User login
CDC: Infants with Zika symptoms should get specialized care
Diagnostic testing of infants with suspected congenital Zika virus should include both immunoglobulin M (IgM) and polymerase chain reaction (PCR) testing, according to initial recommendations from a work group of federal health officials and pediatric specialists.
The group also called for infants with Zika virus symptoms to be transferred to facilities with pediatric subspecialty services.
Officials at the Centers for Disease Control and Prevention, along with pediatric specialists, reviewed the CDC’s existing guidelines for diagnosing, treating, and preventing Zika virus infections in both pregnant mothers and their infants during a 2-day meeting at the agency’s headquarters in Atlanta July 21-22.
“We want to make sure in the evaluation of symptomatic infants that we’re opening up a thorough differential, and that people are thinking about other things, particularly as we know that some of these issues, especially in terms of diagnosis, may be complex,” said Wanda D. Barfield, MD, director of the division of reproductive health at the CDC’s National Center for Chronic Disease Prevention and Health Promotion.
Although final recommendations are forthcoming, the consensus of the work group is that diagnostic testing of infants with suspected congenital Zika virus infection should include IgM and PCR testing of cerebrospinal fluid, urine, saliva and other infant specimens.
Asymptomatic infants should continue to receive care consistent with that of a normal, newborn infant, along with comprehensive physical exams, hearing tests, and head ultrasounds.
Symptomatic infants, however, should undergo different clinical and laboratory evaluations. Any symptomatic infant should be transferred to a pediatric subspecialty facility, which would allow for more specific care and services than would a primary care office. These services would include: neonatal/pediatric intensive care; endocrinologists to treat hypothyroidism, hypoaldosteronism, and growth hormone deficiency; orthopedists to treat arthrogryposis; neurologists to perform neuroimaging and EEGs, and to treat microcephaly and seizures; and other specialists to handle infectious disease, pulmonology, genetics, and feeding issues. These facilities would also offer family and social support, including palliative care options.
Infants with anomalies associated with congenital Zika virus infection should see a primary care physician every month for “routine care and increased surveillance,” said Janet Cragan, MD, of the CDC’s National Center on Birth Defects and Developmental Disorders. These visits should include measurements of weight, length, and head circumference, and should continue through 6 months of age. After that, the frequency of visits can be reevaluated.
During subsequent visits, providers should continue to monitor the infant’s development, as certain abnormalities take longer to become evident. These include sleep issues, excessive irritability, seizures, and “subtle symptoms” such as infantile spasms.
“In terms of coordination of care, there was discussion about the need to close the loop [to] ensure that the needed testing and consultations are done, [and] that the primary physician obtain those results,” Dr. Cragan said.
Similar monitoring – though less complex and stringent – should be done for children with congenital Zika virus infection who do not exhibit abnormalities and may not be symptomatic, the work group recommended. In addition, outpatient care is another critical component in managing congenital Zika virus infections, particularly in children who display no abnormalities at birth. Specifically, hearing evaluations should be performed regularly, and if any abnormalities are found, children should be referred for repeat hearing evaluations.
“We’re talking about a group of infants that we have very little to no information about,” said Kate Russell, MD, of Duke University, Durham, N.C. “Our discussion revolved around what’s known about infants who do have apparent abnormalities and are symptomatic, and trying to infer from that what could be done for these infants with asymptomatic infections.”
Telemedicine is another potentially powerful tool that physicians can use, “particularly in areas where there is limited direct access to pediatric subspecialty care,” Dr. Cragan said. Examples include taking videos of an infant and sending them to a neurologist to determine if symptoms were consistent with Zika, or sharing audio of the crying of Zika-infected children in Brazil so that primary care physicians can compare that with their patients.
“This is an area of very new and growing research and literature, and so this is going to be a continuous process [to] make sure that the people who are going to be seeing these infants on the front line are aware of what’s known,” Dr. Russell said.
Diagnostic testing of infants with suspected congenital Zika virus should include both immunoglobulin M (IgM) and polymerase chain reaction (PCR) testing, according to initial recommendations from a work group of federal health officials and pediatric specialists.
The group also called for infants with Zika virus symptoms to be transferred to facilities with pediatric subspecialty services.
Officials at the Centers for Disease Control and Prevention, along with pediatric specialists, reviewed the CDC’s existing guidelines for diagnosing, treating, and preventing Zika virus infections in both pregnant mothers and their infants during a 2-day meeting at the agency’s headquarters in Atlanta July 21-22.
“We want to make sure in the evaluation of symptomatic infants that we’re opening up a thorough differential, and that people are thinking about other things, particularly as we know that some of these issues, especially in terms of diagnosis, may be complex,” said Wanda D. Barfield, MD, director of the division of reproductive health at the CDC’s National Center for Chronic Disease Prevention and Health Promotion.
Although final recommendations are forthcoming, the consensus of the work group is that diagnostic testing of infants with suspected congenital Zika virus infection should include IgM and PCR testing of cerebrospinal fluid, urine, saliva and other infant specimens.
Asymptomatic infants should continue to receive care consistent with that of a normal, newborn infant, along with comprehensive physical exams, hearing tests, and head ultrasounds.
Symptomatic infants, however, should undergo different clinical and laboratory evaluations. Any symptomatic infant should be transferred to a pediatric subspecialty facility, which would allow for more specific care and services than would a primary care office. These services would include: neonatal/pediatric intensive care; endocrinologists to treat hypothyroidism, hypoaldosteronism, and growth hormone deficiency; orthopedists to treat arthrogryposis; neurologists to perform neuroimaging and EEGs, and to treat microcephaly and seizures; and other specialists to handle infectious disease, pulmonology, genetics, and feeding issues. These facilities would also offer family and social support, including palliative care options.
Infants with anomalies associated with congenital Zika virus infection should see a primary care physician every month for “routine care and increased surveillance,” said Janet Cragan, MD, of the CDC’s National Center on Birth Defects and Developmental Disorders. These visits should include measurements of weight, length, and head circumference, and should continue through 6 months of age. After that, the frequency of visits can be reevaluated.
During subsequent visits, providers should continue to monitor the infant’s development, as certain abnormalities take longer to become evident. These include sleep issues, excessive irritability, seizures, and “subtle symptoms” such as infantile spasms.
“In terms of coordination of care, there was discussion about the need to close the loop [to] ensure that the needed testing and consultations are done, [and] that the primary physician obtain those results,” Dr. Cragan said.
Similar monitoring – though less complex and stringent – should be done for children with congenital Zika virus infection who do not exhibit abnormalities and may not be symptomatic, the work group recommended. In addition, outpatient care is another critical component in managing congenital Zika virus infections, particularly in children who display no abnormalities at birth. Specifically, hearing evaluations should be performed regularly, and if any abnormalities are found, children should be referred for repeat hearing evaluations.
“We’re talking about a group of infants that we have very little to no information about,” said Kate Russell, MD, of Duke University, Durham, N.C. “Our discussion revolved around what’s known about infants who do have apparent abnormalities and are symptomatic, and trying to infer from that what could be done for these infants with asymptomatic infections.”
Telemedicine is another potentially powerful tool that physicians can use, “particularly in areas where there is limited direct access to pediatric subspecialty care,” Dr. Cragan said. Examples include taking videos of an infant and sending them to a neurologist to determine if symptoms were consistent with Zika, or sharing audio of the crying of Zika-infected children in Brazil so that primary care physicians can compare that with their patients.
“This is an area of very new and growing research and literature, and so this is going to be a continuous process [to] make sure that the people who are going to be seeing these infants on the front line are aware of what’s known,” Dr. Russell said.
Diagnostic testing of infants with suspected congenital Zika virus should include both immunoglobulin M (IgM) and polymerase chain reaction (PCR) testing, according to initial recommendations from a work group of federal health officials and pediatric specialists.
The group also called for infants with Zika virus symptoms to be transferred to facilities with pediatric subspecialty services.
Officials at the Centers for Disease Control and Prevention, along with pediatric specialists, reviewed the CDC’s existing guidelines for diagnosing, treating, and preventing Zika virus infections in both pregnant mothers and their infants during a 2-day meeting at the agency’s headquarters in Atlanta July 21-22.
“We want to make sure in the evaluation of symptomatic infants that we’re opening up a thorough differential, and that people are thinking about other things, particularly as we know that some of these issues, especially in terms of diagnosis, may be complex,” said Wanda D. Barfield, MD, director of the division of reproductive health at the CDC’s National Center for Chronic Disease Prevention and Health Promotion.
Although final recommendations are forthcoming, the consensus of the work group is that diagnostic testing of infants with suspected congenital Zika virus infection should include IgM and PCR testing of cerebrospinal fluid, urine, saliva and other infant specimens.
Asymptomatic infants should continue to receive care consistent with that of a normal, newborn infant, along with comprehensive physical exams, hearing tests, and head ultrasounds.
Symptomatic infants, however, should undergo different clinical and laboratory evaluations. Any symptomatic infant should be transferred to a pediatric subspecialty facility, which would allow for more specific care and services than would a primary care office. These services would include: neonatal/pediatric intensive care; endocrinologists to treat hypothyroidism, hypoaldosteronism, and growth hormone deficiency; orthopedists to treat arthrogryposis; neurologists to perform neuroimaging and EEGs, and to treat microcephaly and seizures; and other specialists to handle infectious disease, pulmonology, genetics, and feeding issues. These facilities would also offer family and social support, including palliative care options.
Infants with anomalies associated with congenital Zika virus infection should see a primary care physician every month for “routine care and increased surveillance,” said Janet Cragan, MD, of the CDC’s National Center on Birth Defects and Developmental Disorders. These visits should include measurements of weight, length, and head circumference, and should continue through 6 months of age. After that, the frequency of visits can be reevaluated.
During subsequent visits, providers should continue to monitor the infant’s development, as certain abnormalities take longer to become evident. These include sleep issues, excessive irritability, seizures, and “subtle symptoms” such as infantile spasms.
“In terms of coordination of care, there was discussion about the need to close the loop [to] ensure that the needed testing and consultations are done, [and] that the primary physician obtain those results,” Dr. Cragan said.
Similar monitoring – though less complex and stringent – should be done for children with congenital Zika virus infection who do not exhibit abnormalities and may not be symptomatic, the work group recommended. In addition, outpatient care is another critical component in managing congenital Zika virus infections, particularly in children who display no abnormalities at birth. Specifically, hearing evaluations should be performed regularly, and if any abnormalities are found, children should be referred for repeat hearing evaluations.
“We’re talking about a group of infants that we have very little to no information about,” said Kate Russell, MD, of Duke University, Durham, N.C. “Our discussion revolved around what’s known about infants who do have apparent abnormalities and are symptomatic, and trying to infer from that what could be done for these infants with asymptomatic infections.”
Telemedicine is another potentially powerful tool that physicians can use, “particularly in areas where there is limited direct access to pediatric subspecialty care,” Dr. Cragan said. Examples include taking videos of an infant and sending them to a neurologist to determine if symptoms were consistent with Zika, or sharing audio of the crying of Zika-infected children in Brazil so that primary care physicians can compare that with their patients.
“This is an area of very new and growing research and literature, and so this is going to be a continuous process [to] make sure that the people who are going to be seeing these infants on the front line are aware of what’s known,” Dr. Russell said.
Buprenorphine implants rival daily sublingual buprenorphine for opioid dependence
Using an implant device to administer buprenorphine for adult patients being treated for opioid addiction is a viable alternative to the standard sublingual buprenorphine, a randomized clinical trial published online July 19 suggests.
“An implantable buprenorphine delivery system reduces adherence issues and may improve efficacy,” wrote Richard N. Rosenthal, MD, of the Icahn School of Medicine at Mount Sinai, New York, and his coinvestigators. “Furthermore, buprenorphine implants may reduce the need for sublingual buprenorphine, decreasing its availability for diversion, misuse, and harms.”
Dr. Rosenthal and his coinvestigators enrolled 177 patients from June 2014 to May 2015. The participants were aged 18-65 years, had received a primary diagnosis of opioid addiction, and had been receiving daily 8-mg doses of sublingual buprenorphine at an outpatient clinic for at least 24 weeks. The average age of patients was 39 years, and 40.9% were female. All of the participants were recruited from 21 treatment sites across the United States (JAMA. 2016;316[3]:282-90).
The participants were randomized into one of two cohorts: 87 subjects received buprenorphine implants, and the remaining 90 received sublingual buprenorphine. Those in the former cohort received their implants on the day of randomization, with four subdermal devices implanted along the inner upper arm. The staff involved with implanting and removing the devices did not have anything to do with study evaluation, in order to maintain blinding. Ten urine samples were collected from subjects over the course of the study period, with follow-up visits at 1 week and 2 weeks post treatment.
“The primary efficacy endpoint was the difference in proportion of responders, defined as participants with at least 4 of 6 months without evidence of illicit opioid use (based on urine test and self-report composites) by treatment group” the authors clarified.
Ultimately, 84 of those who received implants and 89 of those receiving sublingual treatment completed the trial, and were included in the primary analysis. Of those, 81 of those receiving implants (96.4%) and 78 of those receiving sublingual buprenorphine (87.6%) responded to treatment.
Based on urine tests and self-reporting, 72 of those with implants (85.7%) maintained their opioid abstinence throughout the study period, compared with 64 of those in the sublingual treatment cohort (71.9%). Additionally, Dr. Rosenthal and his coinvestigators noted that sustained abstinence in months 3-6 was more prominent in the implant cohort. Adverse events not related to the implant site occurred in 42 of the implant subjects (48.3%) and 47 of the sublingual treatment subjects (52.8%).
“To our knowledge, this was the first comparative trial to evaluate efficacy and safety of 6-month buprenorphine implants relative to sublingual buprenorphine in this understudied population of patients clinically stable taking sublingual buprenorphine,” the authors stated. They added that “because there are limited data on patients stable on sublingual buprenorphine, it is important to study maintenance and improvement of stability in patients who achieve good clinical response to initial buprenorphine treatment” with regard to further study.
Dr. Rosenthal and his coinvestigators disclosed receiving grants and nonfinancial support from Braeburn Pharmaceuticals, which was the sole provider of funding for this study. The other investigators also reported financial relationships with other companies.
“Because of the overall high prevalence and marked increases in morbidity and mortality associated with nonmedical prescription opioids and heroin, addressing opioid use disorders is a public health priority in the United States,” wrote Wilson M. Compton, MD, and Nora D. Volkow, MD, in an accompanying editorial (JAMA. 2016;316[3]:277-79).
But a fundamental challenge in addressing the treatment of chronic diseases such as opioid use disorder is nonadherence and discontinuation of care, they wrote. The use of extended-release formulations has been one approach. For example, extended-release naltrexone is administered monthly rather than the “nearly daily dosing necessary for the oral formulations of naltrexone, methadone, and buprenorphine. An additional concern for the agonist medications used for opioid use disorders is their risk of being diverted and misused.”
The results of the study by Richard N. Rosenthal, MD, and his colleagues show clearly that buprenorphine implants are not inferior to buprenorphine administered sublingually. However, as the study authors pointed out, several factors limit the generalizability of their results. For example, the patient population in their study were primarily white, employed, had at least a high school education, and were prescription opioid dependent.
“The approval of this new buprenorphine implant formulation provides a unique new tool to address the complex, often chronic and relapsing opioid use disorders,” Dr. Compton and Dr. Volkow wrote. “This novel implant system may help buttress patients’ decision-making deficits that are a core component of the addiction by making these lifesaving medication adherence decisions far more infrequent.”
Dr. Compton, deputy director of the National Institute on Drug Abuse, reported owning stock in Pfizer, General Electric, and 3M. Dr. Volkow is director of NIDA and reported no disclosures.
“Because of the overall high prevalence and marked increases in morbidity and mortality associated with nonmedical prescription opioids and heroin, addressing opioid use disorders is a public health priority in the United States,” wrote Wilson M. Compton, MD, and Nora D. Volkow, MD, in an accompanying editorial (JAMA. 2016;316[3]:277-79).
But a fundamental challenge in addressing the treatment of chronic diseases such as opioid use disorder is nonadherence and discontinuation of care, they wrote. The use of extended-release formulations has been one approach. For example, extended-release naltrexone is administered monthly rather than the “nearly daily dosing necessary for the oral formulations of naltrexone, methadone, and buprenorphine. An additional concern for the agonist medications used for opioid use disorders is their risk of being diverted and misused.”
The results of the study by Richard N. Rosenthal, MD, and his colleagues show clearly that buprenorphine implants are not inferior to buprenorphine administered sublingually. However, as the study authors pointed out, several factors limit the generalizability of their results. For example, the patient population in their study were primarily white, employed, had at least a high school education, and were prescription opioid dependent.
“The approval of this new buprenorphine implant formulation provides a unique new tool to address the complex, often chronic and relapsing opioid use disorders,” Dr. Compton and Dr. Volkow wrote. “This novel implant system may help buttress patients’ decision-making deficits that are a core component of the addiction by making these lifesaving medication adherence decisions far more infrequent.”
Dr. Compton, deputy director of the National Institute on Drug Abuse, reported owning stock in Pfizer, General Electric, and 3M. Dr. Volkow is director of NIDA and reported no disclosures.
“Because of the overall high prevalence and marked increases in morbidity and mortality associated with nonmedical prescription opioids and heroin, addressing opioid use disorders is a public health priority in the United States,” wrote Wilson M. Compton, MD, and Nora D. Volkow, MD, in an accompanying editorial (JAMA. 2016;316[3]:277-79).
But a fundamental challenge in addressing the treatment of chronic diseases such as opioid use disorder is nonadherence and discontinuation of care, they wrote. The use of extended-release formulations has been one approach. For example, extended-release naltrexone is administered monthly rather than the “nearly daily dosing necessary for the oral formulations of naltrexone, methadone, and buprenorphine. An additional concern for the agonist medications used for opioid use disorders is their risk of being diverted and misused.”
The results of the study by Richard N. Rosenthal, MD, and his colleagues show clearly that buprenorphine implants are not inferior to buprenorphine administered sublingually. However, as the study authors pointed out, several factors limit the generalizability of their results. For example, the patient population in their study were primarily white, employed, had at least a high school education, and were prescription opioid dependent.
“The approval of this new buprenorphine implant formulation provides a unique new tool to address the complex, often chronic and relapsing opioid use disorders,” Dr. Compton and Dr. Volkow wrote. “This novel implant system may help buttress patients’ decision-making deficits that are a core component of the addiction by making these lifesaving medication adherence decisions far more infrequent.”
Dr. Compton, deputy director of the National Institute on Drug Abuse, reported owning stock in Pfizer, General Electric, and 3M. Dr. Volkow is director of NIDA and reported no disclosures.
Using an implant device to administer buprenorphine for adult patients being treated for opioid addiction is a viable alternative to the standard sublingual buprenorphine, a randomized clinical trial published online July 19 suggests.
“An implantable buprenorphine delivery system reduces adherence issues and may improve efficacy,” wrote Richard N. Rosenthal, MD, of the Icahn School of Medicine at Mount Sinai, New York, and his coinvestigators. “Furthermore, buprenorphine implants may reduce the need for sublingual buprenorphine, decreasing its availability for diversion, misuse, and harms.”
Dr. Rosenthal and his coinvestigators enrolled 177 patients from June 2014 to May 2015. The participants were aged 18-65 years, had received a primary diagnosis of opioid addiction, and had been receiving daily 8-mg doses of sublingual buprenorphine at an outpatient clinic for at least 24 weeks. The average age of patients was 39 years, and 40.9% were female. All of the participants were recruited from 21 treatment sites across the United States (JAMA. 2016;316[3]:282-90).
The participants were randomized into one of two cohorts: 87 subjects received buprenorphine implants, and the remaining 90 received sublingual buprenorphine. Those in the former cohort received their implants on the day of randomization, with four subdermal devices implanted along the inner upper arm. The staff involved with implanting and removing the devices did not have anything to do with study evaluation, in order to maintain blinding. Ten urine samples were collected from subjects over the course of the study period, with follow-up visits at 1 week and 2 weeks post treatment.
“The primary efficacy endpoint was the difference in proportion of responders, defined as participants with at least 4 of 6 months without evidence of illicit opioid use (based on urine test and self-report composites) by treatment group” the authors clarified.
Ultimately, 84 of those who received implants and 89 of those receiving sublingual treatment completed the trial, and were included in the primary analysis. Of those, 81 of those receiving implants (96.4%) and 78 of those receiving sublingual buprenorphine (87.6%) responded to treatment.
Based on urine tests and self-reporting, 72 of those with implants (85.7%) maintained their opioid abstinence throughout the study period, compared with 64 of those in the sublingual treatment cohort (71.9%). Additionally, Dr. Rosenthal and his coinvestigators noted that sustained abstinence in months 3-6 was more prominent in the implant cohort. Adverse events not related to the implant site occurred in 42 of the implant subjects (48.3%) and 47 of the sublingual treatment subjects (52.8%).
“To our knowledge, this was the first comparative trial to evaluate efficacy and safety of 6-month buprenorphine implants relative to sublingual buprenorphine in this understudied population of patients clinically stable taking sublingual buprenorphine,” the authors stated. They added that “because there are limited data on patients stable on sublingual buprenorphine, it is important to study maintenance and improvement of stability in patients who achieve good clinical response to initial buprenorphine treatment” with regard to further study.
Dr. Rosenthal and his coinvestigators disclosed receiving grants and nonfinancial support from Braeburn Pharmaceuticals, which was the sole provider of funding for this study. The other investigators also reported financial relationships with other companies.
Using an implant device to administer buprenorphine for adult patients being treated for opioid addiction is a viable alternative to the standard sublingual buprenorphine, a randomized clinical trial published online July 19 suggests.
“An implantable buprenorphine delivery system reduces adherence issues and may improve efficacy,” wrote Richard N. Rosenthal, MD, of the Icahn School of Medicine at Mount Sinai, New York, and his coinvestigators. “Furthermore, buprenorphine implants may reduce the need for sublingual buprenorphine, decreasing its availability for diversion, misuse, and harms.”
Dr. Rosenthal and his coinvestigators enrolled 177 patients from June 2014 to May 2015. The participants were aged 18-65 years, had received a primary diagnosis of opioid addiction, and had been receiving daily 8-mg doses of sublingual buprenorphine at an outpatient clinic for at least 24 weeks. The average age of patients was 39 years, and 40.9% were female. All of the participants were recruited from 21 treatment sites across the United States (JAMA. 2016;316[3]:282-90).
The participants were randomized into one of two cohorts: 87 subjects received buprenorphine implants, and the remaining 90 received sublingual buprenorphine. Those in the former cohort received their implants on the day of randomization, with four subdermal devices implanted along the inner upper arm. The staff involved with implanting and removing the devices did not have anything to do with study evaluation, in order to maintain blinding. Ten urine samples were collected from subjects over the course of the study period, with follow-up visits at 1 week and 2 weeks post treatment.
“The primary efficacy endpoint was the difference in proportion of responders, defined as participants with at least 4 of 6 months without evidence of illicit opioid use (based on urine test and self-report composites) by treatment group” the authors clarified.
Ultimately, 84 of those who received implants and 89 of those receiving sublingual treatment completed the trial, and were included in the primary analysis. Of those, 81 of those receiving implants (96.4%) and 78 of those receiving sublingual buprenorphine (87.6%) responded to treatment.
Based on urine tests and self-reporting, 72 of those with implants (85.7%) maintained their opioid abstinence throughout the study period, compared with 64 of those in the sublingual treatment cohort (71.9%). Additionally, Dr. Rosenthal and his coinvestigators noted that sustained abstinence in months 3-6 was more prominent in the implant cohort. Adverse events not related to the implant site occurred in 42 of the implant subjects (48.3%) and 47 of the sublingual treatment subjects (52.8%).
“To our knowledge, this was the first comparative trial to evaluate efficacy and safety of 6-month buprenorphine implants relative to sublingual buprenorphine in this understudied population of patients clinically stable taking sublingual buprenorphine,” the authors stated. They added that “because there are limited data on patients stable on sublingual buprenorphine, it is important to study maintenance and improvement of stability in patients who achieve good clinical response to initial buprenorphine treatment” with regard to further study.
Dr. Rosenthal and his coinvestigators disclosed receiving grants and nonfinancial support from Braeburn Pharmaceuticals, which was the sole provider of funding for this study. The other investigators also reported financial relationships with other companies.
FROM JAMA
Key clinical point: Buprenorphine implants are no less effective than sublingual buprenorphine, the current standard of care, in managing opioid dependence.
Major finding: 96.4% of those with implants and 87.6% of those with sublingual buprenorphine responded to treatment; over 6 months, 85.7% of those with implants and 71.9% of those receiving sublingual treatment maintained opioid resistance.
Data source: An outpatient, randomized, active-controlled, 24-week, double-blind, double-dummy study of 177 buprenorphine patients at 21 U.S. sites.
Disclosures: The study funded by Braeburn Pharmaceuticals. Dr. Rosenthal and other coinvestigators disclosed receiving grants and nonfinancial support from Braeburn Pharmaceuticals while this study was being conducted. The other investigators also reported financial relationships with other companies.
First female-to-male sexual transmission of Zika virus
A suspected case of sexual transmission of the Zika virus from a female to a male has occurred in New York City, according to the New York City Department of Health and Mental Hygiene (DOHMH).
“This case represents the first reported occurrence of female-to-male sexual transmission of Zika virus,” Alexander Davidson of the DOHMH and his coauthors stated in the CDC’s Morbidity and Mortality Weekly Report. “Current guidance to prevent sexual transmission of Zika virus is based on the assumption that transmission occurs from a male partner to a receptive partner.”
The woman, reportedly in her twenties and not pregnant at the time of infection, had traveled to a region experiencing high volumes of Zika virus transmission. Upon returning home, the woman engaged in condomless vaginal intercourse with her male partner, and subsequently developed symptoms consistent with a Zika virus infection. Three days after symptom onset, her primary care provider took blood and urine samples, from which a Zika virus infection was confirmed. (MMWR Morb Mortal Wkly Rep. 2016 Jul 15. doi: 10.15585/mmwr.mm6528e2)
A week after the sexual encounter, her partner – also in his twenties – began experiencing symptoms of Zika virus infection. Three days after the onset of his symptoms, he went to the same primary care provider as the woman. He confirmed that he had not traveled outside of the United States in the last year, had engaged in condomless vaginal sex with just one individual (the aforementioned female), had no blood on his penis to indicate vaginal bleeding or the presence of open lesions, and had no mosquito bites in the previous week.
“The timing and sequence of events support female-to-male Zika virus transmission through condomless vaginal intercourse,” the coauthors conclude, adding that “virus present in either vaginal fluids or menstrual blood might have been transmitted during exposure to her male partner’s urethral mucosa or undetected abrasions on his penis.”
Both the female and male were tested via real-time reverse transcription–polymerase chain reaction (rRT-PCR), with serum testing done via the Zika immunoglobulin M antibody capture enzyme-linked immunosorbent assay (Zika MAC-ELISA). The rRT-PCR testing showed Zika virus RNA in the woman’s serum, despite being collected three days after the sexual encounter, meaning she was viremic at the time. Studies on nonhuman primates have shown that Zika virus RNA can remain present in vaginal fluid for up to a week, according to the report.
The CDC is cautioning pregnant women against travel to Zika-heavy areas, in particular the 2016 Summer Olympic Games in Rio de Janeiro. Health care providers who receive patients with Zika-like symptoms should ask if the patient has had sexual contact with someone who has traveled to an affected region, if the patient did not travel to such a region.
A suspected case of sexual transmission of the Zika virus from a female to a male has occurred in New York City, according to the New York City Department of Health and Mental Hygiene (DOHMH).
“This case represents the first reported occurrence of female-to-male sexual transmission of Zika virus,” Alexander Davidson of the DOHMH and his coauthors stated in the CDC’s Morbidity and Mortality Weekly Report. “Current guidance to prevent sexual transmission of Zika virus is based on the assumption that transmission occurs from a male partner to a receptive partner.”
The woman, reportedly in her twenties and not pregnant at the time of infection, had traveled to a region experiencing high volumes of Zika virus transmission. Upon returning home, the woman engaged in condomless vaginal intercourse with her male partner, and subsequently developed symptoms consistent with a Zika virus infection. Three days after symptom onset, her primary care provider took blood and urine samples, from which a Zika virus infection was confirmed. (MMWR Morb Mortal Wkly Rep. 2016 Jul 15. doi: 10.15585/mmwr.mm6528e2)
A week after the sexual encounter, her partner – also in his twenties – began experiencing symptoms of Zika virus infection. Three days after the onset of his symptoms, he went to the same primary care provider as the woman. He confirmed that he had not traveled outside of the United States in the last year, had engaged in condomless vaginal sex with just one individual (the aforementioned female), had no blood on his penis to indicate vaginal bleeding or the presence of open lesions, and had no mosquito bites in the previous week.
“The timing and sequence of events support female-to-male Zika virus transmission through condomless vaginal intercourse,” the coauthors conclude, adding that “virus present in either vaginal fluids or menstrual blood might have been transmitted during exposure to her male partner’s urethral mucosa or undetected abrasions on his penis.”
Both the female and male were tested via real-time reverse transcription–polymerase chain reaction (rRT-PCR), with serum testing done via the Zika immunoglobulin M antibody capture enzyme-linked immunosorbent assay (Zika MAC-ELISA). The rRT-PCR testing showed Zika virus RNA in the woman’s serum, despite being collected three days after the sexual encounter, meaning she was viremic at the time. Studies on nonhuman primates have shown that Zika virus RNA can remain present in vaginal fluid for up to a week, according to the report.
The CDC is cautioning pregnant women against travel to Zika-heavy areas, in particular the 2016 Summer Olympic Games in Rio de Janeiro. Health care providers who receive patients with Zika-like symptoms should ask if the patient has had sexual contact with someone who has traveled to an affected region, if the patient did not travel to such a region.
A suspected case of sexual transmission of the Zika virus from a female to a male has occurred in New York City, according to the New York City Department of Health and Mental Hygiene (DOHMH).
“This case represents the first reported occurrence of female-to-male sexual transmission of Zika virus,” Alexander Davidson of the DOHMH and his coauthors stated in the CDC’s Morbidity and Mortality Weekly Report. “Current guidance to prevent sexual transmission of Zika virus is based on the assumption that transmission occurs from a male partner to a receptive partner.”
The woman, reportedly in her twenties and not pregnant at the time of infection, had traveled to a region experiencing high volumes of Zika virus transmission. Upon returning home, the woman engaged in condomless vaginal intercourse with her male partner, and subsequently developed symptoms consistent with a Zika virus infection. Three days after symptom onset, her primary care provider took blood and urine samples, from which a Zika virus infection was confirmed. (MMWR Morb Mortal Wkly Rep. 2016 Jul 15. doi: 10.15585/mmwr.mm6528e2)
A week after the sexual encounter, her partner – also in his twenties – began experiencing symptoms of Zika virus infection. Three days after the onset of his symptoms, he went to the same primary care provider as the woman. He confirmed that he had not traveled outside of the United States in the last year, had engaged in condomless vaginal sex with just one individual (the aforementioned female), had no blood on his penis to indicate vaginal bleeding or the presence of open lesions, and had no mosquito bites in the previous week.
“The timing and sequence of events support female-to-male Zika virus transmission through condomless vaginal intercourse,” the coauthors conclude, adding that “virus present in either vaginal fluids or menstrual blood might have been transmitted during exposure to her male partner’s urethral mucosa or undetected abrasions on his penis.”
Both the female and male were tested via real-time reverse transcription–polymerase chain reaction (rRT-PCR), with serum testing done via the Zika immunoglobulin M antibody capture enzyme-linked immunosorbent assay (Zika MAC-ELISA). The rRT-PCR testing showed Zika virus RNA in the woman’s serum, despite being collected three days after the sexual encounter, meaning she was viremic at the time. Studies on nonhuman primates have shown that Zika virus RNA can remain present in vaginal fluid for up to a week, according to the report.
The CDC is cautioning pregnant women against travel to Zika-heavy areas, in particular the 2016 Summer Olympic Games in Rio de Janeiro. Health care providers who receive patients with Zika-like symptoms should ask if the patient has had sexual contact with someone who has traveled to an affected region, if the patient did not travel to such a region.
FROM MMWR
Prevention of postop GI disorders may reduce ‘failed discharges’ for laparoscopic hernia surgery
Postoperative hospital readmissions after ventral hernia repair may be both predictable and preventable if type of operation and patient characteristics are taken into account, a large retrospective study has found.
Early readmissions, or ‘failed discharges,’ are potentially more preventable than are later readmissions, according to Adam C. Celio, MD, of East Carolina University, Greenville, N.C., and his colleagues. Medical literature “demonstrates that patients readmitted within a few days of discharge return for different reasons than patients who are readmitted later; very early readmissions are likely due to poor coordination of care or inadequate recognition of postdischarge needs, while later readmissions (still within the 30-day window) are more likely due to patient disease or procedural complications.”
Dr. Celio and his coinvestigators examined data from the American College of Surgeons’ National Surgical Quality Improvement Program, looking for patients who underwent either open (n = 9,009) or laparoscopic (n = 3,360) ventral hernia repair (VHR) in 2012. The primary endpoint was defined as readmission within 30 days of discharge following a VHR procedure; early readmission was defined as occurring within 5 days of discharge for laparoscopic VHR and within 9 days of discharge for open VHR, while late readmission was defined as any readmission that occurred after the aforementioned time frames (Surgery. 2016;160[2]:413-17).
The researchers analyzed each readmission to determine its cause among nine classifications: bleeding, cardiovascular events, dehydration, gastrointestinal causes (nausea, emesis, ileus, and bowel obstruction), pain, venous thromboembolic event, wound occurrences (superficial site infection, deep operative site infection, wound disruptions), other infection, and other causes.
Of the 12,369 individuals included in the study, 1,057 (8.5%) were readmitted within 30 days of undergoing VHR. Generally, the patients who had open procedures had higher ASA scores than did those who had laparoscopic surgery, reflecting a greater likelihood of preoperative physical impairment and chronic disease. The researchers then analyzed which patients were mostly likely to be readmitted and the correlation between type of operation, cause of readmission, and timing of readmission (early or late).
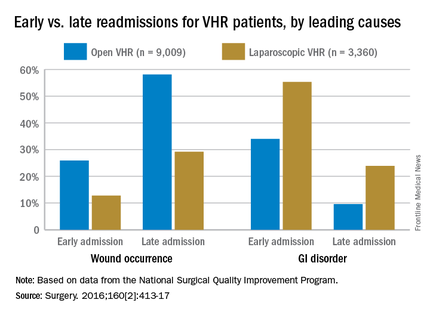
Open-VHR patients were more likely to be readmitted within 30 days than were those who underwent laparoscopic VHR: 9.2% vs. 6.9% readmission rates, respectively (OR 0.73, 95% CI 0.63-0.85). Among all the patients readmitted, wound complications were the most common cause (32.2%), followed by gastrointestinal disorders (14.3%), most which were attributed to emesis or nausea.
Wound complications were more highly associated with late readmission than with early ones, at 52% vs. 23%, respectively (OR 3.68, 95% CI 2.56-5.29). As might be expected, wound complications were more likely to occur in open-VHR patients than in laparoscopic-VHR patients: 49.6% vs. 24.4% (OR 3.05, 95% CI 2.06-4.52).
Of the entire cohort, 279 patients (2.3%) were classified as early readmissions, and gastrointestinal causes were more highly associated with early readmission than with late ones: 39% vs.13% (OR 4.45, 95% CI 3.06-6.47). Gastrointestinal issues occurred more often after laparoscopic VHR than after open VHR: 33% vs. 16% (OR 2.59, 95% CI 1.75-3.84). These early readmissions, occurring within days of discharge, are potentially preventable with a stepped up focus on control of nausea and emesis experienced by these patients, the researchers noted.
A funding source for this study was not disclosed. Dr. Celio and his coauthors did not report any relevant disclosures.
Postoperative hospital readmissions after ventral hernia repair may be both predictable and preventable if type of operation and patient characteristics are taken into account, a large retrospective study has found.
Early readmissions, or ‘failed discharges,’ are potentially more preventable than are later readmissions, according to Adam C. Celio, MD, of East Carolina University, Greenville, N.C., and his colleagues. Medical literature “demonstrates that patients readmitted within a few days of discharge return for different reasons than patients who are readmitted later; very early readmissions are likely due to poor coordination of care or inadequate recognition of postdischarge needs, while later readmissions (still within the 30-day window) are more likely due to patient disease or procedural complications.”
Dr. Celio and his coinvestigators examined data from the American College of Surgeons’ National Surgical Quality Improvement Program, looking for patients who underwent either open (n = 9,009) or laparoscopic (n = 3,360) ventral hernia repair (VHR) in 2012. The primary endpoint was defined as readmission within 30 days of discharge following a VHR procedure; early readmission was defined as occurring within 5 days of discharge for laparoscopic VHR and within 9 days of discharge for open VHR, while late readmission was defined as any readmission that occurred after the aforementioned time frames (Surgery. 2016;160[2]:413-17).
The researchers analyzed each readmission to determine its cause among nine classifications: bleeding, cardiovascular events, dehydration, gastrointestinal causes (nausea, emesis, ileus, and bowel obstruction), pain, venous thromboembolic event, wound occurrences (superficial site infection, deep operative site infection, wound disruptions), other infection, and other causes.
Of the 12,369 individuals included in the study, 1,057 (8.5%) were readmitted within 30 days of undergoing VHR. Generally, the patients who had open procedures had higher ASA scores than did those who had laparoscopic surgery, reflecting a greater likelihood of preoperative physical impairment and chronic disease. The researchers then analyzed which patients were mostly likely to be readmitted and the correlation between type of operation, cause of readmission, and timing of readmission (early or late).

Open-VHR patients were more likely to be readmitted within 30 days than were those who underwent laparoscopic VHR: 9.2% vs. 6.9% readmission rates, respectively (OR 0.73, 95% CI 0.63-0.85). Among all the patients readmitted, wound complications were the most common cause (32.2%), followed by gastrointestinal disorders (14.3%), most which were attributed to emesis or nausea.
Wound complications were more highly associated with late readmission than with early ones, at 52% vs. 23%, respectively (OR 3.68, 95% CI 2.56-5.29). As might be expected, wound complications were more likely to occur in open-VHR patients than in laparoscopic-VHR patients: 49.6% vs. 24.4% (OR 3.05, 95% CI 2.06-4.52).
Of the entire cohort, 279 patients (2.3%) were classified as early readmissions, and gastrointestinal causes were more highly associated with early readmission than with late ones: 39% vs.13% (OR 4.45, 95% CI 3.06-6.47). Gastrointestinal issues occurred more often after laparoscopic VHR than after open VHR: 33% vs. 16% (OR 2.59, 95% CI 1.75-3.84). These early readmissions, occurring within days of discharge, are potentially preventable with a stepped up focus on control of nausea and emesis experienced by these patients, the researchers noted.
A funding source for this study was not disclosed. Dr. Celio and his coauthors did not report any relevant disclosures.
Postoperative hospital readmissions after ventral hernia repair may be both predictable and preventable if type of operation and patient characteristics are taken into account, a large retrospective study has found.
Early readmissions, or ‘failed discharges,’ are potentially more preventable than are later readmissions, according to Adam C. Celio, MD, of East Carolina University, Greenville, N.C., and his colleagues. Medical literature “demonstrates that patients readmitted within a few days of discharge return for different reasons than patients who are readmitted later; very early readmissions are likely due to poor coordination of care or inadequate recognition of postdischarge needs, while later readmissions (still within the 30-day window) are more likely due to patient disease or procedural complications.”
Dr. Celio and his coinvestigators examined data from the American College of Surgeons’ National Surgical Quality Improvement Program, looking for patients who underwent either open (n = 9,009) or laparoscopic (n = 3,360) ventral hernia repair (VHR) in 2012. The primary endpoint was defined as readmission within 30 days of discharge following a VHR procedure; early readmission was defined as occurring within 5 days of discharge for laparoscopic VHR and within 9 days of discharge for open VHR, while late readmission was defined as any readmission that occurred after the aforementioned time frames (Surgery. 2016;160[2]:413-17).
The researchers analyzed each readmission to determine its cause among nine classifications: bleeding, cardiovascular events, dehydration, gastrointestinal causes (nausea, emesis, ileus, and bowel obstruction), pain, venous thromboembolic event, wound occurrences (superficial site infection, deep operative site infection, wound disruptions), other infection, and other causes.
Of the 12,369 individuals included in the study, 1,057 (8.5%) were readmitted within 30 days of undergoing VHR. Generally, the patients who had open procedures had higher ASA scores than did those who had laparoscopic surgery, reflecting a greater likelihood of preoperative physical impairment and chronic disease. The researchers then analyzed which patients were mostly likely to be readmitted and the correlation between type of operation, cause of readmission, and timing of readmission (early or late).

Open-VHR patients were more likely to be readmitted within 30 days than were those who underwent laparoscopic VHR: 9.2% vs. 6.9% readmission rates, respectively (OR 0.73, 95% CI 0.63-0.85). Among all the patients readmitted, wound complications were the most common cause (32.2%), followed by gastrointestinal disorders (14.3%), most which were attributed to emesis or nausea.
Wound complications were more highly associated with late readmission than with early ones, at 52% vs. 23%, respectively (OR 3.68, 95% CI 2.56-5.29). As might be expected, wound complications were more likely to occur in open-VHR patients than in laparoscopic-VHR patients: 49.6% vs. 24.4% (OR 3.05, 95% CI 2.06-4.52).
Of the entire cohort, 279 patients (2.3%) were classified as early readmissions, and gastrointestinal causes were more highly associated with early readmission than with late ones: 39% vs.13% (OR 4.45, 95% CI 3.06-6.47). Gastrointestinal issues occurred more often after laparoscopic VHR than after open VHR: 33% vs. 16% (OR 2.59, 95% CI 1.75-3.84). These early readmissions, occurring within days of discharge, are potentially preventable with a stepped up focus on control of nausea and emesis experienced by these patients, the researchers noted.
A funding source for this study was not disclosed. Dr. Celio and his coauthors did not report any relevant disclosures.
FROM SURGERY
Key clinical point: Postoperative complications and likelihood of readmission differ according to the type of ventral hernia repair procedure (open or laparoscopic).
Major finding: Thirty-day readmissions occurred among 6.9% of patients who had laparoscopic surgery, compared with 9.2% of open-procedure patients; gastrointestinal disorders were more common in early readmissions but wound occurrences were more common in late readmissions.
Data source: Retrospective cohort study of 12,369 ventral hernia repair patients (open and laparoscopic) in 2012.
Disclosures: Funding source not disclosed. Authors did not report any relevant financial disclosures.
CDC forecasts low chance of mosquito-borne Zika infection at Olympics
With only a few weeks left until the 2016 Olympic Games get underway in Rio de Janeiro, officials at the Centers for Disease Control and Prevention are urging anyone traveling to the Olympics to take precautions to avoid contracting Zika virus infection or spreading it when they return home.
But the CDC estimates that there is a low probability of mosquito-borne Zika virus infections during the Olympics because Rio will be experiencing cooler, drier weather then, which typically reduces the mosquito population.
Along with lower mosquito activity, the CDC said that the number of visitors expected in Brazil for the Olympics represents only a fraction of the total travel volume to Zika-affected countries during 2015. The Brazilian Tourism Board is expecting anywhere between 350,000 and 500,000 visitors for the Olympic Games, coming from 207 countries. That represents less than 0.25% of the total travel volume to Zika-affected countries during the entirety of 2015, according to the CDC.
There are 19 countries that the CDC deems susceptible to sustained mosquito-borne transmission of the Zika virus, should the virus enter the country via an attendee of the Olympics (MMWR. 2016 Jul 13. doi: 10.15585/mmwr.mm6528e1).
Of these 19 – none of which are currently experiencing a Zika outbreak – 15 are “not estimated to increase substantially the level of risk above that incurred by the usual aviation travel baseline for these countries.” This leaves Chad, Djibouti, Eritrea, and Yemen at an elevated risk for a Zika outbreak. These four countries “are unique in that they do not have a substantial number of travelers to any country with local Zika virus transmission, except for anticipated travel to the Games,” according to the CDC.
The CDC is urging travelers to take protective measures for their entire stay in Rio and for at least 3 weeks after returning home. The measures include applying mosquito repellent, wearing long-sleeved shirts and long pants, staying in rooms that are air conditioned, and using either screen doors or a mosquito net for additional protection. Additionally, all travelers should take measures to prevent sexual transmission. The CDC continues to advise pregnant women not to travel to the Olympics.
With only a few weeks left until the 2016 Olympic Games get underway in Rio de Janeiro, officials at the Centers for Disease Control and Prevention are urging anyone traveling to the Olympics to take precautions to avoid contracting Zika virus infection or spreading it when they return home.
But the CDC estimates that there is a low probability of mosquito-borne Zika virus infections during the Olympics because Rio will be experiencing cooler, drier weather then, which typically reduces the mosquito population.
Along with lower mosquito activity, the CDC said that the number of visitors expected in Brazil for the Olympics represents only a fraction of the total travel volume to Zika-affected countries during 2015. The Brazilian Tourism Board is expecting anywhere between 350,000 and 500,000 visitors for the Olympic Games, coming from 207 countries. That represents less than 0.25% of the total travel volume to Zika-affected countries during the entirety of 2015, according to the CDC.
There are 19 countries that the CDC deems susceptible to sustained mosquito-borne transmission of the Zika virus, should the virus enter the country via an attendee of the Olympics (MMWR. 2016 Jul 13. doi: 10.15585/mmwr.mm6528e1).
Of these 19 – none of which are currently experiencing a Zika outbreak – 15 are “not estimated to increase substantially the level of risk above that incurred by the usual aviation travel baseline for these countries.” This leaves Chad, Djibouti, Eritrea, and Yemen at an elevated risk for a Zika outbreak. These four countries “are unique in that they do not have a substantial number of travelers to any country with local Zika virus transmission, except for anticipated travel to the Games,” according to the CDC.
The CDC is urging travelers to take protective measures for their entire stay in Rio and for at least 3 weeks after returning home. The measures include applying mosquito repellent, wearing long-sleeved shirts and long pants, staying in rooms that are air conditioned, and using either screen doors or a mosquito net for additional protection. Additionally, all travelers should take measures to prevent sexual transmission. The CDC continues to advise pregnant women not to travel to the Olympics.
With only a few weeks left until the 2016 Olympic Games get underway in Rio de Janeiro, officials at the Centers for Disease Control and Prevention are urging anyone traveling to the Olympics to take precautions to avoid contracting Zika virus infection or spreading it when they return home.
But the CDC estimates that there is a low probability of mosquito-borne Zika virus infections during the Olympics because Rio will be experiencing cooler, drier weather then, which typically reduces the mosquito population.
Along with lower mosquito activity, the CDC said that the number of visitors expected in Brazil for the Olympics represents only a fraction of the total travel volume to Zika-affected countries during 2015. The Brazilian Tourism Board is expecting anywhere between 350,000 and 500,000 visitors for the Olympic Games, coming from 207 countries. That represents less than 0.25% of the total travel volume to Zika-affected countries during the entirety of 2015, according to the CDC.
There are 19 countries that the CDC deems susceptible to sustained mosquito-borne transmission of the Zika virus, should the virus enter the country via an attendee of the Olympics (MMWR. 2016 Jul 13. doi: 10.15585/mmwr.mm6528e1).
Of these 19 – none of which are currently experiencing a Zika outbreak – 15 are “not estimated to increase substantially the level of risk above that incurred by the usual aviation travel baseline for these countries.” This leaves Chad, Djibouti, Eritrea, and Yemen at an elevated risk for a Zika outbreak. These four countries “are unique in that they do not have a substantial number of travelers to any country with local Zika virus transmission, except for anticipated travel to the Games,” according to the CDC.
The CDC is urging travelers to take protective measures for their entire stay in Rio and for at least 3 weeks after returning home. The measures include applying mosquito repellent, wearing long-sleeved shirts and long pants, staying in rooms that are air conditioned, and using either screen doors or a mosquito net for additional protection. Additionally, all travelers should take measures to prevent sexual transmission. The CDC continues to advise pregnant women not to travel to the Olympics.
FROM MMWR
Hepatitis disease burden continues to rise
Although the disease burden of most other prevalent communicable diseases has gone down considerably over the last 25 years, viral hepatitis continues to be a challenge for health care professionals around the world, as incidences of the disease have climbed steadily between 1990 and 2013.
Advances in treating hepatitis A, B, C, and E viruses over the last 25 years have helped to “overcome many barriers to the control and treatment of viral hepatitis in low-income countries and are set to be important components of a new global health strategy,” wrote the study investigators, led by Jeffrey D. Stanaway, MD, of the University of Washington, Seattle. “However, a better understanding of the burden of disease is required to guide these efforts.”
Dr. Stanaway and his coauthors looked at the Global Burden of Disease (GBD) study for data on worldwide morbidity and mortality associated with hepatitis A, B, C, and E viruses, as well as cirrhosis and liver cancer secondary to hepatitis B or C virus. Data collected for the GBD was used to determine disability-adjusted life-years (DALYs), which is a metric calculated by adding up years of life lost (YLL) and years lived with disability (YLD), both of which were also measured by the GBD.
Data showed that worldwide deaths related to viral hepatitis numbered 0.89 million in 1990 (95% uncertainty interval [UI]: 0.86-0.94), but jumped up dramatically to 1.45 million in 2013 (95% UI: 1.38-1.54). Over the same time period, YLLs and YLDs also increased, going from 31.0 million (95% UI: 29.6-32.6) and 0.65 million (95% UI: 0.45-0.89), respectively, in 1990, to 41.6 million (95% UI: 39.1-44.7), and 0.87 million (95% UI: 0.61-1.18), respectively, in 2013. Consequently, DALYs increased from 31.7 million in 1990 (95% UI: 30.2-33.3) to 42.5 million (95% UI: 39.9-45.6).
These figures represent a 34% increase in viral hepatitis disease burden over that period of time. Furthermore, viral hepatitis went from being the 10th leading cause of death in the world in 1990 (95% UI: 10-12) to the seventh leading cause in 2013 (95% UI: 7-8). However, analysis without the data’s demographic trends showed that YLL and YLD rates declined by 20% and 13%, respectively (95% UI: 8-30 and 8-18), while DALY rates dropped by 20% (95% UI: 8-30) with no significant trend detected in age-specific mortality rates, indicating that demographic changes such as population growth may be the biggest factor contributing to viral hepatitis’ growing disease burden.
“HAV is the only hepatitis virus for which DALYs have declined significantly between 1990 and 2013. Some of this decline has been driven by changing population age structures, but most is due to declines in age-specific rates, most likely as a result of vaccination and improvements in water supply and sanitation,” the authors noted.
Dr. Stanaway and his coauthors also urged public health institutions around the world to devote more funding to targeting viral hepatitis, noting that the current state of funding is “disproportionate to [viral hepatitis’] importance as a major cause of death and disability.”
The Bill & Melinda Gates Foundation funded the study. Coauthor Graham S. Cooke, MD, reported being an investigator on trials of hepatitis C virus therapy sponsored by Boehringer Ingelheim, Gilead, Merck, and Bristol-Myers Squibb, and has acted in an advisory role to Merck, Boehringer Ingelheim, Gilead, Janssen, and WHO in relation to viral hepatitis and clinical trials unrelated to this work. Dr. Stanaway and other coauthors did not report any relevant financial disclosures.
Although the disease burden of most other prevalent communicable diseases has gone down considerably over the last 25 years, viral hepatitis continues to be a challenge for health care professionals around the world, as incidences of the disease have climbed steadily between 1990 and 2013.
Advances in treating hepatitis A, B, C, and E viruses over the last 25 years have helped to “overcome many barriers to the control and treatment of viral hepatitis in low-income countries and are set to be important components of a new global health strategy,” wrote the study investigators, led by Jeffrey D. Stanaway, MD, of the University of Washington, Seattle. “However, a better understanding of the burden of disease is required to guide these efforts.”
Dr. Stanaway and his coauthors looked at the Global Burden of Disease (GBD) study for data on worldwide morbidity and mortality associated with hepatitis A, B, C, and E viruses, as well as cirrhosis and liver cancer secondary to hepatitis B or C virus. Data collected for the GBD was used to determine disability-adjusted life-years (DALYs), which is a metric calculated by adding up years of life lost (YLL) and years lived with disability (YLD), both of which were also measured by the GBD.
Data showed that worldwide deaths related to viral hepatitis numbered 0.89 million in 1990 (95% uncertainty interval [UI]: 0.86-0.94), but jumped up dramatically to 1.45 million in 2013 (95% UI: 1.38-1.54). Over the same time period, YLLs and YLDs also increased, going from 31.0 million (95% UI: 29.6-32.6) and 0.65 million (95% UI: 0.45-0.89), respectively, in 1990, to 41.6 million (95% UI: 39.1-44.7), and 0.87 million (95% UI: 0.61-1.18), respectively, in 2013. Consequently, DALYs increased from 31.7 million in 1990 (95% UI: 30.2-33.3) to 42.5 million (95% UI: 39.9-45.6).
These figures represent a 34% increase in viral hepatitis disease burden over that period of time. Furthermore, viral hepatitis went from being the 10th leading cause of death in the world in 1990 (95% UI: 10-12) to the seventh leading cause in 2013 (95% UI: 7-8). However, analysis without the data’s demographic trends showed that YLL and YLD rates declined by 20% and 13%, respectively (95% UI: 8-30 and 8-18), while DALY rates dropped by 20% (95% UI: 8-30) with no significant trend detected in age-specific mortality rates, indicating that demographic changes such as population growth may be the biggest factor contributing to viral hepatitis’ growing disease burden.
“HAV is the only hepatitis virus for which DALYs have declined significantly between 1990 and 2013. Some of this decline has been driven by changing population age structures, but most is due to declines in age-specific rates, most likely as a result of vaccination and improvements in water supply and sanitation,” the authors noted.
Dr. Stanaway and his coauthors also urged public health institutions around the world to devote more funding to targeting viral hepatitis, noting that the current state of funding is “disproportionate to [viral hepatitis’] importance as a major cause of death and disability.”
The Bill & Melinda Gates Foundation funded the study. Coauthor Graham S. Cooke, MD, reported being an investigator on trials of hepatitis C virus therapy sponsored by Boehringer Ingelheim, Gilead, Merck, and Bristol-Myers Squibb, and has acted in an advisory role to Merck, Boehringer Ingelheim, Gilead, Janssen, and WHO in relation to viral hepatitis and clinical trials unrelated to this work. Dr. Stanaway and other coauthors did not report any relevant financial disclosures.
Although the disease burden of most other prevalent communicable diseases has gone down considerably over the last 25 years, viral hepatitis continues to be a challenge for health care professionals around the world, as incidences of the disease have climbed steadily between 1990 and 2013.
Advances in treating hepatitis A, B, C, and E viruses over the last 25 years have helped to “overcome many barriers to the control and treatment of viral hepatitis in low-income countries and are set to be important components of a new global health strategy,” wrote the study investigators, led by Jeffrey D. Stanaway, MD, of the University of Washington, Seattle. “However, a better understanding of the burden of disease is required to guide these efforts.”
Dr. Stanaway and his coauthors looked at the Global Burden of Disease (GBD) study for data on worldwide morbidity and mortality associated with hepatitis A, B, C, and E viruses, as well as cirrhosis and liver cancer secondary to hepatitis B or C virus. Data collected for the GBD was used to determine disability-adjusted life-years (DALYs), which is a metric calculated by adding up years of life lost (YLL) and years lived with disability (YLD), both of which were also measured by the GBD.
Data showed that worldwide deaths related to viral hepatitis numbered 0.89 million in 1990 (95% uncertainty interval [UI]: 0.86-0.94), but jumped up dramatically to 1.45 million in 2013 (95% UI: 1.38-1.54). Over the same time period, YLLs and YLDs also increased, going from 31.0 million (95% UI: 29.6-32.6) and 0.65 million (95% UI: 0.45-0.89), respectively, in 1990, to 41.6 million (95% UI: 39.1-44.7), and 0.87 million (95% UI: 0.61-1.18), respectively, in 2013. Consequently, DALYs increased from 31.7 million in 1990 (95% UI: 30.2-33.3) to 42.5 million (95% UI: 39.9-45.6).
These figures represent a 34% increase in viral hepatitis disease burden over that period of time. Furthermore, viral hepatitis went from being the 10th leading cause of death in the world in 1990 (95% UI: 10-12) to the seventh leading cause in 2013 (95% UI: 7-8). However, analysis without the data’s demographic trends showed that YLL and YLD rates declined by 20% and 13%, respectively (95% UI: 8-30 and 8-18), while DALY rates dropped by 20% (95% UI: 8-30) with no significant trend detected in age-specific mortality rates, indicating that demographic changes such as population growth may be the biggest factor contributing to viral hepatitis’ growing disease burden.
“HAV is the only hepatitis virus for which DALYs have declined significantly between 1990 and 2013. Some of this decline has been driven by changing population age structures, but most is due to declines in age-specific rates, most likely as a result of vaccination and improvements in water supply and sanitation,” the authors noted.
Dr. Stanaway and his coauthors also urged public health institutions around the world to devote more funding to targeting viral hepatitis, noting that the current state of funding is “disproportionate to [viral hepatitis’] importance as a major cause of death and disability.”
The Bill & Melinda Gates Foundation funded the study. Coauthor Graham S. Cooke, MD, reported being an investigator on trials of hepatitis C virus therapy sponsored by Boehringer Ingelheim, Gilead, Merck, and Bristol-Myers Squibb, and has acted in an advisory role to Merck, Boehringer Ingelheim, Gilead, Janssen, and WHO in relation to viral hepatitis and clinical trials unrelated to this work. Dr. Stanaway and other coauthors did not report any relevant financial disclosures.
FROM THE LANCET
Key clinical point: Viral hepatitis increased from 1990 through 2013 despite other communicable diseases decreasing in prevalence, necessitating more effort from the health care community to mitigate the disease burden.
Major finding: Viral hepatitis deaths worldwide were 0.89 million in 1990, and 1.45 million in 2013 (95% uncertainty interval: 0.86-0.94 and 1.38-1.54, respectively).
Data source: Retrospective analysis of data from the Global Burden of Disease study on acute viral hepatitis, cirrhosis, and liver cancer caused by viral hepatitis.
Disclosures: The Bill & Melinda Gates Foundation funded the study. Some coauthors disclosed potential conflicts of interest.
HIV patients with elevated ALT at significant risk of chronic liver disease
Patients with HIV infection should be screened routinely for chronic liver disease if their alanine aminotransferase (ALT) levels are elevated, according to a study published in AIDS Care.
“In HIV-positive individuals, liver disease (mostly due to hepatitis B and C virus [HBV and HCV]) is the third most common cause of mortality after AIDS-related illness and non-AIDS nonviral malignancy,” wrote Sumita Verma, MD, of Brighton and Sussex (U.K.) University Hospital, and her coauthors. “Our aim therefore was to assess prevalence, aetiology, and predictors of nonviral-related chronic liver disease (CLD) in a cohort with HIV monoinfection.”
Investigators recruited 3,872 HIV-positive subjects from a Southeast England teaching hospital between 2005 and 2012, of whom 1,654 (42.7%) were found to be at least two ALT levels above the upper limit of normal. Clinically significant CLD was classified as having at least F2 fibrosis (metavir scoring) on liver biopsy, having a liver stiffness measurement greater than F2 (metavir) on a transient elastography, moderate to severe hepatic steatosis on a liver biopsy, a portal hypertension that was either cirrhotic or noncirrhotic, hepatic decompensation, a liver-related mortality, or any combination of these factors. (AIDS Care. 2016 Ju 5. doi: 10.1080/09540121.2016.1191603)
Of the 1,654 subjects with HIV and elevated ALT levels, 1,047 (27.0%) had elevated ALT levels when tested again after 6 months, with 243 of those (23.2%) having a further radiologic or histologic examination done. The final cohort comprised 222 subjects, of which CLD was found in 147 (66.2%), while only 75 (33.8%) had no CLD despite having elevated ALT and HIV. CLD was determined to be “clinically significant” in one out of every four subjects who had it.
“Potential risk factors for CLD included alcohol use (44.2%), one or more feature of the [metabolic syndrome] (68%) and [antiretroviral] use (74.1%), with 68.7% having [more than one] risk factor [and] serum triglyceride (odds ratio, 1.482; 95% confidence interval. 1.053-2.086; P = .024) was the only independent predictor of CLD,” the investigators noted.
Furthermore, the study shows that “the most striking observation [is] that only 23% with persistently elevated ALT were investigated further, with only 58/1,047 (5.5%) undergoing objective assessment of hepatic fibrosis and 45/1,047 (4.3%) being referred to Hepatology.”
Dr. Verma and her coauthors did not report any relevant financial disclosures.
Patients with HIV infection should be screened routinely for chronic liver disease if their alanine aminotransferase (ALT) levels are elevated, according to a study published in AIDS Care.
“In HIV-positive individuals, liver disease (mostly due to hepatitis B and C virus [HBV and HCV]) is the third most common cause of mortality after AIDS-related illness and non-AIDS nonviral malignancy,” wrote Sumita Verma, MD, of Brighton and Sussex (U.K.) University Hospital, and her coauthors. “Our aim therefore was to assess prevalence, aetiology, and predictors of nonviral-related chronic liver disease (CLD) in a cohort with HIV monoinfection.”
Investigators recruited 3,872 HIV-positive subjects from a Southeast England teaching hospital between 2005 and 2012, of whom 1,654 (42.7%) were found to be at least two ALT levels above the upper limit of normal. Clinically significant CLD was classified as having at least F2 fibrosis (metavir scoring) on liver biopsy, having a liver stiffness measurement greater than F2 (metavir) on a transient elastography, moderate to severe hepatic steatosis on a liver biopsy, a portal hypertension that was either cirrhotic or noncirrhotic, hepatic decompensation, a liver-related mortality, or any combination of these factors. (AIDS Care. 2016 Ju 5. doi: 10.1080/09540121.2016.1191603)
Of the 1,654 subjects with HIV and elevated ALT levels, 1,047 (27.0%) had elevated ALT levels when tested again after 6 months, with 243 of those (23.2%) having a further radiologic or histologic examination done. The final cohort comprised 222 subjects, of which CLD was found in 147 (66.2%), while only 75 (33.8%) had no CLD despite having elevated ALT and HIV. CLD was determined to be “clinically significant” in one out of every four subjects who had it.
“Potential risk factors for CLD included alcohol use (44.2%), one or more feature of the [metabolic syndrome] (68%) and [antiretroviral] use (74.1%), with 68.7% having [more than one] risk factor [and] serum triglyceride (odds ratio, 1.482; 95% confidence interval. 1.053-2.086; P = .024) was the only independent predictor of CLD,” the investigators noted.
Furthermore, the study shows that “the most striking observation [is] that only 23% with persistently elevated ALT were investigated further, with only 58/1,047 (5.5%) undergoing objective assessment of hepatic fibrosis and 45/1,047 (4.3%) being referred to Hepatology.”
Dr. Verma and her coauthors did not report any relevant financial disclosures.
Patients with HIV infection should be screened routinely for chronic liver disease if their alanine aminotransferase (ALT) levels are elevated, according to a study published in AIDS Care.
“In HIV-positive individuals, liver disease (mostly due to hepatitis B and C virus [HBV and HCV]) is the third most common cause of mortality after AIDS-related illness and non-AIDS nonviral malignancy,” wrote Sumita Verma, MD, of Brighton and Sussex (U.K.) University Hospital, and her coauthors. “Our aim therefore was to assess prevalence, aetiology, and predictors of nonviral-related chronic liver disease (CLD) in a cohort with HIV monoinfection.”
Investigators recruited 3,872 HIV-positive subjects from a Southeast England teaching hospital between 2005 and 2012, of whom 1,654 (42.7%) were found to be at least two ALT levels above the upper limit of normal. Clinically significant CLD was classified as having at least F2 fibrosis (metavir scoring) on liver biopsy, having a liver stiffness measurement greater than F2 (metavir) on a transient elastography, moderate to severe hepatic steatosis on a liver biopsy, a portal hypertension that was either cirrhotic or noncirrhotic, hepatic decompensation, a liver-related mortality, or any combination of these factors. (AIDS Care. 2016 Ju 5. doi: 10.1080/09540121.2016.1191603)
Of the 1,654 subjects with HIV and elevated ALT levels, 1,047 (27.0%) had elevated ALT levels when tested again after 6 months, with 243 of those (23.2%) having a further radiologic or histologic examination done. The final cohort comprised 222 subjects, of which CLD was found in 147 (66.2%), while only 75 (33.8%) had no CLD despite having elevated ALT and HIV. CLD was determined to be “clinically significant” in one out of every four subjects who had it.
“Potential risk factors for CLD included alcohol use (44.2%), one or more feature of the [metabolic syndrome] (68%) and [antiretroviral] use (74.1%), with 68.7% having [more than one] risk factor [and] serum triglyceride (odds ratio, 1.482; 95% confidence interval. 1.053-2.086; P = .024) was the only independent predictor of CLD,” the investigators noted.
Furthermore, the study shows that “the most striking observation [is] that only 23% with persistently elevated ALT were investigated further, with only 58/1,047 (5.5%) undergoing objective assessment of hepatic fibrosis and 45/1,047 (4.3%) being referred to Hepatology.”
Dr. Verma and her coauthors did not report any relevant financial disclosures.
FROM AIDS CARE
Key clinical point: Individuals with HIV who also have high levels of alanine aminotransferase (ALT) are at higher risk of having nonviral chronic liver disease.
Major finding: Of 3,872 subjects, 1,047 (27%) had elevated ALT levels; of those 243 (23.2%) were investigated for CLD, and of the 222 finally selected, CLD was found in 66.2%.
Data source: Retrospective, longitudinal cohort study of 3,872 HIV-positive individuals from 2005-2012.
Disclosures: Authors did not report any relevant financial disclosures.
Early preterm birth poses highest risk for recurrence
Women who have had a preterm or early-term birth are at greater risk for an early delivery during a subsequent pregnancy, compared with women whose previous pregnancies have been full term, according to a large cohort study.
“This study confirms that a prior preterm birth increases the risk for a subsequent preterm birth with higher odds among those with a prior early preterm birth. Risks extend to both spontaneous and indicated prior preterm birth,” Juan Yang, PhD, of the California Department of Public Health, and his colleagues wrote (Obstet Gynecol. 2016;128:364-72. doi: 10.1097/AOG.0000000000001506).
The researchers retrospectively examined the records of 163,889 singleton live births that occurred in California from 2005 through 2011 whose mothers had experienced two consecutive pregnancies over that time period that had gestation periods of anywhere between 20 and 44 weeks.
Women were placed into cohorts based on the gestation periods of their first and second pregnancies: fewer than 32 weeks (early preterm), 32-36 weeks (preterm), 37-38 weeks (early term), and 39 weeks or more (full term).
Women whose first pregnancy lasted for fewer than 32 weeks’ gestation – an early preterm birth – were at the highest risk of having another delivery at fewer than 32 weeks in their second pregnancy, compared with women with term pregnancies (adjusted odds ratio, 23.3; 95% confidence interval, 17.2-31.7).
Women with a prior early-term birth (37-38 weeks) had more than a twofold increased risk for a preterm birth, with an adjusted OR of 2.0 for a subsequent birth before 32 weeks and an adjusted OR of 3.0 for a subsequent birth from 32 to 36 weeks of gestation. The adjusted OR for another early-term birth in this group was 2.2.
The researchers wrote that their findings “provide a better understanding of early-term birth and the importance of continuation of gestation beyond 38 weeks of gestation,” noting that “although the association of prior early-term birth with subsequent preterm birth is less strong than prior preterm birth, many more women fall in this category, making this an important risk factor to the overall societal burden of preterm birth.”
The study was funded by the Preterm Birth Initiative-California; the University of California, San Francisco; the March of Dimes Prematurity Research Centers; the Bill and Melinda Gates Foundation; and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The researchers reported having no financial disclosures.
Women who have had a preterm or early-term birth are at greater risk for an early delivery during a subsequent pregnancy, compared with women whose previous pregnancies have been full term, according to a large cohort study.
“This study confirms that a prior preterm birth increases the risk for a subsequent preterm birth with higher odds among those with a prior early preterm birth. Risks extend to both spontaneous and indicated prior preterm birth,” Juan Yang, PhD, of the California Department of Public Health, and his colleagues wrote (Obstet Gynecol. 2016;128:364-72. doi: 10.1097/AOG.0000000000001506).
The researchers retrospectively examined the records of 163,889 singleton live births that occurred in California from 2005 through 2011 whose mothers had experienced two consecutive pregnancies over that time period that had gestation periods of anywhere between 20 and 44 weeks.
Women were placed into cohorts based on the gestation periods of their first and second pregnancies: fewer than 32 weeks (early preterm), 32-36 weeks (preterm), 37-38 weeks (early term), and 39 weeks or more (full term).
Women whose first pregnancy lasted for fewer than 32 weeks’ gestation – an early preterm birth – were at the highest risk of having another delivery at fewer than 32 weeks in their second pregnancy, compared with women with term pregnancies (adjusted odds ratio, 23.3; 95% confidence interval, 17.2-31.7).
Women with a prior early-term birth (37-38 weeks) had more than a twofold increased risk for a preterm birth, with an adjusted OR of 2.0 for a subsequent birth before 32 weeks and an adjusted OR of 3.0 for a subsequent birth from 32 to 36 weeks of gestation. The adjusted OR for another early-term birth in this group was 2.2.
The researchers wrote that their findings “provide a better understanding of early-term birth and the importance of continuation of gestation beyond 38 weeks of gestation,” noting that “although the association of prior early-term birth with subsequent preterm birth is less strong than prior preterm birth, many more women fall in this category, making this an important risk factor to the overall societal burden of preterm birth.”
The study was funded by the Preterm Birth Initiative-California; the University of California, San Francisco; the March of Dimes Prematurity Research Centers; the Bill and Melinda Gates Foundation; and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The researchers reported having no financial disclosures.
Women who have had a preterm or early-term birth are at greater risk for an early delivery during a subsequent pregnancy, compared with women whose previous pregnancies have been full term, according to a large cohort study.
“This study confirms that a prior preterm birth increases the risk for a subsequent preterm birth with higher odds among those with a prior early preterm birth. Risks extend to both spontaneous and indicated prior preterm birth,” Juan Yang, PhD, of the California Department of Public Health, and his colleagues wrote (Obstet Gynecol. 2016;128:364-72. doi: 10.1097/AOG.0000000000001506).
The researchers retrospectively examined the records of 163,889 singleton live births that occurred in California from 2005 through 2011 whose mothers had experienced two consecutive pregnancies over that time period that had gestation periods of anywhere between 20 and 44 weeks.
Women were placed into cohorts based on the gestation periods of their first and second pregnancies: fewer than 32 weeks (early preterm), 32-36 weeks (preterm), 37-38 weeks (early term), and 39 weeks or more (full term).
Women whose first pregnancy lasted for fewer than 32 weeks’ gestation – an early preterm birth – were at the highest risk of having another delivery at fewer than 32 weeks in their second pregnancy, compared with women with term pregnancies (adjusted odds ratio, 23.3; 95% confidence interval, 17.2-31.7).
Women with a prior early-term birth (37-38 weeks) had more than a twofold increased risk for a preterm birth, with an adjusted OR of 2.0 for a subsequent birth before 32 weeks and an adjusted OR of 3.0 for a subsequent birth from 32 to 36 weeks of gestation. The adjusted OR for another early-term birth in this group was 2.2.
The researchers wrote that their findings “provide a better understanding of early-term birth and the importance of continuation of gestation beyond 38 weeks of gestation,” noting that “although the association of prior early-term birth with subsequent preterm birth is less strong than prior preterm birth, many more women fall in this category, making this an important risk factor to the overall societal burden of preterm birth.”
The study was funded by the Preterm Birth Initiative-California; the University of California, San Francisco; the March of Dimes Prematurity Research Centers; the Bill and Melinda Gates Foundation; and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The researchers reported having no financial disclosures.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Earlier preterm or early-term birth is a reliable indicator of a woman’s likelihood to have another early birth.
Major finding: Women with a prior early-term birth had a twofold increased risk for a subsequent birth before 32 weeks’ gestation (adjusted OR, 2.0).
Data source: Retrospective cohort study of 163,889 California women who gave birth to neonates between 20 and 44 weeks of gestation from 2005 to 2011.
Disclosures: The study was funded by the Preterm Birth Initiative-California; the University of California, San Francisco; the March of Dimes Prematurity Research Centers; the Bill and Melinda Gates Foundation; and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The researchers reported having no financial disclosures.
No evidence to show Alzheimer’s or Parkinson’s transmission via blood transfusion
A retrospective study of nearly 1.5 million patients over the course of 44 years showed no evidence to support the claim that neurodegenerative disorders can be transmitted between individuals through blood transfusions.
“Given that the neurodegenerative diseases on the dementia spectrum are relatively common, the misfolded proteins in affected persons have a wide tissue distribution, and the diseases may have a protracted prediagnostic course, potential transmission through transfusion could have important public health implications,” explained study authors led by Gustaf Edgren, MD, PhD, of the Karolinska Institute in Stockholm.
The retrospective cohort study analyzed data on 1,465,845 patients listed in nationwide transfusion registers, all of whom had undergone transfusions between 1968 and 2012 in Sweden, or during 1981-2012 in Denmark. The investigators looked for transfusions in which the donor had a diagnosis – either before or after the transfusion – of Alzheimer’s disease, Parkinson’s disease, or amyotrophic lateral sclerosis, and compared them with transfusions involving two healthy control groups. Creutzfeldt-Jakob disease was not included in the analysis (Ann Intern Med. 2016 Jun 28. doi: 10.7326/M15-2421).
All transfusion recipients were followed from 180 days after the transfusion date in order to minimize the risk of including a patient who had a neurodegenerative disorder that was present but not registered at the time of the transfusion. Follow-ups lasted until either the first diagnosis of a neurodegenerative disorder, death, emigration, or the end of the trial period on Dec. 31, 2012.
If a donor had received a diagnosis of a neurodegenerative disorder within 20 years of the transfusion, all recipients from said donor were considered to be exposed. “The maximum latency of 20 years was used as a compromise between allowing a long preclinical phase – during which donors may still be infectious – and avoiding classifying clearly healthy donors as potentially infectious,” the authors wrote.
Only 42,254 (2.9%) of subjects were deemed to be exposed to a neurodegenerative disorder based on their blood transfusion donor. However, there was no evidence of any increased risk of dementia of any type from exposure to blood from affected donors in any of the recipients over the course of the follow-up period (hazard ratio, 1.04; 95% confidence interval, 0.99-1.09), and similar results were obtained individually for Alzheimer’s disease and Parkinson’s disease. As a control measure, the investigators also tested for hepatitis C transmission before and after transfusions, and were “as expected, readily able to detect transmission of viral hepatitis,” despite finding no traces of neurodegenerative conditions.
“Despite accumulating scientific support for horizontal transmissibility of a range of disorders in the neurodegenerative spectrum through various methods of inoculation in animal models and of variant Creutzfeldt-Jakob disease from human and animal model data, this study provides evidence against blood transfusion as an important route of transmission of neurodegenerative diseases between humans,” the authors concluded.
The authors also added that “given that the study was based on the entire computerized blood banking history in two countries with several decades of follow-up, more comprehensive data are unlikely to be available in the foreseeable future.”
Funding for the study was provided by the Swedish Research Council, the Swedish Heart-Lung Foundation, the Swedish Society for Medical Research, and the Danish Council for Independent Research. One coauthor reported receiving a grant from the Danish Council for Independent Research, and another reported receiving grants from the Swedish Research Council and the Swedish Heart-Lung Foundation during the conduct of the study. No other authors reported any relevant financial disclosures.
A retrospective study of nearly 1.5 million patients over the course of 44 years showed no evidence to support the claim that neurodegenerative disorders can be transmitted between individuals through blood transfusions.
“Given that the neurodegenerative diseases on the dementia spectrum are relatively common, the misfolded proteins in affected persons have a wide tissue distribution, and the diseases may have a protracted prediagnostic course, potential transmission through transfusion could have important public health implications,” explained study authors led by Gustaf Edgren, MD, PhD, of the Karolinska Institute in Stockholm.
The retrospective cohort study analyzed data on 1,465,845 patients listed in nationwide transfusion registers, all of whom had undergone transfusions between 1968 and 2012 in Sweden, or during 1981-2012 in Denmark. The investigators looked for transfusions in which the donor had a diagnosis – either before or after the transfusion – of Alzheimer’s disease, Parkinson’s disease, or amyotrophic lateral sclerosis, and compared them with transfusions involving two healthy control groups. Creutzfeldt-Jakob disease was not included in the analysis (Ann Intern Med. 2016 Jun 28. doi: 10.7326/M15-2421).
All transfusion recipients were followed from 180 days after the transfusion date in order to minimize the risk of including a patient who had a neurodegenerative disorder that was present but not registered at the time of the transfusion. Follow-ups lasted until either the first diagnosis of a neurodegenerative disorder, death, emigration, or the end of the trial period on Dec. 31, 2012.
If a donor had received a diagnosis of a neurodegenerative disorder within 20 years of the transfusion, all recipients from said donor were considered to be exposed. “The maximum latency of 20 years was used as a compromise between allowing a long preclinical phase – during which donors may still be infectious – and avoiding classifying clearly healthy donors as potentially infectious,” the authors wrote.
Only 42,254 (2.9%) of subjects were deemed to be exposed to a neurodegenerative disorder based on their blood transfusion donor. However, there was no evidence of any increased risk of dementia of any type from exposure to blood from affected donors in any of the recipients over the course of the follow-up period (hazard ratio, 1.04; 95% confidence interval, 0.99-1.09), and similar results were obtained individually for Alzheimer’s disease and Parkinson’s disease. As a control measure, the investigators also tested for hepatitis C transmission before and after transfusions, and were “as expected, readily able to detect transmission of viral hepatitis,” despite finding no traces of neurodegenerative conditions.
“Despite accumulating scientific support for horizontal transmissibility of a range of disorders in the neurodegenerative spectrum through various methods of inoculation in animal models and of variant Creutzfeldt-Jakob disease from human and animal model data, this study provides evidence against blood transfusion as an important route of transmission of neurodegenerative diseases between humans,” the authors concluded.
The authors also added that “given that the study was based on the entire computerized blood banking history in two countries with several decades of follow-up, more comprehensive data are unlikely to be available in the foreseeable future.”
Funding for the study was provided by the Swedish Research Council, the Swedish Heart-Lung Foundation, the Swedish Society for Medical Research, and the Danish Council for Independent Research. One coauthor reported receiving a grant from the Danish Council for Independent Research, and another reported receiving grants from the Swedish Research Council and the Swedish Heart-Lung Foundation during the conduct of the study. No other authors reported any relevant financial disclosures.
A retrospective study of nearly 1.5 million patients over the course of 44 years showed no evidence to support the claim that neurodegenerative disorders can be transmitted between individuals through blood transfusions.
“Given that the neurodegenerative diseases on the dementia spectrum are relatively common, the misfolded proteins in affected persons have a wide tissue distribution, and the diseases may have a protracted prediagnostic course, potential transmission through transfusion could have important public health implications,” explained study authors led by Gustaf Edgren, MD, PhD, of the Karolinska Institute in Stockholm.
The retrospective cohort study analyzed data on 1,465,845 patients listed in nationwide transfusion registers, all of whom had undergone transfusions between 1968 and 2012 in Sweden, or during 1981-2012 in Denmark. The investigators looked for transfusions in which the donor had a diagnosis – either before or after the transfusion – of Alzheimer’s disease, Parkinson’s disease, or amyotrophic lateral sclerosis, and compared them with transfusions involving two healthy control groups. Creutzfeldt-Jakob disease was not included in the analysis (Ann Intern Med. 2016 Jun 28. doi: 10.7326/M15-2421).
All transfusion recipients were followed from 180 days after the transfusion date in order to minimize the risk of including a patient who had a neurodegenerative disorder that was present but not registered at the time of the transfusion. Follow-ups lasted until either the first diagnosis of a neurodegenerative disorder, death, emigration, or the end of the trial period on Dec. 31, 2012.
If a donor had received a diagnosis of a neurodegenerative disorder within 20 years of the transfusion, all recipients from said donor were considered to be exposed. “The maximum latency of 20 years was used as a compromise between allowing a long preclinical phase – during which donors may still be infectious – and avoiding classifying clearly healthy donors as potentially infectious,” the authors wrote.
Only 42,254 (2.9%) of subjects were deemed to be exposed to a neurodegenerative disorder based on their blood transfusion donor. However, there was no evidence of any increased risk of dementia of any type from exposure to blood from affected donors in any of the recipients over the course of the follow-up period (hazard ratio, 1.04; 95% confidence interval, 0.99-1.09), and similar results were obtained individually for Alzheimer’s disease and Parkinson’s disease. As a control measure, the investigators also tested for hepatitis C transmission before and after transfusions, and were “as expected, readily able to detect transmission of viral hepatitis,” despite finding no traces of neurodegenerative conditions.
“Despite accumulating scientific support for horizontal transmissibility of a range of disorders in the neurodegenerative spectrum through various methods of inoculation in animal models and of variant Creutzfeldt-Jakob disease from human and animal model data, this study provides evidence against blood transfusion as an important route of transmission of neurodegenerative diseases between humans,” the authors concluded.
The authors also added that “given that the study was based on the entire computerized blood banking history in two countries with several decades of follow-up, more comprehensive data are unlikely to be available in the foreseeable future.”
Funding for the study was provided by the Swedish Research Council, the Swedish Heart-Lung Foundation, the Swedish Society for Medical Research, and the Danish Council for Independent Research. One coauthor reported receiving a grant from the Danish Council for Independent Research, and another reported receiving grants from the Swedish Research Council and the Swedish Heart-Lung Foundation during the conduct of the study. No other authors reported any relevant financial disclosures.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: There are no data to support the notion that human transmission of Alzheimer’s or Parkinson’s diseases can occur via blood transfusions.
Major finding: There was no evidence of any increased risk of dementia of any type from exposure to blood from affected donors in any of the recipients over the course of the follow-up period (HR, 1.04; 95% CI, 0.99-1.09).
Data source: Retrospective case-control study of 1,465,845 transfusion patients in Sweden and Denmark during 1968-2012.
Disclosures: The study was funded by the Swedish Research Council, the Swedish Heart-Lung Foundation, the Swedish Society for Medical Research, and the Danish Council for Independent Research. Two coauthors disclosed receiving grants from the funding institutions.
Primary care management of sepsis survivors does not improve mental health quality of life
Patients who have survived sepsis or septic shock do not receive any significant benefit in the quality of their mental health by receiving primary care management intervention, according to a new study published by JAMA.
“Many survivors of sepsis have multiple medical comorbidities that are typically managed in primary care [but] interventions for managing sepsis sequelae in primary care have not been developed,” states the study, which was led by Jochen Gensichen, MD, of the Institute of General Practice & Family Medicine at Jena (Germany) University Hospital.
“To our knowledge, this is the first large-scale, randomized controlled clinical trial of an intervention to improve outcomes in survivors of sepsis in primary care,” Dr. Gensichen and his coinvestigators added.
The study recruited sepsis and septic shock survivors from nine ICUs across Germany between February 2011 and December 2014, excluding any patients with cognitive impairment, defined as a Telephone Interview of Cognitive Status score no greater than 27. Ultimately, 291 patients aged 18 years or older (mean age of 61.6 years) were selected for inclusion and randomized into cohorts receiving either primary care–based intervention (n = 148) or usual care (n = 143) (JAMA. 2016;315:2703-11. doi: 10.1001/jama.2016.7207).
Those assigned to the usual care cohort received the standard care that their primary care providers would normally carry out, which included “periodic contacts, referrals to specialists, and prescription of medication and therapeutic aids at quantities comparable with those for other populations with multiple chronic conditions.” Those in the other cohort were given active monitoring of symptoms from providers who had been given evidence-based care training and clinical decision support from nurses who underwent training to become case managers. Case managers would take patients through an hour-long face-to-face training on sepsis sequelae within 2-20 days of ICU discharge, along with subsequent follow-up conversations over the phone.
“Case managers monitored patients’ symptoms using validated screening tools to assess critical illness polyneuropathy/myopathy, wasting, neurocognitive deficits, [posttraumatic stress disorder], depressive and pain symptoms, as well as patient self-management behaviors focusing on physical activity and individual self-management goals,” the authors said, noting that case managers would report their results to a consulting physician who “supervised the case managers and provided clinical decision support to the [primary care physicians].”
Baseline Mental Component Summary (MCS) scores were taken for subjects in both cohorts to determine mental health-based quality of life, which averaged 49.1 for the intervention cohort and 49.3 for the control. MCS scores at 6 months’ follow-up were 52.9 for the intervention group (95% confidence interval, 1.05-6.54) and 51.0 for the control group (95% CI, –1.22-4.51), for a mean change of 3.8 in the intervention cohort and 1.6 for the control group. The mean treatment effect was 2.15 (95% CI, –1.79-6.09; P = .28), indicating no significant difference between the two.
“There was no evidence for a differential treatment effect on the study’s primary outcome, postsepsis MCS scores,” the authors concluded. “This finding is similar to those from previous trials of care management interventions following critical illness.”
The authors added that “further research is needed to determine if modified approaches to primary care management may be more effective.”
The study was funded by the Center for Sepsis Control and Care, the German Federal Ministry of Education and Research, and the German Sepsis Society. Dr. Gensichen reported receiving personal fees from the Primary Health Care Foundation and receiving a grant from the German Federal Ministry of Education and Research.
Patients who have survived sepsis or septic shock do not receive any significant benefit in the quality of their mental health by receiving primary care management intervention, according to a new study published by JAMA.
“Many survivors of sepsis have multiple medical comorbidities that are typically managed in primary care [but] interventions for managing sepsis sequelae in primary care have not been developed,” states the study, which was led by Jochen Gensichen, MD, of the Institute of General Practice & Family Medicine at Jena (Germany) University Hospital.
“To our knowledge, this is the first large-scale, randomized controlled clinical trial of an intervention to improve outcomes in survivors of sepsis in primary care,” Dr. Gensichen and his coinvestigators added.
The study recruited sepsis and septic shock survivors from nine ICUs across Germany between February 2011 and December 2014, excluding any patients with cognitive impairment, defined as a Telephone Interview of Cognitive Status score no greater than 27. Ultimately, 291 patients aged 18 years or older (mean age of 61.6 years) were selected for inclusion and randomized into cohorts receiving either primary care–based intervention (n = 148) or usual care (n = 143) (JAMA. 2016;315:2703-11. doi: 10.1001/jama.2016.7207).
Those assigned to the usual care cohort received the standard care that their primary care providers would normally carry out, which included “periodic contacts, referrals to specialists, and prescription of medication and therapeutic aids at quantities comparable with those for other populations with multiple chronic conditions.” Those in the other cohort were given active monitoring of symptoms from providers who had been given evidence-based care training and clinical decision support from nurses who underwent training to become case managers. Case managers would take patients through an hour-long face-to-face training on sepsis sequelae within 2-20 days of ICU discharge, along with subsequent follow-up conversations over the phone.
“Case managers monitored patients’ symptoms using validated screening tools to assess critical illness polyneuropathy/myopathy, wasting, neurocognitive deficits, [posttraumatic stress disorder], depressive and pain symptoms, as well as patient self-management behaviors focusing on physical activity and individual self-management goals,” the authors said, noting that case managers would report their results to a consulting physician who “supervised the case managers and provided clinical decision support to the [primary care physicians].”
Baseline Mental Component Summary (MCS) scores were taken for subjects in both cohorts to determine mental health-based quality of life, which averaged 49.1 for the intervention cohort and 49.3 for the control. MCS scores at 6 months’ follow-up were 52.9 for the intervention group (95% confidence interval, 1.05-6.54) and 51.0 for the control group (95% CI, –1.22-4.51), for a mean change of 3.8 in the intervention cohort and 1.6 for the control group. The mean treatment effect was 2.15 (95% CI, –1.79-6.09; P = .28), indicating no significant difference between the two.
“There was no evidence for a differential treatment effect on the study’s primary outcome, postsepsis MCS scores,” the authors concluded. “This finding is similar to those from previous trials of care management interventions following critical illness.”
The authors added that “further research is needed to determine if modified approaches to primary care management may be more effective.”
The study was funded by the Center for Sepsis Control and Care, the German Federal Ministry of Education and Research, and the German Sepsis Society. Dr. Gensichen reported receiving personal fees from the Primary Health Care Foundation and receiving a grant from the German Federal Ministry of Education and Research.
Patients who have survived sepsis or septic shock do not receive any significant benefit in the quality of their mental health by receiving primary care management intervention, according to a new study published by JAMA.
“Many survivors of sepsis have multiple medical comorbidities that are typically managed in primary care [but] interventions for managing sepsis sequelae in primary care have not been developed,” states the study, which was led by Jochen Gensichen, MD, of the Institute of General Practice & Family Medicine at Jena (Germany) University Hospital.
“To our knowledge, this is the first large-scale, randomized controlled clinical trial of an intervention to improve outcomes in survivors of sepsis in primary care,” Dr. Gensichen and his coinvestigators added.
The study recruited sepsis and septic shock survivors from nine ICUs across Germany between February 2011 and December 2014, excluding any patients with cognitive impairment, defined as a Telephone Interview of Cognitive Status score no greater than 27. Ultimately, 291 patients aged 18 years or older (mean age of 61.6 years) were selected for inclusion and randomized into cohorts receiving either primary care–based intervention (n = 148) or usual care (n = 143) (JAMA. 2016;315:2703-11. doi: 10.1001/jama.2016.7207).
Those assigned to the usual care cohort received the standard care that their primary care providers would normally carry out, which included “periodic contacts, referrals to specialists, and prescription of medication and therapeutic aids at quantities comparable with those for other populations with multiple chronic conditions.” Those in the other cohort were given active monitoring of symptoms from providers who had been given evidence-based care training and clinical decision support from nurses who underwent training to become case managers. Case managers would take patients through an hour-long face-to-face training on sepsis sequelae within 2-20 days of ICU discharge, along with subsequent follow-up conversations over the phone.
“Case managers monitored patients’ symptoms using validated screening tools to assess critical illness polyneuropathy/myopathy, wasting, neurocognitive deficits, [posttraumatic stress disorder], depressive and pain symptoms, as well as patient self-management behaviors focusing on physical activity and individual self-management goals,” the authors said, noting that case managers would report their results to a consulting physician who “supervised the case managers and provided clinical decision support to the [primary care physicians].”
Baseline Mental Component Summary (MCS) scores were taken for subjects in both cohorts to determine mental health-based quality of life, which averaged 49.1 for the intervention cohort and 49.3 for the control. MCS scores at 6 months’ follow-up were 52.9 for the intervention group (95% confidence interval, 1.05-6.54) and 51.0 for the control group (95% CI, –1.22-4.51), for a mean change of 3.8 in the intervention cohort and 1.6 for the control group. The mean treatment effect was 2.15 (95% CI, –1.79-6.09; P = .28), indicating no significant difference between the two.
“There was no evidence for a differential treatment effect on the study’s primary outcome, postsepsis MCS scores,” the authors concluded. “This finding is similar to those from previous trials of care management interventions following critical illness.”
The authors added that “further research is needed to determine if modified approaches to primary care management may be more effective.”
The study was funded by the Center for Sepsis Control and Care, the German Federal Ministry of Education and Research, and the German Sepsis Society. Dr. Gensichen reported receiving personal fees from the Primary Health Care Foundation and receiving a grant from the German Federal Ministry of Education and Research.
FROM JAMA
Key clinical point: Primary care intervention does not improve mental health–related quality of life in survivors of sepsis or septic shock.
Major finding: Mean Mental Component Summary (MCS) scores showed no significant change between the time of ICU discharge (49.1) versus at 6 months postdischarge (52.9) (95% CI, 1.05-6.54), compared with the control group: 49.3 at baseline vs. 51.0 at 6 months follow-up (95% CI, –1.22-4.51).
Data source: A multicenter, unblinded, two-group randomized clinical trial of 291 adult sepsis or septic shock survivors recruited from nine German ICUs from February 2011 through December 2014.
Disclosures: Study funded by the Center for Sepsis Control and Care, the German Federal Ministry of Education and Research, and the German Sepsis Society. Dr. Gensichen reported receiving personal fees from The Primary Health Care Foundation and receiving a grant from the German Federal Ministry of Education and Research.