User login
Extensor Pollicis Longus Ruptures in Distal Radius Fractures: Clinical and Cadaveric Studies With a New Therapeutic Intervention
Distal radius fractures are among the most common upper extremity injuries. A Swedish study noted that 75% of forearm fractures involve the distal radius.1 Extensor pollicis longus (EPL) ruptures are a well-documented complication (0.3% incidence2) of distal radius fractures.
The first description of EPL ruptures is attributed to Duplay in 1876 and was termed drummer boy’s palsy.3 Spontaneous EPL ruptures are often described in the setting of acute or chronic tenosynovitis.4,5 Beginning in the early 1930s, multiple case reports began to connect distal radius fractures with EPL ruptures.6 Although EPL ruptures are rare, their consequences are substantial and typically necessitate reconstructive procedures. Extensor indicis proprius (EIP)-to-EPL tendon transfer has become a common surgical treatment for this complication. Increasing our knowledge of several characteristics associated with this complication may help clinically in preventing EPL ruptures.
Multiple studies have indicated that EPL ruptures occur more often in nondisplaced fractures and often occur between 6 and 8 weeks after injury.2,5,7,8 Several factors are implicated in the etiology of EPL ruptures in distal radius fractures. The classic 1979 study by Engkvist and Lundborg9 showed that the EPL tendon has an area of poor vascularity around the Lister tubercle. Explorations of nondisplaced distal radius fractures have shown an intact extensor retinaculum that allows the tendon to continue to travel through an enclosed space.4,5,7 In the setting of distal radius fracture, hematoma may contribute to tendon ruptures secondary to increased pressure within an intact third dorsal compartment, which further compromises vascularity in this region of the EPL tendon.
Recognition and prevention of an impending EPL rupture may help avoid the significant consequences of this complication. Decompression and release of the third dorsal compartment have been described as constituting a prophylactic surgical option.10,11 Early thumb range of motion is also advocated to help prevent EPL rupture.9 However, results reported in the literature are inconclusive as to the effectiveness of these or indeed any preventive procedures. Dr. Lourie uses a novel technique that involves aspiration of the third dorsal compartment in patients with clinical symptoms associated with impending EPL rupture. Needle decompression, a less invasive option, can be quickly performed in an office, and it is hypothesized that removal of the hematoma may prevent EPL ruptures.
In the present study, we retrospectively reviewed Dr. Lourie’s records of patients with EPL ruptures in association with distal radius fractures to help delineate which radiographic and clinical characteristics identify patients at risk for these ruptures. A cadaveric model of a nondisplaced distal radius fracture was then created in order to simulate a change in third compartment pressures before and after needle decompression. We present preliminary outcomes on a case series of 4 patients who underwent aspiration of the third compartment and who were thought to be at risk for EPL rupture.
Materials and Methods
Institutional review board approval was obtained for this study. From Dr. Lourie’s records, 19 patients treated between 1998 and 2009 were identified as having confirmed or clinically impending EPL ruptures in association with nonoperative treatment of distal radius fractures. Prodromal symptoms that were used to diagnose impending EPL ruptures included pain with resisted active EPL extension, pain with passive flexion of the thumb interphalangeal joint, and localized swelling over the third dorsal compartment of the wrist.5,7,10,12 Eleven patients had complete radiographs, which were reviewed for radiographic characteristics. Posteroanterior (PA) and lateral radiographs of the injured wrist were reviewed. On the PA radiographs, fraction location was measured from the tip of the radial styloid using a line perpendicular to the radial shaft. Fractures were also evaluated for displacement and intra-articular involvement.
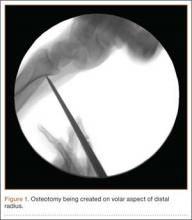
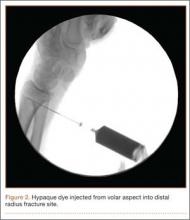
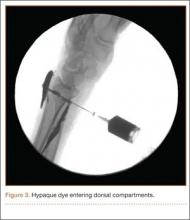
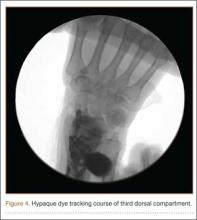
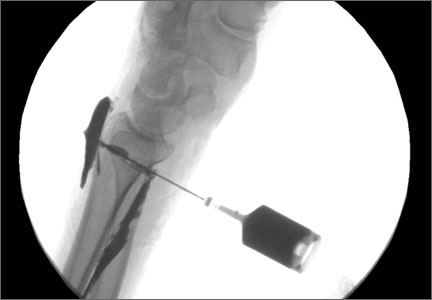
A cadaveric model was developed to evaluate compartment pressures in the EPL sheath in a simulated distal radius fracture. Six fresh-frozen cadaveric forearms were used after being thawed at room temperature. The cadavers were radiographically evaluated to determine that there was no evidence of prior fracture. A Stryker compartment pressure monitoring system (Stryker, Kalamazoo, Michigan) was used to take initial pressure readings in the third dorsal compartment slightly ulnar to the Lister tubercle (preinjection readings). A limited volar approach was then created. Under fluoroscopy, a half-inch osteotome was used to make an extra-articular fracture line in the distal radius, in the region of the Lister tubercle. The osteotomy was a mean of 1.2 cm from the distal aspect of the radius. The osteotomy site was then injected from the volar aspect with 5 mL of radiopaque (Hypaque) dye (Figure 1). Fluid extravasation into the third dorsal compartment was visualized under fluoroscopy (Figures 2–4). The monitor was then reinserted into the EPL sheath, and once again pressures were measured (postinjection readings). An 18-gauge needle was then used to aspirate the compartment just ulnar to the Lister tubercle. Compartment pressures were measured a final time (postaspiration readings). For all readings, 3 pressure measurements were recorded and then averaged. Pressure measurements were compared using t test.
In the office, the third dorsal compartment was aspirated after skin preparation with povidone-iodine. The Lister tubercle is typically palpable along the dorsal distal radius and is aligned with the cleft between the index and long fingers. Aspiration with an 18-gauge needle is performed just ulnar to the Lister tubercle in the EPL sheath, and hematoma is evacuated. The patient is then placed back into a long-arm cast or splint per the clinical situation.
Results
Patient age ranged from 17 to 81 years. Eight (1 male, 7 female) patients sustained an EPL rupture a mean of 46 days after initial trauma (range, 21-118 days). Two patients were treated with a prophylactic EPL transposition secondary to clinically apparent impending rupture, and 4 were treated with prophylactic needle decompression of the third compartment. Ruptures were treated with EIP-to-EPL transfers.
As in other studies, each patient’s radiographs showed a nondisplaced fracture and a transverse fracture line. Six patients also had a longitudinal, intra-articular fracture line that exited in a common spot between the scaphoid and the lunate facet.
Results in our cadaveric model were consistent with those in in vitro decompression of the third dorsal compartment (Table 1). In the cadaver model, mean (SD) initial third dorsal compartment pressure was 0.77 (0.88) mm Hg. Mean (SD) pressure after osteotomy and Hypaque injection was 25.5 (11.11) mm Hg. After simulated therapeutic aspiration, mean (SD) pressure decreased to 1.61 (1.40) mm Hg. Mean change in pressure from after injection to after aspiration was 23.89 mm Hg (P = .000388) (Table 2).
Information from other studies and from Dr. Lourie’s experience was used to identify patients at significant risk for EPL ruptures in association with distal radius fractures. Four patients in Dr. Lourie’s practice between 2004 and 2009 had characteristic findings, including a nondisplaced distal radius fracture, localized swelling over the third dorsal compartment, and pain with resisted active EPL extension. Prophylactic aspiration and hematoma evacuation were performed in this series, yielding a mean hematoma amount of 2 mL (Table 3).
For all 4 patients, aspirations were performed within 2 weeks of injury. Subjectively, these patients described almost immediate pain relief and less discomfort with EPL motion after aspiration. Three of the 4 reported sustained pain relief on close follow-up 7 and 14 days after aspiration. The fourth patient continued to have pain over the third dorsal compartment, though she described it as significantly improved. Her initial fracture contained about 50% dorsal comminution, and she began to have a significant callus response. After 2 months of continued symptoms, and out of concern about consequences of an impending rupture, open decompression and transposition of the EPL were performed. In follow-up over 29 months, this patient continued to do well and had full EPL function. The 3 patients treated with aspiration alone have not had an EPL rupture (range of follow-up, 29-89 months).
Discussion
Distal radius fractures are very common injuries, and treating physicians must attempt to prevent possible complications. EPL tendon ruptures continue to be rare events (incidence, <1%) in association with distal radius fractures. Although statistics vary, studies have found a higher incidence in nondisplaced (vs displaced) distal radius fractures.5,7,10 Ruptures in nondisplaced fractures occur within 2 weeks to 3 years after injury but typically an average of 6 weeks after injury.2,4,7-9 Prodromal symptoms often include tenderness and swelling around the dorsal distal radius region around the Lister tubercle.7,11,12 Patients may complain of pain with active thumb extension or passive thumb range of motion.11 Rupture is indicated by an inability to actively extend the thumb.
Studies have shown that the tendon rupture site is around the Lister tubercle.7 No single cause for EPL ruptures has been confirmed, and the etiology is likely a mix of factors in relation to the clinical situation. Two theories have been espoused for the relation between EPL ruptures and distal radius fractures. The mechanical theory involves a prominent spicule of bone abrading the tendon and subsequently causing rupture.5,9 This seems less likely for nondisplaced fractures. The vascular theory centers on a watershed region of the EPL tendon around the Lister tubercle. Studies have found microangiographic evidence of a 5-mm portion of tendon around the Lister tubercle that has no mesotenon and poor vascularity.7,9 The tendon in this section may be reliant on synovial diffusion for nutrition,7 but hematoma may displace synovial fluid, interfering with tendon nutrition.
Researchers have studied the third dorsal compartment in patients with impending or established ruptures. In a series by Hirasawa and colleagues,7 11 patients with a nondisplaced fracture and a tendon rupture had an intact retinaculum and smooth bony surfaces on the dorsal radius. Periosteal hypertrophy and narrowing of the third compartment were noted. In another series, Helal and colleagues5 reported on 16 patients (nondisplaced and displaced fractures) who had possible EPL ruptures over a 4-year period. In all cases, the extensor retinaculum was intact. Likewise, the 7 patients with EPL ruptures in a series by Bonatz and colleagues4 had an intact extensor retinaculum. On exploration, Bunata10 noted fluid collections, including hematomas, within the sheath, as well as a lack of bony prominences. Simpson12 explored 2 cases of blunt trauma, no fracture, and subsequent EPL rupture. Clinically these 2 patients had swelling in the region of the Lister tubercle, and surgically they were found to have a distended, blood-filled sheath. These ruptures may correlate with nondisplaced distal radius fractures and provide further evidence supporting the vascular theory of ruptures. The combination of intact compartment and volume overload presents a situation akin to compartment syndrome. Acute compartment decompression with needle evacuation would theoretically relieve the vascular insult.
In the study by Helal and colleagues,5 Hypaque injections were given to patients with nondisplaced distal radius fractures. The dye remained in the third dorsal compartment, which implies an intact closed space. That study used a cadaveric model as well, with distal radius osteotomies performed to simulate a nondisplaced distal radius fracture. The authors noted an intact extensor retinaculum in their model. Our cadaver model is similar, except we measured pressures in the third compartment; our model indicated increased compartment pressures within the EPL sheath. Subsequent aspiration in our cadaveric study led to lower pressures in the third dorsal compartment. This cadaveric model implies that needle decompression of the third dorsal compartment may be beneficial in the setting of nondisplaced distal radius fractures and symptoms indicating a compromised EPL.
Splinting is an important factor that may help prevent EPL tendon ruptures after distal radius fractures. Synovial diffusion may be the primary mechanism for delivering nutrition to the EPL tendon.7 A splint that allows thumb metacarpophalangeal and interphalangeal flexion may provide the EPL motion needed for effective synovial nutritional pathways.9 Prodromal symptoms of tendon rupture should then be carefully monitored.
Conclusion
Results of our retrospective review are consistent with previous results elucidating the risk factors for EPL ruptures in association with distal radius fractures. In our patients who sustained EPL ruptures, findings included nondisplaced fractures, about 50% with an intra-articular component. Clinical findings included localized swelling over the third dorsal compartment, pain with resisted active EPL extension, and pain with passive flexion of the thumb interphalangeal joint. The cadaveric portion of this study indicated a significant change in pressure in the third dorsal compartment after aspiration. Preliminary outcomes in this 4-patient series are no EPL ruptures after prophylactic aspiration. Hematoma evacuation after nondisplaced distal radius fractures may become a useful addition to the surgeon’s armamentarium. Studies are needed to determine if needle aspiration of the third dorsal compartment can become an office-based procedure with value in preventing EPL ruptures in the appropriate clinical situation.
1. Alffram PA, Bauer GC. Epidemiology of fractures of the forearm. A biomechanical investigation of bone strength. J Bone Joint Surg Am. 1962;44:105-114.
2. Hove LM. Delayed rupture of the thumb extensor tendon. A 5-year study of 18 consecutive cases. Acta Orthop Scand. 1994;65(2):199-203.
3. Duplay. Rupture sous-cutanee du tendon du long extenseur du pouce, au niveau de la tabatiere anatomique. Bull Et Mem de la Soc de Chir de Paris. 1876.
4. Bonatz E, Kramer TD, Masear VR. Rupture of the extensor pollicis longus tendon. Am J Orthop. 1996;25(2):118-122.
5. Helal B, Chen SC, Iwegbu G. Rupture of the extensor pollicis longus tendon in undisplaced Colles’ type of fracture. Hand. 1982;14(1):41-47.
6. McMaster PE. Late ruptures of extensor and flexor pollicis longus tendons following Colles’ fracture. J Bone Joint Surg Am. 1932;14:93-101.
7. Hirasawa Y, Katsumi Y, Akiyoshi T, Tamai K, Tokioka T. Clinical and microangiographic studies on rupture of the E.P.L. tendon after distal radius fractures. J Hand Surg Br. 1990;15(1):51-57.
8. Björkman A, Jörgsholm P. Rupture of the extensor pollicis longus tendon: a study of aetiological factors. Scand J Plast Reconstruct Surg Hand Surg. 2004;38(1):32-35.
9. Engkvist O, Lundborg G. Rupture of the extensor pollicis longus tendon after fracture of the lower end of the radius—a clinical and microangiographic study. Hand. 1979;11(1):76-86.
10. Bunata RE. Impending rupture of the extensor pollicis longus tendon after a minimally displaced Colles fracture. A case report. J Bone Joint Surg Am. 1983;65(3):401-402.
11. Skoff HD. Postfracture extensor pollicis longus tenosynovitis and tendon rupture: a scientific study and personal series. Am J Orthop. 2003;32(5):245-247.
12. Simpson RG. Delayed rupture of extensor pollicis longus tendon following closed injury. Hand. 1977;9(2):160-161.
Distal radius fractures are among the most common upper extremity injuries. A Swedish study noted that 75% of forearm fractures involve the distal radius.1 Extensor pollicis longus (EPL) ruptures are a well-documented complication (0.3% incidence2) of distal radius fractures.
The first description of EPL ruptures is attributed to Duplay in 1876 and was termed drummer boy’s palsy.3 Spontaneous EPL ruptures are often described in the setting of acute or chronic tenosynovitis.4,5 Beginning in the early 1930s, multiple case reports began to connect distal radius fractures with EPL ruptures.6 Although EPL ruptures are rare, their consequences are substantial and typically necessitate reconstructive procedures. Extensor indicis proprius (EIP)-to-EPL tendon transfer has become a common surgical treatment for this complication. Increasing our knowledge of several characteristics associated with this complication may help clinically in preventing EPL ruptures.
Multiple studies have indicated that EPL ruptures occur more often in nondisplaced fractures and often occur between 6 and 8 weeks after injury.2,5,7,8 Several factors are implicated in the etiology of EPL ruptures in distal radius fractures. The classic 1979 study by Engkvist and Lundborg9 showed that the EPL tendon has an area of poor vascularity around the Lister tubercle. Explorations of nondisplaced distal radius fractures have shown an intact extensor retinaculum that allows the tendon to continue to travel through an enclosed space.4,5,7 In the setting of distal radius fracture, hematoma may contribute to tendon ruptures secondary to increased pressure within an intact third dorsal compartment, which further compromises vascularity in this region of the EPL tendon.
Recognition and prevention of an impending EPL rupture may help avoid the significant consequences of this complication. Decompression and release of the third dorsal compartment have been described as constituting a prophylactic surgical option.10,11 Early thumb range of motion is also advocated to help prevent EPL rupture.9 However, results reported in the literature are inconclusive as to the effectiveness of these or indeed any preventive procedures. Dr. Lourie uses a novel technique that involves aspiration of the third dorsal compartment in patients with clinical symptoms associated with impending EPL rupture. Needle decompression, a less invasive option, can be quickly performed in an office, and it is hypothesized that removal of the hematoma may prevent EPL ruptures.
In the present study, we retrospectively reviewed Dr. Lourie’s records of patients with EPL ruptures in association with distal radius fractures to help delineate which radiographic and clinical characteristics identify patients at risk for these ruptures. A cadaveric model of a nondisplaced distal radius fracture was then created in order to simulate a change in third compartment pressures before and after needle decompression. We present preliminary outcomes on a case series of 4 patients who underwent aspiration of the third compartment and who were thought to be at risk for EPL rupture.
Materials and Methods
Institutional review board approval was obtained for this study. From Dr. Lourie’s records, 19 patients treated between 1998 and 2009 were identified as having confirmed or clinically impending EPL ruptures in association with nonoperative treatment of distal radius fractures. Prodromal symptoms that were used to diagnose impending EPL ruptures included pain with resisted active EPL extension, pain with passive flexion of the thumb interphalangeal joint, and localized swelling over the third dorsal compartment of the wrist.5,7,10,12 Eleven patients had complete radiographs, which were reviewed for radiographic characteristics. Posteroanterior (PA) and lateral radiographs of the injured wrist were reviewed. On the PA radiographs, fraction location was measured from the tip of the radial styloid using a line perpendicular to the radial shaft. Fractures were also evaluated for displacement and intra-articular involvement.
A cadaveric model was developed to evaluate compartment pressures in the EPL sheath in a simulated distal radius fracture. Six fresh-frozen cadaveric forearms were used after being thawed at room temperature. The cadavers were radiographically evaluated to determine that there was no evidence of prior fracture. A Stryker compartment pressure monitoring system (Stryker, Kalamazoo, Michigan) was used to take initial pressure readings in the third dorsal compartment slightly ulnar to the Lister tubercle (preinjection readings). A limited volar approach was then created. Under fluoroscopy, a half-inch osteotome was used to make an extra-articular fracture line in the distal radius, in the region of the Lister tubercle. The osteotomy was a mean of 1.2 cm from the distal aspect of the radius. The osteotomy site was then injected from the volar aspect with 5 mL of radiopaque (Hypaque) dye (Figure 1). Fluid extravasation into the third dorsal compartment was visualized under fluoroscopy (Figures 2–4). The monitor was then reinserted into the EPL sheath, and once again pressures were measured (postinjection readings). An 18-gauge needle was then used to aspirate the compartment just ulnar to the Lister tubercle. Compartment pressures were measured a final time (postaspiration readings). For all readings, 3 pressure measurements were recorded and then averaged. Pressure measurements were compared using t test.
In the office, the third dorsal compartment was aspirated after skin preparation with povidone-iodine. The Lister tubercle is typically palpable along the dorsal distal radius and is aligned with the cleft between the index and long fingers. Aspiration with an 18-gauge needle is performed just ulnar to the Lister tubercle in the EPL sheath, and hematoma is evacuated. The patient is then placed back into a long-arm cast or splint per the clinical situation.
Results
Patient age ranged from 17 to 81 years. Eight (1 male, 7 female) patients sustained an EPL rupture a mean of 46 days after initial trauma (range, 21-118 days). Two patients were treated with a prophylactic EPL transposition secondary to clinically apparent impending rupture, and 4 were treated with prophylactic needle decompression of the third compartment. Ruptures were treated with EIP-to-EPL transfers.
As in other studies, each patient’s radiographs showed a nondisplaced fracture and a transverse fracture line. Six patients also had a longitudinal, intra-articular fracture line that exited in a common spot between the scaphoid and the lunate facet.
Results in our cadaveric model were consistent with those in in vitro decompression of the third dorsal compartment (Table 1). In the cadaver model, mean (SD) initial third dorsal compartment pressure was 0.77 (0.88) mm Hg. Mean (SD) pressure after osteotomy and Hypaque injection was 25.5 (11.11) mm Hg. After simulated therapeutic aspiration, mean (SD) pressure decreased to 1.61 (1.40) mm Hg. Mean change in pressure from after injection to after aspiration was 23.89 mm Hg (P = .000388) (Table 2).
Information from other studies and from Dr. Lourie’s experience was used to identify patients at significant risk for EPL ruptures in association with distal radius fractures. Four patients in Dr. Lourie’s practice between 2004 and 2009 had characteristic findings, including a nondisplaced distal radius fracture, localized swelling over the third dorsal compartment, and pain with resisted active EPL extension. Prophylactic aspiration and hematoma evacuation were performed in this series, yielding a mean hematoma amount of 2 mL (Table 3).
For all 4 patients, aspirations were performed within 2 weeks of injury. Subjectively, these patients described almost immediate pain relief and less discomfort with EPL motion after aspiration. Three of the 4 reported sustained pain relief on close follow-up 7 and 14 days after aspiration. The fourth patient continued to have pain over the third dorsal compartment, though she described it as significantly improved. Her initial fracture contained about 50% dorsal comminution, and she began to have a significant callus response. After 2 months of continued symptoms, and out of concern about consequences of an impending rupture, open decompression and transposition of the EPL were performed. In follow-up over 29 months, this patient continued to do well and had full EPL function. The 3 patients treated with aspiration alone have not had an EPL rupture (range of follow-up, 29-89 months).
Discussion
Distal radius fractures are very common injuries, and treating physicians must attempt to prevent possible complications. EPL tendon ruptures continue to be rare events (incidence, <1%) in association with distal radius fractures. Although statistics vary, studies have found a higher incidence in nondisplaced (vs displaced) distal radius fractures.5,7,10 Ruptures in nondisplaced fractures occur within 2 weeks to 3 years after injury but typically an average of 6 weeks after injury.2,4,7-9 Prodromal symptoms often include tenderness and swelling around the dorsal distal radius region around the Lister tubercle.7,11,12 Patients may complain of pain with active thumb extension or passive thumb range of motion.11 Rupture is indicated by an inability to actively extend the thumb.
Studies have shown that the tendon rupture site is around the Lister tubercle.7 No single cause for EPL ruptures has been confirmed, and the etiology is likely a mix of factors in relation to the clinical situation. Two theories have been espoused for the relation between EPL ruptures and distal radius fractures. The mechanical theory involves a prominent spicule of bone abrading the tendon and subsequently causing rupture.5,9 This seems less likely for nondisplaced fractures. The vascular theory centers on a watershed region of the EPL tendon around the Lister tubercle. Studies have found microangiographic evidence of a 5-mm portion of tendon around the Lister tubercle that has no mesotenon and poor vascularity.7,9 The tendon in this section may be reliant on synovial diffusion for nutrition,7 but hematoma may displace synovial fluid, interfering with tendon nutrition.
Researchers have studied the third dorsal compartment in patients with impending or established ruptures. In a series by Hirasawa and colleagues,7 11 patients with a nondisplaced fracture and a tendon rupture had an intact retinaculum and smooth bony surfaces on the dorsal radius. Periosteal hypertrophy and narrowing of the third compartment were noted. In another series, Helal and colleagues5 reported on 16 patients (nondisplaced and displaced fractures) who had possible EPL ruptures over a 4-year period. In all cases, the extensor retinaculum was intact. Likewise, the 7 patients with EPL ruptures in a series by Bonatz and colleagues4 had an intact extensor retinaculum. On exploration, Bunata10 noted fluid collections, including hematomas, within the sheath, as well as a lack of bony prominences. Simpson12 explored 2 cases of blunt trauma, no fracture, and subsequent EPL rupture. Clinically these 2 patients had swelling in the region of the Lister tubercle, and surgically they were found to have a distended, blood-filled sheath. These ruptures may correlate with nondisplaced distal radius fractures and provide further evidence supporting the vascular theory of ruptures. The combination of intact compartment and volume overload presents a situation akin to compartment syndrome. Acute compartment decompression with needle evacuation would theoretically relieve the vascular insult.
In the study by Helal and colleagues,5 Hypaque injections were given to patients with nondisplaced distal radius fractures. The dye remained in the third dorsal compartment, which implies an intact closed space. That study used a cadaveric model as well, with distal radius osteotomies performed to simulate a nondisplaced distal radius fracture. The authors noted an intact extensor retinaculum in their model. Our cadaver model is similar, except we measured pressures in the third compartment; our model indicated increased compartment pressures within the EPL sheath. Subsequent aspiration in our cadaveric study led to lower pressures in the third dorsal compartment. This cadaveric model implies that needle decompression of the third dorsal compartment may be beneficial in the setting of nondisplaced distal radius fractures and symptoms indicating a compromised EPL.
Splinting is an important factor that may help prevent EPL tendon ruptures after distal radius fractures. Synovial diffusion may be the primary mechanism for delivering nutrition to the EPL tendon.7 A splint that allows thumb metacarpophalangeal and interphalangeal flexion may provide the EPL motion needed for effective synovial nutritional pathways.9 Prodromal symptoms of tendon rupture should then be carefully monitored.
Conclusion
Results of our retrospective review are consistent with previous results elucidating the risk factors for EPL ruptures in association with distal radius fractures. In our patients who sustained EPL ruptures, findings included nondisplaced fractures, about 50% with an intra-articular component. Clinical findings included localized swelling over the third dorsal compartment, pain with resisted active EPL extension, and pain with passive flexion of the thumb interphalangeal joint. The cadaveric portion of this study indicated a significant change in pressure in the third dorsal compartment after aspiration. Preliminary outcomes in this 4-patient series are no EPL ruptures after prophylactic aspiration. Hematoma evacuation after nondisplaced distal radius fractures may become a useful addition to the surgeon’s armamentarium. Studies are needed to determine if needle aspiration of the third dorsal compartment can become an office-based procedure with value in preventing EPL ruptures in the appropriate clinical situation.
Distal radius fractures are among the most common upper extremity injuries. A Swedish study noted that 75% of forearm fractures involve the distal radius.1 Extensor pollicis longus (EPL) ruptures are a well-documented complication (0.3% incidence2) of distal radius fractures.
The first description of EPL ruptures is attributed to Duplay in 1876 and was termed drummer boy’s palsy.3 Spontaneous EPL ruptures are often described in the setting of acute or chronic tenosynovitis.4,5 Beginning in the early 1930s, multiple case reports began to connect distal radius fractures with EPL ruptures.6 Although EPL ruptures are rare, their consequences are substantial and typically necessitate reconstructive procedures. Extensor indicis proprius (EIP)-to-EPL tendon transfer has become a common surgical treatment for this complication. Increasing our knowledge of several characteristics associated with this complication may help clinically in preventing EPL ruptures.
Multiple studies have indicated that EPL ruptures occur more often in nondisplaced fractures and often occur between 6 and 8 weeks after injury.2,5,7,8 Several factors are implicated in the etiology of EPL ruptures in distal radius fractures. The classic 1979 study by Engkvist and Lundborg9 showed that the EPL tendon has an area of poor vascularity around the Lister tubercle. Explorations of nondisplaced distal radius fractures have shown an intact extensor retinaculum that allows the tendon to continue to travel through an enclosed space.4,5,7 In the setting of distal radius fracture, hematoma may contribute to tendon ruptures secondary to increased pressure within an intact third dorsal compartment, which further compromises vascularity in this region of the EPL tendon.
Recognition and prevention of an impending EPL rupture may help avoid the significant consequences of this complication. Decompression and release of the third dorsal compartment have been described as constituting a prophylactic surgical option.10,11 Early thumb range of motion is also advocated to help prevent EPL rupture.9 However, results reported in the literature are inconclusive as to the effectiveness of these or indeed any preventive procedures. Dr. Lourie uses a novel technique that involves aspiration of the third dorsal compartment in patients with clinical symptoms associated with impending EPL rupture. Needle decompression, a less invasive option, can be quickly performed in an office, and it is hypothesized that removal of the hematoma may prevent EPL ruptures.
In the present study, we retrospectively reviewed Dr. Lourie’s records of patients with EPL ruptures in association with distal radius fractures to help delineate which radiographic and clinical characteristics identify patients at risk for these ruptures. A cadaveric model of a nondisplaced distal radius fracture was then created in order to simulate a change in third compartment pressures before and after needle decompression. We present preliminary outcomes on a case series of 4 patients who underwent aspiration of the third compartment and who were thought to be at risk for EPL rupture.
Materials and Methods
Institutional review board approval was obtained for this study. From Dr. Lourie’s records, 19 patients treated between 1998 and 2009 were identified as having confirmed or clinically impending EPL ruptures in association with nonoperative treatment of distal radius fractures. Prodromal symptoms that were used to diagnose impending EPL ruptures included pain with resisted active EPL extension, pain with passive flexion of the thumb interphalangeal joint, and localized swelling over the third dorsal compartment of the wrist.5,7,10,12 Eleven patients had complete radiographs, which were reviewed for radiographic characteristics. Posteroanterior (PA) and lateral radiographs of the injured wrist were reviewed. On the PA radiographs, fraction location was measured from the tip of the radial styloid using a line perpendicular to the radial shaft. Fractures were also evaluated for displacement and intra-articular involvement.
A cadaveric model was developed to evaluate compartment pressures in the EPL sheath in a simulated distal radius fracture. Six fresh-frozen cadaveric forearms were used after being thawed at room temperature. The cadavers were radiographically evaluated to determine that there was no evidence of prior fracture. A Stryker compartment pressure monitoring system (Stryker, Kalamazoo, Michigan) was used to take initial pressure readings in the third dorsal compartment slightly ulnar to the Lister tubercle (preinjection readings). A limited volar approach was then created. Under fluoroscopy, a half-inch osteotome was used to make an extra-articular fracture line in the distal radius, in the region of the Lister tubercle. The osteotomy was a mean of 1.2 cm from the distal aspect of the radius. The osteotomy site was then injected from the volar aspect with 5 mL of radiopaque (Hypaque) dye (Figure 1). Fluid extravasation into the third dorsal compartment was visualized under fluoroscopy (Figures 2–4). The monitor was then reinserted into the EPL sheath, and once again pressures were measured (postinjection readings). An 18-gauge needle was then used to aspirate the compartment just ulnar to the Lister tubercle. Compartment pressures were measured a final time (postaspiration readings). For all readings, 3 pressure measurements were recorded and then averaged. Pressure measurements were compared using t test.
In the office, the third dorsal compartment was aspirated after skin preparation with povidone-iodine. The Lister tubercle is typically palpable along the dorsal distal radius and is aligned with the cleft between the index and long fingers. Aspiration with an 18-gauge needle is performed just ulnar to the Lister tubercle in the EPL sheath, and hematoma is evacuated. The patient is then placed back into a long-arm cast or splint per the clinical situation.
Results
Patient age ranged from 17 to 81 years. Eight (1 male, 7 female) patients sustained an EPL rupture a mean of 46 days after initial trauma (range, 21-118 days). Two patients were treated with a prophylactic EPL transposition secondary to clinically apparent impending rupture, and 4 were treated with prophylactic needle decompression of the third compartment. Ruptures were treated with EIP-to-EPL transfers.
As in other studies, each patient’s radiographs showed a nondisplaced fracture and a transverse fracture line. Six patients also had a longitudinal, intra-articular fracture line that exited in a common spot between the scaphoid and the lunate facet.
Results in our cadaveric model were consistent with those in in vitro decompression of the third dorsal compartment (Table 1). In the cadaver model, mean (SD) initial third dorsal compartment pressure was 0.77 (0.88) mm Hg. Mean (SD) pressure after osteotomy and Hypaque injection was 25.5 (11.11) mm Hg. After simulated therapeutic aspiration, mean (SD) pressure decreased to 1.61 (1.40) mm Hg. Mean change in pressure from after injection to after aspiration was 23.89 mm Hg (P = .000388) (Table 2).
Information from other studies and from Dr. Lourie’s experience was used to identify patients at significant risk for EPL ruptures in association with distal radius fractures. Four patients in Dr. Lourie’s practice between 2004 and 2009 had characteristic findings, including a nondisplaced distal radius fracture, localized swelling over the third dorsal compartment, and pain with resisted active EPL extension. Prophylactic aspiration and hematoma evacuation were performed in this series, yielding a mean hematoma amount of 2 mL (Table 3).
For all 4 patients, aspirations were performed within 2 weeks of injury. Subjectively, these patients described almost immediate pain relief and less discomfort with EPL motion after aspiration. Three of the 4 reported sustained pain relief on close follow-up 7 and 14 days after aspiration. The fourth patient continued to have pain over the third dorsal compartment, though she described it as significantly improved. Her initial fracture contained about 50% dorsal comminution, and she began to have a significant callus response. After 2 months of continued symptoms, and out of concern about consequences of an impending rupture, open decompression and transposition of the EPL were performed. In follow-up over 29 months, this patient continued to do well and had full EPL function. The 3 patients treated with aspiration alone have not had an EPL rupture (range of follow-up, 29-89 months).
Discussion
Distal radius fractures are very common injuries, and treating physicians must attempt to prevent possible complications. EPL tendon ruptures continue to be rare events (incidence, <1%) in association with distal radius fractures. Although statistics vary, studies have found a higher incidence in nondisplaced (vs displaced) distal radius fractures.5,7,10 Ruptures in nondisplaced fractures occur within 2 weeks to 3 years after injury but typically an average of 6 weeks after injury.2,4,7-9 Prodromal symptoms often include tenderness and swelling around the dorsal distal radius region around the Lister tubercle.7,11,12 Patients may complain of pain with active thumb extension or passive thumb range of motion.11 Rupture is indicated by an inability to actively extend the thumb.
Studies have shown that the tendon rupture site is around the Lister tubercle.7 No single cause for EPL ruptures has been confirmed, and the etiology is likely a mix of factors in relation to the clinical situation. Two theories have been espoused for the relation between EPL ruptures and distal radius fractures. The mechanical theory involves a prominent spicule of bone abrading the tendon and subsequently causing rupture.5,9 This seems less likely for nondisplaced fractures. The vascular theory centers on a watershed region of the EPL tendon around the Lister tubercle. Studies have found microangiographic evidence of a 5-mm portion of tendon around the Lister tubercle that has no mesotenon and poor vascularity.7,9 The tendon in this section may be reliant on synovial diffusion for nutrition,7 but hematoma may displace synovial fluid, interfering with tendon nutrition.
Researchers have studied the third dorsal compartment in patients with impending or established ruptures. In a series by Hirasawa and colleagues,7 11 patients with a nondisplaced fracture and a tendon rupture had an intact retinaculum and smooth bony surfaces on the dorsal radius. Periosteal hypertrophy and narrowing of the third compartment were noted. In another series, Helal and colleagues5 reported on 16 patients (nondisplaced and displaced fractures) who had possible EPL ruptures over a 4-year period. In all cases, the extensor retinaculum was intact. Likewise, the 7 patients with EPL ruptures in a series by Bonatz and colleagues4 had an intact extensor retinaculum. On exploration, Bunata10 noted fluid collections, including hematomas, within the sheath, as well as a lack of bony prominences. Simpson12 explored 2 cases of blunt trauma, no fracture, and subsequent EPL rupture. Clinically these 2 patients had swelling in the region of the Lister tubercle, and surgically they were found to have a distended, blood-filled sheath. These ruptures may correlate with nondisplaced distal radius fractures and provide further evidence supporting the vascular theory of ruptures. The combination of intact compartment and volume overload presents a situation akin to compartment syndrome. Acute compartment decompression with needle evacuation would theoretically relieve the vascular insult.
In the study by Helal and colleagues,5 Hypaque injections were given to patients with nondisplaced distal radius fractures. The dye remained in the third dorsal compartment, which implies an intact closed space. That study used a cadaveric model as well, with distal radius osteotomies performed to simulate a nondisplaced distal radius fracture. The authors noted an intact extensor retinaculum in their model. Our cadaver model is similar, except we measured pressures in the third compartment; our model indicated increased compartment pressures within the EPL sheath. Subsequent aspiration in our cadaveric study led to lower pressures in the third dorsal compartment. This cadaveric model implies that needle decompression of the third dorsal compartment may be beneficial in the setting of nondisplaced distal radius fractures and symptoms indicating a compromised EPL.
Splinting is an important factor that may help prevent EPL tendon ruptures after distal radius fractures. Synovial diffusion may be the primary mechanism for delivering nutrition to the EPL tendon.7 A splint that allows thumb metacarpophalangeal and interphalangeal flexion may provide the EPL motion needed for effective synovial nutritional pathways.9 Prodromal symptoms of tendon rupture should then be carefully monitored.
Conclusion
Results of our retrospective review are consistent with previous results elucidating the risk factors for EPL ruptures in association with distal radius fractures. In our patients who sustained EPL ruptures, findings included nondisplaced fractures, about 50% with an intra-articular component. Clinical findings included localized swelling over the third dorsal compartment, pain with resisted active EPL extension, and pain with passive flexion of the thumb interphalangeal joint. The cadaveric portion of this study indicated a significant change in pressure in the third dorsal compartment after aspiration. Preliminary outcomes in this 4-patient series are no EPL ruptures after prophylactic aspiration. Hematoma evacuation after nondisplaced distal radius fractures may become a useful addition to the surgeon’s armamentarium. Studies are needed to determine if needle aspiration of the third dorsal compartment can become an office-based procedure with value in preventing EPL ruptures in the appropriate clinical situation.
1. Alffram PA, Bauer GC. Epidemiology of fractures of the forearm. A biomechanical investigation of bone strength. J Bone Joint Surg Am. 1962;44:105-114.
2. Hove LM. Delayed rupture of the thumb extensor tendon. A 5-year study of 18 consecutive cases. Acta Orthop Scand. 1994;65(2):199-203.
3. Duplay. Rupture sous-cutanee du tendon du long extenseur du pouce, au niveau de la tabatiere anatomique. Bull Et Mem de la Soc de Chir de Paris. 1876.
4. Bonatz E, Kramer TD, Masear VR. Rupture of the extensor pollicis longus tendon. Am J Orthop. 1996;25(2):118-122.
5. Helal B, Chen SC, Iwegbu G. Rupture of the extensor pollicis longus tendon in undisplaced Colles’ type of fracture. Hand. 1982;14(1):41-47.
6. McMaster PE. Late ruptures of extensor and flexor pollicis longus tendons following Colles’ fracture. J Bone Joint Surg Am. 1932;14:93-101.
7. Hirasawa Y, Katsumi Y, Akiyoshi T, Tamai K, Tokioka T. Clinical and microangiographic studies on rupture of the E.P.L. tendon after distal radius fractures. J Hand Surg Br. 1990;15(1):51-57.
8. Björkman A, Jörgsholm P. Rupture of the extensor pollicis longus tendon: a study of aetiological factors. Scand J Plast Reconstruct Surg Hand Surg. 2004;38(1):32-35.
9. Engkvist O, Lundborg G. Rupture of the extensor pollicis longus tendon after fracture of the lower end of the radius—a clinical and microangiographic study. Hand. 1979;11(1):76-86.
10. Bunata RE. Impending rupture of the extensor pollicis longus tendon after a minimally displaced Colles fracture. A case report. J Bone Joint Surg Am. 1983;65(3):401-402.
11. Skoff HD. Postfracture extensor pollicis longus tenosynovitis and tendon rupture: a scientific study and personal series. Am J Orthop. 2003;32(5):245-247.
12. Simpson RG. Delayed rupture of extensor pollicis longus tendon following closed injury. Hand. 1977;9(2):160-161.
1. Alffram PA, Bauer GC. Epidemiology of fractures of the forearm. A biomechanical investigation of bone strength. J Bone Joint Surg Am. 1962;44:105-114.
2. Hove LM. Delayed rupture of the thumb extensor tendon. A 5-year study of 18 consecutive cases. Acta Orthop Scand. 1994;65(2):199-203.
3. Duplay. Rupture sous-cutanee du tendon du long extenseur du pouce, au niveau de la tabatiere anatomique. Bull Et Mem de la Soc de Chir de Paris. 1876.
4. Bonatz E, Kramer TD, Masear VR. Rupture of the extensor pollicis longus tendon. Am J Orthop. 1996;25(2):118-122.
5. Helal B, Chen SC, Iwegbu G. Rupture of the extensor pollicis longus tendon in undisplaced Colles’ type of fracture. Hand. 1982;14(1):41-47.
6. McMaster PE. Late ruptures of extensor and flexor pollicis longus tendons following Colles’ fracture. J Bone Joint Surg Am. 1932;14:93-101.
7. Hirasawa Y, Katsumi Y, Akiyoshi T, Tamai K, Tokioka T. Clinical and microangiographic studies on rupture of the E.P.L. tendon after distal radius fractures. J Hand Surg Br. 1990;15(1):51-57.
8. Björkman A, Jörgsholm P. Rupture of the extensor pollicis longus tendon: a study of aetiological factors. Scand J Plast Reconstruct Surg Hand Surg. 2004;38(1):32-35.
9. Engkvist O, Lundborg G. Rupture of the extensor pollicis longus tendon after fracture of the lower end of the radius—a clinical and microangiographic study. Hand. 1979;11(1):76-86.
10. Bunata RE. Impending rupture of the extensor pollicis longus tendon after a minimally displaced Colles fracture. A case report. J Bone Joint Surg Am. 1983;65(3):401-402.
11. Skoff HD. Postfracture extensor pollicis longus tenosynovitis and tendon rupture: a scientific study and personal series. Am J Orthop. 2003;32(5):245-247.
12. Simpson RG. Delayed rupture of extensor pollicis longus tendon following closed injury. Hand. 1977;9(2):160-161.