User login
Catherine Cooper Nellist is editor of Pediatric News and Ob. Gyn. News. She has more than 30 years of experience reporting, writing, and editing stories about clinical medicine and the U.S. health care industry. Prior to taking the helm of these award-winning publications, Catherine covered major medical research meetings throughout the United States and Canada, and had been editor of Clinical Psychiatry News, and Dermatology News. She joined the company in 1984 after graduating magna cum laude from Dickinson College, Carlisle, Pa., with a BA in English.
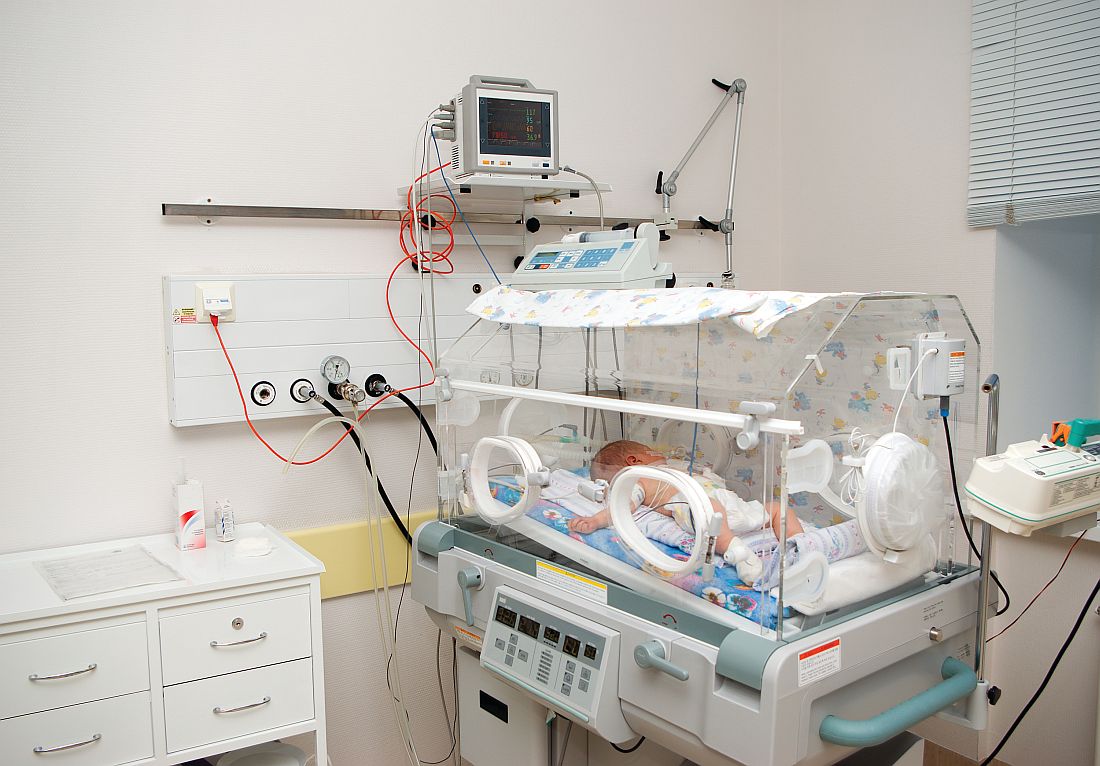
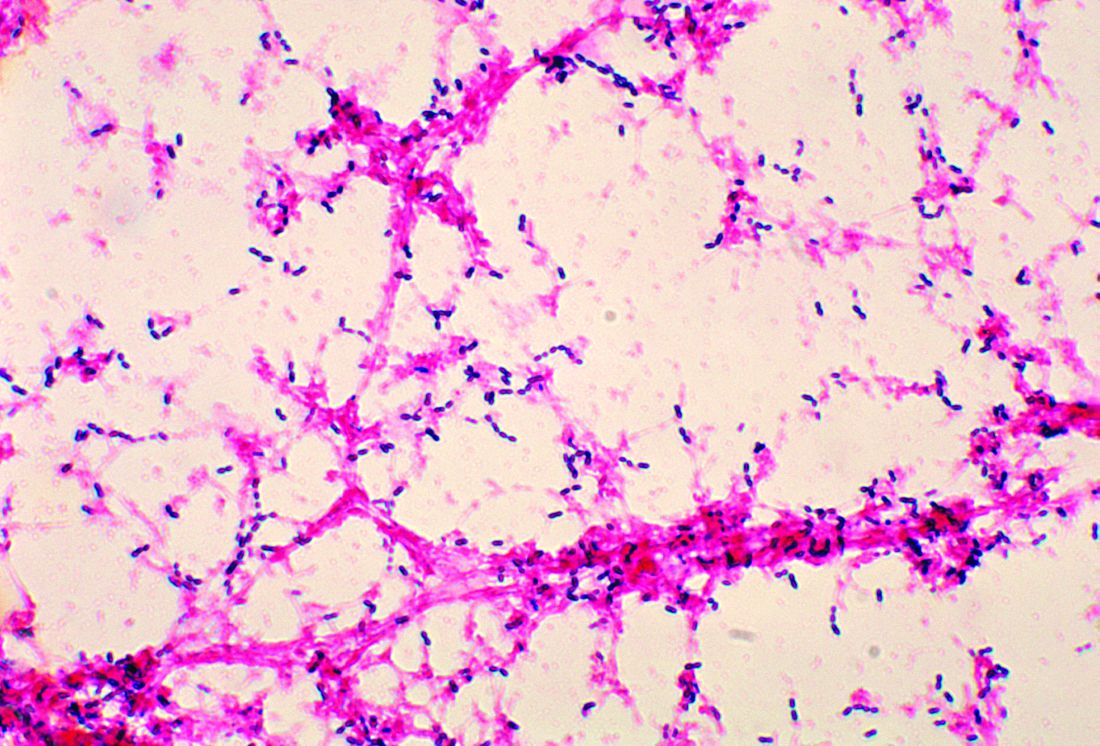
E. coli, GBS account for majority of neonatal bacterial meningitis in Canada
No major shifts appear to have occurred in the bacteria that cause meningitis in Canada, said Lynda Ouchenir, MD, University of Montreal, and her associates.
“There is a paucity of information on the characteristics of neonatal meningitis in the era of infant Haemophilus influenzae type B (Hib) and pneumococcal immunization, maternal group B Streptococcus (GBS) prophylaxis, and emerging antimicrobial resistance,” the researchers said. So, they undertook a retrospective study of infants with onset of bacterial meningitis in the first 90 days of life at seven Canadian hospitals to find out the major pathogens involved and best empirical antibiotics to use.
This substitution of a carbapenem for the cephalosporin was considered prudent if the birth hospitalization was complicated and if the cerebrospinal fluid Gram-stain or the blood culture was suggestive of Gram-negative meningitis, Dr. Ouchenir and her associates said.
Read more at (Pediatrics. 2017;140[1)]:e20170476).
No major shifts appear to have occurred in the bacteria that cause meningitis in Canada, said Lynda Ouchenir, MD, University of Montreal, and her associates.
“There is a paucity of information on the characteristics of neonatal meningitis in the era of infant Haemophilus influenzae type B (Hib) and pneumococcal immunization, maternal group B Streptococcus (GBS) prophylaxis, and emerging antimicrobial resistance,” the researchers said. So, they undertook a retrospective study of infants with onset of bacterial meningitis in the first 90 days of life at seven Canadian hospitals to find out the major pathogens involved and best empirical antibiotics to use.
This substitution of a carbapenem for the cephalosporin was considered prudent if the birth hospitalization was complicated and if the cerebrospinal fluid Gram-stain or the blood culture was suggestive of Gram-negative meningitis, Dr. Ouchenir and her associates said.
Read more at (Pediatrics. 2017;140[1)]:e20170476).
No major shifts appear to have occurred in the bacteria that cause meningitis in Canada, said Lynda Ouchenir, MD, University of Montreal, and her associates.
“There is a paucity of information on the characteristics of neonatal meningitis in the era of infant Haemophilus influenzae type B (Hib) and pneumococcal immunization, maternal group B Streptococcus (GBS) prophylaxis, and emerging antimicrobial resistance,” the researchers said. So, they undertook a retrospective study of infants with onset of bacterial meningitis in the first 90 days of life at seven Canadian hospitals to find out the major pathogens involved and best empirical antibiotics to use.
This substitution of a carbapenem for the cephalosporin was considered prudent if the birth hospitalization was complicated and if the cerebrospinal fluid Gram-stain or the blood culture was suggestive of Gram-negative meningitis, Dr. Ouchenir and her associates said.
Read more at (Pediatrics. 2017;140[1)]:e20170476).
FROM PEDIATRICS
Factors tied to parents’ intent to vaccinate teens for HPV
in a large study, said Kahee A. Mohammed, MD, of Saint Louis (Missouri) University Center for Outcomes Research, and associates.
Messages to boost HPV vaccination rates may need to be targeted based on maternal education, non-Hispanic white ethnicity, and provider recommendations, the researchers said.
Among unvaccinated girls, independent variables predicting parents’ intent to vaccinate teens for HPV were Hispanic race/ethnicity (AOR, 1.57), compared with non-Hispanic whites; mothers with less than a high school diploma (AOR, 1.86), compared with mothers with a college education; and a provider recommendation for HPV vaccine (AOR, 1.38).
Also, mothers with some college education were more likely to intend to vaccinate their sons (AOR, 1.21), but less likely to intend to vaccinate their daughters (AOR, .69) than mothers with a college education.
About 7% of the survey respondents said “not sure/don’t know” regarding their intent to vaccinate their teens. The largest percentage had boys (66%), were non-Hispanic whites (47%), lived in the South (38%), lived above the poverty line (62%), the mother was a college graduate (31%), and had never received a recommendation for HPV vaccination from a health care provider (75%).
“Health care providers should actively engage in discussions with parents about HPV and strongly recommend the vaccine to all eligible patients concurrently with other routinely administered vaccinations to dispel any potential negative assumptions or opinions regarding HPV vaccination, especially among girls,” Dr. Mohammed and his associates said.
Read more at (Prev Chronic Dis. 2017. doi: 10.5888/pcd14.160314).
in a large study, said Kahee A. Mohammed, MD, of Saint Louis (Missouri) University Center for Outcomes Research, and associates.
Messages to boost HPV vaccination rates may need to be targeted based on maternal education, non-Hispanic white ethnicity, and provider recommendations, the researchers said.
Among unvaccinated girls, independent variables predicting parents’ intent to vaccinate teens for HPV were Hispanic race/ethnicity (AOR, 1.57), compared with non-Hispanic whites; mothers with less than a high school diploma (AOR, 1.86), compared with mothers with a college education; and a provider recommendation for HPV vaccine (AOR, 1.38).
Also, mothers with some college education were more likely to intend to vaccinate their sons (AOR, 1.21), but less likely to intend to vaccinate their daughters (AOR, .69) than mothers with a college education.
About 7% of the survey respondents said “not sure/don’t know” regarding their intent to vaccinate their teens. The largest percentage had boys (66%), were non-Hispanic whites (47%), lived in the South (38%), lived above the poverty line (62%), the mother was a college graduate (31%), and had never received a recommendation for HPV vaccination from a health care provider (75%).
“Health care providers should actively engage in discussions with parents about HPV and strongly recommend the vaccine to all eligible patients concurrently with other routinely administered vaccinations to dispel any potential negative assumptions or opinions regarding HPV vaccination, especially among girls,” Dr. Mohammed and his associates said.
Read more at (Prev Chronic Dis. 2017. doi: 10.5888/pcd14.160314).
in a large study, said Kahee A. Mohammed, MD, of Saint Louis (Missouri) University Center for Outcomes Research, and associates.
Messages to boost HPV vaccination rates may need to be targeted based on maternal education, non-Hispanic white ethnicity, and provider recommendations, the researchers said.
Among unvaccinated girls, independent variables predicting parents’ intent to vaccinate teens for HPV were Hispanic race/ethnicity (AOR, 1.57), compared with non-Hispanic whites; mothers with less than a high school diploma (AOR, 1.86), compared with mothers with a college education; and a provider recommendation for HPV vaccine (AOR, 1.38).
Also, mothers with some college education were more likely to intend to vaccinate their sons (AOR, 1.21), but less likely to intend to vaccinate their daughters (AOR, .69) than mothers with a college education.
About 7% of the survey respondents said “not sure/don’t know” regarding their intent to vaccinate their teens. The largest percentage had boys (66%), were non-Hispanic whites (47%), lived in the South (38%), lived above the poverty line (62%), the mother was a college graduate (31%), and had never received a recommendation for HPV vaccination from a health care provider (75%).
“Health care providers should actively engage in discussions with parents about HPV and strongly recommend the vaccine to all eligible patients concurrently with other routinely administered vaccinations to dispel any potential negative assumptions or opinions regarding HPV vaccination, especially among girls,” Dr. Mohammed and his associates said.
Read more at (Prev Chronic Dis. 2017. doi: 10.5888/pcd14.160314).
FROM PREVENTING CHRONIC DISEASE
It matters how you phrase a child’s flu vaccine recommendation
Vaccine hesitant parents were more likely to change their minds about flu vaccine for their children when pediatricians or pediatric nurse practitioners used a presumptive recommendation that their children get the vaccine, pursued the recommendation if the parent was resistant, and combined their recommendation for the flu vaccine with other childhood vaccines, said Annika M. Hofstetter, MD, PhD, of the University of Washington, Seattle, and her associates.
The researchers recruited 17 pediatricians and pediatric nurse practitioners from eight primary care pediatric practices in the Seattle area to take part in 50 videotaped visits with parents during the 2011-2012 and 2013-2014 flu seasons.
(83% vs. 33%; P less than .01), Dr. Hofstetter and her colleagues said. The various communication patterns did not appear to negatively affect the way parents rated their visit experiences.
Read more at Vaccine. 2017;35:2709-15.
Vaccine hesitant parents were more likely to change their minds about flu vaccine for their children when pediatricians or pediatric nurse practitioners used a presumptive recommendation that their children get the vaccine, pursued the recommendation if the parent was resistant, and combined their recommendation for the flu vaccine with other childhood vaccines, said Annika M. Hofstetter, MD, PhD, of the University of Washington, Seattle, and her associates.
The researchers recruited 17 pediatricians and pediatric nurse practitioners from eight primary care pediatric practices in the Seattle area to take part in 50 videotaped visits with parents during the 2011-2012 and 2013-2014 flu seasons.
(83% vs. 33%; P less than .01), Dr. Hofstetter and her colleagues said. The various communication patterns did not appear to negatively affect the way parents rated their visit experiences.
Read more at Vaccine. 2017;35:2709-15.
Vaccine hesitant parents were more likely to change their minds about flu vaccine for their children when pediatricians or pediatric nurse practitioners used a presumptive recommendation that their children get the vaccine, pursued the recommendation if the parent was resistant, and combined their recommendation for the flu vaccine with other childhood vaccines, said Annika M. Hofstetter, MD, PhD, of the University of Washington, Seattle, and her associates.
The researchers recruited 17 pediatricians and pediatric nurse practitioners from eight primary care pediatric practices in the Seattle area to take part in 50 videotaped visits with parents during the 2011-2012 and 2013-2014 flu seasons.
(83% vs. 33%; P less than .01), Dr. Hofstetter and her colleagues said. The various communication patterns did not appear to negatively affect the way parents rated their visit experiences.
Read more at Vaccine. 2017;35:2709-15.
FROM VACCINE
Management of asymptomatic chorioamnionitis-exposed neonates needs revamping
Clinical observation and laboratory evaluation without immediate antibiotic use in asymptomatic chorioamnionitis-exposed neonates prevented neonatal intensive care unit (NICU) admission in two-thirds of these infants, Amanda I. Jan, MD, of the University of Southern California, Los Angeles, and her associates reported in a study.
Since maternal intrapartum antibiotic prophylaxis was introduced, neonatal early-onset sepsis (EOS) rates have dropped considerably, and rates remain low even in chorioamnionitis-exposed infants. Despite these low risks, current American Academy of Pediatrics and Centers for Disease Control and Prevention recommendations still call for a limited laboratory evaluation and immediate empirical antibiotic therapy in all infants exposed to chorioamnionitis, often necessitating NICU admission for IV antibiotics, the researchers noted.
Of the 78 infants admitted to the NICU and put on antibiotics, 76% were treated with antibiotics for more than 72 hours, with a median 7 days of treatment, compared with a median 2 days for nonadmitted infants (P less than .001). Only 85% of admitted infants received any breast milk, compared with 94% of infants in the mother-infant unit (P = .032), and none of the admitted infants were exclusively breastfed.
“When the overall risks of EOS are low, exposure of large numbers of well-appearing infants to even short courses of antibiotics is no longer justified,” Dr. Jan and her associates stated. “The [difference in] cost of a stay in the mother-infant unit for 2 days, compared with a NICU stay, which averaged a week, is substantial. The charge for our NICU is $12,612 per day in contrast to $5,300 per day in the mother-infant unit. The cost savings for the 162 infants who were cared for 2 days in the mother-infant unit, compared with an EOS evaluation and antibiotic therapy in the NICU, totals $2,369,088, or $359,861 per year.
“There were no deaths or morbidities identified in any infant during the study period,” they reported. No infant was readmitted to the study hospital for sepsis after discharge.
Dr. Jan and her associates recommend their alternative management of asymptomatic chorioamnionitis-exposed neonates involving lab evaluations and close clinical observation without immediate antibiotic administration in a mother-infant unit. They believe this prevents unnecessary antibiotic exposure, unnecessarily high hospitalization costs, and disruption of maternal-neonatal bonding and breastfeeding. Additional studies are needed to determine the safety of this approach.
This study received no external funding, and Dr. Jan and her associates reported no relevant financial disclosures.
Dr. Jan and her associates have taken steps in the right direction in altering management of asymptomatic term and near-term newborns with a maternal history of chorioamnionitis to avoid administering empirical antibiotics to all these babies, which is sorely needed as the current American Academy of Pediatrics and Centers for Disease Control and Prevention guidelines are outdated.
However, their alternative plan needs some tweaking. The positive predictive value of abnormal complete blood count or C-reactive protein results is too low to be of use in diagnosing sepsis. “We believe a better approach would be to forgo routine laboratory evaluations among this population altogether and manage them using clinical signs alone.”
They said it was important to state two key caveats. “First, in the immediate postpartum period, mild respiratory distress among term or near-term newborns may be attributable to the physiologic transition, which occurs in all newborn infants. It is not necessary to draw laboratories or start antibiotics on these patients as long as their symptoms improve and resolve within the first 6 hours of life. Second, if newborns with a maternal history of chorioamnionitis are to be monitored for signs of sepsis outside the NICU setting, observations must be frequent (at least hourly for the first 6 hours of life and then every 3 hours for the next 18 hours) and performed by adequately trained medical staff. In the absence of frequent, reliable observation, there is a possibility that the early signs of sepsis will be missed and go untreated with potentially severe consequences.”
This approach, as with any other, needs additional study.
Thomas A. Hooven, MD, and Richard A. Polin, MD, pediatricians at the Columbia University, New York, discussed the study by Jan et al. in a commentary, which is summarized here (Pediatrics. 2017;140[1]:e20171155). They reported that they received no external funding and had no relevant financial disclosures.
Dr. Jan and her associates have taken steps in the right direction in altering management of asymptomatic term and near-term newborns with a maternal history of chorioamnionitis to avoid administering empirical antibiotics to all these babies, which is sorely needed as the current American Academy of Pediatrics and Centers for Disease Control and Prevention guidelines are outdated.
However, their alternative plan needs some tweaking. The positive predictive value of abnormal complete blood count or C-reactive protein results is too low to be of use in diagnosing sepsis. “We believe a better approach would be to forgo routine laboratory evaluations among this population altogether and manage them using clinical signs alone.”
They said it was important to state two key caveats. “First, in the immediate postpartum period, mild respiratory distress among term or near-term newborns may be attributable to the physiologic transition, which occurs in all newborn infants. It is not necessary to draw laboratories or start antibiotics on these patients as long as their symptoms improve and resolve within the first 6 hours of life. Second, if newborns with a maternal history of chorioamnionitis are to be monitored for signs of sepsis outside the NICU setting, observations must be frequent (at least hourly for the first 6 hours of life and then every 3 hours for the next 18 hours) and performed by adequately trained medical staff. In the absence of frequent, reliable observation, there is a possibility that the early signs of sepsis will be missed and go untreated with potentially severe consequences.”
This approach, as with any other, needs additional study.
Thomas A. Hooven, MD, and Richard A. Polin, MD, pediatricians at the Columbia University, New York, discussed the study by Jan et al. in a commentary, which is summarized here (Pediatrics. 2017;140[1]:e20171155). They reported that they received no external funding and had no relevant financial disclosures.
Dr. Jan and her associates have taken steps in the right direction in altering management of asymptomatic term and near-term newborns with a maternal history of chorioamnionitis to avoid administering empirical antibiotics to all these babies, which is sorely needed as the current American Academy of Pediatrics and Centers for Disease Control and Prevention guidelines are outdated.
However, their alternative plan needs some tweaking. The positive predictive value of abnormal complete blood count or C-reactive protein results is too low to be of use in diagnosing sepsis. “We believe a better approach would be to forgo routine laboratory evaluations among this population altogether and manage them using clinical signs alone.”
They said it was important to state two key caveats. “First, in the immediate postpartum period, mild respiratory distress among term or near-term newborns may be attributable to the physiologic transition, which occurs in all newborn infants. It is not necessary to draw laboratories or start antibiotics on these patients as long as their symptoms improve and resolve within the first 6 hours of life. Second, if newborns with a maternal history of chorioamnionitis are to be monitored for signs of sepsis outside the NICU setting, observations must be frequent (at least hourly for the first 6 hours of life and then every 3 hours for the next 18 hours) and performed by adequately trained medical staff. In the absence of frequent, reliable observation, there is a possibility that the early signs of sepsis will be missed and go untreated with potentially severe consequences.”
This approach, as with any other, needs additional study.
Thomas A. Hooven, MD, and Richard A. Polin, MD, pediatricians at the Columbia University, New York, discussed the study by Jan et al. in a commentary, which is summarized here (Pediatrics. 2017;140[1]:e20171155). They reported that they received no external funding and had no relevant financial disclosures.
Clinical observation and laboratory evaluation without immediate antibiotic use in asymptomatic chorioamnionitis-exposed neonates prevented neonatal intensive care unit (NICU) admission in two-thirds of these infants, Amanda I. Jan, MD, of the University of Southern California, Los Angeles, and her associates reported in a study.
Since maternal intrapartum antibiotic prophylaxis was introduced, neonatal early-onset sepsis (EOS) rates have dropped considerably, and rates remain low even in chorioamnionitis-exposed infants. Despite these low risks, current American Academy of Pediatrics and Centers for Disease Control and Prevention recommendations still call for a limited laboratory evaluation and immediate empirical antibiotic therapy in all infants exposed to chorioamnionitis, often necessitating NICU admission for IV antibiotics, the researchers noted.
Of the 78 infants admitted to the NICU and put on antibiotics, 76% were treated with antibiotics for more than 72 hours, with a median 7 days of treatment, compared with a median 2 days for nonadmitted infants (P less than .001). Only 85% of admitted infants received any breast milk, compared with 94% of infants in the mother-infant unit (P = .032), and none of the admitted infants were exclusively breastfed.
“When the overall risks of EOS are low, exposure of large numbers of well-appearing infants to even short courses of antibiotics is no longer justified,” Dr. Jan and her associates stated. “The [difference in] cost of a stay in the mother-infant unit for 2 days, compared with a NICU stay, which averaged a week, is substantial. The charge for our NICU is $12,612 per day in contrast to $5,300 per day in the mother-infant unit. The cost savings for the 162 infants who were cared for 2 days in the mother-infant unit, compared with an EOS evaluation and antibiotic therapy in the NICU, totals $2,369,088, or $359,861 per year.
“There were no deaths or morbidities identified in any infant during the study period,” they reported. No infant was readmitted to the study hospital for sepsis after discharge.
Dr. Jan and her associates recommend their alternative management of asymptomatic chorioamnionitis-exposed neonates involving lab evaluations and close clinical observation without immediate antibiotic administration in a mother-infant unit. They believe this prevents unnecessary antibiotic exposure, unnecessarily high hospitalization costs, and disruption of maternal-neonatal bonding and breastfeeding. Additional studies are needed to determine the safety of this approach.
This study received no external funding, and Dr. Jan and her associates reported no relevant financial disclosures.
Clinical observation and laboratory evaluation without immediate antibiotic use in asymptomatic chorioamnionitis-exposed neonates prevented neonatal intensive care unit (NICU) admission in two-thirds of these infants, Amanda I. Jan, MD, of the University of Southern California, Los Angeles, and her associates reported in a study.
Since maternal intrapartum antibiotic prophylaxis was introduced, neonatal early-onset sepsis (EOS) rates have dropped considerably, and rates remain low even in chorioamnionitis-exposed infants. Despite these low risks, current American Academy of Pediatrics and Centers for Disease Control and Prevention recommendations still call for a limited laboratory evaluation and immediate empirical antibiotic therapy in all infants exposed to chorioamnionitis, often necessitating NICU admission for IV antibiotics, the researchers noted.
Of the 78 infants admitted to the NICU and put on antibiotics, 76% were treated with antibiotics for more than 72 hours, with a median 7 days of treatment, compared with a median 2 days for nonadmitted infants (P less than .001). Only 85% of admitted infants received any breast milk, compared with 94% of infants in the mother-infant unit (P = .032), and none of the admitted infants were exclusively breastfed.
“When the overall risks of EOS are low, exposure of large numbers of well-appearing infants to even short courses of antibiotics is no longer justified,” Dr. Jan and her associates stated. “The [difference in] cost of a stay in the mother-infant unit for 2 days, compared with a NICU stay, which averaged a week, is substantial. The charge for our NICU is $12,612 per day in contrast to $5,300 per day in the mother-infant unit. The cost savings for the 162 infants who were cared for 2 days in the mother-infant unit, compared with an EOS evaluation and antibiotic therapy in the NICU, totals $2,369,088, or $359,861 per year.
“There were no deaths or morbidities identified in any infant during the study period,” they reported. No infant was readmitted to the study hospital for sepsis after discharge.
Dr. Jan and her associates recommend their alternative management of asymptomatic chorioamnionitis-exposed neonates involving lab evaluations and close clinical observation without immediate antibiotic administration in a mother-infant unit. They believe this prevents unnecessary antibiotic exposure, unnecessarily high hospitalization costs, and disruption of maternal-neonatal bonding and breastfeeding. Additional studies are needed to determine the safety of this approach.
This study received no external funding, and Dr. Jan and her associates reported no relevant financial disclosures.
FROM PEDIATRICS
Key clinical point: Alternative management of asymptomatic chorioamnionitis-exposed neonates will prevent unnecessary antibiotic exposure, unnecessarily high hospitalization costs, and disruption of maternal-neonatal bonding and breastfeeding.
Major finding: Of the 240 infants, 67.5% remained well with a routine newborn course in the mother-infant unit and 32.5% subsequently were admitted to the NICU because of abnormal laboratory data, a positive blood culture, or the onset of clinical signs of sepsis.
Data source: A retrospective cohort study of 240 asymptomatic chorioamnionitis-exposed neonates.
Disclosures: This study received no external funding, and Dr. Jan and her associates reported no relevant financial disclosures.
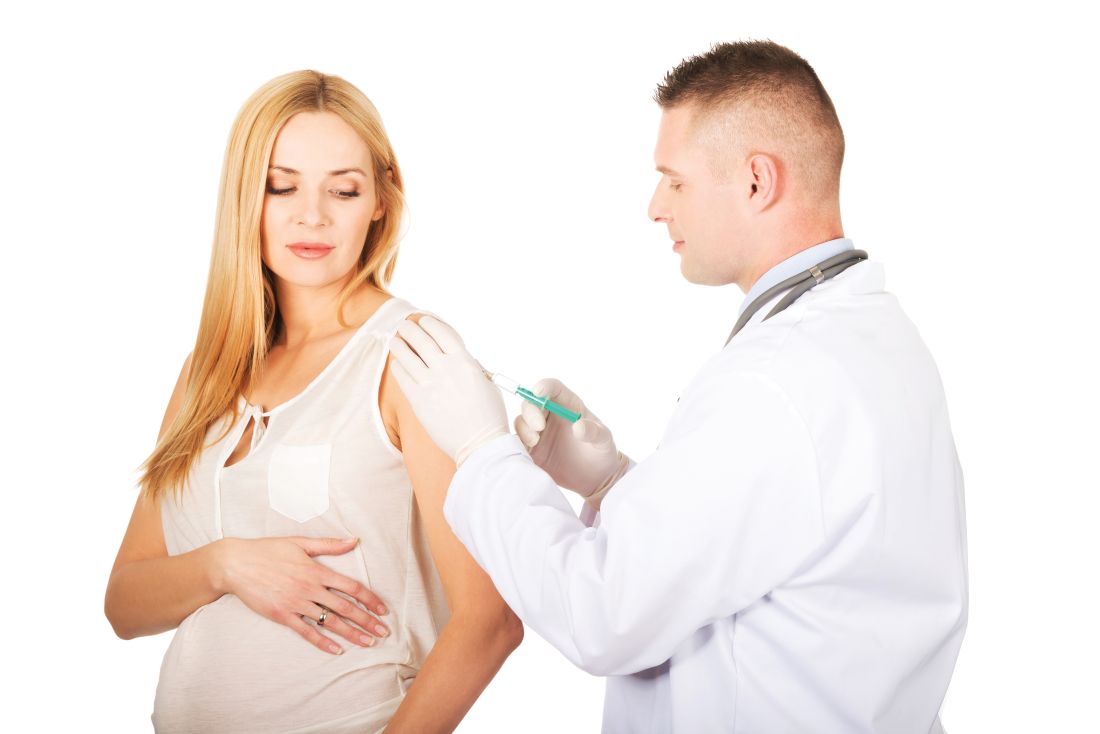
First trimester use of inactivated flu vaccine didn’t cause birth defects
, said Elyse Olshen Kharbanda, MD, of HealthPartners Institute, Minneapolis, and her associates.
Data from seven participating Vaccine Safety Datalink sites in six states were used to identify 52,856 women who received IIV in the first trimester of pregnancy (12% of the study total) and 373,088 not exposed to the flu vaccine in the first trimester (88%). A total of 865 women in the IIV-exposed group had an infant with 1 of the 50 selected major structural defects (1.6 per 100 live births), versus 5,730 in the unexposed group (1.5 per 100 live births).
Among the strengths of the study were the large population, which allowed the researchers to examine subgroups of major structural birth defects; their findings were consistent across all those subgroups. In addition, the investigators were able to exclude women at increased risk for major birth defects because of comorbidities such as diabetes, drug exposures, diagnosed chromosomal abnormalities, or congenital infections. Finally, the study authors were able to exclude women with potential exposure to teratogenic medications.
“Because IIV is currently recommended for all women who will be pregnant during periods of influenza circulation, these data should provide reassurance for women considering first trimester vaccination,” said Dr. Kharbanda and her associates.
Read more in the Journal of Pediatrics (2017 May 24. doi: 10.1016/j.jpeds.2017.04.039).
, said Elyse Olshen Kharbanda, MD, of HealthPartners Institute, Minneapolis, and her associates.
Data from seven participating Vaccine Safety Datalink sites in six states were used to identify 52,856 women who received IIV in the first trimester of pregnancy (12% of the study total) and 373,088 not exposed to the flu vaccine in the first trimester (88%). A total of 865 women in the IIV-exposed group had an infant with 1 of the 50 selected major structural defects (1.6 per 100 live births), versus 5,730 in the unexposed group (1.5 per 100 live births).
Among the strengths of the study were the large population, which allowed the researchers to examine subgroups of major structural birth defects; their findings were consistent across all those subgroups. In addition, the investigators were able to exclude women at increased risk for major birth defects because of comorbidities such as diabetes, drug exposures, diagnosed chromosomal abnormalities, or congenital infections. Finally, the study authors were able to exclude women with potential exposure to teratogenic medications.
“Because IIV is currently recommended for all women who will be pregnant during periods of influenza circulation, these data should provide reassurance for women considering first trimester vaccination,” said Dr. Kharbanda and her associates.
Read more in the Journal of Pediatrics (2017 May 24. doi: 10.1016/j.jpeds.2017.04.039).
, said Elyse Olshen Kharbanda, MD, of HealthPartners Institute, Minneapolis, and her associates.
Data from seven participating Vaccine Safety Datalink sites in six states were used to identify 52,856 women who received IIV in the first trimester of pregnancy (12% of the study total) and 373,088 not exposed to the flu vaccine in the first trimester (88%). A total of 865 women in the IIV-exposed group had an infant with 1 of the 50 selected major structural defects (1.6 per 100 live births), versus 5,730 in the unexposed group (1.5 per 100 live births).
Among the strengths of the study were the large population, which allowed the researchers to examine subgroups of major structural birth defects; their findings were consistent across all those subgroups. In addition, the investigators were able to exclude women at increased risk for major birth defects because of comorbidities such as diabetes, drug exposures, diagnosed chromosomal abnormalities, or congenital infections. Finally, the study authors were able to exclude women with potential exposure to teratogenic medications.
“Because IIV is currently recommended for all women who will be pregnant during periods of influenza circulation, these data should provide reassurance for women considering first trimester vaccination,” said Dr. Kharbanda and her associates.
Read more in the Journal of Pediatrics (2017 May 24. doi: 10.1016/j.jpeds.2017.04.039).
FROM THE JOURNAL OF PEDIATRICS
AAP advises against giving fruit juice to children under 1 year
according to a 2017 policy statement by the American Academy of Pediatrics Section on Gastroenterology, Hepatology, and Nutrition and the Committee on Nutrition.
In addition, no more than 4 ounces of fruit juice per day should be given to toddlers aged 1-3 years, and no more than 4-6 ounces to children aged 4-6 years. For children aged 7-18 years, fruit juice intake should be limited to 8 ounces (Pediatrics. 2017 Jun;139[6]:e20170967).
In fact, the AAP recommends that human milk be the only nutrient for infants up to 6 months of age, or a prepared infant formula for mothers who cannot breastfeed or choose not to breastfeed their infants. In a study of 168 children aged either 2 years or 5 years, consumption of 12 fluid ounces or more per day of fruit juice was associated with short stature and with obesity (Pediatrics. 1997 Jan;99[1]:15-22).
If toddlers are given fruit juice, it should be in a cup rather that a bottle, sippy cup, or box of juice that they can carry around for long periods. Also, infants and toddlers should not be put to bed with a bottle of fruit juice, according to the statement. Prolonged exposure of the teeth to the sugars in juice can result in dental caries.
Fruit juice is sometime erroneously used instead of oral electrolyte solutions to rehydrate infants and young children with gastroenteritis or diarrhea, but the high carbohydrate content of fruit juice “may exceed the intestine’s ability to absorb carbohydrate, resulting in carbohydrate malabsorption. Carbohydrate malabsorption causes osmotic diarrhea, increasing the severity of the diarrhea already present,” according to the statement. Also, if fruit juice is used to replace fluid losses in infants, it may cause hyponatremia.
There are several medical conditions in which it is prudent to determine how much fruit juice is being consumed:
- Overnutrition or undernutrition.
- Chronic diarrhea, excessive flatulence, abdominal pain, and bloating.
- Dental caries.
- Poor or excessive weight gain.
Fruit juice is viewed by parents as nutritious, but toddlers and young children should be encouraged to eat whole fruit instead.
“We know that excessive fruit juice can lead to excessive weight gain and tooth decay,” coauthor Steven A. Abrams, MD, of the University of Texas, Austin, said in a press release. “Pediatricians have a lot of information to share with families on how to provide the proper balance of fresh fruit within their child’s diet.”
The authors said they had no relevant financial conflicts.
The AAP’s new policy statement regarding limiting juice consumption has potential to make a big difference in the prevention of two largely preventable diseases – obesity and dental caries. Recognizing that obesity and dental caries are silent epidemics in the United States, and that overconsumption of sugar is a common risk factor for both of these diseases, the AAP’s new policy statement is overdue. Fruit juice has as much sugar as soda drinks, yet parents feel it is a healthy drink alternative because juice comes from fruit. Parents often introduce juice to their children at a very young age and serve them more juice than is needed. Also, young children commonly consume juice in a sippy cup or bottle, which can lead to dental decay, because the frequent sipping of the juice fuels the acid-producing bacteria that contribute to enamel erosion.
As pediatricians, my colleagues and I are challenged to help children maintain a healthy weight and healthy mouths, and we have long battled the early introduction and overconsumption of juice. Medical and dental health care professionals, along with public health programs, can rally around this new policy statement.
Patricia Braun, MD, is a professor of pediatrics at the University of Colorado, Denver, and a practicing pediatrician at Denver Health. She had no conflicts of interest.
The AAP’s new policy statement regarding limiting juice consumption has potential to make a big difference in the prevention of two largely preventable diseases – obesity and dental caries. Recognizing that obesity and dental caries are silent epidemics in the United States, and that overconsumption of sugar is a common risk factor for both of these diseases, the AAP’s new policy statement is overdue. Fruit juice has as much sugar as soda drinks, yet parents feel it is a healthy drink alternative because juice comes from fruit. Parents often introduce juice to their children at a very young age and serve them more juice than is needed. Also, young children commonly consume juice in a sippy cup or bottle, which can lead to dental decay, because the frequent sipping of the juice fuels the acid-producing bacteria that contribute to enamel erosion.
As pediatricians, my colleagues and I are challenged to help children maintain a healthy weight and healthy mouths, and we have long battled the early introduction and overconsumption of juice. Medical and dental health care professionals, along with public health programs, can rally around this new policy statement.
Patricia Braun, MD, is a professor of pediatrics at the University of Colorado, Denver, and a practicing pediatrician at Denver Health. She had no conflicts of interest.
The AAP’s new policy statement regarding limiting juice consumption has potential to make a big difference in the prevention of two largely preventable diseases – obesity and dental caries. Recognizing that obesity and dental caries are silent epidemics in the United States, and that overconsumption of sugar is a common risk factor for both of these diseases, the AAP’s new policy statement is overdue. Fruit juice has as much sugar as soda drinks, yet parents feel it is a healthy drink alternative because juice comes from fruit. Parents often introduce juice to their children at a very young age and serve them more juice than is needed. Also, young children commonly consume juice in a sippy cup or bottle, which can lead to dental decay, because the frequent sipping of the juice fuels the acid-producing bacteria that contribute to enamel erosion.
As pediatricians, my colleagues and I are challenged to help children maintain a healthy weight and healthy mouths, and we have long battled the early introduction and overconsumption of juice. Medical and dental health care professionals, along with public health programs, can rally around this new policy statement.
Patricia Braun, MD, is a professor of pediatrics at the University of Colorado, Denver, and a practicing pediatrician at Denver Health. She had no conflicts of interest.
according to a 2017 policy statement by the American Academy of Pediatrics Section on Gastroenterology, Hepatology, and Nutrition and the Committee on Nutrition.
In addition, no more than 4 ounces of fruit juice per day should be given to toddlers aged 1-3 years, and no more than 4-6 ounces to children aged 4-6 years. For children aged 7-18 years, fruit juice intake should be limited to 8 ounces (Pediatrics. 2017 Jun;139[6]:e20170967).
In fact, the AAP recommends that human milk be the only nutrient for infants up to 6 months of age, or a prepared infant formula for mothers who cannot breastfeed or choose not to breastfeed their infants. In a study of 168 children aged either 2 years or 5 years, consumption of 12 fluid ounces or more per day of fruit juice was associated with short stature and with obesity (Pediatrics. 1997 Jan;99[1]:15-22).
If toddlers are given fruit juice, it should be in a cup rather that a bottle, sippy cup, or box of juice that they can carry around for long periods. Also, infants and toddlers should not be put to bed with a bottle of fruit juice, according to the statement. Prolonged exposure of the teeth to the sugars in juice can result in dental caries.
Fruit juice is sometime erroneously used instead of oral electrolyte solutions to rehydrate infants and young children with gastroenteritis or diarrhea, but the high carbohydrate content of fruit juice “may exceed the intestine’s ability to absorb carbohydrate, resulting in carbohydrate malabsorption. Carbohydrate malabsorption causes osmotic diarrhea, increasing the severity of the diarrhea already present,” according to the statement. Also, if fruit juice is used to replace fluid losses in infants, it may cause hyponatremia.
There are several medical conditions in which it is prudent to determine how much fruit juice is being consumed:
- Overnutrition or undernutrition.
- Chronic diarrhea, excessive flatulence, abdominal pain, and bloating.
- Dental caries.
- Poor or excessive weight gain.
Fruit juice is viewed by parents as nutritious, but toddlers and young children should be encouraged to eat whole fruit instead.
“We know that excessive fruit juice can lead to excessive weight gain and tooth decay,” coauthor Steven A. Abrams, MD, of the University of Texas, Austin, said in a press release. “Pediatricians have a lot of information to share with families on how to provide the proper balance of fresh fruit within their child’s diet.”
The authors said they had no relevant financial conflicts.
according to a 2017 policy statement by the American Academy of Pediatrics Section on Gastroenterology, Hepatology, and Nutrition and the Committee on Nutrition.
In addition, no more than 4 ounces of fruit juice per day should be given to toddlers aged 1-3 years, and no more than 4-6 ounces to children aged 4-6 years. For children aged 7-18 years, fruit juice intake should be limited to 8 ounces (Pediatrics. 2017 Jun;139[6]:e20170967).
In fact, the AAP recommends that human milk be the only nutrient for infants up to 6 months of age, or a prepared infant formula for mothers who cannot breastfeed or choose not to breastfeed their infants. In a study of 168 children aged either 2 years or 5 years, consumption of 12 fluid ounces or more per day of fruit juice was associated with short stature and with obesity (Pediatrics. 1997 Jan;99[1]:15-22).
If toddlers are given fruit juice, it should be in a cup rather that a bottle, sippy cup, or box of juice that they can carry around for long periods. Also, infants and toddlers should not be put to bed with a bottle of fruit juice, according to the statement. Prolonged exposure of the teeth to the sugars in juice can result in dental caries.
Fruit juice is sometime erroneously used instead of oral electrolyte solutions to rehydrate infants and young children with gastroenteritis or diarrhea, but the high carbohydrate content of fruit juice “may exceed the intestine’s ability to absorb carbohydrate, resulting in carbohydrate malabsorption. Carbohydrate malabsorption causes osmotic diarrhea, increasing the severity of the diarrhea already present,” according to the statement. Also, if fruit juice is used to replace fluid losses in infants, it may cause hyponatremia.
There are several medical conditions in which it is prudent to determine how much fruit juice is being consumed:
- Overnutrition or undernutrition.
- Chronic diarrhea, excessive flatulence, abdominal pain, and bloating.
- Dental caries.
- Poor or excessive weight gain.
Fruit juice is viewed by parents as nutritious, but toddlers and young children should be encouraged to eat whole fruit instead.
“We know that excessive fruit juice can lead to excessive weight gain and tooth decay,” coauthor Steven A. Abrams, MD, of the University of Texas, Austin, said in a press release. “Pediatricians have a lot of information to share with families on how to provide the proper balance of fresh fruit within their child’s diet.”
The authors said they had no relevant financial conflicts.
PCV10 vaccination in Brazil nearly eliminated serotypes
The effect of 10-valent pneumococcal conjugate vaccine (PCV10) after 4 years of routine childhood vaccination in Brazil was near elimination of PCV10 serotypes in a study of over 500 children, said Felipe P.G. Neves, PhD, of the University of California, Berkeley, and his associates.
The emergence of multidrug-resistant (MDR) serotype 6C isolates, however, is of concern, they said.
Of the 284 children who attended a public clinic and 238 children who went to two private clinics in the greater Rio de Janeiro metropolitan area between Sept. 29 and Dec. 5, 2014, 118 (23%) were pneumococcal carriers. Their median age was 2 years and all were under age 6 years. All 118 isolates were susceptible to levofloxacin, rifampicin, and vancomycin; 26 (22%) isolates were MDR, and 14 of the 18 serotype 6C isolates were MDR.
“Serotype 6C has already been reported as having caused meningitis in the pre-PCV10 era in northeastern Brazil. Additionally, following PCV7 implementation, carriage with serotype 6C emerged worldwide and reflected in an increase in IPD [invasive pneumococcal disease] cases,” Dr. Neves and his associates said. “Considering the apparent consistency of MDR 6C as the most common serotype associated with colonization in Brazil after PCV10 universal use, ongoing surveillance to monitor its increase in invasive diseases in Brazil is warranted.”
Read more in Vaccine (2017 May 15;35[21]:2794-800).
The effect of 10-valent pneumococcal conjugate vaccine (PCV10) after 4 years of routine childhood vaccination in Brazil was near elimination of PCV10 serotypes in a study of over 500 children, said Felipe P.G. Neves, PhD, of the University of California, Berkeley, and his associates.
The emergence of multidrug-resistant (MDR) serotype 6C isolates, however, is of concern, they said.
Of the 284 children who attended a public clinic and 238 children who went to two private clinics in the greater Rio de Janeiro metropolitan area between Sept. 29 and Dec. 5, 2014, 118 (23%) were pneumococcal carriers. Their median age was 2 years and all were under age 6 years. All 118 isolates were susceptible to levofloxacin, rifampicin, and vancomycin; 26 (22%) isolates were MDR, and 14 of the 18 serotype 6C isolates were MDR.
“Serotype 6C has already been reported as having caused meningitis in the pre-PCV10 era in northeastern Brazil. Additionally, following PCV7 implementation, carriage with serotype 6C emerged worldwide and reflected in an increase in IPD [invasive pneumococcal disease] cases,” Dr. Neves and his associates said. “Considering the apparent consistency of MDR 6C as the most common serotype associated with colonization in Brazil after PCV10 universal use, ongoing surveillance to monitor its increase in invasive diseases in Brazil is warranted.”
Read more in Vaccine (2017 May 15;35[21]:2794-800).
The effect of 10-valent pneumococcal conjugate vaccine (PCV10) after 4 years of routine childhood vaccination in Brazil was near elimination of PCV10 serotypes in a study of over 500 children, said Felipe P.G. Neves, PhD, of the University of California, Berkeley, and his associates.
The emergence of multidrug-resistant (MDR) serotype 6C isolates, however, is of concern, they said.
Of the 284 children who attended a public clinic and 238 children who went to two private clinics in the greater Rio de Janeiro metropolitan area between Sept. 29 and Dec. 5, 2014, 118 (23%) were pneumococcal carriers. Their median age was 2 years and all were under age 6 years. All 118 isolates were susceptible to levofloxacin, rifampicin, and vancomycin; 26 (22%) isolates were MDR, and 14 of the 18 serotype 6C isolates were MDR.
“Serotype 6C has already been reported as having caused meningitis in the pre-PCV10 era in northeastern Brazil. Additionally, following PCV7 implementation, carriage with serotype 6C emerged worldwide and reflected in an increase in IPD [invasive pneumococcal disease] cases,” Dr. Neves and his associates said. “Considering the apparent consistency of MDR 6C as the most common serotype associated with colonization in Brazil after PCV10 universal use, ongoing surveillance to monitor its increase in invasive diseases in Brazil is warranted.”
Read more in Vaccine (2017 May 15;35[21]:2794-800).
FROM VACCINE
Declines in frequent binge drinking vary in some teen subgroups
The drop in frequent binge drinking (FBD) among adolescents can be attributed to age, period, and cohort effects, but there are variations in certain subgroups of teens, said Joy Bohyun Jang, PhD, and her associates.
The decline in FBD is not as great in teen girls, African American youth, and youth from low socioeconomic backgrounds, so those groups deserve close attention by researchers and clinicians, they said.
The study used an age-period-cohort analysis to examine how these variables affected drinking trends among adolescents, with a particular focus on FBD, which was defined as two or more occasions of consuming at least five alcoholic drinks in a row in the past 2 weeks.
FBD decreased in recent years in all ages during adolescence, suggesting that declines in teen FBD in the past 25 years were “driven by factors influencing all age groups simultaneously as well as influences on particular birth cohorts,” the researchers said. These factors might include greater public efforts to lessen the risk of underage drinking and disapproval of heavy alcohol use among the recent cohorts of teens. “Those born around 1990 had the highest decline of FBD compared with those in the preceding and subsequent cohorts of adolescents.”
But there are variations in FBD among teens by demographics. Boys and those of higher socioeconomic status (SES) showed rapid increases in FBD by age, compared with girls and teens of lower SES, respectively. However, there also has been a convergence in FBD by sex in the more recent time periods because of greater declines in FBD among boys than in girls. Likewise, there is a growing discrepancy by SES in FBD in U.S. teens because higher SES teens were less likely than those from a lower SES to engage in FBD and “the strength of the association is growing in more recent time periods.”
African American youth had the lowest rates of FBD for all the racial groups, yet declines in FBD have been slower among African American youth, compared with white adolescents, since 2007, reported Dr. Jang of the University of Michigan, Ann Arbor, and her associates.
The study was supported by grants from the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and was funded by the National Institutes of Health. Dr. Jang and her associates said they had no relevant financial disclosures.
[email protected]
Teens who drink heavily are more likely to have unprotected sex, perform poorly at school or work, and have problems with their parents. Monitoring the Future data previously have shown that since the late 1990s, the prevalence of binge drinking has dropped to lows of 3%, 10%, and 16% among 8th, 10th, and 12th graders, respectively.
Dr. Jang et al. took a look at Monitoring the Future data to see how age, period, and cohort effects might alter FBD patterns among teens. There was an overall decrease in FBD since the 1990s, with the greatest decline among teens born between 1985 and 1990. It appeared that period and cohort effects drove this decline.
However, some subgroups exhibited differences. “The decline in frequent heavy drinking is not uniform, with female adolescents, black youth, and youth from low-SES backgrounds experiencing a less steep decline.”
“Pediatric primary care providers have an opportunity to screen all adolescents for alcohol use as part of routine annual care and to provide brief prevention and early intervention strategies. Despite the reassuring decline in frequent heavy drinking, it is critical that ongoing efforts address differences in declining rates to avoid exacerbating disparities.”
Justine Wittenauer Welsh, MD, of Emory Adolescent Substance Abuse Treatment Services, Emory University, Atlanta; John Rogers Knight, MD, at the Center for Adolescent Substance Abuse Research, Boston Children’s Hospital; and Scott Evan Hadland, MD, MPH, of Boston University, made these comments in an accompanying editorial (Pediatrics. 2017 May 22;139[6]:e20170932). The authors said they received no funding and have no relevant financial disclosures.
Teens who drink heavily are more likely to have unprotected sex, perform poorly at school or work, and have problems with their parents. Monitoring the Future data previously have shown that since the late 1990s, the prevalence of binge drinking has dropped to lows of 3%, 10%, and 16% among 8th, 10th, and 12th graders, respectively.
Dr. Jang et al. took a look at Monitoring the Future data to see how age, period, and cohort effects might alter FBD patterns among teens. There was an overall decrease in FBD since the 1990s, with the greatest decline among teens born between 1985 and 1990. It appeared that period and cohort effects drove this decline.
However, some subgroups exhibited differences. “The decline in frequent heavy drinking is not uniform, with female adolescents, black youth, and youth from low-SES backgrounds experiencing a less steep decline.”
“Pediatric primary care providers have an opportunity to screen all adolescents for alcohol use as part of routine annual care and to provide brief prevention and early intervention strategies. Despite the reassuring decline in frequent heavy drinking, it is critical that ongoing efforts address differences in declining rates to avoid exacerbating disparities.”
Justine Wittenauer Welsh, MD, of Emory Adolescent Substance Abuse Treatment Services, Emory University, Atlanta; John Rogers Knight, MD, at the Center for Adolescent Substance Abuse Research, Boston Children’s Hospital; and Scott Evan Hadland, MD, MPH, of Boston University, made these comments in an accompanying editorial (Pediatrics. 2017 May 22;139[6]:e20170932). The authors said they received no funding and have no relevant financial disclosures.
Teens who drink heavily are more likely to have unprotected sex, perform poorly at school or work, and have problems with their parents. Monitoring the Future data previously have shown that since the late 1990s, the prevalence of binge drinking has dropped to lows of 3%, 10%, and 16% among 8th, 10th, and 12th graders, respectively.
Dr. Jang et al. took a look at Monitoring the Future data to see how age, period, and cohort effects might alter FBD patterns among teens. There was an overall decrease in FBD since the 1990s, with the greatest decline among teens born between 1985 and 1990. It appeared that period and cohort effects drove this decline.
However, some subgroups exhibited differences. “The decline in frequent heavy drinking is not uniform, with female adolescents, black youth, and youth from low-SES backgrounds experiencing a less steep decline.”
“Pediatric primary care providers have an opportunity to screen all adolescents for alcohol use as part of routine annual care and to provide brief prevention and early intervention strategies. Despite the reassuring decline in frequent heavy drinking, it is critical that ongoing efforts address differences in declining rates to avoid exacerbating disparities.”
Justine Wittenauer Welsh, MD, of Emory Adolescent Substance Abuse Treatment Services, Emory University, Atlanta; John Rogers Knight, MD, at the Center for Adolescent Substance Abuse Research, Boston Children’s Hospital; and Scott Evan Hadland, MD, MPH, of Boston University, made these comments in an accompanying editorial (Pediatrics. 2017 May 22;139[6]:e20170932). The authors said they received no funding and have no relevant financial disclosures.
The drop in frequent binge drinking (FBD) among adolescents can be attributed to age, period, and cohort effects, but there are variations in certain subgroups of teens, said Joy Bohyun Jang, PhD, and her associates.
The decline in FBD is not as great in teen girls, African American youth, and youth from low socioeconomic backgrounds, so those groups deserve close attention by researchers and clinicians, they said.
The study used an age-period-cohort analysis to examine how these variables affected drinking trends among adolescents, with a particular focus on FBD, which was defined as two or more occasions of consuming at least five alcoholic drinks in a row in the past 2 weeks.
FBD decreased in recent years in all ages during adolescence, suggesting that declines in teen FBD in the past 25 years were “driven by factors influencing all age groups simultaneously as well as influences on particular birth cohorts,” the researchers said. These factors might include greater public efforts to lessen the risk of underage drinking and disapproval of heavy alcohol use among the recent cohorts of teens. “Those born around 1990 had the highest decline of FBD compared with those in the preceding and subsequent cohorts of adolescents.”
But there are variations in FBD among teens by demographics. Boys and those of higher socioeconomic status (SES) showed rapid increases in FBD by age, compared with girls and teens of lower SES, respectively. However, there also has been a convergence in FBD by sex in the more recent time periods because of greater declines in FBD among boys than in girls. Likewise, there is a growing discrepancy by SES in FBD in U.S. teens because higher SES teens were less likely than those from a lower SES to engage in FBD and “the strength of the association is growing in more recent time periods.”
African American youth had the lowest rates of FBD for all the racial groups, yet declines in FBD have been slower among African American youth, compared with white adolescents, since 2007, reported Dr. Jang of the University of Michigan, Ann Arbor, and her associates.
The study was supported by grants from the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and was funded by the National Institutes of Health. Dr. Jang and her associates said they had no relevant financial disclosures.
[email protected]
The drop in frequent binge drinking (FBD) among adolescents can be attributed to age, period, and cohort effects, but there are variations in certain subgroups of teens, said Joy Bohyun Jang, PhD, and her associates.
The decline in FBD is not as great in teen girls, African American youth, and youth from low socioeconomic backgrounds, so those groups deserve close attention by researchers and clinicians, they said.
The study used an age-period-cohort analysis to examine how these variables affected drinking trends among adolescents, with a particular focus on FBD, which was defined as two or more occasions of consuming at least five alcoholic drinks in a row in the past 2 weeks.
FBD decreased in recent years in all ages during adolescence, suggesting that declines in teen FBD in the past 25 years were “driven by factors influencing all age groups simultaneously as well as influences on particular birth cohorts,” the researchers said. These factors might include greater public efforts to lessen the risk of underage drinking and disapproval of heavy alcohol use among the recent cohorts of teens. “Those born around 1990 had the highest decline of FBD compared with those in the preceding and subsequent cohorts of adolescents.”
But there are variations in FBD among teens by demographics. Boys and those of higher socioeconomic status (SES) showed rapid increases in FBD by age, compared with girls and teens of lower SES, respectively. However, there also has been a convergence in FBD by sex in the more recent time periods because of greater declines in FBD among boys than in girls. Likewise, there is a growing discrepancy by SES in FBD in U.S. teens because higher SES teens were less likely than those from a lower SES to engage in FBD and “the strength of the association is growing in more recent time periods.”
African American youth had the lowest rates of FBD for all the racial groups, yet declines in FBD have been slower among African American youth, compared with white adolescents, since 2007, reported Dr. Jang of the University of Michigan, Ann Arbor, and her associates.
The study was supported by grants from the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and was funded by the National Institutes of Health. Dr. Jang and her associates said they had no relevant financial disclosures.
[email protected]
FROM PEDIATRICS
Key clinical point: The drop in frequent binge drinking (FBD) among U.S. teens can be attributed to age, period, and cohort effects, but there are variations in certain teen subgroups.
Major finding: FBD decreased in recent years among all ages during adolescence, suggesting that decreases in teen FBD in the past 25 years were “driven by factors influencing all age groups simultaneously as well as influences on particular birth cohorts.”
Data source: A Monitoring the Future study involved 1,065,022 student responses on self-administered questionnaires during 1991-2015 regarding binge drinking.
Disclosures: The study was supported by grants from the National Institutes of Health. Dr. Jang and her associates said they had no relevant financial disclosures.
Combo antifungal/corticosteroid creams contraindicated in children
Don’t use combination antifungal/corticosteroid medications in children or for treating diaper dermatitis, warned Chikoti M. Wheat, MD, of Johns Hopkins University, Baltimore, and her associates.
Lotrisone is a combination product with a high-potency topical corticosteroid, betamethasone dipropionate 0.05% cream, and a topical antifungal agent, clotrimazole 1% cream. The Food and Drug Administration does not recommend it for the population and indication mentioned above, yet it is often used so off-label, Dr. Wheat and her associates said. Mycolog-II, a combination antifungal/corticosteroid with nystatin, an antiyeast agent, and triamcinolone, a medium-potency topical steroid, was taken off the market in 2015, but patients may still have some that they are inclined to use (J Pediatr. 2017. doi: 0.1016/j.jpeds.2017.03.031).
In a study of 9,797 patients aged 0-14 years who were prescribed either Lotrisone or Mycolog-II creams from 2007 to 2014, pediatricians were most likely to prescribe the combination creams (mean, 810 patients), followed by family physicians (mean, 309 patients), and dermatologists (mean, 49 patients). No pediatric dermatologists prescribed either cream.
Why are these combination creams being used?
Sometimes a fungal infection induces an inflammatory dermatitis. In these cases the use of both an antifungal and a corticosteroid with careful monitoring can benefit the patient, the researchers said. “Research, however, has shown that when combination antifungal/corticosteroid agents are used for fungal infections, they not only result in high rates of recurrence of fungal infections but also have greater side effect profiles.”
Also, in 1984, Lotrisone was approved by the FDA for treating tinea corporis, tinea pedis, and tinea cruris in patients older than 12 years. However, lack of efficacy and severe adverse effects including hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, hyperglycemia, hirsutism, delayed growth, and skin atrophy led the FDA to recommend that Lotrisone not be prescribed for patients younger than 17 years and not be prescribed to treat diaper dermatitis.
So what should you do?
Dr. Wheat and her associates recommend a single-agent topical antifungal to treat superficial fungal infection. If there is intense itching due to infection-induced dermatitis, add a low- or medium-potency topical corticosteroid for just 7-10 days.
For diaper dermatitis with a confirmed secondary fungal infection, they recommend a topical antifungal agent in combination with a barrier cream. “If intense inflammatory changes are present, a low- to medium-potency topical corticosteroid can be used up to twice daily and tapered over 1-2 weeks with careful monitoring,” they said.
Dr. Wheat and her associates declared they had no conflicts of interest.
[email protected]
Don’t use combination antifungal/corticosteroid medications in children or for treating diaper dermatitis, warned Chikoti M. Wheat, MD, of Johns Hopkins University, Baltimore, and her associates.
Lotrisone is a combination product with a high-potency topical corticosteroid, betamethasone dipropionate 0.05% cream, and a topical antifungal agent, clotrimazole 1% cream. The Food and Drug Administration does not recommend it for the population and indication mentioned above, yet it is often used so off-label, Dr. Wheat and her associates said. Mycolog-II, a combination antifungal/corticosteroid with nystatin, an antiyeast agent, and triamcinolone, a medium-potency topical steroid, was taken off the market in 2015, but patients may still have some that they are inclined to use (J Pediatr. 2017. doi: 0.1016/j.jpeds.2017.03.031).
In a study of 9,797 patients aged 0-14 years who were prescribed either Lotrisone or Mycolog-II creams from 2007 to 2014, pediatricians were most likely to prescribe the combination creams (mean, 810 patients), followed by family physicians (mean, 309 patients), and dermatologists (mean, 49 patients). No pediatric dermatologists prescribed either cream.
Why are these combination creams being used?
Sometimes a fungal infection induces an inflammatory dermatitis. In these cases the use of both an antifungal and a corticosteroid with careful monitoring can benefit the patient, the researchers said. “Research, however, has shown that when combination antifungal/corticosteroid agents are used for fungal infections, they not only result in high rates of recurrence of fungal infections but also have greater side effect profiles.”
Also, in 1984, Lotrisone was approved by the FDA for treating tinea corporis, tinea pedis, and tinea cruris in patients older than 12 years. However, lack of efficacy and severe adverse effects including hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, hyperglycemia, hirsutism, delayed growth, and skin atrophy led the FDA to recommend that Lotrisone not be prescribed for patients younger than 17 years and not be prescribed to treat diaper dermatitis.
So what should you do?
Dr. Wheat and her associates recommend a single-agent topical antifungal to treat superficial fungal infection. If there is intense itching due to infection-induced dermatitis, add a low- or medium-potency topical corticosteroid for just 7-10 days.
For diaper dermatitis with a confirmed secondary fungal infection, they recommend a topical antifungal agent in combination with a barrier cream. “If intense inflammatory changes are present, a low- to medium-potency topical corticosteroid can be used up to twice daily and tapered over 1-2 weeks with careful monitoring,” they said.
Dr. Wheat and her associates declared they had no conflicts of interest.
[email protected]
Don’t use combination antifungal/corticosteroid medications in children or for treating diaper dermatitis, warned Chikoti M. Wheat, MD, of Johns Hopkins University, Baltimore, and her associates.
Lotrisone is a combination product with a high-potency topical corticosteroid, betamethasone dipropionate 0.05% cream, and a topical antifungal agent, clotrimazole 1% cream. The Food and Drug Administration does not recommend it for the population and indication mentioned above, yet it is often used so off-label, Dr. Wheat and her associates said. Mycolog-II, a combination antifungal/corticosteroid with nystatin, an antiyeast agent, and triamcinolone, a medium-potency topical steroid, was taken off the market in 2015, but patients may still have some that they are inclined to use (J Pediatr. 2017. doi: 0.1016/j.jpeds.2017.03.031).
In a study of 9,797 patients aged 0-14 years who were prescribed either Lotrisone or Mycolog-II creams from 2007 to 2014, pediatricians were most likely to prescribe the combination creams (mean, 810 patients), followed by family physicians (mean, 309 patients), and dermatologists (mean, 49 patients). No pediatric dermatologists prescribed either cream.
Why are these combination creams being used?
Sometimes a fungal infection induces an inflammatory dermatitis. In these cases the use of both an antifungal and a corticosteroid with careful monitoring can benefit the patient, the researchers said. “Research, however, has shown that when combination antifungal/corticosteroid agents are used for fungal infections, they not only result in high rates of recurrence of fungal infections but also have greater side effect profiles.”
Also, in 1984, Lotrisone was approved by the FDA for treating tinea corporis, tinea pedis, and tinea cruris in patients older than 12 years. However, lack of efficacy and severe adverse effects including hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, hyperglycemia, hirsutism, delayed growth, and skin atrophy led the FDA to recommend that Lotrisone not be prescribed for patients younger than 17 years and not be prescribed to treat diaper dermatitis.
So what should you do?
Dr. Wheat and her associates recommend a single-agent topical antifungal to treat superficial fungal infection. If there is intense itching due to infection-induced dermatitis, add a low- or medium-potency topical corticosteroid for just 7-10 days.
For diaper dermatitis with a confirmed secondary fungal infection, they recommend a topical antifungal agent in combination with a barrier cream. “If intense inflammatory changes are present, a low- to medium-potency topical corticosteroid can be used up to twice daily and tapered over 1-2 weeks with careful monitoring,” they said.
Dr. Wheat and her associates declared they had no conflicts of interest.
[email protected]
FROM THE JOURNAL OF PEDIATRICS
Key clinical point:
Major finding: Pediatricians were most likely to prescribe the combination creams (mean, 810 patients), followed by family physicians (mean, 309 patients), dermatologists (mean, 49 patients), and pediatric dermatologists (none).
Data source: A study of 9,797 patients aged 0-14 years who were prescribed either Lotrisone or Mycolog-II creams from 2007 to 2014.
Disclosures: Dr. Wheat and her associates declared they had no conflicts of interest.
Consider S. pyogenes in cases of pediatric intertrigo
Beefy-red, well demarcated lesions in skin folds without satellite lesions are the clinical signs of intertrigo, and Streptococcus pyogenes may be the cause, said Anca Chiriac, MD, PhD, of Apollonia University, Iasi, Romania, and her associates.
Intertrigo is frequently misdiagnosed in young children, and S. pyogenes skin infections often are misdiagnosed or overlooked. A case series of six children under 9 years of age was discussed in which the children presented with intense erythematous patches that often were pruritic occurring around the anus, in and around the umbilicus, on the vulva, in the intertriginous folds of the neck, in the toe web, and in the antecubital fossa.
The skin lesions often were misdiagnosed as contact dermatitis or atopic dermatitis or a fungal infection, and in several cases they were treated with potent corticosteroids, which aggravated the problem, or a topical antifungal, which had no effect. Swabs for bacterial culture identified S. pyogenes, and courses of oral antibiotics such as penicillin, amoxicillin, or ceftriaxone led to rapid improvement.
S. pyogenes skin infections complications include septicemia, arthritis, osteomyelitis, pneumonia, and toxic shock syndrome. “It is our practice to perform urinalysis because of the risk of poststreptococcal glomerulonephritis,” Dr. Chiriac and her associates said. They recommended penicillin or cephalosporin in age-related doses, or erythromycin or clindamycin if children are allergic to penicillin.
Read more at (J Pediatr. 2017 May;184:230-1).
[email protected]
Beefy-red, well demarcated lesions in skin folds without satellite lesions are the clinical signs of intertrigo, and Streptococcus pyogenes may be the cause, said Anca Chiriac, MD, PhD, of Apollonia University, Iasi, Romania, and her associates.
Intertrigo is frequently misdiagnosed in young children, and S. pyogenes skin infections often are misdiagnosed or overlooked. A case series of six children under 9 years of age was discussed in which the children presented with intense erythematous patches that often were pruritic occurring around the anus, in and around the umbilicus, on the vulva, in the intertriginous folds of the neck, in the toe web, and in the antecubital fossa.
The skin lesions often were misdiagnosed as contact dermatitis or atopic dermatitis or a fungal infection, and in several cases they were treated with potent corticosteroids, which aggravated the problem, or a topical antifungal, which had no effect. Swabs for bacterial culture identified S. pyogenes, and courses of oral antibiotics such as penicillin, amoxicillin, or ceftriaxone led to rapid improvement.
S. pyogenes skin infections complications include septicemia, arthritis, osteomyelitis, pneumonia, and toxic shock syndrome. “It is our practice to perform urinalysis because of the risk of poststreptococcal glomerulonephritis,” Dr. Chiriac and her associates said. They recommended penicillin or cephalosporin in age-related doses, or erythromycin or clindamycin if children are allergic to penicillin.
Read more at (J Pediatr. 2017 May;184:230-1).
[email protected]
Beefy-red, well demarcated lesions in skin folds without satellite lesions are the clinical signs of intertrigo, and Streptococcus pyogenes may be the cause, said Anca Chiriac, MD, PhD, of Apollonia University, Iasi, Romania, and her associates.
Intertrigo is frequently misdiagnosed in young children, and S. pyogenes skin infections often are misdiagnosed or overlooked. A case series of six children under 9 years of age was discussed in which the children presented with intense erythematous patches that often were pruritic occurring around the anus, in and around the umbilicus, on the vulva, in the intertriginous folds of the neck, in the toe web, and in the antecubital fossa.
The skin lesions often were misdiagnosed as contact dermatitis or atopic dermatitis or a fungal infection, and in several cases they were treated with potent corticosteroids, which aggravated the problem, or a topical antifungal, which had no effect. Swabs for bacterial culture identified S. pyogenes, and courses of oral antibiotics such as penicillin, amoxicillin, or ceftriaxone led to rapid improvement.
S. pyogenes skin infections complications include septicemia, arthritis, osteomyelitis, pneumonia, and toxic shock syndrome. “It is our practice to perform urinalysis because of the risk of poststreptococcal glomerulonephritis,” Dr. Chiriac and her associates said. They recommended penicillin or cephalosporin in age-related doses, or erythromycin or clindamycin if children are allergic to penicillin.
Read more at (J Pediatr. 2017 May;184:230-1).
[email protected]
FROM THE JOURNAL OF PEDIATRICS