User login
Liraglutide Prevents Ketogenesis in Type 1 Diabetes
ORLANDO – A single injection of liraglutide can prevent ketogenesis in fasting patients with type 1 diabetes who were on basal insulin, findings from a small study have shown.
Husam Ghanim, Ph.D., research associate professor at the State University of New York at Buffalo, presented the results in a late-breaking oral presentation session at the annual meeting of the American Association of Clinical Endocrinologists.
In a previous trial (Diabetes Care. 2016;39:1027-35) of patients with type 1 diabetes who took liraglutide, which does not have Food and Drug Administration approval for use in type 1 diabetes, for 12 weeks, investigators observed decreases in blood glucose levels compared with placebo and decreases in glucagon concentrations following a meal compared with before starting liraglutide. When patients already taking liraglutide and insulin were put on dapagliflozin for 12 weeks, glucagon levels rose more with dapagliflozin compared to placebo, and urinary acetoacetate and beta-hydroxybutyrate (adjusted to creatinine) rose over baseline levels.
Some researchers have hypothesized that liraglutide might stimulate residual beta cells (or beta cell stem cells) in patients with type 1 diabetes to produce insulin, thereby reducing the need for exogenous insulin. Promising data from animal studies suggesting that the drug stimulated residual beta cells were not duplicated in human studies. But some evidence shows it may reduce insulin doses anyway, even in cases of patients with no C-peptide, which means they are not producing any insulin on their own (Diabetes Care 2011. 34:1463-8).
In their study, Dr. Ghanim and his associates therefore wanted to test the effect on glucagon, free fatty acid, and ketone levels of acute administration of liraglutide to patients with type 1 diabetes in an insulinopenic condition. They randomly assigned patients with type 1 diabetes, aged 18-75 years, with undetectable C-peptide and hemoglobin A1c less than 8.5%, to receive an injection of 1.8 mg of liraglutide (n = 8) or placebo (n = 8) the morning after an overnight fast, which continued for the 5 hours of the study.
Patients had their basal insulin dose from the night before but no further insulin unless they were on an infusion pump, which they continued. Subjects were excluded if they were taking a glucagon-like peptide-1 (GLP-1) receptor agonist or a sodium/glucose cotransporter-2 (SGLT2) inhibitor, if they had renal impairment, had type 1 diabetes for less than 1 year, or had various other comorbidities.
The liraglutide group was slightly older than the placebo group (46 vs. 43 years), had a higher HbA1c (7.7% vs. 7.6%), and higher systolic but lower diastolic blood pressure (130/73 vs. 121/78 mm Hg). Body mass index was around 30 kg/m2 for both groups.
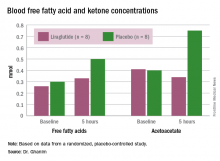
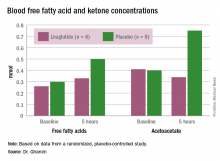
In the placebo group, there was no change in the blood glucose concentrations during the study period, whereas the liraglutide group showed a decrease from a baseline of 175 mg/dL to 135 mg/dL at 5 hours (P less than .05). Glucagon levels were maintained in the placebo group but showed significant suppression from 82 ng/L to 65 ng/L in the liraglutide arm (P less than .05).
“Free fatty acid increased in both groups, but the increase in the placebo arm was significantly higher than that in the liraglutide group,” Dr. Ghanim said. Ketones increased in the placebo group but actually dropped in the liraglutide arm. Ghrelin levels rose by 20% in the placebo group and fell by 10% with liraglutide. Hormone-sensitive lipase decreased about 10% in both arms over the study period.
Dr. Ghanim proposed that since ghrelin is a mediator of lipolysis, possibly the suppression of ghrelin, as well as glucagon, by liraglutide “could contribute to the lower free fatty acid levels, which therefore leads to a lower ketogenic process and reduced ketone bodies.
“With the significant risk of DKA [diabetic ketoacidosis] in type 1 diabetics, especially when you have a drug like an SGLT2 inhibitor, which has been shown to be ketogenic, it is very important to know that liraglutide actually attenuates that response and reduces ketogenesis and therefore reduces the risk of DKA,” he said.
He suggested that these study results should lead to larger randomized trials of GLP-1 receptor agonists and SGLT2 inhibitors, also not approved for use in type 1 diabetes, for use in this population because most of them are not presently well controlled and need additional agents.
Dr. John Miles, professor of both medicine and endocrinology, diabetes, and metabolism at the University of Kansas Medical Center in Kansas City, Kansas, asked Dr. Ghanim why the study subjects did not vomit when receiving the dose of liraglutide. Dr. Ghanim responded that the subjects were not naive to it and had been on it previously.
Session moderator Dr. David Lieb, associate professor of medicine at Eastern Virginia Medical School in Norfolk, said that liraglutide may be a good option for type 1 diabetes patients who are obese and want to lose weight. “I think if there is a drug that can potentially help with glucose control, because liraglutide is not all about causing insulin secretion by the pancreas – it also affects glucagon levels, and it affects appetite and satiety – [so] it may also help with weight loss. I think there’s a role for those sorts of medications in type 1 diabetics on a case-by-case, individual basis,” he said.
However, he wondered if there are any negative effects of suppressing glucagon because patients with type 1 diabetes may be at increased risk for hypoglycemia because of their insulin use, their activities, and their sensitivity to insulin. “Glucagon … allows glucose to be released by the liver,” he said, so (hypothetically) suppressing glucose release may exacerbate hypoglycemia. He said he looks forward to further studies of these drugs for type 1 diabetes and seeing the rate of occurrence of hypoglycemic episodes and how patients respond to them.
There was no funding for the study. Dr. Ghanim and Dr. Lieb reported having no financial disclosures.
ORLANDO – A single injection of liraglutide can prevent ketogenesis in fasting patients with type 1 diabetes who were on basal insulin, findings from a small study have shown.
Husam Ghanim, Ph.D., research associate professor at the State University of New York at Buffalo, presented the results in a late-breaking oral presentation session at the annual meeting of the American Association of Clinical Endocrinologists.
In a previous trial (Diabetes Care. 2016;39:1027-35) of patients with type 1 diabetes who took liraglutide, which does not have Food and Drug Administration approval for use in type 1 diabetes, for 12 weeks, investigators observed decreases in blood glucose levels compared with placebo and decreases in glucagon concentrations following a meal compared with before starting liraglutide. When patients already taking liraglutide and insulin were put on dapagliflozin for 12 weeks, glucagon levels rose more with dapagliflozin compared to placebo, and urinary acetoacetate and beta-hydroxybutyrate (adjusted to creatinine) rose over baseline levels.
Some researchers have hypothesized that liraglutide might stimulate residual beta cells (or beta cell stem cells) in patients with type 1 diabetes to produce insulin, thereby reducing the need for exogenous insulin. Promising data from animal studies suggesting that the drug stimulated residual beta cells were not duplicated in human studies. But some evidence shows it may reduce insulin doses anyway, even in cases of patients with no C-peptide, which means they are not producing any insulin on their own (Diabetes Care 2011. 34:1463-8).
In their study, Dr. Ghanim and his associates therefore wanted to test the effect on glucagon, free fatty acid, and ketone levels of acute administration of liraglutide to patients with type 1 diabetes in an insulinopenic condition. They randomly assigned patients with type 1 diabetes, aged 18-75 years, with undetectable C-peptide and hemoglobin A1c less than 8.5%, to receive an injection of 1.8 mg of liraglutide (n = 8) or placebo (n = 8) the morning after an overnight fast, which continued for the 5 hours of the study.
Patients had their basal insulin dose from the night before but no further insulin unless they were on an infusion pump, which they continued. Subjects were excluded if they were taking a glucagon-like peptide-1 (GLP-1) receptor agonist or a sodium/glucose cotransporter-2 (SGLT2) inhibitor, if they had renal impairment, had type 1 diabetes for less than 1 year, or had various other comorbidities.
The liraglutide group was slightly older than the placebo group (46 vs. 43 years), had a higher HbA1c (7.7% vs. 7.6%), and higher systolic but lower diastolic blood pressure (130/73 vs. 121/78 mm Hg). Body mass index was around 30 kg/m2 for both groups.
In the placebo group, there was no change in the blood glucose concentrations during the study period, whereas the liraglutide group showed a decrease from a baseline of 175 mg/dL to 135 mg/dL at 5 hours (P less than .05). Glucagon levels were maintained in the placebo group but showed significant suppression from 82 ng/L to 65 ng/L in the liraglutide arm (P less than .05).
“Free fatty acid increased in both groups, but the increase in the placebo arm was significantly higher than that in the liraglutide group,” Dr. Ghanim said. Ketones increased in the placebo group but actually dropped in the liraglutide arm. Ghrelin levels rose by 20% in the placebo group and fell by 10% with liraglutide. Hormone-sensitive lipase decreased about 10% in both arms over the study period.
Dr. Ghanim proposed that since ghrelin is a mediator of lipolysis, possibly the suppression of ghrelin, as well as glucagon, by liraglutide “could contribute to the lower free fatty acid levels, which therefore leads to a lower ketogenic process and reduced ketone bodies.
“With the significant risk of DKA [diabetic ketoacidosis] in type 1 diabetics, especially when you have a drug like an SGLT2 inhibitor, which has been shown to be ketogenic, it is very important to know that liraglutide actually attenuates that response and reduces ketogenesis and therefore reduces the risk of DKA,” he said.
He suggested that these study results should lead to larger randomized trials of GLP-1 receptor agonists and SGLT2 inhibitors, also not approved for use in type 1 diabetes, for use in this population because most of them are not presently well controlled and need additional agents.
Dr. John Miles, professor of both medicine and endocrinology, diabetes, and metabolism at the University of Kansas Medical Center in Kansas City, Kansas, asked Dr. Ghanim why the study subjects did not vomit when receiving the dose of liraglutide. Dr. Ghanim responded that the subjects were not naive to it and had been on it previously.
Session moderator Dr. David Lieb, associate professor of medicine at Eastern Virginia Medical School in Norfolk, said that liraglutide may be a good option for type 1 diabetes patients who are obese and want to lose weight. “I think if there is a drug that can potentially help with glucose control, because liraglutide is not all about causing insulin secretion by the pancreas – it also affects glucagon levels, and it affects appetite and satiety – [so] it may also help with weight loss. I think there’s a role for those sorts of medications in type 1 diabetics on a case-by-case, individual basis,” he said.
However, he wondered if there are any negative effects of suppressing glucagon because patients with type 1 diabetes may be at increased risk for hypoglycemia because of their insulin use, their activities, and their sensitivity to insulin. “Glucagon … allows glucose to be released by the liver,” he said, so (hypothetically) suppressing glucose release may exacerbate hypoglycemia. He said he looks forward to further studies of these drugs for type 1 diabetes and seeing the rate of occurrence of hypoglycemic episodes and how patients respond to them.
There was no funding for the study. Dr. Ghanim and Dr. Lieb reported having no financial disclosures.
ORLANDO – A single injection of liraglutide can prevent ketogenesis in fasting patients with type 1 diabetes who were on basal insulin, findings from a small study have shown.
Husam Ghanim, Ph.D., research associate professor at the State University of New York at Buffalo, presented the results in a late-breaking oral presentation session at the annual meeting of the American Association of Clinical Endocrinologists.
In a previous trial (Diabetes Care. 2016;39:1027-35) of patients with type 1 diabetes who took liraglutide, which does not have Food and Drug Administration approval for use in type 1 diabetes, for 12 weeks, investigators observed decreases in blood glucose levels compared with placebo and decreases in glucagon concentrations following a meal compared with before starting liraglutide. When patients already taking liraglutide and insulin were put on dapagliflozin for 12 weeks, glucagon levels rose more with dapagliflozin compared to placebo, and urinary acetoacetate and beta-hydroxybutyrate (adjusted to creatinine) rose over baseline levels.
Some researchers have hypothesized that liraglutide might stimulate residual beta cells (or beta cell stem cells) in patients with type 1 diabetes to produce insulin, thereby reducing the need for exogenous insulin. Promising data from animal studies suggesting that the drug stimulated residual beta cells were not duplicated in human studies. But some evidence shows it may reduce insulin doses anyway, even in cases of patients with no C-peptide, which means they are not producing any insulin on their own (Diabetes Care 2011. 34:1463-8).
In their study, Dr. Ghanim and his associates therefore wanted to test the effect on glucagon, free fatty acid, and ketone levels of acute administration of liraglutide to patients with type 1 diabetes in an insulinopenic condition. They randomly assigned patients with type 1 diabetes, aged 18-75 years, with undetectable C-peptide and hemoglobin A1c less than 8.5%, to receive an injection of 1.8 mg of liraglutide (n = 8) or placebo (n = 8) the morning after an overnight fast, which continued for the 5 hours of the study.
Patients had their basal insulin dose from the night before but no further insulin unless they were on an infusion pump, which they continued. Subjects were excluded if they were taking a glucagon-like peptide-1 (GLP-1) receptor agonist or a sodium/glucose cotransporter-2 (SGLT2) inhibitor, if they had renal impairment, had type 1 diabetes for less than 1 year, or had various other comorbidities.
The liraglutide group was slightly older than the placebo group (46 vs. 43 years), had a higher HbA1c (7.7% vs. 7.6%), and higher systolic but lower diastolic blood pressure (130/73 vs. 121/78 mm Hg). Body mass index was around 30 kg/m2 for both groups.
In the placebo group, there was no change in the blood glucose concentrations during the study period, whereas the liraglutide group showed a decrease from a baseline of 175 mg/dL to 135 mg/dL at 5 hours (P less than .05). Glucagon levels were maintained in the placebo group but showed significant suppression from 82 ng/L to 65 ng/L in the liraglutide arm (P less than .05).
“Free fatty acid increased in both groups, but the increase in the placebo arm was significantly higher than that in the liraglutide group,” Dr. Ghanim said. Ketones increased in the placebo group but actually dropped in the liraglutide arm. Ghrelin levels rose by 20% in the placebo group and fell by 10% with liraglutide. Hormone-sensitive lipase decreased about 10% in both arms over the study period.
Dr. Ghanim proposed that since ghrelin is a mediator of lipolysis, possibly the suppression of ghrelin, as well as glucagon, by liraglutide “could contribute to the lower free fatty acid levels, which therefore leads to a lower ketogenic process and reduced ketone bodies.
“With the significant risk of DKA [diabetic ketoacidosis] in type 1 diabetics, especially when you have a drug like an SGLT2 inhibitor, which has been shown to be ketogenic, it is very important to know that liraglutide actually attenuates that response and reduces ketogenesis and therefore reduces the risk of DKA,” he said.
He suggested that these study results should lead to larger randomized trials of GLP-1 receptor agonists and SGLT2 inhibitors, also not approved for use in type 1 diabetes, for use in this population because most of them are not presently well controlled and need additional agents.
Dr. John Miles, professor of both medicine and endocrinology, diabetes, and metabolism at the University of Kansas Medical Center in Kansas City, Kansas, asked Dr. Ghanim why the study subjects did not vomit when receiving the dose of liraglutide. Dr. Ghanim responded that the subjects were not naive to it and had been on it previously.
Session moderator Dr. David Lieb, associate professor of medicine at Eastern Virginia Medical School in Norfolk, said that liraglutide may be a good option for type 1 diabetes patients who are obese and want to lose weight. “I think if there is a drug that can potentially help with glucose control, because liraglutide is not all about causing insulin secretion by the pancreas – it also affects glucagon levels, and it affects appetite and satiety – [so] it may also help with weight loss. I think there’s a role for those sorts of medications in type 1 diabetics on a case-by-case, individual basis,” he said.
However, he wondered if there are any negative effects of suppressing glucagon because patients with type 1 diabetes may be at increased risk for hypoglycemia because of their insulin use, their activities, and their sensitivity to insulin. “Glucagon … allows glucose to be released by the liver,” he said, so (hypothetically) suppressing glucose release may exacerbate hypoglycemia. He said he looks forward to further studies of these drugs for type 1 diabetes and seeing the rate of occurrence of hypoglycemic episodes and how patients respond to them.
There was no funding for the study. Dr. Ghanim and Dr. Lieb reported having no financial disclosures.
AT AACE 2016
Liraglutide prevents ketogenesis in type 1 diabetes
ORLANDO – A single injection of liraglutide can prevent ketogenesis in fasting patients with type 1 diabetes who were on basal insulin, findings from a small study have shown.
Husam Ghanim, Ph.D., research associate professor at the State University of New York at Buffalo, presented the results in a late-breaking oral presentation session at the annual meeting of the American Association of Clinical Endocrinologists.
In a previous trial (Diabetes Care. 2016;39:1027-35) of patients with type 1 diabetes who took liraglutide, which does not have Food and Drug Administration approval for use in type 1 diabetes, for 12 weeks, investigators observed decreases in blood glucose levels compared with placebo and decreases in glucagon concentrations following a meal compared with before starting liraglutide. When patients already taking liraglutide and insulin were put on dapagliflozin for 12 weeks, glucagon levels rose more with dapagliflozin compared to placebo, and urinary acetoacetate and beta-hydroxybutyrate (adjusted to creatinine) rose over baseline levels.
Some researchers have hypothesized that liraglutide might stimulate residual beta cells (or beta cell stem cells) in patients with type 1 diabetes to produce insulin, thereby reducing the need for exogenous insulin. Promising data from animal studies suggesting that the drug stimulated residual beta cells were not duplicated in human studies. But some evidence shows it may reduce insulin doses anyway, even in cases of patients with no C-peptide, which means they are not producing any insulin on their own (Diabetes Care 2011. 34:1463-8).
In their study, Dr. Ghanim and his associates therefore wanted to test the effect on glucagon, free fatty acid, and ketone levels of acute administration of liraglutide to patients with type 1 diabetes in an insulinopenic condition. They randomly assigned patients with type 1 diabetes, aged 18-75 years, with undetectable C-peptide and hemoglobin A1c less than 8.5%, to receive an injection of 1.8 mg of liraglutide (n = 8) or placebo (n = 8) the morning after an overnight fast, which continued for the 5 hours of the study.
Patients had their basal insulin dose from the night before but no further insulin unless they were on an infusion pump, which they continued. Subjects were excluded if they were taking a glucagon-like peptide-1 (GLP-1) receptor agonist or a sodium/glucose cotransporter-2 (SGLT2) inhibitor, if they had renal impairment, had type 1 diabetes for less than 1 year, or had various other comorbidities.
The liraglutide group was slightly older than the placebo group (46 vs. 43 years), had a higher HbA1c (7.7% vs. 7.6%), and higher systolic but lower diastolic blood pressure (130/73 vs. 121/78 mm Hg). Body mass index was around 30 kg/m2 for both groups.
In the placebo group, there was no change in the blood glucose concentrations during the study period, whereas the liraglutide group showed a decrease from a baseline of 175 mg/dL to 135 mg/dL at 5 hours (P less than .05). Glucagon levels were maintained in the placebo group but showed significant suppression from 82 ng/L to 65 ng/L in the liraglutide arm (P less than .05).
“Free fatty acid increased in both groups, but the increase in the placebo arm was significantly higher than that in the liraglutide group,” Dr. Ghanim said. Ketones increased in the placebo group but actually dropped in the liraglutide arm. Ghrelin levels rose by 20% in the placebo group and fell by 10% with liraglutide. Hormone-sensitive lipase decreased about 10% in both arms over the study period.
Dr. Ghanim proposed that since ghrelin is a mediator of lipolysis, possibly the suppression of ghrelin, as well as glucagon, by liraglutide “could contribute to the lower free fatty acid levels, which therefore leads to a lower ketogenic process and reduced ketone bodies.
“With the significant risk of DKA [diabetic ketoacidosis] in type 1 diabetics, especially when you have a drug like an SGLT2 inhibitor, which has been shown to be ketogenic, it is very important to know that liraglutide actually attenuates that response and reduces ketogenesis and therefore reduces the risk of DKA,” he said.
He suggested that these study results should lead to larger randomized trials of GLP-1 receptor agonists and SGLT2 inhibitors, also not approved for use in type 1 diabetes, for use in this population because most of them are not presently well controlled and need additional agents.
Dr. John Miles, professor of both medicine and endocrinology, diabetes, and metabolism at the University of Kansas Medical Center in Kansas City, Kansas, asked Dr. Ghanim why the study subjects did not vomit when receiving the dose of liraglutide. Dr. Ghanim responded that the subjects were not naive to it and had been on it previously.
Session moderator Dr. David Lieb, associate professor of medicine at Eastern Virginia Medical School in Norfolk, said that liraglutide may be a good option for type 1 diabetes patients who are obese and want to lose weight. “I think if there is a drug that can potentially help with glucose control, because liraglutide is not all about causing insulin secretion by the pancreas – it also affects glucagon levels, and it affects appetite and satiety – [so] it may also help with weight loss. I think there’s a role for those sorts of medications in type 1 diabetics on a case-by-case, individual basis,” he said.
However, he wondered if there are any negative effects of suppressing glucagon because patients with type 1 diabetes may be at increased risk for hypoglycemia because of their insulin use, their activities, and their sensitivity to insulin. “Glucagon … allows glucose to be released by the liver,” he said, so (hypothetically) suppressing glucose release may exacerbate hypoglycemia. He said he looks forward to further studies of these drugs for type 1 diabetes and seeing the rate of occurrence of hypoglycemic episodes and how patients respond to them.
There was no funding for the study. Dr. Ghanim and Dr. Lieb reported having no financial disclosures.
ORLANDO – A single injection of liraglutide can prevent ketogenesis in fasting patients with type 1 diabetes who were on basal insulin, findings from a small study have shown.
Husam Ghanim, Ph.D., research associate professor at the State University of New York at Buffalo, presented the results in a late-breaking oral presentation session at the annual meeting of the American Association of Clinical Endocrinologists.
In a previous trial (Diabetes Care. 2016;39:1027-35) of patients with type 1 diabetes who took liraglutide, which does not have Food and Drug Administration approval for use in type 1 diabetes, for 12 weeks, investigators observed decreases in blood glucose levels compared with placebo and decreases in glucagon concentrations following a meal compared with before starting liraglutide. When patients already taking liraglutide and insulin were put on dapagliflozin for 12 weeks, glucagon levels rose more with dapagliflozin compared to placebo, and urinary acetoacetate and beta-hydroxybutyrate (adjusted to creatinine) rose over baseline levels.
Some researchers have hypothesized that liraglutide might stimulate residual beta cells (or beta cell stem cells) in patients with type 1 diabetes to produce insulin, thereby reducing the need for exogenous insulin. Promising data from animal studies suggesting that the drug stimulated residual beta cells were not duplicated in human studies. But some evidence shows it may reduce insulin doses anyway, even in cases of patients with no C-peptide, which means they are not producing any insulin on their own (Diabetes Care 2011. 34:1463-8).
In their study, Dr. Ghanim and his associates therefore wanted to test the effect on glucagon, free fatty acid, and ketone levels of acute administration of liraglutide to patients with type 1 diabetes in an insulinopenic condition. They randomly assigned patients with type 1 diabetes, aged 18-75 years, with undetectable C-peptide and hemoglobin A1c less than 8.5%, to receive an injection of 1.8 mg of liraglutide (n = 8) or placebo (n = 8) the morning after an overnight fast, which continued for the 5 hours of the study.
Patients had their basal insulin dose from the night before but no further insulin unless they were on an infusion pump, which they continued. Subjects were excluded if they were taking a glucagon-like peptide-1 (GLP-1) receptor agonist or a sodium/glucose cotransporter-2 (SGLT2) inhibitor, if they had renal impairment, had type 1 diabetes for less than 1 year, or had various other comorbidities.
The liraglutide group was slightly older than the placebo group (46 vs. 43 years), had a higher HbA1c (7.7% vs. 7.6%), and higher systolic but lower diastolic blood pressure (130/73 vs. 121/78 mm Hg). Body mass index was around 30 kg/m2 for both groups.
In the placebo group, there was no change in the blood glucose concentrations during the study period, whereas the liraglutide group showed a decrease from a baseline of 175 mg/dL to 135 mg/dL at 5 hours (P less than .05). Glucagon levels were maintained in the placebo group but showed significant suppression from 82 ng/L to 65 ng/L in the liraglutide arm (P less than .05).
“Free fatty acid increased in both groups, but the increase in the placebo arm was significantly higher than that in the liraglutide group,” Dr. Ghanim said. Ketones increased in the placebo group but actually dropped in the liraglutide arm. Ghrelin levels rose by 20% in the placebo group and fell by 10% with liraglutide. Hormone-sensitive lipase decreased about 10% in both arms over the study period.
Dr. Ghanim proposed that since ghrelin is a mediator of lipolysis, possibly the suppression of ghrelin, as well as glucagon, by liraglutide “could contribute to the lower free fatty acid levels, which therefore leads to a lower ketogenic process and reduced ketone bodies.
“With the significant risk of DKA [diabetic ketoacidosis] in type 1 diabetics, especially when you have a drug like an SGLT2 inhibitor, which has been shown to be ketogenic, it is very important to know that liraglutide actually attenuates that response and reduces ketogenesis and therefore reduces the risk of DKA,” he said.
He suggested that these study results should lead to larger randomized trials of GLP-1 receptor agonists and SGLT2 inhibitors, also not approved for use in type 1 diabetes, for use in this population because most of them are not presently well controlled and need additional agents.
Dr. John Miles, professor of both medicine and endocrinology, diabetes, and metabolism at the University of Kansas Medical Center in Kansas City, Kansas, asked Dr. Ghanim why the study subjects did not vomit when receiving the dose of liraglutide. Dr. Ghanim responded that the subjects were not naive to it and had been on it previously.
Session moderator Dr. David Lieb, associate professor of medicine at Eastern Virginia Medical School in Norfolk, said that liraglutide may be a good option for type 1 diabetes patients who are obese and want to lose weight. “I think if there is a drug that can potentially help with glucose control, because liraglutide is not all about causing insulin secretion by the pancreas – it also affects glucagon levels, and it affects appetite and satiety – [so] it may also help with weight loss. I think there’s a role for those sorts of medications in type 1 diabetics on a case-by-case, individual basis,” he said.
However, he wondered if there are any negative effects of suppressing glucagon because patients with type 1 diabetes may be at increased risk for hypoglycemia because of their insulin use, their activities, and their sensitivity to insulin. “Glucagon … allows glucose to be released by the liver,” he said, so (hypothetically) suppressing glucose release may exacerbate hypoglycemia. He said he looks forward to further studies of these drugs for type 1 diabetes and seeing the rate of occurrence of hypoglycemic episodes and how patients respond to them.
There was no funding for the study. Dr. Ghanim and Dr. Lieb reported having no financial disclosures.
ORLANDO – A single injection of liraglutide can prevent ketogenesis in fasting patients with type 1 diabetes who were on basal insulin, findings from a small study have shown.
Husam Ghanim, Ph.D., research associate professor at the State University of New York at Buffalo, presented the results in a late-breaking oral presentation session at the annual meeting of the American Association of Clinical Endocrinologists.
In a previous trial (Diabetes Care. 2016;39:1027-35) of patients with type 1 diabetes who took liraglutide, which does not have Food and Drug Administration approval for use in type 1 diabetes, for 12 weeks, investigators observed decreases in blood glucose levels compared with placebo and decreases in glucagon concentrations following a meal compared with before starting liraglutide. When patients already taking liraglutide and insulin were put on dapagliflozin for 12 weeks, glucagon levels rose more with dapagliflozin compared to placebo, and urinary acetoacetate and beta-hydroxybutyrate (adjusted to creatinine) rose over baseline levels.
Some researchers have hypothesized that liraglutide might stimulate residual beta cells (or beta cell stem cells) in patients with type 1 diabetes to produce insulin, thereby reducing the need for exogenous insulin. Promising data from animal studies suggesting that the drug stimulated residual beta cells were not duplicated in human studies. But some evidence shows it may reduce insulin doses anyway, even in cases of patients with no C-peptide, which means they are not producing any insulin on their own (Diabetes Care 2011. 34:1463-8).
In their study, Dr. Ghanim and his associates therefore wanted to test the effect on glucagon, free fatty acid, and ketone levels of acute administration of liraglutide to patients with type 1 diabetes in an insulinopenic condition. They randomly assigned patients with type 1 diabetes, aged 18-75 years, with undetectable C-peptide and hemoglobin A1c less than 8.5%, to receive an injection of 1.8 mg of liraglutide (n = 8) or placebo (n = 8) the morning after an overnight fast, which continued for the 5 hours of the study.
Patients had their basal insulin dose from the night before but no further insulin unless they were on an infusion pump, which they continued. Subjects were excluded if they were taking a glucagon-like peptide-1 (GLP-1) receptor agonist or a sodium/glucose cotransporter-2 (SGLT2) inhibitor, if they had renal impairment, had type 1 diabetes for less than 1 year, or had various other comorbidities.
The liraglutide group was slightly older than the placebo group (46 vs. 43 years), had a higher HbA1c (7.7% vs. 7.6%), and higher systolic but lower diastolic blood pressure (130/73 vs. 121/78 mm Hg). Body mass index was around 30 kg/m2 for both groups.
In the placebo group, there was no change in the blood glucose concentrations during the study period, whereas the liraglutide group showed a decrease from a baseline of 175 mg/dL to 135 mg/dL at 5 hours (P less than .05). Glucagon levels were maintained in the placebo group but showed significant suppression from 82 ng/L to 65 ng/L in the liraglutide arm (P less than .05).
“Free fatty acid increased in both groups, but the increase in the placebo arm was significantly higher than that in the liraglutide group,” Dr. Ghanim said. Ketones increased in the placebo group but actually dropped in the liraglutide arm. Ghrelin levels rose by 20% in the placebo group and fell by 10% with liraglutide. Hormone-sensitive lipase decreased about 10% in both arms over the study period.
Dr. Ghanim proposed that since ghrelin is a mediator of lipolysis, possibly the suppression of ghrelin, as well as glucagon, by liraglutide “could contribute to the lower free fatty acid levels, which therefore leads to a lower ketogenic process and reduced ketone bodies.
“With the significant risk of DKA [diabetic ketoacidosis] in type 1 diabetics, especially when you have a drug like an SGLT2 inhibitor, which has been shown to be ketogenic, it is very important to know that liraglutide actually attenuates that response and reduces ketogenesis and therefore reduces the risk of DKA,” he said.
He suggested that these study results should lead to larger randomized trials of GLP-1 receptor agonists and SGLT2 inhibitors, also not approved for use in type 1 diabetes, for use in this population because most of them are not presently well controlled and need additional agents.
Dr. John Miles, professor of both medicine and endocrinology, diabetes, and metabolism at the University of Kansas Medical Center in Kansas City, Kansas, asked Dr. Ghanim why the study subjects did not vomit when receiving the dose of liraglutide. Dr. Ghanim responded that the subjects were not naive to it and had been on it previously.
Session moderator Dr. David Lieb, associate professor of medicine at Eastern Virginia Medical School in Norfolk, said that liraglutide may be a good option for type 1 diabetes patients who are obese and want to lose weight. “I think if there is a drug that can potentially help with glucose control, because liraglutide is not all about causing insulin secretion by the pancreas – it also affects glucagon levels, and it affects appetite and satiety – [so] it may also help with weight loss. I think there’s a role for those sorts of medications in type 1 diabetics on a case-by-case, individual basis,” he said.
However, he wondered if there are any negative effects of suppressing glucagon because patients with type 1 diabetes may be at increased risk for hypoglycemia because of their insulin use, their activities, and their sensitivity to insulin. “Glucagon … allows glucose to be released by the liver,” he said, so (hypothetically) suppressing glucose release may exacerbate hypoglycemia. He said he looks forward to further studies of these drugs for type 1 diabetes and seeing the rate of occurrence of hypoglycemic episodes and how patients respond to them.
There was no funding for the study. Dr. Ghanim and Dr. Lieb reported having no financial disclosures.
AT AACE 2016
Key clinical point: Liraglutide suppresses glucagon and ketogenesis in fasting patients with type 1 diabetes.
Major finding: FFA increase was 60% lower on liraglutide than on placebo.
Data source: Randomized, placebo controlled study involving 16 patients.
Disclosures: There was no funding for the study. Dr. Ghanim and Dr. Lieb reported having no financial disclosures.
Starting With Combination Diabetes Therapy Beats Initial Monotherapy
ORLANDO – Whether to start a patient with newly diagnosed type 2 diabetes mellitus on combination therapy or monotherapy should be based on experimentation and observation rather than expert opinion, according to Dr. Alan Garber, president of the American College of Endocrinology and professor of medicine, biochemistry, and molecular and cellular biology at Baylor College of Medicine in Houston.
Monotherapy for type 2 diabetes with stepwise addition of other antihyperglycemic agents has long been the accepted way to initiate therapy in this population. Beginning in the 1990s, investigators began to compare the efficacy of monotherapy with combination therapy, first with metformin and glyburide alone or together, and then testing metformin in combination with glipizide, rosiglitazone, and sitagliptin, he said.
For metformin and glyburide, each agent alone lowered glycated hemoglobin (HbA1c), compared with placebo, but adding one to the other enhanced lowering. Combining the two drugs had the greatest benefit for higher HbA1c entry levels (e.g., HbA1c strata of 9%-9.9% or 10% or greater vs. less than 8%). At the highest-entry HbA1c levels, half doses of each of metformin and glyburide (250 mg/1.25 mg, respectively) were more efficacious than full doses of each (500 mg/2.5 mg). “This is called drug sparing,” he said.
In a trial of metformin and rosiglitazone, the combination was superior to either alone, producing significantly greater mean reductions in HbA1c and in fasting plasma glucose (FPG) at 32 weeks from their respective baselines, again, with greater reductions for higher-entry HbA1c levels. The combination was also better than either drug alone in the speed of reducing HbA1c or FPG, and in the final attained levels.
The combination of metformin and a sulfonylurea presents a risk of hypoglycemia, but Dr. Garber said the results are “much cleaner” using combinations of metformin with agents such as a thiazolidinedione, a dipeptidyl peptidase-4 inhibitor, or a sodium/glucose cotransporter-2 inhibitor.
Also noteworthy are findings from the EDICT (Efficacy and Durability of Initial Combination Therapy for Type 2 Diabetes) trial using insulin-sensitizing and insulin-secreting agents metformin/pioglitazone/exenatide in combination vs. escalating doses of metformin with sequential addition of a sulfonylurea and glargine insulin to treat patients with newly diagnosed type 2 diabetes. Over 2 years, the subjects receiving combination therapy had lower HbA1c, a mean weight loss, compared with weight gain, in the sequential therapy group, and a 7.5-fold lower rate of hypoglycemia, compared with the sequential treatment group (Diabetes Obes Metab. 2015;17:268-75).
Although the agents used in the two treatment strategies were not strictly equivalent, “it’s clear that testing multiple therapeutic mechanisms tends to produce better outcomes than fewer therapeutic mechanisms,” Dr. Garber said. The conclusions are fairly straightforward. “Look for evidence to support what strategies you want to use for your patients’ care.”
Using the Kaiser Permanente database, investigators found that the mean time of having an HbA1c above 8% was 3 years before a second agent was added, and the mean HbA1c was 9%. Many people have ascribed this sort of delay to a problem with the physician. But Dr. Garber said it is more related to patients, who often resist prescriptions for more drugs. So starting with two drugs may produce better efficacy faster as well as overcome the psychological issues of trying to add another one later (Am J Manag Care. 2003;9:213-7).
Session moderator Dr. Daniel Einhorn, medical director of the Scripps Whittier Diabetes Institute in La Jolla, California, raised the possibility of “subtraction therapy, where you start with three agents no matter what, and then if things go well, you subtract. And so you reverse the situation that Alan discussed.” In the patient’s view, “you have a celebration that night instead of a wake,” he said.
Dr. Garber has received honoraria or consulting fees as a member of the advisory boards of Novo Nordisk, Janssen, and Merck. Dr. Einhorn is on the scientific advisory boards of Eli Lilly, Novo Nordisk, Janssen, Boehringer Ingelheim, Sanofi, and Adocia, is a consultant for Halozyme, Glysens, Freedom-Meditech, and Epitracker, and has research funding from Lilly, Novo, Janssen, AstraZeneca, Mannkind, Freedom-Meditech, Merck, Sanofi, and Boehringer Ingelheim.
ORLANDO – Whether to start a patient with newly diagnosed type 2 diabetes mellitus on combination therapy or monotherapy should be based on experimentation and observation rather than expert opinion, according to Dr. Alan Garber, president of the American College of Endocrinology and professor of medicine, biochemistry, and molecular and cellular biology at Baylor College of Medicine in Houston.
Monotherapy for type 2 diabetes with stepwise addition of other antihyperglycemic agents has long been the accepted way to initiate therapy in this population. Beginning in the 1990s, investigators began to compare the efficacy of monotherapy with combination therapy, first with metformin and glyburide alone or together, and then testing metformin in combination with glipizide, rosiglitazone, and sitagliptin, he said.
For metformin and glyburide, each agent alone lowered glycated hemoglobin (HbA1c), compared with placebo, but adding one to the other enhanced lowering. Combining the two drugs had the greatest benefit for higher HbA1c entry levels (e.g., HbA1c strata of 9%-9.9% or 10% or greater vs. less than 8%). At the highest-entry HbA1c levels, half doses of each of metformin and glyburide (250 mg/1.25 mg, respectively) were more efficacious than full doses of each (500 mg/2.5 mg). “This is called drug sparing,” he said.
In a trial of metformin and rosiglitazone, the combination was superior to either alone, producing significantly greater mean reductions in HbA1c and in fasting plasma glucose (FPG) at 32 weeks from their respective baselines, again, with greater reductions for higher-entry HbA1c levels. The combination was also better than either drug alone in the speed of reducing HbA1c or FPG, and in the final attained levels.
The combination of metformin and a sulfonylurea presents a risk of hypoglycemia, but Dr. Garber said the results are “much cleaner” using combinations of metformin with agents such as a thiazolidinedione, a dipeptidyl peptidase-4 inhibitor, or a sodium/glucose cotransporter-2 inhibitor.
Also noteworthy are findings from the EDICT (Efficacy and Durability of Initial Combination Therapy for Type 2 Diabetes) trial using insulin-sensitizing and insulin-secreting agents metformin/pioglitazone/exenatide in combination vs. escalating doses of metformin with sequential addition of a sulfonylurea and glargine insulin to treat patients with newly diagnosed type 2 diabetes. Over 2 years, the subjects receiving combination therapy had lower HbA1c, a mean weight loss, compared with weight gain, in the sequential therapy group, and a 7.5-fold lower rate of hypoglycemia, compared with the sequential treatment group (Diabetes Obes Metab. 2015;17:268-75).
Although the agents used in the two treatment strategies were not strictly equivalent, “it’s clear that testing multiple therapeutic mechanisms tends to produce better outcomes than fewer therapeutic mechanisms,” Dr. Garber said. The conclusions are fairly straightforward. “Look for evidence to support what strategies you want to use for your patients’ care.”
Using the Kaiser Permanente database, investigators found that the mean time of having an HbA1c above 8% was 3 years before a second agent was added, and the mean HbA1c was 9%. Many people have ascribed this sort of delay to a problem with the physician. But Dr. Garber said it is more related to patients, who often resist prescriptions for more drugs. So starting with two drugs may produce better efficacy faster as well as overcome the psychological issues of trying to add another one later (Am J Manag Care. 2003;9:213-7).
Session moderator Dr. Daniel Einhorn, medical director of the Scripps Whittier Diabetes Institute in La Jolla, California, raised the possibility of “subtraction therapy, where you start with three agents no matter what, and then if things go well, you subtract. And so you reverse the situation that Alan discussed.” In the patient’s view, “you have a celebration that night instead of a wake,” he said.
Dr. Garber has received honoraria or consulting fees as a member of the advisory boards of Novo Nordisk, Janssen, and Merck. Dr. Einhorn is on the scientific advisory boards of Eli Lilly, Novo Nordisk, Janssen, Boehringer Ingelheim, Sanofi, and Adocia, is a consultant for Halozyme, Glysens, Freedom-Meditech, and Epitracker, and has research funding from Lilly, Novo, Janssen, AstraZeneca, Mannkind, Freedom-Meditech, Merck, Sanofi, and Boehringer Ingelheim.
ORLANDO – Whether to start a patient with newly diagnosed type 2 diabetes mellitus on combination therapy or monotherapy should be based on experimentation and observation rather than expert opinion, according to Dr. Alan Garber, president of the American College of Endocrinology and professor of medicine, biochemistry, and molecular and cellular biology at Baylor College of Medicine in Houston.
Monotherapy for type 2 diabetes with stepwise addition of other antihyperglycemic agents has long been the accepted way to initiate therapy in this population. Beginning in the 1990s, investigators began to compare the efficacy of monotherapy with combination therapy, first with metformin and glyburide alone or together, and then testing metformin in combination with glipizide, rosiglitazone, and sitagliptin, he said.
For metformin and glyburide, each agent alone lowered glycated hemoglobin (HbA1c), compared with placebo, but adding one to the other enhanced lowering. Combining the two drugs had the greatest benefit for higher HbA1c entry levels (e.g., HbA1c strata of 9%-9.9% or 10% or greater vs. less than 8%). At the highest-entry HbA1c levels, half doses of each of metformin and glyburide (250 mg/1.25 mg, respectively) were more efficacious than full doses of each (500 mg/2.5 mg). “This is called drug sparing,” he said.
In a trial of metformin and rosiglitazone, the combination was superior to either alone, producing significantly greater mean reductions in HbA1c and in fasting plasma glucose (FPG) at 32 weeks from their respective baselines, again, with greater reductions for higher-entry HbA1c levels. The combination was also better than either drug alone in the speed of reducing HbA1c or FPG, and in the final attained levels.
The combination of metformin and a sulfonylurea presents a risk of hypoglycemia, but Dr. Garber said the results are “much cleaner” using combinations of metformin with agents such as a thiazolidinedione, a dipeptidyl peptidase-4 inhibitor, or a sodium/glucose cotransporter-2 inhibitor.
Also noteworthy are findings from the EDICT (Efficacy and Durability of Initial Combination Therapy for Type 2 Diabetes) trial using insulin-sensitizing and insulin-secreting agents metformin/pioglitazone/exenatide in combination vs. escalating doses of metformin with sequential addition of a sulfonylurea and glargine insulin to treat patients with newly diagnosed type 2 diabetes. Over 2 years, the subjects receiving combination therapy had lower HbA1c, a mean weight loss, compared with weight gain, in the sequential therapy group, and a 7.5-fold lower rate of hypoglycemia, compared with the sequential treatment group (Diabetes Obes Metab. 2015;17:268-75).
Although the agents used in the two treatment strategies were not strictly equivalent, “it’s clear that testing multiple therapeutic mechanisms tends to produce better outcomes than fewer therapeutic mechanisms,” Dr. Garber said. The conclusions are fairly straightforward. “Look for evidence to support what strategies you want to use for your patients’ care.”
Using the Kaiser Permanente database, investigators found that the mean time of having an HbA1c above 8% was 3 years before a second agent was added, and the mean HbA1c was 9%. Many people have ascribed this sort of delay to a problem with the physician. But Dr. Garber said it is more related to patients, who often resist prescriptions for more drugs. So starting with two drugs may produce better efficacy faster as well as overcome the psychological issues of trying to add another one later (Am J Manag Care. 2003;9:213-7).
Session moderator Dr. Daniel Einhorn, medical director of the Scripps Whittier Diabetes Institute in La Jolla, California, raised the possibility of “subtraction therapy, where you start with three agents no matter what, and then if things go well, you subtract. And so you reverse the situation that Alan discussed.” In the patient’s view, “you have a celebration that night instead of a wake,” he said.
Dr. Garber has received honoraria or consulting fees as a member of the advisory boards of Novo Nordisk, Janssen, and Merck. Dr. Einhorn is on the scientific advisory boards of Eli Lilly, Novo Nordisk, Janssen, Boehringer Ingelheim, Sanofi, and Adocia, is a consultant for Halozyme, Glysens, Freedom-Meditech, and Epitracker, and has research funding from Lilly, Novo, Janssen, AstraZeneca, Mannkind, Freedom-Meditech, Merck, Sanofi, and Boehringer Ingelheim.
EXPERT ANALYSIS AT AACE 2016
Starting with combination diabetes therapy beats initial monotherapy
ORLANDO – Whether to start a patient with newly diagnosed type 2 diabetes mellitus on combination therapy or monotherapy should be based on experimentation and observation rather than expert opinion, according to Dr. Alan Garber, president of the American College of Endocrinology and professor of medicine, biochemistry, and molecular and cellular biology at Baylor College of Medicine in Houston.
Monotherapy for type 2 diabetes with stepwise addition of other antihyperglycemic agents has long been the accepted way to initiate therapy in this population. Beginning in the 1990s, investigators began to compare the efficacy of monotherapy with combination therapy, first with metformin and glyburide alone or together, and then testing metformin in combination with glipizide, rosiglitazone, and sitagliptin, he said.
For metformin and glyburide, each agent alone lowered glycated hemoglobin (HbA1c), compared with placebo, but adding one to the other enhanced lowering. Combining the two drugs had the greatest benefit for higher HbA1c entry levels (e.g., HbA1c strata of 9%-9.9% or 10% or greater vs. less than 8%). At the highest-entry HbA1c levels, half doses of each of metformin and glyburide (250 mg/1.25 mg, respectively) were more efficacious than full doses of each (500 mg/2.5 mg). “This is called drug sparing,” he said.
In a trial of metformin and rosiglitazone, the combination was superior to either alone, producing significantly greater mean reductions in HbA1c and in fasting plasma glucose (FPG) at 32 weeks from their respective baselines, again, with greater reductions for higher-entry HbA1c levels. The combination was also better than either drug alone in the speed of reducing HbA1c or FPG, and in the final attained levels.
The combination of metformin and a sulfonylurea presents a risk of hypoglycemia, but Dr. Garber said the results are “much cleaner” using combinations of metformin with agents such as a thiazolidinedione, a dipeptidyl peptidase-4 inhibitor, or a sodium/glucose cotransporter-2 inhibitor.
Also noteworthy are findings from the EDICT (Efficacy and Durability of Initial Combination Therapy for Type 2 Diabetes) trial using insulin-sensitizing and insulin-secreting agents metformin/pioglitazone/exenatide in combination vs. escalating doses of metformin with sequential addition of a sulfonylurea and glargine insulin to treat patients with newly diagnosed type 2 diabetes. Over 2 years, the subjects receiving combination therapy had lower HbA1c, a mean weight loss, compared with weight gain, in the sequential therapy group, and a 7.5-fold lower rate of hypoglycemia, compared with the sequential treatment group (Diabetes Obes Metab. 2015;17:268-75).
Although the agents used in the two treatment strategies were not strictly equivalent, “it’s clear that testing multiple therapeutic mechanisms tends to produce better outcomes than fewer therapeutic mechanisms,” Dr. Garber said. The conclusions are fairly straightforward. “Look for evidence to support what strategies you want to use for your patients’ care.”
Using the Kaiser Permanente database, investigators found that the mean time of having an HbA1c above 8% was 3 years before a second agent was added, and the mean HbA1c was 9%. Many people have ascribed this sort of delay to a problem with the physician. But Dr. Garber said it is more related to patients, who often resist prescriptions for more drugs. So starting with two drugs may produce better efficacy faster as well as overcome the psychological issues of trying to add another one later (Am J Manag Care. 2003;9:213-7).
Session moderator Dr. Daniel Einhorn, medical director of the Scripps Whittier Diabetes Institute in La Jolla, California, raised the possibility of “subtraction therapy, where you start with three agents no matter what, and then if things go well, you subtract. And so you reverse the situation that Alan discussed.” In the patient’s view, “you have a celebration that night instead of a wake,” he said.
Dr. Garber has received honoraria or consulting fees as a member of the advisory boards of Novo Nordisk, Janssen, and Merck. Dr. Einhorn is on the scientific advisory boards of Eli Lilly, Novo Nordisk, Janssen, Boehringer Ingelheim, Sanofi, and Adocia, is a consultant for Halozyme, Glysens, Freedom-Meditech, and Epitracker, and has research funding from Lilly, Novo, Janssen, AstraZeneca, Mannkind, Freedom-Meditech, Merck, Sanofi, and Boehringer Ingelheim.
ORLANDO – Whether to start a patient with newly diagnosed type 2 diabetes mellitus on combination therapy or monotherapy should be based on experimentation and observation rather than expert opinion, according to Dr. Alan Garber, president of the American College of Endocrinology and professor of medicine, biochemistry, and molecular and cellular biology at Baylor College of Medicine in Houston.
Monotherapy for type 2 diabetes with stepwise addition of other antihyperglycemic agents has long been the accepted way to initiate therapy in this population. Beginning in the 1990s, investigators began to compare the efficacy of monotherapy with combination therapy, first with metformin and glyburide alone or together, and then testing metformin in combination with glipizide, rosiglitazone, and sitagliptin, he said.
For metformin and glyburide, each agent alone lowered glycated hemoglobin (HbA1c), compared with placebo, but adding one to the other enhanced lowering. Combining the two drugs had the greatest benefit for higher HbA1c entry levels (e.g., HbA1c strata of 9%-9.9% or 10% or greater vs. less than 8%). At the highest-entry HbA1c levels, half doses of each of metformin and glyburide (250 mg/1.25 mg, respectively) were more efficacious than full doses of each (500 mg/2.5 mg). “This is called drug sparing,” he said.
In a trial of metformin and rosiglitazone, the combination was superior to either alone, producing significantly greater mean reductions in HbA1c and in fasting plasma glucose (FPG) at 32 weeks from their respective baselines, again, with greater reductions for higher-entry HbA1c levels. The combination was also better than either drug alone in the speed of reducing HbA1c or FPG, and in the final attained levels.
The combination of metformin and a sulfonylurea presents a risk of hypoglycemia, but Dr. Garber said the results are “much cleaner” using combinations of metformin with agents such as a thiazolidinedione, a dipeptidyl peptidase-4 inhibitor, or a sodium/glucose cotransporter-2 inhibitor.
Also noteworthy are findings from the EDICT (Efficacy and Durability of Initial Combination Therapy for Type 2 Diabetes) trial using insulin-sensitizing and insulin-secreting agents metformin/pioglitazone/exenatide in combination vs. escalating doses of metformin with sequential addition of a sulfonylurea and glargine insulin to treat patients with newly diagnosed type 2 diabetes. Over 2 years, the subjects receiving combination therapy had lower HbA1c, a mean weight loss, compared with weight gain, in the sequential therapy group, and a 7.5-fold lower rate of hypoglycemia, compared with the sequential treatment group (Diabetes Obes Metab. 2015;17:268-75).
Although the agents used in the two treatment strategies were not strictly equivalent, “it’s clear that testing multiple therapeutic mechanisms tends to produce better outcomes than fewer therapeutic mechanisms,” Dr. Garber said. The conclusions are fairly straightforward. “Look for evidence to support what strategies you want to use for your patients’ care.”
Using the Kaiser Permanente database, investigators found that the mean time of having an HbA1c above 8% was 3 years before a second agent was added, and the mean HbA1c was 9%. Many people have ascribed this sort of delay to a problem with the physician. But Dr. Garber said it is more related to patients, who often resist prescriptions for more drugs. So starting with two drugs may produce better efficacy faster as well as overcome the psychological issues of trying to add another one later (Am J Manag Care. 2003;9:213-7).
Session moderator Dr. Daniel Einhorn, medical director of the Scripps Whittier Diabetes Institute in La Jolla, California, raised the possibility of “subtraction therapy, where you start with three agents no matter what, and then if things go well, you subtract. And so you reverse the situation that Alan discussed.” In the patient’s view, “you have a celebration that night instead of a wake,” he said.
Dr. Garber has received honoraria or consulting fees as a member of the advisory boards of Novo Nordisk, Janssen, and Merck. Dr. Einhorn is on the scientific advisory boards of Eli Lilly, Novo Nordisk, Janssen, Boehringer Ingelheim, Sanofi, and Adocia, is a consultant for Halozyme, Glysens, Freedom-Meditech, and Epitracker, and has research funding from Lilly, Novo, Janssen, AstraZeneca, Mannkind, Freedom-Meditech, Merck, Sanofi, and Boehringer Ingelheim.
ORLANDO – Whether to start a patient with newly diagnosed type 2 diabetes mellitus on combination therapy or monotherapy should be based on experimentation and observation rather than expert opinion, according to Dr. Alan Garber, president of the American College of Endocrinology and professor of medicine, biochemistry, and molecular and cellular biology at Baylor College of Medicine in Houston.
Monotherapy for type 2 diabetes with stepwise addition of other antihyperglycemic agents has long been the accepted way to initiate therapy in this population. Beginning in the 1990s, investigators began to compare the efficacy of monotherapy with combination therapy, first with metformin and glyburide alone or together, and then testing metformin in combination with glipizide, rosiglitazone, and sitagliptin, he said.
For metformin and glyburide, each agent alone lowered glycated hemoglobin (HbA1c), compared with placebo, but adding one to the other enhanced lowering. Combining the two drugs had the greatest benefit for higher HbA1c entry levels (e.g., HbA1c strata of 9%-9.9% or 10% or greater vs. less than 8%). At the highest-entry HbA1c levels, half doses of each of metformin and glyburide (250 mg/1.25 mg, respectively) were more efficacious than full doses of each (500 mg/2.5 mg). “This is called drug sparing,” he said.
In a trial of metformin and rosiglitazone, the combination was superior to either alone, producing significantly greater mean reductions in HbA1c and in fasting plasma glucose (FPG) at 32 weeks from their respective baselines, again, with greater reductions for higher-entry HbA1c levels. The combination was also better than either drug alone in the speed of reducing HbA1c or FPG, and in the final attained levels.
The combination of metformin and a sulfonylurea presents a risk of hypoglycemia, but Dr. Garber said the results are “much cleaner” using combinations of metformin with agents such as a thiazolidinedione, a dipeptidyl peptidase-4 inhibitor, or a sodium/glucose cotransporter-2 inhibitor.
Also noteworthy are findings from the EDICT (Efficacy and Durability of Initial Combination Therapy for Type 2 Diabetes) trial using insulin-sensitizing and insulin-secreting agents metformin/pioglitazone/exenatide in combination vs. escalating doses of metformin with sequential addition of a sulfonylurea and glargine insulin to treat patients with newly diagnosed type 2 diabetes. Over 2 years, the subjects receiving combination therapy had lower HbA1c, a mean weight loss, compared with weight gain, in the sequential therapy group, and a 7.5-fold lower rate of hypoglycemia, compared with the sequential treatment group (Diabetes Obes Metab. 2015;17:268-75).
Although the agents used in the two treatment strategies were not strictly equivalent, “it’s clear that testing multiple therapeutic mechanisms tends to produce better outcomes than fewer therapeutic mechanisms,” Dr. Garber said. The conclusions are fairly straightforward. “Look for evidence to support what strategies you want to use for your patients’ care.”
Using the Kaiser Permanente database, investigators found that the mean time of having an HbA1c above 8% was 3 years before a second agent was added, and the mean HbA1c was 9%. Many people have ascribed this sort of delay to a problem with the physician. But Dr. Garber said it is more related to patients, who often resist prescriptions for more drugs. So starting with two drugs may produce better efficacy faster as well as overcome the psychological issues of trying to add another one later (Am J Manag Care. 2003;9:213-7).
Session moderator Dr. Daniel Einhorn, medical director of the Scripps Whittier Diabetes Institute in La Jolla, California, raised the possibility of “subtraction therapy, where you start with three agents no matter what, and then if things go well, you subtract. And so you reverse the situation that Alan discussed.” In the patient’s view, “you have a celebration that night instead of a wake,” he said.
Dr. Garber has received honoraria or consulting fees as a member of the advisory boards of Novo Nordisk, Janssen, and Merck. Dr. Einhorn is on the scientific advisory boards of Eli Lilly, Novo Nordisk, Janssen, Boehringer Ingelheim, Sanofi, and Adocia, is a consultant for Halozyme, Glysens, Freedom-Meditech, and Epitracker, and has research funding from Lilly, Novo, Janssen, AstraZeneca, Mannkind, Freedom-Meditech, Merck, Sanofi, and Boehringer Ingelheim.
EXPERT ANALYSIS AT AACE 2016
RF ablation successfully treats focal adrenal tumors
ORLANDO – Radiofrequency (RF) ablation is a safe and effective procedure for treating focal adrenal tumors in patients who are poor surgical candidates or who refuse adrenalectomy. With a short treatment time and minimal hospital stay, RF ablation can provide rapid clinical and biochemical improvement.
Dr. Lima Lawrence, an internal medicine resident at the University of Illinois at Chicago/Advocate Christ Medical Center in Oak Lawn, presented a case report and a review of the literature during an oral abstract session at the annual meeting of the American Association of Clinical Endocrinologists. The patient was a 65-year-old woman who presented with weight gain, decreased energy, and muscle weakness. On physical exam, she was hypertensive, anxious, obese, and had prominent supraclavicular fat pads. Salivary cortisol and overnight dexamethasone suppression tests were both elevated, and ACTH levels were depressed, confirming the diagnosis of a cortisol-secreting tumor causing adrenal Cushing’s syndrome. Computed tomography (CT) surveillance showed a progressively enlarging right-sided adrenal mass. A peritoneal biopsy revealed a low-grade serous neoplasm of peritoneal origin.
Her medical history included type 2 diabetes, uncontrolled hypertension, mixed connective tissue disease, depression, and total abdominal hysterectomy with bilateral salpingo-oophorectomy for ovarian cancer.
Dr. Lawrence said the patient had been scheduled for adrenalectomy, but it was not performed because of an intraoperative finding of peritoneal studding from what turned out to be metastatic ovarian cancer. Therefore, she underwent CT-guided RF ablation of the adrenal mass using a 14-gauge probe that heated a 3.5-cm ablation zone to 50-60 C for 8-10 minutes to achieve complete tumor necrosis.
The patient showed dramatic “clinical and biochemical improvement,” Dr. Lawrence said. The patient had no procedural complications and no blood loss and was observed for 23 hours before being discharged to home. A CT scan 8 weeks later showed a slightly decreased mass with marked decreased radiographic attenuation post-contrast from 30.2 Hounsfield Units (HU) preoperatively to 17 HU on follow-up.
Potential adverse outcomes using RF ablation include a risk of pneumothorax, hemothorax, and tumor seeding along the catheter track, but this last possibility can be mitigated by continuing to heat the RF probe as it is withdrawn.
Published evidence supports use of RF ablation. “To date there have been no randomized clinical trials comparing the safety, efficacy, and survival benefits of adrenalectomy vs. radio frequency ablation,” she said. It may not be feasible to do a randomized trial. But a review of the literature generally supports the efficacy of the technique although the publications each involved a small series of patients, Dr. Lawrence said in an interview.
A 2003 series (Cancer. 2003;97:554-60) of 15 primary or metastatic adrenal cell carcinomas that were unresectable or were in patients who were not surgical candidates showed nonenhancement and no growth in 8 (53%) at a mean follow-up of 10.3 months. Eight of the 12 tumors of 5 cm or smaller had complete loss of radiographic enhancement and a decrease in size.
From a retrospective series of 13 patients with functional adrenal neoplasms over 7 years, there was 100% resolution of biochemical abnormalities and clinical symptoms at a mean follow-up of 21.2 months. One small pneumothorax and one limited hemothorax occurred, neither of which required hospital admission. There were two instances of transient, self-remitting hypertension associated with the procedures (Radiology. 2011;258:308-16).
In 2015, one group of investigators followed 11 patients for 12 weeks postprocedure. Eight of nine patients with Conn’s syndrome attained normal serum aldosterone levels. One with a nodule close to the inferior vena cava had incomplete ablation. Two of two Cushing’s patients had normal cortisol levels after the procedure (J Vasc Interv Radiol. 2015;26:1459-64).
A retrospective analysis of 16 adrenal metastases showed that 13 (81%) had no local progression over 14 months after ablation. In two of three functional adrenal neoplasms, clinical and biochemical abnormalities resolved (Eur J Radiol. 2012.81:1717-23).
A retrospective series of 10 adrenal metastases showed that one recurred at 7 months after image-guided thermal ablation, with no recurrence of the rest at 26.6 months. There was no tumor recurrence for any of the cases of metastatic disease localized to the RF ablation site (J Vasc Interv Radiol. 2014;25:593-8).
Results were somewhat less good in a retrospective evaluation of 35 patients with unresectable adrenal masses over 9 years. Although 33 of 35 (94%) lost tumor enhancement after the initial adrenal RF ablation, there was local tumor progression in 8 of 35 (23%) patients at a mean follow-up of 30.1 months (Radiology. 2015;277:584-93).
Finally, Dr. Lawrence discussed a systematic literature review on adrenalectomy vs. stereotactic ablative body radiotherapy (SABR) and percutaneous catheter ablation (PCA) in the treatment of adrenal metastases: 30 papers on adrenalectomy on 818 patients; 9 papers on SABR on 178 patients; and 6 papers on PCA, including RF ablation, on 51 patients. The authors concluded that there was “insufficient evidence to determine the best local treatment modality for isolated or limited adrenal metastases.” Adrenalectomy appeared to be a reasonable treatment for suitable patients. SABR was a valid alternative for nonsurgical candidates, but they did not recommend PCA until more long-term outcomes were available (Cancer Treat Rev. 2014;40:838-46).
Dr. Lawrence concurred, based on her case study and literature review. She said RF ablation “offers patients a minimally invasive option for treating focal adrenal tumors” and is a “safe and effective procedure … in patients who are poor surgical candidates or refuse adrenalectomy.” More long-term follow-up studies are needed before RF ablation could replace adrenalectomy, she noted.
ORLANDO – Radiofrequency (RF) ablation is a safe and effective procedure for treating focal adrenal tumors in patients who are poor surgical candidates or who refuse adrenalectomy. With a short treatment time and minimal hospital stay, RF ablation can provide rapid clinical and biochemical improvement.
Dr. Lima Lawrence, an internal medicine resident at the University of Illinois at Chicago/Advocate Christ Medical Center in Oak Lawn, presented a case report and a review of the literature during an oral abstract session at the annual meeting of the American Association of Clinical Endocrinologists. The patient was a 65-year-old woman who presented with weight gain, decreased energy, and muscle weakness. On physical exam, she was hypertensive, anxious, obese, and had prominent supraclavicular fat pads. Salivary cortisol and overnight dexamethasone suppression tests were both elevated, and ACTH levels were depressed, confirming the diagnosis of a cortisol-secreting tumor causing adrenal Cushing’s syndrome. Computed tomography (CT) surveillance showed a progressively enlarging right-sided adrenal mass. A peritoneal biopsy revealed a low-grade serous neoplasm of peritoneal origin.
Her medical history included type 2 diabetes, uncontrolled hypertension, mixed connective tissue disease, depression, and total abdominal hysterectomy with bilateral salpingo-oophorectomy for ovarian cancer.
Dr. Lawrence said the patient had been scheduled for adrenalectomy, but it was not performed because of an intraoperative finding of peritoneal studding from what turned out to be metastatic ovarian cancer. Therefore, she underwent CT-guided RF ablation of the adrenal mass using a 14-gauge probe that heated a 3.5-cm ablation zone to 50-60 C for 8-10 minutes to achieve complete tumor necrosis.
The patient showed dramatic “clinical and biochemical improvement,” Dr. Lawrence said. The patient had no procedural complications and no blood loss and was observed for 23 hours before being discharged to home. A CT scan 8 weeks later showed a slightly decreased mass with marked decreased radiographic attenuation post-contrast from 30.2 Hounsfield Units (HU) preoperatively to 17 HU on follow-up.
Potential adverse outcomes using RF ablation include a risk of pneumothorax, hemothorax, and tumor seeding along the catheter track, but this last possibility can be mitigated by continuing to heat the RF probe as it is withdrawn.
Published evidence supports use of RF ablation. “To date there have been no randomized clinical trials comparing the safety, efficacy, and survival benefits of adrenalectomy vs. radio frequency ablation,” she said. It may not be feasible to do a randomized trial. But a review of the literature generally supports the efficacy of the technique although the publications each involved a small series of patients, Dr. Lawrence said in an interview.
A 2003 series (Cancer. 2003;97:554-60) of 15 primary or metastatic adrenal cell carcinomas that were unresectable or were in patients who were not surgical candidates showed nonenhancement and no growth in 8 (53%) at a mean follow-up of 10.3 months. Eight of the 12 tumors of 5 cm or smaller had complete loss of radiographic enhancement and a decrease in size.
From a retrospective series of 13 patients with functional adrenal neoplasms over 7 years, there was 100% resolution of biochemical abnormalities and clinical symptoms at a mean follow-up of 21.2 months. One small pneumothorax and one limited hemothorax occurred, neither of which required hospital admission. There were two instances of transient, self-remitting hypertension associated with the procedures (Radiology. 2011;258:308-16).
In 2015, one group of investigators followed 11 patients for 12 weeks postprocedure. Eight of nine patients with Conn’s syndrome attained normal serum aldosterone levels. One with a nodule close to the inferior vena cava had incomplete ablation. Two of two Cushing’s patients had normal cortisol levels after the procedure (J Vasc Interv Radiol. 2015;26:1459-64).
A retrospective analysis of 16 adrenal metastases showed that 13 (81%) had no local progression over 14 months after ablation. In two of three functional adrenal neoplasms, clinical and biochemical abnormalities resolved (Eur J Radiol. 2012.81:1717-23).
A retrospective series of 10 adrenal metastases showed that one recurred at 7 months after image-guided thermal ablation, with no recurrence of the rest at 26.6 months. There was no tumor recurrence for any of the cases of metastatic disease localized to the RF ablation site (J Vasc Interv Radiol. 2014;25:593-8).
Results were somewhat less good in a retrospective evaluation of 35 patients with unresectable adrenal masses over 9 years. Although 33 of 35 (94%) lost tumor enhancement after the initial adrenal RF ablation, there was local tumor progression in 8 of 35 (23%) patients at a mean follow-up of 30.1 months (Radiology. 2015;277:584-93).
Finally, Dr. Lawrence discussed a systematic literature review on adrenalectomy vs. stereotactic ablative body radiotherapy (SABR) and percutaneous catheter ablation (PCA) in the treatment of adrenal metastases: 30 papers on adrenalectomy on 818 patients; 9 papers on SABR on 178 patients; and 6 papers on PCA, including RF ablation, on 51 patients. The authors concluded that there was “insufficient evidence to determine the best local treatment modality for isolated or limited adrenal metastases.” Adrenalectomy appeared to be a reasonable treatment for suitable patients. SABR was a valid alternative for nonsurgical candidates, but they did not recommend PCA until more long-term outcomes were available (Cancer Treat Rev. 2014;40:838-46).
Dr. Lawrence concurred, based on her case study and literature review. She said RF ablation “offers patients a minimally invasive option for treating focal adrenal tumors” and is a “safe and effective procedure … in patients who are poor surgical candidates or refuse adrenalectomy.” More long-term follow-up studies are needed before RF ablation could replace adrenalectomy, she noted.
ORLANDO – Radiofrequency (RF) ablation is a safe and effective procedure for treating focal adrenal tumors in patients who are poor surgical candidates or who refuse adrenalectomy. With a short treatment time and minimal hospital stay, RF ablation can provide rapid clinical and biochemical improvement.
Dr. Lima Lawrence, an internal medicine resident at the University of Illinois at Chicago/Advocate Christ Medical Center in Oak Lawn, presented a case report and a review of the literature during an oral abstract session at the annual meeting of the American Association of Clinical Endocrinologists. The patient was a 65-year-old woman who presented with weight gain, decreased energy, and muscle weakness. On physical exam, she was hypertensive, anxious, obese, and had prominent supraclavicular fat pads. Salivary cortisol and overnight dexamethasone suppression tests were both elevated, and ACTH levels were depressed, confirming the diagnosis of a cortisol-secreting tumor causing adrenal Cushing’s syndrome. Computed tomography (CT) surveillance showed a progressively enlarging right-sided adrenal mass. A peritoneal biopsy revealed a low-grade serous neoplasm of peritoneal origin.
Her medical history included type 2 diabetes, uncontrolled hypertension, mixed connective tissue disease, depression, and total abdominal hysterectomy with bilateral salpingo-oophorectomy for ovarian cancer.
Dr. Lawrence said the patient had been scheduled for adrenalectomy, but it was not performed because of an intraoperative finding of peritoneal studding from what turned out to be metastatic ovarian cancer. Therefore, she underwent CT-guided RF ablation of the adrenal mass using a 14-gauge probe that heated a 3.5-cm ablation zone to 50-60 C for 8-10 minutes to achieve complete tumor necrosis.
The patient showed dramatic “clinical and biochemical improvement,” Dr. Lawrence said. The patient had no procedural complications and no blood loss and was observed for 23 hours before being discharged to home. A CT scan 8 weeks later showed a slightly decreased mass with marked decreased radiographic attenuation post-contrast from 30.2 Hounsfield Units (HU) preoperatively to 17 HU on follow-up.
Potential adverse outcomes using RF ablation include a risk of pneumothorax, hemothorax, and tumor seeding along the catheter track, but this last possibility can be mitigated by continuing to heat the RF probe as it is withdrawn.
Published evidence supports use of RF ablation. “To date there have been no randomized clinical trials comparing the safety, efficacy, and survival benefits of adrenalectomy vs. radio frequency ablation,” she said. It may not be feasible to do a randomized trial. But a review of the literature generally supports the efficacy of the technique although the publications each involved a small series of patients, Dr. Lawrence said in an interview.
A 2003 series (Cancer. 2003;97:554-60) of 15 primary or metastatic adrenal cell carcinomas that were unresectable or were in patients who were not surgical candidates showed nonenhancement and no growth in 8 (53%) at a mean follow-up of 10.3 months. Eight of the 12 tumors of 5 cm or smaller had complete loss of radiographic enhancement and a decrease in size.
From a retrospective series of 13 patients with functional adrenal neoplasms over 7 years, there was 100% resolution of biochemical abnormalities and clinical symptoms at a mean follow-up of 21.2 months. One small pneumothorax and one limited hemothorax occurred, neither of which required hospital admission. There were two instances of transient, self-remitting hypertension associated with the procedures (Radiology. 2011;258:308-16).
In 2015, one group of investigators followed 11 patients for 12 weeks postprocedure. Eight of nine patients with Conn’s syndrome attained normal serum aldosterone levels. One with a nodule close to the inferior vena cava had incomplete ablation. Two of two Cushing’s patients had normal cortisol levels after the procedure (J Vasc Interv Radiol. 2015;26:1459-64).
A retrospective analysis of 16 adrenal metastases showed that 13 (81%) had no local progression over 14 months after ablation. In two of three functional adrenal neoplasms, clinical and biochemical abnormalities resolved (Eur J Radiol. 2012.81:1717-23).
A retrospective series of 10 adrenal metastases showed that one recurred at 7 months after image-guided thermal ablation, with no recurrence of the rest at 26.6 months. There was no tumor recurrence for any of the cases of metastatic disease localized to the RF ablation site (J Vasc Interv Radiol. 2014;25:593-8).
Results were somewhat less good in a retrospective evaluation of 35 patients with unresectable adrenal masses over 9 years. Although 33 of 35 (94%) lost tumor enhancement after the initial adrenal RF ablation, there was local tumor progression in 8 of 35 (23%) patients at a mean follow-up of 30.1 months (Radiology. 2015;277:584-93).
Finally, Dr. Lawrence discussed a systematic literature review on adrenalectomy vs. stereotactic ablative body radiotherapy (SABR) and percutaneous catheter ablation (PCA) in the treatment of adrenal metastases: 30 papers on adrenalectomy on 818 patients; 9 papers on SABR on 178 patients; and 6 papers on PCA, including RF ablation, on 51 patients. The authors concluded that there was “insufficient evidence to determine the best local treatment modality for isolated or limited adrenal metastases.” Adrenalectomy appeared to be a reasonable treatment for suitable patients. SABR was a valid alternative for nonsurgical candidates, but they did not recommend PCA until more long-term outcomes were available (Cancer Treat Rev. 2014;40:838-46).
Dr. Lawrence concurred, based on her case study and literature review. She said RF ablation “offers patients a minimally invasive option for treating focal adrenal tumors” and is a “safe and effective procedure … in patients who are poor surgical candidates or refuse adrenalectomy.” More long-term follow-up studies are needed before RF ablation could replace adrenalectomy, she noted.
EXPERT ANALYSIS AT AACE 2016
Initial Monotherapy Safe in Type 2 Diabetes but Must Be Abandoned Quickly if Ineffective
ORLANDO – Starting a patient with newly diagnosed type 2 diabetes mellitus on a single agent is a reasonable approach, but leaving them too long on monotherapy that proves ineffective is insupportable by available data, according to Dr. Richard Pratley.
“It’s not so much whether or not you start with a single drug or with two drugs, but it’s the fact that we don’t advance therapy when appropriate,” he said. Allowing hyperglycemia to persist increases health risks. “We can do better with a combination therapy initially, but we also do better just by advancing therapy sequentially better. Nobody has to spend 17 months with an A1c of eight and a half percent or so,” said Dr. Pratley, director of the Florida Hospital Diabetes Institute and an adjunct professor at the Sanford Burnham Medical Research Institute, both in Orlando.
It is reasonable on several grounds to start with a single agent, but “starting” is the operative word. No matter whether one initiates therapy with one drug or a combination, an essential component of any therapy is lifestyle modification, possibly including medically assisted weight loss, he noted during a point/counterpoint session at the annual meeting of the American Association of Clinical Endocrinologists (AACE).
Guidelines from both the American Diabetes Association and the European Association for the Study of Diabetes recommend starting with metformin and then moving on stepwise to add a second drug, then a third as needed, with each step separated by 3 months. If further therapy is needed, then insulin can be added after another 3-6 months. These organizations have taken the position that metformin “remains the optimal drug for monotherapy,” based on low cost, proven safety, weight neutrality, and possible cardiovascular benefits. However, if the hemoglobin A1c is 9% or greater, initial combination therapy with metformin plus another drug may achieve an HbA1c target more quickly than sequential therapy, he said.
In contrast to the ADA/EASD guidelines, the AACE recommends monotherapy with one of several agents only if the entry HbA1c is less than 7.5%, advancing to dual therapy after 1 month if still not at goal. If the entry HbA1c is at or above 7.5% but less than 9.0%, dual therapy including metformin is advised, moving to triple therapy after 1 month if the patient is still not at goal. Any patient with an entry HbA1c above 9% can start with dual or triple therapy if symptoms are absent, but if symptoms are present, insulin is recommended with or without other agents. “Combination therapy is usually required and should involve agents with complementary mechanisms of action,” according to an AACE consensus statement.
With an entry HbA1c between 7.5% and 9%, the decision to use monotherapy or a combination is a “gray area,” Dr. Pratley said. The arguments in favor of combination therapy are that a single agent may have limited efficacy, multiple metabolic abnormalities contribute to hyperglycemia, drugs with complementary mechanisms of action will work better, and getting to an HbA1c goal faster will improve outcomes. With twelve classes of antihyperglycemic agents, however, to treat type 2 diabetes mellitus (2DM), the combination possibilities are almost endless. Even excluding insulin, there are more than 150 possible recommended two-drug combinations.
Based on several studies, Dr. Pratley countered these arguments, saying that one agent is often enough to reach goal when the entry HbA1c is 7.5-9%. Furthermore, evidence is lacking that early control improves outcomes or the durability of response, and there is no comparative efficacy data on initial combination therapies. Initial combinations limit personalizing therapy because of diminished flexibility of dosing, and attribution of side effects to one agent or another may be blurred.
Since diabetes patients may have comorbidities, polypharmacy for 2DM may present more drug interactions and contribute to adverse effects, aside from the risk of severe hypoglycemia from the antidiabetic drugs themselves. Dr. Pratley said that a higher pill burden may decrease adherence, more drugs add to cost, and insurance companies may cover only stepped care.
Comparing drug prices, he said initial monotherapy with metformin for the 1.7 million newly diagnosed 2DM patients in the United States every year could save $1 billion vs. some combination therapies. He said he found metformin free at one supermarket chain pharmacy, while a bag of potting soil was nearly $5 at a store next door, making metformin “cheaper than dirt.”
Citing a retrospective, observational study of more than 7,200 complete courses of treatment for 2DM (Diabetes Care. 2004;27:1535-40), Dr. Pratley said patients had several years of excess glycemic burden before therapy was intensified, which he called “the real problem.” He noted that initial monotherapy works for patients with HbA1c less than 9.0%, with sequential personalized therapy to minimize side effects and cost. “Clinical inertia must be avoided,” he said, so the key to good initial monotherapy is not to delay adding drugs when necessary.
Session moderator Dr. Daniel Einhorn, medical director of the Scripps Whittier Diabetes Institute in La Jolla, Calif., commented in an interview that Dr. Pratley “made some very cogent arguments for minimizing the number of agents used and that the counter argument more recently for multiple-agent therapy has to be looked at in the context of what insurance companies will accept and the patients can tolerate.” He agreed that sequential therapy “is perfectly reasonable” as long as it is done rapidly, meaning waiting no longer than 3 months to add needed therapy. “If you go quickly in sequential therapy, it’s like the equivalent to starting combination from the beginning,” he said.
Dr. Pratley has received research support from Lexcon, Lilly, Merck, Novo Nordisk, sanofi, and Takeda and has been a consultant for AstraZeneca, Boehringer, GlaxoSmithKline, Lilly, Merck, Novo Nordisk, sanofi, and Takeda. All his honoraria are directed toward Florida Hospital, a nonprofit organization, and Dr. Pratley does not receive direct or indirect compensation for these services.
CORRECTION: An earlier version of this article misidentified the top photo of Dr. Daniel Einhorn.
ORLANDO – Starting a patient with newly diagnosed type 2 diabetes mellitus on a single agent is a reasonable approach, but leaving them too long on monotherapy that proves ineffective is insupportable by available data, according to Dr. Richard Pratley.
“It’s not so much whether or not you start with a single drug or with two drugs, but it’s the fact that we don’t advance therapy when appropriate,” he said. Allowing hyperglycemia to persist increases health risks. “We can do better with a combination therapy initially, but we also do better just by advancing therapy sequentially better. Nobody has to spend 17 months with an A1c of eight and a half percent or so,” said Dr. Pratley, director of the Florida Hospital Diabetes Institute and an adjunct professor at the Sanford Burnham Medical Research Institute, both in Orlando.
It is reasonable on several grounds to start with a single agent, but “starting” is the operative word. No matter whether one initiates therapy with one drug or a combination, an essential component of any therapy is lifestyle modification, possibly including medically assisted weight loss, he noted during a point/counterpoint session at the annual meeting of the American Association of Clinical Endocrinologists (AACE).
Guidelines from both the American Diabetes Association and the European Association for the Study of Diabetes recommend starting with metformin and then moving on stepwise to add a second drug, then a third as needed, with each step separated by 3 months. If further therapy is needed, then insulin can be added after another 3-6 months. These organizations have taken the position that metformin “remains the optimal drug for monotherapy,” based on low cost, proven safety, weight neutrality, and possible cardiovascular benefits. However, if the hemoglobin A1c is 9% or greater, initial combination therapy with metformin plus another drug may achieve an HbA1c target more quickly than sequential therapy, he said.
In contrast to the ADA/EASD guidelines, the AACE recommends monotherapy with one of several agents only if the entry HbA1c is less than 7.5%, advancing to dual therapy after 1 month if still not at goal. If the entry HbA1c is at or above 7.5% but less than 9.0%, dual therapy including metformin is advised, moving to triple therapy after 1 month if the patient is still not at goal. Any patient with an entry HbA1c above 9% can start with dual or triple therapy if symptoms are absent, but if symptoms are present, insulin is recommended with or without other agents. “Combination therapy is usually required and should involve agents with complementary mechanisms of action,” according to an AACE consensus statement.
With an entry HbA1c between 7.5% and 9%, the decision to use monotherapy or a combination is a “gray area,” Dr. Pratley said. The arguments in favor of combination therapy are that a single agent may have limited efficacy, multiple metabolic abnormalities contribute to hyperglycemia, drugs with complementary mechanisms of action will work better, and getting to an HbA1c goal faster will improve outcomes. With twelve classes of antihyperglycemic agents, however, to treat type 2 diabetes mellitus (2DM), the combination possibilities are almost endless. Even excluding insulin, there are more than 150 possible recommended two-drug combinations.
Based on several studies, Dr. Pratley countered these arguments, saying that one agent is often enough to reach goal when the entry HbA1c is 7.5-9%. Furthermore, evidence is lacking that early control improves outcomes or the durability of response, and there is no comparative efficacy data on initial combination therapies. Initial combinations limit personalizing therapy because of diminished flexibility of dosing, and attribution of side effects to one agent or another may be blurred.
Since diabetes patients may have comorbidities, polypharmacy for 2DM may present more drug interactions and contribute to adverse effects, aside from the risk of severe hypoglycemia from the antidiabetic drugs themselves. Dr. Pratley said that a higher pill burden may decrease adherence, more drugs add to cost, and insurance companies may cover only stepped care.
Comparing drug prices, he said initial monotherapy with metformin for the 1.7 million newly diagnosed 2DM patients in the United States every year could save $1 billion vs. some combination therapies. He said he found metformin free at one supermarket chain pharmacy, while a bag of potting soil was nearly $5 at a store next door, making metformin “cheaper than dirt.”
Citing a retrospective, observational study of more than 7,200 complete courses of treatment for 2DM (Diabetes Care. 2004;27:1535-40), Dr. Pratley said patients had several years of excess glycemic burden before therapy was intensified, which he called “the real problem.” He noted that initial monotherapy works for patients with HbA1c less than 9.0%, with sequential personalized therapy to minimize side effects and cost. “Clinical inertia must be avoided,” he said, so the key to good initial monotherapy is not to delay adding drugs when necessary.
Session moderator Dr. Daniel Einhorn, medical director of the Scripps Whittier Diabetes Institute in La Jolla, Calif., commented in an interview that Dr. Pratley “made some very cogent arguments for minimizing the number of agents used and that the counter argument more recently for multiple-agent therapy has to be looked at in the context of what insurance companies will accept and the patients can tolerate.” He agreed that sequential therapy “is perfectly reasonable” as long as it is done rapidly, meaning waiting no longer than 3 months to add needed therapy. “If you go quickly in sequential therapy, it’s like the equivalent to starting combination from the beginning,” he said.
Dr. Pratley has received research support from Lexcon, Lilly, Merck, Novo Nordisk, sanofi, and Takeda and has been a consultant for AstraZeneca, Boehringer, GlaxoSmithKline, Lilly, Merck, Novo Nordisk, sanofi, and Takeda. All his honoraria are directed toward Florida Hospital, a nonprofit organization, and Dr. Pratley does not receive direct or indirect compensation for these services.
CORRECTION: An earlier version of this article misidentified the top photo of Dr. Daniel Einhorn.
ORLANDO – Starting a patient with newly diagnosed type 2 diabetes mellitus on a single agent is a reasonable approach, but leaving them too long on monotherapy that proves ineffective is insupportable by available data, according to Dr. Richard Pratley.
“It’s not so much whether or not you start with a single drug or with two drugs, but it’s the fact that we don’t advance therapy when appropriate,” he said. Allowing hyperglycemia to persist increases health risks. “We can do better with a combination therapy initially, but we also do better just by advancing therapy sequentially better. Nobody has to spend 17 months with an A1c of eight and a half percent or so,” said Dr. Pratley, director of the Florida Hospital Diabetes Institute and an adjunct professor at the Sanford Burnham Medical Research Institute, both in Orlando.
It is reasonable on several grounds to start with a single agent, but “starting” is the operative word. No matter whether one initiates therapy with one drug or a combination, an essential component of any therapy is lifestyle modification, possibly including medically assisted weight loss, he noted during a point/counterpoint session at the annual meeting of the American Association of Clinical Endocrinologists (AACE).
Guidelines from both the American Diabetes Association and the European Association for the Study of Diabetes recommend starting with metformin and then moving on stepwise to add a second drug, then a third as needed, with each step separated by 3 months. If further therapy is needed, then insulin can be added after another 3-6 months. These organizations have taken the position that metformin “remains the optimal drug for monotherapy,” based on low cost, proven safety, weight neutrality, and possible cardiovascular benefits. However, if the hemoglobin A1c is 9% or greater, initial combination therapy with metformin plus another drug may achieve an HbA1c target more quickly than sequential therapy, he said.
In contrast to the ADA/EASD guidelines, the AACE recommends monotherapy with one of several agents only if the entry HbA1c is less than 7.5%, advancing to dual therapy after 1 month if still not at goal. If the entry HbA1c is at or above 7.5% but less than 9.0%, dual therapy including metformin is advised, moving to triple therapy after 1 month if the patient is still not at goal. Any patient with an entry HbA1c above 9% can start with dual or triple therapy if symptoms are absent, but if symptoms are present, insulin is recommended with or without other agents. “Combination therapy is usually required and should involve agents with complementary mechanisms of action,” according to an AACE consensus statement.
With an entry HbA1c between 7.5% and 9%, the decision to use monotherapy or a combination is a “gray area,” Dr. Pratley said. The arguments in favor of combination therapy are that a single agent may have limited efficacy, multiple metabolic abnormalities contribute to hyperglycemia, drugs with complementary mechanisms of action will work better, and getting to an HbA1c goal faster will improve outcomes. With twelve classes of antihyperglycemic agents, however, to treat type 2 diabetes mellitus (2DM), the combination possibilities are almost endless. Even excluding insulin, there are more than 150 possible recommended two-drug combinations.
Based on several studies, Dr. Pratley countered these arguments, saying that one agent is often enough to reach goal when the entry HbA1c is 7.5-9%. Furthermore, evidence is lacking that early control improves outcomes or the durability of response, and there is no comparative efficacy data on initial combination therapies. Initial combinations limit personalizing therapy because of diminished flexibility of dosing, and attribution of side effects to one agent or another may be blurred.
Since diabetes patients may have comorbidities, polypharmacy for 2DM may present more drug interactions and contribute to adverse effects, aside from the risk of severe hypoglycemia from the antidiabetic drugs themselves. Dr. Pratley said that a higher pill burden may decrease adherence, more drugs add to cost, and insurance companies may cover only stepped care.
Comparing drug prices, he said initial monotherapy with metformin for the 1.7 million newly diagnosed 2DM patients in the United States every year could save $1 billion vs. some combination therapies. He said he found metformin free at one supermarket chain pharmacy, while a bag of potting soil was nearly $5 at a store next door, making metformin “cheaper than dirt.”
Citing a retrospective, observational study of more than 7,200 complete courses of treatment for 2DM (Diabetes Care. 2004;27:1535-40), Dr. Pratley said patients had several years of excess glycemic burden before therapy was intensified, which he called “the real problem.” He noted that initial monotherapy works for patients with HbA1c less than 9.0%, with sequential personalized therapy to minimize side effects and cost. “Clinical inertia must be avoided,” he said, so the key to good initial monotherapy is not to delay adding drugs when necessary.
Session moderator Dr. Daniel Einhorn, medical director of the Scripps Whittier Diabetes Institute in La Jolla, Calif., commented in an interview that Dr. Pratley “made some very cogent arguments for minimizing the number of agents used and that the counter argument more recently for multiple-agent therapy has to be looked at in the context of what insurance companies will accept and the patients can tolerate.” He agreed that sequential therapy “is perfectly reasonable” as long as it is done rapidly, meaning waiting no longer than 3 months to add needed therapy. “If you go quickly in sequential therapy, it’s like the equivalent to starting combination from the beginning,” he said.
Dr. Pratley has received research support from Lexcon, Lilly, Merck, Novo Nordisk, sanofi, and Takeda and has been a consultant for AstraZeneca, Boehringer, GlaxoSmithKline, Lilly, Merck, Novo Nordisk, sanofi, and Takeda. All his honoraria are directed toward Florida Hospital, a nonprofit organization, and Dr. Pratley does not receive direct or indirect compensation for these services.
CORRECTION: An earlier version of this article misidentified the top photo of Dr. Daniel Einhorn.
EXPERT ANALYSIS FROM AACE 2016
Initial monotherapy safe in type 2 diabetes but must be abandoned quickly if ineffective
ORLANDO – Starting a patient with newly diagnosed type 2 diabetes mellitus on a single agent is a reasonable approach, but leaving them too long on monotherapy that proves ineffective is insupportable by available data, according to Dr. Richard Pratley.
“It’s not so much whether or not you start with a single drug or with two drugs, but it’s the fact that we don’t advance therapy when appropriate,” he said. Allowing hyperglycemia to persist increases health risks. “We can do better with a combination therapy initially, but we also do better just by advancing therapy sequentially better. Nobody has to spend 17 months with an A1c of eight and a half percent or so,” said Dr. Pratley, director of the Florida Hospital Diabetes Institute and an adjunct professor at the Sanford Burnham Medical Research Institute, both in Orlando.
It is reasonable on several grounds to start with a single agent, but “starting” is the operative word. No matter whether one initiates therapy with one drug or a combination, an essential component of any therapy is lifestyle modification, possibly including medically assisted weight loss, he noted during a point/counterpoint session at the annual meeting of the American Association of Clinical Endocrinologists (AACE).
Guidelines from both the American Diabetes Association and the European Association for the Study of Diabetes recommend starting with metformin and then moving on stepwise to add a second drug, then a third as needed, with each step separated by 3 months. If further therapy is needed, then insulin can be added after another 3-6 months. These organizations have taken the position that metformin “remains the optimal drug for monotherapy,” based on low cost, proven safety, weight neutrality, and possible cardiovascular benefits. However, if the hemoglobin A1c is 9% or greater, initial combination therapy with metformin plus another drug may achieve an HbA1c target more quickly than sequential therapy, he said.
In contrast to the ADA/EASD guidelines, the AACE recommends monotherapy with one of several agents only if the entry HbA1c is less than 7.5%, advancing to dual therapy after 1 month if still not at goal. If the entry HbA1c is at or above 7.5% but less than 9.0%, dual therapy including metformin is advised, moving to triple therapy after 1 month if the patient is still not at goal. Any patient with an entry HbA1c above 9% can start with dual or triple therapy if symptoms are absent, but if symptoms are present, insulin is recommended with or without other agents. “Combination therapy is usually required and should involve agents with complementary mechanisms of action,” according to an AACE consensus statement.
With an entry HbA1c between 7.5% and 9%, the decision to use monotherapy or a combination is a “gray area,” Dr. Pratley said. The arguments in favor of combination therapy are that a single agent may have limited efficacy, multiple metabolic abnormalities contribute to hyperglycemia, drugs with complementary mechanisms of action will work better, and getting to an HbA1c goal faster will improve outcomes. With twelve classes of antihyperglycemic agents, however, to treat type 2 diabetes mellitus (2DM), the combination possibilities are almost endless. Even excluding insulin, there are more than 150 possible recommended two-drug combinations.
Based on several studies, Dr. Pratley countered these arguments, saying that one agent is often enough to reach goal when the entry HbA1c is 7.5-9%. Furthermore, evidence is lacking that early control improves outcomes or the durability of response, and there is no comparative efficacy data on initial combination therapies. Initial combinations limit personalizing therapy because of diminished flexibility of dosing, and attribution of side effects to one agent or another may be blurred.
Since diabetes patients may have comorbidities, polypharmacy for 2DM may present more drug interactions and contribute to adverse effects, aside from the risk of severe hypoglycemia from the antidiabetic drugs themselves. Dr. Pratley said that a higher pill burden may decrease adherence, more drugs add to cost, and insurance companies may cover only stepped care.
Comparing drug prices, he said initial monotherapy with metformin for the 1.7 million newly diagnosed 2DM patients in the United States every year could save $1 billion vs. some combination therapies. He said he found metformin free at one supermarket chain pharmacy, while a bag of potting soil was nearly $5 at a store next door, making metformin “cheaper than dirt.”
Citing a retrospective, observational study of more than 7,200 complete courses of treatment for 2DM (Diabetes Care. 2004;27:1535-40), Dr. Pratley said patients had several years of excess glycemic burden before therapy was intensified, which he called “the real problem.” He noted that initial monotherapy works for patients with HbA1c less than 9.0%, with sequential personalized therapy to minimize side effects and cost. “Clinical inertia must be avoided,” he said, so the key to good initial monotherapy is not to delay adding drugs when necessary.
Session moderator Dr. Daniel Einhorn, medical director of the Scripps Whittier Diabetes Institute in La Jolla, Calif., commented in an interview that Dr. Pratley “made some very cogent arguments for minimizing the number of agents used and that the counter argument more recently for multiple-agent therapy has to be looked at in the context of what insurance companies will accept and the patients can tolerate.” He agreed that sequential therapy “is perfectly reasonable” as long as it is done rapidly, meaning waiting no longer than 3 months to add needed therapy. “If you go quickly in sequential therapy, it’s like the equivalent to starting combination from the beginning,” he said.
Dr. Pratley has received research support from Lexcon, Lilly, Merck, Novo Nordisk, sanofi, and Takeda and has been a consultant for AstraZeneca, Boehringer, GlaxoSmithKline, Lilly, Merck, Novo Nordisk, sanofi, and Takeda. All his honoraria are directed toward Florida Hospital, a nonprofit organization, and Dr. Pratley does not receive direct or indirect compensation for these services.
CORRECTION: An earlier version of this article misidentified the top photo of Dr. Daniel Einhorn.
ORLANDO – Starting a patient with newly diagnosed type 2 diabetes mellitus on a single agent is a reasonable approach, but leaving them too long on monotherapy that proves ineffective is insupportable by available data, according to Dr. Richard Pratley.
“It’s not so much whether or not you start with a single drug or with two drugs, but it’s the fact that we don’t advance therapy when appropriate,” he said. Allowing hyperglycemia to persist increases health risks. “We can do better with a combination therapy initially, but we also do better just by advancing therapy sequentially better. Nobody has to spend 17 months with an A1c of eight and a half percent or so,” said Dr. Pratley, director of the Florida Hospital Diabetes Institute and an adjunct professor at the Sanford Burnham Medical Research Institute, both in Orlando.
It is reasonable on several grounds to start with a single agent, but “starting” is the operative word. No matter whether one initiates therapy with one drug or a combination, an essential component of any therapy is lifestyle modification, possibly including medically assisted weight loss, he noted during a point/counterpoint session at the annual meeting of the American Association of Clinical Endocrinologists (AACE).
Guidelines from both the American Diabetes Association and the European Association for the Study of Diabetes recommend starting with metformin and then moving on stepwise to add a second drug, then a third as needed, with each step separated by 3 months. If further therapy is needed, then insulin can be added after another 3-6 months. These organizations have taken the position that metformin “remains the optimal drug for monotherapy,” based on low cost, proven safety, weight neutrality, and possible cardiovascular benefits. However, if the hemoglobin A1c is 9% or greater, initial combination therapy with metformin plus another drug may achieve an HbA1c target more quickly than sequential therapy, he said.
In contrast to the ADA/EASD guidelines, the AACE recommends monotherapy with one of several agents only if the entry HbA1c is less than 7.5%, advancing to dual therapy after 1 month if still not at goal. If the entry HbA1c is at or above 7.5% but less than 9.0%, dual therapy including metformin is advised, moving to triple therapy after 1 month if the patient is still not at goal. Any patient with an entry HbA1c above 9% can start with dual or triple therapy if symptoms are absent, but if symptoms are present, insulin is recommended with or without other agents. “Combination therapy is usually required and should involve agents with complementary mechanisms of action,” according to an AACE consensus statement.
With an entry HbA1c between 7.5% and 9%, the decision to use monotherapy or a combination is a “gray area,” Dr. Pratley said. The arguments in favor of combination therapy are that a single agent may have limited efficacy, multiple metabolic abnormalities contribute to hyperglycemia, drugs with complementary mechanisms of action will work better, and getting to an HbA1c goal faster will improve outcomes. With twelve classes of antihyperglycemic agents, however, to treat type 2 diabetes mellitus (2DM), the combination possibilities are almost endless. Even excluding insulin, there are more than 150 possible recommended two-drug combinations.
Based on several studies, Dr. Pratley countered these arguments, saying that one agent is often enough to reach goal when the entry HbA1c is 7.5-9%. Furthermore, evidence is lacking that early control improves outcomes or the durability of response, and there is no comparative efficacy data on initial combination therapies. Initial combinations limit personalizing therapy because of diminished flexibility of dosing, and attribution of side effects to one agent or another may be blurred.
Since diabetes patients may have comorbidities, polypharmacy for 2DM may present more drug interactions and contribute to adverse effects, aside from the risk of severe hypoglycemia from the antidiabetic drugs themselves. Dr. Pratley said that a higher pill burden may decrease adherence, more drugs add to cost, and insurance companies may cover only stepped care.
Comparing drug prices, he said initial monotherapy with metformin for the 1.7 million newly diagnosed 2DM patients in the United States every year could save $1 billion vs. some combination therapies. He said he found metformin free at one supermarket chain pharmacy, while a bag of potting soil was nearly $5 at a store next door, making metformin “cheaper than dirt.”
Citing a retrospective, observational study of more than 7,200 complete courses of treatment for 2DM (Diabetes Care. 2004;27:1535-40), Dr. Pratley said patients had several years of excess glycemic burden before therapy was intensified, which he called “the real problem.” He noted that initial monotherapy works for patients with HbA1c less than 9.0%, with sequential personalized therapy to minimize side effects and cost. “Clinical inertia must be avoided,” he said, so the key to good initial monotherapy is not to delay adding drugs when necessary.
Session moderator Dr. Daniel Einhorn, medical director of the Scripps Whittier Diabetes Institute in La Jolla, Calif., commented in an interview that Dr. Pratley “made some very cogent arguments for minimizing the number of agents used and that the counter argument more recently for multiple-agent therapy has to be looked at in the context of what insurance companies will accept and the patients can tolerate.” He agreed that sequential therapy “is perfectly reasonable” as long as it is done rapidly, meaning waiting no longer than 3 months to add needed therapy. “If you go quickly in sequential therapy, it’s like the equivalent to starting combination from the beginning,” he said.
Dr. Pratley has received research support from Lexcon, Lilly, Merck, Novo Nordisk, sanofi, and Takeda and has been a consultant for AstraZeneca, Boehringer, GlaxoSmithKline, Lilly, Merck, Novo Nordisk, sanofi, and Takeda. All his honoraria are directed toward Florida Hospital, a nonprofit organization, and Dr. Pratley does not receive direct or indirect compensation for these services.
CORRECTION: An earlier version of this article misidentified the top photo of Dr. Daniel Einhorn.
ORLANDO – Starting a patient with newly diagnosed type 2 diabetes mellitus on a single agent is a reasonable approach, but leaving them too long on monotherapy that proves ineffective is insupportable by available data, according to Dr. Richard Pratley.
“It’s not so much whether or not you start with a single drug or with two drugs, but it’s the fact that we don’t advance therapy when appropriate,” he said. Allowing hyperglycemia to persist increases health risks. “We can do better with a combination therapy initially, but we also do better just by advancing therapy sequentially better. Nobody has to spend 17 months with an A1c of eight and a half percent or so,” said Dr. Pratley, director of the Florida Hospital Diabetes Institute and an adjunct professor at the Sanford Burnham Medical Research Institute, both in Orlando.
It is reasonable on several grounds to start with a single agent, but “starting” is the operative word. No matter whether one initiates therapy with one drug or a combination, an essential component of any therapy is lifestyle modification, possibly including medically assisted weight loss, he noted during a point/counterpoint session at the annual meeting of the American Association of Clinical Endocrinologists (AACE).
Guidelines from both the American Diabetes Association and the European Association for the Study of Diabetes recommend starting with metformin and then moving on stepwise to add a second drug, then a third as needed, with each step separated by 3 months. If further therapy is needed, then insulin can be added after another 3-6 months. These organizations have taken the position that metformin “remains the optimal drug for monotherapy,” based on low cost, proven safety, weight neutrality, and possible cardiovascular benefits. However, if the hemoglobin A1c is 9% or greater, initial combination therapy with metformin plus another drug may achieve an HbA1c target more quickly than sequential therapy, he said.
In contrast to the ADA/EASD guidelines, the AACE recommends monotherapy with one of several agents only if the entry HbA1c is less than 7.5%, advancing to dual therapy after 1 month if still not at goal. If the entry HbA1c is at or above 7.5% but less than 9.0%, dual therapy including metformin is advised, moving to triple therapy after 1 month if the patient is still not at goal. Any patient with an entry HbA1c above 9% can start with dual or triple therapy if symptoms are absent, but if symptoms are present, insulin is recommended with or without other agents. “Combination therapy is usually required and should involve agents with complementary mechanisms of action,” according to an AACE consensus statement.
With an entry HbA1c between 7.5% and 9%, the decision to use monotherapy or a combination is a “gray area,” Dr. Pratley said. The arguments in favor of combination therapy are that a single agent may have limited efficacy, multiple metabolic abnormalities contribute to hyperglycemia, drugs with complementary mechanisms of action will work better, and getting to an HbA1c goal faster will improve outcomes. With twelve classes of antihyperglycemic agents, however, to treat type 2 diabetes mellitus (2DM), the combination possibilities are almost endless. Even excluding insulin, there are more than 150 possible recommended two-drug combinations.
Based on several studies, Dr. Pratley countered these arguments, saying that one agent is often enough to reach goal when the entry HbA1c is 7.5-9%. Furthermore, evidence is lacking that early control improves outcomes or the durability of response, and there is no comparative efficacy data on initial combination therapies. Initial combinations limit personalizing therapy because of diminished flexibility of dosing, and attribution of side effects to one agent or another may be blurred.
Since diabetes patients may have comorbidities, polypharmacy for 2DM may present more drug interactions and contribute to adverse effects, aside from the risk of severe hypoglycemia from the antidiabetic drugs themselves. Dr. Pratley said that a higher pill burden may decrease adherence, more drugs add to cost, and insurance companies may cover only stepped care.
Comparing drug prices, he said initial monotherapy with metformin for the 1.7 million newly diagnosed 2DM patients in the United States every year could save $1 billion vs. some combination therapies. He said he found metformin free at one supermarket chain pharmacy, while a bag of potting soil was nearly $5 at a store next door, making metformin “cheaper than dirt.”
Citing a retrospective, observational study of more than 7,200 complete courses of treatment for 2DM (Diabetes Care. 2004;27:1535-40), Dr. Pratley said patients had several years of excess glycemic burden before therapy was intensified, which he called “the real problem.” He noted that initial monotherapy works for patients with HbA1c less than 9.0%, with sequential personalized therapy to minimize side effects and cost. “Clinical inertia must be avoided,” he said, so the key to good initial monotherapy is not to delay adding drugs when necessary.
Session moderator Dr. Daniel Einhorn, medical director of the Scripps Whittier Diabetes Institute in La Jolla, Calif., commented in an interview that Dr. Pratley “made some very cogent arguments for minimizing the number of agents used and that the counter argument more recently for multiple-agent therapy has to be looked at in the context of what insurance companies will accept and the patients can tolerate.” He agreed that sequential therapy “is perfectly reasonable” as long as it is done rapidly, meaning waiting no longer than 3 months to add needed therapy. “If you go quickly in sequential therapy, it’s like the equivalent to starting combination from the beginning,” he said.
Dr. Pratley has received research support from Lexcon, Lilly, Merck, Novo Nordisk, sanofi, and Takeda and has been a consultant for AstraZeneca, Boehringer, GlaxoSmithKline, Lilly, Merck, Novo Nordisk, sanofi, and Takeda. All his honoraria are directed toward Florida Hospital, a nonprofit organization, and Dr. Pratley does not receive direct or indirect compensation for these services.
CORRECTION: An earlier version of this article misidentified the top photo of Dr. Daniel Einhorn.
EXPERT ANALYSIS FROM AACE 2016
ASCT still a player for multiple myeloma
Even in this era of novel therapies for multiple myeloma, for patients with newly diagnosed disease, autologous stem cell transplant (ASCT) after chemotherapy provides benefits in terms of disease progression and extent of response, compared with chemotherapy alone. The benefit of ASCT was especially pronounced among certain groups of high-risk patients.
Novel proteasome inhibitors and immunomodulators “have dramatically increased the complete response rate and significantly extended progression-free survival and overall survival in previously untreated multiple myeloma patients,” Dr. Michele Cavo, head of the Seragnoli Institute of Hematology at the University of Bologna School of Medicine in Italy, said at a presscast in advance of the annual meeting of the American Society of Clinical Oncology.
But questions remain about how these newer agents perform, compared with high-dose melphalan (HDM) followed by ASCT, traditionally seen as the standard of care for younger and fit patients with newly diagnosed disease.
EMN02/HO95 is a large, prospective, multicenter, intergroup, randomized phase III study that addresses this question, as well as single vs. double ASCT and the use of consolidation therapy or not. The study includes patients 65 years old or younger, and the trial protocol involves induction therapy with bortezomib (Velcade)–cyclophosphamide-dexamethasone (VCD) and subsequent collection of peripheral blood stem cells.
Patients were then randomly assigned to receive bortezomib-melphalan-prednisone (VMP) or HDM as intensification therapy in centers that had a single ASCT policy. For those centers doing double (tandem) ASCT procedures, the randomization was to VMP vs. HDM + single ASCT vs. HDM + double ASCT.
Patients in each treatment arm then underwent another randomization to consolidation therapy with bortezomib-lenalidomide (Revlimid)–dexamethasone or no consolidation. All patients received lenalidomide maintenance until disease progression or toxicity. At the time of a preliminary analysis of trial data in January 2016, results from the second randomization to consolidation or no consolidation therapy were not yet complete. This first prespecified interim analysis was performed after at least 33% of the required events had occurred.
Early results show ASCT benefit
Early results on 1,266 patients (VMP, n = 512; HDM, n = 754) show that a median progression-free survival (PFS) was not yet reached after a median follow-up of 23.9 months from the first randomization (to VMP vs. HDM+ASCT), the primary endpoint of the trial.
In the overall patient population, patients achieved a significant 24% benefit in PFS when given HDM+ASCT up front (hazard ratio, 0.76 vs. VMP), and this benefit extended to certain patient subgroups, as well.
“PFS benefit with bortezomib-based ASCT was of relevance for patients at high risk of early relapse, in particular for those with revised ISS [International Staging System] stage III and high-risk cytogenetic profiles, who had a relative reduction in the risk of progression or death of 48% and 28%, respectively,” Dr. Cavo said.
Other predictors of longer PFS were ISS stage I (HR, 0.44; 95% confidence interval, 0.28-067; P less than .0001), standard risk cytogenetics (HR, 0.57; 95% CI, 0.41-0.78; P less than .0001), randomization to the HDM+ASCT arm (HR, 0.61; 95% CI, 0.45-0.82; P = .001), and less than 60% bone marrow plasma cells (HR, 0.67; 95% CI, 0.48-0.99; P = .014).
More patients receiving ASCT up front had a significantly greater reduction in tumor volume of at least 90%, as indicated by the composite of very good partial remission, complete response, and stringent complete response, which was achieved in 74.0% in the VMP arm and in 84.4% of the HDM+ASCT arm (P less than .0001).
For patients at low risk of relapse, Dr. Cavo said longer follow up will be needed to compare the different arms of the study, and future analyses will delineate the effects of consolidation or no consolidation therapy and the use of the VMP regimen, compared with single or double ASCT.
ASCO president Dr. Julie Vose said that even with effective novel agents available, older, proven approaches still retain their value. “This study demonstrated that combining the best of both worlds – initial therapy with a novel agent followed by stem cell transplant – resulted in the best patient outcomes,” she said.
Even in this era of novel therapies for multiple myeloma, for patients with newly diagnosed disease, autologous stem cell transplant (ASCT) after chemotherapy provides benefits in terms of disease progression and extent of response, compared with chemotherapy alone. The benefit of ASCT was especially pronounced among certain groups of high-risk patients.
Novel proteasome inhibitors and immunomodulators “have dramatically increased the complete response rate and significantly extended progression-free survival and overall survival in previously untreated multiple myeloma patients,” Dr. Michele Cavo, head of the Seragnoli Institute of Hematology at the University of Bologna School of Medicine in Italy, said at a presscast in advance of the annual meeting of the American Society of Clinical Oncology.
But questions remain about how these newer agents perform, compared with high-dose melphalan (HDM) followed by ASCT, traditionally seen as the standard of care for younger and fit patients with newly diagnosed disease.
EMN02/HO95 is a large, prospective, multicenter, intergroup, randomized phase III study that addresses this question, as well as single vs. double ASCT and the use of consolidation therapy or not. The study includes patients 65 years old or younger, and the trial protocol involves induction therapy with bortezomib (Velcade)–cyclophosphamide-dexamethasone (VCD) and subsequent collection of peripheral blood stem cells.
Patients were then randomly assigned to receive bortezomib-melphalan-prednisone (VMP) or HDM as intensification therapy in centers that had a single ASCT policy. For those centers doing double (tandem) ASCT procedures, the randomization was to VMP vs. HDM + single ASCT vs. HDM + double ASCT.
Patients in each treatment arm then underwent another randomization to consolidation therapy with bortezomib-lenalidomide (Revlimid)–dexamethasone or no consolidation. All patients received lenalidomide maintenance until disease progression or toxicity. At the time of a preliminary analysis of trial data in January 2016, results from the second randomization to consolidation or no consolidation therapy were not yet complete. This first prespecified interim analysis was performed after at least 33% of the required events had occurred.
Early results show ASCT benefit
Early results on 1,266 patients (VMP, n = 512; HDM, n = 754) show that a median progression-free survival (PFS) was not yet reached after a median follow-up of 23.9 months from the first randomization (to VMP vs. HDM+ASCT), the primary endpoint of the trial.
In the overall patient population, patients achieved a significant 24% benefit in PFS when given HDM+ASCT up front (hazard ratio, 0.76 vs. VMP), and this benefit extended to certain patient subgroups, as well.
“PFS benefit with bortezomib-based ASCT was of relevance for patients at high risk of early relapse, in particular for those with revised ISS [International Staging System] stage III and high-risk cytogenetic profiles, who had a relative reduction in the risk of progression or death of 48% and 28%, respectively,” Dr. Cavo said.
Other predictors of longer PFS were ISS stage I (HR, 0.44; 95% confidence interval, 0.28-067; P less than .0001), standard risk cytogenetics (HR, 0.57; 95% CI, 0.41-0.78; P less than .0001), randomization to the HDM+ASCT arm (HR, 0.61; 95% CI, 0.45-0.82; P = .001), and less than 60% bone marrow plasma cells (HR, 0.67; 95% CI, 0.48-0.99; P = .014).
More patients receiving ASCT up front had a significantly greater reduction in tumor volume of at least 90%, as indicated by the composite of very good partial remission, complete response, and stringent complete response, which was achieved in 74.0% in the VMP arm and in 84.4% of the HDM+ASCT arm (P less than .0001).
For patients at low risk of relapse, Dr. Cavo said longer follow up will be needed to compare the different arms of the study, and future analyses will delineate the effects of consolidation or no consolidation therapy and the use of the VMP regimen, compared with single or double ASCT.
ASCO president Dr. Julie Vose said that even with effective novel agents available, older, proven approaches still retain their value. “This study demonstrated that combining the best of both worlds – initial therapy with a novel agent followed by stem cell transplant – resulted in the best patient outcomes,” she said.
Even in this era of novel therapies for multiple myeloma, for patients with newly diagnosed disease, autologous stem cell transplant (ASCT) after chemotherapy provides benefits in terms of disease progression and extent of response, compared with chemotherapy alone. The benefit of ASCT was especially pronounced among certain groups of high-risk patients.
Novel proteasome inhibitors and immunomodulators “have dramatically increased the complete response rate and significantly extended progression-free survival and overall survival in previously untreated multiple myeloma patients,” Dr. Michele Cavo, head of the Seragnoli Institute of Hematology at the University of Bologna School of Medicine in Italy, said at a presscast in advance of the annual meeting of the American Society of Clinical Oncology.
But questions remain about how these newer agents perform, compared with high-dose melphalan (HDM) followed by ASCT, traditionally seen as the standard of care for younger and fit patients with newly diagnosed disease.
EMN02/HO95 is a large, prospective, multicenter, intergroup, randomized phase III study that addresses this question, as well as single vs. double ASCT and the use of consolidation therapy or not. The study includes patients 65 years old or younger, and the trial protocol involves induction therapy with bortezomib (Velcade)–cyclophosphamide-dexamethasone (VCD) and subsequent collection of peripheral blood stem cells.
Patients were then randomly assigned to receive bortezomib-melphalan-prednisone (VMP) or HDM as intensification therapy in centers that had a single ASCT policy. For those centers doing double (tandem) ASCT procedures, the randomization was to VMP vs. HDM + single ASCT vs. HDM + double ASCT.
Patients in each treatment arm then underwent another randomization to consolidation therapy with bortezomib-lenalidomide (Revlimid)–dexamethasone or no consolidation. All patients received lenalidomide maintenance until disease progression or toxicity. At the time of a preliminary analysis of trial data in January 2016, results from the second randomization to consolidation or no consolidation therapy were not yet complete. This first prespecified interim analysis was performed after at least 33% of the required events had occurred.
Early results show ASCT benefit
Early results on 1,266 patients (VMP, n = 512; HDM, n = 754) show that a median progression-free survival (PFS) was not yet reached after a median follow-up of 23.9 months from the first randomization (to VMP vs. HDM+ASCT), the primary endpoint of the trial.
In the overall patient population, patients achieved a significant 24% benefit in PFS when given HDM+ASCT up front (hazard ratio, 0.76 vs. VMP), and this benefit extended to certain patient subgroups, as well.
“PFS benefit with bortezomib-based ASCT was of relevance for patients at high risk of early relapse, in particular for those with revised ISS [International Staging System] stage III and high-risk cytogenetic profiles, who had a relative reduction in the risk of progression or death of 48% and 28%, respectively,” Dr. Cavo said.
Other predictors of longer PFS were ISS stage I (HR, 0.44; 95% confidence interval, 0.28-067; P less than .0001), standard risk cytogenetics (HR, 0.57; 95% CI, 0.41-0.78; P less than .0001), randomization to the HDM+ASCT arm (HR, 0.61; 95% CI, 0.45-0.82; P = .001), and less than 60% bone marrow plasma cells (HR, 0.67; 95% CI, 0.48-0.99; P = .014).
More patients receiving ASCT up front had a significantly greater reduction in tumor volume of at least 90%, as indicated by the composite of very good partial remission, complete response, and stringent complete response, which was achieved in 74.0% in the VMP arm and in 84.4% of the HDM+ASCT arm (P less than .0001).
For patients at low risk of relapse, Dr. Cavo said longer follow up will be needed to compare the different arms of the study, and future analyses will delineate the effects of consolidation or no consolidation therapy and the use of the VMP regimen, compared with single or double ASCT.
ASCO president Dr. Julie Vose said that even with effective novel agents available, older, proven approaches still retain their value. “This study demonstrated that combining the best of both worlds – initial therapy with a novel agent followed by stem cell transplant – resulted in the best patient outcomes,” she said.
FROM THE 2016 ASCO ANNUAL MEETING
Key clinical point: ASCT bested bortezomib for newly diagnosed younger multiple myeloma patients.
Major finding: Multiple myeloma patients showed 24% PFS prolongation with up front HDM+ASCT.
Data source: EMN02/HO95, a prospective, multicenter, intergroup, randomized phase III study of 1,266 patients.
Disclosures: The study was funded by HOVON, the Hemato Oncology Foundation for Adults in the Netherlands. Dr. Cavo disclosed relationships with Janssen, Takeda, Amgen, Bristol-Myers Squibb, and Celgene. Dr. Vose disclosed relationships with Sanofi Aventis, Seattle Genetics, Acerta, Bristol-Myers Squibb, Celgene, Genentech, GlaxoSmithKline, Incyte, Janssen Biotech, Kite Pharma, Pharmacyclics, and Spectrum Pharmaceuticals.
Precision targeting yields better phase I efficacy outcomes
Patients had significantly better outcomes when treatments were selected based on biomarker analysis of their tumors, in a meta-analysis of 346 phase I trials involving 351 study arms and 13,203 cancer patients.
Up to now, the purpose of phase I trials has been to determine safety based on adverse effects and tolerability. “Our analysis really shows that these days it is completely outdated and that with biomarker selection and especially genomic biomarkers, we can reach high response rates even in phase I trials,” Maria Schwaederle, Pharm.D., of the center for personalized cancer therapy at the University of California, San Diego, said in a presscast leading up to the annual meeting of the American Society of Clinical Oncology.
For the meta-analysis, Dr. Schwaederle and her colleagues performed a PubMed search on phase I clinical trials in cancer published in the years 2011-2013. They included studies using a single agent that reported adequate efficacy endpoints for response rate and progression-free survival (PSF). Overall survival was rarely reported and so was excluded.
Personalized therapy was defined as molecular biomarker–based selection of a treatment or at least half the patients in a trial having a specific tumor known to harbor the biomarker. The study compared outcomes based on a personalized strategy with those that were not.
“We think that the results were striking here,” Dr. Schwaederle said. “We can see that personalized therapy that is [based on] biomarker-selected treatments was really associated with significantly higher response rates and progression-free survival.” Multivariable analysis showed that response rates were sixfold higher with the personalized approach (30.6%, n = 58 in the personalized trials, vs. 4.9%, n = 293 in those not personalized; P less than .0001), and median PFS practically doubled (5.7 months, n = 7 in personalized trials vs. 2.95 months, n = 38 in those not personalized; P = .0002).
Most (98.3%) of the personalized arms used targeted agents. However, 76% of the arms using targeted agents did not select patients using a related biomarker and were thus nonpersonalized in their approach to treatment. A subanalysis showed that targeted drugs that were applied in a nontargeted manner had much worse response rates than targeted therapies applied using biomarker-based selection and were equivalent to cytotoxic therapies (personalized approach, 31.1%; nontargeted approach, 5.1%; P less than 0.0001; cytotoxic drugs, 4.7%, P = .63 vs. nonpersonalized approach). The median PFS was also similar for the nonpersonalized and cytotoxic strategies (3.3 vs. 2.5 months, P = .22).
Better targeting with genomic biomarkers
The investigators found that patients selected based on genomic (DNA) biomarkers had almost double the response rates (42%) of patients selected based on protein biomarkers (22.4%, P = .001). The reasons for this difference were not clear. “We might think that the target in the end is a protein, so we would expect maybe to see better results with protein expression,” Dr. Schwaederle said. But she pointed to the example of patients with lung cancer and epidermal growth factor receptor genetic alterations. If looking only for epidermal growth factor receptor protein overexpression, she said, “we wouldn’t see any response, but only the patients that have specific alterations in this gene respond very well.” So in that case, genetic detection appears to be more sensitive than a protein biomarker–based test.
Dr. Schwaederle addressed the question that has often arisen of whether targeted agents are just better therapies. “Our analysis showed that it is not just that the therapies are better but that targeted therapies must be given to the right patients,” she said. “Indeed, when targeted therapies were given to patients without a biomarker selection, the response rates were only about 5%.”
The response rate in the range of 40% using genetic biomarkers in these phase I trials suggests that incorporating such targeted approaches even at this early stage of drug testing may potentially yield useful information on efficacy.
Dr. Don Dizon, presscast moderator and chair of ASCO’s Cancer Communications Committee, said that precision medicine “is here and that we can use patient selection using either changes in a tumor’s DNA, called genomic changes, or even protein biomarkers and do much better than we have done in the past.”
Dr. Schwaederle noted that one limitation of her study is that more recent trials, unpublished at the time of her analysis, could influence the results today.
The study received funding from the Joan and Irwin Jacobs Philanthropic Fund. Two coauthors reported extensive financial relationships with pharmaceutical or other commercial and noncommercial entities.
Patients had significantly better outcomes when treatments were selected based on biomarker analysis of their tumors, in a meta-analysis of 346 phase I trials involving 351 study arms and 13,203 cancer patients.
Up to now, the purpose of phase I trials has been to determine safety based on adverse effects and tolerability. “Our analysis really shows that these days it is completely outdated and that with biomarker selection and especially genomic biomarkers, we can reach high response rates even in phase I trials,” Maria Schwaederle, Pharm.D., of the center for personalized cancer therapy at the University of California, San Diego, said in a presscast leading up to the annual meeting of the American Society of Clinical Oncology.
For the meta-analysis, Dr. Schwaederle and her colleagues performed a PubMed search on phase I clinical trials in cancer published in the years 2011-2013. They included studies using a single agent that reported adequate efficacy endpoints for response rate and progression-free survival (PSF). Overall survival was rarely reported and so was excluded.
Personalized therapy was defined as molecular biomarker–based selection of a treatment or at least half the patients in a trial having a specific tumor known to harbor the biomarker. The study compared outcomes based on a personalized strategy with those that were not.
“We think that the results were striking here,” Dr. Schwaederle said. “We can see that personalized therapy that is [based on] biomarker-selected treatments was really associated with significantly higher response rates and progression-free survival.” Multivariable analysis showed that response rates were sixfold higher with the personalized approach (30.6%, n = 58 in the personalized trials, vs. 4.9%, n = 293 in those not personalized; P less than .0001), and median PFS practically doubled (5.7 months, n = 7 in personalized trials vs. 2.95 months, n = 38 in those not personalized; P = .0002).
Most (98.3%) of the personalized arms used targeted agents. However, 76% of the arms using targeted agents did not select patients using a related biomarker and were thus nonpersonalized in their approach to treatment. A subanalysis showed that targeted drugs that were applied in a nontargeted manner had much worse response rates than targeted therapies applied using biomarker-based selection and were equivalent to cytotoxic therapies (personalized approach, 31.1%; nontargeted approach, 5.1%; P less than 0.0001; cytotoxic drugs, 4.7%, P = .63 vs. nonpersonalized approach). The median PFS was also similar for the nonpersonalized and cytotoxic strategies (3.3 vs. 2.5 months, P = .22).
Better targeting with genomic biomarkers
The investigators found that patients selected based on genomic (DNA) biomarkers had almost double the response rates (42%) of patients selected based on protein biomarkers (22.4%, P = .001). The reasons for this difference were not clear. “We might think that the target in the end is a protein, so we would expect maybe to see better results with protein expression,” Dr. Schwaederle said. But she pointed to the example of patients with lung cancer and epidermal growth factor receptor genetic alterations. If looking only for epidermal growth factor receptor protein overexpression, she said, “we wouldn’t see any response, but only the patients that have specific alterations in this gene respond very well.” So in that case, genetic detection appears to be more sensitive than a protein biomarker–based test.
Dr. Schwaederle addressed the question that has often arisen of whether targeted agents are just better therapies. “Our analysis showed that it is not just that the therapies are better but that targeted therapies must be given to the right patients,” she said. “Indeed, when targeted therapies were given to patients without a biomarker selection, the response rates were only about 5%.”
The response rate in the range of 40% using genetic biomarkers in these phase I trials suggests that incorporating such targeted approaches even at this early stage of drug testing may potentially yield useful information on efficacy.
Dr. Don Dizon, presscast moderator and chair of ASCO’s Cancer Communications Committee, said that precision medicine “is here and that we can use patient selection using either changes in a tumor’s DNA, called genomic changes, or even protein biomarkers and do much better than we have done in the past.”
Dr. Schwaederle noted that one limitation of her study is that more recent trials, unpublished at the time of her analysis, could influence the results today.
The study received funding from the Joan and Irwin Jacobs Philanthropic Fund. Two coauthors reported extensive financial relationships with pharmaceutical or other commercial and noncommercial entities.
Patients had significantly better outcomes when treatments were selected based on biomarker analysis of their tumors, in a meta-analysis of 346 phase I trials involving 351 study arms and 13,203 cancer patients.
Up to now, the purpose of phase I trials has been to determine safety based on adverse effects and tolerability. “Our analysis really shows that these days it is completely outdated and that with biomarker selection and especially genomic biomarkers, we can reach high response rates even in phase I trials,” Maria Schwaederle, Pharm.D., of the center for personalized cancer therapy at the University of California, San Diego, said in a presscast leading up to the annual meeting of the American Society of Clinical Oncology.
For the meta-analysis, Dr. Schwaederle and her colleagues performed a PubMed search on phase I clinical trials in cancer published in the years 2011-2013. They included studies using a single agent that reported adequate efficacy endpoints for response rate and progression-free survival (PSF). Overall survival was rarely reported and so was excluded.
Personalized therapy was defined as molecular biomarker–based selection of a treatment or at least half the patients in a trial having a specific tumor known to harbor the biomarker. The study compared outcomes based on a personalized strategy with those that were not.
“We think that the results were striking here,” Dr. Schwaederle said. “We can see that personalized therapy that is [based on] biomarker-selected treatments was really associated with significantly higher response rates and progression-free survival.” Multivariable analysis showed that response rates were sixfold higher with the personalized approach (30.6%, n = 58 in the personalized trials, vs. 4.9%, n = 293 in those not personalized; P less than .0001), and median PFS practically doubled (5.7 months, n = 7 in personalized trials vs. 2.95 months, n = 38 in those not personalized; P = .0002).
Most (98.3%) of the personalized arms used targeted agents. However, 76% of the arms using targeted agents did not select patients using a related biomarker and were thus nonpersonalized in their approach to treatment. A subanalysis showed that targeted drugs that were applied in a nontargeted manner had much worse response rates than targeted therapies applied using biomarker-based selection and were equivalent to cytotoxic therapies (personalized approach, 31.1%; nontargeted approach, 5.1%; P less than 0.0001; cytotoxic drugs, 4.7%, P = .63 vs. nonpersonalized approach). The median PFS was also similar for the nonpersonalized and cytotoxic strategies (3.3 vs. 2.5 months, P = .22).
Better targeting with genomic biomarkers
The investigators found that patients selected based on genomic (DNA) biomarkers had almost double the response rates (42%) of patients selected based on protein biomarkers (22.4%, P = .001). The reasons for this difference were not clear. “We might think that the target in the end is a protein, so we would expect maybe to see better results with protein expression,” Dr. Schwaederle said. But she pointed to the example of patients with lung cancer and epidermal growth factor receptor genetic alterations. If looking only for epidermal growth factor receptor protein overexpression, she said, “we wouldn’t see any response, but only the patients that have specific alterations in this gene respond very well.” So in that case, genetic detection appears to be more sensitive than a protein biomarker–based test.
Dr. Schwaederle addressed the question that has often arisen of whether targeted agents are just better therapies. “Our analysis showed that it is not just that the therapies are better but that targeted therapies must be given to the right patients,” she said. “Indeed, when targeted therapies were given to patients without a biomarker selection, the response rates were only about 5%.”
The response rate in the range of 40% using genetic biomarkers in these phase I trials suggests that incorporating such targeted approaches even at this early stage of drug testing may potentially yield useful information on efficacy.
Dr. Don Dizon, presscast moderator and chair of ASCO’s Cancer Communications Committee, said that precision medicine “is here and that we can use patient selection using either changes in a tumor’s DNA, called genomic changes, or even protein biomarkers and do much better than we have done in the past.”
Dr. Schwaederle noted that one limitation of her study is that more recent trials, unpublished at the time of her analysis, could influence the results today.
The study received funding from the Joan and Irwin Jacobs Philanthropic Fund. Two coauthors reported extensive financial relationships with pharmaceutical or other commercial and noncommercial entities.
FROM THE 2016 ASCO ANNUAL MEETING
Key clinical point: Precision drug targeting in phase I trials may yield efficacy information.
Major finding: Genomic targeting yielded a 42% response rate vs. 5% without targeting.
Data source: A meta-analysis of 346 phase I trials with 351 treatment arms and 13,203 cancer patients.
Disclosures: The study received funding from the Joan and Irwin Jacobs Philanthropic Fund. Two coauthors reported extensive financial relationships with pharmaceutical or other commercial and noncommercial entities.
Colon tumor side predicts outcomes from therapies
In this election year, the focus on left vs. right has even reached the discussion of colon cancer. In a retrospective analysis of data from the CALGB/SWOG 80405 trial of FOLFIRI or FOLFOX chemotherapy in combination with cetuximab or with bevacizumab as first-line treatment for metastatic colorectal cancer, patients with primary tumors that arose on the left side of the colon (descending and sigmoid colon and rectum; n = 732) survived significantly longer than did patients whose tumors originated on the right side (n = 293), comprising the cecum and ascending colon. And the metastatic disease responded differently to the two biologic agents.
The main cohort of the trial involved only patients with tumors with KRAS wild type gene. Patients were randomly assigned to receive cetuximab or bevacizumab in an open-label fashion. The choice of the FOLFIRI or FOLFOX regimen was at the physician’s discretion.
Although there was no significant difference in overall survival between the two treatment arms, “when we looked at patients whose tumors started on the left side, they had a median overall survival of 33 months compared to 19 months if the cancer began on the right side,” Dr. Alan Venook, professor of medicine at the University of California, San Francisco, said in a presscast leading up to the annual meeting of the American Society of Clinical Oncology.
“This is statistically significant with a hazard ratio [HR] of 1.60 describing the magnitude of difference in these outcomes,” he said.
For patients who received cetuximab, the difference in median overall survival approached 20 months. Patients with left-sided tumors had a median survival of 36.0 months vs. 16.7 months for right-sided tumors (HR = 1.987; 95% CI 1.60-2.46; P less than .001).
“This is really a dramatic finding that I think really was surprising to most of us or all of us given our understanding or belief beforehand that this really was not likely to make a big difference,” Dr. Venook said. The results for cetuximab are in line with a smaller European study, FIRE-3, that found a 22.6 month longer median overall survival for tumors on the left vs. the right.
Although not as large a benefit as seen with cetuximab, patients who received bevacizumab also did better if their tumors arose on the left side (n = 356) than if they arose on the right (n = 150): 31.4 months vs. 24.2 months, respectively (HR = 1.297; 95% CI 1.05-1.60; P = .017).
Including the few tumors found in the transverse colon did not change any of the results, so they were eliminated from analysis.
Agent, side matter for PFS
Data for progression-free survival (PFS) showed differential effects for the two biologic agents, depending on the side of the primary tumor. “We see that bevacizumab seemed to be more helpful on the right side … and cetuximab more helpful on the left side,” Dr. Venook said.
Considering both progression-free and overall survival, bevacizumab was superior to cetuximab (P = .03) for right-sided primaries, and cetuximab was superior for left-sided primaries (P = .04). These results showed a clear interaction of sidedness and the biologic agent used, with a P(interaction) = .003.
Practice implications and biologic explanations
“The 14 months’ improved survival in left vs. right side primary tumors is really striking for patients who present with metastatic disease,” Dr. Venook said. “Cetuximab added to first-line chemotherapy is associated with more favorable outcomes in patients with left-sided primary and appears to add more than bevacizumab to chemotherapy in these patients with left-sided primaries. Patients with right-sided primaries appear to benefit more from bevacizumab.”
He said he believes that the side is a surrogate marker for some biological explanation, and molecular analyses of tumor tissues are now in progress. “Until we have sorted that out, colon cancer originating on the right side should be treated differently than colon cancer on the left side,” he recommended. “And although this is retrospective, these data and other findings [presented at ASCO] and in press suggest that patients with right-sided primary metastatic colon cancer should get little to no benefit from cetuximab.”
He noted that the left and right colon segments arise from different embryonic tissues, the left coming from the hindgut and the right from the midgut. Therefore, based on the behavior of the tumors from this and previous studies as well as the tissues of origin of the gut segments, the left and right colon may have different biologies.
Session moderator Dr. Julie Vose, ASCO president and chief of the oncology/hematology division at the University of Nebraska Medical Center in Omaha, emphasized the role of the federal government in studies of this kind. “I think this study really points out how very large federally funded studies such as this one can really help us to differentiate some of the issues that we need to understand to treat our patients very specifically in a personalized way,” she said. And besides yielding information from the trial at hand, “it definitely is going to help us to generate hypotheses for future studies to hopefully take advantage of this information.”
In this election year, the focus on left vs. right has even reached the discussion of colon cancer. In a retrospective analysis of data from the CALGB/SWOG 80405 trial of FOLFIRI or FOLFOX chemotherapy in combination with cetuximab or with bevacizumab as first-line treatment for metastatic colorectal cancer, patients with primary tumors that arose on the left side of the colon (descending and sigmoid colon and rectum; n = 732) survived significantly longer than did patients whose tumors originated on the right side (n = 293), comprising the cecum and ascending colon. And the metastatic disease responded differently to the two biologic agents.
The main cohort of the trial involved only patients with tumors with KRAS wild type gene. Patients were randomly assigned to receive cetuximab or bevacizumab in an open-label fashion. The choice of the FOLFIRI or FOLFOX regimen was at the physician’s discretion.
Although there was no significant difference in overall survival between the two treatment arms, “when we looked at patients whose tumors started on the left side, they had a median overall survival of 33 months compared to 19 months if the cancer began on the right side,” Dr. Alan Venook, professor of medicine at the University of California, San Francisco, said in a presscast leading up to the annual meeting of the American Society of Clinical Oncology.
“This is statistically significant with a hazard ratio [HR] of 1.60 describing the magnitude of difference in these outcomes,” he said.
For patients who received cetuximab, the difference in median overall survival approached 20 months. Patients with left-sided tumors had a median survival of 36.0 months vs. 16.7 months for right-sided tumors (HR = 1.987; 95% CI 1.60-2.46; P less than .001).
“This is really a dramatic finding that I think really was surprising to most of us or all of us given our understanding or belief beforehand that this really was not likely to make a big difference,” Dr. Venook said. The results for cetuximab are in line with a smaller European study, FIRE-3, that found a 22.6 month longer median overall survival for tumors on the left vs. the right.
Although not as large a benefit as seen with cetuximab, patients who received bevacizumab also did better if their tumors arose on the left side (n = 356) than if they arose on the right (n = 150): 31.4 months vs. 24.2 months, respectively (HR = 1.297; 95% CI 1.05-1.60; P = .017).
Including the few tumors found in the transverse colon did not change any of the results, so they were eliminated from analysis.
Agent, side matter for PFS
Data for progression-free survival (PFS) showed differential effects for the two biologic agents, depending on the side of the primary tumor. “We see that bevacizumab seemed to be more helpful on the right side … and cetuximab more helpful on the left side,” Dr. Venook said.
Considering both progression-free and overall survival, bevacizumab was superior to cetuximab (P = .03) for right-sided primaries, and cetuximab was superior for left-sided primaries (P = .04). These results showed a clear interaction of sidedness and the biologic agent used, with a P(interaction) = .003.
Practice implications and biologic explanations
“The 14 months’ improved survival in left vs. right side primary tumors is really striking for patients who present with metastatic disease,” Dr. Venook said. “Cetuximab added to first-line chemotherapy is associated with more favorable outcomes in patients with left-sided primary and appears to add more than bevacizumab to chemotherapy in these patients with left-sided primaries. Patients with right-sided primaries appear to benefit more from bevacizumab.”
He said he believes that the side is a surrogate marker for some biological explanation, and molecular analyses of tumor tissues are now in progress. “Until we have sorted that out, colon cancer originating on the right side should be treated differently than colon cancer on the left side,” he recommended. “And although this is retrospective, these data and other findings [presented at ASCO] and in press suggest that patients with right-sided primary metastatic colon cancer should get little to no benefit from cetuximab.”
He noted that the left and right colon segments arise from different embryonic tissues, the left coming from the hindgut and the right from the midgut. Therefore, based on the behavior of the tumors from this and previous studies as well as the tissues of origin of the gut segments, the left and right colon may have different biologies.
Session moderator Dr. Julie Vose, ASCO president and chief of the oncology/hematology division at the University of Nebraska Medical Center in Omaha, emphasized the role of the federal government in studies of this kind. “I think this study really points out how very large federally funded studies such as this one can really help us to differentiate some of the issues that we need to understand to treat our patients very specifically in a personalized way,” she said. And besides yielding information from the trial at hand, “it definitely is going to help us to generate hypotheses for future studies to hopefully take advantage of this information.”
In this election year, the focus on left vs. right has even reached the discussion of colon cancer. In a retrospective analysis of data from the CALGB/SWOG 80405 trial of FOLFIRI or FOLFOX chemotherapy in combination with cetuximab or with bevacizumab as first-line treatment for metastatic colorectal cancer, patients with primary tumors that arose on the left side of the colon (descending and sigmoid colon and rectum; n = 732) survived significantly longer than did patients whose tumors originated on the right side (n = 293), comprising the cecum and ascending colon. And the metastatic disease responded differently to the two biologic agents.
The main cohort of the trial involved only patients with tumors with KRAS wild type gene. Patients were randomly assigned to receive cetuximab or bevacizumab in an open-label fashion. The choice of the FOLFIRI or FOLFOX regimen was at the physician’s discretion.
Although there was no significant difference in overall survival between the two treatment arms, “when we looked at patients whose tumors started on the left side, they had a median overall survival of 33 months compared to 19 months if the cancer began on the right side,” Dr. Alan Venook, professor of medicine at the University of California, San Francisco, said in a presscast leading up to the annual meeting of the American Society of Clinical Oncology.
“This is statistically significant with a hazard ratio [HR] of 1.60 describing the magnitude of difference in these outcomes,” he said.
For patients who received cetuximab, the difference in median overall survival approached 20 months. Patients with left-sided tumors had a median survival of 36.0 months vs. 16.7 months for right-sided tumors (HR = 1.987; 95% CI 1.60-2.46; P less than .001).
“This is really a dramatic finding that I think really was surprising to most of us or all of us given our understanding or belief beforehand that this really was not likely to make a big difference,” Dr. Venook said. The results for cetuximab are in line with a smaller European study, FIRE-3, that found a 22.6 month longer median overall survival for tumors on the left vs. the right.
Although not as large a benefit as seen with cetuximab, patients who received bevacizumab also did better if their tumors arose on the left side (n = 356) than if they arose on the right (n = 150): 31.4 months vs. 24.2 months, respectively (HR = 1.297; 95% CI 1.05-1.60; P = .017).
Including the few tumors found in the transverse colon did not change any of the results, so they were eliminated from analysis.
Agent, side matter for PFS
Data for progression-free survival (PFS) showed differential effects for the two biologic agents, depending on the side of the primary tumor. “We see that bevacizumab seemed to be more helpful on the right side … and cetuximab more helpful on the left side,” Dr. Venook said.
Considering both progression-free and overall survival, bevacizumab was superior to cetuximab (P = .03) for right-sided primaries, and cetuximab was superior for left-sided primaries (P = .04). These results showed a clear interaction of sidedness and the biologic agent used, with a P(interaction) = .003.
Practice implications and biologic explanations
“The 14 months’ improved survival in left vs. right side primary tumors is really striking for patients who present with metastatic disease,” Dr. Venook said. “Cetuximab added to first-line chemotherapy is associated with more favorable outcomes in patients with left-sided primary and appears to add more than bevacizumab to chemotherapy in these patients with left-sided primaries. Patients with right-sided primaries appear to benefit more from bevacizumab.”
He said he believes that the side is a surrogate marker for some biological explanation, and molecular analyses of tumor tissues are now in progress. “Until we have sorted that out, colon cancer originating on the right side should be treated differently than colon cancer on the left side,” he recommended. “And although this is retrospective, these data and other findings [presented at ASCO] and in press suggest that patients with right-sided primary metastatic colon cancer should get little to no benefit from cetuximab.”
He noted that the left and right colon segments arise from different embryonic tissues, the left coming from the hindgut and the right from the midgut. Therefore, based on the behavior of the tumors from this and previous studies as well as the tissues of origin of the gut segments, the left and right colon may have different biologies.
Session moderator Dr. Julie Vose, ASCO president and chief of the oncology/hematology division at the University of Nebraska Medical Center in Omaha, emphasized the role of the federal government in studies of this kind. “I think this study really points out how very large federally funded studies such as this one can really help us to differentiate some of the issues that we need to understand to treat our patients very specifically in a personalized way,” she said. And besides yielding information from the trial at hand, “it definitely is going to help us to generate hypotheses for future studies to hopefully take advantage of this information.”
FROM THE 2016 ASCO ANNUAL MEETING
Key clinical point: Cetuximab and bevacizumab have differential effects on overall survival and progression-free survival in metastatic colon cancer depending on which side of the colon the primary tumor originated.
Major finding: Survival with cetuximab was longer for left vs. right primaries.
Data source: Retrospective analysis involving a cohort of 1,085 patients with metastatic colon cancer from the CALGB/SWOG 80405 trial (patients randomized to cetuximab or bevacizumab in combination with FOLFOX or FOLFIRI chemotherapy).
Disclosures: The study received funding and support from Bristol-Myers Squibb, Genentech, and ImClone in collaboration with the National Cancer Institute. Dr. Venook has received compensation and research funding from several companies, including Bristol-Myers Squibb.