User login
Catherine Cooper Nellist is editor of Pediatric News and Ob. Gyn. News. She has more than 30 years of experience reporting, writing, and editing stories about clinical medicine and the U.S. health care industry. Prior to taking the helm of these award-winning publications, Catherine covered major medical research meetings throughout the United States and Canada, and had been editor of Clinical Psychiatry News, and Dermatology News. She joined the company in 1984 after graduating magna cum laude from Dickinson College, Carlisle, Pa., with a BA in English.
Hexavalent hepatitis B vaccination mostly immunogenic 10 years later
results of an Italian study show.
The phase 3 open-label, controlled study included 732 healthy Italian children aged 11-13 years who as infants had received a two-dose primary and booster course with either Hexavac (5 mcg hepatitis B surface antigen [HBsAg]) or Infanrix hexa (10 mcg HBsAg) at 3, 5, and 11 months of age; the last dose was received at least 10 years prior to the challenge dose of a monovalent HB vaccine (HBVaxPro, 5 mcg HBsAg).
Although some of the children had HB surface antigen antibody concentrations below the seroprotection threshold, most of them had an anamnestic response when challenged with the dose of HB vaccine, “indicating the presence of specific immune memory,” the investigators said. There was no evidence of active HB disease in any of the children.
Just what the meaning of the lack of immune memory is, defined as the failure to develop an anamnestic response following an HB vaccine challenge, remains to be determined.
results of an Italian study show.
The phase 3 open-label, controlled study included 732 healthy Italian children aged 11-13 years who as infants had received a two-dose primary and booster course with either Hexavac (5 mcg hepatitis B surface antigen [HBsAg]) or Infanrix hexa (10 mcg HBsAg) at 3, 5, and 11 months of age; the last dose was received at least 10 years prior to the challenge dose of a monovalent HB vaccine (HBVaxPro, 5 mcg HBsAg).
Although some of the children had HB surface antigen antibody concentrations below the seroprotection threshold, most of them had an anamnestic response when challenged with the dose of HB vaccine, “indicating the presence of specific immune memory,” the investigators said. There was no evidence of active HB disease in any of the children.
Just what the meaning of the lack of immune memory is, defined as the failure to develop an anamnestic response following an HB vaccine challenge, remains to be determined.
results of an Italian study show.
The phase 3 open-label, controlled study included 732 healthy Italian children aged 11-13 years who as infants had received a two-dose primary and booster course with either Hexavac (5 mcg hepatitis B surface antigen [HBsAg]) or Infanrix hexa (10 mcg HBsAg) at 3, 5, and 11 months of age; the last dose was received at least 10 years prior to the challenge dose of a monovalent HB vaccine (HBVaxPro, 5 mcg HBsAg).
Although some of the children had HB surface antigen antibody concentrations below the seroprotection threshold, most of them had an anamnestic response when challenged with the dose of HB vaccine, “indicating the presence of specific immune memory,” the investigators said. There was no evidence of active HB disease in any of the children.
Just what the meaning of the lack of immune memory is, defined as the failure to develop an anamnestic response following an HB vaccine challenge, remains to be determined.
FROM VACCINE
If doctors recommend it, teens are more likely to get HPV vaccine
, Brandon Brown, PhD, at the University of California, Riverside, and his associates said.
In a year-long survey of 200 parents’ reasons for agreeing or refusing initial HPV vaccination following practitioner recommendation in a California pediatric practice of six pediatricians, 82% of parents accepted initiation of the HPV series. A significantly higher percentage of parents of male teens did so, compared with parents of female teens (89% vs. 71%; P less than .01), but there were more male children (61.5%) among offspring of the study participants.
Among parents who refused initiation of the HPV vaccine for their adolescents, the most common reason for refusing (53%) and most influential reason (49%) was “I want to learn more about this vaccine,” while 25% said their child was too young to get the vaccine. Some in this latter group said they would vaccinate their child when they were older.
Of the 195 parents who answered a question regarding whether they had a friend or family member diagnosed with cervical cancer, 13% said yes. Of these parents, 92% agreed to have their child get the HPV vaccine.
Read more at Papillomavirus Research (2017 Jan 17. doi: 10.1016/j.pvr.2017.01.002).
, Brandon Brown, PhD, at the University of California, Riverside, and his associates said.
In a year-long survey of 200 parents’ reasons for agreeing or refusing initial HPV vaccination following practitioner recommendation in a California pediatric practice of six pediatricians, 82% of parents accepted initiation of the HPV series. A significantly higher percentage of parents of male teens did so, compared with parents of female teens (89% vs. 71%; P less than .01), but there were more male children (61.5%) among offspring of the study participants.
Among parents who refused initiation of the HPV vaccine for their adolescents, the most common reason for refusing (53%) and most influential reason (49%) was “I want to learn more about this vaccine,” while 25% said their child was too young to get the vaccine. Some in this latter group said they would vaccinate their child when they were older.
Of the 195 parents who answered a question regarding whether they had a friend or family member diagnosed with cervical cancer, 13% said yes. Of these parents, 92% agreed to have their child get the HPV vaccine.
Read more at Papillomavirus Research (2017 Jan 17. doi: 10.1016/j.pvr.2017.01.002).
, Brandon Brown, PhD, at the University of California, Riverside, and his associates said.
In a year-long survey of 200 parents’ reasons for agreeing or refusing initial HPV vaccination following practitioner recommendation in a California pediatric practice of six pediatricians, 82% of parents accepted initiation of the HPV series. A significantly higher percentage of parents of male teens did so, compared with parents of female teens (89% vs. 71%; P less than .01), but there were more male children (61.5%) among offspring of the study participants.
Among parents who refused initiation of the HPV vaccine for their adolescents, the most common reason for refusing (53%) and most influential reason (49%) was “I want to learn more about this vaccine,” while 25% said their child was too young to get the vaccine. Some in this latter group said they would vaccinate their child when they were older.
Of the 195 parents who answered a question regarding whether they had a friend or family member diagnosed with cervical cancer, 13% said yes. Of these parents, 92% agreed to have their child get the HPV vaccine.
Read more at Papillomavirus Research (2017 Jan 17. doi: 10.1016/j.pvr.2017.01.002).
FROM PAPILLOMAVIRUS RESEARCH
Dengue vaccine appears safe, immunogenic in children aged 2-17 years
whether or not they previously have been exposed to the virus, said Xavier Sáez-Llorens, MD, of Hospital del Niño Dr José Renán Esquivel, Panama City, Republic of Panama, and his associates.
Studies show dengue to be the most common mosquito-borne viral disease affecting humans, occurring in 125 countries and causing approximately 100 million symptomatic infections annually. Vector control has not halted the global spread of dengue, necessitating development of vaccines. A vaccine for people aged 9 years and older has been licensed, but there remains the need for a safe and effective vaccine against all four dengue virus serotypes for all ages, said Dr. Sáez-Llorens and his associates.
Takeda’s live tetravalent dengue vaccine, TDV, has an “attenuated [dengue virus serotype] DENV-2 virus strain (TDV-2) and three chimeric (dengue–dengue) viruses containing the premembrane and envelope protein genes of DENV-1, DENV-3, and DENV-4 genetically engineered into the attenuated TDV-2 genome backbone (TDV-1, TDV-3, and TDV-4),” the investigators explained.
In an ongoing, randomized, double-blind, placebo-controlled study in dengue-endemic Panama, the Dominican Republic, and the Philippines, healthy children aged 2-17 years were randomly assigned to receive either one TDV dose at 0 months and one dose at 3 months (group 1); one dose at 0 months (group 2); one dose at 0 months and a booster at 12 months (group 3); or a placebo (group 4). At 6 months, 68% of children were seropositive for all four serotypes after one dose, and 85% were seropositive for all four serotypes after two doses; this occurred regardless of whether the children had previously been exposed to dengue. Group 3 has not yet received its 3-month booster.
The vaccine was well tolerated and safe. In children younger than 6 years, fever and irritability were the only systemic adverse events reported more often with the vaccine than with placebo. In children older than 6 years, only headache and myalgia were more common with the vaccine than with placebo, Dr. Sáez-Llorens and his associates noted.
“These data support phase III evaluation of the efficacy and safety of TDV given in a two-dose schedule 3 months apart, with analyses that take into account baseline age and dengue serostatus,” they concluded.
Read more in The Lancet Infectious Diseases (2017 June; 17[6]:615-25).
whether or not they previously have been exposed to the virus, said Xavier Sáez-Llorens, MD, of Hospital del Niño Dr José Renán Esquivel, Panama City, Republic of Panama, and his associates.
Studies show dengue to be the most common mosquito-borne viral disease affecting humans, occurring in 125 countries and causing approximately 100 million symptomatic infections annually. Vector control has not halted the global spread of dengue, necessitating development of vaccines. A vaccine for people aged 9 years and older has been licensed, but there remains the need for a safe and effective vaccine against all four dengue virus serotypes for all ages, said Dr. Sáez-Llorens and his associates.
Takeda’s live tetravalent dengue vaccine, TDV, has an “attenuated [dengue virus serotype] DENV-2 virus strain (TDV-2) and three chimeric (dengue–dengue) viruses containing the premembrane and envelope protein genes of DENV-1, DENV-3, and DENV-4 genetically engineered into the attenuated TDV-2 genome backbone (TDV-1, TDV-3, and TDV-4),” the investigators explained.
In an ongoing, randomized, double-blind, placebo-controlled study in dengue-endemic Panama, the Dominican Republic, and the Philippines, healthy children aged 2-17 years were randomly assigned to receive either one TDV dose at 0 months and one dose at 3 months (group 1); one dose at 0 months (group 2); one dose at 0 months and a booster at 12 months (group 3); or a placebo (group 4). At 6 months, 68% of children were seropositive for all four serotypes after one dose, and 85% were seropositive for all four serotypes after two doses; this occurred regardless of whether the children had previously been exposed to dengue. Group 3 has not yet received its 3-month booster.
The vaccine was well tolerated and safe. In children younger than 6 years, fever and irritability were the only systemic adverse events reported more often with the vaccine than with placebo. In children older than 6 years, only headache and myalgia were more common with the vaccine than with placebo, Dr. Sáez-Llorens and his associates noted.
“These data support phase III evaluation of the efficacy and safety of TDV given in a two-dose schedule 3 months apart, with analyses that take into account baseline age and dengue serostatus,” they concluded.
Read more in The Lancet Infectious Diseases (2017 June; 17[6]:615-25).
whether or not they previously have been exposed to the virus, said Xavier Sáez-Llorens, MD, of Hospital del Niño Dr José Renán Esquivel, Panama City, Republic of Panama, and his associates.
Studies show dengue to be the most common mosquito-borne viral disease affecting humans, occurring in 125 countries and causing approximately 100 million symptomatic infections annually. Vector control has not halted the global spread of dengue, necessitating development of vaccines. A vaccine for people aged 9 years and older has been licensed, but there remains the need for a safe and effective vaccine against all four dengue virus serotypes for all ages, said Dr. Sáez-Llorens and his associates.
Takeda’s live tetravalent dengue vaccine, TDV, has an “attenuated [dengue virus serotype] DENV-2 virus strain (TDV-2) and three chimeric (dengue–dengue) viruses containing the premembrane and envelope protein genes of DENV-1, DENV-3, and DENV-4 genetically engineered into the attenuated TDV-2 genome backbone (TDV-1, TDV-3, and TDV-4),” the investigators explained.
In an ongoing, randomized, double-blind, placebo-controlled study in dengue-endemic Panama, the Dominican Republic, and the Philippines, healthy children aged 2-17 years were randomly assigned to receive either one TDV dose at 0 months and one dose at 3 months (group 1); one dose at 0 months (group 2); one dose at 0 months and a booster at 12 months (group 3); or a placebo (group 4). At 6 months, 68% of children were seropositive for all four serotypes after one dose, and 85% were seropositive for all four serotypes after two doses; this occurred regardless of whether the children had previously been exposed to dengue. Group 3 has not yet received its 3-month booster.
The vaccine was well tolerated and safe. In children younger than 6 years, fever and irritability were the only systemic adverse events reported more often with the vaccine than with placebo. In children older than 6 years, only headache and myalgia were more common with the vaccine than with placebo, Dr. Sáez-Llorens and his associates noted.
“These data support phase III evaluation of the efficacy and safety of TDV given in a two-dose schedule 3 months apart, with analyses that take into account baseline age and dengue serostatus,” they concluded.
Read more in The Lancet Infectious Diseases (2017 June; 17[6]:615-25).
FROM LANCET INFECTIOUS DISEASES
Consider college immunization requirements when counseling about vaccines
, recommended Allison Noesekabel, a medical student at Wayne State University, Detroit, and Ada M. Fenick, MD, a pediatrician at Yale University, New Haven, Conn.
After identifying the 200 top-ranked U.S. universities in the US News and World Report 2014 National University rankings and sending a survey on university prematriculation immunization requirements (PIRs), the responding 124 universities spanned 44 states and the District of Columbia. Of those who responded, 94% had at least one PIR, 84% had at least two, 63% had at least three, and 16% required all the vaccines as required by any jurisdictional law for attendance at universities (hepatitis B, meningococcus, MMR, TdaP, and varicella). Only 6% of the universities had no immunization requirements.
Of the 121 universities with PIRs, 2% did not allow medical exemptions, 2% did not allow religious exemptions, and 46% did not allow philosophical belief exemptions. Medical exemptions were hardest to obtain for 24% of the 121 universities with PIRs, religious exemptions were the most difficult to obtain at 40%, and philosophical belief exemptions were hardest to obtain at 60% of the universities with PIRs. The most common administrative responses to lack of immunization were “the placement of a hold on registration of classes (89% of universities), additional registration fees (13%), and a hold on student housing (11%),” Ms. Noesekabel and Dr. Frenik reported.
Ms. Noesekabel and Dr. Frenik said they chose highly ranked universities for their study because these were likely to be desirable to vaccine-hesitant families, who tend to be highly educated and affluent. Therefore, the findings of this study may not be representative of all 4-year U.S. colleges, they said.
Read more at Vaccine (2017. doi: 10.1016/j.vaccine.2017.05.038).
, recommended Allison Noesekabel, a medical student at Wayne State University, Detroit, and Ada M. Fenick, MD, a pediatrician at Yale University, New Haven, Conn.
After identifying the 200 top-ranked U.S. universities in the US News and World Report 2014 National University rankings and sending a survey on university prematriculation immunization requirements (PIRs), the responding 124 universities spanned 44 states and the District of Columbia. Of those who responded, 94% had at least one PIR, 84% had at least two, 63% had at least three, and 16% required all the vaccines as required by any jurisdictional law for attendance at universities (hepatitis B, meningococcus, MMR, TdaP, and varicella). Only 6% of the universities had no immunization requirements.
Of the 121 universities with PIRs, 2% did not allow medical exemptions, 2% did not allow religious exemptions, and 46% did not allow philosophical belief exemptions. Medical exemptions were hardest to obtain for 24% of the 121 universities with PIRs, religious exemptions were the most difficult to obtain at 40%, and philosophical belief exemptions were hardest to obtain at 60% of the universities with PIRs. The most common administrative responses to lack of immunization were “the placement of a hold on registration of classes (89% of universities), additional registration fees (13%), and a hold on student housing (11%),” Ms. Noesekabel and Dr. Frenik reported.
Ms. Noesekabel and Dr. Frenik said they chose highly ranked universities for their study because these were likely to be desirable to vaccine-hesitant families, who tend to be highly educated and affluent. Therefore, the findings of this study may not be representative of all 4-year U.S. colleges, they said.
Read more at Vaccine (2017. doi: 10.1016/j.vaccine.2017.05.038).
, recommended Allison Noesekabel, a medical student at Wayne State University, Detroit, and Ada M. Fenick, MD, a pediatrician at Yale University, New Haven, Conn.
After identifying the 200 top-ranked U.S. universities in the US News and World Report 2014 National University rankings and sending a survey on university prematriculation immunization requirements (PIRs), the responding 124 universities spanned 44 states and the District of Columbia. Of those who responded, 94% had at least one PIR, 84% had at least two, 63% had at least three, and 16% required all the vaccines as required by any jurisdictional law for attendance at universities (hepatitis B, meningococcus, MMR, TdaP, and varicella). Only 6% of the universities had no immunization requirements.
Of the 121 universities with PIRs, 2% did not allow medical exemptions, 2% did not allow religious exemptions, and 46% did not allow philosophical belief exemptions. Medical exemptions were hardest to obtain for 24% of the 121 universities with PIRs, religious exemptions were the most difficult to obtain at 40%, and philosophical belief exemptions were hardest to obtain at 60% of the universities with PIRs. The most common administrative responses to lack of immunization were “the placement of a hold on registration of classes (89% of universities), additional registration fees (13%), and a hold on student housing (11%),” Ms. Noesekabel and Dr. Frenik reported.
Ms. Noesekabel and Dr. Frenik said they chose highly ranked universities for their study because these were likely to be desirable to vaccine-hesitant families, who tend to be highly educated and affluent. Therefore, the findings of this study may not be representative of all 4-year U.S. colleges, they said.
Read more at Vaccine (2017. doi: 10.1016/j.vaccine.2017.05.038).
FROM VACCINE
MMR vaccine cut hospitalizations for unrelated respiratory infections
, said Giuseppe La Torre of the Sapienza University of Rome and his associates.
The 2-year retrospective database study included 11,004 children, 21% of whom did not receive the MMR vaccine; 49% received one dose, and 30% received two doses. There were 12 hospitalizations for measles (9 in unvaccinated children, 3 in those who received one dose, and none in those who received two doses), 2 hospitalizations for mumps (1 among vaccinated and 1 among unvaccinated children), and no hospitalizations for rubella (P less than .001).
There were 414 hospitalizations for all infectious diseases, 11% in unvaccinated children, 1.5% in those who had received one dose of vaccine, and 1% in those who had received two doses (P less than .001). MMR vaccine also was highly protective against hospitalizations for all infectious diseases (HR, 0.29).
Of 809 hospitalizations for respiratory diseases, 18% involved children who had not been vaccinated, 4% involved children who had received one dose, and 5.5% involved children vaccinated with two doses (P less than .001). MMR likewise was highly protective against hospitalizations for respiratory diseases (HR, 0.18).
Read more in the journal Human Vaccines & Immunotherapeutics (2017 Jun 12. doi: 10.1080/21645515.2017.1330733).
, said Giuseppe La Torre of the Sapienza University of Rome and his associates.
The 2-year retrospective database study included 11,004 children, 21% of whom did not receive the MMR vaccine; 49% received one dose, and 30% received two doses. There were 12 hospitalizations for measles (9 in unvaccinated children, 3 in those who received one dose, and none in those who received two doses), 2 hospitalizations for mumps (1 among vaccinated and 1 among unvaccinated children), and no hospitalizations for rubella (P less than .001).
There were 414 hospitalizations for all infectious diseases, 11% in unvaccinated children, 1.5% in those who had received one dose of vaccine, and 1% in those who had received two doses (P less than .001). MMR vaccine also was highly protective against hospitalizations for all infectious diseases (HR, 0.29).
Of 809 hospitalizations for respiratory diseases, 18% involved children who had not been vaccinated, 4% involved children who had received one dose, and 5.5% involved children vaccinated with two doses (P less than .001). MMR likewise was highly protective against hospitalizations for respiratory diseases (HR, 0.18).
Read more in the journal Human Vaccines & Immunotherapeutics (2017 Jun 12. doi: 10.1080/21645515.2017.1330733).
, said Giuseppe La Torre of the Sapienza University of Rome and his associates.
The 2-year retrospective database study included 11,004 children, 21% of whom did not receive the MMR vaccine; 49% received one dose, and 30% received two doses. There were 12 hospitalizations for measles (9 in unvaccinated children, 3 in those who received one dose, and none in those who received two doses), 2 hospitalizations for mumps (1 among vaccinated and 1 among unvaccinated children), and no hospitalizations for rubella (P less than .001).
There were 414 hospitalizations for all infectious diseases, 11% in unvaccinated children, 1.5% in those who had received one dose of vaccine, and 1% in those who had received two doses (P less than .001). MMR vaccine also was highly protective against hospitalizations for all infectious diseases (HR, 0.29).
Of 809 hospitalizations for respiratory diseases, 18% involved children who had not been vaccinated, 4% involved children who had received one dose, and 5.5% involved children vaccinated with two doses (P less than .001). MMR likewise was highly protective against hospitalizations for respiratory diseases (HR, 0.18).
Read more in the journal Human Vaccines & Immunotherapeutics (2017 Jun 12. doi: 10.1080/21645515.2017.1330733).
FROM HUMAN VACCINES & IMMUNOTHERAPEUTICS
Teens’ overall tobacco use falls, but e-cigs now most popular product
Twenty percent of surveyed high school students and 7% of middle school students were using some kind of tobacco product in 2016 – and e-cigarettes were the most commonly used product among those groups, according to an analysis from federal researchers.
, although combustible tobacco product use declined in both groups, said Ahmed Jamal, MD, of the Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and his associates. That was determined using data from the 2011-2016 National Youth Tobacco Surveys, which assessed tobacco use in the past 30 days by groups of middle school and high school students numbering from more than 17,000 to almost 25,000.
High school boys had higher use of any tobacco product, two or more tobacco products, cigars, smokeless tobacco, and pipe tobacco than did high school girls. The most commonly used tobacco product among non-Hispanic white (13.7%) and Hispanic high school students (10%) were e-cigarettes; cigars were the most commonly used tobacco product among non-Hispanic black high school students (9.5%).
Seven percent of middle school students had used any tobacco product within the past 30 days, and 3% had used two or more tobacco products. Similar to high school students, e-cigarettes were the most commonly used tobacco product among middle school students (4.3%), followed by cigarettes (2%), cigars (2%), smokeless tobacco (2%), hookahs (2%), pipe tobacco (0.7%), and bidis (0.3%).
Use of any tobacco product among middle school boys was 8.3%, and among middle school girls use was 6%. Hispanic middle school students reported higher use of any tobacco product, were more likely to use two or more tobacco products, and said they used hookahs more often than did non-Hispanic white middle school students.
Looking at the changes from 2011 to 2016, current use of any tobacco product did not change significantly among high school students (falling from 24% to 20%), but there was a reduction in current use of any combustible tobacco product (22%-14%) and in use of two or more tobacco products (12%-9.6%) over this time period.
By product type, there were increases in current use of e-cigarettes (from 1.5% to 11.3%) and hookahs (from 4.1% to 4.8%) among high school students, as well as decreases in current use of cigarettes (from 16% to 8%), cigars (from 12% to 8%), smokeless tobacco (from 8% to 6%), pipe tobacco (from 4% to 1%), and bidis (from 2% to 0.5%).
Among middle school students during 2011-2016, there was a rise in current use of e-cigarettes (from 0.6% to 4%), and in current use of hookahs (from 1% to 2%). There was a drop in current use of any combustible tobacco products (from 6% to 4%), cigarettes (from 4% to 2%), cigars (from 3.5% to 2.2%), and pipe tobacco (from 2% to 0.7%).
“Since February 2014, the Food and Drug Administration’s first national tobacco public education campaign, the Real Cost, has broadcasted tobacco education advertising designed for youths aged 12-17 years; the campaign was associated with an estimated 348,398 U.S. youths who did not initiate cigarette smoking during February 2014–March 2016,” the investigators said. “Continued implementation of these strategies can help prevent and further reduce the use of all forms of tobacco product among U.S. youths.”
Read more in MMWR (2017 Jun 16;66[23]:597-603).
Twenty percent of surveyed high school students and 7% of middle school students were using some kind of tobacco product in 2016 – and e-cigarettes were the most commonly used product among those groups, according to an analysis from federal researchers.
, although combustible tobacco product use declined in both groups, said Ahmed Jamal, MD, of the Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and his associates. That was determined using data from the 2011-2016 National Youth Tobacco Surveys, which assessed tobacco use in the past 30 days by groups of middle school and high school students numbering from more than 17,000 to almost 25,000.
High school boys had higher use of any tobacco product, two or more tobacco products, cigars, smokeless tobacco, and pipe tobacco than did high school girls. The most commonly used tobacco product among non-Hispanic white (13.7%) and Hispanic high school students (10%) were e-cigarettes; cigars were the most commonly used tobacco product among non-Hispanic black high school students (9.5%).
Seven percent of middle school students had used any tobacco product within the past 30 days, and 3% had used two or more tobacco products. Similar to high school students, e-cigarettes were the most commonly used tobacco product among middle school students (4.3%), followed by cigarettes (2%), cigars (2%), smokeless tobacco (2%), hookahs (2%), pipe tobacco (0.7%), and bidis (0.3%).
Use of any tobacco product among middle school boys was 8.3%, and among middle school girls use was 6%. Hispanic middle school students reported higher use of any tobacco product, were more likely to use two or more tobacco products, and said they used hookahs more often than did non-Hispanic white middle school students.
Looking at the changes from 2011 to 2016, current use of any tobacco product did not change significantly among high school students (falling from 24% to 20%), but there was a reduction in current use of any combustible tobacco product (22%-14%) and in use of two or more tobacco products (12%-9.6%) over this time period.
By product type, there were increases in current use of e-cigarettes (from 1.5% to 11.3%) and hookahs (from 4.1% to 4.8%) among high school students, as well as decreases in current use of cigarettes (from 16% to 8%), cigars (from 12% to 8%), smokeless tobacco (from 8% to 6%), pipe tobacco (from 4% to 1%), and bidis (from 2% to 0.5%).
Among middle school students during 2011-2016, there was a rise in current use of e-cigarettes (from 0.6% to 4%), and in current use of hookahs (from 1% to 2%). There was a drop in current use of any combustible tobacco products (from 6% to 4%), cigarettes (from 4% to 2%), cigars (from 3.5% to 2.2%), and pipe tobacco (from 2% to 0.7%).
“Since February 2014, the Food and Drug Administration’s first national tobacco public education campaign, the Real Cost, has broadcasted tobacco education advertising designed for youths aged 12-17 years; the campaign was associated with an estimated 348,398 U.S. youths who did not initiate cigarette smoking during February 2014–March 2016,” the investigators said. “Continued implementation of these strategies can help prevent and further reduce the use of all forms of tobacco product among U.S. youths.”
Read more in MMWR (2017 Jun 16;66[23]:597-603).
Twenty percent of surveyed high school students and 7% of middle school students were using some kind of tobacco product in 2016 – and e-cigarettes were the most commonly used product among those groups, according to an analysis from federal researchers.
, although combustible tobacco product use declined in both groups, said Ahmed Jamal, MD, of the Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and his associates. That was determined using data from the 2011-2016 National Youth Tobacco Surveys, which assessed tobacco use in the past 30 days by groups of middle school and high school students numbering from more than 17,000 to almost 25,000.
High school boys had higher use of any tobacco product, two or more tobacco products, cigars, smokeless tobacco, and pipe tobacco than did high school girls. The most commonly used tobacco product among non-Hispanic white (13.7%) and Hispanic high school students (10%) were e-cigarettes; cigars were the most commonly used tobacco product among non-Hispanic black high school students (9.5%).
Seven percent of middle school students had used any tobacco product within the past 30 days, and 3% had used two or more tobacco products. Similar to high school students, e-cigarettes were the most commonly used tobacco product among middle school students (4.3%), followed by cigarettes (2%), cigars (2%), smokeless tobacco (2%), hookahs (2%), pipe tobacco (0.7%), and bidis (0.3%).
Use of any tobacco product among middle school boys was 8.3%, and among middle school girls use was 6%. Hispanic middle school students reported higher use of any tobacco product, were more likely to use two or more tobacco products, and said they used hookahs more often than did non-Hispanic white middle school students.
Looking at the changes from 2011 to 2016, current use of any tobacco product did not change significantly among high school students (falling from 24% to 20%), but there was a reduction in current use of any combustible tobacco product (22%-14%) and in use of two or more tobacco products (12%-9.6%) over this time period.
By product type, there were increases in current use of e-cigarettes (from 1.5% to 11.3%) and hookahs (from 4.1% to 4.8%) among high school students, as well as decreases in current use of cigarettes (from 16% to 8%), cigars (from 12% to 8%), smokeless tobacco (from 8% to 6%), pipe tobacco (from 4% to 1%), and bidis (from 2% to 0.5%).
Among middle school students during 2011-2016, there was a rise in current use of e-cigarettes (from 0.6% to 4%), and in current use of hookahs (from 1% to 2%). There was a drop in current use of any combustible tobacco products (from 6% to 4%), cigarettes (from 4% to 2%), cigars (from 3.5% to 2.2%), and pipe tobacco (from 2% to 0.7%).
“Since February 2014, the Food and Drug Administration’s first national tobacco public education campaign, the Real Cost, has broadcasted tobacco education advertising designed for youths aged 12-17 years; the campaign was associated with an estimated 348,398 U.S. youths who did not initiate cigarette smoking during February 2014–March 2016,” the investigators said. “Continued implementation of these strategies can help prevent and further reduce the use of all forms of tobacco product among U.S. youths.”
Read more in MMWR (2017 Jun 16;66[23]:597-603).
FROM MMWR
Vaccine delivery costs challenge physicians
, reported Mandy A. Allison, MD, of the University of Colorado at Denver, Aurora, and her associates.
Using the American Academy of Pediatrics’ recommended level of payment for first vaccine administration at that time – $25 – more than three-quarters of the practices surveyed said that all payer types paid less than the cost of vaccine administration, the researchers noted.
Those reporting that payment for vaccine administration was $11 or more was 74% for FFS, 74% for PPOs, 57% for MCOs/HMOs, 37% for CHIP, and 34% for Medicaid, the investigators reported.
In terms of how profit margins for vaccine delivery had changed in the last 3 years, an increase was reported by 25% of pediatric practices (Ped) and 15% of family physician practices (FP), no change was reported by 38% of Ped and 49% of FP, and a decrease by 37% of Ped and 36% of FP practices.
Of those practices that used strategies to reduce vaccine purchase cost or increase payment, 81% Ped and 36% FP used online purchasing discounts, 78% Ped and 49% FP used prompt pay discounts, 65% Ped and 49% FP used bulk order discounts, 69% Ped and 42% FP used group purchasing, and 69% Ped and 33% FP used promotional pricing.
Fewer than half of the practices said that they negotiated with private insurers regarding payment for vaccines (44% Ped, 33% FP) and administration fees (44% Ped, 35% FP).
When asked if they had stopped purchasing one or more pediatric vaccines for financial reasons, the answer was “yes” for 12% of Ped and 23% of FP, “no, but have seriously considered” for 24% of Ped and 26% of FP, and “no” for 64% of Ped and 51% of FP, reported Dr. Allison and her colleagues.
Read more in Academic Pediatrics (2017. doi: 10.1016/j.acap.2017.06.001).
, reported Mandy A. Allison, MD, of the University of Colorado at Denver, Aurora, and her associates.
Using the American Academy of Pediatrics’ recommended level of payment for first vaccine administration at that time – $25 – more than three-quarters of the practices surveyed said that all payer types paid less than the cost of vaccine administration, the researchers noted.
Those reporting that payment for vaccine administration was $11 or more was 74% for FFS, 74% for PPOs, 57% for MCOs/HMOs, 37% for CHIP, and 34% for Medicaid, the investigators reported.
In terms of how profit margins for vaccine delivery had changed in the last 3 years, an increase was reported by 25% of pediatric practices (Ped) and 15% of family physician practices (FP), no change was reported by 38% of Ped and 49% of FP, and a decrease by 37% of Ped and 36% of FP practices.
Of those practices that used strategies to reduce vaccine purchase cost or increase payment, 81% Ped and 36% FP used online purchasing discounts, 78% Ped and 49% FP used prompt pay discounts, 65% Ped and 49% FP used bulk order discounts, 69% Ped and 42% FP used group purchasing, and 69% Ped and 33% FP used promotional pricing.
Fewer than half of the practices said that they negotiated with private insurers regarding payment for vaccines (44% Ped, 33% FP) and administration fees (44% Ped, 35% FP).
When asked if they had stopped purchasing one or more pediatric vaccines for financial reasons, the answer was “yes” for 12% of Ped and 23% of FP, “no, but have seriously considered” for 24% of Ped and 26% of FP, and “no” for 64% of Ped and 51% of FP, reported Dr. Allison and her colleagues.
Read more in Academic Pediatrics (2017. doi: 10.1016/j.acap.2017.06.001).
, reported Mandy A. Allison, MD, of the University of Colorado at Denver, Aurora, and her associates.
Using the American Academy of Pediatrics’ recommended level of payment for first vaccine administration at that time – $25 – more than three-quarters of the practices surveyed said that all payer types paid less than the cost of vaccine administration, the researchers noted.
Those reporting that payment for vaccine administration was $11 or more was 74% for FFS, 74% for PPOs, 57% for MCOs/HMOs, 37% for CHIP, and 34% for Medicaid, the investigators reported.
In terms of how profit margins for vaccine delivery had changed in the last 3 years, an increase was reported by 25% of pediatric practices (Ped) and 15% of family physician practices (FP), no change was reported by 38% of Ped and 49% of FP, and a decrease by 37% of Ped and 36% of FP practices.
Of those practices that used strategies to reduce vaccine purchase cost or increase payment, 81% Ped and 36% FP used online purchasing discounts, 78% Ped and 49% FP used prompt pay discounts, 65% Ped and 49% FP used bulk order discounts, 69% Ped and 42% FP used group purchasing, and 69% Ped and 33% FP used promotional pricing.
Fewer than half of the practices said that they negotiated with private insurers regarding payment for vaccines (44% Ped, 33% FP) and administration fees (44% Ped, 35% FP).
When asked if they had stopped purchasing one or more pediatric vaccines for financial reasons, the answer was “yes” for 12% of Ped and 23% of FP, “no, but have seriously considered” for 24% of Ped and 26% of FP, and “no” for 64% of Ped and 51% of FP, reported Dr. Allison and her colleagues.
Read more in Academic Pediatrics (2017. doi: 10.1016/j.acap.2017.06.001).
FROM ACADEMIC PEDIATRICS
Hexavalent DTaP5-IPV-HB-Hib is immunogenic in series with pentavalent vaccine
against all the hexavalent antigens, said Federico Martinón-Torres, MD, of Hospital Clinico Universitario de Santiago de Compostela, Spain, and his associates.
DTaP5-IPV-HB-Hib combination vaccine has a five-antigen pertussis component and contains polyribosylribitol phosphate–Neisseria meningitidis serogroup B outer membrane protein [PRP-OMP] Hib conjugate, while DTaP5-IPV-Hib contains polyribosylribitol phosphate-tetanus toxoid (PRP-T) Hib conjugate.
The phase III, open-label, single-arm study in 12 Spanish hospitals and health centers included 384 infants who had received only one dose of monovalent hepatitis B vaccine within 3 days of birth prior to starting the study. They then received the hexa/penta/hexa combo vaccines concomitantly with the MCC vaccine. The response rate was 100% against Hib and 98.9% against hepatitis B.
Also, a “robust immune response was induced against all other disease antigens,” said Dr. Martinón-Torres and his colleagues. Coadministration of the combo vaccines with the meningococcal vaccine likewise did not appear to compromise immunogenicity of any of the vaccine antigens.
Use of the hexa/penta/hexa combo vaccine series was mostly safe and well tolerated, the researchers said.
Read more in the journal Vaccine (2017 Jun 17;35[30];3764-72).
against all the hexavalent antigens, said Federico Martinón-Torres, MD, of Hospital Clinico Universitario de Santiago de Compostela, Spain, and his associates.
DTaP5-IPV-HB-Hib combination vaccine has a five-antigen pertussis component and contains polyribosylribitol phosphate–Neisseria meningitidis serogroup B outer membrane protein [PRP-OMP] Hib conjugate, while DTaP5-IPV-Hib contains polyribosylribitol phosphate-tetanus toxoid (PRP-T) Hib conjugate.
The phase III, open-label, single-arm study in 12 Spanish hospitals and health centers included 384 infants who had received only one dose of monovalent hepatitis B vaccine within 3 days of birth prior to starting the study. They then received the hexa/penta/hexa combo vaccines concomitantly with the MCC vaccine. The response rate was 100% against Hib and 98.9% against hepatitis B.
Also, a “robust immune response was induced against all other disease antigens,” said Dr. Martinón-Torres and his colleagues. Coadministration of the combo vaccines with the meningococcal vaccine likewise did not appear to compromise immunogenicity of any of the vaccine antigens.
Use of the hexa/penta/hexa combo vaccine series was mostly safe and well tolerated, the researchers said.
Read more in the journal Vaccine (2017 Jun 17;35[30];3764-72).
against all the hexavalent antigens, said Federico Martinón-Torres, MD, of Hospital Clinico Universitario de Santiago de Compostela, Spain, and his associates.
DTaP5-IPV-HB-Hib combination vaccine has a five-antigen pertussis component and contains polyribosylribitol phosphate–Neisseria meningitidis serogroup B outer membrane protein [PRP-OMP] Hib conjugate, while DTaP5-IPV-Hib contains polyribosylribitol phosphate-tetanus toxoid (PRP-T) Hib conjugate.
The phase III, open-label, single-arm study in 12 Spanish hospitals and health centers included 384 infants who had received only one dose of monovalent hepatitis B vaccine within 3 days of birth prior to starting the study. They then received the hexa/penta/hexa combo vaccines concomitantly with the MCC vaccine. The response rate was 100% against Hib and 98.9% against hepatitis B.
Also, a “robust immune response was induced against all other disease antigens,” said Dr. Martinón-Torres and his colleagues. Coadministration of the combo vaccines with the meningococcal vaccine likewise did not appear to compromise immunogenicity of any of the vaccine antigens.
Use of the hexa/penta/hexa combo vaccine series was mostly safe and well tolerated, the researchers said.
Read more in the journal Vaccine (2017 Jun 17;35[30];3764-72).
FROM VACCINE
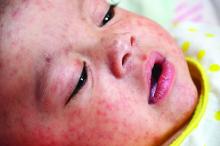
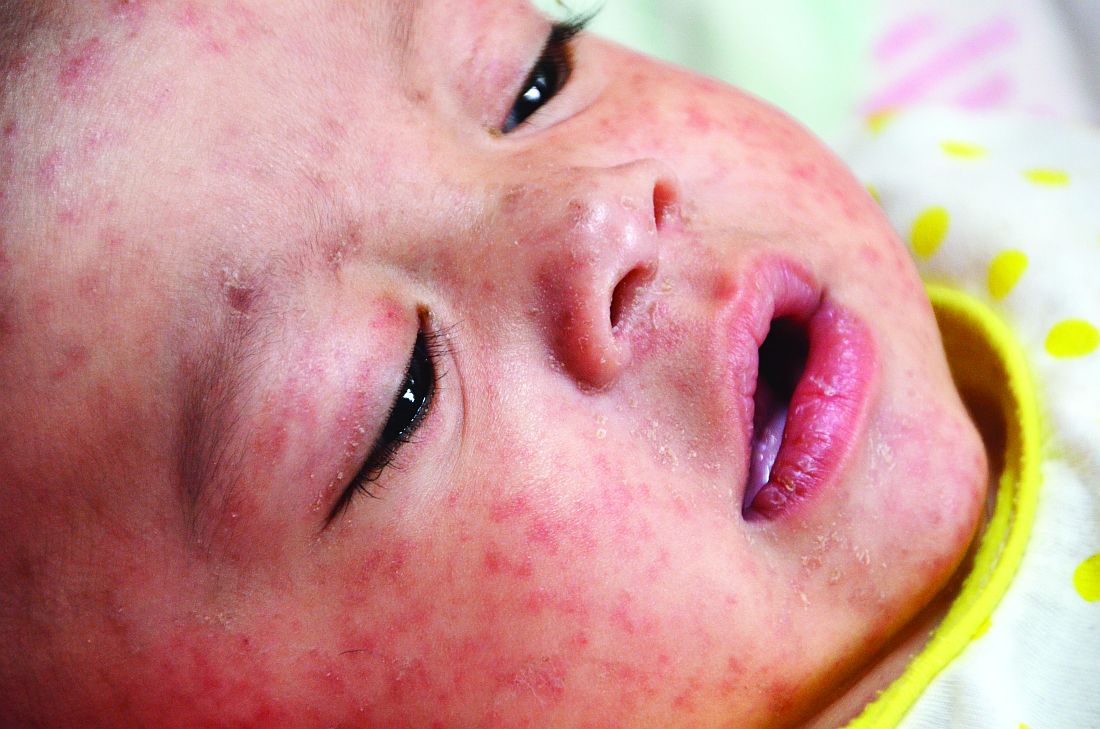
Earlier childhood measles vaccination elevates the risk of vaccine failure
of age or younger, compared with 15 months of age or older, said Sara Carazo Perez, MD, PhD, of Laval University, Quebec City, and her associates.
Studies suggest that measles elimination “requires the maintenance of population immunity above 92%-94%.” So, “the additional protection of 3%-5% of vaccinated children against secondary vaccine failures by postponing the first dose from 12 to 15 months of age would be significant, and the risk would be minimal in countries that achieved elimination” of measles, said Dr. Perez and her colleagues.
The percentage of children who were seronegative for measles after being vaccinated decreased significantly with older age at the first dose, from 8.5% in children vaccinated at 11 months to 3.2%, 2.4%, and 1.5% with vaccination at 12 months, 13-14 months, and 15-22 months, respectively (P less than .001).
Geometric mean concentrations (GMC) after the second dose were highly correlated with GMCs after the first dose.
The investigators then performed multivariable analysis, adjusting for the type of vaccine, the country, and the study. GMCs rose significantly with older age at first dose. Children vaccinated at 11 months had a 23% lower GMC and 30% increased risk of seronegativity than children vaccinated at 12 months.
In children first vaccinated at 13-14 months or 15-22 months, GMCs were 1.21 and 1.37 times greater than in children vaccinated at 12 months, respectively, and their risks for seronegativity were 49% and 71% lower, respectively.
“After two doses, the association between the age at first dose and the GMC was slightly weaker but still significant,” the researchers said.
Read more in the journal Clinical Infectious Diseases (2017 Jun 8. doi: 10.1093/cid/cix510).
of age or younger, compared with 15 months of age or older, said Sara Carazo Perez, MD, PhD, of Laval University, Quebec City, and her associates.
Studies suggest that measles elimination “requires the maintenance of population immunity above 92%-94%.” So, “the additional protection of 3%-5% of vaccinated children against secondary vaccine failures by postponing the first dose from 12 to 15 months of age would be significant, and the risk would be minimal in countries that achieved elimination” of measles, said Dr. Perez and her colleagues.
The percentage of children who were seronegative for measles after being vaccinated decreased significantly with older age at the first dose, from 8.5% in children vaccinated at 11 months to 3.2%, 2.4%, and 1.5% with vaccination at 12 months, 13-14 months, and 15-22 months, respectively (P less than .001).
Geometric mean concentrations (GMC) after the second dose were highly correlated with GMCs after the first dose.
The investigators then performed multivariable analysis, adjusting for the type of vaccine, the country, and the study. GMCs rose significantly with older age at first dose. Children vaccinated at 11 months had a 23% lower GMC and 30% increased risk of seronegativity than children vaccinated at 12 months.
In children first vaccinated at 13-14 months or 15-22 months, GMCs were 1.21 and 1.37 times greater than in children vaccinated at 12 months, respectively, and their risks for seronegativity were 49% and 71% lower, respectively.
“After two doses, the association between the age at first dose and the GMC was slightly weaker but still significant,” the researchers said.
Read more in the journal Clinical Infectious Diseases (2017 Jun 8. doi: 10.1093/cid/cix510).
of age or younger, compared with 15 months of age or older, said Sara Carazo Perez, MD, PhD, of Laval University, Quebec City, and her associates.
Studies suggest that measles elimination “requires the maintenance of population immunity above 92%-94%.” So, “the additional protection of 3%-5% of vaccinated children against secondary vaccine failures by postponing the first dose from 12 to 15 months of age would be significant, and the risk would be minimal in countries that achieved elimination” of measles, said Dr. Perez and her colleagues.
The percentage of children who were seronegative for measles after being vaccinated decreased significantly with older age at the first dose, from 8.5% in children vaccinated at 11 months to 3.2%, 2.4%, and 1.5% with vaccination at 12 months, 13-14 months, and 15-22 months, respectively (P less than .001).
Geometric mean concentrations (GMC) after the second dose were highly correlated with GMCs after the first dose.
The investigators then performed multivariable analysis, adjusting for the type of vaccine, the country, and the study. GMCs rose significantly with older age at first dose. Children vaccinated at 11 months had a 23% lower GMC and 30% increased risk of seronegativity than children vaccinated at 12 months.
In children first vaccinated at 13-14 months or 15-22 months, GMCs were 1.21 and 1.37 times greater than in children vaccinated at 12 months, respectively, and their risks for seronegativity were 49% and 71% lower, respectively.
“After two doses, the association between the age at first dose and the GMC was slightly weaker but still significant,” the researchers said.
Read more in the journal Clinical Infectious Diseases (2017 Jun 8. doi: 10.1093/cid/cix510).
FROM CLINICAL INFECTIOUS DISEASES
Investigational flu vaccine finds way around pyrogenicity problem
aged 5-17 years, Jolanta Airey, MD, of Seqirus in Parkville, Australia, and her associates reported.
In 2010, in Australia and New Zealand, use of a trivalent flu vaccine was associated with unexpected reports of fever and febrile seizures in children aged younger than 9 years. Research into the issue suggested that “degraded RNA fragments delivered by residual lipids activated the release of proinflammatory cytokines, which stimulated the pyrogenic response in children,” the study authors noted. “Increasing the level of sodium taurodeoxycholate (TDOC) to split the B strain in particular resulted in decreased levels of residual lipids and attenuated proinflammatory cytokine signals.”
In a phase III, randomized, observer-blinded study of the two flu vaccines at 32 centers in the United States between September 2015 and June 2016, 1,709 children aged 5-17 years received IIV4, and 569 children the same age received comparator IIV4 (Fluarix Quadrivalent). The two vaccines generated strong immune responses against all the vaccine strains in the children, with the hemagglutination inhibition geometric mean titers similar for all strains and higher for A strains than B strains for both vaccines.
In the 5- to 8-year-old group, fever was reported by 4.5% of those in the IIV4 group and 3.6% of children in the comparator IIV4 group (relative risk, 1.22). In the 9- to 17-year-old group, fever was reported by 2.1% of children in the IIV4 group and 0.8% of those in the comparator IIV4 group (RR, 2.80). Severe fever was reported by 1% or less of any of the groups of children.
Read more in the journal Vaccine (2017 May 9;35[20]:2745-52).
aged 5-17 years, Jolanta Airey, MD, of Seqirus in Parkville, Australia, and her associates reported.
In 2010, in Australia and New Zealand, use of a trivalent flu vaccine was associated with unexpected reports of fever and febrile seizures in children aged younger than 9 years. Research into the issue suggested that “degraded RNA fragments delivered by residual lipids activated the release of proinflammatory cytokines, which stimulated the pyrogenic response in children,” the study authors noted. “Increasing the level of sodium taurodeoxycholate (TDOC) to split the B strain in particular resulted in decreased levels of residual lipids and attenuated proinflammatory cytokine signals.”
In a phase III, randomized, observer-blinded study of the two flu vaccines at 32 centers in the United States between September 2015 and June 2016, 1,709 children aged 5-17 years received IIV4, and 569 children the same age received comparator IIV4 (Fluarix Quadrivalent). The two vaccines generated strong immune responses against all the vaccine strains in the children, with the hemagglutination inhibition geometric mean titers similar for all strains and higher for A strains than B strains for both vaccines.
In the 5- to 8-year-old group, fever was reported by 4.5% of those in the IIV4 group and 3.6% of children in the comparator IIV4 group (relative risk, 1.22). In the 9- to 17-year-old group, fever was reported by 2.1% of children in the IIV4 group and 0.8% of those in the comparator IIV4 group (RR, 2.80). Severe fever was reported by 1% or less of any of the groups of children.
Read more in the journal Vaccine (2017 May 9;35[20]:2745-52).
aged 5-17 years, Jolanta Airey, MD, of Seqirus in Parkville, Australia, and her associates reported.
In 2010, in Australia and New Zealand, use of a trivalent flu vaccine was associated with unexpected reports of fever and febrile seizures in children aged younger than 9 years. Research into the issue suggested that “degraded RNA fragments delivered by residual lipids activated the release of proinflammatory cytokines, which stimulated the pyrogenic response in children,” the study authors noted. “Increasing the level of sodium taurodeoxycholate (TDOC) to split the B strain in particular resulted in decreased levels of residual lipids and attenuated proinflammatory cytokine signals.”
In a phase III, randomized, observer-blinded study of the two flu vaccines at 32 centers in the United States between September 2015 and June 2016, 1,709 children aged 5-17 years received IIV4, and 569 children the same age received comparator IIV4 (Fluarix Quadrivalent). The two vaccines generated strong immune responses against all the vaccine strains in the children, with the hemagglutination inhibition geometric mean titers similar for all strains and higher for A strains than B strains for both vaccines.
In the 5- to 8-year-old group, fever was reported by 4.5% of those in the IIV4 group and 3.6% of children in the comparator IIV4 group (relative risk, 1.22). In the 9- to 17-year-old group, fever was reported by 2.1% of children in the IIV4 group and 0.8% of those in the comparator IIV4 group (RR, 2.80). Severe fever was reported by 1% or less of any of the groups of children.
Read more in the journal Vaccine (2017 May 9;35[20]:2745-52).
FROM VACCINE