User login
Hospitalists Can Improve Healthcare Value
As the nation considers how to reduce healthcare costs, hospitalists can play a crucial role in this effort because they control many healthcare services through routine clinical decisions at the point of care. In fact, the government, payers, and the public now look to hospitalists as essential partners for reining in healthcare costs.[1, 2] The role of hospitalists is even more critical as payers, including Medicare, seek to shift reimbursements from volume to value.[1] Medicare's Value‐Based Purchasing program has already tied a percentage of hospital payments to metrics of quality, patient satisfaction, and cost,[1, 3] and Health and Human Services Secretary Sylvia Burwell announced that by the end of 2018, the goal is to have 50% of Medicare payments tied to quality or value through alternative payment models.[4]
Major opportunities for cost savings exist across the care continuum, particularly in postacute and transitional care, and hospitalist groups are leading innovative models that show promise for coordinating care and improving value.[5] Individual hospitalists are also in a unique position to provide high‐value care for their patients through advocating for appropriate care and leading local initiatives to improve value of care.[6, 7, 8] This commentary article aims to provide practicing hospitalists with a framework to incorporate these strategies into their daily work.
DESIGN STRATEGIES TO COORDINATE CARE
As delivery systems undertake the task of population health management, hospitalists will inevitably play a critical role in facilitating coordination between community, acute, and postacute care. During admission, discharge, and the hospitalization itself, standardizing care pathways for common hospital conditions such as pneumonia and cellulitis can be effective in decreasing utilization and improving clinical outcomes.[9, 10] Intermountain Healthcare in Utah has applied evidence‐based protocols to more than 60 clinical processes, re‐engineering roughly 80% of all care that they deliver.[11] These types of care redesigns and standardization promise to provide better, more efficient, and often safer care for more patients. Hospitalists can play important roles in developing and delivering on these pathways.
In addition, hospital physician discontinuity during admissions may lead to increased resource utilization, costs, and lower patient satisfaction.[12] Therefore, ensuring clear handoffs between inpatient providers, as well as with outpatient providers during transitions in care, is a vital component of delivering high‐value care. Of particular importance is the population of patients frequently readmitted to the hospital. Hospitalists are often well acquainted with these patients, and the myriad of psychosocial, economic, and environmental challenges this vulnerable population faces. Although care coordination programs are increasing in prevalence, data on their cost‐effectiveness are mixed, highlighting the need for testing innovations.[13] Certainly, hospitalists can be leaders adopting and documenting the effectiveness of spreading interventions that have been shown to be promising in improving care transitions at discharge, such as the Care Transitions Intervention, Project RED (Re‐Engineered Discharge), or the Transitional Care Model.[14, 15, 16]
The University of Chicago, through funding from the Centers for Medicare and Medicaid Innovation, is testing the use of a single physician who cares for frequently admitted patients both in and out of the hospital, thereby reducing the costs of coordination.[5] This comprehensivist model depends on physicians seeing patients in the hospital and then in a clinic located in or near the hospital for the subset of patients who stand to benefit most from this continuity. This differs from the old model of having primary care providers (PCPs) see inpatients and outpatients because the comprehensivist's patient panel is enriched with only patients who are at high risk for hospitalization, and thus these physicians have a more direct focus on hospital‐related care and higher daily hospitalized patient censuses, whereas PCPs were seeing fewer and fewer of their patients in the hospital on a daily basis. Evidence concerning the effectiveness of this model is expected by 2016. Hospitalists have also ventured out of the hospital into skilled nursing facilities, specializing in long‐term care.[17] These physicians are helping provide care to the roughly 1.6 million residents of US nursing homes.[17, 18] Preliminary evidence suggests increased physician staffing is associated with decreased hospitalization of nursing home residents.[18]
ADVOCATE FOR APPROPRIATE CARE
Hospitalists can advocate for appropriate care through avoiding low‐value services at the point of care, as well as learning and teaching about value.
Avoiding Low‐Value Services at the Point of Care
The largest contributor to the approximately $750 billion in annual healthcare waste is unnecessary services, which includes overuse, discretionary use beyond benchmarks, and unnecessary choice of higher‐cost services.[19] Drivers of overuse include medical culture, fee‐for‐service payments, patient expectations, and fear of malpractice litigation.[20] For practicing hospitalists, the most substantial motivation for overuse may be a desire to reassure patients and themselves.[21] Unfortunately, patients commonly overestimate the benefits and underestimate the potential harms of testing and treatments.[22] However, clear communication with patients can reduce overuse, underuse, and misuse.[23]
Specific targets for improving appropriate resource utilization may be identified from resources such as Choosing Wisely lists, guidelines, and appropriateness criteria. The Choosing Wisely campaign has brought together an unprecedented number of medical specialty societies to issue top five lists of things that physicians and patients should question (
| Adult Hospital Medicine Recommendations | Pediatric Hospital Medicine Recommendations |
|---|---|
| 1. Do not place, or leave in place, urinary catheters for incontinence or convenience, or monitoring of output for noncritically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days or urologic procedures; use weights instead to monitor diuresis). | 1. Do not order chest radiographs in children with uncomplicated asthma or bronchiolitis. |
| 2. Do not prescribe medications for stress ulcer prophylaxis to medical inpatients unless at high risk for gastrointestinal complication. | 2. Do not routinely use bronchodilators in children with bronchiolitis. |
| 3. Avoid transfusing red blood cells just because hemoglobin levels are below arbitrary thresholds such as 10, 9, or even 8 mg/dL in the absence of symptoms. | 3. Do not use systemic corticosteroids in children under 2 years of age with an uncomplicated lower respiratory tract infection. |
| 4. Avoid overuse/unnecessary use of telemetry monitoring in the hospital, particularly for patients at low risk for adverse cardiac outcomes. | 4. Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy. |
| 5. Do not perform repetitive complete blood count and chemistry testing in the face of clinical and lab stability. | 5. Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. |
As an example of this strategy, 1 multi‐institutional group has started training medical students to augment the traditional subjective‐objective‐assessment‐plan (SOAP) daily template with a value section (SOAP‐V), creating a cognitive forcing function to promote discussion of high‐value care delivery.[28] Physicians could include brief thoughts in this section about why they chose a specific intervention, their consideration of the potential benefits and harms compared to alternatives, how it may incorporate the patient's goals and values, and the known and potential costs of the intervention. Similarly, Flanders and Saint recommend that daily progress notes and sign‐outs include the indication, day of administration, and expected duration of therapy for all antimicrobial treatments, as a mechanism for curbing antimicrobial overuse in hospitalized patients.[29] Likewise, hospitalists can also document whether or not a patient needs routine labs, telemetry, continuous pulse oximetry, or other interventions or monitoring. It is not yet clear how effective this type of strategy will be, and drawbacks include creating longer progress notes and requiring more time for documentation. Another approach would be to work with the electronic health record to flag patients who are scheduled for telemetry or other potentially wasteful practices to inspire a daily practice audit to question whether the patient still meets criteria for such care. This approach acknowledges that patient's clinical status changes, and overcomes the inertia that results in so many therapies being continued despite a need or indication.
Communicating With Patients Who Want Everything
Some patients may be more worried about not getting every possible test, rather than concerns regarding associated costs. This may oftentimes be related to patients routinely overestimating the benefits of testing and treatments while not realizing the many potential downstream harms.[22] The perception is that patient demands frequently drive overtesting, but studies suggest the demanding patient is actually much less common than most physicians think.[30]
The Choosing Wisely campaign features video modules that provide a framework and specific examples for physician‐patient communication around some of the Choosing Wisely recommendations (available at:
Clinicians can explain why they do not believe that a test will help a patient and can share their concerns about the potential harms and downstream consequences of a given test. In addition, Consumer Reports and other groups have created trusted resources for patients that provide clear information for the public about unnecessary testing and services.
Learn and Teach Value
Traditionally, healthcare costs have largely remained hidden from both the public and medical professionals.[31, 32] As a result, hospitalists are generally not aware of the costs associated with their care.[33, 34] Although medical education has historically avoided the topic of healthcare costs,[35] recent calls to teach healthcare value have led to new educational efforts.[35, 36, 37] Future generations of medical professionals will be trained in these skills, but current hospitalists should seek opportunities to improve their knowledge of healthcare value and costs.
Fortunately, several resources can fill this gap. In addition to Choosing Wisely and ACR appropriateness criteria discussed above, newer tools focus on how to operationalize these recommendations with patients. The American College of Physicians (ACP) has launched a high‐value care educational platform that includes clinical recommendations, physician resources, curricula and public policy recommendations, and patient resources to help them understand the benefits, harms, and costs of tests and treatments for common clinical issues (
In an effort to provide frontline clinicians with the knowledge and tools necessary to address healthcare value, we have authored a textbook, Understanding Value‐Based Healthcare.[38] To identify the most promising ways of teaching these concepts, we also host the annual Teaching Value & Choosing Wisely Challenge and convene the Teaching Value in Healthcare Learning Network (bit.ly/teachingvaluenetwork) through our nonprofit, Costs of Care.[39]
In addition, hospitalists can also advocate for greater price transparency to help improve cost awareness and drive more appropriate care. The evidence on the effect of transparent costs in the electronic ordering system is evolving. Historically, efforts to provide diagnostic test prices at time of order led to mixed results,[40] but recent studies show clear benefits in resource utilization related to some form of cost display.[41, 42] This may be because physicians care more about healthcare costs and resource utilization than before. Feldman and colleagues found in a controlled clinical trial at Johns Hopkins that providing the costs of lab tests resulted in substantial decreases of certain lab tests and yielded a net cost reduction (based on 2011 Medicare Allowable Rate) of more than $400,000 at the hospital level during the 6‐month intervention period.[41] A recent systematic review concluded that charge information changed ordering and prescribing behavior in the majority of studies.[42] Some hospitalist programs are developing dashboards for various quality and utilization metrics. Sharing ratings or metrics internally or publically is a powerful way to motivate behavior change.[43]
LEAD LOCAL VALUE INITIATIVES
Hospitalists are ideal leaders of local value initiatives, whether it be through running value‐improvement projects or launching formal high‐value care programs.
Conduct Value‐Improvement Projects
Hospitalists across the country have largely taken the lead on designing value‐improvement pilots, programs, and groups within hospitals. Although value‐improvement projects may be built upon the established structures and techniques for quality improvement, importantly these programs should also include expertise in cost analyses.[8] Furthermore, some traditional quality‐improvement programs have failed to result in actual cost savings[44]; thus, it is not enough to simply rebrand quality improvement with a banner of value. Value‐improvement efforts must overcome the cultural hurdle of more care as better care, as well as pay careful attention to the diplomacy required with value improvement, because reducing costs may result in decreased revenue for certain departments or even decreases in individuals' wages.
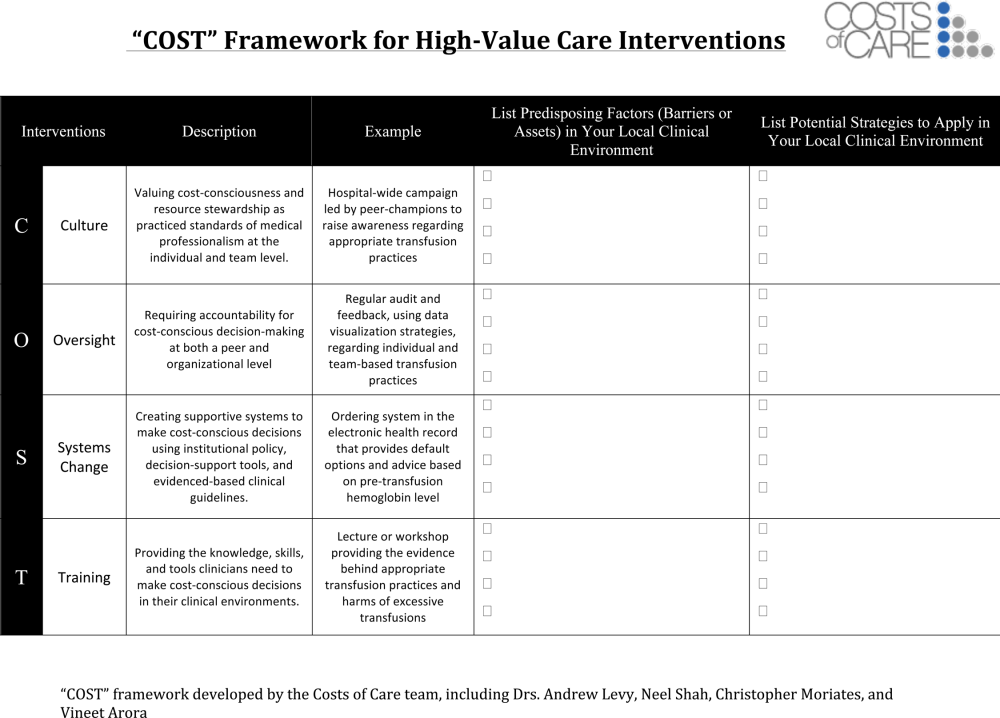
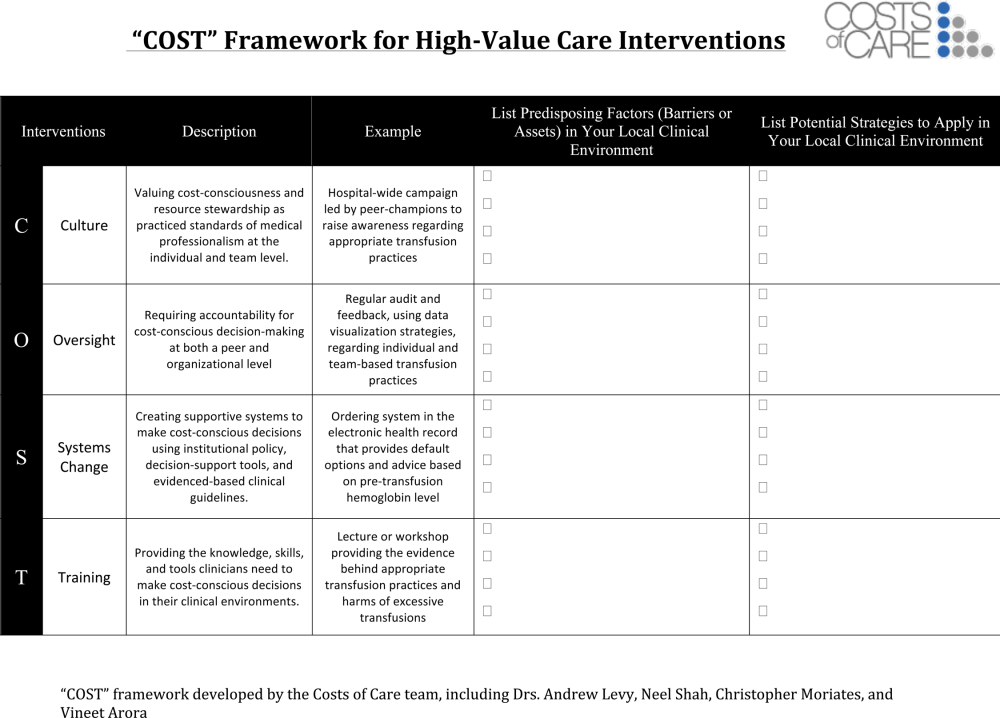
One framework that we have used to guide value‐improvement project design is COST: culture, oversight accountability, system support, and training.[45] This approach leverages principles from implementation science to ensure that value‐improvement projects successfully provide multipronged tactics for overcoming the many barriers to high‐value care delivery. Figure 1 includes a worksheet for individual clinicians or teams to use when initially planning value‐improvement project interventions.[46] The examples in this worksheet come from a successful project at the University of California, San Francisco aimed at improving blood utilization stewardship by supporting adherence to a restrictive transfusion strategy. To address culture, a hospital‐wide campaign was led by physician peer champions to raise awareness about appropriate transfusion practices. This included posters that featured prominent local physician leaders displaying their support for the program. Oversight was provided through regular audit and feedback. Each month the number of patients on the medicine service who received transfusion with a pretransfusion hemoglobin above 8 grams per deciliter was shared at a faculty lunch meeting and shown on a graph included in the quality newsletter that was widely distributed in the hospital. The ordering system in the electronic medical record was eventually modified to include the patient's pretransfusion hemoglobin level at time of transfusion order and to provide default options and advice based on whether or not guidelines would generally recommend transfusion. Hospitalists and resident physicians were trained through multiple lectures and informal teaching settings about the rationale behind the changes and the evidence that supported a restrictive transfusion strategy.
Launch High‐Value Care Programs
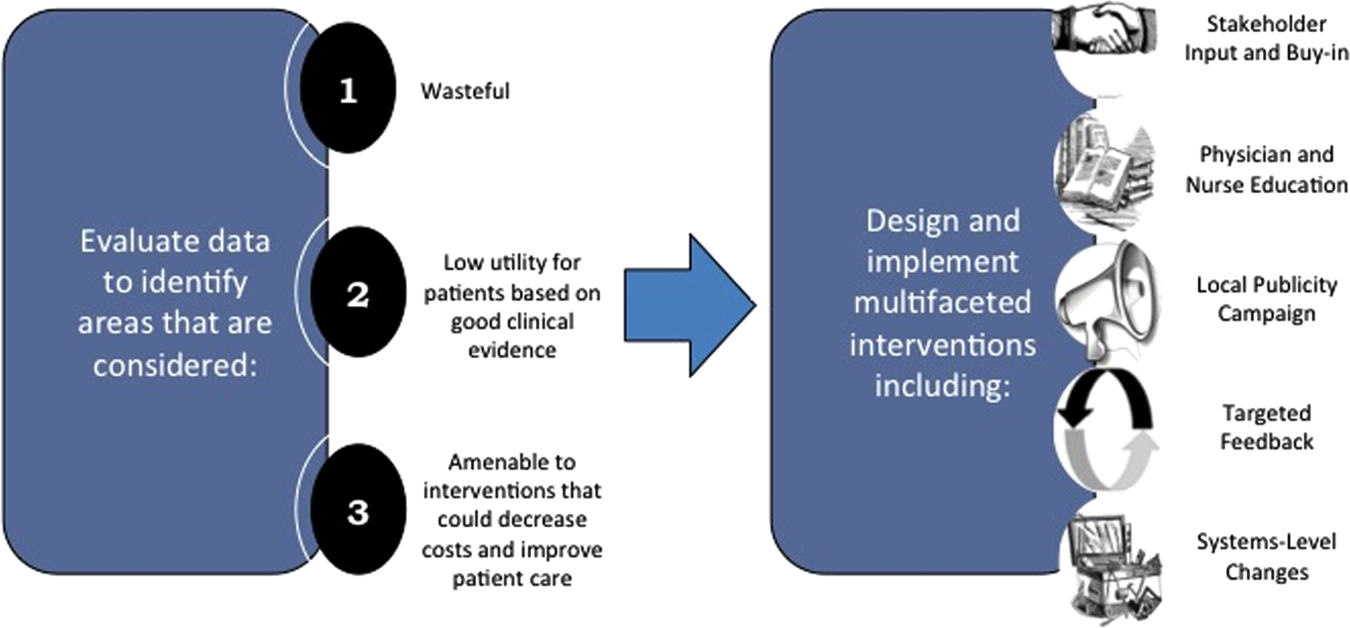
As value‐improvement projects grow, some institutions have created high‐value care programs and infrastructure. In March 2012, the University of California, San Francisco Division of Hospital Medicine launched a high‐value care program to promote healthcare value and clinician engagement.[8] The program was led by clinical hospitalists alongside a financial administrator, and aimed to use financial data to identify areas with clear evidence of waste, create evidence‐based interventions that would simultaneously improve quality while cutting costs, and pair interventions with cost awareness education and culture change efforts. In the first year of this program, 6 projects were launched targeting: (1) nebulizer to inhaler transitions,[47] (2) overuse of proton pump inhibitor stress ulcer prophlaxis,[48] (3) transfusions, (4) telemetry, (5) ionized calcium lab ordering, and (6) repeat inpatient echocardiograms.[8]
Similar hospitalist‐led groups have now formed across the country including the Johns Hopkins High‐Value Care Committee, Johns Hopkins Bayview Physicians for Responsible Ordering, and High‐Value Carolina. These groups are relatively new, and best practices and early lessons are still emerging, but all focus on engaging frontline clinicians in choosing targets and leading multipronged intervention efforts.
What About Financial Incentives?
Hospitalist high‐value care groups thus far have mostly focused on intrinsic motivations for decreasing waste by appealing to hospitalists' sense of professionalism and their commitment to improve patient affordability. When financial incentives are used, it is important that they are well aligned with internal motivations for clinicians to provide the best possible care to their patients. The Institute of Medicine recommends that payments are structured in a way to reward continuous learning and improvement in the provision of best care at lower cost.[19] In the Geisinger Health System in Pennsylvania, physician incentives are designed to reward teamwork and collaboration. For example, endocrinologists' goals are based on good control of glucose levels for all diabetes patients in the system, not just those they see.[49] Moreover, a collaborative approach is encouraged by bringing clinicians together across disciplinary service lines to plan, budget, and evaluate one another's performance. These efforts are partly credited with a 43% reduction in hospitalized days and $100 per member per month in savings among diabetic patients.[50]
Healthcare leaders, Drs. Tom Lee and Toby Cosgrove, have made a number of recommendations for creating incentives that lead to sustainable changes in care delivery[49]: avoid attaching large sums to any single target, watch for conflicts of interest, reward collaboration, and communicate the incentive program and goals clearly to clinicians.
In general, when appropriate extrinsic motivators align or interact synergistically with intrinsic motivation, it can promote high levels of performance and satisfaction.[51]
CONCLUSIONS
Hospitalists are now faced with a responsibility to reduce financial harm and provide high‐value care. To achieve this goal, hospitalist groups are developing innovative models for care across the continuum from hospital to home, and individual hospitalists can advocate for appropriate care and lead value‐improvement initiatives in hospitals. Through existing knowledge and new frameworks and tools that specifically address value, hospitalists can champion value at the bedside and ensure their patients get the best possible care at lower costs.
Disclosures: Drs. Moriates, Shah, and Arora have received grant funding from the ABIM Foundation, and royalties from McGraw‐Hill for the textbook Understanding Value‐Based Healthcare. The authors report no conflicts of interest.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367(4):292–295.
- . Value‐driven health care: implications for hospitals and hospitalists. J Hosp Med. 2009;4(8):507–511.
- , . Hospital value‐based purchasing. J Hosp Med. 2013;8(5):271–277.
- . Setting value‐based payment goals—HHS efforts to improve U.S. health care. N Engl J Med. 2015;372(10):897–899.
- , . Redesigning care for patients at increased hospitalization risk: the Comprehensive Care Physician model. Health Aff Proj Hope. 2014;33(5):770–777.
- , , , et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486–492.
- , , . First, do no (financial) harm. JAMA. 2013;310(6):577–578.
- , , , . Development of a hospital‐based program focused on improving healthcare value. J Hosp Med. 2014;9(10):671–677.
- , , , et al. A controlled trial of a critical pathway for treatment of community‐acquired pneumonia. JAMA. 2000;283(6):749–755.
- , , , , . Evidence‐based care pathway for cellulitis improves process, clinical, and cost outcomes [published online July 28, 2015]. J Hosp Med. doi:10.1002/jhm.2433.
- . The Lean approach to health care: safety, quality, and cost. Institute of Medicine. Available at: http://nam.edu/perspectives‐2012‐the‐lean‐approach‐to‐health‐care‐safety‐quality‐and‐cost/. Accessed September 22, 2015.
- , , , et al. The impact of hospitalist discontinuity on hospital cost, readmissions, and patient satisfaction. J Gen Intern Med. 2014;29(7):1004–1008.
- Congressional Budget Office. Lessons from Medicare's Demonstration Projects on Disease Management, Care Coordination, and Value‐Based Payment. Available at: https://www.cbo.gov/publication/42860. Accessed April 26, 2015.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620.
- . “SNFists” at work: nursing home docs patterned after hospitalists. Mod Healthc. 2012;42(13):32–33.
- , , , . Nursing home physician specialists: a response to the workforce crisis in long‐term care. Ann Intern Med. 2009;150(6):411–413.
- Institute of Medicine. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2012.
- , . The perfect storm of overutilization. JAMA. 2008;299(23):2789–2791.
- , , , et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100–108.
- , . Patients' expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2015;175(2):274–286.
- , , , et al. Enhancing the Use and Quality of Colorectal Cancer Screening. Rockville, MD: Agency for Healthcare Research and Quality; 2010. Available at: http://www.ncbi.nlm.nih.gov/books/NBK44526. Accessed September 30, 2013.
- , , , et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479–485.
- . Teaching Choosing Wisely in medical education and training: the story of a pioneer. The Medical Professionalism Blog. Available at: http://blog.abimfoundation.org/teaching‐choosing‐wisely‐in‐meded. Accessed March 29, 2014.
- American College of Radiology. ACR appropriateness criteria overview. November 2013. Available at: http://www.acr.org/∼/media/ACR/Documents/AppCriteria/Overview.pdf. Accessed March 4, 2014.
- American College of Cardiology Foundation. Appropriate use criteria: what you need to know. Available at: http://www.cardiosource.org/∼/media/Files/Science%20and%20Quality/Quality%20Programs/FOCUS/E1302_AUC_Primer_Update.ashx. Accessed March 4, 2014.
- , , , , . SOAP‐V: applying high‐value care during patient care. The Medical Professionalism Blog. Available at: http://blog.abimfoundation.org/soap‐v‐applying‐high‐value‐care‐during‐patient‐care. Accessed April 3, 2015.
- , . Why does antimicrobial overuse in hospitalized patients persist? JAMA Intern Med. 2014;174(5):661–662.
- . The myth of the demanding patient. JAMA Oncol. 2015;1(1):18–19.
- . The disruptive innovation of price transparency in health care. JAMA. 2013;310(18):1927–1928.
- United States Government Accountability Office. Health Care Price Transparency—Meaningful Price Information Is Difficult for Consumers to Obtain Prior to Receiving Care. Washington, DC: United States Government Accountability Office; 2011:43.
- , , . General pediatric attending physicians' and residents' knowledge of inpatient hospital finances. Pediatrics. 2013;131(6):1072–1080.
- , , . Hospitalists' awareness of patient charges associated with inpatient care. J Hosp Med. 2010;5(5):295–297.
- . Cost consciousness in patient care—what is medical education's responsibility? N Engl J Med. 2010;362(14):1253–1255.
- . Providing high‐value, cost‐conscious care: a critical seventh general competency for physicians. Ann Intern Med. 2011;155(6):386–388.
- , , , . Defining competencies for education in health care value: recommendations from the University of California, San Francisco Center for Healthcare Value Training Initiative. Acad Med. 2015;90(4):421–424.
- , , . Understanding Value‐Based Healthcare. New York: McGraw‐Hill; 2015.
- , , , . Wisdom of the crowd: bright ideas and innovations from the teaching value and choosing wisely challenge. Acad Med. 2015;90(5):624–628.
- , , , et al. Does the computerized display of charges affect inpatient ancillary test utilization? Arch Intern Med. 1997;157(21):2501–2508.
- , , , et al. Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Intern Med. 2013;173(10):903–908.
- , , , . The effect of charge display on cost of care and physician practice behaviors: a systematic review. J Gen Intern Med. 2015;30(6):835–842.
- , , , , , . Closing the Quality Gap: Revisiting the State of the Science. Vol. 5. Public Reporting as a Quality Improvement Strategy. Rockville, MD: Agency for Healthcare Research and Quality; 2012.
- , , , . The savings illusion—why clinical quality improvement fails to deliver bottom‐line results. N Engl J Med. 2011;365(26):e48.
- , , , . Fostering value in clinical practice among future physicians: time to consider COST. Acad Med. 2014;89(11):1440.
- , , , , , . The Teaching Value Workshop. MedEdPORTAL Publications; 2014. Available at: https://www.mededportal.org/publication/9859. Accessed September 22, 2015.
- , , , , . “Nebs no more after 24”: a pilot program to improve the use of appropriate respiratory therapies. JAMA Intern Med. 2013;173(17):1647–1648.
- , , , et al. The development and implementation of a bundled quality improvement initiative to reduce inappropriate stress ulcer prophylaxis. ICU Dir. 2013;4(6):322–325.
- , . Engaging doctors in the health care revolution. Harvard Business Review. June 2014. Available at: http://hbr.org/2014/06/engaging‐doctors‐in‐the‐health‐care‐revolution/ar/1. Accessed July 30, 2014.
- , , . Geisinger Health System: achieving the potential of system integration through innovation, leadership, measurement, and incentives. June 2009. Available at: http://www.commonwealthfund.org/publications/case‐studies/2009/jun/geisinger‐health‐system‐achieving‐the‐potential‐of‐system‐integration. Accessed September 22, 2015.
- Motivational synergy: toward new conceptualizations of intrinsic and extrinsic motivation in the workplace. Hum Resource Manag 1993;3(3):185–201. Available at: http://www.hbs.edu/faculty/Pages/item.aspx?num=2500. Accessed July 31, 2014.
As the nation considers how to reduce healthcare costs, hospitalists can play a crucial role in this effort because they control many healthcare services through routine clinical decisions at the point of care. In fact, the government, payers, and the public now look to hospitalists as essential partners for reining in healthcare costs.[1, 2] The role of hospitalists is even more critical as payers, including Medicare, seek to shift reimbursements from volume to value.[1] Medicare's Value‐Based Purchasing program has already tied a percentage of hospital payments to metrics of quality, patient satisfaction, and cost,[1, 3] and Health and Human Services Secretary Sylvia Burwell announced that by the end of 2018, the goal is to have 50% of Medicare payments tied to quality or value through alternative payment models.[4]
Major opportunities for cost savings exist across the care continuum, particularly in postacute and transitional care, and hospitalist groups are leading innovative models that show promise for coordinating care and improving value.[5] Individual hospitalists are also in a unique position to provide high‐value care for their patients through advocating for appropriate care and leading local initiatives to improve value of care.[6, 7, 8] This commentary article aims to provide practicing hospitalists with a framework to incorporate these strategies into their daily work.
DESIGN STRATEGIES TO COORDINATE CARE
As delivery systems undertake the task of population health management, hospitalists will inevitably play a critical role in facilitating coordination between community, acute, and postacute care. During admission, discharge, and the hospitalization itself, standardizing care pathways for common hospital conditions such as pneumonia and cellulitis can be effective in decreasing utilization and improving clinical outcomes.[9, 10] Intermountain Healthcare in Utah has applied evidence‐based protocols to more than 60 clinical processes, re‐engineering roughly 80% of all care that they deliver.[11] These types of care redesigns and standardization promise to provide better, more efficient, and often safer care for more patients. Hospitalists can play important roles in developing and delivering on these pathways.
In addition, hospital physician discontinuity during admissions may lead to increased resource utilization, costs, and lower patient satisfaction.[12] Therefore, ensuring clear handoffs between inpatient providers, as well as with outpatient providers during transitions in care, is a vital component of delivering high‐value care. Of particular importance is the population of patients frequently readmitted to the hospital. Hospitalists are often well acquainted with these patients, and the myriad of psychosocial, economic, and environmental challenges this vulnerable population faces. Although care coordination programs are increasing in prevalence, data on their cost‐effectiveness are mixed, highlighting the need for testing innovations.[13] Certainly, hospitalists can be leaders adopting and documenting the effectiveness of spreading interventions that have been shown to be promising in improving care transitions at discharge, such as the Care Transitions Intervention, Project RED (Re‐Engineered Discharge), or the Transitional Care Model.[14, 15, 16]
The University of Chicago, through funding from the Centers for Medicare and Medicaid Innovation, is testing the use of a single physician who cares for frequently admitted patients both in and out of the hospital, thereby reducing the costs of coordination.[5] This comprehensivist model depends on physicians seeing patients in the hospital and then in a clinic located in or near the hospital for the subset of patients who stand to benefit most from this continuity. This differs from the old model of having primary care providers (PCPs) see inpatients and outpatients because the comprehensivist's patient panel is enriched with only patients who are at high risk for hospitalization, and thus these physicians have a more direct focus on hospital‐related care and higher daily hospitalized patient censuses, whereas PCPs were seeing fewer and fewer of their patients in the hospital on a daily basis. Evidence concerning the effectiveness of this model is expected by 2016. Hospitalists have also ventured out of the hospital into skilled nursing facilities, specializing in long‐term care.[17] These physicians are helping provide care to the roughly 1.6 million residents of US nursing homes.[17, 18] Preliminary evidence suggests increased physician staffing is associated with decreased hospitalization of nursing home residents.[18]
ADVOCATE FOR APPROPRIATE CARE
Hospitalists can advocate for appropriate care through avoiding low‐value services at the point of care, as well as learning and teaching about value.
Avoiding Low‐Value Services at the Point of Care
The largest contributor to the approximately $750 billion in annual healthcare waste is unnecessary services, which includes overuse, discretionary use beyond benchmarks, and unnecessary choice of higher‐cost services.[19] Drivers of overuse include medical culture, fee‐for‐service payments, patient expectations, and fear of malpractice litigation.[20] For practicing hospitalists, the most substantial motivation for overuse may be a desire to reassure patients and themselves.[21] Unfortunately, patients commonly overestimate the benefits and underestimate the potential harms of testing and treatments.[22] However, clear communication with patients can reduce overuse, underuse, and misuse.[23]
Specific targets for improving appropriate resource utilization may be identified from resources such as Choosing Wisely lists, guidelines, and appropriateness criteria. The Choosing Wisely campaign has brought together an unprecedented number of medical specialty societies to issue top five lists of things that physicians and patients should question (
| Adult Hospital Medicine Recommendations | Pediatric Hospital Medicine Recommendations |
|---|---|
| 1. Do not place, or leave in place, urinary catheters for incontinence or convenience, or monitoring of output for noncritically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days or urologic procedures; use weights instead to monitor diuresis). | 1. Do not order chest radiographs in children with uncomplicated asthma or bronchiolitis. |
| 2. Do not prescribe medications for stress ulcer prophylaxis to medical inpatients unless at high risk for gastrointestinal complication. | 2. Do not routinely use bronchodilators in children with bronchiolitis. |
| 3. Avoid transfusing red blood cells just because hemoglobin levels are below arbitrary thresholds such as 10, 9, or even 8 mg/dL in the absence of symptoms. | 3. Do not use systemic corticosteroids in children under 2 years of age with an uncomplicated lower respiratory tract infection. |
| 4. Avoid overuse/unnecessary use of telemetry monitoring in the hospital, particularly for patients at low risk for adverse cardiac outcomes. | 4. Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy. |
| 5. Do not perform repetitive complete blood count and chemistry testing in the face of clinical and lab stability. | 5. Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. |
As an example of this strategy, 1 multi‐institutional group has started training medical students to augment the traditional subjective‐objective‐assessment‐plan (SOAP) daily template with a value section (SOAP‐V), creating a cognitive forcing function to promote discussion of high‐value care delivery.[28] Physicians could include brief thoughts in this section about why they chose a specific intervention, their consideration of the potential benefits and harms compared to alternatives, how it may incorporate the patient's goals and values, and the known and potential costs of the intervention. Similarly, Flanders and Saint recommend that daily progress notes and sign‐outs include the indication, day of administration, and expected duration of therapy for all antimicrobial treatments, as a mechanism for curbing antimicrobial overuse in hospitalized patients.[29] Likewise, hospitalists can also document whether or not a patient needs routine labs, telemetry, continuous pulse oximetry, or other interventions or monitoring. It is not yet clear how effective this type of strategy will be, and drawbacks include creating longer progress notes and requiring more time for documentation. Another approach would be to work with the electronic health record to flag patients who are scheduled for telemetry or other potentially wasteful practices to inspire a daily practice audit to question whether the patient still meets criteria for such care. This approach acknowledges that patient's clinical status changes, and overcomes the inertia that results in so many therapies being continued despite a need or indication.
Communicating With Patients Who Want Everything
Some patients may be more worried about not getting every possible test, rather than concerns regarding associated costs. This may oftentimes be related to patients routinely overestimating the benefits of testing and treatments while not realizing the many potential downstream harms.[22] The perception is that patient demands frequently drive overtesting, but studies suggest the demanding patient is actually much less common than most physicians think.[30]
The Choosing Wisely campaign features video modules that provide a framework and specific examples for physician‐patient communication around some of the Choosing Wisely recommendations (available at:
Clinicians can explain why they do not believe that a test will help a patient and can share their concerns about the potential harms and downstream consequences of a given test. In addition, Consumer Reports and other groups have created trusted resources for patients that provide clear information for the public about unnecessary testing and services.
Learn and Teach Value
Traditionally, healthcare costs have largely remained hidden from both the public and medical professionals.[31, 32] As a result, hospitalists are generally not aware of the costs associated with their care.[33, 34] Although medical education has historically avoided the topic of healthcare costs,[35] recent calls to teach healthcare value have led to new educational efforts.[35, 36, 37] Future generations of medical professionals will be trained in these skills, but current hospitalists should seek opportunities to improve their knowledge of healthcare value and costs.
Fortunately, several resources can fill this gap. In addition to Choosing Wisely and ACR appropriateness criteria discussed above, newer tools focus on how to operationalize these recommendations with patients. The American College of Physicians (ACP) has launched a high‐value care educational platform that includes clinical recommendations, physician resources, curricula and public policy recommendations, and patient resources to help them understand the benefits, harms, and costs of tests and treatments for common clinical issues (
In an effort to provide frontline clinicians with the knowledge and tools necessary to address healthcare value, we have authored a textbook, Understanding Value‐Based Healthcare.[38] To identify the most promising ways of teaching these concepts, we also host the annual Teaching Value & Choosing Wisely Challenge and convene the Teaching Value in Healthcare Learning Network (bit.ly/teachingvaluenetwork) through our nonprofit, Costs of Care.[39]
In addition, hospitalists can also advocate for greater price transparency to help improve cost awareness and drive more appropriate care. The evidence on the effect of transparent costs in the electronic ordering system is evolving. Historically, efforts to provide diagnostic test prices at time of order led to mixed results,[40] but recent studies show clear benefits in resource utilization related to some form of cost display.[41, 42] This may be because physicians care more about healthcare costs and resource utilization than before. Feldman and colleagues found in a controlled clinical trial at Johns Hopkins that providing the costs of lab tests resulted in substantial decreases of certain lab tests and yielded a net cost reduction (based on 2011 Medicare Allowable Rate) of more than $400,000 at the hospital level during the 6‐month intervention period.[41] A recent systematic review concluded that charge information changed ordering and prescribing behavior in the majority of studies.[42] Some hospitalist programs are developing dashboards for various quality and utilization metrics. Sharing ratings or metrics internally or publically is a powerful way to motivate behavior change.[43]
LEAD LOCAL VALUE INITIATIVES
Hospitalists are ideal leaders of local value initiatives, whether it be through running value‐improvement projects or launching formal high‐value care programs.
Conduct Value‐Improvement Projects
Hospitalists across the country have largely taken the lead on designing value‐improvement pilots, programs, and groups within hospitals. Although value‐improvement projects may be built upon the established structures and techniques for quality improvement, importantly these programs should also include expertise in cost analyses.[8] Furthermore, some traditional quality‐improvement programs have failed to result in actual cost savings[44]; thus, it is not enough to simply rebrand quality improvement with a banner of value. Value‐improvement efforts must overcome the cultural hurdle of more care as better care, as well as pay careful attention to the diplomacy required with value improvement, because reducing costs may result in decreased revenue for certain departments or even decreases in individuals' wages.
One framework that we have used to guide value‐improvement project design is COST: culture, oversight accountability, system support, and training.[45] This approach leverages principles from implementation science to ensure that value‐improvement projects successfully provide multipronged tactics for overcoming the many barriers to high‐value care delivery. Figure 1 includes a worksheet for individual clinicians or teams to use when initially planning value‐improvement project interventions.[46] The examples in this worksheet come from a successful project at the University of California, San Francisco aimed at improving blood utilization stewardship by supporting adherence to a restrictive transfusion strategy. To address culture, a hospital‐wide campaign was led by physician peer champions to raise awareness about appropriate transfusion practices. This included posters that featured prominent local physician leaders displaying their support for the program. Oversight was provided through regular audit and feedback. Each month the number of patients on the medicine service who received transfusion with a pretransfusion hemoglobin above 8 grams per deciliter was shared at a faculty lunch meeting and shown on a graph included in the quality newsletter that was widely distributed in the hospital. The ordering system in the electronic medical record was eventually modified to include the patient's pretransfusion hemoglobin level at time of transfusion order and to provide default options and advice based on whether or not guidelines would generally recommend transfusion. Hospitalists and resident physicians were trained through multiple lectures and informal teaching settings about the rationale behind the changes and the evidence that supported a restrictive transfusion strategy.

Launch High‐Value Care Programs
As value‐improvement projects grow, some institutions have created high‐value care programs and infrastructure. In March 2012, the University of California, San Francisco Division of Hospital Medicine launched a high‐value care program to promote healthcare value and clinician engagement.[8] The program was led by clinical hospitalists alongside a financial administrator, and aimed to use financial data to identify areas with clear evidence of waste, create evidence‐based interventions that would simultaneously improve quality while cutting costs, and pair interventions with cost awareness education and culture change efforts. In the first year of this program, 6 projects were launched targeting: (1) nebulizer to inhaler transitions,[47] (2) overuse of proton pump inhibitor stress ulcer prophlaxis,[48] (3) transfusions, (4) telemetry, (5) ionized calcium lab ordering, and (6) repeat inpatient echocardiograms.[8]
Similar hospitalist‐led groups have now formed across the country including the Johns Hopkins High‐Value Care Committee, Johns Hopkins Bayview Physicians for Responsible Ordering, and High‐Value Carolina. These groups are relatively new, and best practices and early lessons are still emerging, but all focus on engaging frontline clinicians in choosing targets and leading multipronged intervention efforts.
What About Financial Incentives?
Hospitalist high‐value care groups thus far have mostly focused on intrinsic motivations for decreasing waste by appealing to hospitalists' sense of professionalism and their commitment to improve patient affordability. When financial incentives are used, it is important that they are well aligned with internal motivations for clinicians to provide the best possible care to their patients. The Institute of Medicine recommends that payments are structured in a way to reward continuous learning and improvement in the provision of best care at lower cost.[19] In the Geisinger Health System in Pennsylvania, physician incentives are designed to reward teamwork and collaboration. For example, endocrinologists' goals are based on good control of glucose levels for all diabetes patients in the system, not just those they see.[49] Moreover, a collaborative approach is encouraged by bringing clinicians together across disciplinary service lines to plan, budget, and evaluate one another's performance. These efforts are partly credited with a 43% reduction in hospitalized days and $100 per member per month in savings among diabetic patients.[50]
Healthcare leaders, Drs. Tom Lee and Toby Cosgrove, have made a number of recommendations for creating incentives that lead to sustainable changes in care delivery[49]: avoid attaching large sums to any single target, watch for conflicts of interest, reward collaboration, and communicate the incentive program and goals clearly to clinicians.
In general, when appropriate extrinsic motivators align or interact synergistically with intrinsic motivation, it can promote high levels of performance and satisfaction.[51]
CONCLUSIONS
Hospitalists are now faced with a responsibility to reduce financial harm and provide high‐value care. To achieve this goal, hospitalist groups are developing innovative models for care across the continuum from hospital to home, and individual hospitalists can advocate for appropriate care and lead value‐improvement initiatives in hospitals. Through existing knowledge and new frameworks and tools that specifically address value, hospitalists can champion value at the bedside and ensure their patients get the best possible care at lower costs.
Disclosures: Drs. Moriates, Shah, and Arora have received grant funding from the ABIM Foundation, and royalties from McGraw‐Hill for the textbook Understanding Value‐Based Healthcare. The authors report no conflicts of interest.
As the nation considers how to reduce healthcare costs, hospitalists can play a crucial role in this effort because they control many healthcare services through routine clinical decisions at the point of care. In fact, the government, payers, and the public now look to hospitalists as essential partners for reining in healthcare costs.[1, 2] The role of hospitalists is even more critical as payers, including Medicare, seek to shift reimbursements from volume to value.[1] Medicare's Value‐Based Purchasing program has already tied a percentage of hospital payments to metrics of quality, patient satisfaction, and cost,[1, 3] and Health and Human Services Secretary Sylvia Burwell announced that by the end of 2018, the goal is to have 50% of Medicare payments tied to quality or value through alternative payment models.[4]
Major opportunities for cost savings exist across the care continuum, particularly in postacute and transitional care, and hospitalist groups are leading innovative models that show promise for coordinating care and improving value.[5] Individual hospitalists are also in a unique position to provide high‐value care for their patients through advocating for appropriate care and leading local initiatives to improve value of care.[6, 7, 8] This commentary article aims to provide practicing hospitalists with a framework to incorporate these strategies into their daily work.
DESIGN STRATEGIES TO COORDINATE CARE
As delivery systems undertake the task of population health management, hospitalists will inevitably play a critical role in facilitating coordination between community, acute, and postacute care. During admission, discharge, and the hospitalization itself, standardizing care pathways for common hospital conditions such as pneumonia and cellulitis can be effective in decreasing utilization and improving clinical outcomes.[9, 10] Intermountain Healthcare in Utah has applied evidence‐based protocols to more than 60 clinical processes, re‐engineering roughly 80% of all care that they deliver.[11] These types of care redesigns and standardization promise to provide better, more efficient, and often safer care for more patients. Hospitalists can play important roles in developing and delivering on these pathways.
In addition, hospital physician discontinuity during admissions may lead to increased resource utilization, costs, and lower patient satisfaction.[12] Therefore, ensuring clear handoffs between inpatient providers, as well as with outpatient providers during transitions in care, is a vital component of delivering high‐value care. Of particular importance is the population of patients frequently readmitted to the hospital. Hospitalists are often well acquainted with these patients, and the myriad of psychosocial, economic, and environmental challenges this vulnerable population faces. Although care coordination programs are increasing in prevalence, data on their cost‐effectiveness are mixed, highlighting the need for testing innovations.[13] Certainly, hospitalists can be leaders adopting and documenting the effectiveness of spreading interventions that have been shown to be promising in improving care transitions at discharge, such as the Care Transitions Intervention, Project RED (Re‐Engineered Discharge), or the Transitional Care Model.[14, 15, 16]
The University of Chicago, through funding from the Centers for Medicare and Medicaid Innovation, is testing the use of a single physician who cares for frequently admitted patients both in and out of the hospital, thereby reducing the costs of coordination.[5] This comprehensivist model depends on physicians seeing patients in the hospital and then in a clinic located in or near the hospital for the subset of patients who stand to benefit most from this continuity. This differs from the old model of having primary care providers (PCPs) see inpatients and outpatients because the comprehensivist's patient panel is enriched with only patients who are at high risk for hospitalization, and thus these physicians have a more direct focus on hospital‐related care and higher daily hospitalized patient censuses, whereas PCPs were seeing fewer and fewer of their patients in the hospital on a daily basis. Evidence concerning the effectiveness of this model is expected by 2016. Hospitalists have also ventured out of the hospital into skilled nursing facilities, specializing in long‐term care.[17] These physicians are helping provide care to the roughly 1.6 million residents of US nursing homes.[17, 18] Preliminary evidence suggests increased physician staffing is associated with decreased hospitalization of nursing home residents.[18]
ADVOCATE FOR APPROPRIATE CARE
Hospitalists can advocate for appropriate care through avoiding low‐value services at the point of care, as well as learning and teaching about value.
Avoiding Low‐Value Services at the Point of Care
The largest contributor to the approximately $750 billion in annual healthcare waste is unnecessary services, which includes overuse, discretionary use beyond benchmarks, and unnecessary choice of higher‐cost services.[19] Drivers of overuse include medical culture, fee‐for‐service payments, patient expectations, and fear of malpractice litigation.[20] For practicing hospitalists, the most substantial motivation for overuse may be a desire to reassure patients and themselves.[21] Unfortunately, patients commonly overestimate the benefits and underestimate the potential harms of testing and treatments.[22] However, clear communication with patients can reduce overuse, underuse, and misuse.[23]
Specific targets for improving appropriate resource utilization may be identified from resources such as Choosing Wisely lists, guidelines, and appropriateness criteria. The Choosing Wisely campaign has brought together an unprecedented number of medical specialty societies to issue top five lists of things that physicians and patients should question (
| Adult Hospital Medicine Recommendations | Pediatric Hospital Medicine Recommendations |
|---|---|
| 1. Do not place, or leave in place, urinary catheters for incontinence or convenience, or monitoring of output for noncritically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days or urologic procedures; use weights instead to monitor diuresis). | 1. Do not order chest radiographs in children with uncomplicated asthma or bronchiolitis. |
| 2. Do not prescribe medications for stress ulcer prophylaxis to medical inpatients unless at high risk for gastrointestinal complication. | 2. Do not routinely use bronchodilators in children with bronchiolitis. |
| 3. Avoid transfusing red blood cells just because hemoglobin levels are below arbitrary thresholds such as 10, 9, or even 8 mg/dL in the absence of symptoms. | 3. Do not use systemic corticosteroids in children under 2 years of age with an uncomplicated lower respiratory tract infection. |
| 4. Avoid overuse/unnecessary use of telemetry monitoring in the hospital, particularly for patients at low risk for adverse cardiac outcomes. | 4. Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy. |
| 5. Do not perform repetitive complete blood count and chemistry testing in the face of clinical and lab stability. | 5. Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. |
As an example of this strategy, 1 multi‐institutional group has started training medical students to augment the traditional subjective‐objective‐assessment‐plan (SOAP) daily template with a value section (SOAP‐V), creating a cognitive forcing function to promote discussion of high‐value care delivery.[28] Physicians could include brief thoughts in this section about why they chose a specific intervention, their consideration of the potential benefits and harms compared to alternatives, how it may incorporate the patient's goals and values, and the known and potential costs of the intervention. Similarly, Flanders and Saint recommend that daily progress notes and sign‐outs include the indication, day of administration, and expected duration of therapy for all antimicrobial treatments, as a mechanism for curbing antimicrobial overuse in hospitalized patients.[29] Likewise, hospitalists can also document whether or not a patient needs routine labs, telemetry, continuous pulse oximetry, or other interventions or monitoring. It is not yet clear how effective this type of strategy will be, and drawbacks include creating longer progress notes and requiring more time for documentation. Another approach would be to work with the electronic health record to flag patients who are scheduled for telemetry or other potentially wasteful practices to inspire a daily practice audit to question whether the patient still meets criteria for such care. This approach acknowledges that patient's clinical status changes, and overcomes the inertia that results in so many therapies being continued despite a need or indication.
Communicating With Patients Who Want Everything
Some patients may be more worried about not getting every possible test, rather than concerns regarding associated costs. This may oftentimes be related to patients routinely overestimating the benefits of testing and treatments while not realizing the many potential downstream harms.[22] The perception is that patient demands frequently drive overtesting, but studies suggest the demanding patient is actually much less common than most physicians think.[30]
The Choosing Wisely campaign features video modules that provide a framework and specific examples for physician‐patient communication around some of the Choosing Wisely recommendations (available at:
Clinicians can explain why they do not believe that a test will help a patient and can share their concerns about the potential harms and downstream consequences of a given test. In addition, Consumer Reports and other groups have created trusted resources for patients that provide clear information for the public about unnecessary testing and services.
Learn and Teach Value
Traditionally, healthcare costs have largely remained hidden from both the public and medical professionals.[31, 32] As a result, hospitalists are generally not aware of the costs associated with their care.[33, 34] Although medical education has historically avoided the topic of healthcare costs,[35] recent calls to teach healthcare value have led to new educational efforts.[35, 36, 37] Future generations of medical professionals will be trained in these skills, but current hospitalists should seek opportunities to improve their knowledge of healthcare value and costs.
Fortunately, several resources can fill this gap. In addition to Choosing Wisely and ACR appropriateness criteria discussed above, newer tools focus on how to operationalize these recommendations with patients. The American College of Physicians (ACP) has launched a high‐value care educational platform that includes clinical recommendations, physician resources, curricula and public policy recommendations, and patient resources to help them understand the benefits, harms, and costs of tests and treatments for common clinical issues (
In an effort to provide frontline clinicians with the knowledge and tools necessary to address healthcare value, we have authored a textbook, Understanding Value‐Based Healthcare.[38] To identify the most promising ways of teaching these concepts, we also host the annual Teaching Value & Choosing Wisely Challenge and convene the Teaching Value in Healthcare Learning Network (bit.ly/teachingvaluenetwork) through our nonprofit, Costs of Care.[39]
In addition, hospitalists can also advocate for greater price transparency to help improve cost awareness and drive more appropriate care. The evidence on the effect of transparent costs in the electronic ordering system is evolving. Historically, efforts to provide diagnostic test prices at time of order led to mixed results,[40] but recent studies show clear benefits in resource utilization related to some form of cost display.[41, 42] This may be because physicians care more about healthcare costs and resource utilization than before. Feldman and colleagues found in a controlled clinical trial at Johns Hopkins that providing the costs of lab tests resulted in substantial decreases of certain lab tests and yielded a net cost reduction (based on 2011 Medicare Allowable Rate) of more than $400,000 at the hospital level during the 6‐month intervention period.[41] A recent systematic review concluded that charge information changed ordering and prescribing behavior in the majority of studies.[42] Some hospitalist programs are developing dashboards for various quality and utilization metrics. Sharing ratings or metrics internally or publically is a powerful way to motivate behavior change.[43]
LEAD LOCAL VALUE INITIATIVES
Hospitalists are ideal leaders of local value initiatives, whether it be through running value‐improvement projects or launching formal high‐value care programs.
Conduct Value‐Improvement Projects
Hospitalists across the country have largely taken the lead on designing value‐improvement pilots, programs, and groups within hospitals. Although value‐improvement projects may be built upon the established structures and techniques for quality improvement, importantly these programs should also include expertise in cost analyses.[8] Furthermore, some traditional quality‐improvement programs have failed to result in actual cost savings[44]; thus, it is not enough to simply rebrand quality improvement with a banner of value. Value‐improvement efforts must overcome the cultural hurdle of more care as better care, as well as pay careful attention to the diplomacy required with value improvement, because reducing costs may result in decreased revenue for certain departments or even decreases in individuals' wages.
One framework that we have used to guide value‐improvement project design is COST: culture, oversight accountability, system support, and training.[45] This approach leverages principles from implementation science to ensure that value‐improvement projects successfully provide multipronged tactics for overcoming the many barriers to high‐value care delivery. Figure 1 includes a worksheet for individual clinicians or teams to use when initially planning value‐improvement project interventions.[46] The examples in this worksheet come from a successful project at the University of California, San Francisco aimed at improving blood utilization stewardship by supporting adherence to a restrictive transfusion strategy. To address culture, a hospital‐wide campaign was led by physician peer champions to raise awareness about appropriate transfusion practices. This included posters that featured prominent local physician leaders displaying their support for the program. Oversight was provided through regular audit and feedback. Each month the number of patients on the medicine service who received transfusion with a pretransfusion hemoglobin above 8 grams per deciliter was shared at a faculty lunch meeting and shown on a graph included in the quality newsletter that was widely distributed in the hospital. The ordering system in the electronic medical record was eventually modified to include the patient's pretransfusion hemoglobin level at time of transfusion order and to provide default options and advice based on whether or not guidelines would generally recommend transfusion. Hospitalists and resident physicians were trained through multiple lectures and informal teaching settings about the rationale behind the changes and the evidence that supported a restrictive transfusion strategy.

Launch High‐Value Care Programs
As value‐improvement projects grow, some institutions have created high‐value care programs and infrastructure. In March 2012, the University of California, San Francisco Division of Hospital Medicine launched a high‐value care program to promote healthcare value and clinician engagement.[8] The program was led by clinical hospitalists alongside a financial administrator, and aimed to use financial data to identify areas with clear evidence of waste, create evidence‐based interventions that would simultaneously improve quality while cutting costs, and pair interventions with cost awareness education and culture change efforts. In the first year of this program, 6 projects were launched targeting: (1) nebulizer to inhaler transitions,[47] (2) overuse of proton pump inhibitor stress ulcer prophlaxis,[48] (3) transfusions, (4) telemetry, (5) ionized calcium lab ordering, and (6) repeat inpatient echocardiograms.[8]
Similar hospitalist‐led groups have now formed across the country including the Johns Hopkins High‐Value Care Committee, Johns Hopkins Bayview Physicians for Responsible Ordering, and High‐Value Carolina. These groups are relatively new, and best practices and early lessons are still emerging, but all focus on engaging frontline clinicians in choosing targets and leading multipronged intervention efforts.
What About Financial Incentives?
Hospitalist high‐value care groups thus far have mostly focused on intrinsic motivations for decreasing waste by appealing to hospitalists' sense of professionalism and their commitment to improve patient affordability. When financial incentives are used, it is important that they are well aligned with internal motivations for clinicians to provide the best possible care to their patients. The Institute of Medicine recommends that payments are structured in a way to reward continuous learning and improvement in the provision of best care at lower cost.[19] In the Geisinger Health System in Pennsylvania, physician incentives are designed to reward teamwork and collaboration. For example, endocrinologists' goals are based on good control of glucose levels for all diabetes patients in the system, not just those they see.[49] Moreover, a collaborative approach is encouraged by bringing clinicians together across disciplinary service lines to plan, budget, and evaluate one another's performance. These efforts are partly credited with a 43% reduction in hospitalized days and $100 per member per month in savings among diabetic patients.[50]
Healthcare leaders, Drs. Tom Lee and Toby Cosgrove, have made a number of recommendations for creating incentives that lead to sustainable changes in care delivery[49]: avoid attaching large sums to any single target, watch for conflicts of interest, reward collaboration, and communicate the incentive program and goals clearly to clinicians.
In general, when appropriate extrinsic motivators align or interact synergistically with intrinsic motivation, it can promote high levels of performance and satisfaction.[51]
CONCLUSIONS
Hospitalists are now faced with a responsibility to reduce financial harm and provide high‐value care. To achieve this goal, hospitalist groups are developing innovative models for care across the continuum from hospital to home, and individual hospitalists can advocate for appropriate care and lead value‐improvement initiatives in hospitals. Through existing knowledge and new frameworks and tools that specifically address value, hospitalists can champion value at the bedside and ensure their patients get the best possible care at lower costs.
Disclosures: Drs. Moriates, Shah, and Arora have received grant funding from the ABIM Foundation, and royalties from McGraw‐Hill for the textbook Understanding Value‐Based Healthcare. The authors report no conflicts of interest.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367(4):292–295.
- . Value‐driven health care: implications for hospitals and hospitalists. J Hosp Med. 2009;4(8):507–511.
- , . Hospital value‐based purchasing. J Hosp Med. 2013;8(5):271–277.
- . Setting value‐based payment goals—HHS efforts to improve U.S. health care. N Engl J Med. 2015;372(10):897–899.
- , . Redesigning care for patients at increased hospitalization risk: the Comprehensive Care Physician model. Health Aff Proj Hope. 2014;33(5):770–777.
- , , , et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486–492.
- , , . First, do no (financial) harm. JAMA. 2013;310(6):577–578.
- , , , . Development of a hospital‐based program focused on improving healthcare value. J Hosp Med. 2014;9(10):671–677.
- , , , et al. A controlled trial of a critical pathway for treatment of community‐acquired pneumonia. JAMA. 2000;283(6):749–755.
- , , , , . Evidence‐based care pathway for cellulitis improves process, clinical, and cost outcomes [published online July 28, 2015]. J Hosp Med. doi:10.1002/jhm.2433.
- . The Lean approach to health care: safety, quality, and cost. Institute of Medicine. Available at: http://nam.edu/perspectives‐2012‐the‐lean‐approach‐to‐health‐care‐safety‐quality‐and‐cost/. Accessed September 22, 2015.
- , , , et al. The impact of hospitalist discontinuity on hospital cost, readmissions, and patient satisfaction. J Gen Intern Med. 2014;29(7):1004–1008.
- Congressional Budget Office. Lessons from Medicare's Demonstration Projects on Disease Management, Care Coordination, and Value‐Based Payment. Available at: https://www.cbo.gov/publication/42860. Accessed April 26, 2015.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620.
- . “SNFists” at work: nursing home docs patterned after hospitalists. Mod Healthc. 2012;42(13):32–33.
- , , , . Nursing home physician specialists: a response to the workforce crisis in long‐term care. Ann Intern Med. 2009;150(6):411–413.
- Institute of Medicine. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2012.
- , . The perfect storm of overutilization. JAMA. 2008;299(23):2789–2791.
- , , , et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100–108.
- , . Patients' expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2015;175(2):274–286.
- , , , et al. Enhancing the Use and Quality of Colorectal Cancer Screening. Rockville, MD: Agency for Healthcare Research and Quality; 2010. Available at: http://www.ncbi.nlm.nih.gov/books/NBK44526. Accessed September 30, 2013.
- , , , et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479–485.
- . Teaching Choosing Wisely in medical education and training: the story of a pioneer. The Medical Professionalism Blog. Available at: http://blog.abimfoundation.org/teaching‐choosing‐wisely‐in‐meded. Accessed March 29, 2014.
- American College of Radiology. ACR appropriateness criteria overview. November 2013. Available at: http://www.acr.org/∼/media/ACR/Documents/AppCriteria/Overview.pdf. Accessed March 4, 2014.
- American College of Cardiology Foundation. Appropriate use criteria: what you need to know. Available at: http://www.cardiosource.org/∼/media/Files/Science%20and%20Quality/Quality%20Programs/FOCUS/E1302_AUC_Primer_Update.ashx. Accessed March 4, 2014.
- , , , , . SOAP‐V: applying high‐value care during patient care. The Medical Professionalism Blog. Available at: http://blog.abimfoundation.org/soap‐v‐applying‐high‐value‐care‐during‐patient‐care. Accessed April 3, 2015.
- , . Why does antimicrobial overuse in hospitalized patients persist? JAMA Intern Med. 2014;174(5):661–662.
- . The myth of the demanding patient. JAMA Oncol. 2015;1(1):18–19.
- . The disruptive innovation of price transparency in health care. JAMA. 2013;310(18):1927–1928.
- United States Government Accountability Office. Health Care Price Transparency—Meaningful Price Information Is Difficult for Consumers to Obtain Prior to Receiving Care. Washington, DC: United States Government Accountability Office; 2011:43.
- , , . General pediatric attending physicians' and residents' knowledge of inpatient hospital finances. Pediatrics. 2013;131(6):1072–1080.
- , , . Hospitalists' awareness of patient charges associated with inpatient care. J Hosp Med. 2010;5(5):295–297.
- . Cost consciousness in patient care—what is medical education's responsibility? N Engl J Med. 2010;362(14):1253–1255.
- . Providing high‐value, cost‐conscious care: a critical seventh general competency for physicians. Ann Intern Med. 2011;155(6):386–388.
- , , , . Defining competencies for education in health care value: recommendations from the University of California, San Francisco Center for Healthcare Value Training Initiative. Acad Med. 2015;90(4):421–424.
- , , . Understanding Value‐Based Healthcare. New York: McGraw‐Hill; 2015.
- , , , . Wisdom of the crowd: bright ideas and innovations from the teaching value and choosing wisely challenge. Acad Med. 2015;90(5):624–628.
- , , , et al. Does the computerized display of charges affect inpatient ancillary test utilization? Arch Intern Med. 1997;157(21):2501–2508.
- , , , et al. Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Intern Med. 2013;173(10):903–908.
- , , , . The effect of charge display on cost of care and physician practice behaviors: a systematic review. J Gen Intern Med. 2015;30(6):835–842.
- , , , , , . Closing the Quality Gap: Revisiting the State of the Science. Vol. 5. Public Reporting as a Quality Improvement Strategy. Rockville, MD: Agency for Healthcare Research and Quality; 2012.
- , , , . The savings illusion—why clinical quality improvement fails to deliver bottom‐line results. N Engl J Med. 2011;365(26):e48.
- , , , . Fostering value in clinical practice among future physicians: time to consider COST. Acad Med. 2014;89(11):1440.
- , , , , , . The Teaching Value Workshop. MedEdPORTAL Publications; 2014. Available at: https://www.mededportal.org/publication/9859. Accessed September 22, 2015.
- , , , , . “Nebs no more after 24”: a pilot program to improve the use of appropriate respiratory therapies. JAMA Intern Med. 2013;173(17):1647–1648.
- , , , et al. The development and implementation of a bundled quality improvement initiative to reduce inappropriate stress ulcer prophylaxis. ICU Dir. 2013;4(6):322–325.
- , . Engaging doctors in the health care revolution. Harvard Business Review. June 2014. Available at: http://hbr.org/2014/06/engaging‐doctors‐in‐the‐health‐care‐revolution/ar/1. Accessed July 30, 2014.
- , , . Geisinger Health System: achieving the potential of system integration through innovation, leadership, measurement, and incentives. June 2009. Available at: http://www.commonwealthfund.org/publications/case‐studies/2009/jun/geisinger‐health‐system‐achieving‐the‐potential‐of‐system‐integration. Accessed September 22, 2015.
- Motivational synergy: toward new conceptualizations of intrinsic and extrinsic motivation in the workplace. Hum Resource Manag 1993;3(3):185–201. Available at: http://www.hbs.edu/faculty/Pages/item.aspx?num=2500. Accessed July 31, 2014.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367(4):292–295.
- . Value‐driven health care: implications for hospitals and hospitalists. J Hosp Med. 2009;4(8):507–511.
- , . Hospital value‐based purchasing. J Hosp Med. 2013;8(5):271–277.
- . Setting value‐based payment goals—HHS efforts to improve U.S. health care. N Engl J Med. 2015;372(10):897–899.
- , . Redesigning care for patients at increased hospitalization risk: the Comprehensive Care Physician model. Health Aff Proj Hope. 2014;33(5):770–777.
- , , , et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486–492.
- , , . First, do no (financial) harm. JAMA. 2013;310(6):577–578.
- , , , . Development of a hospital‐based program focused on improving healthcare value. J Hosp Med. 2014;9(10):671–677.
- , , , et al. A controlled trial of a critical pathway for treatment of community‐acquired pneumonia. JAMA. 2000;283(6):749–755.
- , , , , . Evidence‐based care pathway for cellulitis improves process, clinical, and cost outcomes [published online July 28, 2015]. J Hosp Med. doi:10.1002/jhm.2433.
- . The Lean approach to health care: safety, quality, and cost. Institute of Medicine. Available at: http://nam.edu/perspectives‐2012‐the‐lean‐approach‐to‐health‐care‐safety‐quality‐and‐cost/. Accessed September 22, 2015.
- , , , et al. The impact of hospitalist discontinuity on hospital cost, readmissions, and patient satisfaction. J Gen Intern Med. 2014;29(7):1004–1008.
- Congressional Budget Office. Lessons from Medicare's Demonstration Projects on Disease Management, Care Coordination, and Value‐Based Payment. Available at: https://www.cbo.gov/publication/42860. Accessed April 26, 2015.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620.
- . “SNFists” at work: nursing home docs patterned after hospitalists. Mod Healthc. 2012;42(13):32–33.
- , , , . Nursing home physician specialists: a response to the workforce crisis in long‐term care. Ann Intern Med. 2009;150(6):411–413.
- Institute of Medicine. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2012.
- , . The perfect storm of overutilization. JAMA. 2008;299(23):2789–2791.
- , , , et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100–108.
- , . Patients' expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2015;175(2):274–286.
- , , , et al. Enhancing the Use and Quality of Colorectal Cancer Screening. Rockville, MD: Agency for Healthcare Research and Quality; 2010. Available at: http://www.ncbi.nlm.nih.gov/books/NBK44526. Accessed September 30, 2013.
- , , , et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479–485.
- . Teaching Choosing Wisely in medical education and training: the story of a pioneer. The Medical Professionalism Blog. Available at: http://blog.abimfoundation.org/teaching‐choosing‐wisely‐in‐meded. Accessed March 29, 2014.
- American College of Radiology. ACR appropriateness criteria overview. November 2013. Available at: http://www.acr.org/∼/media/ACR/Documents/AppCriteria/Overview.pdf. Accessed March 4, 2014.
- American College of Cardiology Foundation. Appropriate use criteria: what you need to know. Available at: http://www.cardiosource.org/∼/media/Files/Science%20and%20Quality/Quality%20Programs/FOCUS/E1302_AUC_Primer_Update.ashx. Accessed March 4, 2014.
- , , , , . SOAP‐V: applying high‐value care during patient care. The Medical Professionalism Blog. Available at: http://blog.abimfoundation.org/soap‐v‐applying‐high‐value‐care‐during‐patient‐care. Accessed April 3, 2015.
- , . Why does antimicrobial overuse in hospitalized patients persist? JAMA Intern Med. 2014;174(5):661–662.
- . The myth of the demanding patient. JAMA Oncol. 2015;1(1):18–19.
- . The disruptive innovation of price transparency in health care. JAMA. 2013;310(18):1927–1928.
- United States Government Accountability Office. Health Care Price Transparency—Meaningful Price Information Is Difficult for Consumers to Obtain Prior to Receiving Care. Washington, DC: United States Government Accountability Office; 2011:43.
- , , . General pediatric attending physicians' and residents' knowledge of inpatient hospital finances. Pediatrics. 2013;131(6):1072–1080.
- , , . Hospitalists' awareness of patient charges associated with inpatient care. J Hosp Med. 2010;5(5):295–297.
- . Cost consciousness in patient care—what is medical education's responsibility? N Engl J Med. 2010;362(14):1253–1255.
- . Providing high‐value, cost‐conscious care: a critical seventh general competency for physicians. Ann Intern Med. 2011;155(6):386–388.
- , , , . Defining competencies for education in health care value: recommendations from the University of California, San Francisco Center for Healthcare Value Training Initiative. Acad Med. 2015;90(4):421–424.
- , , . Understanding Value‐Based Healthcare. New York: McGraw‐Hill; 2015.
- , , , . Wisdom of the crowd: bright ideas and innovations from the teaching value and choosing wisely challenge. Acad Med. 2015;90(5):624–628.
- , , , et al. Does the computerized display of charges affect inpatient ancillary test utilization? Arch Intern Med. 1997;157(21):2501–2508.
- , , , et al. Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Intern Med. 2013;173(10):903–908.
- , , , . The effect of charge display on cost of care and physician practice behaviors: a systematic review. J Gen Intern Med. 2015;30(6):835–842.
- , , , , , . Closing the Quality Gap: Revisiting the State of the Science. Vol. 5. Public Reporting as a Quality Improvement Strategy. Rockville, MD: Agency for Healthcare Research and Quality; 2012.
- , , , . The savings illusion—why clinical quality improvement fails to deliver bottom‐line results. N Engl J Med. 2011;365(26):e48.
- , , , . Fostering value in clinical practice among future physicians: time to consider COST. Acad Med. 2014;89(11):1440.
- , , , , , . The Teaching Value Workshop. MedEdPORTAL Publications; 2014. Available at: https://www.mededportal.org/publication/9859. Accessed September 22, 2015.
- , , , , . “Nebs no more after 24”: a pilot program to improve the use of appropriate respiratory therapies. JAMA Intern Med. 2013;173(17):1647–1648.
- , , , et al. The development and implementation of a bundled quality improvement initiative to reduce inappropriate stress ulcer prophylaxis. ICU Dir. 2013;4(6):322–325.
- , . Engaging doctors in the health care revolution. Harvard Business Review. June 2014. Available at: http://hbr.org/2014/06/engaging‐doctors‐in‐the‐health‐care‐revolution/ar/1. Accessed July 30, 2014.
- , , . Geisinger Health System: achieving the potential of system integration through innovation, leadership, measurement, and incentives. June 2009. Available at: http://www.commonwealthfund.org/publications/case‐studies/2009/jun/geisinger‐health‐system‐achieving‐the‐potential‐of‐system‐integration. Accessed September 22, 2015.
- Motivational synergy: toward new conceptualizations of intrinsic and extrinsic motivation in the workplace. Hum Resource Manag 1993;3(3):185–201. Available at: http://www.hbs.edu/faculty/Pages/item.aspx?num=2500. Accessed July 31, 2014.
© 2015 Society of Hospital Medicine
Striving for Optimal Care/
Hospitalists have a professional obligation to provide the highest quality care for patients and increasingly, hospitalists lead programs to improve quality, value, and patient experience.[1, 2, 3]
The federal government introduced the hospital Value‐Based Purchasing (VBP) program in 2012, initially with 1% of Medicare hospital payments tied to quality indicators. This percentage will continue to grow and the VBP program has expanded to include metrics related to quality, safety, cost‐effectiveness, and patient satisfaction.[4] Hospitals now face significant financial penalties if they do not achieve these benchmarks; thus, remaining up‐to‐date with the literature and the most promising interventions in these arenas is vital for hospitalists.
The goal of this update is to summarize and critique recently published research that has the greatest potential to impact clinical practice in quality, value, and patient experience in hospital medicine. We reviewed articles published between January 2014 and February 2015. To identify articles, we hand‐searched leading journals, continuing medical education collaborative journal reviews (including New England Journal of Medicine Journal Watch and the American College of Physicians Journal Club), the Agency for Healthcare Research and Quality's Patient Safety network, and PubMed. We evaluated articles based on their scientific rigor (peer review, study methodology, site number, and sample size) and applicability to hospital medicine. In this review, we summarize 9 articles that were felt by the authors to have the highest potential for impact on the clinical practice of hospital medicine, as directly related to quality, value, or patient experience. We present each topic with a current quality question that the accompanying article(s) will help address. We summarize each article and its findings and note cautions and implications for practice. The selected articles cover aspects related to patient safety, readmissions, patient satisfaction, and resource utilization, with each of these topics related to specific metrics included in VBP. We presented this update at the 2015 Society of Hospital Medicine national meeting.
IS THERE ANYTHING WE CAN DO TO MAKE HANDOFFS SAFER?
Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):18031812.
Background
With recent changes in resident duty hours and staffing models, the number of clinical handoffs during a patient's hospital stay has been increasing.[5] The omission of critical information and the transfer of erroneous information during handoffs is common, which contributes to preventable medical errors.[6]
Findings
This prospective intervention study of a resident handoff program in 9 hospitals sought to improve communication between healthcare providers and to decrease medical errors. The I‐PASS mnemonic, which stands for illness severity, patient summary, action list, situation awareness, and synthesis by receiver, was introduced to standardize oral and written handoffs. The program also included a 2‐hour workshop, a 1‐hour role‐playing and simulation session, a computer module, a faculty development program, direct observation tools, and a culture change campaign. Medical errors decreased by 23% following the intervention, compared to the preintervention baseline (24.5 vs 18.8 per 100 admissions, P 0.001), and the rate of preventable adverse events dropped by 30% (4.7 vs 3.3 events per 100 admissions, P 0.001), whereas nonpreventable adverse events did not change. Process measures of handoff quality uniformly improved with the intervention. The duration of oral handoffs was approximately 2.5 minutes per patient both before and during the intervention period.
Cautions
Not all of the sites in the study saw significant reductions in medical errors; 3 of the programs did not have significantly improved medical error rates following implementation of the I‐PASS handoff bundle. The study design was not a randomized controlled trial, and thus the pre‐ versus postimplementation analyses cannot draw definitive causal links between the intervention and the observed improvements in safety outcomes. Furthermore, this study was done with pediatric residents, and one cannot assume that the results will translate to practicing hospitalists, who may not benefit as much from a scripted sign‐out.
Implications
A comprehensive handoff program that included the I‐PASS mnemonic along with extensive training, faculty development, and a culture‐change campaign was associated with impressive improvements in patient safety outcomes, without negatively effecting workflow.
WHAT ARE THE COMMON FEATURES OF INTERVENTIONS THAT HAVE SUCCESSFULLY REDUCED READMISSIONS?
Leppin AL, Glonfriddo MR, Kessler M, et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174(7):10951107.
Background
Hospital readmissions are common, costly, and potentially represent a failure to adequately prepare patients for hospital discharge, but efforts to prevent 30‐day readmissions have been mixed.[7] The investigators in this study offer a novel framework, the cumulative complexity model, as a way to conceptualize postdischarge outcomes such as readmission. The model depicts the balance between the patient's workload of managing their illness, including the demands of monitoring treatment and self‐care, and the patient's capacity to handle that workfunctionality, financial/social resources, literacy, and empowerment. Workload‐capacity imbalances (when workload outstrips capacity) may lead to progressively increasing illness and increasing complexity, which contribute to poor patient outcomes like readmissions. Decreasing a patient's workload or increasing their capacity may be effective in reducing readmissions.
Findings
Investigators sought to identify factors associated with successful interventions to reduce 30‐day readmissions, including how the interventions fit into the cumulative complexity model. After performing a comprehensive search of randomized trials of interventions to reduce readmissions, the investigators identified 42 randomized trials with the primary outcome of 30‐day readmission rates. In addition to reviewing intervention characteristics, blinded raters scored interventions based on their effects on reducing or increasing patient workload and reducing or increasing patient capacity for self‐care. Interventions that had several components (eg, pharmacy education, postdischarge phone calls, visiting nurses, health coaches, close primary care follow‐up) were more likely to be successful (1.4 times as likely; P = 0.001), as were interventions that involved 2 or more individuals (1.3 times as likely; P = 0.05). Interventions that were published prior to 2002 were 1.6 times more likely to have reduced readmissions (P = 0.01). When applied to the cumulative complexity model, interventions that sought to augment patient capacity for self‐care were 1.3 times as likely to be successful (P = 0.04), whereas no relationship was found between an intervention's effect on patient workload and readmission.
Cautions
The authors evaluated each intervention based on the degree to which it was likely to affect patient workload and patient capacity. Because a multifaceted intervention may have had components that increased patient workload (eg, more self‐monitoring, appointments) and decreased patient workload (home visits, visiting nurses), the true effect of patient workload on readmissions may not have been optimally analyzed in this study. Additionally, this element of the study relied on a value judgment original to this work. Interventions that are burdensome to some, may be beneficial to those with the capacity and resources to access the care.
Implications
The body of studies reviewed suggests that interventions to reduce 30‐day readmissions are on the whole successful. Their findings are in keeping with past studies demonstrating more successful interventions that are resource‐intensive and multifaceted. Finding successful interventions that are also cost‐effective may be challenging. This article adds the cumulative complexity framework to what we already know about readmissions, highlighting patient capacity to manage the burden of their Illness as a new factor for success. Efforts to deliver patient‐centered education, explore barriers to adherence, and provide health coaching may be more successful than interventions that unwittingly add to the burden of disease treatment (multiple follow‐up appointments, complex medication schedules, and posthospital surveys and patient self‐assessments).
DOES PATIENT ACTIVATION CORRELATE WITH DECREASED RESOURCE USE OR READMISSIONS?
Mitchell SE, Gardiner PM, Sadikova E, et al. Patient activation and 30‐day post discharge hospital utilization. J Gen Intern Med. 2014;29(2):349355.
Background
Patient activation is widely recognized as the knowledge, skills, and confidence a person has in managing their own health or healthcare. Higher patient activation has been associated with improved health outcomes, but the relationship between patient activation and readmission to the hospital within 30 days is unknown.[8]
Findings
Using data from Project RED‐LIT (Re‐Engineered Discharge for patients with low health literacy), a randomized controlled trial conducted at an urban safety‐net hospital, investigators examined the relationship between all unplanned utilization events of hospital services within 30 days of discharge and patient activation, as measured by an abbreviated 8‐item version of the validated Patient Activation Measure (PAM). The PAM uses agreement with statements about a patient's sense of responsibility for his or her own health, confidence in seeking care and following through with medical treatments, and confidence in managing new problems to measure activation. The 695 participants were divided into quartiles based on their PAM score, and the investigators looked at the rates of unplanned utilization events in each group. After adjusting for potential confounders such as gender, age, Charlson Comorbidity Index, insurance, marital status, and education, there remained a significant effect between PAM and 30‐day hospital reutilization. Compared with those who scored in the highest quartile of activation, those in the lowest quartile had 1.75 times the rate of 30‐day reutilization (P 0.001). Those in the second highest and third highest quartile had 1.3 (P = 0.03) and 1.5 times (P 0.001) the rate of reutilization demonstrating a dose‐response relationship between activation and low reutilization.
Cautions
It is as yet unclear how best to apply these results and whether activation is a modifiable risk factor. Can a patient become more activated by providing more education and coaching during their hospital stay? Can providing close follow‐up and home services make a person more confident to manage their own illness? Although early identification of patients with low activation using PAM is being done at many hospitals, there is no study to suggest that targeting these patients can reduce readmission.
Implications
A low level of patient activation appears to be a risk factor for unplanned hospital utilization within 30 days of discharge. Given the increasing financial penalties, many hospitals across the country are using the PAM to determine how much support and which services they provide after discharge. Identifying these patients early in their hospitalization could allow providers to spend more time and attention on preparing them for managing their own illness after discharge. As above, the effects of this intervention on readmissions is as yet unclear.
IS THERE A RELATIONSHIP BETWEEN PATIENT SATISFACTION AND UNDERSTANDING OF THE PLAN OF CARE?
Kebede S, Shihab HM, Berger ZD, et al. Patients' understanding of their hospitalizations and association with satisfaction. JAMA Intern Med. 2014;174(10):16981700.
Background
Effective patient‐physician communication is associated with improved patient satisfaction, care quality, and clinical outcomes.[9] Whether a shared understanding of the plan of care between patients and clinicians affects satisfaction is unknown.
Findings
One hundred seventy‐seven patients who had 2 or more medical conditions, 2 or more medical procedures, and 2 or more days in the hospital were interviewed on the day of discharge. Patients were questioned about their overall understanding of their hospitalization and about specific aspects of their care. They were also asked to provide objective data to measure their understanding of their hospital course by (1) listing their medical diagnoses, (2) identifying indications for medication on discharge paperwork, and (3) listing tests or procedures they underwent from a standard list. Patients were then asked to rate their satisfaction with their hospitalization. Patients' self‐reported understanding was an average of 4.0 (very good) on a 5‐point scale. Their measured understanding scores for medical diagnoses, indications for medications and tests and procedures were 48.9%, 56.2%, and 59.4%, respectively. Factors associated with poor understanding of their hospital course were increasing age, less education, lower household income, black race, and longer length of stay. Patients reported a mean satisfaction of 4.0 (very satisfied). Higher self‐reported understanding was associated with higher patient satisfaction, irrespective of actual understanding.
Cautions
Despite their suboptimal measured understanding of their hospital course, the average patient rated their understanding as very good. This suggests that patients are either poor judges of effective communication or have low expectations for understanding. It also calls into question the relationship between quality of communication and patient satisfaction, because despite their satisfaction, patients' actual understanding was low. There was, however, a clear and positive relationship between patients' perceived understanding and their satisfaction, suggesting that shared understanding remains integral to patient satisfaction.
Implications
Patient satisfaction appears to be tied to patients' perceived understanding of their care, but when tested actual understanding was suboptimal. Further efforts in patient satisfaction should not only focus on the quality of our communication, but on the resulting understanding of our patients.
WHAT ARE UNIVERSAL STRATEGIES TO IMPROVE SATISFACTION AND PATIENT OUTCOMES?
Detsky AS, Krumholz HM. Reducing the trauma of hospitalization. JAMA. 2014;311(21):21692170.
Background
Although high readmission rates are a national problem, a minority of patients treated for common conditions like pneumonia, heart failure, and chronic obstructive pulmonary disease are readmitted for the same problem.[10] This suggests that readmissions may stem not from poor disease management, but from patient vulnerability to illness in the period following hospitalization.
Findings
In this viewpoint opinion article, the authors suggest that the depersonalizing and stressful hospital atmosphere contributes to a transient vulnerability in the period following hospitalization that makes it challenging for patients to care for themselves and their illness. They offer specific strategies for changing the nature of our hospital care to promote healing and to decrease patient stress. The authors suggest promoting personalization through accommodation of family members, and allowing personal clothing and personal dcor in their rooms. Physicians and consultants should make appointments so that patients and families can know when to expect important visits. The authors also focus on the provision of rest and nourishment by reducing nighttime disruption and the elimination on unnecessary restrictive diets. They argue that the hospital is a place of stressful disruptions and surprises, which could all be ameliorated by providing patients with a way to understand the members of their team and their roles as well as through providing a clear schedule for the day. Healthcare providers should not enter a room unannounced, and patients should be given private rooms as much as possible. Last, the authors focus on the elimination of unnecessary tests and procedures such as blood draws, telemetry, and urine cultures and the encouragement of activity by providing activities where patients can gather together outside their rooms.
Cautions
If these changes seem simple, they may not be. Many involve a significant shift in our thinking on how we provide carefrom a focus on disease and provider convenience to a true consideration for the health and peace of mind of our patients. Starting with small steps, such as reductions in phlebotomy and nighttime vital signs checks for the most stable patients and ensuring accommodations for families, may make this long list seem less daunting.
Implications
By promoting factors that affect a patient's well beingrest, nutrition, peace of mindwe may be discharging patients who are better equipped to manage their illness after their hospitalization.
DO HOSPITALISTS OVERTEST, AND IF SO, WHY?
Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100108.
Background
National efforts, such as the Choosing Wisely campaign, seek to decrease overuse of low‐value services.[11] The extent of the problem of overtesting among hospitalists and the underlying drivers for unnecessary testing in this group have not been clearly defined.
Findings
Practicing adult medicine hospitalists across the country were given a questionnaire that included clinical vignettes for common inpatient scenarios: a preoperative evaluation and a syncope workup. Respondents were randomly provided 1 of 4 versions of each vignette, which contained the same clinical information but varied by a family member's request for further testing and by disclosure of the occupation of the family member. For example, in the preoperative evaluation, the vignettes either: (1) provided no details about the patient's son; (2) identified the son as a physician; (3) mentioned the son's request for testing, but did not identify the son as a physician; or (4) identified the son as a physician who requested testing. The syncope vignette versions were structured similarly, except the family member was the patient's wife and she was an attorney. The authors collected 1020 responses from an initial pool of 1500, for a decent 68% response rate. Hospitalists commonly reported overuse of testing, with 52% to 65% of respondents requesting unnecessary testing in the preoperative evaluation scenario, and 82% to 85% in the syncope scenario. The majority of physicians reported that they knew the testing was not clinically indicated based on evidence or guidelines, but were ordering the test due to a desire to reassure the patients or themselves.
Cautions
Responses to clinical vignettes in a survey may not represent actually practices. In addition, all hospitalists surveyed in this study were members of the Society of Hospital Medicine, so may not accurately exemplify all practicing hospitalists.
Implications
Overuse of testing is very common among hospitalists. Although roughly one‐third of respondents incorrectly thought that testing in the given scenarios was supported by the evidence or guidelines, the majority knew that testing was not clinically indicated and reported ordering tests to help reassure their patients or themselves. This suggests evidence‐based medicine approaches to overuse, such as the Choosing Wisely campaign and the emergence of appropriateness criteria, are likely necessary but insufficient to change physician practice patterns. Efforts to decrease overuse will need to engage clinicians and patients in ways that help overcome the attitude that more testing is required to provide reassurance.
DO UNREALISTIC PATIENT EXPECTATIONS ABOUT INTERVENTIONS INFLUENCE DECISION MAKING AND CONTRIBUTE TO OVERUSE?
Hoffmann TC, Del Mar C. Patient expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2015;175(2):274286.
Background
Patient expectations have been implicated as a contributor to overuse of medical interventions. Studies that have measured patients' understanding of the potential benefits and harms of medical treatments and tests have been scattered across the literature.
Findings
This systematic review aggregated all studies that have quantitatively assessed patients' expectations of the benefits and/or harms of any treatment or test. Of more than 15,000 records screened, only 36 articles met the inclusion criteria of describing a study in which participants were asked to provide a quantitative estimate of the expected benefits and/or harms of a treatment, test, or screen. Fourteen of the studies (40%) focused on screening, 15 (43%) on treatment, 3 (9%) on a test, and 3 (9%) on both treatment and screening. Topics included cancer, medications, surgery, cardiovascular disease, and fetal‐maternal medicine. The majority of patients overestimated intervention benefit and underestimated harm, regardless of whether the intervention was a test or a treatment. For example, more than half of participants overestimated benefit for 22 of the 34 outcomes (65%) for which overestimation data were provided, and a majority of participants underestimated harm for 10 of the 15 outcomes (67%) with underestimation data available.
Cautions
This systematic review included a limited number of studies, with varying levels of quality and a lot of heterogeneity, making it difficult to reach clear aggregate conclusions.
Implications
Patients are often overly optimistic about medical interventions and they downplay potential risks, making it more difficult to effectively discourage overuse. Clinicians should clearly understand and communicate realistic expectations for the potential benefits and risks of screening, testing, and medical treatments with patients and the public at large.
HOW BIG OF A PROBLEM IS ANTIBIOTIC OVERUSE IN HOSPITALS AND CAN WE DO BETTER?
Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194200.
Background
Antibiotics are life‐saving therapies, but when used in inappropriate scenarios they can pose many risks.
Findings
This large national database study used the MarketScan Hospital Drug Database and the Centers for Disease Control and Prevention's (CDC) Emerging Infections Program data to explore antibiotic prescribing in hospital patients. More than half of all hospitalized patients (55.7%) received antibiotics during their stay. Half of all treatment antibiotics were prescribed for the treatment of either lower respiratory infections, urinary tract infections, or presumed gram‐positive infections. There was wide variation seen in antibiotic usage across hospital wards. Objective criteria for potential improvement in antimicrobial use were developed and applied at a subset of 36 hospitals. Antibiotic prescribing could be improved in 37.2% of the most common prescription scenarios reviewed, including patients receiving vancomycin or those being treated for a urinary tract infection. The impact of reducing inpatient antibiotic exposure on the incidence of Clostridium difficile colitis was modeled using data from 2 hospitals, revealing that decreasing hospitalized patients' exposure to broad‐spectrum antibiotics by 30% would lead to a 26% reduction in C difficile infections (interquartile range = 15%38%).
Cautions
Some of the estimates in this study are based on a convenience sample of claims and hospital‐based data, thus may not be an accurate representation, particularly when extrapolating to all US hospitals.
Implications
Antibiotic overuse is a rampant problem in hospitals, with many severe downstream effects such as C difficile infections and antimicrobial resistance. All hospital units should have an antibiotic stewardship program and should monitor antibiotic usage.
Lee TC, Frenette C, Jayaraman D, Green L, Pilote L. Antibiotic self‐stewardship: trainee‐led structured antibiotic time‐outs to improve antimicrobial use. Ann Intern Med. 2014;161(10 suppl):S53S58.
Background
The CDC and other groups have called for stewardship programs to address antibiotic overuse.[12] Few interventions have been shown to successfully engage medical trainees in efforts to improve their own antibiotic prescribing practices.
Findings
An antibiotic self‐stewardship program was developed and led by internal medicine residents at Montreal General Hospital. The intervention included a monthly resident education lecture on antimicrobial stewardship and twice‐weekly time‐out audits using a structured electronic checklist. Adherence with auditing was 80%. Total costs for antibiotics decreased from $149,743 CAD to $80,319 CAD, mostly due to an observed reduction in carbapenems. Moxifloxicin use decreased by 1.9 defined daily doses per 1000 patient‐days per month (P = 0.048). Rates of clostridium difficile colitis declined from 24.2 to 19.6 per 10,000 patient‐days, although this trend did not meet statistical significance (incidence rate ratio, 0.8 [confidence interval, 0.5‐1.3]).
Cautions
Although the use of some broader spectrum antibiotics decreased, there was no measurable change in overall antibiotic use, suggesting that physicians may have narrowed antibiotics but did not often completely discontinue them. The time‐series analyses in this study cannot provide causal conclusions between the intervention and outcomes. In fact, carbapenem usage appears to have significantly decreased prior to the implementation of the program, for unclear reasons. The feasibility of this educational intervention outside of a residency program is unclear.
Implications
A combination of education, oversight and frontline clinician engagement in structured time‐outs may be effective, at least in narrowing antibiotic usage. The structured audit checklist developed by these authors is available for free in the supplementary materials of the Annals of Internal Medicine article.
Disclosures: Dr. Moriates has received grant funding from the ABIM Foundation, and royalties from McGraw‐Hill for the textbook Understanding Value‐Based Healthcare. The authors report no conflicts of interest.
- . The role of the hospitalist in quality improvement: systems for improving the care of patients with acute coronary syndrome. J Hosp Med. 2010;5(suppl 4):S1–S7.
- , , , . Impact of hospitalist communication‐skills training on patient‐satisfaction scores. J Hosp Med. 2013;8(6):315–320.
- , , , . Development of a hospital‐based program focused on improving healthcare value. J Hosp Med. 2014;9(10):671–677.
- . Value‐driven health care: implications for hospitals and hospitalists. J Hosp Med. 2009;4(8):507–511.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013;173(8):649–655.
- , , , , . Does housestaff discontinuity of care increase the risk for preventable adverse events? Ann Intern Med. 1994;121(11):866–872.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520–528.
- , , . Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8(5):410–417.
- . Effective physician‐patient communication and health outcomes: a review. CMAJ. 2007;152(9):1423–1433.
- , , , et al. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355–363.
- , , , et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486–492.
- , , , et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194–200.
Hospitalists have a professional obligation to provide the highest quality care for patients and increasingly, hospitalists lead programs to improve quality, value, and patient experience.[1, 2, 3]
The federal government introduced the hospital Value‐Based Purchasing (VBP) program in 2012, initially with 1% of Medicare hospital payments tied to quality indicators. This percentage will continue to grow and the VBP program has expanded to include metrics related to quality, safety, cost‐effectiveness, and patient satisfaction.[4] Hospitals now face significant financial penalties if they do not achieve these benchmarks; thus, remaining up‐to‐date with the literature and the most promising interventions in these arenas is vital for hospitalists.
The goal of this update is to summarize and critique recently published research that has the greatest potential to impact clinical practice in quality, value, and patient experience in hospital medicine. We reviewed articles published between January 2014 and February 2015. To identify articles, we hand‐searched leading journals, continuing medical education collaborative journal reviews (including New England Journal of Medicine Journal Watch and the American College of Physicians Journal Club), the Agency for Healthcare Research and Quality's Patient Safety network, and PubMed. We evaluated articles based on their scientific rigor (peer review, study methodology, site number, and sample size) and applicability to hospital medicine. In this review, we summarize 9 articles that were felt by the authors to have the highest potential for impact on the clinical practice of hospital medicine, as directly related to quality, value, or patient experience. We present each topic with a current quality question that the accompanying article(s) will help address. We summarize each article and its findings and note cautions and implications for practice. The selected articles cover aspects related to patient safety, readmissions, patient satisfaction, and resource utilization, with each of these topics related to specific metrics included in VBP. We presented this update at the 2015 Society of Hospital Medicine national meeting.
IS THERE ANYTHING WE CAN DO TO MAKE HANDOFFS SAFER?
Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):18031812.
Background
With recent changes in resident duty hours and staffing models, the number of clinical handoffs during a patient's hospital stay has been increasing.[5] The omission of critical information and the transfer of erroneous information during handoffs is common, which contributes to preventable medical errors.[6]
Findings
This prospective intervention study of a resident handoff program in 9 hospitals sought to improve communication between healthcare providers and to decrease medical errors. The I‐PASS mnemonic, which stands for illness severity, patient summary, action list, situation awareness, and synthesis by receiver, was introduced to standardize oral and written handoffs. The program also included a 2‐hour workshop, a 1‐hour role‐playing and simulation session, a computer module, a faculty development program, direct observation tools, and a culture change campaign. Medical errors decreased by 23% following the intervention, compared to the preintervention baseline (24.5 vs 18.8 per 100 admissions, P 0.001), and the rate of preventable adverse events dropped by 30% (4.7 vs 3.3 events per 100 admissions, P 0.001), whereas nonpreventable adverse events did not change. Process measures of handoff quality uniformly improved with the intervention. The duration of oral handoffs was approximately 2.5 minutes per patient both before and during the intervention period.
Cautions
Not all of the sites in the study saw significant reductions in medical errors; 3 of the programs did not have significantly improved medical error rates following implementation of the I‐PASS handoff bundle. The study design was not a randomized controlled trial, and thus the pre‐ versus postimplementation analyses cannot draw definitive causal links between the intervention and the observed improvements in safety outcomes. Furthermore, this study was done with pediatric residents, and one cannot assume that the results will translate to practicing hospitalists, who may not benefit as much from a scripted sign‐out.
Implications
A comprehensive handoff program that included the I‐PASS mnemonic along with extensive training, faculty development, and a culture‐change campaign was associated with impressive improvements in patient safety outcomes, without negatively effecting workflow.
WHAT ARE THE COMMON FEATURES OF INTERVENTIONS THAT HAVE SUCCESSFULLY REDUCED READMISSIONS?
Leppin AL, Glonfriddo MR, Kessler M, et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174(7):10951107.
Background
Hospital readmissions are common, costly, and potentially represent a failure to adequately prepare patients for hospital discharge, but efforts to prevent 30‐day readmissions have been mixed.[7] The investigators in this study offer a novel framework, the cumulative complexity model, as a way to conceptualize postdischarge outcomes such as readmission. The model depicts the balance between the patient's workload of managing their illness, including the demands of monitoring treatment and self‐care, and the patient's capacity to handle that workfunctionality, financial/social resources, literacy, and empowerment. Workload‐capacity imbalances (when workload outstrips capacity) may lead to progressively increasing illness and increasing complexity, which contribute to poor patient outcomes like readmissions. Decreasing a patient's workload or increasing their capacity may be effective in reducing readmissions.
Findings
Investigators sought to identify factors associated with successful interventions to reduce 30‐day readmissions, including how the interventions fit into the cumulative complexity model. After performing a comprehensive search of randomized trials of interventions to reduce readmissions, the investigators identified 42 randomized trials with the primary outcome of 30‐day readmission rates. In addition to reviewing intervention characteristics, blinded raters scored interventions based on their effects on reducing or increasing patient workload and reducing or increasing patient capacity for self‐care. Interventions that had several components (eg, pharmacy education, postdischarge phone calls, visiting nurses, health coaches, close primary care follow‐up) were more likely to be successful (1.4 times as likely; P = 0.001), as were interventions that involved 2 or more individuals (1.3 times as likely; P = 0.05). Interventions that were published prior to 2002 were 1.6 times more likely to have reduced readmissions (P = 0.01). When applied to the cumulative complexity model, interventions that sought to augment patient capacity for self‐care were 1.3 times as likely to be successful (P = 0.04), whereas no relationship was found between an intervention's effect on patient workload and readmission.
Cautions
The authors evaluated each intervention based on the degree to which it was likely to affect patient workload and patient capacity. Because a multifaceted intervention may have had components that increased patient workload (eg, more self‐monitoring, appointments) and decreased patient workload (home visits, visiting nurses), the true effect of patient workload on readmissions may not have been optimally analyzed in this study. Additionally, this element of the study relied on a value judgment original to this work. Interventions that are burdensome to some, may be beneficial to those with the capacity and resources to access the care.
Implications
The body of studies reviewed suggests that interventions to reduce 30‐day readmissions are on the whole successful. Their findings are in keeping with past studies demonstrating more successful interventions that are resource‐intensive and multifaceted. Finding successful interventions that are also cost‐effective may be challenging. This article adds the cumulative complexity framework to what we already know about readmissions, highlighting patient capacity to manage the burden of their Illness as a new factor for success. Efforts to deliver patient‐centered education, explore barriers to adherence, and provide health coaching may be more successful than interventions that unwittingly add to the burden of disease treatment (multiple follow‐up appointments, complex medication schedules, and posthospital surveys and patient self‐assessments).
DOES PATIENT ACTIVATION CORRELATE WITH DECREASED RESOURCE USE OR READMISSIONS?
Mitchell SE, Gardiner PM, Sadikova E, et al. Patient activation and 30‐day post discharge hospital utilization. J Gen Intern Med. 2014;29(2):349355.
Background
Patient activation is widely recognized as the knowledge, skills, and confidence a person has in managing their own health or healthcare. Higher patient activation has been associated with improved health outcomes, but the relationship between patient activation and readmission to the hospital within 30 days is unknown.[8]
Findings
Using data from Project RED‐LIT (Re‐Engineered Discharge for patients with low health literacy), a randomized controlled trial conducted at an urban safety‐net hospital, investigators examined the relationship between all unplanned utilization events of hospital services within 30 days of discharge and patient activation, as measured by an abbreviated 8‐item version of the validated Patient Activation Measure (PAM). The PAM uses agreement with statements about a patient's sense of responsibility for his or her own health, confidence in seeking care and following through with medical treatments, and confidence in managing new problems to measure activation. The 695 participants were divided into quartiles based on their PAM score, and the investigators looked at the rates of unplanned utilization events in each group. After adjusting for potential confounders such as gender, age, Charlson Comorbidity Index, insurance, marital status, and education, there remained a significant effect between PAM and 30‐day hospital reutilization. Compared with those who scored in the highest quartile of activation, those in the lowest quartile had 1.75 times the rate of 30‐day reutilization (P 0.001). Those in the second highest and third highest quartile had 1.3 (P = 0.03) and 1.5 times (P 0.001) the rate of reutilization demonstrating a dose‐response relationship between activation and low reutilization.
Cautions
It is as yet unclear how best to apply these results and whether activation is a modifiable risk factor. Can a patient become more activated by providing more education and coaching during their hospital stay? Can providing close follow‐up and home services make a person more confident to manage their own illness? Although early identification of patients with low activation using PAM is being done at many hospitals, there is no study to suggest that targeting these patients can reduce readmission.
Implications
A low level of patient activation appears to be a risk factor for unplanned hospital utilization within 30 days of discharge. Given the increasing financial penalties, many hospitals across the country are using the PAM to determine how much support and which services they provide after discharge. Identifying these patients early in their hospitalization could allow providers to spend more time and attention on preparing them for managing their own illness after discharge. As above, the effects of this intervention on readmissions is as yet unclear.
IS THERE A RELATIONSHIP BETWEEN PATIENT SATISFACTION AND UNDERSTANDING OF THE PLAN OF CARE?
Kebede S, Shihab HM, Berger ZD, et al. Patients' understanding of their hospitalizations and association with satisfaction. JAMA Intern Med. 2014;174(10):16981700.
Background
Effective patient‐physician communication is associated with improved patient satisfaction, care quality, and clinical outcomes.[9] Whether a shared understanding of the plan of care between patients and clinicians affects satisfaction is unknown.
Findings
One hundred seventy‐seven patients who had 2 or more medical conditions, 2 or more medical procedures, and 2 or more days in the hospital were interviewed on the day of discharge. Patients were questioned about their overall understanding of their hospitalization and about specific aspects of their care. They were also asked to provide objective data to measure their understanding of their hospital course by (1) listing their medical diagnoses, (2) identifying indications for medication on discharge paperwork, and (3) listing tests or procedures they underwent from a standard list. Patients were then asked to rate their satisfaction with their hospitalization. Patients' self‐reported understanding was an average of 4.0 (very good) on a 5‐point scale. Their measured understanding scores for medical diagnoses, indications for medications and tests and procedures were 48.9%, 56.2%, and 59.4%, respectively. Factors associated with poor understanding of their hospital course were increasing age, less education, lower household income, black race, and longer length of stay. Patients reported a mean satisfaction of 4.0 (very satisfied). Higher self‐reported understanding was associated with higher patient satisfaction, irrespective of actual understanding.
Cautions
Despite their suboptimal measured understanding of their hospital course, the average patient rated their understanding as very good. This suggests that patients are either poor judges of effective communication or have low expectations for understanding. It also calls into question the relationship between quality of communication and patient satisfaction, because despite their satisfaction, patients' actual understanding was low. There was, however, a clear and positive relationship between patients' perceived understanding and their satisfaction, suggesting that shared understanding remains integral to patient satisfaction.
Implications
Patient satisfaction appears to be tied to patients' perceived understanding of their care, but when tested actual understanding was suboptimal. Further efforts in patient satisfaction should not only focus on the quality of our communication, but on the resulting understanding of our patients.
WHAT ARE UNIVERSAL STRATEGIES TO IMPROVE SATISFACTION AND PATIENT OUTCOMES?
Detsky AS, Krumholz HM. Reducing the trauma of hospitalization. JAMA. 2014;311(21):21692170.
Background
Although high readmission rates are a national problem, a minority of patients treated for common conditions like pneumonia, heart failure, and chronic obstructive pulmonary disease are readmitted for the same problem.[10] This suggests that readmissions may stem not from poor disease management, but from patient vulnerability to illness in the period following hospitalization.
Findings
In this viewpoint opinion article, the authors suggest that the depersonalizing and stressful hospital atmosphere contributes to a transient vulnerability in the period following hospitalization that makes it challenging for patients to care for themselves and their illness. They offer specific strategies for changing the nature of our hospital care to promote healing and to decrease patient stress. The authors suggest promoting personalization through accommodation of family members, and allowing personal clothing and personal dcor in their rooms. Physicians and consultants should make appointments so that patients and families can know when to expect important visits. The authors also focus on the provision of rest and nourishment by reducing nighttime disruption and the elimination on unnecessary restrictive diets. They argue that the hospital is a place of stressful disruptions and surprises, which could all be ameliorated by providing patients with a way to understand the members of their team and their roles as well as through providing a clear schedule for the day. Healthcare providers should not enter a room unannounced, and patients should be given private rooms as much as possible. Last, the authors focus on the elimination of unnecessary tests and procedures such as blood draws, telemetry, and urine cultures and the encouragement of activity by providing activities where patients can gather together outside their rooms.
Cautions
If these changes seem simple, they may not be. Many involve a significant shift in our thinking on how we provide carefrom a focus on disease and provider convenience to a true consideration for the health and peace of mind of our patients. Starting with small steps, such as reductions in phlebotomy and nighttime vital signs checks for the most stable patients and ensuring accommodations for families, may make this long list seem less daunting.
Implications
By promoting factors that affect a patient's well beingrest, nutrition, peace of mindwe may be discharging patients who are better equipped to manage their illness after their hospitalization.
DO HOSPITALISTS OVERTEST, AND IF SO, WHY?
Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100108.
Background
National efforts, such as the Choosing Wisely campaign, seek to decrease overuse of low‐value services.[11] The extent of the problem of overtesting among hospitalists and the underlying drivers for unnecessary testing in this group have not been clearly defined.
Findings
Practicing adult medicine hospitalists across the country were given a questionnaire that included clinical vignettes for common inpatient scenarios: a preoperative evaluation and a syncope workup. Respondents were randomly provided 1 of 4 versions of each vignette, which contained the same clinical information but varied by a family member's request for further testing and by disclosure of the occupation of the family member. For example, in the preoperative evaluation, the vignettes either: (1) provided no details about the patient's son; (2) identified the son as a physician; (3) mentioned the son's request for testing, but did not identify the son as a physician; or (4) identified the son as a physician who requested testing. The syncope vignette versions were structured similarly, except the family member was the patient's wife and she was an attorney. The authors collected 1020 responses from an initial pool of 1500, for a decent 68% response rate. Hospitalists commonly reported overuse of testing, with 52% to 65% of respondents requesting unnecessary testing in the preoperative evaluation scenario, and 82% to 85% in the syncope scenario. The majority of physicians reported that they knew the testing was not clinically indicated based on evidence or guidelines, but were ordering the test due to a desire to reassure the patients or themselves.
Cautions
Responses to clinical vignettes in a survey may not represent actually practices. In addition, all hospitalists surveyed in this study were members of the Society of Hospital Medicine, so may not accurately exemplify all practicing hospitalists.
Implications
Overuse of testing is very common among hospitalists. Although roughly one‐third of respondents incorrectly thought that testing in the given scenarios was supported by the evidence or guidelines, the majority knew that testing was not clinically indicated and reported ordering tests to help reassure their patients or themselves. This suggests evidence‐based medicine approaches to overuse, such as the Choosing Wisely campaign and the emergence of appropriateness criteria, are likely necessary but insufficient to change physician practice patterns. Efforts to decrease overuse will need to engage clinicians and patients in ways that help overcome the attitude that more testing is required to provide reassurance.
DO UNREALISTIC PATIENT EXPECTATIONS ABOUT INTERVENTIONS INFLUENCE DECISION MAKING AND CONTRIBUTE TO OVERUSE?
Hoffmann TC, Del Mar C. Patient expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2015;175(2):274286.
Background
Patient expectations have been implicated as a contributor to overuse of medical interventions. Studies that have measured patients' understanding of the potential benefits and harms of medical treatments and tests have been scattered across the literature.
Findings
This systematic review aggregated all studies that have quantitatively assessed patients' expectations of the benefits and/or harms of any treatment or test. Of more than 15,000 records screened, only 36 articles met the inclusion criteria of describing a study in which participants were asked to provide a quantitative estimate of the expected benefits and/or harms of a treatment, test, or screen. Fourteen of the studies (40%) focused on screening, 15 (43%) on treatment, 3 (9%) on a test, and 3 (9%) on both treatment and screening. Topics included cancer, medications, surgery, cardiovascular disease, and fetal‐maternal medicine. The majority of patients overestimated intervention benefit and underestimated harm, regardless of whether the intervention was a test or a treatment. For example, more than half of participants overestimated benefit for 22 of the 34 outcomes (65%) for which overestimation data were provided, and a majority of participants underestimated harm for 10 of the 15 outcomes (67%) with underestimation data available.
Cautions
This systematic review included a limited number of studies, with varying levels of quality and a lot of heterogeneity, making it difficult to reach clear aggregate conclusions.
Implications
Patients are often overly optimistic about medical interventions and they downplay potential risks, making it more difficult to effectively discourage overuse. Clinicians should clearly understand and communicate realistic expectations for the potential benefits and risks of screening, testing, and medical treatments with patients and the public at large.
HOW BIG OF A PROBLEM IS ANTIBIOTIC OVERUSE IN HOSPITALS AND CAN WE DO BETTER?
Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194200.
Background
Antibiotics are life‐saving therapies, but when used in inappropriate scenarios they can pose many risks.
Findings
This large national database study used the MarketScan Hospital Drug Database and the Centers for Disease Control and Prevention's (CDC) Emerging Infections Program data to explore antibiotic prescribing in hospital patients. More than half of all hospitalized patients (55.7%) received antibiotics during their stay. Half of all treatment antibiotics were prescribed for the treatment of either lower respiratory infections, urinary tract infections, or presumed gram‐positive infections. There was wide variation seen in antibiotic usage across hospital wards. Objective criteria for potential improvement in antimicrobial use were developed and applied at a subset of 36 hospitals. Antibiotic prescribing could be improved in 37.2% of the most common prescription scenarios reviewed, including patients receiving vancomycin or those being treated for a urinary tract infection. The impact of reducing inpatient antibiotic exposure on the incidence of Clostridium difficile colitis was modeled using data from 2 hospitals, revealing that decreasing hospitalized patients' exposure to broad‐spectrum antibiotics by 30% would lead to a 26% reduction in C difficile infections (interquartile range = 15%38%).
Cautions
Some of the estimates in this study are based on a convenience sample of claims and hospital‐based data, thus may not be an accurate representation, particularly when extrapolating to all US hospitals.
Implications
Antibiotic overuse is a rampant problem in hospitals, with many severe downstream effects such as C difficile infections and antimicrobial resistance. All hospital units should have an antibiotic stewardship program and should monitor antibiotic usage.
Lee TC, Frenette C, Jayaraman D, Green L, Pilote L. Antibiotic self‐stewardship: trainee‐led structured antibiotic time‐outs to improve antimicrobial use. Ann Intern Med. 2014;161(10 suppl):S53S58.
Background
The CDC and other groups have called for stewardship programs to address antibiotic overuse.[12] Few interventions have been shown to successfully engage medical trainees in efforts to improve their own antibiotic prescribing practices.
Findings
An antibiotic self‐stewardship program was developed and led by internal medicine residents at Montreal General Hospital. The intervention included a monthly resident education lecture on antimicrobial stewardship and twice‐weekly time‐out audits using a structured electronic checklist. Adherence with auditing was 80%. Total costs for antibiotics decreased from $149,743 CAD to $80,319 CAD, mostly due to an observed reduction in carbapenems. Moxifloxicin use decreased by 1.9 defined daily doses per 1000 patient‐days per month (P = 0.048). Rates of clostridium difficile colitis declined from 24.2 to 19.6 per 10,000 patient‐days, although this trend did not meet statistical significance (incidence rate ratio, 0.8 [confidence interval, 0.5‐1.3]).
Cautions
Although the use of some broader spectrum antibiotics decreased, there was no measurable change in overall antibiotic use, suggesting that physicians may have narrowed antibiotics but did not often completely discontinue them. The time‐series analyses in this study cannot provide causal conclusions between the intervention and outcomes. In fact, carbapenem usage appears to have significantly decreased prior to the implementation of the program, for unclear reasons. The feasibility of this educational intervention outside of a residency program is unclear.
Implications
A combination of education, oversight and frontline clinician engagement in structured time‐outs may be effective, at least in narrowing antibiotic usage. The structured audit checklist developed by these authors is available for free in the supplementary materials of the Annals of Internal Medicine article.
Disclosures: Dr. Moriates has received grant funding from the ABIM Foundation, and royalties from McGraw‐Hill for the textbook Understanding Value‐Based Healthcare. The authors report no conflicts of interest.
Hospitalists have a professional obligation to provide the highest quality care for patients and increasingly, hospitalists lead programs to improve quality, value, and patient experience.[1, 2, 3]
The federal government introduced the hospital Value‐Based Purchasing (VBP) program in 2012, initially with 1% of Medicare hospital payments tied to quality indicators. This percentage will continue to grow and the VBP program has expanded to include metrics related to quality, safety, cost‐effectiveness, and patient satisfaction.[4] Hospitals now face significant financial penalties if they do not achieve these benchmarks; thus, remaining up‐to‐date with the literature and the most promising interventions in these arenas is vital for hospitalists.
The goal of this update is to summarize and critique recently published research that has the greatest potential to impact clinical practice in quality, value, and patient experience in hospital medicine. We reviewed articles published between January 2014 and February 2015. To identify articles, we hand‐searched leading journals, continuing medical education collaborative journal reviews (including New England Journal of Medicine Journal Watch and the American College of Physicians Journal Club), the Agency for Healthcare Research and Quality's Patient Safety network, and PubMed. We evaluated articles based on their scientific rigor (peer review, study methodology, site number, and sample size) and applicability to hospital medicine. In this review, we summarize 9 articles that were felt by the authors to have the highest potential for impact on the clinical practice of hospital medicine, as directly related to quality, value, or patient experience. We present each topic with a current quality question that the accompanying article(s) will help address. We summarize each article and its findings and note cautions and implications for practice. The selected articles cover aspects related to patient safety, readmissions, patient satisfaction, and resource utilization, with each of these topics related to specific metrics included in VBP. We presented this update at the 2015 Society of Hospital Medicine national meeting.
IS THERE ANYTHING WE CAN DO TO MAKE HANDOFFS SAFER?
Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):18031812.
Background
With recent changes in resident duty hours and staffing models, the number of clinical handoffs during a patient's hospital stay has been increasing.[5] The omission of critical information and the transfer of erroneous information during handoffs is common, which contributes to preventable medical errors.[6]
Findings
This prospective intervention study of a resident handoff program in 9 hospitals sought to improve communication between healthcare providers and to decrease medical errors. The I‐PASS mnemonic, which stands for illness severity, patient summary, action list, situation awareness, and synthesis by receiver, was introduced to standardize oral and written handoffs. The program also included a 2‐hour workshop, a 1‐hour role‐playing and simulation session, a computer module, a faculty development program, direct observation tools, and a culture change campaign. Medical errors decreased by 23% following the intervention, compared to the preintervention baseline (24.5 vs 18.8 per 100 admissions, P 0.001), and the rate of preventable adverse events dropped by 30% (4.7 vs 3.3 events per 100 admissions, P 0.001), whereas nonpreventable adverse events did not change. Process measures of handoff quality uniformly improved with the intervention. The duration of oral handoffs was approximately 2.5 minutes per patient both before and during the intervention period.
Cautions
Not all of the sites in the study saw significant reductions in medical errors; 3 of the programs did not have significantly improved medical error rates following implementation of the I‐PASS handoff bundle. The study design was not a randomized controlled trial, and thus the pre‐ versus postimplementation analyses cannot draw definitive causal links between the intervention and the observed improvements in safety outcomes. Furthermore, this study was done with pediatric residents, and one cannot assume that the results will translate to practicing hospitalists, who may not benefit as much from a scripted sign‐out.
Implications
A comprehensive handoff program that included the I‐PASS mnemonic along with extensive training, faculty development, and a culture‐change campaign was associated with impressive improvements in patient safety outcomes, without negatively effecting workflow.
WHAT ARE THE COMMON FEATURES OF INTERVENTIONS THAT HAVE SUCCESSFULLY REDUCED READMISSIONS?
Leppin AL, Glonfriddo MR, Kessler M, et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174(7):10951107.
Background
Hospital readmissions are common, costly, and potentially represent a failure to adequately prepare patients for hospital discharge, but efforts to prevent 30‐day readmissions have been mixed.[7] The investigators in this study offer a novel framework, the cumulative complexity model, as a way to conceptualize postdischarge outcomes such as readmission. The model depicts the balance between the patient's workload of managing their illness, including the demands of monitoring treatment and self‐care, and the patient's capacity to handle that workfunctionality, financial/social resources, literacy, and empowerment. Workload‐capacity imbalances (when workload outstrips capacity) may lead to progressively increasing illness and increasing complexity, which contribute to poor patient outcomes like readmissions. Decreasing a patient's workload or increasing their capacity may be effective in reducing readmissions.
Findings
Investigators sought to identify factors associated with successful interventions to reduce 30‐day readmissions, including how the interventions fit into the cumulative complexity model. After performing a comprehensive search of randomized trials of interventions to reduce readmissions, the investigators identified 42 randomized trials with the primary outcome of 30‐day readmission rates. In addition to reviewing intervention characteristics, blinded raters scored interventions based on their effects on reducing or increasing patient workload and reducing or increasing patient capacity for self‐care. Interventions that had several components (eg, pharmacy education, postdischarge phone calls, visiting nurses, health coaches, close primary care follow‐up) were more likely to be successful (1.4 times as likely; P = 0.001), as were interventions that involved 2 or more individuals (1.3 times as likely; P = 0.05). Interventions that were published prior to 2002 were 1.6 times more likely to have reduced readmissions (P = 0.01). When applied to the cumulative complexity model, interventions that sought to augment patient capacity for self‐care were 1.3 times as likely to be successful (P = 0.04), whereas no relationship was found between an intervention's effect on patient workload and readmission.
Cautions
The authors evaluated each intervention based on the degree to which it was likely to affect patient workload and patient capacity. Because a multifaceted intervention may have had components that increased patient workload (eg, more self‐monitoring, appointments) and decreased patient workload (home visits, visiting nurses), the true effect of patient workload on readmissions may not have been optimally analyzed in this study. Additionally, this element of the study relied on a value judgment original to this work. Interventions that are burdensome to some, may be beneficial to those with the capacity and resources to access the care.
Implications
The body of studies reviewed suggests that interventions to reduce 30‐day readmissions are on the whole successful. Their findings are in keeping with past studies demonstrating more successful interventions that are resource‐intensive and multifaceted. Finding successful interventions that are also cost‐effective may be challenging. This article adds the cumulative complexity framework to what we already know about readmissions, highlighting patient capacity to manage the burden of their Illness as a new factor for success. Efforts to deliver patient‐centered education, explore barriers to adherence, and provide health coaching may be more successful than interventions that unwittingly add to the burden of disease treatment (multiple follow‐up appointments, complex medication schedules, and posthospital surveys and patient self‐assessments).
DOES PATIENT ACTIVATION CORRELATE WITH DECREASED RESOURCE USE OR READMISSIONS?
Mitchell SE, Gardiner PM, Sadikova E, et al. Patient activation and 30‐day post discharge hospital utilization. J Gen Intern Med. 2014;29(2):349355.
Background
Patient activation is widely recognized as the knowledge, skills, and confidence a person has in managing their own health or healthcare. Higher patient activation has been associated with improved health outcomes, but the relationship between patient activation and readmission to the hospital within 30 days is unknown.[8]
Findings
Using data from Project RED‐LIT (Re‐Engineered Discharge for patients with low health literacy), a randomized controlled trial conducted at an urban safety‐net hospital, investigators examined the relationship between all unplanned utilization events of hospital services within 30 days of discharge and patient activation, as measured by an abbreviated 8‐item version of the validated Patient Activation Measure (PAM). The PAM uses agreement with statements about a patient's sense of responsibility for his or her own health, confidence in seeking care and following through with medical treatments, and confidence in managing new problems to measure activation. The 695 participants were divided into quartiles based on their PAM score, and the investigators looked at the rates of unplanned utilization events in each group. After adjusting for potential confounders such as gender, age, Charlson Comorbidity Index, insurance, marital status, and education, there remained a significant effect between PAM and 30‐day hospital reutilization. Compared with those who scored in the highest quartile of activation, those in the lowest quartile had 1.75 times the rate of 30‐day reutilization (P 0.001). Those in the second highest and third highest quartile had 1.3 (P = 0.03) and 1.5 times (P 0.001) the rate of reutilization demonstrating a dose‐response relationship between activation and low reutilization.
Cautions
It is as yet unclear how best to apply these results and whether activation is a modifiable risk factor. Can a patient become more activated by providing more education and coaching during their hospital stay? Can providing close follow‐up and home services make a person more confident to manage their own illness? Although early identification of patients with low activation using PAM is being done at many hospitals, there is no study to suggest that targeting these patients can reduce readmission.
Implications
A low level of patient activation appears to be a risk factor for unplanned hospital utilization within 30 days of discharge. Given the increasing financial penalties, many hospitals across the country are using the PAM to determine how much support and which services they provide after discharge. Identifying these patients early in their hospitalization could allow providers to spend more time and attention on preparing them for managing their own illness after discharge. As above, the effects of this intervention on readmissions is as yet unclear.
IS THERE A RELATIONSHIP BETWEEN PATIENT SATISFACTION AND UNDERSTANDING OF THE PLAN OF CARE?
Kebede S, Shihab HM, Berger ZD, et al. Patients' understanding of their hospitalizations and association with satisfaction. JAMA Intern Med. 2014;174(10):16981700.
Background
Effective patient‐physician communication is associated with improved patient satisfaction, care quality, and clinical outcomes.[9] Whether a shared understanding of the plan of care between patients and clinicians affects satisfaction is unknown.
Findings
One hundred seventy‐seven patients who had 2 or more medical conditions, 2 or more medical procedures, and 2 or more days in the hospital were interviewed on the day of discharge. Patients were questioned about their overall understanding of their hospitalization and about specific aspects of their care. They were also asked to provide objective data to measure their understanding of their hospital course by (1) listing their medical diagnoses, (2) identifying indications for medication on discharge paperwork, and (3) listing tests or procedures they underwent from a standard list. Patients were then asked to rate their satisfaction with their hospitalization. Patients' self‐reported understanding was an average of 4.0 (very good) on a 5‐point scale. Their measured understanding scores for medical diagnoses, indications for medications and tests and procedures were 48.9%, 56.2%, and 59.4%, respectively. Factors associated with poor understanding of their hospital course were increasing age, less education, lower household income, black race, and longer length of stay. Patients reported a mean satisfaction of 4.0 (very satisfied). Higher self‐reported understanding was associated with higher patient satisfaction, irrespective of actual understanding.
Cautions
Despite their suboptimal measured understanding of their hospital course, the average patient rated their understanding as very good. This suggests that patients are either poor judges of effective communication or have low expectations for understanding. It also calls into question the relationship between quality of communication and patient satisfaction, because despite their satisfaction, patients' actual understanding was low. There was, however, a clear and positive relationship between patients' perceived understanding and their satisfaction, suggesting that shared understanding remains integral to patient satisfaction.
Implications
Patient satisfaction appears to be tied to patients' perceived understanding of their care, but when tested actual understanding was suboptimal. Further efforts in patient satisfaction should not only focus on the quality of our communication, but on the resulting understanding of our patients.
WHAT ARE UNIVERSAL STRATEGIES TO IMPROVE SATISFACTION AND PATIENT OUTCOMES?
Detsky AS, Krumholz HM. Reducing the trauma of hospitalization. JAMA. 2014;311(21):21692170.
Background
Although high readmission rates are a national problem, a minority of patients treated for common conditions like pneumonia, heart failure, and chronic obstructive pulmonary disease are readmitted for the same problem.[10] This suggests that readmissions may stem not from poor disease management, but from patient vulnerability to illness in the period following hospitalization.
Findings
In this viewpoint opinion article, the authors suggest that the depersonalizing and stressful hospital atmosphere contributes to a transient vulnerability in the period following hospitalization that makes it challenging for patients to care for themselves and their illness. They offer specific strategies for changing the nature of our hospital care to promote healing and to decrease patient stress. The authors suggest promoting personalization through accommodation of family members, and allowing personal clothing and personal dcor in their rooms. Physicians and consultants should make appointments so that patients and families can know when to expect important visits. The authors also focus on the provision of rest and nourishment by reducing nighttime disruption and the elimination on unnecessary restrictive diets. They argue that the hospital is a place of stressful disruptions and surprises, which could all be ameliorated by providing patients with a way to understand the members of their team and their roles as well as through providing a clear schedule for the day. Healthcare providers should not enter a room unannounced, and patients should be given private rooms as much as possible. Last, the authors focus on the elimination of unnecessary tests and procedures such as blood draws, telemetry, and urine cultures and the encouragement of activity by providing activities where patients can gather together outside their rooms.
Cautions
If these changes seem simple, they may not be. Many involve a significant shift in our thinking on how we provide carefrom a focus on disease and provider convenience to a true consideration for the health and peace of mind of our patients. Starting with small steps, such as reductions in phlebotomy and nighttime vital signs checks for the most stable patients and ensuring accommodations for families, may make this long list seem less daunting.
Implications
By promoting factors that affect a patient's well beingrest, nutrition, peace of mindwe may be discharging patients who are better equipped to manage their illness after their hospitalization.
DO HOSPITALISTS OVERTEST, AND IF SO, WHY?
Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100108.
Background
National efforts, such as the Choosing Wisely campaign, seek to decrease overuse of low‐value services.[11] The extent of the problem of overtesting among hospitalists and the underlying drivers for unnecessary testing in this group have not been clearly defined.
Findings
Practicing adult medicine hospitalists across the country were given a questionnaire that included clinical vignettes for common inpatient scenarios: a preoperative evaluation and a syncope workup. Respondents were randomly provided 1 of 4 versions of each vignette, which contained the same clinical information but varied by a family member's request for further testing and by disclosure of the occupation of the family member. For example, in the preoperative evaluation, the vignettes either: (1) provided no details about the patient's son; (2) identified the son as a physician; (3) mentioned the son's request for testing, but did not identify the son as a physician; or (4) identified the son as a physician who requested testing. The syncope vignette versions were structured similarly, except the family member was the patient's wife and she was an attorney. The authors collected 1020 responses from an initial pool of 1500, for a decent 68% response rate. Hospitalists commonly reported overuse of testing, with 52% to 65% of respondents requesting unnecessary testing in the preoperative evaluation scenario, and 82% to 85% in the syncope scenario. The majority of physicians reported that they knew the testing was not clinically indicated based on evidence or guidelines, but were ordering the test due to a desire to reassure the patients or themselves.
Cautions
Responses to clinical vignettes in a survey may not represent actually practices. In addition, all hospitalists surveyed in this study were members of the Society of Hospital Medicine, so may not accurately exemplify all practicing hospitalists.
Implications
Overuse of testing is very common among hospitalists. Although roughly one‐third of respondents incorrectly thought that testing in the given scenarios was supported by the evidence or guidelines, the majority knew that testing was not clinically indicated and reported ordering tests to help reassure their patients or themselves. This suggests evidence‐based medicine approaches to overuse, such as the Choosing Wisely campaign and the emergence of appropriateness criteria, are likely necessary but insufficient to change physician practice patterns. Efforts to decrease overuse will need to engage clinicians and patients in ways that help overcome the attitude that more testing is required to provide reassurance.
DO UNREALISTIC PATIENT EXPECTATIONS ABOUT INTERVENTIONS INFLUENCE DECISION MAKING AND CONTRIBUTE TO OVERUSE?
Hoffmann TC, Del Mar C. Patient expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2015;175(2):274286.
Background
Patient expectations have been implicated as a contributor to overuse of medical interventions. Studies that have measured patients' understanding of the potential benefits and harms of medical treatments and tests have been scattered across the literature.
Findings
This systematic review aggregated all studies that have quantitatively assessed patients' expectations of the benefits and/or harms of any treatment or test. Of more than 15,000 records screened, only 36 articles met the inclusion criteria of describing a study in which participants were asked to provide a quantitative estimate of the expected benefits and/or harms of a treatment, test, or screen. Fourteen of the studies (40%) focused on screening, 15 (43%) on treatment, 3 (9%) on a test, and 3 (9%) on both treatment and screening. Topics included cancer, medications, surgery, cardiovascular disease, and fetal‐maternal medicine. The majority of patients overestimated intervention benefit and underestimated harm, regardless of whether the intervention was a test or a treatment. For example, more than half of participants overestimated benefit for 22 of the 34 outcomes (65%) for which overestimation data were provided, and a majority of participants underestimated harm for 10 of the 15 outcomes (67%) with underestimation data available.
Cautions
This systematic review included a limited number of studies, with varying levels of quality and a lot of heterogeneity, making it difficult to reach clear aggregate conclusions.
Implications
Patients are often overly optimistic about medical interventions and they downplay potential risks, making it more difficult to effectively discourage overuse. Clinicians should clearly understand and communicate realistic expectations for the potential benefits and risks of screening, testing, and medical treatments with patients and the public at large.
HOW BIG OF A PROBLEM IS ANTIBIOTIC OVERUSE IN HOSPITALS AND CAN WE DO BETTER?
Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194200.
Background
Antibiotics are life‐saving therapies, but when used in inappropriate scenarios they can pose many risks.
Findings
This large national database study used the MarketScan Hospital Drug Database and the Centers for Disease Control and Prevention's (CDC) Emerging Infections Program data to explore antibiotic prescribing in hospital patients. More than half of all hospitalized patients (55.7%) received antibiotics during their stay. Half of all treatment antibiotics were prescribed for the treatment of either lower respiratory infections, urinary tract infections, or presumed gram‐positive infections. There was wide variation seen in antibiotic usage across hospital wards. Objective criteria for potential improvement in antimicrobial use were developed and applied at a subset of 36 hospitals. Antibiotic prescribing could be improved in 37.2% of the most common prescription scenarios reviewed, including patients receiving vancomycin or those being treated for a urinary tract infection. The impact of reducing inpatient antibiotic exposure on the incidence of Clostridium difficile colitis was modeled using data from 2 hospitals, revealing that decreasing hospitalized patients' exposure to broad‐spectrum antibiotics by 30% would lead to a 26% reduction in C difficile infections (interquartile range = 15%38%).
Cautions
Some of the estimates in this study are based on a convenience sample of claims and hospital‐based data, thus may not be an accurate representation, particularly when extrapolating to all US hospitals.
Implications
Antibiotic overuse is a rampant problem in hospitals, with many severe downstream effects such as C difficile infections and antimicrobial resistance. All hospital units should have an antibiotic stewardship program and should monitor antibiotic usage.
Lee TC, Frenette C, Jayaraman D, Green L, Pilote L. Antibiotic self‐stewardship: trainee‐led structured antibiotic time‐outs to improve antimicrobial use. Ann Intern Med. 2014;161(10 suppl):S53S58.
Background
The CDC and other groups have called for stewardship programs to address antibiotic overuse.[12] Few interventions have been shown to successfully engage medical trainees in efforts to improve their own antibiotic prescribing practices.
Findings
An antibiotic self‐stewardship program was developed and led by internal medicine residents at Montreal General Hospital. The intervention included a monthly resident education lecture on antimicrobial stewardship and twice‐weekly time‐out audits using a structured electronic checklist. Adherence with auditing was 80%. Total costs for antibiotics decreased from $149,743 CAD to $80,319 CAD, mostly due to an observed reduction in carbapenems. Moxifloxicin use decreased by 1.9 defined daily doses per 1000 patient‐days per month (P = 0.048). Rates of clostridium difficile colitis declined from 24.2 to 19.6 per 10,000 patient‐days, although this trend did not meet statistical significance (incidence rate ratio, 0.8 [confidence interval, 0.5‐1.3]).
Cautions
Although the use of some broader spectrum antibiotics decreased, there was no measurable change in overall antibiotic use, suggesting that physicians may have narrowed antibiotics but did not often completely discontinue them. The time‐series analyses in this study cannot provide causal conclusions between the intervention and outcomes. In fact, carbapenem usage appears to have significantly decreased prior to the implementation of the program, for unclear reasons. The feasibility of this educational intervention outside of a residency program is unclear.
Implications
A combination of education, oversight and frontline clinician engagement in structured time‐outs may be effective, at least in narrowing antibiotic usage. The structured audit checklist developed by these authors is available for free in the supplementary materials of the Annals of Internal Medicine article.
Disclosures: Dr. Moriates has received grant funding from the ABIM Foundation, and royalties from McGraw‐Hill for the textbook Understanding Value‐Based Healthcare. The authors report no conflicts of interest.
- . The role of the hospitalist in quality improvement: systems for improving the care of patients with acute coronary syndrome. J Hosp Med. 2010;5(suppl 4):S1–S7.
- , , , . Impact of hospitalist communication‐skills training on patient‐satisfaction scores. J Hosp Med. 2013;8(6):315–320.
- , , , . Development of a hospital‐based program focused on improving healthcare value. J Hosp Med. 2014;9(10):671–677.
- . Value‐driven health care: implications for hospitals and hospitalists. J Hosp Med. 2009;4(8):507–511.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013;173(8):649–655.
- , , , , . Does housestaff discontinuity of care increase the risk for preventable adverse events? Ann Intern Med. 1994;121(11):866–872.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520–528.
- , , . Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8(5):410–417.
- . Effective physician‐patient communication and health outcomes: a review. CMAJ. 2007;152(9):1423–1433.
- , , , et al. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355–363.
- , , , et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486–492.
- , , , et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194–200.
- . The role of the hospitalist in quality improvement: systems for improving the care of patients with acute coronary syndrome. J Hosp Med. 2010;5(suppl 4):S1–S7.
- , , , . Impact of hospitalist communication‐skills training on patient‐satisfaction scores. J Hosp Med. 2013;8(6):315–320.
- , , , . Development of a hospital‐based program focused on improving healthcare value. J Hosp Med. 2014;9(10):671–677.
- . Value‐driven health care: implications for hospitals and hospitalists. J Hosp Med. 2009;4(8):507–511.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013;173(8):649–655.
- , , , , . Does housestaff discontinuity of care increase the risk for preventable adverse events? Ann Intern Med. 1994;121(11):866–872.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520–528.
- , , . Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8(5):410–417.
- . Effective physician‐patient communication and health outcomes: a review. CMAJ. 2007;152(9):1423–1433.
- , , , et al. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355–363.
- , , , et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486–492.
- , , , et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194–200.
Nebulized Bronchodilator Instead of MDI
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 54‐year‐old woman presented to the emergency department (ED) with shortness of breath. She reported that her primary care physician diagnosed her with chronic obstructive pulmonary disease (COPD). Her physician had prescribed her an albuterol inhaler to use as needed for shortness of breath. Over the past few weeks she had been trying to use the inhaler, but she noted that it did not seem to help her increasing wheezing, coughing, and sputum production. In the ED, she received continuous albuterol treatments via nebulizer, Solu‐Medrol 125 mg intravenously, antibiotics, and a chest x‐ray. She was admitted to the hospital medicine service for COPD exacerbation and started on nebulized bronchodilator treatments every 4 hours. By the fourth day of her hospital stay, she was discharged to home with an albuterol inhaler, oral prednisone, oral doxycycline, and a follow‐up appointment. Dedicated patient education regarding proper inhaler administration did not occur during hospitalization.
WHY YOU MIGHT THINK NEBULIZED TREATMENTS IN INPATIENTS ARE HELPFUL
Inhaled bronchodilators are a mainstay of therapy for acute obstructive pulmonary diseases, including COPD and asthma exacerbations.[1, 2] Inhaled bronchodilators may be delivered by metered‐dose inhalers (MDIs) or via wet nebulizers powered by compressed air or oxygen. Current practice patterns in EDs and hospital wards tend to favor the use of nebulizers due to many apparent advantages of these devices.[3] For instance, nebulizers do not require any special inhalation technique and can be effectively used by patients at any age.[3, 4] There is also a common perception that nebulizers are more effective, possibly stemming from the assumption that hospitalized patients have already failed their outpatient MDI therapy and an almost mystical belief in the healing power of mist. Moreover, many clinicians have been trained to routinely use nebulizer therapies and may lack sufficient knowledge or comfort about the relative efficacy and equivalence dosing of MDI therapies.
WHY NEBULIZERS ARE NOT BETTER THAN MDIs FOR PATIENTS HOSPITALIZED WITH OBSTRUCTIVE PULMONARY SYMPTOMS
Decades of research support that MDIs are effective, efficient, and less costly (depending on circumstances) than nebulizers for the routine treatment of obstructive pulmonary exacerbations.[3, 4, 5, 6, 7, 8, 9, 10, 11] The clinical effectiveness of MDIs has been shown in studies across populations of adults with acute COPD symptoms,[3, 4, 7, 8] as well as children and adults with asthma exacerbations.[3, 4, 5, 6, 9, 10] A 2005 joint report by the American College of Chest Physicians (ACCP) and the American College of Asthma, Allergy and Immunology (ACAAI), concluded none of the pooled meta‐analyses showed a significant difference between devices in any efficacy outcome in any patient group for each of the clinical settings.[4] Many different outcomes have been investigated, including forced expiratory volumes (FEV), peak flows, symptoms and specific symptom scores, and physical findings.[4]
Compared to MDIs, there are a number of drawbacks to the use of nebulizers: nebulizers are more expensive to buy and maintain, are less portable, and take longer to set up, use, and clean following each use.[12] In addition, nebulizers have been associated with greater increases in heart rate and tremors compared to MDIs, suggesting nebulizers lead to higher systemically absorbed ‐agonist doses.[4]
Of note, nearly all of the clinical effectiveness studies administered MDIs with a valved holding chamber or spacer, facilitating the delivery of drug to the airways.[3, 4] Although valved holding chambers are commonly referred to as a spacer, a true spacer does not have a valve and is rarely used today.[12]
THE EVIDENCE EXAMINING NEBULIZERS VERSUS MDIs IN PATIENTS WITH ASTHMA OR COPD EXACERBATIONS
A 2013 Cochrane review sought to establish the relative efficacy of MDIs with holding chambers versus nebulizers for children and adults who presented to a community setting or emergency department with acute asthma.[6] The review included a total of 1897 children and 729 adults in 39 randomized controlled trials. The authors judged the overall evidence to be of moderate quality. Children with acute asthma treated with MDIs in the ED had shorter lengths of stay in the ED (70 minutes vs 103 minutes), similar peak flow and FEV measurements, lower heart rates, and less tremor compared to children treated with nebulizers.[5, 6] There were no significant differences found between devices for the treatment of adult patients with asthma.[6]
In a separate double‐blind, randomized, placebo‐controlled study evaluating albuterol administered by nebulizer versus MDI with spacer for children <2 years old presenting to an ED with wheezing, the use of MDIs with a spacer and facemask was equally efficacious and may have led to fewer hospital admissions.[10]
Mandelberg et al. performed a double‐blind, randomized, placebo‐controlled trial for unselected adult patients presenting to an ED with obstructive pulmonary symptoms.[8] Patients received either 2 puffs of a placebo MDI with a spacer along with nebulized salbutamol 0.5 mL in 1.5 mL saline solution (n=25), or a salbutamol MDI along with a nebulized placebo saline solution (n=25). Treatments were repeated every 15 minutes up to 3 times, unless side effects occurred. Spirometric measurements were performed following each treatment. No differences were seen between the groups at any point during the study period. The authors concluded, Even in the setting of the unselected group of patient referrals to the [Department of Emergency Medicine] for episodes of severe airflow limitation, the clinical and objective bronchodilator responses to the administration of salbutamol are independent of the method of delivery: MDI with large spacer or aerosol nebulization.[8]
There are surprisingly few studies examining the use of nebulizers versus MDIs in the inpatient setting for both children and adults. Dolovich et al. reviewed 6 studies that included 253 total patients and reported no significant differences in pulmonary function between devices.[4] Based on these findings, the ACCP/ACAAI group recommended both nebulizers and MDIs with spacers/holding chambers are appropriate for use in the inpatient setting. Quality of evidence: good.[4]
WHY USE MDIs FOR INPATIENTS
If MDI and nebulizer treatments are equally effective, why change current practice? The use of MDIs, rather than nebulizers, in hospitals could lead to fewer side effects such as tachycardia, arrhythmias, and tremors. MDIs are also more portable and do not require specialized set‐up. Furthermore, MDI administrations during hospitalization may provide a golden opportunity to have respiratory therapists, pharmacists, or other health professionals spend time teaching patients proper inhaler usage, rather than providing time‐consuming nebulizer treatments.[13] In a recent study, approximately 86% of hospitalized patients with asthma or COPD could not demonstrate appropriate use of an MDI. However, 100% of patients were able to achieve mastery following a short teach‐back session.[14] It is conceivable that transitioning patients to MDIs earlier during hospitalization and providing them with education regarding proper MDI administration could instill confidence in their use of inhalers and result in downstream effects such as shorter lengths of stay, less frequent hospital readmissions, or improved quality of life.
MDI use may result in cost savings in certain settings, although the relative costs of nebulizer versus MDI treatments depends on many institution‐specific factors. Such factors include the institutional policies on who delivers the nebulizer or the MDI and how they are compensated and staffed. For example in the Nebs No More After 24 program initiated at the University of California, San Francisco, the vast majority of the realized cost savings are due to the reduction in respiratory therapist time spent delivering MDIs, which reflects the local policies and compensation structure.[13] Previous inpatient interventions to convert from nebulizers to MDIs also showed cost savings resulting from decreased labor needs.[15] In some hospitals, nurses deliver nebulizer treatments, whereas in others only respiratory therapists are allowed to provide nebulizers. Moreover, whether the MDI can go home with the patient upon discharge depends on whether the hospital has a dispensing pharmacy or not. Formal economic evaluations specific to the local institution are necessary.
WHAT WE SHOULD DO INSTEAD: ENCOURAGE THE USE OF MDIs FOR INPATIENTS
For effective inpatient MDI treatments, MDI technique must be good. Thus, it is vital to enlist the right people to provide proper MDI teaching and supervision. Respiratory therapists are generally trained for this task, and may be complemented by appropriately trained physicians, nurses, or pharmacists. Many institutions have successfully implemented respiratory therapist‐driven protocols for the administration of MDIs, which has led to measurable improvements in the utilization of appropriate respiratory care resources.[15, 16] At University of California, San Francisco, this was accomplished by recruiting respiratory therapists and nurses to help support the transition of patients from nebulizers to MDIs and to provide bedside teaching on proper MDI usage. The institution then launched a Nebs No More After 24 campaign that sought to transition patients from nebulizers to MDIs within 24 hours of hospitalization. This campaign included an educational program for physicians, prepared facilitator guides to assist attending physicians with teaching about the new initiative, publicity efforts including pens and strategically placed posters, and regular feedback regarding nebulizer utilization on the pilot ward. Although the evidence suggests that patients can be started on MDIs immediately upon presentation to the ED, the UCSF campaign focused on transitioning patients within 24 hours so to alleviate concerns about transitions in care between the ED and the medical ward, as well as between overnight and day teams. MDIs are only as or more effective than nebulizers if the correct administration technique is employed. The 24‐hour transition period allows for MDI teaching and transition during regular daytime hours.
Inpatient use of nebulizers may be more appropriate than MDIs for patients with dementia or altered mental status, as well as those in extreme distress resulting in an inability to coordinate inhaler usage. Very low health literacy may be an additional barrier to appropriate MDI teaching and usage.
RECOMMENDATIONS
In patients with obstructive pulmonary symptoms, transition patients from nebulizers to MDIs early in their hospital course, unless the patient is unable to use an inhaler due to altered mental status, dementia, or other circumstances. Ensure that patients are instructed and supervised on proper MDI technique. Enlisting respiratory therapists and appropriately trained staff (pharmacists, nurses, physicians) is key to the successful use of MDIs. Frequency and dosage of MDIs used should be comparable to that of nebulized treatments. Although studies have used a relatively wide range of albuterol MDI dosing, prior programs have determined a dose of albuterol 4 puffs via MDI as being equivalent to the standard albuterol 2.5 mg nebulizer dosage.[17, 18] Some studies have advocated for using a range of 2 to 10 puffs albuterol MDI, with the actual dose based on clinical response.[17] One study in children with mild acute asthma found that 2 puffs of albuterol by MDI was just as effective as higher doses delivered by MDI (610 puffs) or by nebulizer.[19]
CONCLUSION
MDIs with holding chambers are clinically equivalent to nebulizer therapy for the treatment of both children and adults with obstructive pulmonary symptoms, as long as MDI technique and MDI dosing is adequate. This is based on good data in the ED setting but fewer studies in adult inpatients. There are a number of advantages to the use of inpatient MDIs over nebulizers; MDIs are more portable, often less expensive to use, may result in fewer side effects, and will hopefully improve outpatient MDI technique. The delivery of MDIs during hospitalization should be accompanied with patient education regarding proper administration technique.
Disclosure
Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and Liking It on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of COPD. Available at: http://www.goldcopd.org/guidelines‐global‐strategy‐for‐diagnosis‐management.html. Updated January 2015. Accessed September 25, 2014.
- National Heart Lung and Blood Institute. National Asthma Education and Prevention Program. Expert panel report 3: guidelines for the diagnosis and management of asthma. Available at: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Published 2007. Updated April 2012. Accessed September 25, 2014.
- , , , Bronchodilator delivery in acute airflow obstruction. A meta‐analysis. Arch Intern Med. 1997;157(15):1736–1744.
- , , , et al. Device selection and outcomes of aerosol therapy: Evidence‐based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127(1):335–371.
- , Beta‐agonists through metered‐dose inhaler with valved holding chamber versus nebulizer for acute exacerbation of wheezing or asthma in children under 5 years of age: a systematic review with meta‐analysis. J Pediatr. 2004;145(2):172–177.
- , , Holding chambers (spacers) versus nebulisers for beta‐agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
- , , , , Nebulizer vs spacer for bronchodilator delivery in patients hospitalized for acute exacerbations of COPD. Chest. 1989;96(6):1241–1246.
- , , , Nebulized wet aerosol treatment in emergency department—is it essential? Comparison with large spacer device for metered‐dose inhaler. Chest. 1997;112(6):1501–1505.
- , , , , , Randomized controlled trial of salbutamol aerosol therapy via metered dose inhaler‐spacer vs. jet nebulizer in young children with wheezing. Pediatr Pulmonol. 2005;39(5):466–472.
- , , , Nebulizers vs metered‐dose inhalers with spacers for bronchodilator therapy to treat wheezing in children aged 2 to 24 months in a pediatric emergency department. Arch Pediatr Adolesc Med. 2003;157(1):76–80.
- , , , A review and economic evaluation of bronchodilator delivery methods in hospitalized patients. Arch Intern Med. 1996;156(18):2113–2118.
- , Asthma medication delivery: mists and myths. Paediatr Respir Rev. 2013;14(2):112–118.
- , , , , “Nebs no more after 24”: a pilot program to improve the use of appropriate respiratory therapies. JAMA Intern Med. 2013;173(17):1647–1648.
- , , , et al. Misuse of respiratory inhalers in hospitalized patients with asthma or COPD. J Gen Intern Med. 2011;26(6):635–642.
- , , A model for conversion from small volume nebulizer to metered dose inhaler aerosol therapy. Chest. 1992;101(3):634–637.
- , , Physician‐ordered aerosol therapy versus respiratory therapist‐driven aerosol protocol: the effect on resource utilization. Respir Care. 2013;58(3):431–437.
- , , , Automatic replacement of albuterol nebulizer therapy by metered‐dose inhaler and valved holding chamber. Am J Health Syst Pharm. 2005;62(10):1053–1061.
- , , , , The conversion to metered‐dose inhaler with valved holding chamber to administer inhaled albuterol: a pediatric hospital experience. Respir Care. 2008;53(3):338–345.
- , , , , , Comparison of albuterol delivered by a metered dose inhaler with spacer versus a nebulizer in children with mild acute asthma. J Pediatr. 1999;135(1):22–27.
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 54‐year‐old woman presented to the emergency department (ED) with shortness of breath. She reported that her primary care physician diagnosed her with chronic obstructive pulmonary disease (COPD). Her physician had prescribed her an albuterol inhaler to use as needed for shortness of breath. Over the past few weeks she had been trying to use the inhaler, but she noted that it did not seem to help her increasing wheezing, coughing, and sputum production. In the ED, she received continuous albuterol treatments via nebulizer, Solu‐Medrol 125 mg intravenously, antibiotics, and a chest x‐ray. She was admitted to the hospital medicine service for COPD exacerbation and started on nebulized bronchodilator treatments every 4 hours. By the fourth day of her hospital stay, she was discharged to home with an albuterol inhaler, oral prednisone, oral doxycycline, and a follow‐up appointment. Dedicated patient education regarding proper inhaler administration did not occur during hospitalization.
WHY YOU MIGHT THINK NEBULIZED TREATMENTS IN INPATIENTS ARE HELPFUL
Inhaled bronchodilators are a mainstay of therapy for acute obstructive pulmonary diseases, including COPD and asthma exacerbations.[1, 2] Inhaled bronchodilators may be delivered by metered‐dose inhalers (MDIs) or via wet nebulizers powered by compressed air or oxygen. Current practice patterns in EDs and hospital wards tend to favor the use of nebulizers due to many apparent advantages of these devices.[3] For instance, nebulizers do not require any special inhalation technique and can be effectively used by patients at any age.[3, 4] There is also a common perception that nebulizers are more effective, possibly stemming from the assumption that hospitalized patients have already failed their outpatient MDI therapy and an almost mystical belief in the healing power of mist. Moreover, many clinicians have been trained to routinely use nebulizer therapies and may lack sufficient knowledge or comfort about the relative efficacy and equivalence dosing of MDI therapies.
WHY NEBULIZERS ARE NOT BETTER THAN MDIs FOR PATIENTS HOSPITALIZED WITH OBSTRUCTIVE PULMONARY SYMPTOMS
Decades of research support that MDIs are effective, efficient, and less costly (depending on circumstances) than nebulizers for the routine treatment of obstructive pulmonary exacerbations.[3, 4, 5, 6, 7, 8, 9, 10, 11] The clinical effectiveness of MDIs has been shown in studies across populations of adults with acute COPD symptoms,[3, 4, 7, 8] as well as children and adults with asthma exacerbations.[3, 4, 5, 6, 9, 10] A 2005 joint report by the American College of Chest Physicians (ACCP) and the American College of Asthma, Allergy and Immunology (ACAAI), concluded none of the pooled meta‐analyses showed a significant difference between devices in any efficacy outcome in any patient group for each of the clinical settings.[4] Many different outcomes have been investigated, including forced expiratory volumes (FEV), peak flows, symptoms and specific symptom scores, and physical findings.[4]
Compared to MDIs, there are a number of drawbacks to the use of nebulizers: nebulizers are more expensive to buy and maintain, are less portable, and take longer to set up, use, and clean following each use.[12] In addition, nebulizers have been associated with greater increases in heart rate and tremors compared to MDIs, suggesting nebulizers lead to higher systemically absorbed ‐agonist doses.[4]
Of note, nearly all of the clinical effectiveness studies administered MDIs with a valved holding chamber or spacer, facilitating the delivery of drug to the airways.[3, 4] Although valved holding chambers are commonly referred to as a spacer, a true spacer does not have a valve and is rarely used today.[12]
THE EVIDENCE EXAMINING NEBULIZERS VERSUS MDIs IN PATIENTS WITH ASTHMA OR COPD EXACERBATIONS
A 2013 Cochrane review sought to establish the relative efficacy of MDIs with holding chambers versus nebulizers for children and adults who presented to a community setting or emergency department with acute asthma.[6] The review included a total of 1897 children and 729 adults in 39 randomized controlled trials. The authors judged the overall evidence to be of moderate quality. Children with acute asthma treated with MDIs in the ED had shorter lengths of stay in the ED (70 minutes vs 103 minutes), similar peak flow and FEV measurements, lower heart rates, and less tremor compared to children treated with nebulizers.[5, 6] There were no significant differences found between devices for the treatment of adult patients with asthma.[6]
In a separate double‐blind, randomized, placebo‐controlled study evaluating albuterol administered by nebulizer versus MDI with spacer for children <2 years old presenting to an ED with wheezing, the use of MDIs with a spacer and facemask was equally efficacious and may have led to fewer hospital admissions.[10]
Mandelberg et al. performed a double‐blind, randomized, placebo‐controlled trial for unselected adult patients presenting to an ED with obstructive pulmonary symptoms.[8] Patients received either 2 puffs of a placebo MDI with a spacer along with nebulized salbutamol 0.5 mL in 1.5 mL saline solution (n=25), or a salbutamol MDI along with a nebulized placebo saline solution (n=25). Treatments were repeated every 15 minutes up to 3 times, unless side effects occurred. Spirometric measurements were performed following each treatment. No differences were seen between the groups at any point during the study period. The authors concluded, Even in the setting of the unselected group of patient referrals to the [Department of Emergency Medicine] for episodes of severe airflow limitation, the clinical and objective bronchodilator responses to the administration of salbutamol are independent of the method of delivery: MDI with large spacer or aerosol nebulization.[8]
There are surprisingly few studies examining the use of nebulizers versus MDIs in the inpatient setting for both children and adults. Dolovich et al. reviewed 6 studies that included 253 total patients and reported no significant differences in pulmonary function between devices.[4] Based on these findings, the ACCP/ACAAI group recommended both nebulizers and MDIs with spacers/holding chambers are appropriate for use in the inpatient setting. Quality of evidence: good.[4]
WHY USE MDIs FOR INPATIENTS
If MDI and nebulizer treatments are equally effective, why change current practice? The use of MDIs, rather than nebulizers, in hospitals could lead to fewer side effects such as tachycardia, arrhythmias, and tremors. MDIs are also more portable and do not require specialized set‐up. Furthermore, MDI administrations during hospitalization may provide a golden opportunity to have respiratory therapists, pharmacists, or other health professionals spend time teaching patients proper inhaler usage, rather than providing time‐consuming nebulizer treatments.[13] In a recent study, approximately 86% of hospitalized patients with asthma or COPD could not demonstrate appropriate use of an MDI. However, 100% of patients were able to achieve mastery following a short teach‐back session.[14] It is conceivable that transitioning patients to MDIs earlier during hospitalization and providing them with education regarding proper MDI administration could instill confidence in their use of inhalers and result in downstream effects such as shorter lengths of stay, less frequent hospital readmissions, or improved quality of life.
MDI use may result in cost savings in certain settings, although the relative costs of nebulizer versus MDI treatments depends on many institution‐specific factors. Such factors include the institutional policies on who delivers the nebulizer or the MDI and how they are compensated and staffed. For example in the Nebs No More After 24 program initiated at the University of California, San Francisco, the vast majority of the realized cost savings are due to the reduction in respiratory therapist time spent delivering MDIs, which reflects the local policies and compensation structure.[13] Previous inpatient interventions to convert from nebulizers to MDIs also showed cost savings resulting from decreased labor needs.[15] In some hospitals, nurses deliver nebulizer treatments, whereas in others only respiratory therapists are allowed to provide nebulizers. Moreover, whether the MDI can go home with the patient upon discharge depends on whether the hospital has a dispensing pharmacy or not. Formal economic evaluations specific to the local institution are necessary.
WHAT WE SHOULD DO INSTEAD: ENCOURAGE THE USE OF MDIs FOR INPATIENTS
For effective inpatient MDI treatments, MDI technique must be good. Thus, it is vital to enlist the right people to provide proper MDI teaching and supervision. Respiratory therapists are generally trained for this task, and may be complemented by appropriately trained physicians, nurses, or pharmacists. Many institutions have successfully implemented respiratory therapist‐driven protocols for the administration of MDIs, which has led to measurable improvements in the utilization of appropriate respiratory care resources.[15, 16] At University of California, San Francisco, this was accomplished by recruiting respiratory therapists and nurses to help support the transition of patients from nebulizers to MDIs and to provide bedside teaching on proper MDI usage. The institution then launched a Nebs No More After 24 campaign that sought to transition patients from nebulizers to MDIs within 24 hours of hospitalization. This campaign included an educational program for physicians, prepared facilitator guides to assist attending physicians with teaching about the new initiative, publicity efforts including pens and strategically placed posters, and regular feedback regarding nebulizer utilization on the pilot ward. Although the evidence suggests that patients can be started on MDIs immediately upon presentation to the ED, the UCSF campaign focused on transitioning patients within 24 hours so to alleviate concerns about transitions in care between the ED and the medical ward, as well as between overnight and day teams. MDIs are only as or more effective than nebulizers if the correct administration technique is employed. The 24‐hour transition period allows for MDI teaching and transition during regular daytime hours.
Inpatient use of nebulizers may be more appropriate than MDIs for patients with dementia or altered mental status, as well as those in extreme distress resulting in an inability to coordinate inhaler usage. Very low health literacy may be an additional barrier to appropriate MDI teaching and usage.
RECOMMENDATIONS
In patients with obstructive pulmonary symptoms, transition patients from nebulizers to MDIs early in their hospital course, unless the patient is unable to use an inhaler due to altered mental status, dementia, or other circumstances. Ensure that patients are instructed and supervised on proper MDI technique. Enlisting respiratory therapists and appropriately trained staff (pharmacists, nurses, physicians) is key to the successful use of MDIs. Frequency and dosage of MDIs used should be comparable to that of nebulized treatments. Although studies have used a relatively wide range of albuterol MDI dosing, prior programs have determined a dose of albuterol 4 puffs via MDI as being equivalent to the standard albuterol 2.5 mg nebulizer dosage.[17, 18] Some studies have advocated for using a range of 2 to 10 puffs albuterol MDI, with the actual dose based on clinical response.[17] One study in children with mild acute asthma found that 2 puffs of albuterol by MDI was just as effective as higher doses delivered by MDI (610 puffs) or by nebulizer.[19]
CONCLUSION
MDIs with holding chambers are clinically equivalent to nebulizer therapy for the treatment of both children and adults with obstructive pulmonary symptoms, as long as MDI technique and MDI dosing is adequate. This is based on good data in the ED setting but fewer studies in adult inpatients. There are a number of advantages to the use of inpatient MDIs over nebulizers; MDIs are more portable, often less expensive to use, may result in fewer side effects, and will hopefully improve outpatient MDI technique. The delivery of MDIs during hospitalization should be accompanied with patient education regarding proper administration technique.
Disclosure
Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and Liking It on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected]
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 54‐year‐old woman presented to the emergency department (ED) with shortness of breath. She reported that her primary care physician diagnosed her with chronic obstructive pulmonary disease (COPD). Her physician had prescribed her an albuterol inhaler to use as needed for shortness of breath. Over the past few weeks she had been trying to use the inhaler, but she noted that it did not seem to help her increasing wheezing, coughing, and sputum production. In the ED, she received continuous albuterol treatments via nebulizer, Solu‐Medrol 125 mg intravenously, antibiotics, and a chest x‐ray. She was admitted to the hospital medicine service for COPD exacerbation and started on nebulized bronchodilator treatments every 4 hours. By the fourth day of her hospital stay, she was discharged to home with an albuterol inhaler, oral prednisone, oral doxycycline, and a follow‐up appointment. Dedicated patient education regarding proper inhaler administration did not occur during hospitalization.
WHY YOU MIGHT THINK NEBULIZED TREATMENTS IN INPATIENTS ARE HELPFUL
Inhaled bronchodilators are a mainstay of therapy for acute obstructive pulmonary diseases, including COPD and asthma exacerbations.[1, 2] Inhaled bronchodilators may be delivered by metered‐dose inhalers (MDIs) or via wet nebulizers powered by compressed air or oxygen. Current practice patterns in EDs and hospital wards tend to favor the use of nebulizers due to many apparent advantages of these devices.[3] For instance, nebulizers do not require any special inhalation technique and can be effectively used by patients at any age.[3, 4] There is also a common perception that nebulizers are more effective, possibly stemming from the assumption that hospitalized patients have already failed their outpatient MDI therapy and an almost mystical belief in the healing power of mist. Moreover, many clinicians have been trained to routinely use nebulizer therapies and may lack sufficient knowledge or comfort about the relative efficacy and equivalence dosing of MDI therapies.
WHY NEBULIZERS ARE NOT BETTER THAN MDIs FOR PATIENTS HOSPITALIZED WITH OBSTRUCTIVE PULMONARY SYMPTOMS
Decades of research support that MDIs are effective, efficient, and less costly (depending on circumstances) than nebulizers for the routine treatment of obstructive pulmonary exacerbations.[3, 4, 5, 6, 7, 8, 9, 10, 11] The clinical effectiveness of MDIs has been shown in studies across populations of adults with acute COPD symptoms,[3, 4, 7, 8] as well as children and adults with asthma exacerbations.[3, 4, 5, 6, 9, 10] A 2005 joint report by the American College of Chest Physicians (ACCP) and the American College of Asthma, Allergy and Immunology (ACAAI), concluded none of the pooled meta‐analyses showed a significant difference between devices in any efficacy outcome in any patient group for each of the clinical settings.[4] Many different outcomes have been investigated, including forced expiratory volumes (FEV), peak flows, symptoms and specific symptom scores, and physical findings.[4]
Compared to MDIs, there are a number of drawbacks to the use of nebulizers: nebulizers are more expensive to buy and maintain, are less portable, and take longer to set up, use, and clean following each use.[12] In addition, nebulizers have been associated with greater increases in heart rate and tremors compared to MDIs, suggesting nebulizers lead to higher systemically absorbed ‐agonist doses.[4]
Of note, nearly all of the clinical effectiveness studies administered MDIs with a valved holding chamber or spacer, facilitating the delivery of drug to the airways.[3, 4] Although valved holding chambers are commonly referred to as a spacer, a true spacer does not have a valve and is rarely used today.[12]
THE EVIDENCE EXAMINING NEBULIZERS VERSUS MDIs IN PATIENTS WITH ASTHMA OR COPD EXACERBATIONS
A 2013 Cochrane review sought to establish the relative efficacy of MDIs with holding chambers versus nebulizers for children and adults who presented to a community setting or emergency department with acute asthma.[6] The review included a total of 1897 children and 729 adults in 39 randomized controlled trials. The authors judged the overall evidence to be of moderate quality. Children with acute asthma treated with MDIs in the ED had shorter lengths of stay in the ED (70 minutes vs 103 minutes), similar peak flow and FEV measurements, lower heart rates, and less tremor compared to children treated with nebulizers.[5, 6] There were no significant differences found between devices for the treatment of adult patients with asthma.[6]
In a separate double‐blind, randomized, placebo‐controlled study evaluating albuterol administered by nebulizer versus MDI with spacer for children <2 years old presenting to an ED with wheezing, the use of MDIs with a spacer and facemask was equally efficacious and may have led to fewer hospital admissions.[10]
Mandelberg et al. performed a double‐blind, randomized, placebo‐controlled trial for unselected adult patients presenting to an ED with obstructive pulmonary symptoms.[8] Patients received either 2 puffs of a placebo MDI with a spacer along with nebulized salbutamol 0.5 mL in 1.5 mL saline solution (n=25), or a salbutamol MDI along with a nebulized placebo saline solution (n=25). Treatments were repeated every 15 minutes up to 3 times, unless side effects occurred. Spirometric measurements were performed following each treatment. No differences were seen between the groups at any point during the study period. The authors concluded, Even in the setting of the unselected group of patient referrals to the [Department of Emergency Medicine] for episodes of severe airflow limitation, the clinical and objective bronchodilator responses to the administration of salbutamol are independent of the method of delivery: MDI with large spacer or aerosol nebulization.[8]
There are surprisingly few studies examining the use of nebulizers versus MDIs in the inpatient setting for both children and adults. Dolovich et al. reviewed 6 studies that included 253 total patients and reported no significant differences in pulmonary function between devices.[4] Based on these findings, the ACCP/ACAAI group recommended both nebulizers and MDIs with spacers/holding chambers are appropriate for use in the inpatient setting. Quality of evidence: good.[4]
WHY USE MDIs FOR INPATIENTS
If MDI and nebulizer treatments are equally effective, why change current practice? The use of MDIs, rather than nebulizers, in hospitals could lead to fewer side effects such as tachycardia, arrhythmias, and tremors. MDIs are also more portable and do not require specialized set‐up. Furthermore, MDI administrations during hospitalization may provide a golden opportunity to have respiratory therapists, pharmacists, or other health professionals spend time teaching patients proper inhaler usage, rather than providing time‐consuming nebulizer treatments.[13] In a recent study, approximately 86% of hospitalized patients with asthma or COPD could not demonstrate appropriate use of an MDI. However, 100% of patients were able to achieve mastery following a short teach‐back session.[14] It is conceivable that transitioning patients to MDIs earlier during hospitalization and providing them with education regarding proper MDI administration could instill confidence in their use of inhalers and result in downstream effects such as shorter lengths of stay, less frequent hospital readmissions, or improved quality of life.
MDI use may result in cost savings in certain settings, although the relative costs of nebulizer versus MDI treatments depends on many institution‐specific factors. Such factors include the institutional policies on who delivers the nebulizer or the MDI and how they are compensated and staffed. For example in the Nebs No More After 24 program initiated at the University of California, San Francisco, the vast majority of the realized cost savings are due to the reduction in respiratory therapist time spent delivering MDIs, which reflects the local policies and compensation structure.[13] Previous inpatient interventions to convert from nebulizers to MDIs also showed cost savings resulting from decreased labor needs.[15] In some hospitals, nurses deliver nebulizer treatments, whereas in others only respiratory therapists are allowed to provide nebulizers. Moreover, whether the MDI can go home with the patient upon discharge depends on whether the hospital has a dispensing pharmacy or not. Formal economic evaluations specific to the local institution are necessary.
WHAT WE SHOULD DO INSTEAD: ENCOURAGE THE USE OF MDIs FOR INPATIENTS
For effective inpatient MDI treatments, MDI technique must be good. Thus, it is vital to enlist the right people to provide proper MDI teaching and supervision. Respiratory therapists are generally trained for this task, and may be complemented by appropriately trained physicians, nurses, or pharmacists. Many institutions have successfully implemented respiratory therapist‐driven protocols for the administration of MDIs, which has led to measurable improvements in the utilization of appropriate respiratory care resources.[15, 16] At University of California, San Francisco, this was accomplished by recruiting respiratory therapists and nurses to help support the transition of patients from nebulizers to MDIs and to provide bedside teaching on proper MDI usage. The institution then launched a Nebs No More After 24 campaign that sought to transition patients from nebulizers to MDIs within 24 hours of hospitalization. This campaign included an educational program for physicians, prepared facilitator guides to assist attending physicians with teaching about the new initiative, publicity efforts including pens and strategically placed posters, and regular feedback regarding nebulizer utilization on the pilot ward. Although the evidence suggests that patients can be started on MDIs immediately upon presentation to the ED, the UCSF campaign focused on transitioning patients within 24 hours so to alleviate concerns about transitions in care between the ED and the medical ward, as well as between overnight and day teams. MDIs are only as or more effective than nebulizers if the correct administration technique is employed. The 24‐hour transition period allows for MDI teaching and transition during regular daytime hours.
Inpatient use of nebulizers may be more appropriate than MDIs for patients with dementia or altered mental status, as well as those in extreme distress resulting in an inability to coordinate inhaler usage. Very low health literacy may be an additional barrier to appropriate MDI teaching and usage.
RECOMMENDATIONS
In patients with obstructive pulmonary symptoms, transition patients from nebulizers to MDIs early in their hospital course, unless the patient is unable to use an inhaler due to altered mental status, dementia, or other circumstances. Ensure that patients are instructed and supervised on proper MDI technique. Enlisting respiratory therapists and appropriately trained staff (pharmacists, nurses, physicians) is key to the successful use of MDIs. Frequency and dosage of MDIs used should be comparable to that of nebulized treatments. Although studies have used a relatively wide range of albuterol MDI dosing, prior programs have determined a dose of albuterol 4 puffs via MDI as being equivalent to the standard albuterol 2.5 mg nebulizer dosage.[17, 18] Some studies have advocated for using a range of 2 to 10 puffs albuterol MDI, with the actual dose based on clinical response.[17] One study in children with mild acute asthma found that 2 puffs of albuterol by MDI was just as effective as higher doses delivered by MDI (610 puffs) or by nebulizer.[19]
CONCLUSION
MDIs with holding chambers are clinically equivalent to nebulizer therapy for the treatment of both children and adults with obstructive pulmonary symptoms, as long as MDI technique and MDI dosing is adequate. This is based on good data in the ED setting but fewer studies in adult inpatients. There are a number of advantages to the use of inpatient MDIs over nebulizers; MDIs are more portable, often less expensive to use, may result in fewer side effects, and will hopefully improve outpatient MDI technique. The delivery of MDIs during hospitalization should be accompanied with patient education regarding proper administration technique.
Disclosure
Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and Liking It on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of COPD. Available at: http://www.goldcopd.org/guidelines‐global‐strategy‐for‐diagnosis‐management.html. Updated January 2015. Accessed September 25, 2014.
- National Heart Lung and Blood Institute. National Asthma Education and Prevention Program. Expert panel report 3: guidelines for the diagnosis and management of asthma. Available at: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Published 2007. Updated April 2012. Accessed September 25, 2014.
- , , , Bronchodilator delivery in acute airflow obstruction. A meta‐analysis. Arch Intern Med. 1997;157(15):1736–1744.
- , , , et al. Device selection and outcomes of aerosol therapy: Evidence‐based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127(1):335–371.
- , Beta‐agonists through metered‐dose inhaler with valved holding chamber versus nebulizer for acute exacerbation of wheezing or asthma in children under 5 years of age: a systematic review with meta‐analysis. J Pediatr. 2004;145(2):172–177.
- , , Holding chambers (spacers) versus nebulisers for beta‐agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
- , , , , Nebulizer vs spacer for bronchodilator delivery in patients hospitalized for acute exacerbations of COPD. Chest. 1989;96(6):1241–1246.
- , , , Nebulized wet aerosol treatment in emergency department—is it essential? Comparison with large spacer device for metered‐dose inhaler. Chest. 1997;112(6):1501–1505.
- , , , , , Randomized controlled trial of salbutamol aerosol therapy via metered dose inhaler‐spacer vs. jet nebulizer in young children with wheezing. Pediatr Pulmonol. 2005;39(5):466–472.
- , , , Nebulizers vs metered‐dose inhalers with spacers for bronchodilator therapy to treat wheezing in children aged 2 to 24 months in a pediatric emergency department. Arch Pediatr Adolesc Med. 2003;157(1):76–80.
- , , , A review and economic evaluation of bronchodilator delivery methods in hospitalized patients. Arch Intern Med. 1996;156(18):2113–2118.
- , Asthma medication delivery: mists and myths. Paediatr Respir Rev. 2013;14(2):112–118.
- , , , , “Nebs no more after 24”: a pilot program to improve the use of appropriate respiratory therapies. JAMA Intern Med. 2013;173(17):1647–1648.
- , , , et al. Misuse of respiratory inhalers in hospitalized patients with asthma or COPD. J Gen Intern Med. 2011;26(6):635–642.
- , , A model for conversion from small volume nebulizer to metered dose inhaler aerosol therapy. Chest. 1992;101(3):634–637.
- , , Physician‐ordered aerosol therapy versus respiratory therapist‐driven aerosol protocol: the effect on resource utilization. Respir Care. 2013;58(3):431–437.
- , , , Automatic replacement of albuterol nebulizer therapy by metered‐dose inhaler and valved holding chamber. Am J Health Syst Pharm. 2005;62(10):1053–1061.
- , , , , The conversion to metered‐dose inhaler with valved holding chamber to administer inhaled albuterol: a pediatric hospital experience. Respir Care. 2008;53(3):338–345.
- , , , , , Comparison of albuterol delivered by a metered dose inhaler with spacer versus a nebulizer in children with mild acute asthma. J Pediatr. 1999;135(1):22–27.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of COPD. Available at: http://www.goldcopd.org/guidelines‐global‐strategy‐for‐diagnosis‐management.html. Updated January 2015. Accessed September 25, 2014.
- National Heart Lung and Blood Institute. National Asthma Education and Prevention Program. Expert panel report 3: guidelines for the diagnosis and management of asthma. Available at: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Published 2007. Updated April 2012. Accessed September 25, 2014.
- , , , Bronchodilator delivery in acute airflow obstruction. A meta‐analysis. Arch Intern Med. 1997;157(15):1736–1744.
- , , , et al. Device selection and outcomes of aerosol therapy: Evidence‐based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127(1):335–371.
- , Beta‐agonists through metered‐dose inhaler with valved holding chamber versus nebulizer for acute exacerbation of wheezing or asthma in children under 5 years of age: a systematic review with meta‐analysis. J Pediatr. 2004;145(2):172–177.
- , , Holding chambers (spacers) versus nebulisers for beta‐agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
- , , , , Nebulizer vs spacer for bronchodilator delivery in patients hospitalized for acute exacerbations of COPD. Chest. 1989;96(6):1241–1246.
- , , , Nebulized wet aerosol treatment in emergency department—is it essential? Comparison with large spacer device for metered‐dose inhaler. Chest. 1997;112(6):1501–1505.
- , , , , , Randomized controlled trial of salbutamol aerosol therapy via metered dose inhaler‐spacer vs. jet nebulizer in young children with wheezing. Pediatr Pulmonol. 2005;39(5):466–472.
- , , , Nebulizers vs metered‐dose inhalers with spacers for bronchodilator therapy to treat wheezing in children aged 2 to 24 months in a pediatric emergency department. Arch Pediatr Adolesc Med. 2003;157(1):76–80.
- , , , A review and economic evaluation of bronchodilator delivery methods in hospitalized patients. Arch Intern Med. 1996;156(18):2113–2118.
- , Asthma medication delivery: mists and myths. Paediatr Respir Rev. 2013;14(2):112–118.
- , , , , “Nebs no more after 24”: a pilot program to improve the use of appropriate respiratory therapies. JAMA Intern Med. 2013;173(17):1647–1648.
- , , , et al. Misuse of respiratory inhalers in hospitalized patients with asthma or COPD. J Gen Intern Med. 2011;26(6):635–642.
- , , A model for conversion from small volume nebulizer to metered dose inhaler aerosol therapy. Chest. 1992;101(3):634–637.
- , , Physician‐ordered aerosol therapy versus respiratory therapist‐driven aerosol protocol: the effect on resource utilization. Respir Care. 2013;58(3):431–437.
- , , , Automatic replacement of albuterol nebulizer therapy by metered‐dose inhaler and valved holding chamber. Am J Health Syst Pharm. 2005;62(10):1053–1061.
- , , , , The conversion to metered‐dose inhaler with valved holding chamber to administer inhaled albuterol: a pediatric hospital experience. Respir Care. 2008;53(3):338–345.
- , , , , , Comparison of albuterol delivered by a metered dose inhaler with spacer versus a nebulizer in children with mild acute asthma. J Pediatr. 1999;135(1):22–27.
© 2015 Society of Hospital Medicine
When does an adult with headaches need central nervous system imaging?
A 32-year-old woman presents to the clinic for evaluation of headaches, which she describes as pulsatile and throbbing, usually unilateral but involving different sides of the head at different times, and severe, causing her to miss work. They usually last between 12 and 24 hours and are associated with nausea but no vomiting and no changes in vision. They are worse around the time of her menses, have been occurring about twice a month for the past 6 months, and respond to ibuprofen. She thought they were caused by chronic seasonal allergies and sinusitis and has tried antihistamines and nasal irrigation without success. They are not affected by body position, they are not explosive, and they are not brought on by the Valsalva maneuver. She reports no other neurologic or systemic symptoms.
A detailed neurologic examination shows no deficits. However, the patient is concerned, as one of her friends was recently diagnosed with cancer. She requests imaging to “make sure there is no cancer.” Would it be appropriate to order imaging at this time?
No, it would not. Patients who have primary headache disorders without red-flag symptoms should not undergo imaging of the central nervous system (CNS) as part of their initial evaluation.1–4 (The list of potential red-flag symptoms is long but includes new onset after age 50, persistent neurologic changes, systemic symptoms or immunosuppression, sudden onset, progressive pain, positional nature, headaches precipitated by the Valsalva maneuver, and papilledema.)
CNS imaging may be appropriate for patients with features that increase the likelihood of structural diseases such as arteriovenous malformation, aneurysm, tumor, or subarachnoid hemorrhage. This patient, however, does not have worrisome signs or symptoms. Her symptoms are most consistent with migraine headache without aura. In patients with migraine headache without symptoms suggesting structural disease, CNS imaging is unwarranted and may be harmful.
DIAGNOSING MIGRAINE ACCURATELY
Diagnosing migraine headache can be a challenge, and up to half of all patients with migraine may be undiagnosed.5 The proper diagnosis of headache type is critical to the initial evaluation. In diagnosing migraine, one can use the mnemonic POUND4:
- Pulsatile
- One-day duration (4–72 hours)
- Unilateral
- Nausea or vomiting
- Disabling.
If four or five of these features are present, the likelihood ratio that the patient has migraine headache is 24, making it overwhelmingly likely that is the correct diagnosis.4 With three features the likelihood ratio is 3.5. If two or fewer features are present, migraine is much less likely, with a likelihood ratio of 0.41. Thus, patients with classic symptoms of migraine can be confidently and accurately diagnosed without the need for any imaging studies.
The patient in the vignette has all five POUND criteria. If we estimate her pretest probability of migraine headache at 50% (which is actually a conservative estimate—see Guidelines and Choosing Wisely, below), then, utilizing Bayes’ theorem, the likelihood ratio of 24 would result in a 95% probability that her headaches represent migraine.
GUIDELINES AND CHOOSING WISELY
High-quality reviews have found no benefit in performing imaging for primary headache disorders.1–3 This is due, in large part, to the rarity of secondary headache disorders in the primary care setting. In fact, most patients—90% in one study6—presenting to their primary care physicians with headaches meet the diagnostic criteria for migraine.
Significant abnormalities on imaging in patients with migraine headaches are also very rare. In patients with migraine headaches who undergo imaging, the rate of worrisome abnormalities that could lead to a change in management (0.2%) is less than that in the general population at the time of autopsy (0.8%).7
As part of the Choosing Wisely campaign, the American College of Radiology and the American Headache Society recommend against imaging for patients at low risk with migraine headaches. Because of the potential for harm from radiation exposure, the American Headache Society also recommends against computed tomography (CT) for evaluating headaches when magnetic resonance imaging (MRI) is available, except in emergencies.
Lists of tests and treatments that physicians and patients should question and discuss together to make wise decisions are available at www.choosingwisely.org.
HARMS ASSOCIATED WITH CNS IMAGING
Medical tests can be associated with significant harm. Potential harms of head imaging include radiation exposure from CT and false-positive findings. These false-positives, such as the finding of lesions that eventually prove to be benign, may require further testing and cause significant anxiety to the patient.
The effective radiation dose from a CT scan of the head is 2.0 mSv, equivalent to 250 days of background radiation exposure or 100 chest radiographs. Radiation exposure has been linked to increased risk of fatal cancer, and the risks increase with subsequent radiation doses.8
Incidental findings are common on head imaging and often lead to additional medical procedures and workup, without improvements in patient well-being. While the harms of false-positive testing and the finding of benign lesions are difficult to quantify, it is clear that downstream costs can accumulate and that these results cause significant undue worry to the patient.
CLINICAL BOTTOM LINE
Patients with migraine headache who do not have red-flag signs or symptoms are unlikely to benefit from CNS imaging and may experience harm. The rate of abnormalities in this population is not significantly different from that in the general population. A thorough history and physical examination should be done to find the proper diagnosis and to uncover any red-flag symptoms. For migraine headaches that are worsened by identified triggers, those triggers should be addressed before further evaluation is performed. When imaging is needed, physicians should consider minimizing radiation risk by ordering MRI instead of CT.
- Beithon J, Gallenberg M, Johnson K, et al; Institute for Clinical Systems Improvement. Diagnosis and treatment of headache. www.icsi.org/_asset/qwrznq/headache.pdf. Accessed September 5, 2014.
- Frishberg BM, Rosenberg JH, Matchar DB, et al; US Headache Consortium. Evidence-based guidelines in the primary care setting: neuroimaging in patients with nonacute headache. www.aan.com/professionals/practice/pdfs/gl0088.pdf. Accessed September 5, 2014.
- Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000; 55:754–762.
- Detsky ME, McDonald DR, Baerlocher MO, Tomlinson GA, McCrory DC, Booth CM. Does this patient with headache have a migraine or need neuroimaging? JAMA 2006; 296:1274–1283.
- Lipton RB, Diamond S, Reed M, Diamond ML, Stewart WF. Migraine diagnosis and treatment: results from the American Migraine Study II. Headache 2001; 41:638–645.
- Dowson A, Dahlof C, Tepper S, Newman L. Prevalence and diagnosis of migraine in a primary care setting (abstract). Cephalalgia 2002; 22:590–591.
- Frishberg BM. The utility of neuroimaging in the evaluation of headache in patients with normal neurologic examinations. Neurology 1994; 44:1191–1197.
- Semelka RC, Armao DM, Elias J Jr, Huda W. Imaging strategies to reduce the risk of radiation in CT studies, including selective substitution with MRI. J Magn Reson Imaging 2007; 25:900–909.
A 32-year-old woman presents to the clinic for evaluation of headaches, which she describes as pulsatile and throbbing, usually unilateral but involving different sides of the head at different times, and severe, causing her to miss work. They usually last between 12 and 24 hours and are associated with nausea but no vomiting and no changes in vision. They are worse around the time of her menses, have been occurring about twice a month for the past 6 months, and respond to ibuprofen. She thought they were caused by chronic seasonal allergies and sinusitis and has tried antihistamines and nasal irrigation without success. They are not affected by body position, they are not explosive, and they are not brought on by the Valsalva maneuver. She reports no other neurologic or systemic symptoms.
A detailed neurologic examination shows no deficits. However, the patient is concerned, as one of her friends was recently diagnosed with cancer. She requests imaging to “make sure there is no cancer.” Would it be appropriate to order imaging at this time?
No, it would not. Patients who have primary headache disorders without red-flag symptoms should not undergo imaging of the central nervous system (CNS) as part of their initial evaluation.1–4 (The list of potential red-flag symptoms is long but includes new onset after age 50, persistent neurologic changes, systemic symptoms or immunosuppression, sudden onset, progressive pain, positional nature, headaches precipitated by the Valsalva maneuver, and papilledema.)
CNS imaging may be appropriate for patients with features that increase the likelihood of structural diseases such as arteriovenous malformation, aneurysm, tumor, or subarachnoid hemorrhage. This patient, however, does not have worrisome signs or symptoms. Her symptoms are most consistent with migraine headache without aura. In patients with migraine headache without symptoms suggesting structural disease, CNS imaging is unwarranted and may be harmful.
DIAGNOSING MIGRAINE ACCURATELY
Diagnosing migraine headache can be a challenge, and up to half of all patients with migraine may be undiagnosed.5 The proper diagnosis of headache type is critical to the initial evaluation. In diagnosing migraine, one can use the mnemonic POUND4:
- Pulsatile
- One-day duration (4–72 hours)
- Unilateral
- Nausea or vomiting
- Disabling.
If four or five of these features are present, the likelihood ratio that the patient has migraine headache is 24, making it overwhelmingly likely that is the correct diagnosis.4 With three features the likelihood ratio is 3.5. If two or fewer features are present, migraine is much less likely, with a likelihood ratio of 0.41. Thus, patients with classic symptoms of migraine can be confidently and accurately diagnosed without the need for any imaging studies.
The patient in the vignette has all five POUND criteria. If we estimate her pretest probability of migraine headache at 50% (which is actually a conservative estimate—see Guidelines and Choosing Wisely, below), then, utilizing Bayes’ theorem, the likelihood ratio of 24 would result in a 95% probability that her headaches represent migraine.
GUIDELINES AND CHOOSING WISELY
High-quality reviews have found no benefit in performing imaging for primary headache disorders.1–3 This is due, in large part, to the rarity of secondary headache disorders in the primary care setting. In fact, most patients—90% in one study6—presenting to their primary care physicians with headaches meet the diagnostic criteria for migraine.
Significant abnormalities on imaging in patients with migraine headaches are also very rare. In patients with migraine headaches who undergo imaging, the rate of worrisome abnormalities that could lead to a change in management (0.2%) is less than that in the general population at the time of autopsy (0.8%).7
As part of the Choosing Wisely campaign, the American College of Radiology and the American Headache Society recommend against imaging for patients at low risk with migraine headaches. Because of the potential for harm from radiation exposure, the American Headache Society also recommends against computed tomography (CT) for evaluating headaches when magnetic resonance imaging (MRI) is available, except in emergencies.
Lists of tests and treatments that physicians and patients should question and discuss together to make wise decisions are available at www.choosingwisely.org.
HARMS ASSOCIATED WITH CNS IMAGING
Medical tests can be associated with significant harm. Potential harms of head imaging include radiation exposure from CT and false-positive findings. These false-positives, such as the finding of lesions that eventually prove to be benign, may require further testing and cause significant anxiety to the patient.
The effective radiation dose from a CT scan of the head is 2.0 mSv, equivalent to 250 days of background radiation exposure or 100 chest radiographs. Radiation exposure has been linked to increased risk of fatal cancer, and the risks increase with subsequent radiation doses.8
Incidental findings are common on head imaging and often lead to additional medical procedures and workup, without improvements in patient well-being. While the harms of false-positive testing and the finding of benign lesions are difficult to quantify, it is clear that downstream costs can accumulate and that these results cause significant undue worry to the patient.
CLINICAL BOTTOM LINE
Patients with migraine headache who do not have red-flag signs or symptoms are unlikely to benefit from CNS imaging and may experience harm. The rate of abnormalities in this population is not significantly different from that in the general population. A thorough history and physical examination should be done to find the proper diagnosis and to uncover any red-flag symptoms. For migraine headaches that are worsened by identified triggers, those triggers should be addressed before further evaluation is performed. When imaging is needed, physicians should consider minimizing radiation risk by ordering MRI instead of CT.
A 32-year-old woman presents to the clinic for evaluation of headaches, which she describes as pulsatile and throbbing, usually unilateral but involving different sides of the head at different times, and severe, causing her to miss work. They usually last between 12 and 24 hours and are associated with nausea but no vomiting and no changes in vision. They are worse around the time of her menses, have been occurring about twice a month for the past 6 months, and respond to ibuprofen. She thought they were caused by chronic seasonal allergies and sinusitis and has tried antihistamines and nasal irrigation without success. They are not affected by body position, they are not explosive, and they are not brought on by the Valsalva maneuver. She reports no other neurologic or systemic symptoms.
A detailed neurologic examination shows no deficits. However, the patient is concerned, as one of her friends was recently diagnosed with cancer. She requests imaging to “make sure there is no cancer.” Would it be appropriate to order imaging at this time?
No, it would not. Patients who have primary headache disorders without red-flag symptoms should not undergo imaging of the central nervous system (CNS) as part of their initial evaluation.1–4 (The list of potential red-flag symptoms is long but includes new onset after age 50, persistent neurologic changes, systemic symptoms or immunosuppression, sudden onset, progressive pain, positional nature, headaches precipitated by the Valsalva maneuver, and papilledema.)
CNS imaging may be appropriate for patients with features that increase the likelihood of structural diseases such as arteriovenous malformation, aneurysm, tumor, or subarachnoid hemorrhage. This patient, however, does not have worrisome signs or symptoms. Her symptoms are most consistent with migraine headache without aura. In patients with migraine headache without symptoms suggesting structural disease, CNS imaging is unwarranted and may be harmful.
DIAGNOSING MIGRAINE ACCURATELY
Diagnosing migraine headache can be a challenge, and up to half of all patients with migraine may be undiagnosed.5 The proper diagnosis of headache type is critical to the initial evaluation. In diagnosing migraine, one can use the mnemonic POUND4:
- Pulsatile
- One-day duration (4–72 hours)
- Unilateral
- Nausea or vomiting
- Disabling.
If four or five of these features are present, the likelihood ratio that the patient has migraine headache is 24, making it overwhelmingly likely that is the correct diagnosis.4 With three features the likelihood ratio is 3.5. If two or fewer features are present, migraine is much less likely, with a likelihood ratio of 0.41. Thus, patients with classic symptoms of migraine can be confidently and accurately diagnosed without the need for any imaging studies.
The patient in the vignette has all five POUND criteria. If we estimate her pretest probability of migraine headache at 50% (which is actually a conservative estimate—see Guidelines and Choosing Wisely, below), then, utilizing Bayes’ theorem, the likelihood ratio of 24 would result in a 95% probability that her headaches represent migraine.
GUIDELINES AND CHOOSING WISELY
High-quality reviews have found no benefit in performing imaging for primary headache disorders.1–3 This is due, in large part, to the rarity of secondary headache disorders in the primary care setting. In fact, most patients—90% in one study6—presenting to their primary care physicians with headaches meet the diagnostic criteria for migraine.
Significant abnormalities on imaging in patients with migraine headaches are also very rare. In patients with migraine headaches who undergo imaging, the rate of worrisome abnormalities that could lead to a change in management (0.2%) is less than that in the general population at the time of autopsy (0.8%).7
As part of the Choosing Wisely campaign, the American College of Radiology and the American Headache Society recommend against imaging for patients at low risk with migraine headaches. Because of the potential for harm from radiation exposure, the American Headache Society also recommends against computed tomography (CT) for evaluating headaches when magnetic resonance imaging (MRI) is available, except in emergencies.
Lists of tests and treatments that physicians and patients should question and discuss together to make wise decisions are available at www.choosingwisely.org.
HARMS ASSOCIATED WITH CNS IMAGING
Medical tests can be associated with significant harm. Potential harms of head imaging include radiation exposure from CT and false-positive findings. These false-positives, such as the finding of lesions that eventually prove to be benign, may require further testing and cause significant anxiety to the patient.
The effective radiation dose from a CT scan of the head is 2.0 mSv, equivalent to 250 days of background radiation exposure or 100 chest radiographs. Radiation exposure has been linked to increased risk of fatal cancer, and the risks increase with subsequent radiation doses.8
Incidental findings are common on head imaging and often lead to additional medical procedures and workup, without improvements in patient well-being. While the harms of false-positive testing and the finding of benign lesions are difficult to quantify, it is clear that downstream costs can accumulate and that these results cause significant undue worry to the patient.
CLINICAL BOTTOM LINE
Patients with migraine headache who do not have red-flag signs or symptoms are unlikely to benefit from CNS imaging and may experience harm. The rate of abnormalities in this population is not significantly different from that in the general population. A thorough history and physical examination should be done to find the proper diagnosis and to uncover any red-flag symptoms. For migraine headaches that are worsened by identified triggers, those triggers should be addressed before further evaluation is performed. When imaging is needed, physicians should consider minimizing radiation risk by ordering MRI instead of CT.
- Beithon J, Gallenberg M, Johnson K, et al; Institute for Clinical Systems Improvement. Diagnosis and treatment of headache. www.icsi.org/_asset/qwrznq/headache.pdf. Accessed September 5, 2014.
- Frishberg BM, Rosenberg JH, Matchar DB, et al; US Headache Consortium. Evidence-based guidelines in the primary care setting: neuroimaging in patients with nonacute headache. www.aan.com/professionals/practice/pdfs/gl0088.pdf. Accessed September 5, 2014.
- Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000; 55:754–762.
- Detsky ME, McDonald DR, Baerlocher MO, Tomlinson GA, McCrory DC, Booth CM. Does this patient with headache have a migraine or need neuroimaging? JAMA 2006; 296:1274–1283.
- Lipton RB, Diamond S, Reed M, Diamond ML, Stewart WF. Migraine diagnosis and treatment: results from the American Migraine Study II. Headache 2001; 41:638–645.
- Dowson A, Dahlof C, Tepper S, Newman L. Prevalence and diagnosis of migraine in a primary care setting (abstract). Cephalalgia 2002; 22:590–591.
- Frishberg BM. The utility of neuroimaging in the evaluation of headache in patients with normal neurologic examinations. Neurology 1994; 44:1191–1197.
- Semelka RC, Armao DM, Elias J Jr, Huda W. Imaging strategies to reduce the risk of radiation in CT studies, including selective substitution with MRI. J Magn Reson Imaging 2007; 25:900–909.
- Beithon J, Gallenberg M, Johnson K, et al; Institute for Clinical Systems Improvement. Diagnosis and treatment of headache. www.icsi.org/_asset/qwrznq/headache.pdf. Accessed September 5, 2014.
- Frishberg BM, Rosenberg JH, Matchar DB, et al; US Headache Consortium. Evidence-based guidelines in the primary care setting: neuroimaging in patients with nonacute headache. www.aan.com/professionals/practice/pdfs/gl0088.pdf. Accessed September 5, 2014.
- Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000; 55:754–762.
- Detsky ME, McDonald DR, Baerlocher MO, Tomlinson GA, McCrory DC, Booth CM. Does this patient with headache have a migraine or need neuroimaging? JAMA 2006; 296:1274–1283.
- Lipton RB, Diamond S, Reed M, Diamond ML, Stewart WF. Migraine diagnosis and treatment: results from the American Migraine Study II. Headache 2001; 41:638–645.
- Dowson A, Dahlof C, Tepper S, Newman L. Prevalence and diagnosis of migraine in a primary care setting (abstract). Cephalalgia 2002; 22:590–591.
- Frishberg BM. The utility of neuroimaging in the evaluation of headache in patients with normal neurologic examinations. Neurology 1994; 44:1191–1197.
- Semelka RC, Armao DM, Elias J Jr, Huda W. Imaging strategies to reduce the risk of radiation in CT studies, including selective substitution with MRI. J Magn Reson Imaging 2007; 25:900–909.
Hospital High‐Value Care Program
With a United States medical system that spends as much as $750 billion each year on care that does not result in improved health outcomes,[1] many policy initiatives, including the Centers for Medicare and Medicaid Services' Value‐Based Purchasing program, seek to realign hospitals' financial incentives from a focus on production to one on value (quality divided by cost).[2, 3] Professional organizations have now deemed resource stewardship an ethical responsibility for professionalism,[4, 5] and campaigns such as the American Board of Internal Medicine (ABIM) Foundation's Choosing Wisely effort and the American College of Physicians' High‐Value Care platform are calling on frontline clinicians to address unnecessary and wasteful services.[6, 7]
Despite these pressures and initiatives, most physicians lack the knowledge and tools necessary to prioritize the delivery of their own healthcare services according to value.[8, 9, 10] Hospital medicine physicians are unaware of the costs associated with the interventions they order,[10] and the majority of medical training programs lack curricula focused on healthcare costs,[11] creating a large gap between physicians' perceived, desired, and actual knowledge related to costs.[12] Novel frameworks and frontline physician engagement are required if clinicians are to improve the value of the care they deliver.
We describe 1 of our first steps at the University of California, San Francisco (UCSF) to promote high‐value care (HVC) delivery: the creation of a HVC program led by clinicians and administrators focused on identifying and addressing wasteful practices within our hospitalist group. The program aims to (1) use financial and clinical data to identify areas with clear evidence of waste in the hospital, (2) promote evidence‐based interventions that improve both quality of care and value, and (3) pair interventions with evidence‐based cost awareness education to drive culture change. Our experience and inaugural projects provide a model of the key features, inherent challenges, and lessons learned, which may help inform similar efforts.
METHODS
In March 2012, we launched an HVC program within our Division of Hospital Medicine at UCSF Medical Center, a 600‐bed academic medical center in an urban setting. During the 2013 academic year, our division included 45 physicians. The medicine service, comprised of 8 teaching medical ward teams (1 attending, 1 resident, 2 interns, and variable number of medical students), and 1 nonteaching medical ward team (1 attending), admitted 4700 patients that year.
Organizational Framework
The HVC program is co‐led by a UCSF hospitalist (C.M.) and the administrator of the Division of Hospital Medicine (M.N.). Team members include hospitalists, hospital medicine fellows, resident physicians, pharmacists, project coordinators, and other administrators. The team meets in person for 1 hour every month. Project teams and ad hoc subcommittee groups often convene between meetings.
Our HVC program was placed within the infrastructure, and under the leadership, of our already established quality improvement (QI) program at UCSF. Our Division of Hospital Medicine Director of Quality and Safety (M.M.) thus oversees the QI, patient safety, patient experience, and high‐value care efforts.
The HVC program funding is largely in personnel costs. The physician leader (15% effort) is funded by the Division of Hospital Medicine, whereas the administrator is cofunded by both the division and by the medical center (largely through her roles as both division administrator and service line director). An administrative assistant within the division is also assigned to help with administrative tasks. Some additional data gathering and project support comes from existing medical center QI infrastructure, the decision support services unit, and through UCSF's new Center for Healthcare Value. Other ancillary costs for our projects have included publicity, data analytics, and information technology infrastructure. We estimate that the costs of this program are approximately $50,000 to $75,000 annually.
Framework for Identifying Target Projects
Robust Analysis of Costs
We created a framework for identifying, designing, and promoting projects specifically aimed at improving healthcare value (Figure 1). Financial data were used to identify areas with clear evidence of waste in the hospital, areas of high cost with no benefit in health outcomes. We focused particularly on obtaining cost and billing data for our medical service, which provided important insight into potential targets for improvements in value. For example, in 2011, the Division of Hospital Medicine spent more than $1 million annually in direct costs for the administration of nebulized bronchodilator therapies (nebs) to nonintensive care unit patients on the medical service.[13] These high costs, exposed by billing data, were believed to represent potential unnecessary testing and/or procedures. Not every area of high cost was deemed a target for intervention. For example, the use of recombinant factor VIII appeared a necessary expenditure (over $1 million per year) for our patients with hemophilia. Although our efforts focused on reducing waste, it is worth noting that healthcare value can also be increased by improving the delivery of high‐value services.
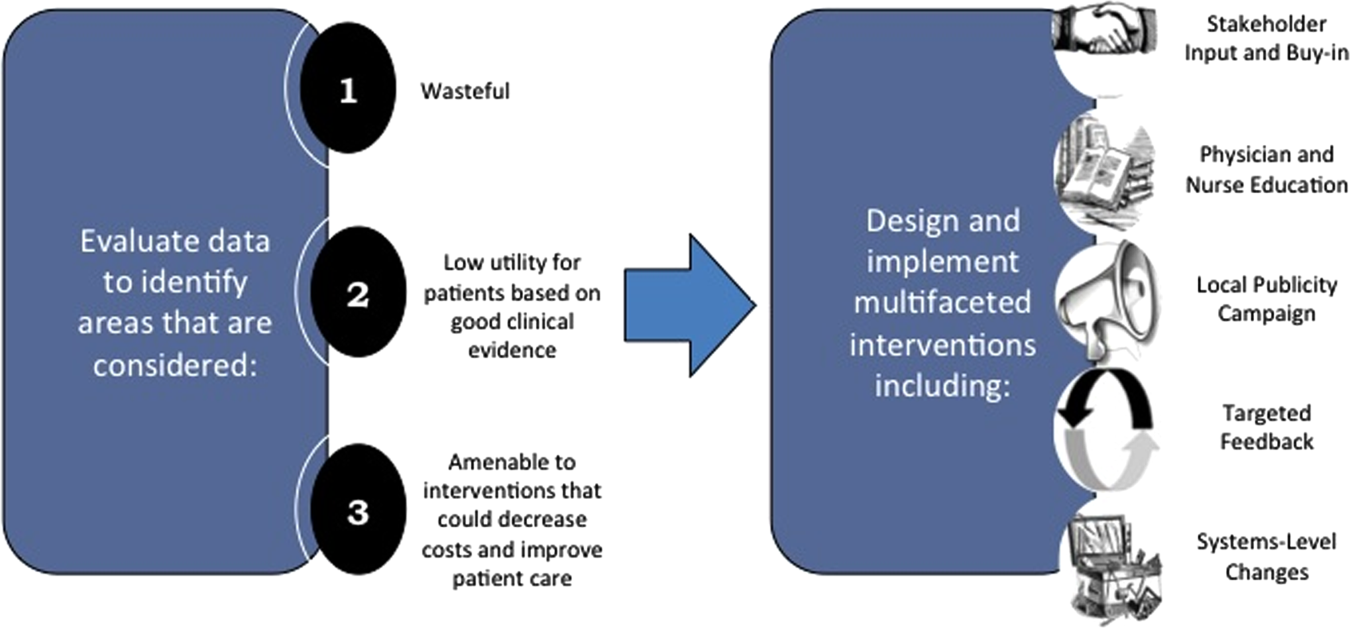
Recognized Benefits in Quality of Care
The program also evaluated the impact of cost reduction efforts on the quality of care, based on a high standard of current evidence. Though value can be improved by interventions that decrease costs while being quality neutral, our group chose to focus first on projects that would simultaneously improve quality while decreasing costs. We felt that this win‐win strategy would help obtain buy‐in from clinicians weary of prior cost‐cutting programs. For example, we pursued interventions aimed at reducing inappropriate gastric stress ulcer prophylaxis, which had the potential to both cut costs and minimize risks of hospital‐acquired pneumonia and Clostridium difficile infections.[14, 15] All proposed HVC targets were vetted through a review of the literature and published guidelines. In general, our initial projects had to be strongly supported by evidence, with high‐quality studies, preferably meta‐analyses or systematic reviews, that displayed the safety of our recommended changes. We reviewed the literature with experts. For example, we met with faculty pulmonologists to discuss the evidence supporting the use of inhalers instead of nebulizers in adults with obstructive pulmonary disease. The goals of our projects were chosen by our HVC committee, based on an analysis of our baseline data and the perceived potential effects of our proposed interventions.
Educational Intervention
Last, we paired interventions with evidence‐based cost awareness education to drive culture change. At UCSF we have an ongoing longitudinal cost‐awareness curriculum for residents, which has previously been described.[16] We took advantage of this educational forum to address gaps in clinician knowledge related to the targeted areas. When launching the initiative to decrease unnecessary inpatient nebulizer usage and improve transitions to inhalers, we utilized the chronic obstructive pulmonary disease case in the cost‐awareness series. Doing so allowed us to both review the evidence behind the effectiveness of inhalers, and introduce our Nebs No More After 24 campaign, which sought to transition adult inpatients with obstructive pulmonary symptoms from nebs to inhalers within 24 hours of admission.[13]
Intervention Strategy
Our general approach has been to design and implement multifaceted interventions, adapted from previous QI literature (Figure 1).[17] Given the importance of frontline clinician engagement to successful project implementation,[18, 19, 20] our interventions are physician‐driven and are vetted by a large group of clinicians prior to launch. The HVC program also explicitly seeks stakeholder input, perspective, and buy‐in prior to implementation. For example, we involved respiratory therapists (RTs) in the design of the Nebs No More After 24 project, thus ensuring that the interventions fit within their workflow and align with their care‐delivery goals.
Local publicity campaigns provide education and reminders for clinicians. Posters, such as the Nebs No More After 24 poster (Figure 2), were hung in physician, nursing, and RT work areas. Pens featuring the catchphrase Nebs No More After 24 were distributed to clinicians.

In addition to presentations to residents through the UCSF cost awareness curriculum, educational presentations were also delivered to attending physicians and to other allied members of the healthcare team (eg, nurses, RTs) during regularly scheduled staff meetings.
The metrics for each of the projects were regularly monitored, and targeted feedback was provided to clinicians. For the Nebs No More After 24 campaign, data for the number of nebs delivered on the target floor were provided to resident physicians during the cost awareness conference each month, and the data were presented to attending hospitalists in the monthly QI newsletter. This academic year, transfusion and telemetry data are presented via the same strategy.
Stakeholder recruitment, education, and promotional campaigns are important to program launches, but to sustain projects over the long‐term, system changes may be necessary. We have pursued changes in the computerized provider order entry (CPOE) system, such as removing nebs from the admission order set or putting a default duration for certain telemetry orders. Systems‐level interventions, although more difficult to achieve, play an important role in creating enduring changes when paired with educational interventions.
RESULTS
During our first 2 years we have initiated ongoing projects directed at 6 major targets (Table 1). Our flagship project, Nebs No More After 24, resulted in a decrease of nebulizer rates by more than 50% on a high‐acuity medical floor, as previously published.[13] We created a financial model that primarily accounted for RT time and pharmaceutical costs, and estimated a savings of approximately $250,000 annually on this single medical ward (see Supporting Information, Table 1, in the online version of this article).[13]
| High‐Value Care Projects | Relevant Baseline Data | Goals of Project | Strategies |
|---|---|---|---|
| |||
| Nebs No More After 24: Improving appropriate use of respiratory services | The medicine service spent $1 million in direct costs on approximately 25,000 nebs for non‐ICU inpatients. | Reduce unnecessary nebs >15% over 9 months. | Removed nebs from admit order set. |
| Improve transitions from nebs to MDIs. | Enlisted RTs and RNs to help with MDI teaching for patients. | ||
| Improve patient self‐administration of MDIs. | Implemented an educational program for medicine physicians. | ||
| Created local publicity: posters, flyers, and pens. | |||
| Provided data feedback to providers. | |||
| Next step: Introduce a CPOE‐linked intervention. | |||
| Improving use of stress ulcer prophylaxis | 77% of ICU patients on acid suppressive therapy; 31% of these patients did not meet criteria for appropriate prophylaxis. | Reduce overuse and inappropriate use of SUP. | A team of pharmacists, nurses, and physicians developed targeted and evidence‐based UCSF guidelines on use of SUP. |
| Developed and implemented a pharmacist‐led intervention to reduce inappropriate SUP in the ICUs that included the following: | |||
| Reminders on admission and discharge from ICU | |||
| Education and awareness initiative for prescribers | |||
| ICU and service champions | |||
| Culture change | |||
| Next step: Incorporate indications in CPOE and work with ICU to incorporate appropriate GI prophylaxis as part of the standard ICU care bundle. | |||
| Blood utilization stewardship | 30% of transfusions on the hospital medicine service are provided to patients with a hemoglobin >8 g/dL. | Decrease units of blood transfused for a hemoglobin >8.0 g/dL by 25%. | Launched an educational campaign for attending and resident physicians. |
| Monthly feedback to residents and attending physicians. | |||
| Next step: Introduce a decision support system in the CPOE for blood transfusion orders in patients with most recent hemoglobin level >8. | |||
| Improving telemetry utilization | 44% of monitored inpatients on the medical service (with length of stay >48 hours) remain on telemetry until discharge. | Decrease by 15% the number of patients (with length of stay >48 hours) who remain on telemetry until discharge. | Implemented an educational campaign for nursing groups and the medicine and cardiology housestaff. |
| Launched a messaging campaign consisting of posters and pocket cards on appropriate telemetry use. | |||
| Designed a feedback campaign with monthly e‐mail to housestaff on their ward team's telemetry use stats. | |||
| Next step: Build a CPOE intervention that asks users to specify an approved indication for telemetry when they order monitoring. The indication then dictates how long the order is active (24, 48, 72 hours or ongoing), and the MD must renew the order after the elapsed time. | |||
| iReduce iCal: ordering ionized calcium only when needed | The medicine service spent $167,000 in direct costs on iCal labs over a year (40% of all calcium lab orders; 42% occurred in non‐ICU patients). | Reduce number of iCal labs drawn on the medicine service by >25% over the course of 6 months. | With the introduction of CPOE, iCal was removed from traditional daily lab order sets. |
| Discussed with lab, renal, and ICU stakeholders. | |||
| Implemented an educational campaign for physicians and nurses. | |||
| Created local publicity: posters and candies. | |||
| Provided data feedback to providers. | |||
| Repeat inpatient echocardiograms | 25% of TTEs are performed within 6 months of a prior; one‐third of these are for inappropriate indications. | Decrease inappropriate repeat TTEs by 25%. | Implemented an educational campaign. |
| Next step: provide the most recent TTE results in the CPOE at time of order, and provide auditing and decision support for repeat TTEs. | |||
The HVC program also provided an arena for collaborating with and supporting value‐based projects launched by other groups, such as the UCSF Medication Outcomes Center's inappropriate gastric stress ulcer prophylaxis program.[21] Our group helped support the development and implementation of evidence‐based clinical practice guidelines, and we assisted educational interventions targeting clinicians. This program resulted in a decrease in inappropriate stress ulcer prophylaxis in intensive care unit patients from 19% to 6.6% within 1 month following implementation.[21]
DISCUSSION
Physicians are increasingly being asked to embrace and lead efforts to improve healthcare value and reduce costs. Our program provides a framework to guide physician‐led initiatives to identify and address areas of healthcare waste.
Challenges and Lessons Learned
Overcoming the Hurdle of More Care as Better Care
Improving the quality of care has traditionally stressed the underuse of beneficial testing and treatments, for example the use of angiotensin‐converting enzyme inhibitors in systolic heart failure. We found that improving quality by curbing overuse was a new idea for many physicians. Traditionally, physicians have struggled with cost reduction programs, feeling that efforts to reduce costs are indifferent to quality of care, and worse, may actually lead to inferior care.[22] The historical separation of most QI and cost reduction programs has likely furthered this sentiment. Our first projects married cost reduction and QI efforts by demonstrating how reducing overuse could provide an opportunity to increase quality and reduce harms from treatments. For example, transitioning from nebs to metered‐dose inhalers offered the chance to provide inpatient inhaler teaching, whereas decreasing proton pump inhibitor use can reduce the incidence of C difficile. By framing these projects as addressing both numerator and denominator of the value equation, we were able to align our cost‐reduction efforts with physicians' traditional notions of QI.
Cost Transparency
If physicians are to play a larger role in cost‐reduction efforts, they need at least a working understanding of fixed and variable costs in healthcare and of institutional prices.[23, 24] Utilization and clear information about costs were used to guide our interventions and ensured that the efforts spent to eliminate waste would result in cost savings. As an example, we learned that decreasing nebulizer use without a corresponding decrease in daily RT staffing would lead to minimal cost savings. These analyses require the support of business, financial, and resource managers in addition to physicians, nurses, project coordinators, and administrators. At many institutions the lack of price and utilization transparency presents a major barrier to the accurate analysis of cost‐reduction efforts.
The Diplomacy of Cost‐Reduction
Because the bulk of healthcare costs go to labor, efforts to reduce cost may lead to reductions in the resources available to certain departments or even to individuals' wages. For example, initiatives aimed at reducing inappropriate diagnostic imaging will affect the radiology department, which is partially paid based on the volume of studies performed.[25] Key stakeholders must be identified early, and project leaders should seek understanding, engagement, and buy‐in from involved parties prior to implementation. There will often be times that support from senior leaders will be needed to negotiate these tricky situations.
Although we benefited from a largely supportive hospital medicine faculty and resident physicians, not all of our proposed projects made it to implementation. Sometimes stakeholder recruitment proved to be difficult. For instance, a proposed project to change the protocol from routine to clinically indicated peripheral intravenous catheter replacement for adult inpatients was met with some resistance by some members of nursing management. We reviewed the literature together and discussed in length the proposal, but ultimately decided that our institution was not ready for this change at this time.
Limitations and Next Steps
Our goal is to provide guidance on exporting the approach of our HVC program to other institutions, but there may be several limitations. First, our strategy relied on several contributing factors that may be unique to our institution. We had engaged frontline physician champions, who may not be available or have the necessary support at other academic or community organizations. Our UCSF cost awareness curriculum provided an educational foundation and framework for our projects. We also had institutional commitment in the form of our medical center division administrator.
Second, there are up‐front costs to running our committee, which are primarily related to personnel funding as described in the Methods. Over the next year we aim to calculate cost‐effectiveness ratios for our projects and overall return on investment for each of our projects, as we have done for the Nebs No More After 24 project (see Supporting Information, Table 1, in the online version of this article). Based on this analysis, the modest upfront costs appear to be easily recouped over the course of the year.
We have anecdotally noted a culture change in the way that our physicians discuss and consider testing. For example, it is common now to hear ward teams on morning rounds consider the costs of testing or discuss the need for prophylactic proton pump inhibitors. An important next step for our HVC program is the building of better data infrastructures for our own electronic health record system to allow us to more quickly, accurately, and comprehensively identify new targets and monitor the progress and sustainability of our projects. The Institute of Medicine has noted that the adoption of technology is a key strategy to creating a continuously learning healthcare system.[1] It is our hope that through consistent audit and feedback of resource utilization we can translate our early gains into sustainable changes in practice.
Furthermore, we hope to target and enact additional organizational changes, including creating CPOE‐linked interventions to help reinforce and further our objectives. We believe that creating systems that make it easier to do the right thing will help the cause of embedding HVC practices throughout our medical center. We have begun to scale some of our projects, such as the Nebs No More After 24 campaign, medical center wide, and ultimately we hope to disseminate successful projects and models beyond our medical center to contribute to the national movement to provide the best care at lower costs.
As discussed above, our interventions are targeted at simultaneous improvements in quality with decreased costs. However, the goal is not to hide our cost interventions behind the banner of quality. We believe that there is a shifting culture that is increasingly ready to accept cost alone as a meaningful patient harm, worthy of interventions on its own merits, assuming that quality and safety remain stable.[26, 27]
CONCLUSIONS
Our HVC program has been successful in promoting improved healthcare value and engaging clinicians in this effort. The program is guided by the use of financial data to identify areas with clear evidence of waste in the hospital, the creation of evidence‐based interventions that improve quality of care while cutting costs, and the pairing of interventions with evidence‐based cost awareness education to drive culture change.
Acknowledgements
The authors acknowledge the following members of the UCSF Division of Hospital Medicine High‐Value Care Committee who have led some of the initiatives mentioned in this article and have directly contributed to Table 1: Dr. Stephanie Rennke, Dr. Alvin Rajkomar, Dr. Nader Najafi, Dr. Steven Ludwin, and Dr. Elizabeth Stewart. Dr. Russ Cucina particularly contributed to the designs and implementation of electronic medical record interventions.
Disclosures: Dr. Moriates received funding from the UCSF Center for Healthcare Value, the Agency for Healthcare Research and Quality (as editor for AHRQ Patient Safety Net), and the ABIM Foundation. Mrs. Novelero received funding from the UCSF Center for Healthcare Value. Dr. Wachter reports serving as the immediate past‐chair of the American Board of Internal Medicine (for which he received a stipend) and is a current member of the ABIM Foundation board; receiving a contract to UCSF from the Agency for Healthcare Research and Quality for editing 2 patient‐safety websites; receiving compensation from John Wiley & Sons for writing a blog; receiving compensation from QuantiaMD for editing and presenting patient safety educational modules; receiving royalties from Lippincott Williams & Wilkins and McGraw‐Hill for writing/editing several books; receiving a stipend and stock/options for serving on the Board of Directors of IPC‐The Hospitalist Company; serving on the scientific advisory boards for PatientSafe Solutions, CRISI, SmartDose, and EarlySense (for which he receives stock options); and holding the Benioff endowed chair in hospital medicine from Marc and Lynne Benioff. He is also a member of the Board of Directors of Salem Hospital, Salem, Oregon, for which he receives travel reimbursement but no compensation. Mr. John Hillman, Mr. Aseem Bharti, and Ms. Claudia Hermann from UCSF Decision Support Services provided financial data support and analyses, and the UCSF Center for Healthcare Value provided resource and financial support.
- Institute of Medicine. Committee on the Learning Health Care System in America. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2012.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367(4):292–295.
- . Making good on ACOs' promise—the final rule for the Medicare Shared Savings Program. N Engl J Med. 2011;365(19):1753–1756.
- . American College of Physicians ethics manual: sixth edition. Ann Intern Med. 2012;156(1 pt 2):73–104.
- ABIM Foundation, American College of Physicians‐American Society of Internal Medicine, European Federation of Internal Medicine. Medical professionalism in the new millennium: a physician charter. Ann Intern Med. 2002;136(3):243–246.
- , . Choosing Wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801.
- , , , . High‐value, cost‐conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154(3):174–180.
- , . Waste not, want not: promoting efficient use of health care resources. Ann Intern Med. 2013;158(1):67–68.
- , , . General pediatric attending physicians' and residents' knowledge of inpatient hospital finances. Pediatrics. 2013;131(6):1072–1080.
- , , . Hospitalists' awareness of patient charges associated with inpatient care. J Hosp Med. 2010;5(5):295–297.
- , , , , . Teaching residents to provide cost‐conscious care: A national survey of residency program directors. JAMA Intern Med. 2014;174(3):470–472.
- , , . Perceived, actual, and desired knowledge regarding medicare billing and reimbursement. J Gen Intern Med. 2006;21(5):466–470.
- , , , , . “Nebs No More After 24”: a pilot program to improve the use of appropriate respiratory therapies. JAMA Intern Med. 2013;173(17):1647–1648.
- , , , . Acid‐suppressive medication use and the risk for hospital‐acquired pneumonia. JAMA. 2009;301(20):2120–2128.
- , , , et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170(9):784–790.
- , , , . The value in the evidence: teaching residents to “choose wisely.” JAMA Intern Med.2013;173(4):308–310.
- , . Evidence‐based quality improvement: the state of the science. Health Aff. 2005;24(1):138–150.
- , , , . The role of physician engagement on the impact of the hospital‐based practice improvement module (PIM). J Hosp Med. 2009;4(8):466–470.
- , . Finding common cause in quality: confronting the physician engagement challenge. Physician Exec. 2008;34(2):26–28, 30–31.
- , . Engaging physicians and leveraging professionalism: a key to success for quality measurement and improvement. JAMA. 2012;308(10):979–980.
- , , et al. The development and implementation of a bundled quality improvement initiative to reduce inappropriate stress ulcer prophylaxis. ICU Dir. 2013;4(6):322–325.
- . Lost in translation: physicians' struggle with cost‐reduction programs. Ann Intern Med. 2011;154(6):430–433.
- , . How to solve the cost crisis in health care. Harv Bus Rev. 2011;89(9):46–52, 54, 56–61 passim.
- , , , . The savings illusion—why clinical quality improvement fails to deliver bottom‐line results. N Engl J Med. 2011;365(26):e48.
- , , , , . Reducing radiology use on an inpatient medical service: choosing wisely. Arch Intern Med. 2012;172(20):1606–1608.
- , , . First, do no (financial) harm. JAMA. 2013;310(6):577–578.
- , , . Full disclosure—out‐of‐pocket costs as side effects. N Engl J Med. 2013;369(16):1484–1486.
With a United States medical system that spends as much as $750 billion each year on care that does not result in improved health outcomes,[1] many policy initiatives, including the Centers for Medicare and Medicaid Services' Value‐Based Purchasing program, seek to realign hospitals' financial incentives from a focus on production to one on value (quality divided by cost).[2, 3] Professional organizations have now deemed resource stewardship an ethical responsibility for professionalism,[4, 5] and campaigns such as the American Board of Internal Medicine (ABIM) Foundation's Choosing Wisely effort and the American College of Physicians' High‐Value Care platform are calling on frontline clinicians to address unnecessary and wasteful services.[6, 7]
Despite these pressures and initiatives, most physicians lack the knowledge and tools necessary to prioritize the delivery of their own healthcare services according to value.[8, 9, 10] Hospital medicine physicians are unaware of the costs associated with the interventions they order,[10] and the majority of medical training programs lack curricula focused on healthcare costs,[11] creating a large gap between physicians' perceived, desired, and actual knowledge related to costs.[12] Novel frameworks and frontline physician engagement are required if clinicians are to improve the value of the care they deliver.
We describe 1 of our first steps at the University of California, San Francisco (UCSF) to promote high‐value care (HVC) delivery: the creation of a HVC program led by clinicians and administrators focused on identifying and addressing wasteful practices within our hospitalist group. The program aims to (1) use financial and clinical data to identify areas with clear evidence of waste in the hospital, (2) promote evidence‐based interventions that improve both quality of care and value, and (3) pair interventions with evidence‐based cost awareness education to drive culture change. Our experience and inaugural projects provide a model of the key features, inherent challenges, and lessons learned, which may help inform similar efforts.
METHODS
In March 2012, we launched an HVC program within our Division of Hospital Medicine at UCSF Medical Center, a 600‐bed academic medical center in an urban setting. During the 2013 academic year, our division included 45 physicians. The medicine service, comprised of 8 teaching medical ward teams (1 attending, 1 resident, 2 interns, and variable number of medical students), and 1 nonteaching medical ward team (1 attending), admitted 4700 patients that year.
Organizational Framework
The HVC program is co‐led by a UCSF hospitalist (C.M.) and the administrator of the Division of Hospital Medicine (M.N.). Team members include hospitalists, hospital medicine fellows, resident physicians, pharmacists, project coordinators, and other administrators. The team meets in person for 1 hour every month. Project teams and ad hoc subcommittee groups often convene between meetings.
Our HVC program was placed within the infrastructure, and under the leadership, of our already established quality improvement (QI) program at UCSF. Our Division of Hospital Medicine Director of Quality and Safety (M.M.) thus oversees the QI, patient safety, patient experience, and high‐value care efforts.
The HVC program funding is largely in personnel costs. The physician leader (15% effort) is funded by the Division of Hospital Medicine, whereas the administrator is cofunded by both the division and by the medical center (largely through her roles as both division administrator and service line director). An administrative assistant within the division is also assigned to help with administrative tasks. Some additional data gathering and project support comes from existing medical center QI infrastructure, the decision support services unit, and through UCSF's new Center for Healthcare Value. Other ancillary costs for our projects have included publicity, data analytics, and information technology infrastructure. We estimate that the costs of this program are approximately $50,000 to $75,000 annually.
Framework for Identifying Target Projects
Robust Analysis of Costs
We created a framework for identifying, designing, and promoting projects specifically aimed at improving healthcare value (Figure 1). Financial data were used to identify areas with clear evidence of waste in the hospital, areas of high cost with no benefit in health outcomes. We focused particularly on obtaining cost and billing data for our medical service, which provided important insight into potential targets for improvements in value. For example, in 2011, the Division of Hospital Medicine spent more than $1 million annually in direct costs for the administration of nebulized bronchodilator therapies (nebs) to nonintensive care unit patients on the medical service.[13] These high costs, exposed by billing data, were believed to represent potential unnecessary testing and/or procedures. Not every area of high cost was deemed a target for intervention. For example, the use of recombinant factor VIII appeared a necessary expenditure (over $1 million per year) for our patients with hemophilia. Although our efforts focused on reducing waste, it is worth noting that healthcare value can also be increased by improving the delivery of high‐value services.

Recognized Benefits in Quality of Care
The program also evaluated the impact of cost reduction efforts on the quality of care, based on a high standard of current evidence. Though value can be improved by interventions that decrease costs while being quality neutral, our group chose to focus first on projects that would simultaneously improve quality while decreasing costs. We felt that this win‐win strategy would help obtain buy‐in from clinicians weary of prior cost‐cutting programs. For example, we pursued interventions aimed at reducing inappropriate gastric stress ulcer prophylaxis, which had the potential to both cut costs and minimize risks of hospital‐acquired pneumonia and Clostridium difficile infections.[14, 15] All proposed HVC targets were vetted through a review of the literature and published guidelines. In general, our initial projects had to be strongly supported by evidence, with high‐quality studies, preferably meta‐analyses or systematic reviews, that displayed the safety of our recommended changes. We reviewed the literature with experts. For example, we met with faculty pulmonologists to discuss the evidence supporting the use of inhalers instead of nebulizers in adults with obstructive pulmonary disease. The goals of our projects were chosen by our HVC committee, based on an analysis of our baseline data and the perceived potential effects of our proposed interventions.
Educational Intervention
Last, we paired interventions with evidence‐based cost awareness education to drive culture change. At UCSF we have an ongoing longitudinal cost‐awareness curriculum for residents, which has previously been described.[16] We took advantage of this educational forum to address gaps in clinician knowledge related to the targeted areas. When launching the initiative to decrease unnecessary inpatient nebulizer usage and improve transitions to inhalers, we utilized the chronic obstructive pulmonary disease case in the cost‐awareness series. Doing so allowed us to both review the evidence behind the effectiveness of inhalers, and introduce our Nebs No More After 24 campaign, which sought to transition adult inpatients with obstructive pulmonary symptoms from nebs to inhalers within 24 hours of admission.[13]
Intervention Strategy
Our general approach has been to design and implement multifaceted interventions, adapted from previous QI literature (Figure 1).[17] Given the importance of frontline clinician engagement to successful project implementation,[18, 19, 20] our interventions are physician‐driven and are vetted by a large group of clinicians prior to launch. The HVC program also explicitly seeks stakeholder input, perspective, and buy‐in prior to implementation. For example, we involved respiratory therapists (RTs) in the design of the Nebs No More After 24 project, thus ensuring that the interventions fit within their workflow and align with their care‐delivery goals.
Local publicity campaigns provide education and reminders for clinicians. Posters, such as the Nebs No More After 24 poster (Figure 2), were hung in physician, nursing, and RT work areas. Pens featuring the catchphrase Nebs No More After 24 were distributed to clinicians.

In addition to presentations to residents through the UCSF cost awareness curriculum, educational presentations were also delivered to attending physicians and to other allied members of the healthcare team (eg, nurses, RTs) during regularly scheduled staff meetings.
The metrics for each of the projects were regularly monitored, and targeted feedback was provided to clinicians. For the Nebs No More After 24 campaign, data for the number of nebs delivered on the target floor were provided to resident physicians during the cost awareness conference each month, and the data were presented to attending hospitalists in the monthly QI newsletter. This academic year, transfusion and telemetry data are presented via the same strategy.
Stakeholder recruitment, education, and promotional campaigns are important to program launches, but to sustain projects over the long‐term, system changes may be necessary. We have pursued changes in the computerized provider order entry (CPOE) system, such as removing nebs from the admission order set or putting a default duration for certain telemetry orders. Systems‐level interventions, although more difficult to achieve, play an important role in creating enduring changes when paired with educational interventions.
RESULTS
During our first 2 years we have initiated ongoing projects directed at 6 major targets (Table 1). Our flagship project, Nebs No More After 24, resulted in a decrease of nebulizer rates by more than 50% on a high‐acuity medical floor, as previously published.[13] We created a financial model that primarily accounted for RT time and pharmaceutical costs, and estimated a savings of approximately $250,000 annually on this single medical ward (see Supporting Information, Table 1, in the online version of this article).[13]
| High‐Value Care Projects | Relevant Baseline Data | Goals of Project | Strategies |
|---|---|---|---|
| |||
| Nebs No More After 24: Improving appropriate use of respiratory services | The medicine service spent $1 million in direct costs on approximately 25,000 nebs for non‐ICU inpatients. | Reduce unnecessary nebs >15% over 9 months. | Removed nebs from admit order set. |
| Improve transitions from nebs to MDIs. | Enlisted RTs and RNs to help with MDI teaching for patients. | ||
| Improve patient self‐administration of MDIs. | Implemented an educational program for medicine physicians. | ||
| Created local publicity: posters, flyers, and pens. | |||
| Provided data feedback to providers. | |||
| Next step: Introduce a CPOE‐linked intervention. | |||
| Improving use of stress ulcer prophylaxis | 77% of ICU patients on acid suppressive therapy; 31% of these patients did not meet criteria for appropriate prophylaxis. | Reduce overuse and inappropriate use of SUP. | A team of pharmacists, nurses, and physicians developed targeted and evidence‐based UCSF guidelines on use of SUP. |
| Developed and implemented a pharmacist‐led intervention to reduce inappropriate SUP in the ICUs that included the following: | |||
| Reminders on admission and discharge from ICU | |||
| Education and awareness initiative for prescribers | |||
| ICU and service champions | |||
| Culture change | |||
| Next step: Incorporate indications in CPOE and work with ICU to incorporate appropriate GI prophylaxis as part of the standard ICU care bundle. | |||
| Blood utilization stewardship | 30% of transfusions on the hospital medicine service are provided to patients with a hemoglobin >8 g/dL. | Decrease units of blood transfused for a hemoglobin >8.0 g/dL by 25%. | Launched an educational campaign for attending and resident physicians. |
| Monthly feedback to residents and attending physicians. | |||
| Next step: Introduce a decision support system in the CPOE for blood transfusion orders in patients with most recent hemoglobin level >8. | |||
| Improving telemetry utilization | 44% of monitored inpatients on the medical service (with length of stay >48 hours) remain on telemetry until discharge. | Decrease by 15% the number of patients (with length of stay >48 hours) who remain on telemetry until discharge. | Implemented an educational campaign for nursing groups and the medicine and cardiology housestaff. |
| Launched a messaging campaign consisting of posters and pocket cards on appropriate telemetry use. | |||
| Designed a feedback campaign with monthly e‐mail to housestaff on their ward team's telemetry use stats. | |||
| Next step: Build a CPOE intervention that asks users to specify an approved indication for telemetry when they order monitoring. The indication then dictates how long the order is active (24, 48, 72 hours or ongoing), and the MD must renew the order after the elapsed time. | |||
| iReduce iCal: ordering ionized calcium only when needed | The medicine service spent $167,000 in direct costs on iCal labs over a year (40% of all calcium lab orders; 42% occurred in non‐ICU patients). | Reduce number of iCal labs drawn on the medicine service by >25% over the course of 6 months. | With the introduction of CPOE, iCal was removed from traditional daily lab order sets. |
| Discussed with lab, renal, and ICU stakeholders. | |||
| Implemented an educational campaign for physicians and nurses. | |||
| Created local publicity: posters and candies. | |||
| Provided data feedback to providers. | |||
| Repeat inpatient echocardiograms | 25% of TTEs are performed within 6 months of a prior; one‐third of these are for inappropriate indications. | Decrease inappropriate repeat TTEs by 25%. | Implemented an educational campaign. |
| Next step: provide the most recent TTE results in the CPOE at time of order, and provide auditing and decision support for repeat TTEs. | |||
The HVC program also provided an arena for collaborating with and supporting value‐based projects launched by other groups, such as the UCSF Medication Outcomes Center's inappropriate gastric stress ulcer prophylaxis program.[21] Our group helped support the development and implementation of evidence‐based clinical practice guidelines, and we assisted educational interventions targeting clinicians. This program resulted in a decrease in inappropriate stress ulcer prophylaxis in intensive care unit patients from 19% to 6.6% within 1 month following implementation.[21]
DISCUSSION
Physicians are increasingly being asked to embrace and lead efforts to improve healthcare value and reduce costs. Our program provides a framework to guide physician‐led initiatives to identify and address areas of healthcare waste.
Challenges and Lessons Learned
Overcoming the Hurdle of More Care as Better Care
Improving the quality of care has traditionally stressed the underuse of beneficial testing and treatments, for example the use of angiotensin‐converting enzyme inhibitors in systolic heart failure. We found that improving quality by curbing overuse was a new idea for many physicians. Traditionally, physicians have struggled with cost reduction programs, feeling that efforts to reduce costs are indifferent to quality of care, and worse, may actually lead to inferior care.[22] The historical separation of most QI and cost reduction programs has likely furthered this sentiment. Our first projects married cost reduction and QI efforts by demonstrating how reducing overuse could provide an opportunity to increase quality and reduce harms from treatments. For example, transitioning from nebs to metered‐dose inhalers offered the chance to provide inpatient inhaler teaching, whereas decreasing proton pump inhibitor use can reduce the incidence of C difficile. By framing these projects as addressing both numerator and denominator of the value equation, we were able to align our cost‐reduction efforts with physicians' traditional notions of QI.
Cost Transparency
If physicians are to play a larger role in cost‐reduction efforts, they need at least a working understanding of fixed and variable costs in healthcare and of institutional prices.[23, 24] Utilization and clear information about costs were used to guide our interventions and ensured that the efforts spent to eliminate waste would result in cost savings. As an example, we learned that decreasing nebulizer use without a corresponding decrease in daily RT staffing would lead to minimal cost savings. These analyses require the support of business, financial, and resource managers in addition to physicians, nurses, project coordinators, and administrators. At many institutions the lack of price and utilization transparency presents a major barrier to the accurate analysis of cost‐reduction efforts.
The Diplomacy of Cost‐Reduction
Because the bulk of healthcare costs go to labor, efforts to reduce cost may lead to reductions in the resources available to certain departments or even to individuals' wages. For example, initiatives aimed at reducing inappropriate diagnostic imaging will affect the radiology department, which is partially paid based on the volume of studies performed.[25] Key stakeholders must be identified early, and project leaders should seek understanding, engagement, and buy‐in from involved parties prior to implementation. There will often be times that support from senior leaders will be needed to negotiate these tricky situations.
Although we benefited from a largely supportive hospital medicine faculty and resident physicians, not all of our proposed projects made it to implementation. Sometimes stakeholder recruitment proved to be difficult. For instance, a proposed project to change the protocol from routine to clinically indicated peripheral intravenous catheter replacement for adult inpatients was met with some resistance by some members of nursing management. We reviewed the literature together and discussed in length the proposal, but ultimately decided that our institution was not ready for this change at this time.
Limitations and Next Steps
Our goal is to provide guidance on exporting the approach of our HVC program to other institutions, but there may be several limitations. First, our strategy relied on several contributing factors that may be unique to our institution. We had engaged frontline physician champions, who may not be available or have the necessary support at other academic or community organizations. Our UCSF cost awareness curriculum provided an educational foundation and framework for our projects. We also had institutional commitment in the form of our medical center division administrator.
Second, there are up‐front costs to running our committee, which are primarily related to personnel funding as described in the Methods. Over the next year we aim to calculate cost‐effectiveness ratios for our projects and overall return on investment for each of our projects, as we have done for the Nebs No More After 24 project (see Supporting Information, Table 1, in the online version of this article). Based on this analysis, the modest upfront costs appear to be easily recouped over the course of the year.
We have anecdotally noted a culture change in the way that our physicians discuss and consider testing. For example, it is common now to hear ward teams on morning rounds consider the costs of testing or discuss the need for prophylactic proton pump inhibitors. An important next step for our HVC program is the building of better data infrastructures for our own electronic health record system to allow us to more quickly, accurately, and comprehensively identify new targets and monitor the progress and sustainability of our projects. The Institute of Medicine has noted that the adoption of technology is a key strategy to creating a continuously learning healthcare system.[1] It is our hope that through consistent audit and feedback of resource utilization we can translate our early gains into sustainable changes in practice.
Furthermore, we hope to target and enact additional organizational changes, including creating CPOE‐linked interventions to help reinforce and further our objectives. We believe that creating systems that make it easier to do the right thing will help the cause of embedding HVC practices throughout our medical center. We have begun to scale some of our projects, such as the Nebs No More After 24 campaign, medical center wide, and ultimately we hope to disseminate successful projects and models beyond our medical center to contribute to the national movement to provide the best care at lower costs.
As discussed above, our interventions are targeted at simultaneous improvements in quality with decreased costs. However, the goal is not to hide our cost interventions behind the banner of quality. We believe that there is a shifting culture that is increasingly ready to accept cost alone as a meaningful patient harm, worthy of interventions on its own merits, assuming that quality and safety remain stable.[26, 27]
CONCLUSIONS
Our HVC program has been successful in promoting improved healthcare value and engaging clinicians in this effort. The program is guided by the use of financial data to identify areas with clear evidence of waste in the hospital, the creation of evidence‐based interventions that improve quality of care while cutting costs, and the pairing of interventions with evidence‐based cost awareness education to drive culture change.
Acknowledgements
The authors acknowledge the following members of the UCSF Division of Hospital Medicine High‐Value Care Committee who have led some of the initiatives mentioned in this article and have directly contributed to Table 1: Dr. Stephanie Rennke, Dr. Alvin Rajkomar, Dr. Nader Najafi, Dr. Steven Ludwin, and Dr. Elizabeth Stewart. Dr. Russ Cucina particularly contributed to the designs and implementation of electronic medical record interventions.
Disclosures: Dr. Moriates received funding from the UCSF Center for Healthcare Value, the Agency for Healthcare Research and Quality (as editor for AHRQ Patient Safety Net), and the ABIM Foundation. Mrs. Novelero received funding from the UCSF Center for Healthcare Value. Dr. Wachter reports serving as the immediate past‐chair of the American Board of Internal Medicine (for which he received a stipend) and is a current member of the ABIM Foundation board; receiving a contract to UCSF from the Agency for Healthcare Research and Quality for editing 2 patient‐safety websites; receiving compensation from John Wiley & Sons for writing a blog; receiving compensation from QuantiaMD for editing and presenting patient safety educational modules; receiving royalties from Lippincott Williams & Wilkins and McGraw‐Hill for writing/editing several books; receiving a stipend and stock/options for serving on the Board of Directors of IPC‐The Hospitalist Company; serving on the scientific advisory boards for PatientSafe Solutions, CRISI, SmartDose, and EarlySense (for which he receives stock options); and holding the Benioff endowed chair in hospital medicine from Marc and Lynne Benioff. He is also a member of the Board of Directors of Salem Hospital, Salem, Oregon, for which he receives travel reimbursement but no compensation. Mr. John Hillman, Mr. Aseem Bharti, and Ms. Claudia Hermann from UCSF Decision Support Services provided financial data support and analyses, and the UCSF Center for Healthcare Value provided resource and financial support.
With a United States medical system that spends as much as $750 billion each year on care that does not result in improved health outcomes,[1] many policy initiatives, including the Centers for Medicare and Medicaid Services' Value‐Based Purchasing program, seek to realign hospitals' financial incentives from a focus on production to one on value (quality divided by cost).[2, 3] Professional organizations have now deemed resource stewardship an ethical responsibility for professionalism,[4, 5] and campaigns such as the American Board of Internal Medicine (ABIM) Foundation's Choosing Wisely effort and the American College of Physicians' High‐Value Care platform are calling on frontline clinicians to address unnecessary and wasteful services.[6, 7]
Despite these pressures and initiatives, most physicians lack the knowledge and tools necessary to prioritize the delivery of their own healthcare services according to value.[8, 9, 10] Hospital medicine physicians are unaware of the costs associated with the interventions they order,[10] and the majority of medical training programs lack curricula focused on healthcare costs,[11] creating a large gap between physicians' perceived, desired, and actual knowledge related to costs.[12] Novel frameworks and frontline physician engagement are required if clinicians are to improve the value of the care they deliver.
We describe 1 of our first steps at the University of California, San Francisco (UCSF) to promote high‐value care (HVC) delivery: the creation of a HVC program led by clinicians and administrators focused on identifying and addressing wasteful practices within our hospitalist group. The program aims to (1) use financial and clinical data to identify areas with clear evidence of waste in the hospital, (2) promote evidence‐based interventions that improve both quality of care and value, and (3) pair interventions with evidence‐based cost awareness education to drive culture change. Our experience and inaugural projects provide a model of the key features, inherent challenges, and lessons learned, which may help inform similar efforts.
METHODS
In March 2012, we launched an HVC program within our Division of Hospital Medicine at UCSF Medical Center, a 600‐bed academic medical center in an urban setting. During the 2013 academic year, our division included 45 physicians. The medicine service, comprised of 8 teaching medical ward teams (1 attending, 1 resident, 2 interns, and variable number of medical students), and 1 nonteaching medical ward team (1 attending), admitted 4700 patients that year.
Organizational Framework
The HVC program is co‐led by a UCSF hospitalist (C.M.) and the administrator of the Division of Hospital Medicine (M.N.). Team members include hospitalists, hospital medicine fellows, resident physicians, pharmacists, project coordinators, and other administrators. The team meets in person for 1 hour every month. Project teams and ad hoc subcommittee groups often convene between meetings.
Our HVC program was placed within the infrastructure, and under the leadership, of our already established quality improvement (QI) program at UCSF. Our Division of Hospital Medicine Director of Quality and Safety (M.M.) thus oversees the QI, patient safety, patient experience, and high‐value care efforts.
The HVC program funding is largely in personnel costs. The physician leader (15% effort) is funded by the Division of Hospital Medicine, whereas the administrator is cofunded by both the division and by the medical center (largely through her roles as both division administrator and service line director). An administrative assistant within the division is also assigned to help with administrative tasks. Some additional data gathering and project support comes from existing medical center QI infrastructure, the decision support services unit, and through UCSF's new Center for Healthcare Value. Other ancillary costs for our projects have included publicity, data analytics, and information technology infrastructure. We estimate that the costs of this program are approximately $50,000 to $75,000 annually.
Framework for Identifying Target Projects
Robust Analysis of Costs
We created a framework for identifying, designing, and promoting projects specifically aimed at improving healthcare value (Figure 1). Financial data were used to identify areas with clear evidence of waste in the hospital, areas of high cost with no benefit in health outcomes. We focused particularly on obtaining cost and billing data for our medical service, which provided important insight into potential targets for improvements in value. For example, in 2011, the Division of Hospital Medicine spent more than $1 million annually in direct costs for the administration of nebulized bronchodilator therapies (nebs) to nonintensive care unit patients on the medical service.[13] These high costs, exposed by billing data, were believed to represent potential unnecessary testing and/or procedures. Not every area of high cost was deemed a target for intervention. For example, the use of recombinant factor VIII appeared a necessary expenditure (over $1 million per year) for our patients with hemophilia. Although our efforts focused on reducing waste, it is worth noting that healthcare value can also be increased by improving the delivery of high‐value services.

Recognized Benefits in Quality of Care
The program also evaluated the impact of cost reduction efforts on the quality of care, based on a high standard of current evidence. Though value can be improved by interventions that decrease costs while being quality neutral, our group chose to focus first on projects that would simultaneously improve quality while decreasing costs. We felt that this win‐win strategy would help obtain buy‐in from clinicians weary of prior cost‐cutting programs. For example, we pursued interventions aimed at reducing inappropriate gastric stress ulcer prophylaxis, which had the potential to both cut costs and minimize risks of hospital‐acquired pneumonia and Clostridium difficile infections.[14, 15] All proposed HVC targets were vetted through a review of the literature and published guidelines. In general, our initial projects had to be strongly supported by evidence, with high‐quality studies, preferably meta‐analyses or systematic reviews, that displayed the safety of our recommended changes. We reviewed the literature with experts. For example, we met with faculty pulmonologists to discuss the evidence supporting the use of inhalers instead of nebulizers in adults with obstructive pulmonary disease. The goals of our projects were chosen by our HVC committee, based on an analysis of our baseline data and the perceived potential effects of our proposed interventions.
Educational Intervention
Last, we paired interventions with evidence‐based cost awareness education to drive culture change. At UCSF we have an ongoing longitudinal cost‐awareness curriculum for residents, which has previously been described.[16] We took advantage of this educational forum to address gaps in clinician knowledge related to the targeted areas. When launching the initiative to decrease unnecessary inpatient nebulizer usage and improve transitions to inhalers, we utilized the chronic obstructive pulmonary disease case in the cost‐awareness series. Doing so allowed us to both review the evidence behind the effectiveness of inhalers, and introduce our Nebs No More After 24 campaign, which sought to transition adult inpatients with obstructive pulmonary symptoms from nebs to inhalers within 24 hours of admission.[13]
Intervention Strategy
Our general approach has been to design and implement multifaceted interventions, adapted from previous QI literature (Figure 1).[17] Given the importance of frontline clinician engagement to successful project implementation,[18, 19, 20] our interventions are physician‐driven and are vetted by a large group of clinicians prior to launch. The HVC program also explicitly seeks stakeholder input, perspective, and buy‐in prior to implementation. For example, we involved respiratory therapists (RTs) in the design of the Nebs No More After 24 project, thus ensuring that the interventions fit within their workflow and align with their care‐delivery goals.
Local publicity campaigns provide education and reminders for clinicians. Posters, such as the Nebs No More After 24 poster (Figure 2), were hung in physician, nursing, and RT work areas. Pens featuring the catchphrase Nebs No More After 24 were distributed to clinicians.

In addition to presentations to residents through the UCSF cost awareness curriculum, educational presentations were also delivered to attending physicians and to other allied members of the healthcare team (eg, nurses, RTs) during regularly scheduled staff meetings.
The metrics for each of the projects were regularly monitored, and targeted feedback was provided to clinicians. For the Nebs No More After 24 campaign, data for the number of nebs delivered on the target floor were provided to resident physicians during the cost awareness conference each month, and the data were presented to attending hospitalists in the monthly QI newsletter. This academic year, transfusion and telemetry data are presented via the same strategy.
Stakeholder recruitment, education, and promotional campaigns are important to program launches, but to sustain projects over the long‐term, system changes may be necessary. We have pursued changes in the computerized provider order entry (CPOE) system, such as removing nebs from the admission order set or putting a default duration for certain telemetry orders. Systems‐level interventions, although more difficult to achieve, play an important role in creating enduring changes when paired with educational interventions.
RESULTS
During our first 2 years we have initiated ongoing projects directed at 6 major targets (Table 1). Our flagship project, Nebs No More After 24, resulted in a decrease of nebulizer rates by more than 50% on a high‐acuity medical floor, as previously published.[13] We created a financial model that primarily accounted for RT time and pharmaceutical costs, and estimated a savings of approximately $250,000 annually on this single medical ward (see Supporting Information, Table 1, in the online version of this article).[13]
| High‐Value Care Projects | Relevant Baseline Data | Goals of Project | Strategies |
|---|---|---|---|
| |||
| Nebs No More After 24: Improving appropriate use of respiratory services | The medicine service spent $1 million in direct costs on approximately 25,000 nebs for non‐ICU inpatients. | Reduce unnecessary nebs >15% over 9 months. | Removed nebs from admit order set. |
| Improve transitions from nebs to MDIs. | Enlisted RTs and RNs to help with MDI teaching for patients. | ||
| Improve patient self‐administration of MDIs. | Implemented an educational program for medicine physicians. | ||
| Created local publicity: posters, flyers, and pens. | |||
| Provided data feedback to providers. | |||
| Next step: Introduce a CPOE‐linked intervention. | |||
| Improving use of stress ulcer prophylaxis | 77% of ICU patients on acid suppressive therapy; 31% of these patients did not meet criteria for appropriate prophylaxis. | Reduce overuse and inappropriate use of SUP. | A team of pharmacists, nurses, and physicians developed targeted and evidence‐based UCSF guidelines on use of SUP. |
| Developed and implemented a pharmacist‐led intervention to reduce inappropriate SUP in the ICUs that included the following: | |||
| Reminders on admission and discharge from ICU | |||
| Education and awareness initiative for prescribers | |||
| ICU and service champions | |||
| Culture change | |||
| Next step: Incorporate indications in CPOE and work with ICU to incorporate appropriate GI prophylaxis as part of the standard ICU care bundle. | |||
| Blood utilization stewardship | 30% of transfusions on the hospital medicine service are provided to patients with a hemoglobin >8 g/dL. | Decrease units of blood transfused for a hemoglobin >8.0 g/dL by 25%. | Launched an educational campaign for attending and resident physicians. |
| Monthly feedback to residents and attending physicians. | |||
| Next step: Introduce a decision support system in the CPOE for blood transfusion orders in patients with most recent hemoglobin level >8. | |||
| Improving telemetry utilization | 44% of monitored inpatients on the medical service (with length of stay >48 hours) remain on telemetry until discharge. | Decrease by 15% the number of patients (with length of stay >48 hours) who remain on telemetry until discharge. | Implemented an educational campaign for nursing groups and the medicine and cardiology housestaff. |
| Launched a messaging campaign consisting of posters and pocket cards on appropriate telemetry use. | |||
| Designed a feedback campaign with monthly e‐mail to housestaff on their ward team's telemetry use stats. | |||
| Next step: Build a CPOE intervention that asks users to specify an approved indication for telemetry when they order monitoring. The indication then dictates how long the order is active (24, 48, 72 hours or ongoing), and the MD must renew the order after the elapsed time. | |||
| iReduce iCal: ordering ionized calcium only when needed | The medicine service spent $167,000 in direct costs on iCal labs over a year (40% of all calcium lab orders; 42% occurred in non‐ICU patients). | Reduce number of iCal labs drawn on the medicine service by >25% over the course of 6 months. | With the introduction of CPOE, iCal was removed from traditional daily lab order sets. |
| Discussed with lab, renal, and ICU stakeholders. | |||
| Implemented an educational campaign for physicians and nurses. | |||
| Created local publicity: posters and candies. | |||
| Provided data feedback to providers. | |||
| Repeat inpatient echocardiograms | 25% of TTEs are performed within 6 months of a prior; one‐third of these are for inappropriate indications. | Decrease inappropriate repeat TTEs by 25%. | Implemented an educational campaign. |
| Next step: provide the most recent TTE results in the CPOE at time of order, and provide auditing and decision support for repeat TTEs. | |||
The HVC program also provided an arena for collaborating with and supporting value‐based projects launched by other groups, such as the UCSF Medication Outcomes Center's inappropriate gastric stress ulcer prophylaxis program.[21] Our group helped support the development and implementation of evidence‐based clinical practice guidelines, and we assisted educational interventions targeting clinicians. This program resulted in a decrease in inappropriate stress ulcer prophylaxis in intensive care unit patients from 19% to 6.6% within 1 month following implementation.[21]
DISCUSSION
Physicians are increasingly being asked to embrace and lead efforts to improve healthcare value and reduce costs. Our program provides a framework to guide physician‐led initiatives to identify and address areas of healthcare waste.
Challenges and Lessons Learned
Overcoming the Hurdle of More Care as Better Care
Improving the quality of care has traditionally stressed the underuse of beneficial testing and treatments, for example the use of angiotensin‐converting enzyme inhibitors in systolic heart failure. We found that improving quality by curbing overuse was a new idea for many physicians. Traditionally, physicians have struggled with cost reduction programs, feeling that efforts to reduce costs are indifferent to quality of care, and worse, may actually lead to inferior care.[22] The historical separation of most QI and cost reduction programs has likely furthered this sentiment. Our first projects married cost reduction and QI efforts by demonstrating how reducing overuse could provide an opportunity to increase quality and reduce harms from treatments. For example, transitioning from nebs to metered‐dose inhalers offered the chance to provide inpatient inhaler teaching, whereas decreasing proton pump inhibitor use can reduce the incidence of C difficile. By framing these projects as addressing both numerator and denominator of the value equation, we were able to align our cost‐reduction efforts with physicians' traditional notions of QI.
Cost Transparency
If physicians are to play a larger role in cost‐reduction efforts, they need at least a working understanding of fixed and variable costs in healthcare and of institutional prices.[23, 24] Utilization and clear information about costs were used to guide our interventions and ensured that the efforts spent to eliminate waste would result in cost savings. As an example, we learned that decreasing nebulizer use without a corresponding decrease in daily RT staffing would lead to minimal cost savings. These analyses require the support of business, financial, and resource managers in addition to physicians, nurses, project coordinators, and administrators. At many institutions the lack of price and utilization transparency presents a major barrier to the accurate analysis of cost‐reduction efforts.
The Diplomacy of Cost‐Reduction
Because the bulk of healthcare costs go to labor, efforts to reduce cost may lead to reductions in the resources available to certain departments or even to individuals' wages. For example, initiatives aimed at reducing inappropriate diagnostic imaging will affect the radiology department, which is partially paid based on the volume of studies performed.[25] Key stakeholders must be identified early, and project leaders should seek understanding, engagement, and buy‐in from involved parties prior to implementation. There will often be times that support from senior leaders will be needed to negotiate these tricky situations.
Although we benefited from a largely supportive hospital medicine faculty and resident physicians, not all of our proposed projects made it to implementation. Sometimes stakeholder recruitment proved to be difficult. For instance, a proposed project to change the protocol from routine to clinically indicated peripheral intravenous catheter replacement for adult inpatients was met with some resistance by some members of nursing management. We reviewed the literature together and discussed in length the proposal, but ultimately decided that our institution was not ready for this change at this time.
Limitations and Next Steps
Our goal is to provide guidance on exporting the approach of our HVC program to other institutions, but there may be several limitations. First, our strategy relied on several contributing factors that may be unique to our institution. We had engaged frontline physician champions, who may not be available or have the necessary support at other academic or community organizations. Our UCSF cost awareness curriculum provided an educational foundation and framework for our projects. We also had institutional commitment in the form of our medical center division administrator.
Second, there are up‐front costs to running our committee, which are primarily related to personnel funding as described in the Methods. Over the next year we aim to calculate cost‐effectiveness ratios for our projects and overall return on investment for each of our projects, as we have done for the Nebs No More After 24 project (see Supporting Information, Table 1, in the online version of this article). Based on this analysis, the modest upfront costs appear to be easily recouped over the course of the year.
We have anecdotally noted a culture change in the way that our physicians discuss and consider testing. For example, it is common now to hear ward teams on morning rounds consider the costs of testing or discuss the need for prophylactic proton pump inhibitors. An important next step for our HVC program is the building of better data infrastructures for our own electronic health record system to allow us to more quickly, accurately, and comprehensively identify new targets and monitor the progress and sustainability of our projects. The Institute of Medicine has noted that the adoption of technology is a key strategy to creating a continuously learning healthcare system.[1] It is our hope that through consistent audit and feedback of resource utilization we can translate our early gains into sustainable changes in practice.
Furthermore, we hope to target and enact additional organizational changes, including creating CPOE‐linked interventions to help reinforce and further our objectives. We believe that creating systems that make it easier to do the right thing will help the cause of embedding HVC practices throughout our medical center. We have begun to scale some of our projects, such as the Nebs No More After 24 campaign, medical center wide, and ultimately we hope to disseminate successful projects and models beyond our medical center to contribute to the national movement to provide the best care at lower costs.
As discussed above, our interventions are targeted at simultaneous improvements in quality with decreased costs. However, the goal is not to hide our cost interventions behind the banner of quality. We believe that there is a shifting culture that is increasingly ready to accept cost alone as a meaningful patient harm, worthy of interventions on its own merits, assuming that quality and safety remain stable.[26, 27]
CONCLUSIONS
Our HVC program has been successful in promoting improved healthcare value and engaging clinicians in this effort. The program is guided by the use of financial data to identify areas with clear evidence of waste in the hospital, the creation of evidence‐based interventions that improve quality of care while cutting costs, and the pairing of interventions with evidence‐based cost awareness education to drive culture change.
Acknowledgements
The authors acknowledge the following members of the UCSF Division of Hospital Medicine High‐Value Care Committee who have led some of the initiatives mentioned in this article and have directly contributed to Table 1: Dr. Stephanie Rennke, Dr. Alvin Rajkomar, Dr. Nader Najafi, Dr. Steven Ludwin, and Dr. Elizabeth Stewart. Dr. Russ Cucina particularly contributed to the designs and implementation of electronic medical record interventions.
Disclosures: Dr. Moriates received funding from the UCSF Center for Healthcare Value, the Agency for Healthcare Research and Quality (as editor for AHRQ Patient Safety Net), and the ABIM Foundation. Mrs. Novelero received funding from the UCSF Center for Healthcare Value. Dr. Wachter reports serving as the immediate past‐chair of the American Board of Internal Medicine (for which he received a stipend) and is a current member of the ABIM Foundation board; receiving a contract to UCSF from the Agency for Healthcare Research and Quality for editing 2 patient‐safety websites; receiving compensation from John Wiley & Sons for writing a blog; receiving compensation from QuantiaMD for editing and presenting patient safety educational modules; receiving royalties from Lippincott Williams & Wilkins and McGraw‐Hill for writing/editing several books; receiving a stipend and stock/options for serving on the Board of Directors of IPC‐The Hospitalist Company; serving on the scientific advisory boards for PatientSafe Solutions, CRISI, SmartDose, and EarlySense (for which he receives stock options); and holding the Benioff endowed chair in hospital medicine from Marc and Lynne Benioff. He is also a member of the Board of Directors of Salem Hospital, Salem, Oregon, for which he receives travel reimbursement but no compensation. Mr. John Hillman, Mr. Aseem Bharti, and Ms. Claudia Hermann from UCSF Decision Support Services provided financial data support and analyses, and the UCSF Center for Healthcare Value provided resource and financial support.
- Institute of Medicine. Committee on the Learning Health Care System in America. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2012.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367(4):292–295.
- . Making good on ACOs' promise—the final rule for the Medicare Shared Savings Program. N Engl J Med. 2011;365(19):1753–1756.
- . American College of Physicians ethics manual: sixth edition. Ann Intern Med. 2012;156(1 pt 2):73–104.
- ABIM Foundation, American College of Physicians‐American Society of Internal Medicine, European Federation of Internal Medicine. Medical professionalism in the new millennium: a physician charter. Ann Intern Med. 2002;136(3):243–246.
- , . Choosing Wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801.
- , , , . High‐value, cost‐conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154(3):174–180.
- , . Waste not, want not: promoting efficient use of health care resources. Ann Intern Med. 2013;158(1):67–68.
- , , . General pediatric attending physicians' and residents' knowledge of inpatient hospital finances. Pediatrics. 2013;131(6):1072–1080.
- , , . Hospitalists' awareness of patient charges associated with inpatient care. J Hosp Med. 2010;5(5):295–297.
- , , , , . Teaching residents to provide cost‐conscious care: A national survey of residency program directors. JAMA Intern Med. 2014;174(3):470–472.
- , , . Perceived, actual, and desired knowledge regarding medicare billing and reimbursement. J Gen Intern Med. 2006;21(5):466–470.
- , , , , . “Nebs No More After 24”: a pilot program to improve the use of appropriate respiratory therapies. JAMA Intern Med. 2013;173(17):1647–1648.
- , , , . Acid‐suppressive medication use and the risk for hospital‐acquired pneumonia. JAMA. 2009;301(20):2120–2128.
- , , , et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170(9):784–790.
- , , , . The value in the evidence: teaching residents to “choose wisely.” JAMA Intern Med.2013;173(4):308–310.
- , . Evidence‐based quality improvement: the state of the science. Health Aff. 2005;24(1):138–150.
- , , , . The role of physician engagement on the impact of the hospital‐based practice improvement module (PIM). J Hosp Med. 2009;4(8):466–470.
- , . Finding common cause in quality: confronting the physician engagement challenge. Physician Exec. 2008;34(2):26–28, 30–31.
- , . Engaging physicians and leveraging professionalism: a key to success for quality measurement and improvement. JAMA. 2012;308(10):979–980.
- , , et al. The development and implementation of a bundled quality improvement initiative to reduce inappropriate stress ulcer prophylaxis. ICU Dir. 2013;4(6):322–325.
- . Lost in translation: physicians' struggle with cost‐reduction programs. Ann Intern Med. 2011;154(6):430–433.
- , . How to solve the cost crisis in health care. Harv Bus Rev. 2011;89(9):46–52, 54, 56–61 passim.
- , , , . The savings illusion—why clinical quality improvement fails to deliver bottom‐line results. N Engl J Med. 2011;365(26):e48.
- , , , , . Reducing radiology use on an inpatient medical service: choosing wisely. Arch Intern Med. 2012;172(20):1606–1608.
- , , . First, do no (financial) harm. JAMA. 2013;310(6):577–578.
- , , . Full disclosure—out‐of‐pocket costs as side effects. N Engl J Med. 2013;369(16):1484–1486.
- Institute of Medicine. Committee on the Learning Health Care System in America. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2012.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367(4):292–295.
- . Making good on ACOs' promise—the final rule for the Medicare Shared Savings Program. N Engl J Med. 2011;365(19):1753–1756.
- . American College of Physicians ethics manual: sixth edition. Ann Intern Med. 2012;156(1 pt 2):73–104.
- ABIM Foundation, American College of Physicians‐American Society of Internal Medicine, European Federation of Internal Medicine. Medical professionalism in the new millennium: a physician charter. Ann Intern Med. 2002;136(3):243–246.
- , . Choosing Wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801.
- , , , . High‐value, cost‐conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154(3):174–180.
- , . Waste not, want not: promoting efficient use of health care resources. Ann Intern Med. 2013;158(1):67–68.
- , , . General pediatric attending physicians' and residents' knowledge of inpatient hospital finances. Pediatrics. 2013;131(6):1072–1080.
- , , . Hospitalists' awareness of patient charges associated with inpatient care. J Hosp Med. 2010;5(5):295–297.
- , , , , . Teaching residents to provide cost‐conscious care: A national survey of residency program directors. JAMA Intern Med. 2014;174(3):470–472.
- , , . Perceived, actual, and desired knowledge regarding medicare billing and reimbursement. J Gen Intern Med. 2006;21(5):466–470.
- , , , , . “Nebs No More After 24”: a pilot program to improve the use of appropriate respiratory therapies. JAMA Intern Med. 2013;173(17):1647–1648.
- , , , . Acid‐suppressive medication use and the risk for hospital‐acquired pneumonia. JAMA. 2009;301(20):2120–2128.
- , , , et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170(9):784–790.
- , , , . The value in the evidence: teaching residents to “choose wisely.” JAMA Intern Med.2013;173(4):308–310.
- , . Evidence‐based quality improvement: the state of the science. Health Aff. 2005;24(1):138–150.
- , , , . The role of physician engagement on the impact of the hospital‐based practice improvement module (PIM). J Hosp Med. 2009;4(8):466–470.
- , . Finding common cause in quality: confronting the physician engagement challenge. Physician Exec. 2008;34(2):26–28, 30–31.
- , . Engaging physicians and leveraging professionalism: a key to success for quality measurement and improvement. JAMA. 2012;308(10):979–980.
- , , et al. The development and implementation of a bundled quality improvement initiative to reduce inappropriate stress ulcer prophylaxis. ICU Dir. 2013;4(6):322–325.
- . Lost in translation: physicians' struggle with cost‐reduction programs. Ann Intern Med. 2011;154(6):430–433.
- , . How to solve the cost crisis in health care. Harv Bus Rev. 2011;89(9):46–52, 54, 56–61 passim.
- , , , . The savings illusion—why clinical quality improvement fails to deliver bottom‐line results. N Engl J Med. 2011;365(26):e48.
- , , , , . Reducing radiology use on an inpatient medical service: choosing wisely. Arch Intern Med. 2012;172(20):1606–1608.
- , , . First, do no (financial) harm. JAMA. 2013;310(6):577–578.
- , , . Full disclosure—out‐of‐pocket costs as side effects. N Engl J Med. 2013;369(16):1484–1486.