User login
Discharge by Noon: Toward a Better Understanding of Benefits and Costs
Targeting “discharge before noon” (DBN) for hospitalized patients has been proposed as a way to improve hospital throughput and patient safety by reducing emergency department (ED) boarding and crowding. In this issue, Kirubarajan et al1 report no association between morning discharge and length of stay (LOS) for either the ED or hospitalization.1 This large (189,781 patients) 7-year study from seven quite different Canadian hospitals adds important data to a literature that remains divided about whether DBN helps or hurts hospital LOS and ED boarding.
Unlike trials reporting interventions to encourage DBN, this observational study was unique in that it took each day as the unit of observation. This method cleverly allowed the authors to examine whether days with more discharges before noon conferred a lower mean ED and inpatient LOS among patients admitted on those days. Their approach appropriately reframes the central issue as one of patient flow.
Kirubarajan et al’s most notable, and perhaps surprising, finding is the lack of association between morning discharge and ED LOS. Computer modeling supports the hypothesis that ED throughput will improve on days with earlier inpatient bed availability.2 Several studies have also noted earlier ED departure times and decreased ED wait times after implementing interventions to promote DBN.3 Why might the authors’ findings contradict previous studies? Their outcomes may in part be due to high ED LOS (>14 hours), exceeding Canadian published targets and reports from the United States.4,5 Problems relating to ED resources, practice, and hospital census may have overwhelmed DBN as factors in boarding. The interpretation of their findings is limited by the authors’ decision to report only ED LOS, rather than including the time between a decision to admit and ED departure (boarding time).
While early studies that focused on interventions to promote DBN noted decreased inpatient LOS after their implementation, later studies found no effect or even an increase in LOS for general internal medicine patients. Concerns have been raised about the confounding effect of concurrent initiatives aimed at improving LOS as well as misaligned incentives to delay discharge to the following morning. As the number of conflicting studies mounts, and with the current report in hand, it is tempting to conclude that for the DBN evidence base as a whole, we are observing random variation around no effect.
With growing doubt about benefits of morning discharge, perhaps we should turn our attention away from the question of how to increase DBN and consider instead why and at what cost. Hospitals are delicate organisms; a singular focus on one metric will undoubtedly impact others. Does the effort to discharge before noon consume valuable morning hours and detract from the care of other patients? Are patients held overnight unnecessarily to comply with DBN? Are there consequences in patient, nursing, or trainee satisfaction? Is bedside teaching affected?
And as concepts of patient-centered care are increasingly valued, we may ask whether DBN is such a concept, or is it rather an increasingly dubious strategy aimed at regularizing hospital operations? The need for a more holistic assessment of “discharge quality” is apparent. Instead of focusing on a particular hour, initiatives should determine the “best, earliest discharge time” for each patient and align multidisciplinary efforts toward this patient-centered goal. Such efforts are already underway in pediatric hospitals, where fixed discharge times are being replaced by discharge milestones embedded into the electronic medical record.6 An instrument to track “discharge readiness” such as this one, paired with ongoing analysis of the barriers to timely discharge, might better facilitate throughput by targeting the entire admission, rather than concentrating pressure on its final hours.
1. Kirubarajan A, Shin S, Fralick M, Kwan Jet al. Morning discharges and patient length-of-stay in inpatient general internal medicine. J Hosp Med. 2021;16(6):334-338. https://doi.org/ 10.12788/jhm.3605
2. Powell ES, Khare RK, Venkatesh AK, Van Roo BD, Adams JG, Reinhardt G. The relationship between inpatient discharge timing and emergency department boarding. J Emerg Med. 2012;42(2):186-196. https://doi.org/10.1016/j.jemermed.2010.06.028
3. Wertheimer B, Jacobs RE, Iturrate E, Bailey M, Hochman K. Discharge before noon: effect on throughput and sustainability. J Hosp Med. 2015;10(10):664-669. https://doi.org/10.1002/jhm.2412
4. Fee C, Burstin H, Maselli JH, Hsia RY. Association of emergency department length of stay with safety-net status. JAMA. 2012;307(5):476-482. https://doi.org/10.1001/jama.2012.41
5. Ontario wait times. Ontario Ministry of Health and Ministry of Long-Term Care. Accessed February 17, 2021. http://www.health.gov.on.ca/en/pro/programs/waittimes/edrs/targets.aspx
6. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
Targeting “discharge before noon” (DBN) for hospitalized patients has been proposed as a way to improve hospital throughput and patient safety by reducing emergency department (ED) boarding and crowding. In this issue, Kirubarajan et al1 report no association between morning discharge and length of stay (LOS) for either the ED or hospitalization.1 This large (189,781 patients) 7-year study from seven quite different Canadian hospitals adds important data to a literature that remains divided about whether DBN helps or hurts hospital LOS and ED boarding.
Unlike trials reporting interventions to encourage DBN, this observational study was unique in that it took each day as the unit of observation. This method cleverly allowed the authors to examine whether days with more discharges before noon conferred a lower mean ED and inpatient LOS among patients admitted on those days. Their approach appropriately reframes the central issue as one of patient flow.
Kirubarajan et al’s most notable, and perhaps surprising, finding is the lack of association between morning discharge and ED LOS. Computer modeling supports the hypothesis that ED throughput will improve on days with earlier inpatient bed availability.2 Several studies have also noted earlier ED departure times and decreased ED wait times after implementing interventions to promote DBN.3 Why might the authors’ findings contradict previous studies? Their outcomes may in part be due to high ED LOS (>14 hours), exceeding Canadian published targets and reports from the United States.4,5 Problems relating to ED resources, practice, and hospital census may have overwhelmed DBN as factors in boarding. The interpretation of their findings is limited by the authors’ decision to report only ED LOS, rather than including the time between a decision to admit and ED departure (boarding time).
While early studies that focused on interventions to promote DBN noted decreased inpatient LOS after their implementation, later studies found no effect or even an increase in LOS for general internal medicine patients. Concerns have been raised about the confounding effect of concurrent initiatives aimed at improving LOS as well as misaligned incentives to delay discharge to the following morning. As the number of conflicting studies mounts, and with the current report in hand, it is tempting to conclude that for the DBN evidence base as a whole, we are observing random variation around no effect.
With growing doubt about benefits of morning discharge, perhaps we should turn our attention away from the question of how to increase DBN and consider instead why and at what cost. Hospitals are delicate organisms; a singular focus on one metric will undoubtedly impact others. Does the effort to discharge before noon consume valuable morning hours and detract from the care of other patients? Are patients held overnight unnecessarily to comply with DBN? Are there consequences in patient, nursing, or trainee satisfaction? Is bedside teaching affected?
And as concepts of patient-centered care are increasingly valued, we may ask whether DBN is such a concept, or is it rather an increasingly dubious strategy aimed at regularizing hospital operations? The need for a more holistic assessment of “discharge quality” is apparent. Instead of focusing on a particular hour, initiatives should determine the “best, earliest discharge time” for each patient and align multidisciplinary efforts toward this patient-centered goal. Such efforts are already underway in pediatric hospitals, where fixed discharge times are being replaced by discharge milestones embedded into the electronic medical record.6 An instrument to track “discharge readiness” such as this one, paired with ongoing analysis of the barriers to timely discharge, might better facilitate throughput by targeting the entire admission, rather than concentrating pressure on its final hours.
Targeting “discharge before noon” (DBN) for hospitalized patients has been proposed as a way to improve hospital throughput and patient safety by reducing emergency department (ED) boarding and crowding. In this issue, Kirubarajan et al1 report no association between morning discharge and length of stay (LOS) for either the ED or hospitalization.1 This large (189,781 patients) 7-year study from seven quite different Canadian hospitals adds important data to a literature that remains divided about whether DBN helps or hurts hospital LOS and ED boarding.
Unlike trials reporting interventions to encourage DBN, this observational study was unique in that it took each day as the unit of observation. This method cleverly allowed the authors to examine whether days with more discharges before noon conferred a lower mean ED and inpatient LOS among patients admitted on those days. Their approach appropriately reframes the central issue as one of patient flow.
Kirubarajan et al’s most notable, and perhaps surprising, finding is the lack of association between morning discharge and ED LOS. Computer modeling supports the hypothesis that ED throughput will improve on days with earlier inpatient bed availability.2 Several studies have also noted earlier ED departure times and decreased ED wait times after implementing interventions to promote DBN.3 Why might the authors’ findings contradict previous studies? Their outcomes may in part be due to high ED LOS (>14 hours), exceeding Canadian published targets and reports from the United States.4,5 Problems relating to ED resources, practice, and hospital census may have overwhelmed DBN as factors in boarding. The interpretation of their findings is limited by the authors’ decision to report only ED LOS, rather than including the time between a decision to admit and ED departure (boarding time).
While early studies that focused on interventions to promote DBN noted decreased inpatient LOS after their implementation, later studies found no effect or even an increase in LOS for general internal medicine patients. Concerns have been raised about the confounding effect of concurrent initiatives aimed at improving LOS as well as misaligned incentives to delay discharge to the following morning. As the number of conflicting studies mounts, and with the current report in hand, it is tempting to conclude that for the DBN evidence base as a whole, we are observing random variation around no effect.
With growing doubt about benefits of morning discharge, perhaps we should turn our attention away from the question of how to increase DBN and consider instead why and at what cost. Hospitals are delicate organisms; a singular focus on one metric will undoubtedly impact others. Does the effort to discharge before noon consume valuable morning hours and detract from the care of other patients? Are patients held overnight unnecessarily to comply with DBN? Are there consequences in patient, nursing, or trainee satisfaction? Is bedside teaching affected?
And as concepts of patient-centered care are increasingly valued, we may ask whether DBN is such a concept, or is it rather an increasingly dubious strategy aimed at regularizing hospital operations? The need for a more holistic assessment of “discharge quality” is apparent. Instead of focusing on a particular hour, initiatives should determine the “best, earliest discharge time” for each patient and align multidisciplinary efforts toward this patient-centered goal. Such efforts are already underway in pediatric hospitals, where fixed discharge times are being replaced by discharge milestones embedded into the electronic medical record.6 An instrument to track “discharge readiness” such as this one, paired with ongoing analysis of the barriers to timely discharge, might better facilitate throughput by targeting the entire admission, rather than concentrating pressure on its final hours.
1. Kirubarajan A, Shin S, Fralick M, Kwan Jet al. Morning discharges and patient length-of-stay in inpatient general internal medicine. J Hosp Med. 2021;16(6):334-338. https://doi.org/ 10.12788/jhm.3605
2. Powell ES, Khare RK, Venkatesh AK, Van Roo BD, Adams JG, Reinhardt G. The relationship between inpatient discharge timing and emergency department boarding. J Emerg Med. 2012;42(2):186-196. https://doi.org/10.1016/j.jemermed.2010.06.028
3. Wertheimer B, Jacobs RE, Iturrate E, Bailey M, Hochman K. Discharge before noon: effect on throughput and sustainability. J Hosp Med. 2015;10(10):664-669. https://doi.org/10.1002/jhm.2412
4. Fee C, Burstin H, Maselli JH, Hsia RY. Association of emergency department length of stay with safety-net status. JAMA. 2012;307(5):476-482. https://doi.org/10.1001/jama.2012.41
5. Ontario wait times. Ontario Ministry of Health and Ministry of Long-Term Care. Accessed February 17, 2021. http://www.health.gov.on.ca/en/pro/programs/waittimes/edrs/targets.aspx
6. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
1. Kirubarajan A, Shin S, Fralick M, Kwan Jet al. Morning discharges and patient length-of-stay in inpatient general internal medicine. J Hosp Med. 2021;16(6):334-338. https://doi.org/ 10.12788/jhm.3605
2. Powell ES, Khare RK, Venkatesh AK, Van Roo BD, Adams JG, Reinhardt G. The relationship between inpatient discharge timing and emergency department boarding. J Emerg Med. 2012;42(2):186-196. https://doi.org/10.1016/j.jemermed.2010.06.028
3. Wertheimer B, Jacobs RE, Iturrate E, Bailey M, Hochman K. Discharge before noon: effect on throughput and sustainability. J Hosp Med. 2015;10(10):664-669. https://doi.org/10.1002/jhm.2412
4. Fee C, Burstin H, Maselli JH, Hsia RY. Association of emergency department length of stay with safety-net status. JAMA. 2012;307(5):476-482. https://doi.org/10.1001/jama.2012.41
5. Ontario wait times. Ontario Ministry of Health and Ministry of Long-Term Care. Accessed February 17, 2021. http://www.health.gov.on.ca/en/pro/programs/waittimes/edrs/targets.aspx
6. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
© 2021 Society of Hospital Medicine
Hospitalist and Internal Medicine Leaders’ Perspectives of Early Discharge Challenges at Academic Medical Centers
The discharge process is a critical bottleneck for efficient patient flow through the hospital. Delayed discharges translate into delays in admissions and other patient transitions, often leading to excess costs, patient dissatisfaction, and even patient harm.1-3 The emergency department is particularly impacted by these delays; bottlenecks there lead to overcrowding, increased overall hospital length of stay, and increased risks for bad outcomes during hospitalization.2
Academic medical centers in particular may struggle with delayed discharges. In a typical teaching hospital, a team composed of an attending physician and housestaff share responsibility for determining the discharge plan. Additionally, clinical teaching activities may affect the process and quality of discharge.4-6
The prevalence and causes of delayed discharges vary greatly.7-9 To improve efficiency around discharge, many hospitals have launched initiatives designed to discharge patients earlier in the day, including goal setting (“discharge by noon”), scheduling discharge appointments, and using quality-improvement methods, such as Lean Methodology (LEAN), to remove inefficiencies within discharge processes.10-12 However, there are few data on the prevalence and effectiveness of different strategies.
The aim of this study was to survey academic hospitalist and general internal medicine physician leaders to elicit their perspectives on the factors contributing to discharge timing and the relative importance and effectiveness of early-discharge initiatives.
METHODS
Study Design, Participants, and Oversight
We obtained a list of 115 university-affiliated hospitals associated with a residency program and, in most cases, a medical school from Vizient Inc. (formerly University HealthSystem Consortium), an alliance of academic medical centers and affiliated hospitals. Each member institution submits clinical data to allow for the benchmarking of outcomes to drive transparency and quality improvement.13 More than 95% of the nation’s academic medical centers and affiliated hospitals participate in this collaborative. Vizient works with members but does not set nor promote quality metrics, such as discharge timeliness. E-mail addresses for hospital medicine physician leaders (eg, division chief) of major academic medical centers were obtained from each institution via publicly available data (eg, the institution’s website). When an institution did not have a hospital medicine section, we identified the division chief of general internal medicine. The University of California, San Francisco Institutional Review Board approved this study.
Survey Development and Domains
We developed a 30-item survey to evaluate 5 main domains of interest: current discharge practices, degree of prioritization of early discharge on the inpatient service, barriers to timely discharge, prevalence and perceived effectiveness of implemented early-discharge initiatives, and barriers to implementation of early-discharge initiatives.
Respondents were first asked to identify their institutions’ goals for discharge time. They were then asked to compare the priority of early-discharge initiatives to other departmental quality-improvement initiatives, such as reducing 30-day readmissions, improving interpreter use, and improving patient satisfaction. Next, respondents were asked to estimate the degree to which clinical or patient factors contributed to delays in discharge. Respondents were then asked whether specific early-discharge initiatives, such as changes to rounding practices or communication interventions, were implemented at their institutions and, if so, the perceived effectiveness of these initiatives at meeting discharge targets. We piloted the questions locally with physicians and researchers prior to finalizing the survey.
Data Collection
We sent surveys via an online platform (Research Electronic Data Capture).14 Nonresponders were sent 2 e-mail reminders and then a follow-up telephone call asking them to complete the survey. Only 1 survey per academic medical center was collected. Any respondent who completed the survey within 2 weeks of receiving it was entered to win a Kindle Fire.
Data Analysis
We summarized survey responses using descriptive statistics. Analysis was completed in IBM SPSS version 22 (Armonk, NY).
RESULTS
Survey Respondent and Institutional Characteristics
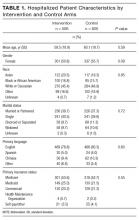
Of the 115 institutions surveyed, we received 61 responses (response rate of 53%), with 39 (64%) respondents from divisions of hospital medicine and 22 (36%) from divisions of general internal medicine. A majority (n = 53; 87%) stated their medicine services have a combination of teaching (with residents) and nonteaching (without residents) teams. Thirty-nine (64%) reported having daily multidisciplinary rounds.
Early Discharge as a Priority
Forty-seven (77%) institutional representatives strongly agreed or agreed that early discharge was a priority, with discharge by noon being the most common target time (n = 23; 38%). Thirty (50%) respondents rated early discharge as more important than improving interpreter use for non-English-speaking patients and equally important as reducing 30-day readmissions (n = 29; 48%) and improving patient satisfaction (n = 27; 44%).
Factors Delaying Discharge
The most common factors perceived as delaying discharge were considered external to the hospital, such as postacute care bed availability or scheduled (eg, ambulance) transport delays (n = 48; 79%), followed by patient factors such as patient transport issues (n = 44; 72%). Less commonly reported were workflow issues, such as competing primary team priorities or case manager bandwidth (n = 38; 62%; Table 1).
Initiatives to Improve Discharge
The most commonly implemented initiatives perceived as effective at improving discharge times were the preemptive identification of early discharges to plan discharge paperwork (n = 34; 56%), communication with patients about anticipated discharge time on the day prior to discharge (n = 29; 48%), and the implementation of additional rounds between physician teams and case managers specifically around discharge planning (n = 28; 46%). Initiatives not commonly implemented included regular audit of and feedback on discharge times to providers and teams (n = 21; 34%), the use of a discharge readiness checklist (n = 26; 43%), incentives such as bonuses or penalties (n = 37; 61%), the use of a whiteboard to indicate discharge times (n = 23; 38%), and dedicated quality-improvement approaches such as LEAN (n = 37; 61%; Table 2).
DISCUSSION
Our study suggests early discharge for medicine patients is a priority among academic institutions. Hospitalist and general internal medicine physician leaders in our study generally attributed delayed discharges to external factors, particularly unavailability of postacute care facilities and transportation delays. Having issues with finding postacute care placements is consistent with previous findings by Selker et al.15 and Carey et al.8 This is despite the 20-year difference between Selker et al.’s study and the current study, reflecting a continued opportunity for improvement, including stronger partnerships with local and regional postacute care facilities to expedite care transition and stronger discharge-planning efforts early in the admission process. Efforts in postacute care placement may be particularly important for Medicaid-insured and uninsured patients.
Our responders, hospitalist and internal medicine physician leaders, did not perceive the additional responsibilities of teaching and supervising trainees to be factors that significantly delayed patient discharge. This is in contrast to previous studies, which attributed delays in discharge to prolonged clinical decision-making related to teaching and supervision.4-6,8 This discrepancy may be due to the fact that we only surveyed single physician leaders at each institution and not residents. Our finding warrants further investigation to understand the degree to which resident skills may impact discharge planning and processes.
Institutions represented in our study have attempted a variety of initiatives promoting earlier discharge, with varying levels of perceived success. Initiatives perceived to be the most effective by hospital leaders centered on 2 main areas: (1) changing individual provider practice and (2) anticipatory discharge preparation. Interestingly, this is in discordance with the main factors labeled as causing delays in discharges, such as obtaining postacute care beds, busy case managers, and competing demands on primary teams. We hypothesize this may be because such changes require organization- or system-level changes and are perceived as more arduous than changes at the individual level. In addition, changes to individual provider behavior may be more cost- and time-effective than more systemic initiatives.
Our findings are consistent with the work published by Wertheimer and colleagues,11 who show that additional afternoon interdisciplinary rounds can help identify patients who may be discharged before noon the next day. In their study, identifying such patients in advance improved the overall early-discharge rate the following day.
Our findings should be interpreted in light of several limitations. Our survey only considers the perspectives of hospitalist and general internal medicine physician leaders at academic medical centers that are part of the Vizient Inc. collaborative. They do not represent all academic or community-based medical centers. Although the perceived effectiveness of some initiatives was high, we did not collect empirical data to support these claims or to determine which initiative had the greatest relative impact on discharge timeliness. Lastly, we did not obtain resident, nursing, or case manager perspectives on discharge practices. Given their roles as frontline providers, we may have missed these alternative perspectives.
Our study shows there is a strong interest in increasing early discharges in an effort to improve hospital throughput and patient flow.
Acknowledgments
The authors thank all participants who completed the survey and Danielle Carrier at Vizient Inc. (formally University HealthSystem Consortium) for her assistance in obtaining data.
Disclosures
Hemali Patel, Margaret Fang, Michelle Mourad, Adrienne Green, Ryan Murphy, and James Harrison report no conflicts of interest. At the time the research was conducted, Robert Wachter reported that he is a member of the Lucian Leape Institute at the National Patient Safety Foundation (no compensation except travel expenses); recently chaired an advisory board to England’s National Health Service (NHS) reviewing the NHS’s digital health strategy (no compensation except travel expenses); has a contract with UCSF from the Agency for Healthcare Research and Quality to edit a patient-safety website; receives compensation from John Wiley & Sons for writing a blog; receives royalties from Lippincott Williams & Wilkins and McGraw-Hill Education for writing and/or editing several books; receives stock options for serving on the board of Acuity Medical Management Systems; receives a yearly stipend for serving on the board of The Doctors Company; serves on the scientific advisory boards for amino.com, PatientSafe Solutions Inc., Twine, and EarlySense (for which he receives stock options); has a small royalty stake in CareWeb, a hospital communication tool developed at UCSF; and holds the Marc and Lynne Benioff Endowed Chair in Hospital Medicine and the Holly Smith Distinguished Professorship in Science and Medicine at UCSF.
1. Khanna S, Boyle J, Good N, Lind J. Impact of admission and discharge peak times on hospital overcrowding. Stud Health Technol Inform. 2011;168:82-88. PubMed
2. White BA, Biddinger PD, Chang Y, Grabowski B, Carignan S, Brown DFM. Boarding Inpatients in the Emergency Department Increases Discharged Patient Length of Stay. J Emerg Med. 2013;44(1):230-235. doi:10.1016/j.jemermed.2012.05.007. PubMed
3. Derlet RW, Richards JR. Overcrowding in the nation’s emergency departments: complex causes and disturbing effects. Ann Emerg Med. 2000;35(1):63-68. PubMed
4. da Silva SA, Valácio RA, Botelho FC, Amaral CFS. Reasons for discharge delays in teaching hospitals. Rev Saúde Pública. 2014;48(2):314-321. doi:10.1590/S0034-8910.2014048004971. PubMed
5. Greysen SR, Schiliro D, Horwitz LI, Curry L, Bradley EH. “Out of Sight, Out of Mind”: Housestaff Perceptions of Quality-Limiting Factors in Discharge Care at Teaching Hospitals. J Hosp Med Off Publ Soc Hosp Med. 2012;7(5):376-381. doi:10.1002/jhm.1928. PubMed
6. Goldman J, Reeves S, Wu R, Silver I, MacMillan K, Kitto S. Medical Residents and Interprofessional Interactions in Discharge: An Ethnographic Exploration of Factors That Affect Negotiation. J Gen Intern Med. 2015;30(10):1454-1460. doi:10.1007/s11606-015-3306-6. PubMed
7. Okoniewska B, Santana MJ, Groshaus H, et al. Barriers to discharge in an acute care medical teaching unit: a qualitative analysis of health providers’ perceptions. J Multidiscip Healthc. 2015;8:83-89. doi:10.2147/JMDH.S72633. PubMed
8. Carey MR, Sheth H, Scott Braithwaite R. A Prospective Study of Reasons for Prolonged Hospitalizations on a General Medicine Teaching Service. J Gen Intern Med. 2005;20(2):108-115. doi:10.1111/j.1525-1497.2005.40269.x. PubMed
9. Kim CS, Hart AL, Paretti RF, et al. Excess Hospitalization Days in an Academic Medical Center: Perceptions of Hospitalists and Discharge Planners. Am J Manag Care. 2011;17(2):e34-e42. http://www.ajmc.com/journals/issue/2011/2011-2-vol17-n2/AJMC_11feb_Kim_WebX_e34to42/. Accessed on October 26, 2016.
10. Gershengorn HB, Kocher R, Factor P. Management Strategies to Effect Change in Intensive Care Units: Lessons from the World of Business. Part II. Quality-Improvement Strategies. Ann Am Thorac Soc. 2014;11(3):444-453. doi:10.1513/AnnalsATS.201311-392AS. PubMed
11. Wertheimer B, Jacobs REA, Bailey M, et al. Discharge before noon: An achievable hospital goal. J Hosp Med. 2014;9(4):210-214. doi:10.1002/jhm.2154. PubMed
12. Manning DM, Tammel KJ, Blegen RN, et al. In-room display of day and time patient is anticipated to leave hospital: a “discharge appointment.” J Hosp Med. 2007;2(1):13-16. doi:10.1002/jhm.146. PubMed
13. Networks for academic medical centers. https://www.vizientinc.com/Our-networks/Networks-for-academic-medical-centers. Accessed on July 13, 2017.
14. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi:10.1016/j.jbi.2008.08.010. PubMed
15. Selker HP, Beshansky JR, Pauker SG, Kassirer JP. The epidemiology of delays in a teaching hospital. The development and use of a tool that detects unnecessary hospital days. Med Care. 1989;27(2):112-129. PubMed
The discharge process is a critical bottleneck for efficient patient flow through the hospital. Delayed discharges translate into delays in admissions and other patient transitions, often leading to excess costs, patient dissatisfaction, and even patient harm.1-3 The emergency department is particularly impacted by these delays; bottlenecks there lead to overcrowding, increased overall hospital length of stay, and increased risks for bad outcomes during hospitalization.2
Academic medical centers in particular may struggle with delayed discharges. In a typical teaching hospital, a team composed of an attending physician and housestaff share responsibility for determining the discharge plan. Additionally, clinical teaching activities may affect the process and quality of discharge.4-6
The prevalence and causes of delayed discharges vary greatly.7-9 To improve efficiency around discharge, many hospitals have launched initiatives designed to discharge patients earlier in the day, including goal setting (“discharge by noon”), scheduling discharge appointments, and using quality-improvement methods, such as Lean Methodology (LEAN), to remove inefficiencies within discharge processes.10-12 However, there are few data on the prevalence and effectiveness of different strategies.
The aim of this study was to survey academic hospitalist and general internal medicine physician leaders to elicit their perspectives on the factors contributing to discharge timing and the relative importance and effectiveness of early-discharge initiatives.
METHODS
Study Design, Participants, and Oversight
We obtained a list of 115 university-affiliated hospitals associated with a residency program and, in most cases, a medical school from Vizient Inc. (formerly University HealthSystem Consortium), an alliance of academic medical centers and affiliated hospitals. Each member institution submits clinical data to allow for the benchmarking of outcomes to drive transparency and quality improvement.13 More than 95% of the nation’s academic medical centers and affiliated hospitals participate in this collaborative. Vizient works with members but does not set nor promote quality metrics, such as discharge timeliness. E-mail addresses for hospital medicine physician leaders (eg, division chief) of major academic medical centers were obtained from each institution via publicly available data (eg, the institution’s website). When an institution did not have a hospital medicine section, we identified the division chief of general internal medicine. The University of California, San Francisco Institutional Review Board approved this study.
Survey Development and Domains
We developed a 30-item survey to evaluate 5 main domains of interest: current discharge practices, degree of prioritization of early discharge on the inpatient service, barriers to timely discharge, prevalence and perceived effectiveness of implemented early-discharge initiatives, and barriers to implementation of early-discharge initiatives.
Respondents were first asked to identify their institutions’ goals for discharge time. They were then asked to compare the priority of early-discharge initiatives to other departmental quality-improvement initiatives, such as reducing 30-day readmissions, improving interpreter use, and improving patient satisfaction. Next, respondents were asked to estimate the degree to which clinical or patient factors contributed to delays in discharge. Respondents were then asked whether specific early-discharge initiatives, such as changes to rounding practices or communication interventions, were implemented at their institutions and, if so, the perceived effectiveness of these initiatives at meeting discharge targets. We piloted the questions locally with physicians and researchers prior to finalizing the survey.
Data Collection
We sent surveys via an online platform (Research Electronic Data Capture).14 Nonresponders were sent 2 e-mail reminders and then a follow-up telephone call asking them to complete the survey. Only 1 survey per academic medical center was collected. Any respondent who completed the survey within 2 weeks of receiving it was entered to win a Kindle Fire.
Data Analysis
We summarized survey responses using descriptive statistics. Analysis was completed in IBM SPSS version 22 (Armonk, NY).
RESULTS
Survey Respondent and Institutional Characteristics
Of the 115 institutions surveyed, we received 61 responses (response rate of 53%), with 39 (64%) respondents from divisions of hospital medicine and 22 (36%) from divisions of general internal medicine. A majority (n = 53; 87%) stated their medicine services have a combination of teaching (with residents) and nonteaching (without residents) teams. Thirty-nine (64%) reported having daily multidisciplinary rounds.
Early Discharge as a Priority
Forty-seven (77%) institutional representatives strongly agreed or agreed that early discharge was a priority, with discharge by noon being the most common target time (n = 23; 38%). Thirty (50%) respondents rated early discharge as more important than improving interpreter use for non-English-speaking patients and equally important as reducing 30-day readmissions (n = 29; 48%) and improving patient satisfaction (n = 27; 44%).
Factors Delaying Discharge
The most common factors perceived as delaying discharge were considered external to the hospital, such as postacute care bed availability or scheduled (eg, ambulance) transport delays (n = 48; 79%), followed by patient factors such as patient transport issues (n = 44; 72%). Less commonly reported were workflow issues, such as competing primary team priorities or case manager bandwidth (n = 38; 62%; Table 1).
Initiatives to Improve Discharge
The most commonly implemented initiatives perceived as effective at improving discharge times were the preemptive identification of early discharges to plan discharge paperwork (n = 34; 56%), communication with patients about anticipated discharge time on the day prior to discharge (n = 29; 48%), and the implementation of additional rounds between physician teams and case managers specifically around discharge planning (n = 28; 46%). Initiatives not commonly implemented included regular audit of and feedback on discharge times to providers and teams (n = 21; 34%), the use of a discharge readiness checklist (n = 26; 43%), incentives such as bonuses or penalties (n = 37; 61%), the use of a whiteboard to indicate discharge times (n = 23; 38%), and dedicated quality-improvement approaches such as LEAN (n = 37; 61%; Table 2).
DISCUSSION
Our study suggests early discharge for medicine patients is a priority among academic institutions. Hospitalist and general internal medicine physician leaders in our study generally attributed delayed discharges to external factors, particularly unavailability of postacute care facilities and transportation delays. Having issues with finding postacute care placements is consistent with previous findings by Selker et al.15 and Carey et al.8 This is despite the 20-year difference between Selker et al.’s study and the current study, reflecting a continued opportunity for improvement, including stronger partnerships with local and regional postacute care facilities to expedite care transition and stronger discharge-planning efforts early in the admission process. Efforts in postacute care placement may be particularly important for Medicaid-insured and uninsured patients.
Our responders, hospitalist and internal medicine physician leaders, did not perceive the additional responsibilities of teaching and supervising trainees to be factors that significantly delayed patient discharge. This is in contrast to previous studies, which attributed delays in discharge to prolonged clinical decision-making related to teaching and supervision.4-6,8 This discrepancy may be due to the fact that we only surveyed single physician leaders at each institution and not residents. Our finding warrants further investigation to understand the degree to which resident skills may impact discharge planning and processes.
Institutions represented in our study have attempted a variety of initiatives promoting earlier discharge, with varying levels of perceived success. Initiatives perceived to be the most effective by hospital leaders centered on 2 main areas: (1) changing individual provider practice and (2) anticipatory discharge preparation. Interestingly, this is in discordance with the main factors labeled as causing delays in discharges, such as obtaining postacute care beds, busy case managers, and competing demands on primary teams. We hypothesize this may be because such changes require organization- or system-level changes and are perceived as more arduous than changes at the individual level. In addition, changes to individual provider behavior may be more cost- and time-effective than more systemic initiatives.
Our findings are consistent with the work published by Wertheimer and colleagues,11 who show that additional afternoon interdisciplinary rounds can help identify patients who may be discharged before noon the next day. In their study, identifying such patients in advance improved the overall early-discharge rate the following day.
Our findings should be interpreted in light of several limitations. Our survey only considers the perspectives of hospitalist and general internal medicine physician leaders at academic medical centers that are part of the Vizient Inc. collaborative. They do not represent all academic or community-based medical centers. Although the perceived effectiveness of some initiatives was high, we did not collect empirical data to support these claims or to determine which initiative had the greatest relative impact on discharge timeliness. Lastly, we did not obtain resident, nursing, or case manager perspectives on discharge practices. Given their roles as frontline providers, we may have missed these alternative perspectives.
Our study shows there is a strong interest in increasing early discharges in an effort to improve hospital throughput and patient flow.
Acknowledgments
The authors thank all participants who completed the survey and Danielle Carrier at Vizient Inc. (formally University HealthSystem Consortium) for her assistance in obtaining data.
Disclosures
Hemali Patel, Margaret Fang, Michelle Mourad, Adrienne Green, Ryan Murphy, and James Harrison report no conflicts of interest. At the time the research was conducted, Robert Wachter reported that he is a member of the Lucian Leape Institute at the National Patient Safety Foundation (no compensation except travel expenses); recently chaired an advisory board to England’s National Health Service (NHS) reviewing the NHS’s digital health strategy (no compensation except travel expenses); has a contract with UCSF from the Agency for Healthcare Research and Quality to edit a patient-safety website; receives compensation from John Wiley & Sons for writing a blog; receives royalties from Lippincott Williams & Wilkins and McGraw-Hill Education for writing and/or editing several books; receives stock options for serving on the board of Acuity Medical Management Systems; receives a yearly stipend for serving on the board of The Doctors Company; serves on the scientific advisory boards for amino.com, PatientSafe Solutions Inc., Twine, and EarlySense (for which he receives stock options); has a small royalty stake in CareWeb, a hospital communication tool developed at UCSF; and holds the Marc and Lynne Benioff Endowed Chair in Hospital Medicine and the Holly Smith Distinguished Professorship in Science and Medicine at UCSF.
The discharge process is a critical bottleneck for efficient patient flow through the hospital. Delayed discharges translate into delays in admissions and other patient transitions, often leading to excess costs, patient dissatisfaction, and even patient harm.1-3 The emergency department is particularly impacted by these delays; bottlenecks there lead to overcrowding, increased overall hospital length of stay, and increased risks for bad outcomes during hospitalization.2
Academic medical centers in particular may struggle with delayed discharges. In a typical teaching hospital, a team composed of an attending physician and housestaff share responsibility for determining the discharge plan. Additionally, clinical teaching activities may affect the process and quality of discharge.4-6
The prevalence and causes of delayed discharges vary greatly.7-9 To improve efficiency around discharge, many hospitals have launched initiatives designed to discharge patients earlier in the day, including goal setting (“discharge by noon”), scheduling discharge appointments, and using quality-improvement methods, such as Lean Methodology (LEAN), to remove inefficiencies within discharge processes.10-12 However, there are few data on the prevalence and effectiveness of different strategies.
The aim of this study was to survey academic hospitalist and general internal medicine physician leaders to elicit their perspectives on the factors contributing to discharge timing and the relative importance and effectiveness of early-discharge initiatives.
METHODS
Study Design, Participants, and Oversight
We obtained a list of 115 university-affiliated hospitals associated with a residency program and, in most cases, a medical school from Vizient Inc. (formerly University HealthSystem Consortium), an alliance of academic medical centers and affiliated hospitals. Each member institution submits clinical data to allow for the benchmarking of outcomes to drive transparency and quality improvement.13 More than 95% of the nation’s academic medical centers and affiliated hospitals participate in this collaborative. Vizient works with members but does not set nor promote quality metrics, such as discharge timeliness. E-mail addresses for hospital medicine physician leaders (eg, division chief) of major academic medical centers were obtained from each institution via publicly available data (eg, the institution’s website). When an institution did not have a hospital medicine section, we identified the division chief of general internal medicine. The University of California, San Francisco Institutional Review Board approved this study.
Survey Development and Domains
We developed a 30-item survey to evaluate 5 main domains of interest: current discharge practices, degree of prioritization of early discharge on the inpatient service, barriers to timely discharge, prevalence and perceived effectiveness of implemented early-discharge initiatives, and barriers to implementation of early-discharge initiatives.
Respondents were first asked to identify their institutions’ goals for discharge time. They were then asked to compare the priority of early-discharge initiatives to other departmental quality-improvement initiatives, such as reducing 30-day readmissions, improving interpreter use, and improving patient satisfaction. Next, respondents were asked to estimate the degree to which clinical or patient factors contributed to delays in discharge. Respondents were then asked whether specific early-discharge initiatives, such as changes to rounding practices or communication interventions, were implemented at their institutions and, if so, the perceived effectiveness of these initiatives at meeting discharge targets. We piloted the questions locally with physicians and researchers prior to finalizing the survey.
Data Collection
We sent surveys via an online platform (Research Electronic Data Capture).14 Nonresponders were sent 2 e-mail reminders and then a follow-up telephone call asking them to complete the survey. Only 1 survey per academic medical center was collected. Any respondent who completed the survey within 2 weeks of receiving it was entered to win a Kindle Fire.
Data Analysis
We summarized survey responses using descriptive statistics. Analysis was completed in IBM SPSS version 22 (Armonk, NY).
RESULTS
Survey Respondent and Institutional Characteristics
Of the 115 institutions surveyed, we received 61 responses (response rate of 53%), with 39 (64%) respondents from divisions of hospital medicine and 22 (36%) from divisions of general internal medicine. A majority (n = 53; 87%) stated their medicine services have a combination of teaching (with residents) and nonteaching (without residents) teams. Thirty-nine (64%) reported having daily multidisciplinary rounds.
Early Discharge as a Priority
Forty-seven (77%) institutional representatives strongly agreed or agreed that early discharge was a priority, with discharge by noon being the most common target time (n = 23; 38%). Thirty (50%) respondents rated early discharge as more important than improving interpreter use for non-English-speaking patients and equally important as reducing 30-day readmissions (n = 29; 48%) and improving patient satisfaction (n = 27; 44%).
Factors Delaying Discharge
The most common factors perceived as delaying discharge were considered external to the hospital, such as postacute care bed availability or scheduled (eg, ambulance) transport delays (n = 48; 79%), followed by patient factors such as patient transport issues (n = 44; 72%). Less commonly reported were workflow issues, such as competing primary team priorities or case manager bandwidth (n = 38; 62%; Table 1).
Initiatives to Improve Discharge
The most commonly implemented initiatives perceived as effective at improving discharge times were the preemptive identification of early discharges to plan discharge paperwork (n = 34; 56%), communication with patients about anticipated discharge time on the day prior to discharge (n = 29; 48%), and the implementation of additional rounds between physician teams and case managers specifically around discharge planning (n = 28; 46%). Initiatives not commonly implemented included regular audit of and feedback on discharge times to providers and teams (n = 21; 34%), the use of a discharge readiness checklist (n = 26; 43%), incentives such as bonuses or penalties (n = 37; 61%), the use of a whiteboard to indicate discharge times (n = 23; 38%), and dedicated quality-improvement approaches such as LEAN (n = 37; 61%; Table 2).
DISCUSSION
Our study suggests early discharge for medicine patients is a priority among academic institutions. Hospitalist and general internal medicine physician leaders in our study generally attributed delayed discharges to external factors, particularly unavailability of postacute care facilities and transportation delays. Having issues with finding postacute care placements is consistent with previous findings by Selker et al.15 and Carey et al.8 This is despite the 20-year difference between Selker et al.’s study and the current study, reflecting a continued opportunity for improvement, including stronger partnerships with local and regional postacute care facilities to expedite care transition and stronger discharge-planning efforts early in the admission process. Efforts in postacute care placement may be particularly important for Medicaid-insured and uninsured patients.
Our responders, hospitalist and internal medicine physician leaders, did not perceive the additional responsibilities of teaching and supervising trainees to be factors that significantly delayed patient discharge. This is in contrast to previous studies, which attributed delays in discharge to prolonged clinical decision-making related to teaching and supervision.4-6,8 This discrepancy may be due to the fact that we only surveyed single physician leaders at each institution and not residents. Our finding warrants further investigation to understand the degree to which resident skills may impact discharge planning and processes.
Institutions represented in our study have attempted a variety of initiatives promoting earlier discharge, with varying levels of perceived success. Initiatives perceived to be the most effective by hospital leaders centered on 2 main areas: (1) changing individual provider practice and (2) anticipatory discharge preparation. Interestingly, this is in discordance with the main factors labeled as causing delays in discharges, such as obtaining postacute care beds, busy case managers, and competing demands on primary teams. We hypothesize this may be because such changes require organization- or system-level changes and are perceived as more arduous than changes at the individual level. In addition, changes to individual provider behavior may be more cost- and time-effective than more systemic initiatives.
Our findings are consistent with the work published by Wertheimer and colleagues,11 who show that additional afternoon interdisciplinary rounds can help identify patients who may be discharged before noon the next day. In their study, identifying such patients in advance improved the overall early-discharge rate the following day.
Our findings should be interpreted in light of several limitations. Our survey only considers the perspectives of hospitalist and general internal medicine physician leaders at academic medical centers that are part of the Vizient Inc. collaborative. They do not represent all academic or community-based medical centers. Although the perceived effectiveness of some initiatives was high, we did not collect empirical data to support these claims or to determine which initiative had the greatest relative impact on discharge timeliness. Lastly, we did not obtain resident, nursing, or case manager perspectives on discharge practices. Given their roles as frontline providers, we may have missed these alternative perspectives.
Our study shows there is a strong interest in increasing early discharges in an effort to improve hospital throughput and patient flow.
Acknowledgments
The authors thank all participants who completed the survey and Danielle Carrier at Vizient Inc. (formally University HealthSystem Consortium) for her assistance in obtaining data.
Disclosures
Hemali Patel, Margaret Fang, Michelle Mourad, Adrienne Green, Ryan Murphy, and James Harrison report no conflicts of interest. At the time the research was conducted, Robert Wachter reported that he is a member of the Lucian Leape Institute at the National Patient Safety Foundation (no compensation except travel expenses); recently chaired an advisory board to England’s National Health Service (NHS) reviewing the NHS’s digital health strategy (no compensation except travel expenses); has a contract with UCSF from the Agency for Healthcare Research and Quality to edit a patient-safety website; receives compensation from John Wiley & Sons for writing a blog; receives royalties from Lippincott Williams & Wilkins and McGraw-Hill Education for writing and/or editing several books; receives stock options for serving on the board of Acuity Medical Management Systems; receives a yearly stipend for serving on the board of The Doctors Company; serves on the scientific advisory boards for amino.com, PatientSafe Solutions Inc., Twine, and EarlySense (for which he receives stock options); has a small royalty stake in CareWeb, a hospital communication tool developed at UCSF; and holds the Marc and Lynne Benioff Endowed Chair in Hospital Medicine and the Holly Smith Distinguished Professorship in Science and Medicine at UCSF.
1. Khanna S, Boyle J, Good N, Lind J. Impact of admission and discharge peak times on hospital overcrowding. Stud Health Technol Inform. 2011;168:82-88. PubMed
2. White BA, Biddinger PD, Chang Y, Grabowski B, Carignan S, Brown DFM. Boarding Inpatients in the Emergency Department Increases Discharged Patient Length of Stay. J Emerg Med. 2013;44(1):230-235. doi:10.1016/j.jemermed.2012.05.007. PubMed
3. Derlet RW, Richards JR. Overcrowding in the nation’s emergency departments: complex causes and disturbing effects. Ann Emerg Med. 2000;35(1):63-68. PubMed
4. da Silva SA, Valácio RA, Botelho FC, Amaral CFS. Reasons for discharge delays in teaching hospitals. Rev Saúde Pública. 2014;48(2):314-321. doi:10.1590/S0034-8910.2014048004971. PubMed
5. Greysen SR, Schiliro D, Horwitz LI, Curry L, Bradley EH. “Out of Sight, Out of Mind”: Housestaff Perceptions of Quality-Limiting Factors in Discharge Care at Teaching Hospitals. J Hosp Med Off Publ Soc Hosp Med. 2012;7(5):376-381. doi:10.1002/jhm.1928. PubMed
6. Goldman J, Reeves S, Wu R, Silver I, MacMillan K, Kitto S. Medical Residents and Interprofessional Interactions in Discharge: An Ethnographic Exploration of Factors That Affect Negotiation. J Gen Intern Med. 2015;30(10):1454-1460. doi:10.1007/s11606-015-3306-6. PubMed
7. Okoniewska B, Santana MJ, Groshaus H, et al. Barriers to discharge in an acute care medical teaching unit: a qualitative analysis of health providers’ perceptions. J Multidiscip Healthc. 2015;8:83-89. doi:10.2147/JMDH.S72633. PubMed
8. Carey MR, Sheth H, Scott Braithwaite R. A Prospective Study of Reasons for Prolonged Hospitalizations on a General Medicine Teaching Service. J Gen Intern Med. 2005;20(2):108-115. doi:10.1111/j.1525-1497.2005.40269.x. PubMed
9. Kim CS, Hart AL, Paretti RF, et al. Excess Hospitalization Days in an Academic Medical Center: Perceptions of Hospitalists and Discharge Planners. Am J Manag Care. 2011;17(2):e34-e42. http://www.ajmc.com/journals/issue/2011/2011-2-vol17-n2/AJMC_11feb_Kim_WebX_e34to42/. Accessed on October 26, 2016.
10. Gershengorn HB, Kocher R, Factor P. Management Strategies to Effect Change in Intensive Care Units: Lessons from the World of Business. Part II. Quality-Improvement Strategies. Ann Am Thorac Soc. 2014;11(3):444-453. doi:10.1513/AnnalsATS.201311-392AS. PubMed
11. Wertheimer B, Jacobs REA, Bailey M, et al. Discharge before noon: An achievable hospital goal. J Hosp Med. 2014;9(4):210-214. doi:10.1002/jhm.2154. PubMed
12. Manning DM, Tammel KJ, Blegen RN, et al. In-room display of day and time patient is anticipated to leave hospital: a “discharge appointment.” J Hosp Med. 2007;2(1):13-16. doi:10.1002/jhm.146. PubMed
13. Networks for academic medical centers. https://www.vizientinc.com/Our-networks/Networks-for-academic-medical-centers. Accessed on July 13, 2017.
14. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi:10.1016/j.jbi.2008.08.010. PubMed
15. Selker HP, Beshansky JR, Pauker SG, Kassirer JP. The epidemiology of delays in a teaching hospital. The development and use of a tool that detects unnecessary hospital days. Med Care. 1989;27(2):112-129. PubMed
1. Khanna S, Boyle J, Good N, Lind J. Impact of admission and discharge peak times on hospital overcrowding. Stud Health Technol Inform. 2011;168:82-88. PubMed
2. White BA, Biddinger PD, Chang Y, Grabowski B, Carignan S, Brown DFM. Boarding Inpatients in the Emergency Department Increases Discharged Patient Length of Stay. J Emerg Med. 2013;44(1):230-235. doi:10.1016/j.jemermed.2012.05.007. PubMed
3. Derlet RW, Richards JR. Overcrowding in the nation’s emergency departments: complex causes and disturbing effects. Ann Emerg Med. 2000;35(1):63-68. PubMed
4. da Silva SA, Valácio RA, Botelho FC, Amaral CFS. Reasons for discharge delays in teaching hospitals. Rev Saúde Pública. 2014;48(2):314-321. doi:10.1590/S0034-8910.2014048004971. PubMed
5. Greysen SR, Schiliro D, Horwitz LI, Curry L, Bradley EH. “Out of Sight, Out of Mind”: Housestaff Perceptions of Quality-Limiting Factors in Discharge Care at Teaching Hospitals. J Hosp Med Off Publ Soc Hosp Med. 2012;7(5):376-381. doi:10.1002/jhm.1928. PubMed
6. Goldman J, Reeves S, Wu R, Silver I, MacMillan K, Kitto S. Medical Residents and Interprofessional Interactions in Discharge: An Ethnographic Exploration of Factors That Affect Negotiation. J Gen Intern Med. 2015;30(10):1454-1460. doi:10.1007/s11606-015-3306-6. PubMed
7. Okoniewska B, Santana MJ, Groshaus H, et al. Barriers to discharge in an acute care medical teaching unit: a qualitative analysis of health providers’ perceptions. J Multidiscip Healthc. 2015;8:83-89. doi:10.2147/JMDH.S72633. PubMed
8. Carey MR, Sheth H, Scott Braithwaite R. A Prospective Study of Reasons for Prolonged Hospitalizations on a General Medicine Teaching Service. J Gen Intern Med. 2005;20(2):108-115. doi:10.1111/j.1525-1497.2005.40269.x. PubMed
9. Kim CS, Hart AL, Paretti RF, et al. Excess Hospitalization Days in an Academic Medical Center: Perceptions of Hospitalists and Discharge Planners. Am J Manag Care. 2011;17(2):e34-e42. http://www.ajmc.com/journals/issue/2011/2011-2-vol17-n2/AJMC_11feb_Kim_WebX_e34to42/. Accessed on October 26, 2016.
10. Gershengorn HB, Kocher R, Factor P. Management Strategies to Effect Change in Intensive Care Units: Lessons from the World of Business. Part II. Quality-Improvement Strategies. Ann Am Thorac Soc. 2014;11(3):444-453. doi:10.1513/AnnalsATS.201311-392AS. PubMed
11. Wertheimer B, Jacobs REA, Bailey M, et al. Discharge before noon: An achievable hospital goal. J Hosp Med. 2014;9(4):210-214. doi:10.1002/jhm.2154. PubMed
12. Manning DM, Tammel KJ, Blegen RN, et al. In-room display of day and time patient is anticipated to leave hospital: a “discharge appointment.” J Hosp Med. 2007;2(1):13-16. doi:10.1002/jhm.146. PubMed
13. Networks for academic medical centers. https://www.vizientinc.com/Our-networks/Networks-for-academic-medical-centers. Accessed on July 13, 2017.
14. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi:10.1016/j.jbi.2008.08.010. PubMed
15. Selker HP, Beshansky JR, Pauker SG, Kassirer JP. The epidemiology of delays in a teaching hospital. The development and use of a tool that detects unnecessary hospital days. Med Care. 1989;27(2):112-129. PubMed
© 2017 Society of Hospital Medicine
Safe and effective bedside thoracentesis: A review of the evidence for practicing clinicians
Pleural effusion can occur in myriad conditions including infection, heart failure, liver disease, and cancer.1 Consequently, physicians from many disciplines routinely encounter both inpatients and outpatients with this diagnosis. Often, evaluation and treatment require thoracentesis to obtain fluid for analysis or symptom relief.
Although historically performed at the bedside without imaging guidance or intraprocedural monitoring, thoracentesis performed in this fashion carries considerable risk of complications. In fact, it has 1 of the highest rates of iatrogenic pneumothorax among bedside procedures.2 However, recent advances in practice and adoption of newer technologies have helped to mitigate risks associated with this procedure. These advances are relevant because approximately 50% of thoracenteses are still performed at the bedside.3 In this review, we aim to identify the most recent key practices that enhance the safety and the effectiveness of thoracentesis for practicing clinicians.
METHODS
Information Sources and Search Strategy
With the assistance of a research librarian, we performed a systematic search of PubMed-indexed articles from January 1, 2000 to September 30, 2015. Articles were identified using search terms such as thoracentesis, pleural effusion, safety, medical error, adverse event, and ultrasound in combination with Boolean operators. Of note, as thoracentesis is indexed as a subgroup of paracentesis in PubMed, this term was also included to increase the sensitivity of the search. The full search strategy is available in the Appendix. Any references cited in this review outside of the date range of our search are provided only to give relevant background information or establish the origin of commonly performed practices.
Study Eligibility and Selection Criteria
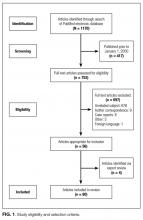
Studies were included if they reported clinical aspects related to thoracentesis. We defined clinical aspects as those strategies that focused on operator training, procedural techniques, technology, management, or prevention of complications. Non-English language articles, animal studies, case reports, conference proceedings, and abstracts were excluded. As our intention was to focus on the contemporary advances related to thoracentesis performance, (eg, ultrasound [US]), our search was limited to studies published after the year 2000. Two authors, Drs. Schildhouse and Lai independently screened studies to determine inclusion, excluding studies with weak methodology, very small sample sizes, and those only tangentially related to our aim. Disagreements regarding study inclusion were resolved by consensus. Drs. Lai, Barsuk, and Mourad identified additional studies by hand review of reference lists and content experts (Figure 1).
Conceptual Framework
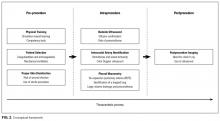
All selected articles were categorized by temporal relationship to thoracentesis as pre-, intra-, or postprocedure. Pre-procedural topics were those outcomes that had been identified and addressed before attempting thoracentesis, such as physician training or perceived risks of harm. Intraprocedural considerations included aspects such as use of bedside US, pleural manometry, and large-volume drainage. Finally, postprocedural factors were those related to evaluation after thoracentesis, such as follow-up imaging. This conceptual framework is outlined in Figure 2.
RESULTS
The PubMed search returned a total of 1170 manuscripts, of which 56 articles met inclusion criteria. Four additional articles were identified by experts and included in the study.4-7 Therefore, 60 articles were identified and included in this review. Study designs included cohort studies, case control studies, systematic reviews, meta-analyses, narrative reviews, consensus guidelines, and randomized controlled trials. A summary of all included articles by topic can be found in the Table.
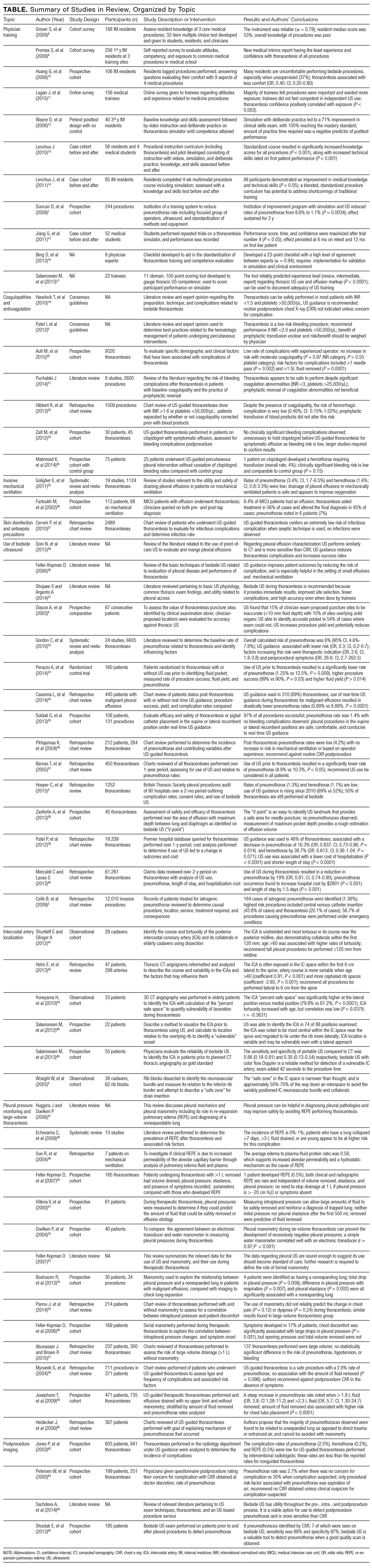
PRE-PROCEDURAL CONSIDERATIONS
Physician Training
Studies indicate that graduate medical education may not adequately prepare clinicians to perform thoracentesis.8 In fact, residents have the least exposure and confidence in performing thoracentesis when compared to other bedside procedures.9,10 In 1 survey, 69% of medical trainees desired more exposure to procedures, and 98% felt that procedural skills were important to master.11 Not surprisingly, then, graduating internal medicine residents perform poorly when assessed on a thoracentesis simulator.12
Supplemental training outside of residency is useful to develop and maintain skills for thoracentesis, such as simulation with direct observation in a zero-risk environment. In 1 study, “simulation-based mastery learning” combined an educational video presentation with repeated, deliberate practice on a simulator until procedural competence was acquired, over two 2-hour sessions. In this study, 40 third-year medicine residents demonstrated a 71% improvement in clinical skills performance after course completion, with 93% achieving a passing score. The remaining 7% also achieved passing scores with extra practice time.12 Others have built upon the concept of simulation-based training. For instance, 2 studies suggest that use of a simulation-based curriculum improved both thoracentesis knowledge and performance skills in a 3-hour session.13,14 Similarly, 1 prospective study reported that a half-day thoracentesis workshop using simulation and 1:1 direct observation successfully lowered pneumothorax rates from 8.6% to 1.8% in a group of practicing clinicians. Notably, additional interventions including use of bedside US, limiting operators to a focused group, and standardization of equipment were also a part of this quality improvement initiative.7 Although repetition is required to gain proficiency when using a simulator, performance and confidence appear to plateau with only 4 simulator trials. In medical students, improvements derived through simulator-based teaching were sustained when retested 6 months following training.15
An instrument to ensure competency is necessary, given variability in procedural experience among both new graduates and practicing physicians,. Our search did not identify any clinically validated tools that adequately assessed thoracentesis performance. However, some have been proposed16 and 1 validated in a simulation environment.12 Regarding the incorporation of US for effusion markup, 1 validated tool used an 11-domain assessment covering knowledge of US machine manipulation, recognition of images with common pleural effusion characteristics, and performance of thoracic US with puncture-site marking on a simulator. When used on 22 participants, scores with the tool could reliably differentiate between novice, intermediate, and advanced groups (P < 0.0001).17
Patient Selection
Coagulopathies and Anticoagulation. Historically, the accepted cutoff for performing thoracentesis is an international normalized ratio (INR) less than 1.5 and a platelet count greater than 50,000/µL. McVay et al.18 first showed in 1991 that use of these cutoffs was associated with low rates of periprocedural bleeding, leading to endorsement in the British Thoracic Society (BTS) Pleural Disease Guideline 2010.19 Other recommendations include the 2012 Society for Interventional Radiology guidelines that endorse correction of an INR greater than 2, or platelets less than 50,000/µL, based almost exclusively on expert opinion.5
However, data suggest that thoracentesis may be safely performed outside these parameters. For instance, a prospective study of approximately 9000 thoracenteses over 12 years found that patients with an INR of 1.5-2.9 or platelets of 20,000 - 49,000/µL experienced rates of bleeding complications similar to those with normal values.20 Similarly, a 2014 review21 found that the overall risk of hemorrhage during thoracentesis in the setting of moderate coagulopathy (defined as an INR of 1.5 - 3 or platelets of 25,000-50,000/µL), was not increased. In 1 retrospective study of more than 1000 procedures, no differences in hemorrhagic events were noted in patients with bleeding diatheses that received prophylactic fresh frozen plasma or platelets vs. those who did not.22 Of note, included studies used a variety of criteria to define a hemorrhagic complication, which included: an isolated 2 g/dL or more decrement in hemoglobin, presence of bloody fluid on repeat tap with associated hemoglobin decrement, rapid re-accumulation of fluid with a hemoglobin decrement, or transfusion of 2 units or more of whole blood.
Whether it is safe to perform thoracentesis on patients taking antiplatelet therapy is less well understood. Although data are limited, a few small-scale studies23,24 suggest that hemorrhagic complications following thoracentesis in patients receiving clopidogrel are comparable to the general population. We found no compelling data regarding the safety of thoracentesis in the setting of direct oral anticoagulants, heparin, low-molecular weight heparin, or intravenous direct thrombin inhibitors. Current practice is to generally avoid thoracentesis while these therapeutic anticoagulants are used.
Invasive mechanical ventilation. Pleural effusion is common in patients in the intensive care unit, including those requiring mechanical ventilation.25 Thoracentesis in this population is clinically important: fluid analysis in 1 study was shown to aid the diagnosis in 45% of cases and changes in treatment in 33%.26 However, clinicians may be reluctant to perform thoracentesis on patients who require mechanical ventilation, given the perception of a greater risk of pneumothorax from positive pressure ventilation.
Despite this concern, a 2011 meta-analysis including 19 studies and more than 1100 patients revealed rates of pneumothorax and hemothorax comparable to nonventilated patients.25 Furthermore, a 2015 prospective study that examined thoracentesis in 1377 mechanically ventilated patients revealed no difference in complication rates as well.20 Therefore, evidence suggests that performance of thoracentesis in mechanically ventilated patients is not contraindicated.
Skin Disinfection and Antisepsis Precautions
The 2010 BTS guidelines list empyema and wound infection as possible complications of thoracentesis.19 However, no data regarding incidence are provided. Additionally, an alcohol-based skin cleanser (such as 2% chlorhexidine gluconate/70% isopropyl alcohol), along with sterile gloves, field, and dressing are suggested as precautionary measures.19 In 1 single-center registry of 2489 thoracenteses performed using alcohol or iodine-based antiseptic and sterile drapes, no postprocedure infections were identified.27 Of note, we did not find other studies (including case reports) that reported either incidence or rate of infectious complications such as wound infection and empyema. In an era of modern skin antiseptics that have effectively reduced complications such as catheter-related bloodstream infection,28 the incidence of this event is thus likely to be low.
INTRAPROCEDURAL CONSIDERATIONS
Use of Bedside Ultrasound
Portable US has particular advantages for evaluation of pleural effusion vs other imaging modalities. Compared with computerized tomography (CT), bedside US offers similar performance but is less costly, avoids both radiation exposure and need for patient transportation, and provides results instantaneously.29,30 Compared to chest x-ray (CXR), US is more sensitive at detecting the presence, volume, and characteristics of pleural fluid30,31 and can be up to 100% sensitive for effusions greater than 100 mL.29 Furthermore, whereas CXR typically requires 200 mL of fluid to be present for detection of an effusion, US can reliably detect as little as 20 mL of fluid.29 When US was used to confirm thoracentesis puncture sites in a study involving 30 physicians of varying experience and 67 consecutive patients, 15% of sites found by clinical exam were inaccurate (less than 10 mm fluid present), 10% were at high risk for organ puncture, and a suitable fluid pocket was found 54% of times when exam could not.4
A 2010 meta-analysis of 24 studies and 6605 thoracenteses estimated the overall rate of pneumothorax at 6%; however, procedures performed with US guidance were associated with a 70% reduced risk of this event (odds ratio, 0.30; 95% confidence interval, 0.20 - 0.70).32 In a 2014 randomized control trial of 160 patients that compared thoracentesis with US guidance for site marking vs no US use, 10 pneumothoraces occurred in the control group vs 1 in the US group (12.5% vs 1.25%, P = 0.009).33 Similarly, another retrospective review of 445 consecutive patients with malignant effusions revealed a pneumothorax rate of 0.97% using US in real time during needle insertion compared to 8.89% for unguided thoracenteses (P < 0.0001).34 Several other studies using US guidance for either site markup or in real time reported similar pneumothorax rates, ranging from 1.1% - 4.8%.35-37 However, it is unclear if real-time US specifically provides an additive effect vs site marking alone, as no studies directly comparing the 2 methods were found.
Benefits of US also include a higher rate of procedural success, with 1 study demonstrating a 99% success rate when using US vs. 90% without (P = 0.030).33 A larger volume of fluid removed has been observed with US use as well, and methods have been described using fluid-pocket depth to guide puncture site localization and maximize drainage.38 Finally, US use for thoracentesis has been associated with lower costs and length of stay.39,40
Intercostal Artery Localization
Although rare (incidence, 0.18%-2%20,21,39), the occurrence of hemothorax following thoracentesis is potentially catastrophic. This serious complication is often caused by laceration of the intercostal artery (ICA) or 1 of its branches during needle insertion.41
While risk of injury is theoretically reduced by needle insertion superior to the rib, studies using cadaver dissection and 3D angiography show significant tortuosity of the ICA.6,41-43 The degree of tortuosity is increased within 6 cm of the midline, in more cephalad rib spaces, and in the elderly (older than 60 years).41-43 Furthermore, 1 cadaveric study also demonstrated the presence of arterial collaterals branching off the ICA at multiple intercostal spaces, ranging between 8 cm and 11 cm from the midline.41 This anatomic variability may explain why some have observed low complication and hemothorax rates with an extreme lateral approach.35 Bedside US with color flow Doppler imaging has been used to identify the ICA, with 88% sensitivity compared to CT imaging while adding little to exam time.44,45 Of note, a 37% drop in the rate of hemothorax was observed in 1 study with routine US guidance alone.39
Pleural Pressure Monitoring and Large-Volume Thoracentesis
While normal intrapleural pressures are approximately -5 to -10 cm H2O,46 the presence of a pleural effusion creates a complex interaction between fluid, compressed lung, and chest wall that can increase these pressures.47 During drainage of an effusion, pleural pressures may rapidly drop, provoking re-expansion pulmonary edema (REPE). While rare (0 -1%), clinically-diagnosed REPE is a serious complication that can lead to rapid respiratory failure and death.20,48 REPE is postulated to be caused by increased capillary permeability resulting from inflammation, driven by rapid re-inflation of the lung when exposed to highly negative intrapleural pressures.47,49
Measurement of intrapleural pressure using a water manometer during thoracentesis may minimize REPE by terminating fluid drainage when intrapleural pressure begins to drop rapidly.50,51 A cutoff of -20 cm H2O has been cited repeatedly as safe since being suggested by Light in 1980, but this is based on animal models.50,52 In 1 prospective study of 185 thoracenteses in which manometry was performed, 15% of patients had intrapleural pressure drop to less than -20 cm H2O (at which point the procedure was terminated) but suffered no REPE.50
Manometry is valuable in the identification of an unexpandable or trapped lung when pleural pressures drop rapidly with only minimal fluid volume removal.47,53 Other findings correlated with an unexpandable lung include a negative opening pressure47 and large fluctuations in pressure during the respiratory cycle.54
While development of symptoms (eg, chest pain, cough, or dyspnea) is often used as a surrogate, the correlation between intrapleural pressure and patient symptoms is inconsistent and not a reliable proxy.55 One study found that 22% of patients with chest pain during thoracentesis had intrapleural pressures lower than -20 cm H2O compared with 8.6% of asymptomatic patients,56 but it is unclear if the association is causal.
Thoracentesis is often performed for symptomatic relief and removal of large fluid volume. However, it remains common to halt fluid removal after 1.5 L, a threshold endorsed by BTS.19 While some investigators have suggested that removal of 2 L or more of pleural fluid does not compromise safety,57,58 a 4- to 5-fold rise in the risk of pneumothorax was noted in 2 studies.20,59 when more than 1.5 L of fluid was removed. The majority of these may be related to pneumothorax ex vacuo, a condition in which fluid is drained from the chest, but the lung is unable to expand and fill the space (eg, “trapped lung”), resulting in a persistent pneumothorax. This condition generally does not require treatment.60 When manometry is employed at 200-mL intervals with termination at an intrapleural pressure of less than 20 mm H2O, drainage of 3 L or more has been reported with low rates of pneumothorax and very low rates of REPE.50,51 However, whether this is cause and effect is unknown because REPE is rare, and more work is needed to determine the role of manometry for its prevention.
POSTPROCEDURAL CONSIDERATIONS
Postprocedure Imaging
Performing an upright CXR following thoracentesis is a practice that remains routinely done by many practitioners to monitor for complications. Such imaging was also endorsed by the American Thoracic Society guidelines.61 However, more recent data question the utility of this practice. Multiple studies have confirmed that post-thoracentesis CXR is unnecessary unless clinical suspicion for pneumothorax or REPE is present.36,58,62,63 The BTS guidelines also advocate this approach.19 Interestingly, a potentially more effective way to screen for postprocedure complications is through bedside US, which has been shown to be more sensitive than CXR in detecting pneumothorax.64 In 1 study of 185 patients, bedside US demonstrated a sensitivity of 88% and a specificity of 97% for diagnosing pneumothorax in patients with adequate quality scans, with positive and negative likelihood ratios of 55 and 0.17, respectively.65
DISCUSSION
Thoracentesis remains a core procedural skill for hospitalists, critical care physicians, and emergency physicians. It is the foundational component when investigating and treating pleural effusions. When the most current training, techniques, and technology are used, data suggest this procedure is safe to perform at the bedside. Our review highlights these strategies and evaluates which aspects might be most applicable to clinical practice.
Our findings have several implications for those who perform this procedure. First, appropriate training is central to procedural safety, and both simulation and direct observation by procedural experts have been shown by multiple investigators to improve knowledge and skill. This training should integrate the use of US in performing a focused thoracic exam.
Second, recommendations regarding coagulopathy and a “safe cutoff” of an INR less than 1.5 or platelets greater than 50,000/µL had limited evidentiary support. Rather, multiple studies suggest no difference in bleeding risk following thoracentesis with an INR as high as 3.0 and platelets greater than 25,000/µL. Furthermore, prophylactic transfusion with fresh frozen plasma or platelets before thoracentesis did not alter bleeding risk and exposes patients to transfusion complications. Thus, routine use of this practice can no longer be recommended. Third, further research is needed to understand the bleeding risk for patients on antiplatelet medications, heparin products, and also direct oral anticoagulants, given the growing popularity in their use and the potential consequences of even temporary cessation. Regarding patients on mechanical ventilation, thoracentesis demonstrated no difference in complication rates vs. the general population, and its performance in this population is encouraged when clinically indicated.
Intraprocedural considerations include the use of bedside US. Due to multiple benefits including effusion characterization, puncture site localization, and significantly lower rates of pneumothorax, the standard of care should be to perform thoracentesis with US guidance. Both use of US to mark an effusion immediately prior to puncture or in real time during needle insertion demonstrated benefit; however, it is unclear if 1 method is superior because no direct comparison studies were found. Further work is needed to investigate this potential.
Our review suggests that the location and course of the ICA is variable, especially near the midline, in the elderly, and in higher intercostal spaces, leaving it vulnerable to laceration. We recommend physicians only attempt thoracentesis at least 6 cm lateral to the midline due to ICA tortuosity and, ideally, 12 cm lateral, to avoid the presence of collaterals. Although only 2 small-scale studies were found pertaining to the use of US in identifying the ICA, we encourage physicians to consider learning how to screen for its presence as a part of their routine thoracic US exam in the area underlying the planned puncture site.
Manometry is beneficial because it can diagnose a nonexpandable lung and allows for pleural pressure monitoring.52,53 A simple U-shaped manometer can be constructed from intravenous tubing included in most thoracentesis kits, which adds little to overall procedure time. While low rates of REPE have been observed when terminating thoracentesis if pressures drop below -20 cm H2O or chest pain develops, neither measure appears to have reliable predictive value, limiting clinical utility. Further work is required to determine if a “safe pressure cutoff” exists. In general, we recommend the use of manometry when a nonexpandable (trapped) lung is suspected, because large drops in intrapleural pressure, a negative opening pressure, and respiratory variation can help confirm the diagnosis and avoid pneumothorax ex vacuo or unnecessary procedures in the future. As this condition appears to be more common in the setting of larger effusions, use of manometry when large-volume thoracenteses are planned is also reasonable.
Postprocedurally, routine imaging after thoracentesis is not recommended unless there is objective concern for complication. When indicated, bedside US is better positioned for this role compared with CXR, because it is more sensitive in detecting pneumothorax, provides instantaneous results, and avoids radiation exposure.
Our review has limitations. First, we searched only for articles between defined time periods, restricted our search to a single database, and excluded non-English articles. This has the potential to introduce selection bias, as nonprimary articles that fall within our time restrictions may cite older studies that are outside our search range. To minimize this effect, we performed a critical review of all included studies, especially nonprimary articles. Second, despite the focus of our search strategy to identify any articles related to patient safety and adverse events, we cannot guarantee that all relevant articles for any particular complication or risk factor were captured given the lack of more specific search terms. Third, although we performed a systematic search of the literature, we did not perform a formal systematic review or formally grade included studies. As the goal of our review was to categorize and operationalize clinical aspects, this approach was necessary, and we acknowledge that the quality of studies is variable. Lastly, we aimed to generate clinical recommendations for physicians performing thoracentesis at the bedside; others reviewing this literature may find or emphasize different aspects relevant to practice outside this setting.
In conclusion, evaluation and treatment of pleural effusions with bedside thoracentesis is an important skill for physicians of many disciplines. The evidence presented in this review will help inform the process and ensure patient safety. Physicians should consider incorporating these recommendations into their practice.
The authors thank Whitney Townsend, MLIS, health sciences informationist, for assistance with serial literature searches.
Disclosure
Nothing to report.
1. Kasper DL. Harrison's Principles of Internal Medicine. 19th ed. New York, NY: McGraw Hill Education; 2015.
2. Celik B, Sahin E, Nadir A, Kaptanoglu M. Iatrogenic pneumothorax: etiology, incidence and risk factors. Thorac Cardiovasc Surg. 2009;57(5):286-290. PubMed
3. Hooper CE, Welham SA, Maskell NA, Soc BT. Pleural procedures and patient safety: a national BTS audit of practice. Thorax. 2015;70(2):189-191. PubMed
4. Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest. 2003;123(2):436-441. PubMed
5. Patel IJ, Davidson JC, Nikolic B, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23(6):727-736. PubMed
6. Wraight WM, Tweedie DJ, Parkin IG. Neurovascular anatomy and variation in the fourth, fifth, and sixth intercostal spaces in the mid-axillary line: a cadaveric study in respect of chest drain insertion. Clin Anat. 2005;18(5):346-349. PubMed
7. Duncan DR, Morgenthaler TI, Ryu JH, Daniels CE. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest. 2009;135(5):1315-1320. PubMed
8. Grover S, Currier PF, Elinoff JM, Mouchantaf KJ, Katz JT, McMahon GT. Development of a test to evaluate residents' knowledge of medical procedures. J Hosp Med. 2009;4(7):430-432. PubMed
9. Promes SB, Chudgar SM, Grochowski CO, et al. Gaps in procedural experience and competency in medical school graduates. Acad Emerg Med. 2009;16 Suppl 2:S58-62. PubMed
10. Huang GC, Smith CC, Gordon CE, et al. Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures. Am J Med. 2006;119(1):71 e17-24. PubMed
11. Lagan J, Cutts L, Zaidi S, Benton I, Rylance J. Are we failing our trainees in providing opportunities to attain procedural confidence? Br J Hosp Med (Lond). 2015;76(2):105-108. PubMed
12. Wayne DB, Barsuk JH, O'Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54. PubMed
13. Lenchus JD. End of the "see one, do one, teach one" era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
14. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. PubMed
15. Jiang G, Chen H, Wang S, et al. Learning curves and long-term outcome of simulation-based thoracentesis training for medical students. BMC Med Educ. 2011;11:39. PubMed
16. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis: preliminary results. Am J Med Qual. 2013;28(3):220-226. PubMed
17. Salamonsen M, McGrath D, Steiler G, Ware R, Colt H, Fielding D. A new instrument to assess physician skill at thoracic ultrasound, including pleural effusion markup. Chest. 2013;144(3):930-934. PubMed
18. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31(2):164-171. PubMed
19. Havelock T, Teoh R, Laws D, Gleeson F, Group BTSPDG. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii61-76. PubMed
20. Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax. 2015;70(2):127-132. PubMed
21. Puchalski J. Thoracentesis and the risks for bleeding: a new era. Curr Opin Pulm Med. 2014;20(4):377-384. PubMed
22. Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest. 2013;144(2):456-463. PubMed
23. Zalt MB, Bechara RI, Parks C, Berkowitz DM. Effect of routine clopidogrel use on bleeding complications after ultrasound-guided thoracentesis. J Bronchology Interv Pulmonol. 2012;19(4):284-287. PubMed
24. Mahmood K, Shofer SL, Moser BK, Argento AC, Smathers EC, Wahidi MM. Hemorrhagic complications of thoracentesis and small-bore chest tube placement in patients taking clopidogrel. Ann Am Thorac Soc. 2014;11(1):73-79. PubMed
25. Goligher EC, Leis JA, Fowler RA, Pinto R, Adhikari NK, Ferguson ND. Utility and safety of draining pleural effusions in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care. 2011;15(1):R46. PubMed
26. Fartoukh M, Azoulay E, Galliot R, et al. Clinically documented pleural effusions in medical ICU patients: how useful is routine thoracentesis? Chest. 2002;121(1):178-184. PubMed
27. Cervini P, Hesley GK, Thompson RL, Sampathkumar P, Knudsen JM. Incidence of infectious complications after an ultrasound-guided intervention. AJR Am J Roentgenol. 2010;195(4):846-850. PubMed
28. Mimoz O, Chopra V, Timsit JF. What's new in catheter-related infection: skin cleansing and skin antisepsis. Intensive Care Med. 2016;42(11):1784-1786. PubMed
29. Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015;10(12):811-816. PubMed
30. Feller-Kopman D. Ultrasound-guided thoracentesis. Chest. 2006;129(6):1709-1714. PubMed
31. Shojaee S, Argento AC. Ultrasound-guided pleural access. Semin Respir Crit Care Med. 2014;35(6):693-705. PubMed
32. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170(4):332-339. PubMed
33. Perazzo A, Gatto P, Barlascini C, Ferrari-Bravo M, Nicolini A. Can ultrasound guidance reduce the risk of pneumothorax following thoracentesis? J Bras Pneumol. 2014;40(1):6-12. PubMed
34. Cavanna L, Mordenti P, Berte R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. PubMed
35. Soldati G, Smargiassi A, Inchingolo R, Sher S, Valente S, Corbo GM. Ultrasound-guided pleural puncture in supine or recumbent lateral position - feasibility study. Multidiscip Respir Med. 2013;8(1):18. PubMed
36. Pihlajamaa K, Bode MK, Puumalainen T, Lehtimaki A, Marjelund S, Tikkakoski T. Pneumothorax and the value of chest radiography after ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(8):828-832. PubMed
37. Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound. 2005;33(9):442-446. PubMed
38. Zanforlin A, Gavelli G, Oboldi D, Galletti S. Ultrasound-guided thoracenthesis: the V-point as a site for optimal drainage positioning. Eur Rev Med Pharmacol Sci. 2013;17(1):25-28. PubMed
39. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40(3):135-141. PubMed
40. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. PubMed
41. Shurtleff E, Olinger A. Posterior intercostal artery tortuosity and collateral branch points: a cadaveric study. Folia Morphol (Warsz). 2012;71(4):245-251. PubMed
42. Helm EJ, Rahman NM, Talakoub O, Fox DL, Gleeson FV. Course and variation of the intercostal artery by CT scan. Chest. 2013;143(3):634-639. PubMed
43. Yoneyama H, Arahata M, Temaru R, Ishizaka S, Minami S. Evaluation of the risk of intercostal artery laceration during thoracentesis in elderly patients by using 3D-CT angiography. Intern Med. 2010;49(4):289-292. PubMed
44. Salamonsen M, Ellis S, Paul E, Steinke K, Fielding D. Thoracic ultrasound demonstrates variable location of the intercostal artery. Respiration. 2012;83(4):323-329. PubMed
45. Salamonsen M, Dobeli K, McGrath D, et al. Physician-performed ultrasound can accurately screen for a vulnerable intercostal artery prior to chest drainage procedures. Respirology. 2013;18(6):942-947. PubMed
46. Grippi MA. Fishman's pulmonary diseases and disorders. Fifth edition. ed. New York: McGraw-Hill Education; 2015.
47. Huggins JT, Doelken P. Pleural manometry. Clin Chest Med. 2006;27(2):229-240. PubMed
48. Echevarria C, Twomey D, Dunning J, Chanda B. Does re-expansion pulmonary oedema exist? Interact Cardiovasc Thorac Surg. 2008;7(3):485-489. PubMed
49. Sue RD, Matthay MA, Ware LB. Hydrostatic mechanisms may contribute to the pathogenesis of human re-expansion pulmonary edema. Intensive Care Med. 2004;30(10):1921-1926. PubMed
50. Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84(5):1656-1661. PubMed
51. Villena V, Lopez-Encuentra A, Pozo F, De-Pablo A, Martin-Escribano P. Measurement of pleural pressure during therapeutic thoracentesis. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1534-1538. PubMed
52. Doelken P, Huggins JT, Pastis NJ, Sahn SA. Pleural manometry: technique and clinical implications. Chest. 2004;126(6):1764-1769. PubMed
53. Feller-Kopman D. Therapeutic thoracentesis: the role of ultrasound and pleural manometry. Curr Opin Pulm Med. 2007;13(4):312-318. PubMed
54. Boshuizen RC, Sinaasappel M, Vincent AD, Goldfinger V, Farag S, van den Heuvel MM. Pleural pressure swing and lung expansion after malignant pleural effusion drainage: the benefits of high-temporal resolution pleural manometry. J Bronchology Interv Pulmonol. 2013;20(3):200-205. PubMed
55. Pannu J, DePew ZS, Mullon JJ, Daniels CE, Hagen CE, Maldonado F. Impact of pleural manometry on the development of chest discomfort during thoracentesis: a symptom-based study. J Bronchology Interv Pulmonol. 2014;21(4):306-313. PubMed
56. Feller-Kopman D, Walkey A, Berkowitz D, Ernst A. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest. 2006;129(6):1556-1560. PubMed
57. Abunasser J, Brown R. Safety of large-volume thoracentesis. Conn Med. 2010;74(1):23-26. PubMed
58. Mynarek G, Brabrand K, Jakobsen JA, Kolbenstvedt A. Complications following ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(5):519-522. PubMed
59. Josephson T, Nordenskjold CA, Larsson J, Rosenberg LU, Kaijser M. Amount drained at ultrasound-guided thoracentesis and risk of pneumothorax. Acta Radiol. 2009;50(1):42-47. PubMed
60. Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest. 2006;130(4):1173-1184. PubMed
61. Sokolowski JW Jr, Burgher LW, Jones FL Jr, Patterson JR, Selecky PA. Guidelines for thoracentesis and needle biopsy of the pleura. This position paper of the American Thoracic Society was adopted by the ATS Board of Directors, June 1988. Am Rev Respir Dis. 1989;140(1):257-258. PubMed
62. Jones PW, Moyers JP, Rogers JT, Rodriguez RM, Lee YC, Light RW. Ultrasound-guided thoracentesis: is it a safer method? Chest. 2003;123(2):418-423. PubMed
63. Petersen WG, Zimmerman R. Limited utility of chest radiograph after thoracentesis. Chest. 2000;117(4):1038-1042. PubMed
64. Sachdeva A, Shepherd RW, Lee HJ. Thoracentesis and thoracic ultrasound: state of the art in 2013. Clin Chest Med. 2013;34(1):1-9. PubMed
65. Shostak E, Brylka D, Krepp J, Pua B, Sanders A. Bedside sonography for detection of postprocedure pneumothorax. J Ultrasound Med. 2013;32(6):1003-1009. PubMed
Pleural effusion can occur in myriad conditions including infection, heart failure, liver disease, and cancer.1 Consequently, physicians from many disciplines routinely encounter both inpatients and outpatients with this diagnosis. Often, evaluation and treatment require thoracentesis to obtain fluid for analysis or symptom relief.
Although historically performed at the bedside without imaging guidance or intraprocedural monitoring, thoracentesis performed in this fashion carries considerable risk of complications. In fact, it has 1 of the highest rates of iatrogenic pneumothorax among bedside procedures.2 However, recent advances in practice and adoption of newer technologies have helped to mitigate risks associated with this procedure. These advances are relevant because approximately 50% of thoracenteses are still performed at the bedside.3 In this review, we aim to identify the most recent key practices that enhance the safety and the effectiveness of thoracentesis for practicing clinicians.
METHODS
Information Sources and Search Strategy
With the assistance of a research librarian, we performed a systematic search of PubMed-indexed articles from January 1, 2000 to September 30, 2015. Articles were identified using search terms such as thoracentesis, pleural effusion, safety, medical error, adverse event, and ultrasound in combination with Boolean operators. Of note, as thoracentesis is indexed as a subgroup of paracentesis in PubMed, this term was also included to increase the sensitivity of the search. The full search strategy is available in the Appendix. Any references cited in this review outside of the date range of our search are provided only to give relevant background information or establish the origin of commonly performed practices.
Study Eligibility and Selection Criteria
Studies were included if they reported clinical aspects related to thoracentesis. We defined clinical aspects as those strategies that focused on operator training, procedural techniques, technology, management, or prevention of complications. Non-English language articles, animal studies, case reports, conference proceedings, and abstracts were excluded. As our intention was to focus on the contemporary advances related to thoracentesis performance, (eg, ultrasound [US]), our search was limited to studies published after the year 2000. Two authors, Drs. Schildhouse and Lai independently screened studies to determine inclusion, excluding studies with weak methodology, very small sample sizes, and those only tangentially related to our aim. Disagreements regarding study inclusion were resolved by consensus. Drs. Lai, Barsuk, and Mourad identified additional studies by hand review of reference lists and content experts (Figure 1).
Conceptual Framework
All selected articles were categorized by temporal relationship to thoracentesis as pre-, intra-, or postprocedure. Pre-procedural topics were those outcomes that had been identified and addressed before attempting thoracentesis, such as physician training or perceived risks of harm. Intraprocedural considerations included aspects such as use of bedside US, pleural manometry, and large-volume drainage. Finally, postprocedural factors were those related to evaluation after thoracentesis, such as follow-up imaging. This conceptual framework is outlined in Figure 2.
RESULTS
The PubMed search returned a total of 1170 manuscripts, of which 56 articles met inclusion criteria. Four additional articles were identified by experts and included in the study.4-7 Therefore, 60 articles were identified and included in this review. Study designs included cohort studies, case control studies, systematic reviews, meta-analyses, narrative reviews, consensus guidelines, and randomized controlled trials. A summary of all included articles by topic can be found in the Table.

PRE-PROCEDURAL CONSIDERATIONS
Physician Training
Studies indicate that graduate medical education may not adequately prepare clinicians to perform thoracentesis.8 In fact, residents have the least exposure and confidence in performing thoracentesis when compared to other bedside procedures.9,10 In 1 survey, 69% of medical trainees desired more exposure to procedures, and 98% felt that procedural skills were important to master.11 Not surprisingly, then, graduating internal medicine residents perform poorly when assessed on a thoracentesis simulator.12
Supplemental training outside of residency is useful to develop and maintain skills for thoracentesis, such as simulation with direct observation in a zero-risk environment. In 1 study, “simulation-based mastery learning” combined an educational video presentation with repeated, deliberate practice on a simulator until procedural competence was acquired, over two 2-hour sessions. In this study, 40 third-year medicine residents demonstrated a 71% improvement in clinical skills performance after course completion, with 93% achieving a passing score. The remaining 7% also achieved passing scores with extra practice time.12 Others have built upon the concept of simulation-based training. For instance, 2 studies suggest that use of a simulation-based curriculum improved both thoracentesis knowledge and performance skills in a 3-hour session.13,14 Similarly, 1 prospective study reported that a half-day thoracentesis workshop using simulation and 1:1 direct observation successfully lowered pneumothorax rates from 8.6% to 1.8% in a group of practicing clinicians. Notably, additional interventions including use of bedside US, limiting operators to a focused group, and standardization of equipment were also a part of this quality improvement initiative.7 Although repetition is required to gain proficiency when using a simulator, performance and confidence appear to plateau with only 4 simulator trials. In medical students, improvements derived through simulator-based teaching were sustained when retested 6 months following training.15
An instrument to ensure competency is necessary, given variability in procedural experience among both new graduates and practicing physicians,. Our search did not identify any clinically validated tools that adequately assessed thoracentesis performance. However, some have been proposed16 and 1 validated in a simulation environment.12 Regarding the incorporation of US for effusion markup, 1 validated tool used an 11-domain assessment covering knowledge of US machine manipulation, recognition of images with common pleural effusion characteristics, and performance of thoracic US with puncture-site marking on a simulator. When used on 22 participants, scores with the tool could reliably differentiate between novice, intermediate, and advanced groups (P < 0.0001).17
Patient Selection
Coagulopathies and Anticoagulation. Historically, the accepted cutoff for performing thoracentesis is an international normalized ratio (INR) less than 1.5 and a platelet count greater than 50,000/µL. McVay et al.18 first showed in 1991 that use of these cutoffs was associated with low rates of periprocedural bleeding, leading to endorsement in the British Thoracic Society (BTS) Pleural Disease Guideline 2010.19 Other recommendations include the 2012 Society for Interventional Radiology guidelines that endorse correction of an INR greater than 2, or platelets less than 50,000/µL, based almost exclusively on expert opinion.5
However, data suggest that thoracentesis may be safely performed outside these parameters. For instance, a prospective study of approximately 9000 thoracenteses over 12 years found that patients with an INR of 1.5-2.9 or platelets of 20,000 - 49,000/µL experienced rates of bleeding complications similar to those with normal values.20 Similarly, a 2014 review21 found that the overall risk of hemorrhage during thoracentesis in the setting of moderate coagulopathy (defined as an INR of 1.5 - 3 or platelets of 25,000-50,000/µL), was not increased. In 1 retrospective study of more than 1000 procedures, no differences in hemorrhagic events were noted in patients with bleeding diatheses that received prophylactic fresh frozen plasma or platelets vs. those who did not.22 Of note, included studies used a variety of criteria to define a hemorrhagic complication, which included: an isolated 2 g/dL or more decrement in hemoglobin, presence of bloody fluid on repeat tap with associated hemoglobin decrement, rapid re-accumulation of fluid with a hemoglobin decrement, or transfusion of 2 units or more of whole blood.
Whether it is safe to perform thoracentesis on patients taking antiplatelet therapy is less well understood. Although data are limited, a few small-scale studies23,24 suggest that hemorrhagic complications following thoracentesis in patients receiving clopidogrel are comparable to the general population. We found no compelling data regarding the safety of thoracentesis in the setting of direct oral anticoagulants, heparin, low-molecular weight heparin, or intravenous direct thrombin inhibitors. Current practice is to generally avoid thoracentesis while these therapeutic anticoagulants are used.
Invasive mechanical ventilation. Pleural effusion is common in patients in the intensive care unit, including those requiring mechanical ventilation.25 Thoracentesis in this population is clinically important: fluid analysis in 1 study was shown to aid the diagnosis in 45% of cases and changes in treatment in 33%.26 However, clinicians may be reluctant to perform thoracentesis on patients who require mechanical ventilation, given the perception of a greater risk of pneumothorax from positive pressure ventilation.
Despite this concern, a 2011 meta-analysis including 19 studies and more than 1100 patients revealed rates of pneumothorax and hemothorax comparable to nonventilated patients.25 Furthermore, a 2015 prospective study that examined thoracentesis in 1377 mechanically ventilated patients revealed no difference in complication rates as well.20 Therefore, evidence suggests that performance of thoracentesis in mechanically ventilated patients is not contraindicated.
Skin Disinfection and Antisepsis Precautions
The 2010 BTS guidelines list empyema and wound infection as possible complications of thoracentesis.19 However, no data regarding incidence are provided. Additionally, an alcohol-based skin cleanser (such as 2% chlorhexidine gluconate/70% isopropyl alcohol), along with sterile gloves, field, and dressing are suggested as precautionary measures.19 In 1 single-center registry of 2489 thoracenteses performed using alcohol or iodine-based antiseptic and sterile drapes, no postprocedure infections were identified.27 Of note, we did not find other studies (including case reports) that reported either incidence or rate of infectious complications such as wound infection and empyema. In an era of modern skin antiseptics that have effectively reduced complications such as catheter-related bloodstream infection,28 the incidence of this event is thus likely to be low.
INTRAPROCEDURAL CONSIDERATIONS
Use of Bedside Ultrasound
Portable US has particular advantages for evaluation of pleural effusion vs other imaging modalities. Compared with computerized tomography (CT), bedside US offers similar performance but is less costly, avoids both radiation exposure and need for patient transportation, and provides results instantaneously.29,30 Compared to chest x-ray (CXR), US is more sensitive at detecting the presence, volume, and characteristics of pleural fluid30,31 and can be up to 100% sensitive for effusions greater than 100 mL.29 Furthermore, whereas CXR typically requires 200 mL of fluid to be present for detection of an effusion, US can reliably detect as little as 20 mL of fluid.29 When US was used to confirm thoracentesis puncture sites in a study involving 30 physicians of varying experience and 67 consecutive patients, 15% of sites found by clinical exam were inaccurate (less than 10 mm fluid present), 10% were at high risk for organ puncture, and a suitable fluid pocket was found 54% of times when exam could not.4
A 2010 meta-analysis of 24 studies and 6605 thoracenteses estimated the overall rate of pneumothorax at 6%; however, procedures performed with US guidance were associated with a 70% reduced risk of this event (odds ratio, 0.30; 95% confidence interval, 0.20 - 0.70).32 In a 2014 randomized control trial of 160 patients that compared thoracentesis with US guidance for site marking vs no US use, 10 pneumothoraces occurred in the control group vs 1 in the US group (12.5% vs 1.25%, P = 0.009).33 Similarly, another retrospective review of 445 consecutive patients with malignant effusions revealed a pneumothorax rate of 0.97% using US in real time during needle insertion compared to 8.89% for unguided thoracenteses (P < 0.0001).34 Several other studies using US guidance for either site markup or in real time reported similar pneumothorax rates, ranging from 1.1% - 4.8%.35-37 However, it is unclear if real-time US specifically provides an additive effect vs site marking alone, as no studies directly comparing the 2 methods were found.
Benefits of US also include a higher rate of procedural success, with 1 study demonstrating a 99% success rate when using US vs. 90% without (P = 0.030).33 A larger volume of fluid removed has been observed with US use as well, and methods have been described using fluid-pocket depth to guide puncture site localization and maximize drainage.38 Finally, US use for thoracentesis has been associated with lower costs and length of stay.39,40
Intercostal Artery Localization
Although rare (incidence, 0.18%-2%20,21,39), the occurrence of hemothorax following thoracentesis is potentially catastrophic. This serious complication is often caused by laceration of the intercostal artery (ICA) or 1 of its branches during needle insertion.41
While risk of injury is theoretically reduced by needle insertion superior to the rib, studies using cadaver dissection and 3D angiography show significant tortuosity of the ICA.6,41-43 The degree of tortuosity is increased within 6 cm of the midline, in more cephalad rib spaces, and in the elderly (older than 60 years).41-43 Furthermore, 1 cadaveric study also demonstrated the presence of arterial collaterals branching off the ICA at multiple intercostal spaces, ranging between 8 cm and 11 cm from the midline.41 This anatomic variability may explain why some have observed low complication and hemothorax rates with an extreme lateral approach.35 Bedside US with color flow Doppler imaging has been used to identify the ICA, with 88% sensitivity compared to CT imaging while adding little to exam time.44,45 Of note, a 37% drop in the rate of hemothorax was observed in 1 study with routine US guidance alone.39
Pleural Pressure Monitoring and Large-Volume Thoracentesis
While normal intrapleural pressures are approximately -5 to -10 cm H2O,46 the presence of a pleural effusion creates a complex interaction between fluid, compressed lung, and chest wall that can increase these pressures.47 During drainage of an effusion, pleural pressures may rapidly drop, provoking re-expansion pulmonary edema (REPE). While rare (0 -1%), clinically-diagnosed REPE is a serious complication that can lead to rapid respiratory failure and death.20,48 REPE is postulated to be caused by increased capillary permeability resulting from inflammation, driven by rapid re-inflation of the lung when exposed to highly negative intrapleural pressures.47,49
Measurement of intrapleural pressure using a water manometer during thoracentesis may minimize REPE by terminating fluid drainage when intrapleural pressure begins to drop rapidly.50,51 A cutoff of -20 cm H2O has been cited repeatedly as safe since being suggested by Light in 1980, but this is based on animal models.50,52 In 1 prospective study of 185 thoracenteses in which manometry was performed, 15% of patients had intrapleural pressure drop to less than -20 cm H2O (at which point the procedure was terminated) but suffered no REPE.50
Manometry is valuable in the identification of an unexpandable or trapped lung when pleural pressures drop rapidly with only minimal fluid volume removal.47,53 Other findings correlated with an unexpandable lung include a negative opening pressure47 and large fluctuations in pressure during the respiratory cycle.54
While development of symptoms (eg, chest pain, cough, or dyspnea) is often used as a surrogate, the correlation between intrapleural pressure and patient symptoms is inconsistent and not a reliable proxy.55 One study found that 22% of patients with chest pain during thoracentesis had intrapleural pressures lower than -20 cm H2O compared with 8.6% of asymptomatic patients,56 but it is unclear if the association is causal.
Thoracentesis is often performed for symptomatic relief and removal of large fluid volume. However, it remains common to halt fluid removal after 1.5 L, a threshold endorsed by BTS.19 While some investigators have suggested that removal of 2 L or more of pleural fluid does not compromise safety,57,58 a 4- to 5-fold rise in the risk of pneumothorax was noted in 2 studies.20,59 when more than 1.5 L of fluid was removed. The majority of these may be related to pneumothorax ex vacuo, a condition in which fluid is drained from the chest, but the lung is unable to expand and fill the space (eg, “trapped lung”), resulting in a persistent pneumothorax. This condition generally does not require treatment.60 When manometry is employed at 200-mL intervals with termination at an intrapleural pressure of less than 20 mm H2O, drainage of 3 L or more has been reported with low rates of pneumothorax and very low rates of REPE.50,51 However, whether this is cause and effect is unknown because REPE is rare, and more work is needed to determine the role of manometry for its prevention.
POSTPROCEDURAL CONSIDERATIONS
Postprocedure Imaging
Performing an upright CXR following thoracentesis is a practice that remains routinely done by many practitioners to monitor for complications. Such imaging was also endorsed by the American Thoracic Society guidelines.61 However, more recent data question the utility of this practice. Multiple studies have confirmed that post-thoracentesis CXR is unnecessary unless clinical suspicion for pneumothorax or REPE is present.36,58,62,63 The BTS guidelines also advocate this approach.19 Interestingly, a potentially more effective way to screen for postprocedure complications is through bedside US, which has been shown to be more sensitive than CXR in detecting pneumothorax.64 In 1 study of 185 patients, bedside US demonstrated a sensitivity of 88% and a specificity of 97% for diagnosing pneumothorax in patients with adequate quality scans, with positive and negative likelihood ratios of 55 and 0.17, respectively.65
DISCUSSION
Thoracentesis remains a core procedural skill for hospitalists, critical care physicians, and emergency physicians. It is the foundational component when investigating and treating pleural effusions. When the most current training, techniques, and technology are used, data suggest this procedure is safe to perform at the bedside. Our review highlights these strategies and evaluates which aspects might be most applicable to clinical practice.
Our findings have several implications for those who perform this procedure. First, appropriate training is central to procedural safety, and both simulation and direct observation by procedural experts have been shown by multiple investigators to improve knowledge and skill. This training should integrate the use of US in performing a focused thoracic exam.
Second, recommendations regarding coagulopathy and a “safe cutoff” of an INR less than 1.5 or platelets greater than 50,000/µL had limited evidentiary support. Rather, multiple studies suggest no difference in bleeding risk following thoracentesis with an INR as high as 3.0 and platelets greater than 25,000/µL. Furthermore, prophylactic transfusion with fresh frozen plasma or platelets before thoracentesis did not alter bleeding risk and exposes patients to transfusion complications. Thus, routine use of this practice can no longer be recommended. Third, further research is needed to understand the bleeding risk for patients on antiplatelet medications, heparin products, and also direct oral anticoagulants, given the growing popularity in their use and the potential consequences of even temporary cessation. Regarding patients on mechanical ventilation, thoracentesis demonstrated no difference in complication rates vs. the general population, and its performance in this population is encouraged when clinically indicated.
Intraprocedural considerations include the use of bedside US. Due to multiple benefits including effusion characterization, puncture site localization, and significantly lower rates of pneumothorax, the standard of care should be to perform thoracentesis with US guidance. Both use of US to mark an effusion immediately prior to puncture or in real time during needle insertion demonstrated benefit; however, it is unclear if 1 method is superior because no direct comparison studies were found. Further work is needed to investigate this potential.
Our review suggests that the location and course of the ICA is variable, especially near the midline, in the elderly, and in higher intercostal spaces, leaving it vulnerable to laceration. We recommend physicians only attempt thoracentesis at least 6 cm lateral to the midline due to ICA tortuosity and, ideally, 12 cm lateral, to avoid the presence of collaterals. Although only 2 small-scale studies were found pertaining to the use of US in identifying the ICA, we encourage physicians to consider learning how to screen for its presence as a part of their routine thoracic US exam in the area underlying the planned puncture site.
Manometry is beneficial because it can diagnose a nonexpandable lung and allows for pleural pressure monitoring.52,53 A simple U-shaped manometer can be constructed from intravenous tubing included in most thoracentesis kits, which adds little to overall procedure time. While low rates of REPE have been observed when terminating thoracentesis if pressures drop below -20 cm H2O or chest pain develops, neither measure appears to have reliable predictive value, limiting clinical utility. Further work is required to determine if a “safe pressure cutoff” exists. In general, we recommend the use of manometry when a nonexpandable (trapped) lung is suspected, because large drops in intrapleural pressure, a negative opening pressure, and respiratory variation can help confirm the diagnosis and avoid pneumothorax ex vacuo or unnecessary procedures in the future. As this condition appears to be more common in the setting of larger effusions, use of manometry when large-volume thoracenteses are planned is also reasonable.
Postprocedurally, routine imaging after thoracentesis is not recommended unless there is objective concern for complication. When indicated, bedside US is better positioned for this role compared with CXR, because it is more sensitive in detecting pneumothorax, provides instantaneous results, and avoids radiation exposure.
Our review has limitations. First, we searched only for articles between defined time periods, restricted our search to a single database, and excluded non-English articles. This has the potential to introduce selection bias, as nonprimary articles that fall within our time restrictions may cite older studies that are outside our search range. To minimize this effect, we performed a critical review of all included studies, especially nonprimary articles. Second, despite the focus of our search strategy to identify any articles related to patient safety and adverse events, we cannot guarantee that all relevant articles for any particular complication or risk factor were captured given the lack of more specific search terms. Third, although we performed a systematic search of the literature, we did not perform a formal systematic review or formally grade included studies. As the goal of our review was to categorize and operationalize clinical aspects, this approach was necessary, and we acknowledge that the quality of studies is variable. Lastly, we aimed to generate clinical recommendations for physicians performing thoracentesis at the bedside; others reviewing this literature may find or emphasize different aspects relevant to practice outside this setting.
In conclusion, evaluation and treatment of pleural effusions with bedside thoracentesis is an important skill for physicians of many disciplines. The evidence presented in this review will help inform the process and ensure patient safety. Physicians should consider incorporating these recommendations into their practice.
The authors thank Whitney Townsend, MLIS, health sciences informationist, for assistance with serial literature searches.
Disclosure
Nothing to report.
Pleural effusion can occur in myriad conditions including infection, heart failure, liver disease, and cancer.1 Consequently, physicians from many disciplines routinely encounter both inpatients and outpatients with this diagnosis. Often, evaluation and treatment require thoracentesis to obtain fluid for analysis or symptom relief.
Although historically performed at the bedside without imaging guidance or intraprocedural monitoring, thoracentesis performed in this fashion carries considerable risk of complications. In fact, it has 1 of the highest rates of iatrogenic pneumothorax among bedside procedures.2 However, recent advances in practice and adoption of newer technologies have helped to mitigate risks associated with this procedure. These advances are relevant because approximately 50% of thoracenteses are still performed at the bedside.3 In this review, we aim to identify the most recent key practices that enhance the safety and the effectiveness of thoracentesis for practicing clinicians.
METHODS
Information Sources and Search Strategy
With the assistance of a research librarian, we performed a systematic search of PubMed-indexed articles from January 1, 2000 to September 30, 2015. Articles were identified using search terms such as thoracentesis, pleural effusion, safety, medical error, adverse event, and ultrasound in combination with Boolean operators. Of note, as thoracentesis is indexed as a subgroup of paracentesis in PubMed, this term was also included to increase the sensitivity of the search. The full search strategy is available in the Appendix. Any references cited in this review outside of the date range of our search are provided only to give relevant background information or establish the origin of commonly performed practices.
Study Eligibility and Selection Criteria
Studies were included if they reported clinical aspects related to thoracentesis. We defined clinical aspects as those strategies that focused on operator training, procedural techniques, technology, management, or prevention of complications. Non-English language articles, animal studies, case reports, conference proceedings, and abstracts were excluded. As our intention was to focus on the contemporary advances related to thoracentesis performance, (eg, ultrasound [US]), our search was limited to studies published after the year 2000. Two authors, Drs. Schildhouse and Lai independently screened studies to determine inclusion, excluding studies with weak methodology, very small sample sizes, and those only tangentially related to our aim. Disagreements regarding study inclusion were resolved by consensus. Drs. Lai, Barsuk, and Mourad identified additional studies by hand review of reference lists and content experts (Figure 1).
Conceptual Framework
All selected articles were categorized by temporal relationship to thoracentesis as pre-, intra-, or postprocedure. Pre-procedural topics were those outcomes that had been identified and addressed before attempting thoracentesis, such as physician training or perceived risks of harm. Intraprocedural considerations included aspects such as use of bedside US, pleural manometry, and large-volume drainage. Finally, postprocedural factors were those related to evaluation after thoracentesis, such as follow-up imaging. This conceptual framework is outlined in Figure 2.
RESULTS
The PubMed search returned a total of 1170 manuscripts, of which 56 articles met inclusion criteria. Four additional articles were identified by experts and included in the study.4-7 Therefore, 60 articles were identified and included in this review. Study designs included cohort studies, case control studies, systematic reviews, meta-analyses, narrative reviews, consensus guidelines, and randomized controlled trials. A summary of all included articles by topic can be found in the Table.

PRE-PROCEDURAL CONSIDERATIONS
Physician Training
Studies indicate that graduate medical education may not adequately prepare clinicians to perform thoracentesis.8 In fact, residents have the least exposure and confidence in performing thoracentesis when compared to other bedside procedures.9,10 In 1 survey, 69% of medical trainees desired more exposure to procedures, and 98% felt that procedural skills were important to master.11 Not surprisingly, then, graduating internal medicine residents perform poorly when assessed on a thoracentesis simulator.12
Supplemental training outside of residency is useful to develop and maintain skills for thoracentesis, such as simulation with direct observation in a zero-risk environment. In 1 study, “simulation-based mastery learning” combined an educational video presentation with repeated, deliberate practice on a simulator until procedural competence was acquired, over two 2-hour sessions. In this study, 40 third-year medicine residents demonstrated a 71% improvement in clinical skills performance after course completion, with 93% achieving a passing score. The remaining 7% also achieved passing scores with extra practice time.12 Others have built upon the concept of simulation-based training. For instance, 2 studies suggest that use of a simulation-based curriculum improved both thoracentesis knowledge and performance skills in a 3-hour session.13,14 Similarly, 1 prospective study reported that a half-day thoracentesis workshop using simulation and 1:1 direct observation successfully lowered pneumothorax rates from 8.6% to 1.8% in a group of practicing clinicians. Notably, additional interventions including use of bedside US, limiting operators to a focused group, and standardization of equipment were also a part of this quality improvement initiative.7 Although repetition is required to gain proficiency when using a simulator, performance and confidence appear to plateau with only 4 simulator trials. In medical students, improvements derived through simulator-based teaching were sustained when retested 6 months following training.15
An instrument to ensure competency is necessary, given variability in procedural experience among both new graduates and practicing physicians,. Our search did not identify any clinically validated tools that adequately assessed thoracentesis performance. However, some have been proposed16 and 1 validated in a simulation environment.12 Regarding the incorporation of US for effusion markup, 1 validated tool used an 11-domain assessment covering knowledge of US machine manipulation, recognition of images with common pleural effusion characteristics, and performance of thoracic US with puncture-site marking on a simulator. When used on 22 participants, scores with the tool could reliably differentiate between novice, intermediate, and advanced groups (P < 0.0001).17
Patient Selection
Coagulopathies and Anticoagulation. Historically, the accepted cutoff for performing thoracentesis is an international normalized ratio (INR) less than 1.5 and a platelet count greater than 50,000/µL. McVay et al.18 first showed in 1991 that use of these cutoffs was associated with low rates of periprocedural bleeding, leading to endorsement in the British Thoracic Society (BTS) Pleural Disease Guideline 2010.19 Other recommendations include the 2012 Society for Interventional Radiology guidelines that endorse correction of an INR greater than 2, or platelets less than 50,000/µL, based almost exclusively on expert opinion.5
However, data suggest that thoracentesis may be safely performed outside these parameters. For instance, a prospective study of approximately 9000 thoracenteses over 12 years found that patients with an INR of 1.5-2.9 or platelets of 20,000 - 49,000/µL experienced rates of bleeding complications similar to those with normal values.20 Similarly, a 2014 review21 found that the overall risk of hemorrhage during thoracentesis in the setting of moderate coagulopathy (defined as an INR of 1.5 - 3 or platelets of 25,000-50,000/µL), was not increased. In 1 retrospective study of more than 1000 procedures, no differences in hemorrhagic events were noted in patients with bleeding diatheses that received prophylactic fresh frozen plasma or platelets vs. those who did not.22 Of note, included studies used a variety of criteria to define a hemorrhagic complication, which included: an isolated 2 g/dL or more decrement in hemoglobin, presence of bloody fluid on repeat tap with associated hemoglobin decrement, rapid re-accumulation of fluid with a hemoglobin decrement, or transfusion of 2 units or more of whole blood.
Whether it is safe to perform thoracentesis on patients taking antiplatelet therapy is less well understood. Although data are limited, a few small-scale studies23,24 suggest that hemorrhagic complications following thoracentesis in patients receiving clopidogrel are comparable to the general population. We found no compelling data regarding the safety of thoracentesis in the setting of direct oral anticoagulants, heparin, low-molecular weight heparin, or intravenous direct thrombin inhibitors. Current practice is to generally avoid thoracentesis while these therapeutic anticoagulants are used.
Invasive mechanical ventilation. Pleural effusion is common in patients in the intensive care unit, including those requiring mechanical ventilation.25 Thoracentesis in this population is clinically important: fluid analysis in 1 study was shown to aid the diagnosis in 45% of cases and changes in treatment in 33%.26 However, clinicians may be reluctant to perform thoracentesis on patients who require mechanical ventilation, given the perception of a greater risk of pneumothorax from positive pressure ventilation.
Despite this concern, a 2011 meta-analysis including 19 studies and more than 1100 patients revealed rates of pneumothorax and hemothorax comparable to nonventilated patients.25 Furthermore, a 2015 prospective study that examined thoracentesis in 1377 mechanically ventilated patients revealed no difference in complication rates as well.20 Therefore, evidence suggests that performance of thoracentesis in mechanically ventilated patients is not contraindicated.
Skin Disinfection and Antisepsis Precautions
The 2010 BTS guidelines list empyema and wound infection as possible complications of thoracentesis.19 However, no data regarding incidence are provided. Additionally, an alcohol-based skin cleanser (such as 2% chlorhexidine gluconate/70% isopropyl alcohol), along with sterile gloves, field, and dressing are suggested as precautionary measures.19 In 1 single-center registry of 2489 thoracenteses performed using alcohol or iodine-based antiseptic and sterile drapes, no postprocedure infections were identified.27 Of note, we did not find other studies (including case reports) that reported either incidence or rate of infectious complications such as wound infection and empyema. In an era of modern skin antiseptics that have effectively reduced complications such as catheter-related bloodstream infection,28 the incidence of this event is thus likely to be low.
INTRAPROCEDURAL CONSIDERATIONS
Use of Bedside Ultrasound
Portable US has particular advantages for evaluation of pleural effusion vs other imaging modalities. Compared with computerized tomography (CT), bedside US offers similar performance but is less costly, avoids both radiation exposure and need for patient transportation, and provides results instantaneously.29,30 Compared to chest x-ray (CXR), US is more sensitive at detecting the presence, volume, and characteristics of pleural fluid30,31 and can be up to 100% sensitive for effusions greater than 100 mL.29 Furthermore, whereas CXR typically requires 200 mL of fluid to be present for detection of an effusion, US can reliably detect as little as 20 mL of fluid.29 When US was used to confirm thoracentesis puncture sites in a study involving 30 physicians of varying experience and 67 consecutive patients, 15% of sites found by clinical exam were inaccurate (less than 10 mm fluid present), 10% were at high risk for organ puncture, and a suitable fluid pocket was found 54% of times when exam could not.4
A 2010 meta-analysis of 24 studies and 6605 thoracenteses estimated the overall rate of pneumothorax at 6%; however, procedures performed with US guidance were associated with a 70% reduced risk of this event (odds ratio, 0.30; 95% confidence interval, 0.20 - 0.70).32 In a 2014 randomized control trial of 160 patients that compared thoracentesis with US guidance for site marking vs no US use, 10 pneumothoraces occurred in the control group vs 1 in the US group (12.5% vs 1.25%, P = 0.009).33 Similarly, another retrospective review of 445 consecutive patients with malignant effusions revealed a pneumothorax rate of 0.97% using US in real time during needle insertion compared to 8.89% for unguided thoracenteses (P < 0.0001).34 Several other studies using US guidance for either site markup or in real time reported similar pneumothorax rates, ranging from 1.1% - 4.8%.35-37 However, it is unclear if real-time US specifically provides an additive effect vs site marking alone, as no studies directly comparing the 2 methods were found.
Benefits of US also include a higher rate of procedural success, with 1 study demonstrating a 99% success rate when using US vs. 90% without (P = 0.030).33 A larger volume of fluid removed has been observed with US use as well, and methods have been described using fluid-pocket depth to guide puncture site localization and maximize drainage.38 Finally, US use for thoracentesis has been associated with lower costs and length of stay.39,40
Intercostal Artery Localization
Although rare (incidence, 0.18%-2%20,21,39), the occurrence of hemothorax following thoracentesis is potentially catastrophic. This serious complication is often caused by laceration of the intercostal artery (ICA) or 1 of its branches during needle insertion.41
While risk of injury is theoretically reduced by needle insertion superior to the rib, studies using cadaver dissection and 3D angiography show significant tortuosity of the ICA.6,41-43 The degree of tortuosity is increased within 6 cm of the midline, in more cephalad rib spaces, and in the elderly (older than 60 years).41-43 Furthermore, 1 cadaveric study also demonstrated the presence of arterial collaterals branching off the ICA at multiple intercostal spaces, ranging between 8 cm and 11 cm from the midline.41 This anatomic variability may explain why some have observed low complication and hemothorax rates with an extreme lateral approach.35 Bedside US with color flow Doppler imaging has been used to identify the ICA, with 88% sensitivity compared to CT imaging while adding little to exam time.44,45 Of note, a 37% drop in the rate of hemothorax was observed in 1 study with routine US guidance alone.39
Pleural Pressure Monitoring and Large-Volume Thoracentesis
While normal intrapleural pressures are approximately -5 to -10 cm H2O,46 the presence of a pleural effusion creates a complex interaction between fluid, compressed lung, and chest wall that can increase these pressures.47 During drainage of an effusion, pleural pressures may rapidly drop, provoking re-expansion pulmonary edema (REPE). While rare (0 -1%), clinically-diagnosed REPE is a serious complication that can lead to rapid respiratory failure and death.20,48 REPE is postulated to be caused by increased capillary permeability resulting from inflammation, driven by rapid re-inflation of the lung when exposed to highly negative intrapleural pressures.47,49
Measurement of intrapleural pressure using a water manometer during thoracentesis may minimize REPE by terminating fluid drainage when intrapleural pressure begins to drop rapidly.50,51 A cutoff of -20 cm H2O has been cited repeatedly as safe since being suggested by Light in 1980, but this is based on animal models.50,52 In 1 prospective study of 185 thoracenteses in which manometry was performed, 15% of patients had intrapleural pressure drop to less than -20 cm H2O (at which point the procedure was terminated) but suffered no REPE.50
Manometry is valuable in the identification of an unexpandable or trapped lung when pleural pressures drop rapidly with only minimal fluid volume removal.47,53 Other findings correlated with an unexpandable lung include a negative opening pressure47 and large fluctuations in pressure during the respiratory cycle.54
While development of symptoms (eg, chest pain, cough, or dyspnea) is often used as a surrogate, the correlation between intrapleural pressure and patient symptoms is inconsistent and not a reliable proxy.55 One study found that 22% of patients with chest pain during thoracentesis had intrapleural pressures lower than -20 cm H2O compared with 8.6% of asymptomatic patients,56 but it is unclear if the association is causal.
Thoracentesis is often performed for symptomatic relief and removal of large fluid volume. However, it remains common to halt fluid removal after 1.5 L, a threshold endorsed by BTS.19 While some investigators have suggested that removal of 2 L or more of pleural fluid does not compromise safety,57,58 a 4- to 5-fold rise in the risk of pneumothorax was noted in 2 studies.20,59 when more than 1.5 L of fluid was removed. The majority of these may be related to pneumothorax ex vacuo, a condition in which fluid is drained from the chest, but the lung is unable to expand and fill the space (eg, “trapped lung”), resulting in a persistent pneumothorax. This condition generally does not require treatment.60 When manometry is employed at 200-mL intervals with termination at an intrapleural pressure of less than 20 mm H2O, drainage of 3 L or more has been reported with low rates of pneumothorax and very low rates of REPE.50,51 However, whether this is cause and effect is unknown because REPE is rare, and more work is needed to determine the role of manometry for its prevention.
POSTPROCEDURAL CONSIDERATIONS
Postprocedure Imaging
Performing an upright CXR following thoracentesis is a practice that remains routinely done by many practitioners to monitor for complications. Such imaging was also endorsed by the American Thoracic Society guidelines.61 However, more recent data question the utility of this practice. Multiple studies have confirmed that post-thoracentesis CXR is unnecessary unless clinical suspicion for pneumothorax or REPE is present.36,58,62,63 The BTS guidelines also advocate this approach.19 Interestingly, a potentially more effective way to screen for postprocedure complications is through bedside US, which has been shown to be more sensitive than CXR in detecting pneumothorax.64 In 1 study of 185 patients, bedside US demonstrated a sensitivity of 88% and a specificity of 97% for diagnosing pneumothorax in patients with adequate quality scans, with positive and negative likelihood ratios of 55 and 0.17, respectively.65
DISCUSSION
Thoracentesis remains a core procedural skill for hospitalists, critical care physicians, and emergency physicians. It is the foundational component when investigating and treating pleural effusions. When the most current training, techniques, and technology are used, data suggest this procedure is safe to perform at the bedside. Our review highlights these strategies and evaluates which aspects might be most applicable to clinical practice.
Our findings have several implications for those who perform this procedure. First, appropriate training is central to procedural safety, and both simulation and direct observation by procedural experts have been shown by multiple investigators to improve knowledge and skill. This training should integrate the use of US in performing a focused thoracic exam.
Second, recommendations regarding coagulopathy and a “safe cutoff” of an INR less than 1.5 or platelets greater than 50,000/µL had limited evidentiary support. Rather, multiple studies suggest no difference in bleeding risk following thoracentesis with an INR as high as 3.0 and platelets greater than 25,000/µL. Furthermore, prophylactic transfusion with fresh frozen plasma or platelets before thoracentesis did not alter bleeding risk and exposes patients to transfusion complications. Thus, routine use of this practice can no longer be recommended. Third, further research is needed to understand the bleeding risk for patients on antiplatelet medications, heparin products, and also direct oral anticoagulants, given the growing popularity in their use and the potential consequences of even temporary cessation. Regarding patients on mechanical ventilation, thoracentesis demonstrated no difference in complication rates vs. the general population, and its performance in this population is encouraged when clinically indicated.
Intraprocedural considerations include the use of bedside US. Due to multiple benefits including effusion characterization, puncture site localization, and significantly lower rates of pneumothorax, the standard of care should be to perform thoracentesis with US guidance. Both use of US to mark an effusion immediately prior to puncture or in real time during needle insertion demonstrated benefit; however, it is unclear if 1 method is superior because no direct comparison studies were found. Further work is needed to investigate this potential.
Our review suggests that the location and course of the ICA is variable, especially near the midline, in the elderly, and in higher intercostal spaces, leaving it vulnerable to laceration. We recommend physicians only attempt thoracentesis at least 6 cm lateral to the midline due to ICA tortuosity and, ideally, 12 cm lateral, to avoid the presence of collaterals. Although only 2 small-scale studies were found pertaining to the use of US in identifying the ICA, we encourage physicians to consider learning how to screen for its presence as a part of their routine thoracic US exam in the area underlying the planned puncture site.
Manometry is beneficial because it can diagnose a nonexpandable lung and allows for pleural pressure monitoring.52,53 A simple U-shaped manometer can be constructed from intravenous tubing included in most thoracentesis kits, which adds little to overall procedure time. While low rates of REPE have been observed when terminating thoracentesis if pressures drop below -20 cm H2O or chest pain develops, neither measure appears to have reliable predictive value, limiting clinical utility. Further work is required to determine if a “safe pressure cutoff” exists. In general, we recommend the use of manometry when a nonexpandable (trapped) lung is suspected, because large drops in intrapleural pressure, a negative opening pressure, and respiratory variation can help confirm the diagnosis and avoid pneumothorax ex vacuo or unnecessary procedures in the future. As this condition appears to be more common in the setting of larger effusions, use of manometry when large-volume thoracenteses are planned is also reasonable.
Postprocedurally, routine imaging after thoracentesis is not recommended unless there is objective concern for complication. When indicated, bedside US is better positioned for this role compared with CXR, because it is more sensitive in detecting pneumothorax, provides instantaneous results, and avoids radiation exposure.
Our review has limitations. First, we searched only for articles between defined time periods, restricted our search to a single database, and excluded non-English articles. This has the potential to introduce selection bias, as nonprimary articles that fall within our time restrictions may cite older studies that are outside our search range. To minimize this effect, we performed a critical review of all included studies, especially nonprimary articles. Second, despite the focus of our search strategy to identify any articles related to patient safety and adverse events, we cannot guarantee that all relevant articles for any particular complication or risk factor were captured given the lack of more specific search terms. Third, although we performed a systematic search of the literature, we did not perform a formal systematic review or formally grade included studies. As the goal of our review was to categorize and operationalize clinical aspects, this approach was necessary, and we acknowledge that the quality of studies is variable. Lastly, we aimed to generate clinical recommendations for physicians performing thoracentesis at the bedside; others reviewing this literature may find or emphasize different aspects relevant to practice outside this setting.
In conclusion, evaluation and treatment of pleural effusions with bedside thoracentesis is an important skill for physicians of many disciplines. The evidence presented in this review will help inform the process and ensure patient safety. Physicians should consider incorporating these recommendations into their practice.
The authors thank Whitney Townsend, MLIS, health sciences informationist, for assistance with serial literature searches.
Disclosure
Nothing to report.
1. Kasper DL. Harrison's Principles of Internal Medicine. 19th ed. New York, NY: McGraw Hill Education; 2015.
2. Celik B, Sahin E, Nadir A, Kaptanoglu M. Iatrogenic pneumothorax: etiology, incidence and risk factors. Thorac Cardiovasc Surg. 2009;57(5):286-290. PubMed
3. Hooper CE, Welham SA, Maskell NA, Soc BT. Pleural procedures and patient safety: a national BTS audit of practice. Thorax. 2015;70(2):189-191. PubMed
4. Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest. 2003;123(2):436-441. PubMed
5. Patel IJ, Davidson JC, Nikolic B, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23(6):727-736. PubMed
6. Wraight WM, Tweedie DJ, Parkin IG. Neurovascular anatomy and variation in the fourth, fifth, and sixth intercostal spaces in the mid-axillary line: a cadaveric study in respect of chest drain insertion. Clin Anat. 2005;18(5):346-349. PubMed
7. Duncan DR, Morgenthaler TI, Ryu JH, Daniels CE. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest. 2009;135(5):1315-1320. PubMed
8. Grover S, Currier PF, Elinoff JM, Mouchantaf KJ, Katz JT, McMahon GT. Development of a test to evaluate residents' knowledge of medical procedures. J Hosp Med. 2009;4(7):430-432. PubMed
9. Promes SB, Chudgar SM, Grochowski CO, et al. Gaps in procedural experience and competency in medical school graduates. Acad Emerg Med. 2009;16 Suppl 2:S58-62. PubMed
10. Huang GC, Smith CC, Gordon CE, et al. Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures. Am J Med. 2006;119(1):71 e17-24. PubMed
11. Lagan J, Cutts L, Zaidi S, Benton I, Rylance J. Are we failing our trainees in providing opportunities to attain procedural confidence? Br J Hosp Med (Lond). 2015;76(2):105-108. PubMed
12. Wayne DB, Barsuk JH, O'Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54. PubMed
13. Lenchus JD. End of the "see one, do one, teach one" era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
14. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. PubMed
15. Jiang G, Chen H, Wang S, et al. Learning curves and long-term outcome of simulation-based thoracentesis training for medical students. BMC Med Educ. 2011;11:39. PubMed
16. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis: preliminary results. Am J Med Qual. 2013;28(3):220-226. PubMed
17. Salamonsen M, McGrath D, Steiler G, Ware R, Colt H, Fielding D. A new instrument to assess physician skill at thoracic ultrasound, including pleural effusion markup. Chest. 2013;144(3):930-934. PubMed
18. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31(2):164-171. PubMed
19. Havelock T, Teoh R, Laws D, Gleeson F, Group BTSPDG. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii61-76. PubMed
20. Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax. 2015;70(2):127-132. PubMed
21. Puchalski J. Thoracentesis and the risks for bleeding: a new era. Curr Opin Pulm Med. 2014;20(4):377-384. PubMed
22. Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest. 2013;144(2):456-463. PubMed
23. Zalt MB, Bechara RI, Parks C, Berkowitz DM. Effect of routine clopidogrel use on bleeding complications after ultrasound-guided thoracentesis. J Bronchology Interv Pulmonol. 2012;19(4):284-287. PubMed
24. Mahmood K, Shofer SL, Moser BK, Argento AC, Smathers EC, Wahidi MM. Hemorrhagic complications of thoracentesis and small-bore chest tube placement in patients taking clopidogrel. Ann Am Thorac Soc. 2014;11(1):73-79. PubMed
25. Goligher EC, Leis JA, Fowler RA, Pinto R, Adhikari NK, Ferguson ND. Utility and safety of draining pleural effusions in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care. 2011;15(1):R46. PubMed
26. Fartoukh M, Azoulay E, Galliot R, et al. Clinically documented pleural effusions in medical ICU patients: how useful is routine thoracentesis? Chest. 2002;121(1):178-184. PubMed
27. Cervini P, Hesley GK, Thompson RL, Sampathkumar P, Knudsen JM. Incidence of infectious complications after an ultrasound-guided intervention. AJR Am J Roentgenol. 2010;195(4):846-850. PubMed
28. Mimoz O, Chopra V, Timsit JF. What's new in catheter-related infection: skin cleansing and skin antisepsis. Intensive Care Med. 2016;42(11):1784-1786. PubMed
29. Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015;10(12):811-816. PubMed
30. Feller-Kopman D. Ultrasound-guided thoracentesis. Chest. 2006;129(6):1709-1714. PubMed
31. Shojaee S, Argento AC. Ultrasound-guided pleural access. Semin Respir Crit Care Med. 2014;35(6):693-705. PubMed
32. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170(4):332-339. PubMed
33. Perazzo A, Gatto P, Barlascini C, Ferrari-Bravo M, Nicolini A. Can ultrasound guidance reduce the risk of pneumothorax following thoracentesis? J Bras Pneumol. 2014;40(1):6-12. PubMed
34. Cavanna L, Mordenti P, Berte R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. PubMed
35. Soldati G, Smargiassi A, Inchingolo R, Sher S, Valente S, Corbo GM. Ultrasound-guided pleural puncture in supine or recumbent lateral position - feasibility study. Multidiscip Respir Med. 2013;8(1):18. PubMed
36. Pihlajamaa K, Bode MK, Puumalainen T, Lehtimaki A, Marjelund S, Tikkakoski T. Pneumothorax and the value of chest radiography after ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(8):828-832. PubMed
37. Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound. 2005;33(9):442-446. PubMed
38. Zanforlin A, Gavelli G, Oboldi D, Galletti S. Ultrasound-guided thoracenthesis: the V-point as a site for optimal drainage positioning. Eur Rev Med Pharmacol Sci. 2013;17(1):25-28. PubMed
39. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40(3):135-141. PubMed
40. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. PubMed
41. Shurtleff E, Olinger A. Posterior intercostal artery tortuosity and collateral branch points: a cadaveric study. Folia Morphol (Warsz). 2012;71(4):245-251. PubMed
42. Helm EJ, Rahman NM, Talakoub O, Fox DL, Gleeson FV. Course and variation of the intercostal artery by CT scan. Chest. 2013;143(3):634-639. PubMed
43. Yoneyama H, Arahata M, Temaru R, Ishizaka S, Minami S. Evaluation of the risk of intercostal artery laceration during thoracentesis in elderly patients by using 3D-CT angiography. Intern Med. 2010;49(4):289-292. PubMed
44. Salamonsen M, Ellis S, Paul E, Steinke K, Fielding D. Thoracic ultrasound demonstrates variable location of the intercostal artery. Respiration. 2012;83(4):323-329. PubMed
45. Salamonsen M, Dobeli K, McGrath D, et al. Physician-performed ultrasound can accurately screen for a vulnerable intercostal artery prior to chest drainage procedures. Respirology. 2013;18(6):942-947. PubMed
46. Grippi MA. Fishman's pulmonary diseases and disorders. Fifth edition. ed. New York: McGraw-Hill Education; 2015.
47. Huggins JT, Doelken P. Pleural manometry. Clin Chest Med. 2006;27(2):229-240. PubMed
48. Echevarria C, Twomey D, Dunning J, Chanda B. Does re-expansion pulmonary oedema exist? Interact Cardiovasc Thorac Surg. 2008;7(3):485-489. PubMed
49. Sue RD, Matthay MA, Ware LB. Hydrostatic mechanisms may contribute to the pathogenesis of human re-expansion pulmonary edema. Intensive Care Med. 2004;30(10):1921-1926. PubMed
50. Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84(5):1656-1661. PubMed
51. Villena V, Lopez-Encuentra A, Pozo F, De-Pablo A, Martin-Escribano P. Measurement of pleural pressure during therapeutic thoracentesis. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1534-1538. PubMed
52. Doelken P, Huggins JT, Pastis NJ, Sahn SA. Pleural manometry: technique and clinical implications. Chest. 2004;126(6):1764-1769. PubMed
53. Feller-Kopman D. Therapeutic thoracentesis: the role of ultrasound and pleural manometry. Curr Opin Pulm Med. 2007;13(4):312-318. PubMed
54. Boshuizen RC, Sinaasappel M, Vincent AD, Goldfinger V, Farag S, van den Heuvel MM. Pleural pressure swing and lung expansion after malignant pleural effusion drainage: the benefits of high-temporal resolution pleural manometry. J Bronchology Interv Pulmonol. 2013;20(3):200-205. PubMed
55. Pannu J, DePew ZS, Mullon JJ, Daniels CE, Hagen CE, Maldonado F. Impact of pleural manometry on the development of chest discomfort during thoracentesis: a symptom-based study. J Bronchology Interv Pulmonol. 2014;21(4):306-313. PubMed
56. Feller-Kopman D, Walkey A, Berkowitz D, Ernst A. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest. 2006;129(6):1556-1560. PubMed
57. Abunasser J, Brown R. Safety of large-volume thoracentesis. Conn Med. 2010;74(1):23-26. PubMed
58. Mynarek G, Brabrand K, Jakobsen JA, Kolbenstvedt A. Complications following ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(5):519-522. PubMed
59. Josephson T, Nordenskjold CA, Larsson J, Rosenberg LU, Kaijser M. Amount drained at ultrasound-guided thoracentesis and risk of pneumothorax. Acta Radiol. 2009;50(1):42-47. PubMed
60. Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest. 2006;130(4):1173-1184. PubMed
61. Sokolowski JW Jr, Burgher LW, Jones FL Jr, Patterson JR, Selecky PA. Guidelines for thoracentesis and needle biopsy of the pleura. This position paper of the American Thoracic Society was adopted by the ATS Board of Directors, June 1988. Am Rev Respir Dis. 1989;140(1):257-258. PubMed
62. Jones PW, Moyers JP, Rogers JT, Rodriguez RM, Lee YC, Light RW. Ultrasound-guided thoracentesis: is it a safer method? Chest. 2003;123(2):418-423. PubMed
63. Petersen WG, Zimmerman R. Limited utility of chest radiograph after thoracentesis. Chest. 2000;117(4):1038-1042. PubMed
64. Sachdeva A, Shepherd RW, Lee HJ. Thoracentesis and thoracic ultrasound: state of the art in 2013. Clin Chest Med. 2013;34(1):1-9. PubMed
65. Shostak E, Brylka D, Krepp J, Pua B, Sanders A. Bedside sonography for detection of postprocedure pneumothorax. J Ultrasound Med. 2013;32(6):1003-1009. PubMed
1. Kasper DL. Harrison's Principles of Internal Medicine. 19th ed. New York, NY: McGraw Hill Education; 2015.
2. Celik B, Sahin E, Nadir A, Kaptanoglu M. Iatrogenic pneumothorax: etiology, incidence and risk factors. Thorac Cardiovasc Surg. 2009;57(5):286-290. PubMed
3. Hooper CE, Welham SA, Maskell NA, Soc BT. Pleural procedures and patient safety: a national BTS audit of practice. Thorax. 2015;70(2):189-191. PubMed
4. Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest. 2003;123(2):436-441. PubMed
5. Patel IJ, Davidson JC, Nikolic B, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23(6):727-736. PubMed
6. Wraight WM, Tweedie DJ, Parkin IG. Neurovascular anatomy and variation in the fourth, fifth, and sixth intercostal spaces in the mid-axillary line: a cadaveric study in respect of chest drain insertion. Clin Anat. 2005;18(5):346-349. PubMed
7. Duncan DR, Morgenthaler TI, Ryu JH, Daniels CE. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest. 2009;135(5):1315-1320. PubMed
8. Grover S, Currier PF, Elinoff JM, Mouchantaf KJ, Katz JT, McMahon GT. Development of a test to evaluate residents' knowledge of medical procedures. J Hosp Med. 2009;4(7):430-432. PubMed
9. Promes SB, Chudgar SM, Grochowski CO, et al. Gaps in procedural experience and competency in medical school graduates. Acad Emerg Med. 2009;16 Suppl 2:S58-62. PubMed
10. Huang GC, Smith CC, Gordon CE, et al. Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures. Am J Med. 2006;119(1):71 e17-24. PubMed
11. Lagan J, Cutts L, Zaidi S, Benton I, Rylance J. Are we failing our trainees in providing opportunities to attain procedural confidence? Br J Hosp Med (Lond). 2015;76(2):105-108. PubMed
12. Wayne DB, Barsuk JH, O'Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54. PubMed
13. Lenchus JD. End of the "see one, do one, teach one" era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
14. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. PubMed
15. Jiang G, Chen H, Wang S, et al. Learning curves and long-term outcome of simulation-based thoracentesis training for medical students. BMC Med Educ. 2011;11:39. PubMed
16. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis: preliminary results. Am J Med Qual. 2013;28(3):220-226. PubMed
17. Salamonsen M, McGrath D, Steiler G, Ware R, Colt H, Fielding D. A new instrument to assess physician skill at thoracic ultrasound, including pleural effusion markup. Chest. 2013;144(3):930-934. PubMed
18. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31(2):164-171. PubMed
19. Havelock T, Teoh R, Laws D, Gleeson F, Group BTSPDG. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii61-76. PubMed
20. Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax. 2015;70(2):127-132. PubMed
21. Puchalski J. Thoracentesis and the risks for bleeding: a new era. Curr Opin Pulm Med. 2014;20(4):377-384. PubMed
22. Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest. 2013;144(2):456-463. PubMed
23. Zalt MB, Bechara RI, Parks C, Berkowitz DM. Effect of routine clopidogrel use on bleeding complications after ultrasound-guided thoracentesis. J Bronchology Interv Pulmonol. 2012;19(4):284-287. PubMed
24. Mahmood K, Shofer SL, Moser BK, Argento AC, Smathers EC, Wahidi MM. Hemorrhagic complications of thoracentesis and small-bore chest tube placement in patients taking clopidogrel. Ann Am Thorac Soc. 2014;11(1):73-79. PubMed
25. Goligher EC, Leis JA, Fowler RA, Pinto R, Adhikari NK, Ferguson ND. Utility and safety of draining pleural effusions in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care. 2011;15(1):R46. PubMed
26. Fartoukh M, Azoulay E, Galliot R, et al. Clinically documented pleural effusions in medical ICU patients: how useful is routine thoracentesis? Chest. 2002;121(1):178-184. PubMed
27. Cervini P, Hesley GK, Thompson RL, Sampathkumar P, Knudsen JM. Incidence of infectious complications after an ultrasound-guided intervention. AJR Am J Roentgenol. 2010;195(4):846-850. PubMed
28. Mimoz O, Chopra V, Timsit JF. What's new in catheter-related infection: skin cleansing and skin antisepsis. Intensive Care Med. 2016;42(11):1784-1786. PubMed
29. Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015;10(12):811-816. PubMed
30. Feller-Kopman D. Ultrasound-guided thoracentesis. Chest. 2006;129(6):1709-1714. PubMed
31. Shojaee S, Argento AC. Ultrasound-guided pleural access. Semin Respir Crit Care Med. 2014;35(6):693-705. PubMed
32. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170(4):332-339. PubMed
33. Perazzo A, Gatto P, Barlascini C, Ferrari-Bravo M, Nicolini A. Can ultrasound guidance reduce the risk of pneumothorax following thoracentesis? J Bras Pneumol. 2014;40(1):6-12. PubMed
34. Cavanna L, Mordenti P, Berte R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. PubMed
35. Soldati G, Smargiassi A, Inchingolo R, Sher S, Valente S, Corbo GM. Ultrasound-guided pleural puncture in supine or recumbent lateral position - feasibility study. Multidiscip Respir Med. 2013;8(1):18. PubMed
36. Pihlajamaa K, Bode MK, Puumalainen T, Lehtimaki A, Marjelund S, Tikkakoski T. Pneumothorax and the value of chest radiography after ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(8):828-832. PubMed
37. Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound. 2005;33(9):442-446. PubMed
38. Zanforlin A, Gavelli G, Oboldi D, Galletti S. Ultrasound-guided thoracenthesis: the V-point as a site for optimal drainage positioning. Eur Rev Med Pharmacol Sci. 2013;17(1):25-28. PubMed
39. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40(3):135-141. PubMed
40. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. PubMed
41. Shurtleff E, Olinger A. Posterior intercostal artery tortuosity and collateral branch points: a cadaveric study. Folia Morphol (Warsz). 2012;71(4):245-251. PubMed
42. Helm EJ, Rahman NM, Talakoub O, Fox DL, Gleeson FV. Course and variation of the intercostal artery by CT scan. Chest. 2013;143(3):634-639. PubMed
43. Yoneyama H, Arahata M, Temaru R, Ishizaka S, Minami S. Evaluation of the risk of intercostal artery laceration during thoracentesis in elderly patients by using 3D-CT angiography. Intern Med. 2010;49(4):289-292. PubMed
44. Salamonsen M, Ellis S, Paul E, Steinke K, Fielding D. Thoracic ultrasound demonstrates variable location of the intercostal artery. Respiration. 2012;83(4):323-329. PubMed
45. Salamonsen M, Dobeli K, McGrath D, et al. Physician-performed ultrasound can accurately screen for a vulnerable intercostal artery prior to chest drainage procedures. Respirology. 2013;18(6):942-947. PubMed
46. Grippi MA. Fishman's pulmonary diseases and disorders. Fifth edition. ed. New York: McGraw-Hill Education; 2015.
47. Huggins JT, Doelken P. Pleural manometry. Clin Chest Med. 2006;27(2):229-240. PubMed
48. Echevarria C, Twomey D, Dunning J, Chanda B. Does re-expansion pulmonary oedema exist? Interact Cardiovasc Thorac Surg. 2008;7(3):485-489. PubMed
49. Sue RD, Matthay MA, Ware LB. Hydrostatic mechanisms may contribute to the pathogenesis of human re-expansion pulmonary edema. Intensive Care Med. 2004;30(10):1921-1926. PubMed
50. Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84(5):1656-1661. PubMed
51. Villena V, Lopez-Encuentra A, Pozo F, De-Pablo A, Martin-Escribano P. Measurement of pleural pressure during therapeutic thoracentesis. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1534-1538. PubMed
52. Doelken P, Huggins JT, Pastis NJ, Sahn SA. Pleural manometry: technique and clinical implications. Chest. 2004;126(6):1764-1769. PubMed
53. Feller-Kopman D. Therapeutic thoracentesis: the role of ultrasound and pleural manometry. Curr Opin Pulm Med. 2007;13(4):312-318. PubMed
54. Boshuizen RC, Sinaasappel M, Vincent AD, Goldfinger V, Farag S, van den Heuvel MM. Pleural pressure swing and lung expansion after malignant pleural effusion drainage: the benefits of high-temporal resolution pleural manometry. J Bronchology Interv Pulmonol. 2013;20(3):200-205. PubMed
55. Pannu J, DePew ZS, Mullon JJ, Daniels CE, Hagen CE, Maldonado F. Impact of pleural manometry on the development of chest discomfort during thoracentesis: a symptom-based study. J Bronchology Interv Pulmonol. 2014;21(4):306-313. PubMed
56. Feller-Kopman D, Walkey A, Berkowitz D, Ernst A. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest. 2006;129(6):1556-1560. PubMed
57. Abunasser J, Brown R. Safety of large-volume thoracentesis. Conn Med. 2010;74(1):23-26. PubMed
58. Mynarek G, Brabrand K, Jakobsen JA, Kolbenstvedt A. Complications following ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(5):519-522. PubMed
59. Josephson T, Nordenskjold CA, Larsson J, Rosenberg LU, Kaijser M. Amount drained at ultrasound-guided thoracentesis and risk of pneumothorax. Acta Radiol. 2009;50(1):42-47. PubMed
60. Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest. 2006;130(4):1173-1184. PubMed
61. Sokolowski JW Jr, Burgher LW, Jones FL Jr, Patterson JR, Selecky PA. Guidelines for thoracentesis and needle biopsy of the pleura. This position paper of the American Thoracic Society was adopted by the ATS Board of Directors, June 1988. Am Rev Respir Dis. 1989;140(1):257-258. PubMed
62. Jones PW, Moyers JP, Rogers JT, Rodriguez RM, Lee YC, Light RW. Ultrasound-guided thoracentesis: is it a safer method? Chest. 2003;123(2):418-423. PubMed
63. Petersen WG, Zimmerman R. Limited utility of chest radiograph after thoracentesis. Chest. 2000;117(4):1038-1042. PubMed
64. Sachdeva A, Shepherd RW, Lee HJ. Thoracentesis and thoracic ultrasound: state of the art in 2013. Clin Chest Med. 2013;34(1):1-9. PubMed
65. Shostak E, Brylka D, Krepp J, Pua B, Sanders A. Bedside sonography for detection of postprocedure pneumothorax. J Ultrasound Med. 2013;32(6):1003-1009. PubMed
© 2017 Society of Hospital Medicine
Standardized attending rounds to improve the patient experience: A pragmatic cluster randomized controlled trial
Patient experience has recently received heightened attention given evidence supporting an association between patient experience and quality of care,1 and the coupling of patient satisfaction to reimbursement rates for Medicare patients.2 Patient experience is often assessed through surveys of patient satisfaction, which correlates with patient perceptions of nurse and physician communication.3 Teaching hospitals introduce variables that may impact communication, including the involvement of multiple levels of care providers and competing patient care vs. educational priorities. Patients admitted to teaching services express decreased satisfaction with coordination and overall care compared with patients on nonteaching services.4
Clinical supervision of trainees on teaching services is primarily achieved through attending rounds (AR), where patients’ clinical presentations and management are discussed with an attending physician. Poor communication during AR may negatively affect the patient experience through inefficient care coordination among the inter-professional care team or through implementation of interventions without patients’ knowledge or input.5-11 Although patient engagement in rounds has been associated with higher patient satisfaction with rounds,12-19 AR and case presentations often occur at a distance from the patient’s bedside.20,21 Furthermore, AR vary in the time allotted per patient and the extent of participation of nurses and other allied health professionals. Standardized bedside rounding processes have been shown to improve efficiency, decrease daily resident work hours,22 and improve nurse-physician teamwork.23
Despite these benefits, recent prospective studies of bedside AR interventions have not improved patient satisfaction with rounds. One involved the implementation of interprofessional patient-centered bedside rounds on a nonteaching service,24 while the other evaluated the impact of integrating athletic principles into multidisciplinary work rounds.25 Work at our institution had sought to develop AR practice recommendations to foster an optimal patient experience, while maintaining provider workflow efficiency, facilitating interdisciplinary communication, and advancing trainee education.26 Using these AR recommendations, we conducted a prospective randomized controlled trial to evaluate the impact of implementing a standardized bedside AR model on patient satisfaction with rounds. We also assessed attending physician and trainee satisfaction with rounds, and perceived and actual AR duration.
METHODS
Setting and Participants
This trial was conducted on the internal medicine teaching service of the University of California San Francisco Medical Center from September 3, 2013 to November 27, 2013. The service is comprised of 8 teams, with a total average daily census of 80 to 90 patients. Teams are comprised of an attending physician, a senior resident (in the second or third year of residency training), 2 interns, and a third- and/or fourth-year medical student.
This trial, which was approved by the University of California, San Francisco Committee on Human Research (UCSF CHR) and was registered with ClinicalTrials.gov (NCT01931553), was classified under Quality Improvement and did not require informed consent of patients or providers.
Intervention Description
We conducted a cluster randomized trial to evaluate the impact of a bundled set of 5 AR practice recommendations, adapted from published work,26 on patient experience, as well as on attending and trainee satisfaction: 1) huddling to establish the rounding schedule and priorities; 2) conducting bedside rounds; 3) integrating bedside nurses; 4) completing real-time order entry using bedside computers; 5) updating the whiteboard in each patient’s room with care plan information.
At the beginning of each month, study investigators (Nader Najafi and Bradley Monash) led a 1.5-hour workshop to train attending physicians and trainees allocated to the intervention arm on the recommended AR practices. Participants also received informational handouts to be referenced during AR. Attending physicians and trainees randomized to the control arm continued usual rounding practices. Control teams were notified that there would be observers on rounds but were not informed of the study aims.
Randomization and Team Assignments
The medicine service was divided into 2 arms, each comprised of 4 teams. Using a coin flip, Cluster 1 (Teams A, B, C and D) was randomized to the intervention, and Cluster 2 (Teams E, F, G and H) was randomized to the control. This design was pragmatically chosen to ensure that 1 team from each arm would admit patients daily. Allocation concealment of attending physicians and trainees was not possible given the nature of the intervention. Patients were blinded to study arm allocation.
MEASURES AND OUTCOMES
Adherence to Practice Recommendations
Thirty premedical students served as volunteer AR auditors. Each auditor received orientation and training in data collection techniques during a single 2-hour workshop. The auditors, blinded to study arm allocation, independently observed morning AR during weekdays and recorded the completion of the following elements as a dichotomous (yes/no) outcome: pre-rounds huddle, participation of nurse in AR, real-time order entry, and whiteboard use. They recorded the duration of AR per day for each team (minutes) and the rounding model for each patient rounding encounter during AR (bedside, hallway, or card flip).23 Bedside rounds were defined as presentation and discussion of the patient care plan in the presence of the patient. Hallway rounds were defined as presentation and discussion of the patient care plan partially outside the patient’s room and partially in the presence of the patient. Card-flip rounds were defined as presentation and discussion of the patient care plan entirely outside of the patient’s room without the team seeing the patient together. Two auditors simultaneously observed a random subset of patient-rounding encounters to evaluate inter-rater reliability, and the concordance between auditor observations was good (Pearson correlation = 0.66).27
Patient-Related Outcomes
The primary outcome was patient satisfaction with AR, assessed using a survey adapted from published work.12,14,28,29 Patients were approached to complete the questionnaire after they had experienced at least 1 AR. Patients were excluded if they were non-English-speaking, unavailable (eg, off the unit for testing or treatment), in isolation, or had impaired mental status. For patients admitted multiple times during the study period, only the first questionnaire was used. Survey questions included patient involvement in decision-making, quality of communication between patient and medicine team, and the perception that the medicine team cared about the patient. Patients were asked to state their level of agreement with each item on a 5-point Likert scale. We obtained data on patient demographics from administrative datasets.
Healthcare Provider Outcomes
Attending physicians and trainees on service for at least 7 consecutive days were sent an electronic survey, adapted from published work.25,30 Questions assessed satisfaction with AR, perceived value of bedside rounds, and extent of patient and nursing involvement.Level of agreement with each item was captured on a continuous scale; 0 = strongly disagree to 100 = strongly agree, or from 0 (far too little) to 100 (far too much), with 50 equating to “about right.” Attending physicians and trainees were also asked to estimate the average duration of AR (in minutes).
Statistical Analyses
Analyses were blinded to study arm allocation and followed intention-to-treat principles. One attending physician crossed over from intervention to control arm; patient surveys associated with this attending (n = 4) were excluded to avoid contamination. No trainees crossed over.
Demographic and clinical characteristics of patients who completed the survey are reported (Appendix). To compare patient satisfaction scores, we used a random-effects regression model to account for correlation among responses within teams within randomized clusters, defining teams by attending physician. As this correlation was negligible and not statistically significant, we did not adjust ordinary linear regression models for clustering. Given observed differences in patient characteristics, we adjusted for a number of covariates (eg, age, gender, insurance payer, race, marital status, trial group arm).
We conducted simple linear regression for attending and trainee satisfaction comparisons between arms, adjusting only for trainee type (eg, resident, intern, and medical student).
We compared the frequency with which intervention and control teams adhered to the 5 recommended AR practices using chi-square tests. We used independent Student’s t tests to compare total duration of AR by teams within each arm, as well as mean time spent per patient.
This trial had a fixed number of arms (n = 2), each of fixed size (n = 600), based on the average monthly inpatient census on the medicine service. This fixed sample size, with 80% power and α = 0.05, will be able to detect a 0.16 difference in patient satisfaction scores between groups.
All analyses were conducted using SAS® v 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
We observed 241 AR involving 1855 patient rounding encounters in the intervention arm and 264 AR involving 1903 patient rounding encounters in the control arm (response rates shown in Figure 1).
Patient Satisfaction and Clinical Outcomes
Five hundred ninety-five patients were allocated to the intervention arm and 605 were allocated to the control arm (Figure 1). Mean age, gender, race, marital status, primary language, and insurance provider did not differ between intervention and control arms (Table 1).
Patients in the intervention arm reported significantly higher satisfaction with AR and felt more cared for by their medicine team (Table 2).
Actual and Perceived Duration of Attending Rounds
The intervention shortened the total duration of AR by 8 minutes on average (143 vs. 151 minutes, P = 0.052) and the time spent per patient by 4 minutes on average (19 vs. 23 minutes, P < 0.001). Despite this, trainees in the intervention arm perceived AR to last longer (mean estimated time: 167 min vs. 152 min, P < 0.001).
Healthcare Provider Outcomes
We observed 79 attending physicians and trainees in the intervention arm and 78 in the control arm, with survey response rates shown in Figure 1. Attending physicians in the intervention and the control arms reported high levels of satisfaction with the quality of AR (Table 2). Attending physicians in the intervention arm were more likely to report an appropriate level of patient involvement and nurse involvement.
Although trainees in the intervention and control arms reported high levels of satisfaction with the quality of AR, trainees in the intervention arm reported lower satisfaction with AR compared with control arm trainees (Table 2). Trainees in the intervention arm reported that AR involved less autonomy, efficiency, and teaching. Trainees in the intervention arm also scored patient involvement more towards the “far too much” end of the scale compared with “about right” in the control arm. However, trainees in the intervention arm perceived nurse involvement closer to “about right,” as opposed to “far too little” in the control arm.
CONCLUSION/DISCUSSION
Training internal medicine teams to adhere to 5 recommended AR practices increased patient satisfaction with AR and the perception that patients were more cared for by their medicine team. Despite the intervention potentially shortening the duration of AR, attending physicians and trainees perceived AR to last longer, and trainee satisfaction with AR decreased.
Teams in the intervention arm adhered to all recommended rounding practices at higher rates than the control teams. Although intervention teams rounded at the bedside 53% of the time, they were encouraged to bedside round only on patients who desired to participate in rounds, were not altered, and for whom the clinical discussion was not too sensitive to occur at the bedside. Of the recommended rounding behaviors, the lowest adherence was seen with whiteboard use.
A major component of the intervention was to move the clinical presentation to the patient’s bedside. Most patients prefer being included in rounds and partaking in trainee education.12-19,28,29,31-33 Patients may also perceive that more time is spent with them during bedside case presentations,14,28 and exposure to providers conferring on their care may enhance patient confidence in the care being delivered.12 Although a recent study of patient-centered bedside rounding on a nonteaching service did not result in increased patient satisfaction,24 teaching services may offer more opportunities for improvement in care coordination and communication.4
Other aspects of the intervention may have contributed to increased patient satisfaction with AR. The pre-rounds huddle may have helped teams prioritize which patients required more time or would benefit most from bedside rounds. The involvement of nurses in AR may have bolstered communication and team dynamics, enhancing the patient’s perception of interprofessional collaboration. Real-time order entry might have led to more efficient implementation of the care plan, and whiteboard use may have helped to keep patients abreast of the care plan.
Patients in the intervention arm felt more cared for by their medicine teams but did not report improvements in communication or in shared decision-making. Prior work highlights that limited patient engagement, activation, and shared decision-making may occur during AR.24,34 Patient-physician communication during AR is challenged by time pressures and competing priorities, including the “need” for trainees to demonstrate their medical knowledge and clinical skills. Efforts that encourage bedside rounding should include communication training with respect to patient engagement and shared decision-making.
Attending physicians reported positive attitudes toward bedside rounding, consistent with prior studies.13,21,31 However, trainees in the intervention arm expressed decreased satisfaction with AR, estimating that AR took longer and reporting too much patient involvement. Prior studies reflect similar bedside-rounding concerns, including perceived workflow inefficiencies, infringement on teaching opportunities, and time constraints.12,20,35 Trainees are under intense time pressures to complete their work, attend educational conferences, and leave the hospital to attend afternoon clinic or to comply with duty-hour restrictions. Trainees value succinctness,12,35,36 so the perception that intervention AR lasted longer likely contributed to trainee dissatisfaction.
Reduced trainee satisfaction with intervention AR may have also stemmed from the perception of decreased autonomy and less teaching, both valued by trainees.20,35,36 The intervention itself reduced trainee autonomy because usual practice at our hospital involves residents deciding where and how to round. Attending physician presence at the bedside during rounds may have further infringed on trainee autonomy if the patient looked to the attending for answers, or if the attending was seen as the AR leader. Attending physicians may mitigate the risk of compromising trainee autonomy by allowing the trainee to speak first, ensuring the trainee is positioned closer to, and at eye level with, the patient, and redirecting patient questions to the trainee as appropriate. Optimizing trainee experience with bedside AR requires preparation and training of attending physicians, who may feel inadequately prepared to lead bedside rounds and conduct bedside teaching.37 Faculty must learn how to preserve team efficiency, create a safe, nonpunitive bedside environment that fosters the trainee-patient relationship, and ensure rounds remain educational.36,38,39
The intervention reduced the average time spent on AR and time spent per patient. Studies examining the relationship between bedside rounding and duration of rounds have yielded mixed results: some have demonstrated no effect of bedside rounds on rounding time,28,40 while others report longer rounding times.37 The pre-rounds huddle and real-time order writing may have enhanced workflow efficiency.
Our study has several limitations. These results reflect the experience of a single large academic medical center and may not be generalizable to other settings. Although overall patient response to the survey was low and may not be representative of the entire patient population, response rates in the intervention and control arms were equivalent. Non-English speaking patients may have preferences that were not reflected in our survey results, and we did not otherwise quantify individual reasons for survey noncompletion. The presence of auditors on AR may have introduced observer bias. There may have been crossover effect; however, observed prevalence of individual practices remained low in the control arm. The 1.5-hour workshop may have inadequately equipped trainees with the complex skills required to lead and participate in bedside rounding, and more training, experience, and feedback may have yielded different results. For instance, residents with more exposure to bedside rounding express greater appreciation of its role in education and patient care.20 While adherence to some of the recommended practices remained low, we did not employ a full range of change-management techniques. Instead, we opted for a “low intensity” intervention (eg, single workshop, handouts) that relied on voluntary adoption by medicine teams and that we hoped other institutions could reproduce. Finally, we did not assess the relative impact of individual rounding behaviors on the measured outcomes.
In conclusion, training medicine teams to adhere to a standardized bedside AR model increased patient satisfaction with rounds. Concomitant trainee dissatisfaction may require further experience and training of attending physicians and trainees to ensure successful adoption.
Acknowledgements
We would like to thank all patients, providers, and trainees who participated in this study. We would also like to acknowledge the following volunteer auditors who observed teams daily: Arianna Abundo, Elahhe Afkhamnejad, Yolanda Banuelos, Laila Fozoun, Soe Yupar Khin, Tam Thien Le, Wing Sum Li, Yaqiao Li, Mengyao Liu, Tzyy-Harn Lo, Shynh-Herng Lo, David Lowe, Danoush Paborji, Sa Nan Park, Urmila Powale, Redha Fouad Qabazard, Monique Quiroz, John-Luke Marcelo Rivera, Manfred Roy Luna Salvador, Tobias Gowen Squier-Roper, Flora Yan Ting, Francesca Natasha T. Tizon, Emily Claire Trautner, Stephen Weiner, Alice Wilson, Kimberly Woo, Bingling J Wu, Johnny Wu, Brenda Yee. Statistical expertise was provided by Joan Hilton from the UCSF Clinical and Translational Science Institute (CTSI), which is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR000004. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Thanks also to Oralia Schatzman, Andrea Mazzini, and Erika Huie for their administrative support, and John Hillman for data-related support. Special thanks to Kirsten Kangelaris and Andrew Auerbach for their valuable feedback throughout, and to Maria Novelero and Robert Wachter for their divisional support of this project.
Disclosure
The authors report no financial conflicts of interest.
1. Doyle C, Lennox L, Bell D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open. 2013;3(1):1-18. PubMed
2. Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) Fact Sheet. August 2013. Centers for Medicare and Medicaid Services (CMS). Baltimore, MD.http://www.hcahpsonline.org/files/August_2013_HCAHPS_Fact_Sheet3.pdf. Accessed December 1, 2015.
3. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17:41-48. PubMed
4. Wray CM, Flores A, Padula WV, Prochaska MT, Meltzer DO, Arora VM. Measuring patient experiences on hospitalist and teaching services: Patient responses to a 30-day postdischarge questionnaire. J Hosp Med. 2016;11(2):99-104. PubMed
5. Bharwani AM, Harris GC, Southwick FS. Perspective: A business school view of medical interprofessional rounds: transforming rounding groups into rounding teams. Acad Med. 2012;87(12):1768-1771. PubMed
6. Chand DV. Observational study using the tools of lean six sigma to improve the efficiency of the resident rounding process. J Grad Med Educ. 2011;3(2):144-150. PubMed
7. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084-1089. PubMed
8. Weber H, Stöckli M, Nübling M, Langewitz WA. Communication during ward rounds in internal medicine. An analysis of patient-nurse-physician interactions using RIAS. Patient Educ Couns. 2007;67(3):343-348. PubMed
9. McMahon GT, Katz JT, Thorndike ME, Levy BD, Loscalzo J. Evaluation of a redesign initiative in an internal-medicine residency. N Engl J Med. 2010;362(14):1304-1311. PubMed
10. Amoss J. Attending rounds: where do we go from here?: comment on “Attending rounds in the current era”. JAMA Intern Med. 2013;173(12):1089-1090. PubMed
11. Curley C, McEachern JE, Speroff T. A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement. Med Care. 1998;36(suppl 8):AS4-A12. PubMed
12. Wang-Cheng RM, Barnas GP, Sigmann P, Riendl PA, Young MJ. Bedside case presentations: why patients like them but learners don’t. J Gen Intern Med. 1989;4(4):284-287. PubMed
13. Chauke, HL, Pattinson RC. Ward rounds—bedside or conference room? S Afr Med J. 2006;96(5):398-400. PubMed
14. Lehmann LS, Brancati FL, Chen MC, Roter D, Dobs AS. The effect of bedside case presentations on patients’ perceptions of their medical care. N Engl J Med. 1997;336(16):336, 1150-1155. PubMed
15. Simons RJ, Baily RG, Zelis R, Zwillich CW. The physiologic and psychological effects of the bedside presentation. N Engl J Med. 1989;321(18):1273-1275. PubMed
16. Wise TN, Feldheim D, Mann LS, Boyle E, Rustgi VK. Patients’ reactions to house staff work rounds. Psychosomatics. 1985;26(8):669-672. PubMed
17. Linfors EW, Neelon FA. Sounding Boards. The case of bedside rounds. N Engl J Med. 1980;303(21):1230-1233. PubMed
18. Nair BR, Coughlan JL, Hensley MJ. Student and patient perspectives on bedside teaching. Med Educ. 1997;31(5):341-346. PubMed
19. Romano J. Patients’ attitudes and behavior in ward round teaching. JAMA. 1941;117(9):664-667.
20. Gonzalo JD, Masters PA, Simons RJ, Chuang CH. Attending rounds and bedside case presentations: medical student and medicine resident experiences and attitudes. Teach Learn Med. 2009;21(2):105-110. PubMed
21. Shoeb M, Khanna R, Fang M, et al. Internal medicine rounding practices and the Accreditation Council for Graduate Medical Education core competencies. J Hosp Med. 2014;9(4):239-243. PubMed
22. Calderon AS, Blackmore CC, Williams BL, et al. Transforming ward rounds through rounding-in-flow. J Grad Med Educ. 2014;6(4):750-755. PubMed
23. Henkin S, Chon TY, Christopherson ML, Halvorsen AJ, Worden LM, Ratelle JT. Improving nurse-physician teamwork through interprofessional bedside rounding. J Multidiscip Healthc. 2016;9:201-205. PubMed
24. O’Leary KJ, Killarney A, Hansen LO, et al. Effect of patient-centred bedside rounds on hospitalised patients’ decision control, activation and satisfaction with care. BMJ Qual Saf. 2016;25:921-928. PubMed
25. Southwick F, Lewis M, Treloar D, et al. Applying athletic principles to medical rounds to improve teaching and patient care. Acad Med. 2014;89(7):1018-1023. PubMed
26. Najafi N, Monash B, Mourad M, et al. Improving attending rounds: Qualitative reflections from multidisciplinary providers. Hosp Pract (1995). 2015;43(3):186-190. PubMed
27. Altman DG. Practical Statistics For Medical Research. Boca Raton, FL: Chapman & Hall/CRC; 2006.
28. Gonzalo JD, Chuang CH, Huang G, Smith C. The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792-798. PubMed
29. Fletcher KE, Rankey DS, Stern DT. Bedside interactions from the other side of the bedrail. J Gen Intern Med. 2005;20(1):58-61. PubMed
30. Gatorounds: Applying Championship Athletic Principles to Healthcare. University of Florida Health. http://gatorounds.med.ufl.edu/surveys/. Accessed March 1, 2013.
31. Gonzalo JD, Heist BS, Duffy BL, et al. The value of bedside rounds: a multicenter qualitative study. Teach Learn Med. 2013;25(4):326-333. PubMed
32. Rogers HD, Carline JD, Paauw DS. Examination room presentations in general internal medicine clinic: patients’ and students’ perceptions. Acad Med. 2003;78(9):945-949. PubMed
33. Fletcher KE, Furney SL, Stern DT. Patients speak: what’s really important about bedside interactions with physician teams. Teach Learn Med. 2007;19(2):120-127. PubMed
34. Satterfield JM, Bereknyei S, Hilton JF, et al. The prevalence of social and behavioral topics and related educational opportunities during attending rounds. Acad Med. 2014; 89(11):1548-1557. PubMed
35. Kroenke K, Simmons JO, Copley JB, Smith C. Attending rounds: a survey of physician attitudes. J Gen Intern Med. 1990;5(3):229-233. PubMed
36. Castiglioni A, Shewchuk RM, Willett LL, Heudebert GR, Centor RM. A pilot study using nominal group technique to assess residents’ perceptions of successful attending rounds. J Gen Intern Med. 2008;23(7):1060-1065. PubMed
37. Crumlish CM, Yialamas MA, McMahon GT. Quantification of bedside teaching by an academic hospitalist group. J Hosp Med. 2009;4(5):304-307. PubMed
38. Gonzalo JD, Wolpaw DR, Lehman E, Chuang CH. Patient-centered interprofessional collaborative care: factors associated with bedside interprofessional rounds. J Gen Intern Med. 2014;29(7):1040-1047. PubMed
39. Roy B, Castiglioni A, Kraemer RR, et al. Using cognitive mapping to define key domains for successful attending rounds. J Gen Intern Med. 2012;27(11):1492-1498. PubMed
40. Bhansali P, Birch S, Campbell JK, et al. A time-motion study of inpatient rounds using a family-centered rounds model. Hosp Pediatr. 2013;3(1):31-38. PubMed
Patient experience has recently received heightened attention given evidence supporting an association between patient experience and quality of care,1 and the coupling of patient satisfaction to reimbursement rates for Medicare patients.2 Patient experience is often assessed through surveys of patient satisfaction, which correlates with patient perceptions of nurse and physician communication.3 Teaching hospitals introduce variables that may impact communication, including the involvement of multiple levels of care providers and competing patient care vs. educational priorities. Patients admitted to teaching services express decreased satisfaction with coordination and overall care compared with patients on nonteaching services.4
Clinical supervision of trainees on teaching services is primarily achieved through attending rounds (AR), where patients’ clinical presentations and management are discussed with an attending physician. Poor communication during AR may negatively affect the patient experience through inefficient care coordination among the inter-professional care team or through implementation of interventions without patients’ knowledge or input.5-11 Although patient engagement in rounds has been associated with higher patient satisfaction with rounds,12-19 AR and case presentations often occur at a distance from the patient’s bedside.20,21 Furthermore, AR vary in the time allotted per patient and the extent of participation of nurses and other allied health professionals. Standardized bedside rounding processes have been shown to improve efficiency, decrease daily resident work hours,22 and improve nurse-physician teamwork.23
Despite these benefits, recent prospective studies of bedside AR interventions have not improved patient satisfaction with rounds. One involved the implementation of interprofessional patient-centered bedside rounds on a nonteaching service,24 while the other evaluated the impact of integrating athletic principles into multidisciplinary work rounds.25 Work at our institution had sought to develop AR practice recommendations to foster an optimal patient experience, while maintaining provider workflow efficiency, facilitating interdisciplinary communication, and advancing trainee education.26 Using these AR recommendations, we conducted a prospective randomized controlled trial to evaluate the impact of implementing a standardized bedside AR model on patient satisfaction with rounds. We also assessed attending physician and trainee satisfaction with rounds, and perceived and actual AR duration.
METHODS
Setting and Participants
This trial was conducted on the internal medicine teaching service of the University of California San Francisco Medical Center from September 3, 2013 to November 27, 2013. The service is comprised of 8 teams, with a total average daily census of 80 to 90 patients. Teams are comprised of an attending physician, a senior resident (in the second or third year of residency training), 2 interns, and a third- and/or fourth-year medical student.
This trial, which was approved by the University of California, San Francisco Committee on Human Research (UCSF CHR) and was registered with ClinicalTrials.gov (NCT01931553), was classified under Quality Improvement and did not require informed consent of patients or providers.
Intervention Description
We conducted a cluster randomized trial to evaluate the impact of a bundled set of 5 AR practice recommendations, adapted from published work,26 on patient experience, as well as on attending and trainee satisfaction: 1) huddling to establish the rounding schedule and priorities; 2) conducting bedside rounds; 3) integrating bedside nurses; 4) completing real-time order entry using bedside computers; 5) updating the whiteboard in each patient’s room with care plan information.
At the beginning of each month, study investigators (Nader Najafi and Bradley Monash) led a 1.5-hour workshop to train attending physicians and trainees allocated to the intervention arm on the recommended AR practices. Participants also received informational handouts to be referenced during AR. Attending physicians and trainees randomized to the control arm continued usual rounding practices. Control teams were notified that there would be observers on rounds but were not informed of the study aims.
Randomization and Team Assignments
The medicine service was divided into 2 arms, each comprised of 4 teams. Using a coin flip, Cluster 1 (Teams A, B, C and D) was randomized to the intervention, and Cluster 2 (Teams E, F, G and H) was randomized to the control. This design was pragmatically chosen to ensure that 1 team from each arm would admit patients daily. Allocation concealment of attending physicians and trainees was not possible given the nature of the intervention. Patients were blinded to study arm allocation.
MEASURES AND OUTCOMES
Adherence to Practice Recommendations
Thirty premedical students served as volunteer AR auditors. Each auditor received orientation and training in data collection techniques during a single 2-hour workshop. The auditors, blinded to study arm allocation, independently observed morning AR during weekdays and recorded the completion of the following elements as a dichotomous (yes/no) outcome: pre-rounds huddle, participation of nurse in AR, real-time order entry, and whiteboard use. They recorded the duration of AR per day for each team (minutes) and the rounding model for each patient rounding encounter during AR (bedside, hallway, or card flip).23 Bedside rounds were defined as presentation and discussion of the patient care plan in the presence of the patient. Hallway rounds were defined as presentation and discussion of the patient care plan partially outside the patient’s room and partially in the presence of the patient. Card-flip rounds were defined as presentation and discussion of the patient care plan entirely outside of the patient’s room without the team seeing the patient together. Two auditors simultaneously observed a random subset of patient-rounding encounters to evaluate inter-rater reliability, and the concordance between auditor observations was good (Pearson correlation = 0.66).27
Patient-Related Outcomes
The primary outcome was patient satisfaction with AR, assessed using a survey adapted from published work.12,14,28,29 Patients were approached to complete the questionnaire after they had experienced at least 1 AR. Patients were excluded if they were non-English-speaking, unavailable (eg, off the unit for testing or treatment), in isolation, or had impaired mental status. For patients admitted multiple times during the study period, only the first questionnaire was used. Survey questions included patient involvement in decision-making, quality of communication between patient and medicine team, and the perception that the medicine team cared about the patient. Patients were asked to state their level of agreement with each item on a 5-point Likert scale. We obtained data on patient demographics from administrative datasets.
Healthcare Provider Outcomes
Attending physicians and trainees on service for at least 7 consecutive days were sent an electronic survey, adapted from published work.25,30 Questions assessed satisfaction with AR, perceived value of bedside rounds, and extent of patient and nursing involvement.Level of agreement with each item was captured on a continuous scale; 0 = strongly disagree to 100 = strongly agree, or from 0 (far too little) to 100 (far too much), with 50 equating to “about right.” Attending physicians and trainees were also asked to estimate the average duration of AR (in minutes).
Statistical Analyses
Analyses were blinded to study arm allocation and followed intention-to-treat principles. One attending physician crossed over from intervention to control arm; patient surveys associated with this attending (n = 4) were excluded to avoid contamination. No trainees crossed over.
Demographic and clinical characteristics of patients who completed the survey are reported (Appendix). To compare patient satisfaction scores, we used a random-effects regression model to account for correlation among responses within teams within randomized clusters, defining teams by attending physician. As this correlation was negligible and not statistically significant, we did not adjust ordinary linear regression models for clustering. Given observed differences in patient characteristics, we adjusted for a number of covariates (eg, age, gender, insurance payer, race, marital status, trial group arm).
We conducted simple linear regression for attending and trainee satisfaction comparisons between arms, adjusting only for trainee type (eg, resident, intern, and medical student).
We compared the frequency with which intervention and control teams adhered to the 5 recommended AR practices using chi-square tests. We used independent Student’s t tests to compare total duration of AR by teams within each arm, as well as mean time spent per patient.
This trial had a fixed number of arms (n = 2), each of fixed size (n = 600), based on the average monthly inpatient census on the medicine service. This fixed sample size, with 80% power and α = 0.05, will be able to detect a 0.16 difference in patient satisfaction scores between groups.
All analyses were conducted using SAS® v 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
We observed 241 AR involving 1855 patient rounding encounters in the intervention arm and 264 AR involving 1903 patient rounding encounters in the control arm (response rates shown in Figure 1).
Patient Satisfaction and Clinical Outcomes
Five hundred ninety-five patients were allocated to the intervention arm and 605 were allocated to the control arm (Figure 1). Mean age, gender, race, marital status, primary language, and insurance provider did not differ between intervention and control arms (Table 1).
Patients in the intervention arm reported significantly higher satisfaction with AR and felt more cared for by their medicine team (Table 2).
Actual and Perceived Duration of Attending Rounds
The intervention shortened the total duration of AR by 8 minutes on average (143 vs. 151 minutes, P = 0.052) and the time spent per patient by 4 minutes on average (19 vs. 23 minutes, P < 0.001). Despite this, trainees in the intervention arm perceived AR to last longer (mean estimated time: 167 min vs. 152 min, P < 0.001).
Healthcare Provider Outcomes
We observed 79 attending physicians and trainees in the intervention arm and 78 in the control arm, with survey response rates shown in Figure 1. Attending physicians in the intervention and the control arms reported high levels of satisfaction with the quality of AR (Table 2). Attending physicians in the intervention arm were more likely to report an appropriate level of patient involvement and nurse involvement.
Although trainees in the intervention and control arms reported high levels of satisfaction with the quality of AR, trainees in the intervention arm reported lower satisfaction with AR compared with control arm trainees (Table 2). Trainees in the intervention arm reported that AR involved less autonomy, efficiency, and teaching. Trainees in the intervention arm also scored patient involvement more towards the “far too much” end of the scale compared with “about right” in the control arm. However, trainees in the intervention arm perceived nurse involvement closer to “about right,” as opposed to “far too little” in the control arm.
CONCLUSION/DISCUSSION
Training internal medicine teams to adhere to 5 recommended AR practices increased patient satisfaction with AR and the perception that patients were more cared for by their medicine team. Despite the intervention potentially shortening the duration of AR, attending physicians and trainees perceived AR to last longer, and trainee satisfaction with AR decreased.
Teams in the intervention arm adhered to all recommended rounding practices at higher rates than the control teams. Although intervention teams rounded at the bedside 53% of the time, they were encouraged to bedside round only on patients who desired to participate in rounds, were not altered, and for whom the clinical discussion was not too sensitive to occur at the bedside. Of the recommended rounding behaviors, the lowest adherence was seen with whiteboard use.
A major component of the intervention was to move the clinical presentation to the patient’s bedside. Most patients prefer being included in rounds and partaking in trainee education.12-19,28,29,31-33 Patients may also perceive that more time is spent with them during bedside case presentations,14,28 and exposure to providers conferring on their care may enhance patient confidence in the care being delivered.12 Although a recent study of patient-centered bedside rounding on a nonteaching service did not result in increased patient satisfaction,24 teaching services may offer more opportunities for improvement in care coordination and communication.4
Other aspects of the intervention may have contributed to increased patient satisfaction with AR. The pre-rounds huddle may have helped teams prioritize which patients required more time or would benefit most from bedside rounds. The involvement of nurses in AR may have bolstered communication and team dynamics, enhancing the patient’s perception of interprofessional collaboration. Real-time order entry might have led to more efficient implementation of the care plan, and whiteboard use may have helped to keep patients abreast of the care plan.
Patients in the intervention arm felt more cared for by their medicine teams but did not report improvements in communication or in shared decision-making. Prior work highlights that limited patient engagement, activation, and shared decision-making may occur during AR.24,34 Patient-physician communication during AR is challenged by time pressures and competing priorities, including the “need” for trainees to demonstrate their medical knowledge and clinical skills. Efforts that encourage bedside rounding should include communication training with respect to patient engagement and shared decision-making.
Attending physicians reported positive attitudes toward bedside rounding, consistent with prior studies.13,21,31 However, trainees in the intervention arm expressed decreased satisfaction with AR, estimating that AR took longer and reporting too much patient involvement. Prior studies reflect similar bedside-rounding concerns, including perceived workflow inefficiencies, infringement on teaching opportunities, and time constraints.12,20,35 Trainees are under intense time pressures to complete their work, attend educational conferences, and leave the hospital to attend afternoon clinic or to comply with duty-hour restrictions. Trainees value succinctness,12,35,36 so the perception that intervention AR lasted longer likely contributed to trainee dissatisfaction.
Reduced trainee satisfaction with intervention AR may have also stemmed from the perception of decreased autonomy and less teaching, both valued by trainees.20,35,36 The intervention itself reduced trainee autonomy because usual practice at our hospital involves residents deciding where and how to round. Attending physician presence at the bedside during rounds may have further infringed on trainee autonomy if the patient looked to the attending for answers, or if the attending was seen as the AR leader. Attending physicians may mitigate the risk of compromising trainee autonomy by allowing the trainee to speak first, ensuring the trainee is positioned closer to, and at eye level with, the patient, and redirecting patient questions to the trainee as appropriate. Optimizing trainee experience with bedside AR requires preparation and training of attending physicians, who may feel inadequately prepared to lead bedside rounds and conduct bedside teaching.37 Faculty must learn how to preserve team efficiency, create a safe, nonpunitive bedside environment that fosters the trainee-patient relationship, and ensure rounds remain educational.36,38,39
The intervention reduced the average time spent on AR and time spent per patient. Studies examining the relationship between bedside rounding and duration of rounds have yielded mixed results: some have demonstrated no effect of bedside rounds on rounding time,28,40 while others report longer rounding times.37 The pre-rounds huddle and real-time order writing may have enhanced workflow efficiency.
Our study has several limitations. These results reflect the experience of a single large academic medical center and may not be generalizable to other settings. Although overall patient response to the survey was low and may not be representative of the entire patient population, response rates in the intervention and control arms were equivalent. Non-English speaking patients may have preferences that were not reflected in our survey results, and we did not otherwise quantify individual reasons for survey noncompletion. The presence of auditors on AR may have introduced observer bias. There may have been crossover effect; however, observed prevalence of individual practices remained low in the control arm. The 1.5-hour workshop may have inadequately equipped trainees with the complex skills required to lead and participate in bedside rounding, and more training, experience, and feedback may have yielded different results. For instance, residents with more exposure to bedside rounding express greater appreciation of its role in education and patient care.20 While adherence to some of the recommended practices remained low, we did not employ a full range of change-management techniques. Instead, we opted for a “low intensity” intervention (eg, single workshop, handouts) that relied on voluntary adoption by medicine teams and that we hoped other institutions could reproduce. Finally, we did not assess the relative impact of individual rounding behaviors on the measured outcomes.
In conclusion, training medicine teams to adhere to a standardized bedside AR model increased patient satisfaction with rounds. Concomitant trainee dissatisfaction may require further experience and training of attending physicians and trainees to ensure successful adoption.
Acknowledgements
We would like to thank all patients, providers, and trainees who participated in this study. We would also like to acknowledge the following volunteer auditors who observed teams daily: Arianna Abundo, Elahhe Afkhamnejad, Yolanda Banuelos, Laila Fozoun, Soe Yupar Khin, Tam Thien Le, Wing Sum Li, Yaqiao Li, Mengyao Liu, Tzyy-Harn Lo, Shynh-Herng Lo, David Lowe, Danoush Paborji, Sa Nan Park, Urmila Powale, Redha Fouad Qabazard, Monique Quiroz, John-Luke Marcelo Rivera, Manfred Roy Luna Salvador, Tobias Gowen Squier-Roper, Flora Yan Ting, Francesca Natasha T. Tizon, Emily Claire Trautner, Stephen Weiner, Alice Wilson, Kimberly Woo, Bingling J Wu, Johnny Wu, Brenda Yee. Statistical expertise was provided by Joan Hilton from the UCSF Clinical and Translational Science Institute (CTSI), which is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR000004. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Thanks also to Oralia Schatzman, Andrea Mazzini, and Erika Huie for their administrative support, and John Hillman for data-related support. Special thanks to Kirsten Kangelaris and Andrew Auerbach for their valuable feedback throughout, and to Maria Novelero and Robert Wachter for their divisional support of this project.
Disclosure
The authors report no financial conflicts of interest.
Patient experience has recently received heightened attention given evidence supporting an association between patient experience and quality of care,1 and the coupling of patient satisfaction to reimbursement rates for Medicare patients.2 Patient experience is often assessed through surveys of patient satisfaction, which correlates with patient perceptions of nurse and physician communication.3 Teaching hospitals introduce variables that may impact communication, including the involvement of multiple levels of care providers and competing patient care vs. educational priorities. Patients admitted to teaching services express decreased satisfaction with coordination and overall care compared with patients on nonteaching services.4
Clinical supervision of trainees on teaching services is primarily achieved through attending rounds (AR), where patients’ clinical presentations and management are discussed with an attending physician. Poor communication during AR may negatively affect the patient experience through inefficient care coordination among the inter-professional care team or through implementation of interventions without patients’ knowledge or input.5-11 Although patient engagement in rounds has been associated with higher patient satisfaction with rounds,12-19 AR and case presentations often occur at a distance from the patient’s bedside.20,21 Furthermore, AR vary in the time allotted per patient and the extent of participation of nurses and other allied health professionals. Standardized bedside rounding processes have been shown to improve efficiency, decrease daily resident work hours,22 and improve nurse-physician teamwork.23
Despite these benefits, recent prospective studies of bedside AR interventions have not improved patient satisfaction with rounds. One involved the implementation of interprofessional patient-centered bedside rounds on a nonteaching service,24 while the other evaluated the impact of integrating athletic principles into multidisciplinary work rounds.25 Work at our institution had sought to develop AR practice recommendations to foster an optimal patient experience, while maintaining provider workflow efficiency, facilitating interdisciplinary communication, and advancing trainee education.26 Using these AR recommendations, we conducted a prospective randomized controlled trial to evaluate the impact of implementing a standardized bedside AR model on patient satisfaction with rounds. We also assessed attending physician and trainee satisfaction with rounds, and perceived and actual AR duration.
METHODS
Setting and Participants
This trial was conducted on the internal medicine teaching service of the University of California San Francisco Medical Center from September 3, 2013 to November 27, 2013. The service is comprised of 8 teams, with a total average daily census of 80 to 90 patients. Teams are comprised of an attending physician, a senior resident (in the second or third year of residency training), 2 interns, and a third- and/or fourth-year medical student.
This trial, which was approved by the University of California, San Francisco Committee on Human Research (UCSF CHR) and was registered with ClinicalTrials.gov (NCT01931553), was classified under Quality Improvement and did not require informed consent of patients or providers.
Intervention Description
We conducted a cluster randomized trial to evaluate the impact of a bundled set of 5 AR practice recommendations, adapted from published work,26 on patient experience, as well as on attending and trainee satisfaction: 1) huddling to establish the rounding schedule and priorities; 2) conducting bedside rounds; 3) integrating bedside nurses; 4) completing real-time order entry using bedside computers; 5) updating the whiteboard in each patient’s room with care plan information.
At the beginning of each month, study investigators (Nader Najafi and Bradley Monash) led a 1.5-hour workshop to train attending physicians and trainees allocated to the intervention arm on the recommended AR practices. Participants also received informational handouts to be referenced during AR. Attending physicians and trainees randomized to the control arm continued usual rounding practices. Control teams were notified that there would be observers on rounds but were not informed of the study aims.
Randomization and Team Assignments
The medicine service was divided into 2 arms, each comprised of 4 teams. Using a coin flip, Cluster 1 (Teams A, B, C and D) was randomized to the intervention, and Cluster 2 (Teams E, F, G and H) was randomized to the control. This design was pragmatically chosen to ensure that 1 team from each arm would admit patients daily. Allocation concealment of attending physicians and trainees was not possible given the nature of the intervention. Patients were blinded to study arm allocation.
MEASURES AND OUTCOMES
Adherence to Practice Recommendations
Thirty premedical students served as volunteer AR auditors. Each auditor received orientation and training in data collection techniques during a single 2-hour workshop. The auditors, blinded to study arm allocation, independently observed morning AR during weekdays and recorded the completion of the following elements as a dichotomous (yes/no) outcome: pre-rounds huddle, participation of nurse in AR, real-time order entry, and whiteboard use. They recorded the duration of AR per day for each team (minutes) and the rounding model for each patient rounding encounter during AR (bedside, hallway, or card flip).23 Bedside rounds were defined as presentation and discussion of the patient care plan in the presence of the patient. Hallway rounds were defined as presentation and discussion of the patient care plan partially outside the patient’s room and partially in the presence of the patient. Card-flip rounds were defined as presentation and discussion of the patient care plan entirely outside of the patient’s room without the team seeing the patient together. Two auditors simultaneously observed a random subset of patient-rounding encounters to evaluate inter-rater reliability, and the concordance between auditor observations was good (Pearson correlation = 0.66).27
Patient-Related Outcomes
The primary outcome was patient satisfaction with AR, assessed using a survey adapted from published work.12,14,28,29 Patients were approached to complete the questionnaire after they had experienced at least 1 AR. Patients were excluded if they were non-English-speaking, unavailable (eg, off the unit for testing or treatment), in isolation, or had impaired mental status. For patients admitted multiple times during the study period, only the first questionnaire was used. Survey questions included patient involvement in decision-making, quality of communication between patient and medicine team, and the perception that the medicine team cared about the patient. Patients were asked to state their level of agreement with each item on a 5-point Likert scale. We obtained data on patient demographics from administrative datasets.
Healthcare Provider Outcomes
Attending physicians and trainees on service for at least 7 consecutive days were sent an electronic survey, adapted from published work.25,30 Questions assessed satisfaction with AR, perceived value of bedside rounds, and extent of patient and nursing involvement.Level of agreement with each item was captured on a continuous scale; 0 = strongly disagree to 100 = strongly agree, or from 0 (far too little) to 100 (far too much), with 50 equating to “about right.” Attending physicians and trainees were also asked to estimate the average duration of AR (in minutes).
Statistical Analyses
Analyses were blinded to study arm allocation and followed intention-to-treat principles. One attending physician crossed over from intervention to control arm; patient surveys associated with this attending (n = 4) were excluded to avoid contamination. No trainees crossed over.
Demographic and clinical characteristics of patients who completed the survey are reported (Appendix). To compare patient satisfaction scores, we used a random-effects regression model to account for correlation among responses within teams within randomized clusters, defining teams by attending physician. As this correlation was negligible and not statistically significant, we did not adjust ordinary linear regression models for clustering. Given observed differences in patient characteristics, we adjusted for a number of covariates (eg, age, gender, insurance payer, race, marital status, trial group arm).
We conducted simple linear regression for attending and trainee satisfaction comparisons between arms, adjusting only for trainee type (eg, resident, intern, and medical student).
We compared the frequency with which intervention and control teams adhered to the 5 recommended AR practices using chi-square tests. We used independent Student’s t tests to compare total duration of AR by teams within each arm, as well as mean time spent per patient.
This trial had a fixed number of arms (n = 2), each of fixed size (n = 600), based on the average monthly inpatient census on the medicine service. This fixed sample size, with 80% power and α = 0.05, will be able to detect a 0.16 difference in patient satisfaction scores between groups.
All analyses were conducted using SAS® v 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
We observed 241 AR involving 1855 patient rounding encounters in the intervention arm and 264 AR involving 1903 patient rounding encounters in the control arm (response rates shown in Figure 1).
Patient Satisfaction and Clinical Outcomes
Five hundred ninety-five patients were allocated to the intervention arm and 605 were allocated to the control arm (Figure 1). Mean age, gender, race, marital status, primary language, and insurance provider did not differ between intervention and control arms (Table 1).
Patients in the intervention arm reported significantly higher satisfaction with AR and felt more cared for by their medicine team (Table 2).
Actual and Perceived Duration of Attending Rounds
The intervention shortened the total duration of AR by 8 minutes on average (143 vs. 151 minutes, P = 0.052) and the time spent per patient by 4 minutes on average (19 vs. 23 minutes, P < 0.001). Despite this, trainees in the intervention arm perceived AR to last longer (mean estimated time: 167 min vs. 152 min, P < 0.001).
Healthcare Provider Outcomes
We observed 79 attending physicians and trainees in the intervention arm and 78 in the control arm, with survey response rates shown in Figure 1. Attending physicians in the intervention and the control arms reported high levels of satisfaction with the quality of AR (Table 2). Attending physicians in the intervention arm were more likely to report an appropriate level of patient involvement and nurse involvement.
Although trainees in the intervention and control arms reported high levels of satisfaction with the quality of AR, trainees in the intervention arm reported lower satisfaction with AR compared with control arm trainees (Table 2). Trainees in the intervention arm reported that AR involved less autonomy, efficiency, and teaching. Trainees in the intervention arm also scored patient involvement more towards the “far too much” end of the scale compared with “about right” in the control arm. However, trainees in the intervention arm perceived nurse involvement closer to “about right,” as opposed to “far too little” in the control arm.
CONCLUSION/DISCUSSION
Training internal medicine teams to adhere to 5 recommended AR practices increased patient satisfaction with AR and the perception that patients were more cared for by their medicine team. Despite the intervention potentially shortening the duration of AR, attending physicians and trainees perceived AR to last longer, and trainee satisfaction with AR decreased.
Teams in the intervention arm adhered to all recommended rounding practices at higher rates than the control teams. Although intervention teams rounded at the bedside 53% of the time, they were encouraged to bedside round only on patients who desired to participate in rounds, were not altered, and for whom the clinical discussion was not too sensitive to occur at the bedside. Of the recommended rounding behaviors, the lowest adherence was seen with whiteboard use.
A major component of the intervention was to move the clinical presentation to the patient’s bedside. Most patients prefer being included in rounds and partaking in trainee education.12-19,28,29,31-33 Patients may also perceive that more time is spent with them during bedside case presentations,14,28 and exposure to providers conferring on their care may enhance patient confidence in the care being delivered.12 Although a recent study of patient-centered bedside rounding on a nonteaching service did not result in increased patient satisfaction,24 teaching services may offer more opportunities for improvement in care coordination and communication.4
Other aspects of the intervention may have contributed to increased patient satisfaction with AR. The pre-rounds huddle may have helped teams prioritize which patients required more time or would benefit most from bedside rounds. The involvement of nurses in AR may have bolstered communication and team dynamics, enhancing the patient’s perception of interprofessional collaboration. Real-time order entry might have led to more efficient implementation of the care plan, and whiteboard use may have helped to keep patients abreast of the care plan.
Patients in the intervention arm felt more cared for by their medicine teams but did not report improvements in communication or in shared decision-making. Prior work highlights that limited patient engagement, activation, and shared decision-making may occur during AR.24,34 Patient-physician communication during AR is challenged by time pressures and competing priorities, including the “need” for trainees to demonstrate their medical knowledge and clinical skills. Efforts that encourage bedside rounding should include communication training with respect to patient engagement and shared decision-making.
Attending physicians reported positive attitudes toward bedside rounding, consistent with prior studies.13,21,31 However, trainees in the intervention arm expressed decreased satisfaction with AR, estimating that AR took longer and reporting too much patient involvement. Prior studies reflect similar bedside-rounding concerns, including perceived workflow inefficiencies, infringement on teaching opportunities, and time constraints.12,20,35 Trainees are under intense time pressures to complete their work, attend educational conferences, and leave the hospital to attend afternoon clinic or to comply with duty-hour restrictions. Trainees value succinctness,12,35,36 so the perception that intervention AR lasted longer likely contributed to trainee dissatisfaction.
Reduced trainee satisfaction with intervention AR may have also stemmed from the perception of decreased autonomy and less teaching, both valued by trainees.20,35,36 The intervention itself reduced trainee autonomy because usual practice at our hospital involves residents deciding where and how to round. Attending physician presence at the bedside during rounds may have further infringed on trainee autonomy if the patient looked to the attending for answers, or if the attending was seen as the AR leader. Attending physicians may mitigate the risk of compromising trainee autonomy by allowing the trainee to speak first, ensuring the trainee is positioned closer to, and at eye level with, the patient, and redirecting patient questions to the trainee as appropriate. Optimizing trainee experience with bedside AR requires preparation and training of attending physicians, who may feel inadequately prepared to lead bedside rounds and conduct bedside teaching.37 Faculty must learn how to preserve team efficiency, create a safe, nonpunitive bedside environment that fosters the trainee-patient relationship, and ensure rounds remain educational.36,38,39
The intervention reduced the average time spent on AR and time spent per patient. Studies examining the relationship between bedside rounding and duration of rounds have yielded mixed results: some have demonstrated no effect of bedside rounds on rounding time,28,40 while others report longer rounding times.37 The pre-rounds huddle and real-time order writing may have enhanced workflow efficiency.
Our study has several limitations. These results reflect the experience of a single large academic medical center and may not be generalizable to other settings. Although overall patient response to the survey was low and may not be representative of the entire patient population, response rates in the intervention and control arms were equivalent. Non-English speaking patients may have preferences that were not reflected in our survey results, and we did not otherwise quantify individual reasons for survey noncompletion. The presence of auditors on AR may have introduced observer bias. There may have been crossover effect; however, observed prevalence of individual practices remained low in the control arm. The 1.5-hour workshop may have inadequately equipped trainees with the complex skills required to lead and participate in bedside rounding, and more training, experience, and feedback may have yielded different results. For instance, residents with more exposure to bedside rounding express greater appreciation of its role in education and patient care.20 While adherence to some of the recommended practices remained low, we did not employ a full range of change-management techniques. Instead, we opted for a “low intensity” intervention (eg, single workshop, handouts) that relied on voluntary adoption by medicine teams and that we hoped other institutions could reproduce. Finally, we did not assess the relative impact of individual rounding behaviors on the measured outcomes.
In conclusion, training medicine teams to adhere to a standardized bedside AR model increased patient satisfaction with rounds. Concomitant trainee dissatisfaction may require further experience and training of attending physicians and trainees to ensure successful adoption.
Acknowledgements
We would like to thank all patients, providers, and trainees who participated in this study. We would also like to acknowledge the following volunteer auditors who observed teams daily: Arianna Abundo, Elahhe Afkhamnejad, Yolanda Banuelos, Laila Fozoun, Soe Yupar Khin, Tam Thien Le, Wing Sum Li, Yaqiao Li, Mengyao Liu, Tzyy-Harn Lo, Shynh-Herng Lo, David Lowe, Danoush Paborji, Sa Nan Park, Urmila Powale, Redha Fouad Qabazard, Monique Quiroz, John-Luke Marcelo Rivera, Manfred Roy Luna Salvador, Tobias Gowen Squier-Roper, Flora Yan Ting, Francesca Natasha T. Tizon, Emily Claire Trautner, Stephen Weiner, Alice Wilson, Kimberly Woo, Bingling J Wu, Johnny Wu, Brenda Yee. Statistical expertise was provided by Joan Hilton from the UCSF Clinical and Translational Science Institute (CTSI), which is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR000004. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Thanks also to Oralia Schatzman, Andrea Mazzini, and Erika Huie for their administrative support, and John Hillman for data-related support. Special thanks to Kirsten Kangelaris and Andrew Auerbach for their valuable feedback throughout, and to Maria Novelero and Robert Wachter for their divisional support of this project.
Disclosure
The authors report no financial conflicts of interest.
1. Doyle C, Lennox L, Bell D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open. 2013;3(1):1-18. PubMed
2. Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) Fact Sheet. August 2013. Centers for Medicare and Medicaid Services (CMS). Baltimore, MD.http://www.hcahpsonline.org/files/August_2013_HCAHPS_Fact_Sheet3.pdf. Accessed December 1, 2015.
3. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17:41-48. PubMed
4. Wray CM, Flores A, Padula WV, Prochaska MT, Meltzer DO, Arora VM. Measuring patient experiences on hospitalist and teaching services: Patient responses to a 30-day postdischarge questionnaire. J Hosp Med. 2016;11(2):99-104. PubMed
5. Bharwani AM, Harris GC, Southwick FS. Perspective: A business school view of medical interprofessional rounds: transforming rounding groups into rounding teams. Acad Med. 2012;87(12):1768-1771. PubMed
6. Chand DV. Observational study using the tools of lean six sigma to improve the efficiency of the resident rounding process. J Grad Med Educ. 2011;3(2):144-150. PubMed
7. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084-1089. PubMed
8. Weber H, Stöckli M, Nübling M, Langewitz WA. Communication during ward rounds in internal medicine. An analysis of patient-nurse-physician interactions using RIAS. Patient Educ Couns. 2007;67(3):343-348. PubMed
9. McMahon GT, Katz JT, Thorndike ME, Levy BD, Loscalzo J. Evaluation of a redesign initiative in an internal-medicine residency. N Engl J Med. 2010;362(14):1304-1311. PubMed
10. Amoss J. Attending rounds: where do we go from here?: comment on “Attending rounds in the current era”. JAMA Intern Med. 2013;173(12):1089-1090. PubMed
11. Curley C, McEachern JE, Speroff T. A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement. Med Care. 1998;36(suppl 8):AS4-A12. PubMed
12. Wang-Cheng RM, Barnas GP, Sigmann P, Riendl PA, Young MJ. Bedside case presentations: why patients like them but learners don’t. J Gen Intern Med. 1989;4(4):284-287. PubMed
13. Chauke, HL, Pattinson RC. Ward rounds—bedside or conference room? S Afr Med J. 2006;96(5):398-400. PubMed
14. Lehmann LS, Brancati FL, Chen MC, Roter D, Dobs AS. The effect of bedside case presentations on patients’ perceptions of their medical care. N Engl J Med. 1997;336(16):336, 1150-1155. PubMed
15. Simons RJ, Baily RG, Zelis R, Zwillich CW. The physiologic and psychological effects of the bedside presentation. N Engl J Med. 1989;321(18):1273-1275. PubMed
16. Wise TN, Feldheim D, Mann LS, Boyle E, Rustgi VK. Patients’ reactions to house staff work rounds. Psychosomatics. 1985;26(8):669-672. PubMed
17. Linfors EW, Neelon FA. Sounding Boards. The case of bedside rounds. N Engl J Med. 1980;303(21):1230-1233. PubMed
18. Nair BR, Coughlan JL, Hensley MJ. Student and patient perspectives on bedside teaching. Med Educ. 1997;31(5):341-346. PubMed
19. Romano J. Patients’ attitudes and behavior in ward round teaching. JAMA. 1941;117(9):664-667.
20. Gonzalo JD, Masters PA, Simons RJ, Chuang CH. Attending rounds and bedside case presentations: medical student and medicine resident experiences and attitudes. Teach Learn Med. 2009;21(2):105-110. PubMed
21. Shoeb M, Khanna R, Fang M, et al. Internal medicine rounding practices and the Accreditation Council for Graduate Medical Education core competencies. J Hosp Med. 2014;9(4):239-243. PubMed
22. Calderon AS, Blackmore CC, Williams BL, et al. Transforming ward rounds through rounding-in-flow. J Grad Med Educ. 2014;6(4):750-755. PubMed
23. Henkin S, Chon TY, Christopherson ML, Halvorsen AJ, Worden LM, Ratelle JT. Improving nurse-physician teamwork through interprofessional bedside rounding. J Multidiscip Healthc. 2016;9:201-205. PubMed
24. O’Leary KJ, Killarney A, Hansen LO, et al. Effect of patient-centred bedside rounds on hospitalised patients’ decision control, activation and satisfaction with care. BMJ Qual Saf. 2016;25:921-928. PubMed
25. Southwick F, Lewis M, Treloar D, et al. Applying athletic principles to medical rounds to improve teaching and patient care. Acad Med. 2014;89(7):1018-1023. PubMed
26. Najafi N, Monash B, Mourad M, et al. Improving attending rounds: Qualitative reflections from multidisciplinary providers. Hosp Pract (1995). 2015;43(3):186-190. PubMed
27. Altman DG. Practical Statistics For Medical Research. Boca Raton, FL: Chapman & Hall/CRC; 2006.
28. Gonzalo JD, Chuang CH, Huang G, Smith C. The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792-798. PubMed
29. Fletcher KE, Rankey DS, Stern DT. Bedside interactions from the other side of the bedrail. J Gen Intern Med. 2005;20(1):58-61. PubMed
30. Gatorounds: Applying Championship Athletic Principles to Healthcare. University of Florida Health. http://gatorounds.med.ufl.edu/surveys/. Accessed March 1, 2013.
31. Gonzalo JD, Heist BS, Duffy BL, et al. The value of bedside rounds: a multicenter qualitative study. Teach Learn Med. 2013;25(4):326-333. PubMed
32. Rogers HD, Carline JD, Paauw DS. Examination room presentations in general internal medicine clinic: patients’ and students’ perceptions. Acad Med. 2003;78(9):945-949. PubMed
33. Fletcher KE, Furney SL, Stern DT. Patients speak: what’s really important about bedside interactions with physician teams. Teach Learn Med. 2007;19(2):120-127. PubMed
34. Satterfield JM, Bereknyei S, Hilton JF, et al. The prevalence of social and behavioral topics and related educational opportunities during attending rounds. Acad Med. 2014; 89(11):1548-1557. PubMed
35. Kroenke K, Simmons JO, Copley JB, Smith C. Attending rounds: a survey of physician attitudes. J Gen Intern Med. 1990;5(3):229-233. PubMed
36. Castiglioni A, Shewchuk RM, Willett LL, Heudebert GR, Centor RM. A pilot study using nominal group technique to assess residents’ perceptions of successful attending rounds. J Gen Intern Med. 2008;23(7):1060-1065. PubMed
37. Crumlish CM, Yialamas MA, McMahon GT. Quantification of bedside teaching by an academic hospitalist group. J Hosp Med. 2009;4(5):304-307. PubMed
38. Gonzalo JD, Wolpaw DR, Lehman E, Chuang CH. Patient-centered interprofessional collaborative care: factors associated with bedside interprofessional rounds. J Gen Intern Med. 2014;29(7):1040-1047. PubMed
39. Roy B, Castiglioni A, Kraemer RR, et al. Using cognitive mapping to define key domains for successful attending rounds. J Gen Intern Med. 2012;27(11):1492-1498. PubMed
40. Bhansali P, Birch S, Campbell JK, et al. A time-motion study of inpatient rounds using a family-centered rounds model. Hosp Pediatr. 2013;3(1):31-38. PubMed
1. Doyle C, Lennox L, Bell D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open. 2013;3(1):1-18. PubMed
2. Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) Fact Sheet. August 2013. Centers for Medicare and Medicaid Services (CMS). Baltimore, MD.http://www.hcahpsonline.org/files/August_2013_HCAHPS_Fact_Sheet3.pdf. Accessed December 1, 2015.
3. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17:41-48. PubMed
4. Wray CM, Flores A, Padula WV, Prochaska MT, Meltzer DO, Arora VM. Measuring patient experiences on hospitalist and teaching services: Patient responses to a 30-day postdischarge questionnaire. J Hosp Med. 2016;11(2):99-104. PubMed
5. Bharwani AM, Harris GC, Southwick FS. Perspective: A business school view of medical interprofessional rounds: transforming rounding groups into rounding teams. Acad Med. 2012;87(12):1768-1771. PubMed
6. Chand DV. Observational study using the tools of lean six sigma to improve the efficiency of the resident rounding process. J Grad Med Educ. 2011;3(2):144-150. PubMed
7. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084-1089. PubMed
8. Weber H, Stöckli M, Nübling M, Langewitz WA. Communication during ward rounds in internal medicine. An analysis of patient-nurse-physician interactions using RIAS. Patient Educ Couns. 2007;67(3):343-348. PubMed
9. McMahon GT, Katz JT, Thorndike ME, Levy BD, Loscalzo J. Evaluation of a redesign initiative in an internal-medicine residency. N Engl J Med. 2010;362(14):1304-1311. PubMed
10. Amoss J. Attending rounds: where do we go from here?: comment on “Attending rounds in the current era”. JAMA Intern Med. 2013;173(12):1089-1090. PubMed
11. Curley C, McEachern JE, Speroff T. A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement. Med Care. 1998;36(suppl 8):AS4-A12. PubMed
12. Wang-Cheng RM, Barnas GP, Sigmann P, Riendl PA, Young MJ. Bedside case presentations: why patients like them but learners don’t. J Gen Intern Med. 1989;4(4):284-287. PubMed
13. Chauke, HL, Pattinson RC. Ward rounds—bedside or conference room? S Afr Med J. 2006;96(5):398-400. PubMed
14. Lehmann LS, Brancati FL, Chen MC, Roter D, Dobs AS. The effect of bedside case presentations on patients’ perceptions of their medical care. N Engl J Med. 1997;336(16):336, 1150-1155. PubMed
15. Simons RJ, Baily RG, Zelis R, Zwillich CW. The physiologic and psychological effects of the bedside presentation. N Engl J Med. 1989;321(18):1273-1275. PubMed
16. Wise TN, Feldheim D, Mann LS, Boyle E, Rustgi VK. Patients’ reactions to house staff work rounds. Psychosomatics. 1985;26(8):669-672. PubMed
17. Linfors EW, Neelon FA. Sounding Boards. The case of bedside rounds. N Engl J Med. 1980;303(21):1230-1233. PubMed
18. Nair BR, Coughlan JL, Hensley MJ. Student and patient perspectives on bedside teaching. Med Educ. 1997;31(5):341-346. PubMed
19. Romano J. Patients’ attitudes and behavior in ward round teaching. JAMA. 1941;117(9):664-667.
20. Gonzalo JD, Masters PA, Simons RJ, Chuang CH. Attending rounds and bedside case presentations: medical student and medicine resident experiences and attitudes. Teach Learn Med. 2009;21(2):105-110. PubMed
21. Shoeb M, Khanna R, Fang M, et al. Internal medicine rounding practices and the Accreditation Council for Graduate Medical Education core competencies. J Hosp Med. 2014;9(4):239-243. PubMed
22. Calderon AS, Blackmore CC, Williams BL, et al. Transforming ward rounds through rounding-in-flow. J Grad Med Educ. 2014;6(4):750-755. PubMed
23. Henkin S, Chon TY, Christopherson ML, Halvorsen AJ, Worden LM, Ratelle JT. Improving nurse-physician teamwork through interprofessional bedside rounding. J Multidiscip Healthc. 2016;9:201-205. PubMed
24. O’Leary KJ, Killarney A, Hansen LO, et al. Effect of patient-centred bedside rounds on hospitalised patients’ decision control, activation and satisfaction with care. BMJ Qual Saf. 2016;25:921-928. PubMed
25. Southwick F, Lewis M, Treloar D, et al. Applying athletic principles to medical rounds to improve teaching and patient care. Acad Med. 2014;89(7):1018-1023. PubMed
26. Najafi N, Monash B, Mourad M, et al. Improving attending rounds: Qualitative reflections from multidisciplinary providers. Hosp Pract (1995). 2015;43(3):186-190. PubMed
27. Altman DG. Practical Statistics For Medical Research. Boca Raton, FL: Chapman & Hall/CRC; 2006.
28. Gonzalo JD, Chuang CH, Huang G, Smith C. The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792-798. PubMed
29. Fletcher KE, Rankey DS, Stern DT. Bedside interactions from the other side of the bedrail. J Gen Intern Med. 2005;20(1):58-61. PubMed
30. Gatorounds: Applying Championship Athletic Principles to Healthcare. University of Florida Health. http://gatorounds.med.ufl.edu/surveys/. Accessed March 1, 2013.
31. Gonzalo JD, Heist BS, Duffy BL, et al. The value of bedside rounds: a multicenter qualitative study. Teach Learn Med. 2013;25(4):326-333. PubMed
32. Rogers HD, Carline JD, Paauw DS. Examination room presentations in general internal medicine clinic: patients’ and students’ perceptions. Acad Med. 2003;78(9):945-949. PubMed
33. Fletcher KE, Furney SL, Stern DT. Patients speak: what’s really important about bedside interactions with physician teams. Teach Learn Med. 2007;19(2):120-127. PubMed
34. Satterfield JM, Bereknyei S, Hilton JF, et al. The prevalence of social and behavioral topics and related educational opportunities during attending rounds. Acad Med. 2014; 89(11):1548-1557. PubMed
35. Kroenke K, Simmons JO, Copley JB, Smith C. Attending rounds: a survey of physician attitudes. J Gen Intern Med. 1990;5(3):229-233. PubMed
36. Castiglioni A, Shewchuk RM, Willett LL, Heudebert GR, Centor RM. A pilot study using nominal group technique to assess residents’ perceptions of successful attending rounds. J Gen Intern Med. 2008;23(7):1060-1065. PubMed
37. Crumlish CM, Yialamas MA, McMahon GT. Quantification of bedside teaching by an academic hospitalist group. J Hosp Med. 2009;4(5):304-307. PubMed
38. Gonzalo JD, Wolpaw DR, Lehman E, Chuang CH. Patient-centered interprofessional collaborative care: factors associated with bedside interprofessional rounds. J Gen Intern Med. 2014;29(7):1040-1047. PubMed
39. Roy B, Castiglioni A, Kraemer RR, et al. Using cognitive mapping to define key domains for successful attending rounds. J Gen Intern Med. 2012;27(11):1492-1498. PubMed
40. Bhansali P, Birch S, Campbell JK, et al. A time-motion study of inpatient rounds using a family-centered rounds model. Hosp Pediatr. 2013;3(1):31-38. PubMed
© 2017 Society of Hospital Medicine
Resident SBML for Thoracentesis
There has been a nationwide shift away from general internists performing bedside thoracenteses and toward referring them to pulmonology and interventional radiology services.[1] Aligning with this trend, the American Board of Internal Medicine now only requires that internal medicine (IM)trained physicians understand the indications, complications, and management of bedside procedures.[2]
However, thoracentesis is still considered a core competency of practicing hospitalists, the fastest growing field within general IM.[3] Furthermore, evidence suggests that thoracenteses done by general internists have high patient satisfaction, reduce hospital length of stay, are more cost‐effective, and are as safe as those done by consultants.[4, 5, 6] It is thus important to understand the reasons for referrals to specialty services and to investigate potential interventions that increase performance of procedures by internists.
In this issue of the Journal of Hospital Medicine, Barsuk and colleagues present a prospective, single‐center study assessing the impact of simulation‐based mastery learning (SBML) on thoracentesis among a randomly selected group of IM residents.[7] They studied how their program influenced simulated skills, procedural self‐confidence, frequency of real‐world performance, and rate and reasons for referral to consultants. The authors compared the latter outcomes to traditionally trained residents and hospitalists, finding that SBML improved skills, self‐confidence, and the relative frequency of general internistperformed procedures. Low confidence and limited time were the primary reasons for referral.
To our knowledge, this is the first study to show that SBML can lead to a clinically and statistically significant change in thoracentesis referral patterns, which may have important implications for hospitalists. Given the inconsistent amount and quality of procedural training across IM residency programs, hospitalists may be increasingly ill prepared to perform thoracentesis and train future generations in its best practices.[2, 8, 9] This study demonstrates that SBML can provide trainees with essential hands‐on skills development and experience that is often missing from traditional training models.
Yet, although SBML seems to affect resident referral patterns, its potential impact on practicing hospitalists is less clear. Hospitalists provide the majority of care for general medicine inpatients around the country, and in this study had a dramatically lower rate of bedside procedure performance than even traditionally trained residents (0.7% vs 14.2$), which makes them vital to any strategy to increase bedside thoracentesis rates.[9] Yet the results by Barsuk et al. suggest that the effect size of SBML on hospitalists may be much smaller than on trainees. First, the primary driver of resident practice change appeared to be increased confidence, but baseline hospitalist confidence was significantly greater than that of traditionally trained residents. Second, it is unclear what, if any, effect SBML would have on the time needed to perform a thoracentesis, which was a major factor for hospitalists referring to consult services. Lastly, given the known decrement in procedural skills over time, the durability and associated costs of longitudinal SBML training are unknown.[10, 11, 12]
The fact that general internistperformed thoracenteses are as safe and more cost‐effective than those performed by consultants is a compelling argument to shift procedures back to the bedside. However, these cost analyses do not account for the opportunity cost for hospitalists, either in lost time spent caring for additional patients or in longer shift lengths. It is important to understand whether and how it can be feasible for general internists to perform more bedside thoracenteses so physician training and resource utilization can be optimized. Whereas confidence and time are likely limiting factors for all general internists, this study suggests that their relative importance may markedly differ between residents and hospitalists, and it is unclear how much the change in confidence resulting from SBML would affect the rates of thoracentesis by generalists beyond practice settings involving trainees. The feasibility, cost, and efficacy of SBML deserve more study in multiple clinical environments to understand its true impact. Ultimately, we suspect that only an intervention addressing procedural time demands will lead to meaningful, sustained increases in general internistperformed thoracenteses.
- , . The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians. Ann Intern Med. 2007;146(5):355–360.
- American Board of Internal Medicine. Internal medicine policies. Available at: http://www.abim.org/certification/policies/internal‐medicine‐subspecialty‐policies/internal‐medicine.aspx. Accessed July 18, 2016.
- Society of Hospital Medicine. SHM core competencies. Available at: http://www.hospitalmedicine.org/Web/Education/Core_Competencies/Web/Education/Core_Competencies.aspx. Accessed July 18, 2016.
- , , , . Patient satisfaction with a hospitalist procedure service: is bedside procedure teaching reassuring to patients? J Hosp Med. 2011;6(4):219–224.
- , , , , . Factors associated with inpatient thoracentesis procedure quality at university hospitals. Jt Comm J Qual Patient Saf. 2016;42(1):34–40.
- , , , , . Thoracentesis outcomes: a 12‐year experience. Thorax. 2015;70(2):127–132.
- , , , et al. The effect of simulation‐based mastery learning on thoracentesis referral patterns. J Hosp Med. 2016;11(11):792–795.
- . What we know about hospitalists. Innovative Thinking website. Available at: http://innovativesolutions.org/innovative‐thinking/what‐we‐know‐about‐hospitalists. Accessed July 18, 2016.
- , , , . Procedural skills training in internal medicine residencies. A survey of program directors. Ann intern Med. 1989;111(11):932–938.
- , , , et al. Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures. Am J Med. 2006;119(1):71:e17–e24.
- . Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains. Acad Med. 2004;79(10 suppl):S70–S81
- , , , et al. Simulation training and its effect on long‐term resident performance in central venous catheterization. Simul Healthc. 2010;5(3):146–151.
There has been a nationwide shift away from general internists performing bedside thoracenteses and toward referring them to pulmonology and interventional radiology services.[1] Aligning with this trend, the American Board of Internal Medicine now only requires that internal medicine (IM)trained physicians understand the indications, complications, and management of bedside procedures.[2]
However, thoracentesis is still considered a core competency of practicing hospitalists, the fastest growing field within general IM.[3] Furthermore, evidence suggests that thoracenteses done by general internists have high patient satisfaction, reduce hospital length of stay, are more cost‐effective, and are as safe as those done by consultants.[4, 5, 6] It is thus important to understand the reasons for referrals to specialty services and to investigate potential interventions that increase performance of procedures by internists.
In this issue of the Journal of Hospital Medicine, Barsuk and colleagues present a prospective, single‐center study assessing the impact of simulation‐based mastery learning (SBML) on thoracentesis among a randomly selected group of IM residents.[7] They studied how their program influenced simulated skills, procedural self‐confidence, frequency of real‐world performance, and rate and reasons for referral to consultants. The authors compared the latter outcomes to traditionally trained residents and hospitalists, finding that SBML improved skills, self‐confidence, and the relative frequency of general internistperformed procedures. Low confidence and limited time were the primary reasons for referral.
To our knowledge, this is the first study to show that SBML can lead to a clinically and statistically significant change in thoracentesis referral patterns, which may have important implications for hospitalists. Given the inconsistent amount and quality of procedural training across IM residency programs, hospitalists may be increasingly ill prepared to perform thoracentesis and train future generations in its best practices.[2, 8, 9] This study demonstrates that SBML can provide trainees with essential hands‐on skills development and experience that is often missing from traditional training models.
Yet, although SBML seems to affect resident referral patterns, its potential impact on practicing hospitalists is less clear. Hospitalists provide the majority of care for general medicine inpatients around the country, and in this study had a dramatically lower rate of bedside procedure performance than even traditionally trained residents (0.7% vs 14.2$), which makes them vital to any strategy to increase bedside thoracentesis rates.[9] Yet the results by Barsuk et al. suggest that the effect size of SBML on hospitalists may be much smaller than on trainees. First, the primary driver of resident practice change appeared to be increased confidence, but baseline hospitalist confidence was significantly greater than that of traditionally trained residents. Second, it is unclear what, if any, effect SBML would have on the time needed to perform a thoracentesis, which was a major factor for hospitalists referring to consult services. Lastly, given the known decrement in procedural skills over time, the durability and associated costs of longitudinal SBML training are unknown.[10, 11, 12]
The fact that general internistperformed thoracenteses are as safe and more cost‐effective than those performed by consultants is a compelling argument to shift procedures back to the bedside. However, these cost analyses do not account for the opportunity cost for hospitalists, either in lost time spent caring for additional patients or in longer shift lengths. It is important to understand whether and how it can be feasible for general internists to perform more bedside thoracenteses so physician training and resource utilization can be optimized. Whereas confidence and time are likely limiting factors for all general internists, this study suggests that their relative importance may markedly differ between residents and hospitalists, and it is unclear how much the change in confidence resulting from SBML would affect the rates of thoracentesis by generalists beyond practice settings involving trainees. The feasibility, cost, and efficacy of SBML deserve more study in multiple clinical environments to understand its true impact. Ultimately, we suspect that only an intervention addressing procedural time demands will lead to meaningful, sustained increases in general internistperformed thoracenteses.
There has been a nationwide shift away from general internists performing bedside thoracenteses and toward referring them to pulmonology and interventional radiology services.[1] Aligning with this trend, the American Board of Internal Medicine now only requires that internal medicine (IM)trained physicians understand the indications, complications, and management of bedside procedures.[2]
However, thoracentesis is still considered a core competency of practicing hospitalists, the fastest growing field within general IM.[3] Furthermore, evidence suggests that thoracenteses done by general internists have high patient satisfaction, reduce hospital length of stay, are more cost‐effective, and are as safe as those done by consultants.[4, 5, 6] It is thus important to understand the reasons for referrals to specialty services and to investigate potential interventions that increase performance of procedures by internists.
In this issue of the Journal of Hospital Medicine, Barsuk and colleagues present a prospective, single‐center study assessing the impact of simulation‐based mastery learning (SBML) on thoracentesis among a randomly selected group of IM residents.[7] They studied how their program influenced simulated skills, procedural self‐confidence, frequency of real‐world performance, and rate and reasons for referral to consultants. The authors compared the latter outcomes to traditionally trained residents and hospitalists, finding that SBML improved skills, self‐confidence, and the relative frequency of general internistperformed procedures. Low confidence and limited time were the primary reasons for referral.
To our knowledge, this is the first study to show that SBML can lead to a clinically and statistically significant change in thoracentesis referral patterns, which may have important implications for hospitalists. Given the inconsistent amount and quality of procedural training across IM residency programs, hospitalists may be increasingly ill prepared to perform thoracentesis and train future generations in its best practices.[2, 8, 9] This study demonstrates that SBML can provide trainees with essential hands‐on skills development and experience that is often missing from traditional training models.
Yet, although SBML seems to affect resident referral patterns, its potential impact on practicing hospitalists is less clear. Hospitalists provide the majority of care for general medicine inpatients around the country, and in this study had a dramatically lower rate of bedside procedure performance than even traditionally trained residents (0.7% vs 14.2$), which makes them vital to any strategy to increase bedside thoracentesis rates.[9] Yet the results by Barsuk et al. suggest that the effect size of SBML on hospitalists may be much smaller than on trainees. First, the primary driver of resident practice change appeared to be increased confidence, but baseline hospitalist confidence was significantly greater than that of traditionally trained residents. Second, it is unclear what, if any, effect SBML would have on the time needed to perform a thoracentesis, which was a major factor for hospitalists referring to consult services. Lastly, given the known decrement in procedural skills over time, the durability and associated costs of longitudinal SBML training are unknown.[10, 11, 12]
The fact that general internistperformed thoracenteses are as safe and more cost‐effective than those performed by consultants is a compelling argument to shift procedures back to the bedside. However, these cost analyses do not account for the opportunity cost for hospitalists, either in lost time spent caring for additional patients or in longer shift lengths. It is important to understand whether and how it can be feasible for general internists to perform more bedside thoracenteses so physician training and resource utilization can be optimized. Whereas confidence and time are likely limiting factors for all general internists, this study suggests that their relative importance may markedly differ between residents and hospitalists, and it is unclear how much the change in confidence resulting from SBML would affect the rates of thoracentesis by generalists beyond practice settings involving trainees. The feasibility, cost, and efficacy of SBML deserve more study in multiple clinical environments to understand its true impact. Ultimately, we suspect that only an intervention addressing procedural time demands will lead to meaningful, sustained increases in general internistperformed thoracenteses.
- , . The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians. Ann Intern Med. 2007;146(5):355–360.
- American Board of Internal Medicine. Internal medicine policies. Available at: http://www.abim.org/certification/policies/internal‐medicine‐subspecialty‐policies/internal‐medicine.aspx. Accessed July 18, 2016.
- Society of Hospital Medicine. SHM core competencies. Available at: http://www.hospitalmedicine.org/Web/Education/Core_Competencies/Web/Education/Core_Competencies.aspx. Accessed July 18, 2016.
- , , , . Patient satisfaction with a hospitalist procedure service: is bedside procedure teaching reassuring to patients? J Hosp Med. 2011;6(4):219–224.
- , , , , . Factors associated with inpatient thoracentesis procedure quality at university hospitals. Jt Comm J Qual Patient Saf. 2016;42(1):34–40.
- , , , , . Thoracentesis outcomes: a 12‐year experience. Thorax. 2015;70(2):127–132.
- , , , et al. The effect of simulation‐based mastery learning on thoracentesis referral patterns. J Hosp Med. 2016;11(11):792–795.
- . What we know about hospitalists. Innovative Thinking website. Available at: http://innovativesolutions.org/innovative‐thinking/what‐we‐know‐about‐hospitalists. Accessed July 18, 2016.
- , , , . Procedural skills training in internal medicine residencies. A survey of program directors. Ann intern Med. 1989;111(11):932–938.
- , , , et al. Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures. Am J Med. 2006;119(1):71:e17–e24.
- . Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains. Acad Med. 2004;79(10 suppl):S70–S81
- , , , et al. Simulation training and its effect on long‐term resident performance in central venous catheterization. Simul Healthc. 2010;5(3):146–151.
- , . The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians. Ann Intern Med. 2007;146(5):355–360.
- American Board of Internal Medicine. Internal medicine policies. Available at: http://www.abim.org/certification/policies/internal‐medicine‐subspecialty‐policies/internal‐medicine.aspx. Accessed July 18, 2016.
- Society of Hospital Medicine. SHM core competencies. Available at: http://www.hospitalmedicine.org/Web/Education/Core_Competencies/Web/Education/Core_Competencies.aspx. Accessed July 18, 2016.
- , , , . Patient satisfaction with a hospitalist procedure service: is bedside procedure teaching reassuring to patients? J Hosp Med. 2011;6(4):219–224.
- , , , , . Factors associated with inpatient thoracentesis procedure quality at university hospitals. Jt Comm J Qual Patient Saf. 2016;42(1):34–40.
- , , , , . Thoracentesis outcomes: a 12‐year experience. Thorax. 2015;70(2):127–132.
- , , , et al. The effect of simulation‐based mastery learning on thoracentesis referral patterns. J Hosp Med. 2016;11(11):792–795.
- . What we know about hospitalists. Innovative Thinking website. Available at: http://innovativesolutions.org/innovative‐thinking/what‐we‐know‐about‐hospitalists. Accessed July 18, 2016.
- , , , . Procedural skills training in internal medicine residencies. A survey of program directors. Ann intern Med. 1989;111(11):932–938.
- , , , et al. Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures. Am J Med. 2006;119(1):71:e17–e24.
- . Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains. Acad Med. 2004;79(10 suppl):S70–S81
- , , , et al. Simulation training and its effect on long‐term resident performance in central venous catheterization. Simul Healthc. 2010;5(3):146–151.
Association Between DCBN and LOS
Slow hospital throughputthe process whereby a patient is admitted, placed in a room, and eventually dischargedcan worsen outcomes if admitted patients are boarded in emergency rooms or postanesthesia units.[1] One potential method to improve throughput is to discharge patients earlier in the day,[2] freeing up available beds and conceivably reducing hospital length of stay (LOS).
To quantify throughput, hospitals are beginning to measure the proportion of patients discharged before noon (DCBN). One study, looking at discharges on a single medical floor in an urban academic medical center, suggested that increasing the percentage of patients discharged by noon decreased observed‐to‐expected LOS in hospitalized medicine patients,[3] and a follow‐up study demonstrated that it was associated with admissions from the emergency department occurring earlier in the day.[4] However, these studies did not adjust for changes in case mix index (CMI) and other patient‐level characteristics that may also have affected these outcomes. Concerns persist that more efforts to discharge patients by noon could inadvertently increase LOS if staff chose to keep patients overnight for an early discharge the following day.
We undertook a retrospective analysis of data from patients discharged from a large academic medical center where an institution‐wide emphasis was placed on discharging more patients by noon. Using these data, we examined the association between discharges before noon and LOS in medical and surgical inpatients.
METHODS
Site and Subjects
Our study was based at the University of California, San Francisco (UCSF) Medical Center, a 400‐bed academic hospital located in San Francisco, California. We examined adult medical and surgical discharges from July 2012 through April 2015. Patients who stayed less than 24 hours or more than 20 days were excluded. Discharges from the hospital medicine service and the following surgical services were included in the analysis: cardiac surgery, colorectal surgery, cardiothoracic surgery, general surgery, gynecologic oncology, gynecology, neurosurgery, orthopedics, otolaryngology, head and neck surgery, plastic surgery, thoracic surgery, urology, and vascular surgery. No exclusions were made based on patient status (eg, observation vs inpatient). UCSF's institutional review board approved our study.
During the time of our study, discharges before noon time became an institutional priority. To this end, rates of DCBN were tracked using retrospective data, and various units undertook efforts such as informal afternoon meetings to prompt planning for the next morning's discharges. These efforts did not differentially affect medical or surgical units or emergent or nonemergent admissions, and no financial incentives or other changes in workflow were in place to increase DCBN rates.
Data Sources
We used the cost accounting system at UCSF (Enterprise Performance System Inc. [EPSI], Chicago, IL) to collect demographic information about each patient, including age, sex, primary race, and primary ethnicity. This system was also used to collect characteristics of each hospitalization including LOS (calculated from admission date time and discharge date time), hospital service at discharge, the discharge attending, discharge disposition of the patient, and the CMI, a marker of the severity of illness of the patient during that hospitalization. EPSI was also used to collect data on the admission type of all patients, either emergent, urgent, or routine, and the insurance status of the patient during that hospitalization.
Data on time of discharge were entered by the discharging nurse or unit assistant to reflect the time the patient left the hospital. Using these data, we defined a before‐noon discharge as one taking place between 8:00 am and 12:00 pm.
Statistical Analysis
Wilcoxon rank sum test and 2 statistics were used to compare baseline characteristics of hospitalizations of patients discharged before and after noon.
We used generalized linear models to assess the association of a discharge before noon on the LOS with gamma models. We accounted for clustering of discharge attendings using generalized estimating equations with exchangeable working correlation and robust standard errors. After the initial unadjusted analyses, covariates were included in the adjusted analysis if they were associated with an LOS at P < 0.05 or reasons of face validity. These variables are shown in Table 1. Because an effort to increase the discharges before noon was started in the 2014 academic year, we added an interaction term between the date of discharge and whether a discharge occurred before noon. The interaction term was included by dividing the study period into time periods corresponding to sequential 6‐month intervals. A new variable was defined by a categorical variable that indicated in which of these time periods a discharge occurred.
| Discharged Before Noon | Discharged After Noon | P Value | |
|---|---|---|---|
| |||
| Median LOS (IQR) | 3.4 (2.25.9) | 3.7 (2.36.3) | <0.0005 |
| Median CMI (IQR) | 1.8 (1.12.4) | 1.7 (1.12.5) | 0.006 |
| Service type, N (%) | |||
| Hospital medicine | 1,919 (29.6) | 11,290 (35.4) | |
| Surgical services | 4,565 (70.4) | 20,591 (64.6) | <0.0005 |
| Discharged before noon, N (%) | 6,484 (16.9) | 31,881 (83.1) | |
| Discharged on weekend, N (%) | |||
| Yes | 1,543 (23.8) | 7,411 (23.3) | |
| No | 4,941 (76.2) | 24,470 (76.8) | 0.34 |
| Discharge disposition, N (%) | |||
| Home with home health | 748 (11.5) | 5,774 (18.1) | |
| Home without home health | 3,997 (61.6) | 17,862 (56.0) | |
| SNF | 837 (12.9) | 3,082 (9.7) | |
| Other | 902 (13.9) | 5,163 (16.2) | <0.0005 |
| 6‐month interval, N (%) | |||
| JulyDecember 2012 | 993 (15.3) | 5,596 (17.6) | |
| JanuaryJune 2013 | 980 (15.1) | 5,721 (17.9) | |
| JulyDecember 2013 | 1,088 (16.8) | 5,690 (17.9) | |
| JanuaryJune 2014 | 1,288 (19.9) | 5,441 (17.1) | |
| JulyDecember 2014 | 1,275 (19.7) | 5,656 (17.7) | |
| JanuaryApril 2015 | 860 (13.3) | 3,777 (11.9) | <0.0005 |
| Age category, N (%) | |||
| 1864 years | 4,177 (64.4) | 20,044 (62.9) | |
| 65+ years | 2,307 (35.6) | 11,837 (37.1) | 0.02 |
| Male, N (%) | 3,274 (50.5) | 15,596 (48.9) | |
| Female, N (%) | 3,210 (49.5) | 16,284 (51.1) | 0.06 |
| Race, N (%) | |||
| White or Caucasian | 4,133 (63.7) | 18,798 (59.0) | |
| African American | 518 (8.0) | 3,020 (9.5) | |
| Asian | 703 (10.8) | 4,052 (12.7) | |
| Other | 1,130 (17.4) | 6,011 (18.9) | <0.0005 |
| Ethnicity, N (%) | |||
| Hispanic or Latino | 691 (10.7) | 3,713 (11.7) | |
| Not Hispanic or Latino | 5,597 (86.3) | 27,209 (85.4) | |
| Unknown/declined | 196 (3.0) | 959 (3.0) | 0.07 |
| Admission type, N (%) | |||
| Elective | 3,494 (53.9) | 13,881 (43.5) | |
| Emergency | 2,047 (31.6) | 12,145 (38.1) | |
| Urgent | 889 (13.7) | 5,459 (17.1) | |
| Other | 54 (0.8) | 396 (1.2) | <0.0005 |
| Payor class, N (%) | |||
| Medicare | 2,648 (40.8) | 13,808 (43.3) | |
| Medi‐Cal | 1,060 (16.4) | 5,913 (18.6) | |
| Commercial | 2,633 (40.6) | 11,242 (35.3) | |
| Other | 143 (2.2) | 918 (2.9) | <0.0005 |
We conducted a sensitivity analysis using propensity scores. The propensity score was based on demographic and clinical variables (as listed in Table 1) that exhibited P < 0.2 in bivariate analysis between the variable and being discharged before noon. We then used the propensity score as a covariate in a generalized linear model of the LOS with a gamma distribution and with generalized estimating equations as described above.
Finally, we performed prespecified secondary subset analyses of patients admitted emergently and nonemergently.
Statistical modeling and analysis was completed using Stata version 13 (StataCorp, College Station, TX).
RESULTS
Patient Demographics and Discharge Before Noon
Our study population comprised 27,983 patients for a total of 38,365 hospitalizations with a median LOS of 3.7 days. We observed 6484 discharges before noon (16.9%) and 31,881 discharges after noon (83.1%). The characteristics of the hospitalizations are shown in Table 1.
Patients who were discharged before noon tended to be younger, white, and discharged with a disposition to home without home health. The median CMI was slightly higher in discharges before noon (1.81, P = 0.006), and elective admissions were more likely than emergent to be discharged before noon (53.9% vs 31.6%, P < 0.0005).
Multivariable Analysis
A discharge before noon was associated with a 4.3% increase in LOS (adjusted odds ratio [OR]: 1.043, 95% confidence interval [CI]: 1.003‐1.086), adjusting for CMI, the service type, discharge on the weekend, discharge disposition, age, sex, ethnicity, race, urgency of admission, payor class, and a full interaction with the date of discharge (in 6‐month intervals). In preplanned subset analyses, the association between longer LOS and DCBN was more pronounced in patients admitted emergently (adjusted OR: 1.14, 95% CI: 1.033‐1.249) and less pronounced for patients not admitted emergently (adjusted OR: 1.03, 95% CI: 0.988‐1.074), although the latter did not meet statistical significance. In patients admitted emergently, this corresponds to approximately a 12‐hour increase in LOS. The interaction term of discharge date and DCBN was significant in the model. In further subset analyses, the association between longer LOS and DCBN was more pronounced in medicine patients (adjusted OR: 1.116, 95% CI: 1.014‐1.228) than in surgical patients (adjusted OR: 1.030, 95% CI: 0.989‐1.074), although the relationship in surgical patients did not meet statistical significance.
We also undertook sensitivity analyses utilizing propensity scores as a covariate in our base multivariable models. Results from these analyses did not differ from the base models and are not presented here. Results also did not differ when comparing discharges before and after the initiation of an attending only service.
DISCUSSION AND CONCLUSION
In our retrospective study of patients discharged from an academic medical center, discharge before noon was associated with a longer LOS, with the effect more pronounced in patients admitted emergently in the hospital. Our results suggest that efforts to discharge patients earlier in the day may have varying degrees of success depending on patient characteristics. Conceivably, elective admissions recover according to predictable plans, allowing for discharges earlier in the day. In contrast, patients discharged from emergent hospitalizations may have ongoing evolution of their care plan, making plans for discharging before noon more challenging.
Our results differ from a previous study,[3] which suggested that increasing the proportion of before‐noon discharges was associated with a fall in observed‐to‐expected LOS. However, observational studies of DCBN are challenging, because the association between early discharge and LOS is potentially bidirectional. One interpretation, for example, is that patients were kept longer in order to be discharged by noon the following day, which for the subgroups of patients admitted emergently corresponded to a roughly 12‐hour increase in LOS. However, it is also plausible that patients who stayed longer also had more time to plan for an early discharge. In either scenario, the ability of managers to utilize LOS as a key metric of throughput efforts may be flawed, and suggests that alternatives (eg, number of patients waiting for beds off unit) may be a more reasonable measure of throughput. Our results have several limitations. As in any observational study, our results are vulnerable to biases from unmeasured covariates that confound the analysis. We caution that a causal relationship between a discharge before noon and LOS cannot be determined from the nature of the study. Our results are also limited in that we were unable to adjust for day‐to‐day hospital capacity and other variables that affect LOS including caregiver and transportation availability, bed capacity at receiving care facilities, and patient consent to discharge. Finally, as a single‐site study, our findings may not be applicable to nonacademic settings.
In conclusion, our observational study discerned an association between discharging patients before noon and longer LOS. We believe our findings suggest a rationale for alternate approaches to measuring an early discharge program's effectiveness, namely, that the evaluation of the success of an early discharge initiative should consider multiple evaluation metrics including the effect on emergency department wait times, intensive care unit or postanesthesia transitions, and on patient reported experiences of care transitions.
Disclosures
Andrew Auerbach, MD, is supported by a K24 grant from the National Heart, Lung, and Blood Institute: K24HL098372. The authors report no conflicts of interest.
- , , , et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16(1):1–10.
- Centers for Medicare 2013.
- , , , et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210–214.
- , , , et al. Discharge before noon: effect on throughput and sustainability. J Hosp Med. 2015;10(10):664–669.
Slow hospital throughputthe process whereby a patient is admitted, placed in a room, and eventually dischargedcan worsen outcomes if admitted patients are boarded in emergency rooms or postanesthesia units.[1] One potential method to improve throughput is to discharge patients earlier in the day,[2] freeing up available beds and conceivably reducing hospital length of stay (LOS).
To quantify throughput, hospitals are beginning to measure the proportion of patients discharged before noon (DCBN). One study, looking at discharges on a single medical floor in an urban academic medical center, suggested that increasing the percentage of patients discharged by noon decreased observed‐to‐expected LOS in hospitalized medicine patients,[3] and a follow‐up study demonstrated that it was associated with admissions from the emergency department occurring earlier in the day.[4] However, these studies did not adjust for changes in case mix index (CMI) and other patient‐level characteristics that may also have affected these outcomes. Concerns persist that more efforts to discharge patients by noon could inadvertently increase LOS if staff chose to keep patients overnight for an early discharge the following day.
We undertook a retrospective analysis of data from patients discharged from a large academic medical center where an institution‐wide emphasis was placed on discharging more patients by noon. Using these data, we examined the association between discharges before noon and LOS in medical and surgical inpatients.
METHODS
Site and Subjects
Our study was based at the University of California, San Francisco (UCSF) Medical Center, a 400‐bed academic hospital located in San Francisco, California. We examined adult medical and surgical discharges from July 2012 through April 2015. Patients who stayed less than 24 hours or more than 20 days were excluded. Discharges from the hospital medicine service and the following surgical services were included in the analysis: cardiac surgery, colorectal surgery, cardiothoracic surgery, general surgery, gynecologic oncology, gynecology, neurosurgery, orthopedics, otolaryngology, head and neck surgery, plastic surgery, thoracic surgery, urology, and vascular surgery. No exclusions were made based on patient status (eg, observation vs inpatient). UCSF's institutional review board approved our study.
During the time of our study, discharges before noon time became an institutional priority. To this end, rates of DCBN were tracked using retrospective data, and various units undertook efforts such as informal afternoon meetings to prompt planning for the next morning's discharges. These efforts did not differentially affect medical or surgical units or emergent or nonemergent admissions, and no financial incentives or other changes in workflow were in place to increase DCBN rates.
Data Sources
We used the cost accounting system at UCSF (Enterprise Performance System Inc. [EPSI], Chicago, IL) to collect demographic information about each patient, including age, sex, primary race, and primary ethnicity. This system was also used to collect characteristics of each hospitalization including LOS (calculated from admission date time and discharge date time), hospital service at discharge, the discharge attending, discharge disposition of the patient, and the CMI, a marker of the severity of illness of the patient during that hospitalization. EPSI was also used to collect data on the admission type of all patients, either emergent, urgent, or routine, and the insurance status of the patient during that hospitalization.
Data on time of discharge were entered by the discharging nurse or unit assistant to reflect the time the patient left the hospital. Using these data, we defined a before‐noon discharge as one taking place between 8:00 am and 12:00 pm.
Statistical Analysis
Wilcoxon rank sum test and 2 statistics were used to compare baseline characteristics of hospitalizations of patients discharged before and after noon.
We used generalized linear models to assess the association of a discharge before noon on the LOS with gamma models. We accounted for clustering of discharge attendings using generalized estimating equations with exchangeable working correlation and robust standard errors. After the initial unadjusted analyses, covariates were included in the adjusted analysis if they were associated with an LOS at P < 0.05 or reasons of face validity. These variables are shown in Table 1. Because an effort to increase the discharges before noon was started in the 2014 academic year, we added an interaction term between the date of discharge and whether a discharge occurred before noon. The interaction term was included by dividing the study period into time periods corresponding to sequential 6‐month intervals. A new variable was defined by a categorical variable that indicated in which of these time periods a discharge occurred.
| Discharged Before Noon | Discharged After Noon | P Value | |
|---|---|---|---|
| |||
| Median LOS (IQR) | 3.4 (2.25.9) | 3.7 (2.36.3) | <0.0005 |
| Median CMI (IQR) | 1.8 (1.12.4) | 1.7 (1.12.5) | 0.006 |
| Service type, N (%) | |||
| Hospital medicine | 1,919 (29.6) | 11,290 (35.4) | |
| Surgical services | 4,565 (70.4) | 20,591 (64.6) | <0.0005 |
| Discharged before noon, N (%) | 6,484 (16.9) | 31,881 (83.1) | |
| Discharged on weekend, N (%) | |||
| Yes | 1,543 (23.8) | 7,411 (23.3) | |
| No | 4,941 (76.2) | 24,470 (76.8) | 0.34 |
| Discharge disposition, N (%) | |||
| Home with home health | 748 (11.5) | 5,774 (18.1) | |
| Home without home health | 3,997 (61.6) | 17,862 (56.0) | |
| SNF | 837 (12.9) | 3,082 (9.7) | |
| Other | 902 (13.9) | 5,163 (16.2) | <0.0005 |
| 6‐month interval, N (%) | |||
| JulyDecember 2012 | 993 (15.3) | 5,596 (17.6) | |
| JanuaryJune 2013 | 980 (15.1) | 5,721 (17.9) | |
| JulyDecember 2013 | 1,088 (16.8) | 5,690 (17.9) | |
| JanuaryJune 2014 | 1,288 (19.9) | 5,441 (17.1) | |
| JulyDecember 2014 | 1,275 (19.7) | 5,656 (17.7) | |
| JanuaryApril 2015 | 860 (13.3) | 3,777 (11.9) | <0.0005 |
| Age category, N (%) | |||
| 1864 years | 4,177 (64.4) | 20,044 (62.9) | |
| 65+ years | 2,307 (35.6) | 11,837 (37.1) | 0.02 |
| Male, N (%) | 3,274 (50.5) | 15,596 (48.9) | |
| Female, N (%) | 3,210 (49.5) | 16,284 (51.1) | 0.06 |
| Race, N (%) | |||
| White or Caucasian | 4,133 (63.7) | 18,798 (59.0) | |
| African American | 518 (8.0) | 3,020 (9.5) | |
| Asian | 703 (10.8) | 4,052 (12.7) | |
| Other | 1,130 (17.4) | 6,011 (18.9) | <0.0005 |
| Ethnicity, N (%) | |||
| Hispanic or Latino | 691 (10.7) | 3,713 (11.7) | |
| Not Hispanic or Latino | 5,597 (86.3) | 27,209 (85.4) | |
| Unknown/declined | 196 (3.0) | 959 (3.0) | 0.07 |
| Admission type, N (%) | |||
| Elective | 3,494 (53.9) | 13,881 (43.5) | |
| Emergency | 2,047 (31.6) | 12,145 (38.1) | |
| Urgent | 889 (13.7) | 5,459 (17.1) | |
| Other | 54 (0.8) | 396 (1.2) | <0.0005 |
| Payor class, N (%) | |||
| Medicare | 2,648 (40.8) | 13,808 (43.3) | |
| Medi‐Cal | 1,060 (16.4) | 5,913 (18.6) | |
| Commercial | 2,633 (40.6) | 11,242 (35.3) | |
| Other | 143 (2.2) | 918 (2.9) | <0.0005 |
We conducted a sensitivity analysis using propensity scores. The propensity score was based on demographic and clinical variables (as listed in Table 1) that exhibited P < 0.2 in bivariate analysis between the variable and being discharged before noon. We then used the propensity score as a covariate in a generalized linear model of the LOS with a gamma distribution and with generalized estimating equations as described above.
Finally, we performed prespecified secondary subset analyses of patients admitted emergently and nonemergently.
Statistical modeling and analysis was completed using Stata version 13 (StataCorp, College Station, TX).
RESULTS
Patient Demographics and Discharge Before Noon
Our study population comprised 27,983 patients for a total of 38,365 hospitalizations with a median LOS of 3.7 days. We observed 6484 discharges before noon (16.9%) and 31,881 discharges after noon (83.1%). The characteristics of the hospitalizations are shown in Table 1.
Patients who were discharged before noon tended to be younger, white, and discharged with a disposition to home without home health. The median CMI was slightly higher in discharges before noon (1.81, P = 0.006), and elective admissions were more likely than emergent to be discharged before noon (53.9% vs 31.6%, P < 0.0005).
Multivariable Analysis
A discharge before noon was associated with a 4.3% increase in LOS (adjusted odds ratio [OR]: 1.043, 95% confidence interval [CI]: 1.003‐1.086), adjusting for CMI, the service type, discharge on the weekend, discharge disposition, age, sex, ethnicity, race, urgency of admission, payor class, and a full interaction with the date of discharge (in 6‐month intervals). In preplanned subset analyses, the association between longer LOS and DCBN was more pronounced in patients admitted emergently (adjusted OR: 1.14, 95% CI: 1.033‐1.249) and less pronounced for patients not admitted emergently (adjusted OR: 1.03, 95% CI: 0.988‐1.074), although the latter did not meet statistical significance. In patients admitted emergently, this corresponds to approximately a 12‐hour increase in LOS. The interaction term of discharge date and DCBN was significant in the model. In further subset analyses, the association between longer LOS and DCBN was more pronounced in medicine patients (adjusted OR: 1.116, 95% CI: 1.014‐1.228) than in surgical patients (adjusted OR: 1.030, 95% CI: 0.989‐1.074), although the relationship in surgical patients did not meet statistical significance.
We also undertook sensitivity analyses utilizing propensity scores as a covariate in our base multivariable models. Results from these analyses did not differ from the base models and are not presented here. Results also did not differ when comparing discharges before and after the initiation of an attending only service.
DISCUSSION AND CONCLUSION
In our retrospective study of patients discharged from an academic medical center, discharge before noon was associated with a longer LOS, with the effect more pronounced in patients admitted emergently in the hospital. Our results suggest that efforts to discharge patients earlier in the day may have varying degrees of success depending on patient characteristics. Conceivably, elective admissions recover according to predictable plans, allowing for discharges earlier in the day. In contrast, patients discharged from emergent hospitalizations may have ongoing evolution of their care plan, making plans for discharging before noon more challenging.
Our results differ from a previous study,[3] which suggested that increasing the proportion of before‐noon discharges was associated with a fall in observed‐to‐expected LOS. However, observational studies of DCBN are challenging, because the association between early discharge and LOS is potentially bidirectional. One interpretation, for example, is that patients were kept longer in order to be discharged by noon the following day, which for the subgroups of patients admitted emergently corresponded to a roughly 12‐hour increase in LOS. However, it is also plausible that patients who stayed longer also had more time to plan for an early discharge. In either scenario, the ability of managers to utilize LOS as a key metric of throughput efforts may be flawed, and suggests that alternatives (eg, number of patients waiting for beds off unit) may be a more reasonable measure of throughput. Our results have several limitations. As in any observational study, our results are vulnerable to biases from unmeasured covariates that confound the analysis. We caution that a causal relationship between a discharge before noon and LOS cannot be determined from the nature of the study. Our results are also limited in that we were unable to adjust for day‐to‐day hospital capacity and other variables that affect LOS including caregiver and transportation availability, bed capacity at receiving care facilities, and patient consent to discharge. Finally, as a single‐site study, our findings may not be applicable to nonacademic settings.
In conclusion, our observational study discerned an association between discharging patients before noon and longer LOS. We believe our findings suggest a rationale for alternate approaches to measuring an early discharge program's effectiveness, namely, that the evaluation of the success of an early discharge initiative should consider multiple evaluation metrics including the effect on emergency department wait times, intensive care unit or postanesthesia transitions, and on patient reported experiences of care transitions.
Disclosures
Andrew Auerbach, MD, is supported by a K24 grant from the National Heart, Lung, and Blood Institute: K24HL098372. The authors report no conflicts of interest.
Slow hospital throughputthe process whereby a patient is admitted, placed in a room, and eventually dischargedcan worsen outcomes if admitted patients are boarded in emergency rooms or postanesthesia units.[1] One potential method to improve throughput is to discharge patients earlier in the day,[2] freeing up available beds and conceivably reducing hospital length of stay (LOS).
To quantify throughput, hospitals are beginning to measure the proportion of patients discharged before noon (DCBN). One study, looking at discharges on a single medical floor in an urban academic medical center, suggested that increasing the percentage of patients discharged by noon decreased observed‐to‐expected LOS in hospitalized medicine patients,[3] and a follow‐up study demonstrated that it was associated with admissions from the emergency department occurring earlier in the day.[4] However, these studies did not adjust for changes in case mix index (CMI) and other patient‐level characteristics that may also have affected these outcomes. Concerns persist that more efforts to discharge patients by noon could inadvertently increase LOS if staff chose to keep patients overnight for an early discharge the following day.
We undertook a retrospective analysis of data from patients discharged from a large academic medical center where an institution‐wide emphasis was placed on discharging more patients by noon. Using these data, we examined the association between discharges before noon and LOS in medical and surgical inpatients.
METHODS
Site and Subjects
Our study was based at the University of California, San Francisco (UCSF) Medical Center, a 400‐bed academic hospital located in San Francisco, California. We examined adult medical and surgical discharges from July 2012 through April 2015. Patients who stayed less than 24 hours or more than 20 days were excluded. Discharges from the hospital medicine service and the following surgical services were included in the analysis: cardiac surgery, colorectal surgery, cardiothoracic surgery, general surgery, gynecologic oncology, gynecology, neurosurgery, orthopedics, otolaryngology, head and neck surgery, plastic surgery, thoracic surgery, urology, and vascular surgery. No exclusions were made based on patient status (eg, observation vs inpatient). UCSF's institutional review board approved our study.
During the time of our study, discharges before noon time became an institutional priority. To this end, rates of DCBN were tracked using retrospective data, and various units undertook efforts such as informal afternoon meetings to prompt planning for the next morning's discharges. These efforts did not differentially affect medical or surgical units or emergent or nonemergent admissions, and no financial incentives or other changes in workflow were in place to increase DCBN rates.
Data Sources
We used the cost accounting system at UCSF (Enterprise Performance System Inc. [EPSI], Chicago, IL) to collect demographic information about each patient, including age, sex, primary race, and primary ethnicity. This system was also used to collect characteristics of each hospitalization including LOS (calculated from admission date time and discharge date time), hospital service at discharge, the discharge attending, discharge disposition of the patient, and the CMI, a marker of the severity of illness of the patient during that hospitalization. EPSI was also used to collect data on the admission type of all patients, either emergent, urgent, or routine, and the insurance status of the patient during that hospitalization.
Data on time of discharge were entered by the discharging nurse or unit assistant to reflect the time the patient left the hospital. Using these data, we defined a before‐noon discharge as one taking place between 8:00 am and 12:00 pm.
Statistical Analysis
Wilcoxon rank sum test and 2 statistics were used to compare baseline characteristics of hospitalizations of patients discharged before and after noon.
We used generalized linear models to assess the association of a discharge before noon on the LOS with gamma models. We accounted for clustering of discharge attendings using generalized estimating equations with exchangeable working correlation and robust standard errors. After the initial unadjusted analyses, covariates were included in the adjusted analysis if they were associated with an LOS at P < 0.05 or reasons of face validity. These variables are shown in Table 1. Because an effort to increase the discharges before noon was started in the 2014 academic year, we added an interaction term between the date of discharge and whether a discharge occurred before noon. The interaction term was included by dividing the study period into time periods corresponding to sequential 6‐month intervals. A new variable was defined by a categorical variable that indicated in which of these time periods a discharge occurred.
| Discharged Before Noon | Discharged After Noon | P Value | |
|---|---|---|---|
| |||
| Median LOS (IQR) | 3.4 (2.25.9) | 3.7 (2.36.3) | <0.0005 |
| Median CMI (IQR) | 1.8 (1.12.4) | 1.7 (1.12.5) | 0.006 |
| Service type, N (%) | |||
| Hospital medicine | 1,919 (29.6) | 11,290 (35.4) | |
| Surgical services | 4,565 (70.4) | 20,591 (64.6) | <0.0005 |
| Discharged before noon, N (%) | 6,484 (16.9) | 31,881 (83.1) | |
| Discharged on weekend, N (%) | |||
| Yes | 1,543 (23.8) | 7,411 (23.3) | |
| No | 4,941 (76.2) | 24,470 (76.8) | 0.34 |
| Discharge disposition, N (%) | |||
| Home with home health | 748 (11.5) | 5,774 (18.1) | |
| Home without home health | 3,997 (61.6) | 17,862 (56.0) | |
| SNF | 837 (12.9) | 3,082 (9.7) | |
| Other | 902 (13.9) | 5,163 (16.2) | <0.0005 |
| 6‐month interval, N (%) | |||
| JulyDecember 2012 | 993 (15.3) | 5,596 (17.6) | |
| JanuaryJune 2013 | 980 (15.1) | 5,721 (17.9) | |
| JulyDecember 2013 | 1,088 (16.8) | 5,690 (17.9) | |
| JanuaryJune 2014 | 1,288 (19.9) | 5,441 (17.1) | |
| JulyDecember 2014 | 1,275 (19.7) | 5,656 (17.7) | |
| JanuaryApril 2015 | 860 (13.3) | 3,777 (11.9) | <0.0005 |
| Age category, N (%) | |||
| 1864 years | 4,177 (64.4) | 20,044 (62.9) | |
| 65+ years | 2,307 (35.6) | 11,837 (37.1) | 0.02 |
| Male, N (%) | 3,274 (50.5) | 15,596 (48.9) | |
| Female, N (%) | 3,210 (49.5) | 16,284 (51.1) | 0.06 |
| Race, N (%) | |||
| White or Caucasian | 4,133 (63.7) | 18,798 (59.0) | |
| African American | 518 (8.0) | 3,020 (9.5) | |
| Asian | 703 (10.8) | 4,052 (12.7) | |
| Other | 1,130 (17.4) | 6,011 (18.9) | <0.0005 |
| Ethnicity, N (%) | |||
| Hispanic or Latino | 691 (10.7) | 3,713 (11.7) | |
| Not Hispanic or Latino | 5,597 (86.3) | 27,209 (85.4) | |
| Unknown/declined | 196 (3.0) | 959 (3.0) | 0.07 |
| Admission type, N (%) | |||
| Elective | 3,494 (53.9) | 13,881 (43.5) | |
| Emergency | 2,047 (31.6) | 12,145 (38.1) | |
| Urgent | 889 (13.7) | 5,459 (17.1) | |
| Other | 54 (0.8) | 396 (1.2) | <0.0005 |
| Payor class, N (%) | |||
| Medicare | 2,648 (40.8) | 13,808 (43.3) | |
| Medi‐Cal | 1,060 (16.4) | 5,913 (18.6) | |
| Commercial | 2,633 (40.6) | 11,242 (35.3) | |
| Other | 143 (2.2) | 918 (2.9) | <0.0005 |
We conducted a sensitivity analysis using propensity scores. The propensity score was based on demographic and clinical variables (as listed in Table 1) that exhibited P < 0.2 in bivariate analysis between the variable and being discharged before noon. We then used the propensity score as a covariate in a generalized linear model of the LOS with a gamma distribution and with generalized estimating equations as described above.
Finally, we performed prespecified secondary subset analyses of patients admitted emergently and nonemergently.
Statistical modeling and analysis was completed using Stata version 13 (StataCorp, College Station, TX).
RESULTS
Patient Demographics and Discharge Before Noon
Our study population comprised 27,983 patients for a total of 38,365 hospitalizations with a median LOS of 3.7 days. We observed 6484 discharges before noon (16.9%) and 31,881 discharges after noon (83.1%). The characteristics of the hospitalizations are shown in Table 1.
Patients who were discharged before noon tended to be younger, white, and discharged with a disposition to home without home health. The median CMI was slightly higher in discharges before noon (1.81, P = 0.006), and elective admissions were more likely than emergent to be discharged before noon (53.9% vs 31.6%, P < 0.0005).
Multivariable Analysis
A discharge before noon was associated with a 4.3% increase in LOS (adjusted odds ratio [OR]: 1.043, 95% confidence interval [CI]: 1.003‐1.086), adjusting for CMI, the service type, discharge on the weekend, discharge disposition, age, sex, ethnicity, race, urgency of admission, payor class, and a full interaction with the date of discharge (in 6‐month intervals). In preplanned subset analyses, the association between longer LOS and DCBN was more pronounced in patients admitted emergently (adjusted OR: 1.14, 95% CI: 1.033‐1.249) and less pronounced for patients not admitted emergently (adjusted OR: 1.03, 95% CI: 0.988‐1.074), although the latter did not meet statistical significance. In patients admitted emergently, this corresponds to approximately a 12‐hour increase in LOS. The interaction term of discharge date and DCBN was significant in the model. In further subset analyses, the association between longer LOS and DCBN was more pronounced in medicine patients (adjusted OR: 1.116, 95% CI: 1.014‐1.228) than in surgical patients (adjusted OR: 1.030, 95% CI: 0.989‐1.074), although the relationship in surgical patients did not meet statistical significance.
We also undertook sensitivity analyses utilizing propensity scores as a covariate in our base multivariable models. Results from these analyses did not differ from the base models and are not presented here. Results also did not differ when comparing discharges before and after the initiation of an attending only service.
DISCUSSION AND CONCLUSION
In our retrospective study of patients discharged from an academic medical center, discharge before noon was associated with a longer LOS, with the effect more pronounced in patients admitted emergently in the hospital. Our results suggest that efforts to discharge patients earlier in the day may have varying degrees of success depending on patient characteristics. Conceivably, elective admissions recover according to predictable plans, allowing for discharges earlier in the day. In contrast, patients discharged from emergent hospitalizations may have ongoing evolution of their care plan, making plans for discharging before noon more challenging.
Our results differ from a previous study,[3] which suggested that increasing the proportion of before‐noon discharges was associated with a fall in observed‐to‐expected LOS. However, observational studies of DCBN are challenging, because the association between early discharge and LOS is potentially bidirectional. One interpretation, for example, is that patients were kept longer in order to be discharged by noon the following day, which for the subgroups of patients admitted emergently corresponded to a roughly 12‐hour increase in LOS. However, it is also plausible that patients who stayed longer also had more time to plan for an early discharge. In either scenario, the ability of managers to utilize LOS as a key metric of throughput efforts may be flawed, and suggests that alternatives (eg, number of patients waiting for beds off unit) may be a more reasonable measure of throughput. Our results have several limitations. As in any observational study, our results are vulnerable to biases from unmeasured covariates that confound the analysis. We caution that a causal relationship between a discharge before noon and LOS cannot be determined from the nature of the study. Our results are also limited in that we were unable to adjust for day‐to‐day hospital capacity and other variables that affect LOS including caregiver and transportation availability, bed capacity at receiving care facilities, and patient consent to discharge. Finally, as a single‐site study, our findings may not be applicable to nonacademic settings.
In conclusion, our observational study discerned an association between discharging patients before noon and longer LOS. We believe our findings suggest a rationale for alternate approaches to measuring an early discharge program's effectiveness, namely, that the evaluation of the success of an early discharge initiative should consider multiple evaluation metrics including the effect on emergency department wait times, intensive care unit or postanesthesia transitions, and on patient reported experiences of care transitions.
Disclosures
Andrew Auerbach, MD, is supported by a K24 grant from the National Heart, Lung, and Blood Institute: K24HL098372. The authors report no conflicts of interest.
- , , , et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16(1):1–10.
- Centers for Medicare 2013.
- , , , et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210–214.
- , , , et al. Discharge before noon: effect on throughput and sustainability. J Hosp Med. 2015;10(10):664–669.
- , , , et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16(1):1–10.
- Centers for Medicare 2013.
- , , , et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210–214.
- , , , et al. Discharge before noon: effect on throughput and sustainability. J Hosp Med. 2015;10(10):664–669.
© 2015 Society of Hospital Medicine
Assessing Discharge Readiness
Widespread evidence suggests that the period around hospitalization remains a vulnerable time for patients. Nearly 20% of patients experience adverse events, including medication errors and hospital readmissions, within 3 weeks of discharge.[1] Multiple factors contribute to adverse events, including the overwhelming volume of information patients receive on their last day in the hospital and fragmented interdisciplinary communication, both among hospital‐based providers and with community providers.[2, 3, 4] A growing body of literature suggests that to ensure patient understanding and a safe transition, discharge planning should start at time of admission. Yet, in the context of high patient volumes and competing priorities, clinicians often postpone discharge planning until they perceive a patient's discharge is imminent. Discharge bundles, designed to improve the safety of hospital discharge, such as those developed by Project BOOST (Better Outcomes by Optimizing Safe Transitions) or Project RED (Re‐Engineered Discharge), are not designed to help providers determine when a patient might be approaching discharge.[5, 6] Early identification of a patient's probable discharge date can provide vital information to inpatient and outpatient teams as they establish comprehensive discharge plans. Accurate discharge‐date predictions allow for effective discharge planning, serving to reduce length of stay (LOS) and consequently improving patient satisfaction and patient safety.[7] However, in the complex world of internal medicine, can clinicians accurately predict the timing of discharge?
A study by Sullivan and colleagues[8] in this issue of the Journal of Hospital Medicine explores a physician's ability to predict hospital discharge. Trainees and attending physicians on general internal medicine wards were asked to predict whether each patient under their care would be discharged on the next day, on the same day, or neither. Discharge predictions were recorded at 3 time points: mornings (79 am), midday (122 pm), or afternoons (57 pm). For predictions of next‐day discharges, the sensitivity (SN) and positive predictive value (PPV) were highest in the afternoon (SN 67%, PPV 69%), whereas for same‐day discharges, accuracy was highest midday (SN 88%, PPV 79%). The authors note that physicians' ability to correctly predict discharges continually improved as time to actual discharge fell.
This study is novel; to our knowledge, no other studies have evaluated the accuracy with which physicians can predict the actual day of discharge. Although this study is particular to a trainee setting and more specific to a single academic medical center, the results are thought provoking. Why are attendings and trainees unable to predict next‐day discharges more accurately? Can we do better? The majority of medical patients are not electively admitted and therefore may have complex and unpredictable courses compared to elective or surgical admissions. Subspecialty consultants may be guiding clinical care and potentially even determining readiness for discharge. Furthermore, the additional responsibilities of teaching and supervising trainees in academic medical centers may further delay discussions and decisions about patient discharges. Another plausible hypothesis, however, is that determination of barriers to discharge and discharge readiness is a clinical skill that is underappreciated and not taught or modeled sufficiently.
If we are to do better at predicting and planning for discharge, we need to build prompts for discharge readiness assessment into our daily work and education of trainees. Although interdisciplinary rounds are typically held in the morning, Wertheimer and colleagues show that additional afternoon interdisciplinary rounds can help identify patients who might be discharged before noon the next day.[9] In their study, identifying such patients in advance improved the overall early discharge rate, moved the average discharge time to earlier in the day, and decreased the observed‐to‐expected LOS, all without any adverse effects on readmissions. We also need more communication between members of the physician care team, especially with subspecialists helping manage care. The authors describe moderate agreement with next‐day and substantial agreement with same‐day discharges between trainees and attendings. Although the authors do not reveal whether trainees or attendings were more accurate, the discrepancy with next‐day discharges is notable. The disagreement suggests a lack of communication between team members about discharge barriers that can hinder planning efforts. Assessing a patient's readiness for and needs upon discharge, and anticipating a patient's disease trajectory, are important clinical skills. Trainees may lack clinical judgment and experience to accurately predict a patient's clinical evolution. As hospitalists, we can role model how to continuously assess patients' discharge needs throughout hospitalization by discussing discharge barriers during daily rounds. As part of transitions of care curricula, in addition to learning about best practices in discharge planning (eg, medication reconciliation, teach back, follow‐up appointments, effective discharge summaries), trainees should be encouraged to conduct structured, daily assessment of discharge readiness and anticipated day of discharge.
Starting the discharge planning process earlier in an admission has the potential to create more thoughtful, efficient, and ultimately safer discharges for our patients. By building discharge readiness assessments into the daily workflow and education curricula, we can prompt trainees and attendings to communicate with interdisciplinary team members and address potential challenges that patients may face in managing their health after discharge. Adequately preparing patients for safe discharges has readmission implications. With Centers for Medicare and Medicaid Services reducing payments to facilities with high rates of readmissions, reducing avoidable readmissions is a priority for all institutions.[10]
We can accomplish safe and early discharges. However, we must get better at accurately assessing our patients' readiness for discharge if we are to take the first step.
Disclosure
Nothing to report.
Widespread evidence suggests that the period around hospitalization remains a vulnerable time for patients. Nearly 20% of patients experience adverse events, including medication errors and hospital readmissions, within 3 weeks of discharge.[1] Multiple factors contribute to adverse events, including the overwhelming volume of information patients receive on their last day in the hospital and fragmented interdisciplinary communication, both among hospital‐based providers and with community providers.[2, 3, 4] A growing body of literature suggests that to ensure patient understanding and a safe transition, discharge planning should start at time of admission. Yet, in the context of high patient volumes and competing priorities, clinicians often postpone discharge planning until they perceive a patient's discharge is imminent. Discharge bundles, designed to improve the safety of hospital discharge, such as those developed by Project BOOST (Better Outcomes by Optimizing Safe Transitions) or Project RED (Re‐Engineered Discharge), are not designed to help providers determine when a patient might be approaching discharge.[5, 6] Early identification of a patient's probable discharge date can provide vital information to inpatient and outpatient teams as they establish comprehensive discharge plans. Accurate discharge‐date predictions allow for effective discharge planning, serving to reduce length of stay (LOS) and consequently improving patient satisfaction and patient safety.[7] However, in the complex world of internal medicine, can clinicians accurately predict the timing of discharge?
A study by Sullivan and colleagues[8] in this issue of the Journal of Hospital Medicine explores a physician's ability to predict hospital discharge. Trainees and attending physicians on general internal medicine wards were asked to predict whether each patient under their care would be discharged on the next day, on the same day, or neither. Discharge predictions were recorded at 3 time points: mornings (79 am), midday (122 pm), or afternoons (57 pm). For predictions of next‐day discharges, the sensitivity (SN) and positive predictive value (PPV) were highest in the afternoon (SN 67%, PPV 69%), whereas for same‐day discharges, accuracy was highest midday (SN 88%, PPV 79%). The authors note that physicians' ability to correctly predict discharges continually improved as time to actual discharge fell.
This study is novel; to our knowledge, no other studies have evaluated the accuracy with which physicians can predict the actual day of discharge. Although this study is particular to a trainee setting and more specific to a single academic medical center, the results are thought provoking. Why are attendings and trainees unable to predict next‐day discharges more accurately? Can we do better? The majority of medical patients are not electively admitted and therefore may have complex and unpredictable courses compared to elective or surgical admissions. Subspecialty consultants may be guiding clinical care and potentially even determining readiness for discharge. Furthermore, the additional responsibilities of teaching and supervising trainees in academic medical centers may further delay discussions and decisions about patient discharges. Another plausible hypothesis, however, is that determination of barriers to discharge and discharge readiness is a clinical skill that is underappreciated and not taught or modeled sufficiently.
If we are to do better at predicting and planning for discharge, we need to build prompts for discharge readiness assessment into our daily work and education of trainees. Although interdisciplinary rounds are typically held in the morning, Wertheimer and colleagues show that additional afternoon interdisciplinary rounds can help identify patients who might be discharged before noon the next day.[9] In their study, identifying such patients in advance improved the overall early discharge rate, moved the average discharge time to earlier in the day, and decreased the observed‐to‐expected LOS, all without any adverse effects on readmissions. We also need more communication between members of the physician care team, especially with subspecialists helping manage care. The authors describe moderate agreement with next‐day and substantial agreement with same‐day discharges between trainees and attendings. Although the authors do not reveal whether trainees or attendings were more accurate, the discrepancy with next‐day discharges is notable. The disagreement suggests a lack of communication between team members about discharge barriers that can hinder planning efforts. Assessing a patient's readiness for and needs upon discharge, and anticipating a patient's disease trajectory, are important clinical skills. Trainees may lack clinical judgment and experience to accurately predict a patient's clinical evolution. As hospitalists, we can role model how to continuously assess patients' discharge needs throughout hospitalization by discussing discharge barriers during daily rounds. As part of transitions of care curricula, in addition to learning about best practices in discharge planning (eg, medication reconciliation, teach back, follow‐up appointments, effective discharge summaries), trainees should be encouraged to conduct structured, daily assessment of discharge readiness and anticipated day of discharge.
Starting the discharge planning process earlier in an admission has the potential to create more thoughtful, efficient, and ultimately safer discharges for our patients. By building discharge readiness assessments into the daily workflow and education curricula, we can prompt trainees and attendings to communicate with interdisciplinary team members and address potential challenges that patients may face in managing their health after discharge. Adequately preparing patients for safe discharges has readmission implications. With Centers for Medicare and Medicaid Services reducing payments to facilities with high rates of readmissions, reducing avoidable readmissions is a priority for all institutions.[10]
We can accomplish safe and early discharges. However, we must get better at accurately assessing our patients' readiness for discharge if we are to take the first step.
Disclosure
Nothing to report.
Widespread evidence suggests that the period around hospitalization remains a vulnerable time for patients. Nearly 20% of patients experience adverse events, including medication errors and hospital readmissions, within 3 weeks of discharge.[1] Multiple factors contribute to adverse events, including the overwhelming volume of information patients receive on their last day in the hospital and fragmented interdisciplinary communication, both among hospital‐based providers and with community providers.[2, 3, 4] A growing body of literature suggests that to ensure patient understanding and a safe transition, discharge planning should start at time of admission. Yet, in the context of high patient volumes and competing priorities, clinicians often postpone discharge planning until they perceive a patient's discharge is imminent. Discharge bundles, designed to improve the safety of hospital discharge, such as those developed by Project BOOST (Better Outcomes by Optimizing Safe Transitions) or Project RED (Re‐Engineered Discharge), are not designed to help providers determine when a patient might be approaching discharge.[5, 6] Early identification of a patient's probable discharge date can provide vital information to inpatient and outpatient teams as they establish comprehensive discharge plans. Accurate discharge‐date predictions allow for effective discharge planning, serving to reduce length of stay (LOS) and consequently improving patient satisfaction and patient safety.[7] However, in the complex world of internal medicine, can clinicians accurately predict the timing of discharge?
A study by Sullivan and colleagues[8] in this issue of the Journal of Hospital Medicine explores a physician's ability to predict hospital discharge. Trainees and attending physicians on general internal medicine wards were asked to predict whether each patient under their care would be discharged on the next day, on the same day, or neither. Discharge predictions were recorded at 3 time points: mornings (79 am), midday (122 pm), or afternoons (57 pm). For predictions of next‐day discharges, the sensitivity (SN) and positive predictive value (PPV) were highest in the afternoon (SN 67%, PPV 69%), whereas for same‐day discharges, accuracy was highest midday (SN 88%, PPV 79%). The authors note that physicians' ability to correctly predict discharges continually improved as time to actual discharge fell.
This study is novel; to our knowledge, no other studies have evaluated the accuracy with which physicians can predict the actual day of discharge. Although this study is particular to a trainee setting and more specific to a single academic medical center, the results are thought provoking. Why are attendings and trainees unable to predict next‐day discharges more accurately? Can we do better? The majority of medical patients are not electively admitted and therefore may have complex and unpredictable courses compared to elective or surgical admissions. Subspecialty consultants may be guiding clinical care and potentially even determining readiness for discharge. Furthermore, the additional responsibilities of teaching and supervising trainees in academic medical centers may further delay discussions and decisions about patient discharges. Another plausible hypothesis, however, is that determination of barriers to discharge and discharge readiness is a clinical skill that is underappreciated and not taught or modeled sufficiently.
If we are to do better at predicting and planning for discharge, we need to build prompts for discharge readiness assessment into our daily work and education of trainees. Although interdisciplinary rounds are typically held in the morning, Wertheimer and colleagues show that additional afternoon interdisciplinary rounds can help identify patients who might be discharged before noon the next day.[9] In their study, identifying such patients in advance improved the overall early discharge rate, moved the average discharge time to earlier in the day, and decreased the observed‐to‐expected LOS, all without any adverse effects on readmissions. We also need more communication between members of the physician care team, especially with subspecialists helping manage care. The authors describe moderate agreement with next‐day and substantial agreement with same‐day discharges between trainees and attendings. Although the authors do not reveal whether trainees or attendings were more accurate, the discrepancy with next‐day discharges is notable. The disagreement suggests a lack of communication between team members about discharge barriers that can hinder planning efforts. Assessing a patient's readiness for and needs upon discharge, and anticipating a patient's disease trajectory, are important clinical skills. Trainees may lack clinical judgment and experience to accurately predict a patient's clinical evolution. As hospitalists, we can role model how to continuously assess patients' discharge needs throughout hospitalization by discussing discharge barriers during daily rounds. As part of transitions of care curricula, in addition to learning about best practices in discharge planning (eg, medication reconciliation, teach back, follow‐up appointments, effective discharge summaries), trainees should be encouraged to conduct structured, daily assessment of discharge readiness and anticipated day of discharge.
Starting the discharge planning process earlier in an admission has the potential to create more thoughtful, efficient, and ultimately safer discharges for our patients. By building discharge readiness assessments into the daily workflow and education curricula, we can prompt trainees and attendings to communicate with interdisciplinary team members and address potential challenges that patients may face in managing their health after discharge. Adequately preparing patients for safe discharges has readmission implications. With Centers for Medicare and Medicaid Services reducing payments to facilities with high rates of readmissions, reducing avoidable readmissions is a priority for all institutions.[10]
We can accomplish safe and early discharges. However, we must get better at accurately assessing our patients' readiness for discharge if we are to take the first step.
Disclosure
Nothing to report.
Striving for Optimal Care/
Hospitalists have a professional obligation to provide the highest quality care for patients and increasingly, hospitalists lead programs to improve quality, value, and patient experience.[1, 2, 3]
The federal government introduced the hospital Value‐Based Purchasing (VBP) program in 2012, initially with 1% of Medicare hospital payments tied to quality indicators. This percentage will continue to grow and the VBP program has expanded to include metrics related to quality, safety, cost‐effectiveness, and patient satisfaction.[4] Hospitals now face significant financial penalties if they do not achieve these benchmarks; thus, remaining up‐to‐date with the literature and the most promising interventions in these arenas is vital for hospitalists.
The goal of this update is to summarize and critique recently published research that has the greatest potential to impact clinical practice in quality, value, and patient experience in hospital medicine. We reviewed articles published between January 2014 and February 2015. To identify articles, we hand‐searched leading journals, continuing medical education collaborative journal reviews (including New England Journal of Medicine Journal Watch and the American College of Physicians Journal Club), the Agency for Healthcare Research and Quality's Patient Safety network, and PubMed. We evaluated articles based on their scientific rigor (peer review, study methodology, site number, and sample size) and applicability to hospital medicine. In this review, we summarize 9 articles that were felt by the authors to have the highest potential for impact on the clinical practice of hospital medicine, as directly related to quality, value, or patient experience. We present each topic with a current quality question that the accompanying article(s) will help address. We summarize each article and its findings and note cautions and implications for practice. The selected articles cover aspects related to patient safety, readmissions, patient satisfaction, and resource utilization, with each of these topics related to specific metrics included in VBP. We presented this update at the 2015 Society of Hospital Medicine national meeting.
IS THERE ANYTHING WE CAN DO TO MAKE HANDOFFS SAFER?
Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):18031812.
Background
With recent changes in resident duty hours and staffing models, the number of clinical handoffs during a patient's hospital stay has been increasing.[5] The omission of critical information and the transfer of erroneous information during handoffs is common, which contributes to preventable medical errors.[6]
Findings
This prospective intervention study of a resident handoff program in 9 hospitals sought to improve communication between healthcare providers and to decrease medical errors. The I‐PASS mnemonic, which stands for illness severity, patient summary, action list, situation awareness, and synthesis by receiver, was introduced to standardize oral and written handoffs. The program also included a 2‐hour workshop, a 1‐hour role‐playing and simulation session, a computer module, a faculty development program, direct observation tools, and a culture change campaign. Medical errors decreased by 23% following the intervention, compared to the preintervention baseline (24.5 vs 18.8 per 100 admissions, P 0.001), and the rate of preventable adverse events dropped by 30% (4.7 vs 3.3 events per 100 admissions, P 0.001), whereas nonpreventable adverse events did not change. Process measures of handoff quality uniformly improved with the intervention. The duration of oral handoffs was approximately 2.5 minutes per patient both before and during the intervention period.
Cautions
Not all of the sites in the study saw significant reductions in medical errors; 3 of the programs did not have significantly improved medical error rates following implementation of the I‐PASS handoff bundle. The study design was not a randomized controlled trial, and thus the pre‐ versus postimplementation analyses cannot draw definitive causal links between the intervention and the observed improvements in safety outcomes. Furthermore, this study was done with pediatric residents, and one cannot assume that the results will translate to practicing hospitalists, who may not benefit as much from a scripted sign‐out.
Implications
A comprehensive handoff program that included the I‐PASS mnemonic along with extensive training, faculty development, and a culture‐change campaign was associated with impressive improvements in patient safety outcomes, without negatively effecting workflow.
WHAT ARE THE COMMON FEATURES OF INTERVENTIONS THAT HAVE SUCCESSFULLY REDUCED READMISSIONS?
Leppin AL, Glonfriddo MR, Kessler M, et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174(7):10951107.
Background
Hospital readmissions are common, costly, and potentially represent a failure to adequately prepare patients for hospital discharge, but efforts to prevent 30‐day readmissions have been mixed.[7] The investigators in this study offer a novel framework, the cumulative complexity model, as a way to conceptualize postdischarge outcomes such as readmission. The model depicts the balance between the patient's workload of managing their illness, including the demands of monitoring treatment and self‐care, and the patient's capacity to handle that workfunctionality, financial/social resources, literacy, and empowerment. Workload‐capacity imbalances (when workload outstrips capacity) may lead to progressively increasing illness and increasing complexity, which contribute to poor patient outcomes like readmissions. Decreasing a patient's workload or increasing their capacity may be effective in reducing readmissions.
Findings
Investigators sought to identify factors associated with successful interventions to reduce 30‐day readmissions, including how the interventions fit into the cumulative complexity model. After performing a comprehensive search of randomized trials of interventions to reduce readmissions, the investigators identified 42 randomized trials with the primary outcome of 30‐day readmission rates. In addition to reviewing intervention characteristics, blinded raters scored interventions based on their effects on reducing or increasing patient workload and reducing or increasing patient capacity for self‐care. Interventions that had several components (eg, pharmacy education, postdischarge phone calls, visiting nurses, health coaches, close primary care follow‐up) were more likely to be successful (1.4 times as likely; P = 0.001), as were interventions that involved 2 or more individuals (1.3 times as likely; P = 0.05). Interventions that were published prior to 2002 were 1.6 times more likely to have reduced readmissions (P = 0.01). When applied to the cumulative complexity model, interventions that sought to augment patient capacity for self‐care were 1.3 times as likely to be successful (P = 0.04), whereas no relationship was found between an intervention's effect on patient workload and readmission.
Cautions
The authors evaluated each intervention based on the degree to which it was likely to affect patient workload and patient capacity. Because a multifaceted intervention may have had components that increased patient workload (eg, more self‐monitoring, appointments) and decreased patient workload (home visits, visiting nurses), the true effect of patient workload on readmissions may not have been optimally analyzed in this study. Additionally, this element of the study relied on a value judgment original to this work. Interventions that are burdensome to some, may be beneficial to those with the capacity and resources to access the care.
Implications
The body of studies reviewed suggests that interventions to reduce 30‐day readmissions are on the whole successful. Their findings are in keeping with past studies demonstrating more successful interventions that are resource‐intensive and multifaceted. Finding successful interventions that are also cost‐effective may be challenging. This article adds the cumulative complexity framework to what we already know about readmissions, highlighting patient capacity to manage the burden of their Illness as a new factor for success. Efforts to deliver patient‐centered education, explore barriers to adherence, and provide health coaching may be more successful than interventions that unwittingly add to the burden of disease treatment (multiple follow‐up appointments, complex medication schedules, and posthospital surveys and patient self‐assessments).
DOES PATIENT ACTIVATION CORRELATE WITH DECREASED RESOURCE USE OR READMISSIONS?
Mitchell SE, Gardiner PM, Sadikova E, et al. Patient activation and 30‐day post discharge hospital utilization. J Gen Intern Med. 2014;29(2):349355.
Background
Patient activation is widely recognized as the knowledge, skills, and confidence a person has in managing their own health or healthcare. Higher patient activation has been associated with improved health outcomes, but the relationship between patient activation and readmission to the hospital within 30 days is unknown.[8]
Findings
Using data from Project RED‐LIT (Re‐Engineered Discharge for patients with low health literacy), a randomized controlled trial conducted at an urban safety‐net hospital, investigators examined the relationship between all unplanned utilization events of hospital services within 30 days of discharge and patient activation, as measured by an abbreviated 8‐item version of the validated Patient Activation Measure (PAM). The PAM uses agreement with statements about a patient's sense of responsibility for his or her own health, confidence in seeking care and following through with medical treatments, and confidence in managing new problems to measure activation. The 695 participants were divided into quartiles based on their PAM score, and the investigators looked at the rates of unplanned utilization events in each group. After adjusting for potential confounders such as gender, age, Charlson Comorbidity Index, insurance, marital status, and education, there remained a significant effect between PAM and 30‐day hospital reutilization. Compared with those who scored in the highest quartile of activation, those in the lowest quartile had 1.75 times the rate of 30‐day reutilization (P 0.001). Those in the second highest and third highest quartile had 1.3 (P = 0.03) and 1.5 times (P 0.001) the rate of reutilization demonstrating a dose‐response relationship between activation and low reutilization.
Cautions
It is as yet unclear how best to apply these results and whether activation is a modifiable risk factor. Can a patient become more activated by providing more education and coaching during their hospital stay? Can providing close follow‐up and home services make a person more confident to manage their own illness? Although early identification of patients with low activation using PAM is being done at many hospitals, there is no study to suggest that targeting these patients can reduce readmission.
Implications
A low level of patient activation appears to be a risk factor for unplanned hospital utilization within 30 days of discharge. Given the increasing financial penalties, many hospitals across the country are using the PAM to determine how much support and which services they provide after discharge. Identifying these patients early in their hospitalization could allow providers to spend more time and attention on preparing them for managing their own illness after discharge. As above, the effects of this intervention on readmissions is as yet unclear.
IS THERE A RELATIONSHIP BETWEEN PATIENT SATISFACTION AND UNDERSTANDING OF THE PLAN OF CARE?
Kebede S, Shihab HM, Berger ZD, et al. Patients' understanding of their hospitalizations and association with satisfaction. JAMA Intern Med. 2014;174(10):16981700.
Background
Effective patient‐physician communication is associated with improved patient satisfaction, care quality, and clinical outcomes.[9] Whether a shared understanding of the plan of care between patients and clinicians affects satisfaction is unknown.
Findings
One hundred seventy‐seven patients who had 2 or more medical conditions, 2 or more medical procedures, and 2 or more days in the hospital were interviewed on the day of discharge. Patients were questioned about their overall understanding of their hospitalization and about specific aspects of their care. They were also asked to provide objective data to measure their understanding of their hospital course by (1) listing their medical diagnoses, (2) identifying indications for medication on discharge paperwork, and (3) listing tests or procedures they underwent from a standard list. Patients were then asked to rate their satisfaction with their hospitalization. Patients' self‐reported understanding was an average of 4.0 (very good) on a 5‐point scale. Their measured understanding scores for medical diagnoses, indications for medications and tests and procedures were 48.9%, 56.2%, and 59.4%, respectively. Factors associated with poor understanding of their hospital course were increasing age, less education, lower household income, black race, and longer length of stay. Patients reported a mean satisfaction of 4.0 (very satisfied). Higher self‐reported understanding was associated with higher patient satisfaction, irrespective of actual understanding.
Cautions
Despite their suboptimal measured understanding of their hospital course, the average patient rated their understanding as very good. This suggests that patients are either poor judges of effective communication or have low expectations for understanding. It also calls into question the relationship between quality of communication and patient satisfaction, because despite their satisfaction, patients' actual understanding was low. There was, however, a clear and positive relationship between patients' perceived understanding and their satisfaction, suggesting that shared understanding remains integral to patient satisfaction.
Implications
Patient satisfaction appears to be tied to patients' perceived understanding of their care, but when tested actual understanding was suboptimal. Further efforts in patient satisfaction should not only focus on the quality of our communication, but on the resulting understanding of our patients.
WHAT ARE UNIVERSAL STRATEGIES TO IMPROVE SATISFACTION AND PATIENT OUTCOMES?
Detsky AS, Krumholz HM. Reducing the trauma of hospitalization. JAMA. 2014;311(21):21692170.
Background
Although high readmission rates are a national problem, a minority of patients treated for common conditions like pneumonia, heart failure, and chronic obstructive pulmonary disease are readmitted for the same problem.[10] This suggests that readmissions may stem not from poor disease management, but from patient vulnerability to illness in the period following hospitalization.
Findings
In this viewpoint opinion article, the authors suggest that the depersonalizing and stressful hospital atmosphere contributes to a transient vulnerability in the period following hospitalization that makes it challenging for patients to care for themselves and their illness. They offer specific strategies for changing the nature of our hospital care to promote healing and to decrease patient stress. The authors suggest promoting personalization through accommodation of family members, and allowing personal clothing and personal dcor in their rooms. Physicians and consultants should make appointments so that patients and families can know when to expect important visits. The authors also focus on the provision of rest and nourishment by reducing nighttime disruption and the elimination on unnecessary restrictive diets. They argue that the hospital is a place of stressful disruptions and surprises, which could all be ameliorated by providing patients with a way to understand the members of their team and their roles as well as through providing a clear schedule for the day. Healthcare providers should not enter a room unannounced, and patients should be given private rooms as much as possible. Last, the authors focus on the elimination of unnecessary tests and procedures such as blood draws, telemetry, and urine cultures and the encouragement of activity by providing activities where patients can gather together outside their rooms.
Cautions
If these changes seem simple, they may not be. Many involve a significant shift in our thinking on how we provide carefrom a focus on disease and provider convenience to a true consideration for the health and peace of mind of our patients. Starting with small steps, such as reductions in phlebotomy and nighttime vital signs checks for the most stable patients and ensuring accommodations for families, may make this long list seem less daunting.
Implications
By promoting factors that affect a patient's well beingrest, nutrition, peace of mindwe may be discharging patients who are better equipped to manage their illness after their hospitalization.
DO HOSPITALISTS OVERTEST, AND IF SO, WHY?
Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100108.
Background
National efforts, such as the Choosing Wisely campaign, seek to decrease overuse of low‐value services.[11] The extent of the problem of overtesting among hospitalists and the underlying drivers for unnecessary testing in this group have not been clearly defined.
Findings
Practicing adult medicine hospitalists across the country were given a questionnaire that included clinical vignettes for common inpatient scenarios: a preoperative evaluation and a syncope workup. Respondents were randomly provided 1 of 4 versions of each vignette, which contained the same clinical information but varied by a family member's request for further testing and by disclosure of the occupation of the family member. For example, in the preoperative evaluation, the vignettes either: (1) provided no details about the patient's son; (2) identified the son as a physician; (3) mentioned the son's request for testing, but did not identify the son as a physician; or (4) identified the son as a physician who requested testing. The syncope vignette versions were structured similarly, except the family member was the patient's wife and she was an attorney. The authors collected 1020 responses from an initial pool of 1500, for a decent 68% response rate. Hospitalists commonly reported overuse of testing, with 52% to 65% of respondents requesting unnecessary testing in the preoperative evaluation scenario, and 82% to 85% in the syncope scenario. The majority of physicians reported that they knew the testing was not clinically indicated based on evidence or guidelines, but were ordering the test due to a desire to reassure the patients or themselves.
Cautions
Responses to clinical vignettes in a survey may not represent actually practices. In addition, all hospitalists surveyed in this study were members of the Society of Hospital Medicine, so may not accurately exemplify all practicing hospitalists.
Implications
Overuse of testing is very common among hospitalists. Although roughly one‐third of respondents incorrectly thought that testing in the given scenarios was supported by the evidence or guidelines, the majority knew that testing was not clinically indicated and reported ordering tests to help reassure their patients or themselves. This suggests evidence‐based medicine approaches to overuse, such as the Choosing Wisely campaign and the emergence of appropriateness criteria, are likely necessary but insufficient to change physician practice patterns. Efforts to decrease overuse will need to engage clinicians and patients in ways that help overcome the attitude that more testing is required to provide reassurance.
DO UNREALISTIC PATIENT EXPECTATIONS ABOUT INTERVENTIONS INFLUENCE DECISION MAKING AND CONTRIBUTE TO OVERUSE?
Hoffmann TC, Del Mar C. Patient expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2015;175(2):274286.
Background
Patient expectations have been implicated as a contributor to overuse of medical interventions. Studies that have measured patients' understanding of the potential benefits and harms of medical treatments and tests have been scattered across the literature.
Findings
This systematic review aggregated all studies that have quantitatively assessed patients' expectations of the benefits and/or harms of any treatment or test. Of more than 15,000 records screened, only 36 articles met the inclusion criteria of describing a study in which participants were asked to provide a quantitative estimate of the expected benefits and/or harms of a treatment, test, or screen. Fourteen of the studies (40%) focused on screening, 15 (43%) on treatment, 3 (9%) on a test, and 3 (9%) on both treatment and screening. Topics included cancer, medications, surgery, cardiovascular disease, and fetal‐maternal medicine. The majority of patients overestimated intervention benefit and underestimated harm, regardless of whether the intervention was a test or a treatment. For example, more than half of participants overestimated benefit for 22 of the 34 outcomes (65%) for which overestimation data were provided, and a majority of participants underestimated harm for 10 of the 15 outcomes (67%) with underestimation data available.
Cautions
This systematic review included a limited number of studies, with varying levels of quality and a lot of heterogeneity, making it difficult to reach clear aggregate conclusions.
Implications
Patients are often overly optimistic about medical interventions and they downplay potential risks, making it more difficult to effectively discourage overuse. Clinicians should clearly understand and communicate realistic expectations for the potential benefits and risks of screening, testing, and medical treatments with patients and the public at large.
HOW BIG OF A PROBLEM IS ANTIBIOTIC OVERUSE IN HOSPITALS AND CAN WE DO BETTER?
Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194200.
Background
Antibiotics are life‐saving therapies, but when used in inappropriate scenarios they can pose many risks.
Findings
This large national database study used the MarketScan Hospital Drug Database and the Centers for Disease Control and Prevention's (CDC) Emerging Infections Program data to explore antibiotic prescribing in hospital patients. More than half of all hospitalized patients (55.7%) received antibiotics during their stay. Half of all treatment antibiotics were prescribed for the treatment of either lower respiratory infections, urinary tract infections, or presumed gram‐positive infections. There was wide variation seen in antibiotic usage across hospital wards. Objective criteria for potential improvement in antimicrobial use were developed and applied at a subset of 36 hospitals. Antibiotic prescribing could be improved in 37.2% of the most common prescription scenarios reviewed, including patients receiving vancomycin or those being treated for a urinary tract infection. The impact of reducing inpatient antibiotic exposure on the incidence of Clostridium difficile colitis was modeled using data from 2 hospitals, revealing that decreasing hospitalized patients' exposure to broad‐spectrum antibiotics by 30% would lead to a 26% reduction in C difficile infections (interquartile range = 15%38%).
Cautions
Some of the estimates in this study are based on a convenience sample of claims and hospital‐based data, thus may not be an accurate representation, particularly when extrapolating to all US hospitals.
Implications
Antibiotic overuse is a rampant problem in hospitals, with many severe downstream effects such as C difficile infections and antimicrobial resistance. All hospital units should have an antibiotic stewardship program and should monitor antibiotic usage.
Lee TC, Frenette C, Jayaraman D, Green L, Pilote L. Antibiotic self‐stewardship: trainee‐led structured antibiotic time‐outs to improve antimicrobial use. Ann Intern Med. 2014;161(10 suppl):S53S58.
Background
The CDC and other groups have called for stewardship programs to address antibiotic overuse.[12] Few interventions have been shown to successfully engage medical trainees in efforts to improve their own antibiotic prescribing practices.
Findings
An antibiotic self‐stewardship program was developed and led by internal medicine residents at Montreal General Hospital. The intervention included a monthly resident education lecture on antimicrobial stewardship and twice‐weekly time‐out audits using a structured electronic checklist. Adherence with auditing was 80%. Total costs for antibiotics decreased from $149,743 CAD to $80,319 CAD, mostly due to an observed reduction in carbapenems. Moxifloxicin use decreased by 1.9 defined daily doses per 1000 patient‐days per month (P = 0.048). Rates of clostridium difficile colitis declined from 24.2 to 19.6 per 10,000 patient‐days, although this trend did not meet statistical significance (incidence rate ratio, 0.8 [confidence interval, 0.5‐1.3]).
Cautions
Although the use of some broader spectrum antibiotics decreased, there was no measurable change in overall antibiotic use, suggesting that physicians may have narrowed antibiotics but did not often completely discontinue them. The time‐series analyses in this study cannot provide causal conclusions between the intervention and outcomes. In fact, carbapenem usage appears to have significantly decreased prior to the implementation of the program, for unclear reasons. The feasibility of this educational intervention outside of a residency program is unclear.
Implications
A combination of education, oversight and frontline clinician engagement in structured time‐outs may be effective, at least in narrowing antibiotic usage. The structured audit checklist developed by these authors is available for free in the supplementary materials of the Annals of Internal Medicine article.
Disclosures: Dr. Moriates has received grant funding from the ABIM Foundation, and royalties from McGraw‐Hill for the textbook Understanding Value‐Based Healthcare. The authors report no conflicts of interest.
- . The role of the hospitalist in quality improvement: systems for improving the care of patients with acute coronary syndrome. J Hosp Med. 2010;5(suppl 4):S1–S7.
- , , , . Impact of hospitalist communication‐skills training on patient‐satisfaction scores. J Hosp Med. 2013;8(6):315–320.
- , , , . Development of a hospital‐based program focused on improving healthcare value. J Hosp Med. 2014;9(10):671–677.
- . Value‐driven health care: implications for hospitals and hospitalists. J Hosp Med. 2009;4(8):507–511.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013;173(8):649–655.
- , , , , . Does housestaff discontinuity of care increase the risk for preventable adverse events? Ann Intern Med. 1994;121(11):866–872.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520–528.
- , , . Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8(5):410–417.
- . Effective physician‐patient communication and health outcomes: a review. CMAJ. 2007;152(9):1423–1433.
- , , , et al. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355–363.
- , , , et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486–492.
- , , , et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194–200.
Hospitalists have a professional obligation to provide the highest quality care for patients and increasingly, hospitalists lead programs to improve quality, value, and patient experience.[1, 2, 3]
The federal government introduced the hospital Value‐Based Purchasing (VBP) program in 2012, initially with 1% of Medicare hospital payments tied to quality indicators. This percentage will continue to grow and the VBP program has expanded to include metrics related to quality, safety, cost‐effectiveness, and patient satisfaction.[4] Hospitals now face significant financial penalties if they do not achieve these benchmarks; thus, remaining up‐to‐date with the literature and the most promising interventions in these arenas is vital for hospitalists.
The goal of this update is to summarize and critique recently published research that has the greatest potential to impact clinical practice in quality, value, and patient experience in hospital medicine. We reviewed articles published between January 2014 and February 2015. To identify articles, we hand‐searched leading journals, continuing medical education collaborative journal reviews (including New England Journal of Medicine Journal Watch and the American College of Physicians Journal Club), the Agency for Healthcare Research and Quality's Patient Safety network, and PubMed. We evaluated articles based on their scientific rigor (peer review, study methodology, site number, and sample size) and applicability to hospital medicine. In this review, we summarize 9 articles that were felt by the authors to have the highest potential for impact on the clinical practice of hospital medicine, as directly related to quality, value, or patient experience. We present each topic with a current quality question that the accompanying article(s) will help address. We summarize each article and its findings and note cautions and implications for practice. The selected articles cover aspects related to patient safety, readmissions, patient satisfaction, and resource utilization, with each of these topics related to specific metrics included in VBP. We presented this update at the 2015 Society of Hospital Medicine national meeting.
IS THERE ANYTHING WE CAN DO TO MAKE HANDOFFS SAFER?
Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):18031812.
Background
With recent changes in resident duty hours and staffing models, the number of clinical handoffs during a patient's hospital stay has been increasing.[5] The omission of critical information and the transfer of erroneous information during handoffs is common, which contributes to preventable medical errors.[6]
Findings
This prospective intervention study of a resident handoff program in 9 hospitals sought to improve communication between healthcare providers and to decrease medical errors. The I‐PASS mnemonic, which stands for illness severity, patient summary, action list, situation awareness, and synthesis by receiver, was introduced to standardize oral and written handoffs. The program also included a 2‐hour workshop, a 1‐hour role‐playing and simulation session, a computer module, a faculty development program, direct observation tools, and a culture change campaign. Medical errors decreased by 23% following the intervention, compared to the preintervention baseline (24.5 vs 18.8 per 100 admissions, P 0.001), and the rate of preventable adverse events dropped by 30% (4.7 vs 3.3 events per 100 admissions, P 0.001), whereas nonpreventable adverse events did not change. Process measures of handoff quality uniformly improved with the intervention. The duration of oral handoffs was approximately 2.5 minutes per patient both before and during the intervention period.
Cautions
Not all of the sites in the study saw significant reductions in medical errors; 3 of the programs did not have significantly improved medical error rates following implementation of the I‐PASS handoff bundle. The study design was not a randomized controlled trial, and thus the pre‐ versus postimplementation analyses cannot draw definitive causal links between the intervention and the observed improvements in safety outcomes. Furthermore, this study was done with pediatric residents, and one cannot assume that the results will translate to practicing hospitalists, who may not benefit as much from a scripted sign‐out.
Implications
A comprehensive handoff program that included the I‐PASS mnemonic along with extensive training, faculty development, and a culture‐change campaign was associated with impressive improvements in patient safety outcomes, without negatively effecting workflow.
WHAT ARE THE COMMON FEATURES OF INTERVENTIONS THAT HAVE SUCCESSFULLY REDUCED READMISSIONS?
Leppin AL, Glonfriddo MR, Kessler M, et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174(7):10951107.
Background
Hospital readmissions are common, costly, and potentially represent a failure to adequately prepare patients for hospital discharge, but efforts to prevent 30‐day readmissions have been mixed.[7] The investigators in this study offer a novel framework, the cumulative complexity model, as a way to conceptualize postdischarge outcomes such as readmission. The model depicts the balance between the patient's workload of managing their illness, including the demands of monitoring treatment and self‐care, and the patient's capacity to handle that workfunctionality, financial/social resources, literacy, and empowerment. Workload‐capacity imbalances (when workload outstrips capacity) may lead to progressively increasing illness and increasing complexity, which contribute to poor patient outcomes like readmissions. Decreasing a patient's workload or increasing their capacity may be effective in reducing readmissions.
Findings
Investigators sought to identify factors associated with successful interventions to reduce 30‐day readmissions, including how the interventions fit into the cumulative complexity model. After performing a comprehensive search of randomized trials of interventions to reduce readmissions, the investigators identified 42 randomized trials with the primary outcome of 30‐day readmission rates. In addition to reviewing intervention characteristics, blinded raters scored interventions based on their effects on reducing or increasing patient workload and reducing or increasing patient capacity for self‐care. Interventions that had several components (eg, pharmacy education, postdischarge phone calls, visiting nurses, health coaches, close primary care follow‐up) were more likely to be successful (1.4 times as likely; P = 0.001), as were interventions that involved 2 or more individuals (1.3 times as likely; P = 0.05). Interventions that were published prior to 2002 were 1.6 times more likely to have reduced readmissions (P = 0.01). When applied to the cumulative complexity model, interventions that sought to augment patient capacity for self‐care were 1.3 times as likely to be successful (P = 0.04), whereas no relationship was found between an intervention's effect on patient workload and readmission.
Cautions
The authors evaluated each intervention based on the degree to which it was likely to affect patient workload and patient capacity. Because a multifaceted intervention may have had components that increased patient workload (eg, more self‐monitoring, appointments) and decreased patient workload (home visits, visiting nurses), the true effect of patient workload on readmissions may not have been optimally analyzed in this study. Additionally, this element of the study relied on a value judgment original to this work. Interventions that are burdensome to some, may be beneficial to those with the capacity and resources to access the care.
Implications
The body of studies reviewed suggests that interventions to reduce 30‐day readmissions are on the whole successful. Their findings are in keeping with past studies demonstrating more successful interventions that are resource‐intensive and multifaceted. Finding successful interventions that are also cost‐effective may be challenging. This article adds the cumulative complexity framework to what we already know about readmissions, highlighting patient capacity to manage the burden of their Illness as a new factor for success. Efforts to deliver patient‐centered education, explore barriers to adherence, and provide health coaching may be more successful than interventions that unwittingly add to the burden of disease treatment (multiple follow‐up appointments, complex medication schedules, and posthospital surveys and patient self‐assessments).
DOES PATIENT ACTIVATION CORRELATE WITH DECREASED RESOURCE USE OR READMISSIONS?
Mitchell SE, Gardiner PM, Sadikova E, et al. Patient activation and 30‐day post discharge hospital utilization. J Gen Intern Med. 2014;29(2):349355.
Background
Patient activation is widely recognized as the knowledge, skills, and confidence a person has in managing their own health or healthcare. Higher patient activation has been associated with improved health outcomes, but the relationship between patient activation and readmission to the hospital within 30 days is unknown.[8]
Findings
Using data from Project RED‐LIT (Re‐Engineered Discharge for patients with low health literacy), a randomized controlled trial conducted at an urban safety‐net hospital, investigators examined the relationship between all unplanned utilization events of hospital services within 30 days of discharge and patient activation, as measured by an abbreviated 8‐item version of the validated Patient Activation Measure (PAM). The PAM uses agreement with statements about a patient's sense of responsibility for his or her own health, confidence in seeking care and following through with medical treatments, and confidence in managing new problems to measure activation. The 695 participants were divided into quartiles based on their PAM score, and the investigators looked at the rates of unplanned utilization events in each group. After adjusting for potential confounders such as gender, age, Charlson Comorbidity Index, insurance, marital status, and education, there remained a significant effect between PAM and 30‐day hospital reutilization. Compared with those who scored in the highest quartile of activation, those in the lowest quartile had 1.75 times the rate of 30‐day reutilization (P 0.001). Those in the second highest and third highest quartile had 1.3 (P = 0.03) and 1.5 times (P 0.001) the rate of reutilization demonstrating a dose‐response relationship between activation and low reutilization.
Cautions
It is as yet unclear how best to apply these results and whether activation is a modifiable risk factor. Can a patient become more activated by providing more education and coaching during their hospital stay? Can providing close follow‐up and home services make a person more confident to manage their own illness? Although early identification of patients with low activation using PAM is being done at many hospitals, there is no study to suggest that targeting these patients can reduce readmission.
Implications
A low level of patient activation appears to be a risk factor for unplanned hospital utilization within 30 days of discharge. Given the increasing financial penalties, many hospitals across the country are using the PAM to determine how much support and which services they provide after discharge. Identifying these patients early in their hospitalization could allow providers to spend more time and attention on preparing them for managing their own illness after discharge. As above, the effects of this intervention on readmissions is as yet unclear.
IS THERE A RELATIONSHIP BETWEEN PATIENT SATISFACTION AND UNDERSTANDING OF THE PLAN OF CARE?
Kebede S, Shihab HM, Berger ZD, et al. Patients' understanding of their hospitalizations and association with satisfaction. JAMA Intern Med. 2014;174(10):16981700.
Background
Effective patient‐physician communication is associated with improved patient satisfaction, care quality, and clinical outcomes.[9] Whether a shared understanding of the plan of care between patients and clinicians affects satisfaction is unknown.
Findings
One hundred seventy‐seven patients who had 2 or more medical conditions, 2 or more medical procedures, and 2 or more days in the hospital were interviewed on the day of discharge. Patients were questioned about their overall understanding of their hospitalization and about specific aspects of their care. They were also asked to provide objective data to measure their understanding of their hospital course by (1) listing their medical diagnoses, (2) identifying indications for medication on discharge paperwork, and (3) listing tests or procedures they underwent from a standard list. Patients were then asked to rate their satisfaction with their hospitalization. Patients' self‐reported understanding was an average of 4.0 (very good) on a 5‐point scale. Their measured understanding scores for medical diagnoses, indications for medications and tests and procedures were 48.9%, 56.2%, and 59.4%, respectively. Factors associated with poor understanding of their hospital course were increasing age, less education, lower household income, black race, and longer length of stay. Patients reported a mean satisfaction of 4.0 (very satisfied). Higher self‐reported understanding was associated with higher patient satisfaction, irrespective of actual understanding.
Cautions
Despite their suboptimal measured understanding of their hospital course, the average patient rated their understanding as very good. This suggests that patients are either poor judges of effective communication or have low expectations for understanding. It also calls into question the relationship between quality of communication and patient satisfaction, because despite their satisfaction, patients' actual understanding was low. There was, however, a clear and positive relationship between patients' perceived understanding and their satisfaction, suggesting that shared understanding remains integral to patient satisfaction.
Implications
Patient satisfaction appears to be tied to patients' perceived understanding of their care, but when tested actual understanding was suboptimal. Further efforts in patient satisfaction should not only focus on the quality of our communication, but on the resulting understanding of our patients.
WHAT ARE UNIVERSAL STRATEGIES TO IMPROVE SATISFACTION AND PATIENT OUTCOMES?
Detsky AS, Krumholz HM. Reducing the trauma of hospitalization. JAMA. 2014;311(21):21692170.
Background
Although high readmission rates are a national problem, a minority of patients treated for common conditions like pneumonia, heart failure, and chronic obstructive pulmonary disease are readmitted for the same problem.[10] This suggests that readmissions may stem not from poor disease management, but from patient vulnerability to illness in the period following hospitalization.
Findings
In this viewpoint opinion article, the authors suggest that the depersonalizing and stressful hospital atmosphere contributes to a transient vulnerability in the period following hospitalization that makes it challenging for patients to care for themselves and their illness. They offer specific strategies for changing the nature of our hospital care to promote healing and to decrease patient stress. The authors suggest promoting personalization through accommodation of family members, and allowing personal clothing and personal dcor in their rooms. Physicians and consultants should make appointments so that patients and families can know when to expect important visits. The authors also focus on the provision of rest and nourishment by reducing nighttime disruption and the elimination on unnecessary restrictive diets. They argue that the hospital is a place of stressful disruptions and surprises, which could all be ameliorated by providing patients with a way to understand the members of their team and their roles as well as through providing a clear schedule for the day. Healthcare providers should not enter a room unannounced, and patients should be given private rooms as much as possible. Last, the authors focus on the elimination of unnecessary tests and procedures such as blood draws, telemetry, and urine cultures and the encouragement of activity by providing activities where patients can gather together outside their rooms.
Cautions
If these changes seem simple, they may not be. Many involve a significant shift in our thinking on how we provide carefrom a focus on disease and provider convenience to a true consideration for the health and peace of mind of our patients. Starting with small steps, such as reductions in phlebotomy and nighttime vital signs checks for the most stable patients and ensuring accommodations for families, may make this long list seem less daunting.
Implications
By promoting factors that affect a patient's well beingrest, nutrition, peace of mindwe may be discharging patients who are better equipped to manage their illness after their hospitalization.
DO HOSPITALISTS OVERTEST, AND IF SO, WHY?
Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100108.
Background
National efforts, such as the Choosing Wisely campaign, seek to decrease overuse of low‐value services.[11] The extent of the problem of overtesting among hospitalists and the underlying drivers for unnecessary testing in this group have not been clearly defined.
Findings
Practicing adult medicine hospitalists across the country were given a questionnaire that included clinical vignettes for common inpatient scenarios: a preoperative evaluation and a syncope workup. Respondents were randomly provided 1 of 4 versions of each vignette, which contained the same clinical information but varied by a family member's request for further testing and by disclosure of the occupation of the family member. For example, in the preoperative evaluation, the vignettes either: (1) provided no details about the patient's son; (2) identified the son as a physician; (3) mentioned the son's request for testing, but did not identify the son as a physician; or (4) identified the son as a physician who requested testing. The syncope vignette versions were structured similarly, except the family member was the patient's wife and she was an attorney. The authors collected 1020 responses from an initial pool of 1500, for a decent 68% response rate. Hospitalists commonly reported overuse of testing, with 52% to 65% of respondents requesting unnecessary testing in the preoperative evaluation scenario, and 82% to 85% in the syncope scenario. The majority of physicians reported that they knew the testing was not clinically indicated based on evidence or guidelines, but were ordering the test due to a desire to reassure the patients or themselves.
Cautions
Responses to clinical vignettes in a survey may not represent actually practices. In addition, all hospitalists surveyed in this study were members of the Society of Hospital Medicine, so may not accurately exemplify all practicing hospitalists.
Implications
Overuse of testing is very common among hospitalists. Although roughly one‐third of respondents incorrectly thought that testing in the given scenarios was supported by the evidence or guidelines, the majority knew that testing was not clinically indicated and reported ordering tests to help reassure their patients or themselves. This suggests evidence‐based medicine approaches to overuse, such as the Choosing Wisely campaign and the emergence of appropriateness criteria, are likely necessary but insufficient to change physician practice patterns. Efforts to decrease overuse will need to engage clinicians and patients in ways that help overcome the attitude that more testing is required to provide reassurance.
DO UNREALISTIC PATIENT EXPECTATIONS ABOUT INTERVENTIONS INFLUENCE DECISION MAKING AND CONTRIBUTE TO OVERUSE?
Hoffmann TC, Del Mar C. Patient expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2015;175(2):274286.
Background
Patient expectations have been implicated as a contributor to overuse of medical interventions. Studies that have measured patients' understanding of the potential benefits and harms of medical treatments and tests have been scattered across the literature.
Findings
This systematic review aggregated all studies that have quantitatively assessed patients' expectations of the benefits and/or harms of any treatment or test. Of more than 15,000 records screened, only 36 articles met the inclusion criteria of describing a study in which participants were asked to provide a quantitative estimate of the expected benefits and/or harms of a treatment, test, or screen. Fourteen of the studies (40%) focused on screening, 15 (43%) on treatment, 3 (9%) on a test, and 3 (9%) on both treatment and screening. Topics included cancer, medications, surgery, cardiovascular disease, and fetal‐maternal medicine. The majority of patients overestimated intervention benefit and underestimated harm, regardless of whether the intervention was a test or a treatment. For example, more than half of participants overestimated benefit for 22 of the 34 outcomes (65%) for which overestimation data were provided, and a majority of participants underestimated harm for 10 of the 15 outcomes (67%) with underestimation data available.
Cautions
This systematic review included a limited number of studies, with varying levels of quality and a lot of heterogeneity, making it difficult to reach clear aggregate conclusions.
Implications
Patients are often overly optimistic about medical interventions and they downplay potential risks, making it more difficult to effectively discourage overuse. Clinicians should clearly understand and communicate realistic expectations for the potential benefits and risks of screening, testing, and medical treatments with patients and the public at large.
HOW BIG OF A PROBLEM IS ANTIBIOTIC OVERUSE IN HOSPITALS AND CAN WE DO BETTER?
Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194200.
Background
Antibiotics are life‐saving therapies, but when used in inappropriate scenarios they can pose many risks.
Findings
This large national database study used the MarketScan Hospital Drug Database and the Centers for Disease Control and Prevention's (CDC) Emerging Infections Program data to explore antibiotic prescribing in hospital patients. More than half of all hospitalized patients (55.7%) received antibiotics during their stay. Half of all treatment antibiotics were prescribed for the treatment of either lower respiratory infections, urinary tract infections, or presumed gram‐positive infections. There was wide variation seen in antibiotic usage across hospital wards. Objective criteria for potential improvement in antimicrobial use were developed and applied at a subset of 36 hospitals. Antibiotic prescribing could be improved in 37.2% of the most common prescription scenarios reviewed, including patients receiving vancomycin or those being treated for a urinary tract infection. The impact of reducing inpatient antibiotic exposure on the incidence of Clostridium difficile colitis was modeled using data from 2 hospitals, revealing that decreasing hospitalized patients' exposure to broad‐spectrum antibiotics by 30% would lead to a 26% reduction in C difficile infections (interquartile range = 15%38%).
Cautions
Some of the estimates in this study are based on a convenience sample of claims and hospital‐based data, thus may not be an accurate representation, particularly when extrapolating to all US hospitals.
Implications
Antibiotic overuse is a rampant problem in hospitals, with many severe downstream effects such as C difficile infections and antimicrobial resistance. All hospital units should have an antibiotic stewardship program and should monitor antibiotic usage.
Lee TC, Frenette C, Jayaraman D, Green L, Pilote L. Antibiotic self‐stewardship: trainee‐led structured antibiotic time‐outs to improve antimicrobial use. Ann Intern Med. 2014;161(10 suppl):S53S58.
Background
The CDC and other groups have called for stewardship programs to address antibiotic overuse.[12] Few interventions have been shown to successfully engage medical trainees in efforts to improve their own antibiotic prescribing practices.
Findings
An antibiotic self‐stewardship program was developed and led by internal medicine residents at Montreal General Hospital. The intervention included a monthly resident education lecture on antimicrobial stewardship and twice‐weekly time‐out audits using a structured electronic checklist. Adherence with auditing was 80%. Total costs for antibiotics decreased from $149,743 CAD to $80,319 CAD, mostly due to an observed reduction in carbapenems. Moxifloxicin use decreased by 1.9 defined daily doses per 1000 patient‐days per month (P = 0.048). Rates of clostridium difficile colitis declined from 24.2 to 19.6 per 10,000 patient‐days, although this trend did not meet statistical significance (incidence rate ratio, 0.8 [confidence interval, 0.5‐1.3]).
Cautions
Although the use of some broader spectrum antibiotics decreased, there was no measurable change in overall antibiotic use, suggesting that physicians may have narrowed antibiotics but did not often completely discontinue them. The time‐series analyses in this study cannot provide causal conclusions between the intervention and outcomes. In fact, carbapenem usage appears to have significantly decreased prior to the implementation of the program, for unclear reasons. The feasibility of this educational intervention outside of a residency program is unclear.
Implications
A combination of education, oversight and frontline clinician engagement in structured time‐outs may be effective, at least in narrowing antibiotic usage. The structured audit checklist developed by these authors is available for free in the supplementary materials of the Annals of Internal Medicine article.
Disclosures: Dr. Moriates has received grant funding from the ABIM Foundation, and royalties from McGraw‐Hill for the textbook Understanding Value‐Based Healthcare. The authors report no conflicts of interest.
Hospitalists have a professional obligation to provide the highest quality care for patients and increasingly, hospitalists lead programs to improve quality, value, and patient experience.[1, 2, 3]
The federal government introduced the hospital Value‐Based Purchasing (VBP) program in 2012, initially with 1% of Medicare hospital payments tied to quality indicators. This percentage will continue to grow and the VBP program has expanded to include metrics related to quality, safety, cost‐effectiveness, and patient satisfaction.[4] Hospitals now face significant financial penalties if they do not achieve these benchmarks; thus, remaining up‐to‐date with the literature and the most promising interventions in these arenas is vital for hospitalists.
The goal of this update is to summarize and critique recently published research that has the greatest potential to impact clinical practice in quality, value, and patient experience in hospital medicine. We reviewed articles published between January 2014 and February 2015. To identify articles, we hand‐searched leading journals, continuing medical education collaborative journal reviews (including New England Journal of Medicine Journal Watch and the American College of Physicians Journal Club), the Agency for Healthcare Research and Quality's Patient Safety network, and PubMed. We evaluated articles based on their scientific rigor (peer review, study methodology, site number, and sample size) and applicability to hospital medicine. In this review, we summarize 9 articles that were felt by the authors to have the highest potential for impact on the clinical practice of hospital medicine, as directly related to quality, value, or patient experience. We present each topic with a current quality question that the accompanying article(s) will help address. We summarize each article and its findings and note cautions and implications for practice. The selected articles cover aspects related to patient safety, readmissions, patient satisfaction, and resource utilization, with each of these topics related to specific metrics included in VBP. We presented this update at the 2015 Society of Hospital Medicine national meeting.
IS THERE ANYTHING WE CAN DO TO MAKE HANDOFFS SAFER?
Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):18031812.
Background
With recent changes in resident duty hours and staffing models, the number of clinical handoffs during a patient's hospital stay has been increasing.[5] The omission of critical information and the transfer of erroneous information during handoffs is common, which contributes to preventable medical errors.[6]
Findings
This prospective intervention study of a resident handoff program in 9 hospitals sought to improve communication between healthcare providers and to decrease medical errors. The I‐PASS mnemonic, which stands for illness severity, patient summary, action list, situation awareness, and synthesis by receiver, was introduced to standardize oral and written handoffs. The program also included a 2‐hour workshop, a 1‐hour role‐playing and simulation session, a computer module, a faculty development program, direct observation tools, and a culture change campaign. Medical errors decreased by 23% following the intervention, compared to the preintervention baseline (24.5 vs 18.8 per 100 admissions, P 0.001), and the rate of preventable adverse events dropped by 30% (4.7 vs 3.3 events per 100 admissions, P 0.001), whereas nonpreventable adverse events did not change. Process measures of handoff quality uniformly improved with the intervention. The duration of oral handoffs was approximately 2.5 minutes per patient both before and during the intervention period.
Cautions
Not all of the sites in the study saw significant reductions in medical errors; 3 of the programs did not have significantly improved medical error rates following implementation of the I‐PASS handoff bundle. The study design was not a randomized controlled trial, and thus the pre‐ versus postimplementation analyses cannot draw definitive causal links between the intervention and the observed improvements in safety outcomes. Furthermore, this study was done with pediatric residents, and one cannot assume that the results will translate to practicing hospitalists, who may not benefit as much from a scripted sign‐out.
Implications
A comprehensive handoff program that included the I‐PASS mnemonic along with extensive training, faculty development, and a culture‐change campaign was associated with impressive improvements in patient safety outcomes, without negatively effecting workflow.
WHAT ARE THE COMMON FEATURES OF INTERVENTIONS THAT HAVE SUCCESSFULLY REDUCED READMISSIONS?
Leppin AL, Glonfriddo MR, Kessler M, et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174(7):10951107.
Background
Hospital readmissions are common, costly, and potentially represent a failure to adequately prepare patients for hospital discharge, but efforts to prevent 30‐day readmissions have been mixed.[7] The investigators in this study offer a novel framework, the cumulative complexity model, as a way to conceptualize postdischarge outcomes such as readmission. The model depicts the balance between the patient's workload of managing their illness, including the demands of monitoring treatment and self‐care, and the patient's capacity to handle that workfunctionality, financial/social resources, literacy, and empowerment. Workload‐capacity imbalances (when workload outstrips capacity) may lead to progressively increasing illness and increasing complexity, which contribute to poor patient outcomes like readmissions. Decreasing a patient's workload or increasing their capacity may be effective in reducing readmissions.
Findings
Investigators sought to identify factors associated with successful interventions to reduce 30‐day readmissions, including how the interventions fit into the cumulative complexity model. After performing a comprehensive search of randomized trials of interventions to reduce readmissions, the investigators identified 42 randomized trials with the primary outcome of 30‐day readmission rates. In addition to reviewing intervention characteristics, blinded raters scored interventions based on their effects on reducing or increasing patient workload and reducing or increasing patient capacity for self‐care. Interventions that had several components (eg, pharmacy education, postdischarge phone calls, visiting nurses, health coaches, close primary care follow‐up) were more likely to be successful (1.4 times as likely; P = 0.001), as were interventions that involved 2 or more individuals (1.3 times as likely; P = 0.05). Interventions that were published prior to 2002 were 1.6 times more likely to have reduced readmissions (P = 0.01). When applied to the cumulative complexity model, interventions that sought to augment patient capacity for self‐care were 1.3 times as likely to be successful (P = 0.04), whereas no relationship was found between an intervention's effect on patient workload and readmission.
Cautions
The authors evaluated each intervention based on the degree to which it was likely to affect patient workload and patient capacity. Because a multifaceted intervention may have had components that increased patient workload (eg, more self‐monitoring, appointments) and decreased patient workload (home visits, visiting nurses), the true effect of patient workload on readmissions may not have been optimally analyzed in this study. Additionally, this element of the study relied on a value judgment original to this work. Interventions that are burdensome to some, may be beneficial to those with the capacity and resources to access the care.
Implications
The body of studies reviewed suggests that interventions to reduce 30‐day readmissions are on the whole successful. Their findings are in keeping with past studies demonstrating more successful interventions that are resource‐intensive and multifaceted. Finding successful interventions that are also cost‐effective may be challenging. This article adds the cumulative complexity framework to what we already know about readmissions, highlighting patient capacity to manage the burden of their Illness as a new factor for success. Efforts to deliver patient‐centered education, explore barriers to adherence, and provide health coaching may be more successful than interventions that unwittingly add to the burden of disease treatment (multiple follow‐up appointments, complex medication schedules, and posthospital surveys and patient self‐assessments).
DOES PATIENT ACTIVATION CORRELATE WITH DECREASED RESOURCE USE OR READMISSIONS?
Mitchell SE, Gardiner PM, Sadikova E, et al. Patient activation and 30‐day post discharge hospital utilization. J Gen Intern Med. 2014;29(2):349355.
Background
Patient activation is widely recognized as the knowledge, skills, and confidence a person has in managing their own health or healthcare. Higher patient activation has been associated with improved health outcomes, but the relationship between patient activation and readmission to the hospital within 30 days is unknown.[8]
Findings
Using data from Project RED‐LIT (Re‐Engineered Discharge for patients with low health literacy), a randomized controlled trial conducted at an urban safety‐net hospital, investigators examined the relationship between all unplanned utilization events of hospital services within 30 days of discharge and patient activation, as measured by an abbreviated 8‐item version of the validated Patient Activation Measure (PAM). The PAM uses agreement with statements about a patient's sense of responsibility for his or her own health, confidence in seeking care and following through with medical treatments, and confidence in managing new problems to measure activation. The 695 participants were divided into quartiles based on their PAM score, and the investigators looked at the rates of unplanned utilization events in each group. After adjusting for potential confounders such as gender, age, Charlson Comorbidity Index, insurance, marital status, and education, there remained a significant effect between PAM and 30‐day hospital reutilization. Compared with those who scored in the highest quartile of activation, those in the lowest quartile had 1.75 times the rate of 30‐day reutilization (P 0.001). Those in the second highest and third highest quartile had 1.3 (P = 0.03) and 1.5 times (P 0.001) the rate of reutilization demonstrating a dose‐response relationship between activation and low reutilization.
Cautions
It is as yet unclear how best to apply these results and whether activation is a modifiable risk factor. Can a patient become more activated by providing more education and coaching during their hospital stay? Can providing close follow‐up and home services make a person more confident to manage their own illness? Although early identification of patients with low activation using PAM is being done at many hospitals, there is no study to suggest that targeting these patients can reduce readmission.
Implications
A low level of patient activation appears to be a risk factor for unplanned hospital utilization within 30 days of discharge. Given the increasing financial penalties, many hospitals across the country are using the PAM to determine how much support and which services they provide after discharge. Identifying these patients early in their hospitalization could allow providers to spend more time and attention on preparing them for managing their own illness after discharge. As above, the effects of this intervention on readmissions is as yet unclear.
IS THERE A RELATIONSHIP BETWEEN PATIENT SATISFACTION AND UNDERSTANDING OF THE PLAN OF CARE?
Kebede S, Shihab HM, Berger ZD, et al. Patients' understanding of their hospitalizations and association with satisfaction. JAMA Intern Med. 2014;174(10):16981700.
Background
Effective patient‐physician communication is associated with improved patient satisfaction, care quality, and clinical outcomes.[9] Whether a shared understanding of the plan of care between patients and clinicians affects satisfaction is unknown.
Findings
One hundred seventy‐seven patients who had 2 or more medical conditions, 2 or more medical procedures, and 2 or more days in the hospital were interviewed on the day of discharge. Patients were questioned about their overall understanding of their hospitalization and about specific aspects of their care. They were also asked to provide objective data to measure their understanding of their hospital course by (1) listing their medical diagnoses, (2) identifying indications for medication on discharge paperwork, and (3) listing tests or procedures they underwent from a standard list. Patients were then asked to rate their satisfaction with their hospitalization. Patients' self‐reported understanding was an average of 4.0 (very good) on a 5‐point scale. Their measured understanding scores for medical diagnoses, indications for medications and tests and procedures were 48.9%, 56.2%, and 59.4%, respectively. Factors associated with poor understanding of their hospital course were increasing age, less education, lower household income, black race, and longer length of stay. Patients reported a mean satisfaction of 4.0 (very satisfied). Higher self‐reported understanding was associated with higher patient satisfaction, irrespective of actual understanding.
Cautions
Despite their suboptimal measured understanding of their hospital course, the average patient rated their understanding as very good. This suggests that patients are either poor judges of effective communication or have low expectations for understanding. It also calls into question the relationship between quality of communication and patient satisfaction, because despite their satisfaction, patients' actual understanding was low. There was, however, a clear and positive relationship between patients' perceived understanding and their satisfaction, suggesting that shared understanding remains integral to patient satisfaction.
Implications
Patient satisfaction appears to be tied to patients' perceived understanding of their care, but when tested actual understanding was suboptimal. Further efforts in patient satisfaction should not only focus on the quality of our communication, but on the resulting understanding of our patients.
WHAT ARE UNIVERSAL STRATEGIES TO IMPROVE SATISFACTION AND PATIENT OUTCOMES?
Detsky AS, Krumholz HM. Reducing the trauma of hospitalization. JAMA. 2014;311(21):21692170.
Background
Although high readmission rates are a national problem, a minority of patients treated for common conditions like pneumonia, heart failure, and chronic obstructive pulmonary disease are readmitted for the same problem.[10] This suggests that readmissions may stem not from poor disease management, but from patient vulnerability to illness in the period following hospitalization.
Findings
In this viewpoint opinion article, the authors suggest that the depersonalizing and stressful hospital atmosphere contributes to a transient vulnerability in the period following hospitalization that makes it challenging for patients to care for themselves and their illness. They offer specific strategies for changing the nature of our hospital care to promote healing and to decrease patient stress. The authors suggest promoting personalization through accommodation of family members, and allowing personal clothing and personal dcor in their rooms. Physicians and consultants should make appointments so that patients and families can know when to expect important visits. The authors also focus on the provision of rest and nourishment by reducing nighttime disruption and the elimination on unnecessary restrictive diets. They argue that the hospital is a place of stressful disruptions and surprises, which could all be ameliorated by providing patients with a way to understand the members of their team and their roles as well as through providing a clear schedule for the day. Healthcare providers should not enter a room unannounced, and patients should be given private rooms as much as possible. Last, the authors focus on the elimination of unnecessary tests and procedures such as blood draws, telemetry, and urine cultures and the encouragement of activity by providing activities where patients can gather together outside their rooms.
Cautions
If these changes seem simple, they may not be. Many involve a significant shift in our thinking on how we provide carefrom a focus on disease and provider convenience to a true consideration for the health and peace of mind of our patients. Starting with small steps, such as reductions in phlebotomy and nighttime vital signs checks for the most stable patients and ensuring accommodations for families, may make this long list seem less daunting.
Implications
By promoting factors that affect a patient's well beingrest, nutrition, peace of mindwe may be discharging patients who are better equipped to manage their illness after their hospitalization.
DO HOSPITALISTS OVERTEST, AND IF SO, WHY?
Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100108.
Background
National efforts, such as the Choosing Wisely campaign, seek to decrease overuse of low‐value services.[11] The extent of the problem of overtesting among hospitalists and the underlying drivers for unnecessary testing in this group have not been clearly defined.
Findings
Practicing adult medicine hospitalists across the country were given a questionnaire that included clinical vignettes for common inpatient scenarios: a preoperative evaluation and a syncope workup. Respondents were randomly provided 1 of 4 versions of each vignette, which contained the same clinical information but varied by a family member's request for further testing and by disclosure of the occupation of the family member. For example, in the preoperative evaluation, the vignettes either: (1) provided no details about the patient's son; (2) identified the son as a physician; (3) mentioned the son's request for testing, but did not identify the son as a physician; or (4) identified the son as a physician who requested testing. The syncope vignette versions were structured similarly, except the family member was the patient's wife and she was an attorney. The authors collected 1020 responses from an initial pool of 1500, for a decent 68% response rate. Hospitalists commonly reported overuse of testing, with 52% to 65% of respondents requesting unnecessary testing in the preoperative evaluation scenario, and 82% to 85% in the syncope scenario. The majority of physicians reported that they knew the testing was not clinically indicated based on evidence or guidelines, but were ordering the test due to a desire to reassure the patients or themselves.
Cautions
Responses to clinical vignettes in a survey may not represent actually practices. In addition, all hospitalists surveyed in this study were members of the Society of Hospital Medicine, so may not accurately exemplify all practicing hospitalists.
Implications
Overuse of testing is very common among hospitalists. Although roughly one‐third of respondents incorrectly thought that testing in the given scenarios was supported by the evidence or guidelines, the majority knew that testing was not clinically indicated and reported ordering tests to help reassure their patients or themselves. This suggests evidence‐based medicine approaches to overuse, such as the Choosing Wisely campaign and the emergence of appropriateness criteria, are likely necessary but insufficient to change physician practice patterns. Efforts to decrease overuse will need to engage clinicians and patients in ways that help overcome the attitude that more testing is required to provide reassurance.
DO UNREALISTIC PATIENT EXPECTATIONS ABOUT INTERVENTIONS INFLUENCE DECISION MAKING AND CONTRIBUTE TO OVERUSE?
Hoffmann TC, Del Mar C. Patient expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2015;175(2):274286.
Background
Patient expectations have been implicated as a contributor to overuse of medical interventions. Studies that have measured patients' understanding of the potential benefits and harms of medical treatments and tests have been scattered across the literature.
Findings
This systematic review aggregated all studies that have quantitatively assessed patients' expectations of the benefits and/or harms of any treatment or test. Of more than 15,000 records screened, only 36 articles met the inclusion criteria of describing a study in which participants were asked to provide a quantitative estimate of the expected benefits and/or harms of a treatment, test, or screen. Fourteen of the studies (40%) focused on screening, 15 (43%) on treatment, 3 (9%) on a test, and 3 (9%) on both treatment and screening. Topics included cancer, medications, surgery, cardiovascular disease, and fetal‐maternal medicine. The majority of patients overestimated intervention benefit and underestimated harm, regardless of whether the intervention was a test or a treatment. For example, more than half of participants overestimated benefit for 22 of the 34 outcomes (65%) for which overestimation data were provided, and a majority of participants underestimated harm for 10 of the 15 outcomes (67%) with underestimation data available.
Cautions
This systematic review included a limited number of studies, with varying levels of quality and a lot of heterogeneity, making it difficult to reach clear aggregate conclusions.
Implications
Patients are often overly optimistic about medical interventions and they downplay potential risks, making it more difficult to effectively discourage overuse. Clinicians should clearly understand and communicate realistic expectations for the potential benefits and risks of screening, testing, and medical treatments with patients and the public at large.
HOW BIG OF A PROBLEM IS ANTIBIOTIC OVERUSE IN HOSPITALS AND CAN WE DO BETTER?
Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194200.
Background
Antibiotics are life‐saving therapies, but when used in inappropriate scenarios they can pose many risks.
Findings
This large national database study used the MarketScan Hospital Drug Database and the Centers for Disease Control and Prevention's (CDC) Emerging Infections Program data to explore antibiotic prescribing in hospital patients. More than half of all hospitalized patients (55.7%) received antibiotics during their stay. Half of all treatment antibiotics were prescribed for the treatment of either lower respiratory infections, urinary tract infections, or presumed gram‐positive infections. There was wide variation seen in antibiotic usage across hospital wards. Objective criteria for potential improvement in antimicrobial use were developed and applied at a subset of 36 hospitals. Antibiotic prescribing could be improved in 37.2% of the most common prescription scenarios reviewed, including patients receiving vancomycin or those being treated for a urinary tract infection. The impact of reducing inpatient antibiotic exposure on the incidence of Clostridium difficile colitis was modeled using data from 2 hospitals, revealing that decreasing hospitalized patients' exposure to broad‐spectrum antibiotics by 30% would lead to a 26% reduction in C difficile infections (interquartile range = 15%38%).
Cautions
Some of the estimates in this study are based on a convenience sample of claims and hospital‐based data, thus may not be an accurate representation, particularly when extrapolating to all US hospitals.
Implications
Antibiotic overuse is a rampant problem in hospitals, with many severe downstream effects such as C difficile infections and antimicrobial resistance. All hospital units should have an antibiotic stewardship program and should monitor antibiotic usage.
Lee TC, Frenette C, Jayaraman D, Green L, Pilote L. Antibiotic self‐stewardship: trainee‐led structured antibiotic time‐outs to improve antimicrobial use. Ann Intern Med. 2014;161(10 suppl):S53S58.
Background
The CDC and other groups have called for stewardship programs to address antibiotic overuse.[12] Few interventions have been shown to successfully engage medical trainees in efforts to improve their own antibiotic prescribing practices.
Findings
An antibiotic self‐stewardship program was developed and led by internal medicine residents at Montreal General Hospital. The intervention included a monthly resident education lecture on antimicrobial stewardship and twice‐weekly time‐out audits using a structured electronic checklist. Adherence with auditing was 80%. Total costs for antibiotics decreased from $149,743 CAD to $80,319 CAD, mostly due to an observed reduction in carbapenems. Moxifloxicin use decreased by 1.9 defined daily doses per 1000 patient‐days per month (P = 0.048). Rates of clostridium difficile colitis declined from 24.2 to 19.6 per 10,000 patient‐days, although this trend did not meet statistical significance (incidence rate ratio, 0.8 [confidence interval, 0.5‐1.3]).
Cautions
Although the use of some broader spectrum antibiotics decreased, there was no measurable change in overall antibiotic use, suggesting that physicians may have narrowed antibiotics but did not often completely discontinue them. The time‐series analyses in this study cannot provide causal conclusions between the intervention and outcomes. In fact, carbapenem usage appears to have significantly decreased prior to the implementation of the program, for unclear reasons. The feasibility of this educational intervention outside of a residency program is unclear.
Implications
A combination of education, oversight and frontline clinician engagement in structured time‐outs may be effective, at least in narrowing antibiotic usage. The structured audit checklist developed by these authors is available for free in the supplementary materials of the Annals of Internal Medicine article.
Disclosures: Dr. Moriates has received grant funding from the ABIM Foundation, and royalties from McGraw‐Hill for the textbook Understanding Value‐Based Healthcare. The authors report no conflicts of interest.
- . The role of the hospitalist in quality improvement: systems for improving the care of patients with acute coronary syndrome. J Hosp Med. 2010;5(suppl 4):S1–S7.
- , , , . Impact of hospitalist communication‐skills training on patient‐satisfaction scores. J Hosp Med. 2013;8(6):315–320.
- , , , . Development of a hospital‐based program focused on improving healthcare value. J Hosp Med. 2014;9(10):671–677.
- . Value‐driven health care: implications for hospitals and hospitalists. J Hosp Med. 2009;4(8):507–511.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013;173(8):649–655.
- , , , , . Does housestaff discontinuity of care increase the risk for preventable adverse events? Ann Intern Med. 1994;121(11):866–872.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520–528.
- , , . Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8(5):410–417.
- . Effective physician‐patient communication and health outcomes: a review. CMAJ. 2007;152(9):1423–1433.
- , , , et al. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355–363.
- , , , et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486–492.
- , , , et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194–200.
- . The role of the hospitalist in quality improvement: systems for improving the care of patients with acute coronary syndrome. J Hosp Med. 2010;5(suppl 4):S1–S7.
- , , , . Impact of hospitalist communication‐skills training on patient‐satisfaction scores. J Hosp Med. 2013;8(6):315–320.
- , , , . Development of a hospital‐based program focused on improving healthcare value. J Hosp Med. 2014;9(10):671–677.
- . Value‐driven health care: implications for hospitals and hospitalists. J Hosp Med. 2009;4(8):507–511.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013;173(8):649–655.
- , , , , . Does housestaff discontinuity of care increase the risk for preventable adverse events? Ann Intern Med. 1994;121(11):866–872.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520–528.
- , , . Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8(5):410–417.
- . Effective physician‐patient communication and health outcomes: a review. CMAJ. 2007;152(9):1423–1433.
- , , , et al. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355–363.
- , , , et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486–492.
- , , , et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194–200.
PCP Communication at Discharge
Transitions of care from hospital to home are high‐risk times for patients.[1, 2] Increasing complexity of hospital admissions and shorter lengths of stay demand more effective coordination of care between hospitalists and outpatient clinicians.[3, 4, 5] Inaccurate, delayed, or incomplete clinical handoversthat is, transfer of information and professional responsibility and accountability[6]can lead to patient harm, and has been recognized as a key cause of preventable morbidity by the World Health Organization and The Joint Commission.[6, 7, 8] Conversely, when done effectively, transitions can result in improved patient health outcomes, reduced readmission rates, and higher patient and provider satisfaction.3
Previous studies note deficits in communication at discharge and primary care provider (PCP) dissatisfaction with discharge practices.[4, 9, 10, 11, 12, 13] In studies at academic medical centers, there were low rates of direct communication between inpatient and outpatient providers, mainly because of providers' belief that the discharge summary was adequate and the presence of significant barriers to direct communication.[14, 15] However, studies have shown that discharge summaries often omit critical information, and often are not available to PCPs in a timely manner.[10, 11, 12, 16] In response, the Society of Hospital Medicine developed a discharge checklist to aide in standardization of safe discharge practices.[1, 5] Discharge summary templates further attempt to improve documentation of patients' hospital courses. An electronic medical record (EMR) system shared by both inpatient and outpatient clinicians should impart several advantages: (1) automated alerts provide timely notification to PCPs regarding admission and discharge, (2) discharge summaries are available to the PCP as soon as they are written, and (3) all patient information pertaining to the hospitalization is available to the PCP.
Although it is plausible that shared EMRs should facilitate transitions of care by streamlining communication between hospitalists and PCPs, guidelines on format and content of PCP communication at discharge in the era of a shared EMR have yet to be defined. In this study, we sought to understand current discharge communication practices and PCP satisfaction within a shared EMR at our institution, and to identify key areas in which communication can be improved.
METHODS
Participants and Setting
We surveyed all resident and attending PCPs (n=124) working in the Division of General Internal Medicine (DGIM) Outpatient Practice at the University of California, San Francisco (UCSF). In June 2012, the outpatient and inpatient practices of UCSF transitioned from having separate medical record systems to a shared EMR (Epic Systems Corp., Verona, WI) where all informationboth inpatient and outpatientis accessible among healthcare professionals. The EMR provides automated notifications of admission and discharge to PCPs, allows for review of inpatient notes, labs, and studies, and immediate access to templated discharge summaries (see Supporting Information, Appendix 1, in the online version of this article). The EMR also enables secure communication between clinicians. At our institution, over 90% of discharge summaries are completed within 24 hours of discharge.[17]
Study Design and Analysis
We developed a survey about the discharge communication practices of inpatient medicine patients based on a previously described survey in the literature (see Supporting Information, Appendix 2, in the online version of this article).[9] The anonymous, 17‐question survey was electronically distributed to resident and attending PCPs at the DGIM practice. The survey was designed to determine: (1) overall PCP satisfaction with current communication practices from the inpatient team at patient discharge, (2) perceived adequacy of automatic discharge notifications, and (3) perception of the types of patients and hospitalizations requiring additional high‐touch communication at discharge.
We analyzed results of our survey using descriptive statistics. Differences in resident and attending responses were analyzed by 2tests.
RESULTS
Seventy‐five of 124 (60%) clinicians (46% residents, 54% attendings) completed the survey. Thirty‐nine (52%) PCPs were satisfied or very satisfied with communication at patient discharge. Although most reported receiving automated discharge notifications (71%), only 39% felt that the notifications plus the discharge summaries were adequate communication for safe transition of care from hospital to community. Fifty‐one percent desired direct contact beyond a discharge summary. There were no differences in preferences on discharge communication between resident and attending PCPs (P>0.05).
Over three‐fourths of PCPs surveyed preferred that for patients with complex hospitalizations (multiple readmissions, multiple active comorbidities, goals of care changes, high‐risk medication changes, time‐sensitive follow‐up needs), an additional e‐mail or verbal communication was needed to augment the information in the discharge summary (Figure 1). Only 31% reported receiving such communication.
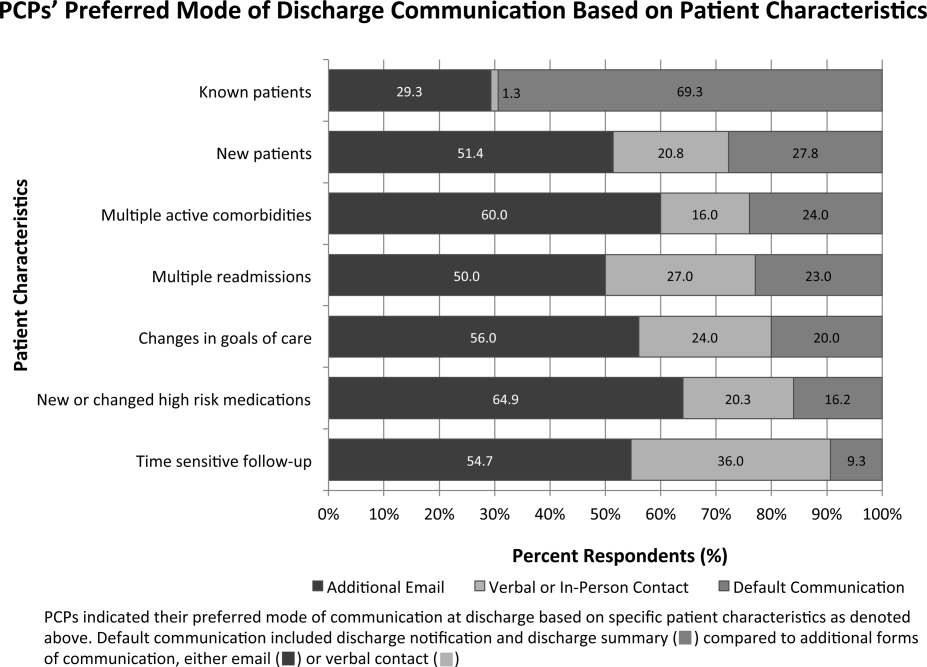
When asked about important items to communicate for safer transitions of care, PCPs reported finding the following elements most critical: (1) medication changes (93%), (2) follow‐up actions for the PCP (88%), and (3) active medical issues (84%) (Figure 2).
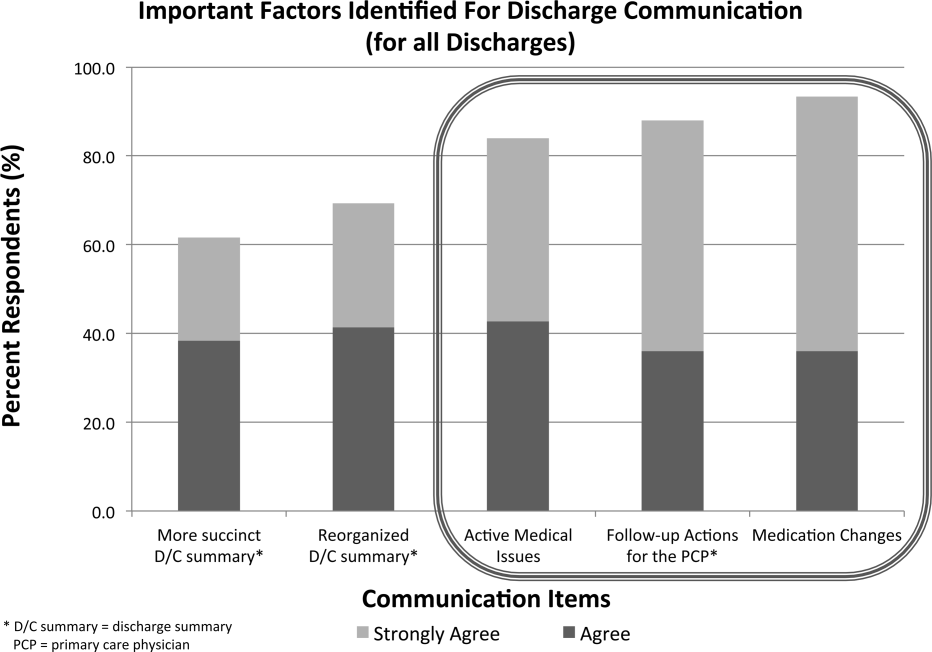
CONCLUSIONS
In the era of shared EMRs, real‐time access to medication lists, pending test results, and discharge summaries should facilitate care transitions at discharge.[18, 19] We conducted a study to determine PCP perceptions of discharge communication after implementation of a shared EMR. We found that although PCPs largely acknowledged timely receipt of automated discharge notifications and discharge summaries, the majority of PCPs felt that most discharges required additional communication to ensure safe transition of care.
Guidelines for discharge communication emphasize timely communication with the PCP, primarily through discharge summaries containing key safety elements.[1, 5, 10] At our institution, we have improved the timeliness and quality of discharge summaries according to guideline recommendations,[17] and conducted quality improvement projects to improve rates of direct communication with PCPs.[9] In addition, the shared EMR provides automated notifications to PCPs when their patients are discharged. Despite these interventions, our survey shows that PCP satisfaction with discharge communication is still inadequate. PCPs desired direct communication that highlights active medical issues, medication changes, and specific follow‐up actions. Although all of these topics are included in our discharge summary template (see Supporting Information, Appendix 1, in the online version of this article), it is possible that the templated discharge summaries lend themselves to longer documents and information overload, as prior studies have documented the desire for more succinct discharge summaries.[18] We also found that automated notifications of discharge were less reliable and useful for PCPs than anticipated. There were several reasons for this: (1) discharge summaries sometimes were sent to PCPs uncoupled from the discharge notification, (2) there were errors with the generation and delivery of automated messages at the rollout of the new system, and (3) PCPs received many other automated system messages, meaning that discharge notifications could be easily missed. These factors all likely contribute to PCPs' desire for high‐touch communication that highlights the most salient aspects of each patient's hospitalization. It is also possible that automated notifications and depersonalized discharge summaries create distance and a less‐collaborative feeling to patient care. PCPs want more direct communication, and desire to play a more active role in inpatient management, especially for complex hospitalizations.[18] This emphasis on direct communication resonates with previous studies conducted before shared EMRs existed.[9, 12, 19]
Our study had several limitations. First, because this is a single‐institution study at a tertiary care academic setting, the results may not be generalizable to all shared EMR settings, and may not reflect all the challenges of communication with the wider community of outpatient providers. One can postulate that inpatient and outpatient clinician relationships are stronger in an academic setting than in other more disparate environments, where direct communication may happen even less frequently. Of note, our low rates of direct communication are consistent with other single‐ and multi‐institution studies, suggesting that our findings are generalizable.[14, 15] Second, our survey is limited in its ability to distinguish those patients who require high‐touch communication and those who do not. Third, although we have used the survey to assess PCP satisfaction in previous studies, it is not a validated instrument, and therefore we cannot reliably say that increasing direct PCP communication would increase their satisfaction around discharge. Last, the PCP‐reported rates of discharge communication are subjective and may be influenced by recall bias. We did not have a systematic way to confirm the actual rates of communication at discharge, which could have occurred through EMR messages, e‐mails, phone calls, or pages.
Although a shared EMR allows for real‐time access to patient data, it does not eliminate PCPs' desire for direct 2‐way dialogue at discharge, especially for complex patients. Key information desired in such communication should include active medical issues, medication changes, and follow‐up needs, which is consistent with prior studies. Standardizing this direct communication process in an efficient way can be challenging. Further elucidation of PCP preferences around which patients necessitate higher‐level communication and preferred methods and timing of communication is needed, as well as determining the most efficient and effective method for hospitalists to provide such communication. Improving communication between hospitalists and PCPs requires not just the presence of a shared EMR, but additional, systematic efforts to engage both inpatient and outpatient clinicians in collaborative care.
Disclosure
Nothing to report.
- , , , et al. Development of a checklist of safe discharge practices for hospital patients. J Hosp Med. 2013;8(8):444–449.
- , , , , The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , et al. Improving patient handovers from hospital to primary care: a systematic review. Ann Intern Med. 2012;157(6):417–428.
- , , , , “Did I do as best as the system would let me?” Healthcare professional views on hospital to home care transitions. J Gen Intern Med. 2012;27(12):1649–1656.
- , , , et al. Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists. J Hosp Med. 2006;1(6):354−660.
- , , , , Improving measurement in clinical handover. Qual Saf Health Care. 2009;18:272–277.
- World Health Organization. Patient safety: action on patient safety: high 5s. 2007. Available at: http://www.who.int/patientsafety/implementation/solutions/high5s/en/index.html. Accessed January 28, 2015.
- The Joint Commission Center for Transforming Healthcare. Hand‐off communications. 2012. Available at: http://www.centerfortransforminghealthcare.org/projects/detail.aspx?Project=1. Accessed January 28, 2015.
- , , , et al. The effect of a resident‐led quality improvement project on improving communication between hospital‐based and outpatient physicians. Am J Med Qual. 2013;28(6):472–479.
- , , , Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- , , , , , Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , Primary care physician attitudes regarding communication with hospitalists. Am J Med. 2001;111(9B):15S–20S.
- , , , et al. Searching for the missing pieces between the hospital and primary care: mapping the patient process during care transitions. BMJ Qual Saf. 2012;21:i97–i105.
- , , , et al. Association of self‐reported hospital discharge handoffs with 30‐day readmissions. JAMA. 2013;173(8):624–629.
- , , , et al. Association of communication between hospital‐based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381–386.
- , , , Effect of discharge summary availability during post‐discharge visits on hospital readmission. J Gen Intern Med. 2002;17(3):186–192.
- , , , , The Housestaff Incentive Program: improving the timeliness and quality of discharge summaries by engaging residents in quality improvement. BMJ Qual Saf. 2013;22(9):768–774.
- , , , et al. A Failure to communicate: a qualitative exploration of care coordination between hospitalists and primary care providers around patient hospitalizations [published online ahead of print October 15, 2014]. J Gen Intern Med. doi: 10.1007/s11606-014-3056-x.
- , , , et al. Pediatric hospitalists and primary care providers: a communication needs assessment. J Hosp Med. 2009;4(3):187–193.
Transitions of care from hospital to home are high‐risk times for patients.[1, 2] Increasing complexity of hospital admissions and shorter lengths of stay demand more effective coordination of care between hospitalists and outpatient clinicians.[3, 4, 5] Inaccurate, delayed, or incomplete clinical handoversthat is, transfer of information and professional responsibility and accountability[6]can lead to patient harm, and has been recognized as a key cause of preventable morbidity by the World Health Organization and The Joint Commission.[6, 7, 8] Conversely, when done effectively, transitions can result in improved patient health outcomes, reduced readmission rates, and higher patient and provider satisfaction.3
Previous studies note deficits in communication at discharge and primary care provider (PCP) dissatisfaction with discharge practices.[4, 9, 10, 11, 12, 13] In studies at academic medical centers, there were low rates of direct communication between inpatient and outpatient providers, mainly because of providers' belief that the discharge summary was adequate and the presence of significant barriers to direct communication.[14, 15] However, studies have shown that discharge summaries often omit critical information, and often are not available to PCPs in a timely manner.[10, 11, 12, 16] In response, the Society of Hospital Medicine developed a discharge checklist to aide in standardization of safe discharge practices.[1, 5] Discharge summary templates further attempt to improve documentation of patients' hospital courses. An electronic medical record (EMR) system shared by both inpatient and outpatient clinicians should impart several advantages: (1) automated alerts provide timely notification to PCPs regarding admission and discharge, (2) discharge summaries are available to the PCP as soon as they are written, and (3) all patient information pertaining to the hospitalization is available to the PCP.
Although it is plausible that shared EMRs should facilitate transitions of care by streamlining communication between hospitalists and PCPs, guidelines on format and content of PCP communication at discharge in the era of a shared EMR have yet to be defined. In this study, we sought to understand current discharge communication practices and PCP satisfaction within a shared EMR at our institution, and to identify key areas in which communication can be improved.
METHODS
Participants and Setting
We surveyed all resident and attending PCPs (n=124) working in the Division of General Internal Medicine (DGIM) Outpatient Practice at the University of California, San Francisco (UCSF). In June 2012, the outpatient and inpatient practices of UCSF transitioned from having separate medical record systems to a shared EMR (Epic Systems Corp., Verona, WI) where all informationboth inpatient and outpatientis accessible among healthcare professionals. The EMR provides automated notifications of admission and discharge to PCPs, allows for review of inpatient notes, labs, and studies, and immediate access to templated discharge summaries (see Supporting Information, Appendix 1, in the online version of this article). The EMR also enables secure communication between clinicians. At our institution, over 90% of discharge summaries are completed within 24 hours of discharge.[17]
Study Design and Analysis
We developed a survey about the discharge communication practices of inpatient medicine patients based on a previously described survey in the literature (see Supporting Information, Appendix 2, in the online version of this article).[9] The anonymous, 17‐question survey was electronically distributed to resident and attending PCPs at the DGIM practice. The survey was designed to determine: (1) overall PCP satisfaction with current communication practices from the inpatient team at patient discharge, (2) perceived adequacy of automatic discharge notifications, and (3) perception of the types of patients and hospitalizations requiring additional high‐touch communication at discharge.
We analyzed results of our survey using descriptive statistics. Differences in resident and attending responses were analyzed by 2tests.
RESULTS
Seventy‐five of 124 (60%) clinicians (46% residents, 54% attendings) completed the survey. Thirty‐nine (52%) PCPs were satisfied or very satisfied with communication at patient discharge. Although most reported receiving automated discharge notifications (71%), only 39% felt that the notifications plus the discharge summaries were adequate communication for safe transition of care from hospital to community. Fifty‐one percent desired direct contact beyond a discharge summary. There were no differences in preferences on discharge communication between resident and attending PCPs (P>0.05).
Over three‐fourths of PCPs surveyed preferred that for patients with complex hospitalizations (multiple readmissions, multiple active comorbidities, goals of care changes, high‐risk medication changes, time‐sensitive follow‐up needs), an additional e‐mail or verbal communication was needed to augment the information in the discharge summary (Figure 1). Only 31% reported receiving such communication.

When asked about important items to communicate for safer transitions of care, PCPs reported finding the following elements most critical: (1) medication changes (93%), (2) follow‐up actions for the PCP (88%), and (3) active medical issues (84%) (Figure 2).

CONCLUSIONS
In the era of shared EMRs, real‐time access to medication lists, pending test results, and discharge summaries should facilitate care transitions at discharge.[18, 19] We conducted a study to determine PCP perceptions of discharge communication after implementation of a shared EMR. We found that although PCPs largely acknowledged timely receipt of automated discharge notifications and discharge summaries, the majority of PCPs felt that most discharges required additional communication to ensure safe transition of care.
Guidelines for discharge communication emphasize timely communication with the PCP, primarily through discharge summaries containing key safety elements.[1, 5, 10] At our institution, we have improved the timeliness and quality of discharge summaries according to guideline recommendations,[17] and conducted quality improvement projects to improve rates of direct communication with PCPs.[9] In addition, the shared EMR provides automated notifications to PCPs when their patients are discharged. Despite these interventions, our survey shows that PCP satisfaction with discharge communication is still inadequate. PCPs desired direct communication that highlights active medical issues, medication changes, and specific follow‐up actions. Although all of these topics are included in our discharge summary template (see Supporting Information, Appendix 1, in the online version of this article), it is possible that the templated discharge summaries lend themselves to longer documents and information overload, as prior studies have documented the desire for more succinct discharge summaries.[18] We also found that automated notifications of discharge were less reliable and useful for PCPs than anticipated. There were several reasons for this: (1) discharge summaries sometimes were sent to PCPs uncoupled from the discharge notification, (2) there were errors with the generation and delivery of automated messages at the rollout of the new system, and (3) PCPs received many other automated system messages, meaning that discharge notifications could be easily missed. These factors all likely contribute to PCPs' desire for high‐touch communication that highlights the most salient aspects of each patient's hospitalization. It is also possible that automated notifications and depersonalized discharge summaries create distance and a less‐collaborative feeling to patient care. PCPs want more direct communication, and desire to play a more active role in inpatient management, especially for complex hospitalizations.[18] This emphasis on direct communication resonates with previous studies conducted before shared EMRs existed.[9, 12, 19]
Our study had several limitations. First, because this is a single‐institution study at a tertiary care academic setting, the results may not be generalizable to all shared EMR settings, and may not reflect all the challenges of communication with the wider community of outpatient providers. One can postulate that inpatient and outpatient clinician relationships are stronger in an academic setting than in other more disparate environments, where direct communication may happen even less frequently. Of note, our low rates of direct communication are consistent with other single‐ and multi‐institution studies, suggesting that our findings are generalizable.[14, 15] Second, our survey is limited in its ability to distinguish those patients who require high‐touch communication and those who do not. Third, although we have used the survey to assess PCP satisfaction in previous studies, it is not a validated instrument, and therefore we cannot reliably say that increasing direct PCP communication would increase their satisfaction around discharge. Last, the PCP‐reported rates of discharge communication are subjective and may be influenced by recall bias. We did not have a systematic way to confirm the actual rates of communication at discharge, which could have occurred through EMR messages, e‐mails, phone calls, or pages.
Although a shared EMR allows for real‐time access to patient data, it does not eliminate PCPs' desire for direct 2‐way dialogue at discharge, especially for complex patients. Key information desired in such communication should include active medical issues, medication changes, and follow‐up needs, which is consistent with prior studies. Standardizing this direct communication process in an efficient way can be challenging. Further elucidation of PCP preferences around which patients necessitate higher‐level communication and preferred methods and timing of communication is needed, as well as determining the most efficient and effective method for hospitalists to provide such communication. Improving communication between hospitalists and PCPs requires not just the presence of a shared EMR, but additional, systematic efforts to engage both inpatient and outpatient clinicians in collaborative care.
Disclosure
Nothing to report.
Transitions of care from hospital to home are high‐risk times for patients.[1, 2] Increasing complexity of hospital admissions and shorter lengths of stay demand more effective coordination of care between hospitalists and outpatient clinicians.[3, 4, 5] Inaccurate, delayed, or incomplete clinical handoversthat is, transfer of information and professional responsibility and accountability[6]can lead to patient harm, and has been recognized as a key cause of preventable morbidity by the World Health Organization and The Joint Commission.[6, 7, 8] Conversely, when done effectively, transitions can result in improved patient health outcomes, reduced readmission rates, and higher patient and provider satisfaction.3
Previous studies note deficits in communication at discharge and primary care provider (PCP) dissatisfaction with discharge practices.[4, 9, 10, 11, 12, 13] In studies at academic medical centers, there were low rates of direct communication between inpatient and outpatient providers, mainly because of providers' belief that the discharge summary was adequate and the presence of significant barriers to direct communication.[14, 15] However, studies have shown that discharge summaries often omit critical information, and often are not available to PCPs in a timely manner.[10, 11, 12, 16] In response, the Society of Hospital Medicine developed a discharge checklist to aide in standardization of safe discharge practices.[1, 5] Discharge summary templates further attempt to improve documentation of patients' hospital courses. An electronic medical record (EMR) system shared by both inpatient and outpatient clinicians should impart several advantages: (1) automated alerts provide timely notification to PCPs regarding admission and discharge, (2) discharge summaries are available to the PCP as soon as they are written, and (3) all patient information pertaining to the hospitalization is available to the PCP.
Although it is plausible that shared EMRs should facilitate transitions of care by streamlining communication between hospitalists and PCPs, guidelines on format and content of PCP communication at discharge in the era of a shared EMR have yet to be defined. In this study, we sought to understand current discharge communication practices and PCP satisfaction within a shared EMR at our institution, and to identify key areas in which communication can be improved.
METHODS
Participants and Setting
We surveyed all resident and attending PCPs (n=124) working in the Division of General Internal Medicine (DGIM) Outpatient Practice at the University of California, San Francisco (UCSF). In June 2012, the outpatient and inpatient practices of UCSF transitioned from having separate medical record systems to a shared EMR (Epic Systems Corp., Verona, WI) where all informationboth inpatient and outpatientis accessible among healthcare professionals. The EMR provides automated notifications of admission and discharge to PCPs, allows for review of inpatient notes, labs, and studies, and immediate access to templated discharge summaries (see Supporting Information, Appendix 1, in the online version of this article). The EMR also enables secure communication between clinicians. At our institution, over 90% of discharge summaries are completed within 24 hours of discharge.[17]
Study Design and Analysis
We developed a survey about the discharge communication practices of inpatient medicine patients based on a previously described survey in the literature (see Supporting Information, Appendix 2, in the online version of this article).[9] The anonymous, 17‐question survey was electronically distributed to resident and attending PCPs at the DGIM practice. The survey was designed to determine: (1) overall PCP satisfaction with current communication practices from the inpatient team at patient discharge, (2) perceived adequacy of automatic discharge notifications, and (3) perception of the types of patients and hospitalizations requiring additional high‐touch communication at discharge.
We analyzed results of our survey using descriptive statistics. Differences in resident and attending responses were analyzed by 2tests.
RESULTS
Seventy‐five of 124 (60%) clinicians (46% residents, 54% attendings) completed the survey. Thirty‐nine (52%) PCPs were satisfied or very satisfied with communication at patient discharge. Although most reported receiving automated discharge notifications (71%), only 39% felt that the notifications plus the discharge summaries were adequate communication for safe transition of care from hospital to community. Fifty‐one percent desired direct contact beyond a discharge summary. There were no differences in preferences on discharge communication between resident and attending PCPs (P>0.05).
Over three‐fourths of PCPs surveyed preferred that for patients with complex hospitalizations (multiple readmissions, multiple active comorbidities, goals of care changes, high‐risk medication changes, time‐sensitive follow‐up needs), an additional e‐mail or verbal communication was needed to augment the information in the discharge summary (Figure 1). Only 31% reported receiving such communication.

When asked about important items to communicate for safer transitions of care, PCPs reported finding the following elements most critical: (1) medication changes (93%), (2) follow‐up actions for the PCP (88%), and (3) active medical issues (84%) (Figure 2).

CONCLUSIONS
In the era of shared EMRs, real‐time access to medication lists, pending test results, and discharge summaries should facilitate care transitions at discharge.[18, 19] We conducted a study to determine PCP perceptions of discharge communication after implementation of a shared EMR. We found that although PCPs largely acknowledged timely receipt of automated discharge notifications and discharge summaries, the majority of PCPs felt that most discharges required additional communication to ensure safe transition of care.
Guidelines for discharge communication emphasize timely communication with the PCP, primarily through discharge summaries containing key safety elements.[1, 5, 10] At our institution, we have improved the timeliness and quality of discharge summaries according to guideline recommendations,[17] and conducted quality improvement projects to improve rates of direct communication with PCPs.[9] In addition, the shared EMR provides automated notifications to PCPs when their patients are discharged. Despite these interventions, our survey shows that PCP satisfaction with discharge communication is still inadequate. PCPs desired direct communication that highlights active medical issues, medication changes, and specific follow‐up actions. Although all of these topics are included in our discharge summary template (see Supporting Information, Appendix 1, in the online version of this article), it is possible that the templated discharge summaries lend themselves to longer documents and information overload, as prior studies have documented the desire for more succinct discharge summaries.[18] We also found that automated notifications of discharge were less reliable and useful for PCPs than anticipated. There were several reasons for this: (1) discharge summaries sometimes were sent to PCPs uncoupled from the discharge notification, (2) there were errors with the generation and delivery of automated messages at the rollout of the new system, and (3) PCPs received many other automated system messages, meaning that discharge notifications could be easily missed. These factors all likely contribute to PCPs' desire for high‐touch communication that highlights the most salient aspects of each patient's hospitalization. It is also possible that automated notifications and depersonalized discharge summaries create distance and a less‐collaborative feeling to patient care. PCPs want more direct communication, and desire to play a more active role in inpatient management, especially for complex hospitalizations.[18] This emphasis on direct communication resonates with previous studies conducted before shared EMRs existed.[9, 12, 19]
Our study had several limitations. First, because this is a single‐institution study at a tertiary care academic setting, the results may not be generalizable to all shared EMR settings, and may not reflect all the challenges of communication with the wider community of outpatient providers. One can postulate that inpatient and outpatient clinician relationships are stronger in an academic setting than in other more disparate environments, where direct communication may happen even less frequently. Of note, our low rates of direct communication are consistent with other single‐ and multi‐institution studies, suggesting that our findings are generalizable.[14, 15] Second, our survey is limited in its ability to distinguish those patients who require high‐touch communication and those who do not. Third, although we have used the survey to assess PCP satisfaction in previous studies, it is not a validated instrument, and therefore we cannot reliably say that increasing direct PCP communication would increase their satisfaction around discharge. Last, the PCP‐reported rates of discharge communication are subjective and may be influenced by recall bias. We did not have a systematic way to confirm the actual rates of communication at discharge, which could have occurred through EMR messages, e‐mails, phone calls, or pages.
Although a shared EMR allows for real‐time access to patient data, it does not eliminate PCPs' desire for direct 2‐way dialogue at discharge, especially for complex patients. Key information desired in such communication should include active medical issues, medication changes, and follow‐up needs, which is consistent with prior studies. Standardizing this direct communication process in an efficient way can be challenging. Further elucidation of PCP preferences around which patients necessitate higher‐level communication and preferred methods and timing of communication is needed, as well as determining the most efficient and effective method for hospitalists to provide such communication. Improving communication between hospitalists and PCPs requires not just the presence of a shared EMR, but additional, systematic efforts to engage both inpatient and outpatient clinicians in collaborative care.
Disclosure
Nothing to report.
- , , , et al. Development of a checklist of safe discharge practices for hospital patients. J Hosp Med. 2013;8(8):444–449.
- , , , , The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , et al. Improving patient handovers from hospital to primary care: a systematic review. Ann Intern Med. 2012;157(6):417–428.
- , , , , “Did I do as best as the system would let me?” Healthcare professional views on hospital to home care transitions. J Gen Intern Med. 2012;27(12):1649–1656.
- , , , et al. Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists. J Hosp Med. 2006;1(6):354−660.
- , , , , Improving measurement in clinical handover. Qual Saf Health Care. 2009;18:272–277.
- World Health Organization. Patient safety: action on patient safety: high 5s. 2007. Available at: http://www.who.int/patientsafety/implementation/solutions/high5s/en/index.html. Accessed January 28, 2015.
- The Joint Commission Center for Transforming Healthcare. Hand‐off communications. 2012. Available at: http://www.centerfortransforminghealthcare.org/projects/detail.aspx?Project=1. Accessed January 28, 2015.
- , , , et al. The effect of a resident‐led quality improvement project on improving communication between hospital‐based and outpatient physicians. Am J Med Qual. 2013;28(6):472–479.
- , , , Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- , , , , , Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , Primary care physician attitudes regarding communication with hospitalists. Am J Med. 2001;111(9B):15S–20S.
- , , , et al. Searching for the missing pieces between the hospital and primary care: mapping the patient process during care transitions. BMJ Qual Saf. 2012;21:i97–i105.
- , , , et al. Association of self‐reported hospital discharge handoffs with 30‐day readmissions. JAMA. 2013;173(8):624–629.
- , , , et al. Association of communication between hospital‐based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381–386.
- , , , Effect of discharge summary availability during post‐discharge visits on hospital readmission. J Gen Intern Med. 2002;17(3):186–192.
- , , , , The Housestaff Incentive Program: improving the timeliness and quality of discharge summaries by engaging residents in quality improvement. BMJ Qual Saf. 2013;22(9):768–774.
- , , , et al. A Failure to communicate: a qualitative exploration of care coordination between hospitalists and primary care providers around patient hospitalizations [published online ahead of print October 15, 2014]. J Gen Intern Med. doi: 10.1007/s11606-014-3056-x.
- , , , et al. Pediatric hospitalists and primary care providers: a communication needs assessment. J Hosp Med. 2009;4(3):187–193.
- , , , et al. Development of a checklist of safe discharge practices for hospital patients. J Hosp Med. 2013;8(8):444–449.
- , , , , The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , et al. Improving patient handovers from hospital to primary care: a systematic review. Ann Intern Med. 2012;157(6):417–428.
- , , , , “Did I do as best as the system would let me?” Healthcare professional views on hospital to home care transitions. J Gen Intern Med. 2012;27(12):1649–1656.
- , , , et al. Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists. J Hosp Med. 2006;1(6):354−660.
- , , , , Improving measurement in clinical handover. Qual Saf Health Care. 2009;18:272–277.
- World Health Organization. Patient safety: action on patient safety: high 5s. 2007. Available at: http://www.who.int/patientsafety/implementation/solutions/high5s/en/index.html. Accessed January 28, 2015.
- The Joint Commission Center for Transforming Healthcare. Hand‐off communications. 2012. Available at: http://www.centerfortransforminghealthcare.org/projects/detail.aspx?Project=1. Accessed January 28, 2015.
- , , , et al. The effect of a resident‐led quality improvement project on improving communication between hospital‐based and outpatient physicians. Am J Med Qual. 2013;28(6):472–479.
- , , , Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- , , , , , Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , Primary care physician attitudes regarding communication with hospitalists. Am J Med. 2001;111(9B):15S–20S.
- , , , et al. Searching for the missing pieces between the hospital and primary care: mapping the patient process during care transitions. BMJ Qual Saf. 2012;21:i97–i105.
- , , , et al. Association of self‐reported hospital discharge handoffs with 30‐day readmissions. JAMA. 2013;173(8):624–629.
- , , , et al. Association of communication between hospital‐based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381–386.
- , , , Effect of discharge summary availability during post‐discharge visits on hospital readmission. J Gen Intern Med. 2002;17(3):186–192.
- , , , , The Housestaff Incentive Program: improving the timeliness and quality of discharge summaries by engaging residents in quality improvement. BMJ Qual Saf. 2013;22(9):768–774.
- , , , et al. A Failure to communicate: a qualitative exploration of care coordination between hospitalists and primary care providers around patient hospitalizations [published online ahead of print October 15, 2014]. J Gen Intern Med. doi: 10.1007/s11606-014-3056-x.
- , , , et al. Pediatric hospitalists and primary care providers: a communication needs assessment. J Hosp Med. 2009;4(3):187–193.
Hospital High‐Value Care Program
With a United States medical system that spends as much as $750 billion each year on care that does not result in improved health outcomes,[1] many policy initiatives, including the Centers for Medicare and Medicaid Services' Value‐Based Purchasing program, seek to realign hospitals' financial incentives from a focus on production to one on value (quality divided by cost).[2, 3] Professional organizations have now deemed resource stewardship an ethical responsibility for professionalism,[4, 5] and campaigns such as the American Board of Internal Medicine (ABIM) Foundation's Choosing Wisely effort and the American College of Physicians' High‐Value Care platform are calling on frontline clinicians to address unnecessary and wasteful services.[6, 7]
Despite these pressures and initiatives, most physicians lack the knowledge and tools necessary to prioritize the delivery of their own healthcare services according to value.[8, 9, 10] Hospital medicine physicians are unaware of the costs associated with the interventions they order,[10] and the majority of medical training programs lack curricula focused on healthcare costs,[11] creating a large gap between physicians' perceived, desired, and actual knowledge related to costs.[12] Novel frameworks and frontline physician engagement are required if clinicians are to improve the value of the care they deliver.
We describe 1 of our first steps at the University of California, San Francisco (UCSF) to promote high‐value care (HVC) delivery: the creation of a HVC program led by clinicians and administrators focused on identifying and addressing wasteful practices within our hospitalist group. The program aims to (1) use financial and clinical data to identify areas with clear evidence of waste in the hospital, (2) promote evidence‐based interventions that improve both quality of care and value, and (3) pair interventions with evidence‐based cost awareness education to drive culture change. Our experience and inaugural projects provide a model of the key features, inherent challenges, and lessons learned, which may help inform similar efforts.
METHODS
In March 2012, we launched an HVC program within our Division of Hospital Medicine at UCSF Medical Center, a 600‐bed academic medical center in an urban setting. During the 2013 academic year, our division included 45 physicians. The medicine service, comprised of 8 teaching medical ward teams (1 attending, 1 resident, 2 interns, and variable number of medical students), and 1 nonteaching medical ward team (1 attending), admitted 4700 patients that year.
Organizational Framework
The HVC program is co‐led by a UCSF hospitalist (C.M.) and the administrator of the Division of Hospital Medicine (M.N.). Team members include hospitalists, hospital medicine fellows, resident physicians, pharmacists, project coordinators, and other administrators. The team meets in person for 1 hour every month. Project teams and ad hoc subcommittee groups often convene between meetings.
Our HVC program was placed within the infrastructure, and under the leadership, of our already established quality improvement (QI) program at UCSF. Our Division of Hospital Medicine Director of Quality and Safety (M.M.) thus oversees the QI, patient safety, patient experience, and high‐value care efforts.
The HVC program funding is largely in personnel costs. The physician leader (15% effort) is funded by the Division of Hospital Medicine, whereas the administrator is cofunded by both the division and by the medical center (largely through her roles as both division administrator and service line director). An administrative assistant within the division is also assigned to help with administrative tasks. Some additional data gathering and project support comes from existing medical center QI infrastructure, the decision support services unit, and through UCSF's new Center for Healthcare Value. Other ancillary costs for our projects have included publicity, data analytics, and information technology infrastructure. We estimate that the costs of this program are approximately $50,000 to $75,000 annually.
Framework for Identifying Target Projects
Robust Analysis of Costs
We created a framework for identifying, designing, and promoting projects specifically aimed at improving healthcare value (Figure 1). Financial data were used to identify areas with clear evidence of waste in the hospital, areas of high cost with no benefit in health outcomes. We focused particularly on obtaining cost and billing data for our medical service, which provided important insight into potential targets for improvements in value. For example, in 2011, the Division of Hospital Medicine spent more than $1 million annually in direct costs for the administration of nebulized bronchodilator therapies (nebs) to nonintensive care unit patients on the medical service.[13] These high costs, exposed by billing data, were believed to represent potential unnecessary testing and/or procedures. Not every area of high cost was deemed a target for intervention. For example, the use of recombinant factor VIII appeared a necessary expenditure (over $1 million per year) for our patients with hemophilia. Although our efforts focused on reducing waste, it is worth noting that healthcare value can also be increased by improving the delivery of high‐value services.
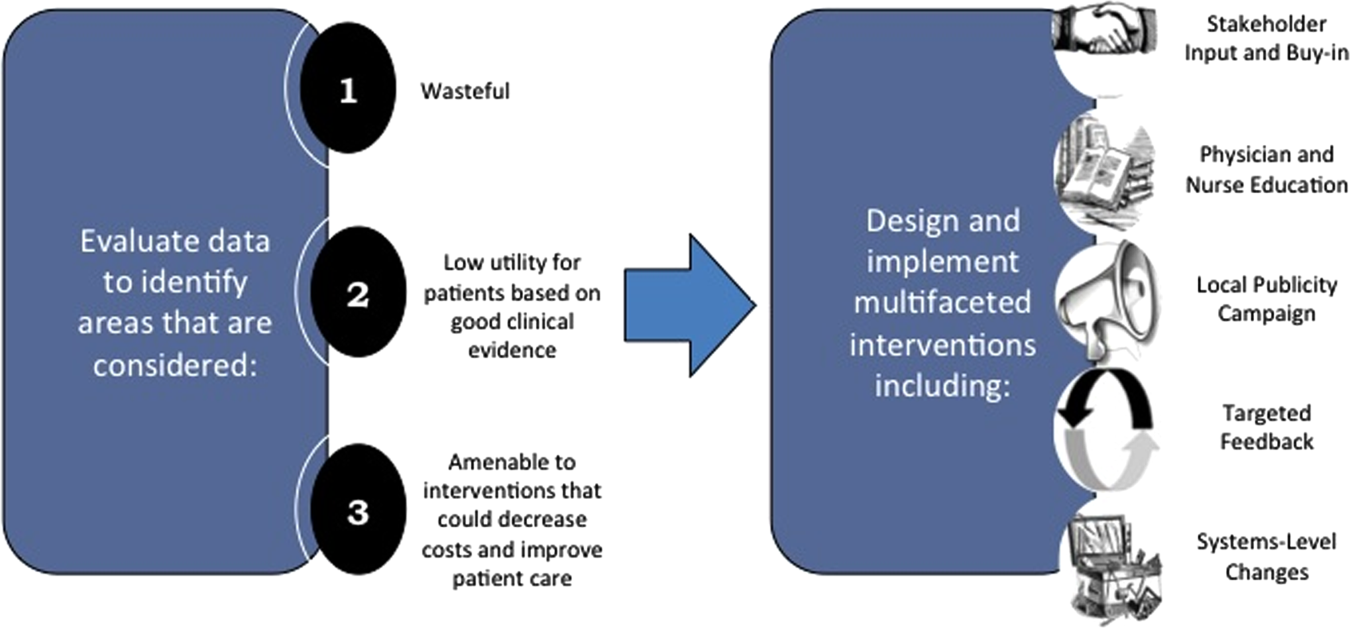
Recognized Benefits in Quality of Care
The program also evaluated the impact of cost reduction efforts on the quality of care, based on a high standard of current evidence. Though value can be improved by interventions that decrease costs while being quality neutral, our group chose to focus first on projects that would simultaneously improve quality while decreasing costs. We felt that this win‐win strategy would help obtain buy‐in from clinicians weary of prior cost‐cutting programs. For example, we pursued interventions aimed at reducing inappropriate gastric stress ulcer prophylaxis, which had the potential to both cut costs and minimize risks of hospital‐acquired pneumonia and Clostridium difficile infections.[14, 15] All proposed HVC targets were vetted through a review of the literature and published guidelines. In general, our initial projects had to be strongly supported by evidence, with high‐quality studies, preferably meta‐analyses or systematic reviews, that displayed the safety of our recommended changes. We reviewed the literature with experts. For example, we met with faculty pulmonologists to discuss the evidence supporting the use of inhalers instead of nebulizers in adults with obstructive pulmonary disease. The goals of our projects were chosen by our HVC committee, based on an analysis of our baseline data and the perceived potential effects of our proposed interventions.
Educational Intervention
Last, we paired interventions with evidence‐based cost awareness education to drive culture change. At UCSF we have an ongoing longitudinal cost‐awareness curriculum for residents, which has previously been described.[16] We took advantage of this educational forum to address gaps in clinician knowledge related to the targeted areas. When launching the initiative to decrease unnecessary inpatient nebulizer usage and improve transitions to inhalers, we utilized the chronic obstructive pulmonary disease case in the cost‐awareness series. Doing so allowed us to both review the evidence behind the effectiveness of inhalers, and introduce our Nebs No More After 24 campaign, which sought to transition adult inpatients with obstructive pulmonary symptoms from nebs to inhalers within 24 hours of admission.[13]
Intervention Strategy
Our general approach has been to design and implement multifaceted interventions, adapted from previous QI literature (Figure 1).[17] Given the importance of frontline clinician engagement to successful project implementation,[18, 19, 20] our interventions are physician‐driven and are vetted by a large group of clinicians prior to launch. The HVC program also explicitly seeks stakeholder input, perspective, and buy‐in prior to implementation. For example, we involved respiratory therapists (RTs) in the design of the Nebs No More After 24 project, thus ensuring that the interventions fit within their workflow and align with their care‐delivery goals.
Local publicity campaigns provide education and reminders for clinicians. Posters, such as the Nebs No More After 24 poster (Figure 2), were hung in physician, nursing, and RT work areas. Pens featuring the catchphrase Nebs No More After 24 were distributed to clinicians.

In addition to presentations to residents through the UCSF cost awareness curriculum, educational presentations were also delivered to attending physicians and to other allied members of the healthcare team (eg, nurses, RTs) during regularly scheduled staff meetings.
The metrics for each of the projects were regularly monitored, and targeted feedback was provided to clinicians. For the Nebs No More After 24 campaign, data for the number of nebs delivered on the target floor were provided to resident physicians during the cost awareness conference each month, and the data were presented to attending hospitalists in the monthly QI newsletter. This academic year, transfusion and telemetry data are presented via the same strategy.
Stakeholder recruitment, education, and promotional campaigns are important to program launches, but to sustain projects over the long‐term, system changes may be necessary. We have pursued changes in the computerized provider order entry (CPOE) system, such as removing nebs from the admission order set or putting a default duration for certain telemetry orders. Systems‐level interventions, although more difficult to achieve, play an important role in creating enduring changes when paired with educational interventions.
RESULTS
During our first 2 years we have initiated ongoing projects directed at 6 major targets (Table 1). Our flagship project, Nebs No More After 24, resulted in a decrease of nebulizer rates by more than 50% on a high‐acuity medical floor, as previously published.[13] We created a financial model that primarily accounted for RT time and pharmaceutical costs, and estimated a savings of approximately $250,000 annually on this single medical ward (see Supporting Information, Table 1, in the online version of this article).[13]
| High‐Value Care Projects | Relevant Baseline Data | Goals of Project | Strategies |
|---|---|---|---|
| |||
| Nebs No More After 24: Improving appropriate use of respiratory services | The medicine service spent $1 million in direct costs on approximately 25,000 nebs for non‐ICU inpatients. | Reduce unnecessary nebs >15% over 9 months. | Removed nebs from admit order set. |
| Improve transitions from nebs to MDIs. | Enlisted RTs and RNs to help with MDI teaching for patients. | ||
| Improve patient self‐administration of MDIs. | Implemented an educational program for medicine physicians. | ||
| Created local publicity: posters, flyers, and pens. | |||
| Provided data feedback to providers. | |||
| Next step: Introduce a CPOE‐linked intervention. | |||
| Improving use of stress ulcer prophylaxis | 77% of ICU patients on acid suppressive therapy; 31% of these patients did not meet criteria for appropriate prophylaxis. | Reduce overuse and inappropriate use of SUP. | A team of pharmacists, nurses, and physicians developed targeted and evidence‐based UCSF guidelines on use of SUP. |
| Developed and implemented a pharmacist‐led intervention to reduce inappropriate SUP in the ICUs that included the following: | |||
| Reminders on admission and discharge from ICU | |||
| Education and awareness initiative for prescribers | |||
| ICU and service champions | |||
| Culture change | |||
| Next step: Incorporate indications in CPOE and work with ICU to incorporate appropriate GI prophylaxis as part of the standard ICU care bundle. | |||
| Blood utilization stewardship | 30% of transfusions on the hospital medicine service are provided to patients with a hemoglobin >8 g/dL. | Decrease units of blood transfused for a hemoglobin >8.0 g/dL by 25%. | Launched an educational campaign for attending and resident physicians. |
| Monthly feedback to residents and attending physicians. | |||
| Next step: Introduce a decision support system in the CPOE for blood transfusion orders in patients with most recent hemoglobin level >8. | |||
| Improving telemetry utilization | 44% of monitored inpatients on the medical service (with length of stay >48 hours) remain on telemetry until discharge. | Decrease by 15% the number of patients (with length of stay >48 hours) who remain on telemetry until discharge. | Implemented an educational campaign for nursing groups and the medicine and cardiology housestaff. |
| Launched a messaging campaign consisting of posters and pocket cards on appropriate telemetry use. | |||
| Designed a feedback campaign with monthly e‐mail to housestaff on their ward team's telemetry use stats. | |||
| Next step: Build a CPOE intervention that asks users to specify an approved indication for telemetry when they order monitoring. The indication then dictates how long the order is active (24, 48, 72 hours or ongoing), and the MD must renew the order after the elapsed time. | |||
| iReduce iCal: ordering ionized calcium only when needed | The medicine service spent $167,000 in direct costs on iCal labs over a year (40% of all calcium lab orders; 42% occurred in non‐ICU patients). | Reduce number of iCal labs drawn on the medicine service by >25% over the course of 6 months. | With the introduction of CPOE, iCal was removed from traditional daily lab order sets. |
| Discussed with lab, renal, and ICU stakeholders. | |||
| Implemented an educational campaign for physicians and nurses. | |||
| Created local publicity: posters and candies. | |||
| Provided data feedback to providers. | |||
| Repeat inpatient echocardiograms | 25% of TTEs are performed within 6 months of a prior; one‐third of these are for inappropriate indications. | Decrease inappropriate repeat TTEs by 25%. | Implemented an educational campaign. |
| Next step: provide the most recent TTE results in the CPOE at time of order, and provide auditing and decision support for repeat TTEs. | |||
The HVC program also provided an arena for collaborating with and supporting value‐based projects launched by other groups, such as the UCSF Medication Outcomes Center's inappropriate gastric stress ulcer prophylaxis program.[21] Our group helped support the development and implementation of evidence‐based clinical practice guidelines, and we assisted educational interventions targeting clinicians. This program resulted in a decrease in inappropriate stress ulcer prophylaxis in intensive care unit patients from 19% to 6.6% within 1 month following implementation.[21]
DISCUSSION
Physicians are increasingly being asked to embrace and lead efforts to improve healthcare value and reduce costs. Our program provides a framework to guide physician‐led initiatives to identify and address areas of healthcare waste.
Challenges and Lessons Learned
Overcoming the Hurdle of More Care as Better Care
Improving the quality of care has traditionally stressed the underuse of beneficial testing and treatments, for example the use of angiotensin‐converting enzyme inhibitors in systolic heart failure. We found that improving quality by curbing overuse was a new idea for many physicians. Traditionally, physicians have struggled with cost reduction programs, feeling that efforts to reduce costs are indifferent to quality of care, and worse, may actually lead to inferior care.[22] The historical separation of most QI and cost reduction programs has likely furthered this sentiment. Our first projects married cost reduction and QI efforts by demonstrating how reducing overuse could provide an opportunity to increase quality and reduce harms from treatments. For example, transitioning from nebs to metered‐dose inhalers offered the chance to provide inpatient inhaler teaching, whereas decreasing proton pump inhibitor use can reduce the incidence of C difficile. By framing these projects as addressing both numerator and denominator of the value equation, we were able to align our cost‐reduction efforts with physicians' traditional notions of QI.
Cost Transparency
If physicians are to play a larger role in cost‐reduction efforts, they need at least a working understanding of fixed and variable costs in healthcare and of institutional prices.[23, 24] Utilization and clear information about costs were used to guide our interventions and ensured that the efforts spent to eliminate waste would result in cost savings. As an example, we learned that decreasing nebulizer use without a corresponding decrease in daily RT staffing would lead to minimal cost savings. These analyses require the support of business, financial, and resource managers in addition to physicians, nurses, project coordinators, and administrators. At many institutions the lack of price and utilization transparency presents a major barrier to the accurate analysis of cost‐reduction efforts.
The Diplomacy of Cost‐Reduction
Because the bulk of healthcare costs go to labor, efforts to reduce cost may lead to reductions in the resources available to certain departments or even to individuals' wages. For example, initiatives aimed at reducing inappropriate diagnostic imaging will affect the radiology department, which is partially paid based on the volume of studies performed.[25] Key stakeholders must be identified early, and project leaders should seek understanding, engagement, and buy‐in from involved parties prior to implementation. There will often be times that support from senior leaders will be needed to negotiate these tricky situations.
Although we benefited from a largely supportive hospital medicine faculty and resident physicians, not all of our proposed projects made it to implementation. Sometimes stakeholder recruitment proved to be difficult. For instance, a proposed project to change the protocol from routine to clinically indicated peripheral intravenous catheter replacement for adult inpatients was met with some resistance by some members of nursing management. We reviewed the literature together and discussed in length the proposal, but ultimately decided that our institution was not ready for this change at this time.
Limitations and Next Steps
Our goal is to provide guidance on exporting the approach of our HVC program to other institutions, but there may be several limitations. First, our strategy relied on several contributing factors that may be unique to our institution. We had engaged frontline physician champions, who may not be available or have the necessary support at other academic or community organizations. Our UCSF cost awareness curriculum provided an educational foundation and framework for our projects. We also had institutional commitment in the form of our medical center division administrator.
Second, there are up‐front costs to running our committee, which are primarily related to personnel funding as described in the Methods. Over the next year we aim to calculate cost‐effectiveness ratios for our projects and overall return on investment for each of our projects, as we have done for the Nebs No More After 24 project (see Supporting Information, Table 1, in the online version of this article). Based on this analysis, the modest upfront costs appear to be easily recouped over the course of the year.
We have anecdotally noted a culture change in the way that our physicians discuss and consider testing. For example, it is common now to hear ward teams on morning rounds consider the costs of testing or discuss the need for prophylactic proton pump inhibitors. An important next step for our HVC program is the building of better data infrastructures for our own electronic health record system to allow us to more quickly, accurately, and comprehensively identify new targets and monitor the progress and sustainability of our projects. The Institute of Medicine has noted that the adoption of technology is a key strategy to creating a continuously learning healthcare system.[1] It is our hope that through consistent audit and feedback of resource utilization we can translate our early gains into sustainable changes in practice.
Furthermore, we hope to target and enact additional organizational changes, including creating CPOE‐linked interventions to help reinforce and further our objectives. We believe that creating systems that make it easier to do the right thing will help the cause of embedding HVC practices throughout our medical center. We have begun to scale some of our projects, such as the Nebs No More After 24 campaign, medical center wide, and ultimately we hope to disseminate successful projects and models beyond our medical center to contribute to the national movement to provide the best care at lower costs.
As discussed above, our interventions are targeted at simultaneous improvements in quality with decreased costs. However, the goal is not to hide our cost interventions behind the banner of quality. We believe that there is a shifting culture that is increasingly ready to accept cost alone as a meaningful patient harm, worthy of interventions on its own merits, assuming that quality and safety remain stable.[26, 27]
CONCLUSIONS
Our HVC program has been successful in promoting improved healthcare value and engaging clinicians in this effort. The program is guided by the use of financial data to identify areas with clear evidence of waste in the hospital, the creation of evidence‐based interventions that improve quality of care while cutting costs, and the pairing of interventions with evidence‐based cost awareness education to drive culture change.
Acknowledgements
The authors acknowledge the following members of the UCSF Division of Hospital Medicine High‐Value Care Committee who have led some of the initiatives mentioned in this article and have directly contributed to Table 1: Dr. Stephanie Rennke, Dr. Alvin Rajkomar, Dr. Nader Najafi, Dr. Steven Ludwin, and Dr. Elizabeth Stewart. Dr. Russ Cucina particularly contributed to the designs and implementation of electronic medical record interventions.
Disclosures: Dr. Moriates received funding from the UCSF Center for Healthcare Value, the Agency for Healthcare Research and Quality (as editor for AHRQ Patient Safety Net), and the ABIM Foundation. Mrs. Novelero received funding from the UCSF Center for Healthcare Value. Dr. Wachter reports serving as the immediate past‐chair of the American Board of Internal Medicine (for which he received a stipend) and is a current member of the ABIM Foundation board; receiving a contract to UCSF from the Agency for Healthcare Research and Quality for editing 2 patient‐safety websites; receiving compensation from John Wiley & Sons for writing a blog; receiving compensation from QuantiaMD for editing and presenting patient safety educational modules; receiving royalties from Lippincott Williams & Wilkins and McGraw‐Hill for writing/editing several books; receiving a stipend and stock/options for serving on the Board of Directors of IPC‐The Hospitalist Company; serving on the scientific advisory boards for PatientSafe Solutions, CRISI, SmartDose, and EarlySense (for which he receives stock options); and holding the Benioff endowed chair in hospital medicine from Marc and Lynne Benioff. He is also a member of the Board of Directors of Salem Hospital, Salem, Oregon, for which he receives travel reimbursement but no compensation. Mr. John Hillman, Mr. Aseem Bharti, and Ms. Claudia Hermann from UCSF Decision Support Services provided financial data support and analyses, and the UCSF Center for Healthcare Value provided resource and financial support.
- Institute of Medicine. Committee on the Learning Health Care System in America. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2012.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367(4):292–295.
- . Making good on ACOs' promise—the final rule for the Medicare Shared Savings Program. N Engl J Med. 2011;365(19):1753–1756.
- . American College of Physicians ethics manual: sixth edition. Ann Intern Med. 2012;156(1 pt 2):73–104.
- ABIM Foundation, American College of Physicians‐American Society of Internal Medicine, European Federation of Internal Medicine. Medical professionalism in the new millennium: a physician charter. Ann Intern Med. 2002;136(3):243–246.
- , . Choosing Wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801.
- , , , . High‐value, cost‐conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154(3):174–180.
- , . Waste not, want not: promoting efficient use of health care resources. Ann Intern Med. 2013;158(1):67–68.
- , , . General pediatric attending physicians' and residents' knowledge of inpatient hospital finances. Pediatrics. 2013;131(6):1072–1080.
- , , . Hospitalists' awareness of patient charges associated with inpatient care. J Hosp Med. 2010;5(5):295–297.
- , , , , . Teaching residents to provide cost‐conscious care: A national survey of residency program directors. JAMA Intern Med. 2014;174(3):470–472.
- , , . Perceived, actual, and desired knowledge regarding medicare billing and reimbursement. J Gen Intern Med. 2006;21(5):466–470.
- , , , , . “Nebs No More After 24”: a pilot program to improve the use of appropriate respiratory therapies. JAMA Intern Med. 2013;173(17):1647–1648.
- , , , . Acid‐suppressive medication use and the risk for hospital‐acquired pneumonia. JAMA. 2009;301(20):2120–2128.
- , , , et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170(9):784–790.
- , , , . The value in the evidence: teaching residents to “choose wisely.” JAMA Intern Med.2013;173(4):308–310.
- , . Evidence‐based quality improvement: the state of the science. Health Aff. 2005;24(1):138–150.
- , , , . The role of physician engagement on the impact of the hospital‐based practice improvement module (PIM). J Hosp Med. 2009;4(8):466–470.
- , . Finding common cause in quality: confronting the physician engagement challenge. Physician Exec. 2008;34(2):26–28, 30–31.
- , . Engaging physicians and leveraging professionalism: a key to success for quality measurement and improvement. JAMA. 2012;308(10):979–980.
- , , et al. The development and implementation of a bundled quality improvement initiative to reduce inappropriate stress ulcer prophylaxis. ICU Dir. 2013;4(6):322–325.
- . Lost in translation: physicians' struggle with cost‐reduction programs. Ann Intern Med. 2011;154(6):430–433.
- , . How to solve the cost crisis in health care. Harv Bus Rev. 2011;89(9):46–52, 54, 56–61 passim.
- , , , . The savings illusion—why clinical quality improvement fails to deliver bottom‐line results. N Engl J Med. 2011;365(26):e48.
- , , , , . Reducing radiology use on an inpatient medical service: choosing wisely. Arch Intern Med. 2012;172(20):1606–1608.
- , , . First, do no (financial) harm. JAMA. 2013;310(6):577–578.
- , , . Full disclosure—out‐of‐pocket costs as side effects. N Engl J Med. 2013;369(16):1484–1486.
With a United States medical system that spends as much as $750 billion each year on care that does not result in improved health outcomes,[1] many policy initiatives, including the Centers for Medicare and Medicaid Services' Value‐Based Purchasing program, seek to realign hospitals' financial incentives from a focus on production to one on value (quality divided by cost).[2, 3] Professional organizations have now deemed resource stewardship an ethical responsibility for professionalism,[4, 5] and campaigns such as the American Board of Internal Medicine (ABIM) Foundation's Choosing Wisely effort and the American College of Physicians' High‐Value Care platform are calling on frontline clinicians to address unnecessary and wasteful services.[6, 7]
Despite these pressures and initiatives, most physicians lack the knowledge and tools necessary to prioritize the delivery of their own healthcare services according to value.[8, 9, 10] Hospital medicine physicians are unaware of the costs associated with the interventions they order,[10] and the majority of medical training programs lack curricula focused on healthcare costs,[11] creating a large gap between physicians' perceived, desired, and actual knowledge related to costs.[12] Novel frameworks and frontline physician engagement are required if clinicians are to improve the value of the care they deliver.
We describe 1 of our first steps at the University of California, San Francisco (UCSF) to promote high‐value care (HVC) delivery: the creation of a HVC program led by clinicians and administrators focused on identifying and addressing wasteful practices within our hospitalist group. The program aims to (1) use financial and clinical data to identify areas with clear evidence of waste in the hospital, (2) promote evidence‐based interventions that improve both quality of care and value, and (3) pair interventions with evidence‐based cost awareness education to drive culture change. Our experience and inaugural projects provide a model of the key features, inherent challenges, and lessons learned, which may help inform similar efforts.
METHODS
In March 2012, we launched an HVC program within our Division of Hospital Medicine at UCSF Medical Center, a 600‐bed academic medical center in an urban setting. During the 2013 academic year, our division included 45 physicians. The medicine service, comprised of 8 teaching medical ward teams (1 attending, 1 resident, 2 interns, and variable number of medical students), and 1 nonteaching medical ward team (1 attending), admitted 4700 patients that year.
Organizational Framework
The HVC program is co‐led by a UCSF hospitalist (C.M.) and the administrator of the Division of Hospital Medicine (M.N.). Team members include hospitalists, hospital medicine fellows, resident physicians, pharmacists, project coordinators, and other administrators. The team meets in person for 1 hour every month. Project teams and ad hoc subcommittee groups often convene between meetings.
Our HVC program was placed within the infrastructure, and under the leadership, of our already established quality improvement (QI) program at UCSF. Our Division of Hospital Medicine Director of Quality and Safety (M.M.) thus oversees the QI, patient safety, patient experience, and high‐value care efforts.
The HVC program funding is largely in personnel costs. The physician leader (15% effort) is funded by the Division of Hospital Medicine, whereas the administrator is cofunded by both the division and by the medical center (largely through her roles as both division administrator and service line director). An administrative assistant within the division is also assigned to help with administrative tasks. Some additional data gathering and project support comes from existing medical center QI infrastructure, the decision support services unit, and through UCSF's new Center for Healthcare Value. Other ancillary costs for our projects have included publicity, data analytics, and information technology infrastructure. We estimate that the costs of this program are approximately $50,000 to $75,000 annually.
Framework for Identifying Target Projects
Robust Analysis of Costs
We created a framework for identifying, designing, and promoting projects specifically aimed at improving healthcare value (Figure 1). Financial data were used to identify areas with clear evidence of waste in the hospital, areas of high cost with no benefit in health outcomes. We focused particularly on obtaining cost and billing data for our medical service, which provided important insight into potential targets for improvements in value. For example, in 2011, the Division of Hospital Medicine spent more than $1 million annually in direct costs for the administration of nebulized bronchodilator therapies (nebs) to nonintensive care unit patients on the medical service.[13] These high costs, exposed by billing data, were believed to represent potential unnecessary testing and/or procedures. Not every area of high cost was deemed a target for intervention. For example, the use of recombinant factor VIII appeared a necessary expenditure (over $1 million per year) for our patients with hemophilia. Although our efforts focused on reducing waste, it is worth noting that healthcare value can also be increased by improving the delivery of high‐value services.

Recognized Benefits in Quality of Care
The program also evaluated the impact of cost reduction efforts on the quality of care, based on a high standard of current evidence. Though value can be improved by interventions that decrease costs while being quality neutral, our group chose to focus first on projects that would simultaneously improve quality while decreasing costs. We felt that this win‐win strategy would help obtain buy‐in from clinicians weary of prior cost‐cutting programs. For example, we pursued interventions aimed at reducing inappropriate gastric stress ulcer prophylaxis, which had the potential to both cut costs and minimize risks of hospital‐acquired pneumonia and Clostridium difficile infections.[14, 15] All proposed HVC targets were vetted through a review of the literature and published guidelines. In general, our initial projects had to be strongly supported by evidence, with high‐quality studies, preferably meta‐analyses or systematic reviews, that displayed the safety of our recommended changes. We reviewed the literature with experts. For example, we met with faculty pulmonologists to discuss the evidence supporting the use of inhalers instead of nebulizers in adults with obstructive pulmonary disease. The goals of our projects were chosen by our HVC committee, based on an analysis of our baseline data and the perceived potential effects of our proposed interventions.
Educational Intervention
Last, we paired interventions with evidence‐based cost awareness education to drive culture change. At UCSF we have an ongoing longitudinal cost‐awareness curriculum for residents, which has previously been described.[16] We took advantage of this educational forum to address gaps in clinician knowledge related to the targeted areas. When launching the initiative to decrease unnecessary inpatient nebulizer usage and improve transitions to inhalers, we utilized the chronic obstructive pulmonary disease case in the cost‐awareness series. Doing so allowed us to both review the evidence behind the effectiveness of inhalers, and introduce our Nebs No More After 24 campaign, which sought to transition adult inpatients with obstructive pulmonary symptoms from nebs to inhalers within 24 hours of admission.[13]
Intervention Strategy
Our general approach has been to design and implement multifaceted interventions, adapted from previous QI literature (Figure 1).[17] Given the importance of frontline clinician engagement to successful project implementation,[18, 19, 20] our interventions are physician‐driven and are vetted by a large group of clinicians prior to launch. The HVC program also explicitly seeks stakeholder input, perspective, and buy‐in prior to implementation. For example, we involved respiratory therapists (RTs) in the design of the Nebs No More After 24 project, thus ensuring that the interventions fit within their workflow and align with their care‐delivery goals.
Local publicity campaigns provide education and reminders for clinicians. Posters, such as the Nebs No More After 24 poster (Figure 2), were hung in physician, nursing, and RT work areas. Pens featuring the catchphrase Nebs No More After 24 were distributed to clinicians.

In addition to presentations to residents through the UCSF cost awareness curriculum, educational presentations were also delivered to attending physicians and to other allied members of the healthcare team (eg, nurses, RTs) during regularly scheduled staff meetings.
The metrics for each of the projects were regularly monitored, and targeted feedback was provided to clinicians. For the Nebs No More After 24 campaign, data for the number of nebs delivered on the target floor were provided to resident physicians during the cost awareness conference each month, and the data were presented to attending hospitalists in the monthly QI newsletter. This academic year, transfusion and telemetry data are presented via the same strategy.
Stakeholder recruitment, education, and promotional campaigns are important to program launches, but to sustain projects over the long‐term, system changes may be necessary. We have pursued changes in the computerized provider order entry (CPOE) system, such as removing nebs from the admission order set or putting a default duration for certain telemetry orders. Systems‐level interventions, although more difficult to achieve, play an important role in creating enduring changes when paired with educational interventions.
RESULTS
During our first 2 years we have initiated ongoing projects directed at 6 major targets (Table 1). Our flagship project, Nebs No More After 24, resulted in a decrease of nebulizer rates by more than 50% on a high‐acuity medical floor, as previously published.[13] We created a financial model that primarily accounted for RT time and pharmaceutical costs, and estimated a savings of approximately $250,000 annually on this single medical ward (see Supporting Information, Table 1, in the online version of this article).[13]
| High‐Value Care Projects | Relevant Baseline Data | Goals of Project | Strategies |
|---|---|---|---|
| |||
| Nebs No More After 24: Improving appropriate use of respiratory services | The medicine service spent $1 million in direct costs on approximately 25,000 nebs for non‐ICU inpatients. | Reduce unnecessary nebs >15% over 9 months. | Removed nebs from admit order set. |
| Improve transitions from nebs to MDIs. | Enlisted RTs and RNs to help with MDI teaching for patients. | ||
| Improve patient self‐administration of MDIs. | Implemented an educational program for medicine physicians. | ||
| Created local publicity: posters, flyers, and pens. | |||
| Provided data feedback to providers. | |||
| Next step: Introduce a CPOE‐linked intervention. | |||
| Improving use of stress ulcer prophylaxis | 77% of ICU patients on acid suppressive therapy; 31% of these patients did not meet criteria for appropriate prophylaxis. | Reduce overuse and inappropriate use of SUP. | A team of pharmacists, nurses, and physicians developed targeted and evidence‐based UCSF guidelines on use of SUP. |
| Developed and implemented a pharmacist‐led intervention to reduce inappropriate SUP in the ICUs that included the following: | |||
| Reminders on admission and discharge from ICU | |||
| Education and awareness initiative for prescribers | |||
| ICU and service champions | |||
| Culture change | |||
| Next step: Incorporate indications in CPOE and work with ICU to incorporate appropriate GI prophylaxis as part of the standard ICU care bundle. | |||
| Blood utilization stewardship | 30% of transfusions on the hospital medicine service are provided to patients with a hemoglobin >8 g/dL. | Decrease units of blood transfused for a hemoglobin >8.0 g/dL by 25%. | Launched an educational campaign for attending and resident physicians. |
| Monthly feedback to residents and attending physicians. | |||
| Next step: Introduce a decision support system in the CPOE for blood transfusion orders in patients with most recent hemoglobin level >8. | |||
| Improving telemetry utilization | 44% of monitored inpatients on the medical service (with length of stay >48 hours) remain on telemetry until discharge. | Decrease by 15% the number of patients (with length of stay >48 hours) who remain on telemetry until discharge. | Implemented an educational campaign for nursing groups and the medicine and cardiology housestaff. |
| Launched a messaging campaign consisting of posters and pocket cards on appropriate telemetry use. | |||
| Designed a feedback campaign with monthly e‐mail to housestaff on their ward team's telemetry use stats. | |||
| Next step: Build a CPOE intervention that asks users to specify an approved indication for telemetry when they order monitoring. The indication then dictates how long the order is active (24, 48, 72 hours or ongoing), and the MD must renew the order after the elapsed time. | |||
| iReduce iCal: ordering ionized calcium only when needed | The medicine service spent $167,000 in direct costs on iCal labs over a year (40% of all calcium lab orders; 42% occurred in non‐ICU patients). | Reduce number of iCal labs drawn on the medicine service by >25% over the course of 6 months. | With the introduction of CPOE, iCal was removed from traditional daily lab order sets. |
| Discussed with lab, renal, and ICU stakeholders. | |||
| Implemented an educational campaign for physicians and nurses. | |||
| Created local publicity: posters and candies. | |||
| Provided data feedback to providers. | |||
| Repeat inpatient echocardiograms | 25% of TTEs are performed within 6 months of a prior; one‐third of these are for inappropriate indications. | Decrease inappropriate repeat TTEs by 25%. | Implemented an educational campaign. |
| Next step: provide the most recent TTE results in the CPOE at time of order, and provide auditing and decision support for repeat TTEs. | |||
The HVC program also provided an arena for collaborating with and supporting value‐based projects launched by other groups, such as the UCSF Medication Outcomes Center's inappropriate gastric stress ulcer prophylaxis program.[21] Our group helped support the development and implementation of evidence‐based clinical practice guidelines, and we assisted educational interventions targeting clinicians. This program resulted in a decrease in inappropriate stress ulcer prophylaxis in intensive care unit patients from 19% to 6.6% within 1 month following implementation.[21]
DISCUSSION
Physicians are increasingly being asked to embrace and lead efforts to improve healthcare value and reduce costs. Our program provides a framework to guide physician‐led initiatives to identify and address areas of healthcare waste.
Challenges and Lessons Learned
Overcoming the Hurdle of More Care as Better Care
Improving the quality of care has traditionally stressed the underuse of beneficial testing and treatments, for example the use of angiotensin‐converting enzyme inhibitors in systolic heart failure. We found that improving quality by curbing overuse was a new idea for many physicians. Traditionally, physicians have struggled with cost reduction programs, feeling that efforts to reduce costs are indifferent to quality of care, and worse, may actually lead to inferior care.[22] The historical separation of most QI and cost reduction programs has likely furthered this sentiment. Our first projects married cost reduction and QI efforts by demonstrating how reducing overuse could provide an opportunity to increase quality and reduce harms from treatments. For example, transitioning from nebs to metered‐dose inhalers offered the chance to provide inpatient inhaler teaching, whereas decreasing proton pump inhibitor use can reduce the incidence of C difficile. By framing these projects as addressing both numerator and denominator of the value equation, we were able to align our cost‐reduction efforts with physicians' traditional notions of QI.
Cost Transparency
If physicians are to play a larger role in cost‐reduction efforts, they need at least a working understanding of fixed and variable costs in healthcare and of institutional prices.[23, 24] Utilization and clear information about costs were used to guide our interventions and ensured that the efforts spent to eliminate waste would result in cost savings. As an example, we learned that decreasing nebulizer use without a corresponding decrease in daily RT staffing would lead to minimal cost savings. These analyses require the support of business, financial, and resource managers in addition to physicians, nurses, project coordinators, and administrators. At many institutions the lack of price and utilization transparency presents a major barrier to the accurate analysis of cost‐reduction efforts.
The Diplomacy of Cost‐Reduction
Because the bulk of healthcare costs go to labor, efforts to reduce cost may lead to reductions in the resources available to certain departments or even to individuals' wages. For example, initiatives aimed at reducing inappropriate diagnostic imaging will affect the radiology department, which is partially paid based on the volume of studies performed.[25] Key stakeholders must be identified early, and project leaders should seek understanding, engagement, and buy‐in from involved parties prior to implementation. There will often be times that support from senior leaders will be needed to negotiate these tricky situations.
Although we benefited from a largely supportive hospital medicine faculty and resident physicians, not all of our proposed projects made it to implementation. Sometimes stakeholder recruitment proved to be difficult. For instance, a proposed project to change the protocol from routine to clinically indicated peripheral intravenous catheter replacement for adult inpatients was met with some resistance by some members of nursing management. We reviewed the literature together and discussed in length the proposal, but ultimately decided that our institution was not ready for this change at this time.
Limitations and Next Steps
Our goal is to provide guidance on exporting the approach of our HVC program to other institutions, but there may be several limitations. First, our strategy relied on several contributing factors that may be unique to our institution. We had engaged frontline physician champions, who may not be available or have the necessary support at other academic or community organizations. Our UCSF cost awareness curriculum provided an educational foundation and framework for our projects. We also had institutional commitment in the form of our medical center division administrator.
Second, there are up‐front costs to running our committee, which are primarily related to personnel funding as described in the Methods. Over the next year we aim to calculate cost‐effectiveness ratios for our projects and overall return on investment for each of our projects, as we have done for the Nebs No More After 24 project (see Supporting Information, Table 1, in the online version of this article). Based on this analysis, the modest upfront costs appear to be easily recouped over the course of the year.
We have anecdotally noted a culture change in the way that our physicians discuss and consider testing. For example, it is common now to hear ward teams on morning rounds consider the costs of testing or discuss the need for prophylactic proton pump inhibitors. An important next step for our HVC program is the building of better data infrastructures for our own electronic health record system to allow us to more quickly, accurately, and comprehensively identify new targets and monitor the progress and sustainability of our projects. The Institute of Medicine has noted that the adoption of technology is a key strategy to creating a continuously learning healthcare system.[1] It is our hope that through consistent audit and feedback of resource utilization we can translate our early gains into sustainable changes in practice.
Furthermore, we hope to target and enact additional organizational changes, including creating CPOE‐linked interventions to help reinforce and further our objectives. We believe that creating systems that make it easier to do the right thing will help the cause of embedding HVC practices throughout our medical center. We have begun to scale some of our projects, such as the Nebs No More After 24 campaign, medical center wide, and ultimately we hope to disseminate successful projects and models beyond our medical center to contribute to the national movement to provide the best care at lower costs.
As discussed above, our interventions are targeted at simultaneous improvements in quality with decreased costs. However, the goal is not to hide our cost interventions behind the banner of quality. We believe that there is a shifting culture that is increasingly ready to accept cost alone as a meaningful patient harm, worthy of interventions on its own merits, assuming that quality and safety remain stable.[26, 27]
CONCLUSIONS
Our HVC program has been successful in promoting improved healthcare value and engaging clinicians in this effort. The program is guided by the use of financial data to identify areas with clear evidence of waste in the hospital, the creation of evidence‐based interventions that improve quality of care while cutting costs, and the pairing of interventions with evidence‐based cost awareness education to drive culture change.
Acknowledgements
The authors acknowledge the following members of the UCSF Division of Hospital Medicine High‐Value Care Committee who have led some of the initiatives mentioned in this article and have directly contributed to Table 1: Dr. Stephanie Rennke, Dr. Alvin Rajkomar, Dr. Nader Najafi, Dr. Steven Ludwin, and Dr. Elizabeth Stewart. Dr. Russ Cucina particularly contributed to the designs and implementation of electronic medical record interventions.
Disclosures: Dr. Moriates received funding from the UCSF Center for Healthcare Value, the Agency for Healthcare Research and Quality (as editor for AHRQ Patient Safety Net), and the ABIM Foundation. Mrs. Novelero received funding from the UCSF Center for Healthcare Value. Dr. Wachter reports serving as the immediate past‐chair of the American Board of Internal Medicine (for which he received a stipend) and is a current member of the ABIM Foundation board; receiving a contract to UCSF from the Agency for Healthcare Research and Quality for editing 2 patient‐safety websites; receiving compensation from John Wiley & Sons for writing a blog; receiving compensation from QuantiaMD for editing and presenting patient safety educational modules; receiving royalties from Lippincott Williams & Wilkins and McGraw‐Hill for writing/editing several books; receiving a stipend and stock/options for serving on the Board of Directors of IPC‐The Hospitalist Company; serving on the scientific advisory boards for PatientSafe Solutions, CRISI, SmartDose, and EarlySense (for which he receives stock options); and holding the Benioff endowed chair in hospital medicine from Marc and Lynne Benioff. He is also a member of the Board of Directors of Salem Hospital, Salem, Oregon, for which he receives travel reimbursement but no compensation. Mr. John Hillman, Mr. Aseem Bharti, and Ms. Claudia Hermann from UCSF Decision Support Services provided financial data support and analyses, and the UCSF Center for Healthcare Value provided resource and financial support.
With a United States medical system that spends as much as $750 billion each year on care that does not result in improved health outcomes,[1] many policy initiatives, including the Centers for Medicare and Medicaid Services' Value‐Based Purchasing program, seek to realign hospitals' financial incentives from a focus on production to one on value (quality divided by cost).[2, 3] Professional organizations have now deemed resource stewardship an ethical responsibility for professionalism,[4, 5] and campaigns such as the American Board of Internal Medicine (ABIM) Foundation's Choosing Wisely effort and the American College of Physicians' High‐Value Care platform are calling on frontline clinicians to address unnecessary and wasteful services.[6, 7]
Despite these pressures and initiatives, most physicians lack the knowledge and tools necessary to prioritize the delivery of their own healthcare services according to value.[8, 9, 10] Hospital medicine physicians are unaware of the costs associated with the interventions they order,[10] and the majority of medical training programs lack curricula focused on healthcare costs,[11] creating a large gap between physicians' perceived, desired, and actual knowledge related to costs.[12] Novel frameworks and frontline physician engagement are required if clinicians are to improve the value of the care they deliver.
We describe 1 of our first steps at the University of California, San Francisco (UCSF) to promote high‐value care (HVC) delivery: the creation of a HVC program led by clinicians and administrators focused on identifying and addressing wasteful practices within our hospitalist group. The program aims to (1) use financial and clinical data to identify areas with clear evidence of waste in the hospital, (2) promote evidence‐based interventions that improve both quality of care and value, and (3) pair interventions with evidence‐based cost awareness education to drive culture change. Our experience and inaugural projects provide a model of the key features, inherent challenges, and lessons learned, which may help inform similar efforts.
METHODS
In March 2012, we launched an HVC program within our Division of Hospital Medicine at UCSF Medical Center, a 600‐bed academic medical center in an urban setting. During the 2013 academic year, our division included 45 physicians. The medicine service, comprised of 8 teaching medical ward teams (1 attending, 1 resident, 2 interns, and variable number of medical students), and 1 nonteaching medical ward team (1 attending), admitted 4700 patients that year.
Organizational Framework
The HVC program is co‐led by a UCSF hospitalist (C.M.) and the administrator of the Division of Hospital Medicine (M.N.). Team members include hospitalists, hospital medicine fellows, resident physicians, pharmacists, project coordinators, and other administrators. The team meets in person for 1 hour every month. Project teams and ad hoc subcommittee groups often convene between meetings.
Our HVC program was placed within the infrastructure, and under the leadership, of our already established quality improvement (QI) program at UCSF. Our Division of Hospital Medicine Director of Quality and Safety (M.M.) thus oversees the QI, patient safety, patient experience, and high‐value care efforts.
The HVC program funding is largely in personnel costs. The physician leader (15% effort) is funded by the Division of Hospital Medicine, whereas the administrator is cofunded by both the division and by the medical center (largely through her roles as both division administrator and service line director). An administrative assistant within the division is also assigned to help with administrative tasks. Some additional data gathering and project support comes from existing medical center QI infrastructure, the decision support services unit, and through UCSF's new Center for Healthcare Value. Other ancillary costs for our projects have included publicity, data analytics, and information technology infrastructure. We estimate that the costs of this program are approximately $50,000 to $75,000 annually.
Framework for Identifying Target Projects
Robust Analysis of Costs
We created a framework for identifying, designing, and promoting projects specifically aimed at improving healthcare value (Figure 1). Financial data were used to identify areas with clear evidence of waste in the hospital, areas of high cost with no benefit in health outcomes. We focused particularly on obtaining cost and billing data for our medical service, which provided important insight into potential targets for improvements in value. For example, in 2011, the Division of Hospital Medicine spent more than $1 million annually in direct costs for the administration of nebulized bronchodilator therapies (nebs) to nonintensive care unit patients on the medical service.[13] These high costs, exposed by billing data, were believed to represent potential unnecessary testing and/or procedures. Not every area of high cost was deemed a target for intervention. For example, the use of recombinant factor VIII appeared a necessary expenditure (over $1 million per year) for our patients with hemophilia. Although our efforts focused on reducing waste, it is worth noting that healthcare value can also be increased by improving the delivery of high‐value services.

Recognized Benefits in Quality of Care
The program also evaluated the impact of cost reduction efforts on the quality of care, based on a high standard of current evidence. Though value can be improved by interventions that decrease costs while being quality neutral, our group chose to focus first on projects that would simultaneously improve quality while decreasing costs. We felt that this win‐win strategy would help obtain buy‐in from clinicians weary of prior cost‐cutting programs. For example, we pursued interventions aimed at reducing inappropriate gastric stress ulcer prophylaxis, which had the potential to both cut costs and minimize risks of hospital‐acquired pneumonia and Clostridium difficile infections.[14, 15] All proposed HVC targets were vetted through a review of the literature and published guidelines. In general, our initial projects had to be strongly supported by evidence, with high‐quality studies, preferably meta‐analyses or systematic reviews, that displayed the safety of our recommended changes. We reviewed the literature with experts. For example, we met with faculty pulmonologists to discuss the evidence supporting the use of inhalers instead of nebulizers in adults with obstructive pulmonary disease. The goals of our projects were chosen by our HVC committee, based on an analysis of our baseline data and the perceived potential effects of our proposed interventions.
Educational Intervention
Last, we paired interventions with evidence‐based cost awareness education to drive culture change. At UCSF we have an ongoing longitudinal cost‐awareness curriculum for residents, which has previously been described.[16] We took advantage of this educational forum to address gaps in clinician knowledge related to the targeted areas. When launching the initiative to decrease unnecessary inpatient nebulizer usage and improve transitions to inhalers, we utilized the chronic obstructive pulmonary disease case in the cost‐awareness series. Doing so allowed us to both review the evidence behind the effectiveness of inhalers, and introduce our Nebs No More After 24 campaign, which sought to transition adult inpatients with obstructive pulmonary symptoms from nebs to inhalers within 24 hours of admission.[13]
Intervention Strategy
Our general approach has been to design and implement multifaceted interventions, adapted from previous QI literature (Figure 1).[17] Given the importance of frontline clinician engagement to successful project implementation,[18, 19, 20] our interventions are physician‐driven and are vetted by a large group of clinicians prior to launch. The HVC program also explicitly seeks stakeholder input, perspective, and buy‐in prior to implementation. For example, we involved respiratory therapists (RTs) in the design of the Nebs No More After 24 project, thus ensuring that the interventions fit within their workflow and align with their care‐delivery goals.
Local publicity campaigns provide education and reminders for clinicians. Posters, such as the Nebs No More After 24 poster (Figure 2), were hung in physician, nursing, and RT work areas. Pens featuring the catchphrase Nebs No More After 24 were distributed to clinicians.

In addition to presentations to residents through the UCSF cost awareness curriculum, educational presentations were also delivered to attending physicians and to other allied members of the healthcare team (eg, nurses, RTs) during regularly scheduled staff meetings.
The metrics for each of the projects were regularly monitored, and targeted feedback was provided to clinicians. For the Nebs No More After 24 campaign, data for the number of nebs delivered on the target floor were provided to resident physicians during the cost awareness conference each month, and the data were presented to attending hospitalists in the monthly QI newsletter. This academic year, transfusion and telemetry data are presented via the same strategy.
Stakeholder recruitment, education, and promotional campaigns are important to program launches, but to sustain projects over the long‐term, system changes may be necessary. We have pursued changes in the computerized provider order entry (CPOE) system, such as removing nebs from the admission order set or putting a default duration for certain telemetry orders. Systems‐level interventions, although more difficult to achieve, play an important role in creating enduring changes when paired with educational interventions.
RESULTS
During our first 2 years we have initiated ongoing projects directed at 6 major targets (Table 1). Our flagship project, Nebs No More After 24, resulted in a decrease of nebulizer rates by more than 50% on a high‐acuity medical floor, as previously published.[13] We created a financial model that primarily accounted for RT time and pharmaceutical costs, and estimated a savings of approximately $250,000 annually on this single medical ward (see Supporting Information, Table 1, in the online version of this article).[13]
| High‐Value Care Projects | Relevant Baseline Data | Goals of Project | Strategies |
|---|---|---|---|
| |||
| Nebs No More After 24: Improving appropriate use of respiratory services | The medicine service spent $1 million in direct costs on approximately 25,000 nebs for non‐ICU inpatients. | Reduce unnecessary nebs >15% over 9 months. | Removed nebs from admit order set. |
| Improve transitions from nebs to MDIs. | Enlisted RTs and RNs to help with MDI teaching for patients. | ||
| Improve patient self‐administration of MDIs. | Implemented an educational program for medicine physicians. | ||
| Created local publicity: posters, flyers, and pens. | |||
| Provided data feedback to providers. | |||
| Next step: Introduce a CPOE‐linked intervention. | |||
| Improving use of stress ulcer prophylaxis | 77% of ICU patients on acid suppressive therapy; 31% of these patients did not meet criteria for appropriate prophylaxis. | Reduce overuse and inappropriate use of SUP. | A team of pharmacists, nurses, and physicians developed targeted and evidence‐based UCSF guidelines on use of SUP. |
| Developed and implemented a pharmacist‐led intervention to reduce inappropriate SUP in the ICUs that included the following: | |||
| Reminders on admission and discharge from ICU | |||
| Education and awareness initiative for prescribers | |||
| ICU and service champions | |||
| Culture change | |||
| Next step: Incorporate indications in CPOE and work with ICU to incorporate appropriate GI prophylaxis as part of the standard ICU care bundle. | |||
| Blood utilization stewardship | 30% of transfusions on the hospital medicine service are provided to patients with a hemoglobin >8 g/dL. | Decrease units of blood transfused for a hemoglobin >8.0 g/dL by 25%. | Launched an educational campaign for attending and resident physicians. |
| Monthly feedback to residents and attending physicians. | |||
| Next step: Introduce a decision support system in the CPOE for blood transfusion orders in patients with most recent hemoglobin level >8. | |||
| Improving telemetry utilization | 44% of monitored inpatients on the medical service (with length of stay >48 hours) remain on telemetry until discharge. | Decrease by 15% the number of patients (with length of stay >48 hours) who remain on telemetry until discharge. | Implemented an educational campaign for nursing groups and the medicine and cardiology housestaff. |
| Launched a messaging campaign consisting of posters and pocket cards on appropriate telemetry use. | |||
| Designed a feedback campaign with monthly e‐mail to housestaff on their ward team's telemetry use stats. | |||
| Next step: Build a CPOE intervention that asks users to specify an approved indication for telemetry when they order monitoring. The indication then dictates how long the order is active (24, 48, 72 hours or ongoing), and the MD must renew the order after the elapsed time. | |||
| iReduce iCal: ordering ionized calcium only when needed | The medicine service spent $167,000 in direct costs on iCal labs over a year (40% of all calcium lab orders; 42% occurred in non‐ICU patients). | Reduce number of iCal labs drawn on the medicine service by >25% over the course of 6 months. | With the introduction of CPOE, iCal was removed from traditional daily lab order sets. |
| Discussed with lab, renal, and ICU stakeholders. | |||
| Implemented an educational campaign for physicians and nurses. | |||
| Created local publicity: posters and candies. | |||
| Provided data feedback to providers. | |||
| Repeat inpatient echocardiograms | 25% of TTEs are performed within 6 months of a prior; one‐third of these are for inappropriate indications. | Decrease inappropriate repeat TTEs by 25%. | Implemented an educational campaign. |
| Next step: provide the most recent TTE results in the CPOE at time of order, and provide auditing and decision support for repeat TTEs. | |||
The HVC program also provided an arena for collaborating with and supporting value‐based projects launched by other groups, such as the UCSF Medication Outcomes Center's inappropriate gastric stress ulcer prophylaxis program.[21] Our group helped support the development and implementation of evidence‐based clinical practice guidelines, and we assisted educational interventions targeting clinicians. This program resulted in a decrease in inappropriate stress ulcer prophylaxis in intensive care unit patients from 19% to 6.6% within 1 month following implementation.[21]
DISCUSSION
Physicians are increasingly being asked to embrace and lead efforts to improve healthcare value and reduce costs. Our program provides a framework to guide physician‐led initiatives to identify and address areas of healthcare waste.
Challenges and Lessons Learned
Overcoming the Hurdle of More Care as Better Care
Improving the quality of care has traditionally stressed the underuse of beneficial testing and treatments, for example the use of angiotensin‐converting enzyme inhibitors in systolic heart failure. We found that improving quality by curbing overuse was a new idea for many physicians. Traditionally, physicians have struggled with cost reduction programs, feeling that efforts to reduce costs are indifferent to quality of care, and worse, may actually lead to inferior care.[22] The historical separation of most QI and cost reduction programs has likely furthered this sentiment. Our first projects married cost reduction and QI efforts by demonstrating how reducing overuse could provide an opportunity to increase quality and reduce harms from treatments. For example, transitioning from nebs to metered‐dose inhalers offered the chance to provide inpatient inhaler teaching, whereas decreasing proton pump inhibitor use can reduce the incidence of C difficile. By framing these projects as addressing both numerator and denominator of the value equation, we were able to align our cost‐reduction efforts with physicians' traditional notions of QI.
Cost Transparency
If physicians are to play a larger role in cost‐reduction efforts, they need at least a working understanding of fixed and variable costs in healthcare and of institutional prices.[23, 24] Utilization and clear information about costs were used to guide our interventions and ensured that the efforts spent to eliminate waste would result in cost savings. As an example, we learned that decreasing nebulizer use without a corresponding decrease in daily RT staffing would lead to minimal cost savings. These analyses require the support of business, financial, and resource managers in addition to physicians, nurses, project coordinators, and administrators. At many institutions the lack of price and utilization transparency presents a major barrier to the accurate analysis of cost‐reduction efforts.
The Diplomacy of Cost‐Reduction
Because the bulk of healthcare costs go to labor, efforts to reduce cost may lead to reductions in the resources available to certain departments or even to individuals' wages. For example, initiatives aimed at reducing inappropriate diagnostic imaging will affect the radiology department, which is partially paid based on the volume of studies performed.[25] Key stakeholders must be identified early, and project leaders should seek understanding, engagement, and buy‐in from involved parties prior to implementation. There will often be times that support from senior leaders will be needed to negotiate these tricky situations.
Although we benefited from a largely supportive hospital medicine faculty and resident physicians, not all of our proposed projects made it to implementation. Sometimes stakeholder recruitment proved to be difficult. For instance, a proposed project to change the protocol from routine to clinically indicated peripheral intravenous catheter replacement for adult inpatients was met with some resistance by some members of nursing management. We reviewed the literature together and discussed in length the proposal, but ultimately decided that our institution was not ready for this change at this time.
Limitations and Next Steps
Our goal is to provide guidance on exporting the approach of our HVC program to other institutions, but there may be several limitations. First, our strategy relied on several contributing factors that may be unique to our institution. We had engaged frontline physician champions, who may not be available or have the necessary support at other academic or community organizations. Our UCSF cost awareness curriculum provided an educational foundation and framework for our projects. We also had institutional commitment in the form of our medical center division administrator.
Second, there are up‐front costs to running our committee, which are primarily related to personnel funding as described in the Methods. Over the next year we aim to calculate cost‐effectiveness ratios for our projects and overall return on investment for each of our projects, as we have done for the Nebs No More After 24 project (see Supporting Information, Table 1, in the online version of this article). Based on this analysis, the modest upfront costs appear to be easily recouped over the course of the year.
We have anecdotally noted a culture change in the way that our physicians discuss and consider testing. For example, it is common now to hear ward teams on morning rounds consider the costs of testing or discuss the need for prophylactic proton pump inhibitors. An important next step for our HVC program is the building of better data infrastructures for our own electronic health record system to allow us to more quickly, accurately, and comprehensively identify new targets and monitor the progress and sustainability of our projects. The Institute of Medicine has noted that the adoption of technology is a key strategy to creating a continuously learning healthcare system.[1] It is our hope that through consistent audit and feedback of resource utilization we can translate our early gains into sustainable changes in practice.
Furthermore, we hope to target and enact additional organizational changes, including creating CPOE‐linked interventions to help reinforce and further our objectives. We believe that creating systems that make it easier to do the right thing will help the cause of embedding HVC practices throughout our medical center. We have begun to scale some of our projects, such as the Nebs No More After 24 campaign, medical center wide, and ultimately we hope to disseminate successful projects and models beyond our medical center to contribute to the national movement to provide the best care at lower costs.
As discussed above, our interventions are targeted at simultaneous improvements in quality with decreased costs. However, the goal is not to hide our cost interventions behind the banner of quality. We believe that there is a shifting culture that is increasingly ready to accept cost alone as a meaningful patient harm, worthy of interventions on its own merits, assuming that quality and safety remain stable.[26, 27]
CONCLUSIONS
Our HVC program has been successful in promoting improved healthcare value and engaging clinicians in this effort. The program is guided by the use of financial data to identify areas with clear evidence of waste in the hospital, the creation of evidence‐based interventions that improve quality of care while cutting costs, and the pairing of interventions with evidence‐based cost awareness education to drive culture change.
Acknowledgements
The authors acknowledge the following members of the UCSF Division of Hospital Medicine High‐Value Care Committee who have led some of the initiatives mentioned in this article and have directly contributed to Table 1: Dr. Stephanie Rennke, Dr. Alvin Rajkomar, Dr. Nader Najafi, Dr. Steven Ludwin, and Dr. Elizabeth Stewart. Dr. Russ Cucina particularly contributed to the designs and implementation of electronic medical record interventions.
Disclosures: Dr. Moriates received funding from the UCSF Center for Healthcare Value, the Agency for Healthcare Research and Quality (as editor for AHRQ Patient Safety Net), and the ABIM Foundation. Mrs. Novelero received funding from the UCSF Center for Healthcare Value. Dr. Wachter reports serving as the immediate past‐chair of the American Board of Internal Medicine (for which he received a stipend) and is a current member of the ABIM Foundation board; receiving a contract to UCSF from the Agency for Healthcare Research and Quality for editing 2 patient‐safety websites; receiving compensation from John Wiley & Sons for writing a blog; receiving compensation from QuantiaMD for editing and presenting patient safety educational modules; receiving royalties from Lippincott Williams & Wilkins and McGraw‐Hill for writing/editing several books; receiving a stipend and stock/options for serving on the Board of Directors of IPC‐The Hospitalist Company; serving on the scientific advisory boards for PatientSafe Solutions, CRISI, SmartDose, and EarlySense (for which he receives stock options); and holding the Benioff endowed chair in hospital medicine from Marc and Lynne Benioff. He is also a member of the Board of Directors of Salem Hospital, Salem, Oregon, for which he receives travel reimbursement but no compensation. Mr. John Hillman, Mr. Aseem Bharti, and Ms. Claudia Hermann from UCSF Decision Support Services provided financial data support and analyses, and the UCSF Center for Healthcare Value provided resource and financial support.
- Institute of Medicine. Committee on the Learning Health Care System in America. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2012.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367(4):292–295.
- . Making good on ACOs' promise—the final rule for the Medicare Shared Savings Program. N Engl J Med. 2011;365(19):1753–1756.
- . American College of Physicians ethics manual: sixth edition. Ann Intern Med. 2012;156(1 pt 2):73–104.
- ABIM Foundation, American College of Physicians‐American Society of Internal Medicine, European Federation of Internal Medicine. Medical professionalism in the new millennium: a physician charter. Ann Intern Med. 2002;136(3):243–246.
- , . Choosing Wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801.
- , , , . High‐value, cost‐conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154(3):174–180.
- , . Waste not, want not: promoting efficient use of health care resources. Ann Intern Med. 2013;158(1):67–68.
- , , . General pediatric attending physicians' and residents' knowledge of inpatient hospital finances. Pediatrics. 2013;131(6):1072–1080.
- , , . Hospitalists' awareness of patient charges associated with inpatient care. J Hosp Med. 2010;5(5):295–297.
- , , , , . Teaching residents to provide cost‐conscious care: A national survey of residency program directors. JAMA Intern Med. 2014;174(3):470–472.
- , , . Perceived, actual, and desired knowledge regarding medicare billing and reimbursement. J Gen Intern Med. 2006;21(5):466–470.
- , , , , . “Nebs No More After 24”: a pilot program to improve the use of appropriate respiratory therapies. JAMA Intern Med. 2013;173(17):1647–1648.
- , , , . Acid‐suppressive medication use and the risk for hospital‐acquired pneumonia. JAMA. 2009;301(20):2120–2128.
- , , , et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170(9):784–790.
- , , , . The value in the evidence: teaching residents to “choose wisely.” JAMA Intern Med.2013;173(4):308–310.
- , . Evidence‐based quality improvement: the state of the science. Health Aff. 2005;24(1):138–150.
- , , , . The role of physician engagement on the impact of the hospital‐based practice improvement module (PIM). J Hosp Med. 2009;4(8):466–470.
- , . Finding common cause in quality: confronting the physician engagement challenge. Physician Exec. 2008;34(2):26–28, 30–31.
- , . Engaging physicians and leveraging professionalism: a key to success for quality measurement and improvement. JAMA. 2012;308(10):979–980.
- , , et al. The development and implementation of a bundled quality improvement initiative to reduce inappropriate stress ulcer prophylaxis. ICU Dir. 2013;4(6):322–325.
- . Lost in translation: physicians' struggle with cost‐reduction programs. Ann Intern Med. 2011;154(6):430–433.
- , . How to solve the cost crisis in health care. Harv Bus Rev. 2011;89(9):46–52, 54, 56–61 passim.
- , , , . The savings illusion—why clinical quality improvement fails to deliver bottom‐line results. N Engl J Med. 2011;365(26):e48.
- , , , , . Reducing radiology use on an inpatient medical service: choosing wisely. Arch Intern Med. 2012;172(20):1606–1608.
- , , . First, do no (financial) harm. JAMA. 2013;310(6):577–578.
- , , . Full disclosure—out‐of‐pocket costs as side effects. N Engl J Med. 2013;369(16):1484–1486.
- Institute of Medicine. Committee on the Learning Health Care System in America. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2012.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367(4):292–295.
- . Making good on ACOs' promise—the final rule for the Medicare Shared Savings Program. N Engl J Med. 2011;365(19):1753–1756.
- . American College of Physicians ethics manual: sixth edition. Ann Intern Med. 2012;156(1 pt 2):73–104.
- ABIM Foundation, American College of Physicians‐American Society of Internal Medicine, European Federation of Internal Medicine. Medical professionalism in the new millennium: a physician charter. Ann Intern Med. 2002;136(3):243–246.
- , . Choosing Wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801.
- , , , . High‐value, cost‐conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154(3):174–180.
- , . Waste not, want not: promoting efficient use of health care resources. Ann Intern Med. 2013;158(1):67–68.
- , , . General pediatric attending physicians' and residents' knowledge of inpatient hospital finances. Pediatrics. 2013;131(6):1072–1080.
- , , . Hospitalists' awareness of patient charges associated with inpatient care. J Hosp Med. 2010;5(5):295–297.
- , , , , . Teaching residents to provide cost‐conscious care: A national survey of residency program directors. JAMA Intern Med. 2014;174(3):470–472.
- , , . Perceived, actual, and desired knowledge regarding medicare billing and reimbursement. J Gen Intern Med. 2006;21(5):466–470.
- , , , , . “Nebs No More After 24”: a pilot program to improve the use of appropriate respiratory therapies. JAMA Intern Med. 2013;173(17):1647–1648.
- , , , . Acid‐suppressive medication use and the risk for hospital‐acquired pneumonia. JAMA. 2009;301(20):2120–2128.
- , , , et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170(9):784–790.
- , , , . The value in the evidence: teaching residents to “choose wisely.” JAMA Intern Med.2013;173(4):308–310.
- , . Evidence‐based quality improvement: the state of the science. Health Aff. 2005;24(1):138–150.
- , , , . The role of physician engagement on the impact of the hospital‐based practice improvement module (PIM). J Hosp Med. 2009;4(8):466–470.
- , . Finding common cause in quality: confronting the physician engagement challenge. Physician Exec. 2008;34(2):26–28, 30–31.
- , . Engaging physicians and leveraging professionalism: a key to success for quality measurement and improvement. JAMA. 2012;308(10):979–980.
- , , et al. The development and implementation of a bundled quality improvement initiative to reduce inappropriate stress ulcer prophylaxis. ICU Dir. 2013;4(6):322–325.
- . Lost in translation: physicians' struggle with cost‐reduction programs. Ann Intern Med. 2011;154(6):430–433.
- , . How to solve the cost crisis in health care. Harv Bus Rev. 2011;89(9):46–52, 54, 56–61 passim.
- , , , . The savings illusion—why clinical quality improvement fails to deliver bottom‐line results. N Engl J Med. 2011;365(26):e48.
- , , , , . Reducing radiology use on an inpatient medical service: choosing wisely. Arch Intern Med. 2012;172(20):1606–1608.
- , , . First, do no (financial) harm. JAMA. 2013;310(6):577–578.
- , , . Full disclosure—out‐of‐pocket costs as side effects. N Engl J Med. 2013;369(16):1484–1486.