User login
Delayed Antimicrobial Administration
In‐hospital administration of antimicrobials is often subject to controls and policies designed to limit the indiscriminant use of antimicrobials in situations where they are not warranted, to control costs, and to reduce the potential for the development of resistant microorganismsa major public health threat and patient safety concern. These controls and policies may include strategies such as limiting the choices of antimicrobials on formulary, rotational schedules of available antimicrobials, and antimicrobial approval processes.16 Antimicrobial approval processes commonly require that the bedside clinician obtain permission from a secondary source to administer a particular antimicrobial. This approval process may take the form of submitting written justification forms and/or direct telephone/fax requests to an Infectious Disease specialist, Clinical Pharmacist, or other surrogate prior to release of the antibiotic from the pharmacy. While these approval processes and other strategies have been shown to reduce the development of resistance,7, 8 improve outcomes,8 and provide education and revision of antimicrobial choice that may be more appropriate for the patient and suspected infection,9 they have the potential to delay the administration of the necessary antimicrobial by adding additional steps to the sequence of ordering, obtaining, and administering the medication. While it is certainly desirable to control the indiscriminant use of these medications, delays in antimicrobial administration may, in turn, worsen outcomes, thus counteracting the beneficial effects of control policies. The early and timely administration of appropriate broad spectrum antimicrobials chosen to cover the most expected organisms has been consistently shown to improve outcomes1027 and has been cited as an essential element of the Surviving Sepsis Campaign (
At our institution, the antimicrobial approval process requires written justification forms and/or a call or fax to an Infectious Disease specialist or Clinical Pharmacist prior to the antimicrobial being released from the pharmacy. We hypothesized that this process is associated with significant delays in patients receiving their prescribed antimicrobials when a restricted drug was chosen. The antibiotic approval process at our institution allows 1‐time stat doses of restricted antimicrobials to be ordered without preapproval at night (10 PM to 8 AM), but not during the day. This allowed us to compare the time‐to‐administration of restricted antimicrobials to their unrestricted counterparts and to themselves during this exempt time period.
Methods
Study Design
This was a single‐institution retrospective cohort study. We included all patients admitted to Johns Hopkins Hospital units utilizing the hospital's computerized provider order entry system (CPOE) who had stat orders placed for any of 24 preselected commonly ordered intravenous antimicrobials over a 1‐year period between November 1, 2005 and October 31, 2006. We did not include oral medications that were similarly subject to the approval process since we anticipated that the amount of time required to prepare the drug in the pharmacy and to deliver it to the patient would be systematically different than for intravenous medications. The CPOE system captures time of administration of all drugs (as charted by the nurse) in addition to the time of order entry by the provider. Institutional guidelines dictate that when drugs are ordered stat, they should be administered within 30 minutes of the order.
Classification of Antibiotics
Particular antimicrobials (24 different drugs) were chosen prospectively to include a broad range of commonly ordered drugs available at our hospital in intravenous form (Table 1). For this analysis, we only considered the first dose of the prescribed antimicrobial, since subsequent doses are not generally ordered stat and the timing of administration is clinically less important than the timing of the initial dose. Additionally, for patients who had multiple orders for stat antimicrobials during the course of hospitalization, we only included the first stat order in analysis.
| Restricted Antimicrobials | Unrestricted Antimicrobials |
|---|---|
| Ampicillin/sulbactam | Acyclovir |
| Azithromycin | Amphotericin |
| Cefepime | Ampicillin |
| Ciprofloxacin | Cefazolin |
| Fluconazole | Ceftriaxone |
| Gatifloxacin | Cephalexin |
| Linezolid | Clindamycin |
| Meropenem | Doxycycline |
| Moxifloxacin | Ertapenem |
| Vancomycin | Gentamicin |
| Metronidazole | |
| Oxacillin | |
| Tobramycin | |
| Trimethoprim/sulfamethoxazole |
For the purposes of the analysis, we defined antimicrobials as restricted or unrestricted. At our institution, restricted antimicrobials require either written or telephone approval from an Infectious Disease physician or Clinical Pharmacist. Obtaining written approval involves filling out a form that indicates the choice of agent (including route and dose) for an approved indication (eg, vancomycin for proven methicillin‐resistant Staphylococcus aureus bacteremia) and faxing this form to the hospital pharmacy. In other cases, approval requires a phone conversation with an Infectious Disease clinical fellow or a Clinical Pharmacist. None of these processes can be initiated or executed from within the CPOE system itself.
At our institution, the first dose of any restricted antimicrobial may be administered without going through the approval process if it is prescribed in the overnight hours defined as between 10 PM and 8 AM (Table 2). Retroactive approval is required the following day if the antimicrobial is to be continued.
| Restricted Antimicrobial (eg, Vancomycin) | Unrestricted Antimicrobial (eg, Cefazolin) | |
|---|---|---|
| Daytime order (8 AM to 10 PM) | Approval required | No approval required |
| Nighttime order (10 PM to 8 AM) | Exempted from approval for first dose (approval required during the next daytime period) | No approval required |
Data Acquisition
All antibiotic administration data were extracted electronically from the CPOE system. During the time of the study, not all hospital units utilized this system. Although CPOE was in place for all general medical wards, the intensive care units and many surgical services were not using CPOE at the time of the study. Based on limitations of the data entered into our CPOE system, many relevant clinical variables were not available for this study, such as clinical diagnoses or the level of care (eg, intensive care unit vs step‐down unit, vs regular nursing floor) at the time of the order.
Outcome Measures
We defined time‐to‐administration as the period of time between order entry and administration of the drug to the patient, as charted by the nurse in the CPOE system. However, nursing policy allows nurses to document the time of administration as the exact time of the order provided that the drug is delivered within 1 hour of a stat order, whereas all administration times beyond 1 hour of the order are expected to be documented precisely. This nursing policy eliminated our ability to determine whether antimicrobials were administered within 30 minutes of the order, and limited the value of examining time‐to‐administration as a continuous variable, but allowed us to reliably identify delays of >1 hour or >2 hours presuming that the nurse‐charted administration times were accurate. As our primary outcome, we defined delay in administration as when the antimicrobial was administered >1 hour from time of order. We examined delays of >2 hours as a secondary outcome. Antimicrobials ordered stat but not delivered within 4 hours of the order were excluded from analysis based on the recognition that extended delays might have resulted from changes in clinical circumstances or errors in documentation. Similarly, antimicrobials charted as being delivered prior to the order were excluded.
Statistical Analyses
We used the likelihood‐ratio chi‐square test to determine 2‐sided P values for differences between proportions. Logistic regression models were used to derive odds ratios (ORs) and to adjust for covariables. We adjusted for weekday versus weekend orders, patient characteristics including sex, age, white versus non‐white ethnicity and orders placed on medicine versus non‐medicine (eg, surgical or obstetrical) wards. Analyses were conducted in JMP 5.1 (SAS Institute, Cary, NC).
Results
In total, 3337 orders for stat antimicrobials were written during the study period, of which 86 (2.6%) were excluded based on being outside the specified 4‐hour window. This left a total of 3251 orders in 3251 discrete patients for analysis.
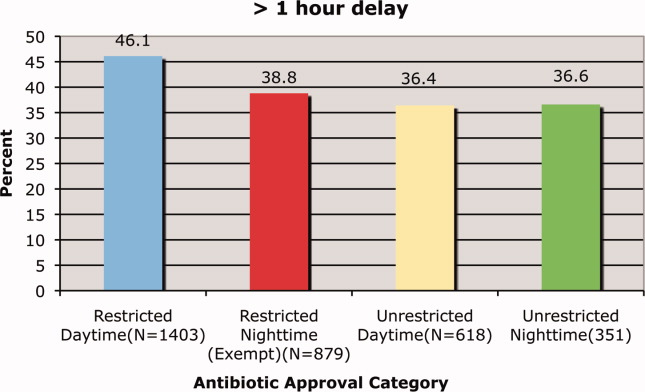
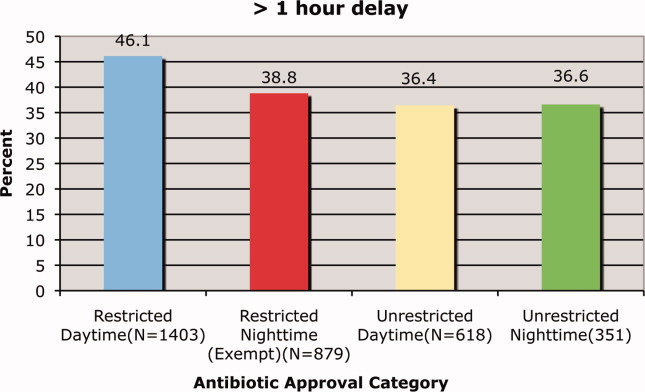
We found that a statistically significantly higher percentage of delays in antimicrobial administration when the antimicrobial was restricted as compared to unrestricted. This was the case for both our primary outcome of a >1 hour delay (Figure 1) and our secondary outcome of a delay of >2 hours (Figure 2). For restricted antimicrobial, delays of >1 hr occurred with 46.1% of orders during the day and with 38.8% of orders at night (when exempt from approval), P < 0.001. For unrestricted antimicrobials, delays of >1 hr occurred in 36.4% and 36.6% of instances, respectively (P = 0.57). The odds ratio for a delay in administration of 1 hour for restricted antimicrobials was 1.49 (95% CI = 1.23‐1.82).
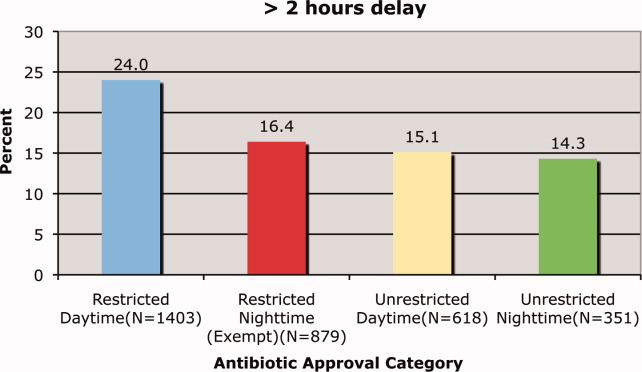
Delays beyond 2 hours occurred 24.0% of the time for restricted antimicrobials during the day versus 16.4% at night. Unrestricted antimicrobials were delayed >2 hrs only 15.1% and 14.3% of the time for day and night periods, respectively (P = 0.35). The odds ratio for a two‐hour or greater delay was 1.78 (95% CI = 1.39‐2.21), P < 0.0001 when the antimicrobial was restricted.
These odds ratios and statistical significance were unchanged by adjustment for primary service (medicine vs. non‐medicine), age, sex, ethnicity or whether the order was placed on a weekend or weekday (data not shown).
Discussion
We found that our institution's antimicrobial approval process was associated with statistically significant delays in the administration of antimicrobials that were ordered stat by the prescribing clinician. These delays were evident both when comparing restricted antimicrobials to unrestricted ones and when these restricted antimicrobials were compared to themselves during the overnight time period when they were temporarily exempt from the approval process. This suggests that the delay is associated with the approval process itself and not the specific drug or the time of day. We also found that over one‐third of all stat antimicrobial orders were not carried out in the within one hour. This rate approached nearly 50% for restricted drugs ordered stat. This high baseline rate for all stat‐ordered antimicrobials underscores system challenges that seem to be exacerbated when restricted antimicrobials are chosen.
We do not know if the delays we observed resulted in patient harm. Indeed it is possible, if not likely, that patient care at our institution is improved by the judicious use of certain antimicrobials, even if the process required to enforce their use may result in delayed antimicrobial administration in some instances. Since we did not collect clinical information on baseline diagnoses or severity of illness, and we did not have information on clinical outcomes, we cannot determine whether the clinical delays we observed might have caused harm. Determining the impact these approval policies have on patient outcomes would require a separate study designed to collect the necessary clinical data to answer that question.
An additional limitation was that we did not ascertain the indications for the antimicrobials to determine whether they truly needed to be given stat. We suspect that antimicrobials are sometimes ordered stat even when the infection being treated is not likely to be serious or life‐threatening. Additionally, since we relied on the nurse‐charted time of administration, it is possible that in some instances there was a charted delay in administration when in reality the patient received the antimicrobial in a timely fashion. In urgent situations, the nurses may be too busy to document that the medication was given until long after the dose is given, and this may result in inaccuracies in the charted administration time. However, this type of documentation error would be expected to affect restricted and unrestricted antimicrobials similarly and would be unlikely to result in a systematic bias.
Because we conducted this study at a single institution, the results may not be applicable to other medical institutions, especially since restriction policies and antimicrobial approval processes vary from hospital to hospital. The burden of delays may be related to the number of restricted antimicrobials on formulary, the types of antimicrobials restricted, the number of steps required to have them released from the pharmacy, whether the approval process is initiated from within the order entry system, and other factors that may streamline or hamper the approval process.
In our institution, there are several steps in the process, any of which might contribute to the delay. Faxed approval sheets may take time to arrive to and be acted upon by the pharmacy, errant pages may delay communication between the provider and the person providing approval, and there may be delays in the final approval being relayed to the pharmacy by the individual providing approval. In fact, an alternative explanation for the observed administration delays is that once ordered in CPOE, the prescribing physicians themselves are slow in initiating the approval process. While this is certainly possible, especially given the stresses surrounding the management of a seriously ill patient on the general ward, this still suggests that having to go through the approval process may impact the process of care.
Other possible explanations for the delays observed when the restrictive antimicrobial policy was in effect may include pharmacy staffing. Since the workload in the pharmacy would be expected to be greater during the day, when more patient care activity is occurring such as clinics and operating rooms, this increased workload may have slowed down the pharmacy filling the orders. However, such human resource‐workload imbalances would also be expected to slow most pharmacy processes and should lead to delays in filling the orders for other medications including the unrestricted antimicrobials. We did not track other non‐antimicrobial medications to examine their patterns of delay. Nursing workload also varies between day and night but the time period where the antimicrobial administration delays occurred is the time when nursing is favorably staffed unlike the night when nurse to patient ratios are low. It is possible that despite better nurse to patient ratios during the day, the workload‐to‐nursing ratio remains high and contributes to delays in administration of otherwise stat‐ordered antimicrobials. Again, it is unclear why this would disproportionately affect the restricted class of antimicrobials.
We do not advocate the abandonment of antimicrobial control policies. The process described here is very institution‐specific and while its benefits are proven, energy should be channeled where appropriate to facilitate this process. These policies are clearly necessary to help reduce costs, limit the unwarranted use of these drugs, and slow the proliferation of ever more resistant strains of microorganisms. However, we do advocate careful consideration of the components of the approval process itself, ensuring that delays in antimicrobial administration are kept to a minimum and are avoided altogether in critically ill patients. One way to accomplish this might be to not require approval for the first administration of a stat antibiotic, but to require approval for subsequent doses. Our institution's overnight exempt period data suggest that this would eliminate the incremental delays incurred by the approval process itself. As important, our results show that even for unrestricted antibiotics, we fall short of achieving recommended best practices, highlighting the challenges inherent to carrying out multi‐step clinical tasks in an efficient fashion.
- .Antimicrobial stewardship.Am J Med.2006;119:S53–S61.
- .Restrictive antibiotic policies are appropriate in intensive care units.Crit Care Med.2003;31:S25–S28.
- ,,, et al.Effects of requiring prior authorization for selected anti‐microbials: expenditures, susceptibilities and clinical outcomes.Clin Infect Dis.1997;25:230–239.
- ,,, et al.Class restriction of cephalosporin use to control total cephaslosporin resistance in nosocomial Klebsiella.JAMA.1998;280:1233–1237.
- ,,, et al.Rotation and restricted use of antibiotics in a medical intensive care unit: impact on the incidence of ventilator associated pneumonia caused by antibiotic resistant Gram‐negative bacteremia.Am J Respir Crit Care Med.2001;63(3 Pt 1):837–843.
- ,,, et al.Impact of rotation of empiric antibiotic schedules on infectious mortality in an intensive care unit.CCM.2001;29:1101–1108.
- ,,, et al.Antimicrobial use control measures to prevent and control antimicrobial resistance in US hospitals.Infect Control Hosp Epidemiol.2006;27:1088–1095.
- ,,,,.Cost‐effectiveness of prospective and continuous parenteral antibiotic control: experience at the Palo Alto Veterans Affairs Medical Center from 1987 to 1989.Am J Med.1991;90:439–444.
- ,.Programmatic role of the infectious diseases physician in controlling antimicrobial costs in the hospital.Clin Infect Dis.1997;24:471–485.
- ,.Antimicrobial stewardship and the role of pharmacokinetics–pharmacodynamics in the modern antibiotic era.Diagn Microbiol Infect Dis.2007;57(3 Suppl.):77S–83S.
- ,,,,,.Improving inpatient antibiotic prescribing: insights from participation in a national collaborative.Jt Comm J Qual Improv.2001;27:387–402.
- ,.Managing antibiotic resistance.N Engl J Med.2000;343:1961–1963.
- ,.Antimicrobial stewardship programs in health care systems.Clin Microbiol Rev.2005;18(4):638–656.
- ,,,,,,,,,,.Interventions to improve antibiotic prescribing practices for hospital inpatients.Cochrane Database Syst Rev.2005;4:CD003543.
- ,,,,.Evaluation of an antimicrobial stewardship program at a pediatric teaching hospital.Pediatr Infect Dis J.2008;27(2):106–111.
- .The utility of hospital antibiograms as tools for guiding empiric therapy and tracking resistance. Insights from the Society of Infectious Diseases Pharmacists.Pharmacotheray.2007;27(9):1306–1312.
- ,,,.Strategies to contain the emergence of antimicrobial resistance: a systematic review of effectiveness and cost‐effectiveness.J Health Serv Res Policy.2002;7(2):111–117.
- ,,,,,,,,,,,,;Infectious Diseases Society of America;Society for Healthcare Epidemiology of America.Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship.Clin Infect Dis.2007;44(2):159–177.
- ,,,, andthe Healthcare Infection Control Practices Advisory Committee. Management of Multi‐Drug Reistsant Organisms in Healthcare Settings: 2006. Available at: http://www.cdc.gov/ncidod/dhqp/pdf/ar/MDROGuideline2006.pdf. Accessed May 19,2008.
- ,,.Gram‐negative bacteremia. IV Re‐evaluation of clinical features and treatment in 612 patients.Am J Med.1980;68:344–355.
- ,,,.Gram‐negative bacteremia. III. Reassessment of etiology, epidemiology and ecology in 612 patients.Am J Med.1980;68:332–343.
- ,,,.Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients.Chest.1999;115:462–474.
- .Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. ICU‐Acquired Pneumonia Study Group.Intensive Care Med.1996;22:387–394.
- ,,,,.The value of routine microbial investigation in ventilator‐associated pneumonia.Am J Respir Crit Care Med.1997;156:196–200.
- ,,, et al.Impact of BAL data on the therapy and outcome of ventilator‐associated pneumonia.Chest.1997;111:676–685.
- ,.The influence of mini‐BAL cultures on patient outcomes: implications for the antibiotic management of ventilator associated pneumonia.Chest.1998;113:412–420.
- ,,,,.The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting.Chest.2000;118:146–155.
In‐hospital administration of antimicrobials is often subject to controls and policies designed to limit the indiscriminant use of antimicrobials in situations where they are not warranted, to control costs, and to reduce the potential for the development of resistant microorganismsa major public health threat and patient safety concern. These controls and policies may include strategies such as limiting the choices of antimicrobials on formulary, rotational schedules of available antimicrobials, and antimicrobial approval processes.16 Antimicrobial approval processes commonly require that the bedside clinician obtain permission from a secondary source to administer a particular antimicrobial. This approval process may take the form of submitting written justification forms and/or direct telephone/fax requests to an Infectious Disease specialist, Clinical Pharmacist, or other surrogate prior to release of the antibiotic from the pharmacy. While these approval processes and other strategies have been shown to reduce the development of resistance,7, 8 improve outcomes,8 and provide education and revision of antimicrobial choice that may be more appropriate for the patient and suspected infection,9 they have the potential to delay the administration of the necessary antimicrobial by adding additional steps to the sequence of ordering, obtaining, and administering the medication. While it is certainly desirable to control the indiscriminant use of these medications, delays in antimicrobial administration may, in turn, worsen outcomes, thus counteracting the beneficial effects of control policies. The early and timely administration of appropriate broad spectrum antimicrobials chosen to cover the most expected organisms has been consistently shown to improve outcomes1027 and has been cited as an essential element of the Surviving Sepsis Campaign (
At our institution, the antimicrobial approval process requires written justification forms and/or a call or fax to an Infectious Disease specialist or Clinical Pharmacist prior to the antimicrobial being released from the pharmacy. We hypothesized that this process is associated with significant delays in patients receiving their prescribed antimicrobials when a restricted drug was chosen. The antibiotic approval process at our institution allows 1‐time stat doses of restricted antimicrobials to be ordered without preapproval at night (10 PM to 8 AM), but not during the day. This allowed us to compare the time‐to‐administration of restricted antimicrobials to their unrestricted counterparts and to themselves during this exempt time period.
Methods
Study Design
This was a single‐institution retrospective cohort study. We included all patients admitted to Johns Hopkins Hospital units utilizing the hospital's computerized provider order entry system (CPOE) who had stat orders placed for any of 24 preselected commonly ordered intravenous antimicrobials over a 1‐year period between November 1, 2005 and October 31, 2006. We did not include oral medications that were similarly subject to the approval process since we anticipated that the amount of time required to prepare the drug in the pharmacy and to deliver it to the patient would be systematically different than for intravenous medications. The CPOE system captures time of administration of all drugs (as charted by the nurse) in addition to the time of order entry by the provider. Institutional guidelines dictate that when drugs are ordered stat, they should be administered within 30 minutes of the order.
Classification of Antibiotics
Particular antimicrobials (24 different drugs) were chosen prospectively to include a broad range of commonly ordered drugs available at our hospital in intravenous form (Table 1). For this analysis, we only considered the first dose of the prescribed antimicrobial, since subsequent doses are not generally ordered stat and the timing of administration is clinically less important than the timing of the initial dose. Additionally, for patients who had multiple orders for stat antimicrobials during the course of hospitalization, we only included the first stat order in analysis.
| Restricted Antimicrobials | Unrestricted Antimicrobials |
|---|---|
| Ampicillin/sulbactam | Acyclovir |
| Azithromycin | Amphotericin |
| Cefepime | Ampicillin |
| Ciprofloxacin | Cefazolin |
| Fluconazole | Ceftriaxone |
| Gatifloxacin | Cephalexin |
| Linezolid | Clindamycin |
| Meropenem | Doxycycline |
| Moxifloxacin | Ertapenem |
| Vancomycin | Gentamicin |
| Metronidazole | |
| Oxacillin | |
| Tobramycin | |
| Trimethoprim/sulfamethoxazole |
For the purposes of the analysis, we defined antimicrobials as restricted or unrestricted. At our institution, restricted antimicrobials require either written or telephone approval from an Infectious Disease physician or Clinical Pharmacist. Obtaining written approval involves filling out a form that indicates the choice of agent (including route and dose) for an approved indication (eg, vancomycin for proven methicillin‐resistant Staphylococcus aureus bacteremia) and faxing this form to the hospital pharmacy. In other cases, approval requires a phone conversation with an Infectious Disease clinical fellow or a Clinical Pharmacist. None of these processes can be initiated or executed from within the CPOE system itself.
At our institution, the first dose of any restricted antimicrobial may be administered without going through the approval process if it is prescribed in the overnight hours defined as between 10 PM and 8 AM (Table 2). Retroactive approval is required the following day if the antimicrobial is to be continued.
| Restricted Antimicrobial (eg, Vancomycin) | Unrestricted Antimicrobial (eg, Cefazolin) | |
|---|---|---|
| Daytime order (8 AM to 10 PM) | Approval required | No approval required |
| Nighttime order (10 PM to 8 AM) | Exempted from approval for first dose (approval required during the next daytime period) | No approval required |
Data Acquisition
All antibiotic administration data were extracted electronically from the CPOE system. During the time of the study, not all hospital units utilized this system. Although CPOE was in place for all general medical wards, the intensive care units and many surgical services were not using CPOE at the time of the study. Based on limitations of the data entered into our CPOE system, many relevant clinical variables were not available for this study, such as clinical diagnoses or the level of care (eg, intensive care unit vs step‐down unit, vs regular nursing floor) at the time of the order.
Outcome Measures
We defined time‐to‐administration as the period of time between order entry and administration of the drug to the patient, as charted by the nurse in the CPOE system. However, nursing policy allows nurses to document the time of administration as the exact time of the order provided that the drug is delivered within 1 hour of a stat order, whereas all administration times beyond 1 hour of the order are expected to be documented precisely. This nursing policy eliminated our ability to determine whether antimicrobials were administered within 30 minutes of the order, and limited the value of examining time‐to‐administration as a continuous variable, but allowed us to reliably identify delays of >1 hour or >2 hours presuming that the nurse‐charted administration times were accurate. As our primary outcome, we defined delay in administration as when the antimicrobial was administered >1 hour from time of order. We examined delays of >2 hours as a secondary outcome. Antimicrobials ordered stat but not delivered within 4 hours of the order were excluded from analysis based on the recognition that extended delays might have resulted from changes in clinical circumstances or errors in documentation. Similarly, antimicrobials charted as being delivered prior to the order were excluded.
Statistical Analyses
We used the likelihood‐ratio chi‐square test to determine 2‐sided P values for differences between proportions. Logistic regression models were used to derive odds ratios (ORs) and to adjust for covariables. We adjusted for weekday versus weekend orders, patient characteristics including sex, age, white versus non‐white ethnicity and orders placed on medicine versus non‐medicine (eg, surgical or obstetrical) wards. Analyses were conducted in JMP 5.1 (SAS Institute, Cary, NC).
Results
In total, 3337 orders for stat antimicrobials were written during the study period, of which 86 (2.6%) were excluded based on being outside the specified 4‐hour window. This left a total of 3251 orders in 3251 discrete patients for analysis.
We found that a statistically significantly higher percentage of delays in antimicrobial administration when the antimicrobial was restricted as compared to unrestricted. This was the case for both our primary outcome of a >1 hour delay (Figure 1) and our secondary outcome of a delay of >2 hours (Figure 2). For restricted antimicrobial, delays of >1 hr occurred with 46.1% of orders during the day and with 38.8% of orders at night (when exempt from approval), P < 0.001. For unrestricted antimicrobials, delays of >1 hr occurred in 36.4% and 36.6% of instances, respectively (P = 0.57). The odds ratio for a delay in administration of 1 hour for restricted antimicrobials was 1.49 (95% CI = 1.23‐1.82).


Delays beyond 2 hours occurred 24.0% of the time for restricted antimicrobials during the day versus 16.4% at night. Unrestricted antimicrobials were delayed >2 hrs only 15.1% and 14.3% of the time for day and night periods, respectively (P = 0.35). The odds ratio for a two‐hour or greater delay was 1.78 (95% CI = 1.39‐2.21), P < 0.0001 when the antimicrobial was restricted.
These odds ratios and statistical significance were unchanged by adjustment for primary service (medicine vs. non‐medicine), age, sex, ethnicity or whether the order was placed on a weekend or weekday (data not shown).
Discussion
We found that our institution's antimicrobial approval process was associated with statistically significant delays in the administration of antimicrobials that were ordered stat by the prescribing clinician. These delays were evident both when comparing restricted antimicrobials to unrestricted ones and when these restricted antimicrobials were compared to themselves during the overnight time period when they were temporarily exempt from the approval process. This suggests that the delay is associated with the approval process itself and not the specific drug or the time of day. We also found that over one‐third of all stat antimicrobial orders were not carried out in the within one hour. This rate approached nearly 50% for restricted drugs ordered stat. This high baseline rate for all stat‐ordered antimicrobials underscores system challenges that seem to be exacerbated when restricted antimicrobials are chosen.
We do not know if the delays we observed resulted in patient harm. Indeed it is possible, if not likely, that patient care at our institution is improved by the judicious use of certain antimicrobials, even if the process required to enforce their use may result in delayed antimicrobial administration in some instances. Since we did not collect clinical information on baseline diagnoses or severity of illness, and we did not have information on clinical outcomes, we cannot determine whether the clinical delays we observed might have caused harm. Determining the impact these approval policies have on patient outcomes would require a separate study designed to collect the necessary clinical data to answer that question.
An additional limitation was that we did not ascertain the indications for the antimicrobials to determine whether they truly needed to be given stat. We suspect that antimicrobials are sometimes ordered stat even when the infection being treated is not likely to be serious or life‐threatening. Additionally, since we relied on the nurse‐charted time of administration, it is possible that in some instances there was a charted delay in administration when in reality the patient received the antimicrobial in a timely fashion. In urgent situations, the nurses may be too busy to document that the medication was given until long after the dose is given, and this may result in inaccuracies in the charted administration time. However, this type of documentation error would be expected to affect restricted and unrestricted antimicrobials similarly and would be unlikely to result in a systematic bias.
Because we conducted this study at a single institution, the results may not be applicable to other medical institutions, especially since restriction policies and antimicrobial approval processes vary from hospital to hospital. The burden of delays may be related to the number of restricted antimicrobials on formulary, the types of antimicrobials restricted, the number of steps required to have them released from the pharmacy, whether the approval process is initiated from within the order entry system, and other factors that may streamline or hamper the approval process.
In our institution, there are several steps in the process, any of which might contribute to the delay. Faxed approval sheets may take time to arrive to and be acted upon by the pharmacy, errant pages may delay communication between the provider and the person providing approval, and there may be delays in the final approval being relayed to the pharmacy by the individual providing approval. In fact, an alternative explanation for the observed administration delays is that once ordered in CPOE, the prescribing physicians themselves are slow in initiating the approval process. While this is certainly possible, especially given the stresses surrounding the management of a seriously ill patient on the general ward, this still suggests that having to go through the approval process may impact the process of care.
Other possible explanations for the delays observed when the restrictive antimicrobial policy was in effect may include pharmacy staffing. Since the workload in the pharmacy would be expected to be greater during the day, when more patient care activity is occurring such as clinics and operating rooms, this increased workload may have slowed down the pharmacy filling the orders. However, such human resource‐workload imbalances would also be expected to slow most pharmacy processes and should lead to delays in filling the orders for other medications including the unrestricted antimicrobials. We did not track other non‐antimicrobial medications to examine their patterns of delay. Nursing workload also varies between day and night but the time period where the antimicrobial administration delays occurred is the time when nursing is favorably staffed unlike the night when nurse to patient ratios are low. It is possible that despite better nurse to patient ratios during the day, the workload‐to‐nursing ratio remains high and contributes to delays in administration of otherwise stat‐ordered antimicrobials. Again, it is unclear why this would disproportionately affect the restricted class of antimicrobials.
We do not advocate the abandonment of antimicrobial control policies. The process described here is very institution‐specific and while its benefits are proven, energy should be channeled where appropriate to facilitate this process. These policies are clearly necessary to help reduce costs, limit the unwarranted use of these drugs, and slow the proliferation of ever more resistant strains of microorganisms. However, we do advocate careful consideration of the components of the approval process itself, ensuring that delays in antimicrobial administration are kept to a minimum and are avoided altogether in critically ill patients. One way to accomplish this might be to not require approval for the first administration of a stat antibiotic, but to require approval for subsequent doses. Our institution's overnight exempt period data suggest that this would eliminate the incremental delays incurred by the approval process itself. As important, our results show that even for unrestricted antibiotics, we fall short of achieving recommended best practices, highlighting the challenges inherent to carrying out multi‐step clinical tasks in an efficient fashion.
In‐hospital administration of antimicrobials is often subject to controls and policies designed to limit the indiscriminant use of antimicrobials in situations where they are not warranted, to control costs, and to reduce the potential for the development of resistant microorganismsa major public health threat and patient safety concern. These controls and policies may include strategies such as limiting the choices of antimicrobials on formulary, rotational schedules of available antimicrobials, and antimicrobial approval processes.16 Antimicrobial approval processes commonly require that the bedside clinician obtain permission from a secondary source to administer a particular antimicrobial. This approval process may take the form of submitting written justification forms and/or direct telephone/fax requests to an Infectious Disease specialist, Clinical Pharmacist, or other surrogate prior to release of the antibiotic from the pharmacy. While these approval processes and other strategies have been shown to reduce the development of resistance,7, 8 improve outcomes,8 and provide education and revision of antimicrobial choice that may be more appropriate for the patient and suspected infection,9 they have the potential to delay the administration of the necessary antimicrobial by adding additional steps to the sequence of ordering, obtaining, and administering the medication. While it is certainly desirable to control the indiscriminant use of these medications, delays in antimicrobial administration may, in turn, worsen outcomes, thus counteracting the beneficial effects of control policies. The early and timely administration of appropriate broad spectrum antimicrobials chosen to cover the most expected organisms has been consistently shown to improve outcomes1027 and has been cited as an essential element of the Surviving Sepsis Campaign (
At our institution, the antimicrobial approval process requires written justification forms and/or a call or fax to an Infectious Disease specialist or Clinical Pharmacist prior to the antimicrobial being released from the pharmacy. We hypothesized that this process is associated with significant delays in patients receiving their prescribed antimicrobials when a restricted drug was chosen. The antibiotic approval process at our institution allows 1‐time stat doses of restricted antimicrobials to be ordered without preapproval at night (10 PM to 8 AM), but not during the day. This allowed us to compare the time‐to‐administration of restricted antimicrobials to their unrestricted counterparts and to themselves during this exempt time period.
Methods
Study Design
This was a single‐institution retrospective cohort study. We included all patients admitted to Johns Hopkins Hospital units utilizing the hospital's computerized provider order entry system (CPOE) who had stat orders placed for any of 24 preselected commonly ordered intravenous antimicrobials over a 1‐year period between November 1, 2005 and October 31, 2006. We did not include oral medications that were similarly subject to the approval process since we anticipated that the amount of time required to prepare the drug in the pharmacy and to deliver it to the patient would be systematically different than for intravenous medications. The CPOE system captures time of administration of all drugs (as charted by the nurse) in addition to the time of order entry by the provider. Institutional guidelines dictate that when drugs are ordered stat, they should be administered within 30 minutes of the order.
Classification of Antibiotics
Particular antimicrobials (24 different drugs) were chosen prospectively to include a broad range of commonly ordered drugs available at our hospital in intravenous form (Table 1). For this analysis, we only considered the first dose of the prescribed antimicrobial, since subsequent doses are not generally ordered stat and the timing of administration is clinically less important than the timing of the initial dose. Additionally, for patients who had multiple orders for stat antimicrobials during the course of hospitalization, we only included the first stat order in analysis.
| Restricted Antimicrobials | Unrestricted Antimicrobials |
|---|---|
| Ampicillin/sulbactam | Acyclovir |
| Azithromycin | Amphotericin |
| Cefepime | Ampicillin |
| Ciprofloxacin | Cefazolin |
| Fluconazole | Ceftriaxone |
| Gatifloxacin | Cephalexin |
| Linezolid | Clindamycin |
| Meropenem | Doxycycline |
| Moxifloxacin | Ertapenem |
| Vancomycin | Gentamicin |
| Metronidazole | |
| Oxacillin | |
| Tobramycin | |
| Trimethoprim/sulfamethoxazole |
For the purposes of the analysis, we defined antimicrobials as restricted or unrestricted. At our institution, restricted antimicrobials require either written or telephone approval from an Infectious Disease physician or Clinical Pharmacist. Obtaining written approval involves filling out a form that indicates the choice of agent (including route and dose) for an approved indication (eg, vancomycin for proven methicillin‐resistant Staphylococcus aureus bacteremia) and faxing this form to the hospital pharmacy. In other cases, approval requires a phone conversation with an Infectious Disease clinical fellow or a Clinical Pharmacist. None of these processes can be initiated or executed from within the CPOE system itself.
At our institution, the first dose of any restricted antimicrobial may be administered without going through the approval process if it is prescribed in the overnight hours defined as between 10 PM and 8 AM (Table 2). Retroactive approval is required the following day if the antimicrobial is to be continued.
| Restricted Antimicrobial (eg, Vancomycin) | Unrestricted Antimicrobial (eg, Cefazolin) | |
|---|---|---|
| Daytime order (8 AM to 10 PM) | Approval required | No approval required |
| Nighttime order (10 PM to 8 AM) | Exempted from approval for first dose (approval required during the next daytime period) | No approval required |
Data Acquisition
All antibiotic administration data were extracted electronically from the CPOE system. During the time of the study, not all hospital units utilized this system. Although CPOE was in place for all general medical wards, the intensive care units and many surgical services were not using CPOE at the time of the study. Based on limitations of the data entered into our CPOE system, many relevant clinical variables were not available for this study, such as clinical diagnoses or the level of care (eg, intensive care unit vs step‐down unit, vs regular nursing floor) at the time of the order.
Outcome Measures
We defined time‐to‐administration as the period of time between order entry and administration of the drug to the patient, as charted by the nurse in the CPOE system. However, nursing policy allows nurses to document the time of administration as the exact time of the order provided that the drug is delivered within 1 hour of a stat order, whereas all administration times beyond 1 hour of the order are expected to be documented precisely. This nursing policy eliminated our ability to determine whether antimicrobials were administered within 30 minutes of the order, and limited the value of examining time‐to‐administration as a continuous variable, but allowed us to reliably identify delays of >1 hour or >2 hours presuming that the nurse‐charted administration times were accurate. As our primary outcome, we defined delay in administration as when the antimicrobial was administered >1 hour from time of order. We examined delays of >2 hours as a secondary outcome. Antimicrobials ordered stat but not delivered within 4 hours of the order were excluded from analysis based on the recognition that extended delays might have resulted from changes in clinical circumstances or errors in documentation. Similarly, antimicrobials charted as being delivered prior to the order were excluded.
Statistical Analyses
We used the likelihood‐ratio chi‐square test to determine 2‐sided P values for differences between proportions. Logistic regression models were used to derive odds ratios (ORs) and to adjust for covariables. We adjusted for weekday versus weekend orders, patient characteristics including sex, age, white versus non‐white ethnicity and orders placed on medicine versus non‐medicine (eg, surgical or obstetrical) wards. Analyses were conducted in JMP 5.1 (SAS Institute, Cary, NC).
Results
In total, 3337 orders for stat antimicrobials were written during the study period, of which 86 (2.6%) were excluded based on being outside the specified 4‐hour window. This left a total of 3251 orders in 3251 discrete patients for analysis.
We found that a statistically significantly higher percentage of delays in antimicrobial administration when the antimicrobial was restricted as compared to unrestricted. This was the case for both our primary outcome of a >1 hour delay (Figure 1) and our secondary outcome of a delay of >2 hours (Figure 2). For restricted antimicrobial, delays of >1 hr occurred with 46.1% of orders during the day and with 38.8% of orders at night (when exempt from approval), P < 0.001. For unrestricted antimicrobials, delays of >1 hr occurred in 36.4% and 36.6% of instances, respectively (P = 0.57). The odds ratio for a delay in administration of 1 hour for restricted antimicrobials was 1.49 (95% CI = 1.23‐1.82).


Delays beyond 2 hours occurred 24.0% of the time for restricted antimicrobials during the day versus 16.4% at night. Unrestricted antimicrobials were delayed >2 hrs only 15.1% and 14.3% of the time for day and night periods, respectively (P = 0.35). The odds ratio for a two‐hour or greater delay was 1.78 (95% CI = 1.39‐2.21), P < 0.0001 when the antimicrobial was restricted.
These odds ratios and statistical significance were unchanged by adjustment for primary service (medicine vs. non‐medicine), age, sex, ethnicity or whether the order was placed on a weekend or weekday (data not shown).
Discussion
We found that our institution's antimicrobial approval process was associated with statistically significant delays in the administration of antimicrobials that were ordered stat by the prescribing clinician. These delays were evident both when comparing restricted antimicrobials to unrestricted ones and when these restricted antimicrobials were compared to themselves during the overnight time period when they were temporarily exempt from the approval process. This suggests that the delay is associated with the approval process itself and not the specific drug or the time of day. We also found that over one‐third of all stat antimicrobial orders were not carried out in the within one hour. This rate approached nearly 50% for restricted drugs ordered stat. This high baseline rate for all stat‐ordered antimicrobials underscores system challenges that seem to be exacerbated when restricted antimicrobials are chosen.
We do not know if the delays we observed resulted in patient harm. Indeed it is possible, if not likely, that patient care at our institution is improved by the judicious use of certain antimicrobials, even if the process required to enforce their use may result in delayed antimicrobial administration in some instances. Since we did not collect clinical information on baseline diagnoses or severity of illness, and we did not have information on clinical outcomes, we cannot determine whether the clinical delays we observed might have caused harm. Determining the impact these approval policies have on patient outcomes would require a separate study designed to collect the necessary clinical data to answer that question.
An additional limitation was that we did not ascertain the indications for the antimicrobials to determine whether they truly needed to be given stat. We suspect that antimicrobials are sometimes ordered stat even when the infection being treated is not likely to be serious or life‐threatening. Additionally, since we relied on the nurse‐charted time of administration, it is possible that in some instances there was a charted delay in administration when in reality the patient received the antimicrobial in a timely fashion. In urgent situations, the nurses may be too busy to document that the medication was given until long after the dose is given, and this may result in inaccuracies in the charted administration time. However, this type of documentation error would be expected to affect restricted and unrestricted antimicrobials similarly and would be unlikely to result in a systematic bias.
Because we conducted this study at a single institution, the results may not be applicable to other medical institutions, especially since restriction policies and antimicrobial approval processes vary from hospital to hospital. The burden of delays may be related to the number of restricted antimicrobials on formulary, the types of antimicrobials restricted, the number of steps required to have them released from the pharmacy, whether the approval process is initiated from within the order entry system, and other factors that may streamline or hamper the approval process.
In our institution, there are several steps in the process, any of which might contribute to the delay. Faxed approval sheets may take time to arrive to and be acted upon by the pharmacy, errant pages may delay communication between the provider and the person providing approval, and there may be delays in the final approval being relayed to the pharmacy by the individual providing approval. In fact, an alternative explanation for the observed administration delays is that once ordered in CPOE, the prescribing physicians themselves are slow in initiating the approval process. While this is certainly possible, especially given the stresses surrounding the management of a seriously ill patient on the general ward, this still suggests that having to go through the approval process may impact the process of care.
Other possible explanations for the delays observed when the restrictive antimicrobial policy was in effect may include pharmacy staffing. Since the workload in the pharmacy would be expected to be greater during the day, when more patient care activity is occurring such as clinics and operating rooms, this increased workload may have slowed down the pharmacy filling the orders. However, such human resource‐workload imbalances would also be expected to slow most pharmacy processes and should lead to delays in filling the orders for other medications including the unrestricted antimicrobials. We did not track other non‐antimicrobial medications to examine their patterns of delay. Nursing workload also varies between day and night but the time period where the antimicrobial administration delays occurred is the time when nursing is favorably staffed unlike the night when nurse to patient ratios are low. It is possible that despite better nurse to patient ratios during the day, the workload‐to‐nursing ratio remains high and contributes to delays in administration of otherwise stat‐ordered antimicrobials. Again, it is unclear why this would disproportionately affect the restricted class of antimicrobials.
We do not advocate the abandonment of antimicrobial control policies. The process described here is very institution‐specific and while its benefits are proven, energy should be channeled where appropriate to facilitate this process. These policies are clearly necessary to help reduce costs, limit the unwarranted use of these drugs, and slow the proliferation of ever more resistant strains of microorganisms. However, we do advocate careful consideration of the components of the approval process itself, ensuring that delays in antimicrobial administration are kept to a minimum and are avoided altogether in critically ill patients. One way to accomplish this might be to not require approval for the first administration of a stat antibiotic, but to require approval for subsequent doses. Our institution's overnight exempt period data suggest that this would eliminate the incremental delays incurred by the approval process itself. As important, our results show that even for unrestricted antibiotics, we fall short of achieving recommended best practices, highlighting the challenges inherent to carrying out multi‐step clinical tasks in an efficient fashion.
- .Antimicrobial stewardship.Am J Med.2006;119:S53–S61.
- .Restrictive antibiotic policies are appropriate in intensive care units.Crit Care Med.2003;31:S25–S28.
- ,,, et al.Effects of requiring prior authorization for selected anti‐microbials: expenditures, susceptibilities and clinical outcomes.Clin Infect Dis.1997;25:230–239.
- ,,, et al.Class restriction of cephalosporin use to control total cephaslosporin resistance in nosocomial Klebsiella.JAMA.1998;280:1233–1237.
- ,,, et al.Rotation and restricted use of antibiotics in a medical intensive care unit: impact on the incidence of ventilator associated pneumonia caused by antibiotic resistant Gram‐negative bacteremia.Am J Respir Crit Care Med.2001;63(3 Pt 1):837–843.
- ,,, et al.Impact of rotation of empiric antibiotic schedules on infectious mortality in an intensive care unit.CCM.2001;29:1101–1108.
- ,,, et al.Antimicrobial use control measures to prevent and control antimicrobial resistance in US hospitals.Infect Control Hosp Epidemiol.2006;27:1088–1095.
- ,,,,.Cost‐effectiveness of prospective and continuous parenteral antibiotic control: experience at the Palo Alto Veterans Affairs Medical Center from 1987 to 1989.Am J Med.1991;90:439–444.
- ,.Programmatic role of the infectious diseases physician in controlling antimicrobial costs in the hospital.Clin Infect Dis.1997;24:471–485.
- ,.Antimicrobial stewardship and the role of pharmacokinetics–pharmacodynamics in the modern antibiotic era.Diagn Microbiol Infect Dis.2007;57(3 Suppl.):77S–83S.
- ,,,,,.Improving inpatient antibiotic prescribing: insights from participation in a national collaborative.Jt Comm J Qual Improv.2001;27:387–402.
- ,.Managing antibiotic resistance.N Engl J Med.2000;343:1961–1963.
- ,.Antimicrobial stewardship programs in health care systems.Clin Microbiol Rev.2005;18(4):638–656.
- ,,,,,,,,,,.Interventions to improve antibiotic prescribing practices for hospital inpatients.Cochrane Database Syst Rev.2005;4:CD003543.
- ,,,,.Evaluation of an antimicrobial stewardship program at a pediatric teaching hospital.Pediatr Infect Dis J.2008;27(2):106–111.
- .The utility of hospital antibiograms as tools for guiding empiric therapy and tracking resistance. Insights from the Society of Infectious Diseases Pharmacists.Pharmacotheray.2007;27(9):1306–1312.
- ,,,.Strategies to contain the emergence of antimicrobial resistance: a systematic review of effectiveness and cost‐effectiveness.J Health Serv Res Policy.2002;7(2):111–117.
- ,,,,,,,,,,,,;Infectious Diseases Society of America;Society for Healthcare Epidemiology of America.Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship.Clin Infect Dis.2007;44(2):159–177.
- ,,,, andthe Healthcare Infection Control Practices Advisory Committee. Management of Multi‐Drug Reistsant Organisms in Healthcare Settings: 2006. Available at: http://www.cdc.gov/ncidod/dhqp/pdf/ar/MDROGuideline2006.pdf. Accessed May 19,2008.
- ,,.Gram‐negative bacteremia. IV Re‐evaluation of clinical features and treatment in 612 patients.Am J Med.1980;68:344–355.
- ,,,.Gram‐negative bacteremia. III. Reassessment of etiology, epidemiology and ecology in 612 patients.Am J Med.1980;68:332–343.
- ,,,.Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients.Chest.1999;115:462–474.
- .Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. ICU‐Acquired Pneumonia Study Group.Intensive Care Med.1996;22:387–394.
- ,,,,.The value of routine microbial investigation in ventilator‐associated pneumonia.Am J Respir Crit Care Med.1997;156:196–200.
- ,,, et al.Impact of BAL data on the therapy and outcome of ventilator‐associated pneumonia.Chest.1997;111:676–685.
- ,.The influence of mini‐BAL cultures on patient outcomes: implications for the antibiotic management of ventilator associated pneumonia.Chest.1998;113:412–420.
- ,,,,.The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting.Chest.2000;118:146–155.
- .Antimicrobial stewardship.Am J Med.2006;119:S53–S61.
- .Restrictive antibiotic policies are appropriate in intensive care units.Crit Care Med.2003;31:S25–S28.
- ,,, et al.Effects of requiring prior authorization for selected anti‐microbials: expenditures, susceptibilities and clinical outcomes.Clin Infect Dis.1997;25:230–239.
- ,,, et al.Class restriction of cephalosporin use to control total cephaslosporin resistance in nosocomial Klebsiella.JAMA.1998;280:1233–1237.
- ,,, et al.Rotation and restricted use of antibiotics in a medical intensive care unit: impact on the incidence of ventilator associated pneumonia caused by antibiotic resistant Gram‐negative bacteremia.Am J Respir Crit Care Med.2001;63(3 Pt 1):837–843.
- ,,, et al.Impact of rotation of empiric antibiotic schedules on infectious mortality in an intensive care unit.CCM.2001;29:1101–1108.
- ,,, et al.Antimicrobial use control measures to prevent and control antimicrobial resistance in US hospitals.Infect Control Hosp Epidemiol.2006;27:1088–1095.
- ,,,,.Cost‐effectiveness of prospective and continuous parenteral antibiotic control: experience at the Palo Alto Veterans Affairs Medical Center from 1987 to 1989.Am J Med.1991;90:439–444.
- ,.Programmatic role of the infectious diseases physician in controlling antimicrobial costs in the hospital.Clin Infect Dis.1997;24:471–485.
- ,.Antimicrobial stewardship and the role of pharmacokinetics–pharmacodynamics in the modern antibiotic era.Diagn Microbiol Infect Dis.2007;57(3 Suppl.):77S–83S.
- ,,,,,.Improving inpatient antibiotic prescribing: insights from participation in a national collaborative.Jt Comm J Qual Improv.2001;27:387–402.
- ,.Managing antibiotic resistance.N Engl J Med.2000;343:1961–1963.
- ,.Antimicrobial stewardship programs in health care systems.Clin Microbiol Rev.2005;18(4):638–656.
- ,,,,,,,,,,.Interventions to improve antibiotic prescribing practices for hospital inpatients.Cochrane Database Syst Rev.2005;4:CD003543.
- ,,,,.Evaluation of an antimicrobial stewardship program at a pediatric teaching hospital.Pediatr Infect Dis J.2008;27(2):106–111.
- .The utility of hospital antibiograms as tools for guiding empiric therapy and tracking resistance. Insights from the Society of Infectious Diseases Pharmacists.Pharmacotheray.2007;27(9):1306–1312.
- ,,,.Strategies to contain the emergence of antimicrobial resistance: a systematic review of effectiveness and cost‐effectiveness.J Health Serv Res Policy.2002;7(2):111–117.
- ,,,,,,,,,,,,;Infectious Diseases Society of America;Society for Healthcare Epidemiology of America.Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship.Clin Infect Dis.2007;44(2):159–177.
- ,,,, andthe Healthcare Infection Control Practices Advisory Committee. Management of Multi‐Drug Reistsant Organisms in Healthcare Settings: 2006. Available at: http://www.cdc.gov/ncidod/dhqp/pdf/ar/MDROGuideline2006.pdf. Accessed May 19,2008.
- ,,.Gram‐negative bacteremia. IV Re‐evaluation of clinical features and treatment in 612 patients.Am J Med.1980;68:344–355.
- ,,,.Gram‐negative bacteremia. III. Reassessment of etiology, epidemiology and ecology in 612 patients.Am J Med.1980;68:332–343.
- ,,,.Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients.Chest.1999;115:462–474.
- .Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. ICU‐Acquired Pneumonia Study Group.Intensive Care Med.1996;22:387–394.
- ,,,,.The value of routine microbial investigation in ventilator‐associated pneumonia.Am J Respir Crit Care Med.1997;156:196–200.
- ,,, et al.Impact of BAL data on the therapy and outcome of ventilator‐associated pneumonia.Chest.1997;111:676–685.
- ,.The influence of mini‐BAL cultures on patient outcomes: implications for the antibiotic management of ventilator associated pneumonia.Chest.1998;113:412–420.
- ,,,,.The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting.Chest.2000;118:146–155.
Copyright © 2010 Society of Hospital Medicine