User login
Concurrent Beau Lines, Onychomadesis, and Retronychia Following Scurvy
Beau lines are palpable transverse depressions on the dorsal aspect of the nail plate that result from a temporary slowing of nail plate production by the proximal nail matrix. Onychomadesis is a separation of the proximal nail plate from the distal nail plate leading to shedding of the nail. It occurs due to a complete growth arrest in the nail matrix and is thought to be on a continuum with Beau lines. The etiologies of these 2 conditions overlap and include trauma, inflammatory diseases, systemic illnesses, hereditary conditions, and infections.1-5 In almost all cases of both conditions, normal nail plate production ensues upon identification and removal of the inciting agent or recuperation from the causal illness.3,4,6 Beau lines will move distally as the nail grows out and can be clipped. In onychomadesis, the affected nails will be shed with time. Resolution of these nail defects can be estimated from average nail growth rates (1 mm/mo for fingernails and 2–3 mm/mo for toenails).7
Retronychia is defined as a proximal ingrowing of the nail plate into the ventral surface of the proximal nail fold.4,6 It is thought to occur via vertical progression of the nail plate into the proximal nail fold, repetitive nail matrix trauma, or shearing forces, resulting in inflammation that leads to nail plate stacking.8,9 Although conservative treatment using topical corticosteroids may be attempted, proximal nail plate avulsion typically is required for treatment.10
Braswell et al1 suggested a unifying hypothesis for a common pathophysiologic basis for these 3 conditions; that is, nail matrix injury results in slowing and/or cessation of nail plate production, followed by recommencement of nail plate production by the nail matrix after removal of the insult. We report a case of a patient presenting with concurrent Beau lines, onychomadesis, and retronychia following scurvy, thus supporting the hypothesis that these 3 nail conditions lie on a continuum.
Case Report
A 41-year-old woman with a history of thyroiditis, gastroesophageal reflux disease, endometriosis, osteoarthritis, gastric ulcer, pancreatitis, fatty liver, and polycystic ovarian syndrome presented with lines on the toenails and no growth of the right second toenail of several months’ duration. She denied any pain or prior trauma to the nails, participation in sports activities, or wearing tight or high-heeled shoes. She had presented 6 months prior for evaluation of perifollicular erythema on the posterior thighs, legs, and abdomen, as well as gingival bleeding.11 At that time, one of the authors (S.R.L.) found that she was vitamin C deficient, and a diagnosis of scurvy was made. The rash and gingival bleeding resolved with vitamin C supplementation.11
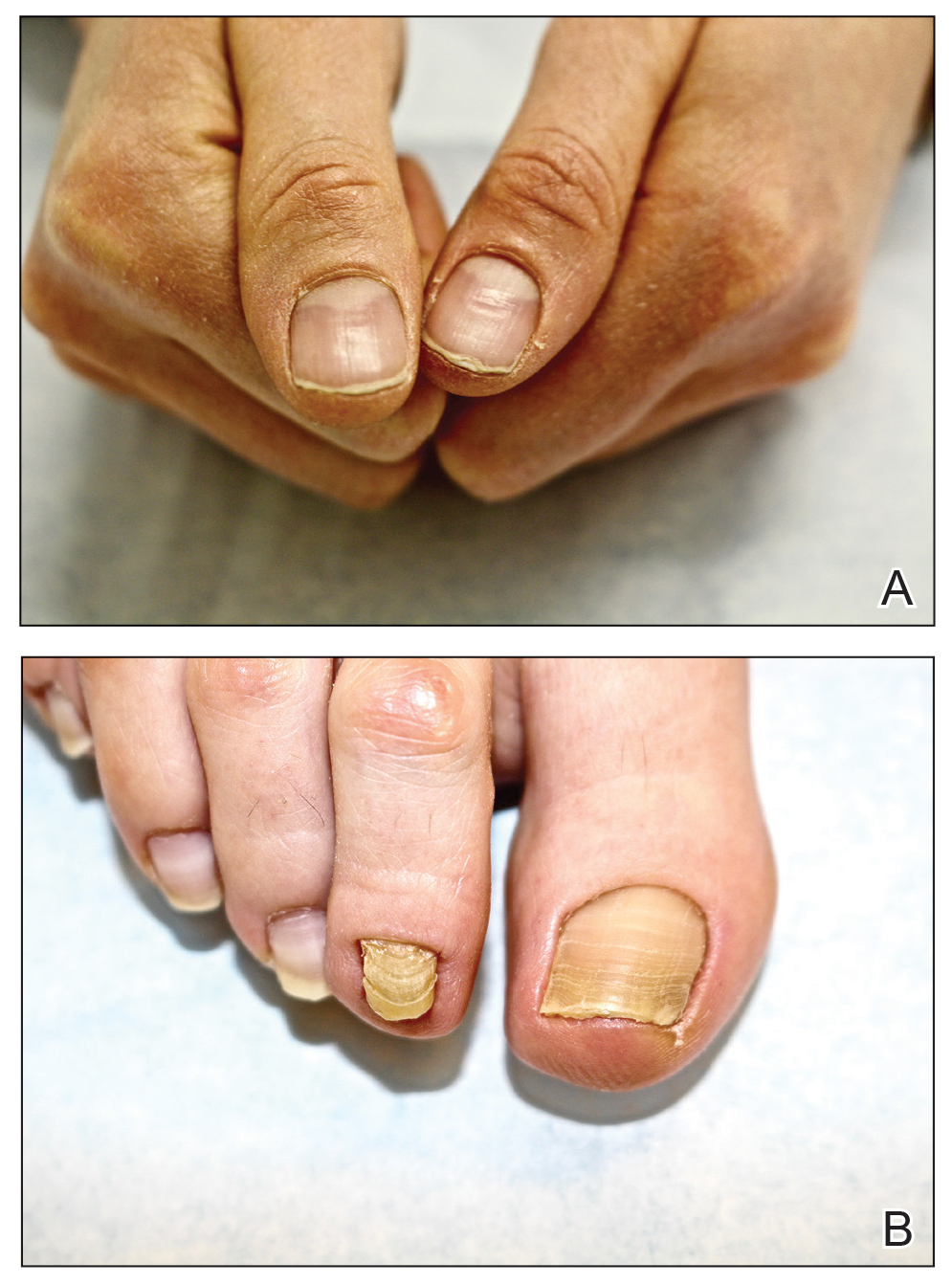
At the current presentation, physical examination revealed transverse grooves involving several fingernails but most evident on the left thumbnail (Figure, A). The grooves did not span the entire breadth of the nail, which was consistent with Beau lines. Several toenails had parallel transverse grooves spanning the entire width of the nail plate such that the proximal nail plate was discontinuous with the distal nail plate, which was consistent with onychomadesis (Figure, B). The right second toenail was yellow and thickened with layered nail plates, indicative of retronychia (Figure, B). Histopathology of a nail plate clipping from the right second toenail was negative for fungal hyphae, and a radiograph was negative for bony changes or exostosis.
Comment
The nail matrix is responsible for nail plate production, and the newly formed nail plate then moves outward over the nail bed. It is hypothesized that the pathophysiologic basis for Beau lines, onychomadesis, and retronychia lies on a continuum such that all 3 conditions are caused by an insult to the nail matrix that results in slowing and/or halting of nail plate growth. Beau lines result from slowing or disruption in cell growth from the nail matrix, whereas onychomadesis is associated with a complete halt in nail plate production.1,3 In retronychia, the new nail growing from the matrix pushes the old one upward, interrupting the longitudinal growth of the nail and leading to nail plate stacking.10
Our patient presented with concurrent Beau lines, onychomadesis, and retronychia. Although Beau lines and onychomadesis have been reported together in some instances,12-14 retronychia is not commonly reported with either of these conditions. The exact incidence of each condition has not been studied, but Beau lines are relatively common, onychomadesis is less common, and retronychia is seen infrequently; therefore, the concurrent presentation of these 3 conditions in the same patient is exceedingly rare. Thus, it was most likely that one etiology accounted for all 3 nail findings.
Because the patient had been diagnosed with scurvy 6 months prior to presentation, we hypothesized that the associated vitamin C deficiency caused a systemic insult to the nail matrix, which resulted in cessation of nail growth. The mechanism of nail matrix arrest in the setting of systemic disease is thought to be due to inhibition of cellular proliferation or a change in the quality of the newly manufactured nail plate, which becomes thinner and more dystrophic.15 Vitamin C (ascorbic acid) deficiency causes scurvy, which is characterized by cutaneous signs such as perifollicular hemorrhage and purpura, corkscrew hairs, bruising, gingivitis, arthralgia, and impaired wound healing.16 These clinical manifestations are due to impaired collagen synthesis and disordered connective tissue. Ascorbic acid also is involved in fatty acid transport, neurotransmitter synthesis, prostaglandin metabolism, and nitric oxide synthesis.17 Ascorbic acid has not been studied for its role in nail plate synthesis18; however, given the role that ascorbic acid plays in a myriad of biologic processes, the deficiency associated with scurvy likely had a considerable systemic effect in our patient that halted nail plate synthesis and resulted in the concurrent presentation of Beau lines, onychomadesis, and retronychia.
- Braswell MA, Daniel CR III, Brodell RT. Beau lines, onychomadesis, and retronychia: a unifying hypothesis. J Am Acad Dermatol. 2015;73:849-855.
- Lipner SR. Onychomadesis following a fish pedicure. JAMA Dermatol. 2018;154:1091-1092.
- Bettoli V, Zauli S, Toni G, et al. Onychomadesis following hand, foot, and mouth disease: a case report from Italy and review of the literature. Int J Dermatol. 2013;52:728-730.
- Lawry M, Daniel CR III. Nails in systemic disease. In: Scher RK, Daniel CR III, eds. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Oxford, England: Elsevier Saunders; 2005:147-176.
- Lipner SR, Scher RK. Evaluation of nail lines: color and shape hold clues. Cleve Clin J Med. 2016;83:385.
- Rich P. Nail signs and symptoms. In: Scher RK, Daniel CR III, eds. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Oxford, England: Elsevier Saunders; 2005:1-6.
- Lipner SR, Scher RK. Nail growth evaluation and factors affecting nail growth. In: Humbert P, Fanian F, Maibach H, et al, eds. Agache’s Measuring the Skin. Cham, Switzerland: Springer; 2017:1-15.
- de Berker DA, Richert B, Duhard E, et al. Retronychia: proximal ingrowing of the nail plate. J Am Acad Dermatol. 2008;58:978-983.
- Wortsman X, Wortsman J, Guerrero R, et al. Anatomical changes in retronychia and onychomadesis detected using ultrasound. Dermatol Surg. 2010;36:1615-1620.
- Piraccini BM, Richert B, de Berker DA, et al. Retronychia in children, adolescents, and young adults: a case series. J Am Acad Dermatol. 2014;70:388-390.
- Lipner S. A classic case of scurvy. Lancet. 2018;392:431.
- Jacobsen L, Zimmerman S, Lohr J. Nail findings in hand-foot-and-mouth disease. Pediatr Infect Dis J. 2015;34:449-450.
- Damevska K, Gocev G, Pollozhani N, et al. Onychomadesis following cutaneous vasculitis. Acta Dermatovenerol Croat. 2017;25:77-79.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand‐foot‐mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Weismann K. J.H.S Beau and his descriptions of transverse depressions on nails. Br J Dermatol. 1977;97:571-572.
- Abdullah M, Jamil RT, Attia FN. Vitamin C (ascorbic acid). Treasure Island, FL: StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK499877/. Updated October 21, 2019. Accessed February 24, 2020.
- Pazirandeh S, Burns DL. Overview of water-soluble vitamins. UpToDate. https://www.uptodate.com/contents/overview-of-water-soluble-vitamins. Updated January 29, 2020. Accessed February 24, 2020.
- Scheinfeld N, Dahdah MJ, Scher RK. Vitamins and minerals: their role in nail health and disease. J Drugs Dermatol. 2007;6:782-787.
Beau lines are palpable transverse depressions on the dorsal aspect of the nail plate that result from a temporary slowing of nail plate production by the proximal nail matrix. Onychomadesis is a separation of the proximal nail plate from the distal nail plate leading to shedding of the nail. It occurs due to a complete growth arrest in the nail matrix and is thought to be on a continuum with Beau lines. The etiologies of these 2 conditions overlap and include trauma, inflammatory diseases, systemic illnesses, hereditary conditions, and infections.1-5 In almost all cases of both conditions, normal nail plate production ensues upon identification and removal of the inciting agent or recuperation from the causal illness.3,4,6 Beau lines will move distally as the nail grows out and can be clipped. In onychomadesis, the affected nails will be shed with time. Resolution of these nail defects can be estimated from average nail growth rates (1 mm/mo for fingernails and 2–3 mm/mo for toenails).7
Retronychia is defined as a proximal ingrowing of the nail plate into the ventral surface of the proximal nail fold.4,6 It is thought to occur via vertical progression of the nail plate into the proximal nail fold, repetitive nail matrix trauma, or shearing forces, resulting in inflammation that leads to nail plate stacking.8,9 Although conservative treatment using topical corticosteroids may be attempted, proximal nail plate avulsion typically is required for treatment.10
Braswell et al1 suggested a unifying hypothesis for a common pathophysiologic basis for these 3 conditions; that is, nail matrix injury results in slowing and/or cessation of nail plate production, followed by recommencement of nail plate production by the nail matrix after removal of the insult. We report a case of a patient presenting with concurrent Beau lines, onychomadesis, and retronychia following scurvy, thus supporting the hypothesis that these 3 nail conditions lie on a continuum.
Case Report
A 41-year-old woman with a history of thyroiditis, gastroesophageal reflux disease, endometriosis, osteoarthritis, gastric ulcer, pancreatitis, fatty liver, and polycystic ovarian syndrome presented with lines on the toenails and no growth of the right second toenail of several months’ duration. She denied any pain or prior trauma to the nails, participation in sports activities, or wearing tight or high-heeled shoes. She had presented 6 months prior for evaluation of perifollicular erythema on the posterior thighs, legs, and abdomen, as well as gingival bleeding.11 At that time, one of the authors (S.R.L.) found that she was vitamin C deficient, and a diagnosis of scurvy was made. The rash and gingival bleeding resolved with vitamin C supplementation.11
At the current presentation, physical examination revealed transverse grooves involving several fingernails but most evident on the left thumbnail (Figure, A). The grooves did not span the entire breadth of the nail, which was consistent with Beau lines. Several toenails had parallel transverse grooves spanning the entire width of the nail plate such that the proximal nail plate was discontinuous with the distal nail plate, which was consistent with onychomadesis (Figure, B). The right second toenail was yellow and thickened with layered nail plates, indicative of retronychia (Figure, B). Histopathology of a nail plate clipping from the right second toenail was negative for fungal hyphae, and a radiograph was negative for bony changes or exostosis.

Comment
The nail matrix is responsible for nail plate production, and the newly formed nail plate then moves outward over the nail bed. It is hypothesized that the pathophysiologic basis for Beau lines, onychomadesis, and retronychia lies on a continuum such that all 3 conditions are caused by an insult to the nail matrix that results in slowing and/or halting of nail plate growth. Beau lines result from slowing or disruption in cell growth from the nail matrix, whereas onychomadesis is associated with a complete halt in nail plate production.1,3 In retronychia, the new nail growing from the matrix pushes the old one upward, interrupting the longitudinal growth of the nail and leading to nail plate stacking.10
Our patient presented with concurrent Beau lines, onychomadesis, and retronychia. Although Beau lines and onychomadesis have been reported together in some instances,12-14 retronychia is not commonly reported with either of these conditions. The exact incidence of each condition has not been studied, but Beau lines are relatively common, onychomadesis is less common, and retronychia is seen infrequently; therefore, the concurrent presentation of these 3 conditions in the same patient is exceedingly rare. Thus, it was most likely that one etiology accounted for all 3 nail findings.
Because the patient had been diagnosed with scurvy 6 months prior to presentation, we hypothesized that the associated vitamin C deficiency caused a systemic insult to the nail matrix, which resulted in cessation of nail growth. The mechanism of nail matrix arrest in the setting of systemic disease is thought to be due to inhibition of cellular proliferation or a change in the quality of the newly manufactured nail plate, which becomes thinner and more dystrophic.15 Vitamin C (ascorbic acid) deficiency causes scurvy, which is characterized by cutaneous signs such as perifollicular hemorrhage and purpura, corkscrew hairs, bruising, gingivitis, arthralgia, and impaired wound healing.16 These clinical manifestations are due to impaired collagen synthesis and disordered connective tissue. Ascorbic acid also is involved in fatty acid transport, neurotransmitter synthesis, prostaglandin metabolism, and nitric oxide synthesis.17 Ascorbic acid has not been studied for its role in nail plate synthesis18; however, given the role that ascorbic acid plays in a myriad of biologic processes, the deficiency associated with scurvy likely had a considerable systemic effect in our patient that halted nail plate synthesis and resulted in the concurrent presentation of Beau lines, onychomadesis, and retronychia.
Beau lines are palpable transverse depressions on the dorsal aspect of the nail plate that result from a temporary slowing of nail plate production by the proximal nail matrix. Onychomadesis is a separation of the proximal nail plate from the distal nail plate leading to shedding of the nail. It occurs due to a complete growth arrest in the nail matrix and is thought to be on a continuum with Beau lines. The etiologies of these 2 conditions overlap and include trauma, inflammatory diseases, systemic illnesses, hereditary conditions, and infections.1-5 In almost all cases of both conditions, normal nail plate production ensues upon identification and removal of the inciting agent or recuperation from the causal illness.3,4,6 Beau lines will move distally as the nail grows out and can be clipped. In onychomadesis, the affected nails will be shed with time. Resolution of these nail defects can be estimated from average nail growth rates (1 mm/mo for fingernails and 2–3 mm/mo for toenails).7
Retronychia is defined as a proximal ingrowing of the nail plate into the ventral surface of the proximal nail fold.4,6 It is thought to occur via vertical progression of the nail plate into the proximal nail fold, repetitive nail matrix trauma, or shearing forces, resulting in inflammation that leads to nail plate stacking.8,9 Although conservative treatment using topical corticosteroids may be attempted, proximal nail plate avulsion typically is required for treatment.10
Braswell et al1 suggested a unifying hypothesis for a common pathophysiologic basis for these 3 conditions; that is, nail matrix injury results in slowing and/or cessation of nail plate production, followed by recommencement of nail plate production by the nail matrix after removal of the insult. We report a case of a patient presenting with concurrent Beau lines, onychomadesis, and retronychia following scurvy, thus supporting the hypothesis that these 3 nail conditions lie on a continuum.
Case Report
A 41-year-old woman with a history of thyroiditis, gastroesophageal reflux disease, endometriosis, osteoarthritis, gastric ulcer, pancreatitis, fatty liver, and polycystic ovarian syndrome presented with lines on the toenails and no growth of the right second toenail of several months’ duration. She denied any pain or prior trauma to the nails, participation in sports activities, or wearing tight or high-heeled shoes. She had presented 6 months prior for evaluation of perifollicular erythema on the posterior thighs, legs, and abdomen, as well as gingival bleeding.11 At that time, one of the authors (S.R.L.) found that she was vitamin C deficient, and a diagnosis of scurvy was made. The rash and gingival bleeding resolved with vitamin C supplementation.11
At the current presentation, physical examination revealed transverse grooves involving several fingernails but most evident on the left thumbnail (Figure, A). The grooves did not span the entire breadth of the nail, which was consistent with Beau lines. Several toenails had parallel transverse grooves spanning the entire width of the nail plate such that the proximal nail plate was discontinuous with the distal nail plate, which was consistent with onychomadesis (Figure, B). The right second toenail was yellow and thickened with layered nail plates, indicative of retronychia (Figure, B). Histopathology of a nail plate clipping from the right second toenail was negative for fungal hyphae, and a radiograph was negative for bony changes or exostosis.

Comment
The nail matrix is responsible for nail plate production, and the newly formed nail plate then moves outward over the nail bed. It is hypothesized that the pathophysiologic basis for Beau lines, onychomadesis, and retronychia lies on a continuum such that all 3 conditions are caused by an insult to the nail matrix that results in slowing and/or halting of nail plate growth. Beau lines result from slowing or disruption in cell growth from the nail matrix, whereas onychomadesis is associated with a complete halt in nail plate production.1,3 In retronychia, the new nail growing from the matrix pushes the old one upward, interrupting the longitudinal growth of the nail and leading to nail plate stacking.10
Our patient presented with concurrent Beau lines, onychomadesis, and retronychia. Although Beau lines and onychomadesis have been reported together in some instances,12-14 retronychia is not commonly reported with either of these conditions. The exact incidence of each condition has not been studied, but Beau lines are relatively common, onychomadesis is less common, and retronychia is seen infrequently; therefore, the concurrent presentation of these 3 conditions in the same patient is exceedingly rare. Thus, it was most likely that one etiology accounted for all 3 nail findings.
Because the patient had been diagnosed with scurvy 6 months prior to presentation, we hypothesized that the associated vitamin C deficiency caused a systemic insult to the nail matrix, which resulted in cessation of nail growth. The mechanism of nail matrix arrest in the setting of systemic disease is thought to be due to inhibition of cellular proliferation or a change in the quality of the newly manufactured nail plate, which becomes thinner and more dystrophic.15 Vitamin C (ascorbic acid) deficiency causes scurvy, which is characterized by cutaneous signs such as perifollicular hemorrhage and purpura, corkscrew hairs, bruising, gingivitis, arthralgia, and impaired wound healing.16 These clinical manifestations are due to impaired collagen synthesis and disordered connective tissue. Ascorbic acid also is involved in fatty acid transport, neurotransmitter synthesis, prostaglandin metabolism, and nitric oxide synthesis.17 Ascorbic acid has not been studied for its role in nail plate synthesis18; however, given the role that ascorbic acid plays in a myriad of biologic processes, the deficiency associated with scurvy likely had a considerable systemic effect in our patient that halted nail plate synthesis and resulted in the concurrent presentation of Beau lines, onychomadesis, and retronychia.
- Braswell MA, Daniel CR III, Brodell RT. Beau lines, onychomadesis, and retronychia: a unifying hypothesis. J Am Acad Dermatol. 2015;73:849-855.
- Lipner SR. Onychomadesis following a fish pedicure. JAMA Dermatol. 2018;154:1091-1092.
- Bettoli V, Zauli S, Toni G, et al. Onychomadesis following hand, foot, and mouth disease: a case report from Italy and review of the literature. Int J Dermatol. 2013;52:728-730.
- Lawry M, Daniel CR III. Nails in systemic disease. In: Scher RK, Daniel CR III, eds. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Oxford, England: Elsevier Saunders; 2005:147-176.
- Lipner SR, Scher RK. Evaluation of nail lines: color and shape hold clues. Cleve Clin J Med. 2016;83:385.
- Rich P. Nail signs and symptoms. In: Scher RK, Daniel CR III, eds. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Oxford, England: Elsevier Saunders; 2005:1-6.
- Lipner SR, Scher RK. Nail growth evaluation and factors affecting nail growth. In: Humbert P, Fanian F, Maibach H, et al, eds. Agache’s Measuring the Skin. Cham, Switzerland: Springer; 2017:1-15.
- de Berker DA, Richert B, Duhard E, et al. Retronychia: proximal ingrowing of the nail plate. J Am Acad Dermatol. 2008;58:978-983.
- Wortsman X, Wortsman J, Guerrero R, et al. Anatomical changes in retronychia and onychomadesis detected using ultrasound. Dermatol Surg. 2010;36:1615-1620.
- Piraccini BM, Richert B, de Berker DA, et al. Retronychia in children, adolescents, and young adults: a case series. J Am Acad Dermatol. 2014;70:388-390.
- Lipner S. A classic case of scurvy. Lancet. 2018;392:431.
- Jacobsen L, Zimmerman S, Lohr J. Nail findings in hand-foot-and-mouth disease. Pediatr Infect Dis J. 2015;34:449-450.
- Damevska K, Gocev G, Pollozhani N, et al. Onychomadesis following cutaneous vasculitis. Acta Dermatovenerol Croat. 2017;25:77-79.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand‐foot‐mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Weismann K. J.H.S Beau and his descriptions of transverse depressions on nails. Br J Dermatol. 1977;97:571-572.
- Abdullah M, Jamil RT, Attia FN. Vitamin C (ascorbic acid). Treasure Island, FL: StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK499877/. Updated October 21, 2019. Accessed February 24, 2020.
- Pazirandeh S, Burns DL. Overview of water-soluble vitamins. UpToDate. https://www.uptodate.com/contents/overview-of-water-soluble-vitamins. Updated January 29, 2020. Accessed February 24, 2020.
- Scheinfeld N, Dahdah MJ, Scher RK. Vitamins and minerals: their role in nail health and disease. J Drugs Dermatol. 2007;6:782-787.
- Braswell MA, Daniel CR III, Brodell RT. Beau lines, onychomadesis, and retronychia: a unifying hypothesis. J Am Acad Dermatol. 2015;73:849-855.
- Lipner SR. Onychomadesis following a fish pedicure. JAMA Dermatol. 2018;154:1091-1092.
- Bettoli V, Zauli S, Toni G, et al. Onychomadesis following hand, foot, and mouth disease: a case report from Italy and review of the literature. Int J Dermatol. 2013;52:728-730.
- Lawry M, Daniel CR III. Nails in systemic disease. In: Scher RK, Daniel CR III, eds. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Oxford, England: Elsevier Saunders; 2005:147-176.
- Lipner SR, Scher RK. Evaluation of nail lines: color and shape hold clues. Cleve Clin J Med. 2016;83:385.
- Rich P. Nail signs and symptoms. In: Scher RK, Daniel CR III, eds. Nails: Diagnosis, Therapy, Surgery. 3rd ed. Oxford, England: Elsevier Saunders; 2005:1-6.
- Lipner SR, Scher RK. Nail growth evaluation and factors affecting nail growth. In: Humbert P, Fanian F, Maibach H, et al, eds. Agache’s Measuring the Skin. Cham, Switzerland: Springer; 2017:1-15.
- de Berker DA, Richert B, Duhard E, et al. Retronychia: proximal ingrowing of the nail plate. J Am Acad Dermatol. 2008;58:978-983.
- Wortsman X, Wortsman J, Guerrero R, et al. Anatomical changes in retronychia and onychomadesis detected using ultrasound. Dermatol Surg. 2010;36:1615-1620.
- Piraccini BM, Richert B, de Berker DA, et al. Retronychia in children, adolescents, and young adults: a case series. J Am Acad Dermatol. 2014;70:388-390.
- Lipner S. A classic case of scurvy. Lancet. 2018;392:431.
- Jacobsen L, Zimmerman S, Lohr J. Nail findings in hand-foot-and-mouth disease. Pediatr Infect Dis J. 2015;34:449-450.
- Damevska K, Gocev G, Pollozhani N, et al. Onychomadesis following cutaneous vasculitis. Acta Dermatovenerol Croat. 2017;25:77-79.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand‐foot‐mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Weismann K. J.H.S Beau and his descriptions of transverse depressions on nails. Br J Dermatol. 1977;97:571-572.
- Abdullah M, Jamil RT, Attia FN. Vitamin C (ascorbic acid). Treasure Island, FL: StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK499877/. Updated October 21, 2019. Accessed February 24, 2020.
- Pazirandeh S, Burns DL. Overview of water-soluble vitamins. UpToDate. https://www.uptodate.com/contents/overview-of-water-soluble-vitamins. Updated January 29, 2020. Accessed February 24, 2020.
- Scheinfeld N, Dahdah MJ, Scher RK. Vitamins and minerals: their role in nail health and disease. J Drugs Dermatol. 2007;6:782-787.
Practice Points
- Beau lines, onychomadesis, and retronychia are nail conditions with distinct clinical findings.
- Beau lines and onychomadesis may be seen concurrently following trauma, inflammatory diseases, systemic illnesses, hereditary conditions, and infections.
- Retronychia shares a common pathophysiology with Beau lines and onychomadesis, and all reflect slowing or cessation of nail plate production.
Optimizing Topical Therapy for Onychomycosis: The Importance of Patient Education
Onychomycosis is a fungal infection of the nail unit due to dermatophytes, yeasts, and nondermatophyte molds (NDMs). It accounts for approximately 50% of all nail disorders seen in clinical practice and is estimated to affect 10% to 12% of the US population.1,2 Oral medications approved by the US Food and Drug Administration (FDA) include terbinafine and itraconazole, which have demonstrated good efficacy in treating onychomycosis but are associated with potential drug-drug interactions and systemic side effects.3,4 Although liver failure associated with these drugs is rare,5 many patients are anxious about systemic adverse events and therefore prefer to use topical therapies for onychomycosis.
Many patients desire topical therapy but not every patient is an appropriate candidate. Patients who will likely respond well to topical therapy include those with superficial onychomycosis, distal lateral subungual onychomycosis that involves less than 50% of the nail plate surface area (without matrix involvement and a nail plate thickness less than 2 mm), and only up to 3 or 4 nails affected.6 In patients who have contraindications to oral therapy, topical therapy may be the only treatment option. To maximize efficacy of FDA-approved topical agents for onychomycosis therapy, patient education is of utmost importance. Failure to properly counsel the patient on proper medication application may result in decreased antifungal efficacy; poor patient compliance due to lack of improvement; and progression of disease, leading to increased onychodystrophy and pain.
Before initiating therapy, patients should be counseled that treatment with topical drugs is long, requiring daily application of the medication for 6 months for fingernails and 12 months for toenails, based on average nail growth rates (2–3 mm per month for fingernails; 1 mm per month for toenails).7 Patients also are advised to avoid nail polish application during the course of therapy, as clinical trials were performed without nail polish and the true efficacy with nail polish is unknown.8-10 Because patients who have had onychomycosis for shorter durations generally have better cure rates than those who have disease for longer durations, it is prudent to initiate topical therapy as early as possible.11,12 Treating the feet with an antifungal while treating the nails for onychomycosis further enhances efficacy.13,14 There are 3 FDA-approved topical therapies for onychomycosis: ciclopirox nail lacquer 8%, efinaconazole solution 10%, and tavaborole solution 5%.15-17
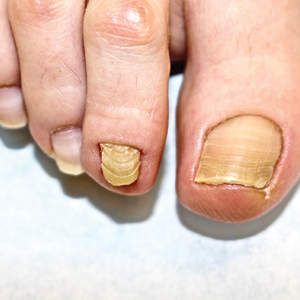
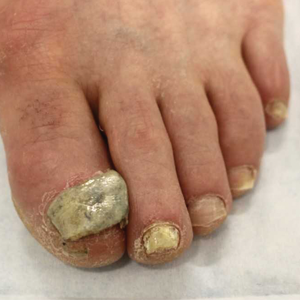
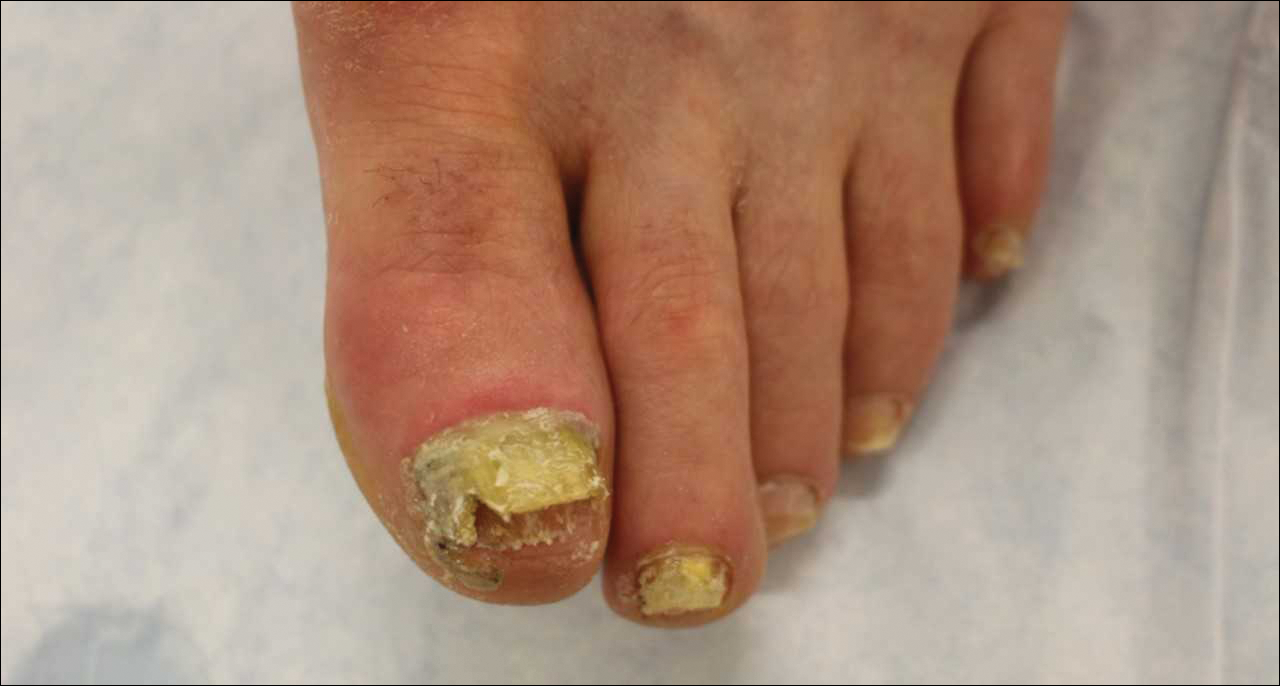
Ciclopirox is a hydroxypyridone with broad-spectrum antimicrobial activity against dermatophytes, NDMs, yeasts, and bacteria. Its mechanism of action is to chelate polyvalent cations, such as Fe3+, and inhibit fungal metal-dependent enzymes responsible for the degradation of toxic metabolites.15 Ciclopirox nail lacquer 8% was FDA approved for the treatment of onychomycosis in 1999, making it the first topical approved for this purpose. Its indication is for immunocompetent patients with mild to moderate onychomycosis (Trichophyton rubrum) without lunula involvement, with mycologic cure rates of 29% to 36% and complete cure rates of 5.5% to 8.5% (toenails).15 It is the only FDA-approved topical treatment for both fingernails and toenails. Using a brush applicator, it is applied daily to the nail plate and its undersurface, hyponychium, and 5 mm of the surrounding skin. It is important to counsel the patient to remove the lacquer from the nail plate weekly because failure to do so will result in accumulation of numerous layers of medication, such that the active drug cannot reach the site of infection (Figures 1 and 2). The nail plate also should be trimmed and filed weekly by the patient, with monthly clipping/debridement by a physician recommended.6,15
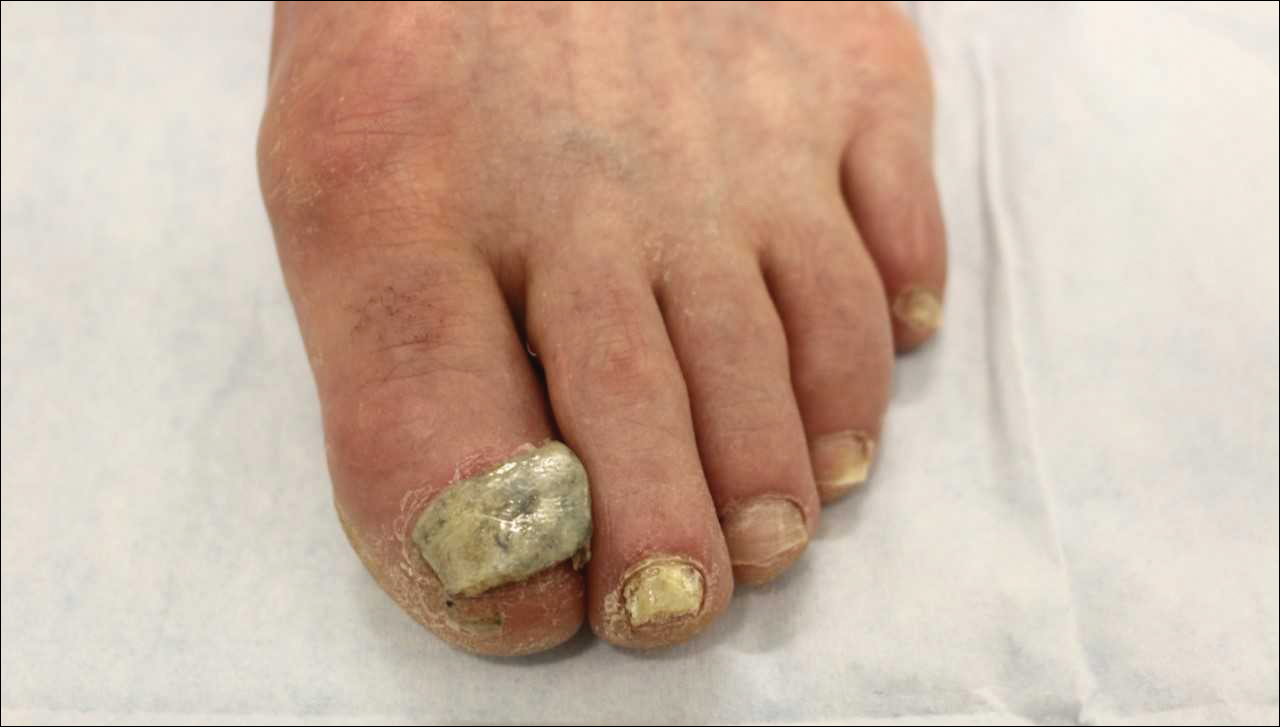
Efinaconazole is a triazole with antifungal activity against dermatophytes, NDMs, and Candida species. Its mechanism of action is inhibition of lanosterol 14α-demethylase, an enzyme involved in the biosynthesis of ergosterol, which is a component of the fungal cell membrane. Efinaconazole solution 10% was FDA approved in June 2014 for the treatment of toenail onychomycosis due to T rubrum and Trichophyton mentagrophytes, with package insert mycologic cure rates of 53.4% to 55.2% and complete cure rates of 15.2% to 17.8%.9,16 It is applied with a brush applicator to the nail plate, as well as its undersurface, nail folds, and hyponychium. Two drops are recommended for the great toenail and one drop for all other toenails, and no removal of the solution or debridement is required.6,16
Tavaborole is a benzoxaborole with antifungal activity against dermatophytes, NDMs, and yeasts. Its mechanism of action is inhibition of fungal aminoacyl transfer RNA synthetase, thus impeding protein synthesis.18 Tavaborole solution 5% was FDA approved in July 2014 for the treatment of toenail onychomycosis due to T rubrum and T mentagrophytes, with mycologic cure rates of 31.1% and 35.9% and complete cure rates of 6.5% and 9.1%, respectively.11,17 It is applied with a glass pointed-tip dropper to the nail plate, such that the entire nail is covered as well as under the nail tip. No removal of the solution or debridement is required.17
Topical therapies for onychomycosis require long treatment durations, thus excellent compliance and adherence to the treatment protocol are vital to maximize efficacy. Dermatologists who prescribe ciclopirox nail lacquer 8% should counsel patients to remove the lacquer with alcohol weekly, such that the antifungal penetrates the nail plate to reach the site of infection. Monthly debridement also must be clarified before initiating therapy. With all topical therapy for onychomycosis, it is important to treat early, treat concurrently for tinea pedis, and avoid use of nail polish so that patients have the best possible cure rates.
- Lipner SR, Scher RK. Onychomycosis: diagnosis and therapy. In: Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Rai M, eds. Medical Mycology: Current Trends and Future Prospects. Boca Raton, FL: CRC Press; 2015:28.
- Scher RK, Rich P, Pariser D, et al. The epidemiology, etiology, and pathophysiology of onychomycosis. Semin Cutan Med Surg. 2013;32(2 suppl 1):S2-S4.
- Lamisil [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 1997.
- Sporanox [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2001.
- National Institutes of Health. Terbinafine. LiverTox website. https://livertox.nlm.nih.gov/Terbinafine.htm. Accessed November 7, 2018.
- Lipner SR, Scher RK. Onychomycosis: topical therapy and devices. In: Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Cham, Switzerland: Springer International Publishing; 2018:173-184.
- Lipner SR, Scher RK. Nail growth evaluation and factors affecting nail growth. In: Humbert P, Fanian F, Maibach HI, et al, eds. Agache’s Measuring the Skin. 2nd ed. Berlin, Germany: Springer; 2017:867-881.
- Gupta AK, Elewski BE, Sugarman JL, et al. The efficacy and safety of efinaconazole 10% solution for treatment of mild to moderate onychomycosis: a pooled analysis of two phase 3 randomized trials. J Drugs Dermatol. 2014;13:815-820.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
- Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies. J Am Acad Dermatol. 2015;73:62-69.
- Rich P. Efinaconazole topical solution, 10%: the benefits of treating onychomycosis early. J Drugs Dermatol. 2015;14:58-62.
- Lipner SR, Scher RK. Efinaconazole 10% topical solution for the topical treatment of onychomycosis of the toenail. Expert Rev Clin Pharmacol. 2015;8:719-731.
- Del Rosso JQ. Onychomycosis of toenails and post-hoc analyses with efinaconazole 10% solution once-daily treatment: impact of disease severity and other concomitant associated factors on selection of therapy and therapeutic outcomes. J Clin Aesthet Dermatol. 2016;9:42.
- Lipner SR, Scher RK. Management of onychomycosis and co-existing tinea pedis. J Drugs Dermatol. 2015;14:492-494.
- Penlac [package insert]. Berwyn, PA: Dermik Laboratories; 2004.
- Jublia [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals, LLC; 2014.
- Kerydin [package insert]. Palo Alto, CA: Anacor Pharmaceuticals, Inc; 2014.
- Rock FL, Mao W, Yaremchuk A, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759-1761.
Onychomycosis is a fungal infection of the nail unit due to dermatophytes, yeasts, and nondermatophyte molds (NDMs). It accounts for approximately 50% of all nail disorders seen in clinical practice and is estimated to affect 10% to 12% of the US population.1,2 Oral medications approved by the US Food and Drug Administration (FDA) include terbinafine and itraconazole, which have demonstrated good efficacy in treating onychomycosis but are associated with potential drug-drug interactions and systemic side effects.3,4 Although liver failure associated with these drugs is rare,5 many patients are anxious about systemic adverse events and therefore prefer to use topical therapies for onychomycosis.
Many patients desire topical therapy but not every patient is an appropriate candidate. Patients who will likely respond well to topical therapy include those with superficial onychomycosis, distal lateral subungual onychomycosis that involves less than 50% of the nail plate surface area (without matrix involvement and a nail plate thickness less than 2 mm), and only up to 3 or 4 nails affected.6 In patients who have contraindications to oral therapy, topical therapy may be the only treatment option. To maximize efficacy of FDA-approved topical agents for onychomycosis therapy, patient education is of utmost importance. Failure to properly counsel the patient on proper medication application may result in decreased antifungal efficacy; poor patient compliance due to lack of improvement; and progression of disease, leading to increased onychodystrophy and pain.
Before initiating therapy, patients should be counseled that treatment with topical drugs is long, requiring daily application of the medication for 6 months for fingernails and 12 months for toenails, based on average nail growth rates (2–3 mm per month for fingernails; 1 mm per month for toenails).7 Patients also are advised to avoid nail polish application during the course of therapy, as clinical trials were performed without nail polish and the true efficacy with nail polish is unknown.8-10 Because patients who have had onychomycosis for shorter durations generally have better cure rates than those who have disease for longer durations, it is prudent to initiate topical therapy as early as possible.11,12 Treating the feet with an antifungal while treating the nails for onychomycosis further enhances efficacy.13,14 There are 3 FDA-approved topical therapies for onychomycosis: ciclopirox nail lacquer 8%, efinaconazole solution 10%, and tavaborole solution 5%.15-17
Ciclopirox is a hydroxypyridone with broad-spectrum antimicrobial activity against dermatophytes, NDMs, yeasts, and bacteria. Its mechanism of action is to chelate polyvalent cations, such as Fe3+, and inhibit fungal metal-dependent enzymes responsible for the degradation of toxic metabolites.15 Ciclopirox nail lacquer 8% was FDA approved for the treatment of onychomycosis in 1999, making it the first topical approved for this purpose. Its indication is for immunocompetent patients with mild to moderate onychomycosis (Trichophyton rubrum) without lunula involvement, with mycologic cure rates of 29% to 36% and complete cure rates of 5.5% to 8.5% (toenails).15 It is the only FDA-approved topical treatment for both fingernails and toenails. Using a brush applicator, it is applied daily to the nail plate and its undersurface, hyponychium, and 5 mm of the surrounding skin. It is important to counsel the patient to remove the lacquer from the nail plate weekly because failure to do so will result in accumulation of numerous layers of medication, such that the active drug cannot reach the site of infection (Figures 1 and 2). The nail plate also should be trimmed and filed weekly by the patient, with monthly clipping/debridement by a physician recommended.6,15


Efinaconazole is a triazole with antifungal activity against dermatophytes, NDMs, and Candida species. Its mechanism of action is inhibition of lanosterol 14α-demethylase, an enzyme involved in the biosynthesis of ergosterol, which is a component of the fungal cell membrane. Efinaconazole solution 10% was FDA approved in June 2014 for the treatment of toenail onychomycosis due to T rubrum and Trichophyton mentagrophytes, with package insert mycologic cure rates of 53.4% to 55.2% and complete cure rates of 15.2% to 17.8%.9,16 It is applied with a brush applicator to the nail plate, as well as its undersurface, nail folds, and hyponychium. Two drops are recommended for the great toenail and one drop for all other toenails, and no removal of the solution or debridement is required.6,16
Tavaborole is a benzoxaborole with antifungal activity against dermatophytes, NDMs, and yeasts. Its mechanism of action is inhibition of fungal aminoacyl transfer RNA synthetase, thus impeding protein synthesis.18 Tavaborole solution 5% was FDA approved in July 2014 for the treatment of toenail onychomycosis due to T rubrum and T mentagrophytes, with mycologic cure rates of 31.1% and 35.9% and complete cure rates of 6.5% and 9.1%, respectively.11,17 It is applied with a glass pointed-tip dropper to the nail plate, such that the entire nail is covered as well as under the nail tip. No removal of the solution or debridement is required.17
Topical therapies for onychomycosis require long treatment durations, thus excellent compliance and adherence to the treatment protocol are vital to maximize efficacy. Dermatologists who prescribe ciclopirox nail lacquer 8% should counsel patients to remove the lacquer with alcohol weekly, such that the antifungal penetrates the nail plate to reach the site of infection. Monthly debridement also must be clarified before initiating therapy. With all topical therapy for onychomycosis, it is important to treat early, treat concurrently for tinea pedis, and avoid use of nail polish so that patients have the best possible cure rates.
Onychomycosis is a fungal infection of the nail unit due to dermatophytes, yeasts, and nondermatophyte molds (NDMs). It accounts for approximately 50% of all nail disorders seen in clinical practice and is estimated to affect 10% to 12% of the US population.1,2 Oral medications approved by the US Food and Drug Administration (FDA) include terbinafine and itraconazole, which have demonstrated good efficacy in treating onychomycosis but are associated with potential drug-drug interactions and systemic side effects.3,4 Although liver failure associated with these drugs is rare,5 many patients are anxious about systemic adverse events and therefore prefer to use topical therapies for onychomycosis.
Many patients desire topical therapy but not every patient is an appropriate candidate. Patients who will likely respond well to topical therapy include those with superficial onychomycosis, distal lateral subungual onychomycosis that involves less than 50% of the nail plate surface area (without matrix involvement and a nail plate thickness less than 2 mm), and only up to 3 or 4 nails affected.6 In patients who have contraindications to oral therapy, topical therapy may be the only treatment option. To maximize efficacy of FDA-approved topical agents for onychomycosis therapy, patient education is of utmost importance. Failure to properly counsel the patient on proper medication application may result in decreased antifungal efficacy; poor patient compliance due to lack of improvement; and progression of disease, leading to increased onychodystrophy and pain.
Before initiating therapy, patients should be counseled that treatment with topical drugs is long, requiring daily application of the medication for 6 months for fingernails and 12 months for toenails, based on average nail growth rates (2–3 mm per month for fingernails; 1 mm per month for toenails).7 Patients also are advised to avoid nail polish application during the course of therapy, as clinical trials were performed without nail polish and the true efficacy with nail polish is unknown.8-10 Because patients who have had onychomycosis for shorter durations generally have better cure rates than those who have disease for longer durations, it is prudent to initiate topical therapy as early as possible.11,12 Treating the feet with an antifungal while treating the nails for onychomycosis further enhances efficacy.13,14 There are 3 FDA-approved topical therapies for onychomycosis: ciclopirox nail lacquer 8%, efinaconazole solution 10%, and tavaborole solution 5%.15-17
Ciclopirox is a hydroxypyridone with broad-spectrum antimicrobial activity against dermatophytes, NDMs, yeasts, and bacteria. Its mechanism of action is to chelate polyvalent cations, such as Fe3+, and inhibit fungal metal-dependent enzymes responsible for the degradation of toxic metabolites.15 Ciclopirox nail lacquer 8% was FDA approved for the treatment of onychomycosis in 1999, making it the first topical approved for this purpose. Its indication is for immunocompetent patients with mild to moderate onychomycosis (Trichophyton rubrum) without lunula involvement, with mycologic cure rates of 29% to 36% and complete cure rates of 5.5% to 8.5% (toenails).15 It is the only FDA-approved topical treatment for both fingernails and toenails. Using a brush applicator, it is applied daily to the nail plate and its undersurface, hyponychium, and 5 mm of the surrounding skin. It is important to counsel the patient to remove the lacquer from the nail plate weekly because failure to do so will result in accumulation of numerous layers of medication, such that the active drug cannot reach the site of infection (Figures 1 and 2). The nail plate also should be trimmed and filed weekly by the patient, with monthly clipping/debridement by a physician recommended.6,15


Efinaconazole is a triazole with antifungal activity against dermatophytes, NDMs, and Candida species. Its mechanism of action is inhibition of lanosterol 14α-demethylase, an enzyme involved in the biosynthesis of ergosterol, which is a component of the fungal cell membrane. Efinaconazole solution 10% was FDA approved in June 2014 for the treatment of toenail onychomycosis due to T rubrum and Trichophyton mentagrophytes, with package insert mycologic cure rates of 53.4% to 55.2% and complete cure rates of 15.2% to 17.8%.9,16 It is applied with a brush applicator to the nail plate, as well as its undersurface, nail folds, and hyponychium. Two drops are recommended for the great toenail and one drop for all other toenails, and no removal of the solution or debridement is required.6,16
Tavaborole is a benzoxaborole with antifungal activity against dermatophytes, NDMs, and yeasts. Its mechanism of action is inhibition of fungal aminoacyl transfer RNA synthetase, thus impeding protein synthesis.18 Tavaborole solution 5% was FDA approved in July 2014 for the treatment of toenail onychomycosis due to T rubrum and T mentagrophytes, with mycologic cure rates of 31.1% and 35.9% and complete cure rates of 6.5% and 9.1%, respectively.11,17 It is applied with a glass pointed-tip dropper to the nail plate, such that the entire nail is covered as well as under the nail tip. No removal of the solution or debridement is required.17
Topical therapies for onychomycosis require long treatment durations, thus excellent compliance and adherence to the treatment protocol are vital to maximize efficacy. Dermatologists who prescribe ciclopirox nail lacquer 8% should counsel patients to remove the lacquer with alcohol weekly, such that the antifungal penetrates the nail plate to reach the site of infection. Monthly debridement also must be clarified before initiating therapy. With all topical therapy for onychomycosis, it is important to treat early, treat concurrently for tinea pedis, and avoid use of nail polish so that patients have the best possible cure rates.
- Lipner SR, Scher RK. Onychomycosis: diagnosis and therapy. In: Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Rai M, eds. Medical Mycology: Current Trends and Future Prospects. Boca Raton, FL: CRC Press; 2015:28.
- Scher RK, Rich P, Pariser D, et al. The epidemiology, etiology, and pathophysiology of onychomycosis. Semin Cutan Med Surg. 2013;32(2 suppl 1):S2-S4.
- Lamisil [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 1997.
- Sporanox [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2001.
- National Institutes of Health. Terbinafine. LiverTox website. https://livertox.nlm.nih.gov/Terbinafine.htm. Accessed November 7, 2018.
- Lipner SR, Scher RK. Onychomycosis: topical therapy and devices. In: Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Cham, Switzerland: Springer International Publishing; 2018:173-184.
- Lipner SR, Scher RK. Nail growth evaluation and factors affecting nail growth. In: Humbert P, Fanian F, Maibach HI, et al, eds. Agache’s Measuring the Skin. 2nd ed. Berlin, Germany: Springer; 2017:867-881.
- Gupta AK, Elewski BE, Sugarman JL, et al. The efficacy and safety of efinaconazole 10% solution for treatment of mild to moderate onychomycosis: a pooled analysis of two phase 3 randomized trials. J Drugs Dermatol. 2014;13:815-820.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
- Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies. J Am Acad Dermatol. 2015;73:62-69.
- Rich P. Efinaconazole topical solution, 10%: the benefits of treating onychomycosis early. J Drugs Dermatol. 2015;14:58-62.
- Lipner SR, Scher RK. Efinaconazole 10% topical solution for the topical treatment of onychomycosis of the toenail. Expert Rev Clin Pharmacol. 2015;8:719-731.
- Del Rosso JQ. Onychomycosis of toenails and post-hoc analyses with efinaconazole 10% solution once-daily treatment: impact of disease severity and other concomitant associated factors on selection of therapy and therapeutic outcomes. J Clin Aesthet Dermatol. 2016;9:42.
- Lipner SR, Scher RK. Management of onychomycosis and co-existing tinea pedis. J Drugs Dermatol. 2015;14:492-494.
- Penlac [package insert]. Berwyn, PA: Dermik Laboratories; 2004.
- Jublia [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals, LLC; 2014.
- Kerydin [package insert]. Palo Alto, CA: Anacor Pharmaceuticals, Inc; 2014.
- Rock FL, Mao W, Yaremchuk A, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759-1761.
- Lipner SR, Scher RK. Onychomycosis: diagnosis and therapy. In: Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Rai M, eds. Medical Mycology: Current Trends and Future Prospects. Boca Raton, FL: CRC Press; 2015:28.
- Scher RK, Rich P, Pariser D, et al. The epidemiology, etiology, and pathophysiology of onychomycosis. Semin Cutan Med Surg. 2013;32(2 suppl 1):S2-S4.
- Lamisil [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 1997.
- Sporanox [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2001.
- National Institutes of Health. Terbinafine. LiverTox website. https://livertox.nlm.nih.gov/Terbinafine.htm. Accessed November 7, 2018.
- Lipner SR, Scher RK. Onychomycosis: topical therapy and devices. In: Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Cham, Switzerland: Springer International Publishing; 2018:173-184.
- Lipner SR, Scher RK. Nail growth evaluation and factors affecting nail growth. In: Humbert P, Fanian F, Maibach HI, et al, eds. Agache’s Measuring the Skin. 2nd ed. Berlin, Germany: Springer; 2017:867-881.
- Gupta AK, Elewski BE, Sugarman JL, et al. The efficacy and safety of efinaconazole 10% solution for treatment of mild to moderate onychomycosis: a pooled analysis of two phase 3 randomized trials. J Drugs Dermatol. 2014;13:815-820.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
- Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies. J Am Acad Dermatol. 2015;73:62-69.
- Rich P. Efinaconazole topical solution, 10%: the benefits of treating onychomycosis early. J Drugs Dermatol. 2015;14:58-62.
- Lipner SR, Scher RK. Efinaconazole 10% topical solution for the topical treatment of onychomycosis of the toenail. Expert Rev Clin Pharmacol. 2015;8:719-731.
- Del Rosso JQ. Onychomycosis of toenails and post-hoc analyses with efinaconazole 10% solution once-daily treatment: impact of disease severity and other concomitant associated factors on selection of therapy and therapeutic outcomes. J Clin Aesthet Dermatol. 2016;9:42.
- Lipner SR, Scher RK. Management of onychomycosis and co-existing tinea pedis. J Drugs Dermatol. 2015;14:492-494.
- Penlac [package insert]. Berwyn, PA: Dermik Laboratories; 2004.
- Jublia [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals, LLC; 2014.
- Kerydin [package insert]. Palo Alto, CA: Anacor Pharmaceuticals, Inc; 2014.
- Rock FL, Mao W, Yaremchuk A, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759-1761.