User login
In reply: Menstrual manipulation
In Reply: Thank you for reading our article. Although the focus was geared more toward a comparison of different means of menstrual manipulation, we appreciate your comments on oral contraceptives and the link to premenopausal breast cancer.
As you noted, oral contraceptives have been linked to an increased risk of breast cancer, both in your meta-analysis1 and again more recently in a prospective study of 116,608 female nurses from 25 to 42 years of age.2 Interestingly, data from the latter study suggested that different formulations of oral contraceptives may pose different risks, and specifically that the use of triphasic preparations with levonorgestrel as the progestin had the highest risk. However, there is otherwise a paucity of data regarding the risk of specific formulations. There is currently no evidence of an association between oral contraceptive use and death from breast cancer, nor is there evidence that longer use of an oral contraceptive increases one’s risk of death from breast cancer.3
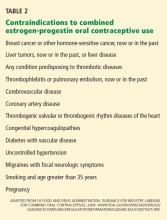
Oral contraceptives have also been associated with a reduced risk of ovarian cancer,4 and they appear to protect against death from ovarian cancer and uterine cancer.3 Therefore, the clinician must consider the individual patient before making treatment recommendations, taking into account personal risk factors and other health concerns. (For a full list of contraindications to oral contraceptives, please refer to Table 2 in our original article.) Further guidelines may also be obtained from the “US Medical Eligibility Criteria for Contraceptive Use 2010,” issued by the US Centers for Disease Control and Prevention in May 2010,5 which delineates the eligibility criteria for initiating and continuing specific contraceptive methods, including oral contraceptives.
Thank you again for sharing your concerns. We appreciate the opportunity to clarify this important point.
- Kahlenborn C, Modugno F, Potter DM, et al. Oral contraceptive use as a risk factor for premenopausal breast cancer: a meta-analysis. Mayo Clin Proc 2006; 81:1290–1302.
- Hunter DJ, Colditz GA, Hankinson SE, et al. Oral contraceptive use and breast cancer: a prospective study of young women. Cancer Epidemiol Biomarkers Prev 2010; 19:2496–2502.
- Vessey M, Yeates D, Flynn S. Factors affecting mortality in a large cohort study with special reference to oral contraceptive use. Contraception 2010; 82:221–229.
- Lurie G, Thompson P, McDuffie KE, et al. Association of estrogen and progestin potency of oral contraceptives with ovarian carcinoma risk. Obstet Gynecol 2007; 109:597–607.
- Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, US Centers for Disease Control and Prevention (CDC), Farr S, et al. US medical eligibility criteria for contraceptive use, 2010: adapted from the World Health Organization medical eligibility criteria for contraceptive use, 4th edition. MMWR Recomm Rep 2010; 59:1–86.
In Reply: Thank you for reading our article. Although the focus was geared more toward a comparison of different means of menstrual manipulation, we appreciate your comments on oral contraceptives and the link to premenopausal breast cancer.
As you noted, oral contraceptives have been linked to an increased risk of breast cancer, both in your meta-analysis1 and again more recently in a prospective study of 116,608 female nurses from 25 to 42 years of age.2 Interestingly, data from the latter study suggested that different formulations of oral contraceptives may pose different risks, and specifically that the use of triphasic preparations with levonorgestrel as the progestin had the highest risk. However, there is otherwise a paucity of data regarding the risk of specific formulations. There is currently no evidence of an association between oral contraceptive use and death from breast cancer, nor is there evidence that longer use of an oral contraceptive increases one’s risk of death from breast cancer.3
Oral contraceptives have also been associated with a reduced risk of ovarian cancer,4 and they appear to protect against death from ovarian cancer and uterine cancer.3 Therefore, the clinician must consider the individual patient before making treatment recommendations, taking into account personal risk factors and other health concerns. (For a full list of contraindications to oral contraceptives, please refer to Table 2 in our original article.) Further guidelines may also be obtained from the “US Medical Eligibility Criteria for Contraceptive Use 2010,” issued by the US Centers for Disease Control and Prevention in May 2010,5 which delineates the eligibility criteria for initiating and continuing specific contraceptive methods, including oral contraceptives.
Thank you again for sharing your concerns. We appreciate the opportunity to clarify this important point.
In Reply: Thank you for reading our article. Although the focus was geared more toward a comparison of different means of menstrual manipulation, we appreciate your comments on oral contraceptives and the link to premenopausal breast cancer.
As you noted, oral contraceptives have been linked to an increased risk of breast cancer, both in your meta-analysis1 and again more recently in a prospective study of 116,608 female nurses from 25 to 42 years of age.2 Interestingly, data from the latter study suggested that different formulations of oral contraceptives may pose different risks, and specifically that the use of triphasic preparations with levonorgestrel as the progestin had the highest risk. However, there is otherwise a paucity of data regarding the risk of specific formulations. There is currently no evidence of an association between oral contraceptive use and death from breast cancer, nor is there evidence that longer use of an oral contraceptive increases one’s risk of death from breast cancer.3
Oral contraceptives have also been associated with a reduced risk of ovarian cancer,4 and they appear to protect against death from ovarian cancer and uterine cancer.3 Therefore, the clinician must consider the individual patient before making treatment recommendations, taking into account personal risk factors and other health concerns. (For a full list of contraindications to oral contraceptives, please refer to Table 2 in our original article.) Further guidelines may also be obtained from the “US Medical Eligibility Criteria for Contraceptive Use 2010,” issued by the US Centers for Disease Control and Prevention in May 2010,5 which delineates the eligibility criteria for initiating and continuing specific contraceptive methods, including oral contraceptives.
Thank you again for sharing your concerns. We appreciate the opportunity to clarify this important point.
- Kahlenborn C, Modugno F, Potter DM, et al. Oral contraceptive use as a risk factor for premenopausal breast cancer: a meta-analysis. Mayo Clin Proc 2006; 81:1290–1302.
- Hunter DJ, Colditz GA, Hankinson SE, et al. Oral contraceptive use and breast cancer: a prospective study of young women. Cancer Epidemiol Biomarkers Prev 2010; 19:2496–2502.
- Vessey M, Yeates D, Flynn S. Factors affecting mortality in a large cohort study with special reference to oral contraceptive use. Contraception 2010; 82:221–229.
- Lurie G, Thompson P, McDuffie KE, et al. Association of estrogen and progestin potency of oral contraceptives with ovarian carcinoma risk. Obstet Gynecol 2007; 109:597–607.
- Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, US Centers for Disease Control and Prevention (CDC), Farr S, et al. US medical eligibility criteria for contraceptive use, 2010: adapted from the World Health Organization medical eligibility criteria for contraceptive use, 4th edition. MMWR Recomm Rep 2010; 59:1–86.
- Kahlenborn C, Modugno F, Potter DM, et al. Oral contraceptive use as a risk factor for premenopausal breast cancer: a meta-analysis. Mayo Clin Proc 2006; 81:1290–1302.
- Hunter DJ, Colditz GA, Hankinson SE, et al. Oral contraceptive use and breast cancer: a prospective study of young women. Cancer Epidemiol Biomarkers Prev 2010; 19:2496–2502.
- Vessey M, Yeates D, Flynn S. Factors affecting mortality in a large cohort study with special reference to oral contraceptive use. Contraception 2010; 82:221–229.
- Lurie G, Thompson P, McDuffie KE, et al. Association of estrogen and progestin potency of oral contraceptives with ovarian carcinoma risk. Obstet Gynecol 2007; 109:597–607.
- Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, US Centers for Disease Control and Prevention (CDC), Farr S, et al. US medical eligibility criteria for contraceptive use, 2010: adapted from the World Health Organization medical eligibility criteria for contraceptive use, 4th edition. MMWR Recomm Rep 2010; 59:1–86.
Menstrual manipulation: Options for suppressing the cycle
If they wish, women can have more control over when and if they menstruate. By using hormonal contraceptives in extended or continuous regimens, they can have their period less often, a practice called menstrual manipulation or menstrual suppression.
Actually, with the help of their clinicians, women have been doing this for years. But now that several products have been approved by the US Food and Drug Administration (FDA) specifically for use in extended or continuous regimens, the practice has become more widely accepted.
Reasons for suppressing menstrual flow range from avoiding bleeding during a particular event (eg, a wedding, graduation, or sports competition) to finding relief from dysmenorrhea or reducing or eliminating menstruation in the treatment of endometriosis, migraine, and other medical conditions exacerbated by hormonal changes around the time of menses.1 Alternatively, some women may practice menstrual manipulation for no other reason than to simply avoid menstruation.
MENSTRUAL DISORDERS ARE TROUBLESOME, COMMON
Each year in the United States, menstrual disorders such as dysmenorrhea (painful menstruation), menorrhagia (excessive or frequent menstruation), metrorrhagia (irregular menstruation), menometrorrhagia (excessive and irregular menstruation), and premenstrual syndrome affect nearly 2.5 million women age 18 to 50 years.2 Menstrual disorders are the leading cause of gynecologic morbidity in the United States, outnumbering adnexal masses (the second most common cause) by a factor of three.2 In addition, these disorders extend into the workplace, costing US industry about 8% of its total wage bill.3
A BRIEF HISTORY OF CONTRACEPTIVE DEVELOPMENT
The idea of using progestins for birth control was first advanced in the 1950s by Dr. Gregory Pincus, who proposed a regimen of 21 days of active drug followed by 7 drug-free days to allow withdrawal bleeding, mimicking the natural cycle.4 This “21/7” regimen was designed to follow the lunar cycle in the hope it would be, in the words of Dr. John Rock, “a morally permissible variant of the rhythm method,”5 thereby making it acceptable to women, clinicians, and the Catholic Church.
In 1977, Loudon et al6 reported the results of a study in which women took active pills for 84 days instead of 21 days, which reduced the frequency of menstruation to every 3 months. Since then, extending the active pills beyond 21 days to avoid menses and other hormone-withdrawal symptoms has become popular in clinical practice, and many studies have investigated the extended or continuous use of oral and other forms of contraception to delay menses.7–18
CURRENT METHODS OF MENSTRUAL MANIPULATION
A variety of available products prevent conception by altering the menstrual cycle:
- Oral estrogen-progestin contraceptive pills
- A drug-releasing intrauterine device
- Depot medroxyprogesterone acetate injections
- A contraceptive vaginal ring
- An implantable etonogestrel contraceptive.
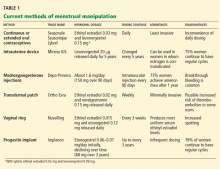
Their use in menstrual manipulation is summarized in Table 1.
Oral contraceptive pills
The most common way to manipulate the menstrual cycle is to extend the time between hormone-free weeks in an oral contraceptive regimen.
If the patient is young, you can prescribe a monophasic 21/7 oral contraceptive and tell her to take one active pill every day for 21 days and then start a new pack and keep taking active pills for up to 84 consecutive days, skipping the placebo pills until she wants to have her menstrual period. She can choose which week to have it: if the scheduled 12th week of an extended-cycle oral contraceptive regimen is inconvenient, she can plan it for week 10, or week 9, or whichever week is convenient.
The rationale for using an 84-day (12-week) cycle is that it still provides four periods per year, alleviating fears of hypertrophic endometrium.19
In this scenario, unscheduled or breakthrough bleeding can be managed by taking a “double-up pill” from a spare pack on any day breakthrough bleeding occurs and until it resolves. Menstrual periods should not be planned for intervals shorter than 21 days, owing to the risk of ovulation. Missed days of pills or use of placebo pills should also not exceed 7 days to prevent escape ovulation. 20
In some women with endometriosis and other medical reasons, continuous oral contraception with no placebo week can be prescribed.
Unfortunately, the downside to suppressing withdrawal bleeding is unscheduled or “breakthrough” bleeding. The best way to treat this unscheduled bleeding is not known. Patients who are not sexually active can be reassured that the goal of an atrophic endometrium can still be achieved, with resultant pill amenorrhea (particularly useful for those with severe dysmenorrhea or other reasons to want to avoid flow). Patients could also try to manage flow by periodically taking a 3- to 5-day break from hormone-containing pills to allow flow. They can also try switching to another oral contraceptive that has a different progestin that would spiral the arterioles of the endometrium more tightly and thus more aggressively induce atrophy.13,17,21 For instance, levonorgestrel is 10 to 20 times more potent than norethindrone. Choosing a pill with a higher monophasic dosing of levonorgestrel or a similar progestin may minimize unscheduled bleeding.
Currently, several oral contraceptives are approved for use in an extended regimen.
Seasonale was the first oral contraceptive marketed in the United States with an extended active regimen.22 It comes in a pack of 84 pills containing ethinyl estradiol 0.03 mg and levonorgestrel 0.15 mg, plus 7 placebo pills.
Seasonique is similar to Seasonale, but instead of placebo pills it has seven pills that contain ethinyl estradiol 0.010 mg.
Lybrel is a low-dose combination containing ethinyl estradiol 0.02 mg and levonorgestrel 0.09 mg. Packaged as an entire year’s worth of active pills to be taken continuously for 365 days without a placebo phase or pillfree interval,23 it is the only FDA-approved continuous oral contraceptive available in the United States.
An intrauterine device
Intrauterine devices were originally developed as contraceptives. The addition of a progestin to these devices has been shown to reduce heavy menstrual bleeding by up to 90%.24,25
Mirena IUS, a levonorgestrel-releasing device, is the only medicated intrauterine device that is currently available in the United States. (“IUS” stands for “intrauterine system.”) It was recently approved by the FDA to treat heavy menstrual bleeding in women who use intrauterine contraception as their method of pregnancy prevention.26 About 50% of women who use this device develop amenorrhea within 6 months of insertion, while 25% report oligomenorrhea.27
The Mirena device can be left in the uterus for up to 5 years. It may be a good choice for inducing amenorrhea in women with hemostatic disorders or in whom estrogen either is contraindicated or causes health concerns.18 The copper intrauterine device (Paragard; Duramed Pharmaceuticals Inc., Pomona, NY) remains a viable option for those who cannot or do not tolerate hormonal therapy. However, Mirena may provide less unscheduled bleeding than the copper intrauterine device.
Depot medroxyprogesterone acetate injections
Depo-Provera (depot medroxyprogesterone acetate) injections are given at 90-day intervals. 28 This contraceptive method inhibits ovulation and decidualizes the endometrium, thereby reducing or eliminating uterine bleeding. 29
While new users may initially experience excessive prolonged bleeding (10 or more days) while shedding their existing lining, the rate of amenorrhea has been shown to increase over time as the lining atrophies.30 Thus, prolonged use of this agent reduces the frequency of menstruation as well as menstruation-related symptoms.
Depot medroxyprogesterone acetate is ideal for patients whose menstrual periods pose a significant hygiene problem (eg, developmentally challenged girls). In our experience, the injections can be given at shorter intervals to induce atrophy of the endometrium quickly. In this scenario, the clinician might give an injection every 4 to 6 weeks for two or three doses to induce amenorrhea and then return to every-12-week dosing.
The main risk when using medroxyprogesterone injections to induce amenorrhea is the potential for bone loss. Users of this method have been shown to have lower mean bone mineral density31–33 and significantly higher levels of biomarkers of bone formation and resorption32,34 than nonusers. However, these changes are similar to those seen in breastfeeding women,35 are reversible with cessation, 36 and are not associated with increased fracture risk.37 In adolescent girls, pregnancy poses similar risks to the bones, with longerterm consequences.
Medroxyprogesterone can also stimulate appetite, causing 10 to 20 kg of weight gain in adolescents and women who are already obese and have trouble with appetite regulation.38 Slender users tend not to gain weight, however.
Given this information, depot medroxyprogesterone acetate appears to be a cost-effective contraceptive option that should be considered in the context of the clinical situation and preference of each patient.
Transdermal contraceptive patch
Ortho Evra, a transdermal patch, is designed to deliver ethinyl estradiol 0.02 mg and norelgestromin 0.150 mg daily.39 It is usually applied weekly for 3 weeks, followed by a patch-free week to induce regular monthly withdrawal bleeding.
Extended use of the patch to manipulate menstruation is an off-label use. In the only trial evaluating extended use of the patch, amenorrhea occurred in 12% of users, but unscheduled bleeding and spotting were common. 16
Although there is some evidence that the long-term use of the patch may increase the risk of venous thromboembolism,40,41 the risk in women who use the patch has been found to be similar to that in women using an oral contraceptive.42 However, serum ethinyl estradiol levels have been found to be higher with the use of the weekly patch than with oral contraceptives or the contraceptive vaginal ring39; as a result, many physicians are hesitant to recommend its continuous use.
Pending further data about the safety profile of this contraceptive, the World Health Organization Medical Eligibility Criteria for Contraceptive Use suggest that the same guidelines for the prescription of combination oral contraceptives should also apply to the patch.43
Contraceptive vaginal ring
NuvaRing, a contraceptive vaginal ring, releases a daily dose of ethinyl estradiol 0.015 mg and etonogestrel 0.12 mg.10 It is inserted, left in for 21 days, and then removed and left out for 7 days, during which withdrawal bleeding occurs.10
Vaginal administration has been shown to allow low, continuous dosing, which results in more stable serum concentrations than with the patch or oral contraceptives.39 In the only trial comparing an extended vaginal ring regimen and the traditional 28-day regimen, extended use resulted in fewer overall days of bleeding than monthly use, but with more unscheduled spotting.15
The most common side effects include headache, vaginitis, and leukorrhea,44 but there is no evidence of bacteriologic or cytologic changes in the cervicovaginal epithelium, even with extended use.45,46
Etonogestrel implantable contraceptive
Implanon, a single-rod progestin implant, is available in the United States and elsewhere. It is placed subdermally in the inner upper arm and provides contraception for as long as 3 years.
Implanon contains 68 mg of the progestin etonogestrel, which it slowly releases over time, initially at 0.06 to 0.07 mg/day, decreasing to 0.035 to 0.045 mg/day at the end of the first year, to 0.03 to 0.04 mg/day at the end of the second year, and then to 0.025 to 0.03 mg/day at the end of the third year.47
The amount of vaginal bleeding associated with the use of the implant is generally modest, but the pattern tends to be unpredictable. 48 In addition, because amenorrhea is reported as a side effect in only 22% of women during the first 2 years of its use,48 the progestin implant is a less satisfactory means of menstrual suppression than the other methods discussed above.
BENEFITS OF MENSTRUAL MANIPULATION
Menstrual manipulation has a number of benefits in terms of both overall health and lifestyle.
For most women, using a long-acting hormonal contraceptive carries low risks and substantial health benefits. Women who take oral contraceptives are less likely to develop osteoporosis, ovarian or endometrial cancer, benign breast changes, or pelvic inflammatory disease. 49 Long-term use of an oral contraceptive can also preserve fertility by reducing and delaying the incidence of endometriosis,50 and is effective at treating acne vulgaris, which tends to be common among patients with polycystic ovary syndrome.51,52 In addition, this practice can be used to reduce overall blood loss, an application that is particularly important in women with a bleeding diathesis such as von Willebrand disease, who frequently suffer from menorrhagia.53
Reduced menstruation may also prove more convenient during particular occasions, such as vacations and athletic activities. Specifically, it may be useful to women serving in the military. In a study by Schneider et al,54 a cohort of 83 female cadets reported a significant perceived impact of premenstrual and menstrual-related symptoms on academic, physical, and military activities, as well as difficulties in obtaining, changing, and disposing of menstrual materials in a military setting. Likewise, reduced menstrual frequency or amenorrhea may play an important role in female athletes, who reportedly use oral contraceptives to control premenstrual symptoms, to protect bone health, and to manipulate the menstrual cycle in order to maximize performance.55
Adolescent girls are another group who may benefit from reduced or absent menses, once they have reached near-final height. By practicing menstrual suppression, girls can avoid dysmenorrhea and the inconvenience of menstruation during the school day, when their access to painkillers, sanitary pads or tampons, and a change of clothes may be limited. 56 Clinicians who discuss with teenage patients the benefits of innovative hormonal contraceptive schedules that reduce menstrual frequency may be able to improve the quality of life for these young women.
In a very short girl just after menarche, care must be taken not to start a hormonal method too early so as not to prematurely close epiphyses and stunt final height; after menarche, most girls still have 1 to 4 inches of potential growth. For a young lady 4 feet 11 inches tall, that extra inch may be important.
Finally, menstrual manipulation may also find a niche among the developmentally challenged. Women with cognitive impairment and physical disabilities may have difficulty with hygienic practice around menses. For a number of years, contraceptives have been used to manage menstrual hygiene in patients with catamenial (ie, menstrual) epilepsy, and to address caregiver concerns in women with severe mental retardation, with improved behavior noted in some patients.57–59 In this setting, an agent that suppresses menses and also provides contraception, especially for those girls and women at risk of abuse, may offer substantial benefits.
DISADVANTAGES OF MENSTRUAL MANIPULATION
Rates of adverse events and of discontinuation of extended and continuous oral contraceptive regimens are comparable with those reported for cyclic regimens, except for higher rates of breakthrough bleeding.
In a trial of continuous oral contraceptive use in more than 2,000 patients, 396 (18.5%) withdrew from the study as a result of bothersome uterine bleeding.60 However, while breakthrough bleeding often occurs during the first few months of extended oral contraceptive use, it usually decreases with each successive cycle of therapy and is comparable to that reported by patients on the conventional oral contraceptive regimen by the fourth extended cycle.12
CONTRACEPTIVE EFFICACY
The efficacy of extended and continuous oral contraceptive regimens is comparable with that of cyclic regimens.12,60,61 One reason for this may be better adherence to continuous regimens: women using this regimen have been shown to miss fewer pills than those on a cyclic regimen, especially during the critical first week of the pill pack.21
Several studies have shown that some women ovulate during the standard 21/7 oral contraceptive regimen even if they do not miss any pills or take pills off-schedule, putting them at greater risk of pregnancy.62 Large studies evaluating the efficacy of an extendedcycle regimen have shown a pregnancy rate during the 1-year study period that was either comparable with61 or lower than12,60 rates with standard regimens.
Heterosexual couples need to be advised to use condoms to further reduce the already low failure rate and to prevent sexually transmitted diseases.
ACCEPTABILITY OF MENSTRUAL MANIPULATION
Ever since the earliest trial of an extended oral contraceptive regimen, participants have expressed a favorable response to the resulting decrease in menstrual frequency; in the 1977 study by Loudon et al,6 patients on the extended regimen cited infrequent periods (82%), fewer menstrual problems (20%), and easier pill-taking (19%) as favorable features.
In 1999, den Tonkelaar and Oddens63 surveyed 1,300 Dutch women about their preferred frequency of menstruation and found that about 70% between the ages of 15 and 49 preferred a frequency of between every 3 months and never. A similar survey in the United States indicated that 58% preferred a bleeding frequency of either every 3 months or never to more frequent periods.64
While patients find menstrual manipulation generally acceptable, clinician approval has been more varied. Loudon et al reported that “the doctors and nurses on the clinic staff were less enthusiastic about this regimen than the volunteers themselves.”6 In a survey of 222 clinicians,65 90% of responders reported ever having prescribed extended or continuous dosing regimens to adolescents, and 33% reported that extended cycles made up more than 10% of their total oral contraceptive prescriptions.
Myths and misperceptions about menstrual manipulation abound. Many clinicians believe that routine use of an extended or continuous oral contraceptive regimen is inadvisable, despite the lack of evidence to support this notion.66 Therefore, many care providers need more education about the practice and benefits of menstrual manipulation.
THE RIGHT METHOD FOR THE RIGHT PATIENT
Manipulation and suppression of menstruation through continuous or extended use of oral contraceptives or by other means may have a number of advantages to women, including fewer menstrual-related syndromes, reduced absenteeism from work or school, and greater overall satisfaction.
For women whose goal is to reduce but not necessarily to eliminate monthly bleeding, the cyclic use of estrogen-progestin contraception (rather than progestins alone or continuous use of combined hormonal preparations) is suggested.
Clinicians should not overestimate the risks of oral contraceptives and other hormonal methods, but rather educate themselves so that they can utilize menstrual manipulation safely to match the individual patient’s needs.
- Association of Reproductive Health Professionals. Extended and continuous use of contraceptives to reduce menstruation. September 2004. http://www.arhp.org/publications-and-resources/clinical-proceedings/reduce-menses. Accessed May 17, 2010.
- Kjerulff KH, Erickson BA, Langenberg PW. Chronic gynecological conditions reported by US women: findings from the National Health Interview Survey, 1984 to 1992. Am J Public Health 1996; 86:195–199.
- Thomas SL, Ellertson C. Nuisance or natural and healthy: should monthly menstruation be optional for women? Lancet 2000; 355:922–924.
- Connell EB. Contraception in the prepill era. Contraception 1999; 59(suppl 1):7S–10S.
- Marks LV. Sexual chemistry: a history of the contraceptive pill. New Haven, CT: Yale University Press, 2001.
- Loudon NB, Foxwell M, Potts DM, Guild AL, Short RV. Acceptability of an oral contraceptive that reduces the frequency of menstruation: the tri-cycle pill regimen. Br Med J 1977; 2:487–490.
- Sulak PJ, Cressman BE, Waldrop E, Holleman S, Kuehl TJ. Extending the duration of active oral contraceptive pills to manage hormone withdrawal symptoms. Obstet Gynecol 1997; 89:179–183.
- Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception 1997; 56:341–352.
- Miller L, Notter KM. Menstrual reduction with extended use of combination oral contraceptive pills: randomized controlled trial. Obstet Gynecol 2001; 98:771–778.
- Mulders TM, Dieben TO. Use of the novel combined contraceptive vaginal ring NuvaRing for ovulation inhibition. Fertil Steril 2001; 75:865–870.
- Stanford JB, Mikolajczyk RT. Mechanisms of action of intrauterine devices: update and estimation of postfertilization effects. Am J Obstet Gynecol 2002; 187:1699–1708.
- Anderson FD, Hait H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception 2003; 68:89–96.
- Miller L, Hughes JP. Continuous combination oral contraceptive pills to eliminate withdrawal bleeding: a randomized trial. Obstet Gynecol 2003; 101:653–661.
- Sillem M, Schneidereit R, Heithecker R, Mueck AO. Use of an oral contraceptive containing drospirenone in an extended regimen. Eur J Contracept Reprod Health Care 2003; 8:162–169.
- Miller L, Verhoeven CH, Hout J. Extended regimens of the contraceptive vaginal ring: a randomized trial. Obstet Gynecol 2005; 106:473–482.
- Stewart FH, Kaunitz AM, Laguardia KD, Karvois DL, Fisher AC, Friedman AJ. Extended use of transdermal norelgestromin/ethinyl estradiol: a randomized trial. Obstet Gynecol 2005; 105:1389–1396.
- Sulak PJ, Kuehl TJ, Coffee A, Willis S. Prospective analysis of occurrence and management of breakthrough bleeding during an extended oral contraceptive regimen. Am J Obstet Gynecol 2006; 195:935–941.
- Lukes AS, Reardon B, Arepally G. Use of the levonorgestrel– releasing intrauterine system in women with hemostatic disorders. Fertil Steril 2008; 90:673–677.
- Anderson FD, Feldman R, Reape KZ. Endometrial effects of a 91-day extended-regimen oral contraceptive with low-dose estrogen in place of placebo. Contraception 2008; 77:91–96.
- Wright KP, Johnson JV. Evaluation of extended and continuous use oral contraceptives. Ther Clin Risk Manag 2008; 4:905–911.
- Edelman AB, Gallo MF, Jensen JT, Nichols MD, Schulz KF, Grimes DA. Continuous or extended cycle vs. cyclic use of combined oral contraceptives for contraception. Cochrane Database Syst Rev 2005; 3:CD004695.
- Sulak PJ, Kuehl TJ, Ortiz M, Shull BL. Acceptance of altering the standard 21-day/7-day oral contraceptive regimen to delay menses and reduce hormone withdrawal symptoms. Am J Obstet Gynecol 2002; 186:1142–1149.
- Turok D. The quest for better contraception: future methods. Obstet Gynecol Clin North Am 2007; 34:137–166.
- Bergqvist A, Rybo G. Treatment of menorrhagia with intrauterine release of progesterone. Br J Obstet Gynaecol 1983; 90:255–258.
- Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception 1994; 49:56–72.
- US Food and Drug Administration. FDA Approves Additional Use for IUD Mirena to Treat Heavy Menstrual Bleeding in IUD Users. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm184747.htm. Accessed May 17, 2010.
- Hidalgo M, Bahamondes L, Perrotti M, Diaz J, Dantas-Monteiro C, Petta C. Bleeding patterns and clinical performance of the levonorgestrel-releasing intrauterine system (Mirena) up to two years. Contraception 2002; 65:129–132.
- Schwallie PC, Assenzo JR. Contraceptive use—efficacy study utilizing medroxyprogesterone acetate administered as an intramuscular injection once every 90 days. Fertil Steril 1973; 24:331–339.
- Kaunitz AM. Injectable contraception. New and existing options. Obstet Gynecol Clin North Am 2000; 27:741–780.
- Mainwaring R, Hales HA, Stevenson K, et al. Metabolic parameter, bleeding, and weight changes in US women using progestin only contraceptives. Contraception 1995; 51:149–153.
- Curtis KM, Martins SL. Progestogen-only contraception and bone mineral density: a systematic review. Contraception 2006; 73:470–487.
- Shaarawy M, El-Mallah SY, Seoudi S, Hassan M, Mohsen IA. Effects of the long-term use of depot medroxyprogesterone acetate as hormonal contraceptive on bone mineral density and biochemical markers of bone remodeling. Contraception 2006; 74:297–302.
- Cromer BA, Bonny AE, Stager M, et al. Bone mineral density in adolescent females using injectable or oral contraceptives: a 24-month prospective study. Fertil Steril 2008; 90:2060–2067.
- Rome E, Ziegler J, Secic M, et al. Bone biochemical markers in adolescent girls using either depot medroxyprogesterone acetate or an oral contraceptive. J Pediatr Adolesc Gynecol 2004; 17:373–377.
- More C, Bettembuk P, Bhattoa HP, Balogh A. The effects of pregnancy and lactation on bone mineral density. Osteoporos Int 2001; 12:732–737.
- Kaunitz AM, Miller PD, Rice VM, Ross D, McClung MR. Bone mineral density in women aged 25-35 years receiving depot medroxyprogesterone acetate: recovery following discontinuation. Contraception 2006; 74:90–99.
- Guilbert ER, Brown JP, Kaunitz AM, et al. The use of depot-medroxyprogesterone acetate in contraception and its potential impact on skeletal health. Contraception 2009; 79:167–177.
- Bonny AE, Ziegler J, Harvey R, Debanne SM, Secic M, Cromer BA. Weight gain in obese and nonobese adolescent girls initiating depot medroxyprogesterone, oral contraceptive pills, or no hormonal contraceptive method. Arch Pediatr Adolesc Med 2006; 160:40–45.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005; 72:168–174.
- Douketis JD, Ginsberg JS, Holbrook A, Crowther M, Duku EK, Burrows RF. A reevaluation of the risk for venous thromboembolism with the use of oral contraceptives and hormone replacement therapy. Arch Intern Med 1997; 157:1522–1530.
- Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol 2007; 109:339–346.
- Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2007; 76:4–7.
- World Health Organization. Medical Eligibility Criteria for Contraceptive Use. 3rd ed. Geneva: Reproductive Health and Research, World Health Organization; 2004.
- Dieben TO, Roumen FJ, Apter D. Efficacy, cycle control, and user acceptability of a novel combined contraceptive vaginal ring. Obstet Gynecol 2002; 100:585–593.
- Davies GC, Feng LX, Newton JR, Dieben TO, Coelingh-Bennink HJ. The effects of a combined contraceptive vaginal ring releasing ethinyloestradiol and 3-ketodesogestrel on vaginal flora. Contraception 1992; 45:511–518.
- Roumen FJ, Boon ME, van Velzen D, Dieben TO, Coelingh Bennink HJ. The cervico-vaginal epithelium during 20 cycles’ use of a combined contraceptive vaginal ring. Hum Reprod 1996; 11:2443–2448.
- Wenzl R, van Beek A, Schnabel P, Huber J. Pharmacokinetics of etonogestrel released from the contraceptive implant Implanon. Contraception 1998; 58:283–288.
- Darney P, Patel A, Rosen K, Shapiro LS, Kaunitz AM. Safety and efficacy of a single-rod etonogestrel implant (Implanon): results from 11 international clinical trials. Fertil Steril 2009; 91:1646–1653.
- Jensen JT, Speroff L. Health benefits of oral contraceptives. Obstet Gynecol Clin North Am 2000; 27:705–721.
- Seracchioli R, Mabrouk M, Frascà C, et al. Long-term cyclic and continuous oral contraceptive therapy and endometrioma recurrence: a randomized controlled trial. Fertil Steril 2010; 93:52–56.
- Falsetti L, Dordoni D, Gastaldi C, Gastaldi A. A new association of ethinylestradiol (0.035 mg) cyproterone acetate (2 mg) in the therapy of polycystic ovary syndrome. Acta Eur Fertil 1986; 17:19–25.
- Koltun W, Lucky AW, Thiboutot D, et al. Efficacy and safety of 3 mg drospirenone/20 mcg ethinylestradiol oral contraceptive administered in 24/4 regimen in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled trial. Contraception 2008; 77:249–256.
- Kadir RA, Sabin CA, Pollard D, Lee CA, Economides DL. Quality of life during menstruation in patients with inherited bleeding disorders. Haemophilia 1998; 4:836–841.
- Schneider MB, Fisher M, Friedman SB, Bijur PE, Toffler AP. Menstrual and premenstrual issues in female military cadets: a unique population with significant concerns. J Pediatr Adolesc Gynecol 1999; 12:195–201.
- Bennell K, White S, Crossley K. The oral contraceptive pill: a revolution for sportswomen? Br J Sports Med 1999; 33:231–238.
- Kaplowitz PB, Oberfield SE. Reexamination of the age limit for defining when puberty is precocious in girls in the United States: implications for evaluation and treatment. Drug and Therapeutics and Executive Committees of the Lawson Wilkins Pediatric Endocrine Society. Pediatrics 1999; 104:936–941.
- Roxburgh DR, West MJ. The use of norethisterone to suppress menstruation in the intellectually severely retarded woman. Med J Aust 1973; 2:310–313.
- Egan TM, Siegert RJ, Fairley NA. Use of hormonal contraceptives in an institutional setting: reasons for use, consent and safety in women with psychiatric and intellectual disabilities. N Z Med J 1993; 106:338–341.
- Pillai M, O’Brien K, Hill E. The levonorgestrel intrauterine system (Mirena) for the treatment of menstrual problems in adolescents with medical disorders, or physical or learning disabilities. BJOG 2010; 117:216–221.
- Archer DF, Jensen JT, Johnson JV, Borisute H, Grubb GS, Constantine GD. Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results. Contraception 2006; 74:439–445.
- Kroll R, Reape KZ, Margolis M. The efficacy and safety of a low-dose, 91-day, extended-regimen oral contraceptive with continuous ethinyl estradiol. Contraception 2010; 81:41–48.
- Archer DF. Menstrual-cycle-related symptoms: a review of the rationale for continuous use of oral contraceptives. Contraception 2006; 74:359–366.
- den Tonkelaar I, Oddens BJ. Preferred frequency and characteristics of menstrual bleeding in relation to reproductive status, oral contraceptive use, and hormone replacement therapy use. Contraception 1999; 59:357–362.
- Edelman A, Lew R, Cwiak C, Nichols M, Jensen J. Acceptability of contraceptive-induced amenorrhea in a racially diverse group of US women. Contraception 2007; 75:450–453.
- Gerschultz KL, Sucato GS, Hennon TR, Murray PJ, Gold MA. Extended cycling of combined hormonal contraceptives in adolescents: physician views and prescribing practices. J Adolesc Health 2007; 40:151–157.
- Frankovich RJ, Lebrun CM. Menstrual cycle, contraception, and performance. Clin Sports Med 2000; 19:251–271.
- Speroff L, Darney PD. A Clinical Guide for Contraception. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2005.
- Kaunitz AM. Long-acting contraceptive options. Int J Fertil Menopausal Stud 1996; 41:69–76.
- US Food and Drug Administration. Guidance for Industry Labeling for Combined Oral Contraceptives, 2004. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm075075.pdfAccessed May 17, 2010.
If they wish, women can have more control over when and if they menstruate. By using hormonal contraceptives in extended or continuous regimens, they can have their period less often, a practice called menstrual manipulation or menstrual suppression.
Actually, with the help of their clinicians, women have been doing this for years. But now that several products have been approved by the US Food and Drug Administration (FDA) specifically for use in extended or continuous regimens, the practice has become more widely accepted.
Reasons for suppressing menstrual flow range from avoiding bleeding during a particular event (eg, a wedding, graduation, or sports competition) to finding relief from dysmenorrhea or reducing or eliminating menstruation in the treatment of endometriosis, migraine, and other medical conditions exacerbated by hormonal changes around the time of menses.1 Alternatively, some women may practice menstrual manipulation for no other reason than to simply avoid menstruation.
MENSTRUAL DISORDERS ARE TROUBLESOME, COMMON
Each year in the United States, menstrual disorders such as dysmenorrhea (painful menstruation), menorrhagia (excessive or frequent menstruation), metrorrhagia (irregular menstruation), menometrorrhagia (excessive and irregular menstruation), and premenstrual syndrome affect nearly 2.5 million women age 18 to 50 years.2 Menstrual disorders are the leading cause of gynecologic morbidity in the United States, outnumbering adnexal masses (the second most common cause) by a factor of three.2 In addition, these disorders extend into the workplace, costing US industry about 8% of its total wage bill.3
A BRIEF HISTORY OF CONTRACEPTIVE DEVELOPMENT
The idea of using progestins for birth control was first advanced in the 1950s by Dr. Gregory Pincus, who proposed a regimen of 21 days of active drug followed by 7 drug-free days to allow withdrawal bleeding, mimicking the natural cycle.4 This “21/7” regimen was designed to follow the lunar cycle in the hope it would be, in the words of Dr. John Rock, “a morally permissible variant of the rhythm method,”5 thereby making it acceptable to women, clinicians, and the Catholic Church.
In 1977, Loudon et al6 reported the results of a study in which women took active pills for 84 days instead of 21 days, which reduced the frequency of menstruation to every 3 months. Since then, extending the active pills beyond 21 days to avoid menses and other hormone-withdrawal symptoms has become popular in clinical practice, and many studies have investigated the extended or continuous use of oral and other forms of contraception to delay menses.7–18
CURRENT METHODS OF MENSTRUAL MANIPULATION
A variety of available products prevent conception by altering the menstrual cycle:
- Oral estrogen-progestin contraceptive pills
- A drug-releasing intrauterine device
- Depot medroxyprogesterone acetate injections
- A contraceptive vaginal ring
- An implantable etonogestrel contraceptive.
Their use in menstrual manipulation is summarized in Table 1.
Oral contraceptive pills
The most common way to manipulate the menstrual cycle is to extend the time between hormone-free weeks in an oral contraceptive regimen.
If the patient is young, you can prescribe a monophasic 21/7 oral contraceptive and tell her to take one active pill every day for 21 days and then start a new pack and keep taking active pills for up to 84 consecutive days, skipping the placebo pills until she wants to have her menstrual period. She can choose which week to have it: if the scheduled 12th week of an extended-cycle oral contraceptive regimen is inconvenient, she can plan it for week 10, or week 9, or whichever week is convenient.
The rationale for using an 84-day (12-week) cycle is that it still provides four periods per year, alleviating fears of hypertrophic endometrium.19
In this scenario, unscheduled or breakthrough bleeding can be managed by taking a “double-up pill” from a spare pack on any day breakthrough bleeding occurs and until it resolves. Menstrual periods should not be planned for intervals shorter than 21 days, owing to the risk of ovulation. Missed days of pills or use of placebo pills should also not exceed 7 days to prevent escape ovulation. 20
In some women with endometriosis and other medical reasons, continuous oral contraception with no placebo week can be prescribed.
Unfortunately, the downside to suppressing withdrawal bleeding is unscheduled or “breakthrough” bleeding. The best way to treat this unscheduled bleeding is not known. Patients who are not sexually active can be reassured that the goal of an atrophic endometrium can still be achieved, with resultant pill amenorrhea (particularly useful for those with severe dysmenorrhea or other reasons to want to avoid flow). Patients could also try to manage flow by periodically taking a 3- to 5-day break from hormone-containing pills to allow flow. They can also try switching to another oral contraceptive that has a different progestin that would spiral the arterioles of the endometrium more tightly and thus more aggressively induce atrophy.13,17,21 For instance, levonorgestrel is 10 to 20 times more potent than norethindrone. Choosing a pill with a higher monophasic dosing of levonorgestrel or a similar progestin may minimize unscheduled bleeding.
Currently, several oral contraceptives are approved for use in an extended regimen.
Seasonale was the first oral contraceptive marketed in the United States with an extended active regimen.22 It comes in a pack of 84 pills containing ethinyl estradiol 0.03 mg and levonorgestrel 0.15 mg, plus 7 placebo pills.
Seasonique is similar to Seasonale, but instead of placebo pills it has seven pills that contain ethinyl estradiol 0.010 mg.
Lybrel is a low-dose combination containing ethinyl estradiol 0.02 mg and levonorgestrel 0.09 mg. Packaged as an entire year’s worth of active pills to be taken continuously for 365 days without a placebo phase or pillfree interval,23 it is the only FDA-approved continuous oral contraceptive available in the United States.
An intrauterine device
Intrauterine devices were originally developed as contraceptives. The addition of a progestin to these devices has been shown to reduce heavy menstrual bleeding by up to 90%.24,25
Mirena IUS, a levonorgestrel-releasing device, is the only medicated intrauterine device that is currently available in the United States. (“IUS” stands for “intrauterine system.”) It was recently approved by the FDA to treat heavy menstrual bleeding in women who use intrauterine contraception as their method of pregnancy prevention.26 About 50% of women who use this device develop amenorrhea within 6 months of insertion, while 25% report oligomenorrhea.27
The Mirena device can be left in the uterus for up to 5 years. It may be a good choice for inducing amenorrhea in women with hemostatic disorders or in whom estrogen either is contraindicated or causes health concerns.18 The copper intrauterine device (Paragard; Duramed Pharmaceuticals Inc., Pomona, NY) remains a viable option for those who cannot or do not tolerate hormonal therapy. However, Mirena may provide less unscheduled bleeding than the copper intrauterine device.
Depot medroxyprogesterone acetate injections
Depo-Provera (depot medroxyprogesterone acetate) injections are given at 90-day intervals. 28 This contraceptive method inhibits ovulation and decidualizes the endometrium, thereby reducing or eliminating uterine bleeding. 29
While new users may initially experience excessive prolonged bleeding (10 or more days) while shedding their existing lining, the rate of amenorrhea has been shown to increase over time as the lining atrophies.30 Thus, prolonged use of this agent reduces the frequency of menstruation as well as menstruation-related symptoms.
Depot medroxyprogesterone acetate is ideal for patients whose menstrual periods pose a significant hygiene problem (eg, developmentally challenged girls). In our experience, the injections can be given at shorter intervals to induce atrophy of the endometrium quickly. In this scenario, the clinician might give an injection every 4 to 6 weeks for two or three doses to induce amenorrhea and then return to every-12-week dosing.
The main risk when using medroxyprogesterone injections to induce amenorrhea is the potential for bone loss. Users of this method have been shown to have lower mean bone mineral density31–33 and significantly higher levels of biomarkers of bone formation and resorption32,34 than nonusers. However, these changes are similar to those seen in breastfeeding women,35 are reversible with cessation, 36 and are not associated with increased fracture risk.37 In adolescent girls, pregnancy poses similar risks to the bones, with longerterm consequences.
Medroxyprogesterone can also stimulate appetite, causing 10 to 20 kg of weight gain in adolescents and women who are already obese and have trouble with appetite regulation.38 Slender users tend not to gain weight, however.
Given this information, depot medroxyprogesterone acetate appears to be a cost-effective contraceptive option that should be considered in the context of the clinical situation and preference of each patient.
Transdermal contraceptive patch
Ortho Evra, a transdermal patch, is designed to deliver ethinyl estradiol 0.02 mg and norelgestromin 0.150 mg daily.39 It is usually applied weekly for 3 weeks, followed by a patch-free week to induce regular monthly withdrawal bleeding.
Extended use of the patch to manipulate menstruation is an off-label use. In the only trial evaluating extended use of the patch, amenorrhea occurred in 12% of users, but unscheduled bleeding and spotting were common. 16
Although there is some evidence that the long-term use of the patch may increase the risk of venous thromboembolism,40,41 the risk in women who use the patch has been found to be similar to that in women using an oral contraceptive.42 However, serum ethinyl estradiol levels have been found to be higher with the use of the weekly patch than with oral contraceptives or the contraceptive vaginal ring39; as a result, many physicians are hesitant to recommend its continuous use.
Pending further data about the safety profile of this contraceptive, the World Health Organization Medical Eligibility Criteria for Contraceptive Use suggest that the same guidelines for the prescription of combination oral contraceptives should also apply to the patch.43
Contraceptive vaginal ring
NuvaRing, a contraceptive vaginal ring, releases a daily dose of ethinyl estradiol 0.015 mg and etonogestrel 0.12 mg.10 It is inserted, left in for 21 days, and then removed and left out for 7 days, during which withdrawal bleeding occurs.10
Vaginal administration has been shown to allow low, continuous dosing, which results in more stable serum concentrations than with the patch or oral contraceptives.39 In the only trial comparing an extended vaginal ring regimen and the traditional 28-day regimen, extended use resulted in fewer overall days of bleeding than monthly use, but with more unscheduled spotting.15
The most common side effects include headache, vaginitis, and leukorrhea,44 but there is no evidence of bacteriologic or cytologic changes in the cervicovaginal epithelium, even with extended use.45,46
Etonogestrel implantable contraceptive
Implanon, a single-rod progestin implant, is available in the United States and elsewhere. It is placed subdermally in the inner upper arm and provides contraception for as long as 3 years.
Implanon contains 68 mg of the progestin etonogestrel, which it slowly releases over time, initially at 0.06 to 0.07 mg/day, decreasing to 0.035 to 0.045 mg/day at the end of the first year, to 0.03 to 0.04 mg/day at the end of the second year, and then to 0.025 to 0.03 mg/day at the end of the third year.47
The amount of vaginal bleeding associated with the use of the implant is generally modest, but the pattern tends to be unpredictable. 48 In addition, because amenorrhea is reported as a side effect in only 22% of women during the first 2 years of its use,48 the progestin implant is a less satisfactory means of menstrual suppression than the other methods discussed above.
BENEFITS OF MENSTRUAL MANIPULATION
Menstrual manipulation has a number of benefits in terms of both overall health and lifestyle.
For most women, using a long-acting hormonal contraceptive carries low risks and substantial health benefits. Women who take oral contraceptives are less likely to develop osteoporosis, ovarian or endometrial cancer, benign breast changes, or pelvic inflammatory disease. 49 Long-term use of an oral contraceptive can also preserve fertility by reducing and delaying the incidence of endometriosis,50 and is effective at treating acne vulgaris, which tends to be common among patients with polycystic ovary syndrome.51,52 In addition, this practice can be used to reduce overall blood loss, an application that is particularly important in women with a bleeding diathesis such as von Willebrand disease, who frequently suffer from menorrhagia.53
Reduced menstruation may also prove more convenient during particular occasions, such as vacations and athletic activities. Specifically, it may be useful to women serving in the military. In a study by Schneider et al,54 a cohort of 83 female cadets reported a significant perceived impact of premenstrual and menstrual-related symptoms on academic, physical, and military activities, as well as difficulties in obtaining, changing, and disposing of menstrual materials in a military setting. Likewise, reduced menstrual frequency or amenorrhea may play an important role in female athletes, who reportedly use oral contraceptives to control premenstrual symptoms, to protect bone health, and to manipulate the menstrual cycle in order to maximize performance.55
Adolescent girls are another group who may benefit from reduced or absent menses, once they have reached near-final height. By practicing menstrual suppression, girls can avoid dysmenorrhea and the inconvenience of menstruation during the school day, when their access to painkillers, sanitary pads or tampons, and a change of clothes may be limited. 56 Clinicians who discuss with teenage patients the benefits of innovative hormonal contraceptive schedules that reduce menstrual frequency may be able to improve the quality of life for these young women.
In a very short girl just after menarche, care must be taken not to start a hormonal method too early so as not to prematurely close epiphyses and stunt final height; after menarche, most girls still have 1 to 4 inches of potential growth. For a young lady 4 feet 11 inches tall, that extra inch may be important.
Finally, menstrual manipulation may also find a niche among the developmentally challenged. Women with cognitive impairment and physical disabilities may have difficulty with hygienic practice around menses. For a number of years, contraceptives have been used to manage menstrual hygiene in patients with catamenial (ie, menstrual) epilepsy, and to address caregiver concerns in women with severe mental retardation, with improved behavior noted in some patients.57–59 In this setting, an agent that suppresses menses and also provides contraception, especially for those girls and women at risk of abuse, may offer substantial benefits.
DISADVANTAGES OF MENSTRUAL MANIPULATION
Rates of adverse events and of discontinuation of extended and continuous oral contraceptive regimens are comparable with those reported for cyclic regimens, except for higher rates of breakthrough bleeding.
In a trial of continuous oral contraceptive use in more than 2,000 patients, 396 (18.5%) withdrew from the study as a result of bothersome uterine bleeding.60 However, while breakthrough bleeding often occurs during the first few months of extended oral contraceptive use, it usually decreases with each successive cycle of therapy and is comparable to that reported by patients on the conventional oral contraceptive regimen by the fourth extended cycle.12
CONTRACEPTIVE EFFICACY
The efficacy of extended and continuous oral contraceptive regimens is comparable with that of cyclic regimens.12,60,61 One reason for this may be better adherence to continuous regimens: women using this regimen have been shown to miss fewer pills than those on a cyclic regimen, especially during the critical first week of the pill pack.21
Several studies have shown that some women ovulate during the standard 21/7 oral contraceptive regimen even if they do not miss any pills or take pills off-schedule, putting them at greater risk of pregnancy.62 Large studies evaluating the efficacy of an extendedcycle regimen have shown a pregnancy rate during the 1-year study period that was either comparable with61 or lower than12,60 rates with standard regimens.
Heterosexual couples need to be advised to use condoms to further reduce the already low failure rate and to prevent sexually transmitted diseases.
ACCEPTABILITY OF MENSTRUAL MANIPULATION
Ever since the earliest trial of an extended oral contraceptive regimen, participants have expressed a favorable response to the resulting decrease in menstrual frequency; in the 1977 study by Loudon et al,6 patients on the extended regimen cited infrequent periods (82%), fewer menstrual problems (20%), and easier pill-taking (19%) as favorable features.
In 1999, den Tonkelaar and Oddens63 surveyed 1,300 Dutch women about their preferred frequency of menstruation and found that about 70% between the ages of 15 and 49 preferred a frequency of between every 3 months and never. A similar survey in the United States indicated that 58% preferred a bleeding frequency of either every 3 months or never to more frequent periods.64
While patients find menstrual manipulation generally acceptable, clinician approval has been more varied. Loudon et al reported that “the doctors and nurses on the clinic staff were less enthusiastic about this regimen than the volunteers themselves.”6 In a survey of 222 clinicians,65 90% of responders reported ever having prescribed extended or continuous dosing regimens to adolescents, and 33% reported that extended cycles made up more than 10% of their total oral contraceptive prescriptions.
Myths and misperceptions about menstrual manipulation abound. Many clinicians believe that routine use of an extended or continuous oral contraceptive regimen is inadvisable, despite the lack of evidence to support this notion.66 Therefore, many care providers need more education about the practice and benefits of menstrual manipulation.
THE RIGHT METHOD FOR THE RIGHT PATIENT
Manipulation and suppression of menstruation through continuous or extended use of oral contraceptives or by other means may have a number of advantages to women, including fewer menstrual-related syndromes, reduced absenteeism from work or school, and greater overall satisfaction.
For women whose goal is to reduce but not necessarily to eliminate monthly bleeding, the cyclic use of estrogen-progestin contraception (rather than progestins alone or continuous use of combined hormonal preparations) is suggested.
Clinicians should not overestimate the risks of oral contraceptives and other hormonal methods, but rather educate themselves so that they can utilize menstrual manipulation safely to match the individual patient’s needs.
If they wish, women can have more control over when and if they menstruate. By using hormonal contraceptives in extended or continuous regimens, they can have their period less often, a practice called menstrual manipulation or menstrual suppression.
Actually, with the help of their clinicians, women have been doing this for years. But now that several products have been approved by the US Food and Drug Administration (FDA) specifically for use in extended or continuous regimens, the practice has become more widely accepted.
Reasons for suppressing menstrual flow range from avoiding bleeding during a particular event (eg, a wedding, graduation, or sports competition) to finding relief from dysmenorrhea or reducing or eliminating menstruation in the treatment of endometriosis, migraine, and other medical conditions exacerbated by hormonal changes around the time of menses.1 Alternatively, some women may practice menstrual manipulation for no other reason than to simply avoid menstruation.
MENSTRUAL DISORDERS ARE TROUBLESOME, COMMON
Each year in the United States, menstrual disorders such as dysmenorrhea (painful menstruation), menorrhagia (excessive or frequent menstruation), metrorrhagia (irregular menstruation), menometrorrhagia (excessive and irregular menstruation), and premenstrual syndrome affect nearly 2.5 million women age 18 to 50 years.2 Menstrual disorders are the leading cause of gynecologic morbidity in the United States, outnumbering adnexal masses (the second most common cause) by a factor of three.2 In addition, these disorders extend into the workplace, costing US industry about 8% of its total wage bill.3
A BRIEF HISTORY OF CONTRACEPTIVE DEVELOPMENT
The idea of using progestins for birth control was first advanced in the 1950s by Dr. Gregory Pincus, who proposed a regimen of 21 days of active drug followed by 7 drug-free days to allow withdrawal bleeding, mimicking the natural cycle.4 This “21/7” regimen was designed to follow the lunar cycle in the hope it would be, in the words of Dr. John Rock, “a morally permissible variant of the rhythm method,”5 thereby making it acceptable to women, clinicians, and the Catholic Church.
In 1977, Loudon et al6 reported the results of a study in which women took active pills for 84 days instead of 21 days, which reduced the frequency of menstruation to every 3 months. Since then, extending the active pills beyond 21 days to avoid menses and other hormone-withdrawal symptoms has become popular in clinical practice, and many studies have investigated the extended or continuous use of oral and other forms of contraception to delay menses.7–18
CURRENT METHODS OF MENSTRUAL MANIPULATION
A variety of available products prevent conception by altering the menstrual cycle:
- Oral estrogen-progestin contraceptive pills
- A drug-releasing intrauterine device
- Depot medroxyprogesterone acetate injections
- A contraceptive vaginal ring
- An implantable etonogestrel contraceptive.
Their use in menstrual manipulation is summarized in Table 1.
Oral contraceptive pills
The most common way to manipulate the menstrual cycle is to extend the time between hormone-free weeks in an oral contraceptive regimen.
If the patient is young, you can prescribe a monophasic 21/7 oral contraceptive and tell her to take one active pill every day for 21 days and then start a new pack and keep taking active pills for up to 84 consecutive days, skipping the placebo pills until she wants to have her menstrual period. She can choose which week to have it: if the scheduled 12th week of an extended-cycle oral contraceptive regimen is inconvenient, she can plan it for week 10, or week 9, or whichever week is convenient.
The rationale for using an 84-day (12-week) cycle is that it still provides four periods per year, alleviating fears of hypertrophic endometrium.19
In this scenario, unscheduled or breakthrough bleeding can be managed by taking a “double-up pill” from a spare pack on any day breakthrough bleeding occurs and until it resolves. Menstrual periods should not be planned for intervals shorter than 21 days, owing to the risk of ovulation. Missed days of pills or use of placebo pills should also not exceed 7 days to prevent escape ovulation. 20
In some women with endometriosis and other medical reasons, continuous oral contraception with no placebo week can be prescribed.
Unfortunately, the downside to suppressing withdrawal bleeding is unscheduled or “breakthrough” bleeding. The best way to treat this unscheduled bleeding is not known. Patients who are not sexually active can be reassured that the goal of an atrophic endometrium can still be achieved, with resultant pill amenorrhea (particularly useful for those with severe dysmenorrhea or other reasons to want to avoid flow). Patients could also try to manage flow by periodically taking a 3- to 5-day break from hormone-containing pills to allow flow. They can also try switching to another oral contraceptive that has a different progestin that would spiral the arterioles of the endometrium more tightly and thus more aggressively induce atrophy.13,17,21 For instance, levonorgestrel is 10 to 20 times more potent than norethindrone. Choosing a pill with a higher monophasic dosing of levonorgestrel or a similar progestin may minimize unscheduled bleeding.
Currently, several oral contraceptives are approved for use in an extended regimen.
Seasonale was the first oral contraceptive marketed in the United States with an extended active regimen.22 It comes in a pack of 84 pills containing ethinyl estradiol 0.03 mg and levonorgestrel 0.15 mg, plus 7 placebo pills.
Seasonique is similar to Seasonale, but instead of placebo pills it has seven pills that contain ethinyl estradiol 0.010 mg.
Lybrel is a low-dose combination containing ethinyl estradiol 0.02 mg and levonorgestrel 0.09 mg. Packaged as an entire year’s worth of active pills to be taken continuously for 365 days without a placebo phase or pillfree interval,23 it is the only FDA-approved continuous oral contraceptive available in the United States.
An intrauterine device
Intrauterine devices were originally developed as contraceptives. The addition of a progestin to these devices has been shown to reduce heavy menstrual bleeding by up to 90%.24,25
Mirena IUS, a levonorgestrel-releasing device, is the only medicated intrauterine device that is currently available in the United States. (“IUS” stands for “intrauterine system.”) It was recently approved by the FDA to treat heavy menstrual bleeding in women who use intrauterine contraception as their method of pregnancy prevention.26 About 50% of women who use this device develop amenorrhea within 6 months of insertion, while 25% report oligomenorrhea.27
The Mirena device can be left in the uterus for up to 5 years. It may be a good choice for inducing amenorrhea in women with hemostatic disorders or in whom estrogen either is contraindicated or causes health concerns.18 The copper intrauterine device (Paragard; Duramed Pharmaceuticals Inc., Pomona, NY) remains a viable option for those who cannot or do not tolerate hormonal therapy. However, Mirena may provide less unscheduled bleeding than the copper intrauterine device.
Depot medroxyprogesterone acetate injections
Depo-Provera (depot medroxyprogesterone acetate) injections are given at 90-day intervals. 28 This contraceptive method inhibits ovulation and decidualizes the endometrium, thereby reducing or eliminating uterine bleeding. 29
While new users may initially experience excessive prolonged bleeding (10 or more days) while shedding their existing lining, the rate of amenorrhea has been shown to increase over time as the lining atrophies.30 Thus, prolonged use of this agent reduces the frequency of menstruation as well as menstruation-related symptoms.
Depot medroxyprogesterone acetate is ideal for patients whose menstrual periods pose a significant hygiene problem (eg, developmentally challenged girls). In our experience, the injections can be given at shorter intervals to induce atrophy of the endometrium quickly. In this scenario, the clinician might give an injection every 4 to 6 weeks for two or three doses to induce amenorrhea and then return to every-12-week dosing.
The main risk when using medroxyprogesterone injections to induce amenorrhea is the potential for bone loss. Users of this method have been shown to have lower mean bone mineral density31–33 and significantly higher levels of biomarkers of bone formation and resorption32,34 than nonusers. However, these changes are similar to those seen in breastfeeding women,35 are reversible with cessation, 36 and are not associated with increased fracture risk.37 In adolescent girls, pregnancy poses similar risks to the bones, with longerterm consequences.
Medroxyprogesterone can also stimulate appetite, causing 10 to 20 kg of weight gain in adolescents and women who are already obese and have trouble with appetite regulation.38 Slender users tend not to gain weight, however.
Given this information, depot medroxyprogesterone acetate appears to be a cost-effective contraceptive option that should be considered in the context of the clinical situation and preference of each patient.
Transdermal contraceptive patch
Ortho Evra, a transdermal patch, is designed to deliver ethinyl estradiol 0.02 mg and norelgestromin 0.150 mg daily.39 It is usually applied weekly for 3 weeks, followed by a patch-free week to induce regular monthly withdrawal bleeding.
Extended use of the patch to manipulate menstruation is an off-label use. In the only trial evaluating extended use of the patch, amenorrhea occurred in 12% of users, but unscheduled bleeding and spotting were common. 16
Although there is some evidence that the long-term use of the patch may increase the risk of venous thromboembolism,40,41 the risk in women who use the patch has been found to be similar to that in women using an oral contraceptive.42 However, serum ethinyl estradiol levels have been found to be higher with the use of the weekly patch than with oral contraceptives or the contraceptive vaginal ring39; as a result, many physicians are hesitant to recommend its continuous use.
Pending further data about the safety profile of this contraceptive, the World Health Organization Medical Eligibility Criteria for Contraceptive Use suggest that the same guidelines for the prescription of combination oral contraceptives should also apply to the patch.43
Contraceptive vaginal ring
NuvaRing, a contraceptive vaginal ring, releases a daily dose of ethinyl estradiol 0.015 mg and etonogestrel 0.12 mg.10 It is inserted, left in for 21 days, and then removed and left out for 7 days, during which withdrawal bleeding occurs.10
Vaginal administration has been shown to allow low, continuous dosing, which results in more stable serum concentrations than with the patch or oral contraceptives.39 In the only trial comparing an extended vaginal ring regimen and the traditional 28-day regimen, extended use resulted in fewer overall days of bleeding than monthly use, but with more unscheduled spotting.15
The most common side effects include headache, vaginitis, and leukorrhea,44 but there is no evidence of bacteriologic or cytologic changes in the cervicovaginal epithelium, even with extended use.45,46
Etonogestrel implantable contraceptive
Implanon, a single-rod progestin implant, is available in the United States and elsewhere. It is placed subdermally in the inner upper arm and provides contraception for as long as 3 years.
Implanon contains 68 mg of the progestin etonogestrel, which it slowly releases over time, initially at 0.06 to 0.07 mg/day, decreasing to 0.035 to 0.045 mg/day at the end of the first year, to 0.03 to 0.04 mg/day at the end of the second year, and then to 0.025 to 0.03 mg/day at the end of the third year.47
The amount of vaginal bleeding associated with the use of the implant is generally modest, but the pattern tends to be unpredictable. 48 In addition, because amenorrhea is reported as a side effect in only 22% of women during the first 2 years of its use,48 the progestin implant is a less satisfactory means of menstrual suppression than the other methods discussed above.
BENEFITS OF MENSTRUAL MANIPULATION
Menstrual manipulation has a number of benefits in terms of both overall health and lifestyle.
For most women, using a long-acting hormonal contraceptive carries low risks and substantial health benefits. Women who take oral contraceptives are less likely to develop osteoporosis, ovarian or endometrial cancer, benign breast changes, or pelvic inflammatory disease. 49 Long-term use of an oral contraceptive can also preserve fertility by reducing and delaying the incidence of endometriosis,50 and is effective at treating acne vulgaris, which tends to be common among patients with polycystic ovary syndrome.51,52 In addition, this practice can be used to reduce overall blood loss, an application that is particularly important in women with a bleeding diathesis such as von Willebrand disease, who frequently suffer from menorrhagia.53
Reduced menstruation may also prove more convenient during particular occasions, such as vacations and athletic activities. Specifically, it may be useful to women serving in the military. In a study by Schneider et al,54 a cohort of 83 female cadets reported a significant perceived impact of premenstrual and menstrual-related symptoms on academic, physical, and military activities, as well as difficulties in obtaining, changing, and disposing of menstrual materials in a military setting. Likewise, reduced menstrual frequency or amenorrhea may play an important role in female athletes, who reportedly use oral contraceptives to control premenstrual symptoms, to protect bone health, and to manipulate the menstrual cycle in order to maximize performance.55
Adolescent girls are another group who may benefit from reduced or absent menses, once they have reached near-final height. By practicing menstrual suppression, girls can avoid dysmenorrhea and the inconvenience of menstruation during the school day, when their access to painkillers, sanitary pads or tampons, and a change of clothes may be limited. 56 Clinicians who discuss with teenage patients the benefits of innovative hormonal contraceptive schedules that reduce menstrual frequency may be able to improve the quality of life for these young women.
In a very short girl just after menarche, care must be taken not to start a hormonal method too early so as not to prematurely close epiphyses and stunt final height; after menarche, most girls still have 1 to 4 inches of potential growth. For a young lady 4 feet 11 inches tall, that extra inch may be important.
Finally, menstrual manipulation may also find a niche among the developmentally challenged. Women with cognitive impairment and physical disabilities may have difficulty with hygienic practice around menses. For a number of years, contraceptives have been used to manage menstrual hygiene in patients with catamenial (ie, menstrual) epilepsy, and to address caregiver concerns in women with severe mental retardation, with improved behavior noted in some patients.57–59 In this setting, an agent that suppresses menses and also provides contraception, especially for those girls and women at risk of abuse, may offer substantial benefits.
DISADVANTAGES OF MENSTRUAL MANIPULATION
Rates of adverse events and of discontinuation of extended and continuous oral contraceptive regimens are comparable with those reported for cyclic regimens, except for higher rates of breakthrough bleeding.
In a trial of continuous oral contraceptive use in more than 2,000 patients, 396 (18.5%) withdrew from the study as a result of bothersome uterine bleeding.60 However, while breakthrough bleeding often occurs during the first few months of extended oral contraceptive use, it usually decreases with each successive cycle of therapy and is comparable to that reported by patients on the conventional oral contraceptive regimen by the fourth extended cycle.12
CONTRACEPTIVE EFFICACY
The efficacy of extended and continuous oral contraceptive regimens is comparable with that of cyclic regimens.12,60,61 One reason for this may be better adherence to continuous regimens: women using this regimen have been shown to miss fewer pills than those on a cyclic regimen, especially during the critical first week of the pill pack.21
Several studies have shown that some women ovulate during the standard 21/7 oral contraceptive regimen even if they do not miss any pills or take pills off-schedule, putting them at greater risk of pregnancy.62 Large studies evaluating the efficacy of an extendedcycle regimen have shown a pregnancy rate during the 1-year study period that was either comparable with61 or lower than12,60 rates with standard regimens.
Heterosexual couples need to be advised to use condoms to further reduce the already low failure rate and to prevent sexually transmitted diseases.
ACCEPTABILITY OF MENSTRUAL MANIPULATION
Ever since the earliest trial of an extended oral contraceptive regimen, participants have expressed a favorable response to the resulting decrease in menstrual frequency; in the 1977 study by Loudon et al,6 patients on the extended regimen cited infrequent periods (82%), fewer menstrual problems (20%), and easier pill-taking (19%) as favorable features.
In 1999, den Tonkelaar and Oddens63 surveyed 1,300 Dutch women about their preferred frequency of menstruation and found that about 70% between the ages of 15 and 49 preferred a frequency of between every 3 months and never. A similar survey in the United States indicated that 58% preferred a bleeding frequency of either every 3 months or never to more frequent periods.64
While patients find menstrual manipulation generally acceptable, clinician approval has been more varied. Loudon et al reported that “the doctors and nurses on the clinic staff were less enthusiastic about this regimen than the volunteers themselves.”6 In a survey of 222 clinicians,65 90% of responders reported ever having prescribed extended or continuous dosing regimens to adolescents, and 33% reported that extended cycles made up more than 10% of their total oral contraceptive prescriptions.
Myths and misperceptions about menstrual manipulation abound. Many clinicians believe that routine use of an extended or continuous oral contraceptive regimen is inadvisable, despite the lack of evidence to support this notion.66 Therefore, many care providers need more education about the practice and benefits of menstrual manipulation.
THE RIGHT METHOD FOR THE RIGHT PATIENT
Manipulation and suppression of menstruation through continuous or extended use of oral contraceptives or by other means may have a number of advantages to women, including fewer menstrual-related syndromes, reduced absenteeism from work or school, and greater overall satisfaction.
For women whose goal is to reduce but not necessarily to eliminate monthly bleeding, the cyclic use of estrogen-progestin contraception (rather than progestins alone or continuous use of combined hormonal preparations) is suggested.
Clinicians should not overestimate the risks of oral contraceptives and other hormonal methods, but rather educate themselves so that they can utilize menstrual manipulation safely to match the individual patient’s needs.
- Association of Reproductive Health Professionals. Extended and continuous use of contraceptives to reduce menstruation. September 2004. http://www.arhp.org/publications-and-resources/clinical-proceedings/reduce-menses. Accessed May 17, 2010.
- Kjerulff KH, Erickson BA, Langenberg PW. Chronic gynecological conditions reported by US women: findings from the National Health Interview Survey, 1984 to 1992. Am J Public Health 1996; 86:195–199.
- Thomas SL, Ellertson C. Nuisance or natural and healthy: should monthly menstruation be optional for women? Lancet 2000; 355:922–924.
- Connell EB. Contraception in the prepill era. Contraception 1999; 59(suppl 1):7S–10S.
- Marks LV. Sexual chemistry: a history of the contraceptive pill. New Haven, CT: Yale University Press, 2001.
- Loudon NB, Foxwell M, Potts DM, Guild AL, Short RV. Acceptability of an oral contraceptive that reduces the frequency of menstruation: the tri-cycle pill regimen. Br Med J 1977; 2:487–490.
- Sulak PJ, Cressman BE, Waldrop E, Holleman S, Kuehl TJ. Extending the duration of active oral contraceptive pills to manage hormone withdrawal symptoms. Obstet Gynecol 1997; 89:179–183.
- Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception 1997; 56:341–352.
- Miller L, Notter KM. Menstrual reduction with extended use of combination oral contraceptive pills: randomized controlled trial. Obstet Gynecol 2001; 98:771–778.
- Mulders TM, Dieben TO. Use of the novel combined contraceptive vaginal ring NuvaRing for ovulation inhibition. Fertil Steril 2001; 75:865–870.
- Stanford JB, Mikolajczyk RT. Mechanisms of action of intrauterine devices: update and estimation of postfertilization effects. Am J Obstet Gynecol 2002; 187:1699–1708.
- Anderson FD, Hait H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception 2003; 68:89–96.
- Miller L, Hughes JP. Continuous combination oral contraceptive pills to eliminate withdrawal bleeding: a randomized trial. Obstet Gynecol 2003; 101:653–661.
- Sillem M, Schneidereit R, Heithecker R, Mueck AO. Use of an oral contraceptive containing drospirenone in an extended regimen. Eur J Contracept Reprod Health Care 2003; 8:162–169.
- Miller L, Verhoeven CH, Hout J. Extended regimens of the contraceptive vaginal ring: a randomized trial. Obstet Gynecol 2005; 106:473–482.
- Stewart FH, Kaunitz AM, Laguardia KD, Karvois DL, Fisher AC, Friedman AJ. Extended use of transdermal norelgestromin/ethinyl estradiol: a randomized trial. Obstet Gynecol 2005; 105:1389–1396.
- Sulak PJ, Kuehl TJ, Coffee A, Willis S. Prospective analysis of occurrence and management of breakthrough bleeding during an extended oral contraceptive regimen. Am J Obstet Gynecol 2006; 195:935–941.
- Lukes AS, Reardon B, Arepally G. Use of the levonorgestrel– releasing intrauterine system in women with hemostatic disorders. Fertil Steril 2008; 90:673–677.
- Anderson FD, Feldman R, Reape KZ. Endometrial effects of a 91-day extended-regimen oral contraceptive with low-dose estrogen in place of placebo. Contraception 2008; 77:91–96.
- Wright KP, Johnson JV. Evaluation of extended and continuous use oral contraceptives. Ther Clin Risk Manag 2008; 4:905–911.
- Edelman AB, Gallo MF, Jensen JT, Nichols MD, Schulz KF, Grimes DA. Continuous or extended cycle vs. cyclic use of combined oral contraceptives for contraception. Cochrane Database Syst Rev 2005; 3:CD004695.
- Sulak PJ, Kuehl TJ, Ortiz M, Shull BL. Acceptance of altering the standard 21-day/7-day oral contraceptive regimen to delay menses and reduce hormone withdrawal symptoms. Am J Obstet Gynecol 2002; 186:1142–1149.
- Turok D. The quest for better contraception: future methods. Obstet Gynecol Clin North Am 2007; 34:137–166.
- Bergqvist A, Rybo G. Treatment of menorrhagia with intrauterine release of progesterone. Br J Obstet Gynaecol 1983; 90:255–258.
- Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception 1994; 49:56–72.
- US Food and Drug Administration. FDA Approves Additional Use for IUD Mirena to Treat Heavy Menstrual Bleeding in IUD Users. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm184747.htm. Accessed May 17, 2010.
- Hidalgo M, Bahamondes L, Perrotti M, Diaz J, Dantas-Monteiro C, Petta C. Bleeding patterns and clinical performance of the levonorgestrel-releasing intrauterine system (Mirena) up to two years. Contraception 2002; 65:129–132.
- Schwallie PC, Assenzo JR. Contraceptive use—efficacy study utilizing medroxyprogesterone acetate administered as an intramuscular injection once every 90 days. Fertil Steril 1973; 24:331–339.
- Kaunitz AM. Injectable contraception. New and existing options. Obstet Gynecol Clin North Am 2000; 27:741–780.
- Mainwaring R, Hales HA, Stevenson K, et al. Metabolic parameter, bleeding, and weight changes in US women using progestin only contraceptives. Contraception 1995; 51:149–153.
- Curtis KM, Martins SL. Progestogen-only contraception and bone mineral density: a systematic review. Contraception 2006; 73:470–487.
- Shaarawy M, El-Mallah SY, Seoudi S, Hassan M, Mohsen IA. Effects of the long-term use of depot medroxyprogesterone acetate as hormonal contraceptive on bone mineral density and biochemical markers of bone remodeling. Contraception 2006; 74:297–302.
- Cromer BA, Bonny AE, Stager M, et al. Bone mineral density in adolescent females using injectable or oral contraceptives: a 24-month prospective study. Fertil Steril 2008; 90:2060–2067.
- Rome E, Ziegler J, Secic M, et al. Bone biochemical markers in adolescent girls using either depot medroxyprogesterone acetate or an oral contraceptive. J Pediatr Adolesc Gynecol 2004; 17:373–377.
- More C, Bettembuk P, Bhattoa HP, Balogh A. The effects of pregnancy and lactation on bone mineral density. Osteoporos Int 2001; 12:732–737.
- Kaunitz AM, Miller PD, Rice VM, Ross D, McClung MR. Bone mineral density in women aged 25-35 years receiving depot medroxyprogesterone acetate: recovery following discontinuation. Contraception 2006; 74:90–99.
- Guilbert ER, Brown JP, Kaunitz AM, et al. The use of depot-medroxyprogesterone acetate in contraception and its potential impact on skeletal health. Contraception 2009; 79:167–177.
- Bonny AE, Ziegler J, Harvey R, Debanne SM, Secic M, Cromer BA. Weight gain in obese and nonobese adolescent girls initiating depot medroxyprogesterone, oral contraceptive pills, or no hormonal contraceptive method. Arch Pediatr Adolesc Med 2006; 160:40–45.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005; 72:168–174.
- Douketis JD, Ginsberg JS, Holbrook A, Crowther M, Duku EK, Burrows RF. A reevaluation of the risk for venous thromboembolism with the use of oral contraceptives and hormone replacement therapy. Arch Intern Med 1997; 157:1522–1530.
- Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol 2007; 109:339–346.
- Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2007; 76:4–7.
- World Health Organization. Medical Eligibility Criteria for Contraceptive Use. 3rd ed. Geneva: Reproductive Health and Research, World Health Organization; 2004.
- Dieben TO, Roumen FJ, Apter D. Efficacy, cycle control, and user acceptability of a novel combined contraceptive vaginal ring. Obstet Gynecol 2002; 100:585–593.
- Davies GC, Feng LX, Newton JR, Dieben TO, Coelingh-Bennink HJ. The effects of a combined contraceptive vaginal ring releasing ethinyloestradiol and 3-ketodesogestrel on vaginal flora. Contraception 1992; 45:511–518.
- Roumen FJ, Boon ME, van Velzen D, Dieben TO, Coelingh Bennink HJ. The cervico-vaginal epithelium during 20 cycles’ use of a combined contraceptive vaginal ring. Hum Reprod 1996; 11:2443–2448.
- Wenzl R, van Beek A, Schnabel P, Huber J. Pharmacokinetics of etonogestrel released from the contraceptive implant Implanon. Contraception 1998; 58:283–288.
- Darney P, Patel A, Rosen K, Shapiro LS, Kaunitz AM. Safety and efficacy of a single-rod etonogestrel implant (Implanon): results from 11 international clinical trials. Fertil Steril 2009; 91:1646–1653.
- Jensen JT, Speroff L. Health benefits of oral contraceptives. Obstet Gynecol Clin North Am 2000; 27:705–721.
- Seracchioli R, Mabrouk M, Frascà C, et al. Long-term cyclic and continuous oral contraceptive therapy and endometrioma recurrence: a randomized controlled trial. Fertil Steril 2010; 93:52–56.
- Falsetti L, Dordoni D, Gastaldi C, Gastaldi A. A new association of ethinylestradiol (0.035 mg) cyproterone acetate (2 mg) in the therapy of polycystic ovary syndrome. Acta Eur Fertil 1986; 17:19–25.
- Koltun W, Lucky AW, Thiboutot D, et al. Efficacy and safety of 3 mg drospirenone/20 mcg ethinylestradiol oral contraceptive administered in 24/4 regimen in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled trial. Contraception 2008; 77:249–256.
- Kadir RA, Sabin CA, Pollard D, Lee CA, Economides DL. Quality of life during menstruation in patients with inherited bleeding disorders. Haemophilia 1998; 4:836–841.
- Schneider MB, Fisher M, Friedman SB, Bijur PE, Toffler AP. Menstrual and premenstrual issues in female military cadets: a unique population with significant concerns. J Pediatr Adolesc Gynecol 1999; 12:195–201.
- Bennell K, White S, Crossley K. The oral contraceptive pill: a revolution for sportswomen? Br J Sports Med 1999; 33:231–238.
- Kaplowitz PB, Oberfield SE. Reexamination of the age limit for defining when puberty is precocious in girls in the United States: implications for evaluation and treatment. Drug and Therapeutics and Executive Committees of the Lawson Wilkins Pediatric Endocrine Society. Pediatrics 1999; 104:936–941.
- Roxburgh DR, West MJ. The use of norethisterone to suppress menstruation in the intellectually severely retarded woman. Med J Aust 1973; 2:310–313.
- Egan TM, Siegert RJ, Fairley NA. Use of hormonal contraceptives in an institutional setting: reasons for use, consent and safety in women with psychiatric and intellectual disabilities. N Z Med J 1993; 106:338–341.
- Pillai M, O’Brien K, Hill E. The levonorgestrel intrauterine system (Mirena) for the treatment of menstrual problems in adolescents with medical disorders, or physical or learning disabilities. BJOG 2010; 117:216–221.
- Archer DF, Jensen JT, Johnson JV, Borisute H, Grubb GS, Constantine GD. Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results. Contraception 2006; 74:439–445.
- Kroll R, Reape KZ, Margolis M. The efficacy and safety of a low-dose, 91-day, extended-regimen oral contraceptive with continuous ethinyl estradiol. Contraception 2010; 81:41–48.
- Archer DF. Menstrual-cycle-related symptoms: a review of the rationale for continuous use of oral contraceptives. Contraception 2006; 74:359–366.
- den Tonkelaar I, Oddens BJ. Preferred frequency and characteristics of menstrual bleeding in relation to reproductive status, oral contraceptive use, and hormone replacement therapy use. Contraception 1999; 59:357–362.
- Edelman A, Lew R, Cwiak C, Nichols M, Jensen J. Acceptability of contraceptive-induced amenorrhea in a racially diverse group of US women. Contraception 2007; 75:450–453.
- Gerschultz KL, Sucato GS, Hennon TR, Murray PJ, Gold MA. Extended cycling of combined hormonal contraceptives in adolescents: physician views and prescribing practices. J Adolesc Health 2007; 40:151–157.
- Frankovich RJ, Lebrun CM. Menstrual cycle, contraception, and performance. Clin Sports Med 2000; 19:251–271.
- Speroff L, Darney PD. A Clinical Guide for Contraception. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2005.
- Kaunitz AM. Long-acting contraceptive options. Int J Fertil Menopausal Stud 1996; 41:69–76.
- US Food and Drug Administration. Guidance for Industry Labeling for Combined Oral Contraceptives, 2004. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm075075.pdfAccessed May 17, 2010.
- Association of Reproductive Health Professionals. Extended and continuous use of contraceptives to reduce menstruation. September 2004. http://www.arhp.org/publications-and-resources/clinical-proceedings/reduce-menses. Accessed May 17, 2010.
- Kjerulff KH, Erickson BA, Langenberg PW. Chronic gynecological conditions reported by US women: findings from the National Health Interview Survey, 1984 to 1992. Am J Public Health 1996; 86:195–199.
- Thomas SL, Ellertson C. Nuisance or natural and healthy: should monthly menstruation be optional for women? Lancet 2000; 355:922–924.
- Connell EB. Contraception in the prepill era. Contraception 1999; 59(suppl 1):7S–10S.
- Marks LV. Sexual chemistry: a history of the contraceptive pill. New Haven, CT: Yale University Press, 2001.
- Loudon NB, Foxwell M, Potts DM, Guild AL, Short RV. Acceptability of an oral contraceptive that reduces the frequency of menstruation: the tri-cycle pill regimen. Br Med J 1977; 2:487–490.
- Sulak PJ, Cressman BE, Waldrop E, Holleman S, Kuehl TJ. Extending the duration of active oral contraceptive pills to manage hormone withdrawal symptoms. Obstet Gynecol 1997; 89:179–183.
- Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception 1997; 56:341–352.
- Miller L, Notter KM. Menstrual reduction with extended use of combination oral contraceptive pills: randomized controlled trial. Obstet Gynecol 2001; 98:771–778.
- Mulders TM, Dieben TO. Use of the novel combined contraceptive vaginal ring NuvaRing for ovulation inhibition. Fertil Steril 2001; 75:865–870.
- Stanford JB, Mikolajczyk RT. Mechanisms of action of intrauterine devices: update and estimation of postfertilization effects. Am J Obstet Gynecol 2002; 187:1699–1708.
- Anderson FD, Hait H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception 2003; 68:89–96.
- Miller L, Hughes JP. Continuous combination oral contraceptive pills to eliminate withdrawal bleeding: a randomized trial. Obstet Gynecol 2003; 101:653–661.
- Sillem M, Schneidereit R, Heithecker R, Mueck AO. Use of an oral contraceptive containing drospirenone in an extended regimen. Eur J Contracept Reprod Health Care 2003; 8:162–169.
- Miller L, Verhoeven CH, Hout J. Extended regimens of the contraceptive vaginal ring: a randomized trial. Obstet Gynecol 2005; 106:473–482.
- Stewart FH, Kaunitz AM, Laguardia KD, Karvois DL, Fisher AC, Friedman AJ. Extended use of transdermal norelgestromin/ethinyl estradiol: a randomized trial. Obstet Gynecol 2005; 105:1389–1396.
- Sulak PJ, Kuehl TJ, Coffee A, Willis S. Prospective analysis of occurrence and management of breakthrough bleeding during an extended oral contraceptive regimen. Am J Obstet Gynecol 2006; 195:935–941.
- Lukes AS, Reardon B, Arepally G. Use of the levonorgestrel– releasing intrauterine system in women with hemostatic disorders. Fertil Steril 2008; 90:673–677.
- Anderson FD, Feldman R, Reape KZ. Endometrial effects of a 91-day extended-regimen oral contraceptive with low-dose estrogen in place of placebo. Contraception 2008; 77:91–96.
- Wright KP, Johnson JV. Evaluation of extended and continuous use oral contraceptives. Ther Clin Risk Manag 2008; 4:905–911.
- Edelman AB, Gallo MF, Jensen JT, Nichols MD, Schulz KF, Grimes DA. Continuous or extended cycle vs. cyclic use of combined oral contraceptives for contraception. Cochrane Database Syst Rev 2005; 3:CD004695.
- Sulak PJ, Kuehl TJ, Ortiz M, Shull BL. Acceptance of altering the standard 21-day/7-day oral contraceptive regimen to delay menses and reduce hormone withdrawal symptoms. Am J Obstet Gynecol 2002; 186:1142–1149.
- Turok D. The quest for better contraception: future methods. Obstet Gynecol Clin North Am 2007; 34:137–166.
- Bergqvist A, Rybo G. Treatment of menorrhagia with intrauterine release of progesterone. Br J Obstet Gynaecol 1983; 90:255–258.
- Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception 1994; 49:56–72.
- US Food and Drug Administration. FDA Approves Additional Use for IUD Mirena to Treat Heavy Menstrual Bleeding in IUD Users. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm184747.htm. Accessed May 17, 2010.
- Hidalgo M, Bahamondes L, Perrotti M, Diaz J, Dantas-Monteiro C, Petta C. Bleeding patterns and clinical performance of the levonorgestrel-releasing intrauterine system (Mirena) up to two years. Contraception 2002; 65:129–132.
- Schwallie PC, Assenzo JR. Contraceptive use—efficacy study utilizing medroxyprogesterone acetate administered as an intramuscular injection once every 90 days. Fertil Steril 1973; 24:331–339.
- Kaunitz AM. Injectable contraception. New and existing options. Obstet Gynecol Clin North Am 2000; 27:741–780.
- Mainwaring R, Hales HA, Stevenson K, et al. Metabolic parameter, bleeding, and weight changes in US women using progestin only contraceptives. Contraception 1995; 51:149–153.
- Curtis KM, Martins SL. Progestogen-only contraception and bone mineral density: a systematic review. Contraception 2006; 73:470–487.
- Shaarawy M, El-Mallah SY, Seoudi S, Hassan M, Mohsen IA. Effects of the long-term use of depot medroxyprogesterone acetate as hormonal contraceptive on bone mineral density and biochemical markers of bone remodeling. Contraception 2006; 74:297–302.
- Cromer BA, Bonny AE, Stager M, et al. Bone mineral density in adolescent females using injectable or oral contraceptives: a 24-month prospective study. Fertil Steril 2008; 90:2060–2067.
- Rome E, Ziegler J, Secic M, et al. Bone biochemical markers in adolescent girls using either depot medroxyprogesterone acetate or an oral contraceptive. J Pediatr Adolesc Gynecol 2004; 17:373–377.
- More C, Bettembuk P, Bhattoa HP, Balogh A. The effects of pregnancy and lactation on bone mineral density. Osteoporos Int 2001; 12:732–737.
- Kaunitz AM, Miller PD, Rice VM, Ross D, McClung MR. Bone mineral density in women aged 25-35 years receiving depot medroxyprogesterone acetate: recovery following discontinuation. Contraception 2006; 74:90–99.
- Guilbert ER, Brown JP, Kaunitz AM, et al. The use of depot-medroxyprogesterone acetate in contraception and its potential impact on skeletal health. Contraception 2009; 79:167–177.
- Bonny AE, Ziegler J, Harvey R, Debanne SM, Secic M, Cromer BA. Weight gain in obese and nonobese adolescent girls initiating depot medroxyprogesterone, oral contraceptive pills, or no hormonal contraceptive method. Arch Pediatr Adolesc Med 2006; 160:40–45.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005; 72:168–174.
- Douketis JD, Ginsberg JS, Holbrook A, Crowther M, Duku EK, Burrows RF. A reevaluation of the risk for venous thromboembolism with the use of oral contraceptives and hormone replacement therapy. Arch Intern Med 1997; 157:1522–1530.
- Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol 2007; 109:339–346.
- Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2007; 76:4–7.
- World Health Organization. Medical Eligibility Criteria for Contraceptive Use. 3rd ed. Geneva: Reproductive Health and Research, World Health Organization; 2004.
- Dieben TO, Roumen FJ, Apter D. Efficacy, cycle control, and user acceptability of a novel combined contraceptive vaginal ring. Obstet Gynecol 2002; 100:585–593.
- Davies GC, Feng LX, Newton JR, Dieben TO, Coelingh-Bennink HJ. The effects of a combined contraceptive vaginal ring releasing ethinyloestradiol and 3-ketodesogestrel on vaginal flora. Contraception 1992; 45:511–518.
- Roumen FJ, Boon ME, van Velzen D, Dieben TO, Coelingh Bennink HJ. The cervico-vaginal epithelium during 20 cycles’ use of a combined contraceptive vaginal ring. Hum Reprod 1996; 11:2443–2448.
- Wenzl R, van Beek A, Schnabel P, Huber J. Pharmacokinetics of etonogestrel released from the contraceptive implant Implanon. Contraception 1998; 58:283–288.
- Darney P, Patel A, Rosen K, Shapiro LS, Kaunitz AM. Safety and efficacy of a single-rod etonogestrel implant (Implanon): results from 11 international clinical trials. Fertil Steril 2009; 91:1646–1653.
- Jensen JT, Speroff L. Health benefits of oral contraceptives. Obstet Gynecol Clin North Am 2000; 27:705–721.
- Seracchioli R, Mabrouk M, Frascà C, et al. Long-term cyclic and continuous oral contraceptive therapy and endometrioma recurrence: a randomized controlled trial. Fertil Steril 2010; 93:52–56.
- Falsetti L, Dordoni D, Gastaldi C, Gastaldi A. A new association of ethinylestradiol (0.035 mg) cyproterone acetate (2 mg) in the therapy of polycystic ovary syndrome. Acta Eur Fertil 1986; 17:19–25.
- Koltun W, Lucky AW, Thiboutot D, et al. Efficacy and safety of 3 mg drospirenone/20 mcg ethinylestradiol oral contraceptive administered in 24/4 regimen in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled trial. Contraception 2008; 77:249–256.
- Kadir RA, Sabin CA, Pollard D, Lee CA, Economides DL. Quality of life during menstruation in patients with inherited bleeding disorders. Haemophilia 1998; 4:836–841.
- Schneider MB, Fisher M, Friedman SB, Bijur PE, Toffler AP. Menstrual and premenstrual issues in female military cadets: a unique population with significant concerns. J Pediatr Adolesc Gynecol 1999; 12:195–201.
- Bennell K, White S, Crossley K. The oral contraceptive pill: a revolution for sportswomen? Br J Sports Med 1999; 33:231–238.
- Kaplowitz PB, Oberfield SE. Reexamination of the age limit for defining when puberty is precocious in girls in the United States: implications for evaluation and treatment. Drug and Therapeutics and Executive Committees of the Lawson Wilkins Pediatric Endocrine Society. Pediatrics 1999; 104:936–941.
- Roxburgh DR, West MJ. The use of norethisterone to suppress menstruation in the intellectually severely retarded woman. Med J Aust 1973; 2:310–313.
- Egan TM, Siegert RJ, Fairley NA. Use of hormonal contraceptives in an institutional setting: reasons for use, consent and safety in women with psychiatric and intellectual disabilities. N Z Med J 1993; 106:338–341.
- Pillai M, O’Brien K, Hill E. The levonorgestrel intrauterine system (Mirena) for the treatment of menstrual problems in adolescents with medical disorders, or physical or learning disabilities. BJOG 2010; 117:216–221.
- Archer DF, Jensen JT, Johnson JV, Borisute H, Grubb GS, Constantine GD. Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results. Contraception 2006; 74:439–445.
- Kroll R, Reape KZ, Margolis M. The efficacy and safety of a low-dose, 91-day, extended-regimen oral contraceptive with continuous ethinyl estradiol. Contraception 2010; 81:41–48.
- Archer DF. Menstrual-cycle-related symptoms: a review of the rationale for continuous use of oral contraceptives. Contraception 2006; 74:359–366.
- den Tonkelaar I, Oddens BJ. Preferred frequency and characteristics of menstrual bleeding in relation to reproductive status, oral contraceptive use, and hormone replacement therapy use. Contraception 1999; 59:357–362.
- Edelman A, Lew R, Cwiak C, Nichols M, Jensen J. Acceptability of contraceptive-induced amenorrhea in a racially diverse group of US women. Contraception 2007; 75:450–453.
- Gerschultz KL, Sucato GS, Hennon TR, Murray PJ, Gold MA. Extended cycling of combined hormonal contraceptives in adolescents: physician views and prescribing practices. J Adolesc Health 2007; 40:151–157.
- Frankovich RJ, Lebrun CM. Menstrual cycle, contraception, and performance. Clin Sports Med 2000; 19:251–271.
- Speroff L, Darney PD. A Clinical Guide for Contraception. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2005.
- Kaunitz AM. Long-acting contraceptive options. Int J Fertil Menopausal Stud 1996; 41:69–76.
- US Food and Drug Administration. Guidance for Industry Labeling for Combined Oral Contraceptives, 2004. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm075075.pdfAccessed May 17, 2010.
KEY POINTS
- The options for menstrual manipulation are extended or continuous regimens of oral, transdermal, or vaginal hormonal contraceptives; a levonorgestrel-releasing intrauterine device; a progestin implant; and depot medroxyprogesterone injections.
- Benefits include fewer menstrual-related syndromes, less absenteeism from work or school, and greater overall satisfaction. Medical indications for it are conditions exacerbated by hormonal changes around the time of menses.
- The main disadvantage is a higher rate of breakthrough bleeding.
- Myths and misperceptions about menstrual manipulation persist; some physicians believe it is somehow inadvisable.