User login
Risk Factors Predicting Cellulitis Diagnosis in a Prospective Cohort Undergoing Dermatology Consultation in the Emergency Department
Cellulitis is an infection of the skin and skin-associated structures characterized by redness, warmth, swelling, and pain of the affected area. Cellulitis most commonly occurs in middle-aged and older adults and frequently affects the lower extremities.1 Serious complications of cellulitis such as bacteremia, metastatic infection, and sepsis are rare, and most cases of cellulitis in patients with normal vital signs and mental status can be managed with outpatient treatment.2
Diagnosis of cellulitis can be confounded by a number of similarly presenting conditions collectively known as pseudocellulitis, such as venous stasis dermatitis and deep vein thrombosis.1 Misdiagnosis of cellulitis is common, with rates exceeding 30% among hospitalized patients initially diagnosed with cellulitis.3,4 Dermatology or infectious disease assessment is considered the diagnostic gold standard for cellulitis4,5 but is not always readily available, especially in resource-constrained settings.
Most cases of uncomplicated cellulitis can be managed with outpatient treatment, especially because serious complications are rare. Frequent misdiagnosis leads to repeat or unnecessary hospitalization and antibiosis. Exceptions necessitating hospitalization usually are predicated on signs of systemic infection, severe immunocompromised states, or failure of prior outpatient therapy.6 Such presentations can be distinguished by corresponding notable historical or examination factors, such as vital sign abnormalities suggesting systemic infection or history of malignancy leading to an immunocompromised state.
We sought to evaluate factors leading to the diagnosis of cellulitis in a cohort of patients with uncomplicated presentations receiving dermatology consultation to emphasize findings indicative of cellulitis in the absence of clinical or historical factors suggestive of other conditions necessitating hospitalization, such as systemic infection.
Methods
Study Participants—A prospective cohort study of patients presenting to an emergency department (ED) between October 2012 and January 2017 at an urban academic medical center in Boston, Massachusetts, was conducted with approval of study design and procedures by the relevant institutional review board. Patients older than 18 years were eligible for inclusion if given an initial diagnosis of cellulitis by an ED physician. Patients were excluded if incarcerated, pregnant, or unable to provide informed consent. Other exclusion criteria includedinfections overlying temporary or permanent indwelling hardware, animal or human bites, or sites of recent surgery (within the prior 4 weeks); preceding antibiotic treatment for more than 24 hours; or clinical or radiographic evidence of complications requiring alternative management such as osteomyelitis or abscess. Patients presenting with an elevated heart rate (>100 beats per minute) or body temperature (>100.5 °F [38.1 °C]) also were excluded. Eligible patients were enrolled upon providing written informed consent, and no remuneration was offered for participation.
Dermatology Consultation Intervention—A random subset of enrolled patients received dermatology consultation within 24 hours of presentation. Consultation consisted of a patient interview and physical examination with care recommendations to relevant ED and inpatient teams. Consultations confirmed the presence or absence of cellulitis as the primary outcome and also noted the presence of any pseudocellulitis diagnoses either occurring concomitantly with or mimicking cellulitis as a secondary outcome.
Statistical Analysis—Patient characteristics were analyzed to identify factors independently associated with the diagnosis of cellulitis in cases affecting the lower extremities. Factors were recorded with categorical variables reported as counts and percentages and continuous variables as means and standard deviations. Univariate analyses between categorical variables or discretized continuous variables and cellulitis diagnosis were conducted via Fisher exact test to identify a preliminary set of potential risk factors. Continuous variables were discretized at multiple incremental values with the discretization most significantly associated with cellulitis diagnosis selected as a preliminary risk factor. Multivariate analyses involved using any objective preliminary factor meeting a significance threshold of P<.1 in univariate comparisons in a multivariate logistic regression model for prediction of cellulitis diagnosis with corresponding calculation of odds ratios with confidence intervals and receiver operating characteristic. Factors with confidence intervals that excluded 1 were considered significant independent predictors of cellulitis. Analyses were performed using Python version 3.8 (Python Software Foundation).
Results
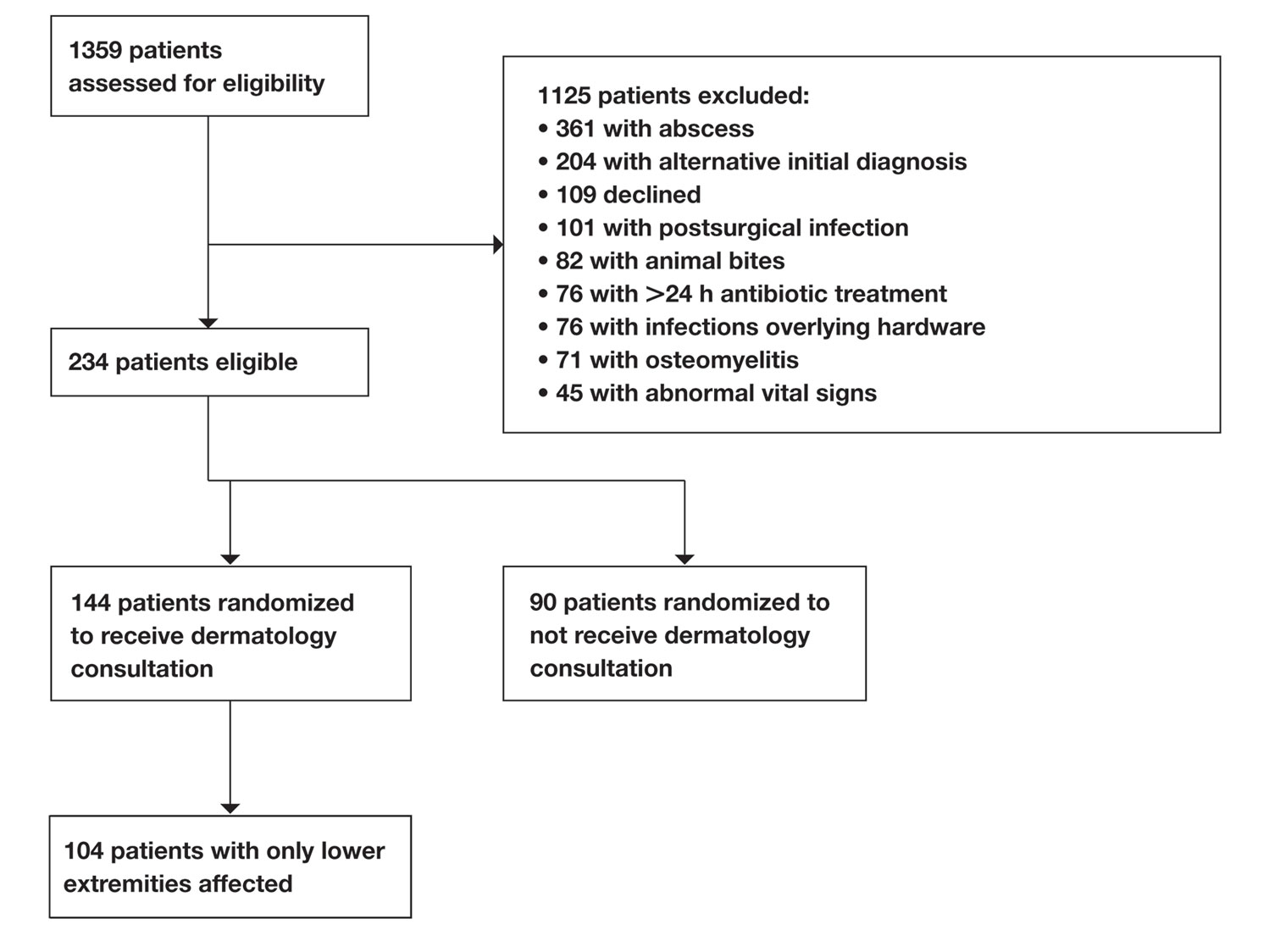
Of 1359 patients screened for eligibility, 104 patients with presumed lower extremity cellulitis undergoing dermatology consultation were included in this study (Figure). The mean patient age (SD) was 60.4 (19.2) years, and 63.5% of patients were male. In the study population, 63 (60.6%) patients received a final diagnosis of cellulitis. The most common pseudocellulitis diagnosis identified was venous stasis dermatitis, which occurred in 12 (11.5%) patients with concomitant cellulitis and in 12 (11.5%) patients mimicking cellulitis (Table).
Univariate comparisons revealed a diverse set of historical, examination, and laboratory factors associated with cellulitis diagnosis. Diagnosis of cellulitis was associated with unilateral presentation, recent trauma to the affected site, and history of cellulitis or onychomycosis. Diagnosis of cellulitis also was associated with elevated white blood cell count, absolute neutrophil count, C-reactive protein, body mass index, hematocrit, and platelet count; age less than 75 years; and lower serum sodium and serum chloride levels. These were the independent factors included in the multivariate analysis, which consisted of a logistic regression model for prediction of cellulitis (eTable).
Multivariate logistic regression on all preliminary factors significantly associated with cellulitis diagnosis in univariate comparisons demonstrated leukocytosis, which was defined as having a white blood cell count exceeding 11,000/μL, unilateral presentation, history of onychomycosis, and trauma to the affected site as significant independent predictors of cellulitis diagnosis; history of cellulitis approached significance (eTable). Unilateral presentation and leukocytosis were the strongest predictors; having either of these factors had a sensitivity of 93.7% and a negative predictive value of 76.5%.

Comment
Importance of Identifying Pseudocellulitis—Successful diagnosis of cellulitis can be confounded by pseudocellulitis that can present concomitantly with or in lieu of cellulitis itself. Although cellulitis mostly affects the lower extremities in adults, pseudocellulitis also was common in this study population of patients with suspected lower extremity cellulitis, occurring both as a mimicker and concomitantly with cellulitis with substantial frequency. Notably, among patients with both venous stasis dermatitis and cellulitis diagnosed, most patients (n=10/12; 83.3%) had unilateral presentations of cellulitis as evidenced by signs and symptoms more notably affecting one lower extremity than the other. These findings suggest that certain pseudocellulitis diagnoses may predispose patients to cellulitis by disrupting the skin barrier, leading to bacterial infiltration; however, these pseudocellulitis diagnoses typically affect both lower extremities equally,1 and asymmetric involvement suggests the presence of overlying cellulitis. Furthermore, the most common pseudocellulitis entities found, such as venous stasis dermatitis, hematoma, and eczema, do not benefit from antibiotic treatment and require alternative therapy.1 Successful discrimination of these pseudocellulitis entities is critical to bolster proper antibiotic stewardship and discourage unnecessary hospitalization.
Independent Predictors of Cellulitis—Unilateral presentation and leukocytosis each emerged as strong independent predictors of cellulitis diagnosis in this study. Having either of these factors furthermore demonstrated high sensitivity and negative predictive value for cellulitis diagnosis. Other notable risk factors were history of onychomycosis, cellulitis, and trauma to the affected site. Prior studies have identified similar historical factors as predisposing patients to cellulitis.7-9 Interestingly, warmth of the affected area on physical examination emerged as strongly associated with cellulitis but was not included in the final predictive model because of its subjective determination. These factors may be especially important in diagnosing cellulitis in patients without concerning vital signs and with concomitant or prior pseudocellulitis.
Study Limitations—This study was limited to patients with uncomplicated presentations to emphasize discrimination of factors associated with cellulitis in the absence of suggestive signs of infection, such as vital sign abnormalities. Signs such as fever and tachypnea have been previously correlated to outpatient treatment failure and necessity for hospitalization.10-12 This study instead focused on patients without concerning vital signs to reduce confounding by such factors in more severe presentations that heighten suspicion for infection and increase likelihood of additional treatment measures. For such patients, suggestive historical factors, such as those discovered in this study, should be considered instead. Interestingly, increased age did not emerge as a significant predictor in this population in contrast to other predictive models that included patients with vital sign abnormalities. Notably, older patients tend to have more variable vital signs, especially in response to physiologic stressors such as infection.13 As such, age may serve as a proxy for vital sign abnormalities to some degree in such predictive models, leading to heightened suspicion for infection in older patients. This study demonstrated that in the absence of concerning vital signs, historical rather than demographic factors are more predictive of cellulitis.
Conclusion
Unilateral presentation and leukocytosis emerged as strong independent predictors of lower extremity cellulitis in patients with uncomplicated presentations. Having either of these factors had a sensitivity of 93.7% and a negative predictive value of 76.5%. Other factors such as history of cellulitis, onychomycosis, and recent trauma to the affected site emerged as additional predictors. These historical, examination, and laboratory characteristics may be especially useful for successful diagnosis of cellulitis in varied practice settings, including outpatient clinics and EDs.
- Raff AB, Kroshinsky D. Cellulitis: a review. JAMA. 2016;316:325-337.
- Gunderson CG, Cherry BM, Fisher A. Do patients with cellulitis need to be hospitalized? a systematic review and meta-analysis of mortality rates of inpatients with cellulitis. J Gen Intern Med. 2018;33:1553-1560.
- Ko LN, Garza-Mayers AC, St. John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- David CV, Chira S, Eells SJ, et al. Diagnostic accuracy in patients admitted to hospitals with cellulitis. Dermatol Online J. 2011;17:1.
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062.
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147-159.
- Björnsdóttir S, Gottfredsson M, Thórisdóttir AS, et al. Risk factors for acute cellulitis of the lower limb: a prospective case-control study. Clin Infect Dis. 2005;41:1416-1422.
- Roujeau JC, Sigurgeirsson B, Korting HC, et al. Chronic dermatomycoses of the foot as risk factors for acute bacterial cellulitis of the leg: a case-control study. Dermatology. 2004;209:301-307.
- McNamara DR, Tleyjeh IM, Berbari EF, et al. A predictive model of recurrent lower extremity cellulitis in a population-based cohort. Arch Intern Med. 2007;167:709-715.
- Yadav K, Suh KN, Eagles D, et al. Predictors of oral antibiotic treatment failure for nonpurulent skin and soft tissue infections in the emergency department. Acad Emerg Med. 2019;26:51-59.
- Peterson D, McLeod S, Woolfrey K, et al. Predictors of failure of empiric outpatient antibiotic therapy in emergency department patients with uncomplicated cellulitis. Acad Emerg Med. 2014;21:526-531.
- Volz KA, Canham L, Kaplan E, et al. Identifying patients with cellulitis who are likely to require inpatient admission after a stay in an ED observation unit. Am J Emerg Med. 2013;31:360-364.
- Chester JG, Rudolph JL. Vital signs in older patients: age-related changes. J Am Med Dir Assoc. 2011;12:337-343.
Cellulitis is an infection of the skin and skin-associated structures characterized by redness, warmth, swelling, and pain of the affected area. Cellulitis most commonly occurs in middle-aged and older adults and frequently affects the lower extremities.1 Serious complications of cellulitis such as bacteremia, metastatic infection, and sepsis are rare, and most cases of cellulitis in patients with normal vital signs and mental status can be managed with outpatient treatment.2
Diagnosis of cellulitis can be confounded by a number of similarly presenting conditions collectively known as pseudocellulitis, such as venous stasis dermatitis and deep vein thrombosis.1 Misdiagnosis of cellulitis is common, with rates exceeding 30% among hospitalized patients initially diagnosed with cellulitis.3,4 Dermatology or infectious disease assessment is considered the diagnostic gold standard for cellulitis4,5 but is not always readily available, especially in resource-constrained settings.
Most cases of uncomplicated cellulitis can be managed with outpatient treatment, especially because serious complications are rare. Frequent misdiagnosis leads to repeat or unnecessary hospitalization and antibiosis. Exceptions necessitating hospitalization usually are predicated on signs of systemic infection, severe immunocompromised states, or failure of prior outpatient therapy.6 Such presentations can be distinguished by corresponding notable historical or examination factors, such as vital sign abnormalities suggesting systemic infection or history of malignancy leading to an immunocompromised state.
We sought to evaluate factors leading to the diagnosis of cellulitis in a cohort of patients with uncomplicated presentations receiving dermatology consultation to emphasize findings indicative of cellulitis in the absence of clinical or historical factors suggestive of other conditions necessitating hospitalization, such as systemic infection.
Methods
Study Participants—A prospective cohort study of patients presenting to an emergency department (ED) between October 2012 and January 2017 at an urban academic medical center in Boston, Massachusetts, was conducted with approval of study design and procedures by the relevant institutional review board. Patients older than 18 years were eligible for inclusion if given an initial diagnosis of cellulitis by an ED physician. Patients were excluded if incarcerated, pregnant, or unable to provide informed consent. Other exclusion criteria includedinfections overlying temporary or permanent indwelling hardware, animal or human bites, or sites of recent surgery (within the prior 4 weeks); preceding antibiotic treatment for more than 24 hours; or clinical or radiographic evidence of complications requiring alternative management such as osteomyelitis or abscess. Patients presenting with an elevated heart rate (>100 beats per minute) or body temperature (>100.5 °F [38.1 °C]) also were excluded. Eligible patients were enrolled upon providing written informed consent, and no remuneration was offered for participation.
Dermatology Consultation Intervention—A random subset of enrolled patients received dermatology consultation within 24 hours of presentation. Consultation consisted of a patient interview and physical examination with care recommendations to relevant ED and inpatient teams. Consultations confirmed the presence or absence of cellulitis as the primary outcome and also noted the presence of any pseudocellulitis diagnoses either occurring concomitantly with or mimicking cellulitis as a secondary outcome.
Statistical Analysis—Patient characteristics were analyzed to identify factors independently associated with the diagnosis of cellulitis in cases affecting the lower extremities. Factors were recorded with categorical variables reported as counts and percentages and continuous variables as means and standard deviations. Univariate analyses between categorical variables or discretized continuous variables and cellulitis diagnosis were conducted via Fisher exact test to identify a preliminary set of potential risk factors. Continuous variables were discretized at multiple incremental values with the discretization most significantly associated with cellulitis diagnosis selected as a preliminary risk factor. Multivariate analyses involved using any objective preliminary factor meeting a significance threshold of P<.1 in univariate comparisons in a multivariate logistic regression model for prediction of cellulitis diagnosis with corresponding calculation of odds ratios with confidence intervals and receiver operating characteristic. Factors with confidence intervals that excluded 1 were considered significant independent predictors of cellulitis. Analyses were performed using Python version 3.8 (Python Software Foundation).
Results
Of 1359 patients screened for eligibility, 104 patients with presumed lower extremity cellulitis undergoing dermatology consultation were included in this study (Figure). The mean patient age (SD) was 60.4 (19.2) years, and 63.5% of patients were male. In the study population, 63 (60.6%) patients received a final diagnosis of cellulitis. The most common pseudocellulitis diagnosis identified was venous stasis dermatitis, which occurred in 12 (11.5%) patients with concomitant cellulitis and in 12 (11.5%) patients mimicking cellulitis (Table).

Univariate comparisons revealed a diverse set of historical, examination, and laboratory factors associated with cellulitis diagnosis. Diagnosis of cellulitis was associated with unilateral presentation, recent trauma to the affected site, and history of cellulitis or onychomycosis. Diagnosis of cellulitis also was associated with elevated white blood cell count, absolute neutrophil count, C-reactive protein, body mass index, hematocrit, and platelet count; age less than 75 years; and lower serum sodium and serum chloride levels. These were the independent factors included in the multivariate analysis, which consisted of a logistic regression model for prediction of cellulitis (eTable).

Multivariate logistic regression on all preliminary factors significantly associated with cellulitis diagnosis in univariate comparisons demonstrated leukocytosis, which was defined as having a white blood cell count exceeding 11,000/μL, unilateral presentation, history of onychomycosis, and trauma to the affected site as significant independent predictors of cellulitis diagnosis; history of cellulitis approached significance (eTable). Unilateral presentation and leukocytosis were the strongest predictors; having either of these factors had a sensitivity of 93.7% and a negative predictive value of 76.5%.

Comment
Importance of Identifying Pseudocellulitis—Successful diagnosis of cellulitis can be confounded by pseudocellulitis that can present concomitantly with or in lieu of cellulitis itself. Although cellulitis mostly affects the lower extremities in adults, pseudocellulitis also was common in this study population of patients with suspected lower extremity cellulitis, occurring both as a mimicker and concomitantly with cellulitis with substantial frequency. Notably, among patients with both venous stasis dermatitis and cellulitis diagnosed, most patients (n=10/12; 83.3%) had unilateral presentations of cellulitis as evidenced by signs and symptoms more notably affecting one lower extremity than the other. These findings suggest that certain pseudocellulitis diagnoses may predispose patients to cellulitis by disrupting the skin barrier, leading to bacterial infiltration; however, these pseudocellulitis diagnoses typically affect both lower extremities equally,1 and asymmetric involvement suggests the presence of overlying cellulitis. Furthermore, the most common pseudocellulitis entities found, such as venous stasis dermatitis, hematoma, and eczema, do not benefit from antibiotic treatment and require alternative therapy.1 Successful discrimination of these pseudocellulitis entities is critical to bolster proper antibiotic stewardship and discourage unnecessary hospitalization.
Independent Predictors of Cellulitis—Unilateral presentation and leukocytosis each emerged as strong independent predictors of cellulitis diagnosis in this study. Having either of these factors furthermore demonstrated high sensitivity and negative predictive value for cellulitis diagnosis. Other notable risk factors were history of onychomycosis, cellulitis, and trauma to the affected site. Prior studies have identified similar historical factors as predisposing patients to cellulitis.7-9 Interestingly, warmth of the affected area on physical examination emerged as strongly associated with cellulitis but was not included in the final predictive model because of its subjective determination. These factors may be especially important in diagnosing cellulitis in patients without concerning vital signs and with concomitant or prior pseudocellulitis.
Study Limitations—This study was limited to patients with uncomplicated presentations to emphasize discrimination of factors associated with cellulitis in the absence of suggestive signs of infection, such as vital sign abnormalities. Signs such as fever and tachypnea have been previously correlated to outpatient treatment failure and necessity for hospitalization.10-12 This study instead focused on patients without concerning vital signs to reduce confounding by such factors in more severe presentations that heighten suspicion for infection and increase likelihood of additional treatment measures. For such patients, suggestive historical factors, such as those discovered in this study, should be considered instead. Interestingly, increased age did not emerge as a significant predictor in this population in contrast to other predictive models that included patients with vital sign abnormalities. Notably, older patients tend to have more variable vital signs, especially in response to physiologic stressors such as infection.13 As such, age may serve as a proxy for vital sign abnormalities to some degree in such predictive models, leading to heightened suspicion for infection in older patients. This study demonstrated that in the absence of concerning vital signs, historical rather than demographic factors are more predictive of cellulitis.
Conclusion
Unilateral presentation and leukocytosis emerged as strong independent predictors of lower extremity cellulitis in patients with uncomplicated presentations. Having either of these factors had a sensitivity of 93.7% and a negative predictive value of 76.5%. Other factors such as history of cellulitis, onychomycosis, and recent trauma to the affected site emerged as additional predictors. These historical, examination, and laboratory characteristics may be especially useful for successful diagnosis of cellulitis in varied practice settings, including outpatient clinics and EDs.
Cellulitis is an infection of the skin and skin-associated structures characterized by redness, warmth, swelling, and pain of the affected area. Cellulitis most commonly occurs in middle-aged and older adults and frequently affects the lower extremities.1 Serious complications of cellulitis such as bacteremia, metastatic infection, and sepsis are rare, and most cases of cellulitis in patients with normal vital signs and mental status can be managed with outpatient treatment.2
Diagnosis of cellulitis can be confounded by a number of similarly presenting conditions collectively known as pseudocellulitis, such as venous stasis dermatitis and deep vein thrombosis.1 Misdiagnosis of cellulitis is common, with rates exceeding 30% among hospitalized patients initially diagnosed with cellulitis.3,4 Dermatology or infectious disease assessment is considered the diagnostic gold standard for cellulitis4,5 but is not always readily available, especially in resource-constrained settings.
Most cases of uncomplicated cellulitis can be managed with outpatient treatment, especially because serious complications are rare. Frequent misdiagnosis leads to repeat or unnecessary hospitalization and antibiosis. Exceptions necessitating hospitalization usually are predicated on signs of systemic infection, severe immunocompromised states, or failure of prior outpatient therapy.6 Such presentations can be distinguished by corresponding notable historical or examination factors, such as vital sign abnormalities suggesting systemic infection or history of malignancy leading to an immunocompromised state.
We sought to evaluate factors leading to the diagnosis of cellulitis in a cohort of patients with uncomplicated presentations receiving dermatology consultation to emphasize findings indicative of cellulitis in the absence of clinical or historical factors suggestive of other conditions necessitating hospitalization, such as systemic infection.
Methods
Study Participants—A prospective cohort study of patients presenting to an emergency department (ED) between October 2012 and January 2017 at an urban academic medical center in Boston, Massachusetts, was conducted with approval of study design and procedures by the relevant institutional review board. Patients older than 18 years were eligible for inclusion if given an initial diagnosis of cellulitis by an ED physician. Patients were excluded if incarcerated, pregnant, or unable to provide informed consent. Other exclusion criteria includedinfections overlying temporary or permanent indwelling hardware, animal or human bites, or sites of recent surgery (within the prior 4 weeks); preceding antibiotic treatment for more than 24 hours; or clinical or radiographic evidence of complications requiring alternative management such as osteomyelitis or abscess. Patients presenting with an elevated heart rate (>100 beats per minute) or body temperature (>100.5 °F [38.1 °C]) also were excluded. Eligible patients were enrolled upon providing written informed consent, and no remuneration was offered for participation.
Dermatology Consultation Intervention—A random subset of enrolled patients received dermatology consultation within 24 hours of presentation. Consultation consisted of a patient interview and physical examination with care recommendations to relevant ED and inpatient teams. Consultations confirmed the presence or absence of cellulitis as the primary outcome and also noted the presence of any pseudocellulitis diagnoses either occurring concomitantly with or mimicking cellulitis as a secondary outcome.
Statistical Analysis—Patient characteristics were analyzed to identify factors independently associated with the diagnosis of cellulitis in cases affecting the lower extremities. Factors were recorded with categorical variables reported as counts and percentages and continuous variables as means and standard deviations. Univariate analyses between categorical variables or discretized continuous variables and cellulitis diagnosis were conducted via Fisher exact test to identify a preliminary set of potential risk factors. Continuous variables were discretized at multiple incremental values with the discretization most significantly associated with cellulitis diagnosis selected as a preliminary risk factor. Multivariate analyses involved using any objective preliminary factor meeting a significance threshold of P<.1 in univariate comparisons in a multivariate logistic regression model for prediction of cellulitis diagnosis with corresponding calculation of odds ratios with confidence intervals and receiver operating characteristic. Factors with confidence intervals that excluded 1 were considered significant independent predictors of cellulitis. Analyses were performed using Python version 3.8 (Python Software Foundation).
Results
Of 1359 patients screened for eligibility, 104 patients with presumed lower extremity cellulitis undergoing dermatology consultation were included in this study (Figure). The mean patient age (SD) was 60.4 (19.2) years, and 63.5% of patients were male. In the study population, 63 (60.6%) patients received a final diagnosis of cellulitis. The most common pseudocellulitis diagnosis identified was venous stasis dermatitis, which occurred in 12 (11.5%) patients with concomitant cellulitis and in 12 (11.5%) patients mimicking cellulitis (Table).

Univariate comparisons revealed a diverse set of historical, examination, and laboratory factors associated with cellulitis diagnosis. Diagnosis of cellulitis was associated with unilateral presentation, recent trauma to the affected site, and history of cellulitis or onychomycosis. Diagnosis of cellulitis also was associated with elevated white blood cell count, absolute neutrophil count, C-reactive protein, body mass index, hematocrit, and platelet count; age less than 75 years; and lower serum sodium and serum chloride levels. These were the independent factors included in the multivariate analysis, which consisted of a logistic regression model for prediction of cellulitis (eTable).

Multivariate logistic regression on all preliminary factors significantly associated with cellulitis diagnosis in univariate comparisons demonstrated leukocytosis, which was defined as having a white blood cell count exceeding 11,000/μL, unilateral presentation, history of onychomycosis, and trauma to the affected site as significant independent predictors of cellulitis diagnosis; history of cellulitis approached significance (eTable). Unilateral presentation and leukocytosis were the strongest predictors; having either of these factors had a sensitivity of 93.7% and a negative predictive value of 76.5%.

Comment
Importance of Identifying Pseudocellulitis—Successful diagnosis of cellulitis can be confounded by pseudocellulitis that can present concomitantly with or in lieu of cellulitis itself. Although cellulitis mostly affects the lower extremities in adults, pseudocellulitis also was common in this study population of patients with suspected lower extremity cellulitis, occurring both as a mimicker and concomitantly with cellulitis with substantial frequency. Notably, among patients with both venous stasis dermatitis and cellulitis diagnosed, most patients (n=10/12; 83.3%) had unilateral presentations of cellulitis as evidenced by signs and symptoms more notably affecting one lower extremity than the other. These findings suggest that certain pseudocellulitis diagnoses may predispose patients to cellulitis by disrupting the skin barrier, leading to bacterial infiltration; however, these pseudocellulitis diagnoses typically affect both lower extremities equally,1 and asymmetric involvement suggests the presence of overlying cellulitis. Furthermore, the most common pseudocellulitis entities found, such as venous stasis dermatitis, hematoma, and eczema, do not benefit from antibiotic treatment and require alternative therapy.1 Successful discrimination of these pseudocellulitis entities is critical to bolster proper antibiotic stewardship and discourage unnecessary hospitalization.
Independent Predictors of Cellulitis—Unilateral presentation and leukocytosis each emerged as strong independent predictors of cellulitis diagnosis in this study. Having either of these factors furthermore demonstrated high sensitivity and negative predictive value for cellulitis diagnosis. Other notable risk factors were history of onychomycosis, cellulitis, and trauma to the affected site. Prior studies have identified similar historical factors as predisposing patients to cellulitis.7-9 Interestingly, warmth of the affected area on physical examination emerged as strongly associated with cellulitis but was not included in the final predictive model because of its subjective determination. These factors may be especially important in diagnosing cellulitis in patients without concerning vital signs and with concomitant or prior pseudocellulitis.
Study Limitations—This study was limited to patients with uncomplicated presentations to emphasize discrimination of factors associated with cellulitis in the absence of suggestive signs of infection, such as vital sign abnormalities. Signs such as fever and tachypnea have been previously correlated to outpatient treatment failure and necessity for hospitalization.10-12 This study instead focused on patients without concerning vital signs to reduce confounding by such factors in more severe presentations that heighten suspicion for infection and increase likelihood of additional treatment measures. For such patients, suggestive historical factors, such as those discovered in this study, should be considered instead. Interestingly, increased age did not emerge as a significant predictor in this population in contrast to other predictive models that included patients with vital sign abnormalities. Notably, older patients tend to have more variable vital signs, especially in response to physiologic stressors such as infection.13 As such, age may serve as a proxy for vital sign abnormalities to some degree in such predictive models, leading to heightened suspicion for infection in older patients. This study demonstrated that in the absence of concerning vital signs, historical rather than demographic factors are more predictive of cellulitis.
Conclusion
Unilateral presentation and leukocytosis emerged as strong independent predictors of lower extremity cellulitis in patients with uncomplicated presentations. Having either of these factors had a sensitivity of 93.7% and a negative predictive value of 76.5%. Other factors such as history of cellulitis, onychomycosis, and recent trauma to the affected site emerged as additional predictors. These historical, examination, and laboratory characteristics may be especially useful for successful diagnosis of cellulitis in varied practice settings, including outpatient clinics and EDs.
- Raff AB, Kroshinsky D. Cellulitis: a review. JAMA. 2016;316:325-337.
- Gunderson CG, Cherry BM, Fisher A. Do patients with cellulitis need to be hospitalized? a systematic review and meta-analysis of mortality rates of inpatients with cellulitis. J Gen Intern Med. 2018;33:1553-1560.
- Ko LN, Garza-Mayers AC, St. John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- David CV, Chira S, Eells SJ, et al. Diagnostic accuracy in patients admitted to hospitals with cellulitis. Dermatol Online J. 2011;17:1.
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062.
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147-159.
- Björnsdóttir S, Gottfredsson M, Thórisdóttir AS, et al. Risk factors for acute cellulitis of the lower limb: a prospective case-control study. Clin Infect Dis. 2005;41:1416-1422.
- Roujeau JC, Sigurgeirsson B, Korting HC, et al. Chronic dermatomycoses of the foot as risk factors for acute bacterial cellulitis of the leg: a case-control study. Dermatology. 2004;209:301-307.
- McNamara DR, Tleyjeh IM, Berbari EF, et al. A predictive model of recurrent lower extremity cellulitis in a population-based cohort. Arch Intern Med. 2007;167:709-715.
- Yadav K, Suh KN, Eagles D, et al. Predictors of oral antibiotic treatment failure for nonpurulent skin and soft tissue infections in the emergency department. Acad Emerg Med. 2019;26:51-59.
- Peterson D, McLeod S, Woolfrey K, et al. Predictors of failure of empiric outpatient antibiotic therapy in emergency department patients with uncomplicated cellulitis. Acad Emerg Med. 2014;21:526-531.
- Volz KA, Canham L, Kaplan E, et al. Identifying patients with cellulitis who are likely to require inpatient admission after a stay in an ED observation unit. Am J Emerg Med. 2013;31:360-364.
- Chester JG, Rudolph JL. Vital signs in older patients: age-related changes. J Am Med Dir Assoc. 2011;12:337-343.
- Raff AB, Kroshinsky D. Cellulitis: a review. JAMA. 2016;316:325-337.
- Gunderson CG, Cherry BM, Fisher A. Do patients with cellulitis need to be hospitalized? a systematic review and meta-analysis of mortality rates of inpatients with cellulitis. J Gen Intern Med. 2018;33:1553-1560.
- Ko LN, Garza-Mayers AC, St. John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- David CV, Chira S, Eells SJ, et al. Diagnostic accuracy in patients admitted to hospitals with cellulitis. Dermatol Online J. 2011;17:1.
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062.
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147-159.
- Björnsdóttir S, Gottfredsson M, Thórisdóttir AS, et al. Risk factors for acute cellulitis of the lower limb: a prospective case-control study. Clin Infect Dis. 2005;41:1416-1422.
- Roujeau JC, Sigurgeirsson B, Korting HC, et al. Chronic dermatomycoses of the foot as risk factors for acute bacterial cellulitis of the leg: a case-control study. Dermatology. 2004;209:301-307.
- McNamara DR, Tleyjeh IM, Berbari EF, et al. A predictive model of recurrent lower extremity cellulitis in a population-based cohort. Arch Intern Med. 2007;167:709-715.
- Yadav K, Suh KN, Eagles D, et al. Predictors of oral antibiotic treatment failure for nonpurulent skin and soft tissue infections in the emergency department. Acad Emerg Med. 2019;26:51-59.
- Peterson D, McLeod S, Woolfrey K, et al. Predictors of failure of empiric outpatient antibiotic therapy in emergency department patients with uncomplicated cellulitis. Acad Emerg Med. 2014;21:526-531.
- Volz KA, Canham L, Kaplan E, et al. Identifying patients with cellulitis who are likely to require inpatient admission after a stay in an ED observation unit. Am J Emerg Med. 2013;31:360-364.
- Chester JG, Rudolph JL. Vital signs in older patients: age-related changes. J Am Med Dir Assoc. 2011;12:337-343.
Practice Points
- Unilateral involvement and leukocytosis are both highly predictive of lower extremity cellulitis in uncomplicated presentations.
- Historical factors such as history of onychomycosis and trauma to the affected site are more predictive of lower extremity cellulitis than demographic factors such as age in uncomplicated presentations of cellulitis.
Update on Calciphylaxis Etiopathogenesis, Diagnosis, and Management
Calciphylaxis, also known as calcific uremic arteriolopathy, is a painful skin condition classically seen in patients with end-stage renal disease (ESRD), particularly those on chronic dialysis.1,2 It also has increasingly been reported in patients with normal renal function and calcium and phosphate homeostasis.3,4 Effective diagnosis and management of calciphylaxis remains challenging for physicians.2,5 The condition is characterized by tissue ischemia caused by calcification of cutaneous arteriolar vessels. As a result, calciphylaxis is associated with high mortality rates, ranging from 60% to 80%.5,6 Excruciating pain and nonhealing ulcers often lead to recurrent hospitalizations and infectious complications,7 and poor nutritional status, chronic pain, depression, and insomnia can further complicate recovery and lead to poor quality of life.8
We provide an update on calciphylaxis etiopathogenesis, diagnosis, and management. We also highlight some challenges faced in managing this potentially fatal condition.
Epidemiology
Calciphylaxis is considered a rare dermatosis with an estimated annual incidence of 1% to 4% in ESRD patients on dialysis. Recent data suggest that incidence of calciphylaxis is rising,5,7,9 which may stem from an increased use of calcium-based phosphate binders, an actual rise in disease incidence, and/or increased recognition of the disease.5 It is difficult to estimate the exact disease burden of calciphylaxis because the diagnostic criteria are not well defined, often leading to missed or delayed diagnosis.3,10 Furthermore, there is no centralized registry for calciphylaxis cases.3
Etiology and Pathogenesis
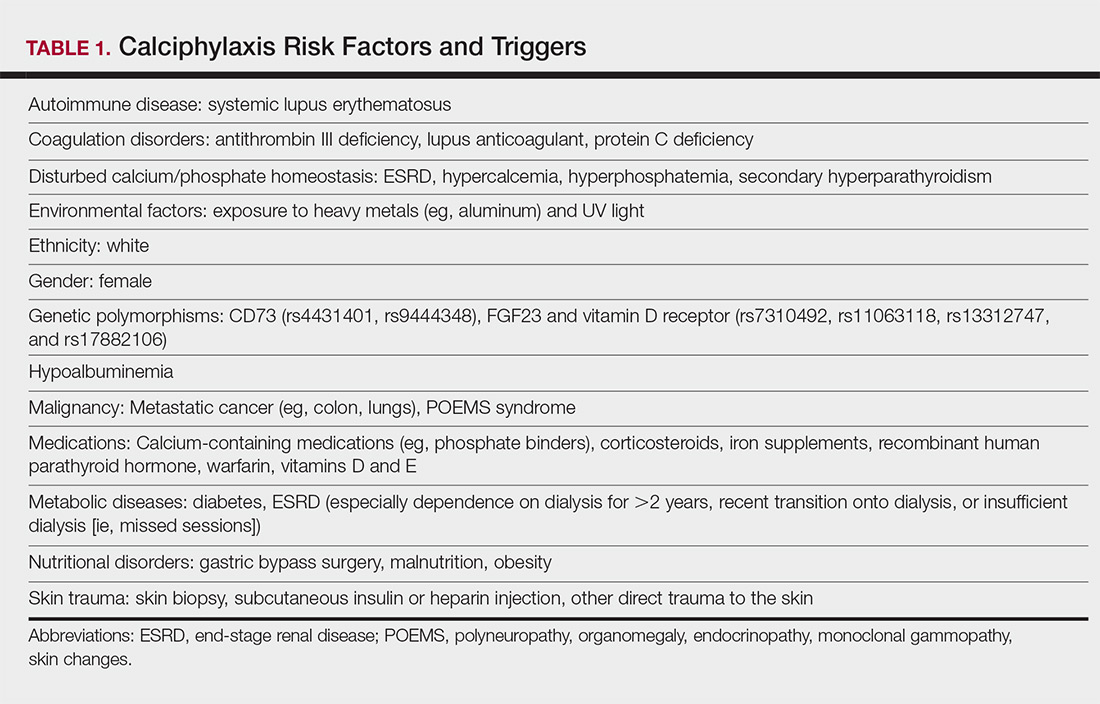
Calciphylaxis is thought to have a multifactorial etiology with the exact cause or trigger unknown.7 A long list of risk factors and triggers is associated with the condition (Table 1). Calciphylaxis primarily affects small arteries (40–600 μm in diameter) that become calcified due to an imbalance between inhibitors and promoters of calcification.2,11 Fetuin-A and matrix Gla protein inhibit vascular calcification and are downregulated in calciphylaxis.12,13 Dysfunctional calcium, phosphate, and parathyroid hormone regulatory pathways provide an increased substrate for the process of calcification, which causes endothelial damage and microthrombosis, resulting in tissue ischemia and infarction.14,15 Notably, there is growing interest in the role of vitamin K in the pathogenesis of calciphylaxis. Vitamin K inhibits vascular calcification, possibly by increasing the circulating levels of carboxylated matrix Gla protein.16
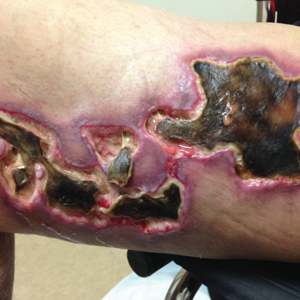
Clinical Features
Calciphylaxis is most commonly seen on the legs, abdomen, and buttocks.2 Patients with ESRD commonly develop proximal lesions affecting adipose-rich sites and have a poor prognosis. Distal lesions are more common in patients with nonuremic calciphylaxis, and mortality rates are lower in this population.2
Early lesions present as painful skin nodules or indurated plaques that often are rock-hard or firm to palpation with overlying mottling or a livedoid pattern (Figure, A). Early lesions progress from livedo reticularis to livedo racemosa and then to retiform purpura (Figure, B). Purpuric lesions later evolve into black eschars (Figure, C), then to necrotic, ulcerated, malodorous plaques or nodules in later stages of the disease (Figure, D). Lesions also may develop a gangrenous sclerotic appearance.2,5
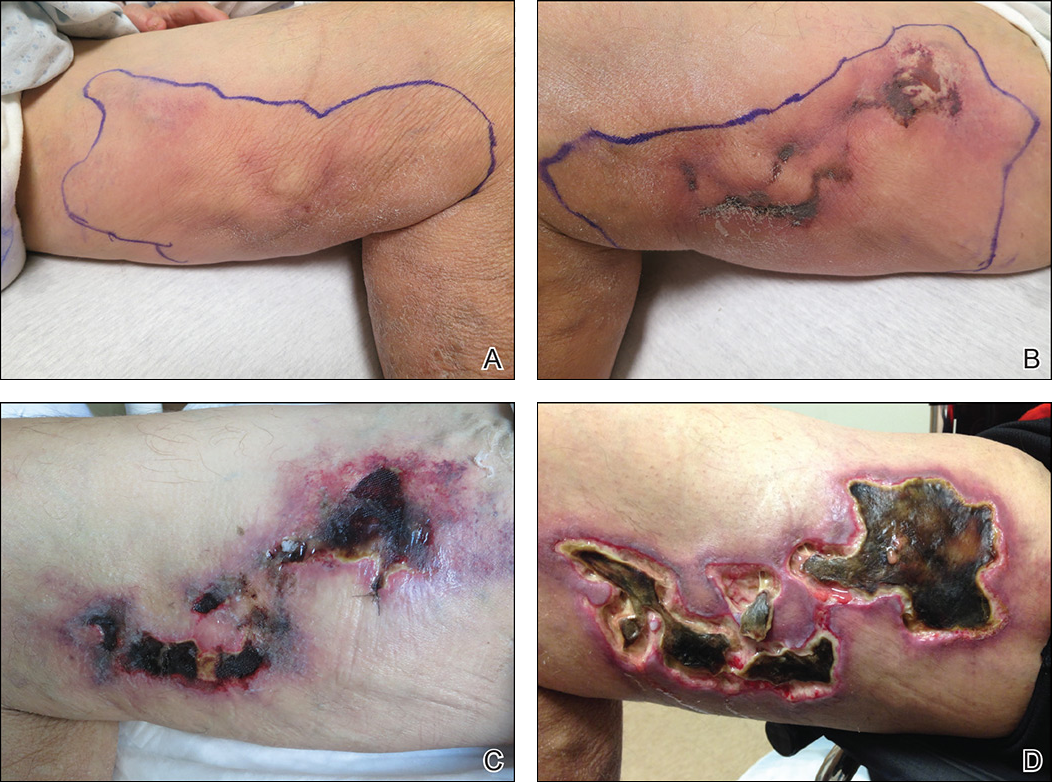
Although most patients with calciphylaxis have ESRD, nonuremic patients also can develop the disease. Those with calciphylaxis who do not have renal dysfunction frequently have other risk factors for the disease and often report another notable health problem in the weeks or months prior to presentation.4 More than half of patients with calciphylaxis become bedridden or require use of a wheelchair.17 Pain is characteristically severe throughout the course of the disease; it may even precede the appearance of the skin lesions.18 Because the pain is associated with ischemia, it tends to be relatively refractory to treatment with opioids. Rare extracutaneous vascular calcifications may lead to visual impairment, gastrointestinal tract bleeding, and myopathy.5,9,19,20
Diagnosis
Considering the high morbidity and mortality associated with calciphylaxis, it is important to provide accurate and timely diagnosis; however, there currently are no validated diagnostic criteria for calciphylaxis. Careful correlation of clinical and histologic findings is required. Calciphylaxis biopsies have demonstrated medial calcification and proliferation of the intima of small- to medium-sized arteries.21 Lobular and septal panniculitis and extravascular soft-tissue calcification, particularly stippled calcification of the eccrine sweat glands, also has been seen.2,22 Special calcium stains (eg, von Kossa, Alizarin red) increase the sensitivity of biopsy by highlighting subtle areas of intravascular and extravascular calcification.5,23 Sufficient sampling of subcutaneous tissue and specimen evaluation by an experienced dermatopathologist are necessary to ensure proper interpretation of the histologic findings.
Despite these measures, skin biopsies may be nondiagnostic or falsely negative; therefore, when there is high clinical suspicion, it may be appropriate to move forward with a presumptive diagnosis of calciphylaxis even if the histologic findings are nondiagnostic.1,9,24 It also is worth noting that localized progression and ulceration may occur following skin biopsy, such that biopsy may even be contraindicated in certain cases (eg, penile calciphylaxis).
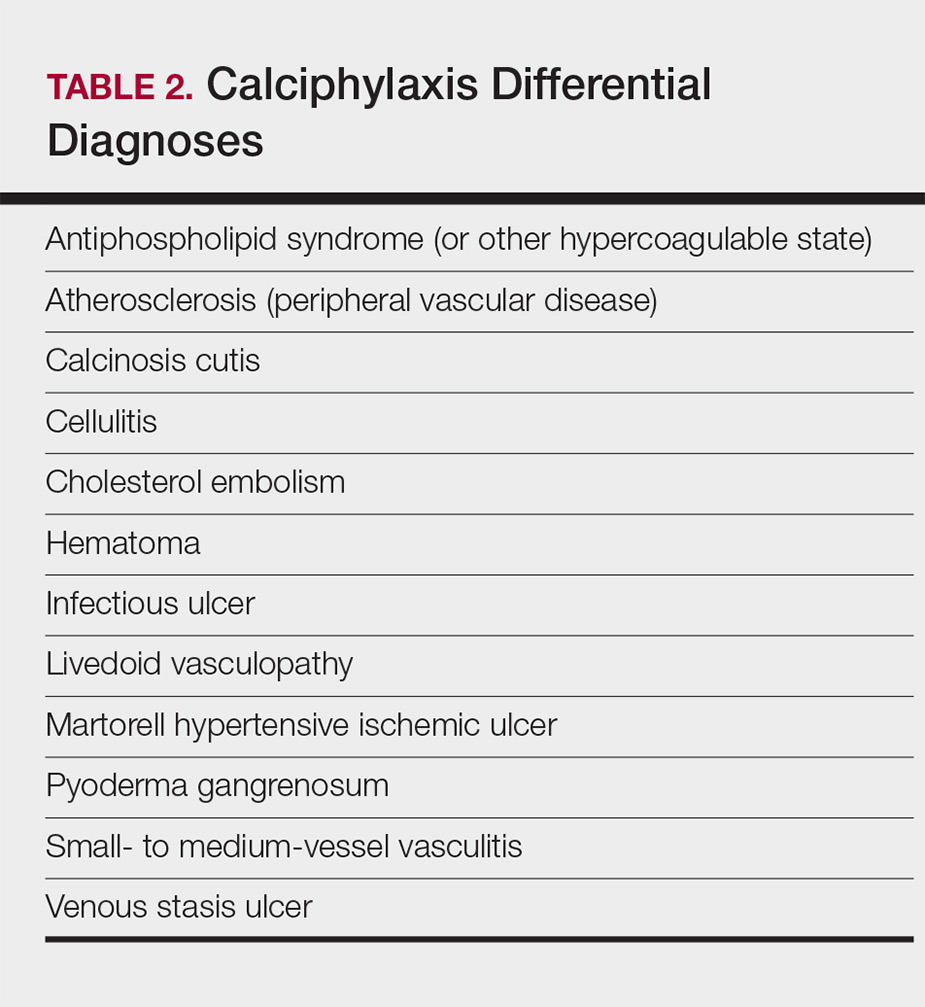
Standard laboratory workup for calciphylaxis includes evaluation for associated risk factors as well as exclusion of other conditions in the differential diagnosis (Table 2). Blood tests to evaluate for risk factors include liver and renal function tests, a complete metabolic panel, parathyroid hormone level, and serum albumin level.5 Elevated calcium and phosphate levels may signal disturbed calcium and phosphate homeostasis but are neither sensitive nor specific for the diagnosis.25 Complete blood cell count, blood cultures, thorough hypercoagulability workup (including but not limited to antiphospholipid antibodies, proteins C and S, factor V Leiden, antithrombin III, homocysteine, methylenetetrahydrofolate reductase mutation, and cryoglobulins), rheumatoid factor, antineutrophil cytoplasmic antibodies, and antinuclear antibody testing may be relevant to help identify contributing factors or mimickers of calciphylaxis.5 Various imaging modalities also have been used to evaluate for the presence of soft-tissue calcification in areas of suspected calciphylaxis, including radiography, mammography, computed tomography, ultrasonography, nuclear bone scintigraphy, and spectroscopy.2,26,27 Unfortunately, there currently is no standardized reproducible imaging modality for reliable diagnosis of calciphylaxis. Ultimately, histologic and radiographic findings should always be interpreted in the context of relevant clinical findings.2,9
Prevention
Reduction of the net calcium phosphorus product may help reduce the risk of calciphylaxis in ESRD patients, which can be accomplished by using non–calcium-phosphate binders, adequate dialysis, and restricting use of vitamin D and vitamin K antagonists.2,5 There are limited data regarding the benefits of using bisphosphonates and cinacalcet in ESRD patients on dialysis to prevent calciphylaxis.28,29
Management
Management of calciphylaxis is multifactorial. Besides dermatology and nephrology, specialists in pain management, wound care, plastic surgery, and nutrition are critical partners in management.1,5,9,30 Nephrologists can help optimize calcium and phosphate balance and ensure adequate dialysis. Pain specialists can aid in creating aggressive multiagent pain regimens that target the neuropathic/ischemic and physical aspects of calciphylaxis pain. When appropriate, nutrition specialists can help establish high-protein, low-phosphorus diets, and wound specialists can provide access to advanced wound dressings and adjunctive hyperbaric oxygen therapy. Plastic surgeons can provide conservative debridement procedures in a subset of patients, usually those with distal stable disease.
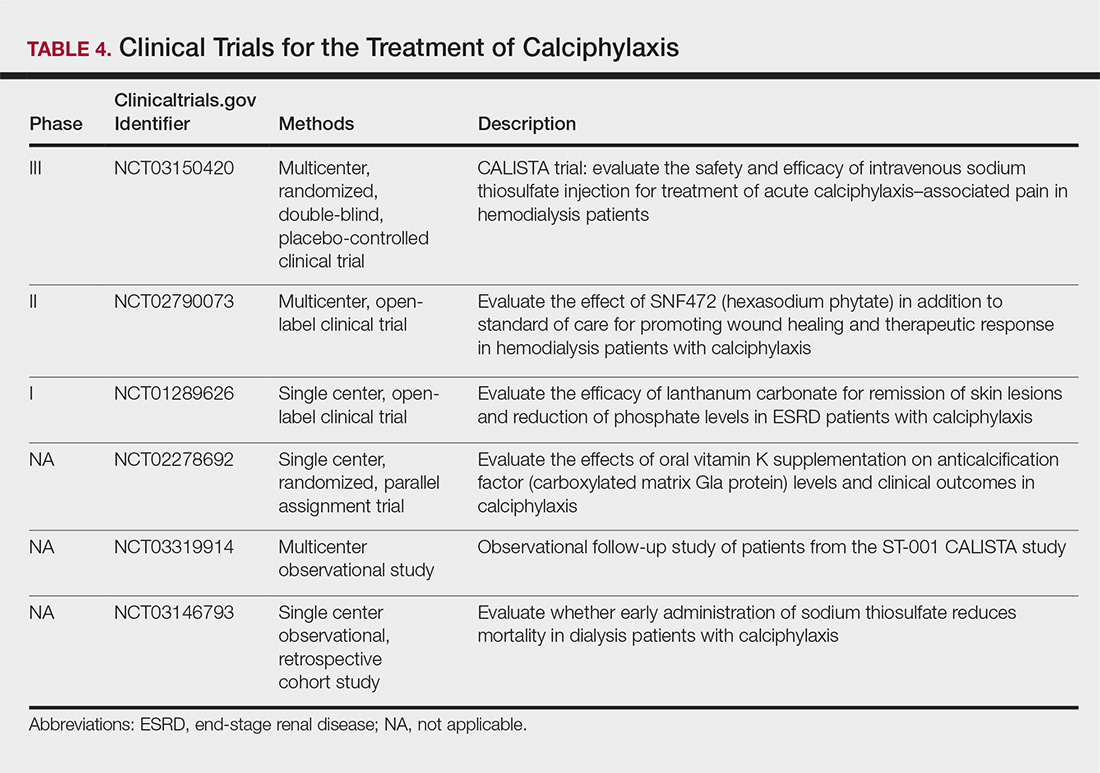
The limited understanding of the etiopathogenesis of calciphylaxis and the lack of data on its management are reflected in the limited treatment options for the disease (Table 3).2,5,9 There are no formal algorithms for the treatment of calciphylaxis. Therapeutic trials are scarce, and most of the current treatment recommendations are based on small retrospective reports or case series. Sodium thiosulfate has been the most widely used treatment option since 2004, when its use in calciphylaxis was first reported.31 Sodium thiosulfate chelates calcium and is thought to have antioxidant and vasodilatory properties.32 There are a few promising clinical trials and large-scale studies (Table 4) that aim to evaluate the efficacy of existing treatments (eg, sodium thiosulfate) as well as novel treatment options such as lanthanum carbonate, SNF472 (hexasodium phytate), and vitamin K.33-36

Prognosis
Calciphylaxis is a potentially fatal condition with a poor prognosis and a median survival rate of approximately 1 year following the appearance of skin lesions.37-39 Patients with proximal lesions and those on peritoneal dialysis (as opposed to hemodialysis) have a worse prognosis.40 Mortality rates are estimated to be 30% at 6 months, 50% at 12 months, and 80% at 2 years, with sepsis secondary to infection of cutaneous ulcers being the leading cause of death.37-39 The impact of calciphylaxis on patient quality of life and activities of daily living is severe.8,17
Future Directions
Multi-institution cohort studies and collaborative registries are needed to provide updated information related to the epidemiology, diagnosis, treatment, morbidity, and mortality associated with calciphylaxis and to help formulate evidence-based diagnostic criteria. Radiographic and histologic studies, as well as other tools for early and accurate diagnosis of calciphylaxis, should be studied for feasibility, accuracy, and reproducibility. The incidence of nonuremic calciphylaxis points toward pathogenic pathways besides those based on the bone-mineral axis. Basic science research directed at improving understanding of the pathophysiology of calciphylaxis would be helpful in devising new treatment strategies targeting these pathways. Establishment of a collaborative, multi-institutional calciphylaxis working group would enable experts to formulate therapeutic guidelines based on current evidence. Such a group could facilitate initiation of large prospective studies to establish the efficacy of existing and new treatment modalities for calciphylaxis. A working group within the Society for Dermatology Hospitalists has been tasked with addressing these issues and is currently establishing a multicenter calciphylaxis database.
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146.
- Nigwekar SU, Thadhani RI, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Davis JM. The relationship between obesity and calciphylaxis: a review of the literature. Ostomy Wound Manage. 2016;62:12-18.
- Bajaj R, Courbebaisse M, Kroshinsky D, et al. Calciphylaxis in patients with normal renal function: a case series and systematic review. Mayo Clin Proc. 2018;93:1202-1212.
- Hafner J, Keusch G, Wahl C, et al. Uremic small-artery disease with medial calcification and intimal hyperplasia (so-called calciphylaxis): a complication of chronic renal failure and benefit from parathyroidectomy. J Am Acad Dermatol. 1995;33:954-962.
- Jeong HS, Dominguez AR. Calciphylaxis: controversies in pathogenesis, diagnosis and treatment. Am J Med Sci. 2016;351:217-227.
- Westphal SG, Plumb T. Calciphylaxis. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2018. https://www.ncbi.nlm.nih.gov/books/NBK519020. Accessed November 12, 2018.
- Riemer CA, El-Azhary RA, Wu KL, et al. Underreported use of palliative care and patient-reported outcome measures to address reduced quality of life in patients with calciphylaxis: a systematic review. Br J Dermatol. 2017;177:1510-1518.
- Nigwekar SU. Calciphylaxis. Curr Opin Nephrol Hypertens. 2017;26:276-281.
- Fine A, Fontaine B. Calciphylaxis: the beginning of the end? Perit Dial Int. 2008;28:268-270.
- Lin WT, Chao CM. Tumoral calcinosis in renal failure. QJM. 2014;107:387.
- Schafer C, Heiss A, Schwarz A, et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357-366.
- Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78-81.
- Bleyer AJ, Choi M, Igwemezie B, et al. A case control study of proximal calciphylaxis. Am J Kidney Dis. 1998;32:376-383.
- Ahmed S, O’Neill KD, Hood AF, et al. Calciphylaxis is associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am J Kidney Dis. 2001;37:267-276.
- Nigwekar SU, Bloch DB, Nazarian RM, et al. Vitamin K-dependent carboxylation of matrix gla protein influences the risk of calciphylaxis. J Am Soc Nephrol. 2017;28:1717-1722.
- Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-579.
- Polizzotto MN, Bryan T, Ashby MA, et al. Symptomatic management of calciphylaxis: a case series and review of the literature. J Pain Symptom Manage. 2006;32:186-190.
- Gupta N, Haq KF, Mahajan S, et al. Gastrointestinal bleeding secondary to calciphylaxis. Am J Case Rep. 2015;16:818-822.
- Edelstein CL, Wickham MK, Kirby PA. Systemic calciphylaxis presenting as a painful, proximal myopathy. Postgrad Med J. 1992;68:209-211.
- Mochel MC, Arakari RY, Wang G, et al. Cutaneous calciphylaxis: a retrospective histopathologic evaluation. Am J Dermatopathol. 2013;35:582-586.
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802.
- Cassius C, Moguelet P, Monfort JB, et al. Calciphylaxis in haemodialysed patients: diagnostic value of calcifications in cutaneous biopsy. Br J Dermatol. 2018;178:292-293.
- Sreedhar A, Sheikh HA, Scagliotti CJ, et al. Advanced-stage calciphylaxis: think before you punch. Cleve Clin J Med. 2016;83:562-564.
- Brandenburg VM, Kramann R, Rothe H, et al. Calcific uraemic arteriolopathy (calciphylaxis): data from a large nation-wide registry. Nephrol Dial Transplant. 2017;32:126-132.
- Paul S, Rabito CA, Vedak P, et al. The role of bone scintigraphy in the diagnosis of calciphylaxis. JAMA Dermatol. 2017;153:101-103.
- Shmidt E, Murthy NS, Knudsen JM, et al. Net-like pattern of calcification on plain soft-tissue radiographs in patients with calciphylaxis. J Am Acad Dermatol. 2012;67:1296-1301.
- EVOLVE Trial Investigators; Chertow GM, Block GA, Correa-Rotter R, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482-2494.
- Rogers NM, Teubner DJO, Coates PT. Calcific uremic arteriolopathy: advances in pathogenesis and treatment. Semin Dial. 2007;20:150-157.
- Nigwekar SU. Multidisciplinary approach to calcific uremic arteriolopathy. Curr Opin Nephrol Hypertens. 2015;24:531-537.
- Cicone JS, Petronis JB, Embert CD, et al. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104-1108.
- Chen NX, O’Neill K, Akl NK, et al. Adipocyte induced arterial calcification is prevented with sodium thiosulfate. Biochem Biophys Res Commun. 2014;449:151-156.
- Chan MR, Ghandour F, Murali NS, et al. Pilot study of the effect of lanthanum carbonate in patients with calciphylaxis: a Wisconsin Network for Health Research (WiNHR) study. J Nephrol Ther. 2014;4:1000162.
- Perelló J, Gómez M, Ferrer MD, et al. SNF472, a novel inhibitor of vascular calcification, could be administered during hemodialysis to attain potentially therapeutic phytate levels. J Nephrol. 2018;31:287-296.
- Christiadi D, Singer RF. Calciphylaxis in a dialysis patient successfully treated with high-dose vitamin K supplementation. Clin Kidney J. 2018;11:528-529.
- Caluwe R, Vandecasteele S, Van Vlem B, et al. Vitamin K2 supplementation in haemodialysis patients: a randomized dose-finding study. Nephrol Dial Transplant. 2014;29:1385-1390.
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394.
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217.
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429.
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241.
Calciphylaxis, also known as calcific uremic arteriolopathy, is a painful skin condition classically seen in patients with end-stage renal disease (ESRD), particularly those on chronic dialysis.1,2 It also has increasingly been reported in patients with normal renal function and calcium and phosphate homeostasis.3,4 Effective diagnosis and management of calciphylaxis remains challenging for physicians.2,5 The condition is characterized by tissue ischemia caused by calcification of cutaneous arteriolar vessels. As a result, calciphylaxis is associated with high mortality rates, ranging from 60% to 80%.5,6 Excruciating pain and nonhealing ulcers often lead to recurrent hospitalizations and infectious complications,7 and poor nutritional status, chronic pain, depression, and insomnia can further complicate recovery and lead to poor quality of life.8
We provide an update on calciphylaxis etiopathogenesis, diagnosis, and management. We also highlight some challenges faced in managing this potentially fatal condition.
Epidemiology
Calciphylaxis is considered a rare dermatosis with an estimated annual incidence of 1% to 4% in ESRD patients on dialysis. Recent data suggest that incidence of calciphylaxis is rising,5,7,9 which may stem from an increased use of calcium-based phosphate binders, an actual rise in disease incidence, and/or increased recognition of the disease.5 It is difficult to estimate the exact disease burden of calciphylaxis because the diagnostic criteria are not well defined, often leading to missed or delayed diagnosis.3,10 Furthermore, there is no centralized registry for calciphylaxis cases.3
Etiology and Pathogenesis
Calciphylaxis is thought to have a multifactorial etiology with the exact cause or trigger unknown.7 A long list of risk factors and triggers is associated with the condition (Table 1). Calciphylaxis primarily affects small arteries (40–600 μm in diameter) that become calcified due to an imbalance between inhibitors and promoters of calcification.2,11 Fetuin-A and matrix Gla protein inhibit vascular calcification and are downregulated in calciphylaxis.12,13 Dysfunctional calcium, phosphate, and parathyroid hormone regulatory pathways provide an increased substrate for the process of calcification, which causes endothelial damage and microthrombosis, resulting in tissue ischemia and infarction.14,15 Notably, there is growing interest in the role of vitamin K in the pathogenesis of calciphylaxis. Vitamin K inhibits vascular calcification, possibly by increasing the circulating levels of carboxylated matrix Gla protein.16

Clinical Features
Calciphylaxis is most commonly seen on the legs, abdomen, and buttocks.2 Patients with ESRD commonly develop proximal lesions affecting adipose-rich sites and have a poor prognosis. Distal lesions are more common in patients with nonuremic calciphylaxis, and mortality rates are lower in this population.2
Early lesions present as painful skin nodules or indurated plaques that often are rock-hard or firm to palpation with overlying mottling or a livedoid pattern (Figure, A). Early lesions progress from livedo reticularis to livedo racemosa and then to retiform purpura (Figure, B). Purpuric lesions later evolve into black eschars (Figure, C), then to necrotic, ulcerated, malodorous plaques or nodules in later stages of the disease (Figure, D). Lesions also may develop a gangrenous sclerotic appearance.2,5

Although most patients with calciphylaxis have ESRD, nonuremic patients also can develop the disease. Those with calciphylaxis who do not have renal dysfunction frequently have other risk factors for the disease and often report another notable health problem in the weeks or months prior to presentation.4 More than half of patients with calciphylaxis become bedridden or require use of a wheelchair.17 Pain is characteristically severe throughout the course of the disease; it may even precede the appearance of the skin lesions.18 Because the pain is associated with ischemia, it tends to be relatively refractory to treatment with opioids. Rare extracutaneous vascular calcifications may lead to visual impairment, gastrointestinal tract bleeding, and myopathy.5,9,19,20
Diagnosis
Considering the high morbidity and mortality associated with calciphylaxis, it is important to provide accurate and timely diagnosis; however, there currently are no validated diagnostic criteria for calciphylaxis. Careful correlation of clinical and histologic findings is required. Calciphylaxis biopsies have demonstrated medial calcification and proliferation of the intima of small- to medium-sized arteries.21 Lobular and septal panniculitis and extravascular soft-tissue calcification, particularly stippled calcification of the eccrine sweat glands, also has been seen.2,22 Special calcium stains (eg, von Kossa, Alizarin red) increase the sensitivity of biopsy by highlighting subtle areas of intravascular and extravascular calcification.5,23 Sufficient sampling of subcutaneous tissue and specimen evaluation by an experienced dermatopathologist are necessary to ensure proper interpretation of the histologic findings.
Despite these measures, skin biopsies may be nondiagnostic or falsely negative; therefore, when there is high clinical suspicion, it may be appropriate to move forward with a presumptive diagnosis of calciphylaxis even if the histologic findings are nondiagnostic.1,9,24 It also is worth noting that localized progression and ulceration may occur following skin biopsy, such that biopsy may even be contraindicated in certain cases (eg, penile calciphylaxis).
Standard laboratory workup for calciphylaxis includes evaluation for associated risk factors as well as exclusion of other conditions in the differential diagnosis (Table 2). Blood tests to evaluate for risk factors include liver and renal function tests, a complete metabolic panel, parathyroid hormone level, and serum albumin level.5 Elevated calcium and phosphate levels may signal disturbed calcium and phosphate homeostasis but are neither sensitive nor specific for the diagnosis.25 Complete blood cell count, blood cultures, thorough hypercoagulability workup (including but not limited to antiphospholipid antibodies, proteins C and S, factor V Leiden, antithrombin III, homocysteine, methylenetetrahydrofolate reductase mutation, and cryoglobulins), rheumatoid factor, antineutrophil cytoplasmic antibodies, and antinuclear antibody testing may be relevant to help identify contributing factors or mimickers of calciphylaxis.5 Various imaging modalities also have been used to evaluate for the presence of soft-tissue calcification in areas of suspected calciphylaxis, including radiography, mammography, computed tomography, ultrasonography, nuclear bone scintigraphy, and spectroscopy.2,26,27 Unfortunately, there currently is no standardized reproducible imaging modality for reliable diagnosis of calciphylaxis. Ultimately, histologic and radiographic findings should always be interpreted in the context of relevant clinical findings.2,9

Prevention
Reduction of the net calcium phosphorus product may help reduce the risk of calciphylaxis in ESRD patients, which can be accomplished by using non–calcium-phosphate binders, adequate dialysis, and restricting use of vitamin D and vitamin K antagonists.2,5 There are limited data regarding the benefits of using bisphosphonates and cinacalcet in ESRD patients on dialysis to prevent calciphylaxis.28,29
Management
Management of calciphylaxis is multifactorial. Besides dermatology and nephrology, specialists in pain management, wound care, plastic surgery, and nutrition are critical partners in management.1,5,9,30 Nephrologists can help optimize calcium and phosphate balance and ensure adequate dialysis. Pain specialists can aid in creating aggressive multiagent pain regimens that target the neuropathic/ischemic and physical aspects of calciphylaxis pain. When appropriate, nutrition specialists can help establish high-protein, low-phosphorus diets, and wound specialists can provide access to advanced wound dressings and adjunctive hyperbaric oxygen therapy. Plastic surgeons can provide conservative debridement procedures in a subset of patients, usually those with distal stable disease.
The limited understanding of the etiopathogenesis of calciphylaxis and the lack of data on its management are reflected in the limited treatment options for the disease (Table 3).2,5,9 There are no formal algorithms for the treatment of calciphylaxis. Therapeutic trials are scarce, and most of the current treatment recommendations are based on small retrospective reports or case series. Sodium thiosulfate has been the most widely used treatment option since 2004, when its use in calciphylaxis was first reported.31 Sodium thiosulfate chelates calcium and is thought to have antioxidant and vasodilatory properties.32 There are a few promising clinical trials and large-scale studies (Table 4) that aim to evaluate the efficacy of existing treatments (eg, sodium thiosulfate) as well as novel treatment options such as lanthanum carbonate, SNF472 (hexasodium phytate), and vitamin K.33-36


Prognosis
Calciphylaxis is a potentially fatal condition with a poor prognosis and a median survival rate of approximately 1 year following the appearance of skin lesions.37-39 Patients with proximal lesions and those on peritoneal dialysis (as opposed to hemodialysis) have a worse prognosis.40 Mortality rates are estimated to be 30% at 6 months, 50% at 12 months, and 80% at 2 years, with sepsis secondary to infection of cutaneous ulcers being the leading cause of death.37-39 The impact of calciphylaxis on patient quality of life and activities of daily living is severe.8,17
Future Directions
Multi-institution cohort studies and collaborative registries are needed to provide updated information related to the epidemiology, diagnosis, treatment, morbidity, and mortality associated with calciphylaxis and to help formulate evidence-based diagnostic criteria. Radiographic and histologic studies, as well as other tools for early and accurate diagnosis of calciphylaxis, should be studied for feasibility, accuracy, and reproducibility. The incidence of nonuremic calciphylaxis points toward pathogenic pathways besides those based on the bone-mineral axis. Basic science research directed at improving understanding of the pathophysiology of calciphylaxis would be helpful in devising new treatment strategies targeting these pathways. Establishment of a collaborative, multi-institutional calciphylaxis working group would enable experts to formulate therapeutic guidelines based on current evidence. Such a group could facilitate initiation of large prospective studies to establish the efficacy of existing and new treatment modalities for calciphylaxis. A working group within the Society for Dermatology Hospitalists has been tasked with addressing these issues and is currently establishing a multicenter calciphylaxis database.
Calciphylaxis, also known as calcific uremic arteriolopathy, is a painful skin condition classically seen in patients with end-stage renal disease (ESRD), particularly those on chronic dialysis.1,2 It also has increasingly been reported in patients with normal renal function and calcium and phosphate homeostasis.3,4 Effective diagnosis and management of calciphylaxis remains challenging for physicians.2,5 The condition is characterized by tissue ischemia caused by calcification of cutaneous arteriolar vessels. As a result, calciphylaxis is associated with high mortality rates, ranging from 60% to 80%.5,6 Excruciating pain and nonhealing ulcers often lead to recurrent hospitalizations and infectious complications,7 and poor nutritional status, chronic pain, depression, and insomnia can further complicate recovery and lead to poor quality of life.8
We provide an update on calciphylaxis etiopathogenesis, diagnosis, and management. We also highlight some challenges faced in managing this potentially fatal condition.
Epidemiology
Calciphylaxis is considered a rare dermatosis with an estimated annual incidence of 1% to 4% in ESRD patients on dialysis. Recent data suggest that incidence of calciphylaxis is rising,5,7,9 which may stem from an increased use of calcium-based phosphate binders, an actual rise in disease incidence, and/or increased recognition of the disease.5 It is difficult to estimate the exact disease burden of calciphylaxis because the diagnostic criteria are not well defined, often leading to missed or delayed diagnosis.3,10 Furthermore, there is no centralized registry for calciphylaxis cases.3
Etiology and Pathogenesis
Calciphylaxis is thought to have a multifactorial etiology with the exact cause or trigger unknown.7 A long list of risk factors and triggers is associated with the condition (Table 1). Calciphylaxis primarily affects small arteries (40–600 μm in diameter) that become calcified due to an imbalance between inhibitors and promoters of calcification.2,11 Fetuin-A and matrix Gla protein inhibit vascular calcification and are downregulated in calciphylaxis.12,13 Dysfunctional calcium, phosphate, and parathyroid hormone regulatory pathways provide an increased substrate for the process of calcification, which causes endothelial damage and microthrombosis, resulting in tissue ischemia and infarction.14,15 Notably, there is growing interest in the role of vitamin K in the pathogenesis of calciphylaxis. Vitamin K inhibits vascular calcification, possibly by increasing the circulating levels of carboxylated matrix Gla protein.16

Clinical Features
Calciphylaxis is most commonly seen on the legs, abdomen, and buttocks.2 Patients with ESRD commonly develop proximal lesions affecting adipose-rich sites and have a poor prognosis. Distal lesions are more common in patients with nonuremic calciphylaxis, and mortality rates are lower in this population.2
Early lesions present as painful skin nodules or indurated plaques that often are rock-hard or firm to palpation with overlying mottling or a livedoid pattern (Figure, A). Early lesions progress from livedo reticularis to livedo racemosa and then to retiform purpura (Figure, B). Purpuric lesions later evolve into black eschars (Figure, C), then to necrotic, ulcerated, malodorous plaques or nodules in later stages of the disease (Figure, D). Lesions also may develop a gangrenous sclerotic appearance.2,5

Although most patients with calciphylaxis have ESRD, nonuremic patients also can develop the disease. Those with calciphylaxis who do not have renal dysfunction frequently have other risk factors for the disease and often report another notable health problem in the weeks or months prior to presentation.4 More than half of patients with calciphylaxis become bedridden or require use of a wheelchair.17 Pain is characteristically severe throughout the course of the disease; it may even precede the appearance of the skin lesions.18 Because the pain is associated with ischemia, it tends to be relatively refractory to treatment with opioids. Rare extracutaneous vascular calcifications may lead to visual impairment, gastrointestinal tract bleeding, and myopathy.5,9,19,20
Diagnosis
Considering the high morbidity and mortality associated with calciphylaxis, it is important to provide accurate and timely diagnosis; however, there currently are no validated diagnostic criteria for calciphylaxis. Careful correlation of clinical and histologic findings is required. Calciphylaxis biopsies have demonstrated medial calcification and proliferation of the intima of small- to medium-sized arteries.21 Lobular and septal panniculitis and extravascular soft-tissue calcification, particularly stippled calcification of the eccrine sweat glands, also has been seen.2,22 Special calcium stains (eg, von Kossa, Alizarin red) increase the sensitivity of biopsy by highlighting subtle areas of intravascular and extravascular calcification.5,23 Sufficient sampling of subcutaneous tissue and specimen evaluation by an experienced dermatopathologist are necessary to ensure proper interpretation of the histologic findings.
Despite these measures, skin biopsies may be nondiagnostic or falsely negative; therefore, when there is high clinical suspicion, it may be appropriate to move forward with a presumptive diagnosis of calciphylaxis even if the histologic findings are nondiagnostic.1,9,24 It also is worth noting that localized progression and ulceration may occur following skin biopsy, such that biopsy may even be contraindicated in certain cases (eg, penile calciphylaxis).
Standard laboratory workup for calciphylaxis includes evaluation for associated risk factors as well as exclusion of other conditions in the differential diagnosis (Table 2). Blood tests to evaluate for risk factors include liver and renal function tests, a complete metabolic panel, parathyroid hormone level, and serum albumin level.5 Elevated calcium and phosphate levels may signal disturbed calcium and phosphate homeostasis but are neither sensitive nor specific for the diagnosis.25 Complete blood cell count, blood cultures, thorough hypercoagulability workup (including but not limited to antiphospholipid antibodies, proteins C and S, factor V Leiden, antithrombin III, homocysteine, methylenetetrahydrofolate reductase mutation, and cryoglobulins), rheumatoid factor, antineutrophil cytoplasmic antibodies, and antinuclear antibody testing may be relevant to help identify contributing factors or mimickers of calciphylaxis.5 Various imaging modalities also have been used to evaluate for the presence of soft-tissue calcification in areas of suspected calciphylaxis, including radiography, mammography, computed tomography, ultrasonography, nuclear bone scintigraphy, and spectroscopy.2,26,27 Unfortunately, there currently is no standardized reproducible imaging modality for reliable diagnosis of calciphylaxis. Ultimately, histologic and radiographic findings should always be interpreted in the context of relevant clinical findings.2,9

Prevention
Reduction of the net calcium phosphorus product may help reduce the risk of calciphylaxis in ESRD patients, which can be accomplished by using non–calcium-phosphate binders, adequate dialysis, and restricting use of vitamin D and vitamin K antagonists.2,5 There are limited data regarding the benefits of using bisphosphonates and cinacalcet in ESRD patients on dialysis to prevent calciphylaxis.28,29
Management
Management of calciphylaxis is multifactorial. Besides dermatology and nephrology, specialists in pain management, wound care, plastic surgery, and nutrition are critical partners in management.1,5,9,30 Nephrologists can help optimize calcium and phosphate balance and ensure adequate dialysis. Pain specialists can aid in creating aggressive multiagent pain regimens that target the neuropathic/ischemic and physical aspects of calciphylaxis pain. When appropriate, nutrition specialists can help establish high-protein, low-phosphorus diets, and wound specialists can provide access to advanced wound dressings and adjunctive hyperbaric oxygen therapy. Plastic surgeons can provide conservative debridement procedures in a subset of patients, usually those with distal stable disease.
The limited understanding of the etiopathogenesis of calciphylaxis and the lack of data on its management are reflected in the limited treatment options for the disease (Table 3).2,5,9 There are no formal algorithms for the treatment of calciphylaxis. Therapeutic trials are scarce, and most of the current treatment recommendations are based on small retrospective reports or case series. Sodium thiosulfate has been the most widely used treatment option since 2004, when its use in calciphylaxis was first reported.31 Sodium thiosulfate chelates calcium and is thought to have antioxidant and vasodilatory properties.32 There are a few promising clinical trials and large-scale studies (Table 4) that aim to evaluate the efficacy of existing treatments (eg, sodium thiosulfate) as well as novel treatment options such as lanthanum carbonate, SNF472 (hexasodium phytate), and vitamin K.33-36


Prognosis
Calciphylaxis is a potentially fatal condition with a poor prognosis and a median survival rate of approximately 1 year following the appearance of skin lesions.37-39 Patients with proximal lesions and those on peritoneal dialysis (as opposed to hemodialysis) have a worse prognosis.40 Mortality rates are estimated to be 30% at 6 months, 50% at 12 months, and 80% at 2 years, with sepsis secondary to infection of cutaneous ulcers being the leading cause of death.37-39 The impact of calciphylaxis on patient quality of life and activities of daily living is severe.8,17
Future Directions
Multi-institution cohort studies and collaborative registries are needed to provide updated information related to the epidemiology, diagnosis, treatment, morbidity, and mortality associated with calciphylaxis and to help formulate evidence-based diagnostic criteria. Radiographic and histologic studies, as well as other tools for early and accurate diagnosis of calciphylaxis, should be studied for feasibility, accuracy, and reproducibility. The incidence of nonuremic calciphylaxis points toward pathogenic pathways besides those based on the bone-mineral axis. Basic science research directed at improving understanding of the pathophysiology of calciphylaxis would be helpful in devising new treatment strategies targeting these pathways. Establishment of a collaborative, multi-institutional calciphylaxis working group would enable experts to formulate therapeutic guidelines based on current evidence. Such a group could facilitate initiation of large prospective studies to establish the efficacy of existing and new treatment modalities for calciphylaxis. A working group within the Society for Dermatology Hospitalists has been tasked with addressing these issues and is currently establishing a multicenter calciphylaxis database.
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146.
- Nigwekar SU, Thadhani RI, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Davis JM. The relationship between obesity and calciphylaxis: a review of the literature. Ostomy Wound Manage. 2016;62:12-18.
- Bajaj R, Courbebaisse M, Kroshinsky D, et al. Calciphylaxis in patients with normal renal function: a case series and systematic review. Mayo Clin Proc. 2018;93:1202-1212.
- Hafner J, Keusch G, Wahl C, et al. Uremic small-artery disease with medial calcification and intimal hyperplasia (so-called calciphylaxis): a complication of chronic renal failure and benefit from parathyroidectomy. J Am Acad Dermatol. 1995;33:954-962.
- Jeong HS, Dominguez AR. Calciphylaxis: controversies in pathogenesis, diagnosis and treatment. Am J Med Sci. 2016;351:217-227.
- Westphal SG, Plumb T. Calciphylaxis. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2018. https://www.ncbi.nlm.nih.gov/books/NBK519020. Accessed November 12, 2018.
- Riemer CA, El-Azhary RA, Wu KL, et al. Underreported use of palliative care and patient-reported outcome measures to address reduced quality of life in patients with calciphylaxis: a systematic review. Br J Dermatol. 2017;177:1510-1518.
- Nigwekar SU. Calciphylaxis. Curr Opin Nephrol Hypertens. 2017;26:276-281.
- Fine A, Fontaine B. Calciphylaxis: the beginning of the end? Perit Dial Int. 2008;28:268-270.
- Lin WT, Chao CM. Tumoral calcinosis in renal failure. QJM. 2014;107:387.
- Schafer C, Heiss A, Schwarz A, et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357-366.
- Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78-81.
- Bleyer AJ, Choi M, Igwemezie B, et al. A case control study of proximal calciphylaxis. Am J Kidney Dis. 1998;32:376-383.
- Ahmed S, O’Neill KD, Hood AF, et al. Calciphylaxis is associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am J Kidney Dis. 2001;37:267-276.
- Nigwekar SU, Bloch DB, Nazarian RM, et al. Vitamin K-dependent carboxylation of matrix gla protein influences the risk of calciphylaxis. J Am Soc Nephrol. 2017;28:1717-1722.
- Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-579.
- Polizzotto MN, Bryan T, Ashby MA, et al. Symptomatic management of calciphylaxis: a case series and review of the literature. J Pain Symptom Manage. 2006;32:186-190.
- Gupta N, Haq KF, Mahajan S, et al. Gastrointestinal bleeding secondary to calciphylaxis. Am J Case Rep. 2015;16:818-822.
- Edelstein CL, Wickham MK, Kirby PA. Systemic calciphylaxis presenting as a painful, proximal myopathy. Postgrad Med J. 1992;68:209-211.
- Mochel MC, Arakari RY, Wang G, et al. Cutaneous calciphylaxis: a retrospective histopathologic evaluation. Am J Dermatopathol. 2013;35:582-586.
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802.
- Cassius C, Moguelet P, Monfort JB, et al. Calciphylaxis in haemodialysed patients: diagnostic value of calcifications in cutaneous biopsy. Br J Dermatol. 2018;178:292-293.
- Sreedhar A, Sheikh HA, Scagliotti CJ, et al. Advanced-stage calciphylaxis: think before you punch. Cleve Clin J Med. 2016;83:562-564.
- Brandenburg VM, Kramann R, Rothe H, et al. Calcific uraemic arteriolopathy (calciphylaxis): data from a large nation-wide registry. Nephrol Dial Transplant. 2017;32:126-132.
- Paul S, Rabito CA, Vedak P, et al. The role of bone scintigraphy in the diagnosis of calciphylaxis. JAMA Dermatol. 2017;153:101-103.
- Shmidt E, Murthy NS, Knudsen JM, et al. Net-like pattern of calcification on plain soft-tissue radiographs in patients with calciphylaxis. J Am Acad Dermatol. 2012;67:1296-1301.
- EVOLVE Trial Investigators; Chertow GM, Block GA, Correa-Rotter R, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482-2494.
- Rogers NM, Teubner DJO, Coates PT. Calcific uremic arteriolopathy: advances in pathogenesis and treatment. Semin Dial. 2007;20:150-157.
- Nigwekar SU. Multidisciplinary approach to calcific uremic arteriolopathy. Curr Opin Nephrol Hypertens. 2015;24:531-537.
- Cicone JS, Petronis JB, Embert CD, et al. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104-1108.
- Chen NX, O’Neill K, Akl NK, et al. Adipocyte induced arterial calcification is prevented with sodium thiosulfate. Biochem Biophys Res Commun. 2014;449:151-156.
- Chan MR, Ghandour F, Murali NS, et al. Pilot study of the effect of lanthanum carbonate in patients with calciphylaxis: a Wisconsin Network for Health Research (WiNHR) study. J Nephrol Ther. 2014;4:1000162.
- Perelló J, Gómez M, Ferrer MD, et al. SNF472, a novel inhibitor of vascular calcification, could be administered during hemodialysis to attain potentially therapeutic phytate levels. J Nephrol. 2018;31:287-296.
- Christiadi D, Singer RF. Calciphylaxis in a dialysis patient successfully treated with high-dose vitamin K supplementation. Clin Kidney J. 2018;11:528-529.
- Caluwe R, Vandecasteele S, Van Vlem B, et al. Vitamin K2 supplementation in haemodialysis patients: a randomized dose-finding study. Nephrol Dial Transplant. 2014;29:1385-1390.
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394.
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217.
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429.
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241.
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146.
- Nigwekar SU, Thadhani RI, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Davis JM. The relationship between obesity and calciphylaxis: a review of the literature. Ostomy Wound Manage. 2016;62:12-18.
- Bajaj R, Courbebaisse M, Kroshinsky D, et al. Calciphylaxis in patients with normal renal function: a case series and systematic review. Mayo Clin Proc. 2018;93:1202-1212.
- Hafner J, Keusch G, Wahl C, et al. Uremic small-artery disease with medial calcification and intimal hyperplasia (so-called calciphylaxis): a complication of chronic renal failure and benefit from parathyroidectomy. J Am Acad Dermatol. 1995;33:954-962.
- Jeong HS, Dominguez AR. Calciphylaxis: controversies in pathogenesis, diagnosis and treatment. Am J Med Sci. 2016;351:217-227.
- Westphal SG, Plumb T. Calciphylaxis. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2018. https://www.ncbi.nlm.nih.gov/books/NBK519020. Accessed November 12, 2018.
- Riemer CA, El-Azhary RA, Wu KL, et al. Underreported use of palliative care and patient-reported outcome measures to address reduced quality of life in patients with calciphylaxis: a systematic review. Br J Dermatol. 2017;177:1510-1518.
- Nigwekar SU. Calciphylaxis. Curr Opin Nephrol Hypertens. 2017;26:276-281.
- Fine A, Fontaine B. Calciphylaxis: the beginning of the end? Perit Dial Int. 2008;28:268-270.
- Lin WT, Chao CM. Tumoral calcinosis in renal failure. QJM. 2014;107:387.
- Schafer C, Heiss A, Schwarz A, et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357-366.
- Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78-81.
- Bleyer AJ, Choi M, Igwemezie B, et al. A case control study of proximal calciphylaxis. Am J Kidney Dis. 1998;32:376-383.
- Ahmed S, O’Neill KD, Hood AF, et al. Calciphylaxis is associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am J Kidney Dis. 2001;37:267-276.
- Nigwekar SU, Bloch DB, Nazarian RM, et al. Vitamin K-dependent carboxylation of matrix gla protein influences the risk of calciphylaxis. J Am Soc Nephrol. 2017;28:1717-1722.
- Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-579.
- Polizzotto MN, Bryan T, Ashby MA, et al. Symptomatic management of calciphylaxis: a case series and review of the literature. J Pain Symptom Manage. 2006;32:186-190.
- Gupta N, Haq KF, Mahajan S, et al. Gastrointestinal bleeding secondary to calciphylaxis. Am J Case Rep. 2015;16:818-822.
- Edelstein CL, Wickham MK, Kirby PA. Systemic calciphylaxis presenting as a painful, proximal myopathy. Postgrad Med J. 1992;68:209-211.
- Mochel MC, Arakari RY, Wang G, et al. Cutaneous calciphylaxis: a retrospective histopathologic evaluation. Am J Dermatopathol. 2013;35:582-586.
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802.
- Cassius C, Moguelet P, Monfort JB, et al. Calciphylaxis in haemodialysed patients: diagnostic value of calcifications in cutaneous biopsy. Br J Dermatol. 2018;178:292-293.
- Sreedhar A, Sheikh HA, Scagliotti CJ, et al. Advanced-stage calciphylaxis: think before you punch. Cleve Clin J Med. 2016;83:562-564.
- Brandenburg VM, Kramann R, Rothe H, et al. Calcific uraemic arteriolopathy (calciphylaxis): data from a large nation-wide registry. Nephrol Dial Transplant. 2017;32:126-132.
- Paul S, Rabito CA, Vedak P, et al. The role of bone scintigraphy in the diagnosis of calciphylaxis. JAMA Dermatol. 2017;153:101-103.
- Shmidt E, Murthy NS, Knudsen JM, et al. Net-like pattern of calcification on plain soft-tissue radiographs in patients with calciphylaxis. J Am Acad Dermatol. 2012;67:1296-1301.
- EVOLVE Trial Investigators; Chertow GM, Block GA, Correa-Rotter R, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482-2494.
- Rogers NM, Teubner DJO, Coates PT. Calcific uremic arteriolopathy: advances in pathogenesis and treatment. Semin Dial. 2007;20:150-157.
- Nigwekar SU. Multidisciplinary approach to calcific uremic arteriolopathy. Curr Opin Nephrol Hypertens. 2015;24:531-537.
- Cicone JS, Petronis JB, Embert CD, et al. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104-1108.
- Chen NX, O’Neill K, Akl NK, et al. Adipocyte induced arterial calcification is prevented with sodium thiosulfate. Biochem Biophys Res Commun. 2014;449:151-156.
- Chan MR, Ghandour F, Murali NS, et al. Pilot study of the effect of lanthanum carbonate in patients with calciphylaxis: a Wisconsin Network for Health Research (WiNHR) study. J Nephrol Ther. 2014;4:1000162.
- Perelló J, Gómez M, Ferrer MD, et al. SNF472, a novel inhibitor of vascular calcification, could be administered during hemodialysis to attain potentially therapeutic phytate levels. J Nephrol. 2018;31:287-296.
- Christiadi D, Singer RF. Calciphylaxis in a dialysis patient successfully treated with high-dose vitamin K supplementation. Clin Kidney J. 2018;11:528-529.
- Caluwe R, Vandecasteele S, Van Vlem B, et al. Vitamin K2 supplementation in haemodialysis patients: a randomized dose-finding study. Nephrol Dial Transplant. 2014;29:1385-1390.
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394.
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217.
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429.
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241.
Practice Points
- Maintain a high index of suspicion for calciphylaxis in patients with end-stage renal disease on chronic dialysis presenting with severely painful livedoid plaques or retiform purpura, particularly in fat-rich body sites.
- Skin biopsies may be limited by biopsy site, inadequate biopsy depth, missed areas of microcalcification, and absence of definitive histologic criteria. Special calcium stains and review by an experienced dermatopathologist may lower the rate of false-negative biopsies.
- In cases where the most likely clinical diagnosis is calciphylaxis, treatment should be initiated even if definitive histopathology findings are lacking.
- Treatment should be multimodal, including elimination of risk factors, intravenous sodium thiosulfate, agents addressing calcium-phosphate metabolism, and surgical debridement, if indicated.