User login
Adding pertuzumab shows benefit in ERBB2-positive breast cancer
Combination pertuzumab, trastuzumab, and docetaxel provided better responses than did placebo, trastuzumab, and docetaxel in Asian patients with ERBB2-positive early or locally advanced breast cancer, according to a phase 3 trial.
The safety profile of the combination regimen was similar between the treatment arms and in accordance with that of pertuzumab alone, reported Zhimin Shao, MD, of Fudan (Shanghai) University Cancer Center, and colleagues. The study was published in JAMA Oncology.
The randomized, placebo-controlled, phase 3 PEONY study included 329 women with ERBB2-positive early or locally advanced disease. The effects of adding pertuzumab to trastuzumab and docetaxel was studied in 23 centers throughout Asia.
Prior to surgery, study subjects in the treatment arm received intravenous pertuzumab at a loading dose of 840 mg followed by 420 mg, trastuzumab at a loading dose of 8 mg/kg followed by 6 mg/kg, and 75 mg/m2 of docetaxel, while patients in the placebo arm received placebo, trastuzumab, and docetaxel. Both regimens were administered every 3 weeks for a total of four cycles.
Post surgery, study patients received intravenous fluorouracil, cyclophosphamide, and epirubicin for a total of 3 cycles, followed by pertuzumab plus trastuzumab or placebo plus trastuzumab for a total of 13 cycles.
The primary outcome was the total pathologic complete response rate assessed at the completion of surgery.
After analysis, the researchers found that total pathologic complete response rates were significantly higher for patients in the pertuzumab arm (39.3%) compared with the placebo arm (21.8%) (difference, 17.5%; P = .001).
With respect to safety, the rates of common adverse events were similar between the groups, with the exception of diarrhea (38.5% in the pertuzumab arm vs. 16.4% in the placebo arm). The incidences of serious toxicities were slightly higher in the pertuzumab arm (10.1%) compared with the placebo arm (8.2%).
“Of the most common grade 3 or higher adverse events, there was a higher incidence of neutropenia in the pertuzumab group (38.1% vs. 32.7%),” they reported.
The researchers acknowledged a key limitation of the study was the short duration of follow-up. As a result, some secondary outcome data were immature at the time of the analysis.
“The PEONY trial adds to the totality of the data showing the benefit of pertuzumab and trastuzumab with chemotherapy in ERBB2-positive early breast cancer,” they concluded.
The authors reported financial affiliations with F. Hoffmann-La Roche Ltd., which funded the study, and Genentech.
SOURCE: Shao Z et al. JAMA Oncol. 2019 Oct 24. doi: 10.1001/jamaoncol.2019.3692.
Combination pertuzumab, trastuzumab, and docetaxel provided better responses than did placebo, trastuzumab, and docetaxel in Asian patients with ERBB2-positive early or locally advanced breast cancer, according to a phase 3 trial.
The safety profile of the combination regimen was similar between the treatment arms and in accordance with that of pertuzumab alone, reported Zhimin Shao, MD, of Fudan (Shanghai) University Cancer Center, and colleagues. The study was published in JAMA Oncology.
The randomized, placebo-controlled, phase 3 PEONY study included 329 women with ERBB2-positive early or locally advanced disease. The effects of adding pertuzumab to trastuzumab and docetaxel was studied in 23 centers throughout Asia.
Prior to surgery, study subjects in the treatment arm received intravenous pertuzumab at a loading dose of 840 mg followed by 420 mg, trastuzumab at a loading dose of 8 mg/kg followed by 6 mg/kg, and 75 mg/m2 of docetaxel, while patients in the placebo arm received placebo, trastuzumab, and docetaxel. Both regimens were administered every 3 weeks for a total of four cycles.
Post surgery, study patients received intravenous fluorouracil, cyclophosphamide, and epirubicin for a total of 3 cycles, followed by pertuzumab plus trastuzumab or placebo plus trastuzumab for a total of 13 cycles.
The primary outcome was the total pathologic complete response rate assessed at the completion of surgery.
After analysis, the researchers found that total pathologic complete response rates were significantly higher for patients in the pertuzumab arm (39.3%) compared with the placebo arm (21.8%) (difference, 17.5%; P = .001).
With respect to safety, the rates of common adverse events were similar between the groups, with the exception of diarrhea (38.5% in the pertuzumab arm vs. 16.4% in the placebo arm). The incidences of serious toxicities were slightly higher in the pertuzumab arm (10.1%) compared with the placebo arm (8.2%).
“Of the most common grade 3 or higher adverse events, there was a higher incidence of neutropenia in the pertuzumab group (38.1% vs. 32.7%),” they reported.
The researchers acknowledged a key limitation of the study was the short duration of follow-up. As a result, some secondary outcome data were immature at the time of the analysis.
“The PEONY trial adds to the totality of the data showing the benefit of pertuzumab and trastuzumab with chemotherapy in ERBB2-positive early breast cancer,” they concluded.
The authors reported financial affiliations with F. Hoffmann-La Roche Ltd., which funded the study, and Genentech.
SOURCE: Shao Z et al. JAMA Oncol. 2019 Oct 24. doi: 10.1001/jamaoncol.2019.3692.
Combination pertuzumab, trastuzumab, and docetaxel provided better responses than did placebo, trastuzumab, and docetaxel in Asian patients with ERBB2-positive early or locally advanced breast cancer, according to a phase 3 trial.
The safety profile of the combination regimen was similar between the treatment arms and in accordance with that of pertuzumab alone, reported Zhimin Shao, MD, of Fudan (Shanghai) University Cancer Center, and colleagues. The study was published in JAMA Oncology.
The randomized, placebo-controlled, phase 3 PEONY study included 329 women with ERBB2-positive early or locally advanced disease. The effects of adding pertuzumab to trastuzumab and docetaxel was studied in 23 centers throughout Asia.
Prior to surgery, study subjects in the treatment arm received intravenous pertuzumab at a loading dose of 840 mg followed by 420 mg, trastuzumab at a loading dose of 8 mg/kg followed by 6 mg/kg, and 75 mg/m2 of docetaxel, while patients in the placebo arm received placebo, trastuzumab, and docetaxel. Both regimens were administered every 3 weeks for a total of four cycles.
Post surgery, study patients received intravenous fluorouracil, cyclophosphamide, and epirubicin for a total of 3 cycles, followed by pertuzumab plus trastuzumab or placebo plus trastuzumab for a total of 13 cycles.
The primary outcome was the total pathologic complete response rate assessed at the completion of surgery.
After analysis, the researchers found that total pathologic complete response rates were significantly higher for patients in the pertuzumab arm (39.3%) compared with the placebo arm (21.8%) (difference, 17.5%; P = .001).
With respect to safety, the rates of common adverse events were similar between the groups, with the exception of diarrhea (38.5% in the pertuzumab arm vs. 16.4% in the placebo arm). The incidences of serious toxicities were slightly higher in the pertuzumab arm (10.1%) compared with the placebo arm (8.2%).
“Of the most common grade 3 or higher adverse events, there was a higher incidence of neutropenia in the pertuzumab group (38.1% vs. 32.7%),” they reported.
The researchers acknowledged a key limitation of the study was the short duration of follow-up. As a result, some secondary outcome data were immature at the time of the analysis.
“The PEONY trial adds to the totality of the data showing the benefit of pertuzumab and trastuzumab with chemotherapy in ERBB2-positive early breast cancer,” they concluded.
The authors reported financial affiliations with F. Hoffmann-La Roche Ltd., which funded the study, and Genentech.
SOURCE: Shao Z et al. JAMA Oncol. 2019 Oct 24. doi: 10.1001/jamaoncol.2019.3692.
FROM JAMA ONCOLOGY
MMS linked with better survival in early-stage melanoma
according to a retrospective cohort study.
In the study, which was published in JAMA Dermatology, patients who underwent MMS had a “modest survival advantage” when compared with those who were treated with WME, the approach recommended for treatment of invasive melanoma without nodal or extralymphatic metastases in national guidelines, reported the investigators.
“We sought herein to investigate the association of the type of surgical excision – WME or MMS – with overall survival for cases of American Joint Committee on Cancer Cancer Staging Manual 8th edition (AJCC-8) stage I invasive melanoma,” wrote Shayan Cheraghlou, of Yale University, New Haven, Conn., and colleagues.
The researchers identified a total of 70,319 patients diagnosed with stage I invasive melanoma between Jan. 1, 2004, and Dec. 31, 2014. Data were collected from the National Cancer Database, including 3,234 (4.6%) and 67,085 (95.4%) patients who underwent MMS and WME, respectively. The median age of patients in the cohort was 57 years; 47.7% were female, and almost 97% were white.
In the survival analysis, the team adjusted for clinical and tumor-specific variables and conducted a matched analysis using propensity scores. The primary outcome measured was overall survival.
After analysis, the researchers found that MMS was associated with modestly better overall survival when compared with WME after adjustments (hazard ratio, 0.86; 95% confidence interval, 0.76-0.97). In the propensity score–matched analysis, a similar modest survival advantage was seen for patients who underwent MMS (hazard ratio, 0.82; 95% CI, 0.68-0.98).
“Significant differences in treatment practices based on the treatment facility were noted, with academic facilities more than twice as likely as nonacademic facilities to use MMS,” they wrote.
The researchers acknowledged a key limitation of the study was the use of a convenience sample, as opposed to a population-based sample. As a result, the generalizability of the findings may be limited to certain treatment facilities.
“These data suggest that MMS is an effective approach compared with WME for AJCC-8 stage I invasive melanoma,” they concluded.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: Cheraghlou S et al. JAMA Dermatol. 2019 Sep 25. doi: 10.1001/jamadermatol.2019.2890.
While controversial historically, evidence showing benefit for Mohs micrographic surgery (MMS) in patients with melanoma has been reported. The findings from the current study add to the body of retrospective data suggesting improved survival for those with early-stage disease.
The survival benefit found by Cheraghlou et al., “although relatively novel,” is not surprising. Previous population-based and database studies have demonstrated a nonsignificant trend toward a survival advantage in patients with early-stage melanoma. In addition, no survival disadvantages have been reported in any other stage of malignancy.
The primary advantage of MMS is the ability of the surgery to allow for full tumor resection. Reducing the likelihood of recurrence and ensuring local control is maximized remain key strategies to ensuring survival in patients with melanoma.
Database studies have limitations, and care should be taken not to overinterpret the results of a study with two groups of patients that are disproportionate in size. As the authors of the study note, their results support the need for prospective studies to compare surgical melanoma treatments. And until those studies can be done, “the weight of existing evidence suggests that MMS is a safe and effective treatment for melanoma.”
These comments are adapted from an accompanying editorial (JAMA Dermatol. 2019 Sep 25. doi: 10.1001/jamadermatol.2019.2622) by Ian Maher, MD, professor and director of dermatologic surgery at the University of Minnesota, Minneapolis. He reported having no conflicts of interest.
While controversial historically, evidence showing benefit for Mohs micrographic surgery (MMS) in patients with melanoma has been reported. The findings from the current study add to the body of retrospective data suggesting improved survival for those with early-stage disease.
The survival benefit found by Cheraghlou et al., “although relatively novel,” is not surprising. Previous population-based and database studies have demonstrated a nonsignificant trend toward a survival advantage in patients with early-stage melanoma. In addition, no survival disadvantages have been reported in any other stage of malignancy.
The primary advantage of MMS is the ability of the surgery to allow for full tumor resection. Reducing the likelihood of recurrence and ensuring local control is maximized remain key strategies to ensuring survival in patients with melanoma.
Database studies have limitations, and care should be taken not to overinterpret the results of a study with two groups of patients that are disproportionate in size. As the authors of the study note, their results support the need for prospective studies to compare surgical melanoma treatments. And until those studies can be done, “the weight of existing evidence suggests that MMS is a safe and effective treatment for melanoma.”
These comments are adapted from an accompanying editorial (JAMA Dermatol. 2019 Sep 25. doi: 10.1001/jamadermatol.2019.2622) by Ian Maher, MD, professor and director of dermatologic surgery at the University of Minnesota, Minneapolis. He reported having no conflicts of interest.
While controversial historically, evidence showing benefit for Mohs micrographic surgery (MMS) in patients with melanoma has been reported. The findings from the current study add to the body of retrospective data suggesting improved survival for those with early-stage disease.
The survival benefit found by Cheraghlou et al., “although relatively novel,” is not surprising. Previous population-based and database studies have demonstrated a nonsignificant trend toward a survival advantage in patients with early-stage melanoma. In addition, no survival disadvantages have been reported in any other stage of malignancy.
The primary advantage of MMS is the ability of the surgery to allow for full tumor resection. Reducing the likelihood of recurrence and ensuring local control is maximized remain key strategies to ensuring survival in patients with melanoma.
Database studies have limitations, and care should be taken not to overinterpret the results of a study with two groups of patients that are disproportionate in size. As the authors of the study note, their results support the need for prospective studies to compare surgical melanoma treatments. And until those studies can be done, “the weight of existing evidence suggests that MMS is a safe and effective treatment for melanoma.”
These comments are adapted from an accompanying editorial (JAMA Dermatol. 2019 Sep 25. doi: 10.1001/jamadermatol.2019.2622) by Ian Maher, MD, professor and director of dermatologic surgery at the University of Minnesota, Minneapolis. He reported having no conflicts of interest.
according to a retrospective cohort study.
In the study, which was published in JAMA Dermatology, patients who underwent MMS had a “modest survival advantage” when compared with those who were treated with WME, the approach recommended for treatment of invasive melanoma without nodal or extralymphatic metastases in national guidelines, reported the investigators.
“We sought herein to investigate the association of the type of surgical excision – WME or MMS – with overall survival for cases of American Joint Committee on Cancer Cancer Staging Manual 8th edition (AJCC-8) stage I invasive melanoma,” wrote Shayan Cheraghlou, of Yale University, New Haven, Conn., and colleagues.
The researchers identified a total of 70,319 patients diagnosed with stage I invasive melanoma between Jan. 1, 2004, and Dec. 31, 2014. Data were collected from the National Cancer Database, including 3,234 (4.6%) and 67,085 (95.4%) patients who underwent MMS and WME, respectively. The median age of patients in the cohort was 57 years; 47.7% were female, and almost 97% were white.
In the survival analysis, the team adjusted for clinical and tumor-specific variables and conducted a matched analysis using propensity scores. The primary outcome measured was overall survival.
After analysis, the researchers found that MMS was associated with modestly better overall survival when compared with WME after adjustments (hazard ratio, 0.86; 95% confidence interval, 0.76-0.97). In the propensity score–matched analysis, a similar modest survival advantage was seen for patients who underwent MMS (hazard ratio, 0.82; 95% CI, 0.68-0.98).
“Significant differences in treatment practices based on the treatment facility were noted, with academic facilities more than twice as likely as nonacademic facilities to use MMS,” they wrote.
The researchers acknowledged a key limitation of the study was the use of a convenience sample, as opposed to a population-based sample. As a result, the generalizability of the findings may be limited to certain treatment facilities.
“These data suggest that MMS is an effective approach compared with WME for AJCC-8 stage I invasive melanoma,” they concluded.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: Cheraghlou S et al. JAMA Dermatol. 2019 Sep 25. doi: 10.1001/jamadermatol.2019.2890.
according to a retrospective cohort study.
In the study, which was published in JAMA Dermatology, patients who underwent MMS had a “modest survival advantage” when compared with those who were treated with WME, the approach recommended for treatment of invasive melanoma without nodal or extralymphatic metastases in national guidelines, reported the investigators.
“We sought herein to investigate the association of the type of surgical excision – WME or MMS – with overall survival for cases of American Joint Committee on Cancer Cancer Staging Manual 8th edition (AJCC-8) stage I invasive melanoma,” wrote Shayan Cheraghlou, of Yale University, New Haven, Conn., and colleagues.
The researchers identified a total of 70,319 patients diagnosed with stage I invasive melanoma between Jan. 1, 2004, and Dec. 31, 2014. Data were collected from the National Cancer Database, including 3,234 (4.6%) and 67,085 (95.4%) patients who underwent MMS and WME, respectively. The median age of patients in the cohort was 57 years; 47.7% were female, and almost 97% were white.
In the survival analysis, the team adjusted for clinical and tumor-specific variables and conducted a matched analysis using propensity scores. The primary outcome measured was overall survival.
After analysis, the researchers found that MMS was associated with modestly better overall survival when compared with WME after adjustments (hazard ratio, 0.86; 95% confidence interval, 0.76-0.97). In the propensity score–matched analysis, a similar modest survival advantage was seen for patients who underwent MMS (hazard ratio, 0.82; 95% CI, 0.68-0.98).
“Significant differences in treatment practices based on the treatment facility were noted, with academic facilities more than twice as likely as nonacademic facilities to use MMS,” they wrote.
The researchers acknowledged a key limitation of the study was the use of a convenience sample, as opposed to a population-based sample. As a result, the generalizability of the findings may be limited to certain treatment facilities.
“These data suggest that MMS is an effective approach compared with WME for AJCC-8 stage I invasive melanoma,” they concluded.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: Cheraghlou S et al. JAMA Dermatol. 2019 Sep 25. doi: 10.1001/jamadermatol.2019.2890.
FROM JAMA DERMATOLOGY
SEER analysis reveals medication adherence factors in newly diagnosed myeloma
Black race, polypharmacy, and increasing age were associated with poor adherence to lenalidomide in older patients with newly-diagnosed multiple myeloma, according to an analysis of Surveillance, Epidemiology, and End Results (SEER)–Medicare linked data.
The objective of the study was to examine factors affecting adherence in older adults who received lenalidomide.
Of 793 patients diagnosed and treated between 2007 and 2014, 302 (38%) had poor adherence to lenalidomide, reported Hira Mian, MD, of McMaster University, Hamilton, Ont., and colleagues. The findings were published in Clinical Lymphoma, Myeloma & Leukemia.
The researchers studied patients 65 years and older who had received at least two lenalidomide prescriptions in the first year following diagnosis. Only patients who filled a prescription for lenalidomide within 60 days of a myeloma diagnosis were included.
The median age of the patients was 73 years; 43% were aged 75 years or older. Most of the patients included in the analysis were white.
The medication possession ratio, defined as the “ratio of the number of days the patient had pills in their possession to the number of days in the observation period,” was used to evaluate adherence to therapy. A ratio of less than 90% was deemed poor adherence by the researchers.
After analysis, the researchers found that black race (adjusted odds ratio, 1.72; P = .022), polypharmacy (aOR, 1.04 per drug; P = .008), and increasing age (aOR, 1.03 per year; P = .024) were all significantly associated with poor adherence to lenalidomide.
The mean medication possession ratio among study patients was 89.5%. Overall, 38% of patients in the study had poor adherence to lenalidomide, while just 7% of patients in the study had a medication possession ratio of 100%, indicating “perfect adherence.”
There was a trend toward inferior overall survival among patients with poor adherence to lenalidomide, but it was not statistically significant (hazard ratio 1.10, 95% confidence interval, 0.88-1.38).
“Our study emphasizes the need for both better clinical monitoring of adherence and for future prospective studies in accurately understanding the rates and predictors of adherence while simultaneously developing strategies for improving adherence for patients that are at high risk of nonadherence,” the researchers wrote.
The National Institutes of Health funded the study. No conflicts of interest were reported.
SOURCE: Mian H et al. Clin Lymphoma Myeloma Leuk. 2019 Oct 9. doi: 10.1016/j.clml.2019.09.618.
Black race, polypharmacy, and increasing age were associated with poor adherence to lenalidomide in older patients with newly-diagnosed multiple myeloma, according to an analysis of Surveillance, Epidemiology, and End Results (SEER)–Medicare linked data.
The objective of the study was to examine factors affecting adherence in older adults who received lenalidomide.
Of 793 patients diagnosed and treated between 2007 and 2014, 302 (38%) had poor adherence to lenalidomide, reported Hira Mian, MD, of McMaster University, Hamilton, Ont., and colleagues. The findings were published in Clinical Lymphoma, Myeloma & Leukemia.
The researchers studied patients 65 years and older who had received at least two lenalidomide prescriptions in the first year following diagnosis. Only patients who filled a prescription for lenalidomide within 60 days of a myeloma diagnosis were included.
The median age of the patients was 73 years; 43% were aged 75 years or older. Most of the patients included in the analysis were white.
The medication possession ratio, defined as the “ratio of the number of days the patient had pills in their possession to the number of days in the observation period,” was used to evaluate adherence to therapy. A ratio of less than 90% was deemed poor adherence by the researchers.
After analysis, the researchers found that black race (adjusted odds ratio, 1.72; P = .022), polypharmacy (aOR, 1.04 per drug; P = .008), and increasing age (aOR, 1.03 per year; P = .024) were all significantly associated with poor adherence to lenalidomide.
The mean medication possession ratio among study patients was 89.5%. Overall, 38% of patients in the study had poor adherence to lenalidomide, while just 7% of patients in the study had a medication possession ratio of 100%, indicating “perfect adherence.”
There was a trend toward inferior overall survival among patients with poor adherence to lenalidomide, but it was not statistically significant (hazard ratio 1.10, 95% confidence interval, 0.88-1.38).
“Our study emphasizes the need for both better clinical monitoring of adherence and for future prospective studies in accurately understanding the rates and predictors of adherence while simultaneously developing strategies for improving adherence for patients that are at high risk of nonadherence,” the researchers wrote.
The National Institutes of Health funded the study. No conflicts of interest were reported.
SOURCE: Mian H et al. Clin Lymphoma Myeloma Leuk. 2019 Oct 9. doi: 10.1016/j.clml.2019.09.618.
Black race, polypharmacy, and increasing age were associated with poor adherence to lenalidomide in older patients with newly-diagnosed multiple myeloma, according to an analysis of Surveillance, Epidemiology, and End Results (SEER)–Medicare linked data.
The objective of the study was to examine factors affecting adherence in older adults who received lenalidomide.
Of 793 patients diagnosed and treated between 2007 and 2014, 302 (38%) had poor adherence to lenalidomide, reported Hira Mian, MD, of McMaster University, Hamilton, Ont., and colleagues. The findings were published in Clinical Lymphoma, Myeloma & Leukemia.
The researchers studied patients 65 years and older who had received at least two lenalidomide prescriptions in the first year following diagnosis. Only patients who filled a prescription for lenalidomide within 60 days of a myeloma diagnosis were included.
The median age of the patients was 73 years; 43% were aged 75 years or older. Most of the patients included in the analysis were white.
The medication possession ratio, defined as the “ratio of the number of days the patient had pills in their possession to the number of days in the observation period,” was used to evaluate adherence to therapy. A ratio of less than 90% was deemed poor adherence by the researchers.
After analysis, the researchers found that black race (adjusted odds ratio, 1.72; P = .022), polypharmacy (aOR, 1.04 per drug; P = .008), and increasing age (aOR, 1.03 per year; P = .024) were all significantly associated with poor adherence to lenalidomide.
The mean medication possession ratio among study patients was 89.5%. Overall, 38% of patients in the study had poor adherence to lenalidomide, while just 7% of patients in the study had a medication possession ratio of 100%, indicating “perfect adherence.”
There was a trend toward inferior overall survival among patients with poor adherence to lenalidomide, but it was not statistically significant (hazard ratio 1.10, 95% confidence interval, 0.88-1.38).
“Our study emphasizes the need for both better clinical monitoring of adherence and for future prospective studies in accurately understanding the rates and predictors of adherence while simultaneously developing strategies for improving adherence for patients that are at high risk of nonadherence,” the researchers wrote.
The National Institutes of Health funded the study. No conflicts of interest were reported.
SOURCE: Mian H et al. Clin Lymphoma Myeloma Leuk. 2019 Oct 9. doi: 10.1016/j.clml.2019.09.618.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Pembrolizumab shows promise for relapsed/refractory PMBCL
The programmed death-ligand 1 (PD-L1) inhibitor pembrolizumab showed manageable safety and promising clinical activity in patients with relapsed/refractory primary mediastinal large B-cell lymphoma (PMBCL), according to results from two early-phase studies.
The phase 1b KEYNOTE-013 study included an expansion cohort that evaluated pembrolizumab monotherapy in patients with relapsed/refractory PMBCL. Based on preliminary findings from KEYNOTE-013, the phase 2 KEYNOTE-170 study was initiated to validate these results.
Philippe Armand, MD, PhD, of Dana-Farber Cancer Institute, Boston, and colleagues reported results from 53 patients in KEYNOTE-170 and extended follow-up of 21 patients in KEYNOTE-013. Data from these two trials formed the basis of an accelerated approval by the Food and Drug Administration of pembrolizumab in patients with relapsed/refractory PMBCL in June 2018.
“Frequent amplification and translocation events occur at 9p24.1 in PMBCL, resulting in tumor expression of the programmed cell death-1 (PD-1) ligands PD-L1 and PD-L2. This suggests susceptibility of PMBCL to PD-1 blockade,” the researchers wrote in the Journal of Clinical Oncology.
KEYNOTE-170 included patients with relapsed or refractory disease who were transplant-ineligible and had failed a minimum of two prior lines of treatment. KEYNOTE-013 enrolled patients who relapsed following autologous stem cell transplantation or were ineligible for transplant.
Among patients in KEYNOTE-013 and KEYNOTE-170, the objective response rates were 48% and 45%, respectively. In total, 33% of patients in KEYNOTE-013 and 13% of patients in KEYNOTE-170 achieved a complete response. Among these patients, no disease progression was observed.
The median progression-free survival in KEYNOTE-170 was 5.5 months and 10.4 months in KEYNOTE-013. In KEYNOTE-170, median overall survival was not reached, while in KEYNOTE-013, the median overall survival was 31.4 months.
After a median follow-up time of 29.1 months in KEYNOTE-013 and 12.5 months in KEYNOTE-170, the median duration of response was not reached in either trial, the researchers reported.
With respect to safety, pembrolizumab-related grade 3 or 4 adverse events were observed in 23% and 24% of patients in KEYNOTE-170 and KEYNOTE-013, respectively. The most common adverse event in both trials was neutropenia. No deaths related to pembrolizumab were observed.
Response rates were lower in KEYNOTE-170, compared with KEYNOTE-013, but the researchers noted that longer follow-up could change these results.
“Although the small numbers allow only a tentative hypothesis, they raise the question of whether PD-1 blockade in this setting might resensitize tumors to chemotherapy, as recently suggested. If this can be further validated, it could have profound implication for the management of patients with [relapsed/refractory] PMBCL,” the researchers wrote.
The study was supported by Merck Sharp & Dohme, the Harold and Virginia Lash Foundation, the Leukemia and Lymphoma Society, and the Center for Immuno-Oncology of the Dana-Farber Cancer Institute. The authors reported financial affiliations with Merck Sharp & Dohme and several other companies.
SOURCE: Armand P et al. J Clin Oncol. 2019 Sep 10. doi: 10.1200/JCO.19.01389.
The programmed death-ligand 1 (PD-L1) inhibitor pembrolizumab showed manageable safety and promising clinical activity in patients with relapsed/refractory primary mediastinal large B-cell lymphoma (PMBCL), according to results from two early-phase studies.
The phase 1b KEYNOTE-013 study included an expansion cohort that evaluated pembrolizumab monotherapy in patients with relapsed/refractory PMBCL. Based on preliminary findings from KEYNOTE-013, the phase 2 KEYNOTE-170 study was initiated to validate these results.
Philippe Armand, MD, PhD, of Dana-Farber Cancer Institute, Boston, and colleagues reported results from 53 patients in KEYNOTE-170 and extended follow-up of 21 patients in KEYNOTE-013. Data from these two trials formed the basis of an accelerated approval by the Food and Drug Administration of pembrolizumab in patients with relapsed/refractory PMBCL in June 2018.
“Frequent amplification and translocation events occur at 9p24.1 in PMBCL, resulting in tumor expression of the programmed cell death-1 (PD-1) ligands PD-L1 and PD-L2. This suggests susceptibility of PMBCL to PD-1 blockade,” the researchers wrote in the Journal of Clinical Oncology.
KEYNOTE-170 included patients with relapsed or refractory disease who were transplant-ineligible and had failed a minimum of two prior lines of treatment. KEYNOTE-013 enrolled patients who relapsed following autologous stem cell transplantation or were ineligible for transplant.
Among patients in KEYNOTE-013 and KEYNOTE-170, the objective response rates were 48% and 45%, respectively. In total, 33% of patients in KEYNOTE-013 and 13% of patients in KEYNOTE-170 achieved a complete response. Among these patients, no disease progression was observed.
The median progression-free survival in KEYNOTE-170 was 5.5 months and 10.4 months in KEYNOTE-013. In KEYNOTE-170, median overall survival was not reached, while in KEYNOTE-013, the median overall survival was 31.4 months.
After a median follow-up time of 29.1 months in KEYNOTE-013 and 12.5 months in KEYNOTE-170, the median duration of response was not reached in either trial, the researchers reported.
With respect to safety, pembrolizumab-related grade 3 or 4 adverse events were observed in 23% and 24% of patients in KEYNOTE-170 and KEYNOTE-013, respectively. The most common adverse event in both trials was neutropenia. No deaths related to pembrolizumab were observed.
Response rates were lower in KEYNOTE-170, compared with KEYNOTE-013, but the researchers noted that longer follow-up could change these results.
“Although the small numbers allow only a tentative hypothesis, they raise the question of whether PD-1 blockade in this setting might resensitize tumors to chemotherapy, as recently suggested. If this can be further validated, it could have profound implication for the management of patients with [relapsed/refractory] PMBCL,” the researchers wrote.
The study was supported by Merck Sharp & Dohme, the Harold and Virginia Lash Foundation, the Leukemia and Lymphoma Society, and the Center for Immuno-Oncology of the Dana-Farber Cancer Institute. The authors reported financial affiliations with Merck Sharp & Dohme and several other companies.
SOURCE: Armand P et al. J Clin Oncol. 2019 Sep 10. doi: 10.1200/JCO.19.01389.
The programmed death-ligand 1 (PD-L1) inhibitor pembrolizumab showed manageable safety and promising clinical activity in patients with relapsed/refractory primary mediastinal large B-cell lymphoma (PMBCL), according to results from two early-phase studies.
The phase 1b KEYNOTE-013 study included an expansion cohort that evaluated pembrolizumab monotherapy in patients with relapsed/refractory PMBCL. Based on preliminary findings from KEYNOTE-013, the phase 2 KEYNOTE-170 study was initiated to validate these results.
Philippe Armand, MD, PhD, of Dana-Farber Cancer Institute, Boston, and colleagues reported results from 53 patients in KEYNOTE-170 and extended follow-up of 21 patients in KEYNOTE-013. Data from these two trials formed the basis of an accelerated approval by the Food and Drug Administration of pembrolizumab in patients with relapsed/refractory PMBCL in June 2018.
“Frequent amplification and translocation events occur at 9p24.1 in PMBCL, resulting in tumor expression of the programmed cell death-1 (PD-1) ligands PD-L1 and PD-L2. This suggests susceptibility of PMBCL to PD-1 blockade,” the researchers wrote in the Journal of Clinical Oncology.
KEYNOTE-170 included patients with relapsed or refractory disease who were transplant-ineligible and had failed a minimum of two prior lines of treatment. KEYNOTE-013 enrolled patients who relapsed following autologous stem cell transplantation or were ineligible for transplant.
Among patients in KEYNOTE-013 and KEYNOTE-170, the objective response rates were 48% and 45%, respectively. In total, 33% of patients in KEYNOTE-013 and 13% of patients in KEYNOTE-170 achieved a complete response. Among these patients, no disease progression was observed.
The median progression-free survival in KEYNOTE-170 was 5.5 months and 10.4 months in KEYNOTE-013. In KEYNOTE-170, median overall survival was not reached, while in KEYNOTE-013, the median overall survival was 31.4 months.
After a median follow-up time of 29.1 months in KEYNOTE-013 and 12.5 months in KEYNOTE-170, the median duration of response was not reached in either trial, the researchers reported.
With respect to safety, pembrolizumab-related grade 3 or 4 adverse events were observed in 23% and 24% of patients in KEYNOTE-170 and KEYNOTE-013, respectively. The most common adverse event in both trials was neutropenia. No deaths related to pembrolizumab were observed.
Response rates were lower in KEYNOTE-170, compared with KEYNOTE-013, but the researchers noted that longer follow-up could change these results.
“Although the small numbers allow only a tentative hypothesis, they raise the question of whether PD-1 blockade in this setting might resensitize tumors to chemotherapy, as recently suggested. If this can be further validated, it could have profound implication for the management of patients with [relapsed/refractory] PMBCL,” the researchers wrote.
The study was supported by Merck Sharp & Dohme, the Harold and Virginia Lash Foundation, the Leukemia and Lymphoma Society, and the Center for Immuno-Oncology of the Dana-Farber Cancer Institute. The authors reported financial affiliations with Merck Sharp & Dohme and several other companies.
SOURCE: Armand P et al. J Clin Oncol. 2019 Sep 10. doi: 10.1200/JCO.19.01389.
FROM JOURNAL OF CLINICAL ONCOLOGY
Exercise intolerance linked to neurocognitive dysfunction in ALL
Exercise intolerance was associated with worse neurocognitive function in adult survivors of pediatric acute lymphoblastic leukemia (ALL), according to results from a cross-sectional study.
The findings suggest additional research is needed to better understand the effects of increased exercise capacity on neurocognitive performance in these patients.
“We used a clinically assessed cohort of childhood cancer survivors participating in the St. Jude Lifetime cohort study to determine whether exercise intolerance, expressed as decreased oxygen uptake, is associated with neurocognitive impairments in long-term survivors of childhood ALL and evaluated whether exercise intolerance mediates the association between chronic cardiac or pulmonary conditions and neurocognitive impairment,” wrote Nicholas S. Phillips, MD, PhD, of St. Jude Children’s Research Hospital in Memphis, Tenn., and colleagues. The results were published in Cancer.
The cross-sectional cohort study included 341 young adult survivors of pediatric ALL and 288 evaluable control participants. Survivors were recruited from 1980 to 2003.
Eligible participants were 18 years or older, and remained alive for a minimum of 5 years post ALL diagnosis. Control subjects were non–first-degree relatives of ALL survivors.
The researchers evaluated exercise capacity using cardiopulmonary exercise testing, expressed as relative peak volume of oxygen (rpkVO2) max scores. Other tests included self-rated questionnaires, as well as a standardized neuropsychological evaluation.
After analysis, the researchers found that ALL survivors had lower mean rpkVO2 scores, compared with control participants (23.45 vs. 33.03 mL/kg per min; P less than .001).
Survivors also had worse performance on several measures of neurocognitive function, including working memory, verbal intelligence, visual-motor speed, and other math and reading domains, compared with controls (all P less than .001).
The researchers also performed a multivariable analysis and found that a 1-unit metabolic equivalent increase in exercise tolerance was associated with significantly increased performance in some neurocognitive measures, including attention, verbal ability, verbal fluency, motor speed, and academics.
“Our research suggests that a minor improvement in exercise tolerance, such as going from sitting on the couch and watching TV, to walking around the block for 30 minutes a day, can have a significant impact on survivors’ intellectual health,” Dr. Phillips said in a statement.
The researchers noted that recent evidence has shown that structured exercise training may benefit younger survivors. “These studies demonstrate that a low-cost, home-based exercise training program can effectively increase cardiopulmonary fitness during and after completion of therapy,” they wrote.
The team acknowledged a key limitation of the study was the cross-sectional design. As a result, the direction of these associations remains unknown, and warrants future study.
The study was supported by the National Institutes of Health and the American Lebanese Syrian Associated Charities. The researchers did not report conflicts of interest.
SOURCE: Phillips NS et al. Cancer. 2019 Oct 21. doi: 10.1002/cncr.32510.
Exercise intolerance was associated with worse neurocognitive function in adult survivors of pediatric acute lymphoblastic leukemia (ALL), according to results from a cross-sectional study.
The findings suggest additional research is needed to better understand the effects of increased exercise capacity on neurocognitive performance in these patients.
“We used a clinically assessed cohort of childhood cancer survivors participating in the St. Jude Lifetime cohort study to determine whether exercise intolerance, expressed as decreased oxygen uptake, is associated with neurocognitive impairments in long-term survivors of childhood ALL and evaluated whether exercise intolerance mediates the association between chronic cardiac or pulmonary conditions and neurocognitive impairment,” wrote Nicholas S. Phillips, MD, PhD, of St. Jude Children’s Research Hospital in Memphis, Tenn., and colleagues. The results were published in Cancer.
The cross-sectional cohort study included 341 young adult survivors of pediatric ALL and 288 evaluable control participants. Survivors were recruited from 1980 to 2003.
Eligible participants were 18 years or older, and remained alive for a minimum of 5 years post ALL diagnosis. Control subjects were non–first-degree relatives of ALL survivors.
The researchers evaluated exercise capacity using cardiopulmonary exercise testing, expressed as relative peak volume of oxygen (rpkVO2) max scores. Other tests included self-rated questionnaires, as well as a standardized neuropsychological evaluation.
After analysis, the researchers found that ALL survivors had lower mean rpkVO2 scores, compared with control participants (23.45 vs. 33.03 mL/kg per min; P less than .001).
Survivors also had worse performance on several measures of neurocognitive function, including working memory, verbal intelligence, visual-motor speed, and other math and reading domains, compared with controls (all P less than .001).
The researchers also performed a multivariable analysis and found that a 1-unit metabolic equivalent increase in exercise tolerance was associated with significantly increased performance in some neurocognitive measures, including attention, verbal ability, verbal fluency, motor speed, and academics.
“Our research suggests that a minor improvement in exercise tolerance, such as going from sitting on the couch and watching TV, to walking around the block for 30 minutes a day, can have a significant impact on survivors’ intellectual health,” Dr. Phillips said in a statement.
The researchers noted that recent evidence has shown that structured exercise training may benefit younger survivors. “These studies demonstrate that a low-cost, home-based exercise training program can effectively increase cardiopulmonary fitness during and after completion of therapy,” they wrote.
The team acknowledged a key limitation of the study was the cross-sectional design. As a result, the direction of these associations remains unknown, and warrants future study.
The study was supported by the National Institutes of Health and the American Lebanese Syrian Associated Charities. The researchers did not report conflicts of interest.
SOURCE: Phillips NS et al. Cancer. 2019 Oct 21. doi: 10.1002/cncr.32510.
Exercise intolerance was associated with worse neurocognitive function in adult survivors of pediatric acute lymphoblastic leukemia (ALL), according to results from a cross-sectional study.
The findings suggest additional research is needed to better understand the effects of increased exercise capacity on neurocognitive performance in these patients.
“We used a clinically assessed cohort of childhood cancer survivors participating in the St. Jude Lifetime cohort study to determine whether exercise intolerance, expressed as decreased oxygen uptake, is associated with neurocognitive impairments in long-term survivors of childhood ALL and evaluated whether exercise intolerance mediates the association between chronic cardiac or pulmonary conditions and neurocognitive impairment,” wrote Nicholas S. Phillips, MD, PhD, of St. Jude Children’s Research Hospital in Memphis, Tenn., and colleagues. The results were published in Cancer.
The cross-sectional cohort study included 341 young adult survivors of pediatric ALL and 288 evaluable control participants. Survivors were recruited from 1980 to 2003.
Eligible participants were 18 years or older, and remained alive for a minimum of 5 years post ALL diagnosis. Control subjects were non–first-degree relatives of ALL survivors.
The researchers evaluated exercise capacity using cardiopulmonary exercise testing, expressed as relative peak volume of oxygen (rpkVO2) max scores. Other tests included self-rated questionnaires, as well as a standardized neuropsychological evaluation.
After analysis, the researchers found that ALL survivors had lower mean rpkVO2 scores, compared with control participants (23.45 vs. 33.03 mL/kg per min; P less than .001).
Survivors also had worse performance on several measures of neurocognitive function, including working memory, verbal intelligence, visual-motor speed, and other math and reading domains, compared with controls (all P less than .001).
The researchers also performed a multivariable analysis and found that a 1-unit metabolic equivalent increase in exercise tolerance was associated with significantly increased performance in some neurocognitive measures, including attention, verbal ability, verbal fluency, motor speed, and academics.
“Our research suggests that a minor improvement in exercise tolerance, such as going from sitting on the couch and watching TV, to walking around the block for 30 minutes a day, can have a significant impact on survivors’ intellectual health,” Dr. Phillips said in a statement.
The researchers noted that recent evidence has shown that structured exercise training may benefit younger survivors. “These studies demonstrate that a low-cost, home-based exercise training program can effectively increase cardiopulmonary fitness during and after completion of therapy,” they wrote.
The team acknowledged a key limitation of the study was the cross-sectional design. As a result, the direction of these associations remains unknown, and warrants future study.
The study was supported by the National Institutes of Health and the American Lebanese Syrian Associated Charities. The researchers did not report conflicts of interest.
SOURCE: Phillips NS et al. Cancer. 2019 Oct 21. doi: 10.1002/cncr.32510.
FROM CANCER
Ibrutinib linked to hypertension in B-cell malignancies
The incidence and severity of hypertension was considerably higher in patients with B-cell malignancies treated with ibrutinib, according to a retrospective analysis.
Additionally, new or worsening hypertension was associated with a greater risk of major adverse cardiac events (MACE), including stroke, myocardial infarction, and cardiovascular-related death (hazard ratio, 2.17; 95% confidence interval, 1.08-4.38; P = .03).
“Despite ibrutinib’s benefits, cardiotoxicity has emerged as an increasingly important complication of this life-saving therapy,” Tyler Dickerson, PhD, of the Ohio State University, Columbus, and colleagues wrote in Blood.
The researchers retrospectively studied 562 consecutive patients with a lymphoid malignancy who received ibrutinib. Data was collected from patients treated at The Ohio State University’s Comprehensive Cancer Center during 2009-2016.
The mean age of study participants was 63.8 years, with a mean body mass index of 28.0 kg/m2. Most of the patients included in the analysis were men.
The team assessed rates of new or worsening hypertension, as well as rates of other MACE. The observed rates were compared with Framingham Heart Study–predicted incident-hypertension rates. The effects of various antihypertensive drugs on ibrutinib-linked hypertension were also evaluated.
After a median follow-up of 30 months, 78.3% of patients who received ibrutinib had new or worsening hypertension using a systolic blood pressure cutoff of 130 mm Hg. Of these, 84.8% of cases had an “at least probable association with ibrutinib,” they reported.
Among the 215 patients with no baseline hypertension, 71.6% developed hypertension while on ibrutinib, with a mean increase in systolic blood pressure of 13.4 mm Hg. Among the 347 patients with baseline hypertension, 82.4% experienced a worsening of their hypertension.
“This relationship remained even after accounting for ibrutinib dose, and was not attenuated by the use of any specific anti-hypertensive class,” the researchers wrote.
The researchers observed MACE among 93 patients. This included 84 patients with new or worsening hypertension and 9 patients with stable or no hypertension. Most MACE events were of at least probable ibrutinib association, the researchers reported.
Overall, the cumulative incidence of new hypertension at 1 year was 442 per 1,000 person-years in the current study. This value is 12.9-fold higher than the Framingham Heart Study risk–predicted rate of 34 per 1,000 person-years.
“Given the expected continued increase in ibrutinib use, further studies characterizing the mechanisms, treatment, and implications of [hypertension] during ibrutinib use are needed,” the researchers wrote.
The study was funded by the National Institutes of Health, the D. Warren Brown Family Foundation, the Four Winds Foundation, and the Connie Brown CLL Research Fund. The authors reported financial affiliations with Janssen, Pharmacyclics, and other companies.
SOURCE: Dickerson T et al. Blood. 2019 Oct 3. doi: 10.1182/blood.2019000840.
The incidence and severity of hypertension was considerably higher in patients with B-cell malignancies treated with ibrutinib, according to a retrospective analysis.
Additionally, new or worsening hypertension was associated with a greater risk of major adverse cardiac events (MACE), including stroke, myocardial infarction, and cardiovascular-related death (hazard ratio, 2.17; 95% confidence interval, 1.08-4.38; P = .03).
“Despite ibrutinib’s benefits, cardiotoxicity has emerged as an increasingly important complication of this life-saving therapy,” Tyler Dickerson, PhD, of the Ohio State University, Columbus, and colleagues wrote in Blood.
The researchers retrospectively studied 562 consecutive patients with a lymphoid malignancy who received ibrutinib. Data was collected from patients treated at The Ohio State University’s Comprehensive Cancer Center during 2009-2016.
The mean age of study participants was 63.8 years, with a mean body mass index of 28.0 kg/m2. Most of the patients included in the analysis were men.
The team assessed rates of new or worsening hypertension, as well as rates of other MACE. The observed rates were compared with Framingham Heart Study–predicted incident-hypertension rates. The effects of various antihypertensive drugs on ibrutinib-linked hypertension were also evaluated.
After a median follow-up of 30 months, 78.3% of patients who received ibrutinib had new or worsening hypertension using a systolic blood pressure cutoff of 130 mm Hg. Of these, 84.8% of cases had an “at least probable association with ibrutinib,” they reported.
Among the 215 patients with no baseline hypertension, 71.6% developed hypertension while on ibrutinib, with a mean increase in systolic blood pressure of 13.4 mm Hg. Among the 347 patients with baseline hypertension, 82.4% experienced a worsening of their hypertension.
“This relationship remained even after accounting for ibrutinib dose, and was not attenuated by the use of any specific anti-hypertensive class,” the researchers wrote.
The researchers observed MACE among 93 patients. This included 84 patients with new or worsening hypertension and 9 patients with stable or no hypertension. Most MACE events were of at least probable ibrutinib association, the researchers reported.
Overall, the cumulative incidence of new hypertension at 1 year was 442 per 1,000 person-years in the current study. This value is 12.9-fold higher than the Framingham Heart Study risk–predicted rate of 34 per 1,000 person-years.
“Given the expected continued increase in ibrutinib use, further studies characterizing the mechanisms, treatment, and implications of [hypertension] during ibrutinib use are needed,” the researchers wrote.
The study was funded by the National Institutes of Health, the D. Warren Brown Family Foundation, the Four Winds Foundation, and the Connie Brown CLL Research Fund. The authors reported financial affiliations with Janssen, Pharmacyclics, and other companies.
SOURCE: Dickerson T et al. Blood. 2019 Oct 3. doi: 10.1182/blood.2019000840.
The incidence and severity of hypertension was considerably higher in patients with B-cell malignancies treated with ibrutinib, according to a retrospective analysis.
Additionally, new or worsening hypertension was associated with a greater risk of major adverse cardiac events (MACE), including stroke, myocardial infarction, and cardiovascular-related death (hazard ratio, 2.17; 95% confidence interval, 1.08-4.38; P = .03).
“Despite ibrutinib’s benefits, cardiotoxicity has emerged as an increasingly important complication of this life-saving therapy,” Tyler Dickerson, PhD, of the Ohio State University, Columbus, and colleagues wrote in Blood.
The researchers retrospectively studied 562 consecutive patients with a lymphoid malignancy who received ibrutinib. Data was collected from patients treated at The Ohio State University’s Comprehensive Cancer Center during 2009-2016.
The mean age of study participants was 63.8 years, with a mean body mass index of 28.0 kg/m2. Most of the patients included in the analysis were men.
The team assessed rates of new or worsening hypertension, as well as rates of other MACE. The observed rates were compared with Framingham Heart Study–predicted incident-hypertension rates. The effects of various antihypertensive drugs on ibrutinib-linked hypertension were also evaluated.
After a median follow-up of 30 months, 78.3% of patients who received ibrutinib had new or worsening hypertension using a systolic blood pressure cutoff of 130 mm Hg. Of these, 84.8% of cases had an “at least probable association with ibrutinib,” they reported.
Among the 215 patients with no baseline hypertension, 71.6% developed hypertension while on ibrutinib, with a mean increase in systolic blood pressure of 13.4 mm Hg. Among the 347 patients with baseline hypertension, 82.4% experienced a worsening of their hypertension.
“This relationship remained even after accounting for ibrutinib dose, and was not attenuated by the use of any specific anti-hypertensive class,” the researchers wrote.
The researchers observed MACE among 93 patients. This included 84 patients with new or worsening hypertension and 9 patients with stable or no hypertension. Most MACE events were of at least probable ibrutinib association, the researchers reported.
Overall, the cumulative incidence of new hypertension at 1 year was 442 per 1,000 person-years in the current study. This value is 12.9-fold higher than the Framingham Heart Study risk–predicted rate of 34 per 1,000 person-years.
“Given the expected continued increase in ibrutinib use, further studies characterizing the mechanisms, treatment, and implications of [hypertension] during ibrutinib use are needed,” the researchers wrote.
The study was funded by the National Institutes of Health, the D. Warren Brown Family Foundation, the Four Winds Foundation, and the Connie Brown CLL Research Fund. The authors reported financial affiliations with Janssen, Pharmacyclics, and other companies.
SOURCE: Dickerson T et al. Blood. 2019 Oct 3. doi: 10.1182/blood.2019000840.
FROM BLOOD
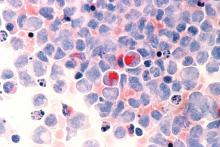
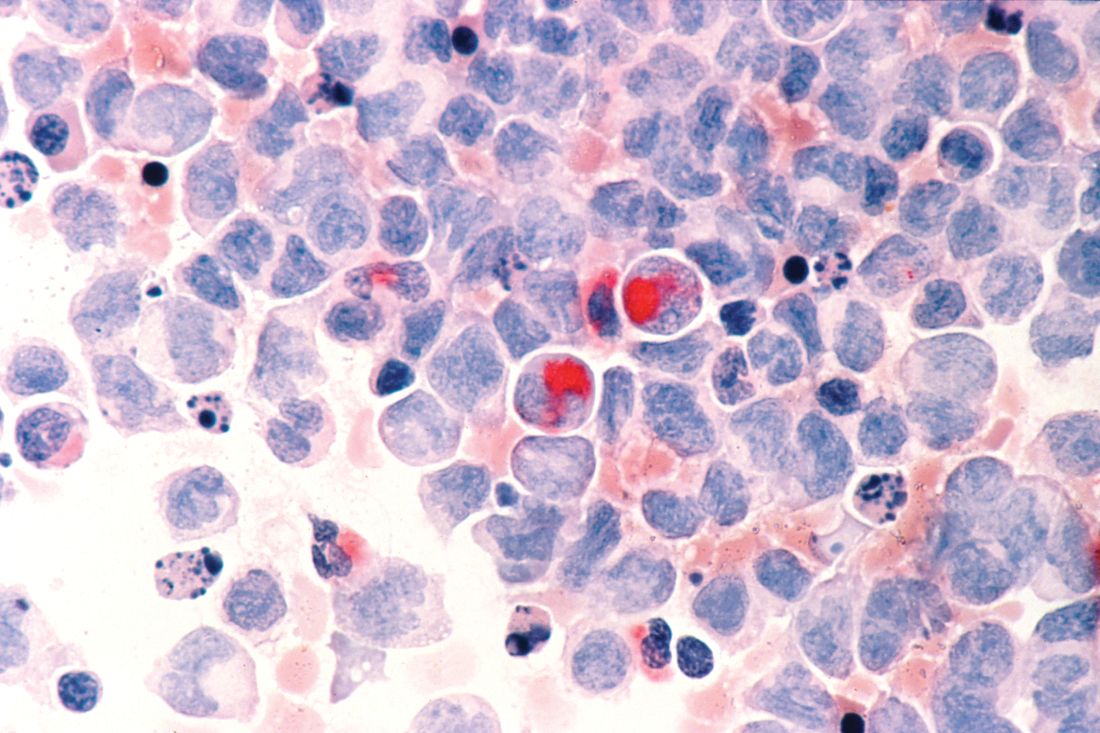
Adverse cytogenetics trump molecular risk in NPM1-mutated AML
A pooled analysis suggests adverse cytogenetics are a key factor negatively impacting outcomes in patients with NPM1mut/FLT3-ITDneg/low acute myeloid leukemia (AML).
In patients with adverse chromosomal abnormalities, NPM1 mutational status was found not to confer a favorable outcome. The findings suggest cytogenetic risk outweighs molecular risk in patients with NPM1 mutations and the FLT3-ITDneg/low genotype.
“Patients carrying adverse-risk cytogenetics shared a virtually identical unfavorable outcome, regardless of whether the otherwise beneficial NPM1mut/FLT3-ITDneg/low status was present. The type of the adverse chromosomal abnormality did not seem to influence this effect, although low numbers might obscure detection of heterogeneity among individual aberrations,” Linus Angenendt, MD, of University Hospital Munster (Germany) and colleagues, wrote in the Journal of Clinical Oncology.
The researchers retrospectively analyzed 2,426 patients with NPM1mut/FLT3-ITDneg/low AML. Of these, 17.6% had an abnormal karyotype, and 3.4% had adverse-risk chromosomal aberrations.
Prior to analysis, individual patient data were pooled from nine international AML study group registries or treatment centers.
After analysis, the researchers found that adverse cytogenetics were associated with inferior complete remission rates (66.3%), compared with in patients with normal karyotype or intermediate-risk cytogenetic abnormalities (87.7% and 86.0%, respectively; P less than .001). The complete remission rates for the NPM1mut/FLT3-ITDneg/low AML adverse cytogenetics group was similar to patients with NPM1wt/FLT3-ITDneg/low and adverse cytogenetic abnormalities (66.3% vs. 57.5%).
Five-year event-free survival rates and overall survival rates were also lower in patients with NPM1mut/FLT3-ITDneg/low AML and adverse cytogenetics, compared with patients with normal karyotype or intermediate-risk cytogenetic abnormalities (P less than .001).
“Even though the combination of an NPM1 mutation with these abnormalities is rare, the prognostic effect of adverse cytogenetics in NPM1mut AML has important implications for postremission treatment decisions, in particular, the current recommendation that patients who are NPM1mut/FLT3-ITDneg/low not receive allogeneic hematopoietic stem cell transplantation (HSCT), given their presumed low risk of relapse might be altered if the adverse karyotype increased the risk,” they wrote.
The type of chromosomal aberration did not appear to impact this effect, but the small sample size may have hindered the ability to detect a difference between different abnormalities, the researchers noted.
One key limitation of the study was the retrospective design. As a result, in patients with an abnormal karyotype, some genetic analyses could have been underutilized.
“These results demand additional validation within prospective trials,” the researchers concluded.
The study was funded by the University of Munster Medical School, the German Research Foundation, the French government, the Ministry of Health of the Czech Republic, and others. The authors reported financial affiliations with numerous pharmaceutical companies.
SOURCE: Angenendt L et al. J Clin Oncol. 2019 Oct 10;37(29):2632-42.
A pooled analysis suggests adverse cytogenetics are a key factor negatively impacting outcomes in patients with NPM1mut/FLT3-ITDneg/low acute myeloid leukemia (AML).
In patients with adverse chromosomal abnormalities, NPM1 mutational status was found not to confer a favorable outcome. The findings suggest cytogenetic risk outweighs molecular risk in patients with NPM1 mutations and the FLT3-ITDneg/low genotype.
“Patients carrying adverse-risk cytogenetics shared a virtually identical unfavorable outcome, regardless of whether the otherwise beneficial NPM1mut/FLT3-ITDneg/low status was present. The type of the adverse chromosomal abnormality did not seem to influence this effect, although low numbers might obscure detection of heterogeneity among individual aberrations,” Linus Angenendt, MD, of University Hospital Munster (Germany) and colleagues, wrote in the Journal of Clinical Oncology.
The researchers retrospectively analyzed 2,426 patients with NPM1mut/FLT3-ITDneg/low AML. Of these, 17.6% had an abnormal karyotype, and 3.4% had adverse-risk chromosomal aberrations.
Prior to analysis, individual patient data were pooled from nine international AML study group registries or treatment centers.
After analysis, the researchers found that adverse cytogenetics were associated with inferior complete remission rates (66.3%), compared with in patients with normal karyotype or intermediate-risk cytogenetic abnormalities (87.7% and 86.0%, respectively; P less than .001). The complete remission rates for the NPM1mut/FLT3-ITDneg/low AML adverse cytogenetics group was similar to patients with NPM1wt/FLT3-ITDneg/low and adverse cytogenetic abnormalities (66.3% vs. 57.5%).
Five-year event-free survival rates and overall survival rates were also lower in patients with NPM1mut/FLT3-ITDneg/low AML and adverse cytogenetics, compared with patients with normal karyotype or intermediate-risk cytogenetic abnormalities (P less than .001).
“Even though the combination of an NPM1 mutation with these abnormalities is rare, the prognostic effect of adverse cytogenetics in NPM1mut AML has important implications for postremission treatment decisions, in particular, the current recommendation that patients who are NPM1mut/FLT3-ITDneg/low not receive allogeneic hematopoietic stem cell transplantation (HSCT), given their presumed low risk of relapse might be altered if the adverse karyotype increased the risk,” they wrote.
The type of chromosomal aberration did not appear to impact this effect, but the small sample size may have hindered the ability to detect a difference between different abnormalities, the researchers noted.
One key limitation of the study was the retrospective design. As a result, in patients with an abnormal karyotype, some genetic analyses could have been underutilized.
“These results demand additional validation within prospective trials,” the researchers concluded.
The study was funded by the University of Munster Medical School, the German Research Foundation, the French government, the Ministry of Health of the Czech Republic, and others. The authors reported financial affiliations with numerous pharmaceutical companies.
SOURCE: Angenendt L et al. J Clin Oncol. 2019 Oct 10;37(29):2632-42.
A pooled analysis suggests adverse cytogenetics are a key factor negatively impacting outcomes in patients with NPM1mut/FLT3-ITDneg/low acute myeloid leukemia (AML).
In patients with adverse chromosomal abnormalities, NPM1 mutational status was found not to confer a favorable outcome. The findings suggest cytogenetic risk outweighs molecular risk in patients with NPM1 mutations and the FLT3-ITDneg/low genotype.
“Patients carrying adverse-risk cytogenetics shared a virtually identical unfavorable outcome, regardless of whether the otherwise beneficial NPM1mut/FLT3-ITDneg/low status was present. The type of the adverse chromosomal abnormality did not seem to influence this effect, although low numbers might obscure detection of heterogeneity among individual aberrations,” Linus Angenendt, MD, of University Hospital Munster (Germany) and colleagues, wrote in the Journal of Clinical Oncology.
The researchers retrospectively analyzed 2,426 patients with NPM1mut/FLT3-ITDneg/low AML. Of these, 17.6% had an abnormal karyotype, and 3.4% had adverse-risk chromosomal aberrations.
Prior to analysis, individual patient data were pooled from nine international AML study group registries or treatment centers.
After analysis, the researchers found that adverse cytogenetics were associated with inferior complete remission rates (66.3%), compared with in patients with normal karyotype or intermediate-risk cytogenetic abnormalities (87.7% and 86.0%, respectively; P less than .001). The complete remission rates for the NPM1mut/FLT3-ITDneg/low AML adverse cytogenetics group was similar to patients with NPM1wt/FLT3-ITDneg/low and adverse cytogenetic abnormalities (66.3% vs. 57.5%).
Five-year event-free survival rates and overall survival rates were also lower in patients with NPM1mut/FLT3-ITDneg/low AML and adverse cytogenetics, compared with patients with normal karyotype or intermediate-risk cytogenetic abnormalities (P less than .001).
“Even though the combination of an NPM1 mutation with these abnormalities is rare, the prognostic effect of adverse cytogenetics in NPM1mut AML has important implications for postremission treatment decisions, in particular, the current recommendation that patients who are NPM1mut/FLT3-ITDneg/low not receive allogeneic hematopoietic stem cell transplantation (HSCT), given their presumed low risk of relapse might be altered if the adverse karyotype increased the risk,” they wrote.
The type of chromosomal aberration did not appear to impact this effect, but the small sample size may have hindered the ability to detect a difference between different abnormalities, the researchers noted.
One key limitation of the study was the retrospective design. As a result, in patients with an abnormal karyotype, some genetic analyses could have been underutilized.
“These results demand additional validation within prospective trials,” the researchers concluded.
The study was funded by the University of Munster Medical School, the German Research Foundation, the French government, the Ministry of Health of the Czech Republic, and others. The authors reported financial affiliations with numerous pharmaceutical companies.
SOURCE: Angenendt L et al. J Clin Oncol. 2019 Oct 10;37(29):2632-42.
REPORTING FROM THE JOURNAL OF CLINICAL ONCOLOGY
Monthly and twice monthly emicizumab dosing safe for children with severe hemophilia A
Administration of twice-monthly or monthly emicizumab appears safe and effective for children with severe hemophilia A without inhibitors, according to a small cohort study.
After 24 weeks of treatment, only one moderate-intensity injection site reaction was reported, but no thrombotic microangiopathy or thromboembolic complications were observed.
The researchers evaluated the efficacy, safety, and pharmacokinetics of emicizumab in Japanese pediatric patients aged less than 12 years with severe hemophilia A without factor VIII inhibitors, wrote Midori Shima, MD, PhD, of Nara Medical University, Kashihara, Japan, and colleagues. The results were published in Haemophilia.
The open-label, nonrandomized study included 13 children who initially received weekly loading doses (3 mg/kg) of subcutaneous emicizumab for 4 weeks. Subsequently, patients received maintenance doses of 3 mg/kg every 2 weeks or 6 mg/kg every 4 weeks until week 24.
At baseline, the median age of patients in the 2- and 4-week dosing cohorts were 6.6 and 4.1 years, respectively. All participants had received factor VIII prophylaxis prior to starting emicizumab, with the exception of one patient.
Among six patients in the twice-monthly dosing cohort, two had no treated bleeding episodes, with an annualized bleeding rate for treated bleeding episodes of 1.3 (95% confidence interval, 0.6-2.9).
Among seven patients in the monthly dosing cohort, five had no treated bleeding episodes, with an annualized bleeding rate for treated bleeding episodes of 0.7 (95% CI, 0.2-2.6).
Caregivers completed a preference survey after the first 16 weeks of treatment, and “all reported a preference for emicizumab prophylaxis over the patient’s previous haemophilia treatment.” They cited the lower frequency of treatment and easier route of administration for favoring emicizumab.
With respect to pharmacokinetics, mean steady-state trough levels were within acceptable limits based on previous studies. No patients tested positive for anti-emicizumab antibodies.
The small sample size and nonrandomized design were key limitations of the study.
The results “confirm the appropriateness” of applying the every 2-week and every 4-week regimens of emicizumab in pediatric patients with hemophilia A without inhibitors, the researchers wrote.
The authors reported having financial affiliations with Chugai Pharmaceutical Co., which funded the study, and other companies.
SOURCE: Shima M et al. Haemophilia. 2019 Sep 12. doi: 10.1111/hae.13848.
Administration of twice-monthly or monthly emicizumab appears safe and effective for children with severe hemophilia A without inhibitors, according to a small cohort study.
After 24 weeks of treatment, only one moderate-intensity injection site reaction was reported, but no thrombotic microangiopathy or thromboembolic complications were observed.
The researchers evaluated the efficacy, safety, and pharmacokinetics of emicizumab in Japanese pediatric patients aged less than 12 years with severe hemophilia A without factor VIII inhibitors, wrote Midori Shima, MD, PhD, of Nara Medical University, Kashihara, Japan, and colleagues. The results were published in Haemophilia.
The open-label, nonrandomized study included 13 children who initially received weekly loading doses (3 mg/kg) of subcutaneous emicizumab for 4 weeks. Subsequently, patients received maintenance doses of 3 mg/kg every 2 weeks or 6 mg/kg every 4 weeks until week 24.
At baseline, the median age of patients in the 2- and 4-week dosing cohorts were 6.6 and 4.1 years, respectively. All participants had received factor VIII prophylaxis prior to starting emicizumab, with the exception of one patient.
Among six patients in the twice-monthly dosing cohort, two had no treated bleeding episodes, with an annualized bleeding rate for treated bleeding episodes of 1.3 (95% confidence interval, 0.6-2.9).
Among seven patients in the monthly dosing cohort, five had no treated bleeding episodes, with an annualized bleeding rate for treated bleeding episodes of 0.7 (95% CI, 0.2-2.6).
Caregivers completed a preference survey after the first 16 weeks of treatment, and “all reported a preference for emicizumab prophylaxis over the patient’s previous haemophilia treatment.” They cited the lower frequency of treatment and easier route of administration for favoring emicizumab.
With respect to pharmacokinetics, mean steady-state trough levels were within acceptable limits based on previous studies. No patients tested positive for anti-emicizumab antibodies.
The small sample size and nonrandomized design were key limitations of the study.
The results “confirm the appropriateness” of applying the every 2-week and every 4-week regimens of emicizumab in pediatric patients with hemophilia A without inhibitors, the researchers wrote.
The authors reported having financial affiliations with Chugai Pharmaceutical Co., which funded the study, and other companies.
SOURCE: Shima M et al. Haemophilia. 2019 Sep 12. doi: 10.1111/hae.13848.
Administration of twice-monthly or monthly emicizumab appears safe and effective for children with severe hemophilia A without inhibitors, according to a small cohort study.
After 24 weeks of treatment, only one moderate-intensity injection site reaction was reported, but no thrombotic microangiopathy or thromboembolic complications were observed.
The researchers evaluated the efficacy, safety, and pharmacokinetics of emicizumab in Japanese pediatric patients aged less than 12 years with severe hemophilia A without factor VIII inhibitors, wrote Midori Shima, MD, PhD, of Nara Medical University, Kashihara, Japan, and colleagues. The results were published in Haemophilia.
The open-label, nonrandomized study included 13 children who initially received weekly loading doses (3 mg/kg) of subcutaneous emicizumab for 4 weeks. Subsequently, patients received maintenance doses of 3 mg/kg every 2 weeks or 6 mg/kg every 4 weeks until week 24.
At baseline, the median age of patients in the 2- and 4-week dosing cohorts were 6.6 and 4.1 years, respectively. All participants had received factor VIII prophylaxis prior to starting emicizumab, with the exception of one patient.
Among six patients in the twice-monthly dosing cohort, two had no treated bleeding episodes, with an annualized bleeding rate for treated bleeding episodes of 1.3 (95% confidence interval, 0.6-2.9).
Among seven patients in the monthly dosing cohort, five had no treated bleeding episodes, with an annualized bleeding rate for treated bleeding episodes of 0.7 (95% CI, 0.2-2.6).
Caregivers completed a preference survey after the first 16 weeks of treatment, and “all reported a preference for emicizumab prophylaxis over the patient’s previous haemophilia treatment.” They cited the lower frequency of treatment and easier route of administration for favoring emicizumab.
With respect to pharmacokinetics, mean steady-state trough levels were within acceptable limits based on previous studies. No patients tested positive for anti-emicizumab antibodies.
The small sample size and nonrandomized design were key limitations of the study.
The results “confirm the appropriateness” of applying the every 2-week and every 4-week regimens of emicizumab in pediatric patients with hemophilia A without inhibitors, the researchers wrote.
The authors reported having financial affiliations with Chugai Pharmaceutical Co., which funded the study, and other companies.
SOURCE: Shima M et al. Haemophilia. 2019 Sep 12. doi: 10.1111/hae.13848.
FROM HAEMOPHILIA
New consensus recommendations on bleeding in acquired hemophilia
New consensus statements, released by a group of 36 experts, provide specific recommendations related to monitoring bleeding and assessing efficacy of treatment in patients with acquired hemophilia.
A global survey was developed by a nine-member steering committee with expertise in the hemostatic management of patients with acquired hemophilia. The Delphi methodology was used to obtain consensus on a list of statements on the location-specific treatment of bleeding in acquired hemophilia.
“The initial survey was circulated via email for refinement and was formally corroborated at a face-to-face meeting,” wrote Andreas Tiede, MD, PhD, of Hannover (Germany) Medical School and fellow experts. The report is published in Haemophilia.
The key areas outlined include the initial management of bleeding, and management of location-specific bleeding, including urological, gastrointestinal, muscle, and pharyngeal bleeds, as well as intracranial and postpartum hemorrhage.
If an expert hematologist is not available, and the bleeding event is life‐threatening, the emergency physician should initiate treatment in accordance with local or national recommendations, according to the initial management guidelines.
With respect to urological bleeds, the best interval for evaluating successful achievement of hemostasis is every 6-12 hours. The experts also reported that, if first-line hemostatic therapy is not effective, more intensive treatment should be considered every 6-12 hours.
In the management of intracranial hemorrhage, the frequency of clinical evaluation is subject to the particular scenario, and it can vary from every 2 hours (for clinical assessment) to every 24 hours (for imaging studies), they wrote.
If initial hemostatic treatment is not effective, more intensive therapy should be considered every 6 hours, they recommended.
“The statement addressing optimal frequency for assessing hemostasis in intracranial bleeds was the subject of much deliberation among the steering committee regarding timing of assessment,” the experts acknowledged.
The geographic diversity and global representation of expert participants were major strengths of these recommendations. However, these statements did not consider socioeconomic parameters or geopolitical differences that could affect patient care. As a result, they may not be applicable to all patient populations.
The manuscript was funded by Novo Nordisk AG. The authors reported having financial affiliations with Novo Nordisk and several other companies.
SOURCE: Tiede A et al. Haemophilia. 2019 Sep 13. doi: 10.1111/hae.13844.
New consensus statements, released by a group of 36 experts, provide specific recommendations related to monitoring bleeding and assessing efficacy of treatment in patients with acquired hemophilia.
A global survey was developed by a nine-member steering committee with expertise in the hemostatic management of patients with acquired hemophilia. The Delphi methodology was used to obtain consensus on a list of statements on the location-specific treatment of bleeding in acquired hemophilia.
“The initial survey was circulated via email for refinement and was formally corroborated at a face-to-face meeting,” wrote Andreas Tiede, MD, PhD, of Hannover (Germany) Medical School and fellow experts. The report is published in Haemophilia.
The key areas outlined include the initial management of bleeding, and management of location-specific bleeding, including urological, gastrointestinal, muscle, and pharyngeal bleeds, as well as intracranial and postpartum hemorrhage.
If an expert hematologist is not available, and the bleeding event is life‐threatening, the emergency physician should initiate treatment in accordance with local or national recommendations, according to the initial management guidelines.
With respect to urological bleeds, the best interval for evaluating successful achievement of hemostasis is every 6-12 hours. The experts also reported that, if first-line hemostatic therapy is not effective, more intensive treatment should be considered every 6-12 hours.
In the management of intracranial hemorrhage, the frequency of clinical evaluation is subject to the particular scenario, and it can vary from every 2 hours (for clinical assessment) to every 24 hours (for imaging studies), they wrote.
If initial hemostatic treatment is not effective, more intensive therapy should be considered every 6 hours, they recommended.
“The statement addressing optimal frequency for assessing hemostasis in intracranial bleeds was the subject of much deliberation among the steering committee regarding timing of assessment,” the experts acknowledged.
The geographic diversity and global representation of expert participants were major strengths of these recommendations. However, these statements did not consider socioeconomic parameters or geopolitical differences that could affect patient care. As a result, they may not be applicable to all patient populations.
The manuscript was funded by Novo Nordisk AG. The authors reported having financial affiliations with Novo Nordisk and several other companies.
SOURCE: Tiede A et al. Haemophilia. 2019 Sep 13. doi: 10.1111/hae.13844.
New consensus statements, released by a group of 36 experts, provide specific recommendations related to monitoring bleeding and assessing efficacy of treatment in patients with acquired hemophilia.
A global survey was developed by a nine-member steering committee with expertise in the hemostatic management of patients with acquired hemophilia. The Delphi methodology was used to obtain consensus on a list of statements on the location-specific treatment of bleeding in acquired hemophilia.
“The initial survey was circulated via email for refinement and was formally corroborated at a face-to-face meeting,” wrote Andreas Tiede, MD, PhD, of Hannover (Germany) Medical School and fellow experts. The report is published in Haemophilia.
The key areas outlined include the initial management of bleeding, and management of location-specific bleeding, including urological, gastrointestinal, muscle, and pharyngeal bleeds, as well as intracranial and postpartum hemorrhage.
If an expert hematologist is not available, and the bleeding event is life‐threatening, the emergency physician should initiate treatment in accordance with local or national recommendations, according to the initial management guidelines.
With respect to urological bleeds, the best interval for evaluating successful achievement of hemostasis is every 6-12 hours. The experts also reported that, if first-line hemostatic therapy is not effective, more intensive treatment should be considered every 6-12 hours.
In the management of intracranial hemorrhage, the frequency of clinical evaluation is subject to the particular scenario, and it can vary from every 2 hours (for clinical assessment) to every 24 hours (for imaging studies), they wrote.
If initial hemostatic treatment is not effective, more intensive therapy should be considered every 6 hours, they recommended.
“The statement addressing optimal frequency for assessing hemostasis in intracranial bleeds was the subject of much deliberation among the steering committee regarding timing of assessment,” the experts acknowledged.
The geographic diversity and global representation of expert participants were major strengths of these recommendations. However, these statements did not consider socioeconomic parameters or geopolitical differences that could affect patient care. As a result, they may not be applicable to all patient populations.
The manuscript was funded by Novo Nordisk AG. The authors reported having financial affiliations with Novo Nordisk and several other companies.
SOURCE: Tiede A et al. Haemophilia. 2019 Sep 13. doi: 10.1111/hae.13844.
FROM HAEMOPHILIA
Eltrombopag elicits positive responses in secondary ITP
Eltrombopag showed good safety and promising clinical activity in patients with immune thrombocytopenia (ITP) secondary to chronic lymphoproliferative disorders, according to results from a phase 2 trial.
Carlo Visco, MD, of the University of Verona (Italy), and colleagues investigated the efficacy and safety of eltrombopag in increasing platelet counts in patients with ITP that was secondary to chronic lymphoproliferative disorders. The findings were published in Blood.
The single-arm, open-label study included 18 patients with ITP secondary to chronic lymphocytic leukemia (14), Waldenstrom macroglobulinemia (2), and classical Hodgkin lymphoma (2). The median age at baseline was 70 years (range, 43-83 years), and all patients were previously treated with ITP.
Study participants were recruited from seven Italian centers from September 2012 to November 2015. Eligible participants were enrolled into an extension phase if a response was observed.
Study patients received oral eltrombopag at 50 mg daily, up to a maximum of 150 mg daily. At weeks 4 and 24, the median dose was 50 mg (ranges, 25-100 mg and 25-150 mg, respectively), with a median total exposure time of 16 months.
At 4 weeks, the researchers found that the platelet response rate was 78%, with a complete response rate of 50%.
After 24 weeks of therapy, the platelet response rate was 59%, with a complete response rate of 30%.
With respect to safety, the therapy was well tolerated, with no adverse events higher than grade 2 reported.
Fifteen patients discontinued therapy: eight due to loss of response, six for disease progression or death, and one for inefficacy and protocol violation, they reported.
The researchers acknowledged two key limitations of the study: the small sample size and lack of a comparison group. “Further prospective studies comparing eltrombopag to standard of care are needed to confirm our findings on the efficacy of this treatment and also to expand our knowledge on its safety, including the potential increased risk of thrombosis,” they wrote.
The study was funded by the Hematology Project Foundation, Vicenza. The authors reported financial affiliations with Amgen, Argenx, and Novartis.
SOURCE: Visco C et al. Blood. 2019 Sep 30. doi: 10.1182/blood.2019001617.
Eltrombopag showed good safety and promising clinical activity in patients with immune thrombocytopenia (ITP) secondary to chronic lymphoproliferative disorders, according to results from a phase 2 trial.
Carlo Visco, MD, of the University of Verona (Italy), and colleagues investigated the efficacy and safety of eltrombopag in increasing platelet counts in patients with ITP that was secondary to chronic lymphoproliferative disorders. The findings were published in Blood.
The single-arm, open-label study included 18 patients with ITP secondary to chronic lymphocytic leukemia (14), Waldenstrom macroglobulinemia (2), and classical Hodgkin lymphoma (2). The median age at baseline was 70 years (range, 43-83 years), and all patients were previously treated with ITP.
Study participants were recruited from seven Italian centers from September 2012 to November 2015. Eligible participants were enrolled into an extension phase if a response was observed.
Study patients received oral eltrombopag at 50 mg daily, up to a maximum of 150 mg daily. At weeks 4 and 24, the median dose was 50 mg (ranges, 25-100 mg and 25-150 mg, respectively), with a median total exposure time of 16 months.
At 4 weeks, the researchers found that the platelet response rate was 78%, with a complete response rate of 50%.
After 24 weeks of therapy, the platelet response rate was 59%, with a complete response rate of 30%.
With respect to safety, the therapy was well tolerated, with no adverse events higher than grade 2 reported.
Fifteen patients discontinued therapy: eight due to loss of response, six for disease progression or death, and one for inefficacy and protocol violation, they reported.
The researchers acknowledged two key limitations of the study: the small sample size and lack of a comparison group. “Further prospective studies comparing eltrombopag to standard of care are needed to confirm our findings on the efficacy of this treatment and also to expand our knowledge on its safety, including the potential increased risk of thrombosis,” they wrote.
The study was funded by the Hematology Project Foundation, Vicenza. The authors reported financial affiliations with Amgen, Argenx, and Novartis.
SOURCE: Visco C et al. Blood. 2019 Sep 30. doi: 10.1182/blood.2019001617.
Eltrombopag showed good safety and promising clinical activity in patients with immune thrombocytopenia (ITP) secondary to chronic lymphoproliferative disorders, according to results from a phase 2 trial.
Carlo Visco, MD, of the University of Verona (Italy), and colleagues investigated the efficacy and safety of eltrombopag in increasing platelet counts in patients with ITP that was secondary to chronic lymphoproliferative disorders. The findings were published in Blood.
The single-arm, open-label study included 18 patients with ITP secondary to chronic lymphocytic leukemia (14), Waldenstrom macroglobulinemia (2), and classical Hodgkin lymphoma (2). The median age at baseline was 70 years (range, 43-83 years), and all patients were previously treated with ITP.
Study participants were recruited from seven Italian centers from September 2012 to November 2015. Eligible participants were enrolled into an extension phase if a response was observed.
Study patients received oral eltrombopag at 50 mg daily, up to a maximum of 150 mg daily. At weeks 4 and 24, the median dose was 50 mg (ranges, 25-100 mg and 25-150 mg, respectively), with a median total exposure time of 16 months.
At 4 weeks, the researchers found that the platelet response rate was 78%, with a complete response rate of 50%.
After 24 weeks of therapy, the platelet response rate was 59%, with a complete response rate of 30%.
With respect to safety, the therapy was well tolerated, with no adverse events higher than grade 2 reported.
Fifteen patients discontinued therapy: eight due to loss of response, six for disease progression or death, and one for inefficacy and protocol violation, they reported.
The researchers acknowledged two key limitations of the study: the small sample size and lack of a comparison group. “Further prospective studies comparing eltrombopag to standard of care are needed to confirm our findings on the efficacy of this treatment and also to expand our knowledge on its safety, including the potential increased risk of thrombosis,” they wrote.
The study was funded by the Hematology Project Foundation, Vicenza. The authors reported financial affiliations with Amgen, Argenx, and Novartis.
SOURCE: Visco C et al. Blood. 2019 Sep 30. doi: 10.1182/blood.2019001617.
FROM BLOOD