User login
Reversible Cutaneous Side Effects of Vismodegib Treatment
To the Editor:
Vismodegib, a first-in-class inhibitor of the hedgehog signaling pathway, is useful in the treatment of advanced basal cell carcinomas (BCCs).1 Common side effects of vismodegib include alopecia (58%), muscle spasms (71%), and dysgeusia (71%).2 Some of these side effects have been hypothesized to be mechanism related.3,4 Keratoacanthomas have been reported to occur after vismodegib treatment of BCC.5 We report 3 cases illustrating reversible cutaneous side effects of vismodegib: alopecia, follicular dermatitis, and drug hypersensitivity reaction.
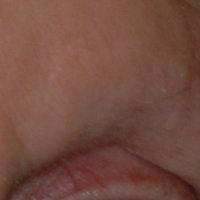
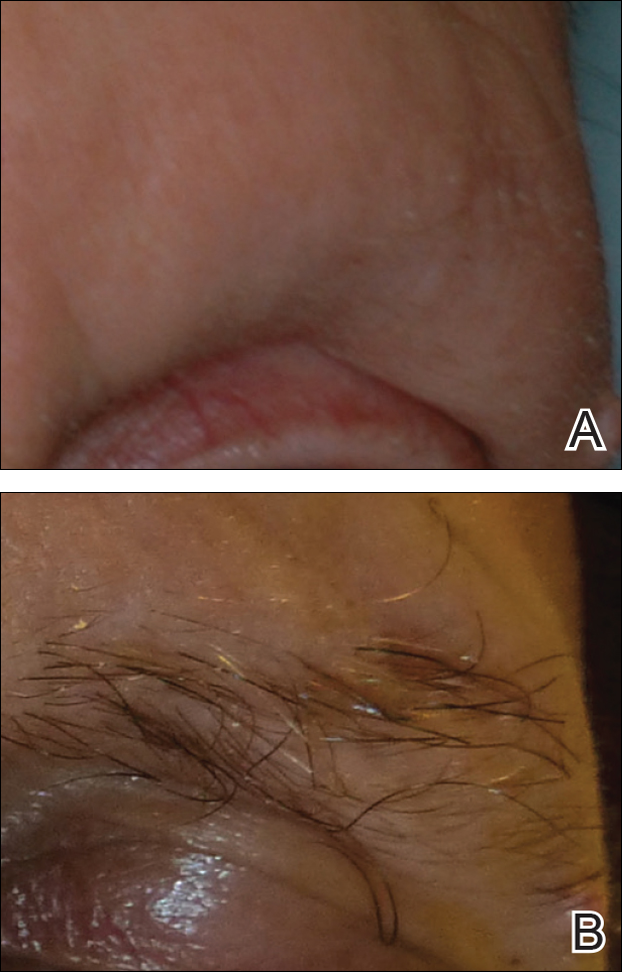
A 53-year-old man with a locally advanced BCC of the right medial canthus began experiencing progressive and diffuse hair loss on the beard area, parietal scalp, eyelashes, and eyebrows after 2 months of vismodegib treatment. At 12 months of treatment, he had complete loss of eyelashes and eyebrows (Figure, A). After vismodegib was discontinued due to disease progression, all of his hair began regrowing within several months, with complete hair regrowth observed at 20 months after the last dose (Figure, B).
A 55-year-old man with several locally advanced BCCs developed new-onset mildly pruritic, acneform lesions on the chest and back after 4 months of vismodegib treatment. Biopsy of the lesions showed a folliculocentric mixed dermal infiltrate. The patient did not have a history of follicular dermatitis. The dermatitis resolved several months after onset without treatment, despite continued vismodegib.
A 55-year-old man with locally advanced BCCs developed erythematous dermal plaques on the arms and chest after 2 months of vismodegib treatment. Lesions were asymptomatic. He was not using any other medications and did not have any contact allergen exposures. Punch biopsy showed superficial and deep perivascular dermatitis with occasional eosinophils, consistent with drug hypersensitivity. Although lesions spontaneously resolved without treatment after 1 month, he experienced a couple more bouts of these lesions over the next year. He continued vismodegib for 2 years without return of this eruption.
The average time frame for hair regrowth after vismodegib cessation has not been characterized and awaits future larger studies. The frequency of follicular dermatitis and drug eruption also has not been determined and may require careful observation by dermatologists in larger numbers of treated patients.
Because the hedgehog pathway is critical for normal hair follicle function, follicle-based toxicities of vismodegib including alopecia and folliculitis could be hypothesized to reflect effective blockade of the pathway.6 Currently, there are no data that these changes correlate with tumor response.
Although alopecia is a recognized side effect of vismodegib, regrowth has not been previously reported.1,2 Knowledge of the reversibility of alopecia as well as other toxicities has the potential to influence patient decision-making on drug initiation and adherence.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Chang AL, Solomon JA, Hainsworth JD, et al. Expanded access study of patients with advanced basal cell carcinoma treated with the Hedgehog pathway inhibitor, vismodegib. J Am Acad Dermatol. 2014;70:60-69.
- St-Jacques B, Dassule HR, Karavanova I, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058-1068.
- Hall JM, Bell ML, Finger TE. Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev Biol. 2003;255:263-277.
- Aasi S, Silkiss R, Tang JY, et al. New onset of keratoacanthomas after vismodegib treatment for locally advanced basal cell carcinomas: a report of 2 cases. JAMA Dermatol. 2013;149:242-243.
- Rittie L, Stoll SW, Kang S, et al. Hedgehog signaling maintains hair follicle stem cell phenotype in young and aged human skin. Aging Cell. 2009;8:738-751.
To the Editor:
Vismodegib, a first-in-class inhibitor of the hedgehog signaling pathway, is useful in the treatment of advanced basal cell carcinomas (BCCs).1 Common side effects of vismodegib include alopecia (58%), muscle spasms (71%), and dysgeusia (71%).2 Some of these side effects have been hypothesized to be mechanism related.3,4 Keratoacanthomas have been reported to occur after vismodegib treatment of BCC.5 We report 3 cases illustrating reversible cutaneous side effects of vismodegib: alopecia, follicular dermatitis, and drug hypersensitivity reaction.
A 53-year-old man with a locally advanced BCC of the right medial canthus began experiencing progressive and diffuse hair loss on the beard area, parietal scalp, eyelashes, and eyebrows after 2 months of vismodegib treatment. At 12 months of treatment, he had complete loss of eyelashes and eyebrows (Figure, A). After vismodegib was discontinued due to disease progression, all of his hair began regrowing within several months, with complete hair regrowth observed at 20 months after the last dose (Figure, B).

A 55-year-old man with several locally advanced BCCs developed new-onset mildly pruritic, acneform lesions on the chest and back after 4 months of vismodegib treatment. Biopsy of the lesions showed a folliculocentric mixed dermal infiltrate. The patient did not have a history of follicular dermatitis. The dermatitis resolved several months after onset without treatment, despite continued vismodegib.
A 55-year-old man with locally advanced BCCs developed erythematous dermal plaques on the arms and chest after 2 months of vismodegib treatment. Lesions were asymptomatic. He was not using any other medications and did not have any contact allergen exposures. Punch biopsy showed superficial and deep perivascular dermatitis with occasional eosinophils, consistent with drug hypersensitivity. Although lesions spontaneously resolved without treatment after 1 month, he experienced a couple more bouts of these lesions over the next year. He continued vismodegib for 2 years without return of this eruption.
The average time frame for hair regrowth after vismodegib cessation has not been characterized and awaits future larger studies. The frequency of follicular dermatitis and drug eruption also has not been determined and may require careful observation by dermatologists in larger numbers of treated patients.
Because the hedgehog pathway is critical for normal hair follicle function, follicle-based toxicities of vismodegib including alopecia and folliculitis could be hypothesized to reflect effective blockade of the pathway.6 Currently, there are no data that these changes correlate with tumor response.
Although alopecia is a recognized side effect of vismodegib, regrowth has not been previously reported.1,2 Knowledge of the reversibility of alopecia as well as other toxicities has the potential to influence patient decision-making on drug initiation and adherence.
To the Editor:
Vismodegib, a first-in-class inhibitor of the hedgehog signaling pathway, is useful in the treatment of advanced basal cell carcinomas (BCCs).1 Common side effects of vismodegib include alopecia (58%), muscle spasms (71%), and dysgeusia (71%).2 Some of these side effects have been hypothesized to be mechanism related.3,4 Keratoacanthomas have been reported to occur after vismodegib treatment of BCC.5 We report 3 cases illustrating reversible cutaneous side effects of vismodegib: alopecia, follicular dermatitis, and drug hypersensitivity reaction.
A 53-year-old man with a locally advanced BCC of the right medial canthus began experiencing progressive and diffuse hair loss on the beard area, parietal scalp, eyelashes, and eyebrows after 2 months of vismodegib treatment. At 12 months of treatment, he had complete loss of eyelashes and eyebrows (Figure, A). After vismodegib was discontinued due to disease progression, all of his hair began regrowing within several months, with complete hair regrowth observed at 20 months after the last dose (Figure, B).

A 55-year-old man with several locally advanced BCCs developed new-onset mildly pruritic, acneform lesions on the chest and back after 4 months of vismodegib treatment. Biopsy of the lesions showed a folliculocentric mixed dermal infiltrate. The patient did not have a history of follicular dermatitis. The dermatitis resolved several months after onset without treatment, despite continued vismodegib.
A 55-year-old man with locally advanced BCCs developed erythematous dermal plaques on the arms and chest after 2 months of vismodegib treatment. Lesions were asymptomatic. He was not using any other medications and did not have any contact allergen exposures. Punch biopsy showed superficial and deep perivascular dermatitis with occasional eosinophils, consistent with drug hypersensitivity. Although lesions spontaneously resolved without treatment after 1 month, he experienced a couple more bouts of these lesions over the next year. He continued vismodegib for 2 years without return of this eruption.
The average time frame for hair regrowth after vismodegib cessation has not been characterized and awaits future larger studies. The frequency of follicular dermatitis and drug eruption also has not been determined and may require careful observation by dermatologists in larger numbers of treated patients.
Because the hedgehog pathway is critical for normal hair follicle function, follicle-based toxicities of vismodegib including alopecia and folliculitis could be hypothesized to reflect effective blockade of the pathway.6 Currently, there are no data that these changes correlate with tumor response.
Although alopecia is a recognized side effect of vismodegib, regrowth has not been previously reported.1,2 Knowledge of the reversibility of alopecia as well as other toxicities has the potential to influence patient decision-making on drug initiation and adherence.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Chang AL, Solomon JA, Hainsworth JD, et al. Expanded access study of patients with advanced basal cell carcinoma treated with the Hedgehog pathway inhibitor, vismodegib. J Am Acad Dermatol. 2014;70:60-69.
- St-Jacques B, Dassule HR, Karavanova I, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058-1068.
- Hall JM, Bell ML, Finger TE. Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev Biol. 2003;255:263-277.
- Aasi S, Silkiss R, Tang JY, et al. New onset of keratoacanthomas after vismodegib treatment for locally advanced basal cell carcinomas: a report of 2 cases. JAMA Dermatol. 2013;149:242-243.
- Rittie L, Stoll SW, Kang S, et al. Hedgehog signaling maintains hair follicle stem cell phenotype in young and aged human skin. Aging Cell. 2009;8:738-751.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Chang AL, Solomon JA, Hainsworth JD, et al. Expanded access study of patients with advanced basal cell carcinoma treated with the Hedgehog pathway inhibitor, vismodegib. J Am Acad Dermatol. 2014;70:60-69.
- St-Jacques B, Dassule HR, Karavanova I, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058-1068.
- Hall JM, Bell ML, Finger TE. Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev Biol. 2003;255:263-277.
- Aasi S, Silkiss R, Tang JY, et al. New onset of keratoacanthomas after vismodegib treatment for locally advanced basal cell carcinomas: a report of 2 cases. JAMA Dermatol. 2013;149:242-243.
- Rittie L, Stoll SW, Kang S, et al. Hedgehog signaling maintains hair follicle stem cell phenotype in young and aged human skin. Aging Cell. 2009;8:738-751.
Practice Points
- Hair loss is a common late side effect of vismodegib usage and is reversible, but regrowth takes many months.
- Mild folliculitis that resolves spontaneously has been observed in patients using vismodegib.
- Dermal hypersensitivity has been observed in patients on vismodegib, though the exact frequency of this type of dermatitis is not known.