User login
‘Universal’ CAR T cell may overcome limitations
FROM THE AACR ANNUAL MEETING
A differently engineered chimeric antigen receptor (CAR) T cell promises to overcome major limitations of current CAR T cell therapies. Rather than engineer the CAR T cells to have a receptor that recognizes specific tumor antigens one at a time and requiring different CAR T cells for every antigen, this technique engineers a T cell receptor that can bind one invariant end of a bifunctional molecule. The molecule is constructed such that the other end can bind to whatever tumor cell surface marker is of interest. In this way, the CAR T cells can be constructed once and be directed to various tumor markers.
Standard CAR T cells are engineered to express on their surfaces receptors that recognize a specific antigen. These cells have been used up to now to recognize and kill tumor cells – for example, B cell leukemias carrying the pan-B cell marker CD19. The CAR T cells and their progeny, including memory T cells, remain in the body and continue to carry out their functions, potentially providing immune surveillance in case cancer cells arise again. But they uniquely recognize just CD19 – a problem, in that they kill even normal B cells, so-called off-target toxicity.
Beyond the unique specificity of standard CAR T cells, Philip Low, Ph.D., director of the Center for Drug Discovery at Purdue University in West Lafayette, Indiana, said these cells have three major limitations. First, they may lyse tumor cells so rapidly that a systemic tumor lysis syndrome or “cytokine storm” occurs. Second, the persisting CAR T cells can kill normal cells – for example, ones directed against CD19 killing normal B cells. Third, tumor cells have unstable genomes, leading to tumor heterogeneity, with some cells potentially losing the targeted antigens and therefore becoming “invisible” to the CAR T cells.
“So what we have done is basically designed a solution to all three, and we call it a universal CAR T cell because of its ability, with the help of an adapter molecule, to recognize all of these mutated tumor cells within a heterogeneous tumor,” he said at the annual meeting of the American Association for Cancer Research. The key was to make a CAR T cell with a surface receptor that binds to the dye fluorescein. Then fluorescein is coupled through a short linker to a molecule that binds specifically to a molecule expressed on tumor cells. In this way the CAR T cell can be made to interact with any tumor cell, depending on what is coupled to the fluorescein. The technique is analogous to a socket wrench. Every socket has the same size hole that the ratchet handle fits into regardless of the size of the “business end” of the sockets, which recognize different size nuts.
Dr. Low gave an example of folic acid, for which he says a receptor is overexpressed on about 40% of human tumors but almost never on normal cells. “We link fluorescein to the vitamin folic acid,” he said. CAR T cells are injected into an animal, and nothing happens unless a folate-fluorescein conjugate is also injected. “As soon as we inject folate-fluorescein, the folate binds to the tumor cell surface, the fluorescein part of the folate-fluorescein binds to the CAR T cell, this forces a very tight interaction between the engineered T cell and the cancer cell, and we found it leads to melting away of the tumor,” he said.
This technique addresses the three major problems with standard CAR T cell therapy. By titrating the binding affinity, concentration, and rate of administration of the fluorescein conjugate, the rate of tumor killing can be controlled, mitigating tumor lysis syndrome. Plus, normal cells may be spared if the parameters are adjusted so that the conjugate binds only to cells with high levels of the target molecule, such as tumor cells.
Because its low molecular weight, the bi-specific conjugate rapidly disappears from the circulation, and the cell killing can be terminated, allowing normal cells to regenerate – for example, in the case of normal B cells that carry CD19. Since CAR T cells generate progeny that stay in the body, the progeny remain “dormant” but are ready to be activated again by addition of the conjugate to attack tumor cells if they arise.
A major issue is dealing with tumor heterogeneity; Dr. Low’s method seems to address that, as well. “We have tumor-specific ligands for over 90% of all human cancers,” he said. “Within another couple of months we’ll have them for 100%.”
Tumors typically contain lots of hypoxic cells, and hypoxic cells overexpress carbonic anhydrase-9. “Virtually every tumor has large fractions of the tumor mass that overexpress carbonic anhydrase-9, and we have a ligand that binds very specifically to that,” Dr. Low said.
To address the problem of tumor heterogeneity, with different mutations within different areas of the tumor or over time because of genetic instability in the cells, Dr. Low said, “We have a cocktail of about five of these small molecules… they are inexpensive to produce… and they clear very rapidly… and with the cocktail we can hit nearly all cancer cells, even in heterogeneous cancers.”
One limitation, as with standard CAR T cell therapy, is that the technique will still depend on using an individual patient’s T cells to modify through use of a lentiviral vector, so there would not be a universal, off-the-shelf T cell to use for everyone.
The technique and materials have been tested only in animals so far, using tumor-specific ligands for the folate receptor, a prostate-specific membrane antigen, and an antigen overexpressed on neuroendocrine tumors. Dr. Low has intentions to move the technology into human trials. He said the bridging molecules exist in highly purified form, and CAR T cell technology has already been developed by others. “Today we see great success in animal models and have no reason to believe that it won’t translate at least to a good extent to the clinic,” he said. Still, he expects some obstacles along the way and is willing to partner with others working on similar problems as well as large pharmaceutical companies.
The research has been supported by Endocyte, a company that Dr. Low founded and for which he is Chief Scientific Officer and a member of the board of directors. He has filed two patents on the technology, which are held by Purdue University and licensed to Endocyte.
FROM THE AACR ANNUAL MEETING
A differently engineered chimeric antigen receptor (CAR) T cell promises to overcome major limitations of current CAR T cell therapies. Rather than engineer the CAR T cells to have a receptor that recognizes specific tumor antigens one at a time and requiring different CAR T cells for every antigen, this technique engineers a T cell receptor that can bind one invariant end of a bifunctional molecule. The molecule is constructed such that the other end can bind to whatever tumor cell surface marker is of interest. In this way, the CAR T cells can be constructed once and be directed to various tumor markers.
Standard CAR T cells are engineered to express on their surfaces receptors that recognize a specific antigen. These cells have been used up to now to recognize and kill tumor cells – for example, B cell leukemias carrying the pan-B cell marker CD19. The CAR T cells and their progeny, including memory T cells, remain in the body and continue to carry out their functions, potentially providing immune surveillance in case cancer cells arise again. But they uniquely recognize just CD19 – a problem, in that they kill even normal B cells, so-called off-target toxicity.
Beyond the unique specificity of standard CAR T cells, Philip Low, Ph.D., director of the Center for Drug Discovery at Purdue University in West Lafayette, Indiana, said these cells have three major limitations. First, they may lyse tumor cells so rapidly that a systemic tumor lysis syndrome or “cytokine storm” occurs. Second, the persisting CAR T cells can kill normal cells – for example, ones directed against CD19 killing normal B cells. Third, tumor cells have unstable genomes, leading to tumor heterogeneity, with some cells potentially losing the targeted antigens and therefore becoming “invisible” to the CAR T cells.
“So what we have done is basically designed a solution to all three, and we call it a universal CAR T cell because of its ability, with the help of an adapter molecule, to recognize all of these mutated tumor cells within a heterogeneous tumor,” he said at the annual meeting of the American Association for Cancer Research. The key was to make a CAR T cell with a surface receptor that binds to the dye fluorescein. Then fluorescein is coupled through a short linker to a molecule that binds specifically to a molecule expressed on tumor cells. In this way the CAR T cell can be made to interact with any tumor cell, depending on what is coupled to the fluorescein. The technique is analogous to a socket wrench. Every socket has the same size hole that the ratchet handle fits into regardless of the size of the “business end” of the sockets, which recognize different size nuts.
Dr. Low gave an example of folic acid, for which he says a receptor is overexpressed on about 40% of human tumors but almost never on normal cells. “We link fluorescein to the vitamin folic acid,” he said. CAR T cells are injected into an animal, and nothing happens unless a folate-fluorescein conjugate is also injected. “As soon as we inject folate-fluorescein, the folate binds to the tumor cell surface, the fluorescein part of the folate-fluorescein binds to the CAR T cell, this forces a very tight interaction between the engineered T cell and the cancer cell, and we found it leads to melting away of the tumor,” he said.
This technique addresses the three major problems with standard CAR T cell therapy. By titrating the binding affinity, concentration, and rate of administration of the fluorescein conjugate, the rate of tumor killing can be controlled, mitigating tumor lysis syndrome. Plus, normal cells may be spared if the parameters are adjusted so that the conjugate binds only to cells with high levels of the target molecule, such as tumor cells.
Because its low molecular weight, the bi-specific conjugate rapidly disappears from the circulation, and the cell killing can be terminated, allowing normal cells to regenerate – for example, in the case of normal B cells that carry CD19. Since CAR T cells generate progeny that stay in the body, the progeny remain “dormant” but are ready to be activated again by addition of the conjugate to attack tumor cells if they arise.
A major issue is dealing with tumor heterogeneity; Dr. Low’s method seems to address that, as well. “We have tumor-specific ligands for over 90% of all human cancers,” he said. “Within another couple of months we’ll have them for 100%.”
Tumors typically contain lots of hypoxic cells, and hypoxic cells overexpress carbonic anhydrase-9. “Virtually every tumor has large fractions of the tumor mass that overexpress carbonic anhydrase-9, and we have a ligand that binds very specifically to that,” Dr. Low said.
To address the problem of tumor heterogeneity, with different mutations within different areas of the tumor or over time because of genetic instability in the cells, Dr. Low said, “We have a cocktail of about five of these small molecules… they are inexpensive to produce… and they clear very rapidly… and with the cocktail we can hit nearly all cancer cells, even in heterogeneous cancers.”
One limitation, as with standard CAR T cell therapy, is that the technique will still depend on using an individual patient’s T cells to modify through use of a lentiviral vector, so there would not be a universal, off-the-shelf T cell to use for everyone.
The technique and materials have been tested only in animals so far, using tumor-specific ligands for the folate receptor, a prostate-specific membrane antigen, and an antigen overexpressed on neuroendocrine tumors. Dr. Low has intentions to move the technology into human trials. He said the bridging molecules exist in highly purified form, and CAR T cell technology has already been developed by others. “Today we see great success in animal models and have no reason to believe that it won’t translate at least to a good extent to the clinic,” he said. Still, he expects some obstacles along the way and is willing to partner with others working on similar problems as well as large pharmaceutical companies.
The research has been supported by Endocyte, a company that Dr. Low founded and for which he is Chief Scientific Officer and a member of the board of directors. He has filed two patents on the technology, which are held by Purdue University and licensed to Endocyte.
FROM THE AACR ANNUAL MEETING
A differently engineered chimeric antigen receptor (CAR) T cell promises to overcome major limitations of current CAR T cell therapies. Rather than engineer the CAR T cells to have a receptor that recognizes specific tumor antigens one at a time and requiring different CAR T cells for every antigen, this technique engineers a T cell receptor that can bind one invariant end of a bifunctional molecule. The molecule is constructed such that the other end can bind to whatever tumor cell surface marker is of interest. In this way, the CAR T cells can be constructed once and be directed to various tumor markers.
Standard CAR T cells are engineered to express on their surfaces receptors that recognize a specific antigen. These cells have been used up to now to recognize and kill tumor cells – for example, B cell leukemias carrying the pan-B cell marker CD19. The CAR T cells and their progeny, including memory T cells, remain in the body and continue to carry out their functions, potentially providing immune surveillance in case cancer cells arise again. But they uniquely recognize just CD19 – a problem, in that they kill even normal B cells, so-called off-target toxicity.
Beyond the unique specificity of standard CAR T cells, Philip Low, Ph.D., director of the Center for Drug Discovery at Purdue University in West Lafayette, Indiana, said these cells have three major limitations. First, they may lyse tumor cells so rapidly that a systemic tumor lysis syndrome or “cytokine storm” occurs. Second, the persisting CAR T cells can kill normal cells – for example, ones directed against CD19 killing normal B cells. Third, tumor cells have unstable genomes, leading to tumor heterogeneity, with some cells potentially losing the targeted antigens and therefore becoming “invisible” to the CAR T cells.
“So what we have done is basically designed a solution to all three, and we call it a universal CAR T cell because of its ability, with the help of an adapter molecule, to recognize all of these mutated tumor cells within a heterogeneous tumor,” he said at the annual meeting of the American Association for Cancer Research. The key was to make a CAR T cell with a surface receptor that binds to the dye fluorescein. Then fluorescein is coupled through a short linker to a molecule that binds specifically to a molecule expressed on tumor cells. In this way the CAR T cell can be made to interact with any tumor cell, depending on what is coupled to the fluorescein. The technique is analogous to a socket wrench. Every socket has the same size hole that the ratchet handle fits into regardless of the size of the “business end” of the sockets, which recognize different size nuts.
Dr. Low gave an example of folic acid, for which he says a receptor is overexpressed on about 40% of human tumors but almost never on normal cells. “We link fluorescein to the vitamin folic acid,” he said. CAR T cells are injected into an animal, and nothing happens unless a folate-fluorescein conjugate is also injected. “As soon as we inject folate-fluorescein, the folate binds to the tumor cell surface, the fluorescein part of the folate-fluorescein binds to the CAR T cell, this forces a very tight interaction between the engineered T cell and the cancer cell, and we found it leads to melting away of the tumor,” he said.
This technique addresses the three major problems with standard CAR T cell therapy. By titrating the binding affinity, concentration, and rate of administration of the fluorescein conjugate, the rate of tumor killing can be controlled, mitigating tumor lysis syndrome. Plus, normal cells may be spared if the parameters are adjusted so that the conjugate binds only to cells with high levels of the target molecule, such as tumor cells.
Because its low molecular weight, the bi-specific conjugate rapidly disappears from the circulation, and the cell killing can be terminated, allowing normal cells to regenerate – for example, in the case of normal B cells that carry CD19. Since CAR T cells generate progeny that stay in the body, the progeny remain “dormant” but are ready to be activated again by addition of the conjugate to attack tumor cells if they arise.
A major issue is dealing with tumor heterogeneity; Dr. Low’s method seems to address that, as well. “We have tumor-specific ligands for over 90% of all human cancers,” he said. “Within another couple of months we’ll have them for 100%.”
Tumors typically contain lots of hypoxic cells, and hypoxic cells overexpress carbonic anhydrase-9. “Virtually every tumor has large fractions of the tumor mass that overexpress carbonic anhydrase-9, and we have a ligand that binds very specifically to that,” Dr. Low said.
To address the problem of tumor heterogeneity, with different mutations within different areas of the tumor or over time because of genetic instability in the cells, Dr. Low said, “We have a cocktail of about five of these small molecules… they are inexpensive to produce… and they clear very rapidly… and with the cocktail we can hit nearly all cancer cells, even in heterogeneous cancers.”
One limitation, as with standard CAR T cell therapy, is that the technique will still depend on using an individual patient’s T cells to modify through use of a lentiviral vector, so there would not be a universal, off-the-shelf T cell to use for everyone.
The technique and materials have been tested only in animals so far, using tumor-specific ligands for the folate receptor, a prostate-specific membrane antigen, and an antigen overexpressed on neuroendocrine tumors. Dr. Low has intentions to move the technology into human trials. He said the bridging molecules exist in highly purified form, and CAR T cell technology has already been developed by others. “Today we see great success in animal models and have no reason to believe that it won’t translate at least to a good extent to the clinic,” he said. Still, he expects some obstacles along the way and is willing to partner with others working on similar problems as well as large pharmaceutical companies.
The research has been supported by Endocyte, a company that Dr. Low founded and for which he is Chief Scientific Officer and a member of the board of directors. He has filed two patents on the technology, which are held by Purdue University and licensed to Endocyte.
Oral bacteria linked with pancreatic cancer
Two species of oral bacteria – Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans – have been linked with an elevated risk of developing pancreatic cancer. This follows previous studies linking poor oral health in general to an increased risk of pancreatic cancer.
“Each [species] is associated with more than 50% higher likelihood to develop pancreatic cancer,” Jiyoung Ahn, Ph.D., of New York University Langone Medical Center, said during a news conference at the annual meeting of the American Association for Cancer Research.
“The findings suggest that carriage of these bacteria is related to subsequent development of pancreatic cancer,” said Dr. Ahn. Given the poor survival rate for pancreatic cancer of 5% at 5 years, identifying risk factors for its development may have implications for screening tools and prevention if the associations are found to be causal.
Employing a prospective, nested case-control study design, Dr. Ahn used samples and data from the Cancer Prevention Study II and the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial cohorts. Both of those studies enrolled healthy people and followed them for several years looking for a variety of outcomes, including cancer. Researchers performed genomic analysis on oral wash samples collected at the beginning of the studies to identify the bacterial species present. Samples from 361 people who eventually developed pancreatic cancer were matched to 371 controls.
After adjustment for covariates of age, race, sex, smoking status, alcohol consumption, body mass index, and diabetes, logistic regression analysis showed that the presence of P. gingivalis in the samples was associated with a 59% increased risk (adjusted odds ratio [OR] = 1.59; 95% confidence interval [CI] 1.15, 2.20) of developing pancreatic cancer, and the presence of A. actinomycetemcomitans was associated with a 119% increased risk (OR = 2.19; 95% CI 1.15, 4.15). The presence of these bacteria remained a risk factor even after the deletion of samples from people who developed pancreatic cancer within the 2 years after collection of their samples to eliminate the possibility that early pancreatic cancers were affecting the populations of bacteria present.
Greater relative abundance of Fusobacteria species was associated with a lower risk (OR per percent increased abundance = 0.92; 95% CI 0.87, 0.98).
“Why this is important is because this is the first evidence suggesting the oral bacteria and pancreatic cancer relationship,” Dr. Ahn said, adding that she foresees eventual clinical applications. “In the long run, knowing these bacteria, we can see who’s more likely to develop pancreatic cancer, and in the more long run, we see that maybe we can control these bacteria ... to prevent pancreatic cancer.”
So far, she said no mechanisms are known to account for the association between bacteria and pancreatic cancer risk although several have been proposed. She said a next step is to collect pancreatic tissue to see if oral bacteria are traveling to the pancreas.
Dr. Ahn noted that a major limitation of the study was that the populations involved in the cohorts from which the oral wash samples were obtained were mainly non-Hispanic whites, so the findings may not be generalizable to other groups.
Dr. Ahn had no disclosures.
Two species of oral bacteria – Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans – have been linked with an elevated risk of developing pancreatic cancer. This follows previous studies linking poor oral health in general to an increased risk of pancreatic cancer.
“Each [species] is associated with more than 50% higher likelihood to develop pancreatic cancer,” Jiyoung Ahn, Ph.D., of New York University Langone Medical Center, said during a news conference at the annual meeting of the American Association for Cancer Research.
“The findings suggest that carriage of these bacteria is related to subsequent development of pancreatic cancer,” said Dr. Ahn. Given the poor survival rate for pancreatic cancer of 5% at 5 years, identifying risk factors for its development may have implications for screening tools and prevention if the associations are found to be causal.
Employing a prospective, nested case-control study design, Dr. Ahn used samples and data from the Cancer Prevention Study II and the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial cohorts. Both of those studies enrolled healthy people and followed them for several years looking for a variety of outcomes, including cancer. Researchers performed genomic analysis on oral wash samples collected at the beginning of the studies to identify the bacterial species present. Samples from 361 people who eventually developed pancreatic cancer were matched to 371 controls.
After adjustment for covariates of age, race, sex, smoking status, alcohol consumption, body mass index, and diabetes, logistic regression analysis showed that the presence of P. gingivalis in the samples was associated with a 59% increased risk (adjusted odds ratio [OR] = 1.59; 95% confidence interval [CI] 1.15, 2.20) of developing pancreatic cancer, and the presence of A. actinomycetemcomitans was associated with a 119% increased risk (OR = 2.19; 95% CI 1.15, 4.15). The presence of these bacteria remained a risk factor even after the deletion of samples from people who developed pancreatic cancer within the 2 years after collection of their samples to eliminate the possibility that early pancreatic cancers were affecting the populations of bacteria present.
Greater relative abundance of Fusobacteria species was associated with a lower risk (OR per percent increased abundance = 0.92; 95% CI 0.87, 0.98).
“Why this is important is because this is the first evidence suggesting the oral bacteria and pancreatic cancer relationship,” Dr. Ahn said, adding that she foresees eventual clinical applications. “In the long run, knowing these bacteria, we can see who’s more likely to develop pancreatic cancer, and in the more long run, we see that maybe we can control these bacteria ... to prevent pancreatic cancer.”
So far, she said no mechanisms are known to account for the association between bacteria and pancreatic cancer risk although several have been proposed. She said a next step is to collect pancreatic tissue to see if oral bacteria are traveling to the pancreas.
Dr. Ahn noted that a major limitation of the study was that the populations involved in the cohorts from which the oral wash samples were obtained were mainly non-Hispanic whites, so the findings may not be generalizable to other groups.
Dr. Ahn had no disclosures.
Two species of oral bacteria – Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans – have been linked with an elevated risk of developing pancreatic cancer. This follows previous studies linking poor oral health in general to an increased risk of pancreatic cancer.
“Each [species] is associated with more than 50% higher likelihood to develop pancreatic cancer,” Jiyoung Ahn, Ph.D., of New York University Langone Medical Center, said during a news conference at the annual meeting of the American Association for Cancer Research.
“The findings suggest that carriage of these bacteria is related to subsequent development of pancreatic cancer,” said Dr. Ahn. Given the poor survival rate for pancreatic cancer of 5% at 5 years, identifying risk factors for its development may have implications for screening tools and prevention if the associations are found to be causal.
Employing a prospective, nested case-control study design, Dr. Ahn used samples and data from the Cancer Prevention Study II and the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial cohorts. Both of those studies enrolled healthy people and followed them for several years looking for a variety of outcomes, including cancer. Researchers performed genomic analysis on oral wash samples collected at the beginning of the studies to identify the bacterial species present. Samples from 361 people who eventually developed pancreatic cancer were matched to 371 controls.
After adjustment for covariates of age, race, sex, smoking status, alcohol consumption, body mass index, and diabetes, logistic regression analysis showed that the presence of P. gingivalis in the samples was associated with a 59% increased risk (adjusted odds ratio [OR] = 1.59; 95% confidence interval [CI] 1.15, 2.20) of developing pancreatic cancer, and the presence of A. actinomycetemcomitans was associated with a 119% increased risk (OR = 2.19; 95% CI 1.15, 4.15). The presence of these bacteria remained a risk factor even after the deletion of samples from people who developed pancreatic cancer within the 2 years after collection of their samples to eliminate the possibility that early pancreatic cancers were affecting the populations of bacteria present.
Greater relative abundance of Fusobacteria species was associated with a lower risk (OR per percent increased abundance = 0.92; 95% CI 0.87, 0.98).
“Why this is important is because this is the first evidence suggesting the oral bacteria and pancreatic cancer relationship,” Dr. Ahn said, adding that she foresees eventual clinical applications. “In the long run, knowing these bacteria, we can see who’s more likely to develop pancreatic cancer, and in the more long run, we see that maybe we can control these bacteria ... to prevent pancreatic cancer.”
So far, she said no mechanisms are known to account for the association between bacteria and pancreatic cancer risk although several have been proposed. She said a next step is to collect pancreatic tissue to see if oral bacteria are traveling to the pancreas.
Dr. Ahn noted that a major limitation of the study was that the populations involved in the cohorts from which the oral wash samples were obtained were mainly non-Hispanic whites, so the findings may not be generalizable to other groups.
Dr. Ahn had no disclosures.
FROM THE AACR ANNUAL MEETING
Key clinical point: Two oral bacteria are associated with increased pancreatic cancer risk.
Major finding: Bacteria are linked to over 50% increased pancreatic cancer risk.
Data source: Prospective nested case-control study of 361 incident pancreatic cancer cases and 371 matched controls.
Disclosures: Dr. Ahn had no disclosures.
Merkel cell carcinoma responds to first-line pembrolizumab
First-line therapy with pembrolizumab is associated with frequent and durable responses in patients with advanced Merkel cell carcinoma (MCC), according to a study presented at the annual meeting of the American Association for Cancer Research.
The objective response rate wad 56% among 25 patients with MCC who had received at least one dose of pembrolizumab in a phase II study, Dr. Paul Nghiem said at a press conference during the meeting.
This rare but aggressive skin cancer has been notoriously hard to treat with typical chemotherapy of platinum compounds and etoposide, with half of tumors progressing by 3 months and 90% progressing by 10 months. MCC is about three times more likely to kill a patient compared with melanoma.
Given that about 40% of the 2,000 cases per year develop advanced disease, pembrolizumab has the potential to be an advance over chemotherapy although at this point no therapy is approved by the Food and Drug Administration for MCC advanced disease. The disease may be caused by ultraviolet light exposure in older individuals or with immune suppression. In about 80% of cases, Merkel cell polyomavirus (MCPyV) is present, but the virus is ubiquitous in the environment, with widespread exposure in the population without ill effects for most people.
Pembrolizumab acts to block the interaction of PD-1 on tumor-specific or MCPyV-specific T cells with its ligand, PD-L1, on tumor cells or antigen-presenting cells, thus allowing the T cells to recognize the tumors and enhance tumor cell killing.
In a phase II multicenter, single-arm, open-label trial, patients with unresectable or metastatic disease who had never before received any systemic therapy received pembrolizumab 2 mg/kg IV every 3 weeks for up to 2 years. Tumor evaluations were done every 9 or 12 weeks with a median follow-up of 7.6 months.
Of the 25 patients who had received at least one dose and had at least one radiological assessment, there was an objective response rate of 56% (14/25), including 4 complete, 10 partial, and 1 unconfirmed partial response. One patient had stable disease, and nine had progressive disease.
“By the first scan at 3 months most patients have found where they were going to go,” said Dr. Nghiem, professor of medicine in dermatology at the University of Washington, Seattle. “Several had failed early, but by the time of the first scan at 3 months there were often very profound and complete responses, and a lot of the partial responses just remain very steady over time.”
With the caveat that this was not a randomized trial, he said the progression-free survival (PFS) among responders was 67% at 6 months and the median PFS was 9 months, compared to a historical median overall survival with chemotherapy of 9.5 months after metastatic diagnosis. Among responders, 86% continue to have excellent disease control more than 6 months after starting therapy.
Objective responses in this small study appeared to be associated with positive viral status of the tumors. Among patients with virus-positive tumors, 62% (10/16) had objective responses vs. 44% (4/9) in whom the tumors were virus negative. “That was not a statistically significant difference … but maybe suggests, like head and neck [cancers], there may be a better story there for virus positive,” Dr. Nghiem said. PD-L1 expression in the tumors did not predict responses.
In response to a question during the news conference about the high response rate compared to responses to therapy of most other solid tumors, Dr. Nghiem pointed out, “Historically, this has been a very immune-associated cancer … If you had CD8 T cells infiltrating into this cancer at any reasonable number, among 300 patients not one died of the disease.” Unleashing the immune system in this trial through PD-1 blockade may therefore help to explain the good outcomes.
Adverse events were managed with corticosteroid treatment and discontinuing the drug. Two patients who had severe drug-related toxicities improved after steroids and stopping the drug. But, nonetheless, they have ongoing antitumor responses several months after stopping pembrolizumab.
The investigators are currently expanding the trial and recruiting more patients.
The study was simultaneously published online in the New England Journal of Medicine (2016 April 19. doi: 10.1056/NEJMoa1603702).
The study was supported in part by Merck. Dr. Nghiem is a consultant to EMD Serono and has grant/research support from Bristol-Myers Squibb.
First-line therapy with pembrolizumab is associated with frequent and durable responses in patients with advanced Merkel cell carcinoma (MCC), according to a study presented at the annual meeting of the American Association for Cancer Research.
The objective response rate wad 56% among 25 patients with MCC who had received at least one dose of pembrolizumab in a phase II study, Dr. Paul Nghiem said at a press conference during the meeting.
This rare but aggressive skin cancer has been notoriously hard to treat with typical chemotherapy of platinum compounds and etoposide, with half of tumors progressing by 3 months and 90% progressing by 10 months. MCC is about three times more likely to kill a patient compared with melanoma.
Given that about 40% of the 2,000 cases per year develop advanced disease, pembrolizumab has the potential to be an advance over chemotherapy although at this point no therapy is approved by the Food and Drug Administration for MCC advanced disease. The disease may be caused by ultraviolet light exposure in older individuals or with immune suppression. In about 80% of cases, Merkel cell polyomavirus (MCPyV) is present, but the virus is ubiquitous in the environment, with widespread exposure in the population without ill effects for most people.
Pembrolizumab acts to block the interaction of PD-1 on tumor-specific or MCPyV-specific T cells with its ligand, PD-L1, on tumor cells or antigen-presenting cells, thus allowing the T cells to recognize the tumors and enhance tumor cell killing.
In a phase II multicenter, single-arm, open-label trial, patients with unresectable or metastatic disease who had never before received any systemic therapy received pembrolizumab 2 mg/kg IV every 3 weeks for up to 2 years. Tumor evaluations were done every 9 or 12 weeks with a median follow-up of 7.6 months.
Of the 25 patients who had received at least one dose and had at least one radiological assessment, there was an objective response rate of 56% (14/25), including 4 complete, 10 partial, and 1 unconfirmed partial response. One patient had stable disease, and nine had progressive disease.
“By the first scan at 3 months most patients have found where they were going to go,” said Dr. Nghiem, professor of medicine in dermatology at the University of Washington, Seattle. “Several had failed early, but by the time of the first scan at 3 months there were often very profound and complete responses, and a lot of the partial responses just remain very steady over time.”
With the caveat that this was not a randomized trial, he said the progression-free survival (PFS) among responders was 67% at 6 months and the median PFS was 9 months, compared to a historical median overall survival with chemotherapy of 9.5 months after metastatic diagnosis. Among responders, 86% continue to have excellent disease control more than 6 months after starting therapy.
Objective responses in this small study appeared to be associated with positive viral status of the tumors. Among patients with virus-positive tumors, 62% (10/16) had objective responses vs. 44% (4/9) in whom the tumors were virus negative. “That was not a statistically significant difference … but maybe suggests, like head and neck [cancers], there may be a better story there for virus positive,” Dr. Nghiem said. PD-L1 expression in the tumors did not predict responses.
In response to a question during the news conference about the high response rate compared to responses to therapy of most other solid tumors, Dr. Nghiem pointed out, “Historically, this has been a very immune-associated cancer … If you had CD8 T cells infiltrating into this cancer at any reasonable number, among 300 patients not one died of the disease.” Unleashing the immune system in this trial through PD-1 blockade may therefore help to explain the good outcomes.
Adverse events were managed with corticosteroid treatment and discontinuing the drug. Two patients who had severe drug-related toxicities improved after steroids and stopping the drug. But, nonetheless, they have ongoing antitumor responses several months after stopping pembrolizumab.
The investigators are currently expanding the trial and recruiting more patients.
The study was simultaneously published online in the New England Journal of Medicine (2016 April 19. doi: 10.1056/NEJMoa1603702).
The study was supported in part by Merck. Dr. Nghiem is a consultant to EMD Serono and has grant/research support from Bristol-Myers Squibb.
First-line therapy with pembrolizumab is associated with frequent and durable responses in patients with advanced Merkel cell carcinoma (MCC), according to a study presented at the annual meeting of the American Association for Cancer Research.
The objective response rate wad 56% among 25 patients with MCC who had received at least one dose of pembrolizumab in a phase II study, Dr. Paul Nghiem said at a press conference during the meeting.
This rare but aggressive skin cancer has been notoriously hard to treat with typical chemotherapy of platinum compounds and etoposide, with half of tumors progressing by 3 months and 90% progressing by 10 months. MCC is about three times more likely to kill a patient compared with melanoma.
Given that about 40% of the 2,000 cases per year develop advanced disease, pembrolizumab has the potential to be an advance over chemotherapy although at this point no therapy is approved by the Food and Drug Administration for MCC advanced disease. The disease may be caused by ultraviolet light exposure in older individuals or with immune suppression. In about 80% of cases, Merkel cell polyomavirus (MCPyV) is present, but the virus is ubiquitous in the environment, with widespread exposure in the population without ill effects for most people.
Pembrolizumab acts to block the interaction of PD-1 on tumor-specific or MCPyV-specific T cells with its ligand, PD-L1, on tumor cells or antigen-presenting cells, thus allowing the T cells to recognize the tumors and enhance tumor cell killing.
In a phase II multicenter, single-arm, open-label trial, patients with unresectable or metastatic disease who had never before received any systemic therapy received pembrolizumab 2 mg/kg IV every 3 weeks for up to 2 years. Tumor evaluations were done every 9 or 12 weeks with a median follow-up of 7.6 months.
Of the 25 patients who had received at least one dose and had at least one radiological assessment, there was an objective response rate of 56% (14/25), including 4 complete, 10 partial, and 1 unconfirmed partial response. One patient had stable disease, and nine had progressive disease.
“By the first scan at 3 months most patients have found where they were going to go,” said Dr. Nghiem, professor of medicine in dermatology at the University of Washington, Seattle. “Several had failed early, but by the time of the first scan at 3 months there were often very profound and complete responses, and a lot of the partial responses just remain very steady over time.”
With the caveat that this was not a randomized trial, he said the progression-free survival (PFS) among responders was 67% at 6 months and the median PFS was 9 months, compared to a historical median overall survival with chemotherapy of 9.5 months after metastatic diagnosis. Among responders, 86% continue to have excellent disease control more than 6 months after starting therapy.
Objective responses in this small study appeared to be associated with positive viral status of the tumors. Among patients with virus-positive tumors, 62% (10/16) had objective responses vs. 44% (4/9) in whom the tumors were virus negative. “That was not a statistically significant difference … but maybe suggests, like head and neck [cancers], there may be a better story there for virus positive,” Dr. Nghiem said. PD-L1 expression in the tumors did not predict responses.
In response to a question during the news conference about the high response rate compared to responses to therapy of most other solid tumors, Dr. Nghiem pointed out, “Historically, this has been a very immune-associated cancer … If you had CD8 T cells infiltrating into this cancer at any reasonable number, among 300 patients not one died of the disease.” Unleashing the immune system in this trial through PD-1 blockade may therefore help to explain the good outcomes.
Adverse events were managed with corticosteroid treatment and discontinuing the drug. Two patients who had severe drug-related toxicities improved after steroids and stopping the drug. But, nonetheless, they have ongoing antitumor responses several months after stopping pembrolizumab.
The investigators are currently expanding the trial and recruiting more patients.
The study was simultaneously published online in the New England Journal of Medicine (2016 April 19. doi: 10.1056/NEJMoa1603702).
The study was supported in part by Merck. Dr. Nghiem is a consultant to EMD Serono and has grant/research support from Bristol-Myers Squibb.
FROM THE AACR ANNUAL MEETING
Key clinical point: Rare Merkel cell carcinoma responds well to immunotherapy with pembrolizumab.
Major finding: The response rate was 56%, all occurring by 3 months.
Data source: Phase II multicenter, single-arm, open-label trial of 25 patients (NCT02267603).
Disclosures: The study was supported in part by Merck. Dr. Nghiem is a consultant to EMD Serono and has grant/research support from Bristol-Myers Squibb.
MammaPrint bests clinical factors in sparing patients from chemotherapy
A large multinational European trial shows that genetic analysis of breast tumors can spare women unnecessary adjuvant chemotherapy, even when clinical factors indicate a high risk of recurrence.
Using MammaPrint, a 70-gene signature test, led to a 14% absolute reduction in the use of chemotherapy, compared with a clinical strategy, according to the highly anticipated results of the Phase III Microarray in Node-negative and 1 to 3 positive lymph node Disease may Avoid Chemo Therapy (MINDACT) trial, presented at the annual meeting of the American Association for Cancer Research.
While three decades of clinical trials have shown survival benefits of adjuvant chemotherapy, it carries later risks. “And it will happen when the survival gain associated with adjuvant chemotherapy is small, in the range of 2%-3% – it happens in good prognosis patients – and is counterbalanced by the long-term severe risks of adjuvant chemotherapy,” said Dr. Martine Piccart, director of medicine at the Jules Bordet Institute in Brussels, and principal investigator for the trial.
Risks include secondary cancers, cardiotoxicity, early menopause, and a decline in cognitive function, as well as negative socioeconomic effects. So avoiding chemotherapy when outcomes would be nearly the same without it is a reasonable goal.
To investigate the best method to determine which breast cancer patients could do just as well without adjuvant chemotherapy, European investigators at 111 centers in 9 countries screened 11,288 patients with early-stage disease and enrolled 6,693 in the MINDACT trial. Speaking at a news conference during the annual meeting, Dr. Piccart said MINDACT compared tumor genetic testing with clinical and pathology parameters to gauge the risk of disease recurrence and is the only trial to date to compare the utility of the two strategies.
MammaPrint is a genetic test (G) of early stage breast cancer tumors that produces a 70-gene signature indicating a high or low 10-year risk of metastatic recurrence. The Adjuvant! Online tool takes into account clinical factors and pathology markers, including a patient’s age and comorbidities and a tumor’s estrogen receptor status, grade, size, and the number of positive lymph nodes, to calculate a ten-year “clinical risk” (C) of negative outcomes with or without adjuvant chemotherapy or hormonal therapy.
After surgery, tissue samples underwent local pathology examination to determine tumor stage and nodal, hormone receptor, and HER2 status. Samples were also sent to a central facility for MammaPrint testing. For women with low-risk tumors by both tests, no chemotherapy was indicated. For high-risk tumors by both tests, chemotherapy was prescribed. But those with discordant results were randomized equally to receive chemotherapy or not.
MammaPrint better predictor than clinical factors
The median age of patients at enrollment was 55 years, 80% of tumors were node-negative, 58% were T1, 88% hormone receptor–positive, and 10% HER2-positive. At a median follow-up of 5 years, 362 (5.4%) women had distant metastases or had died.
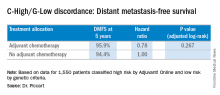
Among 3,356 patients classified as high risk based on the Adjuvant! Online test, there were 1,550 classified by genetic criteria as low risk (C-High/G-Low), including 48% with positive nodes. For this C-High/G-Low group, the 5-year distant metastasis-free survival was greater than 94% regardless of adjuvant chemotherapy and was not statistically different for the two groups. These are patients who would normally be prescribed adjuvant chemotherapy if based solely on the C criteria.
Going by MammaPrint test results would thereby reduce chemotherapy prescriptions by 46% (1,550/3,356) for the study cohort. Considering the entire initial 11,288 patients screened, the genomic strategy would lead to a 14% absolute reduction in chemotherapy, compared with the clinical strategy, according to Dr. Piccart. “MINDACT results provide level 1A evidence of the clinical utility of MammaPrint for assessing the lack of a clinically relevant chemotherapy benefit in the clinically high-risk population,” she said.
When asked about the use of MammaPrint in the United States, she said it is more common in Europe and other places in the world, whereas the use of Oncotype DX genetic testing seems to be more prevalent in the U.S.
News conference moderator Dr. Nancy Davidson, director of the University of Pittsburgh Cancer Institute, said that both tests are used at her institution. “I would say Oncotype is probably the dominant test that’s ordered,” she said. “But there are folks who are big fans of MammaPrint, and they’re going to be very excited to see these major results come out from this trial to help us to discuss this further and to think about how to guide our thinking and our patients.”
Entities and funding sources involved in the study included Adjuvant! Online, Agendia, Novartis, Roche, Sanofi-Aventis, Veridex, and Eli Lilly. Dr. Piccart disclosed that she is a board member of Radius, and a consultant for AstraZeneca, Eli Lilly, Invivus, Merck Sharp & Dohme, Novartis, Pfizer, Roche-Genentech, Synthon, Debiopharm, and PharmaMar. The Jules Bordet Institute receives grant/research funds from most companies in the field.
A large multinational European trial shows that genetic analysis of breast tumors can spare women unnecessary adjuvant chemotherapy, even when clinical factors indicate a high risk of recurrence.
Using MammaPrint, a 70-gene signature test, led to a 14% absolute reduction in the use of chemotherapy, compared with a clinical strategy, according to the highly anticipated results of the Phase III Microarray in Node-negative and 1 to 3 positive lymph node Disease may Avoid Chemo Therapy (MINDACT) trial, presented at the annual meeting of the American Association for Cancer Research.
While three decades of clinical trials have shown survival benefits of adjuvant chemotherapy, it carries later risks. “And it will happen when the survival gain associated with adjuvant chemotherapy is small, in the range of 2%-3% – it happens in good prognosis patients – and is counterbalanced by the long-term severe risks of adjuvant chemotherapy,” said Dr. Martine Piccart, director of medicine at the Jules Bordet Institute in Brussels, and principal investigator for the trial.
Risks include secondary cancers, cardiotoxicity, early menopause, and a decline in cognitive function, as well as negative socioeconomic effects. So avoiding chemotherapy when outcomes would be nearly the same without it is a reasonable goal.
To investigate the best method to determine which breast cancer patients could do just as well without adjuvant chemotherapy, European investigators at 111 centers in 9 countries screened 11,288 patients with early-stage disease and enrolled 6,693 in the MINDACT trial. Speaking at a news conference during the annual meeting, Dr. Piccart said MINDACT compared tumor genetic testing with clinical and pathology parameters to gauge the risk of disease recurrence and is the only trial to date to compare the utility of the two strategies.
MammaPrint is a genetic test (G) of early stage breast cancer tumors that produces a 70-gene signature indicating a high or low 10-year risk of metastatic recurrence. The Adjuvant! Online tool takes into account clinical factors and pathology markers, including a patient’s age and comorbidities and a tumor’s estrogen receptor status, grade, size, and the number of positive lymph nodes, to calculate a ten-year “clinical risk” (C) of negative outcomes with or without adjuvant chemotherapy or hormonal therapy.
After surgery, tissue samples underwent local pathology examination to determine tumor stage and nodal, hormone receptor, and HER2 status. Samples were also sent to a central facility for MammaPrint testing. For women with low-risk tumors by both tests, no chemotherapy was indicated. For high-risk tumors by both tests, chemotherapy was prescribed. But those with discordant results were randomized equally to receive chemotherapy or not.
MammaPrint better predictor than clinical factors
The median age of patients at enrollment was 55 years, 80% of tumors were node-negative, 58% were T1, 88% hormone receptor–positive, and 10% HER2-positive. At a median follow-up of 5 years, 362 (5.4%) women had distant metastases or had died.
Among 3,356 patients classified as high risk based on the Adjuvant! Online test, there were 1,550 classified by genetic criteria as low risk (C-High/G-Low), including 48% with positive nodes. For this C-High/G-Low group, the 5-year distant metastasis-free survival was greater than 94% regardless of adjuvant chemotherapy and was not statistically different for the two groups. These are patients who would normally be prescribed adjuvant chemotherapy if based solely on the C criteria.
Going by MammaPrint test results would thereby reduce chemotherapy prescriptions by 46% (1,550/3,356) for the study cohort. Considering the entire initial 11,288 patients screened, the genomic strategy would lead to a 14% absolute reduction in chemotherapy, compared with the clinical strategy, according to Dr. Piccart. “MINDACT results provide level 1A evidence of the clinical utility of MammaPrint for assessing the lack of a clinically relevant chemotherapy benefit in the clinically high-risk population,” she said.
When asked about the use of MammaPrint in the United States, she said it is more common in Europe and other places in the world, whereas the use of Oncotype DX genetic testing seems to be more prevalent in the U.S.
News conference moderator Dr. Nancy Davidson, director of the University of Pittsburgh Cancer Institute, said that both tests are used at her institution. “I would say Oncotype is probably the dominant test that’s ordered,” she said. “But there are folks who are big fans of MammaPrint, and they’re going to be very excited to see these major results come out from this trial to help us to discuss this further and to think about how to guide our thinking and our patients.”
Entities and funding sources involved in the study included Adjuvant! Online, Agendia, Novartis, Roche, Sanofi-Aventis, Veridex, and Eli Lilly. Dr. Piccart disclosed that she is a board member of Radius, and a consultant for AstraZeneca, Eli Lilly, Invivus, Merck Sharp & Dohme, Novartis, Pfizer, Roche-Genentech, Synthon, Debiopharm, and PharmaMar. The Jules Bordet Institute receives grant/research funds from most companies in the field.
A large multinational European trial shows that genetic analysis of breast tumors can spare women unnecessary adjuvant chemotherapy, even when clinical factors indicate a high risk of recurrence.
Using MammaPrint, a 70-gene signature test, led to a 14% absolute reduction in the use of chemotherapy, compared with a clinical strategy, according to the highly anticipated results of the Phase III Microarray in Node-negative and 1 to 3 positive lymph node Disease may Avoid Chemo Therapy (MINDACT) trial, presented at the annual meeting of the American Association for Cancer Research.
While three decades of clinical trials have shown survival benefits of adjuvant chemotherapy, it carries later risks. “And it will happen when the survival gain associated with adjuvant chemotherapy is small, in the range of 2%-3% – it happens in good prognosis patients – and is counterbalanced by the long-term severe risks of adjuvant chemotherapy,” said Dr. Martine Piccart, director of medicine at the Jules Bordet Institute in Brussels, and principal investigator for the trial.
Risks include secondary cancers, cardiotoxicity, early menopause, and a decline in cognitive function, as well as negative socioeconomic effects. So avoiding chemotherapy when outcomes would be nearly the same without it is a reasonable goal.
To investigate the best method to determine which breast cancer patients could do just as well without adjuvant chemotherapy, European investigators at 111 centers in 9 countries screened 11,288 patients with early-stage disease and enrolled 6,693 in the MINDACT trial. Speaking at a news conference during the annual meeting, Dr. Piccart said MINDACT compared tumor genetic testing with clinical and pathology parameters to gauge the risk of disease recurrence and is the only trial to date to compare the utility of the two strategies.
MammaPrint is a genetic test (G) of early stage breast cancer tumors that produces a 70-gene signature indicating a high or low 10-year risk of metastatic recurrence. The Adjuvant! Online tool takes into account clinical factors and pathology markers, including a patient’s age and comorbidities and a tumor’s estrogen receptor status, grade, size, and the number of positive lymph nodes, to calculate a ten-year “clinical risk” (C) of negative outcomes with or without adjuvant chemotherapy or hormonal therapy.
After surgery, tissue samples underwent local pathology examination to determine tumor stage and nodal, hormone receptor, and HER2 status. Samples were also sent to a central facility for MammaPrint testing. For women with low-risk tumors by both tests, no chemotherapy was indicated. For high-risk tumors by both tests, chemotherapy was prescribed. But those with discordant results were randomized equally to receive chemotherapy or not.
MammaPrint better predictor than clinical factors
The median age of patients at enrollment was 55 years, 80% of tumors were node-negative, 58% were T1, 88% hormone receptor–positive, and 10% HER2-positive. At a median follow-up of 5 years, 362 (5.4%) women had distant metastases or had died.
Among 3,356 patients classified as high risk based on the Adjuvant! Online test, there were 1,550 classified by genetic criteria as low risk (C-High/G-Low), including 48% with positive nodes. For this C-High/G-Low group, the 5-year distant metastasis-free survival was greater than 94% regardless of adjuvant chemotherapy and was not statistically different for the two groups. These are patients who would normally be prescribed adjuvant chemotherapy if based solely on the C criteria.
Going by MammaPrint test results would thereby reduce chemotherapy prescriptions by 46% (1,550/3,356) for the study cohort. Considering the entire initial 11,288 patients screened, the genomic strategy would lead to a 14% absolute reduction in chemotherapy, compared with the clinical strategy, according to Dr. Piccart. “MINDACT results provide level 1A evidence of the clinical utility of MammaPrint for assessing the lack of a clinically relevant chemotherapy benefit in the clinically high-risk population,” she said.
When asked about the use of MammaPrint in the United States, she said it is more common in Europe and other places in the world, whereas the use of Oncotype DX genetic testing seems to be more prevalent in the U.S.
News conference moderator Dr. Nancy Davidson, director of the University of Pittsburgh Cancer Institute, said that both tests are used at her institution. “I would say Oncotype is probably the dominant test that’s ordered,” she said. “But there are folks who are big fans of MammaPrint, and they’re going to be very excited to see these major results come out from this trial to help us to discuss this further and to think about how to guide our thinking and our patients.”
Entities and funding sources involved in the study included Adjuvant! Online, Agendia, Novartis, Roche, Sanofi-Aventis, Veridex, and Eli Lilly. Dr. Piccart disclosed that she is a board member of Radius, and a consultant for AstraZeneca, Eli Lilly, Invivus, Merck Sharp & Dohme, Novartis, Pfizer, Roche-Genentech, Synthon, Debiopharm, and PharmaMar. The Jules Bordet Institute receives grant/research funds from most companies in the field.
FROM THE AACR ANNUAL MEETING
Key clinical point: MammaPrint bests Adjuvant! Online for sparing low-risk patients from chemotherapy.
Major finding: Genotyping results in a 14% reduction in the use of chemotherapy, compared with a clinical strategy.
Data source: Phase III randomized controlled study of 6,693 women with early stage breast cancer.
Disclosures: Entities and funding sources involved in the study include Adjuvant! Online, Agendia, Novartis, Roche, Sanofi-Aventis, Veridex, and Eli Lilly. Dr. Piccart disclosed that she is a board member of Radius, and a consultant for AstraZeneca, Eli Lilly, Invivus, Merck Sharp & Dohme, Novartis, Pfizer, Roche-Genentech, Synthon, Debiopharm, and PharmaMar. The Jules Bordet Institute receives grant/research funds from most companies in the field.
Targeting gene rearrangements shows promise in early study
Entrectinib, an investigational drug that targets several abnormal fusion proteins, showed antitumor activity and was safe in patients with several different types of advanced solid tumors. The patients had never before been exposed to drugs targeting these same genetic alterations.
“Responses can be very rapid and durable … which include colorectal, primary brain tumor, astrocytoma, fibrosarcoma, lung, and mammary analog secretory carcinoma,” Dr. Alexander Drilon of Memorial Sloan Kettering Cancer Center in New York said in a news conference at the annual meeting of the American Association for Cancer Research. “Dramatic intracranial activity … has been demonstrated both in primary brain tumor and also in metastatic.”
NTRK1/2/3, ROS1, and ALK gene–rearranged cancers produce fusion proteins that are ligand independent for their activity and thus constitutively active, driving tumor growth. Entrectinib is a pan-TRK, ROS1, ALK tyrosine kinase inhibitor that targets the abnormal fusion protein products of the genes, is highly potent at low concentrations, and has been designed to cross the blood-brain barrier (BBB). The targeted proteins are present across multiple cancers and are especially prevalent (greater than 80%) among some rare adult and pediatric cancers.
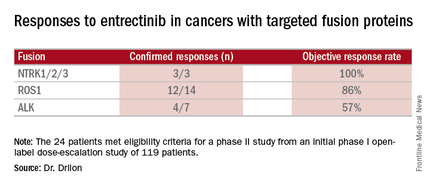
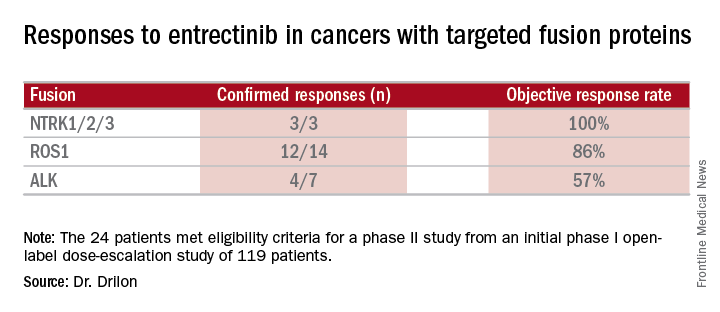
Combined data on 119 patients in two phase I trials established 600 mg orally once daily as the recommended dose to go into phase II trials. Among the 24 patients meeting eligibility criteria for a phase II trial (presence of the targeted gene fusions in their tumors, no prior treatment against these targets, and treatment at or above 600 mg daily), the confirmed response rate was 79% (19/24). Most were partial responses in terms of tumor shrinkage, but two patients had complete responses. Response rates appeared to vary according to the specific fusion protein defect.
All three cases of CNS disease with NTRK-rearranged cancers had intracranial responses, demonstrating that the drug crosses the BBB and is active. In one case, a 46-year-old man with brain metastases heavily pretreated for non–small cell lung cancer with an NTRK1 rearrangement experienced a dramatic response.
“The patient at that point was actually on hospice and was doing extremely poorly on supplemental oxygen,” Dr. Drilon said. “Within a few weeks, the patient had a dramatic clinical response to therapy … At day 26 there was almost a 50% reduction in tumor burden.” At day 317 scans showed he had a complete intracranial response to entrectinib, but he still has visceral disease on therapy past 1 year.
Responses often occurred within the first month of therapy, and many persisted for several months without disease progression, with one patient being followed for more than 2 years with clinical benefit. Nineteen of 24 patients have been on the therapy for more than 6 months, and the therapy appears to be safe and well tolerated.
Commenting on this study and others targeting specific genetic alterations leading to cancer, Dr. Louis Weiner, director of the Georgetown Lombardi Comprehensive Cancer Center in Washington, said, “You’re seeing a series of clinical trials described that aren’t necessarily targeting people with a particular cancer but rather people who have cancers characterized by particular molecular abnormalities.” Not all cancers will have identified molecular abnormalities driving them. “However, I think where you have these drivers, the proper thing to do is not to worry about whether [a drug] works in a given disease but rather whether it works for people with that particular abnormality,” he said.
For the future, the investigators plan a phase II trial called STARTRK-2. It is a multicenter, open-label, global basket study to include any solid tumors with the targeted rearrangements.
Dr. Drilon disclosed ties with Ignyta, which funded the study, and has received research funding from Foundation Medicine. Dr. Weiner disclosed ties with several pharmaceutical companies.
Entrectinib, an investigational drug that targets several abnormal fusion proteins, showed antitumor activity and was safe in patients with several different types of advanced solid tumors. The patients had never before been exposed to drugs targeting these same genetic alterations.
“Responses can be very rapid and durable … which include colorectal, primary brain tumor, astrocytoma, fibrosarcoma, lung, and mammary analog secretory carcinoma,” Dr. Alexander Drilon of Memorial Sloan Kettering Cancer Center in New York said in a news conference at the annual meeting of the American Association for Cancer Research. “Dramatic intracranial activity … has been demonstrated both in primary brain tumor and also in metastatic.”
NTRK1/2/3, ROS1, and ALK gene–rearranged cancers produce fusion proteins that are ligand independent for their activity and thus constitutively active, driving tumor growth. Entrectinib is a pan-TRK, ROS1, ALK tyrosine kinase inhibitor that targets the abnormal fusion protein products of the genes, is highly potent at low concentrations, and has been designed to cross the blood-brain barrier (BBB). The targeted proteins are present across multiple cancers and are especially prevalent (greater than 80%) among some rare adult and pediatric cancers.

Combined data on 119 patients in two phase I trials established 600 mg orally once daily as the recommended dose to go into phase II trials. Among the 24 patients meeting eligibility criteria for a phase II trial (presence of the targeted gene fusions in their tumors, no prior treatment against these targets, and treatment at or above 600 mg daily), the confirmed response rate was 79% (19/24). Most were partial responses in terms of tumor shrinkage, but two patients had complete responses. Response rates appeared to vary according to the specific fusion protein defect.
All three cases of CNS disease with NTRK-rearranged cancers had intracranial responses, demonstrating that the drug crosses the BBB and is active. In one case, a 46-year-old man with brain metastases heavily pretreated for non–small cell lung cancer with an NTRK1 rearrangement experienced a dramatic response.
“The patient at that point was actually on hospice and was doing extremely poorly on supplemental oxygen,” Dr. Drilon said. “Within a few weeks, the patient had a dramatic clinical response to therapy … At day 26 there was almost a 50% reduction in tumor burden.” At day 317 scans showed he had a complete intracranial response to entrectinib, but he still has visceral disease on therapy past 1 year.
Responses often occurred within the first month of therapy, and many persisted for several months without disease progression, with one patient being followed for more than 2 years with clinical benefit. Nineteen of 24 patients have been on the therapy for more than 6 months, and the therapy appears to be safe and well tolerated.
Commenting on this study and others targeting specific genetic alterations leading to cancer, Dr. Louis Weiner, director of the Georgetown Lombardi Comprehensive Cancer Center in Washington, said, “You’re seeing a series of clinical trials described that aren’t necessarily targeting people with a particular cancer but rather people who have cancers characterized by particular molecular abnormalities.” Not all cancers will have identified molecular abnormalities driving them. “However, I think where you have these drivers, the proper thing to do is not to worry about whether [a drug] works in a given disease but rather whether it works for people with that particular abnormality,” he said.
For the future, the investigators plan a phase II trial called STARTRK-2. It is a multicenter, open-label, global basket study to include any solid tumors with the targeted rearrangements.
Dr. Drilon disclosed ties with Ignyta, which funded the study, and has received research funding from Foundation Medicine. Dr. Weiner disclosed ties with several pharmaceutical companies.
Entrectinib, an investigational drug that targets several abnormal fusion proteins, showed antitumor activity and was safe in patients with several different types of advanced solid tumors. The patients had never before been exposed to drugs targeting these same genetic alterations.
“Responses can be very rapid and durable … which include colorectal, primary brain tumor, astrocytoma, fibrosarcoma, lung, and mammary analog secretory carcinoma,” Dr. Alexander Drilon of Memorial Sloan Kettering Cancer Center in New York said in a news conference at the annual meeting of the American Association for Cancer Research. “Dramatic intracranial activity … has been demonstrated both in primary brain tumor and also in metastatic.”
NTRK1/2/3, ROS1, and ALK gene–rearranged cancers produce fusion proteins that are ligand independent for their activity and thus constitutively active, driving tumor growth. Entrectinib is a pan-TRK, ROS1, ALK tyrosine kinase inhibitor that targets the abnormal fusion protein products of the genes, is highly potent at low concentrations, and has been designed to cross the blood-brain barrier (BBB). The targeted proteins are present across multiple cancers and are especially prevalent (greater than 80%) among some rare adult and pediatric cancers.

Combined data on 119 patients in two phase I trials established 600 mg orally once daily as the recommended dose to go into phase II trials. Among the 24 patients meeting eligibility criteria for a phase II trial (presence of the targeted gene fusions in their tumors, no prior treatment against these targets, and treatment at or above 600 mg daily), the confirmed response rate was 79% (19/24). Most were partial responses in terms of tumor shrinkage, but two patients had complete responses. Response rates appeared to vary according to the specific fusion protein defect.
All three cases of CNS disease with NTRK-rearranged cancers had intracranial responses, demonstrating that the drug crosses the BBB and is active. In one case, a 46-year-old man with brain metastases heavily pretreated for non–small cell lung cancer with an NTRK1 rearrangement experienced a dramatic response.
“The patient at that point was actually on hospice and was doing extremely poorly on supplemental oxygen,” Dr. Drilon said. “Within a few weeks, the patient had a dramatic clinical response to therapy … At day 26 there was almost a 50% reduction in tumor burden.” At day 317 scans showed he had a complete intracranial response to entrectinib, but he still has visceral disease on therapy past 1 year.
Responses often occurred within the first month of therapy, and many persisted for several months without disease progression, with one patient being followed for more than 2 years with clinical benefit. Nineteen of 24 patients have been on the therapy for more than 6 months, and the therapy appears to be safe and well tolerated.
Commenting on this study and others targeting specific genetic alterations leading to cancer, Dr. Louis Weiner, director of the Georgetown Lombardi Comprehensive Cancer Center in Washington, said, “You’re seeing a series of clinical trials described that aren’t necessarily targeting people with a particular cancer but rather people who have cancers characterized by particular molecular abnormalities.” Not all cancers will have identified molecular abnormalities driving them. “However, I think where you have these drivers, the proper thing to do is not to worry about whether [a drug] works in a given disease but rather whether it works for people with that particular abnormality,” he said.
For the future, the investigators plan a phase II trial called STARTRK-2. It is a multicenter, open-label, global basket study to include any solid tumors with the targeted rearrangements.
Dr. Drilon disclosed ties with Ignyta, which funded the study, and has received research funding from Foundation Medicine. Dr. Weiner disclosed ties with several pharmaceutical companies.
FROM THE AACR ANNUAL MEETING
Key clinical point: Entrectinib showed antitumor activity including intracranial responses.
Major finding: Patients with the targeted abnormalities had a 79% response rate.
Data source: Twenty-four patients meeting eligibility criteria for a phase II study from an initial phase I open-label dose-escalation study of 119 patients.
Disclosures: Dr. Drilon disclosed ties with Ignyta, which funded the study, and has received research funding from Foundation Medicine. Dr. Weiner disclosed ties with several pharmaceutical companies.
Engineered helper T cells targeting MAGE-A3 produced some clinical responses
CD4+ T cells genetically engineered to recognize MAGE-A3, a protein found on many types of tumor cells but not on normal cells, were safely given to patients with metastatic cancers, and some of the patients had clinical responses.
Whereas the majority of current adoptive T-cell therapies have used genetically modified unpurified T cells or CD8+ (cytotoxic) T cells, Yong-Chen William Lu, Ph.D., of the surgery branch of the National Cancer Institute conducted a phase I trial of CD4+ (helper) T cells to evaluate their contribution to tumor killing. Preclinical and clinical studies indicated that CD4+ T cells can induce tumor regression.
Dr. Lu explained the individualized process of making the T cells for infusion, during a news conference at the annual meeting of the American Association for Cancer Research. First, T cells are harvested from the peripheral blood of a patient, from which CD4+ T cells are isolated. Using a retroviral vector, scientists insert the gene for a T-cell receptor that recognizes MAGE-A3 in conjunction with the HLA molecule DPB1*0401. In this way, the cells can recognize the tumor antigen in anyone of that HLA type. Then the cells carrying the engineered T-cell receptor are expanded in vitro and infused into the patient.
DPB1*0401 was chosen as the HLA genotype because “it is present in 60% of the Caucasian population. It is the highest one,” Dr. Lu said. MAGE-A3 is expressed on cells during fetal development and is later lost on normal tissues. But several tumor types, including some melanomas and some urothelial, esophageal, and cervical cancers reexpress this protein, making it a good target for immunotherapy.
The trial included patients of the HLA DPB1*0401 type with metastatic cancer that is MAGE-A3 positive. Patients had received at least one first-line treatment for metastatic disease and had been nonresponders or had had a recurrence of the tumor.
This phase I trial involved 14 patients in nine different dose cohorts, receiving from 10 million engineered T cells at the lowest dose to 78-103 billion cells in the highest cohort. Three patients in the middle and highest dosing cohorts achieved partial responses, one each with cervical, esophageal, and urothelial tumors. The partial response lasted 4 months for the patient with esophageal cancer and is ongoing at 7 months for the urothelial and 15 months for the cervical cancer patients.
In response to a question from the audience about adverse effects, Dr. Lu said patients had short-term elevations of circulating cytokines, “which only lasted for a few days,” and a fever lasting 1-2 weeks.
In summary, he said, “We think this first [CD4+] engineered T-cells therapy targeting MAGE-A3 is safe and has shown early clinical response.” Phase II trials are now beginning.
The approach of this immunotherapy method is different from that involved in using checkpoint inhibitors, “which essentially release the brakes of the immune system so the T cells can do their job of attacking the cancer,” commented Dr. Louis Weiner, director of the Georgetown Lombardi Cancer Center in Washington, D.C. “This is actually not trying to release brakes but essentially press on the accelerator, if you will, by using an expanded tumor-specific population of T cells that will then be able to hone in and attack the cancer.” He envisioned a time in the future when this approach could be combined with checkpoint inhibitors for enhanced antitumor effects.
Dr, Weiner also noted that the T cells with receptors engineered to recognize MAGE-A3 are more specific for tumors, compared with approaches using chimeric antigen receptor T cells to treat certain hematologic malignancies. In that setting the chimeric antigen receptor T cells recognize and eliminate, for example, not only malignant B cells bearing a pan-B cell marker but also all normal B cells.
However, in the question and answer period, Dr. Lu said one problem with his approach is the possibility that not all cells in a tumor may express MAGE-A3.
There was no commercial funding of the study. Dr. Lu had no relevant financial disclosures. Dr. Weiner is an adviser, review panel member, or consultant, or has other relationships, with numerous pharmaceutical companies.
CD4+ T cells genetically engineered to recognize MAGE-A3, a protein found on many types of tumor cells but not on normal cells, were safely given to patients with metastatic cancers, and some of the patients had clinical responses.
Whereas the majority of current adoptive T-cell therapies have used genetically modified unpurified T cells or CD8+ (cytotoxic) T cells, Yong-Chen William Lu, Ph.D., of the surgery branch of the National Cancer Institute conducted a phase I trial of CD4+ (helper) T cells to evaluate their contribution to tumor killing. Preclinical and clinical studies indicated that CD4+ T cells can induce tumor regression.
Dr. Lu explained the individualized process of making the T cells for infusion, during a news conference at the annual meeting of the American Association for Cancer Research. First, T cells are harvested from the peripheral blood of a patient, from which CD4+ T cells are isolated. Using a retroviral vector, scientists insert the gene for a T-cell receptor that recognizes MAGE-A3 in conjunction with the HLA molecule DPB1*0401. In this way, the cells can recognize the tumor antigen in anyone of that HLA type. Then the cells carrying the engineered T-cell receptor are expanded in vitro and infused into the patient.
DPB1*0401 was chosen as the HLA genotype because “it is present in 60% of the Caucasian population. It is the highest one,” Dr. Lu said. MAGE-A3 is expressed on cells during fetal development and is later lost on normal tissues. But several tumor types, including some melanomas and some urothelial, esophageal, and cervical cancers reexpress this protein, making it a good target for immunotherapy.
The trial included patients of the HLA DPB1*0401 type with metastatic cancer that is MAGE-A3 positive. Patients had received at least one first-line treatment for metastatic disease and had been nonresponders or had had a recurrence of the tumor.
This phase I trial involved 14 patients in nine different dose cohorts, receiving from 10 million engineered T cells at the lowest dose to 78-103 billion cells in the highest cohort. Three patients in the middle and highest dosing cohorts achieved partial responses, one each with cervical, esophageal, and urothelial tumors. The partial response lasted 4 months for the patient with esophageal cancer and is ongoing at 7 months for the urothelial and 15 months for the cervical cancer patients.
In response to a question from the audience about adverse effects, Dr. Lu said patients had short-term elevations of circulating cytokines, “which only lasted for a few days,” and a fever lasting 1-2 weeks.
In summary, he said, “We think this first [CD4+] engineered T-cells therapy targeting MAGE-A3 is safe and has shown early clinical response.” Phase II trials are now beginning.
The approach of this immunotherapy method is different from that involved in using checkpoint inhibitors, “which essentially release the brakes of the immune system so the T cells can do their job of attacking the cancer,” commented Dr. Louis Weiner, director of the Georgetown Lombardi Cancer Center in Washington, D.C. “This is actually not trying to release brakes but essentially press on the accelerator, if you will, by using an expanded tumor-specific population of T cells that will then be able to hone in and attack the cancer.” He envisioned a time in the future when this approach could be combined with checkpoint inhibitors for enhanced antitumor effects.
Dr, Weiner also noted that the T cells with receptors engineered to recognize MAGE-A3 are more specific for tumors, compared with approaches using chimeric antigen receptor T cells to treat certain hematologic malignancies. In that setting the chimeric antigen receptor T cells recognize and eliminate, for example, not only malignant B cells bearing a pan-B cell marker but also all normal B cells.
However, in the question and answer period, Dr. Lu said one problem with his approach is the possibility that not all cells in a tumor may express MAGE-A3.
There was no commercial funding of the study. Dr. Lu had no relevant financial disclosures. Dr. Weiner is an adviser, review panel member, or consultant, or has other relationships, with numerous pharmaceutical companies.
CD4+ T cells genetically engineered to recognize MAGE-A3, a protein found on many types of tumor cells but not on normal cells, were safely given to patients with metastatic cancers, and some of the patients had clinical responses.
Whereas the majority of current adoptive T-cell therapies have used genetically modified unpurified T cells or CD8+ (cytotoxic) T cells, Yong-Chen William Lu, Ph.D., of the surgery branch of the National Cancer Institute conducted a phase I trial of CD4+ (helper) T cells to evaluate their contribution to tumor killing. Preclinical and clinical studies indicated that CD4+ T cells can induce tumor regression.
Dr. Lu explained the individualized process of making the T cells for infusion, during a news conference at the annual meeting of the American Association for Cancer Research. First, T cells are harvested from the peripheral blood of a patient, from which CD4+ T cells are isolated. Using a retroviral vector, scientists insert the gene for a T-cell receptor that recognizes MAGE-A3 in conjunction with the HLA molecule DPB1*0401. In this way, the cells can recognize the tumor antigen in anyone of that HLA type. Then the cells carrying the engineered T-cell receptor are expanded in vitro and infused into the patient.
DPB1*0401 was chosen as the HLA genotype because “it is present in 60% of the Caucasian population. It is the highest one,” Dr. Lu said. MAGE-A3 is expressed on cells during fetal development and is later lost on normal tissues. But several tumor types, including some melanomas and some urothelial, esophageal, and cervical cancers reexpress this protein, making it a good target for immunotherapy.
The trial included patients of the HLA DPB1*0401 type with metastatic cancer that is MAGE-A3 positive. Patients had received at least one first-line treatment for metastatic disease and had been nonresponders or had had a recurrence of the tumor.
This phase I trial involved 14 patients in nine different dose cohorts, receiving from 10 million engineered T cells at the lowest dose to 78-103 billion cells in the highest cohort. Three patients in the middle and highest dosing cohorts achieved partial responses, one each with cervical, esophageal, and urothelial tumors. The partial response lasted 4 months for the patient with esophageal cancer and is ongoing at 7 months for the urothelial and 15 months for the cervical cancer patients.
In response to a question from the audience about adverse effects, Dr. Lu said patients had short-term elevations of circulating cytokines, “which only lasted for a few days,” and a fever lasting 1-2 weeks.
In summary, he said, “We think this first [CD4+] engineered T-cells therapy targeting MAGE-A3 is safe and has shown early clinical response.” Phase II trials are now beginning.
The approach of this immunotherapy method is different from that involved in using checkpoint inhibitors, “which essentially release the brakes of the immune system so the T cells can do their job of attacking the cancer,” commented Dr. Louis Weiner, director of the Georgetown Lombardi Cancer Center in Washington, D.C. “This is actually not trying to release brakes but essentially press on the accelerator, if you will, by using an expanded tumor-specific population of T cells that will then be able to hone in and attack the cancer.” He envisioned a time in the future when this approach could be combined with checkpoint inhibitors for enhanced antitumor effects.
Dr, Weiner also noted that the T cells with receptors engineered to recognize MAGE-A3 are more specific for tumors, compared with approaches using chimeric antigen receptor T cells to treat certain hematologic malignancies. In that setting the chimeric antigen receptor T cells recognize and eliminate, for example, not only malignant B cells bearing a pan-B cell marker but also all normal B cells.
However, in the question and answer period, Dr. Lu said one problem with his approach is the possibility that not all cells in a tumor may express MAGE-A3.
There was no commercial funding of the study. Dr. Lu had no relevant financial disclosures. Dr. Weiner is an adviser, review panel member, or consultant, or has other relationships, with numerous pharmaceutical companies.
FROM THE AACR ANNUAL MEETING
Key clinical point: Engineered helper T cells safely target tumors and produce clinical responses.
Major finding: Three of 14 patients with different tumor types had partial tumor responses when given T cells with receptors engineered to recognize a common tumor antigen, MAGE-A3.
Data source: A phase I dose-escalating study of 14 patients.
Disclosures: There was no commercial funding of the study. Dr. Lu had no relevant financial disclosures. Dr. Weiner is an adviser, review panel member, or consultant, or has other relationships, with numerous pharmaceutical companies.
Study found long-term benefit of nivolumab for advanced melanoma patients
One-third of a group of heavily pretreated patients with advanced melanoma who received nivolumab monotherapy were alive 5 years after starting the drug, a phase I trial showed. Survival plateaued at 34% by 48 months, after which time disease control was maintained.
“What it does demonstrate is the importance of the durability of clinical benefit to patients [who] now we’re measuring in terms of years, as well as the memory aspect of how the immunologic memory can translate into benefit,” said Dr. Stephen Hodi, director of the melanoma center at Dana-Farber Cancer Institute in Boston during a news conference at the annual meeting of the American Association for Cancer Research.
Nivolumab is an immune checkpoint inhibitor that blocks PD-1, allowing enhanced antitumor immune responses. It is approved as a first-line agent for advanced melanoma, alone or in combination with ipilimumab. The approved dose for monotherapy is 3 mg/kg every 2 weeks.
The study randomized 107 patients (median age, 61 years; 67% male) to one of five doses ranging from 0.1 mg/kg to 10 mg/kg IV given every 2 weeks for up to 96 weeks. The patients had advanced melanoma at any site and had been on one to five lines of systemic therapy prior to entering the trial but could not have received any immune checkpoint inhibitors. They had treated and stable brain metastases and an ECOG (Eastern Cooperative Oncology Group) performance status of 0, 1, or 2. The minimum follow-up was 45 months.
Among all the patients, the median overall survival was 17.3 months (95% confidence interval, 12.5-37.8 months), and for those patients receiving 3-mg/kg doses, the median survival was 20.3 months (95% CI, 7.2 months and no upper confidence level reached). The Kaplan-Meier survival curves for all patients and those receiving the 3-mg/kg dose began to level off at about 36 months, and were essentially flat at 34% for all patients as a group from 48 months out to 5 years.
Five patients who achieved disease control but later had progressive disease were retreated with their original doses of nivolumab after being off the drug for more than 100 days. “In all five patients, disease control was obtained again, and this disease control was again durable,” Dr. Hodi reported.
Nivolumab monotherapy was safe and well tolerated. No study deaths or new safety signals were observed. The most common drug-related adverse effects were any grade of fatigue (29.9% of all patients; 47.1% of patients at the 3-mg/kg dose) and any grade of rash (23.4% and 11.8%, respectively). Although there were some grade 3-4 adverse events, adverse reactions led to study discontinuation for only 10.3% of the total patient group and 5.9% of patients on the 3-mg/kg dose.
Calling the study findings “very compelling,” conference moderator Dr. Louis Weiner, director of the Georgetown Lombardi Comprehensive Cancer Center in Washington, D.C., identified what he said is a key point of the study – the length of benefit. “What distinguishes immunotherapy at this point when it’s effective from other forms of cancer treatment is the durability of the benefit,” he said. “People who have good responses really seem to be protected against the disease returning in many cases.”
He asked Dr. Hodi if he would expect even better disease control results if nivolumab were combined with a different checkpoint inhibitor, such as ipilimumab. Dr. Hodi replied that it is reasonable to think that agents working through different mechanisms of action should produce even better results.
A reporter in the audience asked if it was Dr. Hodi’s practice to test tumors for PD-1 expression before using nivolumab. He replied that he and his colleagues do not routinely test for PD-1 because there have been observations that some tumors that test negative for the biomarker do respond to the drug.
One-third of a group of heavily pretreated patients with advanced melanoma who received nivolumab monotherapy were alive 5 years after starting the drug, a phase I trial showed. Survival plateaued at 34% by 48 months, after which time disease control was maintained.
“What it does demonstrate is the importance of the durability of clinical benefit to patients [who] now we’re measuring in terms of years, as well as the memory aspect of how the immunologic memory can translate into benefit,” said Dr. Stephen Hodi, director of the melanoma center at Dana-Farber Cancer Institute in Boston during a news conference at the annual meeting of the American Association for Cancer Research.
Nivolumab is an immune checkpoint inhibitor that blocks PD-1, allowing enhanced antitumor immune responses. It is approved as a first-line agent for advanced melanoma, alone or in combination with ipilimumab. The approved dose for monotherapy is 3 mg/kg every 2 weeks.
The study randomized 107 patients (median age, 61 years; 67% male) to one of five doses ranging from 0.1 mg/kg to 10 mg/kg IV given every 2 weeks for up to 96 weeks. The patients had advanced melanoma at any site and had been on one to five lines of systemic therapy prior to entering the trial but could not have received any immune checkpoint inhibitors. They had treated and stable brain metastases and an ECOG (Eastern Cooperative Oncology Group) performance status of 0, 1, or 2. The minimum follow-up was 45 months.
Among all the patients, the median overall survival was 17.3 months (95% confidence interval, 12.5-37.8 months), and for those patients receiving 3-mg/kg doses, the median survival was 20.3 months (95% CI, 7.2 months and no upper confidence level reached). The Kaplan-Meier survival curves for all patients and those receiving the 3-mg/kg dose began to level off at about 36 months, and were essentially flat at 34% for all patients as a group from 48 months out to 5 years.
Five patients who achieved disease control but later had progressive disease were retreated with their original doses of nivolumab after being off the drug for more than 100 days. “In all five patients, disease control was obtained again, and this disease control was again durable,” Dr. Hodi reported.
Nivolumab monotherapy was safe and well tolerated. No study deaths or new safety signals were observed. The most common drug-related adverse effects were any grade of fatigue (29.9% of all patients; 47.1% of patients at the 3-mg/kg dose) and any grade of rash (23.4% and 11.8%, respectively). Although there were some grade 3-4 adverse events, adverse reactions led to study discontinuation for only 10.3% of the total patient group and 5.9% of patients on the 3-mg/kg dose.
Calling the study findings “very compelling,” conference moderator Dr. Louis Weiner, director of the Georgetown Lombardi Comprehensive Cancer Center in Washington, D.C., identified what he said is a key point of the study – the length of benefit. “What distinguishes immunotherapy at this point when it’s effective from other forms of cancer treatment is the durability of the benefit,” he said. “People who have good responses really seem to be protected against the disease returning in many cases.”
He asked Dr. Hodi if he would expect even better disease control results if nivolumab were combined with a different checkpoint inhibitor, such as ipilimumab. Dr. Hodi replied that it is reasonable to think that agents working through different mechanisms of action should produce even better results.
A reporter in the audience asked if it was Dr. Hodi’s practice to test tumors for PD-1 expression before using nivolumab. He replied that he and his colleagues do not routinely test for PD-1 because there have been observations that some tumors that test negative for the biomarker do respond to the drug.
One-third of a group of heavily pretreated patients with advanced melanoma who received nivolumab monotherapy were alive 5 years after starting the drug, a phase I trial showed. Survival plateaued at 34% by 48 months, after which time disease control was maintained.
“What it does demonstrate is the importance of the durability of clinical benefit to patients [who] now we’re measuring in terms of years, as well as the memory aspect of how the immunologic memory can translate into benefit,” said Dr. Stephen Hodi, director of the melanoma center at Dana-Farber Cancer Institute in Boston during a news conference at the annual meeting of the American Association for Cancer Research.
Nivolumab is an immune checkpoint inhibitor that blocks PD-1, allowing enhanced antitumor immune responses. It is approved as a first-line agent for advanced melanoma, alone or in combination with ipilimumab. The approved dose for monotherapy is 3 mg/kg every 2 weeks.
The study randomized 107 patients (median age, 61 years; 67% male) to one of five doses ranging from 0.1 mg/kg to 10 mg/kg IV given every 2 weeks for up to 96 weeks. The patients had advanced melanoma at any site and had been on one to five lines of systemic therapy prior to entering the trial but could not have received any immune checkpoint inhibitors. They had treated and stable brain metastases and an ECOG (Eastern Cooperative Oncology Group) performance status of 0, 1, or 2. The minimum follow-up was 45 months.
Among all the patients, the median overall survival was 17.3 months (95% confidence interval, 12.5-37.8 months), and for those patients receiving 3-mg/kg doses, the median survival was 20.3 months (95% CI, 7.2 months and no upper confidence level reached). The Kaplan-Meier survival curves for all patients and those receiving the 3-mg/kg dose began to level off at about 36 months, and were essentially flat at 34% for all patients as a group from 48 months out to 5 years.
Five patients who achieved disease control but later had progressive disease were retreated with their original doses of nivolumab after being off the drug for more than 100 days. “In all five patients, disease control was obtained again, and this disease control was again durable,” Dr. Hodi reported.
Nivolumab monotherapy was safe and well tolerated. No study deaths or new safety signals were observed. The most common drug-related adverse effects were any grade of fatigue (29.9% of all patients; 47.1% of patients at the 3-mg/kg dose) and any grade of rash (23.4% and 11.8%, respectively). Although there were some grade 3-4 adverse events, adverse reactions led to study discontinuation for only 10.3% of the total patient group and 5.9% of patients on the 3-mg/kg dose.
Calling the study findings “very compelling,” conference moderator Dr. Louis Weiner, director of the Georgetown Lombardi Comprehensive Cancer Center in Washington, D.C., identified what he said is a key point of the study – the length of benefit. “What distinguishes immunotherapy at this point when it’s effective from other forms of cancer treatment is the durability of the benefit,” he said. “People who have good responses really seem to be protected against the disease returning in many cases.”
He asked Dr. Hodi if he would expect even better disease control results if nivolumab were combined with a different checkpoint inhibitor, such as ipilimumab. Dr. Hodi replied that it is reasonable to think that agents working through different mechanisms of action should produce even better results.
A reporter in the audience asked if it was Dr. Hodi’s practice to test tumors for PD-1 expression before using nivolumab. He replied that he and his colleagues do not routinely test for PD-1 because there have been observations that some tumors that test negative for the biomarker do respond to the drug.
FROM THE AACR ANNUAL MEETING
Key clinical point: Heavily pretreated patients with advanced melanoma had long-term responses on nivolumab monotherapy.
Major finding: Nivolumab monotherapy resulted in a 34% overall survival rate at 5 years for melanoma patients who had received extensive prior therapy. Data source: A phase I randomized dose-ranging study involving 107 patients with advanced melanoma.
Disclosures: The study was funded by Bristol-Myers Squibb. Dr. Hodi has received grant/research support from and is a nonpaid consultant to Bristol-Myers. Dr. Weiner is an adviser, review panel member, or consultant or has other relationships with numerous pharmaceutical companies.