User login
Hospital Dermatology: Review of Research in 2023-2024
Inpatient consultative dermatology has advanced as a subspecialty and increasingly gained recognition in recent years. Since its founding in 2009, the Society of Dermatology Hospitalists has fostered research and education in hospital dermatology. Last year, we reviewed the 2022-2023 literature with a focus on developments in severe cutaneous adverse reactions, supportive oncodermatology, cost of inpatient services, and teledermatology.1 In this review, we highlight 3 areas of interest from the 2023-2024 literature: severe cutaneous adverse drug reactions, skin and soft tissue infections, and autoimmune blistering diseases (AIBDs).
Severe Cutaneous Adverse Drug Reactions
Adverse drug reactions are among the most common diagnoses encountered by inpatient dermatology consultants.2,3 Severe cutaneous adverse drug reactions are associated with substantial morbidity and mortality. Efforts to characterize these conditions and standardize their diagnosis and management continue to be a major focus of ongoing research.
A single-center retrospective analysis of 102 cases of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome evaluated differences in clinical manifestations depending on the culprit drug, offering insights into the heterogeneity of DRESS syndrome and the potential for diagnostic uncertainty.4 The shortest median latency was observed in a case caused by penicillin and cephalosporins (12 and 18 days, respectively), while DRESS syndrome secondary to allopurinol had the longest median latency (36 days). Nonsteroidal anti-inflammatory drug–induced DRESS syndrome was associated with the shortest hospital stay (6.5 days), while cephalosporin and vancomycin cases had the highest mortality rates.4
In the first international Delphi consensus study on the diagnostic workup, severity assessment, and management of DRESS syndrome, 54 dermatology and/or allergy experts reached consensus on 93 statements.5 Specific recommendations included basic evaluation with complete blood count with differential, kidney and liver function parameters, and electrocardiogram for all patients with suspected DRESS syndrome, with additional complementary workup considered in patients with evidence of specific organ damage and/or severe disease. In the proposed DRESS syndrome severity grading scheme, laboratory values that reached consensus for inclusion were hemoglobin, neutrophil, and platelet counts and creatinine, transaminases, and alkaline phosphatase levels. Although treatment of DRESS syndrome should be based on assessed disease severity, treatment with corticosteroids should be initiated in all patients with confirmed DRESS syndrome. Cyclosporine, antibodies interfering with the IL-5 axis, and intravenous immunoglobulins can be considered in patients with corticosteroid-refractory DRESS syndrome, and antiviral treatment can be considered in patients with a high serum cytomegalovirus viral load. Regularly following up with laboratory evaluation of involved organs; screening for autoantibodies, thyroid dysfunction, and steroid adverse effects; and offering of psychological support also were consensus recommendations.5
Identifying causative agents in drug hypersensitivity reactions remains challenging. A retrospective cohort study of 48 patients with Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) highlighted the need for a systematic unbiased approach to identifying culprit drugs. Using the RegiSCAR database and algorithm for drug causality for epidermal necrolysis to analyze the cohort, more than half of causative agents were determined to be different from those initially identified by the treating physicians. Nine additional suspected culprit drugs were identified, while 43 drugs initially identified as allergens were exonerated.6
Etiology-associated definitions for blistering reactions in children have been proposed to replace the existing terms Stevens-Johnson syndrome, toxic epidermal necrolysis, and others.7 Investigators in a recent study reclassified cases of SJS and TEN as reactive infectious mucocutaneous eruption (RIME) or drug-induced epidermal necrolysis (DEN), respectively. In RIME cases, Mycoplasma pneumoniae was the most commonly identified trigger, and in DEN cases, anticonvulsants were the most common class of culprit medications. Cases of RIME were less severe and were most often treated with antibiotics, whereas patients with DEN were more likely to receive supportive care, corticosteroids, intravenous immunoglobulins, and other immunosuppressive therapies.7
In addition to causing acute devastating mucocutaneous complications, SJS and TEN have long-lasting effects that require ongoing care. In a cohort of 6552 incident SJS/TEN cases over an 11-year period, survivors of SJS/TEN endured a mean loss of 9.4 years in life expectancy and excess health care expenditures of $3752 per year compared with age- and sex-matched controls. Patients with more severe disease, comorbid malignancy, diabetes, end-stage renal disease, or SJS/TEN sequelae experienced greater loss in life expectancy and lifetime health care expenditures.8 Separately, a qualitative study investigating the psychological impact of SJS/TEN in pediatric patients described sequelae including night terrors, posttraumatic stress disorder, depression, and anxiety for many years after the acute phase. Many patients reported a desire for increased support for their physical and emotional needs following hospital discharge.9
Skin and Soft Tissue Infections: Diagnosis, Management, and Prevention
Dermatology consultation has been shown to be a cost-effective intervention to improve outcomes in hospitalized patients with skin and soft tissue infections.10,11 In particular, cellulitis frequently is misdiagnosed, leading to unnecessary antibiotic use, hospitalizations, and major health care expenditures.12 Recognizing this challenge, researchers have worked to develop objective tools to improve diagnostic accuracy. In a large prospective prognostic validation study, Pulia et al13 found that thermal imaging alone or in combination with the ALT-70 prediction model (asymmetry, leukocytosis, tachycardia, and age ≥70 years) could be used successfully to reduce overdiagnosis of cellulitis. Both thermal imaging and the ALT-70 prediction model demonstrated robust sensitivity (93.5% and 98.8%, respectively) but low specificity (38.4% and 22.0%, respectively, and 53.9% when combined).13
In a systematic review, Kovacs et al14 analyzed case reports of pseudocellulitis caused by chemotherapeutic medications. Of the 81 cases selected, 58 (71.6%) were associated with gemcitabine, with the remaining 23 (28.4%) attributed to pemetrexed. Within this group, two-thirds of the patients received antibiotic treatment prior to receiving the correct diagnosis, and 36% experienced interruptions to their oncologic therapies. In contrast to infectious cellulitis, which tends to be unilateral and associated with elevated erythrocyte sedimentation rate or C-reactive protein, most chemotherapy-induced pseudocellulitis cases occurred bilaterally on the lower extremities, while erythrocyte sedimentation rate and C-reactive protein seldom were elevated.14
Necrotizing soft tissue infections (NSTIs) are severe life-threatening conditions characterized by widespread tissue destruction, signs of systemic toxicity, hemodynamic collapse, organ failure, and high mortality. Surgical inspection along with intraoperative tissue culture is the gold standard for diagnosis. Early detection, prompt surgical intervention, and appropriate antibiotic treatment are essential to reduce mortality and improve outcomes.15 A retrospective study of patients with surgically confirmed NSTIs assessed the incidence and risk factors for recurrence within 1 year following an initial NSTI of the lower extremity. Among 93 included patients, 32 (34.4%) had recurrence within 1 year, and more than half of recurrences occurred in the first 3 months (median, 66 days). The comparison of patients with and without recurrence showed similar proportions of antibiotic prophylaxis use after the first NSTI. There was significantly less compression therapy use (33.3% vs 62.3%; P=.13) and more negative pressure wound therapy use (83.3% vs 63.3%; P=.03) in the recurrence group, though the authors acknowledged that factors such as severity of pain and size of soft tissue defect may have affected the decisions for compression and negative pressure wound therapy.16
Residents of nursing homes are a particularly vulnerable population at high risk for health care–associated infections due to older age and a higher likelihood of having wounds, indwelling medical devices, and/or coexisting conditions.17 One cluster-randomized trial compared universal decolonization with routine-care bathing practices in nursing homes (N=28,956 residents). Decolonization entailed the use of chlorhexidine for all routine bathing and showering and administration of nasal povidone-iodine twice daily for the first 5 days after admission and then twice daily for 5 days every other week. Transfer to a hospital due to infection decreased from 62.9% to 52.2% with decolonization, for a difference in risk ratio of 16.6% (P<.001) compared with routine care. Additionally, the difference in risk ratio of the secondary end point (transfer to a hospital for any reason) was 14.6%. The number needed to treat was 9.7 to prevent 1 infection-related hospitalization and 8.9 to prevent 1 hospitalization for any reason.17
Autoimmune Blistering Diseases
Although rare, AIBDs are potentially life-threatening cutaneous diseases that often require inpatient management. While corticosteroids remain the mainstay of initial AIBD management, rituximab is now well recognized as the steroid-sparing treatment of choice for patients with moderate to severe pemphigus. In a long-term follow-up study of Ritux 318—the trial that led to the US Food and Drug Administration approval of rituximab in the treatment of moderate to severe pemphigus vulgaris—researchers assessed the long-term efficacy and safety of rituximab as a first-line treatment in patients with pemphigus.19 The 5- and 7-year disease-free survival rates without corticosteroid therapy for patients treated with rituximab were 76.7% and 72.1%, respectively, compared with 35.3% and 35.3% in those treated with prednisone alone (P<.001). Fewer serious adverse events were reported in those treated with rituximab plus prednisone compared with those treated with prednisone alone. None of the patients who maintained complete remission off corticosteroid therapy received any additional maintenance infusions of rituximab after the end of the Ritux 3 regimen (1 g of rituximab at day 0 and day 14, then 500 mg at months 12 and 18).19
By contrast, treatment of severe bullous pemphigoid (BP) often is less clear-cut, as no single therapeutic option has been shown to be superior to other immunomodulatory and immunosuppressive regimens, and the medical comorbidities of elderly patients with BP can be limiting. Fortunately, newer therapies with favorable safety profiles have emerged in recent years. In a multicenter retrospective study, 100 patients with BP received omalizumab after previously failing to respond to at least one alternative therapy. Disease control was obtained after a median of 10 days, and complete remission was achieved in 77% of patients in a median time of 3 months.20 In a multicenter retrospective cohort study of 146 patients with BP treated with dupilumab following the atopic dermatitis dosing schedule (one 600-mg dose followed by 300 mg every 2 weeks), disease control was achieved in a median of 14 days, while complete remission was achieved in 35.6% of patients, with 8.9% relapsing during the observation period.21 A retrospective case series of 30 patients with BP treated with dupilumab with maintenance dosing frequency tailored to individual patient response showed complete remission or marked response in 76.7% (23/30) of patients.22 A phase 2/3 randomized controlled trial of dupilumab in BP is currently ongoing (ClinicalTrials.gov identifier NCT04206553).
Pemphigoid gestationis is a rare autoimmune subepidermal bullous dermatosis of pregnancy that may be difficult to distinguish clinically from polymorphic eruption of pregnancy but confers notably different maternal and fetal risks. Researchers developed and validated a scoring system using clinical factors—history of pemphigoid gestationis, primigravidae, timing of rash onset, and specific clinical examination findings—that was able to differentiate between the 2 diseases with 79% sensitivity, 95% specificity, and an area under the curve of 0.93 without the need for advanced immunologic testing.23
Final Thoughts
Highlights of the literature from 2023-2024 demonstrate advancements in hospital-based dermatology as well as ongoing challenges. This year’s review emphasizes key developments in severe cutaneous adverse drug reactions, skin and soft tissue infections, and AIBDs. Continued expansion of knowledge in these areas and others informs patient care and demonstrates the value of dermatologic expertise in the inpatient setting.
- Berk-Krauss J, Micheletti RG. Hospital dermatology: review of research in 2022-2023. Cutis. 2023;112:236-239.
- Falanga V, Schachner LA, Rae V, et al. Dermatologic consultations in the hospital setting. Arch Dermatol. 1994;130:1022-1025.
- Kroshinsky D, Cotliar J, Hughey LC, et al. Association of dermatology consultation with accuracy of cutaneous disorder diagnoses in hospitalized patients: a multicenter analysis. JAMA Dermatol. 2016;152:477-480.
- Blumenthal KG, Alvarez-Arango S, Kroshinsky D, et al. Drug reaction eosinophilia and systemic symptoms: clinical phenotypic patterns according to causative drug. J Am Acad Dermatol. 2024;90:1240-1242.
- Brüggen MC, Walsh S, Ameri MM, et al. Management of adult patients with drug reaction with eosinophilia and systemic symptoms: a Delphi-based international consensus. JAMA Dermatol. 2024;160:37-44.
- Li DJ, Velasquez GA, Romar GA, et al. Assessment of need for improved identification of a culprit drug in Stevens-Johnson syndrome/toxic epidermal necrolysis. JAMA Dermatol. 2023;159:830-836.
- Martinez-Cabriales S, Coulombe J, Aaron M, et al. Preliminary summary and reclassification of cases from the Pediatric Research of Management in Stevens-Johnson syndrome and Epidermonecrolysis (PROMISE) study: a North American, multisite retrospective cohort. J Am Acad Dermatol. 2024;90:635-637.
- Chiu YM, Chiu HY. Lifetime risk, life expectancy, loss-of-life expectancy and lifetime healthcare expenditure for Stevens-Johnson syndrome/toxic epidermal necrolysis in Taiwan: follow-up of a nationwide cohort from 2008 to 2019. Br J Dermatol. 2023;189:553-560.
- Phillips C, Russell E, McNiven A, et al. A qualitative study of psychological morbidity in paediatric survivors of Stevens-Johnson syndrome/toxic epidermal necrolysis. Br J Dermatol. 2024;191:293-295.
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
- Pulia MS, Schwei RJ, Alexandridis R, et al. Validation of thermal imaging and the ALT-70 prediction model to differentiate cellulitis from pseudocellulitis. JAMA Dermatol. 2024;160:511-517.
- Kovacs LD, O’Donoghue M, Cogen AL. Chemotherapy-induced pseudocellulitis without prior radiation exposure: a systematic review. JAMA Dermatol. 2023;159:870-874.
- Yildiz H, Yombi JC. Necrotizing soft-tissue infections. comment. N Engl J Med. 2018;378:970.
- Traineau H, Charpentier C, Lepeule R, et al. First-year recurrence rate of skin and soft tissue infections following an initial necrotizing soft tissue infection of the lower extremities: a retrospective cohort study of 93 patients. J Am Acad Dermatol. 2023;88:1360-1363.
- Miller LG, McKinnell JA, Singh RD, et al. Decolonization in nursing homes to prevent infection and hospitalization. N Engl J Med. 2023;389:1766-1777.
- Joly P, Maho-Vaillant M, Prost-Squarcioni C, et al; French Study Group on Autoimmune Bullous Skin Diseases. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389:2031-2040.
- Tedbirt B, Maho-Vaillant M, Houivet E, et al; French Reference Center for Autoimmune Blistering Diseases MALIBUL. Sustained remission without corticosteroids among patients with pemphigus who had rituximab as first-line therapy: follow-up of the Ritux 3 Trial. JAMA Dermatol. 2024;160:290-296.
- Chebani R, Lombart F, Chaby G, et al; French Study Group on Autoimmune Bullous Diseases. Omalizumab in the treatment of bullous pemphigoid resistant to first-line therapy: a French national multicentre retrospective study of 100 patients. Br J Dermatol. 2024;190:258-265.
- Zhao L, Wang Q, Liang G, et al. Evaluation of dupilumab in patients with bullous pemphigoid. JAMA Dermatol. 2023;159:953-960.
- Miller AC, Temiz LA, Adjei S, et al. Treatment of bullous pemphigoid with dupilumab: a case series of 30 patients. J Drugs Dermatol. 2024;23:E144-E148.
- Xie F, Davis DMR, Baban F, et al. Development and multicenter international validation of a diagnostic tool to differentiate between pemphigoid gestationis and polymorphic eruption of pregnancy. J Am Acad Dermatol. 2023;89:106-113.
Inpatient consultative dermatology has advanced as a subspecialty and increasingly gained recognition in recent years. Since its founding in 2009, the Society of Dermatology Hospitalists has fostered research and education in hospital dermatology. Last year, we reviewed the 2022-2023 literature with a focus on developments in severe cutaneous adverse reactions, supportive oncodermatology, cost of inpatient services, and teledermatology.1 In this review, we highlight 3 areas of interest from the 2023-2024 literature: severe cutaneous adverse drug reactions, skin and soft tissue infections, and autoimmune blistering diseases (AIBDs).
Severe Cutaneous Adverse Drug Reactions
Adverse drug reactions are among the most common diagnoses encountered by inpatient dermatology consultants.2,3 Severe cutaneous adverse drug reactions are associated with substantial morbidity and mortality. Efforts to characterize these conditions and standardize their diagnosis and management continue to be a major focus of ongoing research.
A single-center retrospective analysis of 102 cases of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome evaluated differences in clinical manifestations depending on the culprit drug, offering insights into the heterogeneity of DRESS syndrome and the potential for diagnostic uncertainty.4 The shortest median latency was observed in a case caused by penicillin and cephalosporins (12 and 18 days, respectively), while DRESS syndrome secondary to allopurinol had the longest median latency (36 days). Nonsteroidal anti-inflammatory drug–induced DRESS syndrome was associated with the shortest hospital stay (6.5 days), while cephalosporin and vancomycin cases had the highest mortality rates.4
In the first international Delphi consensus study on the diagnostic workup, severity assessment, and management of DRESS syndrome, 54 dermatology and/or allergy experts reached consensus on 93 statements.5 Specific recommendations included basic evaluation with complete blood count with differential, kidney and liver function parameters, and electrocardiogram for all patients with suspected DRESS syndrome, with additional complementary workup considered in patients with evidence of specific organ damage and/or severe disease. In the proposed DRESS syndrome severity grading scheme, laboratory values that reached consensus for inclusion were hemoglobin, neutrophil, and platelet counts and creatinine, transaminases, and alkaline phosphatase levels. Although treatment of DRESS syndrome should be based on assessed disease severity, treatment with corticosteroids should be initiated in all patients with confirmed DRESS syndrome. Cyclosporine, antibodies interfering with the IL-5 axis, and intravenous immunoglobulins can be considered in patients with corticosteroid-refractory DRESS syndrome, and antiviral treatment can be considered in patients with a high serum cytomegalovirus viral load. Regularly following up with laboratory evaluation of involved organs; screening for autoantibodies, thyroid dysfunction, and steroid adverse effects; and offering of psychological support also were consensus recommendations.5
Identifying causative agents in drug hypersensitivity reactions remains challenging. A retrospective cohort study of 48 patients with Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) highlighted the need for a systematic unbiased approach to identifying culprit drugs. Using the RegiSCAR database and algorithm for drug causality for epidermal necrolysis to analyze the cohort, more than half of causative agents were determined to be different from those initially identified by the treating physicians. Nine additional suspected culprit drugs were identified, while 43 drugs initially identified as allergens were exonerated.6
Etiology-associated definitions for blistering reactions in children have been proposed to replace the existing terms Stevens-Johnson syndrome, toxic epidermal necrolysis, and others.7 Investigators in a recent study reclassified cases of SJS and TEN as reactive infectious mucocutaneous eruption (RIME) or drug-induced epidermal necrolysis (DEN), respectively. In RIME cases, Mycoplasma pneumoniae was the most commonly identified trigger, and in DEN cases, anticonvulsants were the most common class of culprit medications. Cases of RIME were less severe and were most often treated with antibiotics, whereas patients with DEN were more likely to receive supportive care, corticosteroids, intravenous immunoglobulins, and other immunosuppressive therapies.7
In addition to causing acute devastating mucocutaneous complications, SJS and TEN have long-lasting effects that require ongoing care. In a cohort of 6552 incident SJS/TEN cases over an 11-year period, survivors of SJS/TEN endured a mean loss of 9.4 years in life expectancy and excess health care expenditures of $3752 per year compared with age- and sex-matched controls. Patients with more severe disease, comorbid malignancy, diabetes, end-stage renal disease, or SJS/TEN sequelae experienced greater loss in life expectancy and lifetime health care expenditures.8 Separately, a qualitative study investigating the psychological impact of SJS/TEN in pediatric patients described sequelae including night terrors, posttraumatic stress disorder, depression, and anxiety for many years after the acute phase. Many patients reported a desire for increased support for their physical and emotional needs following hospital discharge.9
Skin and Soft Tissue Infections: Diagnosis, Management, and Prevention
Dermatology consultation has been shown to be a cost-effective intervention to improve outcomes in hospitalized patients with skin and soft tissue infections.10,11 In particular, cellulitis frequently is misdiagnosed, leading to unnecessary antibiotic use, hospitalizations, and major health care expenditures.12 Recognizing this challenge, researchers have worked to develop objective tools to improve diagnostic accuracy. In a large prospective prognostic validation study, Pulia et al13 found that thermal imaging alone or in combination with the ALT-70 prediction model (asymmetry, leukocytosis, tachycardia, and age ≥70 years) could be used successfully to reduce overdiagnosis of cellulitis. Both thermal imaging and the ALT-70 prediction model demonstrated robust sensitivity (93.5% and 98.8%, respectively) but low specificity (38.4% and 22.0%, respectively, and 53.9% when combined).13
In a systematic review, Kovacs et al14 analyzed case reports of pseudocellulitis caused by chemotherapeutic medications. Of the 81 cases selected, 58 (71.6%) were associated with gemcitabine, with the remaining 23 (28.4%) attributed to pemetrexed. Within this group, two-thirds of the patients received antibiotic treatment prior to receiving the correct diagnosis, and 36% experienced interruptions to their oncologic therapies. In contrast to infectious cellulitis, which tends to be unilateral and associated with elevated erythrocyte sedimentation rate or C-reactive protein, most chemotherapy-induced pseudocellulitis cases occurred bilaterally on the lower extremities, while erythrocyte sedimentation rate and C-reactive protein seldom were elevated.14
Necrotizing soft tissue infections (NSTIs) are severe life-threatening conditions characterized by widespread tissue destruction, signs of systemic toxicity, hemodynamic collapse, organ failure, and high mortality. Surgical inspection along with intraoperative tissue culture is the gold standard for diagnosis. Early detection, prompt surgical intervention, and appropriate antibiotic treatment are essential to reduce mortality and improve outcomes.15 A retrospective study of patients with surgically confirmed NSTIs assessed the incidence and risk factors for recurrence within 1 year following an initial NSTI of the lower extremity. Among 93 included patients, 32 (34.4%) had recurrence within 1 year, and more than half of recurrences occurred in the first 3 months (median, 66 days). The comparison of patients with and without recurrence showed similar proportions of antibiotic prophylaxis use after the first NSTI. There was significantly less compression therapy use (33.3% vs 62.3%; P=.13) and more negative pressure wound therapy use (83.3% vs 63.3%; P=.03) in the recurrence group, though the authors acknowledged that factors such as severity of pain and size of soft tissue defect may have affected the decisions for compression and negative pressure wound therapy.16
Residents of nursing homes are a particularly vulnerable population at high risk for health care–associated infections due to older age and a higher likelihood of having wounds, indwelling medical devices, and/or coexisting conditions.17 One cluster-randomized trial compared universal decolonization with routine-care bathing practices in nursing homes (N=28,956 residents). Decolonization entailed the use of chlorhexidine for all routine bathing and showering and administration of nasal povidone-iodine twice daily for the first 5 days after admission and then twice daily for 5 days every other week. Transfer to a hospital due to infection decreased from 62.9% to 52.2% with decolonization, for a difference in risk ratio of 16.6% (P<.001) compared with routine care. Additionally, the difference in risk ratio of the secondary end point (transfer to a hospital for any reason) was 14.6%. The number needed to treat was 9.7 to prevent 1 infection-related hospitalization and 8.9 to prevent 1 hospitalization for any reason.17
Autoimmune Blistering Diseases
Although rare, AIBDs are potentially life-threatening cutaneous diseases that often require inpatient management. While corticosteroids remain the mainstay of initial AIBD management, rituximab is now well recognized as the steroid-sparing treatment of choice for patients with moderate to severe pemphigus. In a long-term follow-up study of Ritux 318—the trial that led to the US Food and Drug Administration approval of rituximab in the treatment of moderate to severe pemphigus vulgaris—researchers assessed the long-term efficacy and safety of rituximab as a first-line treatment in patients with pemphigus.19 The 5- and 7-year disease-free survival rates without corticosteroid therapy for patients treated with rituximab were 76.7% and 72.1%, respectively, compared with 35.3% and 35.3% in those treated with prednisone alone (P<.001). Fewer serious adverse events were reported in those treated with rituximab plus prednisone compared with those treated with prednisone alone. None of the patients who maintained complete remission off corticosteroid therapy received any additional maintenance infusions of rituximab after the end of the Ritux 3 regimen (1 g of rituximab at day 0 and day 14, then 500 mg at months 12 and 18).19
By contrast, treatment of severe bullous pemphigoid (BP) often is less clear-cut, as no single therapeutic option has been shown to be superior to other immunomodulatory and immunosuppressive regimens, and the medical comorbidities of elderly patients with BP can be limiting. Fortunately, newer therapies with favorable safety profiles have emerged in recent years. In a multicenter retrospective study, 100 patients with BP received omalizumab after previously failing to respond to at least one alternative therapy. Disease control was obtained after a median of 10 days, and complete remission was achieved in 77% of patients in a median time of 3 months.20 In a multicenter retrospective cohort study of 146 patients with BP treated with dupilumab following the atopic dermatitis dosing schedule (one 600-mg dose followed by 300 mg every 2 weeks), disease control was achieved in a median of 14 days, while complete remission was achieved in 35.6% of patients, with 8.9% relapsing during the observation period.21 A retrospective case series of 30 patients with BP treated with dupilumab with maintenance dosing frequency tailored to individual patient response showed complete remission or marked response in 76.7% (23/30) of patients.22 A phase 2/3 randomized controlled trial of dupilumab in BP is currently ongoing (ClinicalTrials.gov identifier NCT04206553).
Pemphigoid gestationis is a rare autoimmune subepidermal bullous dermatosis of pregnancy that may be difficult to distinguish clinically from polymorphic eruption of pregnancy but confers notably different maternal and fetal risks. Researchers developed and validated a scoring system using clinical factors—history of pemphigoid gestationis, primigravidae, timing of rash onset, and specific clinical examination findings—that was able to differentiate between the 2 diseases with 79% sensitivity, 95% specificity, and an area under the curve of 0.93 without the need for advanced immunologic testing.23
Final Thoughts
Highlights of the literature from 2023-2024 demonstrate advancements in hospital-based dermatology as well as ongoing challenges. This year’s review emphasizes key developments in severe cutaneous adverse drug reactions, skin and soft tissue infections, and AIBDs. Continued expansion of knowledge in these areas and others informs patient care and demonstrates the value of dermatologic expertise in the inpatient setting.
Inpatient consultative dermatology has advanced as a subspecialty and increasingly gained recognition in recent years. Since its founding in 2009, the Society of Dermatology Hospitalists has fostered research and education in hospital dermatology. Last year, we reviewed the 2022-2023 literature with a focus on developments in severe cutaneous adverse reactions, supportive oncodermatology, cost of inpatient services, and teledermatology.1 In this review, we highlight 3 areas of interest from the 2023-2024 literature: severe cutaneous adverse drug reactions, skin and soft tissue infections, and autoimmune blistering diseases (AIBDs).
Severe Cutaneous Adverse Drug Reactions
Adverse drug reactions are among the most common diagnoses encountered by inpatient dermatology consultants.2,3 Severe cutaneous adverse drug reactions are associated with substantial morbidity and mortality. Efforts to characterize these conditions and standardize their diagnosis and management continue to be a major focus of ongoing research.
A single-center retrospective analysis of 102 cases of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome evaluated differences in clinical manifestations depending on the culprit drug, offering insights into the heterogeneity of DRESS syndrome and the potential for diagnostic uncertainty.4 The shortest median latency was observed in a case caused by penicillin and cephalosporins (12 and 18 days, respectively), while DRESS syndrome secondary to allopurinol had the longest median latency (36 days). Nonsteroidal anti-inflammatory drug–induced DRESS syndrome was associated with the shortest hospital stay (6.5 days), while cephalosporin and vancomycin cases had the highest mortality rates.4
In the first international Delphi consensus study on the diagnostic workup, severity assessment, and management of DRESS syndrome, 54 dermatology and/or allergy experts reached consensus on 93 statements.5 Specific recommendations included basic evaluation with complete blood count with differential, kidney and liver function parameters, and electrocardiogram for all patients with suspected DRESS syndrome, with additional complementary workup considered in patients with evidence of specific organ damage and/or severe disease. In the proposed DRESS syndrome severity grading scheme, laboratory values that reached consensus for inclusion were hemoglobin, neutrophil, and platelet counts and creatinine, transaminases, and alkaline phosphatase levels. Although treatment of DRESS syndrome should be based on assessed disease severity, treatment with corticosteroids should be initiated in all patients with confirmed DRESS syndrome. Cyclosporine, antibodies interfering with the IL-5 axis, and intravenous immunoglobulins can be considered in patients with corticosteroid-refractory DRESS syndrome, and antiviral treatment can be considered in patients with a high serum cytomegalovirus viral load. Regularly following up with laboratory evaluation of involved organs; screening for autoantibodies, thyroid dysfunction, and steroid adverse effects; and offering of psychological support also were consensus recommendations.5
Identifying causative agents in drug hypersensitivity reactions remains challenging. A retrospective cohort study of 48 patients with Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) highlighted the need for a systematic unbiased approach to identifying culprit drugs. Using the RegiSCAR database and algorithm for drug causality for epidermal necrolysis to analyze the cohort, more than half of causative agents were determined to be different from those initially identified by the treating physicians. Nine additional suspected culprit drugs were identified, while 43 drugs initially identified as allergens were exonerated.6
Etiology-associated definitions for blistering reactions in children have been proposed to replace the existing terms Stevens-Johnson syndrome, toxic epidermal necrolysis, and others.7 Investigators in a recent study reclassified cases of SJS and TEN as reactive infectious mucocutaneous eruption (RIME) or drug-induced epidermal necrolysis (DEN), respectively. In RIME cases, Mycoplasma pneumoniae was the most commonly identified trigger, and in DEN cases, anticonvulsants were the most common class of culprit medications. Cases of RIME were less severe and were most often treated with antibiotics, whereas patients with DEN were more likely to receive supportive care, corticosteroids, intravenous immunoglobulins, and other immunosuppressive therapies.7
In addition to causing acute devastating mucocutaneous complications, SJS and TEN have long-lasting effects that require ongoing care. In a cohort of 6552 incident SJS/TEN cases over an 11-year period, survivors of SJS/TEN endured a mean loss of 9.4 years in life expectancy and excess health care expenditures of $3752 per year compared with age- and sex-matched controls. Patients with more severe disease, comorbid malignancy, diabetes, end-stage renal disease, or SJS/TEN sequelae experienced greater loss in life expectancy and lifetime health care expenditures.8 Separately, a qualitative study investigating the psychological impact of SJS/TEN in pediatric patients described sequelae including night terrors, posttraumatic stress disorder, depression, and anxiety for many years after the acute phase. Many patients reported a desire for increased support for their physical and emotional needs following hospital discharge.9
Skin and Soft Tissue Infections: Diagnosis, Management, and Prevention
Dermatology consultation has been shown to be a cost-effective intervention to improve outcomes in hospitalized patients with skin and soft tissue infections.10,11 In particular, cellulitis frequently is misdiagnosed, leading to unnecessary antibiotic use, hospitalizations, and major health care expenditures.12 Recognizing this challenge, researchers have worked to develop objective tools to improve diagnostic accuracy. In a large prospective prognostic validation study, Pulia et al13 found that thermal imaging alone or in combination with the ALT-70 prediction model (asymmetry, leukocytosis, tachycardia, and age ≥70 years) could be used successfully to reduce overdiagnosis of cellulitis. Both thermal imaging and the ALT-70 prediction model demonstrated robust sensitivity (93.5% and 98.8%, respectively) but low specificity (38.4% and 22.0%, respectively, and 53.9% when combined).13
In a systematic review, Kovacs et al14 analyzed case reports of pseudocellulitis caused by chemotherapeutic medications. Of the 81 cases selected, 58 (71.6%) were associated with gemcitabine, with the remaining 23 (28.4%) attributed to pemetrexed. Within this group, two-thirds of the patients received antibiotic treatment prior to receiving the correct diagnosis, and 36% experienced interruptions to their oncologic therapies. In contrast to infectious cellulitis, which tends to be unilateral and associated with elevated erythrocyte sedimentation rate or C-reactive protein, most chemotherapy-induced pseudocellulitis cases occurred bilaterally on the lower extremities, while erythrocyte sedimentation rate and C-reactive protein seldom were elevated.14
Necrotizing soft tissue infections (NSTIs) are severe life-threatening conditions characterized by widespread tissue destruction, signs of systemic toxicity, hemodynamic collapse, organ failure, and high mortality. Surgical inspection along with intraoperative tissue culture is the gold standard for diagnosis. Early detection, prompt surgical intervention, and appropriate antibiotic treatment are essential to reduce mortality and improve outcomes.15 A retrospective study of patients with surgically confirmed NSTIs assessed the incidence and risk factors for recurrence within 1 year following an initial NSTI of the lower extremity. Among 93 included patients, 32 (34.4%) had recurrence within 1 year, and more than half of recurrences occurred in the first 3 months (median, 66 days). The comparison of patients with and without recurrence showed similar proportions of antibiotic prophylaxis use after the first NSTI. There was significantly less compression therapy use (33.3% vs 62.3%; P=.13) and more negative pressure wound therapy use (83.3% vs 63.3%; P=.03) in the recurrence group, though the authors acknowledged that factors such as severity of pain and size of soft tissue defect may have affected the decisions for compression and negative pressure wound therapy.16
Residents of nursing homes are a particularly vulnerable population at high risk for health care–associated infections due to older age and a higher likelihood of having wounds, indwelling medical devices, and/or coexisting conditions.17 One cluster-randomized trial compared universal decolonization with routine-care bathing practices in nursing homes (N=28,956 residents). Decolonization entailed the use of chlorhexidine for all routine bathing and showering and administration of nasal povidone-iodine twice daily for the first 5 days after admission and then twice daily for 5 days every other week. Transfer to a hospital due to infection decreased from 62.9% to 52.2% with decolonization, for a difference in risk ratio of 16.6% (P<.001) compared with routine care. Additionally, the difference in risk ratio of the secondary end point (transfer to a hospital for any reason) was 14.6%. The number needed to treat was 9.7 to prevent 1 infection-related hospitalization and 8.9 to prevent 1 hospitalization for any reason.17
Autoimmune Blistering Diseases
Although rare, AIBDs are potentially life-threatening cutaneous diseases that often require inpatient management. While corticosteroids remain the mainstay of initial AIBD management, rituximab is now well recognized as the steroid-sparing treatment of choice for patients with moderate to severe pemphigus. In a long-term follow-up study of Ritux 318—the trial that led to the US Food and Drug Administration approval of rituximab in the treatment of moderate to severe pemphigus vulgaris—researchers assessed the long-term efficacy and safety of rituximab as a first-line treatment in patients with pemphigus.19 The 5- and 7-year disease-free survival rates without corticosteroid therapy for patients treated with rituximab were 76.7% and 72.1%, respectively, compared with 35.3% and 35.3% in those treated with prednisone alone (P<.001). Fewer serious adverse events were reported in those treated with rituximab plus prednisone compared with those treated with prednisone alone. None of the patients who maintained complete remission off corticosteroid therapy received any additional maintenance infusions of rituximab after the end of the Ritux 3 regimen (1 g of rituximab at day 0 and day 14, then 500 mg at months 12 and 18).19
By contrast, treatment of severe bullous pemphigoid (BP) often is less clear-cut, as no single therapeutic option has been shown to be superior to other immunomodulatory and immunosuppressive regimens, and the medical comorbidities of elderly patients with BP can be limiting. Fortunately, newer therapies with favorable safety profiles have emerged in recent years. In a multicenter retrospective study, 100 patients with BP received omalizumab after previously failing to respond to at least one alternative therapy. Disease control was obtained after a median of 10 days, and complete remission was achieved in 77% of patients in a median time of 3 months.20 In a multicenter retrospective cohort study of 146 patients with BP treated with dupilumab following the atopic dermatitis dosing schedule (one 600-mg dose followed by 300 mg every 2 weeks), disease control was achieved in a median of 14 days, while complete remission was achieved in 35.6% of patients, with 8.9% relapsing during the observation period.21 A retrospective case series of 30 patients with BP treated with dupilumab with maintenance dosing frequency tailored to individual patient response showed complete remission or marked response in 76.7% (23/30) of patients.22 A phase 2/3 randomized controlled trial of dupilumab in BP is currently ongoing (ClinicalTrials.gov identifier NCT04206553).
Pemphigoid gestationis is a rare autoimmune subepidermal bullous dermatosis of pregnancy that may be difficult to distinguish clinically from polymorphic eruption of pregnancy but confers notably different maternal and fetal risks. Researchers developed and validated a scoring system using clinical factors—history of pemphigoid gestationis, primigravidae, timing of rash onset, and specific clinical examination findings—that was able to differentiate between the 2 diseases with 79% sensitivity, 95% specificity, and an area under the curve of 0.93 without the need for advanced immunologic testing.23
Final Thoughts
Highlights of the literature from 2023-2024 demonstrate advancements in hospital-based dermatology as well as ongoing challenges. This year’s review emphasizes key developments in severe cutaneous adverse drug reactions, skin and soft tissue infections, and AIBDs. Continued expansion of knowledge in these areas and others informs patient care and demonstrates the value of dermatologic expertise in the inpatient setting.
- Berk-Krauss J, Micheletti RG. Hospital dermatology: review of research in 2022-2023. Cutis. 2023;112:236-239.
- Falanga V, Schachner LA, Rae V, et al. Dermatologic consultations in the hospital setting. Arch Dermatol. 1994;130:1022-1025.
- Kroshinsky D, Cotliar J, Hughey LC, et al. Association of dermatology consultation with accuracy of cutaneous disorder diagnoses in hospitalized patients: a multicenter analysis. JAMA Dermatol. 2016;152:477-480.
- Blumenthal KG, Alvarez-Arango S, Kroshinsky D, et al. Drug reaction eosinophilia and systemic symptoms: clinical phenotypic patterns according to causative drug. J Am Acad Dermatol. 2024;90:1240-1242.
- Brüggen MC, Walsh S, Ameri MM, et al. Management of adult patients with drug reaction with eosinophilia and systemic symptoms: a Delphi-based international consensus. JAMA Dermatol. 2024;160:37-44.
- Li DJ, Velasquez GA, Romar GA, et al. Assessment of need for improved identification of a culprit drug in Stevens-Johnson syndrome/toxic epidermal necrolysis. JAMA Dermatol. 2023;159:830-836.
- Martinez-Cabriales S, Coulombe J, Aaron M, et al. Preliminary summary and reclassification of cases from the Pediatric Research of Management in Stevens-Johnson syndrome and Epidermonecrolysis (PROMISE) study: a North American, multisite retrospective cohort. J Am Acad Dermatol. 2024;90:635-637.
- Chiu YM, Chiu HY. Lifetime risk, life expectancy, loss-of-life expectancy and lifetime healthcare expenditure for Stevens-Johnson syndrome/toxic epidermal necrolysis in Taiwan: follow-up of a nationwide cohort from 2008 to 2019. Br J Dermatol. 2023;189:553-560.
- Phillips C, Russell E, McNiven A, et al. A qualitative study of psychological morbidity in paediatric survivors of Stevens-Johnson syndrome/toxic epidermal necrolysis. Br J Dermatol. 2024;191:293-295.
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
- Pulia MS, Schwei RJ, Alexandridis R, et al. Validation of thermal imaging and the ALT-70 prediction model to differentiate cellulitis from pseudocellulitis. JAMA Dermatol. 2024;160:511-517.
- Kovacs LD, O’Donoghue M, Cogen AL. Chemotherapy-induced pseudocellulitis without prior radiation exposure: a systematic review. JAMA Dermatol. 2023;159:870-874.
- Yildiz H, Yombi JC. Necrotizing soft-tissue infections. comment. N Engl J Med. 2018;378:970.
- Traineau H, Charpentier C, Lepeule R, et al. First-year recurrence rate of skin and soft tissue infections following an initial necrotizing soft tissue infection of the lower extremities: a retrospective cohort study of 93 patients. J Am Acad Dermatol. 2023;88:1360-1363.
- Miller LG, McKinnell JA, Singh RD, et al. Decolonization in nursing homes to prevent infection and hospitalization. N Engl J Med. 2023;389:1766-1777.
- Joly P, Maho-Vaillant M, Prost-Squarcioni C, et al; French Study Group on Autoimmune Bullous Skin Diseases. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389:2031-2040.
- Tedbirt B, Maho-Vaillant M, Houivet E, et al; French Reference Center for Autoimmune Blistering Diseases MALIBUL. Sustained remission without corticosteroids among patients with pemphigus who had rituximab as first-line therapy: follow-up of the Ritux 3 Trial. JAMA Dermatol. 2024;160:290-296.
- Chebani R, Lombart F, Chaby G, et al; French Study Group on Autoimmune Bullous Diseases. Omalizumab in the treatment of bullous pemphigoid resistant to first-line therapy: a French national multicentre retrospective study of 100 patients. Br J Dermatol. 2024;190:258-265.
- Zhao L, Wang Q, Liang G, et al. Evaluation of dupilumab in patients with bullous pemphigoid. JAMA Dermatol. 2023;159:953-960.
- Miller AC, Temiz LA, Adjei S, et al. Treatment of bullous pemphigoid with dupilumab: a case series of 30 patients. J Drugs Dermatol. 2024;23:E144-E148.
- Xie F, Davis DMR, Baban F, et al. Development and multicenter international validation of a diagnostic tool to differentiate between pemphigoid gestationis and polymorphic eruption of pregnancy. J Am Acad Dermatol. 2023;89:106-113.
- Berk-Krauss J, Micheletti RG. Hospital dermatology: review of research in 2022-2023. Cutis. 2023;112:236-239.
- Falanga V, Schachner LA, Rae V, et al. Dermatologic consultations in the hospital setting. Arch Dermatol. 1994;130:1022-1025.
- Kroshinsky D, Cotliar J, Hughey LC, et al. Association of dermatology consultation with accuracy of cutaneous disorder diagnoses in hospitalized patients: a multicenter analysis. JAMA Dermatol. 2016;152:477-480.
- Blumenthal KG, Alvarez-Arango S, Kroshinsky D, et al. Drug reaction eosinophilia and systemic symptoms: clinical phenotypic patterns according to causative drug. J Am Acad Dermatol. 2024;90:1240-1242.
- Brüggen MC, Walsh S, Ameri MM, et al. Management of adult patients with drug reaction with eosinophilia and systemic symptoms: a Delphi-based international consensus. JAMA Dermatol. 2024;160:37-44.
- Li DJ, Velasquez GA, Romar GA, et al. Assessment of need for improved identification of a culprit drug in Stevens-Johnson syndrome/toxic epidermal necrolysis. JAMA Dermatol. 2023;159:830-836.
- Martinez-Cabriales S, Coulombe J, Aaron M, et al. Preliminary summary and reclassification of cases from the Pediatric Research of Management in Stevens-Johnson syndrome and Epidermonecrolysis (PROMISE) study: a North American, multisite retrospective cohort. J Am Acad Dermatol. 2024;90:635-637.
- Chiu YM, Chiu HY. Lifetime risk, life expectancy, loss-of-life expectancy and lifetime healthcare expenditure for Stevens-Johnson syndrome/toxic epidermal necrolysis in Taiwan: follow-up of a nationwide cohort from 2008 to 2019. Br J Dermatol. 2023;189:553-560.
- Phillips C, Russell E, McNiven A, et al. A qualitative study of psychological morbidity in paediatric survivors of Stevens-Johnson syndrome/toxic epidermal necrolysis. Br J Dermatol. 2024;191:293-295.
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
- Pulia MS, Schwei RJ, Alexandridis R, et al. Validation of thermal imaging and the ALT-70 prediction model to differentiate cellulitis from pseudocellulitis. JAMA Dermatol. 2024;160:511-517.
- Kovacs LD, O’Donoghue M, Cogen AL. Chemotherapy-induced pseudocellulitis without prior radiation exposure: a systematic review. JAMA Dermatol. 2023;159:870-874.
- Yildiz H, Yombi JC. Necrotizing soft-tissue infections. comment. N Engl J Med. 2018;378:970.
- Traineau H, Charpentier C, Lepeule R, et al. First-year recurrence rate of skin and soft tissue infections following an initial necrotizing soft tissue infection of the lower extremities: a retrospective cohort study of 93 patients. J Am Acad Dermatol. 2023;88:1360-1363.
- Miller LG, McKinnell JA, Singh RD, et al. Decolonization in nursing homes to prevent infection and hospitalization. N Engl J Med. 2023;389:1766-1777.
- Joly P, Maho-Vaillant M, Prost-Squarcioni C, et al; French Study Group on Autoimmune Bullous Skin Diseases. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389:2031-2040.
- Tedbirt B, Maho-Vaillant M, Houivet E, et al; French Reference Center for Autoimmune Blistering Diseases MALIBUL. Sustained remission without corticosteroids among patients with pemphigus who had rituximab as first-line therapy: follow-up of the Ritux 3 Trial. JAMA Dermatol. 2024;160:290-296.
- Chebani R, Lombart F, Chaby G, et al; French Study Group on Autoimmune Bullous Diseases. Omalizumab in the treatment of bullous pemphigoid resistant to first-line therapy: a French national multicentre retrospective study of 100 patients. Br J Dermatol. 2024;190:258-265.
- Zhao L, Wang Q, Liang G, et al. Evaluation of dupilumab in patients with bullous pemphigoid. JAMA Dermatol. 2023;159:953-960.
- Miller AC, Temiz LA, Adjei S, et al. Treatment of bullous pemphigoid with dupilumab: a case series of 30 patients. J Drugs Dermatol. 2024;23:E144-E148.
- Xie F, Davis DMR, Baban F, et al. Development and multicenter international validation of a diagnostic tool to differentiate between pemphigoid gestationis and polymorphic eruption of pregnancy. J Am Acad Dermatol. 2023;89:106-113.
Practice Points
- An international Delphi study reached consensus on 93 statements regarding workup, severity assessment, and management of DRESS syndrome.
- In nursing homes, universal decolonization with chlorhexidine and nasal iodophor greatly reduced the risk for hospital transfers due to infection compared to routine care.
- Rituximab as the first-line therapy for pemphigus vulgaris is associated with long-term sustained complete remission without corticosteroid therapy.
- Dupilumab and omalizumab are emerging safe and effective treatment options for bullous pemphigoid.
Inpatient Management of Hidradenitis Suppurativa: A Delphi Consensus Study
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition that affects approximately 0.1% of the US population.1,2 Severe disease or HS flares can lead patients to seek care through the emergency department (ED), with some requiring inpatient admission. 3 Inpatient hospitalization of patients with HS has increased over the last 2 decades, and patients with HS utilize emergency and inpatient care more frequently than those with other dermatologic conditions.4,5 Minority patients and those of lower socioeconomic status are more likely to present to the ED for HS management due to limited access to care and other existing comorbid conditions. 4 In a 2022 study of the Nationwide Readmissions Database, the authors looked at hospital readmission rates of patients with HS compared with those with heart failure—both patient populations with chronic debilitating conditions. Results indicated that the hospital readmission rates for patients with HS surpassed those of patients with heart failure for that year, highlighting the need for improved inpatient management of HS.6
Patients with HS present to the ED with severe pain, fever, wound care, or the need for surgical intervention. The ED and inpatient hospital setting are locations in which physicians may not be as familiar with the diagnosis or treatment of HS, specifically flares or severe disease. 7 The inpatient care setting provides access to certain resources that can be challenging to obtain in the outpatient clinical setting, such as social workers and pain specialists, but also can prove challenging in obtaining other resources for HS management, such as advanced medical therapies. Given the increase in hospital- based care for HS and lack of widespread inpatient access to dermatology and HS experts, consensus recommendations for management of HS in the acute hospital setting would be beneficial. In our study, we sought to generate a collection of expert consensus statements providers can refer to when managing patients with HS in the inpatient setting.
Methods
The study team at the Wake Forest University School of Medicine (Winston-Salem, North Carolina)(M.N., R.P., L.C.S.) developed an initial set of consensus statements based on current published HS treatment guidelines,8,9 publications on management of inpatient HS,3 published supportive care guidelines for Stevens-Johnson syndrome, 10 and personal clinical experience in managing inpatient HS, which resulted in 50 statements organized into the following categories: overall care, wound care, genital care, pain management, infection control, medical management, surgical management, nutrition, and transitional care guidelines. This study was approved by the Wake Forest University institutional review board (IRB00084257).
Participant Recruitment—Dermatologists were identified for participation in the study based on membership in the Society of Dermatology Hospitalists and the Hidradenitis Suppurativa Foundation or authorship of publications relevant to HS or inpatient dermatology. Dermatologists from larger academic institutions with HS specialty clinics and inpatient dermatology services also were identified. Participants were invited via email and could suggest other experts for inclusion. A total of 31 dermatologists were invited to participate in the study, with 26 agreeing to participate. All participating dermatologists were practicing in the United States.
Delphi Study—In the first round of the Delphi study, the participants were sent an online survey via REDCap in which they were asked to rank the appropriateness of each of the proposed 50 guideline statements on a scale of 1 (very inappropriate) to 9 (very appropriate). Participants also were able to provide commentary and feedback on each of the statements. Survey results were analyzed using the RAND/ UCLA Appropriateness Method.11 For each statement, the median rating for appropriateness, interpercentile range (IPR), IPR adjusted for symmetry, and disagreement index (DI) were calculated (DI=IPR/IPR adjusted for symmetry). The 30th and 70th percentiles were used in the DI calculation as the upper and lower limits, respectively. A median rating for appropriateness of 1.0 to 3.9 was considered “inappropriate,” 4.0 to 6.9 was considered “uncertain appropriateness,” and 7.0 to 9.0 was “appropriate.” A DI value greater than or equal to 1 indicated a lack of consensus regarding the appropriateness of the statement. Following each round, participants received a copy of their responses along with the group median rank of each statement. Statements that did not reach consensus in the first Delphi round were revised based on feedback received by the participants, and a second survey with 14 statements was sent via REDCap 2 weeks later. The RAND/UCLA Appropriateness Method also was applied to this second Delphi round. After the second survey, participants received a copy of anonymized comments regarding the consensus statements and were allowed to provide additional final commentary to be included in the discussion of these recommendations.
Results
Twenty-six dermatologists completed the first-round survey, and 24 participants completed the second-round survey. All participants self-identified as having expertise in either HS (n=22 [85%]) or inpatient dermatology (n=17 [65%]), and 13 (50%) participants self-identified as experts in both HS and inpatient dermatology. All participants, except 1, were affiliated with an academic health system with inpatient dermatology services. The average length of time in practice as a dermatologist was 10 years (median, 9 years [range, 3–27 years]).
Of the 50 initial proposed consensus statements, 26 (52%) achieved consensus after the first round; 21 statements revealed DI calculations that did not achieve consensus. Two statements achieved consensus but received median ratings for appropriateness, indicating uncertain appropriateness; because of this, 1 statement was removed and 1 was revised based on participant feedback, resulting in 13 revised statements (eTable 1). Controversial topics in the consensus process included obtaining wound cultures and meaningful culture data interpretation, use of specific biologic medications in the inpatient setting, and use of intravenous ertapenem. Participant responses to these topics are discussed in detail below. Of these secondround statements, all achieved consensus. The final set of consensus statements can be found in eTable 2.
Comment
Our Delphi consensus study combined the expertise of both dermatologists who care for patients with HS and those with inpatient dermatology experience to produce a set of recommendations for the management of HS in the hospital care setting. A strength of this study is inclusion of many national leaders in both HS and inpatient dermatology, with some participants having developed the previously published HS treatment guidelines and others having participated in inpatient dermatology Delphi studies.8-10 The expertise is further strengthened by the geographically diverse institutional representation within the United States.
The final consensus recommendations included 40 statements covering a range of patient care issues, including use of appropriate inpatient subspecialists (care team), supportive care measures (wound care, pain control, genital care), disease-oriented treatment (medical management, surgical management), inpatient complications (infection control, nutrition), and successful transition back to outpatient management (transitional care). These recommendations are meant to serve as a resource for providers to consider when taking care of inpatient HS flares, recognizing that the complexity and individual circumstances of each patient are unique.
Delphi Consensus Recommendations Compared to Prior Guidelines—Several recommendations in the current study align with the previously published North American clinical management guidelines for HS.8,9 Our recommendations agree with prior guidelines on the importance of disease staging and pain assessment using validated assessment tools as well as screening for HS comorbidities. There also is agreement in the potential benefit of involving pain specialists in the development of a comprehensive pain management plan. The inpatient care setting provides a unique opportunity to engage multiple specialists and collaborate on patient care in a timely manner. Our recommendations regarding surgical care also align with established guidelines in recommending incision and drainage as an acute bedside procedure best utilized for symptom relief in inflamed abscesses and relegating most other surgical management to the outpatient setting. Wound care recommendations also are similar, with our expert participants agreeing on individualizing dressing choices based on wound characteristics. A benefit of inpatient wound care is access to skilled nursing for dressing changes and potentially improved access to more sophisticated dressing materials. Our recommendations differ from the prior guidelines in our focus on severe HS, HS flares, and HS complications, which constitute the majority of inpatient disease management. We provide additional guidance on management of secondary infections, perianal fistulous disease, and importantly transitional care to optimize discharge planning.
Differing Opinions in Our Analysis—Despite the success of our Delphi consensus process, there were some differing opinions regarding certain aspects of inpatient HS management, which is to be expected given the lack of strong evidence-based research to support some of the recommended practices. There were differing opinions on the utility of wound culture data, with some participants feeling culture data could help with antibiotic susceptibility and resistance patterns, while others felt wound cultures represent bacterial colonization or biofilm formation.
Initial consensus statements in the first Delphi round were created for individual biologic medications but did not achieve consensus, and feedback on the use of biologics in the inpatient environment was mixed, largely due to logistic and insurance issues. Many participants felt biologic medication cost, difficulty obtaining inpatient reimbursement, health care resource utilization, and availability of biologics in different hospital systems prevented recommending the use of specific biologics during hospitalization. The one exception was in the case of a hospitalized patient who was already receiving infliximab for HS: there was consensus on ensuring the patient dosing was maximized, if appropriate, to 10 mg/kg.12 Ertapenem use also was controversial, with some participants using it as a bridge therapy to either outpatient biologic use or surgery, while others felt it was onerous and difficult to establish reliable access to secure intravenous administration and regular dosing once the patient left the inpatient setting.13 Others said they have experienced objections from infectious disease colleagues on the use of intravenous antibiotics, citing antibiotic stewardship concerns.
Patient Care in the Inpatient Setting—Prior literature suggests patients admitted as inpatients for HS tend to be of lower socioeconomic status and are admitted to larger urban teaching hospitals.14,15 Patients with lower socioeconomic status have increased difficulty accessing health care resources; therefore, inpatient admission serves as an opportunity to provide a holistic HS assessment and coordinate resources for chronic outpatient management.
Study Limitations—This Delphi consensus study has some limitations. The existing literature on inpatient management of HS is limited, challenging our ability to assess the extent to which these published recommendations are already being implemented. Additionally, the study included HS and inpatient dermatology experts from the United States, which means the recommendations may not be generalizable to other countries. Most participants practiced dermatology at large tertiary care academic medical centers, which may limit the ability to implement recommendations in all US inpatient care settings such as small community-based hospitals; however, many of the supportive care guidelines such as pain control, wound care, nutritional support, and social work should be achievable in most inpatient care settings.
Conclusion
Given the increase in inpatient and ED health care utilization for HS, there is an urgent need for expert consensus recommendations on inpatient management of this unique patient population, which requires complex multidisciplinary care. Our recommendations are a resource for providers to utilize and potentially improve the standard of care we provide these patients.
Acknowledgment—We thank the Wake Forest University Clinical and Translational Science Institute (Winston- Salem, North Carolina) for providing statistical help.
- Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764.
- Ingram JR. The epidemiology of hidradenitis suppurativa. Br J Dermatol. 2020;183:990-998. doi:10.1111/bjd.19435
- Charrow A, Savage KT, Flood K, et al. Hidradenitis suppurativa for the dermatologic hospitalist. Cutis. 2019;104:276-280.
- Anzaldi L, Perkins JA, Byrd AS, et al. Characterizing inpatient hospitalizations for hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2020;82:510-513. doi:10.1016/j.jaad.2019.09.019
- Khalsa A, Liu G, Kirby JS. Increased utilization of emergency department and inpatient care by patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73:609-614. doi:10.1016/j.jaad.2015.06.053
- Edigin E, Kaul S, Eseaton PO, et al. At 180 days hidradenitis suppurativa readmission rate is comparable to heart failure: analysis of the nationwide readmissions database. J Am Acad Dermatol. 2022;87:188-192. doi:10.1016/j.jaad.2021.06.894
- Kirby JS, Miller JJ, Adams DR, et al. Health care utilization patterns and costs for patients with hidradenitis suppurativa. JAMA Dermatol. 2014;150:937-944. doi:10.1001/jamadermatol.2014.691
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016/j .jaad.2019.02.067
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101. doi:10.1016/j.jaad.2019.02.068
- Seminario-Vidal L, Kroshinsky D, Malachowski SJ, et al. Society of Dermatology Hospitalists supportive care guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults. J Am Acad Dermatol. 2020;82:1553-1567. doi:10.1016/j .jaad.2020.02.066
- Fitch K, Bernstein SJ, Burnand B, et al. The RAND/UCLA Appropriateness Method: User’s Manual. Rand; 2001.
- Oskardmay AN, Miles JA, Sayed CJ. Determining the optimal dose of infliximab for treatment of hidradenitis suppurativa. J Am Acad Dermatol. 2019;81:702-708. doi:10.1016/j.jaad.2019.05.022
- Join-Lambert O, Coignard-Biehler H, Jais JP, et al. Efficacy of ertapenem in severe hidradenitis suppurativa: a pilot study in a cohort of 30 consecutive patients. J Antimicrob Chemother. 2016;71:513-520. doi:10.1093/jac/dkv361
- Khanna R, Whang KA, Huang AH, et al. Inpatient burden of hidradenitis suppurativa in the United States: analysis of the 2016 National Inpatient Sample. J Dermatolog Treat. 2022;33:1150-1152. doi:10.1080/09 546634.2020.1773380
- Patel A, Patel A, Solanki D, et al. Hidradenitis suppurativa in the United States: insights from the national inpatient sample (2008-2017) on contemporary trends in demographics, hospitalization rates, chronic comorbid conditions, and mortality. Cureus. 2022;14:E24755. doi:10.7759/cureus.24755
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition that affects approximately 0.1% of the US population.1,2 Severe disease or HS flares can lead patients to seek care through the emergency department (ED), with some requiring inpatient admission. 3 Inpatient hospitalization of patients with HS has increased over the last 2 decades, and patients with HS utilize emergency and inpatient care more frequently than those with other dermatologic conditions.4,5 Minority patients and those of lower socioeconomic status are more likely to present to the ED for HS management due to limited access to care and other existing comorbid conditions. 4 In a 2022 study of the Nationwide Readmissions Database, the authors looked at hospital readmission rates of patients with HS compared with those with heart failure—both patient populations with chronic debilitating conditions. Results indicated that the hospital readmission rates for patients with HS surpassed those of patients with heart failure for that year, highlighting the need for improved inpatient management of HS.6
Patients with HS present to the ED with severe pain, fever, wound care, or the need for surgical intervention. The ED and inpatient hospital setting are locations in which physicians may not be as familiar with the diagnosis or treatment of HS, specifically flares or severe disease. 7 The inpatient care setting provides access to certain resources that can be challenging to obtain in the outpatient clinical setting, such as social workers and pain specialists, but also can prove challenging in obtaining other resources for HS management, such as advanced medical therapies. Given the increase in hospital- based care for HS and lack of widespread inpatient access to dermatology and HS experts, consensus recommendations for management of HS in the acute hospital setting would be beneficial. In our study, we sought to generate a collection of expert consensus statements providers can refer to when managing patients with HS in the inpatient setting.
Methods
The study team at the Wake Forest University School of Medicine (Winston-Salem, North Carolina)(M.N., R.P., L.C.S.) developed an initial set of consensus statements based on current published HS treatment guidelines,8,9 publications on management of inpatient HS,3 published supportive care guidelines for Stevens-Johnson syndrome, 10 and personal clinical experience in managing inpatient HS, which resulted in 50 statements organized into the following categories: overall care, wound care, genital care, pain management, infection control, medical management, surgical management, nutrition, and transitional care guidelines. This study was approved by the Wake Forest University institutional review board (IRB00084257).
Participant Recruitment—Dermatologists were identified for participation in the study based on membership in the Society of Dermatology Hospitalists and the Hidradenitis Suppurativa Foundation or authorship of publications relevant to HS or inpatient dermatology. Dermatologists from larger academic institutions with HS specialty clinics and inpatient dermatology services also were identified. Participants were invited via email and could suggest other experts for inclusion. A total of 31 dermatologists were invited to participate in the study, with 26 agreeing to participate. All participating dermatologists were practicing in the United States.
Delphi Study—In the first round of the Delphi study, the participants were sent an online survey via REDCap in which they were asked to rank the appropriateness of each of the proposed 50 guideline statements on a scale of 1 (very inappropriate) to 9 (very appropriate). Participants also were able to provide commentary and feedback on each of the statements. Survey results were analyzed using the RAND/ UCLA Appropriateness Method.11 For each statement, the median rating for appropriateness, interpercentile range (IPR), IPR adjusted for symmetry, and disagreement index (DI) were calculated (DI=IPR/IPR adjusted for symmetry). The 30th and 70th percentiles were used in the DI calculation as the upper and lower limits, respectively. A median rating for appropriateness of 1.0 to 3.9 was considered “inappropriate,” 4.0 to 6.9 was considered “uncertain appropriateness,” and 7.0 to 9.0 was “appropriate.” A DI value greater than or equal to 1 indicated a lack of consensus regarding the appropriateness of the statement. Following each round, participants received a copy of their responses along with the group median rank of each statement. Statements that did not reach consensus in the first Delphi round were revised based on feedback received by the participants, and a second survey with 14 statements was sent via REDCap 2 weeks later. The RAND/UCLA Appropriateness Method also was applied to this second Delphi round. After the second survey, participants received a copy of anonymized comments regarding the consensus statements and were allowed to provide additional final commentary to be included in the discussion of these recommendations.
Results
Twenty-six dermatologists completed the first-round survey, and 24 participants completed the second-round survey. All participants self-identified as having expertise in either HS (n=22 [85%]) or inpatient dermatology (n=17 [65%]), and 13 (50%) participants self-identified as experts in both HS and inpatient dermatology. All participants, except 1, were affiliated with an academic health system with inpatient dermatology services. The average length of time in practice as a dermatologist was 10 years (median, 9 years [range, 3–27 years]).
Of the 50 initial proposed consensus statements, 26 (52%) achieved consensus after the first round; 21 statements revealed DI calculations that did not achieve consensus. Two statements achieved consensus but received median ratings for appropriateness, indicating uncertain appropriateness; because of this, 1 statement was removed and 1 was revised based on participant feedback, resulting in 13 revised statements (eTable 1). Controversial topics in the consensus process included obtaining wound cultures and meaningful culture data interpretation, use of specific biologic medications in the inpatient setting, and use of intravenous ertapenem. Participant responses to these topics are discussed in detail below. Of these secondround statements, all achieved consensus. The final set of consensus statements can be found in eTable 2.
Comment
Our Delphi consensus study combined the expertise of both dermatologists who care for patients with HS and those with inpatient dermatology experience to produce a set of recommendations for the management of HS in the hospital care setting. A strength of this study is inclusion of many national leaders in both HS and inpatient dermatology, with some participants having developed the previously published HS treatment guidelines and others having participated in inpatient dermatology Delphi studies.8-10 The expertise is further strengthened by the geographically diverse institutional representation within the United States.
The final consensus recommendations included 40 statements covering a range of patient care issues, including use of appropriate inpatient subspecialists (care team), supportive care measures (wound care, pain control, genital care), disease-oriented treatment (medical management, surgical management), inpatient complications (infection control, nutrition), and successful transition back to outpatient management (transitional care). These recommendations are meant to serve as a resource for providers to consider when taking care of inpatient HS flares, recognizing that the complexity and individual circumstances of each patient are unique.
Delphi Consensus Recommendations Compared to Prior Guidelines—Several recommendations in the current study align with the previously published North American clinical management guidelines for HS.8,9 Our recommendations agree with prior guidelines on the importance of disease staging and pain assessment using validated assessment tools as well as screening for HS comorbidities. There also is agreement in the potential benefit of involving pain specialists in the development of a comprehensive pain management plan. The inpatient care setting provides a unique opportunity to engage multiple specialists and collaborate on patient care in a timely manner. Our recommendations regarding surgical care also align with established guidelines in recommending incision and drainage as an acute bedside procedure best utilized for symptom relief in inflamed abscesses and relegating most other surgical management to the outpatient setting. Wound care recommendations also are similar, with our expert participants agreeing on individualizing dressing choices based on wound characteristics. A benefit of inpatient wound care is access to skilled nursing for dressing changes and potentially improved access to more sophisticated dressing materials. Our recommendations differ from the prior guidelines in our focus on severe HS, HS flares, and HS complications, which constitute the majority of inpatient disease management. We provide additional guidance on management of secondary infections, perianal fistulous disease, and importantly transitional care to optimize discharge planning.
Differing Opinions in Our Analysis—Despite the success of our Delphi consensus process, there were some differing opinions regarding certain aspects of inpatient HS management, which is to be expected given the lack of strong evidence-based research to support some of the recommended practices. There were differing opinions on the utility of wound culture data, with some participants feeling culture data could help with antibiotic susceptibility and resistance patterns, while others felt wound cultures represent bacterial colonization or biofilm formation.
Initial consensus statements in the first Delphi round were created for individual biologic medications but did not achieve consensus, and feedback on the use of biologics in the inpatient environment was mixed, largely due to logistic and insurance issues. Many participants felt biologic medication cost, difficulty obtaining inpatient reimbursement, health care resource utilization, and availability of biologics in different hospital systems prevented recommending the use of specific biologics during hospitalization. The one exception was in the case of a hospitalized patient who was already receiving infliximab for HS: there was consensus on ensuring the patient dosing was maximized, if appropriate, to 10 mg/kg.12 Ertapenem use also was controversial, with some participants using it as a bridge therapy to either outpatient biologic use or surgery, while others felt it was onerous and difficult to establish reliable access to secure intravenous administration and regular dosing once the patient left the inpatient setting.13 Others said they have experienced objections from infectious disease colleagues on the use of intravenous antibiotics, citing antibiotic stewardship concerns.
Patient Care in the Inpatient Setting—Prior literature suggests patients admitted as inpatients for HS tend to be of lower socioeconomic status and are admitted to larger urban teaching hospitals.14,15 Patients with lower socioeconomic status have increased difficulty accessing health care resources; therefore, inpatient admission serves as an opportunity to provide a holistic HS assessment and coordinate resources for chronic outpatient management.
Study Limitations—This Delphi consensus study has some limitations. The existing literature on inpatient management of HS is limited, challenging our ability to assess the extent to which these published recommendations are already being implemented. Additionally, the study included HS and inpatient dermatology experts from the United States, which means the recommendations may not be generalizable to other countries. Most participants practiced dermatology at large tertiary care academic medical centers, which may limit the ability to implement recommendations in all US inpatient care settings such as small community-based hospitals; however, many of the supportive care guidelines such as pain control, wound care, nutritional support, and social work should be achievable in most inpatient care settings.
Conclusion
Given the increase in inpatient and ED health care utilization for HS, there is an urgent need for expert consensus recommendations on inpatient management of this unique patient population, which requires complex multidisciplinary care. Our recommendations are a resource for providers to utilize and potentially improve the standard of care we provide these patients.
Acknowledgment—We thank the Wake Forest University Clinical and Translational Science Institute (Winston- Salem, North Carolina) for providing statistical help.
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition that affects approximately 0.1% of the US population.1,2 Severe disease or HS flares can lead patients to seek care through the emergency department (ED), with some requiring inpatient admission. 3 Inpatient hospitalization of patients with HS has increased over the last 2 decades, and patients with HS utilize emergency and inpatient care more frequently than those with other dermatologic conditions.4,5 Minority patients and those of lower socioeconomic status are more likely to present to the ED for HS management due to limited access to care and other existing comorbid conditions. 4 In a 2022 study of the Nationwide Readmissions Database, the authors looked at hospital readmission rates of patients with HS compared with those with heart failure—both patient populations with chronic debilitating conditions. Results indicated that the hospital readmission rates for patients with HS surpassed those of patients with heart failure for that year, highlighting the need for improved inpatient management of HS.6
Patients with HS present to the ED with severe pain, fever, wound care, or the need for surgical intervention. The ED and inpatient hospital setting are locations in which physicians may not be as familiar with the diagnosis or treatment of HS, specifically flares or severe disease. 7 The inpatient care setting provides access to certain resources that can be challenging to obtain in the outpatient clinical setting, such as social workers and pain specialists, but also can prove challenging in obtaining other resources for HS management, such as advanced medical therapies. Given the increase in hospital- based care for HS and lack of widespread inpatient access to dermatology and HS experts, consensus recommendations for management of HS in the acute hospital setting would be beneficial. In our study, we sought to generate a collection of expert consensus statements providers can refer to when managing patients with HS in the inpatient setting.
Methods
The study team at the Wake Forest University School of Medicine (Winston-Salem, North Carolina)(M.N., R.P., L.C.S.) developed an initial set of consensus statements based on current published HS treatment guidelines,8,9 publications on management of inpatient HS,3 published supportive care guidelines for Stevens-Johnson syndrome, 10 and personal clinical experience in managing inpatient HS, which resulted in 50 statements organized into the following categories: overall care, wound care, genital care, pain management, infection control, medical management, surgical management, nutrition, and transitional care guidelines. This study was approved by the Wake Forest University institutional review board (IRB00084257).
Participant Recruitment—Dermatologists were identified for participation in the study based on membership in the Society of Dermatology Hospitalists and the Hidradenitis Suppurativa Foundation or authorship of publications relevant to HS or inpatient dermatology. Dermatologists from larger academic institutions with HS specialty clinics and inpatient dermatology services also were identified. Participants were invited via email and could suggest other experts for inclusion. A total of 31 dermatologists were invited to participate in the study, with 26 agreeing to participate. All participating dermatologists were practicing in the United States.
Delphi Study—In the first round of the Delphi study, the participants were sent an online survey via REDCap in which they were asked to rank the appropriateness of each of the proposed 50 guideline statements on a scale of 1 (very inappropriate) to 9 (very appropriate). Participants also were able to provide commentary and feedback on each of the statements. Survey results were analyzed using the RAND/ UCLA Appropriateness Method.11 For each statement, the median rating for appropriateness, interpercentile range (IPR), IPR adjusted for symmetry, and disagreement index (DI) were calculated (DI=IPR/IPR adjusted for symmetry). The 30th and 70th percentiles were used in the DI calculation as the upper and lower limits, respectively. A median rating for appropriateness of 1.0 to 3.9 was considered “inappropriate,” 4.0 to 6.9 was considered “uncertain appropriateness,” and 7.0 to 9.0 was “appropriate.” A DI value greater than or equal to 1 indicated a lack of consensus regarding the appropriateness of the statement. Following each round, participants received a copy of their responses along with the group median rank of each statement. Statements that did not reach consensus in the first Delphi round were revised based on feedback received by the participants, and a second survey with 14 statements was sent via REDCap 2 weeks later. The RAND/UCLA Appropriateness Method also was applied to this second Delphi round. After the second survey, participants received a copy of anonymized comments regarding the consensus statements and were allowed to provide additional final commentary to be included in the discussion of these recommendations.
Results
Twenty-six dermatologists completed the first-round survey, and 24 participants completed the second-round survey. All participants self-identified as having expertise in either HS (n=22 [85%]) or inpatient dermatology (n=17 [65%]), and 13 (50%) participants self-identified as experts in both HS and inpatient dermatology. All participants, except 1, were affiliated with an academic health system with inpatient dermatology services. The average length of time in practice as a dermatologist was 10 years (median, 9 years [range, 3–27 years]).
Of the 50 initial proposed consensus statements, 26 (52%) achieved consensus after the first round; 21 statements revealed DI calculations that did not achieve consensus. Two statements achieved consensus but received median ratings for appropriateness, indicating uncertain appropriateness; because of this, 1 statement was removed and 1 was revised based on participant feedback, resulting in 13 revised statements (eTable 1). Controversial topics in the consensus process included obtaining wound cultures and meaningful culture data interpretation, use of specific biologic medications in the inpatient setting, and use of intravenous ertapenem. Participant responses to these topics are discussed in detail below. Of these secondround statements, all achieved consensus. The final set of consensus statements can be found in eTable 2.
Comment
Our Delphi consensus study combined the expertise of both dermatologists who care for patients with HS and those with inpatient dermatology experience to produce a set of recommendations for the management of HS in the hospital care setting. A strength of this study is inclusion of many national leaders in both HS and inpatient dermatology, with some participants having developed the previously published HS treatment guidelines and others having participated in inpatient dermatology Delphi studies.8-10 The expertise is further strengthened by the geographically diverse institutional representation within the United States.
The final consensus recommendations included 40 statements covering a range of patient care issues, including use of appropriate inpatient subspecialists (care team), supportive care measures (wound care, pain control, genital care), disease-oriented treatment (medical management, surgical management), inpatient complications (infection control, nutrition), and successful transition back to outpatient management (transitional care). These recommendations are meant to serve as a resource for providers to consider when taking care of inpatient HS flares, recognizing that the complexity and individual circumstances of each patient are unique.
Delphi Consensus Recommendations Compared to Prior Guidelines—Several recommendations in the current study align with the previously published North American clinical management guidelines for HS.8,9 Our recommendations agree with prior guidelines on the importance of disease staging and pain assessment using validated assessment tools as well as screening for HS comorbidities. There also is agreement in the potential benefit of involving pain specialists in the development of a comprehensive pain management plan. The inpatient care setting provides a unique opportunity to engage multiple specialists and collaborate on patient care in a timely manner. Our recommendations regarding surgical care also align with established guidelines in recommending incision and drainage as an acute bedside procedure best utilized for symptom relief in inflamed abscesses and relegating most other surgical management to the outpatient setting. Wound care recommendations also are similar, with our expert participants agreeing on individualizing dressing choices based on wound characteristics. A benefit of inpatient wound care is access to skilled nursing for dressing changes and potentially improved access to more sophisticated dressing materials. Our recommendations differ from the prior guidelines in our focus on severe HS, HS flares, and HS complications, which constitute the majority of inpatient disease management. We provide additional guidance on management of secondary infections, perianal fistulous disease, and importantly transitional care to optimize discharge planning.
Differing Opinions in Our Analysis—Despite the success of our Delphi consensus process, there were some differing opinions regarding certain aspects of inpatient HS management, which is to be expected given the lack of strong evidence-based research to support some of the recommended practices. There were differing opinions on the utility of wound culture data, with some participants feeling culture data could help with antibiotic susceptibility and resistance patterns, while others felt wound cultures represent bacterial colonization or biofilm formation.
Initial consensus statements in the first Delphi round were created for individual biologic medications but did not achieve consensus, and feedback on the use of biologics in the inpatient environment was mixed, largely due to logistic and insurance issues. Many participants felt biologic medication cost, difficulty obtaining inpatient reimbursement, health care resource utilization, and availability of biologics in different hospital systems prevented recommending the use of specific biologics during hospitalization. The one exception was in the case of a hospitalized patient who was already receiving infliximab for HS: there was consensus on ensuring the patient dosing was maximized, if appropriate, to 10 mg/kg.12 Ertapenem use also was controversial, with some participants using it as a bridge therapy to either outpatient biologic use or surgery, while others felt it was onerous and difficult to establish reliable access to secure intravenous administration and regular dosing once the patient left the inpatient setting.13 Others said they have experienced objections from infectious disease colleagues on the use of intravenous antibiotics, citing antibiotic stewardship concerns.
Patient Care in the Inpatient Setting—Prior literature suggests patients admitted as inpatients for HS tend to be of lower socioeconomic status and are admitted to larger urban teaching hospitals.14,15 Patients with lower socioeconomic status have increased difficulty accessing health care resources; therefore, inpatient admission serves as an opportunity to provide a holistic HS assessment and coordinate resources for chronic outpatient management.
Study Limitations—This Delphi consensus study has some limitations. The existing literature on inpatient management of HS is limited, challenging our ability to assess the extent to which these published recommendations are already being implemented. Additionally, the study included HS and inpatient dermatology experts from the United States, which means the recommendations may not be generalizable to other countries. Most participants practiced dermatology at large tertiary care academic medical centers, which may limit the ability to implement recommendations in all US inpatient care settings such as small community-based hospitals; however, many of the supportive care guidelines such as pain control, wound care, nutritional support, and social work should be achievable in most inpatient care settings.
Conclusion
Given the increase in inpatient and ED health care utilization for HS, there is an urgent need for expert consensus recommendations on inpatient management of this unique patient population, which requires complex multidisciplinary care. Our recommendations are a resource for providers to utilize and potentially improve the standard of care we provide these patients.
Acknowledgment—We thank the Wake Forest University Clinical and Translational Science Institute (Winston- Salem, North Carolina) for providing statistical help.
- Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764.
- Ingram JR. The epidemiology of hidradenitis suppurativa. Br J Dermatol. 2020;183:990-998. doi:10.1111/bjd.19435
- Charrow A, Savage KT, Flood K, et al. Hidradenitis suppurativa for the dermatologic hospitalist. Cutis. 2019;104:276-280.
- Anzaldi L, Perkins JA, Byrd AS, et al. Characterizing inpatient hospitalizations for hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2020;82:510-513. doi:10.1016/j.jaad.2019.09.019
- Khalsa A, Liu G, Kirby JS. Increased utilization of emergency department and inpatient care by patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73:609-614. doi:10.1016/j.jaad.2015.06.053
- Edigin E, Kaul S, Eseaton PO, et al. At 180 days hidradenitis suppurativa readmission rate is comparable to heart failure: analysis of the nationwide readmissions database. J Am Acad Dermatol. 2022;87:188-192. doi:10.1016/j.jaad.2021.06.894
- Kirby JS, Miller JJ, Adams DR, et al. Health care utilization patterns and costs for patients with hidradenitis suppurativa. JAMA Dermatol. 2014;150:937-944. doi:10.1001/jamadermatol.2014.691
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016/j .jaad.2019.02.067
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101. doi:10.1016/j.jaad.2019.02.068
- Seminario-Vidal L, Kroshinsky D, Malachowski SJ, et al. Society of Dermatology Hospitalists supportive care guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults. J Am Acad Dermatol. 2020;82:1553-1567. doi:10.1016/j .jaad.2020.02.066
- Fitch K, Bernstein SJ, Burnand B, et al. The RAND/UCLA Appropriateness Method: User’s Manual. Rand; 2001.
- Oskardmay AN, Miles JA, Sayed CJ. Determining the optimal dose of infliximab for treatment of hidradenitis suppurativa. J Am Acad Dermatol. 2019;81:702-708. doi:10.1016/j.jaad.2019.05.022
- Join-Lambert O, Coignard-Biehler H, Jais JP, et al. Efficacy of ertapenem in severe hidradenitis suppurativa: a pilot study in a cohort of 30 consecutive patients. J Antimicrob Chemother. 2016;71:513-520. doi:10.1093/jac/dkv361
- Khanna R, Whang KA, Huang AH, et al. Inpatient burden of hidradenitis suppurativa in the United States: analysis of the 2016 National Inpatient Sample. J Dermatolog Treat. 2022;33:1150-1152. doi:10.1080/09 546634.2020.1773380
- Patel A, Patel A, Solanki D, et al. Hidradenitis suppurativa in the United States: insights from the national inpatient sample (2008-2017) on contemporary trends in demographics, hospitalization rates, chronic comorbid conditions, and mortality. Cureus. 2022;14:E24755. doi:10.7759/cureus.24755
- Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764.
- Ingram JR. The epidemiology of hidradenitis suppurativa. Br J Dermatol. 2020;183:990-998. doi:10.1111/bjd.19435
- Charrow A, Savage KT, Flood K, et al. Hidradenitis suppurativa for the dermatologic hospitalist. Cutis. 2019;104:276-280.
- Anzaldi L, Perkins JA, Byrd AS, et al. Characterizing inpatient hospitalizations for hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2020;82:510-513. doi:10.1016/j.jaad.2019.09.019
- Khalsa A, Liu G, Kirby JS. Increased utilization of emergency department and inpatient care by patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73:609-614. doi:10.1016/j.jaad.2015.06.053
- Edigin E, Kaul S, Eseaton PO, et al. At 180 days hidradenitis suppurativa readmission rate is comparable to heart failure: analysis of the nationwide readmissions database. J Am Acad Dermatol. 2022;87:188-192. doi:10.1016/j.jaad.2021.06.894
- Kirby JS, Miller JJ, Adams DR, et al. Health care utilization patterns and costs for patients with hidradenitis suppurativa. JAMA Dermatol. 2014;150:937-944. doi:10.1001/jamadermatol.2014.691
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016/j .jaad.2019.02.067
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101. doi:10.1016/j.jaad.2019.02.068
- Seminario-Vidal L, Kroshinsky D, Malachowski SJ, et al. Society of Dermatology Hospitalists supportive care guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults. J Am Acad Dermatol. 2020;82:1553-1567. doi:10.1016/j .jaad.2020.02.066
- Fitch K, Bernstein SJ, Burnand B, et al. The RAND/UCLA Appropriateness Method: User’s Manual. Rand; 2001.
- Oskardmay AN, Miles JA, Sayed CJ. Determining the optimal dose of infliximab for treatment of hidradenitis suppurativa. J Am Acad Dermatol. 2019;81:702-708. doi:10.1016/j.jaad.2019.05.022
- Join-Lambert O, Coignard-Biehler H, Jais JP, et al. Efficacy of ertapenem in severe hidradenitis suppurativa: a pilot study in a cohort of 30 consecutive patients. J Antimicrob Chemother. 2016;71:513-520. doi:10.1093/jac/dkv361
- Khanna R, Whang KA, Huang AH, et al. Inpatient burden of hidradenitis suppurativa in the United States: analysis of the 2016 National Inpatient Sample. J Dermatolog Treat. 2022;33:1150-1152. doi:10.1080/09 546634.2020.1773380
- Patel A, Patel A, Solanki D, et al. Hidradenitis suppurativa in the United States: insights from the national inpatient sample (2008-2017) on contemporary trends in demographics, hospitalization rates, chronic comorbid conditions, and mortality. Cureus. 2022;14:E24755. doi:10.7759/cureus.24755
Practice Points
- Given the increase in hospital-based care for hidradenitis suppurativa (HS) and the lack of widespread inpatient access to dermatology and HS experts, consensus recommendations for management of HS in the acute hospital setting would be beneficial.
- Our Delphi study yielded 40 statements that reached consensus covering a range of patient care issues (eg, appropriate inpatient subspecialists [care team]), supportive care measures (wound care, pain control, genital care), disease-oriented treatment (medical management, surgical management), inpatient complications (infection control, nutrition), and successful transition to outpatient management (transitional care).
- These recommendations serve as an important resource for providers caring for inpatients with HS and represent a successful collaboration between inpatient dermatology and HS experts.
Hospital Dermatology: Review of Research in 2022-2023
Dermatologists improve the diagnostic accuracy and quality of care of patients in the hospital setting. They help shorten the length of stay, improve outpatient follow-up, and reduce the rate of hospital readmission.1 Medicare beneficiaries hospitalized with skin conditions at institutions with a dermatology hospitalist—a provider with a specialty interest in inpatient dermatology—have 24% lower odds of risk-adjusted 30-day mortality and 12% lower odds of risk-adjusted 30-day readmissions.2
In the last year, research among the dermatology hospitalist community has actively contributed to our understanding of challenging inpatient skin diseases and has identified new ways in which dermatologists can contribute to the care of hospitalized patients. In this review, we highlight 4 areas of focus from the published literature in 2022-2023—severe cutaneous adverse reactions, supportive oncodermatology, cost of inpatient services, and teledermatology.
Severe Cutaneous Adverse Reactions: Old and New
Severe cutaneous adverse reactions to medications frequently are encountered in the inpatient setting. Dermatology hospitalists are well positioned to phenotype these reactions, drawing insights that aid in identifying, characterizing, risk stratifying, and managing these conditions, which have considerable morbidity and mortality.
A recent 20-year retrospective review of cases of acute generalized exanthematous pustulosis (N=340) across 10 academic systems—the largest to date—improves our understanding of the features of this rare entity.3 The authors found that acute generalized exanthematous pustulosis most often is triggered by β-lactam and other antibiotics (75.5%) and is accompanied by fever (49.7%), neutrophilia (85.1%), and eosinophilia (52.1%). Kidney and liver involvement occur in less than 10% of cases, and mortality rates are low but not zero, with an all-cause 30-day mortality rate of 3.5%.3
In a multi-institutional retrospective study of 68 patients diagnosed with DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome, Sharma et al4 developed a scoring system to identify those at greatest risk for DRESS recurrence. Variables associated with recurrence including younger age, female sex, and features considered atypical for DRESS syndrome—nonmorbilliform rash; absence of facial edema; antinuclear antibody positivity; medication class other than antibiotic, antigout, or antiseizure—were used to develop a “ReDRESS” score. This predictive model had a sensitivity of 73% and specificity of 83% for predicting DRESS recurrence.4
Another case series characterized SCoRCH (sudden conjunctivitis, lymphopenia, sunburnlike rash, and hemodynamic changes), a newly described hypersensitivity reaction to trimethoprim-sulfamethoxazole.5 The onset of this reaction typically occurs 4 to 11 days after initiation of trimethoprim-sulfamethoxazole but can occur as quickly as 1 day following re-exposure. Patients are systemically ill with fever, hypotension, tachycardia, acute renal insufficiency, and transaminitis, and they have a diffuse sunburnlike erythema without scale, facial edema, and conjunctivitis. It is thought this distinct hypersensitivity reaction may be mediated by IL-6, which has a role in triggering a sepsislike physiology, with vasodilation, hypotension, and edema.5
A systematic review and meta-analysis found that sulfonamides remain the most prominent cause of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN).6 A case-control study described SJS/TEN presentations triggered by Mycoplasma, advocating for routine Mycoplasma screening, especially in patients without a clear medication culprit. Mycoplasma-induced cases carried statistically lower rates of mortality (0%) compared with medication-induced cases (22.5%).7 Another prospective open-label study evaluated SJS/TEN management by randomizing 25 patients to receive either combination therapy with methylprednisolone plus a tumor necrosis factor α inhibitor or methylprednisolone alone.8 Anti–tumor necrosis factor therapy was associated with a shorter length of initial steroid treatment and duration of the acute stage, hospitalization, and time to re-epithelialization8; however, as in a prior randomized unblinded trial,9 there was no difference in mortality between the 2 groups.
There is limited high-quality evidence to support the use of any systemic immunomodulator to decrease SJS/TEN–related mortality.10 A Cochrane systematic review highlighted the many limitations of the available data due to variations in presentation, assessment, and management.11 Because SJS/TEN is rare, powering studies based on mortality is infeasible; the authors calculated that 2872 participants were needed to detect a 50% mortality reduction among those with SCORTEN (severity-of-illness score for TEN) scores of 0 to 1.11 Therefore, collaborative efforts using appropriate outcomes measures (eg, time to re-epithelialization, length of hospital stay), standardized terminology and dosing regimens, and adaptive trial designs are needed. Consensus-derived assessment and treatment protocols could help account for variation, ensure consistency in treatment, and enable head-to-head comparisons. Members of the Society of Dermatology Hospitalists are working on efforts to standardize terminology and validate outcomes measures needed for future studies.12
Supportive Oncodermatology: A New Frontier
With the advent of immune checkpoint inhibitors (ICIs) for a growing number of cancers, dermatologists have become critical to identifying and managing cutaneous immune-related adverse events (cirAEs). Recent findings have demonstrated that dermatology input improves patient outcomes, not only regarding the treatment of dermatoses but also by augmenting cancer-related survival. One group found that patients with cirAEs who were evaluated by a dermatologist had improved progression-free (hazard ratio, 0.69; 95% CI, 0.54-0.87; P=.002) and overall survival rates (hazard ratio, 0.62; 95% CI, 0.45-0.84; P=.002), controlling for cirAE severity, age, sex, cancer type, and ICI subtype. Patients who were under the care of a dermatologist also were more likely to resume ICI therapy following an interruption (odds ratio, 10.52; 95% CI, 5.15-21.48; P<.001).13 Dermatologists help to optimize skin-directed and targeted therapies, such as dupilumab, minimizing exposure to systemic immunosuppression in these complex patients.14
Supportive oncodermatologists also have made important observations on how cirAEs relate to other adverse events and prognosis. A review of 628 patients found that almost half of those with cirAEs had co-occurring noncutaneous immune-related adverse events, most commonly pulmonary. Psoriasiform eruptions were most frequently associated with noncutaneous immune-related adverse events, and cutaneous reactions frequently preceded the development of systemic manifestations, serving as a clinical biomarker to provide prognostic information.15 A review of 95 patients found that spongiotic and lichenoid interface reactions were associated with decreased mortality rates, whereas vacuolar interface and perivascular dermatitis were associated with increased mortality.16
As with severe cutaneous adverse events, dermatology input has been critical for accurately phenotyping and risk stratifying these novel reactions. The dermatologist’s skill set is necessary for optimizing skin-directed and targeted therapies while minimizing systemic immunosuppression, thereby improving patient outcomes with respect to rash, cancer response, and survival.
The Cost of Inpatient Skin Disease
Hospitalizations account for approximately half of all health care expenditures, and hospital readmission, seen as a measure of the quality of health care delivery, can double this cost.17 Identifying and developing protocols for addressing patients with complex chronic inflammatory disorders is one strategy for improving outcomes and reducing financial burden. Inpatient dermatologists have identified hidradenitis suppurativa as one disease that can benefit from early intervention by dermatologists in the hospital, with its 30-day (17.8%) and 180-day (48.6%) readmission rates being comparable to those of heart failure.18
Following an index emergency department (ED) visit, 17.2% (3484/20,269) of patients with HS have at least 1 return ED visit within 30 days, while only 2.4% (483/20,269) have a dermatology visit within the same time frame.19 Understanding the risk factors for hospital readmission and ED utilization, including severity of illness, the presence of medical comorbidities, health coverage under Medicaid, and receipt of opioids, can allow dermatologists to anticipate those at greatest risk.19 Opportunities exist for cross-specialty interventions to anticipate and address modifiable risk factors. Shorter time to dermatology outpatient follow-up leads to improved clinic attendance and may help reduce ED utilization and hospital readmission.20
Teledermatology: Leveraging Inpatient Expertise
Although the benefit of inpatient dermatologic care is substantial, access to that care is finite. Following the COVID-19 pandemic, there is an increased acceptance of telemedicine and the long-term role it can play in leveraging dermatologic expertise, including meeting the increasing demand for inpatient dermatology care in rural and resource-poor communities.21
Recent studies conducted by dermatology hospitalists have illustrated the value of asynchronous store-and-forward technology in settings lacking access to consultative dermatology.22,23 Stephens et al22 found that expanding provider-to-provider electronic consultation (e-consultation) capacity to an inpatient rehabilitation facility resulted in completed consultations within 1.5 days compared with a 7- to 14-day wait time for patients attending an in-person urgent access dermatology clinic. In another study, the implementation of asynchronous dermatology e-consultations for immunobullous diseases, vasculitis, and herpes zoster resulted in a change in diagnosis 86% of the time, accompanied by at least 1 new systemic or topical therapy recommendation.23
Researchers also identified ways in which teledermatology can be inelegant and proposed specific supplemental data to aid in diagnosis. A review of 126 inpatient e-consultations demonstrated limitations related to the diagnosis of skin and soft-tissue infections. In two-thirds to three-quarters of cases, potentially useful descriptive information was missing, and in 70% (88/126), images were not appropriately focused. The authors developed a detailed checklist to help primary medical teams focus their differential diagnoses.24 A recent pilot study found that supplementation of clinical information with a standardized questionnaire and thermal images improved the accuracy of cellulitis diagnosis. Using this method, there was no difference in accuracy between dermatology hospitalists and other board-certified dermatologists, supporting the notion that any dermatologist can fulfill this need successfully, even without specific inpatient experience.25 Due to the high incidence and cost of cellulitis and related hospital admissions,26 such an intervention could have a considerable financial and patient safety impact.
Final Thoughts
This last year brought many changes to the health care landscape, the recession of a global pandemic, and an increasingly complex health care delivery system. Inpatient dermatologists met these challenges by providing high-quality dermatologic care and practice-modifying research in the areas of severe cutaneous adverse reactions, supportive oncodermatology, hospital readmission, telemedicine, and more, demonstrating the value of dermatologic expertise in the hospital setting.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528.
- Puri P, Pollock BD, Yousif M, et al. Association of Society of Dermatology hospitalist institutions with improved outcomes in Medicare beneficiaries hospitalized for skin disease. J Am Acad Dermatol. 2023;88:1372-1375.
- Creadore A, Desai S, Alloo A, et al. Clinical characteristics, disease course, and outcomes of patients with acute generalized exanthematous pustulosis in the US. JAMA Dermatol. 2022;158:176-183.
- Sharma AN, Murphy K, Shwe S, et al. Predicting DRESS syndrome recurrence—the ReDRESS score. JAMA Dermatol. 2022;158:1445-1447.
- Brian M, Rose EK, Mauskar MM, et al. Sudden conjunctivitis, lymphopenia, and rash combined with hemodynamic changes (SCoRCH) after trimethoprim-sulfamethoxazole use: a case series study of a hypersensitivity reaction. JAMA Dermatol. 2023;159:73-78.
- Lee EY, Knox C, Phillips EJ. Worldwide prevalence of antibiotic-associated Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2023;159:384-392.
- Liew YCC, Choo KJL, Oh CC, et al. Mycoplasma-induced Stevens-Johnson syndrome/toxic epidermal necrolysis: case-control analysis of a cohort managed in a specialized center. J Am Acad Dermatol. 2022;86:811-817.
- Ao S, Gao X, Zhan J, et al. Inhibition of tumor necrosis factor improves conventional steroid therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis in a cohort of patients. J Am Acad Dermatol. 2022;86:1236-1245.
- Wang CW, Yang LY, Chen CB, et al; the Taiwan Severe Cutaneous Adverse Reaction (TSCAR) Consortium. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-996.
- Han JJ, Creadore A, Seminario-Vidal L, et al. Medical management of Stevens-Johnson syndrome/toxic epidermal necrolysis among North American dermatologists. J Am Acad Dermatol. 2022;87:429-431.
- Noe MH, Micheletti RG. Systemic interventions for treatment of Stevens-Johnson syndrome/toxic epidermal necrolysis: summary of a Cochrane review. JAMA Dermatol. 2022;158:1436-1437.
- Waters M, Dobry A, Le ST, et al. Development of a skin-directed scoring system for Stevens-Johnson syndrome and epidermal necrolysis: a Delphi consensus exercise. JAMA Dermatol. 2023;159:772-777.
- Jacoby TV, Shah N, Asdourian MS, et al. Dermatology evaluation for cutaneous immune-related adverse events is associated with improved survival in cancer patients treated with checkpoint inhibition. J Am Acad Dermatol. 2023;88:711-714.
- Said JT, Elman SA, Perez-Chada LM, et al. Treatment of immune checkpoint inhibitor-mediated psoriasis: a systematic review. J Am Acad Dermatol. 2022;87:399-400.
- Asdourian MS, Shah N, Jacoby TV, et al. Evaluating patterns of co-occurrence between cutaneous and noncutaneous immune-related adverse events after immune checkpoint inhibitor therapy. J Am Acad Dermatol. 2023;88:246-249.
- Hirotsu KE, Scott MKD, Marquez C, et al. Histologic subtype of cutaneous immune-related adverse events predicts overall survival in patients receiving immune checkpoint inhibitors. J Am Acad Dermatol. 2022;87:651-653.
- Benbassat J, Taragin M. Hospital readmissions as a measure of quality of health care: advantages and limitations. Arch Intern Med. 2000;160:1074-1081.
- Edigin E, Kaul S, Eseaton PO, et al. At 180 days hidradenitis suppurativa readmission rate is comparable to heart failure: analysis of the nationwide readmissions database. J Am Acad Dermatol. 2022;87:188-192.
- Wang CX, Buss JL, Keller M, et al. Factors associated with dermatologic follow-up vs emergency department return in patients with hidradenitis suppurativa after an initial emergency department visit. JAMA Dermatol. 2022;158:1378-1386.
- Zakaria A, Chang AY, Kim-Lim P, et al. Predictors of postdischarge follow-up attendance among hospitalized dermatology patients: disparities and potential interventions. J Am Acad Dermatol. 2022;87:186-188.
- Arnold JD, Yoon S, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2019;80:425-432. doi:10.1016/j.jaad.2018.06.070
- Stephens MR, Das S, Smith GP. Utilization and outcomes of an asynchronous teledermatology pilot for an inpatient rehabilitation hospital. J Am Acad Dermatol. 2022;87:421-423.
- Ortiz C, Khosravi H, Kettering C, et al. Concordance data for inpatient asynchronous eDermatology consultation for immunobullous disease, zoster, and vasculitis. J Am Acad Dermatol. 2022;86:918-920.
- Salle R, Hua C, Mongereau M, et al. Challenges and limitations of teledermatology for skin and soft-tissue infections: a real-world study of an expert center. J Am Acad Dermatol. 2023;88:457-459.
- Creadore A, Manjaly P, Tkachenko E, et al. The utility of augmented teledermatology to improve dermatologist diagnosis of cellulitis: a cross-sectional study. Arch Dermatol Res. 2023;315:1347-1353.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
Dermatologists improve the diagnostic accuracy and quality of care of patients in the hospital setting. They help shorten the length of stay, improve outpatient follow-up, and reduce the rate of hospital readmission.1 Medicare beneficiaries hospitalized with skin conditions at institutions with a dermatology hospitalist—a provider with a specialty interest in inpatient dermatology—have 24% lower odds of risk-adjusted 30-day mortality and 12% lower odds of risk-adjusted 30-day readmissions.2
In the last year, research among the dermatology hospitalist community has actively contributed to our understanding of challenging inpatient skin diseases and has identified new ways in which dermatologists can contribute to the care of hospitalized patients. In this review, we highlight 4 areas of focus from the published literature in 2022-2023—severe cutaneous adverse reactions, supportive oncodermatology, cost of inpatient services, and teledermatology.
Severe Cutaneous Adverse Reactions: Old and New
Severe cutaneous adverse reactions to medications frequently are encountered in the inpatient setting. Dermatology hospitalists are well positioned to phenotype these reactions, drawing insights that aid in identifying, characterizing, risk stratifying, and managing these conditions, which have considerable morbidity and mortality.
A recent 20-year retrospective review of cases of acute generalized exanthematous pustulosis (N=340) across 10 academic systems—the largest to date—improves our understanding of the features of this rare entity.3 The authors found that acute generalized exanthematous pustulosis most often is triggered by β-lactam and other antibiotics (75.5%) and is accompanied by fever (49.7%), neutrophilia (85.1%), and eosinophilia (52.1%). Kidney and liver involvement occur in less than 10% of cases, and mortality rates are low but not zero, with an all-cause 30-day mortality rate of 3.5%.3
In a multi-institutional retrospective study of 68 patients diagnosed with DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome, Sharma et al4 developed a scoring system to identify those at greatest risk for DRESS recurrence. Variables associated with recurrence including younger age, female sex, and features considered atypical for DRESS syndrome—nonmorbilliform rash; absence of facial edema; antinuclear antibody positivity; medication class other than antibiotic, antigout, or antiseizure—were used to develop a “ReDRESS” score. This predictive model had a sensitivity of 73% and specificity of 83% for predicting DRESS recurrence.4
Another case series characterized SCoRCH (sudden conjunctivitis, lymphopenia, sunburnlike rash, and hemodynamic changes), a newly described hypersensitivity reaction to trimethoprim-sulfamethoxazole.5 The onset of this reaction typically occurs 4 to 11 days after initiation of trimethoprim-sulfamethoxazole but can occur as quickly as 1 day following re-exposure. Patients are systemically ill with fever, hypotension, tachycardia, acute renal insufficiency, and transaminitis, and they have a diffuse sunburnlike erythema without scale, facial edema, and conjunctivitis. It is thought this distinct hypersensitivity reaction may be mediated by IL-6, which has a role in triggering a sepsislike physiology, with vasodilation, hypotension, and edema.5
A systematic review and meta-analysis found that sulfonamides remain the most prominent cause of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN).6 A case-control study described SJS/TEN presentations triggered by Mycoplasma, advocating for routine Mycoplasma screening, especially in patients without a clear medication culprit. Mycoplasma-induced cases carried statistically lower rates of mortality (0%) compared with medication-induced cases (22.5%).7 Another prospective open-label study evaluated SJS/TEN management by randomizing 25 patients to receive either combination therapy with methylprednisolone plus a tumor necrosis factor α inhibitor or methylprednisolone alone.8 Anti–tumor necrosis factor therapy was associated with a shorter length of initial steroid treatment and duration of the acute stage, hospitalization, and time to re-epithelialization8; however, as in a prior randomized unblinded trial,9 there was no difference in mortality between the 2 groups.
There is limited high-quality evidence to support the use of any systemic immunomodulator to decrease SJS/TEN–related mortality.10 A Cochrane systematic review highlighted the many limitations of the available data due to variations in presentation, assessment, and management.11 Because SJS/TEN is rare, powering studies based on mortality is infeasible; the authors calculated that 2872 participants were needed to detect a 50% mortality reduction among those with SCORTEN (severity-of-illness score for TEN) scores of 0 to 1.11 Therefore, collaborative efforts using appropriate outcomes measures (eg, time to re-epithelialization, length of hospital stay), standardized terminology and dosing regimens, and adaptive trial designs are needed. Consensus-derived assessment and treatment protocols could help account for variation, ensure consistency in treatment, and enable head-to-head comparisons. Members of the Society of Dermatology Hospitalists are working on efforts to standardize terminology and validate outcomes measures needed for future studies.12
Supportive Oncodermatology: A New Frontier
With the advent of immune checkpoint inhibitors (ICIs) for a growing number of cancers, dermatologists have become critical to identifying and managing cutaneous immune-related adverse events (cirAEs). Recent findings have demonstrated that dermatology input improves patient outcomes, not only regarding the treatment of dermatoses but also by augmenting cancer-related survival. One group found that patients with cirAEs who were evaluated by a dermatologist had improved progression-free (hazard ratio, 0.69; 95% CI, 0.54-0.87; P=.002) and overall survival rates (hazard ratio, 0.62; 95% CI, 0.45-0.84; P=.002), controlling for cirAE severity, age, sex, cancer type, and ICI subtype. Patients who were under the care of a dermatologist also were more likely to resume ICI therapy following an interruption (odds ratio, 10.52; 95% CI, 5.15-21.48; P<.001).13 Dermatologists help to optimize skin-directed and targeted therapies, such as dupilumab, minimizing exposure to systemic immunosuppression in these complex patients.14
Supportive oncodermatologists also have made important observations on how cirAEs relate to other adverse events and prognosis. A review of 628 patients found that almost half of those with cirAEs had co-occurring noncutaneous immune-related adverse events, most commonly pulmonary. Psoriasiform eruptions were most frequently associated with noncutaneous immune-related adverse events, and cutaneous reactions frequently preceded the development of systemic manifestations, serving as a clinical biomarker to provide prognostic information.15 A review of 95 patients found that spongiotic and lichenoid interface reactions were associated with decreased mortality rates, whereas vacuolar interface and perivascular dermatitis were associated with increased mortality.16
As with severe cutaneous adverse events, dermatology input has been critical for accurately phenotyping and risk stratifying these novel reactions. The dermatologist’s skill set is necessary for optimizing skin-directed and targeted therapies while minimizing systemic immunosuppression, thereby improving patient outcomes with respect to rash, cancer response, and survival.
The Cost of Inpatient Skin Disease
Hospitalizations account for approximately half of all health care expenditures, and hospital readmission, seen as a measure of the quality of health care delivery, can double this cost.17 Identifying and developing protocols for addressing patients with complex chronic inflammatory disorders is one strategy for improving outcomes and reducing financial burden. Inpatient dermatologists have identified hidradenitis suppurativa as one disease that can benefit from early intervention by dermatologists in the hospital, with its 30-day (17.8%) and 180-day (48.6%) readmission rates being comparable to those of heart failure.18
Following an index emergency department (ED) visit, 17.2% (3484/20,269) of patients with HS have at least 1 return ED visit within 30 days, while only 2.4% (483/20,269) have a dermatology visit within the same time frame.19 Understanding the risk factors for hospital readmission and ED utilization, including severity of illness, the presence of medical comorbidities, health coverage under Medicaid, and receipt of opioids, can allow dermatologists to anticipate those at greatest risk.19 Opportunities exist for cross-specialty interventions to anticipate and address modifiable risk factors. Shorter time to dermatology outpatient follow-up leads to improved clinic attendance and may help reduce ED utilization and hospital readmission.20
Teledermatology: Leveraging Inpatient Expertise
Although the benefit of inpatient dermatologic care is substantial, access to that care is finite. Following the COVID-19 pandemic, there is an increased acceptance of telemedicine and the long-term role it can play in leveraging dermatologic expertise, including meeting the increasing demand for inpatient dermatology care in rural and resource-poor communities.21
Recent studies conducted by dermatology hospitalists have illustrated the value of asynchronous store-and-forward technology in settings lacking access to consultative dermatology.22,23 Stephens et al22 found that expanding provider-to-provider electronic consultation (e-consultation) capacity to an inpatient rehabilitation facility resulted in completed consultations within 1.5 days compared with a 7- to 14-day wait time for patients attending an in-person urgent access dermatology clinic. In another study, the implementation of asynchronous dermatology e-consultations for immunobullous diseases, vasculitis, and herpes zoster resulted in a change in diagnosis 86% of the time, accompanied by at least 1 new systemic or topical therapy recommendation.23
Researchers also identified ways in which teledermatology can be inelegant and proposed specific supplemental data to aid in diagnosis. A review of 126 inpatient e-consultations demonstrated limitations related to the diagnosis of skin and soft-tissue infections. In two-thirds to three-quarters of cases, potentially useful descriptive information was missing, and in 70% (88/126), images were not appropriately focused. The authors developed a detailed checklist to help primary medical teams focus their differential diagnoses.24 A recent pilot study found that supplementation of clinical information with a standardized questionnaire and thermal images improved the accuracy of cellulitis diagnosis. Using this method, there was no difference in accuracy between dermatology hospitalists and other board-certified dermatologists, supporting the notion that any dermatologist can fulfill this need successfully, even without specific inpatient experience.25 Due to the high incidence and cost of cellulitis and related hospital admissions,26 such an intervention could have a considerable financial and patient safety impact.
Final Thoughts
This last year brought many changes to the health care landscape, the recession of a global pandemic, and an increasingly complex health care delivery system. Inpatient dermatologists met these challenges by providing high-quality dermatologic care and practice-modifying research in the areas of severe cutaneous adverse reactions, supportive oncodermatology, hospital readmission, telemedicine, and more, demonstrating the value of dermatologic expertise in the hospital setting.
Dermatologists improve the diagnostic accuracy and quality of care of patients in the hospital setting. They help shorten the length of stay, improve outpatient follow-up, and reduce the rate of hospital readmission.1 Medicare beneficiaries hospitalized with skin conditions at institutions with a dermatology hospitalist—a provider with a specialty interest in inpatient dermatology—have 24% lower odds of risk-adjusted 30-day mortality and 12% lower odds of risk-adjusted 30-day readmissions.2
In the last year, research among the dermatology hospitalist community has actively contributed to our understanding of challenging inpatient skin diseases and has identified new ways in which dermatologists can contribute to the care of hospitalized patients. In this review, we highlight 4 areas of focus from the published literature in 2022-2023—severe cutaneous adverse reactions, supportive oncodermatology, cost of inpatient services, and teledermatology.
Severe Cutaneous Adverse Reactions: Old and New
Severe cutaneous adverse reactions to medications frequently are encountered in the inpatient setting. Dermatology hospitalists are well positioned to phenotype these reactions, drawing insights that aid in identifying, characterizing, risk stratifying, and managing these conditions, which have considerable morbidity and mortality.
A recent 20-year retrospective review of cases of acute generalized exanthematous pustulosis (N=340) across 10 academic systems—the largest to date—improves our understanding of the features of this rare entity.3 The authors found that acute generalized exanthematous pustulosis most often is triggered by β-lactam and other antibiotics (75.5%) and is accompanied by fever (49.7%), neutrophilia (85.1%), and eosinophilia (52.1%). Kidney and liver involvement occur in less than 10% of cases, and mortality rates are low but not zero, with an all-cause 30-day mortality rate of 3.5%.3
In a multi-institutional retrospective study of 68 patients diagnosed with DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome, Sharma et al4 developed a scoring system to identify those at greatest risk for DRESS recurrence. Variables associated with recurrence including younger age, female sex, and features considered atypical for DRESS syndrome—nonmorbilliform rash; absence of facial edema; antinuclear antibody positivity; medication class other than antibiotic, antigout, or antiseizure—were used to develop a “ReDRESS” score. This predictive model had a sensitivity of 73% and specificity of 83% for predicting DRESS recurrence.4
Another case series characterized SCoRCH (sudden conjunctivitis, lymphopenia, sunburnlike rash, and hemodynamic changes), a newly described hypersensitivity reaction to trimethoprim-sulfamethoxazole.5 The onset of this reaction typically occurs 4 to 11 days after initiation of trimethoprim-sulfamethoxazole but can occur as quickly as 1 day following re-exposure. Patients are systemically ill with fever, hypotension, tachycardia, acute renal insufficiency, and transaminitis, and they have a diffuse sunburnlike erythema without scale, facial edema, and conjunctivitis. It is thought this distinct hypersensitivity reaction may be mediated by IL-6, which has a role in triggering a sepsislike physiology, with vasodilation, hypotension, and edema.5
A systematic review and meta-analysis found that sulfonamides remain the most prominent cause of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN).6 A case-control study described SJS/TEN presentations triggered by Mycoplasma, advocating for routine Mycoplasma screening, especially in patients without a clear medication culprit. Mycoplasma-induced cases carried statistically lower rates of mortality (0%) compared with medication-induced cases (22.5%).7 Another prospective open-label study evaluated SJS/TEN management by randomizing 25 patients to receive either combination therapy with methylprednisolone plus a tumor necrosis factor α inhibitor or methylprednisolone alone.8 Anti–tumor necrosis factor therapy was associated with a shorter length of initial steroid treatment and duration of the acute stage, hospitalization, and time to re-epithelialization8; however, as in a prior randomized unblinded trial,9 there was no difference in mortality between the 2 groups.
There is limited high-quality evidence to support the use of any systemic immunomodulator to decrease SJS/TEN–related mortality.10 A Cochrane systematic review highlighted the many limitations of the available data due to variations in presentation, assessment, and management.11 Because SJS/TEN is rare, powering studies based on mortality is infeasible; the authors calculated that 2872 participants were needed to detect a 50% mortality reduction among those with SCORTEN (severity-of-illness score for TEN) scores of 0 to 1.11 Therefore, collaborative efforts using appropriate outcomes measures (eg, time to re-epithelialization, length of hospital stay), standardized terminology and dosing regimens, and adaptive trial designs are needed. Consensus-derived assessment and treatment protocols could help account for variation, ensure consistency in treatment, and enable head-to-head comparisons. Members of the Society of Dermatology Hospitalists are working on efforts to standardize terminology and validate outcomes measures needed for future studies.12
Supportive Oncodermatology: A New Frontier
With the advent of immune checkpoint inhibitors (ICIs) for a growing number of cancers, dermatologists have become critical to identifying and managing cutaneous immune-related adverse events (cirAEs). Recent findings have demonstrated that dermatology input improves patient outcomes, not only regarding the treatment of dermatoses but also by augmenting cancer-related survival. One group found that patients with cirAEs who were evaluated by a dermatologist had improved progression-free (hazard ratio, 0.69; 95% CI, 0.54-0.87; P=.002) and overall survival rates (hazard ratio, 0.62; 95% CI, 0.45-0.84; P=.002), controlling for cirAE severity, age, sex, cancer type, and ICI subtype. Patients who were under the care of a dermatologist also were more likely to resume ICI therapy following an interruption (odds ratio, 10.52; 95% CI, 5.15-21.48; P<.001).13 Dermatologists help to optimize skin-directed and targeted therapies, such as dupilumab, minimizing exposure to systemic immunosuppression in these complex patients.14
Supportive oncodermatologists also have made important observations on how cirAEs relate to other adverse events and prognosis. A review of 628 patients found that almost half of those with cirAEs had co-occurring noncutaneous immune-related adverse events, most commonly pulmonary. Psoriasiform eruptions were most frequently associated with noncutaneous immune-related adverse events, and cutaneous reactions frequently preceded the development of systemic manifestations, serving as a clinical biomarker to provide prognostic information.15 A review of 95 patients found that spongiotic and lichenoid interface reactions were associated with decreased mortality rates, whereas vacuolar interface and perivascular dermatitis were associated with increased mortality.16
As with severe cutaneous adverse events, dermatology input has been critical for accurately phenotyping and risk stratifying these novel reactions. The dermatologist’s skill set is necessary for optimizing skin-directed and targeted therapies while minimizing systemic immunosuppression, thereby improving patient outcomes with respect to rash, cancer response, and survival.
The Cost of Inpatient Skin Disease
Hospitalizations account for approximately half of all health care expenditures, and hospital readmission, seen as a measure of the quality of health care delivery, can double this cost.17 Identifying and developing protocols for addressing patients with complex chronic inflammatory disorders is one strategy for improving outcomes and reducing financial burden. Inpatient dermatologists have identified hidradenitis suppurativa as one disease that can benefit from early intervention by dermatologists in the hospital, with its 30-day (17.8%) and 180-day (48.6%) readmission rates being comparable to those of heart failure.18
Following an index emergency department (ED) visit, 17.2% (3484/20,269) of patients with HS have at least 1 return ED visit within 30 days, while only 2.4% (483/20,269) have a dermatology visit within the same time frame.19 Understanding the risk factors for hospital readmission and ED utilization, including severity of illness, the presence of medical comorbidities, health coverage under Medicaid, and receipt of opioids, can allow dermatologists to anticipate those at greatest risk.19 Opportunities exist for cross-specialty interventions to anticipate and address modifiable risk factors. Shorter time to dermatology outpatient follow-up leads to improved clinic attendance and may help reduce ED utilization and hospital readmission.20
Teledermatology: Leveraging Inpatient Expertise
Although the benefit of inpatient dermatologic care is substantial, access to that care is finite. Following the COVID-19 pandemic, there is an increased acceptance of telemedicine and the long-term role it can play in leveraging dermatologic expertise, including meeting the increasing demand for inpatient dermatology care in rural and resource-poor communities.21
Recent studies conducted by dermatology hospitalists have illustrated the value of asynchronous store-and-forward technology in settings lacking access to consultative dermatology.22,23 Stephens et al22 found that expanding provider-to-provider electronic consultation (e-consultation) capacity to an inpatient rehabilitation facility resulted in completed consultations within 1.5 days compared with a 7- to 14-day wait time for patients attending an in-person urgent access dermatology clinic. In another study, the implementation of asynchronous dermatology e-consultations for immunobullous diseases, vasculitis, and herpes zoster resulted in a change in diagnosis 86% of the time, accompanied by at least 1 new systemic or topical therapy recommendation.23
Researchers also identified ways in which teledermatology can be inelegant and proposed specific supplemental data to aid in diagnosis. A review of 126 inpatient e-consultations demonstrated limitations related to the diagnosis of skin and soft-tissue infections. In two-thirds to three-quarters of cases, potentially useful descriptive information was missing, and in 70% (88/126), images were not appropriately focused. The authors developed a detailed checklist to help primary medical teams focus their differential diagnoses.24 A recent pilot study found that supplementation of clinical information with a standardized questionnaire and thermal images improved the accuracy of cellulitis diagnosis. Using this method, there was no difference in accuracy between dermatology hospitalists and other board-certified dermatologists, supporting the notion that any dermatologist can fulfill this need successfully, even without specific inpatient experience.25 Due to the high incidence and cost of cellulitis and related hospital admissions,26 such an intervention could have a considerable financial and patient safety impact.
Final Thoughts
This last year brought many changes to the health care landscape, the recession of a global pandemic, and an increasingly complex health care delivery system. Inpatient dermatologists met these challenges by providing high-quality dermatologic care and practice-modifying research in the areas of severe cutaneous adverse reactions, supportive oncodermatology, hospital readmission, telemedicine, and more, demonstrating the value of dermatologic expertise in the hospital setting.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528.
- Puri P, Pollock BD, Yousif M, et al. Association of Society of Dermatology hospitalist institutions with improved outcomes in Medicare beneficiaries hospitalized for skin disease. J Am Acad Dermatol. 2023;88:1372-1375.
- Creadore A, Desai S, Alloo A, et al. Clinical characteristics, disease course, and outcomes of patients with acute generalized exanthematous pustulosis in the US. JAMA Dermatol. 2022;158:176-183.
- Sharma AN, Murphy K, Shwe S, et al. Predicting DRESS syndrome recurrence—the ReDRESS score. JAMA Dermatol. 2022;158:1445-1447.
- Brian M, Rose EK, Mauskar MM, et al. Sudden conjunctivitis, lymphopenia, and rash combined with hemodynamic changes (SCoRCH) after trimethoprim-sulfamethoxazole use: a case series study of a hypersensitivity reaction. JAMA Dermatol. 2023;159:73-78.
- Lee EY, Knox C, Phillips EJ. Worldwide prevalence of antibiotic-associated Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2023;159:384-392.
- Liew YCC, Choo KJL, Oh CC, et al. Mycoplasma-induced Stevens-Johnson syndrome/toxic epidermal necrolysis: case-control analysis of a cohort managed in a specialized center. J Am Acad Dermatol. 2022;86:811-817.
- Ao S, Gao X, Zhan J, et al. Inhibition of tumor necrosis factor improves conventional steroid therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis in a cohort of patients. J Am Acad Dermatol. 2022;86:1236-1245.
- Wang CW, Yang LY, Chen CB, et al; the Taiwan Severe Cutaneous Adverse Reaction (TSCAR) Consortium. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-996.
- Han JJ, Creadore A, Seminario-Vidal L, et al. Medical management of Stevens-Johnson syndrome/toxic epidermal necrolysis among North American dermatologists. J Am Acad Dermatol. 2022;87:429-431.
- Noe MH, Micheletti RG. Systemic interventions for treatment of Stevens-Johnson syndrome/toxic epidermal necrolysis: summary of a Cochrane review. JAMA Dermatol. 2022;158:1436-1437.
- Waters M, Dobry A, Le ST, et al. Development of a skin-directed scoring system for Stevens-Johnson syndrome and epidermal necrolysis: a Delphi consensus exercise. JAMA Dermatol. 2023;159:772-777.
- Jacoby TV, Shah N, Asdourian MS, et al. Dermatology evaluation for cutaneous immune-related adverse events is associated with improved survival in cancer patients treated with checkpoint inhibition. J Am Acad Dermatol. 2023;88:711-714.
- Said JT, Elman SA, Perez-Chada LM, et al. Treatment of immune checkpoint inhibitor-mediated psoriasis: a systematic review. J Am Acad Dermatol. 2022;87:399-400.
- Asdourian MS, Shah N, Jacoby TV, et al. Evaluating patterns of co-occurrence between cutaneous and noncutaneous immune-related adverse events after immune checkpoint inhibitor therapy. J Am Acad Dermatol. 2023;88:246-249.
- Hirotsu KE, Scott MKD, Marquez C, et al. Histologic subtype of cutaneous immune-related adverse events predicts overall survival in patients receiving immune checkpoint inhibitors. J Am Acad Dermatol. 2022;87:651-653.
- Benbassat J, Taragin M. Hospital readmissions as a measure of quality of health care: advantages and limitations. Arch Intern Med. 2000;160:1074-1081.
- Edigin E, Kaul S, Eseaton PO, et al. At 180 days hidradenitis suppurativa readmission rate is comparable to heart failure: analysis of the nationwide readmissions database. J Am Acad Dermatol. 2022;87:188-192.
- Wang CX, Buss JL, Keller M, et al. Factors associated with dermatologic follow-up vs emergency department return in patients with hidradenitis suppurativa after an initial emergency department visit. JAMA Dermatol. 2022;158:1378-1386.
- Zakaria A, Chang AY, Kim-Lim P, et al. Predictors of postdischarge follow-up attendance among hospitalized dermatology patients: disparities and potential interventions. J Am Acad Dermatol. 2022;87:186-188.
- Arnold JD, Yoon S, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2019;80:425-432. doi:10.1016/j.jaad.2018.06.070
- Stephens MR, Das S, Smith GP. Utilization and outcomes of an asynchronous teledermatology pilot for an inpatient rehabilitation hospital. J Am Acad Dermatol. 2022;87:421-423.
- Ortiz C, Khosravi H, Kettering C, et al. Concordance data for inpatient asynchronous eDermatology consultation for immunobullous disease, zoster, and vasculitis. J Am Acad Dermatol. 2022;86:918-920.
- Salle R, Hua C, Mongereau M, et al. Challenges and limitations of teledermatology for skin and soft-tissue infections: a real-world study of an expert center. J Am Acad Dermatol. 2023;88:457-459.
- Creadore A, Manjaly P, Tkachenko E, et al. The utility of augmented teledermatology to improve dermatologist diagnosis of cellulitis: a cross-sectional study. Arch Dermatol Res. 2023;315:1347-1353.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528.
- Puri P, Pollock BD, Yousif M, et al. Association of Society of Dermatology hospitalist institutions with improved outcomes in Medicare beneficiaries hospitalized for skin disease. J Am Acad Dermatol. 2023;88:1372-1375.
- Creadore A, Desai S, Alloo A, et al. Clinical characteristics, disease course, and outcomes of patients with acute generalized exanthematous pustulosis in the US. JAMA Dermatol. 2022;158:176-183.
- Sharma AN, Murphy K, Shwe S, et al. Predicting DRESS syndrome recurrence—the ReDRESS score. JAMA Dermatol. 2022;158:1445-1447.
- Brian M, Rose EK, Mauskar MM, et al. Sudden conjunctivitis, lymphopenia, and rash combined with hemodynamic changes (SCoRCH) after trimethoprim-sulfamethoxazole use: a case series study of a hypersensitivity reaction. JAMA Dermatol. 2023;159:73-78.
- Lee EY, Knox C, Phillips EJ. Worldwide prevalence of antibiotic-associated Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2023;159:384-392.
- Liew YCC, Choo KJL, Oh CC, et al. Mycoplasma-induced Stevens-Johnson syndrome/toxic epidermal necrolysis: case-control analysis of a cohort managed in a specialized center. J Am Acad Dermatol. 2022;86:811-817.
- Ao S, Gao X, Zhan J, et al. Inhibition of tumor necrosis factor improves conventional steroid therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis in a cohort of patients. J Am Acad Dermatol. 2022;86:1236-1245.
- Wang CW, Yang LY, Chen CB, et al; the Taiwan Severe Cutaneous Adverse Reaction (TSCAR) Consortium. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-996.
- Han JJ, Creadore A, Seminario-Vidal L, et al. Medical management of Stevens-Johnson syndrome/toxic epidermal necrolysis among North American dermatologists. J Am Acad Dermatol. 2022;87:429-431.
- Noe MH, Micheletti RG. Systemic interventions for treatment of Stevens-Johnson syndrome/toxic epidermal necrolysis: summary of a Cochrane review. JAMA Dermatol. 2022;158:1436-1437.
- Waters M, Dobry A, Le ST, et al. Development of a skin-directed scoring system for Stevens-Johnson syndrome and epidermal necrolysis: a Delphi consensus exercise. JAMA Dermatol. 2023;159:772-777.
- Jacoby TV, Shah N, Asdourian MS, et al. Dermatology evaluation for cutaneous immune-related adverse events is associated with improved survival in cancer patients treated with checkpoint inhibition. J Am Acad Dermatol. 2023;88:711-714.
- Said JT, Elman SA, Perez-Chada LM, et al. Treatment of immune checkpoint inhibitor-mediated psoriasis: a systematic review. J Am Acad Dermatol. 2022;87:399-400.
- Asdourian MS, Shah N, Jacoby TV, et al. Evaluating patterns of co-occurrence between cutaneous and noncutaneous immune-related adverse events after immune checkpoint inhibitor therapy. J Am Acad Dermatol. 2023;88:246-249.
- Hirotsu KE, Scott MKD, Marquez C, et al. Histologic subtype of cutaneous immune-related adverse events predicts overall survival in patients receiving immune checkpoint inhibitors. J Am Acad Dermatol. 2022;87:651-653.
- Benbassat J, Taragin M. Hospital readmissions as a measure of quality of health care: advantages and limitations. Arch Intern Med. 2000;160:1074-1081.
- Edigin E, Kaul S, Eseaton PO, et al. At 180 days hidradenitis suppurativa readmission rate is comparable to heart failure: analysis of the nationwide readmissions database. J Am Acad Dermatol. 2022;87:188-192.
- Wang CX, Buss JL, Keller M, et al. Factors associated with dermatologic follow-up vs emergency department return in patients with hidradenitis suppurativa after an initial emergency department visit. JAMA Dermatol. 2022;158:1378-1386.
- Zakaria A, Chang AY, Kim-Lim P, et al. Predictors of postdischarge follow-up attendance among hospitalized dermatology patients: disparities and potential interventions. J Am Acad Dermatol. 2022;87:186-188.
- Arnold JD, Yoon S, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2019;80:425-432. doi:10.1016/j.jaad.2018.06.070
- Stephens MR, Das S, Smith GP. Utilization and outcomes of an asynchronous teledermatology pilot for an inpatient rehabilitation hospital. J Am Acad Dermatol. 2022;87:421-423.
- Ortiz C, Khosravi H, Kettering C, et al. Concordance data for inpatient asynchronous eDermatology consultation for immunobullous disease, zoster, and vasculitis. J Am Acad Dermatol. 2022;86:918-920.
- Salle R, Hua C, Mongereau M, et al. Challenges and limitations of teledermatology for skin and soft-tissue infections: a real-world study of an expert center. J Am Acad Dermatol. 2023;88:457-459.
- Creadore A, Manjaly P, Tkachenko E, et al. The utility of augmented teledermatology to improve dermatologist diagnosis of cellulitis: a cross-sectional study. Arch Dermatol Res. 2023;315:1347-1353.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
Practice Points
- A severe hypersensitivity reaction to trimethoprim-sulfamethoxazole—sudden conjunctivitis, lymphopenia, sunburnlike rash, and hemodynamic changes (SCoRCH)—has been described.
- Patients experiencing cutaneous reactions to immune checkpoint inhibitors have improved progression-free and overall survival rates if evaluated by a dermatologist who can optimize skin-directed and targeted therapies.
- Interventions, including shorter time to dermatology outpatient follow-up, are needed to reduce emergency department utilization by patients with hidradenitis suppurativa.
- Asynchronous store-and-forward dermatology e-consultation is effective for immunobullous diseases, vasculitis, herpes zoster, and cellulitis, demonstrating the utility of teledermatology in the inpatient setting, particularly when standardized data capture tools are used.
Cutaneous Manifestations of COVID-19: Characteristics, Pathogenesis, and the Role of Dermatology in the Pandemic
The virus that causes COVID-19—SARS-CoV-2—has infected more than 128 million individuals, resulting in more than 2.8 million deaths worldwide between December 2019 and April 2021. Disease mortality primarily is driven by hypoxemic respiratory failure and systemic hypercoagulability, resulting in multisystem organ failure.1 With more than 17 million Americans infected, the virus is estimated to have impacted someone within the social circle of nearly every American.2
The COVID-19 pandemic has highlighted resource limitations, delayed elective and preventive care, and rapidly increased the adoption of telemedicine, presenting a host of new challenges to providers in every medical specialty, including dermatology. Although COVID-19 primarily is a respiratory disease, clinical manifestations have been observed in nearly every organ, including the skin. The cutaneous manifestations of COVID-19 provide insight into disease diagnosis, prognosis, and pathophysiology. In this article, we review the cutaneous manifestations of COVID-19 and explore the state of knowledge regarding their pathophysiology and clinical significance. Finally, we discuss the role of dermatology consultants in the care of patients with COVID-19, and the impact of the pandemic on the field of dermatology.
Prevalence of Cutaneous Findings in COVID-19
Early reports characterizing the clinical presentation of patients hospitalized with COVID-19 suggested skin findings associated with the disease were rare. Cohort studies from Europe, China, and New York City in January through March 2020 reported a low prevalence or made no mention of rash.3-7 However, reports from dermatologists in Italy that emerged in May 2020 indicated a substantially higher proportion of cutaneous disease: 18 of 88 (20.4%) hospitalized patients were found to have cutaneous involvement, primarily consisting of erythematous rash, along with some cases of urticarial and vesicular lesions.8 In October 2020, a retrospective cohort study from Spain examining 2761 patients presenting to the emergency department or admitted to the hospital for COVID-19 found that 58 (2.1%) patients had skin lesions attributed to COVID-19.9
The wide range in reported prevalence of skin lesions may be due to variable involvement of dermatologic specialists in patient care, particularly in China.10 Some variation also may be due to variability in the timing of clinical examination, as well as demographic and clinical differences in patient populations. Of note, a multisystem inflammatory disease seen in US children subsequent to infection with COVID-19 has been associated with rash in as many as 74% of cases.11 Although COVID-19 disproportionately impacts people with skin of color, there are few reports of cutaneous manifestations in that population,12 highlighting the challenges of the dermatologic examination in individuals with darker skin and suggesting the prevalence of dermatologic disease in COVID-19 may be greater than reported.
Morphologic Patterns of Cutaneous Involvement in COVID-19
Researchers in Europe and the United States have attempted to classify the cutaneous manifestations of COVID-19. A registry established through the American Academy of Dermatology published a compilation of reports from 31 countries, totaling 716 patient profiles.13 A prospective Spanish study detailed the cutaneous involvement of 375 patients with suspected or confirmed COVID-19.14 Together, these efforts have revealed several distinct patterns of cutaneous involvement associated with COVID-19 (Table).9,15-18
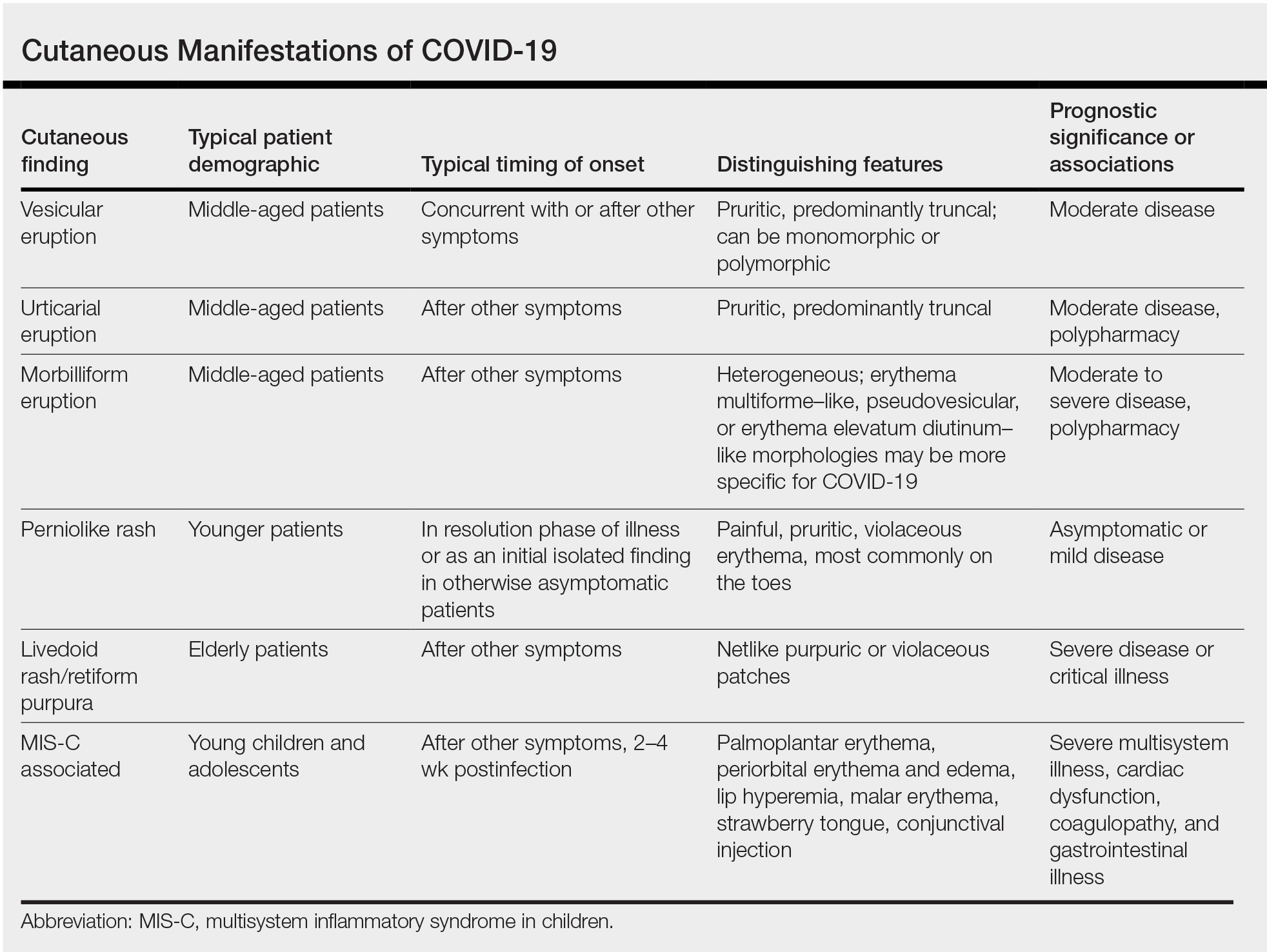
Vesicular Rash
Vesicular rash associated with COVID-19 has been described in several studies and case series8,13,14 and is considered, along with the pseudopernio (or pseudochilblains) morphology, to be one of the more disease-specific patterns in COVID-19.14,18 Vesicular rash appears to comprise roughly one-tenth of all COVID-19–associated rashes.13,14 It usually is described as pruritic, with 72% to 83% of patients reporting itch.13,16
Small monomorphic or polymorphic vesicles predominantly on the trunk and to a lesser extent the extremities and head have been described by multiple authors.14,16 Vesicular rash is most common among middle-aged individuals, with studies reporting median and mean ages ranging from 40.5 to 55 years.9,13,14,16
Vesicular rash develops concurrent with or after other presenting symptoms of COVID-19; in 2 studies, vesicular rash preceded development of other symptoms in only 15% and 5.6% of cases, respectively.13,14 Prognostically, vesicular rash is associated with moderate disease severity.14,16 It may persist for an average of 8 to 10 days.14,16,18
Histopathologic examination reveals basal layer vacuolar degeneration, hyperchromatic keratinocytes, acantholysis, and dyskeratosis.9,16,18
Urticarial Rash
Urticarial lesions represent approximately 7% to 19% of reported COVID-19–associated rashes.9,13,14 Urticarial rashes in patients testing positive for SARS-CoV-2 primarily occur on the trunk.14 The urticaria, which typically last about 1 week,14 are seen most frequently in middle-aged patients (mean/median age, 42–48 years)13,14 and are associated with pruritus, which has been reported in 74% to 92% of patients.13,14 Urticarial lesions typically do not precede other symptoms of COVID-19 and are nonspecific, making them less useful diagnostically.14
Urticaria appears to be associated with more severe COVID-19 illness in several studies, but this finding may be confounded by several factors, including older age, increased tobacco use, and polypharmacy. Of 104 patients with reported urticarial rash and suspected or confirmed COVID-19 across 3 studies, only 1 death was reported.9,13,14
The histopathologic appearance is that of typical hives, demonstrating a perivascular infiltrate of lymphocytes and eosinophils with edema of the upper dermis.9,19
Morbilliform Eruption
Morbilliform eruption is a commonly reported morphology associated with COVID-19, accounting for 20% to 47% of rashes.9,13,14 This categorization may have limited utility from a diagnostic and prognostic perspective, given that morbilliform eruptions are common, nonspecific, and heterogenous and can arise from many causes.9,13,14 Onset of morbilliform eruption appears to coincide with14 or follow13,20,21 the development of other COVID-19–related symptoms, with 5% of patients reporting morbilliform rash as the initial manifestation of infection.13,14 Morbilliform eruptions have been observed to occur in patients with more severe disease.9,13,14
Certain morphologic subtypes, such as erythema multiforme–like, erythema elevatum diutinum–like, or pseudovesicular, may be more specific to COVID-19 infection.14 A small case series highlighted 4 patients with erythema multiforme–like eruptions, 3 of whom also were found to have petechial enanthem occurring after COVID-19 diagnosis; however, the investigators were unable to exclude drug reaction as a potential cause of rash in these patients.22 Another case series of 21 patients with COVID-19 and skin rash described a (primarily) petechial enanthem on the palate in 6 (28.5%) patients.23 It is unclear to what extent oral enanthem may be underrecognized given that some physicians may be disinclined to remove the masks of known COVID-19–positive patients to examine the oral cavity.
The histologic appearance of morbilliform rash seen in association with COVID-19 has been described as spongiotic with interface dermatitis with perivascular lymphocytic inflammation.9,21
COVID Toes, Pseudochilblains Rash, Perniolike Rash, and Acral Erythema/Edema
Of all the rashes associated with COVID-19, COVID toes, or pseudochilblains rash, has perhaps attracted the most attention. The characteristic violaceous erythema on the fingers and/or toes may be itchy or painful, presenting similar to idiopathic cases of pernio (Figure 1).14 The entity has been controversial because of an absence of a clear correlation with a positive SARS-CoV-2 polymerase chain reaction test or antibodies to the virus in a subset of reported cases.24,25 Onset of the rash late in the disease course, generally after symptom resolution in mild or asymptomatic cases, may explain the absence of viral DNA in the nasopharynx by the time of lesion appearance.14,26 Seronegative patients may have cleared SARS-CoV-2 infection before humoral immunity could occur via a strong type 1 interferon response.25
Across 3 studies, perniolike skin lesions constituted 18% to 29% of COVID-19–associated skin findings9,13,14 and persisted for an average of 12 to 14 days.13,14 Perniolike lesions portend a favorable outcome; patients with COVID toes rarely present with systemic symptoms or laboratory or imaging abnormalities9 and less commonly require hospitalization for severe illness. Perniolike lesions have been reported most frequently in younger patients, with a median or mean age of 32 to 35 years.13,14
Histology demonstrates lichenoid dermatitis with perivascular and periadnexal lymphocytic infiltrates.9 Notably, one study observed interface dermatitis of the intraepidermal portion of the acrosyringium, a rare finding in chilblain lupus, in 83% of patients (N=40).25 Direct immunofluorescence demonstrates a vasculopathic pattern, with some patients showing deposition of IgM or IgG, C3, and fibrinogen in dermal blood vessels. Vascular C9 deposits also have been demonstrated on immunohistochemistry.9 Biopsies of perniolike lesions in COVID-19 patients have demonstrated the presence of SARS-CoV-2 RNA,27 have identified SARS-CoV-2 spike protein in endothelial cells on immunohistochemistry, and have visualized intracytoplasmic viral particles in vascular endothelium on electron microscopy.28
Livedoid Rash/Retiform Purpura
Netlike purpuric or violaceous patches signifying vessel damage or occlusion have been seen in association with COVID-19, constituting approximately 6% of COVID-19–associated skin findings in 2 studies.13,14 Livedoid rash (Figure 2) and retiform purpura (Figure 3) are associated with older age and occur primarily in severely ill patients, including those requiring intensive care. In a registry of 716 patients with COVID-19, 100% of patients with retiform purpura were hospitalized, and 82% had acute respiratory distress syndrome.13 In another study, 33% (7/21) of patients with livedoid and necrotic lesions required intensive care, and 10% (2/21) died.14
Livedoid lesions and retiform purpura represent thrombotic disease in the skin due to vasculopathy/coagulopathy. Dermatopathology available through the American Academy of Dermatology registry revealed thrombotic vasculopathy.13 A case series of 4 patients with livedo racemosa and retiform purpura demonstrated pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, and arterioles with complement deposition.29 Livedoid and retiform lesions in the skin may be associated with a COVID-19–induced coagulopathy, a propensity for systemic clotting including pulmonary embolism, which mostly occurs in hospitalized patients with severe illness.30
Multisystem Inflammatory Disease in Children
A hyperinflammatory syndrome similar to Kawasaki disease and toxic shock syndrome associated with mucocutaneous, cardiac, and gastrointestinal manifestations has been reported following COVID-19 infection.31 This syndrome, known as multisystem inflammatory syndrome in children (MIS-C), predominantly affects adolescents and children older than 5 years,11 typically occurs 2 to 4 weeks after infection, and appears to be at least 100-times less common than COVID-19 infection among the same age group.31 Sixty percent31 to 74%11 of affected patients have mucocutaneous involvement, with the most common clinical findings being conjunctival injection, palmoplantar erythema, lip hyperemia, periorbital erythema and edema, strawberry tongue, and malar erythema, respectively.32
Because this condition appears to reflect an immune response to the virus, the majority of cases demonstrate negative SARS-CoV-2 polymerase chain reaction and positive antibody testing.33 Although cutaneous findings are similar to those seen in Kawasaki disease, certain findings have been noted in MIS-C that are not typical of Kawasaki disease, including heliotrope rash–like periorbital edema and erythema as well as erythema infectiosum–like malar erythema and reticulated erythematous eruptions.32
The course of MIS-C can be severe; in one case series of patients presenting with MIS-C, 80% (79/99) required intensive care unit admission, with 10% requiring mechanical ventilation and 2% of patients dying during admission.31 Cardiac dysfunction, coagulopathy, and gastrointestinal symptoms are common.11,31 It has been postulated that a superantigenlike region of the SARS-CoV-2 spike protein, similar to that of staphylococcal enterotoxin B, may underlie MIS-C and account for its similarities to toxic shock syndrome.34 Of note, a similar multisystem inflammatory syndrome associated with COVID-19 also has been described in adults, and it too may present with rash as a cardinal feature.35
Pathophysiology of COVID-19: What the Skin May Reveal About the Disease
The diverse range of cutaneous manifestations in COVID-19 reflects a spectrum of host immunologicresponses to SARS-CoV-2 and may inform the pathophysiology of the disease as well as potential treatment modalities.
Host Response to SARS-CoV-2
The body’s response to viral infection is 2-pronged, involving activation of cellular antiviral defenses mediated by type I and III interferons, as well as recruitment of leukocytes, mobilized by cytokines and chemokines.36,37 Infection with SARS-CoV-2 results in a unique inflammatory response characterized by suppression of interferons, juxtaposed with a rampant proinflammatory cytokine and chemokine response, reminiscent of a cytokine storm. Reflective of this imbalance, a study of 50 COVID-19 patients and 20 healthy controls found decreased natural killer cells and CD3+ T cells in COVID-19 patients, particularly severely or critically ill patients, with an increase in B cells and monocytes.38 This distinctive immune imbalance positions SARS-CoV-2 to thrive in the absence of inhibitory interferon activity while submitting the host to the deleterious effects of a cytokine surge.36
Type I Interferons
The perniolike lesions associated with mild COVID-19 disease14 may represent a robust immune response via effective stimulation of type I interferons (IFN-1). Similar perniolike lesions are observed in Aicardi-Goutières syndrome37 and familial chilblain lupus, hereditary interferonopathies associated with mutations in the TREX1 (three prime repair exonuclease 1) gene and characterized by inappropriate upregulation of IFN-1,39 resulting in chilblains. It has been suggested that perniolike lesions in COVID-19 result from IFN-1 activation—a robust effective immunologic response to the virus.14,26,40
On the other end of the spectrum, patients with severe COVID-19 may have a blunted IFN-1 response and reduced IFN-1–stimulated gene expression.36,38 Notably, low IFN-1 response preceded clinical deterioration and was associated with increased risk for evolution to critical illness.38 Severe disease from COVID-19 also is more commonly observed in older patients and those with comorbidities,1 both of which are known factors associated with depressed IFN-1 function.38,41 Reflective of this disparate IFN-1 response, biopsies of COVID-19 perniosis have demonstrated striking expression of myxovirus resistance protein A (MXA), a marker for IFN-1 signaling in tissue, whereas its expression is absent in COVID-19 livedo/retiform purpura.27
Familial chilblain lupus may be effectively treated by the Janus kinase inhibitor baricitinib,39 which inhibits IFN-1 signaling. Baricitinib recently received emergency use authorization by the US Food and Drug Administration for treatment of severe COVID-19 pneumonia,42,43 hinting to disordered IFN-1 signaling in the COVID-19 pathophysiology.
The impaired IFN-1 response in COVID-19 patients may be due to a unique characteristic of SARS-CoV-2: its ORF3b gene is a potent IFN-1 antagonist. In a series of experiments comparing SARS-CoV-2 to the related virus severe acute respiratory disease coronavirus (which was responsible for an epidemic in 2002), Konno et al44 found that SARS-CoV-2 is more effectively able to downregulate host IFN-1, likely due to premature stop codons on ORF3b that produce a truncated version of the gene with amplified anti–IFN-1 activity.
Cytokine Storm and Coagulation Cascade
This dulled interferon response is juxtaposed with a surge of inflammatory chemokines and cytokines, including IL-6, IL-8, IL-10, and tumor necrosis factor α, impairing innate immunity and leading to end-organ damage. This inflammatory response is associated with the influx of innate immune cells, specifically neutrophils and monocytes, which likely contribute to lung injury in COVID-19 acute respiratory distress syndrome.38 It also is thought to lead to downstream activation of coagulation, with a high incidence of thrombotic events observed in patients with severe COVID-19.1 In a retrospective study of 184 intensive care patients with COVID-19 receiving at least standard doses of thromboprophylaxis, venous thromboembolism occurred in 27% and arterial thrombotic events occurred in 3.7%.45
Livedo racemosa and retiform purpura are cutaneous markers of hypercoagulability, which indicate an increased risk for systemic clotting in COVID-19. A positive feedback loop between the complement and coagulation cascades appears to be important.13,14,29,46-48 In addition, a few studies have reported antiphospholipid antibody positivity in hospitalized COVID-19 patients.49,50
The high incidence of coagulopathy in severe COVID-19 has prompted many institutions to develop aggressive prophylactic anticoagulation protocols. Elevation of proinflammatory cytokines and observation of terminal complement activation in the skin and other organs has led to therapeutic trials of IL-6 inhibitors such as tocilizumab,51 complement inhibitors such as eculizumab, and Janus kinase inhibitors such as ruxolitinib and baricitinib.42,48
COVID Long-Haulers
The long-term effects of immune dysregulation in COVID-19 patients remain to be seen. Viral triggering of autoimmune disease is a well-established phenomenon, seen in DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome and other dermatologic diseases, raising the possibility that dermatologists will see a rising incidence of cutaneous autoimmune disease in the aftermath of the pandemic. Disordered interferon stimulation could lead to increased incidence of interferon-mediated disorders, such as sarcoidosis and other granulomatous diseases. Vasculitislike skin lesions could persist beyond the acute infectious period. Recent data from a registry of 990 COVID-19 cases from 39 countries suggest that COVID-19 perniolike lesions may persist as long as 150 days.52 In a time of many unknowns, these questions serve as a call to action for rigorous data collection, contribution to existing registries for dermatologic manifestations of COVID-19, and long-term follow-up of COVID-19 patients by the dermatology community.
Pandemic Dermatology
The pandemic has posed unprecedented challenges for patient care. The use of hydroxychloroquine as a popular but unproven treatment for COVID-19, 53 particularly early in the pandemic, has resulted in drug shortages for patients with lupus and other autoimmune skin diseases. Meanwhile, the need for patients with complex dermatologic conditions to receive systemic immunosuppression has had to be balanced against the associated risks during a global pandemic. To help dermatologists navigate this dilemma, various subspecialty groups have issued guidelines, including the COVID-19 Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists, which recommends a stepwise approach to shared decision-making with the goal of minimizing both the risk for disease flare and that of infection. The use of systemic steroids and rituximab, as well as the dose of immunosuppression—particularly broad-acting immunosuppression—should be limited where permitted. 54
Rapid adoption of telemedicine and remote monitoring strategies has enabled dermatologists to provide safe and timely care when in-person visits have not been possible, including for patients with confirmed or suspected COVID-19, as well as for hospitalized patients. 55-57 Use of telemedicine has facilitated preservation of personal protective equipment at a time when these important resources have been scarce. For patients with transportation or scheduling barriers, telemedicine has even expanded access to care.
However, this strategy cannot completely replace comprehensive in-person evaluation. Variability in video and photographic quality limits evaluation, while in-person physical examination can reveal subtle morphologic clues necessary for diagnosis. 5 8 Additionally, unequal access to technology may disadvantage some patients. For dermatologists to provide optimal care and continue to contribute accurate and insightful observations into COVID-19, it is essential to be physically present in the clinic and in the hospital when necessary, caring for patients in need of dermatologic expertise. Creative management strategies developed during this time will benefit patients and expand the reach of the specialty . 5 8
Final Thoughts
The COVID-19 pandemic has profoundly challenged the medical community and dermatology is no exception. By documenting and characterizing the diverse cutaneous manifestations of this novel disease, dermatologists have furthered understanding of its pathophysiology and management. By adapting quickly and developing creative ways to deliver care, dermatologists have found ways to contribute, both large and small. As we take stock at this juncture of the pandemic, it is clear there remains much to learn. We hope dermatologists will continue to take an active role in meeting the challenges of this time.
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA . 2020;324:782-793. doi:10.1001/jama.2020.12839
- New York Times . Updated December 23, 2020. Accessed March 22, 2021. https://www.nytimes.com/2020/11/15/us/coronavirus-us-cases-deaths.html
- Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med . 2020;382:1708-1720. doi:10.1056/NEJMoa2002032
- Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med . 2020;288:335-344. doi:https://doi.org/10.1111/joim.13089
- Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu province: a multicenter descriptive study. Clin Infect Dis . 2020;71:706-712. doi:10.1093/cid/ciaa199
- Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med . 2020;382:2372-2374. doi:10.1056/NEJMc2010419
- Sun L, Shen L, Fan J, et al. Clinical features of patients with coronavirus disease 2019 from a designated hospital in Beijing, China. J Med Virol . 2020;92:2055-2066. https://doi.org/10.1002/jmv.25966
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatology Venereol . 2020;34:E212-E213. https://doi.org/10.1111/jdv.16387
- Giavedoni P, Podlipnik S, Pericàs JM, et al. Skin manifestations in COVID-19: prevalence and relationship with disease severity. J Clin Med . 2020;9:3261. doi:10.3390/jcm9103261
- Jimenez-Cauhe J, Ortega-Quijano D, Prieto-Barrios M, et al. Reply to “COVID-19 can present with a rash and be mistaken for dengue”: petechial rash in a patient with COVID-19 infection. J Am Acad Dermatol . 2020;83:E141-E142. doi:10.1016/j.jaad.2020.04.016
- Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med . 2020;383:334-346. doi:10.1056/NEJMoa2021680
- Shinkai K, Bruckner AL. Dermatology and COVID-19. JAMA . 2020;324:1133-1134. doi:10.1001/jama.2020.15276
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol . 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol . 2020;183:71-77. https://doi.org/10.1111/bjd.19163
- Bouaziz JD, Duong TA, Jachiet M, et al. Vascular skin symptoms in COVID-19: a French observational study. J Eur Acad Dermatology Venereol . 2020;34:E451-E452. https://doi.org/10.1111/jdv.16544
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol . 2020;45:872-875. https://doi.org/10.1111/ced.14277
- Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol . 2020;83:E61-E63. doi:10.1016/j.jaad.2020.04.093
- Marzano AV, Genovese G, Fabbrocini G, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: Multicenter case series of 22 patients. J Am Acad Dermatol . 2020;83:280-285. doi:10.1016/j.jaad.2020.04.044
- Fernandez-Nieto D, Ortega-Quijano D, Segurado-Miravalles G, et al. Comment on: cutaneous manifestations in COVID-19: a first perspective. safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol . 2020;34:E252-E254. https://doi.org/10.1111/jdv.16470
- Herrero-Moyano M, Capusan TM, Andreu-Barasoain M, et al. A clinicopathological study of eight patients with COVID-19 pneumonia and a late-onset exanthema. J Eur Acad Dermatol Venereol . 2020;34:E460-E464. https://doi.org/10.1111/jdv.16631
- Rubio-Muniz CA, Puerta-Peñ a M, Falkenhain-L ópez D, et al. The broad spectrum of dermatological manifestations in COVID-19: clinical and histopathological features learned from a series of 34 cases. J Eur Acad Dermatol Venereol . 2020;34:E574-E576. https://doi.org/10.1111/jdv.16734
- Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings. Clin Exp Dermatol . 2020;45:892-895. https://doi.org/10.1111/ced.14281
- Jimenez-Cauhe J, Ortega-Quijano D, de Perosanz-Lobo D, et al. Enanthem in patients with COVID-19 and skin rash. JAMA Dermatol . 2020;156:1134-1136. doi:10.1001/jamadermatol.2020.2550
- Le Cleach L, Dousset L, Assier H, et al. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol . 2020;183:866-874. https://doi.org/10.1111/bjd.19377
- Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic [published online November 25, 2020]. JAMA Dermatol . doi:10.1001/jamadermatol.2020.4324
- Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol . 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Magro CM, Mulvey JJ, Laurence J, et al. The differing pathophysiologies that underlie COVID-19-associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol . 2021;184:141-150. https://doi.org/10.1111/bjd.19415
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol . 2020;183:729-737. doi:10.1111/bjd.19327
- Droesch C, Do MH, DeSancho M, et al. Livedoid and purpuric skin eruptions associated with coagulopathy in severe COVID-19. JAMA Dermatol . 2020;156:1-3. doi:10.1001/jamadermatol.2020.2800
- Asakura H, Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol . 2021;113:45-57. doi:10.1007/s12185-020-03029-y
- Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med . 2020;383:347-358. doi:10.1056/NEJMoa2021756
- Young TK, Shaw KS, Shah JK, et al. Mucocutaneous manifestations of multisystem inflammatory syndrome in children during the COVID-19 pandemic. JAMA Dermatol . 2021;157:207-212. doi:10.1001/jamadermatol.2020.4779
- Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259-269. doi:10.1001/jama.2020.10369
- Cheng MH, Zhang S, Porritt RA, et al. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation.
- Morris SB, Schwartz NG, Patel P, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 Infection—United Kingdom and United States, March–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1450-1456. doi:10.15585/mmwr.mm6940e1
- Blanco-Melo D, Nilsson-Payant BE, Liu W-C, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036.e9-1045.e9. doi:10.1016/j.cell.2020.04.026
- Crow YJ, Manel N. Aicardi–Goutières syndrome and the type I interferonopathies. Nat Rev Immunol. 2015;15:429-440. doi:10.1038/nri3850
- Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718-724. doi:10.1126/science.abc6027
- Zimmermann N, Wolf C, Schwenke R, et al. Assessment of clinical response to janus kinase inhibition in patients with familial chilblain lupus and TREX1 mutation. JAMA Dermatol. 2019;155:342-346. doi:10.1001/jamadermatol.2018.5077
- Hubiche T, Le Duff F, Chiaverini C, et al. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions. Lancet Infect Dis. 2021;21:315-316. doi:10.1016/S1473-3099(20)30518-1
- Agrawal A. Mechanisms and implications of age-associated impaired innate interferon secretion by dendritic cells: a mini-review. Gerontology. 2013;59:421-426. doi:10.1159/000350536
- Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795-807. doi:10.1056/NEJMoa2031994
- US Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization (EUA) of baricitinib. Accessed March 29, 2021. https://www.fda.gov/media/143823/download
- Konno Y, Kimura I, Uriu K, et al. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep. 2020;32:108185. doi:10.1016/j.celrep.2020.108185
- Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke: from the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO). J Vasc Interv Radiol. 2018;29:441-453. doi:10.1016/j.jvir.2017.11.026
- Lo MW, Kemper C, Woodruff TM. COVID-19: complement, coagulation, and collateral damage. J Immunol. 2020;205:1488-1495. doi:10.4049/jimmunol.2000644
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Yan B, Freiwald T, Chauss D, et al. SARS-CoV2 drives JAK1/2-dependent local and systemic complement hyper-activation [published online June 9, 2020]. Res Sq. doi:10.21203/rs.3.rs-33390/v1
- Marietta M, Coluccio V, Luppi M. COVID-19, coagulopathy and venous thromboembolism: more questions than answers. Intern Emerg Med. 2020;15:1375-1387. doi:10.1007/s11739-020-02432-x
- Zuo Y, Estes SK, Ali RA, et al. Prothrombotic antiphospholipid antibodies in COVID-19 [published online June 17, 2020]. medRxiv. doi:10.1101/2020.06.15.20131607
- Lan S-H, Lai C-C, Huang H-T, et al. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56:106103. doi:10.1016/j.ijantimicag.2020.106103
- McMahon D, Gallman A, Hruza G, et al. COVID-19 “long-haulers” in dermatology? duration of dermatologic symptoms in an international registry from 39 countries. Abstract presented at: 29th EADV Congress; October 29, 2020. Accessed March 29, 2020. https://eadvdistribute.m-anage.com/from.storage?image=PXQEdDtICIihN3sM_8nAmh7p_y9AFijhQlf2-_KjrtYgOsOXNVwGxDdti95GZ2Yh0
- Saag MS. Misguided use of hydroxychloroquine for COVID-19: the infusion of politics into science. JAMA. 2020;324:2161-2162. doi:10.1001/jama.2020.22389
- Zahedi Niaki O, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Hammond MI, Sharma TR, Cooper KD, et al. Conducting inpatient dermatology consultations and maintaining resident education in the COVID-19 telemedicine era. J Am Acad Dermatol. 2020;83:E317-E318. doi:10.1016/j.jaad.2020.07.008
- Brunasso AMG, Massone C. Teledermatologic monitoring for chronic cutaneous autoimmune diseases with smartworking during COVID-19 emergency in a tertiary center in Italy. Dermatol Ther. 2020;33:E13495-E13495. doi:10.1111/dth.13695
- Trinidad J, Kroshinsky D, Kaffenberger BH, et al. Telemedicine for inpatient dermatology consultations in response to the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E69-E71. doi:10.1016/j.jaad.2020.04.096
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatology. 2020;156:733-734. doi:10.1001/jamadermatol.2020.1438
The virus that causes COVID-19—SARS-CoV-2—has infected more than 128 million individuals, resulting in more than 2.8 million deaths worldwide between December 2019 and April 2021. Disease mortality primarily is driven by hypoxemic respiratory failure and systemic hypercoagulability, resulting in multisystem organ failure.1 With more than 17 million Americans infected, the virus is estimated to have impacted someone within the social circle of nearly every American.2
The COVID-19 pandemic has highlighted resource limitations, delayed elective and preventive care, and rapidly increased the adoption of telemedicine, presenting a host of new challenges to providers in every medical specialty, including dermatology. Although COVID-19 primarily is a respiratory disease, clinical manifestations have been observed in nearly every organ, including the skin. The cutaneous manifestations of COVID-19 provide insight into disease diagnosis, prognosis, and pathophysiology. In this article, we review the cutaneous manifestations of COVID-19 and explore the state of knowledge regarding their pathophysiology and clinical significance. Finally, we discuss the role of dermatology consultants in the care of patients with COVID-19, and the impact of the pandemic on the field of dermatology.
Prevalence of Cutaneous Findings in COVID-19
Early reports characterizing the clinical presentation of patients hospitalized with COVID-19 suggested skin findings associated with the disease were rare. Cohort studies from Europe, China, and New York City in January through March 2020 reported a low prevalence or made no mention of rash.3-7 However, reports from dermatologists in Italy that emerged in May 2020 indicated a substantially higher proportion of cutaneous disease: 18 of 88 (20.4%) hospitalized patients were found to have cutaneous involvement, primarily consisting of erythematous rash, along with some cases of urticarial and vesicular lesions.8 In October 2020, a retrospective cohort study from Spain examining 2761 patients presenting to the emergency department or admitted to the hospital for COVID-19 found that 58 (2.1%) patients had skin lesions attributed to COVID-19.9
The wide range in reported prevalence of skin lesions may be due to variable involvement of dermatologic specialists in patient care, particularly in China.10 Some variation also may be due to variability in the timing of clinical examination, as well as demographic and clinical differences in patient populations. Of note, a multisystem inflammatory disease seen in US children subsequent to infection with COVID-19 has been associated with rash in as many as 74% of cases.11 Although COVID-19 disproportionately impacts people with skin of color, there are few reports of cutaneous manifestations in that population,12 highlighting the challenges of the dermatologic examination in individuals with darker skin and suggesting the prevalence of dermatologic disease in COVID-19 may be greater than reported.
Morphologic Patterns of Cutaneous Involvement in COVID-19
Researchers in Europe and the United States have attempted to classify the cutaneous manifestations of COVID-19. A registry established through the American Academy of Dermatology published a compilation of reports from 31 countries, totaling 716 patient profiles.13 A prospective Spanish study detailed the cutaneous involvement of 375 patients with suspected or confirmed COVID-19.14 Together, these efforts have revealed several distinct patterns of cutaneous involvement associated with COVID-19 (Table).9,15-18

Vesicular Rash
Vesicular rash associated with COVID-19 has been described in several studies and case series8,13,14 and is considered, along with the pseudopernio (or pseudochilblains) morphology, to be one of the more disease-specific patterns in COVID-19.14,18 Vesicular rash appears to comprise roughly one-tenth of all COVID-19–associated rashes.13,14 It usually is described as pruritic, with 72% to 83% of patients reporting itch.13,16
Small monomorphic or polymorphic vesicles predominantly on the trunk and to a lesser extent the extremities and head have been described by multiple authors.14,16 Vesicular rash is most common among middle-aged individuals, with studies reporting median and mean ages ranging from 40.5 to 55 years.9,13,14,16
Vesicular rash develops concurrent with or after other presenting symptoms of COVID-19; in 2 studies, vesicular rash preceded development of other symptoms in only 15% and 5.6% of cases, respectively.13,14 Prognostically, vesicular rash is associated with moderate disease severity.14,16 It may persist for an average of 8 to 10 days.14,16,18
Histopathologic examination reveals basal layer vacuolar degeneration, hyperchromatic keratinocytes, acantholysis, and dyskeratosis.9,16,18
Urticarial Rash
Urticarial lesions represent approximately 7% to 19% of reported COVID-19–associated rashes.9,13,14 Urticarial rashes in patients testing positive for SARS-CoV-2 primarily occur on the trunk.14 The urticaria, which typically last about 1 week,14 are seen most frequently in middle-aged patients (mean/median age, 42–48 years)13,14 and are associated with pruritus, which has been reported in 74% to 92% of patients.13,14 Urticarial lesions typically do not precede other symptoms of COVID-19 and are nonspecific, making them less useful diagnostically.14
Urticaria appears to be associated with more severe COVID-19 illness in several studies, but this finding may be confounded by several factors, including older age, increased tobacco use, and polypharmacy. Of 104 patients with reported urticarial rash and suspected or confirmed COVID-19 across 3 studies, only 1 death was reported.9,13,14
The histopathologic appearance is that of typical hives, demonstrating a perivascular infiltrate of lymphocytes and eosinophils with edema of the upper dermis.9,19
Morbilliform Eruption
Morbilliform eruption is a commonly reported morphology associated with COVID-19, accounting for 20% to 47% of rashes.9,13,14 This categorization may have limited utility from a diagnostic and prognostic perspective, given that morbilliform eruptions are common, nonspecific, and heterogenous and can arise from many causes.9,13,14 Onset of morbilliform eruption appears to coincide with14 or follow13,20,21 the development of other COVID-19–related symptoms, with 5% of patients reporting morbilliform rash as the initial manifestation of infection.13,14 Morbilliform eruptions have been observed to occur in patients with more severe disease.9,13,14
Certain morphologic subtypes, such as erythema multiforme–like, erythema elevatum diutinum–like, or pseudovesicular, may be more specific to COVID-19 infection.14 A small case series highlighted 4 patients with erythema multiforme–like eruptions, 3 of whom also were found to have petechial enanthem occurring after COVID-19 diagnosis; however, the investigators were unable to exclude drug reaction as a potential cause of rash in these patients.22 Another case series of 21 patients with COVID-19 and skin rash described a (primarily) petechial enanthem on the palate in 6 (28.5%) patients.23 It is unclear to what extent oral enanthem may be underrecognized given that some physicians may be disinclined to remove the masks of known COVID-19–positive patients to examine the oral cavity.
The histologic appearance of morbilliform rash seen in association with COVID-19 has been described as spongiotic with interface dermatitis with perivascular lymphocytic inflammation.9,21
COVID Toes, Pseudochilblains Rash, Perniolike Rash, and Acral Erythema/Edema
Of all the rashes associated with COVID-19, COVID toes, or pseudochilblains rash, has perhaps attracted the most attention. The characteristic violaceous erythema on the fingers and/or toes may be itchy or painful, presenting similar to idiopathic cases of pernio (Figure 1).14 The entity has been controversial because of an absence of a clear correlation with a positive SARS-CoV-2 polymerase chain reaction test or antibodies to the virus in a subset of reported cases.24,25 Onset of the rash late in the disease course, generally after symptom resolution in mild or asymptomatic cases, may explain the absence of viral DNA in the nasopharynx by the time of lesion appearance.14,26 Seronegative patients may have cleared SARS-CoV-2 infection before humoral immunity could occur via a strong type 1 interferon response.25

Across 3 studies, perniolike skin lesions constituted 18% to 29% of COVID-19–associated skin findings9,13,14 and persisted for an average of 12 to 14 days.13,14 Perniolike lesions portend a favorable outcome; patients with COVID toes rarely present with systemic symptoms or laboratory or imaging abnormalities9 and less commonly require hospitalization for severe illness. Perniolike lesions have been reported most frequently in younger patients, with a median or mean age of 32 to 35 years.13,14
Histology demonstrates lichenoid dermatitis with perivascular and periadnexal lymphocytic infiltrates.9 Notably, one study observed interface dermatitis of the intraepidermal portion of the acrosyringium, a rare finding in chilblain lupus, in 83% of patients (N=40).25 Direct immunofluorescence demonstrates a vasculopathic pattern, with some patients showing deposition of IgM or IgG, C3, and fibrinogen in dermal blood vessels. Vascular C9 deposits also have been demonstrated on immunohistochemistry.9 Biopsies of perniolike lesions in COVID-19 patients have demonstrated the presence of SARS-CoV-2 RNA,27 have identified SARS-CoV-2 spike protein in endothelial cells on immunohistochemistry, and have visualized intracytoplasmic viral particles in vascular endothelium on electron microscopy.28
Livedoid Rash/Retiform Purpura
Netlike purpuric or violaceous patches signifying vessel damage or occlusion have been seen in association with COVID-19, constituting approximately 6% of COVID-19–associated skin findings in 2 studies.13,14 Livedoid rash (Figure 2) and retiform purpura (Figure 3) are associated with older age and occur primarily in severely ill patients, including those requiring intensive care. In a registry of 716 patients with COVID-19, 100% of patients with retiform purpura were hospitalized, and 82% had acute respiratory distress syndrome.13 In another study, 33% (7/21) of patients with livedoid and necrotic lesions required intensive care, and 10% (2/21) died.14


Livedoid lesions and retiform purpura represent thrombotic disease in the skin due to vasculopathy/coagulopathy. Dermatopathology available through the American Academy of Dermatology registry revealed thrombotic vasculopathy.13 A case series of 4 patients with livedo racemosa and retiform purpura demonstrated pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, and arterioles with complement deposition.29 Livedoid and retiform lesions in the skin may be associated with a COVID-19–induced coagulopathy, a propensity for systemic clotting including pulmonary embolism, which mostly occurs in hospitalized patients with severe illness.30
Multisystem Inflammatory Disease in Children
A hyperinflammatory syndrome similar to Kawasaki disease and toxic shock syndrome associated with mucocutaneous, cardiac, and gastrointestinal manifestations has been reported following COVID-19 infection.31 This syndrome, known as multisystem inflammatory syndrome in children (MIS-C), predominantly affects adolescents and children older than 5 years,11 typically occurs 2 to 4 weeks after infection, and appears to be at least 100-times less common than COVID-19 infection among the same age group.31 Sixty percent31 to 74%11 of affected patients have mucocutaneous involvement, with the most common clinical findings being conjunctival injection, palmoplantar erythema, lip hyperemia, periorbital erythema and edema, strawberry tongue, and malar erythema, respectively.32
Because this condition appears to reflect an immune response to the virus, the majority of cases demonstrate negative SARS-CoV-2 polymerase chain reaction and positive antibody testing.33 Although cutaneous findings are similar to those seen in Kawasaki disease, certain findings have been noted in MIS-C that are not typical of Kawasaki disease, including heliotrope rash–like periorbital edema and erythema as well as erythema infectiosum–like malar erythema and reticulated erythematous eruptions.32
The course of MIS-C can be severe; in one case series of patients presenting with MIS-C, 80% (79/99) required intensive care unit admission, with 10% requiring mechanical ventilation and 2% of patients dying during admission.31 Cardiac dysfunction, coagulopathy, and gastrointestinal symptoms are common.11,31 It has been postulated that a superantigenlike region of the SARS-CoV-2 spike protein, similar to that of staphylococcal enterotoxin B, may underlie MIS-C and account for its similarities to toxic shock syndrome.34 Of note, a similar multisystem inflammatory syndrome associated with COVID-19 also has been described in adults, and it too may present with rash as a cardinal feature.35
Pathophysiology of COVID-19: What the Skin May Reveal About the Disease
The diverse range of cutaneous manifestations in COVID-19 reflects a spectrum of host immunologicresponses to SARS-CoV-2 and may inform the pathophysiology of the disease as well as potential treatment modalities.
Host Response to SARS-CoV-2
The body’s response to viral infection is 2-pronged, involving activation of cellular antiviral defenses mediated by type I and III interferons, as well as recruitment of leukocytes, mobilized by cytokines and chemokines.36,37 Infection with SARS-CoV-2 results in a unique inflammatory response characterized by suppression of interferons, juxtaposed with a rampant proinflammatory cytokine and chemokine response, reminiscent of a cytokine storm. Reflective of this imbalance, a study of 50 COVID-19 patients and 20 healthy controls found decreased natural killer cells and CD3+ T cells in COVID-19 patients, particularly severely or critically ill patients, with an increase in B cells and monocytes.38 This distinctive immune imbalance positions SARS-CoV-2 to thrive in the absence of inhibitory interferon activity while submitting the host to the deleterious effects of a cytokine surge.36
Type I Interferons
The perniolike lesions associated with mild COVID-19 disease14 may represent a robust immune response via effective stimulation of type I interferons (IFN-1). Similar perniolike lesions are observed in Aicardi-Goutières syndrome37 and familial chilblain lupus, hereditary interferonopathies associated with mutations in the TREX1 (three prime repair exonuclease 1) gene and characterized by inappropriate upregulation of IFN-1,39 resulting in chilblains. It has been suggested that perniolike lesions in COVID-19 result from IFN-1 activation—a robust effective immunologic response to the virus.14,26,40
On the other end of the spectrum, patients with severe COVID-19 may have a blunted IFN-1 response and reduced IFN-1–stimulated gene expression.36,38 Notably, low IFN-1 response preceded clinical deterioration and was associated with increased risk for evolution to critical illness.38 Severe disease from COVID-19 also is more commonly observed in older patients and those with comorbidities,1 both of which are known factors associated with depressed IFN-1 function.38,41 Reflective of this disparate IFN-1 response, biopsies of COVID-19 perniosis have demonstrated striking expression of myxovirus resistance protein A (MXA), a marker for IFN-1 signaling in tissue, whereas its expression is absent in COVID-19 livedo/retiform purpura.27
Familial chilblain lupus may be effectively treated by the Janus kinase inhibitor baricitinib,39 which inhibits IFN-1 signaling. Baricitinib recently received emergency use authorization by the US Food and Drug Administration for treatment of severe COVID-19 pneumonia,42,43 hinting to disordered IFN-1 signaling in the COVID-19 pathophysiology.
The impaired IFN-1 response in COVID-19 patients may be due to a unique characteristic of SARS-CoV-2: its ORF3b gene is a potent IFN-1 antagonist. In a series of experiments comparing SARS-CoV-2 to the related virus severe acute respiratory disease coronavirus (which was responsible for an epidemic in 2002), Konno et al44 found that SARS-CoV-2 is more effectively able to downregulate host IFN-1, likely due to premature stop codons on ORF3b that produce a truncated version of the gene with amplified anti–IFN-1 activity.
Cytokine Storm and Coagulation Cascade
This dulled interferon response is juxtaposed with a surge of inflammatory chemokines and cytokines, including IL-6, IL-8, IL-10, and tumor necrosis factor α, impairing innate immunity and leading to end-organ damage. This inflammatory response is associated with the influx of innate immune cells, specifically neutrophils and monocytes, which likely contribute to lung injury in COVID-19 acute respiratory distress syndrome.38 It also is thought to lead to downstream activation of coagulation, with a high incidence of thrombotic events observed in patients with severe COVID-19.1 In a retrospective study of 184 intensive care patients with COVID-19 receiving at least standard doses of thromboprophylaxis, venous thromboembolism occurred in 27% and arterial thrombotic events occurred in 3.7%.45
Livedo racemosa and retiform purpura are cutaneous markers of hypercoagulability, which indicate an increased risk for systemic clotting in COVID-19. A positive feedback loop between the complement and coagulation cascades appears to be important.13,14,29,46-48 In addition, a few studies have reported antiphospholipid antibody positivity in hospitalized COVID-19 patients.49,50
The high incidence of coagulopathy in severe COVID-19 has prompted many institutions to develop aggressive prophylactic anticoagulation protocols. Elevation of proinflammatory cytokines and observation of terminal complement activation in the skin and other organs has led to therapeutic trials of IL-6 inhibitors such as tocilizumab,51 complement inhibitors such as eculizumab, and Janus kinase inhibitors such as ruxolitinib and baricitinib.42,48
COVID Long-Haulers
The long-term effects of immune dysregulation in COVID-19 patients remain to be seen. Viral triggering of autoimmune disease is a well-established phenomenon, seen in DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome and other dermatologic diseases, raising the possibility that dermatologists will see a rising incidence of cutaneous autoimmune disease in the aftermath of the pandemic. Disordered interferon stimulation could lead to increased incidence of interferon-mediated disorders, such as sarcoidosis and other granulomatous diseases. Vasculitislike skin lesions could persist beyond the acute infectious period. Recent data from a registry of 990 COVID-19 cases from 39 countries suggest that COVID-19 perniolike lesions may persist as long as 150 days.52 In a time of many unknowns, these questions serve as a call to action for rigorous data collection, contribution to existing registries for dermatologic manifestations of COVID-19, and long-term follow-up of COVID-19 patients by the dermatology community.
Pandemic Dermatology
The pandemic has posed unprecedented challenges for patient care. The use of hydroxychloroquine as a popular but unproven treatment for COVID-19, 53 particularly early in the pandemic, has resulted in drug shortages for patients with lupus and other autoimmune skin diseases. Meanwhile, the need for patients with complex dermatologic conditions to receive systemic immunosuppression has had to be balanced against the associated risks during a global pandemic. To help dermatologists navigate this dilemma, various subspecialty groups have issued guidelines, including the COVID-19 Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists, which recommends a stepwise approach to shared decision-making with the goal of minimizing both the risk for disease flare and that of infection. The use of systemic steroids and rituximab, as well as the dose of immunosuppression—particularly broad-acting immunosuppression—should be limited where permitted. 54
Rapid adoption of telemedicine and remote monitoring strategies has enabled dermatologists to provide safe and timely care when in-person visits have not been possible, including for patients with confirmed or suspected COVID-19, as well as for hospitalized patients. 55-57 Use of telemedicine has facilitated preservation of personal protective equipment at a time when these important resources have been scarce. For patients with transportation or scheduling barriers, telemedicine has even expanded access to care.
However, this strategy cannot completely replace comprehensive in-person evaluation. Variability in video and photographic quality limits evaluation, while in-person physical examination can reveal subtle morphologic clues necessary for diagnosis. 5 8 Additionally, unequal access to technology may disadvantage some patients. For dermatologists to provide optimal care and continue to contribute accurate and insightful observations into COVID-19, it is essential to be physically present in the clinic and in the hospital when necessary, caring for patients in need of dermatologic expertise. Creative management strategies developed during this time will benefit patients and expand the reach of the specialty . 5 8
Final Thoughts
The COVID-19 pandemic has profoundly challenged the medical community and dermatology is no exception. By documenting and characterizing the diverse cutaneous manifestations of this novel disease, dermatologists have furthered understanding of its pathophysiology and management. By adapting quickly and developing creative ways to deliver care, dermatologists have found ways to contribute, both large and small. As we take stock at this juncture of the pandemic, it is clear there remains much to learn. We hope dermatologists will continue to take an active role in meeting the challenges of this time.
The virus that causes COVID-19—SARS-CoV-2—has infected more than 128 million individuals, resulting in more than 2.8 million deaths worldwide between December 2019 and April 2021. Disease mortality primarily is driven by hypoxemic respiratory failure and systemic hypercoagulability, resulting in multisystem organ failure.1 With more than 17 million Americans infected, the virus is estimated to have impacted someone within the social circle of nearly every American.2
The COVID-19 pandemic has highlighted resource limitations, delayed elective and preventive care, and rapidly increased the adoption of telemedicine, presenting a host of new challenges to providers in every medical specialty, including dermatology. Although COVID-19 primarily is a respiratory disease, clinical manifestations have been observed in nearly every organ, including the skin. The cutaneous manifestations of COVID-19 provide insight into disease diagnosis, prognosis, and pathophysiology. In this article, we review the cutaneous manifestations of COVID-19 and explore the state of knowledge regarding their pathophysiology and clinical significance. Finally, we discuss the role of dermatology consultants in the care of patients with COVID-19, and the impact of the pandemic on the field of dermatology.
Prevalence of Cutaneous Findings in COVID-19
Early reports characterizing the clinical presentation of patients hospitalized with COVID-19 suggested skin findings associated with the disease were rare. Cohort studies from Europe, China, and New York City in January through March 2020 reported a low prevalence or made no mention of rash.3-7 However, reports from dermatologists in Italy that emerged in May 2020 indicated a substantially higher proportion of cutaneous disease: 18 of 88 (20.4%) hospitalized patients were found to have cutaneous involvement, primarily consisting of erythematous rash, along with some cases of urticarial and vesicular lesions.8 In October 2020, a retrospective cohort study from Spain examining 2761 patients presenting to the emergency department or admitted to the hospital for COVID-19 found that 58 (2.1%) patients had skin lesions attributed to COVID-19.9
The wide range in reported prevalence of skin lesions may be due to variable involvement of dermatologic specialists in patient care, particularly in China.10 Some variation also may be due to variability in the timing of clinical examination, as well as demographic and clinical differences in patient populations. Of note, a multisystem inflammatory disease seen in US children subsequent to infection with COVID-19 has been associated with rash in as many as 74% of cases.11 Although COVID-19 disproportionately impacts people with skin of color, there are few reports of cutaneous manifestations in that population,12 highlighting the challenges of the dermatologic examination in individuals with darker skin and suggesting the prevalence of dermatologic disease in COVID-19 may be greater than reported.
Morphologic Patterns of Cutaneous Involvement in COVID-19
Researchers in Europe and the United States have attempted to classify the cutaneous manifestations of COVID-19. A registry established through the American Academy of Dermatology published a compilation of reports from 31 countries, totaling 716 patient profiles.13 A prospective Spanish study detailed the cutaneous involvement of 375 patients with suspected or confirmed COVID-19.14 Together, these efforts have revealed several distinct patterns of cutaneous involvement associated with COVID-19 (Table).9,15-18

Vesicular Rash
Vesicular rash associated with COVID-19 has been described in several studies and case series8,13,14 and is considered, along with the pseudopernio (or pseudochilblains) morphology, to be one of the more disease-specific patterns in COVID-19.14,18 Vesicular rash appears to comprise roughly one-tenth of all COVID-19–associated rashes.13,14 It usually is described as pruritic, with 72% to 83% of patients reporting itch.13,16
Small monomorphic or polymorphic vesicles predominantly on the trunk and to a lesser extent the extremities and head have been described by multiple authors.14,16 Vesicular rash is most common among middle-aged individuals, with studies reporting median and mean ages ranging from 40.5 to 55 years.9,13,14,16
Vesicular rash develops concurrent with or after other presenting symptoms of COVID-19; in 2 studies, vesicular rash preceded development of other symptoms in only 15% and 5.6% of cases, respectively.13,14 Prognostically, vesicular rash is associated with moderate disease severity.14,16 It may persist for an average of 8 to 10 days.14,16,18
Histopathologic examination reveals basal layer vacuolar degeneration, hyperchromatic keratinocytes, acantholysis, and dyskeratosis.9,16,18
Urticarial Rash
Urticarial lesions represent approximately 7% to 19% of reported COVID-19–associated rashes.9,13,14 Urticarial rashes in patients testing positive for SARS-CoV-2 primarily occur on the trunk.14 The urticaria, which typically last about 1 week,14 are seen most frequently in middle-aged patients (mean/median age, 42–48 years)13,14 and are associated with pruritus, which has been reported in 74% to 92% of patients.13,14 Urticarial lesions typically do not precede other symptoms of COVID-19 and are nonspecific, making them less useful diagnostically.14
Urticaria appears to be associated with more severe COVID-19 illness in several studies, but this finding may be confounded by several factors, including older age, increased tobacco use, and polypharmacy. Of 104 patients with reported urticarial rash and suspected or confirmed COVID-19 across 3 studies, only 1 death was reported.9,13,14
The histopathologic appearance is that of typical hives, demonstrating a perivascular infiltrate of lymphocytes and eosinophils with edema of the upper dermis.9,19
Morbilliform Eruption
Morbilliform eruption is a commonly reported morphology associated with COVID-19, accounting for 20% to 47% of rashes.9,13,14 This categorization may have limited utility from a diagnostic and prognostic perspective, given that morbilliform eruptions are common, nonspecific, and heterogenous and can arise from many causes.9,13,14 Onset of morbilliform eruption appears to coincide with14 or follow13,20,21 the development of other COVID-19–related symptoms, with 5% of patients reporting morbilliform rash as the initial manifestation of infection.13,14 Morbilliform eruptions have been observed to occur in patients with more severe disease.9,13,14
Certain morphologic subtypes, such as erythema multiforme–like, erythema elevatum diutinum–like, or pseudovesicular, may be more specific to COVID-19 infection.14 A small case series highlighted 4 patients with erythema multiforme–like eruptions, 3 of whom also were found to have petechial enanthem occurring after COVID-19 diagnosis; however, the investigators were unable to exclude drug reaction as a potential cause of rash in these patients.22 Another case series of 21 patients with COVID-19 and skin rash described a (primarily) petechial enanthem on the palate in 6 (28.5%) patients.23 It is unclear to what extent oral enanthem may be underrecognized given that some physicians may be disinclined to remove the masks of known COVID-19–positive patients to examine the oral cavity.
The histologic appearance of morbilliform rash seen in association with COVID-19 has been described as spongiotic with interface dermatitis with perivascular lymphocytic inflammation.9,21
COVID Toes, Pseudochilblains Rash, Perniolike Rash, and Acral Erythema/Edema
Of all the rashes associated with COVID-19, COVID toes, or pseudochilblains rash, has perhaps attracted the most attention. The characteristic violaceous erythema on the fingers and/or toes may be itchy or painful, presenting similar to idiopathic cases of pernio (Figure 1).14 The entity has been controversial because of an absence of a clear correlation with a positive SARS-CoV-2 polymerase chain reaction test or antibodies to the virus in a subset of reported cases.24,25 Onset of the rash late in the disease course, generally after symptom resolution in mild or asymptomatic cases, may explain the absence of viral DNA in the nasopharynx by the time of lesion appearance.14,26 Seronegative patients may have cleared SARS-CoV-2 infection before humoral immunity could occur via a strong type 1 interferon response.25

Across 3 studies, perniolike skin lesions constituted 18% to 29% of COVID-19–associated skin findings9,13,14 and persisted for an average of 12 to 14 days.13,14 Perniolike lesions portend a favorable outcome; patients with COVID toes rarely present with systemic symptoms or laboratory or imaging abnormalities9 and less commonly require hospitalization for severe illness. Perniolike lesions have been reported most frequently in younger patients, with a median or mean age of 32 to 35 years.13,14
Histology demonstrates lichenoid dermatitis with perivascular and periadnexal lymphocytic infiltrates.9 Notably, one study observed interface dermatitis of the intraepidermal portion of the acrosyringium, a rare finding in chilblain lupus, in 83% of patients (N=40).25 Direct immunofluorescence demonstrates a vasculopathic pattern, with some patients showing deposition of IgM or IgG, C3, and fibrinogen in dermal blood vessels. Vascular C9 deposits also have been demonstrated on immunohistochemistry.9 Biopsies of perniolike lesions in COVID-19 patients have demonstrated the presence of SARS-CoV-2 RNA,27 have identified SARS-CoV-2 spike protein in endothelial cells on immunohistochemistry, and have visualized intracytoplasmic viral particles in vascular endothelium on electron microscopy.28
Livedoid Rash/Retiform Purpura
Netlike purpuric or violaceous patches signifying vessel damage or occlusion have been seen in association with COVID-19, constituting approximately 6% of COVID-19–associated skin findings in 2 studies.13,14 Livedoid rash (Figure 2) and retiform purpura (Figure 3) are associated with older age and occur primarily in severely ill patients, including those requiring intensive care. In a registry of 716 patients with COVID-19, 100% of patients with retiform purpura were hospitalized, and 82% had acute respiratory distress syndrome.13 In another study, 33% (7/21) of patients with livedoid and necrotic lesions required intensive care, and 10% (2/21) died.14


Livedoid lesions and retiform purpura represent thrombotic disease in the skin due to vasculopathy/coagulopathy. Dermatopathology available through the American Academy of Dermatology registry revealed thrombotic vasculopathy.13 A case series of 4 patients with livedo racemosa and retiform purpura demonstrated pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, and arterioles with complement deposition.29 Livedoid and retiform lesions in the skin may be associated with a COVID-19–induced coagulopathy, a propensity for systemic clotting including pulmonary embolism, which mostly occurs in hospitalized patients with severe illness.30
Multisystem Inflammatory Disease in Children
A hyperinflammatory syndrome similar to Kawasaki disease and toxic shock syndrome associated with mucocutaneous, cardiac, and gastrointestinal manifestations has been reported following COVID-19 infection.31 This syndrome, known as multisystem inflammatory syndrome in children (MIS-C), predominantly affects adolescents and children older than 5 years,11 typically occurs 2 to 4 weeks after infection, and appears to be at least 100-times less common than COVID-19 infection among the same age group.31 Sixty percent31 to 74%11 of affected patients have mucocutaneous involvement, with the most common clinical findings being conjunctival injection, palmoplantar erythema, lip hyperemia, periorbital erythema and edema, strawberry tongue, and malar erythema, respectively.32
Because this condition appears to reflect an immune response to the virus, the majority of cases demonstrate negative SARS-CoV-2 polymerase chain reaction and positive antibody testing.33 Although cutaneous findings are similar to those seen in Kawasaki disease, certain findings have been noted in MIS-C that are not typical of Kawasaki disease, including heliotrope rash–like periorbital edema and erythema as well as erythema infectiosum–like malar erythema and reticulated erythematous eruptions.32
The course of MIS-C can be severe; in one case series of patients presenting with MIS-C, 80% (79/99) required intensive care unit admission, with 10% requiring mechanical ventilation and 2% of patients dying during admission.31 Cardiac dysfunction, coagulopathy, and gastrointestinal symptoms are common.11,31 It has been postulated that a superantigenlike region of the SARS-CoV-2 spike protein, similar to that of staphylococcal enterotoxin B, may underlie MIS-C and account for its similarities to toxic shock syndrome.34 Of note, a similar multisystem inflammatory syndrome associated with COVID-19 also has been described in adults, and it too may present with rash as a cardinal feature.35
Pathophysiology of COVID-19: What the Skin May Reveal About the Disease
The diverse range of cutaneous manifestations in COVID-19 reflects a spectrum of host immunologicresponses to SARS-CoV-2 and may inform the pathophysiology of the disease as well as potential treatment modalities.
Host Response to SARS-CoV-2
The body’s response to viral infection is 2-pronged, involving activation of cellular antiviral defenses mediated by type I and III interferons, as well as recruitment of leukocytes, mobilized by cytokines and chemokines.36,37 Infection with SARS-CoV-2 results in a unique inflammatory response characterized by suppression of interferons, juxtaposed with a rampant proinflammatory cytokine and chemokine response, reminiscent of a cytokine storm. Reflective of this imbalance, a study of 50 COVID-19 patients and 20 healthy controls found decreased natural killer cells and CD3+ T cells in COVID-19 patients, particularly severely or critically ill patients, with an increase in B cells and monocytes.38 This distinctive immune imbalance positions SARS-CoV-2 to thrive in the absence of inhibitory interferon activity while submitting the host to the deleterious effects of a cytokine surge.36
Type I Interferons
The perniolike lesions associated with mild COVID-19 disease14 may represent a robust immune response via effective stimulation of type I interferons (IFN-1). Similar perniolike lesions are observed in Aicardi-Goutières syndrome37 and familial chilblain lupus, hereditary interferonopathies associated with mutations in the TREX1 (three prime repair exonuclease 1) gene and characterized by inappropriate upregulation of IFN-1,39 resulting in chilblains. It has been suggested that perniolike lesions in COVID-19 result from IFN-1 activation—a robust effective immunologic response to the virus.14,26,40
On the other end of the spectrum, patients with severe COVID-19 may have a blunted IFN-1 response and reduced IFN-1–stimulated gene expression.36,38 Notably, low IFN-1 response preceded clinical deterioration and was associated with increased risk for evolution to critical illness.38 Severe disease from COVID-19 also is more commonly observed in older patients and those with comorbidities,1 both of which are known factors associated with depressed IFN-1 function.38,41 Reflective of this disparate IFN-1 response, biopsies of COVID-19 perniosis have demonstrated striking expression of myxovirus resistance protein A (MXA), a marker for IFN-1 signaling in tissue, whereas its expression is absent in COVID-19 livedo/retiform purpura.27
Familial chilblain lupus may be effectively treated by the Janus kinase inhibitor baricitinib,39 which inhibits IFN-1 signaling. Baricitinib recently received emergency use authorization by the US Food and Drug Administration for treatment of severe COVID-19 pneumonia,42,43 hinting to disordered IFN-1 signaling in the COVID-19 pathophysiology.
The impaired IFN-1 response in COVID-19 patients may be due to a unique characteristic of SARS-CoV-2: its ORF3b gene is a potent IFN-1 antagonist. In a series of experiments comparing SARS-CoV-2 to the related virus severe acute respiratory disease coronavirus (which was responsible for an epidemic in 2002), Konno et al44 found that SARS-CoV-2 is more effectively able to downregulate host IFN-1, likely due to premature stop codons on ORF3b that produce a truncated version of the gene with amplified anti–IFN-1 activity.
Cytokine Storm and Coagulation Cascade
This dulled interferon response is juxtaposed with a surge of inflammatory chemokines and cytokines, including IL-6, IL-8, IL-10, and tumor necrosis factor α, impairing innate immunity and leading to end-organ damage. This inflammatory response is associated with the influx of innate immune cells, specifically neutrophils and monocytes, which likely contribute to lung injury in COVID-19 acute respiratory distress syndrome.38 It also is thought to lead to downstream activation of coagulation, with a high incidence of thrombotic events observed in patients with severe COVID-19.1 In a retrospective study of 184 intensive care patients with COVID-19 receiving at least standard doses of thromboprophylaxis, venous thromboembolism occurred in 27% and arterial thrombotic events occurred in 3.7%.45
Livedo racemosa and retiform purpura are cutaneous markers of hypercoagulability, which indicate an increased risk for systemic clotting in COVID-19. A positive feedback loop between the complement and coagulation cascades appears to be important.13,14,29,46-48 In addition, a few studies have reported antiphospholipid antibody positivity in hospitalized COVID-19 patients.49,50
The high incidence of coagulopathy in severe COVID-19 has prompted many institutions to develop aggressive prophylactic anticoagulation protocols. Elevation of proinflammatory cytokines and observation of terminal complement activation in the skin and other organs has led to therapeutic trials of IL-6 inhibitors such as tocilizumab,51 complement inhibitors such as eculizumab, and Janus kinase inhibitors such as ruxolitinib and baricitinib.42,48
COVID Long-Haulers
The long-term effects of immune dysregulation in COVID-19 patients remain to be seen. Viral triggering of autoimmune disease is a well-established phenomenon, seen in DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome and other dermatologic diseases, raising the possibility that dermatologists will see a rising incidence of cutaneous autoimmune disease in the aftermath of the pandemic. Disordered interferon stimulation could lead to increased incidence of interferon-mediated disorders, such as sarcoidosis and other granulomatous diseases. Vasculitislike skin lesions could persist beyond the acute infectious period. Recent data from a registry of 990 COVID-19 cases from 39 countries suggest that COVID-19 perniolike lesions may persist as long as 150 days.52 In a time of many unknowns, these questions serve as a call to action for rigorous data collection, contribution to existing registries for dermatologic manifestations of COVID-19, and long-term follow-up of COVID-19 patients by the dermatology community.
Pandemic Dermatology
The pandemic has posed unprecedented challenges for patient care. The use of hydroxychloroquine as a popular but unproven treatment for COVID-19, 53 particularly early in the pandemic, has resulted in drug shortages for patients with lupus and other autoimmune skin diseases. Meanwhile, the need for patients with complex dermatologic conditions to receive systemic immunosuppression has had to be balanced against the associated risks during a global pandemic. To help dermatologists navigate this dilemma, various subspecialty groups have issued guidelines, including the COVID-19 Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists, which recommends a stepwise approach to shared decision-making with the goal of minimizing both the risk for disease flare and that of infection. The use of systemic steroids and rituximab, as well as the dose of immunosuppression—particularly broad-acting immunosuppression—should be limited where permitted. 54
Rapid adoption of telemedicine and remote monitoring strategies has enabled dermatologists to provide safe and timely care when in-person visits have not been possible, including for patients with confirmed or suspected COVID-19, as well as for hospitalized patients. 55-57 Use of telemedicine has facilitated preservation of personal protective equipment at a time when these important resources have been scarce. For patients with transportation or scheduling barriers, telemedicine has even expanded access to care.
However, this strategy cannot completely replace comprehensive in-person evaluation. Variability in video and photographic quality limits evaluation, while in-person physical examination can reveal subtle morphologic clues necessary for diagnosis. 5 8 Additionally, unequal access to technology may disadvantage some patients. For dermatologists to provide optimal care and continue to contribute accurate and insightful observations into COVID-19, it is essential to be physically present in the clinic and in the hospital when necessary, caring for patients in need of dermatologic expertise. Creative management strategies developed during this time will benefit patients and expand the reach of the specialty . 5 8
Final Thoughts
The COVID-19 pandemic has profoundly challenged the medical community and dermatology is no exception. By documenting and characterizing the diverse cutaneous manifestations of this novel disease, dermatologists have furthered understanding of its pathophysiology and management. By adapting quickly and developing creative ways to deliver care, dermatologists have found ways to contribute, both large and small. As we take stock at this juncture of the pandemic, it is clear there remains much to learn. We hope dermatologists will continue to take an active role in meeting the challenges of this time.
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA . 2020;324:782-793. doi:10.1001/jama.2020.12839
- New York Times . Updated December 23, 2020. Accessed March 22, 2021. https://www.nytimes.com/2020/11/15/us/coronavirus-us-cases-deaths.html
- Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med . 2020;382:1708-1720. doi:10.1056/NEJMoa2002032
- Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med . 2020;288:335-344. doi:https://doi.org/10.1111/joim.13089
- Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu province: a multicenter descriptive study. Clin Infect Dis . 2020;71:706-712. doi:10.1093/cid/ciaa199
- Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med . 2020;382:2372-2374. doi:10.1056/NEJMc2010419
- Sun L, Shen L, Fan J, et al. Clinical features of patients with coronavirus disease 2019 from a designated hospital in Beijing, China. J Med Virol . 2020;92:2055-2066. https://doi.org/10.1002/jmv.25966
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatology Venereol . 2020;34:E212-E213. https://doi.org/10.1111/jdv.16387
- Giavedoni P, Podlipnik S, Pericàs JM, et al. Skin manifestations in COVID-19: prevalence and relationship with disease severity. J Clin Med . 2020;9:3261. doi:10.3390/jcm9103261
- Jimenez-Cauhe J, Ortega-Quijano D, Prieto-Barrios M, et al. Reply to “COVID-19 can present with a rash and be mistaken for dengue”: petechial rash in a patient with COVID-19 infection. J Am Acad Dermatol . 2020;83:E141-E142. doi:10.1016/j.jaad.2020.04.016
- Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med . 2020;383:334-346. doi:10.1056/NEJMoa2021680
- Shinkai K, Bruckner AL. Dermatology and COVID-19. JAMA . 2020;324:1133-1134. doi:10.1001/jama.2020.15276
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol . 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol . 2020;183:71-77. https://doi.org/10.1111/bjd.19163
- Bouaziz JD, Duong TA, Jachiet M, et al. Vascular skin symptoms in COVID-19: a French observational study. J Eur Acad Dermatology Venereol . 2020;34:E451-E452. https://doi.org/10.1111/jdv.16544
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol . 2020;45:872-875. https://doi.org/10.1111/ced.14277
- Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol . 2020;83:E61-E63. doi:10.1016/j.jaad.2020.04.093
- Marzano AV, Genovese G, Fabbrocini G, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: Multicenter case series of 22 patients. J Am Acad Dermatol . 2020;83:280-285. doi:10.1016/j.jaad.2020.04.044
- Fernandez-Nieto D, Ortega-Quijano D, Segurado-Miravalles G, et al. Comment on: cutaneous manifestations in COVID-19: a first perspective. safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol . 2020;34:E252-E254. https://doi.org/10.1111/jdv.16470
- Herrero-Moyano M, Capusan TM, Andreu-Barasoain M, et al. A clinicopathological study of eight patients with COVID-19 pneumonia and a late-onset exanthema. J Eur Acad Dermatol Venereol . 2020;34:E460-E464. https://doi.org/10.1111/jdv.16631
- Rubio-Muniz CA, Puerta-Peñ a M, Falkenhain-L ópez D, et al. The broad spectrum of dermatological manifestations in COVID-19: clinical and histopathological features learned from a series of 34 cases. J Eur Acad Dermatol Venereol . 2020;34:E574-E576. https://doi.org/10.1111/jdv.16734
- Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings. Clin Exp Dermatol . 2020;45:892-895. https://doi.org/10.1111/ced.14281
- Jimenez-Cauhe J, Ortega-Quijano D, de Perosanz-Lobo D, et al. Enanthem in patients with COVID-19 and skin rash. JAMA Dermatol . 2020;156:1134-1136. doi:10.1001/jamadermatol.2020.2550
- Le Cleach L, Dousset L, Assier H, et al. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol . 2020;183:866-874. https://doi.org/10.1111/bjd.19377
- Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic [published online November 25, 2020]. JAMA Dermatol . doi:10.1001/jamadermatol.2020.4324
- Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol . 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Magro CM, Mulvey JJ, Laurence J, et al. The differing pathophysiologies that underlie COVID-19-associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol . 2021;184:141-150. https://doi.org/10.1111/bjd.19415
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol . 2020;183:729-737. doi:10.1111/bjd.19327
- Droesch C, Do MH, DeSancho M, et al. Livedoid and purpuric skin eruptions associated with coagulopathy in severe COVID-19. JAMA Dermatol . 2020;156:1-3. doi:10.1001/jamadermatol.2020.2800
- Asakura H, Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol . 2021;113:45-57. doi:10.1007/s12185-020-03029-y
- Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med . 2020;383:347-358. doi:10.1056/NEJMoa2021756
- Young TK, Shaw KS, Shah JK, et al. Mucocutaneous manifestations of multisystem inflammatory syndrome in children during the COVID-19 pandemic. JAMA Dermatol . 2021;157:207-212. doi:10.1001/jamadermatol.2020.4779
- Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259-269. doi:10.1001/jama.2020.10369
- Cheng MH, Zhang S, Porritt RA, et al. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation.
- Morris SB, Schwartz NG, Patel P, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 Infection—United Kingdom and United States, March–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1450-1456. doi:10.15585/mmwr.mm6940e1
- Blanco-Melo D, Nilsson-Payant BE, Liu W-C, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036.e9-1045.e9. doi:10.1016/j.cell.2020.04.026
- Crow YJ, Manel N. Aicardi–Goutières syndrome and the type I interferonopathies. Nat Rev Immunol. 2015;15:429-440. doi:10.1038/nri3850
- Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718-724. doi:10.1126/science.abc6027
- Zimmermann N, Wolf C, Schwenke R, et al. Assessment of clinical response to janus kinase inhibition in patients with familial chilblain lupus and TREX1 mutation. JAMA Dermatol. 2019;155:342-346. doi:10.1001/jamadermatol.2018.5077
- Hubiche T, Le Duff F, Chiaverini C, et al. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions. Lancet Infect Dis. 2021;21:315-316. doi:10.1016/S1473-3099(20)30518-1
- Agrawal A. Mechanisms and implications of age-associated impaired innate interferon secretion by dendritic cells: a mini-review. Gerontology. 2013;59:421-426. doi:10.1159/000350536
- Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795-807. doi:10.1056/NEJMoa2031994
- US Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization (EUA) of baricitinib. Accessed March 29, 2021. https://www.fda.gov/media/143823/download
- Konno Y, Kimura I, Uriu K, et al. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep. 2020;32:108185. doi:10.1016/j.celrep.2020.108185
- Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke: from the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO). J Vasc Interv Radiol. 2018;29:441-453. doi:10.1016/j.jvir.2017.11.026
- Lo MW, Kemper C, Woodruff TM. COVID-19: complement, coagulation, and collateral damage. J Immunol. 2020;205:1488-1495. doi:10.4049/jimmunol.2000644
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Yan B, Freiwald T, Chauss D, et al. SARS-CoV2 drives JAK1/2-dependent local and systemic complement hyper-activation [published online June 9, 2020]. Res Sq. doi:10.21203/rs.3.rs-33390/v1
- Marietta M, Coluccio V, Luppi M. COVID-19, coagulopathy and venous thromboembolism: more questions than answers. Intern Emerg Med. 2020;15:1375-1387. doi:10.1007/s11739-020-02432-x
- Zuo Y, Estes SK, Ali RA, et al. Prothrombotic antiphospholipid antibodies in COVID-19 [published online June 17, 2020]. medRxiv. doi:10.1101/2020.06.15.20131607
- Lan S-H, Lai C-C, Huang H-T, et al. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56:106103. doi:10.1016/j.ijantimicag.2020.106103
- McMahon D, Gallman A, Hruza G, et al. COVID-19 “long-haulers” in dermatology? duration of dermatologic symptoms in an international registry from 39 countries. Abstract presented at: 29th EADV Congress; October 29, 2020. Accessed March 29, 2020. https://eadvdistribute.m-anage.com/from.storage?image=PXQEdDtICIihN3sM_8nAmh7p_y9AFijhQlf2-_KjrtYgOsOXNVwGxDdti95GZ2Yh0
- Saag MS. Misguided use of hydroxychloroquine for COVID-19: the infusion of politics into science. JAMA. 2020;324:2161-2162. doi:10.1001/jama.2020.22389
- Zahedi Niaki O, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Hammond MI, Sharma TR, Cooper KD, et al. Conducting inpatient dermatology consultations and maintaining resident education in the COVID-19 telemedicine era. J Am Acad Dermatol. 2020;83:E317-E318. doi:10.1016/j.jaad.2020.07.008
- Brunasso AMG, Massone C. Teledermatologic monitoring for chronic cutaneous autoimmune diseases with smartworking during COVID-19 emergency in a tertiary center in Italy. Dermatol Ther. 2020;33:E13495-E13495. doi:10.1111/dth.13695
- Trinidad J, Kroshinsky D, Kaffenberger BH, et al. Telemedicine for inpatient dermatology consultations in response to the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E69-E71. doi:10.1016/j.jaad.2020.04.096
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatology. 2020;156:733-734. doi:10.1001/jamadermatol.2020.1438
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA . 2020;324:782-793. doi:10.1001/jama.2020.12839
- New York Times . Updated December 23, 2020. Accessed March 22, 2021. https://www.nytimes.com/2020/11/15/us/coronavirus-us-cases-deaths.html
- Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med . 2020;382:1708-1720. doi:10.1056/NEJMoa2002032
- Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med . 2020;288:335-344. doi:https://doi.org/10.1111/joim.13089
- Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu province: a multicenter descriptive study. Clin Infect Dis . 2020;71:706-712. doi:10.1093/cid/ciaa199
- Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med . 2020;382:2372-2374. doi:10.1056/NEJMc2010419
- Sun L, Shen L, Fan J, et al. Clinical features of patients with coronavirus disease 2019 from a designated hospital in Beijing, China. J Med Virol . 2020;92:2055-2066. https://doi.org/10.1002/jmv.25966
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatology Venereol . 2020;34:E212-E213. https://doi.org/10.1111/jdv.16387
- Giavedoni P, Podlipnik S, Pericàs JM, et al. Skin manifestations in COVID-19: prevalence and relationship with disease severity. J Clin Med . 2020;9:3261. doi:10.3390/jcm9103261
- Jimenez-Cauhe J, Ortega-Quijano D, Prieto-Barrios M, et al. Reply to “COVID-19 can present with a rash and be mistaken for dengue”: petechial rash in a patient with COVID-19 infection. J Am Acad Dermatol . 2020;83:E141-E142. doi:10.1016/j.jaad.2020.04.016
- Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med . 2020;383:334-346. doi:10.1056/NEJMoa2021680
- Shinkai K, Bruckner AL. Dermatology and COVID-19. JAMA . 2020;324:1133-1134. doi:10.1001/jama.2020.15276
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol . 2020;83:1118-1129. doi:10.1016/j.jaad.2020.06.1016
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol . 2020;183:71-77. https://doi.org/10.1111/bjd.19163
- Bouaziz JD, Duong TA, Jachiet M, et al. Vascular skin symptoms in COVID-19: a French observational study. J Eur Acad Dermatology Venereol . 2020;34:E451-E452. https://doi.org/10.1111/jdv.16544
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol . 2020;45:872-875. https://doi.org/10.1111/ced.14277
- Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol . 2020;83:E61-E63. doi:10.1016/j.jaad.2020.04.093
- Marzano AV, Genovese G, Fabbrocini G, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: Multicenter case series of 22 patients. J Am Acad Dermatol . 2020;83:280-285. doi:10.1016/j.jaad.2020.04.044
- Fernandez-Nieto D, Ortega-Quijano D, Segurado-Miravalles G, et al. Comment on: cutaneous manifestations in COVID-19: a first perspective. safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol . 2020;34:E252-E254. https://doi.org/10.1111/jdv.16470
- Herrero-Moyano M, Capusan TM, Andreu-Barasoain M, et al. A clinicopathological study of eight patients with COVID-19 pneumonia and a late-onset exanthema. J Eur Acad Dermatol Venereol . 2020;34:E460-E464. https://doi.org/10.1111/jdv.16631
- Rubio-Muniz CA, Puerta-Peñ a M, Falkenhain-L ópez D, et al. The broad spectrum of dermatological manifestations in COVID-19: clinical and histopathological features learned from a series of 34 cases. J Eur Acad Dermatol Venereol . 2020;34:E574-E576. https://doi.org/10.1111/jdv.16734
- Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings. Clin Exp Dermatol . 2020;45:892-895. https://doi.org/10.1111/ced.14281
- Jimenez-Cauhe J, Ortega-Quijano D, de Perosanz-Lobo D, et al. Enanthem in patients with COVID-19 and skin rash. JAMA Dermatol . 2020;156:1134-1136. doi:10.1001/jamadermatol.2020.2550
- Le Cleach L, Dousset L, Assier H, et al. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol . 2020;183:866-874. https://doi.org/10.1111/bjd.19377
- Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic [published online November 25, 2020]. JAMA Dermatol . doi:10.1001/jamadermatol.2020.4324
- Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol . 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Magro CM, Mulvey JJ, Laurence J, et al. The differing pathophysiologies that underlie COVID-19-associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol . 2021;184:141-150. https://doi.org/10.1111/bjd.19415
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol . 2020;183:729-737. doi:10.1111/bjd.19327
- Droesch C, Do MH, DeSancho M, et al. Livedoid and purpuric skin eruptions associated with coagulopathy in severe COVID-19. JAMA Dermatol . 2020;156:1-3. doi:10.1001/jamadermatol.2020.2800
- Asakura H, Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol . 2021;113:45-57. doi:10.1007/s12185-020-03029-y
- Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med . 2020;383:347-358. doi:10.1056/NEJMoa2021756
- Young TK, Shaw KS, Shah JK, et al. Mucocutaneous manifestations of multisystem inflammatory syndrome in children during the COVID-19 pandemic. JAMA Dermatol . 2021;157:207-212. doi:10.1001/jamadermatol.2020.4779
- Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259-269. doi:10.1001/jama.2020.10369
- Cheng MH, Zhang S, Porritt RA, et al. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation.
- Morris SB, Schwartz NG, Patel P, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 Infection—United Kingdom and United States, March–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1450-1456. doi:10.15585/mmwr.mm6940e1
- Blanco-Melo D, Nilsson-Payant BE, Liu W-C, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036.e9-1045.e9. doi:10.1016/j.cell.2020.04.026
- Crow YJ, Manel N. Aicardi–Goutières syndrome and the type I interferonopathies. Nat Rev Immunol. 2015;15:429-440. doi:10.1038/nri3850
- Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718-724. doi:10.1126/science.abc6027
- Zimmermann N, Wolf C, Schwenke R, et al. Assessment of clinical response to janus kinase inhibition in patients with familial chilblain lupus and TREX1 mutation. JAMA Dermatol. 2019;155:342-346. doi:10.1001/jamadermatol.2018.5077
- Hubiche T, Le Duff F, Chiaverini C, et al. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions. Lancet Infect Dis. 2021;21:315-316. doi:10.1016/S1473-3099(20)30518-1
- Agrawal A. Mechanisms and implications of age-associated impaired innate interferon secretion by dendritic cells: a mini-review. Gerontology. 2013;59:421-426. doi:10.1159/000350536
- Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795-807. doi:10.1056/NEJMoa2031994
- US Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization (EUA) of baricitinib. Accessed March 29, 2021. https://www.fda.gov/media/143823/download
- Konno Y, Kimura I, Uriu K, et al. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep. 2020;32:108185. doi:10.1016/j.celrep.2020.108185
- Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke: from the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO). J Vasc Interv Radiol. 2018;29:441-453. doi:10.1016/j.jvir.2017.11.026
- Lo MW, Kemper C, Woodruff TM. COVID-19: complement, coagulation, and collateral damage. J Immunol. 2020;205:1488-1495. doi:10.4049/jimmunol.2000644
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Yan B, Freiwald T, Chauss D, et al. SARS-CoV2 drives JAK1/2-dependent local and systemic complement hyper-activation [published online June 9, 2020]. Res Sq. doi:10.21203/rs.3.rs-33390/v1
- Marietta M, Coluccio V, Luppi M. COVID-19, coagulopathy and venous thromboembolism: more questions than answers. Intern Emerg Med. 2020;15:1375-1387. doi:10.1007/s11739-020-02432-x
- Zuo Y, Estes SK, Ali RA, et al. Prothrombotic antiphospholipid antibodies in COVID-19 [published online June 17, 2020]. medRxiv. doi:10.1101/2020.06.15.20131607
- Lan S-H, Lai C-C, Huang H-T, et al. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56:106103. doi:10.1016/j.ijantimicag.2020.106103
- McMahon D, Gallman A, Hruza G, et al. COVID-19 “long-haulers” in dermatology? duration of dermatologic symptoms in an international registry from 39 countries. Abstract presented at: 29th EADV Congress; October 29, 2020. Accessed March 29, 2020. https://eadvdistribute.m-anage.com/from.storage?image=PXQEdDtICIihN3sM_8nAmh7p_y9AFijhQlf2-_KjrtYgOsOXNVwGxDdti95GZ2Yh0
- Saag MS. Misguided use of hydroxychloroquine for COVID-19: the infusion of politics into science. JAMA. 2020;324:2161-2162. doi:10.1001/jama.2020.22389
- Zahedi Niaki O, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Hammond MI, Sharma TR, Cooper KD, et al. Conducting inpatient dermatology consultations and maintaining resident education in the COVID-19 telemedicine era. J Am Acad Dermatol. 2020;83:E317-E318. doi:10.1016/j.jaad.2020.07.008
- Brunasso AMG, Massone C. Teledermatologic monitoring for chronic cutaneous autoimmune diseases with smartworking during COVID-19 emergency in a tertiary center in Italy. Dermatol Ther. 2020;33:E13495-E13495. doi:10.1111/dth.13695
- Trinidad J, Kroshinsky D, Kaffenberger BH, et al. Telemedicine for inpatient dermatology consultations in response to the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E69-E71. doi:10.1016/j.jaad.2020.04.096
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatology. 2020;156:733-734. doi:10.1001/jamadermatol.2020.1438
Practice Points
- Cutaneous manifestations of COVID-19 may reflect the range of host immunologic responses to SARS-CoV-2.
- Perniosis appears to be a late manifestation of COVID-19 associated with a comparatively benign disease course, whereas livedoid or other vasculopathic lesions portend poorer outcomes and may warrant further workup for occult thrombotic disease.
- Maculopapular, vesicular, and urticarial eruptions may be seen in association with COVID-19 but are nonspecific and necessitate a broad differential and workup.
- Challenges posed by the COVID-19 pandemic necessitate creative management strategies for immunosuppression and clinical assessment.
Nutritional Dermatoses in the Hospitalized Patient
The World Health Organization defines malnutrition as deficiencies, excesses, or imbalances in an individual’s intake of energy and/or nutrients.1 This review will focus on undernutrition, which may result from macronutrient or micronutrient deficiencies. Undernutrition in the hospitalized patient is a common yet underrecognized phenomenon, with an estimated prevalence of 20% to 50% worldwide.2 Malnutrition is an independent risk factor for patient morbidity and mortality and has been associated with increased health care costs.3 Nutritional deficiencies may arise from inadequate nutrient intake, abnormal nutrient absorption, or improper nutrient utilization.4 Unfortunately, no standardized algorithm for screening and diagnosing patients with malnutrition exists, making early physical examination findings of utmost importance. Herein, we present a review of acquired nutritional deficiency dermatoses in the inpatient setting.
Protein-Energy Malnutrition
Protein-energy malnutrition (PEM) refers to a set of related disorders that include marasmus, kwashiorkor (KW), and marasmic KW. These conditions frequently are seen in developing countries but also have been reported in developed nations.5 Marasmus occurs from a chronic deficiency of protein and calories. Decreased insulin production and unopposed catabolism result in sarcopenia and loss of bone and subcutaneous fat.6 Affected patients include children who are less than 60% ideal body weight (IBW) without edema or hypoproteinemia.7 Kwashiorkor is the edematous form of PEM that develops from isolated protein deficiency, resulting in edema, diarrhea, and immunosuppression.6 Micronutrient deficiencies, oxidative stress, slow protein catabolism, and excess antidiuretic hormone have been proposed as potential drivers of KW.8 Kwashiorkor affects children between 60% and 80% IBW. Marasmic KW has features of both diseases, including children who are less than 60% IBW but with associated edema and/or hypoproteinemia.9
Although PEM is uncommon in adults, hospitalized patients carry many predisposing risk factors, including infections, malabsorptive conditions, psychiatric disease, and chronic illness (eTable). Patients with chronic infections present with findings consistent with marasmic KW due to lean body mass loss.
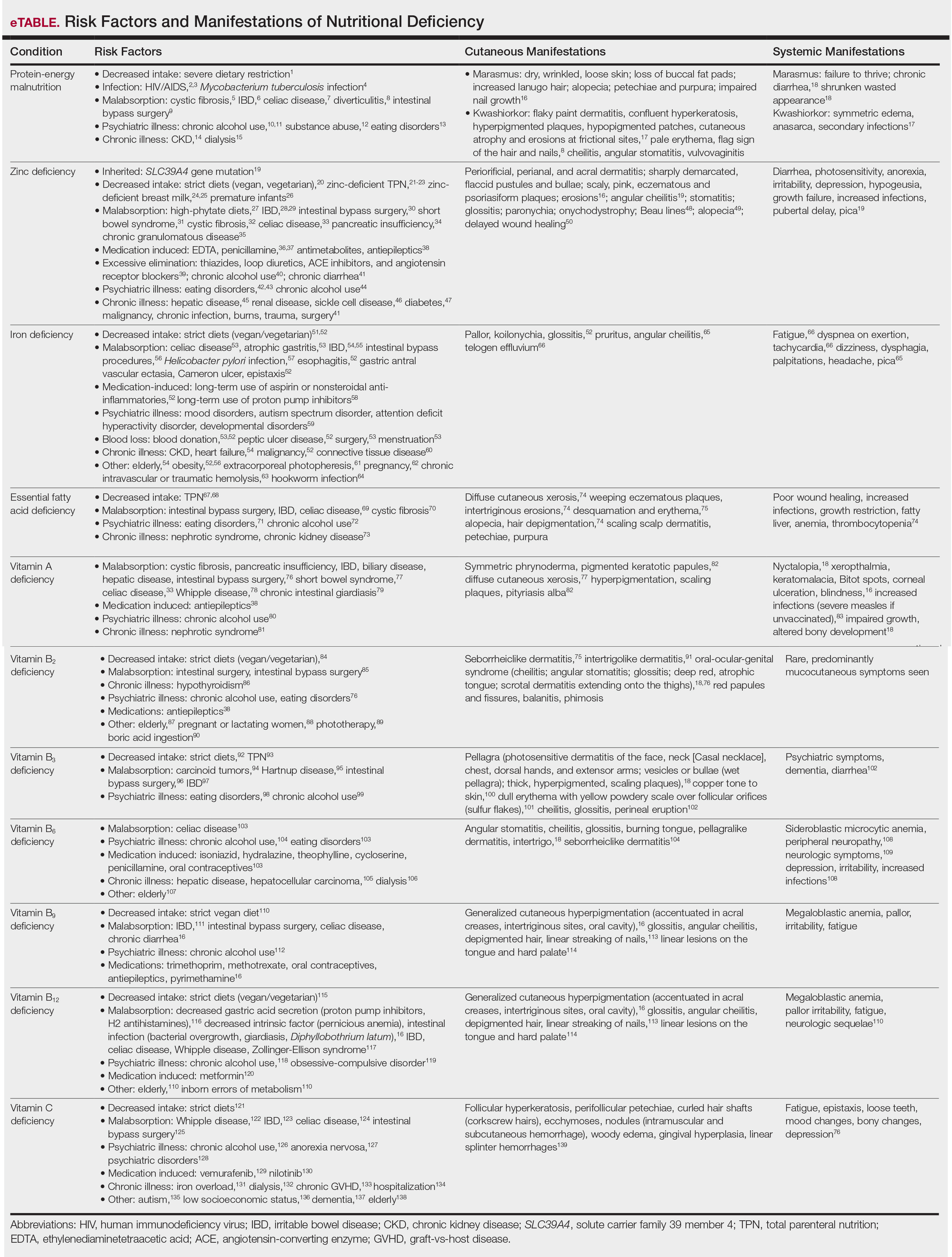
The cutaneous findings in PEM are related to dysmaturation of epidermal keratinocytes and resultant epidermal atrophy.10 Patients with marasmus exhibit dry, wrinkled, loose skin due to subcutaneous fat loss. Emaciated children often lose their buccal fat pads, and reduced perianal adipose may lead to rectal prolapse. Increased lanugo hair may be present on the face, and alopecia of the scalp may occur.6 In KW, cutaneous disease progresses from confluent hyperkeratosis to a dry atrophic epidermis that erodes easily, leaving underlying pale erythema. The resultant pattern is one of hyperpigmented plaques with slightly raised borders, and hypopigmented patches and erosions described as flaky paint dermatitis (Figure 1).5 Lesions appear first in areas of friction. The hair often is dry and brittle; curly hair may straighten and scale.11 Red-yellow to gray-white hypopigmentation may develop, denoting periods of inadequate nutrition. The flag sign describes alternating horizontal bands of hypopigmentation interspersed with bands of pigmented hair. The nails usually are thin and soft and may exhibit the nail flag sign, characterized by horizontal bands of white and red.12 Cheilitis, angular stomatitis, and vulvovaginitis may be present.6
In adults, weight loss and body mass index can be used to assess nutritional status, along with a focused history and physical examination. Complete blood cell count, electrolyte levels, and blood urea nitrogen should be assessed, as hypoglycemia and anemia often accompany PEM.13 In KW, hypoalbuminemia and hypoproteinemia are invariably present. Although prealbumin may be a valid prognostic indicator of disease outcomes and mortality in patients at risk for malnutrition, checking other serum biomarkers remains controversial.14 Focused testing may be warranted in patients with risk factors for chronic infectious processes, such as human immunodeficiency virus or tuberculosis.6 Skin biopsy may solidify the diagnosis of PEM. Hypertrophy of the stratum corneum, atrophy of the stratum spinosum and stratum granulosum, and increased basal layer melanin have been reported.15
Treatment involves initial fluid resuscitation and correction of electrolyte imbalances, followed by nutritional replacement.13 Oral or enteral tube feedings are preferred over total parenteral nutrition (TPN), as they enhance recovery of the gastrointestinal tract.16 Refeeding should occur in small amounts and frequent intervals.5 Skin-directed therapy is aimed at restoring epidermal function and hydration, with regular moisturization and application of barrier creams, such as zinc oxide ointment or petrolatum.10
Zinc Deficiency
Zinc is an essential trace element that provides regulatory, structural, and catalytic functions across multiple biochemical pathways6 and serves as an enzymatic cofactor and key component for numerous transcription factors.17 Zinc is derived from food sources, and its concentration correlates with protein content.18 Zinc is found in both animal and plant-based proteins, albeit with a lower oral bioavailability in the latter. Zinc deficiency may be inherited or acquired. Primary acrodermatitis enteropathica is an autosomal-recessive disorder of the solute carrier family 39 member 4 gene, SLC39A4 (encodes zinc transporter ZIP4 on enterocytes); the result is abnormal zinc absorption from the small intestine.18
Acquired zinc deficiency occurs from decreased dietary zinc intake, impaired intestinal zinc absorption, excessive zinc elimination, or systemic states of high catabolism or low albumin (eTable). Total parenteral nutrition–associated deficiency has arisen when nutritional formulations did not contain trace elements during national shortages or when prolonged TPN was not anticipated and trace elements were removed.19 Zinc levels may already be low in patients with chronic illness or inflammation, so even a short period on TPN can precipitate deficiency.18,19 Diets high in phytate may result in zinc deficiency, as phytate impairs intestinal zinc absorption.20 Approximately 15% of patients with inflammatory bowel disease experienced zinc deficiency worldwide.21 In Crohn disease, zinc deficiency has been associated with active intestinal inflammation, increased risk for hospitalization, surgeries, and disease-related complications.22,23
Medications such as antiepileptics, antimetabolites, or penicillamine may induce zinc deficiency, highlighting the importance of medication review for hospitalized patients (eTable). Catabolic states, frequently encountered in hospitalized patients, increase the risk for zinc deficiency.24 Patients with necrolytic migratory erythema (associated with pancreatic glucagonomas) often experience low serum zinc levels.25
The skin is the third most zinc-abundant tissue in the human body. Within keratinocytes, zinc is critical to normal proliferation and suppression of inflammation.17 Zinc also plays an important role in cutaneous immune function.26 Zinc deficiency presents with sharply demarcated, flaccid pustules and bullae that erode into scaly, pink, eczematous or psoriasiform plaques. Lesions are found preferentially in acral and periorificial sites, often with crusting and exudate. The groin and flexural surfaces may be affected. Erosions often become secondarily impetiginized. Other cutaneous findings include angular cheilitis, stomatitis, glossitis, paronychia, onychodystrophy, generalized alopecia, and delayed wound healing.26 Histopathology of skin lesions is characterized by granular layer loss, epidermal pallor, confluent parakeratosis, spongiosis, dyskeratosis, and psoriasiform hyperplasia.27 Acquired bullous acrodermatitis enteropathica has been reported as a histologic mimicker of pemphigus foliaceous in patients on TPN.28
Diagnosis of zinc deficiency is made by measuring plasma zinc levels. Fasting levels should be drawn in the morning, as they can fluctuate based on the time of day, stress levels, or inflammation.6 Sample hemolysis and anticoagulants high in zinc may falsely elevate plasma zinc. A normal zinc level is greater than 70 µg/dL; however, normal levels do not rule out deficiency.18 Measurement of zinc-dependent enzymes, such as alkaline phosphatase, can be a quick way to assess zinc status. Serum albumin also should be measured; because zinc is carried by albumin in the blood, hypoalbuminemia may result in secondary zinc deficiency.18
Zinc replacement therapy is largely through oral supplementation and should start at 0.5 to 2.0 mg/kg/d in adults with acquired disease.29,30 Zinc sulfate is the most affordable and is the supplement of choice, with 50 mg of elemental zinc per 220 mg of zinc sulfate (~23% elemental zinc).31 Alternative zinc salts, such as zinc gluconate (13% elemental zinc), may be used. Patients with malabsorptive disorders often require parenteral supplementation.32 Clinical symptoms often will resolve within 1 to 2 weeks of supplementation.29 In patients with primary acrodermatitis enteropathica, lifelong supplementation with 3 mg/kg/d elemental zinc should occur.6 Calcium and folate may reduce zinc absorption, while zinc supplementation can interfere with copper and iron absorption.33
Iron Deficiency
Iron is an essential component of the hemoglobin molecule. Iron homeostasis and metabolism are tightly regulated processes that drive erythropoiesis. Only 5% to 10% of dietary iron is absorbed through nutrition, while the remainder is recycled from red cell breakdown. Both normal iron levels and iron deficiency (ID) are defined by age and gender.34 Iron-deficiency anemia (IDA) is one of the most common cause-specific anemias worldwide.35
Fatigue is the most common and earliest symptom of ID. In a single study, pallor was predictive of anemia in hospitalized patients; however, absence of pallor did not rule out anemia.34 Dyspnea on exertion, tachycardia, dysphagia, and pica also may be reported. Cutaneous manifestations include koilonychia (Figure 2), glossitis, pruritus, angular cheilitis, and telogen effluvium. Plummer-Vinson syndrome is characterized by microcytic anemia, glossitis, and dysphagia.
Risk factors for ID include insufficient dietary consumption,36 blood loss, malabsorptive states,37,38 and increased iron requirements (eTable). Patient fragility (eg, elderly, chronic disease) is a newly described risk factor where correction of ID may impact morbidity, mortality, and quality of life.35
Iron deficiency can be present despite a normal hemoglobin level. Serum ferritin and percentage transferrin saturation are key to early identification of IDA.35 Ferritin levels lower than 30 µg/L confirm the diagnosis. Decreased transferrin saturation and increased total iron binding capacity aid in the diagnosis of IDA. Serum ferritin is an acute-phase reactant, and levels may be falsely elevated in the setting of inflammation or infection.
Treatment includes reversing the cause of deficiency and supplementing iron. Calculation of the total iron deficit can help inform iron supplementation. First-line therapy for IDA is oral ferrous sulfate 325 mg (65 mg elemental iron) 3 times daily. Newer studies suggest 40 to 80 mg oral iron should be taken every other day to increase absorption.39 Other iron salts, such as ferrous gluconate (325 mg is equivalent to 38 mg elemental iron), have been used. Iron absorption is enhanced by an acidic environment. Parenteral iron is utilized in patients with uncorrectable blood loss, malabsorption, renal failure, intolerance to oral iron, and nonadherence in those who are unable to receive transfusions. Iron infusions are favored in frail patients, such as the elderly and those with chronic kidney disease or heart failure.35 Multiple parenteral iron formulations exist, and their use should be driven by underlying patient comorbidities and potential risks. Packed red blood cell transfusions should be considered in acute blood loss, hypoxia, or cardiac insufficiency.
Essential Fatty Acid Deficiency
Essential fatty acids (EFAs) including linoleic and α-linolenic acid cannot be synthesized by the human body and must be obtained through diet (mostly plant oils). Essential fatty acids have various functions, including maintaining phospholipid membrane integrity, forming prostaglandins and leukotrienes, and storing energy.40 Essential fatty acids are important in the structure and function of the stratum corneum and are crucial in maintaining epidermal barrier function.41 Increased epidermal permeability and transepidermal water loss may be the first signs of EFA deficiency (EFAD).42
The cutaneous manifestations of EFAD include xerosis, weeping eczematous plaques, and erosions in intertriginous sites. The lesions may progress to widespread desquamation and erythema. With time, the skin can become thick and leathery. Alopecia may occur, and hair may depigment.7 Additional findings include poor wound healing and increased susceptibility to infections.43,44
Essential fatty acid deficiency may occur when dietary fat intake is severely restricted or in malabsorptive states.45,46 It develops in patients on prolonged TPN, typically when receiving fat-restricted nutrition,47,48 as occurs in hypertriglyceridemia.47 Essential fatty acid deficiency has developed in patients on TPN containing EFAs,47 as the introduction of novel intravenous lipid emulsions has resulted in varying proportions of EFA.40 Premature neonates are particularly at risk for EFAD.49
The diagnosis of EFAD involves the measurement of the triene to tetraene ratio. A ratio of more than 0.2 suggests EFAD, but the clinical signs are not seen until the ratio is over 0.4.40 Low plasma levels of linoleic, linolenic, and arachidonic acids also are seen. Elevated liver function tests are supportive of the diagnosis. Biochemical findings typically are seen before cutaneous manifestations.40
Treatment of EFAD includes topical, oral, or intravenous replacement of EFAs. Improvement of EFAD with the application of topical linoleic acid to the skin has been reported.50 Patients receiving TPN should undergo assessment of parenteral lipid emulsion to ensure adequate fatty acid composition.
Vitamin A Deficiency
Vitamin A (retinol) is a fat-soluble vitamin that plays a critical role in keratinization, epithelial proliferation, and cellular differentiation.6 Vitamin A is found in animal products as retinyl esters and in plants as beta-carotene. Vitamin A has 2 clinically important forms: all-trans retinoic acid and 11-cis-retinal. All-trans retinoic acid is involved in cellular differentiation and regulating gene transcription, while 11-cis-retinal is key to rhodopsin generation required for vision. Vitamin A deficiency presents with early ophthalmologic findings, specifically nyctalopia, or delayed adaptation to the dark.51 Xerophthalmia, abnormal conjunctival keratinization, and Bitot spots subsequently develop and may progress to corneal ulceration and blindness.6
Vitamin A deficiency manifests in the skin as follicular hyperkeratosis, or phrynoderma. Notably, numerous other micronutrient deficiencies may result in phrynoderma. Clinically, multiple pigmented keratotic papules of various sizes, many with a central keratinous plug, are distributed symmetrically on the extensor elbows, knees, shoulders, buttocks, and extremities. The skin surrounding these lesions may be scaly and hyperpigmented.52 Generalized xerosis without preceding nyctalopia has been reported.53 Accompanying pityriasis alba may develop.52 Lesions on the face may mimic acne, while lesions on the extremities may simulate a perforating disorder. Histopathology of phrynoderma reveals epidermal hyperkeratosis, follicular hyperkeratosis, and follicular plugging.52
Patients at risk for vitamin A deficiency include those with conditions that affect intestinal fat absorption, underlying psychiatric illness, or chronic disease (eTable). Chronic alcohol use predisposes patients to a multitude of micronutrient deficiencies, including vitamin A deficiency.54 In chronic alcohol use, even mild cutaneous changes may be the first clue to low serum retinol.55
Vitamin A deficiency can be diagnosed by measuring serum retinol levels, with levels lower than 20 µg/dL being diagnostic of deficiency.56 Decreased serum retinol in patients hospitalized with flaring irritable bowel disorder has been repeatedly reported.57-59 Notably, serum retinol concentration does not decline until liver reserves of vitamin A are nearing exhaustion.33
The US Food and Drug Administration requires manufacturers to list retinol activity equivalents on labels. One international unit of retinol is equivalent to 0.3 µg of retinol activity equivalents.60 The treatment of vitamin A deficiency involves high-dose oral supplementation when possible.61 Although dependent on age, the treatment dose for most adults with vitamin A deficiency is 3000 µg (10,000 IU) once daily.
Phrynoderma has been specifically treated with salicylic acid ointment 3% and intramuscular vitamin A.62 Topical urea cream also may treat phrynoderma.63
Vitamin B2
Vitamin B2 (riboflavin) is absorbed in the small intestine and converted into 2 biologically active forms—flavin adenine dinucleotide and flavin mononucleotide—which serve as cofactors in metabolic and oxidation-reduction reactions. Malabsorptive disorders and bowel resection can lead to riboflavin deficiency.64 Other at-risk populations include those with restrictive diets,65 psychiatric illness, or systemic illness (eTable). Riboflavin can be degraded by light (deficiency has been reported after phototherapy for neonatal jaundice66) and following boric acid ingestion.67 Medications, including long-term treatment with antiepileptics, may lead to riboflavin deficiency.68
Riboflavin is critical to maintaining collagen production. Riboflavin deficiency may manifest clinically with extensive seborrheiclike dermatitis,44 intertrigolike dermatitis,69 or oral-ocular-genital syndrome.70 Angular cheilitis may accompany an atrophic tongue that is deep red in color. The scrotum is characteristically involved in men, with confluent dermatitis extending onto the thighs and sparing the midline. Red papules and painful fissures may develop. Balanitis and phimosis have been reported. Testing for riboflavin deficiency should be considered in patients with refractory seborrheic dermatitis.
Riboflavin stores are assessed by the erythrocyte glutathione reductase activity coefficient.44 A level of 1.4 or higher is consistent with deficiency. Serum riboflavin levels, performed after a 12-hour fast, may support the diagnosis but are less sensitive. Patients with glucose-6-phosphate deficiency cannot be assessed via the erythrocyte glutathione reductase activity coefficient and may instead require evaluation of 24-hour urine riboflavin level.44
Vitamin B3
Vitamin B3 (niacin, nicotinamide, nicotinic acid) is found in plant and animal products or can be derived from its amino acid precursor tryptophan. Niacin deficiency results in pellagra, characterized by dermatitis, dementia, and diarrhea.71 The most prominent feature is a symmetrically distributed photosensitive dermatitis of the face, neck (called Casal necklace)(Figure 3), chest, dorsal hands, and extensor arms. The eruption may begin with erythema, vesicles, or bullae (wet pellagra) and evolve into thick, hyperpigmented, scaling plaques.71 The skin may take on a copper tone and become atrophic.72 Dull erythema with overlying yellow powdery scale (called sulfur flakes) at follicular orifices has been described on the nasal bridge.73
Causes of niacin deficiency include malabsorptive conditions, malignancy (including carcinoid tumors), parenteral nutrition, psychiatric disease,74,75 and restrictive diets (eTable).76 Carcinoid tumors divert tryptophan to serotonin resulting in niacin deficiency.77
The diagnosis of niacin deficiency is based on clinical findings and response to supplementation.75 Low niacin urinary metabolites (N-methylnicotinamide and 2-pyridone) may aid in diagnosis.6 Treatment generally includes oral nicotinamide 100 mg every 6 hours; the dose can then be tapered to 50 mg every 8 to 12 hours until symptoms resolve. Severe deficiency may require parenteral nicotinamide 1 g 3 to 4 times daily.75
Vitamin B6
Vitamin B6 (pyridoxine, pyridoxamine, pyridoxal) is found in whole grains and plant and animal products. Vitamin B6 functions as a coenzyme in many metabolic pathways and is involved in the conversion of tryptophan to niacin.44 Absorption requires hydrolysis by intestinal phosphates and transport to the liver for rephosphorylation prior to release in active form.6
Cutaneous findings associated with vitamin B6 deficiency include periorificial and perineal seborrheic dermatitis,78 angular stomatitis, and cheilitis, with associated burning, redness, and tongue edema.6 Vitamin B6 deficiency is a rarely reported cause of burning mouth syndrome.79 Because vitamin B6 is involved in the conversion of tryptophan to niacin, deficiency also may present with pellagralike findings.70 Other clinical symptoms are outlined in the eTable.80,81
Conditions that increase risk for vitamin B6 deficiency are highlighted in the eTable and include malabsorptive disorders; psychiatric illness82; and chronic disease, especially end-stage renal disease.83 Vitamin B6 deficiency associated with chronic alcohol use is due to both inadequate vitamin B6 intake as well as reduced hepatic storage.78 Medications such as isoniazid, hydralazine, and oral contraceptives may decrease vitamin B6 levels (eTable).82
Vitamin B6 can be measured in the plasma as pyridoxal 5′-phosphate. Plasma concentrations of less than 20 nmol/L are suggestive of deficiency.82 Indirect tests include tryptophan and methionine loading.6 The treatment of vitamin B6 deficiency is determined by symptom severity. Recommendations for oral supplementation range from 25 to 600 mg daily.82 Symptoms typically improve on 100 mg daily.6
Vitamins B9 and B12
Deficiencies of vitamins B9 (folic acid, folate) and B12 (cobalamin) have similar clinical presentations. Folate is essential in the metabolism of amino acids, purines, and pyrimidines.6 Cobalamin, found in animal products, is a cofactor for methionine synthase and methylmalonyl-CoA mutase.84 Megaloblastic anemia is the main finding in folate or cobalamin deficiency. Neurologic findings only accompany cobalamin deficiency. Risk factors for folate deficiency include malabsorptive conditions,6 chronic alcohol use,85 and antifolate medication use (eTable).6
Cobalamin absorption requires gastric acid and intrinsic factor binding in the duodenum. Deficiency may occur from strict diets, psychiatric illness, old age,86 decreased gastric acid secretion,87 abnormal intrinsic factor function, or intestinal infections.6
Generalized cutaneous hyperpigmentation may be the first manifestation of vitamins B9 and B12 deficiency.88 Typically accentuated in acral creases and the oral cavity, pigmentation may mimic Addison disease. Hair depigmentation and linear streaking of the nails are reported.84 The tongue becomes painful and red with atrophy of the filiform papillae (Hunter glossitis).78 Linear lesions on the tongue and hard palate may serve as an early sign of cobalamin deficiency.89
Folate deficiency is diagnosed by measuring the plasma folate level; coincidental cobalamin deficiency should be excluded. Deficiency is managed with oral supplementation (when possible) with 1 to 5 mg of folate daily.6 Cobalamin deficiency is based on low serum levels (<150 pg/mL is diagnostic).86 Cobalamin deficiency may take years to develop, as vitamin B12 exists in large body stores.6 Serum methylmalonic acid may be elevated in patients with clinical features but normal-low serum vitamin B12 level.86 Treatment of vitamin B12 deficiency is with oral (2 mg once daily) or parenteral (1 mg every 4 weeks then maintained at once monthly) cyanocobalamin. For patients with neurologic symptoms, intramuscular injection should be given.86 The underlying cause of deficiency must be elucidated and treated.
Vitamin C Deficiency
Vitamin C (ascorbic acid) is an essential cofactor for the hydroxylation of proline and lysine residues in collagen synthesis. Plant-based foods are the main dietary source of vitamin C, and deficiency presents clinically as scurvy. Cutaneous findings include follicular hyperkeratosis, perifollicular petechiae, and curled hair shafts (corkscrew hairs)(Figure 4). Ecchymoses of the lower extremities, forearms, and abdomen may be seen. Nodules representing intramuscular and subcutaneous hemorrhage can be present.90 Woody edema may mimic cellulitis, while lower extremity hemorrhage may mimic vasculitis. Gingival hyperplasia, hemorrhage, and edema may occur,90 along with linear splinter hemorrhages.91
Hypovitaminosis C has been routinely demonstrated in hospitalized patients.92 Scurvy may occur in patients on strict diets,93 chronic alcohol use,94 psychiatric illness,95 or gastrointestinal tract disease (eTable).96-99 Those with low socioeconomic status70 or dementia100 as well as the elderly also are at risk.101 Scurvy has developed in patients with iron overload and those who are on hemodialysis44 as well as in association with nilotinib use.102 Patients with chronic mucous membrane graft-vs-host disease may exhibit vitamin C deficiency.103
Scurvy is a clinical diagnosis. Vitamin C levels normalize quickly with supplementation. Cutaneous biopsy will exhibit follicular hyperkeratosis, perifollicular hemorrhage, and fibrosis.91
Oral ascorbic acid supplementation should be initiated at 500 to 1000 mg daily in adults.104 The cause of deficiency should be identified, and further supplementation should be decided based on patient risk factors. Lifestyle modifications, such as cessation of smoking and chronic alcohol use, is recommended. The diagnosis of scurvy should prompt workup for additional nutrient deficiencies.
Final Thoughts
Dermatologists play an important role in the early recognition of nutritional deficiencies, as cutaneous manifestations often are the first clue to diagnosis. Nutritional deficiencies are common yet underrecognized in the hospitalized patient and serve as an independent risk factor for patient morbidity and mortality.3 Awareness of the cutaneous manifestations of undernutrition as well as the risk factors for nutritional deficiency may expedite diagnosis and supplementation, thereby improving outcomes for hospitalized patients.
- Mehta NM, Corkins MR, Lyman B, et al. Defining pediatric malnutrition: a paradigm shift toward etiology-related definitions. JPEN J Parenter Enteral Nutr. 2013;37:460-481.
- Barker LA, Gout BS, Crowe TC. Hospital malnutrition: prevalence, identification and impact on patients and the healthcare system. Int J Environ Res Public Health. 2011;8:514-527.
- Bharadwaj S, Ginoya S, Tandon P, et al. Malnutrition: laboratory markers vs nutritional assessment. Gastroenterol Rep (Oxf). 2016;4:272-280.
- Basavaraj KH, Seemanthini C, Rashmi R. Diet in dermatology: present perspectives. Indian J Dermatol. 2010;55:205-210.
- Grover Z, Ee LC. Protein energy malnutrition. Pediatr Clin North Am. 2009;56:1055-1068.
- Jen M, Yan AC. Syndromes associated with nutritional deficiency and excess. Clin Dermatol. 2010;28:669-685.
- Lekwuttikarn R, Teng JMC. Cutaneous manifestations of nutritional deficiency. Curr Opin Pediatr. 2018;30:505-513.
- Jaffe AT, Heymann WR. Kwashiorkor/zinc deficiency overlap following partial gastrectomy. Int J Dermatol. 1998;37:134-137.
- Listernick R, Christoffel K, Pace J, et al. Severe primary malnutrition in US children. Am J Dis Child. 1985;139:1157-1160.
- Heilskov S, Rytter MJ, Vestergaard C, et al. Dermatosis in children with oedematous malnutrition (Kwashiorkor): a review of the literature. J Eur Acad Dermatol Venereol. 2014;28:995-1001.
- Bradfield RB. Hair tissue as a medium for the differential diagnosis of protein-calorie malnutrition: a commentary. J Pediatr. 1974;84:294-296.
- Cohen PR. The nail flag sign: case report in a man with diverticulitis and review of dermatology flag sign of the hair, skin, and nails. Cureus. 2018;10:e2929.
- Management of Severe Malnutrition: A Manual for Physicians and Other Senior Health Workers. Geneva, Switzerland: World Health Organization; 1999. https://www.who.int/nutrition/publications/en/manage_severe_malnutrition_eng.pdf. Accessed May 19, 2020.
- Keller U. Nutritional laboratory markers in malnutrition. J Clin Med. 2019;8:775.
- Thavaraj V, Sesikeran B. Histopathological changes in skin of children with clinical protein energy malnutrition before and after recovery. J Trop Pediatr. 1989;35:105-108.
- McClave SA, Heyland DK. The physiologic response and associated clinical benefits from provision of early enteral nutrition. Nutr Clin Pract. 2009;24:305-315.
- Ogawa Y, Kinoshita M, Shimada S, et al. Zinc and skin disorders. Nutrients. 2018;10:199.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007;56:116-124.
- Wiznia LE, Bhansali S, Brinster N, et al. Acquired acrodermatitis enteropathica due to zinc-depleted parenteral nutrition. Pediatr Dermatol. 2019;36:520-523.
- Sandstead HH, Freeland-Graves JH. Dietary phytate, zinc and hidden zinc deficiency. J Trace Elem Med Biol. 2014;28:414-417.
- Vagianos K, Bector S, McConnell J, et al. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2007;31:311-319.
- Schoelmerich J, Becher MS, Hoppe-Seyler P, et al. Zinc and vitamin A deficiency in patients with Crohn’s disease is correlated with activity but not with localization or extent of the disease. Hepatogastroenterology. 1985;32:34-38.
- Siva S, Rubin DT, Gulotta G, et al. Zinc deficiency is associated with poor clinical outcomes in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:152-157.
- Semrad CE. Zinc and intestinal function. Curr Gastroenterol Rep. 1999;1:398-403.
- Sinclair SA, Reynolds NJ. Necrolytic migratory erythema and zinc deficiency. Br J Dermatol. 1997;136:783-785.
- Gammoh NZ, Rink L. Zinc in infection and inflammation. Nutrients. 2017;9:624.
- Gonzalez JR, Botet MV, Sanchez JL. The histopathology of acrodermatitis enteropathica. Am J Dermatopathol. 1982;4:303-311.
- Wu D, Fung MA, Kiuru M, et al. Acquired bullous acrodermatitis enteropathica as a histologic mimic of pemphigus foliaceus in a patient on parenteral nutrition. Dermatol Online J. 2018;24:20.
- Maxfield L, Crane J. Zinc Deficiency. Treasure Island, FL: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK493231/Updated November 14, 2019. Accessed May 19, 2020.
- Macdonald JB, Connolly SM, DiCaudo DJ. Think zinc deficiency: acquired acrodermatitis enteropathica due to poor diet and common medications. Arch Dermatol. 2012;148:961-963.
- Wegmüller R, Tay F, Zeder C, et al. Zinc absorption by young adults from supplemental zinc citrate is comparable with that from zinc gluconate and higher than from zinc oxide. J Nutr. 2014;144:132-136.
- Vick G, Mahmoudizad R, Fiala K. Intravenous zinc therapy for acquired zinc deficiency secondary to gastric bypass surgery: a case report. Dermatol Ther. 2015;28:222-225.
- Ghishan FK, Kiela PR. Vitamins and minerals in inflammatory bowel disease. Gastroenterol Clin North Am. 2017;46:797-808.
- Killip S, Bennett JM, Chambers MD. Iron deficiency anemia. Am Fam Physician. 2007;75:671-678.
- De Franceschi L, Iolascon A, Taher A, et al. Clinical management of iron deficiency anemia in adults: systemic review on advances in diagnosis and treatment. Eur J Intern Med. 2017;42:16-23.
- Haider LM, Schwingshackl L, Hoffmann G, et al. The effect of vegetarian diets on iron status in adults: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2018;58:1359-1374.
- Enani G, Bilgic E, Lebedeva E, et al. The incidence of iron deficiency anemia post-Roux-en-Y gastric bypass and sleeve gastrectomy: a systematic review [published online September 4, 2019]. Surg Endosc. doi:10.1007/s00464-019-07092-3.
- Kaitha S, Bashir M, Ali T. Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol. 2015;6:62-72.
- Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126:1981-1989.
- Gramlich L, Meddings L, Alberda C, et al. Essential fatty acid deficiency in 2015: the impact of novel intravenous lipid emulsions. JPEN J Parenter Enteral Nutr. 2015;39(1 suppl):61S-66S.
- Khnykin D, Miner JH, Jahnsen F. Role of fatty acid transporters in epidermis: implications for health and disease. Dermatoendocrinol. 2011;3:53-61.
- Wright S. Essential fatty acids and the skin. Br J Dermatol. 1991;125:503-515.
- Lakdawala N, Grant-Kels JM. Acrodermatitis caused by nutritional deficiency and metabolic disorders. Clin Dermatol. 2017;35:64-67.
- DiBaise M, Tarleton SM. Hair, nails, and skin: differentiating cutaneous manifestations of micronutrient deficiency. Nutr Clin Pract. 2019;34:490-503.
- Aldámiz-Echevarría L, Bilbao A, Andrade F, et al. Fatty acid deficiency profile in children with food allergy managed with elimination diets. Acta Paediatr. 2008;97:1572-1576.
- Jeppesen PB, Christensen MS, Høy CE, et al. Essential fatty acid deficiency in patients with severe fat malabsorption. Am J Clin Nutr. 1997;65:837-843.
- Roongpisuthipong W, Phanachet P, Roongpisuthipong C, et al. Essential fatty acid deficiency while a patient receiving fat regimen total parenteral nutrition [published online June 14, 2012]. BMJ Case Rep. doi:10.1136/bcr.07.2011.4475.
- Fleming CR, Smith LM, Hodges RE. Essential fatty acid deficiency in adults receiving total parenteral nutrition. Am J Clin Nutr. 1976;29:976-983.
- Cooke RJ, Zee P, Yeh YY. Essential fatty acid status of the premature infant during short-term fat-free parenteral nutrition. J Pediatr Gastroenterol Nutr. 1984;3:446-449.
- Skolnik P, Eaglstein WH, Ziboh VA. Human essential fatty acid deficiency: treatment by topical application of linoleic acid. Arch Dermatol. 1977;113:939-941.
- Vahlquist A. Clinical use of vitamin A and its derivatives—physiological and pharmacological aspects. Clin Exp Dermatol. 1985;10:133-143.
- Ragunatha S, Kumar VJ, Murugesh SB. A clinical study of 125 patients with phrynoderma. Indian J Dermatol. 2011;56:389-392.
- Phanachet P, Shantavasinkul PC, Chantrathammachart P, et al. Unusual manifestation of vitamin A deficiency presenting with generalized xerosis without night blindness. Clin Case Rep. 2018;6:878-882.
- Fuchs J. Alcoholism, malnutrition, vitamin deficiencies, and the skin. Clin Dermatol. 1999;17:457-461.
- Uhoda E, Petit L, Piérard-Franchimont C, et al. Ultraviolet light-enhanced visualization of cutaneous signs of carotene and vitamin A dietary deficiency. Acta Clin Belg. 2004;59:97-101.
- de Pee S, Dary O. Biochemical indicators of vitamin A deficiency: serum retinol and serum retinol binding protein. J Nutr. 2002;132(9 suppl):2895S-2901S.
- Fernandez-Banares F, Abad-Lacruz A, Xiol X, et al. Vitamin status in patients with inflammatory bowel disease. Am J Gastroenterol. 1989;84:744-748.
- Main AN, Mills PR, Russell RI, et al. Vitamin A deficiency in Crohn’s disease. Gut. 1983;24:1169-1175.
- Cobos G, Cornejo C, McMahon P. A case of phrynoderma in a patient with Crohn’s disease. Pediatr Dermatol. 2015;32:234-236.
- Trumbo P, Yates AA, Schlicker S, et al. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001;101:294-301.
- Ross DA. Recommendations for vitamin A supplementation. J Nutr. 2002;132(9 suppl):2902S-2906S.
- Ragunatha S, Jagannath Kumar V, Murugesh SB, et al. Therapeutic response of vitamin A, vitamin B complex, essential fatty acids (EFA) and vitamin E in the treatment of phrynoderma: a randomized controlled study. J Clin Diagn Res. 2014;8:116-118.
- Nakjang Y, Yuttanavivat T. Phrynoderma: a review of 105 cases. J Dermatol. 1988;15:531-534.
- Pinto JT, Zempleni J. Riboflavin. Adv Nutr. 2016;7:973-975.
- Larsson CL, Johansson GK. Dietary intake and nutritional status of young vegans and omnivores in Sweden. Am J Clin Nutr. 2002;76:100-106.
- Gromisch DS, Lopez R, Cole HS, et al. Light (phototherapy)—induced riboflavin deficiency in the neonate. J Pediatr. 1977;90:118-122.
- Pinto J, Huang YP, McConnell RJ, et al. Increased urinary riboflavin excretion resulting from boric acid ingestion. J Lab Clin Med. 1978;92:126-134.
- Soltani D, Ghaffar Pour M, et al. Nutritional aspects of treatment in epileptic patients. Iran J Child Neurol. 2016;10:1-12.
- Roe DA. Riboflavin deficiency: mucocutaneous signs of acute and chronic deficiency. Semin Dermatol. 1991;10:293-295.
- Galimberti F, Mesinkovska NA. Skin findings associated with nutritional deficiencies. Cleve Clin J Med. 2016;83:731-739.
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol. 2002;41:476-481.
- Nogueira A, Duarte AF, Magina S, et al. Pellagra associated with esophageal carcinoma and alcoholism. Dermatol Online J. 2009;15:8.
- Wan P, Moat S, Anstey A. Pellagra: a review with emphasis on photosensitivity. Br J Dermatol. 2011;164:1188-1200.
- Jagielska G, Tomaszewicz-Libudzic EC, Brzozowska A. Pellagra: a rare complication of anorexia nervosa. Eur Child Adolesc Psychiatry. 2007;16:417-420.
- Li R, Yu K, Wang Q, et al. Pellagra secondary to medication and alcoholism: a case report and review of the literature. Nutr Clin Pract. 2016;31:785-789.
- Ladoyanni E, Cheung ST, North J, et al. Pellagra occurring in a patient with atopic dermatitis and food allergy. J Eur Acad Dermatol Venereol. 2007;21:394-396.
- Bell HK, Poston GJ, Vora J, et al. Cutaneous manifestations of the malignant carcinoid syndrome. Br J Dermatol. 2005;152:71-75.
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol. 1986;15:1263-1274.
- Lamey PJ, Hammond A, Allam BF, et al. Vitamin status of patients with burning mouth syndrome and the response to replacement therapy. Br Dent J. 1986;160:81-84.
- Stover PJ, Field MS. Vitamin B-6. Adv Nutr. 2015;6:132-133.
- Gerlach AT, Thomas S, Stawicki SP, et al. Vitamin B6 deficiency: a potential cause of refractory seizures in adults. JPEN J Parenter Enteral Nutr. 2011;35:272-275.
- Spinneker A, Sola R, Lemmen V, et al. Vitamin B6 status, deficiency and its consequences—an overview. Nutr Hosp. 2007;22:7-24.
- Ross EA, Shah GM, Reynolds RD, et al. Vitamin B6 requirements of patients on chronic peritoneal dialysis. Kidney Int. 1989;36:702-706.
- Brescoll J, Daveluy S. A review of vitamin B12 in dermatology. Am J Clin Dermatol. 2015;16:27-33.
- Sanvisens A, Zuluaga P, Pineda M, et al. Folate deficiency in patients seeking treatment of alcohol use disorder. Drug Alcohol Depend. 2017;180:417-422.
- Langan RC, Goodbred AJ. Vitamin B12 deficiency: recognition and management. Am Fam Physician. 2017;96:384-389.
- Bradford GS, Taylor CT. Omeprazole and vitamin B12 deficiency. Ann Pharmacother. 1999;33:641-643.
- Srivastava N, Chand S, Bansal M, et al. Reversible hyperpigmentation as the first manifestation of dietary vitamin B12 deficiency. Indian J Dermatol Venereol Leprol. 2006;72:389-390.
- Graells J, Ojeda RM, Muniesa C, et al. Glossitis with linear lesions: an early sign of vitamin B12 deficiency. J Am Acad Dermatol. 2009;60:498-500.
- Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999;41:895-906; quiz 907-810.
- Shaath T, Fischer R, Goeser M, et al. Scurvy in the present times: vitamin C allergy leading to strict fast food diet. Dermatol Online J. 2016;22:13030/qt50b8w28b.
- Fain O, Pariés J, Jacquart B, et al. Hypovitaminosis C in hospitalized patients. Eur J Intern Med. 2003;14:419-425.
- Ahmad SA, Al Thobiti TA, El Toum M, et al. Florid scurvy in an autistic child on a ketogenic diet [published online November 19, 2018]. Pediatr Emerg Care. doi:10.1097/PEC.0000000000001695.
- Lux-Battistelli C, Battistelli D. Latent scurvy with tiredness and leg pain in alcoholics: an underestimated disease three case reports. Medicine (Baltimore). 2017;96:e8861.
- Christopher K, Tammaro D, Wing EJ. Early scurvy complicating anorexia nervosa. South Med J. 2002;95:1065-1066.
- Berger ML, Siegel DM, Lee EL. Scurvy as an initial manifestation of Whipple’s disease. Ann Intern Med. 1984;101:58-59.
- Imes S, Dinwoodie A, Walker K, et al. Vitamin C status in 137 outpatients with Crohn’s disease. effect of diet counseling. J Clin Gastroenterol. 1986;8:443-446.
- Echeverría Zudaire L, García Cuartero B, Campelo Moreno O, et al. Scurvy associated with celiac disease [in Spanish]. An Esp Pediatr. 2002;57:587.
- Hansen EP, Metzsche C, Henningsen E, et al. Severe scurvy after gastric bypass surgery and a poor postoperative diet. J Clin Med Res. 2012;4:135-137.
- Rivière S, Birlouez-Aragon I, Nourhashémi F, et al. Low plasma vitamin C in Alzheimer patients despite an adequate diet. Int J Geriatr Psychiatry. 1998;13:749-754.
- Bhattacharyya P, Giannoutsos J, Eslick GD, et al. Scurvy: an unrecognized and emerging public health issue in developed economies. Mayo Clin Proc. 2019;94:2594-2597.
- Oak AS, Jaleel T, Fening K, et al. A case of scurvy associated with nilotinib. J Cutan Pathol. 2016;43:725-726.
- Kletzel M, Powers K, Hayes M. Scurvy: a new problem for patients with chronic GVHD involving mucous membranes; an easy problem to resolve. Pediatr Transplant. 2014;18:524-526.
- Maxfield L, Crane JS. Vitamin C Deficiency (Scurvy). Treasure Island, FL: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK493187/. Updated November 19, 2019. Accessed May 19, 2020.
The World Health Organization defines malnutrition as deficiencies, excesses, or imbalances in an individual’s intake of energy and/or nutrients.1 This review will focus on undernutrition, which may result from macronutrient or micronutrient deficiencies. Undernutrition in the hospitalized patient is a common yet underrecognized phenomenon, with an estimated prevalence of 20% to 50% worldwide.2 Malnutrition is an independent risk factor for patient morbidity and mortality and has been associated with increased health care costs.3 Nutritional deficiencies may arise from inadequate nutrient intake, abnormal nutrient absorption, or improper nutrient utilization.4 Unfortunately, no standardized algorithm for screening and diagnosing patients with malnutrition exists, making early physical examination findings of utmost importance. Herein, we present a review of acquired nutritional deficiency dermatoses in the inpatient setting.
Protein-Energy Malnutrition
Protein-energy malnutrition (PEM) refers to a set of related disorders that include marasmus, kwashiorkor (KW), and marasmic KW. These conditions frequently are seen in developing countries but also have been reported in developed nations.5 Marasmus occurs from a chronic deficiency of protein and calories. Decreased insulin production and unopposed catabolism result in sarcopenia and loss of bone and subcutaneous fat.6 Affected patients include children who are less than 60% ideal body weight (IBW) without edema or hypoproteinemia.7 Kwashiorkor is the edematous form of PEM that develops from isolated protein deficiency, resulting in edema, diarrhea, and immunosuppression.6 Micronutrient deficiencies, oxidative stress, slow protein catabolism, and excess antidiuretic hormone have been proposed as potential drivers of KW.8 Kwashiorkor affects children between 60% and 80% IBW. Marasmic KW has features of both diseases, including children who are less than 60% IBW but with associated edema and/or hypoproteinemia.9
Although PEM is uncommon in adults, hospitalized patients carry many predisposing risk factors, including infections, malabsorptive conditions, psychiatric disease, and chronic illness (eTable). Patients with chronic infections present with findings consistent with marasmic KW due to lean body mass loss.

The cutaneous findings in PEM are related to dysmaturation of epidermal keratinocytes and resultant epidermal atrophy.10 Patients with marasmus exhibit dry, wrinkled, loose skin due to subcutaneous fat loss. Emaciated children often lose their buccal fat pads, and reduced perianal adipose may lead to rectal prolapse. Increased lanugo hair may be present on the face, and alopecia of the scalp may occur.6 In KW, cutaneous disease progresses from confluent hyperkeratosis to a dry atrophic epidermis that erodes easily, leaving underlying pale erythema. The resultant pattern is one of hyperpigmented plaques with slightly raised borders, and hypopigmented patches and erosions described as flaky paint dermatitis (Figure 1).5 Lesions appear first in areas of friction. The hair often is dry and brittle; curly hair may straighten and scale.11 Red-yellow to gray-white hypopigmentation may develop, denoting periods of inadequate nutrition. The flag sign describes alternating horizontal bands of hypopigmentation interspersed with bands of pigmented hair. The nails usually are thin and soft and may exhibit the nail flag sign, characterized by horizontal bands of white and red.12 Cheilitis, angular stomatitis, and vulvovaginitis may be present.6

In adults, weight loss and body mass index can be used to assess nutritional status, along with a focused history and physical examination. Complete blood cell count, electrolyte levels, and blood urea nitrogen should be assessed, as hypoglycemia and anemia often accompany PEM.13 In KW, hypoalbuminemia and hypoproteinemia are invariably present. Although prealbumin may be a valid prognostic indicator of disease outcomes and mortality in patients at risk for malnutrition, checking other serum biomarkers remains controversial.14 Focused testing may be warranted in patients with risk factors for chronic infectious processes, such as human immunodeficiency virus or tuberculosis.6 Skin biopsy may solidify the diagnosis of PEM. Hypertrophy of the stratum corneum, atrophy of the stratum spinosum and stratum granulosum, and increased basal layer melanin have been reported.15
Treatment involves initial fluid resuscitation and correction of electrolyte imbalances, followed by nutritional replacement.13 Oral or enteral tube feedings are preferred over total parenteral nutrition (TPN), as they enhance recovery of the gastrointestinal tract.16 Refeeding should occur in small amounts and frequent intervals.5 Skin-directed therapy is aimed at restoring epidermal function and hydration, with regular moisturization and application of barrier creams, such as zinc oxide ointment or petrolatum.10
Zinc Deficiency
Zinc is an essential trace element that provides regulatory, structural, and catalytic functions across multiple biochemical pathways6 and serves as an enzymatic cofactor and key component for numerous transcription factors.17 Zinc is derived from food sources, and its concentration correlates with protein content.18 Zinc is found in both animal and plant-based proteins, albeit with a lower oral bioavailability in the latter. Zinc deficiency may be inherited or acquired. Primary acrodermatitis enteropathica is an autosomal-recessive disorder of the solute carrier family 39 member 4 gene, SLC39A4 (encodes zinc transporter ZIP4 on enterocytes); the result is abnormal zinc absorption from the small intestine.18
Acquired zinc deficiency occurs from decreased dietary zinc intake, impaired intestinal zinc absorption, excessive zinc elimination, or systemic states of high catabolism or low albumin (eTable). Total parenteral nutrition–associated deficiency has arisen when nutritional formulations did not contain trace elements during national shortages or when prolonged TPN was not anticipated and trace elements were removed.19 Zinc levels may already be low in patients with chronic illness or inflammation, so even a short period on TPN can precipitate deficiency.18,19 Diets high in phytate may result in zinc deficiency, as phytate impairs intestinal zinc absorption.20 Approximately 15% of patients with inflammatory bowel disease experienced zinc deficiency worldwide.21 In Crohn disease, zinc deficiency has been associated with active intestinal inflammation, increased risk for hospitalization, surgeries, and disease-related complications.22,23
Medications such as antiepileptics, antimetabolites, or penicillamine may induce zinc deficiency, highlighting the importance of medication review for hospitalized patients (eTable). Catabolic states, frequently encountered in hospitalized patients, increase the risk for zinc deficiency.24 Patients with necrolytic migratory erythema (associated with pancreatic glucagonomas) often experience low serum zinc levels.25
The skin is the third most zinc-abundant tissue in the human body. Within keratinocytes, zinc is critical to normal proliferation and suppression of inflammation.17 Zinc also plays an important role in cutaneous immune function.26 Zinc deficiency presents with sharply demarcated, flaccid pustules and bullae that erode into scaly, pink, eczematous or psoriasiform plaques. Lesions are found preferentially in acral and periorificial sites, often with crusting and exudate. The groin and flexural surfaces may be affected. Erosions often become secondarily impetiginized. Other cutaneous findings include angular cheilitis, stomatitis, glossitis, paronychia, onychodystrophy, generalized alopecia, and delayed wound healing.26 Histopathology of skin lesions is characterized by granular layer loss, epidermal pallor, confluent parakeratosis, spongiosis, dyskeratosis, and psoriasiform hyperplasia.27 Acquired bullous acrodermatitis enteropathica has been reported as a histologic mimicker of pemphigus foliaceous in patients on TPN.28
Diagnosis of zinc deficiency is made by measuring plasma zinc levels. Fasting levels should be drawn in the morning, as they can fluctuate based on the time of day, stress levels, or inflammation.6 Sample hemolysis and anticoagulants high in zinc may falsely elevate plasma zinc. A normal zinc level is greater than 70 µg/dL; however, normal levels do not rule out deficiency.18 Measurement of zinc-dependent enzymes, such as alkaline phosphatase, can be a quick way to assess zinc status. Serum albumin also should be measured; because zinc is carried by albumin in the blood, hypoalbuminemia may result in secondary zinc deficiency.18
Zinc replacement therapy is largely through oral supplementation and should start at 0.5 to 2.0 mg/kg/d in adults with acquired disease.29,30 Zinc sulfate is the most affordable and is the supplement of choice, with 50 mg of elemental zinc per 220 mg of zinc sulfate (~23% elemental zinc).31 Alternative zinc salts, such as zinc gluconate (13% elemental zinc), may be used. Patients with malabsorptive disorders often require parenteral supplementation.32 Clinical symptoms often will resolve within 1 to 2 weeks of supplementation.29 In patients with primary acrodermatitis enteropathica, lifelong supplementation with 3 mg/kg/d elemental zinc should occur.6 Calcium and folate may reduce zinc absorption, while zinc supplementation can interfere with copper and iron absorption.33
Iron Deficiency
Iron is an essential component of the hemoglobin molecule. Iron homeostasis and metabolism are tightly regulated processes that drive erythropoiesis. Only 5% to 10% of dietary iron is absorbed through nutrition, while the remainder is recycled from red cell breakdown. Both normal iron levels and iron deficiency (ID) are defined by age and gender.34 Iron-deficiency anemia (IDA) is one of the most common cause-specific anemias worldwide.35
Fatigue is the most common and earliest symptom of ID. In a single study, pallor was predictive of anemia in hospitalized patients; however, absence of pallor did not rule out anemia.34 Dyspnea on exertion, tachycardia, dysphagia, and pica also may be reported. Cutaneous manifestations include koilonychia (Figure 2), glossitis, pruritus, angular cheilitis, and telogen effluvium. Plummer-Vinson syndrome is characterized by microcytic anemia, glossitis, and dysphagia.

Risk factors for ID include insufficient dietary consumption,36 blood loss, malabsorptive states,37,38 and increased iron requirements (eTable). Patient fragility (eg, elderly, chronic disease) is a newly described risk factor where correction of ID may impact morbidity, mortality, and quality of life.35
Iron deficiency can be present despite a normal hemoglobin level. Serum ferritin and percentage transferrin saturation are key to early identification of IDA.35 Ferritin levels lower than 30 µg/L confirm the diagnosis. Decreased transferrin saturation and increased total iron binding capacity aid in the diagnosis of IDA. Serum ferritin is an acute-phase reactant, and levels may be falsely elevated in the setting of inflammation or infection.
Treatment includes reversing the cause of deficiency and supplementing iron. Calculation of the total iron deficit can help inform iron supplementation. First-line therapy for IDA is oral ferrous sulfate 325 mg (65 mg elemental iron) 3 times daily. Newer studies suggest 40 to 80 mg oral iron should be taken every other day to increase absorption.39 Other iron salts, such as ferrous gluconate (325 mg is equivalent to 38 mg elemental iron), have been used. Iron absorption is enhanced by an acidic environment. Parenteral iron is utilized in patients with uncorrectable blood loss, malabsorption, renal failure, intolerance to oral iron, and nonadherence in those who are unable to receive transfusions. Iron infusions are favored in frail patients, such as the elderly and those with chronic kidney disease or heart failure.35 Multiple parenteral iron formulations exist, and their use should be driven by underlying patient comorbidities and potential risks. Packed red blood cell transfusions should be considered in acute blood loss, hypoxia, or cardiac insufficiency.
Essential Fatty Acid Deficiency
Essential fatty acids (EFAs) including linoleic and α-linolenic acid cannot be synthesized by the human body and must be obtained through diet (mostly plant oils). Essential fatty acids have various functions, including maintaining phospholipid membrane integrity, forming prostaglandins and leukotrienes, and storing energy.40 Essential fatty acids are important in the structure and function of the stratum corneum and are crucial in maintaining epidermal barrier function.41 Increased epidermal permeability and transepidermal water loss may be the first signs of EFA deficiency (EFAD).42
The cutaneous manifestations of EFAD include xerosis, weeping eczematous plaques, and erosions in intertriginous sites. The lesions may progress to widespread desquamation and erythema. With time, the skin can become thick and leathery. Alopecia may occur, and hair may depigment.7 Additional findings include poor wound healing and increased susceptibility to infections.43,44
Essential fatty acid deficiency may occur when dietary fat intake is severely restricted or in malabsorptive states.45,46 It develops in patients on prolonged TPN, typically when receiving fat-restricted nutrition,47,48 as occurs in hypertriglyceridemia.47 Essential fatty acid deficiency has developed in patients on TPN containing EFAs,47 as the introduction of novel intravenous lipid emulsions has resulted in varying proportions of EFA.40 Premature neonates are particularly at risk for EFAD.49
The diagnosis of EFAD involves the measurement of the triene to tetraene ratio. A ratio of more than 0.2 suggests EFAD, but the clinical signs are not seen until the ratio is over 0.4.40 Low plasma levels of linoleic, linolenic, and arachidonic acids also are seen. Elevated liver function tests are supportive of the diagnosis. Biochemical findings typically are seen before cutaneous manifestations.40
Treatment of EFAD includes topical, oral, or intravenous replacement of EFAs. Improvement of EFAD with the application of topical linoleic acid to the skin has been reported.50 Patients receiving TPN should undergo assessment of parenteral lipid emulsion to ensure adequate fatty acid composition.
Vitamin A Deficiency
Vitamin A (retinol) is a fat-soluble vitamin that plays a critical role in keratinization, epithelial proliferation, and cellular differentiation.6 Vitamin A is found in animal products as retinyl esters and in plants as beta-carotene. Vitamin A has 2 clinically important forms: all-trans retinoic acid and 11-cis-retinal. All-trans retinoic acid is involved in cellular differentiation and regulating gene transcription, while 11-cis-retinal is key to rhodopsin generation required for vision. Vitamin A deficiency presents with early ophthalmologic findings, specifically nyctalopia, or delayed adaptation to the dark.51 Xerophthalmia, abnormal conjunctival keratinization, and Bitot spots subsequently develop and may progress to corneal ulceration and blindness.6
Vitamin A deficiency manifests in the skin as follicular hyperkeratosis, or phrynoderma. Notably, numerous other micronutrient deficiencies may result in phrynoderma. Clinically, multiple pigmented keratotic papules of various sizes, many with a central keratinous plug, are distributed symmetrically on the extensor elbows, knees, shoulders, buttocks, and extremities. The skin surrounding these lesions may be scaly and hyperpigmented.52 Generalized xerosis without preceding nyctalopia has been reported.53 Accompanying pityriasis alba may develop.52 Lesions on the face may mimic acne, while lesions on the extremities may simulate a perforating disorder. Histopathology of phrynoderma reveals epidermal hyperkeratosis, follicular hyperkeratosis, and follicular plugging.52
Patients at risk for vitamin A deficiency include those with conditions that affect intestinal fat absorption, underlying psychiatric illness, or chronic disease (eTable). Chronic alcohol use predisposes patients to a multitude of micronutrient deficiencies, including vitamin A deficiency.54 In chronic alcohol use, even mild cutaneous changes may be the first clue to low serum retinol.55
Vitamin A deficiency can be diagnosed by measuring serum retinol levels, with levels lower than 20 µg/dL being diagnostic of deficiency.56 Decreased serum retinol in patients hospitalized with flaring irritable bowel disorder has been repeatedly reported.57-59 Notably, serum retinol concentration does not decline until liver reserves of vitamin A are nearing exhaustion.33
The US Food and Drug Administration requires manufacturers to list retinol activity equivalents on labels. One international unit of retinol is equivalent to 0.3 µg of retinol activity equivalents.60 The treatment of vitamin A deficiency involves high-dose oral supplementation when possible.61 Although dependent on age, the treatment dose for most adults with vitamin A deficiency is 3000 µg (10,000 IU) once daily.
Phrynoderma has been specifically treated with salicylic acid ointment 3% and intramuscular vitamin A.62 Topical urea cream also may treat phrynoderma.63
Vitamin B2
Vitamin B2 (riboflavin) is absorbed in the small intestine and converted into 2 biologically active forms—flavin adenine dinucleotide and flavin mononucleotide—which serve as cofactors in metabolic and oxidation-reduction reactions. Malabsorptive disorders and bowel resection can lead to riboflavin deficiency.64 Other at-risk populations include those with restrictive diets,65 psychiatric illness, or systemic illness (eTable). Riboflavin can be degraded by light (deficiency has been reported after phototherapy for neonatal jaundice66) and following boric acid ingestion.67 Medications, including long-term treatment with antiepileptics, may lead to riboflavin deficiency.68
Riboflavin is critical to maintaining collagen production. Riboflavin deficiency may manifest clinically with extensive seborrheiclike dermatitis,44 intertrigolike dermatitis,69 or oral-ocular-genital syndrome.70 Angular cheilitis may accompany an atrophic tongue that is deep red in color. The scrotum is characteristically involved in men, with confluent dermatitis extending onto the thighs and sparing the midline. Red papules and painful fissures may develop. Balanitis and phimosis have been reported. Testing for riboflavin deficiency should be considered in patients with refractory seborrheic dermatitis.
Riboflavin stores are assessed by the erythrocyte glutathione reductase activity coefficient.44 A level of 1.4 or higher is consistent with deficiency. Serum riboflavin levels, performed after a 12-hour fast, may support the diagnosis but are less sensitive. Patients with glucose-6-phosphate deficiency cannot be assessed via the erythrocyte glutathione reductase activity coefficient and may instead require evaluation of 24-hour urine riboflavin level.44
Vitamin B3
Vitamin B3 (niacin, nicotinamide, nicotinic acid) is found in plant and animal products or can be derived from its amino acid precursor tryptophan. Niacin deficiency results in pellagra, characterized by dermatitis, dementia, and diarrhea.71 The most prominent feature is a symmetrically distributed photosensitive dermatitis of the face, neck (called Casal necklace)(Figure 3), chest, dorsal hands, and extensor arms. The eruption may begin with erythema, vesicles, or bullae (wet pellagra) and evolve into thick, hyperpigmented, scaling plaques.71 The skin may take on a copper tone and become atrophic.72 Dull erythema with overlying yellow powdery scale (called sulfur flakes) at follicular orifices has been described on the nasal bridge.73

Causes of niacin deficiency include malabsorptive conditions, malignancy (including carcinoid tumors), parenteral nutrition, psychiatric disease,74,75 and restrictive diets (eTable).76 Carcinoid tumors divert tryptophan to serotonin resulting in niacin deficiency.77
The diagnosis of niacin deficiency is based on clinical findings and response to supplementation.75 Low niacin urinary metabolites (N-methylnicotinamide and 2-pyridone) may aid in diagnosis.6 Treatment generally includes oral nicotinamide 100 mg every 6 hours; the dose can then be tapered to 50 mg every 8 to 12 hours until symptoms resolve. Severe deficiency may require parenteral nicotinamide 1 g 3 to 4 times daily.75
Vitamin B6
Vitamin B6 (pyridoxine, pyridoxamine, pyridoxal) is found in whole grains and plant and animal products. Vitamin B6 functions as a coenzyme in many metabolic pathways and is involved in the conversion of tryptophan to niacin.44 Absorption requires hydrolysis by intestinal phosphates and transport to the liver for rephosphorylation prior to release in active form.6
Cutaneous findings associated with vitamin B6 deficiency include periorificial and perineal seborrheic dermatitis,78 angular stomatitis, and cheilitis, with associated burning, redness, and tongue edema.6 Vitamin B6 deficiency is a rarely reported cause of burning mouth syndrome.79 Because vitamin B6 is involved in the conversion of tryptophan to niacin, deficiency also may present with pellagralike findings.70 Other clinical symptoms are outlined in the eTable.80,81
Conditions that increase risk for vitamin B6 deficiency are highlighted in the eTable and include malabsorptive disorders; psychiatric illness82; and chronic disease, especially end-stage renal disease.83 Vitamin B6 deficiency associated with chronic alcohol use is due to both inadequate vitamin B6 intake as well as reduced hepatic storage.78 Medications such as isoniazid, hydralazine, and oral contraceptives may decrease vitamin B6 levels (eTable).82
Vitamin B6 can be measured in the plasma as pyridoxal 5′-phosphate. Plasma concentrations of less than 20 nmol/L are suggestive of deficiency.82 Indirect tests include tryptophan and methionine loading.6 The treatment of vitamin B6 deficiency is determined by symptom severity. Recommendations for oral supplementation range from 25 to 600 mg daily.82 Symptoms typically improve on 100 mg daily.6
Vitamins B9 and B12
Deficiencies of vitamins B9 (folic acid, folate) and B12 (cobalamin) have similar clinical presentations. Folate is essential in the metabolism of amino acids, purines, and pyrimidines.6 Cobalamin, found in animal products, is a cofactor for methionine synthase and methylmalonyl-CoA mutase.84 Megaloblastic anemia is the main finding in folate or cobalamin deficiency. Neurologic findings only accompany cobalamin deficiency. Risk factors for folate deficiency include malabsorptive conditions,6 chronic alcohol use,85 and antifolate medication use (eTable).6
Cobalamin absorption requires gastric acid and intrinsic factor binding in the duodenum. Deficiency may occur from strict diets, psychiatric illness, old age,86 decreased gastric acid secretion,87 abnormal intrinsic factor function, or intestinal infections.6
Generalized cutaneous hyperpigmentation may be the first manifestation of vitamins B9 and B12 deficiency.88 Typically accentuated in acral creases and the oral cavity, pigmentation may mimic Addison disease. Hair depigmentation and linear streaking of the nails are reported.84 The tongue becomes painful and red with atrophy of the filiform papillae (Hunter glossitis).78 Linear lesions on the tongue and hard palate may serve as an early sign of cobalamin deficiency.89
Folate deficiency is diagnosed by measuring the plasma folate level; coincidental cobalamin deficiency should be excluded. Deficiency is managed with oral supplementation (when possible) with 1 to 5 mg of folate daily.6 Cobalamin deficiency is based on low serum levels (<150 pg/mL is diagnostic).86 Cobalamin deficiency may take years to develop, as vitamin B12 exists in large body stores.6 Serum methylmalonic acid may be elevated in patients with clinical features but normal-low serum vitamin B12 level.86 Treatment of vitamin B12 deficiency is with oral (2 mg once daily) or parenteral (1 mg every 4 weeks then maintained at once monthly) cyanocobalamin. For patients with neurologic symptoms, intramuscular injection should be given.86 The underlying cause of deficiency must be elucidated and treated.
Vitamin C Deficiency
Vitamin C (ascorbic acid) is an essential cofactor for the hydroxylation of proline and lysine residues in collagen synthesis. Plant-based foods are the main dietary source of vitamin C, and deficiency presents clinically as scurvy. Cutaneous findings include follicular hyperkeratosis, perifollicular petechiae, and curled hair shafts (corkscrew hairs)(Figure 4). Ecchymoses of the lower extremities, forearms, and abdomen may be seen. Nodules representing intramuscular and subcutaneous hemorrhage can be present.90 Woody edema may mimic cellulitis, while lower extremity hemorrhage may mimic vasculitis. Gingival hyperplasia, hemorrhage, and edema may occur,90 along with linear splinter hemorrhages.91

Hypovitaminosis C has been routinely demonstrated in hospitalized patients.92 Scurvy may occur in patients on strict diets,93 chronic alcohol use,94 psychiatric illness,95 or gastrointestinal tract disease (eTable).96-99 Those with low socioeconomic status70 or dementia100 as well as the elderly also are at risk.101 Scurvy has developed in patients with iron overload and those who are on hemodialysis44 as well as in association with nilotinib use.102 Patients with chronic mucous membrane graft-vs-host disease may exhibit vitamin C deficiency.103
Scurvy is a clinical diagnosis. Vitamin C levels normalize quickly with supplementation. Cutaneous biopsy will exhibit follicular hyperkeratosis, perifollicular hemorrhage, and fibrosis.91
Oral ascorbic acid supplementation should be initiated at 500 to 1000 mg daily in adults.104 The cause of deficiency should be identified, and further supplementation should be decided based on patient risk factors. Lifestyle modifications, such as cessation of smoking and chronic alcohol use, is recommended. The diagnosis of scurvy should prompt workup for additional nutrient deficiencies.
Final Thoughts
Dermatologists play an important role in the early recognition of nutritional deficiencies, as cutaneous manifestations often are the first clue to diagnosis. Nutritional deficiencies are common yet underrecognized in the hospitalized patient and serve as an independent risk factor for patient morbidity and mortality.3 Awareness of the cutaneous manifestations of undernutrition as well as the risk factors for nutritional deficiency may expedite diagnosis and supplementation, thereby improving outcomes for hospitalized patients.
The World Health Organization defines malnutrition as deficiencies, excesses, or imbalances in an individual’s intake of energy and/or nutrients.1 This review will focus on undernutrition, which may result from macronutrient or micronutrient deficiencies. Undernutrition in the hospitalized patient is a common yet underrecognized phenomenon, with an estimated prevalence of 20% to 50% worldwide.2 Malnutrition is an independent risk factor for patient morbidity and mortality and has been associated with increased health care costs.3 Nutritional deficiencies may arise from inadequate nutrient intake, abnormal nutrient absorption, or improper nutrient utilization.4 Unfortunately, no standardized algorithm for screening and diagnosing patients with malnutrition exists, making early physical examination findings of utmost importance. Herein, we present a review of acquired nutritional deficiency dermatoses in the inpatient setting.
Protein-Energy Malnutrition
Protein-energy malnutrition (PEM) refers to a set of related disorders that include marasmus, kwashiorkor (KW), and marasmic KW. These conditions frequently are seen in developing countries but also have been reported in developed nations.5 Marasmus occurs from a chronic deficiency of protein and calories. Decreased insulin production and unopposed catabolism result in sarcopenia and loss of bone and subcutaneous fat.6 Affected patients include children who are less than 60% ideal body weight (IBW) without edema or hypoproteinemia.7 Kwashiorkor is the edematous form of PEM that develops from isolated protein deficiency, resulting in edema, diarrhea, and immunosuppression.6 Micronutrient deficiencies, oxidative stress, slow protein catabolism, and excess antidiuretic hormone have been proposed as potential drivers of KW.8 Kwashiorkor affects children between 60% and 80% IBW. Marasmic KW has features of both diseases, including children who are less than 60% IBW but with associated edema and/or hypoproteinemia.9
Although PEM is uncommon in adults, hospitalized patients carry many predisposing risk factors, including infections, malabsorptive conditions, psychiatric disease, and chronic illness (eTable). Patients with chronic infections present with findings consistent with marasmic KW due to lean body mass loss.

The cutaneous findings in PEM are related to dysmaturation of epidermal keratinocytes and resultant epidermal atrophy.10 Patients with marasmus exhibit dry, wrinkled, loose skin due to subcutaneous fat loss. Emaciated children often lose their buccal fat pads, and reduced perianal adipose may lead to rectal prolapse. Increased lanugo hair may be present on the face, and alopecia of the scalp may occur.6 In KW, cutaneous disease progresses from confluent hyperkeratosis to a dry atrophic epidermis that erodes easily, leaving underlying pale erythema. The resultant pattern is one of hyperpigmented plaques with slightly raised borders, and hypopigmented patches and erosions described as flaky paint dermatitis (Figure 1).5 Lesions appear first in areas of friction. The hair often is dry and brittle; curly hair may straighten and scale.11 Red-yellow to gray-white hypopigmentation may develop, denoting periods of inadequate nutrition. The flag sign describes alternating horizontal bands of hypopigmentation interspersed with bands of pigmented hair. The nails usually are thin and soft and may exhibit the nail flag sign, characterized by horizontal bands of white and red.12 Cheilitis, angular stomatitis, and vulvovaginitis may be present.6

In adults, weight loss and body mass index can be used to assess nutritional status, along with a focused history and physical examination. Complete blood cell count, electrolyte levels, and blood urea nitrogen should be assessed, as hypoglycemia and anemia often accompany PEM.13 In KW, hypoalbuminemia and hypoproteinemia are invariably present. Although prealbumin may be a valid prognostic indicator of disease outcomes and mortality in patients at risk for malnutrition, checking other serum biomarkers remains controversial.14 Focused testing may be warranted in patients with risk factors for chronic infectious processes, such as human immunodeficiency virus or tuberculosis.6 Skin biopsy may solidify the diagnosis of PEM. Hypertrophy of the stratum corneum, atrophy of the stratum spinosum and stratum granulosum, and increased basal layer melanin have been reported.15
Treatment involves initial fluid resuscitation and correction of electrolyte imbalances, followed by nutritional replacement.13 Oral or enteral tube feedings are preferred over total parenteral nutrition (TPN), as they enhance recovery of the gastrointestinal tract.16 Refeeding should occur in small amounts and frequent intervals.5 Skin-directed therapy is aimed at restoring epidermal function and hydration, with regular moisturization and application of barrier creams, such as zinc oxide ointment or petrolatum.10
Zinc Deficiency
Zinc is an essential trace element that provides regulatory, structural, and catalytic functions across multiple biochemical pathways6 and serves as an enzymatic cofactor and key component for numerous transcription factors.17 Zinc is derived from food sources, and its concentration correlates with protein content.18 Zinc is found in both animal and plant-based proteins, albeit with a lower oral bioavailability in the latter. Zinc deficiency may be inherited or acquired. Primary acrodermatitis enteropathica is an autosomal-recessive disorder of the solute carrier family 39 member 4 gene, SLC39A4 (encodes zinc transporter ZIP4 on enterocytes); the result is abnormal zinc absorption from the small intestine.18
Acquired zinc deficiency occurs from decreased dietary zinc intake, impaired intestinal zinc absorption, excessive zinc elimination, or systemic states of high catabolism or low albumin (eTable). Total parenteral nutrition–associated deficiency has arisen when nutritional formulations did not contain trace elements during national shortages or when prolonged TPN was not anticipated and trace elements were removed.19 Zinc levels may already be low in patients with chronic illness or inflammation, so even a short period on TPN can precipitate deficiency.18,19 Diets high in phytate may result in zinc deficiency, as phytate impairs intestinal zinc absorption.20 Approximately 15% of patients with inflammatory bowel disease experienced zinc deficiency worldwide.21 In Crohn disease, zinc deficiency has been associated with active intestinal inflammation, increased risk for hospitalization, surgeries, and disease-related complications.22,23
Medications such as antiepileptics, antimetabolites, or penicillamine may induce zinc deficiency, highlighting the importance of medication review for hospitalized patients (eTable). Catabolic states, frequently encountered in hospitalized patients, increase the risk for zinc deficiency.24 Patients with necrolytic migratory erythema (associated with pancreatic glucagonomas) often experience low serum zinc levels.25
The skin is the third most zinc-abundant tissue in the human body. Within keratinocytes, zinc is critical to normal proliferation and suppression of inflammation.17 Zinc also plays an important role in cutaneous immune function.26 Zinc deficiency presents with sharply demarcated, flaccid pustules and bullae that erode into scaly, pink, eczematous or psoriasiform plaques. Lesions are found preferentially in acral and periorificial sites, often with crusting and exudate. The groin and flexural surfaces may be affected. Erosions often become secondarily impetiginized. Other cutaneous findings include angular cheilitis, stomatitis, glossitis, paronychia, onychodystrophy, generalized alopecia, and delayed wound healing.26 Histopathology of skin lesions is characterized by granular layer loss, epidermal pallor, confluent parakeratosis, spongiosis, dyskeratosis, and psoriasiform hyperplasia.27 Acquired bullous acrodermatitis enteropathica has been reported as a histologic mimicker of pemphigus foliaceous in patients on TPN.28
Diagnosis of zinc deficiency is made by measuring plasma zinc levels. Fasting levels should be drawn in the morning, as they can fluctuate based on the time of day, stress levels, or inflammation.6 Sample hemolysis and anticoagulants high in zinc may falsely elevate plasma zinc. A normal zinc level is greater than 70 µg/dL; however, normal levels do not rule out deficiency.18 Measurement of zinc-dependent enzymes, such as alkaline phosphatase, can be a quick way to assess zinc status. Serum albumin also should be measured; because zinc is carried by albumin in the blood, hypoalbuminemia may result in secondary zinc deficiency.18
Zinc replacement therapy is largely through oral supplementation and should start at 0.5 to 2.0 mg/kg/d in adults with acquired disease.29,30 Zinc sulfate is the most affordable and is the supplement of choice, with 50 mg of elemental zinc per 220 mg of zinc sulfate (~23% elemental zinc).31 Alternative zinc salts, such as zinc gluconate (13% elemental zinc), may be used. Patients with malabsorptive disorders often require parenteral supplementation.32 Clinical symptoms often will resolve within 1 to 2 weeks of supplementation.29 In patients with primary acrodermatitis enteropathica, lifelong supplementation with 3 mg/kg/d elemental zinc should occur.6 Calcium and folate may reduce zinc absorption, while zinc supplementation can interfere with copper and iron absorption.33
Iron Deficiency
Iron is an essential component of the hemoglobin molecule. Iron homeostasis and metabolism are tightly regulated processes that drive erythropoiesis. Only 5% to 10% of dietary iron is absorbed through nutrition, while the remainder is recycled from red cell breakdown. Both normal iron levels and iron deficiency (ID) are defined by age and gender.34 Iron-deficiency anemia (IDA) is one of the most common cause-specific anemias worldwide.35
Fatigue is the most common and earliest symptom of ID. In a single study, pallor was predictive of anemia in hospitalized patients; however, absence of pallor did not rule out anemia.34 Dyspnea on exertion, tachycardia, dysphagia, and pica also may be reported. Cutaneous manifestations include koilonychia (Figure 2), glossitis, pruritus, angular cheilitis, and telogen effluvium. Plummer-Vinson syndrome is characterized by microcytic anemia, glossitis, and dysphagia.

Risk factors for ID include insufficient dietary consumption,36 blood loss, malabsorptive states,37,38 and increased iron requirements (eTable). Patient fragility (eg, elderly, chronic disease) is a newly described risk factor where correction of ID may impact morbidity, mortality, and quality of life.35
Iron deficiency can be present despite a normal hemoglobin level. Serum ferritin and percentage transferrin saturation are key to early identification of IDA.35 Ferritin levels lower than 30 µg/L confirm the diagnosis. Decreased transferrin saturation and increased total iron binding capacity aid in the diagnosis of IDA. Serum ferritin is an acute-phase reactant, and levels may be falsely elevated in the setting of inflammation or infection.
Treatment includes reversing the cause of deficiency and supplementing iron. Calculation of the total iron deficit can help inform iron supplementation. First-line therapy for IDA is oral ferrous sulfate 325 mg (65 mg elemental iron) 3 times daily. Newer studies suggest 40 to 80 mg oral iron should be taken every other day to increase absorption.39 Other iron salts, such as ferrous gluconate (325 mg is equivalent to 38 mg elemental iron), have been used. Iron absorption is enhanced by an acidic environment. Parenteral iron is utilized in patients with uncorrectable blood loss, malabsorption, renal failure, intolerance to oral iron, and nonadherence in those who are unable to receive transfusions. Iron infusions are favored in frail patients, such as the elderly and those with chronic kidney disease or heart failure.35 Multiple parenteral iron formulations exist, and their use should be driven by underlying patient comorbidities and potential risks. Packed red blood cell transfusions should be considered in acute blood loss, hypoxia, or cardiac insufficiency.
Essential Fatty Acid Deficiency
Essential fatty acids (EFAs) including linoleic and α-linolenic acid cannot be synthesized by the human body and must be obtained through diet (mostly plant oils). Essential fatty acids have various functions, including maintaining phospholipid membrane integrity, forming prostaglandins and leukotrienes, and storing energy.40 Essential fatty acids are important in the structure and function of the stratum corneum and are crucial in maintaining epidermal barrier function.41 Increased epidermal permeability and transepidermal water loss may be the first signs of EFA deficiency (EFAD).42
The cutaneous manifestations of EFAD include xerosis, weeping eczematous plaques, and erosions in intertriginous sites. The lesions may progress to widespread desquamation and erythema. With time, the skin can become thick and leathery. Alopecia may occur, and hair may depigment.7 Additional findings include poor wound healing and increased susceptibility to infections.43,44
Essential fatty acid deficiency may occur when dietary fat intake is severely restricted or in malabsorptive states.45,46 It develops in patients on prolonged TPN, typically when receiving fat-restricted nutrition,47,48 as occurs in hypertriglyceridemia.47 Essential fatty acid deficiency has developed in patients on TPN containing EFAs,47 as the introduction of novel intravenous lipid emulsions has resulted in varying proportions of EFA.40 Premature neonates are particularly at risk for EFAD.49
The diagnosis of EFAD involves the measurement of the triene to tetraene ratio. A ratio of more than 0.2 suggests EFAD, but the clinical signs are not seen until the ratio is over 0.4.40 Low plasma levels of linoleic, linolenic, and arachidonic acids also are seen. Elevated liver function tests are supportive of the diagnosis. Biochemical findings typically are seen before cutaneous manifestations.40
Treatment of EFAD includes topical, oral, or intravenous replacement of EFAs. Improvement of EFAD with the application of topical linoleic acid to the skin has been reported.50 Patients receiving TPN should undergo assessment of parenteral lipid emulsion to ensure adequate fatty acid composition.
Vitamin A Deficiency
Vitamin A (retinol) is a fat-soluble vitamin that plays a critical role in keratinization, epithelial proliferation, and cellular differentiation.6 Vitamin A is found in animal products as retinyl esters and in plants as beta-carotene. Vitamin A has 2 clinically important forms: all-trans retinoic acid and 11-cis-retinal. All-trans retinoic acid is involved in cellular differentiation and regulating gene transcription, while 11-cis-retinal is key to rhodopsin generation required for vision. Vitamin A deficiency presents with early ophthalmologic findings, specifically nyctalopia, or delayed adaptation to the dark.51 Xerophthalmia, abnormal conjunctival keratinization, and Bitot spots subsequently develop and may progress to corneal ulceration and blindness.6
Vitamin A deficiency manifests in the skin as follicular hyperkeratosis, or phrynoderma. Notably, numerous other micronutrient deficiencies may result in phrynoderma. Clinically, multiple pigmented keratotic papules of various sizes, many with a central keratinous plug, are distributed symmetrically on the extensor elbows, knees, shoulders, buttocks, and extremities. The skin surrounding these lesions may be scaly and hyperpigmented.52 Generalized xerosis without preceding nyctalopia has been reported.53 Accompanying pityriasis alba may develop.52 Lesions on the face may mimic acne, while lesions on the extremities may simulate a perforating disorder. Histopathology of phrynoderma reveals epidermal hyperkeratosis, follicular hyperkeratosis, and follicular plugging.52
Patients at risk for vitamin A deficiency include those with conditions that affect intestinal fat absorption, underlying psychiatric illness, or chronic disease (eTable). Chronic alcohol use predisposes patients to a multitude of micronutrient deficiencies, including vitamin A deficiency.54 In chronic alcohol use, even mild cutaneous changes may be the first clue to low serum retinol.55
Vitamin A deficiency can be diagnosed by measuring serum retinol levels, with levels lower than 20 µg/dL being diagnostic of deficiency.56 Decreased serum retinol in patients hospitalized with flaring irritable bowel disorder has been repeatedly reported.57-59 Notably, serum retinol concentration does not decline until liver reserves of vitamin A are nearing exhaustion.33
The US Food and Drug Administration requires manufacturers to list retinol activity equivalents on labels. One international unit of retinol is equivalent to 0.3 µg of retinol activity equivalents.60 The treatment of vitamin A deficiency involves high-dose oral supplementation when possible.61 Although dependent on age, the treatment dose for most adults with vitamin A deficiency is 3000 µg (10,000 IU) once daily.
Phrynoderma has been specifically treated with salicylic acid ointment 3% and intramuscular vitamin A.62 Topical urea cream also may treat phrynoderma.63
Vitamin B2
Vitamin B2 (riboflavin) is absorbed in the small intestine and converted into 2 biologically active forms—flavin adenine dinucleotide and flavin mononucleotide—which serve as cofactors in metabolic and oxidation-reduction reactions. Malabsorptive disorders and bowel resection can lead to riboflavin deficiency.64 Other at-risk populations include those with restrictive diets,65 psychiatric illness, or systemic illness (eTable). Riboflavin can be degraded by light (deficiency has been reported after phototherapy for neonatal jaundice66) and following boric acid ingestion.67 Medications, including long-term treatment with antiepileptics, may lead to riboflavin deficiency.68
Riboflavin is critical to maintaining collagen production. Riboflavin deficiency may manifest clinically with extensive seborrheiclike dermatitis,44 intertrigolike dermatitis,69 or oral-ocular-genital syndrome.70 Angular cheilitis may accompany an atrophic tongue that is deep red in color. The scrotum is characteristically involved in men, with confluent dermatitis extending onto the thighs and sparing the midline. Red papules and painful fissures may develop. Balanitis and phimosis have been reported. Testing for riboflavin deficiency should be considered in patients with refractory seborrheic dermatitis.
Riboflavin stores are assessed by the erythrocyte glutathione reductase activity coefficient.44 A level of 1.4 or higher is consistent with deficiency. Serum riboflavin levels, performed after a 12-hour fast, may support the diagnosis but are less sensitive. Patients with glucose-6-phosphate deficiency cannot be assessed via the erythrocyte glutathione reductase activity coefficient and may instead require evaluation of 24-hour urine riboflavin level.44
Vitamin B3
Vitamin B3 (niacin, nicotinamide, nicotinic acid) is found in plant and animal products or can be derived from its amino acid precursor tryptophan. Niacin deficiency results in pellagra, characterized by dermatitis, dementia, and diarrhea.71 The most prominent feature is a symmetrically distributed photosensitive dermatitis of the face, neck (called Casal necklace)(Figure 3), chest, dorsal hands, and extensor arms. The eruption may begin with erythema, vesicles, or bullae (wet pellagra) and evolve into thick, hyperpigmented, scaling plaques.71 The skin may take on a copper tone and become atrophic.72 Dull erythema with overlying yellow powdery scale (called sulfur flakes) at follicular orifices has been described on the nasal bridge.73

Causes of niacin deficiency include malabsorptive conditions, malignancy (including carcinoid tumors), parenteral nutrition, psychiatric disease,74,75 and restrictive diets (eTable).76 Carcinoid tumors divert tryptophan to serotonin resulting in niacin deficiency.77
The diagnosis of niacin deficiency is based on clinical findings and response to supplementation.75 Low niacin urinary metabolites (N-methylnicotinamide and 2-pyridone) may aid in diagnosis.6 Treatment generally includes oral nicotinamide 100 mg every 6 hours; the dose can then be tapered to 50 mg every 8 to 12 hours until symptoms resolve. Severe deficiency may require parenteral nicotinamide 1 g 3 to 4 times daily.75
Vitamin B6
Vitamin B6 (pyridoxine, pyridoxamine, pyridoxal) is found in whole grains and plant and animal products. Vitamin B6 functions as a coenzyme in many metabolic pathways and is involved in the conversion of tryptophan to niacin.44 Absorption requires hydrolysis by intestinal phosphates and transport to the liver for rephosphorylation prior to release in active form.6
Cutaneous findings associated with vitamin B6 deficiency include periorificial and perineal seborrheic dermatitis,78 angular stomatitis, and cheilitis, with associated burning, redness, and tongue edema.6 Vitamin B6 deficiency is a rarely reported cause of burning mouth syndrome.79 Because vitamin B6 is involved in the conversion of tryptophan to niacin, deficiency also may present with pellagralike findings.70 Other clinical symptoms are outlined in the eTable.80,81
Conditions that increase risk for vitamin B6 deficiency are highlighted in the eTable and include malabsorptive disorders; psychiatric illness82; and chronic disease, especially end-stage renal disease.83 Vitamin B6 deficiency associated with chronic alcohol use is due to both inadequate vitamin B6 intake as well as reduced hepatic storage.78 Medications such as isoniazid, hydralazine, and oral contraceptives may decrease vitamin B6 levels (eTable).82
Vitamin B6 can be measured in the plasma as pyridoxal 5′-phosphate. Plasma concentrations of less than 20 nmol/L are suggestive of deficiency.82 Indirect tests include tryptophan and methionine loading.6 The treatment of vitamin B6 deficiency is determined by symptom severity. Recommendations for oral supplementation range from 25 to 600 mg daily.82 Symptoms typically improve on 100 mg daily.6
Vitamins B9 and B12
Deficiencies of vitamins B9 (folic acid, folate) and B12 (cobalamin) have similar clinical presentations. Folate is essential in the metabolism of amino acids, purines, and pyrimidines.6 Cobalamin, found in animal products, is a cofactor for methionine synthase and methylmalonyl-CoA mutase.84 Megaloblastic anemia is the main finding in folate or cobalamin deficiency. Neurologic findings only accompany cobalamin deficiency. Risk factors for folate deficiency include malabsorptive conditions,6 chronic alcohol use,85 and antifolate medication use (eTable).6
Cobalamin absorption requires gastric acid and intrinsic factor binding in the duodenum. Deficiency may occur from strict diets, psychiatric illness, old age,86 decreased gastric acid secretion,87 abnormal intrinsic factor function, or intestinal infections.6
Generalized cutaneous hyperpigmentation may be the first manifestation of vitamins B9 and B12 deficiency.88 Typically accentuated in acral creases and the oral cavity, pigmentation may mimic Addison disease. Hair depigmentation and linear streaking of the nails are reported.84 The tongue becomes painful and red with atrophy of the filiform papillae (Hunter glossitis).78 Linear lesions on the tongue and hard palate may serve as an early sign of cobalamin deficiency.89
Folate deficiency is diagnosed by measuring the plasma folate level; coincidental cobalamin deficiency should be excluded. Deficiency is managed with oral supplementation (when possible) with 1 to 5 mg of folate daily.6 Cobalamin deficiency is based on low serum levels (<150 pg/mL is diagnostic).86 Cobalamin deficiency may take years to develop, as vitamin B12 exists in large body stores.6 Serum methylmalonic acid may be elevated in patients with clinical features but normal-low serum vitamin B12 level.86 Treatment of vitamin B12 deficiency is with oral (2 mg once daily) or parenteral (1 mg every 4 weeks then maintained at once monthly) cyanocobalamin. For patients with neurologic symptoms, intramuscular injection should be given.86 The underlying cause of deficiency must be elucidated and treated.
Vitamin C Deficiency
Vitamin C (ascorbic acid) is an essential cofactor for the hydroxylation of proline and lysine residues in collagen synthesis. Plant-based foods are the main dietary source of vitamin C, and deficiency presents clinically as scurvy. Cutaneous findings include follicular hyperkeratosis, perifollicular petechiae, and curled hair shafts (corkscrew hairs)(Figure 4). Ecchymoses of the lower extremities, forearms, and abdomen may be seen. Nodules representing intramuscular and subcutaneous hemorrhage can be present.90 Woody edema may mimic cellulitis, while lower extremity hemorrhage may mimic vasculitis. Gingival hyperplasia, hemorrhage, and edema may occur,90 along with linear splinter hemorrhages.91

Hypovitaminosis C has been routinely demonstrated in hospitalized patients.92 Scurvy may occur in patients on strict diets,93 chronic alcohol use,94 psychiatric illness,95 or gastrointestinal tract disease (eTable).96-99 Those with low socioeconomic status70 or dementia100 as well as the elderly also are at risk.101 Scurvy has developed in patients with iron overload and those who are on hemodialysis44 as well as in association with nilotinib use.102 Patients with chronic mucous membrane graft-vs-host disease may exhibit vitamin C deficiency.103
Scurvy is a clinical diagnosis. Vitamin C levels normalize quickly with supplementation. Cutaneous biopsy will exhibit follicular hyperkeratosis, perifollicular hemorrhage, and fibrosis.91
Oral ascorbic acid supplementation should be initiated at 500 to 1000 mg daily in adults.104 The cause of deficiency should be identified, and further supplementation should be decided based on patient risk factors. Lifestyle modifications, such as cessation of smoking and chronic alcohol use, is recommended. The diagnosis of scurvy should prompt workup for additional nutrient deficiencies.
Final Thoughts
Dermatologists play an important role in the early recognition of nutritional deficiencies, as cutaneous manifestations often are the first clue to diagnosis. Nutritional deficiencies are common yet underrecognized in the hospitalized patient and serve as an independent risk factor for patient morbidity and mortality.3 Awareness of the cutaneous manifestations of undernutrition as well as the risk factors for nutritional deficiency may expedite diagnosis and supplementation, thereby improving outcomes for hospitalized patients.
- Mehta NM, Corkins MR, Lyman B, et al. Defining pediatric malnutrition: a paradigm shift toward etiology-related definitions. JPEN J Parenter Enteral Nutr. 2013;37:460-481.
- Barker LA, Gout BS, Crowe TC. Hospital malnutrition: prevalence, identification and impact on patients and the healthcare system. Int J Environ Res Public Health. 2011;8:514-527.
- Bharadwaj S, Ginoya S, Tandon P, et al. Malnutrition: laboratory markers vs nutritional assessment. Gastroenterol Rep (Oxf). 2016;4:272-280.
- Basavaraj KH, Seemanthini C, Rashmi R. Diet in dermatology: present perspectives. Indian J Dermatol. 2010;55:205-210.
- Grover Z, Ee LC. Protein energy malnutrition. Pediatr Clin North Am. 2009;56:1055-1068.
- Jen M, Yan AC. Syndromes associated with nutritional deficiency and excess. Clin Dermatol. 2010;28:669-685.
- Lekwuttikarn R, Teng JMC. Cutaneous manifestations of nutritional deficiency. Curr Opin Pediatr. 2018;30:505-513.
- Jaffe AT, Heymann WR. Kwashiorkor/zinc deficiency overlap following partial gastrectomy. Int J Dermatol. 1998;37:134-137.
- Listernick R, Christoffel K, Pace J, et al. Severe primary malnutrition in US children. Am J Dis Child. 1985;139:1157-1160.
- Heilskov S, Rytter MJ, Vestergaard C, et al. Dermatosis in children with oedematous malnutrition (Kwashiorkor): a review of the literature. J Eur Acad Dermatol Venereol. 2014;28:995-1001.
- Bradfield RB. Hair tissue as a medium for the differential diagnosis of protein-calorie malnutrition: a commentary. J Pediatr. 1974;84:294-296.
- Cohen PR. The nail flag sign: case report in a man with diverticulitis and review of dermatology flag sign of the hair, skin, and nails. Cureus. 2018;10:e2929.
- Management of Severe Malnutrition: A Manual for Physicians and Other Senior Health Workers. Geneva, Switzerland: World Health Organization; 1999. https://www.who.int/nutrition/publications/en/manage_severe_malnutrition_eng.pdf. Accessed May 19, 2020.
- Keller U. Nutritional laboratory markers in malnutrition. J Clin Med. 2019;8:775.
- Thavaraj V, Sesikeran B. Histopathological changes in skin of children with clinical protein energy malnutrition before and after recovery. J Trop Pediatr. 1989;35:105-108.
- McClave SA, Heyland DK. The physiologic response and associated clinical benefits from provision of early enteral nutrition. Nutr Clin Pract. 2009;24:305-315.
- Ogawa Y, Kinoshita M, Shimada S, et al. Zinc and skin disorders. Nutrients. 2018;10:199.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007;56:116-124.
- Wiznia LE, Bhansali S, Brinster N, et al. Acquired acrodermatitis enteropathica due to zinc-depleted parenteral nutrition. Pediatr Dermatol. 2019;36:520-523.
- Sandstead HH, Freeland-Graves JH. Dietary phytate, zinc and hidden zinc deficiency. J Trace Elem Med Biol. 2014;28:414-417.
- Vagianos K, Bector S, McConnell J, et al. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2007;31:311-319.
- Schoelmerich J, Becher MS, Hoppe-Seyler P, et al. Zinc and vitamin A deficiency in patients with Crohn’s disease is correlated with activity but not with localization or extent of the disease. Hepatogastroenterology. 1985;32:34-38.
- Siva S, Rubin DT, Gulotta G, et al. Zinc deficiency is associated with poor clinical outcomes in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:152-157.
- Semrad CE. Zinc and intestinal function. Curr Gastroenterol Rep. 1999;1:398-403.
- Sinclair SA, Reynolds NJ. Necrolytic migratory erythema and zinc deficiency. Br J Dermatol. 1997;136:783-785.
- Gammoh NZ, Rink L. Zinc in infection and inflammation. Nutrients. 2017;9:624.
- Gonzalez JR, Botet MV, Sanchez JL. The histopathology of acrodermatitis enteropathica. Am J Dermatopathol. 1982;4:303-311.
- Wu D, Fung MA, Kiuru M, et al. Acquired bullous acrodermatitis enteropathica as a histologic mimic of pemphigus foliaceus in a patient on parenteral nutrition. Dermatol Online J. 2018;24:20.
- Maxfield L, Crane J. Zinc Deficiency. Treasure Island, FL: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK493231/Updated November 14, 2019. Accessed May 19, 2020.
- Macdonald JB, Connolly SM, DiCaudo DJ. Think zinc deficiency: acquired acrodermatitis enteropathica due to poor diet and common medications. Arch Dermatol. 2012;148:961-963.
- Wegmüller R, Tay F, Zeder C, et al. Zinc absorption by young adults from supplemental zinc citrate is comparable with that from zinc gluconate and higher than from zinc oxide. J Nutr. 2014;144:132-136.
- Vick G, Mahmoudizad R, Fiala K. Intravenous zinc therapy for acquired zinc deficiency secondary to gastric bypass surgery: a case report. Dermatol Ther. 2015;28:222-225.
- Ghishan FK, Kiela PR. Vitamins and minerals in inflammatory bowel disease. Gastroenterol Clin North Am. 2017;46:797-808.
- Killip S, Bennett JM, Chambers MD. Iron deficiency anemia. Am Fam Physician. 2007;75:671-678.
- De Franceschi L, Iolascon A, Taher A, et al. Clinical management of iron deficiency anemia in adults: systemic review on advances in diagnosis and treatment. Eur J Intern Med. 2017;42:16-23.
- Haider LM, Schwingshackl L, Hoffmann G, et al. The effect of vegetarian diets on iron status in adults: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2018;58:1359-1374.
- Enani G, Bilgic E, Lebedeva E, et al. The incidence of iron deficiency anemia post-Roux-en-Y gastric bypass and sleeve gastrectomy: a systematic review [published online September 4, 2019]. Surg Endosc. doi:10.1007/s00464-019-07092-3.
- Kaitha S, Bashir M, Ali T. Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol. 2015;6:62-72.
- Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126:1981-1989.
- Gramlich L, Meddings L, Alberda C, et al. Essential fatty acid deficiency in 2015: the impact of novel intravenous lipid emulsions. JPEN J Parenter Enteral Nutr. 2015;39(1 suppl):61S-66S.
- Khnykin D, Miner JH, Jahnsen F. Role of fatty acid transporters in epidermis: implications for health and disease. Dermatoendocrinol. 2011;3:53-61.
- Wright S. Essential fatty acids and the skin. Br J Dermatol. 1991;125:503-515.
- Lakdawala N, Grant-Kels JM. Acrodermatitis caused by nutritional deficiency and metabolic disorders. Clin Dermatol. 2017;35:64-67.
- DiBaise M, Tarleton SM. Hair, nails, and skin: differentiating cutaneous manifestations of micronutrient deficiency. Nutr Clin Pract. 2019;34:490-503.
- Aldámiz-Echevarría L, Bilbao A, Andrade F, et al. Fatty acid deficiency profile in children with food allergy managed with elimination diets. Acta Paediatr. 2008;97:1572-1576.
- Jeppesen PB, Christensen MS, Høy CE, et al. Essential fatty acid deficiency in patients with severe fat malabsorption. Am J Clin Nutr. 1997;65:837-843.
- Roongpisuthipong W, Phanachet P, Roongpisuthipong C, et al. Essential fatty acid deficiency while a patient receiving fat regimen total parenteral nutrition [published online June 14, 2012]. BMJ Case Rep. doi:10.1136/bcr.07.2011.4475.
- Fleming CR, Smith LM, Hodges RE. Essential fatty acid deficiency in adults receiving total parenteral nutrition. Am J Clin Nutr. 1976;29:976-983.
- Cooke RJ, Zee P, Yeh YY. Essential fatty acid status of the premature infant during short-term fat-free parenteral nutrition. J Pediatr Gastroenterol Nutr. 1984;3:446-449.
- Skolnik P, Eaglstein WH, Ziboh VA. Human essential fatty acid deficiency: treatment by topical application of linoleic acid. Arch Dermatol. 1977;113:939-941.
- Vahlquist A. Clinical use of vitamin A and its derivatives—physiological and pharmacological aspects. Clin Exp Dermatol. 1985;10:133-143.
- Ragunatha S, Kumar VJ, Murugesh SB. A clinical study of 125 patients with phrynoderma. Indian J Dermatol. 2011;56:389-392.
- Phanachet P, Shantavasinkul PC, Chantrathammachart P, et al. Unusual manifestation of vitamin A deficiency presenting with generalized xerosis without night blindness. Clin Case Rep. 2018;6:878-882.
- Fuchs J. Alcoholism, malnutrition, vitamin deficiencies, and the skin. Clin Dermatol. 1999;17:457-461.
- Uhoda E, Petit L, Piérard-Franchimont C, et al. Ultraviolet light-enhanced visualization of cutaneous signs of carotene and vitamin A dietary deficiency. Acta Clin Belg. 2004;59:97-101.
- de Pee S, Dary O. Biochemical indicators of vitamin A deficiency: serum retinol and serum retinol binding protein. J Nutr. 2002;132(9 suppl):2895S-2901S.
- Fernandez-Banares F, Abad-Lacruz A, Xiol X, et al. Vitamin status in patients with inflammatory bowel disease. Am J Gastroenterol. 1989;84:744-748.
- Main AN, Mills PR, Russell RI, et al. Vitamin A deficiency in Crohn’s disease. Gut. 1983;24:1169-1175.
- Cobos G, Cornejo C, McMahon P. A case of phrynoderma in a patient with Crohn’s disease. Pediatr Dermatol. 2015;32:234-236.
- Trumbo P, Yates AA, Schlicker S, et al. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001;101:294-301.
- Ross DA. Recommendations for vitamin A supplementation. J Nutr. 2002;132(9 suppl):2902S-2906S.
- Ragunatha S, Jagannath Kumar V, Murugesh SB, et al. Therapeutic response of vitamin A, vitamin B complex, essential fatty acids (EFA) and vitamin E in the treatment of phrynoderma: a randomized controlled study. J Clin Diagn Res. 2014;8:116-118.
- Nakjang Y, Yuttanavivat T. Phrynoderma: a review of 105 cases. J Dermatol. 1988;15:531-534.
- Pinto JT, Zempleni J. Riboflavin. Adv Nutr. 2016;7:973-975.
- Larsson CL, Johansson GK. Dietary intake and nutritional status of young vegans and omnivores in Sweden. Am J Clin Nutr. 2002;76:100-106.
- Gromisch DS, Lopez R, Cole HS, et al. Light (phototherapy)—induced riboflavin deficiency in the neonate. J Pediatr. 1977;90:118-122.
- Pinto J, Huang YP, McConnell RJ, et al. Increased urinary riboflavin excretion resulting from boric acid ingestion. J Lab Clin Med. 1978;92:126-134.
- Soltani D, Ghaffar Pour M, et al. Nutritional aspects of treatment in epileptic patients. Iran J Child Neurol. 2016;10:1-12.
- Roe DA. Riboflavin deficiency: mucocutaneous signs of acute and chronic deficiency. Semin Dermatol. 1991;10:293-295.
- Galimberti F, Mesinkovska NA. Skin findings associated with nutritional deficiencies. Cleve Clin J Med. 2016;83:731-739.
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol. 2002;41:476-481.
- Nogueira A, Duarte AF, Magina S, et al. Pellagra associated with esophageal carcinoma and alcoholism. Dermatol Online J. 2009;15:8.
- Wan P, Moat S, Anstey A. Pellagra: a review with emphasis on photosensitivity. Br J Dermatol. 2011;164:1188-1200.
- Jagielska G, Tomaszewicz-Libudzic EC, Brzozowska A. Pellagra: a rare complication of anorexia nervosa. Eur Child Adolesc Psychiatry. 2007;16:417-420.
- Li R, Yu K, Wang Q, et al. Pellagra secondary to medication and alcoholism: a case report and review of the literature. Nutr Clin Pract. 2016;31:785-789.
- Ladoyanni E, Cheung ST, North J, et al. Pellagra occurring in a patient with atopic dermatitis and food allergy. J Eur Acad Dermatol Venereol. 2007;21:394-396.
- Bell HK, Poston GJ, Vora J, et al. Cutaneous manifestations of the malignant carcinoid syndrome. Br J Dermatol. 2005;152:71-75.
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol. 1986;15:1263-1274.
- Lamey PJ, Hammond A, Allam BF, et al. Vitamin status of patients with burning mouth syndrome and the response to replacement therapy. Br Dent J. 1986;160:81-84.
- Stover PJ, Field MS. Vitamin B-6. Adv Nutr. 2015;6:132-133.
- Gerlach AT, Thomas S, Stawicki SP, et al. Vitamin B6 deficiency: a potential cause of refractory seizures in adults. JPEN J Parenter Enteral Nutr. 2011;35:272-275.
- Spinneker A, Sola R, Lemmen V, et al. Vitamin B6 status, deficiency and its consequences—an overview. Nutr Hosp. 2007;22:7-24.
- Ross EA, Shah GM, Reynolds RD, et al. Vitamin B6 requirements of patients on chronic peritoneal dialysis. Kidney Int. 1989;36:702-706.
- Brescoll J, Daveluy S. A review of vitamin B12 in dermatology. Am J Clin Dermatol. 2015;16:27-33.
- Sanvisens A, Zuluaga P, Pineda M, et al. Folate deficiency in patients seeking treatment of alcohol use disorder. Drug Alcohol Depend. 2017;180:417-422.
- Langan RC, Goodbred AJ. Vitamin B12 deficiency: recognition and management. Am Fam Physician. 2017;96:384-389.
- Bradford GS, Taylor CT. Omeprazole and vitamin B12 deficiency. Ann Pharmacother. 1999;33:641-643.
- Srivastava N, Chand S, Bansal M, et al. Reversible hyperpigmentation as the first manifestation of dietary vitamin B12 deficiency. Indian J Dermatol Venereol Leprol. 2006;72:389-390.
- Graells J, Ojeda RM, Muniesa C, et al. Glossitis with linear lesions: an early sign of vitamin B12 deficiency. J Am Acad Dermatol. 2009;60:498-500.
- Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999;41:895-906; quiz 907-810.
- Shaath T, Fischer R, Goeser M, et al. Scurvy in the present times: vitamin C allergy leading to strict fast food diet. Dermatol Online J. 2016;22:13030/qt50b8w28b.
- Fain O, Pariés J, Jacquart B, et al. Hypovitaminosis C in hospitalized patients. Eur J Intern Med. 2003;14:419-425.
- Ahmad SA, Al Thobiti TA, El Toum M, et al. Florid scurvy in an autistic child on a ketogenic diet [published online November 19, 2018]. Pediatr Emerg Care. doi:10.1097/PEC.0000000000001695.
- Lux-Battistelli C, Battistelli D. Latent scurvy with tiredness and leg pain in alcoholics: an underestimated disease three case reports. Medicine (Baltimore). 2017;96:e8861.
- Christopher K, Tammaro D, Wing EJ. Early scurvy complicating anorexia nervosa. South Med J. 2002;95:1065-1066.
- Berger ML, Siegel DM, Lee EL. Scurvy as an initial manifestation of Whipple’s disease. Ann Intern Med. 1984;101:58-59.
- Imes S, Dinwoodie A, Walker K, et al. Vitamin C status in 137 outpatients with Crohn’s disease. effect of diet counseling. J Clin Gastroenterol. 1986;8:443-446.
- Echeverría Zudaire L, García Cuartero B, Campelo Moreno O, et al. Scurvy associated with celiac disease [in Spanish]. An Esp Pediatr. 2002;57:587.
- Hansen EP, Metzsche C, Henningsen E, et al. Severe scurvy after gastric bypass surgery and a poor postoperative diet. J Clin Med Res. 2012;4:135-137.
- Rivière S, Birlouez-Aragon I, Nourhashémi F, et al. Low plasma vitamin C in Alzheimer patients despite an adequate diet. Int J Geriatr Psychiatry. 1998;13:749-754.
- Bhattacharyya P, Giannoutsos J, Eslick GD, et al. Scurvy: an unrecognized and emerging public health issue in developed economies. Mayo Clin Proc. 2019;94:2594-2597.
- Oak AS, Jaleel T, Fening K, et al. A case of scurvy associated with nilotinib. J Cutan Pathol. 2016;43:725-726.
- Kletzel M, Powers K, Hayes M. Scurvy: a new problem for patients with chronic GVHD involving mucous membranes; an easy problem to resolve. Pediatr Transplant. 2014;18:524-526.
- Maxfield L, Crane JS. Vitamin C Deficiency (Scurvy). Treasure Island, FL: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK493187/. Updated November 19, 2019. Accessed May 19, 2020.
- Mehta NM, Corkins MR, Lyman B, et al. Defining pediatric malnutrition: a paradigm shift toward etiology-related definitions. JPEN J Parenter Enteral Nutr. 2013;37:460-481.
- Barker LA, Gout BS, Crowe TC. Hospital malnutrition: prevalence, identification and impact on patients and the healthcare system. Int J Environ Res Public Health. 2011;8:514-527.
- Bharadwaj S, Ginoya S, Tandon P, et al. Malnutrition: laboratory markers vs nutritional assessment. Gastroenterol Rep (Oxf). 2016;4:272-280.
- Basavaraj KH, Seemanthini C, Rashmi R. Diet in dermatology: present perspectives. Indian J Dermatol. 2010;55:205-210.
- Grover Z, Ee LC. Protein energy malnutrition. Pediatr Clin North Am. 2009;56:1055-1068.
- Jen M, Yan AC. Syndromes associated with nutritional deficiency and excess. Clin Dermatol. 2010;28:669-685.
- Lekwuttikarn R, Teng JMC. Cutaneous manifestations of nutritional deficiency. Curr Opin Pediatr. 2018;30:505-513.
- Jaffe AT, Heymann WR. Kwashiorkor/zinc deficiency overlap following partial gastrectomy. Int J Dermatol. 1998;37:134-137.
- Listernick R, Christoffel K, Pace J, et al. Severe primary malnutrition in US children. Am J Dis Child. 1985;139:1157-1160.
- Heilskov S, Rytter MJ, Vestergaard C, et al. Dermatosis in children with oedematous malnutrition (Kwashiorkor): a review of the literature. J Eur Acad Dermatol Venereol. 2014;28:995-1001.
- Bradfield RB. Hair tissue as a medium for the differential diagnosis of protein-calorie malnutrition: a commentary. J Pediatr. 1974;84:294-296.
- Cohen PR. The nail flag sign: case report in a man with diverticulitis and review of dermatology flag sign of the hair, skin, and nails. Cureus. 2018;10:e2929.
- Management of Severe Malnutrition: A Manual for Physicians and Other Senior Health Workers. Geneva, Switzerland: World Health Organization; 1999. https://www.who.int/nutrition/publications/en/manage_severe_malnutrition_eng.pdf. Accessed May 19, 2020.
- Keller U. Nutritional laboratory markers in malnutrition. J Clin Med. 2019;8:775.
- Thavaraj V, Sesikeran B. Histopathological changes in skin of children with clinical protein energy malnutrition before and after recovery. J Trop Pediatr. 1989;35:105-108.
- McClave SA, Heyland DK. The physiologic response and associated clinical benefits from provision of early enteral nutrition. Nutr Clin Pract. 2009;24:305-315.
- Ogawa Y, Kinoshita M, Shimada S, et al. Zinc and skin disorders. Nutrients. 2018;10:199.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007;56:116-124.
- Wiznia LE, Bhansali S, Brinster N, et al. Acquired acrodermatitis enteropathica due to zinc-depleted parenteral nutrition. Pediatr Dermatol. 2019;36:520-523.
- Sandstead HH, Freeland-Graves JH. Dietary phytate, zinc and hidden zinc deficiency. J Trace Elem Med Biol. 2014;28:414-417.
- Vagianos K, Bector S, McConnell J, et al. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2007;31:311-319.
- Schoelmerich J, Becher MS, Hoppe-Seyler P, et al. Zinc and vitamin A deficiency in patients with Crohn’s disease is correlated with activity but not with localization or extent of the disease. Hepatogastroenterology. 1985;32:34-38.
- Siva S, Rubin DT, Gulotta G, et al. Zinc deficiency is associated with poor clinical outcomes in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:152-157.
- Semrad CE. Zinc and intestinal function. Curr Gastroenterol Rep. 1999;1:398-403.
- Sinclair SA, Reynolds NJ. Necrolytic migratory erythema and zinc deficiency. Br J Dermatol. 1997;136:783-785.
- Gammoh NZ, Rink L. Zinc in infection and inflammation. Nutrients. 2017;9:624.
- Gonzalez JR, Botet MV, Sanchez JL. The histopathology of acrodermatitis enteropathica. Am J Dermatopathol. 1982;4:303-311.
- Wu D, Fung MA, Kiuru M, et al. Acquired bullous acrodermatitis enteropathica as a histologic mimic of pemphigus foliaceus in a patient on parenteral nutrition. Dermatol Online J. 2018;24:20.
- Maxfield L, Crane J. Zinc Deficiency. Treasure Island, FL: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK493231/Updated November 14, 2019. Accessed May 19, 2020.
- Macdonald JB, Connolly SM, DiCaudo DJ. Think zinc deficiency: acquired acrodermatitis enteropathica due to poor diet and common medications. Arch Dermatol. 2012;148:961-963.
- Wegmüller R, Tay F, Zeder C, et al. Zinc absorption by young adults from supplemental zinc citrate is comparable with that from zinc gluconate and higher than from zinc oxide. J Nutr. 2014;144:132-136.
- Vick G, Mahmoudizad R, Fiala K. Intravenous zinc therapy for acquired zinc deficiency secondary to gastric bypass surgery: a case report. Dermatol Ther. 2015;28:222-225.
- Ghishan FK, Kiela PR. Vitamins and minerals in inflammatory bowel disease. Gastroenterol Clin North Am. 2017;46:797-808.
- Killip S, Bennett JM, Chambers MD. Iron deficiency anemia. Am Fam Physician. 2007;75:671-678.
- De Franceschi L, Iolascon A, Taher A, et al. Clinical management of iron deficiency anemia in adults: systemic review on advances in diagnosis and treatment. Eur J Intern Med. 2017;42:16-23.
- Haider LM, Schwingshackl L, Hoffmann G, et al. The effect of vegetarian diets on iron status in adults: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2018;58:1359-1374.
- Enani G, Bilgic E, Lebedeva E, et al. The incidence of iron deficiency anemia post-Roux-en-Y gastric bypass and sleeve gastrectomy: a systematic review [published online September 4, 2019]. Surg Endosc. doi:10.1007/s00464-019-07092-3.
- Kaitha S, Bashir M, Ali T. Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol. 2015;6:62-72.
- Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126:1981-1989.
- Gramlich L, Meddings L, Alberda C, et al. Essential fatty acid deficiency in 2015: the impact of novel intravenous lipid emulsions. JPEN J Parenter Enteral Nutr. 2015;39(1 suppl):61S-66S.
- Khnykin D, Miner JH, Jahnsen F. Role of fatty acid transporters in epidermis: implications for health and disease. Dermatoendocrinol. 2011;3:53-61.
- Wright S. Essential fatty acids and the skin. Br J Dermatol. 1991;125:503-515.
- Lakdawala N, Grant-Kels JM. Acrodermatitis caused by nutritional deficiency and metabolic disorders. Clin Dermatol. 2017;35:64-67.
- DiBaise M, Tarleton SM. Hair, nails, and skin: differentiating cutaneous manifestations of micronutrient deficiency. Nutr Clin Pract. 2019;34:490-503.
- Aldámiz-Echevarría L, Bilbao A, Andrade F, et al. Fatty acid deficiency profile in children with food allergy managed with elimination diets. Acta Paediatr. 2008;97:1572-1576.
- Jeppesen PB, Christensen MS, Høy CE, et al. Essential fatty acid deficiency in patients with severe fat malabsorption. Am J Clin Nutr. 1997;65:837-843.
- Roongpisuthipong W, Phanachet P, Roongpisuthipong C, et al. Essential fatty acid deficiency while a patient receiving fat regimen total parenteral nutrition [published online June 14, 2012]. BMJ Case Rep. doi:10.1136/bcr.07.2011.4475.
- Fleming CR, Smith LM, Hodges RE. Essential fatty acid deficiency in adults receiving total parenteral nutrition. Am J Clin Nutr. 1976;29:976-983.
- Cooke RJ, Zee P, Yeh YY. Essential fatty acid status of the premature infant during short-term fat-free parenteral nutrition. J Pediatr Gastroenterol Nutr. 1984;3:446-449.
- Skolnik P, Eaglstein WH, Ziboh VA. Human essential fatty acid deficiency: treatment by topical application of linoleic acid. Arch Dermatol. 1977;113:939-941.
- Vahlquist A. Clinical use of vitamin A and its derivatives—physiological and pharmacological aspects. Clin Exp Dermatol. 1985;10:133-143.
- Ragunatha S, Kumar VJ, Murugesh SB. A clinical study of 125 patients with phrynoderma. Indian J Dermatol. 2011;56:389-392.
- Phanachet P, Shantavasinkul PC, Chantrathammachart P, et al. Unusual manifestation of vitamin A deficiency presenting with generalized xerosis without night blindness. Clin Case Rep. 2018;6:878-882.
- Fuchs J. Alcoholism, malnutrition, vitamin deficiencies, and the skin. Clin Dermatol. 1999;17:457-461.
- Uhoda E, Petit L, Piérard-Franchimont C, et al. Ultraviolet light-enhanced visualization of cutaneous signs of carotene and vitamin A dietary deficiency. Acta Clin Belg. 2004;59:97-101.
- de Pee S, Dary O. Biochemical indicators of vitamin A deficiency: serum retinol and serum retinol binding protein. J Nutr. 2002;132(9 suppl):2895S-2901S.
- Fernandez-Banares F, Abad-Lacruz A, Xiol X, et al. Vitamin status in patients with inflammatory bowel disease. Am J Gastroenterol. 1989;84:744-748.
- Main AN, Mills PR, Russell RI, et al. Vitamin A deficiency in Crohn’s disease. Gut. 1983;24:1169-1175.
- Cobos G, Cornejo C, McMahon P. A case of phrynoderma in a patient with Crohn’s disease. Pediatr Dermatol. 2015;32:234-236.
- Trumbo P, Yates AA, Schlicker S, et al. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001;101:294-301.
- Ross DA. Recommendations for vitamin A supplementation. J Nutr. 2002;132(9 suppl):2902S-2906S.
- Ragunatha S, Jagannath Kumar V, Murugesh SB, et al. Therapeutic response of vitamin A, vitamin B complex, essential fatty acids (EFA) and vitamin E in the treatment of phrynoderma: a randomized controlled study. J Clin Diagn Res. 2014;8:116-118.
- Nakjang Y, Yuttanavivat T. Phrynoderma: a review of 105 cases. J Dermatol. 1988;15:531-534.
- Pinto JT, Zempleni J. Riboflavin. Adv Nutr. 2016;7:973-975.
- Larsson CL, Johansson GK. Dietary intake and nutritional status of young vegans and omnivores in Sweden. Am J Clin Nutr. 2002;76:100-106.
- Gromisch DS, Lopez R, Cole HS, et al. Light (phototherapy)—induced riboflavin deficiency in the neonate. J Pediatr. 1977;90:118-122.
- Pinto J, Huang YP, McConnell RJ, et al. Increased urinary riboflavin excretion resulting from boric acid ingestion. J Lab Clin Med. 1978;92:126-134.
- Soltani D, Ghaffar Pour M, et al. Nutritional aspects of treatment in epileptic patients. Iran J Child Neurol. 2016;10:1-12.
- Roe DA. Riboflavin deficiency: mucocutaneous signs of acute and chronic deficiency. Semin Dermatol. 1991;10:293-295.
- Galimberti F, Mesinkovska NA. Skin findings associated with nutritional deficiencies. Cleve Clin J Med. 2016;83:731-739.
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol. 2002;41:476-481.
- Nogueira A, Duarte AF, Magina S, et al. Pellagra associated with esophageal carcinoma and alcoholism. Dermatol Online J. 2009;15:8.
- Wan P, Moat S, Anstey A. Pellagra: a review with emphasis on photosensitivity. Br J Dermatol. 2011;164:1188-1200.
- Jagielska G, Tomaszewicz-Libudzic EC, Brzozowska A. Pellagra: a rare complication of anorexia nervosa. Eur Child Adolesc Psychiatry. 2007;16:417-420.
- Li R, Yu K, Wang Q, et al. Pellagra secondary to medication and alcoholism: a case report and review of the literature. Nutr Clin Pract. 2016;31:785-789.
- Ladoyanni E, Cheung ST, North J, et al. Pellagra occurring in a patient with atopic dermatitis and food allergy. J Eur Acad Dermatol Venereol. 2007;21:394-396.
- Bell HK, Poston GJ, Vora J, et al. Cutaneous manifestations of the malignant carcinoid syndrome. Br J Dermatol. 2005;152:71-75.
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol. 1986;15:1263-1274.
- Lamey PJ, Hammond A, Allam BF, et al. Vitamin status of patients with burning mouth syndrome and the response to replacement therapy. Br Dent J. 1986;160:81-84.
- Stover PJ, Field MS. Vitamin B-6. Adv Nutr. 2015;6:132-133.
- Gerlach AT, Thomas S, Stawicki SP, et al. Vitamin B6 deficiency: a potential cause of refractory seizures in adults. JPEN J Parenter Enteral Nutr. 2011;35:272-275.
- Spinneker A, Sola R, Lemmen V, et al. Vitamin B6 status, deficiency and its consequences—an overview. Nutr Hosp. 2007;22:7-24.
- Ross EA, Shah GM, Reynolds RD, et al. Vitamin B6 requirements of patients on chronic peritoneal dialysis. Kidney Int. 1989;36:702-706.
- Brescoll J, Daveluy S. A review of vitamin B12 in dermatology. Am J Clin Dermatol. 2015;16:27-33.
- Sanvisens A, Zuluaga P, Pineda M, et al. Folate deficiency in patients seeking treatment of alcohol use disorder. Drug Alcohol Depend. 2017;180:417-422.
- Langan RC, Goodbred AJ. Vitamin B12 deficiency: recognition and management. Am Fam Physician. 2017;96:384-389.
- Bradford GS, Taylor CT. Omeprazole and vitamin B12 deficiency. Ann Pharmacother. 1999;33:641-643.
- Srivastava N, Chand S, Bansal M, et al. Reversible hyperpigmentation as the first manifestation of dietary vitamin B12 deficiency. Indian J Dermatol Venereol Leprol. 2006;72:389-390.
- Graells J, Ojeda RM, Muniesa C, et al. Glossitis with linear lesions: an early sign of vitamin B12 deficiency. J Am Acad Dermatol. 2009;60:498-500.
- Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999;41:895-906; quiz 907-810.
- Shaath T, Fischer R, Goeser M, et al. Scurvy in the present times: vitamin C allergy leading to strict fast food diet. Dermatol Online J. 2016;22:13030/qt50b8w28b.
- Fain O, Pariés J, Jacquart B, et al. Hypovitaminosis C in hospitalized patients. Eur J Intern Med. 2003;14:419-425.
- Ahmad SA, Al Thobiti TA, El Toum M, et al. Florid scurvy in an autistic child on a ketogenic diet [published online November 19, 2018]. Pediatr Emerg Care. doi:10.1097/PEC.0000000000001695.
- Lux-Battistelli C, Battistelli D. Latent scurvy with tiredness and leg pain in alcoholics: an underestimated disease three case reports. Medicine (Baltimore). 2017;96:e8861.
- Christopher K, Tammaro D, Wing EJ. Early scurvy complicating anorexia nervosa. South Med J. 2002;95:1065-1066.
- Berger ML, Siegel DM, Lee EL. Scurvy as an initial manifestation of Whipple’s disease. Ann Intern Med. 1984;101:58-59.
- Imes S, Dinwoodie A, Walker K, et al. Vitamin C status in 137 outpatients with Crohn’s disease. effect of diet counseling. J Clin Gastroenterol. 1986;8:443-446.
- Echeverría Zudaire L, García Cuartero B, Campelo Moreno O, et al. Scurvy associated with celiac disease [in Spanish]. An Esp Pediatr. 2002;57:587.
- Hansen EP, Metzsche C, Henningsen E, et al. Severe scurvy after gastric bypass surgery and a poor postoperative diet. J Clin Med Res. 2012;4:135-137.
- Rivière S, Birlouez-Aragon I, Nourhashémi F, et al. Low plasma vitamin C in Alzheimer patients despite an adequate diet. Int J Geriatr Psychiatry. 1998;13:749-754.
- Bhattacharyya P, Giannoutsos J, Eslick GD, et al. Scurvy: an unrecognized and emerging public health issue in developed economies. Mayo Clin Proc. 2019;94:2594-2597.
- Oak AS, Jaleel T, Fening K, et al. A case of scurvy associated with nilotinib. J Cutan Pathol. 2016;43:725-726.
- Kletzel M, Powers K, Hayes M. Scurvy: a new problem for patients with chronic GVHD involving mucous membranes; an easy problem to resolve. Pediatr Transplant. 2014;18:524-526.
- Maxfield L, Crane JS. Vitamin C Deficiency (Scurvy). Treasure Island, FL: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK493187/. Updated November 19, 2019. Accessed May 19, 2020.
Practice Points
- Nutritional deficiencies are common in hospitalized patients and often go unrecognized.
- Awareness of the risk factors predisposing patients to nutritional deficiencies and the cutaneous manifestations associated with undernutrition can promote early diagnosis.
- When investigating cutaneous findings, undernutrition should be considered in patients with chronic infections, malabsorptive states, psychiatric illness, and strict dietary practices, as well as in those using certain medications.
- Prompt nutritional supplementation can prevent patient morbidity and mortality and reverse skin disease.
Erythematous Plaques and Nodules on the Abdomen and Groin
The Diagnosis: Inflammatory Urothelial Carcinoma
Microscopic examination revealed metastatic carcinoma with extensive dermal lymphatic invasion (Figure). Immunohistochemical stains were positive for p63 and GATA3, markers for urothelial carcinomas, and negative for S-100 and Melan-A, markers for melanoma. Thus, the biopsy was compatible with a diagnosis of urothelial carcinoma. Gram and Grocott-Gomori methenamine-silver stains were negative for bacterial or fungal organisms. An additional 4-mm punch biopsy was performed of the left thigh at the distal-most aspect of the eruption to determine the extent of cutaneous metastasis. Pathology again showed metastatic urothelial carcinoma with extensive dermal lymphatic involvement and overlying epidermal spongiosis.
The patient had a history of bladder cancer diagnosed 1.5 years prior to presentation. It was a high-grade (World Health Organization) urothelial carcinoma that penetrated the bladder muscular wall, focally infiltrating into pericystic fat with multifocal seeding of pericystic lymphatics. It was unresponsive to bacillus Calmette-Guérin therapy. He underwent a cystoprostatectomy and bilateral staging lymph node dissection with clear surgical margins without adjuvant chemotherapy or radiation. He also reported a history of 2 prior cutaneous melanomas that were excised without sentinel lymph node biopsy.
Four months prior to presentation, he developed a mildly pruritic cutaneous eruption on the abdomen that was treated with topical miconazole for presumed tinea cruris without improvement. He also was previously diagnosed with candidiasis of his urostomy and was taking oral fluconazole. The patient was admitted for the abdominal pain and distension, and computed tomography of the abdomen and pelvis revealed peritoneal carcinomatosis resulting in mechanical small bowel obstruction as well as enlarged pelvic and retroperitoneal lymph nodes. Confirmation of metastatic disease via skin biopsy avoided an invasive peritoneal biopsy. He was treated with triamcinolone acetonide ointment 0.1% with moderate relief of pruritus, and a palliative percutaneous endoscopic gastrostomy tube was placed for bowel decompression. The patient's hospital course was complicated by Proteus mirabilis bacteremia requiring cefepime. He was transitioned to home hospice and died 1 month after presentation.
Inflammatory carcinoma, also called carcinoma erysipeloides, is a type of cutaneous metastasis most commonly seen in breast adenocarcinoma. Reported cases secondary to urothelial carcinoma are rare and most often involve the abdomen, groin, and lower extremities.1-5 Clinically, inflammatory carcinoma presents as erythematous indurated patches or plaques with well-defined borders, often with edema, warmth, and tenderness. Its morphologic appearance is due to the obstruction of lymphatic vessels by tumor cells and the release of inflammatory cytokines. Its presentation can mimic other dermatoses such as cellulitis, erysipelas, fungal infection, radiation dermatitis, Majocchi granuloma, or contact dermatitis.6 Cutaneous metastases may be the first clinical manifestations of metastatic disease, and they may occur due to hematogenous and lymphatic spread, direct contiguous tissue invasion, or iatrogenic implantation following surgical excision of the primary tumor. Histologically, nuclear markers GATA3 and p63 stain positively in urothelial carcinomas and are negative in prostatic adenocarcinomas.7,8 Other markers may be used such as cytokeratins 7 and 20, which are cytoplasmic epithelial markers that both stain positive in urothelial neoplasms.9
Inflammatory carcinoma may be treated with radiation or systemic chemotherapy depending on the extent of systemic involvement in the patient; however, its presence portends a poor prognosis. Less than 1% of genitourinary malignancies have cutaneous involvement, and median disease-specific survival is less than 6 months from presentation of the cutaneous metastasis.10 Clinicians faced with a recalcitrant inflammatory cutaneous eruption should maintain a high index of suspicion for cutaneous metastases, particularly in patients with a history of cancer. Early dermatology referral may help establish the diagnosis and guide disease-targeted therapy or goals of care discussions.
- Grace SA, Livingood MR, Boyd AS. Metastatic urothelial carcinoma presenting as carcinoma erysipeloides. J Cutan Pathol. 2017;44:513-515.
- Zangrilli A, Saraceno R, Sarmati L, et al. Erysipeloid cutaneous metastasis from bladder carcinoma. Eur J Dermatol. 2007;17:534-536.
- Chang CP, Lee Y, Shih HJ. Unusual presentation of cutaneous metastasis from bladder urothelial carcinoma. Chin J Cancer Res. 2013;25:362-365.
- Aloi F, Solaroli C, Paradiso M, et al. Inflammatory type cutaneous metastasis of bladder neoplasm: erysipeloid carcinoma [in Italian]. Minerva Urol Nefrol. 1998;50:205-208.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Al Ameer A, Imran M, Kaliyadan F, et al. Carcinoma erysipeloides as a presenting feature of breast carcinoma: a case report and brief review of literature. Indian Dermatol Online J. 2015;6:396-398.
- Chang A, Amin A, Gabrielson E, et al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol. 2012;36:1472-1476.
- Ud Din N, Qureshi A, Mansoor S. Utility of p63 immunohistochemical stain in differentiating urothelial carcinomas from adenocarcinomas of prostate. Indian J Pathol Microbiol. 2011;54:59-62.
- Bassily NH, Vallorosi CJ, Akdas G, et al. Coordinate expression of cytokeratins 7 and 20 in prostate adenocarcinoma and bladder urothelial carcinoma. Am J Clin Pathol. 2000;113:383-388.
- Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026.
The Diagnosis: Inflammatory Urothelial Carcinoma
Microscopic examination revealed metastatic carcinoma with extensive dermal lymphatic invasion (Figure). Immunohistochemical stains were positive for p63 and GATA3, markers for urothelial carcinomas, and negative for S-100 and Melan-A, markers for melanoma. Thus, the biopsy was compatible with a diagnosis of urothelial carcinoma. Gram and Grocott-Gomori methenamine-silver stains were negative for bacterial or fungal organisms. An additional 4-mm punch biopsy was performed of the left thigh at the distal-most aspect of the eruption to determine the extent of cutaneous metastasis. Pathology again showed metastatic urothelial carcinoma with extensive dermal lymphatic involvement and overlying epidermal spongiosis.

The patient had a history of bladder cancer diagnosed 1.5 years prior to presentation. It was a high-grade (World Health Organization) urothelial carcinoma that penetrated the bladder muscular wall, focally infiltrating into pericystic fat with multifocal seeding of pericystic lymphatics. It was unresponsive to bacillus Calmette-Guérin therapy. He underwent a cystoprostatectomy and bilateral staging lymph node dissection with clear surgical margins without adjuvant chemotherapy or radiation. He also reported a history of 2 prior cutaneous melanomas that were excised without sentinel lymph node biopsy.
Four months prior to presentation, he developed a mildly pruritic cutaneous eruption on the abdomen that was treated with topical miconazole for presumed tinea cruris without improvement. He also was previously diagnosed with candidiasis of his urostomy and was taking oral fluconazole. The patient was admitted for the abdominal pain and distension, and computed tomography of the abdomen and pelvis revealed peritoneal carcinomatosis resulting in mechanical small bowel obstruction as well as enlarged pelvic and retroperitoneal lymph nodes. Confirmation of metastatic disease via skin biopsy avoided an invasive peritoneal biopsy. He was treated with triamcinolone acetonide ointment 0.1% with moderate relief of pruritus, and a palliative percutaneous endoscopic gastrostomy tube was placed for bowel decompression. The patient's hospital course was complicated by Proteus mirabilis bacteremia requiring cefepime. He was transitioned to home hospice and died 1 month after presentation.
Inflammatory carcinoma, also called carcinoma erysipeloides, is a type of cutaneous metastasis most commonly seen in breast adenocarcinoma. Reported cases secondary to urothelial carcinoma are rare and most often involve the abdomen, groin, and lower extremities.1-5 Clinically, inflammatory carcinoma presents as erythematous indurated patches or plaques with well-defined borders, often with edema, warmth, and tenderness. Its morphologic appearance is due to the obstruction of lymphatic vessels by tumor cells and the release of inflammatory cytokines. Its presentation can mimic other dermatoses such as cellulitis, erysipelas, fungal infection, radiation dermatitis, Majocchi granuloma, or contact dermatitis.6 Cutaneous metastases may be the first clinical manifestations of metastatic disease, and they may occur due to hematogenous and lymphatic spread, direct contiguous tissue invasion, or iatrogenic implantation following surgical excision of the primary tumor. Histologically, nuclear markers GATA3 and p63 stain positively in urothelial carcinomas and are negative in prostatic adenocarcinomas.7,8 Other markers may be used such as cytokeratins 7 and 20, which are cytoplasmic epithelial markers that both stain positive in urothelial neoplasms.9
Inflammatory carcinoma may be treated with radiation or systemic chemotherapy depending on the extent of systemic involvement in the patient; however, its presence portends a poor prognosis. Less than 1% of genitourinary malignancies have cutaneous involvement, and median disease-specific survival is less than 6 months from presentation of the cutaneous metastasis.10 Clinicians faced with a recalcitrant inflammatory cutaneous eruption should maintain a high index of suspicion for cutaneous metastases, particularly in patients with a history of cancer. Early dermatology referral may help establish the diagnosis and guide disease-targeted therapy or goals of care discussions.
The Diagnosis: Inflammatory Urothelial Carcinoma
Microscopic examination revealed metastatic carcinoma with extensive dermal lymphatic invasion (Figure). Immunohistochemical stains were positive for p63 and GATA3, markers for urothelial carcinomas, and negative for S-100 and Melan-A, markers for melanoma. Thus, the biopsy was compatible with a diagnosis of urothelial carcinoma. Gram and Grocott-Gomori methenamine-silver stains were negative for bacterial or fungal organisms. An additional 4-mm punch biopsy was performed of the left thigh at the distal-most aspect of the eruption to determine the extent of cutaneous metastasis. Pathology again showed metastatic urothelial carcinoma with extensive dermal lymphatic involvement and overlying epidermal spongiosis.

The patient had a history of bladder cancer diagnosed 1.5 years prior to presentation. It was a high-grade (World Health Organization) urothelial carcinoma that penetrated the bladder muscular wall, focally infiltrating into pericystic fat with multifocal seeding of pericystic lymphatics. It was unresponsive to bacillus Calmette-Guérin therapy. He underwent a cystoprostatectomy and bilateral staging lymph node dissection with clear surgical margins without adjuvant chemotherapy or radiation. He also reported a history of 2 prior cutaneous melanomas that were excised without sentinel lymph node biopsy.
Four months prior to presentation, he developed a mildly pruritic cutaneous eruption on the abdomen that was treated with topical miconazole for presumed tinea cruris without improvement. He also was previously diagnosed with candidiasis of his urostomy and was taking oral fluconazole. The patient was admitted for the abdominal pain and distension, and computed tomography of the abdomen and pelvis revealed peritoneal carcinomatosis resulting in mechanical small bowel obstruction as well as enlarged pelvic and retroperitoneal lymph nodes. Confirmation of metastatic disease via skin biopsy avoided an invasive peritoneal biopsy. He was treated with triamcinolone acetonide ointment 0.1% with moderate relief of pruritus, and a palliative percutaneous endoscopic gastrostomy tube was placed for bowel decompression. The patient's hospital course was complicated by Proteus mirabilis bacteremia requiring cefepime. He was transitioned to home hospice and died 1 month after presentation.
Inflammatory carcinoma, also called carcinoma erysipeloides, is a type of cutaneous metastasis most commonly seen in breast adenocarcinoma. Reported cases secondary to urothelial carcinoma are rare and most often involve the abdomen, groin, and lower extremities.1-5 Clinically, inflammatory carcinoma presents as erythematous indurated patches or plaques with well-defined borders, often with edema, warmth, and tenderness. Its morphologic appearance is due to the obstruction of lymphatic vessels by tumor cells and the release of inflammatory cytokines. Its presentation can mimic other dermatoses such as cellulitis, erysipelas, fungal infection, radiation dermatitis, Majocchi granuloma, or contact dermatitis.6 Cutaneous metastases may be the first clinical manifestations of metastatic disease, and they may occur due to hematogenous and lymphatic spread, direct contiguous tissue invasion, or iatrogenic implantation following surgical excision of the primary tumor. Histologically, nuclear markers GATA3 and p63 stain positively in urothelial carcinomas and are negative in prostatic adenocarcinomas.7,8 Other markers may be used such as cytokeratins 7 and 20, which are cytoplasmic epithelial markers that both stain positive in urothelial neoplasms.9
Inflammatory carcinoma may be treated with radiation or systemic chemotherapy depending on the extent of systemic involvement in the patient; however, its presence portends a poor prognosis. Less than 1% of genitourinary malignancies have cutaneous involvement, and median disease-specific survival is less than 6 months from presentation of the cutaneous metastasis.10 Clinicians faced with a recalcitrant inflammatory cutaneous eruption should maintain a high index of suspicion for cutaneous metastases, particularly in patients with a history of cancer. Early dermatology referral may help establish the diagnosis and guide disease-targeted therapy or goals of care discussions.
- Grace SA, Livingood MR, Boyd AS. Metastatic urothelial carcinoma presenting as carcinoma erysipeloides. J Cutan Pathol. 2017;44:513-515.
- Zangrilli A, Saraceno R, Sarmati L, et al. Erysipeloid cutaneous metastasis from bladder carcinoma. Eur J Dermatol. 2007;17:534-536.
- Chang CP, Lee Y, Shih HJ. Unusual presentation of cutaneous metastasis from bladder urothelial carcinoma. Chin J Cancer Res. 2013;25:362-365.
- Aloi F, Solaroli C, Paradiso M, et al. Inflammatory type cutaneous metastasis of bladder neoplasm: erysipeloid carcinoma [in Italian]. Minerva Urol Nefrol. 1998;50:205-208.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Al Ameer A, Imran M, Kaliyadan F, et al. Carcinoma erysipeloides as a presenting feature of breast carcinoma: a case report and brief review of literature. Indian Dermatol Online J. 2015;6:396-398.
- Chang A, Amin A, Gabrielson E, et al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol. 2012;36:1472-1476.
- Ud Din N, Qureshi A, Mansoor S. Utility of p63 immunohistochemical stain in differentiating urothelial carcinomas from adenocarcinomas of prostate. Indian J Pathol Microbiol. 2011;54:59-62.
- Bassily NH, Vallorosi CJ, Akdas G, et al. Coordinate expression of cytokeratins 7 and 20 in prostate adenocarcinoma and bladder urothelial carcinoma. Am J Clin Pathol. 2000;113:383-388.
- Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026.
- Grace SA, Livingood MR, Boyd AS. Metastatic urothelial carcinoma presenting as carcinoma erysipeloides. J Cutan Pathol. 2017;44:513-515.
- Zangrilli A, Saraceno R, Sarmati L, et al. Erysipeloid cutaneous metastasis from bladder carcinoma. Eur J Dermatol. 2007;17:534-536.
- Chang CP, Lee Y, Shih HJ. Unusual presentation of cutaneous metastasis from bladder urothelial carcinoma. Chin J Cancer Res. 2013;25:362-365.
- Aloi F, Solaroli C, Paradiso M, et al. Inflammatory type cutaneous metastasis of bladder neoplasm: erysipeloid carcinoma [in Italian]. Minerva Urol Nefrol. 1998;50:205-208.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Al Ameer A, Imran M, Kaliyadan F, et al. Carcinoma erysipeloides as a presenting feature of breast carcinoma: a case report and brief review of literature. Indian Dermatol Online J. 2015;6:396-398.
- Chang A, Amin A, Gabrielson E, et al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol. 2012;36:1472-1476.
- Ud Din N, Qureshi A, Mansoor S. Utility of p63 immunohistochemical stain in differentiating urothelial carcinomas from adenocarcinomas of prostate. Indian J Pathol Microbiol. 2011;54:59-62.
- Bassily NH, Vallorosi CJ, Akdas G, et al. Coordinate expression of cytokeratins 7 and 20 in prostate adenocarcinoma and bladder urothelial carcinoma. Am J Clin Pathol. 2000;113:383-388.
- Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026.
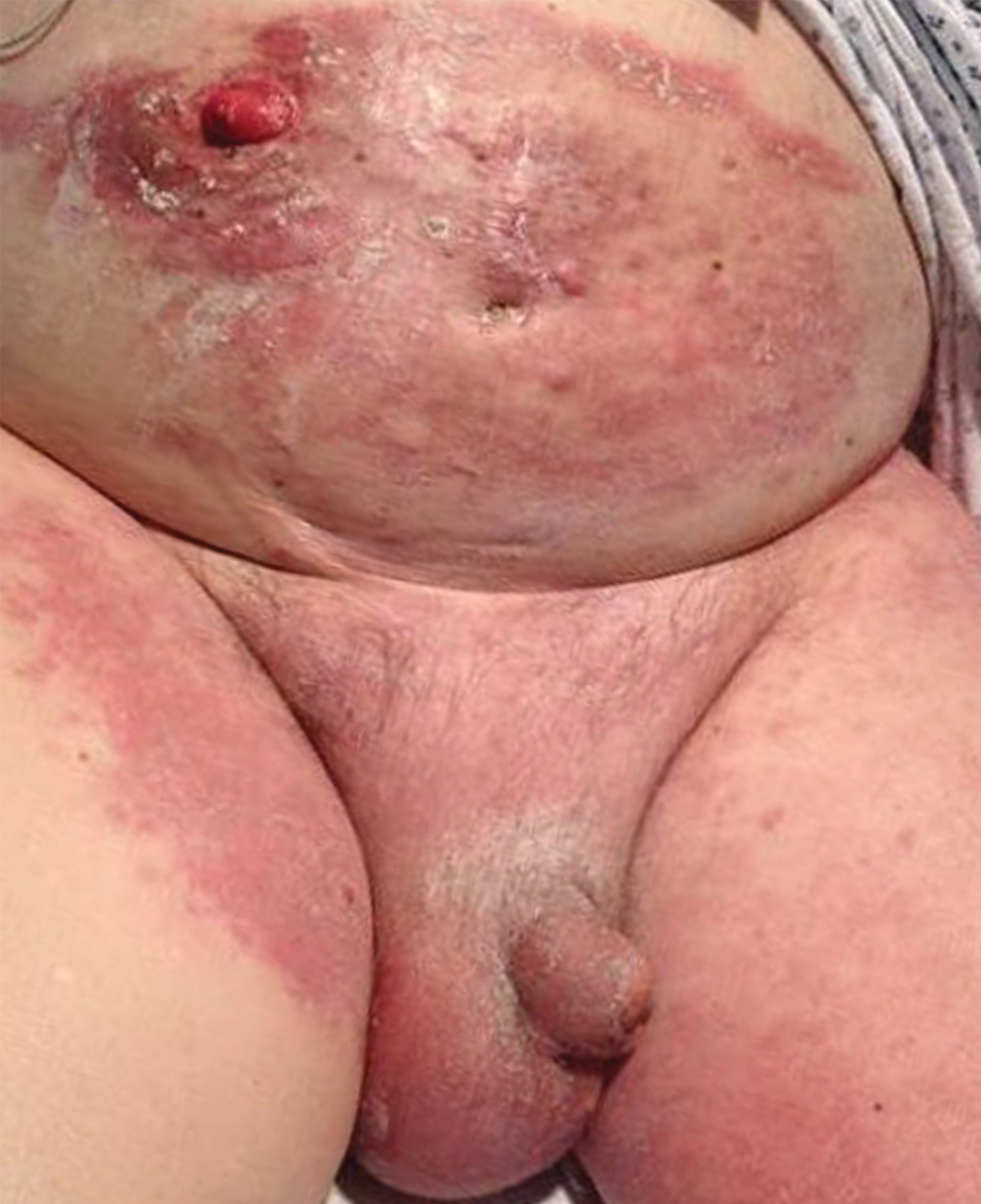
An 82-year-old man presented with acute abdominal pain and distension as well as an abdominal rash of 4 months' duration that was expanding despite treatment with topical miconazole. He had a history of melanoma and bladder cancer treated with cystoprostatectomy. He previously was diagnosed with candidiasis of his urostomy and was taking oral fluconazole. Physical examination revealed a large, well-demarcated, erythematous, smooth plaque covering the entire abdomen, scrotum, penis, inguinal folds, and bilateral upper thighs, with several satellite plaques and firm nodules clustered around the umbilicus. An 8-mm punch biopsy of a periumbilical nodule was performed.
Calciphylaxis: Diagnostic and Treatment Pearls
Update on Calciphylaxis Etiopathogenesis, Diagnosis, and Management
Calciphylaxis, also known as calcific uremic arteriolopathy, is a painful skin condition classically seen in patients with end-stage renal disease (ESRD), particularly those on chronic dialysis.1,2 It also has increasingly been reported in patients with normal renal function and calcium and phosphate homeostasis.3,4 Effective diagnosis and management of calciphylaxis remains challenging for physicians.2,5 The condition is characterized by tissue ischemia caused by calcification of cutaneous arteriolar vessels. As a result, calciphylaxis is associated with high mortality rates, ranging from 60% to 80%.5,6 Excruciating pain and nonhealing ulcers often lead to recurrent hospitalizations and infectious complications,7 and poor nutritional status, chronic pain, depression, and insomnia can further complicate recovery and lead to poor quality of life.8
We provide an update on calciphylaxis etiopathogenesis, diagnosis, and management. We also highlight some challenges faced in managing this potentially fatal condition.
Epidemiology
Calciphylaxis is considered a rare dermatosis with an estimated annual incidence of 1% to 4% in ESRD patients on dialysis. Recent data suggest that incidence of calciphylaxis is rising,5,7,9 which may stem from an increased use of calcium-based phosphate binders, an actual rise in disease incidence, and/or increased recognition of the disease.5 It is difficult to estimate the exact disease burden of calciphylaxis because the diagnostic criteria are not well defined, often leading to missed or delayed diagnosis.3,10 Furthermore, there is no centralized registry for calciphylaxis cases.3
Etiology and Pathogenesis
Calciphylaxis is thought to have a multifactorial etiology with the exact cause or trigger unknown.7 A long list of risk factors and triggers is associated with the condition (Table 1). Calciphylaxis primarily affects small arteries (40–600 μm in diameter) that become calcified due to an imbalance between inhibitors and promoters of calcification.2,11 Fetuin-A and matrix Gla protein inhibit vascular calcification and are downregulated in calciphylaxis.12,13 Dysfunctional calcium, phosphate, and parathyroid hormone regulatory pathways provide an increased substrate for the process of calcification, which causes endothelial damage and microthrombosis, resulting in tissue ischemia and infarction.14,15 Notably, there is growing interest in the role of vitamin K in the pathogenesis of calciphylaxis. Vitamin K inhibits vascular calcification, possibly by increasing the circulating levels of carboxylated matrix Gla protein.16
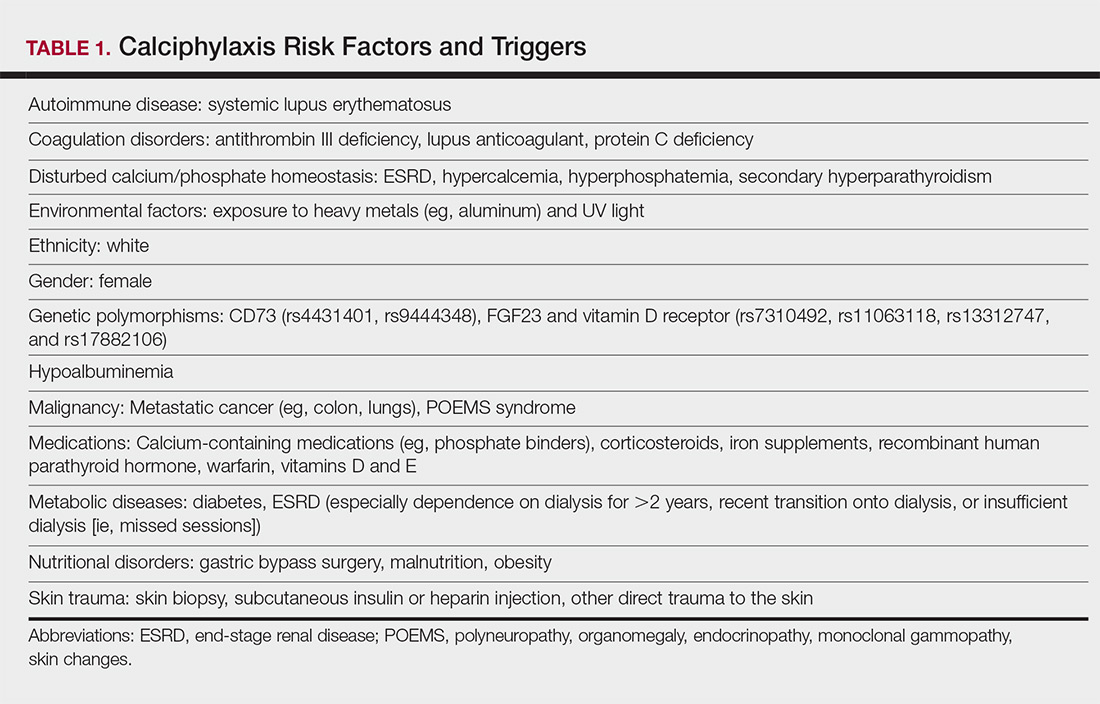
Clinical Features
Calciphylaxis is most commonly seen on the legs, abdomen, and buttocks.2 Patients with ESRD commonly develop proximal lesions affecting adipose-rich sites and have a poor prognosis. Distal lesions are more common in patients with nonuremic calciphylaxis, and mortality rates are lower in this population.2
Early lesions present as painful skin nodules or indurated plaques that often are rock-hard or firm to palpation with overlying mottling or a livedoid pattern (Figure, A). Early lesions progress from livedo reticularis to livedo racemosa and then to retiform purpura (Figure, B). Purpuric lesions later evolve into black eschars (Figure, C), then to necrotic, ulcerated, malodorous plaques or nodules in later stages of the disease (Figure, D). Lesions also may develop a gangrenous sclerotic appearance.2,5
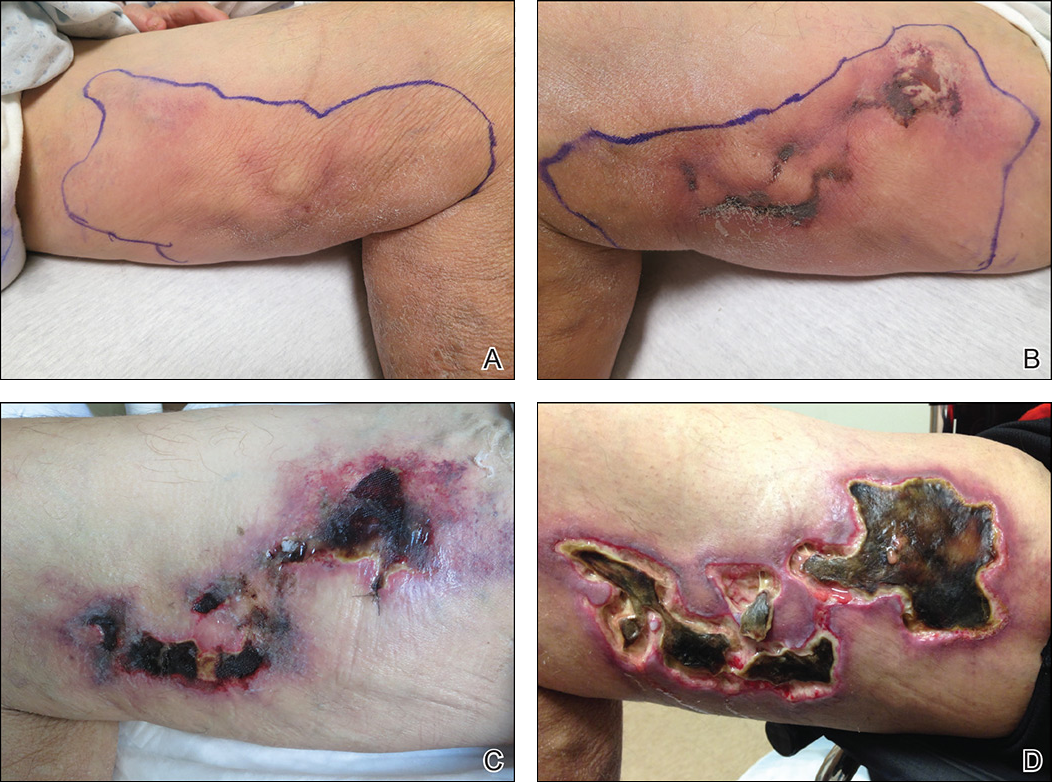
Although most patients with calciphylaxis have ESRD, nonuremic patients also can develop the disease. Those with calciphylaxis who do not have renal dysfunction frequently have other risk factors for the disease and often report another notable health problem in the weeks or months prior to presentation.4 More than half of patients with calciphylaxis become bedridden or require use of a wheelchair.17 Pain is characteristically severe throughout the course of the disease; it may even precede the appearance of the skin lesions.18 Because the pain is associated with ischemia, it tends to be relatively refractory to treatment with opioids. Rare extracutaneous vascular calcifications may lead to visual impairment, gastrointestinal tract bleeding, and myopathy.5,9,19,20
Diagnosis
Considering the high morbidity and mortality associated with calciphylaxis, it is important to provide accurate and timely diagnosis; however, there currently are no validated diagnostic criteria for calciphylaxis. Careful correlation of clinical and histologic findings is required. Calciphylaxis biopsies have demonstrated medial calcification and proliferation of the intima of small- to medium-sized arteries.21 Lobular and septal panniculitis and extravascular soft-tissue calcification, particularly stippled calcification of the eccrine sweat glands, also has been seen.2,22 Special calcium stains (eg, von Kossa, Alizarin red) increase the sensitivity of biopsy by highlighting subtle areas of intravascular and extravascular calcification.5,23 Sufficient sampling of subcutaneous tissue and specimen evaluation by an experienced dermatopathologist are necessary to ensure proper interpretation of the histologic findings.
Despite these measures, skin biopsies may be nondiagnostic or falsely negative; therefore, when there is high clinical suspicion, it may be appropriate to move forward with a presumptive diagnosis of calciphylaxis even if the histologic findings are nondiagnostic.1,9,24 It also is worth noting that localized progression and ulceration may occur following skin biopsy, such that biopsy may even be contraindicated in certain cases (eg, penile calciphylaxis).
Standard laboratory workup for calciphylaxis includes evaluation for associated risk factors as well as exclusion of other conditions in the differential diagnosis (Table 2). Blood tests to evaluate for risk factors include liver and renal function tests, a complete metabolic panel, parathyroid hormone level, and serum albumin level.5 Elevated calcium and phosphate levels may signal disturbed calcium and phosphate homeostasis but are neither sensitive nor specific for the diagnosis.25 Complete blood cell count, blood cultures, thorough hypercoagulability workup (including but not limited to antiphospholipid antibodies, proteins C and S, factor V Leiden, antithrombin III, homocysteine, methylenetetrahydrofolate reductase mutation, and cryoglobulins), rheumatoid factor, antineutrophil cytoplasmic antibodies, and antinuclear antibody testing may be relevant to help identify contributing factors or mimickers of calciphylaxis.5 Various imaging modalities also have been used to evaluate for the presence of soft-tissue calcification in areas of suspected calciphylaxis, including radiography, mammography, computed tomography, ultrasonography, nuclear bone scintigraphy, and spectroscopy.2,26,27 Unfortunately, there currently is no standardized reproducible imaging modality for reliable diagnosis of calciphylaxis. Ultimately, histologic and radiographic findings should always be interpreted in the context of relevant clinical findings.2,9
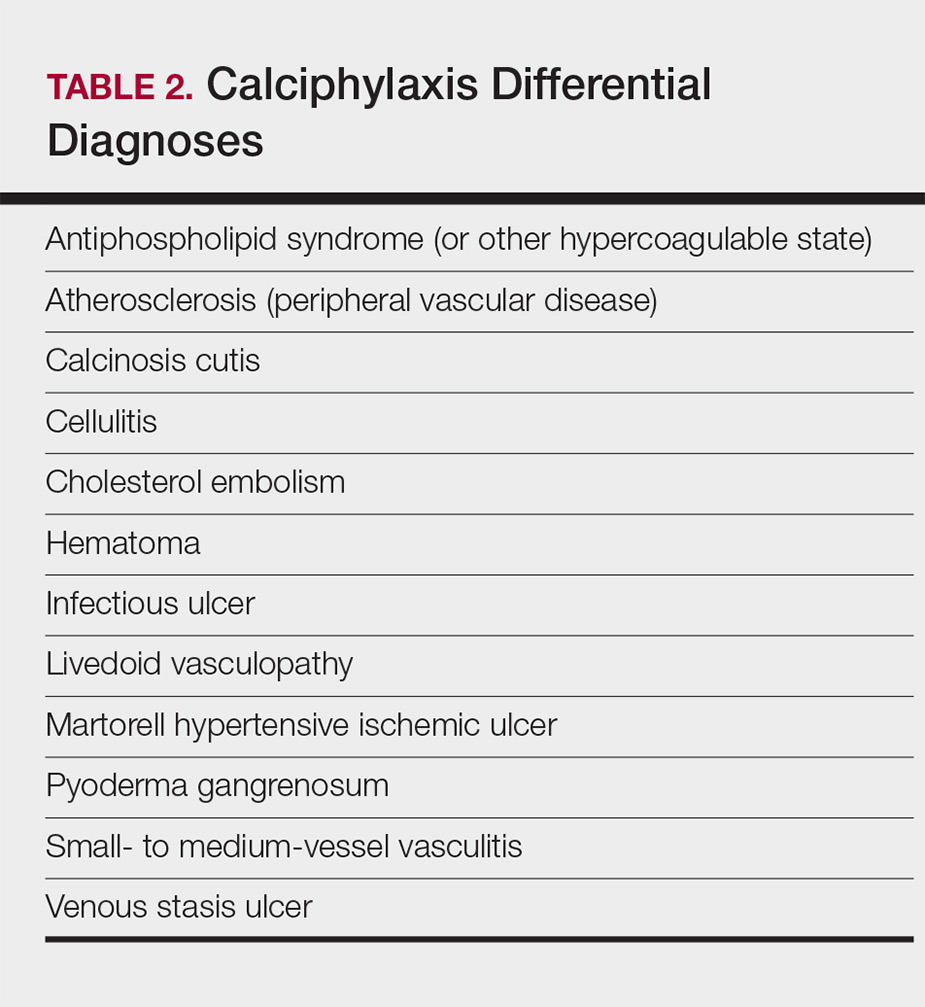
Prevention
Reduction of the net calcium phosphorus product may help reduce the risk of calciphylaxis in ESRD patients, which can be accomplished by using non–calcium-phosphate binders, adequate dialysis, and restricting use of vitamin D and vitamin K antagonists.2,5 There are limited data regarding the benefits of using bisphosphonates and cinacalcet in ESRD patients on dialysis to prevent calciphylaxis.28,29
Management
Management of calciphylaxis is multifactorial. Besides dermatology and nephrology, specialists in pain management, wound care, plastic surgery, and nutrition are critical partners in management.1,5,9,30 Nephrologists can help optimize calcium and phosphate balance and ensure adequate dialysis. Pain specialists can aid in creating aggressive multiagent pain regimens that target the neuropathic/ischemic and physical aspects of calciphylaxis pain. When appropriate, nutrition specialists can help establish high-protein, low-phosphorus diets, and wound specialists can provide access to advanced wound dressings and adjunctive hyperbaric oxygen therapy. Plastic surgeons can provide conservative debridement procedures in a subset of patients, usually those with distal stable disease.
The limited understanding of the etiopathogenesis of calciphylaxis and the lack of data on its management are reflected in the limited treatment options for the disease (Table 3).2,5,9 There are no formal algorithms for the treatment of calciphylaxis. Therapeutic trials are scarce, and most of the current treatment recommendations are based on small retrospective reports or case series. Sodium thiosulfate has been the most widely used treatment option since 2004, when its use in calciphylaxis was first reported.31 Sodium thiosulfate chelates calcium and is thought to have antioxidant and vasodilatory properties.32 There are a few promising clinical trials and large-scale studies (Table 4) that aim to evaluate the efficacy of existing treatments (eg, sodium thiosulfate) as well as novel treatment options such as lanthanum carbonate, SNF472 (hexasodium phytate), and vitamin K.33-36

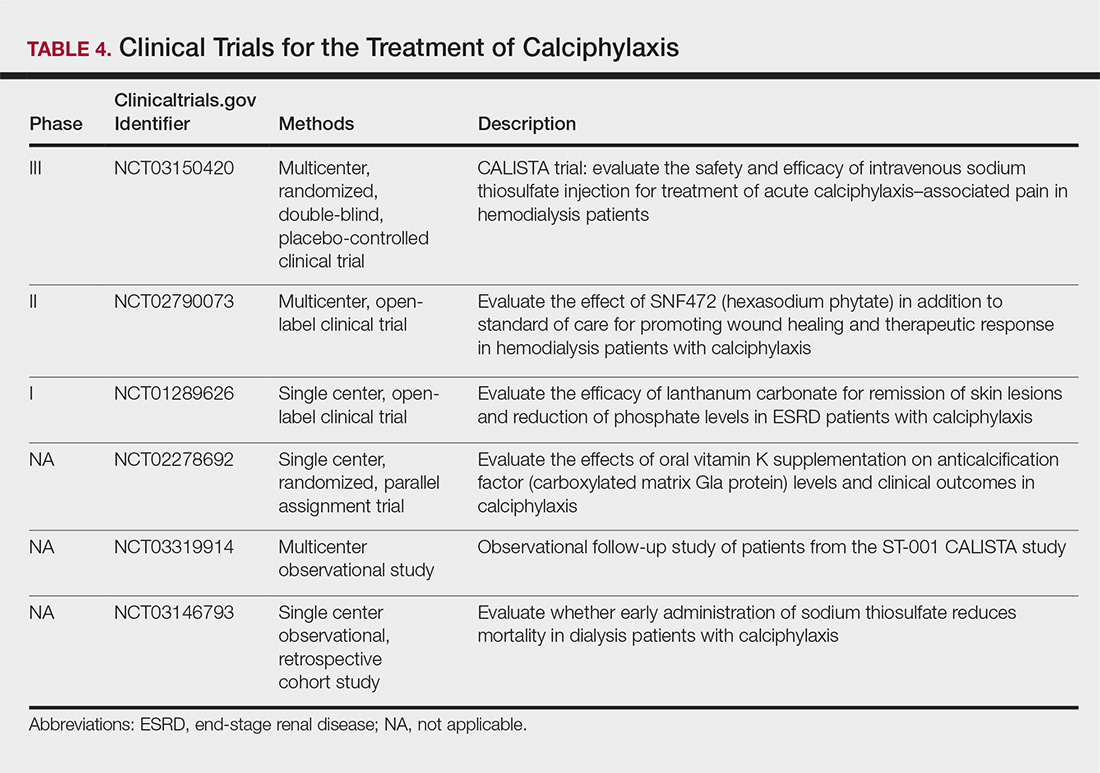
Prognosis
Calciphylaxis is a potentially fatal condition with a poor prognosis and a median survival rate of approximately 1 year following the appearance of skin lesions.37-39 Patients with proximal lesions and those on peritoneal dialysis (as opposed to hemodialysis) have a worse prognosis.40 Mortality rates are estimated to be 30% at 6 months, 50% at 12 months, and 80% at 2 years, with sepsis secondary to infection of cutaneous ulcers being the leading cause of death.37-39 The impact of calciphylaxis on patient quality of life and activities of daily living is severe.8,17
Future Directions
Multi-institution cohort studies and collaborative registries are needed to provide updated information related to the epidemiology, diagnosis, treatment, morbidity, and mortality associated with calciphylaxis and to help formulate evidence-based diagnostic criteria. Radiographic and histologic studies, as well as other tools for early and accurate diagnosis of calciphylaxis, should be studied for feasibility, accuracy, and reproducibility. The incidence of nonuremic calciphylaxis points toward pathogenic pathways besides those based on the bone-mineral axis. Basic science research directed at improving understanding of the pathophysiology of calciphylaxis would be helpful in devising new treatment strategies targeting these pathways. Establishment of a collaborative, multi-institutional calciphylaxis working group would enable experts to formulate therapeutic guidelines based on current evidence. Such a group could facilitate initiation of large prospective studies to establish the efficacy of existing and new treatment modalities for calciphylaxis. A working group within the Society for Dermatology Hospitalists has been tasked with addressing these issues and is currently establishing a multicenter calciphylaxis database.
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146.
- Nigwekar SU, Thadhani RI, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Davis JM. The relationship between obesity and calciphylaxis: a review of the literature. Ostomy Wound Manage. 2016;62:12-18.
- Bajaj R, Courbebaisse M, Kroshinsky D, et al. Calciphylaxis in patients with normal renal function: a case series and systematic review. Mayo Clin Proc. 2018;93:1202-1212.
- Hafner J, Keusch G, Wahl C, et al. Uremic small-artery disease with medial calcification and intimal hyperplasia (so-called calciphylaxis): a complication of chronic renal failure and benefit from parathyroidectomy. J Am Acad Dermatol. 1995;33:954-962.
- Jeong HS, Dominguez AR. Calciphylaxis: controversies in pathogenesis, diagnosis and treatment. Am J Med Sci. 2016;351:217-227.
- Westphal SG, Plumb T. Calciphylaxis. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2018. https://www.ncbi.nlm.nih.gov/books/NBK519020. Accessed November 12, 2018.
- Riemer CA, El-Azhary RA, Wu KL, et al. Underreported use of palliative care and patient-reported outcome measures to address reduced quality of life in patients with calciphylaxis: a systematic review. Br J Dermatol. 2017;177:1510-1518.
- Nigwekar SU. Calciphylaxis. Curr Opin Nephrol Hypertens. 2017;26:276-281.
- Fine A, Fontaine B. Calciphylaxis: the beginning of the end? Perit Dial Int. 2008;28:268-270.
- Lin WT, Chao CM. Tumoral calcinosis in renal failure. QJM. 2014;107:387.
- Schafer C, Heiss A, Schwarz A, et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357-366.
- Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78-81.
- Bleyer AJ, Choi M, Igwemezie B, et al. A case control study of proximal calciphylaxis. Am J Kidney Dis. 1998;32:376-383.
- Ahmed S, O’Neill KD, Hood AF, et al. Calciphylaxis is associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am J Kidney Dis. 2001;37:267-276.
- Nigwekar SU, Bloch DB, Nazarian RM, et al. Vitamin K-dependent carboxylation of matrix gla protein influences the risk of calciphylaxis. J Am Soc Nephrol. 2017;28:1717-1722.
- Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-579.
- Polizzotto MN, Bryan T, Ashby MA, et al. Symptomatic management of calciphylaxis: a case series and review of the literature. J Pain Symptom Manage. 2006;32:186-190.
- Gupta N, Haq KF, Mahajan S, et al. Gastrointestinal bleeding secondary to calciphylaxis. Am J Case Rep. 2015;16:818-822.
- Edelstein CL, Wickham MK, Kirby PA. Systemic calciphylaxis presenting as a painful, proximal myopathy. Postgrad Med J. 1992;68:209-211.
- Mochel MC, Arakari RY, Wang G, et al. Cutaneous calciphylaxis: a retrospective histopathologic evaluation. Am J Dermatopathol. 2013;35:582-586.
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802.
- Cassius C, Moguelet P, Monfort JB, et al. Calciphylaxis in haemodialysed patients: diagnostic value of calcifications in cutaneous biopsy. Br J Dermatol. 2018;178:292-293.
- Sreedhar A, Sheikh HA, Scagliotti CJ, et al. Advanced-stage calciphylaxis: think before you punch. Cleve Clin J Med. 2016;83:562-564.
- Brandenburg VM, Kramann R, Rothe H, et al. Calcific uraemic arteriolopathy (calciphylaxis): data from a large nation-wide registry. Nephrol Dial Transplant. 2017;32:126-132.
- Paul S, Rabito CA, Vedak P, et al. The role of bone scintigraphy in the diagnosis of calciphylaxis. JAMA Dermatol. 2017;153:101-103.
- Shmidt E, Murthy NS, Knudsen JM, et al. Net-like pattern of calcification on plain soft-tissue radiographs in patients with calciphylaxis. J Am Acad Dermatol. 2012;67:1296-1301.
- EVOLVE Trial Investigators; Chertow GM, Block GA, Correa-Rotter R, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482-2494.
- Rogers NM, Teubner DJO, Coates PT. Calcific uremic arteriolopathy: advances in pathogenesis and treatment. Semin Dial. 2007;20:150-157.
- Nigwekar SU. Multidisciplinary approach to calcific uremic arteriolopathy. Curr Opin Nephrol Hypertens. 2015;24:531-537.
- Cicone JS, Petronis JB, Embert CD, et al. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104-1108.
- Chen NX, O’Neill K, Akl NK, et al. Adipocyte induced arterial calcification is prevented with sodium thiosulfate. Biochem Biophys Res Commun. 2014;449:151-156.
- Chan MR, Ghandour F, Murali NS, et al. Pilot study of the effect of lanthanum carbonate in patients with calciphylaxis: a Wisconsin Network for Health Research (WiNHR) study. J Nephrol Ther. 2014;4:1000162.
- Perelló J, Gómez M, Ferrer MD, et al. SNF472, a novel inhibitor of vascular calcification, could be administered during hemodialysis to attain potentially therapeutic phytate levels. J Nephrol. 2018;31:287-296.
- Christiadi D, Singer RF. Calciphylaxis in a dialysis patient successfully treated with high-dose vitamin K supplementation. Clin Kidney J. 2018;11:528-529.
- Caluwe R, Vandecasteele S, Van Vlem B, et al. Vitamin K2 supplementation in haemodialysis patients: a randomized dose-finding study. Nephrol Dial Transplant. 2014;29:1385-1390.
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394.
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217.
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429.
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241.
Calciphylaxis, also known as calcific uremic arteriolopathy, is a painful skin condition classically seen in patients with end-stage renal disease (ESRD), particularly those on chronic dialysis.1,2 It also has increasingly been reported in patients with normal renal function and calcium and phosphate homeostasis.3,4 Effective diagnosis and management of calciphylaxis remains challenging for physicians.2,5 The condition is characterized by tissue ischemia caused by calcification of cutaneous arteriolar vessels. As a result, calciphylaxis is associated with high mortality rates, ranging from 60% to 80%.5,6 Excruciating pain and nonhealing ulcers often lead to recurrent hospitalizations and infectious complications,7 and poor nutritional status, chronic pain, depression, and insomnia can further complicate recovery and lead to poor quality of life.8
We provide an update on calciphylaxis etiopathogenesis, diagnosis, and management. We also highlight some challenges faced in managing this potentially fatal condition.
Epidemiology
Calciphylaxis is considered a rare dermatosis with an estimated annual incidence of 1% to 4% in ESRD patients on dialysis. Recent data suggest that incidence of calciphylaxis is rising,5,7,9 which may stem from an increased use of calcium-based phosphate binders, an actual rise in disease incidence, and/or increased recognition of the disease.5 It is difficult to estimate the exact disease burden of calciphylaxis because the diagnostic criteria are not well defined, often leading to missed or delayed diagnosis.3,10 Furthermore, there is no centralized registry for calciphylaxis cases.3
Etiology and Pathogenesis
Calciphylaxis is thought to have a multifactorial etiology with the exact cause or trigger unknown.7 A long list of risk factors and triggers is associated with the condition (Table 1). Calciphylaxis primarily affects small arteries (40–600 μm in diameter) that become calcified due to an imbalance between inhibitors and promoters of calcification.2,11 Fetuin-A and matrix Gla protein inhibit vascular calcification and are downregulated in calciphylaxis.12,13 Dysfunctional calcium, phosphate, and parathyroid hormone regulatory pathways provide an increased substrate for the process of calcification, which causes endothelial damage and microthrombosis, resulting in tissue ischemia and infarction.14,15 Notably, there is growing interest in the role of vitamin K in the pathogenesis of calciphylaxis. Vitamin K inhibits vascular calcification, possibly by increasing the circulating levels of carboxylated matrix Gla protein.16

Clinical Features
Calciphylaxis is most commonly seen on the legs, abdomen, and buttocks.2 Patients with ESRD commonly develop proximal lesions affecting adipose-rich sites and have a poor prognosis. Distal lesions are more common in patients with nonuremic calciphylaxis, and mortality rates are lower in this population.2
Early lesions present as painful skin nodules or indurated plaques that often are rock-hard or firm to palpation with overlying mottling or a livedoid pattern (Figure, A). Early lesions progress from livedo reticularis to livedo racemosa and then to retiform purpura (Figure, B). Purpuric lesions later evolve into black eschars (Figure, C), then to necrotic, ulcerated, malodorous plaques or nodules in later stages of the disease (Figure, D). Lesions also may develop a gangrenous sclerotic appearance.2,5

Although most patients with calciphylaxis have ESRD, nonuremic patients also can develop the disease. Those with calciphylaxis who do not have renal dysfunction frequently have other risk factors for the disease and often report another notable health problem in the weeks or months prior to presentation.4 More than half of patients with calciphylaxis become bedridden or require use of a wheelchair.17 Pain is characteristically severe throughout the course of the disease; it may even precede the appearance of the skin lesions.18 Because the pain is associated with ischemia, it tends to be relatively refractory to treatment with opioids. Rare extracutaneous vascular calcifications may lead to visual impairment, gastrointestinal tract bleeding, and myopathy.5,9,19,20
Diagnosis
Considering the high morbidity and mortality associated with calciphylaxis, it is important to provide accurate and timely diagnosis; however, there currently are no validated diagnostic criteria for calciphylaxis. Careful correlation of clinical and histologic findings is required. Calciphylaxis biopsies have demonstrated medial calcification and proliferation of the intima of small- to medium-sized arteries.21 Lobular and septal panniculitis and extravascular soft-tissue calcification, particularly stippled calcification of the eccrine sweat glands, also has been seen.2,22 Special calcium stains (eg, von Kossa, Alizarin red) increase the sensitivity of biopsy by highlighting subtle areas of intravascular and extravascular calcification.5,23 Sufficient sampling of subcutaneous tissue and specimen evaluation by an experienced dermatopathologist are necessary to ensure proper interpretation of the histologic findings.
Despite these measures, skin biopsies may be nondiagnostic or falsely negative; therefore, when there is high clinical suspicion, it may be appropriate to move forward with a presumptive diagnosis of calciphylaxis even if the histologic findings are nondiagnostic.1,9,24 It also is worth noting that localized progression and ulceration may occur following skin biopsy, such that biopsy may even be contraindicated in certain cases (eg, penile calciphylaxis).
Standard laboratory workup for calciphylaxis includes evaluation for associated risk factors as well as exclusion of other conditions in the differential diagnosis (Table 2). Blood tests to evaluate for risk factors include liver and renal function tests, a complete metabolic panel, parathyroid hormone level, and serum albumin level.5 Elevated calcium and phosphate levels may signal disturbed calcium and phosphate homeostasis but are neither sensitive nor specific for the diagnosis.25 Complete blood cell count, blood cultures, thorough hypercoagulability workup (including but not limited to antiphospholipid antibodies, proteins C and S, factor V Leiden, antithrombin III, homocysteine, methylenetetrahydrofolate reductase mutation, and cryoglobulins), rheumatoid factor, antineutrophil cytoplasmic antibodies, and antinuclear antibody testing may be relevant to help identify contributing factors or mimickers of calciphylaxis.5 Various imaging modalities also have been used to evaluate for the presence of soft-tissue calcification in areas of suspected calciphylaxis, including radiography, mammography, computed tomography, ultrasonography, nuclear bone scintigraphy, and spectroscopy.2,26,27 Unfortunately, there currently is no standardized reproducible imaging modality for reliable diagnosis of calciphylaxis. Ultimately, histologic and radiographic findings should always be interpreted in the context of relevant clinical findings.2,9

Prevention
Reduction of the net calcium phosphorus product may help reduce the risk of calciphylaxis in ESRD patients, which can be accomplished by using non–calcium-phosphate binders, adequate dialysis, and restricting use of vitamin D and vitamin K antagonists.2,5 There are limited data regarding the benefits of using bisphosphonates and cinacalcet in ESRD patients on dialysis to prevent calciphylaxis.28,29
Management
Management of calciphylaxis is multifactorial. Besides dermatology and nephrology, specialists in pain management, wound care, plastic surgery, and nutrition are critical partners in management.1,5,9,30 Nephrologists can help optimize calcium and phosphate balance and ensure adequate dialysis. Pain specialists can aid in creating aggressive multiagent pain regimens that target the neuropathic/ischemic and physical aspects of calciphylaxis pain. When appropriate, nutrition specialists can help establish high-protein, low-phosphorus diets, and wound specialists can provide access to advanced wound dressings and adjunctive hyperbaric oxygen therapy. Plastic surgeons can provide conservative debridement procedures in a subset of patients, usually those with distal stable disease.
The limited understanding of the etiopathogenesis of calciphylaxis and the lack of data on its management are reflected in the limited treatment options for the disease (Table 3).2,5,9 There are no formal algorithms for the treatment of calciphylaxis. Therapeutic trials are scarce, and most of the current treatment recommendations are based on small retrospective reports or case series. Sodium thiosulfate has been the most widely used treatment option since 2004, when its use in calciphylaxis was first reported.31 Sodium thiosulfate chelates calcium and is thought to have antioxidant and vasodilatory properties.32 There are a few promising clinical trials and large-scale studies (Table 4) that aim to evaluate the efficacy of existing treatments (eg, sodium thiosulfate) as well as novel treatment options such as lanthanum carbonate, SNF472 (hexasodium phytate), and vitamin K.33-36


Prognosis
Calciphylaxis is a potentially fatal condition with a poor prognosis and a median survival rate of approximately 1 year following the appearance of skin lesions.37-39 Patients with proximal lesions and those on peritoneal dialysis (as opposed to hemodialysis) have a worse prognosis.40 Mortality rates are estimated to be 30% at 6 months, 50% at 12 months, and 80% at 2 years, with sepsis secondary to infection of cutaneous ulcers being the leading cause of death.37-39 The impact of calciphylaxis on patient quality of life and activities of daily living is severe.8,17
Future Directions
Multi-institution cohort studies and collaborative registries are needed to provide updated information related to the epidemiology, diagnosis, treatment, morbidity, and mortality associated with calciphylaxis and to help formulate evidence-based diagnostic criteria. Radiographic and histologic studies, as well as other tools for early and accurate diagnosis of calciphylaxis, should be studied for feasibility, accuracy, and reproducibility. The incidence of nonuremic calciphylaxis points toward pathogenic pathways besides those based on the bone-mineral axis. Basic science research directed at improving understanding of the pathophysiology of calciphylaxis would be helpful in devising new treatment strategies targeting these pathways. Establishment of a collaborative, multi-institutional calciphylaxis working group would enable experts to formulate therapeutic guidelines based on current evidence. Such a group could facilitate initiation of large prospective studies to establish the efficacy of existing and new treatment modalities for calciphylaxis. A working group within the Society for Dermatology Hospitalists has been tasked with addressing these issues and is currently establishing a multicenter calciphylaxis database.
Calciphylaxis, also known as calcific uremic arteriolopathy, is a painful skin condition classically seen in patients with end-stage renal disease (ESRD), particularly those on chronic dialysis.1,2 It also has increasingly been reported in patients with normal renal function and calcium and phosphate homeostasis.3,4 Effective diagnosis and management of calciphylaxis remains challenging for physicians.2,5 The condition is characterized by tissue ischemia caused by calcification of cutaneous arteriolar vessels. As a result, calciphylaxis is associated with high mortality rates, ranging from 60% to 80%.5,6 Excruciating pain and nonhealing ulcers often lead to recurrent hospitalizations and infectious complications,7 and poor nutritional status, chronic pain, depression, and insomnia can further complicate recovery and lead to poor quality of life.8
We provide an update on calciphylaxis etiopathogenesis, diagnosis, and management. We also highlight some challenges faced in managing this potentially fatal condition.
Epidemiology
Calciphylaxis is considered a rare dermatosis with an estimated annual incidence of 1% to 4% in ESRD patients on dialysis. Recent data suggest that incidence of calciphylaxis is rising,5,7,9 which may stem from an increased use of calcium-based phosphate binders, an actual rise in disease incidence, and/or increased recognition of the disease.5 It is difficult to estimate the exact disease burden of calciphylaxis because the diagnostic criteria are not well defined, often leading to missed or delayed diagnosis.3,10 Furthermore, there is no centralized registry for calciphylaxis cases.3
Etiology and Pathogenesis
Calciphylaxis is thought to have a multifactorial etiology with the exact cause or trigger unknown.7 A long list of risk factors and triggers is associated with the condition (Table 1). Calciphylaxis primarily affects small arteries (40–600 μm in diameter) that become calcified due to an imbalance between inhibitors and promoters of calcification.2,11 Fetuin-A and matrix Gla protein inhibit vascular calcification and are downregulated in calciphylaxis.12,13 Dysfunctional calcium, phosphate, and parathyroid hormone regulatory pathways provide an increased substrate for the process of calcification, which causes endothelial damage and microthrombosis, resulting in tissue ischemia and infarction.14,15 Notably, there is growing interest in the role of vitamin K in the pathogenesis of calciphylaxis. Vitamin K inhibits vascular calcification, possibly by increasing the circulating levels of carboxylated matrix Gla protein.16

Clinical Features
Calciphylaxis is most commonly seen on the legs, abdomen, and buttocks.2 Patients with ESRD commonly develop proximal lesions affecting adipose-rich sites and have a poor prognosis. Distal lesions are more common in patients with nonuremic calciphylaxis, and mortality rates are lower in this population.2
Early lesions present as painful skin nodules or indurated plaques that often are rock-hard or firm to palpation with overlying mottling or a livedoid pattern (Figure, A). Early lesions progress from livedo reticularis to livedo racemosa and then to retiform purpura (Figure, B). Purpuric lesions later evolve into black eschars (Figure, C), then to necrotic, ulcerated, malodorous plaques or nodules in later stages of the disease (Figure, D). Lesions also may develop a gangrenous sclerotic appearance.2,5

Although most patients with calciphylaxis have ESRD, nonuremic patients also can develop the disease. Those with calciphylaxis who do not have renal dysfunction frequently have other risk factors for the disease and often report another notable health problem in the weeks or months prior to presentation.4 More than half of patients with calciphylaxis become bedridden or require use of a wheelchair.17 Pain is characteristically severe throughout the course of the disease; it may even precede the appearance of the skin lesions.18 Because the pain is associated with ischemia, it tends to be relatively refractory to treatment with opioids. Rare extracutaneous vascular calcifications may lead to visual impairment, gastrointestinal tract bleeding, and myopathy.5,9,19,20
Diagnosis
Considering the high morbidity and mortality associated with calciphylaxis, it is important to provide accurate and timely diagnosis; however, there currently are no validated diagnostic criteria for calciphylaxis. Careful correlation of clinical and histologic findings is required. Calciphylaxis biopsies have demonstrated medial calcification and proliferation of the intima of small- to medium-sized arteries.21 Lobular and septal panniculitis and extravascular soft-tissue calcification, particularly stippled calcification of the eccrine sweat glands, also has been seen.2,22 Special calcium stains (eg, von Kossa, Alizarin red) increase the sensitivity of biopsy by highlighting subtle areas of intravascular and extravascular calcification.5,23 Sufficient sampling of subcutaneous tissue and specimen evaluation by an experienced dermatopathologist are necessary to ensure proper interpretation of the histologic findings.
Despite these measures, skin biopsies may be nondiagnostic or falsely negative; therefore, when there is high clinical suspicion, it may be appropriate to move forward with a presumptive diagnosis of calciphylaxis even if the histologic findings are nondiagnostic.1,9,24 It also is worth noting that localized progression and ulceration may occur following skin biopsy, such that biopsy may even be contraindicated in certain cases (eg, penile calciphylaxis).
Standard laboratory workup for calciphylaxis includes evaluation for associated risk factors as well as exclusion of other conditions in the differential diagnosis (Table 2). Blood tests to evaluate for risk factors include liver and renal function tests, a complete metabolic panel, parathyroid hormone level, and serum albumin level.5 Elevated calcium and phosphate levels may signal disturbed calcium and phosphate homeostasis but are neither sensitive nor specific for the diagnosis.25 Complete blood cell count, blood cultures, thorough hypercoagulability workup (including but not limited to antiphospholipid antibodies, proteins C and S, factor V Leiden, antithrombin III, homocysteine, methylenetetrahydrofolate reductase mutation, and cryoglobulins), rheumatoid factor, antineutrophil cytoplasmic antibodies, and antinuclear antibody testing may be relevant to help identify contributing factors or mimickers of calciphylaxis.5 Various imaging modalities also have been used to evaluate for the presence of soft-tissue calcification in areas of suspected calciphylaxis, including radiography, mammography, computed tomography, ultrasonography, nuclear bone scintigraphy, and spectroscopy.2,26,27 Unfortunately, there currently is no standardized reproducible imaging modality for reliable diagnosis of calciphylaxis. Ultimately, histologic and radiographic findings should always be interpreted in the context of relevant clinical findings.2,9

Prevention
Reduction of the net calcium phosphorus product may help reduce the risk of calciphylaxis in ESRD patients, which can be accomplished by using non–calcium-phosphate binders, adequate dialysis, and restricting use of vitamin D and vitamin K antagonists.2,5 There are limited data regarding the benefits of using bisphosphonates and cinacalcet in ESRD patients on dialysis to prevent calciphylaxis.28,29
Management
Management of calciphylaxis is multifactorial. Besides dermatology and nephrology, specialists in pain management, wound care, plastic surgery, and nutrition are critical partners in management.1,5,9,30 Nephrologists can help optimize calcium and phosphate balance and ensure adequate dialysis. Pain specialists can aid in creating aggressive multiagent pain regimens that target the neuropathic/ischemic and physical aspects of calciphylaxis pain. When appropriate, nutrition specialists can help establish high-protein, low-phosphorus diets, and wound specialists can provide access to advanced wound dressings and adjunctive hyperbaric oxygen therapy. Plastic surgeons can provide conservative debridement procedures in a subset of patients, usually those with distal stable disease.
The limited understanding of the etiopathogenesis of calciphylaxis and the lack of data on its management are reflected in the limited treatment options for the disease (Table 3).2,5,9 There are no formal algorithms for the treatment of calciphylaxis. Therapeutic trials are scarce, and most of the current treatment recommendations are based on small retrospective reports or case series. Sodium thiosulfate has been the most widely used treatment option since 2004, when its use in calciphylaxis was first reported.31 Sodium thiosulfate chelates calcium and is thought to have antioxidant and vasodilatory properties.32 There are a few promising clinical trials and large-scale studies (Table 4) that aim to evaluate the efficacy of existing treatments (eg, sodium thiosulfate) as well as novel treatment options such as lanthanum carbonate, SNF472 (hexasodium phytate), and vitamin K.33-36


Prognosis
Calciphylaxis is a potentially fatal condition with a poor prognosis and a median survival rate of approximately 1 year following the appearance of skin lesions.37-39 Patients with proximal lesions and those on peritoneal dialysis (as opposed to hemodialysis) have a worse prognosis.40 Mortality rates are estimated to be 30% at 6 months, 50% at 12 months, and 80% at 2 years, with sepsis secondary to infection of cutaneous ulcers being the leading cause of death.37-39 The impact of calciphylaxis on patient quality of life and activities of daily living is severe.8,17
Future Directions
Multi-institution cohort studies and collaborative registries are needed to provide updated information related to the epidemiology, diagnosis, treatment, morbidity, and mortality associated with calciphylaxis and to help formulate evidence-based diagnostic criteria. Radiographic and histologic studies, as well as other tools for early and accurate diagnosis of calciphylaxis, should be studied for feasibility, accuracy, and reproducibility. The incidence of nonuremic calciphylaxis points toward pathogenic pathways besides those based on the bone-mineral axis. Basic science research directed at improving understanding of the pathophysiology of calciphylaxis would be helpful in devising new treatment strategies targeting these pathways. Establishment of a collaborative, multi-institutional calciphylaxis working group would enable experts to formulate therapeutic guidelines based on current evidence. Such a group could facilitate initiation of large prospective studies to establish the efficacy of existing and new treatment modalities for calciphylaxis. A working group within the Society for Dermatology Hospitalists has been tasked with addressing these issues and is currently establishing a multicenter calciphylaxis database.
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146.
- Nigwekar SU, Thadhani RI, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Davis JM. The relationship between obesity and calciphylaxis: a review of the literature. Ostomy Wound Manage. 2016;62:12-18.
- Bajaj R, Courbebaisse M, Kroshinsky D, et al. Calciphylaxis in patients with normal renal function: a case series and systematic review. Mayo Clin Proc. 2018;93:1202-1212.
- Hafner J, Keusch G, Wahl C, et al. Uremic small-artery disease with medial calcification and intimal hyperplasia (so-called calciphylaxis): a complication of chronic renal failure and benefit from parathyroidectomy. J Am Acad Dermatol. 1995;33:954-962.
- Jeong HS, Dominguez AR. Calciphylaxis: controversies in pathogenesis, diagnosis and treatment. Am J Med Sci. 2016;351:217-227.
- Westphal SG, Plumb T. Calciphylaxis. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2018. https://www.ncbi.nlm.nih.gov/books/NBK519020. Accessed November 12, 2018.
- Riemer CA, El-Azhary RA, Wu KL, et al. Underreported use of palliative care and patient-reported outcome measures to address reduced quality of life in patients with calciphylaxis: a systematic review. Br J Dermatol. 2017;177:1510-1518.
- Nigwekar SU. Calciphylaxis. Curr Opin Nephrol Hypertens. 2017;26:276-281.
- Fine A, Fontaine B. Calciphylaxis: the beginning of the end? Perit Dial Int. 2008;28:268-270.
- Lin WT, Chao CM. Tumoral calcinosis in renal failure. QJM. 2014;107:387.
- Schafer C, Heiss A, Schwarz A, et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357-366.
- Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78-81.
- Bleyer AJ, Choi M, Igwemezie B, et al. A case control study of proximal calciphylaxis. Am J Kidney Dis. 1998;32:376-383.
- Ahmed S, O’Neill KD, Hood AF, et al. Calciphylaxis is associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am J Kidney Dis. 2001;37:267-276.
- Nigwekar SU, Bloch DB, Nazarian RM, et al. Vitamin K-dependent carboxylation of matrix gla protein influences the risk of calciphylaxis. J Am Soc Nephrol. 2017;28:1717-1722.
- Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-579.
- Polizzotto MN, Bryan T, Ashby MA, et al. Symptomatic management of calciphylaxis: a case series and review of the literature. J Pain Symptom Manage. 2006;32:186-190.
- Gupta N, Haq KF, Mahajan S, et al. Gastrointestinal bleeding secondary to calciphylaxis. Am J Case Rep. 2015;16:818-822.
- Edelstein CL, Wickham MK, Kirby PA. Systemic calciphylaxis presenting as a painful, proximal myopathy. Postgrad Med J. 1992;68:209-211.
- Mochel MC, Arakari RY, Wang G, et al. Cutaneous calciphylaxis: a retrospective histopathologic evaluation. Am J Dermatopathol. 2013;35:582-586.
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802.
- Cassius C, Moguelet P, Monfort JB, et al. Calciphylaxis in haemodialysed patients: diagnostic value of calcifications in cutaneous biopsy. Br J Dermatol. 2018;178:292-293.
- Sreedhar A, Sheikh HA, Scagliotti CJ, et al. Advanced-stage calciphylaxis: think before you punch. Cleve Clin J Med. 2016;83:562-564.
- Brandenburg VM, Kramann R, Rothe H, et al. Calcific uraemic arteriolopathy (calciphylaxis): data from a large nation-wide registry. Nephrol Dial Transplant. 2017;32:126-132.
- Paul S, Rabito CA, Vedak P, et al. The role of bone scintigraphy in the diagnosis of calciphylaxis. JAMA Dermatol. 2017;153:101-103.
- Shmidt E, Murthy NS, Knudsen JM, et al. Net-like pattern of calcification on plain soft-tissue radiographs in patients with calciphylaxis. J Am Acad Dermatol. 2012;67:1296-1301.
- EVOLVE Trial Investigators; Chertow GM, Block GA, Correa-Rotter R, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482-2494.
- Rogers NM, Teubner DJO, Coates PT. Calcific uremic arteriolopathy: advances in pathogenesis and treatment. Semin Dial. 2007;20:150-157.
- Nigwekar SU. Multidisciplinary approach to calcific uremic arteriolopathy. Curr Opin Nephrol Hypertens. 2015;24:531-537.
- Cicone JS, Petronis JB, Embert CD, et al. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104-1108.
- Chen NX, O’Neill K, Akl NK, et al. Adipocyte induced arterial calcification is prevented with sodium thiosulfate. Biochem Biophys Res Commun. 2014;449:151-156.
- Chan MR, Ghandour F, Murali NS, et al. Pilot study of the effect of lanthanum carbonate in patients with calciphylaxis: a Wisconsin Network for Health Research (WiNHR) study. J Nephrol Ther. 2014;4:1000162.
- Perelló J, Gómez M, Ferrer MD, et al. SNF472, a novel inhibitor of vascular calcification, could be administered during hemodialysis to attain potentially therapeutic phytate levels. J Nephrol. 2018;31:287-296.
- Christiadi D, Singer RF. Calciphylaxis in a dialysis patient successfully treated with high-dose vitamin K supplementation. Clin Kidney J. 2018;11:528-529.
- Caluwe R, Vandecasteele S, Van Vlem B, et al. Vitamin K2 supplementation in haemodialysis patients: a randomized dose-finding study. Nephrol Dial Transplant. 2014;29:1385-1390.
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394.
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217.
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429.
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241.
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146.
- Nigwekar SU, Thadhani RI, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Davis JM. The relationship between obesity and calciphylaxis: a review of the literature. Ostomy Wound Manage. 2016;62:12-18.
- Bajaj R, Courbebaisse M, Kroshinsky D, et al. Calciphylaxis in patients with normal renal function: a case series and systematic review. Mayo Clin Proc. 2018;93:1202-1212.
- Hafner J, Keusch G, Wahl C, et al. Uremic small-artery disease with medial calcification and intimal hyperplasia (so-called calciphylaxis): a complication of chronic renal failure and benefit from parathyroidectomy. J Am Acad Dermatol. 1995;33:954-962.
- Jeong HS, Dominguez AR. Calciphylaxis: controversies in pathogenesis, diagnosis and treatment. Am J Med Sci. 2016;351:217-227.
- Westphal SG, Plumb T. Calciphylaxis. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2018. https://www.ncbi.nlm.nih.gov/books/NBK519020. Accessed November 12, 2018.
- Riemer CA, El-Azhary RA, Wu KL, et al. Underreported use of palliative care and patient-reported outcome measures to address reduced quality of life in patients with calciphylaxis: a systematic review. Br J Dermatol. 2017;177:1510-1518.
- Nigwekar SU. Calciphylaxis. Curr Opin Nephrol Hypertens. 2017;26:276-281.
- Fine A, Fontaine B. Calciphylaxis: the beginning of the end? Perit Dial Int. 2008;28:268-270.
- Lin WT, Chao CM. Tumoral calcinosis in renal failure. QJM. 2014;107:387.
- Schafer C, Heiss A, Schwarz A, et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357-366.
- Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78-81.
- Bleyer AJ, Choi M, Igwemezie B, et al. A case control study of proximal calciphylaxis. Am J Kidney Dis. 1998;32:376-383.
- Ahmed S, O’Neill KD, Hood AF, et al. Calciphylaxis is associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am J Kidney Dis. 2001;37:267-276.
- Nigwekar SU, Bloch DB, Nazarian RM, et al. Vitamin K-dependent carboxylation of matrix gla protein influences the risk of calciphylaxis. J Am Soc Nephrol. 2017;28:1717-1722.
- Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-579.
- Polizzotto MN, Bryan T, Ashby MA, et al. Symptomatic management of calciphylaxis: a case series and review of the literature. J Pain Symptom Manage. 2006;32:186-190.
- Gupta N, Haq KF, Mahajan S, et al. Gastrointestinal bleeding secondary to calciphylaxis. Am J Case Rep. 2015;16:818-822.
- Edelstein CL, Wickham MK, Kirby PA. Systemic calciphylaxis presenting as a painful, proximal myopathy. Postgrad Med J. 1992;68:209-211.
- Mochel MC, Arakari RY, Wang G, et al. Cutaneous calciphylaxis: a retrospective histopathologic evaluation. Am J Dermatopathol. 2013;35:582-586.
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802.
- Cassius C, Moguelet P, Monfort JB, et al. Calciphylaxis in haemodialysed patients: diagnostic value of calcifications in cutaneous biopsy. Br J Dermatol. 2018;178:292-293.
- Sreedhar A, Sheikh HA, Scagliotti CJ, et al. Advanced-stage calciphylaxis: think before you punch. Cleve Clin J Med. 2016;83:562-564.
- Brandenburg VM, Kramann R, Rothe H, et al. Calcific uraemic arteriolopathy (calciphylaxis): data from a large nation-wide registry. Nephrol Dial Transplant. 2017;32:126-132.
- Paul S, Rabito CA, Vedak P, et al. The role of bone scintigraphy in the diagnosis of calciphylaxis. JAMA Dermatol. 2017;153:101-103.
- Shmidt E, Murthy NS, Knudsen JM, et al. Net-like pattern of calcification on plain soft-tissue radiographs in patients with calciphylaxis. J Am Acad Dermatol. 2012;67:1296-1301.
- EVOLVE Trial Investigators; Chertow GM, Block GA, Correa-Rotter R, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482-2494.
- Rogers NM, Teubner DJO, Coates PT. Calcific uremic arteriolopathy: advances in pathogenesis and treatment. Semin Dial. 2007;20:150-157.
- Nigwekar SU. Multidisciplinary approach to calcific uremic arteriolopathy. Curr Opin Nephrol Hypertens. 2015;24:531-537.
- Cicone JS, Petronis JB, Embert CD, et al. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104-1108.
- Chen NX, O’Neill K, Akl NK, et al. Adipocyte induced arterial calcification is prevented with sodium thiosulfate. Biochem Biophys Res Commun. 2014;449:151-156.
- Chan MR, Ghandour F, Murali NS, et al. Pilot study of the effect of lanthanum carbonate in patients with calciphylaxis: a Wisconsin Network for Health Research (WiNHR) study. J Nephrol Ther. 2014;4:1000162.
- Perelló J, Gómez M, Ferrer MD, et al. SNF472, a novel inhibitor of vascular calcification, could be administered during hemodialysis to attain potentially therapeutic phytate levels. J Nephrol. 2018;31:287-296.
- Christiadi D, Singer RF. Calciphylaxis in a dialysis patient successfully treated with high-dose vitamin K supplementation. Clin Kidney J. 2018;11:528-529.
- Caluwe R, Vandecasteele S, Van Vlem B, et al. Vitamin K2 supplementation in haemodialysis patients: a randomized dose-finding study. Nephrol Dial Transplant. 2014;29:1385-1390.
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394.
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217.
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429.
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241.
Practice Points
- Maintain a high index of suspicion for calciphylaxis in patients with end-stage renal disease on chronic dialysis presenting with severely painful livedoid plaques or retiform purpura, particularly in fat-rich body sites.
- Skin biopsies may be limited by biopsy site, inadequate biopsy depth, missed areas of microcalcification, and absence of definitive histologic criteria. Special calcium stains and review by an experienced dermatopathologist may lower the rate of false-negative biopsies.
- In cases where the most likely clinical diagnosis is calciphylaxis, treatment should be initiated even if definitive histopathology findings are lacking.
- Treatment should be multimodal, including elimination of risk factors, intravenous sodium thiosulfate, agents addressing calcium-phosphate metabolism, and surgical debridement, if indicated.
Enlarging Red Papulonodule on the Chest
The Diagnosis: Metastatic Renal Cell Carcinoma
Histopathologic examination of the punch biopsy demonstrated epithelioid cells with abundant clear cytoplasm and numerous chicken wire-like vascular channels consistent with a diagnosis of cutaneous metastasis of renal cell carcinoma (RCC)(Figure). Collateral history revealed that 8 years prior, the patient had been diagnosed with clear cell RCC, stage III (T3aN0M0). At that time, he was treated with radical nephrectomy, which was considered curative. He remained disease free until several months prior to the development of the cutaneous lesion when he was found to have pulmonary and cerebral metastases with biopsies showing metastatic RCC. He was treated with lobectomy and Gamma Knife radiation for the lung and cerebral metastases, respectively. His oncologist planned to initiate therapy with the multikinase inhibitor sunitinib, which inhibits vascular endothelial growth factor (VEGF) signaling. Unfortunately, the patient died prior to treatment due to overwhelming tumor burden.
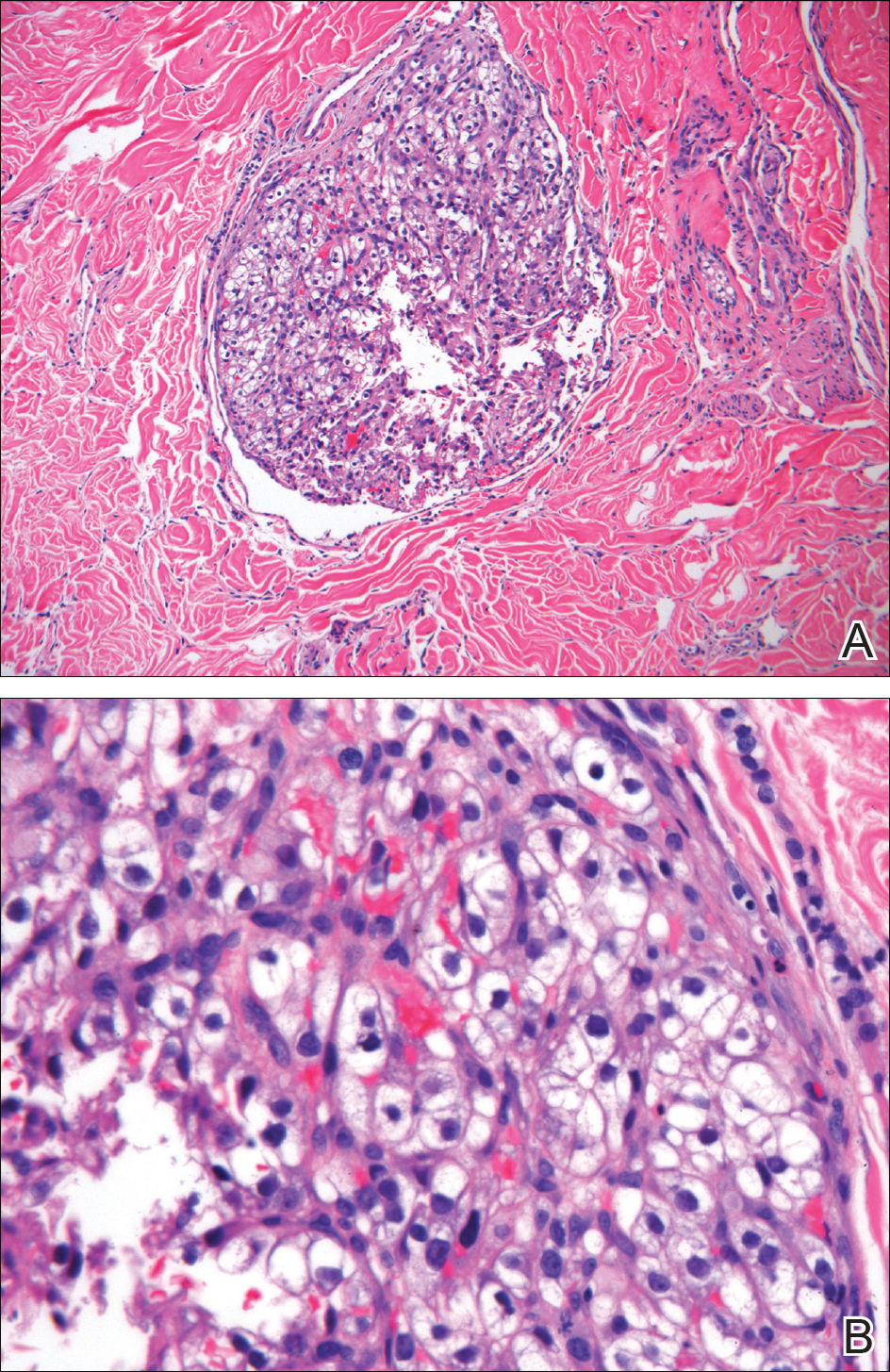
Clear cell RCC, the most common renal malignancy, presents with metastatic disease at the time of diagnosis in 21% of patients.1 An additional 20% of patients with localized disease develop metastases within several years of receiving a nephrectomy without adjuvant therapy, which is standard treatment for stage I to stage III disease.1,2 Metastatic RCC most frequently targets the lungs, bone, liver, and brain, though virtually any organ can be involved. Cutaneous involvement is estimated to occur in 3.3% of RCC cases,3 accounting for only 1.4% of cutaneous metastases overall.4 The risk for developing cutaneous metastases is greatest within 3 years following nephrectomy.3 However, our patient demonstrates that metastasis of RCC to skin can be long delayed (>5 years) despite an initial diagnosis of localized disease.
Cutaneous RCC classically presents as a painless firm papulonodule with a deep red or purple color due to its high vascularity.4 Several retrospective studies have identified the scalp as the most frequent site of cutaneous involvement, followed by the chest, abdomen, and nephrectomy scar.3,4 The differential diagnosis includes other vascular lesions such as pyogenic granuloma, hemangioma, angiosarcoma, bacillary angiomatosis, and Kaposi sarcoma. Diagnosis usually is easily confirmed histologically. Proliferative nests of epithelioid cells with clear cell morphology are surrounded by delicately branching vessels referred to as chicken wire-like vasculature. Immunohistochemical studies demonstrate positivity for pan-cytokeratin, vimentin, and CD-10, and negativity for p63 and cytokeratins 5 and 6, helping to confirm the diagnosis in more challenging cases, especially when there is no known history of primary RCC.5
If cutaneous metastasis of RCC is diagnosed, a chest and abdominal computed tomography scan as well as serum alkaline phosphatase test are warranted, as up to 90% of patients with RCC in the skin have additional lesions in at least 1 other site such as the lungs, bones, or liver.3 Management of metastatic RCC includes surgical excision if a single metastasis is found and either immunotherapy with high-dose IL-2 or an anti-programmed cell death inhibitor. Patients with progressive disease also may receive targeted anti-VEGF inhibitors (eg, axitinib, pazopanib, sunitinib), which have been shown to increase progression-free survival in metastatic RCC.6-8 Interestingly, some evidence suggests severely delayed recurrence of RCC (>5 years following nephrectomy) may predict better response to systemic therapy.9
This case of severely delayed metastasis of RCC 8 years after nephrectomy raises the question of whether routine surveillance for RCC recurrence should continue beyond 5 years. It also underscores the need for further studies to determine the utility of postsurgical adjuvant therapy for localized disease (stages I-III). A randomized clinical trial showed no significant difference in disease-free survival when the multikinase inhibitors sunitinib and sorafenib were used as adjuvant therapy.10 The randomized, placebo-controlled PROTECT trial showed no significant difference in disease-free survival between the VEGF inhibitor pazopanib and placebo when used as adjuvant therapy.11 However, trials are ongoing to investigate a potential survival advantage of adjuvant therapy with the VEGF receptor inhibitor axitinib and the mammalian target of rapamycin inhibitor everolimus.
- Dabestani S, Thorstenson A, Lindblad P, et al. Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: a population-based study. World J Urol. 2016;34:1081-1086.
- Ljungberg B, Campbell SC, Choi HY, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615-621.
- Dorairajan LN, Hemal AK, Aron M, et al. Cutaneous metastases in renal cell carcinoma. Urol Int. 1999;63:164-167.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, pt 1):228-236.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613-620.
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061-1068.
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584-3590.
- Rini BI, Grunwald V, Fishman MN, et al. Axitinib for first-line metastatic renal cell carcinoma (mRCC): overall efficacy and pharmacokinetic (PK) analyses from a randomized phase II study. J Clin Oncol. 2012;30(suppl). doi:10.1200/jco.2012.30.15_suppl.4503.
- Ficarra V, Novara G. Characterizing late recurrence of renal cell carcinoma. Nat Rev Urol. 2013;10:687-689.
- Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial [published online March 9, 2016]. Lancet. 2016;387:2008-2016.
- Motzer RJ, Haas NB, Donskov F, et al; PROTECT investigators. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma [published online September 13, 2017]. J Clin Oncol. 2017;35:3916-3923.
The Diagnosis: Metastatic Renal Cell Carcinoma
Histopathologic examination of the punch biopsy demonstrated epithelioid cells with abundant clear cytoplasm and numerous chicken wire-like vascular channels consistent with a diagnosis of cutaneous metastasis of renal cell carcinoma (RCC)(Figure). Collateral history revealed that 8 years prior, the patient had been diagnosed with clear cell RCC, stage III (T3aN0M0). At that time, he was treated with radical nephrectomy, which was considered curative. He remained disease free until several months prior to the development of the cutaneous lesion when he was found to have pulmonary and cerebral metastases with biopsies showing metastatic RCC. He was treated with lobectomy and Gamma Knife radiation for the lung and cerebral metastases, respectively. His oncologist planned to initiate therapy with the multikinase inhibitor sunitinib, which inhibits vascular endothelial growth factor (VEGF) signaling. Unfortunately, the patient died prior to treatment due to overwhelming tumor burden.

Clear cell RCC, the most common renal malignancy, presents with metastatic disease at the time of diagnosis in 21% of patients.1 An additional 20% of patients with localized disease develop metastases within several years of receiving a nephrectomy without adjuvant therapy, which is standard treatment for stage I to stage III disease.1,2 Metastatic RCC most frequently targets the lungs, bone, liver, and brain, though virtually any organ can be involved. Cutaneous involvement is estimated to occur in 3.3% of RCC cases,3 accounting for only 1.4% of cutaneous metastases overall.4 The risk for developing cutaneous metastases is greatest within 3 years following nephrectomy.3 However, our patient demonstrates that metastasis of RCC to skin can be long delayed (>5 years) despite an initial diagnosis of localized disease.
Cutaneous RCC classically presents as a painless firm papulonodule with a deep red or purple color due to its high vascularity.4 Several retrospective studies have identified the scalp as the most frequent site of cutaneous involvement, followed by the chest, abdomen, and nephrectomy scar.3,4 The differential diagnosis includes other vascular lesions such as pyogenic granuloma, hemangioma, angiosarcoma, bacillary angiomatosis, and Kaposi sarcoma. Diagnosis usually is easily confirmed histologically. Proliferative nests of epithelioid cells with clear cell morphology are surrounded by delicately branching vessels referred to as chicken wire-like vasculature. Immunohistochemical studies demonstrate positivity for pan-cytokeratin, vimentin, and CD-10, and negativity for p63 and cytokeratins 5 and 6, helping to confirm the diagnosis in more challenging cases, especially when there is no known history of primary RCC.5
If cutaneous metastasis of RCC is diagnosed, a chest and abdominal computed tomography scan as well as serum alkaline phosphatase test are warranted, as up to 90% of patients with RCC in the skin have additional lesions in at least 1 other site such as the lungs, bones, or liver.3 Management of metastatic RCC includes surgical excision if a single metastasis is found and either immunotherapy with high-dose IL-2 or an anti-programmed cell death inhibitor. Patients with progressive disease also may receive targeted anti-VEGF inhibitors (eg, axitinib, pazopanib, sunitinib), which have been shown to increase progression-free survival in metastatic RCC.6-8 Interestingly, some evidence suggests severely delayed recurrence of RCC (>5 years following nephrectomy) may predict better response to systemic therapy.9
This case of severely delayed metastasis of RCC 8 years after nephrectomy raises the question of whether routine surveillance for RCC recurrence should continue beyond 5 years. It also underscores the need for further studies to determine the utility of postsurgical adjuvant therapy for localized disease (stages I-III). A randomized clinical trial showed no significant difference in disease-free survival when the multikinase inhibitors sunitinib and sorafenib were used as adjuvant therapy.10 The randomized, placebo-controlled PROTECT trial showed no significant difference in disease-free survival between the VEGF inhibitor pazopanib and placebo when used as adjuvant therapy.11 However, trials are ongoing to investigate a potential survival advantage of adjuvant therapy with the VEGF receptor inhibitor axitinib and the mammalian target of rapamycin inhibitor everolimus.
The Diagnosis: Metastatic Renal Cell Carcinoma
Histopathologic examination of the punch biopsy demonstrated epithelioid cells with abundant clear cytoplasm and numerous chicken wire-like vascular channels consistent with a diagnosis of cutaneous metastasis of renal cell carcinoma (RCC)(Figure). Collateral history revealed that 8 years prior, the patient had been diagnosed with clear cell RCC, stage III (T3aN0M0). At that time, he was treated with radical nephrectomy, which was considered curative. He remained disease free until several months prior to the development of the cutaneous lesion when he was found to have pulmonary and cerebral metastases with biopsies showing metastatic RCC. He was treated with lobectomy and Gamma Knife radiation for the lung and cerebral metastases, respectively. His oncologist planned to initiate therapy with the multikinase inhibitor sunitinib, which inhibits vascular endothelial growth factor (VEGF) signaling. Unfortunately, the patient died prior to treatment due to overwhelming tumor burden.

Clear cell RCC, the most common renal malignancy, presents with metastatic disease at the time of diagnosis in 21% of patients.1 An additional 20% of patients with localized disease develop metastases within several years of receiving a nephrectomy without adjuvant therapy, which is standard treatment for stage I to stage III disease.1,2 Metastatic RCC most frequently targets the lungs, bone, liver, and brain, though virtually any organ can be involved. Cutaneous involvement is estimated to occur in 3.3% of RCC cases,3 accounting for only 1.4% of cutaneous metastases overall.4 The risk for developing cutaneous metastases is greatest within 3 years following nephrectomy.3 However, our patient demonstrates that metastasis of RCC to skin can be long delayed (>5 years) despite an initial diagnosis of localized disease.
Cutaneous RCC classically presents as a painless firm papulonodule with a deep red or purple color due to its high vascularity.4 Several retrospective studies have identified the scalp as the most frequent site of cutaneous involvement, followed by the chest, abdomen, and nephrectomy scar.3,4 The differential diagnosis includes other vascular lesions such as pyogenic granuloma, hemangioma, angiosarcoma, bacillary angiomatosis, and Kaposi sarcoma. Diagnosis usually is easily confirmed histologically. Proliferative nests of epithelioid cells with clear cell morphology are surrounded by delicately branching vessels referred to as chicken wire-like vasculature. Immunohistochemical studies demonstrate positivity for pan-cytokeratin, vimentin, and CD-10, and negativity for p63 and cytokeratins 5 and 6, helping to confirm the diagnosis in more challenging cases, especially when there is no known history of primary RCC.5
If cutaneous metastasis of RCC is diagnosed, a chest and abdominal computed tomography scan as well as serum alkaline phosphatase test are warranted, as up to 90% of patients with RCC in the skin have additional lesions in at least 1 other site such as the lungs, bones, or liver.3 Management of metastatic RCC includes surgical excision if a single metastasis is found and either immunotherapy with high-dose IL-2 or an anti-programmed cell death inhibitor. Patients with progressive disease also may receive targeted anti-VEGF inhibitors (eg, axitinib, pazopanib, sunitinib), which have been shown to increase progression-free survival in metastatic RCC.6-8 Interestingly, some evidence suggests severely delayed recurrence of RCC (>5 years following nephrectomy) may predict better response to systemic therapy.9
This case of severely delayed metastasis of RCC 8 years after nephrectomy raises the question of whether routine surveillance for RCC recurrence should continue beyond 5 years. It also underscores the need for further studies to determine the utility of postsurgical adjuvant therapy for localized disease (stages I-III). A randomized clinical trial showed no significant difference in disease-free survival when the multikinase inhibitors sunitinib and sorafenib were used as adjuvant therapy.10 The randomized, placebo-controlled PROTECT trial showed no significant difference in disease-free survival between the VEGF inhibitor pazopanib and placebo when used as adjuvant therapy.11 However, trials are ongoing to investigate a potential survival advantage of adjuvant therapy with the VEGF receptor inhibitor axitinib and the mammalian target of rapamycin inhibitor everolimus.
- Dabestani S, Thorstenson A, Lindblad P, et al. Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: a population-based study. World J Urol. 2016;34:1081-1086.
- Ljungberg B, Campbell SC, Choi HY, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615-621.
- Dorairajan LN, Hemal AK, Aron M, et al. Cutaneous metastases in renal cell carcinoma. Urol Int. 1999;63:164-167.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, pt 1):228-236.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613-620.
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061-1068.
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584-3590.
- Rini BI, Grunwald V, Fishman MN, et al. Axitinib for first-line metastatic renal cell carcinoma (mRCC): overall efficacy and pharmacokinetic (PK) analyses from a randomized phase II study. J Clin Oncol. 2012;30(suppl). doi:10.1200/jco.2012.30.15_suppl.4503.
- Ficarra V, Novara G. Characterizing late recurrence of renal cell carcinoma. Nat Rev Urol. 2013;10:687-689.
- Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial [published online March 9, 2016]. Lancet. 2016;387:2008-2016.
- Motzer RJ, Haas NB, Donskov F, et al; PROTECT investigators. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma [published online September 13, 2017]. J Clin Oncol. 2017;35:3916-3923.
- Dabestani S, Thorstenson A, Lindblad P, et al. Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: a population-based study. World J Urol. 2016;34:1081-1086.
- Ljungberg B, Campbell SC, Choi HY, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615-621.
- Dorairajan LN, Hemal AK, Aron M, et al. Cutaneous metastases in renal cell carcinoma. Urol Int. 1999;63:164-167.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, pt 1):228-236.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613-620.
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061-1068.
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584-3590.
- Rini BI, Grunwald V, Fishman MN, et al. Axitinib for first-line metastatic renal cell carcinoma (mRCC): overall efficacy and pharmacokinetic (PK) analyses from a randomized phase II study. J Clin Oncol. 2012;30(suppl). doi:10.1200/jco.2012.30.15_suppl.4503.
- Ficarra V, Novara G. Characterizing late recurrence of renal cell carcinoma. Nat Rev Urol. 2013;10:687-689.
- Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial [published online March 9, 2016]. Lancet. 2016;387:2008-2016.
- Motzer RJ, Haas NB, Donskov F, et al; PROTECT investigators. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma [published online September 13, 2017]. J Clin Oncol. 2017;35:3916-3923.
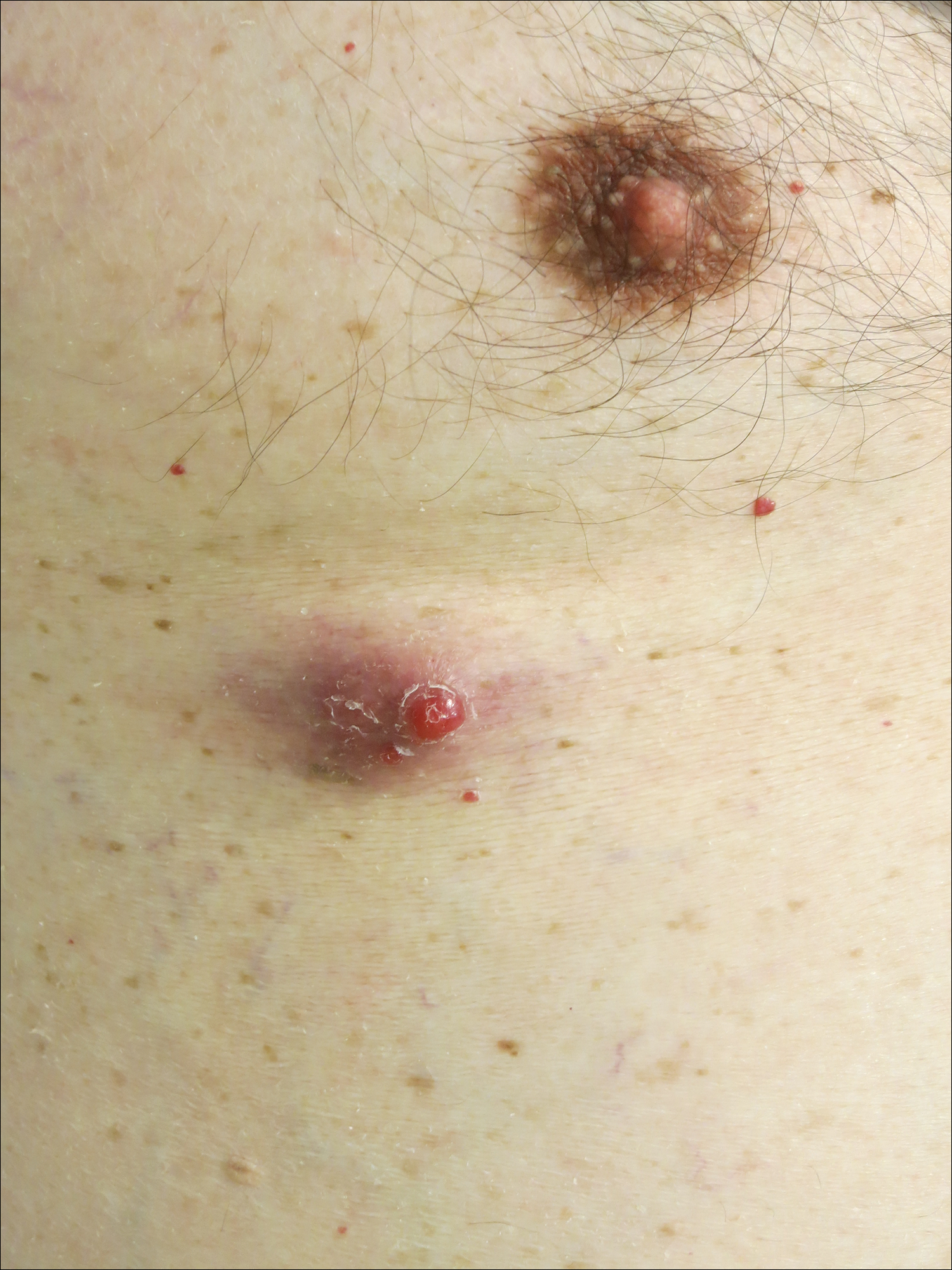
A man in his 60s presented with a subcutaneous nodule on the right side of the chest. Due to impaired mental status, he was unable to describe the precise age of the lesion, but his wife reported it had been present at least several weeks. She recently noted a new, bright red growth on top of the nodule. The lesion was asymptomatic but seemed to be growing in size. Physical examination revealed a 3-cm firm fixed nodule on the right side of the chest with an overlying, exophytic bright red papule. No similar lesions were found elsewhere on physical examination. A punch biopsy of the lesion was performed.
Purple Curvilinear Papules on the Back
The Diagnosis: Blaschkoid Graft-vs-host Disease
The patient had a history of myelodysplastic syndrome and underwent a bone marrow transplant 1 year prior to presentation. She had acute graft-vs-host disease (GVHD) 6 weeks following the transplant, which resolved with high-dose prednisone followed by UVB phototherapy. Skin biopsy demonstrated lichenoid dermatitis with vacuolar degeneration, dyskeratosis, and prominent pigment incontinence (Figure). Based on these findings and her clinical presentation, a diagnosis of blaschkoid GVHD was made.

Although acute GVHD is the result of immunocompetent donor T cells recognizing host tissues as foreign and initiating an immune response, the pathophysiology of chronic GVHD is not well understood.1,2 Theories for disease pathogenesis in chronic GVHD suggest an underlying autoimmune and/or alloreactive process.2-5 The skin often is the first organ affected in acute GVHD, and patients generally present with a pruritic morbilliform eruption that begins on the trunk and spreads to the rest of the body.1,2 Cutaneous manifestations of chronic GVHD may be protean. Lesions can resemble systemic sclerosis or morphea, lichen planus, psoriasis, ichthyosis, and many other conditions.2
The differential diagnosis of linear dermatoses includes herpes zoster, contact dermatitis, lichen striatus (blaschkitis), nevus unius lateris, inflammatory linear verrucous epidermal nevus, and incontinentia pigmenti.6,7 Lichen planus-like chronic GVHD occurring in a linear distribution has been described.6-14 Distinction between dermatomal and blaschkoid processes is diagnostically important. In the case of GVHD, dermatomal distribution may suggest an association between GVHD and prior herpes simplex virus or varicella-zoster virus infection.6,8 Herpesvirus may alter surface antigens of keratinocytes, rendering them targets of donor lymphocytes, and antibodies to viral particles may cross-react with host keratinocyte HLA antigens. It also is possible that dermatomal GVHD may simply be a type of isomorphic response (Köbner phenomenon).8
When cutaneous GVHD follows Blaschko lines, other mechanisms appear to be at play.9-14 It is plausible that these patients have an underlying genetic mosaicism, perhaps the result of a postzygotic mutation, that results in a daughter cell population that expresses surface antigens different from those of the primary cell population found elsewhere in the skin. Donor lymphocytes may selectively react to this mosaic population, leading to the clinical picture of chronic GVHD oriented along Blaschko lines.10,11,13,14
In conclusion, lichenoid linear GVHD following Blaschko lines is an uncommon presentation of chronic GVHD that highlights the heterogeneity of this disease and should be considered in the appropriate clinical setting.
- Ferrara JL, Levine JE, Reddy P, et al. Graft-versus-host disease. Lancet. 2009;373:1550-1561.
- Hymes SR, Alousi AM, Cowen EW. Graft-versus-host disease: part I. pathogenesis and clinical manifestations of graft-versus-host disease. J Am Acad Dermatol. 2012;66:515.e1-515.e18; quiz 533-534.
- Patriarca F, Skert C, Sperotto A, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol. 2006;34:389-396.
- Shimada M, Onizuka M, Machida S, et al. Association of autoimmune disease-related gene polymorphisms with chronic graft-versus-host disease. Br J Haematol. 2007;139:458-463.
- Zhang C, Todorov I, Zhang Z, et al. Donor CD4+ T and B cells in transplants induce chronic graft-versus-host disease with autoimmune manifestations. Blood. 2006;107:2993-3001.
- Freemer CS, Farmer ER, Corio RL, et al. Lichenoid chronic graft-vs-host disease occurring in a dermatomal distribution. Arch Dermatol. 1994;130:70-72.
- Kikuchi A, Okamoto S, Takahashi S, et al. Linear chronic cutaneous graft-versus-host disease. J Am Acad Dermatol. 1997;37:1004-1006.
- Sanli H, Anadolu R, Arat M, et al. Dermatomal lichenoid graft-versus-host disease within herpes zoster scars. Int J Dermatol. 2003;42:562-564.
- Kennedy FE, Hilari H, Ferrer B, et al. Lichenoid chronic graft-vs-host disease following Blaschko lines. ActasDermosifiliogr. 2014;105:89-92.
- Lee SW, Kim YC, Lee E, et al. Linear lichenoid graft versus host disease: an unusual configuration following Blaschko's lines. J Dermatol. 2006;33:583-584.
- Beers B, Kalish RS, Kaye VN, et al. Unilateral linear lichenoid eruption after bone marrow transplantation: an unmasking of tolerance to an abnormal keratinocyte clone? J Am Acad Dermatol. 1993;28(5, pt 2):888-892.
- Wilson B, Lockman D. Linear lichenoid graft-vs-host disease. Arch Dermatol. 1994;130(9):1206-1208.
- Reisfeld PL. Lichenoid chronic graft-vs-host disease. Arch Dermatol. 1994;130:1207-1208.
- Vassallo C, Derlino F, Ripamonti F, et al. Lichenoid cutaneous chronic GvHD following Blaschko lines. Int J Dermatol. 2014;53:473-475.
The Diagnosis: Blaschkoid Graft-vs-host Disease
The patient had a history of myelodysplastic syndrome and underwent a bone marrow transplant 1 year prior to presentation. She had acute graft-vs-host disease (GVHD) 6 weeks following the transplant, which resolved with high-dose prednisone followed by UVB phototherapy. Skin biopsy demonstrated lichenoid dermatitis with vacuolar degeneration, dyskeratosis, and prominent pigment incontinence (Figure). Based on these findings and her clinical presentation, a diagnosis of blaschkoid GVHD was made.

Although acute GVHD is the result of immunocompetent donor T cells recognizing host tissues as foreign and initiating an immune response, the pathophysiology of chronic GVHD is not well understood.1,2 Theories for disease pathogenesis in chronic GVHD suggest an underlying autoimmune and/or alloreactive process.2-5 The skin often is the first organ affected in acute GVHD, and patients generally present with a pruritic morbilliform eruption that begins on the trunk and spreads to the rest of the body.1,2 Cutaneous manifestations of chronic GVHD may be protean. Lesions can resemble systemic sclerosis or morphea, lichen planus, psoriasis, ichthyosis, and many other conditions.2
The differential diagnosis of linear dermatoses includes herpes zoster, contact dermatitis, lichen striatus (blaschkitis), nevus unius lateris, inflammatory linear verrucous epidermal nevus, and incontinentia pigmenti.6,7 Lichen planus-like chronic GVHD occurring in a linear distribution has been described.6-14 Distinction between dermatomal and blaschkoid processes is diagnostically important. In the case of GVHD, dermatomal distribution may suggest an association between GVHD and prior herpes simplex virus or varicella-zoster virus infection.6,8 Herpesvirus may alter surface antigens of keratinocytes, rendering them targets of donor lymphocytes, and antibodies to viral particles may cross-react with host keratinocyte HLA antigens. It also is possible that dermatomal GVHD may simply be a type of isomorphic response (Köbner phenomenon).8
When cutaneous GVHD follows Blaschko lines, other mechanisms appear to be at play.9-14 It is plausible that these patients have an underlying genetic mosaicism, perhaps the result of a postzygotic mutation, that results in a daughter cell population that expresses surface antigens different from those of the primary cell population found elsewhere in the skin. Donor lymphocytes may selectively react to this mosaic population, leading to the clinical picture of chronic GVHD oriented along Blaschko lines.10,11,13,14
In conclusion, lichenoid linear GVHD following Blaschko lines is an uncommon presentation of chronic GVHD that highlights the heterogeneity of this disease and should be considered in the appropriate clinical setting.
The Diagnosis: Blaschkoid Graft-vs-host Disease
The patient had a history of myelodysplastic syndrome and underwent a bone marrow transplant 1 year prior to presentation. She had acute graft-vs-host disease (GVHD) 6 weeks following the transplant, which resolved with high-dose prednisone followed by UVB phototherapy. Skin biopsy demonstrated lichenoid dermatitis with vacuolar degeneration, dyskeratosis, and prominent pigment incontinence (Figure). Based on these findings and her clinical presentation, a diagnosis of blaschkoid GVHD was made.

Although acute GVHD is the result of immunocompetent donor T cells recognizing host tissues as foreign and initiating an immune response, the pathophysiology of chronic GVHD is not well understood.1,2 Theories for disease pathogenesis in chronic GVHD suggest an underlying autoimmune and/or alloreactive process.2-5 The skin often is the first organ affected in acute GVHD, and patients generally present with a pruritic morbilliform eruption that begins on the trunk and spreads to the rest of the body.1,2 Cutaneous manifestations of chronic GVHD may be protean. Lesions can resemble systemic sclerosis or morphea, lichen planus, psoriasis, ichthyosis, and many other conditions.2
The differential diagnosis of linear dermatoses includes herpes zoster, contact dermatitis, lichen striatus (blaschkitis), nevus unius lateris, inflammatory linear verrucous epidermal nevus, and incontinentia pigmenti.6,7 Lichen planus-like chronic GVHD occurring in a linear distribution has been described.6-14 Distinction between dermatomal and blaschkoid processes is diagnostically important. In the case of GVHD, dermatomal distribution may suggest an association between GVHD and prior herpes simplex virus or varicella-zoster virus infection.6,8 Herpesvirus may alter surface antigens of keratinocytes, rendering them targets of donor lymphocytes, and antibodies to viral particles may cross-react with host keratinocyte HLA antigens. It also is possible that dermatomal GVHD may simply be a type of isomorphic response (Köbner phenomenon).8
When cutaneous GVHD follows Blaschko lines, other mechanisms appear to be at play.9-14 It is plausible that these patients have an underlying genetic mosaicism, perhaps the result of a postzygotic mutation, that results in a daughter cell population that expresses surface antigens different from those of the primary cell population found elsewhere in the skin. Donor lymphocytes may selectively react to this mosaic population, leading to the clinical picture of chronic GVHD oriented along Blaschko lines.10,11,13,14
In conclusion, lichenoid linear GVHD following Blaschko lines is an uncommon presentation of chronic GVHD that highlights the heterogeneity of this disease and should be considered in the appropriate clinical setting.
- Ferrara JL, Levine JE, Reddy P, et al. Graft-versus-host disease. Lancet. 2009;373:1550-1561.
- Hymes SR, Alousi AM, Cowen EW. Graft-versus-host disease: part I. pathogenesis and clinical manifestations of graft-versus-host disease. J Am Acad Dermatol. 2012;66:515.e1-515.e18; quiz 533-534.
- Patriarca F, Skert C, Sperotto A, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol. 2006;34:389-396.
- Shimada M, Onizuka M, Machida S, et al. Association of autoimmune disease-related gene polymorphisms with chronic graft-versus-host disease. Br J Haematol. 2007;139:458-463.
- Zhang C, Todorov I, Zhang Z, et al. Donor CD4+ T and B cells in transplants induce chronic graft-versus-host disease with autoimmune manifestations. Blood. 2006;107:2993-3001.
- Freemer CS, Farmer ER, Corio RL, et al. Lichenoid chronic graft-vs-host disease occurring in a dermatomal distribution. Arch Dermatol. 1994;130:70-72.
- Kikuchi A, Okamoto S, Takahashi S, et al. Linear chronic cutaneous graft-versus-host disease. J Am Acad Dermatol. 1997;37:1004-1006.
- Sanli H, Anadolu R, Arat M, et al. Dermatomal lichenoid graft-versus-host disease within herpes zoster scars. Int J Dermatol. 2003;42:562-564.
- Kennedy FE, Hilari H, Ferrer B, et al. Lichenoid chronic graft-vs-host disease following Blaschko lines. ActasDermosifiliogr. 2014;105:89-92.
- Lee SW, Kim YC, Lee E, et al. Linear lichenoid graft versus host disease: an unusual configuration following Blaschko's lines. J Dermatol. 2006;33:583-584.
- Beers B, Kalish RS, Kaye VN, et al. Unilateral linear lichenoid eruption after bone marrow transplantation: an unmasking of tolerance to an abnormal keratinocyte clone? J Am Acad Dermatol. 1993;28(5, pt 2):888-892.
- Wilson B, Lockman D. Linear lichenoid graft-vs-host disease. Arch Dermatol. 1994;130(9):1206-1208.
- Reisfeld PL. Lichenoid chronic graft-vs-host disease. Arch Dermatol. 1994;130:1207-1208.
- Vassallo C, Derlino F, Ripamonti F, et al. Lichenoid cutaneous chronic GvHD following Blaschko lines. Int J Dermatol. 2014;53:473-475.
- Ferrara JL, Levine JE, Reddy P, et al. Graft-versus-host disease. Lancet. 2009;373:1550-1561.
- Hymes SR, Alousi AM, Cowen EW. Graft-versus-host disease: part I. pathogenesis and clinical manifestations of graft-versus-host disease. J Am Acad Dermatol. 2012;66:515.e1-515.e18; quiz 533-534.
- Patriarca F, Skert C, Sperotto A, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol. 2006;34:389-396.
- Shimada M, Onizuka M, Machida S, et al. Association of autoimmune disease-related gene polymorphisms with chronic graft-versus-host disease. Br J Haematol. 2007;139:458-463.
- Zhang C, Todorov I, Zhang Z, et al. Donor CD4+ T and B cells in transplants induce chronic graft-versus-host disease with autoimmune manifestations. Blood. 2006;107:2993-3001.
- Freemer CS, Farmer ER, Corio RL, et al. Lichenoid chronic graft-vs-host disease occurring in a dermatomal distribution. Arch Dermatol. 1994;130:70-72.
- Kikuchi A, Okamoto S, Takahashi S, et al. Linear chronic cutaneous graft-versus-host disease. J Am Acad Dermatol. 1997;37:1004-1006.
- Sanli H, Anadolu R, Arat M, et al. Dermatomal lichenoid graft-versus-host disease within herpes zoster scars. Int J Dermatol. 2003;42:562-564.
- Kennedy FE, Hilari H, Ferrer B, et al. Lichenoid chronic graft-vs-host disease following Blaschko lines. ActasDermosifiliogr. 2014;105:89-92.
- Lee SW, Kim YC, Lee E, et al. Linear lichenoid graft versus host disease: an unusual configuration following Blaschko's lines. J Dermatol. 2006;33:583-584.
- Beers B, Kalish RS, Kaye VN, et al. Unilateral linear lichenoid eruption after bone marrow transplantation: an unmasking of tolerance to an abnormal keratinocyte clone? J Am Acad Dermatol. 1993;28(5, pt 2):888-892.
- Wilson B, Lockman D. Linear lichenoid graft-vs-host disease. Arch Dermatol. 1994;130(9):1206-1208.
- Reisfeld PL. Lichenoid chronic graft-vs-host disease. Arch Dermatol. 1994;130:1207-1208.
- Vassallo C, Derlino F, Ripamonti F, et al. Lichenoid cutaneous chronic GvHD following Blaschko lines. Int J Dermatol. 2014;53:473-475.
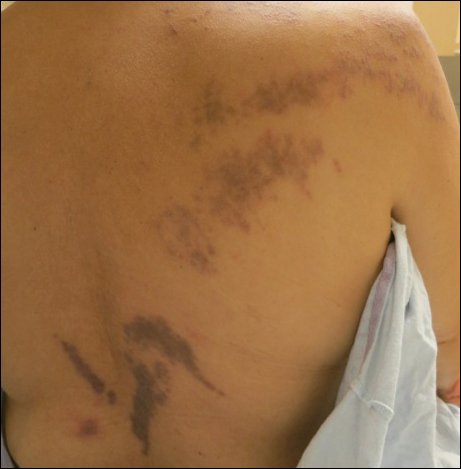
A 56-year-old woman with a history of bone marrow transplant presented for evaluation of a nonpruritic rash of 3 months' duration. Physical examination revealed confluent purple-colored and hyperpigmented papules localized to the back and right arm in a curvilinear pattern. Laboratory results were notable for mildly elevated aspartate transaminase and alanine transaminase levels.