User login
Tips for managing 4 common soft-tissue finger and thumb injuries
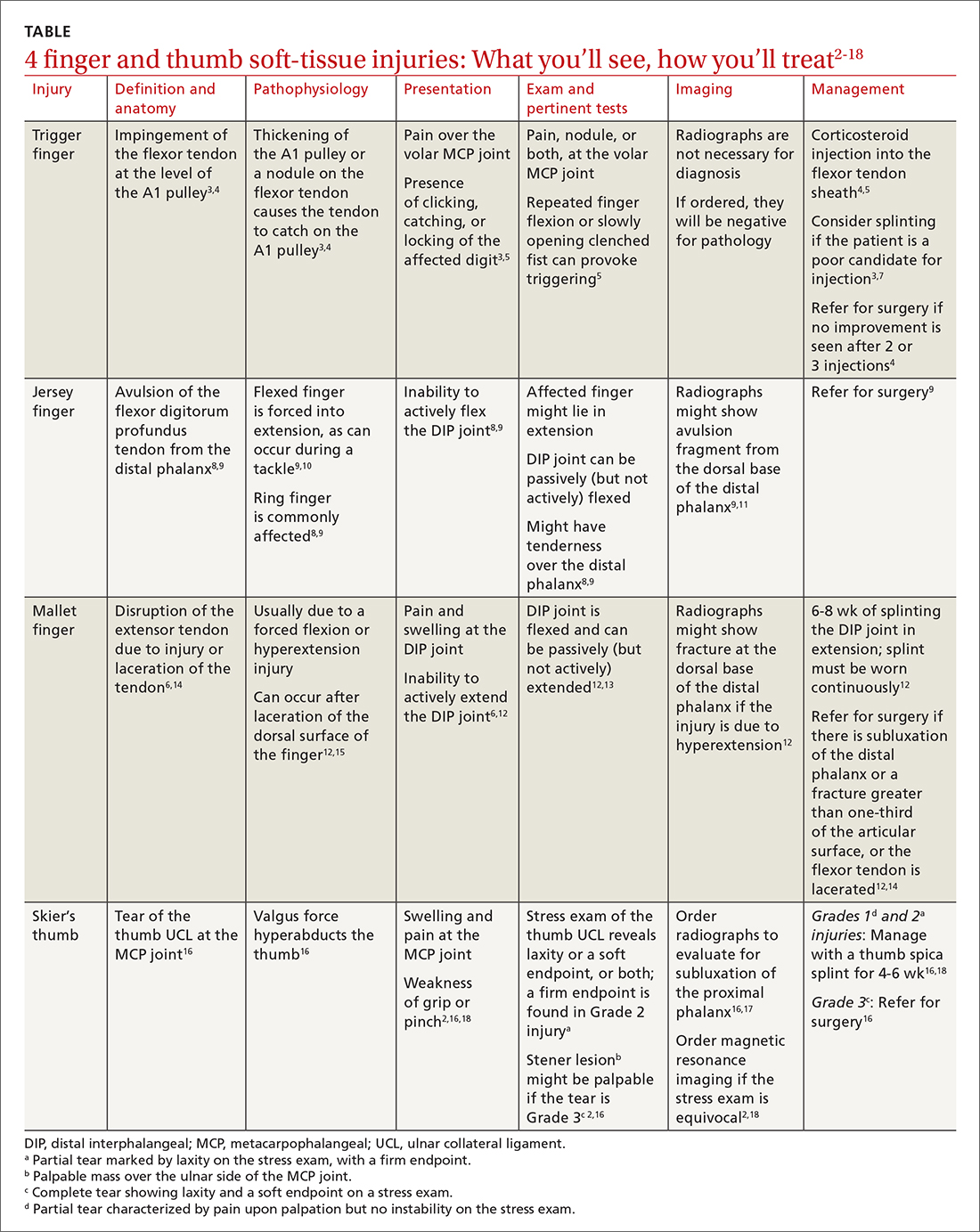
Finger injuries are often seen in the primary care physician’s office. The evidence—and our experience in sports medicine—indicates that many of these injuries can be managed conservatively with bracing or injection; a subset, however, requires surgical referral. In this article, we provide a refresher on finger anatomy (see “A guide to the anatomic structures of the digits of the hand”1,2) and review the diagnosis and management of 4 common soft-tissue finger and thumb injuries in adults: trigger finger, jersey finger, mallet finger, and skier’s thumb (TABLE2-18).
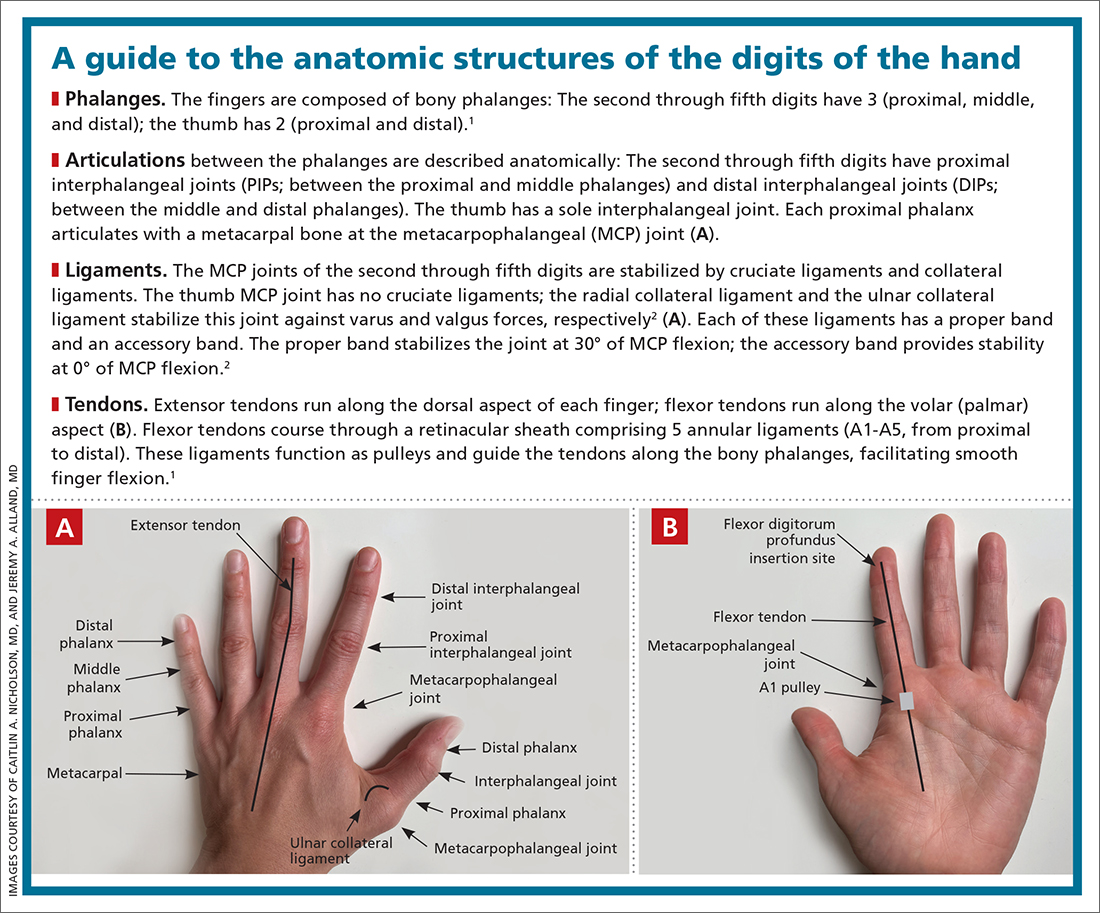
Trigger finger
Also called stenosing flexor tenosynovitis, trigger finger is caused by abnormal flexor tendon movement that results from impingement at the level of the A1 pulley.
Causes and incidence. Impingement usually occurs because of thickening of the A1 pulley but can also be caused by inflammation or a nodule on the flexor tendon.3,4 The A1 pulley at the metacarpal head is the most proximal part of the retinacular sheath and therefore experiences the greatest force upon finger flexion, making it the most common site of inflammation and constriction.4

Trigger finger occurs in 2% to 3% of the general population and in as many as 10% of people with diabetes.5 The condition typically affects the long and ring fingers of the dominant hand; most cases occur in women in the sixth and seventh decades.3-5
Multiple systemic conditions predispose to trigger finger, including endocrine disorders (eg, diabetes, hypothyroidism), inflammatory arthropathies (gout, pseudogout), and autoimmune disorders (rheumatoid arthritis, sarcoidosis).3,5 Diabetes commonly causes bilateral hand and multiple digit involvement, as well as more severe disease.3,5 Occupation is also a risk factor for trigger finger because repetitive movements and manual work can exacerbate triggering.4
Presentation and exam. Patients report pain at the metacarpal head or metacarpophalangeal (MCP) joint, difficulty grasping objects, and, possibly, clicking and catching of the digit and locking of the digit in flexion.3,5
On exam, there might be tenderness at the level of the A1 pulley over the volar MCP joint or a palpable nodule. In severe cases, the proximal interphalangeal (PIP) joint or entire finger can be fixed in flexion.5 Repeated compound finger flexion (eg, closing and opening a fist) or holding a fist for as long as 1 minute and then slowly opening it might provoke triggering.
More than 60% of patients with trigger finger also have carpal tunnel syndrome.5 This makes it important to assess for (1) sensory changes in the distribution of the median nerve and (2) nerve compression, by eliciting Phalen and Tinel signs.4,5
Continue to: Imaging
Imaging. Trigger finger is a clinical diagnosis. Imaging is therefore unnecessary for diagnosis or treatment.5
Treatment. Trigger finger resolves spontaneously in 52% of cases.3 Most patients experience relief in 8 to 12 months.3
First-line treatment is injection of a corticosteroid into the flexor tendon sheath, which often alleviates symptoms.4,5 Injection is performed at the level of the A1 pulley on the palmar surface, just proximal to the MCP joint at the level of the distal palmar crease6 (FIGURE 1). The needle is inserted at an oblique angle until there is an increase in resistance. The needle is then slightly withdrawn to reposition it in the tendon sheath; 0.5 to 1 mL of 50% corticosteroid and 50% local anesthetic without epinephrine is then injected.6
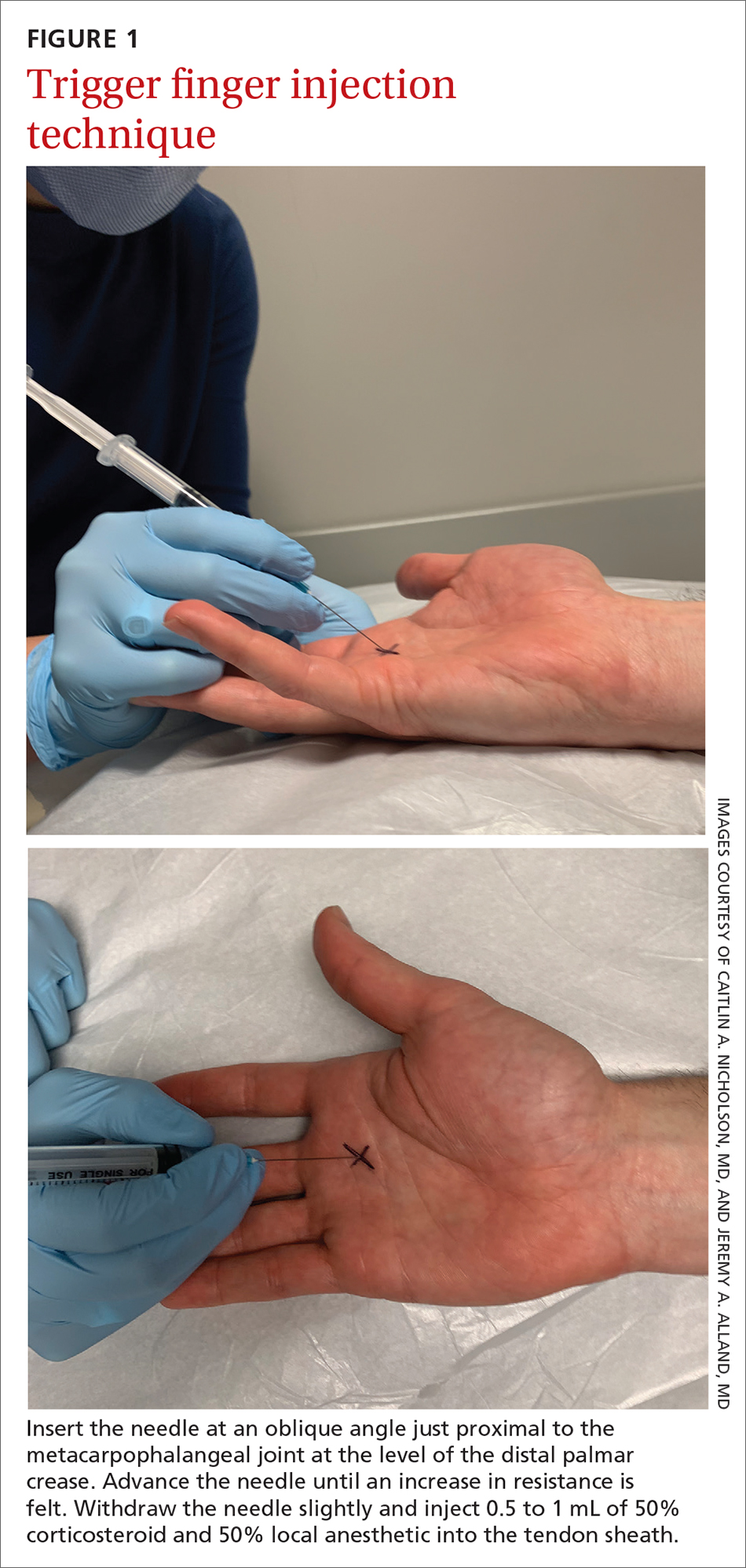
The cure rate of trigger finger is 57% to 70% with 1 injection and 82% to 86% after 2 injections.3,4,19
Many patients experience symptom relief in 1 to 4 weeks after a corticosteroid injection; however, as many as 56% experience repeat triggering within 6 months—often making multiple injections (maximum, 3 per digit) necessary.19,20 Patients who have a longer duration of symptoms, more severe symptoms, and multiple trigger fingers are less likely to experience relief with injections.3,5
Continue to: Splinting is an effective treatment...
Splinting is an effective treatment for patients who cannot undergo corticosteroid injection or surgery. The MCP or PIP joint is immobilized in extension while movement of the distal interphalangeal (DIP) joint is maintained. Instruct the patient that the splint must be worn day and night; splinting is continued for ≥ 6 weeks.21 Splinting relieves symptoms in 47% to 70% of cases and is most effective in patients whose symptoms have been present for < 6 months.3,7
Patients whose trigger finger is locked in flexion and those who have not experienced improvement after 2 or 3 corticosteroid injections should be referred for surgery.4 The surgical cure rate is nearly 100%; only 6% of patients experience repeat triggering 6 to 12 months postoperatively.4,7,22
Jersey finger
Causes and incidence. Jersey finger is caused by avulsion injury to the flexor digitorum profundus (FDP) tendon at its insertion on the distal phalanx.8,9 It occurs when a flexed finger is forced into extension, such as when a football or rugby player grabs another player’s jersey during a tackle.9,10 This action causes the FDP tendon to detach from the distal phalanx, sometimes with a bony fragment.9,11 Once detached, the tendon might retract proximally within the finger or to the palm, with consequent loss of its blood supply.9
Although jersey finger is not as common as the other conditions discussed in this article,9 it is important not to miss this diagnosis because of the risk of chronic disability when it is not treated promptly. Seventy-five percent of cases occur in the ring finger, which is more susceptible to injury because it extends past the other digits in a power grip.8,9
Presentation and exam. On exam, the affected finger lies in slight extension compared to the other digits; the patient is unable to actively flex the DIP joint.8,9 There may be tenderness to palpation over the volar distal phalanx. The retracted FDP tendon might be palpable more proximally in the digit.
Continue to: Imaging
Imaging. Anteroposterior (AP), oblique, and lateral radiographs, although unnecessary for diagnosis, are recommended to assess for an avulsion fragment, associated fracture, or dislocation.9,11 Ultrasonography or magnetic resonance imaging is useful in chronic cases to quantify the degree of tendon retraction.9
Treatment. Refer acute cases of jersey finger for surgical management urgently because most cases require flexor tendon repair within 1 or 2 weeks for a successful outcome.9 Chronic jersey finger, in which injury occurred > 6 weeks before presentation, also requires surgical repair, although not as urgently.9
Complications of jersey finger include flexion contracture at the DIP joint and the so-called quadriga effect, in which the patient is unable to fully flex the fingers adjacent to the injured digit.8 These complications can cause chronic disability in the affected hand, making early diagnosis and referral key to successful treatment.9
Mallet finger
Also called drop finger, mallet finger is a result of loss of active extension at the DIP joint.12,13
Causes and incidence. Mallet finger is a relatively common injury that typically affects the long, ring, or small finger of the dominant hand in young to middle-aged men and older women.12,14,23 The condition is the result of forced flexion or hyperextension injury, which disrupts the extensor tendon.6,14
Continue to: Sudden forced flexion...
Sudden forced flexion of an extended DIP joint during work or sports (eg, catching a ball) is the most common mechanism of injury.12,15 This action causes stretching or tearing of the extensor tendon as well as a possible avulsion fracture of the distal phalanx.13 Mallet finger can also result from a laceration or crush injury of the extensor tendon (open mallet finger) or hyperextension of the DIP joint, causing a fracture at the dorsal base of the distal phalanx.12
Presentation. Through any of the aforementioned mechanisms, the delicate balance between the flexor and extensor tendons is disrupted, causing the patient to present with a flexed DIP joint that can be passively, but not actively, extended.6,12 The DIP joint might also be painful and swollen. Patients whose injury occurred > 4 weeks prior to presentation (chronic mallet finger) might also have a so-called swan-neck deformity, with hyperextension of the PIP joint in the affected finger.12
Imaging. AP, oblique, and lateral radiographs are recommended to assess for bony injury.
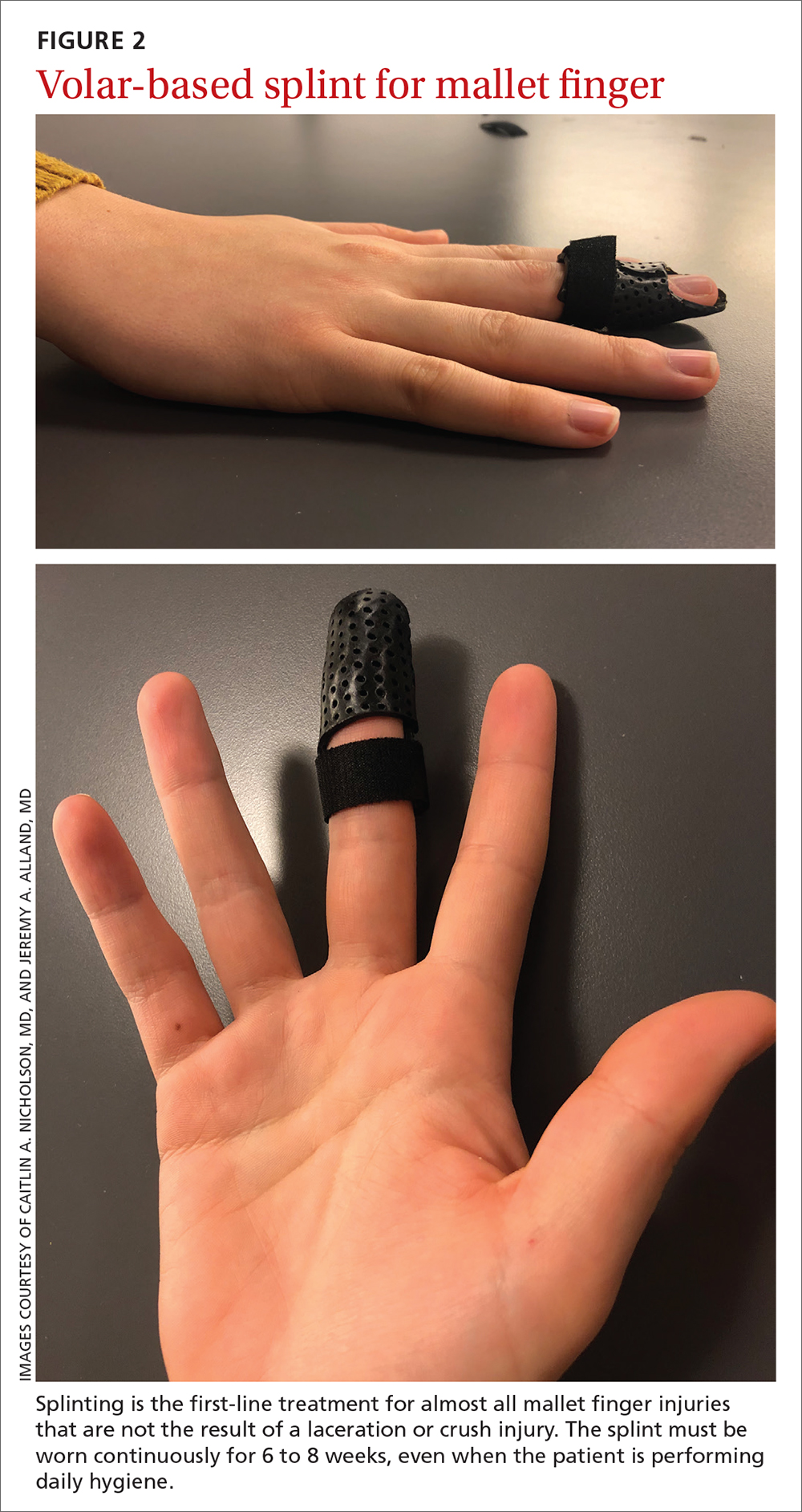
Treatment. Splinting is the first-line treatment for almost all mallet finger injuries that are not the result of a laceration or crush injury. Immobilize the DIP joint in extension for 6 to 8 weeks, with an additional 2 to 4 weeks of splinting at night.6,12 The splint must be worn continuously in the initial 6 to 8 weeks, and the DIP joint should remain in extension—even when the patient is performing daily hygiene.12 It is imperative that patients comply with that period of continuous immobilization; if the DIP joint is allowed to flex, the course of treatment must be restarted.13
Many different types of splints exist; functional outcomes are equivalent across all of them.24,25 In our practice, we manage mallet finger with a volar-based splint (FIGURE 2), which is associated with fewer dermatologic complications and has provided the most success for our patients.23
Continue to: Surgical repair of mallet finger injury...
Surgical repair of mallet finger injury is indicated in any of these situations12,14:
- injury is caused by laceration
- there is volar subluxation of the DIP joint
- more than one-third of the articular surface is involved in an avulsion fracture.
Patients who cannot comply with wearing a splint 24 hours per day or whose occupation precludes wearing a splint at all (eg, surgeons, dentists, musicians) are also surgical candidates.12
Surgical and conservative treatments have similar clinical and functional outcomes, including loss of approximately 5° to 7° of active extension and an increased risk of DIP joint osteoarthritis.12,14,24 Patients with chronic mallet finger can be managed with 6 weeks of splinting initially but will likely require surgery.6,12,13
Skier’s thumb
This relatively common injury is a tear of the ulnar collateral ligament (UCL) at the MCP joint of the thumb.16
Causes and incidence. Skier’s thumb occurs when a valgus force hyperabducts the thumb,16 and is so named because the injury is often seen in recreational skiers who fall while holding a ski pole.15-17 It can also occur in racquet sports when a ball or racquet strikes the ulnar side of thumb.16
Continue to: In chronic cases...
In chronic cases, the UCL can be injured by occupational demands and is termed gamekeeper’s thumb because it was first described in this population, who killed game by breaking the animal's neck between the thumb and index finger against the ground.16,18 A UCL tear causes instability at the thumb MCP joint, which affects a person’s ability to grip and pinch.2,16,18
Presentation. On exam, the affected thumb is swollen and, possibly, bruised. There might be radial deviation and volar subluxation of the proximal phalanx. The ulnar side of the MCP joint is tender to palpation.16 If the distal UCL is torn completely, it can displace proximally and present as a palpable mass over the ulnar side of the MCP joint, known as a Stener lesion.16
Stress testing of the MCP joint is the most important part of the physical exam for skier’s thumb. Stabilize the metacarpal neck and apply a valgus stress on the proximal phalanx at both 0° and 30° of MCP flexion (FIGURE 3), which allows for assessment of both the proper and accessory bands of the UCL.2,16 (A common pitfall during stress testing is to allow the MCP joint to rotate, which can mimic instability.2) Intra-articular local anesthesia might be necessary for this exam because it can be painful.16,18,26 A stress exam should assess for laxity and a soft or firm endpoint; the result should be compared to that of a stress exam on the contralateral side.16,17
Imaging. AP, oblique, and lateral radiographs of the thumb should be obtained to assess for instability, avulsion injury, and associated fracture. Subluxation (volar or radial) or supination of the proximal phalanx relative to the metacarpal on imaging suggests MCP instability of the MCP joint.16,17
If the stress exam is equivocal, magnetic resonance imaging is recommended for further assessment.2,18
Continue to: Stress radiographs...
Stress radiographs (ie, radiographs of the thumb with valgus stress applied at the MCP joint) can aid in diagnosis but are controversial. Some experts think that these stress views can further damage the UCL; others recommend against them because they carry a false-negative rate ≥ 25%.15,16 If you choose to perform stress views, order standard radiographs beforehand to rule out bony injury.17
Treatment. UCL tears are classified as 3 tiers to guide treatment.
- Grade 1 injury (a partial tear) is characterized by pain upon palpation but no instability on the stress exam.
- Grade 2 injury (also a partial tear) is marked by laxity on the stress exam with a firm endpoint.
- Grade 3 injury (complete tear) shows laxity and a soft endpoint on a stress exam16,17; Stener lesions are seen only in grade 3 tears.16,17
Grades 1 and 2 UCL tears without fracture or with a nondisplaced avulsion fracture can be managed nonoperatively by immobilizing the thumb in a spica splint or cast for 4 to 6 weeks.16,18 The MCP joint is immobilized and the interphalangeal joint is allowed to move freely.2,16,17
Grade 3 injuries should be referred to a hand specialist for surgical repair.16 Patients presenting > 12 weeks after acute injury or with a chronic UCL tear should also be referred for surgical repair.16
CORRESPONDENCE
Caitlin A. Nicholson, MD, 1611 West Harrison Street, Suite 300, Chicago, IL 60612; [email protected]
1. Hirt B, Seyhan H, Wagner M, et al. Hand and Wrist Anatomy and Biomechanics: A Comprehensive Guide. Thieme; 2017:57,58,71,72,75-80.
2. Daley D, Geary M, Gaston RG. Thumb metacarpophalangeal ulnar and radial collateral ligament injuries. Clin Sports Med. 2020;39:443-455. doi: 10.1016/j.csm.2019.12.003
3. Gil JA, Hresko AM, Weiss AC. Current concepts in the management of trigger finger in adults. J Am Acad Orthop Surg. 2020;28:e642-e650. doi: 10.5435/JAAOS-D-19-00614
4. Henton J, Jain A, Medhurst C, et al. Adult trigger finger. BMJ. 2012;345:e5743. doi: 10.1136/bmj.e5743
5. Bates T, Dunn J. Trigger finger. Orthobullets [Internet]. Updated December 8, 2021. Accessed April 14, 2022. www.orthobullets.com/hand/6027/trigger-finger
6. Chhabra AB, Deal ND. Soft tissue injuries of the wrist and hand. In: O’Connor FG, Casa DJ, Davis BA, et al. ACSM’s Sports Medicine: A Comprehensive Review. Lippincott Williams & Wilkins; 2012:370-373.
7. Ballard TNS, Kozlow JH. Trigger finger in adults. CMAJ. 2016;188:61. doi: 10.1503/cmaj.150225
8. Vitale M. Jersey finger. Orthobullets [Internet]. Updated May 22, 2021. 2019. Accessed April 15, 2022. www.orthobullets.com/hand/6015/jersey-finger
9. Shapiro LM, Kamal RN. Evaluation and treatment of flexor tendon and pulley injuries in athletes. Clin Sports Med. 2020;39:279-297. doi: 10.1016/j.csm.2019.12.004
10. Goodson A, Morgan M, Rajeswaran G, et al. Current management of Jersey finger in rugby players: case series and literature review. Hand Surg. 2010;15:103-107. doi: 10.1142/S0218810410004710
11. Lapegue F, Andre A, Brun C, et al. Traumatic flexor tendon injuries. Diagn Interv Imaging. 2015;96:1279-1292. doi: 10.1016/j.diii.2015.09.010
12. Bendre AA, Hartigan BJ, Kalainov DM. Mallet finger. J Am Acad Orthop Surg. 2005;13:336-344. doi: 10.5435/00124635-200509000-00007
13. Lamaris GA, Matthew MK. The diagnosis and management of mallet finger injuries. Hand (N Y). 2017;12:223-228. doi: 10.1177/1558944716642763
14. Sheth U. Mallet finger. Orthobullets [Internet]. Updated August 5, 2021. Accessed April 15, 2022. www.orthobullets.com/hand/6014/mallet-finger
15. Weintraub MD, Hansford BG, Stilwill SE, et al. Avulsion injuries of the hand and wrist. Radiographics. 2020;40:163-180. doi: 10.1148/rg.2020190085
16. Avery III DM, Inkellis ER, Carlson MG. Thumb collateral ligament injuries in the athlete. Curr Rev Musculoskelet Med. 2017;10:28-37. doi: 10.1007/s12178-017-9381-z
17. Steffes MJ. Thumb collateral ligament injury. Orthobullets [Internet]. Updated February 18, 2022. Accessed April 15, 2022. www.orthobullets.com/hand/6040/thumb-collateral-ligament-injury
18. Madan SS, Pai DR, Kaur A, et al. Injury to ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7. doi: 10.1111/os.12084
19. Dardas AZ, VandenBerg J, Shen T, et al. Long-term effectiveness of repeat corticosteroid injections for trigger finger. J Hand Surg Am. 2017;42:227-235. doi: 10.1016/j.jhsa.2017.02.001
20. Huisstede BM, Gladdines S, Randsdorp MS, et al. Effectiveness of conservative, surgical, and postsurgical interventions for trigger finger, Dupuytren disease, and de Quervain disease: a systematic review. Arch Phys Med Rehabil. 2018;99:1635-1649.e21. doi: 10.1016/j.apmr.2017.07.014
21. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
22. Fiorini HJ, Tamaoki MJ, Lenza M, et al. Surgery for trigger finger. Cochrane Database Syst Rev. 2018;2:CD009860. doi: 10.1002/14651858.CD009860.pub2
23. Salazar Botero S, Hidalgo Diaz JJ, Benaïda A, et al. Review of acute traumatic closed mallet finger injuries in adults. Arch Plast Surg. 2016;43:134-144. doi: 10.5999/aps.2016.43.2.134
24. Lin JS, Samora JB. Surgical and nonsurgical management of mallet finger: a systematic review. J Hand Surg Am. 2018;43:146-163.e2. doi: 10.1016/j.jhsa.2017.10.004
25. Handoll H, Vaghela MV. Interventions for treating mallet finger injuries. Cochrane Database Syst Rev. 2004;(3):CD004574. doi: 10.1002/14651858.CD004574.pub2
26. Pulos N, Shin AY. Treatment of ulnar collateral ligament injuries of the thumb: a critical analysis review. JBJS Rev. 2017;5:e3. doi: 10.2106/JBJS.RVW.16.00051
Finger injuries are often seen in the primary care physician’s office. The evidence—and our experience in sports medicine—indicates that many of these injuries can be managed conservatively with bracing or injection; a subset, however, requires surgical referral. In this article, we provide a refresher on finger anatomy (see “A guide to the anatomic structures of the digits of the hand”1,2) and review the diagnosis and management of 4 common soft-tissue finger and thumb injuries in adults: trigger finger, jersey finger, mallet finger, and skier’s thumb (TABLE2-18).


Trigger finger
Also called stenosing flexor tenosynovitis, trigger finger is caused by abnormal flexor tendon movement that results from impingement at the level of the A1 pulley.
Causes and incidence. Impingement usually occurs because of thickening of the A1 pulley but can also be caused by inflammation or a nodule on the flexor tendon.3,4 The A1 pulley at the metacarpal head is the most proximal part of the retinacular sheath and therefore experiences the greatest force upon finger flexion, making it the most common site of inflammation and constriction.4

Trigger finger occurs in 2% to 3% of the general population and in as many as 10% of people with diabetes.5 The condition typically affects the long and ring fingers of the dominant hand; most cases occur in women in the sixth and seventh decades.3-5
Multiple systemic conditions predispose to trigger finger, including endocrine disorders (eg, diabetes, hypothyroidism), inflammatory arthropathies (gout, pseudogout), and autoimmune disorders (rheumatoid arthritis, sarcoidosis).3,5 Diabetes commonly causes bilateral hand and multiple digit involvement, as well as more severe disease.3,5 Occupation is also a risk factor for trigger finger because repetitive movements and manual work can exacerbate triggering.4
Presentation and exam. Patients report pain at the metacarpal head or metacarpophalangeal (MCP) joint, difficulty grasping objects, and, possibly, clicking and catching of the digit and locking of the digit in flexion.3,5
On exam, there might be tenderness at the level of the A1 pulley over the volar MCP joint or a palpable nodule. In severe cases, the proximal interphalangeal (PIP) joint or entire finger can be fixed in flexion.5 Repeated compound finger flexion (eg, closing and opening a fist) or holding a fist for as long as 1 minute and then slowly opening it might provoke triggering.
More than 60% of patients with trigger finger also have carpal tunnel syndrome.5 This makes it important to assess for (1) sensory changes in the distribution of the median nerve and (2) nerve compression, by eliciting Phalen and Tinel signs.4,5
Continue to: Imaging
Imaging. Trigger finger is a clinical diagnosis. Imaging is therefore unnecessary for diagnosis or treatment.5
Treatment. Trigger finger resolves spontaneously in 52% of cases.3 Most patients experience relief in 8 to 12 months.3
First-line treatment is injection of a corticosteroid into the flexor tendon sheath, which often alleviates symptoms.4,5 Injection is performed at the level of the A1 pulley on the palmar surface, just proximal to the MCP joint at the level of the distal palmar crease6 (FIGURE 1). The needle is inserted at an oblique angle until there is an increase in resistance. The needle is then slightly withdrawn to reposition it in the tendon sheath; 0.5 to 1 mL of 50% corticosteroid and 50% local anesthetic without epinephrine is then injected.6

The cure rate of trigger finger is 57% to 70% with 1 injection and 82% to 86% after 2 injections.3,4,19
Many patients experience symptom relief in 1 to 4 weeks after a corticosteroid injection; however, as many as 56% experience repeat triggering within 6 months—often making multiple injections (maximum, 3 per digit) necessary.19,20 Patients who have a longer duration of symptoms, more severe symptoms, and multiple trigger fingers are less likely to experience relief with injections.3,5
Continue to: Splinting is an effective treatment...
Splinting is an effective treatment for patients who cannot undergo corticosteroid injection or surgery. The MCP or PIP joint is immobilized in extension while movement of the distal interphalangeal (DIP) joint is maintained. Instruct the patient that the splint must be worn day and night; splinting is continued for ≥ 6 weeks.21 Splinting relieves symptoms in 47% to 70% of cases and is most effective in patients whose symptoms have been present for < 6 months.3,7
Patients whose trigger finger is locked in flexion and those who have not experienced improvement after 2 or 3 corticosteroid injections should be referred for surgery.4 The surgical cure rate is nearly 100%; only 6% of patients experience repeat triggering 6 to 12 months postoperatively.4,7,22
Jersey finger
Causes and incidence. Jersey finger is caused by avulsion injury to the flexor digitorum profundus (FDP) tendon at its insertion on the distal phalanx.8,9 It occurs when a flexed finger is forced into extension, such as when a football or rugby player grabs another player’s jersey during a tackle.9,10 This action causes the FDP tendon to detach from the distal phalanx, sometimes with a bony fragment.9,11 Once detached, the tendon might retract proximally within the finger or to the palm, with consequent loss of its blood supply.9
Although jersey finger is not as common as the other conditions discussed in this article,9 it is important not to miss this diagnosis because of the risk of chronic disability when it is not treated promptly. Seventy-five percent of cases occur in the ring finger, which is more susceptible to injury because it extends past the other digits in a power grip.8,9
Presentation and exam. On exam, the affected finger lies in slight extension compared to the other digits; the patient is unable to actively flex the DIP joint.8,9 There may be tenderness to palpation over the volar distal phalanx. The retracted FDP tendon might be palpable more proximally in the digit.
Continue to: Imaging
Imaging. Anteroposterior (AP), oblique, and lateral radiographs, although unnecessary for diagnosis, are recommended to assess for an avulsion fragment, associated fracture, or dislocation.9,11 Ultrasonography or magnetic resonance imaging is useful in chronic cases to quantify the degree of tendon retraction.9
Treatment. Refer acute cases of jersey finger for surgical management urgently because most cases require flexor tendon repair within 1 or 2 weeks for a successful outcome.9 Chronic jersey finger, in which injury occurred > 6 weeks before presentation, also requires surgical repair, although not as urgently.9
Complications of jersey finger include flexion contracture at the DIP joint and the so-called quadriga effect, in which the patient is unable to fully flex the fingers adjacent to the injured digit.8 These complications can cause chronic disability in the affected hand, making early diagnosis and referral key to successful treatment.9
Mallet finger
Also called drop finger, mallet finger is a result of loss of active extension at the DIP joint.12,13
Causes and incidence. Mallet finger is a relatively common injury that typically affects the long, ring, or small finger of the dominant hand in young to middle-aged men and older women.12,14,23 The condition is the result of forced flexion or hyperextension injury, which disrupts the extensor tendon.6,14
Continue to: Sudden forced flexion...
Sudden forced flexion of an extended DIP joint during work or sports (eg, catching a ball) is the most common mechanism of injury.12,15 This action causes stretching or tearing of the extensor tendon as well as a possible avulsion fracture of the distal phalanx.13 Mallet finger can also result from a laceration or crush injury of the extensor tendon (open mallet finger) or hyperextension of the DIP joint, causing a fracture at the dorsal base of the distal phalanx.12
Presentation. Through any of the aforementioned mechanisms, the delicate balance between the flexor and extensor tendons is disrupted, causing the patient to present with a flexed DIP joint that can be passively, but not actively, extended.6,12 The DIP joint might also be painful and swollen. Patients whose injury occurred > 4 weeks prior to presentation (chronic mallet finger) might also have a so-called swan-neck deformity, with hyperextension of the PIP joint in the affected finger.12
Imaging. AP, oblique, and lateral radiographs are recommended to assess for bony injury.
Treatment. Splinting is the first-line treatment for almost all mallet finger injuries that are not the result of a laceration or crush injury. Immobilize the DIP joint in extension for 6 to 8 weeks, with an additional 2 to 4 weeks of splinting at night.6,12 The splint must be worn continuously in the initial 6 to 8 weeks, and the DIP joint should remain in extension—even when the patient is performing daily hygiene.12 It is imperative that patients comply with that period of continuous immobilization; if the DIP joint is allowed to flex, the course of treatment must be restarted.13
Many different types of splints exist; functional outcomes are equivalent across all of them.24,25 In our practice, we manage mallet finger with a volar-based splint (FIGURE 2), which is associated with fewer dermatologic complications and has provided the most success for our patients.23

Continue to: Surgical repair of mallet finger injury...
Surgical repair of mallet finger injury is indicated in any of these situations12,14:
- injury is caused by laceration
- there is volar subluxation of the DIP joint
- more than one-third of the articular surface is involved in an avulsion fracture.
Patients who cannot comply with wearing a splint 24 hours per day or whose occupation precludes wearing a splint at all (eg, surgeons, dentists, musicians) are also surgical candidates.12
Surgical and conservative treatments have similar clinical and functional outcomes, including loss of approximately 5° to 7° of active extension and an increased risk of DIP joint osteoarthritis.12,14,24 Patients with chronic mallet finger can be managed with 6 weeks of splinting initially but will likely require surgery.6,12,13
Skier’s thumb
This relatively common injury is a tear of the ulnar collateral ligament (UCL) at the MCP joint of the thumb.16
Causes and incidence. Skier’s thumb occurs when a valgus force hyperabducts the thumb,16 and is so named because the injury is often seen in recreational skiers who fall while holding a ski pole.15-17 It can also occur in racquet sports when a ball or racquet strikes the ulnar side of thumb.16
Continue to: In chronic cases...
In chronic cases, the UCL can be injured by occupational demands and is termed gamekeeper’s thumb because it was first described in this population, who killed game by breaking the animal's neck between the thumb and index finger against the ground.16,18 A UCL tear causes instability at the thumb MCP joint, which affects a person’s ability to grip and pinch.2,16,18
Presentation. On exam, the affected thumb is swollen and, possibly, bruised. There might be radial deviation and volar subluxation of the proximal phalanx. The ulnar side of the MCP joint is tender to palpation.16 If the distal UCL is torn completely, it can displace proximally and present as a palpable mass over the ulnar side of the MCP joint, known as a Stener lesion.16
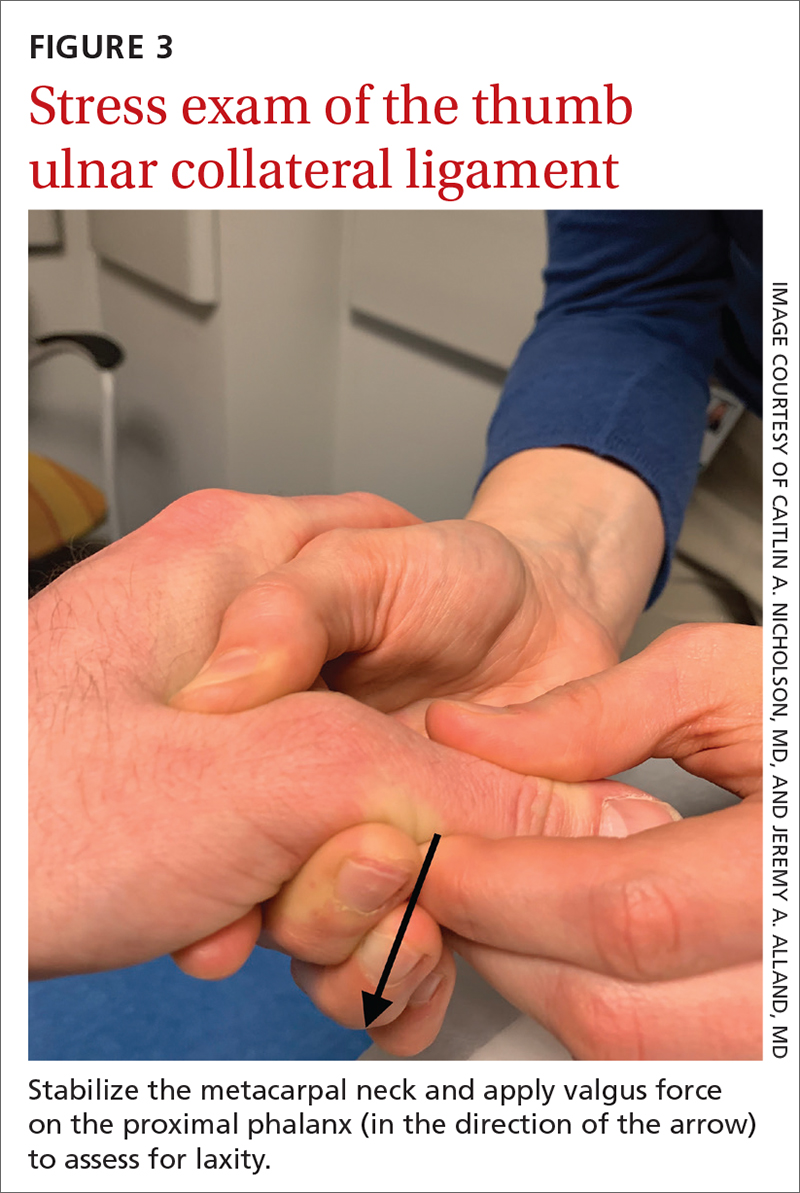
Stress testing of the MCP joint is the most important part of the physical exam for skier’s thumb. Stabilize the metacarpal neck and apply a valgus stress on the proximal phalanx at both 0° and 30° of MCP flexion (FIGURE 3), which allows for assessment of both the proper and accessory bands of the UCL.2,16 (A common pitfall during stress testing is to allow the MCP joint to rotate, which can mimic instability.2) Intra-articular local anesthesia might be necessary for this exam because it can be painful.16,18,26 A stress exam should assess for laxity and a soft or firm endpoint; the result should be compared to that of a stress exam on the contralateral side.16,17

Imaging. AP, oblique, and lateral radiographs of the thumb should be obtained to assess for instability, avulsion injury, and associated fracture. Subluxation (volar or radial) or supination of the proximal phalanx relative to the metacarpal on imaging suggests MCP instability of the MCP joint.16,17
If the stress exam is equivocal, magnetic resonance imaging is recommended for further assessment.2,18
Continue to: Stress radiographs...
Stress radiographs (ie, radiographs of the thumb with valgus stress applied at the MCP joint) can aid in diagnosis but are controversial. Some experts think that these stress views can further damage the UCL; others recommend against them because they carry a false-negative rate ≥ 25%.15,16 If you choose to perform stress views, order standard radiographs beforehand to rule out bony injury.17
Treatment. UCL tears are classified as 3 tiers to guide treatment.
- Grade 1 injury (a partial tear) is characterized by pain upon palpation but no instability on the stress exam.
- Grade 2 injury (also a partial tear) is marked by laxity on the stress exam with a firm endpoint.
- Grade 3 injury (complete tear) shows laxity and a soft endpoint on a stress exam16,17; Stener lesions are seen only in grade 3 tears.16,17
Grades 1 and 2 UCL tears without fracture or with a nondisplaced avulsion fracture can be managed nonoperatively by immobilizing the thumb in a spica splint or cast for 4 to 6 weeks.16,18 The MCP joint is immobilized and the interphalangeal joint is allowed to move freely.2,16,17
Grade 3 injuries should be referred to a hand specialist for surgical repair.16 Patients presenting > 12 weeks after acute injury or with a chronic UCL tear should also be referred for surgical repair.16
CORRESPONDENCE
Caitlin A. Nicholson, MD, 1611 West Harrison Street, Suite 300, Chicago, IL 60612; [email protected]
Finger injuries are often seen in the primary care physician’s office. The evidence—and our experience in sports medicine—indicates that many of these injuries can be managed conservatively with bracing or injection; a subset, however, requires surgical referral. In this article, we provide a refresher on finger anatomy (see “A guide to the anatomic structures of the digits of the hand”1,2) and review the diagnosis and management of 4 common soft-tissue finger and thumb injuries in adults: trigger finger, jersey finger, mallet finger, and skier’s thumb (TABLE2-18).


Trigger finger
Also called stenosing flexor tenosynovitis, trigger finger is caused by abnormal flexor tendon movement that results from impingement at the level of the A1 pulley.
Causes and incidence. Impingement usually occurs because of thickening of the A1 pulley but can also be caused by inflammation or a nodule on the flexor tendon.3,4 The A1 pulley at the metacarpal head is the most proximal part of the retinacular sheath and therefore experiences the greatest force upon finger flexion, making it the most common site of inflammation and constriction.4

Trigger finger occurs in 2% to 3% of the general population and in as many as 10% of people with diabetes.5 The condition typically affects the long and ring fingers of the dominant hand; most cases occur in women in the sixth and seventh decades.3-5
Multiple systemic conditions predispose to trigger finger, including endocrine disorders (eg, diabetes, hypothyroidism), inflammatory arthropathies (gout, pseudogout), and autoimmune disorders (rheumatoid arthritis, sarcoidosis).3,5 Diabetes commonly causes bilateral hand and multiple digit involvement, as well as more severe disease.3,5 Occupation is also a risk factor for trigger finger because repetitive movements and manual work can exacerbate triggering.4
Presentation and exam. Patients report pain at the metacarpal head or metacarpophalangeal (MCP) joint, difficulty grasping objects, and, possibly, clicking and catching of the digit and locking of the digit in flexion.3,5
On exam, there might be tenderness at the level of the A1 pulley over the volar MCP joint or a palpable nodule. In severe cases, the proximal interphalangeal (PIP) joint or entire finger can be fixed in flexion.5 Repeated compound finger flexion (eg, closing and opening a fist) or holding a fist for as long as 1 minute and then slowly opening it might provoke triggering.
More than 60% of patients with trigger finger also have carpal tunnel syndrome.5 This makes it important to assess for (1) sensory changes in the distribution of the median nerve and (2) nerve compression, by eliciting Phalen and Tinel signs.4,5
Continue to: Imaging
Imaging. Trigger finger is a clinical diagnosis. Imaging is therefore unnecessary for diagnosis or treatment.5
Treatment. Trigger finger resolves spontaneously in 52% of cases.3 Most patients experience relief in 8 to 12 months.3
First-line treatment is injection of a corticosteroid into the flexor tendon sheath, which often alleviates symptoms.4,5 Injection is performed at the level of the A1 pulley on the palmar surface, just proximal to the MCP joint at the level of the distal palmar crease6 (FIGURE 1). The needle is inserted at an oblique angle until there is an increase in resistance. The needle is then slightly withdrawn to reposition it in the tendon sheath; 0.5 to 1 mL of 50% corticosteroid and 50% local anesthetic without epinephrine is then injected.6

The cure rate of trigger finger is 57% to 70% with 1 injection and 82% to 86% after 2 injections.3,4,19
Many patients experience symptom relief in 1 to 4 weeks after a corticosteroid injection; however, as many as 56% experience repeat triggering within 6 months—often making multiple injections (maximum, 3 per digit) necessary.19,20 Patients who have a longer duration of symptoms, more severe symptoms, and multiple trigger fingers are less likely to experience relief with injections.3,5
Continue to: Splinting is an effective treatment...
Splinting is an effective treatment for patients who cannot undergo corticosteroid injection or surgery. The MCP or PIP joint is immobilized in extension while movement of the distal interphalangeal (DIP) joint is maintained. Instruct the patient that the splint must be worn day and night; splinting is continued for ≥ 6 weeks.21 Splinting relieves symptoms in 47% to 70% of cases and is most effective in patients whose symptoms have been present for < 6 months.3,7
Patients whose trigger finger is locked in flexion and those who have not experienced improvement after 2 or 3 corticosteroid injections should be referred for surgery.4 The surgical cure rate is nearly 100%; only 6% of patients experience repeat triggering 6 to 12 months postoperatively.4,7,22
Jersey finger
Causes and incidence. Jersey finger is caused by avulsion injury to the flexor digitorum profundus (FDP) tendon at its insertion on the distal phalanx.8,9 It occurs when a flexed finger is forced into extension, such as when a football or rugby player grabs another player’s jersey during a tackle.9,10 This action causes the FDP tendon to detach from the distal phalanx, sometimes with a bony fragment.9,11 Once detached, the tendon might retract proximally within the finger or to the palm, with consequent loss of its blood supply.9
Although jersey finger is not as common as the other conditions discussed in this article,9 it is important not to miss this diagnosis because of the risk of chronic disability when it is not treated promptly. Seventy-five percent of cases occur in the ring finger, which is more susceptible to injury because it extends past the other digits in a power grip.8,9
Presentation and exam. On exam, the affected finger lies in slight extension compared to the other digits; the patient is unable to actively flex the DIP joint.8,9 There may be tenderness to palpation over the volar distal phalanx. The retracted FDP tendon might be palpable more proximally in the digit.
Continue to: Imaging
Imaging. Anteroposterior (AP), oblique, and lateral radiographs, although unnecessary for diagnosis, are recommended to assess for an avulsion fragment, associated fracture, or dislocation.9,11 Ultrasonography or magnetic resonance imaging is useful in chronic cases to quantify the degree of tendon retraction.9
Treatment. Refer acute cases of jersey finger for surgical management urgently because most cases require flexor tendon repair within 1 or 2 weeks for a successful outcome.9 Chronic jersey finger, in which injury occurred > 6 weeks before presentation, also requires surgical repair, although not as urgently.9
Complications of jersey finger include flexion contracture at the DIP joint and the so-called quadriga effect, in which the patient is unable to fully flex the fingers adjacent to the injured digit.8 These complications can cause chronic disability in the affected hand, making early diagnosis and referral key to successful treatment.9
Mallet finger
Also called drop finger, mallet finger is a result of loss of active extension at the DIP joint.12,13
Causes and incidence. Mallet finger is a relatively common injury that typically affects the long, ring, or small finger of the dominant hand in young to middle-aged men and older women.12,14,23 The condition is the result of forced flexion or hyperextension injury, which disrupts the extensor tendon.6,14
Continue to: Sudden forced flexion...
Sudden forced flexion of an extended DIP joint during work or sports (eg, catching a ball) is the most common mechanism of injury.12,15 This action causes stretching or tearing of the extensor tendon as well as a possible avulsion fracture of the distal phalanx.13 Mallet finger can also result from a laceration or crush injury of the extensor tendon (open mallet finger) or hyperextension of the DIP joint, causing a fracture at the dorsal base of the distal phalanx.12
Presentation. Through any of the aforementioned mechanisms, the delicate balance between the flexor and extensor tendons is disrupted, causing the patient to present with a flexed DIP joint that can be passively, but not actively, extended.6,12 The DIP joint might also be painful and swollen. Patients whose injury occurred > 4 weeks prior to presentation (chronic mallet finger) might also have a so-called swan-neck deformity, with hyperextension of the PIP joint in the affected finger.12
Imaging. AP, oblique, and lateral radiographs are recommended to assess for bony injury.
Treatment. Splinting is the first-line treatment for almost all mallet finger injuries that are not the result of a laceration or crush injury. Immobilize the DIP joint in extension for 6 to 8 weeks, with an additional 2 to 4 weeks of splinting at night.6,12 The splint must be worn continuously in the initial 6 to 8 weeks, and the DIP joint should remain in extension—even when the patient is performing daily hygiene.12 It is imperative that patients comply with that period of continuous immobilization; if the DIP joint is allowed to flex, the course of treatment must be restarted.13
Many different types of splints exist; functional outcomes are equivalent across all of them.24,25 In our practice, we manage mallet finger with a volar-based splint (FIGURE 2), which is associated with fewer dermatologic complications and has provided the most success for our patients.23

Continue to: Surgical repair of mallet finger injury...
Surgical repair of mallet finger injury is indicated in any of these situations12,14:
- injury is caused by laceration
- there is volar subluxation of the DIP joint
- more than one-third of the articular surface is involved in an avulsion fracture.
Patients who cannot comply with wearing a splint 24 hours per day or whose occupation precludes wearing a splint at all (eg, surgeons, dentists, musicians) are also surgical candidates.12
Surgical and conservative treatments have similar clinical and functional outcomes, including loss of approximately 5° to 7° of active extension and an increased risk of DIP joint osteoarthritis.12,14,24 Patients with chronic mallet finger can be managed with 6 weeks of splinting initially but will likely require surgery.6,12,13
Skier’s thumb
This relatively common injury is a tear of the ulnar collateral ligament (UCL) at the MCP joint of the thumb.16
Causes and incidence. Skier’s thumb occurs when a valgus force hyperabducts the thumb,16 and is so named because the injury is often seen in recreational skiers who fall while holding a ski pole.15-17 It can also occur in racquet sports when a ball or racquet strikes the ulnar side of thumb.16
Continue to: In chronic cases...
In chronic cases, the UCL can be injured by occupational demands and is termed gamekeeper’s thumb because it was first described in this population, who killed game by breaking the animal's neck between the thumb and index finger against the ground.16,18 A UCL tear causes instability at the thumb MCP joint, which affects a person’s ability to grip and pinch.2,16,18
Presentation. On exam, the affected thumb is swollen and, possibly, bruised. There might be radial deviation and volar subluxation of the proximal phalanx. The ulnar side of the MCP joint is tender to palpation.16 If the distal UCL is torn completely, it can displace proximally and present as a palpable mass over the ulnar side of the MCP joint, known as a Stener lesion.16
Stress testing of the MCP joint is the most important part of the physical exam for skier’s thumb. Stabilize the metacarpal neck and apply a valgus stress on the proximal phalanx at both 0° and 30° of MCP flexion (FIGURE 3), which allows for assessment of both the proper and accessory bands of the UCL.2,16 (A common pitfall during stress testing is to allow the MCP joint to rotate, which can mimic instability.2) Intra-articular local anesthesia might be necessary for this exam because it can be painful.16,18,26 A stress exam should assess for laxity and a soft or firm endpoint; the result should be compared to that of a stress exam on the contralateral side.16,17

Imaging. AP, oblique, and lateral radiographs of the thumb should be obtained to assess for instability, avulsion injury, and associated fracture. Subluxation (volar or radial) or supination of the proximal phalanx relative to the metacarpal on imaging suggests MCP instability of the MCP joint.16,17
If the stress exam is equivocal, magnetic resonance imaging is recommended for further assessment.2,18
Continue to: Stress radiographs...
Stress radiographs (ie, radiographs of the thumb with valgus stress applied at the MCP joint) can aid in diagnosis but are controversial. Some experts think that these stress views can further damage the UCL; others recommend against them because they carry a false-negative rate ≥ 25%.15,16 If you choose to perform stress views, order standard radiographs beforehand to rule out bony injury.17
Treatment. UCL tears are classified as 3 tiers to guide treatment.
- Grade 1 injury (a partial tear) is characterized by pain upon palpation but no instability on the stress exam.
- Grade 2 injury (also a partial tear) is marked by laxity on the stress exam with a firm endpoint.
- Grade 3 injury (complete tear) shows laxity and a soft endpoint on a stress exam16,17; Stener lesions are seen only in grade 3 tears.16,17
Grades 1 and 2 UCL tears without fracture or with a nondisplaced avulsion fracture can be managed nonoperatively by immobilizing the thumb in a spica splint or cast for 4 to 6 weeks.16,18 The MCP joint is immobilized and the interphalangeal joint is allowed to move freely.2,16,17
Grade 3 injuries should be referred to a hand specialist for surgical repair.16 Patients presenting > 12 weeks after acute injury or with a chronic UCL tear should also be referred for surgical repair.16
CORRESPONDENCE
Caitlin A. Nicholson, MD, 1611 West Harrison Street, Suite 300, Chicago, IL 60612; [email protected]
1. Hirt B, Seyhan H, Wagner M, et al. Hand and Wrist Anatomy and Biomechanics: A Comprehensive Guide. Thieme; 2017:57,58,71,72,75-80.
2. Daley D, Geary M, Gaston RG. Thumb metacarpophalangeal ulnar and radial collateral ligament injuries. Clin Sports Med. 2020;39:443-455. doi: 10.1016/j.csm.2019.12.003
3. Gil JA, Hresko AM, Weiss AC. Current concepts in the management of trigger finger in adults. J Am Acad Orthop Surg. 2020;28:e642-e650. doi: 10.5435/JAAOS-D-19-00614
4. Henton J, Jain A, Medhurst C, et al. Adult trigger finger. BMJ. 2012;345:e5743. doi: 10.1136/bmj.e5743
5. Bates T, Dunn J. Trigger finger. Orthobullets [Internet]. Updated December 8, 2021. Accessed April 14, 2022. www.orthobullets.com/hand/6027/trigger-finger
6. Chhabra AB, Deal ND. Soft tissue injuries of the wrist and hand. In: O’Connor FG, Casa DJ, Davis BA, et al. ACSM’s Sports Medicine: A Comprehensive Review. Lippincott Williams & Wilkins; 2012:370-373.
7. Ballard TNS, Kozlow JH. Trigger finger in adults. CMAJ. 2016;188:61. doi: 10.1503/cmaj.150225
8. Vitale M. Jersey finger. Orthobullets [Internet]. Updated May 22, 2021. 2019. Accessed April 15, 2022. www.orthobullets.com/hand/6015/jersey-finger
9. Shapiro LM, Kamal RN. Evaluation and treatment of flexor tendon and pulley injuries in athletes. Clin Sports Med. 2020;39:279-297. doi: 10.1016/j.csm.2019.12.004
10. Goodson A, Morgan M, Rajeswaran G, et al. Current management of Jersey finger in rugby players: case series and literature review. Hand Surg. 2010;15:103-107. doi: 10.1142/S0218810410004710
11. Lapegue F, Andre A, Brun C, et al. Traumatic flexor tendon injuries. Diagn Interv Imaging. 2015;96:1279-1292. doi: 10.1016/j.diii.2015.09.010
12. Bendre AA, Hartigan BJ, Kalainov DM. Mallet finger. J Am Acad Orthop Surg. 2005;13:336-344. doi: 10.5435/00124635-200509000-00007
13. Lamaris GA, Matthew MK. The diagnosis and management of mallet finger injuries. Hand (N Y). 2017;12:223-228. doi: 10.1177/1558944716642763
14. Sheth U. Mallet finger. Orthobullets [Internet]. Updated August 5, 2021. Accessed April 15, 2022. www.orthobullets.com/hand/6014/mallet-finger
15. Weintraub MD, Hansford BG, Stilwill SE, et al. Avulsion injuries of the hand and wrist. Radiographics. 2020;40:163-180. doi: 10.1148/rg.2020190085
16. Avery III DM, Inkellis ER, Carlson MG. Thumb collateral ligament injuries in the athlete. Curr Rev Musculoskelet Med. 2017;10:28-37. doi: 10.1007/s12178-017-9381-z
17. Steffes MJ. Thumb collateral ligament injury. Orthobullets [Internet]. Updated February 18, 2022. Accessed April 15, 2022. www.orthobullets.com/hand/6040/thumb-collateral-ligament-injury
18. Madan SS, Pai DR, Kaur A, et al. Injury to ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7. doi: 10.1111/os.12084
19. Dardas AZ, VandenBerg J, Shen T, et al. Long-term effectiveness of repeat corticosteroid injections for trigger finger. J Hand Surg Am. 2017;42:227-235. doi: 10.1016/j.jhsa.2017.02.001
20. Huisstede BM, Gladdines S, Randsdorp MS, et al. Effectiveness of conservative, surgical, and postsurgical interventions for trigger finger, Dupuytren disease, and de Quervain disease: a systematic review. Arch Phys Med Rehabil. 2018;99:1635-1649.e21. doi: 10.1016/j.apmr.2017.07.014
21. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
22. Fiorini HJ, Tamaoki MJ, Lenza M, et al. Surgery for trigger finger. Cochrane Database Syst Rev. 2018;2:CD009860. doi: 10.1002/14651858.CD009860.pub2
23. Salazar Botero S, Hidalgo Diaz JJ, Benaïda A, et al. Review of acute traumatic closed mallet finger injuries in adults. Arch Plast Surg. 2016;43:134-144. doi: 10.5999/aps.2016.43.2.134
24. Lin JS, Samora JB. Surgical and nonsurgical management of mallet finger: a systematic review. J Hand Surg Am. 2018;43:146-163.e2. doi: 10.1016/j.jhsa.2017.10.004
25. Handoll H, Vaghela MV. Interventions for treating mallet finger injuries. Cochrane Database Syst Rev. 2004;(3):CD004574. doi: 10.1002/14651858.CD004574.pub2
26. Pulos N, Shin AY. Treatment of ulnar collateral ligament injuries of the thumb: a critical analysis review. JBJS Rev. 2017;5:e3. doi: 10.2106/JBJS.RVW.16.00051
1. Hirt B, Seyhan H, Wagner M, et al. Hand and Wrist Anatomy and Biomechanics: A Comprehensive Guide. Thieme; 2017:57,58,71,72,75-80.
2. Daley D, Geary M, Gaston RG. Thumb metacarpophalangeal ulnar and radial collateral ligament injuries. Clin Sports Med. 2020;39:443-455. doi: 10.1016/j.csm.2019.12.003
3. Gil JA, Hresko AM, Weiss AC. Current concepts in the management of trigger finger in adults. J Am Acad Orthop Surg. 2020;28:e642-e650. doi: 10.5435/JAAOS-D-19-00614
4. Henton J, Jain A, Medhurst C, et al. Adult trigger finger. BMJ. 2012;345:e5743. doi: 10.1136/bmj.e5743
5. Bates T, Dunn J. Trigger finger. Orthobullets [Internet]. Updated December 8, 2021. Accessed April 14, 2022. www.orthobullets.com/hand/6027/trigger-finger
6. Chhabra AB, Deal ND. Soft tissue injuries of the wrist and hand. In: O’Connor FG, Casa DJ, Davis BA, et al. ACSM’s Sports Medicine: A Comprehensive Review. Lippincott Williams & Wilkins; 2012:370-373.
7. Ballard TNS, Kozlow JH. Trigger finger in adults. CMAJ. 2016;188:61. doi: 10.1503/cmaj.150225
8. Vitale M. Jersey finger. Orthobullets [Internet]. Updated May 22, 2021. 2019. Accessed April 15, 2022. www.orthobullets.com/hand/6015/jersey-finger
9. Shapiro LM, Kamal RN. Evaluation and treatment of flexor tendon and pulley injuries in athletes. Clin Sports Med. 2020;39:279-297. doi: 10.1016/j.csm.2019.12.004
10. Goodson A, Morgan M, Rajeswaran G, et al. Current management of Jersey finger in rugby players: case series and literature review. Hand Surg. 2010;15:103-107. doi: 10.1142/S0218810410004710
11. Lapegue F, Andre A, Brun C, et al. Traumatic flexor tendon injuries. Diagn Interv Imaging. 2015;96:1279-1292. doi: 10.1016/j.diii.2015.09.010
12. Bendre AA, Hartigan BJ, Kalainov DM. Mallet finger. J Am Acad Orthop Surg. 2005;13:336-344. doi: 10.5435/00124635-200509000-00007
13. Lamaris GA, Matthew MK. The diagnosis and management of mallet finger injuries. Hand (N Y). 2017;12:223-228. doi: 10.1177/1558944716642763
14. Sheth U. Mallet finger. Orthobullets [Internet]. Updated August 5, 2021. Accessed April 15, 2022. www.orthobullets.com/hand/6014/mallet-finger
15. Weintraub MD, Hansford BG, Stilwill SE, et al. Avulsion injuries of the hand and wrist. Radiographics. 2020;40:163-180. doi: 10.1148/rg.2020190085
16. Avery III DM, Inkellis ER, Carlson MG. Thumb collateral ligament injuries in the athlete. Curr Rev Musculoskelet Med. 2017;10:28-37. doi: 10.1007/s12178-017-9381-z
17. Steffes MJ. Thumb collateral ligament injury. Orthobullets [Internet]. Updated February 18, 2022. Accessed April 15, 2022. www.orthobullets.com/hand/6040/thumb-collateral-ligament-injury
18. Madan SS, Pai DR, Kaur A, et al. Injury to ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7. doi: 10.1111/os.12084
19. Dardas AZ, VandenBerg J, Shen T, et al. Long-term effectiveness of repeat corticosteroid injections for trigger finger. J Hand Surg Am. 2017;42:227-235. doi: 10.1016/j.jhsa.2017.02.001
20. Huisstede BM, Gladdines S, Randsdorp MS, et al. Effectiveness of conservative, surgical, and postsurgical interventions for trigger finger, Dupuytren disease, and de Quervain disease: a systematic review. Arch Phys Med Rehabil. 2018;99:1635-1649.e21. doi: 10.1016/j.apmr.2017.07.014
21. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
22. Fiorini HJ, Tamaoki MJ, Lenza M, et al. Surgery for trigger finger. Cochrane Database Syst Rev. 2018;2:CD009860. doi: 10.1002/14651858.CD009860.pub2
23. Salazar Botero S, Hidalgo Diaz JJ, Benaïda A, et al. Review of acute traumatic closed mallet finger injuries in adults. Arch Plast Surg. 2016;43:134-144. doi: 10.5999/aps.2016.43.2.134
24. Lin JS, Samora JB. Surgical and nonsurgical management of mallet finger: a systematic review. J Hand Surg Am. 2018;43:146-163.e2. doi: 10.1016/j.jhsa.2017.10.004
25. Handoll H, Vaghela MV. Interventions for treating mallet finger injuries. Cochrane Database Syst Rev. 2004;(3):CD004574. doi: 10.1002/14651858.CD004574.pub2
26. Pulos N, Shin AY. Treatment of ulnar collateral ligament injuries of the thumb: a critical analysis review. JBJS Rev. 2017;5:e3. doi: 10.2106/JBJS.RVW.16.00051
PRACTICE RECOMMENDATIONS
› Treat trigger finger with a corticosteroid injection into the flexor tendon sheath. A
› Refer a case of jersey finger to a hand surgeon within 1 week after injury for flexor tendon repair. C
› Treat mallet finger with strict distal interphalangeal joint immobilization for 6 to 8 weeks. A
› Treat Grades 1 and 2 skier’s thumb with immobilization in a thumb spica splint or a cast for 4 to 6 weeks. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
43-year-old male • fatigue • unintentional weight loss • pancytopenia • Dx?
THE CASE
A 43-year-old Black male presented to his primary care physician with an 8-month history of progressive fatigue, weakness, and unintentional weight loss. The patient’s history also included antiphospholipid antibody syndrome (APS) with prior deep venous thrombosis/pulmonary embolism for which he was taking warfarin.
At the time of presentation, he reported profound dyspnea on exertion, lightheadedness, dry mouth, low back pain, and worsening nocturia. The remainder of the review of systems was negative. He denied tobacco, alcohol, or illicit drug use or recent travel. His personal and family histories were negative for cancer.
Laboratory data collected during the outpatient visit were notable for a white blood cell count of 2300/mcL (reference range, 4000-11,000/mcL); hemoglobin, 8.6 g/dL (13.5-17.5 g/dL); and platelets, 44,000/mcL (150,000-400,000/mcL). Proteinuria was indicated by a measurement > 500 mg/dL on urine dipstick.
The patient was admitted to the hospital for further work-up of new pancytopenia. His vital signs on admission were notable for tachycardia and a weight of 237 lbs, decreased from 283 lbs 8 months prior. His physical exam revealed dry mucous membranes, bruising of fingertips, and marked lower extremity weakness with preserved sensation. No lymphadenopathy was noted on the admission physical exam.
THE DIAGNOSIS
Inpatient laboratory studies showed elevated inflammatory markers and a positive Coombs test with low haptoglobin. There was no evidence of bacterial or viral infection.
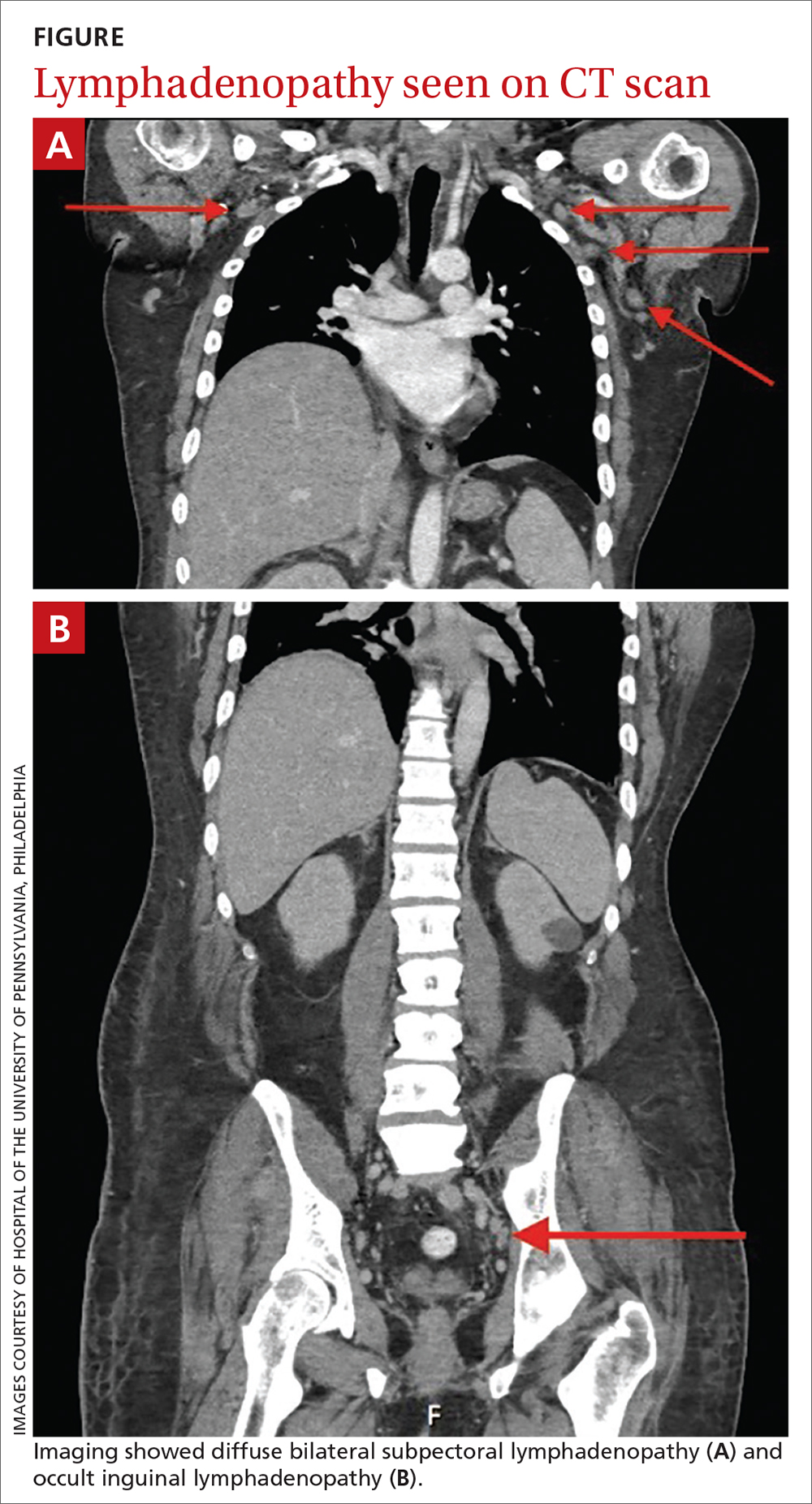
Autoimmune laboratory data included a positive antiphospholipid antibody (ANA) test (1:10,240, diffuse; reference < 1:160), an elevated dsDNA antibody level (800 IU/mL; reference range, 0-99 IU/mL), low complement levels, and antibody titers consistent with the patient’s known APS. Based on these findings, the patient was given a diagnosis of systemic lupus erythematosus (SLE).
DISCUSSION
Lymphadenopathy, revealed by exam or by imaging, in combination with systemic symptoms such as weight loss and fatigue, elicits an extensive differential diagnosis. In the absence of recent exposures, travel, or risk factors for infectious causes, our patient’s work-up was appropriately narrowed to noninfectious etiologies of pancytopenia and lymphadenopathy. At the top of this differential are malignancies—in particular, multiple myeloma and lymphoma—and rheumatologic processes, such as sarcoidosis, connective tissue disease, and SLE.1,2 Ultimately, the combination of autoimmune markers with the pancytopenia and a negative work-up for malignancy confirmed a diagnosis of SLE.
Continue to: SLE classification and generalized lymphadenopathy
SLE classification and generalized lymphadenopathy. SLE is a multisystem inflammatory process with a wide spectrum of clinical presentations. The American College of Rheumatology (ACR) has established validated criteria to aid in the diagnosis of SLE,3 which were most recently updated in 2012 to improve clinical utility. For a diagnosis to be made, at least 1 clinical and 1 immunologic criterion must be present or a renal biopsy must show lupus nephritis.3
Notably, lymphadenopathy is not included in this validated model, despite its occurrence in 25% to 50% of patients with SLE.1,3,4 With this in mind, SLE should be considered in the work-up of generalized lymphadenopathy.
ANA and SLE. Although it is estimated that 30% to 40% of patients with SLE test positive for ANA,5 the presence of ANA also is not part of the diagnostic criteria for SLE. Interestingly, the co-occurrence of the 2 has clinical implications for patients. In particular, patients with SLE and a positive ANA have higher prevalence of thrombosis, valvular disease, thrombocytopenia, and hemolytic anemia, among other complications.5 Although our patient’s presentation of thrombocytopenia and hemolysis clouded the initial work-up, such a combination is consistent with co-presentation of SLE and APS.
Differences in sex, age, and race. SLE is more common in women than in men, with a prevalence ratio of 7:1.6 It is estimated that 65% of patients with SLE experience disease onset between the ages of 16 and 55 years.7
The median age of diagnosis also differs based on sex and race: According to Rus et al,8 the typical age ranges are 37 to 50 years for White women; 50 to 59 for White men; 15 to 44 for Black women; and 45 to 64 for Black men. These estimates of incidence stratified by race, sex, and age can be helpful when evaluating patients with confusing clinical presentations. Our patient’s age was consistent with the median for his sex and race.
Continue to: Our patient
Our patient was started on oral prednisone 60 mg/d with plans for a prolonged taper over 6 months under the close supervision of Rheumatology. His weakness and polyuria began to improve within a month, and lupus-related symptoms resolved within 3 months. His cytopenia also significantly improved, with the exception of refractory thrombocytopenia.
THE TAKEAWAY
SLE is a common diagnosis with multiple presentations. Although lymphadenopathy is not part of the clinical criteria for the diagnosis of SLE, multiple case studies have highlighted its prevalence among affected patients.1,2,4,9-17 APS and antiphospholipid antibodies are also absent in the diagnostic criteria despite being highly associated with SLE. Thus, co-presentation (as well as age and sex) can be helpful with both disease stratification and risk assessment once a diagnosis is made.
CORRESPONDENCE
Isabella Buzzo Bellon Brout, MD, 409 West Broadway, Boston, MA 02127; [email protected]
1. Afzal W, Arab T, Ullah T, et al. Generalized lymphadenopathy as presenting features of systemic lupus erythematosus: case report and review of literature. J Clin Med Res. 2016;8:819-823. doi: 10.14740/jocmr2717w
2. Smith LW, Petri M. Diffuse lymphadenopathy as the presenting manifestation of systemic lupus erythematosus. J Clin Rheumatol. 2013;19:397-399. doi: 10.1097/RHU.0b013e3182a6a924
3. Petri M, Orbai A, Graciela S, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi: 10.1002/art.34473
4. Kitsanou M, Adreopoulou E, Bai MK, et al. Extensive lymphadenopathy as the first clinical manifestation in systemic lupus erythematosus. Lupus. 2000;9:140-143. doi: 10.1191/096120300678828037
5. Unlu O, Zuily S, Erkan D. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur J Rheumatol. 2016;3:75-84. doi: 10.5152/eurjrheum.2015.0085
6. Lahita RG. The role of sex hormones in systemic lupus erythematosus. Curr Opin Rheumatol. 1999;11:352-356. doi: 10.1097/00002281-199909000-00005
7. Rothfield N. Clinical features of systemic lupus erythematosus. In: Kelley WN, Harris ED, Ruddy S, Sledge CB (eds). Textbook of Rheumatology. WB Saunders; 1981.
8. Rus V, Maury EE, Hochberg MC. The epidemiology of systemic lupus erythematosus. In: Wallace DJ, Hahn BH (eds). Dubois’ Lupus Erythematosus. Lippincott Williams and Wilkins; 2002.
9. Biner B, Acunas B, Karasalihoglu S, et al. Systemic lupus erythematosus presenting with generalized lymphadenopathy: a case report. Turk J Pediatr. 2001;43:94-96.
10. Gilmore R, Sin WY. Systemic lupus erythematosus mimicking lymphoma: the relevance of the clinical background in interpreting imaging studies. BMJ Case Rep. 2014;2014:bcr2013201802. doi: 10.1136/bcr-2013-201802
11. Shrestha D, Dhakal AK, Shiva RK, et al. Systemic lupus erythematosus and granulomatous lymphadenopathy. BMC Pediatr. 2013;13:179. doi: 10.1186/1471-2431-13-179
12. Melikoglu MA, Melikoglu M. The clinical importance of lymphadenopathy in systemic lupus erythematosus. Acta Rheumatol Port. 2008;33:402-406.
13. Tamaki K, Morishima S, Nakachi S, et al. An atypical case of late-onset systemic lupus erythematosus with systemic lymphadenopathy and severe autoimmune thrombocytopenia/neutropenia mimicking malignant lymphoma. Int J Hematol. 2017;105:526-531. doi: 10.1007/s12185-016-2126-8
14. Hyami T, Kato T, Moritani S, et al. Systemic lupus erythematosus with abdominal lymphadenopathy. Eur J Dermatol. 2019;29:342-344. doi: 10.1684/ejd.2019.3589
15. Mull ES, Aranez V, Pierce D, et al. Newly diagnosed systemic lupus erythematosus: atypical presentation with focal seizures and long-standing lymphadenopathy. J Clin Rheumatol. 2019;25:e109-e113. doi: 10.1097/RHU.0000000000000681
16. Kassan SS, Moss ML, Reddick RL. Progressive hilar and mediastinal lymphadenopathy in systemic lupus erythematosus on corticosteroid therapy. N Engl J Med. 1976;294:1382-1383. doi: 10.1056/NEJM197606172942506
17. Tuinman PR, Nieuwenhuis MB, Groen E, et al. A young woman with generalized lymphadenopathy. Systemic lupus erythematosus. Neth J Med. 2011;69:284-288.
THE CASE
A 43-year-old Black male presented to his primary care physician with an 8-month history of progressive fatigue, weakness, and unintentional weight loss. The patient’s history also included antiphospholipid antibody syndrome (APS) with prior deep venous thrombosis/pulmonary embolism for which he was taking warfarin.
At the time of presentation, he reported profound dyspnea on exertion, lightheadedness, dry mouth, low back pain, and worsening nocturia. The remainder of the review of systems was negative. He denied tobacco, alcohol, or illicit drug use or recent travel. His personal and family histories were negative for cancer.
Laboratory data collected during the outpatient visit were notable for a white blood cell count of 2300/mcL (reference range, 4000-11,000/mcL); hemoglobin, 8.6 g/dL (13.5-17.5 g/dL); and platelets, 44,000/mcL (150,000-400,000/mcL). Proteinuria was indicated by a measurement > 500 mg/dL on urine dipstick.
The patient was admitted to the hospital for further work-up of new pancytopenia. His vital signs on admission were notable for tachycardia and a weight of 237 lbs, decreased from 283 lbs 8 months prior. His physical exam revealed dry mucous membranes, bruising of fingertips, and marked lower extremity weakness with preserved sensation. No lymphadenopathy was noted on the admission physical exam.
THE DIAGNOSIS
Inpatient laboratory studies showed elevated inflammatory markers and a positive Coombs test with low haptoglobin. There was no evidence of bacterial or viral infection.

Autoimmune laboratory data included a positive antiphospholipid antibody (ANA) test (1:10,240, diffuse; reference < 1:160), an elevated dsDNA antibody level (800 IU/mL; reference range, 0-99 IU/mL), low complement levels, and antibody titers consistent with the patient’s known APS. Based on these findings, the patient was given a diagnosis of systemic lupus erythematosus (SLE).
DISCUSSION
Lymphadenopathy, revealed by exam or by imaging, in combination with systemic symptoms such as weight loss and fatigue, elicits an extensive differential diagnosis. In the absence of recent exposures, travel, or risk factors for infectious causes, our patient’s work-up was appropriately narrowed to noninfectious etiologies of pancytopenia and lymphadenopathy. At the top of this differential are malignancies—in particular, multiple myeloma and lymphoma—and rheumatologic processes, such as sarcoidosis, connective tissue disease, and SLE.1,2 Ultimately, the combination of autoimmune markers with the pancytopenia and a negative work-up for malignancy confirmed a diagnosis of SLE.
Continue to: SLE classification and generalized lymphadenopathy
SLE classification and generalized lymphadenopathy. SLE is a multisystem inflammatory process with a wide spectrum of clinical presentations. The American College of Rheumatology (ACR) has established validated criteria to aid in the diagnosis of SLE,3 which were most recently updated in 2012 to improve clinical utility. For a diagnosis to be made, at least 1 clinical and 1 immunologic criterion must be present or a renal biopsy must show lupus nephritis.3
Notably, lymphadenopathy is not included in this validated model, despite its occurrence in 25% to 50% of patients with SLE.1,3,4 With this in mind, SLE should be considered in the work-up of generalized lymphadenopathy.
ANA and SLE. Although it is estimated that 30% to 40% of patients with SLE test positive for ANA,5 the presence of ANA also is not part of the diagnostic criteria for SLE. Interestingly, the co-occurrence of the 2 has clinical implications for patients. In particular, patients with SLE and a positive ANA have higher prevalence of thrombosis, valvular disease, thrombocytopenia, and hemolytic anemia, among other complications.5 Although our patient’s presentation of thrombocytopenia and hemolysis clouded the initial work-up, such a combination is consistent with co-presentation of SLE and APS.
Differences in sex, age, and race. SLE is more common in women than in men, with a prevalence ratio of 7:1.6 It is estimated that 65% of patients with SLE experience disease onset between the ages of 16 and 55 years.7
The median age of diagnosis also differs based on sex and race: According to Rus et al,8 the typical age ranges are 37 to 50 years for White women; 50 to 59 for White men; 15 to 44 for Black women; and 45 to 64 for Black men. These estimates of incidence stratified by race, sex, and age can be helpful when evaluating patients with confusing clinical presentations. Our patient’s age was consistent with the median for his sex and race.
Continue to: Our patient
Our patient was started on oral prednisone 60 mg/d with plans for a prolonged taper over 6 months under the close supervision of Rheumatology. His weakness and polyuria began to improve within a month, and lupus-related symptoms resolved within 3 months. His cytopenia also significantly improved, with the exception of refractory thrombocytopenia.
THE TAKEAWAY
SLE is a common diagnosis with multiple presentations. Although lymphadenopathy is not part of the clinical criteria for the diagnosis of SLE, multiple case studies have highlighted its prevalence among affected patients.1,2,4,9-17 APS and antiphospholipid antibodies are also absent in the diagnostic criteria despite being highly associated with SLE. Thus, co-presentation (as well as age and sex) can be helpful with both disease stratification and risk assessment once a diagnosis is made.
CORRESPONDENCE
Isabella Buzzo Bellon Brout, MD, 409 West Broadway, Boston, MA 02127; [email protected]
THE CASE
A 43-year-old Black male presented to his primary care physician with an 8-month history of progressive fatigue, weakness, and unintentional weight loss. The patient’s history also included antiphospholipid antibody syndrome (APS) with prior deep venous thrombosis/pulmonary embolism for which he was taking warfarin.
At the time of presentation, he reported profound dyspnea on exertion, lightheadedness, dry mouth, low back pain, and worsening nocturia. The remainder of the review of systems was negative. He denied tobacco, alcohol, or illicit drug use or recent travel. His personal and family histories were negative for cancer.
Laboratory data collected during the outpatient visit were notable for a white blood cell count of 2300/mcL (reference range, 4000-11,000/mcL); hemoglobin, 8.6 g/dL (13.5-17.5 g/dL); and platelets, 44,000/mcL (150,000-400,000/mcL). Proteinuria was indicated by a measurement > 500 mg/dL on urine dipstick.
The patient was admitted to the hospital for further work-up of new pancytopenia. His vital signs on admission were notable for tachycardia and a weight of 237 lbs, decreased from 283 lbs 8 months prior. His physical exam revealed dry mucous membranes, bruising of fingertips, and marked lower extremity weakness with preserved sensation. No lymphadenopathy was noted on the admission physical exam.
THE DIAGNOSIS
Inpatient laboratory studies showed elevated inflammatory markers and a positive Coombs test with low haptoglobin. There was no evidence of bacterial or viral infection.

Autoimmune laboratory data included a positive antiphospholipid antibody (ANA) test (1:10,240, diffuse; reference < 1:160), an elevated dsDNA antibody level (800 IU/mL; reference range, 0-99 IU/mL), low complement levels, and antibody titers consistent with the patient’s known APS. Based on these findings, the patient was given a diagnosis of systemic lupus erythematosus (SLE).
DISCUSSION
Lymphadenopathy, revealed by exam or by imaging, in combination with systemic symptoms such as weight loss and fatigue, elicits an extensive differential diagnosis. In the absence of recent exposures, travel, or risk factors for infectious causes, our patient’s work-up was appropriately narrowed to noninfectious etiologies of pancytopenia and lymphadenopathy. At the top of this differential are malignancies—in particular, multiple myeloma and lymphoma—and rheumatologic processes, such as sarcoidosis, connective tissue disease, and SLE.1,2 Ultimately, the combination of autoimmune markers with the pancytopenia and a negative work-up for malignancy confirmed a diagnosis of SLE.
Continue to: SLE classification and generalized lymphadenopathy
SLE classification and generalized lymphadenopathy. SLE is a multisystem inflammatory process with a wide spectrum of clinical presentations. The American College of Rheumatology (ACR) has established validated criteria to aid in the diagnosis of SLE,3 which were most recently updated in 2012 to improve clinical utility. For a diagnosis to be made, at least 1 clinical and 1 immunologic criterion must be present or a renal biopsy must show lupus nephritis.3
Notably, lymphadenopathy is not included in this validated model, despite its occurrence in 25% to 50% of patients with SLE.1,3,4 With this in mind, SLE should be considered in the work-up of generalized lymphadenopathy.
ANA and SLE. Although it is estimated that 30% to 40% of patients with SLE test positive for ANA,5 the presence of ANA also is not part of the diagnostic criteria for SLE. Interestingly, the co-occurrence of the 2 has clinical implications for patients. In particular, patients with SLE and a positive ANA have higher prevalence of thrombosis, valvular disease, thrombocytopenia, and hemolytic anemia, among other complications.5 Although our patient’s presentation of thrombocytopenia and hemolysis clouded the initial work-up, such a combination is consistent with co-presentation of SLE and APS.
Differences in sex, age, and race. SLE is more common in women than in men, with a prevalence ratio of 7:1.6 It is estimated that 65% of patients with SLE experience disease onset between the ages of 16 and 55 years.7
The median age of diagnosis also differs based on sex and race: According to Rus et al,8 the typical age ranges are 37 to 50 years for White women; 50 to 59 for White men; 15 to 44 for Black women; and 45 to 64 for Black men. These estimates of incidence stratified by race, sex, and age can be helpful when evaluating patients with confusing clinical presentations. Our patient’s age was consistent with the median for his sex and race.
Continue to: Our patient
Our patient was started on oral prednisone 60 mg/d with plans for a prolonged taper over 6 months under the close supervision of Rheumatology. His weakness and polyuria began to improve within a month, and lupus-related symptoms resolved within 3 months. His cytopenia also significantly improved, with the exception of refractory thrombocytopenia.
THE TAKEAWAY
SLE is a common diagnosis with multiple presentations. Although lymphadenopathy is not part of the clinical criteria for the diagnosis of SLE, multiple case studies have highlighted its prevalence among affected patients.1,2,4,9-17 APS and antiphospholipid antibodies are also absent in the diagnostic criteria despite being highly associated with SLE. Thus, co-presentation (as well as age and sex) can be helpful with both disease stratification and risk assessment once a diagnosis is made.
CORRESPONDENCE
Isabella Buzzo Bellon Brout, MD, 409 West Broadway, Boston, MA 02127; [email protected]
1. Afzal W, Arab T, Ullah T, et al. Generalized lymphadenopathy as presenting features of systemic lupus erythematosus: case report and review of literature. J Clin Med Res. 2016;8:819-823. doi: 10.14740/jocmr2717w
2. Smith LW, Petri M. Diffuse lymphadenopathy as the presenting manifestation of systemic lupus erythematosus. J Clin Rheumatol. 2013;19:397-399. doi: 10.1097/RHU.0b013e3182a6a924
3. Petri M, Orbai A, Graciela S, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi: 10.1002/art.34473
4. Kitsanou M, Adreopoulou E, Bai MK, et al. Extensive lymphadenopathy as the first clinical manifestation in systemic lupus erythematosus. Lupus. 2000;9:140-143. doi: 10.1191/096120300678828037
5. Unlu O, Zuily S, Erkan D. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur J Rheumatol. 2016;3:75-84. doi: 10.5152/eurjrheum.2015.0085
6. Lahita RG. The role of sex hormones in systemic lupus erythematosus. Curr Opin Rheumatol. 1999;11:352-356. doi: 10.1097/00002281-199909000-00005
7. Rothfield N. Clinical features of systemic lupus erythematosus. In: Kelley WN, Harris ED, Ruddy S, Sledge CB (eds). Textbook of Rheumatology. WB Saunders; 1981.
8. Rus V, Maury EE, Hochberg MC. The epidemiology of systemic lupus erythematosus. In: Wallace DJ, Hahn BH (eds). Dubois’ Lupus Erythematosus. Lippincott Williams and Wilkins; 2002.
9. Biner B, Acunas B, Karasalihoglu S, et al. Systemic lupus erythematosus presenting with generalized lymphadenopathy: a case report. Turk J Pediatr. 2001;43:94-96.
10. Gilmore R, Sin WY. Systemic lupus erythematosus mimicking lymphoma: the relevance of the clinical background in interpreting imaging studies. BMJ Case Rep. 2014;2014:bcr2013201802. doi: 10.1136/bcr-2013-201802
11. Shrestha D, Dhakal AK, Shiva RK, et al. Systemic lupus erythematosus and granulomatous lymphadenopathy. BMC Pediatr. 2013;13:179. doi: 10.1186/1471-2431-13-179
12. Melikoglu MA, Melikoglu M. The clinical importance of lymphadenopathy in systemic lupus erythematosus. Acta Rheumatol Port. 2008;33:402-406.
13. Tamaki K, Morishima S, Nakachi S, et al. An atypical case of late-onset systemic lupus erythematosus with systemic lymphadenopathy and severe autoimmune thrombocytopenia/neutropenia mimicking malignant lymphoma. Int J Hematol. 2017;105:526-531. doi: 10.1007/s12185-016-2126-8
14. Hyami T, Kato T, Moritani S, et al. Systemic lupus erythematosus with abdominal lymphadenopathy. Eur J Dermatol. 2019;29:342-344. doi: 10.1684/ejd.2019.3589
15. Mull ES, Aranez V, Pierce D, et al. Newly diagnosed systemic lupus erythematosus: atypical presentation with focal seizures and long-standing lymphadenopathy. J Clin Rheumatol. 2019;25:e109-e113. doi: 10.1097/RHU.0000000000000681
16. Kassan SS, Moss ML, Reddick RL. Progressive hilar and mediastinal lymphadenopathy in systemic lupus erythematosus on corticosteroid therapy. N Engl J Med. 1976;294:1382-1383. doi: 10.1056/NEJM197606172942506
17. Tuinman PR, Nieuwenhuis MB, Groen E, et al. A young woman with generalized lymphadenopathy. Systemic lupus erythematosus. Neth J Med. 2011;69:284-288.
1. Afzal W, Arab T, Ullah T, et al. Generalized lymphadenopathy as presenting features of systemic lupus erythematosus: case report and review of literature. J Clin Med Res. 2016;8:819-823. doi: 10.14740/jocmr2717w
2. Smith LW, Petri M. Diffuse lymphadenopathy as the presenting manifestation of systemic lupus erythematosus. J Clin Rheumatol. 2013;19:397-399. doi: 10.1097/RHU.0b013e3182a6a924
3. Petri M, Orbai A, Graciela S, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi: 10.1002/art.34473
4. Kitsanou M, Adreopoulou E, Bai MK, et al. Extensive lymphadenopathy as the first clinical manifestation in systemic lupus erythematosus. Lupus. 2000;9:140-143. doi: 10.1191/096120300678828037
5. Unlu O, Zuily S, Erkan D. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur J Rheumatol. 2016;3:75-84. doi: 10.5152/eurjrheum.2015.0085
6. Lahita RG. The role of sex hormones in systemic lupus erythematosus. Curr Opin Rheumatol. 1999;11:352-356. doi: 10.1097/00002281-199909000-00005
7. Rothfield N. Clinical features of systemic lupus erythematosus. In: Kelley WN, Harris ED, Ruddy S, Sledge CB (eds). Textbook of Rheumatology. WB Saunders; 1981.
8. Rus V, Maury EE, Hochberg MC. The epidemiology of systemic lupus erythematosus. In: Wallace DJ, Hahn BH (eds). Dubois’ Lupus Erythematosus. Lippincott Williams and Wilkins; 2002.
9. Biner B, Acunas B, Karasalihoglu S, et al. Systemic lupus erythematosus presenting with generalized lymphadenopathy: a case report. Turk J Pediatr. 2001;43:94-96.
10. Gilmore R, Sin WY. Systemic lupus erythematosus mimicking lymphoma: the relevance of the clinical background in interpreting imaging studies. BMJ Case Rep. 2014;2014:bcr2013201802. doi: 10.1136/bcr-2013-201802
11. Shrestha D, Dhakal AK, Shiva RK, et al. Systemic lupus erythematosus and granulomatous lymphadenopathy. BMC Pediatr. 2013;13:179. doi: 10.1186/1471-2431-13-179
12. Melikoglu MA, Melikoglu M. The clinical importance of lymphadenopathy in systemic lupus erythematosus. Acta Rheumatol Port. 2008;33:402-406.
13. Tamaki K, Morishima S, Nakachi S, et al. An atypical case of late-onset systemic lupus erythematosus with systemic lymphadenopathy and severe autoimmune thrombocytopenia/neutropenia mimicking malignant lymphoma. Int J Hematol. 2017;105:526-531. doi: 10.1007/s12185-016-2126-8
14. Hyami T, Kato T, Moritani S, et al. Systemic lupus erythematosus with abdominal lymphadenopathy. Eur J Dermatol. 2019;29:342-344. doi: 10.1684/ejd.2019.3589
15. Mull ES, Aranez V, Pierce D, et al. Newly diagnosed systemic lupus erythematosus: atypical presentation with focal seizures and long-standing lymphadenopathy. J Clin Rheumatol. 2019;25:e109-e113. doi: 10.1097/RHU.0000000000000681
16. Kassan SS, Moss ML, Reddick RL. Progressive hilar and mediastinal lymphadenopathy in systemic lupus erythematosus on corticosteroid therapy. N Engl J Med. 1976;294:1382-1383. doi: 10.1056/NEJM197606172942506
17. Tuinman PR, Nieuwenhuis MB, Groen E, et al. A young woman with generalized lymphadenopathy. Systemic lupus erythematosus. Neth J Med. 2011;69:284-288.