User login
Vaping: The new wave of nicotine addiction
Electronic cigarettes and other “vaping” devices have been increasing in popularity among youth and adults since their introduction in the US market in 2007.1 This increase is partially driven by a public perception that vaping is harmless, or at least less harmful than cigarette smoking.2 Vaping fans also argue that current smokers can use vaping as nicotine replacement therapy to help them quit smoking.3
We disagree. Research on the health effects of vaping, though still limited, is accumulating rapidly and making it increasingly clear that this habit is far from harmless. For youth, it is a gateway to addiction to nicotine and other substances. Whether it can help people quit smoking remains to be seen. And recent months have seen reports of serious respiratory illnesses and even deaths linked to vaping.4
In December 2016, the US Surgeon General warned that e-cigarette use among youth and young adults in the United States represents a “major public health concern,”5 and that more adolescents and young adults are now vaping than smoking conventional tobacco products.
This article reviews the issue of vaping in the United States, as well as available evidence regarding its safety.
YOUTH AT RISK
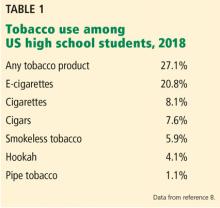
Retail sales of e-cigarettes and vaping devices approach an annual $7 billion.6 A 2014–2015 survey found that 2.4% of the general US population were current users of e-cigarettes, and 8.5% had tried them at least once.3
In 2014, for the first time, e-cigarette use became more common among US youth than traditional cigarettes.5
The odds of taking up vaping are higher among minority youth in the United States, particularly Hispanics.9 This trend is particularly worrisome because several longitudinal studies have shown that adolescents who use e-cigarettes are 3 times as likely to eventually become smokers of traditional cigarettes compared with adolescents who do not use e-cigarettes.10–12
If US youth continue smoking at the current rate, 5.6 million of the current population under age 18, or 1 of every 13, will die early of a smoking-related illness.13
RECENT OUTBREAK OF VAPING-ASSOCIATED LUNG INJURY
As of November 5, 2019, there had been 2,051 cases of vaping-associated lung injury in 49 states (all except Alaska), the District of Columbia, and 1 US territory reported to the US Centers for Disease Control and Prevention (CDC), with 39 confirmed deaths.4 The reported cases include respiratory injury including acute eosinophilic pneumonia, organizing pneumonia, acute respiratory distress syndrome, and hypersensitivity pneumonitis.14
Most of these patients had been vaping tetrahydrocannabinol (THC), though many used both nicotine- and THC-containing products, and others used products containing nicotine exclusively.4 Thus, it is difficult to identify the exact substance or substances that may be contributing to this sudden outbreak among vape users, and many different product sources are currently under investigation.
One substance that may be linked to the epidemic is vitamin E acetate, which the New York State Department of Health has detected in high levels in cannabis vaping cartridges used by patients who developed lung injury.15 The US Food and Drug Administration (FDA) is continuing to analyze vape cartridge samples submitted by affected patients to look for other chemicals that can contribute to the development of serious pulmonary illness.
WHAT IS AN E-CIGARETTE? WHAT IS A VAPE PEN?
Vape pens consist of similar elements but are not necessarily similar in appearance to a conventional cigarette, and may look more like a pen or a USB flash drive. In fact, the Juul device is recharged by plugging it into a USB port.
Vaping devices have many street names, including e-cigs, e-hookahs, vape pens, mods, vapes, and tank systems.
The first US patent application for a device resembling a modern e-cigarette was filed in 1963, but the product never made it to the market.16 Instead, the first commercially successful e-cigarette was created in Beijing in 2003 and introduced to US markets in 2007.
Newer-generation devices have larger batteries and can heat the liquid to higher temperatures, releasing more nicotine and forming additional toxicants such as formaldehyde. Devices lack standardization in terms of design, capacity for safely holding e-liquid, packaging of the e-liquid, and features designed to minimize hazards of use.
Not just nicotine
Many devices are designed for use with other drugs, including THC.17 In a 2018 study, 10.9% of college students reported vaping marijuana in the past 30 days, up from 5.2% in 2017.18
Other substances are being vaped as well.19 In theory, any heat-stable psychoactive recreational drug could be aerosolized and vaped. There are increasing reports of e-liquids containing recreational drugs such as synthetic cannabinoid receptor agonists, crack cocaine, LSD, and methamphetamine.17
Freedom, rebellion, glamour
Sales have risen rapidly since 2007 with widespread advertising on television and in print publications for popular brands, often featuring celebrities.20 Spending on advertising for e-cigarettes and vape devices rose from $6.4 million in 2011 to $115 million in 2014—and that was before the advent of Juul (see below).21
Marketing campaigns for vaping devices mimic the themes previously used successfully by the tobacco industry, eg, freedom, rebellion, and glamour. They also make unsubstantiated claims about health benefits and smoking cessation, though initial websites contained endorsements from physicians, similar to the strategies of tobacco companies in old cigarette ads. Cigarette ads have been prohibited since 1971—but not e-cigarette ads. Moreover, vaping products appear as product placements in television shows and movies, with advocacy groups on social media.22
By law, buyers have to be 18 or 21
Vaping devices can be purchased at vape shops, convenience stores, gas stations, and over the Internet; up to 50% of sales are conducted online.24
Fruit flavors are popular
Zhu et al25 estimated that 7,700 unique vaping flavors exist, with fruit and candy flavors predominating. The most popular flavors are tobacco and mint, followed by fruit, dessert and candy flavors, alcoholic flavors (strawberry daiquiri, margarita), and food flavors.25 These flavors have been associated with higher usage in youth, leading to increased risk of nicotine addiction.26
WHAT IS JUUL?
The Juul device (Juul Labs, www.juul.com) was developed in 2015 by 2 Stanford University graduates. Their goal was to produce a more satisfying and cigarette-like vaping experience, specifically by increasing the amount of nicotine delivered while maintaining smooth and pleasant inhalation. They created an e-liquid that could be vaporized effectively at lower temperatures.27
While more than 400 brands of vaping devices are currently available in the United States,3 Juul has held the largest market share since 2017,28 an estimated 72.1% as of August 2018.29 The surge in popularity of this particular brand is attributed to its trendy design that is similar in size and appearance to a USB flash drive,29 and its offering of sweet flavors such as “crème brûlée” and “cool mint.”
On April 24, 2018, in view of growing concern about the popularity of Juul products among youth, the FDA requested that the company submit documents regarding its marketing tactics, as well as research on the effects of this marketing on product design and public health impact, and information about adverse experiences and complaints.30 The company was forced to change its marketing to appeal less to youth. Now it offers only 3 flavors: “Virginia tobacco,” “classic tobacco,” and “menthol,” although off-brand pods containing a variety of flavors are still available. And some pods are refillable, so users can essentially vape any substance they want.
Although the Juul device delivers a strong dose of nicotine, it is small and therefore easy to hide from parents and teachers, and widespread use has been reported among youth in middle and high schools. Hoodies, hemp jewelry, and backpacks have been designed to hide the devices and allow for easy, hands-free use. YouTube searches for terms such as “Juul,” “hiding Juul at school,” and “Juul in class,” yield thousands of results.31 A 2017 survey reported that 8% of Americans age 15 to 24 had used Juul in the month prior to the survey.32 “To juul” has become a verb.
Each Juul starter kit contains the rechargeable inhalation device plus 4 flavored pods. In the United States, each Juul pod contains nearly as much nicotine as 1 pack of 20 cigarettes in a concentration of 3% or 5%. (Israel and Europe have forced the company to replace the 5% nicotine pods with 1.7% nicotine pods.33) A starter kit costs $49.99, and additional packs of 4 flavored liquid cartridges or pods cost $15.99.34 Other brands of vape pens cost between $15 and $35, and 10-mL bottles of e-liquid cost approximately $7.
What is ‘dripping’?
Hard-core vapers seeking a more intense experience are taking their vaping devices apart and modifying them for “dripping,” ie, directly dripping vape liquids onto the heated coils for inhalation. In a survey, 1 in 4 high school students using vape devices also used them for dripping, citing desires for a thicker cloud of vapor, more intense flavor, “a stronger throat hit,” curiosity, and other reasons.35 Dripping involves higher temperatures, which leads to higher amounts of nicotine delivered, along with more formaldehyde, acetaldehyde, and acetone (see below).36
BAD THINGS IN E-LIQUID AEROSOL
Studies of vape liquids consistently confirm the presence of toxic substances in the resulting vape aerosol.37–40 Depending on the combination of flavorings and solvents in a given e-liquid, a variety of chemicals can be detected in the aerosol from various vaping devices. Chemicals that may be detected include known irritants of respiratory mucosa, as well as various carcinogens. The list includes:
- Organic volatile compounds such as propylene glycol, glycerin, and toluene
- Aldehydes such as formaldehyde (released when propylene glycol is heated to high temperatures), acetaldehyde, and benzaldehyde
- Acetone and acrolein
- Carcinogenic nitrosamines
- Polycyclic aromatic hydrocarbons
- Particulate matter
- Metals including chromium, cadmium, nickel, and lead; and particles of copper, nickel, and silver have been found in electronic nicotine delivery system aerosol in higher levels than in conventional cigarette smoke.41
The specific chemicals detected can vary greatly between brands, even when the flavoring and nicotine content are equivalent, which frequently results in inconsistent and conflicting study findings. The chemicals detected also vary with the voltage or power used to generate the aerosol. Different flavors may carry varying levels of risk; for example, mint- and menthol-flavored e-cigarettes were shown to expose users to dangerous levels of pulegone, a carcinogenic compound banned as a food additive in 2018.42 The concentrations of some of these chemicals are sufficiently high to be of toxicologic concern; for example, one study reported the presence of benzaldehyde in e-cigarette aerosol at twice the workplace exposure limit.43
Biologic effects
In an in vitro study,44 57% of e-liquids studied were found to be cytotoxic to human pulmonary fibroblasts, lung epithelial cells, and human embryonic stem cells. Fruit-flavored e-liquids in particular caused a significant increase in DNA fragmentation. Cell cultures treated with e-cigarette liquids showed increased oxidative stress, reduced cell proliferation, and increased DNA damage,44 which may have implications for carcinogenic risk.
In another study,45 exposure to e-cigarette aerosol as well as conventional cigarette smoke resulted in suppression of genes related to immune and inflammatory response in respiratory epithelial cells. All genes with decreased expression after exposure to conventional cigarette smoke also showed decreased expression with exposure to e-cigarette smoke, which the study authors suggested could lead to immune suppression at the level of the nasal mucosa. Diacetyl and acetoin, chemicals found in certain flavorings, have been linked to bronchiolitis obliterans, or “popcorn lung.”46
Nicotine is not benign
The nicotine itself in many vaping liquids should also not be underestimated. Nicotine has harmful neurocognitive effects and addictive properties, particularly in the developing brains of adolescents and young adults.47 Nicotine exposure during adolescence negatively affects memory, attention, and emotional regulation,48 as well as executive functioning, reward processing, and learning.49
The brain undergoes major structural remodeling in adolescence, and nicotine acetylcholine receptors regulate neural maturation. Early exposure to nicotine disrupts this process, leading to poor executive functioning, difficulty learning, decreased memory, and issues with reward processing.
Fetal exposure, if nicotine products are used during pregnancy, has also been linked to adverse consequences such as deficits in attention and cognition, behavioral effects, and sudden infant death syndrome.5
Much to learn about toxicity
Partly because vaping devices have been available to US consumers only since 2007, limited evidence is available regarding the long-term effects of exposure to the aerosol from these devices in humans.1 Many of the studies mentioned above were in vitro studies or conducted in mouse models. Differences in device design and the composition of the e-liquid among device brands pose a challenge for developing well-designed studies of the long-term health effects of e-cigarette and vape use. Additionally, devices may have different health impacts when used to vape cannabis or other drugs besides nicotine, which requires further investigation.
E-CIGARETTES AND SMOKING CESSATION
Conventional cigarette smoking is a major public health threat, as tobacco use is responsible for 480,000 deaths annually in the United States.50
And smoking is extremely difficult to quit: as many as 80% of smokers who attempt to quit resume smoking within the first month.51 The chance of successfully quitting improves by over 50% if the individual undergoes nicotine replacement therapy, and it improves even more with counseling.50
There are currently 5 types of FDA-approved nicotine replacement therapy products (gum, patch, lozenge, inhaler, nasal spray) to help with smoking cessation. In addition, 2 non-nicotine prescription drugs (varenicline and bupropion) have been approved for treating tobacco dependence.
Can vaping devices be added to the list of nicotine replacement therapy products? Although some manufacturers try to brand their devices as smoking cessation aids, in one study,52 one-third of e-cigarette users said they had either never used conventional cigarettes or had formerly smoked them.
Bullen et al53 randomized smokers interested in quitting to receive either e-cigarettes, nicotine patches, or placebo (nicotine-free) e-cigarettes and followed them for 6 months. Rates of tobacco cessation were less than predicted for the entire study population, resulting in insufficient power to determine the superiority of any single method, but the study authors concluded that nicotine e-cigarettes were “modestly effective” at helping smokers quit, and that abstinence rates may be similar to those with nicotine patches.53
Hajek et al54 randomized 886 smokers to e-cigarette or nicotine replacement products of their choice. After 1 year, 18% of e-cigarette users had stopped smoking, compared with 9.9% of nicotine replacement product users. However, 80% of the e-cigarette users were still using e-cigarettes after 1 year, while only 9% of nicotine replacement product users were still using nicotine replacement therapy products after 1 year.
While quitting conventional cigarette smoking altogether has widely established health benefits, little is known about the health benefits of transitioning from conventional cigarette smoking to reduced conventional cigarette smoking with concomitant use of e-cigarettes.
Campagna et al55 found no beneficial health effects in smokers who partially substituted conventional cigarettes for e-cigarettes.
Many studies found that smokers use e-cigarettes to maintain their habit instead of quitting entirely.56 It has been suggested that any slight increase in effectiveness in smoking cessation by using e-cigarettes compared with other nicotine replacement products could be linked to satisfying of the habitual smoking actions, such as inhaling and bringing the hand to the mouth,24 which are absent when using other nicotine replacement methods such as a nicotine patch.
As with safety information, long-term outcomes regarding the use of vape devices for smoking cessation have not been yet established, as this option is still relatively new.
VAPING AS A GATEWAY DRUG
Another worrisome trend involving electronic nicotine delivery systems is their marketing and branding, which appear to be aimed directly at adolescents and young adults. Juul and other similar products cannot be sold to anyone under the age of 18 (or 21 in 18 states, including California, Massachusetts, New York, and now Ohio). Despite this, Juul and similar products continue to increase in popularity among middle school and high school students.57
While smoking cessation and health improvement are cited as reasons for vaping among middle-aged and older adults, adolescents and young adults more often cite flavor, enjoyment, peer use, and curiosity as reasons for use.
Adolescents are more likely to report interest in trying a vape product flavored with menthol or fruit than tobacco, and commonly hold the belief that fruit-flavored e-cigarettes are less harmful than tobacco-flavored e-cigarettes.58 Harrell et al59 polled youth and young adults who used flavored e-cigarettes, and 78% said they would no longer use the product if their preferred flavor were not available. In September 2019, Michigan became the first state to ban the sale of flavored e-cigarettes in stores and online. Similar bills have been introduced in California, Massachusetts, and New York.60
Myths and misperceptions abound among youth regarding smoking vs vaping. Young people view regular cigarette smoking negatively, as causing cancer, bad breath, and asthma exacerbations. Meanwhile, they believe marijuana is safer and less addictive than traditional cigarette smoking.61 Youth exposed to e-cigarette advertisements viewed e-cigarettes as healthier, more enjoyable, “cool,” safe, and fun.61 The overall public health impact of increasing initiation of smoking, particularly among youth and young adults, should not be underestimated.
SECONDHAND VAPE AND OTHER EXPOSURE RISKS
Cigarette smoking has been banned in many public places, in view of a large body of scientific evidence about the harmful effects of secondhand smoke. Advocates for allowing vaping in public places say that vaping emissions do not harm bystanders, but evidence is insufficient to support this claim.62 One study showed that passive exposure to e-cigarette aerosol generated increases in serum levels of cotinine (a nicotine metabolite) similar to those with passive exposure to conventional cigarette smoke.5
Accidental nicotine poisoning in children as a result of ingesting e-cigarette liquid is also a major concern,63 particularly with sweet flavors such as bubblegum or cheesecake that may be attractive to children.
Calls to US poison control centers with respect to e-cigarettes and vaping increased from 1 per month in September 2010 to 215 in February 2014, with 51% involving children under age 5.64 This trend resulted in the Child Nicotine Poisoning Prevention Act, which passed in 2015 and went into effect in 2016, requiring packaging that is difficult to open for children under age 5.5
Device malfunctions or battery failures have led to explosions that have resulted in substantial injuries to users, as well as house and car fires.49
HOW DO WE DISCOURAGE ADOLESCENT USE?
There are currently no established treatment approaches for adolescents who have become addicted to vaping. A review of the literature regarding treatment modalities used to address adolescent use of tobacco and marijuana provides insight that options such as nicotine replacement therapy and counseling modalities such as cognitive behavioral therapy may be helpful in treating teen vaping addiction. However, more research is needed to determine the effectiveness of these treatments in youth addicted to vaping.
Given that youth who vape even once are more likely to try other types of tobacco, we recommend that parents and healthcare providers start conversations by asking what the young person has seen or heard about vaping. Young people can also be asked what they think the school’s response should be: Do they think vaping should be banned in public places, as cigarettes have been banned? What about the carbon footprint? What are their thoughts on the plastic waste, batteries, and other toxins generated by the e-cigarette industry?
New US laws ban the sale of e-cigarettes and vaping devices to minors in stores and online. These policies are modeled in many cases on environmental control policies that have been previously employed to reduce tobacco use, particularly by youth. For example, changing laws to mandate sales only to individuals age 21 and older in all states can help to decrease access to these products among middle school and high school students.
As with tobacco cessation, education will not be enough. Support of legislation that bans vaping in public places, increases pricing to discourage adolescent use, and other measures used successfully to decrease conventional cigarette smoking can be deployed to decrease the public health impact of e-cigarettes. We recommend further regulation of specific harmful chemicals and clear, detailed ingredient labeling to increase consumer understanding of the risks associated with these products. Additionally, we recommend eliminating flavored e-cigarettes, which are the most appealing type for young users, and raising prices of e-cigarettes and similar products to discourage use by youth.
If current cigarette smokers want to use e-cigarettes to quit, we recommend that clinicians counsel them to eventually completely stop use of traditional cigarettes and switch to using e-cigarettes, instead of becoming a dual user of both types of products or using e-cigarettes indefinitely. After making that switch, they should then work to gradually taper usage and nicotine addiction by reducing the amount of nicotine in the e-liquid. Clinicians should ask patients about use of e-cigarettes and vaping devices specifically, and should counsel nonsmokers to avoid initiation of use.
EVIDENCE OF HARM CONTINUES TO EMERGE
Data about respiratory effects, secondhand exposure, and long-term smoking cessation efficacy are still limited, and it remains as yet unknown what combinations of solvents, flavorings, and nicotine in a given e-liquid will result in the most harmful or least harmful effects. In addition, while much of the information about the safety of these components has been obtained using in vitro or mouse models, increasing reports of serious respiratory illness and rising numbers of deaths linked to vaping make it clear that these findings likely translate to harmful effects in humans.
E-cigarettes may ultimately prove to be less harmful than traditional cigarettes, but it seems likely that with further time and research, serious health risks of e-cigarette use will continue to emerge.
- Sood A, Kesic M, Hernandez M. Electronic cigarettes: one size does not fit all. J Allergy Clin Immunol 2018; 141(6):1973-1982. doi:10.1016/j.jaci.2018.02.029
- Romijnders K, van Osch L, de Vries H, Talhout R. Perceptions and reasons regarding e-cigarette use among users and non-users: a narrative literature review. Int J Environ Res Public Health 2018; 15(6):1190. doi:10.3390/ijerph15061190
- Zhu S, Zhuang Y-L, Wong S, Cummins SE, Tedeschi GJ. E-cigarette use and associated changes in population smoking cessation: evidence from US current population surveys. BMJ 2017; 358:j3262. doi:10.1136/bmj.j3262
- Centers for Disease Control and Prevention. Outbreak of lung injury associated with e-cigarette use, or vaping. Updated November 5, 2019. www.cdc.gov/tobacco/basic_information/e-Cigarettes/severe-Lung-Disease.html. Accessed November 14, 2019.
- US Public Health Services, US Department of Health and Human Services. E-cigarette use among youth and young adults: a report of the Surgeon General. Rockville, MD, U.S. Department of Health and Human Services, 2016. https://e-cigarettes.surgeongeneral.gov/documents/2016_sgr_full_report_non-508.pdf. Accessed November 14, 2019.
- Thomas K, Kaplan S. E-cigarettes went unchecked in 10 years of federal inaction. New York Times Oct 14, 2019; updated November 1, 2019. www.nytimes.com/2019/10/14/health/vaping-e-cigarettes-fda.html.
- Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA. Notes from the field: use of electronic cigarettes and any tobacco product among middle and high school students—United States, 2011–2018. MMWR Morb Mortal Wkly Rep 2018; 67(45):1276–1277. doi:10.15585/mmwr.mm6745a5
- Gentzke A, Creamer M, Cullen K, et al; Centers for Disease Control and Prevention. Vital signs: tobacco product use among middle and high school students—United States, 2011–2018. MMWR Morb Mortal Wkly Rep 2019; 68(6):157–164. doi:10.15585/mmwr.mm6806e1
- Hammig B, Daniel-Dobbs P, Blunt-Vinti H. Electronic cigarette initiation among minority youth in the United States. Am J Drug Alcohol Abuse 2017; 43(3):306–310. doi:10.1080/00952990.2016.1203926
- Primack BA, Soneji S, Stoolmiller M, Fine MJ, Sargent JD. Progression to traditional cigarette smoking after electronic cigarette use among U.S. adolescents and young adults. JAMA Pediatr 2015; 169(11):1018–1023. doi:10.1001/jamapediatrics.2015.1742
- Leventhal AM, Strong DR, Kirkpatrick MG, et al. Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence. JAMA 2015; 314(7):700–707. doi:10.1001/jama.2015.8950
- Wills TA, Knight R, Sargent JD, Gibbons FX, Pagano I, Williams RJ. Longitudinal study of e-cigarette use and onset of cigarette smoking among high school students in Hawaii. Tob Control 2016; 26(1):34–39. doi:10.1136/tobaccocontrol-2015-052705
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The health consequences of smoking—50 years of progress: a report of the Surgeon General. Atlanta: Centers for Disease Control and Prevention, 2014. www.ncbi.nlm.nih.gov/books/NBK179276/pdf/Bookshelf_NBK179276.pdf. Accessed November 14, 2019.
- Christiani DC. Vaping-induced lung injury. N Engl J Med 2019; Sept 6. Epub ahead of print. doi:10.1056/NEJMe1912032
- Neel J, Aubrey A. Vitamin E suspected in serious lung problems among people who vaped cannabis. NPR Sept 5, 2019. www.npr.org/sections/health-shots/2019/09/05/758005409/vitamin-e-suspected-in-serious-lung-problems-among-people-who-vaped-cannabis. Accessed November 14, 2019.
- White A. Plans for the first e-cigarette went up in smoke 50 years ago. Smithsonian Magazine December 2018. www.smithsonianmag.com/innovation/plans-for-first-e-cigarette-went-up-in-smoke-50-years-ago-180970730.
- Blundell MS, Dargan PI, Wood DM. The dark cloud of recreational drugs and vaping. QJM 2018; 111(3):145–148. doi:10.1093/qjmed/hcx049
- Schulenberg JE, Johnston LD, O’Malley PM, Bachman JG, Miech RA, Patrick ME. Monitoring the future: national survey results on drug use, 1975–2018. 2018 Volume 2. College students & adults ages 19–60. www.monitoringthefuture.org/pubs/monographs/mtf-vol2_2018.pdf. Accessed November 14, 2019.
- Eggers ME, Lee YO, Jackson J, Wiley JL, Porter J, Nonnemaker JM. Youth use of electronic vapor products and blunts for administering cannabis. Addict Behav 2017; 70:79-82. doi:10.1016/j.addbeh.2017.02.020
- Regan AK, Promoff G, Dube SR, Arrazola R. Electronic nicotine delivery systems: adult use and awareness of the “e-cigarette”in the USA. Tob Control 2013; 22(1):19–23. doi:10.1136/tobaccocontrol-2011-050044
- Centers for Disease Control and Prevention. E-cigarette ads and youth. www.cdc.gov/vitalsigns/ecigarette-ads/index.html.
- Noel JK, Rees VW, Connolly GN. Electronic cigarettes: a new “tobacco” industry? Tob Control 2011; 20(1):81. doi:10.1136/tc.2010.038562
- US Food and Drug Administration. Deeming tobacco products to be subject to the federal food, drug, and cosmetic act, as amended by the family smoking prevention and tobacco control act; restrictions on the sale and distribution of tobacco products and required warning statements for tobacco products. Federal Register 2016; 81(90), May 10, 2016. www.govinfo.gov/content/pkg/FR-2016-05-10/pdf/2016-10685.pdf. Accessed November 14, 2019.
- Rom O, Pecorelli A, Valacchi G, Reznick AZ. Are e-cigarettes a safe and good alternative to cigarette smoking? Ann NY Acad Sci 2015; 1340:65–74. doi:10.1111/nyas.12609
- Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control 2014; 23(suppl 3):iii3-iii9. doi:10.1136/tobaccocontrol-2014-051670
- Kong G, Morean ME, Cavallo DA, Camenga DR, Krishnan-Sarin S. Reasons for electronic cigarette experimentation and discontinuation among adolescents and young adults. Nicotine Tob Res 2015; 17(7):847–854. doi:10.1093/ntr/ntu257
- Baca MC. How two Stanford grads aimed for big tech glory and got big tobacco instead. Updated September 4, 2019. The Washington Post September 4, 2019. www.washingtonpost.com/technology/2019/09/04/how-two-stanford-grads-aimed-big-tech-glory-got-big-tobacco-instead. Accessed November 14, 2019.
- Huang J, Duan Z, Kwok J, et al. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control 2019; 28(2):146–151. doi:10.1136/tobaccocontrol-2018-054382
- Walley SC, Wilson KM, Winickoff JP, Groner J. A public health crisis: electronic cigarettes, vape, and JUUL. Pediatrics 2019; 143(6):pii:e20182741. doi:10.1542/peds.2018-2741
- Zernike K. F.D.A. cracks down on “juuling” among teenagers. The New York Times April 24, 2018. www.nytimes.com/2018/04/24/health/fda-e-cigarettes-minors-juul.html. Accessed November 14, 2019.
- Ramamurthi D, Chau C, Jackler RK. JUUL and other stealth vaporisers: hiding the habit from parents and teachers. Tob Control 2018 Sep 15; pii:tobaccocontrol-2018-054455. doi:10.1136/tobaccocontrol-2018-054455. [Epub ahead of print]
- Willett JG, Bennett M, Hair EC, et al. Recognition, use and perceptions of JUUL among youth and young adults. Tob Control 2019; 28(1):115–116. doi:10.1136/tobaccocontrol-2018-054273
- Kaplan S. Juul’s new product: less nicotine, more intense vapor. New York Times Nov 27, 2018. www.nytimes.com/2018/11/27/health/juul-ecigarettes-nicotine.html.
- JUUL Labs. JUULpods. www.juul.com/shop/pods. Accessed November 14, 2019.
- Krishnan-Sarin S, Morean M, Kong G, et al. E-cigarettes and “dripping” among high-school youth. Pediatrics 2017; 139(3):pii:e20163224. doi:10.1542/peds.2016-3224
- Kosmider L, Sobczak A, Fik M, et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res 2014; 16(10):1319–1326. doi:10.1093/ntr/ntu078
- Rawlinson C, Martin S, Frosina J, Wright C. Chemical characterisation of aerosols emitted by electronic cigarettes using thermal desorption-gas chromatography-time of flight mass spectrometry. J Chromatogr A 2017; 1497:144–154. doi:10.1016/j.chroma.2017.02.050
- Lee MS, LeBouf RF, Son YS, Koutrakis P, Christiani DC. Nicotine, aerosol particles, carbonyls and volatile organic compounds in tobacco- and menthol-flavored e-cigarettes. Environ Health 2017; 16(1):42. doi:10.1186/s12940-017-0249-x
- Williams M, Bozhilov K, Ghai S, Talbot P. Elements including metals in the atomizer and aerosol of disposable electronic cigarettes and electronic hookahs. PLoS One 2017; 12(4):e0175430. doi:10.1371/journal.pone.0175430.
- Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 2014; 23(2):133–139. doi:10.1136/tobaccocontrol-2012-050859
- Drope J, Cahn Z, Kennedy R, et al. Key issues surrounding the health impacts of electronic nicotine delivery systems (ENDS) and other sources of nicotine. CA Cancer J Clin 2017; 67(6):449–471. doi:10.3322/caac.21413
- Jabba SV, Jordt SE. Risk analysis for the carcinogen pulegone in mint- and menthol-flavored e-cigarettes and smokeless tobacco products. JAMA Intern Med 2019 Sep 16 [Epub ahead of print]. doi:10.1001/jamainternmed.2019.3649
- Tierney PA, Karpinsky CD, Brown JE, Luo W, Pankow JF. Flavour chemicals in electronic cigarette fluids. Tob Control 2016; 25(e1):e10–e15. doi:10.1136/tobaccocontrol-2014-052175
- Behar RZ, Wang Y, Talbot P. Comparing the cytotoxicity of electronic cigarette fluids, aerosols and solvents. Tob Control 2017; 27(3):325–333. doi:10.1136/tobaccocontrol-2016-053472
- Martin EM, Clapp PW, Rebuli ME, et al. E-cigarette use results in suppression of immune and inflammatory-response genes in nasal epithelial cells similar to cigarette smoke. Am J Physiol Lung Cell Mol Physiol 2016; 311(1):L135–L144. doi:10.1152/ajplung.00170.2016
- Holden VK, Hines SE. Update on flavoring-induced lung disease. Curr Opin Pulm Med 2016;22(2):158–164. doi:10.1097/MCP.0000000000000250
- Siqueira L; Committee on Substance Use and Prevention. Nicotine and tobacco as substances of abuse in children and adolescents. Pediatrics 2017; 139(1):pii:e20163436. doi:10.1542/peds.2016-3436
- England LJ, Bunnell RE, Pechacek TF, Tong VT, McAfee TA. Nicotine and the developing human: a neglected element in the electronic cigarette debate. Am J Prev Med 2015; 49(2):286–293. doi:10.1016/j.amepre.2015.01.015
- Modesto-Lowe V, Alvarado C. E-cigs…are they cool? Talking to teens about e-cigarettes. Clin Pediatr (Phila) 2017; 51(10):947–952. doi:10.1177/0009922817705188
- Prochaska JJ, Benowitz NL. The past, present, and future of nicotine addiction therapy. Annu Rev Med 2017; 67:467–486. doi:10.1146/annurev-med-111314-033712
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004; 99(1):29–38. doi:10.1111/j.1360-0443.2004.00540.x
- McMillen RC, Gottlieb MA, Shaefer RM, Winickoff JP, Klein JD. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res 2015;17(10):119_1202. doi:10.1093/ntr/ntu213
- Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet 2013; 382(9905):1629–1637. doi:10.1016/S0140-6736(13)61842-5
- Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine replacement therapy. N Engl J Med 2019; 380(7):629–637. doi:10.1056/NEJMoa1808779
- Campagna D, Cibella F, Caponnetto P, et al. Changes in breathomics from a 1-year randomized smoking cessation trial of electronic cigarettes. Eur J Clin Invest 2016; 46(8):698–706. doi:10.1111/eci.12651
- Rehan HS, Maini J, Hungin APS. Vaping versus smoking: a quest for efficacy and safety of e-cigarette. Curr Drug Saf 2018; 13(2):92–101. doi:10.2174/1574886313666180227110556
- Zernike K. ‘I can’t stop’: schools struggle with vaping explosion. New York Times April 2, 2018. www.nytimes.com/2018/04/02/health/vaping-ecigarettes-addiction-teen.html.
- Pepper JK, Ribisl KM, Brewer NT. Adolescents’ interest in trying flavoured e-cigarettes. Tob Control 2016; 25(suppl 2):ii62–ii66. doi:10.1136/tobaccocontrol-2016-053174
- Harrell MB, Loukas A, Jackson CD, Marti CN, Perry CL. Flavored tobacco product use among youth and young adults: what if flavors didn’t exist? Tob Regul Sci 2017; 3(2):168–173. doi:10.18001/TRS.3.2.4
- Smith M. Amid vaping crackdown, Michigan to ban sale of flavored e-cigarettes. New York Times Sept 4, 2019. www.nytimes.com/2019/09/04/us/michigan-vaping.html?module=inline.
- Roditis ML, Halpern-Felsher B. Adolescents’ perceptions of risks and benefits of conventional cigarettes, e-cigarettes, and marijuana: a qualitative analysis. J Adolesc Health 2015; 57(2):179–185. doi:10.1016/j.jadohealth.2015.04.002
- Chapman S, Daube M, Maziak W. Should e-cigarette use be permitted in smoke-free public places? No. Tob Control 2017; 26(e1):e3–e4. doi:10.1136/tobaccocontrol-2016-053359
- Marcham CL, Springston JP. Electronic cigarettes in the indoor environment. Rev Env Health 2019; 34(2):105–124. doi:10.1515/reveh-2019-0012
- Chatham-Stephens K, Law R, Taylor E, et al; Centers for Disease Control and Prevention. Notes from the field: calls to poison centers for exposures to electronic cigarettes—United States, September 2010–September 2014. MMWR Morb Mortal Wkly Report 2014; 63(13):292–293. pmid:24699766
Electronic cigarettes and other “vaping” devices have been increasing in popularity among youth and adults since their introduction in the US market in 2007.1 This increase is partially driven by a public perception that vaping is harmless, or at least less harmful than cigarette smoking.2 Vaping fans also argue that current smokers can use vaping as nicotine replacement therapy to help them quit smoking.3
We disagree. Research on the health effects of vaping, though still limited, is accumulating rapidly and making it increasingly clear that this habit is far from harmless. For youth, it is a gateway to addiction to nicotine and other substances. Whether it can help people quit smoking remains to be seen. And recent months have seen reports of serious respiratory illnesses and even deaths linked to vaping.4
In December 2016, the US Surgeon General warned that e-cigarette use among youth and young adults in the United States represents a “major public health concern,”5 and that more adolescents and young adults are now vaping than smoking conventional tobacco products.
This article reviews the issue of vaping in the United States, as well as available evidence regarding its safety.
YOUTH AT RISK
Retail sales of e-cigarettes and vaping devices approach an annual $7 billion.6 A 2014–2015 survey found that 2.4% of the general US population were current users of e-cigarettes, and 8.5% had tried them at least once.3
In 2014, for the first time, e-cigarette use became more common among US youth than traditional cigarettes.5
The odds of taking up vaping are higher among minority youth in the United States, particularly Hispanics.9 This trend is particularly worrisome because several longitudinal studies have shown that adolescents who use e-cigarettes are 3 times as likely to eventually become smokers of traditional cigarettes compared with adolescents who do not use e-cigarettes.10–12
If US youth continue smoking at the current rate, 5.6 million of the current population under age 18, or 1 of every 13, will die early of a smoking-related illness.13
RECENT OUTBREAK OF VAPING-ASSOCIATED LUNG INJURY
As of November 5, 2019, there had been 2,051 cases of vaping-associated lung injury in 49 states (all except Alaska), the District of Columbia, and 1 US territory reported to the US Centers for Disease Control and Prevention (CDC), with 39 confirmed deaths.4 The reported cases include respiratory injury including acute eosinophilic pneumonia, organizing pneumonia, acute respiratory distress syndrome, and hypersensitivity pneumonitis.14
Most of these patients had been vaping tetrahydrocannabinol (THC), though many used both nicotine- and THC-containing products, and others used products containing nicotine exclusively.4 Thus, it is difficult to identify the exact substance or substances that may be contributing to this sudden outbreak among vape users, and many different product sources are currently under investigation.
One substance that may be linked to the epidemic is vitamin E acetate, which the New York State Department of Health has detected in high levels in cannabis vaping cartridges used by patients who developed lung injury.15 The US Food and Drug Administration (FDA) is continuing to analyze vape cartridge samples submitted by affected patients to look for other chemicals that can contribute to the development of serious pulmonary illness.
WHAT IS AN E-CIGARETTE? WHAT IS A VAPE PEN?
Vape pens consist of similar elements but are not necessarily similar in appearance to a conventional cigarette, and may look more like a pen or a USB flash drive. In fact, the Juul device is recharged by plugging it into a USB port.
Vaping devices have many street names, including e-cigs, e-hookahs, vape pens, mods, vapes, and tank systems.
The first US patent application for a device resembling a modern e-cigarette was filed in 1963, but the product never made it to the market.16 Instead, the first commercially successful e-cigarette was created in Beijing in 2003 and introduced to US markets in 2007.
Newer-generation devices have larger batteries and can heat the liquid to higher temperatures, releasing more nicotine and forming additional toxicants such as formaldehyde. Devices lack standardization in terms of design, capacity for safely holding e-liquid, packaging of the e-liquid, and features designed to minimize hazards of use.
Not just nicotine
Many devices are designed for use with other drugs, including THC.17 In a 2018 study, 10.9% of college students reported vaping marijuana in the past 30 days, up from 5.2% in 2017.18
Other substances are being vaped as well.19 In theory, any heat-stable psychoactive recreational drug could be aerosolized and vaped. There are increasing reports of e-liquids containing recreational drugs such as synthetic cannabinoid receptor agonists, crack cocaine, LSD, and methamphetamine.17
Freedom, rebellion, glamour
Sales have risen rapidly since 2007 with widespread advertising on television and in print publications for popular brands, often featuring celebrities.20 Spending on advertising for e-cigarettes and vape devices rose from $6.4 million in 2011 to $115 million in 2014—and that was before the advent of Juul (see below).21
Marketing campaigns for vaping devices mimic the themes previously used successfully by the tobacco industry, eg, freedom, rebellion, and glamour. They also make unsubstantiated claims about health benefits and smoking cessation, though initial websites contained endorsements from physicians, similar to the strategies of tobacco companies in old cigarette ads. Cigarette ads have been prohibited since 1971—but not e-cigarette ads. Moreover, vaping products appear as product placements in television shows and movies, with advocacy groups on social media.22
By law, buyers have to be 18 or 21
Vaping devices can be purchased at vape shops, convenience stores, gas stations, and over the Internet; up to 50% of sales are conducted online.24
Fruit flavors are popular
Zhu et al25 estimated that 7,700 unique vaping flavors exist, with fruit and candy flavors predominating. The most popular flavors are tobacco and mint, followed by fruit, dessert and candy flavors, alcoholic flavors (strawberry daiquiri, margarita), and food flavors.25 These flavors have been associated with higher usage in youth, leading to increased risk of nicotine addiction.26
WHAT IS JUUL?
The Juul device (Juul Labs, www.juul.com) was developed in 2015 by 2 Stanford University graduates. Their goal was to produce a more satisfying and cigarette-like vaping experience, specifically by increasing the amount of nicotine delivered while maintaining smooth and pleasant inhalation. They created an e-liquid that could be vaporized effectively at lower temperatures.27
While more than 400 brands of vaping devices are currently available in the United States,3 Juul has held the largest market share since 2017,28 an estimated 72.1% as of August 2018.29 The surge in popularity of this particular brand is attributed to its trendy design that is similar in size and appearance to a USB flash drive,29 and its offering of sweet flavors such as “crème brûlée” and “cool mint.”
On April 24, 2018, in view of growing concern about the popularity of Juul products among youth, the FDA requested that the company submit documents regarding its marketing tactics, as well as research on the effects of this marketing on product design and public health impact, and information about adverse experiences and complaints.30 The company was forced to change its marketing to appeal less to youth. Now it offers only 3 flavors: “Virginia tobacco,” “classic tobacco,” and “menthol,” although off-brand pods containing a variety of flavors are still available. And some pods are refillable, so users can essentially vape any substance they want.
Although the Juul device delivers a strong dose of nicotine, it is small and therefore easy to hide from parents and teachers, and widespread use has been reported among youth in middle and high schools. Hoodies, hemp jewelry, and backpacks have been designed to hide the devices and allow for easy, hands-free use. YouTube searches for terms such as “Juul,” “hiding Juul at school,” and “Juul in class,” yield thousands of results.31 A 2017 survey reported that 8% of Americans age 15 to 24 had used Juul in the month prior to the survey.32 “To juul” has become a verb.
Each Juul starter kit contains the rechargeable inhalation device plus 4 flavored pods. In the United States, each Juul pod contains nearly as much nicotine as 1 pack of 20 cigarettes in a concentration of 3% or 5%. (Israel and Europe have forced the company to replace the 5% nicotine pods with 1.7% nicotine pods.33) A starter kit costs $49.99, and additional packs of 4 flavored liquid cartridges or pods cost $15.99.34 Other brands of vape pens cost between $15 and $35, and 10-mL bottles of e-liquid cost approximately $7.
What is ‘dripping’?
Hard-core vapers seeking a more intense experience are taking their vaping devices apart and modifying them for “dripping,” ie, directly dripping vape liquids onto the heated coils for inhalation. In a survey, 1 in 4 high school students using vape devices also used them for dripping, citing desires for a thicker cloud of vapor, more intense flavor, “a stronger throat hit,” curiosity, and other reasons.35 Dripping involves higher temperatures, which leads to higher amounts of nicotine delivered, along with more formaldehyde, acetaldehyde, and acetone (see below).36
BAD THINGS IN E-LIQUID AEROSOL
Studies of vape liquids consistently confirm the presence of toxic substances in the resulting vape aerosol.37–40 Depending on the combination of flavorings and solvents in a given e-liquid, a variety of chemicals can be detected in the aerosol from various vaping devices. Chemicals that may be detected include known irritants of respiratory mucosa, as well as various carcinogens. The list includes:
- Organic volatile compounds such as propylene glycol, glycerin, and toluene
- Aldehydes such as formaldehyde (released when propylene glycol is heated to high temperatures), acetaldehyde, and benzaldehyde
- Acetone and acrolein
- Carcinogenic nitrosamines
- Polycyclic aromatic hydrocarbons
- Particulate matter
- Metals including chromium, cadmium, nickel, and lead; and particles of copper, nickel, and silver have been found in electronic nicotine delivery system aerosol in higher levels than in conventional cigarette smoke.41
The specific chemicals detected can vary greatly between brands, even when the flavoring and nicotine content are equivalent, which frequently results in inconsistent and conflicting study findings. The chemicals detected also vary with the voltage or power used to generate the aerosol. Different flavors may carry varying levels of risk; for example, mint- and menthol-flavored e-cigarettes were shown to expose users to dangerous levels of pulegone, a carcinogenic compound banned as a food additive in 2018.42 The concentrations of some of these chemicals are sufficiently high to be of toxicologic concern; for example, one study reported the presence of benzaldehyde in e-cigarette aerosol at twice the workplace exposure limit.43
Biologic effects
In an in vitro study,44 57% of e-liquids studied were found to be cytotoxic to human pulmonary fibroblasts, lung epithelial cells, and human embryonic stem cells. Fruit-flavored e-liquids in particular caused a significant increase in DNA fragmentation. Cell cultures treated with e-cigarette liquids showed increased oxidative stress, reduced cell proliferation, and increased DNA damage,44 which may have implications for carcinogenic risk.
In another study,45 exposure to e-cigarette aerosol as well as conventional cigarette smoke resulted in suppression of genes related to immune and inflammatory response in respiratory epithelial cells. All genes with decreased expression after exposure to conventional cigarette smoke also showed decreased expression with exposure to e-cigarette smoke, which the study authors suggested could lead to immune suppression at the level of the nasal mucosa. Diacetyl and acetoin, chemicals found in certain flavorings, have been linked to bronchiolitis obliterans, or “popcorn lung.”46
Nicotine is not benign
The nicotine itself in many vaping liquids should also not be underestimated. Nicotine has harmful neurocognitive effects and addictive properties, particularly in the developing brains of adolescents and young adults.47 Nicotine exposure during adolescence negatively affects memory, attention, and emotional regulation,48 as well as executive functioning, reward processing, and learning.49
The brain undergoes major structural remodeling in adolescence, and nicotine acetylcholine receptors regulate neural maturation. Early exposure to nicotine disrupts this process, leading to poor executive functioning, difficulty learning, decreased memory, and issues with reward processing.
Fetal exposure, if nicotine products are used during pregnancy, has also been linked to adverse consequences such as deficits in attention and cognition, behavioral effects, and sudden infant death syndrome.5
Much to learn about toxicity
Partly because vaping devices have been available to US consumers only since 2007, limited evidence is available regarding the long-term effects of exposure to the aerosol from these devices in humans.1 Many of the studies mentioned above were in vitro studies or conducted in mouse models. Differences in device design and the composition of the e-liquid among device brands pose a challenge for developing well-designed studies of the long-term health effects of e-cigarette and vape use. Additionally, devices may have different health impacts when used to vape cannabis or other drugs besides nicotine, which requires further investigation.
E-CIGARETTES AND SMOKING CESSATION
Conventional cigarette smoking is a major public health threat, as tobacco use is responsible for 480,000 deaths annually in the United States.50
And smoking is extremely difficult to quit: as many as 80% of smokers who attempt to quit resume smoking within the first month.51 The chance of successfully quitting improves by over 50% if the individual undergoes nicotine replacement therapy, and it improves even more with counseling.50
There are currently 5 types of FDA-approved nicotine replacement therapy products (gum, patch, lozenge, inhaler, nasal spray) to help with smoking cessation. In addition, 2 non-nicotine prescription drugs (varenicline and bupropion) have been approved for treating tobacco dependence.
Can vaping devices be added to the list of nicotine replacement therapy products? Although some manufacturers try to brand their devices as smoking cessation aids, in one study,52 one-third of e-cigarette users said they had either never used conventional cigarettes or had formerly smoked them.
Bullen et al53 randomized smokers interested in quitting to receive either e-cigarettes, nicotine patches, or placebo (nicotine-free) e-cigarettes and followed them for 6 months. Rates of tobacco cessation were less than predicted for the entire study population, resulting in insufficient power to determine the superiority of any single method, but the study authors concluded that nicotine e-cigarettes were “modestly effective” at helping smokers quit, and that abstinence rates may be similar to those with nicotine patches.53
Hajek et al54 randomized 886 smokers to e-cigarette or nicotine replacement products of their choice. After 1 year, 18% of e-cigarette users had stopped smoking, compared with 9.9% of nicotine replacement product users. However, 80% of the e-cigarette users were still using e-cigarettes after 1 year, while only 9% of nicotine replacement product users were still using nicotine replacement therapy products after 1 year.
While quitting conventional cigarette smoking altogether has widely established health benefits, little is known about the health benefits of transitioning from conventional cigarette smoking to reduced conventional cigarette smoking with concomitant use of e-cigarettes.
Campagna et al55 found no beneficial health effects in smokers who partially substituted conventional cigarettes for e-cigarettes.
Many studies found that smokers use e-cigarettes to maintain their habit instead of quitting entirely.56 It has been suggested that any slight increase in effectiveness in smoking cessation by using e-cigarettes compared with other nicotine replacement products could be linked to satisfying of the habitual smoking actions, such as inhaling and bringing the hand to the mouth,24 which are absent when using other nicotine replacement methods such as a nicotine patch.
As with safety information, long-term outcomes regarding the use of vape devices for smoking cessation have not been yet established, as this option is still relatively new.
VAPING AS A GATEWAY DRUG
Another worrisome trend involving electronic nicotine delivery systems is their marketing and branding, which appear to be aimed directly at adolescents and young adults. Juul and other similar products cannot be sold to anyone under the age of 18 (or 21 in 18 states, including California, Massachusetts, New York, and now Ohio). Despite this, Juul and similar products continue to increase in popularity among middle school and high school students.57
While smoking cessation and health improvement are cited as reasons for vaping among middle-aged and older adults, adolescents and young adults more often cite flavor, enjoyment, peer use, and curiosity as reasons for use.
Adolescents are more likely to report interest in trying a vape product flavored with menthol or fruit than tobacco, and commonly hold the belief that fruit-flavored e-cigarettes are less harmful than tobacco-flavored e-cigarettes.58 Harrell et al59 polled youth and young adults who used flavored e-cigarettes, and 78% said they would no longer use the product if their preferred flavor were not available. In September 2019, Michigan became the first state to ban the sale of flavored e-cigarettes in stores and online. Similar bills have been introduced in California, Massachusetts, and New York.60
Myths and misperceptions abound among youth regarding smoking vs vaping. Young people view regular cigarette smoking negatively, as causing cancer, bad breath, and asthma exacerbations. Meanwhile, they believe marijuana is safer and less addictive than traditional cigarette smoking.61 Youth exposed to e-cigarette advertisements viewed e-cigarettes as healthier, more enjoyable, “cool,” safe, and fun.61 The overall public health impact of increasing initiation of smoking, particularly among youth and young adults, should not be underestimated.
SECONDHAND VAPE AND OTHER EXPOSURE RISKS
Cigarette smoking has been banned in many public places, in view of a large body of scientific evidence about the harmful effects of secondhand smoke. Advocates for allowing vaping in public places say that vaping emissions do not harm bystanders, but evidence is insufficient to support this claim.62 One study showed that passive exposure to e-cigarette aerosol generated increases in serum levels of cotinine (a nicotine metabolite) similar to those with passive exposure to conventional cigarette smoke.5
Accidental nicotine poisoning in children as a result of ingesting e-cigarette liquid is also a major concern,63 particularly with sweet flavors such as bubblegum or cheesecake that may be attractive to children.
Calls to US poison control centers with respect to e-cigarettes and vaping increased from 1 per month in September 2010 to 215 in February 2014, with 51% involving children under age 5.64 This trend resulted in the Child Nicotine Poisoning Prevention Act, which passed in 2015 and went into effect in 2016, requiring packaging that is difficult to open for children under age 5.5
Device malfunctions or battery failures have led to explosions that have resulted in substantial injuries to users, as well as house and car fires.49
HOW DO WE DISCOURAGE ADOLESCENT USE?
There are currently no established treatment approaches for adolescents who have become addicted to vaping. A review of the literature regarding treatment modalities used to address adolescent use of tobacco and marijuana provides insight that options such as nicotine replacement therapy and counseling modalities such as cognitive behavioral therapy may be helpful in treating teen vaping addiction. However, more research is needed to determine the effectiveness of these treatments in youth addicted to vaping.
Given that youth who vape even once are more likely to try other types of tobacco, we recommend that parents and healthcare providers start conversations by asking what the young person has seen or heard about vaping. Young people can also be asked what they think the school’s response should be: Do they think vaping should be banned in public places, as cigarettes have been banned? What about the carbon footprint? What are their thoughts on the plastic waste, batteries, and other toxins generated by the e-cigarette industry?
New US laws ban the sale of e-cigarettes and vaping devices to minors in stores and online. These policies are modeled in many cases on environmental control policies that have been previously employed to reduce tobacco use, particularly by youth. For example, changing laws to mandate sales only to individuals age 21 and older in all states can help to decrease access to these products among middle school and high school students.
As with tobacco cessation, education will not be enough. Support of legislation that bans vaping in public places, increases pricing to discourage adolescent use, and other measures used successfully to decrease conventional cigarette smoking can be deployed to decrease the public health impact of e-cigarettes. We recommend further regulation of specific harmful chemicals and clear, detailed ingredient labeling to increase consumer understanding of the risks associated with these products. Additionally, we recommend eliminating flavored e-cigarettes, which are the most appealing type for young users, and raising prices of e-cigarettes and similar products to discourage use by youth.
If current cigarette smokers want to use e-cigarettes to quit, we recommend that clinicians counsel them to eventually completely stop use of traditional cigarettes and switch to using e-cigarettes, instead of becoming a dual user of both types of products or using e-cigarettes indefinitely. After making that switch, they should then work to gradually taper usage and nicotine addiction by reducing the amount of nicotine in the e-liquid. Clinicians should ask patients about use of e-cigarettes and vaping devices specifically, and should counsel nonsmokers to avoid initiation of use.
EVIDENCE OF HARM CONTINUES TO EMERGE
Data about respiratory effects, secondhand exposure, and long-term smoking cessation efficacy are still limited, and it remains as yet unknown what combinations of solvents, flavorings, and nicotine in a given e-liquid will result in the most harmful or least harmful effects. In addition, while much of the information about the safety of these components has been obtained using in vitro or mouse models, increasing reports of serious respiratory illness and rising numbers of deaths linked to vaping make it clear that these findings likely translate to harmful effects in humans.
E-cigarettes may ultimately prove to be less harmful than traditional cigarettes, but it seems likely that with further time and research, serious health risks of e-cigarette use will continue to emerge.
Electronic cigarettes and other “vaping” devices have been increasing in popularity among youth and adults since their introduction in the US market in 2007.1 This increase is partially driven by a public perception that vaping is harmless, or at least less harmful than cigarette smoking.2 Vaping fans also argue that current smokers can use vaping as nicotine replacement therapy to help them quit smoking.3
We disagree. Research on the health effects of vaping, though still limited, is accumulating rapidly and making it increasingly clear that this habit is far from harmless. For youth, it is a gateway to addiction to nicotine and other substances. Whether it can help people quit smoking remains to be seen. And recent months have seen reports of serious respiratory illnesses and even deaths linked to vaping.4
In December 2016, the US Surgeon General warned that e-cigarette use among youth and young adults in the United States represents a “major public health concern,”5 and that more adolescents and young adults are now vaping than smoking conventional tobacco products.
This article reviews the issue of vaping in the United States, as well as available evidence regarding its safety.
YOUTH AT RISK
Retail sales of e-cigarettes and vaping devices approach an annual $7 billion.6 A 2014–2015 survey found that 2.4% of the general US population were current users of e-cigarettes, and 8.5% had tried them at least once.3
In 2014, for the first time, e-cigarette use became more common among US youth than traditional cigarettes.5
The odds of taking up vaping are higher among minority youth in the United States, particularly Hispanics.9 This trend is particularly worrisome because several longitudinal studies have shown that adolescents who use e-cigarettes are 3 times as likely to eventually become smokers of traditional cigarettes compared with adolescents who do not use e-cigarettes.10–12
If US youth continue smoking at the current rate, 5.6 million of the current population under age 18, or 1 of every 13, will die early of a smoking-related illness.13
RECENT OUTBREAK OF VAPING-ASSOCIATED LUNG INJURY
As of November 5, 2019, there had been 2,051 cases of vaping-associated lung injury in 49 states (all except Alaska), the District of Columbia, and 1 US territory reported to the US Centers for Disease Control and Prevention (CDC), with 39 confirmed deaths.4 The reported cases include respiratory injury including acute eosinophilic pneumonia, organizing pneumonia, acute respiratory distress syndrome, and hypersensitivity pneumonitis.14
Most of these patients had been vaping tetrahydrocannabinol (THC), though many used both nicotine- and THC-containing products, and others used products containing nicotine exclusively.4 Thus, it is difficult to identify the exact substance or substances that may be contributing to this sudden outbreak among vape users, and many different product sources are currently under investigation.
One substance that may be linked to the epidemic is vitamin E acetate, which the New York State Department of Health has detected in high levels in cannabis vaping cartridges used by patients who developed lung injury.15 The US Food and Drug Administration (FDA) is continuing to analyze vape cartridge samples submitted by affected patients to look for other chemicals that can contribute to the development of serious pulmonary illness.
WHAT IS AN E-CIGARETTE? WHAT IS A VAPE PEN?
Vape pens consist of similar elements but are not necessarily similar in appearance to a conventional cigarette, and may look more like a pen or a USB flash drive. In fact, the Juul device is recharged by plugging it into a USB port.
Vaping devices have many street names, including e-cigs, e-hookahs, vape pens, mods, vapes, and tank systems.
The first US patent application for a device resembling a modern e-cigarette was filed in 1963, but the product never made it to the market.16 Instead, the first commercially successful e-cigarette was created in Beijing in 2003 and introduced to US markets in 2007.
Newer-generation devices have larger batteries and can heat the liquid to higher temperatures, releasing more nicotine and forming additional toxicants such as formaldehyde. Devices lack standardization in terms of design, capacity for safely holding e-liquid, packaging of the e-liquid, and features designed to minimize hazards of use.
Not just nicotine
Many devices are designed for use with other drugs, including THC.17 In a 2018 study, 10.9% of college students reported vaping marijuana in the past 30 days, up from 5.2% in 2017.18
Other substances are being vaped as well.19 In theory, any heat-stable psychoactive recreational drug could be aerosolized and vaped. There are increasing reports of e-liquids containing recreational drugs such as synthetic cannabinoid receptor agonists, crack cocaine, LSD, and methamphetamine.17
Freedom, rebellion, glamour
Sales have risen rapidly since 2007 with widespread advertising on television and in print publications for popular brands, often featuring celebrities.20 Spending on advertising for e-cigarettes and vape devices rose from $6.4 million in 2011 to $115 million in 2014—and that was before the advent of Juul (see below).21
Marketing campaigns for vaping devices mimic the themes previously used successfully by the tobacco industry, eg, freedom, rebellion, and glamour. They also make unsubstantiated claims about health benefits and smoking cessation, though initial websites contained endorsements from physicians, similar to the strategies of tobacco companies in old cigarette ads. Cigarette ads have been prohibited since 1971—but not e-cigarette ads. Moreover, vaping products appear as product placements in television shows and movies, with advocacy groups on social media.22
By law, buyers have to be 18 or 21
Vaping devices can be purchased at vape shops, convenience stores, gas stations, and over the Internet; up to 50% of sales are conducted online.24
Fruit flavors are popular
Zhu et al25 estimated that 7,700 unique vaping flavors exist, with fruit and candy flavors predominating. The most popular flavors are tobacco and mint, followed by fruit, dessert and candy flavors, alcoholic flavors (strawberry daiquiri, margarita), and food flavors.25 These flavors have been associated with higher usage in youth, leading to increased risk of nicotine addiction.26
WHAT IS JUUL?
The Juul device (Juul Labs, www.juul.com) was developed in 2015 by 2 Stanford University graduates. Their goal was to produce a more satisfying and cigarette-like vaping experience, specifically by increasing the amount of nicotine delivered while maintaining smooth and pleasant inhalation. They created an e-liquid that could be vaporized effectively at lower temperatures.27
While more than 400 brands of vaping devices are currently available in the United States,3 Juul has held the largest market share since 2017,28 an estimated 72.1% as of August 2018.29 The surge in popularity of this particular brand is attributed to its trendy design that is similar in size and appearance to a USB flash drive,29 and its offering of sweet flavors such as “crème brûlée” and “cool mint.”
On April 24, 2018, in view of growing concern about the popularity of Juul products among youth, the FDA requested that the company submit documents regarding its marketing tactics, as well as research on the effects of this marketing on product design and public health impact, and information about adverse experiences and complaints.30 The company was forced to change its marketing to appeal less to youth. Now it offers only 3 flavors: “Virginia tobacco,” “classic tobacco,” and “menthol,” although off-brand pods containing a variety of flavors are still available. And some pods are refillable, so users can essentially vape any substance they want.
Although the Juul device delivers a strong dose of nicotine, it is small and therefore easy to hide from parents and teachers, and widespread use has been reported among youth in middle and high schools. Hoodies, hemp jewelry, and backpacks have been designed to hide the devices and allow for easy, hands-free use. YouTube searches for terms such as “Juul,” “hiding Juul at school,” and “Juul in class,” yield thousands of results.31 A 2017 survey reported that 8% of Americans age 15 to 24 had used Juul in the month prior to the survey.32 “To juul” has become a verb.
Each Juul starter kit contains the rechargeable inhalation device plus 4 flavored pods. In the United States, each Juul pod contains nearly as much nicotine as 1 pack of 20 cigarettes in a concentration of 3% or 5%. (Israel and Europe have forced the company to replace the 5% nicotine pods with 1.7% nicotine pods.33) A starter kit costs $49.99, and additional packs of 4 flavored liquid cartridges or pods cost $15.99.34 Other brands of vape pens cost between $15 and $35, and 10-mL bottles of e-liquid cost approximately $7.
What is ‘dripping’?
Hard-core vapers seeking a more intense experience are taking their vaping devices apart and modifying them for “dripping,” ie, directly dripping vape liquids onto the heated coils for inhalation. In a survey, 1 in 4 high school students using vape devices also used them for dripping, citing desires for a thicker cloud of vapor, more intense flavor, “a stronger throat hit,” curiosity, and other reasons.35 Dripping involves higher temperatures, which leads to higher amounts of nicotine delivered, along with more formaldehyde, acetaldehyde, and acetone (see below).36
BAD THINGS IN E-LIQUID AEROSOL
Studies of vape liquids consistently confirm the presence of toxic substances in the resulting vape aerosol.37–40 Depending on the combination of flavorings and solvents in a given e-liquid, a variety of chemicals can be detected in the aerosol from various vaping devices. Chemicals that may be detected include known irritants of respiratory mucosa, as well as various carcinogens. The list includes:
- Organic volatile compounds such as propylene glycol, glycerin, and toluene
- Aldehydes such as formaldehyde (released when propylene glycol is heated to high temperatures), acetaldehyde, and benzaldehyde
- Acetone and acrolein
- Carcinogenic nitrosamines
- Polycyclic aromatic hydrocarbons
- Particulate matter
- Metals including chromium, cadmium, nickel, and lead; and particles of copper, nickel, and silver have been found in electronic nicotine delivery system aerosol in higher levels than in conventional cigarette smoke.41
The specific chemicals detected can vary greatly between brands, even when the flavoring and nicotine content are equivalent, which frequently results in inconsistent and conflicting study findings. The chemicals detected also vary with the voltage or power used to generate the aerosol. Different flavors may carry varying levels of risk; for example, mint- and menthol-flavored e-cigarettes were shown to expose users to dangerous levels of pulegone, a carcinogenic compound banned as a food additive in 2018.42 The concentrations of some of these chemicals are sufficiently high to be of toxicologic concern; for example, one study reported the presence of benzaldehyde in e-cigarette aerosol at twice the workplace exposure limit.43
Biologic effects
In an in vitro study,44 57% of e-liquids studied were found to be cytotoxic to human pulmonary fibroblasts, lung epithelial cells, and human embryonic stem cells. Fruit-flavored e-liquids in particular caused a significant increase in DNA fragmentation. Cell cultures treated with e-cigarette liquids showed increased oxidative stress, reduced cell proliferation, and increased DNA damage,44 which may have implications for carcinogenic risk.
In another study,45 exposure to e-cigarette aerosol as well as conventional cigarette smoke resulted in suppression of genes related to immune and inflammatory response in respiratory epithelial cells. All genes with decreased expression after exposure to conventional cigarette smoke also showed decreased expression with exposure to e-cigarette smoke, which the study authors suggested could lead to immune suppression at the level of the nasal mucosa. Diacetyl and acetoin, chemicals found in certain flavorings, have been linked to bronchiolitis obliterans, or “popcorn lung.”46
Nicotine is not benign
The nicotine itself in many vaping liquids should also not be underestimated. Nicotine has harmful neurocognitive effects and addictive properties, particularly in the developing brains of adolescents and young adults.47 Nicotine exposure during adolescence negatively affects memory, attention, and emotional regulation,48 as well as executive functioning, reward processing, and learning.49
The brain undergoes major structural remodeling in adolescence, and nicotine acetylcholine receptors regulate neural maturation. Early exposure to nicotine disrupts this process, leading to poor executive functioning, difficulty learning, decreased memory, and issues with reward processing.
Fetal exposure, if nicotine products are used during pregnancy, has also been linked to adverse consequences such as deficits in attention and cognition, behavioral effects, and sudden infant death syndrome.5
Much to learn about toxicity
Partly because vaping devices have been available to US consumers only since 2007, limited evidence is available regarding the long-term effects of exposure to the aerosol from these devices in humans.1 Many of the studies mentioned above were in vitro studies or conducted in mouse models. Differences in device design and the composition of the e-liquid among device brands pose a challenge for developing well-designed studies of the long-term health effects of e-cigarette and vape use. Additionally, devices may have different health impacts when used to vape cannabis or other drugs besides nicotine, which requires further investigation.
E-CIGARETTES AND SMOKING CESSATION
Conventional cigarette smoking is a major public health threat, as tobacco use is responsible for 480,000 deaths annually in the United States.50
And smoking is extremely difficult to quit: as many as 80% of smokers who attempt to quit resume smoking within the first month.51 The chance of successfully quitting improves by over 50% if the individual undergoes nicotine replacement therapy, and it improves even more with counseling.50
There are currently 5 types of FDA-approved nicotine replacement therapy products (gum, patch, lozenge, inhaler, nasal spray) to help with smoking cessation. In addition, 2 non-nicotine prescription drugs (varenicline and bupropion) have been approved for treating tobacco dependence.
Can vaping devices be added to the list of nicotine replacement therapy products? Although some manufacturers try to brand their devices as smoking cessation aids, in one study,52 one-third of e-cigarette users said they had either never used conventional cigarettes or had formerly smoked them.
Bullen et al53 randomized smokers interested in quitting to receive either e-cigarettes, nicotine patches, or placebo (nicotine-free) e-cigarettes and followed them for 6 months. Rates of tobacco cessation were less than predicted for the entire study population, resulting in insufficient power to determine the superiority of any single method, but the study authors concluded that nicotine e-cigarettes were “modestly effective” at helping smokers quit, and that abstinence rates may be similar to those with nicotine patches.53
Hajek et al54 randomized 886 smokers to e-cigarette or nicotine replacement products of their choice. After 1 year, 18% of e-cigarette users had stopped smoking, compared with 9.9% of nicotine replacement product users. However, 80% of the e-cigarette users were still using e-cigarettes after 1 year, while only 9% of nicotine replacement product users were still using nicotine replacement therapy products after 1 year.
While quitting conventional cigarette smoking altogether has widely established health benefits, little is known about the health benefits of transitioning from conventional cigarette smoking to reduced conventional cigarette smoking with concomitant use of e-cigarettes.
Campagna et al55 found no beneficial health effects in smokers who partially substituted conventional cigarettes for e-cigarettes.
Many studies found that smokers use e-cigarettes to maintain their habit instead of quitting entirely.56 It has been suggested that any slight increase in effectiveness in smoking cessation by using e-cigarettes compared with other nicotine replacement products could be linked to satisfying of the habitual smoking actions, such as inhaling and bringing the hand to the mouth,24 which are absent when using other nicotine replacement methods such as a nicotine patch.
As with safety information, long-term outcomes regarding the use of vape devices for smoking cessation have not been yet established, as this option is still relatively new.
VAPING AS A GATEWAY DRUG
Another worrisome trend involving electronic nicotine delivery systems is their marketing and branding, which appear to be aimed directly at adolescents and young adults. Juul and other similar products cannot be sold to anyone under the age of 18 (or 21 in 18 states, including California, Massachusetts, New York, and now Ohio). Despite this, Juul and similar products continue to increase in popularity among middle school and high school students.57
While smoking cessation and health improvement are cited as reasons for vaping among middle-aged and older adults, adolescents and young adults more often cite flavor, enjoyment, peer use, and curiosity as reasons for use.
Adolescents are more likely to report interest in trying a vape product flavored with menthol or fruit than tobacco, and commonly hold the belief that fruit-flavored e-cigarettes are less harmful than tobacco-flavored e-cigarettes.58 Harrell et al59 polled youth and young adults who used flavored e-cigarettes, and 78% said they would no longer use the product if their preferred flavor were not available. In September 2019, Michigan became the first state to ban the sale of flavored e-cigarettes in stores and online. Similar bills have been introduced in California, Massachusetts, and New York.60
Myths and misperceptions abound among youth regarding smoking vs vaping. Young people view regular cigarette smoking negatively, as causing cancer, bad breath, and asthma exacerbations. Meanwhile, they believe marijuana is safer and less addictive than traditional cigarette smoking.61 Youth exposed to e-cigarette advertisements viewed e-cigarettes as healthier, more enjoyable, “cool,” safe, and fun.61 The overall public health impact of increasing initiation of smoking, particularly among youth and young adults, should not be underestimated.
SECONDHAND VAPE AND OTHER EXPOSURE RISKS
Cigarette smoking has been banned in many public places, in view of a large body of scientific evidence about the harmful effects of secondhand smoke. Advocates for allowing vaping in public places say that vaping emissions do not harm bystanders, but evidence is insufficient to support this claim.62 One study showed that passive exposure to e-cigarette aerosol generated increases in serum levels of cotinine (a nicotine metabolite) similar to those with passive exposure to conventional cigarette smoke.5
Accidental nicotine poisoning in children as a result of ingesting e-cigarette liquid is also a major concern,63 particularly with sweet flavors such as bubblegum or cheesecake that may be attractive to children.
Calls to US poison control centers with respect to e-cigarettes and vaping increased from 1 per month in September 2010 to 215 in February 2014, with 51% involving children under age 5.64 This trend resulted in the Child Nicotine Poisoning Prevention Act, which passed in 2015 and went into effect in 2016, requiring packaging that is difficult to open for children under age 5.5
Device malfunctions or battery failures have led to explosions that have resulted in substantial injuries to users, as well as house and car fires.49
HOW DO WE DISCOURAGE ADOLESCENT USE?
There are currently no established treatment approaches for adolescents who have become addicted to vaping. A review of the literature regarding treatment modalities used to address adolescent use of tobacco and marijuana provides insight that options such as nicotine replacement therapy and counseling modalities such as cognitive behavioral therapy may be helpful in treating teen vaping addiction. However, more research is needed to determine the effectiveness of these treatments in youth addicted to vaping.
Given that youth who vape even once are more likely to try other types of tobacco, we recommend that parents and healthcare providers start conversations by asking what the young person has seen or heard about vaping. Young people can also be asked what they think the school’s response should be: Do they think vaping should be banned in public places, as cigarettes have been banned? What about the carbon footprint? What are their thoughts on the plastic waste, batteries, and other toxins generated by the e-cigarette industry?
New US laws ban the sale of e-cigarettes and vaping devices to minors in stores and online. These policies are modeled in many cases on environmental control policies that have been previously employed to reduce tobacco use, particularly by youth. For example, changing laws to mandate sales only to individuals age 21 and older in all states can help to decrease access to these products among middle school and high school students.
As with tobacco cessation, education will not be enough. Support of legislation that bans vaping in public places, increases pricing to discourage adolescent use, and other measures used successfully to decrease conventional cigarette smoking can be deployed to decrease the public health impact of e-cigarettes. We recommend further regulation of specific harmful chemicals and clear, detailed ingredient labeling to increase consumer understanding of the risks associated with these products. Additionally, we recommend eliminating flavored e-cigarettes, which are the most appealing type for young users, and raising prices of e-cigarettes and similar products to discourage use by youth.
If current cigarette smokers want to use e-cigarettes to quit, we recommend that clinicians counsel them to eventually completely stop use of traditional cigarettes and switch to using e-cigarettes, instead of becoming a dual user of both types of products or using e-cigarettes indefinitely. After making that switch, they should then work to gradually taper usage and nicotine addiction by reducing the amount of nicotine in the e-liquid. Clinicians should ask patients about use of e-cigarettes and vaping devices specifically, and should counsel nonsmokers to avoid initiation of use.
EVIDENCE OF HARM CONTINUES TO EMERGE
Data about respiratory effects, secondhand exposure, and long-term smoking cessation efficacy are still limited, and it remains as yet unknown what combinations of solvents, flavorings, and nicotine in a given e-liquid will result in the most harmful or least harmful effects. In addition, while much of the information about the safety of these components has been obtained using in vitro or mouse models, increasing reports of serious respiratory illness and rising numbers of deaths linked to vaping make it clear that these findings likely translate to harmful effects in humans.
E-cigarettes may ultimately prove to be less harmful than traditional cigarettes, but it seems likely that with further time and research, serious health risks of e-cigarette use will continue to emerge.
- Sood A, Kesic M, Hernandez M. Electronic cigarettes: one size does not fit all. J Allergy Clin Immunol 2018; 141(6):1973-1982. doi:10.1016/j.jaci.2018.02.029
- Romijnders K, van Osch L, de Vries H, Talhout R. Perceptions and reasons regarding e-cigarette use among users and non-users: a narrative literature review. Int J Environ Res Public Health 2018; 15(6):1190. doi:10.3390/ijerph15061190
- Zhu S, Zhuang Y-L, Wong S, Cummins SE, Tedeschi GJ. E-cigarette use and associated changes in population smoking cessation: evidence from US current population surveys. BMJ 2017; 358:j3262. doi:10.1136/bmj.j3262
- Centers for Disease Control and Prevention. Outbreak of lung injury associated with e-cigarette use, or vaping. Updated November 5, 2019. www.cdc.gov/tobacco/basic_information/e-Cigarettes/severe-Lung-Disease.html. Accessed November 14, 2019.
- US Public Health Services, US Department of Health and Human Services. E-cigarette use among youth and young adults: a report of the Surgeon General. Rockville, MD, U.S. Department of Health and Human Services, 2016. https://e-cigarettes.surgeongeneral.gov/documents/2016_sgr_full_report_non-508.pdf. Accessed November 14, 2019.
- Thomas K, Kaplan S. E-cigarettes went unchecked in 10 years of federal inaction. New York Times Oct 14, 2019; updated November 1, 2019. www.nytimes.com/2019/10/14/health/vaping-e-cigarettes-fda.html.
- Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA. Notes from the field: use of electronic cigarettes and any tobacco product among middle and high school students—United States, 2011–2018. MMWR Morb Mortal Wkly Rep 2018; 67(45):1276–1277. doi:10.15585/mmwr.mm6745a5
- Gentzke A, Creamer M, Cullen K, et al; Centers for Disease Control and Prevention. Vital signs: tobacco product use among middle and high school students—United States, 2011–2018. MMWR Morb Mortal Wkly Rep 2019; 68(6):157–164. doi:10.15585/mmwr.mm6806e1
- Hammig B, Daniel-Dobbs P, Blunt-Vinti H. Electronic cigarette initiation among minority youth in the United States. Am J Drug Alcohol Abuse 2017; 43(3):306–310. doi:10.1080/00952990.2016.1203926
- Primack BA, Soneji S, Stoolmiller M, Fine MJ, Sargent JD. Progression to traditional cigarette smoking after electronic cigarette use among U.S. adolescents and young adults. JAMA Pediatr 2015; 169(11):1018–1023. doi:10.1001/jamapediatrics.2015.1742
- Leventhal AM, Strong DR, Kirkpatrick MG, et al. Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence. JAMA 2015; 314(7):700–707. doi:10.1001/jama.2015.8950
- Wills TA, Knight R, Sargent JD, Gibbons FX, Pagano I, Williams RJ. Longitudinal study of e-cigarette use and onset of cigarette smoking among high school students in Hawaii. Tob Control 2016; 26(1):34–39. doi:10.1136/tobaccocontrol-2015-052705
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The health consequences of smoking—50 years of progress: a report of the Surgeon General. Atlanta: Centers for Disease Control and Prevention, 2014. www.ncbi.nlm.nih.gov/books/NBK179276/pdf/Bookshelf_NBK179276.pdf. Accessed November 14, 2019.
- Christiani DC. Vaping-induced lung injury. N Engl J Med 2019; Sept 6. Epub ahead of print. doi:10.1056/NEJMe1912032
- Neel J, Aubrey A. Vitamin E suspected in serious lung problems among people who vaped cannabis. NPR Sept 5, 2019. www.npr.org/sections/health-shots/2019/09/05/758005409/vitamin-e-suspected-in-serious-lung-problems-among-people-who-vaped-cannabis. Accessed November 14, 2019.
- White A. Plans for the first e-cigarette went up in smoke 50 years ago. Smithsonian Magazine December 2018. www.smithsonianmag.com/innovation/plans-for-first-e-cigarette-went-up-in-smoke-50-years-ago-180970730.
- Blundell MS, Dargan PI, Wood DM. The dark cloud of recreational drugs and vaping. QJM 2018; 111(3):145–148. doi:10.1093/qjmed/hcx049
- Schulenberg JE, Johnston LD, O’Malley PM, Bachman JG, Miech RA, Patrick ME. Monitoring the future: national survey results on drug use, 1975–2018. 2018 Volume 2. College students & adults ages 19–60. www.monitoringthefuture.org/pubs/monographs/mtf-vol2_2018.pdf. Accessed November 14, 2019.
- Eggers ME, Lee YO, Jackson J, Wiley JL, Porter J, Nonnemaker JM. Youth use of electronic vapor products and blunts for administering cannabis. Addict Behav 2017; 70:79-82. doi:10.1016/j.addbeh.2017.02.020
- Regan AK, Promoff G, Dube SR, Arrazola R. Electronic nicotine delivery systems: adult use and awareness of the “e-cigarette”in the USA. Tob Control 2013; 22(1):19–23. doi:10.1136/tobaccocontrol-2011-050044
- Centers for Disease Control and Prevention. E-cigarette ads and youth. www.cdc.gov/vitalsigns/ecigarette-ads/index.html.
- Noel JK, Rees VW, Connolly GN. Electronic cigarettes: a new “tobacco” industry? Tob Control 2011; 20(1):81. doi:10.1136/tc.2010.038562
- US Food and Drug Administration. Deeming tobacco products to be subject to the federal food, drug, and cosmetic act, as amended by the family smoking prevention and tobacco control act; restrictions on the sale and distribution of tobacco products and required warning statements for tobacco products. Federal Register 2016; 81(90), May 10, 2016. www.govinfo.gov/content/pkg/FR-2016-05-10/pdf/2016-10685.pdf. Accessed November 14, 2019.
- Rom O, Pecorelli A, Valacchi G, Reznick AZ. Are e-cigarettes a safe and good alternative to cigarette smoking? Ann NY Acad Sci 2015; 1340:65–74. doi:10.1111/nyas.12609
- Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control 2014; 23(suppl 3):iii3-iii9. doi:10.1136/tobaccocontrol-2014-051670
- Kong G, Morean ME, Cavallo DA, Camenga DR, Krishnan-Sarin S. Reasons for electronic cigarette experimentation and discontinuation among adolescents and young adults. Nicotine Tob Res 2015; 17(7):847–854. doi:10.1093/ntr/ntu257
- Baca MC. How two Stanford grads aimed for big tech glory and got big tobacco instead. Updated September 4, 2019. The Washington Post September 4, 2019. www.washingtonpost.com/technology/2019/09/04/how-two-stanford-grads-aimed-big-tech-glory-got-big-tobacco-instead. Accessed November 14, 2019.
- Huang J, Duan Z, Kwok J, et al. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control 2019; 28(2):146–151. doi:10.1136/tobaccocontrol-2018-054382
- Walley SC, Wilson KM, Winickoff JP, Groner J. A public health crisis: electronic cigarettes, vape, and JUUL. Pediatrics 2019; 143(6):pii:e20182741. doi:10.1542/peds.2018-2741
- Zernike K. F.D.A. cracks down on “juuling” among teenagers. The New York Times April 24, 2018. www.nytimes.com/2018/04/24/health/fda-e-cigarettes-minors-juul.html. Accessed November 14, 2019.
- Ramamurthi D, Chau C, Jackler RK. JUUL and other stealth vaporisers: hiding the habit from parents and teachers. Tob Control 2018 Sep 15; pii:tobaccocontrol-2018-054455. doi:10.1136/tobaccocontrol-2018-054455. [Epub ahead of print]
- Willett JG, Bennett M, Hair EC, et al. Recognition, use and perceptions of JUUL among youth and young adults. Tob Control 2019; 28(1):115–116. doi:10.1136/tobaccocontrol-2018-054273
- Kaplan S. Juul’s new product: less nicotine, more intense vapor. New York Times Nov 27, 2018. www.nytimes.com/2018/11/27/health/juul-ecigarettes-nicotine.html.
- JUUL Labs. JUULpods. www.juul.com/shop/pods. Accessed November 14, 2019.
- Krishnan-Sarin S, Morean M, Kong G, et al. E-cigarettes and “dripping” among high-school youth. Pediatrics 2017; 139(3):pii:e20163224. doi:10.1542/peds.2016-3224
- Kosmider L, Sobczak A, Fik M, et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res 2014; 16(10):1319–1326. doi:10.1093/ntr/ntu078
- Rawlinson C, Martin S, Frosina J, Wright C. Chemical characterisation of aerosols emitted by electronic cigarettes using thermal desorption-gas chromatography-time of flight mass spectrometry. J Chromatogr A 2017; 1497:144–154. doi:10.1016/j.chroma.2017.02.050
- Lee MS, LeBouf RF, Son YS, Koutrakis P, Christiani DC. Nicotine, aerosol particles, carbonyls and volatile organic compounds in tobacco- and menthol-flavored e-cigarettes. Environ Health 2017; 16(1):42. doi:10.1186/s12940-017-0249-x
- Williams M, Bozhilov K, Ghai S, Talbot P. Elements including metals in the atomizer and aerosol of disposable electronic cigarettes and electronic hookahs. PLoS One 2017; 12(4):e0175430. doi:10.1371/journal.pone.0175430.
- Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 2014; 23(2):133–139. doi:10.1136/tobaccocontrol-2012-050859
- Drope J, Cahn Z, Kennedy R, et al. Key issues surrounding the health impacts of electronic nicotine delivery systems (ENDS) and other sources of nicotine. CA Cancer J Clin 2017; 67(6):449–471. doi:10.3322/caac.21413
- Jabba SV, Jordt SE. Risk analysis for the carcinogen pulegone in mint- and menthol-flavored e-cigarettes and smokeless tobacco products. JAMA Intern Med 2019 Sep 16 [Epub ahead of print]. doi:10.1001/jamainternmed.2019.3649
- Tierney PA, Karpinsky CD, Brown JE, Luo W, Pankow JF. Flavour chemicals in electronic cigarette fluids. Tob Control 2016; 25(e1):e10–e15. doi:10.1136/tobaccocontrol-2014-052175
- Behar RZ, Wang Y, Talbot P. Comparing the cytotoxicity of electronic cigarette fluids, aerosols and solvents. Tob Control 2017; 27(3):325–333. doi:10.1136/tobaccocontrol-2016-053472
- Martin EM, Clapp PW, Rebuli ME, et al. E-cigarette use results in suppression of immune and inflammatory-response genes in nasal epithelial cells similar to cigarette smoke. Am J Physiol Lung Cell Mol Physiol 2016; 311(1):L135–L144. doi:10.1152/ajplung.00170.2016
- Holden VK, Hines SE. Update on flavoring-induced lung disease. Curr Opin Pulm Med 2016;22(2):158–164. doi:10.1097/MCP.0000000000000250
- Siqueira L; Committee on Substance Use and Prevention. Nicotine and tobacco as substances of abuse in children and adolescents. Pediatrics 2017; 139(1):pii:e20163436. doi:10.1542/peds.2016-3436
- England LJ, Bunnell RE, Pechacek TF, Tong VT, McAfee TA. Nicotine and the developing human: a neglected element in the electronic cigarette debate. Am J Prev Med 2015; 49(2):286–293. doi:10.1016/j.amepre.2015.01.015
- Modesto-Lowe V, Alvarado C. E-cigs…are they cool? Talking to teens about e-cigarettes. Clin Pediatr (Phila) 2017; 51(10):947–952. doi:10.1177/0009922817705188
- Prochaska JJ, Benowitz NL. The past, present, and future of nicotine addiction therapy. Annu Rev Med 2017; 67:467–486. doi:10.1146/annurev-med-111314-033712
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004; 99(1):29–38. doi:10.1111/j.1360-0443.2004.00540.x
- McMillen RC, Gottlieb MA, Shaefer RM, Winickoff JP, Klein JD. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res 2015;17(10):119_1202. doi:10.1093/ntr/ntu213
- Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet 2013; 382(9905):1629–1637. doi:10.1016/S0140-6736(13)61842-5
- Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine replacement therapy. N Engl J Med 2019; 380(7):629–637. doi:10.1056/NEJMoa1808779
- Campagna D, Cibella F, Caponnetto P, et al. Changes in breathomics from a 1-year randomized smoking cessation trial of electronic cigarettes. Eur J Clin Invest 2016; 46(8):698–706. doi:10.1111/eci.12651
- Rehan HS, Maini J, Hungin APS. Vaping versus smoking: a quest for efficacy and safety of e-cigarette. Curr Drug Saf 2018; 13(2):92–101. doi:10.2174/1574886313666180227110556
- Zernike K. ‘I can’t stop’: schools struggle with vaping explosion. New York Times April 2, 2018. www.nytimes.com/2018/04/02/health/vaping-ecigarettes-addiction-teen.html.
- Pepper JK, Ribisl KM, Brewer NT. Adolescents’ interest in trying flavoured e-cigarettes. Tob Control 2016; 25(suppl 2):ii62–ii66. doi:10.1136/tobaccocontrol-2016-053174
- Harrell MB, Loukas A, Jackson CD, Marti CN, Perry CL. Flavored tobacco product use among youth and young adults: what if flavors didn’t exist? Tob Regul Sci 2017; 3(2):168–173. doi:10.18001/TRS.3.2.4
- Smith M. Amid vaping crackdown, Michigan to ban sale of flavored e-cigarettes. New York Times Sept 4, 2019. www.nytimes.com/2019/09/04/us/michigan-vaping.html?module=inline.
- Roditis ML, Halpern-Felsher B. Adolescents’ perceptions of risks and benefits of conventional cigarettes, e-cigarettes, and marijuana: a qualitative analysis. J Adolesc Health 2015; 57(2):179–185. doi:10.1016/j.jadohealth.2015.04.002
- Chapman S, Daube M, Maziak W. Should e-cigarette use be permitted in smoke-free public places? No. Tob Control 2017; 26(e1):e3–e4. doi:10.1136/tobaccocontrol-2016-053359
- Marcham CL, Springston JP. Electronic cigarettes in the indoor environment. Rev Env Health 2019; 34(2):105–124. doi:10.1515/reveh-2019-0012
- Chatham-Stephens K, Law R, Taylor E, et al; Centers for Disease Control and Prevention. Notes from the field: calls to poison centers for exposures to electronic cigarettes—United States, September 2010–September 2014. MMWR Morb Mortal Wkly Report 2014; 63(13):292–293. pmid:24699766
- Sood A, Kesic M, Hernandez M. Electronic cigarettes: one size does not fit all. J Allergy Clin Immunol 2018; 141(6):1973-1982. doi:10.1016/j.jaci.2018.02.029
- Romijnders K, van Osch L, de Vries H, Talhout R. Perceptions and reasons regarding e-cigarette use among users and non-users: a narrative literature review. Int J Environ Res Public Health 2018; 15(6):1190. doi:10.3390/ijerph15061190
- Zhu S, Zhuang Y-L, Wong S, Cummins SE, Tedeschi GJ. E-cigarette use and associated changes in population smoking cessation: evidence from US current population surveys. BMJ 2017; 358:j3262. doi:10.1136/bmj.j3262
- Centers for Disease Control and Prevention. Outbreak of lung injury associated with e-cigarette use, or vaping. Updated November 5, 2019. www.cdc.gov/tobacco/basic_information/e-Cigarettes/severe-Lung-Disease.html. Accessed November 14, 2019.
- US Public Health Services, US Department of Health and Human Services. E-cigarette use among youth and young adults: a report of the Surgeon General. Rockville, MD, U.S. Department of Health and Human Services, 2016. https://e-cigarettes.surgeongeneral.gov/documents/2016_sgr_full_report_non-508.pdf. Accessed November 14, 2019.
- Thomas K, Kaplan S. E-cigarettes went unchecked in 10 years of federal inaction. New York Times Oct 14, 2019; updated November 1, 2019. www.nytimes.com/2019/10/14/health/vaping-e-cigarettes-fda.html.
- Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA. Notes from the field: use of electronic cigarettes and any tobacco product among middle and high school students—United States, 2011–2018. MMWR Morb Mortal Wkly Rep 2018; 67(45):1276–1277. doi:10.15585/mmwr.mm6745a5
- Gentzke A, Creamer M, Cullen K, et al; Centers for Disease Control and Prevention. Vital signs: tobacco product use among middle and high school students—United States, 2011–2018. MMWR Morb Mortal Wkly Rep 2019; 68(6):157–164. doi:10.15585/mmwr.mm6806e1
- Hammig B, Daniel-Dobbs P, Blunt-Vinti H. Electronic cigarette initiation among minority youth in the United States. Am J Drug Alcohol Abuse 2017; 43(3):306–310. doi:10.1080/00952990.2016.1203926
- Primack BA, Soneji S, Stoolmiller M, Fine MJ, Sargent JD. Progression to traditional cigarette smoking after electronic cigarette use among U.S. adolescents and young adults. JAMA Pediatr 2015; 169(11):1018–1023. doi:10.1001/jamapediatrics.2015.1742
- Leventhal AM, Strong DR, Kirkpatrick MG, et al. Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence. JAMA 2015; 314(7):700–707. doi:10.1001/jama.2015.8950
- Wills TA, Knight R, Sargent JD, Gibbons FX, Pagano I, Williams RJ. Longitudinal study of e-cigarette use and onset of cigarette smoking among high school students in Hawaii. Tob Control 2016; 26(1):34–39. doi:10.1136/tobaccocontrol-2015-052705
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The health consequences of smoking—50 years of progress: a report of the Surgeon General. Atlanta: Centers for Disease Control and Prevention, 2014. www.ncbi.nlm.nih.gov/books/NBK179276/pdf/Bookshelf_NBK179276.pdf. Accessed November 14, 2019.
- Christiani DC. Vaping-induced lung injury. N Engl J Med 2019; Sept 6. Epub ahead of print. doi:10.1056/NEJMe1912032
- Neel J, Aubrey A. Vitamin E suspected in serious lung problems among people who vaped cannabis. NPR Sept 5, 2019. www.npr.org/sections/health-shots/2019/09/05/758005409/vitamin-e-suspected-in-serious-lung-problems-among-people-who-vaped-cannabis. Accessed November 14, 2019.
- White A. Plans for the first e-cigarette went up in smoke 50 years ago. Smithsonian Magazine December 2018. www.smithsonianmag.com/innovation/plans-for-first-e-cigarette-went-up-in-smoke-50-years-ago-180970730.
- Blundell MS, Dargan PI, Wood DM. The dark cloud of recreational drugs and vaping. QJM 2018; 111(3):145–148. doi:10.1093/qjmed/hcx049
- Schulenberg JE, Johnston LD, O’Malley PM, Bachman JG, Miech RA, Patrick ME. Monitoring the future: national survey results on drug use, 1975–2018. 2018 Volume 2. College students & adults ages 19–60. www.monitoringthefuture.org/pubs/monographs/mtf-vol2_2018.pdf. Accessed November 14, 2019.
- Eggers ME, Lee YO, Jackson J, Wiley JL, Porter J, Nonnemaker JM. Youth use of electronic vapor products and blunts for administering cannabis. Addict Behav 2017; 70:79-82. doi:10.1016/j.addbeh.2017.02.020
- Regan AK, Promoff G, Dube SR, Arrazola R. Electronic nicotine delivery systems: adult use and awareness of the “e-cigarette”in the USA. Tob Control 2013; 22(1):19–23. doi:10.1136/tobaccocontrol-2011-050044
- Centers for Disease Control and Prevention. E-cigarette ads and youth. www.cdc.gov/vitalsigns/ecigarette-ads/index.html.
- Noel JK, Rees VW, Connolly GN. Electronic cigarettes: a new “tobacco” industry? Tob Control 2011; 20(1):81. doi:10.1136/tc.2010.038562
- US Food and Drug Administration. Deeming tobacco products to be subject to the federal food, drug, and cosmetic act, as amended by the family smoking prevention and tobacco control act; restrictions on the sale and distribution of tobacco products and required warning statements for tobacco products. Federal Register 2016; 81(90), May 10, 2016. www.govinfo.gov/content/pkg/FR-2016-05-10/pdf/2016-10685.pdf. Accessed November 14, 2019.
- Rom O, Pecorelli A, Valacchi G, Reznick AZ. Are e-cigarettes a safe and good alternative to cigarette smoking? Ann NY Acad Sci 2015; 1340:65–74. doi:10.1111/nyas.12609
- Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control 2014; 23(suppl 3):iii3-iii9. doi:10.1136/tobaccocontrol-2014-051670
- Kong G, Morean ME, Cavallo DA, Camenga DR, Krishnan-Sarin S. Reasons for electronic cigarette experimentation and discontinuation among adolescents and young adults. Nicotine Tob Res 2015; 17(7):847–854. doi:10.1093/ntr/ntu257
- Baca MC. How two Stanford grads aimed for big tech glory and got big tobacco instead. Updated September 4, 2019. The Washington Post September 4, 2019. www.washingtonpost.com/technology/2019/09/04/how-two-stanford-grads-aimed-big-tech-glory-got-big-tobacco-instead. Accessed November 14, 2019.
- Huang J, Duan Z, Kwok J, et al. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control 2019; 28(2):146–151. doi:10.1136/tobaccocontrol-2018-054382
- Walley SC, Wilson KM, Winickoff JP, Groner J. A public health crisis: electronic cigarettes, vape, and JUUL. Pediatrics 2019; 143(6):pii:e20182741. doi:10.1542/peds.2018-2741
- Zernike K. F.D.A. cracks down on “juuling” among teenagers. The New York Times April 24, 2018. www.nytimes.com/2018/04/24/health/fda-e-cigarettes-minors-juul.html. Accessed November 14, 2019.
- Ramamurthi D, Chau C, Jackler RK. JUUL and other stealth vaporisers: hiding the habit from parents and teachers. Tob Control 2018 Sep 15; pii:tobaccocontrol-2018-054455. doi:10.1136/tobaccocontrol-2018-054455. [Epub ahead of print]
- Willett JG, Bennett M, Hair EC, et al. Recognition, use and perceptions of JUUL among youth and young adults. Tob Control 2019; 28(1):115–116. doi:10.1136/tobaccocontrol-2018-054273
- Kaplan S. Juul’s new product: less nicotine, more intense vapor. New York Times Nov 27, 2018. www.nytimes.com/2018/11/27/health/juul-ecigarettes-nicotine.html.
- JUUL Labs. JUULpods. www.juul.com/shop/pods. Accessed November 14, 2019.
- Krishnan-Sarin S, Morean M, Kong G, et al. E-cigarettes and “dripping” among high-school youth. Pediatrics 2017; 139(3):pii:e20163224. doi:10.1542/peds.2016-3224
- Kosmider L, Sobczak A, Fik M, et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res 2014; 16(10):1319–1326. doi:10.1093/ntr/ntu078
- Rawlinson C, Martin S, Frosina J, Wright C. Chemical characterisation of aerosols emitted by electronic cigarettes using thermal desorption-gas chromatography-time of flight mass spectrometry. J Chromatogr A 2017; 1497:144–154. doi:10.1016/j.chroma.2017.02.050
- Lee MS, LeBouf RF, Son YS, Koutrakis P, Christiani DC. Nicotine, aerosol particles, carbonyls and volatile organic compounds in tobacco- and menthol-flavored e-cigarettes. Environ Health 2017; 16(1):42. doi:10.1186/s12940-017-0249-x
- Williams M, Bozhilov K, Ghai S, Talbot P. Elements including metals in the atomizer and aerosol of disposable electronic cigarettes and electronic hookahs. PLoS One 2017; 12(4):e0175430. doi:10.1371/journal.pone.0175430.
- Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 2014; 23(2):133–139. doi:10.1136/tobaccocontrol-2012-050859
- Drope J, Cahn Z, Kennedy R, et al. Key issues surrounding the health impacts of electronic nicotine delivery systems (ENDS) and other sources of nicotine. CA Cancer J Clin 2017; 67(6):449–471. doi:10.3322/caac.21413
- Jabba SV, Jordt SE. Risk analysis for the carcinogen pulegone in mint- and menthol-flavored e-cigarettes and smokeless tobacco products. JAMA Intern Med 2019 Sep 16 [Epub ahead of print]. doi:10.1001/jamainternmed.2019.3649
- Tierney PA, Karpinsky CD, Brown JE, Luo W, Pankow JF. Flavour chemicals in electronic cigarette fluids. Tob Control 2016; 25(e1):e10–e15. doi:10.1136/tobaccocontrol-2014-052175
- Behar RZ, Wang Y, Talbot P. Comparing the cytotoxicity of electronic cigarette fluids, aerosols and solvents. Tob Control 2017; 27(3):325–333. doi:10.1136/tobaccocontrol-2016-053472
- Martin EM, Clapp PW, Rebuli ME, et al. E-cigarette use results in suppression of immune and inflammatory-response genes in nasal epithelial cells similar to cigarette smoke. Am J Physiol Lung Cell Mol Physiol 2016; 311(1):L135–L144. doi:10.1152/ajplung.00170.2016
- Holden VK, Hines SE. Update on flavoring-induced lung disease. Curr Opin Pulm Med 2016;22(2):158–164. doi:10.1097/MCP.0000000000000250
- Siqueira L; Committee on Substance Use and Prevention. Nicotine and tobacco as substances of abuse in children and adolescents. Pediatrics 2017; 139(1):pii:e20163436. doi:10.1542/peds.2016-3436
- England LJ, Bunnell RE, Pechacek TF, Tong VT, McAfee TA. Nicotine and the developing human: a neglected element in the electronic cigarette debate. Am J Prev Med 2015; 49(2):286–293. doi:10.1016/j.amepre.2015.01.015
- Modesto-Lowe V, Alvarado C. E-cigs…are they cool? Talking to teens about e-cigarettes. Clin Pediatr (Phila) 2017; 51(10):947–952. doi:10.1177/0009922817705188
- Prochaska JJ, Benowitz NL. The past, present, and future of nicotine addiction therapy. Annu Rev Med 2017; 67:467–486. doi:10.1146/annurev-med-111314-033712
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004; 99(1):29–38. doi:10.1111/j.1360-0443.2004.00540.x
- McMillen RC, Gottlieb MA, Shaefer RM, Winickoff JP, Klein JD. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res 2015;17(10):119_1202. doi:10.1093/ntr/ntu213
- Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet 2013; 382(9905):1629–1637. doi:10.1016/S0140-6736(13)61842-5
- Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine replacement therapy. N Engl J Med 2019; 380(7):629–637. doi:10.1056/NEJMoa1808779
- Campagna D, Cibella F, Caponnetto P, et al. Changes in breathomics from a 1-year randomized smoking cessation trial of electronic cigarettes. Eur J Clin Invest 2016; 46(8):698–706. doi:10.1111/eci.12651
- Rehan HS, Maini J, Hungin APS. Vaping versus smoking: a quest for efficacy and safety of e-cigarette. Curr Drug Saf 2018; 13(2):92–101. doi:10.2174/1574886313666180227110556
- Zernike K. ‘I can’t stop’: schools struggle with vaping explosion. New York Times April 2, 2018. www.nytimes.com/2018/04/02/health/vaping-ecigarettes-addiction-teen.html.
- Pepper JK, Ribisl KM, Brewer NT. Adolescents’ interest in trying flavoured e-cigarettes. Tob Control 2016; 25(suppl 2):ii62–ii66. doi:10.1136/tobaccocontrol-2016-053174
- Harrell MB, Loukas A, Jackson CD, Marti CN, Perry CL. Flavored tobacco product use among youth and young adults: what if flavors didn’t exist? Tob Regul Sci 2017; 3(2):168–173. doi:10.18001/TRS.3.2.4
- Smith M. Amid vaping crackdown, Michigan to ban sale of flavored e-cigarettes. New York Times Sept 4, 2019. www.nytimes.com/2019/09/04/us/michigan-vaping.html?module=inline.
- Roditis ML, Halpern-Felsher B. Adolescents’ perceptions of risks and benefits of conventional cigarettes, e-cigarettes, and marijuana: a qualitative analysis. J Adolesc Health 2015; 57(2):179–185. doi:10.1016/j.jadohealth.2015.04.002
- Chapman S, Daube M, Maziak W. Should e-cigarette use be permitted in smoke-free public places? No. Tob Control 2017; 26(e1):e3–e4. doi:10.1136/tobaccocontrol-2016-053359
- Marcham CL, Springston JP. Electronic cigarettes in the indoor environment. Rev Env Health 2019; 34(2):105–124. doi:10.1515/reveh-2019-0012
- Chatham-Stephens K, Law R, Taylor E, et al; Centers for Disease Control and Prevention. Notes from the field: calls to poison centers for exposures to electronic cigarettes—United States, September 2010–September 2014. MMWR Morb Mortal Wkly Report 2014; 63(13):292–293. pmid:24699766
KEY POINTS
- Vaping is a common gateway to tobacco and marijuana use for adolescents and adults.
- The Juul vaping device delivers high nicotine concentrations that may pose a higher risk of nicotine addiction.
- Vaping has had unintended consequences that include poisoning of children who swallowed liquid nicotine, fires and explosions from defective batteries in the devices, and effects on the developing brain.
- Vaping is associated with respiratory illness and, in rare cases, death, likely due to vaporized agents introduced into the lungs. Small amounts of heavy metals, acetone, and other carcinogenic compounds in the vaping aerosol may cause lung damage.
Recognizing, managing medical consequences of eating disorders in primary care
Eating disorders are debilitating biopsychosocial illnesses associated with serious medical illness and a high risk of death.1
Primary care physicians are often the first to see young women who have these problems, diagnose them, and start their evaluation and treatment.2–4 Many patients require acute medical interventions as well as long-term care for chronic medical issues. Therefore, primary care physicians play essential front-line and long-term roles in the multidisciplinary treatment team.
DEFINITIONS OF EATING DISORDERS HAVE CHANGED
Several problems existed in the category of eating disorders in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-4) and in the DSM-4 Text Revision (DSM-4-TR). These problems have been addressed in the fifth edition (DSM-5), released in 2013.5
One problem in the earlier editions was that many patients referred for treatment of eating disorders—more than 50% in one study6—did not meet the criteria for anorexia nervosa or bulimia nervosa and thus had to be categorized as having “eating disorder not otherwise specified.” Further, the earlier editions did not recognize that young children and adolescent males can be affected.7
Eating disorders are now recognized as an equal-opportunity disease, with all ethnic and socioeconomic groups affected. Children can run into medical trouble with even a small amount of weight loss or falling off the growth curve. Moreover, children and adolescents do not “experience” their bodies in the same way adults do; they may lack the vocabulary for eating-disorder thoughts.
For these reasons, the definitions of eating disorders have changed in the DSM-5.5
Anorexia nervosa. Older editions of the DSM listed amenorrhea as a criterion. This has been eliminated in DSM-5, since amenorrhea does not necessarily predict medical risk or treatment outcome; also, it is not applicable to males or premenorrheal girls and postmenopausal women.8 In addition, the requirement of low weight is now defined in the context of “age, sex, developmental trajectory, and physical health,” rather than the old threshold of 85% of expected weight.9
What remains unchanged is that anorexia nervosa is still characterized by self-starvation in order to maintain an abnormally low body weight, along with an intense fear of being fat and a disturbed self-image.
Bulimia nervosa. In both the old and the new editions of the DSM, bulimia nervosa is characterized by episodes of binge eating followed by inappropriate compensatory behaviors to avoid weight gain, such as vomiting, laxative abuse, diuretic abuse, and overexercise. In DSM-5, bulimia nervosa no longer has subtypes and requires only one binge per week with compensatory behavior, for at least 3 months. This change was based on the finding that there is no clear difference in psychopathology or treatment outcome between patients with one and two binge-purge episodes a week.10
“Eating disorder not otherwise specified” was a wastebasket category, lumping all those who did not meet the criteria for anorexia nervosa or bulimia nervosa or who did not neatly fit into a specific category.10 In DSM-5, subcategories were designed to help distinguish different treatment needs and outcomes between various subtypes.
Binge-eating disorder, one of the new subcategories, is characterized by binge eating without inappropriate compensatory behaviors.9 Patients with binge-eating disorder are often obese, have greater functional impairment, and are more likely to develop components of metabolic syndrome than obese patients without eating disorders.11
Avoidant/restrictive food intake disorder is another new DSM-5 diagnosis, characterized by failure to meet nutritional needs for reasons other than weight control. Reasons include disinterest in eating, dislike of sensory characteristics of food, or avoidance of consequences of eating. This disorder replaces the category “feeding disorder of infancy or early childhood,” since the condition can also occur in adolescents and adults.12
Other new diagnoses are:
- Atypical anorexia nervosa (if the patient is not underweight)
- Purging disorder
- Subthreshold bulimia nervosa (if the patient has < 1 episode per week or has had them for < 3 months)
- Subthreshold binge eating disorder (< 1 time a week or < 3 months)
- Night eating syndrome
- Pica and rumination disorder.
Regardless of the diagnostic label, the medical evaluation and treatment of anyone with an eating disorder should be tailored to the specific behaviors of the eating disorder. Medical complications can be subdivided into those from starvation, from purging, and from refeeding.
MEDICAL COMPLICATIONS OF STARVATION
Cardiovascular effects of starvation
Malnutrition and starvation have multiple adverse effects on the heart.
Electrophysiologic effects. Sinus bradycardia (< 60 bpm) and hypotension are common cardiac manifestations of starvation.13 Bradycardia has been attributed to an adaptive increase in parasympathetic vagal tone.14 QTc prolongation is also seen in patients with malnutrition.15
Together, these electrocardiographic abnormalities predispose the patient to ventricular arrhythmia and sudden cardiac death.16 The risk of ventricular arrhythmia is particularly relevant when treating psychiatric symptoms, since antipsychotics and tricyclic antidepressants are among several drug classes that can cause further QTc prolongation (Table 1).17,18
In patients with QTc prolongation, bradycardia, or both, the standard of care involves acute hospitalization for refeeding using continuous telemetric monitoring until normal rhythm is restored and the heart rate is above 40 at night and 50 by day.4,19
Structural changes. Starvation also causes structural changes in the heart. Loss of lean body mass can reduce cardiac muscle mass, compromise cardiac output, and lead to mitral valve prolapse.20 These changes are fully reversible with restored nutrition and regaining of heart mass.21,22
Effects of starvation on the brain
Starvation can affect brain structure and cognitive function. Undernourished patients have reduced volumes of white and gray matter, a change that can occur within months. Cortical volumes may increase with weight gain, but a reduction in gray matter volume may not be completely reversible.23
Furthermore, starvation impairs cognitive functions that are needed to stop eating-disorder behaviors; namely, decision-making, emotional control, regulation of appetite, and reward path-ways. Therefore, undernourished patients may not have sufficient insight into the disease to be able to make the best choices for recovery. This finding lends support for using the Maudsley method in adolescents, in which parents take control of their child’s eating until the child can maintain a healthy weight.24
Gastrointestinal consequences of starvation
Patients with malnutrition have prolonged gastric emptying and colonic transit time with solid foods.25 They often complain of early satiety, abdominal pain, bloating, and constipation, all symptoms that complicate the refeeding process. A prokinetic such as metoclopramide (Reglan), given 1 hour before meals and at bedtime, may provide some relief from gastrointestinal symptoms.26
Patients may also experience transient lactose or fructose intolerance after prolonged starvation. Taking a lactase supplement (eg, Lactaid 1–10 tabs) before consuming dairy products and dextrose (contained in candies such as Smarties) before eating fruit or fructose-containing foods can sometimes partially relieve symptoms. In general, gastrointestinal function returns over time as nutritional status improves.
Patients with severe or prolonged starvation can develop steatosis accompanied by elevated levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). In reports of starvation-induced steatosis, liver enzyme levels rapidly normalize with nutritional rehabilitation.27
Endocrine consequences of starvation
Amenorrhea. Dysregulation of the hypothalamic-pituitary-gonadal axis is a major endocrine complication of nutritional in-sufficiency. Weight loss disrupts the normal pulsatile secretion of gonadotropin-releasing hormone, reduces secretion of luteinizing hormone and follicle-stimulating hormone, and decreases estrogen levels.28 Leptin deficiency likely plays a role in suppressing gonadotropin secretion with subsequent development of amenorrhea. With weight gain, levels of leptin and gonadotropins normalize and menstruation eventually returns.29,30
Hypothyroidism. Starvation can also lead to dysregulation of the hypothalamic-pituitary-thyroid axis. Typically, the concentration of triiodothyronine (T3) is reduced, the ratio of thyroxine (T4) to T3 is elevated, and thyroid-stimulating hormone (TSH) is close to or within the normal range, creating a euthyroid sick syndrome. In eating disorders, this thyroid disturbance is a result of starvation and resolves with weight restoration. Therefore, thyroid hormone replacement therapy is not medically indicated.28
Osteoporosis. Amenorrhea resulting from low estrogen levels in undernourished patients can raise the risk of osteoporosis and fractures, particularly in patients with a low body mass index. Osteopenia results from a negative balance between bone deposition and resorption.
Lack of bone deposition can be especially problematic when disordered eating occurs during peak bone mass development, ie, ages 11 to 14 for girls, and ages 15 to 17 for boys.31,32 Even a 5% to 10% decrease in bone deposition can result in significant risk of osteopenia.33 However, after age 30, bone resorption is a greater contributor.34
Does hormone therapy correct bone loss? Given the association between estrogen deficiency and bone loss, estrogen supplementation was expected to be an effective treatment for bone loss in patients with eating disorders.35 Also, the restoration of menses through hormone replacement may give underweight patients a false sense of achieving a “healthy” weight.36
Golden et al37 prospectively studied 50 adolescents and found no significant difference in bone mineral density at 1 year of follow-up between patients treated with estrogen and those who received only standard nutritional therapy. However, increased bone mineral density was achieved in adolescents with anorexia nervosa treated with transdermally administered estrogen dosed to mimic physiologic pubertal levels.38
Klibanski et al39 found that hormone therapy resulted in a 4% gain in bone density in an extremely low-weight subset of women with anorexia nervosa (< 70% of ideal body weight), whereas similar patients in the control group lost 20%. However, in all groups, only weight gain correlated with bone gain in women who were within 70% of their ideal body weight.
Divasta et al40 evaluated 60 girls and women ages 13 to 27 with anorexia nervosa, randomized to receive either placebo or dehydroepiandrosterone combined with an estrogen-progestin oral contraceptive, and followed for 18 months. As in the study by Klibanski et al,39 bone loss was prevented in the treatment group, but significant bone gain occurred only in the context of weight gain.
The bottom line is that only weight gain has resulted in significant increases in bone density in patients with anorexia nervosa, and hormone therapy without weight gain has not been shown to increase bone density effectively in this population. Although calcium and vitamin D in oral therapeutic doses through foods or through supplementation are required for bone gain, the combination is not enough to augment bone density in the absence of weight gain.37 Although not curative, weight gain is currently the best option for treating bone loss, and no single pharmacologic treatment is effective.
COMPLICATIONS OF PURGING
Oral complications of purging
Patients who purge by vomiting are at risk of complications from exposure of the esophagus, pharynx, and mouth to acidic gastric contents.
Dental problems. Over time, contact with gastric acid wears down enamel on the lingual and occlusal surfaces of teeth, resulting in dental caries and periodontal disease. Until they can give up purging, patients should be instructed to rinse with mouthwash or water immediately after vomiting to reduce the acidity in the mouth.41,42 We recommend that patients not brush their teeth after vomiting, because brushing can deliver acid to otherwise unreachable surfaces and thus worsen tooth erosion. For patients who are determined to brush after vomiting, a bicarbonate toothpaste might mitigate harm.42
Sialadenosis (hypertrophy of the salivary glands) is another consequence of repeated vomiting, with elevated salivary amylase. Both the size of the glands and the salivary amylase level generally normalize on their own after vomiting is stopped, but parotitis can take up to a year to resolve. Similar to smoker’s cough, parotitis may acutely worsen when the patient abruptly stops vomiting and may worsen before it improves.
To reduce discomfort, patients can use hot compresses or sugarless hard candies.44 However, the latter should not be substituted as a chronic habit in a patient with disordered eating. Patients need to be reassured that the swelling is not permanent, since they often interpret it as having fat cheeks (the “chipmunk sign”).
Hypokalemia, metabolic alkalosis, renal dysfunction
Chronic vomiting can cause electrolyte and acid-base imbalances, the most worrisome of which is hypokalemia. With repeated vomiting, loss of potassium and gastric acid causes metabolic alkalosis with hypokalemia, hypochloremia, and hypomagnesemia. Loss of water and the resultant volume contraction activates the renin-angiotensin-aldosterone system, and elevated aldosterone further decreases serum potassium.
In patients with eating disorders, who often have other factors contributing to electrolyte imbalance, vomiting-induced hypokalemia heightens the risk of cardiac arrhythmias.43
Hypokalemia can also cause rhabdomyolysis and kidney damage.41,43 Prolonged hypokalemia and reduced kidney perfusion in the setting of volume depletion causes acute kidney injury and impaired concentrating ability of the renal tubules. Hypovolemia can cause prerenal azotemia and increases the risk for nephrolithiasis and nephrocalcinosis.44,45
When a patient stops vomiting, elevated aldosterone from prior hypovolemia results in water retention and can manifest in significant edema associated with hypochloremic alkalosis. This condition, known as pseudo-Bartter syndrome, usually resolves without treatment. In the meantime, salt restriction and leg elevation can help reduce edema.26
Laxative abuse: A mode of purging
Many patients with eating disorders abuse laxatives to lose weight or to prevent weight gain. Believing that laxatives will prevent calorie absorption, patients commonly take them to compensate for caloric intake (eg, during a binge episode). The immediate weight loss, albeit artificial, is highly reinforcing for an eating-disorder patient. In some cases, patients with eating disorders also abuse laxatives to self-treat the constipation that results from chronic starvation.46
Over time, tolerance to laxatives develops, and patients use increasingly larger doses. This can lead to activation of the renin-angiotensin-aldosterone system.47 Patients interpret the resultant edema as true weight gain and again take laxatives to get rid of it. If laxatives are stopped abruptly, the patient may need inpatient and outpatient support for the resultant fluid shifts.
Gastrointestinal complications of laxative abuse include reflex hypofunction of the bowel, malabsorption, steatorrhea, and gastrointestinal bleeding.47 Reflex hypofunction during laxative withdrawal is a consequence of the bowel becoming tolerant of laxatives.48 Cathartic colon syndrome is a rare complication characterized by loss of the normal haustral markings and slowed or absent peristalsis in segments of the colon.49
Systemically, the major risk of laxative abuse relates to electrolyte and acid-base imbalance. Loss of potassium and water in the stool can cause hypokalemia and metabolic alkalosis.48 The disturbances caused by laxative abuse are similar to those caused by vomiting and diuretic use and have the same treatment.
The most important component of treating laxative abuse is giving patients realistic expectations to help them tolerate temporary discomfort and to help manage the edema and fluid shifts that can happen acutely with shifting of fluid into the intracellular space. In extreme cases, this may need to be managed in the hospital. To help relieve the initial anxiety, doctors should emphasize that any bloating the patient experiences is not true weight gain and will go away within a few days to weeks. In addition, explaining that laxatives reduce nutrient absorption only minimally may lessen the temptation to resume taking them.48
Diuretic abuse: Another form of purging
Diuretic abuse is yet another mode of purging, with its own set of medical complications. Like laxatives, diuretics are not effective weight-loss agents, and the weight reduction they cause is only temporary.
As with vomiting, there is a compensatory activation of the renin-angiotensin-aldosterone system, and therefore subsequent fluid intake will lead to water retention, which encourages further diuretic use.41 Diuretics can also contribute to hypokalemia, hypomagnesemia, hypochloremia, and metabolic alkalosis.
Ipecac abuse can lead to heart failure
Ipecac syrup has long been used to induce vomiting, but this practice has become much less common since ipecac has become harder to obtain in the United States.50 The emetine base contained in ipecac binds irreversibly to cardiac and skeletal muscle. With continued use, irreversible cardiomyopathy develops and can lead to heart failure. Treatment should include supportive care and immediate cessation of ipecac use.
Diabetic patients may skip insulin to lose weight
Patients with diabetes, especially those with type 1 that begins in childhood, are at greater risk of eating disorders over time.51 They may withhold insulin to lose weight, a practice referred to in the nonmedical literature as “diabulimia,” and they seem particularly more likely to develop bulimia nervosa than those without diabetes.52
The medical prognosis is poor for patients with diabetes who develop eating disorders and do not receive intensive treatment.51 In addition, if a diabetic patient on an insulin pump becomes depressed in addition to having an eating disorder, careful monitoring for suicidal thoughts and a rapid follow-up with mental health services are in order.
REFEEDING SYNDROME
When refeeding is started, a high glucose load stimulates insulin secretion, resulting in cellular uptake of phosphorus along with potassium, magnesium, and glucose. In addition, total body phosphorus is depleted by the increased demand for adenosine triphosphate and 2,3-diphosphoglycerate for cellular metabolism.
When liver enzyme levels increase, the astute clinician will closely monitor the patient for evidence of refeeding syndrome. In a child, adolescent, or young adult, the standard of care is inpatient monitoring for acute stabilization.4,19
Hypophosphatemia is the hallmark of refeeding syndrome, although hypomagnesemia, hypokalemia, and hypoglycemia can also occur.53 In addition, sodium and water retention can lead to fluid overload, with shifting of fluid into the intracellular space, resulting in dependent edema.
Cardiovascular complications are the most worrisome manifestations of refeeding syndrome. Electrolyte shifts and increased fluid volume can cause arrhythmias and heart failure. Furthermore, severely undernourished patients may have reduced myocardial mass as well as electrocardiographic abnormalities associated with starvation, which further increase their vulnerability to electrolyte shifts and fluid retention during refeeding.15
Other manifestations of refeeding syndrome include delirium, seizures, rhabdomyolysis, and respiratory failure. In the most extreme cases, refeeding syndrome causes sudden death.53
Fortunately, refeeding syndrome is easily preventable and treatable when recognized early. Electrolytes and cardiovascular and renal function must be carefully monitored, especially during the first week of nutritional restoration.53 In patients with extremely low body mass (< 70% of ideal body weight) or with precipitous weight loss, close monitoring of the complete metabolic panel including electrolytes, AST, ALT, calcium, magnesium, and phosphorus may be required to detect changes that can affect cardiac status. Specific suggestions for refeeding are discussed below and in Table 2.45
ACUTE CARE OF PATIENTS WITH EATING DISORDERS
Refeeding in the inpatient setting
The decision to hospitalize an eating-disorder patient is based on the current or potential risk of serious medical complications and the likelihood of success at home. Medical criteria for hospital admission are outlined in Table 3.4,54
In refeeding undernourished patients, the challenge is to maximize weight gain while preventing refeeding syndrome. Undernourished patients are generally hypometabolic at baseline but become hypermetabolic once refeeding begins.
How many calories should refeeding start with? The traditional principle of “start low and go slow” has been recently challenged.55 Starting at 1,200 kcal/day or less in the typical patient can result in failure to gain weight or even in weight loss in the first week of refeeding.56 The goal is to achieve a weight gain of 0.2 kg/day while the patient is in the hospital. Thus, we start higher, and to date we have seen no cases of life-threatening refeeding syndrome. In all patients who need hospitalization or who are beginning the refeeding process as outpatients, caloric intake should be started at 1,500 to 2,000 kcal/day.45,57 However, for exceptionally low-weight patients, intake may be started lower.
In Australia, patients are started at 1,900 kcal/day.56 All patients in one program there receive nasogastric feeding initially in an intensive care unit and then are moved to a regular nursing floor where they graduate to full oral feeding as they improve cardiovascularly and behaviorally. In the United States, some programs use nasogastric feeding at night for caloric restoration; our program and others use nasogastric feeding as a behavioral modification strategy for patients who refuse food or supplements by mouth.
Phosphorus supplementation. Many centers give phosphorus supplements preventively. In our center, we give potassium phosphate (Neutra-Phos) 500 mg orally twice daily for 5 days, and we have seen no life-threatening cases of refeeding syndrome with that regimen. Other centers give phosphorus supplements in a dose of 250 mg orally twice a day for 5 days, while still others only supplement phosphorus reactively once a deficit has been identified. The latter method requires daily blood draws for monitoring and is reactive rather than proactive. Further studies can help clarify the optimal dosing and timing of phosphorus supplementation.
Managing fluid balance. Fluid-loading these patients may tip them over the edge into refeeding syndrome. Except in cases of shock, patients with eating disorders should not be given intravenous fluids, as it is safer to rehydrate and feed them orally. Electrolyte imbalances can be corrected orally with no need for intravenous supplementation. To avoid fluid overload, fluids can be started at 1,500 mL to 2,000 mL per day, with strict monitoring of intake and output. Fluids are liberalized if ALT and AST levels remain normal and to gradually correct orthostatic hypotension; caloric fluids are ideal to help address energy needs and improve bradycardia.
Laboratory monitoring. On admission, a urinalysis, complete blood cell count, complete metabolic panel, TSH, erythrocyte sedimentation rate, serum magnesium, and phosphorus should be obtained.26 In addition, continuous electrocardiographic recording should begin on admission.45 Inpatient use of a telemetry bed helps identify extreme tachycardia with arrhythmia, as well as profound bradycardia.45,56
Some protocols call for daily laboratory monitoring, although that degree of testing is less cost-effective. If initial results are normal, clinical judgment can be used on when to repeat laboratory evaluation. For instance, patients with edema require repeat complete metabolic panels to assess for elevated ALT and AST, electrolyte imbalances, and other abnormalities.
Signs of refeeding syndrome include tachycardia, hepatosplenomegaly, peripheral edema, altered mental status, and electrolyte disturbances, specifically, acute or severe hypophosphatemia or hypokalemia.26,45 If refeeding syndrome is suspected, the rate of caloric intake should be reduced or not advanced, fluid intake should be urgently reassessed for volume overload, and supportive care with close monitoring should be provided.
KNOWLEDGE SAVES LIVES
Eating disorders can lead to potentially life-threatening medical complications that require attentive care by the primary care clinician and subspecialist. Without thoughtful consideration, it is easy for even a caring medical team to unintentionally enable patients with these illnesses or to cause active harm in the case of underrecognized pathology.58
Acute medical stabilization on an inpatient unit trained to recognize pathology and treat sequelae can be lifesaving. Arming patients and families with medical knowledge, as provided in the Academy for Eating Disorders’ brochure, “Critical Points for Early Recognition and Medical Risk Management in the Care of Individuals with Eating Disorders”59 can help save patients’ lives.
- Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch Gen Psychiatry 2011; 68:724–731.
- Walsh JM, Wheat ME, Freund K. Detection, evaluation, and treatment of eating disorders the role of the primary care physician. J Gen Intern Med 2000; 15:577–590.
- American Academy of Pediatrics; Committee on Adolescence. Identifying and treating eating disorders. Pediatrics 2003; 111:204–211.
- Rosen DS; American Academy of Pediatrics Committee on Adolescence. Identification and management of eating disorders in children and adolescents. Pediatrics 2010; 126:1240–1253.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Arlington, VA: American Psychiatric Publishing, Incorporated; 2013.
- Eddy KT, Celio Doyle A, Hoste RR, Herzog DB, le Grange D. Eating disorder not otherwise specified in adolescents. J Am Acad Child Adolesc Psychiatry 2008; 47:156–164.
- Muise AM, Stein DG, Arbess G. Eating disorders in adolescent boys: a review of the adolescent and young adult literature. J Adolesc Health 2003; 33:427–435.
- Attia E, Roberto CA. Should amenorrhea be a diagnostic criterion for anorexia nervosa? Int J Eat Disord 2009; 42:581–589.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, fifth edition. http://dsm.psychiatryonline.org/content.aspx?bookid=556§ionid=41101776#103439089. Accessed January 31, 2014.
- Wilfley DE, Bishop ME, Wilson GT, Agras WS. Classification of eating disorders: toward DSM-V. Int J Eat Disord 2007; 40:S123–S129.
- Wonderlich SA, Gordon KH, Mitchell JE, Crosby RD, Engel SG. The validity and clinical utility of binge eating disorder. Int J Eat Disord 2009; 42:687–705.
- Ornstein RM, Rosen DS, Mammel KA, et al. Distribution of eating disorders in children and adolescents using the proposed DSM-5 criteria for feeding and eating disorders. J Adolesc Health 2013: 53:303–305.
- Winston AP, Stafford PJ. Cardiovascular effects of anorexia nervosa. Eur Eat Disord Rev 2000; 8:117–125.
- Galetta F, Franzoni F, Prattichizzo F, Rolla M, Santoro G, Pentimone F. Heart rate variability and left ventricular diastolic function in anorexia nervosa. J Adolesc Health 2003; 32:416–421.
- McCallum K, Bermudez O, Ohlemeyer C, Tyson E, Portilla M, Ferdman B. How should the clinician evaluate and manage the cardiovascular complications of anorexia nervosa? Eat Disord 2006; 14:73–80.
- Akhtar M. Clinical spectrum of ventricular tachycardia. Circulation 1990; 82:1561–1573.
- Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics 2013; 54:1–13.
- The University of Arizona Center for Education and Research on Therapeutics. QT Drug Lists. http://crediblemeds.org/everyone/compos-ite-list-all-qtdrugs/?rf=US. Accessed January 31, 2014.
- Rome ES, Ammerman S. Medical complications of eating disorders: an update. J Adolesc Health 2003; 33:418–426.
- Romano C, Chinali M, Pasanisi F, et al. Reduced hemodynamic load and cardiac hypotrophy in patients with anorexia nervosa. Am J Clin Nutr 2003; 77:308–312.
- Shamim T, Golden NH, Arden M, Filiberto L, Shenker IR. Resolution of vital sign instability: an objective measure of medical stability in anorexia nervosa. J Adolesc Health 2003; 32:73–77.
- Mont L, Castro J, Herreros B, et al. Reversibility of cardiac abnormalities in adolescents with anorexia nervosa after weight recovery. J Am Acad Child Adolesc Psychiatry 2003; 42:808–813.
- Roberto CA, Mayer LE, Brickman AM, et al. Brain tissue volume changes following weight gain in adults with anorexia nervosa. Int J Eat Disord 2011; 44:406–411.
- Treasure J, Russell G. The case for early intervention in anorexia nervosa: theoretical exploration of maintaining factors. Br J Psychiatry 2011; 199:5–7.
- Hadley SJ, Walsh BT. Gastrointestinal disturbances in anorexia nervosa and bulimia nervosa. Curr Drug Targets CNS Neurol Disord 2003; 2:1–9.
- Yager J, Andersen AE. Clinical practice. Anorexia nervosa. N Engl J Med 2005; 353:1481–1488.
- De Caprio C, Alfano A, Senatore I, Zarrella L, Pasanisi F, Contaldo F. Severe acute liver damage in anorexia nervosa: two case reports. Nutrition 2006; 22:572–575.
- Lawson EA, Klibanski A. Endocrine abnormalities in anorexia nervosa. Nat Clin Pract Endocrinol Metab 2008; 4:407–414.
- Holtkamp K, Mika C, Grzella I, et al. Reproductive function during weight gain in anorexia nervosa. Leptin represents a metabolic gate to gonadotropin secretion. J Neural Transm 2003; 110:427–435.
- Golden NH, Jacobson MS, Schebendach J, Solanto MV, Hertz SM, Shenker IR. Resumption of menses in anorexia nervosa. Arch Pediatr Adolesc Med 1997; 151:16–21.
- Soyka LA, Misra M, Frenchman A, et al. Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab 2002; 87:4177–4185.
- Misra M, Klibanski A. Bone metabolism in adolescents with anorexia nervosa. J Endocrinol Invest 2011; 34:324–332.
- Recker RR, Davies KM, Hinders SM, Heaney RP, Stegman MR, Kimmel DB. Bone gain in young adult women. JAMA 1992; 268:2403–2408.
- Biller BM, Saxe V, Herzog DB, Rosenthal DI, Holzman S, Klibanski A. Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinol Metab 1989; 68:548–554.
- Hergenroeder AC, Smith EO, Shypailo R, Jones LA, Klish WJ, Ellis K. Bone mineral changes in young women with hypothalamic amenorrhea treated with oral contraceptives, medroxyprogesterone, or placebo over 12 months. Am J Obstet Gynecol 1997; 176:1017–1025.
- Sim LA, McGovern L, Elamin MB, Swiglo BA, Erwin PJ, Montori VM. Effect on bone health of estrogen preparations in premenopausal women with anorexia nervosa: a systematic review and meta-analyses. Int J Eat Disord 2010; 43:218–225.
- Golden NH, Lanzkowsky L, Schebendach J, Palestro CJ, Jacobson MS, Shenker IR. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol 2002; 15:135–143.
- Misra M, Katzman D, Miller KK, et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res 2011; 26:2430–2438.
- Klibanski A, Biller BM, Schoenfeld DA, Herzog DB, Saxe VC. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab 1995; 80:898–904.
- Divasta AD, Feldman HA, Giancaterino C, Rosen CJ, Leboff MS, Gordon CM. The effect of gonadal and adrenal steroid therapy on skeletal health in adolescents and young women with anorexia nervosa. Metabolism 2012; 61:1010–1020.
- Mehler PS. Medical complications of bulimia nervosa and their treatments. Int J Eat Disord 2011; 44:95–104.
- Milosevic A. Eating disorders and the dentist. Br Dent J 1999; 186:109–113.
- Greenfeld D, Mickley D, Quinlan DM, Roloff P. Hypokalemia in outpatients with eating disorders. Am J Psychiatry 1995; 152:60–63.
- Bouquegneau A, Dubois BE, Krzesinski JM, Delanaye P. Anorexia nervosa and the kidney. Am J Kidney Dis 2012; 60:299–307.
- Auron M, Rome E. Anorexia nervosa and bulimia nervosa: what the hospitalist needs to know about CPT 269.9, or nutritional insufficiency. ACP Hospitalist 2011 Sept:28–45.
- Steffen KJ, Mitchell JE, Roerig JL, Lancaster KL. The eating disorders medicine cabinet revisited: a clinician’s guide to ipecac and laxatives. Int J Eat Disord 2007; 40:360–368.
- Roerig JL, Steffen KJ, Mitchell JE, Zunker C. Laxative abuse: epidemiology, diagnosis and management. Drugs 2010; 70:1487–1503.
- Mitchell JE, Boutacoff LI. Laxative abuse complicating bulimia: medical and treatment implications. Int J Eat Disord 1986; 5:325–334.
- Joo JS, Ehrenpreis ED, Gonzalez L, et al. Alterations in colonic anatomy induced by chronic stimulant laxatives: the cathartic colon revisited. J Clin Gastroenterol 1998; 26:283–286.
- Drugs.com. Ipecac syrup. www.drugs.com/monograph/ipecac-syrup.html. Accessed January 31, 2014.
- Peveler RC, Bryden KS, Neil HA, et al. The relationship of disordered eating habits and attitudes to clinical outcomes in young adult females with type 1 diabetes. Diabetes Care 2005; 28:84–88.
- Mannucci E, Rotella F, Ricca V, Moretti S, Placidi GF, Rotella CM. Eating disorders in patients with type 1 diabetes: a meta-analysis. J Endocrinol Invest 2005; 28:417–419.
- Crook MA, Hally V, Panteli JV. The importance of the refeeding syndrome. Nutrition 2001; 17:632–637.
- Fisher M, Golden NH, Katzman DK, et al. Eating disorders in adolescents: a background paper. J Adolesc Health 1995; 16:420–437.
- Kohn MR, Madden S, Clarke SD. Refeeding in anorexia nervosa: increased safety and efficiency through understanding the pathophysiology of protein calorie malnutrition. Curr Opin Pediatr 2011; 23:390–394.
- Garber AK, Michihata N, Hetnal K, Shafer MA, Moscicki AB. A prospective examination of weight gain in hospitalized adolescents with anorexia nervosa on a recommended refeeding protocol. J Adolesc Health 2012; 50:24–29.
- Whitelaw M, Gilbertson H, Lam PY, Sawyer SM. Does aggressive refeeding in hospitalized adolescents with anorexia nervosa result in increased hypophosphatemia? J Adolesc Health 2010; 46:577–582.
- Treasure J, Crane A, McKnight R, Buchanan E, Wolfe M. First do no harm: iatrogenic maintaining factors in anorexia nervosa. Eur Eat Disord Rev 2011; 19:296–302.
- Academy for Eating Disorders (AED). Critical points for early recognition and medical risk management in the care of individuals with eating disorders. http://www.aedweb.org/AM/Template.cfm?Section=Medical_Care_Standards&Template=/CM/ContentDisplay.cfm&ContentID=2413. Accessed January 31, 2014.
Eating disorders are debilitating biopsychosocial illnesses associated with serious medical illness and a high risk of death.1
Primary care physicians are often the first to see young women who have these problems, diagnose them, and start their evaluation and treatment.2–4 Many patients require acute medical interventions as well as long-term care for chronic medical issues. Therefore, primary care physicians play essential front-line and long-term roles in the multidisciplinary treatment team.
DEFINITIONS OF EATING DISORDERS HAVE CHANGED
Several problems existed in the category of eating disorders in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-4) and in the DSM-4 Text Revision (DSM-4-TR). These problems have been addressed in the fifth edition (DSM-5), released in 2013.5
One problem in the earlier editions was that many patients referred for treatment of eating disorders—more than 50% in one study6—did not meet the criteria for anorexia nervosa or bulimia nervosa and thus had to be categorized as having “eating disorder not otherwise specified.” Further, the earlier editions did not recognize that young children and adolescent males can be affected.7
Eating disorders are now recognized as an equal-opportunity disease, with all ethnic and socioeconomic groups affected. Children can run into medical trouble with even a small amount of weight loss or falling off the growth curve. Moreover, children and adolescents do not “experience” their bodies in the same way adults do; they may lack the vocabulary for eating-disorder thoughts.
For these reasons, the definitions of eating disorders have changed in the DSM-5.5
Anorexia nervosa. Older editions of the DSM listed amenorrhea as a criterion. This has been eliminated in DSM-5, since amenorrhea does not necessarily predict medical risk or treatment outcome; also, it is not applicable to males or premenorrheal girls and postmenopausal women.8 In addition, the requirement of low weight is now defined in the context of “age, sex, developmental trajectory, and physical health,” rather than the old threshold of 85% of expected weight.9
What remains unchanged is that anorexia nervosa is still characterized by self-starvation in order to maintain an abnormally low body weight, along with an intense fear of being fat and a disturbed self-image.
Bulimia nervosa. In both the old and the new editions of the DSM, bulimia nervosa is characterized by episodes of binge eating followed by inappropriate compensatory behaviors to avoid weight gain, such as vomiting, laxative abuse, diuretic abuse, and overexercise. In DSM-5, bulimia nervosa no longer has subtypes and requires only one binge per week with compensatory behavior, for at least 3 months. This change was based on the finding that there is no clear difference in psychopathology or treatment outcome between patients with one and two binge-purge episodes a week.10
“Eating disorder not otherwise specified” was a wastebasket category, lumping all those who did not meet the criteria for anorexia nervosa or bulimia nervosa or who did not neatly fit into a specific category.10 In DSM-5, subcategories were designed to help distinguish different treatment needs and outcomes between various subtypes.
Binge-eating disorder, one of the new subcategories, is characterized by binge eating without inappropriate compensatory behaviors.9 Patients with binge-eating disorder are often obese, have greater functional impairment, and are more likely to develop components of metabolic syndrome than obese patients without eating disorders.11
Avoidant/restrictive food intake disorder is another new DSM-5 diagnosis, characterized by failure to meet nutritional needs for reasons other than weight control. Reasons include disinterest in eating, dislike of sensory characteristics of food, or avoidance of consequences of eating. This disorder replaces the category “feeding disorder of infancy or early childhood,” since the condition can also occur in adolescents and adults.12
Other new diagnoses are:
- Atypical anorexia nervosa (if the patient is not underweight)
- Purging disorder
- Subthreshold bulimia nervosa (if the patient has < 1 episode per week or has had them for < 3 months)
- Subthreshold binge eating disorder (< 1 time a week or < 3 months)
- Night eating syndrome
- Pica and rumination disorder.
Regardless of the diagnostic label, the medical evaluation and treatment of anyone with an eating disorder should be tailored to the specific behaviors of the eating disorder. Medical complications can be subdivided into those from starvation, from purging, and from refeeding.
MEDICAL COMPLICATIONS OF STARVATION
Cardiovascular effects of starvation
Malnutrition and starvation have multiple adverse effects on the heart.
Electrophysiologic effects. Sinus bradycardia (< 60 bpm) and hypotension are common cardiac manifestations of starvation.13 Bradycardia has been attributed to an adaptive increase in parasympathetic vagal tone.14 QTc prolongation is also seen in patients with malnutrition.15
Together, these electrocardiographic abnormalities predispose the patient to ventricular arrhythmia and sudden cardiac death.16 The risk of ventricular arrhythmia is particularly relevant when treating psychiatric symptoms, since antipsychotics and tricyclic antidepressants are among several drug classes that can cause further QTc prolongation (Table 1).17,18
In patients with QTc prolongation, bradycardia, or both, the standard of care involves acute hospitalization for refeeding using continuous telemetric monitoring until normal rhythm is restored and the heart rate is above 40 at night and 50 by day.4,19
Structural changes. Starvation also causes structural changes in the heart. Loss of lean body mass can reduce cardiac muscle mass, compromise cardiac output, and lead to mitral valve prolapse.20 These changes are fully reversible with restored nutrition and regaining of heart mass.21,22
Effects of starvation on the brain
Starvation can affect brain structure and cognitive function. Undernourished patients have reduced volumes of white and gray matter, a change that can occur within months. Cortical volumes may increase with weight gain, but a reduction in gray matter volume may not be completely reversible.23
Furthermore, starvation impairs cognitive functions that are needed to stop eating-disorder behaviors; namely, decision-making, emotional control, regulation of appetite, and reward path-ways. Therefore, undernourished patients may not have sufficient insight into the disease to be able to make the best choices for recovery. This finding lends support for using the Maudsley method in adolescents, in which parents take control of their child’s eating until the child can maintain a healthy weight.24
Gastrointestinal consequences of starvation
Patients with malnutrition have prolonged gastric emptying and colonic transit time with solid foods.25 They often complain of early satiety, abdominal pain, bloating, and constipation, all symptoms that complicate the refeeding process. A prokinetic such as metoclopramide (Reglan), given 1 hour before meals and at bedtime, may provide some relief from gastrointestinal symptoms.26
Patients may also experience transient lactose or fructose intolerance after prolonged starvation. Taking a lactase supplement (eg, Lactaid 1–10 tabs) before consuming dairy products and dextrose (contained in candies such as Smarties) before eating fruit or fructose-containing foods can sometimes partially relieve symptoms. In general, gastrointestinal function returns over time as nutritional status improves.
Patients with severe or prolonged starvation can develop steatosis accompanied by elevated levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). In reports of starvation-induced steatosis, liver enzyme levels rapidly normalize with nutritional rehabilitation.27
Endocrine consequences of starvation
Amenorrhea. Dysregulation of the hypothalamic-pituitary-gonadal axis is a major endocrine complication of nutritional in-sufficiency. Weight loss disrupts the normal pulsatile secretion of gonadotropin-releasing hormone, reduces secretion of luteinizing hormone and follicle-stimulating hormone, and decreases estrogen levels.28 Leptin deficiency likely plays a role in suppressing gonadotropin secretion with subsequent development of amenorrhea. With weight gain, levels of leptin and gonadotropins normalize and menstruation eventually returns.29,30
Hypothyroidism. Starvation can also lead to dysregulation of the hypothalamic-pituitary-thyroid axis. Typically, the concentration of triiodothyronine (T3) is reduced, the ratio of thyroxine (T4) to T3 is elevated, and thyroid-stimulating hormone (TSH) is close to or within the normal range, creating a euthyroid sick syndrome. In eating disorders, this thyroid disturbance is a result of starvation and resolves with weight restoration. Therefore, thyroid hormone replacement therapy is not medically indicated.28
Osteoporosis. Amenorrhea resulting from low estrogen levels in undernourished patients can raise the risk of osteoporosis and fractures, particularly in patients with a low body mass index. Osteopenia results from a negative balance between bone deposition and resorption.
Lack of bone deposition can be especially problematic when disordered eating occurs during peak bone mass development, ie, ages 11 to 14 for girls, and ages 15 to 17 for boys.31,32 Even a 5% to 10% decrease in bone deposition can result in significant risk of osteopenia.33 However, after age 30, bone resorption is a greater contributor.34
Does hormone therapy correct bone loss? Given the association between estrogen deficiency and bone loss, estrogen supplementation was expected to be an effective treatment for bone loss in patients with eating disorders.35 Also, the restoration of menses through hormone replacement may give underweight patients a false sense of achieving a “healthy” weight.36
Golden et al37 prospectively studied 50 adolescents and found no significant difference in bone mineral density at 1 year of follow-up between patients treated with estrogen and those who received only standard nutritional therapy. However, increased bone mineral density was achieved in adolescents with anorexia nervosa treated with transdermally administered estrogen dosed to mimic physiologic pubertal levels.38
Klibanski et al39 found that hormone therapy resulted in a 4% gain in bone density in an extremely low-weight subset of women with anorexia nervosa (< 70% of ideal body weight), whereas similar patients in the control group lost 20%. However, in all groups, only weight gain correlated with bone gain in women who were within 70% of their ideal body weight.
Divasta et al40 evaluated 60 girls and women ages 13 to 27 with anorexia nervosa, randomized to receive either placebo or dehydroepiandrosterone combined with an estrogen-progestin oral contraceptive, and followed for 18 months. As in the study by Klibanski et al,39 bone loss was prevented in the treatment group, but significant bone gain occurred only in the context of weight gain.
The bottom line is that only weight gain has resulted in significant increases in bone density in patients with anorexia nervosa, and hormone therapy without weight gain has not been shown to increase bone density effectively in this population. Although calcium and vitamin D in oral therapeutic doses through foods or through supplementation are required for bone gain, the combination is not enough to augment bone density in the absence of weight gain.37 Although not curative, weight gain is currently the best option for treating bone loss, and no single pharmacologic treatment is effective.
COMPLICATIONS OF PURGING
Oral complications of purging
Patients who purge by vomiting are at risk of complications from exposure of the esophagus, pharynx, and mouth to acidic gastric contents.
Dental problems. Over time, contact with gastric acid wears down enamel on the lingual and occlusal surfaces of teeth, resulting in dental caries and periodontal disease. Until they can give up purging, patients should be instructed to rinse with mouthwash or water immediately after vomiting to reduce the acidity in the mouth.41,42 We recommend that patients not brush their teeth after vomiting, because brushing can deliver acid to otherwise unreachable surfaces and thus worsen tooth erosion. For patients who are determined to brush after vomiting, a bicarbonate toothpaste might mitigate harm.42
Sialadenosis (hypertrophy of the salivary glands) is another consequence of repeated vomiting, with elevated salivary amylase. Both the size of the glands and the salivary amylase level generally normalize on their own after vomiting is stopped, but parotitis can take up to a year to resolve. Similar to smoker’s cough, parotitis may acutely worsen when the patient abruptly stops vomiting and may worsen before it improves.
To reduce discomfort, patients can use hot compresses or sugarless hard candies.44 However, the latter should not be substituted as a chronic habit in a patient with disordered eating. Patients need to be reassured that the swelling is not permanent, since they often interpret it as having fat cheeks (the “chipmunk sign”).
Hypokalemia, metabolic alkalosis, renal dysfunction
Chronic vomiting can cause electrolyte and acid-base imbalances, the most worrisome of which is hypokalemia. With repeated vomiting, loss of potassium and gastric acid causes metabolic alkalosis with hypokalemia, hypochloremia, and hypomagnesemia. Loss of water and the resultant volume contraction activates the renin-angiotensin-aldosterone system, and elevated aldosterone further decreases serum potassium.
In patients with eating disorders, who often have other factors contributing to electrolyte imbalance, vomiting-induced hypokalemia heightens the risk of cardiac arrhythmias.43
Hypokalemia can also cause rhabdomyolysis and kidney damage.41,43 Prolonged hypokalemia and reduced kidney perfusion in the setting of volume depletion causes acute kidney injury and impaired concentrating ability of the renal tubules. Hypovolemia can cause prerenal azotemia and increases the risk for nephrolithiasis and nephrocalcinosis.44,45
When a patient stops vomiting, elevated aldosterone from prior hypovolemia results in water retention and can manifest in significant edema associated with hypochloremic alkalosis. This condition, known as pseudo-Bartter syndrome, usually resolves without treatment. In the meantime, salt restriction and leg elevation can help reduce edema.26
Laxative abuse: A mode of purging
Many patients with eating disorders abuse laxatives to lose weight or to prevent weight gain. Believing that laxatives will prevent calorie absorption, patients commonly take them to compensate for caloric intake (eg, during a binge episode). The immediate weight loss, albeit artificial, is highly reinforcing for an eating-disorder patient. In some cases, patients with eating disorders also abuse laxatives to self-treat the constipation that results from chronic starvation.46
Over time, tolerance to laxatives develops, and patients use increasingly larger doses. This can lead to activation of the renin-angiotensin-aldosterone system.47 Patients interpret the resultant edema as true weight gain and again take laxatives to get rid of it. If laxatives are stopped abruptly, the patient may need inpatient and outpatient support for the resultant fluid shifts.
Gastrointestinal complications of laxative abuse include reflex hypofunction of the bowel, malabsorption, steatorrhea, and gastrointestinal bleeding.47 Reflex hypofunction during laxative withdrawal is a consequence of the bowel becoming tolerant of laxatives.48 Cathartic colon syndrome is a rare complication characterized by loss of the normal haustral markings and slowed or absent peristalsis in segments of the colon.49
Systemically, the major risk of laxative abuse relates to electrolyte and acid-base imbalance. Loss of potassium and water in the stool can cause hypokalemia and metabolic alkalosis.48 The disturbances caused by laxative abuse are similar to those caused by vomiting and diuretic use and have the same treatment.
The most important component of treating laxative abuse is giving patients realistic expectations to help them tolerate temporary discomfort and to help manage the edema and fluid shifts that can happen acutely with shifting of fluid into the intracellular space. In extreme cases, this may need to be managed in the hospital. To help relieve the initial anxiety, doctors should emphasize that any bloating the patient experiences is not true weight gain and will go away within a few days to weeks. In addition, explaining that laxatives reduce nutrient absorption only minimally may lessen the temptation to resume taking them.48
Diuretic abuse: Another form of purging
Diuretic abuse is yet another mode of purging, with its own set of medical complications. Like laxatives, diuretics are not effective weight-loss agents, and the weight reduction they cause is only temporary.
As with vomiting, there is a compensatory activation of the renin-angiotensin-aldosterone system, and therefore subsequent fluid intake will lead to water retention, which encourages further diuretic use.41 Diuretics can also contribute to hypokalemia, hypomagnesemia, hypochloremia, and metabolic alkalosis.
Ipecac abuse can lead to heart failure
Ipecac syrup has long been used to induce vomiting, but this practice has become much less common since ipecac has become harder to obtain in the United States.50 The emetine base contained in ipecac binds irreversibly to cardiac and skeletal muscle. With continued use, irreversible cardiomyopathy develops and can lead to heart failure. Treatment should include supportive care and immediate cessation of ipecac use.
Diabetic patients may skip insulin to lose weight
Patients with diabetes, especially those with type 1 that begins in childhood, are at greater risk of eating disorders over time.51 They may withhold insulin to lose weight, a practice referred to in the nonmedical literature as “diabulimia,” and they seem particularly more likely to develop bulimia nervosa than those without diabetes.52
The medical prognosis is poor for patients with diabetes who develop eating disorders and do not receive intensive treatment.51 In addition, if a diabetic patient on an insulin pump becomes depressed in addition to having an eating disorder, careful monitoring for suicidal thoughts and a rapid follow-up with mental health services are in order.
REFEEDING SYNDROME
When refeeding is started, a high glucose load stimulates insulin secretion, resulting in cellular uptake of phosphorus along with potassium, magnesium, and glucose. In addition, total body phosphorus is depleted by the increased demand for adenosine triphosphate and 2,3-diphosphoglycerate for cellular metabolism.
When liver enzyme levels increase, the astute clinician will closely monitor the patient for evidence of refeeding syndrome. In a child, adolescent, or young adult, the standard of care is inpatient monitoring for acute stabilization.4,19
Hypophosphatemia is the hallmark of refeeding syndrome, although hypomagnesemia, hypokalemia, and hypoglycemia can also occur.53 In addition, sodium and water retention can lead to fluid overload, with shifting of fluid into the intracellular space, resulting in dependent edema.
Cardiovascular complications are the most worrisome manifestations of refeeding syndrome. Electrolyte shifts and increased fluid volume can cause arrhythmias and heart failure. Furthermore, severely undernourished patients may have reduced myocardial mass as well as electrocardiographic abnormalities associated with starvation, which further increase their vulnerability to electrolyte shifts and fluid retention during refeeding.15
Other manifestations of refeeding syndrome include delirium, seizures, rhabdomyolysis, and respiratory failure. In the most extreme cases, refeeding syndrome causes sudden death.53
Fortunately, refeeding syndrome is easily preventable and treatable when recognized early. Electrolytes and cardiovascular and renal function must be carefully monitored, especially during the first week of nutritional restoration.53 In patients with extremely low body mass (< 70% of ideal body weight) or with precipitous weight loss, close monitoring of the complete metabolic panel including electrolytes, AST, ALT, calcium, magnesium, and phosphorus may be required to detect changes that can affect cardiac status. Specific suggestions for refeeding are discussed below and in Table 2.45
ACUTE CARE OF PATIENTS WITH EATING DISORDERS
Refeeding in the inpatient setting
The decision to hospitalize an eating-disorder patient is based on the current or potential risk of serious medical complications and the likelihood of success at home. Medical criteria for hospital admission are outlined in Table 3.4,54
In refeeding undernourished patients, the challenge is to maximize weight gain while preventing refeeding syndrome. Undernourished patients are generally hypometabolic at baseline but become hypermetabolic once refeeding begins.
How many calories should refeeding start with? The traditional principle of “start low and go slow” has been recently challenged.55 Starting at 1,200 kcal/day or less in the typical patient can result in failure to gain weight or even in weight loss in the first week of refeeding.56 The goal is to achieve a weight gain of 0.2 kg/day while the patient is in the hospital. Thus, we start higher, and to date we have seen no cases of life-threatening refeeding syndrome. In all patients who need hospitalization or who are beginning the refeeding process as outpatients, caloric intake should be started at 1,500 to 2,000 kcal/day.45,57 However, for exceptionally low-weight patients, intake may be started lower.
In Australia, patients are started at 1,900 kcal/day.56 All patients in one program there receive nasogastric feeding initially in an intensive care unit and then are moved to a regular nursing floor where they graduate to full oral feeding as they improve cardiovascularly and behaviorally. In the United States, some programs use nasogastric feeding at night for caloric restoration; our program and others use nasogastric feeding as a behavioral modification strategy for patients who refuse food or supplements by mouth.
Phosphorus supplementation. Many centers give phosphorus supplements preventively. In our center, we give potassium phosphate (Neutra-Phos) 500 mg orally twice daily for 5 days, and we have seen no life-threatening cases of refeeding syndrome with that regimen. Other centers give phosphorus supplements in a dose of 250 mg orally twice a day for 5 days, while still others only supplement phosphorus reactively once a deficit has been identified. The latter method requires daily blood draws for monitoring and is reactive rather than proactive. Further studies can help clarify the optimal dosing and timing of phosphorus supplementation.
Managing fluid balance. Fluid-loading these patients may tip them over the edge into refeeding syndrome. Except in cases of shock, patients with eating disorders should not be given intravenous fluids, as it is safer to rehydrate and feed them orally. Electrolyte imbalances can be corrected orally with no need for intravenous supplementation. To avoid fluid overload, fluids can be started at 1,500 mL to 2,000 mL per day, with strict monitoring of intake and output. Fluids are liberalized if ALT and AST levels remain normal and to gradually correct orthostatic hypotension; caloric fluids are ideal to help address energy needs and improve bradycardia.
Laboratory monitoring. On admission, a urinalysis, complete blood cell count, complete metabolic panel, TSH, erythrocyte sedimentation rate, serum magnesium, and phosphorus should be obtained.26 In addition, continuous electrocardiographic recording should begin on admission.45 Inpatient use of a telemetry bed helps identify extreme tachycardia with arrhythmia, as well as profound bradycardia.45,56
Some protocols call for daily laboratory monitoring, although that degree of testing is less cost-effective. If initial results are normal, clinical judgment can be used on when to repeat laboratory evaluation. For instance, patients with edema require repeat complete metabolic panels to assess for elevated ALT and AST, electrolyte imbalances, and other abnormalities.
Signs of refeeding syndrome include tachycardia, hepatosplenomegaly, peripheral edema, altered mental status, and electrolyte disturbances, specifically, acute or severe hypophosphatemia or hypokalemia.26,45 If refeeding syndrome is suspected, the rate of caloric intake should be reduced or not advanced, fluid intake should be urgently reassessed for volume overload, and supportive care with close monitoring should be provided.
KNOWLEDGE SAVES LIVES
Eating disorders can lead to potentially life-threatening medical complications that require attentive care by the primary care clinician and subspecialist. Without thoughtful consideration, it is easy for even a caring medical team to unintentionally enable patients with these illnesses or to cause active harm in the case of underrecognized pathology.58
Acute medical stabilization on an inpatient unit trained to recognize pathology and treat sequelae can be lifesaving. Arming patients and families with medical knowledge, as provided in the Academy for Eating Disorders’ brochure, “Critical Points for Early Recognition and Medical Risk Management in the Care of Individuals with Eating Disorders”59 can help save patients’ lives.
Eating disorders are debilitating biopsychosocial illnesses associated with serious medical illness and a high risk of death.1
Primary care physicians are often the first to see young women who have these problems, diagnose them, and start their evaluation and treatment.2–4 Many patients require acute medical interventions as well as long-term care for chronic medical issues. Therefore, primary care physicians play essential front-line and long-term roles in the multidisciplinary treatment team.
DEFINITIONS OF EATING DISORDERS HAVE CHANGED
Several problems existed in the category of eating disorders in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-4) and in the DSM-4 Text Revision (DSM-4-TR). These problems have been addressed in the fifth edition (DSM-5), released in 2013.5
One problem in the earlier editions was that many patients referred for treatment of eating disorders—more than 50% in one study6—did not meet the criteria for anorexia nervosa or bulimia nervosa and thus had to be categorized as having “eating disorder not otherwise specified.” Further, the earlier editions did not recognize that young children and adolescent males can be affected.7
Eating disorders are now recognized as an equal-opportunity disease, with all ethnic and socioeconomic groups affected. Children can run into medical trouble with even a small amount of weight loss or falling off the growth curve. Moreover, children and adolescents do not “experience” their bodies in the same way adults do; they may lack the vocabulary for eating-disorder thoughts.
For these reasons, the definitions of eating disorders have changed in the DSM-5.5
Anorexia nervosa. Older editions of the DSM listed amenorrhea as a criterion. This has been eliminated in DSM-5, since amenorrhea does not necessarily predict medical risk or treatment outcome; also, it is not applicable to males or premenorrheal girls and postmenopausal women.8 In addition, the requirement of low weight is now defined in the context of “age, sex, developmental trajectory, and physical health,” rather than the old threshold of 85% of expected weight.9
What remains unchanged is that anorexia nervosa is still characterized by self-starvation in order to maintain an abnormally low body weight, along with an intense fear of being fat and a disturbed self-image.
Bulimia nervosa. In both the old and the new editions of the DSM, bulimia nervosa is characterized by episodes of binge eating followed by inappropriate compensatory behaviors to avoid weight gain, such as vomiting, laxative abuse, diuretic abuse, and overexercise. In DSM-5, bulimia nervosa no longer has subtypes and requires only one binge per week with compensatory behavior, for at least 3 months. This change was based on the finding that there is no clear difference in psychopathology or treatment outcome between patients with one and two binge-purge episodes a week.10
“Eating disorder not otherwise specified” was a wastebasket category, lumping all those who did not meet the criteria for anorexia nervosa or bulimia nervosa or who did not neatly fit into a specific category.10 In DSM-5, subcategories were designed to help distinguish different treatment needs and outcomes between various subtypes.
Binge-eating disorder, one of the new subcategories, is characterized by binge eating without inappropriate compensatory behaviors.9 Patients with binge-eating disorder are often obese, have greater functional impairment, and are more likely to develop components of metabolic syndrome than obese patients without eating disorders.11
Avoidant/restrictive food intake disorder is another new DSM-5 diagnosis, characterized by failure to meet nutritional needs for reasons other than weight control. Reasons include disinterest in eating, dislike of sensory characteristics of food, or avoidance of consequences of eating. This disorder replaces the category “feeding disorder of infancy or early childhood,” since the condition can also occur in adolescents and adults.12
Other new diagnoses are:
- Atypical anorexia nervosa (if the patient is not underweight)
- Purging disorder
- Subthreshold bulimia nervosa (if the patient has < 1 episode per week or has had them for < 3 months)
- Subthreshold binge eating disorder (< 1 time a week or < 3 months)
- Night eating syndrome
- Pica and rumination disorder.
Regardless of the diagnostic label, the medical evaluation and treatment of anyone with an eating disorder should be tailored to the specific behaviors of the eating disorder. Medical complications can be subdivided into those from starvation, from purging, and from refeeding.
MEDICAL COMPLICATIONS OF STARVATION
Cardiovascular effects of starvation
Malnutrition and starvation have multiple adverse effects on the heart.
Electrophysiologic effects. Sinus bradycardia (< 60 bpm) and hypotension are common cardiac manifestations of starvation.13 Bradycardia has been attributed to an adaptive increase in parasympathetic vagal tone.14 QTc prolongation is also seen in patients with malnutrition.15
Together, these electrocardiographic abnormalities predispose the patient to ventricular arrhythmia and sudden cardiac death.16 The risk of ventricular arrhythmia is particularly relevant when treating psychiatric symptoms, since antipsychotics and tricyclic antidepressants are among several drug classes that can cause further QTc prolongation (Table 1).17,18
In patients with QTc prolongation, bradycardia, or both, the standard of care involves acute hospitalization for refeeding using continuous telemetric monitoring until normal rhythm is restored and the heart rate is above 40 at night and 50 by day.4,19
Structural changes. Starvation also causes structural changes in the heart. Loss of lean body mass can reduce cardiac muscle mass, compromise cardiac output, and lead to mitral valve prolapse.20 These changes are fully reversible with restored nutrition and regaining of heart mass.21,22
Effects of starvation on the brain
Starvation can affect brain structure and cognitive function. Undernourished patients have reduced volumes of white and gray matter, a change that can occur within months. Cortical volumes may increase with weight gain, but a reduction in gray matter volume may not be completely reversible.23
Furthermore, starvation impairs cognitive functions that are needed to stop eating-disorder behaviors; namely, decision-making, emotional control, regulation of appetite, and reward path-ways. Therefore, undernourished patients may not have sufficient insight into the disease to be able to make the best choices for recovery. This finding lends support for using the Maudsley method in adolescents, in which parents take control of their child’s eating until the child can maintain a healthy weight.24
Gastrointestinal consequences of starvation
Patients with malnutrition have prolonged gastric emptying and colonic transit time with solid foods.25 They often complain of early satiety, abdominal pain, bloating, and constipation, all symptoms that complicate the refeeding process. A prokinetic such as metoclopramide (Reglan), given 1 hour before meals and at bedtime, may provide some relief from gastrointestinal symptoms.26
Patients may also experience transient lactose or fructose intolerance after prolonged starvation. Taking a lactase supplement (eg, Lactaid 1–10 tabs) before consuming dairy products and dextrose (contained in candies such as Smarties) before eating fruit or fructose-containing foods can sometimes partially relieve symptoms. In general, gastrointestinal function returns over time as nutritional status improves.
Patients with severe or prolonged starvation can develop steatosis accompanied by elevated levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). In reports of starvation-induced steatosis, liver enzyme levels rapidly normalize with nutritional rehabilitation.27
Endocrine consequences of starvation
Amenorrhea. Dysregulation of the hypothalamic-pituitary-gonadal axis is a major endocrine complication of nutritional in-sufficiency. Weight loss disrupts the normal pulsatile secretion of gonadotropin-releasing hormone, reduces secretion of luteinizing hormone and follicle-stimulating hormone, and decreases estrogen levels.28 Leptin deficiency likely plays a role in suppressing gonadotropin secretion with subsequent development of amenorrhea. With weight gain, levels of leptin and gonadotropins normalize and menstruation eventually returns.29,30
Hypothyroidism. Starvation can also lead to dysregulation of the hypothalamic-pituitary-thyroid axis. Typically, the concentration of triiodothyronine (T3) is reduced, the ratio of thyroxine (T4) to T3 is elevated, and thyroid-stimulating hormone (TSH) is close to or within the normal range, creating a euthyroid sick syndrome. In eating disorders, this thyroid disturbance is a result of starvation and resolves with weight restoration. Therefore, thyroid hormone replacement therapy is not medically indicated.28
Osteoporosis. Amenorrhea resulting from low estrogen levels in undernourished patients can raise the risk of osteoporosis and fractures, particularly in patients with a low body mass index. Osteopenia results from a negative balance between bone deposition and resorption.
Lack of bone deposition can be especially problematic when disordered eating occurs during peak bone mass development, ie, ages 11 to 14 for girls, and ages 15 to 17 for boys.31,32 Even a 5% to 10% decrease in bone deposition can result in significant risk of osteopenia.33 However, after age 30, bone resorption is a greater contributor.34
Does hormone therapy correct bone loss? Given the association between estrogen deficiency and bone loss, estrogen supplementation was expected to be an effective treatment for bone loss in patients with eating disorders.35 Also, the restoration of menses through hormone replacement may give underweight patients a false sense of achieving a “healthy” weight.36
Golden et al37 prospectively studied 50 adolescents and found no significant difference in bone mineral density at 1 year of follow-up between patients treated with estrogen and those who received only standard nutritional therapy. However, increased bone mineral density was achieved in adolescents with anorexia nervosa treated with transdermally administered estrogen dosed to mimic physiologic pubertal levels.38
Klibanski et al39 found that hormone therapy resulted in a 4% gain in bone density in an extremely low-weight subset of women with anorexia nervosa (< 70% of ideal body weight), whereas similar patients in the control group lost 20%. However, in all groups, only weight gain correlated with bone gain in women who were within 70% of their ideal body weight.
Divasta et al40 evaluated 60 girls and women ages 13 to 27 with anorexia nervosa, randomized to receive either placebo or dehydroepiandrosterone combined with an estrogen-progestin oral contraceptive, and followed for 18 months. As in the study by Klibanski et al,39 bone loss was prevented in the treatment group, but significant bone gain occurred only in the context of weight gain.
The bottom line is that only weight gain has resulted in significant increases in bone density in patients with anorexia nervosa, and hormone therapy without weight gain has not been shown to increase bone density effectively in this population. Although calcium and vitamin D in oral therapeutic doses through foods or through supplementation are required for bone gain, the combination is not enough to augment bone density in the absence of weight gain.37 Although not curative, weight gain is currently the best option for treating bone loss, and no single pharmacologic treatment is effective.
COMPLICATIONS OF PURGING
Oral complications of purging
Patients who purge by vomiting are at risk of complications from exposure of the esophagus, pharynx, and mouth to acidic gastric contents.
Dental problems. Over time, contact with gastric acid wears down enamel on the lingual and occlusal surfaces of teeth, resulting in dental caries and periodontal disease. Until they can give up purging, patients should be instructed to rinse with mouthwash or water immediately after vomiting to reduce the acidity in the mouth.41,42 We recommend that patients not brush their teeth after vomiting, because brushing can deliver acid to otherwise unreachable surfaces and thus worsen tooth erosion. For patients who are determined to brush after vomiting, a bicarbonate toothpaste might mitigate harm.42
Sialadenosis (hypertrophy of the salivary glands) is another consequence of repeated vomiting, with elevated salivary amylase. Both the size of the glands and the salivary amylase level generally normalize on their own after vomiting is stopped, but parotitis can take up to a year to resolve. Similar to smoker’s cough, parotitis may acutely worsen when the patient abruptly stops vomiting and may worsen before it improves.
To reduce discomfort, patients can use hot compresses or sugarless hard candies.44 However, the latter should not be substituted as a chronic habit in a patient with disordered eating. Patients need to be reassured that the swelling is not permanent, since they often interpret it as having fat cheeks (the “chipmunk sign”).
Hypokalemia, metabolic alkalosis, renal dysfunction
Chronic vomiting can cause electrolyte and acid-base imbalances, the most worrisome of which is hypokalemia. With repeated vomiting, loss of potassium and gastric acid causes metabolic alkalosis with hypokalemia, hypochloremia, and hypomagnesemia. Loss of water and the resultant volume contraction activates the renin-angiotensin-aldosterone system, and elevated aldosterone further decreases serum potassium.
In patients with eating disorders, who often have other factors contributing to electrolyte imbalance, vomiting-induced hypokalemia heightens the risk of cardiac arrhythmias.43
Hypokalemia can also cause rhabdomyolysis and kidney damage.41,43 Prolonged hypokalemia and reduced kidney perfusion in the setting of volume depletion causes acute kidney injury and impaired concentrating ability of the renal tubules. Hypovolemia can cause prerenal azotemia and increases the risk for nephrolithiasis and nephrocalcinosis.44,45
When a patient stops vomiting, elevated aldosterone from prior hypovolemia results in water retention and can manifest in significant edema associated with hypochloremic alkalosis. This condition, known as pseudo-Bartter syndrome, usually resolves without treatment. In the meantime, salt restriction and leg elevation can help reduce edema.26
Laxative abuse: A mode of purging
Many patients with eating disorders abuse laxatives to lose weight or to prevent weight gain. Believing that laxatives will prevent calorie absorption, patients commonly take them to compensate for caloric intake (eg, during a binge episode). The immediate weight loss, albeit artificial, is highly reinforcing for an eating-disorder patient. In some cases, patients with eating disorders also abuse laxatives to self-treat the constipation that results from chronic starvation.46
Over time, tolerance to laxatives develops, and patients use increasingly larger doses. This can lead to activation of the renin-angiotensin-aldosterone system.47 Patients interpret the resultant edema as true weight gain and again take laxatives to get rid of it. If laxatives are stopped abruptly, the patient may need inpatient and outpatient support for the resultant fluid shifts.
Gastrointestinal complications of laxative abuse include reflex hypofunction of the bowel, malabsorption, steatorrhea, and gastrointestinal bleeding.47 Reflex hypofunction during laxative withdrawal is a consequence of the bowel becoming tolerant of laxatives.48 Cathartic colon syndrome is a rare complication characterized by loss of the normal haustral markings and slowed or absent peristalsis in segments of the colon.49
Systemically, the major risk of laxative abuse relates to electrolyte and acid-base imbalance. Loss of potassium and water in the stool can cause hypokalemia and metabolic alkalosis.48 The disturbances caused by laxative abuse are similar to those caused by vomiting and diuretic use and have the same treatment.
The most important component of treating laxative abuse is giving patients realistic expectations to help them tolerate temporary discomfort and to help manage the edema and fluid shifts that can happen acutely with shifting of fluid into the intracellular space. In extreme cases, this may need to be managed in the hospital. To help relieve the initial anxiety, doctors should emphasize that any bloating the patient experiences is not true weight gain and will go away within a few days to weeks. In addition, explaining that laxatives reduce nutrient absorption only minimally may lessen the temptation to resume taking them.48
Diuretic abuse: Another form of purging
Diuretic abuse is yet another mode of purging, with its own set of medical complications. Like laxatives, diuretics are not effective weight-loss agents, and the weight reduction they cause is only temporary.
As with vomiting, there is a compensatory activation of the renin-angiotensin-aldosterone system, and therefore subsequent fluid intake will lead to water retention, which encourages further diuretic use.41 Diuretics can also contribute to hypokalemia, hypomagnesemia, hypochloremia, and metabolic alkalosis.
Ipecac abuse can lead to heart failure
Ipecac syrup has long been used to induce vomiting, but this practice has become much less common since ipecac has become harder to obtain in the United States.50 The emetine base contained in ipecac binds irreversibly to cardiac and skeletal muscle. With continued use, irreversible cardiomyopathy develops and can lead to heart failure. Treatment should include supportive care and immediate cessation of ipecac use.
Diabetic patients may skip insulin to lose weight
Patients with diabetes, especially those with type 1 that begins in childhood, are at greater risk of eating disorders over time.51 They may withhold insulin to lose weight, a practice referred to in the nonmedical literature as “diabulimia,” and they seem particularly more likely to develop bulimia nervosa than those without diabetes.52
The medical prognosis is poor for patients with diabetes who develop eating disorders and do not receive intensive treatment.51 In addition, if a diabetic patient on an insulin pump becomes depressed in addition to having an eating disorder, careful monitoring for suicidal thoughts and a rapid follow-up with mental health services are in order.
REFEEDING SYNDROME
When refeeding is started, a high glucose load stimulates insulin secretion, resulting in cellular uptake of phosphorus along with potassium, magnesium, and glucose. In addition, total body phosphorus is depleted by the increased demand for adenosine triphosphate and 2,3-diphosphoglycerate for cellular metabolism.
When liver enzyme levels increase, the astute clinician will closely monitor the patient for evidence of refeeding syndrome. In a child, adolescent, or young adult, the standard of care is inpatient monitoring for acute stabilization.4,19
Hypophosphatemia is the hallmark of refeeding syndrome, although hypomagnesemia, hypokalemia, and hypoglycemia can also occur.53 In addition, sodium and water retention can lead to fluid overload, with shifting of fluid into the intracellular space, resulting in dependent edema.
Cardiovascular complications are the most worrisome manifestations of refeeding syndrome. Electrolyte shifts and increased fluid volume can cause arrhythmias and heart failure. Furthermore, severely undernourished patients may have reduced myocardial mass as well as electrocardiographic abnormalities associated with starvation, which further increase their vulnerability to electrolyte shifts and fluid retention during refeeding.15
Other manifestations of refeeding syndrome include delirium, seizures, rhabdomyolysis, and respiratory failure. In the most extreme cases, refeeding syndrome causes sudden death.53
Fortunately, refeeding syndrome is easily preventable and treatable when recognized early. Electrolytes and cardiovascular and renal function must be carefully monitored, especially during the first week of nutritional restoration.53 In patients with extremely low body mass (< 70% of ideal body weight) or with precipitous weight loss, close monitoring of the complete metabolic panel including electrolytes, AST, ALT, calcium, magnesium, and phosphorus may be required to detect changes that can affect cardiac status. Specific suggestions for refeeding are discussed below and in Table 2.45
ACUTE CARE OF PATIENTS WITH EATING DISORDERS
Refeeding in the inpatient setting
The decision to hospitalize an eating-disorder patient is based on the current or potential risk of serious medical complications and the likelihood of success at home. Medical criteria for hospital admission are outlined in Table 3.4,54
In refeeding undernourished patients, the challenge is to maximize weight gain while preventing refeeding syndrome. Undernourished patients are generally hypometabolic at baseline but become hypermetabolic once refeeding begins.
How many calories should refeeding start with? The traditional principle of “start low and go slow” has been recently challenged.55 Starting at 1,200 kcal/day or less in the typical patient can result in failure to gain weight or even in weight loss in the first week of refeeding.56 The goal is to achieve a weight gain of 0.2 kg/day while the patient is in the hospital. Thus, we start higher, and to date we have seen no cases of life-threatening refeeding syndrome. In all patients who need hospitalization or who are beginning the refeeding process as outpatients, caloric intake should be started at 1,500 to 2,000 kcal/day.45,57 However, for exceptionally low-weight patients, intake may be started lower.
In Australia, patients are started at 1,900 kcal/day.56 All patients in one program there receive nasogastric feeding initially in an intensive care unit and then are moved to a regular nursing floor where they graduate to full oral feeding as they improve cardiovascularly and behaviorally. In the United States, some programs use nasogastric feeding at night for caloric restoration; our program and others use nasogastric feeding as a behavioral modification strategy for patients who refuse food or supplements by mouth.
Phosphorus supplementation. Many centers give phosphorus supplements preventively. In our center, we give potassium phosphate (Neutra-Phos) 500 mg orally twice daily for 5 days, and we have seen no life-threatening cases of refeeding syndrome with that regimen. Other centers give phosphorus supplements in a dose of 250 mg orally twice a day for 5 days, while still others only supplement phosphorus reactively once a deficit has been identified. The latter method requires daily blood draws for monitoring and is reactive rather than proactive. Further studies can help clarify the optimal dosing and timing of phosphorus supplementation.
Managing fluid balance. Fluid-loading these patients may tip them over the edge into refeeding syndrome. Except in cases of shock, patients with eating disorders should not be given intravenous fluids, as it is safer to rehydrate and feed them orally. Electrolyte imbalances can be corrected orally with no need for intravenous supplementation. To avoid fluid overload, fluids can be started at 1,500 mL to 2,000 mL per day, with strict monitoring of intake and output. Fluids are liberalized if ALT and AST levels remain normal and to gradually correct orthostatic hypotension; caloric fluids are ideal to help address energy needs and improve bradycardia.
Laboratory monitoring. On admission, a urinalysis, complete blood cell count, complete metabolic panel, TSH, erythrocyte sedimentation rate, serum magnesium, and phosphorus should be obtained.26 In addition, continuous electrocardiographic recording should begin on admission.45 Inpatient use of a telemetry bed helps identify extreme tachycardia with arrhythmia, as well as profound bradycardia.45,56
Some protocols call for daily laboratory monitoring, although that degree of testing is less cost-effective. If initial results are normal, clinical judgment can be used on when to repeat laboratory evaluation. For instance, patients with edema require repeat complete metabolic panels to assess for elevated ALT and AST, electrolyte imbalances, and other abnormalities.
Signs of refeeding syndrome include tachycardia, hepatosplenomegaly, peripheral edema, altered mental status, and electrolyte disturbances, specifically, acute or severe hypophosphatemia or hypokalemia.26,45 If refeeding syndrome is suspected, the rate of caloric intake should be reduced or not advanced, fluid intake should be urgently reassessed for volume overload, and supportive care with close monitoring should be provided.
KNOWLEDGE SAVES LIVES
Eating disorders can lead to potentially life-threatening medical complications that require attentive care by the primary care clinician and subspecialist. Without thoughtful consideration, it is easy for even a caring medical team to unintentionally enable patients with these illnesses or to cause active harm in the case of underrecognized pathology.58
Acute medical stabilization on an inpatient unit trained to recognize pathology and treat sequelae can be lifesaving. Arming patients and families with medical knowledge, as provided in the Academy for Eating Disorders’ brochure, “Critical Points for Early Recognition and Medical Risk Management in the Care of Individuals with Eating Disorders”59 can help save patients’ lives.
- Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch Gen Psychiatry 2011; 68:724–731.
- Walsh JM, Wheat ME, Freund K. Detection, evaluation, and treatment of eating disorders the role of the primary care physician. J Gen Intern Med 2000; 15:577–590.
- American Academy of Pediatrics; Committee on Adolescence. Identifying and treating eating disorders. Pediatrics 2003; 111:204–211.
- Rosen DS; American Academy of Pediatrics Committee on Adolescence. Identification and management of eating disorders in children and adolescents. Pediatrics 2010; 126:1240–1253.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Arlington, VA: American Psychiatric Publishing, Incorporated; 2013.
- Eddy KT, Celio Doyle A, Hoste RR, Herzog DB, le Grange D. Eating disorder not otherwise specified in adolescents. J Am Acad Child Adolesc Psychiatry 2008; 47:156–164.
- Muise AM, Stein DG, Arbess G. Eating disorders in adolescent boys: a review of the adolescent and young adult literature. J Adolesc Health 2003; 33:427–435.
- Attia E, Roberto CA. Should amenorrhea be a diagnostic criterion for anorexia nervosa? Int J Eat Disord 2009; 42:581–589.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, fifth edition. http://dsm.psychiatryonline.org/content.aspx?bookid=556§ionid=41101776#103439089. Accessed January 31, 2014.
- Wilfley DE, Bishop ME, Wilson GT, Agras WS. Classification of eating disorders: toward DSM-V. Int J Eat Disord 2007; 40:S123–S129.
- Wonderlich SA, Gordon KH, Mitchell JE, Crosby RD, Engel SG. The validity and clinical utility of binge eating disorder. Int J Eat Disord 2009; 42:687–705.
- Ornstein RM, Rosen DS, Mammel KA, et al. Distribution of eating disorders in children and adolescents using the proposed DSM-5 criteria for feeding and eating disorders. J Adolesc Health 2013: 53:303–305.
- Winston AP, Stafford PJ. Cardiovascular effects of anorexia nervosa. Eur Eat Disord Rev 2000; 8:117–125.
- Galetta F, Franzoni F, Prattichizzo F, Rolla M, Santoro G, Pentimone F. Heart rate variability and left ventricular diastolic function in anorexia nervosa. J Adolesc Health 2003; 32:416–421.
- McCallum K, Bermudez O, Ohlemeyer C, Tyson E, Portilla M, Ferdman B. How should the clinician evaluate and manage the cardiovascular complications of anorexia nervosa? Eat Disord 2006; 14:73–80.
- Akhtar M. Clinical spectrum of ventricular tachycardia. Circulation 1990; 82:1561–1573.
- Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics 2013; 54:1–13.
- The University of Arizona Center for Education and Research on Therapeutics. QT Drug Lists. http://crediblemeds.org/everyone/compos-ite-list-all-qtdrugs/?rf=US. Accessed January 31, 2014.
- Rome ES, Ammerman S. Medical complications of eating disorders: an update. J Adolesc Health 2003; 33:418–426.
- Romano C, Chinali M, Pasanisi F, et al. Reduced hemodynamic load and cardiac hypotrophy in patients with anorexia nervosa. Am J Clin Nutr 2003; 77:308–312.
- Shamim T, Golden NH, Arden M, Filiberto L, Shenker IR. Resolution of vital sign instability: an objective measure of medical stability in anorexia nervosa. J Adolesc Health 2003; 32:73–77.
- Mont L, Castro J, Herreros B, et al. Reversibility of cardiac abnormalities in adolescents with anorexia nervosa after weight recovery. J Am Acad Child Adolesc Psychiatry 2003; 42:808–813.
- Roberto CA, Mayer LE, Brickman AM, et al. Brain tissue volume changes following weight gain in adults with anorexia nervosa. Int J Eat Disord 2011; 44:406–411.
- Treasure J, Russell G. The case for early intervention in anorexia nervosa: theoretical exploration of maintaining factors. Br J Psychiatry 2011; 199:5–7.
- Hadley SJ, Walsh BT. Gastrointestinal disturbances in anorexia nervosa and bulimia nervosa. Curr Drug Targets CNS Neurol Disord 2003; 2:1–9.
- Yager J, Andersen AE. Clinical practice. Anorexia nervosa. N Engl J Med 2005; 353:1481–1488.
- De Caprio C, Alfano A, Senatore I, Zarrella L, Pasanisi F, Contaldo F. Severe acute liver damage in anorexia nervosa: two case reports. Nutrition 2006; 22:572–575.
- Lawson EA, Klibanski A. Endocrine abnormalities in anorexia nervosa. Nat Clin Pract Endocrinol Metab 2008; 4:407–414.
- Holtkamp K, Mika C, Grzella I, et al. Reproductive function during weight gain in anorexia nervosa. Leptin represents a metabolic gate to gonadotropin secretion. J Neural Transm 2003; 110:427–435.
- Golden NH, Jacobson MS, Schebendach J, Solanto MV, Hertz SM, Shenker IR. Resumption of menses in anorexia nervosa. Arch Pediatr Adolesc Med 1997; 151:16–21.
- Soyka LA, Misra M, Frenchman A, et al. Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab 2002; 87:4177–4185.
- Misra M, Klibanski A. Bone metabolism in adolescents with anorexia nervosa. J Endocrinol Invest 2011; 34:324–332.
- Recker RR, Davies KM, Hinders SM, Heaney RP, Stegman MR, Kimmel DB. Bone gain in young adult women. JAMA 1992; 268:2403–2408.
- Biller BM, Saxe V, Herzog DB, Rosenthal DI, Holzman S, Klibanski A. Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinol Metab 1989; 68:548–554.
- Hergenroeder AC, Smith EO, Shypailo R, Jones LA, Klish WJ, Ellis K. Bone mineral changes in young women with hypothalamic amenorrhea treated with oral contraceptives, medroxyprogesterone, or placebo over 12 months. Am J Obstet Gynecol 1997; 176:1017–1025.
- Sim LA, McGovern L, Elamin MB, Swiglo BA, Erwin PJ, Montori VM. Effect on bone health of estrogen preparations in premenopausal women with anorexia nervosa: a systematic review and meta-analyses. Int J Eat Disord 2010; 43:218–225.
- Golden NH, Lanzkowsky L, Schebendach J, Palestro CJ, Jacobson MS, Shenker IR. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol 2002; 15:135–143.
- Misra M, Katzman D, Miller KK, et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res 2011; 26:2430–2438.
- Klibanski A, Biller BM, Schoenfeld DA, Herzog DB, Saxe VC. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab 1995; 80:898–904.
- Divasta AD, Feldman HA, Giancaterino C, Rosen CJ, Leboff MS, Gordon CM. The effect of gonadal and adrenal steroid therapy on skeletal health in adolescents and young women with anorexia nervosa. Metabolism 2012; 61:1010–1020.
- Mehler PS. Medical complications of bulimia nervosa and their treatments. Int J Eat Disord 2011; 44:95–104.
- Milosevic A. Eating disorders and the dentist. Br Dent J 1999; 186:109–113.
- Greenfeld D, Mickley D, Quinlan DM, Roloff P. Hypokalemia in outpatients with eating disorders. Am J Psychiatry 1995; 152:60–63.
- Bouquegneau A, Dubois BE, Krzesinski JM, Delanaye P. Anorexia nervosa and the kidney. Am J Kidney Dis 2012; 60:299–307.
- Auron M, Rome E. Anorexia nervosa and bulimia nervosa: what the hospitalist needs to know about CPT 269.9, or nutritional insufficiency. ACP Hospitalist 2011 Sept:28–45.
- Steffen KJ, Mitchell JE, Roerig JL, Lancaster KL. The eating disorders medicine cabinet revisited: a clinician’s guide to ipecac and laxatives. Int J Eat Disord 2007; 40:360–368.
- Roerig JL, Steffen KJ, Mitchell JE, Zunker C. Laxative abuse: epidemiology, diagnosis and management. Drugs 2010; 70:1487–1503.
- Mitchell JE, Boutacoff LI. Laxative abuse complicating bulimia: medical and treatment implications. Int J Eat Disord 1986; 5:325–334.
- Joo JS, Ehrenpreis ED, Gonzalez L, et al. Alterations in colonic anatomy induced by chronic stimulant laxatives: the cathartic colon revisited. J Clin Gastroenterol 1998; 26:283–286.
- Drugs.com. Ipecac syrup. www.drugs.com/monograph/ipecac-syrup.html. Accessed January 31, 2014.
- Peveler RC, Bryden KS, Neil HA, et al. The relationship of disordered eating habits and attitudes to clinical outcomes in young adult females with type 1 diabetes. Diabetes Care 2005; 28:84–88.
- Mannucci E, Rotella F, Ricca V, Moretti S, Placidi GF, Rotella CM. Eating disorders in patients with type 1 diabetes: a meta-analysis. J Endocrinol Invest 2005; 28:417–419.
- Crook MA, Hally V, Panteli JV. The importance of the refeeding syndrome. Nutrition 2001; 17:632–637.
- Fisher M, Golden NH, Katzman DK, et al. Eating disorders in adolescents: a background paper. J Adolesc Health 1995; 16:420–437.
- Kohn MR, Madden S, Clarke SD. Refeeding in anorexia nervosa: increased safety and efficiency through understanding the pathophysiology of protein calorie malnutrition. Curr Opin Pediatr 2011; 23:390–394.
- Garber AK, Michihata N, Hetnal K, Shafer MA, Moscicki AB. A prospective examination of weight gain in hospitalized adolescents with anorexia nervosa on a recommended refeeding protocol. J Adolesc Health 2012; 50:24–29.
- Whitelaw M, Gilbertson H, Lam PY, Sawyer SM. Does aggressive refeeding in hospitalized adolescents with anorexia nervosa result in increased hypophosphatemia? J Adolesc Health 2010; 46:577–582.
- Treasure J, Crane A, McKnight R, Buchanan E, Wolfe M. First do no harm: iatrogenic maintaining factors in anorexia nervosa. Eur Eat Disord Rev 2011; 19:296–302.
- Academy for Eating Disorders (AED). Critical points for early recognition and medical risk management in the care of individuals with eating disorders. http://www.aedweb.org/AM/Template.cfm?Section=Medical_Care_Standards&Template=/CM/ContentDisplay.cfm&ContentID=2413. Accessed January 31, 2014.
- Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch Gen Psychiatry 2011; 68:724–731.
- Walsh JM, Wheat ME, Freund K. Detection, evaluation, and treatment of eating disorders the role of the primary care physician. J Gen Intern Med 2000; 15:577–590.
- American Academy of Pediatrics; Committee on Adolescence. Identifying and treating eating disorders. Pediatrics 2003; 111:204–211.
- Rosen DS; American Academy of Pediatrics Committee on Adolescence. Identification and management of eating disorders in children and adolescents. Pediatrics 2010; 126:1240–1253.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Arlington, VA: American Psychiatric Publishing, Incorporated; 2013.
- Eddy KT, Celio Doyle A, Hoste RR, Herzog DB, le Grange D. Eating disorder not otherwise specified in adolescents. J Am Acad Child Adolesc Psychiatry 2008; 47:156–164.
- Muise AM, Stein DG, Arbess G. Eating disorders in adolescent boys: a review of the adolescent and young adult literature. J Adolesc Health 2003; 33:427–435.
- Attia E, Roberto CA. Should amenorrhea be a diagnostic criterion for anorexia nervosa? Int J Eat Disord 2009; 42:581–589.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, fifth edition. http://dsm.psychiatryonline.org/content.aspx?bookid=556§ionid=41101776#103439089. Accessed January 31, 2014.
- Wilfley DE, Bishop ME, Wilson GT, Agras WS. Classification of eating disorders: toward DSM-V. Int J Eat Disord 2007; 40:S123–S129.
- Wonderlich SA, Gordon KH, Mitchell JE, Crosby RD, Engel SG. The validity and clinical utility of binge eating disorder. Int J Eat Disord 2009; 42:687–705.
- Ornstein RM, Rosen DS, Mammel KA, et al. Distribution of eating disorders in children and adolescents using the proposed DSM-5 criteria for feeding and eating disorders. J Adolesc Health 2013: 53:303–305.
- Winston AP, Stafford PJ. Cardiovascular effects of anorexia nervosa. Eur Eat Disord Rev 2000; 8:117–125.
- Galetta F, Franzoni F, Prattichizzo F, Rolla M, Santoro G, Pentimone F. Heart rate variability and left ventricular diastolic function in anorexia nervosa. J Adolesc Health 2003; 32:416–421.
- McCallum K, Bermudez O, Ohlemeyer C, Tyson E, Portilla M, Ferdman B. How should the clinician evaluate and manage the cardiovascular complications of anorexia nervosa? Eat Disord 2006; 14:73–80.
- Akhtar M. Clinical spectrum of ventricular tachycardia. Circulation 1990; 82:1561–1573.
- Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics 2013; 54:1–13.
- The University of Arizona Center for Education and Research on Therapeutics. QT Drug Lists. http://crediblemeds.org/everyone/compos-ite-list-all-qtdrugs/?rf=US. Accessed January 31, 2014.
- Rome ES, Ammerman S. Medical complications of eating disorders: an update. J Adolesc Health 2003; 33:418–426.
- Romano C, Chinali M, Pasanisi F, et al. Reduced hemodynamic load and cardiac hypotrophy in patients with anorexia nervosa. Am J Clin Nutr 2003; 77:308–312.
- Shamim T, Golden NH, Arden M, Filiberto L, Shenker IR. Resolution of vital sign instability: an objective measure of medical stability in anorexia nervosa. J Adolesc Health 2003; 32:73–77.
- Mont L, Castro J, Herreros B, et al. Reversibility of cardiac abnormalities in adolescents with anorexia nervosa after weight recovery. J Am Acad Child Adolesc Psychiatry 2003; 42:808–813.
- Roberto CA, Mayer LE, Brickman AM, et al. Brain tissue volume changes following weight gain in adults with anorexia nervosa. Int J Eat Disord 2011; 44:406–411.
- Treasure J, Russell G. The case for early intervention in anorexia nervosa: theoretical exploration of maintaining factors. Br J Psychiatry 2011; 199:5–7.
- Hadley SJ, Walsh BT. Gastrointestinal disturbances in anorexia nervosa and bulimia nervosa. Curr Drug Targets CNS Neurol Disord 2003; 2:1–9.
- Yager J, Andersen AE. Clinical practice. Anorexia nervosa. N Engl J Med 2005; 353:1481–1488.
- De Caprio C, Alfano A, Senatore I, Zarrella L, Pasanisi F, Contaldo F. Severe acute liver damage in anorexia nervosa: two case reports. Nutrition 2006; 22:572–575.
- Lawson EA, Klibanski A. Endocrine abnormalities in anorexia nervosa. Nat Clin Pract Endocrinol Metab 2008; 4:407–414.
- Holtkamp K, Mika C, Grzella I, et al. Reproductive function during weight gain in anorexia nervosa. Leptin represents a metabolic gate to gonadotropin secretion. J Neural Transm 2003; 110:427–435.
- Golden NH, Jacobson MS, Schebendach J, Solanto MV, Hertz SM, Shenker IR. Resumption of menses in anorexia nervosa. Arch Pediatr Adolesc Med 1997; 151:16–21.
- Soyka LA, Misra M, Frenchman A, et al. Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab 2002; 87:4177–4185.
- Misra M, Klibanski A. Bone metabolism in adolescents with anorexia nervosa. J Endocrinol Invest 2011; 34:324–332.
- Recker RR, Davies KM, Hinders SM, Heaney RP, Stegman MR, Kimmel DB. Bone gain in young adult women. JAMA 1992; 268:2403–2408.
- Biller BM, Saxe V, Herzog DB, Rosenthal DI, Holzman S, Klibanski A. Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinol Metab 1989; 68:548–554.
- Hergenroeder AC, Smith EO, Shypailo R, Jones LA, Klish WJ, Ellis K. Bone mineral changes in young women with hypothalamic amenorrhea treated with oral contraceptives, medroxyprogesterone, or placebo over 12 months. Am J Obstet Gynecol 1997; 176:1017–1025.
- Sim LA, McGovern L, Elamin MB, Swiglo BA, Erwin PJ, Montori VM. Effect on bone health of estrogen preparations in premenopausal women with anorexia nervosa: a systematic review and meta-analyses. Int J Eat Disord 2010; 43:218–225.
- Golden NH, Lanzkowsky L, Schebendach J, Palestro CJ, Jacobson MS, Shenker IR. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol 2002; 15:135–143.
- Misra M, Katzman D, Miller KK, et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res 2011; 26:2430–2438.
- Klibanski A, Biller BM, Schoenfeld DA, Herzog DB, Saxe VC. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab 1995; 80:898–904.
- Divasta AD, Feldman HA, Giancaterino C, Rosen CJ, Leboff MS, Gordon CM. The effect of gonadal and adrenal steroid therapy on skeletal health in adolescents and young women with anorexia nervosa. Metabolism 2012; 61:1010–1020.
- Mehler PS. Medical complications of bulimia nervosa and their treatments. Int J Eat Disord 2011; 44:95–104.
- Milosevic A. Eating disorders and the dentist. Br Dent J 1999; 186:109–113.
- Greenfeld D, Mickley D, Quinlan DM, Roloff P. Hypokalemia in outpatients with eating disorders. Am J Psychiatry 1995; 152:60–63.
- Bouquegneau A, Dubois BE, Krzesinski JM, Delanaye P. Anorexia nervosa and the kidney. Am J Kidney Dis 2012; 60:299–307.
- Auron M, Rome E. Anorexia nervosa and bulimia nervosa: what the hospitalist needs to know about CPT 269.9, or nutritional insufficiency. ACP Hospitalist 2011 Sept:28–45.
- Steffen KJ, Mitchell JE, Roerig JL, Lancaster KL. The eating disorders medicine cabinet revisited: a clinician’s guide to ipecac and laxatives. Int J Eat Disord 2007; 40:360–368.
- Roerig JL, Steffen KJ, Mitchell JE, Zunker C. Laxative abuse: epidemiology, diagnosis and management. Drugs 2010; 70:1487–1503.
- Mitchell JE, Boutacoff LI. Laxative abuse complicating bulimia: medical and treatment implications. Int J Eat Disord 1986; 5:325–334.
- Joo JS, Ehrenpreis ED, Gonzalez L, et al. Alterations in colonic anatomy induced by chronic stimulant laxatives: the cathartic colon revisited. J Clin Gastroenterol 1998; 26:283–286.
- Drugs.com. Ipecac syrup. www.drugs.com/monograph/ipecac-syrup.html. Accessed January 31, 2014.
- Peveler RC, Bryden KS, Neil HA, et al. The relationship of disordered eating habits and attitudes to clinical outcomes in young adult females with type 1 diabetes. Diabetes Care 2005; 28:84–88.
- Mannucci E, Rotella F, Ricca V, Moretti S, Placidi GF, Rotella CM. Eating disorders in patients with type 1 diabetes: a meta-analysis. J Endocrinol Invest 2005; 28:417–419.
- Crook MA, Hally V, Panteli JV. The importance of the refeeding syndrome. Nutrition 2001; 17:632–637.
- Fisher M, Golden NH, Katzman DK, et al. Eating disorders in adolescents: a background paper. J Adolesc Health 1995; 16:420–437.
- Kohn MR, Madden S, Clarke SD. Refeeding in anorexia nervosa: increased safety and efficiency through understanding the pathophysiology of protein calorie malnutrition. Curr Opin Pediatr 2011; 23:390–394.
- Garber AK, Michihata N, Hetnal K, Shafer MA, Moscicki AB. A prospective examination of weight gain in hospitalized adolescents with anorexia nervosa on a recommended refeeding protocol. J Adolesc Health 2012; 50:24–29.
- Whitelaw M, Gilbertson H, Lam PY, Sawyer SM. Does aggressive refeeding in hospitalized adolescents with anorexia nervosa result in increased hypophosphatemia? J Adolesc Health 2010; 46:577–582.
- Treasure J, Crane A, McKnight R, Buchanan E, Wolfe M. First do no harm: iatrogenic maintaining factors in anorexia nervosa. Eur Eat Disord Rev 2011; 19:296–302.
- Academy for Eating Disorders (AED). Critical points for early recognition and medical risk management in the care of individuals with eating disorders. http://www.aedweb.org/AM/Template.cfm?Section=Medical_Care_Standards&Template=/CM/ContentDisplay.cfm&ContentID=2413. Accessed January 31, 2014.
KEY POINTS
- The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), released in 2013, has updated the criteria for some eating disorders and has added some new disorders.
- Starvation can cause cardiac, cerebral, gastrointestinal, and endocrine problems.
- Purging can lead to problems with oral health, electrolyte imbalances, and even renal failure.
- Refeeding poses the risk of refeeding syndrome, with fluid overload and electrolyte imbalances. Many patients undergoing refeeding are best managed in the hospital.
In reply: Menstrual manipulation
In Reply: Thank you for reading our article. Although the focus was geared more toward a comparison of different means of menstrual manipulation, we appreciate your comments on oral contraceptives and the link to premenopausal breast cancer.
As you noted, oral contraceptives have been linked to an increased risk of breast cancer, both in your meta-analysis1 and again more recently in a prospective study of 116,608 female nurses from 25 to 42 years of age.2 Interestingly, data from the latter study suggested that different formulations of oral contraceptives may pose different risks, and specifically that the use of triphasic preparations with levonorgestrel as the progestin had the highest risk. However, there is otherwise a paucity of data regarding the risk of specific formulations. There is currently no evidence of an association between oral contraceptive use and death from breast cancer, nor is there evidence that longer use of an oral contraceptive increases one’s risk of death from breast cancer.3
Oral contraceptives have also been associated with a reduced risk of ovarian cancer,4 and they appear to protect against death from ovarian cancer and uterine cancer.3 Therefore, the clinician must consider the individual patient before making treatment recommendations, taking into account personal risk factors and other health concerns. (For a full list of contraindications to oral contraceptives, please refer to Table 2 in our original article.) Further guidelines may also be obtained from the “US Medical Eligibility Criteria for Contraceptive Use 2010,” issued by the US Centers for Disease Control and Prevention in May 2010,5 which delineates the eligibility criteria for initiating and continuing specific contraceptive methods, including oral contraceptives.
Thank you again for sharing your concerns. We appreciate the opportunity to clarify this important point.
- Kahlenborn C, Modugno F, Potter DM, et al. Oral contraceptive use as a risk factor for premenopausal breast cancer: a meta-analysis. Mayo Clin Proc 2006; 81:1290–1302.
- Hunter DJ, Colditz GA, Hankinson SE, et al. Oral contraceptive use and breast cancer: a prospective study of young women. Cancer Epidemiol Biomarkers Prev 2010; 19:2496–2502.
- Vessey M, Yeates D, Flynn S. Factors affecting mortality in a large cohort study with special reference to oral contraceptive use. Contraception 2010; 82:221–229.
- Lurie G, Thompson P, McDuffie KE, et al. Association of estrogen and progestin potency of oral contraceptives with ovarian carcinoma risk. Obstet Gynecol 2007; 109:597–607.
- Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, US Centers for Disease Control and Prevention (CDC), Farr S, et al. US medical eligibility criteria for contraceptive use, 2010: adapted from the World Health Organization medical eligibility criteria for contraceptive use, 4th edition. MMWR Recomm Rep 2010; 59:1–86.
In Reply: Thank you for reading our article. Although the focus was geared more toward a comparison of different means of menstrual manipulation, we appreciate your comments on oral contraceptives and the link to premenopausal breast cancer.
As you noted, oral contraceptives have been linked to an increased risk of breast cancer, both in your meta-analysis1 and again more recently in a prospective study of 116,608 female nurses from 25 to 42 years of age.2 Interestingly, data from the latter study suggested that different formulations of oral contraceptives may pose different risks, and specifically that the use of triphasic preparations with levonorgestrel as the progestin had the highest risk. However, there is otherwise a paucity of data regarding the risk of specific formulations. There is currently no evidence of an association between oral contraceptive use and death from breast cancer, nor is there evidence that longer use of an oral contraceptive increases one’s risk of death from breast cancer.3
Oral contraceptives have also been associated with a reduced risk of ovarian cancer,4 and they appear to protect against death from ovarian cancer and uterine cancer.3 Therefore, the clinician must consider the individual patient before making treatment recommendations, taking into account personal risk factors and other health concerns. (For a full list of contraindications to oral contraceptives, please refer to Table 2 in our original article.) Further guidelines may also be obtained from the “US Medical Eligibility Criteria for Contraceptive Use 2010,” issued by the US Centers for Disease Control and Prevention in May 2010,5 which delineates the eligibility criteria for initiating and continuing specific contraceptive methods, including oral contraceptives.
Thank you again for sharing your concerns. We appreciate the opportunity to clarify this important point.
In Reply: Thank you for reading our article. Although the focus was geared more toward a comparison of different means of menstrual manipulation, we appreciate your comments on oral contraceptives and the link to premenopausal breast cancer.
As you noted, oral contraceptives have been linked to an increased risk of breast cancer, both in your meta-analysis1 and again more recently in a prospective study of 116,608 female nurses from 25 to 42 years of age.2 Interestingly, data from the latter study suggested that different formulations of oral contraceptives may pose different risks, and specifically that the use of triphasic preparations with levonorgestrel as the progestin had the highest risk. However, there is otherwise a paucity of data regarding the risk of specific formulations. There is currently no evidence of an association between oral contraceptive use and death from breast cancer, nor is there evidence that longer use of an oral contraceptive increases one’s risk of death from breast cancer.3
Oral contraceptives have also been associated with a reduced risk of ovarian cancer,4 and they appear to protect against death from ovarian cancer and uterine cancer.3 Therefore, the clinician must consider the individual patient before making treatment recommendations, taking into account personal risk factors and other health concerns. (For a full list of contraindications to oral contraceptives, please refer to Table 2 in our original article.) Further guidelines may also be obtained from the “US Medical Eligibility Criteria for Contraceptive Use 2010,” issued by the US Centers for Disease Control and Prevention in May 2010,5 which delineates the eligibility criteria for initiating and continuing specific contraceptive methods, including oral contraceptives.
Thank you again for sharing your concerns. We appreciate the opportunity to clarify this important point.
- Kahlenborn C, Modugno F, Potter DM, et al. Oral contraceptive use as a risk factor for premenopausal breast cancer: a meta-analysis. Mayo Clin Proc 2006; 81:1290–1302.
- Hunter DJ, Colditz GA, Hankinson SE, et al. Oral contraceptive use and breast cancer: a prospective study of young women. Cancer Epidemiol Biomarkers Prev 2010; 19:2496–2502.
- Vessey M, Yeates D, Flynn S. Factors affecting mortality in a large cohort study with special reference to oral contraceptive use. Contraception 2010; 82:221–229.
- Lurie G, Thompson P, McDuffie KE, et al. Association of estrogen and progestin potency of oral contraceptives with ovarian carcinoma risk. Obstet Gynecol 2007; 109:597–607.
- Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, US Centers for Disease Control and Prevention (CDC), Farr S, et al. US medical eligibility criteria for contraceptive use, 2010: adapted from the World Health Organization medical eligibility criteria for contraceptive use, 4th edition. MMWR Recomm Rep 2010; 59:1–86.
- Kahlenborn C, Modugno F, Potter DM, et al. Oral contraceptive use as a risk factor for premenopausal breast cancer: a meta-analysis. Mayo Clin Proc 2006; 81:1290–1302.
- Hunter DJ, Colditz GA, Hankinson SE, et al. Oral contraceptive use and breast cancer: a prospective study of young women. Cancer Epidemiol Biomarkers Prev 2010; 19:2496–2502.
- Vessey M, Yeates D, Flynn S. Factors affecting mortality in a large cohort study with special reference to oral contraceptive use. Contraception 2010; 82:221–229.
- Lurie G, Thompson P, McDuffie KE, et al. Association of estrogen and progestin potency of oral contraceptives with ovarian carcinoma risk. Obstet Gynecol 2007; 109:597–607.
- Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, US Centers for Disease Control and Prevention (CDC), Farr S, et al. US medical eligibility criteria for contraceptive use, 2010: adapted from the World Health Organization medical eligibility criteria for contraceptive use, 4th edition. MMWR Recomm Rep 2010; 59:1–86.