User login
Large Hyperpigmented Nodule on the Leg
The Diagnosis: Dermatofibroma
Dermatofibroma (DF) is a commonly encountered lesion. Although usually a straightforward clinical diagnosis, histopathological diagnosis is sometimes required. Conventional histologic findings of DF are hyperkeratosis, induction of the epidermis with acanthosis, and basal layer hyperpigmentation.1,2 Within the dermis there usually is proliferation of fibroblasts, histiocytes, and blood vessels that sometimes spares the overlying papillary dermis. Nomenclature of specific variants may be assigned based on the predominant component (eg, nodular subepidermal fibrosis, histiocytoma, sclerosing hemangioma) or histologic findings (eg, fibrocollagenous, sclerotic, cellular, histiocytic, lipidized, angiomatous, aneurysmal, clear cell, monster cell, myxoid, keloidal, palisading, osteoclastic, epithelioid).3-5 Of the histologic variants, fibrocollagenous is most common, but knowledge of other variants is important for accurate diagnosis, especially to exclude malignancy.
The sclerosing hemangioma variant of DF may pre-sent a diagnostic dilemma. In addition to typical features of DF, pseudovascular spaces, abundant hemosiderin, and reactive-appearing spindled cells are histologically demonstrated. The marked sclerosis and pigment deposition may mimic a blue nevus, and the dilated pseudovascular spaces may be reminiscent of a vascular neoplasm such as angiosarcoma or Kaposi sarcoma. However, the presence of characteristic features such as peripheral collagen trapping and overlying epidermal hyperplasia provide important clues for correct diagnosis.
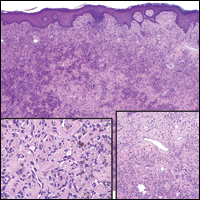
Angiosarcomas (Figure 1) are malignant neoplasms with vascular differentiation. Cutaneous angiosarcomas present as purple plaques or nodules on the head and/or neck in elderly individuals as well as in patients with chronic lymphedema or prior radiation exposure.6-9 They are aggressive neoplasms with high rates of recurrence and metastases. Microscopically, the tumor is composed of anastomosing vascular channels lined by atypical endothelial cells with a multilayered appearance. There is frequent red blood cell extravasation, and substantial hemosiderin deposition may be noted in long-standing lesions. Neoplastic cells are positive for vascular markers (CD34, CD31, ETS-related gene transcription factor). Notably, cases associated with radiation exposure and chronic lymphedema are positive for MYC.10

Blue nevi (Figure 2) are benign melanocytic tumors that occur most frequently in children but may pre-sent in any age group. Clinical presentation is a blue to black, slightly raised papule that may be found on any site of the body. Biopsy typically shows a wedge-shaped infiltrate of spindled melanocytes with elongated dendritic processes in a sclerotic collagenous stroma. There frequently is a striking population of heavily pigmented melanophages. The melanocytes are positive for melanoma antigen recognized by T cells (MART-1)/melan-A, S-100, and transcription factor SOX-10. In contrast to other benign nevi, human melanoma black-45 will be positive in the dermal component.
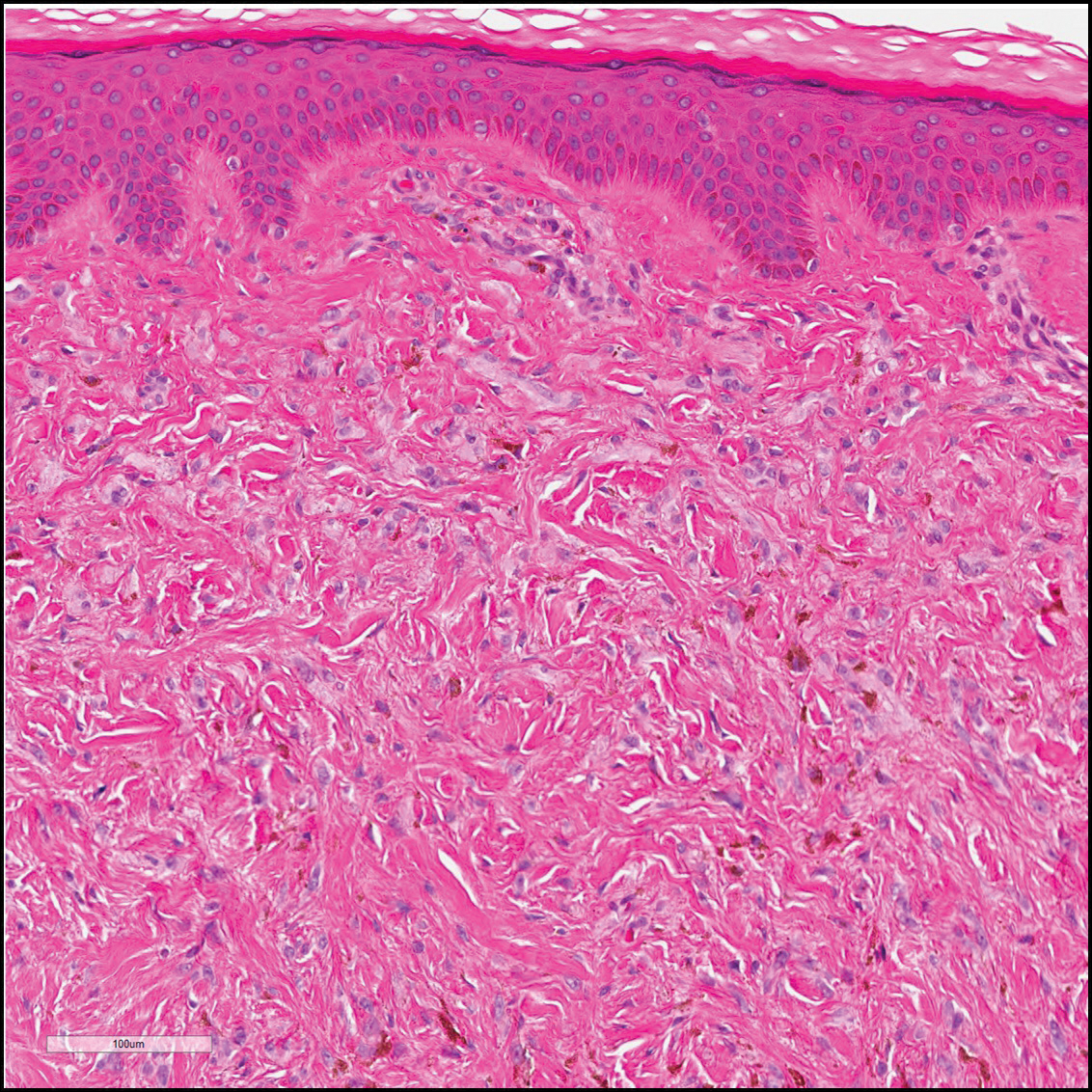
Dermatofibrosarcoma protuberans (Figure 3) is a dermal-based tumor of intermediate malignant potential with a high rate of local recurrence and potential for sarcomatous transformation. Dermatofibrosarcoma protuberans most commonly presents in young adults as firm, pink to brown plaques and can occur on any site of the body. Histologically, they show a dermal proliferation of spindled cells that infiltrate in a storiform fashion into the subcutaneous adipose tissue,11 which imparts a honeycomb or Swiss cheese pattern. The tumor characteristically demonstrates positive staining for CD34. Loss of CD34 staining, increased mitoses, nuclear atypia, and fascicular growth are features suggestive of sarcomatous transformation.11,12 Dermatofibrosarcoma protuberans is associated with chromosomal abnormalities of chromosomes 17 and 22, resulting in COL1A1 (collagen type 1 alpha 1 chain) and PDGF-β (platelet-derived growth factor subunit B) gene fusion.13
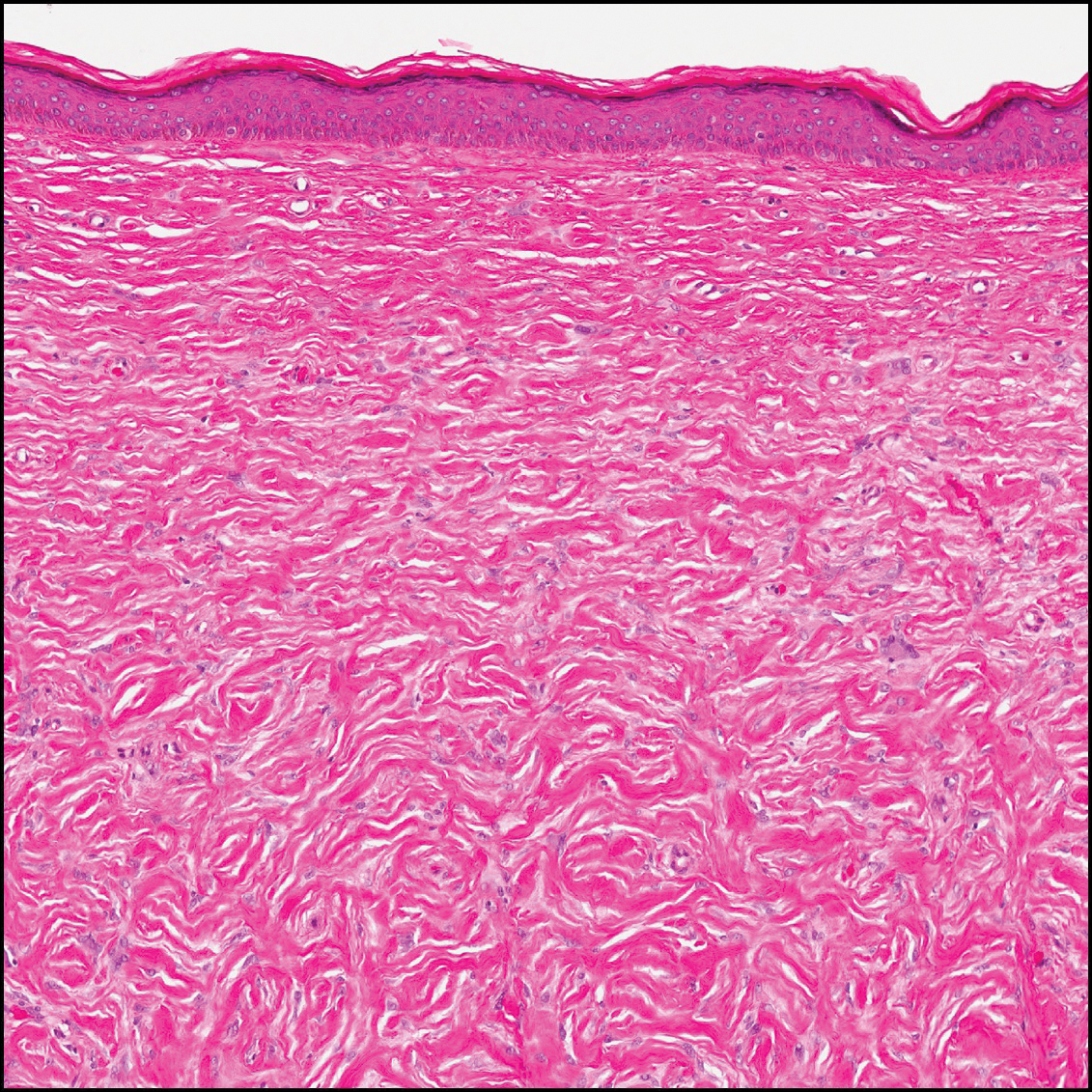
Sclerotic fibromas (also known as storiform collagenomas)(Figure 4) may represent regressed DFs and are frequently associated with prior trauma to the affected area.14,15 They usually appear as flesh-colored papules or nodules on the face and trunk. The presence of multiple sclerotic fibromas is associated with Cowden syndrome.16,17 Histologically, the lesions present as well-demarcated, nonencapsulated, dermal nodules composed of a storiform or whorled arrangement of collagen with spindled fibroblasts. The sclerotic collagen bundles often are separated by small clefts imparting a plywoodlike pattern.16
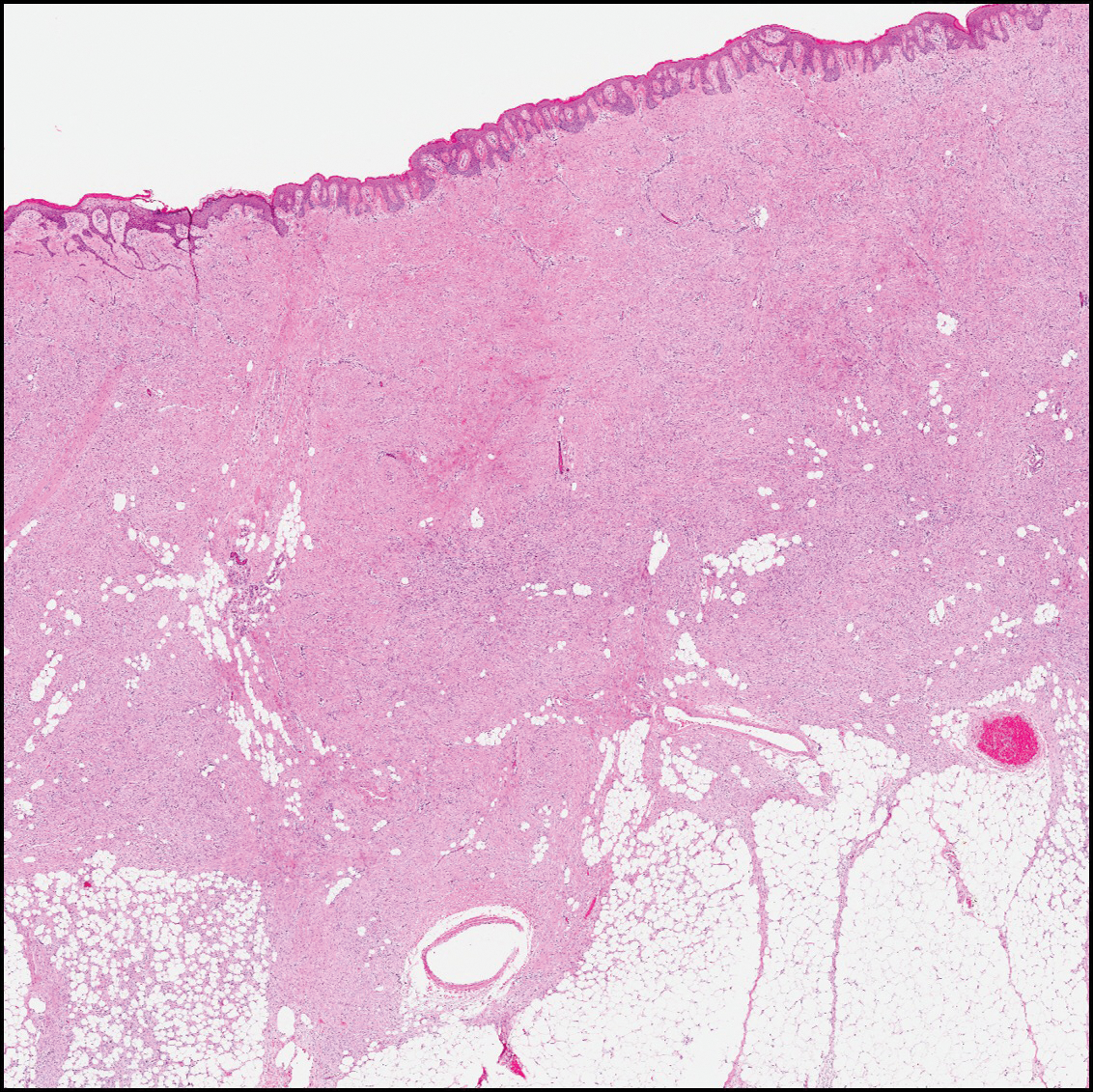
The differential diagnosis for DF expands once atypical clinical and histopathological findings are present. In this case, the nodule was much larger and darker than the usual appearance of DF (3-10 mm).2,4 Given the lesion's nodularity, the clinical dimple sign on lateral compression could not be seen. On biopsy, the predominance of blood vessels and sclerosis further complicated the diagnostic picture. In unusual cases such as this one, correlation of clinical history, histology, and immunophenotype is ever important.
- Zeidi M, North JP. Sebaceous induction in dermatofibroma: a common feature of dermatofibromas on the shoulder. J Cutan Pathol. 2015;42:400-405.
- Şenel E, Yuyucu Karabulut Y, Doğruer S¸enel S. Clinical, histopathological, dermatoscopic and digital microscopic features of dermatofibroma: a retrospective analysis of 200 lesions. J Eur Acad Dermatol Venereol. 2015;29:1958-1966.
- Vilanova JR, Flint A. The morphological variations of fibrous histiocytomas. J Cutan Pathol. 1974;1:155-164.
- Han TY, Chang HS, Lee JH, et al. A clinical and histopathological study of 122 cases of dermatofibroma (benign fibrous histiocytoma)[published online May 27, 2011]. Ann Dermatol. 2011;23:185-192.
- Alves JVP, Matos DM, Barreiros HF, et al. Variants of dermatofibroma--a histopathological study. An Bras Dermatol. 2014;89:472-477.
- Rosai J, Sumner HW, Major MC, et al. Angiosarcoma of the skin: a clinicopathologic and fine structural study. Hum Pathol. 1976;7:83-109.
- Haustein UF. Angiosarcoma of the face and scalp. Int J Dermatol. 1991;30:851-856.
- Stewart FW, Treves N. Lymphangiosarcoma in postmastectomy lymphedema: a report of six cases in elephantiasis chirurgica. Cancer. 1948;1:64-81.
- Goette DK, Detlefs RL. Postirradiation angiosarcoma. J Am Acad Dermatol. 1985;12(5 pt 2):922-926.
- Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34-39.
- Voth H, Landsberg J, Hinz T, et al. Management of dermatofibrosarcoma protuberans with fibrosarcomatous transformation: an evidence-based review of the literature. J Eur Acad Dermatol Venereol. 2011;25:1385-1391.
- Goldblum JR. CD34 positivity in fibrosarcomas which arise in dermatofibrosarcoma protuberans. Arch Pathol Lab Med. 1995;119:238-241.
- Patel KU, Szabo SS, Hernandez VS, et al. Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays. Hum Pathol. 2008;39:184-193.
- Sohn IB, Hwang SM, Lee SH, et al. Dermatofibroma with sclerotic areas resembling a sclerotic fibroma of the skin. J Cutan Pathol. 2002;29:44-47.
- Pujol RM, de Castro F, Schroeter AL, et al. Solitary sclerotic fibroma of the skin: a sclerotic dermatofibroma? Am J Dermatopathol. 1996;18:620-624.
- Requena L, Gutiérrez J, Sánchez Yus E. Multiple sclerotic fibromas of the skin: a cutaneous marker of Cowden's disease. J Cutan Pathol. 1992;19:346-351.
- Weary PE, Gorlin RJ, Gentry WC Jr, et al. Multiple hamartoma syndrome (Cowden's disease). Arch Dermatol. 1972;106:682-690.
The Diagnosis: Dermatofibroma
Dermatofibroma (DF) is a commonly encountered lesion. Although usually a straightforward clinical diagnosis, histopathological diagnosis is sometimes required. Conventional histologic findings of DF are hyperkeratosis, induction of the epidermis with acanthosis, and basal layer hyperpigmentation.1,2 Within the dermis there usually is proliferation of fibroblasts, histiocytes, and blood vessels that sometimes spares the overlying papillary dermis. Nomenclature of specific variants may be assigned based on the predominant component (eg, nodular subepidermal fibrosis, histiocytoma, sclerosing hemangioma) or histologic findings (eg, fibrocollagenous, sclerotic, cellular, histiocytic, lipidized, angiomatous, aneurysmal, clear cell, monster cell, myxoid, keloidal, palisading, osteoclastic, epithelioid).3-5 Of the histologic variants, fibrocollagenous is most common, but knowledge of other variants is important for accurate diagnosis, especially to exclude malignancy.
The sclerosing hemangioma variant of DF may pre-sent a diagnostic dilemma. In addition to typical features of DF, pseudovascular spaces, abundant hemosiderin, and reactive-appearing spindled cells are histologically demonstrated. The marked sclerosis and pigment deposition may mimic a blue nevus, and the dilated pseudovascular spaces may be reminiscent of a vascular neoplasm such as angiosarcoma or Kaposi sarcoma. However, the presence of characteristic features such as peripheral collagen trapping and overlying epidermal hyperplasia provide important clues for correct diagnosis.
Angiosarcomas (Figure 1) are malignant neoplasms with vascular differentiation. Cutaneous angiosarcomas present as purple plaques or nodules on the head and/or neck in elderly individuals as well as in patients with chronic lymphedema or prior radiation exposure.6-9 They are aggressive neoplasms with high rates of recurrence and metastases. Microscopically, the tumor is composed of anastomosing vascular channels lined by atypical endothelial cells with a multilayered appearance. There is frequent red blood cell extravasation, and substantial hemosiderin deposition may be noted in long-standing lesions. Neoplastic cells are positive for vascular markers (CD34, CD31, ETS-related gene transcription factor). Notably, cases associated with radiation exposure and chronic lymphedema are positive for MYC.10

Blue nevi (Figure 2) are benign melanocytic tumors that occur most frequently in children but may pre-sent in any age group. Clinical presentation is a blue to black, slightly raised papule that may be found on any site of the body. Biopsy typically shows a wedge-shaped infiltrate of spindled melanocytes with elongated dendritic processes in a sclerotic collagenous stroma. There frequently is a striking population of heavily pigmented melanophages. The melanocytes are positive for melanoma antigen recognized by T cells (MART-1)/melan-A, S-100, and transcription factor SOX-10. In contrast to other benign nevi, human melanoma black-45 will be positive in the dermal component.

Dermatofibrosarcoma protuberans (Figure 3) is a dermal-based tumor of intermediate malignant potential with a high rate of local recurrence and potential for sarcomatous transformation. Dermatofibrosarcoma protuberans most commonly presents in young adults as firm, pink to brown plaques and can occur on any site of the body. Histologically, they show a dermal proliferation of spindled cells that infiltrate in a storiform fashion into the subcutaneous adipose tissue,11 which imparts a honeycomb or Swiss cheese pattern. The tumor characteristically demonstrates positive staining for CD34. Loss of CD34 staining, increased mitoses, nuclear atypia, and fascicular growth are features suggestive of sarcomatous transformation.11,12 Dermatofibrosarcoma protuberans is associated with chromosomal abnormalities of chromosomes 17 and 22, resulting in COL1A1 (collagen type 1 alpha 1 chain) and PDGF-β (platelet-derived growth factor subunit B) gene fusion.13

Sclerotic fibromas (also known as storiform collagenomas)(Figure 4) may represent regressed DFs and are frequently associated with prior trauma to the affected area.14,15 They usually appear as flesh-colored papules or nodules on the face and trunk. The presence of multiple sclerotic fibromas is associated with Cowden syndrome.16,17 Histologically, the lesions present as well-demarcated, nonencapsulated, dermal nodules composed of a storiform or whorled arrangement of collagen with spindled fibroblasts. The sclerotic collagen bundles often are separated by small clefts imparting a plywoodlike pattern.16

The differential diagnosis for DF expands once atypical clinical and histopathological findings are present. In this case, the nodule was much larger and darker than the usual appearance of DF (3-10 mm).2,4 Given the lesion's nodularity, the clinical dimple sign on lateral compression could not be seen. On biopsy, the predominance of blood vessels and sclerosis further complicated the diagnostic picture. In unusual cases such as this one, correlation of clinical history, histology, and immunophenotype is ever important.
The Diagnosis: Dermatofibroma
Dermatofibroma (DF) is a commonly encountered lesion. Although usually a straightforward clinical diagnosis, histopathological diagnosis is sometimes required. Conventional histologic findings of DF are hyperkeratosis, induction of the epidermis with acanthosis, and basal layer hyperpigmentation.1,2 Within the dermis there usually is proliferation of fibroblasts, histiocytes, and blood vessels that sometimes spares the overlying papillary dermis. Nomenclature of specific variants may be assigned based on the predominant component (eg, nodular subepidermal fibrosis, histiocytoma, sclerosing hemangioma) or histologic findings (eg, fibrocollagenous, sclerotic, cellular, histiocytic, lipidized, angiomatous, aneurysmal, clear cell, monster cell, myxoid, keloidal, palisading, osteoclastic, epithelioid).3-5 Of the histologic variants, fibrocollagenous is most common, but knowledge of other variants is important for accurate diagnosis, especially to exclude malignancy.
The sclerosing hemangioma variant of DF may pre-sent a diagnostic dilemma. In addition to typical features of DF, pseudovascular spaces, abundant hemosiderin, and reactive-appearing spindled cells are histologically demonstrated. The marked sclerosis and pigment deposition may mimic a blue nevus, and the dilated pseudovascular spaces may be reminiscent of a vascular neoplasm such as angiosarcoma or Kaposi sarcoma. However, the presence of characteristic features such as peripheral collagen trapping and overlying epidermal hyperplasia provide important clues for correct diagnosis.
Angiosarcomas (Figure 1) are malignant neoplasms with vascular differentiation. Cutaneous angiosarcomas present as purple plaques or nodules on the head and/or neck in elderly individuals as well as in patients with chronic lymphedema or prior radiation exposure.6-9 They are aggressive neoplasms with high rates of recurrence and metastases. Microscopically, the tumor is composed of anastomosing vascular channels lined by atypical endothelial cells with a multilayered appearance. There is frequent red blood cell extravasation, and substantial hemosiderin deposition may be noted in long-standing lesions. Neoplastic cells are positive for vascular markers (CD34, CD31, ETS-related gene transcription factor). Notably, cases associated with radiation exposure and chronic lymphedema are positive for MYC.10

Blue nevi (Figure 2) are benign melanocytic tumors that occur most frequently in children but may pre-sent in any age group. Clinical presentation is a blue to black, slightly raised papule that may be found on any site of the body. Biopsy typically shows a wedge-shaped infiltrate of spindled melanocytes with elongated dendritic processes in a sclerotic collagenous stroma. There frequently is a striking population of heavily pigmented melanophages. The melanocytes are positive for melanoma antigen recognized by T cells (MART-1)/melan-A, S-100, and transcription factor SOX-10. In contrast to other benign nevi, human melanoma black-45 will be positive in the dermal component.

Dermatofibrosarcoma protuberans (Figure 3) is a dermal-based tumor of intermediate malignant potential with a high rate of local recurrence and potential for sarcomatous transformation. Dermatofibrosarcoma protuberans most commonly presents in young adults as firm, pink to brown plaques and can occur on any site of the body. Histologically, they show a dermal proliferation of spindled cells that infiltrate in a storiform fashion into the subcutaneous adipose tissue,11 which imparts a honeycomb or Swiss cheese pattern. The tumor characteristically demonstrates positive staining for CD34. Loss of CD34 staining, increased mitoses, nuclear atypia, and fascicular growth are features suggestive of sarcomatous transformation.11,12 Dermatofibrosarcoma protuberans is associated with chromosomal abnormalities of chromosomes 17 and 22, resulting in COL1A1 (collagen type 1 alpha 1 chain) and PDGF-β (platelet-derived growth factor subunit B) gene fusion.13

Sclerotic fibromas (also known as storiform collagenomas)(Figure 4) may represent regressed DFs and are frequently associated with prior trauma to the affected area.14,15 They usually appear as flesh-colored papules or nodules on the face and trunk. The presence of multiple sclerotic fibromas is associated with Cowden syndrome.16,17 Histologically, the lesions present as well-demarcated, nonencapsulated, dermal nodules composed of a storiform or whorled arrangement of collagen with spindled fibroblasts. The sclerotic collagen bundles often are separated by small clefts imparting a plywoodlike pattern.16

The differential diagnosis for DF expands once atypical clinical and histopathological findings are present. In this case, the nodule was much larger and darker than the usual appearance of DF (3-10 mm).2,4 Given the lesion's nodularity, the clinical dimple sign on lateral compression could not be seen. On biopsy, the predominance of blood vessels and sclerosis further complicated the diagnostic picture. In unusual cases such as this one, correlation of clinical history, histology, and immunophenotype is ever important.
- Zeidi M, North JP. Sebaceous induction in dermatofibroma: a common feature of dermatofibromas on the shoulder. J Cutan Pathol. 2015;42:400-405.
- Şenel E, Yuyucu Karabulut Y, Doğruer S¸enel S. Clinical, histopathological, dermatoscopic and digital microscopic features of dermatofibroma: a retrospective analysis of 200 lesions. J Eur Acad Dermatol Venereol. 2015;29:1958-1966.
- Vilanova JR, Flint A. The morphological variations of fibrous histiocytomas. J Cutan Pathol. 1974;1:155-164.
- Han TY, Chang HS, Lee JH, et al. A clinical and histopathological study of 122 cases of dermatofibroma (benign fibrous histiocytoma)[published online May 27, 2011]. Ann Dermatol. 2011;23:185-192.
- Alves JVP, Matos DM, Barreiros HF, et al. Variants of dermatofibroma--a histopathological study. An Bras Dermatol. 2014;89:472-477.
- Rosai J, Sumner HW, Major MC, et al. Angiosarcoma of the skin: a clinicopathologic and fine structural study. Hum Pathol. 1976;7:83-109.
- Haustein UF. Angiosarcoma of the face and scalp. Int J Dermatol. 1991;30:851-856.
- Stewart FW, Treves N. Lymphangiosarcoma in postmastectomy lymphedema: a report of six cases in elephantiasis chirurgica. Cancer. 1948;1:64-81.
- Goette DK, Detlefs RL. Postirradiation angiosarcoma. J Am Acad Dermatol. 1985;12(5 pt 2):922-926.
- Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34-39.
- Voth H, Landsberg J, Hinz T, et al. Management of dermatofibrosarcoma protuberans with fibrosarcomatous transformation: an evidence-based review of the literature. J Eur Acad Dermatol Venereol. 2011;25:1385-1391.
- Goldblum JR. CD34 positivity in fibrosarcomas which arise in dermatofibrosarcoma protuberans. Arch Pathol Lab Med. 1995;119:238-241.
- Patel KU, Szabo SS, Hernandez VS, et al. Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays. Hum Pathol. 2008;39:184-193.
- Sohn IB, Hwang SM, Lee SH, et al. Dermatofibroma with sclerotic areas resembling a sclerotic fibroma of the skin. J Cutan Pathol. 2002;29:44-47.
- Pujol RM, de Castro F, Schroeter AL, et al. Solitary sclerotic fibroma of the skin: a sclerotic dermatofibroma? Am J Dermatopathol. 1996;18:620-624.
- Requena L, Gutiérrez J, Sánchez Yus E. Multiple sclerotic fibromas of the skin: a cutaneous marker of Cowden's disease. J Cutan Pathol. 1992;19:346-351.
- Weary PE, Gorlin RJ, Gentry WC Jr, et al. Multiple hamartoma syndrome (Cowden's disease). Arch Dermatol. 1972;106:682-690.
- Zeidi M, North JP. Sebaceous induction in dermatofibroma: a common feature of dermatofibromas on the shoulder. J Cutan Pathol. 2015;42:400-405.
- Şenel E, Yuyucu Karabulut Y, Doğruer S¸enel S. Clinical, histopathological, dermatoscopic and digital microscopic features of dermatofibroma: a retrospective analysis of 200 lesions. J Eur Acad Dermatol Venereol. 2015;29:1958-1966.
- Vilanova JR, Flint A. The morphological variations of fibrous histiocytomas. J Cutan Pathol. 1974;1:155-164.
- Han TY, Chang HS, Lee JH, et al. A clinical and histopathological study of 122 cases of dermatofibroma (benign fibrous histiocytoma)[published online May 27, 2011]. Ann Dermatol. 2011;23:185-192.
- Alves JVP, Matos DM, Barreiros HF, et al. Variants of dermatofibroma--a histopathological study. An Bras Dermatol. 2014;89:472-477.
- Rosai J, Sumner HW, Major MC, et al. Angiosarcoma of the skin: a clinicopathologic and fine structural study. Hum Pathol. 1976;7:83-109.
- Haustein UF. Angiosarcoma of the face and scalp. Int J Dermatol. 1991;30:851-856.
- Stewart FW, Treves N. Lymphangiosarcoma in postmastectomy lymphedema: a report of six cases in elephantiasis chirurgica. Cancer. 1948;1:64-81.
- Goette DK, Detlefs RL. Postirradiation angiosarcoma. J Am Acad Dermatol. 1985;12(5 pt 2):922-926.
- Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34-39.
- Voth H, Landsberg J, Hinz T, et al. Management of dermatofibrosarcoma protuberans with fibrosarcomatous transformation: an evidence-based review of the literature. J Eur Acad Dermatol Venereol. 2011;25:1385-1391.
- Goldblum JR. CD34 positivity in fibrosarcomas which arise in dermatofibrosarcoma protuberans. Arch Pathol Lab Med. 1995;119:238-241.
- Patel KU, Szabo SS, Hernandez VS, et al. Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays. Hum Pathol. 2008;39:184-193.
- Sohn IB, Hwang SM, Lee SH, et al. Dermatofibroma with sclerotic areas resembling a sclerotic fibroma of the skin. J Cutan Pathol. 2002;29:44-47.
- Pujol RM, de Castro F, Schroeter AL, et al. Solitary sclerotic fibroma of the skin: a sclerotic dermatofibroma? Am J Dermatopathol. 1996;18:620-624.
- Requena L, Gutiérrez J, Sánchez Yus E. Multiple sclerotic fibromas of the skin: a cutaneous marker of Cowden's disease. J Cutan Pathol. 1992;19:346-351.
- Weary PE, Gorlin RJ, Gentry WC Jr, et al. Multiple hamartoma syndrome (Cowden's disease). Arch Dermatol. 1972;106:682-690.

A 61-year-old woman presented with a 2.5-cm hyperpigmented exophytic nodule on the anterior aspect of the left shin of approximately 2 years' duration. The patient initially noticed a small lesion following a bee sting, but it subsequently grew over the ensuing 2 years. A shave biopsy was obtained.