User login
FDA Approves DNA Stool Test for CRC Screening; Medicare Considers Coverage
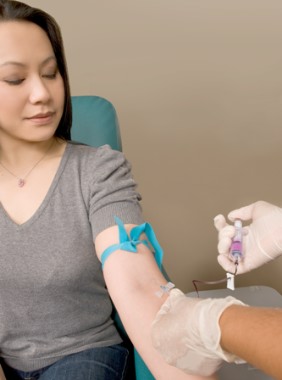
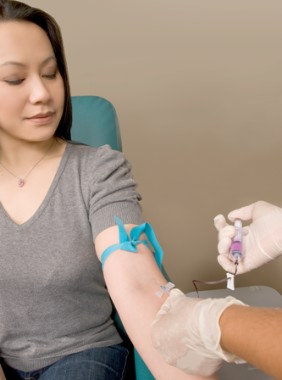
The Food and Drug Administration has approved another noninvasive alternative to colonoscopy for screening for colorectal cancer, this one a stool-based test that detects the presence of red blood cells and DNA mutations.
And the same day as the FDA approval, the Centers for Medicare & Medicaid Services said that it was starting to review the technology with an eye toward approving it for Medicare beneficiaries.
The Cologuard, made by Exact Sciences of Madison, Wisc., is prescribed by a physician. It is mailed to the patient, who collects a stool sample and sends it to Exact Sciences for analysis. The $599 diagnostic detects hemoglobin and certain mutations associated with colorectal cancer in the DNA of cells shed by advanced adenomas as stool moves through the large intestine and rectum. Results – which are simply positive or negative for precancerous polyps or cancer – are reported by the prescribing physician. Patients with positive results are advised to undergo a diagnostic colonoscopy.
The test is not a substitute for colonoscopy; it is an alternative to fecal occult testing, and in trials, the Cologuard "detected more cancers than a commonly used fecal occult test," said Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostics and Radiological Health at the FDA’s Center for Devices and Radiological Health, in a statement.
The FDA pointed out that approval does not change the currently recommended colorectal cancer screening protocols. Stool DNA testing is not recommended by the United States Preventive Services Task Force, for instance. Among other guidelines, the USPSTF recommends adults aged 50-75 years, at average risk for colon cancer, be screened using fecal occult blood testing, sigmoidoscopy, or colonoscopy.
"This approval offers patients and physicians another option to screen for colorectal cancer," said Dr. Gutierrez.
Dr. David A. Ahlquist, a Mayo Clinic gastroenterologist who was a coinventor of the test, said in a statement issued by Exact Sciences that the clinical studies had "proven that this noninvasive test is highly sensitive in detecting both early stage colorectal cancer and the most advanced precancerous polyps most likely to develop into cancer."
According to the FDA, in the 10,000-patient study, Cologuard accurately detected cancers and advanced adenomas more often than did the fecal occult test. Cologuard detected 92% of colorectal cancers and 42% of advanced adenomas; fecal occult screening detected 74% of cancers and 24% of advanced adenomas. Cologuard, however, was less accurate than fecal occult was at correctly identifying subjects negative for colorectal cancer or advanced adenomas.
For the first time ever, the CMS also proposed coverage of a product on the same day as it was approved, said Dr. Patrick Conway, chief medical officer and deputy administrator for innovation and quality at CMS, in a statement. The CMS analysis "will provide timely access for Medicare beneficiaries to an innovative screening test to help in the early detection of colorectal cancer," said Dr. Conway.
The agency is proposing to cover Cologuard once every 3 years for Medicare beneficiaries who are aged 50-85 years, asymptomatic (no signs or symptoms of colorectal disease including but not limited to lower gastrointestinal pain, blood in stool, positive guaiac fecal occult blood test or fecal immunochemical test), and are at average risk of developing colorectal cancer.
The CMS is accepting comments until Sept. 11, 2014. It did not give a timeline for when it would make a final coverage decision.
Dr. Ahlquist and the Mayo Clinic will share equity and royalties from Exact Sciences.
On Twitter @aliciaault
The Food and Drug Administration has approved another noninvasive alternative to colonoscopy for screening for colorectal cancer, this one a stool-based test that detects the presence of red blood cells and DNA mutations.
And the same day as the FDA approval, the Centers for Medicare & Medicaid Services said that it was starting to review the technology with an eye toward approving it for Medicare beneficiaries.
The Cologuard, made by Exact Sciences of Madison, Wisc., is prescribed by a physician. It is mailed to the patient, who collects a stool sample and sends it to Exact Sciences for analysis. The $599 diagnostic detects hemoglobin and certain mutations associated with colorectal cancer in the DNA of cells shed by advanced adenomas as stool moves through the large intestine and rectum. Results – which are simply positive or negative for precancerous polyps or cancer – are reported by the prescribing physician. Patients with positive results are advised to undergo a diagnostic colonoscopy.
The test is not a substitute for colonoscopy; it is an alternative to fecal occult testing, and in trials, the Cologuard "detected more cancers than a commonly used fecal occult test," said Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostics and Radiological Health at the FDA’s Center for Devices and Radiological Health, in a statement.
The FDA pointed out that approval does not change the currently recommended colorectal cancer screening protocols. Stool DNA testing is not recommended by the United States Preventive Services Task Force, for instance. Among other guidelines, the USPSTF recommends adults aged 50-75 years, at average risk for colon cancer, be screened using fecal occult blood testing, sigmoidoscopy, or colonoscopy.
"This approval offers patients and physicians another option to screen for colorectal cancer," said Dr. Gutierrez.
Dr. David A. Ahlquist, a Mayo Clinic gastroenterologist who was a coinventor of the test, said in a statement issued by Exact Sciences that the clinical studies had "proven that this noninvasive test is highly sensitive in detecting both early stage colorectal cancer and the most advanced precancerous polyps most likely to develop into cancer."
According to the FDA, in the 10,000-patient study, Cologuard accurately detected cancers and advanced adenomas more often than did the fecal occult test. Cologuard detected 92% of colorectal cancers and 42% of advanced adenomas; fecal occult screening detected 74% of cancers and 24% of advanced adenomas. Cologuard, however, was less accurate than fecal occult was at correctly identifying subjects negative for colorectal cancer or advanced adenomas.
For the first time ever, the CMS also proposed coverage of a product on the same day as it was approved, said Dr. Patrick Conway, chief medical officer and deputy administrator for innovation and quality at CMS, in a statement. The CMS analysis "will provide timely access for Medicare beneficiaries to an innovative screening test to help in the early detection of colorectal cancer," said Dr. Conway.
The agency is proposing to cover Cologuard once every 3 years for Medicare beneficiaries who are aged 50-85 years, asymptomatic (no signs or symptoms of colorectal disease including but not limited to lower gastrointestinal pain, blood in stool, positive guaiac fecal occult blood test or fecal immunochemical test), and are at average risk of developing colorectal cancer.
The CMS is accepting comments until Sept. 11, 2014. It did not give a timeline for when it would make a final coverage decision.
Dr. Ahlquist and the Mayo Clinic will share equity and royalties from Exact Sciences.
On Twitter @aliciaault
The Food and Drug Administration has approved another noninvasive alternative to colonoscopy for screening for colorectal cancer, this one a stool-based test that detects the presence of red blood cells and DNA mutations.
And the same day as the FDA approval, the Centers for Medicare & Medicaid Services said that it was starting to review the technology with an eye toward approving it for Medicare beneficiaries.
The Cologuard, made by Exact Sciences of Madison, Wisc., is prescribed by a physician. It is mailed to the patient, who collects a stool sample and sends it to Exact Sciences for analysis. The $599 diagnostic detects hemoglobin and certain mutations associated with colorectal cancer in the DNA of cells shed by advanced adenomas as stool moves through the large intestine and rectum. Results – which are simply positive or negative for precancerous polyps or cancer – are reported by the prescribing physician. Patients with positive results are advised to undergo a diagnostic colonoscopy.
The test is not a substitute for colonoscopy; it is an alternative to fecal occult testing, and in trials, the Cologuard "detected more cancers than a commonly used fecal occult test," said Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostics and Radiological Health at the FDA’s Center for Devices and Radiological Health, in a statement.
The FDA pointed out that approval does not change the currently recommended colorectal cancer screening protocols. Stool DNA testing is not recommended by the United States Preventive Services Task Force, for instance. Among other guidelines, the USPSTF recommends adults aged 50-75 years, at average risk for colon cancer, be screened using fecal occult blood testing, sigmoidoscopy, or colonoscopy.
"This approval offers patients and physicians another option to screen for colorectal cancer," said Dr. Gutierrez.
Dr. David A. Ahlquist, a Mayo Clinic gastroenterologist who was a coinventor of the test, said in a statement issued by Exact Sciences that the clinical studies had "proven that this noninvasive test is highly sensitive in detecting both early stage colorectal cancer and the most advanced precancerous polyps most likely to develop into cancer."
According to the FDA, in the 10,000-patient study, Cologuard accurately detected cancers and advanced adenomas more often than did the fecal occult test. Cologuard detected 92% of colorectal cancers and 42% of advanced adenomas; fecal occult screening detected 74% of cancers and 24% of advanced adenomas. Cologuard, however, was less accurate than fecal occult was at correctly identifying subjects negative for colorectal cancer or advanced adenomas.
For the first time ever, the CMS also proposed coverage of a product on the same day as it was approved, said Dr. Patrick Conway, chief medical officer and deputy administrator for innovation and quality at CMS, in a statement. The CMS analysis "will provide timely access for Medicare beneficiaries to an innovative screening test to help in the early detection of colorectal cancer," said Dr. Conway.
The agency is proposing to cover Cologuard once every 3 years for Medicare beneficiaries who are aged 50-85 years, asymptomatic (no signs or symptoms of colorectal disease including but not limited to lower gastrointestinal pain, blood in stool, positive guaiac fecal occult blood test or fecal immunochemical test), and are at average risk of developing colorectal cancer.
The CMS is accepting comments until Sept. 11, 2014. It did not give a timeline for when it would make a final coverage decision.
Dr. Ahlquist and the Mayo Clinic will share equity and royalties from Exact Sciences.
On Twitter @aliciaault
FDA okays DNA stool test for CRC screening; Medicare considers coverage
The Food and Drug Administration has approved another noninvasive alternative to colonoscopy for screening for colorectal cancer, this one a stool-based test that detects the presence of red blood cells and DNA mutations.
And the same day as the FDA approval, the Centers for Medicare & Medicaid Services said that it was starting to review the technology with an eye toward approving it for Medicare beneficiaries.
The Cologuard, made by Exact Sciences of Madison, Wisc., is prescribed by a physician. It is mailed to the patient, who collects a stool sample and sends it to Exact Sciences for analysis. The $599 diagnostic detects hemoglobin and certain mutations associated with colorectal cancer in the DNA of cells shed by advanced adenomas as stool moves through the large intestine and rectum. Results – which are simply positive or negative for precancerous polyps or cancer – are reported by the prescribing physician. Patients with positive results are advised to undergo a diagnostic colonoscopy.
The test is not a substitute for colonoscopy; it is an alternative to fecal occult testing, and in trials, the Cologuard "detected more cancers than a commonly used fecal occult test," said Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostics and Radiological Health at the FDA’s Center for Devices and Radiological Health, in a statement.
The FDA pointed out that approval does not change the currently recommended colorectal cancer screening protocols. Stool DNA testing is not recommended by the United States Preventive Services Task Force, for instance. Among other guidelines, the USPSTF recommends adults aged 50-75 years, at average risk for colon cancer, be screened using fecal occult blood testing, sigmoidoscopy, or colonoscopy.
"This approval offers patients and physicians another option to screen for colorectal cancer," said Dr. Gutierrez.
Dr. David A. Ahlquist, a Mayo Clinic gastroenterologist who was a coinventor of the test, said in a statement issued by Exact Sciences that the clinical studies had "proven that this noninvasive test is highly sensitive in detecting both early stage colorectal cancer and the most advanced precancerous polyps most likely to develop into cancer."
According to the FDA, in the 10,000-patient study, Cologuard accurately detected cancers and advanced adenomas more often than did the fecal occult test. Cologuard detected 92% of colorectal cancers and 42% of advanced adenomas; fecal occult screening detected 74% of cancers and 24% of advanced adenomas. Cologuard, however, was less accurate than fecal occult was at correctly identifying subjects negative for colorectal cancer or advanced adenomas.
For the first time ever, the CMS also proposed coverage of a product on the same day as it was approved, said Dr. Patrick Conway, chief medical officer and deputy administrator for innovation and quality at CMS, in a statement. The CMS analysis "will provide timely access for Medicare beneficiaries to an innovative screening test to help in the early detection of colorectal cancer," said Dr. Conway.
The agency is proposing to cover Cologuard once every 3 years for Medicare beneficiaries who are aged 50-85 years, asymptomatic (no signs or symptoms of colorectal disease including but not limited to lower gastrointestinal pain, blood in stool, positive guaiac fecal occult blood test or fecal immunochemical test), and are at average risk of developing colorectal cancer.
The CMS is accepting comments until Sept. 11, 2014. It did not give a timeline for when it would make a final coverage decision.
Dr. Ahlquist and the Mayo Clinic will share equity and royalties from Exact Sciences.
On Twitter @aliciaault
The Food and Drug Administration has approved another noninvasive alternative to colonoscopy for screening for colorectal cancer, this one a stool-based test that detects the presence of red blood cells and DNA mutations.
And the same day as the FDA approval, the Centers for Medicare & Medicaid Services said that it was starting to review the technology with an eye toward approving it for Medicare beneficiaries.
The Cologuard, made by Exact Sciences of Madison, Wisc., is prescribed by a physician. It is mailed to the patient, who collects a stool sample and sends it to Exact Sciences for analysis. The $599 diagnostic detects hemoglobin and certain mutations associated with colorectal cancer in the DNA of cells shed by advanced adenomas as stool moves through the large intestine and rectum. Results – which are simply positive or negative for precancerous polyps or cancer – are reported by the prescribing physician. Patients with positive results are advised to undergo a diagnostic colonoscopy.
The test is not a substitute for colonoscopy; it is an alternative to fecal occult testing, and in trials, the Cologuard "detected more cancers than a commonly used fecal occult test," said Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostics and Radiological Health at the FDA’s Center for Devices and Radiological Health, in a statement.
The FDA pointed out that approval does not change the currently recommended colorectal cancer screening protocols. Stool DNA testing is not recommended by the United States Preventive Services Task Force, for instance. Among other guidelines, the USPSTF recommends adults aged 50-75 years, at average risk for colon cancer, be screened using fecal occult blood testing, sigmoidoscopy, or colonoscopy.
"This approval offers patients and physicians another option to screen for colorectal cancer," said Dr. Gutierrez.
Dr. David A. Ahlquist, a Mayo Clinic gastroenterologist who was a coinventor of the test, said in a statement issued by Exact Sciences that the clinical studies had "proven that this noninvasive test is highly sensitive in detecting both early stage colorectal cancer and the most advanced precancerous polyps most likely to develop into cancer."
According to the FDA, in the 10,000-patient study, Cologuard accurately detected cancers and advanced adenomas more often than did the fecal occult test. Cologuard detected 92% of colorectal cancers and 42% of advanced adenomas; fecal occult screening detected 74% of cancers and 24% of advanced adenomas. Cologuard, however, was less accurate than fecal occult was at correctly identifying subjects negative for colorectal cancer or advanced adenomas.
For the first time ever, the CMS also proposed coverage of a product on the same day as it was approved, said Dr. Patrick Conway, chief medical officer and deputy administrator for innovation and quality at CMS, in a statement. The CMS analysis "will provide timely access for Medicare beneficiaries to an innovative screening test to help in the early detection of colorectal cancer," said Dr. Conway.
The agency is proposing to cover Cologuard once every 3 years for Medicare beneficiaries who are aged 50-85 years, asymptomatic (no signs or symptoms of colorectal disease including but not limited to lower gastrointestinal pain, blood in stool, positive guaiac fecal occult blood test or fecal immunochemical test), and are at average risk of developing colorectal cancer.
The CMS is accepting comments until Sept. 11, 2014. It did not give a timeline for when it would make a final coverage decision.
Dr. Ahlquist and the Mayo Clinic will share equity and royalties from Exact Sciences.
On Twitter @aliciaault
The Food and Drug Administration has approved another noninvasive alternative to colonoscopy for screening for colorectal cancer, this one a stool-based test that detects the presence of red blood cells and DNA mutations.
And the same day as the FDA approval, the Centers for Medicare & Medicaid Services said that it was starting to review the technology with an eye toward approving it for Medicare beneficiaries.
The Cologuard, made by Exact Sciences of Madison, Wisc., is prescribed by a physician. It is mailed to the patient, who collects a stool sample and sends it to Exact Sciences for analysis. The $599 diagnostic detects hemoglobin and certain mutations associated with colorectal cancer in the DNA of cells shed by advanced adenomas as stool moves through the large intestine and rectum. Results – which are simply positive or negative for precancerous polyps or cancer – are reported by the prescribing physician. Patients with positive results are advised to undergo a diagnostic colonoscopy.
The test is not a substitute for colonoscopy; it is an alternative to fecal occult testing, and in trials, the Cologuard "detected more cancers than a commonly used fecal occult test," said Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostics and Radiological Health at the FDA’s Center for Devices and Radiological Health, in a statement.
The FDA pointed out that approval does not change the currently recommended colorectal cancer screening protocols. Stool DNA testing is not recommended by the United States Preventive Services Task Force, for instance. Among other guidelines, the USPSTF recommends adults aged 50-75 years, at average risk for colon cancer, be screened using fecal occult blood testing, sigmoidoscopy, or colonoscopy.
"This approval offers patients and physicians another option to screen for colorectal cancer," said Dr. Gutierrez.
Dr. David A. Ahlquist, a Mayo Clinic gastroenterologist who was a coinventor of the test, said in a statement issued by Exact Sciences that the clinical studies had "proven that this noninvasive test is highly sensitive in detecting both early stage colorectal cancer and the most advanced precancerous polyps most likely to develop into cancer."
According to the FDA, in the 10,000-patient study, Cologuard accurately detected cancers and advanced adenomas more often than did the fecal occult test. Cologuard detected 92% of colorectal cancers and 42% of advanced adenomas; fecal occult screening detected 74% of cancers and 24% of advanced adenomas. Cologuard, however, was less accurate than fecal occult was at correctly identifying subjects negative for colorectal cancer or advanced adenomas.
For the first time ever, the CMS also proposed coverage of a product on the same day as it was approved, said Dr. Patrick Conway, chief medical officer and deputy administrator for innovation and quality at CMS, in a statement. The CMS analysis "will provide timely access for Medicare beneficiaries to an innovative screening test to help in the early detection of colorectal cancer," said Dr. Conway.
The agency is proposing to cover Cologuard once every 3 years for Medicare beneficiaries who are aged 50-85 years, asymptomatic (no signs or symptoms of colorectal disease including but not limited to lower gastrointestinal pain, blood in stool, positive guaiac fecal occult blood test or fecal immunochemical test), and are at average risk of developing colorectal cancer.
The CMS is accepting comments until Sept. 11, 2014. It did not give a timeline for when it would make a final coverage decision.
Dr. Ahlquist and the Mayo Clinic will share equity and royalties from Exact Sciences.
On Twitter @aliciaault
FDA approves canagliflozin/metformin combination
The Food and Drug Administration has approved Invokamet, a fixed-dose combination of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, and metformin, for type 2 diabetes.
Janssen Pharmaceuticals, which will market Invokamet in the United States, says that it’s the first such combination approved by the FDA. Specifically, the indication is "as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus who are not adequately controlled by treatment that includes either canagliflozin or metformin, or who are already being treated with both canagliflozin and metformin as separate medications."
The drug will be available in four dosage combinations containing canagliflozin 50 mg or 150 mg and metformin 500 mg or 1000 mg. The recommended dose is twice daily.
Canagliflozin was originally approved by the FDA in March 2013. During three phase III trials of canagliflozin plus metformin, the combination "lowered blood sugar and, in pre-specified secondary endpoints, was associated with significant reductions in body weight and systolic blood pressure," according to the statement. The most common adverse events were female genital mycotic infections, urinary tract infections, and increased urination. The most common adverse reactions with metformin were diarrhea, nausea/vomiting, flatulence, asthenia, indigestion, abdominal discomfort, and headache. Canagliflozin can increase the risk of hypoglycemia when combined with insulin or a sulfonylurea. The company says that lower doses of those therapies may be required to minimize the risk of hypoglycemia when used in combination with Invokamet.
There is also a boxed warning on the potential for lactic acidosis.
On Twitter @aliciaault
The Food and Drug Administration has approved Invokamet, a fixed-dose combination of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, and metformin, for type 2 diabetes.
Janssen Pharmaceuticals, which will market Invokamet in the United States, says that it’s the first such combination approved by the FDA. Specifically, the indication is "as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus who are not adequately controlled by treatment that includes either canagliflozin or metformin, or who are already being treated with both canagliflozin and metformin as separate medications."
The drug will be available in four dosage combinations containing canagliflozin 50 mg or 150 mg and metformin 500 mg or 1000 mg. The recommended dose is twice daily.
Canagliflozin was originally approved by the FDA in March 2013. During three phase III trials of canagliflozin plus metformin, the combination "lowered blood sugar and, in pre-specified secondary endpoints, was associated with significant reductions in body weight and systolic blood pressure," according to the statement. The most common adverse events were female genital mycotic infections, urinary tract infections, and increased urination. The most common adverse reactions with metformin were diarrhea, nausea/vomiting, flatulence, asthenia, indigestion, abdominal discomfort, and headache. Canagliflozin can increase the risk of hypoglycemia when combined with insulin or a sulfonylurea. The company says that lower doses of those therapies may be required to minimize the risk of hypoglycemia when used in combination with Invokamet.
There is also a boxed warning on the potential for lactic acidosis.
On Twitter @aliciaault
The Food and Drug Administration has approved Invokamet, a fixed-dose combination of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, and metformin, for type 2 diabetes.
Janssen Pharmaceuticals, which will market Invokamet in the United States, says that it’s the first such combination approved by the FDA. Specifically, the indication is "as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus who are not adequately controlled by treatment that includes either canagliflozin or metformin, or who are already being treated with both canagliflozin and metformin as separate medications."
The drug will be available in four dosage combinations containing canagliflozin 50 mg or 150 mg and metformin 500 mg or 1000 mg. The recommended dose is twice daily.
Canagliflozin was originally approved by the FDA in March 2013. During three phase III trials of canagliflozin plus metformin, the combination "lowered blood sugar and, in pre-specified secondary endpoints, was associated with significant reductions in body weight and systolic blood pressure," according to the statement. The most common adverse events were female genital mycotic infections, urinary tract infections, and increased urination. The most common adverse reactions with metformin were diarrhea, nausea/vomiting, flatulence, asthenia, indigestion, abdominal discomfort, and headache. Canagliflozin can increase the risk of hypoglycemia when combined with insulin or a sulfonylurea. The company says that lower doses of those therapies may be required to minimize the risk of hypoglycemia when used in combination with Invokamet.
There is also a boxed warning on the potential for lactic acidosis.
On Twitter @aliciaault
Medicare will cover TMVR, with conditions
As expected, the Centers for Medicare & Medicaid Services says it will cover transcatheter mitral valve repair procedures, subject to conditions.
The final decision, issued Aug. 7, came not long after the agency proposed in May to pay for the procedure under its coverage with evidence development policy.
Despite the conditions, the decision opens up a potentially wide universe of people who could receive transcatheter mitral valve repair (TMVR).
According to the CMS, mitral regurgitation is the most common type of heart valve insufficiency in the United States. In 2000, 2-3 million Americans had the condition, and that number is expected to double by 2030. The standard treatment is open surgical repair, but there are many people who are not suitable candidates.
Only one TMVR device is currently approved by the Food and Drug Administration – Abbott Vascular’s MitraClip, approved in October 2013 – but others are in development.
The request to consider coverage of TMVR was initiated by the Society of Thoracic Surgeons, the American College of Cardiology Foundation, the Society for Cardiovascular Angiography and Interventions, and the American Association for Thoracic Surgery.
The agency said TMVR would now be covered for the treatment of significant symptomatic degenerative mitral regurgitation, when furnished according to an FDA-approved indication and when certain conditions are met.
Among those:
• TMVR must be performed by an interventional cardiologist or a cardiothoracic surgeon.
• Both a cardiothoracic surgeon experienced in mitral valve surgery and a cardiologist experienced in mitral valve disease have independently examined the patient face to face, evaluated the patient’s suitability for mitral valve surgery, and made a determination of prohibitive risk, and both physicians have documented the rationale for their clinical judgment, and the rationale is available to the heart team.
• The patient (preoperatively and postoperatively) is under the care of a heart team: a cohesive, multidisciplinary team of medical professionals.
• The procedure has to be done in a hospital with specific infrastructure, including a valvular heart disease surgical program, a cardiac catheterization lab, noninvasive imaging expertise, an intensive care facility with personnel who have experience with patients who have undergone open-heart valve procedures, and space to accommodate cases that might have complications.
The agency also imposed volume requirements. The surgical program must perform at least 25 mitral valve surgical procedures for severe mitral regurgitation annually, 10 of which must be mitral valve repairs. The interventional cardiology program has to perform more than 1,000 catheterizations per year.
The heart team and hospital also have to participate in a prospective, national audited registry that accepts all manufactured devices, follows the patient for at least 1 year, and tracks all-cause mortality, stroke, repeat mitral valve surgery or other mitral procedures, worsening mitral regurgitation, transient ischemic events, major vascular events, renal complications, functional capacity, and, quality of life.
On Twitter @aliciaault
As expected, the Centers for Medicare & Medicaid Services says it will cover transcatheter mitral valve repair procedures, subject to conditions.
The final decision, issued Aug. 7, came not long after the agency proposed in May to pay for the procedure under its coverage with evidence development policy.
Despite the conditions, the decision opens up a potentially wide universe of people who could receive transcatheter mitral valve repair (TMVR).
According to the CMS, mitral regurgitation is the most common type of heart valve insufficiency in the United States. In 2000, 2-3 million Americans had the condition, and that number is expected to double by 2030. The standard treatment is open surgical repair, but there are many people who are not suitable candidates.
Only one TMVR device is currently approved by the Food and Drug Administration – Abbott Vascular’s MitraClip, approved in October 2013 – but others are in development.
The request to consider coverage of TMVR was initiated by the Society of Thoracic Surgeons, the American College of Cardiology Foundation, the Society for Cardiovascular Angiography and Interventions, and the American Association for Thoracic Surgery.
The agency said TMVR would now be covered for the treatment of significant symptomatic degenerative mitral regurgitation, when furnished according to an FDA-approved indication and when certain conditions are met.
Among those:
• TMVR must be performed by an interventional cardiologist or a cardiothoracic surgeon.
• Both a cardiothoracic surgeon experienced in mitral valve surgery and a cardiologist experienced in mitral valve disease have independently examined the patient face to face, evaluated the patient’s suitability for mitral valve surgery, and made a determination of prohibitive risk, and both physicians have documented the rationale for their clinical judgment, and the rationale is available to the heart team.
• The patient (preoperatively and postoperatively) is under the care of a heart team: a cohesive, multidisciplinary team of medical professionals.
• The procedure has to be done in a hospital with specific infrastructure, including a valvular heart disease surgical program, a cardiac catheterization lab, noninvasive imaging expertise, an intensive care facility with personnel who have experience with patients who have undergone open-heart valve procedures, and space to accommodate cases that might have complications.
The agency also imposed volume requirements. The surgical program must perform at least 25 mitral valve surgical procedures for severe mitral regurgitation annually, 10 of which must be mitral valve repairs. The interventional cardiology program has to perform more than 1,000 catheterizations per year.
The heart team and hospital also have to participate in a prospective, national audited registry that accepts all manufactured devices, follows the patient for at least 1 year, and tracks all-cause mortality, stroke, repeat mitral valve surgery or other mitral procedures, worsening mitral regurgitation, transient ischemic events, major vascular events, renal complications, functional capacity, and, quality of life.
On Twitter @aliciaault
As expected, the Centers for Medicare & Medicaid Services says it will cover transcatheter mitral valve repair procedures, subject to conditions.
The final decision, issued Aug. 7, came not long after the agency proposed in May to pay for the procedure under its coverage with evidence development policy.
Despite the conditions, the decision opens up a potentially wide universe of people who could receive transcatheter mitral valve repair (TMVR).
According to the CMS, mitral regurgitation is the most common type of heart valve insufficiency in the United States. In 2000, 2-3 million Americans had the condition, and that number is expected to double by 2030. The standard treatment is open surgical repair, but there are many people who are not suitable candidates.
Only one TMVR device is currently approved by the Food and Drug Administration – Abbott Vascular’s MitraClip, approved in October 2013 – but others are in development.
The request to consider coverage of TMVR was initiated by the Society of Thoracic Surgeons, the American College of Cardiology Foundation, the Society for Cardiovascular Angiography and Interventions, and the American Association for Thoracic Surgery.
The agency said TMVR would now be covered for the treatment of significant symptomatic degenerative mitral regurgitation, when furnished according to an FDA-approved indication and when certain conditions are met.
Among those:
• TMVR must be performed by an interventional cardiologist or a cardiothoracic surgeon.
• Both a cardiothoracic surgeon experienced in mitral valve surgery and a cardiologist experienced in mitral valve disease have independently examined the patient face to face, evaluated the patient’s suitability for mitral valve surgery, and made a determination of prohibitive risk, and both physicians have documented the rationale for their clinical judgment, and the rationale is available to the heart team.
• The patient (preoperatively and postoperatively) is under the care of a heart team: a cohesive, multidisciplinary team of medical professionals.
• The procedure has to be done in a hospital with specific infrastructure, including a valvular heart disease surgical program, a cardiac catheterization lab, noninvasive imaging expertise, an intensive care facility with personnel who have experience with patients who have undergone open-heart valve procedures, and space to accommodate cases that might have complications.
The agency also imposed volume requirements. The surgical program must perform at least 25 mitral valve surgical procedures for severe mitral regurgitation annually, 10 of which must be mitral valve repairs. The interventional cardiology program has to perform more than 1,000 catheterizations per year.
The heart team and hospital also have to participate in a prospective, national audited registry that accepts all manufactured devices, follows the patient for at least 1 year, and tracks all-cause mortality, stroke, repeat mitral valve surgery or other mitral procedures, worsening mitral regurgitation, transient ischemic events, major vascular events, renal complications, functional capacity, and, quality of life.
On Twitter @aliciaault
VA health care reform signed into law
President Obama has signed a bill that promises to give veterans faster access to medical care, in part by reimbursing services given by physicians and hospitals outside the Veterans Affairs health care system.
The Veterans Access, Choice and Accountability Act of 2014 makes $10 billion available through the "Veterans Choice Fund." The money is available until the funds are spent or until August 2017, whichever comes first.
Under the act, the VA is required to authorize non-VA care for any veteran who is enrolled in the VA system as of Aug. 1 or who is a newly discharged combat veteran if they can’t get an appointment at a VA medical facility within 30 days, or if they live more than 40 miles from the nearest VA medical facility.
The law also will take a closer look at care delivered within the VA, by requiring an independent assessment and establishing a congressional commission that will evaluate access to care throughout the system. It also provides $5 billion to hire new physicians and other medical staff to help increase access to care.
"This will not and cannot be the end of our effort," said President Obama at a signing ceremony Aug. 7 at Ft. Belvoir, Va. He also said that change would not happen overnight. "Implementing this law will take time," said the president.
Physician groups praised the legislation when lawmakers reached a compromise on the act, leading to passage by the House July 30, followed by the Senate July 31.
The American Medical Association supported the bill "because it is an important step to connecting veterans with physicians who can help them right now," AMA President Robert M. Wah said in a statement.
There are a number of reasons to be pleased with the law, added Dr. Reid Blackwelder, president of the American Academy of Family Physicians. Payment will be negotiated at rates not to exceed Medicare, except in very rural areas, he noted in a statement. The law also establishes 1,500 new residency slots in the VA system, with priority given to primary care and mental health.
"While the challenges associated with the current and future physician workforce within the VA will take time to fully resolve, this legislation will allow America’s civilian physicians to fill the void right away, ensuring that those who have served their country will no longer have to wait for primary care," said Dr. Blackwelder.
The American Psychiatric Association also applauded the increase in residency slots, and it welcomed the news that the VA will be able to prioritize hiring each year based on its identification of the five biggest staffing shortage areas. "In 2012, the VA Office of the Inspector General identified recruiting and retaining psychiatrists as the VA’s greatest challenge in the mental health area," said APA President Paul Summergrad in a statement.
"This legislation puts in place several actions to directly address that shortage, and will result in many more veterans having timely access to needed psychiatric services," he said.
The two congressmen who helped shepherd the legislation through the House and Senate also said they were gratified.
"I am pleased President Obama has finally recognized what we have been telling administration officials for years: that VA’s widespread and systemic lack of accountability is jeopardizing the health of veterans and contributing to all of the department’s most pressing problems," said Rep. Jeff Miller (R-Fla.), chairman of the House Committee on Veterans Affairs, in a statement.
"To prevent history from repeating itself, President Obama must become personally involved in solving VA’s many problems," Rep. Miller said. "A good place for him to start would be to meet with family members and veterans who have been struck by the VA scandal, order the department to cooperate with the congressional committees investigating VA, and force [the Department of Defense] and VA to work together to establish a joint electronic health record integrated across all DoD and VA components."
"In a dysfunctional Congress, I’m glad we accomplished something significant for veterans," Sen. Bernie Sanders (I-Vt.) said in a statement. This legislation will go a long way toward ending unacceptably long waiting times for veterans to access health care, and allow the VA the resources to hire the doctors, nurses, and other medical staff it needs to address these problems over the long term."
On Twitter @aliciaault
President Obama has signed a bill that promises to give veterans faster access to medical care, in part by reimbursing services given by physicians and hospitals outside the Veterans Affairs health care system.
The Veterans Access, Choice and Accountability Act of 2014 makes $10 billion available through the "Veterans Choice Fund." The money is available until the funds are spent or until August 2017, whichever comes first.
Under the act, the VA is required to authorize non-VA care for any veteran who is enrolled in the VA system as of Aug. 1 or who is a newly discharged combat veteran if they can’t get an appointment at a VA medical facility within 30 days, or if they live more than 40 miles from the nearest VA medical facility.
The law also will take a closer look at care delivered within the VA, by requiring an independent assessment and establishing a congressional commission that will evaluate access to care throughout the system. It also provides $5 billion to hire new physicians and other medical staff to help increase access to care.
"This will not and cannot be the end of our effort," said President Obama at a signing ceremony Aug. 7 at Ft. Belvoir, Va. He also said that change would not happen overnight. "Implementing this law will take time," said the president.
Physician groups praised the legislation when lawmakers reached a compromise on the act, leading to passage by the House July 30, followed by the Senate July 31.
The American Medical Association supported the bill "because it is an important step to connecting veterans with physicians who can help them right now," AMA President Robert M. Wah said in a statement.
There are a number of reasons to be pleased with the law, added Dr. Reid Blackwelder, president of the American Academy of Family Physicians. Payment will be negotiated at rates not to exceed Medicare, except in very rural areas, he noted in a statement. The law also establishes 1,500 new residency slots in the VA system, with priority given to primary care and mental health.
"While the challenges associated with the current and future physician workforce within the VA will take time to fully resolve, this legislation will allow America’s civilian physicians to fill the void right away, ensuring that those who have served their country will no longer have to wait for primary care," said Dr. Blackwelder.
The American Psychiatric Association also applauded the increase in residency slots, and it welcomed the news that the VA will be able to prioritize hiring each year based on its identification of the five biggest staffing shortage areas. "In 2012, the VA Office of the Inspector General identified recruiting and retaining psychiatrists as the VA’s greatest challenge in the mental health area," said APA President Paul Summergrad in a statement.
"This legislation puts in place several actions to directly address that shortage, and will result in many more veterans having timely access to needed psychiatric services," he said.
The two congressmen who helped shepherd the legislation through the House and Senate also said they were gratified.
"I am pleased President Obama has finally recognized what we have been telling administration officials for years: that VA’s widespread and systemic lack of accountability is jeopardizing the health of veterans and contributing to all of the department’s most pressing problems," said Rep. Jeff Miller (R-Fla.), chairman of the House Committee on Veterans Affairs, in a statement.
"To prevent history from repeating itself, President Obama must become personally involved in solving VA’s many problems," Rep. Miller said. "A good place for him to start would be to meet with family members and veterans who have been struck by the VA scandal, order the department to cooperate with the congressional committees investigating VA, and force [the Department of Defense] and VA to work together to establish a joint electronic health record integrated across all DoD and VA components."
"In a dysfunctional Congress, I’m glad we accomplished something significant for veterans," Sen. Bernie Sanders (I-Vt.) said in a statement. This legislation will go a long way toward ending unacceptably long waiting times for veterans to access health care, and allow the VA the resources to hire the doctors, nurses, and other medical staff it needs to address these problems over the long term."
On Twitter @aliciaault
President Obama has signed a bill that promises to give veterans faster access to medical care, in part by reimbursing services given by physicians and hospitals outside the Veterans Affairs health care system.
The Veterans Access, Choice and Accountability Act of 2014 makes $10 billion available through the "Veterans Choice Fund." The money is available until the funds are spent or until August 2017, whichever comes first.
Under the act, the VA is required to authorize non-VA care for any veteran who is enrolled in the VA system as of Aug. 1 or who is a newly discharged combat veteran if they can’t get an appointment at a VA medical facility within 30 days, or if they live more than 40 miles from the nearest VA medical facility.
The law also will take a closer look at care delivered within the VA, by requiring an independent assessment and establishing a congressional commission that will evaluate access to care throughout the system. It also provides $5 billion to hire new physicians and other medical staff to help increase access to care.
"This will not and cannot be the end of our effort," said President Obama at a signing ceremony Aug. 7 at Ft. Belvoir, Va. He also said that change would not happen overnight. "Implementing this law will take time," said the president.
Physician groups praised the legislation when lawmakers reached a compromise on the act, leading to passage by the House July 30, followed by the Senate July 31.
The American Medical Association supported the bill "because it is an important step to connecting veterans with physicians who can help them right now," AMA President Robert M. Wah said in a statement.
There are a number of reasons to be pleased with the law, added Dr. Reid Blackwelder, president of the American Academy of Family Physicians. Payment will be negotiated at rates not to exceed Medicare, except in very rural areas, he noted in a statement. The law also establishes 1,500 new residency slots in the VA system, with priority given to primary care and mental health.
"While the challenges associated with the current and future physician workforce within the VA will take time to fully resolve, this legislation will allow America’s civilian physicians to fill the void right away, ensuring that those who have served their country will no longer have to wait for primary care," said Dr. Blackwelder.
The American Psychiatric Association also applauded the increase in residency slots, and it welcomed the news that the VA will be able to prioritize hiring each year based on its identification of the five biggest staffing shortage areas. "In 2012, the VA Office of the Inspector General identified recruiting and retaining psychiatrists as the VA’s greatest challenge in the mental health area," said APA President Paul Summergrad in a statement.
"This legislation puts in place several actions to directly address that shortage, and will result in many more veterans having timely access to needed psychiatric services," he said.
The two congressmen who helped shepherd the legislation through the House and Senate also said they were gratified.
"I am pleased President Obama has finally recognized what we have been telling administration officials for years: that VA’s widespread and systemic lack of accountability is jeopardizing the health of veterans and contributing to all of the department’s most pressing problems," said Rep. Jeff Miller (R-Fla.), chairman of the House Committee on Veterans Affairs, in a statement.
"To prevent history from repeating itself, President Obama must become personally involved in solving VA’s many problems," Rep. Miller said. "A good place for him to start would be to meet with family members and veterans who have been struck by the VA scandal, order the department to cooperate with the congressional committees investigating VA, and force [the Department of Defense] and VA to work together to establish a joint electronic health record integrated across all DoD and VA components."
"In a dysfunctional Congress, I’m glad we accomplished something significant for veterans," Sen. Bernie Sanders (I-Vt.) said in a statement. This legislation will go a long way toward ending unacceptably long waiting times for veterans to access health care, and allow the VA the resources to hire the doctors, nurses, and other medical staff it needs to address these problems over the long term."
On Twitter @aliciaault
ABIM’s changes to MOC barely quell unrest
The American Board of Internal Medicine is making several changes to its maintenance of certification requirements, but, so far, it is not enough to quell the still-growing tide of anger and resentment against the process.
The ABIM announced the changes just ahead of a July 15 "summit" with 26 specialty societies that receive certification through the organization, and outlined them in a detailed letter that was sent to internal medicine diplomates on July 28. That correspondence also responded in detail to a May 7 letter sent by the American College of Physicians (ACP) on behalf of 14 medical societies, enumerating their concerns about the process.
The Endocrine Society, which attended the July 15 meeting, said that it left with many of the concerns it came in with. In a statement issued shortly thereafter, the Society called on the ABIM to suspend the maintenance of certification (MOC) requirements until it conducted "a formal analysis of all possible unintended consequences of the new MOC requirements."
Among the unintended consequences it says might result: If endocrinologists have to spend more time on MOC, they might spend less time with patients or on research, or they may even leave the field all together. That will put a dent in access to care when there is an increasing need, according to a letter from the Society to the ABIM in early June.
The American Association of Clinical Endocrinologists (AACE) also warned ABIM that by "monopolizing physician time," MOC could end up harming patient care. The group also said that many endocrinologists who previously did not have to recertify (so-called "grandfathers") might leave practice rather than go through the current process. In a letter to ABIM in late June, AACE also called on the Board to suspend the new requirements until there was a better understanding of how it might affect the endocrinology workforce. The ABIM should also "refrain from publicly reporting that a physician does not meet the MOC requirements," said Dr. R. Mack Harrell, AACE’s president, in that letter.
Meanwhile, 3,000 or so physicians have signed on to a "Pledge of Non-Compliance" with the ABIM’s requirements. The pledge was organized by Dr. Paul Teirstein, chief of cardiology and director of interventional cardiology at the Scripps Clinic, La Jolla, Calif., and his colleagues who have formed Physicians for Certification Change, an anti-MOC organization. Dr. Teirstein also launched a petition drive in March to overhaul the MOC process. That petition had about 18,000 signees at press time.
There are several other organizations hoping to derail or significantly change the ABIM’s requirements, including the Association of American Physicians and Surgeons, Change Board Recertification, and Docs4PatientCare.
In addition, many of the 26 professional societies whose members are certified by the ABIM have also been very organized in presenting their discontent.
The ABIM understands the frustration, said Dr. Richard Baron, ABIM president and CEO, in an interview. "One of the things that people have said is that we’re not listening," he said. That is one reason why the ABIM decided to hold what he called a "listening session" on July 15. "We’re a learning organization," he added, noting that the ABIM will "continue to evolve the program," based on the feedback it receives from individuals and from professional societies, among others.
Dr. Baron also acknowledges that by presenting an entirely new MOC process this year, "our timing was not propitious," given the many challenges physicians now face. The ABIM outlined those pressures in the July 28 letter, counting among them "dealing with the Affordable Care Act," fulfilling meaningful use requirements, and responding to changes in payment and practice models, including team-based care.
Dr. Steven E. Weinberger, executive vice president and CEO of the ACP agrees that the ABIM’s timing was pretty poor. He said that there had been a "low to medium level of concern" about MOC for years, but the new requirements announced in January catalyzed the complaints and were "an important tipping point." Added Dr. Weinberger, "To some extent, this was in part the straw that broke the camel’s back."
The ABIM is making a handful of changes to address some of the concerns, including "broadening the kinds of educational activities that can count for self-assessment of knowledge (Part 2)," said Dr. Baron. That is, the ABIM will be broadening the continuing medical education that will count toward the MOC requirements.
There have also been a huge number of complaints about the self-evaluation of practice assessment. There will be less direct data collection by the ABIM and more of a focus "on improvement activities doctors may already be doing in their practice," Dr. Baron said.
Among other things, ABIM is also looking at changes to the secure exam and whether it needs to change how it describes on its website whether physicians are meeting MOC requirements.
Critics are still not satisfied.
"This is nothing more than offering band-aids for gaping wounds," said Dr. Jonathan Weiss, an internist in Middletown, N.Y. "This is the ABIM trying to throw some bones to quiet down the rabble, given the unexpectedly strong pushback they are trying to deal with," he said, adding that he believes that the current process "is too flawed to be fixed."
Dr. Ron Benbassat, an internist in Beverly Hills, Calif., and a founder of Change Board Certification, goes even further. "No one is drinking the ABIM’s Kool-Aid," he said. "We’re on the right side of the truth here, and they know it," he said, adding, "The momentum is increasing and I believe we’re reaching the tipping point. As to what form it will take – widespread noncompliance or political or legal – I don’t have the answer. But we’re getting there."
Dr. Teirstein was a bit more muted in his criticism. "They are certainly taking many good steps. But, the devil will be in the details." He, like many physicians, said that he still sees the ABIM’s fees as a big problem. "To reestablish credibility, ABIM will need to roll back its fee schedule," said Dr. Teirstein.
The ABIM charges $1,940 for a 10-year basic internal medicine certification, which includes a secure examination for each specialty the diplomate chooses to maintain, access to all ABIM self-evaluation products, and any CME credit a physician can claim through completion of an ABIM module. There are basically four requirements: MOC participants take a secure exam; they have to complete a self-assessment of knowledge; they have to assess their practice by applying quality measurement and using the resulting data to improve their practice; and they must assess patient safety and/or include the "patient voice," which could, for instance, consist of a survey of patient satisfaction.
The fee is $2,060 for a focused practice in hospital medicine for 10 years, and $2,560 for any subspecialty for the 10 years.
But it’s more than just a financial cost, say physicians. There’s also an opportunity cost, said Dr. Eric Green, chair of the MOC Task Force at the Society of General Internal Medicine. "When you are doing MOC, what are you not doing?" he asks.
"It’s about a process that seems arbitrary, a little bit burdensome, and perhaps not achieving what we all want, which is the best care for our patients," said Dr. Christopher White, professor and chairman of medicine, the Ochsner Medical Center, New Orleans, in an interview.
Dr. White, who is a founding member of Physicians for Certification Change, has signed the Pledge of Non-Compliance. He is currently certified, and at age 63, wonders if it’s worth the time and effort to recertify in 2019, when he is next due. "It is a good idea that we continually educate physicians and that physicians have continuous improvement," said Dr. White, but he said he questions whether the ABIM process is the best way. "There’s no evidence that this works, or there’s value," he said.
The American College of Cardiology has offered to partner with the ABIM in getting some answers to the evidence question, said ACC President Patrick O’Gara in an interview. "I’m hopeful that our offer to partner to do research will be taken seriously," he said. Although more evidence will "go a long way toward answering this question about relevance," it would be a long-term project, said Dr. O’Gara, who is also director of clinical cardiology at Brigham and Women’s Hospital, Boston.
The ABIM said in its July 28 letter that extensive evidence does exist – with more than half of the studies coming from non-ABIM researchers – but that maybe it hasn’t done the best job of communicating that to diplomates. However, it does say it welcomes partnering on new projects "and a broader discussion" of potential research agendas.
Many say that they are in favor of requiring lifelong learning, but that the ABIM has gone about it the wrong way.
The ACC would like to find a way "to modify the methodology" ABIM is using to achieve that goal, Dr. O’Gara said. Cost is also a big concern for cardiologists, and especially subspecialists, he said. An interventional cardiologist, for instance, not only has to pay for and pass the general cardiology exam, but also a secure board exam in the subspecialty. In an ACC survey this spring, 90% of the 4,400 respondents said they were concerned about the cost of MOC.
The Society for Cardiovascular Angiography and Interventions (SCAI) is concerned about the impact on its members, too, and has formed a work group to "develop an action plan to spur MOC reform," according to a statement. "We believe that maintaining physician competence is of the highest importance," said Dr. Alan Yeung, chair of the MOC Working Group, in the statement. "It is the current process and products for facilitating maintenance of board certification that are not acceptable."
"The problem is not the lofty goals of maintenance of certification, it’s the implementation of it," agreed Dr. Green. The SGIM is also concerned about the one-size-fits-all approach that ABIM seems to be using. The SGIM has a lot of members who are administrators or physician scientists, and the MOC modules as constructed currently aren’t necessarily meaningful to their practice, said Dr. Green, who is also the program director for the internal medicine residency at Mercy Catholic Medical Center, Philadelphia.
Dr. Weinberger of the ACP said that his organization also wants to see more customization of the secure board exam "so that it’s more relevant to a particular physician’s practice."
Many who attended the ABIM’s July 15 meeting said that they felt like the organization had heard their concerns. But they are still waiting to see what will happen next.
The changes already announced "are a very good start," said Dr. Weinberger, who said that the ACP hopes that the ABIM board might make more changes at its meeting in August. "This is clearly a work in evolution," he said, adding that the ACP would continue to work with ABIM.
Likewise, Dr. Green said that the SGIM would continue to work in cooperation with the ABIM. The question is how much the ABIM will use the input from the subspecialty societies, he said.
"I left the [July 15] meeting with the impression that they are generally interested in receiving this information, in processing it, and in determining with their board what’s the best pathway forward," said Dr. O’Gara.
Dr. White, however, is a bit more skeptical. "I think the ABIM is going to have to be a lot less arrogant," he said, calling for more accountability by the organization.
ABIM’s changes to MOC
The new set of changes to the maintenance of certification process announced by the American Board of Internal Medicine represented the first time the organization had responded to the many concerns being voiced by individual physicians and representatives of the 26 societies that receive certification through the ABIM.
The changes were contained in a July 28 letter. Among other things, the ABIM said that it will be more transparent in how it governs, and how it reports its income and expenses, and that it will set up a more formal process for communication among those specialty societies and the ABIM’s boards. The group also said that in August, its board will "discuss website language for ‘meeting MOC requirements’."
The ABIM agreed with a recommendation from the American College of Physicians and 14 other medical societies that it should convene a conference dedicated to addressing the concerns about MOC and that it should focus on working with various societies to identify problems and solutions.
The organization also said it is open to research projects that can help determine whether MOC has any impact on the physician workforce.
Some other changes:
• The ABIM is streamlining the process for validating products from other organizations that can be eligible for credit toward Part 2 (medical knowledge). The ABIM will accept products that are Accreditation Council for Continuing Medical Education–accredited, and it will align its standards with the American Medical Association’s PRA "Category 1 standards, journal-based continuing medical education (CME), test item writing, and Internet point of care learning." The revised program is expected to be available later in 2014.
• If a diplomate takes an exam before his due date and fails, he will have an additional year to pass before being reported as "not certified" or "not meeting MOC requirements."
• Each ABIM specialty board will decide whether underlying certifications are required in each tertiary specialty and conjoint board. A decision is expected by 2015.
• The ABIM acknowledges that the "patient survey" requirement has been confusing. The name will be changed to the "patient voice" and will be designed to ensure that physicians are doing their best to incorporate that voice into their work. There will be four pathways for completing the requirement, including a survey. Specialty boards will determine which options are the most appropriate for each specialty. The deadline for the completion of the patient voice requirement is 2018.
• The organization said it will look into options for discounting its fees for diplomates who want to complete some of their MOC requirements with other groups. However, it said that it might not be able to offer a very deep discount. "We will commit to researching and brainstorming other fee structures," said the ABIM.
On Twitter @aliciaault
The American Board of Internal Medicine is making several changes to its maintenance of certification requirements, but, so far, it is not enough to quell the still-growing tide of anger and resentment against the process.
The ABIM announced the changes just ahead of a July 15 "summit" with 26 specialty societies that receive certification through the organization, and outlined them in a detailed letter that was sent to internal medicine diplomates on July 28. That correspondence also responded in detail to a May 7 letter sent by the American College of Physicians (ACP) on behalf of 14 medical societies, enumerating their concerns about the process.
The Endocrine Society, which attended the July 15 meeting, said that it left with many of the concerns it came in with. In a statement issued shortly thereafter, the Society called on the ABIM to suspend the maintenance of certification (MOC) requirements until it conducted "a formal analysis of all possible unintended consequences of the new MOC requirements."
Among the unintended consequences it says might result: If endocrinologists have to spend more time on MOC, they might spend less time with patients or on research, or they may even leave the field all together. That will put a dent in access to care when there is an increasing need, according to a letter from the Society to the ABIM in early June.
The American Association of Clinical Endocrinologists (AACE) also warned ABIM that by "monopolizing physician time," MOC could end up harming patient care. The group also said that many endocrinologists who previously did not have to recertify (so-called "grandfathers") might leave practice rather than go through the current process. In a letter to ABIM in late June, AACE also called on the Board to suspend the new requirements until there was a better understanding of how it might affect the endocrinology workforce. The ABIM should also "refrain from publicly reporting that a physician does not meet the MOC requirements," said Dr. R. Mack Harrell, AACE’s president, in that letter.
Meanwhile, 3,000 or so physicians have signed on to a "Pledge of Non-Compliance" with the ABIM’s requirements. The pledge was organized by Dr. Paul Teirstein, chief of cardiology and director of interventional cardiology at the Scripps Clinic, La Jolla, Calif., and his colleagues who have formed Physicians for Certification Change, an anti-MOC organization. Dr. Teirstein also launched a petition drive in March to overhaul the MOC process. That petition had about 18,000 signees at press time.
There are several other organizations hoping to derail or significantly change the ABIM’s requirements, including the Association of American Physicians and Surgeons, Change Board Recertification, and Docs4PatientCare.
In addition, many of the 26 professional societies whose members are certified by the ABIM have also been very organized in presenting their discontent.
The ABIM understands the frustration, said Dr. Richard Baron, ABIM president and CEO, in an interview. "One of the things that people have said is that we’re not listening," he said. That is one reason why the ABIM decided to hold what he called a "listening session" on July 15. "We’re a learning organization," he added, noting that the ABIM will "continue to evolve the program," based on the feedback it receives from individuals and from professional societies, among others.
Dr. Baron also acknowledges that by presenting an entirely new MOC process this year, "our timing was not propitious," given the many challenges physicians now face. The ABIM outlined those pressures in the July 28 letter, counting among them "dealing with the Affordable Care Act," fulfilling meaningful use requirements, and responding to changes in payment and practice models, including team-based care.
Dr. Steven E. Weinberger, executive vice president and CEO of the ACP agrees that the ABIM’s timing was pretty poor. He said that there had been a "low to medium level of concern" about MOC for years, but the new requirements announced in January catalyzed the complaints and were "an important tipping point." Added Dr. Weinberger, "To some extent, this was in part the straw that broke the camel’s back."
The ABIM is making a handful of changes to address some of the concerns, including "broadening the kinds of educational activities that can count for self-assessment of knowledge (Part 2)," said Dr. Baron. That is, the ABIM will be broadening the continuing medical education that will count toward the MOC requirements.
There have also been a huge number of complaints about the self-evaluation of practice assessment. There will be less direct data collection by the ABIM and more of a focus "on improvement activities doctors may already be doing in their practice," Dr. Baron said.
Among other things, ABIM is also looking at changes to the secure exam and whether it needs to change how it describes on its website whether physicians are meeting MOC requirements.
Critics are still not satisfied.
"This is nothing more than offering band-aids for gaping wounds," said Dr. Jonathan Weiss, an internist in Middletown, N.Y. "This is the ABIM trying to throw some bones to quiet down the rabble, given the unexpectedly strong pushback they are trying to deal with," he said, adding that he believes that the current process "is too flawed to be fixed."
Dr. Ron Benbassat, an internist in Beverly Hills, Calif., and a founder of Change Board Certification, goes even further. "No one is drinking the ABIM’s Kool-Aid," he said. "We’re on the right side of the truth here, and they know it," he said, adding, "The momentum is increasing and I believe we’re reaching the tipping point. As to what form it will take – widespread noncompliance or political or legal – I don’t have the answer. But we’re getting there."
Dr. Teirstein was a bit more muted in his criticism. "They are certainly taking many good steps. But, the devil will be in the details." He, like many physicians, said that he still sees the ABIM’s fees as a big problem. "To reestablish credibility, ABIM will need to roll back its fee schedule," said Dr. Teirstein.
The ABIM charges $1,940 for a 10-year basic internal medicine certification, which includes a secure examination for each specialty the diplomate chooses to maintain, access to all ABIM self-evaluation products, and any CME credit a physician can claim through completion of an ABIM module. There are basically four requirements: MOC participants take a secure exam; they have to complete a self-assessment of knowledge; they have to assess their practice by applying quality measurement and using the resulting data to improve their practice; and they must assess patient safety and/or include the "patient voice," which could, for instance, consist of a survey of patient satisfaction.
The fee is $2,060 for a focused practice in hospital medicine for 10 years, and $2,560 for any subspecialty for the 10 years.
But it’s more than just a financial cost, say physicians. There’s also an opportunity cost, said Dr. Eric Green, chair of the MOC Task Force at the Society of General Internal Medicine. "When you are doing MOC, what are you not doing?" he asks.
"It’s about a process that seems arbitrary, a little bit burdensome, and perhaps not achieving what we all want, which is the best care for our patients," said Dr. Christopher White, professor and chairman of medicine, the Ochsner Medical Center, New Orleans, in an interview.
Dr. White, who is a founding member of Physicians for Certification Change, has signed the Pledge of Non-Compliance. He is currently certified, and at age 63, wonders if it’s worth the time and effort to recertify in 2019, when he is next due. "It is a good idea that we continually educate physicians and that physicians have continuous improvement," said Dr. White, but he said he questions whether the ABIM process is the best way. "There’s no evidence that this works, or there’s value," he said.
The American College of Cardiology has offered to partner with the ABIM in getting some answers to the evidence question, said ACC President Patrick O’Gara in an interview. "I’m hopeful that our offer to partner to do research will be taken seriously," he said. Although more evidence will "go a long way toward answering this question about relevance," it would be a long-term project, said Dr. O’Gara, who is also director of clinical cardiology at Brigham and Women’s Hospital, Boston.
The ABIM said in its July 28 letter that extensive evidence does exist – with more than half of the studies coming from non-ABIM researchers – but that maybe it hasn’t done the best job of communicating that to diplomates. However, it does say it welcomes partnering on new projects "and a broader discussion" of potential research agendas.
Many say that they are in favor of requiring lifelong learning, but that the ABIM has gone about it the wrong way.
The ACC would like to find a way "to modify the methodology" ABIM is using to achieve that goal, Dr. O’Gara said. Cost is also a big concern for cardiologists, and especially subspecialists, he said. An interventional cardiologist, for instance, not only has to pay for and pass the general cardiology exam, but also a secure board exam in the subspecialty. In an ACC survey this spring, 90% of the 4,400 respondents said they were concerned about the cost of MOC.
The Society for Cardiovascular Angiography and Interventions (SCAI) is concerned about the impact on its members, too, and has formed a work group to "develop an action plan to spur MOC reform," according to a statement. "We believe that maintaining physician competence is of the highest importance," said Dr. Alan Yeung, chair of the MOC Working Group, in the statement. "It is the current process and products for facilitating maintenance of board certification that are not acceptable."
"The problem is not the lofty goals of maintenance of certification, it’s the implementation of it," agreed Dr. Green. The SGIM is also concerned about the one-size-fits-all approach that ABIM seems to be using. The SGIM has a lot of members who are administrators or physician scientists, and the MOC modules as constructed currently aren’t necessarily meaningful to their practice, said Dr. Green, who is also the program director for the internal medicine residency at Mercy Catholic Medical Center, Philadelphia.
Dr. Weinberger of the ACP said that his organization also wants to see more customization of the secure board exam "so that it’s more relevant to a particular physician’s practice."
Many who attended the ABIM’s July 15 meeting said that they felt like the organization had heard their concerns. But they are still waiting to see what will happen next.
The changes already announced "are a very good start," said Dr. Weinberger, who said that the ACP hopes that the ABIM board might make more changes at its meeting in August. "This is clearly a work in evolution," he said, adding that the ACP would continue to work with ABIM.
Likewise, Dr. Green said that the SGIM would continue to work in cooperation with the ABIM. The question is how much the ABIM will use the input from the subspecialty societies, he said.
"I left the [July 15] meeting with the impression that they are generally interested in receiving this information, in processing it, and in determining with their board what’s the best pathway forward," said Dr. O’Gara.
Dr. White, however, is a bit more skeptical. "I think the ABIM is going to have to be a lot less arrogant," he said, calling for more accountability by the organization.
ABIM’s changes to MOC
The new set of changes to the maintenance of certification process announced by the American Board of Internal Medicine represented the first time the organization had responded to the many concerns being voiced by individual physicians and representatives of the 26 societies that receive certification through the ABIM.
The changes were contained in a July 28 letter. Among other things, the ABIM said that it will be more transparent in how it governs, and how it reports its income and expenses, and that it will set up a more formal process for communication among those specialty societies and the ABIM’s boards. The group also said that in August, its board will "discuss website language for ‘meeting MOC requirements’."
The ABIM agreed with a recommendation from the American College of Physicians and 14 other medical societies that it should convene a conference dedicated to addressing the concerns about MOC and that it should focus on working with various societies to identify problems and solutions.
The organization also said it is open to research projects that can help determine whether MOC has any impact on the physician workforce.
Some other changes:
• The ABIM is streamlining the process for validating products from other organizations that can be eligible for credit toward Part 2 (medical knowledge). The ABIM will accept products that are Accreditation Council for Continuing Medical Education–accredited, and it will align its standards with the American Medical Association’s PRA "Category 1 standards, journal-based continuing medical education (CME), test item writing, and Internet point of care learning." The revised program is expected to be available later in 2014.
• If a diplomate takes an exam before his due date and fails, he will have an additional year to pass before being reported as "not certified" or "not meeting MOC requirements."
• Each ABIM specialty board will decide whether underlying certifications are required in each tertiary specialty and conjoint board. A decision is expected by 2015.
• The ABIM acknowledges that the "patient survey" requirement has been confusing. The name will be changed to the "patient voice" and will be designed to ensure that physicians are doing their best to incorporate that voice into their work. There will be four pathways for completing the requirement, including a survey. Specialty boards will determine which options are the most appropriate for each specialty. The deadline for the completion of the patient voice requirement is 2018.
• The organization said it will look into options for discounting its fees for diplomates who want to complete some of their MOC requirements with other groups. However, it said that it might not be able to offer a very deep discount. "We will commit to researching and brainstorming other fee structures," said the ABIM.
On Twitter @aliciaault
The American Board of Internal Medicine is making several changes to its maintenance of certification requirements, but, so far, it is not enough to quell the still-growing tide of anger and resentment against the process.
The ABIM announced the changes just ahead of a July 15 "summit" with 26 specialty societies that receive certification through the organization, and outlined them in a detailed letter that was sent to internal medicine diplomates on July 28. That correspondence also responded in detail to a May 7 letter sent by the American College of Physicians (ACP) on behalf of 14 medical societies, enumerating their concerns about the process.
The Endocrine Society, which attended the July 15 meeting, said that it left with many of the concerns it came in with. In a statement issued shortly thereafter, the Society called on the ABIM to suspend the maintenance of certification (MOC) requirements until it conducted "a formal analysis of all possible unintended consequences of the new MOC requirements."
Among the unintended consequences it says might result: If endocrinologists have to spend more time on MOC, they might spend less time with patients or on research, or they may even leave the field all together. That will put a dent in access to care when there is an increasing need, according to a letter from the Society to the ABIM in early June.
The American Association of Clinical Endocrinologists (AACE) also warned ABIM that by "monopolizing physician time," MOC could end up harming patient care. The group also said that many endocrinologists who previously did not have to recertify (so-called "grandfathers") might leave practice rather than go through the current process. In a letter to ABIM in late June, AACE also called on the Board to suspend the new requirements until there was a better understanding of how it might affect the endocrinology workforce. The ABIM should also "refrain from publicly reporting that a physician does not meet the MOC requirements," said Dr. R. Mack Harrell, AACE’s president, in that letter.
Meanwhile, 3,000 or so physicians have signed on to a "Pledge of Non-Compliance" with the ABIM’s requirements. The pledge was organized by Dr. Paul Teirstein, chief of cardiology and director of interventional cardiology at the Scripps Clinic, La Jolla, Calif., and his colleagues who have formed Physicians for Certification Change, an anti-MOC organization. Dr. Teirstein also launched a petition drive in March to overhaul the MOC process. That petition had about 18,000 signees at press time.
There are several other organizations hoping to derail or significantly change the ABIM’s requirements, including the Association of American Physicians and Surgeons, Change Board Recertification, and Docs4PatientCare.
In addition, many of the 26 professional societies whose members are certified by the ABIM have also been very organized in presenting their discontent.
The ABIM understands the frustration, said Dr. Richard Baron, ABIM president and CEO, in an interview. "One of the things that people have said is that we’re not listening," he said. That is one reason why the ABIM decided to hold what he called a "listening session" on July 15. "We’re a learning organization," he added, noting that the ABIM will "continue to evolve the program," based on the feedback it receives from individuals and from professional societies, among others.
Dr. Baron also acknowledges that by presenting an entirely new MOC process this year, "our timing was not propitious," given the many challenges physicians now face. The ABIM outlined those pressures in the July 28 letter, counting among them "dealing with the Affordable Care Act," fulfilling meaningful use requirements, and responding to changes in payment and practice models, including team-based care.
Dr. Steven E. Weinberger, executive vice president and CEO of the ACP agrees that the ABIM’s timing was pretty poor. He said that there had been a "low to medium level of concern" about MOC for years, but the new requirements announced in January catalyzed the complaints and were "an important tipping point." Added Dr. Weinberger, "To some extent, this was in part the straw that broke the camel’s back."
The ABIM is making a handful of changes to address some of the concerns, including "broadening the kinds of educational activities that can count for self-assessment of knowledge (Part 2)," said Dr. Baron. That is, the ABIM will be broadening the continuing medical education that will count toward the MOC requirements.
There have also been a huge number of complaints about the self-evaluation of practice assessment. There will be less direct data collection by the ABIM and more of a focus "on improvement activities doctors may already be doing in their practice," Dr. Baron said.
Among other things, ABIM is also looking at changes to the secure exam and whether it needs to change how it describes on its website whether physicians are meeting MOC requirements.
Critics are still not satisfied.
"This is nothing more than offering band-aids for gaping wounds," said Dr. Jonathan Weiss, an internist in Middletown, N.Y. "This is the ABIM trying to throw some bones to quiet down the rabble, given the unexpectedly strong pushback they are trying to deal with," he said, adding that he believes that the current process "is too flawed to be fixed."
Dr. Ron Benbassat, an internist in Beverly Hills, Calif., and a founder of Change Board Certification, goes even further. "No one is drinking the ABIM’s Kool-Aid," he said. "We’re on the right side of the truth here, and they know it," he said, adding, "The momentum is increasing and I believe we’re reaching the tipping point. As to what form it will take – widespread noncompliance or political or legal – I don’t have the answer. But we’re getting there."
Dr. Teirstein was a bit more muted in his criticism. "They are certainly taking many good steps. But, the devil will be in the details." He, like many physicians, said that he still sees the ABIM’s fees as a big problem. "To reestablish credibility, ABIM will need to roll back its fee schedule," said Dr. Teirstein.
The ABIM charges $1,940 for a 10-year basic internal medicine certification, which includes a secure examination for each specialty the diplomate chooses to maintain, access to all ABIM self-evaluation products, and any CME credit a physician can claim through completion of an ABIM module. There are basically four requirements: MOC participants take a secure exam; they have to complete a self-assessment of knowledge; they have to assess their practice by applying quality measurement and using the resulting data to improve their practice; and they must assess patient safety and/or include the "patient voice," which could, for instance, consist of a survey of patient satisfaction.
The fee is $2,060 for a focused practice in hospital medicine for 10 years, and $2,560 for any subspecialty for the 10 years.
But it’s more than just a financial cost, say physicians. There’s also an opportunity cost, said Dr. Eric Green, chair of the MOC Task Force at the Society of General Internal Medicine. "When you are doing MOC, what are you not doing?" he asks.
"It’s about a process that seems arbitrary, a little bit burdensome, and perhaps not achieving what we all want, which is the best care for our patients," said Dr. Christopher White, professor and chairman of medicine, the Ochsner Medical Center, New Orleans, in an interview.
Dr. White, who is a founding member of Physicians for Certification Change, has signed the Pledge of Non-Compliance. He is currently certified, and at age 63, wonders if it’s worth the time and effort to recertify in 2019, when he is next due. "It is a good idea that we continually educate physicians and that physicians have continuous improvement," said Dr. White, but he said he questions whether the ABIM process is the best way. "There’s no evidence that this works, or there’s value," he said.
The American College of Cardiology has offered to partner with the ABIM in getting some answers to the evidence question, said ACC President Patrick O’Gara in an interview. "I’m hopeful that our offer to partner to do research will be taken seriously," he said. Although more evidence will "go a long way toward answering this question about relevance," it would be a long-term project, said Dr. O’Gara, who is also director of clinical cardiology at Brigham and Women’s Hospital, Boston.
The ABIM said in its July 28 letter that extensive evidence does exist – with more than half of the studies coming from non-ABIM researchers – but that maybe it hasn’t done the best job of communicating that to diplomates. However, it does say it welcomes partnering on new projects "and a broader discussion" of potential research agendas.
Many say that they are in favor of requiring lifelong learning, but that the ABIM has gone about it the wrong way.
The ACC would like to find a way "to modify the methodology" ABIM is using to achieve that goal, Dr. O’Gara said. Cost is also a big concern for cardiologists, and especially subspecialists, he said. An interventional cardiologist, for instance, not only has to pay for and pass the general cardiology exam, but also a secure board exam in the subspecialty. In an ACC survey this spring, 90% of the 4,400 respondents said they were concerned about the cost of MOC.
The Society for Cardiovascular Angiography and Interventions (SCAI) is concerned about the impact on its members, too, and has formed a work group to "develop an action plan to spur MOC reform," according to a statement. "We believe that maintaining physician competence is of the highest importance," said Dr. Alan Yeung, chair of the MOC Working Group, in the statement. "It is the current process and products for facilitating maintenance of board certification that are not acceptable."
"The problem is not the lofty goals of maintenance of certification, it’s the implementation of it," agreed Dr. Green. The SGIM is also concerned about the one-size-fits-all approach that ABIM seems to be using. The SGIM has a lot of members who are administrators or physician scientists, and the MOC modules as constructed currently aren’t necessarily meaningful to their practice, said Dr. Green, who is also the program director for the internal medicine residency at Mercy Catholic Medical Center, Philadelphia.
Dr. Weinberger of the ACP said that his organization also wants to see more customization of the secure board exam "so that it’s more relevant to a particular physician’s practice."
Many who attended the ABIM’s July 15 meeting said that they felt like the organization had heard their concerns. But they are still waiting to see what will happen next.
The changes already announced "are a very good start," said Dr. Weinberger, who said that the ACP hopes that the ABIM board might make more changes at its meeting in August. "This is clearly a work in evolution," he said, adding that the ACP would continue to work with ABIM.
Likewise, Dr. Green said that the SGIM would continue to work in cooperation with the ABIM. The question is how much the ABIM will use the input from the subspecialty societies, he said.
"I left the [July 15] meeting with the impression that they are generally interested in receiving this information, in processing it, and in determining with their board what’s the best pathway forward," said Dr. O’Gara.
Dr. White, however, is a bit more skeptical. "I think the ABIM is going to have to be a lot less arrogant," he said, calling for more accountability by the organization.
ABIM’s changes to MOC
The new set of changes to the maintenance of certification process announced by the American Board of Internal Medicine represented the first time the organization had responded to the many concerns being voiced by individual physicians and representatives of the 26 societies that receive certification through the ABIM.
The changes were contained in a July 28 letter. Among other things, the ABIM said that it will be more transparent in how it governs, and how it reports its income and expenses, and that it will set up a more formal process for communication among those specialty societies and the ABIM’s boards. The group also said that in August, its board will "discuss website language for ‘meeting MOC requirements’."
The ABIM agreed with a recommendation from the American College of Physicians and 14 other medical societies that it should convene a conference dedicated to addressing the concerns about MOC and that it should focus on working with various societies to identify problems and solutions.
The organization also said it is open to research projects that can help determine whether MOC has any impact on the physician workforce.
Some other changes:
• The ABIM is streamlining the process for validating products from other organizations that can be eligible for credit toward Part 2 (medical knowledge). The ABIM will accept products that are Accreditation Council for Continuing Medical Education–accredited, and it will align its standards with the American Medical Association’s PRA "Category 1 standards, journal-based continuing medical education (CME), test item writing, and Internet point of care learning." The revised program is expected to be available later in 2014.
• If a diplomate takes an exam before his due date and fails, he will have an additional year to pass before being reported as "not certified" or "not meeting MOC requirements."
• Each ABIM specialty board will decide whether underlying certifications are required in each tertiary specialty and conjoint board. A decision is expected by 2015.
• The ABIM acknowledges that the "patient survey" requirement has been confusing. The name will be changed to the "patient voice" and will be designed to ensure that physicians are doing their best to incorporate that voice into their work. There will be four pathways for completing the requirement, including a survey. Specialty boards will determine which options are the most appropriate for each specialty. The deadline for the completion of the patient voice requirement is 2018.
• The organization said it will look into options for discounting its fees for diplomates who want to complete some of their MOC requirements with other groups. However, it said that it might not be able to offer a very deep discount. "We will commit to researching and brainstorming other fee structures," said the ABIM.
On Twitter @aliciaault
FDA approves oritavancin for skin infections
The Food and Drug Administration has approved the injectable antibiotic oritavancin (Orbactiv) to treat adults with acute bacterial skin and skin structure infections that might be caused by gram-positive bacteria, including methicillin-resistant Staphylococcus aureus.
Oritavancin, which is manufactured and sold by The Medicines Company of Parsippany, N.J., is given in a single 3-hour infusion, after which the treatment regimen is considered complete.
Oritavancin is the third new antibacterial drug approved by the FDA in 2014 to treat acute bacterial skin and skin structure infections (ABSSSIs). The agency approved dalbavancin (Dalvance) in May and tedizolid (Sivextro) in June.
"The approval of several new antibacterial drugs this year demonstrates that we are making progress in increasing the availability of treatment options for patients and physicians," said Dr. Edward Cox, director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research, in a statement. "However, more work is needed in this area, and the FDA remains a committed partner to help promote the development of antibacterial drugs," he added.
Like dalbavancin and tedizolid, oritavancin was given priority review by the agency because it is intended to treat a serious or life-threatening condition. All three drugs also qualify for an additional 5 years of marketing exclusivity.
In a statement issued by The Medicines Company, several clinicians said that they welcomed the addition of oritavancin to their treatment arsenal. "With a single-dose treatment regimen, Orbactiv may help reduce the dosing burden seen with antibiotics given as multiple intravenous administrations to patients with these infections," said Dr. G. Ralph Corey, professor of medicine and infectious diseases at Duke University, Durham, N.C.
"The growing challenge of antibiotic resistance in the U.S. has had a significant impact on the clinical management decisions in the emergency department," said Dr. Charles Pollack, professor of emergency medicine at the University of Pennsylvania, Philadelphia. A single, once-only IV therapy such as Orbactiv offers the option to administer a single treatment in the outpatient setting for patients with skin infections caused by gram-positive bacteria likely due to MRSA [methicillin-resistant Staphylococcus aureus]."
The FDA based its approval on the results of the SOLO I and SOLO II clinical studies. The randomized, double-blind, multicenter trials compared a single 1,200-mg IV dose to 7-10 days of twice-daily vancomycin (1 g or 15 mg/kg) in 1,987 patients. The trials also included a subset of 405 patients with documented MRSA. Oritavancin was equivalent to vancomycin in these trials.
The most common side effects were headache, nausea, vomiting, diarrhea, and the formation of skin and soft tissue abscesses on arms and legs, according to the FDA. The drug’s label also includes a warning regarding interference with coagulation tests and interaction with warfarin.
Oritavancin also is under review in Europe for the treatment of complicated skin and soft tissue infections.
On Twitter @aliciaault
The Food and Drug Administration has approved the injectable antibiotic oritavancin (Orbactiv) to treat adults with acute bacterial skin and skin structure infections that might be caused by gram-positive bacteria, including methicillin-resistant Staphylococcus aureus.
Oritavancin, which is manufactured and sold by The Medicines Company of Parsippany, N.J., is given in a single 3-hour infusion, after which the treatment regimen is considered complete.
Oritavancin is the third new antibacterial drug approved by the FDA in 2014 to treat acute bacterial skin and skin structure infections (ABSSSIs). The agency approved dalbavancin (Dalvance) in May and tedizolid (Sivextro) in June.
"The approval of several new antibacterial drugs this year demonstrates that we are making progress in increasing the availability of treatment options for patients and physicians," said Dr. Edward Cox, director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research, in a statement. "However, more work is needed in this area, and the FDA remains a committed partner to help promote the development of antibacterial drugs," he added.
Like dalbavancin and tedizolid, oritavancin was given priority review by the agency because it is intended to treat a serious or life-threatening condition. All three drugs also qualify for an additional 5 years of marketing exclusivity.
In a statement issued by The Medicines Company, several clinicians said that they welcomed the addition of oritavancin to their treatment arsenal. "With a single-dose treatment regimen, Orbactiv may help reduce the dosing burden seen with antibiotics given as multiple intravenous administrations to patients with these infections," said Dr. G. Ralph Corey, professor of medicine and infectious diseases at Duke University, Durham, N.C.
"The growing challenge of antibiotic resistance in the U.S. has had a significant impact on the clinical management decisions in the emergency department," said Dr. Charles Pollack, professor of emergency medicine at the University of Pennsylvania, Philadelphia. A single, once-only IV therapy such as Orbactiv offers the option to administer a single treatment in the outpatient setting for patients with skin infections caused by gram-positive bacteria likely due to MRSA [methicillin-resistant Staphylococcus aureus]."
The FDA based its approval on the results of the SOLO I and SOLO II clinical studies. The randomized, double-blind, multicenter trials compared a single 1,200-mg IV dose to 7-10 days of twice-daily vancomycin (1 g or 15 mg/kg) in 1,987 patients. The trials also included a subset of 405 patients with documented MRSA. Oritavancin was equivalent to vancomycin in these trials.
The most common side effects were headache, nausea, vomiting, diarrhea, and the formation of skin and soft tissue abscesses on arms and legs, according to the FDA. The drug’s label also includes a warning regarding interference with coagulation tests and interaction with warfarin.
Oritavancin also is under review in Europe for the treatment of complicated skin and soft tissue infections.
On Twitter @aliciaault
The Food and Drug Administration has approved the injectable antibiotic oritavancin (Orbactiv) to treat adults with acute bacterial skin and skin structure infections that might be caused by gram-positive bacteria, including methicillin-resistant Staphylococcus aureus.
Oritavancin, which is manufactured and sold by The Medicines Company of Parsippany, N.J., is given in a single 3-hour infusion, after which the treatment regimen is considered complete.
Oritavancin is the third new antibacterial drug approved by the FDA in 2014 to treat acute bacterial skin and skin structure infections (ABSSSIs). The agency approved dalbavancin (Dalvance) in May and tedizolid (Sivextro) in June.
"The approval of several new antibacterial drugs this year demonstrates that we are making progress in increasing the availability of treatment options for patients and physicians," said Dr. Edward Cox, director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research, in a statement. "However, more work is needed in this area, and the FDA remains a committed partner to help promote the development of antibacterial drugs," he added.
Like dalbavancin and tedizolid, oritavancin was given priority review by the agency because it is intended to treat a serious or life-threatening condition. All three drugs also qualify for an additional 5 years of marketing exclusivity.
In a statement issued by The Medicines Company, several clinicians said that they welcomed the addition of oritavancin to their treatment arsenal. "With a single-dose treatment regimen, Orbactiv may help reduce the dosing burden seen with antibiotics given as multiple intravenous administrations to patients with these infections," said Dr. G. Ralph Corey, professor of medicine and infectious diseases at Duke University, Durham, N.C.
"The growing challenge of antibiotic resistance in the U.S. has had a significant impact on the clinical management decisions in the emergency department," said Dr. Charles Pollack, professor of emergency medicine at the University of Pennsylvania, Philadelphia. A single, once-only IV therapy such as Orbactiv offers the option to administer a single treatment in the outpatient setting for patients with skin infections caused by gram-positive bacteria likely due to MRSA [methicillin-resistant Staphylococcus aureus]."
The FDA based its approval on the results of the SOLO I and SOLO II clinical studies. The randomized, double-blind, multicenter trials compared a single 1,200-mg IV dose to 7-10 days of twice-daily vancomycin (1 g or 15 mg/kg) in 1,987 patients. The trials also included a subset of 405 patients with documented MRSA. Oritavancin was equivalent to vancomycin in these trials.
The most common side effects were headache, nausea, vomiting, diarrhea, and the formation of skin and soft tissue abscesses on arms and legs, according to the FDA. The drug’s label also includes a warning regarding interference with coagulation tests and interaction with warfarin.
Oritavancin also is under review in Europe for the treatment of complicated skin and soft tissue infections.
On Twitter @aliciaault
2015 outpatient proposal focuses on bundled pay
The Centers for Medicare & Medicaid Services’ proposed rule on outpatient department and ambulatory surgery center payment for 2015 expands the agency’s focus on bundling pay for device-related procedures.
The Hospital Outpatient Prospective Payment System (OPPS) rule also continues the same payment rate for outpatient drug delivery such as chemotherapy. That payment rate has been a source of disappointment for oncologists.
The agency is proposing again in 2015 to continue paying average sales price plus 6% for non–pass through drugs and biologicals that are administered under Part B of Medicare.
The rule published on July 14 covers payment for 4,000 hospitals, including general acute care hospitals, inpatient rehabilitation facilities, inpatient psychiatric facilities, long-term acute care hospitals, children’s hospitals, and cancer hospitals. It also applies to 5,300 ambulatory surgery centers (ASCs) that participate in Medicare.
Overall, the government is proposing to increase payments to outpatient departments by 2%. The CMS expects to pay out $57 billion for outpatient services in 2015. Payments to ASCs will increase just over 1% to $4 billion.
The agency is proposing to expand its Comprehensive Ambulatory Payment Classification (APC) policy, which was first discussed in its 2014 rule. The idea is to give a single Medicare payment and require a single beneficiary copayment for the entire hospital stay for a group of 28 procedures, including pacemaker insertion, implantation of neurostimulators, and stereotactic radiosurgery.
In GI procedures and stents, CPT codes 43274 and 43276, endoscopic retrograde cholangiopancreatography with stent placement into the biliary or pancreatic duct including dilation, guide wire passage, and sphincterotomy were reassigned to APC 0384. The single, bundled payments would begin in 2015.
The proposed rule also contains adjustments to both the Hospital Outpatient Quality Reporting Program and the ASC Quality Reporting Program. On the hospital side, the CMS is proposing to remove three quality measures, stating that performance has been uniformly high among reporting facilities. Those measures are aspirin at arrival (cardiac care), timing of prophylaxis antibiotics, and prophylactic antibiotic selection for surgical patients. The agency is proposing to add a claims-based measure – facility 7-day risk-standardized hospital visit rate after outpatient colonoscopy – for 2017 and beyond.
For ASCs, the agency is proposing to continue its effort to align measures with the hospital program. In 2015, ASCs will be required to report on the 7-day risk-standardized visit rate after outpatient colonoscopy measure.
The CMS is accepting comments on the proposed rule until Sept. 2, 2014. A final rule will be issued by Nov. 1.
On Twitter @aliciaault
The Centers for Medicare & Medicaid Services’ proposed rule on outpatient department and ambulatory surgery center payment for 2015 expands the agency’s focus on bundling pay for device-related procedures.
The Hospital Outpatient Prospective Payment System (OPPS) rule also continues the same payment rate for outpatient drug delivery such as chemotherapy. That payment rate has been a source of disappointment for oncologists.
The agency is proposing again in 2015 to continue paying average sales price plus 6% for non–pass through drugs and biologicals that are administered under Part B of Medicare.
The rule published on July 14 covers payment for 4,000 hospitals, including general acute care hospitals, inpatient rehabilitation facilities, inpatient psychiatric facilities, long-term acute care hospitals, children’s hospitals, and cancer hospitals. It also applies to 5,300 ambulatory surgery centers (ASCs) that participate in Medicare.
Overall, the government is proposing to increase payments to outpatient departments by 2%. The CMS expects to pay out $57 billion for outpatient services in 2015. Payments to ASCs will increase just over 1% to $4 billion.
The agency is proposing to expand its Comprehensive Ambulatory Payment Classification (APC) policy, which was first discussed in its 2014 rule. The idea is to give a single Medicare payment and require a single beneficiary copayment for the entire hospital stay for a group of 28 procedures, including pacemaker insertion, implantation of neurostimulators, and stereotactic radiosurgery.
In GI procedures and stents, CPT codes 43274 and 43276, endoscopic retrograde cholangiopancreatography with stent placement into the biliary or pancreatic duct including dilation, guide wire passage, and sphincterotomy were reassigned to APC 0384. The single, bundled payments would begin in 2015.
The proposed rule also contains adjustments to both the Hospital Outpatient Quality Reporting Program and the ASC Quality Reporting Program. On the hospital side, the CMS is proposing to remove three quality measures, stating that performance has been uniformly high among reporting facilities. Those measures are aspirin at arrival (cardiac care), timing of prophylaxis antibiotics, and prophylactic antibiotic selection for surgical patients. The agency is proposing to add a claims-based measure – facility 7-day risk-standardized hospital visit rate after outpatient colonoscopy – for 2017 and beyond.
For ASCs, the agency is proposing to continue its effort to align measures with the hospital program. In 2015, ASCs will be required to report on the 7-day risk-standardized visit rate after outpatient colonoscopy measure.
The CMS is accepting comments on the proposed rule until Sept. 2, 2014. A final rule will be issued by Nov. 1.
On Twitter @aliciaault
The Centers for Medicare & Medicaid Services’ proposed rule on outpatient department and ambulatory surgery center payment for 2015 expands the agency’s focus on bundling pay for device-related procedures.
The Hospital Outpatient Prospective Payment System (OPPS) rule also continues the same payment rate for outpatient drug delivery such as chemotherapy. That payment rate has been a source of disappointment for oncologists.
The agency is proposing again in 2015 to continue paying average sales price plus 6% for non–pass through drugs and biologicals that are administered under Part B of Medicare.
The rule published on July 14 covers payment for 4,000 hospitals, including general acute care hospitals, inpatient rehabilitation facilities, inpatient psychiatric facilities, long-term acute care hospitals, children’s hospitals, and cancer hospitals. It also applies to 5,300 ambulatory surgery centers (ASCs) that participate in Medicare.
Overall, the government is proposing to increase payments to outpatient departments by 2%. The CMS expects to pay out $57 billion for outpatient services in 2015. Payments to ASCs will increase just over 1% to $4 billion.
The agency is proposing to expand its Comprehensive Ambulatory Payment Classification (APC) policy, which was first discussed in its 2014 rule. The idea is to give a single Medicare payment and require a single beneficiary copayment for the entire hospital stay for a group of 28 procedures, including pacemaker insertion, implantation of neurostimulators, and stereotactic radiosurgery.
In GI procedures and stents, CPT codes 43274 and 43276, endoscopic retrograde cholangiopancreatography with stent placement into the biliary or pancreatic duct including dilation, guide wire passage, and sphincterotomy were reassigned to APC 0384. The single, bundled payments would begin in 2015.
The proposed rule also contains adjustments to both the Hospital Outpatient Quality Reporting Program and the ASC Quality Reporting Program. On the hospital side, the CMS is proposing to remove three quality measures, stating that performance has been uniformly high among reporting facilities. Those measures are aspirin at arrival (cardiac care), timing of prophylaxis antibiotics, and prophylactic antibiotic selection for surgical patients. The agency is proposing to add a claims-based measure – facility 7-day risk-standardized hospital visit rate after outpatient colonoscopy – for 2017 and beyond.
For ASCs, the agency is proposing to continue its effort to align measures with the hospital program. In 2015, ASCs will be required to report on the 7-day risk-standardized visit rate after outpatient colonoscopy measure.
The CMS is accepting comments on the proposed rule until Sept. 2, 2014. A final rule will be issued by Nov. 1.
On Twitter @aliciaault
CMS Reconsidering Coverage of HIV Screening for all Medicare Beneficiaries
The Centers for Medicare & Medicaid Services says it is considering extending coverage of HIV screening to all Medicare beneficiaries, not just those at risk.
The agency announced on Aug. 4 that it was starting a new analysis, which was prompted by a request from the HIV Testing Reimbursement Working Group and the Federal AIDS Policy Partnership. Those groups include the American Academy of HIV Medicine, GMHC, the HIV Medicine Association, the National Alliance of State and Territorial AIDS Directors, the National Association of County and City Health Officials, and the National Viral Hepatitis Roundtable.
Medicare has covered routine HIV testing as a preventive service since 2009 for adolescents, pregnant women, and adults who are at risk for infection. That coverage decision was made in light of the U.S. Preventive Services Task Force grade A recommendation that such screening was supported by the evidence.
In 2013, the USPTF revised that recommendation, saying that all adolescents and adults aged 15-65 years should be screened, regardless of their risk. All pregnant women should still continue to be screened, said the Task Force. Adolescents younger than 15 years and adults older than 65 years who are at increased risk of HIV infection should also receive screening, it added.
Because the screening recommendation had an A grade, it must be covered by private insurers under the Affordable Care Act.
But the CMS has not updated its policy, which means that "HIV testing coverage for Medicare beneficiaries is limited to those who are perceived to be or identify themselves as at risk and to pregnant women," said the letter from the HIV Testing Reimbursement Working Group and the Federal AIDS Policy Partnership.
"This coverage limitation does not reflect the current science or HHS’s own best practices for HIV medicine," they said.
The groups said that at least 16% of people with the infection, or 181,400 individuals, are unaware that they are HIV positive and that half of all new infections are transmitted by someone who did not know their status. They also noted that about 17% of the Medicare population is young and disabled; with routine testing, they would be more likely to receive earlier diagnosis and treatment.
"It is important for CMS to make a clear statement that HIV infection is an important issue impacting Medicare beneficiaries," added the organizations, noting that, "While only about 3% of those living with HIV are estimated to be 65 or older, the CDC estimates that by 2017 more than half of those living with HIV will be over 50 years old, approaching Medicare eligibility."
The agency is accepting public comments on expanding coverage of screening until Sept. 3.
Instructions on submitting public comments can be found at http://www.cms.gov/Medicare/Coverage/InfoExchange/publiccomments.html.
The CMS said that it expects to issue a proposed decision in Feb. 2015, and to complete its analysis by May next year.
On Twitter @aliciaault
The Centers for Medicare & Medicaid Services says it is considering extending coverage of HIV screening to all Medicare beneficiaries, not just those at risk.
The agency announced on Aug. 4 that it was starting a new analysis, which was prompted by a request from the HIV Testing Reimbursement Working Group and the Federal AIDS Policy Partnership. Those groups include the American Academy of HIV Medicine, GMHC, the HIV Medicine Association, the National Alliance of State and Territorial AIDS Directors, the National Association of County and City Health Officials, and the National Viral Hepatitis Roundtable.
Medicare has covered routine HIV testing as a preventive service since 2009 for adolescents, pregnant women, and adults who are at risk for infection. That coverage decision was made in light of the U.S. Preventive Services Task Force grade A recommendation that such screening was supported by the evidence.
In 2013, the USPTF revised that recommendation, saying that all adolescents and adults aged 15-65 years should be screened, regardless of their risk. All pregnant women should still continue to be screened, said the Task Force. Adolescents younger than 15 years and adults older than 65 years who are at increased risk of HIV infection should also receive screening, it added.
Because the screening recommendation had an A grade, it must be covered by private insurers under the Affordable Care Act.
But the CMS has not updated its policy, which means that "HIV testing coverage for Medicare beneficiaries is limited to those who are perceived to be or identify themselves as at risk and to pregnant women," said the letter from the HIV Testing Reimbursement Working Group and the Federal AIDS Policy Partnership.
"This coverage limitation does not reflect the current science or HHS’s own best practices for HIV medicine," they said.
The groups said that at least 16% of people with the infection, or 181,400 individuals, are unaware that they are HIV positive and that half of all new infections are transmitted by someone who did not know their status. They also noted that about 17% of the Medicare population is young and disabled; with routine testing, they would be more likely to receive earlier diagnosis and treatment.
"It is important for CMS to make a clear statement that HIV infection is an important issue impacting Medicare beneficiaries," added the organizations, noting that, "While only about 3% of those living with HIV are estimated to be 65 or older, the CDC estimates that by 2017 more than half of those living with HIV will be over 50 years old, approaching Medicare eligibility."
The agency is accepting public comments on expanding coverage of screening until Sept. 3.
Instructions on submitting public comments can be found at http://www.cms.gov/Medicare/Coverage/InfoExchange/publiccomments.html.
The CMS said that it expects to issue a proposed decision in Feb. 2015, and to complete its analysis by May next year.
On Twitter @aliciaault
The Centers for Medicare & Medicaid Services says it is considering extending coverage of HIV screening to all Medicare beneficiaries, not just those at risk.
The agency announced on Aug. 4 that it was starting a new analysis, which was prompted by a request from the HIV Testing Reimbursement Working Group and the Federal AIDS Policy Partnership. Those groups include the American Academy of HIV Medicine, GMHC, the HIV Medicine Association, the National Alliance of State and Territorial AIDS Directors, the National Association of County and City Health Officials, and the National Viral Hepatitis Roundtable.
Medicare has covered routine HIV testing as a preventive service since 2009 for adolescents, pregnant women, and adults who are at risk for infection. That coverage decision was made in light of the U.S. Preventive Services Task Force grade A recommendation that such screening was supported by the evidence.
In 2013, the USPTF revised that recommendation, saying that all adolescents and adults aged 15-65 years should be screened, regardless of their risk. All pregnant women should still continue to be screened, said the Task Force. Adolescents younger than 15 years and adults older than 65 years who are at increased risk of HIV infection should also receive screening, it added.
Because the screening recommendation had an A grade, it must be covered by private insurers under the Affordable Care Act.
But the CMS has not updated its policy, which means that "HIV testing coverage for Medicare beneficiaries is limited to those who are perceived to be or identify themselves as at risk and to pregnant women," said the letter from the HIV Testing Reimbursement Working Group and the Federal AIDS Policy Partnership.
"This coverage limitation does not reflect the current science or HHS’s own best practices for HIV medicine," they said.
The groups said that at least 16% of people with the infection, or 181,400 individuals, are unaware that they are HIV positive and that half of all new infections are transmitted by someone who did not know their status. They also noted that about 17% of the Medicare population is young and disabled; with routine testing, they would be more likely to receive earlier diagnosis and treatment.
"It is important for CMS to make a clear statement that HIV infection is an important issue impacting Medicare beneficiaries," added the organizations, noting that, "While only about 3% of those living with HIV are estimated to be 65 or older, the CDC estimates that by 2017 more than half of those living with HIV will be over 50 years old, approaching Medicare eligibility."
The agency is accepting public comments on expanding coverage of screening until Sept. 3.
Instructions on submitting public comments can be found at http://www.cms.gov/Medicare/Coverage/InfoExchange/publiccomments.html.
The CMS said that it expects to issue a proposed decision in Feb. 2015, and to complete its analysis by May next year.
On Twitter @aliciaault
CMS reconsidering coverage of HIV screening for all Medicare beneficiaries
The Centers for Medicare & Medicaid Services says it is considering extending coverage of HIV screening to all Medicare beneficiaries, not just those at risk.
The agency announced on Aug. 4 that it was starting a new analysis, which was prompted by a request from the HIV Testing Reimbursement Working Group and the Federal AIDS Policy Partnership. Those groups include the American Academy of HIV Medicine, GMHC, the HIV Medicine Association, the National Alliance of State and Territorial AIDS Directors, the National Association of County and City Health Officials, and the National Viral Hepatitis Roundtable.
Medicare has covered routine HIV testing as a preventive service since 2009 for adolescents, pregnant women, and adults who are at risk for infection. That coverage decision was made in light of the U.S. Preventive Services Task Force grade A recommendation that such screening was supported by the evidence.
In 2013, the USPTF revised that recommendation, saying that all adolescents and adults aged 15-65 years should be screened, regardless of their risk. All pregnant women should still continue to be screened, said the Task Force. Adolescents younger than 15 years and adults older than 65 years who are at increased risk of HIV infection should also receive screening, it added.
Because the screening recommendation had an A grade, it must be covered by private insurers under the Affordable Care Act.
But the CMS has not updated its policy, which means that "HIV testing coverage for Medicare beneficiaries is limited to those who are perceived to be or identify themselves as at risk and to pregnant women," said the letter from the HIV Testing Reimbursement Working Group and the Federal AIDS Policy Partnership.
"This coverage limitation does not reflect the current science or HHS’s own best practices for HIV medicine," they said.
The groups said that at least 16% of people with the infection, or 181,400 individuals, are unaware that they are HIV positive and that half of all new infections are transmitted by someone who did not know their status. They also noted that about 17% of the Medicare population is young and disabled; with routine testing, they would be more likely to receive earlier diagnosis and treatment.
"It is important for CMS to make a clear statement that HIV infection is an important issue impacting Medicare beneficiaries," added the organizations, noting that, "While only about 3% of those living with HIV are estimated to be 65 or older, the CDC estimates that by 2017 more than half of those living with HIV will be over 50 years old, approaching Medicare eligibility."
The agency is accepting public comments on expanding coverage of screening until Sept. 3.
The CMS said that it expects to issue a proposed decision in Feb. 2015, and to complete its analysis by May next year.
On Twitter @aliciaault
The Centers for Medicare & Medicaid Services says it is considering extending coverage of HIV screening to all Medicare beneficiaries, not just those at risk.
The agency announced on Aug. 4 that it was starting a new analysis, which was prompted by a request from the HIV Testing Reimbursement Working Group and the Federal AIDS Policy Partnership. Those groups include the American Academy of HIV Medicine, GMHC, the HIV Medicine Association, the National Alliance of State and Territorial AIDS Directors, the National Association of County and City Health Officials, and the National Viral Hepatitis Roundtable.
Medicare has covered routine HIV testing as a preventive service since 2009 for adolescents, pregnant women, and adults who are at risk for infection. That coverage decision was made in light of the U.S. Preventive Services Task Force grade A recommendation that such screening was supported by the evidence.
In 2013, the USPTF revised that recommendation, saying that all adolescents and adults aged 15-65 years should be screened, regardless of their risk. All pregnant women should still continue to be screened, said the Task Force. Adolescents younger than 15 years and adults older than 65 years who are at increased risk of HIV infection should also receive screening, it added.
Because the screening recommendation had an A grade, it must be covered by private insurers under the Affordable Care Act.
But the CMS has not updated its policy, which means that "HIV testing coverage for Medicare beneficiaries is limited to those who are perceived to be or identify themselves as at risk and to pregnant women," said the letter from the HIV Testing Reimbursement Working Group and the Federal AIDS Policy Partnership.
"This coverage limitation does not reflect the current science or HHS’s own best practices for HIV medicine," they said.
The groups said that at least 16% of people with the infection, or 181,400 individuals, are unaware that they are HIV positive and that half of all new infections are transmitted by someone who did not know their status. They also noted that about 17% of the Medicare population is young and disabled; with routine testing, they would be more likely to receive earlier diagnosis and treatment.
"It is important for CMS to make a clear statement that HIV infection is an important issue impacting Medicare beneficiaries," added the organizations, noting that, "While only about 3% of those living with HIV are estimated to be 65 or older, the CDC estimates that by 2017 more than half of those living with HIV will be over 50 years old, approaching Medicare eligibility."
The agency is accepting public comments on expanding coverage of screening until Sept. 3.
The CMS said that it expects to issue a proposed decision in Feb. 2015, and to complete its analysis by May next year.
On Twitter @aliciaault
The Centers for Medicare & Medicaid Services says it is considering extending coverage of HIV screening to all Medicare beneficiaries, not just those at risk.
The agency announced on Aug. 4 that it was starting a new analysis, which was prompted by a request from the HIV Testing Reimbursement Working Group and the Federal AIDS Policy Partnership. Those groups include the American Academy of HIV Medicine, GMHC, the HIV Medicine Association, the National Alliance of State and Territorial AIDS Directors, the National Association of County and City Health Officials, and the National Viral Hepatitis Roundtable.
Medicare has covered routine HIV testing as a preventive service since 2009 for adolescents, pregnant women, and adults who are at risk for infection. That coverage decision was made in light of the U.S. Preventive Services Task Force grade A recommendation that such screening was supported by the evidence.
In 2013, the USPTF revised that recommendation, saying that all adolescents and adults aged 15-65 years should be screened, regardless of their risk. All pregnant women should still continue to be screened, said the Task Force. Adolescents younger than 15 years and adults older than 65 years who are at increased risk of HIV infection should also receive screening, it added.
Because the screening recommendation had an A grade, it must be covered by private insurers under the Affordable Care Act.
But the CMS has not updated its policy, which means that "HIV testing coverage for Medicare beneficiaries is limited to those who are perceived to be or identify themselves as at risk and to pregnant women," said the letter from the HIV Testing Reimbursement Working Group and the Federal AIDS Policy Partnership.
"This coverage limitation does not reflect the current science or HHS’s own best practices for HIV medicine," they said.
The groups said that at least 16% of people with the infection, or 181,400 individuals, are unaware that they are HIV positive and that half of all new infections are transmitted by someone who did not know their status. They also noted that about 17% of the Medicare population is young and disabled; with routine testing, they would be more likely to receive earlier diagnosis and treatment.
"It is important for CMS to make a clear statement that HIV infection is an important issue impacting Medicare beneficiaries," added the organizations, noting that, "While only about 3% of those living with HIV are estimated to be 65 or older, the CDC estimates that by 2017 more than half of those living with HIV will be over 50 years old, approaching Medicare eligibility."
The agency is accepting public comments on expanding coverage of screening until Sept. 3.
The CMS said that it expects to issue a proposed decision in Feb. 2015, and to complete its analysis by May next year.
On Twitter @aliciaault