User login
Effect of Multidisciplinary Transitional Pain Service on Health Care Use and Costs Following Orthopedic Surgery
Opioid use disorder (OUD) is a significant cause of morbidity, mortality, and health care costs in the US.1,2 Surgery can be the inciting cause for exposure to an opioid; as many as 23% of patients develop chronic OUD following surgery.3,4 Patients with a history of substance use, mood disorders, anxiety, or previous chronic opioid use (COU) are at risk for relapse, dose escalation, and poor pain control after high-risk surgery, such as orthopedic joint procedures.5 Recently focus has been on identifying high-risk patients before orthopedic joint surgery and implementing evidence-based strategies that reduce the postoperative incidence of COU.
A transitional pain service (TPS) has been shown to reduce COU for high-risk surgical patients in different health care settings.6-9 The TPS model bundles multiple interventions that can be applied to patients at high risk for COU within a health care system. This includes individually tailored programs for preoperative education or pain management planning, use of multimodal analgesia (including regional or neuraxial techniques or nonopioid systemic medications), application of nonpharmacologic modalities (such as cognitive-based intervention), and a coordinated approach to postdischarge instructions and transitions of care. These interventions are coordinated by a multidisciplinary clinical service consisting of anesthesiologists and advanced practice clinicians with specialization in acute pain management and opioid tapering, nurse care coordinators, and psychologists with expertise in cognitive behavioral therapy.
TPS has been shown to reduce the incidence of COU for patients undergoing orthopedic joint surgery, but its impact on health care use and costs is unknown.6-9 The TPS intervention is resource intensive and increases the use of health care for preoperative education or pain management, which may increase the burden of costs. However, reducing long-term COU may reduce the use of health care for COU- and OUD-related complications, leading to cost savings. This study evaluated whether the TPS intervention influenced health care use and cost for inpatient, outpatient, or pharmacy services during the year following orthopedic joint surgery compared with that of the standard pain management care for procedures that place patients at high risk for COU. We used a difference-in-differences (DID) analysis to estimate this intervention effect, using multivariable regression models that controlled for unobserved time trends and cohort characteristics.
METHODS
This was a quasi-experimental study of patients who underwent orthopedic joint surgery and associated procedures at high risk for COU at the Veterans Affairs Salt Lake City Healthcare System (VASLCHS) between January 2016 through April 2020. The pre-TPS period between January 2016 through December 2017 was compared with the post-TPS period between January 2018 to September 2019. The control patient cohort was selected from 5 geographically diverse VA health care systems throughout the US: Eastern Colorado, Central Plains (Nebraska), White River Junction (Vermont), North Florida/South Georgia, and Portland (Oregon). By sampling health care costs from VA medical centers (VAMCs) across these different regions, our control group was generalizable to veterans receiving orthopedic joint surgery across the US. This study used data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse, a repository of nearly all clinical and administrative data found in electronic health records for VA-provided care and fee-basis care paid for by the VA.10 All data were hosted and analyzed in the VA Informatics and Computing Infrastructure (VINCI) workspace. The University of Utah Institutional Review Board and the VASLCHS Office of Research and Development approved the protocol for this study.
TPS Intervention
The VASLCHS TPS has already been described in detail elsewhere.6,7 Briefly, patients at high risk for COU at the VASLCHS were enrolled in the TPS program before surgery for total knee, hip, or shoulder arthroplasty or rotator cuff procedures. The TPS service consists of an anesthesiologist and advanced practice clinician with specialization in acute pain management and opioid tapering, a psychologist with expertise in cognitive behavioral therapy, and 3 nurse care coordinators. These TPS practitioners work together to provide preoperative education, including setting expectations regarding postoperative pain, recommending nonopioid pain management strategies, and providing guidance regarding the appropriate use of opioids for surgical pain. Individual pain plans were developed and implemented for the perioperative period. After surgery, the TPS provided recommendations and support for nonopioid pain therapies and opioid tapers. Patients were followed by the TPS team for at least 12 months after surgery. At a minimum, the goals set by TPS included cessation of all opioid use for prior nonopioid users (NOU) by 90 days after surgery and the return to baseline opioid use or lower for prior COU patients by 90 days after surgery. The TPS also encouraged and supported opioid tapering among COU patients to reduce or completely stop opioid use after surgery.
Patient Cohorts
Veterans having primary or revision total knee, hip, or shoulder arthroplasty or rotator cuff repair between January 1, 2016, and September 30, 2019, at the aforementioned VAMCs were included in the study. Patients who had any hospitalization within 90 days pre- or postindex surgery or who died within 8 months after surgery were excluded from analysis. Patients who had multiple surgeries during the index inpatient visit or within 90 days after the index surgery also were excluded. Comorbid conditions for mental health and substance use were identified using the International Classification of Diseases, 10th revision Clinical Modification (ICD-10) codes or 9th revision equivalent grouped by Clinical Classifications Software Refined (CCS-R).11 Preoperative exposure to clinically relevant pharmacotherapy (ie, agents associated with prolonged opioid use and nonopioid adjuvants) was captured using VA outpatient prescription records (eAppendix 1).
Outcome Variables
Outcome variables included health care use and costs during 1-year pre- and postperiods from the date of surgery. VA health care costs for outpatient, inpatient, and pharmacy services for direct patient care were collected from the Managerial Cost Accounting System, an activity-based cost allocation system that generates estimates of the cost of individual VA hospital stays, health care encounters, and medications. Health care use was defined as the number of encounters for each visit type in the Managerial Cost Accounting System. All costs were adjusted to 2019 US dollars, using the Personal Consumption Expenditures price index for health care services.15
A set of sociodemographic variables including sex, age at surgery, race and ethnicity, rurality, military branch (Army, Air Force, Marine Corps, Navy, and other), and service connectivity were included as covariates in our regression models.
Statistical Analyses
Descriptive analyses were used to evaluate differences in baseline patient sociodemographic and clinical characteristics between pre- and postperiods for TPS intervention and control cohorts using 2-sample t tests for continuous variables and χ2 tests for categorical variables. We summarized unadjusted health care use and costs for outpatient, inpatient, and pharmacy visits and compared the pre- and postintervention periods using the Mann-Whitney test. Both mean (SD) and median (IQR) were considered, reflecting the skewed distribution of the outcome variables.
We used a DID approach to assess the intervention effect while minimizing confounding from the nonrandom sample. The DID approach controls for unobserved differences between VAMCs that are related to both the intervention and outcomes while controlling for trends over time that could affect outcomes across clinics. To implement the DID approach, we included 3 key independent variables in our regression models: (1) an indicator for whether the observation occurred in the postintervention period; (2) an indicator for whether the patient was exposed to the TPS intervention; and (3) the interaction between these 2 variables.
For cost outcomes, we used multivariable generalized linear models with a log link and a Poisson or Υ family. We analyzed inpatient costs using a 2-part generalized linear model because only 17% to 20% of patients had ≥ 1 inpatient visit. We used multivariable negative binomial regression for health care use outcomes. Demographic and clinical covariates described earlier were included in the regression models to control for differences in the composition of patient groups and clinics that could lead to confounding bias.
RESULTS
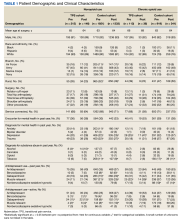
Of the 4954 patients included in our study cohort, 3545 (71.6%) were in the NOU group and 1409 (28.4%) were in the COU group. Among the NOU cohort, 361 patients were in the intervention group and 3184 in the control group. Among the COU cohort, 149 patients were in the intervention group and 1260 in the control group (Table 1). Most patients were male, White race, with a mean (SD) age of 64 (11) years. The most common orthopedic procedure was total knee arthroplasty, followed by total hip arthroplasty. Among both NOU and COU cohorts, patients’ characteristics were similar between the pre- and postintervention period among either TPS or control cohort.
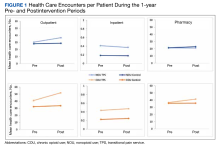
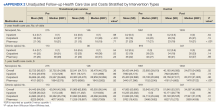
Figures 1 and 2 and eAppendix 2 depict unadjusted per-person average outpatient, inpatient, and pharmacy visits and costs incurred during the 1-year pre- and postintervention periods for the NOU and COU cohorts. Average total health care follow-up costs ranged from $40,000 to $53,000 for NOU and from $47,000 to $82,000 for COU cohort. Cost for outpatient visits accounted for about 70% of the average total costs, followed by costs for inpatient visits of about 20%, and costs for pharmacy for the remaining.
For the NOU cohort, the number of health care encounters remained fairly stable between periods except for the outpatient visits among the TPS group. The TPS group experienced an increase in mean outpatient visits in the postperiod: 30 vs 37 visits (23%) (
Table 2 summarizes the results from the multivariable DID analyses for the outpatient, inpatient, and pharmacy visit and cost outcomes. Here, the estimated effect of the TPS intervention is the coefficient from the interaction between the postintervention and TPS exposure indicator variables. This coefficient was calculated as the difference in the outcome before and after the TPS intervention among the TPS group minus the difference in the outcome before and after the TPS intervention among the control group. For the NOU cohort, TPS was associated with an increase in the use of outpatient health care (mean [SD] increase of 6.9 [2] visits; P < .001) after the surgery with no statistically significant effect on outpatient costs (mean [SD] increase of $2787 [$3749]; P = .55). There was no statistically significant effect of TPS on the use of inpatient visits or pharmacy, but a decrease in costs for inpatient visits among those who had at least 1 inpatient visit (mean [SD] decrease of $12,170 [$6100]; P = .02). For the COU cohort, TPS had no statistically significant impact on the use of outpatient, inpatient, or pharmacy or the corresponding costs.
DISCUSSION
TPS is a multidisciplinary approach to perioperative pain management that has been shown to reduce both the quantity and duration of opioid use among orthopedic surgery patients.6,7 Although the cost burden of providing TPS services to prevent COU is borne by the individual health care system, it is unclear whether this expense is offset by lower long-term medical costs and health care use for COU- and OUD-related complications. In this study focused on a veteran population undergoing orthopedic joint procedures, a DID analysis of cost and health care use showed that TPS, which has been shown to reduce COU for high-risk surgical patients, can be implemented without increasing the overall costs to the VA health care system during the 1 year following surgery, even with increased outpatient visits. For NOU patients, there was no difference in outpatient visit costs or pharmacy costs over 12 months after surgery, although there was a significant reduction in subsequent inpatient costs over the same period. Further, there was no difference in outpatient, inpatient, or pharmacy costs after surgery for COU patients. These findings suggest that TPS can be a cost-effective approach to reduce opioid use among patients undergoing orthopedic joint surgery in VAMCs.
The costs of managing COU after surgery are substantial. Prior reports have shown that adjusted total health care costs are 1.6 to 2.5 times higher for previously NOU patients with new COU after major surgery than those for such patients without persistent use.16 The 1-year costs associated with new COU in this prior study ranged between $7944 and $17,702 after inpatient surgery and between $5598 and $12,834 after outpatient index surgery, depending on the payer, which are in line with the cost differences found in our current study. Another report among patients with COU following orthopedic joint replacement showed that they had higher use of inpatient, emergency department, and ambulance/paramedic services in the 12 months following their surgery than did those without persistent use.17 Although these results highlight the impact that COU plays in driving increased costs after major surgery, there have been limited studies focused on interventions that can neutralize the costs associated with opioid misuse after surgery. To our knowledge, our study is the first analysis to show the impact of using an intervention such as TPS to reduce postoperative opioid use on health care use and cost.
Although a rigorous and comprehensive return on investment analysis was beyond the scope of this analysis, these results may have several implications for other health care systems and hospitals that wish to invest in a multidisciplinary perioperative pain management program such as TPS but may be reluctant due to the upfront investment. First, the increased number of patient follow-up visits needed during TPS seems to be more than offset by the reduction in opioid use and associated complications that may occur after surgery. Second, TPS did not seem to be associated with an increase in overall health care costs during the 1-year follow-up period. Together, these results indicate that the return on investment for a TPS approach to perioperative pain management in which optimal patient-centered outcomes are achieved without increasing long-term costs to a health care system may be positive.
Limitations
This study has several limitations. First, this was a quasi-experimental observational study, and the associations we identified between intervention and outcomes should not be assumed to demonstrate causality. Although our DID analysis controlled for an array of demographic and clinical characteristics, differences in medical costs and health care use between the 2 cohorts might be driven by unobserved confounding variables.
Our study also was limited to veterans who received medical care at the VA, and results may not be generalizable to other non-VA health care systems or to veterans with Medicare insurance who have dual benefits. While our finding on health care use and costs may be incomplete because of the uncaptured health care use outside the VA, our DID analysis helped reduce unobserved bias because the absence of data outside of VA care applies to both TPS and control groups. Further, the total costs of operating a TPS program at any given institution will depend on the size of the hospital and volume of surgical patients who meet criteria for enrollment. However, the relative differences in health care use and costs may be extrapolated to patients undergoing orthopedic surgery in other types of academic and community-based health care systems.
Furthermore, this analysis focused primarily on COU and NOU patients undergoing orthopedic joint surgery. While this represents a high-risk population for OUD, the costs and health care use associated with delivering the TPS intervention to other types of surgical procedures may be significantly different. All costs in this analysis were based on 2019 estimates and do not account for the potential inflation over the past several years. Nonmonetary costs to the patient and per-person average total intervention costs were not included in the study. However, we assumed that costs associated with TPS and standard of care would have increased to an equivalent degree over the same period. Further, the average cost of TPS per patient (approximately $900) is relatively small compared with the average annual costs during 1-year pre- and postoperative periods and was not expected to have a significant effect on the analysis.
Conclusions
We found that the significant reduction in COU seen in previous studies following the implementation of TPS was not accompanied by increased health care costs.6,7 When considering the other costs of long-term opioid use, such as abuse potential, overdose, death, and increased disability, implementation of a TPS service has the potential to improve patient quality of life while reducing other health-related costs. Health care systems should consider the implementation of similar multidisciplinary approaches to perioperative pain management to improve outcomes after orthopedic joint surgery and other high-risk procedures.
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. doi:10.15585/mmwr.mm655051e1
2. Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901-906. doi:10.1097/MLR.0000000000000625
3. Jiang X, Orton M, Feng R, et al. Chronic opioid usage in surgical patients in a large academic center. Ann Surg. 2017;265(4):722-727. doi:10.1097/SLA.0000000000001780
4. Johnson SP, Chung KC, Zhong L, et al. Risk of prolonged opioid use among opioid-naive patients following common hand surgery procedures. J Hand Surg Am. 2016;41(10):947-957, e3. doi:10.1016/j.jhsa.2016.07.113
5. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504. doi:10.1001/jamasurg.2017.0504
6. Buys MJ, Bayless K, Romesser J, et al. Multidisciplinary transitional pain service for the veteran population. Fed Pract. 2020;37(10):472-478. doi:10.12788/fp.0053
7. Buys MJ, Bayless K, Romesser J, et al. Opioid use among veterans undergoing major joint surgery managed by a multidisciplinary transitional pain service. Reg Anesth Pain Med. 2020;45(11):847-852. doi:10.1136/rapm-2020-101797
8. Huang A, Katz J, Clarke H. Ensuring safe prescribing of controlled substances for pain following surgery by developing a transitional pain service. Pain Manag. 2015;5(2):97-105. doi:10.2217/pmt.15.7
9. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695-702. doi:10.2147/JPR.S91924
10. Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
11. Agency for Healthcare Research and Quality. Clinical Classifications Software Refined (CCSR). Updated December 9, 2022. Accessed October 30, 2023. www.hcup-us.ahrq.gov/toolssoftware/ccsr/ccs_refined.jsp
12. Mosher HJ, Richardson KK, Lund BC. The 1-year treatment course of new opioid recipients in Veterans Health Administration. Pain Med. 2016;17(7):1282-1291. doi:10.1093/pm/pnw058
13. Hadlandsmyth K, Mosher HJ, Vander Weg MW, O’Shea AM, McCoy KD, Lund BC. Utility of accumulated opioid supply days and individual patient factors in predicting probability of transitioning to long-term opioid use: an observational study in the Veterans Health Administration. Pharmacol Res Perspect. 2020;8(2):e00571. doi:10.1002/prp2.571
14. Pagé MG, Kudrina I, Zomahoun HTV, et al. Relative frequency and risk factors for long-term opioid therapy following surgery and trauma among adults: a systematic review protocol. Syst Rev. 2018;7(1):97. doi:10.1186/s13643-018-0760-3
15. US. Bureau of Economic Analysis. Price indexes for personal consumption expenditures by major type of product. Accessed October 30, 2023. https://apps.bea.gov/iTable/?reqid=19&step=3&isuri=1&nipa_table_list=64&categories=survey
16. Brummett CM, Evans-Shields J, England C, et al. Increased health care costs associated with new persistent opioid use after major surgery in opioid-naive patients. J Manag Care Spec Pharm. 2021;27(6):760-771. doi:10.18553/jmcp.2021.20507
17. Gold LS, Strassels SA, Hansen RN. Health care costs and utilization in patients receiving prescriptions for long-acting opioids for acute postsurgical pain. Clin J Pain. 2016;32(9):747-754. doi:10.1097/ajp.0000000000000322
Opioid use disorder (OUD) is a significant cause of morbidity, mortality, and health care costs in the US.1,2 Surgery can be the inciting cause for exposure to an opioid; as many as 23% of patients develop chronic OUD following surgery.3,4 Patients with a history of substance use, mood disorders, anxiety, or previous chronic opioid use (COU) are at risk for relapse, dose escalation, and poor pain control after high-risk surgery, such as orthopedic joint procedures.5 Recently focus has been on identifying high-risk patients before orthopedic joint surgery and implementing evidence-based strategies that reduce the postoperative incidence of COU.
A transitional pain service (TPS) has been shown to reduce COU for high-risk surgical patients in different health care settings.6-9 The TPS model bundles multiple interventions that can be applied to patients at high risk for COU within a health care system. This includes individually tailored programs for preoperative education or pain management planning, use of multimodal analgesia (including regional or neuraxial techniques or nonopioid systemic medications), application of nonpharmacologic modalities (such as cognitive-based intervention), and a coordinated approach to postdischarge instructions and transitions of care. These interventions are coordinated by a multidisciplinary clinical service consisting of anesthesiologists and advanced practice clinicians with specialization in acute pain management and opioid tapering, nurse care coordinators, and psychologists with expertise in cognitive behavioral therapy.
TPS has been shown to reduce the incidence of COU for patients undergoing orthopedic joint surgery, but its impact on health care use and costs is unknown.6-9 The TPS intervention is resource intensive and increases the use of health care for preoperative education or pain management, which may increase the burden of costs. However, reducing long-term COU may reduce the use of health care for COU- and OUD-related complications, leading to cost savings. This study evaluated whether the TPS intervention influenced health care use and cost for inpatient, outpatient, or pharmacy services during the year following orthopedic joint surgery compared with that of the standard pain management care for procedures that place patients at high risk for COU. We used a difference-in-differences (DID) analysis to estimate this intervention effect, using multivariable regression models that controlled for unobserved time trends and cohort characteristics.
METHODS
This was a quasi-experimental study of patients who underwent orthopedic joint surgery and associated procedures at high risk for COU at the Veterans Affairs Salt Lake City Healthcare System (VASLCHS) between January 2016 through April 2020. The pre-TPS period between January 2016 through December 2017 was compared with the post-TPS period between January 2018 to September 2019. The control patient cohort was selected from 5 geographically diverse VA health care systems throughout the US: Eastern Colorado, Central Plains (Nebraska), White River Junction (Vermont), North Florida/South Georgia, and Portland (Oregon). By sampling health care costs from VA medical centers (VAMCs) across these different regions, our control group was generalizable to veterans receiving orthopedic joint surgery across the US. This study used data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse, a repository of nearly all clinical and administrative data found in electronic health records for VA-provided care and fee-basis care paid for by the VA.10 All data were hosted and analyzed in the VA Informatics and Computing Infrastructure (VINCI) workspace. The University of Utah Institutional Review Board and the VASLCHS Office of Research and Development approved the protocol for this study.
TPS Intervention
The VASLCHS TPS has already been described in detail elsewhere.6,7 Briefly, patients at high risk for COU at the VASLCHS were enrolled in the TPS program before surgery for total knee, hip, or shoulder arthroplasty or rotator cuff procedures. The TPS service consists of an anesthesiologist and advanced practice clinician with specialization in acute pain management and opioid tapering, a psychologist with expertise in cognitive behavioral therapy, and 3 nurse care coordinators. These TPS practitioners work together to provide preoperative education, including setting expectations regarding postoperative pain, recommending nonopioid pain management strategies, and providing guidance regarding the appropriate use of opioids for surgical pain. Individual pain plans were developed and implemented for the perioperative period. After surgery, the TPS provided recommendations and support for nonopioid pain therapies and opioid tapers. Patients were followed by the TPS team for at least 12 months after surgery. At a minimum, the goals set by TPS included cessation of all opioid use for prior nonopioid users (NOU) by 90 days after surgery and the return to baseline opioid use or lower for prior COU patients by 90 days after surgery. The TPS also encouraged and supported opioid tapering among COU patients to reduce or completely stop opioid use after surgery.
Patient Cohorts
Veterans having primary or revision total knee, hip, or shoulder arthroplasty or rotator cuff repair between January 1, 2016, and September 30, 2019, at the aforementioned VAMCs were included in the study. Patients who had any hospitalization within 90 days pre- or postindex surgery or who died within 8 months after surgery were excluded from analysis. Patients who had multiple surgeries during the index inpatient visit or within 90 days after the index surgery also were excluded. Comorbid conditions for mental health and substance use were identified using the International Classification of Diseases, 10th revision Clinical Modification (ICD-10) codes or 9th revision equivalent grouped by Clinical Classifications Software Refined (CCS-R).11 Preoperative exposure to clinically relevant pharmacotherapy (ie, agents associated with prolonged opioid use and nonopioid adjuvants) was captured using VA outpatient prescription records (eAppendix 1).
Outcome Variables
Outcome variables included health care use and costs during 1-year pre- and postperiods from the date of surgery. VA health care costs for outpatient, inpatient, and pharmacy services for direct patient care were collected from the Managerial Cost Accounting System, an activity-based cost allocation system that generates estimates of the cost of individual VA hospital stays, health care encounters, and medications. Health care use was defined as the number of encounters for each visit type in the Managerial Cost Accounting System. All costs were adjusted to 2019 US dollars, using the Personal Consumption Expenditures price index for health care services.15
A set of sociodemographic variables including sex, age at surgery, race and ethnicity, rurality, military branch (Army, Air Force, Marine Corps, Navy, and other), and service connectivity were included as covariates in our regression models.
Statistical Analyses
Descriptive analyses were used to evaluate differences in baseline patient sociodemographic and clinical characteristics between pre- and postperiods for TPS intervention and control cohorts using 2-sample t tests for continuous variables and χ2 tests for categorical variables. We summarized unadjusted health care use and costs for outpatient, inpatient, and pharmacy visits and compared the pre- and postintervention periods using the Mann-Whitney test. Both mean (SD) and median (IQR) were considered, reflecting the skewed distribution of the outcome variables.
We used a DID approach to assess the intervention effect while minimizing confounding from the nonrandom sample. The DID approach controls for unobserved differences between VAMCs that are related to both the intervention and outcomes while controlling for trends over time that could affect outcomes across clinics. To implement the DID approach, we included 3 key independent variables in our regression models: (1) an indicator for whether the observation occurred in the postintervention period; (2) an indicator for whether the patient was exposed to the TPS intervention; and (3) the interaction between these 2 variables.
For cost outcomes, we used multivariable generalized linear models with a log link and a Poisson or Υ family. We analyzed inpatient costs using a 2-part generalized linear model because only 17% to 20% of patients had ≥ 1 inpatient visit. We used multivariable negative binomial regression for health care use outcomes. Demographic and clinical covariates described earlier were included in the regression models to control for differences in the composition of patient groups and clinics that could lead to confounding bias.
RESULTS
Of the 4954 patients included in our study cohort, 3545 (71.6%) were in the NOU group and 1409 (28.4%) were in the COU group. Among the NOU cohort, 361 patients were in the intervention group and 3184 in the control group. Among the COU cohort, 149 patients were in the intervention group and 1260 in the control group (Table 1). Most patients were male, White race, with a mean (SD) age of 64 (11) years. The most common orthopedic procedure was total knee arthroplasty, followed by total hip arthroplasty. Among both NOU and COU cohorts, patients’ characteristics were similar between the pre- and postintervention period among either TPS or control cohort.
Figures 1 and 2 and eAppendix 2 depict unadjusted per-person average outpatient, inpatient, and pharmacy visits and costs incurred during the 1-year pre- and postintervention periods for the NOU and COU cohorts. Average total health care follow-up costs ranged from $40,000 to $53,000 for NOU and from $47,000 to $82,000 for COU cohort. Cost for outpatient visits accounted for about 70% of the average total costs, followed by costs for inpatient visits of about 20%, and costs for pharmacy for the remaining.
For the NOU cohort, the number of health care encounters remained fairly stable between periods except for the outpatient visits among the TPS group. The TPS group experienced an increase in mean outpatient visits in the postperiod: 30 vs 37 visits (23%) (
Table 2 summarizes the results from the multivariable DID analyses for the outpatient, inpatient, and pharmacy visit and cost outcomes. Here, the estimated effect of the TPS intervention is the coefficient from the interaction between the postintervention and TPS exposure indicator variables. This coefficient was calculated as the difference in the outcome before and after the TPS intervention among the TPS group minus the difference in the outcome before and after the TPS intervention among the control group. For the NOU cohort, TPS was associated with an increase in the use of outpatient health care (mean [SD] increase of 6.9 [2] visits; P < .001) after the surgery with no statistically significant effect on outpatient costs (mean [SD] increase of $2787 [$3749]; P = .55). There was no statistically significant effect of TPS on the use of inpatient visits or pharmacy, but a decrease in costs for inpatient visits among those who had at least 1 inpatient visit (mean [SD] decrease of $12,170 [$6100]; P = .02). For the COU cohort, TPS had no statistically significant impact on the use of outpatient, inpatient, or pharmacy or the corresponding costs.
DISCUSSION
TPS is a multidisciplinary approach to perioperative pain management that has been shown to reduce both the quantity and duration of opioid use among orthopedic surgery patients.6,7 Although the cost burden of providing TPS services to prevent COU is borne by the individual health care system, it is unclear whether this expense is offset by lower long-term medical costs and health care use for COU- and OUD-related complications. In this study focused on a veteran population undergoing orthopedic joint procedures, a DID analysis of cost and health care use showed that TPS, which has been shown to reduce COU for high-risk surgical patients, can be implemented without increasing the overall costs to the VA health care system during the 1 year following surgery, even with increased outpatient visits. For NOU patients, there was no difference in outpatient visit costs or pharmacy costs over 12 months after surgery, although there was a significant reduction in subsequent inpatient costs over the same period. Further, there was no difference in outpatient, inpatient, or pharmacy costs after surgery for COU patients. These findings suggest that TPS can be a cost-effective approach to reduce opioid use among patients undergoing orthopedic joint surgery in VAMCs.
The costs of managing COU after surgery are substantial. Prior reports have shown that adjusted total health care costs are 1.6 to 2.5 times higher for previously NOU patients with new COU after major surgery than those for such patients without persistent use.16 The 1-year costs associated with new COU in this prior study ranged between $7944 and $17,702 after inpatient surgery and between $5598 and $12,834 after outpatient index surgery, depending on the payer, which are in line with the cost differences found in our current study. Another report among patients with COU following orthopedic joint replacement showed that they had higher use of inpatient, emergency department, and ambulance/paramedic services in the 12 months following their surgery than did those without persistent use.17 Although these results highlight the impact that COU plays in driving increased costs after major surgery, there have been limited studies focused on interventions that can neutralize the costs associated with opioid misuse after surgery. To our knowledge, our study is the first analysis to show the impact of using an intervention such as TPS to reduce postoperative opioid use on health care use and cost.
Although a rigorous and comprehensive return on investment analysis was beyond the scope of this analysis, these results may have several implications for other health care systems and hospitals that wish to invest in a multidisciplinary perioperative pain management program such as TPS but may be reluctant due to the upfront investment. First, the increased number of patient follow-up visits needed during TPS seems to be more than offset by the reduction in opioid use and associated complications that may occur after surgery. Second, TPS did not seem to be associated with an increase in overall health care costs during the 1-year follow-up period. Together, these results indicate that the return on investment for a TPS approach to perioperative pain management in which optimal patient-centered outcomes are achieved without increasing long-term costs to a health care system may be positive.
Limitations
This study has several limitations. First, this was a quasi-experimental observational study, and the associations we identified between intervention and outcomes should not be assumed to demonstrate causality. Although our DID analysis controlled for an array of demographic and clinical characteristics, differences in medical costs and health care use between the 2 cohorts might be driven by unobserved confounding variables.
Our study also was limited to veterans who received medical care at the VA, and results may not be generalizable to other non-VA health care systems or to veterans with Medicare insurance who have dual benefits. While our finding on health care use and costs may be incomplete because of the uncaptured health care use outside the VA, our DID analysis helped reduce unobserved bias because the absence of data outside of VA care applies to both TPS and control groups. Further, the total costs of operating a TPS program at any given institution will depend on the size of the hospital and volume of surgical patients who meet criteria for enrollment. However, the relative differences in health care use and costs may be extrapolated to patients undergoing orthopedic surgery in other types of academic and community-based health care systems.
Furthermore, this analysis focused primarily on COU and NOU patients undergoing orthopedic joint surgery. While this represents a high-risk population for OUD, the costs and health care use associated with delivering the TPS intervention to other types of surgical procedures may be significantly different. All costs in this analysis were based on 2019 estimates and do not account for the potential inflation over the past several years. Nonmonetary costs to the patient and per-person average total intervention costs were not included in the study. However, we assumed that costs associated with TPS and standard of care would have increased to an equivalent degree over the same period. Further, the average cost of TPS per patient (approximately $900) is relatively small compared with the average annual costs during 1-year pre- and postoperative periods and was not expected to have a significant effect on the analysis.
Conclusions
We found that the significant reduction in COU seen in previous studies following the implementation of TPS was not accompanied by increased health care costs.6,7 When considering the other costs of long-term opioid use, such as abuse potential, overdose, death, and increased disability, implementation of a TPS service has the potential to improve patient quality of life while reducing other health-related costs. Health care systems should consider the implementation of similar multidisciplinary approaches to perioperative pain management to improve outcomes after orthopedic joint surgery and other high-risk procedures.
Opioid use disorder (OUD) is a significant cause of morbidity, mortality, and health care costs in the US.1,2 Surgery can be the inciting cause for exposure to an opioid; as many as 23% of patients develop chronic OUD following surgery.3,4 Patients with a history of substance use, mood disorders, anxiety, or previous chronic opioid use (COU) are at risk for relapse, dose escalation, and poor pain control after high-risk surgery, such as orthopedic joint procedures.5 Recently focus has been on identifying high-risk patients before orthopedic joint surgery and implementing evidence-based strategies that reduce the postoperative incidence of COU.
A transitional pain service (TPS) has been shown to reduce COU for high-risk surgical patients in different health care settings.6-9 The TPS model bundles multiple interventions that can be applied to patients at high risk for COU within a health care system. This includes individually tailored programs for preoperative education or pain management planning, use of multimodal analgesia (including regional or neuraxial techniques or nonopioid systemic medications), application of nonpharmacologic modalities (such as cognitive-based intervention), and a coordinated approach to postdischarge instructions and transitions of care. These interventions are coordinated by a multidisciplinary clinical service consisting of anesthesiologists and advanced practice clinicians with specialization in acute pain management and opioid tapering, nurse care coordinators, and psychologists with expertise in cognitive behavioral therapy.
TPS has been shown to reduce the incidence of COU for patients undergoing orthopedic joint surgery, but its impact on health care use and costs is unknown.6-9 The TPS intervention is resource intensive and increases the use of health care for preoperative education or pain management, which may increase the burden of costs. However, reducing long-term COU may reduce the use of health care for COU- and OUD-related complications, leading to cost savings. This study evaluated whether the TPS intervention influenced health care use and cost for inpatient, outpatient, or pharmacy services during the year following orthopedic joint surgery compared with that of the standard pain management care for procedures that place patients at high risk for COU. We used a difference-in-differences (DID) analysis to estimate this intervention effect, using multivariable regression models that controlled for unobserved time trends and cohort characteristics.
METHODS
This was a quasi-experimental study of patients who underwent orthopedic joint surgery and associated procedures at high risk for COU at the Veterans Affairs Salt Lake City Healthcare System (VASLCHS) between January 2016 through April 2020. The pre-TPS period between January 2016 through December 2017 was compared with the post-TPS period between January 2018 to September 2019. The control patient cohort was selected from 5 geographically diverse VA health care systems throughout the US: Eastern Colorado, Central Plains (Nebraska), White River Junction (Vermont), North Florida/South Georgia, and Portland (Oregon). By sampling health care costs from VA medical centers (VAMCs) across these different regions, our control group was generalizable to veterans receiving orthopedic joint surgery across the US. This study used data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse, a repository of nearly all clinical and administrative data found in electronic health records for VA-provided care and fee-basis care paid for by the VA.10 All data were hosted and analyzed in the VA Informatics and Computing Infrastructure (VINCI) workspace. The University of Utah Institutional Review Board and the VASLCHS Office of Research and Development approved the protocol for this study.
TPS Intervention
The VASLCHS TPS has already been described in detail elsewhere.6,7 Briefly, patients at high risk for COU at the VASLCHS were enrolled in the TPS program before surgery for total knee, hip, or shoulder arthroplasty or rotator cuff procedures. The TPS service consists of an anesthesiologist and advanced practice clinician with specialization in acute pain management and opioid tapering, a psychologist with expertise in cognitive behavioral therapy, and 3 nurse care coordinators. These TPS practitioners work together to provide preoperative education, including setting expectations regarding postoperative pain, recommending nonopioid pain management strategies, and providing guidance regarding the appropriate use of opioids for surgical pain. Individual pain plans were developed and implemented for the perioperative period. After surgery, the TPS provided recommendations and support for nonopioid pain therapies and opioid tapers. Patients were followed by the TPS team for at least 12 months after surgery. At a minimum, the goals set by TPS included cessation of all opioid use for prior nonopioid users (NOU) by 90 days after surgery and the return to baseline opioid use or lower for prior COU patients by 90 days after surgery. The TPS also encouraged and supported opioid tapering among COU patients to reduce or completely stop opioid use after surgery.
Patient Cohorts
Veterans having primary or revision total knee, hip, or shoulder arthroplasty or rotator cuff repair between January 1, 2016, and September 30, 2019, at the aforementioned VAMCs were included in the study. Patients who had any hospitalization within 90 days pre- or postindex surgery or who died within 8 months after surgery were excluded from analysis. Patients who had multiple surgeries during the index inpatient visit or within 90 days after the index surgery also were excluded. Comorbid conditions for mental health and substance use were identified using the International Classification of Diseases, 10th revision Clinical Modification (ICD-10) codes or 9th revision equivalent grouped by Clinical Classifications Software Refined (CCS-R).11 Preoperative exposure to clinically relevant pharmacotherapy (ie, agents associated with prolonged opioid use and nonopioid adjuvants) was captured using VA outpatient prescription records (eAppendix 1).
Outcome Variables
Outcome variables included health care use and costs during 1-year pre- and postperiods from the date of surgery. VA health care costs for outpatient, inpatient, and pharmacy services for direct patient care were collected from the Managerial Cost Accounting System, an activity-based cost allocation system that generates estimates of the cost of individual VA hospital stays, health care encounters, and medications. Health care use was defined as the number of encounters for each visit type in the Managerial Cost Accounting System. All costs were adjusted to 2019 US dollars, using the Personal Consumption Expenditures price index for health care services.15
A set of sociodemographic variables including sex, age at surgery, race and ethnicity, rurality, military branch (Army, Air Force, Marine Corps, Navy, and other), and service connectivity were included as covariates in our regression models.
Statistical Analyses
Descriptive analyses were used to evaluate differences in baseline patient sociodemographic and clinical characteristics between pre- and postperiods for TPS intervention and control cohorts using 2-sample t tests for continuous variables and χ2 tests for categorical variables. We summarized unadjusted health care use and costs for outpatient, inpatient, and pharmacy visits and compared the pre- and postintervention periods using the Mann-Whitney test. Both mean (SD) and median (IQR) were considered, reflecting the skewed distribution of the outcome variables.
We used a DID approach to assess the intervention effect while minimizing confounding from the nonrandom sample. The DID approach controls for unobserved differences between VAMCs that are related to both the intervention and outcomes while controlling for trends over time that could affect outcomes across clinics. To implement the DID approach, we included 3 key independent variables in our regression models: (1) an indicator for whether the observation occurred in the postintervention period; (2) an indicator for whether the patient was exposed to the TPS intervention; and (3) the interaction between these 2 variables.
For cost outcomes, we used multivariable generalized linear models with a log link and a Poisson or Υ family. We analyzed inpatient costs using a 2-part generalized linear model because only 17% to 20% of patients had ≥ 1 inpatient visit. We used multivariable negative binomial regression for health care use outcomes. Demographic and clinical covariates described earlier were included in the regression models to control for differences in the composition of patient groups and clinics that could lead to confounding bias.
RESULTS
Of the 4954 patients included in our study cohort, 3545 (71.6%) were in the NOU group and 1409 (28.4%) were in the COU group. Among the NOU cohort, 361 patients were in the intervention group and 3184 in the control group. Among the COU cohort, 149 patients were in the intervention group and 1260 in the control group (Table 1). Most patients were male, White race, with a mean (SD) age of 64 (11) years. The most common orthopedic procedure was total knee arthroplasty, followed by total hip arthroplasty. Among both NOU and COU cohorts, patients’ characteristics were similar between the pre- and postintervention period among either TPS or control cohort.
Figures 1 and 2 and eAppendix 2 depict unadjusted per-person average outpatient, inpatient, and pharmacy visits and costs incurred during the 1-year pre- and postintervention periods for the NOU and COU cohorts. Average total health care follow-up costs ranged from $40,000 to $53,000 for NOU and from $47,000 to $82,000 for COU cohort. Cost for outpatient visits accounted for about 70% of the average total costs, followed by costs for inpatient visits of about 20%, and costs for pharmacy for the remaining.
For the NOU cohort, the number of health care encounters remained fairly stable between periods except for the outpatient visits among the TPS group. The TPS group experienced an increase in mean outpatient visits in the postperiod: 30 vs 37 visits (23%) (
Table 2 summarizes the results from the multivariable DID analyses for the outpatient, inpatient, and pharmacy visit and cost outcomes. Here, the estimated effect of the TPS intervention is the coefficient from the interaction between the postintervention and TPS exposure indicator variables. This coefficient was calculated as the difference in the outcome before and after the TPS intervention among the TPS group minus the difference in the outcome before and after the TPS intervention among the control group. For the NOU cohort, TPS was associated with an increase in the use of outpatient health care (mean [SD] increase of 6.9 [2] visits; P < .001) after the surgery with no statistically significant effect on outpatient costs (mean [SD] increase of $2787 [$3749]; P = .55). There was no statistically significant effect of TPS on the use of inpatient visits or pharmacy, but a decrease in costs for inpatient visits among those who had at least 1 inpatient visit (mean [SD] decrease of $12,170 [$6100]; P = .02). For the COU cohort, TPS had no statistically significant impact on the use of outpatient, inpatient, or pharmacy or the corresponding costs.
DISCUSSION
TPS is a multidisciplinary approach to perioperative pain management that has been shown to reduce both the quantity and duration of opioid use among orthopedic surgery patients.6,7 Although the cost burden of providing TPS services to prevent COU is borne by the individual health care system, it is unclear whether this expense is offset by lower long-term medical costs and health care use for COU- and OUD-related complications. In this study focused on a veteran population undergoing orthopedic joint procedures, a DID analysis of cost and health care use showed that TPS, which has been shown to reduce COU for high-risk surgical patients, can be implemented without increasing the overall costs to the VA health care system during the 1 year following surgery, even with increased outpatient visits. For NOU patients, there was no difference in outpatient visit costs or pharmacy costs over 12 months after surgery, although there was a significant reduction in subsequent inpatient costs over the same period. Further, there was no difference in outpatient, inpatient, or pharmacy costs after surgery for COU patients. These findings suggest that TPS can be a cost-effective approach to reduce opioid use among patients undergoing orthopedic joint surgery in VAMCs.
The costs of managing COU after surgery are substantial. Prior reports have shown that adjusted total health care costs are 1.6 to 2.5 times higher for previously NOU patients with new COU after major surgery than those for such patients without persistent use.16 The 1-year costs associated with new COU in this prior study ranged between $7944 and $17,702 after inpatient surgery and between $5598 and $12,834 after outpatient index surgery, depending on the payer, which are in line with the cost differences found in our current study. Another report among patients with COU following orthopedic joint replacement showed that they had higher use of inpatient, emergency department, and ambulance/paramedic services in the 12 months following their surgery than did those without persistent use.17 Although these results highlight the impact that COU plays in driving increased costs after major surgery, there have been limited studies focused on interventions that can neutralize the costs associated with opioid misuse after surgery. To our knowledge, our study is the first analysis to show the impact of using an intervention such as TPS to reduce postoperative opioid use on health care use and cost.
Although a rigorous and comprehensive return on investment analysis was beyond the scope of this analysis, these results may have several implications for other health care systems and hospitals that wish to invest in a multidisciplinary perioperative pain management program such as TPS but may be reluctant due to the upfront investment. First, the increased number of patient follow-up visits needed during TPS seems to be more than offset by the reduction in opioid use and associated complications that may occur after surgery. Second, TPS did not seem to be associated with an increase in overall health care costs during the 1-year follow-up period. Together, these results indicate that the return on investment for a TPS approach to perioperative pain management in which optimal patient-centered outcomes are achieved without increasing long-term costs to a health care system may be positive.
Limitations
This study has several limitations. First, this was a quasi-experimental observational study, and the associations we identified between intervention and outcomes should not be assumed to demonstrate causality. Although our DID analysis controlled for an array of demographic and clinical characteristics, differences in medical costs and health care use between the 2 cohorts might be driven by unobserved confounding variables.
Our study also was limited to veterans who received medical care at the VA, and results may not be generalizable to other non-VA health care systems or to veterans with Medicare insurance who have dual benefits. While our finding on health care use and costs may be incomplete because of the uncaptured health care use outside the VA, our DID analysis helped reduce unobserved bias because the absence of data outside of VA care applies to both TPS and control groups. Further, the total costs of operating a TPS program at any given institution will depend on the size of the hospital and volume of surgical patients who meet criteria for enrollment. However, the relative differences in health care use and costs may be extrapolated to patients undergoing orthopedic surgery in other types of academic and community-based health care systems.
Furthermore, this analysis focused primarily on COU and NOU patients undergoing orthopedic joint surgery. While this represents a high-risk population for OUD, the costs and health care use associated with delivering the TPS intervention to other types of surgical procedures may be significantly different. All costs in this analysis were based on 2019 estimates and do not account for the potential inflation over the past several years. Nonmonetary costs to the patient and per-person average total intervention costs were not included in the study. However, we assumed that costs associated with TPS and standard of care would have increased to an equivalent degree over the same period. Further, the average cost of TPS per patient (approximately $900) is relatively small compared with the average annual costs during 1-year pre- and postoperative periods and was not expected to have a significant effect on the analysis.
Conclusions
We found that the significant reduction in COU seen in previous studies following the implementation of TPS was not accompanied by increased health care costs.6,7 When considering the other costs of long-term opioid use, such as abuse potential, overdose, death, and increased disability, implementation of a TPS service has the potential to improve patient quality of life while reducing other health-related costs. Health care systems should consider the implementation of similar multidisciplinary approaches to perioperative pain management to improve outcomes after orthopedic joint surgery and other high-risk procedures.
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. doi:10.15585/mmwr.mm655051e1
2. Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901-906. doi:10.1097/MLR.0000000000000625
3. Jiang X, Orton M, Feng R, et al. Chronic opioid usage in surgical patients in a large academic center. Ann Surg. 2017;265(4):722-727. doi:10.1097/SLA.0000000000001780
4. Johnson SP, Chung KC, Zhong L, et al. Risk of prolonged opioid use among opioid-naive patients following common hand surgery procedures. J Hand Surg Am. 2016;41(10):947-957, e3. doi:10.1016/j.jhsa.2016.07.113
5. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504. doi:10.1001/jamasurg.2017.0504
6. Buys MJ, Bayless K, Romesser J, et al. Multidisciplinary transitional pain service for the veteran population. Fed Pract. 2020;37(10):472-478. doi:10.12788/fp.0053
7. Buys MJ, Bayless K, Romesser J, et al. Opioid use among veterans undergoing major joint surgery managed by a multidisciplinary transitional pain service. Reg Anesth Pain Med. 2020;45(11):847-852. doi:10.1136/rapm-2020-101797
8. Huang A, Katz J, Clarke H. Ensuring safe prescribing of controlled substances for pain following surgery by developing a transitional pain service. Pain Manag. 2015;5(2):97-105. doi:10.2217/pmt.15.7
9. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695-702. doi:10.2147/JPR.S91924
10. Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
11. Agency for Healthcare Research and Quality. Clinical Classifications Software Refined (CCSR). Updated December 9, 2022. Accessed October 30, 2023. www.hcup-us.ahrq.gov/toolssoftware/ccsr/ccs_refined.jsp
12. Mosher HJ, Richardson KK, Lund BC. The 1-year treatment course of new opioid recipients in Veterans Health Administration. Pain Med. 2016;17(7):1282-1291. doi:10.1093/pm/pnw058
13. Hadlandsmyth K, Mosher HJ, Vander Weg MW, O’Shea AM, McCoy KD, Lund BC. Utility of accumulated opioid supply days and individual patient factors in predicting probability of transitioning to long-term opioid use: an observational study in the Veterans Health Administration. Pharmacol Res Perspect. 2020;8(2):e00571. doi:10.1002/prp2.571
14. Pagé MG, Kudrina I, Zomahoun HTV, et al. Relative frequency and risk factors for long-term opioid therapy following surgery and trauma among adults: a systematic review protocol. Syst Rev. 2018;7(1):97. doi:10.1186/s13643-018-0760-3
15. US. Bureau of Economic Analysis. Price indexes for personal consumption expenditures by major type of product. Accessed October 30, 2023. https://apps.bea.gov/iTable/?reqid=19&step=3&isuri=1&nipa_table_list=64&categories=survey
16. Brummett CM, Evans-Shields J, England C, et al. Increased health care costs associated with new persistent opioid use after major surgery in opioid-naive patients. J Manag Care Spec Pharm. 2021;27(6):760-771. doi:10.18553/jmcp.2021.20507
17. Gold LS, Strassels SA, Hansen RN. Health care costs and utilization in patients receiving prescriptions for long-acting opioids for acute postsurgical pain. Clin J Pain. 2016;32(9):747-754. doi:10.1097/ajp.0000000000000322
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. doi:10.15585/mmwr.mm655051e1
2. Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901-906. doi:10.1097/MLR.0000000000000625
3. Jiang X, Orton M, Feng R, et al. Chronic opioid usage in surgical patients in a large academic center. Ann Surg. 2017;265(4):722-727. doi:10.1097/SLA.0000000000001780
4. Johnson SP, Chung KC, Zhong L, et al. Risk of prolonged opioid use among opioid-naive patients following common hand surgery procedures. J Hand Surg Am. 2016;41(10):947-957, e3. doi:10.1016/j.jhsa.2016.07.113
5. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504. doi:10.1001/jamasurg.2017.0504
6. Buys MJ, Bayless K, Romesser J, et al. Multidisciplinary transitional pain service for the veteran population. Fed Pract. 2020;37(10):472-478. doi:10.12788/fp.0053
7. Buys MJ, Bayless K, Romesser J, et al. Opioid use among veterans undergoing major joint surgery managed by a multidisciplinary transitional pain service. Reg Anesth Pain Med. 2020;45(11):847-852. doi:10.1136/rapm-2020-101797
8. Huang A, Katz J, Clarke H. Ensuring safe prescribing of controlled substances for pain following surgery by developing a transitional pain service. Pain Manag. 2015;5(2):97-105. doi:10.2217/pmt.15.7
9. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695-702. doi:10.2147/JPR.S91924
10. Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
11. Agency for Healthcare Research and Quality. Clinical Classifications Software Refined (CCSR). Updated December 9, 2022. Accessed October 30, 2023. www.hcup-us.ahrq.gov/toolssoftware/ccsr/ccs_refined.jsp
12. Mosher HJ, Richardson KK, Lund BC. The 1-year treatment course of new opioid recipients in Veterans Health Administration. Pain Med. 2016;17(7):1282-1291. doi:10.1093/pm/pnw058
13. Hadlandsmyth K, Mosher HJ, Vander Weg MW, O’Shea AM, McCoy KD, Lund BC. Utility of accumulated opioid supply days and individual patient factors in predicting probability of transitioning to long-term opioid use: an observational study in the Veterans Health Administration. Pharmacol Res Perspect. 2020;8(2):e00571. doi:10.1002/prp2.571
14. Pagé MG, Kudrina I, Zomahoun HTV, et al. Relative frequency and risk factors for long-term opioid therapy following surgery and trauma among adults: a systematic review protocol. Syst Rev. 2018;7(1):97. doi:10.1186/s13643-018-0760-3
15. US. Bureau of Economic Analysis. Price indexes for personal consumption expenditures by major type of product. Accessed October 30, 2023. https://apps.bea.gov/iTable/?reqid=19&step=3&isuri=1&nipa_table_list=64&categories=survey
16. Brummett CM, Evans-Shields J, England C, et al. Increased health care costs associated with new persistent opioid use after major surgery in opioid-naive patients. J Manag Care Spec Pharm. 2021;27(6):760-771. doi:10.18553/jmcp.2021.20507
17. Gold LS, Strassels SA, Hansen RN. Health care costs and utilization in patients receiving prescriptions for long-acting opioids for acute postsurgical pain. Clin J Pain. 2016;32(9):747-754. doi:10.1097/ajp.0000000000000322
Multidisciplinary Transitional Pain Service for the Veteran Population
Despite advancements in techniques, postsurgical pain continues to be a prominent part of the patient experience. Often this experience can lead to developing chronic postsurgical pain that interferes with quality of life after the expected time to recovery.1-3 As many as 14% of patients who undergo surgery without any history of opioid use develop chronic opioid use that persists after recovery from their operation.4-8 For patients with existing chronic opioid use or a history of substance use disorder (SUD), surgeons, primary care providers, or addiction providers often do not provide sufficient presurgical planning or postsurgical coordination of care. This lack of pain care coordination can increase the risk of inadequate pain control, opioid use escalation, or SUD relapse after surgery.
Convincing arguments have been made that a perioperative surgical home can improve significantly the quality of perioperative care.9-14 This report describes our experience implementing a perioperative surgical home at the US Department of Veterans Affairs (VA) Salt Lake City VA Medical Center (SLCVAMC), focusing on pain management extending from the preoperative period until 6 months or more after surgery. This type of Transitional Pain Service (TPS) has been described previously.15-17 Our service differs from those described previously by enrolling all patients before surgery rather than select postsurgical enrollment of only patients with a history of opioid use or SUD or patients who struggle with persistent postsurgical pain.
Methods
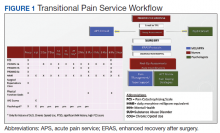
In January 2018, we developed and implemented a new TPS at the SLCVAMC. The transitional pain team consisted of an anesthesiologist with specialization in acute pain management, a nurse practitioner (NP) with experience in both acute and chronic pain management, 2 nurse care coordinators, and a psychologist (Figure 1). Before implementation, a needs assessment took place with these key stakeholders and others at SLCVAMC to identify the following specific goals of the TPS: (1) reduce pain through pharmacologic and nonpharmacologic interventions; (2) eliminate new chronic opioid use in previously nonopioid user (NOU) patients; (3) address chronic opioid use in previous chronic opioid users (COUs) by providing support for opioid taper and alternative analgesic therapies for their chronic pain conditions; and (4) improve continuity of care by close coordination with the surgical team, primary care providers (PCPs), and mental health or chronic pain providers as needed.
Once these TPS goals were defined, the Consolidated Framework for Implementation Research (CFIR) guided the implementation. CFIR is a theory-based implementation framework consisting of 5 domains: intervention characteristics, inner setting, outer setting, characteristics of individuals, and process. These domains were used to identify barriers and facilitators during the early implementation process and helped refine TPS as it was put into clinical practice.
Patient Selection
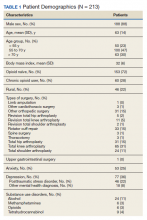
During the initial implementation of TPS, enrollment was limited to patients scheduled for elective primary or revision knee, hip, or shoulder replacement as well as rotator cuff repair surgery. But as the TPS workflow became established after iterative refinement, we expanded the program to enroll patients with established risk factors for OUD having other types of surgery (Table 1). The diagnosis of risk factors, such as history of SUD, chronic opioid use, or significant mental health disorders (ie, history of suicidal ideation or attempt, posttraumatic stress disorder, and inpatient psychiatric care) were confirmed through both in-person interviews and electronic health record (EHR) documentation. The overall goal was to identify all at-risk patients as soon as they were indicated for surgery, to allow time for evaluation, education, developing an individualized pain plan, and opioid taper prior to surgery if indicated.
Preoperative Procedures
Once identified, patients were contacted by a TPS team member and invited to attend a onetime 90-minute presurgical expectations class held at SLCVAMC. The education curriculum was developed by the whole team, and classes were taught primarily by the TPS psychologist. The class included education about expectations for postoperative pain, available analgesic therapies, opioid education, appropriate use of opioids, and the effect of psychological factors on pain. Pain coping strategies were introduced using a mindfulness-based intervention (MBI) and the Acceptance and Commitment Therapy (ACT) matrix. Classes were offered multiple times a week to help maximize convenience for patients and were separate from the anesthesia preoperative evaluation. Patients attended class only once. High-risk patients (patients with chronic opioid therapy, recent history of or current SUDs, significant comorbid mental health issues) were encouraged to attend this class one-on-one with the TPS psychologist rather than in the group setting, so individual attention to mental health and SUD issues could be addressed directly.
Baseline history, morphine equivalent daily dose (MEDD), and patient-reported outcomes using measures from the Patient-Reported Outcome Measurement System (PROMIS) for pain intensity (PROMIS 3a), pain interference (PROMIS 6b), and physical function (PROMIS 8b), and a pain-catastrophizing scale (PCS) score were obtained on all patients.18 PROMIS measures are validated questionnaires developed with the National Institutes of Health to standardize and quantify patient-reported outcomes in many domains.19 Patients with a history of SUD or COU met with the anesthesiologist and/or NP, and a personalized pain plan was developed that included preoperative opioid taper, buprenorphine use strategy, or opioid-free strategies.
Hospital Procedures
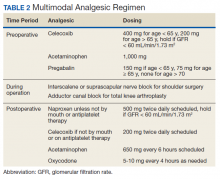
On the day of surgery, the TPS team met with the patient preoperatively and implemented an individualized pain plan that included multimodal analgesic techniques with nonsteroidal anti-inflammatory drugs, acetaminophen, gabapentinoids, and regional anesthesia, where appropriate (Table 2). Enhanced recovery after surgery protocols were developed in conjunction with the surgeons to include local infiltration analgesia by the surgeon, postoperative multimodal analgesic strategies, and intensive physical therapy starting the day of surgery for inpatient procedures.
After surgery, the TPS team followed up with patients daily and provided recommendations for analgesic therapies. Patients were offered daily sessions with the psychologist to reinforce and practice nonpharmacologic pain-coping strategies, such as meditation and relaxation. Prior to patient discharge, the TPS team provided recommendations for discharge medications and an opioid taper plan. For some patients taking buprenorphine before surgery who had stopped this therapy prior to or during their hospital stay, TPS providers transitioned them back to buprenorphine before discharge.
Postoperative Procedures
Patients were called by the nurse care coordinators at postdischarge days 2, 7, 10, 14, 21, 28, and then monthly for ≥ 6 months. For patients who had not stopped opioid use or returned to their preoperative baseline opioid dose, weekly calls were made until opioid taper goals were achieved. At each call, nurses collected PROMIS scores for the previous 24 hours, the most recent 24-hour MEDD, the date of last opioid use, and the number of remaining opioid tablets after opioid cessation. In addition, nurses provided active listening and supportive care and encouragement as well as care coordination for issues related to rehabilitation facilities, physical therapy, transportation, medication questions, and wound questions. Nurses notified the anesthesiologist or NP when patients were unable to taper opioid use or had poor pain control as indicated by their PROMIS scores, opioid use, or directly expressed by the patient.
The TPS team prescribed alternative analgesic therapies, opioid taper plans, and communicated with surgeons and primary care providers if limited continued opioid therapy was recommended. Individual sessions with the psychologist were available to patients after discharge with a focus on ACT-matrix therapy and consultation with long-term mental health and/or substance abuse providers as indicated. Frequent communication and care coordination were maintained with the surgical team, the PCP, and other providers on the mental health or chronic pain services. This care coordination often included postsurgical joint clinic appointments in which TPS providers and nurses would be present with the surgeon or the PCP.
For patients with inadequately treated chronic pain conditions or who required long-term opioid tapers, we developed a combined clinic with the TPS and Anesthesia Chronic Pain group. This clinic allows patients to be seen by both services in the same setting, allowing a warm handoff by TPS to the chronic pain team.
Heath and Decision Support Tools
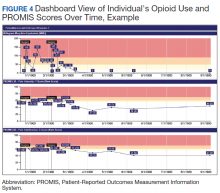
An electronic dashboard registry of surgical episodes managed by TPS was developed to achieve clinical, administrative, and quality improvement goals. The dashboard registry consists of surgical episode data, opioid doses, patient-reported outcomes, and clinical decision-making processes. Custom-built note templates capture pertinent data through embedded data labels, called health factors. Data are captured as part of routine clinical care, recorded in Computerized Patient Record System as health factors. They are available in the VA Corporate Data Warehouse as structured data. Workflows are executed daily to keep the dashboard registry current, clean, and able to process new data. Information displays direct daily clinical workflow and support point-of-care clinical decision making (Figures 2, 3, and 4). Data are aggregated across patient-care encounters and allow nurse care coordinators to concisely review pertinent patient data prior to delivering care. These data include surgical history, comorbidities, timeline of opioid use, and PROMIS scores during their course of recovery. This system allows TPS to optimize care delivery by providing longitudinal data across the surgical episode, thereby reducing the time needed to review records. Secondary purposes of captured data include measuring clinic performance and quality improvement to improve care delivery.
Results
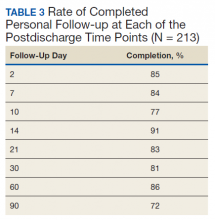
The TPS intervention was implemented January 1, 2018. Two-hundred thirteen patients were enrolled between January and December 2018, which included 60 (28%) patients with a history of chronic opioid use and 153 (72%) patients who were considered opioid naïve. A total of 99% of patients had ≥ 1 successful follow-up within 14 days after discharge, 96% had ≥ 1 follow-up between 14 and 30 days after surgery, and 72% had completed personal follow-up 90 days after discharge (Table 3). For patients who TPS was unable to contact in person or by phone, 90-day MEDD was obtained using prescription and Controlled Substance Database reviews. The protocol for this retrospective analysis was approved by the University of Utah Institutional Review Board and the VA Research Review Committee.
By 90 days after surgery, 26 (43.3%) COUs were off opioids completely, 17 (28.3%) had decreased their opioid dose from their preoperative baseline MEDD (120 [SD, 108] vs 55 [SD, 45]), 14 (23.3%) returned to their baseline dose, and 3 (5%) increased from their baseline dose. Of the 153 patients who were NOUs before surgery, only 1 (0.7%) was taking opioids after 90 days. TPS continued to work closely with the patient and their PCP and that patient was finally able to stop opioid use 262 days after discharge. Ten patients had an additional surgery within 90 days of the initial surgery. Of these, 6 were COU, of whom 3 stopped all opioids by 90 days from their original surgery, 2 had no change in MEDD at 90 days, and 1 had a lower MEDD at 90 days. Of the 4 NOU who had additional surgery, all were off opioids by 90 days from the original surgery.
Although difficult to quantify, a meaningful outcome of TPS has been to improve satisfaction substantially among health care providers caring for complex patients at risk for chronic opioid abuse. This group includes the many members of the surgical team, PCPs, and addiction specialists who appreciate the close care coordination and assistance in caring for patients with difficult issues, especially with opioid tapers or SUDs. We also have noticed changes in prescribing practices among surgeons and PCPs for their patients who are not part of TPS.
Discussion
With any new clinical service, there are obstacles and challenges. TPS requires a considerable investment in personnel, and currently no mechanism is in place for obtaining payment for many of the provided services. We were fortunate the VA Whole Health Initiative, the VA Office of Rural Health, and the VA Centers of Innovation provided support for the development, implementation, and pilot evaluation of TPS. After we presented our initial results to hospital leadership, we also received hospital support to expand TPS service to include a total of 4 nurse care coordinators and 2 psychologists. We are currently performing a cost analysis of the service but recognize that this model may be difficult to reproduce at other institutions without a change in reimbursement standards.
Developing a working relationship with the surgical and primary care services required a concerted effort from the TPS team and a number of months to become effective. As most veterans receive primary care, mental health care, and surgical care within the VA system, this model lends itself to close care coordination. Initially there was skepticism about TPS recommendations to reduce opioid use, especially from PCPs who had cared for complex patients over many years. But this uncertainty went away as we showed evidence of close patient follow-up and detailed communication. TPS soon became the designated service for both primary care and surgical providers who were otherwise uncomfortable with how to approach opioid tapers and nonopioid pain strategies. In fact, a substantial portion of our referrals now come directly from the PCP who is referring a high-risk patient for evaluation for surgery rather than from the surgeons, and joint visits with TPS and primary care have become commonplace.
Challenges abound when working with patients with substance abuse history, opioid use history, high anxiety, significant pain catastrophizing, and those who have had previous negative experiences with surgery. We have found that the most important facet of our service comes from the amount of time and effort team members, especially the nurses, spend helping patients. Much of the nurses' work focuses on nonpain-related issues, such as assisting patients with finding transportation, housing issues, questions about medications, help scheduling appointments, etc. Through this concerted effort, patients gain trust in TPS providers and are willing to listen to and experiment with our recommendations. Many patients who were initially extremely unreceptive to the presurgery education asked for our support weeks after surgery to help with postsurgery pain.
Another challenge we continue to experience comes from the success of the program.
Conclusions
The multidisciplinary TPS supports greater preoperative to postoperative longitudinal care for surgical patients. This endeavor has resulted in better patient preparation before surgery and improved care coordination after surgery, with specific improvements in appropriate use of opioid medications and smooth transitions of care for patients with ongoing and complex needs. Development of sophisticated note templates and customized health information technology allows for accurate follow-through and data gathering for quality improvement, facilitating data-driven improvements and proving value to the facility.
Given that TPS is a multidisciplinary program with multiple interventions, it is difficult to pinpoint which specific aspects of TPS are most effective in achieving success. For example, although we have little doubt that the work our psychologists do with our patients is beneficial and even essential for the success we have had with some of our most difficult patients, it is less clear whether it matters if they use mindfulness, ACT matrix, or cognitive behavioral therapy. We think that an important part of TPS is the frequent human interaction with a caring individual. Therefore, as TPS continues to grow, maintaining the ability to provide frequent personal interaction is a priority.
The role of opioids in acute pain deserves further scrutiny. In 2018, with TPS use of opioids after orthopedic surgery decreased by > 40% from the previous year. Despite this more restricted use of opioids, pain interference and physical function scores indicated that surgical patients do not seem to experience increased pain or reduced physical function. In addition, stopping opioid use for COUs did not seem to affect the quality of recovery, pain, or physical function. Future prospective controlled studies of TPS are needed to confirm these findings and identify which aspects of TPS are most effective in improving functional recovery of patients. Also, more evidence is needed to determine the appropriateness or need for opioids in acute postsurgical pain.
TPS has expanded to include all surgical specialties. Given the high burden and limited resources, we have chosen to focus on patients at higher risk for chronic postsurgical pain by type of surgery (eg, thoracotomy, open abdominal, limb amputation, major joint surgery) and/or history of substance abuse or chronic opioid use. To better direct scarce resources where it would be of most benefit, we are now enrolling only NOUs without other risk factors postoperatively if they request a refill of opioids or are otherwise struggling with pain control after surgery. Whether this approach affects the success we had in the first year in preventing new COUs after surgery remains to be seen.
It is unlikely that any single model of a perioperative surgical home will fit the needs of the many different types of medical systems that exist. The TPS model fits well in large hospital systems, like the VA, where patients receive most of their care within the same system. However, it seems to us that the optimal TPS program in any health system will provide education, support, and care coordination beginning preoperatively to prepare the patient for surgery and then to facilitate care coordination to transition patients back to their PCPs or on to specialized chronic care.
Acknowledgments
We would like to acknowledge the contributions of Candice Harmon, RN; David Merrill, RN; Amy Beckstead, RN, who have provided invaluable assistance with establishing the TPS program at the VA Salt Lake City and helping with the evaluation process.
Funding for the implementation and evaluation of the TPS was received from the VA Whole Health Initiative, the VA Center of Innovation, the VA Office of Rural Health, and National Institutes of Health Grant UL1TR002538.
1. Ilfeld BM, Madison SJ, Suresh PJ. Persistent postmastectomy pain and pain-related physical and emotional functioning with and without a continuous paravertebral nerve block: a prospective 1-year follow-up assessment of a randomized, triple-masked, placebo-controlled study. Ann Surg Oncol. 2015;22(6):2017-2025. doi:10.1245/s10434-014-4248-7
2. Richebé P, Capdevila X, Rivat C. Persistent postsurgical pain. Anesthesiology. 2018;129(3):590-607. doi:10.1097/aln.0000000000002238
3. Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393(10180):1537-1546. doi:10.1016/s0140-6736(19)30352-6
4. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surgery. 2017;152(6):e170504-e170504. doi:10.1001/jamasurg.2017.0504
5. Swenson CW, Kamdar NS, Seiler K, Morgan DM, Lin P, As-Sanie S. Definition development and prevalence of new persistent opioid use following hysterectomy. Am J Obstet Gynecol. 2018;219(5):486.e1-486.e7. doi:10.1016/j.ajog.2018.06.010
6. Bartels K, Fernandez-Bustamante A, McWilliams SK, Hopfer CJ, Mikulich-Gilbertson SK. Long-term opioid use after inpatient surgery - a retrospective cohort study. Drug Alcohol Depend. 2018;187:61-65. doi:10.1016/j.drugalcdep.2018.02.013
7. Bedard N, DeMik D, Dowdle S, Callaghan J. Trends and risk factors for prolonged opioid use after unicompartmental knee arthroplasty. Bone Joint J. 2018;100-B(1)(suppl A):62-67. doi:10.1302/0301-620x.100b1.bjj-2017-0547.r1
8. Politzer CS, Kildow BJ, Goltz DE, Green CL, Bolognesi MP, Seyler T. Trends in opioid utilization before and after total knee arthroplasty. J Arthroplasty. 2018;33(7S):S147-S153.e1. doi:10.1016/j.arth.2017.10.060
9. Mariano ER, Walters TL, Kim ET, Kain ZN. Why the perioperative surgical home makes sense for Veterans Affairs health care. Anesth Analg. 2015;120(5):1163-1166. doi:10.1213/ane.0000000000000712
10. Walters TL, Howard SK, Kou A, et al. Design and implementation of a perioperative surgical home at a Veterans Affairs hospital. Semin Cardiothorac Vasc Anesth. 2016;20(2):133-140. doi:10.1177/1089253215607066
11. Walters TL, Mariano ER, Clark DJ. Perioperative surgical home and the integral role of pain medicine. Pain Med. 2015;16(9):1666-1672. doi:10.1111/pme.12796
12. Vetter TR, Kain ZN. Role of the perioperative surgical home in optimizing the perioperative use of opioids. Anesth Analg. 2017;125(5):1653-1657. doi:10.1213/ane.0000000000002280
13. Shafer SL. Anesthesia & Analgesia’s 2015 collection on the perioperative surgical home. Anesth Analg. 2015;120(5):966-967. doi:10.1213/ane.0000000000000696
14. Wenzel JT, Schwenk ES, Baratta JL, Viscusi ER. Managing opioid-tolerant patients in the perioperative surgical home. Anesthesiol Clin. 2016;34(2):287-301. doi:10.1016/j.anclin.2016.01.005
15. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695-702. doi:10.2147/jpr.s91924
16. Tiippana E, Hamunen K, Heiskanen T, Nieminen T, Kalso E, Kontinen VK. New approach for treatment of prolonged postoperative pain: APS Out-Patient Clinic. Scand J Pain. 2016;12(1):19-24. doi:10.1016/j.sjpain.2016.02.008
17. Katz J, Weinrib AZ, Clarke H. Chronic postsurgical pain: from risk factor identification to multidisciplinary management at the Toronto General Hospital Transitional Pain Service. Can J Pain. 2019;3(2):49-58. doi:10.1080/24740527.2019.1574537
18. Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7(4):524-532. doi:10.1037/1040-3590.7.4.524
19. HealthMeasures. Intro to PROMIS. https://www.healthmeasures.net/explore-measurement-systems/promis. Accessed September 28, 2020.
Despite advancements in techniques, postsurgical pain continues to be a prominent part of the patient experience. Often this experience can lead to developing chronic postsurgical pain that interferes with quality of life after the expected time to recovery.1-3 As many as 14% of patients who undergo surgery without any history of opioid use develop chronic opioid use that persists after recovery from their operation.4-8 For patients with existing chronic opioid use or a history of substance use disorder (SUD), surgeons, primary care providers, or addiction providers often do not provide sufficient presurgical planning or postsurgical coordination of care. This lack of pain care coordination can increase the risk of inadequate pain control, opioid use escalation, or SUD relapse after surgery.
Convincing arguments have been made that a perioperative surgical home can improve significantly the quality of perioperative care.9-14 This report describes our experience implementing a perioperative surgical home at the US Department of Veterans Affairs (VA) Salt Lake City VA Medical Center (SLCVAMC), focusing on pain management extending from the preoperative period until 6 months or more after surgery. This type of Transitional Pain Service (TPS) has been described previously.15-17 Our service differs from those described previously by enrolling all patients before surgery rather than select postsurgical enrollment of only patients with a history of opioid use or SUD or patients who struggle with persistent postsurgical pain.
Methods
In January 2018, we developed and implemented a new TPS at the SLCVAMC. The transitional pain team consisted of an anesthesiologist with specialization in acute pain management, a nurse practitioner (NP) with experience in both acute and chronic pain management, 2 nurse care coordinators, and a psychologist (Figure 1). Before implementation, a needs assessment took place with these key stakeholders and others at SLCVAMC to identify the following specific goals of the TPS: (1) reduce pain through pharmacologic and nonpharmacologic interventions; (2) eliminate new chronic opioid use in previously nonopioid user (NOU) patients; (3) address chronic opioid use in previous chronic opioid users (COUs) by providing support for opioid taper and alternative analgesic therapies for their chronic pain conditions; and (4) improve continuity of care by close coordination with the surgical team, primary care providers (PCPs), and mental health or chronic pain providers as needed.
Once these TPS goals were defined, the Consolidated Framework for Implementation Research (CFIR) guided the implementation. CFIR is a theory-based implementation framework consisting of 5 domains: intervention characteristics, inner setting, outer setting, characteristics of individuals, and process. These domains were used to identify barriers and facilitators during the early implementation process and helped refine TPS as it was put into clinical practice.
Patient Selection
During the initial implementation of TPS, enrollment was limited to patients scheduled for elective primary or revision knee, hip, or shoulder replacement as well as rotator cuff repair surgery. But as the TPS workflow became established after iterative refinement, we expanded the program to enroll patients with established risk factors for OUD having other types of surgery (Table 1). The diagnosis of risk factors, such as history of SUD, chronic opioid use, or significant mental health disorders (ie, history of suicidal ideation or attempt, posttraumatic stress disorder, and inpatient psychiatric care) were confirmed through both in-person interviews and electronic health record (EHR) documentation. The overall goal was to identify all at-risk patients as soon as they were indicated for surgery, to allow time for evaluation, education, developing an individualized pain plan, and opioid taper prior to surgery if indicated.
Preoperative Procedures
Once identified, patients were contacted by a TPS team member and invited to attend a onetime 90-minute presurgical expectations class held at SLCVAMC. The education curriculum was developed by the whole team, and classes were taught primarily by the TPS psychologist. The class included education about expectations for postoperative pain, available analgesic therapies, opioid education, appropriate use of opioids, and the effect of psychological factors on pain. Pain coping strategies were introduced using a mindfulness-based intervention (MBI) and the Acceptance and Commitment Therapy (ACT) matrix. Classes were offered multiple times a week to help maximize convenience for patients and were separate from the anesthesia preoperative evaluation. Patients attended class only once. High-risk patients (patients with chronic opioid therapy, recent history of or current SUDs, significant comorbid mental health issues) were encouraged to attend this class one-on-one with the TPS psychologist rather than in the group setting, so individual attention to mental health and SUD issues could be addressed directly.
Baseline history, morphine equivalent daily dose (MEDD), and patient-reported outcomes using measures from the Patient-Reported Outcome Measurement System (PROMIS) for pain intensity (PROMIS 3a), pain interference (PROMIS 6b), and physical function (PROMIS 8b), and a pain-catastrophizing scale (PCS) score were obtained on all patients.18 PROMIS measures are validated questionnaires developed with the National Institutes of Health to standardize and quantify patient-reported outcomes in many domains.19 Patients with a history of SUD or COU met with the anesthesiologist and/or NP, and a personalized pain plan was developed that included preoperative opioid taper, buprenorphine use strategy, or opioid-free strategies.
Hospital Procedures
On the day of surgery, the TPS team met with the patient preoperatively and implemented an individualized pain plan that included multimodal analgesic techniques with nonsteroidal anti-inflammatory drugs, acetaminophen, gabapentinoids, and regional anesthesia, where appropriate (Table 2). Enhanced recovery after surgery protocols were developed in conjunction with the surgeons to include local infiltration analgesia by the surgeon, postoperative multimodal analgesic strategies, and intensive physical therapy starting the day of surgery for inpatient procedures.
After surgery, the TPS team followed up with patients daily and provided recommendations for analgesic therapies. Patients were offered daily sessions with the psychologist to reinforce and practice nonpharmacologic pain-coping strategies, such as meditation and relaxation. Prior to patient discharge, the TPS team provided recommendations for discharge medications and an opioid taper plan. For some patients taking buprenorphine before surgery who had stopped this therapy prior to or during their hospital stay, TPS providers transitioned them back to buprenorphine before discharge.
Postoperative Procedures
Patients were called by the nurse care coordinators at postdischarge days 2, 7, 10, 14, 21, 28, and then monthly for ≥ 6 months. For patients who had not stopped opioid use or returned to their preoperative baseline opioid dose, weekly calls were made until opioid taper goals were achieved. At each call, nurses collected PROMIS scores for the previous 24 hours, the most recent 24-hour MEDD, the date of last opioid use, and the number of remaining opioid tablets after opioid cessation. In addition, nurses provided active listening and supportive care and encouragement as well as care coordination for issues related to rehabilitation facilities, physical therapy, transportation, medication questions, and wound questions. Nurses notified the anesthesiologist or NP when patients were unable to taper opioid use or had poor pain control as indicated by their PROMIS scores, opioid use, or directly expressed by the patient.
The TPS team prescribed alternative analgesic therapies, opioid taper plans, and communicated with surgeons and primary care providers if limited continued opioid therapy was recommended. Individual sessions with the psychologist were available to patients after discharge with a focus on ACT-matrix therapy and consultation with long-term mental health and/or substance abuse providers as indicated. Frequent communication and care coordination were maintained with the surgical team, the PCP, and other providers on the mental health or chronic pain services. This care coordination often included postsurgical joint clinic appointments in which TPS providers and nurses would be present with the surgeon or the PCP.
For patients with inadequately treated chronic pain conditions or who required long-term opioid tapers, we developed a combined clinic with the TPS and Anesthesia Chronic Pain group. This clinic allows patients to be seen by both services in the same setting, allowing a warm handoff by TPS to the chronic pain team.
Heath and Decision Support Tools
An electronic dashboard registry of surgical episodes managed by TPS was developed to achieve clinical, administrative, and quality improvement goals. The dashboard registry consists of surgical episode data, opioid doses, patient-reported outcomes, and clinical decision-making processes. Custom-built note templates capture pertinent data through embedded data labels, called health factors. Data are captured as part of routine clinical care, recorded in Computerized Patient Record System as health factors. They are available in the VA Corporate Data Warehouse as structured data. Workflows are executed daily to keep the dashboard registry current, clean, and able to process new data. Information displays direct daily clinical workflow and support point-of-care clinical decision making (Figures 2, 3, and 4). Data are aggregated across patient-care encounters and allow nurse care coordinators to concisely review pertinent patient data prior to delivering care. These data include surgical history, comorbidities, timeline of opioid use, and PROMIS scores during their course of recovery. This system allows TPS to optimize care delivery by providing longitudinal data across the surgical episode, thereby reducing the time needed to review records. Secondary purposes of captured data include measuring clinic performance and quality improvement to improve care delivery.
Results
The TPS intervention was implemented January 1, 2018. Two-hundred thirteen patients were enrolled between January and December 2018, which included 60 (28%) patients with a history of chronic opioid use and 153 (72%) patients who were considered opioid naïve. A total of 99% of patients had ≥ 1 successful follow-up within 14 days after discharge, 96% had ≥ 1 follow-up between 14 and 30 days after surgery, and 72% had completed personal follow-up 90 days after discharge (Table 3). For patients who TPS was unable to contact in person or by phone, 90-day MEDD was obtained using prescription and Controlled Substance Database reviews. The protocol for this retrospective analysis was approved by the University of Utah Institutional Review Board and the VA Research Review Committee.
By 90 days after surgery, 26 (43.3%) COUs were off opioids completely, 17 (28.3%) had decreased their opioid dose from their preoperative baseline MEDD (120 [SD, 108] vs 55 [SD, 45]), 14 (23.3%) returned to their baseline dose, and 3 (5%) increased from their baseline dose. Of the 153 patients who were NOUs before surgery, only 1 (0.7%) was taking opioids after 90 days. TPS continued to work closely with the patient and their PCP and that patient was finally able to stop opioid use 262 days after discharge. Ten patients had an additional surgery within 90 days of the initial surgery. Of these, 6 were COU, of whom 3 stopped all opioids by 90 days from their original surgery, 2 had no change in MEDD at 90 days, and 1 had a lower MEDD at 90 days. Of the 4 NOU who had additional surgery, all were off opioids by 90 days from the original surgery.
Although difficult to quantify, a meaningful outcome of TPS has been to improve satisfaction substantially among health care providers caring for complex patients at risk for chronic opioid abuse. This group includes the many members of the surgical team, PCPs, and addiction specialists who appreciate the close care coordination and assistance in caring for patients with difficult issues, especially with opioid tapers or SUDs. We also have noticed changes in prescribing practices among surgeons and PCPs for their patients who are not part of TPS.
Discussion
With any new clinical service, there are obstacles and challenges. TPS requires a considerable investment in personnel, and currently no mechanism is in place for obtaining payment for many of the provided services. We were fortunate the VA Whole Health Initiative, the VA Office of Rural Health, and the VA Centers of Innovation provided support for the development, implementation, and pilot evaluation of TPS. After we presented our initial results to hospital leadership, we also received hospital support to expand TPS service to include a total of 4 nurse care coordinators and 2 psychologists. We are currently performing a cost analysis of the service but recognize that this model may be difficult to reproduce at other institutions without a change in reimbursement standards.
Developing a working relationship with the surgical and primary care services required a concerted effort from the TPS team and a number of months to become effective. As most veterans receive primary care, mental health care, and surgical care within the VA system, this model lends itself to close care coordination. Initially there was skepticism about TPS recommendations to reduce opioid use, especially from PCPs who had cared for complex patients over many years. But this uncertainty went away as we showed evidence of close patient follow-up and detailed communication. TPS soon became the designated service for both primary care and surgical providers who were otherwise uncomfortable with how to approach opioid tapers and nonopioid pain strategies. In fact, a substantial portion of our referrals now come directly from the PCP who is referring a high-risk patient for evaluation for surgery rather than from the surgeons, and joint visits with TPS and primary care have become commonplace.
Challenges abound when working with patients with substance abuse history, opioid use history, high anxiety, significant pain catastrophizing, and those who have had previous negative experiences with surgery. We have found that the most important facet of our service comes from the amount of time and effort team members, especially the nurses, spend helping patients. Much of the nurses' work focuses on nonpain-related issues, such as assisting patients with finding transportation, housing issues, questions about medications, help scheduling appointments, etc. Through this concerted effort, patients gain trust in TPS providers and are willing to listen to and experiment with our recommendations. Many patients who were initially extremely unreceptive to the presurgery education asked for our support weeks after surgery to help with postsurgery pain.
Another challenge we continue to experience comes from the success of the program.
Conclusions
The multidisciplinary TPS supports greater preoperative to postoperative longitudinal care for surgical patients. This endeavor has resulted in better patient preparation before surgery and improved care coordination after surgery, with specific improvements in appropriate use of opioid medications and smooth transitions of care for patients with ongoing and complex needs. Development of sophisticated note templates and customized health information technology allows for accurate follow-through and data gathering for quality improvement, facilitating data-driven improvements and proving value to the facility.
Given that TPS is a multidisciplinary program with multiple interventions, it is difficult to pinpoint which specific aspects of TPS are most effective in achieving success. For example, although we have little doubt that the work our psychologists do with our patients is beneficial and even essential for the success we have had with some of our most difficult patients, it is less clear whether it matters if they use mindfulness, ACT matrix, or cognitive behavioral therapy. We think that an important part of TPS is the frequent human interaction with a caring individual. Therefore, as TPS continues to grow, maintaining the ability to provide frequent personal interaction is a priority.
The role of opioids in acute pain deserves further scrutiny. In 2018, with TPS use of opioids after orthopedic surgery decreased by > 40% from the previous year. Despite this more restricted use of opioids, pain interference and physical function scores indicated that surgical patients do not seem to experience increased pain or reduced physical function. In addition, stopping opioid use for COUs did not seem to affect the quality of recovery, pain, or physical function. Future prospective controlled studies of TPS are needed to confirm these findings and identify which aspects of TPS are most effective in improving functional recovery of patients. Also, more evidence is needed to determine the appropriateness or need for opioids in acute postsurgical pain.
TPS has expanded to include all surgical specialties. Given the high burden and limited resources, we have chosen to focus on patients at higher risk for chronic postsurgical pain by type of surgery (eg, thoracotomy, open abdominal, limb amputation, major joint surgery) and/or history of substance abuse or chronic opioid use. To better direct scarce resources where it would be of most benefit, we are now enrolling only NOUs without other risk factors postoperatively if they request a refill of opioids or are otherwise struggling with pain control after surgery. Whether this approach affects the success we had in the first year in preventing new COUs after surgery remains to be seen.
It is unlikely that any single model of a perioperative surgical home will fit the needs of the many different types of medical systems that exist. The TPS model fits well in large hospital systems, like the VA, where patients receive most of their care within the same system. However, it seems to us that the optimal TPS program in any health system will provide education, support, and care coordination beginning preoperatively to prepare the patient for surgery and then to facilitate care coordination to transition patients back to their PCPs or on to specialized chronic care.
Acknowledgments
We would like to acknowledge the contributions of Candice Harmon, RN; David Merrill, RN; Amy Beckstead, RN, who have provided invaluable assistance with establishing the TPS program at the VA Salt Lake City and helping with the evaluation process.
Funding for the implementation and evaluation of the TPS was received from the VA Whole Health Initiative, the VA Center of Innovation, the VA Office of Rural Health, and National Institutes of Health Grant UL1TR002538.
Despite advancements in techniques, postsurgical pain continues to be a prominent part of the patient experience. Often this experience can lead to developing chronic postsurgical pain that interferes with quality of life after the expected time to recovery.1-3 As many as 14% of patients who undergo surgery without any history of opioid use develop chronic opioid use that persists after recovery from their operation.4-8 For patients with existing chronic opioid use or a history of substance use disorder (SUD), surgeons, primary care providers, or addiction providers often do not provide sufficient presurgical planning or postsurgical coordination of care. This lack of pain care coordination can increase the risk of inadequate pain control, opioid use escalation, or SUD relapse after surgery.
Convincing arguments have been made that a perioperative surgical home can improve significantly the quality of perioperative care.9-14 This report describes our experience implementing a perioperative surgical home at the US Department of Veterans Affairs (VA) Salt Lake City VA Medical Center (SLCVAMC), focusing on pain management extending from the preoperative period until 6 months or more after surgery. This type of Transitional Pain Service (TPS) has been described previously.15-17 Our service differs from those described previously by enrolling all patients before surgery rather than select postsurgical enrollment of only patients with a history of opioid use or SUD or patients who struggle with persistent postsurgical pain.
Methods
In January 2018, we developed and implemented a new TPS at the SLCVAMC. The transitional pain team consisted of an anesthesiologist with specialization in acute pain management, a nurse practitioner (NP) with experience in both acute and chronic pain management, 2 nurse care coordinators, and a psychologist (Figure 1). Before implementation, a needs assessment took place with these key stakeholders and others at SLCVAMC to identify the following specific goals of the TPS: (1) reduce pain through pharmacologic and nonpharmacologic interventions; (2) eliminate new chronic opioid use in previously nonopioid user (NOU) patients; (3) address chronic opioid use in previous chronic opioid users (COUs) by providing support for opioid taper and alternative analgesic therapies for their chronic pain conditions; and (4) improve continuity of care by close coordination with the surgical team, primary care providers (PCPs), and mental health or chronic pain providers as needed.
Once these TPS goals were defined, the Consolidated Framework for Implementation Research (CFIR) guided the implementation. CFIR is a theory-based implementation framework consisting of 5 domains: intervention characteristics, inner setting, outer setting, characteristics of individuals, and process. These domains were used to identify barriers and facilitators during the early implementation process and helped refine TPS as it was put into clinical practice.
Patient Selection
During the initial implementation of TPS, enrollment was limited to patients scheduled for elective primary or revision knee, hip, or shoulder replacement as well as rotator cuff repair surgery. But as the TPS workflow became established after iterative refinement, we expanded the program to enroll patients with established risk factors for OUD having other types of surgery (Table 1). The diagnosis of risk factors, such as history of SUD, chronic opioid use, or significant mental health disorders (ie, history of suicidal ideation or attempt, posttraumatic stress disorder, and inpatient psychiatric care) were confirmed through both in-person interviews and electronic health record (EHR) documentation. The overall goal was to identify all at-risk patients as soon as they were indicated for surgery, to allow time for evaluation, education, developing an individualized pain plan, and opioid taper prior to surgery if indicated.
Preoperative Procedures
Once identified, patients were contacted by a TPS team member and invited to attend a onetime 90-minute presurgical expectations class held at SLCVAMC. The education curriculum was developed by the whole team, and classes were taught primarily by the TPS psychologist. The class included education about expectations for postoperative pain, available analgesic therapies, opioid education, appropriate use of opioids, and the effect of psychological factors on pain. Pain coping strategies were introduced using a mindfulness-based intervention (MBI) and the Acceptance and Commitment Therapy (ACT) matrix. Classes were offered multiple times a week to help maximize convenience for patients and were separate from the anesthesia preoperative evaluation. Patients attended class only once. High-risk patients (patients with chronic opioid therapy, recent history of or current SUDs, significant comorbid mental health issues) were encouraged to attend this class one-on-one with the TPS psychologist rather than in the group setting, so individual attention to mental health and SUD issues could be addressed directly.
Baseline history, morphine equivalent daily dose (MEDD), and patient-reported outcomes using measures from the Patient-Reported Outcome Measurement System (PROMIS) for pain intensity (PROMIS 3a), pain interference (PROMIS 6b), and physical function (PROMIS 8b), and a pain-catastrophizing scale (PCS) score were obtained on all patients.18 PROMIS measures are validated questionnaires developed with the National Institutes of Health to standardize and quantify patient-reported outcomes in many domains.19 Patients with a history of SUD or COU met with the anesthesiologist and/or NP, and a personalized pain plan was developed that included preoperative opioid taper, buprenorphine use strategy, or opioid-free strategies.
Hospital Procedures
On the day of surgery, the TPS team met with the patient preoperatively and implemented an individualized pain plan that included multimodal analgesic techniques with nonsteroidal anti-inflammatory drugs, acetaminophen, gabapentinoids, and regional anesthesia, where appropriate (Table 2). Enhanced recovery after surgery protocols were developed in conjunction with the surgeons to include local infiltration analgesia by the surgeon, postoperative multimodal analgesic strategies, and intensive physical therapy starting the day of surgery for inpatient procedures.
After surgery, the TPS team followed up with patients daily and provided recommendations for analgesic therapies. Patients were offered daily sessions with the psychologist to reinforce and practice nonpharmacologic pain-coping strategies, such as meditation and relaxation. Prior to patient discharge, the TPS team provided recommendations for discharge medications and an opioid taper plan. For some patients taking buprenorphine before surgery who had stopped this therapy prior to or during their hospital stay, TPS providers transitioned them back to buprenorphine before discharge.
Postoperative Procedures
Patients were called by the nurse care coordinators at postdischarge days 2, 7, 10, 14, 21, 28, and then monthly for ≥ 6 months. For patients who had not stopped opioid use or returned to their preoperative baseline opioid dose, weekly calls were made until opioid taper goals were achieved. At each call, nurses collected PROMIS scores for the previous 24 hours, the most recent 24-hour MEDD, the date of last opioid use, and the number of remaining opioid tablets after opioid cessation. In addition, nurses provided active listening and supportive care and encouragement as well as care coordination for issues related to rehabilitation facilities, physical therapy, transportation, medication questions, and wound questions. Nurses notified the anesthesiologist or NP when patients were unable to taper opioid use or had poor pain control as indicated by their PROMIS scores, opioid use, or directly expressed by the patient.
The TPS team prescribed alternative analgesic therapies, opioid taper plans, and communicated with surgeons and primary care providers if limited continued opioid therapy was recommended. Individual sessions with the psychologist were available to patients after discharge with a focus on ACT-matrix therapy and consultation with long-term mental health and/or substance abuse providers as indicated. Frequent communication and care coordination were maintained with the surgical team, the PCP, and other providers on the mental health or chronic pain services. This care coordination often included postsurgical joint clinic appointments in which TPS providers and nurses would be present with the surgeon or the PCP.
For patients with inadequately treated chronic pain conditions or who required long-term opioid tapers, we developed a combined clinic with the TPS and Anesthesia Chronic Pain group. This clinic allows patients to be seen by both services in the same setting, allowing a warm handoff by TPS to the chronic pain team.
Heath and Decision Support Tools
An electronic dashboard registry of surgical episodes managed by TPS was developed to achieve clinical, administrative, and quality improvement goals. The dashboard registry consists of surgical episode data, opioid doses, patient-reported outcomes, and clinical decision-making processes. Custom-built note templates capture pertinent data through embedded data labels, called health factors. Data are captured as part of routine clinical care, recorded in Computerized Patient Record System as health factors. They are available in the VA Corporate Data Warehouse as structured data. Workflows are executed daily to keep the dashboard registry current, clean, and able to process new data. Information displays direct daily clinical workflow and support point-of-care clinical decision making (Figures 2, 3, and 4). Data are aggregated across patient-care encounters and allow nurse care coordinators to concisely review pertinent patient data prior to delivering care. These data include surgical history, comorbidities, timeline of opioid use, and PROMIS scores during their course of recovery. This system allows TPS to optimize care delivery by providing longitudinal data across the surgical episode, thereby reducing the time needed to review records. Secondary purposes of captured data include measuring clinic performance and quality improvement to improve care delivery.
Results
The TPS intervention was implemented January 1, 2018. Two-hundred thirteen patients were enrolled between January and December 2018, which included 60 (28%) patients with a history of chronic opioid use and 153 (72%) patients who were considered opioid naïve. A total of 99% of patients had ≥ 1 successful follow-up within 14 days after discharge, 96% had ≥ 1 follow-up between 14 and 30 days after surgery, and 72% had completed personal follow-up 90 days after discharge (Table 3). For patients who TPS was unable to contact in person or by phone, 90-day MEDD was obtained using prescription and Controlled Substance Database reviews. The protocol for this retrospective analysis was approved by the University of Utah Institutional Review Board and the VA Research Review Committee.
By 90 days after surgery, 26 (43.3%) COUs were off opioids completely, 17 (28.3%) had decreased their opioid dose from their preoperative baseline MEDD (120 [SD, 108] vs 55 [SD, 45]), 14 (23.3%) returned to their baseline dose, and 3 (5%) increased from their baseline dose. Of the 153 patients who were NOUs before surgery, only 1 (0.7%) was taking opioids after 90 days. TPS continued to work closely with the patient and their PCP and that patient was finally able to stop opioid use 262 days after discharge. Ten patients had an additional surgery within 90 days of the initial surgery. Of these, 6 were COU, of whom 3 stopped all opioids by 90 days from their original surgery, 2 had no change in MEDD at 90 days, and 1 had a lower MEDD at 90 days. Of the 4 NOU who had additional surgery, all were off opioids by 90 days from the original surgery.
Although difficult to quantify, a meaningful outcome of TPS has been to improve satisfaction substantially among health care providers caring for complex patients at risk for chronic opioid abuse. This group includes the many members of the surgical team, PCPs, and addiction specialists who appreciate the close care coordination and assistance in caring for patients with difficult issues, especially with opioid tapers or SUDs. We also have noticed changes in prescribing practices among surgeons and PCPs for their patients who are not part of TPS.
Discussion
With any new clinical service, there are obstacles and challenges. TPS requires a considerable investment in personnel, and currently no mechanism is in place for obtaining payment for many of the provided services. We were fortunate the VA Whole Health Initiative, the VA Office of Rural Health, and the VA Centers of Innovation provided support for the development, implementation, and pilot evaluation of TPS. After we presented our initial results to hospital leadership, we also received hospital support to expand TPS service to include a total of 4 nurse care coordinators and 2 psychologists. We are currently performing a cost analysis of the service but recognize that this model may be difficult to reproduce at other institutions without a change in reimbursement standards.
Developing a working relationship with the surgical and primary care services required a concerted effort from the TPS team and a number of months to become effective. As most veterans receive primary care, mental health care, and surgical care within the VA system, this model lends itself to close care coordination. Initially there was skepticism about TPS recommendations to reduce opioid use, especially from PCPs who had cared for complex patients over many years. But this uncertainty went away as we showed evidence of close patient follow-up and detailed communication. TPS soon became the designated service for both primary care and surgical providers who were otherwise uncomfortable with how to approach opioid tapers and nonopioid pain strategies. In fact, a substantial portion of our referrals now come directly from the PCP who is referring a high-risk patient for evaluation for surgery rather than from the surgeons, and joint visits with TPS and primary care have become commonplace.
Challenges abound when working with patients with substance abuse history, opioid use history, high anxiety, significant pain catastrophizing, and those who have had previous negative experiences with surgery. We have found that the most important facet of our service comes from the amount of time and effort team members, especially the nurses, spend helping patients. Much of the nurses' work focuses on nonpain-related issues, such as assisting patients with finding transportation, housing issues, questions about medications, help scheduling appointments, etc. Through this concerted effort, patients gain trust in TPS providers and are willing to listen to and experiment with our recommendations. Many patients who were initially extremely unreceptive to the presurgery education asked for our support weeks after surgery to help with postsurgery pain.
Another challenge we continue to experience comes from the success of the program.
Conclusions
The multidisciplinary TPS supports greater preoperative to postoperative longitudinal care for surgical patients. This endeavor has resulted in better patient preparation before surgery and improved care coordination after surgery, with specific improvements in appropriate use of opioid medications and smooth transitions of care for patients with ongoing and complex needs. Development of sophisticated note templates and customized health information technology allows for accurate follow-through and data gathering for quality improvement, facilitating data-driven improvements and proving value to the facility.
Given that TPS is a multidisciplinary program with multiple interventions, it is difficult to pinpoint which specific aspects of TPS are most effective in achieving success. For example, although we have little doubt that the work our psychologists do with our patients is beneficial and even essential for the success we have had with some of our most difficult patients, it is less clear whether it matters if they use mindfulness, ACT matrix, or cognitive behavioral therapy. We think that an important part of TPS is the frequent human interaction with a caring individual. Therefore, as TPS continues to grow, maintaining the ability to provide frequent personal interaction is a priority.
The role of opioids in acute pain deserves further scrutiny. In 2018, with TPS use of opioids after orthopedic surgery decreased by > 40% from the previous year. Despite this more restricted use of opioids, pain interference and physical function scores indicated that surgical patients do not seem to experience increased pain or reduced physical function. In addition, stopping opioid use for COUs did not seem to affect the quality of recovery, pain, or physical function. Future prospective controlled studies of TPS are needed to confirm these findings and identify which aspects of TPS are most effective in improving functional recovery of patients. Also, more evidence is needed to determine the appropriateness or need for opioids in acute postsurgical pain.
TPS has expanded to include all surgical specialties. Given the high burden and limited resources, we have chosen to focus on patients at higher risk for chronic postsurgical pain by type of surgery (eg, thoracotomy, open abdominal, limb amputation, major joint surgery) and/or history of substance abuse or chronic opioid use. To better direct scarce resources where it would be of most benefit, we are now enrolling only NOUs without other risk factors postoperatively if they request a refill of opioids or are otherwise struggling with pain control after surgery. Whether this approach affects the success we had in the first year in preventing new COUs after surgery remains to be seen.
It is unlikely that any single model of a perioperative surgical home will fit the needs of the many different types of medical systems that exist. The TPS model fits well in large hospital systems, like the VA, where patients receive most of their care within the same system. However, it seems to us that the optimal TPS program in any health system will provide education, support, and care coordination beginning preoperatively to prepare the patient for surgery and then to facilitate care coordination to transition patients back to their PCPs or on to specialized chronic care.
Acknowledgments
We would like to acknowledge the contributions of Candice Harmon, RN; David Merrill, RN; Amy Beckstead, RN, who have provided invaluable assistance with establishing the TPS program at the VA Salt Lake City and helping with the evaluation process.
Funding for the implementation and evaluation of the TPS was received from the VA Whole Health Initiative, the VA Center of Innovation, the VA Office of Rural Health, and National Institutes of Health Grant UL1TR002538.
1. Ilfeld BM, Madison SJ, Suresh PJ. Persistent postmastectomy pain and pain-related physical and emotional functioning with and without a continuous paravertebral nerve block: a prospective 1-year follow-up assessment of a randomized, triple-masked, placebo-controlled study. Ann Surg Oncol. 2015;22(6):2017-2025. doi:10.1245/s10434-014-4248-7
2. Richebé P, Capdevila X, Rivat C. Persistent postsurgical pain. Anesthesiology. 2018;129(3):590-607. doi:10.1097/aln.0000000000002238
3. Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393(10180):1537-1546. doi:10.1016/s0140-6736(19)30352-6
4. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surgery. 2017;152(6):e170504-e170504. doi:10.1001/jamasurg.2017.0504
5. Swenson CW, Kamdar NS, Seiler K, Morgan DM, Lin P, As-Sanie S. Definition development and prevalence of new persistent opioid use following hysterectomy. Am J Obstet Gynecol. 2018;219(5):486.e1-486.e7. doi:10.1016/j.ajog.2018.06.010
6. Bartels K, Fernandez-Bustamante A, McWilliams SK, Hopfer CJ, Mikulich-Gilbertson SK. Long-term opioid use after inpatient surgery - a retrospective cohort study. Drug Alcohol Depend. 2018;187:61-65. doi:10.1016/j.drugalcdep.2018.02.013
7. Bedard N, DeMik D, Dowdle S, Callaghan J. Trends and risk factors for prolonged opioid use after unicompartmental knee arthroplasty. Bone Joint J. 2018;100-B(1)(suppl A):62-67. doi:10.1302/0301-620x.100b1.bjj-2017-0547.r1
8. Politzer CS, Kildow BJ, Goltz DE, Green CL, Bolognesi MP, Seyler T. Trends in opioid utilization before and after total knee arthroplasty. J Arthroplasty. 2018;33(7S):S147-S153.e1. doi:10.1016/j.arth.2017.10.060
9. Mariano ER, Walters TL, Kim ET, Kain ZN. Why the perioperative surgical home makes sense for Veterans Affairs health care. Anesth Analg. 2015;120(5):1163-1166. doi:10.1213/ane.0000000000000712
10. Walters TL, Howard SK, Kou A, et al. Design and implementation of a perioperative surgical home at a Veterans Affairs hospital. Semin Cardiothorac Vasc Anesth. 2016;20(2):133-140. doi:10.1177/1089253215607066
11. Walters TL, Mariano ER, Clark DJ. Perioperative surgical home and the integral role of pain medicine. Pain Med. 2015;16(9):1666-1672. doi:10.1111/pme.12796
12. Vetter TR, Kain ZN. Role of the perioperative surgical home in optimizing the perioperative use of opioids. Anesth Analg. 2017;125(5):1653-1657. doi:10.1213/ane.0000000000002280
13. Shafer SL. Anesthesia & Analgesia’s 2015 collection on the perioperative surgical home. Anesth Analg. 2015;120(5):966-967. doi:10.1213/ane.0000000000000696
14. Wenzel JT, Schwenk ES, Baratta JL, Viscusi ER. Managing opioid-tolerant patients in the perioperative surgical home. Anesthesiol Clin. 2016;34(2):287-301. doi:10.1016/j.anclin.2016.01.005
15. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695-702. doi:10.2147/jpr.s91924
16. Tiippana E, Hamunen K, Heiskanen T, Nieminen T, Kalso E, Kontinen VK. New approach for treatment of prolonged postoperative pain: APS Out-Patient Clinic. Scand J Pain. 2016;12(1):19-24. doi:10.1016/j.sjpain.2016.02.008
17. Katz J, Weinrib AZ, Clarke H. Chronic postsurgical pain: from risk factor identification to multidisciplinary management at the Toronto General Hospital Transitional Pain Service. Can J Pain. 2019;3(2):49-58. doi:10.1080/24740527.2019.1574537
18. Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7(4):524-532. doi:10.1037/1040-3590.7.4.524
19. HealthMeasures. Intro to PROMIS. https://www.healthmeasures.net/explore-measurement-systems/promis. Accessed September 28, 2020.
1. Ilfeld BM, Madison SJ, Suresh PJ. Persistent postmastectomy pain and pain-related physical and emotional functioning with and without a continuous paravertebral nerve block: a prospective 1-year follow-up assessment of a randomized, triple-masked, placebo-controlled study. Ann Surg Oncol. 2015;22(6):2017-2025. doi:10.1245/s10434-014-4248-7
2. Richebé P, Capdevila X, Rivat C. Persistent postsurgical pain. Anesthesiology. 2018;129(3):590-607. doi:10.1097/aln.0000000000002238
3. Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393(10180):1537-1546. doi:10.1016/s0140-6736(19)30352-6
4. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surgery. 2017;152(6):e170504-e170504. doi:10.1001/jamasurg.2017.0504
5. Swenson CW, Kamdar NS, Seiler K, Morgan DM, Lin P, As-Sanie S. Definition development and prevalence of new persistent opioid use following hysterectomy. Am J Obstet Gynecol. 2018;219(5):486.e1-486.e7. doi:10.1016/j.ajog.2018.06.010
6. Bartels K, Fernandez-Bustamante A, McWilliams SK, Hopfer CJ, Mikulich-Gilbertson SK. Long-term opioid use after inpatient surgery - a retrospective cohort study. Drug Alcohol Depend. 2018;187:61-65. doi:10.1016/j.drugalcdep.2018.02.013
7. Bedard N, DeMik D, Dowdle S, Callaghan J. Trends and risk factors for prolonged opioid use after unicompartmental knee arthroplasty. Bone Joint J. 2018;100-B(1)(suppl A):62-67. doi:10.1302/0301-620x.100b1.bjj-2017-0547.r1
8. Politzer CS, Kildow BJ, Goltz DE, Green CL, Bolognesi MP, Seyler T. Trends in opioid utilization before and after total knee arthroplasty. J Arthroplasty. 2018;33(7S):S147-S153.e1. doi:10.1016/j.arth.2017.10.060
9. Mariano ER, Walters TL, Kim ET, Kain ZN. Why the perioperative surgical home makes sense for Veterans Affairs health care. Anesth Analg. 2015;120(5):1163-1166. doi:10.1213/ane.0000000000000712
10. Walters TL, Howard SK, Kou A, et al. Design and implementation of a perioperative surgical home at a Veterans Affairs hospital. Semin Cardiothorac Vasc Anesth. 2016;20(2):133-140. doi:10.1177/1089253215607066
11. Walters TL, Mariano ER, Clark DJ. Perioperative surgical home and the integral role of pain medicine. Pain Med. 2015;16(9):1666-1672. doi:10.1111/pme.12796
12. Vetter TR, Kain ZN. Role of the perioperative surgical home in optimizing the perioperative use of opioids. Anesth Analg. 2017;125(5):1653-1657. doi:10.1213/ane.0000000000002280
13. Shafer SL. Anesthesia & Analgesia’s 2015 collection on the perioperative surgical home. Anesth Analg. 2015;120(5):966-967. doi:10.1213/ane.0000000000000696
14. Wenzel JT, Schwenk ES, Baratta JL, Viscusi ER. Managing opioid-tolerant patients in the perioperative surgical home. Anesthesiol Clin. 2016;34(2):287-301. doi:10.1016/j.anclin.2016.01.005
15. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695-702. doi:10.2147/jpr.s91924
16. Tiippana E, Hamunen K, Heiskanen T, Nieminen T, Kalso E, Kontinen VK. New approach for treatment of prolonged postoperative pain: APS Out-Patient Clinic. Scand J Pain. 2016;12(1):19-24. doi:10.1016/j.sjpain.2016.02.008
17. Katz J, Weinrib AZ, Clarke H. Chronic postsurgical pain: from risk factor identification to multidisciplinary management at the Toronto General Hospital Transitional Pain Service. Can J Pain. 2019;3(2):49-58. doi:10.1080/24740527.2019.1574537
18. Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7(4):524-532. doi:10.1037/1040-3590.7.4.524
19. HealthMeasures. Intro to PROMIS. https://www.healthmeasures.net/explore-measurement-systems/promis. Accessed September 28, 2020.