User login
Patent foramen ovale and cryptogenic stroke: Many unanswered questions
Your patient has had an ischemic stroke, and so far you have found no obvious cause such as atrial fibrillation or carotid disease. Should you look for a patent foramen ovale (PFO)? And if you find it, what should you do?
This scenario continues to challenge primary care physicians and subspecialists and requires an understanding of the relationship between PFO and cryptogenic stroke, as well as familiarity with current data on the safety and effectiveness of the management options. PFO is known to be associated with cryptogenic stroke, but many questions remain, including:
- How can we tell if PFO is a culprit (“pathologic”) or an innocent bystander (“incidental”) in a patient who has had a cryptogenic stroke?
- Should stroke patients receive different medical therapy if they have a PFO? In particular, should they receive warfarin in addition to aspirin? And what about the novel oral anticoagulants?
- Which patients should undergo percutaneous closure of the PFO?
- Should we even be looking for PFO in stroke patients at this point, if we cannot say with certainty what we should do if we find it?
WHY IS THIS IMPORTANT?
Cerebrovascular disease is common and costly. The estimated yearly incidence of stroke in the United States is 795,000 events, at a cost of nearly $30 billion.1 The incidence of stroke in Europe is more than 1 million annually.2
During the diagnostic evaluation of stroke or transient ischemic attack (TIA), PFO is occasionally discovered incidentally by echocardiography. The management decisions that follow often fall to the primary care physician, who must decipher the conflicting data currently available and explain the options to the patient.
Although reviews have been published on this subject,3 several newer key trials and data on risk stratification warrant consideration.
DEFINITIONS
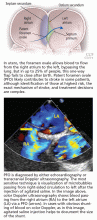
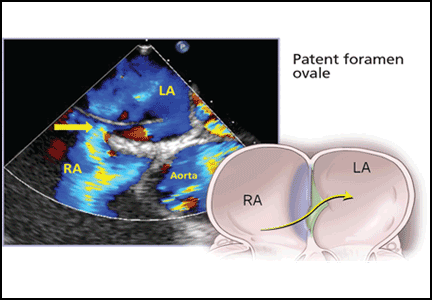
PFO is the failure of the septum primum to fuse with the septum secundum, so that a communication remains between the atria (Figure 1). The diagnosis is commonly made by echocardiography, when agitated saline is injected into the venous system and bubbles can be seen in the left atrium within three to five cardiac cycles (see video).
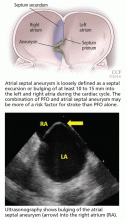
Atrial septal aneurysm is loosely defined as a septal excursion or bulging of at least 10 to 15 mm into the left and right atria during the cardiac cycle (Figure 2). The combination of PFO and atrial septal aneurysm may be more of a risk factor for stroke than PFO alone (see discussion below).
Cryptogenic stroke. The diagnostic workup of stroke fails to elucidate a clear cause in up to 40% of cases, which are thus called cryptogenic.4 The workup varies, but typically includes a search for a cardioembolic source and for atherosclerotic disease. Embolic sources are evaluated for by electrocardiography, transthoracic echocardiography, and possibly imaging of the aortic arch. Evaluation for atherosclerotic disease of the intracranial and extracranial arteries includes magnetic resonance angiography or, if that is unavailable, computed tomographic angiography or carotid Doppler ultrasonography. If no source is found, long-term cardiac monitoring may be used to detect paroxysmal atrial fibrillation, which may be more common than previously thought.
PFO AND CRYPTOGENIC STROKE ARE COMMON
As noted, there are approximately 800,000 strokes every year in the United States. If 25% to 40% of them are cryptogenic (the true prevalence warrants more evaluation),4,5 then 200,000 to 320,000 strokes are cryptogenic.
Autopsy studies indicate that 25% of the general population have a PFO, and if the prevalence is the same in people with cryptogenic stroke, that would equal 80,000 people with both cryptogenic stroke and PFO every year. However, the prevalence of PFO in patients with cryptogenic stroke appears to be significantly higher than in the general population.6 Although these numbers are crude estimates, they provide some insight into the prevalence of this clinical presentation.
HOW ARE CRYPTOGENIC STROKE AND PFO RELATED?
The exact relationship between PFO and cryptogenic stroke is unknown, although cases have been reported of thrombus in transit through a PFO, supporting paradoxical embolism as the plausible cause in stroke patients with PFO.7–9
There is clear evidence that the two conditions are associated by more than chance. Homma and Sacco6 reported that, in several studies, 93 (46%) of 202 patients under age 55 with cryptogenic stroke had PFOs, compared with 29 (11%) of 271 controls (P < .05 in all studies).6
In their evaluation of 23 case-control studies, Alsheikh-Ali et al10 found that the summary odds ratio (OR) for PFO in cryptogenic stroke vs PFO in control patients was 2.9 (95% confidence interval [CI] 2.1–4), largely driven by an OR of 5.1 (3.3–7.8) in those under age 55. Through Bayesian probability theory, this correlated with only a 33% probability that PFO in a patient with cryptogenic stroke was an innocent bystander rather than the culprit.10
IS PFO A RISK FACTOR FOR STROKE?
One of the more puzzling aspects of the relationship of PFO to cryptogenic stroke is that despite a clear association, there is little evidence that the relationship is causal.
Di Tullio et al11 followed 1,100 people who had no history of stroke and found that the risk of a first stroke in those with a PFO was not significantly higher than in those without a PFO, regardless of age, sex, or ethnic or racial group. At 80 months, the hazard ratio of stroke in people who had a PFO was 1.64 (95% CI 0.87–3.09).11 The findings were similar at 11 years, with a hazard ratio of 1.10 (95% CI 0.64–1.91).12
A prospective study of 585 patients found a similar risk of stroke in those with and without a PFO, with a hazard ratio of 1.46 (95% CI 0.74–2.88; P = .28).13
These prospective trials suggest that although previous studies have found a higher prevalence of PFOs in patients with cryptogenic stroke than in patients without stroke, there appears to be very little if any increased risk from baseline for a first stroke or TIA.
The lack of statistical significance in these trials should be interpreted with some caution, as a small increased risk is difficult to show if the event rate is low (approximately 10% of patients had events over 11 years in the study by Di Tullio et al12).
HOW DO WE KNOW IF A PFO IS A CULPRIT OR BYSTANDER?
Unfortunately, this is largely unanswered, though experts have suggested that echocardiographic features of the PFO, radiographic characteristics of the stroke, and clinical features of the patient may provide useful information.
‘High-risk’ features on echocardiography
Certain features of PFO may portend a high risk of cerebrovascular events. Both right-to-left shunting at rest and septal hypermobility were found in one study14 to be more common in patients with a PFO who had a stroke or TIA than in patients with a PFO but no cerebrovascular events. Also, patients who had these features and had a stroke had a higher risk of recurrence than stroke patients without these features (12.5% vs 4.3%, P = .05).14
Septal hypermobility and shunting at rest are easily diagnosed by echocardiography, and detecting these “high-risk” features would be useful if they could identify patients who would benefit from special therapy, such as percutaneous closure of the PFO.
Unfortunately, when investigators looked at these features in subgroup analysis of the major randomized controlled trials of percutaneous closure vs medical therapy, the results were mixed.
CLOSURE 1 (the Evaluation of the STARFlex Septal Closure System in Patients With a Stroke and/or Transient Ischemic Attack Due to Presumed Paradoxical Embolism Through a Patent Foramen Ovale)15 found percutaneous closure to be no better than medical therapy, regardless of shunt size or the presence of atrial septal aneurysm.
Similarly, the PC trial (Clinical Trial Comparing Percutaneous Closure of Patent Foramen Ovale Using the Amplatzer PFO Occluder With Medical Treatment in Patients With Cryptogenic Embolism)16 found no statistically significant benefit of closure in those with atrial septal aneurysm.
In contrast, the RESPECT trial (Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment)17 showed percutaneous closure to be beneficial in patients with atrial septal aneurysm or large shunt.
Radiographic characteristics of the stroke
Another area of interest in trying to identify culprit PFOs is the radiographic characteristics of the stroke.
In a study comparing patients with stroke related to atrial fibrillation vs patients with cryptogenic stroke and a known PFO, those in the latter group were more likely to have a single cortical infarction (34.2% vs 3.1%; P < .001) or multiple small scattered lesions (23.1% vs 5.9%; P < .01).18 Similarly, in a large database of patients with cryptogenic stroke and known PFO status, a superficially located stroke was associated with the presence of PFO (OR 1.54; P < .0001).19
Although these findings do not tell us with certainty that a patient’s PFO was the cause of his or her stroke, they provide guidance when dealing with the uncertainty of how to manage a patient with PFO. They may be useful in clinical practice, for example, when discussing treatment options with a young patient with cryptogenic stroke who has no risk factors and a superficial single infarct and who is found to have a PFO with a right-to-left shunting at rest.
Patient characteristics
Kent et al20 developed a 10-point index (the RoPE score) in an attempt to assign a probability to whether a stroke was PFO-related. Points were assigned for patients who were younger, who had a cortical stroke on neuroimaging, and who did not have diabetes, hypertension, smoking, or prior stroke or TIA. Patients with cryptogenic stroke with a higher RoPE score were more likely to have a PFO and thus had a higher likelihood that the index event was related to PFO. Of note, the patients with the highest likelihood of PFO-related stroke were the least likely to have a recurrence (RoPE score of 9 to 10; PFO-attributable fraction 88%; estimated 2-year recurrence rate 2%; 95% CI 0%–4%), whereas those with a low RoPE score have more traditional risk factors for stroke and thus are more likely to have a recurrence (RoPE 0 to 3; estimated 2-year recurrence rate 20%; 95% CI 12%–28%).20
Again, this sheds light on a difficulty faced by randomized controlled trials: the patients who may benefit from closure of a PFO may very well be those with the lowest recurrence rates without intervention.
The RoPE index was examined in an attempt to validate previously described morphologic criteria of “high-risk” PFO,21 though none of the previously described “high-risk” echocardiographic features (large physiologic size, hypermobile septum, shunt at rest) were more common in the group with presumed PFO-attributable stroke (RoPE score > 6). This underscores the difficulty of distinguishing pathologic PFO from incidental PFO.
KEY TREATMENT CONSIDERATIONS FOR SECONDARY PREVENTION
Given the complicated relationship between PFO and cryptogenic stroke, there has been much debate over management strategies. The three options are surgical closure, percutaneous closure with a device, and medical therapy. The goal of all three is to prevent the recurrence of stroke or TIA.
Surgical closure has largely been supplanted by percutaneous closure, but is still done in specific situations such as when a PFO is found incidentally on transesophageal echocardiography during surgery for another cardiac condition. The data on such cases22 tend to support the argument that asymptomatic PFOs in the general population have a relatively benign natural history.
Thus, the two key questions about management that warrant discussion are: is anticoagulation superior to antiplatelet therapy? And is percutaneous closure superior to medical management?
Anticoagulant vs antiplatelet therapy
Whether to treat with aspirin or with a vitamin K antagonist has been a subject of debate, although there is no strong evidence to suggest that anticoagulation is superior to antiplatelet therapy.
The concern that aspirin alone is insufficient in some patients stems from a study by Mas et al,23 who followed 581 patients with cryptogenic stroke who had a PFO alone, a PFO with an atrial septal aneurysm, or neither. The rate of stroke recurrence at 4 years on aspirin therapy was 2.3% in those with a PFO alone, 15.2% in those with a PFO with an atrial septal aneurysm, and 4.2% in those with neither.
Many have concluded that aspirin therapy does not sufficiently protect those with both PFO and atrial septal aneurysm, given the high recurrence rate in this group. This might lead to the suggestion that anticoagulation could be of benefit in these patients.
However, the Patent Foramen Ovale in Cryptogenic Stroke Study (PiCSS)24 and the Spanish Multicenter Study Into Right-to-Left Shunt in Cryptogenic Stroke (CODICIA)25 found similar recurrence rates in patients with PFO and atrial septal aneurysm compared with those with only PFO. In these two studies, recurrence rates were similar regardless of whether patients were taking aspirin or warfarin.
In a study that followed 140 consecutive patients with both stroke and PFO, those treated in a nonrandomized fashion with antiplatelet agents had no difference in the recurrence rate compared with those treated with anticoagulation.26
Although uncertainty remains because no head-to-head randomized controlled trial has been done, some patients with PFO have other indications for anticoagulation, most commonly atrial fibrillation and venous thromboembolic disease.
There are currently no data on the use of novel oral anticoagulants in this setting.
Is percutaneous closure better than medical therapy?
When cryptogenic stroke is treated with antiplatelet therapy or anticoagulation therapy, the recurrence rate is the same whether or not the patient has a PFO.23–25 The belief that medical therapy offers adequate secondary protection is supported by a meta-analysis of 15 studies that found no increased risk of recurrent ischemic events in those with a PFO on medical therapy (antiplatelet or anticoagulant) vs those without a PFO (relative risk 1.1, 95% CI 0.8–1.5).27
Despite the conflicting evidence, percutaneous closure of PFO is still performed, mostly on a case-by-case basis. This has been supported by an apparent benefit in observational studies.
A systematic review of 52 single-arm studies and 7 comparative nonrandomized studies of patients with PFO and cryptogenic stroke found the rate of recurrent stroke to be 0.36 per 100 person-years with percutaneous closure vs 2.53 per 100 person-years with medical therapy.28 However, three long-awaited randomized controlled trials (CLOSURE 1, the PC trial, and RESPECT) failed to show a significant reduction in primary end points with percutaneous closure vs standard medical therapy.15–17
These trials had several limitations: event rates were low, medical therapy varied by provider, and enrollment was slowed by out-of-study percutaneous closure in patients perceived to be at high risk (though, as discussed above, high risk is difficult to determine).
Intention-to-treat analysis in RESPECT showed no benefit from percutaneous closure, but a favorable outcome was noted with closure in as-treated analysis (HR 0.27; 95% CI 0.1–0.75; P = .007) and per-protocol analysis (HR 0.37; 95% CI 0.14–0.96; P = .03) of the 980 randomized patients.17 This suggests some benefit, as does the CLOSURE 1 trial, in which 3 of the 12 recurrent strokes in the percutaneous closure group occurred before the device was implanted.15
The low event rates in these studies prompted several meta-analyses.29–35 However, only two suggested a benefit of percutaneous closure over medical therapy. In one recent meta-analysis,29 observational study data suggested benefit from percutaneous closure, whereas three randomized controlled trials failed to show a statistically significant benefit.
The conclusions of the meta-analyses must be interpreted with caution because of inherent differences in the randomized controlled trials, including the closure device used, inclusion criteria, study end points, and variations in medical therapy.
Devices differ
A meta-analysis by Khan et al35 showed a benefit of percutaneous closure when evaluating only studies using the Amplatzer PFO occluder (AGA Medical), as in RESPECT and the PC trial.35 As data accumulate, it is important to remember that there are differences between devices. Ongoing trials continue to investigate the Amplatzer device (NCT01550588) and the GORE HELEX Septal Occluder/GORE Septal Occluder (Gore Medical) (NCT00738894).
In another meta-analysis, Pineda et al31 found a benefit with closure in the as-treated analysis using data from all three randomized controlled trials (OR 0.62; 95% CI 0.41–0.94; P = .02).31 Although paradoxical embolism through the PFO as the mechanism of stroke has been questioned, this finding suggests that actual closure of a PFO may protect against further events, presumably by preventing paradoxical embolism.
Different closure devices have different side effects. The incidence of atrial fibrillation with the CardioSEAL STARFlex device (NMT Medical) is higher than with medical therapy (used in the CLOSURE trial15), whereas this risk was not statistically significantly increased in the PC trial16 and RESPECT,17 which used the Amplatzer device.
Benefit in those with atrial septal aneurysm?
Percutaneous closure has been shown to be safe and effective in patients with PFO and atrial septal aneurysm.36 There was some benefit of closure over medical therapy in a subgroup analysis from RESPECT in these patients, with a HR of 0.19 (95% CI 0.04–0.87, P = .02),17 although this was not seen in either CLOSURE 1 or the PC trial.
WHAT ARE THE RISKS OF PERCUTANEOUS CLOSURE?
Minor complications of percutaneous closure include bleeding, atrial arrhythmias, device embolization and fracture, and complications related to vascular access. Major complications include hemorrhage requiring transfusion, need for surgery, cardiac tamponade, pulmonary embolism, and death.
The cumulative rate of major complications in 10 observational studies was 1.5%, and the rate of minor complications was 7.9%.37 The RESPECT investigators reported a serious adverse event in 4.2% of patients (ranging in severity from chest tightness to cardiac tamponade).17
Another possible consequence of percutaneous closure is the need for chronic anticoagulation because of the increased risk of postprocedural atrial fibrillation seen in meta-analyses,29,31,32 though this may be device-specific.32
Percutaneous closure was considered successful—ie, to have nearly or completely eliminated shunting of blood through the defect—at 6 months of follow-up in 95.9% of patients in the PC trial, 93.5% in RESPECT, and 86.1% in CLOSURE 1.15–17
WHAT SHOULD WE BE DOING IN DAILY PRACTICE?
Give aspirin. Aspirin is effective in secondary stroke prevention, and data suggest that patients with PFO and cryptogenic stroke who receive aspirin therapy alone have a similar risk of recurrent events as patients without PFO.
Give warfarin if indicated. Evidence is insufficient to recommend vitamin K antagonist therapy in all patients with PFO and cryptogenic stroke. However, coexisting conditions that warrant anticoagulation must be taken into account.
Individualize. Given the lack of evidence to definitively guide management of patients with cryptogenic stroke and PFO, we need to individualize our approach, taking into account patient preferences, bleeding risk, ability to tolerate procedures, and the likelihood that the PFO is at fault.
No definitive answer on PFO closure. The most recent data suggest that closure may be beneficial, but key questions remain: Who will benefit? And what is the ideal medical therapy? Optimal management will only be established by the continued enrollment of appropriate patients into ongoing clinical trials.
Another question is whether it is possible to perform a randomized controlled trial with enough patients to definitively prove whether percutaneous closure is superior to medical therapy. Recent experience would suggest not.
In the meantime, we have some guidance from the American Heart Association and the American Stroke Association Council on Stroke38 based on the limited evidence available.
Consider patient preference. The physician should present the options to the patient in a balanced manner to enable him or her to make an informed decision. Patients can also be encouraged to seek additional information at websites such as www.stroke.org and www.nlm.nih.gov.
Referral to an interventional cardiologist for evaluation for closure is reasonable in patients with recurrent stroke, medication failure, complicated atrial septal anatomy such as PFO with aneurysm or large shunt, concurrent thromboembolic disease, or contraindications to anticoagulation.
MORE WORK NEEDED
Areas for further study include further identifying the characteristics of patients with PFO and cryptogenic stroke that might indicate who would benefit from percutaneous closure, elucidating the mechanism of stroke in these patients, and determining whether routine stroke evaluation should include echocardiography with a bubble study if there is no change in management based on the finding of PFO.39
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220.
- Truelsen T, Piechowski-JóŸwiak B, Bonita R, Mathers C, Bogousslavsky J, Boysen G. Stroke incidence and prevalence in Europe: a review of available data. Eur J Neurol 2006; 13:581–598.
- Furlan AJ. Patent foramen ovale and stroke: to close or not to close? Cleve Clin J Med 2007; 74(suppl 1):S118–S120.
- Sacco RL, Ellenberg JH, Mohr JP, et al. Infarcts of undetermined cause: the NINCDS Stroke Data Bank. Ann Neurol 1989; 25:382–390.
- Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke 2001; 32:2559–2566.
- Homma S, Sacco RL. Patent foramen ovale and stroke. Circulation 2005; 112:1063–1072.
- Sattiraju S, Masri SC, Liao K, Missov E. Three-dimensional transesophageal echocardiography of a thrombus entrapped by a patent foramen ovale. Ann Thorac Surg 2012; 94:e101–e102.
- Schreiter SW, Phillips JH. Thromboembolus traversing a patent foramen ovale: resolution with anticoagulation. J Am Soc Echocardiogr 1994; 7:659–662.
- Hust MH, Staiger M, Braun B. Migration of paradoxic embolus through a patent foramen ovale diagnosed by echocardiography: successful thrombolysis. Am Heart J 1995; 129:620–622.
- Alsheikh-Ali AA, Thaler DE, Kent DM. Patent foramen ovale in cryptogenic stroke: incidental or pathogenic? Stroke 2009; 40:2349–2355.
- Di Tullio MR, Sacco RL, Sciacca RR, Jin Z, Homma S. Patent foramen ovale and the risk of ischemic stroke in a multiethnic population. J Am Coll Cardiol 2007; 49:797–802.
- Di Tullio MR, Jin Z, Russo C, et al. Patent foramen ovale, subclinical cerebrovascular disease, and ischemic stroke in a population-based cohort. J Am Coll Cardiol 2013; 62:35–41.
- Meissner I, Khandheria BK, Heit JA, et al. Patent foramen ovale: innocent or guilty? Evidence from a prospective population-based study. J Am Coll Cardiol 2006; 47:440–445.
- De Castro S, Cartoni D, Fiorelli M, et al. Morphological and functional characteristics of patent foramen ovale and their embolic implications. Stroke 2000; 31:2407–2413.
- Furlan AJ, Reisman M, Massaro J, et al; CLOSURE I Investigators. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med 2012; 366:991–999.
- Meier B, Kalesan B, Mattle HP, et al; PC Trial Investigators. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med 2013; 368:1083–1091.
- Carroll JD, Saver JL, Thaler DE, et al; RESPECT Investigators. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med 2013; 368:1092–1100.
- Kim BJ, Sohn H, Sun BJ, et al. Imaging characteristics of ischemic strokes related to patent foramen ovale. Stroke 2013; 44:3350–3356.
- Thaler DE, Ruthazer R, Di Angelantonio E, et al. Neuroimaging findings in cryptogenic stroke patients with and without patent foramen ovale. Stroke 2013; 44:675–680.
- Kent DM, Ruthazer R, Weimar C, et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology 2013; 81:619–625.
- Wessler BS, Thaler DE, Ruthazer R, et al. Transesophageal echocardiography in cryptogenic stroke and patent foramen ovale: analysis of putative high-risk features from the risk of paradoxical embolism database. Circ Cardiovasc Imaging 2014; 7:125–131.
- Krasuski RA, Hart SA, Allen D, et al. Prevalence and repair of intraoperatively diagnosed patent foramen ovale and association with perioperative outcomes and long-term survival. JAMA 2009; 302:290–297.
- Mas JL, Arquizan C, Lamy C, et al; Patent Foramen Ovale and Atrial Septal Aneurysm Study Group. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med 2001; 345:1740–1746.
- Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP; PFO in Cryptogenic Stroke Study (PICSS) Investigators. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke Study. Circulation 2002; 105:2625–2631.
- Serena J, Marti-Fàbregas J, Santamarina E, et al; CODICIA, Right-to-Left Shunt in Cryptogenic Stroke Study; Stroke Project of the Cerebrovascular Diseases Study Group, Spanish Society of Neurology. Recurrent stroke and massive right-to-left shunt: results from the prospective Spanish multicenter (CODICIA) study. Stroke 2008; 39:3131–3136.
- Bogousslavsky J, Garazi S, Jeanrenaud X, Aebischer N, Van Melle G. Stroke recurrence in patients with patent foramen ovale: the Lausanne Study. Lausanne Stroke with Paradoxal Embolism Study Group. Neurology 1996; 46:1301–1305.
- Almekhlafi MA, Wilton SB, Rabi DM, Ghali WA, Lorenzetti DL, Hill MD. Recurrent cerebral ischemia in medically treated patent foramen ovale: a meta-analysis. Neurology 2009; 73:89–97.
- Kitsios GD, Dahabreh IJ, Abu Dabrh AM, Thaler DE, Kent DM. Patent foramen ovale closure and medical treatments for secondary stroke prevention: a systematic review of observational and randomized evidence. Stroke 2012; 43:422–431.
- Wolfrum M, Froehlich GM, Knapp G, et al. Stroke prevention by percutaneous closure of patent foramen ovale: a systematic review and meta-analysis. Heart 2014; 100:389–395.
- Rengifo-Moreno P, Palacios IF, Junpaparp P, Witzke CF, Morris DL, Romero-Corral A. Patent foramen ovale transcatheter closure vs medical therapy on recurrent vascular events: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J 2013; 34:3342–3352.
- Pineda AM, Nascimento FO, Yang SC, Kirtane AJ, Sommer RJ, Beohar N. A meta-analysis of transcatheter closure of patent foramen ovale versus medical therapy for prevention of recurrent thromboembolic events in patients with cryptogenic cerebrovascular events. Catheter Cardiovasc Interv 2013; 82:968–975.
- Kwong JS, Lam YY, Yu CM. Percutaneous closure of patent foramen ovale for cryptogenic stroke: a meta-analysis of randomized controlled trials. Int J Cardiol 2013; 168:4132–4148.
- Ntaios G, Papavasileiou V, Makaritsis K, Michel P. PFO closure vs medical therapy in cryptogenic stroke or transient ischemic attack: a systematic review and meta-analysis. Int J Cardiol 2013; 169:101–105.
- Nagaraja V, Raval J, Eslick GD, Burgess D, Denniss AR. Is transcatheter closure better than medical therapy for cryptogenic stroke with patent foramen ovale? A meta-analysis of randomised trials. Heart Lung Circ 2013; 22:903–909.
- Khan AR, Bin Abdulhak AA, Sheikh MA, et al. Device closure of patent foramen ovale versus medical therapy in cryptogenic stroke: a systematic review and meta-analysis. JACC Cardiovasc Interv 2013; 6:1316–1323.
- Wahl A, Krumsdorf U, Meier B, et al. Transcatheter treatment of atrial septal aneurysm associated with patent foramen ovale for prevention of recurrent paradoxical embolism in high-risk patients. J Am Coll Cardiol 2005; 45:377–380.
- Khairy P, O’Donnell CP, Landzberg MJ. Transcatheter closure versus medical therapy of patent foramen ovale and presumed paradoxical thromboemboli: a systematic review. Ann Intern Med 2003; 139:753–760.
- Sacco RL, Adams R, Albers G, et al; American Heart Association; American Stroke Association Council on Stroke; Council on Cardiovascular Radiology and Intervention; American Academy of Neurology. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke 2006; 37:577–617.
- Rana BS, Thomas MR, Calvert PA, Monaghan MJ, Hildick-Smith D. Echocardiographic evaluation of patent foramen ovale prior to device closure. JACC Cardiovasc Imaging 2010; 3:749–760.
Your patient has had an ischemic stroke, and so far you have found no obvious cause such as atrial fibrillation or carotid disease. Should you look for a patent foramen ovale (PFO)? And if you find it, what should you do?
This scenario continues to challenge primary care physicians and subspecialists and requires an understanding of the relationship between PFO and cryptogenic stroke, as well as familiarity with current data on the safety and effectiveness of the management options. PFO is known to be associated with cryptogenic stroke, but many questions remain, including:
- How can we tell if PFO is a culprit (“pathologic”) or an innocent bystander (“incidental”) in a patient who has had a cryptogenic stroke?
- Should stroke patients receive different medical therapy if they have a PFO? In particular, should they receive warfarin in addition to aspirin? And what about the novel oral anticoagulants?
- Which patients should undergo percutaneous closure of the PFO?
- Should we even be looking for PFO in stroke patients at this point, if we cannot say with certainty what we should do if we find it?
WHY IS THIS IMPORTANT?
Cerebrovascular disease is common and costly. The estimated yearly incidence of stroke in the United States is 795,000 events, at a cost of nearly $30 billion.1 The incidence of stroke in Europe is more than 1 million annually.2
During the diagnostic evaluation of stroke or transient ischemic attack (TIA), PFO is occasionally discovered incidentally by echocardiography. The management decisions that follow often fall to the primary care physician, who must decipher the conflicting data currently available and explain the options to the patient.
Although reviews have been published on this subject,3 several newer key trials and data on risk stratification warrant consideration.
DEFINITIONS
PFO is the failure of the septum primum to fuse with the septum secundum, so that a communication remains between the atria (Figure 1). The diagnosis is commonly made by echocardiography, when agitated saline is injected into the venous system and bubbles can be seen in the left atrium within three to five cardiac cycles (see video).
Atrial septal aneurysm is loosely defined as a septal excursion or bulging of at least 10 to 15 mm into the left and right atria during the cardiac cycle (Figure 2). The combination of PFO and atrial septal aneurysm may be more of a risk factor for stroke than PFO alone (see discussion below).
Cryptogenic stroke. The diagnostic workup of stroke fails to elucidate a clear cause in up to 40% of cases, which are thus called cryptogenic.4 The workup varies, but typically includes a search for a cardioembolic source and for atherosclerotic disease. Embolic sources are evaluated for by electrocardiography, transthoracic echocardiography, and possibly imaging of the aortic arch. Evaluation for atherosclerotic disease of the intracranial and extracranial arteries includes magnetic resonance angiography or, if that is unavailable, computed tomographic angiography or carotid Doppler ultrasonography. If no source is found, long-term cardiac monitoring may be used to detect paroxysmal atrial fibrillation, which may be more common than previously thought.
PFO AND CRYPTOGENIC STROKE ARE COMMON
As noted, there are approximately 800,000 strokes every year in the United States. If 25% to 40% of them are cryptogenic (the true prevalence warrants more evaluation),4,5 then 200,000 to 320,000 strokes are cryptogenic.
Autopsy studies indicate that 25% of the general population have a PFO, and if the prevalence is the same in people with cryptogenic stroke, that would equal 80,000 people with both cryptogenic stroke and PFO every year. However, the prevalence of PFO in patients with cryptogenic stroke appears to be significantly higher than in the general population.6 Although these numbers are crude estimates, they provide some insight into the prevalence of this clinical presentation.
HOW ARE CRYPTOGENIC STROKE AND PFO RELATED?
The exact relationship between PFO and cryptogenic stroke is unknown, although cases have been reported of thrombus in transit through a PFO, supporting paradoxical embolism as the plausible cause in stroke patients with PFO.7–9
There is clear evidence that the two conditions are associated by more than chance. Homma and Sacco6 reported that, in several studies, 93 (46%) of 202 patients under age 55 with cryptogenic stroke had PFOs, compared with 29 (11%) of 271 controls (P < .05 in all studies).6
In their evaluation of 23 case-control studies, Alsheikh-Ali et al10 found that the summary odds ratio (OR) for PFO in cryptogenic stroke vs PFO in control patients was 2.9 (95% confidence interval [CI] 2.1–4), largely driven by an OR of 5.1 (3.3–7.8) in those under age 55. Through Bayesian probability theory, this correlated with only a 33% probability that PFO in a patient with cryptogenic stroke was an innocent bystander rather than the culprit.10
IS PFO A RISK FACTOR FOR STROKE?
One of the more puzzling aspects of the relationship of PFO to cryptogenic stroke is that despite a clear association, there is little evidence that the relationship is causal.
Di Tullio et al11 followed 1,100 people who had no history of stroke and found that the risk of a first stroke in those with a PFO was not significantly higher than in those without a PFO, regardless of age, sex, or ethnic or racial group. At 80 months, the hazard ratio of stroke in people who had a PFO was 1.64 (95% CI 0.87–3.09).11 The findings were similar at 11 years, with a hazard ratio of 1.10 (95% CI 0.64–1.91).12
A prospective study of 585 patients found a similar risk of stroke in those with and without a PFO, with a hazard ratio of 1.46 (95% CI 0.74–2.88; P = .28).13
These prospective trials suggest that although previous studies have found a higher prevalence of PFOs in patients with cryptogenic stroke than in patients without stroke, there appears to be very little if any increased risk from baseline for a first stroke or TIA.
The lack of statistical significance in these trials should be interpreted with some caution, as a small increased risk is difficult to show if the event rate is low (approximately 10% of patients had events over 11 years in the study by Di Tullio et al12).
HOW DO WE KNOW IF A PFO IS A CULPRIT OR BYSTANDER?
Unfortunately, this is largely unanswered, though experts have suggested that echocardiographic features of the PFO, radiographic characteristics of the stroke, and clinical features of the patient may provide useful information.
‘High-risk’ features on echocardiography
Certain features of PFO may portend a high risk of cerebrovascular events. Both right-to-left shunting at rest and septal hypermobility were found in one study14 to be more common in patients with a PFO who had a stroke or TIA than in patients with a PFO but no cerebrovascular events. Also, patients who had these features and had a stroke had a higher risk of recurrence than stroke patients without these features (12.5% vs 4.3%, P = .05).14
Septal hypermobility and shunting at rest are easily diagnosed by echocardiography, and detecting these “high-risk” features would be useful if they could identify patients who would benefit from special therapy, such as percutaneous closure of the PFO.
Unfortunately, when investigators looked at these features in subgroup analysis of the major randomized controlled trials of percutaneous closure vs medical therapy, the results were mixed.
CLOSURE 1 (the Evaluation of the STARFlex Septal Closure System in Patients With a Stroke and/or Transient Ischemic Attack Due to Presumed Paradoxical Embolism Through a Patent Foramen Ovale)15 found percutaneous closure to be no better than medical therapy, regardless of shunt size or the presence of atrial septal aneurysm.
Similarly, the PC trial (Clinical Trial Comparing Percutaneous Closure of Patent Foramen Ovale Using the Amplatzer PFO Occluder With Medical Treatment in Patients With Cryptogenic Embolism)16 found no statistically significant benefit of closure in those with atrial septal aneurysm.
In contrast, the RESPECT trial (Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment)17 showed percutaneous closure to be beneficial in patients with atrial septal aneurysm or large shunt.
Radiographic characteristics of the stroke
Another area of interest in trying to identify culprit PFOs is the radiographic characteristics of the stroke.
In a study comparing patients with stroke related to atrial fibrillation vs patients with cryptogenic stroke and a known PFO, those in the latter group were more likely to have a single cortical infarction (34.2% vs 3.1%; P < .001) or multiple small scattered lesions (23.1% vs 5.9%; P < .01).18 Similarly, in a large database of patients with cryptogenic stroke and known PFO status, a superficially located stroke was associated with the presence of PFO (OR 1.54; P < .0001).19
Although these findings do not tell us with certainty that a patient’s PFO was the cause of his or her stroke, they provide guidance when dealing with the uncertainty of how to manage a patient with PFO. They may be useful in clinical practice, for example, when discussing treatment options with a young patient with cryptogenic stroke who has no risk factors and a superficial single infarct and who is found to have a PFO with a right-to-left shunting at rest.
Patient characteristics
Kent et al20 developed a 10-point index (the RoPE score) in an attempt to assign a probability to whether a stroke was PFO-related. Points were assigned for patients who were younger, who had a cortical stroke on neuroimaging, and who did not have diabetes, hypertension, smoking, or prior stroke or TIA. Patients with cryptogenic stroke with a higher RoPE score were more likely to have a PFO and thus had a higher likelihood that the index event was related to PFO. Of note, the patients with the highest likelihood of PFO-related stroke were the least likely to have a recurrence (RoPE score of 9 to 10; PFO-attributable fraction 88%; estimated 2-year recurrence rate 2%; 95% CI 0%–4%), whereas those with a low RoPE score have more traditional risk factors for stroke and thus are more likely to have a recurrence (RoPE 0 to 3; estimated 2-year recurrence rate 20%; 95% CI 12%–28%).20
Again, this sheds light on a difficulty faced by randomized controlled trials: the patients who may benefit from closure of a PFO may very well be those with the lowest recurrence rates without intervention.
The RoPE index was examined in an attempt to validate previously described morphologic criteria of “high-risk” PFO,21 though none of the previously described “high-risk” echocardiographic features (large physiologic size, hypermobile septum, shunt at rest) were more common in the group with presumed PFO-attributable stroke (RoPE score > 6). This underscores the difficulty of distinguishing pathologic PFO from incidental PFO.
KEY TREATMENT CONSIDERATIONS FOR SECONDARY PREVENTION
Given the complicated relationship between PFO and cryptogenic stroke, there has been much debate over management strategies. The three options are surgical closure, percutaneous closure with a device, and medical therapy. The goal of all three is to prevent the recurrence of stroke or TIA.
Surgical closure has largely been supplanted by percutaneous closure, but is still done in specific situations such as when a PFO is found incidentally on transesophageal echocardiography during surgery for another cardiac condition. The data on such cases22 tend to support the argument that asymptomatic PFOs in the general population have a relatively benign natural history.
Thus, the two key questions about management that warrant discussion are: is anticoagulation superior to antiplatelet therapy? And is percutaneous closure superior to medical management?
Anticoagulant vs antiplatelet therapy
Whether to treat with aspirin or with a vitamin K antagonist has been a subject of debate, although there is no strong evidence to suggest that anticoagulation is superior to antiplatelet therapy.
The concern that aspirin alone is insufficient in some patients stems from a study by Mas et al,23 who followed 581 patients with cryptogenic stroke who had a PFO alone, a PFO with an atrial septal aneurysm, or neither. The rate of stroke recurrence at 4 years on aspirin therapy was 2.3% in those with a PFO alone, 15.2% in those with a PFO with an atrial septal aneurysm, and 4.2% in those with neither.
Many have concluded that aspirin therapy does not sufficiently protect those with both PFO and atrial septal aneurysm, given the high recurrence rate in this group. This might lead to the suggestion that anticoagulation could be of benefit in these patients.
However, the Patent Foramen Ovale in Cryptogenic Stroke Study (PiCSS)24 and the Spanish Multicenter Study Into Right-to-Left Shunt in Cryptogenic Stroke (CODICIA)25 found similar recurrence rates in patients with PFO and atrial septal aneurysm compared with those with only PFO. In these two studies, recurrence rates were similar regardless of whether patients were taking aspirin or warfarin.
In a study that followed 140 consecutive patients with both stroke and PFO, those treated in a nonrandomized fashion with antiplatelet agents had no difference in the recurrence rate compared with those treated with anticoagulation.26
Although uncertainty remains because no head-to-head randomized controlled trial has been done, some patients with PFO have other indications for anticoagulation, most commonly atrial fibrillation and venous thromboembolic disease.
There are currently no data on the use of novel oral anticoagulants in this setting.
Is percutaneous closure better than medical therapy?
When cryptogenic stroke is treated with antiplatelet therapy or anticoagulation therapy, the recurrence rate is the same whether or not the patient has a PFO.23–25 The belief that medical therapy offers adequate secondary protection is supported by a meta-analysis of 15 studies that found no increased risk of recurrent ischemic events in those with a PFO on medical therapy (antiplatelet or anticoagulant) vs those without a PFO (relative risk 1.1, 95% CI 0.8–1.5).27
Despite the conflicting evidence, percutaneous closure of PFO is still performed, mostly on a case-by-case basis. This has been supported by an apparent benefit in observational studies.
A systematic review of 52 single-arm studies and 7 comparative nonrandomized studies of patients with PFO and cryptogenic stroke found the rate of recurrent stroke to be 0.36 per 100 person-years with percutaneous closure vs 2.53 per 100 person-years with medical therapy.28 However, three long-awaited randomized controlled trials (CLOSURE 1, the PC trial, and RESPECT) failed to show a significant reduction in primary end points with percutaneous closure vs standard medical therapy.15–17
These trials had several limitations: event rates were low, medical therapy varied by provider, and enrollment was slowed by out-of-study percutaneous closure in patients perceived to be at high risk (though, as discussed above, high risk is difficult to determine).
Intention-to-treat analysis in RESPECT showed no benefit from percutaneous closure, but a favorable outcome was noted with closure in as-treated analysis (HR 0.27; 95% CI 0.1–0.75; P = .007) and per-protocol analysis (HR 0.37; 95% CI 0.14–0.96; P = .03) of the 980 randomized patients.17 This suggests some benefit, as does the CLOSURE 1 trial, in which 3 of the 12 recurrent strokes in the percutaneous closure group occurred before the device was implanted.15
The low event rates in these studies prompted several meta-analyses.29–35 However, only two suggested a benefit of percutaneous closure over medical therapy. In one recent meta-analysis,29 observational study data suggested benefit from percutaneous closure, whereas three randomized controlled trials failed to show a statistically significant benefit.
The conclusions of the meta-analyses must be interpreted with caution because of inherent differences in the randomized controlled trials, including the closure device used, inclusion criteria, study end points, and variations in medical therapy.
Devices differ
A meta-analysis by Khan et al35 showed a benefit of percutaneous closure when evaluating only studies using the Amplatzer PFO occluder (AGA Medical), as in RESPECT and the PC trial.35 As data accumulate, it is important to remember that there are differences between devices. Ongoing trials continue to investigate the Amplatzer device (NCT01550588) and the GORE HELEX Septal Occluder/GORE Septal Occluder (Gore Medical) (NCT00738894).
In another meta-analysis, Pineda et al31 found a benefit with closure in the as-treated analysis using data from all three randomized controlled trials (OR 0.62; 95% CI 0.41–0.94; P = .02).31 Although paradoxical embolism through the PFO as the mechanism of stroke has been questioned, this finding suggests that actual closure of a PFO may protect against further events, presumably by preventing paradoxical embolism.
Different closure devices have different side effects. The incidence of atrial fibrillation with the CardioSEAL STARFlex device (NMT Medical) is higher than with medical therapy (used in the CLOSURE trial15), whereas this risk was not statistically significantly increased in the PC trial16 and RESPECT,17 which used the Amplatzer device.
Benefit in those with atrial septal aneurysm?
Percutaneous closure has been shown to be safe and effective in patients with PFO and atrial septal aneurysm.36 There was some benefit of closure over medical therapy in a subgroup analysis from RESPECT in these patients, with a HR of 0.19 (95% CI 0.04–0.87, P = .02),17 although this was not seen in either CLOSURE 1 or the PC trial.
WHAT ARE THE RISKS OF PERCUTANEOUS CLOSURE?
Minor complications of percutaneous closure include bleeding, atrial arrhythmias, device embolization and fracture, and complications related to vascular access. Major complications include hemorrhage requiring transfusion, need for surgery, cardiac tamponade, pulmonary embolism, and death.
The cumulative rate of major complications in 10 observational studies was 1.5%, and the rate of minor complications was 7.9%.37 The RESPECT investigators reported a serious adverse event in 4.2% of patients (ranging in severity from chest tightness to cardiac tamponade).17
Another possible consequence of percutaneous closure is the need for chronic anticoagulation because of the increased risk of postprocedural atrial fibrillation seen in meta-analyses,29,31,32 though this may be device-specific.32
Percutaneous closure was considered successful—ie, to have nearly or completely eliminated shunting of blood through the defect—at 6 months of follow-up in 95.9% of patients in the PC trial, 93.5% in RESPECT, and 86.1% in CLOSURE 1.15–17
WHAT SHOULD WE BE DOING IN DAILY PRACTICE?
Give aspirin. Aspirin is effective in secondary stroke prevention, and data suggest that patients with PFO and cryptogenic stroke who receive aspirin therapy alone have a similar risk of recurrent events as patients without PFO.
Give warfarin if indicated. Evidence is insufficient to recommend vitamin K antagonist therapy in all patients with PFO and cryptogenic stroke. However, coexisting conditions that warrant anticoagulation must be taken into account.
Individualize. Given the lack of evidence to definitively guide management of patients with cryptogenic stroke and PFO, we need to individualize our approach, taking into account patient preferences, bleeding risk, ability to tolerate procedures, and the likelihood that the PFO is at fault.
No definitive answer on PFO closure. The most recent data suggest that closure may be beneficial, but key questions remain: Who will benefit? And what is the ideal medical therapy? Optimal management will only be established by the continued enrollment of appropriate patients into ongoing clinical trials.
Another question is whether it is possible to perform a randomized controlled trial with enough patients to definitively prove whether percutaneous closure is superior to medical therapy. Recent experience would suggest not.
In the meantime, we have some guidance from the American Heart Association and the American Stroke Association Council on Stroke38 based on the limited evidence available.
Consider patient preference. The physician should present the options to the patient in a balanced manner to enable him or her to make an informed decision. Patients can also be encouraged to seek additional information at websites such as www.stroke.org and www.nlm.nih.gov.
Referral to an interventional cardiologist for evaluation for closure is reasonable in patients with recurrent stroke, medication failure, complicated atrial septal anatomy such as PFO with aneurysm or large shunt, concurrent thromboembolic disease, or contraindications to anticoagulation.
MORE WORK NEEDED
Areas for further study include further identifying the characteristics of patients with PFO and cryptogenic stroke that might indicate who would benefit from percutaneous closure, elucidating the mechanism of stroke in these patients, and determining whether routine stroke evaluation should include echocardiography with a bubble study if there is no change in management based on the finding of PFO.39
Your patient has had an ischemic stroke, and so far you have found no obvious cause such as atrial fibrillation or carotid disease. Should you look for a patent foramen ovale (PFO)? And if you find it, what should you do?
This scenario continues to challenge primary care physicians and subspecialists and requires an understanding of the relationship between PFO and cryptogenic stroke, as well as familiarity with current data on the safety and effectiveness of the management options. PFO is known to be associated with cryptogenic stroke, but many questions remain, including:
- How can we tell if PFO is a culprit (“pathologic”) or an innocent bystander (“incidental”) in a patient who has had a cryptogenic stroke?
- Should stroke patients receive different medical therapy if they have a PFO? In particular, should they receive warfarin in addition to aspirin? And what about the novel oral anticoagulants?
- Which patients should undergo percutaneous closure of the PFO?
- Should we even be looking for PFO in stroke patients at this point, if we cannot say with certainty what we should do if we find it?
WHY IS THIS IMPORTANT?
Cerebrovascular disease is common and costly. The estimated yearly incidence of stroke in the United States is 795,000 events, at a cost of nearly $30 billion.1 The incidence of stroke in Europe is more than 1 million annually.2
During the diagnostic evaluation of stroke or transient ischemic attack (TIA), PFO is occasionally discovered incidentally by echocardiography. The management decisions that follow often fall to the primary care physician, who must decipher the conflicting data currently available and explain the options to the patient.
Although reviews have been published on this subject,3 several newer key trials and data on risk stratification warrant consideration.
DEFINITIONS
PFO is the failure of the septum primum to fuse with the septum secundum, so that a communication remains between the atria (Figure 1). The diagnosis is commonly made by echocardiography, when agitated saline is injected into the venous system and bubbles can be seen in the left atrium within three to five cardiac cycles (see video).
Atrial septal aneurysm is loosely defined as a septal excursion or bulging of at least 10 to 15 mm into the left and right atria during the cardiac cycle (Figure 2). The combination of PFO and atrial septal aneurysm may be more of a risk factor for stroke than PFO alone (see discussion below).
Cryptogenic stroke. The diagnostic workup of stroke fails to elucidate a clear cause in up to 40% of cases, which are thus called cryptogenic.4 The workup varies, but typically includes a search for a cardioembolic source and for atherosclerotic disease. Embolic sources are evaluated for by electrocardiography, transthoracic echocardiography, and possibly imaging of the aortic arch. Evaluation for atherosclerotic disease of the intracranial and extracranial arteries includes magnetic resonance angiography or, if that is unavailable, computed tomographic angiography or carotid Doppler ultrasonography. If no source is found, long-term cardiac monitoring may be used to detect paroxysmal atrial fibrillation, which may be more common than previously thought.
PFO AND CRYPTOGENIC STROKE ARE COMMON
As noted, there are approximately 800,000 strokes every year in the United States. If 25% to 40% of them are cryptogenic (the true prevalence warrants more evaluation),4,5 then 200,000 to 320,000 strokes are cryptogenic.
Autopsy studies indicate that 25% of the general population have a PFO, and if the prevalence is the same in people with cryptogenic stroke, that would equal 80,000 people with both cryptogenic stroke and PFO every year. However, the prevalence of PFO in patients with cryptogenic stroke appears to be significantly higher than in the general population.6 Although these numbers are crude estimates, they provide some insight into the prevalence of this clinical presentation.
HOW ARE CRYPTOGENIC STROKE AND PFO RELATED?
The exact relationship between PFO and cryptogenic stroke is unknown, although cases have been reported of thrombus in transit through a PFO, supporting paradoxical embolism as the plausible cause in stroke patients with PFO.7–9
There is clear evidence that the two conditions are associated by more than chance. Homma and Sacco6 reported that, in several studies, 93 (46%) of 202 patients under age 55 with cryptogenic stroke had PFOs, compared with 29 (11%) of 271 controls (P < .05 in all studies).6
In their evaluation of 23 case-control studies, Alsheikh-Ali et al10 found that the summary odds ratio (OR) for PFO in cryptogenic stroke vs PFO in control patients was 2.9 (95% confidence interval [CI] 2.1–4), largely driven by an OR of 5.1 (3.3–7.8) in those under age 55. Through Bayesian probability theory, this correlated with only a 33% probability that PFO in a patient with cryptogenic stroke was an innocent bystander rather than the culprit.10
IS PFO A RISK FACTOR FOR STROKE?
One of the more puzzling aspects of the relationship of PFO to cryptogenic stroke is that despite a clear association, there is little evidence that the relationship is causal.
Di Tullio et al11 followed 1,100 people who had no history of stroke and found that the risk of a first stroke in those with a PFO was not significantly higher than in those without a PFO, regardless of age, sex, or ethnic or racial group. At 80 months, the hazard ratio of stroke in people who had a PFO was 1.64 (95% CI 0.87–3.09).11 The findings were similar at 11 years, with a hazard ratio of 1.10 (95% CI 0.64–1.91).12
A prospective study of 585 patients found a similar risk of stroke in those with and without a PFO, with a hazard ratio of 1.46 (95% CI 0.74–2.88; P = .28).13
These prospective trials suggest that although previous studies have found a higher prevalence of PFOs in patients with cryptogenic stroke than in patients without stroke, there appears to be very little if any increased risk from baseline for a first stroke or TIA.
The lack of statistical significance in these trials should be interpreted with some caution, as a small increased risk is difficult to show if the event rate is low (approximately 10% of patients had events over 11 years in the study by Di Tullio et al12).
HOW DO WE KNOW IF A PFO IS A CULPRIT OR BYSTANDER?
Unfortunately, this is largely unanswered, though experts have suggested that echocardiographic features of the PFO, radiographic characteristics of the stroke, and clinical features of the patient may provide useful information.
‘High-risk’ features on echocardiography
Certain features of PFO may portend a high risk of cerebrovascular events. Both right-to-left shunting at rest and septal hypermobility were found in one study14 to be more common in patients with a PFO who had a stroke or TIA than in patients with a PFO but no cerebrovascular events. Also, patients who had these features and had a stroke had a higher risk of recurrence than stroke patients without these features (12.5% vs 4.3%, P = .05).14
Septal hypermobility and shunting at rest are easily diagnosed by echocardiography, and detecting these “high-risk” features would be useful if they could identify patients who would benefit from special therapy, such as percutaneous closure of the PFO.
Unfortunately, when investigators looked at these features in subgroup analysis of the major randomized controlled trials of percutaneous closure vs medical therapy, the results were mixed.
CLOSURE 1 (the Evaluation of the STARFlex Septal Closure System in Patients With a Stroke and/or Transient Ischemic Attack Due to Presumed Paradoxical Embolism Through a Patent Foramen Ovale)15 found percutaneous closure to be no better than medical therapy, regardless of shunt size or the presence of atrial septal aneurysm.
Similarly, the PC trial (Clinical Trial Comparing Percutaneous Closure of Patent Foramen Ovale Using the Amplatzer PFO Occluder With Medical Treatment in Patients With Cryptogenic Embolism)16 found no statistically significant benefit of closure in those with atrial septal aneurysm.
In contrast, the RESPECT trial (Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment)17 showed percutaneous closure to be beneficial in patients with atrial septal aneurysm or large shunt.
Radiographic characteristics of the stroke
Another area of interest in trying to identify culprit PFOs is the radiographic characteristics of the stroke.
In a study comparing patients with stroke related to atrial fibrillation vs patients with cryptogenic stroke and a known PFO, those in the latter group were more likely to have a single cortical infarction (34.2% vs 3.1%; P < .001) or multiple small scattered lesions (23.1% vs 5.9%; P < .01).18 Similarly, in a large database of patients with cryptogenic stroke and known PFO status, a superficially located stroke was associated with the presence of PFO (OR 1.54; P < .0001).19
Although these findings do not tell us with certainty that a patient’s PFO was the cause of his or her stroke, they provide guidance when dealing with the uncertainty of how to manage a patient with PFO. They may be useful in clinical practice, for example, when discussing treatment options with a young patient with cryptogenic stroke who has no risk factors and a superficial single infarct and who is found to have a PFO with a right-to-left shunting at rest.
Patient characteristics
Kent et al20 developed a 10-point index (the RoPE score) in an attempt to assign a probability to whether a stroke was PFO-related. Points were assigned for patients who were younger, who had a cortical stroke on neuroimaging, and who did not have diabetes, hypertension, smoking, or prior stroke or TIA. Patients with cryptogenic stroke with a higher RoPE score were more likely to have a PFO and thus had a higher likelihood that the index event was related to PFO. Of note, the patients with the highest likelihood of PFO-related stroke were the least likely to have a recurrence (RoPE score of 9 to 10; PFO-attributable fraction 88%; estimated 2-year recurrence rate 2%; 95% CI 0%–4%), whereas those with a low RoPE score have more traditional risk factors for stroke and thus are more likely to have a recurrence (RoPE 0 to 3; estimated 2-year recurrence rate 20%; 95% CI 12%–28%).20
Again, this sheds light on a difficulty faced by randomized controlled trials: the patients who may benefit from closure of a PFO may very well be those with the lowest recurrence rates without intervention.
The RoPE index was examined in an attempt to validate previously described morphologic criteria of “high-risk” PFO,21 though none of the previously described “high-risk” echocardiographic features (large physiologic size, hypermobile septum, shunt at rest) were more common in the group with presumed PFO-attributable stroke (RoPE score > 6). This underscores the difficulty of distinguishing pathologic PFO from incidental PFO.
KEY TREATMENT CONSIDERATIONS FOR SECONDARY PREVENTION
Given the complicated relationship between PFO and cryptogenic stroke, there has been much debate over management strategies. The three options are surgical closure, percutaneous closure with a device, and medical therapy. The goal of all three is to prevent the recurrence of stroke or TIA.
Surgical closure has largely been supplanted by percutaneous closure, but is still done in specific situations such as when a PFO is found incidentally on transesophageal echocardiography during surgery for another cardiac condition. The data on such cases22 tend to support the argument that asymptomatic PFOs in the general population have a relatively benign natural history.
Thus, the two key questions about management that warrant discussion are: is anticoagulation superior to antiplatelet therapy? And is percutaneous closure superior to medical management?
Anticoagulant vs antiplatelet therapy
Whether to treat with aspirin or with a vitamin K antagonist has been a subject of debate, although there is no strong evidence to suggest that anticoagulation is superior to antiplatelet therapy.
The concern that aspirin alone is insufficient in some patients stems from a study by Mas et al,23 who followed 581 patients with cryptogenic stroke who had a PFO alone, a PFO with an atrial septal aneurysm, or neither. The rate of stroke recurrence at 4 years on aspirin therapy was 2.3% in those with a PFO alone, 15.2% in those with a PFO with an atrial septal aneurysm, and 4.2% in those with neither.
Many have concluded that aspirin therapy does not sufficiently protect those with both PFO and atrial septal aneurysm, given the high recurrence rate in this group. This might lead to the suggestion that anticoagulation could be of benefit in these patients.
However, the Patent Foramen Ovale in Cryptogenic Stroke Study (PiCSS)24 and the Spanish Multicenter Study Into Right-to-Left Shunt in Cryptogenic Stroke (CODICIA)25 found similar recurrence rates in patients with PFO and atrial septal aneurysm compared with those with only PFO. In these two studies, recurrence rates were similar regardless of whether patients were taking aspirin or warfarin.
In a study that followed 140 consecutive patients with both stroke and PFO, those treated in a nonrandomized fashion with antiplatelet agents had no difference in the recurrence rate compared with those treated with anticoagulation.26
Although uncertainty remains because no head-to-head randomized controlled trial has been done, some patients with PFO have other indications for anticoagulation, most commonly atrial fibrillation and venous thromboembolic disease.
There are currently no data on the use of novel oral anticoagulants in this setting.
Is percutaneous closure better than medical therapy?
When cryptogenic stroke is treated with antiplatelet therapy or anticoagulation therapy, the recurrence rate is the same whether or not the patient has a PFO.23–25 The belief that medical therapy offers adequate secondary protection is supported by a meta-analysis of 15 studies that found no increased risk of recurrent ischemic events in those with a PFO on medical therapy (antiplatelet or anticoagulant) vs those without a PFO (relative risk 1.1, 95% CI 0.8–1.5).27
Despite the conflicting evidence, percutaneous closure of PFO is still performed, mostly on a case-by-case basis. This has been supported by an apparent benefit in observational studies.
A systematic review of 52 single-arm studies and 7 comparative nonrandomized studies of patients with PFO and cryptogenic stroke found the rate of recurrent stroke to be 0.36 per 100 person-years with percutaneous closure vs 2.53 per 100 person-years with medical therapy.28 However, three long-awaited randomized controlled trials (CLOSURE 1, the PC trial, and RESPECT) failed to show a significant reduction in primary end points with percutaneous closure vs standard medical therapy.15–17
These trials had several limitations: event rates were low, medical therapy varied by provider, and enrollment was slowed by out-of-study percutaneous closure in patients perceived to be at high risk (though, as discussed above, high risk is difficult to determine).
Intention-to-treat analysis in RESPECT showed no benefit from percutaneous closure, but a favorable outcome was noted with closure in as-treated analysis (HR 0.27; 95% CI 0.1–0.75; P = .007) and per-protocol analysis (HR 0.37; 95% CI 0.14–0.96; P = .03) of the 980 randomized patients.17 This suggests some benefit, as does the CLOSURE 1 trial, in which 3 of the 12 recurrent strokes in the percutaneous closure group occurred before the device was implanted.15
The low event rates in these studies prompted several meta-analyses.29–35 However, only two suggested a benefit of percutaneous closure over medical therapy. In one recent meta-analysis,29 observational study data suggested benefit from percutaneous closure, whereas three randomized controlled trials failed to show a statistically significant benefit.
The conclusions of the meta-analyses must be interpreted with caution because of inherent differences in the randomized controlled trials, including the closure device used, inclusion criteria, study end points, and variations in medical therapy.
Devices differ
A meta-analysis by Khan et al35 showed a benefit of percutaneous closure when evaluating only studies using the Amplatzer PFO occluder (AGA Medical), as in RESPECT and the PC trial.35 As data accumulate, it is important to remember that there are differences between devices. Ongoing trials continue to investigate the Amplatzer device (NCT01550588) and the GORE HELEX Septal Occluder/GORE Septal Occluder (Gore Medical) (NCT00738894).
In another meta-analysis, Pineda et al31 found a benefit with closure in the as-treated analysis using data from all three randomized controlled trials (OR 0.62; 95% CI 0.41–0.94; P = .02).31 Although paradoxical embolism through the PFO as the mechanism of stroke has been questioned, this finding suggests that actual closure of a PFO may protect against further events, presumably by preventing paradoxical embolism.
Different closure devices have different side effects. The incidence of atrial fibrillation with the CardioSEAL STARFlex device (NMT Medical) is higher than with medical therapy (used in the CLOSURE trial15), whereas this risk was not statistically significantly increased in the PC trial16 and RESPECT,17 which used the Amplatzer device.
Benefit in those with atrial septal aneurysm?
Percutaneous closure has been shown to be safe and effective in patients with PFO and atrial septal aneurysm.36 There was some benefit of closure over medical therapy in a subgroup analysis from RESPECT in these patients, with a HR of 0.19 (95% CI 0.04–0.87, P = .02),17 although this was not seen in either CLOSURE 1 or the PC trial.
WHAT ARE THE RISKS OF PERCUTANEOUS CLOSURE?
Minor complications of percutaneous closure include bleeding, atrial arrhythmias, device embolization and fracture, and complications related to vascular access. Major complications include hemorrhage requiring transfusion, need for surgery, cardiac tamponade, pulmonary embolism, and death.
The cumulative rate of major complications in 10 observational studies was 1.5%, and the rate of minor complications was 7.9%.37 The RESPECT investigators reported a serious adverse event in 4.2% of patients (ranging in severity from chest tightness to cardiac tamponade).17
Another possible consequence of percutaneous closure is the need for chronic anticoagulation because of the increased risk of postprocedural atrial fibrillation seen in meta-analyses,29,31,32 though this may be device-specific.32
Percutaneous closure was considered successful—ie, to have nearly or completely eliminated shunting of blood through the defect—at 6 months of follow-up in 95.9% of patients in the PC trial, 93.5% in RESPECT, and 86.1% in CLOSURE 1.15–17
WHAT SHOULD WE BE DOING IN DAILY PRACTICE?
Give aspirin. Aspirin is effective in secondary stroke prevention, and data suggest that patients with PFO and cryptogenic stroke who receive aspirin therapy alone have a similar risk of recurrent events as patients without PFO.
Give warfarin if indicated. Evidence is insufficient to recommend vitamin K antagonist therapy in all patients with PFO and cryptogenic stroke. However, coexisting conditions that warrant anticoagulation must be taken into account.
Individualize. Given the lack of evidence to definitively guide management of patients with cryptogenic stroke and PFO, we need to individualize our approach, taking into account patient preferences, bleeding risk, ability to tolerate procedures, and the likelihood that the PFO is at fault.
No definitive answer on PFO closure. The most recent data suggest that closure may be beneficial, but key questions remain: Who will benefit? And what is the ideal medical therapy? Optimal management will only be established by the continued enrollment of appropriate patients into ongoing clinical trials.
Another question is whether it is possible to perform a randomized controlled trial with enough patients to definitively prove whether percutaneous closure is superior to medical therapy. Recent experience would suggest not.
In the meantime, we have some guidance from the American Heart Association and the American Stroke Association Council on Stroke38 based on the limited evidence available.
Consider patient preference. The physician should present the options to the patient in a balanced manner to enable him or her to make an informed decision. Patients can also be encouraged to seek additional information at websites such as www.stroke.org and www.nlm.nih.gov.
Referral to an interventional cardiologist for evaluation for closure is reasonable in patients with recurrent stroke, medication failure, complicated atrial septal anatomy such as PFO with aneurysm or large shunt, concurrent thromboembolic disease, or contraindications to anticoagulation.
MORE WORK NEEDED
Areas for further study include further identifying the characteristics of patients with PFO and cryptogenic stroke that might indicate who would benefit from percutaneous closure, elucidating the mechanism of stroke in these patients, and determining whether routine stroke evaluation should include echocardiography with a bubble study if there is no change in management based on the finding of PFO.39
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220.
- Truelsen T, Piechowski-JóŸwiak B, Bonita R, Mathers C, Bogousslavsky J, Boysen G. Stroke incidence and prevalence in Europe: a review of available data. Eur J Neurol 2006; 13:581–598.
- Furlan AJ. Patent foramen ovale and stroke: to close or not to close? Cleve Clin J Med 2007; 74(suppl 1):S118–S120.
- Sacco RL, Ellenberg JH, Mohr JP, et al. Infarcts of undetermined cause: the NINCDS Stroke Data Bank. Ann Neurol 1989; 25:382–390.
- Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke 2001; 32:2559–2566.
- Homma S, Sacco RL. Patent foramen ovale and stroke. Circulation 2005; 112:1063–1072.
- Sattiraju S, Masri SC, Liao K, Missov E. Three-dimensional transesophageal echocardiography of a thrombus entrapped by a patent foramen ovale. Ann Thorac Surg 2012; 94:e101–e102.
- Schreiter SW, Phillips JH. Thromboembolus traversing a patent foramen ovale: resolution with anticoagulation. J Am Soc Echocardiogr 1994; 7:659–662.
- Hust MH, Staiger M, Braun B. Migration of paradoxic embolus through a patent foramen ovale diagnosed by echocardiography: successful thrombolysis. Am Heart J 1995; 129:620–622.
- Alsheikh-Ali AA, Thaler DE, Kent DM. Patent foramen ovale in cryptogenic stroke: incidental or pathogenic? Stroke 2009; 40:2349–2355.
- Di Tullio MR, Sacco RL, Sciacca RR, Jin Z, Homma S. Patent foramen ovale and the risk of ischemic stroke in a multiethnic population. J Am Coll Cardiol 2007; 49:797–802.
- Di Tullio MR, Jin Z, Russo C, et al. Patent foramen ovale, subclinical cerebrovascular disease, and ischemic stroke in a population-based cohort. J Am Coll Cardiol 2013; 62:35–41.
- Meissner I, Khandheria BK, Heit JA, et al. Patent foramen ovale: innocent or guilty? Evidence from a prospective population-based study. J Am Coll Cardiol 2006; 47:440–445.
- De Castro S, Cartoni D, Fiorelli M, et al. Morphological and functional characteristics of patent foramen ovale and their embolic implications. Stroke 2000; 31:2407–2413.
- Furlan AJ, Reisman M, Massaro J, et al; CLOSURE I Investigators. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med 2012; 366:991–999.
- Meier B, Kalesan B, Mattle HP, et al; PC Trial Investigators. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med 2013; 368:1083–1091.
- Carroll JD, Saver JL, Thaler DE, et al; RESPECT Investigators. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med 2013; 368:1092–1100.
- Kim BJ, Sohn H, Sun BJ, et al. Imaging characteristics of ischemic strokes related to patent foramen ovale. Stroke 2013; 44:3350–3356.
- Thaler DE, Ruthazer R, Di Angelantonio E, et al. Neuroimaging findings in cryptogenic stroke patients with and without patent foramen ovale. Stroke 2013; 44:675–680.
- Kent DM, Ruthazer R, Weimar C, et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology 2013; 81:619–625.
- Wessler BS, Thaler DE, Ruthazer R, et al. Transesophageal echocardiography in cryptogenic stroke and patent foramen ovale: analysis of putative high-risk features from the risk of paradoxical embolism database. Circ Cardiovasc Imaging 2014; 7:125–131.
- Krasuski RA, Hart SA, Allen D, et al. Prevalence and repair of intraoperatively diagnosed patent foramen ovale and association with perioperative outcomes and long-term survival. JAMA 2009; 302:290–297.
- Mas JL, Arquizan C, Lamy C, et al; Patent Foramen Ovale and Atrial Septal Aneurysm Study Group. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med 2001; 345:1740–1746.
- Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP; PFO in Cryptogenic Stroke Study (PICSS) Investigators. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke Study. Circulation 2002; 105:2625–2631.
- Serena J, Marti-Fàbregas J, Santamarina E, et al; CODICIA, Right-to-Left Shunt in Cryptogenic Stroke Study; Stroke Project of the Cerebrovascular Diseases Study Group, Spanish Society of Neurology. Recurrent stroke and massive right-to-left shunt: results from the prospective Spanish multicenter (CODICIA) study. Stroke 2008; 39:3131–3136.
- Bogousslavsky J, Garazi S, Jeanrenaud X, Aebischer N, Van Melle G. Stroke recurrence in patients with patent foramen ovale: the Lausanne Study. Lausanne Stroke with Paradoxal Embolism Study Group. Neurology 1996; 46:1301–1305.
- Almekhlafi MA, Wilton SB, Rabi DM, Ghali WA, Lorenzetti DL, Hill MD. Recurrent cerebral ischemia in medically treated patent foramen ovale: a meta-analysis. Neurology 2009; 73:89–97.
- Kitsios GD, Dahabreh IJ, Abu Dabrh AM, Thaler DE, Kent DM. Patent foramen ovale closure and medical treatments for secondary stroke prevention: a systematic review of observational and randomized evidence. Stroke 2012; 43:422–431.
- Wolfrum M, Froehlich GM, Knapp G, et al. Stroke prevention by percutaneous closure of patent foramen ovale: a systematic review and meta-analysis. Heart 2014; 100:389–395.
- Rengifo-Moreno P, Palacios IF, Junpaparp P, Witzke CF, Morris DL, Romero-Corral A. Patent foramen ovale transcatheter closure vs medical therapy on recurrent vascular events: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J 2013; 34:3342–3352.
- Pineda AM, Nascimento FO, Yang SC, Kirtane AJ, Sommer RJ, Beohar N. A meta-analysis of transcatheter closure of patent foramen ovale versus medical therapy for prevention of recurrent thromboembolic events in patients with cryptogenic cerebrovascular events. Catheter Cardiovasc Interv 2013; 82:968–975.
- Kwong JS, Lam YY, Yu CM. Percutaneous closure of patent foramen ovale for cryptogenic stroke: a meta-analysis of randomized controlled trials. Int J Cardiol 2013; 168:4132–4148.
- Ntaios G, Papavasileiou V, Makaritsis K, Michel P. PFO closure vs medical therapy in cryptogenic stroke or transient ischemic attack: a systematic review and meta-analysis. Int J Cardiol 2013; 169:101–105.
- Nagaraja V, Raval J, Eslick GD, Burgess D, Denniss AR. Is transcatheter closure better than medical therapy for cryptogenic stroke with patent foramen ovale? A meta-analysis of randomised trials. Heart Lung Circ 2013; 22:903–909.
- Khan AR, Bin Abdulhak AA, Sheikh MA, et al. Device closure of patent foramen ovale versus medical therapy in cryptogenic stroke: a systematic review and meta-analysis. JACC Cardiovasc Interv 2013; 6:1316–1323.
- Wahl A, Krumsdorf U, Meier B, et al. Transcatheter treatment of atrial septal aneurysm associated with patent foramen ovale for prevention of recurrent paradoxical embolism in high-risk patients. J Am Coll Cardiol 2005; 45:377–380.
- Khairy P, O’Donnell CP, Landzberg MJ. Transcatheter closure versus medical therapy of patent foramen ovale and presumed paradoxical thromboemboli: a systematic review. Ann Intern Med 2003; 139:753–760.
- Sacco RL, Adams R, Albers G, et al; American Heart Association; American Stroke Association Council on Stroke; Council on Cardiovascular Radiology and Intervention; American Academy of Neurology. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke 2006; 37:577–617.
- Rana BS, Thomas MR, Calvert PA, Monaghan MJ, Hildick-Smith D. Echocardiographic evaluation of patent foramen ovale prior to device closure. JACC Cardiovasc Imaging 2010; 3:749–760.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220.
- Truelsen T, Piechowski-JóŸwiak B, Bonita R, Mathers C, Bogousslavsky J, Boysen G. Stroke incidence and prevalence in Europe: a review of available data. Eur J Neurol 2006; 13:581–598.
- Furlan AJ. Patent foramen ovale and stroke: to close or not to close? Cleve Clin J Med 2007; 74(suppl 1):S118–S120.
- Sacco RL, Ellenberg JH, Mohr JP, et al. Infarcts of undetermined cause: the NINCDS Stroke Data Bank. Ann Neurol 1989; 25:382–390.
- Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke 2001; 32:2559–2566.
- Homma S, Sacco RL. Patent foramen ovale and stroke. Circulation 2005; 112:1063–1072.
- Sattiraju S, Masri SC, Liao K, Missov E. Three-dimensional transesophageal echocardiography of a thrombus entrapped by a patent foramen ovale. Ann Thorac Surg 2012; 94:e101–e102.
- Schreiter SW, Phillips JH. Thromboembolus traversing a patent foramen ovale: resolution with anticoagulation. J Am Soc Echocardiogr 1994; 7:659–662.
- Hust MH, Staiger M, Braun B. Migration of paradoxic embolus through a patent foramen ovale diagnosed by echocardiography: successful thrombolysis. Am Heart J 1995; 129:620–622.
- Alsheikh-Ali AA, Thaler DE, Kent DM. Patent foramen ovale in cryptogenic stroke: incidental or pathogenic? Stroke 2009; 40:2349–2355.
- Di Tullio MR, Sacco RL, Sciacca RR, Jin Z, Homma S. Patent foramen ovale and the risk of ischemic stroke in a multiethnic population. J Am Coll Cardiol 2007; 49:797–802.
- Di Tullio MR, Jin Z, Russo C, et al. Patent foramen ovale, subclinical cerebrovascular disease, and ischemic stroke in a population-based cohort. J Am Coll Cardiol 2013; 62:35–41.
- Meissner I, Khandheria BK, Heit JA, et al. Patent foramen ovale: innocent or guilty? Evidence from a prospective population-based study. J Am Coll Cardiol 2006; 47:440–445.
- De Castro S, Cartoni D, Fiorelli M, et al. Morphological and functional characteristics of patent foramen ovale and their embolic implications. Stroke 2000; 31:2407–2413.
- Furlan AJ, Reisman M, Massaro J, et al; CLOSURE I Investigators. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med 2012; 366:991–999.
- Meier B, Kalesan B, Mattle HP, et al; PC Trial Investigators. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med 2013; 368:1083–1091.
- Carroll JD, Saver JL, Thaler DE, et al; RESPECT Investigators. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med 2013; 368:1092–1100.
- Kim BJ, Sohn H, Sun BJ, et al. Imaging characteristics of ischemic strokes related to patent foramen ovale. Stroke 2013; 44:3350–3356.
- Thaler DE, Ruthazer R, Di Angelantonio E, et al. Neuroimaging findings in cryptogenic stroke patients with and without patent foramen ovale. Stroke 2013; 44:675–680.
- Kent DM, Ruthazer R, Weimar C, et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology 2013; 81:619–625.
- Wessler BS, Thaler DE, Ruthazer R, et al. Transesophageal echocardiography in cryptogenic stroke and patent foramen ovale: analysis of putative high-risk features from the risk of paradoxical embolism database. Circ Cardiovasc Imaging 2014; 7:125–131.
- Krasuski RA, Hart SA, Allen D, et al. Prevalence and repair of intraoperatively diagnosed patent foramen ovale and association with perioperative outcomes and long-term survival. JAMA 2009; 302:290–297.
- Mas JL, Arquizan C, Lamy C, et al; Patent Foramen Ovale and Atrial Septal Aneurysm Study Group. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med 2001; 345:1740–1746.
- Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP; PFO in Cryptogenic Stroke Study (PICSS) Investigators. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke Study. Circulation 2002; 105:2625–2631.
- Serena J, Marti-Fàbregas J, Santamarina E, et al; CODICIA, Right-to-Left Shunt in Cryptogenic Stroke Study; Stroke Project of the Cerebrovascular Diseases Study Group, Spanish Society of Neurology. Recurrent stroke and massive right-to-left shunt: results from the prospective Spanish multicenter (CODICIA) study. Stroke 2008; 39:3131–3136.
- Bogousslavsky J, Garazi S, Jeanrenaud X, Aebischer N, Van Melle G. Stroke recurrence in patients with patent foramen ovale: the Lausanne Study. Lausanne Stroke with Paradoxal Embolism Study Group. Neurology 1996; 46:1301–1305.
- Almekhlafi MA, Wilton SB, Rabi DM, Ghali WA, Lorenzetti DL, Hill MD. Recurrent cerebral ischemia in medically treated patent foramen ovale: a meta-analysis. Neurology 2009; 73:89–97.
- Kitsios GD, Dahabreh IJ, Abu Dabrh AM, Thaler DE, Kent DM. Patent foramen ovale closure and medical treatments for secondary stroke prevention: a systematic review of observational and randomized evidence. Stroke 2012; 43:422–431.
- Wolfrum M, Froehlich GM, Knapp G, et al. Stroke prevention by percutaneous closure of patent foramen ovale: a systematic review and meta-analysis. Heart 2014; 100:389–395.
- Rengifo-Moreno P, Palacios IF, Junpaparp P, Witzke CF, Morris DL, Romero-Corral A. Patent foramen ovale transcatheter closure vs medical therapy on recurrent vascular events: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J 2013; 34:3342–3352.
- Pineda AM, Nascimento FO, Yang SC, Kirtane AJ, Sommer RJ, Beohar N. A meta-analysis of transcatheter closure of patent foramen ovale versus medical therapy for prevention of recurrent thromboembolic events in patients with cryptogenic cerebrovascular events. Catheter Cardiovasc Interv 2013; 82:968–975.
- Kwong JS, Lam YY, Yu CM. Percutaneous closure of patent foramen ovale for cryptogenic stroke: a meta-analysis of randomized controlled trials. Int J Cardiol 2013; 168:4132–4148.
- Ntaios G, Papavasileiou V, Makaritsis K, Michel P. PFO closure vs medical therapy in cryptogenic stroke or transient ischemic attack: a systematic review and meta-analysis. Int J Cardiol 2013; 169:101–105.
- Nagaraja V, Raval J, Eslick GD, Burgess D, Denniss AR. Is transcatheter closure better than medical therapy for cryptogenic stroke with patent foramen ovale? A meta-analysis of randomised trials. Heart Lung Circ 2013; 22:903–909.
- Khan AR, Bin Abdulhak AA, Sheikh MA, et al. Device closure of patent foramen ovale versus medical therapy in cryptogenic stroke: a systematic review and meta-analysis. JACC Cardiovasc Interv 2013; 6:1316–1323.
- Wahl A, Krumsdorf U, Meier B, et al. Transcatheter treatment of atrial septal aneurysm associated with patent foramen ovale for prevention of recurrent paradoxical embolism in high-risk patients. J Am Coll Cardiol 2005; 45:377–380.
- Khairy P, O’Donnell CP, Landzberg MJ. Transcatheter closure versus medical therapy of patent foramen ovale and presumed paradoxical thromboemboli: a systematic review. Ann Intern Med 2003; 139:753–760.
- Sacco RL, Adams R, Albers G, et al; American Heart Association; American Stroke Association Council on Stroke; Council on Cardiovascular Radiology and Intervention; American Academy of Neurology. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke 2006; 37:577–617.
- Rana BS, Thomas MR, Calvert PA, Monaghan MJ, Hildick-Smith D. Echocardiographic evaluation of patent foramen ovale prior to device closure. JACC Cardiovasc Imaging 2010; 3:749–760.
KEY POINTS
- PFO is present in up to 25% of the general population, and it is even more common in young patients with cryptogenic stroke.
- PFO has not been shown to cause stroke or to significantly increase the risk of recurrent cerebrovascular events in patients treated with antiplatelet drugs.
- In patients with PFO, atrial septal aneurysm and large shunt size may confer increased risk of stroke.
- There is still no definitive evidence that closure of PFO is better than medical therapy in all patients with PFO and cryptogenic stroke.