User login
Adherence and persistence problems affect RA triple therapy more than TNFi combination therapy
SAN FRANCISCO – Patients who initiate triple therapy for rheumatoid arthritis appear to have lower persistence in filling the prescriptions and lower adherence to the regimen even when prescriptions are dispensed for the drugs, compared with users of combination therapy with a tumor necrosis factor inhibitor plus methotrexate in two Veterans Affairs studies.
The studies also suggest that the similar efficacy and patient adherence for both triple therapy and combination therapy with a tumor necrosis factor inhibitor (TNFi) plus methotrexate that have been observed in randomized controlled trials (RCTs) do not accurately portray what occurs in real-world clinical practice.
In one study, Brian C. Sauer, Ph.D., of the University of Utah, Salt Lake City, and coinvestigators utilized the historical data available for utilized the historical data available for 4,364 U.S. veterans over 28 years of age treated for RA from 2006 through 2012. Patients needed to be enrolled with Veterans Affairs for about 6 months prior to and 12 months after the initiation of the triple therapy. The triple therapy involved 3,204 patients. The three drugs could be initiated concomitantly, in an approach that is typical of an RCT design, or could be introduced at different times, with the requirement of an overlapping period of use of all three. For the combination therapy used in 1,160 patients, the two drugs needed to overlap by at least 1 day. When various algorithms that accounted for clinician-mandated shifts in drug use according to patient response to treatment were used, the persistence outcomes could be calculated, with patients judged to be adherent or nonadherent to either treatment regimen.
At 12 months, the rate of persistence in staying on triple therapy ranged from 29% to 50% and for TNFi/methotrexate from 42% to 59%, according to three definitions of persistence the investigators used. Adherence of greater than 80% during the year to the regimens occurred in 17% on triple therapy and 24% on TNFi/methotrexate. The advantage for the combination therapy persisted when factors likely to affect treatment choice and persistence were accounted for in propensity scoring of 1,143 patients from each group.
“We did not evaluate early side effects. Our study focused on 1-year persistence. Similar early drop-off between the groups and RCTs suggest that the treatments have similar tolerability. The fact that patients on triple therapy appeared to be sicker also suggests that providers are possibly concerned about TNF [inhibitor] side effects in the population treated with triple therapy,” Dr. Sauer said at the annual meeting of the American College of Rheumatology.
The additional complexity of triple therapy might also have contributed to the lower adherence, he said.
A second, similar VA study that was presented at the meeting examined patients who had an inadequate response to methotrexate monotherapy found similar results in a comparison of 2,125 patients who added a TNFi to methotrexate with 171 patients who added sulfasalazine and hydroxychloroquine, a scenario that has been tested in RCTs. In this study, 18% persisted in staying on triple therapy over a year, compared with 43% on TNFi/methotrexate, while 12-month adherence rates were 11% and 25%, respectively.
Dr. Sauer reported financial disclosures involving research grant support from Amgen.
SAN FRANCISCO – Patients who initiate triple therapy for rheumatoid arthritis appear to have lower persistence in filling the prescriptions and lower adherence to the regimen even when prescriptions are dispensed for the drugs, compared with users of combination therapy with a tumor necrosis factor inhibitor plus methotrexate in two Veterans Affairs studies.
The studies also suggest that the similar efficacy and patient adherence for both triple therapy and combination therapy with a tumor necrosis factor inhibitor (TNFi) plus methotrexate that have been observed in randomized controlled trials (RCTs) do not accurately portray what occurs in real-world clinical practice.
In one study, Brian C. Sauer, Ph.D., of the University of Utah, Salt Lake City, and coinvestigators utilized the historical data available for utilized the historical data available for 4,364 U.S. veterans over 28 years of age treated for RA from 2006 through 2012. Patients needed to be enrolled with Veterans Affairs for about 6 months prior to and 12 months after the initiation of the triple therapy. The triple therapy involved 3,204 patients. The three drugs could be initiated concomitantly, in an approach that is typical of an RCT design, or could be introduced at different times, with the requirement of an overlapping period of use of all three. For the combination therapy used in 1,160 patients, the two drugs needed to overlap by at least 1 day. When various algorithms that accounted for clinician-mandated shifts in drug use according to patient response to treatment were used, the persistence outcomes could be calculated, with patients judged to be adherent or nonadherent to either treatment regimen.
At 12 months, the rate of persistence in staying on triple therapy ranged from 29% to 50% and for TNFi/methotrexate from 42% to 59%, according to three definitions of persistence the investigators used. Adherence of greater than 80% during the year to the regimens occurred in 17% on triple therapy and 24% on TNFi/methotrexate. The advantage for the combination therapy persisted when factors likely to affect treatment choice and persistence were accounted for in propensity scoring of 1,143 patients from each group.
“We did not evaluate early side effects. Our study focused on 1-year persistence. Similar early drop-off between the groups and RCTs suggest that the treatments have similar tolerability. The fact that patients on triple therapy appeared to be sicker also suggests that providers are possibly concerned about TNF [inhibitor] side effects in the population treated with triple therapy,” Dr. Sauer said at the annual meeting of the American College of Rheumatology.
The additional complexity of triple therapy might also have contributed to the lower adherence, he said.
A second, similar VA study that was presented at the meeting examined patients who had an inadequate response to methotrexate monotherapy found similar results in a comparison of 2,125 patients who added a TNFi to methotrexate with 171 patients who added sulfasalazine and hydroxychloroquine, a scenario that has been tested in RCTs. In this study, 18% persisted in staying on triple therapy over a year, compared with 43% on TNFi/methotrexate, while 12-month adherence rates were 11% and 25%, respectively.
Dr. Sauer reported financial disclosures involving research grant support from Amgen.
SAN FRANCISCO – Patients who initiate triple therapy for rheumatoid arthritis appear to have lower persistence in filling the prescriptions and lower adherence to the regimen even when prescriptions are dispensed for the drugs, compared with users of combination therapy with a tumor necrosis factor inhibitor plus methotrexate in two Veterans Affairs studies.
The studies also suggest that the similar efficacy and patient adherence for both triple therapy and combination therapy with a tumor necrosis factor inhibitor (TNFi) plus methotrexate that have been observed in randomized controlled trials (RCTs) do not accurately portray what occurs in real-world clinical practice.
In one study, Brian C. Sauer, Ph.D., of the University of Utah, Salt Lake City, and coinvestigators utilized the historical data available for utilized the historical data available for 4,364 U.S. veterans over 28 years of age treated for RA from 2006 through 2012. Patients needed to be enrolled with Veterans Affairs for about 6 months prior to and 12 months after the initiation of the triple therapy. The triple therapy involved 3,204 patients. The three drugs could be initiated concomitantly, in an approach that is typical of an RCT design, or could be introduced at different times, with the requirement of an overlapping period of use of all three. For the combination therapy used in 1,160 patients, the two drugs needed to overlap by at least 1 day. When various algorithms that accounted for clinician-mandated shifts in drug use according to patient response to treatment were used, the persistence outcomes could be calculated, with patients judged to be adherent or nonadherent to either treatment regimen.
At 12 months, the rate of persistence in staying on triple therapy ranged from 29% to 50% and for TNFi/methotrexate from 42% to 59%, according to three definitions of persistence the investigators used. Adherence of greater than 80% during the year to the regimens occurred in 17% on triple therapy and 24% on TNFi/methotrexate. The advantage for the combination therapy persisted when factors likely to affect treatment choice and persistence were accounted for in propensity scoring of 1,143 patients from each group.
“We did not evaluate early side effects. Our study focused on 1-year persistence. Similar early drop-off between the groups and RCTs suggest that the treatments have similar tolerability. The fact that patients on triple therapy appeared to be sicker also suggests that providers are possibly concerned about TNF [inhibitor] side effects in the population treated with triple therapy,” Dr. Sauer said at the annual meeting of the American College of Rheumatology.
The additional complexity of triple therapy might also have contributed to the lower adherence, he said.
A second, similar VA study that was presented at the meeting examined patients who had an inadequate response to methotrexate monotherapy found similar results in a comparison of 2,125 patients who added a TNFi to methotrexate with 171 patients who added sulfasalazine and hydroxychloroquine, a scenario that has been tested in RCTs. In this study, 18% persisted in staying on triple therapy over a year, compared with 43% on TNFi/methotrexate, while 12-month adherence rates were 11% and 25%, respectively.
Dr. Sauer reported financial disclosures involving research grant support from Amgen.
Key clinical point: Poor adherence and persistence may occur with triple therapy for RA over the course of a year.
Major finding: The rate of persistence over a year in staying on triple therapy ranged from 29% to 50% and for TNFi/methotrexate from 42% to 59%.
Data source: Veterans Affairs clinical and administrative data.
Disclosures: Dr. Sauer reported financial disclosures involving research grant support from Amgen.
Real-world use of triple therapy for RA differs from clinical trial use
SAN FRANCISCO – Triple therapy for rheumatoid arthritis has shone in clinical trials, but its adoption by rheumatologists in the trenches of patient care has been lukewarm.
The challenges facing triple therapy were highlighted in two presentations at the annual meeting of the American College of Rheumatology.
In the first, Dr. Jeffrey Sparks noted that findings from several randomized clinical trials have established the noninferiority of triple therapy with methotrexate (MTX), sulfasazine (SUL), and hydroxychloroquine (HCQ), compared with the combination of MTX and tumor necrosis factor inhibitor. Triple therapy also yields comparable quality of life and is delivered at less cost. Reflecting the evidence, triple therapy is an American College of Rheumatology–recommended option for first-line therapy for high disease activity and poor prognosis, and following failure of nonbiologic disease-modifying antirheumatic drugs (DMARDs). “The many options available add to the complexity of RA treatment decisions. It is unclear whether data supporting triple-therapy use have affected prescribing patterns in typical practice in the United States,” said Dr. Sparks of the division of rheumatology, immunology, and allergy at Brigham and Women’s Hospital, Harvard Medical School, Boston.
Longitudinal data available in the Aetna U.S. insurance claims and Veterans Administration databases allowed a look at the issue. The databases were scrutinized from mid-2009 to mid-2014, and from 2006 through 2012, respectively. The age range was broad – 18 years and older. Of the 24,576 eligible subjects identified in the Aetna insurance claims database, the initial treatment was intensified in 2,920 (11.9%) subjects. Almost all (n = 2,739) involved biologic DMARDs, with only 181 (0.7%) intensifying to triple therapy. The frequency of triple therapy was unrelated to calendar time. Use of triple therapy was not associated with patients’ sex, household income, antimicrobial or opioid use, hospitalization for serious infection, and/or comorbidities.
“Triple therapy was infrequently used for RA treatment in this large nationwide study, reflecting typical clinical practice. Use of steroids and nonsteroidal anti-inflammatory drugs prior to initial nonbiologic DMARD treatment, and geographic location, with more frequent use in the northeastern U.S.A., were associated with triple therapy use,” said Dr. Sparks.
Clinician reluctance to move from the conventional therapy and problems with patient adherence are likely factors in the stalled adoption of triple therapy, Dr. Sparks speculated.
During a separate presentation, Dr. Grant W. Cannon said that the disconnection between randomized controlled trial (RCT) data and the reality of clinical practice also extends to the pattern of drug use in triple therapy. In the VA population, the pattern of introduction of MTX, SUL, and HCQ spanned all possible combinations including the concomitant introduction typical of a RCT. The RCT pattern was infrequent, involving only 20% of the patients. The remaining 80% of cases involved the various patterns of drug introduction, with 57% of patients treated by sequential introduction of the individual DMARDs. No appreciable differences in baseline characteristics were apparent.
Calculation of persistence on therapy revealed that patients were significantly less likely to stay on triple therapy when it was introduced in the RCT style of administering all three DMARDs concomitantly (P less than .01). Adherence to therapy was also worse, although not statistically significantly (P = .096).
“Understanding provider practices with the initiation of triple-drug therapy is important as these practices might impact treatment effectiveness,” said Dr. Cannon of the division of rheumatology, Salt Lake City VA Medical Center and University of Utah, Salt Lake City. “Since clinical response and patient experience during sequential DMARD initiation may affect decisions to progress to triple therapy, RCT results describing the benefits of triple therapy may not generalize to clinical practice.”
SAN FRANCISCO – Triple therapy for rheumatoid arthritis has shone in clinical trials, but its adoption by rheumatologists in the trenches of patient care has been lukewarm.
The challenges facing triple therapy were highlighted in two presentations at the annual meeting of the American College of Rheumatology.
In the first, Dr. Jeffrey Sparks noted that findings from several randomized clinical trials have established the noninferiority of triple therapy with methotrexate (MTX), sulfasazine (SUL), and hydroxychloroquine (HCQ), compared with the combination of MTX and tumor necrosis factor inhibitor. Triple therapy also yields comparable quality of life and is delivered at less cost. Reflecting the evidence, triple therapy is an American College of Rheumatology–recommended option for first-line therapy for high disease activity and poor prognosis, and following failure of nonbiologic disease-modifying antirheumatic drugs (DMARDs). “The many options available add to the complexity of RA treatment decisions. It is unclear whether data supporting triple-therapy use have affected prescribing patterns in typical practice in the United States,” said Dr. Sparks of the division of rheumatology, immunology, and allergy at Brigham and Women’s Hospital, Harvard Medical School, Boston.
Longitudinal data available in the Aetna U.S. insurance claims and Veterans Administration databases allowed a look at the issue. The databases were scrutinized from mid-2009 to mid-2014, and from 2006 through 2012, respectively. The age range was broad – 18 years and older. Of the 24,576 eligible subjects identified in the Aetna insurance claims database, the initial treatment was intensified in 2,920 (11.9%) subjects. Almost all (n = 2,739) involved biologic DMARDs, with only 181 (0.7%) intensifying to triple therapy. The frequency of triple therapy was unrelated to calendar time. Use of triple therapy was not associated with patients’ sex, household income, antimicrobial or opioid use, hospitalization for serious infection, and/or comorbidities.
“Triple therapy was infrequently used for RA treatment in this large nationwide study, reflecting typical clinical practice. Use of steroids and nonsteroidal anti-inflammatory drugs prior to initial nonbiologic DMARD treatment, and geographic location, with more frequent use in the northeastern U.S.A., were associated with triple therapy use,” said Dr. Sparks.
Clinician reluctance to move from the conventional therapy and problems with patient adherence are likely factors in the stalled adoption of triple therapy, Dr. Sparks speculated.
During a separate presentation, Dr. Grant W. Cannon said that the disconnection between randomized controlled trial (RCT) data and the reality of clinical practice also extends to the pattern of drug use in triple therapy. In the VA population, the pattern of introduction of MTX, SUL, and HCQ spanned all possible combinations including the concomitant introduction typical of a RCT. The RCT pattern was infrequent, involving only 20% of the patients. The remaining 80% of cases involved the various patterns of drug introduction, with 57% of patients treated by sequential introduction of the individual DMARDs. No appreciable differences in baseline characteristics were apparent.
Calculation of persistence on therapy revealed that patients were significantly less likely to stay on triple therapy when it was introduced in the RCT style of administering all three DMARDs concomitantly (P less than .01). Adherence to therapy was also worse, although not statistically significantly (P = .096).
“Understanding provider practices with the initiation of triple-drug therapy is important as these practices might impact treatment effectiveness,” said Dr. Cannon of the division of rheumatology, Salt Lake City VA Medical Center and University of Utah, Salt Lake City. “Since clinical response and patient experience during sequential DMARD initiation may affect decisions to progress to triple therapy, RCT results describing the benefits of triple therapy may not generalize to clinical practice.”
SAN FRANCISCO – Triple therapy for rheumatoid arthritis has shone in clinical trials, but its adoption by rheumatologists in the trenches of patient care has been lukewarm.
The challenges facing triple therapy were highlighted in two presentations at the annual meeting of the American College of Rheumatology.
In the first, Dr. Jeffrey Sparks noted that findings from several randomized clinical trials have established the noninferiority of triple therapy with methotrexate (MTX), sulfasazine (SUL), and hydroxychloroquine (HCQ), compared with the combination of MTX and tumor necrosis factor inhibitor. Triple therapy also yields comparable quality of life and is delivered at less cost. Reflecting the evidence, triple therapy is an American College of Rheumatology–recommended option for first-line therapy for high disease activity and poor prognosis, and following failure of nonbiologic disease-modifying antirheumatic drugs (DMARDs). “The many options available add to the complexity of RA treatment decisions. It is unclear whether data supporting triple-therapy use have affected prescribing patterns in typical practice in the United States,” said Dr. Sparks of the division of rheumatology, immunology, and allergy at Brigham and Women’s Hospital, Harvard Medical School, Boston.
Longitudinal data available in the Aetna U.S. insurance claims and Veterans Administration databases allowed a look at the issue. The databases were scrutinized from mid-2009 to mid-2014, and from 2006 through 2012, respectively. The age range was broad – 18 years and older. Of the 24,576 eligible subjects identified in the Aetna insurance claims database, the initial treatment was intensified in 2,920 (11.9%) subjects. Almost all (n = 2,739) involved biologic DMARDs, with only 181 (0.7%) intensifying to triple therapy. The frequency of triple therapy was unrelated to calendar time. Use of triple therapy was not associated with patients’ sex, household income, antimicrobial or opioid use, hospitalization for serious infection, and/or comorbidities.
“Triple therapy was infrequently used for RA treatment in this large nationwide study, reflecting typical clinical practice. Use of steroids and nonsteroidal anti-inflammatory drugs prior to initial nonbiologic DMARD treatment, and geographic location, with more frequent use in the northeastern U.S.A., were associated with triple therapy use,” said Dr. Sparks.
Clinician reluctance to move from the conventional therapy and problems with patient adherence are likely factors in the stalled adoption of triple therapy, Dr. Sparks speculated.
During a separate presentation, Dr. Grant W. Cannon said that the disconnection between randomized controlled trial (RCT) data and the reality of clinical practice also extends to the pattern of drug use in triple therapy. In the VA population, the pattern of introduction of MTX, SUL, and HCQ spanned all possible combinations including the concomitant introduction typical of a RCT. The RCT pattern was infrequent, involving only 20% of the patients. The remaining 80% of cases involved the various patterns of drug introduction, with 57% of patients treated by sequential introduction of the individual DMARDs. No appreciable differences in baseline characteristics were apparent.
Calculation of persistence on therapy revealed that patients were significantly less likely to stay on triple therapy when it was introduced in the RCT style of administering all three DMARDs concomitantly (P less than .01). Adherence to therapy was also worse, although not statistically significantly (P = .096).
“Understanding provider practices with the initiation of triple-drug therapy is important as these practices might impact treatment effectiveness,” said Dr. Cannon of the division of rheumatology, Salt Lake City VA Medical Center and University of Utah, Salt Lake City. “Since clinical response and patient experience during sequential DMARD initiation may affect decisions to progress to triple therapy, RCT results describing the benefits of triple therapy may not generalize to clinical practice.”
AT THE ACR ANNUAL MEETING
Key clinical point: Triple therapy for rheumatoid arthritis is being underutilized.
Major finding: The noninferiority of triple therapy for rheumatoid arthritis evident from randomized controlled trials has not translated into widespread use of the regimen.
Data source: Veterans’ Affairs clinical and administrative data and insurance claims data.
Disclosures: Dr. Sparks reported an honorarium for a presentation to the study funder. Dr. Cannon reported financial disclosures involving research grant support from Amgen.
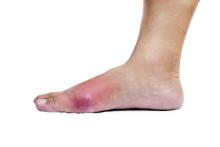
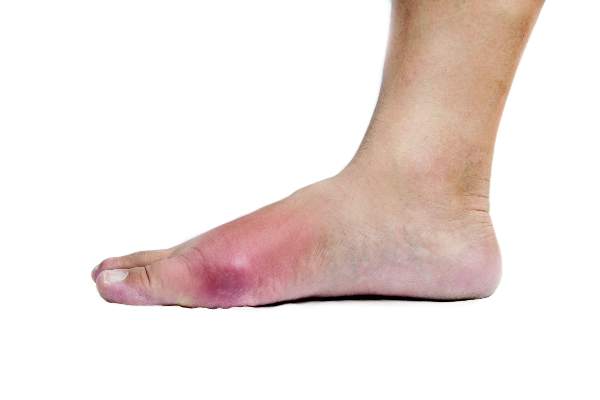
ACR: Study confirms potential genetic basis for poor response to allopurinol in gout
SAN FRANCISCO – Patients with gout who fail to sufficiently lower their serum urate level despite adherence to a regimen of allopurinol 300 mg/day may have a genetic polymorphism affecting their response to the medication, according to new findings presented at the annual meeting of the American College of Rheumatology.
“ABCG2 genotyping may identify patients who will not reach target serum urate levels on standard allopurinol doses,” said study presenter and principal investigator Dr. Lisa K. Stamp of the University of Otago, Christchurch, New Zealand.
Sustained lowering of serum urate (SU) to less than 6 mg/dL – achieved most commonly by inhibition of xanthine oxidase using allopurinol – is crucial for the long-term management of gout. Yet, in clinical practice, less than half of patients treated with allopurinol achieve this SU target. This is usually because of poor adherence or an inappropriately low dose of the drug. But, in some patients, the response can be poor even despite allopurinol 300 mg daily. The reason for failure of allopurinol treatment in these poor responders has been unknown.
“It is important to consider genes that might help predict response or toxicity. To date, pharmacogenetic studies of allopurinol have focused on adverse effects. However, we were more interested in predicting response. The ability to predict poor response is important because it can influence drug choice and shorten the time to achieve target SU,” Dr. Stamp said.
The present study helps to validate the results of a previous genomewide association study in which the single nucleotide polymorphism (SNP) rs2231142 in ABCG2 was associated with poor response to allopurinol (Clin Pharmacol Ther. 2015 May;97[5]:518-25). Adherence to allopurinol was not examined in that study, so Dr. Stamp and her colleagues set out to make sure that it was not a confounder. The current study examined the SNP’s association with allopurinol response more closely in 264 gout patients who were well characterized phenotypically. All were being treated with allopurinol and all adhered to their treatment regimen. Overall, 120 were deemed good responders, with SU of 6 mg/dL or less on daily allopurinol of 300 mg or less, and 68 were poor responders, with SU of 6 mg/dL or higher despite a daily dose of allopurinol exceeding 300 mg. Another 76 patients were nonadherent/nonclassifiable. The poor responders tended to be younger and were more likely to be male, nonwhite, and heavier than their counterparts who responded well.
Genotyping for the rs2231142 SNP revealed a greater preponderance of the minor genotype in poor responders, compared with good responders (39% vs. 18%). This genotype was significantly associated with poor response after adjusting for age, sex, body-mass index, and ethnicity (odds ratio, 2.91; 95% confidence interval, 1.71-5.17; P = .00015). The mechanism – still to be proven – may involve reduced urate excretion from the gut.
There may not be a case for screening for the genotype just yet, according to Dr. Christopher M. Burns, a rheumatologist at Dartmouth Hitchcock Medical Center, Lebanon, N.H., who was not involved in the study. He said “it’s hard to see why you would screen for this variant, as it doesn’t account for all resistance, and you would still end up pushing the allopurinol dose up to achieve a SU of less than 6.0 mg/dL anyway. Since it’s not an adverse event warning signal, but simply one marker of possibly requiring a higher allopurinol dose, and allopurinol is an inexpensive drug, why would you check it? At least, that’s my sense. The findings may be more important simply for a better understanding of urate metabolism and the mechanism of action of allopurinol and oxypurinol.”
Dr. Stamp reported a financial disclosure with AstraZeneca.
SAN FRANCISCO – Patients with gout who fail to sufficiently lower their serum urate level despite adherence to a regimen of allopurinol 300 mg/day may have a genetic polymorphism affecting their response to the medication, according to new findings presented at the annual meeting of the American College of Rheumatology.
“ABCG2 genotyping may identify patients who will not reach target serum urate levels on standard allopurinol doses,” said study presenter and principal investigator Dr. Lisa K. Stamp of the University of Otago, Christchurch, New Zealand.
Sustained lowering of serum urate (SU) to less than 6 mg/dL – achieved most commonly by inhibition of xanthine oxidase using allopurinol – is crucial for the long-term management of gout. Yet, in clinical practice, less than half of patients treated with allopurinol achieve this SU target. This is usually because of poor adherence or an inappropriately low dose of the drug. But, in some patients, the response can be poor even despite allopurinol 300 mg daily. The reason for failure of allopurinol treatment in these poor responders has been unknown.
“It is important to consider genes that might help predict response or toxicity. To date, pharmacogenetic studies of allopurinol have focused on adverse effects. However, we were more interested in predicting response. The ability to predict poor response is important because it can influence drug choice and shorten the time to achieve target SU,” Dr. Stamp said.
The present study helps to validate the results of a previous genomewide association study in which the single nucleotide polymorphism (SNP) rs2231142 in ABCG2 was associated with poor response to allopurinol (Clin Pharmacol Ther. 2015 May;97[5]:518-25). Adherence to allopurinol was not examined in that study, so Dr. Stamp and her colleagues set out to make sure that it was not a confounder. The current study examined the SNP’s association with allopurinol response more closely in 264 gout patients who were well characterized phenotypically. All were being treated with allopurinol and all adhered to their treatment regimen. Overall, 120 were deemed good responders, with SU of 6 mg/dL or less on daily allopurinol of 300 mg or less, and 68 were poor responders, with SU of 6 mg/dL or higher despite a daily dose of allopurinol exceeding 300 mg. Another 76 patients were nonadherent/nonclassifiable. The poor responders tended to be younger and were more likely to be male, nonwhite, and heavier than their counterparts who responded well.
Genotyping for the rs2231142 SNP revealed a greater preponderance of the minor genotype in poor responders, compared with good responders (39% vs. 18%). This genotype was significantly associated with poor response after adjusting for age, sex, body-mass index, and ethnicity (odds ratio, 2.91; 95% confidence interval, 1.71-5.17; P = .00015). The mechanism – still to be proven – may involve reduced urate excretion from the gut.
There may not be a case for screening for the genotype just yet, according to Dr. Christopher M. Burns, a rheumatologist at Dartmouth Hitchcock Medical Center, Lebanon, N.H., who was not involved in the study. He said “it’s hard to see why you would screen for this variant, as it doesn’t account for all resistance, and you would still end up pushing the allopurinol dose up to achieve a SU of less than 6.0 mg/dL anyway. Since it’s not an adverse event warning signal, but simply one marker of possibly requiring a higher allopurinol dose, and allopurinol is an inexpensive drug, why would you check it? At least, that’s my sense. The findings may be more important simply for a better understanding of urate metabolism and the mechanism of action of allopurinol and oxypurinol.”
Dr. Stamp reported a financial disclosure with AstraZeneca.
SAN FRANCISCO – Patients with gout who fail to sufficiently lower their serum urate level despite adherence to a regimen of allopurinol 300 mg/day may have a genetic polymorphism affecting their response to the medication, according to new findings presented at the annual meeting of the American College of Rheumatology.
“ABCG2 genotyping may identify patients who will not reach target serum urate levels on standard allopurinol doses,” said study presenter and principal investigator Dr. Lisa K. Stamp of the University of Otago, Christchurch, New Zealand.
Sustained lowering of serum urate (SU) to less than 6 mg/dL – achieved most commonly by inhibition of xanthine oxidase using allopurinol – is crucial for the long-term management of gout. Yet, in clinical practice, less than half of patients treated with allopurinol achieve this SU target. This is usually because of poor adherence or an inappropriately low dose of the drug. But, in some patients, the response can be poor even despite allopurinol 300 mg daily. The reason for failure of allopurinol treatment in these poor responders has been unknown.
“It is important to consider genes that might help predict response or toxicity. To date, pharmacogenetic studies of allopurinol have focused on adverse effects. However, we were more interested in predicting response. The ability to predict poor response is important because it can influence drug choice and shorten the time to achieve target SU,” Dr. Stamp said.
The present study helps to validate the results of a previous genomewide association study in which the single nucleotide polymorphism (SNP) rs2231142 in ABCG2 was associated with poor response to allopurinol (Clin Pharmacol Ther. 2015 May;97[5]:518-25). Adherence to allopurinol was not examined in that study, so Dr. Stamp and her colleagues set out to make sure that it was not a confounder. The current study examined the SNP’s association with allopurinol response more closely in 264 gout patients who were well characterized phenotypically. All were being treated with allopurinol and all adhered to their treatment regimen. Overall, 120 were deemed good responders, with SU of 6 mg/dL or less on daily allopurinol of 300 mg or less, and 68 were poor responders, with SU of 6 mg/dL or higher despite a daily dose of allopurinol exceeding 300 mg. Another 76 patients were nonadherent/nonclassifiable. The poor responders tended to be younger and were more likely to be male, nonwhite, and heavier than their counterparts who responded well.
Genotyping for the rs2231142 SNP revealed a greater preponderance of the minor genotype in poor responders, compared with good responders (39% vs. 18%). This genotype was significantly associated with poor response after adjusting for age, sex, body-mass index, and ethnicity (odds ratio, 2.91; 95% confidence interval, 1.71-5.17; P = .00015). The mechanism – still to be proven – may involve reduced urate excretion from the gut.
There may not be a case for screening for the genotype just yet, according to Dr. Christopher M. Burns, a rheumatologist at Dartmouth Hitchcock Medical Center, Lebanon, N.H., who was not involved in the study. He said “it’s hard to see why you would screen for this variant, as it doesn’t account for all resistance, and you would still end up pushing the allopurinol dose up to achieve a SU of less than 6.0 mg/dL anyway. Since it’s not an adverse event warning signal, but simply one marker of possibly requiring a higher allopurinol dose, and allopurinol is an inexpensive drug, why would you check it? At least, that’s my sense. The findings may be more important simply for a better understanding of urate metabolism and the mechanism of action of allopurinol and oxypurinol.”
Dr. Stamp reported a financial disclosure with AstraZeneca.
AT THE ACR ANNUAL MEETING
Key clinical point: Screening could identify gout patients who require a dose adjustment of allopurinol.
Major finding: Presence of the rs2231142 SNP in the ABCG2 gene was significantly associated with poor response to allopurinol after adjusting for age, sex, body-mass index, and ethnicity (OR, 2.91; 95% CI, 1.71-5.17; P = .00015).
Data source: An analysis of 264 gout patients participating in clinical trials with allopurinol.
Disclosures: Dr. Stamp reported a financial disclosure with AstraZeneca.
EEG detects early conversion to Alzheimer’s disease
WASHINGTON – Electroencephalography combined with novel software can distinguish mild cognitive impairment (MCI) from prodromal Alzheimer’s disease years before clinical symptoms become evident, according to a longitudinal assessment of patient data from a memory clinic in Iceland.
Monitoring electrophysiology could be used to select subjects for clinical trials who have yet to, but likely will, develop Alzheimer’s, said the investigators, led by Kristinn Johnsen, Ph.D., chief scientific officer at MentisCura, an Icelandic company developing the technology.
The software applied statistical pattern recognition to a large set of electroencephalography (EEG) features gathered from 169 individuals with MCI from the Memory Clinic at National University Hospital, Reykjavik, Iceland, over the course of 10 years’ follow-up to predict conversion from MCI to Alzheimer’s within 3-4 years, with a sensitivity of 87% and specificity of 79%.
During follow-up, 68 were diagnosed with AD after varying times ranging from 1 to nearly 10 years. Another 56 subjects had MCI that remained stable for at least 3 years, the researchers noted in a poster presented at the Alzheimer’s Association International Conference 2015.
A total of 24 subjects developed other dementias, and 21 were lost to follow-up. The groups were similar at baseline in gender, age in the mid-70s, and Mini-Mental State Examination score.
A Kaplan-Meier plot of the patients who were pegged as being likely to convert based on the stratification index revealed a curve similar to that of patients who had Alzheimer’s at baseline, with divergence of the curves occurring only after 3-4 years. In contrast, a Kaplan-Meier plot of the conversion of the full MCI cohort to Alzheimer’s separated from the curve of patients who had Alzheimer’s at baseline almost immediately.
The EEG software is already in routine clinical use in Iceland, with more than 700 patients with suspicion of dementia having been examined, according to Thora Thorgilsdottir of MentisCura. The technology has not yet been approved by the Food and Drug Administration and is available in the United States only for investigational purposes.
WASHINGTON – Electroencephalography combined with novel software can distinguish mild cognitive impairment (MCI) from prodromal Alzheimer’s disease years before clinical symptoms become evident, according to a longitudinal assessment of patient data from a memory clinic in Iceland.
Monitoring electrophysiology could be used to select subjects for clinical trials who have yet to, but likely will, develop Alzheimer’s, said the investigators, led by Kristinn Johnsen, Ph.D., chief scientific officer at MentisCura, an Icelandic company developing the technology.
The software applied statistical pattern recognition to a large set of electroencephalography (EEG) features gathered from 169 individuals with MCI from the Memory Clinic at National University Hospital, Reykjavik, Iceland, over the course of 10 years’ follow-up to predict conversion from MCI to Alzheimer’s within 3-4 years, with a sensitivity of 87% and specificity of 79%.
During follow-up, 68 were diagnosed with AD after varying times ranging from 1 to nearly 10 years. Another 56 subjects had MCI that remained stable for at least 3 years, the researchers noted in a poster presented at the Alzheimer’s Association International Conference 2015.
A total of 24 subjects developed other dementias, and 21 were lost to follow-up. The groups were similar at baseline in gender, age in the mid-70s, and Mini-Mental State Examination score.
A Kaplan-Meier plot of the patients who were pegged as being likely to convert based on the stratification index revealed a curve similar to that of patients who had Alzheimer’s at baseline, with divergence of the curves occurring only after 3-4 years. In contrast, a Kaplan-Meier plot of the conversion of the full MCI cohort to Alzheimer’s separated from the curve of patients who had Alzheimer’s at baseline almost immediately.
The EEG software is already in routine clinical use in Iceland, with more than 700 patients with suspicion of dementia having been examined, according to Thora Thorgilsdottir of MentisCura. The technology has not yet been approved by the Food and Drug Administration and is available in the United States only for investigational purposes.
WASHINGTON – Electroencephalography combined with novel software can distinguish mild cognitive impairment (MCI) from prodromal Alzheimer’s disease years before clinical symptoms become evident, according to a longitudinal assessment of patient data from a memory clinic in Iceland.
Monitoring electrophysiology could be used to select subjects for clinical trials who have yet to, but likely will, develop Alzheimer’s, said the investigators, led by Kristinn Johnsen, Ph.D., chief scientific officer at MentisCura, an Icelandic company developing the technology.
The software applied statistical pattern recognition to a large set of electroencephalography (EEG) features gathered from 169 individuals with MCI from the Memory Clinic at National University Hospital, Reykjavik, Iceland, over the course of 10 years’ follow-up to predict conversion from MCI to Alzheimer’s within 3-4 years, with a sensitivity of 87% and specificity of 79%.
During follow-up, 68 were diagnosed with AD after varying times ranging from 1 to nearly 10 years. Another 56 subjects had MCI that remained stable for at least 3 years, the researchers noted in a poster presented at the Alzheimer’s Association International Conference 2015.
A total of 24 subjects developed other dementias, and 21 were lost to follow-up. The groups were similar at baseline in gender, age in the mid-70s, and Mini-Mental State Examination score.
A Kaplan-Meier plot of the patients who were pegged as being likely to convert based on the stratification index revealed a curve similar to that of patients who had Alzheimer’s at baseline, with divergence of the curves occurring only after 3-4 years. In contrast, a Kaplan-Meier plot of the conversion of the full MCI cohort to Alzheimer’s separated from the curve of patients who had Alzheimer’s at baseline almost immediately.
The EEG software is already in routine clinical use in Iceland, with more than 700 patients with suspicion of dementia having been examined, according to Thora Thorgilsdottir of MentisCura. The technology has not yet been approved by the Food and Drug Administration and is available in the United States only for investigational purposes.
AT AAIC 2015
Key clinical point: Early conversion from mild cognitive inhibition to clinical Alzheimer’s disease can be detected via electroencephalography.
Major finding: EEG of individuals with MCI predicted conversion to Alzheimer’s within 3-4 years, with a sensitivity of 87% and specificity of 79%.
Data source: Single-institute, prospective, follow-up study of 169 patients.
Disclosures: Dr. Johnsen is chief scientific officer at MentisCura, which sponsored the study.
Cognitive benefits apparent with commercially available supplement
WASHINGTON – A randomized, double-blind, placebo-controlled trial has supported the purported cognitive boosting capability of the dietary supplement Alpha BRAIN. While the trial was small, it’s a start, and lends credence to the cognitive boost claimed by the commercially available pills.
“From a science and medicine standpoint, we felt this was an interesting study, both because it would allow us to examine if Alpha BRAIN was indeed efficacious as a cognitive enhancer, and also due to the complete dearth of rigorous scientific and methodologically sound studies that have been performed on similar supplements to date,” principal investigator Todd Solomon, Ph.D., of Boston University, said in an email.
At the Alzheimer’s Association International Conference 2015, Dr. Solomon presented a study of 63 participants aged 18-35 years who had a 2-week placebo run-in before 30 were double-blind randomized to Alpha BRAIN (Onnit Labs, Austin, Tex.) and 33 to placebo. A battery of neuropsychological tests (Wechsler Memory Scale–Fourth Edition, Delis-Kaplan Executive Function System, California Verbal Learning Test-Second Edition, Trail Making Test parts A and B, and Paced Auditory Serial Addition Test) was run before and at the end of the 6-week study. The two groups were similar at baseline demographically concerning age, estimated IQ, gender, level of education (the majority were currently enrolled college students), and race (most were white). Nearly everyone followed the manufacturer’s instructions, with over 90% in each group taking the real or placebo preparation on schedule for the 6 weeks.
At baseline, both groups were similar in all the cognitive measures. Four participants in the Alpha BRAIN arm and three in the placebo arm dropped out during the next 6 weeks. At trial’s end, both groups showed neuropsychological improvements. Those randomized to receive Alpha BRAIN were significantly better on tasks of delayed verbal recall (P = .01) and executive functioning (P = .05), compared with placebo.
The results are “encouraging,” Dr. Solomon said. Still, the small number of subjects and the brevity of the trial rule out solid conclusions. That will come with similarly designed trials that involve more subjects and run longer.
“As supplements in the United States are not regulated by any governing body, we felt it was important to support a company that was willing to hold themselves to the same type of standards promoted by the Food and Drug Administration for testing the efficacy of experimental compounds,” Dr. Solomon said in an email.
Onnit Labs funded the study. Dr. Solomon had no disclosures. One coauthor is an employee of Onnit Labs, the maker of Alpha BRAIN.
WASHINGTON – A randomized, double-blind, placebo-controlled trial has supported the purported cognitive boosting capability of the dietary supplement Alpha BRAIN. While the trial was small, it’s a start, and lends credence to the cognitive boost claimed by the commercially available pills.
“From a science and medicine standpoint, we felt this was an interesting study, both because it would allow us to examine if Alpha BRAIN was indeed efficacious as a cognitive enhancer, and also due to the complete dearth of rigorous scientific and methodologically sound studies that have been performed on similar supplements to date,” principal investigator Todd Solomon, Ph.D., of Boston University, said in an email.
At the Alzheimer’s Association International Conference 2015, Dr. Solomon presented a study of 63 participants aged 18-35 years who had a 2-week placebo run-in before 30 were double-blind randomized to Alpha BRAIN (Onnit Labs, Austin, Tex.) and 33 to placebo. A battery of neuropsychological tests (Wechsler Memory Scale–Fourth Edition, Delis-Kaplan Executive Function System, California Verbal Learning Test-Second Edition, Trail Making Test parts A and B, and Paced Auditory Serial Addition Test) was run before and at the end of the 6-week study. The two groups were similar at baseline demographically concerning age, estimated IQ, gender, level of education (the majority were currently enrolled college students), and race (most were white). Nearly everyone followed the manufacturer’s instructions, with over 90% in each group taking the real or placebo preparation on schedule for the 6 weeks.
At baseline, both groups were similar in all the cognitive measures. Four participants in the Alpha BRAIN arm and three in the placebo arm dropped out during the next 6 weeks. At trial’s end, both groups showed neuropsychological improvements. Those randomized to receive Alpha BRAIN were significantly better on tasks of delayed verbal recall (P = .01) and executive functioning (P = .05), compared with placebo.
The results are “encouraging,” Dr. Solomon said. Still, the small number of subjects and the brevity of the trial rule out solid conclusions. That will come with similarly designed trials that involve more subjects and run longer.
“As supplements in the United States are not regulated by any governing body, we felt it was important to support a company that was willing to hold themselves to the same type of standards promoted by the Food and Drug Administration for testing the efficacy of experimental compounds,” Dr. Solomon said in an email.
Onnit Labs funded the study. Dr. Solomon had no disclosures. One coauthor is an employee of Onnit Labs, the maker of Alpha BRAIN.
WASHINGTON – A randomized, double-blind, placebo-controlled trial has supported the purported cognitive boosting capability of the dietary supplement Alpha BRAIN. While the trial was small, it’s a start, and lends credence to the cognitive boost claimed by the commercially available pills.
“From a science and medicine standpoint, we felt this was an interesting study, both because it would allow us to examine if Alpha BRAIN was indeed efficacious as a cognitive enhancer, and also due to the complete dearth of rigorous scientific and methodologically sound studies that have been performed on similar supplements to date,” principal investigator Todd Solomon, Ph.D., of Boston University, said in an email.
At the Alzheimer’s Association International Conference 2015, Dr. Solomon presented a study of 63 participants aged 18-35 years who had a 2-week placebo run-in before 30 were double-blind randomized to Alpha BRAIN (Onnit Labs, Austin, Tex.) and 33 to placebo. A battery of neuropsychological tests (Wechsler Memory Scale–Fourth Edition, Delis-Kaplan Executive Function System, California Verbal Learning Test-Second Edition, Trail Making Test parts A and B, and Paced Auditory Serial Addition Test) was run before and at the end of the 6-week study. The two groups were similar at baseline demographically concerning age, estimated IQ, gender, level of education (the majority were currently enrolled college students), and race (most were white). Nearly everyone followed the manufacturer’s instructions, with over 90% in each group taking the real or placebo preparation on schedule for the 6 weeks.
At baseline, both groups were similar in all the cognitive measures. Four participants in the Alpha BRAIN arm and three in the placebo arm dropped out during the next 6 weeks. At trial’s end, both groups showed neuropsychological improvements. Those randomized to receive Alpha BRAIN were significantly better on tasks of delayed verbal recall (P = .01) and executive functioning (P = .05), compared with placebo.
The results are “encouraging,” Dr. Solomon said. Still, the small number of subjects and the brevity of the trial rule out solid conclusions. That will come with similarly designed trials that involve more subjects and run longer.
“As supplements in the United States are not regulated by any governing body, we felt it was important to support a company that was willing to hold themselves to the same type of standards promoted by the Food and Drug Administration for testing the efficacy of experimental compounds,” Dr. Solomon said in an email.
Onnit Labs funded the study. Dr. Solomon had no disclosures. One coauthor is an employee of Onnit Labs, the maker of Alpha BRAIN.
AT AAIC 2015
Key clinical point: A small randomized, placebo controlled study has shown verbal memory benefits with Alpha BRAIN.
Major finding: Six weeks of Alpha BRAIN use improved verbal memory and executive function.
Data source: Single-institute, prospective, follow-up study of 169 patients.
Disclosures: Onnit Labs funded the study. Dr. Solomon had no disclosures. One coauthor is an employee of Onnit Labs, the maker of Alpha BRAIN.
Overgeneral Autobiographical Memory Predicts Adolescent Depression
Overgeneral autobiographical memory – the tendency to categorize memories rather than to remember specific events in response to a cue – which has long been pegged as a risk factor for depression in adults, also is a risk factor of depression in adolescents, a study of 277 adolescents has shown.
The study involved adolescents aged 10-18 years who were part of the Early Prediction of Adolescent Depression study – an ongoing longitudinal study of depression in children of parents with recurrent unipolar depression. Data on autobiographical memory in response to verbal cues of prior positive and negative experiences were collected at baseline and at intervals during the approximately 12-month follow-up. The data were analyzed to tease out the association of overgeneral autobiographical memory (OGM) with depression.
Clinical interviews at baseline categorized the subjects as no disorder, depressive disorder, anxiety disorder, and externalizing disorder, reported Adhip Rawal, Ph.D., and Frances Rice, Ph.D., of University College London (J. Am. Acad. Child Adolesc. Psychiatry 2012;51:518-27).
OGM to "negative cues" was more likely among adolescents who were depressed at baseline than in those with no disorder, anxiety disorder, and externalizing disorder. In those who were not depressed at baseline, OGM was associated with the onset of depression disorder and symptoms at follow-up, but not with development of anxiety disorder or externalizing disorders. Adolescents who were depressed at baseline and at follow-up were no more likely to display OGM that those who were no longer depressed after 1-year, they said.
Age, IQ, and depressive symptoms were not correlated with OGM.
"The present results showed that increased overgenerality to negative cues predicted the occurrence of depressive disorder in adolescents free from depressive disorder at baseline, independent of age, IQ, and depressive symptoms," Dr. Rawal and Dr. Rice wrote.
When asked about the results, Filip Raes, Ph.D., said that seeing findings found in adults being replicated in adolescents "says something about the robustness of the phenomenon of OGM."
"What makes their findings important is that they found evidence for OGM’s predictive value for new onsets of clinical depressive episodes," Dr. Raes, a clinical psychologist and professor of psychology at the University of Leuven (Belgium) and a leading researcher in the area of OGM, said in the interview. "As rightly noted by the authors, OGM may constitute an important target for preventive interventions."
Limitations of the study included the absence of an examination of the influence of gender, the relatively short follow-up period, the possibility that episodes of depression were missed in the periods between assessments, and lack of a control for prior episodes of depression in the years before the study.
Dr. Rawal and Dr. Rice reported no potential conflicts of interest.
Overgeneral autobiographical memory – the tendency to categorize memories rather than to remember specific events in response to a cue – which has long been pegged as a risk factor for depression in adults, also is a risk factor of depression in adolescents, a study of 277 adolescents has shown.
The study involved adolescents aged 10-18 years who were part of the Early Prediction of Adolescent Depression study – an ongoing longitudinal study of depression in children of parents with recurrent unipolar depression. Data on autobiographical memory in response to verbal cues of prior positive and negative experiences were collected at baseline and at intervals during the approximately 12-month follow-up. The data were analyzed to tease out the association of overgeneral autobiographical memory (OGM) with depression.
Clinical interviews at baseline categorized the subjects as no disorder, depressive disorder, anxiety disorder, and externalizing disorder, reported Adhip Rawal, Ph.D., and Frances Rice, Ph.D., of University College London (J. Am. Acad. Child Adolesc. Psychiatry 2012;51:518-27).
OGM to "negative cues" was more likely among adolescents who were depressed at baseline than in those with no disorder, anxiety disorder, and externalizing disorder. In those who were not depressed at baseline, OGM was associated with the onset of depression disorder and symptoms at follow-up, but not with development of anxiety disorder or externalizing disorders. Adolescents who were depressed at baseline and at follow-up were no more likely to display OGM that those who were no longer depressed after 1-year, they said.
Age, IQ, and depressive symptoms were not correlated with OGM.
"The present results showed that increased overgenerality to negative cues predicted the occurrence of depressive disorder in adolescents free from depressive disorder at baseline, independent of age, IQ, and depressive symptoms," Dr. Rawal and Dr. Rice wrote.
When asked about the results, Filip Raes, Ph.D., said that seeing findings found in adults being replicated in adolescents "says something about the robustness of the phenomenon of OGM."
"What makes their findings important is that they found evidence for OGM’s predictive value for new onsets of clinical depressive episodes," Dr. Raes, a clinical psychologist and professor of psychology at the University of Leuven (Belgium) and a leading researcher in the area of OGM, said in the interview. "As rightly noted by the authors, OGM may constitute an important target for preventive interventions."
Limitations of the study included the absence of an examination of the influence of gender, the relatively short follow-up period, the possibility that episodes of depression were missed in the periods between assessments, and lack of a control for prior episodes of depression in the years before the study.
Dr. Rawal and Dr. Rice reported no potential conflicts of interest.
Overgeneral autobiographical memory – the tendency to categorize memories rather than to remember specific events in response to a cue – which has long been pegged as a risk factor for depression in adults, also is a risk factor of depression in adolescents, a study of 277 adolescents has shown.
The study involved adolescents aged 10-18 years who were part of the Early Prediction of Adolescent Depression study – an ongoing longitudinal study of depression in children of parents with recurrent unipolar depression. Data on autobiographical memory in response to verbal cues of prior positive and negative experiences were collected at baseline and at intervals during the approximately 12-month follow-up. The data were analyzed to tease out the association of overgeneral autobiographical memory (OGM) with depression.
Clinical interviews at baseline categorized the subjects as no disorder, depressive disorder, anxiety disorder, and externalizing disorder, reported Adhip Rawal, Ph.D., and Frances Rice, Ph.D., of University College London (J. Am. Acad. Child Adolesc. Psychiatry 2012;51:518-27).
OGM to "negative cues" was more likely among adolescents who were depressed at baseline than in those with no disorder, anxiety disorder, and externalizing disorder. In those who were not depressed at baseline, OGM was associated with the onset of depression disorder and symptoms at follow-up, but not with development of anxiety disorder or externalizing disorders. Adolescents who were depressed at baseline and at follow-up were no more likely to display OGM that those who were no longer depressed after 1-year, they said.
Age, IQ, and depressive symptoms were not correlated with OGM.
"The present results showed that increased overgenerality to negative cues predicted the occurrence of depressive disorder in adolescents free from depressive disorder at baseline, independent of age, IQ, and depressive symptoms," Dr. Rawal and Dr. Rice wrote.
When asked about the results, Filip Raes, Ph.D., said that seeing findings found in adults being replicated in adolescents "says something about the robustness of the phenomenon of OGM."
"What makes their findings important is that they found evidence for OGM’s predictive value for new onsets of clinical depressive episodes," Dr. Raes, a clinical psychologist and professor of psychology at the University of Leuven (Belgium) and a leading researcher in the area of OGM, said in the interview. "As rightly noted by the authors, OGM may constitute an important target for preventive interventions."
Limitations of the study included the absence of an examination of the influence of gender, the relatively short follow-up period, the possibility that episodes of depression were missed in the periods between assessments, and lack of a control for prior episodes of depression in the years before the study.
Dr. Rawal and Dr. Rice reported no potential conflicts of interest.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF CHILD AND ADOLESCENT PSYCHIATRY
Major Finding: Adolescents with depression onset were more overgeneral to negative cues at baseline than were adolescents who did not develop depression (P = .03). When investigators controlled for baseline depressive symptoms, overgenerality to negative cues kept predicting depressive disorder at follow-up.
Data Source: The data are from 277 adolescents aged 10-18 years at familial risk for depression who were part of the Early Prediction of Adolescent Depression study.
Disclosures: Dr. Rawal and Dr. Rice reported no potential conflicts of interest.
Online CBT for Depression, GAD Is Effective for Most
Internet-based cognitive-behavioral therapy is a treatment approach that can benefit patients with anxiety and depression, researchers report.
The study, conducted by Matthew Sunderland, Ph.D., and colleagues, bolsters the case for the web-based approach as a way of overcoming treatment obstacles such as the lack of rural-based services and wait time (Behavior Res. and Therapy 2012;50:374-80).
Dr. Sunderland and his colleagues have been engaged in creating a web-based option for general practitioners and other front-line workers to treat patients, freeing therapy time for those patients in need of more intensive treatment. Hindering this goal has been a lack of information on the "trajectory of change" of patients during the treatment course, reported Dr. Sunderland of the University of New South Wales, Sydney.
The investigators studied 663 patients who completed an online cognitive-behavioral therapy (CBT) course for depression (n=302; 58% female) and generalized anxiety disorder (GAD) (n=361; 74% female). The patients’ average age was 43. The primary outcome measure was the Kessler-10 psychological distress scale. The time course of depression and GAD was respectively determined using the Patient Health Questionnaire-9 and GAD-7 instruments. Levels of functional impairment over the previous 30 days were measured using the World Health Organization Disability Assessment Schedule 2.0.
Most (75%-80%) patients with either psychological disorder displayed progressive and clinically significant improvements over the period of the six online course lessons, corroborating previous studies.
But the rest were "low responders," whose initial improvement leveled off and even slightly regressed in the later sessions, indicating to the researchers that Internet CBT for anxiety and depression "may be better suited as an initial step within a stepped care model for treatment."
When asked about the results, Erik Andersson, M.Sc., of the department of clinical neuroscience, Karolinska Institutet, Stockholm, said his overall impression is that they are impressive. "The rate of responders is high and much better than, for example, pharmacological treatments. Of course, one could wish they had more predictor variables, such as genetic data," he said. "That is an important issue for future research."
He also said the results should be compared to traditional live therapy.
What factors determine the good and low responses remain to be clarified.
"That is indeed the billion dollar question," Mr. Andersson said. "The authors have found some predictive variables, but it is always hard to tell on an individual level. Some patients really surprise you as a clinician. In my view, the ability to predict treatment success beforehand is still impossible to decide on an individual level. But this article gives some guidance in the clinical decision process.
Gerhard Andersson, Ph.D., said in an interview that it is unclear whether the low responders would indeed benefit from another form of therapy. "My impression is that there is a group (albeit small) who is less likely to benefit from therapy regardless what form of psychotherapy, said Dr. Andersson, of the department of behavioural sciences and learning at Linköping University, Sweden. "For depression, it is possible that combined treatment may lead to more responders, such as if Internet CBT and medication are combined."
Dr. Sunderland reported several study limitations, including the absence of a control group and the inability to look into the kinds of barriers that might have prevented people from benefiting from online CBT courses. "Further research with more robust experimental designs is required to address some of the questions regarding the defining features of the sub-classes raised in the current study," they wrote.
Dr. Sunderland reported no relevant financial conflicts of interest.
Internet-based cognitive-behavioral therapy is a treatment approach that can benefit patients with anxiety and depression, researchers report.
The study, conducted by Matthew Sunderland, Ph.D., and colleagues, bolsters the case for the web-based approach as a way of overcoming treatment obstacles such as the lack of rural-based services and wait time (Behavior Res. and Therapy 2012;50:374-80).
Dr. Sunderland and his colleagues have been engaged in creating a web-based option for general practitioners and other front-line workers to treat patients, freeing therapy time for those patients in need of more intensive treatment. Hindering this goal has been a lack of information on the "trajectory of change" of patients during the treatment course, reported Dr. Sunderland of the University of New South Wales, Sydney.
The investigators studied 663 patients who completed an online cognitive-behavioral therapy (CBT) course for depression (n=302; 58% female) and generalized anxiety disorder (GAD) (n=361; 74% female). The patients’ average age was 43. The primary outcome measure was the Kessler-10 psychological distress scale. The time course of depression and GAD was respectively determined using the Patient Health Questionnaire-9 and GAD-7 instruments. Levels of functional impairment over the previous 30 days were measured using the World Health Organization Disability Assessment Schedule 2.0.
Most (75%-80%) patients with either psychological disorder displayed progressive and clinically significant improvements over the period of the six online course lessons, corroborating previous studies.
But the rest were "low responders," whose initial improvement leveled off and even slightly regressed in the later sessions, indicating to the researchers that Internet CBT for anxiety and depression "may be better suited as an initial step within a stepped care model for treatment."
When asked about the results, Erik Andersson, M.Sc., of the department of clinical neuroscience, Karolinska Institutet, Stockholm, said his overall impression is that they are impressive. "The rate of responders is high and much better than, for example, pharmacological treatments. Of course, one could wish they had more predictor variables, such as genetic data," he said. "That is an important issue for future research."
He also said the results should be compared to traditional live therapy.
What factors determine the good and low responses remain to be clarified.
"That is indeed the billion dollar question," Mr. Andersson said. "The authors have found some predictive variables, but it is always hard to tell on an individual level. Some patients really surprise you as a clinician. In my view, the ability to predict treatment success beforehand is still impossible to decide on an individual level. But this article gives some guidance in the clinical decision process.
Gerhard Andersson, Ph.D., said in an interview that it is unclear whether the low responders would indeed benefit from another form of therapy. "My impression is that there is a group (albeit small) who is less likely to benefit from therapy regardless what form of psychotherapy, said Dr. Andersson, of the department of behavioural sciences and learning at Linköping University, Sweden. "For depression, it is possible that combined treatment may lead to more responders, such as if Internet CBT and medication are combined."
Dr. Sunderland reported several study limitations, including the absence of a control group and the inability to look into the kinds of barriers that might have prevented people from benefiting from online CBT courses. "Further research with more robust experimental designs is required to address some of the questions regarding the defining features of the sub-classes raised in the current study," they wrote.
Dr. Sunderland reported no relevant financial conflicts of interest.
Internet-based cognitive-behavioral therapy is a treatment approach that can benefit patients with anxiety and depression, researchers report.
The study, conducted by Matthew Sunderland, Ph.D., and colleagues, bolsters the case for the web-based approach as a way of overcoming treatment obstacles such as the lack of rural-based services and wait time (Behavior Res. and Therapy 2012;50:374-80).
Dr. Sunderland and his colleagues have been engaged in creating a web-based option for general practitioners and other front-line workers to treat patients, freeing therapy time for those patients in need of more intensive treatment. Hindering this goal has been a lack of information on the "trajectory of change" of patients during the treatment course, reported Dr. Sunderland of the University of New South Wales, Sydney.
The investigators studied 663 patients who completed an online cognitive-behavioral therapy (CBT) course for depression (n=302; 58% female) and generalized anxiety disorder (GAD) (n=361; 74% female). The patients’ average age was 43. The primary outcome measure was the Kessler-10 psychological distress scale. The time course of depression and GAD was respectively determined using the Patient Health Questionnaire-9 and GAD-7 instruments. Levels of functional impairment over the previous 30 days were measured using the World Health Organization Disability Assessment Schedule 2.0.
Most (75%-80%) patients with either psychological disorder displayed progressive and clinically significant improvements over the period of the six online course lessons, corroborating previous studies.
But the rest were "low responders," whose initial improvement leveled off and even slightly regressed in the later sessions, indicating to the researchers that Internet CBT for anxiety and depression "may be better suited as an initial step within a stepped care model for treatment."
When asked about the results, Erik Andersson, M.Sc., of the department of clinical neuroscience, Karolinska Institutet, Stockholm, said his overall impression is that they are impressive. "The rate of responders is high and much better than, for example, pharmacological treatments. Of course, one could wish they had more predictor variables, such as genetic data," he said. "That is an important issue for future research."
He also said the results should be compared to traditional live therapy.
What factors determine the good and low responses remain to be clarified.
"That is indeed the billion dollar question," Mr. Andersson said. "The authors have found some predictive variables, but it is always hard to tell on an individual level. Some patients really surprise you as a clinician. In my view, the ability to predict treatment success beforehand is still impossible to decide on an individual level. But this article gives some guidance in the clinical decision process.
Gerhard Andersson, Ph.D., said in an interview that it is unclear whether the low responders would indeed benefit from another form of therapy. "My impression is that there is a group (albeit small) who is less likely to benefit from therapy regardless what form of psychotherapy, said Dr. Andersson, of the department of behavioural sciences and learning at Linköping University, Sweden. "For depression, it is possible that combined treatment may lead to more responders, such as if Internet CBT and medication are combined."
Dr. Sunderland reported several study limitations, including the absence of a control group and the inability to look into the kinds of barriers that might have prevented people from benefiting from online CBT courses. "Further research with more robust experimental designs is required to address some of the questions regarding the defining features of the sub-classes raised in the current study," they wrote.
Dr. Sunderland reported no relevant financial conflicts of interest.
FROM BEHAVIOUR RESEARCH AND THERAPY
Major Finding: Among patients described as "responders," 75% of those with depression and 80% with generalized anxiety disorder showed improvement across all six online lessons.
Data Source: The investigators studied 663 patients who completed an Internet CBT course for depression and GAD.
Disclosures: Dr. Sunderland reported no relevant financial conflicts of interest.