User login
Taking vaccines to the next level via mucosal immunity
Vaccines are marvelous, and there are many well documented success stories, including rotavirus (RV) vaccines, where a live vaccine is administered to the gastrointestinal mucosa via oral drops. Antigens presented at the mucosal/epithelial surface not only induce systemic serum IgG – as do injectable vaccines – but also induce secretory IgA (sIgA), which is most helpful in diseases that directly affect the mucosa.

Mucosal vs. systemic immunity
Antibody being present on mucosal surfaces (point of initial pathogen contact) has a chance to neutralize the pathogen before it gains a foothold. Pathogen-specific mucosal lymphoid elements (e.g. in Peyer’s patches in the gut) also appear critical for optimal protection.1 The presence of both mucosal immune elements means that infection is severely limited or at times entirely prevented. So virus entering the GI tract causes minimal to no gut lining injury. Hence, there is no or mostly reduced vomiting/diarrhea. A downside of mucosally-administered live vaccines is that preexisting antibody to the vaccine antigens can reduce or block vaccine virus replication in the vaccinee, blunting or preventing protection. Note: Preexisting antibody also affects injectable live vaccines, such as the measles vaccine, similarly.
Classic injectable live or nonlive vaccines provide their most potent protection via systemic cellular responses antibody and/or antibodies in serum and extracellular fluid (ECF) where IgG and IgM are in highest concentrations. So even successful injectable vaccines still allow mucosal infection to start but then intercept further spread and prevent most of the downstream damage (think pertussis) or neutralize an infection-generated toxin (pertussis or tetanus). It usually is only after infection-induced damage occurs that systemic IgG and IgM gain better access to respiratory epithelial surfaces, but still only at a fraction of circulating concentrations. Indeed, pertussis vaccine–induced systemic immunity allows the pathogen to attack and replicate in/on host surface cells, causing toxin release and variable amounts of local mucosal injury/inflammation before vaccine-induced systemic immunity gains adequate access to the pathogen and/or to its toxin which may enter systemic circulation.
Live attenuated influenza vaccine (LAIV) induces mucosal immunity
Another “standard” vaccine that induces mucosal immunity – LAIV – was developed to improve on protection afforded by injectable influenza vaccines (IIVs), but LAIV has had hiccups in the United States. One example is several years of negligible protection against H1N1 disease. As long as LAIV’s vaccine strain had reasonably matched the circulating strains, LAIV worked at least as well as injectable influenza vaccine, and even offered some cross-protection against mildly mismatched strains. But after a number of years of LAIV use, vaccine effectiveness in the United States vs. H1N1 strains appeared to fade due to previously undetected but significant changes in the circulating H1N1 strain. The lesson is that mucosal immunity’s advantages are lost if too much change occurs in the pathogen target for sIgA and mucosally-associated lymphoid tissue cells (MALT)).
Other vaccines likely need to induce mucosal immunity
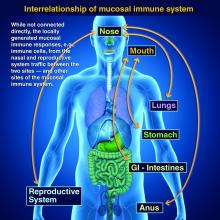
Protection at the mucosal level will likely be needed for success against norovirus, parainfluenza, respiratory syncytial virus (RSV), Neisseria gonorrhea, and chlamydia. Another helpful aspect of mucosal immunity is that immune cells and sIgA not only reside on the mucosa where the antigen was originally presented, but there is also a reasonable chance that these components will traffic to other mucosal surfaces.2
So intranasal vaccine could be expected to protect distant mucosal surfaces (urogenital, GI, and respiratory), leading to vaccine-induced systemic antibody plus mucosal immunity (sIGA and MALT responses) at each site.
Let’s look at a novel “two-site” chlamydia vaccine
Recently a phase 1 chlamydia vaccine that used a novel two-pronged administration site/schedule was successful at inducing both mucosal and systemic immunity in a proof-of-concept study – achieving the best of both worlds.3 This may be a template for vaccines in years to come. British investigators studied 50 healthy women aged 19-45 years in a double-blind, parallel, randomized, placebo-controlled trial that used a recombinant chlamydia protein subunit antigen (CTH522). The vaccine schedule involved three injectable priming doses followed soon thereafter by two intranasal boosting doses. There were three groups:
1. CTH522 adjuvanted with CAF01 liposomes (CTH522:CAF01).
2. CTH522 adjuvanted with aluminum hydroxide (CTH522:AH).
3. Placebo (saline).
The intramuscular (IM) priming schedule was 0, 1, and 4 months. The intranasal vaccine booster doses or placebo were given at 4.5 and 5 months. No related serious adverse reactions occurred. For injectable dosing, the most frequent adverse event was mild local injection-site reactions in all subjects in both vaccine groups vs. in 60% of placebo recipients (P = .053). The adjuvants were the likely cause for local reactions. Intranasal doses had local reactions in 47% of both vaccine groups and 60% of placebo recipients; P = 1.000).
Both vaccines produced systemic IgG seroconversion (including neutralizing antibody) plus small amounts of IgG in the nasal cavity and genital tract in all vaccine recipients; no placebo recipient seroconverted. Interestingly, liposomally-adjuvanted vaccine produced a more rapid systemic IgG response and higher serum titers than the alum-adjuvanted vaccine. Likewise, the IM liposomal vaccine also induced higher but still small mucosal IgG antibody responses (P = .0091). Intranasal IM-induced IgG titers were not boosted by later intranasal vaccine dosing.
Subjects getting liposomal vaccine (but not alum vaccine or placebo) boosters had detectable sIgA titers in both nasal and genital tract secretions. Liposomal vaccine recipients also had fivefold to sixfold higher median titers than alum vaccine recipients after the priming dose, and these higher titers persisted to the end of the study. All liposomal vaccine recipients developed antichlamydial cell-mediated responses vs. 57% alum-adjuvanted vaccine recipients. (P = .01). So both use of two-site dosing and the liposomal adjuvant appeared critical to better responses.
In summary
While this candidate vaccine has hurdles to overcome before coming into routine use, the proof-of-principle that a combination injectable-intranasal vaccine schedule can induce robust systemic and mucosal immunity when given with an appropriate adjuvant is very promising. Adding more vaccines to the schedule then becomes an issue, but that is one of those “good” problems we can deal with later.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines, receives funding from GlaxoSmithKline for studies on pneumococcal and rotavirus vaccines, and from Pfizer for a study on pneumococcal vaccine on which Dr. Harrison is a sub-investigator. The hospital also receives Centers for Disease Control and Prevention funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus, and also for rotavirus. Email Dr. Harrison at [email protected].
References
1. PLOS Biology. 2012 Sep 1. doi: 10.1371/journal.pbio.1001397.
2. Mucosal Immunity in the Human Female Reproductive Tract in “Mucosal Immunology,” 4th ed., Volume 2 (Cambridge, MA: Academic Press, 2015, pp. 2097-124).
3. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30279-8.
Vaccines are marvelous, and there are many well documented success stories, including rotavirus (RV) vaccines, where a live vaccine is administered to the gastrointestinal mucosa via oral drops. Antigens presented at the mucosal/epithelial surface not only induce systemic serum IgG – as do injectable vaccines – but also induce secretory IgA (sIgA), which is most helpful in diseases that directly affect the mucosa.

Mucosal vs. systemic immunity
Antibody being present on mucosal surfaces (point of initial pathogen contact) has a chance to neutralize the pathogen before it gains a foothold. Pathogen-specific mucosal lymphoid elements (e.g. in Peyer’s patches in the gut) also appear critical for optimal protection.1 The presence of both mucosal immune elements means that infection is severely limited or at times entirely prevented. So virus entering the GI tract causes minimal to no gut lining injury. Hence, there is no or mostly reduced vomiting/diarrhea. A downside of mucosally-administered live vaccines is that preexisting antibody to the vaccine antigens can reduce or block vaccine virus replication in the vaccinee, blunting or preventing protection. Note: Preexisting antibody also affects injectable live vaccines, such as the measles vaccine, similarly.
Classic injectable live or nonlive vaccines provide their most potent protection via systemic cellular responses antibody and/or antibodies in serum and extracellular fluid (ECF) where IgG and IgM are in highest concentrations. So even successful injectable vaccines still allow mucosal infection to start but then intercept further spread and prevent most of the downstream damage (think pertussis) or neutralize an infection-generated toxin (pertussis or tetanus). It usually is only after infection-induced damage occurs that systemic IgG and IgM gain better access to respiratory epithelial surfaces, but still only at a fraction of circulating concentrations. Indeed, pertussis vaccine–induced systemic immunity allows the pathogen to attack and replicate in/on host surface cells, causing toxin release and variable amounts of local mucosal injury/inflammation before vaccine-induced systemic immunity gains adequate access to the pathogen and/or to its toxin which may enter systemic circulation.
Live attenuated influenza vaccine (LAIV) induces mucosal immunity
Another “standard” vaccine that induces mucosal immunity – LAIV – was developed to improve on protection afforded by injectable influenza vaccines (IIVs), but LAIV has had hiccups in the United States. One example is several years of negligible protection against H1N1 disease. As long as LAIV’s vaccine strain had reasonably matched the circulating strains, LAIV worked at least as well as injectable influenza vaccine, and even offered some cross-protection against mildly mismatched strains. But after a number of years of LAIV use, vaccine effectiveness in the United States vs. H1N1 strains appeared to fade due to previously undetected but significant changes in the circulating H1N1 strain. The lesson is that mucosal immunity’s advantages are lost if too much change occurs in the pathogen target for sIgA and mucosally-associated lymphoid tissue cells (MALT)).
Other vaccines likely need to induce mucosal immunity
Protection at the mucosal level will likely be needed for success against norovirus, parainfluenza, respiratory syncytial virus (RSV), Neisseria gonorrhea, and chlamydia. Another helpful aspect of mucosal immunity is that immune cells and sIgA not only reside on the mucosa where the antigen was originally presented, but there is also a reasonable chance that these components will traffic to other mucosal surfaces.2
So intranasal vaccine could be expected to protect distant mucosal surfaces (urogenital, GI, and respiratory), leading to vaccine-induced systemic antibody plus mucosal immunity (sIGA and MALT responses) at each site.
Let’s look at a novel “two-site” chlamydia vaccine
Recently a phase 1 chlamydia vaccine that used a novel two-pronged administration site/schedule was successful at inducing both mucosal and systemic immunity in a proof-of-concept study – achieving the best of both worlds.3 This may be a template for vaccines in years to come. British investigators studied 50 healthy women aged 19-45 years in a double-blind, parallel, randomized, placebo-controlled trial that used a recombinant chlamydia protein subunit antigen (CTH522). The vaccine schedule involved three injectable priming doses followed soon thereafter by two intranasal boosting doses. There were three groups:
1. CTH522 adjuvanted with CAF01 liposomes (CTH522:CAF01).
2. CTH522 adjuvanted with aluminum hydroxide (CTH522:AH).
3. Placebo (saline).
The intramuscular (IM) priming schedule was 0, 1, and 4 months. The intranasal vaccine booster doses or placebo were given at 4.5 and 5 months. No related serious adverse reactions occurred. For injectable dosing, the most frequent adverse event was mild local injection-site reactions in all subjects in both vaccine groups vs. in 60% of placebo recipients (P = .053). The adjuvants were the likely cause for local reactions. Intranasal doses had local reactions in 47% of both vaccine groups and 60% of placebo recipients; P = 1.000).
Both vaccines produced systemic IgG seroconversion (including neutralizing antibody) plus small amounts of IgG in the nasal cavity and genital tract in all vaccine recipients; no placebo recipient seroconverted. Interestingly, liposomally-adjuvanted vaccine produced a more rapid systemic IgG response and higher serum titers than the alum-adjuvanted vaccine. Likewise, the IM liposomal vaccine also induced higher but still small mucosal IgG antibody responses (P = .0091). Intranasal IM-induced IgG titers were not boosted by later intranasal vaccine dosing.
Subjects getting liposomal vaccine (but not alum vaccine or placebo) boosters had detectable sIgA titers in both nasal and genital tract secretions. Liposomal vaccine recipients also had fivefold to sixfold higher median titers than alum vaccine recipients after the priming dose, and these higher titers persisted to the end of the study. All liposomal vaccine recipients developed antichlamydial cell-mediated responses vs. 57% alum-adjuvanted vaccine recipients. (P = .01). So both use of two-site dosing and the liposomal adjuvant appeared critical to better responses.
In summary
While this candidate vaccine has hurdles to overcome before coming into routine use, the proof-of-principle that a combination injectable-intranasal vaccine schedule can induce robust systemic and mucosal immunity when given with an appropriate adjuvant is very promising. Adding more vaccines to the schedule then becomes an issue, but that is one of those “good” problems we can deal with later.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines, receives funding from GlaxoSmithKline for studies on pneumococcal and rotavirus vaccines, and from Pfizer for a study on pneumococcal vaccine on which Dr. Harrison is a sub-investigator. The hospital also receives Centers for Disease Control and Prevention funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus, and also for rotavirus. Email Dr. Harrison at [email protected].
References
1. PLOS Biology. 2012 Sep 1. doi: 10.1371/journal.pbio.1001397.
2. Mucosal Immunity in the Human Female Reproductive Tract in “Mucosal Immunology,” 4th ed., Volume 2 (Cambridge, MA: Academic Press, 2015, pp. 2097-124).
3. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30279-8.
Vaccines are marvelous, and there are many well documented success stories, including rotavirus (RV) vaccines, where a live vaccine is administered to the gastrointestinal mucosa via oral drops. Antigens presented at the mucosal/epithelial surface not only induce systemic serum IgG – as do injectable vaccines – but also induce secretory IgA (sIgA), which is most helpful in diseases that directly affect the mucosa.

Mucosal vs. systemic immunity
Antibody being present on mucosal surfaces (point of initial pathogen contact) has a chance to neutralize the pathogen before it gains a foothold. Pathogen-specific mucosal lymphoid elements (e.g. in Peyer’s patches in the gut) also appear critical for optimal protection.1 The presence of both mucosal immune elements means that infection is severely limited or at times entirely prevented. So virus entering the GI tract causes minimal to no gut lining injury. Hence, there is no or mostly reduced vomiting/diarrhea. A downside of mucosally-administered live vaccines is that preexisting antibody to the vaccine antigens can reduce or block vaccine virus replication in the vaccinee, blunting or preventing protection. Note: Preexisting antibody also affects injectable live vaccines, such as the measles vaccine, similarly.
Classic injectable live or nonlive vaccines provide their most potent protection via systemic cellular responses antibody and/or antibodies in serum and extracellular fluid (ECF) where IgG and IgM are in highest concentrations. So even successful injectable vaccines still allow mucosal infection to start but then intercept further spread and prevent most of the downstream damage (think pertussis) or neutralize an infection-generated toxin (pertussis or tetanus). It usually is only after infection-induced damage occurs that systemic IgG and IgM gain better access to respiratory epithelial surfaces, but still only at a fraction of circulating concentrations. Indeed, pertussis vaccine–induced systemic immunity allows the pathogen to attack and replicate in/on host surface cells, causing toxin release and variable amounts of local mucosal injury/inflammation before vaccine-induced systemic immunity gains adequate access to the pathogen and/or to its toxin which may enter systemic circulation.
Live attenuated influenza vaccine (LAIV) induces mucosal immunity
Another “standard” vaccine that induces mucosal immunity – LAIV – was developed to improve on protection afforded by injectable influenza vaccines (IIVs), but LAIV has had hiccups in the United States. One example is several years of negligible protection against H1N1 disease. As long as LAIV’s vaccine strain had reasonably matched the circulating strains, LAIV worked at least as well as injectable influenza vaccine, and even offered some cross-protection against mildly mismatched strains. But after a number of years of LAIV use, vaccine effectiveness in the United States vs. H1N1 strains appeared to fade due to previously undetected but significant changes in the circulating H1N1 strain. The lesson is that mucosal immunity’s advantages are lost if too much change occurs in the pathogen target for sIgA and mucosally-associated lymphoid tissue cells (MALT)).
Other vaccines likely need to induce mucosal immunity
Protection at the mucosal level will likely be needed for success against norovirus, parainfluenza, respiratory syncytial virus (RSV), Neisseria gonorrhea, and chlamydia. Another helpful aspect of mucosal immunity is that immune cells and sIgA not only reside on the mucosa where the antigen was originally presented, but there is also a reasonable chance that these components will traffic to other mucosal surfaces.2
So intranasal vaccine could be expected to protect distant mucosal surfaces (urogenital, GI, and respiratory), leading to vaccine-induced systemic antibody plus mucosal immunity (sIGA and MALT responses) at each site.
Let’s look at a novel “two-site” chlamydia vaccine
Recently a phase 1 chlamydia vaccine that used a novel two-pronged administration site/schedule was successful at inducing both mucosal and systemic immunity in a proof-of-concept study – achieving the best of both worlds.3 This may be a template for vaccines in years to come. British investigators studied 50 healthy women aged 19-45 years in a double-blind, parallel, randomized, placebo-controlled trial that used a recombinant chlamydia protein subunit antigen (CTH522). The vaccine schedule involved three injectable priming doses followed soon thereafter by two intranasal boosting doses. There were three groups:
1. CTH522 adjuvanted with CAF01 liposomes (CTH522:CAF01).
2. CTH522 adjuvanted with aluminum hydroxide (CTH522:AH).
3. Placebo (saline).
The intramuscular (IM) priming schedule was 0, 1, and 4 months. The intranasal vaccine booster doses or placebo were given at 4.5 and 5 months. No related serious adverse reactions occurred. For injectable dosing, the most frequent adverse event was mild local injection-site reactions in all subjects in both vaccine groups vs. in 60% of placebo recipients (P = .053). The adjuvants were the likely cause for local reactions. Intranasal doses had local reactions in 47% of both vaccine groups and 60% of placebo recipients; P = 1.000).
Both vaccines produced systemic IgG seroconversion (including neutralizing antibody) plus small amounts of IgG in the nasal cavity and genital tract in all vaccine recipients; no placebo recipient seroconverted. Interestingly, liposomally-adjuvanted vaccine produced a more rapid systemic IgG response and higher serum titers than the alum-adjuvanted vaccine. Likewise, the IM liposomal vaccine also induced higher but still small mucosal IgG antibody responses (P = .0091). Intranasal IM-induced IgG titers were not boosted by later intranasal vaccine dosing.
Subjects getting liposomal vaccine (but not alum vaccine or placebo) boosters had detectable sIgA titers in both nasal and genital tract secretions. Liposomal vaccine recipients also had fivefold to sixfold higher median titers than alum vaccine recipients after the priming dose, and these higher titers persisted to the end of the study. All liposomal vaccine recipients developed antichlamydial cell-mediated responses vs. 57% alum-adjuvanted vaccine recipients. (P = .01). So both use of two-site dosing and the liposomal adjuvant appeared critical to better responses.
In summary
While this candidate vaccine has hurdles to overcome before coming into routine use, the proof-of-principle that a combination injectable-intranasal vaccine schedule can induce robust systemic and mucosal immunity when given with an appropriate adjuvant is very promising. Adding more vaccines to the schedule then becomes an issue, but that is one of those “good” problems we can deal with later.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines, receives funding from GlaxoSmithKline for studies on pneumococcal and rotavirus vaccines, and from Pfizer for a study on pneumococcal vaccine on which Dr. Harrison is a sub-investigator. The hospital also receives Centers for Disease Control and Prevention funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus, and also for rotavirus. Email Dr. Harrison at [email protected].
References
1. PLOS Biology. 2012 Sep 1. doi: 10.1371/journal.pbio.1001397.
2. Mucosal Immunity in the Human Female Reproductive Tract in “Mucosal Immunology,” 4th ed., Volume 2 (Cambridge, MA: Academic Press, 2015, pp. 2097-124).
3. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30279-8.
Unreliable Herd Immunity Leads to More Measles
The Centers for Disease Control and Prevention's summary of the alarming 118 U.S. cases of measles in 2011 reports that nearly all were caused by scattered inadvertent measles introduction from measles-endemic countries. This importation resulted from U.S. residents returning or immigrants coming from endemic countries. A dozen or so imported cases of measles are not unexpected or new. Every year, cases of imported measles occur.
So why is there an increase in the number of transmitted cases in the United States? Increased vulnerability to ongoing transmission is now possible because herd immunity has become unreliable.
Herd immunity in the past was often discussed in the context of protecting the less than 5% of the community who are too young (less than 12 months old) to receive MMR vaccine or who have true contraindications to vaccine. For measles, reliable herd immunity requires approximately 90% of the community to be immune to measles. To achieve this, we need 95% of the community immunized because approximately 5% of immunized children fail to become immune from a single immunization.
This became clear during the 1990s measles outbreaks and led to a recommendation for two doses, the second dose at 4–6 years of age. That controlled measles outbreaks until the past 2 years, when measles has been increasingly reported. Partly, this is due to the increase in the number of countries with endemic measles, including developed countries, most notably France, as reported in MMWR (2011:60;666-8). So the number of imported cases likely increased. But if herd immunity is strong, secondary cases should not be frequent.
What is new, and has directly led to many cases, is an increase in the number of geographic clusters of unvaccinated children due to parents delaying or refusing measles vaccine. In those areas, secondary cases are occurring at a rate not seen in decades. It's not that the overall national measles immunization rate is that much lower. The overall rate of one dose of MMR vaccine is near 90%, and two-dose coverage is around 80%.
The problem is that the extra geographically clustered 5% who choose to delay or avoid MMR vaccine permit transmission from imported cases mostly among unvaccinated children. In those areas, herd immunity is broken. Just this relatively small shift in local immunization density breaks down herd immunity to measles.
Why is it so? Measles is one of the three most contagious infections, being transmitted via airborne particles. Further, during the first 3 days of measles the presentation is a nonspecific febrile upper respiratory tract infection. So persons with measles often do not restrict their activity and may expose many people via normal activities or even in the reception rooms at medical facilities.
It is not until the third day or perhaps the fourth day of contagion and fever that the classic symptoms of cough, coryza, and nonpurulent conjunctivitis (the 3 C's) begin to appear, along with the beginnings of a maculopapular rash starting on the head. Because most clinicians under 60 years of age have little if any experience with measles, measles may go initially undiagnosed. This leads to additional exposures.
So imported measles added to focal weak spots in herd immunity to measles is the mechanism for increasing measles cases in the United States. Now children whose parents choose to avoid measles vaccine because the disease “is gone” or because of unfounded fears of adverse effects are no longer safe from disease. Note that 105 of the 118 (89%) cases were unvaccinated. And parents who would wish to vaccinate their children but cannot because of age or true contraindication also can no longer rely on herd immunity to protect their children.
This is a call to action. First, we should continue to be strong advocates for on-time MMR vaccination. We are unlikely to convince adamant antivaccine parents, but perhaps we can sway those who are merely conflicted by the false and discredited information promulgated by antivaccine groups.
Second, each of us needs to be aware of whether measles has occurred in our practice area, or in areas where our patients are planning to travel. If there is an expectation of possible exposure, consider administering the second MMR dose anytime more than 1 month after the first dose. And if the child is 9–12 months of age, consider giving a first dose prior to the usual 12 months of age. This will not be a valid dose per current Advisory Committee on Immunization Practices (ACIP) and American Academy of Pediatrics recommendations, but it may save the child from an illness with risks for both immediate and long-term severe pulmonary or neurological complications.
Third, don't miss the disease if it shows up in your patient. Be hypervigilant for the three C's plus high fever, and classic morbilliform rash.
Measles is in the air and until herd immunity is restored, expect cases in every major city in the United States. Hopefully, we as pediatric clinicians, in partnership with our local health departments, can make a difference and minimize these outbreaks.
The Centers for Disease Control and Prevention's summary of the alarming 118 U.S. cases of measles in 2011 reports that nearly all were caused by scattered inadvertent measles introduction from measles-endemic countries. This importation resulted from U.S. residents returning or immigrants coming from endemic countries. A dozen or so imported cases of measles are not unexpected or new. Every year, cases of imported measles occur.
So why is there an increase in the number of transmitted cases in the United States? Increased vulnerability to ongoing transmission is now possible because herd immunity has become unreliable.
Herd immunity in the past was often discussed in the context of protecting the less than 5% of the community who are too young (less than 12 months old) to receive MMR vaccine or who have true contraindications to vaccine. For measles, reliable herd immunity requires approximately 90% of the community to be immune to measles. To achieve this, we need 95% of the community immunized because approximately 5% of immunized children fail to become immune from a single immunization.
This became clear during the 1990s measles outbreaks and led to a recommendation for two doses, the second dose at 4–6 years of age. That controlled measles outbreaks until the past 2 years, when measles has been increasingly reported. Partly, this is due to the increase in the number of countries with endemic measles, including developed countries, most notably France, as reported in MMWR (2011:60;666-8). So the number of imported cases likely increased. But if herd immunity is strong, secondary cases should not be frequent.
What is new, and has directly led to many cases, is an increase in the number of geographic clusters of unvaccinated children due to parents delaying or refusing measles vaccine. In those areas, secondary cases are occurring at a rate not seen in decades. It's not that the overall national measles immunization rate is that much lower. The overall rate of one dose of MMR vaccine is near 90%, and two-dose coverage is around 80%.
The problem is that the extra geographically clustered 5% who choose to delay or avoid MMR vaccine permit transmission from imported cases mostly among unvaccinated children. In those areas, herd immunity is broken. Just this relatively small shift in local immunization density breaks down herd immunity to measles.
Why is it so? Measles is one of the three most contagious infections, being transmitted via airborne particles. Further, during the first 3 days of measles the presentation is a nonspecific febrile upper respiratory tract infection. So persons with measles often do not restrict their activity and may expose many people via normal activities or even in the reception rooms at medical facilities.
It is not until the third day or perhaps the fourth day of contagion and fever that the classic symptoms of cough, coryza, and nonpurulent conjunctivitis (the 3 C's) begin to appear, along with the beginnings of a maculopapular rash starting on the head. Because most clinicians under 60 years of age have little if any experience with measles, measles may go initially undiagnosed. This leads to additional exposures.
So imported measles added to focal weak spots in herd immunity to measles is the mechanism for increasing measles cases in the United States. Now children whose parents choose to avoid measles vaccine because the disease “is gone” or because of unfounded fears of adverse effects are no longer safe from disease. Note that 105 of the 118 (89%) cases were unvaccinated. And parents who would wish to vaccinate their children but cannot because of age or true contraindication also can no longer rely on herd immunity to protect their children.
This is a call to action. First, we should continue to be strong advocates for on-time MMR vaccination. We are unlikely to convince adamant antivaccine parents, but perhaps we can sway those who are merely conflicted by the false and discredited information promulgated by antivaccine groups.
Second, each of us needs to be aware of whether measles has occurred in our practice area, or in areas where our patients are planning to travel. If there is an expectation of possible exposure, consider administering the second MMR dose anytime more than 1 month after the first dose. And if the child is 9–12 months of age, consider giving a first dose prior to the usual 12 months of age. This will not be a valid dose per current Advisory Committee on Immunization Practices (ACIP) and American Academy of Pediatrics recommendations, but it may save the child from an illness with risks for both immediate and long-term severe pulmonary or neurological complications.
Third, don't miss the disease if it shows up in your patient. Be hypervigilant for the three C's plus high fever, and classic morbilliform rash.
Measles is in the air and until herd immunity is restored, expect cases in every major city in the United States. Hopefully, we as pediatric clinicians, in partnership with our local health departments, can make a difference and minimize these outbreaks.
The Centers for Disease Control and Prevention's summary of the alarming 118 U.S. cases of measles in 2011 reports that nearly all were caused by scattered inadvertent measles introduction from measles-endemic countries. This importation resulted from U.S. residents returning or immigrants coming from endemic countries. A dozen or so imported cases of measles are not unexpected or new. Every year, cases of imported measles occur.
So why is there an increase in the number of transmitted cases in the United States? Increased vulnerability to ongoing transmission is now possible because herd immunity has become unreliable.
Herd immunity in the past was often discussed in the context of protecting the less than 5% of the community who are too young (less than 12 months old) to receive MMR vaccine or who have true contraindications to vaccine. For measles, reliable herd immunity requires approximately 90% of the community to be immune to measles. To achieve this, we need 95% of the community immunized because approximately 5% of immunized children fail to become immune from a single immunization.
This became clear during the 1990s measles outbreaks and led to a recommendation for two doses, the second dose at 4–6 years of age. That controlled measles outbreaks until the past 2 years, when measles has been increasingly reported. Partly, this is due to the increase in the number of countries with endemic measles, including developed countries, most notably France, as reported in MMWR (2011:60;666-8). So the number of imported cases likely increased. But if herd immunity is strong, secondary cases should not be frequent.
What is new, and has directly led to many cases, is an increase in the number of geographic clusters of unvaccinated children due to parents delaying or refusing measles vaccine. In those areas, secondary cases are occurring at a rate not seen in decades. It's not that the overall national measles immunization rate is that much lower. The overall rate of one dose of MMR vaccine is near 90%, and two-dose coverage is around 80%.
The problem is that the extra geographically clustered 5% who choose to delay or avoid MMR vaccine permit transmission from imported cases mostly among unvaccinated children. In those areas, herd immunity is broken. Just this relatively small shift in local immunization density breaks down herd immunity to measles.
Why is it so? Measles is one of the three most contagious infections, being transmitted via airborne particles. Further, during the first 3 days of measles the presentation is a nonspecific febrile upper respiratory tract infection. So persons with measles often do not restrict their activity and may expose many people via normal activities or even in the reception rooms at medical facilities.
It is not until the third day or perhaps the fourth day of contagion and fever that the classic symptoms of cough, coryza, and nonpurulent conjunctivitis (the 3 C's) begin to appear, along with the beginnings of a maculopapular rash starting on the head. Because most clinicians under 60 years of age have little if any experience with measles, measles may go initially undiagnosed. This leads to additional exposures.
So imported measles added to focal weak spots in herd immunity to measles is the mechanism for increasing measles cases in the United States. Now children whose parents choose to avoid measles vaccine because the disease “is gone” or because of unfounded fears of adverse effects are no longer safe from disease. Note that 105 of the 118 (89%) cases were unvaccinated. And parents who would wish to vaccinate their children but cannot because of age or true contraindication also can no longer rely on herd immunity to protect their children.
This is a call to action. First, we should continue to be strong advocates for on-time MMR vaccination. We are unlikely to convince adamant antivaccine parents, but perhaps we can sway those who are merely conflicted by the false and discredited information promulgated by antivaccine groups.
Second, each of us needs to be aware of whether measles has occurred in our practice area, or in areas where our patients are planning to travel. If there is an expectation of possible exposure, consider administering the second MMR dose anytime more than 1 month after the first dose. And if the child is 9–12 months of age, consider giving a first dose prior to the usual 12 months of age. This will not be a valid dose per current Advisory Committee on Immunization Practices (ACIP) and American Academy of Pediatrics recommendations, but it may save the child from an illness with risks for both immediate and long-term severe pulmonary or neurological complications.
Third, don't miss the disease if it shows up in your patient. Be hypervigilant for the three C's plus high fever, and classic morbilliform rash.
Measles is in the air and until herd immunity is restored, expect cases in every major city in the United States. Hopefully, we as pediatric clinicians, in partnership with our local health departments, can make a difference and minimize these outbreaks.
Time to Expand Definition of a Travel Vaccine
Recent outbreaks of measles in western Europe and of pertussis here in the United States suggest that we consider expanding our definition of a “travel vaccine.”
We typically think of travel vaccines as those that aren't routinely given to children (or adults) but that are given only to our patients who travel to developing countries that lack our standards of medical care. But now that there are large measles outbreaks in places like France and Belgium and pertussis in California and elsewhere in the United States, I think we need to start routinely asking patients about travel plans and ensure that they are fully immunized with the measles-mumps-rubella (MMR) and diphtheria-tetanus-acellular pertussis (DTaP) or tetanus-diphtheria-acellular pertussis (Tdap) vaccines if they aren't already.
This includes accelerating MMR immunization for children younger than 1 year who will be traveling. It appears that not all health care providers are aware of this particular recommendation from the American Academy of Pediatrics' Red Book: While MMR is recommended for routine use in children at age 12 through 15 months with a booster at age 4-6 years, those aged 6 through 11 months who are traveling anywhere outside the United States are advised to receive one dose of MMR vaccine prior to their trip (Red Book;2009:444-55). For these 6- through 11-month-old children, this travel dose is not “valid,” meaning it doesn't officially count toward requirements for school attendance, but it is still in their best interests.
The Advisory Committee of Immunization Practices (ACIP) recommends: “Because serologic response to the measles component of the vaccine varies among infants aged 6–11 months, infants vaccinated before age 12 months should be revaccinated on or after the first birthday with 1 dose of MMR vaccine followed by a second dose at least 28 days later” (MMWR 1998;47[RR-8]:1-57).
This recommendation applies to ANY travel outside the United States except Canada or Australia, not just developing countries. According to the World Health Organization, as of April 18 more than 6,500 measles cases were reported from 33 countries in Europe. France has now passed 5,000 cases of measles and looks to be heading for a record year. It appears that nearly all of the cases in France have been among children with no vaccine doses. They have had at least two deaths – one from encephalitis and one from pneumonia.
There are two other major pockets. One near Belgium that seems to be associated with a religious group of vaccine refusers, while we're not sure what's behind another outbreak near the Spanish border. Other countries that have seen upticks in measles cases include Germany, the former Yugoslav Republic of Macedonia, the Netherlands, Norway, Romania, the Russian Federation, Switzerland, and the United Kingdom.
Measles cases have also been reported in the United States, including 29 during January-February 2011. Of those, 28 were import-associated (either imported or linked to an imported cases), of which 16 were actually imported. Of 13 imported cases among U.S. residents, 7 were children aged 6-23 months, all of whom had traveled internationally. Four of those children were hospitalized for measles-related complications: two with diarrhea and dehydration, one with persistent fever, and one with pneumonia. All four recovered (MMWR 2011;60:397-400).
The diagnosis had been delayed in three of the seven, presumably because measles had not been considered in the differential diagnosis of rash illness, even with a history of international travel. There's an obvious clinical lesson here.
None of those 7 had received MMR vaccine, and only 3 of 47 children aged 6-23 months with imported measles during 2001-2010 had received MMR vaccine. The reasons for nonvaccination of children often are unknown, but contributing to these might be a lack of perceived risk for severe measles. The frequency of imported measles among children aged 6-23 months also suggests that parents and clinicians might not be aware of recommendations to administer MMR vaccine to children as young as age 6 months when they are living or traveling abroad. Likewise, some aren't aware that they should give a second dose to any who have only one MMR dose more than 28 days prior. This “travel dose” can be given to a 13-month-old who had their first dose at 12 months of age. In fact, the parents of one of these 2011 measles patients had asked their pediatrician about vaccination for their child before traveling and were advised that it was unnecessary.
Travelers to the WHO European Region should be aware that measles is endemic in several countries of that region, which was the source of 39% of U.S. measles imports during 2005-2008, according to the Centers for Disease Control and Prevention.
Pertussis is the other vaccine-preventable disease that has been popping up lately and for which we need to consider vaccinating patients who may be traveling to affected areas, even within the United States. As of April 13, the California Department of Public Health reported ongoing pertussis activity, with 733 cases in 2011 for a rate of 6.5/100,000 population. There were 9,273 cases with onset in 2010, or 2.37/100,000, the highest incidence reported in the state since 1958.
Of the 755 hospitalized cases in 2010, more than half (55%) were infants younger than 3 months of age and nearly three-quarters (72%) were infants less than 6 months of age. Of the 10 deaths, 9 were infants.
So far in 2011, the highest rates of pertussis in California have been in the counties of Amador (86/100,000), Sonoma (32.5), and Santa Clara (23.5). Have a patient traveling to California who hasn't received a DTaP within 10 years and never received a Tdap booster? There is no longer a duration limit since the last Td dose. Just go ahead and give the Tdap.
And while we're on the subject, I wanted to mention that I chaired a committee for the Pediatric Infectious Diseases Society that has just published a position statement regarding personal belief exemption from immunization mandates. This document is aimed at helping pediatricians and family physicians who live in states that have laws allowing such exemptions, by providing a resource to support you medicolegally when facing parents who attempt to use misguided laws to avoid immunizing their children. It is available at www.pids.org/news/238-pid-position-statement-on-pbes.html
Recent outbreaks of measles in western Europe and of pertussis here in the United States suggest that we consider expanding our definition of a “travel vaccine.”
We typically think of travel vaccines as those that aren't routinely given to children (or adults) but that are given only to our patients who travel to developing countries that lack our standards of medical care. But now that there are large measles outbreaks in places like France and Belgium and pertussis in California and elsewhere in the United States, I think we need to start routinely asking patients about travel plans and ensure that they are fully immunized with the measles-mumps-rubella (MMR) and diphtheria-tetanus-acellular pertussis (DTaP) or tetanus-diphtheria-acellular pertussis (Tdap) vaccines if they aren't already.
This includes accelerating MMR immunization for children younger than 1 year who will be traveling. It appears that not all health care providers are aware of this particular recommendation from the American Academy of Pediatrics' Red Book: While MMR is recommended for routine use in children at age 12 through 15 months with a booster at age 4-6 years, those aged 6 through 11 months who are traveling anywhere outside the United States are advised to receive one dose of MMR vaccine prior to their trip (Red Book;2009:444-55). For these 6- through 11-month-old children, this travel dose is not “valid,” meaning it doesn't officially count toward requirements for school attendance, but it is still in their best interests.
The Advisory Committee of Immunization Practices (ACIP) recommends: “Because serologic response to the measles component of the vaccine varies among infants aged 6–11 months, infants vaccinated before age 12 months should be revaccinated on or after the first birthday with 1 dose of MMR vaccine followed by a second dose at least 28 days later” (MMWR 1998;47[RR-8]:1-57).
This recommendation applies to ANY travel outside the United States except Canada or Australia, not just developing countries. According to the World Health Organization, as of April 18 more than 6,500 measles cases were reported from 33 countries in Europe. France has now passed 5,000 cases of measles and looks to be heading for a record year. It appears that nearly all of the cases in France have been among children with no vaccine doses. They have had at least two deaths – one from encephalitis and one from pneumonia.
There are two other major pockets. One near Belgium that seems to be associated with a religious group of vaccine refusers, while we're not sure what's behind another outbreak near the Spanish border. Other countries that have seen upticks in measles cases include Germany, the former Yugoslav Republic of Macedonia, the Netherlands, Norway, Romania, the Russian Federation, Switzerland, and the United Kingdom.
Measles cases have also been reported in the United States, including 29 during January-February 2011. Of those, 28 were import-associated (either imported or linked to an imported cases), of which 16 were actually imported. Of 13 imported cases among U.S. residents, 7 were children aged 6-23 months, all of whom had traveled internationally. Four of those children were hospitalized for measles-related complications: two with diarrhea and dehydration, one with persistent fever, and one with pneumonia. All four recovered (MMWR 2011;60:397-400).
The diagnosis had been delayed in three of the seven, presumably because measles had not been considered in the differential diagnosis of rash illness, even with a history of international travel. There's an obvious clinical lesson here.
None of those 7 had received MMR vaccine, and only 3 of 47 children aged 6-23 months with imported measles during 2001-2010 had received MMR vaccine. The reasons for nonvaccination of children often are unknown, but contributing to these might be a lack of perceived risk for severe measles. The frequency of imported measles among children aged 6-23 months also suggests that parents and clinicians might not be aware of recommendations to administer MMR vaccine to children as young as age 6 months when they are living or traveling abroad. Likewise, some aren't aware that they should give a second dose to any who have only one MMR dose more than 28 days prior. This “travel dose” can be given to a 13-month-old who had their first dose at 12 months of age. In fact, the parents of one of these 2011 measles patients had asked their pediatrician about vaccination for their child before traveling and were advised that it was unnecessary.
Travelers to the WHO European Region should be aware that measles is endemic in several countries of that region, which was the source of 39% of U.S. measles imports during 2005-2008, according to the Centers for Disease Control and Prevention.
Pertussis is the other vaccine-preventable disease that has been popping up lately and for which we need to consider vaccinating patients who may be traveling to affected areas, even within the United States. As of April 13, the California Department of Public Health reported ongoing pertussis activity, with 733 cases in 2011 for a rate of 6.5/100,000 population. There were 9,273 cases with onset in 2010, or 2.37/100,000, the highest incidence reported in the state since 1958.
Of the 755 hospitalized cases in 2010, more than half (55%) were infants younger than 3 months of age and nearly three-quarters (72%) were infants less than 6 months of age. Of the 10 deaths, 9 were infants.
So far in 2011, the highest rates of pertussis in California have been in the counties of Amador (86/100,000), Sonoma (32.5), and Santa Clara (23.5). Have a patient traveling to California who hasn't received a DTaP within 10 years and never received a Tdap booster? There is no longer a duration limit since the last Td dose. Just go ahead and give the Tdap.
And while we're on the subject, I wanted to mention that I chaired a committee for the Pediatric Infectious Diseases Society that has just published a position statement regarding personal belief exemption from immunization mandates. This document is aimed at helping pediatricians and family physicians who live in states that have laws allowing such exemptions, by providing a resource to support you medicolegally when facing parents who attempt to use misguided laws to avoid immunizing their children. It is available at www.pids.org/news/238-pid-position-statement-on-pbes.html
Recent outbreaks of measles in western Europe and of pertussis here in the United States suggest that we consider expanding our definition of a “travel vaccine.”
We typically think of travel vaccines as those that aren't routinely given to children (or adults) but that are given only to our patients who travel to developing countries that lack our standards of medical care. But now that there are large measles outbreaks in places like France and Belgium and pertussis in California and elsewhere in the United States, I think we need to start routinely asking patients about travel plans and ensure that they are fully immunized with the measles-mumps-rubella (MMR) and diphtheria-tetanus-acellular pertussis (DTaP) or tetanus-diphtheria-acellular pertussis (Tdap) vaccines if they aren't already.
This includes accelerating MMR immunization for children younger than 1 year who will be traveling. It appears that not all health care providers are aware of this particular recommendation from the American Academy of Pediatrics' Red Book: While MMR is recommended for routine use in children at age 12 through 15 months with a booster at age 4-6 years, those aged 6 through 11 months who are traveling anywhere outside the United States are advised to receive one dose of MMR vaccine prior to their trip (Red Book;2009:444-55). For these 6- through 11-month-old children, this travel dose is not “valid,” meaning it doesn't officially count toward requirements for school attendance, but it is still in their best interests.
The Advisory Committee of Immunization Practices (ACIP) recommends: “Because serologic response to the measles component of the vaccine varies among infants aged 6–11 months, infants vaccinated before age 12 months should be revaccinated on or after the first birthday with 1 dose of MMR vaccine followed by a second dose at least 28 days later” (MMWR 1998;47[RR-8]:1-57).
This recommendation applies to ANY travel outside the United States except Canada or Australia, not just developing countries. According to the World Health Organization, as of April 18 more than 6,500 measles cases were reported from 33 countries in Europe. France has now passed 5,000 cases of measles and looks to be heading for a record year. It appears that nearly all of the cases in France have been among children with no vaccine doses. They have had at least two deaths – one from encephalitis and one from pneumonia.
There are two other major pockets. One near Belgium that seems to be associated with a religious group of vaccine refusers, while we're not sure what's behind another outbreak near the Spanish border. Other countries that have seen upticks in measles cases include Germany, the former Yugoslav Republic of Macedonia, the Netherlands, Norway, Romania, the Russian Federation, Switzerland, and the United Kingdom.
Measles cases have also been reported in the United States, including 29 during January-February 2011. Of those, 28 were import-associated (either imported or linked to an imported cases), of which 16 were actually imported. Of 13 imported cases among U.S. residents, 7 were children aged 6-23 months, all of whom had traveled internationally. Four of those children were hospitalized for measles-related complications: two with diarrhea and dehydration, one with persistent fever, and one with pneumonia. All four recovered (MMWR 2011;60:397-400).
The diagnosis had been delayed in three of the seven, presumably because measles had not been considered in the differential diagnosis of rash illness, even with a history of international travel. There's an obvious clinical lesson here.
None of those 7 had received MMR vaccine, and only 3 of 47 children aged 6-23 months with imported measles during 2001-2010 had received MMR vaccine. The reasons for nonvaccination of children often are unknown, but contributing to these might be a lack of perceived risk for severe measles. The frequency of imported measles among children aged 6-23 months also suggests that parents and clinicians might not be aware of recommendations to administer MMR vaccine to children as young as age 6 months when they are living or traveling abroad. Likewise, some aren't aware that they should give a second dose to any who have only one MMR dose more than 28 days prior. This “travel dose” can be given to a 13-month-old who had their first dose at 12 months of age. In fact, the parents of one of these 2011 measles patients had asked their pediatrician about vaccination for their child before traveling and were advised that it was unnecessary.
Travelers to the WHO European Region should be aware that measles is endemic in several countries of that region, which was the source of 39% of U.S. measles imports during 2005-2008, according to the Centers for Disease Control and Prevention.
Pertussis is the other vaccine-preventable disease that has been popping up lately and for which we need to consider vaccinating patients who may be traveling to affected areas, even within the United States. As of April 13, the California Department of Public Health reported ongoing pertussis activity, with 733 cases in 2011 for a rate of 6.5/100,000 population. There were 9,273 cases with onset in 2010, or 2.37/100,000, the highest incidence reported in the state since 1958.
Of the 755 hospitalized cases in 2010, more than half (55%) were infants younger than 3 months of age and nearly three-quarters (72%) were infants less than 6 months of age. Of the 10 deaths, 9 were infants.
So far in 2011, the highest rates of pertussis in California have been in the counties of Amador (86/100,000), Sonoma (32.5), and Santa Clara (23.5). Have a patient traveling to California who hasn't received a DTaP within 10 years and never received a Tdap booster? There is no longer a duration limit since the last Td dose. Just go ahead and give the Tdap.
And while we're on the subject, I wanted to mention that I chaired a committee for the Pediatric Infectious Diseases Society that has just published a position statement regarding personal belief exemption from immunization mandates. This document is aimed at helping pediatricians and family physicians who live in states that have laws allowing such exemptions, by providing a resource to support you medicolegally when facing parents who attempt to use misguided laws to avoid immunizing their children. It is available at www.pids.org/news/238-pid-position-statement-on-pbes.html
Protecting the Young Against Pertussis
The current pertussis outbreak occurring in California clearly demonstrates that we need to make a greater effort to vaccinate adults in order to protect infants too young to be completely vaccinated.
To quote the 2010 editorial by Dr. Alfred DeMaria Jr. and Dr. Susan Lett (Clin. Infect. Dis. 2010;50:1346-8), “If it does take a village to raise a child, then that village should be fully immunized against pertussis.”
Between January and July of this year, the California Department of Public Health received reports of a total 1,337 confirmed or probable cases of pertussis, which represents a fourfold increase from the 258 cases reported during the first half of 2009. If these rates persist throughout 2010, California will have its highest annual rate of pertussis since 1963 and the most cases reported since 1958, according the Centers for Disease Control and Prevention (MMWR 2010; 59:817).
During this outbreak, the CDPH expanded recommendations to off-label situations, including vaccination of those who are pregnant, older than 65 years, and aged 7-10 years.
As we've seen in the past, infants younger than 6 months of age—too young to have received the recommended three protective diphtheria-tetanus-acellular pertussis (DTaP) doses yet—are bearing the brunt of the illness, accounting for 89% of all the California cases. Disease incidence in children younger than 1 year of age was 38.5 cases per 100,000 population vs. 3.4 per 100,000 for all ages.
Of 634 case reports with available data, 105 (17%) were hospitalized, with 63% being younger than 3 months old. And, sadly, all six of the pertussis deaths reported as of July 13, 2010, were in previously healthy infants aged younger than 2 months at disease onset.
These deaths could have been prevented. A 2006-2008 study in the Netherlands demonstrated why the so-called “cocooning” effect really works. Of 560 not recently immunized household contacts of 164 hospitalized infants who were tested for Bordetella pertussis infection, 53% were infected and 14% had no symptoms. Among 96 households for which the most likely source of infection was established, 41% were siblings, 38% were mothers, and 17% were fathers.
The authors concluded that maintaining or boosting immunity to pertussis in parents and relatives could prevent 35%-55% of infant cases (Clin. Infect. Dis. 2010;50:1339-45).
The adolescent/adult tetanus-diphtheria-acellular pertussis vaccine (Tdap) has now been recommended for all adults as a replacement for the old Td vaccine. In practice, however, beyond the adolescent years, most adults receive it only if both they require tetanus prevention and the provider is aware of recent changes in the immunization recommendations.
As clinicians caring for children, we routinely vaccinate children as old as 6 years of age with DTaP and 10- to 18-year-olds with Tdap. But I believe we also have a role in helping to ensure that our youngest patients are protected by encouraging their adult contacts to be immunized with Tdap.
Certainly, most family physicians and med-ped (combined internal medicine and pediatrics) physicians are already doing this. Pediatricians who feel comfortable vaccinating parents/adult caregivers in their offices have a great opportunity, but others could still recommend that parents get the booster from their personal physician or a local health department clinic. And don't forget to suggest pertussis immunization for other adults who come into regular contact with the young infant, including grandparents and babysitters. Some health departments offer a price reduction if they're told that the Tdap is to protect a new infant in your family.
Pregnant women are a special situation. The U.S. Advisory Committee on Immunization Practices (ACIP) recommends pertussis immunization for women prior to conception and after birth if they have not received it within the past 2 years. The ACIP did not recommend Tdap for routine use during pregnancy because there is too little safety and efficacy data (MMWR 2008; 57[RR-4]:1-51).
However, the American College of Obstetricians and Gynecologists suggests vaccinating pregnant women if the risk is felt to be higher than the undefined risks of vaccine (Obstet. Gynecol. 2009;114:398-400). The American Academy of Pediatrics, for its part, recommends Tdap for pregnant adolescents in the same way as for nonpregnant adolescents (Pediatrics 2006; 117:965-78).
Dr. DeMaria and Dr. Lett also went on to write in their editorial that—when Tdap is given to pregnant women in the second or third trimester—counseling and administration is recommended.
Pediatricians might consider suggesting pertussis immunization to pregnant women who come in to “pediatrician shop,” and to those who have their older children accompanying them.
By the time this column is published, I will have a new grandchild. During talks with my son, it became clear to me that the cocooning concept has not reached enough health care professionals. I advised him that he, my daughter-in-law, and other in-laws receive Tdap before the baby's birth to maximize the chance of protection. My wife made sure she got hers.
In my view, Tdap for adult contacts is just as important as making sure the crib and car seat you buy for your baby are safe. Here's a potentially lethal disease that's resurgent in parts of the country, and we have a tool to protect our newborns against it. Shouldn't we make every effort to do so?
The current pertussis outbreak occurring in California clearly demonstrates that we need to make a greater effort to vaccinate adults in order to protect infants too young to be completely vaccinated.
To quote the 2010 editorial by Dr. Alfred DeMaria Jr. and Dr. Susan Lett (Clin. Infect. Dis. 2010;50:1346-8), “If it does take a village to raise a child, then that village should be fully immunized against pertussis.”
Between January and July of this year, the California Department of Public Health received reports of a total 1,337 confirmed or probable cases of pertussis, which represents a fourfold increase from the 258 cases reported during the first half of 2009. If these rates persist throughout 2010, California will have its highest annual rate of pertussis since 1963 and the most cases reported since 1958, according the Centers for Disease Control and Prevention (MMWR 2010; 59:817).
During this outbreak, the CDPH expanded recommendations to off-label situations, including vaccination of those who are pregnant, older than 65 years, and aged 7-10 years.
As we've seen in the past, infants younger than 6 months of age—too young to have received the recommended three protective diphtheria-tetanus-acellular pertussis (DTaP) doses yet—are bearing the brunt of the illness, accounting for 89% of all the California cases. Disease incidence in children younger than 1 year of age was 38.5 cases per 100,000 population vs. 3.4 per 100,000 for all ages.
Of 634 case reports with available data, 105 (17%) were hospitalized, with 63% being younger than 3 months old. And, sadly, all six of the pertussis deaths reported as of July 13, 2010, were in previously healthy infants aged younger than 2 months at disease onset.
These deaths could have been prevented. A 2006-2008 study in the Netherlands demonstrated why the so-called “cocooning” effect really works. Of 560 not recently immunized household contacts of 164 hospitalized infants who were tested for Bordetella pertussis infection, 53% were infected and 14% had no symptoms. Among 96 households for which the most likely source of infection was established, 41% were siblings, 38% were mothers, and 17% were fathers.
The authors concluded that maintaining or boosting immunity to pertussis in parents and relatives could prevent 35%-55% of infant cases (Clin. Infect. Dis. 2010;50:1339-45).
The adolescent/adult tetanus-diphtheria-acellular pertussis vaccine (Tdap) has now been recommended for all adults as a replacement for the old Td vaccine. In practice, however, beyond the adolescent years, most adults receive it only if both they require tetanus prevention and the provider is aware of recent changes in the immunization recommendations.
As clinicians caring for children, we routinely vaccinate children as old as 6 years of age with DTaP and 10- to 18-year-olds with Tdap. But I believe we also have a role in helping to ensure that our youngest patients are protected by encouraging their adult contacts to be immunized with Tdap.
Certainly, most family physicians and med-ped (combined internal medicine and pediatrics) physicians are already doing this. Pediatricians who feel comfortable vaccinating parents/adult caregivers in their offices have a great opportunity, but others could still recommend that parents get the booster from their personal physician or a local health department clinic. And don't forget to suggest pertussis immunization for other adults who come into regular contact with the young infant, including grandparents and babysitters. Some health departments offer a price reduction if they're told that the Tdap is to protect a new infant in your family.
Pregnant women are a special situation. The U.S. Advisory Committee on Immunization Practices (ACIP) recommends pertussis immunization for women prior to conception and after birth if they have not received it within the past 2 years. The ACIP did not recommend Tdap for routine use during pregnancy because there is too little safety and efficacy data (MMWR 2008; 57[RR-4]:1-51).
However, the American College of Obstetricians and Gynecologists suggests vaccinating pregnant women if the risk is felt to be higher than the undefined risks of vaccine (Obstet. Gynecol. 2009;114:398-400). The American Academy of Pediatrics, for its part, recommends Tdap for pregnant adolescents in the same way as for nonpregnant adolescents (Pediatrics 2006; 117:965-78).
Dr. DeMaria and Dr. Lett also went on to write in their editorial that—when Tdap is given to pregnant women in the second or third trimester—counseling and administration is recommended.
Pediatricians might consider suggesting pertussis immunization to pregnant women who come in to “pediatrician shop,” and to those who have their older children accompanying them.
By the time this column is published, I will have a new grandchild. During talks with my son, it became clear to me that the cocooning concept has not reached enough health care professionals. I advised him that he, my daughter-in-law, and other in-laws receive Tdap before the baby's birth to maximize the chance of protection. My wife made sure she got hers.
In my view, Tdap for adult contacts is just as important as making sure the crib and car seat you buy for your baby are safe. Here's a potentially lethal disease that's resurgent in parts of the country, and we have a tool to protect our newborns against it. Shouldn't we make every effort to do so?
The current pertussis outbreak occurring in California clearly demonstrates that we need to make a greater effort to vaccinate adults in order to protect infants too young to be completely vaccinated.
To quote the 2010 editorial by Dr. Alfred DeMaria Jr. and Dr. Susan Lett (Clin. Infect. Dis. 2010;50:1346-8), “If it does take a village to raise a child, then that village should be fully immunized against pertussis.”
Between January and July of this year, the California Department of Public Health received reports of a total 1,337 confirmed or probable cases of pertussis, which represents a fourfold increase from the 258 cases reported during the first half of 2009. If these rates persist throughout 2010, California will have its highest annual rate of pertussis since 1963 and the most cases reported since 1958, according the Centers for Disease Control and Prevention (MMWR 2010; 59:817).
During this outbreak, the CDPH expanded recommendations to off-label situations, including vaccination of those who are pregnant, older than 65 years, and aged 7-10 years.
As we've seen in the past, infants younger than 6 months of age—too young to have received the recommended three protective diphtheria-tetanus-acellular pertussis (DTaP) doses yet—are bearing the brunt of the illness, accounting for 89% of all the California cases. Disease incidence in children younger than 1 year of age was 38.5 cases per 100,000 population vs. 3.4 per 100,000 for all ages.
Of 634 case reports with available data, 105 (17%) were hospitalized, with 63% being younger than 3 months old. And, sadly, all six of the pertussis deaths reported as of July 13, 2010, were in previously healthy infants aged younger than 2 months at disease onset.
These deaths could have been prevented. A 2006-2008 study in the Netherlands demonstrated why the so-called “cocooning” effect really works. Of 560 not recently immunized household contacts of 164 hospitalized infants who were tested for Bordetella pertussis infection, 53% were infected and 14% had no symptoms. Among 96 households for which the most likely source of infection was established, 41% were siblings, 38% were mothers, and 17% were fathers.
The authors concluded that maintaining or boosting immunity to pertussis in parents and relatives could prevent 35%-55% of infant cases (Clin. Infect. Dis. 2010;50:1339-45).
The adolescent/adult tetanus-diphtheria-acellular pertussis vaccine (Tdap) has now been recommended for all adults as a replacement for the old Td vaccine. In practice, however, beyond the adolescent years, most adults receive it only if both they require tetanus prevention and the provider is aware of recent changes in the immunization recommendations.
As clinicians caring for children, we routinely vaccinate children as old as 6 years of age with DTaP and 10- to 18-year-olds with Tdap. But I believe we also have a role in helping to ensure that our youngest patients are protected by encouraging their adult contacts to be immunized with Tdap.
Certainly, most family physicians and med-ped (combined internal medicine and pediatrics) physicians are already doing this. Pediatricians who feel comfortable vaccinating parents/adult caregivers in their offices have a great opportunity, but others could still recommend that parents get the booster from their personal physician or a local health department clinic. And don't forget to suggest pertussis immunization for other adults who come into regular contact with the young infant, including grandparents and babysitters. Some health departments offer a price reduction if they're told that the Tdap is to protect a new infant in your family.
Pregnant women are a special situation. The U.S. Advisory Committee on Immunization Practices (ACIP) recommends pertussis immunization for women prior to conception and after birth if they have not received it within the past 2 years. The ACIP did not recommend Tdap for routine use during pregnancy because there is too little safety and efficacy data (MMWR 2008; 57[RR-4]:1-51).
However, the American College of Obstetricians and Gynecologists suggests vaccinating pregnant women if the risk is felt to be higher than the undefined risks of vaccine (Obstet. Gynecol. 2009;114:398-400). The American Academy of Pediatrics, for its part, recommends Tdap for pregnant adolescents in the same way as for nonpregnant adolescents (Pediatrics 2006; 117:965-78).
Dr. DeMaria and Dr. Lett also went on to write in their editorial that—when Tdap is given to pregnant women in the second or third trimester—counseling and administration is recommended.
Pediatricians might consider suggesting pertussis immunization to pregnant women who come in to “pediatrician shop,” and to those who have their older children accompanying them.
By the time this column is published, I will have a new grandchild. During talks with my son, it became clear to me that the cocooning concept has not reached enough health care professionals. I advised him that he, my daughter-in-law, and other in-laws receive Tdap before the baby's birth to maximize the chance of protection. My wife made sure she got hers.
In my view, Tdap for adult contacts is just as important as making sure the crib and car seat you buy for your baby are safe. Here's a potentially lethal disease that's resurgent in parts of the country, and we have a tool to protect our newborns against it. Shouldn't we make every effort to do so?
Should We Consider Giving MMR Earlier?
Parents' concern that children receive too many vaccines too soon can result in delay or avoidance of vaccination, with the measles-mumps-rubella vaccine often being delayed. However, a recent study showed no neurologic harm from on-time receipt of all the recommended vaccines—including MMR—from the Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices, and children with on-time receipt of vaccines performed better on select neurologic testing than those delaying vaccine. Another study showed that children lose maternally derived measles antibody protection as early as 1 month of age.
The study by Dr. Michael J. Smith and Dr. Charles R. Woods of the University of Louisville (Ky.) addressed the “too many vaccines too close together” issue. Using publicly available Vaccine Safety Datalink data from a previous study on thimerosal exposure and neuropsychological outcomes, the authors found that getting all recommended vaccines per the ACIP recommended schedule was associated with better—not worse—performance on selected neurologic outcomes at age 7-10 years, even when such factors as socioeconomic status were controlled for (Pediatrics 2010;125:1134-41). Importantly, there were no statistically significant differences favoring the less-vaccinated children. The authors concluded—and, I agree—that these data add reassurance for parents who are concerned that children receive too many vaccines too soon.
In the other study, Belgian investigators measured measles antibodies in mothers and persistence of the maternal antibody transferred to infants (BMJ 2010;340:c1626[doi:10.1136/bmj.c1626]). They found that the 86 women with antibody from measles vaccine had significantly lower, yet still protective, measles IgG titers, being one-quarter as high as in 120 mothers with antibody from previous measles infection, and that cord blood and initial infant titers correlated with maternal titers.
Of concern is that maternally endowed measles antibody disappeared at a median of 3.8 months in infants of previously measles-infected mothers (only a few infants had antibody at 6 months of age), and at nearly 1 month of age in infants of vaccinated women (none had antibody at 6 months). Thus infants became vulnerable to measles even earlier than previously reported. If maternal antibody is from vaccine, their infants are susceptible for the 9-14 months just prior to the MMR if it is administered at 12-15 months of age.
While waning maternally endowed antibody by 6 months of age is expected for most infections, measles had seemed different. In the 1970s-1980s, MMR was given at 15 months of age. This was because maternal antibody reportedly persisted up to 12 months and prevented a vaccine “take” if the mothers' antibody came from measles infection (J. Pediatr 1977; 91:715-8).hA later report showed waning antibody sooner when mothers' immunity came from measles vaccine: no antibody in 71% of 9-month-olds and 95% of 12-month-olds Maediatrics 1995;96:447-50).httis set the stage for the earlier 12-month MMR option. Now we have increasing evidence of even younger age for disappearance of the vaccine-interfering yet protective antibody to measles.
These data also have implications for the infant traveler. Although MMR isn't currently licensed for infants less than 1 year of age, data like these are the rationale for the Redbook recommendation that MMR be given to infants at 6 months of age or older who will be traveling to measles-endemic countries or during measles outbreaks. Of note, this is considered an “invalid” dose and the 12- to 15-month dose is still needed to attend school.
It might surprise some that Switzerland is now a measles-endemic country apparently due to its low 71% measles immunization rate. In fact, the per capita Swiss measles attack rate is similar to Somalia's. This shows that developed countries will have reemergent measles if herd immunity is lost.
I think we can make a case for studying earlier MMR dosing, particularly with measles outbreaks occurring in the United States, and imported cases potentially now coming from developed countries. If herd immunity (greater than 90% immunized) is in place, the infants' gap in measles protection may not be so worrisome. But as MMR immunization rates decline and become particularly low in some pockets in our country, concern increases over potential larger outbreaks. Studies to evaluate MMR at age 9 months could be the first step. If the vaccine were effective, we could narrow the measles-vulnerable window and vaccinate at the 9-month wellness visit.
Parents' concern that children receive too many vaccines too soon can result in delay or avoidance of vaccination, with the measles-mumps-rubella vaccine often being delayed. However, a recent study showed no neurologic harm from on-time receipt of all the recommended vaccines—including MMR—from the Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices, and children with on-time receipt of vaccines performed better on select neurologic testing than those delaying vaccine. Another study showed that children lose maternally derived measles antibody protection as early as 1 month of age.
The study by Dr. Michael J. Smith and Dr. Charles R. Woods of the University of Louisville (Ky.) addressed the “too many vaccines too close together” issue. Using publicly available Vaccine Safety Datalink data from a previous study on thimerosal exposure and neuropsychological outcomes, the authors found that getting all recommended vaccines per the ACIP recommended schedule was associated with better—not worse—performance on selected neurologic outcomes at age 7-10 years, even when such factors as socioeconomic status were controlled for (Pediatrics 2010;125:1134-41). Importantly, there were no statistically significant differences favoring the less-vaccinated children. The authors concluded—and, I agree—that these data add reassurance for parents who are concerned that children receive too many vaccines too soon.
In the other study, Belgian investigators measured measles antibodies in mothers and persistence of the maternal antibody transferred to infants (BMJ 2010;340:c1626[doi:10.1136/bmj.c1626]). They found that the 86 women with antibody from measles vaccine had significantly lower, yet still protective, measles IgG titers, being one-quarter as high as in 120 mothers with antibody from previous measles infection, and that cord blood and initial infant titers correlated with maternal titers.
Of concern is that maternally endowed measles antibody disappeared at a median of 3.8 months in infants of previously measles-infected mothers (only a few infants had antibody at 6 months of age), and at nearly 1 month of age in infants of vaccinated women (none had antibody at 6 months). Thus infants became vulnerable to measles even earlier than previously reported. If maternal antibody is from vaccine, their infants are susceptible for the 9-14 months just prior to the MMR if it is administered at 12-15 months of age.
While waning maternally endowed antibody by 6 months of age is expected for most infections, measles had seemed different. In the 1970s-1980s, MMR was given at 15 months of age. This was because maternal antibody reportedly persisted up to 12 months and prevented a vaccine “take” if the mothers' antibody came from measles infection (J. Pediatr 1977; 91:715-8).hA later report showed waning antibody sooner when mothers' immunity came from measles vaccine: no antibody in 71% of 9-month-olds and 95% of 12-month-olds Maediatrics 1995;96:447-50).httis set the stage for the earlier 12-month MMR option. Now we have increasing evidence of even younger age for disappearance of the vaccine-interfering yet protective antibody to measles.
These data also have implications for the infant traveler. Although MMR isn't currently licensed for infants less than 1 year of age, data like these are the rationale for the Redbook recommendation that MMR be given to infants at 6 months of age or older who will be traveling to measles-endemic countries or during measles outbreaks. Of note, this is considered an “invalid” dose and the 12- to 15-month dose is still needed to attend school.
It might surprise some that Switzerland is now a measles-endemic country apparently due to its low 71% measles immunization rate. In fact, the per capita Swiss measles attack rate is similar to Somalia's. This shows that developed countries will have reemergent measles if herd immunity is lost.
I think we can make a case for studying earlier MMR dosing, particularly with measles outbreaks occurring in the United States, and imported cases potentially now coming from developed countries. If herd immunity (greater than 90% immunized) is in place, the infants' gap in measles protection may not be so worrisome. But as MMR immunization rates decline and become particularly low in some pockets in our country, concern increases over potential larger outbreaks. Studies to evaluate MMR at age 9 months could be the first step. If the vaccine were effective, we could narrow the measles-vulnerable window and vaccinate at the 9-month wellness visit.
Parents' concern that children receive too many vaccines too soon can result in delay or avoidance of vaccination, with the measles-mumps-rubella vaccine often being delayed. However, a recent study showed no neurologic harm from on-time receipt of all the recommended vaccines—including MMR—from the Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices, and children with on-time receipt of vaccines performed better on select neurologic testing than those delaying vaccine. Another study showed that children lose maternally derived measles antibody protection as early as 1 month of age.
The study by Dr. Michael J. Smith and Dr. Charles R. Woods of the University of Louisville (Ky.) addressed the “too many vaccines too close together” issue. Using publicly available Vaccine Safety Datalink data from a previous study on thimerosal exposure and neuropsychological outcomes, the authors found that getting all recommended vaccines per the ACIP recommended schedule was associated with better—not worse—performance on selected neurologic outcomes at age 7-10 years, even when such factors as socioeconomic status were controlled for (Pediatrics 2010;125:1134-41). Importantly, there were no statistically significant differences favoring the less-vaccinated children. The authors concluded—and, I agree—that these data add reassurance for parents who are concerned that children receive too many vaccines too soon.
In the other study, Belgian investigators measured measles antibodies in mothers and persistence of the maternal antibody transferred to infants (BMJ 2010;340:c1626[doi:10.1136/bmj.c1626]). They found that the 86 women with antibody from measles vaccine had significantly lower, yet still protective, measles IgG titers, being one-quarter as high as in 120 mothers with antibody from previous measles infection, and that cord blood and initial infant titers correlated with maternal titers.
Of concern is that maternally endowed measles antibody disappeared at a median of 3.8 months in infants of previously measles-infected mothers (only a few infants had antibody at 6 months of age), and at nearly 1 month of age in infants of vaccinated women (none had antibody at 6 months). Thus infants became vulnerable to measles even earlier than previously reported. If maternal antibody is from vaccine, their infants are susceptible for the 9-14 months just prior to the MMR if it is administered at 12-15 months of age.
While waning maternally endowed antibody by 6 months of age is expected for most infections, measles had seemed different. In the 1970s-1980s, MMR was given at 15 months of age. This was because maternal antibody reportedly persisted up to 12 months and prevented a vaccine “take” if the mothers' antibody came from measles infection (J. Pediatr 1977; 91:715-8).hA later report showed waning antibody sooner when mothers' immunity came from measles vaccine: no antibody in 71% of 9-month-olds and 95% of 12-month-olds Maediatrics 1995;96:447-50).httis set the stage for the earlier 12-month MMR option. Now we have increasing evidence of even younger age for disappearance of the vaccine-interfering yet protective antibody to measles.
These data also have implications for the infant traveler. Although MMR isn't currently licensed for infants less than 1 year of age, data like these are the rationale for the Redbook recommendation that MMR be given to infants at 6 months of age or older who will be traveling to measles-endemic countries or during measles outbreaks. Of note, this is considered an “invalid” dose and the 12- to 15-month dose is still needed to attend school.
It might surprise some that Switzerland is now a measles-endemic country apparently due to its low 71% measles immunization rate. In fact, the per capita Swiss measles attack rate is similar to Somalia's. This shows that developed countries will have reemergent measles if herd immunity is lost.
I think we can make a case for studying earlier MMR dosing, particularly with measles outbreaks occurring in the United States, and imported cases potentially now coming from developed countries. If herd immunity (greater than 90% immunized) is in place, the infants' gap in measles protection may not be so worrisome. But as MMR immunization rates decline and become particularly low in some pockets in our country, concern increases over potential larger outbreaks. Studies to evaluate MMR at age 9 months could be the first step. If the vaccine were effective, we could narrow the measles-vulnerable window and vaccinate at the 9-month wellness visit.
Factors Affecting HPV Immunization
Could it be that our own cultural affiliations and beliefs might affect our patients' willingness to accept the human papillomavirus vaccine? A fascinating new study suggests just that.
To me, HPV vaccine should be a no-brainer. It protects against 60%-70% of cervical cancers, and is as safe as any other available vaccine. Yet, only about 40% of young females recommended to receive the vaccine have done so thus far. Why?
It may be in part because it is one of the most expensive vaccines in our repertoire, but it's covered by the Vaccines for Children program and now by most third-party payers. And it's not just a matter of 11- to 12-year-olds not getting vaccinated overall. In my area, only about two-thirds of adolescents who get the tetanus-diphtheria-acellular pertussis booster are concurrently receiving the HPV vaccine. It seems that they are refusing it specifically.
The HPV vaccine has been the object of misinformation and is controversial. Some people argue that it is unsafe or that it encourages young females to be more sexually active.
But a recent study actually suggests that girls getting HPV vaccine may be more cautious about sexual activity (Br. J. Cancer 2009;101:1502-4), yet the incorrect beliefs persist.
We hope that families will accept our advice on matters when they have concerns, but another new study sheds light on why families might not.
Yale University law professor Dan M. Kahan and his associates randomly surveyed 1,538 U.S. adults from a database of 40,000 scholarly public opinion poll respondents regarding their views on the HPV vaccine.
Individuals with cultural values favoring “authority” and/or “individualism” perceived the vaccine as risky, in part because they believed it would lead girls to engage in unsafe sex. But those favoring gender equality and/or community/government involvement in basic health care were more likely to see the vaccine as low risk and high benefit (Law Hum. Behav. 2010 Jan. 14 [doi:10.1007/s10979-009-9201-0
We all have suspected this to be the case, but now there are data to support that suspicion. Now here's the really interesting part: The researchers designed fictional “experts” who appeared to either share or oppose the respondents' cultural values. When views about HPV vaccines came from experts who respondents believed shared their values, they were more willing to accept the information. But when the views came from experts whom they perceived held values different from theirs, the subjects did not accept the experts' information.
So, when proauthority/individualism experts asserted the vaccine was risky, proauthority/individualism respondents agreed with them. When the egalitarian/procommunity experts argued that it was safe, egalitarian/procommunity respondents also agreed with them, solidifying overall disagreement about use of the vaccine.
However, when proauthority/individualism experts asserted that the vaccine was safe, proauthority/individualism respondents (who originally thought the vaccine was risky) moderated their original viewpoints, because the information came from experts who they perceived shared their values.
This held true for the opposite scenario, too: If egalitarian/procommunity experts argued the vaccine was risky, egalitarian/procommunity respondents shifted their belief toward its being risky.
As clinicians, we'd like to believe that our patients respect and trust us. But it's possible that when it comes to controversial recommendations, they may resist what we say if they don't identify enough with us based on our apparent values. If it is clear that our patient's family holds values widely disparate from ours, it might be helpful to utilize another more culturally congruent health professional in our practice to counsel about vaccination. This would vary by practice and from case to case, but could include people of similar race, religion, political viewpoint, or even regional accent.
Studies suggest that patients sometimes choose physicians to match their values. But with Medicaid and managed care, that may not always be possible. Using this type of approach may have more impact.
Surveys and discussion groups by the CDC suggest that scare tactics and scientific data may not successfully modify the opinion of parents who are disinclined toward vaccination (and I think most of us have the same experience). However, I did want to briefly mention recent data regarding HPV transmission in young adults that took me by surprise and may be persuasive for some patients.
Dr. Ann N. Burchell and her associates at McGill University, Montreal, evaluated female college/university students (aged 18-24 years) in self-described “stable” relationships exclusively with one male partner. The 263 couples had engaged in vaginal sex for a median of 3.9 months. HPV was detected in 64% of the couples. In 41% of the couples, both partners had the same HPV type. This risk of having the same strain was nearly four times more than what would be found by testing two random individuals. Also, oncogenic HPV-16 was the most common type, detected in 22% of couples (Epidemiology 2010;21:31-7).
In other words, one partner frequently came into the relationship with HPV and quickly transmitted it to the other. I was startled by the transmission frequency in these young adult females, who considered themselves in stable relationships. It suggests that acquisition is not just in early adolescence (although the risk of persistence is higher in that age group) and that catch-up immunization may be more important than some have thought. Perhaps these data won't convince all of your patients to get the HPV vaccine, but it may be helpful in some who are in their late teens or precollege age.
Could it be that our own cultural affiliations and beliefs might affect our patients' willingness to accept the human papillomavirus vaccine? A fascinating new study suggests just that.
To me, HPV vaccine should be a no-brainer. It protects against 60%-70% of cervical cancers, and is as safe as any other available vaccine. Yet, only about 40% of young females recommended to receive the vaccine have done so thus far. Why?
It may be in part because it is one of the most expensive vaccines in our repertoire, but it's covered by the Vaccines for Children program and now by most third-party payers. And it's not just a matter of 11- to 12-year-olds not getting vaccinated overall. In my area, only about two-thirds of adolescents who get the tetanus-diphtheria-acellular pertussis booster are concurrently receiving the HPV vaccine. It seems that they are refusing it specifically.
The HPV vaccine has been the object of misinformation and is controversial. Some people argue that it is unsafe or that it encourages young females to be more sexually active.
But a recent study actually suggests that girls getting HPV vaccine may be more cautious about sexual activity (Br. J. Cancer 2009;101:1502-4), yet the incorrect beliefs persist.
We hope that families will accept our advice on matters when they have concerns, but another new study sheds light on why families might not.
Yale University law professor Dan M. Kahan and his associates randomly surveyed 1,538 U.S. adults from a database of 40,000 scholarly public opinion poll respondents regarding their views on the HPV vaccine.
Individuals with cultural values favoring “authority” and/or “individualism” perceived the vaccine as risky, in part because they believed it would lead girls to engage in unsafe sex. But those favoring gender equality and/or community/government involvement in basic health care were more likely to see the vaccine as low risk and high benefit (Law Hum. Behav. 2010 Jan. 14 [doi:10.1007/s10979-009-9201-0
We all have suspected this to be the case, but now there are data to support that suspicion. Now here's the really interesting part: The researchers designed fictional “experts” who appeared to either share or oppose the respondents' cultural values. When views about HPV vaccines came from experts who respondents believed shared their values, they were more willing to accept the information. But when the views came from experts whom they perceived held values different from theirs, the subjects did not accept the experts' information.
So, when proauthority/individualism experts asserted the vaccine was risky, proauthority/individualism respondents agreed with them. When the egalitarian/procommunity experts argued that it was safe, egalitarian/procommunity respondents also agreed with them, solidifying overall disagreement about use of the vaccine.
However, when proauthority/individualism experts asserted that the vaccine was safe, proauthority/individualism respondents (who originally thought the vaccine was risky) moderated their original viewpoints, because the information came from experts who they perceived shared their values.
This held true for the opposite scenario, too: If egalitarian/procommunity experts argued the vaccine was risky, egalitarian/procommunity respondents shifted their belief toward its being risky.
As clinicians, we'd like to believe that our patients respect and trust us. But it's possible that when it comes to controversial recommendations, they may resist what we say if they don't identify enough with us based on our apparent values. If it is clear that our patient's family holds values widely disparate from ours, it might be helpful to utilize another more culturally congruent health professional in our practice to counsel about vaccination. This would vary by practice and from case to case, but could include people of similar race, religion, political viewpoint, or even regional accent.
Studies suggest that patients sometimes choose physicians to match their values. But with Medicaid and managed care, that may not always be possible. Using this type of approach may have more impact.
Surveys and discussion groups by the CDC suggest that scare tactics and scientific data may not successfully modify the opinion of parents who are disinclined toward vaccination (and I think most of us have the same experience). However, I did want to briefly mention recent data regarding HPV transmission in young adults that took me by surprise and may be persuasive for some patients.
Dr. Ann N. Burchell and her associates at McGill University, Montreal, evaluated female college/university students (aged 18-24 years) in self-described “stable” relationships exclusively with one male partner. The 263 couples had engaged in vaginal sex for a median of 3.9 months. HPV was detected in 64% of the couples. In 41% of the couples, both partners had the same HPV type. This risk of having the same strain was nearly four times more than what would be found by testing two random individuals. Also, oncogenic HPV-16 was the most common type, detected in 22% of couples (Epidemiology 2010;21:31-7).
In other words, one partner frequently came into the relationship with HPV and quickly transmitted it to the other. I was startled by the transmission frequency in these young adult females, who considered themselves in stable relationships. It suggests that acquisition is not just in early adolescence (although the risk of persistence is higher in that age group) and that catch-up immunization may be more important than some have thought. Perhaps these data won't convince all of your patients to get the HPV vaccine, but it may be helpful in some who are in their late teens or precollege age.
Could it be that our own cultural affiliations and beliefs might affect our patients' willingness to accept the human papillomavirus vaccine? A fascinating new study suggests just that.
To me, HPV vaccine should be a no-brainer. It protects against 60%-70% of cervical cancers, and is as safe as any other available vaccine. Yet, only about 40% of young females recommended to receive the vaccine have done so thus far. Why?
It may be in part because it is one of the most expensive vaccines in our repertoire, but it's covered by the Vaccines for Children program and now by most third-party payers. And it's not just a matter of 11- to 12-year-olds not getting vaccinated overall. In my area, only about two-thirds of adolescents who get the tetanus-diphtheria-acellular pertussis booster are concurrently receiving the HPV vaccine. It seems that they are refusing it specifically.
The HPV vaccine has been the object of misinformation and is controversial. Some people argue that it is unsafe or that it encourages young females to be more sexually active.
But a recent study actually suggests that girls getting HPV vaccine may be more cautious about sexual activity (Br. J. Cancer 2009;101:1502-4), yet the incorrect beliefs persist.
We hope that families will accept our advice on matters when they have concerns, but another new study sheds light on why families might not.
Yale University law professor Dan M. Kahan and his associates randomly surveyed 1,538 U.S. adults from a database of 40,000 scholarly public opinion poll respondents regarding their views on the HPV vaccine.
Individuals with cultural values favoring “authority” and/or “individualism” perceived the vaccine as risky, in part because they believed it would lead girls to engage in unsafe sex. But those favoring gender equality and/or community/government involvement in basic health care were more likely to see the vaccine as low risk and high benefit (Law Hum. Behav. 2010 Jan. 14 [doi:10.1007/s10979-009-9201-0
We all have suspected this to be the case, but now there are data to support that suspicion. Now here's the really interesting part: The researchers designed fictional “experts” who appeared to either share or oppose the respondents' cultural values. When views about HPV vaccines came from experts who respondents believed shared their values, they were more willing to accept the information. But when the views came from experts whom they perceived held values different from theirs, the subjects did not accept the experts' information.
So, when proauthority/individualism experts asserted the vaccine was risky, proauthority/individualism respondents agreed with them. When the egalitarian/procommunity experts argued that it was safe, egalitarian/procommunity respondents also agreed with them, solidifying overall disagreement about use of the vaccine.
However, when proauthority/individualism experts asserted that the vaccine was safe, proauthority/individualism respondents (who originally thought the vaccine was risky) moderated their original viewpoints, because the information came from experts who they perceived shared their values.
This held true for the opposite scenario, too: If egalitarian/procommunity experts argued the vaccine was risky, egalitarian/procommunity respondents shifted their belief toward its being risky.
As clinicians, we'd like to believe that our patients respect and trust us. But it's possible that when it comes to controversial recommendations, they may resist what we say if they don't identify enough with us based on our apparent values. If it is clear that our patient's family holds values widely disparate from ours, it might be helpful to utilize another more culturally congruent health professional in our practice to counsel about vaccination. This would vary by practice and from case to case, but could include people of similar race, religion, political viewpoint, or even regional accent.
Studies suggest that patients sometimes choose physicians to match their values. But with Medicaid and managed care, that may not always be possible. Using this type of approach may have more impact.
Surveys and discussion groups by the CDC suggest that scare tactics and scientific data may not successfully modify the opinion of parents who are disinclined toward vaccination (and I think most of us have the same experience). However, I did want to briefly mention recent data regarding HPV transmission in young adults that took me by surprise and may be persuasive for some patients.
Dr. Ann N. Burchell and her associates at McGill University, Montreal, evaluated female college/university students (aged 18-24 years) in self-described “stable” relationships exclusively with one male partner. The 263 couples had engaged in vaginal sex for a median of 3.9 months. HPV was detected in 64% of the couples. In 41% of the couples, both partners had the same HPV type. This risk of having the same strain was nearly four times more than what would be found by testing two random individuals. Also, oncogenic HPV-16 was the most common type, detected in 22% of couples (Epidemiology 2010;21:31-7).
In other words, one partner frequently came into the relationship with HPV and quickly transmitted it to the other. I was startled by the transmission frequency in these young adult females, who considered themselves in stable relationships. It suggests that acquisition is not just in early adolescence (although the risk of persistence is higher in that age group) and that catch-up immunization may be more important than some have thought. Perhaps these data won't convince all of your patients to get the HPV vaccine, but it may be helpful in some who are in their late teens or precollege age.
Flu Season Throws Some Clinical Curveballs
This year's influenza season, while mild so far, comes with a few of Mother Nature's curveballs that will impact our approach to prevention and treatment.
Normally, peak influenza activity hits by mid-January, and as of mid-January this year, the Centers for Disease Control and Prevention (CDC) had reported influenza in 49 of the 50 states. However, only one state (Virginia) has had widespread influenza activity, 5 have had regional activity, and 10 have had local disease activity. Sporadic activity has been reported in 33 states, the District of Columbia, and Puerto Rico. But at this writing in early February, we're just now seeing a notable increase in influenza-like illnesses and culture documentation that both influenza A and B have arrived here in Kansas City.
This late start sends a clear message about prevention: It's not too late to vaccinate. All children aged 6 months and older now are recommended to receive influenza vaccination. But because infants younger than 6 months are not eligible for influenza vaccine and antiviral medications are not indicated for those younger than 1 year, a “cocoon” strategy is best for infants. This approach works by immunizing the persons most in contact with infants—mostly family members, but ideally also the day care personnel, babysitters, etc., thereby creating a “zone of protection” around the child.
The CDC's Advisory Committee on Immunization Practices (ACIP) is moving toward a universal recommendation for all persons over age 6 months to receive the influenza vaccine. Expect that recommendation to be made within the next year. In the meantime, recent data suggest that cross-protection and protection in general is likely to be superior with intranasal vaccine, compared with injected vaccine. Unfortunately, the intranasal vaccine (FluMist) is not approved for use in children under 2 years old or adults older than 50 years. I'd like all health care staff to be able to receive it, and I wish the ACIP would recommend its use in the 50-plus age group, despite current labeling.
To date it appears that this season's influenza vaccines match the circulating A strains, while the influenza B match may not be quite as good. However, it's still too early to predict for certain because the number of isolates is small and so far mostly from only three states.
With regard to influenza treatment, the circulating strains thus far are presenting us with a clinical conundrum: For the last 2 years, we've been told to stop using rimantadine and amantadine because they don't work on influenza A (they were never effective for influenza B), and to restrict antiviral therapy to two available products, oseltamivir and zanamivir. Now we find that we need to partially reverse course. This year, two-thirds of typed circulating strains are H1N1 strains that are resistant to oseltamivir but surprisingly susceptible to rimantadine/amantadine.
Of the strains currently circulating, one-quarter is influenza B and is still susceptible to oseltamivir and zanamivir. Less than 10% of all circulating strains have been H3N2, and these also are still susceptible to oseltamivir and zanamivir, but resistant to rimantadine/amantadine, similar to last year. So far, the proportions of types A vs. B in Kansas City have been the same as the proportions reported nationally by the CDC.
So here's how it could work clinically: If the patient presents within 48 hours of fever onset and a rapid antigen test shows influenza B, you can proceed as in the last 2 years and treat with oseltamivir or zanamivir.
But if it's influenza A, it gets tricky: About 90% of the influenza As—the H1N1s—will be susceptible to rimantadine and resistant to oseltamivir, but the reverse is true for the 10% or so that are H3N2s. So for influenza A, it seems reasonable to offer rimantadine but explain that there's a 10% chance it won't work. Amantadine also is an option, although it has more frequent and often more severe side effects.
If the patient desires 100% certainty, the CDC says to consider both antivirals—rimantadine plus oseltamivir. We don't have prospective controlled data for using these two together, because this particular problem previously was not on our radar screen. Doing so also doubles the cost of treatment.
And here's another odd twist: Zanamivir, the neuraminidase-inhibitor cousin of oseltamivir, is still active against all circulating strains we've seen so far, including those that are resistant to oseltamivir. The problem with zanamivir, though, is that it's not approved in children under 7 years of age. Also, it is administered via rotahaler (also called a diskhaler), which can be tricky to manipulate. But if your patient is skilled in or capable of using this device, zanamivir is another option.
Remember, though, that these antiviral drugs are likely to reduce the duration of illness in otherwise normal influenza patients only if started within 2 days of fever onset, so the earlier we can intervene, the better. One study showed that starting oseltamivir within the first 12 hours of fever reduced illness by 3 days (41%) more than starting it at 48 hours of fever.
To be able to distinguish among the H1 and H3 influenza A strains, the most widely available tool is multiplex polymerase chain reaction. However, this can be expensive, ranging from $600 to $1,200 depending on the lab. Despite the conundrum posed by this year's A-strain divergent resistance, I don't think that these tests are worth the cost in outpatients. Consider such testing, however, in hospitalized patients or those at high risk for influenza complications, such as immunocompromised patients.
You can keep track of changes in influenza activity or resistance at www.cdc.gov/fluwww2a.cdc.gov/HAN/ArchiveSys/ViewMsgV.asp?AlertNum=00279
This year's influenza season, while mild so far, comes with a few of Mother Nature's curveballs that will impact our approach to prevention and treatment.
Normally, peak influenza activity hits by mid-January, and as of mid-January this year, the Centers for Disease Control and Prevention (CDC) had reported influenza in 49 of the 50 states. However, only one state (Virginia) has had widespread influenza activity, 5 have had regional activity, and 10 have had local disease activity. Sporadic activity has been reported in 33 states, the District of Columbia, and Puerto Rico. But at this writing in early February, we're just now seeing a notable increase in influenza-like illnesses and culture documentation that both influenza A and B have arrived here in Kansas City.
This late start sends a clear message about prevention: It's not too late to vaccinate. All children aged 6 months and older now are recommended to receive influenza vaccination. But because infants younger than 6 months are not eligible for influenza vaccine and antiviral medications are not indicated for those younger than 1 year, a “cocoon” strategy is best for infants. This approach works by immunizing the persons most in contact with infants—mostly family members, but ideally also the day care personnel, babysitters, etc., thereby creating a “zone of protection” around the child.
The CDC's Advisory Committee on Immunization Practices (ACIP) is moving toward a universal recommendation for all persons over age 6 months to receive the influenza vaccine. Expect that recommendation to be made within the next year. In the meantime, recent data suggest that cross-protection and protection in general is likely to be superior with intranasal vaccine, compared with injected vaccine. Unfortunately, the intranasal vaccine (FluMist) is not approved for use in children under 2 years old or adults older than 50 years. I'd like all health care staff to be able to receive it, and I wish the ACIP would recommend its use in the 50-plus age group, despite current labeling.
To date it appears that this season's influenza vaccines match the circulating A strains, while the influenza B match may not be quite as good. However, it's still too early to predict for certain because the number of isolates is small and so far mostly from only three states.
With regard to influenza treatment, the circulating strains thus far are presenting us with a clinical conundrum: For the last 2 years, we've been told to stop using rimantadine and amantadine because they don't work on influenza A (they were never effective for influenza B), and to restrict antiviral therapy to two available products, oseltamivir and zanamivir. Now we find that we need to partially reverse course. This year, two-thirds of typed circulating strains are H1N1 strains that are resistant to oseltamivir but surprisingly susceptible to rimantadine/amantadine.
Of the strains currently circulating, one-quarter is influenza B and is still susceptible to oseltamivir and zanamivir. Less than 10% of all circulating strains have been H3N2, and these also are still susceptible to oseltamivir and zanamivir, but resistant to rimantadine/amantadine, similar to last year. So far, the proportions of types A vs. B in Kansas City have been the same as the proportions reported nationally by the CDC.
So here's how it could work clinically: If the patient presents within 48 hours of fever onset and a rapid antigen test shows influenza B, you can proceed as in the last 2 years and treat with oseltamivir or zanamivir.
But if it's influenza A, it gets tricky: About 90% of the influenza As—the H1N1s—will be susceptible to rimantadine and resistant to oseltamivir, but the reverse is true for the 10% or so that are H3N2s. So for influenza A, it seems reasonable to offer rimantadine but explain that there's a 10% chance it won't work. Amantadine also is an option, although it has more frequent and often more severe side effects.
If the patient desires 100% certainty, the CDC says to consider both antivirals—rimantadine plus oseltamivir. We don't have prospective controlled data for using these two together, because this particular problem previously was not on our radar screen. Doing so also doubles the cost of treatment.
And here's another odd twist: Zanamivir, the neuraminidase-inhibitor cousin of oseltamivir, is still active against all circulating strains we've seen so far, including those that are resistant to oseltamivir. The problem with zanamivir, though, is that it's not approved in children under 7 years of age. Also, it is administered via rotahaler (also called a diskhaler), which can be tricky to manipulate. But if your patient is skilled in or capable of using this device, zanamivir is another option.
Remember, though, that these antiviral drugs are likely to reduce the duration of illness in otherwise normal influenza patients only if started within 2 days of fever onset, so the earlier we can intervene, the better. One study showed that starting oseltamivir within the first 12 hours of fever reduced illness by 3 days (41%) more than starting it at 48 hours of fever.
To be able to distinguish among the H1 and H3 influenza A strains, the most widely available tool is multiplex polymerase chain reaction. However, this can be expensive, ranging from $600 to $1,200 depending on the lab. Despite the conundrum posed by this year's A-strain divergent resistance, I don't think that these tests are worth the cost in outpatients. Consider such testing, however, in hospitalized patients or those at high risk for influenza complications, such as immunocompromised patients.
You can keep track of changes in influenza activity or resistance at www.cdc.gov/fluwww2a.cdc.gov/HAN/ArchiveSys/ViewMsgV.asp?AlertNum=00279
This year's influenza season, while mild so far, comes with a few of Mother Nature's curveballs that will impact our approach to prevention and treatment.
Normally, peak influenza activity hits by mid-January, and as of mid-January this year, the Centers for Disease Control and Prevention (CDC) had reported influenza in 49 of the 50 states. However, only one state (Virginia) has had widespread influenza activity, 5 have had regional activity, and 10 have had local disease activity. Sporadic activity has been reported in 33 states, the District of Columbia, and Puerto Rico. But at this writing in early February, we're just now seeing a notable increase in influenza-like illnesses and culture documentation that both influenza A and B have arrived here in Kansas City.
This late start sends a clear message about prevention: It's not too late to vaccinate. All children aged 6 months and older now are recommended to receive influenza vaccination. But because infants younger than 6 months are not eligible for influenza vaccine and antiviral medications are not indicated for those younger than 1 year, a “cocoon” strategy is best for infants. This approach works by immunizing the persons most in contact with infants—mostly family members, but ideally also the day care personnel, babysitters, etc., thereby creating a “zone of protection” around the child.
The CDC's Advisory Committee on Immunization Practices (ACIP) is moving toward a universal recommendation for all persons over age 6 months to receive the influenza vaccine. Expect that recommendation to be made within the next year. In the meantime, recent data suggest that cross-protection and protection in general is likely to be superior with intranasal vaccine, compared with injected vaccine. Unfortunately, the intranasal vaccine (FluMist) is not approved for use in children under 2 years old or adults older than 50 years. I'd like all health care staff to be able to receive it, and I wish the ACIP would recommend its use in the 50-plus age group, despite current labeling.
To date it appears that this season's influenza vaccines match the circulating A strains, while the influenza B match may not be quite as good. However, it's still too early to predict for certain because the number of isolates is small and so far mostly from only three states.
With regard to influenza treatment, the circulating strains thus far are presenting us with a clinical conundrum: For the last 2 years, we've been told to stop using rimantadine and amantadine because they don't work on influenza A (they were never effective for influenza B), and to restrict antiviral therapy to two available products, oseltamivir and zanamivir. Now we find that we need to partially reverse course. This year, two-thirds of typed circulating strains are H1N1 strains that are resistant to oseltamivir but surprisingly susceptible to rimantadine/amantadine.
Of the strains currently circulating, one-quarter is influenza B and is still susceptible to oseltamivir and zanamivir. Less than 10% of all circulating strains have been H3N2, and these also are still susceptible to oseltamivir and zanamivir, but resistant to rimantadine/amantadine, similar to last year. So far, the proportions of types A vs. B in Kansas City have been the same as the proportions reported nationally by the CDC.
So here's how it could work clinically: If the patient presents within 48 hours of fever onset and a rapid antigen test shows influenza B, you can proceed as in the last 2 years and treat with oseltamivir or zanamivir.
But if it's influenza A, it gets tricky: About 90% of the influenza As—the H1N1s—will be susceptible to rimantadine and resistant to oseltamivir, but the reverse is true for the 10% or so that are H3N2s. So for influenza A, it seems reasonable to offer rimantadine but explain that there's a 10% chance it won't work. Amantadine also is an option, although it has more frequent and often more severe side effects.
If the patient desires 100% certainty, the CDC says to consider both antivirals—rimantadine plus oseltamivir. We don't have prospective controlled data for using these two together, because this particular problem previously was not on our radar screen. Doing so also doubles the cost of treatment.
And here's another odd twist: Zanamivir, the neuraminidase-inhibitor cousin of oseltamivir, is still active against all circulating strains we've seen so far, including those that are resistant to oseltamivir. The problem with zanamivir, though, is that it's not approved in children under 7 years of age. Also, it is administered via rotahaler (also called a diskhaler), which can be tricky to manipulate. But if your patient is skilled in or capable of using this device, zanamivir is another option.
Remember, though, that these antiviral drugs are likely to reduce the duration of illness in otherwise normal influenza patients only if started within 2 days of fever onset, so the earlier we can intervene, the better. One study showed that starting oseltamivir within the first 12 hours of fever reduced illness by 3 days (41%) more than starting it at 48 hours of fever.
To be able to distinguish among the H1 and H3 influenza A strains, the most widely available tool is multiplex polymerase chain reaction. However, this can be expensive, ranging from $600 to $1,200 depending on the lab. Despite the conundrum posed by this year's A-strain divergent resistance, I don't think that these tests are worth the cost in outpatients. Consider such testing, however, in hospitalized patients or those at high risk for influenza complications, such as immunocompromised patients.
You can keep track of changes in influenza activity or resistance at www.cdc.gov/fluwww2a.cdc.gov/HAN/ArchiveSys/ViewMsgV.asp?AlertNum=00279
The Pitfalls in Diagnosing and Treating Mono
Mononucleosis is no stranger to most clinicians, who know it is most often caused by Epstein-Barr virus. Still, it presents diagnostic and management difficulties.
Consider a 12-year-old with 4 days of fever, headache, severe sore throat, and fatigue. Your exam detects bilateral, mildly tender, swollen (greater than 1 cm) anterior cervical lymph nodes and white tonsillar exudate, but no splenomegaly, which you know is only present in about 50% of children with EBV. Other EBV signs, such as supraorbital edema or maculopapular rash are absent, although they are seen in about 15%–20% of cases. A negative rapid streptococcal antigen and throat culture point to a virus (although 5%–25% of patients with EBV can have concomitant group A streptococcus). Now, how do you go about confirming EBV?
▸ Pitfall 1. Laboratory confirmation is unlikely until at least the second week of EBV illness. It is tempting to order serology (monospot-like test or EBV-specific serology) plus a CBC when the patient feels lousy and parents want answers. But keep in mind that in the first week negative serology doesn't rule out EBV and complete blood count results are usually nonspecific.
Not until the second week (or maybe even later), after illness onset, does the picture become clearer. At this point, nonspecific viral illnesses will usually have resolved and EBV infection becomes more likely if fever, sore throat, and cervical adenopathy continue (although they may be diminished), while fatigue is increasing. Splenomegaly also may develop in the interim with more generalized symmetrically bilateral adenopathy (groin, axilla, or posterior cervical).
Now, a CBC could suggest EBV mono via lymphocytosis (greater than 50% lymphocytes), and more than 10% atypical lymphocytes. In the case above, this result would allow a correct clinical diagnosis of EBV even without serology 90% of the time. However, not all patients with EBV will have this CBC result.
▸ Pitfall 2. Monospot-like tests have limitations. When the CBC is not sufficiently consistent with EBV but the clinical picture still suggests EBV mono in the second week of illness or later, then it's time for a nonspecific but quick and inexpensive serology—a monospot-like test. Contrary to what its name suggests—and to what one might believe—it does not detect EBV-specific antibody. Rather, it detects heterophile antibody, a low-affinity, highly cross-reactive IgM produced when EBV infects uncommitted B cells. Some of this nonspecific antibody cross-reacts with membrane antigens on mammalian red blood cells. (“Heterophile” refers to the cross-species affinity).
Monospot-like tests may not turn positive for up to 4 weeks. Moreover, children younger than 8 years are less likely to ever produce heterophile antibody, so the test isn't useful in that age group. In addition, a positive monospot isn't always caused by currently active mononucleosis. A rare individual can have persistent heterophile antibody years after recovery.
Also, some individuals who had EBV mono in the past may have a positive monospot because of amnestic responses while ill with an alternative virus, such as rubella. Other causes of false-positive monospots include malaria, autoimmune hepatitis, systemic lupus erythematosus, leukemia, pancreatic cancer, or, rarely, primary HIV infection (Am. J. Med. 2001;111:192-4). Of course, primary HIV is far less common than EBV, but should be kept in mind.
Still, EBV mono is the most likely diagnosis in a patient with a positive monospot who has had the classic symptoms.
▸ Pitfall 3. Specific EBV serology panels can be confusing. An EBV-specific antibody panel is the next step in the persistently ill patient with a negative monospot test. It not only nails down the diagnosis but also can tell us where the patient is in the course of infection. Depending on the laboratory, either three or four antibodies are included in the EBV panel:
The first is IgM to viral capsid antigen (VCA). It is initially positive in the second or third week of infection. It usually wanes by 2–4 weeks and may not develop at all in young children.
Next is IgG to VCA. It is initially positive in second to fourth week of infection and detectable for life.
Third is an antibody to early antigen (EA). It is usually present during EBV replication. (This is the one that some labs omit.)
Fourth is an antibody to EBV nuclear antigen (EBNA). Its presence coincides with recovery and arises beyond 6 weeks.
A positive IgM to only VCA confirms that the patient is early in course of EBV mono. A positive IgG to VCA, with or without a positive IgM to VCA, is also diagnostic of currently active mono.
However, if EBNA antibody is present, EBV is NOT the likely cause of the current problem. I use an EBNA mnemonic, “EB Not Active.” Occasionally an anti-EBNA-positive patient is entering recovery even if they don't feel well quite yet. We can assure them that they will feel better soon.
If EBV serology indicates recovery from a past EBV infection (positive for both IgG to VCA and anti-EBNA) or it is completely negative, a different cause for current symptoms could be sought by testing for cytomegalovirus, adenovirus, or Toxoplasma gondii.
EBV-mono patients should expect to have symptoms for at least 4–6 weeks before recovery. Reactivation may occur, but is nearly always asymptomatic or involves short-lived nonspecific symptoms. Chronic mono is so rare as to not be considered in primary care.
▸ Pitfall 4. Avoid having the patient stay too long on bed rest. Patients infected with EBV should be on bed rest only for the highly febrile stage, usually less than a week. We no longer recommend that they stay home from school or away from routine activities while riding out mononucleosis. Once the fever goes away, encourage patients to return to as much activity as their energy level will allow. The important exception is to refrain from contact sports as long as the spleen is palpable (and perhaps a little longer) to minimize chance of splenic rupture. I tell athletes to hang up the current sports season.
Patients kept in bed too long have more difficulty readjusting to normal life routines. Some may even experience clinical depression. It's important to consider how a patient with mono is coping psychologically when fatigue remains the main complaint.
▸ Pitfall 5. Active treatment is not usually helpful. Unfortunately, antivirals such as acyclovir don't work. Current consensus is not to give patients corticosteroids during acute mononucleosis. Steroids were postulated to speed recovery, and subjective mood improvement is possible due to the “steroid high” effect. However, in controlled trials they do not improve recovery other than reducing pain in first 12 hours (Cochrane Database Syst. Rev. 2006;3:CD004 402).
Further, steroids kill off defensive T cells that hold EBV-driven expansion of potentially malignant B cells in check. Such an imbalance could lead to later lymphoma. Although I don't think this is a huge risk, transient symptom relief does not seem worth the risk to me and I don't believe it's something we should do routinely. However, there are a few exceptions: The risk/benefit ratio changes in favor of corticosteroids if tonsillar swelling compromises the airway, or if there are other life-threatening EBV complications such as severe thrombocytopenia, neutropenia, or encephalitis.
But for uncomplicated EBV-mono, our best tools are ibuprofen, supportive care, and the tincture of time.
Mononucleosis is no stranger to most clinicians, who know it is most often caused by Epstein-Barr virus. Still, it presents diagnostic and management difficulties.
Consider a 12-year-old with 4 days of fever, headache, severe sore throat, and fatigue. Your exam detects bilateral, mildly tender, swollen (greater than 1 cm) anterior cervical lymph nodes and white tonsillar exudate, but no splenomegaly, which you know is only present in about 50% of children with EBV. Other EBV signs, such as supraorbital edema or maculopapular rash are absent, although they are seen in about 15%–20% of cases. A negative rapid streptococcal antigen and throat culture point to a virus (although 5%–25% of patients with EBV can have concomitant group A streptococcus). Now, how do you go about confirming EBV?
▸ Pitfall 1. Laboratory confirmation is unlikely until at least the second week of EBV illness. It is tempting to order serology (monospot-like test or EBV-specific serology) plus a CBC when the patient feels lousy and parents want answers. But keep in mind that in the first week negative serology doesn't rule out EBV and complete blood count results are usually nonspecific.
Not until the second week (or maybe even later), after illness onset, does the picture become clearer. At this point, nonspecific viral illnesses will usually have resolved and EBV infection becomes more likely if fever, sore throat, and cervical adenopathy continue (although they may be diminished), while fatigue is increasing. Splenomegaly also may develop in the interim with more generalized symmetrically bilateral adenopathy (groin, axilla, or posterior cervical).
Now, a CBC could suggest EBV mono via lymphocytosis (greater than 50% lymphocytes), and more than 10% atypical lymphocytes. In the case above, this result would allow a correct clinical diagnosis of EBV even without serology 90% of the time. However, not all patients with EBV will have this CBC result.
▸ Pitfall 2. Monospot-like tests have limitations. When the CBC is not sufficiently consistent with EBV but the clinical picture still suggests EBV mono in the second week of illness or later, then it's time for a nonspecific but quick and inexpensive serology—a monospot-like test. Contrary to what its name suggests—and to what one might believe—it does not detect EBV-specific antibody. Rather, it detects heterophile antibody, a low-affinity, highly cross-reactive IgM produced when EBV infects uncommitted B cells. Some of this nonspecific antibody cross-reacts with membrane antigens on mammalian red blood cells. (“Heterophile” refers to the cross-species affinity).
Monospot-like tests may not turn positive for up to 4 weeks. Moreover, children younger than 8 years are less likely to ever produce heterophile antibody, so the test isn't useful in that age group. In addition, a positive monospot isn't always caused by currently active mononucleosis. A rare individual can have persistent heterophile antibody years after recovery.
Also, some individuals who had EBV mono in the past may have a positive monospot because of amnestic responses while ill with an alternative virus, such as rubella. Other causes of false-positive monospots include malaria, autoimmune hepatitis, systemic lupus erythematosus, leukemia, pancreatic cancer, or, rarely, primary HIV infection (Am. J. Med. 2001;111:192-4). Of course, primary HIV is far less common than EBV, but should be kept in mind.
Still, EBV mono is the most likely diagnosis in a patient with a positive monospot who has had the classic symptoms.
▸ Pitfall 3. Specific EBV serology panels can be confusing. An EBV-specific antibody panel is the next step in the persistently ill patient with a negative monospot test. It not only nails down the diagnosis but also can tell us where the patient is in the course of infection. Depending on the laboratory, either three or four antibodies are included in the EBV panel:
The first is IgM to viral capsid antigen (VCA). It is initially positive in the second or third week of infection. It usually wanes by 2–4 weeks and may not develop at all in young children.
Next is IgG to VCA. It is initially positive in second to fourth week of infection and detectable for life.
Third is an antibody to early antigen (EA). It is usually present during EBV replication. (This is the one that some labs omit.)
Fourth is an antibody to EBV nuclear antigen (EBNA). Its presence coincides with recovery and arises beyond 6 weeks.
A positive IgM to only VCA confirms that the patient is early in course of EBV mono. A positive IgG to VCA, with or without a positive IgM to VCA, is also diagnostic of currently active mono.
However, if EBNA antibody is present, EBV is NOT the likely cause of the current problem. I use an EBNA mnemonic, “EB Not Active.” Occasionally an anti-EBNA-positive patient is entering recovery even if they don't feel well quite yet. We can assure them that they will feel better soon.
If EBV serology indicates recovery from a past EBV infection (positive for both IgG to VCA and anti-EBNA) or it is completely negative, a different cause for current symptoms could be sought by testing for cytomegalovirus, adenovirus, or Toxoplasma gondii.
EBV-mono patients should expect to have symptoms for at least 4–6 weeks before recovery. Reactivation may occur, but is nearly always asymptomatic or involves short-lived nonspecific symptoms. Chronic mono is so rare as to not be considered in primary care.
▸ Pitfall 4. Avoid having the patient stay too long on bed rest. Patients infected with EBV should be on bed rest only for the highly febrile stage, usually less than a week. We no longer recommend that they stay home from school or away from routine activities while riding out mononucleosis. Once the fever goes away, encourage patients to return to as much activity as their energy level will allow. The important exception is to refrain from contact sports as long as the spleen is palpable (and perhaps a little longer) to minimize chance of splenic rupture. I tell athletes to hang up the current sports season.
Patients kept in bed too long have more difficulty readjusting to normal life routines. Some may even experience clinical depression. It's important to consider how a patient with mono is coping psychologically when fatigue remains the main complaint.
▸ Pitfall 5. Active treatment is not usually helpful. Unfortunately, antivirals such as acyclovir don't work. Current consensus is not to give patients corticosteroids during acute mononucleosis. Steroids were postulated to speed recovery, and subjective mood improvement is possible due to the “steroid high” effect. However, in controlled trials they do not improve recovery other than reducing pain in first 12 hours (Cochrane Database Syst. Rev. 2006;3:CD004 402).
Further, steroids kill off defensive T cells that hold EBV-driven expansion of potentially malignant B cells in check. Such an imbalance could lead to later lymphoma. Although I don't think this is a huge risk, transient symptom relief does not seem worth the risk to me and I don't believe it's something we should do routinely. However, there are a few exceptions: The risk/benefit ratio changes in favor of corticosteroids if tonsillar swelling compromises the airway, or if there are other life-threatening EBV complications such as severe thrombocytopenia, neutropenia, or encephalitis.
But for uncomplicated EBV-mono, our best tools are ibuprofen, supportive care, and the tincture of time.
Mononucleosis is no stranger to most clinicians, who know it is most often caused by Epstein-Barr virus. Still, it presents diagnostic and management difficulties.
Consider a 12-year-old with 4 days of fever, headache, severe sore throat, and fatigue. Your exam detects bilateral, mildly tender, swollen (greater than 1 cm) anterior cervical lymph nodes and white tonsillar exudate, but no splenomegaly, which you know is only present in about 50% of children with EBV. Other EBV signs, such as supraorbital edema or maculopapular rash are absent, although they are seen in about 15%–20% of cases. A negative rapid streptococcal antigen and throat culture point to a virus (although 5%–25% of patients with EBV can have concomitant group A streptococcus). Now, how do you go about confirming EBV?
▸ Pitfall 1. Laboratory confirmation is unlikely until at least the second week of EBV illness. It is tempting to order serology (monospot-like test or EBV-specific serology) plus a CBC when the patient feels lousy and parents want answers. But keep in mind that in the first week negative serology doesn't rule out EBV and complete blood count results are usually nonspecific.
Not until the second week (or maybe even later), after illness onset, does the picture become clearer. At this point, nonspecific viral illnesses will usually have resolved and EBV infection becomes more likely if fever, sore throat, and cervical adenopathy continue (although they may be diminished), while fatigue is increasing. Splenomegaly also may develop in the interim with more generalized symmetrically bilateral adenopathy (groin, axilla, or posterior cervical).
Now, a CBC could suggest EBV mono via lymphocytosis (greater than 50% lymphocytes), and more than 10% atypical lymphocytes. In the case above, this result would allow a correct clinical diagnosis of EBV even without serology 90% of the time. However, not all patients with EBV will have this CBC result.
▸ Pitfall 2. Monospot-like tests have limitations. When the CBC is not sufficiently consistent with EBV but the clinical picture still suggests EBV mono in the second week of illness or later, then it's time for a nonspecific but quick and inexpensive serology—a monospot-like test. Contrary to what its name suggests—and to what one might believe—it does not detect EBV-specific antibody. Rather, it detects heterophile antibody, a low-affinity, highly cross-reactive IgM produced when EBV infects uncommitted B cells. Some of this nonspecific antibody cross-reacts with membrane antigens on mammalian red blood cells. (“Heterophile” refers to the cross-species affinity).
Monospot-like tests may not turn positive for up to 4 weeks. Moreover, children younger than 8 years are less likely to ever produce heterophile antibody, so the test isn't useful in that age group. In addition, a positive monospot isn't always caused by currently active mononucleosis. A rare individual can have persistent heterophile antibody years after recovery.
Also, some individuals who had EBV mono in the past may have a positive monospot because of amnestic responses while ill with an alternative virus, such as rubella. Other causes of false-positive monospots include malaria, autoimmune hepatitis, systemic lupus erythematosus, leukemia, pancreatic cancer, or, rarely, primary HIV infection (Am. J. Med. 2001;111:192-4). Of course, primary HIV is far less common than EBV, but should be kept in mind.
Still, EBV mono is the most likely diagnosis in a patient with a positive monospot who has had the classic symptoms.
▸ Pitfall 3. Specific EBV serology panels can be confusing. An EBV-specific antibody panel is the next step in the persistently ill patient with a negative monospot test. It not only nails down the diagnosis but also can tell us where the patient is in the course of infection. Depending on the laboratory, either three or four antibodies are included in the EBV panel:
The first is IgM to viral capsid antigen (VCA). It is initially positive in the second or third week of infection. It usually wanes by 2–4 weeks and may not develop at all in young children.
Next is IgG to VCA. It is initially positive in second to fourth week of infection and detectable for life.
Third is an antibody to early antigen (EA). It is usually present during EBV replication. (This is the one that some labs omit.)
Fourth is an antibody to EBV nuclear antigen (EBNA). Its presence coincides with recovery and arises beyond 6 weeks.
A positive IgM to only VCA confirms that the patient is early in course of EBV mono. A positive IgG to VCA, with or without a positive IgM to VCA, is also diagnostic of currently active mono.
However, if EBNA antibody is present, EBV is NOT the likely cause of the current problem. I use an EBNA mnemonic, “EB Not Active.” Occasionally an anti-EBNA-positive patient is entering recovery even if they don't feel well quite yet. We can assure them that they will feel better soon.
If EBV serology indicates recovery from a past EBV infection (positive for both IgG to VCA and anti-EBNA) or it is completely negative, a different cause for current symptoms could be sought by testing for cytomegalovirus, adenovirus, or Toxoplasma gondii.
EBV-mono patients should expect to have symptoms for at least 4–6 weeks before recovery. Reactivation may occur, but is nearly always asymptomatic or involves short-lived nonspecific symptoms. Chronic mono is so rare as to not be considered in primary care.
▸ Pitfall 4. Avoid having the patient stay too long on bed rest. Patients infected with EBV should be on bed rest only for the highly febrile stage, usually less than a week. We no longer recommend that they stay home from school or away from routine activities while riding out mononucleosis. Once the fever goes away, encourage patients to return to as much activity as their energy level will allow. The important exception is to refrain from contact sports as long as the spleen is palpable (and perhaps a little longer) to minimize chance of splenic rupture. I tell athletes to hang up the current sports season.
Patients kept in bed too long have more difficulty readjusting to normal life routines. Some may even experience clinical depression. It's important to consider how a patient with mono is coping psychologically when fatigue remains the main complaint.
▸ Pitfall 5. Active treatment is not usually helpful. Unfortunately, antivirals such as acyclovir don't work. Current consensus is not to give patients corticosteroids during acute mononucleosis. Steroids were postulated to speed recovery, and subjective mood improvement is possible due to the “steroid high” effect. However, in controlled trials they do not improve recovery other than reducing pain in first 12 hours (Cochrane Database Syst. Rev. 2006;3:CD004 402).
Further, steroids kill off defensive T cells that hold EBV-driven expansion of potentially malignant B cells in check. Such an imbalance could lead to later lymphoma. Although I don't think this is a huge risk, transient symptom relief does not seem worth the risk to me and I don't believe it's something we should do routinely. However, there are a few exceptions: The risk/benefit ratio changes in favor of corticosteroids if tonsillar swelling compromises the airway, or if there are other life-threatening EBV complications such as severe thrombocytopenia, neutropenia, or encephalitis.
But for uncomplicated EBV-mono, our best tools are ibuprofen, supportive care, and the tincture of time.
Let's Reexamine the Treatment of URIs
The data from three recent studies should prompt us to reexamine our approach to the management of upper respiratory infections in children.
Guidelines from the American Academy of Pediatrics recommend antimicrobial treatment for children with upper respiratory symptoms lasting longer than 10-14 days or for those with severe symptoms, including a high fever and toxicity (Pediatrics 2001;108:798-808). The Sinus and Allergy Health Partnership Guidelines—to which I contributed—also advised antimicrobial treatment for children with signs and symptoms of viral upper respiratory infection (URI) for more than 10 days or worsening symptoms after 5-7 days (Int. J. Pediatr. Otorhinolaryngol. 2002;63:1-13).
Now data suggest that we perhaps should consider antibiotic treatment only for children whose symptoms are worsening after 10 days.
The recommendation to treat rhinorrhea beyond 10 days with antibiotics as presumptive bacterial sinusitis requires a subjective judgment, and is based on small data sets.
This is problematic in an era in which we're trying to limit antimicrobial use to times when there is definite benefit. It's also been difficult to follow in practice, because parents often bring a child in who has had symptoms for fewer than 10 days. We're not supposed to treat at that point unless they have acute toxicity, but there can be a lot of real or perceived pressure to prescribe.
In fact, the 10-day rule appears to derive from a 40-year-old study on rhinovirus in adults (JAMA 1967;202;494-500). Surprisingly, it wasn't until earlier this year that good data became available regarding the symptom profile of colds in otherwise healthy school-aged children. In that study, which utilized nasopharyngeal aspirates and symptom diaries, 73% of 81 children with colds continued to be symptomatic 10 days after onset (Pediatr. Infect. Dis. J. 2008;27:8-11).
These new findings suggest we've probably been overtreating a proportion of school-aged children—for bacterial sinusitis—when they actually have had mild to moderate upper respiratory symptoms. Further, these data should provide reassurance that we're not putting such patients at risk for invasive complications if we don't treat before 10 days of illness, as long as they do not fit the acute severe criteria or the symptoms aren't getting rapidly worse.
Data from another recent study suggest that children with acute sinusitis who are destined to develop subperiosteal orbital abscess (SPA) typically do so well before 10 days of rhinorrhea. In this 10-year retrospective chart review from a tertiary pediatric center, 39 children required operative drainage for SPA, with only a mean of 1.6 days of antibiotics prediagnosis in just 10 (26%). Of the 28 children presenting with fever, the mean duration was 2.5 days. Only 28 had rhinorrhea/mucoid discharge, and that for a mean duration of 3.9 days (Int. J. Pediatr. Otorhinolaryngol. 2007;71:1003-6). Thus, complications arose in the first days of symptoms, even among those children on antibiotics.
Since it's not feasible—or wise—to give antibiotics to every child with cold symptoms in order to prevent SPA, the authors concluded that “SPA may not be a preventable complication of acute sinusitis in children” using standard oral antibiotics. Indeed, this paper suggests that children destined to develop complications are by and large not the ones who appear in your office with mild symptoms at days 4 to 7.
If the child has high fever and facial pain or swelling, there's little question you're going to treat. But for those without clear signs of toxicity or rapidly progressing disease, complications seem unlikely after 4 days.
A third study, of pneumococcal mastoiditis complicating acute otitis media (AOM), suggests that severe complications of URIs in children are becoming more difficult to treat with our usual oral drugs because of the emergence of multidrug-resistant pneumococcal serotype 19A, a strain that is not included in the 7-valent pneumococcal conjugate vaccine (PCV7).
Among 41 children with pneumococcal mastoiditis (mean age 23 months, range 3 months-12 years) who were seen at Texas Children's Hospital, Houston, between January 2005 and June 2007, 19 cases were caused by 19A. That strain was responsible for all cases of pneumococcal mastoiditis seen in 2006 and 2007, compared with just three of six seen between 2004 and 2005, and just one of two in 2003 (Pediatrics 2008;122:34-9).
Even more worrisome, all of the children with 19A mastoiditis had SPA, compared with only 2 of the 22 children with non-19A mastoiditis. Mastoidectomy was required in 17 of the 19A group (89%) compared with just 10 (45%) of those with non-19A strains. Thirteen of the 19A isolates (68%) were resistant to all antibiotics tested routinely.
These data correspond to what I've been seeing at my institution. We're seeing less otitis and sinusitis overall since the introduction of PCV7 in 2000. A concern in the last 2-3 years is that the incidence of difficult-to-treat pneumococcal mastoiditis—nearly all due to 19A—has risen among the difficult-to-treat AOM that does occur. In fact, I'm now seeing as much serious invasive pneumococcal disease as before PCV7 was licensed, nearly half due to 19A.
I believe there are two messages here. First, if you withhold antibiotics for 10 days in a nontoxic child with rhinorrhea, according to the guidelines, you probably aren't putting him or her at any greater risk for complicated sinus disease; even treating then is likely to overtreat a proportion of children. Second, we may need a new strategy for persistent or complicated AOM when 19A is the pathogen. These cases may not even respond to clindamycin or three doses of ceftriaxone and may require linezolid or a quinolone (JAMA 2007;298:1772-8) despite the new Food and Drug Administration black box warning on quinolones, usually along with a subspecialty consultation.
But there is hope on the horizon. Wyeth Pharmaceuticals, which partially funded the Texas mastoiditis study, announced at the end of May that the FDA has granted fast-track designation to the company's investigational 13-valent pneumococcal conjugate vaccine for infants and toddlers. That vaccine contains 19A as well as serotypes 1 and 3, the most common causes of empyema.
It's becoming obvious that we will need to stay ahead of the game from now on. Ongoing surveillance will be critical as we move forward.
I have no current disclosures for any products mentioned in this article.
The data from three recent studies should prompt us to reexamine our approach to the management of upper respiratory infections in children.
Guidelines from the American Academy of Pediatrics recommend antimicrobial treatment for children with upper respiratory symptoms lasting longer than 10-14 days or for those with severe symptoms, including a high fever and toxicity (Pediatrics 2001;108:798-808). The Sinus and Allergy Health Partnership Guidelines—to which I contributed—also advised antimicrobial treatment for children with signs and symptoms of viral upper respiratory infection (URI) for more than 10 days or worsening symptoms after 5-7 days (Int. J. Pediatr. Otorhinolaryngol. 2002;63:1-13).
Now data suggest that we perhaps should consider antibiotic treatment only for children whose symptoms are worsening after 10 days.
The recommendation to treat rhinorrhea beyond 10 days with antibiotics as presumptive bacterial sinusitis requires a subjective judgment, and is based on small data sets.
This is problematic in an era in which we're trying to limit antimicrobial use to times when there is definite benefit. It's also been difficult to follow in practice, because parents often bring a child in who has had symptoms for fewer than 10 days. We're not supposed to treat at that point unless they have acute toxicity, but there can be a lot of real or perceived pressure to prescribe.
In fact, the 10-day rule appears to derive from a 40-year-old study on rhinovirus in adults (JAMA 1967;202;494-500). Surprisingly, it wasn't until earlier this year that good data became available regarding the symptom profile of colds in otherwise healthy school-aged children. In that study, which utilized nasopharyngeal aspirates and symptom diaries, 73% of 81 children with colds continued to be symptomatic 10 days after onset (Pediatr. Infect. Dis. J. 2008;27:8-11).
These new findings suggest we've probably been overtreating a proportion of school-aged children—for bacterial sinusitis—when they actually have had mild to moderate upper respiratory symptoms. Further, these data should provide reassurance that we're not putting such patients at risk for invasive complications if we don't treat before 10 days of illness, as long as they do not fit the acute severe criteria or the symptoms aren't getting rapidly worse.
Data from another recent study suggest that children with acute sinusitis who are destined to develop subperiosteal orbital abscess (SPA) typically do so well before 10 days of rhinorrhea. In this 10-year retrospective chart review from a tertiary pediatric center, 39 children required operative drainage for SPA, with only a mean of 1.6 days of antibiotics prediagnosis in just 10 (26%). Of the 28 children presenting with fever, the mean duration was 2.5 days. Only 28 had rhinorrhea/mucoid discharge, and that for a mean duration of 3.9 days (Int. J. Pediatr. Otorhinolaryngol. 2007;71:1003-6). Thus, complications arose in the first days of symptoms, even among those children on antibiotics.
Since it's not feasible—or wise—to give antibiotics to every child with cold symptoms in order to prevent SPA, the authors concluded that “SPA may not be a preventable complication of acute sinusitis in children” using standard oral antibiotics. Indeed, this paper suggests that children destined to develop complications are by and large not the ones who appear in your office with mild symptoms at days 4 to 7.
If the child has high fever and facial pain or swelling, there's little question you're going to treat. But for those without clear signs of toxicity or rapidly progressing disease, complications seem unlikely after 4 days.
A third study, of pneumococcal mastoiditis complicating acute otitis media (AOM), suggests that severe complications of URIs in children are becoming more difficult to treat with our usual oral drugs because of the emergence of multidrug-resistant pneumococcal serotype 19A, a strain that is not included in the 7-valent pneumococcal conjugate vaccine (PCV7).
Among 41 children with pneumococcal mastoiditis (mean age 23 months, range 3 months-12 years) who were seen at Texas Children's Hospital, Houston, between January 2005 and June 2007, 19 cases were caused by 19A. That strain was responsible for all cases of pneumococcal mastoiditis seen in 2006 and 2007, compared with just three of six seen between 2004 and 2005, and just one of two in 2003 (Pediatrics 2008;122:34-9).
Even more worrisome, all of the children with 19A mastoiditis had SPA, compared with only 2 of the 22 children with non-19A mastoiditis. Mastoidectomy was required in 17 of the 19A group (89%) compared with just 10 (45%) of those with non-19A strains. Thirteen of the 19A isolates (68%) were resistant to all antibiotics tested routinely.
These data correspond to what I've been seeing at my institution. We're seeing less otitis and sinusitis overall since the introduction of PCV7 in 2000. A concern in the last 2-3 years is that the incidence of difficult-to-treat pneumococcal mastoiditis—nearly all due to 19A—has risen among the difficult-to-treat AOM that does occur. In fact, I'm now seeing as much serious invasive pneumococcal disease as before PCV7 was licensed, nearly half due to 19A.
I believe there are two messages here. First, if you withhold antibiotics for 10 days in a nontoxic child with rhinorrhea, according to the guidelines, you probably aren't putting him or her at any greater risk for complicated sinus disease; even treating then is likely to overtreat a proportion of children. Second, we may need a new strategy for persistent or complicated AOM when 19A is the pathogen. These cases may not even respond to clindamycin or three doses of ceftriaxone and may require linezolid or a quinolone (JAMA 2007;298:1772-8) despite the new Food and Drug Administration black box warning on quinolones, usually along with a subspecialty consultation.
But there is hope on the horizon. Wyeth Pharmaceuticals, which partially funded the Texas mastoiditis study, announced at the end of May that the FDA has granted fast-track designation to the company's investigational 13-valent pneumococcal conjugate vaccine for infants and toddlers. That vaccine contains 19A as well as serotypes 1 and 3, the most common causes of empyema.
It's becoming obvious that we will need to stay ahead of the game from now on. Ongoing surveillance will be critical as we move forward.
I have no current disclosures for any products mentioned in this article.
The data from three recent studies should prompt us to reexamine our approach to the management of upper respiratory infections in children.
Guidelines from the American Academy of Pediatrics recommend antimicrobial treatment for children with upper respiratory symptoms lasting longer than 10-14 days or for those with severe symptoms, including a high fever and toxicity (Pediatrics 2001;108:798-808). The Sinus and Allergy Health Partnership Guidelines—to which I contributed—also advised antimicrobial treatment for children with signs and symptoms of viral upper respiratory infection (URI) for more than 10 days or worsening symptoms after 5-7 days (Int. J. Pediatr. Otorhinolaryngol. 2002;63:1-13).
Now data suggest that we perhaps should consider antibiotic treatment only for children whose symptoms are worsening after 10 days.
The recommendation to treat rhinorrhea beyond 10 days with antibiotics as presumptive bacterial sinusitis requires a subjective judgment, and is based on small data sets.
This is problematic in an era in which we're trying to limit antimicrobial use to times when there is definite benefit. It's also been difficult to follow in practice, because parents often bring a child in who has had symptoms for fewer than 10 days. We're not supposed to treat at that point unless they have acute toxicity, but there can be a lot of real or perceived pressure to prescribe.
In fact, the 10-day rule appears to derive from a 40-year-old study on rhinovirus in adults (JAMA 1967;202;494-500). Surprisingly, it wasn't until earlier this year that good data became available regarding the symptom profile of colds in otherwise healthy school-aged children. In that study, which utilized nasopharyngeal aspirates and symptom diaries, 73% of 81 children with colds continued to be symptomatic 10 days after onset (Pediatr. Infect. Dis. J. 2008;27:8-11).
These new findings suggest we've probably been overtreating a proportion of school-aged children—for bacterial sinusitis—when they actually have had mild to moderate upper respiratory symptoms. Further, these data should provide reassurance that we're not putting such patients at risk for invasive complications if we don't treat before 10 days of illness, as long as they do not fit the acute severe criteria or the symptoms aren't getting rapidly worse.
Data from another recent study suggest that children with acute sinusitis who are destined to develop subperiosteal orbital abscess (SPA) typically do so well before 10 days of rhinorrhea. In this 10-year retrospective chart review from a tertiary pediatric center, 39 children required operative drainage for SPA, with only a mean of 1.6 days of antibiotics prediagnosis in just 10 (26%). Of the 28 children presenting with fever, the mean duration was 2.5 days. Only 28 had rhinorrhea/mucoid discharge, and that for a mean duration of 3.9 days (Int. J. Pediatr. Otorhinolaryngol. 2007;71:1003-6). Thus, complications arose in the first days of symptoms, even among those children on antibiotics.
Since it's not feasible—or wise—to give antibiotics to every child with cold symptoms in order to prevent SPA, the authors concluded that “SPA may not be a preventable complication of acute sinusitis in children” using standard oral antibiotics. Indeed, this paper suggests that children destined to develop complications are by and large not the ones who appear in your office with mild symptoms at days 4 to 7.
If the child has high fever and facial pain or swelling, there's little question you're going to treat. But for those without clear signs of toxicity or rapidly progressing disease, complications seem unlikely after 4 days.
A third study, of pneumococcal mastoiditis complicating acute otitis media (AOM), suggests that severe complications of URIs in children are becoming more difficult to treat with our usual oral drugs because of the emergence of multidrug-resistant pneumococcal serotype 19A, a strain that is not included in the 7-valent pneumococcal conjugate vaccine (PCV7).
Among 41 children with pneumococcal mastoiditis (mean age 23 months, range 3 months-12 years) who were seen at Texas Children's Hospital, Houston, between January 2005 and June 2007, 19 cases were caused by 19A. That strain was responsible for all cases of pneumococcal mastoiditis seen in 2006 and 2007, compared with just three of six seen between 2004 and 2005, and just one of two in 2003 (Pediatrics 2008;122:34-9).
Even more worrisome, all of the children with 19A mastoiditis had SPA, compared with only 2 of the 22 children with non-19A mastoiditis. Mastoidectomy was required in 17 of the 19A group (89%) compared with just 10 (45%) of those with non-19A strains. Thirteen of the 19A isolates (68%) were resistant to all antibiotics tested routinely.
These data correspond to what I've been seeing at my institution. We're seeing less otitis and sinusitis overall since the introduction of PCV7 in 2000. A concern in the last 2-3 years is that the incidence of difficult-to-treat pneumococcal mastoiditis—nearly all due to 19A—has risen among the difficult-to-treat AOM that does occur. In fact, I'm now seeing as much serious invasive pneumococcal disease as before PCV7 was licensed, nearly half due to 19A.
I believe there are two messages here. First, if you withhold antibiotics for 10 days in a nontoxic child with rhinorrhea, according to the guidelines, you probably aren't putting him or her at any greater risk for complicated sinus disease; even treating then is likely to overtreat a proportion of children. Second, we may need a new strategy for persistent or complicated AOM when 19A is the pathogen. These cases may not even respond to clindamycin or three doses of ceftriaxone and may require linezolid or a quinolone (JAMA 2007;298:1772-8) despite the new Food and Drug Administration black box warning on quinolones, usually along with a subspecialty consultation.
But there is hope on the horizon. Wyeth Pharmaceuticals, which partially funded the Texas mastoiditis study, announced at the end of May that the FDA has granted fast-track designation to the company's investigational 13-valent pneumococcal conjugate vaccine for infants and toddlers. That vaccine contains 19A as well as serotypes 1 and 3, the most common causes of empyema.
It's becoming obvious that we will need to stay ahead of the game from now on. Ongoing surveillance will be critical as we move forward.
I have no current disclosures for any products mentioned in this article.
Adenovirus Serotype 14: One of Nature's Pathogen Cycles
Acute respiratory disease associated with emerging adenovirus serotype 14 that caused nine deaths last fall in the United States is a development worth noting, but there seems little reason to fear this strain will lead to larger ongoing outbreaks of “killer colds.”
In fact, it is probably part of a natural life cycle that has been going on for millennia. We're only learning of these mutations in recent years because of active surveillance that the Centers for Disease Control and Prevention now routinely conducts at sentinel sites around the country. When the system detects something noteworthy, the findings are published in the Morbidity and Mortality Weekly Report (MMWR). Media are mining the MMWR for stories, we're seeing frequent infectious disease stories with alarmist headlines. We should be prepared to explain them to worried parents of patients.
As we know, adenovirus typically isn't a life-threatening problem. In 99% of cases it's a self-limited infection that causes conjunctivitis, rhinorrhea, exudative pharyngitis, and/or fever for 3–8 days.
Adenovirus serotype 14 (Ad14) does appear to be a bit different, though: In May 2006, a 12-day-old infant in New York died of respiratory illness caused by “Ad14.” From March to June 2007, a total of 140 additional cases of confirmed Ad14 respiratory illness were identified in clusters of patients in Oregon, Washington, and Texas. Of those, 38% were hospitalized and 5% died (MMWR 2007;56:1181–4). Deaths were due to progressive pneumonias, not colds.
It's possible that Ad14 produces less frequent disease in young children than in the elderly, considering the relative ages of Ad14 patients. Among 12 cases with available medical information in Oregon, 11 (92%) of non-type-14 adenovirus patients were younger than 5 years, compared with only 5 (17%) out of 30 cases of Ad14. There was, however, one death in a 1-month-old in the Ad14 group.
But Ad14 is not new. It was initially described in 1955, and was associated with epidemic of acute respiratory disease in military recruits in Europe in 1969. According to the CDC, in 2001–2002 it was reported to be associated with approximately 8% of all respiratory adenoviral infections in the pediatric ward of a Taiwan Hospital.
However, because Ad14 hasn't been circulating in a while—it's just one of at least 42 different adenovirus strains—the current population isn't likely to be immune. While most healthy individuals are still able to mount an immune response to it, certain susceptible people will become more ill, including the very old, the very young, and those with compromised immune systems; perhaps some healthy people will have a genetic predisposition that makes them more vulnerable.
Indeed, a single nucleotide polymorphism (SNP)—one change in the DNA of a key gene—can have a dramatic effect on how a person responds to environmental or infectious triggers. Consider as an example the case of a disease that we are more familiar with than Ad14, respiratory syncytial virus (RSV) in children. Hospitalization and more severe symptoms have been demonstrated with one SNP (Pediatr. Infect. Dis. J. 2007;26:1094–8), and it's likely that similar mechanisms explain some of the variation in disease severity with other viruses as well. We're just beginning to learn about these mechanisms within the innate immune system.
In the meantime, we might want to consider obtaining viral cultures—commercially available testing systems do include adenovirus—in hospitalized pneumonia patients who do not have positive RSV or influenza rapid tests and who do not improve quickly despite appropriate supportive and perhaps empiric antibiotic therapy.
Even though we don't have a commonly used effective antiviral for adenovirus, such as oseltamivir for influenza, it can be important to be aware of cases of severe adenovirus occurring outside of the surveillance network. While Ad14 is a recent culprit, other serotypes also have been implicated in sporadic outbreaks. Also, if you have a firm viral diagnosis, you don't need to keep escalating broad-spectrum antibiotics. Adenovirus can initially mimic the high fever, leukocytosis, and ill-appearing presentation of bacterial pneumonia, particularly in young children. The x-ray findings usually are bilateral and patchy initially, but the infiltrates can become dense and appear more “bacterial” as time goes on.
Another reason to culture for adenovirus is its potential to mimic Kawasaki disease, with the nonpurulent conjunctivitis, red throat, mucositis, high fever, and swollen lymph nodes. If you can confirm that the child actually has adenovirus during the Kawasaki work-up, you can save thousands of dollars that would otherwise be spent on intravenous immune globulin (IVIG) therapy. Of course, to be really useful, you'll need to get the viral cultures early, because current culture techniques—shell vial or standard—require anywhere from 48 hours to 7 days for results to develop. Because the window of effective use of IVIG in Kawasaki disease is within 10 days of the onset of fever, getting viral cultures more than 5 days into the fever may not give you time to make the diagnosis before you will need to empirically use IVIG.
New multiplex polymerase chain reaction (PCR) technology should improve on that situation in the near future. Already in use at some teaching institutions, multiplex PCR improves the diagnostic capacity of traditional PCR by amplifying target sequences of multiple viruses all at once. The technology allows you to order a panel of 17–20 different viral tests in one batch and get the results back in a day (J. Clin. Microbiol. 2007;45:2965–70), at a cost of not much more than the $150-$200 for the current viral panel of just 6 or 7.
Another new technology on the horizon—flocked nasal swabs—will make it easier to obtain the sample from the child. Currently approved for use in adults, the swabs are made with perpendicular nylon fibers that allow you to collect epithelial cells and surrounding pathogens with a few simple twirls in the nares, a technique far more comfortable for the patient than a nasal wash. Data from the company's abstracts suggest that the sample you get from the swab is equivalent to that from the nasal wash (information available at www.copanusa.com
These new modalities together should make viral testing as simple as taking a throat culture for the group A streptococcus bacterium, and allow us to obtain timely information that is more pertinent while the child is still sick. But at the same time we need to remind our patients—and ourselves—that in the vast majority of cases we're not talking about a “killer” disease, even with adenovirus.
Acute respiratory disease associated with emerging adenovirus serotype 14 that caused nine deaths last fall in the United States is a development worth noting, but there seems little reason to fear this strain will lead to larger ongoing outbreaks of “killer colds.”
In fact, it is probably part of a natural life cycle that has been going on for millennia. We're only learning of these mutations in recent years because of active surveillance that the Centers for Disease Control and Prevention now routinely conducts at sentinel sites around the country. When the system detects something noteworthy, the findings are published in the Morbidity and Mortality Weekly Report (MMWR). Media are mining the MMWR for stories, we're seeing frequent infectious disease stories with alarmist headlines. We should be prepared to explain them to worried parents of patients.
As we know, adenovirus typically isn't a life-threatening problem. In 99% of cases it's a self-limited infection that causes conjunctivitis, rhinorrhea, exudative pharyngitis, and/or fever for 3–8 days.
Adenovirus serotype 14 (Ad14) does appear to be a bit different, though: In May 2006, a 12-day-old infant in New York died of respiratory illness caused by “Ad14.” From March to June 2007, a total of 140 additional cases of confirmed Ad14 respiratory illness were identified in clusters of patients in Oregon, Washington, and Texas. Of those, 38% were hospitalized and 5% died (MMWR 2007;56:1181–4). Deaths were due to progressive pneumonias, not colds.
It's possible that Ad14 produces less frequent disease in young children than in the elderly, considering the relative ages of Ad14 patients. Among 12 cases with available medical information in Oregon, 11 (92%) of non-type-14 adenovirus patients were younger than 5 years, compared with only 5 (17%) out of 30 cases of Ad14. There was, however, one death in a 1-month-old in the Ad14 group.
But Ad14 is not new. It was initially described in 1955, and was associated with epidemic of acute respiratory disease in military recruits in Europe in 1969. According to the CDC, in 2001–2002 it was reported to be associated with approximately 8% of all respiratory adenoviral infections in the pediatric ward of a Taiwan Hospital.
However, because Ad14 hasn't been circulating in a while—it's just one of at least 42 different adenovirus strains—the current population isn't likely to be immune. While most healthy individuals are still able to mount an immune response to it, certain susceptible people will become more ill, including the very old, the very young, and those with compromised immune systems; perhaps some healthy people will have a genetic predisposition that makes them more vulnerable.
Indeed, a single nucleotide polymorphism (SNP)—one change in the DNA of a key gene—can have a dramatic effect on how a person responds to environmental or infectious triggers. Consider as an example the case of a disease that we are more familiar with than Ad14, respiratory syncytial virus (RSV) in children. Hospitalization and more severe symptoms have been demonstrated with one SNP (Pediatr. Infect. Dis. J. 2007;26:1094–8), and it's likely that similar mechanisms explain some of the variation in disease severity with other viruses as well. We're just beginning to learn about these mechanisms within the innate immune system.
In the meantime, we might want to consider obtaining viral cultures—commercially available testing systems do include adenovirus—in hospitalized pneumonia patients who do not have positive RSV or influenza rapid tests and who do not improve quickly despite appropriate supportive and perhaps empiric antibiotic therapy.
Even though we don't have a commonly used effective antiviral for adenovirus, such as oseltamivir for influenza, it can be important to be aware of cases of severe adenovirus occurring outside of the surveillance network. While Ad14 is a recent culprit, other serotypes also have been implicated in sporadic outbreaks. Also, if you have a firm viral diagnosis, you don't need to keep escalating broad-spectrum antibiotics. Adenovirus can initially mimic the high fever, leukocytosis, and ill-appearing presentation of bacterial pneumonia, particularly in young children. The x-ray findings usually are bilateral and patchy initially, but the infiltrates can become dense and appear more “bacterial” as time goes on.
Another reason to culture for adenovirus is its potential to mimic Kawasaki disease, with the nonpurulent conjunctivitis, red throat, mucositis, high fever, and swollen lymph nodes. If you can confirm that the child actually has adenovirus during the Kawasaki work-up, you can save thousands of dollars that would otherwise be spent on intravenous immune globulin (IVIG) therapy. Of course, to be really useful, you'll need to get the viral cultures early, because current culture techniques—shell vial or standard—require anywhere from 48 hours to 7 days for results to develop. Because the window of effective use of IVIG in Kawasaki disease is within 10 days of the onset of fever, getting viral cultures more than 5 days into the fever may not give you time to make the diagnosis before you will need to empirically use IVIG.
New multiplex polymerase chain reaction (PCR) technology should improve on that situation in the near future. Already in use at some teaching institutions, multiplex PCR improves the diagnostic capacity of traditional PCR by amplifying target sequences of multiple viruses all at once. The technology allows you to order a panel of 17–20 different viral tests in one batch and get the results back in a day (J. Clin. Microbiol. 2007;45:2965–70), at a cost of not much more than the $150-$200 for the current viral panel of just 6 or 7.
Another new technology on the horizon—flocked nasal swabs—will make it easier to obtain the sample from the child. Currently approved for use in adults, the swabs are made with perpendicular nylon fibers that allow you to collect epithelial cells and surrounding pathogens with a few simple twirls in the nares, a technique far more comfortable for the patient than a nasal wash. Data from the company's abstracts suggest that the sample you get from the swab is equivalent to that from the nasal wash (information available at www.copanusa.com
These new modalities together should make viral testing as simple as taking a throat culture for the group A streptococcus bacterium, and allow us to obtain timely information that is more pertinent while the child is still sick. But at the same time we need to remind our patients—and ourselves—that in the vast majority of cases we're not talking about a “killer” disease, even with adenovirus.
Acute respiratory disease associated with emerging adenovirus serotype 14 that caused nine deaths last fall in the United States is a development worth noting, but there seems little reason to fear this strain will lead to larger ongoing outbreaks of “killer colds.”
In fact, it is probably part of a natural life cycle that has been going on for millennia. We're only learning of these mutations in recent years because of active surveillance that the Centers for Disease Control and Prevention now routinely conducts at sentinel sites around the country. When the system detects something noteworthy, the findings are published in the Morbidity and Mortality Weekly Report (MMWR). Media are mining the MMWR for stories, we're seeing frequent infectious disease stories with alarmist headlines. We should be prepared to explain them to worried parents of patients.
As we know, adenovirus typically isn't a life-threatening problem. In 99% of cases it's a self-limited infection that causes conjunctivitis, rhinorrhea, exudative pharyngitis, and/or fever for 3–8 days.
Adenovirus serotype 14 (Ad14) does appear to be a bit different, though: In May 2006, a 12-day-old infant in New York died of respiratory illness caused by “Ad14.” From March to June 2007, a total of 140 additional cases of confirmed Ad14 respiratory illness were identified in clusters of patients in Oregon, Washington, and Texas. Of those, 38% were hospitalized and 5% died (MMWR 2007;56:1181–4). Deaths were due to progressive pneumonias, not colds.
It's possible that Ad14 produces less frequent disease in young children than in the elderly, considering the relative ages of Ad14 patients. Among 12 cases with available medical information in Oregon, 11 (92%) of non-type-14 adenovirus patients were younger than 5 years, compared with only 5 (17%) out of 30 cases of Ad14. There was, however, one death in a 1-month-old in the Ad14 group.
But Ad14 is not new. It was initially described in 1955, and was associated with epidemic of acute respiratory disease in military recruits in Europe in 1969. According to the CDC, in 2001–2002 it was reported to be associated with approximately 8% of all respiratory adenoviral infections in the pediatric ward of a Taiwan Hospital.
However, because Ad14 hasn't been circulating in a while—it's just one of at least 42 different adenovirus strains—the current population isn't likely to be immune. While most healthy individuals are still able to mount an immune response to it, certain susceptible people will become more ill, including the very old, the very young, and those with compromised immune systems; perhaps some healthy people will have a genetic predisposition that makes them more vulnerable.
Indeed, a single nucleotide polymorphism (SNP)—one change in the DNA of a key gene—can have a dramatic effect on how a person responds to environmental or infectious triggers. Consider as an example the case of a disease that we are more familiar with than Ad14, respiratory syncytial virus (RSV) in children. Hospitalization and more severe symptoms have been demonstrated with one SNP (Pediatr. Infect. Dis. J. 2007;26:1094–8), and it's likely that similar mechanisms explain some of the variation in disease severity with other viruses as well. We're just beginning to learn about these mechanisms within the innate immune system.
In the meantime, we might want to consider obtaining viral cultures—commercially available testing systems do include adenovirus—in hospitalized pneumonia patients who do not have positive RSV or influenza rapid tests and who do not improve quickly despite appropriate supportive and perhaps empiric antibiotic therapy.
Even though we don't have a commonly used effective antiviral for adenovirus, such as oseltamivir for influenza, it can be important to be aware of cases of severe adenovirus occurring outside of the surveillance network. While Ad14 is a recent culprit, other serotypes also have been implicated in sporadic outbreaks. Also, if you have a firm viral diagnosis, you don't need to keep escalating broad-spectrum antibiotics. Adenovirus can initially mimic the high fever, leukocytosis, and ill-appearing presentation of bacterial pneumonia, particularly in young children. The x-ray findings usually are bilateral and patchy initially, but the infiltrates can become dense and appear more “bacterial” as time goes on.
Another reason to culture for adenovirus is its potential to mimic Kawasaki disease, with the nonpurulent conjunctivitis, red throat, mucositis, high fever, and swollen lymph nodes. If you can confirm that the child actually has adenovirus during the Kawasaki work-up, you can save thousands of dollars that would otherwise be spent on intravenous immune globulin (IVIG) therapy. Of course, to be really useful, you'll need to get the viral cultures early, because current culture techniques—shell vial or standard—require anywhere from 48 hours to 7 days for results to develop. Because the window of effective use of IVIG in Kawasaki disease is within 10 days of the onset of fever, getting viral cultures more than 5 days into the fever may not give you time to make the diagnosis before you will need to empirically use IVIG.
New multiplex polymerase chain reaction (PCR) technology should improve on that situation in the near future. Already in use at some teaching institutions, multiplex PCR improves the diagnostic capacity of traditional PCR by amplifying target sequences of multiple viruses all at once. The technology allows you to order a panel of 17–20 different viral tests in one batch and get the results back in a day (J. Clin. Microbiol. 2007;45:2965–70), at a cost of not much more than the $150-$200 for the current viral panel of just 6 or 7.
Another new technology on the horizon—flocked nasal swabs—will make it easier to obtain the sample from the child. Currently approved for use in adults, the swabs are made with perpendicular nylon fibers that allow you to collect epithelial cells and surrounding pathogens with a few simple twirls in the nares, a technique far more comfortable for the patient than a nasal wash. Data from the company's abstracts suggest that the sample you get from the swab is equivalent to that from the nasal wash (information available at www.copanusa.com
These new modalities together should make viral testing as simple as taking a throat culture for the group A streptococcus bacterium, and allow us to obtain timely information that is more pertinent while the child is still sick. But at the same time we need to remind our patients—and ourselves—that in the vast majority of cases we're not talking about a “killer” disease, even with adenovirus.