on the same day of a visit and that the physician would be reimbursed for the cost of the device plus a fee for placing it. And, physicians should be able to counsel patients without thought of potential oversight from an employer or anyone else.
But, she said, "We are very far away from that right now."
On Twitter @aliciaault