User login
Mismatch Between Process and Outcome Measures for Hospital-Acquired Venous Thromboembolism in a Surgical Cohort
From Tufts Medical Center, Boston, MA.
Abstract
- Objective: Audits at our academic medical center revealed near 100% compliance with protocols for perioperative venous thromboembolism (VTE) prophylaxis, but recent National Surgical Quality Improvement Program data demonstrated a higher than expected incidence of VTE (observed/expected = 1.32). The objective of this study was to identify potential causes of this discrepancy.
- Design: Retrospective case-control study.
- Setting: Urban academic medical center with high case-mix indices (Medicare approximately 2.4, non-Medicare approximately 2.0).
- Participants: 102 surgical inpatients with VTE (September 2012 to October 2015) matched with controls for age, gender, and type of procedure.
- Measurements: Prevalence of common VTE risk factors, length of stay, number of procedures, index operation times, and postoperative bed rest > 12 hours were assessed. Utilization of and compliance with our VTE risk assessment tool was also investigated.
- Results: Cases underwent more procedures and had longer lengths of stay and index procedures than controls. In addition, cases were more likely to have had > 12 hours of postoperative bed rest and central venous access than controls. Cases had more infections and were more likely to have severe lung disease, thrombophilia, and a history of prior VTE than controls. No differences in body mass index, tobacco use, current or previous malignancy, or VTE risk assessment form use were observed. Overall, care complexity and risk factors were equally important in determining VTE incidence. Our analyses also revealed lack of strict adherence to our VTE risk stratification protocol and frequent use of suboptimal prophylactic regimens.
- Conclusion: Well-accepted risk factors and overall care complexity determine VTE risk. Preventing VTE in high-risk patients requires assiduous attention to detail in VTE risk assessment and in delivery of optimal prophylaxis. Patients at especially high risk may require customized prophylactic regimens.
Keywords: hospital-acquired venous thromboembolic disease; VTE prophylaxis, surgical patients.
Deep vein thrombosis (DVT) and pulmonary embolism (PE) are well-recognized causes of morbidity and mortality in surgical patients. Between 350,000 and 600,000 cases of venous thromboembolism (VTE) occur each year in the United States, and it is responsible for approximately 10% of preventable in-hospital fatalities.1-3 Given VTE’s impact on patients and the healthcare system and the fact that it is preventable, intense effort has been focused on developing more effective prophylactic measures to decrease its incidence.2-4 In 2008, the surgeon general issued a “call to action” for increased efforts to prevent VTE.5
The American College of Chest Physicians (ACCP) guidelines subcategorize patients based on type of surgery. In addition, the ACCP guidelines support the use of a Caprini-based scoring system to aid in risk stratification and improve clinical decision-making (
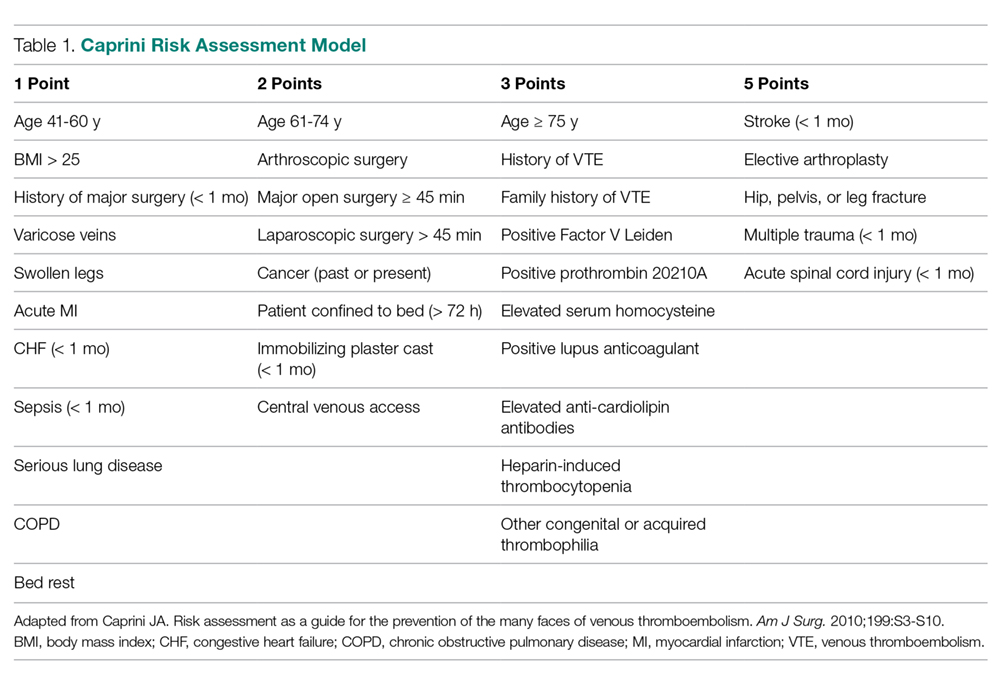
Our hospital, a 350-bed academic medical center in downtown Boston, MA, serving a diverse population with a very high case-mix index (2.4 Medicare and 2.0 non-Medicare), has strict protocols for VTE prophylaxis consistent with the ACCP guidelines and based on the Surgical Care Improvement Project (SCIP) measures published in 2006.10 The SCIP mandates allow for considerable surgeon discretion in the use of chemoprophylaxis for neurosurgical cases and general and orthopedic surgery cases deemed to be at high risk for bleeding. In addition, SCIP requires only that prophylaxis be initiated within 24 hours of surgical end time. Although recent audits revealed nearly 100% compliance with SCIP-mandated protocols, National Surgical Quality Improvement Program (NSQIP) data showed that the incidence of VTE events at our institution was higher than expected (observed/expected [O/E] = 1.32).
In order to determine the reasons for this mismatch between process and outcome performance, we investigated whether there were characteristics of our patient population that contributed to the higher than expected rates of VTE, and we scrutinized our VTE prophylaxis protocol to determine if there were aspects of our process that were also contributory.
Methods
Study Sample
This is a retrospective case-control study of surgical inpatients at our hospital during the period September 2012 to October 2015. Cases were identified as patients diagnosed with a VTE (DVT or PE). Controls were identified from a pool of surgical patients whose courses were not complicated by VTE during the same time frame as the cases and who were matched as closely as possible by procedure code, age, and gender.
Variables
Patient and hospital course variables that were analyzed included demographics, comorbidities, length of stay, number of procedures, index operation times, duration of postoperative bed rest, use of mechanical prophylaxis, and type of chemoprophylaxis and time frame within which it was initiated. Data were collected via chart review using International Classification of Diseases-9 and -10 codes to identify surgical cases within the allotted time period who were diagnosed with VTE. Demographic variables included age, sex, and ethnicity. Comorbidities included hypertension, diabetes, coronary artery disease, serious lung disease, previous or current malignancy, documented hypercoagulable state, and previous history of VTE. Body mass index (BMI) was also recorded. The aforementioned disease-specific variables were not matched between the case and control groups, as this data was obtained retrospectively during data collection.
Analysis
Associations between case and matched control were analyzed using the paired t-test for continuous variables and McNemar’s test for categorical variables. P values < 0.05 were considered statistically significant. SAS Enterprise Guide 7.15 (Cary, NC) was used for all statistical analyses.
The requirement for informed consent was waived by our Institutional Review Board, as the study was initially deemed to be a quality improvement project, and all data used for this report were de-identified.
Results
Our retrospective case-control analysis included a sample of 102 surgical patients whose courses were complicated by VTE between September 2012 and October 2015. The cases were distributed among 6 different surgical categories (Figure 1): trauma (20%), cancer (10%), cardiovascular (21%), noncancer neurosurgery (28%), elective orthopedics (11%), and miscellaneous general surgery (10%). 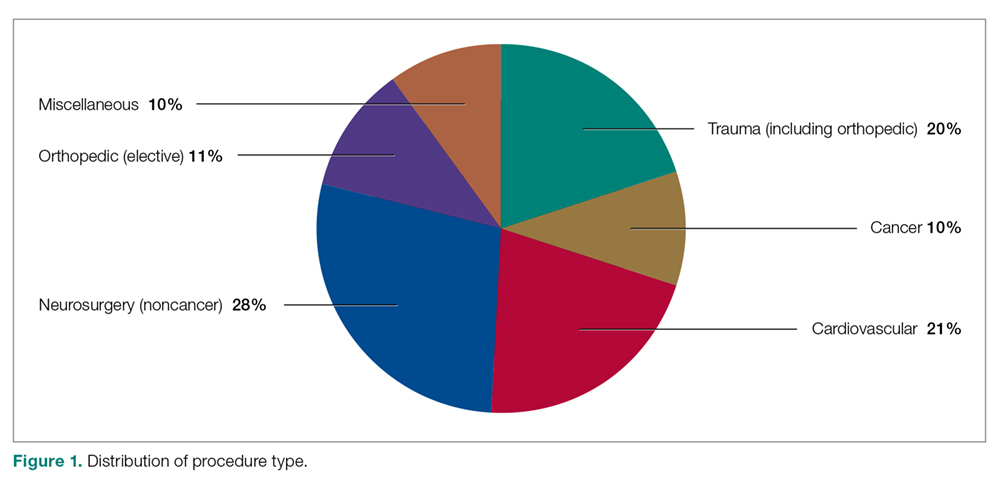
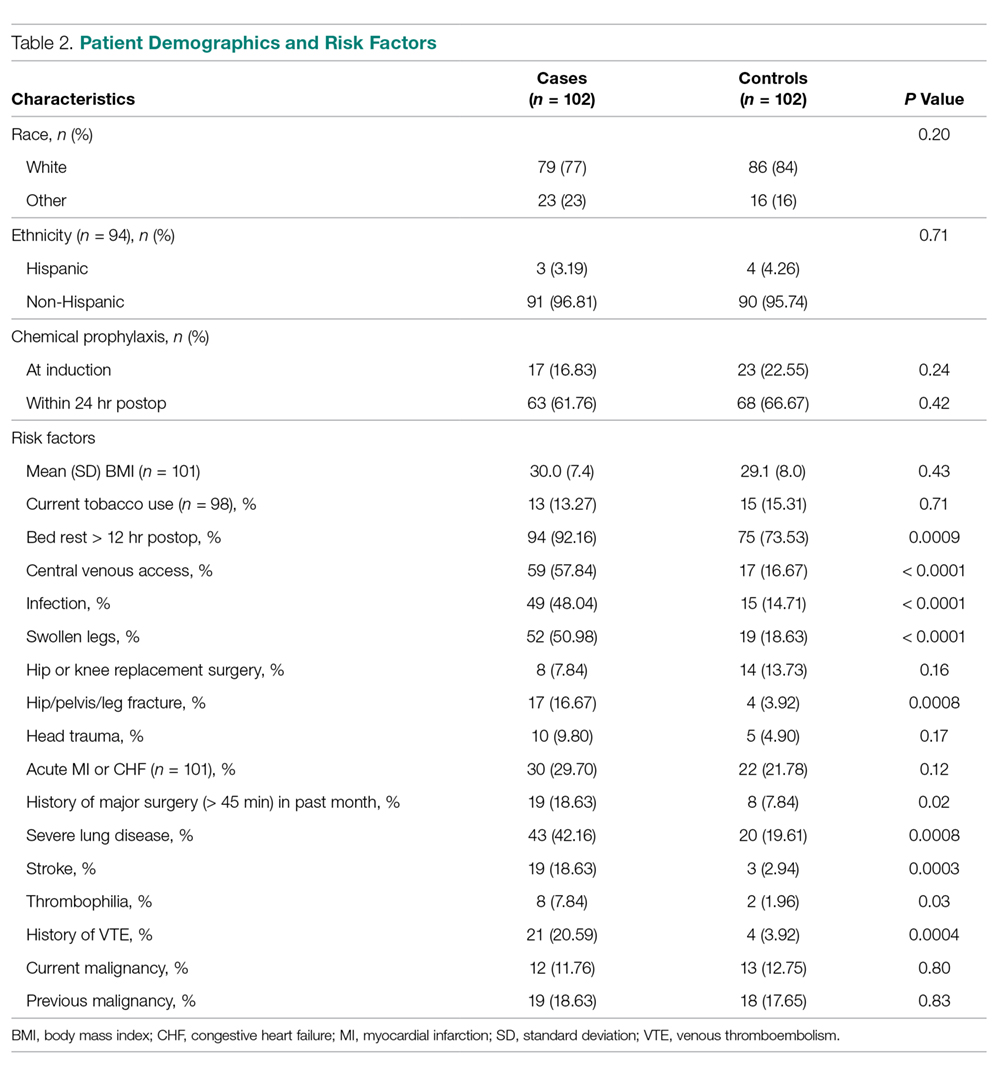
Comparisons between cases and controls in terms of patient demographics and risk factors are shown in Table 2. No statistically significant difference was observed in ethnicity or race between the 2 groups. Overall, cases had more hip/pelvis/leg fractures at presentation (P = 0.0008). The case group also had higher proportions of patients with postoperative bed rest greater than 12 hours (P = 0.009), central venous access (P < 0.0001), infection (P < 0.0001), and lower extremity edema documented during the hospitalization prior to development of DVT (P < 0.0001). Additionally, cases had significantly greater rates of previous VTE (P = 0.0004), inherited or acquired thrombophilia (P = 0.03), history of stroke (P = 0.0003), and severe lung disease, including pneumonia (P = 0.0008). No significant differences were noted between cases and matched controls in BMI (P = 0.43), current tobacco use (P = 0.71), current malignancy (P = 0.80), previous malignancy (P = 0.83), head trauma (P = 0.17), or acute cardiac disease (myocardial infarction or congestive heart failure; P = 0.12).
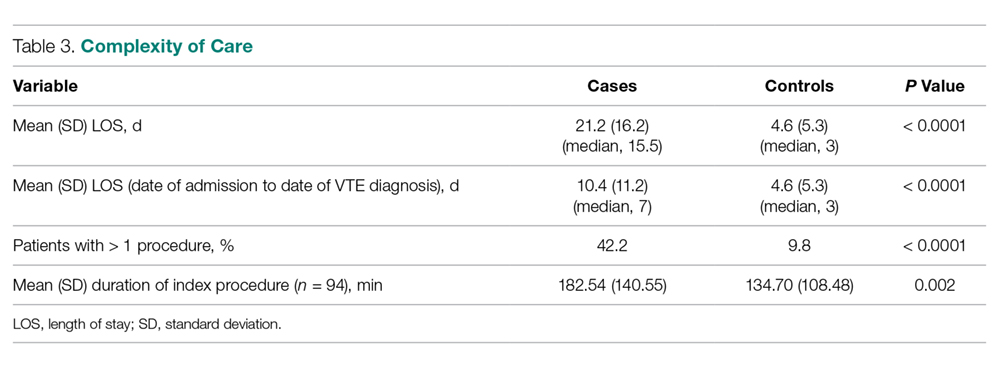
Variables felt to indicate overall complexity of hospital course for cases as compared to controls are outlined in Table 3. Cases were found to have significantly longer lengths of stay (median, 15.5 days versus 3 days, P < 0.0001). To account for the possibility that the development of VTE contributed to the increased length of stay in the cases, we also looked at the duration between admission date and the date of VTE diagnosis and determined that cases still had a longer length of stay when this was accounted for (median, 7 days versus 3 days, P < 0.0001). A much higher proportion of cases underwent more than 1 procedure compared to controls (P < 0.0001), and cases had significantly longer index operations as compared to controls (P = 0.002).
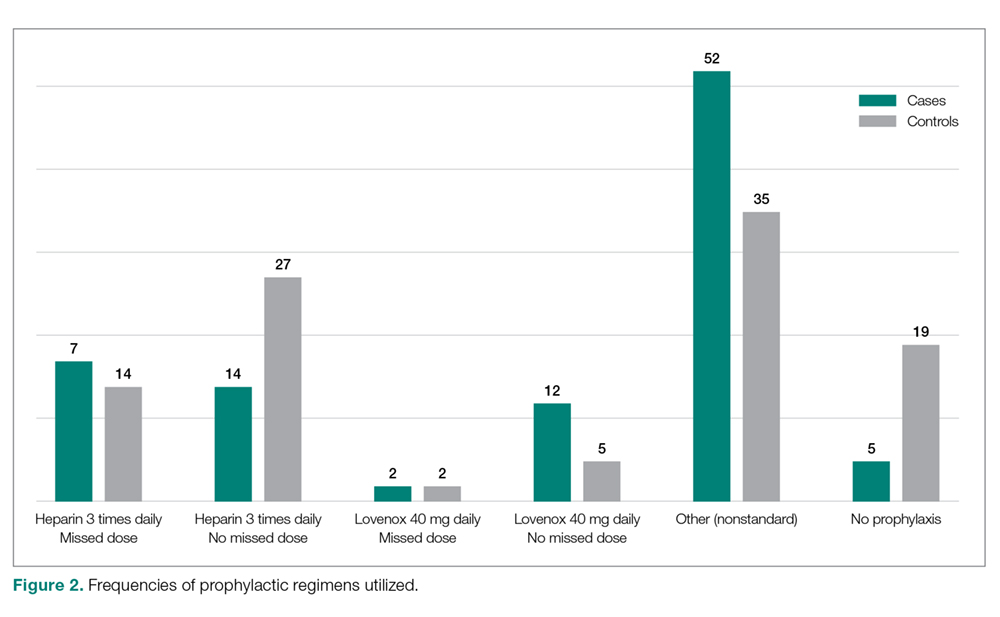
Seventeen cases received heparin on induction during their index procedure, compared to 23 controls (P = 0.24). Additionally, 63 cases began a prophylaxis regimen within 24 hours of surgery end time, compared to 68 controls (P = 0.24). The chemoprophylactic regimens utilized in cases and in controls are summarized in Figure 2. Of note, only 26 cases and 32 controls received standard prophylactic regimens with no missed doses (heparin 5000 units 3 times daily or enoxaparin 40 mg daily). Additionally, in over half of cases and a third of controls, nonstandard regimens were ordered. Examples of nonstandard regimens included nonstandard heparin or enoxaparin doses, low-dose warfarin, or aspirin alone. In most cases, nonstandard regimens were justified on the basis of high risk for bleeding.
Mechanical prophylaxis with pneumatic sequential compression devices (SCDs) was ordered in 93 (91%) cases and 87 (85%) controls; however, we were unable to accurately document uniform compliance in the use of these devices.
With regard to evaluation of our process measures, we found only 17% of cases and controls combined actually had a VTE risk assessment in their chart, and when it was present, it was often incomplete or was completed inaccurately.
Discussion
The goal of this study was to identify factors (patient characteristics and/or processes of care) that may be contributing to the higher than expected incidence of VTE events at our medical center, despite internal audits suggesting near perfect compliance with SCIP-mandated protocols. We found that in addition to usual risk factors for VTE, an overarching theme of our case cohort was their high complexity of illness. At baseline, these patients had significantly greater rates of stroke, thrombophilia, severe lung disease, infection, and history of VTE than controls. Moreover, the hospital courses of cases were significantly more complex than those of controls, as these patients had more procedures, longer lengths of stay and longer index operations, higher rates of postoperative bed rest exceeding 12 hours, and more prevalent central venous access than controls (Table 2). Several of these risk factors have been found to contribute to VTE development despite compliance with prophylaxis protocols.
Cassidy et al reviewed a cohort of nontrauma general surgery patients who developed VTE despite receiving appropriate prophylaxis and found that both multiple operations and emergency procedures contributed to the failure of VTE prophylaxis.11 Similarly, Wang et al identified several independent risk factors for VTE despite thromboprophylaxis, including central venous access and infection, as well as intensive care unit admission, hospitalization for cranial surgery, and admission from a long-term care facility.12 While our study did not capture some of these additional factors considered by Wang et al, the presence of risk factors not captured in traditional assessment tools suggests that additional consideration for complex patients is warranted.
In addition to these nonmodifiable patient characteristics, aspects of our VTE prophylaxis processes likely contributed to the higher than expected rate of VTE. While the electronic medical record at our institution does contain a VTE risk assessment tool based on the Caprini score, we found it often is not used at all or is used incorrectly/incompletely, which likely reflects the fact that physicians are neither prompted nor required to complete the assessment prior to prescribing VTE prophylaxis.
There is a significant body of evidence demonstrating that mandatory computerized VTE risk assessments can effectively reduce VTE rates and that improved outcomes occur shortly after implementation. Cassidy et al demonstrated the benefits of instituting a hospital-wide, mandatory, Caprini-based computerized VTE risk assessment that provides prophylaxis/early ambulation recommendations. Two years after implementing this system, they observed an 84% reduction in DVTs (P < 0.001) and a 55% reduction in PEs (P < 0.001).13 Nimeri et al had similarly impressive success, achieving a reduction in their NSQIP O/E for PE/DVT in general surgery from 6.00 in 2010 to 0.82 (for DVTs) and 0.78 (for PEs) 5 years after implementation of mandatory VTE risk assessment (though they noted that the most dramatic reduction occurred 1 year after implementation).14 Additionally, a recent systematic review and meta-analysis by Borab et al found computerized VTE risk assessments to be associated with a significant decrease in VTE events.15
The risk assessment tool used at our institution is qualitative in nature, and current literature suggests that employing a more quantitative tool may yield improved outcomes. Numerous studies have highlighted the importance of identifying patients at very high risk for VTE, as higher risk may necessitate more careful consideration of their prophylactic regimens. Obi et al found patients with Caprini scores higher than 8 to be at significantly greater risk of developing VTE compared to patients with scores of 7 or 8. Also, patients with scores of 7 or 8 were significantly more likely to have a VTE compared to those with scores of 5 or 6.16 In another study, Lobastov et al identified Caprini scores of 11 or higher as representing an extremely high-risk category for which standard prophylaxis regimens may not be effective.17 Thus, while having mandatory risk assessment has been shown to dramatically decrease VTE incidence, it is important to consider the magnitude of the numerical risk score. This is of particular importance at medical centers with high case-mix indices where patients at the highest risk might need to be managed with different prophylactic guidelines.
Another notable aspect of the process at our hospital was the great variation in the types of prophylactic regimens ordered, and the adherence to what was ordered. Only 25.5% of patients were maintained on a standard prophylactic regimen with no missed doses (heparin 5000 every 8 hours or enoxaparin 40 mg daily). Thus, the vast majority of the patients who went on to develop VTE either were prescribed a nontraditional prophylaxis regimen or missed doses of standard agents. The need for secondary surgical procedures or other invasive interventions may explain many, but not all, of the missed doses.
The timing of prophylaxis initiation for our patients was also found to deviate from accepted standards. Only 16.8% of cases received prophylaxis upon induction of anesthesia, and furthermore, 38% of cases did not receive any anticoagulation within 24 hours of their index operation. While this variability in prophylaxis implementation was acceptable within the SCIP guidelines based on “high risk for bleeding” or other considerations, it likely contributed to our suboptimal outcomes. The variations and interruptions in prophylactic regimens speak to barriers that have previously been reported as contributing factors to noncompliance with VTE prophylaxis.18
Given these known barriers and the observed underutilization and improper use of our risk assessment tool, we have recently changed our surgical admission order sets such that a mandatory quantitative risk assessment must be done for every surgical patient at the time of admission/operation before other orders can be completed. Following completion of the assessment, the physician will be presented with an appropriate standard regimen based on the individual patient’s risk assessment. Early results of our VTE quality improvement project have been satisfying: in the most recent NSQIP semi-annual report, our O/E for VTE was 0.74, placing us in the first decile. Some of these early reports may simply be the product of the Hawthorne effect; however, we are encouraged by the early improvements seen in other research. While we are hopeful that these changes will result in sustainable improvements in outcomes, patients at extremely high risk may require novel weight-based or otherwise customized aggressive prophylactic regimens. Such regimens have already been proposed for arthroplasty and other high-risk patients.
Future research may identify other risk factors not captured by traditional risk assessments. In addition, research should continue to explore the use and efficacy of standard prophylactic regimens in these populations to help determine if they are sufficient. Currently, weight-based low-molecular-weight heparin dosing and alternative regimens employing fondaparinux are under investigation for very-high-risk patients.19
There were several limitations to the present study. First, due to the retrospective design of our study, we could collect only data that had been uniformly recorded in the charts throughout the study period. Second, we were unable to accurately assess compliance with mechanical prophylaxis. While our chart review showed that the vast majority of cases and controls were ordered to have mechanical prophylaxis, it is impossible to document how often these devices were used appropriately in a retrospective analysis. Anecdotal observation suggests that once patients are out of post-anesthesia or critical care units, SCD use is not standardized. The inability to measure compliance precisely may be leading to an overestimation of our compliance with prophylaxis. Finally, because our study included only patients who underwent surgery at our hospital, our observations may not be generalizable outside our institution.
Conclusion
Our study findings reinforce the importance of attention to detail in VTE risk assessment and in ordering and administering VTE prophylactic regimens, especially in high-risk surgical patients. While we adhered to the SCIP-mandated prophylaxis requirements, the complexity of our patients and our lack of a truly standardized approach to risk assessment and prophylactic regimens resulted in suboptimal outcomes. Stricter and more quantitative mandatory VTE risk assessment, along with highly standardized VTE prophylaxis regimens, are required to achieve optimal outcomes.
Corresponding author: Jason C. DeGiovanni, MS, BA, [email protected].
Financial disclosures: None.
1. Spyropoulos AC, Hussein M, Lin J, et al. Rates of symptomatic venous thromboembolism in US surgical patients: a retrospective administrative database study. J Thromb Thrombolysis. 2009;28:458-464.
2. Deitzelzweig SB, Johnson BH, Lin J, et al. Prevalence of clinical venous thromboembolism in the USA: Current trends and future projections. Am J Hematol. 2011;86:217-220.
3. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979-1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711-1717.
4. Guyatt GH, Akl EA, Crowther M, et al. Introduction to the ninth edition: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):48S-52S.
5. Office of the Surgeon General; National Heart, Lung, and Blood Institute. The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville, MD: Office of the Surgeon General; 2008. www.ncbi.nlm.nih.gov/books/NBK44178/. Accessed May 2, 2019.
6. Pannucci CJ, Swistun L, MacDonald JK, et al. Individualized venous thromboembolism risk stratification using the 2005 Caprini score to identify the benefits and harms of chemoprophylaxis in surgical patients: a meta-analysis. Ann Surg. 2017;265:1094-1102.
7. Caprini JA, Arcelus JI, Hasty JH, et al. Clinical assessment of venous thromboembolic risk in surgical patients. Semin Thromb Hemost. 1991;17(suppl 3):304-312.
8. Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199:S3-S10.
9. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e227S-e277S.
10. The Joint Commission. Surgical Care Improvement Project (SCIP) Measure Information Form (Version 2.1c). www.jointcommission.org/surgical_care_improvement_project_scip_measure_information_form_version_21c/. Accessed June 22, 2016.
11. Cassidy MR, Macht RD, Rosenkranz P, et al. Patterns of failure of a standardized perioperative venous thromboembolism prophylaxis protocol. J Am Coll Surg. 2016;222:1074-1081.
12. Wang TF, Wong CA, Milligan PE, et al. Risk factors for inpatient venous thromboembolism despite thromboprophylaxis. Thromb Res. 2014;133:25-29.
13. Cassidy MR, Rosenkranz P, McAneny D. Reducing postoperative venous thromboembolism complications with a standardized risk-stratified prophylaxis protocol and mobilization program. J Am Coll Surg. 2014;218:1095-1104.
14. Nimeri AA, Gamaleldin MM, McKenna KL, et al. Reduction of venous thromboembolism in surgical patients using a mandatory risk-scoring system: 5-year follow-up of an American College of Surgeons National Quality Improvement Program. Clin Appl Thromb Hemost. 2017;23:392-396.
15. Borab ZM, Lanni MA, Tecce MG, et al. Use of computerized clinical decision support systems to prevent venous thromboembolism in surgical patients: a systematic review and meta-analysis. JAMA Surg. 2017;152:638–645.
16. Obi AT, Pannucci CJ, Nackashi A, et al. Validation of the Caprini venous thromboembolism risk assessment model in critically ill surgical patients. JAMA Surg. 2015;150:941-948.
17. Lobastov K, Barinov V, Schastlivtsev I, et al. Validation of the Caprini risk assessment model for venous thromboembolism in high-risk surgical patients in the background of standard prophylaxis. J Vasc Surg Venous Lymphat Disord. 2016;4:153-160.
18. Kakkar AK, Cohen AT, Tapson VF, et al. Venous thromboembolism risk and prophylaxis in the acute care hospital setting (ENDORSE survey): findings in surgical patients. Ann Surg. 2010;251:330-338.
19. Smythe MA, Priziola J, Dobesh PP, et al. Guidance for the practical management of the heparin anticoagulants in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41:165-186.
From Tufts Medical Center, Boston, MA.
Abstract
- Objective: Audits at our academic medical center revealed near 100% compliance with protocols for perioperative venous thromboembolism (VTE) prophylaxis, but recent National Surgical Quality Improvement Program data demonstrated a higher than expected incidence of VTE (observed/expected = 1.32). The objective of this study was to identify potential causes of this discrepancy.
- Design: Retrospective case-control study.
- Setting: Urban academic medical center with high case-mix indices (Medicare approximately 2.4, non-Medicare approximately 2.0).
- Participants: 102 surgical inpatients with VTE (September 2012 to October 2015) matched with controls for age, gender, and type of procedure.
- Measurements: Prevalence of common VTE risk factors, length of stay, number of procedures, index operation times, and postoperative bed rest > 12 hours were assessed. Utilization of and compliance with our VTE risk assessment tool was also investigated.
- Results: Cases underwent more procedures and had longer lengths of stay and index procedures than controls. In addition, cases were more likely to have had > 12 hours of postoperative bed rest and central venous access than controls. Cases had more infections and were more likely to have severe lung disease, thrombophilia, and a history of prior VTE than controls. No differences in body mass index, tobacco use, current or previous malignancy, or VTE risk assessment form use were observed. Overall, care complexity and risk factors were equally important in determining VTE incidence. Our analyses also revealed lack of strict adherence to our VTE risk stratification protocol and frequent use of suboptimal prophylactic regimens.
- Conclusion: Well-accepted risk factors and overall care complexity determine VTE risk. Preventing VTE in high-risk patients requires assiduous attention to detail in VTE risk assessment and in delivery of optimal prophylaxis. Patients at especially high risk may require customized prophylactic regimens.
Keywords: hospital-acquired venous thromboembolic disease; VTE prophylaxis, surgical patients.
Deep vein thrombosis (DVT) and pulmonary embolism (PE) are well-recognized causes of morbidity and mortality in surgical patients. Between 350,000 and 600,000 cases of venous thromboembolism (VTE) occur each year in the United States, and it is responsible for approximately 10% of preventable in-hospital fatalities.1-3 Given VTE’s impact on patients and the healthcare system and the fact that it is preventable, intense effort has been focused on developing more effective prophylactic measures to decrease its incidence.2-4 In 2008, the surgeon general issued a “call to action” for increased efforts to prevent VTE.5
The American College of Chest Physicians (ACCP) guidelines subcategorize patients based on type of surgery. In addition, the ACCP guidelines support the use of a Caprini-based scoring system to aid in risk stratification and improve clinical decision-making (

Our hospital, a 350-bed academic medical center in downtown Boston, MA, serving a diverse population with a very high case-mix index (2.4 Medicare and 2.0 non-Medicare), has strict protocols for VTE prophylaxis consistent with the ACCP guidelines and based on the Surgical Care Improvement Project (SCIP) measures published in 2006.10 The SCIP mandates allow for considerable surgeon discretion in the use of chemoprophylaxis for neurosurgical cases and general and orthopedic surgery cases deemed to be at high risk for bleeding. In addition, SCIP requires only that prophylaxis be initiated within 24 hours of surgical end time. Although recent audits revealed nearly 100% compliance with SCIP-mandated protocols, National Surgical Quality Improvement Program (NSQIP) data showed that the incidence of VTE events at our institution was higher than expected (observed/expected [O/E] = 1.32).
In order to determine the reasons for this mismatch between process and outcome performance, we investigated whether there were characteristics of our patient population that contributed to the higher than expected rates of VTE, and we scrutinized our VTE prophylaxis protocol to determine if there were aspects of our process that were also contributory.
Methods
Study Sample
This is a retrospective case-control study of surgical inpatients at our hospital during the period September 2012 to October 2015. Cases were identified as patients diagnosed with a VTE (DVT or PE). Controls were identified from a pool of surgical patients whose courses were not complicated by VTE during the same time frame as the cases and who were matched as closely as possible by procedure code, age, and gender.
Variables
Patient and hospital course variables that were analyzed included demographics, comorbidities, length of stay, number of procedures, index operation times, duration of postoperative bed rest, use of mechanical prophylaxis, and type of chemoprophylaxis and time frame within which it was initiated. Data were collected via chart review using International Classification of Diseases-9 and -10 codes to identify surgical cases within the allotted time period who were diagnosed with VTE. Demographic variables included age, sex, and ethnicity. Comorbidities included hypertension, diabetes, coronary artery disease, serious lung disease, previous or current malignancy, documented hypercoagulable state, and previous history of VTE. Body mass index (BMI) was also recorded. The aforementioned disease-specific variables were not matched between the case and control groups, as this data was obtained retrospectively during data collection.
Analysis
Associations between case and matched control were analyzed using the paired t-test for continuous variables and McNemar’s test for categorical variables. P values < 0.05 were considered statistically significant. SAS Enterprise Guide 7.15 (Cary, NC) was used for all statistical analyses.
The requirement for informed consent was waived by our Institutional Review Board, as the study was initially deemed to be a quality improvement project, and all data used for this report were de-identified.
Results
Our retrospective case-control analysis included a sample of 102 surgical patients whose courses were complicated by VTE between September 2012 and October 2015. The cases were distributed among 6 different surgical categories (Figure 1): trauma (20%), cancer (10%), cardiovascular (21%), noncancer neurosurgery (28%), elective orthopedics (11%), and miscellaneous general surgery (10%). 
Comparisons between cases and controls in terms of patient demographics and risk factors are shown in Table 2. No statistically significant difference was observed in ethnicity or race between the 2 groups. Overall, cases had more hip/pelvis/leg fractures at presentation (P = 0.0008). The case group also had higher proportions of patients with postoperative bed rest greater than 12 hours (P = 0.009), central venous access (P < 0.0001), infection (P < 0.0001), and lower extremity edema documented during the hospitalization prior to development of DVT (P < 0.0001). Additionally, cases had significantly greater rates of previous VTE (P = 0.0004), inherited or acquired thrombophilia (P = 0.03), history of stroke (P = 0.0003), and severe lung disease, including pneumonia (P = 0.0008). No significant differences were noted between cases and matched controls in BMI (P = 0.43), current tobacco use (P = 0.71), current malignancy (P = 0.80), previous malignancy (P = 0.83), head trauma (P = 0.17), or acute cardiac disease (myocardial infarction or congestive heart failure; P = 0.12).

Variables felt to indicate overall complexity of hospital course for cases as compared to controls are outlined in Table 3. Cases were found to have significantly longer lengths of stay (median, 15.5 days versus 3 days, P < 0.0001). To account for the possibility that the development of VTE contributed to the increased length of stay in the cases, we also looked at the duration between admission date and the date of VTE diagnosis and determined that cases still had a longer length of stay when this was accounted for (median, 7 days versus 3 days, P < 0.0001). A much higher proportion of cases underwent more than 1 procedure compared to controls (P < 0.0001), and cases had significantly longer index operations as compared to controls (P = 0.002).

Seventeen cases received heparin on induction during their index procedure, compared to 23 controls (P = 0.24). Additionally, 63 cases began a prophylaxis regimen within 24 hours of surgery end time, compared to 68 controls (P = 0.24). The chemoprophylactic regimens utilized in cases and in controls are summarized in Figure 2. Of note, only 26 cases and 32 controls received standard prophylactic regimens with no missed doses (heparin 5000 units 3 times daily or enoxaparin 40 mg daily). Additionally, in over half of cases and a third of controls, nonstandard regimens were ordered. Examples of nonstandard regimens included nonstandard heparin or enoxaparin doses, low-dose warfarin, or aspirin alone. In most cases, nonstandard regimens were justified on the basis of high risk for bleeding.

Mechanical prophylaxis with pneumatic sequential compression devices (SCDs) was ordered in 93 (91%) cases and 87 (85%) controls; however, we were unable to accurately document uniform compliance in the use of these devices.
With regard to evaluation of our process measures, we found only 17% of cases and controls combined actually had a VTE risk assessment in their chart, and when it was present, it was often incomplete or was completed inaccurately.
Discussion
The goal of this study was to identify factors (patient characteristics and/or processes of care) that may be contributing to the higher than expected incidence of VTE events at our medical center, despite internal audits suggesting near perfect compliance with SCIP-mandated protocols. We found that in addition to usual risk factors for VTE, an overarching theme of our case cohort was their high complexity of illness. At baseline, these patients had significantly greater rates of stroke, thrombophilia, severe lung disease, infection, and history of VTE than controls. Moreover, the hospital courses of cases were significantly more complex than those of controls, as these patients had more procedures, longer lengths of stay and longer index operations, higher rates of postoperative bed rest exceeding 12 hours, and more prevalent central venous access than controls (Table 2). Several of these risk factors have been found to contribute to VTE development despite compliance with prophylaxis protocols.
Cassidy et al reviewed a cohort of nontrauma general surgery patients who developed VTE despite receiving appropriate prophylaxis and found that both multiple operations and emergency procedures contributed to the failure of VTE prophylaxis.11 Similarly, Wang et al identified several independent risk factors for VTE despite thromboprophylaxis, including central venous access and infection, as well as intensive care unit admission, hospitalization for cranial surgery, and admission from a long-term care facility.12 While our study did not capture some of these additional factors considered by Wang et al, the presence of risk factors not captured in traditional assessment tools suggests that additional consideration for complex patients is warranted.
In addition to these nonmodifiable patient characteristics, aspects of our VTE prophylaxis processes likely contributed to the higher than expected rate of VTE. While the electronic medical record at our institution does contain a VTE risk assessment tool based on the Caprini score, we found it often is not used at all or is used incorrectly/incompletely, which likely reflects the fact that physicians are neither prompted nor required to complete the assessment prior to prescribing VTE prophylaxis.
There is a significant body of evidence demonstrating that mandatory computerized VTE risk assessments can effectively reduce VTE rates and that improved outcomes occur shortly after implementation. Cassidy et al demonstrated the benefits of instituting a hospital-wide, mandatory, Caprini-based computerized VTE risk assessment that provides prophylaxis/early ambulation recommendations. Two years after implementing this system, they observed an 84% reduction in DVTs (P < 0.001) and a 55% reduction in PEs (P < 0.001).13 Nimeri et al had similarly impressive success, achieving a reduction in their NSQIP O/E for PE/DVT in general surgery from 6.00 in 2010 to 0.82 (for DVTs) and 0.78 (for PEs) 5 years after implementation of mandatory VTE risk assessment (though they noted that the most dramatic reduction occurred 1 year after implementation).14 Additionally, a recent systematic review and meta-analysis by Borab et al found computerized VTE risk assessments to be associated with a significant decrease in VTE events.15
The risk assessment tool used at our institution is qualitative in nature, and current literature suggests that employing a more quantitative tool may yield improved outcomes. Numerous studies have highlighted the importance of identifying patients at very high risk for VTE, as higher risk may necessitate more careful consideration of their prophylactic regimens. Obi et al found patients with Caprini scores higher than 8 to be at significantly greater risk of developing VTE compared to patients with scores of 7 or 8. Also, patients with scores of 7 or 8 were significantly more likely to have a VTE compared to those with scores of 5 or 6.16 In another study, Lobastov et al identified Caprini scores of 11 or higher as representing an extremely high-risk category for which standard prophylaxis regimens may not be effective.17 Thus, while having mandatory risk assessment has been shown to dramatically decrease VTE incidence, it is important to consider the magnitude of the numerical risk score. This is of particular importance at medical centers with high case-mix indices where patients at the highest risk might need to be managed with different prophylactic guidelines.
Another notable aspect of the process at our hospital was the great variation in the types of prophylactic regimens ordered, and the adherence to what was ordered. Only 25.5% of patients were maintained on a standard prophylactic regimen with no missed doses (heparin 5000 every 8 hours or enoxaparin 40 mg daily). Thus, the vast majority of the patients who went on to develop VTE either were prescribed a nontraditional prophylaxis regimen or missed doses of standard agents. The need for secondary surgical procedures or other invasive interventions may explain many, but not all, of the missed doses.
The timing of prophylaxis initiation for our patients was also found to deviate from accepted standards. Only 16.8% of cases received prophylaxis upon induction of anesthesia, and furthermore, 38% of cases did not receive any anticoagulation within 24 hours of their index operation. While this variability in prophylaxis implementation was acceptable within the SCIP guidelines based on “high risk for bleeding” or other considerations, it likely contributed to our suboptimal outcomes. The variations and interruptions in prophylactic regimens speak to barriers that have previously been reported as contributing factors to noncompliance with VTE prophylaxis.18
Given these known barriers and the observed underutilization and improper use of our risk assessment tool, we have recently changed our surgical admission order sets such that a mandatory quantitative risk assessment must be done for every surgical patient at the time of admission/operation before other orders can be completed. Following completion of the assessment, the physician will be presented with an appropriate standard regimen based on the individual patient’s risk assessment. Early results of our VTE quality improvement project have been satisfying: in the most recent NSQIP semi-annual report, our O/E for VTE was 0.74, placing us in the first decile. Some of these early reports may simply be the product of the Hawthorne effect; however, we are encouraged by the early improvements seen in other research. While we are hopeful that these changes will result in sustainable improvements in outcomes, patients at extremely high risk may require novel weight-based or otherwise customized aggressive prophylactic regimens. Such regimens have already been proposed for arthroplasty and other high-risk patients.
Future research may identify other risk factors not captured by traditional risk assessments. In addition, research should continue to explore the use and efficacy of standard prophylactic regimens in these populations to help determine if they are sufficient. Currently, weight-based low-molecular-weight heparin dosing and alternative regimens employing fondaparinux are under investigation for very-high-risk patients.19
There were several limitations to the present study. First, due to the retrospective design of our study, we could collect only data that had been uniformly recorded in the charts throughout the study period. Second, we were unable to accurately assess compliance with mechanical prophylaxis. While our chart review showed that the vast majority of cases and controls were ordered to have mechanical prophylaxis, it is impossible to document how often these devices were used appropriately in a retrospective analysis. Anecdotal observation suggests that once patients are out of post-anesthesia or critical care units, SCD use is not standardized. The inability to measure compliance precisely may be leading to an overestimation of our compliance with prophylaxis. Finally, because our study included only patients who underwent surgery at our hospital, our observations may not be generalizable outside our institution.
Conclusion
Our study findings reinforce the importance of attention to detail in VTE risk assessment and in ordering and administering VTE prophylactic regimens, especially in high-risk surgical patients. While we adhered to the SCIP-mandated prophylaxis requirements, the complexity of our patients and our lack of a truly standardized approach to risk assessment and prophylactic regimens resulted in suboptimal outcomes. Stricter and more quantitative mandatory VTE risk assessment, along with highly standardized VTE prophylaxis regimens, are required to achieve optimal outcomes.
Corresponding author: Jason C. DeGiovanni, MS, BA, [email protected].
Financial disclosures: None.
From Tufts Medical Center, Boston, MA.
Abstract
- Objective: Audits at our academic medical center revealed near 100% compliance with protocols for perioperative venous thromboembolism (VTE) prophylaxis, but recent National Surgical Quality Improvement Program data demonstrated a higher than expected incidence of VTE (observed/expected = 1.32). The objective of this study was to identify potential causes of this discrepancy.
- Design: Retrospective case-control study.
- Setting: Urban academic medical center with high case-mix indices (Medicare approximately 2.4, non-Medicare approximately 2.0).
- Participants: 102 surgical inpatients with VTE (September 2012 to October 2015) matched with controls for age, gender, and type of procedure.
- Measurements: Prevalence of common VTE risk factors, length of stay, number of procedures, index operation times, and postoperative bed rest > 12 hours were assessed. Utilization of and compliance with our VTE risk assessment tool was also investigated.
- Results: Cases underwent more procedures and had longer lengths of stay and index procedures than controls. In addition, cases were more likely to have had > 12 hours of postoperative bed rest and central venous access than controls. Cases had more infections and were more likely to have severe lung disease, thrombophilia, and a history of prior VTE than controls. No differences in body mass index, tobacco use, current or previous malignancy, or VTE risk assessment form use were observed. Overall, care complexity and risk factors were equally important in determining VTE incidence. Our analyses also revealed lack of strict adherence to our VTE risk stratification protocol and frequent use of suboptimal prophylactic regimens.
- Conclusion: Well-accepted risk factors and overall care complexity determine VTE risk. Preventing VTE in high-risk patients requires assiduous attention to detail in VTE risk assessment and in delivery of optimal prophylaxis. Patients at especially high risk may require customized prophylactic regimens.
Keywords: hospital-acquired venous thromboembolic disease; VTE prophylaxis, surgical patients.
Deep vein thrombosis (DVT) and pulmonary embolism (PE) are well-recognized causes of morbidity and mortality in surgical patients. Between 350,000 and 600,000 cases of venous thromboembolism (VTE) occur each year in the United States, and it is responsible for approximately 10% of preventable in-hospital fatalities.1-3 Given VTE’s impact on patients and the healthcare system and the fact that it is preventable, intense effort has been focused on developing more effective prophylactic measures to decrease its incidence.2-4 In 2008, the surgeon general issued a “call to action” for increased efforts to prevent VTE.5
The American College of Chest Physicians (ACCP) guidelines subcategorize patients based on type of surgery. In addition, the ACCP guidelines support the use of a Caprini-based scoring system to aid in risk stratification and improve clinical decision-making (

Our hospital, a 350-bed academic medical center in downtown Boston, MA, serving a diverse population with a very high case-mix index (2.4 Medicare and 2.0 non-Medicare), has strict protocols for VTE prophylaxis consistent with the ACCP guidelines and based on the Surgical Care Improvement Project (SCIP) measures published in 2006.10 The SCIP mandates allow for considerable surgeon discretion in the use of chemoprophylaxis for neurosurgical cases and general and orthopedic surgery cases deemed to be at high risk for bleeding. In addition, SCIP requires only that prophylaxis be initiated within 24 hours of surgical end time. Although recent audits revealed nearly 100% compliance with SCIP-mandated protocols, National Surgical Quality Improvement Program (NSQIP) data showed that the incidence of VTE events at our institution was higher than expected (observed/expected [O/E] = 1.32).
In order to determine the reasons for this mismatch between process and outcome performance, we investigated whether there were characteristics of our patient population that contributed to the higher than expected rates of VTE, and we scrutinized our VTE prophylaxis protocol to determine if there were aspects of our process that were also contributory.
Methods
Study Sample
This is a retrospective case-control study of surgical inpatients at our hospital during the period September 2012 to October 2015. Cases were identified as patients diagnosed with a VTE (DVT or PE). Controls were identified from a pool of surgical patients whose courses were not complicated by VTE during the same time frame as the cases and who were matched as closely as possible by procedure code, age, and gender.
Variables
Patient and hospital course variables that were analyzed included demographics, comorbidities, length of stay, number of procedures, index operation times, duration of postoperative bed rest, use of mechanical prophylaxis, and type of chemoprophylaxis and time frame within which it was initiated. Data were collected via chart review using International Classification of Diseases-9 and -10 codes to identify surgical cases within the allotted time period who were diagnosed with VTE. Demographic variables included age, sex, and ethnicity. Comorbidities included hypertension, diabetes, coronary artery disease, serious lung disease, previous or current malignancy, documented hypercoagulable state, and previous history of VTE. Body mass index (BMI) was also recorded. The aforementioned disease-specific variables were not matched between the case and control groups, as this data was obtained retrospectively during data collection.
Analysis
Associations between case and matched control were analyzed using the paired t-test for continuous variables and McNemar’s test for categorical variables. P values < 0.05 were considered statistically significant. SAS Enterprise Guide 7.15 (Cary, NC) was used for all statistical analyses.
The requirement for informed consent was waived by our Institutional Review Board, as the study was initially deemed to be a quality improvement project, and all data used for this report were de-identified.
Results
Our retrospective case-control analysis included a sample of 102 surgical patients whose courses were complicated by VTE between September 2012 and October 2015. The cases were distributed among 6 different surgical categories (Figure 1): trauma (20%), cancer (10%), cardiovascular (21%), noncancer neurosurgery (28%), elective orthopedics (11%), and miscellaneous general surgery (10%). 
Comparisons between cases and controls in terms of patient demographics and risk factors are shown in Table 2. No statistically significant difference was observed in ethnicity or race between the 2 groups. Overall, cases had more hip/pelvis/leg fractures at presentation (P = 0.0008). The case group also had higher proportions of patients with postoperative bed rest greater than 12 hours (P = 0.009), central venous access (P < 0.0001), infection (P < 0.0001), and lower extremity edema documented during the hospitalization prior to development of DVT (P < 0.0001). Additionally, cases had significantly greater rates of previous VTE (P = 0.0004), inherited or acquired thrombophilia (P = 0.03), history of stroke (P = 0.0003), and severe lung disease, including pneumonia (P = 0.0008). No significant differences were noted between cases and matched controls in BMI (P = 0.43), current tobacco use (P = 0.71), current malignancy (P = 0.80), previous malignancy (P = 0.83), head trauma (P = 0.17), or acute cardiac disease (myocardial infarction or congestive heart failure; P = 0.12).

Variables felt to indicate overall complexity of hospital course for cases as compared to controls are outlined in Table 3. Cases were found to have significantly longer lengths of stay (median, 15.5 days versus 3 days, P < 0.0001). To account for the possibility that the development of VTE contributed to the increased length of stay in the cases, we also looked at the duration between admission date and the date of VTE diagnosis and determined that cases still had a longer length of stay when this was accounted for (median, 7 days versus 3 days, P < 0.0001). A much higher proportion of cases underwent more than 1 procedure compared to controls (P < 0.0001), and cases had significantly longer index operations as compared to controls (P = 0.002).

Seventeen cases received heparin on induction during their index procedure, compared to 23 controls (P = 0.24). Additionally, 63 cases began a prophylaxis regimen within 24 hours of surgery end time, compared to 68 controls (P = 0.24). The chemoprophylactic regimens utilized in cases and in controls are summarized in Figure 2. Of note, only 26 cases and 32 controls received standard prophylactic regimens with no missed doses (heparin 5000 units 3 times daily or enoxaparin 40 mg daily). Additionally, in over half of cases and a third of controls, nonstandard regimens were ordered. Examples of nonstandard regimens included nonstandard heparin or enoxaparin doses, low-dose warfarin, or aspirin alone. In most cases, nonstandard regimens were justified on the basis of high risk for bleeding.

Mechanical prophylaxis with pneumatic sequential compression devices (SCDs) was ordered in 93 (91%) cases and 87 (85%) controls; however, we were unable to accurately document uniform compliance in the use of these devices.
With regard to evaluation of our process measures, we found only 17% of cases and controls combined actually had a VTE risk assessment in their chart, and when it was present, it was often incomplete or was completed inaccurately.
Discussion
The goal of this study was to identify factors (patient characteristics and/or processes of care) that may be contributing to the higher than expected incidence of VTE events at our medical center, despite internal audits suggesting near perfect compliance with SCIP-mandated protocols. We found that in addition to usual risk factors for VTE, an overarching theme of our case cohort was their high complexity of illness. At baseline, these patients had significantly greater rates of stroke, thrombophilia, severe lung disease, infection, and history of VTE than controls. Moreover, the hospital courses of cases were significantly more complex than those of controls, as these patients had more procedures, longer lengths of stay and longer index operations, higher rates of postoperative bed rest exceeding 12 hours, and more prevalent central venous access than controls (Table 2). Several of these risk factors have been found to contribute to VTE development despite compliance with prophylaxis protocols.
Cassidy et al reviewed a cohort of nontrauma general surgery patients who developed VTE despite receiving appropriate prophylaxis and found that both multiple operations and emergency procedures contributed to the failure of VTE prophylaxis.11 Similarly, Wang et al identified several independent risk factors for VTE despite thromboprophylaxis, including central venous access and infection, as well as intensive care unit admission, hospitalization for cranial surgery, and admission from a long-term care facility.12 While our study did not capture some of these additional factors considered by Wang et al, the presence of risk factors not captured in traditional assessment tools suggests that additional consideration for complex patients is warranted.
In addition to these nonmodifiable patient characteristics, aspects of our VTE prophylaxis processes likely contributed to the higher than expected rate of VTE. While the electronic medical record at our institution does contain a VTE risk assessment tool based on the Caprini score, we found it often is not used at all or is used incorrectly/incompletely, which likely reflects the fact that physicians are neither prompted nor required to complete the assessment prior to prescribing VTE prophylaxis.
There is a significant body of evidence demonstrating that mandatory computerized VTE risk assessments can effectively reduce VTE rates and that improved outcomes occur shortly after implementation. Cassidy et al demonstrated the benefits of instituting a hospital-wide, mandatory, Caprini-based computerized VTE risk assessment that provides prophylaxis/early ambulation recommendations. Two years after implementing this system, they observed an 84% reduction in DVTs (P < 0.001) and a 55% reduction in PEs (P < 0.001).13 Nimeri et al had similarly impressive success, achieving a reduction in their NSQIP O/E for PE/DVT in general surgery from 6.00 in 2010 to 0.82 (for DVTs) and 0.78 (for PEs) 5 years after implementation of mandatory VTE risk assessment (though they noted that the most dramatic reduction occurred 1 year after implementation).14 Additionally, a recent systematic review and meta-analysis by Borab et al found computerized VTE risk assessments to be associated with a significant decrease in VTE events.15
The risk assessment tool used at our institution is qualitative in nature, and current literature suggests that employing a more quantitative tool may yield improved outcomes. Numerous studies have highlighted the importance of identifying patients at very high risk for VTE, as higher risk may necessitate more careful consideration of their prophylactic regimens. Obi et al found patients with Caprini scores higher than 8 to be at significantly greater risk of developing VTE compared to patients with scores of 7 or 8. Also, patients with scores of 7 or 8 were significantly more likely to have a VTE compared to those with scores of 5 or 6.16 In another study, Lobastov et al identified Caprini scores of 11 or higher as representing an extremely high-risk category for which standard prophylaxis regimens may not be effective.17 Thus, while having mandatory risk assessment has been shown to dramatically decrease VTE incidence, it is important to consider the magnitude of the numerical risk score. This is of particular importance at medical centers with high case-mix indices where patients at the highest risk might need to be managed with different prophylactic guidelines.
Another notable aspect of the process at our hospital was the great variation in the types of prophylactic regimens ordered, and the adherence to what was ordered. Only 25.5% of patients were maintained on a standard prophylactic regimen with no missed doses (heparin 5000 every 8 hours or enoxaparin 40 mg daily). Thus, the vast majority of the patients who went on to develop VTE either were prescribed a nontraditional prophylaxis regimen or missed doses of standard agents. The need for secondary surgical procedures or other invasive interventions may explain many, but not all, of the missed doses.
The timing of prophylaxis initiation for our patients was also found to deviate from accepted standards. Only 16.8% of cases received prophylaxis upon induction of anesthesia, and furthermore, 38% of cases did not receive any anticoagulation within 24 hours of their index operation. While this variability in prophylaxis implementation was acceptable within the SCIP guidelines based on “high risk for bleeding” or other considerations, it likely contributed to our suboptimal outcomes. The variations and interruptions in prophylactic regimens speak to barriers that have previously been reported as contributing factors to noncompliance with VTE prophylaxis.18
Given these known barriers and the observed underutilization and improper use of our risk assessment tool, we have recently changed our surgical admission order sets such that a mandatory quantitative risk assessment must be done for every surgical patient at the time of admission/operation before other orders can be completed. Following completion of the assessment, the physician will be presented with an appropriate standard regimen based on the individual patient’s risk assessment. Early results of our VTE quality improvement project have been satisfying: in the most recent NSQIP semi-annual report, our O/E for VTE was 0.74, placing us in the first decile. Some of these early reports may simply be the product of the Hawthorne effect; however, we are encouraged by the early improvements seen in other research. While we are hopeful that these changes will result in sustainable improvements in outcomes, patients at extremely high risk may require novel weight-based or otherwise customized aggressive prophylactic regimens. Such regimens have already been proposed for arthroplasty and other high-risk patients.
Future research may identify other risk factors not captured by traditional risk assessments. In addition, research should continue to explore the use and efficacy of standard prophylactic regimens in these populations to help determine if they are sufficient. Currently, weight-based low-molecular-weight heparin dosing and alternative regimens employing fondaparinux are under investigation for very-high-risk patients.19
There were several limitations to the present study. First, due to the retrospective design of our study, we could collect only data that had been uniformly recorded in the charts throughout the study period. Second, we were unable to accurately assess compliance with mechanical prophylaxis. While our chart review showed that the vast majority of cases and controls were ordered to have mechanical prophylaxis, it is impossible to document how often these devices were used appropriately in a retrospective analysis. Anecdotal observation suggests that once patients are out of post-anesthesia or critical care units, SCD use is not standardized. The inability to measure compliance precisely may be leading to an overestimation of our compliance with prophylaxis. Finally, because our study included only patients who underwent surgery at our hospital, our observations may not be generalizable outside our institution.
Conclusion
Our study findings reinforce the importance of attention to detail in VTE risk assessment and in ordering and administering VTE prophylactic regimens, especially in high-risk surgical patients. While we adhered to the SCIP-mandated prophylaxis requirements, the complexity of our patients and our lack of a truly standardized approach to risk assessment and prophylactic regimens resulted in suboptimal outcomes. Stricter and more quantitative mandatory VTE risk assessment, along with highly standardized VTE prophylaxis regimens, are required to achieve optimal outcomes.
Corresponding author: Jason C. DeGiovanni, MS, BA, [email protected].
Financial disclosures: None.
1. Spyropoulos AC, Hussein M, Lin J, et al. Rates of symptomatic venous thromboembolism in US surgical patients: a retrospective administrative database study. J Thromb Thrombolysis. 2009;28:458-464.
2. Deitzelzweig SB, Johnson BH, Lin J, et al. Prevalence of clinical venous thromboembolism in the USA: Current trends and future projections. Am J Hematol. 2011;86:217-220.
3. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979-1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711-1717.
4. Guyatt GH, Akl EA, Crowther M, et al. Introduction to the ninth edition: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):48S-52S.
5. Office of the Surgeon General; National Heart, Lung, and Blood Institute. The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville, MD: Office of the Surgeon General; 2008. www.ncbi.nlm.nih.gov/books/NBK44178/. Accessed May 2, 2019.
6. Pannucci CJ, Swistun L, MacDonald JK, et al. Individualized venous thromboembolism risk stratification using the 2005 Caprini score to identify the benefits and harms of chemoprophylaxis in surgical patients: a meta-analysis. Ann Surg. 2017;265:1094-1102.
7. Caprini JA, Arcelus JI, Hasty JH, et al. Clinical assessment of venous thromboembolic risk in surgical patients. Semin Thromb Hemost. 1991;17(suppl 3):304-312.
8. Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199:S3-S10.
9. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e227S-e277S.
10. The Joint Commission. Surgical Care Improvement Project (SCIP) Measure Information Form (Version 2.1c). www.jointcommission.org/surgical_care_improvement_project_scip_measure_information_form_version_21c/. Accessed June 22, 2016.
11. Cassidy MR, Macht RD, Rosenkranz P, et al. Patterns of failure of a standardized perioperative venous thromboembolism prophylaxis protocol. J Am Coll Surg. 2016;222:1074-1081.
12. Wang TF, Wong CA, Milligan PE, et al. Risk factors for inpatient venous thromboembolism despite thromboprophylaxis. Thromb Res. 2014;133:25-29.
13. Cassidy MR, Rosenkranz P, McAneny D. Reducing postoperative venous thromboembolism complications with a standardized risk-stratified prophylaxis protocol and mobilization program. J Am Coll Surg. 2014;218:1095-1104.
14. Nimeri AA, Gamaleldin MM, McKenna KL, et al. Reduction of venous thromboembolism in surgical patients using a mandatory risk-scoring system: 5-year follow-up of an American College of Surgeons National Quality Improvement Program. Clin Appl Thromb Hemost. 2017;23:392-396.
15. Borab ZM, Lanni MA, Tecce MG, et al. Use of computerized clinical decision support systems to prevent venous thromboembolism in surgical patients: a systematic review and meta-analysis. JAMA Surg. 2017;152:638–645.
16. Obi AT, Pannucci CJ, Nackashi A, et al. Validation of the Caprini venous thromboembolism risk assessment model in critically ill surgical patients. JAMA Surg. 2015;150:941-948.
17. Lobastov K, Barinov V, Schastlivtsev I, et al. Validation of the Caprini risk assessment model for venous thromboembolism in high-risk surgical patients in the background of standard prophylaxis. J Vasc Surg Venous Lymphat Disord. 2016;4:153-160.
18. Kakkar AK, Cohen AT, Tapson VF, et al. Venous thromboembolism risk and prophylaxis in the acute care hospital setting (ENDORSE survey): findings in surgical patients. Ann Surg. 2010;251:330-338.
19. Smythe MA, Priziola J, Dobesh PP, et al. Guidance for the practical management of the heparin anticoagulants in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41:165-186.
1. Spyropoulos AC, Hussein M, Lin J, et al. Rates of symptomatic venous thromboembolism in US surgical patients: a retrospective administrative database study. J Thromb Thrombolysis. 2009;28:458-464.
2. Deitzelzweig SB, Johnson BH, Lin J, et al. Prevalence of clinical venous thromboembolism in the USA: Current trends and future projections. Am J Hematol. 2011;86:217-220.
3. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979-1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711-1717.
4. Guyatt GH, Akl EA, Crowther M, et al. Introduction to the ninth edition: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):48S-52S.
5. Office of the Surgeon General; National Heart, Lung, and Blood Institute. The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville, MD: Office of the Surgeon General; 2008. www.ncbi.nlm.nih.gov/books/NBK44178/. Accessed May 2, 2019.
6. Pannucci CJ, Swistun L, MacDonald JK, et al. Individualized venous thromboembolism risk stratification using the 2005 Caprini score to identify the benefits and harms of chemoprophylaxis in surgical patients: a meta-analysis. Ann Surg. 2017;265:1094-1102.
7. Caprini JA, Arcelus JI, Hasty JH, et al. Clinical assessment of venous thromboembolic risk in surgical patients. Semin Thromb Hemost. 1991;17(suppl 3):304-312.
8. Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199:S3-S10.
9. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e227S-e277S.
10. The Joint Commission. Surgical Care Improvement Project (SCIP) Measure Information Form (Version 2.1c). www.jointcommission.org/surgical_care_improvement_project_scip_measure_information_form_version_21c/. Accessed June 22, 2016.
11. Cassidy MR, Macht RD, Rosenkranz P, et al. Patterns of failure of a standardized perioperative venous thromboembolism prophylaxis protocol. J Am Coll Surg. 2016;222:1074-1081.
12. Wang TF, Wong CA, Milligan PE, et al. Risk factors for inpatient venous thromboembolism despite thromboprophylaxis. Thromb Res. 2014;133:25-29.
13. Cassidy MR, Rosenkranz P, McAneny D. Reducing postoperative venous thromboembolism complications with a standardized risk-stratified prophylaxis protocol and mobilization program. J Am Coll Surg. 2014;218:1095-1104.
14. Nimeri AA, Gamaleldin MM, McKenna KL, et al. Reduction of venous thromboembolism in surgical patients using a mandatory risk-scoring system: 5-year follow-up of an American College of Surgeons National Quality Improvement Program. Clin Appl Thromb Hemost. 2017;23:392-396.
15. Borab ZM, Lanni MA, Tecce MG, et al. Use of computerized clinical decision support systems to prevent venous thromboembolism in surgical patients: a systematic review and meta-analysis. JAMA Surg. 2017;152:638–645.
16. Obi AT, Pannucci CJ, Nackashi A, et al. Validation of the Caprini venous thromboembolism risk assessment model in critically ill surgical patients. JAMA Surg. 2015;150:941-948.
17. Lobastov K, Barinov V, Schastlivtsev I, et al. Validation of the Caprini risk assessment model for venous thromboembolism in high-risk surgical patients in the background of standard prophylaxis. J Vasc Surg Venous Lymphat Disord. 2016;4:153-160.
18. Kakkar AK, Cohen AT, Tapson VF, et al. Venous thromboembolism risk and prophylaxis in the acute care hospital setting (ENDORSE survey): findings in surgical patients. Ann Surg. 2010;251:330-338.
19. Smythe MA, Priziola J, Dobesh PP, et al. Guidance for the practical management of the heparin anticoagulants in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41:165-186.