User login
Modifiable Factors Associated with Quality of Bowel Preparation Among Hospitalized Patients Undergoing Colonoscopy
Inadequate bowel preparation (IBP) at the time of inpatient colonoscopy is common and associated with increased length of stay and cost of care.1 The factors that contribute to IBP can be categorized into those that are modifiable and those that are nonmodifiable. While many factors have been associated with IBP, studies have been limited by small sample size or have combined inpatient/outpatient populations, thus limiting generalizability.1-5 Moreover, most factors associated with IBP, such as socioeconomic status, male gender, increased age, and comorbidities, are nonmodifiable. No studies have explicitly focused on modifiable risk factors, such as medication use, colonoscopy timing, or assessed the potential impact of modifying these factors.
In a large, multihospital system, we examine the frequency of IBP among inpatients undergoing colonoscopy along with factors associated with IBP. We attempted to identify
METHODS
Potential Predictors of IBP
Demographic data such as patient age, gender, ethnicity, body mass index (BMI), and insurance/payor status were obtained from the electronic health record (EHR). International Classification of Disease 9th and 10th revision, Clinical Modifications (ICD-9/10-CM) codes were used to obtain patient comorbidities including diabetes, coronary artery disease, heart failure, cirrhosis, gastroparesis, hypothyroidism, inflammatory bowel disease, constipation, stroke, dementia, dysphagia, and nausea/vomiting. Use of opioid medications within three days before colonoscopy was extracted from the medication administration record. These variables were chosen as biologically plausible modifiers of bowel preparation or had previously been assessed in the literature.1-6 The name and volume, classified as 4 L (GoLytely®) and < 4 liters (MoviPrep®) of bowel preparation, time of day when colonoscopy was performed, solid diet the day prior to colonoscopy, type of sedation used (conscious sedation or general anesthesia), and total colonoscopy time (defined as the time from scope insertion to removal) was recorded. Hospitalization-related variables, including the number of hospitalizations in the year before the current hospitalization, the year in which the colonoscopy was performed, and the number of days from admission to colonoscopy, were also obtained from the EHR.
Outcome Measures
An internally validated natural language algorithm, using Structured Queried Language was used to search through colonoscopy reports to identify adequacy of bowel preparation. ProVation® software allows the gastroenterologist to use some terms to describe bowel preparation in a drop-down menu format. In addition to the Aronchik scale (which allows the gastroenterologist to rate bowel preparation on a five-point scale: “excellent,” “good,” “fair,” “poor,” and “inadequate”) it also allows the provider to use terms such as “adequate” or “adequate to detect polyps >5 mm” as well as “unsatisfactory.”7 Mirroring prior literature, bowel preparation quality was classified into “adequate” and “inadequate”; “good” and “excellent” on the Aronchik scale were categorized as adequate as was the term “adequate” in any form; “fair,” “poor,” or “inadequate” on the Aronchik scale were classified as inadequate as was the term “unsatisfactory.” We evaluated the hospital length of stay (LOS) as a secondary outcome measure.
Statistical Analysis
After describing the frequency of IBP, the quality of bowel preparation (adequate vs inadequate) was compared based on the predictors described above. Categorical variables were reported as frequencies with percentages and continuous variables were reported as medians with 25th-75th percentile values. The significance of the difference between the proportion or median values of those who had inadequate versus adequate bowel preparation was assessed. Two-sided chi-square analysis was used to assess the significance of differences between categorical variables and the Wilcoxon Rank-Sum test was used to assess the significance of differences between continuous variables.
Multivariate logistic regression analysis was performed to assess factors associated with hospital predictors and outcomes, after adjusting for all the aforementioned factors and clustering the effect based on the endoscopist. To evaluate the potential impact of modifiable factors on IBP, we performed counterfactual analysis, in which the observed distribution was compared to a hypothetical population in which all the modifiable risk factors were optimal.
RESULTS
Overall, 8,819 patients were included in our study population. They had a median age of 64 [53-76] years; 50.5% were female and 51% had an IBP. Patient characteristics and rates of IBP are presented in Table 1.
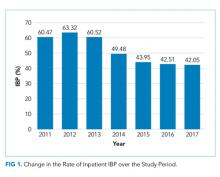
In unadjusted analyses, with regards to modifiable factors, opiate use within three days of colonoscopy was associated with a higher rate of IBP (55.4% vs 47.3%, P <.001), as was a lower volume (<4L) bowel preparation (55.3% vs 50.4%, P = .003). IBP was less frequent when colonoscopy was performed before noon vs afternoon (50.3% vs 57.4%, P < .001), and when patients were documented to receive a clear liquid diet or nil per os vs a solid diet the day prior to colonoscopy (50.3% vs 57.4%, P < .001). Overall bowel preparation quality improved over time (Figure 1). Median LOS was five [3-11] days. Patients who had IBP on their initial colonoscopy had a LOS one day longer than patients without IBP (six days vs five days, P < .001).
Multivariate Analysis
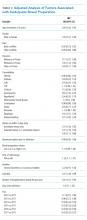
Table 2 shows the results of the multivariate analysis. The following modifiable factors were associated with IBP: opiate used within three days of the procedure (OR 1.31; 95% CI 1.8, 1.45), having the colonoscopy performed after12:00
Potential Impact of Modifiable Variables
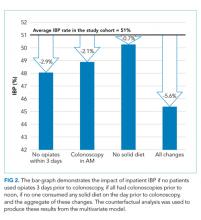
We conducted a counterfactual analysis based on a multivariate model to assess the impact of each modifiable risk factor on the IBP rate (Figure 1). In the included study population, 44.9% received an opiate, 39.3% had a colonoscopy after 12:00
DISCUSSION
In this large, multihospital cohort, IBP was documented in half (51%) of 8,819 inpatient colonoscopies performed. Nonmodifiable patient characteristics independently associated with IBP were age, male gender, white race, Medicare and Medicaid insurance, nausea/vomiting, dysphagia, and gastroparesis. Modifiable factors included not consuming opiates within three days of colonoscopy, avoidance of a solid diet the day prior to colonoscopy and performing the colonoscopy before noon. The volume of bowel preparation consumed was not associated with IBP. In a counterfactual analysis, we found that if all three modifiable factors were optimized, the predicted rate of IBP would drop to 45%.
Many studies, including our analysis, have shown significant differences between the frequency of IBP in inpatient versus outpatient bowel preparations.8-11 Therefore, it is crucial to study IBP in these settings separately. Three single-institution studies, including a total of 898 patients, have identified risk factors for inpatient IBP. Individual studies ranged in size from 130 to 524 patients with rates of IBP ranging from 22%-57%.1-3 They found IBP to be associated with increasing age, lower income, ASA Grade >3, diabetes, coronary artery disease (CAD), nausea or vomiting, BMI >25, and chronic constipation. Modifiable factors included opiates, afternoon procedures, and runway times >6 hours.
We also found IBP to be associated with increasing age and male gender. However, we found no association with diabetes, chronic constipation, CAD or BMI. As we were able to adjust for a wider variety of variables, it is possible that we were able to account for residual confounding better than previous studies. For example, we found that having nausea/vomiting, dysphagia, and gastroparesis was associated with IBP. Gastroparesis with associated nausea and vomiting may be the mechanism by which diabetes increases the risk for IBP. Further studies are needed to assess if interventions or alternative bowel cleansing in these patients can result in improved IBP. Finally, in contrast to studies with smaller cohorts which found that lower volume bowel preps improved IBP in the right colon,4,12 we found no association between IBP based and volume of bowel preparation consumed. Our impact analysis suggests that avoidance of opiates for at least three days before colonoscopy, avoidance of solid diet on the day before colonoscopy and performing all colonoscopies before noon would
The factors mentioned above may not always be amenable to modification. For example, for patients with active gastrointestinal bleeding, postponing colonoscopy by one day for the sake of maintaining a patient on a clear diet may not be feasible. Similarly, performing colonoscopies in the morning is highly dependent on endoscopy suite availability and hospital logistics. Denying opiates to patients experiencing severe pain is not ethical. In many scenarios, however, these variables could be modified, and institutional efforts to support these practices could yield considerable savings. Future prospective studies are needed to verify the real impact of these changes.
Further discussion is needed to contextualize the finding that colonoscopies scheduled in the afternoon are associated with improved bowel preparation quality. Previous research—albeit in the outpatient setting—has demonstrated 11.8 hours as the maximum upper time limit for the time elapsed between the end of bowel preparation to colonoscopy.14 Another study found an inverse relationship between the quality of bowel preparation and the time after completion of the bowel preparation.15 This makes sense from a physiological perspective as delaying the time between completion of bowel preparation, and the procedure allows chyme from the small intestine to reaccumulate in the colon. Anecdotally, at our institution as well as at many others, the bowel preparations are ordered to start in the evening to allow the consumption of complete bowel preparation by midnight. As a result of this practice, only patients who have their colonoscopies scheduled before noon fall within the optimal period of 11.8 hours. In the outpatient setting, the use of split preparations has led to the obliteration of the difference in the quality of bowel preparation between morning and afternoon colonoscopies.16 Prospective trials are needed to evaluate the use of split preparations to improve the quality of afternoon inpatient colonoscopies.
Few other strategies have been shown to mitigate IBP in the inpatient setting. In a small randomized controlled trial, Ergen et al. found that providing an educational booklet improved inpatient bowel preparation as measured by the Boston Bowel Preparation Scale.17 In a quasi-experimental design, Yadlapati et al. found that an automated split-dose bowel preparation resulted in decreased IBP, fewer repeated procedures, shorter LOS, and lower hospital cost.18 Our study adds to these tools by identifying three additional risk factors which could be optimized for inpatients. Because our findings are observational, they should be subjected to prospective trials. Our study also calls into question the impact of bowel preparation volume. We found no difference in the rate of IBP between low and large volume preparations. It is possible that other factors are more important than the specific preparation employed.
Interestingly, we found that IBP declined substantially in 2014 and continued to decline after that. The year was the most influential risk factor for IBP (on par with gastroparesis). The reason for this is unclear, as rates of our modifiable risk factors did not differ substantially by year. Other possibilities include improved access (including weekend access) to endoscopy coinciding with the development of a new endoscopy facility and use of integrated irrigation pump system instead of the use of manual syringes for flushing.
Our study has many strengths. It is by far the most extensive study of bowel preparation quality in inpatients to date and the only one that has included patient, procedural and bowel preparation characteristics. The study also has several significant limitations. This is a single center study, which could limit generalizability. Nonetheless, it was conducted within a health system with multiple hospitals in different parts of the United States (Ohio and Florida) and included a broad population mix with differing levels of acuity. The retrospective nature of the assessment precludes establishing causation. However, we mitigated confounding by adjusting for a wide variety of factors, and there is a plausible physiological mechanism for each of the factors we studied. Also, the retrospective nature of our study predisposes our data to omissions and misrepresentations during the documentation process. This is especially true with the use of ICD codes.19 Inaccuracies in coding are likely to bias toward the null, so observed associations may be an underestimate of the true association.
Our inability to ascertain if a patient completed the prescribed bowel preparation limited our ability to detect what may be a significant risk factor. Lastly, while clinically relevant, the Aronchik scale used to identify adequate from IBP has never been validated though it is frequently utilized and cited in the bowel preparation literature.20
CONCLUSIONS
In this large retrospective study evaluating bowel preparation quality in inpatients undergoing colonoscopy, we found that more than half of the patients have IBP and that IBP was associated with an extra day of hospitalization. Our study identifies those patients at highest risk and identifies modifiable risk factors for IBP. Specifically, we found that abstinence from opiates or solid diet before the colonoscopy, along with performing colonoscopies before noon were associated with improved outcomes. Prospective studies are needed to confirm the effects of these interventions on bowel preparation quality.
Disclosures
Carol A Burke, MD has received research funding from Ferring Pharmaceuticals. Other authors have no conflicts of interest to disclose.
1. Yadlapati R, Johnston ER, Gregory DL, Ciolino JD, Cooper A, Keswani RN. Predictors of inadequate inpatient colonoscopy preparation and its association with hospital length of stay and costs. Dig Dis Sci. 2015;60(11):3482-3490. doi: 10.1007/s10620-015-3761-2. PubMed
2. Jawa H, Mosli M, Alsamadani W, et al. Predictors of inadequate bowel preparation for inpatient colonoscopy. Turk J Gastroenterol. 2017;28(6):460-464. doi: 10.5152/tjg.2017.17196. PubMed
3. Mcnabb-Baltar J, Dorreen A, Dhahab HA, et al. Age is the only predictor of poor bowel preparation in the hospitalized patient. Can J Gastroenterol Hepatol. 2016;2016:1-5. doi: 10.1155/2016/2139264. PubMed
4. Rotondano G, Rispo A, Bottiglieri ME, et al. Tu1503 Quality of bowel cleansing in hospitalized patients is not worse than that of outpatients undergoing colonoscopy: results of a multicenter prospective regional study. Gastrointest Endosc. 2014;79(5):AB564. doi: 10.1016/j.gie.2014.02.949. PubMed
5. Ness R. Predictors of inadequate bowel preparation for colonoscopy. Am J Gastroenterol. 2001;96(6):1797-1802. doi: 10.1016/s0002-9270(01)02437-6. PubMed
6. Johnson DA, Barkun AN, Cohen LB, et al. Optimizing adequacy of bowel cleansing for colonoscopy: recommendations from the us multi-society task force on colorectal cancer. Gastroenterology. 2014;147(4):903-924. doi: 10.1053/j.gastro.2014.07.002. PubMed
7. Aronchick CA, Lipshutz WH, Wright SH, et al. A novel tableted purgative for colonoscopic preparation: efficacy and safety comparisons with Colyte and Fleet Phospho-Soda. Gastrointest Endosc. 2000;52(3):346-352. doi: 10.1067/mge.2000.108480. PubMed
8. Froehlich F, Wietlisbach V, Gonvers J-J, Burnand B, Vader J-P. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61(3):378-384. doi: 10.1016/s0016-5107(04)02776-2. PubMed
9. Sarvepalli S, Garber A, Rizk M, et al. 923 adjusted comparison of commercial bowel preparations based on inadequacy of bowel preparation in outpatient settings. Gastrointest Endosc. 2018;87(6):AB127. doi: 10.1016/j.gie.2018.04.1331.
10. Hendry PO, Jenkins JT, Diament RH. The impact of poor bowel preparation on colonoscopy: a prospective single center study of 10 571 colonoscopies. Colorectal Dis. 2007;9(8):745-748. doi: 10.1111/j.1463-1318.2007.01220.x. PubMed
11. Lebwohl B, Wang TC, Neugut AI. Socioeconomic and other predictors of colonoscopy preparation quality. Dig Dis Sci. 2010;55(7):2014-2020. doi: 10.1007/s10620-009-1079-7. PubMed
12. Chorev N, Chadad B, Segal N, et al. Preparation for colonoscopy in hospitalized patients. Dig Dis Sci. 2007;52(3):835-839. doi: 10.1007/s10620-006-9591-5. PubMed
13. Weiss AJ. Overview of Hospital Stays in the United States, 2012. HCUP Statistical Brief #180. Rockville, MD: Agency for Healthcare Research and Quality; 2014. PubMed
14. Kojecky V, Matous J, Keil R, et al. The optimal bowel preparation intervals before colonoscopy: a randomized study comparing polyethylene glycol and low-volume solutions. Dig Liver Dis. 2018;50(3):271-276. doi: 10.1016/j.dld.2017.10.010. PubMed
15. Siddiqui AA, Yang K, Spechler SJ, et al. Duration of the interval between the completion of bowel preparation and the start of colonoscopy predicts bowel-preparation quality. Gastrointest Endosc. 2009;69(3):700-706. doi: 10.1016/j.gie.2008.09.047. PubMed
16. Eun CS, Han DS, Hyun YS, et al. The timing of bowel preparation is more important than the timing of colonoscopy in determining the quality of bowel cleansing. Dig Dis Sci. 2010;56(2):539-544. doi: 10.1007/s10620-010-1457-1. PubMed
17. Ergen WF, Pasricha T, Hubbard FJ, et al. Providing hospitalized patients with an educational booklet increases the quality of colonoscopy bowel preparation. Clin Gastroenterol Hepatol. 2016;14(6):858-864. doi: 10.1016/j.cgh.2015.11.015. PubMed
18. Yadlapati R, Johnston ER, Gluskin AB, et al. An automated inpatient split-dose bowel preparation system improves colonoscopy quality and reduces repeat procedures. J Clin Gastroenterol. 2018;52(8):709-714. doi: 10.1097/mcg.0000000000000849. PubMed
19. Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. The accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43(5):480-485. doi: 10.1097/01.mlr.0000160417.39497.a9. PubMed
20. Parmar R, Martel M, Rostom A, Barkun AN. Validated scales for colon cleansing: a systematic review. J Clin Gastroenterol. 2016;111(2):197-204. doi: 10.1038/ajg.2015.417. PubMed
Inadequate bowel preparation (IBP) at the time of inpatient colonoscopy is common and associated with increased length of stay and cost of care.1 The factors that contribute to IBP can be categorized into those that are modifiable and those that are nonmodifiable. While many factors have been associated with IBP, studies have been limited by small sample size or have combined inpatient/outpatient populations, thus limiting generalizability.1-5 Moreover, most factors associated with IBP, such as socioeconomic status, male gender, increased age, and comorbidities, are nonmodifiable. No studies have explicitly focused on modifiable risk factors, such as medication use, colonoscopy timing, or assessed the potential impact of modifying these factors.
In a large, multihospital system, we examine the frequency of IBP among inpatients undergoing colonoscopy along with factors associated with IBP. We attempted to identify
METHODS
Potential Predictors of IBP
Demographic data such as patient age, gender, ethnicity, body mass index (BMI), and insurance/payor status were obtained from the electronic health record (EHR). International Classification of Disease 9th and 10th revision, Clinical Modifications (ICD-9/10-CM) codes were used to obtain patient comorbidities including diabetes, coronary artery disease, heart failure, cirrhosis, gastroparesis, hypothyroidism, inflammatory bowel disease, constipation, stroke, dementia, dysphagia, and nausea/vomiting. Use of opioid medications within three days before colonoscopy was extracted from the medication administration record. These variables were chosen as biologically plausible modifiers of bowel preparation or had previously been assessed in the literature.1-6 The name and volume, classified as 4 L (GoLytely®) and < 4 liters (MoviPrep®) of bowel preparation, time of day when colonoscopy was performed, solid diet the day prior to colonoscopy, type of sedation used (conscious sedation or general anesthesia), and total colonoscopy time (defined as the time from scope insertion to removal) was recorded. Hospitalization-related variables, including the number of hospitalizations in the year before the current hospitalization, the year in which the colonoscopy was performed, and the number of days from admission to colonoscopy, were also obtained from the EHR.
Outcome Measures
An internally validated natural language algorithm, using Structured Queried Language was used to search through colonoscopy reports to identify adequacy of bowel preparation. ProVation® software allows the gastroenterologist to use some terms to describe bowel preparation in a drop-down menu format. In addition to the Aronchik scale (which allows the gastroenterologist to rate bowel preparation on a five-point scale: “excellent,” “good,” “fair,” “poor,” and “inadequate”) it also allows the provider to use terms such as “adequate” or “adequate to detect polyps >5 mm” as well as “unsatisfactory.”7 Mirroring prior literature, bowel preparation quality was classified into “adequate” and “inadequate”; “good” and “excellent” on the Aronchik scale were categorized as adequate as was the term “adequate” in any form; “fair,” “poor,” or “inadequate” on the Aronchik scale were classified as inadequate as was the term “unsatisfactory.” We evaluated the hospital length of stay (LOS) as a secondary outcome measure.
Statistical Analysis
After describing the frequency of IBP, the quality of bowel preparation (adequate vs inadequate) was compared based on the predictors described above. Categorical variables were reported as frequencies with percentages and continuous variables were reported as medians with 25th-75th percentile values. The significance of the difference between the proportion or median values of those who had inadequate versus adequate bowel preparation was assessed. Two-sided chi-square analysis was used to assess the significance of differences between categorical variables and the Wilcoxon Rank-Sum test was used to assess the significance of differences between continuous variables.
Multivariate logistic regression analysis was performed to assess factors associated with hospital predictors and outcomes, after adjusting for all the aforementioned factors and clustering the effect based on the endoscopist. To evaluate the potential impact of modifiable factors on IBP, we performed counterfactual analysis, in which the observed distribution was compared to a hypothetical population in which all the modifiable risk factors were optimal.
RESULTS
Overall, 8,819 patients were included in our study population. They had a median age of 64 [53-76] years; 50.5% were female and 51% had an IBP. Patient characteristics and rates of IBP are presented in Table 1.
In unadjusted analyses, with regards to modifiable factors, opiate use within three days of colonoscopy was associated with a higher rate of IBP (55.4% vs 47.3%, P <.001), as was a lower volume (<4L) bowel preparation (55.3% vs 50.4%, P = .003). IBP was less frequent when colonoscopy was performed before noon vs afternoon (50.3% vs 57.4%, P < .001), and when patients were documented to receive a clear liquid diet or nil per os vs a solid diet the day prior to colonoscopy (50.3% vs 57.4%, P < .001). Overall bowel preparation quality improved over time (Figure 1). Median LOS was five [3-11] days. Patients who had IBP on their initial colonoscopy had a LOS one day longer than patients without IBP (six days vs five days, P < .001).
Multivariate Analysis
Table 2 shows the results of the multivariate analysis. The following modifiable factors were associated with IBP: opiate used within three days of the procedure (OR 1.31; 95% CI 1.8, 1.45), having the colonoscopy performed after12:00
Potential Impact of Modifiable Variables
We conducted a counterfactual analysis based on a multivariate model to assess the impact of each modifiable risk factor on the IBP rate (Figure 1). In the included study population, 44.9% received an opiate, 39.3% had a colonoscopy after 12:00
DISCUSSION
In this large, multihospital cohort, IBP was documented in half (51%) of 8,819 inpatient colonoscopies performed. Nonmodifiable patient characteristics independently associated with IBP were age, male gender, white race, Medicare and Medicaid insurance, nausea/vomiting, dysphagia, and gastroparesis. Modifiable factors included not consuming opiates within three days of colonoscopy, avoidance of a solid diet the day prior to colonoscopy and performing the colonoscopy before noon. The volume of bowel preparation consumed was not associated with IBP. In a counterfactual analysis, we found that if all three modifiable factors were optimized, the predicted rate of IBP would drop to 45%.
Many studies, including our analysis, have shown significant differences between the frequency of IBP in inpatient versus outpatient bowel preparations.8-11 Therefore, it is crucial to study IBP in these settings separately. Three single-institution studies, including a total of 898 patients, have identified risk factors for inpatient IBP. Individual studies ranged in size from 130 to 524 patients with rates of IBP ranging from 22%-57%.1-3 They found IBP to be associated with increasing age, lower income, ASA Grade >3, diabetes, coronary artery disease (CAD), nausea or vomiting, BMI >25, and chronic constipation. Modifiable factors included opiates, afternoon procedures, and runway times >6 hours.
We also found IBP to be associated with increasing age and male gender. However, we found no association with diabetes, chronic constipation, CAD or BMI. As we were able to adjust for a wider variety of variables, it is possible that we were able to account for residual confounding better than previous studies. For example, we found that having nausea/vomiting, dysphagia, and gastroparesis was associated with IBP. Gastroparesis with associated nausea and vomiting may be the mechanism by which diabetes increases the risk for IBP. Further studies are needed to assess if interventions or alternative bowel cleansing in these patients can result in improved IBP. Finally, in contrast to studies with smaller cohorts which found that lower volume bowel preps improved IBP in the right colon,4,12 we found no association between IBP based and volume of bowel preparation consumed. Our impact analysis suggests that avoidance of opiates for at least three days before colonoscopy, avoidance of solid diet on the day before colonoscopy and performing all colonoscopies before noon would
The factors mentioned above may not always be amenable to modification. For example, for patients with active gastrointestinal bleeding, postponing colonoscopy by one day for the sake of maintaining a patient on a clear diet may not be feasible. Similarly, performing colonoscopies in the morning is highly dependent on endoscopy suite availability and hospital logistics. Denying opiates to patients experiencing severe pain is not ethical. In many scenarios, however, these variables could be modified, and institutional efforts to support these practices could yield considerable savings. Future prospective studies are needed to verify the real impact of these changes.
Further discussion is needed to contextualize the finding that colonoscopies scheduled in the afternoon are associated with improved bowel preparation quality. Previous research—albeit in the outpatient setting—has demonstrated 11.8 hours as the maximum upper time limit for the time elapsed between the end of bowel preparation to colonoscopy.14 Another study found an inverse relationship between the quality of bowel preparation and the time after completion of the bowel preparation.15 This makes sense from a physiological perspective as delaying the time between completion of bowel preparation, and the procedure allows chyme from the small intestine to reaccumulate in the colon. Anecdotally, at our institution as well as at many others, the bowel preparations are ordered to start in the evening to allow the consumption of complete bowel preparation by midnight. As a result of this practice, only patients who have their colonoscopies scheduled before noon fall within the optimal period of 11.8 hours. In the outpatient setting, the use of split preparations has led to the obliteration of the difference in the quality of bowel preparation between morning and afternoon colonoscopies.16 Prospective trials are needed to evaluate the use of split preparations to improve the quality of afternoon inpatient colonoscopies.
Few other strategies have been shown to mitigate IBP in the inpatient setting. In a small randomized controlled trial, Ergen et al. found that providing an educational booklet improved inpatient bowel preparation as measured by the Boston Bowel Preparation Scale.17 In a quasi-experimental design, Yadlapati et al. found that an automated split-dose bowel preparation resulted in decreased IBP, fewer repeated procedures, shorter LOS, and lower hospital cost.18 Our study adds to these tools by identifying three additional risk factors which could be optimized for inpatients. Because our findings are observational, they should be subjected to prospective trials. Our study also calls into question the impact of bowel preparation volume. We found no difference in the rate of IBP between low and large volume preparations. It is possible that other factors are more important than the specific preparation employed.
Interestingly, we found that IBP declined substantially in 2014 and continued to decline after that. The year was the most influential risk factor for IBP (on par with gastroparesis). The reason for this is unclear, as rates of our modifiable risk factors did not differ substantially by year. Other possibilities include improved access (including weekend access) to endoscopy coinciding with the development of a new endoscopy facility and use of integrated irrigation pump system instead of the use of manual syringes for flushing.
Our study has many strengths. It is by far the most extensive study of bowel preparation quality in inpatients to date and the only one that has included patient, procedural and bowel preparation characteristics. The study also has several significant limitations. This is a single center study, which could limit generalizability. Nonetheless, it was conducted within a health system with multiple hospitals in different parts of the United States (Ohio and Florida) and included a broad population mix with differing levels of acuity. The retrospective nature of the assessment precludes establishing causation. However, we mitigated confounding by adjusting for a wide variety of factors, and there is a plausible physiological mechanism for each of the factors we studied. Also, the retrospective nature of our study predisposes our data to omissions and misrepresentations during the documentation process. This is especially true with the use of ICD codes.19 Inaccuracies in coding are likely to bias toward the null, so observed associations may be an underestimate of the true association.
Our inability to ascertain if a patient completed the prescribed bowel preparation limited our ability to detect what may be a significant risk factor. Lastly, while clinically relevant, the Aronchik scale used to identify adequate from IBP has never been validated though it is frequently utilized and cited in the bowel preparation literature.20
CONCLUSIONS
In this large retrospective study evaluating bowel preparation quality in inpatients undergoing colonoscopy, we found that more than half of the patients have IBP and that IBP was associated with an extra day of hospitalization. Our study identifies those patients at highest risk and identifies modifiable risk factors for IBP. Specifically, we found that abstinence from opiates or solid diet before the colonoscopy, along with performing colonoscopies before noon were associated with improved outcomes. Prospective studies are needed to confirm the effects of these interventions on bowel preparation quality.
Disclosures
Carol A Burke, MD has received research funding from Ferring Pharmaceuticals. Other authors have no conflicts of interest to disclose.
Inadequate bowel preparation (IBP) at the time of inpatient colonoscopy is common and associated with increased length of stay and cost of care.1 The factors that contribute to IBP can be categorized into those that are modifiable and those that are nonmodifiable. While many factors have been associated with IBP, studies have been limited by small sample size or have combined inpatient/outpatient populations, thus limiting generalizability.1-5 Moreover, most factors associated with IBP, such as socioeconomic status, male gender, increased age, and comorbidities, are nonmodifiable. No studies have explicitly focused on modifiable risk factors, such as medication use, colonoscopy timing, or assessed the potential impact of modifying these factors.
In a large, multihospital system, we examine the frequency of IBP among inpatients undergoing colonoscopy along with factors associated with IBP. We attempted to identify
METHODS
Potential Predictors of IBP
Demographic data such as patient age, gender, ethnicity, body mass index (BMI), and insurance/payor status were obtained from the electronic health record (EHR). International Classification of Disease 9th and 10th revision, Clinical Modifications (ICD-9/10-CM) codes were used to obtain patient comorbidities including diabetes, coronary artery disease, heart failure, cirrhosis, gastroparesis, hypothyroidism, inflammatory bowel disease, constipation, stroke, dementia, dysphagia, and nausea/vomiting. Use of opioid medications within three days before colonoscopy was extracted from the medication administration record. These variables were chosen as biologically plausible modifiers of bowel preparation or had previously been assessed in the literature.1-6 The name and volume, classified as 4 L (GoLytely®) and < 4 liters (MoviPrep®) of bowel preparation, time of day when colonoscopy was performed, solid diet the day prior to colonoscopy, type of sedation used (conscious sedation or general anesthesia), and total colonoscopy time (defined as the time from scope insertion to removal) was recorded. Hospitalization-related variables, including the number of hospitalizations in the year before the current hospitalization, the year in which the colonoscopy was performed, and the number of days from admission to colonoscopy, were also obtained from the EHR.
Outcome Measures
An internally validated natural language algorithm, using Structured Queried Language was used to search through colonoscopy reports to identify adequacy of bowel preparation. ProVation® software allows the gastroenterologist to use some terms to describe bowel preparation in a drop-down menu format. In addition to the Aronchik scale (which allows the gastroenterologist to rate bowel preparation on a five-point scale: “excellent,” “good,” “fair,” “poor,” and “inadequate”) it also allows the provider to use terms such as “adequate” or “adequate to detect polyps >5 mm” as well as “unsatisfactory.”7 Mirroring prior literature, bowel preparation quality was classified into “adequate” and “inadequate”; “good” and “excellent” on the Aronchik scale were categorized as adequate as was the term “adequate” in any form; “fair,” “poor,” or “inadequate” on the Aronchik scale were classified as inadequate as was the term “unsatisfactory.” We evaluated the hospital length of stay (LOS) as a secondary outcome measure.
Statistical Analysis
After describing the frequency of IBP, the quality of bowel preparation (adequate vs inadequate) was compared based on the predictors described above. Categorical variables were reported as frequencies with percentages and continuous variables were reported as medians with 25th-75th percentile values. The significance of the difference between the proportion or median values of those who had inadequate versus adequate bowel preparation was assessed. Two-sided chi-square analysis was used to assess the significance of differences between categorical variables and the Wilcoxon Rank-Sum test was used to assess the significance of differences between continuous variables.
Multivariate logistic regression analysis was performed to assess factors associated with hospital predictors and outcomes, after adjusting for all the aforementioned factors and clustering the effect based on the endoscopist. To evaluate the potential impact of modifiable factors on IBP, we performed counterfactual analysis, in which the observed distribution was compared to a hypothetical population in which all the modifiable risk factors were optimal.
RESULTS
Overall, 8,819 patients were included in our study population. They had a median age of 64 [53-76] years; 50.5% were female and 51% had an IBP. Patient characteristics and rates of IBP are presented in Table 1.
In unadjusted analyses, with regards to modifiable factors, opiate use within three days of colonoscopy was associated with a higher rate of IBP (55.4% vs 47.3%, P <.001), as was a lower volume (<4L) bowel preparation (55.3% vs 50.4%, P = .003). IBP was less frequent when colonoscopy was performed before noon vs afternoon (50.3% vs 57.4%, P < .001), and when patients were documented to receive a clear liquid diet or nil per os vs a solid diet the day prior to colonoscopy (50.3% vs 57.4%, P < .001). Overall bowel preparation quality improved over time (Figure 1). Median LOS was five [3-11] days. Patients who had IBP on their initial colonoscopy had a LOS one day longer than patients without IBP (six days vs five days, P < .001).
Multivariate Analysis
Table 2 shows the results of the multivariate analysis. The following modifiable factors were associated with IBP: opiate used within three days of the procedure (OR 1.31; 95% CI 1.8, 1.45), having the colonoscopy performed after12:00
Potential Impact of Modifiable Variables
We conducted a counterfactual analysis based on a multivariate model to assess the impact of each modifiable risk factor on the IBP rate (Figure 1). In the included study population, 44.9% received an opiate, 39.3% had a colonoscopy after 12:00
DISCUSSION
In this large, multihospital cohort, IBP was documented in half (51%) of 8,819 inpatient colonoscopies performed. Nonmodifiable patient characteristics independently associated with IBP were age, male gender, white race, Medicare and Medicaid insurance, nausea/vomiting, dysphagia, and gastroparesis. Modifiable factors included not consuming opiates within three days of colonoscopy, avoidance of a solid diet the day prior to colonoscopy and performing the colonoscopy before noon. The volume of bowel preparation consumed was not associated with IBP. In a counterfactual analysis, we found that if all three modifiable factors were optimized, the predicted rate of IBP would drop to 45%.
Many studies, including our analysis, have shown significant differences between the frequency of IBP in inpatient versus outpatient bowel preparations.8-11 Therefore, it is crucial to study IBP in these settings separately. Three single-institution studies, including a total of 898 patients, have identified risk factors for inpatient IBP. Individual studies ranged in size from 130 to 524 patients with rates of IBP ranging from 22%-57%.1-3 They found IBP to be associated with increasing age, lower income, ASA Grade >3, diabetes, coronary artery disease (CAD), nausea or vomiting, BMI >25, and chronic constipation. Modifiable factors included opiates, afternoon procedures, and runway times >6 hours.
We also found IBP to be associated with increasing age and male gender. However, we found no association with diabetes, chronic constipation, CAD or BMI. As we were able to adjust for a wider variety of variables, it is possible that we were able to account for residual confounding better than previous studies. For example, we found that having nausea/vomiting, dysphagia, and gastroparesis was associated with IBP. Gastroparesis with associated nausea and vomiting may be the mechanism by which diabetes increases the risk for IBP. Further studies are needed to assess if interventions or alternative bowel cleansing in these patients can result in improved IBP. Finally, in contrast to studies with smaller cohorts which found that lower volume bowel preps improved IBP in the right colon,4,12 we found no association between IBP based and volume of bowel preparation consumed. Our impact analysis suggests that avoidance of opiates for at least three days before colonoscopy, avoidance of solid diet on the day before colonoscopy and performing all colonoscopies before noon would
The factors mentioned above may not always be amenable to modification. For example, for patients with active gastrointestinal bleeding, postponing colonoscopy by one day for the sake of maintaining a patient on a clear diet may not be feasible. Similarly, performing colonoscopies in the morning is highly dependent on endoscopy suite availability and hospital logistics. Denying opiates to patients experiencing severe pain is not ethical. In many scenarios, however, these variables could be modified, and institutional efforts to support these practices could yield considerable savings. Future prospective studies are needed to verify the real impact of these changes.
Further discussion is needed to contextualize the finding that colonoscopies scheduled in the afternoon are associated with improved bowel preparation quality. Previous research—albeit in the outpatient setting—has demonstrated 11.8 hours as the maximum upper time limit for the time elapsed between the end of bowel preparation to colonoscopy.14 Another study found an inverse relationship between the quality of bowel preparation and the time after completion of the bowel preparation.15 This makes sense from a physiological perspective as delaying the time between completion of bowel preparation, and the procedure allows chyme from the small intestine to reaccumulate in the colon. Anecdotally, at our institution as well as at many others, the bowel preparations are ordered to start in the evening to allow the consumption of complete bowel preparation by midnight. As a result of this practice, only patients who have their colonoscopies scheduled before noon fall within the optimal period of 11.8 hours. In the outpatient setting, the use of split preparations has led to the obliteration of the difference in the quality of bowel preparation between morning and afternoon colonoscopies.16 Prospective trials are needed to evaluate the use of split preparations to improve the quality of afternoon inpatient colonoscopies.
Few other strategies have been shown to mitigate IBP in the inpatient setting. In a small randomized controlled trial, Ergen et al. found that providing an educational booklet improved inpatient bowel preparation as measured by the Boston Bowel Preparation Scale.17 In a quasi-experimental design, Yadlapati et al. found that an automated split-dose bowel preparation resulted in decreased IBP, fewer repeated procedures, shorter LOS, and lower hospital cost.18 Our study adds to these tools by identifying three additional risk factors which could be optimized for inpatients. Because our findings are observational, they should be subjected to prospective trials. Our study also calls into question the impact of bowel preparation volume. We found no difference in the rate of IBP between low and large volume preparations. It is possible that other factors are more important than the specific preparation employed.
Interestingly, we found that IBP declined substantially in 2014 and continued to decline after that. The year was the most influential risk factor for IBP (on par with gastroparesis). The reason for this is unclear, as rates of our modifiable risk factors did not differ substantially by year. Other possibilities include improved access (including weekend access) to endoscopy coinciding with the development of a new endoscopy facility and use of integrated irrigation pump system instead of the use of manual syringes for flushing.
Our study has many strengths. It is by far the most extensive study of bowel preparation quality in inpatients to date and the only one that has included patient, procedural and bowel preparation characteristics. The study also has several significant limitations. This is a single center study, which could limit generalizability. Nonetheless, it was conducted within a health system with multiple hospitals in different parts of the United States (Ohio and Florida) and included a broad population mix with differing levels of acuity. The retrospective nature of the assessment precludes establishing causation. However, we mitigated confounding by adjusting for a wide variety of factors, and there is a plausible physiological mechanism for each of the factors we studied. Also, the retrospective nature of our study predisposes our data to omissions and misrepresentations during the documentation process. This is especially true with the use of ICD codes.19 Inaccuracies in coding are likely to bias toward the null, so observed associations may be an underestimate of the true association.
Our inability to ascertain if a patient completed the prescribed bowel preparation limited our ability to detect what may be a significant risk factor. Lastly, while clinically relevant, the Aronchik scale used to identify adequate from IBP has never been validated though it is frequently utilized and cited in the bowel preparation literature.20
CONCLUSIONS
In this large retrospective study evaluating bowel preparation quality in inpatients undergoing colonoscopy, we found that more than half of the patients have IBP and that IBP was associated with an extra day of hospitalization. Our study identifies those patients at highest risk and identifies modifiable risk factors for IBP. Specifically, we found that abstinence from opiates or solid diet before the colonoscopy, along with performing colonoscopies before noon were associated with improved outcomes. Prospective studies are needed to confirm the effects of these interventions on bowel preparation quality.
Disclosures
Carol A Burke, MD has received research funding from Ferring Pharmaceuticals. Other authors have no conflicts of interest to disclose.
1. Yadlapati R, Johnston ER, Gregory DL, Ciolino JD, Cooper A, Keswani RN. Predictors of inadequate inpatient colonoscopy preparation and its association with hospital length of stay and costs. Dig Dis Sci. 2015;60(11):3482-3490. doi: 10.1007/s10620-015-3761-2. PubMed
2. Jawa H, Mosli M, Alsamadani W, et al. Predictors of inadequate bowel preparation for inpatient colonoscopy. Turk J Gastroenterol. 2017;28(6):460-464. doi: 10.5152/tjg.2017.17196. PubMed
3. Mcnabb-Baltar J, Dorreen A, Dhahab HA, et al. Age is the only predictor of poor bowel preparation in the hospitalized patient. Can J Gastroenterol Hepatol. 2016;2016:1-5. doi: 10.1155/2016/2139264. PubMed
4. Rotondano G, Rispo A, Bottiglieri ME, et al. Tu1503 Quality of bowel cleansing in hospitalized patients is not worse than that of outpatients undergoing colonoscopy: results of a multicenter prospective regional study. Gastrointest Endosc. 2014;79(5):AB564. doi: 10.1016/j.gie.2014.02.949. PubMed
5. Ness R. Predictors of inadequate bowel preparation for colonoscopy. Am J Gastroenterol. 2001;96(6):1797-1802. doi: 10.1016/s0002-9270(01)02437-6. PubMed
6. Johnson DA, Barkun AN, Cohen LB, et al. Optimizing adequacy of bowel cleansing for colonoscopy: recommendations from the us multi-society task force on colorectal cancer. Gastroenterology. 2014;147(4):903-924. doi: 10.1053/j.gastro.2014.07.002. PubMed
7. Aronchick CA, Lipshutz WH, Wright SH, et al. A novel tableted purgative for colonoscopic preparation: efficacy and safety comparisons with Colyte and Fleet Phospho-Soda. Gastrointest Endosc. 2000;52(3):346-352. doi: 10.1067/mge.2000.108480. PubMed
8. Froehlich F, Wietlisbach V, Gonvers J-J, Burnand B, Vader J-P. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61(3):378-384. doi: 10.1016/s0016-5107(04)02776-2. PubMed
9. Sarvepalli S, Garber A, Rizk M, et al. 923 adjusted comparison of commercial bowel preparations based on inadequacy of bowel preparation in outpatient settings. Gastrointest Endosc. 2018;87(6):AB127. doi: 10.1016/j.gie.2018.04.1331.
10. Hendry PO, Jenkins JT, Diament RH. The impact of poor bowel preparation on colonoscopy: a prospective single center study of 10 571 colonoscopies. Colorectal Dis. 2007;9(8):745-748. doi: 10.1111/j.1463-1318.2007.01220.x. PubMed
11. Lebwohl B, Wang TC, Neugut AI. Socioeconomic and other predictors of colonoscopy preparation quality. Dig Dis Sci. 2010;55(7):2014-2020. doi: 10.1007/s10620-009-1079-7. PubMed
12. Chorev N, Chadad B, Segal N, et al. Preparation for colonoscopy in hospitalized patients. Dig Dis Sci. 2007;52(3):835-839. doi: 10.1007/s10620-006-9591-5. PubMed
13. Weiss AJ. Overview of Hospital Stays in the United States, 2012. HCUP Statistical Brief #180. Rockville, MD: Agency for Healthcare Research and Quality; 2014. PubMed
14. Kojecky V, Matous J, Keil R, et al. The optimal bowel preparation intervals before colonoscopy: a randomized study comparing polyethylene glycol and low-volume solutions. Dig Liver Dis. 2018;50(3):271-276. doi: 10.1016/j.dld.2017.10.010. PubMed
15. Siddiqui AA, Yang K, Spechler SJ, et al. Duration of the interval between the completion of bowel preparation and the start of colonoscopy predicts bowel-preparation quality. Gastrointest Endosc. 2009;69(3):700-706. doi: 10.1016/j.gie.2008.09.047. PubMed
16. Eun CS, Han DS, Hyun YS, et al. The timing of bowel preparation is more important than the timing of colonoscopy in determining the quality of bowel cleansing. Dig Dis Sci. 2010;56(2):539-544. doi: 10.1007/s10620-010-1457-1. PubMed
17. Ergen WF, Pasricha T, Hubbard FJ, et al. Providing hospitalized patients with an educational booklet increases the quality of colonoscopy bowel preparation. Clin Gastroenterol Hepatol. 2016;14(6):858-864. doi: 10.1016/j.cgh.2015.11.015. PubMed
18. Yadlapati R, Johnston ER, Gluskin AB, et al. An automated inpatient split-dose bowel preparation system improves colonoscopy quality and reduces repeat procedures. J Clin Gastroenterol. 2018;52(8):709-714. doi: 10.1097/mcg.0000000000000849. PubMed
19. Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. The accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43(5):480-485. doi: 10.1097/01.mlr.0000160417.39497.a9. PubMed
20. Parmar R, Martel M, Rostom A, Barkun AN. Validated scales for colon cleansing: a systematic review. J Clin Gastroenterol. 2016;111(2):197-204. doi: 10.1038/ajg.2015.417. PubMed
1. Yadlapati R, Johnston ER, Gregory DL, Ciolino JD, Cooper A, Keswani RN. Predictors of inadequate inpatient colonoscopy preparation and its association with hospital length of stay and costs. Dig Dis Sci. 2015;60(11):3482-3490. doi: 10.1007/s10620-015-3761-2. PubMed
2. Jawa H, Mosli M, Alsamadani W, et al. Predictors of inadequate bowel preparation for inpatient colonoscopy. Turk J Gastroenterol. 2017;28(6):460-464. doi: 10.5152/tjg.2017.17196. PubMed
3. Mcnabb-Baltar J, Dorreen A, Dhahab HA, et al. Age is the only predictor of poor bowel preparation in the hospitalized patient. Can J Gastroenterol Hepatol. 2016;2016:1-5. doi: 10.1155/2016/2139264. PubMed
4. Rotondano G, Rispo A, Bottiglieri ME, et al. Tu1503 Quality of bowel cleansing in hospitalized patients is not worse than that of outpatients undergoing colonoscopy: results of a multicenter prospective regional study. Gastrointest Endosc. 2014;79(5):AB564. doi: 10.1016/j.gie.2014.02.949. PubMed
5. Ness R. Predictors of inadequate bowel preparation for colonoscopy. Am J Gastroenterol. 2001;96(6):1797-1802. doi: 10.1016/s0002-9270(01)02437-6. PubMed
6. Johnson DA, Barkun AN, Cohen LB, et al. Optimizing adequacy of bowel cleansing for colonoscopy: recommendations from the us multi-society task force on colorectal cancer. Gastroenterology. 2014;147(4):903-924. doi: 10.1053/j.gastro.2014.07.002. PubMed
7. Aronchick CA, Lipshutz WH, Wright SH, et al. A novel tableted purgative for colonoscopic preparation: efficacy and safety comparisons with Colyte and Fleet Phospho-Soda. Gastrointest Endosc. 2000;52(3):346-352. doi: 10.1067/mge.2000.108480. PubMed
8. Froehlich F, Wietlisbach V, Gonvers J-J, Burnand B, Vader J-P. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61(3):378-384. doi: 10.1016/s0016-5107(04)02776-2. PubMed
9. Sarvepalli S, Garber A, Rizk M, et al. 923 adjusted comparison of commercial bowel preparations based on inadequacy of bowel preparation in outpatient settings. Gastrointest Endosc. 2018;87(6):AB127. doi: 10.1016/j.gie.2018.04.1331.
10. Hendry PO, Jenkins JT, Diament RH. The impact of poor bowel preparation on colonoscopy: a prospective single center study of 10 571 colonoscopies. Colorectal Dis. 2007;9(8):745-748. doi: 10.1111/j.1463-1318.2007.01220.x. PubMed
11. Lebwohl B, Wang TC, Neugut AI. Socioeconomic and other predictors of colonoscopy preparation quality. Dig Dis Sci. 2010;55(7):2014-2020. doi: 10.1007/s10620-009-1079-7. PubMed
12. Chorev N, Chadad B, Segal N, et al. Preparation for colonoscopy in hospitalized patients. Dig Dis Sci. 2007;52(3):835-839. doi: 10.1007/s10620-006-9591-5. PubMed
13. Weiss AJ. Overview of Hospital Stays in the United States, 2012. HCUP Statistical Brief #180. Rockville, MD: Agency for Healthcare Research and Quality; 2014. PubMed
14. Kojecky V, Matous J, Keil R, et al. The optimal bowel preparation intervals before colonoscopy: a randomized study comparing polyethylene glycol and low-volume solutions. Dig Liver Dis. 2018;50(3):271-276. doi: 10.1016/j.dld.2017.10.010. PubMed
15. Siddiqui AA, Yang K, Spechler SJ, et al. Duration of the interval between the completion of bowel preparation and the start of colonoscopy predicts bowel-preparation quality. Gastrointest Endosc. 2009;69(3):700-706. doi: 10.1016/j.gie.2008.09.047. PubMed
16. Eun CS, Han DS, Hyun YS, et al. The timing of bowel preparation is more important than the timing of colonoscopy in determining the quality of bowel cleansing. Dig Dis Sci. 2010;56(2):539-544. doi: 10.1007/s10620-010-1457-1. PubMed
17. Ergen WF, Pasricha T, Hubbard FJ, et al. Providing hospitalized patients with an educational booklet increases the quality of colonoscopy bowel preparation. Clin Gastroenterol Hepatol. 2016;14(6):858-864. doi: 10.1016/j.cgh.2015.11.015. PubMed
18. Yadlapati R, Johnston ER, Gluskin AB, et al. An automated inpatient split-dose bowel preparation system improves colonoscopy quality and reduces repeat procedures. J Clin Gastroenterol. 2018;52(8):709-714. doi: 10.1097/mcg.0000000000000849. PubMed
19. Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. The accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43(5):480-485. doi: 10.1097/01.mlr.0000160417.39497.a9. PubMed
20. Parmar R, Martel M, Rostom A, Barkun AN. Validated scales for colon cleansing: a systematic review. J Clin Gastroenterol. 2016;111(2):197-204. doi: 10.1038/ajg.2015.417. PubMed
© 2019 Society of Hospital Medicine
Gallstones: Watch and wait, or intervene?
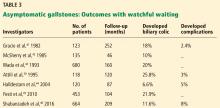
The prevalence of gallstones is approximately 10% to 15% of the adult US population.1,2 Most cases are asymptomatic, as gallstones are usually discovered incidentally during routine imaging for other abdominal conditions, and only about 20% of patients with asymptomatic gallstones develop clinically significant complications.2,3
Nevertheless, gallstones carry significant healthcare costs. In 2004, the median inpatient cost for any gallstone-related disease was $11,584, with an overall annual cost of $6.2 billion.4,5
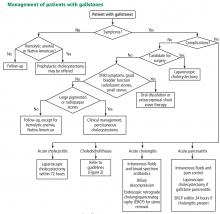
Laparoscopic cholecystectomy is the standard treatment for symptomatic cholelithiasis. For asymptomatic cholelithasis, the usual approach is expectant management (“watch and wait”), but prophylactic cholecystectomy may be an option in certain patients at high risk.
CHEMICAL COMPOSITION
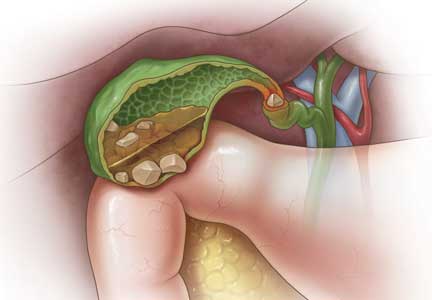
Gallstones can be classified into 2 main categories based on their predominant chemical composition: cholesterol or pigment.
Cholesterol gallstones
About 75% of gallstones are composed of cholesterol.3,4 In the past, this type of stone was thought to be caused by gallbladder inflammation, bile stasis, and absorption of bile salts from damaged mucosa. However, it is now known that cholesterol gallstones are the result of biliary supersaturation caused by cholesterol hypersecretion into the gallbladder, gallbladder hypomotility, accelerated cholesterol nucleation and crystallization, and mucin gel accumulation.
Pigment gallstones
Black pigment gallstones account for 10% to 15% of all gallstones.6 They are caused by chronic hemolysis in association with supersaturation of bile with calcium hydrogen bilirubinate, along with deposition of calcium carbonate, phosphate, and inorganic salts.7
Brown pigment stones, accounting for 5% to 10% of all gallstones,6 are caused by infection in the obstructed bile ducts, where bacteria that produce beta-glucuronidase, phospholipase, and slime contribute to formation of the stone.8,9
RISK FACTORS FOR GALLSTONES
Age. After age 40, the risk increases dramatically, with an incidence 4 times higher for those ages 40 to 69 than in younger people.10
Female sex. Women of reproductive age are 4 times more likely to develop gallstones than men, but this gap narrows after menopause.11 The higher risk is attributed to female sex hormones, pregnancy, and oral contraceptive use. Estrogen decreases secretion of bile salts and increases secretion of cholesterol into the gallbladder, which leads to cholesterol supersaturation. Progesterone acts synergistically by causing hypomobility of the gallbladder, which in turn leads to bile stasis.12,13
Ethnicity. The risk is higher in Mexican Americans and Native Americans than in other ethnic groups.14
Rapid weight loss, such as after bariatric surgery, occurs from decreased caloric intake and promotes bile stasis, while lipolysis increases cholesterol mobilization and secretion into the gallbladder. This creates an environment conducive to bile supersaturation with cholesterol, leading to gallstone formation.
Chronic hemolytic disorders carry an increased risk of developing calcium bilirubinate stones due to increased excretion of bilirubin during hemolysis.
Obesity and diabetes mellitus are both attributed to insulin resistance. Obesity also increases bile stasis and cholesterol saturation.
CLINICAL PRESENTATION OF GALLSTONES (CHOLELITHIASIS)
Most patients with gallstones (cholelithiasis) experience no symptoms. Their gallstones are often discovered incidentally during imaging tests for unrelated or unexplained abdominal symptoms. Most patients with asymptomatic gallstones remain symptom-free, while about 20% develop gallstone-related symptoms.2,3
Abdominal pain is the most common symptom. The phrase biliary colic—suggesting pain that is fluctuating in nature—appears ubiquitously in the medical literature, but it does not correctly characterize the pain associated with gallstones.
Most patients with gallstone symptoms describe a constant and often severe pain in the right upper abdomen, epigastrium, or both, often persisting for 30 to 120 minutes. Symptoms are frequently reported in the epigastrium when only visceral pain fibers are stimulated due to gallbladder distention. This is usually called midline pain; however, pain occurs in the back and right shoulder in up to 60% of patients, with involvement of somatic fibers.15,16 Gallstone pain is not relieved by change of position or passage of stool or gas.
Onset of symptoms more than an hour after eating or in the late evening or at night also very strongly suggests biliary pain. Patients with a history of biliary pain are more likely to experience it again, with a 69% chance of developing recurrent pain within 2 years.17
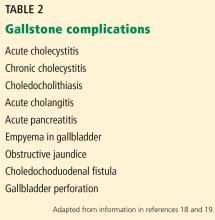
GALLSTONE-RELATED COMPLICATIONS
Acute gallbladder inflammation (cholecystitis)
Gallbladder inflammation (cholecystitis) is the most common complication, occurring in up to 10% of symptomatic cases. Many patients with acute cholecystitis present with right upper quadrant pain that may be accompanied by anorexia, nausea, or vomiting. Inspiratory arrest on deep palpation of the right upper quadrant (Murphy sign) has a specificity of 79% to 96% for acute cholecystitis.20 Markers of systemic inflammation such as fever, elevated white blood cell count, and elevated C-reactive protein are highly suggestive of acute cholecystitis.20,21
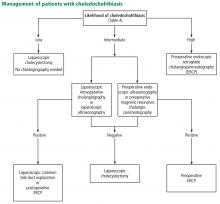
Bile duct stones (choledocholithiasis)
Bile duct stones (choledocholithiasis) are detected in 3.4% to 12% of patients with gallstones.22,23 Most stones in the common bile duct migrate there from the gallbladder via the cystic duct. Less commonly, primary duct stones form in the duct due to biliary stasis. Removing the gallbladder does not completely eliminate the risk of bile duct stones, as stones can remain or recur after surgery.
Bile duct stones can obstruct the common bile duct, which disrupts normal bile flow and leads to jaundice. Other symptoms may include pruritus, right upper quadrant pain, nausea, and vomiting. Serum levels of bilirubin, aspartate aminotransferase, alanine aminotransferase (ALT), and alkaline phosphatase are usually high.24
Acute bacterial infection (cholangitis)
Acute bacterial infection of the biliary system (cholangitis) is usually associated with obstruction of the common bile duct. Common symptoms of acute cholangitis include right upper quadrant pain, fever, and jaundice (Charcot triad), and these are present in about 50% to 75% of cases.21 In severe cases, patients can develop altered mental status and septicemic shock in addition to the Charcot triad, a condition called the Reynold pentad. White blood cell counts and serum levels of C-reactive protein, bilirubin, aminotransferases, and alkaline phosphatase are usually elevated.21
Pancreatitis
Approximately 4% to 8% of patients with gallstones develop inflammation of the pancreas (pancreatitis).25 The diagnosis of acute pancreatitis requires at least 2 of the following:26,27
- Abdominal pain (typically epigastric, often radiating to the back)
- Amylase or lipase levels at least 3 times above the normal limit
- Imaging findings that suggest acute pancreatitis.
Gallstone-related pancreatitis should be considered if the ALT level is greater than 150 U/mL, which has a 97% specificity for gallstone-related pancreatitis.28
ABDOMINAL ULTRASONOGRAPHY FOR DIAGNOSIS
Transabdominal ultrasonography, with a sensitivity of 84% to 89% and a specificity of up to 99%, is the test of choice for detecting gallstones.29 The characteristic findings of acute cholecystitis on ultrasonography include enlargement of the gallbladder, thickening of the gallbladder wall, presence of pericholecystic fluid, and tenderness elicited by the ultrasound probe over the gallbladder (sonographic Murphy sign).
Scintigraphy as a second test
Acute cholecystitis is primarily a clinical diagnosis and typically does not require additional imaging beyond ultrasonography. When there is discordance between clinical and ultrasonographic findings, the most accurate second imaging test is scintigraphy of the biliary tract, usually performed with technetium-labeled hydroxy iminodiacetic acid. Given intravenously, the radionuclide is rapidly taken up by the liver and then secreted into the bile. In acute cholecystitis, the cystic duct is functionally occluded and the isotope does not enter the gallbladder, creating an imaging void compared with a normal appearance.
Scintigraphy is more sensitive than abdominal ultrasonography, with a sensitivity of up to 97% vs 81% to 88%, respectively.29,30 The tests have about equal specificity.
Even though scintigraphy is more sensitive, abdominal ultrasonography is often the initial test for patients with suspected acute cholecystitis because it is more widely available, takes less time, does not involve radiation exposure, and can assess for the presence or absence of gallstones and dilation of the intra- and extrahepatic bile ducts.
Looking for stones in the common bile duct
When acute cholangitis due to choledocholithiasis is suspected, abdominal ultrasonography is a prudent initial test to look for gallstones or biliary dilation suggesting obstruction by stones in the common bile duct. Abdominal ultrasonography has only a 22% to 55% sensitivity for visualizing stones in the common bile duct, but it has a 77% to 87% sensitivity for detecting common bile duct dilation, a surrogate marker of stones.31
The normal bile duct diameter ranges from 3 to 6 mm, although mild dilation is often seen in older patients or after cholecystectomy or Roux-en-Y gastric bypass surgery.32,33 Bile duct dilation of up to 10 mm can be considered normal in patients after cholecystectomy.34 A normal-appearing bile duct on ultrasonography has a negative predictive value of 95% for excluding common bile duct stones.31
Endoscopic ultrasonography (EUS), magnetic resonance cholangiopancreatography (MRCP), and endoscopic retrograde cholangiopancreatography (ERCP) have similar sensitivity (89%–94%, 85%–92%, and 89%–93%, respectively) and specificity (94%–95%, 93%–97%, and 100%, respectively) for detecting common bile duct stones.35–37 EUS is superior to MRCP in detecting stones smaller than 6 mm.38
ERCP should be reserved for managing rather than diagnosing common bile duct stones because of the risk of pancreatitis and perforation. Patients undergoing cholecystectomy who are suspected of having choledocholithiasis may undergo intraoperative cholangiography or laparoscopic common bile duct ultrasonography.
WATCH AND WAIT, OR INTERVENE?
Asymptomatic gallstones
Standard treatment for these patients is expectant management. Cholecystectomy is not recommended for patients with asymptomatic gallstones.47 Nevertheless, some patients may benefit from prophylactic cholecystectomy. We and others48 suggest considering cholecystectomy in the following patients.
Patients with chronic hemolytic anemia (including children with sickle cell anemia and spherocytosis). These patients have a higher risk of developing calcium bilirubinate stones, and cholecystectomy has improved outcomes.49 It should be noted that most of these data come from pediatric populations and have been extrapolated to adults.
Native Americans, who have a higher risk of gallbladder cancer if they have gallstones.2,50
Conversely, calcification of the gallbladder wall (“porcelain gallbladder”) is no longer considered an absolute indication for cholecystectomy. This condition was thought to be associated with a high rate of gallbladder carcinoma, but analyses of larger, more recent data sets found much smaller risks.51,52 Further, cholecystectomy in these patients was found to be associated with high rates of postoperative complications. Thus, prophylactic cholecystectomy is no longer recommended in asymptomatic cases of porcelain gallbladder.
In addition, concomitant cholecystectomy in patients undergoing bariatric surgery is no longer considered the therapeutic standard. Historically, cholecystectomy was performed in these patients because of the increased risk of gallstones associated with rapid weight loss after surgery. However, research now weighs against concomitant cholecystectomy with bariatric surgery and most other abdominal surgeries for asymptomatic gallstones.53
Laparoscopic surgery for symptomatic gallstones
For patients experiencing acute cholecystitis, laparoscopic cholecystectomy within 72 hours is recommended.48 There were safety concerns regarding higher rates of morbidity and conversion from laparoscopic to open cholecystectomy in patients who underwent surgery before the acute cholecystitis episode had settled. However, a large meta-analysis found no significant difference between early and delayed laparoscopic cholecystectomy in bile duct injury or conversion rates.54 Further, early cholecystectomy—defined as within 1 week of symptom onset—has been found to reduce gallstone-related complications, shorten hospital stays, and lower costs.55–57 If the patient cannot undergo surgery, percutaneous cholecystotomy or novel endoscopic gallbladder drainage interventions can be used.
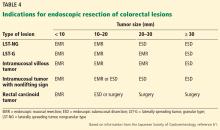
Several variables predict the presence of bile duct stones in patients who have symptoms (Table 4). Based on these predictors, the ASGE classifies the probabilities as low (< 10%), intermediate (10% to 50%), and high (> 50%)31:
- High-risk patients should undergo preoperative ERCP and stone extraction if needed
- Intermediate-risk patients should undergo preoperative imaging with EUS or MRCP or intraoperative bile duct evaluation, depending on the availability, costs, and local expertise.
Patients with associated cholangitis should be given intravenous fluids and broad-spectrum antibiotics. Biliary decompression should be done as early as possible to decrease the risk of morbidity and mortality. For acute cholangitis, ERCP is the treatment of choice.25
Patients with acute gallstone pancreatitis should receive conservative management with intravenous isotonic solutions and pain control, followed by laparoscopic cholecystectomy.48
The timing of laparoscopic cholecystectomy in acute gallstone pancreatitis has been debated. Studies conducted during the era of open cholecystectomy reported similar or worse outcomes if cholecystectomy was done sooner rather than later.
However, in 1999, Uhl et al58 reported that 48 of 77 patients admitted with acute gallstone pancreatitis were able to undergo laparoscopic cholecystectomy during the same admission. Success rates were 85% (30 of 35 patients) in those with mild disease and 62% (8 of 13 patients) in those with severe disease. They concluded laparoscopic cholecystectomy could be safely performed within 7 days in patients with mild disease, whereas in severe disease at least 3 weeks should elapse because of the risk of infection.
In a randomized trial published in 2010, Aboulian et al59 reported that hospital length of stay (the primary end point) was shorter in 25 patients who underwent laparoscopic cholecystectomy early (within 48 hours of admission) than in 25 patients who underwent surgery after abdominal pain had resolved and laboratory enzymes showed a normalizing trend, 3.5 vs 5.8 days (P = .0016). Rates of perioperative complications and need for conversion to open surgery were similar between the 2 groups.
If there is associated cholangitis, patients should also be given broad-spectrum antibiotics and should undergo ERCP within 24 hours of admission.25–27
SUMMARY
Gallstones are common in US adults. Abdominal ultrasonography is the diagnostic imaging test of choice to detect gallbladder stones and assess for findings suggestive of acute cholecystitis and dilation of the common bile duct. Fortunately, most gallstones are asymptomatic and can usually be managed expectantly. In patients who have symptoms or have gallstone complications, laparoscopic cholecystectomy is the standard of care.
- Schirmer BD, Winters KL, Edlich RF. Cholelithiasis and cholecystitis. J Long Term Eff Med Implants 2005; 15(3):329–338. doi:10.1615/JLongTermEffMedImplants.v15.i3.90
- Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver 2012; 6(2):172–187. doi:10.5009/gnl.2012.6.2.172
- Lee JY, Keane MG, Pereira S. Diagnosis and treatment of gallstone disease. Practitioner 2015; 259(1783):15–19.
- Russo MW, Wei JT, Thiny MT, et al. Digestive and liver diseases statistics, 2004. Gastroenterology 2004; 126(5):1448–1453. doi:10.1053/j.gastro.2004.01.025
- Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology 2009; 136(2):376–386. doi:10.1053/j.gastro.2008.12.015
- Cariati A. Gallstone classification in Western countries. Indian J Surg 2015; 77(suppl 2):376–380. doi.org/10.1007/s12262-013-0847-y
- Carey MC. Pathogenesis of gallstones. Am J Surg 1993; 165(4):410–419. doi:10.1016/S0002-9610(05)80932-8
- Lammert F, Gurusamy K, Ko CW, et al. Gallstones. Nat Rev Dis Primers 2016; 2:16024. doi:10.1038/nrdp.2016.24
- Stewart L, Oesterle AL, Erdan I, Griffiss JM, Way LW. Pathogenesis of pigment gallstones in Western societies: the central role of bacteria. J Gastrointest Surg 2002; 6(6):891–904.
- Barbara L, Sama C, Morselli Labate AM, et al. A population study on the prevalence of gallstone disease: the Sirmione Study. Hepatology 1987; 7(5):913–917. doi:10.1002/hep.1840070520
- Sood S, Winn T, Ibrahim S, et al. Natural history of asymptomatic gallstones: differential behaviour in male and female subjects. Med J Malaysia 2015; 70(6):341–345.
- Maringhini A, Ciambra M, Baccelliere P, et al. Biliary sludge and gallstones in pregnancy: incidence, risk factors, and natural history. Ann Intern Med 1993; 119(2):116–120. doi:10.7326/0003-4819-119-2-199307150-00004
- Etminan M, Delaney JA, Bressler B, Brophy JM. Oral contraceptives and the risk of gallbladder disease: a comparative safety study. CMAJ 2011; 183(8):899–904. doi:10.1503/cmaj.110161
- Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology 1999; 117(3):632–639.
- Festi D, Sottili S, Colecchia A, et al. Clinical manifestations of gallstone disease: evidence from the multicenter Italian study on cholelithiasis (MICOL). Hepatology 1999; 30(4):839–846. doi:10.1002/hep.510300401
- Berhane T, Vetrhus M, Hausken T, Olafsson S, Sondenaa K. Pain attacks in non-complicated and complicated gallstone disease have a characteristic pattern and are accompanied by dyspepsia in most patients: the results of a prospective study. Scand J Gastroenterol 2006; 41(1):93–101. doi:10.1080/00365520510023990
- Thistle JL, Cleary PA, Lachin JM, Tyor MP, Hersh T. The natural history of cholelithiasis: the National Cooperative Gallstone Study. Ann Intern Med 1984; 101(2):171–175. doi:10.7326/0003-4819-101-2-171
- Friedman GD. Natural history of asymptomatic and symptomatic gallstones. Am J Surg 1993; 165(4):399–404. doi:0.1016/S0002-9610(05)80930-4
- Friedman GD, Raviola CA, Fireman B. Prognosis of gallstones with mild or no symptoms: 25 years of follow-up in a health maintenance organization. J Clin Epidemiol 1989; 42(2):127–136. doi:10.1016/0895-4356(89)90086-3
- Hirota M, Takada T, Kawarada Y, et al. Diagnostic criteria and severity assessment of acute cholecystitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg 2007; 14(1):78–82. doi:10.1007/s00534-006-1159-4
- Miura F, Takada T, Kawarada Y, et al. Flowcharts for the diagnosis and treatment of acute cholangitis and cholecystitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg 2007; 14(1):27–34. doi:10.1007/s00534-006-1153-x
- Koo KP, Traverso LW. Do preoperative indicators predict the presence of common bile duct stones during laparoscopic cholecystectomy? Am J Surg 1996; 171(5):495–499. doi:10.1016/S0002-9610(97)89611-0
- Collins C, Maguire D, Ireland A, Fitzgerald E, O’Sullivan GC. A prospective study of common bile duct calculi in patients undergoing laparoscopic cholecystectomy: natural history of choledocholithiasis revisited. Ann Surg 2004; 239(1):28–33. doi:10.1097/01.sla.0000103069.00170.9c
- Costi R, Gnocchi A, Di Mario F, Sarli L. Diagnosis and management of choledocholithiasis in the golden age of imaging, endoscopy and laparoscopy. World J Gastroenterol 2014; 20(37):13382–13401. doi:10.3748/wjg.v20.i37.13382
- European Association for the Study of the Liver (EASL). EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. J Hepatol 2016; 65(1):146–181. doi:10.1016/j.jhep.2016.03.005
- Greenberg JA, Hsu J, Bawazeer M, et al. Clinical practice guideline: management of acute pancreatitis. Can J Surg 2016; 59 (2):128–140. doi:10.1503/cjs.015015
- Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013; 108(9):1400–1416. doi:10.1038/ajg.2013.218
- Moolla Z, Anderson F, Thomson SR. Use of amylase and alanine transaminase to predict acute gallstone pancreatitis in a population with high HIV prevalence. World J Surg 2013; 37(1):156–161. doi:10.1007/s00268-012-1801-z
- Shea JA, Berlin JA, Escarce JJ, et al. Revised estimates of diagnostic test sensitivity and specificity in suspected biliary tract disease. Arch Intern Med 1994; 154(22):2573–2581. doi:10.1001/archinte.1994.00420220069008
- Kiewiet JJ, Leeuwenburgh MM, Bipat S, et al. A systematic review and meta-analysis of diagnostic performance of imaging in acute cholecystitis. Radiology 2012; 264(3):708–720. doi:10.1148/radiol.12111561
- ASGE Standards of Practice Committee; Maple JT, Ben-Menachem T, Anderson MA, et al. The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointest Endosc 2010; 71(1):1–9. doi:10.1016/j.gie.2009.09.041
- Bachar GN, Cohen M, Belenky A, Atar E, Gideon S. Effect of aging on the adult extrahepatic bile duct: a sonographic study. J Ultrasound Med 2003; 22(9):879–885. doi:10.7863/jum.2003.22.9.879
- El-Hayek K, Timratana P, Meranda J, Shimizu H, Eldar S, Chand B. Post Roux-en-Y gastric bypass biliary dilation: natural process or significant entity? J Gastrointest Surg 2012; 16(12):2185–2189. doi:10.1007/s11605-012-2058-4
- Park SM, Kim WS, Bae IH, et al. Common bile duct dilatation after cholecystectomy: a one-year prospective study. J Korean Surg Soc 2012; 83(2):97–101. doi:10.4174/jkss.2012.83.2.97
- Tse F, Liu L, Barkun AN, Armstrong D, Moayyedi P. EUS: a meta-analysis of test performance in suspected choledocholithiasis. Gastrointest Endosc 2008; 67(2):235–244. doi:10.1016/j.gie.2007.09.047
- Verma D, Kapadia A, Eisen GM, Adler DG. EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc 2006; 64(2):248–254. doi:10.1016/j.gie.2005.12.038
- Tseng LJ, Jao YT, Mo LR, Lin RC. Over-the-wire US catheter probe as an adjunct to ERCP in the detection of choledocholithiasis. Gastrointest Endosc 2001; 54(6):720–723. doi:10.1067/mge.2001.119255
- Kondo S, Isayama H, Akahane M, et al. Detection of common bile duct stones: comparison between endoscopic ultrasonography, magnetic resonance cholangiography, and helical-computed-tomographic cholangiography. Eur J Radiol 2005; 54(2):271–275. doi:10.1016/j.ejrad.2004.07.007
- Attili AF, De Santis A, Capri R, Repice AM, Maselli S. The natural history of gallstones: the GREPCO experience. The GREPCO Group. Hepatology 1995; 21(3):656–660. doi:10.1016/0270-9139(95)90514-6
- Sakorafas GH, Milingos D, Peros G. Asymptomatic cholelithiasis: is cholecystectomy really needed? A critical reappraisal 15 years after the introduction of laparoscopic cholecystectomy. Dig Dis Sci 2007; 52(5):1313–1325. doi:10.1007/s10620-006-9107-3
- Gracie WA, Ransohoff DF. The natural history of silent gallstones: the innocent gallstone is not a myth. N Engl J Med 1982; 307(13):798–800. doi:10.1056/NEJM198209233071305
- McSherry CK, Ferstenberg H, Calhoun WF, Lahman E, Virshup M. The natural history of diagnosed gallstone disease in symptomatic and asymptomatic patients. Ann Surg 1985; 202(1):59–63. doi:10.1097/00000658-198507000-00009
- Wada K, Wada K, Imamura T. Natural course of asymptomatic gallstone disease. Nihon Rinsho 1993; 51(7):1737–1743. Japanese.
- Halldestam I, Enell EL, Kullman E, Borch K. Development of symptoms and complications in individuals with asymptomatic gallstones. Br J Surg 2004; 91(6):734–738. doi:10.1002/bjs.4547
- Festi D, Reggiani ML, Attili AF, et al. Natural history of gallstone disease: expectant management or active treatment? Results from a population-based cohort study. J Gastroenterol Hepatol 2010; 25(4):719–724. doi:10.1111/j.1440-1746.2009.06146.x
- Shabanzadeh DM, Sorensen LT, Jorgensen T. A prediction rule for risk stratification of incidentally discovered gallstones: results from a large cohort study. Gastroenterology 2016; 150(1):156–167e1. doi:10.1053/j.gastro.2015.09.002
- Overby DW, Apelgren KN, Richardson W, Fanelli R; Society of American Gastrointestinal and Endoscopic Surgeons. SAGES guidelines for the clinical application of laparoscopic biliary tract surgery. Surg Endosc 2010; 24(10):2368–2386. doi:10.1007/s00464-010-1268-7
- Abraham S, Rivero HG, Erlikh IV, Griffith LF, Kondamudi VK. Surgical and nonsurgical management of gallstones. Am Fam Physician 2014; 89(10):795–802.
- Currò G,, Iapichino G, Lorenzini C, Palmeri R, Cucinotta E. Laparoscopic cholecystectomy in children with chronic hemolytic anemia. Is the outcome related to the timing of the procedure? Surg Endosc 2006; 20(2):252–255. doi:10.1007/s00464-005-0318-z
- Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol 2014; 6:99–109. doi:10.2147/CLEP.S37357
- Chen GL, Akmal Y, DiFronzo AL, Vuong B, O’Connor V. Porcelain gallbladder: no longer an indication for prophylactic cholecystectomy. Am Surg 2015; 81(10):936–940.
- Schnelldorfer T. Porcelain gallbladder: a benign process or concern for malignancy? J Gastrointest Surg 2013; 17(6):1161–1168. doi:10.1007/s11605-013-2170-0
- Warschkow R, Tarantino I, Ukegjini K, et al. Concomitant cholecystectomy during laparoscopic Roux-en-Y gastric bypass in obese patients is not justified: a meta-analysis. Obes Surg 2013; 23(3)3979–408. doi:10.1007/s11695-012-0852-4
- Gurusamy K, Samraj K, Gluud C, Wilson E, Davidson BR. Meta-analysis of randomized controlled trials on the safety and effectiveness of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Br J Surg 2010; 97(2):141–150. doi:10.1002/bjs.6870
- Papi C, Catarci M, D’Ambrosio L, et al. Timing of cholecystectomy for acute calculous cholecystitis: a meta-analysis. Am J Gastroenterol 2004; 99(1):147–155. doi:10.1046/j.1572-0241.2003.04002.x
- Gurusamy KS, Davidson C, Gluud C, Davidson BR. Early versus delayed laparoscopic cholecystectomy for people with acute cholecystitis. Cochrane Database Syst Rev 2013; 6:CD005440. doi:10.1002/14651858
- Menahem B, Mulliri A, Fohlen A, Guittet L, Alves A, Lubrano J. Delayed laparoscopic cholecystectomy increases the total hospital stay compared to an early laparoscopic cholecystectomy after acute cholecystitis: an updated meta-analysis of randomized controlled trials. HPB (Oxford) 2015; 17(10):857–862. doi:10.1111/hpb.12449
- Uhl W, Müller CA, Krähenbühl L, Schmid SW, Schölzel S, Büchler MW. Acute gallstone pancreatitis: timing of laparoscopic cholecystectomy in mild and severe disease. Surg Endosc 1999; 13(11):1070–1076. doi:10.1007/s004649901175
- Aboulian A, Chan T, Yaghoubian A, et al. Early cholecystectomy safely decreases hospital stay in patients with mild gallstone pancreatitis: a randomized prospective study. Ann Surg 2010(4): 251:615–619. doi:10.1097/SLA.0b013e3181c38f1f
The prevalence of gallstones is approximately 10% to 15% of the adult US population.1,2 Most cases are asymptomatic, as gallstones are usually discovered incidentally during routine imaging for other abdominal conditions, and only about 20% of patients with asymptomatic gallstones develop clinically significant complications.2,3
Nevertheless, gallstones carry significant healthcare costs. In 2004, the median inpatient cost for any gallstone-related disease was $11,584, with an overall annual cost of $6.2 billion.4,5
Laparoscopic cholecystectomy is the standard treatment for symptomatic cholelithiasis. For asymptomatic cholelithasis, the usual approach is expectant management (“watch and wait”), but prophylactic cholecystectomy may be an option in certain patients at high risk.
CHEMICAL COMPOSITION
Gallstones can be classified into 2 main categories based on their predominant chemical composition: cholesterol or pigment.
Cholesterol gallstones
About 75% of gallstones are composed of cholesterol.3,4 In the past, this type of stone was thought to be caused by gallbladder inflammation, bile stasis, and absorption of bile salts from damaged mucosa. However, it is now known that cholesterol gallstones are the result of biliary supersaturation caused by cholesterol hypersecretion into the gallbladder, gallbladder hypomotility, accelerated cholesterol nucleation and crystallization, and mucin gel accumulation.
Pigment gallstones
Black pigment gallstones account for 10% to 15% of all gallstones.6 They are caused by chronic hemolysis in association with supersaturation of bile with calcium hydrogen bilirubinate, along with deposition of calcium carbonate, phosphate, and inorganic salts.7
Brown pigment stones, accounting for 5% to 10% of all gallstones,6 are caused by infection in the obstructed bile ducts, where bacteria that produce beta-glucuronidase, phospholipase, and slime contribute to formation of the stone.8,9
RISK FACTORS FOR GALLSTONES
Age. After age 40, the risk increases dramatically, with an incidence 4 times higher for those ages 40 to 69 than in younger people.10
Female sex. Women of reproductive age are 4 times more likely to develop gallstones than men, but this gap narrows after menopause.11 The higher risk is attributed to female sex hormones, pregnancy, and oral contraceptive use. Estrogen decreases secretion of bile salts and increases secretion of cholesterol into the gallbladder, which leads to cholesterol supersaturation. Progesterone acts synergistically by causing hypomobility of the gallbladder, which in turn leads to bile stasis.12,13
Ethnicity. The risk is higher in Mexican Americans and Native Americans than in other ethnic groups.14
Rapid weight loss, such as after bariatric surgery, occurs from decreased caloric intake and promotes bile stasis, while lipolysis increases cholesterol mobilization and secretion into the gallbladder. This creates an environment conducive to bile supersaturation with cholesterol, leading to gallstone formation.
Chronic hemolytic disorders carry an increased risk of developing calcium bilirubinate stones due to increased excretion of bilirubin during hemolysis.
Obesity and diabetes mellitus are both attributed to insulin resistance. Obesity also increases bile stasis and cholesterol saturation.
CLINICAL PRESENTATION OF GALLSTONES (CHOLELITHIASIS)
Most patients with gallstones (cholelithiasis) experience no symptoms. Their gallstones are often discovered incidentally during imaging tests for unrelated or unexplained abdominal symptoms. Most patients with asymptomatic gallstones remain symptom-free, while about 20% develop gallstone-related symptoms.2,3
Abdominal pain is the most common symptom. The phrase biliary colic—suggesting pain that is fluctuating in nature—appears ubiquitously in the medical literature, but it does not correctly characterize the pain associated with gallstones.
Most patients with gallstone symptoms describe a constant and often severe pain in the right upper abdomen, epigastrium, or both, often persisting for 30 to 120 minutes. Symptoms are frequently reported in the epigastrium when only visceral pain fibers are stimulated due to gallbladder distention. This is usually called midline pain; however, pain occurs in the back and right shoulder in up to 60% of patients, with involvement of somatic fibers.15,16 Gallstone pain is not relieved by change of position or passage of stool or gas.
Onset of symptoms more than an hour after eating or in the late evening or at night also very strongly suggests biliary pain. Patients with a history of biliary pain are more likely to experience it again, with a 69% chance of developing recurrent pain within 2 years.17
GALLSTONE-RELATED COMPLICATIONS
Acute gallbladder inflammation (cholecystitis)
Gallbladder inflammation (cholecystitis) is the most common complication, occurring in up to 10% of symptomatic cases. Many patients with acute cholecystitis present with right upper quadrant pain that may be accompanied by anorexia, nausea, or vomiting. Inspiratory arrest on deep palpation of the right upper quadrant (Murphy sign) has a specificity of 79% to 96% for acute cholecystitis.20 Markers of systemic inflammation such as fever, elevated white blood cell count, and elevated C-reactive protein are highly suggestive of acute cholecystitis.20,21
Bile duct stones (choledocholithiasis)
Bile duct stones (choledocholithiasis) are detected in 3.4% to 12% of patients with gallstones.22,23 Most stones in the common bile duct migrate there from the gallbladder via the cystic duct. Less commonly, primary duct stones form in the duct due to biliary stasis. Removing the gallbladder does not completely eliminate the risk of bile duct stones, as stones can remain or recur after surgery.
Bile duct stones can obstruct the common bile duct, which disrupts normal bile flow and leads to jaundice. Other symptoms may include pruritus, right upper quadrant pain, nausea, and vomiting. Serum levels of bilirubin, aspartate aminotransferase, alanine aminotransferase (ALT), and alkaline phosphatase are usually high.24
Acute bacterial infection (cholangitis)
Acute bacterial infection of the biliary system (cholangitis) is usually associated with obstruction of the common bile duct. Common symptoms of acute cholangitis include right upper quadrant pain, fever, and jaundice (Charcot triad), and these are present in about 50% to 75% of cases.21 In severe cases, patients can develop altered mental status and septicemic shock in addition to the Charcot triad, a condition called the Reynold pentad. White blood cell counts and serum levels of C-reactive protein, bilirubin, aminotransferases, and alkaline phosphatase are usually elevated.21
Pancreatitis
Approximately 4% to 8% of patients with gallstones develop inflammation of the pancreas (pancreatitis).25 The diagnosis of acute pancreatitis requires at least 2 of the following:26,27
- Abdominal pain (typically epigastric, often radiating to the back)
- Amylase or lipase levels at least 3 times above the normal limit
- Imaging findings that suggest acute pancreatitis.
Gallstone-related pancreatitis should be considered if the ALT level is greater than 150 U/mL, which has a 97% specificity for gallstone-related pancreatitis.28
ABDOMINAL ULTRASONOGRAPHY FOR DIAGNOSIS
Transabdominal ultrasonography, with a sensitivity of 84% to 89% and a specificity of up to 99%, is the test of choice for detecting gallstones.29 The characteristic findings of acute cholecystitis on ultrasonography include enlargement of the gallbladder, thickening of the gallbladder wall, presence of pericholecystic fluid, and tenderness elicited by the ultrasound probe over the gallbladder (sonographic Murphy sign).
Scintigraphy as a second test
Acute cholecystitis is primarily a clinical diagnosis and typically does not require additional imaging beyond ultrasonography. When there is discordance between clinical and ultrasonographic findings, the most accurate second imaging test is scintigraphy of the biliary tract, usually performed with technetium-labeled hydroxy iminodiacetic acid. Given intravenously, the radionuclide is rapidly taken up by the liver and then secreted into the bile. In acute cholecystitis, the cystic duct is functionally occluded and the isotope does not enter the gallbladder, creating an imaging void compared with a normal appearance.
Scintigraphy is more sensitive than abdominal ultrasonography, with a sensitivity of up to 97% vs 81% to 88%, respectively.29,30 The tests have about equal specificity.
Even though scintigraphy is more sensitive, abdominal ultrasonography is often the initial test for patients with suspected acute cholecystitis because it is more widely available, takes less time, does not involve radiation exposure, and can assess for the presence or absence of gallstones and dilation of the intra- and extrahepatic bile ducts.
Looking for stones in the common bile duct
When acute cholangitis due to choledocholithiasis is suspected, abdominal ultrasonography is a prudent initial test to look for gallstones or biliary dilation suggesting obstruction by stones in the common bile duct. Abdominal ultrasonography has only a 22% to 55% sensitivity for visualizing stones in the common bile duct, but it has a 77% to 87% sensitivity for detecting common bile duct dilation, a surrogate marker of stones.31
The normal bile duct diameter ranges from 3 to 6 mm, although mild dilation is often seen in older patients or after cholecystectomy or Roux-en-Y gastric bypass surgery.32,33 Bile duct dilation of up to 10 mm can be considered normal in patients after cholecystectomy.34 A normal-appearing bile duct on ultrasonography has a negative predictive value of 95% for excluding common bile duct stones.31
Endoscopic ultrasonography (EUS), magnetic resonance cholangiopancreatography (MRCP), and endoscopic retrograde cholangiopancreatography (ERCP) have similar sensitivity (89%–94%, 85%–92%, and 89%–93%, respectively) and specificity (94%–95%, 93%–97%, and 100%, respectively) for detecting common bile duct stones.35–37 EUS is superior to MRCP in detecting stones smaller than 6 mm.38
ERCP should be reserved for managing rather than diagnosing common bile duct stones because of the risk of pancreatitis and perforation. Patients undergoing cholecystectomy who are suspected of having choledocholithiasis may undergo intraoperative cholangiography or laparoscopic common bile duct ultrasonography.
WATCH AND WAIT, OR INTERVENE?
Asymptomatic gallstones
Standard treatment for these patients is expectant management. Cholecystectomy is not recommended for patients with asymptomatic gallstones.47 Nevertheless, some patients may benefit from prophylactic cholecystectomy. We and others48 suggest considering cholecystectomy in the following patients.
Patients with chronic hemolytic anemia (including children with sickle cell anemia and spherocytosis). These patients have a higher risk of developing calcium bilirubinate stones, and cholecystectomy has improved outcomes.49 It should be noted that most of these data come from pediatric populations and have been extrapolated to adults.
Native Americans, who have a higher risk of gallbladder cancer if they have gallstones.2,50
Conversely, calcification of the gallbladder wall (“porcelain gallbladder”) is no longer considered an absolute indication for cholecystectomy. This condition was thought to be associated with a high rate of gallbladder carcinoma, but analyses of larger, more recent data sets found much smaller risks.51,52 Further, cholecystectomy in these patients was found to be associated with high rates of postoperative complications. Thus, prophylactic cholecystectomy is no longer recommended in asymptomatic cases of porcelain gallbladder.
In addition, concomitant cholecystectomy in patients undergoing bariatric surgery is no longer considered the therapeutic standard. Historically, cholecystectomy was performed in these patients because of the increased risk of gallstones associated with rapid weight loss after surgery. However, research now weighs against concomitant cholecystectomy with bariatric surgery and most other abdominal surgeries for asymptomatic gallstones.53
Laparoscopic surgery for symptomatic gallstones
For patients experiencing acute cholecystitis, laparoscopic cholecystectomy within 72 hours is recommended.48 There were safety concerns regarding higher rates of morbidity and conversion from laparoscopic to open cholecystectomy in patients who underwent surgery before the acute cholecystitis episode had settled. However, a large meta-analysis found no significant difference between early and delayed laparoscopic cholecystectomy in bile duct injury or conversion rates.54 Further, early cholecystectomy—defined as within 1 week of symptom onset—has been found to reduce gallstone-related complications, shorten hospital stays, and lower costs.55–57 If the patient cannot undergo surgery, percutaneous cholecystotomy or novel endoscopic gallbladder drainage interventions can be used.
Several variables predict the presence of bile duct stones in patients who have symptoms (Table 4). Based on these predictors, the ASGE classifies the probabilities as low (< 10%), intermediate (10% to 50%), and high (> 50%)31:
- High-risk patients should undergo preoperative ERCP and stone extraction if needed
- Intermediate-risk patients should undergo preoperative imaging with EUS or MRCP or intraoperative bile duct evaluation, depending on the availability, costs, and local expertise.
Patients with associated cholangitis should be given intravenous fluids and broad-spectrum antibiotics. Biliary decompression should be done as early as possible to decrease the risk of morbidity and mortality. For acute cholangitis, ERCP is the treatment of choice.25
Patients with acute gallstone pancreatitis should receive conservative management with intravenous isotonic solutions and pain control, followed by laparoscopic cholecystectomy.48
The timing of laparoscopic cholecystectomy in acute gallstone pancreatitis has been debated. Studies conducted during the era of open cholecystectomy reported similar or worse outcomes if cholecystectomy was done sooner rather than later.
However, in 1999, Uhl et al58 reported that 48 of 77 patients admitted with acute gallstone pancreatitis were able to undergo laparoscopic cholecystectomy during the same admission. Success rates were 85% (30 of 35 patients) in those with mild disease and 62% (8 of 13 patients) in those with severe disease. They concluded laparoscopic cholecystectomy could be safely performed within 7 days in patients with mild disease, whereas in severe disease at least 3 weeks should elapse because of the risk of infection.
In a randomized trial published in 2010, Aboulian et al59 reported that hospital length of stay (the primary end point) was shorter in 25 patients who underwent laparoscopic cholecystectomy early (within 48 hours of admission) than in 25 patients who underwent surgery after abdominal pain had resolved and laboratory enzymes showed a normalizing trend, 3.5 vs 5.8 days (P = .0016). Rates of perioperative complications and need for conversion to open surgery were similar between the 2 groups.
If there is associated cholangitis, patients should also be given broad-spectrum antibiotics and should undergo ERCP within 24 hours of admission.25–27
SUMMARY
Gallstones are common in US adults. Abdominal ultrasonography is the diagnostic imaging test of choice to detect gallbladder stones and assess for findings suggestive of acute cholecystitis and dilation of the common bile duct. Fortunately, most gallstones are asymptomatic and can usually be managed expectantly. In patients who have symptoms or have gallstone complications, laparoscopic cholecystectomy is the standard of care.
The prevalence of gallstones is approximately 10% to 15% of the adult US population.1,2 Most cases are asymptomatic, as gallstones are usually discovered incidentally during routine imaging for other abdominal conditions, and only about 20% of patients with asymptomatic gallstones develop clinically significant complications.2,3
Nevertheless, gallstones carry significant healthcare costs. In 2004, the median inpatient cost for any gallstone-related disease was $11,584, with an overall annual cost of $6.2 billion.4,5
Laparoscopic cholecystectomy is the standard treatment for symptomatic cholelithiasis. For asymptomatic cholelithasis, the usual approach is expectant management (“watch and wait”), but prophylactic cholecystectomy may be an option in certain patients at high risk.
CHEMICAL COMPOSITION
Gallstones can be classified into 2 main categories based on their predominant chemical composition: cholesterol or pigment.
Cholesterol gallstones
About 75% of gallstones are composed of cholesterol.3,4 In the past, this type of stone was thought to be caused by gallbladder inflammation, bile stasis, and absorption of bile salts from damaged mucosa. However, it is now known that cholesterol gallstones are the result of biliary supersaturation caused by cholesterol hypersecretion into the gallbladder, gallbladder hypomotility, accelerated cholesterol nucleation and crystallization, and mucin gel accumulation.
Pigment gallstones
Black pigment gallstones account for 10% to 15% of all gallstones.6 They are caused by chronic hemolysis in association with supersaturation of bile with calcium hydrogen bilirubinate, along with deposition of calcium carbonate, phosphate, and inorganic salts.7
Brown pigment stones, accounting for 5% to 10% of all gallstones,6 are caused by infection in the obstructed bile ducts, where bacteria that produce beta-glucuronidase, phospholipase, and slime contribute to formation of the stone.8,9
RISK FACTORS FOR GALLSTONES
Age. After age 40, the risk increases dramatically, with an incidence 4 times higher for those ages 40 to 69 than in younger people.10
Female sex. Women of reproductive age are 4 times more likely to develop gallstones than men, but this gap narrows after menopause.11 The higher risk is attributed to female sex hormones, pregnancy, and oral contraceptive use. Estrogen decreases secretion of bile salts and increases secretion of cholesterol into the gallbladder, which leads to cholesterol supersaturation. Progesterone acts synergistically by causing hypomobility of the gallbladder, which in turn leads to bile stasis.12,13
Ethnicity. The risk is higher in Mexican Americans and Native Americans than in other ethnic groups.14
Rapid weight loss, such as after bariatric surgery, occurs from decreased caloric intake and promotes bile stasis, while lipolysis increases cholesterol mobilization and secretion into the gallbladder. This creates an environment conducive to bile supersaturation with cholesterol, leading to gallstone formation.
Chronic hemolytic disorders carry an increased risk of developing calcium bilirubinate stones due to increased excretion of bilirubin during hemolysis.
Obesity and diabetes mellitus are both attributed to insulin resistance. Obesity also increases bile stasis and cholesterol saturation.
CLINICAL PRESENTATION OF GALLSTONES (CHOLELITHIASIS)
Most patients with gallstones (cholelithiasis) experience no symptoms. Their gallstones are often discovered incidentally during imaging tests for unrelated or unexplained abdominal symptoms. Most patients with asymptomatic gallstones remain symptom-free, while about 20% develop gallstone-related symptoms.2,3
Abdominal pain is the most common symptom. The phrase biliary colic—suggesting pain that is fluctuating in nature—appears ubiquitously in the medical literature, but it does not correctly characterize the pain associated with gallstones.
Most patients with gallstone symptoms describe a constant and often severe pain in the right upper abdomen, epigastrium, or both, often persisting for 30 to 120 minutes. Symptoms are frequently reported in the epigastrium when only visceral pain fibers are stimulated due to gallbladder distention. This is usually called midline pain; however, pain occurs in the back and right shoulder in up to 60% of patients, with involvement of somatic fibers.15,16 Gallstone pain is not relieved by change of position or passage of stool or gas.
Onset of symptoms more than an hour after eating or in the late evening or at night also very strongly suggests biliary pain. Patients with a history of biliary pain are more likely to experience it again, with a 69% chance of developing recurrent pain within 2 years.17
GALLSTONE-RELATED COMPLICATIONS
Acute gallbladder inflammation (cholecystitis)
Gallbladder inflammation (cholecystitis) is the most common complication, occurring in up to 10% of symptomatic cases. Many patients with acute cholecystitis present with right upper quadrant pain that may be accompanied by anorexia, nausea, or vomiting. Inspiratory arrest on deep palpation of the right upper quadrant (Murphy sign) has a specificity of 79% to 96% for acute cholecystitis.20 Markers of systemic inflammation such as fever, elevated white blood cell count, and elevated C-reactive protein are highly suggestive of acute cholecystitis.20,21
Bile duct stones (choledocholithiasis)
Bile duct stones (choledocholithiasis) are detected in 3.4% to 12% of patients with gallstones.22,23 Most stones in the common bile duct migrate there from the gallbladder via the cystic duct. Less commonly, primary duct stones form in the duct due to biliary stasis. Removing the gallbladder does not completely eliminate the risk of bile duct stones, as stones can remain or recur after surgery.
Bile duct stones can obstruct the common bile duct, which disrupts normal bile flow and leads to jaundice. Other symptoms may include pruritus, right upper quadrant pain, nausea, and vomiting. Serum levels of bilirubin, aspartate aminotransferase, alanine aminotransferase (ALT), and alkaline phosphatase are usually high.24
Acute bacterial infection (cholangitis)
Acute bacterial infection of the biliary system (cholangitis) is usually associated with obstruction of the common bile duct. Common symptoms of acute cholangitis include right upper quadrant pain, fever, and jaundice (Charcot triad), and these are present in about 50% to 75% of cases.21 In severe cases, patients can develop altered mental status and septicemic shock in addition to the Charcot triad, a condition called the Reynold pentad. White blood cell counts and serum levels of C-reactive protein, bilirubin, aminotransferases, and alkaline phosphatase are usually elevated.21
Pancreatitis
Approximately 4% to 8% of patients with gallstones develop inflammation of the pancreas (pancreatitis).25 The diagnosis of acute pancreatitis requires at least 2 of the following:26,27
- Abdominal pain (typically epigastric, often radiating to the back)
- Amylase or lipase levels at least 3 times above the normal limit
- Imaging findings that suggest acute pancreatitis.
Gallstone-related pancreatitis should be considered if the ALT level is greater than 150 U/mL, which has a 97% specificity for gallstone-related pancreatitis.28
ABDOMINAL ULTRASONOGRAPHY FOR DIAGNOSIS
Transabdominal ultrasonography, with a sensitivity of 84% to 89% and a specificity of up to 99%, is the test of choice for detecting gallstones.29 The characteristic findings of acute cholecystitis on ultrasonography include enlargement of the gallbladder, thickening of the gallbladder wall, presence of pericholecystic fluid, and tenderness elicited by the ultrasound probe over the gallbladder (sonographic Murphy sign).
Scintigraphy as a second test
Acute cholecystitis is primarily a clinical diagnosis and typically does not require additional imaging beyond ultrasonography. When there is discordance between clinical and ultrasonographic findings, the most accurate second imaging test is scintigraphy of the biliary tract, usually performed with technetium-labeled hydroxy iminodiacetic acid. Given intravenously, the radionuclide is rapidly taken up by the liver and then secreted into the bile. In acute cholecystitis, the cystic duct is functionally occluded and the isotope does not enter the gallbladder, creating an imaging void compared with a normal appearance.
Scintigraphy is more sensitive than abdominal ultrasonography, with a sensitivity of up to 97% vs 81% to 88%, respectively.29,30 The tests have about equal specificity.
Even though scintigraphy is more sensitive, abdominal ultrasonography is often the initial test for patients with suspected acute cholecystitis because it is more widely available, takes less time, does not involve radiation exposure, and can assess for the presence or absence of gallstones and dilation of the intra- and extrahepatic bile ducts.
Looking for stones in the common bile duct
When acute cholangitis due to choledocholithiasis is suspected, abdominal ultrasonography is a prudent initial test to look for gallstones or biliary dilation suggesting obstruction by stones in the common bile duct. Abdominal ultrasonography has only a 22% to 55% sensitivity for visualizing stones in the common bile duct, but it has a 77% to 87% sensitivity for detecting common bile duct dilation, a surrogate marker of stones.31
The normal bile duct diameter ranges from 3 to 6 mm, although mild dilation is often seen in older patients or after cholecystectomy or Roux-en-Y gastric bypass surgery.32,33 Bile duct dilation of up to 10 mm can be considered normal in patients after cholecystectomy.34 A normal-appearing bile duct on ultrasonography has a negative predictive value of 95% for excluding common bile duct stones.31
Endoscopic ultrasonography (EUS), magnetic resonance cholangiopancreatography (MRCP), and endoscopic retrograde cholangiopancreatography (ERCP) have similar sensitivity (89%–94%, 85%–92%, and 89%–93%, respectively) and specificity (94%–95%, 93%–97%, and 100%, respectively) for detecting common bile duct stones.35–37 EUS is superior to MRCP in detecting stones smaller than 6 mm.38
ERCP should be reserved for managing rather than diagnosing common bile duct stones because of the risk of pancreatitis and perforation. Patients undergoing cholecystectomy who are suspected of having choledocholithiasis may undergo intraoperative cholangiography or laparoscopic common bile duct ultrasonography.
WATCH AND WAIT, OR INTERVENE?
Asymptomatic gallstones
Standard treatment for these patients is expectant management. Cholecystectomy is not recommended for patients with asymptomatic gallstones.47 Nevertheless, some patients may benefit from prophylactic cholecystectomy. We and others48 suggest considering cholecystectomy in the following patients.
Patients with chronic hemolytic anemia (including children with sickle cell anemia and spherocytosis). These patients have a higher risk of developing calcium bilirubinate stones, and cholecystectomy has improved outcomes.49 It should be noted that most of these data come from pediatric populations and have been extrapolated to adults.
Native Americans, who have a higher risk of gallbladder cancer if they have gallstones.2,50
Conversely, calcification of the gallbladder wall (“porcelain gallbladder”) is no longer considered an absolute indication for cholecystectomy. This condition was thought to be associated with a high rate of gallbladder carcinoma, but analyses of larger, more recent data sets found much smaller risks.51,52 Further, cholecystectomy in these patients was found to be associated with high rates of postoperative complications. Thus, prophylactic cholecystectomy is no longer recommended in asymptomatic cases of porcelain gallbladder.
In addition, concomitant cholecystectomy in patients undergoing bariatric surgery is no longer considered the therapeutic standard. Historically, cholecystectomy was performed in these patients because of the increased risk of gallstones associated with rapid weight loss after surgery. However, research now weighs against concomitant cholecystectomy with bariatric surgery and most other abdominal surgeries for asymptomatic gallstones.53
Laparoscopic surgery for symptomatic gallstones
For patients experiencing acute cholecystitis, laparoscopic cholecystectomy within 72 hours is recommended.48 There were safety concerns regarding higher rates of morbidity and conversion from laparoscopic to open cholecystectomy in patients who underwent surgery before the acute cholecystitis episode had settled. However, a large meta-analysis found no significant difference between early and delayed laparoscopic cholecystectomy in bile duct injury or conversion rates.54 Further, early cholecystectomy—defined as within 1 week of symptom onset—has been found to reduce gallstone-related complications, shorten hospital stays, and lower costs.55–57 If the patient cannot undergo surgery, percutaneous cholecystotomy or novel endoscopic gallbladder drainage interventions can be used.
Several variables predict the presence of bile duct stones in patients who have symptoms (Table 4). Based on these predictors, the ASGE classifies the probabilities as low (< 10%), intermediate (10% to 50%), and high (> 50%)31:
- High-risk patients should undergo preoperative ERCP and stone extraction if needed
- Intermediate-risk patients should undergo preoperative imaging with EUS or MRCP or intraoperative bile duct evaluation, depending on the availability, costs, and local expertise.
Patients with associated cholangitis should be given intravenous fluids and broad-spectrum antibiotics. Biliary decompression should be done as early as possible to decrease the risk of morbidity and mortality. For acute cholangitis, ERCP is the treatment of choice.25
Patients with acute gallstone pancreatitis should receive conservative management with intravenous isotonic solutions and pain control, followed by laparoscopic cholecystectomy.48
The timing of laparoscopic cholecystectomy in acute gallstone pancreatitis has been debated. Studies conducted during the era of open cholecystectomy reported similar or worse outcomes if cholecystectomy was done sooner rather than later.
However, in 1999, Uhl et al58 reported that 48 of 77 patients admitted with acute gallstone pancreatitis were able to undergo laparoscopic cholecystectomy during the same admission. Success rates were 85% (30 of 35 patients) in those with mild disease and 62% (8 of 13 patients) in those with severe disease. They concluded laparoscopic cholecystectomy could be safely performed within 7 days in patients with mild disease, whereas in severe disease at least 3 weeks should elapse because of the risk of infection.
In a randomized trial published in 2010, Aboulian et al59 reported that hospital length of stay (the primary end point) was shorter in 25 patients who underwent laparoscopic cholecystectomy early (within 48 hours of admission) than in 25 patients who underwent surgery after abdominal pain had resolved and laboratory enzymes showed a normalizing trend, 3.5 vs 5.8 days (P = .0016). Rates of perioperative complications and need for conversion to open surgery were similar between the 2 groups.
If there is associated cholangitis, patients should also be given broad-spectrum antibiotics and should undergo ERCP within 24 hours of admission.25–27
SUMMARY
Gallstones are common in US adults. Abdominal ultrasonography is the diagnostic imaging test of choice to detect gallbladder stones and assess for findings suggestive of acute cholecystitis and dilation of the common bile duct. Fortunately, most gallstones are asymptomatic and can usually be managed expectantly. In patients who have symptoms or have gallstone complications, laparoscopic cholecystectomy is the standard of care.
- Schirmer BD, Winters KL, Edlich RF. Cholelithiasis and cholecystitis. J Long Term Eff Med Implants 2005; 15(3):329–338. doi:10.1615/JLongTermEffMedImplants.v15.i3.90
- Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver 2012; 6(2):172–187. doi:10.5009/gnl.2012.6.2.172
- Lee JY, Keane MG, Pereira S. Diagnosis and treatment of gallstone disease. Practitioner 2015; 259(1783):15–19.
- Russo MW, Wei JT, Thiny MT, et al. Digestive and liver diseases statistics, 2004. Gastroenterology 2004; 126(5):1448–1453. doi:10.1053/j.gastro.2004.01.025
- Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology 2009; 136(2):376–386. doi:10.1053/j.gastro.2008.12.015
- Cariati A. Gallstone classification in Western countries. Indian J Surg 2015; 77(suppl 2):376–380. doi.org/10.1007/s12262-013-0847-y
- Carey MC. Pathogenesis of gallstones. Am J Surg 1993; 165(4):410–419. doi:10.1016/S0002-9610(05)80932-8
- Lammert F, Gurusamy K, Ko CW, et al. Gallstones. Nat Rev Dis Primers 2016; 2:16024. doi:10.1038/nrdp.2016.24
- Stewart L, Oesterle AL, Erdan I, Griffiss JM, Way LW. Pathogenesis of pigment gallstones in Western societies: the central role of bacteria. J Gastrointest Surg 2002; 6(6):891–904.
- Barbara L, Sama C, Morselli Labate AM, et al. A population study on the prevalence of gallstone disease: the Sirmione Study. Hepatology 1987; 7(5):913–917. doi:10.1002/hep.1840070520
- Sood S, Winn T, Ibrahim S, et al. Natural history of asymptomatic gallstones: differential behaviour in male and female subjects. Med J Malaysia 2015; 70(6):341–345.
- Maringhini A, Ciambra M, Baccelliere P, et al. Biliary sludge and gallstones in pregnancy: incidence, risk factors, and natural history. Ann Intern Med 1993; 119(2):116–120. doi:10.7326/0003-4819-119-2-199307150-00004
- Etminan M, Delaney JA, Bressler B, Brophy JM. Oral contraceptives and the risk of gallbladder disease: a comparative safety study. CMAJ 2011; 183(8):899–904. doi:10.1503/cmaj.110161
- Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology 1999; 117(3):632–639.
- Festi D, Sottili S, Colecchia A, et al. Clinical manifestations of gallstone disease: evidence from the multicenter Italian study on cholelithiasis (MICOL). Hepatology 1999; 30(4):839–846. doi:10.1002/hep.510300401
- Berhane T, Vetrhus M, Hausken T, Olafsson S, Sondenaa K. Pain attacks in non-complicated and complicated gallstone disease have a characteristic pattern and are accompanied by dyspepsia in most patients: the results of a prospective study. Scand J Gastroenterol 2006; 41(1):93–101. doi:10.1080/00365520510023990
- Thistle JL, Cleary PA, Lachin JM, Tyor MP, Hersh T. The natural history of cholelithiasis: the National Cooperative Gallstone Study. Ann Intern Med 1984; 101(2):171–175. doi:10.7326/0003-4819-101-2-171
- Friedman GD. Natural history of asymptomatic and symptomatic gallstones. Am J Surg 1993; 165(4):399–404. doi:0.1016/S0002-9610(05)80930-4
- Friedman GD, Raviola CA, Fireman B. Prognosis of gallstones with mild or no symptoms: 25 years of follow-up in a health maintenance organization. J Clin Epidemiol 1989; 42(2):127–136. doi:10.1016/0895-4356(89)90086-3
- Hirota M, Takada T, Kawarada Y, et al. Diagnostic criteria and severity assessment of acute cholecystitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg 2007; 14(1):78–82. doi:10.1007/s00534-006-1159-4
- Miura F, Takada T, Kawarada Y, et al. Flowcharts for the diagnosis and treatment of acute cholangitis and cholecystitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg 2007; 14(1):27–34. doi:10.1007/s00534-006-1153-x
- Koo KP, Traverso LW. Do preoperative indicators predict the presence of common bile duct stones during laparoscopic cholecystectomy? Am J Surg 1996; 171(5):495–499. doi:10.1016/S0002-9610(97)89611-0
- Collins C, Maguire D, Ireland A, Fitzgerald E, O’Sullivan GC. A prospective study of common bile duct calculi in patients undergoing laparoscopic cholecystectomy: natural history of choledocholithiasis revisited. Ann Surg 2004; 239(1):28–33. doi:10.1097/01.sla.0000103069.00170.9c
- Costi R, Gnocchi A, Di Mario F, Sarli L. Diagnosis and management of choledocholithiasis in the golden age of imaging, endoscopy and laparoscopy. World J Gastroenterol 2014; 20(37):13382–13401. doi:10.3748/wjg.v20.i37.13382
- European Association for the Study of the Liver (EASL). EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. J Hepatol 2016; 65(1):146–181. doi:10.1016/j.jhep.2016.03.005
- Greenberg JA, Hsu J, Bawazeer M, et al. Clinical practice guideline: management of acute pancreatitis. Can J Surg 2016; 59 (2):128–140. doi:10.1503/cjs.015015
- Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013; 108(9):1400–1416. doi:10.1038/ajg.2013.218
- Moolla Z, Anderson F, Thomson SR. Use of amylase and alanine transaminase to predict acute gallstone pancreatitis in a population with high HIV prevalence. World J Surg 2013; 37(1):156–161. doi:10.1007/s00268-012-1801-z
- Shea JA, Berlin JA, Escarce JJ, et al. Revised estimates of diagnostic test sensitivity and specificity in suspected biliary tract disease. Arch Intern Med 1994; 154(22):2573–2581. doi:10.1001/archinte.1994.00420220069008
- Kiewiet JJ, Leeuwenburgh MM, Bipat S, et al. A systematic review and meta-analysis of diagnostic performance of imaging in acute cholecystitis. Radiology 2012; 264(3):708–720. doi:10.1148/radiol.12111561
- ASGE Standards of Practice Committee; Maple JT, Ben-Menachem T, Anderson MA, et al. The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointest Endosc 2010; 71(1):1–9. doi:10.1016/j.gie.2009.09.041
- Bachar GN, Cohen M, Belenky A, Atar E, Gideon S. Effect of aging on the adult extrahepatic bile duct: a sonographic study. J Ultrasound Med 2003; 22(9):879–885. doi:10.7863/jum.2003.22.9.879
- El-Hayek K, Timratana P, Meranda J, Shimizu H, Eldar S, Chand B. Post Roux-en-Y gastric bypass biliary dilation: natural process or significant entity? J Gastrointest Surg 2012; 16(12):2185–2189. doi:10.1007/s11605-012-2058-4
- Park SM, Kim WS, Bae IH, et al. Common bile duct dilatation after cholecystectomy: a one-year prospective study. J Korean Surg Soc 2012; 83(2):97–101. doi:10.4174/jkss.2012.83.2.97
- Tse F, Liu L, Barkun AN, Armstrong D, Moayyedi P. EUS: a meta-analysis of test performance in suspected choledocholithiasis. Gastrointest Endosc 2008; 67(2):235–244. doi:10.1016/j.gie.2007.09.047
- Verma D, Kapadia A, Eisen GM, Adler DG. EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc 2006; 64(2):248–254. doi:10.1016/j.gie.2005.12.038
- Tseng LJ, Jao YT, Mo LR, Lin RC. Over-the-wire US catheter probe as an adjunct to ERCP in the detection of choledocholithiasis. Gastrointest Endosc 2001; 54(6):720–723. doi:10.1067/mge.2001.119255
- Kondo S, Isayama H, Akahane M, et al. Detection of common bile duct stones: comparison between endoscopic ultrasonography, magnetic resonance cholangiography, and helical-computed-tomographic cholangiography. Eur J Radiol 2005; 54(2):271–275. doi:10.1016/j.ejrad.2004.07.007
- Attili AF, De Santis A, Capri R, Repice AM, Maselli S. The natural history of gallstones: the GREPCO experience. The GREPCO Group. Hepatology 1995; 21(3):656–660. doi:10.1016/0270-9139(95)90514-6
- Sakorafas GH, Milingos D, Peros G. Asymptomatic cholelithiasis: is cholecystectomy really needed? A critical reappraisal 15 years after the introduction of laparoscopic cholecystectomy. Dig Dis Sci 2007; 52(5):1313–1325. doi:10.1007/s10620-006-9107-3
- Gracie WA, Ransohoff DF. The natural history of silent gallstones: the innocent gallstone is not a myth. N Engl J Med 1982; 307(13):798–800. doi:10.1056/NEJM198209233071305
- McSherry CK, Ferstenberg H, Calhoun WF, Lahman E, Virshup M. The natural history of diagnosed gallstone disease in symptomatic and asymptomatic patients. Ann Surg 1985; 202(1):59–63. doi:10.1097/00000658-198507000-00009
- Wada K, Wada K, Imamura T. Natural course of asymptomatic gallstone disease. Nihon Rinsho 1993; 51(7):1737–1743. Japanese.
- Halldestam I, Enell EL, Kullman E, Borch K. Development of symptoms and complications in individuals with asymptomatic gallstones. Br J Surg 2004; 91(6):734–738. doi:10.1002/bjs.4547
- Festi D, Reggiani ML, Attili AF, et al. Natural history of gallstone disease: expectant management or active treatment? Results from a population-based cohort study. J Gastroenterol Hepatol 2010; 25(4):719–724. doi:10.1111/j.1440-1746.2009.06146.x
- Shabanzadeh DM, Sorensen LT, Jorgensen T. A prediction rule for risk stratification of incidentally discovered gallstones: results from a large cohort study. Gastroenterology 2016; 150(1):156–167e1. doi:10.1053/j.gastro.2015.09.002
- Overby DW, Apelgren KN, Richardson W, Fanelli R; Society of American Gastrointestinal and Endoscopic Surgeons. SAGES guidelines for the clinical application of laparoscopic biliary tract surgery. Surg Endosc 2010; 24(10):2368–2386. doi:10.1007/s00464-010-1268-7
- Abraham S, Rivero HG, Erlikh IV, Griffith LF, Kondamudi VK. Surgical and nonsurgical management of gallstones. Am Fam Physician 2014; 89(10):795–802.
- Currò G,, Iapichino G, Lorenzini C, Palmeri R, Cucinotta E. Laparoscopic cholecystectomy in children with chronic hemolytic anemia. Is the outcome related to the timing of the procedure? Surg Endosc 2006; 20(2):252–255. doi:10.1007/s00464-005-0318-z
- Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol 2014; 6:99–109. doi:10.2147/CLEP.S37357
- Chen GL, Akmal Y, DiFronzo AL, Vuong B, O’Connor V. Porcelain gallbladder: no longer an indication for prophylactic cholecystectomy. Am Surg 2015; 81(10):936–940.
- Schnelldorfer T. Porcelain gallbladder: a benign process or concern for malignancy? J Gastrointest Surg 2013; 17(6):1161–1168. doi:10.1007/s11605-013-2170-0
- Warschkow R, Tarantino I, Ukegjini K, et al. Concomitant cholecystectomy during laparoscopic Roux-en-Y gastric bypass in obese patients is not justified: a meta-analysis. Obes Surg 2013; 23(3)3979–408. doi:10.1007/s11695-012-0852-4
- Gurusamy K, Samraj K, Gluud C, Wilson E, Davidson BR. Meta-analysis of randomized controlled trials on the safety and effectiveness of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Br J Surg 2010; 97(2):141–150. doi:10.1002/bjs.6870
- Papi C, Catarci M, D’Ambrosio L, et al. Timing of cholecystectomy for acute calculous cholecystitis: a meta-analysis. Am J Gastroenterol 2004; 99(1):147–155. doi:10.1046/j.1572-0241.2003.04002.x
- Gurusamy KS, Davidson C, Gluud C, Davidson BR. Early versus delayed laparoscopic cholecystectomy for people with acute cholecystitis. Cochrane Database Syst Rev 2013; 6:CD005440. doi:10.1002/14651858
- Menahem B, Mulliri A, Fohlen A, Guittet L, Alves A, Lubrano J. Delayed laparoscopic cholecystectomy increases the total hospital stay compared to an early laparoscopic cholecystectomy after acute cholecystitis: an updated meta-analysis of randomized controlled trials. HPB (Oxford) 2015; 17(10):857–862. doi:10.1111/hpb.12449
- Uhl W, Müller CA, Krähenbühl L, Schmid SW, Schölzel S, Büchler MW. Acute gallstone pancreatitis: timing of laparoscopic cholecystectomy in mild and severe disease. Surg Endosc 1999; 13(11):1070–1076. doi:10.1007/s004649901175
- Aboulian A, Chan T, Yaghoubian A, et al. Early cholecystectomy safely decreases hospital stay in patients with mild gallstone pancreatitis: a randomized prospective study. Ann Surg 2010(4): 251:615–619. doi:10.1097/SLA.0b013e3181c38f1f
- Schirmer BD, Winters KL, Edlich RF. Cholelithiasis and cholecystitis. J Long Term Eff Med Implants 2005; 15(3):329–338. doi:10.1615/JLongTermEffMedImplants.v15.i3.90
- Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver 2012; 6(2):172–187. doi:10.5009/gnl.2012.6.2.172
- Lee JY, Keane MG, Pereira S. Diagnosis and treatment of gallstone disease. Practitioner 2015; 259(1783):15–19.
- Russo MW, Wei JT, Thiny MT, et al. Digestive and liver diseases statistics, 2004. Gastroenterology 2004; 126(5):1448–1453. doi:10.1053/j.gastro.2004.01.025
- Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology 2009; 136(2):376–386. doi:10.1053/j.gastro.2008.12.015
- Cariati A. Gallstone classification in Western countries. Indian J Surg 2015; 77(suppl 2):376–380. doi.org/10.1007/s12262-013-0847-y
- Carey MC. Pathogenesis of gallstones. Am J Surg 1993; 165(4):410–419. doi:10.1016/S0002-9610(05)80932-8
- Lammert F, Gurusamy K, Ko CW, et al. Gallstones. Nat Rev Dis Primers 2016; 2:16024. doi:10.1038/nrdp.2016.24
- Stewart L, Oesterle AL, Erdan I, Griffiss JM, Way LW. Pathogenesis of pigment gallstones in Western societies: the central role of bacteria. J Gastrointest Surg 2002; 6(6):891–904.
- Barbara L, Sama C, Morselli Labate AM, et al. A population study on the prevalence of gallstone disease: the Sirmione Study. Hepatology 1987; 7(5):913–917. doi:10.1002/hep.1840070520
- Sood S, Winn T, Ibrahim S, et al. Natural history of asymptomatic gallstones: differential behaviour in male and female subjects. Med J Malaysia 2015; 70(6):341–345.
- Maringhini A, Ciambra M, Baccelliere P, et al. Biliary sludge and gallstones in pregnancy: incidence, risk factors, and natural history. Ann Intern Med 1993; 119(2):116–120. doi:10.7326/0003-4819-119-2-199307150-00004
- Etminan M, Delaney JA, Bressler B, Brophy JM. Oral contraceptives and the risk of gallbladder disease: a comparative safety study. CMAJ 2011; 183(8):899–904. doi:10.1503/cmaj.110161
- Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology 1999; 117(3):632–639.
- Festi D, Sottili S, Colecchia A, et al. Clinical manifestations of gallstone disease: evidence from the multicenter Italian study on cholelithiasis (MICOL). Hepatology 1999; 30(4):839–846. doi:10.1002/hep.510300401
- Berhane T, Vetrhus M, Hausken T, Olafsson S, Sondenaa K. Pain attacks in non-complicated and complicated gallstone disease have a characteristic pattern and are accompanied by dyspepsia in most patients: the results of a prospective study. Scand J Gastroenterol 2006; 41(1):93–101. doi:10.1080/00365520510023990
- Thistle JL, Cleary PA, Lachin JM, Tyor MP, Hersh T. The natural history of cholelithiasis: the National Cooperative Gallstone Study. Ann Intern Med 1984; 101(2):171–175. doi:10.7326/0003-4819-101-2-171
- Friedman GD. Natural history of asymptomatic and symptomatic gallstones. Am J Surg 1993; 165(4):399–404. doi:0.1016/S0002-9610(05)80930-4
- Friedman GD, Raviola CA, Fireman B. Prognosis of gallstones with mild or no symptoms: 25 years of follow-up in a health maintenance organization. J Clin Epidemiol 1989; 42(2):127–136. doi:10.1016/0895-4356(89)90086-3
- Hirota M, Takada T, Kawarada Y, et al. Diagnostic criteria and severity assessment of acute cholecystitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg 2007; 14(1):78–82. doi:10.1007/s00534-006-1159-4
- Miura F, Takada T, Kawarada Y, et al. Flowcharts for the diagnosis and treatment of acute cholangitis and cholecystitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg 2007; 14(1):27–34. doi:10.1007/s00534-006-1153-x
- Koo KP, Traverso LW. Do preoperative indicators predict the presence of common bile duct stones during laparoscopic cholecystectomy? Am J Surg 1996; 171(5):495–499. doi:10.1016/S0002-9610(97)89611-0
- Collins C, Maguire D, Ireland A, Fitzgerald E, O’Sullivan GC. A prospective study of common bile duct calculi in patients undergoing laparoscopic cholecystectomy: natural history of choledocholithiasis revisited. Ann Surg 2004; 239(1):28–33. doi:10.1097/01.sla.0000103069.00170.9c
- Costi R, Gnocchi A, Di Mario F, Sarli L. Diagnosis and management of choledocholithiasis in the golden age of imaging, endoscopy and laparoscopy. World J Gastroenterol 2014; 20(37):13382–13401. doi:10.3748/wjg.v20.i37.13382
- European Association for the Study of the Liver (EASL). EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. J Hepatol 2016; 65(1):146–181. doi:10.1016/j.jhep.2016.03.005
- Greenberg JA, Hsu J, Bawazeer M, et al. Clinical practice guideline: management of acute pancreatitis. Can J Surg 2016; 59 (2):128–140. doi:10.1503/cjs.015015
- Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013; 108(9):1400–1416. doi:10.1038/ajg.2013.218
- Moolla Z, Anderson F, Thomson SR. Use of amylase and alanine transaminase to predict acute gallstone pancreatitis in a population with high HIV prevalence. World J Surg 2013; 37(1):156–161. doi:10.1007/s00268-012-1801-z
- Shea JA, Berlin JA, Escarce JJ, et al. Revised estimates of diagnostic test sensitivity and specificity in suspected biliary tract disease. Arch Intern Med 1994; 154(22):2573–2581. doi:10.1001/archinte.1994.00420220069008
- Kiewiet JJ, Leeuwenburgh MM, Bipat S, et al. A systematic review and meta-analysis of diagnostic performance of imaging in acute cholecystitis. Radiology 2012; 264(3):708–720. doi:10.1148/radiol.12111561
- ASGE Standards of Practice Committee; Maple JT, Ben-Menachem T, Anderson MA, et al. The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointest Endosc 2010; 71(1):1–9. doi:10.1016/j.gie.2009.09.041
- Bachar GN, Cohen M, Belenky A, Atar E, Gideon S. Effect of aging on the adult extrahepatic bile duct: a sonographic study. J Ultrasound Med 2003; 22(9):879–885. doi:10.7863/jum.2003.22.9.879
- El-Hayek K, Timratana P, Meranda J, Shimizu H, Eldar S, Chand B. Post Roux-en-Y gastric bypass biliary dilation: natural process or significant entity? J Gastrointest Surg 2012; 16(12):2185–2189. doi:10.1007/s11605-012-2058-4
- Park SM, Kim WS, Bae IH, et al. Common bile duct dilatation after cholecystectomy: a one-year prospective study. J Korean Surg Soc 2012; 83(2):97–101. doi:10.4174/jkss.2012.83.2.97
- Tse F, Liu L, Barkun AN, Armstrong D, Moayyedi P. EUS: a meta-analysis of test performance in suspected choledocholithiasis. Gastrointest Endosc 2008; 67(2):235–244. doi:10.1016/j.gie.2007.09.047
- Verma D, Kapadia A, Eisen GM, Adler DG. EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc 2006; 64(2):248–254. doi:10.1016/j.gie.2005.12.038
- Tseng LJ, Jao YT, Mo LR, Lin RC. Over-the-wire US catheter probe as an adjunct to ERCP in the detection of choledocholithiasis. Gastrointest Endosc 2001; 54(6):720–723. doi:10.1067/mge.2001.119255
- Kondo S, Isayama H, Akahane M, et al. Detection of common bile duct stones: comparison between endoscopic ultrasonography, magnetic resonance cholangiography, and helical-computed-tomographic cholangiography. Eur J Radiol 2005; 54(2):271–275. doi:10.1016/j.ejrad.2004.07.007
- Attili AF, De Santis A, Capri R, Repice AM, Maselli S. The natural history of gallstones: the GREPCO experience. The GREPCO Group. Hepatology 1995; 21(3):656–660. doi:10.1016/0270-9139(95)90514-6
- Sakorafas GH, Milingos D, Peros G. Asymptomatic cholelithiasis: is cholecystectomy really needed? A critical reappraisal 15 years after the introduction of laparoscopic cholecystectomy. Dig Dis Sci 2007; 52(5):1313–1325. doi:10.1007/s10620-006-9107-3
- Gracie WA, Ransohoff DF. The natural history of silent gallstones: the innocent gallstone is not a myth. N Engl J Med 1982; 307(13):798–800. doi:10.1056/NEJM198209233071305
- McSherry CK, Ferstenberg H, Calhoun WF, Lahman E, Virshup M. The natural history of diagnosed gallstone disease in symptomatic and asymptomatic patients. Ann Surg 1985; 202(1):59–63. doi:10.1097/00000658-198507000-00009
- Wada K, Wada K, Imamura T. Natural course of asymptomatic gallstone disease. Nihon Rinsho 1993; 51(7):1737–1743. Japanese.
- Halldestam I, Enell EL, Kullman E, Borch K. Development of symptoms and complications in individuals with asymptomatic gallstones. Br J Surg 2004; 91(6):734–738. doi:10.1002/bjs.4547
- Festi D, Reggiani ML, Attili AF, et al. Natural history of gallstone disease: expectant management or active treatment? Results from a population-based cohort study. J Gastroenterol Hepatol 2010; 25(4):719–724. doi:10.1111/j.1440-1746.2009.06146.x
- Shabanzadeh DM, Sorensen LT, Jorgensen T. A prediction rule for risk stratification of incidentally discovered gallstones: results from a large cohort study. Gastroenterology 2016; 150(1):156–167e1. doi:10.1053/j.gastro.2015.09.002
- Overby DW, Apelgren KN, Richardson W, Fanelli R; Society of American Gastrointestinal and Endoscopic Surgeons. SAGES guidelines for the clinical application of laparoscopic biliary tract surgery. Surg Endosc 2010; 24(10):2368–2386. doi:10.1007/s00464-010-1268-7
- Abraham S, Rivero HG, Erlikh IV, Griffith LF, Kondamudi VK. Surgical and nonsurgical management of gallstones. Am Fam Physician 2014; 89(10):795–802.
- Currò G,, Iapichino G, Lorenzini C, Palmeri R, Cucinotta E. Laparoscopic cholecystectomy in children with chronic hemolytic anemia. Is the outcome related to the timing of the procedure? Surg Endosc 2006; 20(2):252–255. doi:10.1007/s00464-005-0318-z
- Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol 2014; 6:99–109. doi:10.2147/CLEP.S37357
- Chen GL, Akmal Y, DiFronzo AL, Vuong B, O’Connor V. Porcelain gallbladder: no longer an indication for prophylactic cholecystectomy. Am Surg 2015; 81(10):936–940.
- Schnelldorfer T. Porcelain gallbladder: a benign process or concern for malignancy? J Gastrointest Surg 2013; 17(6):1161–1168. doi:10.1007/s11605-013-2170-0
- Warschkow R, Tarantino I, Ukegjini K, et al. Concomitant cholecystectomy during laparoscopic Roux-en-Y gastric bypass in obese patients is not justified: a meta-analysis. Obes Surg 2013; 23(3)3979–408. doi:10.1007/s11695-012-0852-4
- Gurusamy K, Samraj K, Gluud C, Wilson E, Davidson BR. Meta-analysis of randomized controlled trials on the safety and effectiveness of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Br J Surg 2010; 97(2):141–150. doi:10.1002/bjs.6870
- Papi C, Catarci M, D’Ambrosio L, et al. Timing of cholecystectomy for acute calculous cholecystitis: a meta-analysis. Am J Gastroenterol 2004; 99(1):147–155. doi:10.1046/j.1572-0241.2003.04002.x
- Gurusamy KS, Davidson C, Gluud C, Davidson BR. Early versus delayed laparoscopic cholecystectomy for people with acute cholecystitis. Cochrane Database Syst Rev 2013; 6:CD005440. doi:10.1002/14651858
- Menahem B, Mulliri A, Fohlen A, Guittet L, Alves A, Lubrano J. Delayed laparoscopic cholecystectomy increases the total hospital stay compared to an early laparoscopic cholecystectomy after acute cholecystitis: an updated meta-analysis of randomized controlled trials. HPB (Oxford) 2015; 17(10):857–862. doi:10.1111/hpb.12449
- Uhl W, Müller CA, Krähenbühl L, Schmid SW, Schölzel S, Büchler MW. Acute gallstone pancreatitis: timing of laparoscopic cholecystectomy in mild and severe disease. Surg Endosc 1999; 13(11):1070–1076. doi:10.1007/s004649901175
- Aboulian A, Chan T, Yaghoubian A, et al. Early cholecystectomy safely decreases hospital stay in patients with mild gallstone pancreatitis: a randomized prospective study. Ann Surg 2010(4): 251:615–619. doi:10.1097/SLA.0b013e3181c38f1f
KEY POINTS
- Abdominal pain is the primary symptom associated with gallstones.
- Abdominal ultrasonography is the diagnostic test of choice to detect gallstones and assess for findings suggestive of acute cholecystitis and dilation of the common bile duct.
- First-line therapy for asymptomatic gallstones is expectant management.
- First-line therapy for symptomatic gallstones is cholecystectomy.
A minimally invasive treatment for early GI cancers
The treatment of early esophageal, gastric, and colorectal cancer is changing.1 For many years, surgery was the mainstay of treatment for early-stage gastrointestinal cancer. Unfortunately, surgery leads to significant loss of function of the organ, resulting in increased morbidity and decreased quality of life.2
Endoscopic techniques, particularly endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), have been developed and are widely used in Japan, where gastrointestinal cancer is more common than in the West. This article reviews the indications, complications, and outcomes of ESD for early gastrointestinal neoplasms, so that readers will recognize the subset of patients who would benefit from ESD in a Western setting.
ENDOSCOPIC MUCOSAL RESECTION AND SUBMUCOSAL DISSECTION
Since the first therapeutic polypectomy was performed in Japan in 1974, several endoscopic techniques for tumor resection have been developed.3
EMR, one of the most successful and widely used techniques, involves elevating the lesion either with submucosal injection of a solution or with cap suction, and then removing it with a snare.4 Most lesions smaller than 20 mm can be removed in one piece (en bloc).5 Larger lesions are removed in multiple pieces (ie, piecemeal). Unfortunately, some fibrotic lesions, which are usually difficult to lift, cannot be completely removed by EMR.
ESD was first performed in the late 1990s with the aim of overcoming the limitations of EMR in resecting large or fibrotic tumors en bloc.6,7 Since then, ESD technique has been standardized and training centers have been created, especially in Asia, where it is widely used for treatment of early gastric cancer.3,8–10 Since 2012 it has been covered by the Japanese National Health Insurance for treatment of early gastric cancer, and since 2014 for treatment of colorectal malignant tumors measuring 2 to 5 cm.11
Adoption of ESD has been slow in Western countries, where many patients are still referred for surgery or undergo EMR for removal of superficial neoplasms. Reasons for this slow adoption are that gastric cancer is much less common in Western countries, and also that ESD demands a high level of technical skill, is difficult to learn, and is expensive.3,12,13 However, small groups of Western endoscopists have become interested and are advocating it, first studying it on their own and then training in a Japanese center and learning from experts performing the procedure.
Therefore, in a Western setting, ESD should be performed in specialized endoscopy centers and offered to selected patients.1
CANDIDATES SHOULD HAVE EARLY-STAGE, SUPERFICIAL TUMORS
Ideal candidates for endoscopic resection are patients who have early cancer with a negligible risk of lymph node metastasis, such as cancer limited to the mucosa (stage T1a).7 Therefore, to determine the best treatment for a patient with a newly diagnosed gastrointestinal neoplasm, it is mandatory to estimate the depth of invasion.
The depth of invasion is directly correlated with lymph node involvement, which is ultimately the main predictive factor for long-term adverse outcomes of gastrointestinal tumors.4,14–17 Accurate multidisciplinary preprocedure estimations are mandatory, as incorrect evaluations may result in inappropriate therapy and residual cancer.18
Other factors that have been used to predict lymph node involvement include tumor size, macroscopic appearance, histologic differentiation, and lymphatic and vascular involvement.19 Some of these factors can be assessed by special endoscopic techniques (chromoendoscopy and narrow-band imaging with magnifying endoscopy) that allow accurate real-time estimation of the depth of invasion of the lesion.5,17,20–27 Evaluation of microsurface and microvascular arrangements is especially useful for determining the feasibility of ESD in gastric tumors, evaluation of intracapillary loops is useful in esophageal lesions, and assessment of mucosal pit patterns is useful for colorectal lesions.21–29
Endoscopic ultrasonography is another tool that has been used to estimate the depth of the tumor. Although it can differentiate between definite intramucosal and definite submucosal invasive cancers, its ability to confirm minute submucosal invasion is limited. Its use as the sole tumor staging modality is not encouraged, and it should always be used in conjunction with endoscopic evaluation.18
Though the aforementioned factors help stratify patients, pathologic staging is the best predictor of lymph node metastasis. ESD provides adequate specimens for accurate pathologic evaluation, as it removes lesions en bloc.30
All patients found to have risk factors for lymph node metastasis on endoscopic, ultrasonographic, or pathologic analysis should be referred for surgical evaluation.9,19,31,32
ENDOSCOPIC SUBMUCOSAL DISSECTION
Before the procedure, the patient’s physicians need to do the following:
Determine the best type of intervention (EMR, ESD, ablation, surgery) for the specific lesion.3 A multidisciplinary approach is encouraged, with involvement of the internist, gastroenterologist, and surgeon.
Plan for anesthesia, additional consultations, pre- and postprocedural hospital admission, and need for special equipment.33
During the procedure
Define the lateral extent of the lesion using magnification chromoendoscopy or narrow-band imaging. In the stomach, a biopsy sample should be taken from the worst-looking segment and from normal-looking mucosa. Multiple biopsies should be avoided to prevent subsequent fibrosis.33 In the colon, biopsy should be avoided.34
Identify and circumferentially mark the target lesion. Cautery or argon plasma coagulation can be used for making markings at a distance of 5 to 10 mm from the edges.33 This is done to recognize the borders of the lesion, because they can become distorted after submucosal injection.14 This step is unnecessary in colorectal cases, as tumor margins can be adequately visualized after chromoendoscopy.16,35
Lift the lesion by injecting saline, 0.5% hyaluronate, or glycerin to create a submucosal fluid cushion.19,33
Perform a circumferential incision lateral to the mucosal margins to allow for a normal tissue margin.33 Partial incision is performed for esophageal and colorectal ESD to avoid fluid leakage from the submucosal layer, achieving a sustained submucosal lift and safer dissection.16
Submucosal dissection. The submucosal layer is dissected with an electrocautery knife until the lesion is completely removed. Dissection should be done carefully to keep the submucosal plane.33 Hemoclips or hemostat forceps can be used to control visible bleeding. The resected specimen is then stretched and fixed to a board using small pins for further histopathologic evaluation.35
Postprocedural monitoring. All patients should be admitted for overnight observation. Those who undergo gastric ESD should receive high-dose acid suppression, and the next day they can be started on a liquid diet.19
STOMACH CANCER
Indications for ESD for stomach cancer in the East
The incidence of gastric cancer is higher in Japan and Korea, where widespread screening programs have led to early identification and early treatment of this disease.36
Pathology studies37 of samples from patients with gastric cancer identified the following as risk factors for lymph node metastasis, which would make ESD unsuitable:
- Undifferentiated type
- Tumors larger than 2 cm
- Lymphatic or venous involvement
- Submucosal invasion
- Ulcerative change.
Based on these findings, the situations in which there was no risk of lymph node involvement (ie, when none of the above factors are present) were accepted as absolute indications for endoscopic resection of early gastric cancer.38 Further histologic studies identified a subset of patients with lesions with very low risk of lymph node metastasis, which outweighed the risk of surgery. Based on these findings, expanded criteria for gastric ESD were proposed,39,40 and the Japanese gastric cancer treatment guidelines now include these expanded preoperative indications9,17 (Table 1).
The Japanese Gastric Cancer Association has proposed a treatment algorithm based on the histopathologic evaluation after resection (Figure 2).9
Outcomes
In the largest series of patients who underwent curative ESD for early gastric cancer, the 5-year survival rate was 92.6%, the 5-year disease-specific survival rate was 99.9%, and the 5-year relative survival rate was 105%.41
Similarly, in a Japanese population-based survival analysis, the relative 5-year survival rate for localized gastric cancer was 94.4%.42 Rates of en bloc resection and complete resection with ESD are higher than those with EMR, resulting in a lower risk of local recurrence in selected patients who undergo ESD.8,43,44
Although rare, local recurrence after curative gastric ESD has been reported.45 The annual incidence of local recurrence has been estimated to be 0.84%.46
ESD entails a shorter hospital stay and requires fewer resources than surgery, resulting in lower medical costs (Table 2).44 Additionally, as endoscopic resection is associated with less morbidity, fewer procedure-related adverse events, and fewer complications, ESD could be used as the standard treatment for early gastric cancer.47,48
The Western perspective on endoscopic submucosal dissection for gastric cancer
Since the prevalence of gastric cancer in Western countries is significantly lower than in Japan and Korea, local data and experience are scarce. However, experts performing ESD in the West have adopted the indications of the Japan Gastroenterological Endoscopy Society. The European Society of Gastrointestinal Endoscopy recommends ESD for excision of most superficial gastric neoplasms, with EMR being preferred only in lesions smaller than 15 mm, Paris classification 0 or IIA.5,32
Patients with gastric lesions measuring 15 mm or larger should undergo high-quality endoscopy, preferably chromoendoscopy, to evaluate the mucosal patterns and determine the depth of invasion. If superficial involvement is confirmed, other imaging techniques are not routinely recommended.5 A surgery consult is also recommended.
ESOPHAGEAL CANCER
Indications for ESD for esophageal cancer in the East
Due to the success of ESD for early gastric cancer, this technique is now also used for superficial esophageal neoplasms.19,49 It should be done in a specialized center, as it is more technically difficult than gastric ESD: the esophageal lumen is narrow, the wall is thin, and the esophagus moves with respiration and heartbeat.50 A multidisciplinary approach including an endoscopist, a surgeon, and a pathologist is highly recommended for evaluation and treatment.
EMR is preferred for removal of mucosal cancer, in view of its safety profile and success rates. ESD can be considered in cases of lesions larger than 15 mm, poorly lifting tumors, and those with the possibility of submucosal invasion (Table 3).5,45,49,51
Circumference involvement is critical when determining eligible candidates, as a defect involving more than three-fourths of the esophageal circumference can lead to esophageal strictures.52 Controlled prospective studies have shown promising results from giving intralesional and oral steroids to prevent stricture after ESD, which could potentially overcome this size limitation.53,54
Outcomes for esophageal cancer
ESD has been shown to be safe and effective, achieving en bloc resection in 85% to 100% of patients.19,51 Its advantages over EMR include en bloc resection, complete resection, and high curative rates, resulting in higher recurrence-free survival.2,55,56 Although the incidence of complications such as bleeding, perforation, and stricture formation are higher with ESD, patients usually recover uneventfully.2,19,20
ESD in the esophagus: The Western perspective
As data on the efficacy of EMR vs ESD for the treatment of Barrett esophagus with adenocarcinoma are limited, EMR is the gold standard endoscopic technique for removal of visible esophageal dysplastic lesions.5,51,57 ESD can be considered for tumors larger than 15 mm, for poorly lifting lesions, and if there is suspicion of submucosal invasion.5
Patients should be evaluated by an experienced endoscopist, using an advanced imaging technique such as narrow-band imaging or chromoendoscopy. If suspicious features are found, endoscopic ultrasonography should be considered to confirm submucosal invasion or lymph node involvement.5
COLORECTAL CANCER
Indications for ESD for colorectal cancer in the East
Colon cancer is one of the leading causes of cancer-related deaths worldwide.58 Since ESD has been found to be effective and safe in treating gastric cancer, it has also been used to remove large colorectal tumors.59 However, ESD is not universally accepted in the treatment of colorectal neoplasms due to its greater technical difficulty, longer procedural time, and higher risk of perforating the thinner colonic wall compared with EMR.21,60
Outcomes for colorectal cancer
Tumor size of 50 mm or larger is a risk factor for complications, while a high procedure volume at the center is a protective factor.60
Endoscopic treatment of colorectal cancer: The Western perspective
EMR is the gold standard for removal of superficial colorectal lesions. However, ESD can be considered if there is suspicion of superficial submucosal invasion, especially for lesions larger than 20 mm that cannot be resected en bloc by EMR.32 ESD can also be used for fibrotic lesions not amenable to complete EMR removal, or as a salvage procedure after recurrence after EMR.67 Proper selection of cases is critical.1
Patients who have a superficial colonic lesion should be evaluated by means of high-definition endoscopy and chromoendoscopy to assess the mucosal pattern and establish feasibility of endoscopic resection. If submucosal invasion is suspected, staging with endoscopic ultrasonography or magnetic resonance imaging should be considered.5
FOLLOW-UP AFTER ESD
Endoscopic surveillance after the procedure is recommended, given the persistent risk of metachronous cancer after curative ESD due to its organ-sparing quality.68 Surveillance endoscopy aims to achieve early detection and subsequent endoscopic resection of metachronous lesions.
Histopathologic evaluation assessing the presence of malignant cells in the margins of a resected sample is mandatory for determining the next step in treatment. If margins are negative, follow-up endoscopy can be done every 6 to 12 months. If margins are positive, the approach includes surgery, reattempting ESD or endoscopic surveillance in 3 or 6 months.3,32 Although the surveillance strategy varies according to individual risk of metachronous cancer, it should be continued indefinitely.68
COMPLICATIONS OF ESD
The most common procedure-related complications of ESD are bleeding, perforation, and stricture. Most intraprocedural adverse events can be managed endoscopically.69
Bleeding
Most bleeding occurs during the procedure or early after it and can be controlled with electrocautery.49,69 No episodes of massive bleeding, defined as causing clinical symptoms and requiring transfusion or surgery, have been reported.20,43,55
In gastric ESD, delayed bleeding rates have ranged from 0 to 15.6%.69 Bleeding may be prevented with endoscopic coagulation of visible vessels after dissection has been completed and by proton pump inhibitor therapy.70,71 Excessive coagulation should be avoided to lower the risk of perforation.33
In colorectal ESD the bleeding rate has been reported to be 2.2%; applying coagulation to an area where a blood vessel is suspected before cutting (precoagulation) may prevent subsequent bleeding.21
Perforation
For gastric ESD, perforation rates range from 1.2% to 5.2%.69 Esophageal perforation rates can be up to 4%.49 In colorectal ESD, perforation rates have been reported to be 1.6% to 6.6%.60,72
Although most of the cases were successfully managed with conservative treatment, some required emergency surgery.60,73
Strictures
In a case series of 532 patients undergoing gastric ESD, stricture was reported in 5 patients, all of whom presented with obstructive symptoms.74 Risk factors for post-ESD gastric stenosis are a mucosal defect with a circumferential extent of more than three-fourths or a longitudinal extent of more than 5 cm.75
Strictures are common after esophageal ESD, with rates ranging from 2% to 26%. The risk is higher when longer segments are removed or circumferential resection is performed. As previously mentioned, this complication may be reduced with ingestion or injection of steroids after the procedure.53,54
Surprisingly, ESD of large colorectal lesions involving more than three-fourths of the circumference of the rectum is rarely complicated by stenosis.76
LIMITATIONS OF ESD
ESD requires a high level of technical skill, is time-consuming, and has a higher rate of complications than conventional endoscopic resection. A standardized ESD training system is needed, as the procedure is more difficult than EMR. Training in porcine models has been shown to confer competency in ESD in a Western setting.13,16,33
Colorectal ESD is an even more challenging procedure, given the potential for complications related to its anatomy. Training centers in Japan usually have their trainees first master gastric ESD, then assist in more than 20 colorectal ESDs conducted by experienced endoscopists, and accomplish 30 cases before performing the procedure safely and independently.
As the incidence of gastric cancer is low in Western countries, trainees may also begin with lower rectal lesions, which are easier to remove.77 Incorporation of ESD in the West would require a clear treatment algorithm. It is a complex procedure, with higher rates of complications, a prolonged learning curve, and prolonged procedure time. Therefore, it should be performed in specialized centers and under the special situations discussed here to ensure that the benefits for the patients outweigh the risks.
VALUE OF ENDOSCOPIC SUBMUCOSAL DISSECTION
The optimal method for resecting gastrointestinal neoplasms should be safe, cost-effective, and quick and should also completely remove the lesion. The best treatment strategy takes into account the characteristics of the lesion and the comorbidities and wishes of the patient. Internists should be aware of the multiple options available to achieve the best outcome for the patient.1
Endoscopic resection of superficial gastrointestinal neoplasms, including EMR and ESD, has been a subject of increasing interest due to its minimally invasive and potentially curative character. However, cancer can recur after endoscopic resection because the procedure is organ-sparing.
ESD allows resection of early gastrointestinal tumors with a minimally invasive technique. It can achieve higher curative resection rates and lower recurrence rates compared with EMR. Compared with surgery, ESD leads to less morbidity, fewer procedure-related complications, and lower medical costs. Indications should be rigorously followed to achieve successful treatments in selected patients.
Multiple variables have to be taken into account when deciding which treatment is best, such as tumor characteristics, the patient’s baseline condition, physician expertise, and hospital resources.48 Less-invasive treatments may improve the prognosis of patients. No matter the approach, patients should be treated in specialized treatment centers.
Internal medicine physicians should be aware of the advances in treatments for early gastrointestinal cancer so appropriate options can be considered.
- Burgess NG, Bourke MJ. Endoscopic resection of colorectal lesions: the narrowing divide between East and West. Dig Endosc 2016; 28:296–305.
- Kim DH, Jung HY, Gong EJ, et al. Endoscopic and oncologic outcomes of endoscopic resection for superficial esophageal neoplasm. Gut Liver 2015; 9:470–477.
- Draganov PV, Gotoda T, Chavalitdhamrong D, Wallace MB. Techniques of endoscopic submucosal dissection: application for the Western endoscopist? Gastrointest Endosc 2013; 78:677–688.
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14:101–112.
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47:829–854.
- Farhat S, Chaussade S, Ponchon T, et al; SFED ESD Study Group. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy 2011; 43:664–670.
- Gotoda T, Jung H. Endoscopic resection (endoscopic mucosal resection/endoscopic submucosal dissection) for early gastric cancer. Dig Endosc 2013; 25(suppl 1):55–63.
- Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 2009; 69:1228–1235.
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011; 14:113–123.
- Ono H. Endoscopic submucosal dissection for early gastric cancer. Chin J Dig Dis 2005; 6:119–121.
- Watanabe T, Itabashi M, Shimada Y, et al; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 2015; 20:207–239.
- Oyama T, Yahagi N, Ponchon T, Kiesslich T, Berr F. How to establish endoscopic submucosal dissection in Western countries. World J Gastroenterol 2015; 21:11209–11220.
- Bhatt A, Abe S, Kumaravel A, et al. SU1575 Western skill training in endoscopic submucosal dissection (ESD)—an international remote video based study—the WEST ESD Study. Gastrointest Endosc 2015; 81(suppl):AB335–AB336.
- Sano T, Sasako M, Kinoshita T, Maruyama K. Recurrence of early gastric cancer follow-up of 1475 patients and review of the Japanese literature. Cancer 1993; 72:3174–3178.
- Japan Esophageal Society. Japanese classification of esophageal cancer, tenth edition: part I. Esophagus 2009; 6:1–25.
- Bhatt A, Abe S, Kumaravel A, Vargo J, Saito Y. Indications and techniques for endoscopic submucosal dissection. Am J Gastroenterol 2015; 110:784–791.
- Eleftheriadis N, Inoue H, Ikeda H, et al. Definition and staging of early esophageal, gastric and colorectal cancer. J Tumor 2014; 2:161–178.
- Yoshinaga S, Oda I, Nonaka S, Kushima R, Saito Y. Endoscopic ultrasound using ultrasound probes for the diagnosis of early esophageal and gastric cancers. World J Gastrointest Endosc 2012; 4:218–226.
- Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D; ESMO Guidelines Working Group. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi51–vi56.
- Higuchi K, Tanabe S, Azuma M, et al. A phase II study of endoscopic submucosal dissection for superficial esophageal neoplasms (KDOG 0901). Gastrointest Endosc 2013; 78:704–710.
- Sakamoto T, Mori G, Yamada M, et al. Endoscopic submucosal dissection for colorectal neoplasms: a review. World J Gastroenterol 2014; 20:16153–16158.
- Ohta A, Tominaga K, Sakai Y. Efficacy of magnifying colonoscopy for the diagnosis of colorectal neoplasia: comparison with histopathological findings. Dig Endosc 2004; 16:308–314.
- Katagiri A, Fu K, Sano Y, et al. Narrow band imaging with magnifying colonoscopy as diagnostic tool for predicting histology of early colorectal neoplasia. Aliment Pharmacol Ther 2008; 27:1269–1274.
- Fu KI, Kato S, Sano Y, et al. Staging of early colorectal cancers: magnifying colonoscopy versus endoscopic ultrasonography for estimation of depth of invasion. Dig Dis Sci 2008; 53:1886–1892.
- Uraoka T, Saito Y, Ikematsu H, Yamamoto K, Sano Y. Sano’s capillary pattern classification for narrow-band imaging of early colorectal lesions. Dig Endosc 2011; 23(suppl 1):112–115.
- Ikematsu H, Matsuda T, Emura F, et al. Efficacy of capillary pattern type IIIA/IIIB by magnifying narrow band imaging for estimating depth of invasion of early colorectal neoplasms. BMC Gastroenterol 2010;10:33.
- Matsuda T, Fujii T, Saito Y, et al. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol 2008; 103:2700–2706.
- Sato H, Inoue H, Ikeda H, et al. Utility of intrapapillary capillary loops seen on magnifying narrow-band imaging in estimating invasive depth of esophageal squamous cell carcinoma. Endoscopy 2015; 8:122–128.
- Muto M, Yao K, Kaise M, et al. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G). Dig Endosc 2016; 28:379–393.
- Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; European Society for Medical Oncology (ESMO); European Society of Surgical Oncology (ESSO); European Society of Radiotherapy and Oncology (ESTRO). Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi57–vi63.
- Kuwano H, Nishimura Y, Ohtsu A, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus. April 2007 edition: part I - Edited by the Japan Esophageal Society. Esophagus 2008; 5:61–73.
- Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 2015; 27:417–434.
- Gotoda T, Ho KY, Soetikno R, Kaltenbach T, Draganov P. Gastric ESD: current status and future directions of devices and training. Gastrointest Endosc Clin North Am 2014; 24:213–233.
- Saito Y, Sakamoto T, Nakajima T, Matsuda T. Colorectal ESD: current indications and latest technical advances. Gastrointest Endosc Clin N Am 2014; 24:245–255.
- Saito Y, Otake Y, Sakamoto T, et al. Indications for and technical aspects of colorectal endoscopic submucosal dissection. Gut Liver 2013; 7:263–269.
- Saragoni L. Upgrading the definition of early gastric cancer: better staging means more appropriate treatment. Cancer Biol Med 2015; 12:355–361.
- Tsujitani S, Oka S, Saito H, et al. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery 1999; 125:148–154.
- Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc 2003; 57:567–579.
- Hirasawa T, Gotoda T, Miyata S, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009; 12:148–152.
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000; 3:219–225.
- Suzuki H, Oda I, Abe S, et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer 2016; 19:198–205.
- Matsuda T, Ajiki W, Marugame T, Ioka A, Tsukuma H, Sobue T; Research Group of Population-Based Cancer Registries of Japan. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol 2011; 41:40–51.
- Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc 2011; 74:485–493.
- Kim Y, Kim YW, Choi IJ, et al. Cost comparison between surgical treatments and endoscopic submucosal dissection in patients with early gastric cancer in Korea. Gut Liver 2015; 9:174–180.
- Abe S, Oda I, Nakajima T, et al. A case of local recurrence and distant metastasis following curative endoscopic submucosal dissection of early gastric cancer. Gastric Cancer 2015; 18:188–192.
- Hahn KY, Park JC, Kim EH, et al. Incidence and impact of scheduled endoscopic surveillance on recurrence after curative endoscopic resection for early gastric cancer. Gastrointest Endosc 2016; 84:628–638.e1.
- Wang S, Zhang Z, Liu M, Li S, Jiang C. Endoscopic resection compared with gastrectomy to treat early gastric cancer: a systematic review and meta-analysis. PLoS One 2015; 10:e0144774.
- Kondo A, de Moura EG, Bernardo WM, et al. Endoscopy vs surgery in the treatment of early gastric cancer: systematic review. World J Gastroenterol 2015; 21:13177–13187.
- Kothari S, Kaul V. Endoscopic mucosal resection and endoscopic submucosal dissection for endoscopic therapy of Barrett’s esophagus-related neoplasia. Gastroenterol Clin North Am 2015; 44:317–335.
- Yamashita T, Zeniya A, Ishii H, et al. Endoscopic mucosal resection using a cap-fitted panendoscope and endoscopic submucosal dissection as optimal endoscopic procedures for superficial esophageal carcinoma. Surg Endosc 2011; 25:2541–2546.
- Kagemoto K, Oka S, Tanaka S, et al. Clinical outcomes of endoscopic submucosal dissection for superficial Barrett’s adenocarcinoma. Gastrointest Endosc 2014; 80:239–245.
- Katada C, Muto M, Manabe T, Boku N, Ohtsu A, Yoshida S. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc 2003; 57:165–169.
- Hanaoka N, Ishihara R, Takeuchi Y, et al. 1139: A single session of intralesional steroid injection to prevent esophageal stricture after endoscopic submucosal dissection for esophageal squamous cell carcinoma. Gastrointest Endosc 2012; 75(suppl):AB175.
- Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc 2011; 73:1115–1121.
- Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009; 70:860–866.
- Katada C, Muto M, Manabe T, Ohtsu A, Yoshida S. Local recurrence of squamous-cell carcinoma of the esophagus after EMR. Gastrointest Endosc 2005; 61:219–225.
- Hirasawa K, Kokawa A, Oka H, et al. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc 2010; 72:960–966.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
- Nakajima T, Saito Y, Tanaka S, et al. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc 2013; 27:3262–3770.
- Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010; 72:1217–1225.
- Tanaka S, Saitoh Y, Matsuda T, et al; Japanese Society of Gastroenterology. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol 2015; 50:252–260.
- Oka S, Tanaka S, Saito Y, et al; Colorectal Endoscopic Resection Standardization Implementation Working Group of the Japanese Society for Cancer of the Colon and Rectum, Tokyo, Japan. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol 2015; 110:697–707.
- Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc 2010; 24:343–352.
- Makazu M, Sakamoto T, So E, et al. Relationship between indeterminate or positive lateral margin and local recurrence after endoscopic resection of colorectal polyps. Endosc Int Open 2015; 3:E252–E257.
- Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014; 46:388–402.
- Fujiya M, Tanaka K, Dokoshi T, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc 2015; 81:583–595.
- Rahmi G, Tanaka S, Ohara Y, et al. Efficacy of endoscopic submucosal dissection for residual or recurrent superficial colorectal tumors after endoscopic mucosal resection. J Dig Dis 2015; 16:14–21.
- Abe S, Oda I, Suzuki H, et al. Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy 2015; 47:1113–1118.
- Oda I, Suzuki H, Nonaka S, Yoshinaga S. Complications of gastric endoscopic submucosal dissection. Dig Endosc 2013; 25(suppl 1):71–78.
- Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection—an analysis of risk factors. Endoscopy 2008; 40:179–183.
- Uedo N, Takeuchi Y, Yamada T, et al. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol 2007; 102:1610–1616.
- Hayashi N, Tanaka S, Nishiyama S, et al. Predictors of incomplete resection and perforation associated with endoscopic submucosal dissection for colorectal tumors. Gastrointest Endosc 2014; 79:427–435.
- Suzuki H, Oda I, Sekiguchi M, et al. Management and associated factors of delayed perforation after gastric endoscopic submucosal dissection. World J Gastroenterol 2015; 21:12635–12643.
- Tsunada S, Ogata S, Mannen K, et al. Case series of endoscopic balloon dilation to treat a stricture caused by circumferential resection of the gastric antrum by endoscopic submucosal dissection. Gastrointest Endosc 2008; 67:979–983.
- Coda S, Oda I, Gotoda T, Yokoi C, Kikuchi T, Ono H. Risk factors for cardiac and pyloric stenosis after endoscopic submucosal dissection, and efficacy of endoscopic balloon dilation treatment. Endoscopy 2009; 41:421–426.
- Abe S, Sakamoto T, Takamaru H, et al. Stenosis rates after endoscopic submucosal dissection of large rectal tumors involving greater than three quarters of the luminal circumference. Surg Endosc 2016; 30:5459–5464.
- Sakamoto T, Saito Y, Fukunaga S, Nakajima T, Matsuda T. Learning curve associated with colorectal endoscopic submucosal dissection for endoscopists experienced in gastric endoscopic submucosal dissection. Dis Colon Rectum 2011; 54:1307–1312.
The treatment of early esophageal, gastric, and colorectal cancer is changing.1 For many years, surgery was the mainstay of treatment for early-stage gastrointestinal cancer. Unfortunately, surgery leads to significant loss of function of the organ, resulting in increased morbidity and decreased quality of life.2
Endoscopic techniques, particularly endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), have been developed and are widely used in Japan, where gastrointestinal cancer is more common than in the West. This article reviews the indications, complications, and outcomes of ESD for early gastrointestinal neoplasms, so that readers will recognize the subset of patients who would benefit from ESD in a Western setting.
ENDOSCOPIC MUCOSAL RESECTION AND SUBMUCOSAL DISSECTION
Since the first therapeutic polypectomy was performed in Japan in 1974, several endoscopic techniques for tumor resection have been developed.3
EMR, one of the most successful and widely used techniques, involves elevating the lesion either with submucosal injection of a solution or with cap suction, and then removing it with a snare.4 Most lesions smaller than 20 mm can be removed in one piece (en bloc).5 Larger lesions are removed in multiple pieces (ie, piecemeal). Unfortunately, some fibrotic lesions, which are usually difficult to lift, cannot be completely removed by EMR.
ESD was first performed in the late 1990s with the aim of overcoming the limitations of EMR in resecting large or fibrotic tumors en bloc.6,7 Since then, ESD technique has been standardized and training centers have been created, especially in Asia, where it is widely used for treatment of early gastric cancer.3,8–10 Since 2012 it has been covered by the Japanese National Health Insurance for treatment of early gastric cancer, and since 2014 for treatment of colorectal malignant tumors measuring 2 to 5 cm.11
Adoption of ESD has been slow in Western countries, where many patients are still referred for surgery or undergo EMR for removal of superficial neoplasms. Reasons for this slow adoption are that gastric cancer is much less common in Western countries, and also that ESD demands a high level of technical skill, is difficult to learn, and is expensive.3,12,13 However, small groups of Western endoscopists have become interested and are advocating it, first studying it on their own and then training in a Japanese center and learning from experts performing the procedure.
Therefore, in a Western setting, ESD should be performed in specialized endoscopy centers and offered to selected patients.1
CANDIDATES SHOULD HAVE EARLY-STAGE, SUPERFICIAL TUMORS
Ideal candidates for endoscopic resection are patients who have early cancer with a negligible risk of lymph node metastasis, such as cancer limited to the mucosa (stage T1a).7 Therefore, to determine the best treatment for a patient with a newly diagnosed gastrointestinal neoplasm, it is mandatory to estimate the depth of invasion.
The depth of invasion is directly correlated with lymph node involvement, which is ultimately the main predictive factor for long-term adverse outcomes of gastrointestinal tumors.4,14–17 Accurate multidisciplinary preprocedure estimations are mandatory, as incorrect evaluations may result in inappropriate therapy and residual cancer.18
Other factors that have been used to predict lymph node involvement include tumor size, macroscopic appearance, histologic differentiation, and lymphatic and vascular involvement.19 Some of these factors can be assessed by special endoscopic techniques (chromoendoscopy and narrow-band imaging with magnifying endoscopy) that allow accurate real-time estimation of the depth of invasion of the lesion.5,17,20–27 Evaluation of microsurface and microvascular arrangements is especially useful for determining the feasibility of ESD in gastric tumors, evaluation of intracapillary loops is useful in esophageal lesions, and assessment of mucosal pit patterns is useful for colorectal lesions.21–29
Endoscopic ultrasonography is another tool that has been used to estimate the depth of the tumor. Although it can differentiate between definite intramucosal and definite submucosal invasive cancers, its ability to confirm minute submucosal invasion is limited. Its use as the sole tumor staging modality is not encouraged, and it should always be used in conjunction with endoscopic evaluation.18
Though the aforementioned factors help stratify patients, pathologic staging is the best predictor of lymph node metastasis. ESD provides adequate specimens for accurate pathologic evaluation, as it removes lesions en bloc.30
All patients found to have risk factors for lymph node metastasis on endoscopic, ultrasonographic, or pathologic analysis should be referred for surgical evaluation.9,19,31,32
ENDOSCOPIC SUBMUCOSAL DISSECTION
Before the procedure, the patient’s physicians need to do the following:
Determine the best type of intervention (EMR, ESD, ablation, surgery) for the specific lesion.3 A multidisciplinary approach is encouraged, with involvement of the internist, gastroenterologist, and surgeon.
Plan for anesthesia, additional consultations, pre- and postprocedural hospital admission, and need for special equipment.33
During the procedure
Define the lateral extent of the lesion using magnification chromoendoscopy or narrow-band imaging. In the stomach, a biopsy sample should be taken from the worst-looking segment and from normal-looking mucosa. Multiple biopsies should be avoided to prevent subsequent fibrosis.33 In the colon, biopsy should be avoided.34
Identify and circumferentially mark the target lesion. Cautery or argon plasma coagulation can be used for making markings at a distance of 5 to 10 mm from the edges.33 This is done to recognize the borders of the lesion, because they can become distorted after submucosal injection.14 This step is unnecessary in colorectal cases, as tumor margins can be adequately visualized after chromoendoscopy.16,35
Lift the lesion by injecting saline, 0.5% hyaluronate, or glycerin to create a submucosal fluid cushion.19,33
Perform a circumferential incision lateral to the mucosal margins to allow for a normal tissue margin.33 Partial incision is performed for esophageal and colorectal ESD to avoid fluid leakage from the submucosal layer, achieving a sustained submucosal lift and safer dissection.16
Submucosal dissection. The submucosal layer is dissected with an electrocautery knife until the lesion is completely removed. Dissection should be done carefully to keep the submucosal plane.33 Hemoclips or hemostat forceps can be used to control visible bleeding. The resected specimen is then stretched and fixed to a board using small pins for further histopathologic evaluation.35
Postprocedural monitoring. All patients should be admitted for overnight observation. Those who undergo gastric ESD should receive high-dose acid suppression, and the next day they can be started on a liquid diet.19
STOMACH CANCER
Indications for ESD for stomach cancer in the East
The incidence of gastric cancer is higher in Japan and Korea, where widespread screening programs have led to early identification and early treatment of this disease.36
Pathology studies37 of samples from patients with gastric cancer identified the following as risk factors for lymph node metastasis, which would make ESD unsuitable:
- Undifferentiated type
- Tumors larger than 2 cm
- Lymphatic or venous involvement
- Submucosal invasion
- Ulcerative change.
Based on these findings, the situations in which there was no risk of lymph node involvement (ie, when none of the above factors are present) were accepted as absolute indications for endoscopic resection of early gastric cancer.38 Further histologic studies identified a subset of patients with lesions with very low risk of lymph node metastasis, which outweighed the risk of surgery. Based on these findings, expanded criteria for gastric ESD were proposed,39,40 and the Japanese gastric cancer treatment guidelines now include these expanded preoperative indications9,17 (Table 1).
The Japanese Gastric Cancer Association has proposed a treatment algorithm based on the histopathologic evaluation after resection (Figure 2).9
Outcomes
In the largest series of patients who underwent curative ESD for early gastric cancer, the 5-year survival rate was 92.6%, the 5-year disease-specific survival rate was 99.9%, and the 5-year relative survival rate was 105%.41
Similarly, in a Japanese population-based survival analysis, the relative 5-year survival rate for localized gastric cancer was 94.4%.42 Rates of en bloc resection and complete resection with ESD are higher than those with EMR, resulting in a lower risk of local recurrence in selected patients who undergo ESD.8,43,44
Although rare, local recurrence after curative gastric ESD has been reported.45 The annual incidence of local recurrence has been estimated to be 0.84%.46
ESD entails a shorter hospital stay and requires fewer resources than surgery, resulting in lower medical costs (Table 2).44 Additionally, as endoscopic resection is associated with less morbidity, fewer procedure-related adverse events, and fewer complications, ESD could be used as the standard treatment for early gastric cancer.47,48
The Western perspective on endoscopic submucosal dissection for gastric cancer
Since the prevalence of gastric cancer in Western countries is significantly lower than in Japan and Korea, local data and experience are scarce. However, experts performing ESD in the West have adopted the indications of the Japan Gastroenterological Endoscopy Society. The European Society of Gastrointestinal Endoscopy recommends ESD for excision of most superficial gastric neoplasms, with EMR being preferred only in lesions smaller than 15 mm, Paris classification 0 or IIA.5,32
Patients with gastric lesions measuring 15 mm or larger should undergo high-quality endoscopy, preferably chromoendoscopy, to evaluate the mucosal patterns and determine the depth of invasion. If superficial involvement is confirmed, other imaging techniques are not routinely recommended.5 A surgery consult is also recommended.
ESOPHAGEAL CANCER
Indications for ESD for esophageal cancer in the East
Due to the success of ESD for early gastric cancer, this technique is now also used for superficial esophageal neoplasms.19,49 It should be done in a specialized center, as it is more technically difficult than gastric ESD: the esophageal lumen is narrow, the wall is thin, and the esophagus moves with respiration and heartbeat.50 A multidisciplinary approach including an endoscopist, a surgeon, and a pathologist is highly recommended for evaluation and treatment.
EMR is preferred for removal of mucosal cancer, in view of its safety profile and success rates. ESD can be considered in cases of lesions larger than 15 mm, poorly lifting tumors, and those with the possibility of submucosal invasion (Table 3).5,45,49,51
Circumference involvement is critical when determining eligible candidates, as a defect involving more than three-fourths of the esophageal circumference can lead to esophageal strictures.52 Controlled prospective studies have shown promising results from giving intralesional and oral steroids to prevent stricture after ESD, which could potentially overcome this size limitation.53,54
Outcomes for esophageal cancer
ESD has been shown to be safe and effective, achieving en bloc resection in 85% to 100% of patients.19,51 Its advantages over EMR include en bloc resection, complete resection, and high curative rates, resulting in higher recurrence-free survival.2,55,56 Although the incidence of complications such as bleeding, perforation, and stricture formation are higher with ESD, patients usually recover uneventfully.2,19,20
ESD in the esophagus: The Western perspective
As data on the efficacy of EMR vs ESD for the treatment of Barrett esophagus with adenocarcinoma are limited, EMR is the gold standard endoscopic technique for removal of visible esophageal dysplastic lesions.5,51,57 ESD can be considered for tumors larger than 15 mm, for poorly lifting lesions, and if there is suspicion of submucosal invasion.5
Patients should be evaluated by an experienced endoscopist, using an advanced imaging technique such as narrow-band imaging or chromoendoscopy. If suspicious features are found, endoscopic ultrasonography should be considered to confirm submucosal invasion or lymph node involvement.5
COLORECTAL CANCER
Indications for ESD for colorectal cancer in the East
Colon cancer is one of the leading causes of cancer-related deaths worldwide.58 Since ESD has been found to be effective and safe in treating gastric cancer, it has also been used to remove large colorectal tumors.59 However, ESD is not universally accepted in the treatment of colorectal neoplasms due to its greater technical difficulty, longer procedural time, and higher risk of perforating the thinner colonic wall compared with EMR.21,60
Outcomes for colorectal cancer
Tumor size of 50 mm or larger is a risk factor for complications, while a high procedure volume at the center is a protective factor.60
Endoscopic treatment of colorectal cancer: The Western perspective
EMR is the gold standard for removal of superficial colorectal lesions. However, ESD can be considered if there is suspicion of superficial submucosal invasion, especially for lesions larger than 20 mm that cannot be resected en bloc by EMR.32 ESD can also be used for fibrotic lesions not amenable to complete EMR removal, or as a salvage procedure after recurrence after EMR.67 Proper selection of cases is critical.1
Patients who have a superficial colonic lesion should be evaluated by means of high-definition endoscopy and chromoendoscopy to assess the mucosal pattern and establish feasibility of endoscopic resection. If submucosal invasion is suspected, staging with endoscopic ultrasonography or magnetic resonance imaging should be considered.5
FOLLOW-UP AFTER ESD
Endoscopic surveillance after the procedure is recommended, given the persistent risk of metachronous cancer after curative ESD due to its organ-sparing quality.68 Surveillance endoscopy aims to achieve early detection and subsequent endoscopic resection of metachronous lesions.
Histopathologic evaluation assessing the presence of malignant cells in the margins of a resected sample is mandatory for determining the next step in treatment. If margins are negative, follow-up endoscopy can be done every 6 to 12 months. If margins are positive, the approach includes surgery, reattempting ESD or endoscopic surveillance in 3 or 6 months.3,32 Although the surveillance strategy varies according to individual risk of metachronous cancer, it should be continued indefinitely.68
COMPLICATIONS OF ESD
The most common procedure-related complications of ESD are bleeding, perforation, and stricture. Most intraprocedural adverse events can be managed endoscopically.69
Bleeding
Most bleeding occurs during the procedure or early after it and can be controlled with electrocautery.49,69 No episodes of massive bleeding, defined as causing clinical symptoms and requiring transfusion or surgery, have been reported.20,43,55
In gastric ESD, delayed bleeding rates have ranged from 0 to 15.6%.69 Bleeding may be prevented with endoscopic coagulation of visible vessels after dissection has been completed and by proton pump inhibitor therapy.70,71 Excessive coagulation should be avoided to lower the risk of perforation.33
In colorectal ESD the bleeding rate has been reported to be 2.2%; applying coagulation to an area where a blood vessel is suspected before cutting (precoagulation) may prevent subsequent bleeding.21
Perforation
For gastric ESD, perforation rates range from 1.2% to 5.2%.69 Esophageal perforation rates can be up to 4%.49 In colorectal ESD, perforation rates have been reported to be 1.6% to 6.6%.60,72
Although most of the cases were successfully managed with conservative treatment, some required emergency surgery.60,73
Strictures
In a case series of 532 patients undergoing gastric ESD, stricture was reported in 5 patients, all of whom presented with obstructive symptoms.74 Risk factors for post-ESD gastric stenosis are a mucosal defect with a circumferential extent of more than three-fourths or a longitudinal extent of more than 5 cm.75
Strictures are common after esophageal ESD, with rates ranging from 2% to 26%. The risk is higher when longer segments are removed or circumferential resection is performed. As previously mentioned, this complication may be reduced with ingestion or injection of steroids after the procedure.53,54
Surprisingly, ESD of large colorectal lesions involving more than three-fourths of the circumference of the rectum is rarely complicated by stenosis.76
LIMITATIONS OF ESD
ESD requires a high level of technical skill, is time-consuming, and has a higher rate of complications than conventional endoscopic resection. A standardized ESD training system is needed, as the procedure is more difficult than EMR. Training in porcine models has been shown to confer competency in ESD in a Western setting.13,16,33
Colorectal ESD is an even more challenging procedure, given the potential for complications related to its anatomy. Training centers in Japan usually have their trainees first master gastric ESD, then assist in more than 20 colorectal ESDs conducted by experienced endoscopists, and accomplish 30 cases before performing the procedure safely and independently.
As the incidence of gastric cancer is low in Western countries, trainees may also begin with lower rectal lesions, which are easier to remove.77 Incorporation of ESD in the West would require a clear treatment algorithm. It is a complex procedure, with higher rates of complications, a prolonged learning curve, and prolonged procedure time. Therefore, it should be performed in specialized centers and under the special situations discussed here to ensure that the benefits for the patients outweigh the risks.
VALUE OF ENDOSCOPIC SUBMUCOSAL DISSECTION
The optimal method for resecting gastrointestinal neoplasms should be safe, cost-effective, and quick and should also completely remove the lesion. The best treatment strategy takes into account the characteristics of the lesion and the comorbidities and wishes of the patient. Internists should be aware of the multiple options available to achieve the best outcome for the patient.1
Endoscopic resection of superficial gastrointestinal neoplasms, including EMR and ESD, has been a subject of increasing interest due to its minimally invasive and potentially curative character. However, cancer can recur after endoscopic resection because the procedure is organ-sparing.
ESD allows resection of early gastrointestinal tumors with a minimally invasive technique. It can achieve higher curative resection rates and lower recurrence rates compared with EMR. Compared with surgery, ESD leads to less morbidity, fewer procedure-related complications, and lower medical costs. Indications should be rigorously followed to achieve successful treatments in selected patients.
Multiple variables have to be taken into account when deciding which treatment is best, such as tumor characteristics, the patient’s baseline condition, physician expertise, and hospital resources.48 Less-invasive treatments may improve the prognosis of patients. No matter the approach, patients should be treated in specialized treatment centers.
Internal medicine physicians should be aware of the advances in treatments for early gastrointestinal cancer so appropriate options can be considered.
The treatment of early esophageal, gastric, and colorectal cancer is changing.1 For many years, surgery was the mainstay of treatment for early-stage gastrointestinal cancer. Unfortunately, surgery leads to significant loss of function of the organ, resulting in increased morbidity and decreased quality of life.2
Endoscopic techniques, particularly endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), have been developed and are widely used in Japan, where gastrointestinal cancer is more common than in the West. This article reviews the indications, complications, and outcomes of ESD for early gastrointestinal neoplasms, so that readers will recognize the subset of patients who would benefit from ESD in a Western setting.
ENDOSCOPIC MUCOSAL RESECTION AND SUBMUCOSAL DISSECTION
Since the first therapeutic polypectomy was performed in Japan in 1974, several endoscopic techniques for tumor resection have been developed.3
EMR, one of the most successful and widely used techniques, involves elevating the lesion either with submucosal injection of a solution or with cap suction, and then removing it with a snare.4 Most lesions smaller than 20 mm can be removed in one piece (en bloc).5 Larger lesions are removed in multiple pieces (ie, piecemeal). Unfortunately, some fibrotic lesions, which are usually difficult to lift, cannot be completely removed by EMR.
ESD was first performed in the late 1990s with the aim of overcoming the limitations of EMR in resecting large or fibrotic tumors en bloc.6,7 Since then, ESD technique has been standardized and training centers have been created, especially in Asia, where it is widely used for treatment of early gastric cancer.3,8–10 Since 2012 it has been covered by the Japanese National Health Insurance for treatment of early gastric cancer, and since 2014 for treatment of colorectal malignant tumors measuring 2 to 5 cm.11
Adoption of ESD has been slow in Western countries, where many patients are still referred for surgery or undergo EMR for removal of superficial neoplasms. Reasons for this slow adoption are that gastric cancer is much less common in Western countries, and also that ESD demands a high level of technical skill, is difficult to learn, and is expensive.3,12,13 However, small groups of Western endoscopists have become interested and are advocating it, first studying it on their own and then training in a Japanese center and learning from experts performing the procedure.
Therefore, in a Western setting, ESD should be performed in specialized endoscopy centers and offered to selected patients.1
CANDIDATES SHOULD HAVE EARLY-STAGE, SUPERFICIAL TUMORS
Ideal candidates for endoscopic resection are patients who have early cancer with a negligible risk of lymph node metastasis, such as cancer limited to the mucosa (stage T1a).7 Therefore, to determine the best treatment for a patient with a newly diagnosed gastrointestinal neoplasm, it is mandatory to estimate the depth of invasion.
The depth of invasion is directly correlated with lymph node involvement, which is ultimately the main predictive factor for long-term adverse outcomes of gastrointestinal tumors.4,14–17 Accurate multidisciplinary preprocedure estimations are mandatory, as incorrect evaluations may result in inappropriate therapy and residual cancer.18
Other factors that have been used to predict lymph node involvement include tumor size, macroscopic appearance, histologic differentiation, and lymphatic and vascular involvement.19 Some of these factors can be assessed by special endoscopic techniques (chromoendoscopy and narrow-band imaging with magnifying endoscopy) that allow accurate real-time estimation of the depth of invasion of the lesion.5,17,20–27 Evaluation of microsurface and microvascular arrangements is especially useful for determining the feasibility of ESD in gastric tumors, evaluation of intracapillary loops is useful in esophageal lesions, and assessment of mucosal pit patterns is useful for colorectal lesions.21–29
Endoscopic ultrasonography is another tool that has been used to estimate the depth of the tumor. Although it can differentiate between definite intramucosal and definite submucosal invasive cancers, its ability to confirm minute submucosal invasion is limited. Its use as the sole tumor staging modality is not encouraged, and it should always be used in conjunction with endoscopic evaluation.18
Though the aforementioned factors help stratify patients, pathologic staging is the best predictor of lymph node metastasis. ESD provides adequate specimens for accurate pathologic evaluation, as it removes lesions en bloc.30
All patients found to have risk factors for lymph node metastasis on endoscopic, ultrasonographic, or pathologic analysis should be referred for surgical evaluation.9,19,31,32
ENDOSCOPIC SUBMUCOSAL DISSECTION
Before the procedure, the patient’s physicians need to do the following:
Determine the best type of intervention (EMR, ESD, ablation, surgery) for the specific lesion.3 A multidisciplinary approach is encouraged, with involvement of the internist, gastroenterologist, and surgeon.
Plan for anesthesia, additional consultations, pre- and postprocedural hospital admission, and need for special equipment.33
During the procedure
Define the lateral extent of the lesion using magnification chromoendoscopy or narrow-band imaging. In the stomach, a biopsy sample should be taken from the worst-looking segment and from normal-looking mucosa. Multiple biopsies should be avoided to prevent subsequent fibrosis.33 In the colon, biopsy should be avoided.34
Identify and circumferentially mark the target lesion. Cautery or argon plasma coagulation can be used for making markings at a distance of 5 to 10 mm from the edges.33 This is done to recognize the borders of the lesion, because they can become distorted after submucosal injection.14 This step is unnecessary in colorectal cases, as tumor margins can be adequately visualized after chromoendoscopy.16,35
Lift the lesion by injecting saline, 0.5% hyaluronate, or glycerin to create a submucosal fluid cushion.19,33
Perform a circumferential incision lateral to the mucosal margins to allow for a normal tissue margin.33 Partial incision is performed for esophageal and colorectal ESD to avoid fluid leakage from the submucosal layer, achieving a sustained submucosal lift and safer dissection.16
Submucosal dissection. The submucosal layer is dissected with an electrocautery knife until the lesion is completely removed. Dissection should be done carefully to keep the submucosal plane.33 Hemoclips or hemostat forceps can be used to control visible bleeding. The resected specimen is then stretched and fixed to a board using small pins for further histopathologic evaluation.35
Postprocedural monitoring. All patients should be admitted for overnight observation. Those who undergo gastric ESD should receive high-dose acid suppression, and the next day they can be started on a liquid diet.19
STOMACH CANCER
Indications for ESD for stomach cancer in the East
The incidence of gastric cancer is higher in Japan and Korea, where widespread screening programs have led to early identification and early treatment of this disease.36
Pathology studies37 of samples from patients with gastric cancer identified the following as risk factors for lymph node metastasis, which would make ESD unsuitable:
- Undifferentiated type
- Tumors larger than 2 cm
- Lymphatic or venous involvement
- Submucosal invasion
- Ulcerative change.
Based on these findings, the situations in which there was no risk of lymph node involvement (ie, when none of the above factors are present) were accepted as absolute indications for endoscopic resection of early gastric cancer.38 Further histologic studies identified a subset of patients with lesions with very low risk of lymph node metastasis, which outweighed the risk of surgery. Based on these findings, expanded criteria for gastric ESD were proposed,39,40 and the Japanese gastric cancer treatment guidelines now include these expanded preoperative indications9,17 (Table 1).
The Japanese Gastric Cancer Association has proposed a treatment algorithm based on the histopathologic evaluation after resection (Figure 2).9
Outcomes
In the largest series of patients who underwent curative ESD for early gastric cancer, the 5-year survival rate was 92.6%, the 5-year disease-specific survival rate was 99.9%, and the 5-year relative survival rate was 105%.41
Similarly, in a Japanese population-based survival analysis, the relative 5-year survival rate for localized gastric cancer was 94.4%.42 Rates of en bloc resection and complete resection with ESD are higher than those with EMR, resulting in a lower risk of local recurrence in selected patients who undergo ESD.8,43,44
Although rare, local recurrence after curative gastric ESD has been reported.45 The annual incidence of local recurrence has been estimated to be 0.84%.46
ESD entails a shorter hospital stay and requires fewer resources than surgery, resulting in lower medical costs (Table 2).44 Additionally, as endoscopic resection is associated with less morbidity, fewer procedure-related adverse events, and fewer complications, ESD could be used as the standard treatment for early gastric cancer.47,48
The Western perspective on endoscopic submucosal dissection for gastric cancer
Since the prevalence of gastric cancer in Western countries is significantly lower than in Japan and Korea, local data and experience are scarce. However, experts performing ESD in the West have adopted the indications of the Japan Gastroenterological Endoscopy Society. The European Society of Gastrointestinal Endoscopy recommends ESD for excision of most superficial gastric neoplasms, with EMR being preferred only in lesions smaller than 15 mm, Paris classification 0 or IIA.5,32
Patients with gastric lesions measuring 15 mm or larger should undergo high-quality endoscopy, preferably chromoendoscopy, to evaluate the mucosal patterns and determine the depth of invasion. If superficial involvement is confirmed, other imaging techniques are not routinely recommended.5 A surgery consult is also recommended.
ESOPHAGEAL CANCER
Indications for ESD for esophageal cancer in the East
Due to the success of ESD for early gastric cancer, this technique is now also used for superficial esophageal neoplasms.19,49 It should be done in a specialized center, as it is more technically difficult than gastric ESD: the esophageal lumen is narrow, the wall is thin, and the esophagus moves with respiration and heartbeat.50 A multidisciplinary approach including an endoscopist, a surgeon, and a pathologist is highly recommended for evaluation and treatment.
EMR is preferred for removal of mucosal cancer, in view of its safety profile and success rates. ESD can be considered in cases of lesions larger than 15 mm, poorly lifting tumors, and those with the possibility of submucosal invasion (Table 3).5,45,49,51
Circumference involvement is critical when determining eligible candidates, as a defect involving more than three-fourths of the esophageal circumference can lead to esophageal strictures.52 Controlled prospective studies have shown promising results from giving intralesional and oral steroids to prevent stricture after ESD, which could potentially overcome this size limitation.53,54
Outcomes for esophageal cancer
ESD has been shown to be safe and effective, achieving en bloc resection in 85% to 100% of patients.19,51 Its advantages over EMR include en bloc resection, complete resection, and high curative rates, resulting in higher recurrence-free survival.2,55,56 Although the incidence of complications such as bleeding, perforation, and stricture formation are higher with ESD, patients usually recover uneventfully.2,19,20
ESD in the esophagus: The Western perspective
As data on the efficacy of EMR vs ESD for the treatment of Barrett esophagus with adenocarcinoma are limited, EMR is the gold standard endoscopic technique for removal of visible esophageal dysplastic lesions.5,51,57 ESD can be considered for tumors larger than 15 mm, for poorly lifting lesions, and if there is suspicion of submucosal invasion.5
Patients should be evaluated by an experienced endoscopist, using an advanced imaging technique such as narrow-band imaging or chromoendoscopy. If suspicious features are found, endoscopic ultrasonography should be considered to confirm submucosal invasion or lymph node involvement.5
COLORECTAL CANCER
Indications for ESD for colorectal cancer in the East
Colon cancer is one of the leading causes of cancer-related deaths worldwide.58 Since ESD has been found to be effective and safe in treating gastric cancer, it has also been used to remove large colorectal tumors.59 However, ESD is not universally accepted in the treatment of colorectal neoplasms due to its greater technical difficulty, longer procedural time, and higher risk of perforating the thinner colonic wall compared with EMR.21,60
Outcomes for colorectal cancer
Tumor size of 50 mm or larger is a risk factor for complications, while a high procedure volume at the center is a protective factor.60
Endoscopic treatment of colorectal cancer: The Western perspective
EMR is the gold standard for removal of superficial colorectal lesions. However, ESD can be considered if there is suspicion of superficial submucosal invasion, especially for lesions larger than 20 mm that cannot be resected en bloc by EMR.32 ESD can also be used for fibrotic lesions not amenable to complete EMR removal, or as a salvage procedure after recurrence after EMR.67 Proper selection of cases is critical.1
Patients who have a superficial colonic lesion should be evaluated by means of high-definition endoscopy and chromoendoscopy to assess the mucosal pattern and establish feasibility of endoscopic resection. If submucosal invasion is suspected, staging with endoscopic ultrasonography or magnetic resonance imaging should be considered.5
FOLLOW-UP AFTER ESD
Endoscopic surveillance after the procedure is recommended, given the persistent risk of metachronous cancer after curative ESD due to its organ-sparing quality.68 Surveillance endoscopy aims to achieve early detection and subsequent endoscopic resection of metachronous lesions.
Histopathologic evaluation assessing the presence of malignant cells in the margins of a resected sample is mandatory for determining the next step in treatment. If margins are negative, follow-up endoscopy can be done every 6 to 12 months. If margins are positive, the approach includes surgery, reattempting ESD or endoscopic surveillance in 3 or 6 months.3,32 Although the surveillance strategy varies according to individual risk of metachronous cancer, it should be continued indefinitely.68
COMPLICATIONS OF ESD
The most common procedure-related complications of ESD are bleeding, perforation, and stricture. Most intraprocedural adverse events can be managed endoscopically.69
Bleeding
Most bleeding occurs during the procedure or early after it and can be controlled with electrocautery.49,69 No episodes of massive bleeding, defined as causing clinical symptoms and requiring transfusion or surgery, have been reported.20,43,55
In gastric ESD, delayed bleeding rates have ranged from 0 to 15.6%.69 Bleeding may be prevented with endoscopic coagulation of visible vessels after dissection has been completed and by proton pump inhibitor therapy.70,71 Excessive coagulation should be avoided to lower the risk of perforation.33
In colorectal ESD the bleeding rate has been reported to be 2.2%; applying coagulation to an area where a blood vessel is suspected before cutting (precoagulation) may prevent subsequent bleeding.21
Perforation
For gastric ESD, perforation rates range from 1.2% to 5.2%.69 Esophageal perforation rates can be up to 4%.49 In colorectal ESD, perforation rates have been reported to be 1.6% to 6.6%.60,72
Although most of the cases were successfully managed with conservative treatment, some required emergency surgery.60,73
Strictures
In a case series of 532 patients undergoing gastric ESD, stricture was reported in 5 patients, all of whom presented with obstructive symptoms.74 Risk factors for post-ESD gastric stenosis are a mucosal defect with a circumferential extent of more than three-fourths or a longitudinal extent of more than 5 cm.75
Strictures are common after esophageal ESD, with rates ranging from 2% to 26%. The risk is higher when longer segments are removed or circumferential resection is performed. As previously mentioned, this complication may be reduced with ingestion or injection of steroids after the procedure.53,54
Surprisingly, ESD of large colorectal lesions involving more than three-fourths of the circumference of the rectum is rarely complicated by stenosis.76
LIMITATIONS OF ESD
ESD requires a high level of technical skill, is time-consuming, and has a higher rate of complications than conventional endoscopic resection. A standardized ESD training system is needed, as the procedure is more difficult than EMR. Training in porcine models has been shown to confer competency in ESD in a Western setting.13,16,33
Colorectal ESD is an even more challenging procedure, given the potential for complications related to its anatomy. Training centers in Japan usually have their trainees first master gastric ESD, then assist in more than 20 colorectal ESDs conducted by experienced endoscopists, and accomplish 30 cases before performing the procedure safely and independently.
As the incidence of gastric cancer is low in Western countries, trainees may also begin with lower rectal lesions, which are easier to remove.77 Incorporation of ESD in the West would require a clear treatment algorithm. It is a complex procedure, with higher rates of complications, a prolonged learning curve, and prolonged procedure time. Therefore, it should be performed in specialized centers and under the special situations discussed here to ensure that the benefits for the patients outweigh the risks.
VALUE OF ENDOSCOPIC SUBMUCOSAL DISSECTION
The optimal method for resecting gastrointestinal neoplasms should be safe, cost-effective, and quick and should also completely remove the lesion. The best treatment strategy takes into account the characteristics of the lesion and the comorbidities and wishes of the patient. Internists should be aware of the multiple options available to achieve the best outcome for the patient.1
Endoscopic resection of superficial gastrointestinal neoplasms, including EMR and ESD, has been a subject of increasing interest due to its minimally invasive and potentially curative character. However, cancer can recur after endoscopic resection because the procedure is organ-sparing.
ESD allows resection of early gastrointestinal tumors with a minimally invasive technique. It can achieve higher curative resection rates and lower recurrence rates compared with EMR. Compared with surgery, ESD leads to less morbidity, fewer procedure-related complications, and lower medical costs. Indications should be rigorously followed to achieve successful treatments in selected patients.
Multiple variables have to be taken into account when deciding which treatment is best, such as tumor characteristics, the patient’s baseline condition, physician expertise, and hospital resources.48 Less-invasive treatments may improve the prognosis of patients. No matter the approach, patients should be treated in specialized treatment centers.
Internal medicine physicians should be aware of the advances in treatments for early gastrointestinal cancer so appropriate options can be considered.
- Burgess NG, Bourke MJ. Endoscopic resection of colorectal lesions: the narrowing divide between East and West. Dig Endosc 2016; 28:296–305.
- Kim DH, Jung HY, Gong EJ, et al. Endoscopic and oncologic outcomes of endoscopic resection for superficial esophageal neoplasm. Gut Liver 2015; 9:470–477.
- Draganov PV, Gotoda T, Chavalitdhamrong D, Wallace MB. Techniques of endoscopic submucosal dissection: application for the Western endoscopist? Gastrointest Endosc 2013; 78:677–688.
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14:101–112.
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47:829–854.
- Farhat S, Chaussade S, Ponchon T, et al; SFED ESD Study Group. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy 2011; 43:664–670.
- Gotoda T, Jung H. Endoscopic resection (endoscopic mucosal resection/endoscopic submucosal dissection) for early gastric cancer. Dig Endosc 2013; 25(suppl 1):55–63.
- Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 2009; 69:1228–1235.
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011; 14:113–123.
- Ono H. Endoscopic submucosal dissection for early gastric cancer. Chin J Dig Dis 2005; 6:119–121.
- Watanabe T, Itabashi M, Shimada Y, et al; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 2015; 20:207–239.
- Oyama T, Yahagi N, Ponchon T, Kiesslich T, Berr F. How to establish endoscopic submucosal dissection in Western countries. World J Gastroenterol 2015; 21:11209–11220.
- Bhatt A, Abe S, Kumaravel A, et al. SU1575 Western skill training in endoscopic submucosal dissection (ESD)—an international remote video based study—the WEST ESD Study. Gastrointest Endosc 2015; 81(suppl):AB335–AB336.
- Sano T, Sasako M, Kinoshita T, Maruyama K. Recurrence of early gastric cancer follow-up of 1475 patients and review of the Japanese literature. Cancer 1993; 72:3174–3178.
- Japan Esophageal Society. Japanese classification of esophageal cancer, tenth edition: part I. Esophagus 2009; 6:1–25.
- Bhatt A, Abe S, Kumaravel A, Vargo J, Saito Y. Indications and techniques for endoscopic submucosal dissection. Am J Gastroenterol 2015; 110:784–791.
- Eleftheriadis N, Inoue H, Ikeda H, et al. Definition and staging of early esophageal, gastric and colorectal cancer. J Tumor 2014; 2:161–178.
- Yoshinaga S, Oda I, Nonaka S, Kushima R, Saito Y. Endoscopic ultrasound using ultrasound probes for the diagnosis of early esophageal and gastric cancers. World J Gastrointest Endosc 2012; 4:218–226.
- Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D; ESMO Guidelines Working Group. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi51–vi56.
- Higuchi K, Tanabe S, Azuma M, et al. A phase II study of endoscopic submucosal dissection for superficial esophageal neoplasms (KDOG 0901). Gastrointest Endosc 2013; 78:704–710.
- Sakamoto T, Mori G, Yamada M, et al. Endoscopic submucosal dissection for colorectal neoplasms: a review. World J Gastroenterol 2014; 20:16153–16158.
- Ohta A, Tominaga K, Sakai Y. Efficacy of magnifying colonoscopy for the diagnosis of colorectal neoplasia: comparison with histopathological findings. Dig Endosc 2004; 16:308–314.
- Katagiri A, Fu K, Sano Y, et al. Narrow band imaging with magnifying colonoscopy as diagnostic tool for predicting histology of early colorectal neoplasia. Aliment Pharmacol Ther 2008; 27:1269–1274.
- Fu KI, Kato S, Sano Y, et al. Staging of early colorectal cancers: magnifying colonoscopy versus endoscopic ultrasonography for estimation of depth of invasion. Dig Dis Sci 2008; 53:1886–1892.
- Uraoka T, Saito Y, Ikematsu H, Yamamoto K, Sano Y. Sano’s capillary pattern classification for narrow-band imaging of early colorectal lesions. Dig Endosc 2011; 23(suppl 1):112–115.
- Ikematsu H, Matsuda T, Emura F, et al. Efficacy of capillary pattern type IIIA/IIIB by magnifying narrow band imaging for estimating depth of invasion of early colorectal neoplasms. BMC Gastroenterol 2010;10:33.
- Matsuda T, Fujii T, Saito Y, et al. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol 2008; 103:2700–2706.
- Sato H, Inoue H, Ikeda H, et al. Utility of intrapapillary capillary loops seen on magnifying narrow-band imaging in estimating invasive depth of esophageal squamous cell carcinoma. Endoscopy 2015; 8:122–128.
- Muto M, Yao K, Kaise M, et al. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G). Dig Endosc 2016; 28:379–393.
- Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; European Society for Medical Oncology (ESMO); European Society of Surgical Oncology (ESSO); European Society of Radiotherapy and Oncology (ESTRO). Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi57–vi63.
- Kuwano H, Nishimura Y, Ohtsu A, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus. April 2007 edition: part I - Edited by the Japan Esophageal Society. Esophagus 2008; 5:61–73.
- Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 2015; 27:417–434.
- Gotoda T, Ho KY, Soetikno R, Kaltenbach T, Draganov P. Gastric ESD: current status and future directions of devices and training. Gastrointest Endosc Clin North Am 2014; 24:213–233.
- Saito Y, Sakamoto T, Nakajima T, Matsuda T. Colorectal ESD: current indications and latest technical advances. Gastrointest Endosc Clin N Am 2014; 24:245–255.
- Saito Y, Otake Y, Sakamoto T, et al. Indications for and technical aspects of colorectal endoscopic submucosal dissection. Gut Liver 2013; 7:263–269.
- Saragoni L. Upgrading the definition of early gastric cancer: better staging means more appropriate treatment. Cancer Biol Med 2015; 12:355–361.
- Tsujitani S, Oka S, Saito H, et al. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery 1999; 125:148–154.
- Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc 2003; 57:567–579.
- Hirasawa T, Gotoda T, Miyata S, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009; 12:148–152.
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000; 3:219–225.
- Suzuki H, Oda I, Abe S, et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer 2016; 19:198–205.
- Matsuda T, Ajiki W, Marugame T, Ioka A, Tsukuma H, Sobue T; Research Group of Population-Based Cancer Registries of Japan. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol 2011; 41:40–51.
- Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc 2011; 74:485–493.
- Kim Y, Kim YW, Choi IJ, et al. Cost comparison between surgical treatments and endoscopic submucosal dissection in patients with early gastric cancer in Korea. Gut Liver 2015; 9:174–180.
- Abe S, Oda I, Nakajima T, et al. A case of local recurrence and distant metastasis following curative endoscopic submucosal dissection of early gastric cancer. Gastric Cancer 2015; 18:188–192.
- Hahn KY, Park JC, Kim EH, et al. Incidence and impact of scheduled endoscopic surveillance on recurrence after curative endoscopic resection for early gastric cancer. Gastrointest Endosc 2016; 84:628–638.e1.
- Wang S, Zhang Z, Liu M, Li S, Jiang C. Endoscopic resection compared with gastrectomy to treat early gastric cancer: a systematic review and meta-analysis. PLoS One 2015; 10:e0144774.
- Kondo A, de Moura EG, Bernardo WM, et al. Endoscopy vs surgery in the treatment of early gastric cancer: systematic review. World J Gastroenterol 2015; 21:13177–13187.
- Kothari S, Kaul V. Endoscopic mucosal resection and endoscopic submucosal dissection for endoscopic therapy of Barrett’s esophagus-related neoplasia. Gastroenterol Clin North Am 2015; 44:317–335.
- Yamashita T, Zeniya A, Ishii H, et al. Endoscopic mucosal resection using a cap-fitted panendoscope and endoscopic submucosal dissection as optimal endoscopic procedures for superficial esophageal carcinoma. Surg Endosc 2011; 25:2541–2546.
- Kagemoto K, Oka S, Tanaka S, et al. Clinical outcomes of endoscopic submucosal dissection for superficial Barrett’s adenocarcinoma. Gastrointest Endosc 2014; 80:239–245.
- Katada C, Muto M, Manabe T, Boku N, Ohtsu A, Yoshida S. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc 2003; 57:165–169.
- Hanaoka N, Ishihara R, Takeuchi Y, et al. 1139: A single session of intralesional steroid injection to prevent esophageal stricture after endoscopic submucosal dissection for esophageal squamous cell carcinoma. Gastrointest Endosc 2012; 75(suppl):AB175.
- Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc 2011; 73:1115–1121.
- Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009; 70:860–866.
- Katada C, Muto M, Manabe T, Ohtsu A, Yoshida S. Local recurrence of squamous-cell carcinoma of the esophagus after EMR. Gastrointest Endosc 2005; 61:219–225.
- Hirasawa K, Kokawa A, Oka H, et al. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc 2010; 72:960–966.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
- Nakajima T, Saito Y, Tanaka S, et al. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc 2013; 27:3262–3770.
- Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010; 72:1217–1225.
- Tanaka S, Saitoh Y, Matsuda T, et al; Japanese Society of Gastroenterology. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol 2015; 50:252–260.
- Oka S, Tanaka S, Saito Y, et al; Colorectal Endoscopic Resection Standardization Implementation Working Group of the Japanese Society for Cancer of the Colon and Rectum, Tokyo, Japan. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol 2015; 110:697–707.
- Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc 2010; 24:343–352.
- Makazu M, Sakamoto T, So E, et al. Relationship between indeterminate or positive lateral margin and local recurrence after endoscopic resection of colorectal polyps. Endosc Int Open 2015; 3:E252–E257.
- Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014; 46:388–402.
- Fujiya M, Tanaka K, Dokoshi T, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc 2015; 81:583–595.
- Rahmi G, Tanaka S, Ohara Y, et al. Efficacy of endoscopic submucosal dissection for residual or recurrent superficial colorectal tumors after endoscopic mucosal resection. J Dig Dis 2015; 16:14–21.
- Abe S, Oda I, Suzuki H, et al. Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy 2015; 47:1113–1118.
- Oda I, Suzuki H, Nonaka S, Yoshinaga S. Complications of gastric endoscopic submucosal dissection. Dig Endosc 2013; 25(suppl 1):71–78.
- Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection—an analysis of risk factors. Endoscopy 2008; 40:179–183.
- Uedo N, Takeuchi Y, Yamada T, et al. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol 2007; 102:1610–1616.
- Hayashi N, Tanaka S, Nishiyama S, et al. Predictors of incomplete resection and perforation associated with endoscopic submucosal dissection for colorectal tumors. Gastrointest Endosc 2014; 79:427–435.
- Suzuki H, Oda I, Sekiguchi M, et al. Management and associated factors of delayed perforation after gastric endoscopic submucosal dissection. World J Gastroenterol 2015; 21:12635–12643.
- Tsunada S, Ogata S, Mannen K, et al. Case series of endoscopic balloon dilation to treat a stricture caused by circumferential resection of the gastric antrum by endoscopic submucosal dissection. Gastrointest Endosc 2008; 67:979–983.
- Coda S, Oda I, Gotoda T, Yokoi C, Kikuchi T, Ono H. Risk factors for cardiac and pyloric stenosis after endoscopic submucosal dissection, and efficacy of endoscopic balloon dilation treatment. Endoscopy 2009; 41:421–426.
- Abe S, Sakamoto T, Takamaru H, et al. Stenosis rates after endoscopic submucosal dissection of large rectal tumors involving greater than three quarters of the luminal circumference. Surg Endosc 2016; 30:5459–5464.
- Sakamoto T, Saito Y, Fukunaga S, Nakajima T, Matsuda T. Learning curve associated with colorectal endoscopic submucosal dissection for endoscopists experienced in gastric endoscopic submucosal dissection. Dis Colon Rectum 2011; 54:1307–1312.
- Burgess NG, Bourke MJ. Endoscopic resection of colorectal lesions: the narrowing divide between East and West. Dig Endosc 2016; 28:296–305.
- Kim DH, Jung HY, Gong EJ, et al. Endoscopic and oncologic outcomes of endoscopic resection for superficial esophageal neoplasm. Gut Liver 2015; 9:470–477.
- Draganov PV, Gotoda T, Chavalitdhamrong D, Wallace MB. Techniques of endoscopic submucosal dissection: application for the Western endoscopist? Gastrointest Endosc 2013; 78:677–688.
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14:101–112.
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47:829–854.
- Farhat S, Chaussade S, Ponchon T, et al; SFED ESD Study Group. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy 2011; 43:664–670.
- Gotoda T, Jung H. Endoscopic resection (endoscopic mucosal resection/endoscopic submucosal dissection) for early gastric cancer. Dig Endosc 2013; 25(suppl 1):55–63.
- Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 2009; 69:1228–1235.
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011; 14:113–123.
- Ono H. Endoscopic submucosal dissection for early gastric cancer. Chin J Dig Dis 2005; 6:119–121.
- Watanabe T, Itabashi M, Shimada Y, et al; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 2015; 20:207–239.
- Oyama T, Yahagi N, Ponchon T, Kiesslich T, Berr F. How to establish endoscopic submucosal dissection in Western countries. World J Gastroenterol 2015; 21:11209–11220.
- Bhatt A, Abe S, Kumaravel A, et al. SU1575 Western skill training in endoscopic submucosal dissection (ESD)—an international remote video based study—the WEST ESD Study. Gastrointest Endosc 2015; 81(suppl):AB335–AB336.
- Sano T, Sasako M, Kinoshita T, Maruyama K. Recurrence of early gastric cancer follow-up of 1475 patients and review of the Japanese literature. Cancer 1993; 72:3174–3178.
- Japan Esophageal Society. Japanese classification of esophageal cancer, tenth edition: part I. Esophagus 2009; 6:1–25.
- Bhatt A, Abe S, Kumaravel A, Vargo J, Saito Y. Indications and techniques for endoscopic submucosal dissection. Am J Gastroenterol 2015; 110:784–791.
- Eleftheriadis N, Inoue H, Ikeda H, et al. Definition and staging of early esophageal, gastric and colorectal cancer. J Tumor 2014; 2:161–178.
- Yoshinaga S, Oda I, Nonaka S, Kushima R, Saito Y. Endoscopic ultrasound using ultrasound probes for the diagnosis of early esophageal and gastric cancers. World J Gastrointest Endosc 2012; 4:218–226.
- Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D; ESMO Guidelines Working Group. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi51–vi56.
- Higuchi K, Tanabe S, Azuma M, et al. A phase II study of endoscopic submucosal dissection for superficial esophageal neoplasms (KDOG 0901). Gastrointest Endosc 2013; 78:704–710.
- Sakamoto T, Mori G, Yamada M, et al. Endoscopic submucosal dissection for colorectal neoplasms: a review. World J Gastroenterol 2014; 20:16153–16158.
- Ohta A, Tominaga K, Sakai Y. Efficacy of magnifying colonoscopy for the diagnosis of colorectal neoplasia: comparison with histopathological findings. Dig Endosc 2004; 16:308–314.
- Katagiri A, Fu K, Sano Y, et al. Narrow band imaging with magnifying colonoscopy as diagnostic tool for predicting histology of early colorectal neoplasia. Aliment Pharmacol Ther 2008; 27:1269–1274.
- Fu KI, Kato S, Sano Y, et al. Staging of early colorectal cancers: magnifying colonoscopy versus endoscopic ultrasonography for estimation of depth of invasion. Dig Dis Sci 2008; 53:1886–1892.
- Uraoka T, Saito Y, Ikematsu H, Yamamoto K, Sano Y. Sano’s capillary pattern classification for narrow-band imaging of early colorectal lesions. Dig Endosc 2011; 23(suppl 1):112–115.
- Ikematsu H, Matsuda T, Emura F, et al. Efficacy of capillary pattern type IIIA/IIIB by magnifying narrow band imaging for estimating depth of invasion of early colorectal neoplasms. BMC Gastroenterol 2010;10:33.
- Matsuda T, Fujii T, Saito Y, et al. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol 2008; 103:2700–2706.
- Sato H, Inoue H, Ikeda H, et al. Utility of intrapapillary capillary loops seen on magnifying narrow-band imaging in estimating invasive depth of esophageal squamous cell carcinoma. Endoscopy 2015; 8:122–128.
- Muto M, Yao K, Kaise M, et al. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G). Dig Endosc 2016; 28:379–393.
- Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; European Society for Medical Oncology (ESMO); European Society of Surgical Oncology (ESSO); European Society of Radiotherapy and Oncology (ESTRO). Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi57–vi63.
- Kuwano H, Nishimura Y, Ohtsu A, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus. April 2007 edition: part I - Edited by the Japan Esophageal Society. Esophagus 2008; 5:61–73.
- Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 2015; 27:417–434.
- Gotoda T, Ho KY, Soetikno R, Kaltenbach T, Draganov P. Gastric ESD: current status and future directions of devices and training. Gastrointest Endosc Clin North Am 2014; 24:213–233.
- Saito Y, Sakamoto T, Nakajima T, Matsuda T. Colorectal ESD: current indications and latest technical advances. Gastrointest Endosc Clin N Am 2014; 24:245–255.
- Saito Y, Otake Y, Sakamoto T, et al. Indications for and technical aspects of colorectal endoscopic submucosal dissection. Gut Liver 2013; 7:263–269.
- Saragoni L. Upgrading the definition of early gastric cancer: better staging means more appropriate treatment. Cancer Biol Med 2015; 12:355–361.
- Tsujitani S, Oka S, Saito H, et al. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery 1999; 125:148–154.
- Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc 2003; 57:567–579.
- Hirasawa T, Gotoda T, Miyata S, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009; 12:148–152.
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000; 3:219–225.
- Suzuki H, Oda I, Abe S, et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer 2016; 19:198–205.
- Matsuda T, Ajiki W, Marugame T, Ioka A, Tsukuma H, Sobue T; Research Group of Population-Based Cancer Registries of Japan. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol 2011; 41:40–51.
- Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc 2011; 74:485–493.
- Kim Y, Kim YW, Choi IJ, et al. Cost comparison between surgical treatments and endoscopic submucosal dissection in patients with early gastric cancer in Korea. Gut Liver 2015; 9:174–180.
- Abe S, Oda I, Nakajima T, et al. A case of local recurrence and distant metastasis following curative endoscopic submucosal dissection of early gastric cancer. Gastric Cancer 2015; 18:188–192.
- Hahn KY, Park JC, Kim EH, et al. Incidence and impact of scheduled endoscopic surveillance on recurrence after curative endoscopic resection for early gastric cancer. Gastrointest Endosc 2016; 84:628–638.e1.
- Wang S, Zhang Z, Liu M, Li S, Jiang C. Endoscopic resection compared with gastrectomy to treat early gastric cancer: a systematic review and meta-analysis. PLoS One 2015; 10:e0144774.
- Kondo A, de Moura EG, Bernardo WM, et al. Endoscopy vs surgery in the treatment of early gastric cancer: systematic review. World J Gastroenterol 2015; 21:13177–13187.
- Kothari S, Kaul V. Endoscopic mucosal resection and endoscopic submucosal dissection for endoscopic therapy of Barrett’s esophagus-related neoplasia. Gastroenterol Clin North Am 2015; 44:317–335.
- Yamashita T, Zeniya A, Ishii H, et al. Endoscopic mucosal resection using a cap-fitted panendoscope and endoscopic submucosal dissection as optimal endoscopic procedures for superficial esophageal carcinoma. Surg Endosc 2011; 25:2541–2546.
- Kagemoto K, Oka S, Tanaka S, et al. Clinical outcomes of endoscopic submucosal dissection for superficial Barrett’s adenocarcinoma. Gastrointest Endosc 2014; 80:239–245.
- Katada C, Muto M, Manabe T, Boku N, Ohtsu A, Yoshida S. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc 2003; 57:165–169.
- Hanaoka N, Ishihara R, Takeuchi Y, et al. 1139: A single session of intralesional steroid injection to prevent esophageal stricture after endoscopic submucosal dissection for esophageal squamous cell carcinoma. Gastrointest Endosc 2012; 75(suppl):AB175.
- Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc 2011; 73:1115–1121.
- Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009; 70:860–866.
- Katada C, Muto M, Manabe T, Ohtsu A, Yoshida S. Local recurrence of squamous-cell carcinoma of the esophagus after EMR. Gastrointest Endosc 2005; 61:219–225.
- Hirasawa K, Kokawa A, Oka H, et al. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc 2010; 72:960–966.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
- Nakajima T, Saito Y, Tanaka S, et al. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc 2013; 27:3262–3770.
- Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010; 72:1217–1225.
- Tanaka S, Saitoh Y, Matsuda T, et al; Japanese Society of Gastroenterology. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol 2015; 50:252–260.
- Oka S, Tanaka S, Saito Y, et al; Colorectal Endoscopic Resection Standardization Implementation Working Group of the Japanese Society for Cancer of the Colon and Rectum, Tokyo, Japan. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol 2015; 110:697–707.
- Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc 2010; 24:343–352.
- Makazu M, Sakamoto T, So E, et al. Relationship between indeterminate or positive lateral margin and local recurrence after endoscopic resection of colorectal polyps. Endosc Int Open 2015; 3:E252–E257.
- Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014; 46:388–402.
- Fujiya M, Tanaka K, Dokoshi T, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc 2015; 81:583–595.
- Rahmi G, Tanaka S, Ohara Y, et al. Efficacy of endoscopic submucosal dissection for residual or recurrent superficial colorectal tumors after endoscopic mucosal resection. J Dig Dis 2015; 16:14–21.
- Abe S, Oda I, Suzuki H, et al. Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy 2015; 47:1113–1118.
- Oda I, Suzuki H, Nonaka S, Yoshinaga S. Complications of gastric endoscopic submucosal dissection. Dig Endosc 2013; 25(suppl 1):71–78.
- Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection—an analysis of risk factors. Endoscopy 2008; 40:179–183.
- Uedo N, Takeuchi Y, Yamada T, et al. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol 2007; 102:1610–1616.
- Hayashi N, Tanaka S, Nishiyama S, et al. Predictors of incomplete resection and perforation associated with endoscopic submucosal dissection for colorectal tumors. Gastrointest Endosc 2014; 79:427–435.
- Suzuki H, Oda I, Sekiguchi M, et al. Management and associated factors of delayed perforation after gastric endoscopic submucosal dissection. World J Gastroenterol 2015; 21:12635–12643.
- Tsunada S, Ogata S, Mannen K, et al. Case series of endoscopic balloon dilation to treat a stricture caused by circumferential resection of the gastric antrum by endoscopic submucosal dissection. Gastrointest Endosc 2008; 67:979–983.
- Coda S, Oda I, Gotoda T, Yokoi C, Kikuchi T, Ono H. Risk factors for cardiac and pyloric stenosis after endoscopic submucosal dissection, and efficacy of endoscopic balloon dilation treatment. Endoscopy 2009; 41:421–426.
- Abe S, Sakamoto T, Takamaru H, et al. Stenosis rates after endoscopic submucosal dissection of large rectal tumors involving greater than three quarters of the luminal circumference. Surg Endosc 2016; 30:5459–5464.
- Sakamoto T, Saito Y, Fukunaga S, Nakajima T, Matsuda T. Learning curve associated with colorectal endoscopic submucosal dissection for endoscopists experienced in gastric endoscopic submucosal dissection. Dis Colon Rectum 2011; 54:1307–1312.
KEY POINTS
- ESD is a minimally invasive endoscopic technique with curative potential for patients with superficial GI neoplasia.
- ESD preserves the integrity of the organ while achieving curative resection of large neoplasms.
- ESD is indicated rather than surgery in patients with early GI lesions with a negligible risk of lymph node metastasis.
- Complications of the procedure include bleeding, perforation, and stenosis. Most of these respond to endoscopic treatment.
- Successful ESD requires supportive teamwork among internists, gastroenterologists, pathologists, and surgeons.