User login
Sweat Regeneration Following CO2 Fractionated Laser Therapy
To the Editor:
It is not uncommon for patients with extensive dermal scarring to overheat due to the inability to regulate body temperature through evaporative heat loss, as lack of perspiration in areas of prior full-thickness skin injury is well known. One of the authors (C.M.H.) previously reported a case of a patient with considerable hypertrophic scarring after surviving an episode of toxic epidermal necrolysis that was likely precipitated by lamotrigine.1 The patient initially presented to our clinic in consultation for laser therapy to improve the pliability and cosmetic appearance of the scars; however, approximately 3 weeks after initiating treatment with a fractional CO2 laser, the patient noticed perspiration in areas where she once lacked the ability to perspire as well as improved functionality.1 It was speculated that scar remodeling stimulated by the CO2 fractional laser allowed new connections to form between eccrine ducts in the dermis and epidermis.2
These findings are even more notable in light of a study by Rittié et al3 that suggested the primary appendages of the skin involved in human wound healing are the eccrine sweat glands. The investigators were able to demonstrate that eccrine sweat glands are major contributors in reepithelialization and wound healing in humans; therefore, it is possible that stimulating these glands with the CO2 laser may promote enhanced reepithelialization in addition to the reestablishment of perspiration and wound healing.3 Considering inadequate wound repair represents a substantial disturbance to the patient and health care system, this finding offers promise as a potential means to decrease morbidity in patients with dermal scarring from burns and traumatic injuries. We have since evaluated and treated 3 patients who demonstrated sweat regeneration following treatment with the fractional CO2 laser (Table).
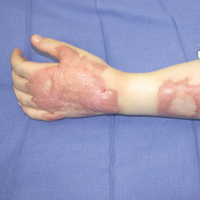
A 42-year-old man was our first patient to demonstrate functional scar improvement following bone marrow transplant for acute lymphoblastic leukemia complicated by chronic sclerodermoid graft-versus-host disease and subsequent extensive scarring on the chest and arms. Approximately 2 weeks after the first treatment with the fractional CO2 laser, the patient began to notice the presence of sweat beads in the treated areas. In addition to the reestablishment of perspiration, the patient had perceived increased mobility with improved pliability and “softness” (as described by family members) in treated areas likely related to scar remodeling.
A 36-year-old wounded army veteran presented with burns to the face, arms, and chest affecting 49% of the body surface area. After only 1 treatment, the patient reported that he could subjectively tolerate 10°F more ambient temperature and work all day outside in south Texas when heat intolerance previously would allow him to work only 2 to 3 hours. Additionally, he noted increased mobility and chest wall expansion, which in combination contributed to overall increased exercise tolerance and enhanced quality of life.
A 35-year-old US Marine and firefighter with burns primarily on the chest and arms involving 35% body surface area experienced increased exercise tolerance and sweat regeneration, particularly on the chest after a single treatment with the fractional CO2 laser but continued to experience improvement after a total of 3 treatments. Additionally, the cosmetic improvement was so substantial that the physician (C.M.H) had to review older photographs to verify the location of the scars.
We have now treated 3 patients with various mechanisms of injury and extensive scarring who noticed improved heat tolerance from sweat regeneration following fractional CO2 laser therapy. At this point, we only have anecdotal evidence of subjective functional improvement, and further research is warranted to elucidate the exact mechanism of action to support our findings.
- Neiner J, Whittemore D, Hivnor C. Buried alive: functional eccrine coils buried under scar tissue? J Am Acad Dermatol. 2011;65:661-663.
- Waibel J, Beer K, Narurkar V, et al. Preliminary observations on fractional ablative resurfacing devices: clinical impressions. J Drugs Dermatol. 2009;8:481-485.
- Rittié L, Sachs D, Orringer J, et al. Eccrine sweat glands are major contributors to reepithelialization of human wounds. Am J Pathol. 2013;1:163-171.
To the Editor:
It is not uncommon for patients with extensive dermal scarring to overheat due to the inability to regulate body temperature through evaporative heat loss, as lack of perspiration in areas of prior full-thickness skin injury is well known. One of the authors (C.M.H.) previously reported a case of a patient with considerable hypertrophic scarring after surviving an episode of toxic epidermal necrolysis that was likely precipitated by lamotrigine.1 The patient initially presented to our clinic in consultation for laser therapy to improve the pliability and cosmetic appearance of the scars; however, approximately 3 weeks after initiating treatment with a fractional CO2 laser, the patient noticed perspiration in areas where she once lacked the ability to perspire as well as improved functionality.1 It was speculated that scar remodeling stimulated by the CO2 fractional laser allowed new connections to form between eccrine ducts in the dermis and epidermis.2
These findings are even more notable in light of a study by Rittié et al3 that suggested the primary appendages of the skin involved in human wound healing are the eccrine sweat glands. The investigators were able to demonstrate that eccrine sweat glands are major contributors in reepithelialization and wound healing in humans; therefore, it is possible that stimulating these glands with the CO2 laser may promote enhanced reepithelialization in addition to the reestablishment of perspiration and wound healing.3 Considering inadequate wound repair represents a substantial disturbance to the patient and health care system, this finding offers promise as a potential means to decrease morbidity in patients with dermal scarring from burns and traumatic injuries. We have since evaluated and treated 3 patients who demonstrated sweat regeneration following treatment with the fractional CO2 laser (Table).

A 42-year-old man was our first patient to demonstrate functional scar improvement following bone marrow transplant for acute lymphoblastic leukemia complicated by chronic sclerodermoid graft-versus-host disease and subsequent extensive scarring on the chest and arms. Approximately 2 weeks after the first treatment with the fractional CO2 laser, the patient began to notice the presence of sweat beads in the treated areas. In addition to the reestablishment of perspiration, the patient had perceived increased mobility with improved pliability and “softness” (as described by family members) in treated areas likely related to scar remodeling.
A 36-year-old wounded army veteran presented with burns to the face, arms, and chest affecting 49% of the body surface area. After only 1 treatment, the patient reported that he could subjectively tolerate 10°F more ambient temperature and work all day outside in south Texas when heat intolerance previously would allow him to work only 2 to 3 hours. Additionally, he noted increased mobility and chest wall expansion, which in combination contributed to overall increased exercise tolerance and enhanced quality of life.
A 35-year-old US Marine and firefighter with burns primarily on the chest and arms involving 35% body surface area experienced increased exercise tolerance and sweat regeneration, particularly on the chest after a single treatment with the fractional CO2 laser but continued to experience improvement after a total of 3 treatments. Additionally, the cosmetic improvement was so substantial that the physician (C.M.H) had to review older photographs to verify the location of the scars.
We have now treated 3 patients with various mechanisms of injury and extensive scarring who noticed improved heat tolerance from sweat regeneration following fractional CO2 laser therapy. At this point, we only have anecdotal evidence of subjective functional improvement, and further research is warranted to elucidate the exact mechanism of action to support our findings.
To the Editor:
It is not uncommon for patients with extensive dermal scarring to overheat due to the inability to regulate body temperature through evaporative heat loss, as lack of perspiration in areas of prior full-thickness skin injury is well known. One of the authors (C.M.H.) previously reported a case of a patient with considerable hypertrophic scarring after surviving an episode of toxic epidermal necrolysis that was likely precipitated by lamotrigine.1 The patient initially presented to our clinic in consultation for laser therapy to improve the pliability and cosmetic appearance of the scars; however, approximately 3 weeks after initiating treatment with a fractional CO2 laser, the patient noticed perspiration in areas where she once lacked the ability to perspire as well as improved functionality.1 It was speculated that scar remodeling stimulated by the CO2 fractional laser allowed new connections to form between eccrine ducts in the dermis and epidermis.2
These findings are even more notable in light of a study by Rittié et al3 that suggested the primary appendages of the skin involved in human wound healing are the eccrine sweat glands. The investigators were able to demonstrate that eccrine sweat glands are major contributors in reepithelialization and wound healing in humans; therefore, it is possible that stimulating these glands with the CO2 laser may promote enhanced reepithelialization in addition to the reestablishment of perspiration and wound healing.3 Considering inadequate wound repair represents a substantial disturbance to the patient and health care system, this finding offers promise as a potential means to decrease morbidity in patients with dermal scarring from burns and traumatic injuries. We have since evaluated and treated 3 patients who demonstrated sweat regeneration following treatment with the fractional CO2 laser (Table).

A 42-year-old man was our first patient to demonstrate functional scar improvement following bone marrow transplant for acute lymphoblastic leukemia complicated by chronic sclerodermoid graft-versus-host disease and subsequent extensive scarring on the chest and arms. Approximately 2 weeks after the first treatment with the fractional CO2 laser, the patient began to notice the presence of sweat beads in the treated areas. In addition to the reestablishment of perspiration, the patient had perceived increased mobility with improved pliability and “softness” (as described by family members) in treated areas likely related to scar remodeling.
A 36-year-old wounded army veteran presented with burns to the face, arms, and chest affecting 49% of the body surface area. After only 1 treatment, the patient reported that he could subjectively tolerate 10°F more ambient temperature and work all day outside in south Texas when heat intolerance previously would allow him to work only 2 to 3 hours. Additionally, he noted increased mobility and chest wall expansion, which in combination contributed to overall increased exercise tolerance and enhanced quality of life.
A 35-year-old US Marine and firefighter with burns primarily on the chest and arms involving 35% body surface area experienced increased exercise tolerance and sweat regeneration, particularly on the chest after a single treatment with the fractional CO2 laser but continued to experience improvement after a total of 3 treatments. Additionally, the cosmetic improvement was so substantial that the physician (C.M.H) had to review older photographs to verify the location of the scars.
We have now treated 3 patients with various mechanisms of injury and extensive scarring who noticed improved heat tolerance from sweat regeneration following fractional CO2 laser therapy. At this point, we only have anecdotal evidence of subjective functional improvement, and further research is warranted to elucidate the exact mechanism of action to support our findings.
- Neiner J, Whittemore D, Hivnor C. Buried alive: functional eccrine coils buried under scar tissue? J Am Acad Dermatol. 2011;65:661-663.
- Waibel J, Beer K, Narurkar V, et al. Preliminary observations on fractional ablative resurfacing devices: clinical impressions. J Drugs Dermatol. 2009;8:481-485.
- Rittié L, Sachs D, Orringer J, et al. Eccrine sweat glands are major contributors to reepithelialization of human wounds. Am J Pathol. 2013;1:163-171.
- Neiner J, Whittemore D, Hivnor C. Buried alive: functional eccrine coils buried under scar tissue? J Am Acad Dermatol. 2011;65:661-663.
- Waibel J, Beer K, Narurkar V, et al. Preliminary observations on fractional ablative resurfacing devices: clinical impressions. J Drugs Dermatol. 2009;8:481-485.
- Rittié L, Sachs D, Orringer J, et al. Eccrine sweat glands are major contributors to reepithelialization of human wounds. Am J Pathol. 2013;1:163-171.
Practice Points
- Treatment of dermal scarring with fractional CO2 laser may contribute to eccrine sweat gland regeneration during the remodeling process in addition to increased skin pliability.
- Sweat regeneration has been demonstrated following treatment with fractional CO2 laser in patients with extensive scarring; this case shows sweat regeneration secondary to burns and chronic sclerodermoid graft-versus-host disease.
Management of Trauma and Burn Scars: The Dermatologist’s Role in Expanding Patient Access to Care
Hypertrophic scarring secondary to trauma, burns, and surgical interventions is a major source of morbidity worldwide and often is mechanically, aesthetically, and symptomatically debilitating. Modern advances in acute trauma care protocols have resulted in survival rates greater than 90% in both civilian and military populations.1,2 Patients with wounds that have historically proven fatal are now surviving and are confronted with the long-term sequelae of their injuries. With more than 52,000 service members injured in military engagements from 2001 to 2015 and 8.5 million civilians presenting annually with injury patterns at risk for hypertrophic scarring, it is paramount that we ensure access to safe and effective long-term scar care.2,3
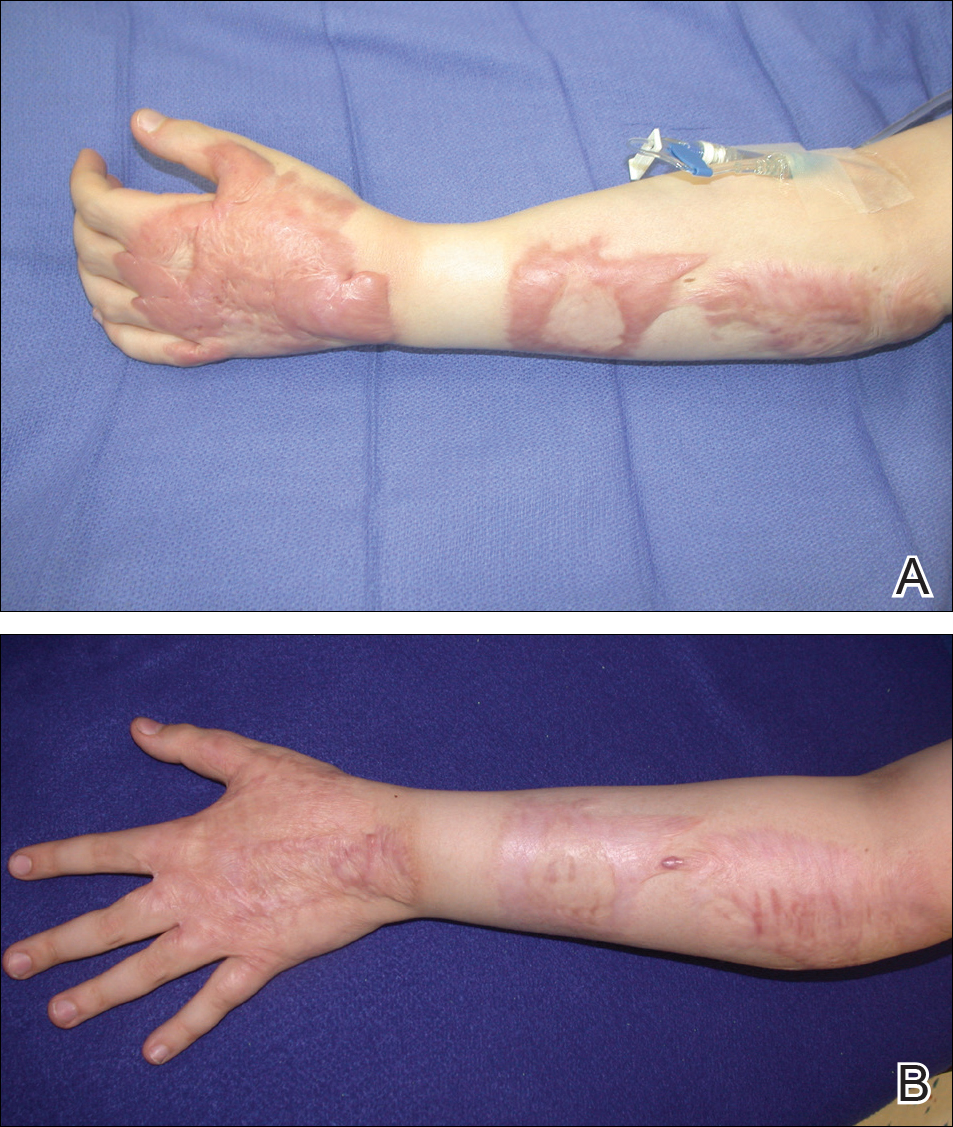
At its simplest level, hypertrophic scarring is believed to result from a disequilibrium between collagen production and degradation. This failure to properly transition through the stages of wound healing results in bothersome symptoms, a disfigured appearance, and mechanical dysfunction of the skin (Figure, A). Decreased elasticity and extensibility, increased dermal thickness, and scar contractures impair patient range of motion and functional mobility. Those affected commonly experience varying degrees of pruritus and dysesthesia along the scar. Combined with aesthetic variations in pigmentation, erythema, texture, and thickness, hypertrophic scarring often leads to long-term psychosocial impairment and decreased health-related quality of life.4
Treatment Approach
Treatment of hypertrophic scars requires a multimodal approach due to the spectrum of associated concerns and the natural recalcitrance of the scar to therapy. Protocols should be tailored to the individual but generally begin with tissue-conserving surgical interventions followed by selective photothermolysis of the scar vasculature. Subsequently, deep and superficial ablative fractional laser (AFL) treatment and local pharmacotherapy also are employed. Treatment can be accomplished in the outpatient setting under local anesthesia in a serial fashion. In the authors’ experience, these therapies behave in a synergistic fashion, achieving outcomes that far exceed the sum of their parts, often obviating the need for scar excision in the majority of cases (Figure, B).
Tissue-Conserving Surgical Intervention
Z-plasty is an indispensable surgical tool due to its ability to lengthen scars and reduce wound tension. Treatment is easily customizable to the patient and can be performed using the individual or multiple Z-plasty techniques. Undermining and step-off correction while suturing allow the physician to lower raised scars, elevate depressed scars, and obscure scar presence by minimizing the straight lines that draw the eye to the scar. Z-plasties rely on the creation and transposition of 2 triangular flaps and permit a 75% increase in length along the desired tension vector. As such, Z-plasties decrease wound tension and facilitate scar maturation.
Selective Photothermolysis of the Vasculature
Although there are several devices available to treat vascular and immature hypertrophic scars, the majority of studies have been conducted with the 595-nm pulsed dye laser. By preferentially heating oxyhemoglobin within the dermal microvasculature, the pulsed dye laser irreparably injures the vascular endothelium. The subsequent tissue hypoxia and collagen fiber heating results in collagen fiber realignment, normalization of collagen subtypes, and neocollagenesis.5 Pulsed dye laser therapy most effectively reduces erythema and pruritus; however, improvements in scar volume, pliability, and elasticity also have been reported.5 When targeting the fine vasculature of the scar, thermal confinement is critical to prevent injury to the surrounding dermis. As such, pulse widths of 0.45 to 1.5 milliseconds are routinely utilized with a fluence just sufficient to elicit transient purpura lasting 3 to 5 seconds. Employing a spot size of 7 to 10 mm, typical fluences range from 4.5 to 6.5 J/cm2. Engagement of the dynamic cooling device reduces the risk for complications, allowing the patient to proceed to the next step in their therapy regimen: the AFL.
Ablative Fractional Laser
The AFL creates a pixilated pattern of injury throughout the epidermis and dermis of the treatment area. Ablative fractional laser platforms include the 10,600-nm CO2 and 2940-nm erbium-doped YAG lasers, both targeting intracellular water. The AFL vaporizes columns of tissue, leaving minute vertical channels with narrow rims of protein coagulation referred to as microscopic treatment zones (MTZs).6 Scar collagen analysis after AFL treatment has shown a profile resembling unaffected skin.7 Consistently, patients report improvements in stiffness, range of motion, pain, pruritus, pigmentation, and erythema.Physician observers also have reported similar improvements in these end points.8,9 Recently, interim data from a prospective controlled trial were presented showing objective improvements in dermal thickness, elasticity, and extensibility after 3 treatments with the CO2 AFL.6 The UltraPulse CO2 laser (Lumenis) is the most well-studied and widely available AFL for scar therapy and as such we will outline common treatment parameters with this device. Of note, treatment end points may be generalized to any AFL.
The DeepFX UltraPulse configuration is utilized to achieve deep AFL therapy and has a fixed pulse width of 0.8 milliseconds, slightly less than the thermal relaxation time of the skin. The diameter of the MTZs is 120 µm, and MTZ density for scar treatment ranges from 1% to 10% with a goal depth of at least 80% of scar thickness. Maximal penetration of the AFL is 4 mm, which is directly proportional to fluence. The goal of deep AFL is the removal of scar tissue to facilitate remodeling and neocollagenesis. Superficial fractional ablation can then be achieved utilizing the ActiveFX UltraPulse configuration generating a 1.3-mm MTZ spot size. We commonly use a treatment level of 3 (82% density). Typical treatment energy ranges from 80 to 125 mJ, which correlates with depths of approximately 50 to 115 µm. With both configurations, the size and shape of the treatment area can be customized to the scar. In addition, frequency may be adjusted to control the speed of treatment while balancing the risk of bulk heating. The goal of superficial AFL is to minimize scar surface irregularities and ensure blending of deep AFL treatment. Once AFL treatment is complete, local pharmacotherapy can then be employed.
Pharmacotherapy
Intralesional corticosteroids have long represented the standard of care for hypertrophic scars, with concentrations between 2.5 and 40 mg/mL that are titrated to scar thickness and location to avoid unwanted atrophy. Visual blanching of the scar represents the clinical end point for treatment. Corticosteroids act by inhibiting fibroblast proliferation and enhancing collagen degradation.10 5-Fluorouracil (5-FU) also is used in scar management. In addition to inhibiting fibroblast proliferation and inducing fibroblast apoptosis, 5-FU inhibits myofibroblast proliferation, which is helpful in the prevention and treatment of scar contracture.11 As monotherapy, weekly injections with 1 to 3 mL of 50 mg/mL 5-FU has been safe and effective. Combination intralesional corticosteroid and 5-FU therapy has been reported and is associated with improved scar regression, reduced reoccurrence, and fewer side effects.11 In our experience, a 1:1 suspension is effective with appropriate titration of the corticosteroid component. Although less well defined, topical application of pharmacotherapy and massage to the newly created MTZs appears beneficial and offers another option for delivery of corticosteroids and 5-FU, in addition to a number of promising medications such as bimatoprost, poly-L-lactic acid, timolol, and rapamycin.12
Conclusion
Advances in laser surgery and our understanding of wound healing have created a paradigm shift in the treatment approach to trauma and burn scars. In lieu of extensive scar excisions, the summarized multimodal regimen emphasizing tissue conservation and autologous remodeling is gaining favor in the military, academic medical centers, and scar centers of excellence, but patients are finding local access to care difficult. Dermatologists are uniquely positioned to cost-effectively deliver this care in the outpatient setting utilizing devices and techniques they already possess. With the end goal of optimization of functional, symptomatic, and aesthetic state of the patient, it is critical that dermatologists seize this opportunity to truly make a difference for the military and civilian patients that need it most.
- American Burn Association, National Burn Repository. 2015 National burn repository report of data from 2005-2014. http://www.ameriburn.org/2015NBRAnnualReport.pdf. Accessed May 10, 2017.
- Centers for Disease Control and Prevention. 2013 National hospital ambulatory medical care survey emergency department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2013_ed_web_tables.pdf. Accessed May 10, 2017.
- Fischer H. A guide to U.S. Military casualty statistics: Operation Freedom’s Sentinel, Operation Inherent Resolve, Operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom. Congressional Research Service website. https://fas.org/sgp/crs/natsec/RS22452.pdf. Published August 7, 2015. Accessed May 10, 2017.
- Van Loey NE, Van Son MJ. Psychopathology and psychological problems in patients with burn scars: epidemiology and management. Am J Clin Dermatol. 2003;4:245-272.
- Vrijman C, van Drooge AM, Limpens J, et al. Laser and intense pulsed light therapy for the treatment of hypertrophic scars: a systematic review. Br J Dermatol. 2011;165:934-942.
- Miletta N, Lee K, Siwy K, et al. Objective improvement in burn scars after treatment with fractionated CO2 laser. Paper presented at: American Society for Laser Medicine and Surgery 36th Annual Conference; April 1-3, 2016; Boston, MA.
- Ozog DM, Liu A, Chaffins ML, et al. Evaluation of clinical results, histological architecture, and collagen expression following treatment of mature burn scars with a fraction carbon dioxide laser. JAMA Dermatol. 2013;149:50-57.
- Levi B, Ibrahim A, Mathews K, et al. The use of CO2 fractional photothermolysis for the treatment of burn scars. J Burn Care Res. 2016;37:106-114.
- van Drooge AM, Vrijman C, van der Veen W, et al. A randomized controlled pilot study on ablative fractional CO2 laser for consecutive patients presenting with various scar types. Dermatol Surg. 2015;41:371-377.
- Wang XQ, Lui YK, Wang ZY, et al. Antimitotic drug injections and radiotherapy: a review of the effectiveness of treatment for hypertrophic scars and keloids. Int J Low Extrem Wounds. 2008;7:151-159.
- Gupta S, Kalra A. Efficacy and safety of intralesional 5-fluorouracil in the treatment of keloids. Dermatology. 2002;204:130-132.
- Haedersdal M, Erlendsson AM, Paasch U, et al. Translational medicine in the field of AFL (AFXL)-assisted drug delivery: a critical review from basics to current clinical status. J Am Acad Dermatol. 2016;74:981-1004.
Hypertrophic scarring secondary to trauma, burns, and surgical interventions is a major source of morbidity worldwide and often is mechanically, aesthetically, and symptomatically debilitating. Modern advances in acute trauma care protocols have resulted in survival rates greater than 90% in both civilian and military populations.1,2 Patients with wounds that have historically proven fatal are now surviving and are confronted with the long-term sequelae of their injuries. With more than 52,000 service members injured in military engagements from 2001 to 2015 and 8.5 million civilians presenting annually with injury patterns at risk for hypertrophic scarring, it is paramount that we ensure access to safe and effective long-term scar care.2,3
At its simplest level, hypertrophic scarring is believed to result from a disequilibrium between collagen production and degradation. This failure to properly transition through the stages of wound healing results in bothersome symptoms, a disfigured appearance, and mechanical dysfunction of the skin (Figure, A). Decreased elasticity and extensibility, increased dermal thickness, and scar contractures impair patient range of motion and functional mobility. Those affected commonly experience varying degrees of pruritus and dysesthesia along the scar. Combined with aesthetic variations in pigmentation, erythema, texture, and thickness, hypertrophic scarring often leads to long-term psychosocial impairment and decreased health-related quality of life.4

Treatment Approach
Treatment of hypertrophic scars requires a multimodal approach due to the spectrum of associated concerns and the natural recalcitrance of the scar to therapy. Protocols should be tailored to the individual but generally begin with tissue-conserving surgical interventions followed by selective photothermolysis of the scar vasculature. Subsequently, deep and superficial ablative fractional laser (AFL) treatment and local pharmacotherapy also are employed. Treatment can be accomplished in the outpatient setting under local anesthesia in a serial fashion. In the authors’ experience, these therapies behave in a synergistic fashion, achieving outcomes that far exceed the sum of their parts, often obviating the need for scar excision in the majority of cases (Figure, B).
Tissue-Conserving Surgical Intervention
Z-plasty is an indispensable surgical tool due to its ability to lengthen scars and reduce wound tension. Treatment is easily customizable to the patient and can be performed using the individual or multiple Z-plasty techniques. Undermining and step-off correction while suturing allow the physician to lower raised scars, elevate depressed scars, and obscure scar presence by minimizing the straight lines that draw the eye to the scar. Z-plasties rely on the creation and transposition of 2 triangular flaps and permit a 75% increase in length along the desired tension vector. As such, Z-plasties decrease wound tension and facilitate scar maturation.
Selective Photothermolysis of the Vasculature
Although there are several devices available to treat vascular and immature hypertrophic scars, the majority of studies have been conducted with the 595-nm pulsed dye laser. By preferentially heating oxyhemoglobin within the dermal microvasculature, the pulsed dye laser irreparably injures the vascular endothelium. The subsequent tissue hypoxia and collagen fiber heating results in collagen fiber realignment, normalization of collagen subtypes, and neocollagenesis.5 Pulsed dye laser therapy most effectively reduces erythema and pruritus; however, improvements in scar volume, pliability, and elasticity also have been reported.5 When targeting the fine vasculature of the scar, thermal confinement is critical to prevent injury to the surrounding dermis. As such, pulse widths of 0.45 to 1.5 milliseconds are routinely utilized with a fluence just sufficient to elicit transient purpura lasting 3 to 5 seconds. Employing a spot size of 7 to 10 mm, typical fluences range from 4.5 to 6.5 J/cm2. Engagement of the dynamic cooling device reduces the risk for complications, allowing the patient to proceed to the next step in their therapy regimen: the AFL.
Ablative Fractional Laser
The AFL creates a pixilated pattern of injury throughout the epidermis and dermis of the treatment area. Ablative fractional laser platforms include the 10,600-nm CO2 and 2940-nm erbium-doped YAG lasers, both targeting intracellular water. The AFL vaporizes columns of tissue, leaving minute vertical channels with narrow rims of protein coagulation referred to as microscopic treatment zones (MTZs).6 Scar collagen analysis after AFL treatment has shown a profile resembling unaffected skin.7 Consistently, patients report improvements in stiffness, range of motion, pain, pruritus, pigmentation, and erythema.Physician observers also have reported similar improvements in these end points.8,9 Recently, interim data from a prospective controlled trial were presented showing objective improvements in dermal thickness, elasticity, and extensibility after 3 treatments with the CO2 AFL.6 The UltraPulse CO2 laser (Lumenis) is the most well-studied and widely available AFL for scar therapy and as such we will outline common treatment parameters with this device. Of note, treatment end points may be generalized to any AFL.
The DeepFX UltraPulse configuration is utilized to achieve deep AFL therapy and has a fixed pulse width of 0.8 milliseconds, slightly less than the thermal relaxation time of the skin. The diameter of the MTZs is 120 µm, and MTZ density for scar treatment ranges from 1% to 10% with a goal depth of at least 80% of scar thickness. Maximal penetration of the AFL is 4 mm, which is directly proportional to fluence. The goal of deep AFL is the removal of scar tissue to facilitate remodeling and neocollagenesis. Superficial fractional ablation can then be achieved utilizing the ActiveFX UltraPulse configuration generating a 1.3-mm MTZ spot size. We commonly use a treatment level of 3 (82% density). Typical treatment energy ranges from 80 to 125 mJ, which correlates with depths of approximately 50 to 115 µm. With both configurations, the size and shape of the treatment area can be customized to the scar. In addition, frequency may be adjusted to control the speed of treatment while balancing the risk of bulk heating. The goal of superficial AFL is to minimize scar surface irregularities and ensure blending of deep AFL treatment. Once AFL treatment is complete, local pharmacotherapy can then be employed.
Pharmacotherapy
Intralesional corticosteroids have long represented the standard of care for hypertrophic scars, with concentrations between 2.5 and 40 mg/mL that are titrated to scar thickness and location to avoid unwanted atrophy. Visual blanching of the scar represents the clinical end point for treatment. Corticosteroids act by inhibiting fibroblast proliferation and enhancing collagen degradation.10 5-Fluorouracil (5-FU) also is used in scar management. In addition to inhibiting fibroblast proliferation and inducing fibroblast apoptosis, 5-FU inhibits myofibroblast proliferation, which is helpful in the prevention and treatment of scar contracture.11 As monotherapy, weekly injections with 1 to 3 mL of 50 mg/mL 5-FU has been safe and effective. Combination intralesional corticosteroid and 5-FU therapy has been reported and is associated with improved scar regression, reduced reoccurrence, and fewer side effects.11 In our experience, a 1:1 suspension is effective with appropriate titration of the corticosteroid component. Although less well defined, topical application of pharmacotherapy and massage to the newly created MTZs appears beneficial and offers another option for delivery of corticosteroids and 5-FU, in addition to a number of promising medications such as bimatoprost, poly-L-lactic acid, timolol, and rapamycin.12
Conclusion
Advances in laser surgery and our understanding of wound healing have created a paradigm shift in the treatment approach to trauma and burn scars. In lieu of extensive scar excisions, the summarized multimodal regimen emphasizing tissue conservation and autologous remodeling is gaining favor in the military, academic medical centers, and scar centers of excellence, but patients are finding local access to care difficult. Dermatologists are uniquely positioned to cost-effectively deliver this care in the outpatient setting utilizing devices and techniques they already possess. With the end goal of optimization of functional, symptomatic, and aesthetic state of the patient, it is critical that dermatologists seize this opportunity to truly make a difference for the military and civilian patients that need it most.
Hypertrophic scarring secondary to trauma, burns, and surgical interventions is a major source of morbidity worldwide and often is mechanically, aesthetically, and symptomatically debilitating. Modern advances in acute trauma care protocols have resulted in survival rates greater than 90% in both civilian and military populations.1,2 Patients with wounds that have historically proven fatal are now surviving and are confronted with the long-term sequelae of their injuries. With more than 52,000 service members injured in military engagements from 2001 to 2015 and 8.5 million civilians presenting annually with injury patterns at risk for hypertrophic scarring, it is paramount that we ensure access to safe and effective long-term scar care.2,3
At its simplest level, hypertrophic scarring is believed to result from a disequilibrium between collagen production and degradation. This failure to properly transition through the stages of wound healing results in bothersome symptoms, a disfigured appearance, and mechanical dysfunction of the skin (Figure, A). Decreased elasticity and extensibility, increased dermal thickness, and scar contractures impair patient range of motion and functional mobility. Those affected commonly experience varying degrees of pruritus and dysesthesia along the scar. Combined with aesthetic variations in pigmentation, erythema, texture, and thickness, hypertrophic scarring often leads to long-term psychosocial impairment and decreased health-related quality of life.4

Treatment Approach
Treatment of hypertrophic scars requires a multimodal approach due to the spectrum of associated concerns and the natural recalcitrance of the scar to therapy. Protocols should be tailored to the individual but generally begin with tissue-conserving surgical interventions followed by selective photothermolysis of the scar vasculature. Subsequently, deep and superficial ablative fractional laser (AFL) treatment and local pharmacotherapy also are employed. Treatment can be accomplished in the outpatient setting under local anesthesia in a serial fashion. In the authors’ experience, these therapies behave in a synergistic fashion, achieving outcomes that far exceed the sum of their parts, often obviating the need for scar excision in the majority of cases (Figure, B).
Tissue-Conserving Surgical Intervention
Z-plasty is an indispensable surgical tool due to its ability to lengthen scars and reduce wound tension. Treatment is easily customizable to the patient and can be performed using the individual or multiple Z-plasty techniques. Undermining and step-off correction while suturing allow the physician to lower raised scars, elevate depressed scars, and obscure scar presence by minimizing the straight lines that draw the eye to the scar. Z-plasties rely on the creation and transposition of 2 triangular flaps and permit a 75% increase in length along the desired tension vector. As such, Z-plasties decrease wound tension and facilitate scar maturation.
Selective Photothermolysis of the Vasculature
Although there are several devices available to treat vascular and immature hypertrophic scars, the majority of studies have been conducted with the 595-nm pulsed dye laser. By preferentially heating oxyhemoglobin within the dermal microvasculature, the pulsed dye laser irreparably injures the vascular endothelium. The subsequent tissue hypoxia and collagen fiber heating results in collagen fiber realignment, normalization of collagen subtypes, and neocollagenesis.5 Pulsed dye laser therapy most effectively reduces erythema and pruritus; however, improvements in scar volume, pliability, and elasticity also have been reported.5 When targeting the fine vasculature of the scar, thermal confinement is critical to prevent injury to the surrounding dermis. As such, pulse widths of 0.45 to 1.5 milliseconds are routinely utilized with a fluence just sufficient to elicit transient purpura lasting 3 to 5 seconds. Employing a spot size of 7 to 10 mm, typical fluences range from 4.5 to 6.5 J/cm2. Engagement of the dynamic cooling device reduces the risk for complications, allowing the patient to proceed to the next step in their therapy regimen: the AFL.
Ablative Fractional Laser
The AFL creates a pixilated pattern of injury throughout the epidermis and dermis of the treatment area. Ablative fractional laser platforms include the 10,600-nm CO2 and 2940-nm erbium-doped YAG lasers, both targeting intracellular water. The AFL vaporizes columns of tissue, leaving minute vertical channels with narrow rims of protein coagulation referred to as microscopic treatment zones (MTZs).6 Scar collagen analysis after AFL treatment has shown a profile resembling unaffected skin.7 Consistently, patients report improvements in stiffness, range of motion, pain, pruritus, pigmentation, and erythema.Physician observers also have reported similar improvements in these end points.8,9 Recently, interim data from a prospective controlled trial were presented showing objective improvements in dermal thickness, elasticity, and extensibility after 3 treatments with the CO2 AFL.6 The UltraPulse CO2 laser (Lumenis) is the most well-studied and widely available AFL for scar therapy and as such we will outline common treatment parameters with this device. Of note, treatment end points may be generalized to any AFL.
The DeepFX UltraPulse configuration is utilized to achieve deep AFL therapy and has a fixed pulse width of 0.8 milliseconds, slightly less than the thermal relaxation time of the skin. The diameter of the MTZs is 120 µm, and MTZ density for scar treatment ranges from 1% to 10% with a goal depth of at least 80% of scar thickness. Maximal penetration of the AFL is 4 mm, which is directly proportional to fluence. The goal of deep AFL is the removal of scar tissue to facilitate remodeling and neocollagenesis. Superficial fractional ablation can then be achieved utilizing the ActiveFX UltraPulse configuration generating a 1.3-mm MTZ spot size. We commonly use a treatment level of 3 (82% density). Typical treatment energy ranges from 80 to 125 mJ, which correlates with depths of approximately 50 to 115 µm. With both configurations, the size and shape of the treatment area can be customized to the scar. In addition, frequency may be adjusted to control the speed of treatment while balancing the risk of bulk heating. The goal of superficial AFL is to minimize scar surface irregularities and ensure blending of deep AFL treatment. Once AFL treatment is complete, local pharmacotherapy can then be employed.
Pharmacotherapy
Intralesional corticosteroids have long represented the standard of care for hypertrophic scars, with concentrations between 2.5 and 40 mg/mL that are titrated to scar thickness and location to avoid unwanted atrophy. Visual blanching of the scar represents the clinical end point for treatment. Corticosteroids act by inhibiting fibroblast proliferation and enhancing collagen degradation.10 5-Fluorouracil (5-FU) also is used in scar management. In addition to inhibiting fibroblast proliferation and inducing fibroblast apoptosis, 5-FU inhibits myofibroblast proliferation, which is helpful in the prevention and treatment of scar contracture.11 As monotherapy, weekly injections with 1 to 3 mL of 50 mg/mL 5-FU has been safe and effective. Combination intralesional corticosteroid and 5-FU therapy has been reported and is associated with improved scar regression, reduced reoccurrence, and fewer side effects.11 In our experience, a 1:1 suspension is effective with appropriate titration of the corticosteroid component. Although less well defined, topical application of pharmacotherapy and massage to the newly created MTZs appears beneficial and offers another option for delivery of corticosteroids and 5-FU, in addition to a number of promising medications such as bimatoprost, poly-L-lactic acid, timolol, and rapamycin.12
Conclusion
Advances in laser surgery and our understanding of wound healing have created a paradigm shift in the treatment approach to trauma and burn scars. In lieu of extensive scar excisions, the summarized multimodal regimen emphasizing tissue conservation and autologous remodeling is gaining favor in the military, academic medical centers, and scar centers of excellence, but patients are finding local access to care difficult. Dermatologists are uniquely positioned to cost-effectively deliver this care in the outpatient setting utilizing devices and techniques they already possess. With the end goal of optimization of functional, symptomatic, and aesthetic state of the patient, it is critical that dermatologists seize this opportunity to truly make a difference for the military and civilian patients that need it most.
- American Burn Association, National Burn Repository. 2015 National burn repository report of data from 2005-2014. http://www.ameriburn.org/2015NBRAnnualReport.pdf. Accessed May 10, 2017.
- Centers for Disease Control and Prevention. 2013 National hospital ambulatory medical care survey emergency department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2013_ed_web_tables.pdf. Accessed May 10, 2017.
- Fischer H. A guide to U.S. Military casualty statistics: Operation Freedom’s Sentinel, Operation Inherent Resolve, Operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom. Congressional Research Service website. https://fas.org/sgp/crs/natsec/RS22452.pdf. Published August 7, 2015. Accessed May 10, 2017.
- Van Loey NE, Van Son MJ. Psychopathology and psychological problems in patients with burn scars: epidemiology and management. Am J Clin Dermatol. 2003;4:245-272.
- Vrijman C, van Drooge AM, Limpens J, et al. Laser and intense pulsed light therapy for the treatment of hypertrophic scars: a systematic review. Br J Dermatol. 2011;165:934-942.
- Miletta N, Lee K, Siwy K, et al. Objective improvement in burn scars after treatment with fractionated CO2 laser. Paper presented at: American Society for Laser Medicine and Surgery 36th Annual Conference; April 1-3, 2016; Boston, MA.
- Ozog DM, Liu A, Chaffins ML, et al. Evaluation of clinical results, histological architecture, and collagen expression following treatment of mature burn scars with a fraction carbon dioxide laser. JAMA Dermatol. 2013;149:50-57.
- Levi B, Ibrahim A, Mathews K, et al. The use of CO2 fractional photothermolysis for the treatment of burn scars. J Burn Care Res. 2016;37:106-114.
- van Drooge AM, Vrijman C, van der Veen W, et al. A randomized controlled pilot study on ablative fractional CO2 laser for consecutive patients presenting with various scar types. Dermatol Surg. 2015;41:371-377.
- Wang XQ, Lui YK, Wang ZY, et al. Antimitotic drug injections and radiotherapy: a review of the effectiveness of treatment for hypertrophic scars and keloids. Int J Low Extrem Wounds. 2008;7:151-159.
- Gupta S, Kalra A. Efficacy and safety of intralesional 5-fluorouracil in the treatment of keloids. Dermatology. 2002;204:130-132.
- Haedersdal M, Erlendsson AM, Paasch U, et al. Translational medicine in the field of AFL (AFXL)-assisted drug delivery: a critical review from basics to current clinical status. J Am Acad Dermatol. 2016;74:981-1004.
- American Burn Association, National Burn Repository. 2015 National burn repository report of data from 2005-2014. http://www.ameriburn.org/2015NBRAnnualReport.pdf. Accessed May 10, 2017.
- Centers for Disease Control and Prevention. 2013 National hospital ambulatory medical care survey emergency department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2013_ed_web_tables.pdf. Accessed May 10, 2017.
- Fischer H. A guide to U.S. Military casualty statistics: Operation Freedom’s Sentinel, Operation Inherent Resolve, Operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom. Congressional Research Service website. https://fas.org/sgp/crs/natsec/RS22452.pdf. Published August 7, 2015. Accessed May 10, 2017.
- Van Loey NE, Van Son MJ. Psychopathology and psychological problems in patients with burn scars: epidemiology and management. Am J Clin Dermatol. 2003;4:245-272.
- Vrijman C, van Drooge AM, Limpens J, et al. Laser and intense pulsed light therapy for the treatment of hypertrophic scars: a systematic review. Br J Dermatol. 2011;165:934-942.
- Miletta N, Lee K, Siwy K, et al. Objective improvement in burn scars after treatment with fractionated CO2 laser. Paper presented at: American Society for Laser Medicine and Surgery 36th Annual Conference; April 1-3, 2016; Boston, MA.
- Ozog DM, Liu A, Chaffins ML, et al. Evaluation of clinical results, histological architecture, and collagen expression following treatment of mature burn scars with a fraction carbon dioxide laser. JAMA Dermatol. 2013;149:50-57.
- Levi B, Ibrahim A, Mathews K, et al. The use of CO2 fractional photothermolysis for the treatment of burn scars. J Burn Care Res. 2016;37:106-114.
- van Drooge AM, Vrijman C, van der Veen W, et al. A randomized controlled pilot study on ablative fractional CO2 laser for consecutive patients presenting with various scar types. Dermatol Surg. 2015;41:371-377.
- Wang XQ, Lui YK, Wang ZY, et al. Antimitotic drug injections and radiotherapy: a review of the effectiveness of treatment for hypertrophic scars and keloids. Int J Low Extrem Wounds. 2008;7:151-159.
- Gupta S, Kalra A. Efficacy and safety of intralesional 5-fluorouracil in the treatment of keloids. Dermatology. 2002;204:130-132.
- Haedersdal M, Erlendsson AM, Paasch U, et al. Translational medicine in the field of AFL (AFXL)-assisted drug delivery: a critical review from basics to current clinical status. J Am Acad Dermatol. 2016;74:981-1004.
Practice Points
- Burn and trauma scarring represents a major source of morbidity in both the civilian and military populations worldwide and often is mechanically, aesthetically, and symptomatically debilitating.
- Advances in laser surgery and our understanding of wound healing have resulted in a scar therapy paradigm shift from large scar excisions and repair to a multimodal, tissue-conserving approach that relies on remodeling of the existing tissue.
- Dermatologists are uniquely positioned to increase patient access to cost-effective, outpatient-based burn and trauma scar care utilizing devices and techniques that they currently possess.