User login
Diagnostic Errors in Hospitalized Patients
Abstract
Diagnostic errors in hospitalized patients are a leading cause of preventable morbidity and mortality. Significant challenges in defining and measuring diagnostic errors and underlying process failure points have led to considerable variability in reported rates of diagnostic errors and adverse outcomes. In this article, we explore the diagnostic process and its discrete components, emphasizing the centrality of the patient in decision-making as well as the continuous nature of the process. We review the incidence of diagnostic errors in hospitalized patients and different methodological approaches that have been used to arrive at these estimates. We discuss different but interdependent provider- and system-related process-failure points that lead to diagnostic errors. We examine specific challenges related to measurement of diagnostic errors and describe traditional and novel approaches that are being used to obtain the most precise estimates. Finally, we examine various patient-, provider-, and organizational-level interventions that have been proposed to improve diagnostic safety in hospitalized patients.
Keywords: diagnostic error, hospital medicine, patient safety.
Diagnosis is defined as a “pre-existing set of categories agreed upon by the medical profession to designate a specific condition.”1 The diagnostic process involves obtaining a clinical history, performing a physical examination, conducting diagnostic testing, and consulting with other clinical providers to gather data that are relevant to understanding the underlying disease processes. This exercise involves generating hypotheses and updating prior probabilities as more information and evidence become available. Throughout this process of information gathering, integration, and interpretation, there is an ongoing assessment of whether sufficient and necessary knowledge has been obtained to make an accurate diagnosis and provide appropriate treatment.2
Diagnostic error is defined as a missed opportunity to make a timely diagnosis as part of this iterative process, including the failure of communicating the diagnosis to the patient in a timely manner.3 It can be categorized as a missed, delayed, or incorrect diagnosis based on available evidence at the time. Establishing the correct diagnosis has important implications. A timely and precise diagnosis ensures the patient the highest probability of having a positive health outcome that reflects an appropriate understanding of underlying disease processes and is consistent with their overall goals of care.3 When diagnostic errors occur, they can cause patient harm. Adverse events due to medical errors, including diagnostic errors, are estimated to be the third leading cause of death in the United States.4 Most people will experience at least 1 diagnostic error in their lifetime. In the 2015 National Academy of Medicine report Improving Diagnosis in Healthcare, diagnostic errors were identified as a major hazard as well as an opportunity to improve patient outcomes.2
Diagnostic errors during hospitalizations are especially concerning, as they are more likely to be implicated in a wider spectrum of harm, including permanent disability and death. This has become even more relevant for hospital medicine physicians and other clinical providers as they encounter increasing cognitive and administrative workloads, rising dissatisfaction and burnout, and unique obstacles such as night-time scheduling.5
Incidence of Diagnostic Errors in Hospitalized Patients
Several methodological approaches have been used to estimate the incidence of diagnostic errors in hospitalized patients. These include retrospective reviews of a sample of all hospital admissions, evaluations of selected adverse outcomes including autopsy studies, patient and provider surveys, and malpractice claims. Laboratory testing audits and secondary reviews in other diagnostic subspecialities (eg, radiology, pathology, and microbiology) are also essential to improving diagnostic performance in these specialized fields, which in turn affects overall hospital diagnostic error rates.6-8 These diverse approaches provide unique insights regarding our ability to assess the degree to which potential harms, ranging from temporary impairment to permanent disability, to death, are attributable to different failure points in the diagnostic process.
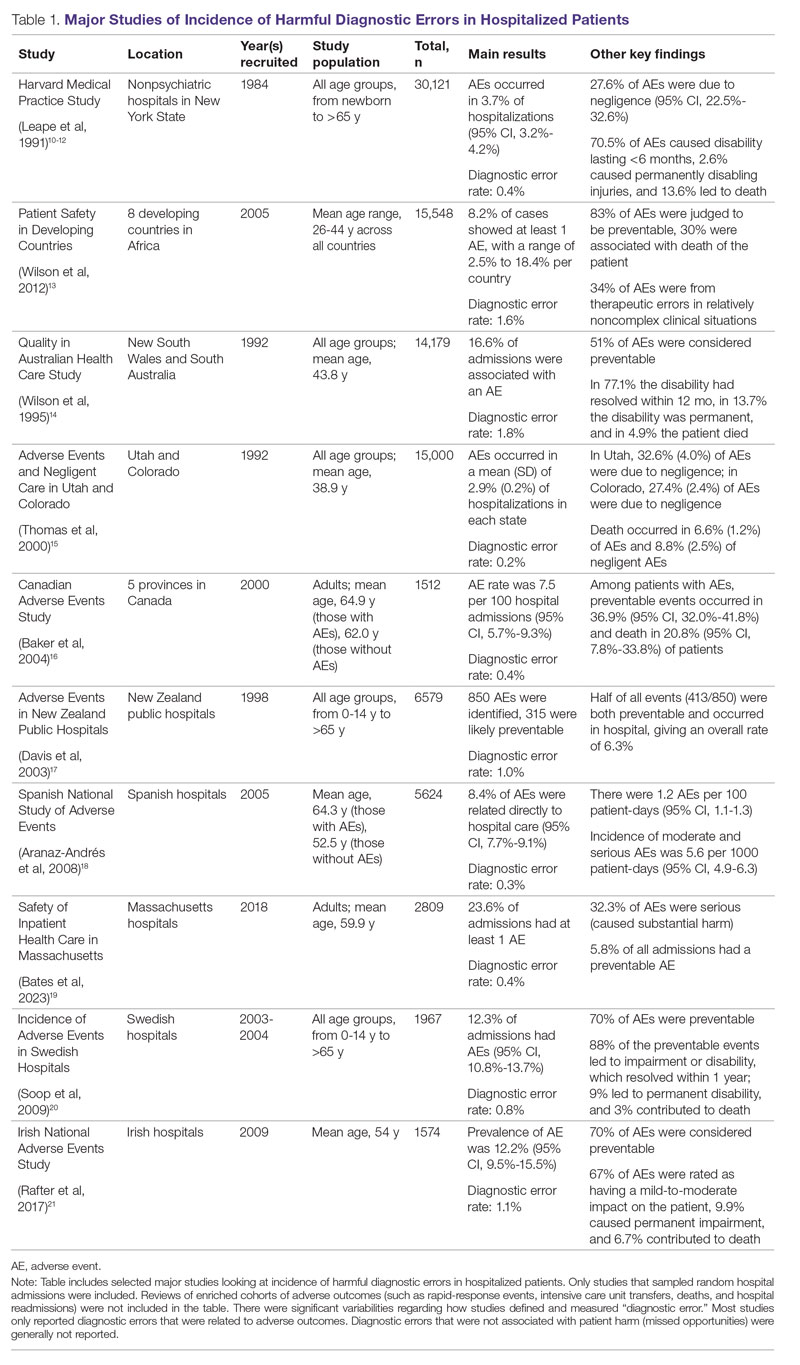
Large retrospective chart reviews of random hospital admissions remain the most accurate way to determine the overall incidence of diagnostic errors in hospitalized patients.9 The Harvard Medical Practice Study, published in 1991, laid the groundwork for measuring the incidence of adverse events in hospitalized patients and assessing their relation to medical error, negligence, and disability. Reviewing 30,121 randomly selected records from 51 randomly selected acute care hospitals in New York State, the study found that adverse events occurred in 3.7% of hospitalizations, diagnostic errors accounted for 13.8% of these events, and these errors were likely attributable to negligence in 74.7% of cases. The study not only outlined individual-level process failures, but also focused attention on some of the systemic causes, setting the agenda for quality improvement research in hospital-based care for years to come.10-12 A recent systematic review and meta-analysis of 22 hospital admission studies found a pooled rate of 0.7% (95% CI, 0.5%-1.1%) for harmful diagnostic errors.9 It found significant variations in the rates of adverse events, diagnostic errors, and range of diagnoses that were missed. This was primarily because of variabilities in pre-test probabilities in detecting diagnostic errors in these specific cohorts, as well as due to heterogeneity in study definitions and methodologies, especially regarding how they defined and measured “diagnostic error.” The analysis, however, did not account for diagnostic errors that were not related to patient harm (missed opportunities); therefore, it likely significantly underestimated the true incidence of diagnostic errors in these study populations. Table 1 summarizes some of key studies that have examined the incidence of harmful diagnostic errors in hospitalized patients.9-21
The chief limitation of reviewing random hospital admissions is that, since overall rates of diagnostic errors are still relatively low, a large number of case reviews are required to identify a sufficient sample of adverse outcomes to gain a meaningful understanding of the underlying process failure points and develop tools for remediation. Patient and provider surveys or data from malpractice claims can be high-yield starting points for research on process errors.22,23 Reviews of enriched cohorts of adverse outcomes, such as rapid-response events, intensive care unit (ICU) transfers, deaths, and hospital readmissions, can be an efficient way to identify process failures that lead to greatest harm. Depending on the research approach and the types of underlying patient populations sampled, rates of diagnostic errors in these high-risk groups have been estimated to be approximately 5% to 20%, or even higher.6,24-31 For example, a retrospective study of 391 cases of unplanned 7-day readmissions found that 5.6% of cases contained at least 1 diagnostic error during the index admission.32 In a study conducted at 6 Belgian acute-care hospitals, 56% of patients requiring an unplanned transfer to a higher level of care were determined to have had an adverse event, and of these adverse events, 12.4% of cases were associated with errors in diagnosis.29 A systematic review of 16 hospital-based studies estimated that 3.1% of all inpatient deaths were likely preventable, which corresponded to 22,165 deaths annually in the United States.30 Another such review of 31 autopsy studies reported that 28% of autopsied ICU patients had at least 1 misdiagnosis; of these diagnostic errors, 8% were classified as potentially lethal, and 15% were considered major but not lethal.31 Significant drawbacks of such enriched cohort studies, however, are their poor generalizability and inability to detect failure points that do not lead to patient harm (near-miss events).33
Causes of Diagnostic Errors in Hospitalized Patients
All aspects of the diagnostic process are susceptible to errors. These errors stem from a variety of faulty processes, including failure of the patient to engage with the health care system (eg, due to lack of insurance or transportation, or delay in seeking care); failure in information gathering (eg, missed history or exam findings, ordering wrong tests, laboratory errors); failure in information interpretation (eg, exam finding or test result misinterpretation); inaccurate hypothesis generation (eg, due to suboptimal prioritization or weighing of supporting evidence); and failure in communication (eg, with other team members or with the patient).2,34 Reasons for diagnostic process failures vary widely across different health care settings. While clinician assessment errors (eg, failure to consider or alternatively overweigh competing diagnoses) and errors in testing and the monitoring phase (eg, failure to order or follow up diagnostic tests) can lead to a majority of diagnostic errors in some patient populations, in other settings, social (eg, poor health literacy, punitive cultural practices) and economic factors (eg, lack of access to appropriate diagnostic tests or to specialty expertise) play a more prominent role.34,35
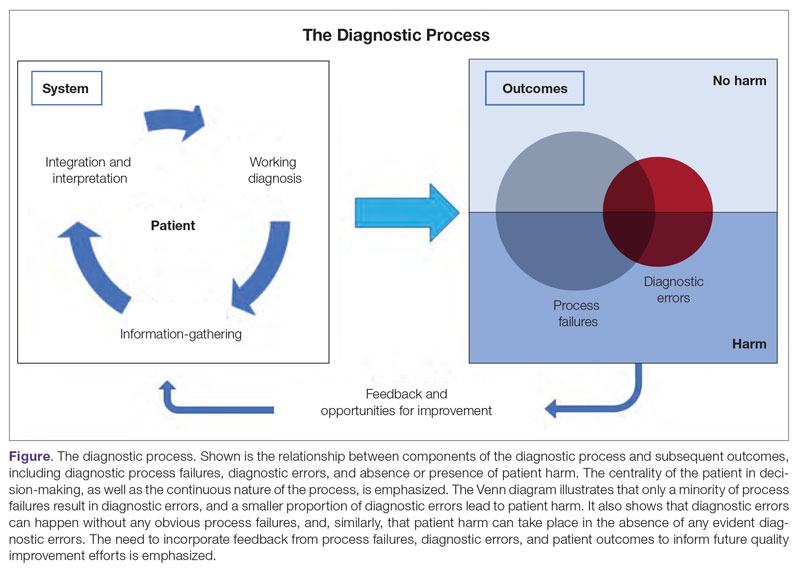
The Figure describes the relationship between components of the diagnostic process and subsequent outcomes, including diagnostic process failures, diagnostic errors, and absence or presence of patient harm.2,36,37 It reemphasizes the centrality of the patient in decision-making and the continuous nature of the process. The Figure also illustrates that only a minority of process failures result in diagnostic errors, and a smaller proportion of diagnostic errors actually lead to patient harm. Conversely, it also shows that diagnostic errors can happen without any obvious process-failure points, and, similarly, patient harm can take place in the absence of any evident diagnostic errors.36-38 Finally, it highlights the need to incorporate feedback from process failures, diagnostic errors, and favorable and unfavorable patient outcomes in order to inform future quality improvement efforts and research.
A significant proportion of diagnostic errors are due to system-related vulnerabilities, such as limitations in availability, adoption or quality of work force training, health informatics resources, and diagnostic capabilities. Lack of institutional culture that promotes safety and transparency also predisposes to diagnostic errors.39,40 The other major domain of process failures is related to cognitive errors in clinician decision-making. Anchoring, confirmation bias, availability bias, and base-rate neglect are some of the common cognitive biases that, along with personality traits (aversion to risk or ambiguity, overconfidence) and affective biases (influence of emotion on decision-making), often determine the degree of utilization of resources and the possibility of suboptimal diagnostic performance.41,42 Further, implicit biases related to age, race, gender, and sexual orientation contribute to disparities in access to health care and outcomes.43 In a large number of cases of preventable adverse outcomes, however, there are multiple interdependent individual and system-related failure points that lead to diagnostic error and patient harm.6,32
Challenges in Defining and Measuring Diagnostic Errors
In order to develop effective, evidence-based interventions to reduce diagnostic errors in hospitalized patients, it is essential to be able to first operationally define, and then accurately measure, diagnostic errors and the process failures that contribute to these errors in a standardized way that is reproducible across different settings.6,44 There are a number of obstacles in this endeavor.
A fundamental problem is that establishing a diagnosis is not a single act but a process. Patterns of symptoms and clinical presentations often differ for the same disease. Information required to make a diagnosis is usually gathered in stages, where the clinician obtains additional data, while considering many possibilities, of which 1 may be ultimately correct. Diagnoses evolve over time and in different care settings. “The most likely diagnosis” is not always the same as “the final correct diagnosis.” Moreover, the diagnostic process is influenced by patients’ individual clinical courses and preferences over time. This makes determination of missed, delayed, or incorrect diagnoses challenging.45,46
For hospitalized patients, generally the goal is to first rule out more serious and acute conditions (eg, pulmonary embolism or stroke), even if their probability is rather low. Conversely, a diagnosis that appears less consequential if delayed (eg, chronic anemia of unclear etiology) might not be pursued on an urgent basis, and is often left to outpatient providers to examine, but still may manifest in downstream harm (eg, delayed diagnosis of gastrointestinal malignancy or recurrent admissions for heart failure due to missed iron-deficiency anemia). Therefore, coming up with disease diagnosis likelihoods in hindsight may turn out to be highly subjective and not always accurate. This can be particularly difficult when clinician and other team deliberations are not recorded in their entirety.47
Another hurdle in the practice of diagnostic medicine is to preserve the balance between underdiagnosing versus pursuing overly aggressive diagnostic approaches. Conducting laboratory, imaging, or other diagnostic studies without a clear shared understanding of how they would affect clinical decision-making (eg, use of prostate-specific antigen to detect prostate cancer) not only leads to increased costs but can also delay appropriate care. Worse, subsequent unnecessary diagnostic tests and treatments can sometimes lead to serious harm.48,49
Finally, retrospective reviews by clinicians are subject to multiple potential limitations that include failure to create well-defined research questions, poorly developed inclusion and exclusion criteria, and issues related to inter- and intra-rater reliability.50 These methodological deficiencies can occur despite following "best practice" guidelines during the study planning, execution, and analysis phases. They further add to the challenge of defining and measuring diagnostic errors.47
Strategies to Improve Measurement of Diagnostic Errors
Development of new methodologies to reliably measure diagnostic errors is an area of active research. The advancement of uniform and universally agreed-upon frameworks to define and identify process failure points and diagnostic errors would help reduce measurement error and support development and testing of interventions that could be generalizable across different health care settings. To more accurately define and measure diagnostic errors, several novel approaches have been proposed (Table 2).
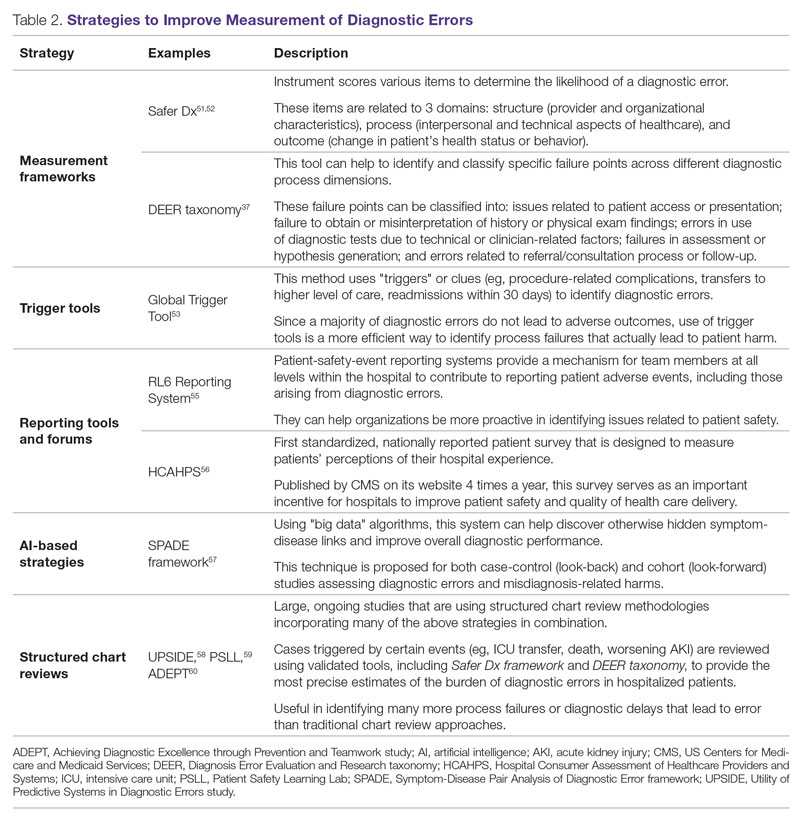
The Safer Dx framework is an all-round tool developed to advance the discipline of measuring diagnostic errors. For an episode of care under review, the instrument scores various items to determine the likelihood of a diagnostic error. These items evaluate multiple dimensions affecting diagnostic performance and measurements across 3 broad domains: structure (provider and organizational characteristics—from everyone involved with patient care, to computing infrastructure, to policies and regulations), process (elements of the patient-provider encounter, diagnostic test performance and follow-up, and subspecialty- and referral-specific factors), and outcome (establishing accurate and timely diagnosis as opposed to missed, delayed, or incorrect diagnosis). This instrument has been revised and can be further modified by a variety of stakeholders, including clinicians, health care organizations, and policymakers, to identify potential diagnostic errors in a standardized way for patient safety and quality improvement research.51,52
Use of standardized tools, such as the Diagnosis Error Evaluation and Research (DEER) taxonomy, can help to identify and classify specific failure points across different diagnostic process dimensions.37 These failure points can be classified into: issues related to patient presentation or access to health care; failure to obtain or misinterpretation of history or physical exam findings; errors in use of diagnostics tests due to technical or clinician-related factors; failures in appropriate weighing of evidence and hypothesis generation; errors associated with referral or consultation process; and failure to monitor the patient or obtain timely follow-up.34 The DEER taxonomy can also be modified based on specific research questions and study populations. Further, it can be recategorized to correspond to Safer Dx framework diagnostic process dimensions to provide insights into reasons for specific process failures and to develop new interventions to mitigate errors and patient harm.6
Since a majority of diagnostic errors do not lead to actual harm, use of “triggers” or clues (eg, procedure-related complications, patient falls, transfers to a higher level of care, readmissions within 30 days) can be a more efficient method to identify diagnostic errors and adverse events that do cause harm. The Global Trigger Tool, developed by the Institute for Healthcare Improvement, uses this strategy. This tool has been shown to identify a significantly higher number of serious adverse events than comparable methods.53 This facilitates selection and development of strategies at the institutional level that are most likely to improve patient outcomes.24
Encouraging and facilitating voluntary or prompted reporting from patients and clinicians can also play an important role in capturing diagnostic errors. Patients and clinicians are not only the key stakeholders but are also uniquely placed within the diagnostic process to detect and report potential errors.25,54 Patient-safety-event reporting systems, such as RL6, play a vital role in reporting near-misses and adverse events. These systems provide a mechanism for team members at all levels within the hospital to contribute toward reporting patient adverse events, including those arising from diagnostic errors.55 The Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey is the first standardized, nationally reported patient survey designed to measure patients’ perceptions of their hospital experience. The US Centers for Medicare and Medicaid Services (CMS) publishes HCAHPS results on its website 4 times a year, which serves as an important incentive for hospitals to improve patient safety and quality of health care delivery.56
Another novel approach links multiple symptoms to a range of target diseases using the Symptom-Disease Pair Analysis of Diagnostic Error (SPADE) framework. Using “big data” technologies, this technique can help discover otherwise hidden symptom-disease links and improve overall diagnostic performance. This approach is proposed for both case-control (look-back) and cohort (look-forward) studies assessing diagnostic errors and misdiagnosis-related harms. For example, starting with a known diagnosis with high potential for harm (eg, stroke), the “look-back” approach can be used to identify high-risk symptoms (eg, dizziness, vertigo). In the “look-forward” approach, a single symptom or exposure risk factor known to be frequently misdiagnosed (eg, dizziness) can be analyzed to identify potential adverse disease outcomes (eg, stroke, migraine).57
Many large ongoing studies looking at diagnostic errors among hospitalized patients, such as Utility of Predictive Systems to identify Inpatient Diagnostic Errors (UPSIDE),58 Patient Safety Learning Lab (PSLL),59 and Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT),60 are using structured chart review methodologies incorporating many of the above strategies in combination. Cases triggered by certain events (eg, ICU transfer, death, rapid response event, new or worsening acute kidney injury) are reviewed using validated tools, including Safer Dx framework and DEER taxonomy, to provide the most precise estimates of the burden of diagnostic errors in hospitalized patients. These estimates may be much higher than previously predicted using traditional chart review approaches.6,24 For example, a recently published study of 2809 random admissions in 11 Massachusetts hospitals identified 978 adverse events but only 10 diagnostic errors (diagnostic error rate, 0.4%).19 This was likely because the trigger method used in the study did not specifically examine the diagnostic process as critically as done by the Safer Dx framework and DEER taxonomy tools, thereby underestimating the total number of diagnostic errors. Further, these ongoing studies (eg, UPSIDE, ADEPT) aim to employ new and upcoming advanced machine-learning methods to create models that can improve overall diagnostic performance. This would pave the way to test and build novel, efficient, and scalable interventions to reduce diagnostic errors and improve patient outcomes.
Strategies to Improve Diagnostic Safety in Hospitalized Patients
Disease-specific biomedical research, as well as advances in laboratory, imaging, and other technologies, play a critical role in improving diagnostic accuracy. However, these technical approaches do not address many of the broader clinician- and system-level failure points and opportunities for improvement. Various patient-, provider-, and organizational-level interventions that could make diagnostic processes more resilient and reduce the risk of error and patient harm have been proposed.61
Among these strategies are approaches to empower patients and their families. Fostering therapeutic relationships between patients and members of the care team is essential to reducing diagnostic errors.62 Facilitating timely access to health records, ensuring transparency in decision making, and tailoring communication strategies to patients’ cultural and educational backgrounds can reduce harm.63 Similarly, at the system level, enhancing communication among different providers by use of tools such as structured handoffs can prevent communication breakdowns and facilitate positive outcomes.64
Interventions targeted at individual health care providers, such as educational programs to improve content-specific knowledge, can enhance diagnostic performance. Regular feedback, strategies to enhance equity, and fostering an environment where all providers are actively encouraged to think critically and participate in the diagnostic process (training programs to use “diagnostic time-outs” and making it a “team sport”) can improve clinical reasoning.65,66 Use of standardized patients can help identify individual-level cognitive failure points and facilitate creation of new interventions to improve clinical decision-making processes.67
Novel health information technologies can further augment these efforts. These include effective documentation by maintaining dynamic and accurate patient histories, problem lists, and medication lists68-70; use of electronic health record–based algorithms to identify potential diagnostic delays for serious conditions71,72; use of telemedicine technologies to improve accessibility and coordination73; application of mobile health and wearable technologies to facilitate data-gathering and care delivery74,75; and use of computerized decision-support tools, including applications to interpret electrocardiograms, imaging studies, and other diagnostic tests.76
Use of precision medicine, powered by new artificial intelligence (AI) tools, is becoming more widespread. Algorithms powered by AI can augment and sometimes even outperform clinician decision-making in areas such as oncology, radiology, and primary care.77 Creation of large biobanks like the All of Us research program can be used to study thousands of environmental and genetic risk factors and health conditions simultaneously, and help identify specific treatments that work best for people of different backgrounds.78 Active research in these areas holds great promise in terms of how and when we diagnose diseases and make appropriate preventative and treatment decisions. Significant scientific, ethical, and regulatory challenges will need to be overcome before these technologies can address some of the most complex problems in health care.79
Finally, diagnostic performance is affected by the external environment, including the functioning of the medical liability system. Diagnostic errors that lead to patient harm are a leading cause of malpractice claims.80 Developing a legal environment, in collaboration with patient advocacy groups and health care organizations, that promotes and facilitates timely disclosure of diagnostic errors could decrease the incentive to hide errors, advance care processes, and improve outcomes.81,82
Conclusion
The burden of diagnostic errors in hospitalized patients is unacceptably high and remains an underemphasized cause of preventable morbidity and mortality. Diagnostic errors often result from a breakdown in multiple interdependent processes that involve patient-, provider-, and system-level factors. Significant challenges remain in defining and identifying diagnostic errors as well as underlying process-failure points. The most effective interventions to reduce diagnostic errors will require greater patient participation in the diagnostic process and a mix of evidence-based interventions that promote individual-provider excellence as well as system-level changes. Further research and collaboration among various stakeholders should help improve diagnostic safety for hospitalized patients.
Corresponding author: Abhishek Goyal, MD, MPH; [email protected]
Disclosures: Dr. Dalal disclosed receiving income ≥ $250 from MayaMD.
1. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499. doi:10.1001/archinte.165.13.1493
2. National Academies of Sciences, Engineering, and Medicine. 2015. Improving Diagnosis in Health Care. The National Academies Press. doi:10.17226/21794
3. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
4. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139. doi:10.1136/bmj.i2139
5. Flanders SA, Centor B, Weber V, McGinn T, Desalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit. J Gen Intern Med. 2009;24(5):636-641. doi:10.1007/s11606-009-0944-6
6. Griffin JA, Carr K, Bersani K, et al. Analyzing diagnostic errors in the acute setting: a process-driven approach. Diagnosis (Berl). 2021;9(1):77-88. doi:10.1515/dx-2021-0033
7. Itri JN, Tappouni RR, McEachern RO, Pesch AJ, Patel SH. Fundamentals of diagnostic error in imaging. RadioGraphics. 2018;38(6):1845-1865. doi:10.1148/rg.2018180021
8. Hammerling JA. A Review of medical errors in laboratory diagnostics and where we are today. Lab Med. 2012;43(2):41-44. doi:10.1309/LM6ER9WJR1IHQAUY
9. Gunderson CG, Bilan VP, Holleck JL, et al. Prevalence of harmful diagnostic errors in hospitalised adults: a systematic review and meta-analysis. BMJ Qual Saf. 2020;29(12):1008-1018. doi:10.1136/bmjqs-2019-010822
10. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi:10.1056/NEJM199102073240604
11. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324(6):377-384. doi:10.1056/NEJM199102073240605
12. Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. doi:10.1056/NEJM199107253250405
13. Wilson RM, Michel P, Olsen S, et al. Patient safety in developing countries: retrospective estimation of scale and nature of harm to patients in hospital. BMJ. 2012;344:e832. doi:10.1136/bmj.e832
14. Wilson RM, Runciman WB, Gibberd RW, Harrison BT, Newby L, Hamilton JD. The Quality in Australian Health Care Study. Med J Aust. 1995;163(9):458-471. doi:10.5694/j.1326-5377.1995.tb124691.x
15. Thomas EJ, Studdert DM, Burstin HR, et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38(3):261-271. doi:10.1097/00005650-200003000-00003
16. Baker GR, Norton PG, Flintoft V, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ. 2004;170(11):1678-1686. doi:10.1503/cmaj.1040498
17. Davis P, Lay-Yee R, Briant R, Ali W, Scott A, Schug S. Adverse events in New Zealand public hospitals II: preventability and clinical context. N Z Med J. 2003;116(1183):U624.
18. Aranaz-Andrés JM, Aibar-Remón C, Vitaller-Murillo J, et al. Incidence of adverse events related to health care in Spain: results of the Spanish National Study of Adverse Events. J Epidemiol Community Health. 2008;62(12):1022-1029. doi:10.1136/jech.2007.065227
19. Bates DW, Levine DM, Salmasian H, et al. The safety of inpatient health care. N Engl J Med. 2023;388(2):142-153. doi:10.1056/NEJMsa2206117
20. Soop M, Fryksmark U, Köster M, Haglund B. The incidence of adverse events in Swedish hospitals: a retrospective medical record review study. Int J Qual Health Care. 2009;21(4):285-291. doi:10.1093/intqhc/mzp025
21. Rafter N, Hickey A, Conroy RM, et al. The Irish National Adverse Events Study (INAES): the frequency and nature of adverse events in Irish hospitals—a retrospective record review study. BMJ Qual Saf. 2017;26(2):111-119. doi:10.1136/bmjqs-2015-004828
22. Blendon RJ, DesRoches CM, Brodie M, et al. Views of practicing physicians and the public on medical errors. N Engl J Med. 2002;347(24):1933-1940. doi:10.1056/NEJMsa022151
23. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680. doi:10.1136/bmjqs-2012-001550
24. Malik MA, Motta-Calderon D, Piniella N, et al. A structured approach to EHR surveillance of diagnostic error in acute care: an exploratory analysis of two institutionally-defined case cohorts. Diagnosis (Berl). 2022;9(4):446-457. doi:10.1515/dx-2022-0032
25. Graber ML. The incidence of diagnostic error in medicine. BMJ Qual Saf. 2013;22(suppl 2):ii21-ii27. doi:10.1136/bmjqs-2012-001615
26. Bergl PA, Taneja A, El-Kareh R, Singh H, Nanchal RS. Frequency, risk factors, causes, and consequences of diagnostic errors in critically ill medical patients: a retrospective cohort study. Crit Care Med. 2019;47(11):e902-e910. doi:10.1097/CCM.0000000000003976
27. Hogan H, Healey F, Neale G, Thomson R, Vincent C, Black N. Preventable deaths due to problems in care in English acute hospitals: a retrospective case record review study. BMJ Qual Saf. 2012;21(9):737-745. doi:10.1136/bmjqs-2011-001159
28. Bergl PA, Nanchal RS, Singh H. Diagnostic error in the critically ill: defining the problem and exploring next steps to advance intensive care unit safety. Ann Am Thorac Soc. 2018;15(8):903-907. doi:10.1513/AnnalsATS.201801-068PS
29. Marquet K, Claes N, De Troy E, et al. One fourth of unplanned transfers to a higher level of care are associated with a highly preventable adverse event: a patient record review in six Belgian hospitals. Crit Care Med. 2015;43(5):1053-1061. doi:10.1097/CCM.0000000000000932
30. Rodwin BA, Bilan VP, Merchant NB, et al. Rate of preventable mortality in hospitalized patients: a systematic review and meta-analysis. J Gen Intern Med. 2020;35(7):2099-2106. doi:10.1007/s11606-019-05592-5
31. Winters B, Custer J, Galvagno SM, et al. Diagnostic errors in the intensive care unit: a systematic review of autopsy studies. BMJ Qual Saf. 2012;21(11):894-902. doi:10.1136/bmjqs-2012-000803
32. Raffel KE, Kantor MA, Barish P, et al. Prevalence and characterisation of diagnostic error among 7-day all-cause hospital medicine readmissions: a retrospective cohort study. BMJ Qual Saf. 2020;29(12):971-979. doi:10.1136/bmjqs-2020-010896
33. Weingart SN, Pagovich O, Sands DZ, et al. What can hospitalized patients tell us about adverse events? learning from patient-reported incidents. J Gen Intern Med. 2005;20(9):830-836. doi:10.1111/j.1525-1497.2005.0180.x
34. Schiff GD, Hasan O, Kim S, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169(20):1881-1887. doi:10.1001/archinternmed.2009.333
35. Singh H, Schiff GD, Graber ML, Onakpoya I, Thompson MJ. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
36. Schiff GD, Leape LL. Commentary: how can we make diagnosis safer? Acad Med J Assoc Am Med Coll. 2012;87(2):135-138. doi:10.1097/ACM.0b013e31823f711c
37. Schiff GD, Kim S, Abrams R, et al. Diagnosing diagnosis errors: lessons from a multi-institutional collaborative project. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Volume 2: Concepts and Methodology. AHRQ Publication No. 05-0021-2. Agency for Healthcare Research and Quality (US); 2005. Accessed January 16, 2023. http://www.ncbi.nlm.nih.gov/books/NBK20492/
38. Newman-Toker DE. A unified conceptual model for diagnostic errors: underdiagnosis, overdiagnosis, and misdiagnosis. Diagnosis (Berl). 2014;1(1):43-48. doi:10.1515/dx-2013-0027
39. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, Firipis M, Wanni Arachchige Dona S, Watts JJ. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
40. Gupta A, Harrod M, Quinn M, et al. Mind the overlap: how system problems contribute to cognitive failure and diagnostic errors. Diagnosis (Berl). 2018;5(3):151-156. doi:10.1515/dx-2018-0014
41. Saposnik G, Redelmeier D, Ruff CC, Tobler PN. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16:138. doi:10.1186/s12911-016-0377-1
42. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78(8):775-780. doi: 10.1097/00001888-200308000-00003
43. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504-1510. doi:10.1007/s11606-013-2441-1
44. Zwaan L, Singh H. The challenges in defining and measuring diagnostic error. Diagnosis (Ber). 2015;2(2):97-103. doi:10.1515/dx-2014-0069
45. Arkes HR, Wortmann RL, Saville PD, Harkness AR. Hindsight bias among physicians weighing the likelihood of diagnoses. J Appl Psychol. 1981;66(2):252-254.
46. Singh H. Editorial: Helping health care organizations to define diagnostic errors as missed opportunities in diagnosis. Jt Comm J Qual Patient Saf. 2014;40(3):99-101. doi:10.1016/s1553-7250(14)40012-6
47. Vassar M, Holzmann M. The retrospective chart review: important methodological considerations. J Educ Eval Health Prof. 2013;10:12. doi:10.3352/jeehp.2013.10.12
48. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605-613. doi:10.1093/jnci/djq099
49. Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. doi:10.1136/bmj.e3502
50. Hayward RA, Hofer TP. Estimating hospital deaths due to medical errors: preventability is in the eye of the reviewer. JAMA. 2001;286(4):415-420. doi:10.1001/jama.286.4.415
51. Singh H, Sittig DF. Advancing the science of measurement of diagnostic errors in healthcare: the Safer Dx framework. BMJ Qual Saf. 2015;24(2):103-110. doi:10.1136/bmjqs-2014-003675
52. Singh H, Khanna A, Spitzmueller C, Meyer AND. Recommendations for using the Revised Safer Dx Instrument to help measure and improve diagnostic safety. Diagnosis (Berl). 2019;6(4):315-323. doi:10.1515/dx-2019-0012
53. Classen DC, Resar R, Griffin F, et al. “Global trigger tool” shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood). 2011;30(4):581-589. doi:10.1377/hlthaff.2011.0190
54. Schiff GD. Minimizing diagnostic error: the importance of follow-up and feedback. Am J Med. 2008;121(5 suppl):S38-S42. doi:10.1016/j.amjmed.2008.02.004
55. Mitchell I, Schuster A, Smith K, Pronovost P, Wu A. Patient safety incident reporting: a qualitative study of thoughts and perceptions of experts 15 years after “To Err is Human.” BMJ Qual Saf. 2016;25(2):92-99. doi:10.1136/bmjqs-2015-004405
56. Mazurenko O, Collum T, Ferdinand A, Menachemi N. Predictors of hospital patient satisfaction as measured by HCAHPS: a systematic review. J Healthc Manag. 2017;62(4):272-283. doi:10.1097/JHM-D-15-00050
57. Liberman AL, Newman-Toker DE. Symptom-Disease Pair Analysis of Diagnostic Error (SPADE): a conceptual framework and methodological approach for unearthing misdiagnosis-related harms using big data. BMJ Qual Saf. 2018;27(7):557-566. doi:10.1136/bmjqs-2017-007032
58. Utility of Predictive Systems to Identify Inpatient Diagnostic Errors: the UPSIDE study. NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/search/rpoHXlEAcEudQV3B9ld8iw/project-details/10020962
59. Overview of Patient Safety Learning Laboratory (PSLL) Projects. Agency for Healthcare Research and Quality. Accessed January 14, 2023. https://www.ahrq.gov/patient-safety/resources/learning-lab/index.html
60. Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT). NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/project-details/10642576
61. Zwaan L, Singh H. Diagnostic error in hospitals: finding forests not just the big trees. BMJ Qual Saf. 2020;29(12):961-964. doi:10.1136/bmjqs-2020-011099
62. Longtin Y, Sax H, Leape LL, Sheridan SE, Donaldson L, Pittet D. Patient participation: current knowledge and applicability to patient safety. Mayo Clin Proc. 2010;85(1):53-62. doi:10.4065/mcp.2009.0248
63. Murphy DR, Singh H, Berlin L. Communication breakdowns and diagnostic errors: a radiology perspective. Diagnosis (Berl). 2014;1(4):253-261. doi:10.1515/dx-2014-0035
64. Singh H, Naik AD, Rao R, Petersen LA. Reducing diagnostic errors through effective communication: harnessing the power of information technology. J Gen Intern Med. 2008;23(4):489-494. doi:10.1007/s11606-007-0393-z
65. Singh H, Connor DM, Dhaliwal G. Five strategies for clinicians to advance diagnostic excellence. BMJ. 2022;376:e068044. doi:10.1136/bmj-2021-068044
66. Yale S, Cohen S, Bordini BJ. Diagnostic time-outs to improve diagnosis. Crit Care Clin. 2022;38(2):185-194. doi:10.1016/j.ccc.2021.11.008
67. Schwartz A, Peskin S, Spiro A, Weiner SJ. Impact of unannounced standardized patient audit and feedback on care, documentation, and costs: an experiment and claims analysis. J Gen Intern Med. 2021;36(1):27-34. doi:10.1007/s11606-020-05965-1
68. Carpenter JD, Gorman PN. Using medication list—problem list mismatches as markers of potential error. Proc AMIA Symp. 2002:106-110.
69. Hron JD, Manzi S, Dionne R, et al. Electronic medication reconciliation and medication errors. Int J Qual Health Care. 2015;27(4):314-319. doi:10.1093/intqhc/mzv046
70. Graber ML, Siegal D, Riah H, Johnston D, Kenyon K. Electronic health record–related events in medical malpractice claims. J Patient Saf. 2019;15(2):77-85. doi:10.1097/PTS.0000000000000240
71. Murphy DR, Wu L, Thomas EJ, Forjuoh SN, Meyer AND, Singh H. Electronic trigger-based intervention to reduce delays in diagnostic evaluation for cancer: a cluster randomized controlled trial. J Clin Oncol. 2015;33(31):3560-3567. doi:10.1200/JCO.2015.61.1301
72. Singh H, Giardina TD, Forjuoh SN, et al. Electronic health record-based surveillance of diagnostic errors in primary care. BMJ Qual Saf. 2012;21(2):93-100. doi:10.1136/bmjqs-2011-000304
73. Armaignac DL, Saxena A, Rubens M, et al. Impact of telemedicine on mortality, length of stay, and cost among patients in progressive care units: experience from a large healthcare system. Crit Care Med. 2018;46(5):728-735. doi:10.1097/CCM.0000000000002994
74. MacKinnon GE, Brittain EL. Mobile health technologies in cardiopulmonary disease. Chest. 2020;157(3):654-664. doi:10.1016/j.chest.2019.10.015
75. DeVore AD, Wosik J, Hernandez AF. The future of wearables in heart failure patients. JACC Heart Fail. 2019;7(11):922-932. doi:10.1016/j.jchf.2019.08.008
76. Tsai TL, Fridsma DB, Gatti G. Computer decision support as a source of interpretation error: the case of electrocardiograms. J Am Med Inform Assoc. 2003;10(5):478-483. doi:10.1197/jamia.M1279
77. Lin SY, Mahoney MR, Sinsky CA. Ten ways artificial intelligence will transform primary care. J Gen Intern Med. 2019;34(8):1626-1630. doi:10.1007/s11606-019-05035-1
78. Ramirez AH, Gebo KA, Harris PA. Progress with the All Of Us research program: opening access for researchers. JAMA. 2021;325(24):2441-2442. doi:10.1001/jama.2021.7702
79. Johnson KB, Wei W, Weeraratne D, et al. Precision medicine, AI, and the future of personalized health care. Clin Transl Sci. 2021;14(1):86-93. doi:10.1111/cts.12884
80. Gupta A, Snyder A, Kachalia A, Flanders S, Saint S, Chopra V. Malpractice claims related to diagnostic errors in the hospital. BMJ Qual Saf. 2017;27(1):bmjqs-2017-006774. doi:10.1136/bmjqs-2017-006774
81. Renkema E, Broekhuis M, Ahaus K. Conditions that influence the impact of malpractice litigation risk on physicians’ behavior regarding patient safety. BMC Health Serv Res. 2014;14(1):38. doi:10.1186/1472-6963-14-38
82. Kachalia A, Mello MM, Nallamothu BK, Studdert DM. Legal and policy interventions to improve patient safety. Circulation. 2016;133(7):661-671. doi:10.1161/CIRCULATIONAHA.115.015880
Abstract
Diagnostic errors in hospitalized patients are a leading cause of preventable morbidity and mortality. Significant challenges in defining and measuring diagnostic errors and underlying process failure points have led to considerable variability in reported rates of diagnostic errors and adverse outcomes. In this article, we explore the diagnostic process and its discrete components, emphasizing the centrality of the patient in decision-making as well as the continuous nature of the process. We review the incidence of diagnostic errors in hospitalized patients and different methodological approaches that have been used to arrive at these estimates. We discuss different but interdependent provider- and system-related process-failure points that lead to diagnostic errors. We examine specific challenges related to measurement of diagnostic errors and describe traditional and novel approaches that are being used to obtain the most precise estimates. Finally, we examine various patient-, provider-, and organizational-level interventions that have been proposed to improve diagnostic safety in hospitalized patients.
Keywords: diagnostic error, hospital medicine, patient safety.
Diagnosis is defined as a “pre-existing set of categories agreed upon by the medical profession to designate a specific condition.”1 The diagnostic process involves obtaining a clinical history, performing a physical examination, conducting diagnostic testing, and consulting with other clinical providers to gather data that are relevant to understanding the underlying disease processes. This exercise involves generating hypotheses and updating prior probabilities as more information and evidence become available. Throughout this process of information gathering, integration, and interpretation, there is an ongoing assessment of whether sufficient and necessary knowledge has been obtained to make an accurate diagnosis and provide appropriate treatment.2
Diagnostic error is defined as a missed opportunity to make a timely diagnosis as part of this iterative process, including the failure of communicating the diagnosis to the patient in a timely manner.3 It can be categorized as a missed, delayed, or incorrect diagnosis based on available evidence at the time. Establishing the correct diagnosis has important implications. A timely and precise diagnosis ensures the patient the highest probability of having a positive health outcome that reflects an appropriate understanding of underlying disease processes and is consistent with their overall goals of care.3 When diagnostic errors occur, they can cause patient harm. Adverse events due to medical errors, including diagnostic errors, are estimated to be the third leading cause of death in the United States.4 Most people will experience at least 1 diagnostic error in their lifetime. In the 2015 National Academy of Medicine report Improving Diagnosis in Healthcare, diagnostic errors were identified as a major hazard as well as an opportunity to improve patient outcomes.2
Diagnostic errors during hospitalizations are especially concerning, as they are more likely to be implicated in a wider spectrum of harm, including permanent disability and death. This has become even more relevant for hospital medicine physicians and other clinical providers as they encounter increasing cognitive and administrative workloads, rising dissatisfaction and burnout, and unique obstacles such as night-time scheduling.5
Incidence of Diagnostic Errors in Hospitalized Patients
Several methodological approaches have been used to estimate the incidence of diagnostic errors in hospitalized patients. These include retrospective reviews of a sample of all hospital admissions, evaluations of selected adverse outcomes including autopsy studies, patient and provider surveys, and malpractice claims. Laboratory testing audits and secondary reviews in other diagnostic subspecialities (eg, radiology, pathology, and microbiology) are also essential to improving diagnostic performance in these specialized fields, which in turn affects overall hospital diagnostic error rates.6-8 These diverse approaches provide unique insights regarding our ability to assess the degree to which potential harms, ranging from temporary impairment to permanent disability, to death, are attributable to different failure points in the diagnostic process.
Large retrospective chart reviews of random hospital admissions remain the most accurate way to determine the overall incidence of diagnostic errors in hospitalized patients.9 The Harvard Medical Practice Study, published in 1991, laid the groundwork for measuring the incidence of adverse events in hospitalized patients and assessing their relation to medical error, negligence, and disability. Reviewing 30,121 randomly selected records from 51 randomly selected acute care hospitals in New York State, the study found that adverse events occurred in 3.7% of hospitalizations, diagnostic errors accounted for 13.8% of these events, and these errors were likely attributable to negligence in 74.7% of cases. The study not only outlined individual-level process failures, but also focused attention on some of the systemic causes, setting the agenda for quality improvement research in hospital-based care for years to come.10-12 A recent systematic review and meta-analysis of 22 hospital admission studies found a pooled rate of 0.7% (95% CI, 0.5%-1.1%) for harmful diagnostic errors.9 It found significant variations in the rates of adverse events, diagnostic errors, and range of diagnoses that were missed. This was primarily because of variabilities in pre-test probabilities in detecting diagnostic errors in these specific cohorts, as well as due to heterogeneity in study definitions and methodologies, especially regarding how they defined and measured “diagnostic error.” The analysis, however, did not account for diagnostic errors that were not related to patient harm (missed opportunities); therefore, it likely significantly underestimated the true incidence of diagnostic errors in these study populations. Table 1 summarizes some of key studies that have examined the incidence of harmful diagnostic errors in hospitalized patients.9-21

The chief limitation of reviewing random hospital admissions is that, since overall rates of diagnostic errors are still relatively low, a large number of case reviews are required to identify a sufficient sample of adverse outcomes to gain a meaningful understanding of the underlying process failure points and develop tools for remediation. Patient and provider surveys or data from malpractice claims can be high-yield starting points for research on process errors.22,23 Reviews of enriched cohorts of adverse outcomes, such as rapid-response events, intensive care unit (ICU) transfers, deaths, and hospital readmissions, can be an efficient way to identify process failures that lead to greatest harm. Depending on the research approach and the types of underlying patient populations sampled, rates of diagnostic errors in these high-risk groups have been estimated to be approximately 5% to 20%, or even higher.6,24-31 For example, a retrospective study of 391 cases of unplanned 7-day readmissions found that 5.6% of cases contained at least 1 diagnostic error during the index admission.32 In a study conducted at 6 Belgian acute-care hospitals, 56% of patients requiring an unplanned transfer to a higher level of care were determined to have had an adverse event, and of these adverse events, 12.4% of cases were associated with errors in diagnosis.29 A systematic review of 16 hospital-based studies estimated that 3.1% of all inpatient deaths were likely preventable, which corresponded to 22,165 deaths annually in the United States.30 Another such review of 31 autopsy studies reported that 28% of autopsied ICU patients had at least 1 misdiagnosis; of these diagnostic errors, 8% were classified as potentially lethal, and 15% were considered major but not lethal.31 Significant drawbacks of such enriched cohort studies, however, are their poor generalizability and inability to detect failure points that do not lead to patient harm (near-miss events).33
Causes of Diagnostic Errors in Hospitalized Patients
All aspects of the diagnostic process are susceptible to errors. These errors stem from a variety of faulty processes, including failure of the patient to engage with the health care system (eg, due to lack of insurance or transportation, or delay in seeking care); failure in information gathering (eg, missed history or exam findings, ordering wrong tests, laboratory errors); failure in information interpretation (eg, exam finding or test result misinterpretation); inaccurate hypothesis generation (eg, due to suboptimal prioritization or weighing of supporting evidence); and failure in communication (eg, with other team members or with the patient).2,34 Reasons for diagnostic process failures vary widely across different health care settings. While clinician assessment errors (eg, failure to consider or alternatively overweigh competing diagnoses) and errors in testing and the monitoring phase (eg, failure to order or follow up diagnostic tests) can lead to a majority of diagnostic errors in some patient populations, in other settings, social (eg, poor health literacy, punitive cultural practices) and economic factors (eg, lack of access to appropriate diagnostic tests or to specialty expertise) play a more prominent role.34,35
The Figure describes the relationship between components of the diagnostic process and subsequent outcomes, including diagnostic process failures, diagnostic errors, and absence or presence of patient harm.2,36,37 It reemphasizes the centrality of the patient in decision-making and the continuous nature of the process. The Figure also illustrates that only a minority of process failures result in diagnostic errors, and a smaller proportion of diagnostic errors actually lead to patient harm. Conversely, it also shows that diagnostic errors can happen without any obvious process-failure points, and, similarly, patient harm can take place in the absence of any evident diagnostic errors.36-38 Finally, it highlights the need to incorporate feedback from process failures, diagnostic errors, and favorable and unfavorable patient outcomes in order to inform future quality improvement efforts and research.

A significant proportion of diagnostic errors are due to system-related vulnerabilities, such as limitations in availability, adoption or quality of work force training, health informatics resources, and diagnostic capabilities. Lack of institutional culture that promotes safety and transparency also predisposes to diagnostic errors.39,40 The other major domain of process failures is related to cognitive errors in clinician decision-making. Anchoring, confirmation bias, availability bias, and base-rate neglect are some of the common cognitive biases that, along with personality traits (aversion to risk or ambiguity, overconfidence) and affective biases (influence of emotion on decision-making), often determine the degree of utilization of resources and the possibility of suboptimal diagnostic performance.41,42 Further, implicit biases related to age, race, gender, and sexual orientation contribute to disparities in access to health care and outcomes.43 In a large number of cases of preventable adverse outcomes, however, there are multiple interdependent individual and system-related failure points that lead to diagnostic error and patient harm.6,32
Challenges in Defining and Measuring Diagnostic Errors
In order to develop effective, evidence-based interventions to reduce diagnostic errors in hospitalized patients, it is essential to be able to first operationally define, and then accurately measure, diagnostic errors and the process failures that contribute to these errors in a standardized way that is reproducible across different settings.6,44 There are a number of obstacles in this endeavor.
A fundamental problem is that establishing a diagnosis is not a single act but a process. Patterns of symptoms and clinical presentations often differ for the same disease. Information required to make a diagnosis is usually gathered in stages, where the clinician obtains additional data, while considering many possibilities, of which 1 may be ultimately correct. Diagnoses evolve over time and in different care settings. “The most likely diagnosis” is not always the same as “the final correct diagnosis.” Moreover, the diagnostic process is influenced by patients’ individual clinical courses and preferences over time. This makes determination of missed, delayed, or incorrect diagnoses challenging.45,46
For hospitalized patients, generally the goal is to first rule out more serious and acute conditions (eg, pulmonary embolism or stroke), even if their probability is rather low. Conversely, a diagnosis that appears less consequential if delayed (eg, chronic anemia of unclear etiology) might not be pursued on an urgent basis, and is often left to outpatient providers to examine, but still may manifest in downstream harm (eg, delayed diagnosis of gastrointestinal malignancy or recurrent admissions for heart failure due to missed iron-deficiency anemia). Therefore, coming up with disease diagnosis likelihoods in hindsight may turn out to be highly subjective and not always accurate. This can be particularly difficult when clinician and other team deliberations are not recorded in their entirety.47
Another hurdle in the practice of diagnostic medicine is to preserve the balance between underdiagnosing versus pursuing overly aggressive diagnostic approaches. Conducting laboratory, imaging, or other diagnostic studies without a clear shared understanding of how they would affect clinical decision-making (eg, use of prostate-specific antigen to detect prostate cancer) not only leads to increased costs but can also delay appropriate care. Worse, subsequent unnecessary diagnostic tests and treatments can sometimes lead to serious harm.48,49
Finally, retrospective reviews by clinicians are subject to multiple potential limitations that include failure to create well-defined research questions, poorly developed inclusion and exclusion criteria, and issues related to inter- and intra-rater reliability.50 These methodological deficiencies can occur despite following "best practice" guidelines during the study planning, execution, and analysis phases. They further add to the challenge of defining and measuring diagnostic errors.47
Strategies to Improve Measurement of Diagnostic Errors
Development of new methodologies to reliably measure diagnostic errors is an area of active research. The advancement of uniform and universally agreed-upon frameworks to define and identify process failure points and diagnostic errors would help reduce measurement error and support development and testing of interventions that could be generalizable across different health care settings. To more accurately define and measure diagnostic errors, several novel approaches have been proposed (Table 2).

The Safer Dx framework is an all-round tool developed to advance the discipline of measuring diagnostic errors. For an episode of care under review, the instrument scores various items to determine the likelihood of a diagnostic error. These items evaluate multiple dimensions affecting diagnostic performance and measurements across 3 broad domains: structure (provider and organizational characteristics—from everyone involved with patient care, to computing infrastructure, to policies and regulations), process (elements of the patient-provider encounter, diagnostic test performance and follow-up, and subspecialty- and referral-specific factors), and outcome (establishing accurate and timely diagnosis as opposed to missed, delayed, or incorrect diagnosis). This instrument has been revised and can be further modified by a variety of stakeholders, including clinicians, health care organizations, and policymakers, to identify potential diagnostic errors in a standardized way for patient safety and quality improvement research.51,52
Use of standardized tools, such as the Diagnosis Error Evaluation and Research (DEER) taxonomy, can help to identify and classify specific failure points across different diagnostic process dimensions.37 These failure points can be classified into: issues related to patient presentation or access to health care; failure to obtain or misinterpretation of history or physical exam findings; errors in use of diagnostics tests due to technical or clinician-related factors; failures in appropriate weighing of evidence and hypothesis generation; errors associated with referral or consultation process; and failure to monitor the patient or obtain timely follow-up.34 The DEER taxonomy can also be modified based on specific research questions and study populations. Further, it can be recategorized to correspond to Safer Dx framework diagnostic process dimensions to provide insights into reasons for specific process failures and to develop new interventions to mitigate errors and patient harm.6
Since a majority of diagnostic errors do not lead to actual harm, use of “triggers” or clues (eg, procedure-related complications, patient falls, transfers to a higher level of care, readmissions within 30 days) can be a more efficient method to identify diagnostic errors and adverse events that do cause harm. The Global Trigger Tool, developed by the Institute for Healthcare Improvement, uses this strategy. This tool has been shown to identify a significantly higher number of serious adverse events than comparable methods.53 This facilitates selection and development of strategies at the institutional level that are most likely to improve patient outcomes.24
Encouraging and facilitating voluntary or prompted reporting from patients and clinicians can also play an important role in capturing diagnostic errors. Patients and clinicians are not only the key stakeholders but are also uniquely placed within the diagnostic process to detect and report potential errors.25,54 Patient-safety-event reporting systems, such as RL6, play a vital role in reporting near-misses and adverse events. These systems provide a mechanism for team members at all levels within the hospital to contribute toward reporting patient adverse events, including those arising from diagnostic errors.55 The Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey is the first standardized, nationally reported patient survey designed to measure patients’ perceptions of their hospital experience. The US Centers for Medicare and Medicaid Services (CMS) publishes HCAHPS results on its website 4 times a year, which serves as an important incentive for hospitals to improve patient safety and quality of health care delivery.56
Another novel approach links multiple symptoms to a range of target diseases using the Symptom-Disease Pair Analysis of Diagnostic Error (SPADE) framework. Using “big data” technologies, this technique can help discover otherwise hidden symptom-disease links and improve overall diagnostic performance. This approach is proposed for both case-control (look-back) and cohort (look-forward) studies assessing diagnostic errors and misdiagnosis-related harms. For example, starting with a known diagnosis with high potential for harm (eg, stroke), the “look-back” approach can be used to identify high-risk symptoms (eg, dizziness, vertigo). In the “look-forward” approach, a single symptom or exposure risk factor known to be frequently misdiagnosed (eg, dizziness) can be analyzed to identify potential adverse disease outcomes (eg, stroke, migraine).57
Many large ongoing studies looking at diagnostic errors among hospitalized patients, such as Utility of Predictive Systems to identify Inpatient Diagnostic Errors (UPSIDE),58 Patient Safety Learning Lab (PSLL),59 and Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT),60 are using structured chart review methodologies incorporating many of the above strategies in combination. Cases triggered by certain events (eg, ICU transfer, death, rapid response event, new or worsening acute kidney injury) are reviewed using validated tools, including Safer Dx framework and DEER taxonomy, to provide the most precise estimates of the burden of diagnostic errors in hospitalized patients. These estimates may be much higher than previously predicted using traditional chart review approaches.6,24 For example, a recently published study of 2809 random admissions in 11 Massachusetts hospitals identified 978 adverse events but only 10 diagnostic errors (diagnostic error rate, 0.4%).19 This was likely because the trigger method used in the study did not specifically examine the diagnostic process as critically as done by the Safer Dx framework and DEER taxonomy tools, thereby underestimating the total number of diagnostic errors. Further, these ongoing studies (eg, UPSIDE, ADEPT) aim to employ new and upcoming advanced machine-learning methods to create models that can improve overall diagnostic performance. This would pave the way to test and build novel, efficient, and scalable interventions to reduce diagnostic errors and improve patient outcomes.
Strategies to Improve Diagnostic Safety in Hospitalized Patients
Disease-specific biomedical research, as well as advances in laboratory, imaging, and other technologies, play a critical role in improving diagnostic accuracy. However, these technical approaches do not address many of the broader clinician- and system-level failure points and opportunities for improvement. Various patient-, provider-, and organizational-level interventions that could make diagnostic processes more resilient and reduce the risk of error and patient harm have been proposed.61
Among these strategies are approaches to empower patients and their families. Fostering therapeutic relationships between patients and members of the care team is essential to reducing diagnostic errors.62 Facilitating timely access to health records, ensuring transparency in decision making, and tailoring communication strategies to patients’ cultural and educational backgrounds can reduce harm.63 Similarly, at the system level, enhancing communication among different providers by use of tools such as structured handoffs can prevent communication breakdowns and facilitate positive outcomes.64
Interventions targeted at individual health care providers, such as educational programs to improve content-specific knowledge, can enhance diagnostic performance. Regular feedback, strategies to enhance equity, and fostering an environment where all providers are actively encouraged to think critically and participate in the diagnostic process (training programs to use “diagnostic time-outs” and making it a “team sport”) can improve clinical reasoning.65,66 Use of standardized patients can help identify individual-level cognitive failure points and facilitate creation of new interventions to improve clinical decision-making processes.67
Novel health information technologies can further augment these efforts. These include effective documentation by maintaining dynamic and accurate patient histories, problem lists, and medication lists68-70; use of electronic health record–based algorithms to identify potential diagnostic delays for serious conditions71,72; use of telemedicine technologies to improve accessibility and coordination73; application of mobile health and wearable technologies to facilitate data-gathering and care delivery74,75; and use of computerized decision-support tools, including applications to interpret electrocardiograms, imaging studies, and other diagnostic tests.76
Use of precision medicine, powered by new artificial intelligence (AI) tools, is becoming more widespread. Algorithms powered by AI can augment and sometimes even outperform clinician decision-making in areas such as oncology, radiology, and primary care.77 Creation of large biobanks like the All of Us research program can be used to study thousands of environmental and genetic risk factors and health conditions simultaneously, and help identify specific treatments that work best for people of different backgrounds.78 Active research in these areas holds great promise in terms of how and when we diagnose diseases and make appropriate preventative and treatment decisions. Significant scientific, ethical, and regulatory challenges will need to be overcome before these technologies can address some of the most complex problems in health care.79
Finally, diagnostic performance is affected by the external environment, including the functioning of the medical liability system. Diagnostic errors that lead to patient harm are a leading cause of malpractice claims.80 Developing a legal environment, in collaboration with patient advocacy groups and health care organizations, that promotes and facilitates timely disclosure of diagnostic errors could decrease the incentive to hide errors, advance care processes, and improve outcomes.81,82
Conclusion
The burden of diagnostic errors in hospitalized patients is unacceptably high and remains an underemphasized cause of preventable morbidity and mortality. Diagnostic errors often result from a breakdown in multiple interdependent processes that involve patient-, provider-, and system-level factors. Significant challenges remain in defining and identifying diagnostic errors as well as underlying process-failure points. The most effective interventions to reduce diagnostic errors will require greater patient participation in the diagnostic process and a mix of evidence-based interventions that promote individual-provider excellence as well as system-level changes. Further research and collaboration among various stakeholders should help improve diagnostic safety for hospitalized patients.
Corresponding author: Abhishek Goyal, MD, MPH; [email protected]
Disclosures: Dr. Dalal disclosed receiving income ≥ $250 from MayaMD.
Abstract
Diagnostic errors in hospitalized patients are a leading cause of preventable morbidity and mortality. Significant challenges in defining and measuring diagnostic errors and underlying process failure points have led to considerable variability in reported rates of diagnostic errors and adverse outcomes. In this article, we explore the diagnostic process and its discrete components, emphasizing the centrality of the patient in decision-making as well as the continuous nature of the process. We review the incidence of diagnostic errors in hospitalized patients and different methodological approaches that have been used to arrive at these estimates. We discuss different but interdependent provider- and system-related process-failure points that lead to diagnostic errors. We examine specific challenges related to measurement of diagnostic errors and describe traditional and novel approaches that are being used to obtain the most precise estimates. Finally, we examine various patient-, provider-, and organizational-level interventions that have been proposed to improve diagnostic safety in hospitalized patients.
Keywords: diagnostic error, hospital medicine, patient safety.
Diagnosis is defined as a “pre-existing set of categories agreed upon by the medical profession to designate a specific condition.”1 The diagnostic process involves obtaining a clinical history, performing a physical examination, conducting diagnostic testing, and consulting with other clinical providers to gather data that are relevant to understanding the underlying disease processes. This exercise involves generating hypotheses and updating prior probabilities as more information and evidence become available. Throughout this process of information gathering, integration, and interpretation, there is an ongoing assessment of whether sufficient and necessary knowledge has been obtained to make an accurate diagnosis and provide appropriate treatment.2
Diagnostic error is defined as a missed opportunity to make a timely diagnosis as part of this iterative process, including the failure of communicating the diagnosis to the patient in a timely manner.3 It can be categorized as a missed, delayed, or incorrect diagnosis based on available evidence at the time. Establishing the correct diagnosis has important implications. A timely and precise diagnosis ensures the patient the highest probability of having a positive health outcome that reflects an appropriate understanding of underlying disease processes and is consistent with their overall goals of care.3 When diagnostic errors occur, they can cause patient harm. Adverse events due to medical errors, including diagnostic errors, are estimated to be the third leading cause of death in the United States.4 Most people will experience at least 1 diagnostic error in their lifetime. In the 2015 National Academy of Medicine report Improving Diagnosis in Healthcare, diagnostic errors were identified as a major hazard as well as an opportunity to improve patient outcomes.2
Diagnostic errors during hospitalizations are especially concerning, as they are more likely to be implicated in a wider spectrum of harm, including permanent disability and death. This has become even more relevant for hospital medicine physicians and other clinical providers as they encounter increasing cognitive and administrative workloads, rising dissatisfaction and burnout, and unique obstacles such as night-time scheduling.5
Incidence of Diagnostic Errors in Hospitalized Patients
Several methodological approaches have been used to estimate the incidence of diagnostic errors in hospitalized patients. These include retrospective reviews of a sample of all hospital admissions, evaluations of selected adverse outcomes including autopsy studies, patient and provider surveys, and malpractice claims. Laboratory testing audits and secondary reviews in other diagnostic subspecialities (eg, radiology, pathology, and microbiology) are also essential to improving diagnostic performance in these specialized fields, which in turn affects overall hospital diagnostic error rates.6-8 These diverse approaches provide unique insights regarding our ability to assess the degree to which potential harms, ranging from temporary impairment to permanent disability, to death, are attributable to different failure points in the diagnostic process.
Large retrospective chart reviews of random hospital admissions remain the most accurate way to determine the overall incidence of diagnostic errors in hospitalized patients.9 The Harvard Medical Practice Study, published in 1991, laid the groundwork for measuring the incidence of adverse events in hospitalized patients and assessing their relation to medical error, negligence, and disability. Reviewing 30,121 randomly selected records from 51 randomly selected acute care hospitals in New York State, the study found that adverse events occurred in 3.7% of hospitalizations, diagnostic errors accounted for 13.8% of these events, and these errors were likely attributable to negligence in 74.7% of cases. The study not only outlined individual-level process failures, but also focused attention on some of the systemic causes, setting the agenda for quality improvement research in hospital-based care for years to come.10-12 A recent systematic review and meta-analysis of 22 hospital admission studies found a pooled rate of 0.7% (95% CI, 0.5%-1.1%) for harmful diagnostic errors.9 It found significant variations in the rates of adverse events, diagnostic errors, and range of diagnoses that were missed. This was primarily because of variabilities in pre-test probabilities in detecting diagnostic errors in these specific cohorts, as well as due to heterogeneity in study definitions and methodologies, especially regarding how they defined and measured “diagnostic error.” The analysis, however, did not account for diagnostic errors that were not related to patient harm (missed opportunities); therefore, it likely significantly underestimated the true incidence of diagnostic errors in these study populations. Table 1 summarizes some of key studies that have examined the incidence of harmful diagnostic errors in hospitalized patients.9-21

The chief limitation of reviewing random hospital admissions is that, since overall rates of diagnostic errors are still relatively low, a large number of case reviews are required to identify a sufficient sample of adverse outcomes to gain a meaningful understanding of the underlying process failure points and develop tools for remediation. Patient and provider surveys or data from malpractice claims can be high-yield starting points for research on process errors.22,23 Reviews of enriched cohorts of adverse outcomes, such as rapid-response events, intensive care unit (ICU) transfers, deaths, and hospital readmissions, can be an efficient way to identify process failures that lead to greatest harm. Depending on the research approach and the types of underlying patient populations sampled, rates of diagnostic errors in these high-risk groups have been estimated to be approximately 5% to 20%, or even higher.6,24-31 For example, a retrospective study of 391 cases of unplanned 7-day readmissions found that 5.6% of cases contained at least 1 diagnostic error during the index admission.32 In a study conducted at 6 Belgian acute-care hospitals, 56% of patients requiring an unplanned transfer to a higher level of care were determined to have had an adverse event, and of these adverse events, 12.4% of cases were associated with errors in diagnosis.29 A systematic review of 16 hospital-based studies estimated that 3.1% of all inpatient deaths were likely preventable, which corresponded to 22,165 deaths annually in the United States.30 Another such review of 31 autopsy studies reported that 28% of autopsied ICU patients had at least 1 misdiagnosis; of these diagnostic errors, 8% were classified as potentially lethal, and 15% were considered major but not lethal.31 Significant drawbacks of such enriched cohort studies, however, are their poor generalizability and inability to detect failure points that do not lead to patient harm (near-miss events).33
Causes of Diagnostic Errors in Hospitalized Patients
All aspects of the diagnostic process are susceptible to errors. These errors stem from a variety of faulty processes, including failure of the patient to engage with the health care system (eg, due to lack of insurance or transportation, or delay in seeking care); failure in information gathering (eg, missed history or exam findings, ordering wrong tests, laboratory errors); failure in information interpretation (eg, exam finding or test result misinterpretation); inaccurate hypothesis generation (eg, due to suboptimal prioritization or weighing of supporting evidence); and failure in communication (eg, with other team members or with the patient).2,34 Reasons for diagnostic process failures vary widely across different health care settings. While clinician assessment errors (eg, failure to consider or alternatively overweigh competing diagnoses) and errors in testing and the monitoring phase (eg, failure to order or follow up diagnostic tests) can lead to a majority of diagnostic errors in some patient populations, in other settings, social (eg, poor health literacy, punitive cultural practices) and economic factors (eg, lack of access to appropriate diagnostic tests or to specialty expertise) play a more prominent role.34,35
The Figure describes the relationship between components of the diagnostic process and subsequent outcomes, including diagnostic process failures, diagnostic errors, and absence or presence of patient harm.2,36,37 It reemphasizes the centrality of the patient in decision-making and the continuous nature of the process. The Figure also illustrates that only a minority of process failures result in diagnostic errors, and a smaller proportion of diagnostic errors actually lead to patient harm. Conversely, it also shows that diagnostic errors can happen without any obvious process-failure points, and, similarly, patient harm can take place in the absence of any evident diagnostic errors.36-38 Finally, it highlights the need to incorporate feedback from process failures, diagnostic errors, and favorable and unfavorable patient outcomes in order to inform future quality improvement efforts and research.

A significant proportion of diagnostic errors are due to system-related vulnerabilities, such as limitations in availability, adoption or quality of work force training, health informatics resources, and diagnostic capabilities. Lack of institutional culture that promotes safety and transparency also predisposes to diagnostic errors.39,40 The other major domain of process failures is related to cognitive errors in clinician decision-making. Anchoring, confirmation bias, availability bias, and base-rate neglect are some of the common cognitive biases that, along with personality traits (aversion to risk or ambiguity, overconfidence) and affective biases (influence of emotion on decision-making), often determine the degree of utilization of resources and the possibility of suboptimal diagnostic performance.41,42 Further, implicit biases related to age, race, gender, and sexual orientation contribute to disparities in access to health care and outcomes.43 In a large number of cases of preventable adverse outcomes, however, there are multiple interdependent individual and system-related failure points that lead to diagnostic error and patient harm.6,32
Challenges in Defining and Measuring Diagnostic Errors
In order to develop effective, evidence-based interventions to reduce diagnostic errors in hospitalized patients, it is essential to be able to first operationally define, and then accurately measure, diagnostic errors and the process failures that contribute to these errors in a standardized way that is reproducible across different settings.6,44 There are a number of obstacles in this endeavor.
A fundamental problem is that establishing a diagnosis is not a single act but a process. Patterns of symptoms and clinical presentations often differ for the same disease. Information required to make a diagnosis is usually gathered in stages, where the clinician obtains additional data, while considering many possibilities, of which 1 may be ultimately correct. Diagnoses evolve over time and in different care settings. “The most likely diagnosis” is not always the same as “the final correct diagnosis.” Moreover, the diagnostic process is influenced by patients’ individual clinical courses and preferences over time. This makes determination of missed, delayed, or incorrect diagnoses challenging.45,46
For hospitalized patients, generally the goal is to first rule out more serious and acute conditions (eg, pulmonary embolism or stroke), even if their probability is rather low. Conversely, a diagnosis that appears less consequential if delayed (eg, chronic anemia of unclear etiology) might not be pursued on an urgent basis, and is often left to outpatient providers to examine, but still may manifest in downstream harm (eg, delayed diagnosis of gastrointestinal malignancy or recurrent admissions for heart failure due to missed iron-deficiency anemia). Therefore, coming up with disease diagnosis likelihoods in hindsight may turn out to be highly subjective and not always accurate. This can be particularly difficult when clinician and other team deliberations are not recorded in their entirety.47
Another hurdle in the practice of diagnostic medicine is to preserve the balance between underdiagnosing versus pursuing overly aggressive diagnostic approaches. Conducting laboratory, imaging, or other diagnostic studies without a clear shared understanding of how they would affect clinical decision-making (eg, use of prostate-specific antigen to detect prostate cancer) not only leads to increased costs but can also delay appropriate care. Worse, subsequent unnecessary diagnostic tests and treatments can sometimes lead to serious harm.48,49
Finally, retrospective reviews by clinicians are subject to multiple potential limitations that include failure to create well-defined research questions, poorly developed inclusion and exclusion criteria, and issues related to inter- and intra-rater reliability.50 These methodological deficiencies can occur despite following "best practice" guidelines during the study planning, execution, and analysis phases. They further add to the challenge of defining and measuring diagnostic errors.47
Strategies to Improve Measurement of Diagnostic Errors
Development of new methodologies to reliably measure diagnostic errors is an area of active research. The advancement of uniform and universally agreed-upon frameworks to define and identify process failure points and diagnostic errors would help reduce measurement error and support development and testing of interventions that could be generalizable across different health care settings. To more accurately define and measure diagnostic errors, several novel approaches have been proposed (Table 2).

The Safer Dx framework is an all-round tool developed to advance the discipline of measuring diagnostic errors. For an episode of care under review, the instrument scores various items to determine the likelihood of a diagnostic error. These items evaluate multiple dimensions affecting diagnostic performance and measurements across 3 broad domains: structure (provider and organizational characteristics—from everyone involved with patient care, to computing infrastructure, to policies and regulations), process (elements of the patient-provider encounter, diagnostic test performance and follow-up, and subspecialty- and referral-specific factors), and outcome (establishing accurate and timely diagnosis as opposed to missed, delayed, or incorrect diagnosis). This instrument has been revised and can be further modified by a variety of stakeholders, including clinicians, health care organizations, and policymakers, to identify potential diagnostic errors in a standardized way for patient safety and quality improvement research.51,52
Use of standardized tools, such as the Diagnosis Error Evaluation and Research (DEER) taxonomy, can help to identify and classify specific failure points across different diagnostic process dimensions.37 These failure points can be classified into: issues related to patient presentation or access to health care; failure to obtain or misinterpretation of history or physical exam findings; errors in use of diagnostics tests due to technical or clinician-related factors; failures in appropriate weighing of evidence and hypothesis generation; errors associated with referral or consultation process; and failure to monitor the patient or obtain timely follow-up.34 The DEER taxonomy can also be modified based on specific research questions and study populations. Further, it can be recategorized to correspond to Safer Dx framework diagnostic process dimensions to provide insights into reasons for specific process failures and to develop new interventions to mitigate errors and patient harm.6
Since a majority of diagnostic errors do not lead to actual harm, use of “triggers” or clues (eg, procedure-related complications, patient falls, transfers to a higher level of care, readmissions within 30 days) can be a more efficient method to identify diagnostic errors and adverse events that do cause harm. The Global Trigger Tool, developed by the Institute for Healthcare Improvement, uses this strategy. This tool has been shown to identify a significantly higher number of serious adverse events than comparable methods.53 This facilitates selection and development of strategies at the institutional level that are most likely to improve patient outcomes.24
Encouraging and facilitating voluntary or prompted reporting from patients and clinicians can also play an important role in capturing diagnostic errors. Patients and clinicians are not only the key stakeholders but are also uniquely placed within the diagnostic process to detect and report potential errors.25,54 Patient-safety-event reporting systems, such as RL6, play a vital role in reporting near-misses and adverse events. These systems provide a mechanism for team members at all levels within the hospital to contribute toward reporting patient adverse events, including those arising from diagnostic errors.55 The Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey is the first standardized, nationally reported patient survey designed to measure patients’ perceptions of their hospital experience. The US Centers for Medicare and Medicaid Services (CMS) publishes HCAHPS results on its website 4 times a year, which serves as an important incentive for hospitals to improve patient safety and quality of health care delivery.56
Another novel approach links multiple symptoms to a range of target diseases using the Symptom-Disease Pair Analysis of Diagnostic Error (SPADE) framework. Using “big data” technologies, this technique can help discover otherwise hidden symptom-disease links and improve overall diagnostic performance. This approach is proposed for both case-control (look-back) and cohort (look-forward) studies assessing diagnostic errors and misdiagnosis-related harms. For example, starting with a known diagnosis with high potential for harm (eg, stroke), the “look-back” approach can be used to identify high-risk symptoms (eg, dizziness, vertigo). In the “look-forward” approach, a single symptom or exposure risk factor known to be frequently misdiagnosed (eg, dizziness) can be analyzed to identify potential adverse disease outcomes (eg, stroke, migraine).57
Many large ongoing studies looking at diagnostic errors among hospitalized patients, such as Utility of Predictive Systems to identify Inpatient Diagnostic Errors (UPSIDE),58 Patient Safety Learning Lab (PSLL),59 and Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT),60 are using structured chart review methodologies incorporating many of the above strategies in combination. Cases triggered by certain events (eg, ICU transfer, death, rapid response event, new or worsening acute kidney injury) are reviewed using validated tools, including Safer Dx framework and DEER taxonomy, to provide the most precise estimates of the burden of diagnostic errors in hospitalized patients. These estimates may be much higher than previously predicted using traditional chart review approaches.6,24 For example, a recently published study of 2809 random admissions in 11 Massachusetts hospitals identified 978 adverse events but only 10 diagnostic errors (diagnostic error rate, 0.4%).19 This was likely because the trigger method used in the study did not specifically examine the diagnostic process as critically as done by the Safer Dx framework and DEER taxonomy tools, thereby underestimating the total number of diagnostic errors. Further, these ongoing studies (eg, UPSIDE, ADEPT) aim to employ new and upcoming advanced machine-learning methods to create models that can improve overall diagnostic performance. This would pave the way to test and build novel, efficient, and scalable interventions to reduce diagnostic errors and improve patient outcomes.
Strategies to Improve Diagnostic Safety in Hospitalized Patients
Disease-specific biomedical research, as well as advances in laboratory, imaging, and other technologies, play a critical role in improving diagnostic accuracy. However, these technical approaches do not address many of the broader clinician- and system-level failure points and opportunities for improvement. Various patient-, provider-, and organizational-level interventions that could make diagnostic processes more resilient and reduce the risk of error and patient harm have been proposed.61
Among these strategies are approaches to empower patients and their families. Fostering therapeutic relationships between patients and members of the care team is essential to reducing diagnostic errors.62 Facilitating timely access to health records, ensuring transparency in decision making, and tailoring communication strategies to patients’ cultural and educational backgrounds can reduce harm.63 Similarly, at the system level, enhancing communication among different providers by use of tools such as structured handoffs can prevent communication breakdowns and facilitate positive outcomes.64
Interventions targeted at individual health care providers, such as educational programs to improve content-specific knowledge, can enhance diagnostic performance. Regular feedback, strategies to enhance equity, and fostering an environment where all providers are actively encouraged to think critically and participate in the diagnostic process (training programs to use “diagnostic time-outs” and making it a “team sport”) can improve clinical reasoning.65,66 Use of standardized patients can help identify individual-level cognitive failure points and facilitate creation of new interventions to improve clinical decision-making processes.67
Novel health information technologies can further augment these efforts. These include effective documentation by maintaining dynamic and accurate patient histories, problem lists, and medication lists68-70; use of electronic health record–based algorithms to identify potential diagnostic delays for serious conditions71,72; use of telemedicine technologies to improve accessibility and coordination73; application of mobile health and wearable technologies to facilitate data-gathering and care delivery74,75; and use of computerized decision-support tools, including applications to interpret electrocardiograms, imaging studies, and other diagnostic tests.76
Use of precision medicine, powered by new artificial intelligence (AI) tools, is becoming more widespread. Algorithms powered by AI can augment and sometimes even outperform clinician decision-making in areas such as oncology, radiology, and primary care.77 Creation of large biobanks like the All of Us research program can be used to study thousands of environmental and genetic risk factors and health conditions simultaneously, and help identify specific treatments that work best for people of different backgrounds.78 Active research in these areas holds great promise in terms of how and when we diagnose diseases and make appropriate preventative and treatment decisions. Significant scientific, ethical, and regulatory challenges will need to be overcome before these technologies can address some of the most complex problems in health care.79
Finally, diagnostic performance is affected by the external environment, including the functioning of the medical liability system. Diagnostic errors that lead to patient harm are a leading cause of malpractice claims.80 Developing a legal environment, in collaboration with patient advocacy groups and health care organizations, that promotes and facilitates timely disclosure of diagnostic errors could decrease the incentive to hide errors, advance care processes, and improve outcomes.81,82
Conclusion
The burden of diagnostic errors in hospitalized patients is unacceptably high and remains an underemphasized cause of preventable morbidity and mortality. Diagnostic errors often result from a breakdown in multiple interdependent processes that involve patient-, provider-, and system-level factors. Significant challenges remain in defining and identifying diagnostic errors as well as underlying process-failure points. The most effective interventions to reduce diagnostic errors will require greater patient participation in the diagnostic process and a mix of evidence-based interventions that promote individual-provider excellence as well as system-level changes. Further research and collaboration among various stakeholders should help improve diagnostic safety for hospitalized patients.
Corresponding author: Abhishek Goyal, MD, MPH; [email protected]
Disclosures: Dr. Dalal disclosed receiving income ≥ $250 from MayaMD.
1. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499. doi:10.1001/archinte.165.13.1493
2. National Academies of Sciences, Engineering, and Medicine. 2015. Improving Diagnosis in Health Care. The National Academies Press. doi:10.17226/21794
3. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
4. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139. doi:10.1136/bmj.i2139
5. Flanders SA, Centor B, Weber V, McGinn T, Desalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit. J Gen Intern Med. 2009;24(5):636-641. doi:10.1007/s11606-009-0944-6
6. Griffin JA, Carr K, Bersani K, et al. Analyzing diagnostic errors in the acute setting: a process-driven approach. Diagnosis (Berl). 2021;9(1):77-88. doi:10.1515/dx-2021-0033
7. Itri JN, Tappouni RR, McEachern RO, Pesch AJ, Patel SH. Fundamentals of diagnostic error in imaging. RadioGraphics. 2018;38(6):1845-1865. doi:10.1148/rg.2018180021
8. Hammerling JA. A Review of medical errors in laboratory diagnostics and where we are today. Lab Med. 2012;43(2):41-44. doi:10.1309/LM6ER9WJR1IHQAUY
9. Gunderson CG, Bilan VP, Holleck JL, et al. Prevalence of harmful diagnostic errors in hospitalised adults: a systematic review and meta-analysis. BMJ Qual Saf. 2020;29(12):1008-1018. doi:10.1136/bmjqs-2019-010822
10. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi:10.1056/NEJM199102073240604
11. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324(6):377-384. doi:10.1056/NEJM199102073240605
12. Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. doi:10.1056/NEJM199107253250405
13. Wilson RM, Michel P, Olsen S, et al. Patient safety in developing countries: retrospective estimation of scale and nature of harm to patients in hospital. BMJ. 2012;344:e832. doi:10.1136/bmj.e832
14. Wilson RM, Runciman WB, Gibberd RW, Harrison BT, Newby L, Hamilton JD. The Quality in Australian Health Care Study. Med J Aust. 1995;163(9):458-471. doi:10.5694/j.1326-5377.1995.tb124691.x
15. Thomas EJ, Studdert DM, Burstin HR, et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38(3):261-271. doi:10.1097/00005650-200003000-00003
16. Baker GR, Norton PG, Flintoft V, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ. 2004;170(11):1678-1686. doi:10.1503/cmaj.1040498
17. Davis P, Lay-Yee R, Briant R, Ali W, Scott A, Schug S. Adverse events in New Zealand public hospitals II: preventability and clinical context. N Z Med J. 2003;116(1183):U624.
18. Aranaz-Andrés JM, Aibar-Remón C, Vitaller-Murillo J, et al. Incidence of adverse events related to health care in Spain: results of the Spanish National Study of Adverse Events. J Epidemiol Community Health. 2008;62(12):1022-1029. doi:10.1136/jech.2007.065227
19. Bates DW, Levine DM, Salmasian H, et al. The safety of inpatient health care. N Engl J Med. 2023;388(2):142-153. doi:10.1056/NEJMsa2206117
20. Soop M, Fryksmark U, Köster M, Haglund B. The incidence of adverse events in Swedish hospitals: a retrospective medical record review study. Int J Qual Health Care. 2009;21(4):285-291. doi:10.1093/intqhc/mzp025
21. Rafter N, Hickey A, Conroy RM, et al. The Irish National Adverse Events Study (INAES): the frequency and nature of adverse events in Irish hospitals—a retrospective record review study. BMJ Qual Saf. 2017;26(2):111-119. doi:10.1136/bmjqs-2015-004828
22. Blendon RJ, DesRoches CM, Brodie M, et al. Views of practicing physicians and the public on medical errors. N Engl J Med. 2002;347(24):1933-1940. doi:10.1056/NEJMsa022151
23. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680. doi:10.1136/bmjqs-2012-001550
24. Malik MA, Motta-Calderon D, Piniella N, et al. A structured approach to EHR surveillance of diagnostic error in acute care: an exploratory analysis of two institutionally-defined case cohorts. Diagnosis (Berl). 2022;9(4):446-457. doi:10.1515/dx-2022-0032
25. Graber ML. The incidence of diagnostic error in medicine. BMJ Qual Saf. 2013;22(suppl 2):ii21-ii27. doi:10.1136/bmjqs-2012-001615
26. Bergl PA, Taneja A, El-Kareh R, Singh H, Nanchal RS. Frequency, risk factors, causes, and consequences of diagnostic errors in critically ill medical patients: a retrospective cohort study. Crit Care Med. 2019;47(11):e902-e910. doi:10.1097/CCM.0000000000003976
27. Hogan H, Healey F, Neale G, Thomson R, Vincent C, Black N. Preventable deaths due to problems in care in English acute hospitals: a retrospective case record review study. BMJ Qual Saf. 2012;21(9):737-745. doi:10.1136/bmjqs-2011-001159
28. Bergl PA, Nanchal RS, Singh H. Diagnostic error in the critically ill: defining the problem and exploring next steps to advance intensive care unit safety. Ann Am Thorac Soc. 2018;15(8):903-907. doi:10.1513/AnnalsATS.201801-068PS
29. Marquet K, Claes N, De Troy E, et al. One fourth of unplanned transfers to a higher level of care are associated with a highly preventable adverse event: a patient record review in six Belgian hospitals. Crit Care Med. 2015;43(5):1053-1061. doi:10.1097/CCM.0000000000000932
30. Rodwin BA, Bilan VP, Merchant NB, et al. Rate of preventable mortality in hospitalized patients: a systematic review and meta-analysis. J Gen Intern Med. 2020;35(7):2099-2106. doi:10.1007/s11606-019-05592-5
31. Winters B, Custer J, Galvagno SM, et al. Diagnostic errors in the intensive care unit: a systematic review of autopsy studies. BMJ Qual Saf. 2012;21(11):894-902. doi:10.1136/bmjqs-2012-000803
32. Raffel KE, Kantor MA, Barish P, et al. Prevalence and characterisation of diagnostic error among 7-day all-cause hospital medicine readmissions: a retrospective cohort study. BMJ Qual Saf. 2020;29(12):971-979. doi:10.1136/bmjqs-2020-010896
33. Weingart SN, Pagovich O, Sands DZ, et al. What can hospitalized patients tell us about adverse events? learning from patient-reported incidents. J Gen Intern Med. 2005;20(9):830-836. doi:10.1111/j.1525-1497.2005.0180.x
34. Schiff GD, Hasan O, Kim S, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169(20):1881-1887. doi:10.1001/archinternmed.2009.333
35. Singh H, Schiff GD, Graber ML, Onakpoya I, Thompson MJ. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
36. Schiff GD, Leape LL. Commentary: how can we make diagnosis safer? Acad Med J Assoc Am Med Coll. 2012;87(2):135-138. doi:10.1097/ACM.0b013e31823f711c
37. Schiff GD, Kim S, Abrams R, et al. Diagnosing diagnosis errors: lessons from a multi-institutional collaborative project. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Volume 2: Concepts and Methodology. AHRQ Publication No. 05-0021-2. Agency for Healthcare Research and Quality (US); 2005. Accessed January 16, 2023. http://www.ncbi.nlm.nih.gov/books/NBK20492/
38. Newman-Toker DE. A unified conceptual model for diagnostic errors: underdiagnosis, overdiagnosis, and misdiagnosis. Diagnosis (Berl). 2014;1(1):43-48. doi:10.1515/dx-2013-0027
39. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, Firipis M, Wanni Arachchige Dona S, Watts JJ. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
40. Gupta A, Harrod M, Quinn M, et al. Mind the overlap: how system problems contribute to cognitive failure and diagnostic errors. Diagnosis (Berl). 2018;5(3):151-156. doi:10.1515/dx-2018-0014
41. Saposnik G, Redelmeier D, Ruff CC, Tobler PN. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16:138. doi:10.1186/s12911-016-0377-1
42. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78(8):775-780. doi: 10.1097/00001888-200308000-00003
43. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504-1510. doi:10.1007/s11606-013-2441-1
44. Zwaan L, Singh H. The challenges in defining and measuring diagnostic error. Diagnosis (Ber). 2015;2(2):97-103. doi:10.1515/dx-2014-0069
45. Arkes HR, Wortmann RL, Saville PD, Harkness AR. Hindsight bias among physicians weighing the likelihood of diagnoses. J Appl Psychol. 1981;66(2):252-254.
46. Singh H. Editorial: Helping health care organizations to define diagnostic errors as missed opportunities in diagnosis. Jt Comm J Qual Patient Saf. 2014;40(3):99-101. doi:10.1016/s1553-7250(14)40012-6
47. Vassar M, Holzmann M. The retrospective chart review: important methodological considerations. J Educ Eval Health Prof. 2013;10:12. doi:10.3352/jeehp.2013.10.12
48. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605-613. doi:10.1093/jnci/djq099
49. Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. doi:10.1136/bmj.e3502
50. Hayward RA, Hofer TP. Estimating hospital deaths due to medical errors: preventability is in the eye of the reviewer. JAMA. 2001;286(4):415-420. doi:10.1001/jama.286.4.415
51. Singh H, Sittig DF. Advancing the science of measurement of diagnostic errors in healthcare: the Safer Dx framework. BMJ Qual Saf. 2015;24(2):103-110. doi:10.1136/bmjqs-2014-003675
52. Singh H, Khanna A, Spitzmueller C, Meyer AND. Recommendations for using the Revised Safer Dx Instrument to help measure and improve diagnostic safety. Diagnosis (Berl). 2019;6(4):315-323. doi:10.1515/dx-2019-0012
53. Classen DC, Resar R, Griffin F, et al. “Global trigger tool” shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood). 2011;30(4):581-589. doi:10.1377/hlthaff.2011.0190
54. Schiff GD. Minimizing diagnostic error: the importance of follow-up and feedback. Am J Med. 2008;121(5 suppl):S38-S42. doi:10.1016/j.amjmed.2008.02.004
55. Mitchell I, Schuster A, Smith K, Pronovost P, Wu A. Patient safety incident reporting: a qualitative study of thoughts and perceptions of experts 15 years after “To Err is Human.” BMJ Qual Saf. 2016;25(2):92-99. doi:10.1136/bmjqs-2015-004405
56. Mazurenko O, Collum T, Ferdinand A, Menachemi N. Predictors of hospital patient satisfaction as measured by HCAHPS: a systematic review. J Healthc Manag. 2017;62(4):272-283. doi:10.1097/JHM-D-15-00050
57. Liberman AL, Newman-Toker DE. Symptom-Disease Pair Analysis of Diagnostic Error (SPADE): a conceptual framework and methodological approach for unearthing misdiagnosis-related harms using big data. BMJ Qual Saf. 2018;27(7):557-566. doi:10.1136/bmjqs-2017-007032
58. Utility of Predictive Systems to Identify Inpatient Diagnostic Errors: the UPSIDE study. NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/search/rpoHXlEAcEudQV3B9ld8iw/project-details/10020962
59. Overview of Patient Safety Learning Laboratory (PSLL) Projects. Agency for Healthcare Research and Quality. Accessed January 14, 2023. https://www.ahrq.gov/patient-safety/resources/learning-lab/index.html
60. Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT). NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/project-details/10642576
61. Zwaan L, Singh H. Diagnostic error in hospitals: finding forests not just the big trees. BMJ Qual Saf. 2020;29(12):961-964. doi:10.1136/bmjqs-2020-011099
62. Longtin Y, Sax H, Leape LL, Sheridan SE, Donaldson L, Pittet D. Patient participation: current knowledge and applicability to patient safety. Mayo Clin Proc. 2010;85(1):53-62. doi:10.4065/mcp.2009.0248
63. Murphy DR, Singh H, Berlin L. Communication breakdowns and diagnostic errors: a radiology perspective. Diagnosis (Berl). 2014;1(4):253-261. doi:10.1515/dx-2014-0035
64. Singh H, Naik AD, Rao R, Petersen LA. Reducing diagnostic errors through effective communication: harnessing the power of information technology. J Gen Intern Med. 2008;23(4):489-494. doi:10.1007/s11606-007-0393-z
65. Singh H, Connor DM, Dhaliwal G. Five strategies for clinicians to advance diagnostic excellence. BMJ. 2022;376:e068044. doi:10.1136/bmj-2021-068044
66. Yale S, Cohen S, Bordini BJ. Diagnostic time-outs to improve diagnosis. Crit Care Clin. 2022;38(2):185-194. doi:10.1016/j.ccc.2021.11.008
67. Schwartz A, Peskin S, Spiro A, Weiner SJ. Impact of unannounced standardized patient audit and feedback on care, documentation, and costs: an experiment and claims analysis. J Gen Intern Med. 2021;36(1):27-34. doi:10.1007/s11606-020-05965-1
68. Carpenter JD, Gorman PN. Using medication list—problem list mismatches as markers of potential error. Proc AMIA Symp. 2002:106-110.
69. Hron JD, Manzi S, Dionne R, et al. Electronic medication reconciliation and medication errors. Int J Qual Health Care. 2015;27(4):314-319. doi:10.1093/intqhc/mzv046
70. Graber ML, Siegal D, Riah H, Johnston D, Kenyon K. Electronic health record–related events in medical malpractice claims. J Patient Saf. 2019;15(2):77-85. doi:10.1097/PTS.0000000000000240
71. Murphy DR, Wu L, Thomas EJ, Forjuoh SN, Meyer AND, Singh H. Electronic trigger-based intervention to reduce delays in diagnostic evaluation for cancer: a cluster randomized controlled trial. J Clin Oncol. 2015;33(31):3560-3567. doi:10.1200/JCO.2015.61.1301
72. Singh H, Giardina TD, Forjuoh SN, et al. Electronic health record-based surveillance of diagnostic errors in primary care. BMJ Qual Saf. 2012;21(2):93-100. doi:10.1136/bmjqs-2011-000304
73. Armaignac DL, Saxena A, Rubens M, et al. Impact of telemedicine on mortality, length of stay, and cost among patients in progressive care units: experience from a large healthcare system. Crit Care Med. 2018;46(5):728-735. doi:10.1097/CCM.0000000000002994
74. MacKinnon GE, Brittain EL. Mobile health technologies in cardiopulmonary disease. Chest. 2020;157(3):654-664. doi:10.1016/j.chest.2019.10.015
75. DeVore AD, Wosik J, Hernandez AF. The future of wearables in heart failure patients. JACC Heart Fail. 2019;7(11):922-932. doi:10.1016/j.jchf.2019.08.008
76. Tsai TL, Fridsma DB, Gatti G. Computer decision support as a source of interpretation error: the case of electrocardiograms. J Am Med Inform Assoc. 2003;10(5):478-483. doi:10.1197/jamia.M1279
77. Lin SY, Mahoney MR, Sinsky CA. Ten ways artificial intelligence will transform primary care. J Gen Intern Med. 2019;34(8):1626-1630. doi:10.1007/s11606-019-05035-1
78. Ramirez AH, Gebo KA, Harris PA. Progress with the All Of Us research program: opening access for researchers. JAMA. 2021;325(24):2441-2442. doi:10.1001/jama.2021.7702
79. Johnson KB, Wei W, Weeraratne D, et al. Precision medicine, AI, and the future of personalized health care. Clin Transl Sci. 2021;14(1):86-93. doi:10.1111/cts.12884
80. Gupta A, Snyder A, Kachalia A, Flanders S, Saint S, Chopra V. Malpractice claims related to diagnostic errors in the hospital. BMJ Qual Saf. 2017;27(1):bmjqs-2017-006774. doi:10.1136/bmjqs-2017-006774
81. Renkema E, Broekhuis M, Ahaus K. Conditions that influence the impact of malpractice litigation risk on physicians’ behavior regarding patient safety. BMC Health Serv Res. 2014;14(1):38. doi:10.1186/1472-6963-14-38
82. Kachalia A, Mello MM, Nallamothu BK, Studdert DM. Legal and policy interventions to improve patient safety. Circulation. 2016;133(7):661-671. doi:10.1161/CIRCULATIONAHA.115.015880
1. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499. doi:10.1001/archinte.165.13.1493
2. National Academies of Sciences, Engineering, and Medicine. 2015. Improving Diagnosis in Health Care. The National Academies Press. doi:10.17226/21794
3. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
4. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139. doi:10.1136/bmj.i2139
5. Flanders SA, Centor B, Weber V, McGinn T, Desalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit. J Gen Intern Med. 2009;24(5):636-641. doi:10.1007/s11606-009-0944-6
6. Griffin JA, Carr K, Bersani K, et al. Analyzing diagnostic errors in the acute setting: a process-driven approach. Diagnosis (Berl). 2021;9(1):77-88. doi:10.1515/dx-2021-0033
7. Itri JN, Tappouni RR, McEachern RO, Pesch AJ, Patel SH. Fundamentals of diagnostic error in imaging. RadioGraphics. 2018;38(6):1845-1865. doi:10.1148/rg.2018180021
8. Hammerling JA. A Review of medical errors in laboratory diagnostics and where we are today. Lab Med. 2012;43(2):41-44. doi:10.1309/LM6ER9WJR1IHQAUY
9. Gunderson CG, Bilan VP, Holleck JL, et al. Prevalence of harmful diagnostic errors in hospitalised adults: a systematic review and meta-analysis. BMJ Qual Saf. 2020;29(12):1008-1018. doi:10.1136/bmjqs-2019-010822
10. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi:10.1056/NEJM199102073240604
11. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324(6):377-384. doi:10.1056/NEJM199102073240605
12. Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. doi:10.1056/NEJM199107253250405
13. Wilson RM, Michel P, Olsen S, et al. Patient safety in developing countries: retrospective estimation of scale and nature of harm to patients in hospital. BMJ. 2012;344:e832. doi:10.1136/bmj.e832
14. Wilson RM, Runciman WB, Gibberd RW, Harrison BT, Newby L, Hamilton JD. The Quality in Australian Health Care Study. Med J Aust. 1995;163(9):458-471. doi:10.5694/j.1326-5377.1995.tb124691.x
15. Thomas EJ, Studdert DM, Burstin HR, et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38(3):261-271. doi:10.1097/00005650-200003000-00003
16. Baker GR, Norton PG, Flintoft V, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ. 2004;170(11):1678-1686. doi:10.1503/cmaj.1040498
17. Davis P, Lay-Yee R, Briant R, Ali W, Scott A, Schug S. Adverse events in New Zealand public hospitals II: preventability and clinical context. N Z Med J. 2003;116(1183):U624.
18. Aranaz-Andrés JM, Aibar-Remón C, Vitaller-Murillo J, et al. Incidence of adverse events related to health care in Spain: results of the Spanish National Study of Adverse Events. J Epidemiol Community Health. 2008;62(12):1022-1029. doi:10.1136/jech.2007.065227
19. Bates DW, Levine DM, Salmasian H, et al. The safety of inpatient health care. N Engl J Med. 2023;388(2):142-153. doi:10.1056/NEJMsa2206117
20. Soop M, Fryksmark U, Köster M, Haglund B. The incidence of adverse events in Swedish hospitals: a retrospective medical record review study. Int J Qual Health Care. 2009;21(4):285-291. doi:10.1093/intqhc/mzp025
21. Rafter N, Hickey A, Conroy RM, et al. The Irish National Adverse Events Study (INAES): the frequency and nature of adverse events in Irish hospitals—a retrospective record review study. BMJ Qual Saf. 2017;26(2):111-119. doi:10.1136/bmjqs-2015-004828
22. Blendon RJ, DesRoches CM, Brodie M, et al. Views of practicing physicians and the public on medical errors. N Engl J Med. 2002;347(24):1933-1940. doi:10.1056/NEJMsa022151
23. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680. doi:10.1136/bmjqs-2012-001550
24. Malik MA, Motta-Calderon D, Piniella N, et al. A structured approach to EHR surveillance of diagnostic error in acute care: an exploratory analysis of two institutionally-defined case cohorts. Diagnosis (Berl). 2022;9(4):446-457. doi:10.1515/dx-2022-0032
25. Graber ML. The incidence of diagnostic error in medicine. BMJ Qual Saf. 2013;22(suppl 2):ii21-ii27. doi:10.1136/bmjqs-2012-001615
26. Bergl PA, Taneja A, El-Kareh R, Singh H, Nanchal RS. Frequency, risk factors, causes, and consequences of diagnostic errors in critically ill medical patients: a retrospective cohort study. Crit Care Med. 2019;47(11):e902-e910. doi:10.1097/CCM.0000000000003976
27. Hogan H, Healey F, Neale G, Thomson R, Vincent C, Black N. Preventable deaths due to problems in care in English acute hospitals: a retrospective case record review study. BMJ Qual Saf. 2012;21(9):737-745. doi:10.1136/bmjqs-2011-001159
28. Bergl PA, Nanchal RS, Singh H. Diagnostic error in the critically ill: defining the problem and exploring next steps to advance intensive care unit safety. Ann Am Thorac Soc. 2018;15(8):903-907. doi:10.1513/AnnalsATS.201801-068PS
29. Marquet K, Claes N, De Troy E, et al. One fourth of unplanned transfers to a higher level of care are associated with a highly preventable adverse event: a patient record review in six Belgian hospitals. Crit Care Med. 2015;43(5):1053-1061. doi:10.1097/CCM.0000000000000932
30. Rodwin BA, Bilan VP, Merchant NB, et al. Rate of preventable mortality in hospitalized patients: a systematic review and meta-analysis. J Gen Intern Med. 2020;35(7):2099-2106. doi:10.1007/s11606-019-05592-5
31. Winters B, Custer J, Galvagno SM, et al. Diagnostic errors in the intensive care unit: a systematic review of autopsy studies. BMJ Qual Saf. 2012;21(11):894-902. doi:10.1136/bmjqs-2012-000803
32. Raffel KE, Kantor MA, Barish P, et al. Prevalence and characterisation of diagnostic error among 7-day all-cause hospital medicine readmissions: a retrospective cohort study. BMJ Qual Saf. 2020;29(12):971-979. doi:10.1136/bmjqs-2020-010896
33. Weingart SN, Pagovich O, Sands DZ, et al. What can hospitalized patients tell us about adverse events? learning from patient-reported incidents. J Gen Intern Med. 2005;20(9):830-836. doi:10.1111/j.1525-1497.2005.0180.x
34. Schiff GD, Hasan O, Kim S, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169(20):1881-1887. doi:10.1001/archinternmed.2009.333
35. Singh H, Schiff GD, Graber ML, Onakpoya I, Thompson MJ. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
36. Schiff GD, Leape LL. Commentary: how can we make diagnosis safer? Acad Med J Assoc Am Med Coll. 2012;87(2):135-138. doi:10.1097/ACM.0b013e31823f711c
37. Schiff GD, Kim S, Abrams R, et al. Diagnosing diagnosis errors: lessons from a multi-institutional collaborative project. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Volume 2: Concepts and Methodology. AHRQ Publication No. 05-0021-2. Agency for Healthcare Research and Quality (US); 2005. Accessed January 16, 2023. http://www.ncbi.nlm.nih.gov/books/NBK20492/
38. Newman-Toker DE. A unified conceptual model for diagnostic errors: underdiagnosis, overdiagnosis, and misdiagnosis. Diagnosis (Berl). 2014;1(1):43-48. doi:10.1515/dx-2013-0027
39. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, Firipis M, Wanni Arachchige Dona S, Watts JJ. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
40. Gupta A, Harrod M, Quinn M, et al. Mind the overlap: how system problems contribute to cognitive failure and diagnostic errors. Diagnosis (Berl). 2018;5(3):151-156. doi:10.1515/dx-2018-0014
41. Saposnik G, Redelmeier D, Ruff CC, Tobler PN. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16:138. doi:10.1186/s12911-016-0377-1
42. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78(8):775-780. doi: 10.1097/00001888-200308000-00003
43. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504-1510. doi:10.1007/s11606-013-2441-1
44. Zwaan L, Singh H. The challenges in defining and measuring diagnostic error. Diagnosis (Ber). 2015;2(2):97-103. doi:10.1515/dx-2014-0069
45. Arkes HR, Wortmann RL, Saville PD, Harkness AR. Hindsight bias among physicians weighing the likelihood of diagnoses. J Appl Psychol. 1981;66(2):252-254.
46. Singh H. Editorial: Helping health care organizations to define diagnostic errors as missed opportunities in diagnosis. Jt Comm J Qual Patient Saf. 2014;40(3):99-101. doi:10.1016/s1553-7250(14)40012-6
47. Vassar M, Holzmann M. The retrospective chart review: important methodological considerations. J Educ Eval Health Prof. 2013;10:12. doi:10.3352/jeehp.2013.10.12
48. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605-613. doi:10.1093/jnci/djq099
49. Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. doi:10.1136/bmj.e3502
50. Hayward RA, Hofer TP. Estimating hospital deaths due to medical errors: preventability is in the eye of the reviewer. JAMA. 2001;286(4):415-420. doi:10.1001/jama.286.4.415
51. Singh H, Sittig DF. Advancing the science of measurement of diagnostic errors in healthcare: the Safer Dx framework. BMJ Qual Saf. 2015;24(2):103-110. doi:10.1136/bmjqs-2014-003675
52. Singh H, Khanna A, Spitzmueller C, Meyer AND. Recommendations for using the Revised Safer Dx Instrument to help measure and improve diagnostic safety. Diagnosis (Berl). 2019;6(4):315-323. doi:10.1515/dx-2019-0012
53. Classen DC, Resar R, Griffin F, et al. “Global trigger tool” shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood). 2011;30(4):581-589. doi:10.1377/hlthaff.2011.0190
54. Schiff GD. Minimizing diagnostic error: the importance of follow-up and feedback. Am J Med. 2008;121(5 suppl):S38-S42. doi:10.1016/j.amjmed.2008.02.004
55. Mitchell I, Schuster A, Smith K, Pronovost P, Wu A. Patient safety incident reporting: a qualitative study of thoughts and perceptions of experts 15 years after “To Err is Human.” BMJ Qual Saf. 2016;25(2):92-99. doi:10.1136/bmjqs-2015-004405
56. Mazurenko O, Collum T, Ferdinand A, Menachemi N. Predictors of hospital patient satisfaction as measured by HCAHPS: a systematic review. J Healthc Manag. 2017;62(4):272-283. doi:10.1097/JHM-D-15-00050
57. Liberman AL, Newman-Toker DE. Symptom-Disease Pair Analysis of Diagnostic Error (SPADE): a conceptual framework and methodological approach for unearthing misdiagnosis-related harms using big data. BMJ Qual Saf. 2018;27(7):557-566. doi:10.1136/bmjqs-2017-007032
58. Utility of Predictive Systems to Identify Inpatient Diagnostic Errors: the UPSIDE study. NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/search/rpoHXlEAcEudQV3B9ld8iw/project-details/10020962
59. Overview of Patient Safety Learning Laboratory (PSLL) Projects. Agency for Healthcare Research and Quality. Accessed January 14, 2023. https://www.ahrq.gov/patient-safety/resources/learning-lab/index.html
60. Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT). NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/project-details/10642576
61. Zwaan L, Singh H. Diagnostic error in hospitals: finding forests not just the big trees. BMJ Qual Saf. 2020;29(12):961-964. doi:10.1136/bmjqs-2020-011099
62. Longtin Y, Sax H, Leape LL, Sheridan SE, Donaldson L, Pittet D. Patient participation: current knowledge and applicability to patient safety. Mayo Clin Proc. 2010;85(1):53-62. doi:10.4065/mcp.2009.0248
63. Murphy DR, Singh H, Berlin L. Communication breakdowns and diagnostic errors: a radiology perspective. Diagnosis (Berl). 2014;1(4):253-261. doi:10.1515/dx-2014-0035
64. Singh H, Naik AD, Rao R, Petersen LA. Reducing diagnostic errors through effective communication: harnessing the power of information technology. J Gen Intern Med. 2008;23(4):489-494. doi:10.1007/s11606-007-0393-z
65. Singh H, Connor DM, Dhaliwal G. Five strategies for clinicians to advance diagnostic excellence. BMJ. 2022;376:e068044. doi:10.1136/bmj-2021-068044
66. Yale S, Cohen S, Bordini BJ. Diagnostic time-outs to improve diagnosis. Crit Care Clin. 2022;38(2):185-194. doi:10.1016/j.ccc.2021.11.008
67. Schwartz A, Peskin S, Spiro A, Weiner SJ. Impact of unannounced standardized patient audit and feedback on care, documentation, and costs: an experiment and claims analysis. J Gen Intern Med. 2021;36(1):27-34. doi:10.1007/s11606-020-05965-1
68. Carpenter JD, Gorman PN. Using medication list—problem list mismatches as markers of potential error. Proc AMIA Symp. 2002:106-110.
69. Hron JD, Manzi S, Dionne R, et al. Electronic medication reconciliation and medication errors. Int J Qual Health Care. 2015;27(4):314-319. doi:10.1093/intqhc/mzv046
70. Graber ML, Siegal D, Riah H, Johnston D, Kenyon K. Electronic health record–related events in medical malpractice claims. J Patient Saf. 2019;15(2):77-85. doi:10.1097/PTS.0000000000000240
71. Murphy DR, Wu L, Thomas EJ, Forjuoh SN, Meyer AND, Singh H. Electronic trigger-based intervention to reduce delays in diagnostic evaluation for cancer: a cluster randomized controlled trial. J Clin Oncol. 2015;33(31):3560-3567. doi:10.1200/JCO.2015.61.1301
72. Singh H, Giardina TD, Forjuoh SN, et al. Electronic health record-based surveillance of diagnostic errors in primary care. BMJ Qual Saf. 2012;21(2):93-100. doi:10.1136/bmjqs-2011-000304
73. Armaignac DL, Saxena A, Rubens M, et al. Impact of telemedicine on mortality, length of stay, and cost among patients in progressive care units: experience from a large healthcare system. Crit Care Med. 2018;46(5):728-735. doi:10.1097/CCM.0000000000002994
74. MacKinnon GE, Brittain EL. Mobile health technologies in cardiopulmonary disease. Chest. 2020;157(3):654-664. doi:10.1016/j.chest.2019.10.015
75. DeVore AD, Wosik J, Hernandez AF. The future of wearables in heart failure patients. JACC Heart Fail. 2019;7(11):922-932. doi:10.1016/j.jchf.2019.08.008
76. Tsai TL, Fridsma DB, Gatti G. Computer decision support as a source of interpretation error: the case of electrocardiograms. J Am Med Inform Assoc. 2003;10(5):478-483. doi:10.1197/jamia.M1279
77. Lin SY, Mahoney MR, Sinsky CA. Ten ways artificial intelligence will transform primary care. J Gen Intern Med. 2019;34(8):1626-1630. doi:10.1007/s11606-019-05035-1
78. Ramirez AH, Gebo KA, Harris PA. Progress with the All Of Us research program: opening access for researchers. JAMA. 2021;325(24):2441-2442. doi:10.1001/jama.2021.7702
79. Johnson KB, Wei W, Weeraratne D, et al. Precision medicine, AI, and the future of personalized health care. Clin Transl Sci. 2021;14(1):86-93. doi:10.1111/cts.12884
80. Gupta A, Snyder A, Kachalia A, Flanders S, Saint S, Chopra V. Malpractice claims related to diagnostic errors in the hospital. BMJ Qual Saf. 2017;27(1):bmjqs-2017-006774. doi:10.1136/bmjqs-2017-006774
81. Renkema E, Broekhuis M, Ahaus K. Conditions that influence the impact of malpractice litigation risk on physicians’ behavior regarding patient safety. BMC Health Serv Res. 2014;14(1):38. doi:10.1186/1472-6963-14-38
82. Kachalia A, Mello MM, Nallamothu BK, Studdert DM. Legal and policy interventions to improve patient safety. Circulation. 2016;133(7):661-671. doi:10.1161/CIRCULATIONAHA.115.015880
Opportunities and Challenges for Improving the Patient Experience in the Acute and Post–Acute Care Setting Using Patient Portals: The Patient’s Perspective
To realize the vision of patient-centered care, efforts are focusing on engaging patients and “care partners,” often a family caregiver, by using patient-facing technologies.1-4 Web-based patient portals linked to the electronic health record (EHR) provide patients and care partners with the ability to access personal health information online and to communicate with clinicians. In recent years, institutions have been increasing patient portal offerings to improve the patient experience, promote safety, and optimize healthcare delivery.5-7
DRIVERS OF ADOPTION
The adoption of patient portals has been driven by federal incentive programs (Meaningful Use), efforts by the Center for Medicare and Medicaid Services, and the Office of the National Coordinator for Health Information Technology to improve patient outcomes and the transition toward value-based reimbursement.2,8,9 The vast majority of use has been in ambulatory settings; use for acute care is nascent at best.10 Among hospitalized patients, few bring an internet-enabled computer or mobile device to access personal health records online.11 However, evidence suggests that care partners will use portals on behalf of acutely ill patients.4 As the Caregiver Advise, Record, Enable Act is implemented, hospitals will be required to identify patients’ care partners during hospitalization, inform them when the patient is ready for discharge, and provide self-management instructions during the transition home.12 In this context, understanding how best to leverage acute care patient portals will be important to institutions, clinicians, and vendors.
CURRENT KNOWLEDGE
The literature regarding acute care patient portals is rapidly growing.4,10 Hospitalized patients have unmet information and communication needs, and hospital-based clinicians struggle to meet these needs in a timely manner.13-15 In general, patients feel that using a mobile device to access personal health records has the potential to improve their experience.11 Early studies suggest that acute care patient portals can promote patient-centered communication and collaboration during hospitalization, including in intensive care settings.4,16,17 Furthermore, the use of acute care patient portals can improve perception of safety and quality, decrease anxiety, and increase understanding of health conditions.3,14 Although early evidence is promising, considerable knowledge gaps exist regarding patient outcomes over the acute episode of care.10,18
OUTSTANDING QUESTIONS
A clear area of interest is accessing acute care patient portals via mobile technology to engage patients during recovery from hospitalization.4,11 Although we do not yet know whether use during care transitions will favorably impact outcomes, given the high rate of harm after discharge, this seems likely.19 The few studies evaluating the effect on validated measures of engagement (Patient Activation Measure) and hospital readmissions have not shown demonstrable improvement to date.20,21 Clearly, optimizing acute care patient portals with regard to patient-clinician communication, as well as the type, timing, and format of information delivered, will be necessary to maximize value.4,22
From the patient’s perspective, there is much we can learn.23 Is the information that is presented pertinent, timely, and easy to understand? Will the use of portals detract from face-to-face interactions? Does greater transparency foster more accountability? Achieving an appropriate balance of digital health-information sharing for hospitalized patients is challenging given the sensitivity of patient data when diagnoses are uncertain and treatments are in flux.4,24 These questions must be answered as hospitals implement acute care patient portals.
ACUTE CARE PATIENT PORTAL TASK FORCE
To start addressing knowledge gaps, we established a task force of 21 leading researchers, informatics and policy experts, and clinical leaders. The Acute Care Patient Portal Task Force was a subgroup of the Libretto Consortium, a collaboration of 4 academic medical centers established by the Gordon and Betty Moore Foundation to design, develop, and implement technologies to engage patients, care partners, and providers in preventing harm in hospital settings. Initially, we were challenged with assessing stakeholders’ perspectives from early adopter institutions. We learned that acute care patient portals must offer an integrated experience across care settings, humanize the patient-clinician relationship, enable equitable access, and align with institutional strategy to promote sustainability.19
Cognitive Support
The opportunities identified include acclimatizing and assimilating to the hospital environment (reviewing policies and patient rights) and facilitating self-education and preparation by linking to personal health information and providing structured guidance at transitions.4 For example, a care partner of an incapacitated patient may watch a video to orient to the intensive care unit, navigate educational content linked to the patient’s admission diagnosis (pneumonia) entered in the EHR, view the timing of an upcoming imaging study (chest computed tomography scan), and complete a standardized checklist prior to discharge.
The main challenges we identified include ensuring accuracy of hospital-, unit-, and patient-level information, addressing information overload, configuring notification and display settings to optimize the user experience, presenting information at an appropriate health literacy level,4,21 and addressing security and privacy concerns when expanding access to family members.24
Respect and Boundaries
Opportunities identified include supporting individual learning styles by using interactive features of mobile devices to improve comprehension for visual, auditory, and tactile learners and reinforcing learning through the use of various types of digital media.25-27 For example, a visual learner may view a video tutorial for a newly prescribed medication. A tactile learner may prefer to use interactive graphical displays that exploit multidimensional touch capabilities of mobile devices to learn about active conditions or an upcoming procedure. An auditory learner may choose to use intelligent personal assistants to navigate their plan of care (“Hey Siri, what is my schedule for today?”). By addressing the learning preferences of patients and time constraints of clinicians, institutions can use acute care patient portals to promote more respectful interactions and collaborative decision-making during important care processes, such as obtaining surgical consent.28,29
We also identified opportunities to facilitate personalization by tailoring educational content and by enabling the use of patient-generated health data collected from wearable devices. For example, patients may prefer to interact with a virtual advocate to review discharge instructions (“Louis” in Project Re-Engineered Discharge) when personalized to their demographics and health literacy level.30-32 Patients may choose to upload step counts from wearable devices so that clinicians can monitor activity goals in preparation for discharge and while recovering afterwards. When supported in these ways, acute care patient portals allow patients to have more meaningful interactions with clinicians about diagnoses, treatments, prognosis, and goals for recovery.
The main challenges we identified include balancing interactions with technology and clinicians, ensuring clinicians understand how patients from different socioeconomic backgrounds use existing and newer technology to enhance self-management, assessing health and technology literacy, and understanding individual preferences for sharing patient-generated health data. Importantly, we must remain vigilant that patients will express concern about overdependence on technology, especially if it detracts from in-person interaction; our panelists emphasized that technology should never replace “human touch.”
Patient and Family Empowerment
The opportunities identified include promoting patient-centered communication by supporting a real-time and asynchronous dialogue among patients, care partners, and care team members (including ambulatory clinicians) while minimizing conversational silos4,33; displaying names, roles, and pictures of all care team members4,34; fostering transparency by sharing clinician documentation in progress notes and sign-outs35; ensuring accountability for a single plan of care spanning shift changes and handoffs, and providing a mechanism to enable real-time feedback.
Hospitalization can be a vulnerable and isolating experience, perpetuated by a lack of timely and coordinated communication with the care team. We identified opportunities to mitigate anxiety by promoting shared understanding when questions require input from multiple clinicians, when team members change, or when patients wish to communicate with their longitudinal ambulatory providers.4,34 For example, inviting patients to review clinicians’ progress notes should stimulate more open and meaningful communication.35 Furthermore, requesting that patients state their wishes, preferences, and goals could improve overall concordance with care team members.36,37 Empowering patients and care partners to voice their concerns, particularly those related to miscommunication, may mitigate harm propagated by handoffs, shift work, and weekend coverage.38,39 While reporting safety concerns represents a novel mechanism to augment medical-error reporting by clinicians alone,23,40 this strategy will be most effective when aligned with standardized communication initiatives (I-PASS) that have been proven to reduce medical errors and preventable adverse events and are being implemented nationally.41 Finally, by leveraging tools that facilitate instantaneous feedback, patients can be empowered to react to their plan (ranking skilled nursing facility options) as it is developed.
The main challenges we identified include managing expectations regarding the use of communication tools, accurately and reliably identifying care team members in the EHR,34 acknowledging patients as equal partners, ensuring patients receive a consistent message about diagnoses and therapies during handoffs and when multiple consultants have conflicting opinions about the plan,37 and addressing patient concerns fairly and respectfully.
RECOMMENDATIONS AND CONCLUSIONS
In summary, the patient-centered themes we identified serve as guiding principles for institutions, clinicians, and vendors who wish to use patient portals to improve the acute and postacute care patient experience. One central message resonates: Patients do not simply want access to their health information and the ability to communicate with the clinicians who furnish this information; they want to feel supported, respected, and empowered when doing so. It is only through partnership with patients and their advocates that we can fully realize the impact of digital technologies when patients are in their most vulnerable state.
Acknowledgments
The authors thank their colleagues and the patient and family advocates who contributed to this body of work as part of the Acute Care Patient Portal Task Force and conference: Brittany Couture; Ronen Rozenblum, PhD, MPH; Jennifer Prey, MPhil, MS, PhD; Kristin O’Reilly, RN, BSN, MPH; Patricia Q. Bourie, RN, MS, Cindy Dwyer, RN, BSN,S; Ryan Greysen, MD, MHS, MA; Jeffery Smith, MPP; Michael Gropper, MD, PhD; Patricia Dykes, RN, PhD; Martha B. Carnie; Jeffrey W. Mello; and Jane Webster.
Disclosure
Anuj K. Dalal, MD, David W. Bates, MD, MSc, and Sarah Collins, RN, PhD, are responsible for the conception or design of the work; acquisition, analysis, or interpretation of data; drafting the work or revising it critically for important intellectual content; and final approval of the version to be published. The authors agree to be accountable for all aspects of the work and to ensure that questions related to the accuracy or integrity of the work are appropriately investigated and resolved. This work was supported by a grant from the Gordon and Betty Moore Foundation ([GBMF] #4993). GBMF had no role in the design or conduct of the study; the collection, analysis, or interpretation of data; or preparation or review of the manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of GBMF. The authors report no conflicts of interest.
1. Sarkar U, Bates DW. Care partners and online patient portals. JAMA. 2014;311(4):357-358. PubMed
2. Grando MA, Rozenblum R, Bates DW, eds. Information Technology for Patient Empowerment in Healthcare, 1st Edition. Berlin: Walter de Gruyter Inc.; 2015.
3. Kelly MM, Hoonakker PLT, Dean SM. Using an inpatient portal to engage families in pediatric hospital care. J Am Med Inform Assoc. 2016;24(1):153-161. PubMed
4. Dalal AK, Dykes PC, Collins S, et al. A web-based, patient-centered toolkit to engage patients and caregivers in the acute care setting: A preliminary evaluation. J Am Med Inform Assoc. 2016;23(1):80-87. PubMed
5. Prey JE, Restaino S, Vawdrey DK. Providing hospital patients with access to their medical records. AMIA Annu Symp Proc. 2014;2014:1884-1893. PubMed
6. Herrin J, Harris KG, Kenward K, Hines S, Joshi MS, Frosch DL. Patient and family engagement: A survey of US hospital practices. BMJ Qual Saf. 2016;25(3):182-189. PubMed
7. Tom JO, Mangione-Smith R, Solomon C, Grossman DC. Integrated personal health record use: Association with parent-reported care experiences. Pediatrics. 2012;130(1):e183-e190. PubMed
8. Centers for Medicare & Medicaid Services (CMS), HHS. Medicare and Medicaid Programs; Electronic Health Record Incentive Program-Stage 2. Federal Register Final Rule. Sect. 170; 2012. https://www.federalregister.gov/documents/2012/03/07/2012-4443/medicare-and-medicaid-programs-electronic-health-record-incentive-program-stage-2. Accessed March 1, 2017.
9. Centers for Medicare & Medicaid Services (CMS), HHS. Medicare program; merit-based incentive payment system (MIPS) and alternative payment model (APM) incentive under the physician fee schedule, and criteria for physician-focused payment models. Final rule with comment period. Fed Regist. 2016;81(214):77008-77831. PubMed
10. Prey JE, Woollen J, Wilcox L, et al. Patient engagement in the inpatient setting: A systematic review. J Am Med Informat Assoc. 2014;21(4):742-750. PubMed
11. Ludwin S, Greysen SR. Use of smartphones and mobile devices in hospitalized patients: Untapped opportunities for inpatient engagement. J Hosp Med. 2015;10(7):459-461. PubMed
12. Coleman EA. Family caregivers as partners in care transitions: The caregiver advise record and enable act. J Hosp Med. 2016;11(12):883-885. PubMed
13. Kaziunas E, Hanauer DA, Ackerman MS, Choi SW. Identifying unmet informational needs in the inpatient setting to increase patient and caregiver engagement in the context of pediatric hematopoietic stem cell transplantation. J Am Med Inform Assoc. 2016;23(1):94-104. PubMed
14. Woollen J, Prey J, Wilcox L, et al. Patient experiences using an inpatient personal health record. Appl Clin Inform. 2016;7(2):446-460. PubMed
15. Irizarry T, DeVito Dabbs A, Curran CR. Patient portals and patient engagement: A state of the science review. J Med Internet Res. 2015;17(6):e148. doi:10.2196/jmir.4255. PubMed
16. Vawdrey DK, Wilcox LG, Collins SA, et al. A tablet computer application for patients to participate in their hospital care. AMIA Annu Symp Proc. 2011;2011:1428-1435. PubMed
17. Collins SA, Rozenblum R, Leung WY, et al. Acute care patient portals: A qualitative study of stakeholder perspectives on current practices. J Am Med Inform Assoc. 2016;24(e1):e9-e17. PubMed
18. Berger Z, Flickinger TE, Pfoh E, Martinez KA, Dy SM. Promoting engagement by patients and families to reduce adverse events in acute care settings: A systematic review. BMJ Qual Saf. 2014;23(7):548-555. PubMed
19. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. PubMed
20. Griffin A, Skinner A, Thornhill J, Weinberger M. Patient Portals: Who uses them? What features do they use? And do they reduce hospital readmissions? Appl Clin Inform. 2016;7(2):489-501. PubMed
21. O’Leary KJ, Lohman ME, Culver E, Killarney A, Randy Smith G Jr, Liebovitz DM. The effect of tablet computers with a mobile patient portal application on hospitalized patients’ knowledge and activation. J Am Med Inform Assoc. 2016;23(1):159-165. PubMed
22. O’Leary KJ, Sharma RK, Killarney A, et al. Patients’ and Healthcare Providers’ Perceptions of a Mobile Portal Application for Hospitalized Patients. BMC Med Inform Decis Mak. 2016;16(1):123. PubMed
23. Pell JM, Mancuso M, Limon S, Oman K, Lin CT. Patient access to electronic health records during hospitalization. JAMA Intern Med. 2015;175(5):856-858. PubMed
24. Brown SM, Aboumatar HJ, Francis L, et al. Balancing digital information-sharing and patient privacy when engaging families in the intensive care unit. J Am Med Inform Assoc. 2016;23(5):995-1000. PubMed
25. Krishna S, Francisco BD, Balas EA, et al. Internet-enabled interactive multimedia asthma education program: A randomized trial. Pediatrics. 2003;111(3):503-510. PubMed
26. Fox MP. A systematic review of the literature reporting on studies that examined the impact of interactive, computer-based patient education programs. Patient Educ Couns. 2009;77(1):6-13. PubMed
27. Morgan ER, Laing K, McCarthy J, McCrate F, Seal MD. Using tablet-based technology in patient education about systemic therapy options for early-stage breast cancer: A pilot study. Curr Oncol. 2015;22(5):e364-e369. PubMed
28. Nehme J, El-Khani U, Chow A, Hakky S, Ahmed AR, Purkayastha S. The use of multimedia consent programs for surgical procedures: A systematic review. Surg Innov. 2013;20(1):13-23. PubMed
29. Waller A, Forshaw K, Carey M, et al. Optimizing patient preparation and surgical experience using eHealth technology. JMIR Med Inform. 2015;3(3):e29. PubMed
30. Abbott MB, Shaw P. Virtual nursing avatars: Nurse roles and evolving concepts of care. Online J Issues Nurs. 2016;21(3):7. PubMed
31. Cawthon C, Walia S, Osborn CY, Niesner KJ, Schnipper JL, Kripalani S. Improving care transitions: The patient perspective. J Health Commun. 2012;17 Suppl 3:312-324. PubMed
32. Bickmore TW, Pfeifer LM, Byron D, et al. Usability of conversational agents by patients with inadequate health literacy: Evidence from two clinical trials. J Health Commun. 2010;15 Suppl 2:197-210. PubMed
33. 2017;376(20):1905-1907. N Engl J Med.42. Mandl KD, Kohane IS. A 21st-century health IT system—creating a real-world information economy. PubMed
34. 2014;371(19):1803-1812.N Engl J Med41. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. PubMed
35. 2016;24(1):153-161.J Am Med Inform Assoc.40. Kelly MM, Hoonakker PLT, Dean SM. Using an inpatient portal to engage families in pediatric hospital care. PubMed
36. 2017;171(4):372-381.JAMA Pediatr.39. Khan A, Coffey M, Litterer KP, et al. Families as partners in hospital error and adverse event surveillance. PubMed
37. 2017;17(4):389-402.Acad Pediatr.38. Khan A, Baird J, Rogers JE, et al. Parent and provider experience and shared understanding after a family-centered nighttime communication intervention. PubMed
38. 2016;6(6):319-329.Hosp Pediatr. 37. Khan A, Rogers JE, Forster CS, Furtak SL, Schuster MA, Landrigan CP. Communication and shared understanding between parents and resident-physicians at night. PubMed
39. 2016;11(9):615-619.J Hosp Med36. Figueroa JF, Schnipper JL, McNally K, Stade D, Lipsitz SR, Dalal AK. How often are hospitalized patients and providers on the same page with regard to the patient’s primary recovery goal for hospitalization? PubMed
40. 2013;8(7):414-417.J Hosp Med.35. Feldman HJ, Walker J, Li J, Delbanco T. OpenNotes: Hospitalists’ challenge and opportunity. PubMed
41. 2016;11(5):381-385.J Hosp Med.34. Dalal AK, Schnipper JL. Care team identification in the electronic health record: A critical first step for patient-centered communication.PubMed
42. 2016;24(e1):e178-e184.J Am Med Inform Assoc.33. Dalal AK, Schnipper J, Massaro A, et al. A web-based and mobile patient-centered “microblog” messaging platform to improve care team communication in acute care. PubMed
To realize the vision of patient-centered care, efforts are focusing on engaging patients and “care partners,” often a family caregiver, by using patient-facing technologies.1-4 Web-based patient portals linked to the electronic health record (EHR) provide patients and care partners with the ability to access personal health information online and to communicate with clinicians. In recent years, institutions have been increasing patient portal offerings to improve the patient experience, promote safety, and optimize healthcare delivery.5-7
DRIVERS OF ADOPTION
The adoption of patient portals has been driven by federal incentive programs (Meaningful Use), efforts by the Center for Medicare and Medicaid Services, and the Office of the National Coordinator for Health Information Technology to improve patient outcomes and the transition toward value-based reimbursement.2,8,9 The vast majority of use has been in ambulatory settings; use for acute care is nascent at best.10 Among hospitalized patients, few bring an internet-enabled computer or mobile device to access personal health records online.11 However, evidence suggests that care partners will use portals on behalf of acutely ill patients.4 As the Caregiver Advise, Record, Enable Act is implemented, hospitals will be required to identify patients’ care partners during hospitalization, inform them when the patient is ready for discharge, and provide self-management instructions during the transition home.12 In this context, understanding how best to leverage acute care patient portals will be important to institutions, clinicians, and vendors.
CURRENT KNOWLEDGE
The literature regarding acute care patient portals is rapidly growing.4,10 Hospitalized patients have unmet information and communication needs, and hospital-based clinicians struggle to meet these needs in a timely manner.13-15 In general, patients feel that using a mobile device to access personal health records has the potential to improve their experience.11 Early studies suggest that acute care patient portals can promote patient-centered communication and collaboration during hospitalization, including in intensive care settings.4,16,17 Furthermore, the use of acute care patient portals can improve perception of safety and quality, decrease anxiety, and increase understanding of health conditions.3,14 Although early evidence is promising, considerable knowledge gaps exist regarding patient outcomes over the acute episode of care.10,18
OUTSTANDING QUESTIONS
A clear area of interest is accessing acute care patient portals via mobile technology to engage patients during recovery from hospitalization.4,11 Although we do not yet know whether use during care transitions will favorably impact outcomes, given the high rate of harm after discharge, this seems likely.19 The few studies evaluating the effect on validated measures of engagement (Patient Activation Measure) and hospital readmissions have not shown demonstrable improvement to date.20,21 Clearly, optimizing acute care patient portals with regard to patient-clinician communication, as well as the type, timing, and format of information delivered, will be necessary to maximize value.4,22
From the patient’s perspective, there is much we can learn.23 Is the information that is presented pertinent, timely, and easy to understand? Will the use of portals detract from face-to-face interactions? Does greater transparency foster more accountability? Achieving an appropriate balance of digital health-information sharing for hospitalized patients is challenging given the sensitivity of patient data when diagnoses are uncertain and treatments are in flux.4,24 These questions must be answered as hospitals implement acute care patient portals.
ACUTE CARE PATIENT PORTAL TASK FORCE
To start addressing knowledge gaps, we established a task force of 21 leading researchers, informatics and policy experts, and clinical leaders. The Acute Care Patient Portal Task Force was a subgroup of the Libretto Consortium, a collaboration of 4 academic medical centers established by the Gordon and Betty Moore Foundation to design, develop, and implement technologies to engage patients, care partners, and providers in preventing harm in hospital settings. Initially, we were challenged with assessing stakeholders’ perspectives from early adopter institutions. We learned that acute care patient portals must offer an integrated experience across care settings, humanize the patient-clinician relationship, enable equitable access, and align with institutional strategy to promote sustainability.19
Cognitive Support
The opportunities identified include acclimatizing and assimilating to the hospital environment (reviewing policies and patient rights) and facilitating self-education and preparation by linking to personal health information and providing structured guidance at transitions.4 For example, a care partner of an incapacitated patient may watch a video to orient to the intensive care unit, navigate educational content linked to the patient’s admission diagnosis (pneumonia) entered in the EHR, view the timing of an upcoming imaging study (chest computed tomography scan), and complete a standardized checklist prior to discharge.
The main challenges we identified include ensuring accuracy of hospital-, unit-, and patient-level information, addressing information overload, configuring notification and display settings to optimize the user experience, presenting information at an appropriate health literacy level,4,21 and addressing security and privacy concerns when expanding access to family members.24
Respect and Boundaries
Opportunities identified include supporting individual learning styles by using interactive features of mobile devices to improve comprehension for visual, auditory, and tactile learners and reinforcing learning through the use of various types of digital media.25-27 For example, a visual learner may view a video tutorial for a newly prescribed medication. A tactile learner may prefer to use interactive graphical displays that exploit multidimensional touch capabilities of mobile devices to learn about active conditions or an upcoming procedure. An auditory learner may choose to use intelligent personal assistants to navigate their plan of care (“Hey Siri, what is my schedule for today?”). By addressing the learning preferences of patients and time constraints of clinicians, institutions can use acute care patient portals to promote more respectful interactions and collaborative decision-making during important care processes, such as obtaining surgical consent.28,29
We also identified opportunities to facilitate personalization by tailoring educational content and by enabling the use of patient-generated health data collected from wearable devices. For example, patients may prefer to interact with a virtual advocate to review discharge instructions (“Louis” in Project Re-Engineered Discharge) when personalized to their demographics and health literacy level.30-32 Patients may choose to upload step counts from wearable devices so that clinicians can monitor activity goals in preparation for discharge and while recovering afterwards. When supported in these ways, acute care patient portals allow patients to have more meaningful interactions with clinicians about diagnoses, treatments, prognosis, and goals for recovery.
The main challenges we identified include balancing interactions with technology and clinicians, ensuring clinicians understand how patients from different socioeconomic backgrounds use existing and newer technology to enhance self-management, assessing health and technology literacy, and understanding individual preferences for sharing patient-generated health data. Importantly, we must remain vigilant that patients will express concern about overdependence on technology, especially if it detracts from in-person interaction; our panelists emphasized that technology should never replace “human touch.”
Patient and Family Empowerment
The opportunities identified include promoting patient-centered communication by supporting a real-time and asynchronous dialogue among patients, care partners, and care team members (including ambulatory clinicians) while minimizing conversational silos4,33; displaying names, roles, and pictures of all care team members4,34; fostering transparency by sharing clinician documentation in progress notes and sign-outs35; ensuring accountability for a single plan of care spanning shift changes and handoffs, and providing a mechanism to enable real-time feedback.
Hospitalization can be a vulnerable and isolating experience, perpetuated by a lack of timely and coordinated communication with the care team. We identified opportunities to mitigate anxiety by promoting shared understanding when questions require input from multiple clinicians, when team members change, or when patients wish to communicate with their longitudinal ambulatory providers.4,34 For example, inviting patients to review clinicians’ progress notes should stimulate more open and meaningful communication.35 Furthermore, requesting that patients state their wishes, preferences, and goals could improve overall concordance with care team members.36,37 Empowering patients and care partners to voice their concerns, particularly those related to miscommunication, may mitigate harm propagated by handoffs, shift work, and weekend coverage.38,39 While reporting safety concerns represents a novel mechanism to augment medical-error reporting by clinicians alone,23,40 this strategy will be most effective when aligned with standardized communication initiatives (I-PASS) that have been proven to reduce medical errors and preventable adverse events and are being implemented nationally.41 Finally, by leveraging tools that facilitate instantaneous feedback, patients can be empowered to react to their plan (ranking skilled nursing facility options) as it is developed.
The main challenges we identified include managing expectations regarding the use of communication tools, accurately and reliably identifying care team members in the EHR,34 acknowledging patients as equal partners, ensuring patients receive a consistent message about diagnoses and therapies during handoffs and when multiple consultants have conflicting opinions about the plan,37 and addressing patient concerns fairly and respectfully.
RECOMMENDATIONS AND CONCLUSIONS
In summary, the patient-centered themes we identified serve as guiding principles for institutions, clinicians, and vendors who wish to use patient portals to improve the acute and postacute care patient experience. One central message resonates: Patients do not simply want access to their health information and the ability to communicate with the clinicians who furnish this information; they want to feel supported, respected, and empowered when doing so. It is only through partnership with patients and their advocates that we can fully realize the impact of digital technologies when patients are in their most vulnerable state.
Acknowledgments
The authors thank their colleagues and the patient and family advocates who contributed to this body of work as part of the Acute Care Patient Portal Task Force and conference: Brittany Couture; Ronen Rozenblum, PhD, MPH; Jennifer Prey, MPhil, MS, PhD; Kristin O’Reilly, RN, BSN, MPH; Patricia Q. Bourie, RN, MS, Cindy Dwyer, RN, BSN,S; Ryan Greysen, MD, MHS, MA; Jeffery Smith, MPP; Michael Gropper, MD, PhD; Patricia Dykes, RN, PhD; Martha B. Carnie; Jeffrey W. Mello; and Jane Webster.
Disclosure
Anuj K. Dalal, MD, David W. Bates, MD, MSc, and Sarah Collins, RN, PhD, are responsible for the conception or design of the work; acquisition, analysis, or interpretation of data; drafting the work or revising it critically for important intellectual content; and final approval of the version to be published. The authors agree to be accountable for all aspects of the work and to ensure that questions related to the accuracy or integrity of the work are appropriately investigated and resolved. This work was supported by a grant from the Gordon and Betty Moore Foundation ([GBMF] #4993). GBMF had no role in the design or conduct of the study; the collection, analysis, or interpretation of data; or preparation or review of the manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of GBMF. The authors report no conflicts of interest.
To realize the vision of patient-centered care, efforts are focusing on engaging patients and “care partners,” often a family caregiver, by using patient-facing technologies.1-4 Web-based patient portals linked to the electronic health record (EHR) provide patients and care partners with the ability to access personal health information online and to communicate with clinicians. In recent years, institutions have been increasing patient portal offerings to improve the patient experience, promote safety, and optimize healthcare delivery.5-7
DRIVERS OF ADOPTION
The adoption of patient portals has been driven by federal incentive programs (Meaningful Use), efforts by the Center for Medicare and Medicaid Services, and the Office of the National Coordinator for Health Information Technology to improve patient outcomes and the transition toward value-based reimbursement.2,8,9 The vast majority of use has been in ambulatory settings; use for acute care is nascent at best.10 Among hospitalized patients, few bring an internet-enabled computer or mobile device to access personal health records online.11 However, evidence suggests that care partners will use portals on behalf of acutely ill patients.4 As the Caregiver Advise, Record, Enable Act is implemented, hospitals will be required to identify patients’ care partners during hospitalization, inform them when the patient is ready for discharge, and provide self-management instructions during the transition home.12 In this context, understanding how best to leverage acute care patient portals will be important to institutions, clinicians, and vendors.
CURRENT KNOWLEDGE
The literature regarding acute care patient portals is rapidly growing.4,10 Hospitalized patients have unmet information and communication needs, and hospital-based clinicians struggle to meet these needs in a timely manner.13-15 In general, patients feel that using a mobile device to access personal health records has the potential to improve their experience.11 Early studies suggest that acute care patient portals can promote patient-centered communication and collaboration during hospitalization, including in intensive care settings.4,16,17 Furthermore, the use of acute care patient portals can improve perception of safety and quality, decrease anxiety, and increase understanding of health conditions.3,14 Although early evidence is promising, considerable knowledge gaps exist regarding patient outcomes over the acute episode of care.10,18
OUTSTANDING QUESTIONS
A clear area of interest is accessing acute care patient portals via mobile technology to engage patients during recovery from hospitalization.4,11 Although we do not yet know whether use during care transitions will favorably impact outcomes, given the high rate of harm after discharge, this seems likely.19 The few studies evaluating the effect on validated measures of engagement (Patient Activation Measure) and hospital readmissions have not shown demonstrable improvement to date.20,21 Clearly, optimizing acute care patient portals with regard to patient-clinician communication, as well as the type, timing, and format of information delivered, will be necessary to maximize value.4,22
From the patient’s perspective, there is much we can learn.23 Is the information that is presented pertinent, timely, and easy to understand? Will the use of portals detract from face-to-face interactions? Does greater transparency foster more accountability? Achieving an appropriate balance of digital health-information sharing for hospitalized patients is challenging given the sensitivity of patient data when diagnoses are uncertain and treatments are in flux.4,24 These questions must be answered as hospitals implement acute care patient portals.
ACUTE CARE PATIENT PORTAL TASK FORCE
To start addressing knowledge gaps, we established a task force of 21 leading researchers, informatics and policy experts, and clinical leaders. The Acute Care Patient Portal Task Force was a subgroup of the Libretto Consortium, a collaboration of 4 academic medical centers established by the Gordon and Betty Moore Foundation to design, develop, and implement technologies to engage patients, care partners, and providers in preventing harm in hospital settings. Initially, we were challenged with assessing stakeholders’ perspectives from early adopter institutions. We learned that acute care patient portals must offer an integrated experience across care settings, humanize the patient-clinician relationship, enable equitable access, and align with institutional strategy to promote sustainability.19
Cognitive Support
The opportunities identified include acclimatizing and assimilating to the hospital environment (reviewing policies and patient rights) and facilitating self-education and preparation by linking to personal health information and providing structured guidance at transitions.4 For example, a care partner of an incapacitated patient may watch a video to orient to the intensive care unit, navigate educational content linked to the patient’s admission diagnosis (pneumonia) entered in the EHR, view the timing of an upcoming imaging study (chest computed tomography scan), and complete a standardized checklist prior to discharge.
The main challenges we identified include ensuring accuracy of hospital-, unit-, and patient-level information, addressing information overload, configuring notification and display settings to optimize the user experience, presenting information at an appropriate health literacy level,4,21 and addressing security and privacy concerns when expanding access to family members.24
Respect and Boundaries
Opportunities identified include supporting individual learning styles by using interactive features of mobile devices to improve comprehension for visual, auditory, and tactile learners and reinforcing learning through the use of various types of digital media.25-27 For example, a visual learner may view a video tutorial for a newly prescribed medication. A tactile learner may prefer to use interactive graphical displays that exploit multidimensional touch capabilities of mobile devices to learn about active conditions or an upcoming procedure. An auditory learner may choose to use intelligent personal assistants to navigate their plan of care (“Hey Siri, what is my schedule for today?”). By addressing the learning preferences of patients and time constraints of clinicians, institutions can use acute care patient portals to promote more respectful interactions and collaborative decision-making during important care processes, such as obtaining surgical consent.28,29
We also identified opportunities to facilitate personalization by tailoring educational content and by enabling the use of patient-generated health data collected from wearable devices. For example, patients may prefer to interact with a virtual advocate to review discharge instructions (“Louis” in Project Re-Engineered Discharge) when personalized to their demographics and health literacy level.30-32 Patients may choose to upload step counts from wearable devices so that clinicians can monitor activity goals in preparation for discharge and while recovering afterwards. When supported in these ways, acute care patient portals allow patients to have more meaningful interactions with clinicians about diagnoses, treatments, prognosis, and goals for recovery.
The main challenges we identified include balancing interactions with technology and clinicians, ensuring clinicians understand how patients from different socioeconomic backgrounds use existing and newer technology to enhance self-management, assessing health and technology literacy, and understanding individual preferences for sharing patient-generated health data. Importantly, we must remain vigilant that patients will express concern about overdependence on technology, especially if it detracts from in-person interaction; our panelists emphasized that technology should never replace “human touch.”
Patient and Family Empowerment
The opportunities identified include promoting patient-centered communication by supporting a real-time and asynchronous dialogue among patients, care partners, and care team members (including ambulatory clinicians) while minimizing conversational silos4,33; displaying names, roles, and pictures of all care team members4,34; fostering transparency by sharing clinician documentation in progress notes and sign-outs35; ensuring accountability for a single plan of care spanning shift changes and handoffs, and providing a mechanism to enable real-time feedback.
Hospitalization can be a vulnerable and isolating experience, perpetuated by a lack of timely and coordinated communication with the care team. We identified opportunities to mitigate anxiety by promoting shared understanding when questions require input from multiple clinicians, when team members change, or when patients wish to communicate with their longitudinal ambulatory providers.4,34 For example, inviting patients to review clinicians’ progress notes should stimulate more open and meaningful communication.35 Furthermore, requesting that patients state their wishes, preferences, and goals could improve overall concordance with care team members.36,37 Empowering patients and care partners to voice their concerns, particularly those related to miscommunication, may mitigate harm propagated by handoffs, shift work, and weekend coverage.38,39 While reporting safety concerns represents a novel mechanism to augment medical-error reporting by clinicians alone,23,40 this strategy will be most effective when aligned with standardized communication initiatives (I-PASS) that have been proven to reduce medical errors and preventable adverse events and are being implemented nationally.41 Finally, by leveraging tools that facilitate instantaneous feedback, patients can be empowered to react to their plan (ranking skilled nursing facility options) as it is developed.
The main challenges we identified include managing expectations regarding the use of communication tools, accurately and reliably identifying care team members in the EHR,34 acknowledging patients as equal partners, ensuring patients receive a consistent message about diagnoses and therapies during handoffs and when multiple consultants have conflicting opinions about the plan,37 and addressing patient concerns fairly and respectfully.
RECOMMENDATIONS AND CONCLUSIONS
In summary, the patient-centered themes we identified serve as guiding principles for institutions, clinicians, and vendors who wish to use patient portals to improve the acute and postacute care patient experience. One central message resonates: Patients do not simply want access to their health information and the ability to communicate with the clinicians who furnish this information; they want to feel supported, respected, and empowered when doing so. It is only through partnership with patients and their advocates that we can fully realize the impact of digital technologies when patients are in their most vulnerable state.
Acknowledgments
The authors thank their colleagues and the patient and family advocates who contributed to this body of work as part of the Acute Care Patient Portal Task Force and conference: Brittany Couture; Ronen Rozenblum, PhD, MPH; Jennifer Prey, MPhil, MS, PhD; Kristin O’Reilly, RN, BSN, MPH; Patricia Q. Bourie, RN, MS, Cindy Dwyer, RN, BSN,S; Ryan Greysen, MD, MHS, MA; Jeffery Smith, MPP; Michael Gropper, MD, PhD; Patricia Dykes, RN, PhD; Martha B. Carnie; Jeffrey W. Mello; and Jane Webster.
Disclosure
Anuj K. Dalal, MD, David W. Bates, MD, MSc, and Sarah Collins, RN, PhD, are responsible for the conception or design of the work; acquisition, analysis, or interpretation of data; drafting the work or revising it critically for important intellectual content; and final approval of the version to be published. The authors agree to be accountable for all aspects of the work and to ensure that questions related to the accuracy or integrity of the work are appropriately investigated and resolved. This work was supported by a grant from the Gordon and Betty Moore Foundation ([GBMF] #4993). GBMF had no role in the design or conduct of the study; the collection, analysis, or interpretation of data; or preparation or review of the manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of GBMF. The authors report no conflicts of interest.
1. Sarkar U, Bates DW. Care partners and online patient portals. JAMA. 2014;311(4):357-358. PubMed
2. Grando MA, Rozenblum R, Bates DW, eds. Information Technology for Patient Empowerment in Healthcare, 1st Edition. Berlin: Walter de Gruyter Inc.; 2015.
3. Kelly MM, Hoonakker PLT, Dean SM. Using an inpatient portal to engage families in pediatric hospital care. J Am Med Inform Assoc. 2016;24(1):153-161. PubMed
4. Dalal AK, Dykes PC, Collins S, et al. A web-based, patient-centered toolkit to engage patients and caregivers in the acute care setting: A preliminary evaluation. J Am Med Inform Assoc. 2016;23(1):80-87. PubMed
5. Prey JE, Restaino S, Vawdrey DK. Providing hospital patients with access to their medical records. AMIA Annu Symp Proc. 2014;2014:1884-1893. PubMed
6. Herrin J, Harris KG, Kenward K, Hines S, Joshi MS, Frosch DL. Patient and family engagement: A survey of US hospital practices. BMJ Qual Saf. 2016;25(3):182-189. PubMed
7. Tom JO, Mangione-Smith R, Solomon C, Grossman DC. Integrated personal health record use: Association with parent-reported care experiences. Pediatrics. 2012;130(1):e183-e190. PubMed
8. Centers for Medicare & Medicaid Services (CMS), HHS. Medicare and Medicaid Programs; Electronic Health Record Incentive Program-Stage 2. Federal Register Final Rule. Sect. 170; 2012. https://www.federalregister.gov/documents/2012/03/07/2012-4443/medicare-and-medicaid-programs-electronic-health-record-incentive-program-stage-2. Accessed March 1, 2017.
9. Centers for Medicare & Medicaid Services (CMS), HHS. Medicare program; merit-based incentive payment system (MIPS) and alternative payment model (APM) incentive under the physician fee schedule, and criteria for physician-focused payment models. Final rule with comment period. Fed Regist. 2016;81(214):77008-77831. PubMed
10. Prey JE, Woollen J, Wilcox L, et al. Patient engagement in the inpatient setting: A systematic review. J Am Med Informat Assoc. 2014;21(4):742-750. PubMed
11. Ludwin S, Greysen SR. Use of smartphones and mobile devices in hospitalized patients: Untapped opportunities for inpatient engagement. J Hosp Med. 2015;10(7):459-461. PubMed
12. Coleman EA. Family caregivers as partners in care transitions: The caregiver advise record and enable act. J Hosp Med. 2016;11(12):883-885. PubMed
13. Kaziunas E, Hanauer DA, Ackerman MS, Choi SW. Identifying unmet informational needs in the inpatient setting to increase patient and caregiver engagement in the context of pediatric hematopoietic stem cell transplantation. J Am Med Inform Assoc. 2016;23(1):94-104. PubMed
14. Woollen J, Prey J, Wilcox L, et al. Patient experiences using an inpatient personal health record. Appl Clin Inform. 2016;7(2):446-460. PubMed
15. Irizarry T, DeVito Dabbs A, Curran CR. Patient portals and patient engagement: A state of the science review. J Med Internet Res. 2015;17(6):e148. doi:10.2196/jmir.4255. PubMed
16. Vawdrey DK, Wilcox LG, Collins SA, et al. A tablet computer application for patients to participate in their hospital care. AMIA Annu Symp Proc. 2011;2011:1428-1435. PubMed
17. Collins SA, Rozenblum R, Leung WY, et al. Acute care patient portals: A qualitative study of stakeholder perspectives on current practices. J Am Med Inform Assoc. 2016;24(e1):e9-e17. PubMed
18. Berger Z, Flickinger TE, Pfoh E, Martinez KA, Dy SM. Promoting engagement by patients and families to reduce adverse events in acute care settings: A systematic review. BMJ Qual Saf. 2014;23(7):548-555. PubMed
19. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. PubMed
20. Griffin A, Skinner A, Thornhill J, Weinberger M. Patient Portals: Who uses them? What features do they use? And do they reduce hospital readmissions? Appl Clin Inform. 2016;7(2):489-501. PubMed
21. O’Leary KJ, Lohman ME, Culver E, Killarney A, Randy Smith G Jr, Liebovitz DM. The effect of tablet computers with a mobile patient portal application on hospitalized patients’ knowledge and activation. J Am Med Inform Assoc. 2016;23(1):159-165. PubMed
22. O’Leary KJ, Sharma RK, Killarney A, et al. Patients’ and Healthcare Providers’ Perceptions of a Mobile Portal Application for Hospitalized Patients. BMC Med Inform Decis Mak. 2016;16(1):123. PubMed
23. Pell JM, Mancuso M, Limon S, Oman K, Lin CT. Patient access to electronic health records during hospitalization. JAMA Intern Med. 2015;175(5):856-858. PubMed
24. Brown SM, Aboumatar HJ, Francis L, et al. Balancing digital information-sharing and patient privacy when engaging families in the intensive care unit. J Am Med Inform Assoc. 2016;23(5):995-1000. PubMed
25. Krishna S, Francisco BD, Balas EA, et al. Internet-enabled interactive multimedia asthma education program: A randomized trial. Pediatrics. 2003;111(3):503-510. PubMed
26. Fox MP. A systematic review of the literature reporting on studies that examined the impact of interactive, computer-based patient education programs. Patient Educ Couns. 2009;77(1):6-13. PubMed
27. Morgan ER, Laing K, McCarthy J, McCrate F, Seal MD. Using tablet-based technology in patient education about systemic therapy options for early-stage breast cancer: A pilot study. Curr Oncol. 2015;22(5):e364-e369. PubMed
28. Nehme J, El-Khani U, Chow A, Hakky S, Ahmed AR, Purkayastha S. The use of multimedia consent programs for surgical procedures: A systematic review. Surg Innov. 2013;20(1):13-23. PubMed
29. Waller A, Forshaw K, Carey M, et al. Optimizing patient preparation and surgical experience using eHealth technology. JMIR Med Inform. 2015;3(3):e29. PubMed
30. Abbott MB, Shaw P. Virtual nursing avatars: Nurse roles and evolving concepts of care. Online J Issues Nurs. 2016;21(3):7. PubMed
31. Cawthon C, Walia S, Osborn CY, Niesner KJ, Schnipper JL, Kripalani S. Improving care transitions: The patient perspective. J Health Commun. 2012;17 Suppl 3:312-324. PubMed
32. Bickmore TW, Pfeifer LM, Byron D, et al. Usability of conversational agents by patients with inadequate health literacy: Evidence from two clinical trials. J Health Commun. 2010;15 Suppl 2:197-210. PubMed
33. 2017;376(20):1905-1907. N Engl J Med.42. Mandl KD, Kohane IS. A 21st-century health IT system—creating a real-world information economy. PubMed
34. 2014;371(19):1803-1812.N Engl J Med41. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. PubMed
35. 2016;24(1):153-161.J Am Med Inform Assoc.40. Kelly MM, Hoonakker PLT, Dean SM. Using an inpatient portal to engage families in pediatric hospital care. PubMed
36. 2017;171(4):372-381.JAMA Pediatr.39. Khan A, Coffey M, Litterer KP, et al. Families as partners in hospital error and adverse event surveillance. PubMed
37. 2017;17(4):389-402.Acad Pediatr.38. Khan A, Baird J, Rogers JE, et al. Parent and provider experience and shared understanding after a family-centered nighttime communication intervention. PubMed
38. 2016;6(6):319-329.Hosp Pediatr. 37. Khan A, Rogers JE, Forster CS, Furtak SL, Schuster MA, Landrigan CP. Communication and shared understanding between parents and resident-physicians at night. PubMed
39. 2016;11(9):615-619.J Hosp Med36. Figueroa JF, Schnipper JL, McNally K, Stade D, Lipsitz SR, Dalal AK. How often are hospitalized patients and providers on the same page with regard to the patient’s primary recovery goal for hospitalization? PubMed
40. 2013;8(7):414-417.J Hosp Med.35. Feldman HJ, Walker J, Li J, Delbanco T. OpenNotes: Hospitalists’ challenge and opportunity. PubMed
41. 2016;11(5):381-385.J Hosp Med.34. Dalal AK, Schnipper JL. Care team identification in the electronic health record: A critical first step for patient-centered communication.PubMed
42. 2016;24(e1):e178-e184.J Am Med Inform Assoc.33. Dalal AK, Schnipper J, Massaro A, et al. A web-based and mobile patient-centered “microblog” messaging platform to improve care team communication in acute care. PubMed
1. Sarkar U, Bates DW. Care partners and online patient portals. JAMA. 2014;311(4):357-358. PubMed
2. Grando MA, Rozenblum R, Bates DW, eds. Information Technology for Patient Empowerment in Healthcare, 1st Edition. Berlin: Walter de Gruyter Inc.; 2015.
3. Kelly MM, Hoonakker PLT, Dean SM. Using an inpatient portal to engage families in pediatric hospital care. J Am Med Inform Assoc. 2016;24(1):153-161. PubMed
4. Dalal AK, Dykes PC, Collins S, et al. A web-based, patient-centered toolkit to engage patients and caregivers in the acute care setting: A preliminary evaluation. J Am Med Inform Assoc. 2016;23(1):80-87. PubMed
5. Prey JE, Restaino S, Vawdrey DK. Providing hospital patients with access to their medical records. AMIA Annu Symp Proc. 2014;2014:1884-1893. PubMed
6. Herrin J, Harris KG, Kenward K, Hines S, Joshi MS, Frosch DL. Patient and family engagement: A survey of US hospital practices. BMJ Qual Saf. 2016;25(3):182-189. PubMed
7. Tom JO, Mangione-Smith R, Solomon C, Grossman DC. Integrated personal health record use: Association with parent-reported care experiences. Pediatrics. 2012;130(1):e183-e190. PubMed
8. Centers for Medicare & Medicaid Services (CMS), HHS. Medicare and Medicaid Programs; Electronic Health Record Incentive Program-Stage 2. Federal Register Final Rule. Sect. 170; 2012. https://www.federalregister.gov/documents/2012/03/07/2012-4443/medicare-and-medicaid-programs-electronic-health-record-incentive-program-stage-2. Accessed March 1, 2017.
9. Centers for Medicare & Medicaid Services (CMS), HHS. Medicare program; merit-based incentive payment system (MIPS) and alternative payment model (APM) incentive under the physician fee schedule, and criteria for physician-focused payment models. Final rule with comment period. Fed Regist. 2016;81(214):77008-77831. PubMed
10. Prey JE, Woollen J, Wilcox L, et al. Patient engagement in the inpatient setting: A systematic review. J Am Med Informat Assoc. 2014;21(4):742-750. PubMed
11. Ludwin S, Greysen SR. Use of smartphones and mobile devices in hospitalized patients: Untapped opportunities for inpatient engagement. J Hosp Med. 2015;10(7):459-461. PubMed
12. Coleman EA. Family caregivers as partners in care transitions: The caregiver advise record and enable act. J Hosp Med. 2016;11(12):883-885. PubMed
13. Kaziunas E, Hanauer DA, Ackerman MS, Choi SW. Identifying unmet informational needs in the inpatient setting to increase patient and caregiver engagement in the context of pediatric hematopoietic stem cell transplantation. J Am Med Inform Assoc. 2016;23(1):94-104. PubMed
14. Woollen J, Prey J, Wilcox L, et al. Patient experiences using an inpatient personal health record. Appl Clin Inform. 2016;7(2):446-460. PubMed
15. Irizarry T, DeVito Dabbs A, Curran CR. Patient portals and patient engagement: A state of the science review. J Med Internet Res. 2015;17(6):e148. doi:10.2196/jmir.4255. PubMed
16. Vawdrey DK, Wilcox LG, Collins SA, et al. A tablet computer application for patients to participate in their hospital care. AMIA Annu Symp Proc. 2011;2011:1428-1435. PubMed
17. Collins SA, Rozenblum R, Leung WY, et al. Acute care patient portals: A qualitative study of stakeholder perspectives on current practices. J Am Med Inform Assoc. 2016;24(e1):e9-e17. PubMed
18. Berger Z, Flickinger TE, Pfoh E, Martinez KA, Dy SM. Promoting engagement by patients and families to reduce adverse events in acute care settings: A systematic review. BMJ Qual Saf. 2014;23(7):548-555. PubMed
19. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. PubMed
20. Griffin A, Skinner A, Thornhill J, Weinberger M. Patient Portals: Who uses them? What features do they use? And do they reduce hospital readmissions? Appl Clin Inform. 2016;7(2):489-501. PubMed
21. O’Leary KJ, Lohman ME, Culver E, Killarney A, Randy Smith G Jr, Liebovitz DM. The effect of tablet computers with a mobile patient portal application on hospitalized patients’ knowledge and activation. J Am Med Inform Assoc. 2016;23(1):159-165. PubMed
22. O’Leary KJ, Sharma RK, Killarney A, et al. Patients’ and Healthcare Providers’ Perceptions of a Mobile Portal Application for Hospitalized Patients. BMC Med Inform Decis Mak. 2016;16(1):123. PubMed
23. Pell JM, Mancuso M, Limon S, Oman K, Lin CT. Patient access to electronic health records during hospitalization. JAMA Intern Med. 2015;175(5):856-858. PubMed
24. Brown SM, Aboumatar HJ, Francis L, et al. Balancing digital information-sharing and patient privacy when engaging families in the intensive care unit. J Am Med Inform Assoc. 2016;23(5):995-1000. PubMed
25. Krishna S, Francisco BD, Balas EA, et al. Internet-enabled interactive multimedia asthma education program: A randomized trial. Pediatrics. 2003;111(3):503-510. PubMed
26. Fox MP. A systematic review of the literature reporting on studies that examined the impact of interactive, computer-based patient education programs. Patient Educ Couns. 2009;77(1):6-13. PubMed
27. Morgan ER, Laing K, McCarthy J, McCrate F, Seal MD. Using tablet-based technology in patient education about systemic therapy options for early-stage breast cancer: A pilot study. Curr Oncol. 2015;22(5):e364-e369. PubMed
28. Nehme J, El-Khani U, Chow A, Hakky S, Ahmed AR, Purkayastha S. The use of multimedia consent programs for surgical procedures: A systematic review. Surg Innov. 2013;20(1):13-23. PubMed
29. Waller A, Forshaw K, Carey M, et al. Optimizing patient preparation and surgical experience using eHealth technology. JMIR Med Inform. 2015;3(3):e29. PubMed
30. Abbott MB, Shaw P. Virtual nursing avatars: Nurse roles and evolving concepts of care. Online J Issues Nurs. 2016;21(3):7. PubMed
31. Cawthon C, Walia S, Osborn CY, Niesner KJ, Schnipper JL, Kripalani S. Improving care transitions: The patient perspective. J Health Commun. 2012;17 Suppl 3:312-324. PubMed
32. Bickmore TW, Pfeifer LM, Byron D, et al. Usability of conversational agents by patients with inadequate health literacy: Evidence from two clinical trials. J Health Commun. 2010;15 Suppl 2:197-210. PubMed
33. 2017;376(20):1905-1907. N Engl J Med.42. Mandl KD, Kohane IS. A 21st-century health IT system—creating a real-world information economy. PubMed
34. 2014;371(19):1803-1812.N Engl J Med41. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. PubMed
35. 2016;24(1):153-161.J Am Med Inform Assoc.40. Kelly MM, Hoonakker PLT, Dean SM. Using an inpatient portal to engage families in pediatric hospital care. PubMed
36. 2017;171(4):372-381.JAMA Pediatr.39. Khan A, Coffey M, Litterer KP, et al. Families as partners in hospital error and adverse event surveillance. PubMed
37. 2017;17(4):389-402.Acad Pediatr.38. Khan A, Baird J, Rogers JE, et al. Parent and provider experience and shared understanding after a family-centered nighttime communication intervention. PubMed
38. 2016;6(6):319-329.Hosp Pediatr. 37. Khan A, Rogers JE, Forster CS, Furtak SL, Schuster MA, Landrigan CP. Communication and shared understanding between parents and resident-physicians at night. PubMed
39. 2016;11(9):615-619.J Hosp Med36. Figueroa JF, Schnipper JL, McNally K, Stade D, Lipsitz SR, Dalal AK. How often are hospitalized patients and providers on the same page with regard to the patient’s primary recovery goal for hospitalization? PubMed
40. 2013;8(7):414-417.J Hosp Med.35. Feldman HJ, Walker J, Li J, Delbanco T. OpenNotes: Hospitalists’ challenge and opportunity. PubMed
41. 2016;11(5):381-385.J Hosp Med.34. Dalal AK, Schnipper JL. Care team identification in the electronic health record: A critical first step for patient-centered communication.PubMed
42. 2016;24(e1):e178-e184.J Am Med Inform Assoc.33. Dalal AK, Schnipper J, Massaro A, et al. A web-based and mobile patient-centered “microblog” messaging platform to improve care team communication in acute care. PubMed
© 2017 Society of Hospital Medicine
Discordance Between Patient and Provider
Patient‐centered care has been recognized by the Institute of Medicine as an essential aim of the US healthcare system.[1] A fundamental component of patient‐centered care is to engage patients and caregivers in establishing preferences, needs, values, and overall goals regarding their care.[1] Prior studies have shown that delivering high‐quality patient‐centered care is associated with improved health outcomes, and in some cases, reduced costs.[2, 3, 4, 5, 6, 7] Payors, including the Centers for Medicare and Medicaid Services under the Hospital Value‐Based Purchasing program, are increasingly tying payments to measures of patient experience.[8, 9] As more emphasis is placed on public reporting of these patient‐reported outcomes, healthcare organizations are investing in efforts to engage patients and caregivers, including efforts at establishing patients' preferences for care.[10]
In the acute care setting, a prerequisite for high‐quality patient‐centered care is identifying a patient's primary goal for recovery and then delivering care consistent with that goal.[11, 12, 13] Haberle et al. previously validated patients' most common goals for recovery in the hospital setting into 7 broad categories: (1) be cured, (2) live longer, (3) improve or maintain health, (4) be comfortable, (5) accomplish a particular life goal, (6) provide support for a family member, or (7) other.[13] When providers' understanding of these recovery goals are not concordant with the patient's stated goals, patients may receive care inconsistent with their preferences; it is not uncommon for patients to receive aggressive curative treatments (eg, cardiopulmonary resuscitation) when they have expressed otherwise.[14] On the other hand, when patient goals and priorities are clearly established, patients may have better outcomes.[15] For example, earlier conversations about patient goals and priorities in serious illness can lead to realistic expectations of treatment, enhanced goal‐concordant care, improved quality of life, higher patient satisfaction, more and earlier hospice care, fewer hospitalizations, better patient and family coping, reduced burden of decision making for families, and improved bereavement outcomes.[16, 17, 18]
Although previous studies have suggested poor patient‐physician concordance with regard to the patient's plan of care,[19, 20, 21, 22, 23, 24] there are limited data regarding providers' understanding of the patient's primary recovery goal during hospitalization. The purpose of this study was to identify the patients' Haberle goal, and then determine the degree of concordance among patients and key hospital providers regarding this goal.
METHODS
Study Setting
The Partners Human Research Committee approved the study. The study was conducted on an oncology and medical intensive care unit (MICU) at a major academic medical center in Boston, Massachusetts. The oncology unit was comprised of 2 non‐localized medical teams caring for patients admitted to that unit. The MICU was comprised of a single localized medical team. Medical teams working on these units consisted of a first responder (eg, intern or a physician assistant [PA]), medical residents, and an attending physician. Both units had dedicated nursing staff.
Study Participants
All adult patients (>17 years of age) admitted to the oncology and MICU units during the study period (November 2013 through May 2014) were eligible. These units were chosen because these patients are typically complex and have multiple medical comorbidities longer lengths of stay, and many procedures and tests. In addition, a standard method for asking patients to identify a primary recovery goal for hospitalization aligned well with ongoing institutional efforts to engage these patients in goals of care discussions.
Research assistants identified all patients admitted to each study unit for at least 48 hours and approached them in a random order with a daily target of 2 to 3 patients. Only patients who demonstrated capacity (determined by medical team), or had a legally designated healthcare proxy (who spoke English and was available to participate on their behalf) were included. Research assistants then approached the patient's nurse and a physician provider (defined for this study as housestaff physician, PA, or attending) from the primary medical team to participate in the interview (within 24 hours of patient's interview). We excluded eligible patients who did not have capacity or an available caregiver or declined to participate.
Data Collection Instrument and Interviews
Research assistants administered a validated questionnaire developed by Haberle et al. to participants after 48 hours into the patient's admission to provide time to establish mutual understanding of the diagnosis and prognosis.[13] We asked patients (or the designated healthcare proxy) to select their single, most important Haberle goal (see above). Specifically, as in the original validation study,[13] patients or proxies were asked the following question: Please tell me your most important goal of care for this hospitalization. If they did not understand this question, we asked a follow‐up question: What are you expecting will be accomplished during this hospitalization? Within 24 hours of the patient/proxy interview, we independently asked the patient's nurse and physician to select what they thought was the patient's most important goal for recovery using the same questionnaire, adapted for providers. In each case, all participants were blinded to the responses of others.
Measures
We measured the frequency that each participant (patient/proxy, nurse, and physician) selected a specific Haberle recovery goal across all patients. We measured the rate of pairwise concordance by recovery goal for each participant dyad (patient/proxy‐nurse, patient/proxy‐physician, and nurse‐physician). Finally, we calculated the frequency of cases for which all 3 participants selected the same recovery goal.
Statistical Analyses
Descriptive statistics were used to report patient demographic data. The frequencies of selected responses were calculated and reported as percentages for each type of participant. The differences in rate of responses for each Haberle goal were compared across each participant group using 2 analysis. We then performed 2‐way Kappa statistical tests to measure inter‐rater agreement for each dyad.
RESULTS
Of 1436 patients (882 oncology, 554 MICU) hospitalized during the study period, 341(156 oncology, 185 MICU) were admitted for 48 hours. Of 914 potentially eligible patients (617 oncology, 297 MICU), 191 (112 oncology and 79 MICU) were approached to participate based on our sampling strategy; of these, 8 (2 oncology and 6 MICU) did not have capacity (and no proxy was available) and 2 (1 oncology and 1 MICU) declined. Of the remaining 181 patients (109 oncology and 72 MICU), we obtained a completed questionnaire from all 3 interviewees on 109 (60.2% response rate).
Of the 109 study patients, 52 (47.7%) and 57 (52.3%) were admitted to the oncology and medical intensive care units, respectively (Table 1). Patients were predominantly middle aged, Caucasian, English‐speaking, and college‐educated. Healthcare proxies were frequently interviewed on behalf of patients in the MICU. Housestaff physicians were more often interviewed in the MICU, and PAs were interviewed only on oncology units. Compared to patient responders, nonresponders tended to be male and were admitted to oncology units (see Supporting Table 1 in the online version of this article).
| Characteristics | All Patients | Admitted to Medical Intensive Care Units | Admitted to Oncology Units |
|---|---|---|---|
| |||
| Total, no. (%) | 109 (100%) | 57 (52.3%) | 52 (47.7%) |
| Gender, no. (%) | |||
| Male | 55 (50.5%) | 28 (49.1%) | 26 (50.0%) |
| Female | 54 (49.5%) | 29 (50.9%) | 26 (50.0%) |
| Age, y, mean SD | 59.4 14 | 59.7 15 | 59.1 13 |
| Median | 61 | 61 | 60 |
| Range | 2188 | 2188 | 2285 |
| Race, no. (%) | |||
| White | 103 (94.5%) | 53 (93.0%) | 50 (96.2%) |
| Other | 6 (5.5%) | 4 (7.0%) | 2 (3.8%) |
| Language, no. (%) | |||
| English | 106 (97.2%) | 56 (98.1%) | 50 (96.2%) |
| Other | 3 (2.8%) | 1 (1.9%) | 2 (3.8%) |
| Education level, no. (%) | |||
| Less than high school | 30 (27.5%) | 17 (29.8%) | 13 (25.0%) |
| High school diploma | 27 (24.5%) | 18 (31.6%) | 9 (17.3%) |
| Some college or beyond | 52 (47.7%) | 22 (38.6%) | 30 (57.7%) |
| Patient or caregiver interviewed, no. (%) | |||
| Patient | 68 (62.4%) | 27 (47.4%) | 48 (92.3%) |
| Caregiver | 41 (37.6%) | 30 (52.6%) | 4 (7.7%) |
| Nurse interviewed, no. (unique) | 109 (75) | 57 (42) | 52 (33) |
| Physician provider interviewed, no. (%); no. unique | |||
| Attending | 27 (24.8%); 20 | 15 (26.3%); 10 | 12 (23.1%); 10 |
| Housestaff | 48 (44.0%); 39 | 42 (73.7%); 33 | 6 (11.5%); 6 |
| Physician assistant | 34 (31.2%); 25 | 0 (0%); 0 | 34 (65.4%); 25 |
The frequencies of selected Haberle recovery goals by participant type across all patients are listed in Table 2. Patients (or proxies) most often selected be cured (46.8%). Assigned nurses and physicians more commonly selected improve or maintain health (38.5% and 46.8%, respectively). Be comfortable was selected by nurses and physicians more frequently than by patients (16.5%, 16.5%, and 8.3%, respectively). The rate of responses for each Haberle goal was significantly different across all respondent groups (P 0.0001). The frequencies of selected Haberle goals were not significantly different between patients or proxies (P = 0.67), or for patients admitted to the MICU compared to oncology units (P = 0.64).
| Haberle Recovery Goal | Patient/Caregiver, no. (%), n = 109 | Physician Provider, no. (%), n = 109* | Nurse, no. (%), n = 109 |
|---|---|---|---|
| |||
| Be cured | 51 (46.8%) | 20 (18.3%) | 20 (18.3%) |
| Be comfortable | 9 (8.3%) | 18 (16.5%) | 18 (16.5%) |
| Improve or maintain health | 32 (29.4%) | 42 (38.5%) | 51 (46.8%) |
| Live longer | 14 (12.8%) | 21 (19.3%) | 12 (11%) |
| Accomplish personal goal | 2 (1.8%) | 0 (0%) | 3 (2.8%) |
| Provide support for family | 1 (0.9%) | 1 (0.9%) | 1 (0.9%) |
| Other | 0 (0%) | 7 (6.4%) | 4 (3.7%) |
Inter‐rater agreement was poor to slight for the 3 participant dyads (kappa 0.09 [0.03‐0.19], 0.19 [0.08‐0.30], and 0.20 [0.08‐0.32] for patient‐physician, patient‐nurse, and nurse‐physician, respectively). The 3 participants selected the identical recovery goal in 22 (20.2%) cases, and each selected a distinct recovery goal in 32 (29.4%) cases. Pairwise concordance between nurses and physicians was 39.4%. There were no significant differences in agreement between patients admitted to the MICU compared to oncology units (P = 0.09).
DISCUSSION
We observed poor to slight concordance among patients and key hospital providers with regard to identifying the patient's primary recovery goal during acute hospitalization. The majority of patients (or proxies), chose be cured, whereas the majority of hospital providers chose improve or maintain health. Patients were twice as likely to select be cured and half as likely to choose be comfortable compared to nurses or physicians. Strikingly, the patient (or proxy), nurse, and physician identified the same recovery goal in just 20% of cases. These findings were similar for patients admitted to either the MICU or oncology units or when healthcare proxies participated on behalf of the patient (eg, when incapacitated in the MICU).
There are many reasons why hospital providers may not correctly identify the patients' primary recovery goals. First, we do not routinely ask patients to identify recovery goals upon admission in a structured and standardized manner. In fact, clinicians often do not elicit patients' needs, concerns, and expectations regarding their care in general.[25] Second, even when recovery goals are elicited at admission, they may not be communicated effectively to all members of the care team. This could be due to geographically non‐localized teams (although we did not observe a statistically significant difference between regionalized MICU and nonregionalized oncology care units), frequent provider‐to‐provider handoffs, and siloed electronic communication (eg, email, alphanumeric pages) regarding goals of care that inevitably leaves out key providers.[26] Third, healthcare proxies who are involved in decision making on the patient's behalf may not always be available to meet with the care team in person; consequently, their input may not be considered in a timely manner or reliably communicated to all members of the care team. We observed a large discrepancy in how often patients chose be cured compared to their hospital providers. This could be explained by clinicians' unwillingness to disclose bad news or divulge accurate prognostic information that causes patients to feel depressed or lose hope, particularly for those patients with the worst prognoses.[16, 27, 28] Patients may lack sophisticated knowledge of their conditions for a variety of reasons, including low health literacy, at times choosing to hope for the best even when it is not realistic. Additionally, there may be more subtle differences in what patients and hospital providers consider the primary recovery goal in context of the main reason for hospitalization and underlying medical illness. For example, a patient with metastatic lung cancer hospitalized with recurrent postobstructive pneumonia may choose be cured as his/her primary recovery goal (thinking of the pneumonia), whereas physicians may choose improve/maintain health or comfort (thinking of the cancer). We also cannot exclude the possibility that sometimes when patients state be cured and clinicians state improve health as the primary goal, that they are really saying the same thing in different ways. However, these are 2 different constructs (cure may not be possible for many patients) that may deserve an explicit discussion for patients to have realistic expectations for their health following hospitalization.
In short, our results underscore the importance of having an open and honest dialog with patients and caregivers throughout hospitalization, and the need to provide education about the potential futility of excessive care in situations where appropriate. Simply following patients' goals without discussing their feasibility and the consequences of aggressive treatments may result in unnecessary morbidity and misuse of healthcare resources. Once goals are clearly established, communicated, and refined in hospitalized patients with serious illness, there is much reason to believe that ongoing conversation will favorably impact outcomes.[29]
We found few studies that rigorously quantified the rate of concordance of hospital recovery goals among patients and key hospital providers; however, studies that measured overall plan of care agreement have demonstrated suboptimal concordance.[20, 30, 31] Shin et al. found significant underestimation of cancer patients' needs and poor concordance between patients and oncologists in assessing perceived needs of supportive care.[20] It is also notable that nurses and physicians had low levels of concordance in our study. O'Leary and colleagues found that nurses and physicians did not reliably communicate and often did not agree on the plan of care for hospitalized patients.[30] Although geographic regionalization of care teams and multidisciplinary rounds can improve the likelihood that key members of the care team are on the same page with regard to the plan of care, there is still much room for improvement.[26, 32, 33, 34] For example, although nurses and physicians in our study independently selected individual recovery goals with similar frequencies (Table 2), we observed suboptimal concordance between nurses and providers (36.8%) for specific patients, including on our regionalized care unit (MICU). This may be due to the reasons described above.
There are several implications of these findings. As payors continue to shift payments toward value‐based metrics, largely determined by patient experience and adequate advance care planning,[9] our findings suggest that more effort should be focused on delivering care consistent with patients' primary recovery goals. As a first step, healthcare organizations can focus on efforts to systematically identify and communicate recovery goals to all members of the care team, ensuring that patients' preferences, needs, and values are captured. In addition, as innovation in patient engagement and care delivery using Web‐based and mobile technology continues to grow,[35] using these tools to capture key goals for hospitalization and recovery can play an essential role. For example, as electronic health record vendors and institutions start to implement patient portals in the acute care setting, they should consider how to configure these tools to capture key goals for hospitalization and recovery, and then communicate them to the care team; preliminary work in this area is promising.[10]
Our study has several limitations to generalizability. First, the study was conducted on 2 services (MICU and oncology) at a single institution using a sampling strategy where research assistants enrolled 2 to 3 patients per day. Although the sampling was random, the availability of patients and proxies to be interviewed may have led to selection bias. Second, the sample size was small. Third, the patients who participated were predominantly white, English‐speaking, and well educated, possibly a consequence of our sampling strategy. However, this fact makes our findings more striking; although cultural and language barriers were generally not present in our study population, large discrepancies in goal concordance still existed. Fourth, in instances when patients were unable to participate themselves, we interviewed their healthcare proxy; therefore, it is possible that the proxies' responses did not reflect those of the patient. However, we note that concordance rates did not significantly differ between the 2 services despite the fact that the proportion of proxy interviews was much higher in the MICU. Similarly, we cannot exclude the possibility that patients altered their stated goals in the presence of proxies, but patients were given the option to be interviewed alone. Patients may also have misunderstood the timing of the goals (during this hospitalization as opposed to long term), although research assistants made every effort to clarify this during the interviews. Finally, our data‐collection instrument was previously validated in hospitalized general medicine patients and not oncology or MICU patients, and it has not been used to directly ask clinicians to identify patients' recovery goals. However, there is no reason to suspect that it could not be used for this purpose in critical care as well as noncritical care settings, as the survey was developed by a multidisciplinary team that included medical professionals and was validated by clinicians who successfully identified a single, very broad goal (eg, be cured) in each case.
CONCLUSION
We report poor to slight concordance among hospitalized patients and key hospital providers with regard to the main recovery goal. Future studies should assess whether patient satisfaction and experience is adversely impacted by patient‐provider discordance regarding key recovery goals. Additionally, institutions may consider future efforts to elicit and communicate patients' primary recovery goals more effectively to all members of the care team, and address discrepancies as soon as they are discovered.
Disclosures
This work was supported by a grant from the Gordon and Betty Moore Foundation (GBMF) (grant GBMF3914). GBMF had no role in the design or conduct of the study; collection, analysis, or interpretation of data; or preparation or review of the manuscript. The authors report no conflicts of interest.
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy of Sciences; 2001.
- , , , , , . The effects of physician communications skills on patient satisfaction; recall, and adherence. J Chronic Dis. 1984;37(9–10):755–764.
- , , , et al. Observational study of effect of patient centredness and positive approach on outcomes of general practice consultations. BMJ. 2001;323(7318):908–911.
- . Reengineering hospital discharge: a protocol to improve patient safety, reduce costs, and boost patient satisfaction. Am J Med Qual. 2009;24(4):344–346.
- , , , , . Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41–48.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921–1931.
- , , . Enhanced support for shared decision making reduced costs of care for patients with preference‐sensitive conditions. Health Aff (Millwood). 2013;32(2):285–293.
- Centers for Medicare and Medicaid Services. Medicare program; hospital inpatient value‐based purchasing program. Final rule. Fed Regist. 2011;76(88):26490–26547.
- Centers for Medicare and Medicaid Services. CMS begins implementation of key payment legislation. Available at: https://www.cms.gov/Newsroom/MediaReleaseDatabase/Press‐releases/2015‐Press‐releases‐items/2015‐07‐08.html. Published July 8, 2015.
- , , , et al. A web‐based, patient‐centered toolkit to engage patients and caregivers in the acute care setting: a preliminary evaluation [published online August 2, 2015]. J Am Med Informatics Assoc. doi: 10.1093/jamia/ocv093.
- , , , et al. Effectiveness trial of an intensive communication structure for families of long‐stay ICU patients. Chest. 2010;138(6):1340–1348.
- , , , . Understanding goals of care statements and preferences among patients and their surrogates in the medical ICU. J Hosp Palliat Nurs. 2012;14(2):126–132.
- , , , . Goals of care among hospitalized patients: a validation study. Am J Hosp Palliat Care. 2011;28(5):335–341.
- , , , et al. Factors associated with use of cardiopulmonary resuscitation in seriously ill hospitalized adults. JAMA. 1999;282(24):2333–2339.
- , , , , . End‐of‐life discussions, goal attainment, and distress at the end of life: Predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol. 2010;28(7):1203–1208.
- , . Reasons why physicians do not have discussions about poor prognosis, why it matters, and what can be improved. J Clin Oncol. 2012;30(22):2715–2717.
- , , , et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673.
- , , , . Prior advance care planning is associated with less decisional conflict among surrogates for critically ill patients. Ann Am Thorac Soc. 2015;12(10):1528–1533.
- , , , et al. Hospitalized patients' understanding of their plan of care. Mayo Clin Proc. 2010;85(1):47–52.
- , , , et al. Discordance in perceived needs between patients and physicians in oncology practice: a nationwide survey in Korea. J Clin Oncol. 2011;29(33):4424–4429.
- , , , , , . Leveraging standards to support patient‐centric interdisciplinary plans of care. AMIA Annu Symp Proc. 2011;2011:356–363.
- , . Discordance between physician and patient self‐rated health and all‐cause mortality. Ochsner J. 2011;11(3):232–240.
- , , , , , . Determinants of discordance between patients and physicians in their assessment of lupus disease activity. J Rheumatol. 2003;30(9):1967–1976.
- , , , , , . Predictors of discordance between physicians' and patients' appraisals of health‐related quality of life in atrial fibrillation patients: Findings from the Angiotensin II Antagonist in Paroxysmal Atrial Fibrillation Trial. Am Heart J. 2013;166(3):589–596.
- , , , et al. Uncovering the blind spot of patient satisfaction: an international survey. BMJ Qual Saf. 2011;20(11):959–965.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88–93.
- , , , . Discrepancies between patient and physician estimates for the success of stem cell transplantation. JAMA. 2001;285(8):1034–1038.
- , , , , , . Optimistic expectations and survival after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2003;9(6):389–396.
- , , , et al. Development of the Serious Illness Care Program: a randomised controlled trial of a palliative care communication intervention. BMJ Open. 2015;5(10):e009032.
- , , , et al. Patterns of nurse‐physician communication and agreement on the plan of care. Qual Saf Health Care. 2010;19(3):195–199.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812.
- , , , et al. Reorganizing a hospital ward as an accountable care unit. J Hosp Med. 2015;10(1):36–40.
- , , , . Interdisciplinary teamwork in hospitals: a review and practical recommendations for improvement. J Hosp Med. 2012;7(1):48–54.
- , , , , . An evaluation of mobile health application tools. JMIR mHealth uHealth. 2014;2(2):e19.
Patient‐centered care has been recognized by the Institute of Medicine as an essential aim of the US healthcare system.[1] A fundamental component of patient‐centered care is to engage patients and caregivers in establishing preferences, needs, values, and overall goals regarding their care.[1] Prior studies have shown that delivering high‐quality patient‐centered care is associated with improved health outcomes, and in some cases, reduced costs.[2, 3, 4, 5, 6, 7] Payors, including the Centers for Medicare and Medicaid Services under the Hospital Value‐Based Purchasing program, are increasingly tying payments to measures of patient experience.[8, 9] As more emphasis is placed on public reporting of these patient‐reported outcomes, healthcare organizations are investing in efforts to engage patients and caregivers, including efforts at establishing patients' preferences for care.[10]
In the acute care setting, a prerequisite for high‐quality patient‐centered care is identifying a patient's primary goal for recovery and then delivering care consistent with that goal.[11, 12, 13] Haberle et al. previously validated patients' most common goals for recovery in the hospital setting into 7 broad categories: (1) be cured, (2) live longer, (3) improve or maintain health, (4) be comfortable, (5) accomplish a particular life goal, (6) provide support for a family member, or (7) other.[13] When providers' understanding of these recovery goals are not concordant with the patient's stated goals, patients may receive care inconsistent with their preferences; it is not uncommon for patients to receive aggressive curative treatments (eg, cardiopulmonary resuscitation) when they have expressed otherwise.[14] On the other hand, when patient goals and priorities are clearly established, patients may have better outcomes.[15] For example, earlier conversations about patient goals and priorities in serious illness can lead to realistic expectations of treatment, enhanced goal‐concordant care, improved quality of life, higher patient satisfaction, more and earlier hospice care, fewer hospitalizations, better patient and family coping, reduced burden of decision making for families, and improved bereavement outcomes.[16, 17, 18]
Although previous studies have suggested poor patient‐physician concordance with regard to the patient's plan of care,[19, 20, 21, 22, 23, 24] there are limited data regarding providers' understanding of the patient's primary recovery goal during hospitalization. The purpose of this study was to identify the patients' Haberle goal, and then determine the degree of concordance among patients and key hospital providers regarding this goal.
METHODS
Study Setting
The Partners Human Research Committee approved the study. The study was conducted on an oncology and medical intensive care unit (MICU) at a major academic medical center in Boston, Massachusetts. The oncology unit was comprised of 2 non‐localized medical teams caring for patients admitted to that unit. The MICU was comprised of a single localized medical team. Medical teams working on these units consisted of a first responder (eg, intern or a physician assistant [PA]), medical residents, and an attending physician. Both units had dedicated nursing staff.
Study Participants
All adult patients (>17 years of age) admitted to the oncology and MICU units during the study period (November 2013 through May 2014) were eligible. These units were chosen because these patients are typically complex and have multiple medical comorbidities longer lengths of stay, and many procedures and tests. In addition, a standard method for asking patients to identify a primary recovery goal for hospitalization aligned well with ongoing institutional efforts to engage these patients in goals of care discussions.
Research assistants identified all patients admitted to each study unit for at least 48 hours and approached them in a random order with a daily target of 2 to 3 patients. Only patients who demonstrated capacity (determined by medical team), or had a legally designated healthcare proxy (who spoke English and was available to participate on their behalf) were included. Research assistants then approached the patient's nurse and a physician provider (defined for this study as housestaff physician, PA, or attending) from the primary medical team to participate in the interview (within 24 hours of patient's interview). We excluded eligible patients who did not have capacity or an available caregiver or declined to participate.
Data Collection Instrument and Interviews
Research assistants administered a validated questionnaire developed by Haberle et al. to participants after 48 hours into the patient's admission to provide time to establish mutual understanding of the diagnosis and prognosis.[13] We asked patients (or the designated healthcare proxy) to select their single, most important Haberle goal (see above). Specifically, as in the original validation study,[13] patients or proxies were asked the following question: Please tell me your most important goal of care for this hospitalization. If they did not understand this question, we asked a follow‐up question: What are you expecting will be accomplished during this hospitalization? Within 24 hours of the patient/proxy interview, we independently asked the patient's nurse and physician to select what they thought was the patient's most important goal for recovery using the same questionnaire, adapted for providers. In each case, all participants were blinded to the responses of others.
Measures
We measured the frequency that each participant (patient/proxy, nurse, and physician) selected a specific Haberle recovery goal across all patients. We measured the rate of pairwise concordance by recovery goal for each participant dyad (patient/proxy‐nurse, patient/proxy‐physician, and nurse‐physician). Finally, we calculated the frequency of cases for which all 3 participants selected the same recovery goal.
Statistical Analyses
Descriptive statistics were used to report patient demographic data. The frequencies of selected responses were calculated and reported as percentages for each type of participant. The differences in rate of responses for each Haberle goal were compared across each participant group using 2 analysis. We then performed 2‐way Kappa statistical tests to measure inter‐rater agreement for each dyad.
RESULTS
Of 1436 patients (882 oncology, 554 MICU) hospitalized during the study period, 341(156 oncology, 185 MICU) were admitted for 48 hours. Of 914 potentially eligible patients (617 oncology, 297 MICU), 191 (112 oncology and 79 MICU) were approached to participate based on our sampling strategy; of these, 8 (2 oncology and 6 MICU) did not have capacity (and no proxy was available) and 2 (1 oncology and 1 MICU) declined. Of the remaining 181 patients (109 oncology and 72 MICU), we obtained a completed questionnaire from all 3 interviewees on 109 (60.2% response rate).
Of the 109 study patients, 52 (47.7%) and 57 (52.3%) were admitted to the oncology and medical intensive care units, respectively (Table 1). Patients were predominantly middle aged, Caucasian, English‐speaking, and college‐educated. Healthcare proxies were frequently interviewed on behalf of patients in the MICU. Housestaff physicians were more often interviewed in the MICU, and PAs were interviewed only on oncology units. Compared to patient responders, nonresponders tended to be male and were admitted to oncology units (see Supporting Table 1 in the online version of this article).
| Characteristics | All Patients | Admitted to Medical Intensive Care Units | Admitted to Oncology Units |
|---|---|---|---|
| |||
| Total, no. (%) | 109 (100%) | 57 (52.3%) | 52 (47.7%) |
| Gender, no. (%) | |||
| Male | 55 (50.5%) | 28 (49.1%) | 26 (50.0%) |
| Female | 54 (49.5%) | 29 (50.9%) | 26 (50.0%) |
| Age, y, mean SD | 59.4 14 | 59.7 15 | 59.1 13 |
| Median | 61 | 61 | 60 |
| Range | 2188 | 2188 | 2285 |
| Race, no. (%) | |||
| White | 103 (94.5%) | 53 (93.0%) | 50 (96.2%) |
| Other | 6 (5.5%) | 4 (7.0%) | 2 (3.8%) |
| Language, no. (%) | |||
| English | 106 (97.2%) | 56 (98.1%) | 50 (96.2%) |
| Other | 3 (2.8%) | 1 (1.9%) | 2 (3.8%) |
| Education level, no. (%) | |||
| Less than high school | 30 (27.5%) | 17 (29.8%) | 13 (25.0%) |
| High school diploma | 27 (24.5%) | 18 (31.6%) | 9 (17.3%) |
| Some college or beyond | 52 (47.7%) | 22 (38.6%) | 30 (57.7%) |
| Patient or caregiver interviewed, no. (%) | |||
| Patient | 68 (62.4%) | 27 (47.4%) | 48 (92.3%) |
| Caregiver | 41 (37.6%) | 30 (52.6%) | 4 (7.7%) |
| Nurse interviewed, no. (unique) | 109 (75) | 57 (42) | 52 (33) |
| Physician provider interviewed, no. (%); no. unique | |||
| Attending | 27 (24.8%); 20 | 15 (26.3%); 10 | 12 (23.1%); 10 |
| Housestaff | 48 (44.0%); 39 | 42 (73.7%); 33 | 6 (11.5%); 6 |
| Physician assistant | 34 (31.2%); 25 | 0 (0%); 0 | 34 (65.4%); 25 |
The frequencies of selected Haberle recovery goals by participant type across all patients are listed in Table 2. Patients (or proxies) most often selected be cured (46.8%). Assigned nurses and physicians more commonly selected improve or maintain health (38.5% and 46.8%, respectively). Be comfortable was selected by nurses and physicians more frequently than by patients (16.5%, 16.5%, and 8.3%, respectively). The rate of responses for each Haberle goal was significantly different across all respondent groups (P 0.0001). The frequencies of selected Haberle goals were not significantly different between patients or proxies (P = 0.67), or for patients admitted to the MICU compared to oncology units (P = 0.64).
| Haberle Recovery Goal | Patient/Caregiver, no. (%), n = 109 | Physician Provider, no. (%), n = 109* | Nurse, no. (%), n = 109 |
|---|---|---|---|
| |||
| Be cured | 51 (46.8%) | 20 (18.3%) | 20 (18.3%) |
| Be comfortable | 9 (8.3%) | 18 (16.5%) | 18 (16.5%) |
| Improve or maintain health | 32 (29.4%) | 42 (38.5%) | 51 (46.8%) |
| Live longer | 14 (12.8%) | 21 (19.3%) | 12 (11%) |
| Accomplish personal goal | 2 (1.8%) | 0 (0%) | 3 (2.8%) |
| Provide support for family | 1 (0.9%) | 1 (0.9%) | 1 (0.9%) |
| Other | 0 (0%) | 7 (6.4%) | 4 (3.7%) |
Inter‐rater agreement was poor to slight for the 3 participant dyads (kappa 0.09 [0.03‐0.19], 0.19 [0.08‐0.30], and 0.20 [0.08‐0.32] for patient‐physician, patient‐nurse, and nurse‐physician, respectively). The 3 participants selected the identical recovery goal in 22 (20.2%) cases, and each selected a distinct recovery goal in 32 (29.4%) cases. Pairwise concordance between nurses and physicians was 39.4%. There were no significant differences in agreement between patients admitted to the MICU compared to oncology units (P = 0.09).
DISCUSSION
We observed poor to slight concordance among patients and key hospital providers with regard to identifying the patient's primary recovery goal during acute hospitalization. The majority of patients (or proxies), chose be cured, whereas the majority of hospital providers chose improve or maintain health. Patients were twice as likely to select be cured and half as likely to choose be comfortable compared to nurses or physicians. Strikingly, the patient (or proxy), nurse, and physician identified the same recovery goal in just 20% of cases. These findings were similar for patients admitted to either the MICU or oncology units or when healthcare proxies participated on behalf of the patient (eg, when incapacitated in the MICU).
There are many reasons why hospital providers may not correctly identify the patients' primary recovery goals. First, we do not routinely ask patients to identify recovery goals upon admission in a structured and standardized manner. In fact, clinicians often do not elicit patients' needs, concerns, and expectations regarding their care in general.[25] Second, even when recovery goals are elicited at admission, they may not be communicated effectively to all members of the care team. This could be due to geographically non‐localized teams (although we did not observe a statistically significant difference between regionalized MICU and nonregionalized oncology care units), frequent provider‐to‐provider handoffs, and siloed electronic communication (eg, email, alphanumeric pages) regarding goals of care that inevitably leaves out key providers.[26] Third, healthcare proxies who are involved in decision making on the patient's behalf may not always be available to meet with the care team in person; consequently, their input may not be considered in a timely manner or reliably communicated to all members of the care team. We observed a large discrepancy in how often patients chose be cured compared to their hospital providers. This could be explained by clinicians' unwillingness to disclose bad news or divulge accurate prognostic information that causes patients to feel depressed or lose hope, particularly for those patients with the worst prognoses.[16, 27, 28] Patients may lack sophisticated knowledge of their conditions for a variety of reasons, including low health literacy, at times choosing to hope for the best even when it is not realistic. Additionally, there may be more subtle differences in what patients and hospital providers consider the primary recovery goal in context of the main reason for hospitalization and underlying medical illness. For example, a patient with metastatic lung cancer hospitalized with recurrent postobstructive pneumonia may choose be cured as his/her primary recovery goal (thinking of the pneumonia), whereas physicians may choose improve/maintain health or comfort (thinking of the cancer). We also cannot exclude the possibility that sometimes when patients state be cured and clinicians state improve health as the primary goal, that they are really saying the same thing in different ways. However, these are 2 different constructs (cure may not be possible for many patients) that may deserve an explicit discussion for patients to have realistic expectations for their health following hospitalization.
In short, our results underscore the importance of having an open and honest dialog with patients and caregivers throughout hospitalization, and the need to provide education about the potential futility of excessive care in situations where appropriate. Simply following patients' goals without discussing their feasibility and the consequences of aggressive treatments may result in unnecessary morbidity and misuse of healthcare resources. Once goals are clearly established, communicated, and refined in hospitalized patients with serious illness, there is much reason to believe that ongoing conversation will favorably impact outcomes.[29]
We found few studies that rigorously quantified the rate of concordance of hospital recovery goals among patients and key hospital providers; however, studies that measured overall plan of care agreement have demonstrated suboptimal concordance.[20, 30, 31] Shin et al. found significant underestimation of cancer patients' needs and poor concordance between patients and oncologists in assessing perceived needs of supportive care.[20] It is also notable that nurses and physicians had low levels of concordance in our study. O'Leary and colleagues found that nurses and physicians did not reliably communicate and often did not agree on the plan of care for hospitalized patients.[30] Although geographic regionalization of care teams and multidisciplinary rounds can improve the likelihood that key members of the care team are on the same page with regard to the plan of care, there is still much room for improvement.[26, 32, 33, 34] For example, although nurses and physicians in our study independently selected individual recovery goals with similar frequencies (Table 2), we observed suboptimal concordance between nurses and providers (36.8%) for specific patients, including on our regionalized care unit (MICU). This may be due to the reasons described above.
There are several implications of these findings. As payors continue to shift payments toward value‐based metrics, largely determined by patient experience and adequate advance care planning,[9] our findings suggest that more effort should be focused on delivering care consistent with patients' primary recovery goals. As a first step, healthcare organizations can focus on efforts to systematically identify and communicate recovery goals to all members of the care team, ensuring that patients' preferences, needs, and values are captured. In addition, as innovation in patient engagement and care delivery using Web‐based and mobile technology continues to grow,[35] using these tools to capture key goals for hospitalization and recovery can play an essential role. For example, as electronic health record vendors and institutions start to implement patient portals in the acute care setting, they should consider how to configure these tools to capture key goals for hospitalization and recovery, and then communicate them to the care team; preliminary work in this area is promising.[10]
Our study has several limitations to generalizability. First, the study was conducted on 2 services (MICU and oncology) at a single institution using a sampling strategy where research assistants enrolled 2 to 3 patients per day. Although the sampling was random, the availability of patients and proxies to be interviewed may have led to selection bias. Second, the sample size was small. Third, the patients who participated were predominantly white, English‐speaking, and well educated, possibly a consequence of our sampling strategy. However, this fact makes our findings more striking; although cultural and language barriers were generally not present in our study population, large discrepancies in goal concordance still existed. Fourth, in instances when patients were unable to participate themselves, we interviewed their healthcare proxy; therefore, it is possible that the proxies' responses did not reflect those of the patient. However, we note that concordance rates did not significantly differ between the 2 services despite the fact that the proportion of proxy interviews was much higher in the MICU. Similarly, we cannot exclude the possibility that patients altered their stated goals in the presence of proxies, but patients were given the option to be interviewed alone. Patients may also have misunderstood the timing of the goals (during this hospitalization as opposed to long term), although research assistants made every effort to clarify this during the interviews. Finally, our data‐collection instrument was previously validated in hospitalized general medicine patients and not oncology or MICU patients, and it has not been used to directly ask clinicians to identify patients' recovery goals. However, there is no reason to suspect that it could not be used for this purpose in critical care as well as noncritical care settings, as the survey was developed by a multidisciplinary team that included medical professionals and was validated by clinicians who successfully identified a single, very broad goal (eg, be cured) in each case.
CONCLUSION
We report poor to slight concordance among hospitalized patients and key hospital providers with regard to the main recovery goal. Future studies should assess whether patient satisfaction and experience is adversely impacted by patient‐provider discordance regarding key recovery goals. Additionally, institutions may consider future efforts to elicit and communicate patients' primary recovery goals more effectively to all members of the care team, and address discrepancies as soon as they are discovered.
Disclosures
This work was supported by a grant from the Gordon and Betty Moore Foundation (GBMF) (grant GBMF3914). GBMF had no role in the design or conduct of the study; collection, analysis, or interpretation of data; or preparation or review of the manuscript. The authors report no conflicts of interest.
Patient‐centered care has been recognized by the Institute of Medicine as an essential aim of the US healthcare system.[1] A fundamental component of patient‐centered care is to engage patients and caregivers in establishing preferences, needs, values, and overall goals regarding their care.[1] Prior studies have shown that delivering high‐quality patient‐centered care is associated with improved health outcomes, and in some cases, reduced costs.[2, 3, 4, 5, 6, 7] Payors, including the Centers for Medicare and Medicaid Services under the Hospital Value‐Based Purchasing program, are increasingly tying payments to measures of patient experience.[8, 9] As more emphasis is placed on public reporting of these patient‐reported outcomes, healthcare organizations are investing in efforts to engage patients and caregivers, including efforts at establishing patients' preferences for care.[10]
In the acute care setting, a prerequisite for high‐quality patient‐centered care is identifying a patient's primary goal for recovery and then delivering care consistent with that goal.[11, 12, 13] Haberle et al. previously validated patients' most common goals for recovery in the hospital setting into 7 broad categories: (1) be cured, (2) live longer, (3) improve or maintain health, (4) be comfortable, (5) accomplish a particular life goal, (6) provide support for a family member, or (7) other.[13] When providers' understanding of these recovery goals are not concordant with the patient's stated goals, patients may receive care inconsistent with their preferences; it is not uncommon for patients to receive aggressive curative treatments (eg, cardiopulmonary resuscitation) when they have expressed otherwise.[14] On the other hand, when patient goals and priorities are clearly established, patients may have better outcomes.[15] For example, earlier conversations about patient goals and priorities in serious illness can lead to realistic expectations of treatment, enhanced goal‐concordant care, improved quality of life, higher patient satisfaction, more and earlier hospice care, fewer hospitalizations, better patient and family coping, reduced burden of decision making for families, and improved bereavement outcomes.[16, 17, 18]
Although previous studies have suggested poor patient‐physician concordance with regard to the patient's plan of care,[19, 20, 21, 22, 23, 24] there are limited data regarding providers' understanding of the patient's primary recovery goal during hospitalization. The purpose of this study was to identify the patients' Haberle goal, and then determine the degree of concordance among patients and key hospital providers regarding this goal.
METHODS
Study Setting
The Partners Human Research Committee approved the study. The study was conducted on an oncology and medical intensive care unit (MICU) at a major academic medical center in Boston, Massachusetts. The oncology unit was comprised of 2 non‐localized medical teams caring for patients admitted to that unit. The MICU was comprised of a single localized medical team. Medical teams working on these units consisted of a first responder (eg, intern or a physician assistant [PA]), medical residents, and an attending physician. Both units had dedicated nursing staff.
Study Participants
All adult patients (>17 years of age) admitted to the oncology and MICU units during the study period (November 2013 through May 2014) were eligible. These units were chosen because these patients are typically complex and have multiple medical comorbidities longer lengths of stay, and many procedures and tests. In addition, a standard method for asking patients to identify a primary recovery goal for hospitalization aligned well with ongoing institutional efforts to engage these patients in goals of care discussions.
Research assistants identified all patients admitted to each study unit for at least 48 hours and approached them in a random order with a daily target of 2 to 3 patients. Only patients who demonstrated capacity (determined by medical team), or had a legally designated healthcare proxy (who spoke English and was available to participate on their behalf) were included. Research assistants then approached the patient's nurse and a physician provider (defined for this study as housestaff physician, PA, or attending) from the primary medical team to participate in the interview (within 24 hours of patient's interview). We excluded eligible patients who did not have capacity or an available caregiver or declined to participate.
Data Collection Instrument and Interviews
Research assistants administered a validated questionnaire developed by Haberle et al. to participants after 48 hours into the patient's admission to provide time to establish mutual understanding of the diagnosis and prognosis.[13] We asked patients (or the designated healthcare proxy) to select their single, most important Haberle goal (see above). Specifically, as in the original validation study,[13] patients or proxies were asked the following question: Please tell me your most important goal of care for this hospitalization. If they did not understand this question, we asked a follow‐up question: What are you expecting will be accomplished during this hospitalization? Within 24 hours of the patient/proxy interview, we independently asked the patient's nurse and physician to select what they thought was the patient's most important goal for recovery using the same questionnaire, adapted for providers. In each case, all participants were blinded to the responses of others.
Measures
We measured the frequency that each participant (patient/proxy, nurse, and physician) selected a specific Haberle recovery goal across all patients. We measured the rate of pairwise concordance by recovery goal for each participant dyad (patient/proxy‐nurse, patient/proxy‐physician, and nurse‐physician). Finally, we calculated the frequency of cases for which all 3 participants selected the same recovery goal.
Statistical Analyses
Descriptive statistics were used to report patient demographic data. The frequencies of selected responses were calculated and reported as percentages for each type of participant. The differences in rate of responses for each Haberle goal were compared across each participant group using 2 analysis. We then performed 2‐way Kappa statistical tests to measure inter‐rater agreement for each dyad.
RESULTS
Of 1436 patients (882 oncology, 554 MICU) hospitalized during the study period, 341(156 oncology, 185 MICU) were admitted for 48 hours. Of 914 potentially eligible patients (617 oncology, 297 MICU), 191 (112 oncology and 79 MICU) were approached to participate based on our sampling strategy; of these, 8 (2 oncology and 6 MICU) did not have capacity (and no proxy was available) and 2 (1 oncology and 1 MICU) declined. Of the remaining 181 patients (109 oncology and 72 MICU), we obtained a completed questionnaire from all 3 interviewees on 109 (60.2% response rate).
Of the 109 study patients, 52 (47.7%) and 57 (52.3%) were admitted to the oncology and medical intensive care units, respectively (Table 1). Patients were predominantly middle aged, Caucasian, English‐speaking, and college‐educated. Healthcare proxies were frequently interviewed on behalf of patients in the MICU. Housestaff physicians were more often interviewed in the MICU, and PAs were interviewed only on oncology units. Compared to patient responders, nonresponders tended to be male and were admitted to oncology units (see Supporting Table 1 in the online version of this article).
| Characteristics | All Patients | Admitted to Medical Intensive Care Units | Admitted to Oncology Units |
|---|---|---|---|
| |||
| Total, no. (%) | 109 (100%) | 57 (52.3%) | 52 (47.7%) |
| Gender, no. (%) | |||
| Male | 55 (50.5%) | 28 (49.1%) | 26 (50.0%) |
| Female | 54 (49.5%) | 29 (50.9%) | 26 (50.0%) |
| Age, y, mean SD | 59.4 14 | 59.7 15 | 59.1 13 |
| Median | 61 | 61 | 60 |
| Range | 2188 | 2188 | 2285 |
| Race, no. (%) | |||
| White | 103 (94.5%) | 53 (93.0%) | 50 (96.2%) |
| Other | 6 (5.5%) | 4 (7.0%) | 2 (3.8%) |
| Language, no. (%) | |||
| English | 106 (97.2%) | 56 (98.1%) | 50 (96.2%) |
| Other | 3 (2.8%) | 1 (1.9%) | 2 (3.8%) |
| Education level, no. (%) | |||
| Less than high school | 30 (27.5%) | 17 (29.8%) | 13 (25.0%) |
| High school diploma | 27 (24.5%) | 18 (31.6%) | 9 (17.3%) |
| Some college or beyond | 52 (47.7%) | 22 (38.6%) | 30 (57.7%) |
| Patient or caregiver interviewed, no. (%) | |||
| Patient | 68 (62.4%) | 27 (47.4%) | 48 (92.3%) |
| Caregiver | 41 (37.6%) | 30 (52.6%) | 4 (7.7%) |
| Nurse interviewed, no. (unique) | 109 (75) | 57 (42) | 52 (33) |
| Physician provider interviewed, no. (%); no. unique | |||
| Attending | 27 (24.8%); 20 | 15 (26.3%); 10 | 12 (23.1%); 10 |
| Housestaff | 48 (44.0%); 39 | 42 (73.7%); 33 | 6 (11.5%); 6 |
| Physician assistant | 34 (31.2%); 25 | 0 (0%); 0 | 34 (65.4%); 25 |
The frequencies of selected Haberle recovery goals by participant type across all patients are listed in Table 2. Patients (or proxies) most often selected be cured (46.8%). Assigned nurses and physicians more commonly selected improve or maintain health (38.5% and 46.8%, respectively). Be comfortable was selected by nurses and physicians more frequently than by patients (16.5%, 16.5%, and 8.3%, respectively). The rate of responses for each Haberle goal was significantly different across all respondent groups (P 0.0001). The frequencies of selected Haberle goals were not significantly different between patients or proxies (P = 0.67), or for patients admitted to the MICU compared to oncology units (P = 0.64).
| Haberle Recovery Goal | Patient/Caregiver, no. (%), n = 109 | Physician Provider, no. (%), n = 109* | Nurse, no. (%), n = 109 |
|---|---|---|---|
| |||
| Be cured | 51 (46.8%) | 20 (18.3%) | 20 (18.3%) |
| Be comfortable | 9 (8.3%) | 18 (16.5%) | 18 (16.5%) |
| Improve or maintain health | 32 (29.4%) | 42 (38.5%) | 51 (46.8%) |
| Live longer | 14 (12.8%) | 21 (19.3%) | 12 (11%) |
| Accomplish personal goal | 2 (1.8%) | 0 (0%) | 3 (2.8%) |
| Provide support for family | 1 (0.9%) | 1 (0.9%) | 1 (0.9%) |
| Other | 0 (0%) | 7 (6.4%) | 4 (3.7%) |
Inter‐rater agreement was poor to slight for the 3 participant dyads (kappa 0.09 [0.03‐0.19], 0.19 [0.08‐0.30], and 0.20 [0.08‐0.32] for patient‐physician, patient‐nurse, and nurse‐physician, respectively). The 3 participants selected the identical recovery goal in 22 (20.2%) cases, and each selected a distinct recovery goal in 32 (29.4%) cases. Pairwise concordance between nurses and physicians was 39.4%. There were no significant differences in agreement between patients admitted to the MICU compared to oncology units (P = 0.09).
DISCUSSION
We observed poor to slight concordance among patients and key hospital providers with regard to identifying the patient's primary recovery goal during acute hospitalization. The majority of patients (or proxies), chose be cured, whereas the majority of hospital providers chose improve or maintain health. Patients were twice as likely to select be cured and half as likely to choose be comfortable compared to nurses or physicians. Strikingly, the patient (or proxy), nurse, and physician identified the same recovery goal in just 20% of cases. These findings were similar for patients admitted to either the MICU or oncology units or when healthcare proxies participated on behalf of the patient (eg, when incapacitated in the MICU).
There are many reasons why hospital providers may not correctly identify the patients' primary recovery goals. First, we do not routinely ask patients to identify recovery goals upon admission in a structured and standardized manner. In fact, clinicians often do not elicit patients' needs, concerns, and expectations regarding their care in general.[25] Second, even when recovery goals are elicited at admission, they may not be communicated effectively to all members of the care team. This could be due to geographically non‐localized teams (although we did not observe a statistically significant difference between regionalized MICU and nonregionalized oncology care units), frequent provider‐to‐provider handoffs, and siloed electronic communication (eg, email, alphanumeric pages) regarding goals of care that inevitably leaves out key providers.[26] Third, healthcare proxies who are involved in decision making on the patient's behalf may not always be available to meet with the care team in person; consequently, their input may not be considered in a timely manner or reliably communicated to all members of the care team. We observed a large discrepancy in how often patients chose be cured compared to their hospital providers. This could be explained by clinicians' unwillingness to disclose bad news or divulge accurate prognostic information that causes patients to feel depressed or lose hope, particularly for those patients with the worst prognoses.[16, 27, 28] Patients may lack sophisticated knowledge of their conditions for a variety of reasons, including low health literacy, at times choosing to hope for the best even when it is not realistic. Additionally, there may be more subtle differences in what patients and hospital providers consider the primary recovery goal in context of the main reason for hospitalization and underlying medical illness. For example, a patient with metastatic lung cancer hospitalized with recurrent postobstructive pneumonia may choose be cured as his/her primary recovery goal (thinking of the pneumonia), whereas physicians may choose improve/maintain health or comfort (thinking of the cancer). We also cannot exclude the possibility that sometimes when patients state be cured and clinicians state improve health as the primary goal, that they are really saying the same thing in different ways. However, these are 2 different constructs (cure may not be possible for many patients) that may deserve an explicit discussion for patients to have realistic expectations for their health following hospitalization.
In short, our results underscore the importance of having an open and honest dialog with patients and caregivers throughout hospitalization, and the need to provide education about the potential futility of excessive care in situations where appropriate. Simply following patients' goals without discussing their feasibility and the consequences of aggressive treatments may result in unnecessary morbidity and misuse of healthcare resources. Once goals are clearly established, communicated, and refined in hospitalized patients with serious illness, there is much reason to believe that ongoing conversation will favorably impact outcomes.[29]
We found few studies that rigorously quantified the rate of concordance of hospital recovery goals among patients and key hospital providers; however, studies that measured overall plan of care agreement have demonstrated suboptimal concordance.[20, 30, 31] Shin et al. found significant underestimation of cancer patients' needs and poor concordance between patients and oncologists in assessing perceived needs of supportive care.[20] It is also notable that nurses and physicians had low levels of concordance in our study. O'Leary and colleagues found that nurses and physicians did not reliably communicate and often did not agree on the plan of care for hospitalized patients.[30] Although geographic regionalization of care teams and multidisciplinary rounds can improve the likelihood that key members of the care team are on the same page with regard to the plan of care, there is still much room for improvement.[26, 32, 33, 34] For example, although nurses and physicians in our study independently selected individual recovery goals with similar frequencies (Table 2), we observed suboptimal concordance between nurses and providers (36.8%) for specific patients, including on our regionalized care unit (MICU). This may be due to the reasons described above.
There are several implications of these findings. As payors continue to shift payments toward value‐based metrics, largely determined by patient experience and adequate advance care planning,[9] our findings suggest that more effort should be focused on delivering care consistent with patients' primary recovery goals. As a first step, healthcare organizations can focus on efforts to systematically identify and communicate recovery goals to all members of the care team, ensuring that patients' preferences, needs, and values are captured. In addition, as innovation in patient engagement and care delivery using Web‐based and mobile technology continues to grow,[35] using these tools to capture key goals for hospitalization and recovery can play an essential role. For example, as electronic health record vendors and institutions start to implement patient portals in the acute care setting, they should consider how to configure these tools to capture key goals for hospitalization and recovery, and then communicate them to the care team; preliminary work in this area is promising.[10]
Our study has several limitations to generalizability. First, the study was conducted on 2 services (MICU and oncology) at a single institution using a sampling strategy where research assistants enrolled 2 to 3 patients per day. Although the sampling was random, the availability of patients and proxies to be interviewed may have led to selection bias. Second, the sample size was small. Third, the patients who participated were predominantly white, English‐speaking, and well educated, possibly a consequence of our sampling strategy. However, this fact makes our findings more striking; although cultural and language barriers were generally not present in our study population, large discrepancies in goal concordance still existed. Fourth, in instances when patients were unable to participate themselves, we interviewed their healthcare proxy; therefore, it is possible that the proxies' responses did not reflect those of the patient. However, we note that concordance rates did not significantly differ between the 2 services despite the fact that the proportion of proxy interviews was much higher in the MICU. Similarly, we cannot exclude the possibility that patients altered their stated goals in the presence of proxies, but patients were given the option to be interviewed alone. Patients may also have misunderstood the timing of the goals (during this hospitalization as opposed to long term), although research assistants made every effort to clarify this during the interviews. Finally, our data‐collection instrument was previously validated in hospitalized general medicine patients and not oncology or MICU patients, and it has not been used to directly ask clinicians to identify patients' recovery goals. However, there is no reason to suspect that it could not be used for this purpose in critical care as well as noncritical care settings, as the survey was developed by a multidisciplinary team that included medical professionals and was validated by clinicians who successfully identified a single, very broad goal (eg, be cured) in each case.
CONCLUSION
We report poor to slight concordance among hospitalized patients and key hospital providers with regard to the main recovery goal. Future studies should assess whether patient satisfaction and experience is adversely impacted by patient‐provider discordance regarding key recovery goals. Additionally, institutions may consider future efforts to elicit and communicate patients' primary recovery goals more effectively to all members of the care team, and address discrepancies as soon as they are discovered.
Disclosures
This work was supported by a grant from the Gordon and Betty Moore Foundation (GBMF) (grant GBMF3914). GBMF had no role in the design or conduct of the study; collection, analysis, or interpretation of data; or preparation or review of the manuscript. The authors report no conflicts of interest.
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy of Sciences; 2001.
- , , , , , . The effects of physician communications skills on patient satisfaction; recall, and adherence. J Chronic Dis. 1984;37(9–10):755–764.
- , , , et al. Observational study of effect of patient centredness and positive approach on outcomes of general practice consultations. BMJ. 2001;323(7318):908–911.
- . Reengineering hospital discharge: a protocol to improve patient safety, reduce costs, and boost patient satisfaction. Am J Med Qual. 2009;24(4):344–346.
- , , , , . Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41–48.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921–1931.
- , , . Enhanced support for shared decision making reduced costs of care for patients with preference‐sensitive conditions. Health Aff (Millwood). 2013;32(2):285–293.
- Centers for Medicare and Medicaid Services. Medicare program; hospital inpatient value‐based purchasing program. Final rule. Fed Regist. 2011;76(88):26490–26547.
- Centers for Medicare and Medicaid Services. CMS begins implementation of key payment legislation. Available at: https://www.cms.gov/Newsroom/MediaReleaseDatabase/Press‐releases/2015‐Press‐releases‐items/2015‐07‐08.html. Published July 8, 2015.
- , , , et al. A web‐based, patient‐centered toolkit to engage patients and caregivers in the acute care setting: a preliminary evaluation [published online August 2, 2015]. J Am Med Informatics Assoc. doi: 10.1093/jamia/ocv093.
- , , , et al. Effectiveness trial of an intensive communication structure for families of long‐stay ICU patients. Chest. 2010;138(6):1340–1348.
- , , , . Understanding goals of care statements and preferences among patients and their surrogates in the medical ICU. J Hosp Palliat Nurs. 2012;14(2):126–132.
- , , , . Goals of care among hospitalized patients: a validation study. Am J Hosp Palliat Care. 2011;28(5):335–341.
- , , , et al. Factors associated with use of cardiopulmonary resuscitation in seriously ill hospitalized adults. JAMA. 1999;282(24):2333–2339.
- , , , , . End‐of‐life discussions, goal attainment, and distress at the end of life: Predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol. 2010;28(7):1203–1208.
- , . Reasons why physicians do not have discussions about poor prognosis, why it matters, and what can be improved. J Clin Oncol. 2012;30(22):2715–2717.
- , , , et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673.
- , , , . Prior advance care planning is associated with less decisional conflict among surrogates for critically ill patients. Ann Am Thorac Soc. 2015;12(10):1528–1533.
- , , , et al. Hospitalized patients' understanding of their plan of care. Mayo Clin Proc. 2010;85(1):47–52.
- , , , et al. Discordance in perceived needs between patients and physicians in oncology practice: a nationwide survey in Korea. J Clin Oncol. 2011;29(33):4424–4429.
- , , , , , . Leveraging standards to support patient‐centric interdisciplinary plans of care. AMIA Annu Symp Proc. 2011;2011:356–363.
- , . Discordance between physician and patient self‐rated health and all‐cause mortality. Ochsner J. 2011;11(3):232–240.
- , , , , , . Determinants of discordance between patients and physicians in their assessment of lupus disease activity. J Rheumatol. 2003;30(9):1967–1976.
- , , , , , . Predictors of discordance between physicians' and patients' appraisals of health‐related quality of life in atrial fibrillation patients: Findings from the Angiotensin II Antagonist in Paroxysmal Atrial Fibrillation Trial. Am Heart J. 2013;166(3):589–596.
- , , , et al. Uncovering the blind spot of patient satisfaction: an international survey. BMJ Qual Saf. 2011;20(11):959–965.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88–93.
- , , , . Discrepancies between patient and physician estimates for the success of stem cell transplantation. JAMA. 2001;285(8):1034–1038.
- , , , , , . Optimistic expectations and survival after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2003;9(6):389–396.
- , , , et al. Development of the Serious Illness Care Program: a randomised controlled trial of a palliative care communication intervention. BMJ Open. 2015;5(10):e009032.
- , , , et al. Patterns of nurse‐physician communication and agreement on the plan of care. Qual Saf Health Care. 2010;19(3):195–199.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812.
- , , , et al. Reorganizing a hospital ward as an accountable care unit. J Hosp Med. 2015;10(1):36–40.
- , , , . Interdisciplinary teamwork in hospitals: a review and practical recommendations for improvement. J Hosp Med. 2012;7(1):48–54.
- , , , , . An evaluation of mobile health application tools. JMIR mHealth uHealth. 2014;2(2):e19.
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy of Sciences; 2001.
- , , , , , . The effects of physician communications skills on patient satisfaction; recall, and adherence. J Chronic Dis. 1984;37(9–10):755–764.
- , , , et al. Observational study of effect of patient centredness and positive approach on outcomes of general practice consultations. BMJ. 2001;323(7318):908–911.
- . Reengineering hospital discharge: a protocol to improve patient safety, reduce costs, and boost patient satisfaction. Am J Med Qual. 2009;24(4):344–346.
- , , , , . Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41–48.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921–1931.
- , , . Enhanced support for shared decision making reduced costs of care for patients with preference‐sensitive conditions. Health Aff (Millwood). 2013;32(2):285–293.
- Centers for Medicare and Medicaid Services. Medicare program; hospital inpatient value‐based purchasing program. Final rule. Fed Regist. 2011;76(88):26490–26547.
- Centers for Medicare and Medicaid Services. CMS begins implementation of key payment legislation. Available at: https://www.cms.gov/Newsroom/MediaReleaseDatabase/Press‐releases/2015‐Press‐releases‐items/2015‐07‐08.html. Published July 8, 2015.
- , , , et al. A web‐based, patient‐centered toolkit to engage patients and caregivers in the acute care setting: a preliminary evaluation [published online August 2, 2015]. J Am Med Informatics Assoc. doi: 10.1093/jamia/ocv093.
- , , , et al. Effectiveness trial of an intensive communication structure for families of long‐stay ICU patients. Chest. 2010;138(6):1340–1348.
- , , , . Understanding goals of care statements and preferences among patients and their surrogates in the medical ICU. J Hosp Palliat Nurs. 2012;14(2):126–132.
- , , , . Goals of care among hospitalized patients: a validation study. Am J Hosp Palliat Care. 2011;28(5):335–341.
- , , , et al. Factors associated with use of cardiopulmonary resuscitation in seriously ill hospitalized adults. JAMA. 1999;282(24):2333–2339.
- , , , , . End‐of‐life discussions, goal attainment, and distress at the end of life: Predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol. 2010;28(7):1203–1208.
- , . Reasons why physicians do not have discussions about poor prognosis, why it matters, and what can be improved. J Clin Oncol. 2012;30(22):2715–2717.
- , , , et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673.
- , , , . Prior advance care planning is associated with less decisional conflict among surrogates for critically ill patients. Ann Am Thorac Soc. 2015;12(10):1528–1533.
- , , , et al. Hospitalized patients' understanding of their plan of care. Mayo Clin Proc. 2010;85(1):47–52.
- , , , et al. Discordance in perceived needs between patients and physicians in oncology practice: a nationwide survey in Korea. J Clin Oncol. 2011;29(33):4424–4429.
- , , , , , . Leveraging standards to support patient‐centric interdisciplinary plans of care. AMIA Annu Symp Proc. 2011;2011:356–363.
- , . Discordance between physician and patient self‐rated health and all‐cause mortality. Ochsner J. 2011;11(3):232–240.
- , , , , , . Determinants of discordance between patients and physicians in their assessment of lupus disease activity. J Rheumatol. 2003;30(9):1967–1976.
- , , , , , . Predictors of discordance between physicians' and patients' appraisals of health‐related quality of life in atrial fibrillation patients: Findings from the Angiotensin II Antagonist in Paroxysmal Atrial Fibrillation Trial. Am Heart J. 2013;166(3):589–596.
- , , , et al. Uncovering the blind spot of patient satisfaction: an international survey. BMJ Qual Saf. 2011;20(11):959–965.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88–93.
- , , , . Discrepancies between patient and physician estimates for the success of stem cell transplantation. JAMA. 2001;285(8):1034–1038.
- , , , , , . Optimistic expectations and survival after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2003;9(6):389–396.
- , , , et al. Development of the Serious Illness Care Program: a randomised controlled trial of a palliative care communication intervention. BMJ Open. 2015;5(10):e009032.
- , , , et al. Patterns of nurse‐physician communication and agreement on the plan of care. Qual Saf Health Care. 2010;19(3):195–199.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812.
- , , , et al. Reorganizing a hospital ward as an accountable care unit. J Hosp Med. 2015;10(1):36–40.
- , , , . Interdisciplinary teamwork in hospitals: a review and practical recommendations for improvement. J Hosp Med. 2012;7(1):48–54.
- , , , , . An evaluation of mobile health application tools. JMIR mHealth uHealth. 2014;2(2):e19.
Care Team Identification
Patient‐centered communication is a strategy that is used to promote shared understanding of the plan of care among providers and patients.[1, 2, 3] Caring for hospitalized patients is a collaborative effort that requires seamless patient‐centered communication among a rapidly changing care team to safely progress a patient from admission through discharge. Yet, hospitals continue to struggle with improving the complex and increasingly electronic conversation patterns among care team members and patients to achieve effective patient‐centered communication.[4, 5] When members of the care team operate in this environment, patients often receive conflicting information regarding their plan of care, medications, and test results. Ineffective communication can lead to a suboptimal patient experience, additional costs, medical errors, and preventable adverse events.[6, 7, 8, 9, 10]
A critical first step to improving patient‐centered communication is identifying the care team.[11, 12] Accurate and reliable identification of all care team members is a pressing information need; it is fundamental to efficiently conveying information about the plan of care to those who know the patient the best, must make timely decisions, or will assume care once the patient leaves the hospital.[13] Furthermore, it has implications for engaging patients more meaningfully in their care.[14, 15, 16, 17] Ideally, the process of identifying an individual caring for the patient in a specific role is quickly and reliably determined from the electronic health record, the single source of truth where any provider can quickly identify other team members. This source of truth can be updated manually when individual members assign and remove themselves from the care team, or automatically when accessing the patient's record, writing a note, placing an order, or adding a patient to a coverage list. When providers correctly identify other team members in this way, hospital paging directories and secure messaging tools that link to the electronic health record become more effective at supporting care team communication.[18]
In general, the process of identifying care teams is difficult,[19] and maintaining role assignments in the electronic health record is equally challenging. Vawdrey et al. previously reported that care team lists are inaccurate and cannot be used to reliably identify other members at any given moment.[18] The inability to identify team members often leads to incorrectly routed pages, e‐mail messages, and phone calls.[20] Consequently, the potential to reliably manage the care team and improve electronic communication remains untapped, rendering team collaboration and care coordination less effective.[18, 21, 22]
In recent years, the trend toward restructuring inpatient teamsgeographical localization, structured communication interventions, teamwork training, and interdisciplinary roundswould seem to diminish the need for electronic care team identification, as those efforts have already made a positive impact with regard to interprofessional communication and collaboration, team satisfaction, and adverse events.[23, 24, 25, 26] Nonetheless, interdisciplinary teamwork, though critically important for patient‐centered communication, does not completely obviate the need for accurate and reliable care team identification.[26] Although care teams are statically located on units, the plan of care is dynamic; it evolves when the patient's status changes, when new information becomes available, and when key longitudinal providers (eg, primary care physician, subspecialty consultant) make recommendations. Thus, information conveyed as a team on rounds quickly becomes out of date, requiring additional forms of communication. Furthermore, due to frequent ad hoc coverage among team members, the identity of providers covering the patient at any given moment is often not clear.[27] This is particularly problematic for nonunit‐based providers who try to communicate with unit‐based care team members. These providers, in particular, have valuable knowledge and insight that can aid the primary team in decision making.[28, 29] However, they typically do not participate in rounds, often waste time identifying responsible providers,[20] and may communicate their recommendations directly with the patient without discussing with the primary team. These factors in part explain why geographic localization has shown limited improvement in shared understanding of the plan of care.[23]
From the perspective of patients and caregivers, identification of the providers entering and leaving their room is also challenging; only 11% to 51% of patients identify their providers correctly.[30] This adds to confusion regarding who is responsible for which aspects of the patient's care and can negatively affect the perception of the quality of care received.[31] Use of whiteboards has been shown to improve the proportion of patients who could identify key providers,[32] but these are not reliably updated and generally cannot accommodate all team members. When face cards are used, patients and caregivers report that they are more likely to identify their providers correctly.[14, 33, 34] However, potential confusion may ensue when another provider assumes care of the patient in the same role. Finally, use of technology to display team members at the bedside is typically a feature that patients like and can improve identification of care team members.[14, 15, 16] Yet, patient engagement technologies are not readily available in the hospital setting,[35] and ideally should be linked to the electronic health record, which again must be reliably updated.[11, 12, 15, 16]
If care team identification is so critical for delivering effective patient‐centered communication, why is maintaining role assignment problematic? At the individual level, reasons include discontinuity of the care team due to changing clinical rotations and intrateam coverage, shift‐based schedules, and lack of awareness and underutilization of functionality. Additionally, clinicians may have different ways to maintain lists of patients. At the institutional level, functionality to enforce role assignment when accessing patient records may be disabled (to avoid perceived burdens on clinical staff or nonclinical personnel who require access for administrative functions). Finally, electronic health record vendors currently have no incentive to adopt functionality that supports more effective care coordination across settings.[22]
However, more than technical solutions and policy changes are required; care team identification in the electronic health record requires a change in institutional culture. Maintaining an accurate relationship to each patient requires work without tangible benefitsthe benefits accrue only when everyone else identifies their role on the teama tragedy of the commons. This can be illustrated by our own experience. We conducted a quality improvement initiative (Table 1) as a part of 2 concurrent research initiatives that serve to promote patient‐centered communication:[12] PCORI (Patient‐Centered Outcomes Research Institute Transitions), the goal of which is to improve care transitions within the Partners' Pioneer Accountable Care Organization; and the PROSPECT (Promoting Respect and Ongoing Safety Through Patient‐Centeredness, Engagement, Communication, and Technology) project, an initiative funded by the Gordon and Betty Moore Foundation to eliminate preventable harms in the acute care environment.[29] Our goal was to electronically manage the care team with a high degree of fidelity. We enhanced a home‐grown application, which was developed to improve management of team lists for inpatient providers, accessible from our electronic health record, to facilitate role assignment. Specifically, we leveraged existing care processes (eg, nursing log‐on to the electronic medication administration system) to automatically assign certain providers to the care team at change of shift, added functionality to make it easy to assign a provider to all patients on a list for a defined period of time, and encouraged providers to assign their role by demonstrating benefits including quick access to patient‐specific group e‐mail and secure messaging tools (Table 1, Key Facets). The initiative was well‐received by most disciplines, but uptake was suboptimal. Our research assistants routinely assigned residents and others to the care team because our proactive attempts at advertising and reinforcing use of the application failed to reach a critical mass. Most did not see immediate benefits because it was an added step to their busy day, had other methods of managing team lists, and only saw benefit if everyone else participated. Key facets of our care team identification initiative, successes, and challenges are outlined in Table 1.
| Key Facets | Successes | Challenges |
|---|---|---|
| Linked electronic role assignment to administrative processes and clinical workflows | Leveraged existing processes to identify attending provider by routinely reviewing online schedules Linked role assignment to electronic medication administration system sign‐in process for nurses at the start of their shift |
Difficult to generate buy‐in from administrators and specific clinician groups to incorporate routine use of role assignment functionality into existing and/or new workflows No institutional policy mandating role assignments for members of extended care team |
| Incorporated default functionality to specify length of role assignment (eg, stop date) | Used by trainees (residents, fellows) to automate team list role assignments for a prespecified period of time according to online schedules | Underutilized by subspecialty consultants, many of who were unaware or did not fully appreciate the added value of this functionality Research assistants regularly verified that default role assignments were accurately maintained for trainees |
| Linked role assignment to patient‐specific group e‐mail and messaging tools | Clinicians acknowledged clear efficiency benefits (eg, automated patient identification within messages, correct routing of e‐mails) Used by specific members of the care team tasked with facilitating coordination of care (eg, nurse practitioner trained as discharge advocate for research study) |
Difficult to promote use of patient‐specific messaging, particularly for nonunit‐based providers (eg, consultants, primary care physicians) Required access to an application not typically used for clinical messaging Difficult to change culture of network e‐mail use for clinical messaging |
| Advertised new functionality and demonstrated potential efficiencies for care team communication | Unit‐based clinicians (hospitalists, nurses, housestaff) typically understood benefits when demonstrated and were easier to engage | Some nonunit‐based clinicians (eg, consulting attendings, primary care physicians) did not see benefits and/or were difficult to engage |
| Some nonunit‐based provider groups (eg, social workers, nutritionists, subspecialty fellows) considered the initiative worthwhile, and were open to learning about new functionality to improve communication | Clinicians had several options for managing team lists prior to implementation of new electronic health record | |
| Institutional effort toward implementing new electronic health record detracted from efforts at demonstrating enhanced functionality of existing applications |
There were a few glimmers of hope, however. On several PROSPECT units, we displayed team members on a tablet‐based patient portal so that patients would recognize their providers.[11, 17, 36] Similar to recent work by O'Leary et al.,[14] patients on PROSPECT units were able to correctly identify several care team members, but regularly asked why other providers (eg, consulting fellow) were not listed. Those providers asked the same question, and some eventually learned to assign their role via the application. As part of PROSEPCT, we visited other institutions and learned of an effort to display team members on high‐definition televisions in the patient's room. Several providers, wondering why they were not listed, learned to assign their role and their picture then appeared. Social pressure was the driving force.
Coincidently, we recently implemented a new electronic health record at our institution. Anecdotally, although no formal policy was established, many providers (eg, attendings, first responders, nurses, care coordinators, and other unit‐based providers) appear to be assigning their roles. Other providers (eg, dieticians, physical therapists, residents) also assign their role, but often fail to end role assignments upon completing their rotation or when the patient transfers to another service. Finally, even when actively involved, most subspecialists still do not designate their role. Despite these gaps and inconsistencies, we have made progress toward improving care team identification. The reasons for this progress are straightforward; during required training for the new electronic health record, all inpatient providers were taught to assign their role on the treatment team when assuming care of patients and now have 1 option for managing team lists. However, most providers were not trained to end their role assignments, and many have learned that role assignment is not required to access the patient's record; functionality to enforce this was disabled. Based on lessons learned from our experience,[12] we offer several strategies that hospitalists can employ to improve care team identification in the electronic health record (Table 2).
| Goal | Strategies to Achieve Goal |
|---|---|
| Identify and/or establish reliable processes that administrative staff can use to ensure accurate care team role assignments | Identify databases that serve as the source of truth for provider schedules and routinely access those databases |
| Access resident scheduling application (eg, Amion) that is routinely updated by training program staff | |
| Work with clinical and administrative staff to maintain care team role assignments | |
| Engage affiliated ambulatory practices to ensure patient's primary care physician is updated in the electronic health record | |
| Engage admissions office to improve reliability of attending assignments based on online clinical schedules when patients are admitted | |
| Integrate role assignment into established workflows for specific provider groups when administrative processes not feasible | Link routine care processes to care team role assignment |
| Train nurses, interns, physician assistants to assign role on care team when assuming care of patient at shift change | |
| Train residents, fellows to use default functionality to automatically assign their role on care team at the beginning of a clinical rotation | |
| Demonstrate value of maintaining role assignments in the electronic health record to the unit‐based care team | Emphasize how accurate and reliable care team role assignment can facilitate correct routing of information (eg, test results, discharge summaries) |
| Helps to maintain patient coverage lists (eg, fellows, consultants, social workers) | |
| Facilitates patient‐specific communication (eg, via group email and messaging tools linked to the electronic health record's care team functionality) | |
| Align with concurrent institutional initiatives that enforce or incentivize care team role assignment | Mandate role assignment when writing a note, placing an order, or adding a patient to a coverage list in the electronic health record |
| Provide patients and caregivers the ability to identify the care team via patient portalcreates social pressure for those providers who do not identify themselves on the care team | |
| Incentivize providers to maintain role assignments during patient's hospitalization in order to receive notifications if patients are readmitted | |
| Automate role assignments for all members of the care team whenever possible | Work with clinical informatics/emnformation system staff to determine feasibility of linking online scheduling systems or log‐in process to other systems routinely accessed by specific providers to automatically assign/unassign specific providers at the beginning/end of a shift (eg, nurses automatically assigned to care team when they access the electronic medication administration record system at beginning of shift) |
| Explore availability of default functionality to assign and unassign providers to and from the care team in a specific role by team, service, or unit‐based patient lists | |
| Require a stop time/date for role assignments or set a default if none entered |
In the future, care team identification in the electronic health record can be automated by integrating directly with electronic workflows, online scheduling applications, and provider directories. Hospitals could then leverage care team lists to facilitate patient‐centered communication via secure web‐based and mobile messaging applications configured to simultaneously update all team members (eg, group messaging apps, microblogs).[11, 37, 38] By synchronizing with the electronic health record, role assignments can be automatically updated via these applications, further increasing fidelity of care team identification.[12] Finally, as hospitals implement acute care patient portals, team lists can be leveraged to display all care team members correctly so that patients and caregivers can communicate more easily with providers.[17] The potential ramifications for patient‐centered communication are tremendous.
Disclosures
This work was funded by the Patient‐Centered Outcomes Research Institute and the Gordon and Betty Moore Foundation (GBMF3914). The authors report no conflicts of interest.
- . Patient‐centered communication. Annu Rev Nurs Res. 1999;17:85–104.
- , , , . Facilitating patient‐centered cancer communication: a road map. Patient Educ Couns. 2009;77:319–321.
- , . Patient‐Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering. NIH Publication No. 07–6225. Bethesda, MD: National Cancer Institute; 2007.
- . When conversation is better than computation. J Am Med Inform Assoc. 2000;7:277–286.
- . Communication systems in healthcare. Clin Biochem Rev. 2006;27:89–98.
- , , , et al. The nature of adverse events in hospitalized patients. results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324:377–384.
- , , , et al. A look into the nature and causes of human errors in the intensive care unit. Crit Care Med. 1995;23:294–300.
- , , . Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79:186–194.
- , . Interdisciplinary communication: an uncharted source of medical error? J Crit Care. 2006;21:236–242; discussion 242.
- , , . Quantifying the economic impact of communication inefficiencies in U.S. hospitals. J Healthc Manag. 2010;55:265–281; discussion 281–282.
- , , , . Transforming the acute care environment: a web‐based patient‐centered toolkit [abstract]. J Hosp Med. 2014;9(suppl 2):694.
- , , , et al. Creating a culture of patient‐centered care team communication at a large academic medical center [Abstract]. J Hosp Med. 2015;10 (suppl 2). Available at: http://www.shmabstracts.com/abstract/creating‐a‐culture‐of‐patient‐centered‐care‐team‐communication‐at‐a‐large‐academic‐medical‐center. Accessed April 24, 2015.
- , , , , . Perceived information needs and communication difficulties of inpatient physicians and nurses. Proc AMIA Symp. 2001:453–457.
- , , , , , . The effect of tablet computers with a mobile patient portal application on hospitalized patients' knowledge and activation [published online June 15, 2015]. J Am Med Inform Assoc. doi: 10.1093/jamia/ocv058.
- , , , , . Bedside information technology to support patient‐centered care. Int J Med Inform. 2012;81(7):442–451.
- , , , et al. Building and testing a patient‐centric electronic bedside communication center. J Gerontol Nurs. 2013;39:15–19.
- , , , et al. A web‐based, patient‐centered toolkit to engage patients and caregivers in the acute care setting: a preliminary evaluation [published online August 2, 2015]. J Am Med Inform Assoc. doi: 10.1093/jamia/ocv093.
- , , , et al. Awareness of the care team in electronic health records. Appl Clin Inform. 2011;2:395–405.
- , , , , , . Teamwork on inpatient medical units: assessing attitudes and barriers. Qual Saf Health Care. 2010;19:117–121.
- , , , et al. Frequency and clinical importance of pages sent to the wrong physician. Arch Intern Med. 2009;169:1072–1073.
- , . Patient experiences with coordination of care: the benefit of continuity and primary care physician as referral source. J Gen Intern Med. 2009;24:170–177.
- , , , , . Are electronic medical records helpful for care coordination? Experiences of physician practices. J Gen Intern Med. 2010;25:177–185.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24:1223–1227.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6:88–93.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171:678–684.
- , , , ; High Performance Teams and the Hospital of the Future Project Team. Interdisciplinary teamwork in hospitals: a review and practical recommendations for improvement. J Hosp Med. 2012;7:48–54.
- , , . Intrateam coverage is common, intrateam handoffs are not. J Hosp Med. 2014;9:734–736.
- . A primary care physician's ideal transitions of care? Where's the evidence? J Hosp Med. 2013;8:472–477.
- , , , , . Primary care physician communication at hospital discharge reduces medication discrepancies. J Hosp Med. 2013;8:672–677.
- , . Let's “face” it: time to introduce yourself to patients. J Hosp Med. 2014;9:199–200.
- , , . Patient perceptions of coordinated care: the importance of organized communication in hospitals. J Healthc Qual. 1999;21:18–23.
- , , , . Patient whiteboards to improve patient‐centred care in the hospital. Postgrad Med J. 2013;89:604–609.
- , , , , , . Effect of a face sheet tool on medical team provider identification and family satisfaction. J Hosp Med. 2014;9:186–188.
- , , , , , . The impact of facecards on patients' knowledge, satisfaction, trust, and agreement with hospital physicians: a pilot study. J Hosp Med. 2014;9:137–141.
- , , , et al. Patient engagement in the inpatient setting: a systematic review. J Am Med Inform Assoc. 2014;21:742–750.
- PROSPECT: Promoting Respect and Ongoing Safety Through Patient‐centeredness, Engagement, Communication, and Technology. Available at: http://www.partners.org/cird/PROSPECT/Index.htm. Accessed May 3, 2015.
- , , , , , . Smarter hospital communication: secure smartphone text messaging improves provider satisfaction and perception of efficacy, workflow. J Hosp Med. 2014;9:573–578.
- , , , et al. Engaging patients, providers, and institutional stakeholders in developing a patient‐centered microblog. Paper presented at: Proceeding of the American Medical Informatics Association Annual Fall Symposium; November 16–19, 2014; Washington, DC.
Patient‐centered communication is a strategy that is used to promote shared understanding of the plan of care among providers and patients.[1, 2, 3] Caring for hospitalized patients is a collaborative effort that requires seamless patient‐centered communication among a rapidly changing care team to safely progress a patient from admission through discharge. Yet, hospitals continue to struggle with improving the complex and increasingly electronic conversation patterns among care team members and patients to achieve effective patient‐centered communication.[4, 5] When members of the care team operate in this environment, patients often receive conflicting information regarding their plan of care, medications, and test results. Ineffective communication can lead to a suboptimal patient experience, additional costs, medical errors, and preventable adverse events.[6, 7, 8, 9, 10]
A critical first step to improving patient‐centered communication is identifying the care team.[11, 12] Accurate and reliable identification of all care team members is a pressing information need; it is fundamental to efficiently conveying information about the plan of care to those who know the patient the best, must make timely decisions, or will assume care once the patient leaves the hospital.[13] Furthermore, it has implications for engaging patients more meaningfully in their care.[14, 15, 16, 17] Ideally, the process of identifying an individual caring for the patient in a specific role is quickly and reliably determined from the electronic health record, the single source of truth where any provider can quickly identify other team members. This source of truth can be updated manually when individual members assign and remove themselves from the care team, or automatically when accessing the patient's record, writing a note, placing an order, or adding a patient to a coverage list. When providers correctly identify other team members in this way, hospital paging directories and secure messaging tools that link to the electronic health record become more effective at supporting care team communication.[18]
In general, the process of identifying care teams is difficult,[19] and maintaining role assignments in the electronic health record is equally challenging. Vawdrey et al. previously reported that care team lists are inaccurate and cannot be used to reliably identify other members at any given moment.[18] The inability to identify team members often leads to incorrectly routed pages, e‐mail messages, and phone calls.[20] Consequently, the potential to reliably manage the care team and improve electronic communication remains untapped, rendering team collaboration and care coordination less effective.[18, 21, 22]
In recent years, the trend toward restructuring inpatient teamsgeographical localization, structured communication interventions, teamwork training, and interdisciplinary roundswould seem to diminish the need for electronic care team identification, as those efforts have already made a positive impact with regard to interprofessional communication and collaboration, team satisfaction, and adverse events.[23, 24, 25, 26] Nonetheless, interdisciplinary teamwork, though critically important for patient‐centered communication, does not completely obviate the need for accurate and reliable care team identification.[26] Although care teams are statically located on units, the plan of care is dynamic; it evolves when the patient's status changes, when new information becomes available, and when key longitudinal providers (eg, primary care physician, subspecialty consultant) make recommendations. Thus, information conveyed as a team on rounds quickly becomes out of date, requiring additional forms of communication. Furthermore, due to frequent ad hoc coverage among team members, the identity of providers covering the patient at any given moment is often not clear.[27] This is particularly problematic for nonunit‐based providers who try to communicate with unit‐based care team members. These providers, in particular, have valuable knowledge and insight that can aid the primary team in decision making.[28, 29] However, they typically do not participate in rounds, often waste time identifying responsible providers,[20] and may communicate their recommendations directly with the patient without discussing with the primary team. These factors in part explain why geographic localization has shown limited improvement in shared understanding of the plan of care.[23]
From the perspective of patients and caregivers, identification of the providers entering and leaving their room is also challenging; only 11% to 51% of patients identify their providers correctly.[30] This adds to confusion regarding who is responsible for which aspects of the patient's care and can negatively affect the perception of the quality of care received.[31] Use of whiteboards has been shown to improve the proportion of patients who could identify key providers,[32] but these are not reliably updated and generally cannot accommodate all team members. When face cards are used, patients and caregivers report that they are more likely to identify their providers correctly.[14, 33, 34] However, potential confusion may ensue when another provider assumes care of the patient in the same role. Finally, use of technology to display team members at the bedside is typically a feature that patients like and can improve identification of care team members.[14, 15, 16] Yet, patient engagement technologies are not readily available in the hospital setting,[35] and ideally should be linked to the electronic health record, which again must be reliably updated.[11, 12, 15, 16]
If care team identification is so critical for delivering effective patient‐centered communication, why is maintaining role assignment problematic? At the individual level, reasons include discontinuity of the care team due to changing clinical rotations and intrateam coverage, shift‐based schedules, and lack of awareness and underutilization of functionality. Additionally, clinicians may have different ways to maintain lists of patients. At the institutional level, functionality to enforce role assignment when accessing patient records may be disabled (to avoid perceived burdens on clinical staff or nonclinical personnel who require access for administrative functions). Finally, electronic health record vendors currently have no incentive to adopt functionality that supports more effective care coordination across settings.[22]
However, more than technical solutions and policy changes are required; care team identification in the electronic health record requires a change in institutional culture. Maintaining an accurate relationship to each patient requires work without tangible benefitsthe benefits accrue only when everyone else identifies their role on the teama tragedy of the commons. This can be illustrated by our own experience. We conducted a quality improvement initiative (Table 1) as a part of 2 concurrent research initiatives that serve to promote patient‐centered communication:[12] PCORI (Patient‐Centered Outcomes Research Institute Transitions), the goal of which is to improve care transitions within the Partners' Pioneer Accountable Care Organization; and the PROSPECT (Promoting Respect and Ongoing Safety Through Patient‐Centeredness, Engagement, Communication, and Technology) project, an initiative funded by the Gordon and Betty Moore Foundation to eliminate preventable harms in the acute care environment.[29] Our goal was to electronically manage the care team with a high degree of fidelity. We enhanced a home‐grown application, which was developed to improve management of team lists for inpatient providers, accessible from our electronic health record, to facilitate role assignment. Specifically, we leveraged existing care processes (eg, nursing log‐on to the electronic medication administration system) to automatically assign certain providers to the care team at change of shift, added functionality to make it easy to assign a provider to all patients on a list for a defined period of time, and encouraged providers to assign their role by demonstrating benefits including quick access to patient‐specific group e‐mail and secure messaging tools (Table 1, Key Facets). The initiative was well‐received by most disciplines, but uptake was suboptimal. Our research assistants routinely assigned residents and others to the care team because our proactive attempts at advertising and reinforcing use of the application failed to reach a critical mass. Most did not see immediate benefits because it was an added step to their busy day, had other methods of managing team lists, and only saw benefit if everyone else participated. Key facets of our care team identification initiative, successes, and challenges are outlined in Table 1.
| Key Facets | Successes | Challenges |
|---|---|---|
| Linked electronic role assignment to administrative processes and clinical workflows | Leveraged existing processes to identify attending provider by routinely reviewing online schedules Linked role assignment to electronic medication administration system sign‐in process for nurses at the start of their shift |
Difficult to generate buy‐in from administrators and specific clinician groups to incorporate routine use of role assignment functionality into existing and/or new workflows No institutional policy mandating role assignments for members of extended care team |
| Incorporated default functionality to specify length of role assignment (eg, stop date) | Used by trainees (residents, fellows) to automate team list role assignments for a prespecified period of time according to online schedules | Underutilized by subspecialty consultants, many of who were unaware or did not fully appreciate the added value of this functionality Research assistants regularly verified that default role assignments were accurately maintained for trainees |
| Linked role assignment to patient‐specific group e‐mail and messaging tools | Clinicians acknowledged clear efficiency benefits (eg, automated patient identification within messages, correct routing of e‐mails) Used by specific members of the care team tasked with facilitating coordination of care (eg, nurse practitioner trained as discharge advocate for research study) |
Difficult to promote use of patient‐specific messaging, particularly for nonunit‐based providers (eg, consultants, primary care physicians) Required access to an application not typically used for clinical messaging Difficult to change culture of network e‐mail use for clinical messaging |
| Advertised new functionality and demonstrated potential efficiencies for care team communication | Unit‐based clinicians (hospitalists, nurses, housestaff) typically understood benefits when demonstrated and were easier to engage | Some nonunit‐based clinicians (eg, consulting attendings, primary care physicians) did not see benefits and/or were difficult to engage |
| Some nonunit‐based provider groups (eg, social workers, nutritionists, subspecialty fellows) considered the initiative worthwhile, and were open to learning about new functionality to improve communication | Clinicians had several options for managing team lists prior to implementation of new electronic health record | |
| Institutional effort toward implementing new electronic health record detracted from efforts at demonstrating enhanced functionality of existing applications |
There were a few glimmers of hope, however. On several PROSPECT units, we displayed team members on a tablet‐based patient portal so that patients would recognize their providers.[11, 17, 36] Similar to recent work by O'Leary et al.,[14] patients on PROSPECT units were able to correctly identify several care team members, but regularly asked why other providers (eg, consulting fellow) were not listed. Those providers asked the same question, and some eventually learned to assign their role via the application. As part of PROSEPCT, we visited other institutions and learned of an effort to display team members on high‐definition televisions in the patient's room. Several providers, wondering why they were not listed, learned to assign their role and their picture then appeared. Social pressure was the driving force.
Coincidently, we recently implemented a new electronic health record at our institution. Anecdotally, although no formal policy was established, many providers (eg, attendings, first responders, nurses, care coordinators, and other unit‐based providers) appear to be assigning their roles. Other providers (eg, dieticians, physical therapists, residents) also assign their role, but often fail to end role assignments upon completing their rotation or when the patient transfers to another service. Finally, even when actively involved, most subspecialists still do not designate their role. Despite these gaps and inconsistencies, we have made progress toward improving care team identification. The reasons for this progress are straightforward; during required training for the new electronic health record, all inpatient providers were taught to assign their role on the treatment team when assuming care of patients and now have 1 option for managing team lists. However, most providers were not trained to end their role assignments, and many have learned that role assignment is not required to access the patient's record; functionality to enforce this was disabled. Based on lessons learned from our experience,[12] we offer several strategies that hospitalists can employ to improve care team identification in the electronic health record (Table 2).
| Goal | Strategies to Achieve Goal |
|---|---|
| Identify and/or establish reliable processes that administrative staff can use to ensure accurate care team role assignments | Identify databases that serve as the source of truth for provider schedules and routinely access those databases |
| Access resident scheduling application (eg, Amion) that is routinely updated by training program staff | |
| Work with clinical and administrative staff to maintain care team role assignments | |
| Engage affiliated ambulatory practices to ensure patient's primary care physician is updated in the electronic health record | |
| Engage admissions office to improve reliability of attending assignments based on online clinical schedules when patients are admitted | |
| Integrate role assignment into established workflows for specific provider groups when administrative processes not feasible | Link routine care processes to care team role assignment |
| Train nurses, interns, physician assistants to assign role on care team when assuming care of patient at shift change | |
| Train residents, fellows to use default functionality to automatically assign their role on care team at the beginning of a clinical rotation | |
| Demonstrate value of maintaining role assignments in the electronic health record to the unit‐based care team | Emphasize how accurate and reliable care team role assignment can facilitate correct routing of information (eg, test results, discharge summaries) |
| Helps to maintain patient coverage lists (eg, fellows, consultants, social workers) | |
| Facilitates patient‐specific communication (eg, via group email and messaging tools linked to the electronic health record's care team functionality) | |
| Align with concurrent institutional initiatives that enforce or incentivize care team role assignment | Mandate role assignment when writing a note, placing an order, or adding a patient to a coverage list in the electronic health record |
| Provide patients and caregivers the ability to identify the care team via patient portalcreates social pressure for those providers who do not identify themselves on the care team | |
| Incentivize providers to maintain role assignments during patient's hospitalization in order to receive notifications if patients are readmitted | |
| Automate role assignments for all members of the care team whenever possible | Work with clinical informatics/emnformation system staff to determine feasibility of linking online scheduling systems or log‐in process to other systems routinely accessed by specific providers to automatically assign/unassign specific providers at the beginning/end of a shift (eg, nurses automatically assigned to care team when they access the electronic medication administration record system at beginning of shift) |
| Explore availability of default functionality to assign and unassign providers to and from the care team in a specific role by team, service, or unit‐based patient lists | |
| Require a stop time/date for role assignments or set a default if none entered |
In the future, care team identification in the electronic health record can be automated by integrating directly with electronic workflows, online scheduling applications, and provider directories. Hospitals could then leverage care team lists to facilitate patient‐centered communication via secure web‐based and mobile messaging applications configured to simultaneously update all team members (eg, group messaging apps, microblogs).[11, 37, 38] By synchronizing with the electronic health record, role assignments can be automatically updated via these applications, further increasing fidelity of care team identification.[12] Finally, as hospitals implement acute care patient portals, team lists can be leveraged to display all care team members correctly so that patients and caregivers can communicate more easily with providers.[17] The potential ramifications for patient‐centered communication are tremendous.
Disclosures
This work was funded by the Patient‐Centered Outcomes Research Institute and the Gordon and Betty Moore Foundation (GBMF3914). The authors report no conflicts of interest.
Patient‐centered communication is a strategy that is used to promote shared understanding of the plan of care among providers and patients.[1, 2, 3] Caring for hospitalized patients is a collaborative effort that requires seamless patient‐centered communication among a rapidly changing care team to safely progress a patient from admission through discharge. Yet, hospitals continue to struggle with improving the complex and increasingly electronic conversation patterns among care team members and patients to achieve effective patient‐centered communication.[4, 5] When members of the care team operate in this environment, patients often receive conflicting information regarding their plan of care, medications, and test results. Ineffective communication can lead to a suboptimal patient experience, additional costs, medical errors, and preventable adverse events.[6, 7, 8, 9, 10]
A critical first step to improving patient‐centered communication is identifying the care team.[11, 12] Accurate and reliable identification of all care team members is a pressing information need; it is fundamental to efficiently conveying information about the plan of care to those who know the patient the best, must make timely decisions, or will assume care once the patient leaves the hospital.[13] Furthermore, it has implications for engaging patients more meaningfully in their care.[14, 15, 16, 17] Ideally, the process of identifying an individual caring for the patient in a specific role is quickly and reliably determined from the electronic health record, the single source of truth where any provider can quickly identify other team members. This source of truth can be updated manually when individual members assign and remove themselves from the care team, or automatically when accessing the patient's record, writing a note, placing an order, or adding a patient to a coverage list. When providers correctly identify other team members in this way, hospital paging directories and secure messaging tools that link to the electronic health record become more effective at supporting care team communication.[18]
In general, the process of identifying care teams is difficult,[19] and maintaining role assignments in the electronic health record is equally challenging. Vawdrey et al. previously reported that care team lists are inaccurate and cannot be used to reliably identify other members at any given moment.[18] The inability to identify team members often leads to incorrectly routed pages, e‐mail messages, and phone calls.[20] Consequently, the potential to reliably manage the care team and improve electronic communication remains untapped, rendering team collaboration and care coordination less effective.[18, 21, 22]
In recent years, the trend toward restructuring inpatient teamsgeographical localization, structured communication interventions, teamwork training, and interdisciplinary roundswould seem to diminish the need for electronic care team identification, as those efforts have already made a positive impact with regard to interprofessional communication and collaboration, team satisfaction, and adverse events.[23, 24, 25, 26] Nonetheless, interdisciplinary teamwork, though critically important for patient‐centered communication, does not completely obviate the need for accurate and reliable care team identification.[26] Although care teams are statically located on units, the plan of care is dynamic; it evolves when the patient's status changes, when new information becomes available, and when key longitudinal providers (eg, primary care physician, subspecialty consultant) make recommendations. Thus, information conveyed as a team on rounds quickly becomes out of date, requiring additional forms of communication. Furthermore, due to frequent ad hoc coverage among team members, the identity of providers covering the patient at any given moment is often not clear.[27] This is particularly problematic for nonunit‐based providers who try to communicate with unit‐based care team members. These providers, in particular, have valuable knowledge and insight that can aid the primary team in decision making.[28, 29] However, they typically do not participate in rounds, often waste time identifying responsible providers,[20] and may communicate their recommendations directly with the patient without discussing with the primary team. These factors in part explain why geographic localization has shown limited improvement in shared understanding of the plan of care.[23]
From the perspective of patients and caregivers, identification of the providers entering and leaving their room is also challenging; only 11% to 51% of patients identify their providers correctly.[30] This adds to confusion regarding who is responsible for which aspects of the patient's care and can negatively affect the perception of the quality of care received.[31] Use of whiteboards has been shown to improve the proportion of patients who could identify key providers,[32] but these are not reliably updated and generally cannot accommodate all team members. When face cards are used, patients and caregivers report that they are more likely to identify their providers correctly.[14, 33, 34] However, potential confusion may ensue when another provider assumes care of the patient in the same role. Finally, use of technology to display team members at the bedside is typically a feature that patients like and can improve identification of care team members.[14, 15, 16] Yet, patient engagement technologies are not readily available in the hospital setting,[35] and ideally should be linked to the electronic health record, which again must be reliably updated.[11, 12, 15, 16]
If care team identification is so critical for delivering effective patient‐centered communication, why is maintaining role assignment problematic? At the individual level, reasons include discontinuity of the care team due to changing clinical rotations and intrateam coverage, shift‐based schedules, and lack of awareness and underutilization of functionality. Additionally, clinicians may have different ways to maintain lists of patients. At the institutional level, functionality to enforce role assignment when accessing patient records may be disabled (to avoid perceived burdens on clinical staff or nonclinical personnel who require access for administrative functions). Finally, electronic health record vendors currently have no incentive to adopt functionality that supports more effective care coordination across settings.[22]
However, more than technical solutions and policy changes are required; care team identification in the electronic health record requires a change in institutional culture. Maintaining an accurate relationship to each patient requires work without tangible benefitsthe benefits accrue only when everyone else identifies their role on the teama tragedy of the commons. This can be illustrated by our own experience. We conducted a quality improvement initiative (Table 1) as a part of 2 concurrent research initiatives that serve to promote patient‐centered communication:[12] PCORI (Patient‐Centered Outcomes Research Institute Transitions), the goal of which is to improve care transitions within the Partners' Pioneer Accountable Care Organization; and the PROSPECT (Promoting Respect and Ongoing Safety Through Patient‐Centeredness, Engagement, Communication, and Technology) project, an initiative funded by the Gordon and Betty Moore Foundation to eliminate preventable harms in the acute care environment.[29] Our goal was to electronically manage the care team with a high degree of fidelity. We enhanced a home‐grown application, which was developed to improve management of team lists for inpatient providers, accessible from our electronic health record, to facilitate role assignment. Specifically, we leveraged existing care processes (eg, nursing log‐on to the electronic medication administration system) to automatically assign certain providers to the care team at change of shift, added functionality to make it easy to assign a provider to all patients on a list for a defined period of time, and encouraged providers to assign their role by demonstrating benefits including quick access to patient‐specific group e‐mail and secure messaging tools (Table 1, Key Facets). The initiative was well‐received by most disciplines, but uptake was suboptimal. Our research assistants routinely assigned residents and others to the care team because our proactive attempts at advertising and reinforcing use of the application failed to reach a critical mass. Most did not see immediate benefits because it was an added step to their busy day, had other methods of managing team lists, and only saw benefit if everyone else participated. Key facets of our care team identification initiative, successes, and challenges are outlined in Table 1.
| Key Facets | Successes | Challenges |
|---|---|---|
| Linked electronic role assignment to administrative processes and clinical workflows | Leveraged existing processes to identify attending provider by routinely reviewing online schedules Linked role assignment to electronic medication administration system sign‐in process for nurses at the start of their shift |
Difficult to generate buy‐in from administrators and specific clinician groups to incorporate routine use of role assignment functionality into existing and/or new workflows No institutional policy mandating role assignments for members of extended care team |
| Incorporated default functionality to specify length of role assignment (eg, stop date) | Used by trainees (residents, fellows) to automate team list role assignments for a prespecified period of time according to online schedules | Underutilized by subspecialty consultants, many of who were unaware or did not fully appreciate the added value of this functionality Research assistants regularly verified that default role assignments were accurately maintained for trainees |
| Linked role assignment to patient‐specific group e‐mail and messaging tools | Clinicians acknowledged clear efficiency benefits (eg, automated patient identification within messages, correct routing of e‐mails) Used by specific members of the care team tasked with facilitating coordination of care (eg, nurse practitioner trained as discharge advocate for research study) |
Difficult to promote use of patient‐specific messaging, particularly for nonunit‐based providers (eg, consultants, primary care physicians) Required access to an application not typically used for clinical messaging Difficult to change culture of network e‐mail use for clinical messaging |
| Advertised new functionality and demonstrated potential efficiencies for care team communication | Unit‐based clinicians (hospitalists, nurses, housestaff) typically understood benefits when demonstrated and were easier to engage | Some nonunit‐based clinicians (eg, consulting attendings, primary care physicians) did not see benefits and/or were difficult to engage |
| Some nonunit‐based provider groups (eg, social workers, nutritionists, subspecialty fellows) considered the initiative worthwhile, and were open to learning about new functionality to improve communication | Clinicians had several options for managing team lists prior to implementation of new electronic health record | |
| Institutional effort toward implementing new electronic health record detracted from efforts at demonstrating enhanced functionality of existing applications |
There were a few glimmers of hope, however. On several PROSPECT units, we displayed team members on a tablet‐based patient portal so that patients would recognize their providers.[11, 17, 36] Similar to recent work by O'Leary et al.,[14] patients on PROSPECT units were able to correctly identify several care team members, but regularly asked why other providers (eg, consulting fellow) were not listed. Those providers asked the same question, and some eventually learned to assign their role via the application. As part of PROSEPCT, we visited other institutions and learned of an effort to display team members on high‐definition televisions in the patient's room. Several providers, wondering why they were not listed, learned to assign their role and their picture then appeared. Social pressure was the driving force.
Coincidently, we recently implemented a new electronic health record at our institution. Anecdotally, although no formal policy was established, many providers (eg, attendings, first responders, nurses, care coordinators, and other unit‐based providers) appear to be assigning their roles. Other providers (eg, dieticians, physical therapists, residents) also assign their role, but often fail to end role assignments upon completing their rotation or when the patient transfers to another service. Finally, even when actively involved, most subspecialists still do not designate their role. Despite these gaps and inconsistencies, we have made progress toward improving care team identification. The reasons for this progress are straightforward; during required training for the new electronic health record, all inpatient providers were taught to assign their role on the treatment team when assuming care of patients and now have 1 option for managing team lists. However, most providers were not trained to end their role assignments, and many have learned that role assignment is not required to access the patient's record; functionality to enforce this was disabled. Based on lessons learned from our experience,[12] we offer several strategies that hospitalists can employ to improve care team identification in the electronic health record (Table 2).
| Goal | Strategies to Achieve Goal |
|---|---|
| Identify and/or establish reliable processes that administrative staff can use to ensure accurate care team role assignments | Identify databases that serve as the source of truth for provider schedules and routinely access those databases |
| Access resident scheduling application (eg, Amion) that is routinely updated by training program staff | |
| Work with clinical and administrative staff to maintain care team role assignments | |
| Engage affiliated ambulatory practices to ensure patient's primary care physician is updated in the electronic health record | |
| Engage admissions office to improve reliability of attending assignments based on online clinical schedules when patients are admitted | |
| Integrate role assignment into established workflows for specific provider groups when administrative processes not feasible | Link routine care processes to care team role assignment |
| Train nurses, interns, physician assistants to assign role on care team when assuming care of patient at shift change | |
| Train residents, fellows to use default functionality to automatically assign their role on care team at the beginning of a clinical rotation | |
| Demonstrate value of maintaining role assignments in the electronic health record to the unit‐based care team | Emphasize how accurate and reliable care team role assignment can facilitate correct routing of information (eg, test results, discharge summaries) |
| Helps to maintain patient coverage lists (eg, fellows, consultants, social workers) | |
| Facilitates patient‐specific communication (eg, via group email and messaging tools linked to the electronic health record's care team functionality) | |
| Align with concurrent institutional initiatives that enforce or incentivize care team role assignment | Mandate role assignment when writing a note, placing an order, or adding a patient to a coverage list in the electronic health record |
| Provide patients and caregivers the ability to identify the care team via patient portalcreates social pressure for those providers who do not identify themselves on the care team | |
| Incentivize providers to maintain role assignments during patient's hospitalization in order to receive notifications if patients are readmitted | |
| Automate role assignments for all members of the care team whenever possible | Work with clinical informatics/emnformation system staff to determine feasibility of linking online scheduling systems or log‐in process to other systems routinely accessed by specific providers to automatically assign/unassign specific providers at the beginning/end of a shift (eg, nurses automatically assigned to care team when they access the electronic medication administration record system at beginning of shift) |
| Explore availability of default functionality to assign and unassign providers to and from the care team in a specific role by team, service, or unit‐based patient lists | |
| Require a stop time/date for role assignments or set a default if none entered |
In the future, care team identification in the electronic health record can be automated by integrating directly with electronic workflows, online scheduling applications, and provider directories. Hospitals could then leverage care team lists to facilitate patient‐centered communication via secure web‐based and mobile messaging applications configured to simultaneously update all team members (eg, group messaging apps, microblogs).[11, 37, 38] By synchronizing with the electronic health record, role assignments can be automatically updated via these applications, further increasing fidelity of care team identification.[12] Finally, as hospitals implement acute care patient portals, team lists can be leveraged to display all care team members correctly so that patients and caregivers can communicate more easily with providers.[17] The potential ramifications for patient‐centered communication are tremendous.
Disclosures
This work was funded by the Patient‐Centered Outcomes Research Institute and the Gordon and Betty Moore Foundation (GBMF3914). The authors report no conflicts of interest.
- . Patient‐centered communication. Annu Rev Nurs Res. 1999;17:85–104.
- , , , . Facilitating patient‐centered cancer communication: a road map. Patient Educ Couns. 2009;77:319–321.
- , . Patient‐Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering. NIH Publication No. 07–6225. Bethesda, MD: National Cancer Institute; 2007.
- . When conversation is better than computation. J Am Med Inform Assoc. 2000;7:277–286.
- . Communication systems in healthcare. Clin Biochem Rev. 2006;27:89–98.
- , , , et al. The nature of adverse events in hospitalized patients. results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324:377–384.
- , , , et al. A look into the nature and causes of human errors in the intensive care unit. Crit Care Med. 1995;23:294–300.
- , , . Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79:186–194.
- , . Interdisciplinary communication: an uncharted source of medical error? J Crit Care. 2006;21:236–242; discussion 242.
- , , . Quantifying the economic impact of communication inefficiencies in U.S. hospitals. J Healthc Manag. 2010;55:265–281; discussion 281–282.
- , , , . Transforming the acute care environment: a web‐based patient‐centered toolkit [abstract]. J Hosp Med. 2014;9(suppl 2):694.
- , , , et al. Creating a culture of patient‐centered care team communication at a large academic medical center [Abstract]. J Hosp Med. 2015;10 (suppl 2). Available at: http://www.shmabstracts.com/abstract/creating‐a‐culture‐of‐patient‐centered‐care‐team‐communication‐at‐a‐large‐academic‐medical‐center. Accessed April 24, 2015.
- , , , , . Perceived information needs and communication difficulties of inpatient physicians and nurses. Proc AMIA Symp. 2001:453–457.
- , , , , , . The effect of tablet computers with a mobile patient portal application on hospitalized patients' knowledge and activation [published online June 15, 2015]. J Am Med Inform Assoc. doi: 10.1093/jamia/ocv058.
- , , , , . Bedside information technology to support patient‐centered care. Int J Med Inform. 2012;81(7):442–451.
- , , , et al. Building and testing a patient‐centric electronic bedside communication center. J Gerontol Nurs. 2013;39:15–19.
- , , , et al. A web‐based, patient‐centered toolkit to engage patients and caregivers in the acute care setting: a preliminary evaluation [published online August 2, 2015]. J Am Med Inform Assoc. doi: 10.1093/jamia/ocv093.
- , , , et al. Awareness of the care team in electronic health records. Appl Clin Inform. 2011;2:395–405.
- , , , , , . Teamwork on inpatient medical units: assessing attitudes and barriers. Qual Saf Health Care. 2010;19:117–121.
- , , , et al. Frequency and clinical importance of pages sent to the wrong physician. Arch Intern Med. 2009;169:1072–1073.
- , . Patient experiences with coordination of care: the benefit of continuity and primary care physician as referral source. J Gen Intern Med. 2009;24:170–177.
- , , , , . Are electronic medical records helpful for care coordination? Experiences of physician practices. J Gen Intern Med. 2010;25:177–185.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24:1223–1227.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6:88–93.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171:678–684.
- , , , ; High Performance Teams and the Hospital of the Future Project Team. Interdisciplinary teamwork in hospitals: a review and practical recommendations for improvement. J Hosp Med. 2012;7:48–54.
- , , . Intrateam coverage is common, intrateam handoffs are not. J Hosp Med. 2014;9:734–736.
- . A primary care physician's ideal transitions of care? Where's the evidence? J Hosp Med. 2013;8:472–477.
- , , , , . Primary care physician communication at hospital discharge reduces medication discrepancies. J Hosp Med. 2013;8:672–677.
- , . Let's “face” it: time to introduce yourself to patients. J Hosp Med. 2014;9:199–200.
- , , . Patient perceptions of coordinated care: the importance of organized communication in hospitals. J Healthc Qual. 1999;21:18–23.
- , , , . Patient whiteboards to improve patient‐centred care in the hospital. Postgrad Med J. 2013;89:604–609.
- , , , , , . Effect of a face sheet tool on medical team provider identification and family satisfaction. J Hosp Med. 2014;9:186–188.
- , , , , , . The impact of facecards on patients' knowledge, satisfaction, trust, and agreement with hospital physicians: a pilot study. J Hosp Med. 2014;9:137–141.
- , , , et al. Patient engagement in the inpatient setting: a systematic review. J Am Med Inform Assoc. 2014;21:742–750.
- PROSPECT: Promoting Respect and Ongoing Safety Through Patient‐centeredness, Engagement, Communication, and Technology. Available at: http://www.partners.org/cird/PROSPECT/Index.htm. Accessed May 3, 2015.
- , , , , , . Smarter hospital communication: secure smartphone text messaging improves provider satisfaction and perception of efficacy, workflow. J Hosp Med. 2014;9:573–578.
- , , , et al. Engaging patients, providers, and institutional stakeholders in developing a patient‐centered microblog. Paper presented at: Proceeding of the American Medical Informatics Association Annual Fall Symposium; November 16–19, 2014; Washington, DC.
- . Patient‐centered communication. Annu Rev Nurs Res. 1999;17:85–104.
- , , , . Facilitating patient‐centered cancer communication: a road map. Patient Educ Couns. 2009;77:319–321.
- , . Patient‐Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering. NIH Publication No. 07–6225. Bethesda, MD: National Cancer Institute; 2007.
- . When conversation is better than computation. J Am Med Inform Assoc. 2000;7:277–286.
- . Communication systems in healthcare. Clin Biochem Rev. 2006;27:89–98.
- , , , et al. The nature of adverse events in hospitalized patients. results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324:377–384.
- , , , et al. A look into the nature and causes of human errors in the intensive care unit. Crit Care Med. 1995;23:294–300.
- , , . Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79:186–194.
- , . Interdisciplinary communication: an uncharted source of medical error? J Crit Care. 2006;21:236–242; discussion 242.
- , , . Quantifying the economic impact of communication inefficiencies in U.S. hospitals. J Healthc Manag. 2010;55:265–281; discussion 281–282.
- , , , . Transforming the acute care environment: a web‐based patient‐centered toolkit [abstract]. J Hosp Med. 2014;9(suppl 2):694.
- , , , et al. Creating a culture of patient‐centered care team communication at a large academic medical center [Abstract]. J Hosp Med. 2015;10 (suppl 2). Available at: http://www.shmabstracts.com/abstract/creating‐a‐culture‐of‐patient‐centered‐care‐team‐communication‐at‐a‐large‐academic‐medical‐center. Accessed April 24, 2015.
- , , , , . Perceived information needs and communication difficulties of inpatient physicians and nurses. Proc AMIA Symp. 2001:453–457.
- , , , , , . The effect of tablet computers with a mobile patient portal application on hospitalized patients' knowledge and activation [published online June 15, 2015]. J Am Med Inform Assoc. doi: 10.1093/jamia/ocv058.
- , , , , . Bedside information technology to support patient‐centered care. Int J Med Inform. 2012;81(7):442–451.
- , , , et al. Building and testing a patient‐centric electronic bedside communication center. J Gerontol Nurs. 2013;39:15–19.
- , , , et al. A web‐based, patient‐centered toolkit to engage patients and caregivers in the acute care setting: a preliminary evaluation [published online August 2, 2015]. J Am Med Inform Assoc. doi: 10.1093/jamia/ocv093.
- , , , et al. Awareness of the care team in electronic health records. Appl Clin Inform. 2011;2:395–405.
- , , , , , . Teamwork on inpatient medical units: assessing attitudes and barriers. Qual Saf Health Care. 2010;19:117–121.
- , , , et al. Frequency and clinical importance of pages sent to the wrong physician. Arch Intern Med. 2009;169:1072–1073.
- , . Patient experiences with coordination of care: the benefit of continuity and primary care physician as referral source. J Gen Intern Med. 2009;24:170–177.
- , , , , . Are electronic medical records helpful for care coordination? Experiences of physician practices. J Gen Intern Med. 2010;25:177–185.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24:1223–1227.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6:88–93.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171:678–684.
- , , , ; High Performance Teams and the Hospital of the Future Project Team. Interdisciplinary teamwork in hospitals: a review and practical recommendations for improvement. J Hosp Med. 2012;7:48–54.
- , , . Intrateam coverage is common, intrateam handoffs are not. J Hosp Med. 2014;9:734–736.
- . A primary care physician's ideal transitions of care? Where's the evidence? J Hosp Med. 2013;8:472–477.
- , , , , . Primary care physician communication at hospital discharge reduces medication discrepancies. J Hosp Med. 2013;8:672–677.
- , . Let's “face” it: time to introduce yourself to patients. J Hosp Med. 2014;9:199–200.
- , , . Patient perceptions of coordinated care: the importance of organized communication in hospitals. J Healthc Qual. 1999;21:18–23.
- , , , . Patient whiteboards to improve patient‐centred care in the hospital. Postgrad Med J. 2013;89:604–609.
- , , , , , . Effect of a face sheet tool on medical team provider identification and family satisfaction. J Hosp Med. 2014;9:186–188.
- , , , , , . The impact of facecards on patients' knowledge, satisfaction, trust, and agreement with hospital physicians: a pilot study. J Hosp Med. 2014;9:137–141.
- , , , et al. Patient engagement in the inpatient setting: a systematic review. J Am Med Inform Assoc. 2014;21:742–750.
- PROSPECT: Promoting Respect and Ongoing Safety Through Patient‐centeredness, Engagement, Communication, and Technology. Available at: http://www.partners.org/cird/PROSPECT/Index.htm. Accessed May 3, 2015.
- , , , , , . Smarter hospital communication: secure smartphone text messaging improves provider satisfaction and perception of efficacy, workflow. J Hosp Med. 2014;9:573–578.
- , , , et al. Engaging patients, providers, and institutional stakeholders in developing a patient‐centered microblog. Paper presented at: Proceeding of the American Medical Informatics Association Annual Fall Symposium; November 16–19, 2014; Washington, DC.
Actionability of TPAD Results
Effective communication between inpatient and primary care physicians (PCPs) is essential for safe, high‐quality transitions. Unfortunately, PCPs are often not meaningfully engaged in this process; communication is frequently challenging or nonexistent.[1, 2] Instead, information is suboptimally conveyed via lengthy, disorganized discharge summaries.[3] Consequently, timely knowledge is not transferred to PCPs, who instead must seek out and identify actionable information themselves. These deficiencies can lead to misinterpretation of information and patient harm.[4]
An important component of ideal transitions[5] is timely communication of results of tests pending at discharge (TPADs). TPADs are variably documented in discharge summaries, and physician awareness about them is strikingly poor.[3, 6, 7] Communication about TPADs should convey rationales for ordering tests and necessary actions to take in response to finalized results. Most often, this knowledge resides with the inpatient team.
Health information technology (HIT) is an effective strategy for improving test‐result management. We implemented an automated system that notifies inpatient attendings and PCPs of TPAD results via email and demonstrated increased awareness by these physicians at the time of required action.[8, 9] Nevertheless, without timely knowledge transfer, attendings and PCPs may have differing opinions regarding which TPAD results require action. We conducted a secondary analysis of survey respondents from our original clustered randomized controlled trial to measure the degree of agreement between inpatient and ambulatory physicians regarding actionability of TPAD results.
METHODS
The methods of our original study are described elsewhere.[9] In that study, the attending and PCP of each patient were independently surveyed (via email and then by fax if the electronic survey was not completed) to determine their awareness of finalized TPAD results, and to identify actionable results and the types of actions taken (or that would need to be taken). Discharge summaries were available in our electronic medical record (EMR) within 24 hours of discharge. Network physicians (affiliated with Partners HealthCare, Inc.) had access to all components of the EMR, including the discharge summary and test results. Non‐network PCPs were faxed discharge summaries within 48 hours of discharge per institutional policies. For this study, we identified all patients for whom the attending and PCP completed the survey and answered questions about TPAD actionability. We then compared the identified TPADs listed by the attending and PCP in that survey.
RESULTS
We enrolled 441 patients in our original study. We sent 441 surveys to 117 attendings and 353 surveys to 273 PCPs. Eighty‐eight patients did not have an identified PCP. We received 275 responses from 83 attendings (62% response rate), and 152 responses from 112 PCPs (43% response rate). Patient and physician characteristics are reported elsewhere.[9]
For this analysis, we identified the 98 patients (aged 6018 years, 44 male, 52 Caucasian, 46 non‐Caucasian, 85 network, 13 non‐network) cared for by 46 attendings (aged 4411 years, 33 male, 22 hospitalists, 24 nonhospitalists) and 79 PCPs (aged 4512.5, 33 male, 66 network, 13 non‐network) for whom we received completed surveys from both physicians. For 59 patients, both thought none of the TPAD results were actionable. For 12 patients, both thought at least 1 was actionable, and they identified the same actionable TPAD result for all 12. Overall, attendings and PCPs agreed on actionability in 72.5% (71/98) (Kappa 0.29, 95% confidence interval: 0.09‐0.50). Table 1 shows the type of action taken by responsible providers. There were 9 patients (9%) for whom the attending alone thought at least 1 TPAD result was actionable; of these, subsequent attending‐initiated communication occurred in 77.8% (7/9). There were 18 patients (18%) for whom the PCP alone thought at least 1 TPAD result was actionable; of these, subsequent PCP‐initiated communication occurred in 77.8% (14/18). Table 2 shows concordance of actionable TPAD by type. In instances of disagreement, the attending frequently reported microbiology TPADs (eg, culture data, viral serologies) as actionable, whereas the PCP reported all TPAD types (eg, culture data, colon biopsy, vitamin D, magnetic resonance imaging) as actionable.
| Inpatient Attending‐Initiated Action(s)a | PCP‐Initiated Action(s)a | |
|---|---|---|
| ||
| Patient was notifiedb | 11.1% (1/9) | 66.7% (12/18) |
| Subspecialist was contacted | 33.3% (3/9) | 16.7% (3/18) |
| PCP or inpatient team contacted | 33.3% (3/9) | 16.7% (3/18) |
| Further testing/modified treatment | 11.1% (1/9) | 33.3% (6/18) |
| Referred to ambulatory visit/emergency room | 0% (0/9) | 11.1% (2/18) |
| Documentation | 11.1% (1/9) | 16.7% (3/18) |
| Type of TPAD | Attending and PCP Agreed on Identity of Actionable TPADa | Attending and PCP Disagreed on Identity of Actionable TPADa | ||
|---|---|---|---|---|
| TPAD Identified | No TPAD Identified, n=59 | TPAD Identified by Attending Only | TPAD Identified by PCP Only | |
| ||||
| Microbiologyb | 25% (3/12) | N/A | 56% (5/9) | 17% (3/18) |
| Pathologyc | 17% (2/12) | N/A | 0% (0/9) | 17% (3/18) |
| Chemistry and hematologyd | 58% (7/12) | N/A | 11% (1/9) | 22% (4/18) |
| Radiologye | 0% (0/12) | N/A | 11% (1/9) | 39% (5/18) |
| Unclassified (left blank) | 0% (0/12) | N/A | 22% (1/9) | 17% (3/18) |
DISCUSSION
We found fair agreement between attendings and PCPs regarding actionability of TPAD results. In 27 patients (27.5%), either the attending or PCP considered TPAD results actionable when the other did not. Possible explanations for this include different thresholds for taking action (eg, inpatient physicians may view vitamin D levels as acceptable within broader ranges than PCPs, and PCPs may view negative results as actionable if they need to contact the patient whereas attendings may not), varying clinical context (eg, rationale for why microbiology culture data is actionable), and varying practices for escalating care (eg, referring patients back to the hospital).
Our study was limited by small sample size and low PCP response rate. Nonetheless, the findings suggest that poor concordance between inpatient and ambulatory physicians will persist without tools that promote more effective communication. Greater awareness alone may be insufficient to mitigate consequences of missed TPAD results if physicians are not on the same page regarding which results require action.
To better engage PCPs, healthcare systems require HIT infrastructure that facilitates seamless care team communication across care settings.[2] When optimally configured, HIT can facilitate greater PCP involvement in postdischarge communication. For example, our system promoted subsequent postdischarge communication in 78% of initial discordance in TPAD actionability; however, most of it was not between the attending and the PCP. Thus, improvements could be made to facilitate more effective communication among key inpatient and ambulatory providers. Furthermore, when configured to facilitate conversation among these providers regarding the discharge care plan throughout a patient's entire hospital course, HIT can promote effective knowledge transfer by virtue of adding clinical context to test ordering and follow‐up. Additional work is needed to understand whether such communication clarifies contingencies and facilitates appropriate postdischarge action. Nevertheless, current electronic solutions (eg, passive placement into results in‐baskets) will likely be ineffective because they do not reliably improve awareness and active communication about context, rationale, interpretation, suggested action, or transfer of responsibility.
In summary, discrepancies in TPAD actionability by inpatient and ambulatory providers still exist, even when awareness of TPAD results is improved by HIT. By fostering more effective communication among key care‐team members across care settings, HIT could mitigate the consequences of suboptimal care transitions. With regard to TPAD results, this may favorably impact unnecessary testing, diagnostic and therapeutic delays, and medical errors.
Disclosures: This article is based on research funded through AHRQ grant #R21HS018229; the authors have no other disclosures or conflicts or interest.
- , , , et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5:385–391.
- . A primary care physician's ideal transitions of care—where's the evidence? J Hosp Med. 2013;8(8):472–477.
- , , , et al. Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138:161–167.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2012;8(2):102–109.
- , , , et al. Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow‐up providers. J Gen Intern Med. 2009;24(9):1002–1006.
- , , , et al. Patient safety concerns rising from test results that return after hospital discharge. Ann Intern Med. 2005;143:121–128.
- , , , et al. Design and implementation of an automated email notification system for results of tests pending at discharge. J Am Med Inform Assoc. 2012;19(4):523–528.
- , , , et al. Impact of an automated email notification system for results of rest pending at discharge: a cluster‐randomized controlled trial [published online ahead of print October 23, 2013]. J Am Med Inform Assoc. doi:10.1136/amiajnl‐2013‐002030.
Effective communication between inpatient and primary care physicians (PCPs) is essential for safe, high‐quality transitions. Unfortunately, PCPs are often not meaningfully engaged in this process; communication is frequently challenging or nonexistent.[1, 2] Instead, information is suboptimally conveyed via lengthy, disorganized discharge summaries.[3] Consequently, timely knowledge is not transferred to PCPs, who instead must seek out and identify actionable information themselves. These deficiencies can lead to misinterpretation of information and patient harm.[4]
An important component of ideal transitions[5] is timely communication of results of tests pending at discharge (TPADs). TPADs are variably documented in discharge summaries, and physician awareness about them is strikingly poor.[3, 6, 7] Communication about TPADs should convey rationales for ordering tests and necessary actions to take in response to finalized results. Most often, this knowledge resides with the inpatient team.
Health information technology (HIT) is an effective strategy for improving test‐result management. We implemented an automated system that notifies inpatient attendings and PCPs of TPAD results via email and demonstrated increased awareness by these physicians at the time of required action.[8, 9] Nevertheless, without timely knowledge transfer, attendings and PCPs may have differing opinions regarding which TPAD results require action. We conducted a secondary analysis of survey respondents from our original clustered randomized controlled trial to measure the degree of agreement between inpatient and ambulatory physicians regarding actionability of TPAD results.
METHODS
The methods of our original study are described elsewhere.[9] In that study, the attending and PCP of each patient were independently surveyed (via email and then by fax if the electronic survey was not completed) to determine their awareness of finalized TPAD results, and to identify actionable results and the types of actions taken (or that would need to be taken). Discharge summaries were available in our electronic medical record (EMR) within 24 hours of discharge. Network physicians (affiliated with Partners HealthCare, Inc.) had access to all components of the EMR, including the discharge summary and test results. Non‐network PCPs were faxed discharge summaries within 48 hours of discharge per institutional policies. For this study, we identified all patients for whom the attending and PCP completed the survey and answered questions about TPAD actionability. We then compared the identified TPADs listed by the attending and PCP in that survey.
RESULTS
We enrolled 441 patients in our original study. We sent 441 surveys to 117 attendings and 353 surveys to 273 PCPs. Eighty‐eight patients did not have an identified PCP. We received 275 responses from 83 attendings (62% response rate), and 152 responses from 112 PCPs (43% response rate). Patient and physician characteristics are reported elsewhere.[9]
For this analysis, we identified the 98 patients (aged 6018 years, 44 male, 52 Caucasian, 46 non‐Caucasian, 85 network, 13 non‐network) cared for by 46 attendings (aged 4411 years, 33 male, 22 hospitalists, 24 nonhospitalists) and 79 PCPs (aged 4512.5, 33 male, 66 network, 13 non‐network) for whom we received completed surveys from both physicians. For 59 patients, both thought none of the TPAD results were actionable. For 12 patients, both thought at least 1 was actionable, and they identified the same actionable TPAD result for all 12. Overall, attendings and PCPs agreed on actionability in 72.5% (71/98) (Kappa 0.29, 95% confidence interval: 0.09‐0.50). Table 1 shows the type of action taken by responsible providers. There were 9 patients (9%) for whom the attending alone thought at least 1 TPAD result was actionable; of these, subsequent attending‐initiated communication occurred in 77.8% (7/9). There were 18 patients (18%) for whom the PCP alone thought at least 1 TPAD result was actionable; of these, subsequent PCP‐initiated communication occurred in 77.8% (14/18). Table 2 shows concordance of actionable TPAD by type. In instances of disagreement, the attending frequently reported microbiology TPADs (eg, culture data, viral serologies) as actionable, whereas the PCP reported all TPAD types (eg, culture data, colon biopsy, vitamin D, magnetic resonance imaging) as actionable.
| Inpatient Attending‐Initiated Action(s)a | PCP‐Initiated Action(s)a | |
|---|---|---|
| ||
| Patient was notifiedb | 11.1% (1/9) | 66.7% (12/18) |
| Subspecialist was contacted | 33.3% (3/9) | 16.7% (3/18) |
| PCP or inpatient team contacted | 33.3% (3/9) | 16.7% (3/18) |
| Further testing/modified treatment | 11.1% (1/9) | 33.3% (6/18) |
| Referred to ambulatory visit/emergency room | 0% (0/9) | 11.1% (2/18) |
| Documentation | 11.1% (1/9) | 16.7% (3/18) |
| Type of TPAD | Attending and PCP Agreed on Identity of Actionable TPADa | Attending and PCP Disagreed on Identity of Actionable TPADa | ||
|---|---|---|---|---|
| TPAD Identified | No TPAD Identified, n=59 | TPAD Identified by Attending Only | TPAD Identified by PCP Only | |
| ||||
| Microbiologyb | 25% (3/12) | N/A | 56% (5/9) | 17% (3/18) |
| Pathologyc | 17% (2/12) | N/A | 0% (0/9) | 17% (3/18) |
| Chemistry and hematologyd | 58% (7/12) | N/A | 11% (1/9) | 22% (4/18) |
| Radiologye | 0% (0/12) | N/A | 11% (1/9) | 39% (5/18) |
| Unclassified (left blank) | 0% (0/12) | N/A | 22% (1/9) | 17% (3/18) |
DISCUSSION
We found fair agreement between attendings and PCPs regarding actionability of TPAD results. In 27 patients (27.5%), either the attending or PCP considered TPAD results actionable when the other did not. Possible explanations for this include different thresholds for taking action (eg, inpatient physicians may view vitamin D levels as acceptable within broader ranges than PCPs, and PCPs may view negative results as actionable if they need to contact the patient whereas attendings may not), varying clinical context (eg, rationale for why microbiology culture data is actionable), and varying practices for escalating care (eg, referring patients back to the hospital).
Our study was limited by small sample size and low PCP response rate. Nonetheless, the findings suggest that poor concordance between inpatient and ambulatory physicians will persist without tools that promote more effective communication. Greater awareness alone may be insufficient to mitigate consequences of missed TPAD results if physicians are not on the same page regarding which results require action.
To better engage PCPs, healthcare systems require HIT infrastructure that facilitates seamless care team communication across care settings.[2] When optimally configured, HIT can facilitate greater PCP involvement in postdischarge communication. For example, our system promoted subsequent postdischarge communication in 78% of initial discordance in TPAD actionability; however, most of it was not between the attending and the PCP. Thus, improvements could be made to facilitate more effective communication among key inpatient and ambulatory providers. Furthermore, when configured to facilitate conversation among these providers regarding the discharge care plan throughout a patient's entire hospital course, HIT can promote effective knowledge transfer by virtue of adding clinical context to test ordering and follow‐up. Additional work is needed to understand whether such communication clarifies contingencies and facilitates appropriate postdischarge action. Nevertheless, current electronic solutions (eg, passive placement into results in‐baskets) will likely be ineffective because they do not reliably improve awareness and active communication about context, rationale, interpretation, suggested action, or transfer of responsibility.
In summary, discrepancies in TPAD actionability by inpatient and ambulatory providers still exist, even when awareness of TPAD results is improved by HIT. By fostering more effective communication among key care‐team members across care settings, HIT could mitigate the consequences of suboptimal care transitions. With regard to TPAD results, this may favorably impact unnecessary testing, diagnostic and therapeutic delays, and medical errors.
Disclosures: This article is based on research funded through AHRQ grant #R21HS018229; the authors have no other disclosures or conflicts or interest.
Effective communication between inpatient and primary care physicians (PCPs) is essential for safe, high‐quality transitions. Unfortunately, PCPs are often not meaningfully engaged in this process; communication is frequently challenging or nonexistent.[1, 2] Instead, information is suboptimally conveyed via lengthy, disorganized discharge summaries.[3] Consequently, timely knowledge is not transferred to PCPs, who instead must seek out and identify actionable information themselves. These deficiencies can lead to misinterpretation of information and patient harm.[4]
An important component of ideal transitions[5] is timely communication of results of tests pending at discharge (TPADs). TPADs are variably documented in discharge summaries, and physician awareness about them is strikingly poor.[3, 6, 7] Communication about TPADs should convey rationales for ordering tests and necessary actions to take in response to finalized results. Most often, this knowledge resides with the inpatient team.
Health information technology (HIT) is an effective strategy for improving test‐result management. We implemented an automated system that notifies inpatient attendings and PCPs of TPAD results via email and demonstrated increased awareness by these physicians at the time of required action.[8, 9] Nevertheless, without timely knowledge transfer, attendings and PCPs may have differing opinions regarding which TPAD results require action. We conducted a secondary analysis of survey respondents from our original clustered randomized controlled trial to measure the degree of agreement between inpatient and ambulatory physicians regarding actionability of TPAD results.
METHODS
The methods of our original study are described elsewhere.[9] In that study, the attending and PCP of each patient were independently surveyed (via email and then by fax if the electronic survey was not completed) to determine their awareness of finalized TPAD results, and to identify actionable results and the types of actions taken (or that would need to be taken). Discharge summaries were available in our electronic medical record (EMR) within 24 hours of discharge. Network physicians (affiliated with Partners HealthCare, Inc.) had access to all components of the EMR, including the discharge summary and test results. Non‐network PCPs were faxed discharge summaries within 48 hours of discharge per institutional policies. For this study, we identified all patients for whom the attending and PCP completed the survey and answered questions about TPAD actionability. We then compared the identified TPADs listed by the attending and PCP in that survey.
RESULTS
We enrolled 441 patients in our original study. We sent 441 surveys to 117 attendings and 353 surveys to 273 PCPs. Eighty‐eight patients did not have an identified PCP. We received 275 responses from 83 attendings (62% response rate), and 152 responses from 112 PCPs (43% response rate). Patient and physician characteristics are reported elsewhere.[9]
For this analysis, we identified the 98 patients (aged 6018 years, 44 male, 52 Caucasian, 46 non‐Caucasian, 85 network, 13 non‐network) cared for by 46 attendings (aged 4411 years, 33 male, 22 hospitalists, 24 nonhospitalists) and 79 PCPs (aged 4512.5, 33 male, 66 network, 13 non‐network) for whom we received completed surveys from both physicians. For 59 patients, both thought none of the TPAD results were actionable. For 12 patients, both thought at least 1 was actionable, and they identified the same actionable TPAD result for all 12. Overall, attendings and PCPs agreed on actionability in 72.5% (71/98) (Kappa 0.29, 95% confidence interval: 0.09‐0.50). Table 1 shows the type of action taken by responsible providers. There were 9 patients (9%) for whom the attending alone thought at least 1 TPAD result was actionable; of these, subsequent attending‐initiated communication occurred in 77.8% (7/9). There were 18 patients (18%) for whom the PCP alone thought at least 1 TPAD result was actionable; of these, subsequent PCP‐initiated communication occurred in 77.8% (14/18). Table 2 shows concordance of actionable TPAD by type. In instances of disagreement, the attending frequently reported microbiology TPADs (eg, culture data, viral serologies) as actionable, whereas the PCP reported all TPAD types (eg, culture data, colon biopsy, vitamin D, magnetic resonance imaging) as actionable.
| Inpatient Attending‐Initiated Action(s)a | PCP‐Initiated Action(s)a | |
|---|---|---|
| ||
| Patient was notifiedb | 11.1% (1/9) | 66.7% (12/18) |
| Subspecialist was contacted | 33.3% (3/9) | 16.7% (3/18) |
| PCP or inpatient team contacted | 33.3% (3/9) | 16.7% (3/18) |
| Further testing/modified treatment | 11.1% (1/9) | 33.3% (6/18) |
| Referred to ambulatory visit/emergency room | 0% (0/9) | 11.1% (2/18) |
| Documentation | 11.1% (1/9) | 16.7% (3/18) |
| Type of TPAD | Attending and PCP Agreed on Identity of Actionable TPADa | Attending and PCP Disagreed on Identity of Actionable TPADa | ||
|---|---|---|---|---|
| TPAD Identified | No TPAD Identified, n=59 | TPAD Identified by Attending Only | TPAD Identified by PCP Only | |
| ||||
| Microbiologyb | 25% (3/12) | N/A | 56% (5/9) | 17% (3/18) |
| Pathologyc | 17% (2/12) | N/A | 0% (0/9) | 17% (3/18) |
| Chemistry and hematologyd | 58% (7/12) | N/A | 11% (1/9) | 22% (4/18) |
| Radiologye | 0% (0/12) | N/A | 11% (1/9) | 39% (5/18) |
| Unclassified (left blank) | 0% (0/12) | N/A | 22% (1/9) | 17% (3/18) |
DISCUSSION
We found fair agreement between attendings and PCPs regarding actionability of TPAD results. In 27 patients (27.5%), either the attending or PCP considered TPAD results actionable when the other did not. Possible explanations for this include different thresholds for taking action (eg, inpatient physicians may view vitamin D levels as acceptable within broader ranges than PCPs, and PCPs may view negative results as actionable if they need to contact the patient whereas attendings may not), varying clinical context (eg, rationale for why microbiology culture data is actionable), and varying practices for escalating care (eg, referring patients back to the hospital).
Our study was limited by small sample size and low PCP response rate. Nonetheless, the findings suggest that poor concordance between inpatient and ambulatory physicians will persist without tools that promote more effective communication. Greater awareness alone may be insufficient to mitigate consequences of missed TPAD results if physicians are not on the same page regarding which results require action.
To better engage PCPs, healthcare systems require HIT infrastructure that facilitates seamless care team communication across care settings.[2] When optimally configured, HIT can facilitate greater PCP involvement in postdischarge communication. For example, our system promoted subsequent postdischarge communication in 78% of initial discordance in TPAD actionability; however, most of it was not between the attending and the PCP. Thus, improvements could be made to facilitate more effective communication among key inpatient and ambulatory providers. Furthermore, when configured to facilitate conversation among these providers regarding the discharge care plan throughout a patient's entire hospital course, HIT can promote effective knowledge transfer by virtue of adding clinical context to test ordering and follow‐up. Additional work is needed to understand whether such communication clarifies contingencies and facilitates appropriate postdischarge action. Nevertheless, current electronic solutions (eg, passive placement into results in‐baskets) will likely be ineffective because they do not reliably improve awareness and active communication about context, rationale, interpretation, suggested action, or transfer of responsibility.
In summary, discrepancies in TPAD actionability by inpatient and ambulatory providers still exist, even when awareness of TPAD results is improved by HIT. By fostering more effective communication among key care‐team members across care settings, HIT could mitigate the consequences of suboptimal care transitions. With regard to TPAD results, this may favorably impact unnecessary testing, diagnostic and therapeutic delays, and medical errors.
Disclosures: This article is based on research funded through AHRQ grant #R21HS018229; the authors have no other disclosures or conflicts or interest.
- , , , et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5:385–391.
- . A primary care physician's ideal transitions of care—where's the evidence? J Hosp Med. 2013;8(8):472–477.
- , , , et al. Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138:161–167.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2012;8(2):102–109.
- , , , et al. Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow‐up providers. J Gen Intern Med. 2009;24(9):1002–1006.
- , , , et al. Patient safety concerns rising from test results that return after hospital discharge. Ann Intern Med. 2005;143:121–128.
- , , , et al. Design and implementation of an automated email notification system for results of tests pending at discharge. J Am Med Inform Assoc. 2012;19(4):523–528.
- , , , et al. Impact of an automated email notification system for results of rest pending at discharge: a cluster‐randomized controlled trial [published online ahead of print October 23, 2013]. J Am Med Inform Assoc. doi:10.1136/amiajnl‐2013‐002030.
- , , , et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5:385–391.
- . A primary care physician's ideal transitions of care—where's the evidence? J Hosp Med. 2013;8(8):472–477.
- , , , et al. Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138:161–167.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2012;8(2):102–109.
- , , , et al. Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow‐up providers. J Gen Intern Med. 2009;24(9):1002–1006.
- , , , et al. Patient safety concerns rising from test results that return after hospital discharge. Ann Intern Med. 2005;143:121–128.
- , , , et al. Design and implementation of an automated email notification system for results of tests pending at discharge. J Am Med Inform Assoc. 2012;19(4):523–528.
- , , , et al. Impact of an automated email notification system for results of rest pending at discharge: a cluster‐randomized controlled trial [published online ahead of print October 23, 2013]. J Am Med Inform Assoc. doi:10.1136/amiajnl‐2013‐002030.
Continuing Medical Education Program in
If you wish to receive credit for this activity, which beginson the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to
www.blackwellpublishing.com/cme . -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which beginson the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to
www.blackwellpublishing.com/cme . -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which beginson the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to
www.blackwellpublishing.com/cme . -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
Pending Tests at Discharge
The period following discharge is a vulnerable time for patientsthe prevalence of medical errors related to this transition is high and has important patient safety and medico‐legal ramifications.13 Factors contributing to this vulnerability include complexity of hospitalized patients, shorter lengths of stay, and increased discontinuity of care. Hospitalists have recognized this threat to patient safety and have worked toward improving information exchange between inpatient and outpatient providers at hospital discharge.46 Nonetheless, the evidence suggests that more work is necessary. A recent study found that discharge summaries are often incomplete, and do not contain important information requiring follow‐up, such as pending tests.7 Additionally, a review by Kripalani et al. characterizing information deficits at hospital discharge found few interventions which specifically improve communication of pending tests at hospital discharge.8
In a prior study we determined that 41% of patients left the hospital before all laboratory and radiology test results were finalized. Of these results, 9.4% were potentially actionable and could have altered management. Physicians were aware of only 38% of post‐discharge test results.9 This awareness gap is a consequence of several factors including the lack of systems to track and alert providers of test results finalized post discharge. Also, it is unclear who is responsible for pending tests at discharge, since these tests are ordered by the inpatient physicians but often reported in the time period between hospital discharge and the patient's first follow‐up appointment with the primary care physician (PCP). Because responsibility is not explicitly made in the final communication between physicians at discharge, such test results may not be reviewed in a timely manner, potentially resulting in delays in treatment, a need for readmission, or other unfavorable outcomes.
Even in integrated health systems with advanced electronic health records, missed test results which result in treatment delays remain prevalent.10, 11 Test result management applications aid clinicians in reviewing and acting upon results as they become available and such systems may provide solutions to this problem. At Partners Healthcare in Boston, the Results Manager (RM) application was developed to help clinicians in the ambulatory setting safely, reliably, and efficiently review and act upon test results. The application enables clinicians to prioritize test results, utilize guidelines, and generate letters to patients. This system also prompts physicians to set reminders for future testing.12 In a 2.5‐year study evaluating the impact of this intervention, PCPs at 26 adult primary care practices were able to expedite communication of outpatient laboratory and imaging test results to patients with the help of RM. Patients of physicians who participated in the project reported greater satisfaction with test result communication and with information provided about their condition than did a control group of similar patients.13 RM has not yet been studied in the inpatient setting or at care transitions. We describe an attempt at modifying the Partners RM application to help inpatient physicians manage pending tests at hospital discharge.
Methods
Study Setting and Participants
We piloted our application at 2 major academic medical centers (hospitals A and B) associated with Partners Healthcare, an integrated regional health delivery network in eastern Massachusetts, from October 2004 to March 2005. Both centers use the longitudinal medical record (LMR), the electronic medical record (EMR), for nearly all ambulatory practices. The LMR is an internally developed full‐featured EMR, including a repository of laboratory and radiology reports, discharge summaries, ambulatory care notes, medication lists, problem lists, coded allergies, and other patient data. Both centers also have their own inpatient results viewing and order entry systems which provide clinicians caring for patients in the hospital the ability to review results and write orders. Although possible, clinicians caring for patients in the inpatient setting do not routinely access LMR to view test results. Inpatient physician use of the LMR is generally limited to review of the outpatient record, medication lists, and ambulatory notes at admission.
At hospital A, the hospitalist attending physician is typically responsible for all communication to outpatient physicians at discharge, as well as for follow‐up on all test results that return after discharge. Hospital B has 2 types of hospitalist services. One is staffed only by hospitalist and nonhospitalist attending physicians. Nonhospitalist attending physicians were excluded because they care for their own patients in the inpatient and ambulatory setting and typically use RM to manage test results. The other hospitalist service at hospital B is a teaching service consisting of an attending physician, resident, and interns. For this service, the resident is responsible for communication at discharge and follow‐up on all pending tests. For purposes of this study inpatient physicians refers to those physicians responsible for communication with PCPs and follow‐up on pending tests. All inpatient physicians were eligible to participate during the study period.
Test Result Management Application
RM was originally developed by Partners Healthcare to improve timely review and appropriate management of test results in the ambulatory setting. RM was developed for and vetted by primarily ambulatory physicians. The application is browser‐based, provider‐centric, and embedded in the LMR to help ambulatory clinicians review and act upon test results in a safe, reliable, and efficient manner. Although RM has access to all inpatient and outpatient data in the Partners Clinical Data Repository (CDR), given the volume of inpatient tests ordered, hospital‐based results are suppressed by default to limit inundating ambulatory clinicians' queues. Therefore, users of RM only receive results of laboratory and radiology tests ordered in the ambulatory setting. They can track these tests for specific patients for a designated period of time by placing the patient on a watch list. Finally, RM incorporates extensive decision support features to classify the degree of abnormality for each result, presents guidelines to help clinicians manage abnormal results, allows clinicians to generate result letters to patients using predefined, context‐sensitive templates, and prompts physicians to set reminders for future testing. Because RM was developed from the ambulatory perspective, there was limited input from hospitalist physicians with regard to inpatient workflow in the original design of the module.12 See Figure 1 for a screen shot of RM and a description of its features.
figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
For purposes of this pilot, we modified RM to allow results of tests ordered in the inpatient setting to be available for viewing (Hospitalist Results Manager, HRM). This feature was turned on only for inpatient physicians as previously defined. Inpatient tests, including pending tests at discharge, continued to be suppressed from PCP's RM queue (however, any physician could access a patient's test result(s) directly from the Partners CDR). Inpatient physicians could track laboratory and radiology results finalized after discharge by keeping discharged patients on their HRM watch list for a designated period of time. The finalized results would become available for review in their HRM queue and abnormal results were displayed prominently at the top of this queue. Inpatient physicians were trained to use HRM in a series of meetings and demonstrations. Although HRM could be accessed from inpatient clinical workstations, it was not part of the inpatient clinical information system.
Surveys
Study surveys were developed and refined through an iterative process and pilot tested among inpatient physicians at both centers for clarity. We surveyed inpatient physicians five months after HRM implementation. Inpatient physicians were asked how often they used HRM, what barriers they faced (respondents asked to quantify agreement to statements on a 5‐point Likert scale), and which elements of an ideal system they would prefer. Finally, we solicited comments regarding perceived obstacles and suggestions for improvement. Because HRM was targeted to inpatient physicians, and because RM has been evaluated from the ambulatory perspective in a prior study,13 PCPs were not surveyed. See Supporting Information Appendix for the survey instrument used in the study.
Results
A total of 35 inpatient physicians participated in the pilot. Among 649 patients discharged during the study period, there were 1075 tests pending of which 555 were subsequently flagged as abnormal in HRM. Study surveys were sent to the 35 inpatient physician participants and 29 were completed, including partial responses (83% survey response rate). The 35 inpatient physician participants had the following characteristics: 22 were male, 13 were female; 21 were trainees and 14 were nontrainees/faculty. All 21 trainees were PGY2s. Nontrainees and faculty varied in experience level (PGY 15: 5, PGY 610: 7, PGY 1120: 1, PGY 21+: 1). Of 29 survey respondents, 7 were from hospital A and 22 were from hospital B; 19 were trainees and 10 were nontrainees/faculty. Of the 6 nonrespondents, 2 were from hospital A and 4 were from hospital B; 2 were trainees and 4 were nontrainees/faculty.
Table 1 shows the results of our survey of inpatient physicians regarding usage of HRM. Of 29 survey respondents, 14 (48%) reported never using HRM. Thirteen (45%) reported using HRM 1 to 2 times per week. None of the respondents used it more than 4 times per week. The frequency of usage was similar for hospitals A and B. Table 2 details barriers to using HRM. Twenty‐three inpatient physicians (79%) reported barriers. Seventeen (59%) thought that results in their HRM queue were not clinically relevant, 16 (55%) felt that HRM did not fit into their daily workflow, 14 (48%) had limited time to use HRM, and 12 (41%) noted that too many results in their HRM queue were on other physician's patients. Seven (24%) reported operational issues and 3 (10%) reported technical issues prohibiting use of HRM. With regard to preferred elements of an ideal results manager system, 21 (72%) inpatient physician respondents wanted to receive notification of abnormal and clinician‐designated pending test results. Four (14%) wanted to receive only abnormal results and 1 (3%) wanted to receive all results. Twenty‐seven (93%) physicians agreed that an ideally designed computerized test result management application would be valuable for managing pending tests at discharge.
| Frequency | Number of Inpatient Physicians Using HRM, n (%) | ||
|---|---|---|---|
| Overall | Hospital A | Hospital B | |
| |||
| Never | 14 (48) | 3 (43) | 11 (50) |
| 12 times per week | 13 (45) | 3 (43) | 10 (45.5) |
| 34 times per week | 2 (7) | 1 (14) | 1 (4.5) |
| 57 times per week | 0 | 0 | 0 |
| >7 times per week | 0 | 0 | 0 |
| Barrier | Overall, n (%) | Hospital A, n (%) | Hospital B, n (%) |
|---|---|---|---|
| |||
| Forgot to use HRM | 23 (79) | 7 (100) | 16 (73) |
| Results not clinically relevant | 17 (59) | 7 (100) | 10 (45) |
| Did not fit daily workflow | 16 (55) | 7 (100) | 9 (41) |
| Too little time to use HRM | 14 (48) | 6 (86) | 8 (46) |
| Results on others' patients | 12 (41) | 6 (86) | 6 (27) |
| HRM was difficult to use | 7 (24) | 2 (29) | 5 (23) |
| Had technical difficulties | 3 (10) | 0 (0) | 3 (14) |
Table 3 provides comments from inpatient physician respondents regarding obstacles prohibiting use of HRM and suggestions for future systems.
|
| Suggestions |
| Would be more useful if accessible from (the inpatient clinical information system). |
| Email notification (would have been useful). |
| At time of discharge, if there is a way to find pending labs at discharge, this would be of great utility. |
| Linking responsibility for follow‐up to test ordering (would have been useful). |
| Smarter system for filtering results so less important results are filtered out (is desirable). |
| Can the system be tied into PCP's email somehow? |
| Obstacles |
| Blood cultures, abnormal films can be difficult and time‐consuming to look up. |
| A big problem is results that automatically trigger even though they're not clinically relevant. |
| Keeping a record of patients that left with tests pending (is often difficult to do). |
| Addressing pending results is very time consuming. |
Discussion
We describe a pilot implementation of a computerized application for the management of pending tests at hospital discharge. From responses to post‐implementation surveys, we were able to identify multiple factors prohibiting successful implementation of the application. These observations may help inform future interventions and evaluations.
Almost half of inpatient physicians reported never using HRM despite training and reminders. The feedback provided by physicians in our study suggested that HRM was not ideally designed from an inpatient physician perspective. We discovered several barriers to its use: (1) HRM overburdened physicians with clinically irrelevant test results, suggesting that more robust filtering of abnormal but low importance test results may be required (eg, a borderline electrolyte abnormality or low but stable hematocrit); (2) HRM did not integrate well into inpatient workflowthe system was not integrated into the inpatient results viewing and computerized physician order entry (CPOE) applications, and therefore required an extra step to access; (3) there was no mechanism of alerting inpatient physicians that finalized test results were available for viewing in their HRM queues (eg, by email or by an alert in the inpatient computer system); (4) because responsibility for these results was unclear, most inpatient physicians had no formal method of managing them, and for many, using HRM represented an additional task; and finally (5) several physicians commented on finding results in their HRM queue that belonged to other physician's patients, implying that the hospital databases were inaccurate in identifying the discharging physician or that rotation schedules, and therefore patient responsibility, had changed in the intervening period. Table 4 summarizes the advantages and respective limitations of features of HRM available to inpatient physicians.
| Advantages | Limitations |
|---|---|
| |
| Creates a physician‐managed queue of pending test results by patient | Does not provide alert or push notification when new results available for patients |
| Filters test results by severity with most critical results appearing at the top of the queue | Severity filter set for outpatients; not restrictive enough for post‐discharge period, resulting in excessive alerting |
| Independent, voluntary acknowledgement of results by user | Active acknowledgment not required; no audit trail, feedback, or escalation if result not acknowledged |
| Embedded within LMR (the ambulatory EMR) | LMR not routinely used by many inpatient physicians |
| Offers patient communication tools (eg, pre‐populated patient results letter) | Tools not optimized for post‐discharge test result communication by inpatient physicians (eg, a tool for PCP result notification and acknowledgment) |
In the literature, there is little information regarding optimal features of a test result management system for transitions from the inpatient to ambulatory care setting. Prior studies outline important functions for results management systems developed for noninpatient sites of care, including the ambulatory and emergency room setting.12, 14, 15 These include a method of prioritizing by degree of abnormality, the ability to reliably and efficiently act upon results, and an automated alerting system for abnormal results. Findings from our study provide insight in defining core functions for result management systems which focus on transitions from the inpatient to ambulatory care setting. These functions include tight integration with applications used by inpatient physicians, clear assignment of responsibility for test results finalized after hospital discharge (as well as a mechanism to reassign responsibility), automated alerts to responsible providers of test results finalized post‐discharge, and ways to automatically filter test results to avoid over‐burdening physicians with clinically irrelevant results.
Almost all surveyed inpatient physicians agreed that an ideally designed electronic post‐discharge results management system would be valuable. For such systems to be successfully adopted, we offer several principles to help guide future work. These include: (1) clarifying responsibility at the time a test is ordered and again at discharge, (2) understanding workflow and communication patterns among inpatient and outpatient clinicians, and (3) integrating technological solutions into existing systems to minimize workflow disruptions. For example, if the primary responsibility for post‐discharge result follow‐up lies with the ordering physician, the system should be integrated within the EMR most often used by inpatient physicians and become part of inpatient physician workflow. If the system depends on administrative databases to identify the responsible providers, these must be accurate. Alternatively, in organizations with computerized provider order entry, responsibility for the result could be assigned when the test is ordered and confirmed at discharge (ie, the results management system would be integrated into the discharge order such that pending tests are reviewed at the time of discharge). The discharging physician should have the ability to assign responsibility for each pending test and select preferred mode(s) of notification once its result is finalized (eg, e‐mail, alphanumeric page, etc.). The system should have the ability to generate an automatic notification to the inpatient and PCP (and perhaps other designated providers involved in the patient's inpatient care), but it should not burden busy clinicians with unnecessary alerts and warnings. Finally, the rules by which results are prioritized must be robust enough to filter out less urgent results, and should be modified to reflect the severity of illness of recently discharged patients. In essence, in consideration of the time constraints of busy clinicians, an ideal results management system should achieve automated notification of test results while minimizing the risk of alert fatigue from the potentially large volume of alerts generated.
Our study has several important limitations. First, although our survey response rate was high, the sample size of actual participants was small. Second, because the study was conducted in 2 similar, tertiary care academic centers, it may not generalize to other settings (we note that hospital B included a nonteaching service similar to those in nonacademic medical centers). This may be particularly true in assessing the importance of specific barriers to use of results management systems, which may vary at different institutions. Third, the representation of survey respondents were skeweda majority of the responses were from trainees (all post‐graduate level [PGY] level 2) and from hospital B. Fourth, we did not actively monitor physician interaction with the test result management application, and therefore, we depended heavily on physician recollection of use of the system when responding to surveys. Finally, we did not convene focus groups of key individuals with regard to the factors facilitating or prohibiting adoption of the system. Use of semi‐structured, key informant interviews (ie, focus groups) before and after implementation of an electronic results management application, have been shown to be effective in evaluating potential barriers and facilitators of adoption.16 Focus groups of and/or interviews with inpatient and PCPs, physician extenders, and housestaff could have been useful to better characterize the potential barriers and facilitators of adoption noted by survey respondents in our study.
In summary, we offer several lessons from our attempt to implement a system to manage pending tests at hospital discharge. The success of implementing future systems to address this patient safety concern will rely on accurately assigning responsibility for these test results, integrating the system within clinical information systems commonly used by the inpatient physician, addressing workflow issues and time constraints, maximizing appropriateness of alerting, and minimizing alert fatigue.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138(3):161–167.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18(8):646–651.
- .Key legal principles for hospitalists.Am J Med.2001;111(9B):5S–9S.
- ,,.Passing the clinical baton: 6 principles to guide the hospitalist.Am J Med.2001;111(9B):36S–39S.
- ,.Lost in transition: challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;141(7):533–536.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,,, et al.Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow‐up providers.J Gen Intern Med.2009;24(9):1002–1006.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: Implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143(2):121–128.
- ,,.The continuing problem of missed test results in an integrated health system with an advanced electronic medical record.Jt Comm J Qual Patient Saf.2007;33(8):485–492.
- ,.The frequency of missed test results and associated treatment delays in a highly computerized health system.BMC Fam Pract.2007;8:32.
- ,,,,.Design and implementation of a comprehensive outpatient results manager.J Biomed Inform.2003;36(1–2):80–91.
- ,,, et al.Impact of an automated test results management system on patients' satisfaction about test result communication.Arch Intern Med.2007;167(20):2233–2239.
- ,,,,,.“I wish I had seen this test result earlier!”: dissatisfaction with test result management systems in primary care.Arch Intern Med.2004;164(20):2223–2228.
- ,,.Potential impact of a computerized system to report late‐arriving laboratory results in the emergency department.Pediatr Emerg Care.2000;16(5):313–315.
- ,,, et al.Electronic results management in pediatric ambulatory care: Qualitative assessment.Pediatrics.2009;123Suppl 2:S85–S91.
The period following discharge is a vulnerable time for patientsthe prevalence of medical errors related to this transition is high and has important patient safety and medico‐legal ramifications.13 Factors contributing to this vulnerability include complexity of hospitalized patients, shorter lengths of stay, and increased discontinuity of care. Hospitalists have recognized this threat to patient safety and have worked toward improving information exchange between inpatient and outpatient providers at hospital discharge.46 Nonetheless, the evidence suggests that more work is necessary. A recent study found that discharge summaries are often incomplete, and do not contain important information requiring follow‐up, such as pending tests.7 Additionally, a review by Kripalani et al. characterizing information deficits at hospital discharge found few interventions which specifically improve communication of pending tests at hospital discharge.8
In a prior study we determined that 41% of patients left the hospital before all laboratory and radiology test results were finalized. Of these results, 9.4% were potentially actionable and could have altered management. Physicians were aware of only 38% of post‐discharge test results.9 This awareness gap is a consequence of several factors including the lack of systems to track and alert providers of test results finalized post discharge. Also, it is unclear who is responsible for pending tests at discharge, since these tests are ordered by the inpatient physicians but often reported in the time period between hospital discharge and the patient's first follow‐up appointment with the primary care physician (PCP). Because responsibility is not explicitly made in the final communication between physicians at discharge, such test results may not be reviewed in a timely manner, potentially resulting in delays in treatment, a need for readmission, or other unfavorable outcomes.
Even in integrated health systems with advanced electronic health records, missed test results which result in treatment delays remain prevalent.10, 11 Test result management applications aid clinicians in reviewing and acting upon results as they become available and such systems may provide solutions to this problem. At Partners Healthcare in Boston, the Results Manager (RM) application was developed to help clinicians in the ambulatory setting safely, reliably, and efficiently review and act upon test results. The application enables clinicians to prioritize test results, utilize guidelines, and generate letters to patients. This system also prompts physicians to set reminders for future testing.12 In a 2.5‐year study evaluating the impact of this intervention, PCPs at 26 adult primary care practices were able to expedite communication of outpatient laboratory and imaging test results to patients with the help of RM. Patients of physicians who participated in the project reported greater satisfaction with test result communication and with information provided about their condition than did a control group of similar patients.13 RM has not yet been studied in the inpatient setting or at care transitions. We describe an attempt at modifying the Partners RM application to help inpatient physicians manage pending tests at hospital discharge.
Methods
Study Setting and Participants
We piloted our application at 2 major academic medical centers (hospitals A and B) associated with Partners Healthcare, an integrated regional health delivery network in eastern Massachusetts, from October 2004 to March 2005. Both centers use the longitudinal medical record (LMR), the electronic medical record (EMR), for nearly all ambulatory practices. The LMR is an internally developed full‐featured EMR, including a repository of laboratory and radiology reports, discharge summaries, ambulatory care notes, medication lists, problem lists, coded allergies, and other patient data. Both centers also have their own inpatient results viewing and order entry systems which provide clinicians caring for patients in the hospital the ability to review results and write orders. Although possible, clinicians caring for patients in the inpatient setting do not routinely access LMR to view test results. Inpatient physician use of the LMR is generally limited to review of the outpatient record, medication lists, and ambulatory notes at admission.
At hospital A, the hospitalist attending physician is typically responsible for all communication to outpatient physicians at discharge, as well as for follow‐up on all test results that return after discharge. Hospital B has 2 types of hospitalist services. One is staffed only by hospitalist and nonhospitalist attending physicians. Nonhospitalist attending physicians were excluded because they care for their own patients in the inpatient and ambulatory setting and typically use RM to manage test results. The other hospitalist service at hospital B is a teaching service consisting of an attending physician, resident, and interns. For this service, the resident is responsible for communication at discharge and follow‐up on all pending tests. For purposes of this study inpatient physicians refers to those physicians responsible for communication with PCPs and follow‐up on pending tests. All inpatient physicians were eligible to participate during the study period.
Test Result Management Application
RM was originally developed by Partners Healthcare to improve timely review and appropriate management of test results in the ambulatory setting. RM was developed for and vetted by primarily ambulatory physicians. The application is browser‐based, provider‐centric, and embedded in the LMR to help ambulatory clinicians review and act upon test results in a safe, reliable, and efficient manner. Although RM has access to all inpatient and outpatient data in the Partners Clinical Data Repository (CDR), given the volume of inpatient tests ordered, hospital‐based results are suppressed by default to limit inundating ambulatory clinicians' queues. Therefore, users of RM only receive results of laboratory and radiology tests ordered in the ambulatory setting. They can track these tests for specific patients for a designated period of time by placing the patient on a watch list. Finally, RM incorporates extensive decision support features to classify the degree of abnormality for each result, presents guidelines to help clinicians manage abnormal results, allows clinicians to generate result letters to patients using predefined, context‐sensitive templates, and prompts physicians to set reminders for future testing. Because RM was developed from the ambulatory perspective, there was limited input from hospitalist physicians with regard to inpatient workflow in the original design of the module.12 See Figure 1 for a screen shot of RM and a description of its features.

figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
For purposes of this pilot, we modified RM to allow results of tests ordered in the inpatient setting to be available for viewing (Hospitalist Results Manager, HRM). This feature was turned on only for inpatient physicians as previously defined. Inpatient tests, including pending tests at discharge, continued to be suppressed from PCP's RM queue (however, any physician could access a patient's test result(s) directly from the Partners CDR). Inpatient physicians could track laboratory and radiology results finalized after discharge by keeping discharged patients on their HRM watch list for a designated period of time. The finalized results would become available for review in their HRM queue and abnormal results were displayed prominently at the top of this queue. Inpatient physicians were trained to use HRM in a series of meetings and demonstrations. Although HRM could be accessed from inpatient clinical workstations, it was not part of the inpatient clinical information system.
Surveys
Study surveys were developed and refined through an iterative process and pilot tested among inpatient physicians at both centers for clarity. We surveyed inpatient physicians five months after HRM implementation. Inpatient physicians were asked how often they used HRM, what barriers they faced (respondents asked to quantify agreement to statements on a 5‐point Likert scale), and which elements of an ideal system they would prefer. Finally, we solicited comments regarding perceived obstacles and suggestions for improvement. Because HRM was targeted to inpatient physicians, and because RM has been evaluated from the ambulatory perspective in a prior study,13 PCPs were not surveyed. See Supporting Information Appendix for the survey instrument used in the study.
Results
A total of 35 inpatient physicians participated in the pilot. Among 649 patients discharged during the study period, there were 1075 tests pending of which 555 were subsequently flagged as abnormal in HRM. Study surveys were sent to the 35 inpatient physician participants and 29 were completed, including partial responses (83% survey response rate). The 35 inpatient physician participants had the following characteristics: 22 were male, 13 were female; 21 were trainees and 14 were nontrainees/faculty. All 21 trainees were PGY2s. Nontrainees and faculty varied in experience level (PGY 15: 5, PGY 610: 7, PGY 1120: 1, PGY 21+: 1). Of 29 survey respondents, 7 were from hospital A and 22 were from hospital B; 19 were trainees and 10 were nontrainees/faculty. Of the 6 nonrespondents, 2 were from hospital A and 4 were from hospital B; 2 were trainees and 4 were nontrainees/faculty.
Table 1 shows the results of our survey of inpatient physicians regarding usage of HRM. Of 29 survey respondents, 14 (48%) reported never using HRM. Thirteen (45%) reported using HRM 1 to 2 times per week. None of the respondents used it more than 4 times per week. The frequency of usage was similar for hospitals A and B. Table 2 details barriers to using HRM. Twenty‐three inpatient physicians (79%) reported barriers. Seventeen (59%) thought that results in their HRM queue were not clinically relevant, 16 (55%) felt that HRM did not fit into their daily workflow, 14 (48%) had limited time to use HRM, and 12 (41%) noted that too many results in their HRM queue were on other physician's patients. Seven (24%) reported operational issues and 3 (10%) reported technical issues prohibiting use of HRM. With regard to preferred elements of an ideal results manager system, 21 (72%) inpatient physician respondents wanted to receive notification of abnormal and clinician‐designated pending test results. Four (14%) wanted to receive only abnormal results and 1 (3%) wanted to receive all results. Twenty‐seven (93%) physicians agreed that an ideally designed computerized test result management application would be valuable for managing pending tests at discharge.
| Frequency | Number of Inpatient Physicians Using HRM, n (%) | ||
|---|---|---|---|
| Overall | Hospital A | Hospital B | |
| |||
| Never | 14 (48) | 3 (43) | 11 (50) |
| 12 times per week | 13 (45) | 3 (43) | 10 (45.5) |
| 34 times per week | 2 (7) | 1 (14) | 1 (4.5) |
| 57 times per week | 0 | 0 | 0 |
| >7 times per week | 0 | 0 | 0 |
| Barrier | Overall, n (%) | Hospital A, n (%) | Hospital B, n (%) |
|---|---|---|---|
| |||
| Forgot to use HRM | 23 (79) | 7 (100) | 16 (73) |
| Results not clinically relevant | 17 (59) | 7 (100) | 10 (45) |
| Did not fit daily workflow | 16 (55) | 7 (100) | 9 (41) |
| Too little time to use HRM | 14 (48) | 6 (86) | 8 (46) |
| Results on others' patients | 12 (41) | 6 (86) | 6 (27) |
| HRM was difficult to use | 7 (24) | 2 (29) | 5 (23) |
| Had technical difficulties | 3 (10) | 0 (0) | 3 (14) |
Table 3 provides comments from inpatient physician respondents regarding obstacles prohibiting use of HRM and suggestions for future systems.
|
| Suggestions |
| Would be more useful if accessible from (the inpatient clinical information system). |
| Email notification (would have been useful). |
| At time of discharge, if there is a way to find pending labs at discharge, this would be of great utility. |
| Linking responsibility for follow‐up to test ordering (would have been useful). |
| Smarter system for filtering results so less important results are filtered out (is desirable). |
| Can the system be tied into PCP's email somehow? |
| Obstacles |
| Blood cultures, abnormal films can be difficult and time‐consuming to look up. |
| A big problem is results that automatically trigger even though they're not clinically relevant. |
| Keeping a record of patients that left with tests pending (is often difficult to do). |
| Addressing pending results is very time consuming. |
Discussion
We describe a pilot implementation of a computerized application for the management of pending tests at hospital discharge. From responses to post‐implementation surveys, we were able to identify multiple factors prohibiting successful implementation of the application. These observations may help inform future interventions and evaluations.
Almost half of inpatient physicians reported never using HRM despite training and reminders. The feedback provided by physicians in our study suggested that HRM was not ideally designed from an inpatient physician perspective. We discovered several barriers to its use: (1) HRM overburdened physicians with clinically irrelevant test results, suggesting that more robust filtering of abnormal but low importance test results may be required (eg, a borderline electrolyte abnormality or low but stable hematocrit); (2) HRM did not integrate well into inpatient workflowthe system was not integrated into the inpatient results viewing and computerized physician order entry (CPOE) applications, and therefore required an extra step to access; (3) there was no mechanism of alerting inpatient physicians that finalized test results were available for viewing in their HRM queues (eg, by email or by an alert in the inpatient computer system); (4) because responsibility for these results was unclear, most inpatient physicians had no formal method of managing them, and for many, using HRM represented an additional task; and finally (5) several physicians commented on finding results in their HRM queue that belonged to other physician's patients, implying that the hospital databases were inaccurate in identifying the discharging physician or that rotation schedules, and therefore patient responsibility, had changed in the intervening period. Table 4 summarizes the advantages and respective limitations of features of HRM available to inpatient physicians.
| Advantages | Limitations |
|---|---|
| |
| Creates a physician‐managed queue of pending test results by patient | Does not provide alert or push notification when new results available for patients |
| Filters test results by severity with most critical results appearing at the top of the queue | Severity filter set for outpatients; not restrictive enough for post‐discharge period, resulting in excessive alerting |
| Independent, voluntary acknowledgement of results by user | Active acknowledgment not required; no audit trail, feedback, or escalation if result not acknowledged |
| Embedded within LMR (the ambulatory EMR) | LMR not routinely used by many inpatient physicians |
| Offers patient communication tools (eg, pre‐populated patient results letter) | Tools not optimized for post‐discharge test result communication by inpatient physicians (eg, a tool for PCP result notification and acknowledgment) |
In the literature, there is little information regarding optimal features of a test result management system for transitions from the inpatient to ambulatory care setting. Prior studies outline important functions for results management systems developed for noninpatient sites of care, including the ambulatory and emergency room setting.12, 14, 15 These include a method of prioritizing by degree of abnormality, the ability to reliably and efficiently act upon results, and an automated alerting system for abnormal results. Findings from our study provide insight in defining core functions for result management systems which focus on transitions from the inpatient to ambulatory care setting. These functions include tight integration with applications used by inpatient physicians, clear assignment of responsibility for test results finalized after hospital discharge (as well as a mechanism to reassign responsibility), automated alerts to responsible providers of test results finalized post‐discharge, and ways to automatically filter test results to avoid over‐burdening physicians with clinically irrelevant results.
Almost all surveyed inpatient physicians agreed that an ideally designed electronic post‐discharge results management system would be valuable. For such systems to be successfully adopted, we offer several principles to help guide future work. These include: (1) clarifying responsibility at the time a test is ordered and again at discharge, (2) understanding workflow and communication patterns among inpatient and outpatient clinicians, and (3) integrating technological solutions into existing systems to minimize workflow disruptions. For example, if the primary responsibility for post‐discharge result follow‐up lies with the ordering physician, the system should be integrated within the EMR most often used by inpatient physicians and become part of inpatient physician workflow. If the system depends on administrative databases to identify the responsible providers, these must be accurate. Alternatively, in organizations with computerized provider order entry, responsibility for the result could be assigned when the test is ordered and confirmed at discharge (ie, the results management system would be integrated into the discharge order such that pending tests are reviewed at the time of discharge). The discharging physician should have the ability to assign responsibility for each pending test and select preferred mode(s) of notification once its result is finalized (eg, e‐mail, alphanumeric page, etc.). The system should have the ability to generate an automatic notification to the inpatient and PCP (and perhaps other designated providers involved in the patient's inpatient care), but it should not burden busy clinicians with unnecessary alerts and warnings. Finally, the rules by which results are prioritized must be robust enough to filter out less urgent results, and should be modified to reflect the severity of illness of recently discharged patients. In essence, in consideration of the time constraints of busy clinicians, an ideal results management system should achieve automated notification of test results while minimizing the risk of alert fatigue from the potentially large volume of alerts generated.
Our study has several important limitations. First, although our survey response rate was high, the sample size of actual participants was small. Second, because the study was conducted in 2 similar, tertiary care academic centers, it may not generalize to other settings (we note that hospital B included a nonteaching service similar to those in nonacademic medical centers). This may be particularly true in assessing the importance of specific barriers to use of results management systems, which may vary at different institutions. Third, the representation of survey respondents were skeweda majority of the responses were from trainees (all post‐graduate level [PGY] level 2) and from hospital B. Fourth, we did not actively monitor physician interaction with the test result management application, and therefore, we depended heavily on physician recollection of use of the system when responding to surveys. Finally, we did not convene focus groups of key individuals with regard to the factors facilitating or prohibiting adoption of the system. Use of semi‐structured, key informant interviews (ie, focus groups) before and after implementation of an electronic results management application, have been shown to be effective in evaluating potential barriers and facilitators of adoption.16 Focus groups of and/or interviews with inpatient and PCPs, physician extenders, and housestaff could have been useful to better characterize the potential barriers and facilitators of adoption noted by survey respondents in our study.
In summary, we offer several lessons from our attempt to implement a system to manage pending tests at hospital discharge. The success of implementing future systems to address this patient safety concern will rely on accurately assigning responsibility for these test results, integrating the system within clinical information systems commonly used by the inpatient physician, addressing workflow issues and time constraints, maximizing appropriateness of alerting, and minimizing alert fatigue.
The period following discharge is a vulnerable time for patientsthe prevalence of medical errors related to this transition is high and has important patient safety and medico‐legal ramifications.13 Factors contributing to this vulnerability include complexity of hospitalized patients, shorter lengths of stay, and increased discontinuity of care. Hospitalists have recognized this threat to patient safety and have worked toward improving information exchange between inpatient and outpatient providers at hospital discharge.46 Nonetheless, the evidence suggests that more work is necessary. A recent study found that discharge summaries are often incomplete, and do not contain important information requiring follow‐up, such as pending tests.7 Additionally, a review by Kripalani et al. characterizing information deficits at hospital discharge found few interventions which specifically improve communication of pending tests at hospital discharge.8
In a prior study we determined that 41% of patients left the hospital before all laboratory and radiology test results were finalized. Of these results, 9.4% were potentially actionable and could have altered management. Physicians were aware of only 38% of post‐discharge test results.9 This awareness gap is a consequence of several factors including the lack of systems to track and alert providers of test results finalized post discharge. Also, it is unclear who is responsible for pending tests at discharge, since these tests are ordered by the inpatient physicians but often reported in the time period between hospital discharge and the patient's first follow‐up appointment with the primary care physician (PCP). Because responsibility is not explicitly made in the final communication between physicians at discharge, such test results may not be reviewed in a timely manner, potentially resulting in delays in treatment, a need for readmission, or other unfavorable outcomes.
Even in integrated health systems with advanced electronic health records, missed test results which result in treatment delays remain prevalent.10, 11 Test result management applications aid clinicians in reviewing and acting upon results as they become available and such systems may provide solutions to this problem. At Partners Healthcare in Boston, the Results Manager (RM) application was developed to help clinicians in the ambulatory setting safely, reliably, and efficiently review and act upon test results. The application enables clinicians to prioritize test results, utilize guidelines, and generate letters to patients. This system also prompts physicians to set reminders for future testing.12 In a 2.5‐year study evaluating the impact of this intervention, PCPs at 26 adult primary care practices were able to expedite communication of outpatient laboratory and imaging test results to patients with the help of RM. Patients of physicians who participated in the project reported greater satisfaction with test result communication and with information provided about their condition than did a control group of similar patients.13 RM has not yet been studied in the inpatient setting or at care transitions. We describe an attempt at modifying the Partners RM application to help inpatient physicians manage pending tests at hospital discharge.
Methods
Study Setting and Participants
We piloted our application at 2 major academic medical centers (hospitals A and B) associated with Partners Healthcare, an integrated regional health delivery network in eastern Massachusetts, from October 2004 to March 2005. Both centers use the longitudinal medical record (LMR), the electronic medical record (EMR), for nearly all ambulatory practices. The LMR is an internally developed full‐featured EMR, including a repository of laboratory and radiology reports, discharge summaries, ambulatory care notes, medication lists, problem lists, coded allergies, and other patient data. Both centers also have their own inpatient results viewing and order entry systems which provide clinicians caring for patients in the hospital the ability to review results and write orders. Although possible, clinicians caring for patients in the inpatient setting do not routinely access LMR to view test results. Inpatient physician use of the LMR is generally limited to review of the outpatient record, medication lists, and ambulatory notes at admission.
At hospital A, the hospitalist attending physician is typically responsible for all communication to outpatient physicians at discharge, as well as for follow‐up on all test results that return after discharge. Hospital B has 2 types of hospitalist services. One is staffed only by hospitalist and nonhospitalist attending physicians. Nonhospitalist attending physicians were excluded because they care for their own patients in the inpatient and ambulatory setting and typically use RM to manage test results. The other hospitalist service at hospital B is a teaching service consisting of an attending physician, resident, and interns. For this service, the resident is responsible for communication at discharge and follow‐up on all pending tests. For purposes of this study inpatient physicians refers to those physicians responsible for communication with PCPs and follow‐up on pending tests. All inpatient physicians were eligible to participate during the study period.
Test Result Management Application
RM was originally developed by Partners Healthcare to improve timely review and appropriate management of test results in the ambulatory setting. RM was developed for and vetted by primarily ambulatory physicians. The application is browser‐based, provider‐centric, and embedded in the LMR to help ambulatory clinicians review and act upon test results in a safe, reliable, and efficient manner. Although RM has access to all inpatient and outpatient data in the Partners Clinical Data Repository (CDR), given the volume of inpatient tests ordered, hospital‐based results are suppressed by default to limit inundating ambulatory clinicians' queues. Therefore, users of RM only receive results of laboratory and radiology tests ordered in the ambulatory setting. They can track these tests for specific patients for a designated period of time by placing the patient on a watch list. Finally, RM incorporates extensive decision support features to classify the degree of abnormality for each result, presents guidelines to help clinicians manage abnormal results, allows clinicians to generate result letters to patients using predefined, context‐sensitive templates, and prompts physicians to set reminders for future testing. Because RM was developed from the ambulatory perspective, there was limited input from hospitalist physicians with regard to inpatient workflow in the original design of the module.12 See Figure 1 for a screen shot of RM and a description of its features.

figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
For purposes of this pilot, we modified RM to allow results of tests ordered in the inpatient setting to be available for viewing (Hospitalist Results Manager, HRM). This feature was turned on only for inpatient physicians as previously defined. Inpatient tests, including pending tests at discharge, continued to be suppressed from PCP's RM queue (however, any physician could access a patient's test result(s) directly from the Partners CDR). Inpatient physicians could track laboratory and radiology results finalized after discharge by keeping discharged patients on their HRM watch list for a designated period of time. The finalized results would become available for review in their HRM queue and abnormal results were displayed prominently at the top of this queue. Inpatient physicians were trained to use HRM in a series of meetings and demonstrations. Although HRM could be accessed from inpatient clinical workstations, it was not part of the inpatient clinical information system.
Surveys
Study surveys were developed and refined through an iterative process and pilot tested among inpatient physicians at both centers for clarity. We surveyed inpatient physicians five months after HRM implementation. Inpatient physicians were asked how often they used HRM, what barriers they faced (respondents asked to quantify agreement to statements on a 5‐point Likert scale), and which elements of an ideal system they would prefer. Finally, we solicited comments regarding perceived obstacles and suggestions for improvement. Because HRM was targeted to inpatient physicians, and because RM has been evaluated from the ambulatory perspective in a prior study,13 PCPs were not surveyed. See Supporting Information Appendix for the survey instrument used in the study.
Results
A total of 35 inpatient physicians participated in the pilot. Among 649 patients discharged during the study period, there were 1075 tests pending of which 555 were subsequently flagged as abnormal in HRM. Study surveys were sent to the 35 inpatient physician participants and 29 were completed, including partial responses (83% survey response rate). The 35 inpatient physician participants had the following characteristics: 22 were male, 13 were female; 21 were trainees and 14 were nontrainees/faculty. All 21 trainees were PGY2s. Nontrainees and faculty varied in experience level (PGY 15: 5, PGY 610: 7, PGY 1120: 1, PGY 21+: 1). Of 29 survey respondents, 7 were from hospital A and 22 were from hospital B; 19 were trainees and 10 were nontrainees/faculty. Of the 6 nonrespondents, 2 were from hospital A and 4 were from hospital B; 2 were trainees and 4 were nontrainees/faculty.
Table 1 shows the results of our survey of inpatient physicians regarding usage of HRM. Of 29 survey respondents, 14 (48%) reported never using HRM. Thirteen (45%) reported using HRM 1 to 2 times per week. None of the respondents used it more than 4 times per week. The frequency of usage was similar for hospitals A and B. Table 2 details barriers to using HRM. Twenty‐three inpatient physicians (79%) reported barriers. Seventeen (59%) thought that results in their HRM queue were not clinically relevant, 16 (55%) felt that HRM did not fit into their daily workflow, 14 (48%) had limited time to use HRM, and 12 (41%) noted that too many results in their HRM queue were on other physician's patients. Seven (24%) reported operational issues and 3 (10%) reported technical issues prohibiting use of HRM. With regard to preferred elements of an ideal results manager system, 21 (72%) inpatient physician respondents wanted to receive notification of abnormal and clinician‐designated pending test results. Four (14%) wanted to receive only abnormal results and 1 (3%) wanted to receive all results. Twenty‐seven (93%) physicians agreed that an ideally designed computerized test result management application would be valuable for managing pending tests at discharge.
| Frequency | Number of Inpatient Physicians Using HRM, n (%) | ||
|---|---|---|---|
| Overall | Hospital A | Hospital B | |
| |||
| Never | 14 (48) | 3 (43) | 11 (50) |
| 12 times per week | 13 (45) | 3 (43) | 10 (45.5) |
| 34 times per week | 2 (7) | 1 (14) | 1 (4.5) |
| 57 times per week | 0 | 0 | 0 |
| >7 times per week | 0 | 0 | 0 |
| Barrier | Overall, n (%) | Hospital A, n (%) | Hospital B, n (%) |
|---|---|---|---|
| |||
| Forgot to use HRM | 23 (79) | 7 (100) | 16 (73) |
| Results not clinically relevant | 17 (59) | 7 (100) | 10 (45) |
| Did not fit daily workflow | 16 (55) | 7 (100) | 9 (41) |
| Too little time to use HRM | 14 (48) | 6 (86) | 8 (46) |
| Results on others' patients | 12 (41) | 6 (86) | 6 (27) |
| HRM was difficult to use | 7 (24) | 2 (29) | 5 (23) |
| Had technical difficulties | 3 (10) | 0 (0) | 3 (14) |
Table 3 provides comments from inpatient physician respondents regarding obstacles prohibiting use of HRM and suggestions for future systems.
|
| Suggestions |
| Would be more useful if accessible from (the inpatient clinical information system). |
| Email notification (would have been useful). |
| At time of discharge, if there is a way to find pending labs at discharge, this would be of great utility. |
| Linking responsibility for follow‐up to test ordering (would have been useful). |
| Smarter system for filtering results so less important results are filtered out (is desirable). |
| Can the system be tied into PCP's email somehow? |
| Obstacles |
| Blood cultures, abnormal films can be difficult and time‐consuming to look up. |
| A big problem is results that automatically trigger even though they're not clinically relevant. |
| Keeping a record of patients that left with tests pending (is often difficult to do). |
| Addressing pending results is very time consuming. |
Discussion
We describe a pilot implementation of a computerized application for the management of pending tests at hospital discharge. From responses to post‐implementation surveys, we were able to identify multiple factors prohibiting successful implementation of the application. These observations may help inform future interventions and evaluations.
Almost half of inpatient physicians reported never using HRM despite training and reminders. The feedback provided by physicians in our study suggested that HRM was not ideally designed from an inpatient physician perspective. We discovered several barriers to its use: (1) HRM overburdened physicians with clinically irrelevant test results, suggesting that more robust filtering of abnormal but low importance test results may be required (eg, a borderline electrolyte abnormality or low but stable hematocrit); (2) HRM did not integrate well into inpatient workflowthe system was not integrated into the inpatient results viewing and computerized physician order entry (CPOE) applications, and therefore required an extra step to access; (3) there was no mechanism of alerting inpatient physicians that finalized test results were available for viewing in their HRM queues (eg, by email or by an alert in the inpatient computer system); (4) because responsibility for these results was unclear, most inpatient physicians had no formal method of managing them, and for many, using HRM represented an additional task; and finally (5) several physicians commented on finding results in their HRM queue that belonged to other physician's patients, implying that the hospital databases were inaccurate in identifying the discharging physician or that rotation schedules, and therefore patient responsibility, had changed in the intervening period. Table 4 summarizes the advantages and respective limitations of features of HRM available to inpatient physicians.
| Advantages | Limitations |
|---|---|
| |
| Creates a physician‐managed queue of pending test results by patient | Does not provide alert or push notification when new results available for patients |
| Filters test results by severity with most critical results appearing at the top of the queue | Severity filter set for outpatients; not restrictive enough for post‐discharge period, resulting in excessive alerting |
| Independent, voluntary acknowledgement of results by user | Active acknowledgment not required; no audit trail, feedback, or escalation if result not acknowledged |
| Embedded within LMR (the ambulatory EMR) | LMR not routinely used by many inpatient physicians |
| Offers patient communication tools (eg, pre‐populated patient results letter) | Tools not optimized for post‐discharge test result communication by inpatient physicians (eg, a tool for PCP result notification and acknowledgment) |
In the literature, there is little information regarding optimal features of a test result management system for transitions from the inpatient to ambulatory care setting. Prior studies outline important functions for results management systems developed for noninpatient sites of care, including the ambulatory and emergency room setting.12, 14, 15 These include a method of prioritizing by degree of abnormality, the ability to reliably and efficiently act upon results, and an automated alerting system for abnormal results. Findings from our study provide insight in defining core functions for result management systems which focus on transitions from the inpatient to ambulatory care setting. These functions include tight integration with applications used by inpatient physicians, clear assignment of responsibility for test results finalized after hospital discharge (as well as a mechanism to reassign responsibility), automated alerts to responsible providers of test results finalized post‐discharge, and ways to automatically filter test results to avoid over‐burdening physicians with clinically irrelevant results.
Almost all surveyed inpatient physicians agreed that an ideally designed electronic post‐discharge results management system would be valuable. For such systems to be successfully adopted, we offer several principles to help guide future work. These include: (1) clarifying responsibility at the time a test is ordered and again at discharge, (2) understanding workflow and communication patterns among inpatient and outpatient clinicians, and (3) integrating technological solutions into existing systems to minimize workflow disruptions. For example, if the primary responsibility for post‐discharge result follow‐up lies with the ordering physician, the system should be integrated within the EMR most often used by inpatient physicians and become part of inpatient physician workflow. If the system depends on administrative databases to identify the responsible providers, these must be accurate. Alternatively, in organizations with computerized provider order entry, responsibility for the result could be assigned when the test is ordered and confirmed at discharge (ie, the results management system would be integrated into the discharge order such that pending tests are reviewed at the time of discharge). The discharging physician should have the ability to assign responsibility for each pending test and select preferred mode(s) of notification once its result is finalized (eg, e‐mail, alphanumeric page, etc.). The system should have the ability to generate an automatic notification to the inpatient and PCP (and perhaps other designated providers involved in the patient's inpatient care), but it should not burden busy clinicians with unnecessary alerts and warnings. Finally, the rules by which results are prioritized must be robust enough to filter out less urgent results, and should be modified to reflect the severity of illness of recently discharged patients. In essence, in consideration of the time constraints of busy clinicians, an ideal results management system should achieve automated notification of test results while minimizing the risk of alert fatigue from the potentially large volume of alerts generated.
Our study has several important limitations. First, although our survey response rate was high, the sample size of actual participants was small. Second, because the study was conducted in 2 similar, tertiary care academic centers, it may not generalize to other settings (we note that hospital B included a nonteaching service similar to those in nonacademic medical centers). This may be particularly true in assessing the importance of specific barriers to use of results management systems, which may vary at different institutions. Third, the representation of survey respondents were skeweda majority of the responses were from trainees (all post‐graduate level [PGY] level 2) and from hospital B. Fourth, we did not actively monitor physician interaction with the test result management application, and therefore, we depended heavily on physician recollection of use of the system when responding to surveys. Finally, we did not convene focus groups of key individuals with regard to the factors facilitating or prohibiting adoption of the system. Use of semi‐structured, key informant interviews (ie, focus groups) before and after implementation of an electronic results management application, have been shown to be effective in evaluating potential barriers and facilitators of adoption.16 Focus groups of and/or interviews with inpatient and PCPs, physician extenders, and housestaff could have been useful to better characterize the potential barriers and facilitators of adoption noted by survey respondents in our study.
In summary, we offer several lessons from our attempt to implement a system to manage pending tests at hospital discharge. The success of implementing future systems to address this patient safety concern will rely on accurately assigning responsibility for these test results, integrating the system within clinical information systems commonly used by the inpatient physician, addressing workflow issues and time constraints, maximizing appropriateness of alerting, and minimizing alert fatigue.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138(3):161–167.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18(8):646–651.
- .Key legal principles for hospitalists.Am J Med.2001;111(9B):5S–9S.
- ,,.Passing the clinical baton: 6 principles to guide the hospitalist.Am J Med.2001;111(9B):36S–39S.
- ,.Lost in transition: challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;141(7):533–536.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,,, et al.Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow‐up providers.J Gen Intern Med.2009;24(9):1002–1006.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: Implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143(2):121–128.
- ,,.The continuing problem of missed test results in an integrated health system with an advanced electronic medical record.Jt Comm J Qual Patient Saf.2007;33(8):485–492.
- ,.The frequency of missed test results and associated treatment delays in a highly computerized health system.BMC Fam Pract.2007;8:32.
- ,,,,.Design and implementation of a comprehensive outpatient results manager.J Biomed Inform.2003;36(1–2):80–91.
- ,,, et al.Impact of an automated test results management system on patients' satisfaction about test result communication.Arch Intern Med.2007;167(20):2233–2239.
- ,,,,,.“I wish I had seen this test result earlier!”: dissatisfaction with test result management systems in primary care.Arch Intern Med.2004;164(20):2223–2228.
- ,,.Potential impact of a computerized system to report late‐arriving laboratory results in the emergency department.Pediatr Emerg Care.2000;16(5):313–315.
- ,,, et al.Electronic results management in pediatric ambulatory care: Qualitative assessment.Pediatrics.2009;123Suppl 2:S85–S91.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138(3):161–167.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18(8):646–651.
- .Key legal principles for hospitalists.Am J Med.2001;111(9B):5S–9S.
- ,,.Passing the clinical baton: 6 principles to guide the hospitalist.Am J Med.2001;111(9B):36S–39S.
- ,.Lost in transition: challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;141(7):533–536.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,,, et al.Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow‐up providers.J Gen Intern Med.2009;24(9):1002–1006.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: Implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143(2):121–128.
- ,,.The continuing problem of missed test results in an integrated health system with an advanced electronic medical record.Jt Comm J Qual Patient Saf.2007;33(8):485–492.
- ,.The frequency of missed test results and associated treatment delays in a highly computerized health system.BMC Fam Pract.2007;8:32.
- ,,,,.Design and implementation of a comprehensive outpatient results manager.J Biomed Inform.2003;36(1–2):80–91.
- ,,, et al.Impact of an automated test results management system on patients' satisfaction about test result communication.Arch Intern Med.2007;167(20):2233–2239.
- ,,,,,.“I wish I had seen this test result earlier!”: dissatisfaction with test result management systems in primary care.Arch Intern Med.2004;164(20):2223–2228.
- ,,.Potential impact of a computerized system to report late‐arriving laboratory results in the emergency department.Pediatr Emerg Care.2000;16(5):313–315.
- ,,, et al.Electronic results management in pediatric ambulatory care: Qualitative assessment.Pediatrics.2009;123Suppl 2:S85–S91.
Copyright © 2010 Society of Hospital Medicine