User login
Modifiable Factors Associated with Quality of Bowel Preparation Among Hospitalized Patients Undergoing Colonoscopy
Inadequate bowel preparation (IBP) at the time of inpatient colonoscopy is common and associated with increased length of stay and cost of care.1 The factors that contribute to IBP can be categorized into those that are modifiable and those that are nonmodifiable. While many factors have been associated with IBP, studies have been limited by small sample size or have combined inpatient/outpatient populations, thus limiting generalizability.1-5 Moreover, most factors associated with IBP, such as socioeconomic status, male gender, increased age, and comorbidities, are nonmodifiable. No studies have explicitly focused on modifiable risk factors, such as medication use, colonoscopy timing, or assessed the potential impact of modifying these factors.
In a large, multihospital system, we examine the frequency of IBP among inpatients undergoing colonoscopy along with factors associated with IBP. We attempted to identify
METHODS
Potential Predictors of IBP
Demographic data such as patient age, gender, ethnicity, body mass index (BMI), and insurance/payor status were obtained from the electronic health record (EHR). International Classification of Disease 9th and 10th revision, Clinical Modifications (ICD-9/10-CM) codes were used to obtain patient comorbidities including diabetes, coronary artery disease, heart failure, cirrhosis, gastroparesis, hypothyroidism, inflammatory bowel disease, constipation, stroke, dementia, dysphagia, and nausea/vomiting. Use of opioid medications within three days before colonoscopy was extracted from the medication administration record. These variables were chosen as biologically plausible modifiers of bowel preparation or had previously been assessed in the literature.1-6 The name and volume, classified as 4 L (GoLytely®) and < 4 liters (MoviPrep®) of bowel preparation, time of day when colonoscopy was performed, solid diet the day prior to colonoscopy, type of sedation used (conscious sedation or general anesthesia), and total colonoscopy time (defined as the time from scope insertion to removal) was recorded. Hospitalization-related variables, including the number of hospitalizations in the year before the current hospitalization, the year in which the colonoscopy was performed, and the number of days from admission to colonoscopy, were also obtained from the EHR.
Outcome Measures
An internally validated natural language algorithm, using Structured Queried Language was used to search through colonoscopy reports to identify adequacy of bowel preparation. ProVation® software allows the gastroenterologist to use some terms to describe bowel preparation in a drop-down menu format. In addition to the Aronchik scale (which allows the gastroenterologist to rate bowel preparation on a five-point scale: “excellent,” “good,” “fair,” “poor,” and “inadequate”) it also allows the provider to use terms such as “adequate” or “adequate to detect polyps >5 mm” as well as “unsatisfactory.”7 Mirroring prior literature, bowel preparation quality was classified into “adequate” and “inadequate”; “good” and “excellent” on the Aronchik scale were categorized as adequate as was the term “adequate” in any form; “fair,” “poor,” or “inadequate” on the Aronchik scale were classified as inadequate as was the term “unsatisfactory.” We evaluated the hospital length of stay (LOS) as a secondary outcome measure.
Statistical Analysis
After describing the frequency of IBP, the quality of bowel preparation (adequate vs inadequate) was compared based on the predictors described above. Categorical variables were reported as frequencies with percentages and continuous variables were reported as medians with 25th-75th percentile values. The significance of the difference between the proportion or median values of those who had inadequate versus adequate bowel preparation was assessed. Two-sided chi-square analysis was used to assess the significance of differences between categorical variables and the Wilcoxon Rank-Sum test was used to assess the significance of differences between continuous variables.
Multivariate logistic regression analysis was performed to assess factors associated with hospital predictors and outcomes, after adjusting for all the aforementioned factors and clustering the effect based on the endoscopist. To evaluate the potential impact of modifiable factors on IBP, we performed counterfactual analysis, in which the observed distribution was compared to a hypothetical population in which all the modifiable risk factors were optimal.
RESULTS
Overall, 8,819 patients were included in our study population. They had a median age of 64 [53-76] years; 50.5% were female and 51% had an IBP. Patient characteristics and rates of IBP are presented in Table 1.
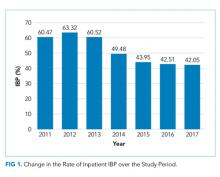
In unadjusted analyses, with regards to modifiable factors, opiate use within three days of colonoscopy was associated with a higher rate of IBP (55.4% vs 47.3%, P <.001), as was a lower volume (<4L) bowel preparation (55.3% vs 50.4%, P = .003). IBP was less frequent when colonoscopy was performed before noon vs afternoon (50.3% vs 57.4%, P < .001), and when patients were documented to receive a clear liquid diet or nil per os vs a solid diet the day prior to colonoscopy (50.3% vs 57.4%, P < .001). Overall bowel preparation quality improved over time (Figure 1). Median LOS was five [3-11] days. Patients who had IBP on their initial colonoscopy had a LOS one day longer than patients without IBP (six days vs five days, P < .001).
Multivariate Analysis
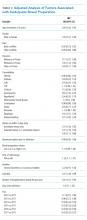
Table 2 shows the results of the multivariate analysis. The following modifiable factors were associated with IBP: opiate used within three days of the procedure (OR 1.31; 95% CI 1.8, 1.45), having the colonoscopy performed after12:00
Potential Impact of Modifiable Variables
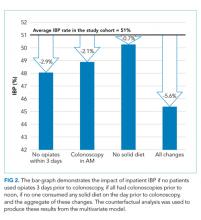
We conducted a counterfactual analysis based on a multivariate model to assess the impact of each modifiable risk factor on the IBP rate (Figure 1). In the included study population, 44.9% received an opiate, 39.3% had a colonoscopy after 12:00
DISCUSSION
In this large, multihospital cohort, IBP was documented in half (51%) of 8,819 inpatient colonoscopies performed. Nonmodifiable patient characteristics independently associated with IBP were age, male gender, white race, Medicare and Medicaid insurance, nausea/vomiting, dysphagia, and gastroparesis. Modifiable factors included not consuming opiates within three days of colonoscopy, avoidance of a solid diet the day prior to colonoscopy and performing the colonoscopy before noon. The volume of bowel preparation consumed was not associated with IBP. In a counterfactual analysis, we found that if all three modifiable factors were optimized, the predicted rate of IBP would drop to 45%.
Many studies, including our analysis, have shown significant differences between the frequency of IBP in inpatient versus outpatient bowel preparations.8-11 Therefore, it is crucial to study IBP in these settings separately. Three single-institution studies, including a total of 898 patients, have identified risk factors for inpatient IBP. Individual studies ranged in size from 130 to 524 patients with rates of IBP ranging from 22%-57%.1-3 They found IBP to be associated with increasing age, lower income, ASA Grade >3, diabetes, coronary artery disease (CAD), nausea or vomiting, BMI >25, and chronic constipation. Modifiable factors included opiates, afternoon procedures, and runway times >6 hours.
We also found IBP to be associated with increasing age and male gender. However, we found no association with diabetes, chronic constipation, CAD or BMI. As we were able to adjust for a wider variety of variables, it is possible that we were able to account for residual confounding better than previous studies. For example, we found that having nausea/vomiting, dysphagia, and gastroparesis was associated with IBP. Gastroparesis with associated nausea and vomiting may be the mechanism by which diabetes increases the risk for IBP. Further studies are needed to assess if interventions or alternative bowel cleansing in these patients can result in improved IBP. Finally, in contrast to studies with smaller cohorts which found that lower volume bowel preps improved IBP in the right colon,4,12 we found no association between IBP based and volume of bowel preparation consumed. Our impact analysis suggests that avoidance of opiates for at least three days before colonoscopy, avoidance of solid diet on the day before colonoscopy and performing all colonoscopies before noon would
The factors mentioned above may not always be amenable to modification. For example, for patients with active gastrointestinal bleeding, postponing colonoscopy by one day for the sake of maintaining a patient on a clear diet may not be feasible. Similarly, performing colonoscopies in the morning is highly dependent on endoscopy suite availability and hospital logistics. Denying opiates to patients experiencing severe pain is not ethical. In many scenarios, however, these variables could be modified, and institutional efforts to support these practices could yield considerable savings. Future prospective studies are needed to verify the real impact of these changes.
Further discussion is needed to contextualize the finding that colonoscopies scheduled in the afternoon are associated with improved bowel preparation quality. Previous research—albeit in the outpatient setting—has demonstrated 11.8 hours as the maximum upper time limit for the time elapsed between the end of bowel preparation to colonoscopy.14 Another study found an inverse relationship between the quality of bowel preparation and the time after completion of the bowel preparation.15 This makes sense from a physiological perspective as delaying the time between completion of bowel preparation, and the procedure allows chyme from the small intestine to reaccumulate in the colon. Anecdotally, at our institution as well as at many others, the bowel preparations are ordered to start in the evening to allow the consumption of complete bowel preparation by midnight. As a result of this practice, only patients who have their colonoscopies scheduled before noon fall within the optimal period of 11.8 hours. In the outpatient setting, the use of split preparations has led to the obliteration of the difference in the quality of bowel preparation between morning and afternoon colonoscopies.16 Prospective trials are needed to evaluate the use of split preparations to improve the quality of afternoon inpatient colonoscopies.
Few other strategies have been shown to mitigate IBP in the inpatient setting. In a small randomized controlled trial, Ergen et al. found that providing an educational booklet improved inpatient bowel preparation as measured by the Boston Bowel Preparation Scale.17 In a quasi-experimental design, Yadlapati et al. found that an automated split-dose bowel preparation resulted in decreased IBP, fewer repeated procedures, shorter LOS, and lower hospital cost.18 Our study adds to these tools by identifying three additional risk factors which could be optimized for inpatients. Because our findings are observational, they should be subjected to prospective trials. Our study also calls into question the impact of bowel preparation volume. We found no difference in the rate of IBP between low and large volume preparations. It is possible that other factors are more important than the specific preparation employed.
Interestingly, we found that IBP declined substantially in 2014 and continued to decline after that. The year was the most influential risk factor for IBP (on par with gastroparesis). The reason for this is unclear, as rates of our modifiable risk factors did not differ substantially by year. Other possibilities include improved access (including weekend access) to endoscopy coinciding with the development of a new endoscopy facility and use of integrated irrigation pump system instead of the use of manual syringes for flushing.
Our study has many strengths. It is by far the most extensive study of bowel preparation quality in inpatients to date and the only one that has included patient, procedural and bowel preparation characteristics. The study also has several significant limitations. This is a single center study, which could limit generalizability. Nonetheless, it was conducted within a health system with multiple hospitals in different parts of the United States (Ohio and Florida) and included a broad population mix with differing levels of acuity. The retrospective nature of the assessment precludes establishing causation. However, we mitigated confounding by adjusting for a wide variety of factors, and there is a plausible physiological mechanism for each of the factors we studied. Also, the retrospective nature of our study predisposes our data to omissions and misrepresentations during the documentation process. This is especially true with the use of ICD codes.19 Inaccuracies in coding are likely to bias toward the null, so observed associations may be an underestimate of the true association.
Our inability to ascertain if a patient completed the prescribed bowel preparation limited our ability to detect what may be a significant risk factor. Lastly, while clinically relevant, the Aronchik scale used to identify adequate from IBP has never been validated though it is frequently utilized and cited in the bowel preparation literature.20
CONCLUSIONS
In this large retrospective study evaluating bowel preparation quality in inpatients undergoing colonoscopy, we found that more than half of the patients have IBP and that IBP was associated with an extra day of hospitalization. Our study identifies those patients at highest risk and identifies modifiable risk factors for IBP. Specifically, we found that abstinence from opiates or solid diet before the colonoscopy, along with performing colonoscopies before noon were associated with improved outcomes. Prospective studies are needed to confirm the effects of these interventions on bowel preparation quality.
Disclosures
Carol A Burke, MD has received research funding from Ferring Pharmaceuticals. Other authors have no conflicts of interest to disclose.
1. Yadlapati R, Johnston ER, Gregory DL, Ciolino JD, Cooper A, Keswani RN. Predictors of inadequate inpatient colonoscopy preparation and its association with hospital length of stay and costs. Dig Dis Sci. 2015;60(11):3482-3490. doi: 10.1007/s10620-015-3761-2. PubMed
2. Jawa H, Mosli M, Alsamadani W, et al. Predictors of inadequate bowel preparation for inpatient colonoscopy. Turk J Gastroenterol. 2017;28(6):460-464. doi: 10.5152/tjg.2017.17196. PubMed
3. Mcnabb-Baltar J, Dorreen A, Dhahab HA, et al. Age is the only predictor of poor bowel preparation in the hospitalized patient. Can J Gastroenterol Hepatol. 2016;2016:1-5. doi: 10.1155/2016/2139264. PubMed
4. Rotondano G, Rispo A, Bottiglieri ME, et al. Tu1503 Quality of bowel cleansing in hospitalized patients is not worse than that of outpatients undergoing colonoscopy: results of a multicenter prospective regional study. Gastrointest Endosc. 2014;79(5):AB564. doi: 10.1016/j.gie.2014.02.949. PubMed
5. Ness R. Predictors of inadequate bowel preparation for colonoscopy. Am J Gastroenterol. 2001;96(6):1797-1802. doi: 10.1016/s0002-9270(01)02437-6. PubMed
6. Johnson DA, Barkun AN, Cohen LB, et al. Optimizing adequacy of bowel cleansing for colonoscopy: recommendations from the us multi-society task force on colorectal cancer. Gastroenterology. 2014;147(4):903-924. doi: 10.1053/j.gastro.2014.07.002. PubMed
7. Aronchick CA, Lipshutz WH, Wright SH, et al. A novel tableted purgative for colonoscopic preparation: efficacy and safety comparisons with Colyte and Fleet Phospho-Soda. Gastrointest Endosc. 2000;52(3):346-352. doi: 10.1067/mge.2000.108480. PubMed
8. Froehlich F, Wietlisbach V, Gonvers J-J, Burnand B, Vader J-P. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61(3):378-384. doi: 10.1016/s0016-5107(04)02776-2. PubMed
9. Sarvepalli S, Garber A, Rizk M, et al. 923 adjusted comparison of commercial bowel preparations based on inadequacy of bowel preparation in outpatient settings. Gastrointest Endosc. 2018;87(6):AB127. doi: 10.1016/j.gie.2018.04.1331.
10. Hendry PO, Jenkins JT, Diament RH. The impact of poor bowel preparation on colonoscopy: a prospective single center study of 10 571 colonoscopies. Colorectal Dis. 2007;9(8):745-748. doi: 10.1111/j.1463-1318.2007.01220.x. PubMed
11. Lebwohl B, Wang TC, Neugut AI. Socioeconomic and other predictors of colonoscopy preparation quality. Dig Dis Sci. 2010;55(7):2014-2020. doi: 10.1007/s10620-009-1079-7. PubMed
12. Chorev N, Chadad B, Segal N, et al. Preparation for colonoscopy in hospitalized patients. Dig Dis Sci. 2007;52(3):835-839. doi: 10.1007/s10620-006-9591-5. PubMed
13. Weiss AJ. Overview of Hospital Stays in the United States, 2012. HCUP Statistical Brief #180. Rockville, MD: Agency for Healthcare Research and Quality; 2014. PubMed
14. Kojecky V, Matous J, Keil R, et al. The optimal bowel preparation intervals before colonoscopy: a randomized study comparing polyethylene glycol and low-volume solutions. Dig Liver Dis. 2018;50(3):271-276. doi: 10.1016/j.dld.2017.10.010. PubMed
15. Siddiqui AA, Yang K, Spechler SJ, et al. Duration of the interval between the completion of bowel preparation and the start of colonoscopy predicts bowel-preparation quality. Gastrointest Endosc. 2009;69(3):700-706. doi: 10.1016/j.gie.2008.09.047. PubMed
16. Eun CS, Han DS, Hyun YS, et al. The timing of bowel preparation is more important than the timing of colonoscopy in determining the quality of bowel cleansing. Dig Dis Sci. 2010;56(2):539-544. doi: 10.1007/s10620-010-1457-1. PubMed
17. Ergen WF, Pasricha T, Hubbard FJ, et al. Providing hospitalized patients with an educational booklet increases the quality of colonoscopy bowel preparation. Clin Gastroenterol Hepatol. 2016;14(6):858-864. doi: 10.1016/j.cgh.2015.11.015. PubMed
18. Yadlapati R, Johnston ER, Gluskin AB, et al. An automated inpatient split-dose bowel preparation system improves colonoscopy quality and reduces repeat procedures. J Clin Gastroenterol. 2018;52(8):709-714. doi: 10.1097/mcg.0000000000000849. PubMed
19. Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. The accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43(5):480-485. doi: 10.1097/01.mlr.0000160417.39497.a9. PubMed
20. Parmar R, Martel M, Rostom A, Barkun AN. Validated scales for colon cleansing: a systematic review. J Clin Gastroenterol. 2016;111(2):197-204. doi: 10.1038/ajg.2015.417. PubMed
Inadequate bowel preparation (IBP) at the time of inpatient colonoscopy is common and associated with increased length of stay and cost of care.1 The factors that contribute to IBP can be categorized into those that are modifiable and those that are nonmodifiable. While many factors have been associated with IBP, studies have been limited by small sample size or have combined inpatient/outpatient populations, thus limiting generalizability.1-5 Moreover, most factors associated with IBP, such as socioeconomic status, male gender, increased age, and comorbidities, are nonmodifiable. No studies have explicitly focused on modifiable risk factors, such as medication use, colonoscopy timing, or assessed the potential impact of modifying these factors.
In a large, multihospital system, we examine the frequency of IBP among inpatients undergoing colonoscopy along with factors associated with IBP. We attempted to identify
METHODS
Potential Predictors of IBP
Demographic data such as patient age, gender, ethnicity, body mass index (BMI), and insurance/payor status were obtained from the electronic health record (EHR). International Classification of Disease 9th and 10th revision, Clinical Modifications (ICD-9/10-CM) codes were used to obtain patient comorbidities including diabetes, coronary artery disease, heart failure, cirrhosis, gastroparesis, hypothyroidism, inflammatory bowel disease, constipation, stroke, dementia, dysphagia, and nausea/vomiting. Use of opioid medications within three days before colonoscopy was extracted from the medication administration record. These variables were chosen as biologically plausible modifiers of bowel preparation or had previously been assessed in the literature.1-6 The name and volume, classified as 4 L (GoLytely®) and < 4 liters (MoviPrep®) of bowel preparation, time of day when colonoscopy was performed, solid diet the day prior to colonoscopy, type of sedation used (conscious sedation or general anesthesia), and total colonoscopy time (defined as the time from scope insertion to removal) was recorded. Hospitalization-related variables, including the number of hospitalizations in the year before the current hospitalization, the year in which the colonoscopy was performed, and the number of days from admission to colonoscopy, were also obtained from the EHR.
Outcome Measures
An internally validated natural language algorithm, using Structured Queried Language was used to search through colonoscopy reports to identify adequacy of bowel preparation. ProVation® software allows the gastroenterologist to use some terms to describe bowel preparation in a drop-down menu format. In addition to the Aronchik scale (which allows the gastroenterologist to rate bowel preparation on a five-point scale: “excellent,” “good,” “fair,” “poor,” and “inadequate”) it also allows the provider to use terms such as “adequate” or “adequate to detect polyps >5 mm” as well as “unsatisfactory.”7 Mirroring prior literature, bowel preparation quality was classified into “adequate” and “inadequate”; “good” and “excellent” on the Aronchik scale were categorized as adequate as was the term “adequate” in any form; “fair,” “poor,” or “inadequate” on the Aronchik scale were classified as inadequate as was the term “unsatisfactory.” We evaluated the hospital length of stay (LOS) as a secondary outcome measure.
Statistical Analysis
After describing the frequency of IBP, the quality of bowel preparation (adequate vs inadequate) was compared based on the predictors described above. Categorical variables were reported as frequencies with percentages and continuous variables were reported as medians with 25th-75th percentile values. The significance of the difference between the proportion or median values of those who had inadequate versus adequate bowel preparation was assessed. Two-sided chi-square analysis was used to assess the significance of differences between categorical variables and the Wilcoxon Rank-Sum test was used to assess the significance of differences between continuous variables.
Multivariate logistic regression analysis was performed to assess factors associated with hospital predictors and outcomes, after adjusting for all the aforementioned factors and clustering the effect based on the endoscopist. To evaluate the potential impact of modifiable factors on IBP, we performed counterfactual analysis, in which the observed distribution was compared to a hypothetical population in which all the modifiable risk factors were optimal.
RESULTS
Overall, 8,819 patients were included in our study population. They had a median age of 64 [53-76] years; 50.5% were female and 51% had an IBP. Patient characteristics and rates of IBP are presented in Table 1.
In unadjusted analyses, with regards to modifiable factors, opiate use within three days of colonoscopy was associated with a higher rate of IBP (55.4% vs 47.3%, P <.001), as was a lower volume (<4L) bowel preparation (55.3% vs 50.4%, P = .003). IBP was less frequent when colonoscopy was performed before noon vs afternoon (50.3% vs 57.4%, P < .001), and when patients were documented to receive a clear liquid diet or nil per os vs a solid diet the day prior to colonoscopy (50.3% vs 57.4%, P < .001). Overall bowel preparation quality improved over time (Figure 1). Median LOS was five [3-11] days. Patients who had IBP on their initial colonoscopy had a LOS one day longer than patients without IBP (six days vs five days, P < .001).
Multivariate Analysis
Table 2 shows the results of the multivariate analysis. The following modifiable factors were associated with IBP: opiate used within three days of the procedure (OR 1.31; 95% CI 1.8, 1.45), having the colonoscopy performed after12:00
Potential Impact of Modifiable Variables
We conducted a counterfactual analysis based on a multivariate model to assess the impact of each modifiable risk factor on the IBP rate (Figure 1). In the included study population, 44.9% received an opiate, 39.3% had a colonoscopy after 12:00
DISCUSSION
In this large, multihospital cohort, IBP was documented in half (51%) of 8,819 inpatient colonoscopies performed. Nonmodifiable patient characteristics independently associated with IBP were age, male gender, white race, Medicare and Medicaid insurance, nausea/vomiting, dysphagia, and gastroparesis. Modifiable factors included not consuming opiates within three days of colonoscopy, avoidance of a solid diet the day prior to colonoscopy and performing the colonoscopy before noon. The volume of bowel preparation consumed was not associated with IBP. In a counterfactual analysis, we found that if all three modifiable factors were optimized, the predicted rate of IBP would drop to 45%.
Many studies, including our analysis, have shown significant differences between the frequency of IBP in inpatient versus outpatient bowel preparations.8-11 Therefore, it is crucial to study IBP in these settings separately. Three single-institution studies, including a total of 898 patients, have identified risk factors for inpatient IBP. Individual studies ranged in size from 130 to 524 patients with rates of IBP ranging from 22%-57%.1-3 They found IBP to be associated with increasing age, lower income, ASA Grade >3, diabetes, coronary artery disease (CAD), nausea or vomiting, BMI >25, and chronic constipation. Modifiable factors included opiates, afternoon procedures, and runway times >6 hours.
We also found IBP to be associated with increasing age and male gender. However, we found no association with diabetes, chronic constipation, CAD or BMI. As we were able to adjust for a wider variety of variables, it is possible that we were able to account for residual confounding better than previous studies. For example, we found that having nausea/vomiting, dysphagia, and gastroparesis was associated with IBP. Gastroparesis with associated nausea and vomiting may be the mechanism by which diabetes increases the risk for IBP. Further studies are needed to assess if interventions or alternative bowel cleansing in these patients can result in improved IBP. Finally, in contrast to studies with smaller cohorts which found that lower volume bowel preps improved IBP in the right colon,4,12 we found no association between IBP based and volume of bowel preparation consumed. Our impact analysis suggests that avoidance of opiates for at least three days before colonoscopy, avoidance of solid diet on the day before colonoscopy and performing all colonoscopies before noon would
The factors mentioned above may not always be amenable to modification. For example, for patients with active gastrointestinal bleeding, postponing colonoscopy by one day for the sake of maintaining a patient on a clear diet may not be feasible. Similarly, performing colonoscopies in the morning is highly dependent on endoscopy suite availability and hospital logistics. Denying opiates to patients experiencing severe pain is not ethical. In many scenarios, however, these variables could be modified, and institutional efforts to support these practices could yield considerable savings. Future prospective studies are needed to verify the real impact of these changes.
Further discussion is needed to contextualize the finding that colonoscopies scheduled in the afternoon are associated with improved bowel preparation quality. Previous research—albeit in the outpatient setting—has demonstrated 11.8 hours as the maximum upper time limit for the time elapsed between the end of bowel preparation to colonoscopy.14 Another study found an inverse relationship between the quality of bowel preparation and the time after completion of the bowel preparation.15 This makes sense from a physiological perspective as delaying the time between completion of bowel preparation, and the procedure allows chyme from the small intestine to reaccumulate in the colon. Anecdotally, at our institution as well as at many others, the bowel preparations are ordered to start in the evening to allow the consumption of complete bowel preparation by midnight. As a result of this practice, only patients who have their colonoscopies scheduled before noon fall within the optimal period of 11.8 hours. In the outpatient setting, the use of split preparations has led to the obliteration of the difference in the quality of bowel preparation between morning and afternoon colonoscopies.16 Prospective trials are needed to evaluate the use of split preparations to improve the quality of afternoon inpatient colonoscopies.
Few other strategies have been shown to mitigate IBP in the inpatient setting. In a small randomized controlled trial, Ergen et al. found that providing an educational booklet improved inpatient bowel preparation as measured by the Boston Bowel Preparation Scale.17 In a quasi-experimental design, Yadlapati et al. found that an automated split-dose bowel preparation resulted in decreased IBP, fewer repeated procedures, shorter LOS, and lower hospital cost.18 Our study adds to these tools by identifying three additional risk factors which could be optimized for inpatients. Because our findings are observational, they should be subjected to prospective trials. Our study also calls into question the impact of bowel preparation volume. We found no difference in the rate of IBP between low and large volume preparations. It is possible that other factors are more important than the specific preparation employed.
Interestingly, we found that IBP declined substantially in 2014 and continued to decline after that. The year was the most influential risk factor for IBP (on par with gastroparesis). The reason for this is unclear, as rates of our modifiable risk factors did not differ substantially by year. Other possibilities include improved access (including weekend access) to endoscopy coinciding with the development of a new endoscopy facility and use of integrated irrigation pump system instead of the use of manual syringes for flushing.
Our study has many strengths. It is by far the most extensive study of bowel preparation quality in inpatients to date and the only one that has included patient, procedural and bowel preparation characteristics. The study also has several significant limitations. This is a single center study, which could limit generalizability. Nonetheless, it was conducted within a health system with multiple hospitals in different parts of the United States (Ohio and Florida) and included a broad population mix with differing levels of acuity. The retrospective nature of the assessment precludes establishing causation. However, we mitigated confounding by adjusting for a wide variety of factors, and there is a plausible physiological mechanism for each of the factors we studied. Also, the retrospective nature of our study predisposes our data to omissions and misrepresentations during the documentation process. This is especially true with the use of ICD codes.19 Inaccuracies in coding are likely to bias toward the null, so observed associations may be an underestimate of the true association.
Our inability to ascertain if a patient completed the prescribed bowel preparation limited our ability to detect what may be a significant risk factor. Lastly, while clinically relevant, the Aronchik scale used to identify adequate from IBP has never been validated though it is frequently utilized and cited in the bowel preparation literature.20
CONCLUSIONS
In this large retrospective study evaluating bowel preparation quality in inpatients undergoing colonoscopy, we found that more than half of the patients have IBP and that IBP was associated with an extra day of hospitalization. Our study identifies those patients at highest risk and identifies modifiable risk factors for IBP. Specifically, we found that abstinence from opiates or solid diet before the colonoscopy, along with performing colonoscopies before noon were associated with improved outcomes. Prospective studies are needed to confirm the effects of these interventions on bowel preparation quality.
Disclosures
Carol A Burke, MD has received research funding from Ferring Pharmaceuticals. Other authors have no conflicts of interest to disclose.
Inadequate bowel preparation (IBP) at the time of inpatient colonoscopy is common and associated with increased length of stay and cost of care.1 The factors that contribute to IBP can be categorized into those that are modifiable and those that are nonmodifiable. While many factors have been associated with IBP, studies have been limited by small sample size or have combined inpatient/outpatient populations, thus limiting generalizability.1-5 Moreover, most factors associated with IBP, such as socioeconomic status, male gender, increased age, and comorbidities, are nonmodifiable. No studies have explicitly focused on modifiable risk factors, such as medication use, colonoscopy timing, or assessed the potential impact of modifying these factors.
In a large, multihospital system, we examine the frequency of IBP among inpatients undergoing colonoscopy along with factors associated with IBP. We attempted to identify
METHODS
Potential Predictors of IBP
Demographic data such as patient age, gender, ethnicity, body mass index (BMI), and insurance/payor status were obtained from the electronic health record (EHR). International Classification of Disease 9th and 10th revision, Clinical Modifications (ICD-9/10-CM) codes were used to obtain patient comorbidities including diabetes, coronary artery disease, heart failure, cirrhosis, gastroparesis, hypothyroidism, inflammatory bowel disease, constipation, stroke, dementia, dysphagia, and nausea/vomiting. Use of opioid medications within three days before colonoscopy was extracted from the medication administration record. These variables were chosen as biologically plausible modifiers of bowel preparation or had previously been assessed in the literature.1-6 The name and volume, classified as 4 L (GoLytely®) and < 4 liters (MoviPrep®) of bowel preparation, time of day when colonoscopy was performed, solid diet the day prior to colonoscopy, type of sedation used (conscious sedation or general anesthesia), and total colonoscopy time (defined as the time from scope insertion to removal) was recorded. Hospitalization-related variables, including the number of hospitalizations in the year before the current hospitalization, the year in which the colonoscopy was performed, and the number of days from admission to colonoscopy, were also obtained from the EHR.
Outcome Measures
An internally validated natural language algorithm, using Structured Queried Language was used to search through colonoscopy reports to identify adequacy of bowel preparation. ProVation® software allows the gastroenterologist to use some terms to describe bowel preparation in a drop-down menu format. In addition to the Aronchik scale (which allows the gastroenterologist to rate bowel preparation on a five-point scale: “excellent,” “good,” “fair,” “poor,” and “inadequate”) it also allows the provider to use terms such as “adequate” or “adequate to detect polyps >5 mm” as well as “unsatisfactory.”7 Mirroring prior literature, bowel preparation quality was classified into “adequate” and “inadequate”; “good” and “excellent” on the Aronchik scale were categorized as adequate as was the term “adequate” in any form; “fair,” “poor,” or “inadequate” on the Aronchik scale were classified as inadequate as was the term “unsatisfactory.” We evaluated the hospital length of stay (LOS) as a secondary outcome measure.
Statistical Analysis
After describing the frequency of IBP, the quality of bowel preparation (adequate vs inadequate) was compared based on the predictors described above. Categorical variables were reported as frequencies with percentages and continuous variables were reported as medians with 25th-75th percentile values. The significance of the difference between the proportion or median values of those who had inadequate versus adequate bowel preparation was assessed. Two-sided chi-square analysis was used to assess the significance of differences between categorical variables and the Wilcoxon Rank-Sum test was used to assess the significance of differences between continuous variables.
Multivariate logistic regression analysis was performed to assess factors associated with hospital predictors and outcomes, after adjusting for all the aforementioned factors and clustering the effect based on the endoscopist. To evaluate the potential impact of modifiable factors on IBP, we performed counterfactual analysis, in which the observed distribution was compared to a hypothetical population in which all the modifiable risk factors were optimal.
RESULTS
Overall, 8,819 patients were included in our study population. They had a median age of 64 [53-76] years; 50.5% were female and 51% had an IBP. Patient characteristics and rates of IBP are presented in Table 1.
In unadjusted analyses, with regards to modifiable factors, opiate use within three days of colonoscopy was associated with a higher rate of IBP (55.4% vs 47.3%, P <.001), as was a lower volume (<4L) bowel preparation (55.3% vs 50.4%, P = .003). IBP was less frequent when colonoscopy was performed before noon vs afternoon (50.3% vs 57.4%, P < .001), and when patients were documented to receive a clear liquid diet or nil per os vs a solid diet the day prior to colonoscopy (50.3% vs 57.4%, P < .001). Overall bowel preparation quality improved over time (Figure 1). Median LOS was five [3-11] days. Patients who had IBP on their initial colonoscopy had a LOS one day longer than patients without IBP (six days vs five days, P < .001).
Multivariate Analysis
Table 2 shows the results of the multivariate analysis. The following modifiable factors were associated with IBP: opiate used within three days of the procedure (OR 1.31; 95% CI 1.8, 1.45), having the colonoscopy performed after12:00
Potential Impact of Modifiable Variables
We conducted a counterfactual analysis based on a multivariate model to assess the impact of each modifiable risk factor on the IBP rate (Figure 1). In the included study population, 44.9% received an opiate, 39.3% had a colonoscopy after 12:00
DISCUSSION
In this large, multihospital cohort, IBP was documented in half (51%) of 8,819 inpatient colonoscopies performed. Nonmodifiable patient characteristics independently associated with IBP were age, male gender, white race, Medicare and Medicaid insurance, nausea/vomiting, dysphagia, and gastroparesis. Modifiable factors included not consuming opiates within three days of colonoscopy, avoidance of a solid diet the day prior to colonoscopy and performing the colonoscopy before noon. The volume of bowel preparation consumed was not associated with IBP. In a counterfactual analysis, we found that if all three modifiable factors were optimized, the predicted rate of IBP would drop to 45%.
Many studies, including our analysis, have shown significant differences between the frequency of IBP in inpatient versus outpatient bowel preparations.8-11 Therefore, it is crucial to study IBP in these settings separately. Three single-institution studies, including a total of 898 patients, have identified risk factors for inpatient IBP. Individual studies ranged in size from 130 to 524 patients with rates of IBP ranging from 22%-57%.1-3 They found IBP to be associated with increasing age, lower income, ASA Grade >3, diabetes, coronary artery disease (CAD), nausea or vomiting, BMI >25, and chronic constipation. Modifiable factors included opiates, afternoon procedures, and runway times >6 hours.
We also found IBP to be associated with increasing age and male gender. However, we found no association with diabetes, chronic constipation, CAD or BMI. As we were able to adjust for a wider variety of variables, it is possible that we were able to account for residual confounding better than previous studies. For example, we found that having nausea/vomiting, dysphagia, and gastroparesis was associated with IBP. Gastroparesis with associated nausea and vomiting may be the mechanism by which diabetes increases the risk for IBP. Further studies are needed to assess if interventions or alternative bowel cleansing in these patients can result in improved IBP. Finally, in contrast to studies with smaller cohorts which found that lower volume bowel preps improved IBP in the right colon,4,12 we found no association between IBP based and volume of bowel preparation consumed. Our impact analysis suggests that avoidance of opiates for at least three days before colonoscopy, avoidance of solid diet on the day before colonoscopy and performing all colonoscopies before noon would
The factors mentioned above may not always be amenable to modification. For example, for patients with active gastrointestinal bleeding, postponing colonoscopy by one day for the sake of maintaining a patient on a clear diet may not be feasible. Similarly, performing colonoscopies in the morning is highly dependent on endoscopy suite availability and hospital logistics. Denying opiates to patients experiencing severe pain is not ethical. In many scenarios, however, these variables could be modified, and institutional efforts to support these practices could yield considerable savings. Future prospective studies are needed to verify the real impact of these changes.
Further discussion is needed to contextualize the finding that colonoscopies scheduled in the afternoon are associated with improved bowel preparation quality. Previous research—albeit in the outpatient setting—has demonstrated 11.8 hours as the maximum upper time limit for the time elapsed between the end of bowel preparation to colonoscopy.14 Another study found an inverse relationship between the quality of bowel preparation and the time after completion of the bowel preparation.15 This makes sense from a physiological perspective as delaying the time between completion of bowel preparation, and the procedure allows chyme from the small intestine to reaccumulate in the colon. Anecdotally, at our institution as well as at many others, the bowel preparations are ordered to start in the evening to allow the consumption of complete bowel preparation by midnight. As a result of this practice, only patients who have their colonoscopies scheduled before noon fall within the optimal period of 11.8 hours. In the outpatient setting, the use of split preparations has led to the obliteration of the difference in the quality of bowel preparation between morning and afternoon colonoscopies.16 Prospective trials are needed to evaluate the use of split preparations to improve the quality of afternoon inpatient colonoscopies.
Few other strategies have been shown to mitigate IBP in the inpatient setting. In a small randomized controlled trial, Ergen et al. found that providing an educational booklet improved inpatient bowel preparation as measured by the Boston Bowel Preparation Scale.17 In a quasi-experimental design, Yadlapati et al. found that an automated split-dose bowel preparation resulted in decreased IBP, fewer repeated procedures, shorter LOS, and lower hospital cost.18 Our study adds to these tools by identifying three additional risk factors which could be optimized for inpatients. Because our findings are observational, they should be subjected to prospective trials. Our study also calls into question the impact of bowel preparation volume. We found no difference in the rate of IBP between low and large volume preparations. It is possible that other factors are more important than the specific preparation employed.
Interestingly, we found that IBP declined substantially in 2014 and continued to decline after that. The year was the most influential risk factor for IBP (on par with gastroparesis). The reason for this is unclear, as rates of our modifiable risk factors did not differ substantially by year. Other possibilities include improved access (including weekend access) to endoscopy coinciding with the development of a new endoscopy facility and use of integrated irrigation pump system instead of the use of manual syringes for flushing.
Our study has many strengths. It is by far the most extensive study of bowel preparation quality in inpatients to date and the only one that has included patient, procedural and bowel preparation characteristics. The study also has several significant limitations. This is a single center study, which could limit generalizability. Nonetheless, it was conducted within a health system with multiple hospitals in different parts of the United States (Ohio and Florida) and included a broad population mix with differing levels of acuity. The retrospective nature of the assessment precludes establishing causation. However, we mitigated confounding by adjusting for a wide variety of factors, and there is a plausible physiological mechanism for each of the factors we studied. Also, the retrospective nature of our study predisposes our data to omissions and misrepresentations during the documentation process. This is especially true with the use of ICD codes.19 Inaccuracies in coding are likely to bias toward the null, so observed associations may be an underestimate of the true association.
Our inability to ascertain if a patient completed the prescribed bowel preparation limited our ability to detect what may be a significant risk factor. Lastly, while clinically relevant, the Aronchik scale used to identify adequate from IBP has never been validated though it is frequently utilized and cited in the bowel preparation literature.20
CONCLUSIONS
In this large retrospective study evaluating bowel preparation quality in inpatients undergoing colonoscopy, we found that more than half of the patients have IBP and that IBP was associated with an extra day of hospitalization. Our study identifies those patients at highest risk and identifies modifiable risk factors for IBP. Specifically, we found that abstinence from opiates or solid diet before the colonoscopy, along with performing colonoscopies before noon were associated with improved outcomes. Prospective studies are needed to confirm the effects of these interventions on bowel preparation quality.
Disclosures
Carol A Burke, MD has received research funding from Ferring Pharmaceuticals. Other authors have no conflicts of interest to disclose.
1. Yadlapati R, Johnston ER, Gregory DL, Ciolino JD, Cooper A, Keswani RN. Predictors of inadequate inpatient colonoscopy preparation and its association with hospital length of stay and costs. Dig Dis Sci. 2015;60(11):3482-3490. doi: 10.1007/s10620-015-3761-2. PubMed
2. Jawa H, Mosli M, Alsamadani W, et al. Predictors of inadequate bowel preparation for inpatient colonoscopy. Turk J Gastroenterol. 2017;28(6):460-464. doi: 10.5152/tjg.2017.17196. PubMed
3. Mcnabb-Baltar J, Dorreen A, Dhahab HA, et al. Age is the only predictor of poor bowel preparation in the hospitalized patient. Can J Gastroenterol Hepatol. 2016;2016:1-5. doi: 10.1155/2016/2139264. PubMed
4. Rotondano G, Rispo A, Bottiglieri ME, et al. Tu1503 Quality of bowel cleansing in hospitalized patients is not worse than that of outpatients undergoing colonoscopy: results of a multicenter prospective regional study. Gastrointest Endosc. 2014;79(5):AB564. doi: 10.1016/j.gie.2014.02.949. PubMed
5. Ness R. Predictors of inadequate bowel preparation for colonoscopy. Am J Gastroenterol. 2001;96(6):1797-1802. doi: 10.1016/s0002-9270(01)02437-6. PubMed
6. Johnson DA, Barkun AN, Cohen LB, et al. Optimizing adequacy of bowel cleansing for colonoscopy: recommendations from the us multi-society task force on colorectal cancer. Gastroenterology. 2014;147(4):903-924. doi: 10.1053/j.gastro.2014.07.002. PubMed
7. Aronchick CA, Lipshutz WH, Wright SH, et al. A novel tableted purgative for colonoscopic preparation: efficacy and safety comparisons with Colyte and Fleet Phospho-Soda. Gastrointest Endosc. 2000;52(3):346-352. doi: 10.1067/mge.2000.108480. PubMed
8. Froehlich F, Wietlisbach V, Gonvers J-J, Burnand B, Vader J-P. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61(3):378-384. doi: 10.1016/s0016-5107(04)02776-2. PubMed
9. Sarvepalli S, Garber A, Rizk M, et al. 923 adjusted comparison of commercial bowel preparations based on inadequacy of bowel preparation in outpatient settings. Gastrointest Endosc. 2018;87(6):AB127. doi: 10.1016/j.gie.2018.04.1331.
10. Hendry PO, Jenkins JT, Diament RH. The impact of poor bowel preparation on colonoscopy: a prospective single center study of 10 571 colonoscopies. Colorectal Dis. 2007;9(8):745-748. doi: 10.1111/j.1463-1318.2007.01220.x. PubMed
11. Lebwohl B, Wang TC, Neugut AI. Socioeconomic and other predictors of colonoscopy preparation quality. Dig Dis Sci. 2010;55(7):2014-2020. doi: 10.1007/s10620-009-1079-7. PubMed
12. Chorev N, Chadad B, Segal N, et al. Preparation for colonoscopy in hospitalized patients. Dig Dis Sci. 2007;52(3):835-839. doi: 10.1007/s10620-006-9591-5. PubMed
13. Weiss AJ. Overview of Hospital Stays in the United States, 2012. HCUP Statistical Brief #180. Rockville, MD: Agency for Healthcare Research and Quality; 2014. PubMed
14. Kojecky V, Matous J, Keil R, et al. The optimal bowel preparation intervals before colonoscopy: a randomized study comparing polyethylene glycol and low-volume solutions. Dig Liver Dis. 2018;50(3):271-276. doi: 10.1016/j.dld.2017.10.010. PubMed
15. Siddiqui AA, Yang K, Spechler SJ, et al. Duration of the interval between the completion of bowel preparation and the start of colonoscopy predicts bowel-preparation quality. Gastrointest Endosc. 2009;69(3):700-706. doi: 10.1016/j.gie.2008.09.047. PubMed
16. Eun CS, Han DS, Hyun YS, et al. The timing of bowel preparation is more important than the timing of colonoscopy in determining the quality of bowel cleansing. Dig Dis Sci. 2010;56(2):539-544. doi: 10.1007/s10620-010-1457-1. PubMed
17. Ergen WF, Pasricha T, Hubbard FJ, et al. Providing hospitalized patients with an educational booklet increases the quality of colonoscopy bowel preparation. Clin Gastroenterol Hepatol. 2016;14(6):858-864. doi: 10.1016/j.cgh.2015.11.015. PubMed
18. Yadlapati R, Johnston ER, Gluskin AB, et al. An automated inpatient split-dose bowel preparation system improves colonoscopy quality and reduces repeat procedures. J Clin Gastroenterol. 2018;52(8):709-714. doi: 10.1097/mcg.0000000000000849. PubMed
19. Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. The accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43(5):480-485. doi: 10.1097/01.mlr.0000160417.39497.a9. PubMed
20. Parmar R, Martel M, Rostom A, Barkun AN. Validated scales for colon cleansing: a systematic review. J Clin Gastroenterol. 2016;111(2):197-204. doi: 10.1038/ajg.2015.417. PubMed
1. Yadlapati R, Johnston ER, Gregory DL, Ciolino JD, Cooper A, Keswani RN. Predictors of inadequate inpatient colonoscopy preparation and its association with hospital length of stay and costs. Dig Dis Sci. 2015;60(11):3482-3490. doi: 10.1007/s10620-015-3761-2. PubMed
2. Jawa H, Mosli M, Alsamadani W, et al. Predictors of inadequate bowel preparation for inpatient colonoscopy. Turk J Gastroenterol. 2017;28(6):460-464. doi: 10.5152/tjg.2017.17196. PubMed
3. Mcnabb-Baltar J, Dorreen A, Dhahab HA, et al. Age is the only predictor of poor bowel preparation in the hospitalized patient. Can J Gastroenterol Hepatol. 2016;2016:1-5. doi: 10.1155/2016/2139264. PubMed
4. Rotondano G, Rispo A, Bottiglieri ME, et al. Tu1503 Quality of bowel cleansing in hospitalized patients is not worse than that of outpatients undergoing colonoscopy: results of a multicenter prospective regional study. Gastrointest Endosc. 2014;79(5):AB564. doi: 10.1016/j.gie.2014.02.949. PubMed
5. Ness R. Predictors of inadequate bowel preparation for colonoscopy. Am J Gastroenterol. 2001;96(6):1797-1802. doi: 10.1016/s0002-9270(01)02437-6. PubMed
6. Johnson DA, Barkun AN, Cohen LB, et al. Optimizing adequacy of bowel cleansing for colonoscopy: recommendations from the us multi-society task force on colorectal cancer. Gastroenterology. 2014;147(4):903-924. doi: 10.1053/j.gastro.2014.07.002. PubMed
7. Aronchick CA, Lipshutz WH, Wright SH, et al. A novel tableted purgative for colonoscopic preparation: efficacy and safety comparisons with Colyte and Fleet Phospho-Soda. Gastrointest Endosc. 2000;52(3):346-352. doi: 10.1067/mge.2000.108480. PubMed
8. Froehlich F, Wietlisbach V, Gonvers J-J, Burnand B, Vader J-P. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61(3):378-384. doi: 10.1016/s0016-5107(04)02776-2. PubMed
9. Sarvepalli S, Garber A, Rizk M, et al. 923 adjusted comparison of commercial bowel preparations based on inadequacy of bowel preparation in outpatient settings. Gastrointest Endosc. 2018;87(6):AB127. doi: 10.1016/j.gie.2018.04.1331.
10. Hendry PO, Jenkins JT, Diament RH. The impact of poor bowel preparation on colonoscopy: a prospective single center study of 10 571 colonoscopies. Colorectal Dis. 2007;9(8):745-748. doi: 10.1111/j.1463-1318.2007.01220.x. PubMed
11. Lebwohl B, Wang TC, Neugut AI. Socioeconomic and other predictors of colonoscopy preparation quality. Dig Dis Sci. 2010;55(7):2014-2020. doi: 10.1007/s10620-009-1079-7. PubMed
12. Chorev N, Chadad B, Segal N, et al. Preparation for colonoscopy in hospitalized patients. Dig Dis Sci. 2007;52(3):835-839. doi: 10.1007/s10620-006-9591-5. PubMed
13. Weiss AJ. Overview of Hospital Stays in the United States, 2012. HCUP Statistical Brief #180. Rockville, MD: Agency for Healthcare Research and Quality; 2014. PubMed
14. Kojecky V, Matous J, Keil R, et al. The optimal bowel preparation intervals before colonoscopy: a randomized study comparing polyethylene glycol and low-volume solutions. Dig Liver Dis. 2018;50(3):271-276. doi: 10.1016/j.dld.2017.10.010. PubMed
15. Siddiqui AA, Yang K, Spechler SJ, et al. Duration of the interval between the completion of bowel preparation and the start of colonoscopy predicts bowel-preparation quality. Gastrointest Endosc. 2009;69(3):700-706. doi: 10.1016/j.gie.2008.09.047. PubMed
16. Eun CS, Han DS, Hyun YS, et al. The timing of bowel preparation is more important than the timing of colonoscopy in determining the quality of bowel cleansing. Dig Dis Sci. 2010;56(2):539-544. doi: 10.1007/s10620-010-1457-1. PubMed
17. Ergen WF, Pasricha T, Hubbard FJ, et al. Providing hospitalized patients with an educational booklet increases the quality of colonoscopy bowel preparation. Clin Gastroenterol Hepatol. 2016;14(6):858-864. doi: 10.1016/j.cgh.2015.11.015. PubMed
18. Yadlapati R, Johnston ER, Gluskin AB, et al. An automated inpatient split-dose bowel preparation system improves colonoscopy quality and reduces repeat procedures. J Clin Gastroenterol. 2018;52(8):709-714. doi: 10.1097/mcg.0000000000000849. PubMed
19. Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. The accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43(5):480-485. doi: 10.1097/01.mlr.0000160417.39497.a9. PubMed
20. Parmar R, Martel M, Rostom A, Barkun AN. Validated scales for colon cleansing: a systematic review. J Clin Gastroenterol. 2016;111(2):197-204. doi: 10.1038/ajg.2015.417. PubMed
© 2019 Society of Hospital Medicine