User login
Perform urine culture if UTI suspected despite absence of pyuria
Urine cultures should be performed in all children clinically suspected of having a urinary tract infection (UTI), results of a study in Pediatrics suggest.
That’s because pyuria may be absent in some children with certain uropathogens.
“Clinicians rely heavily on the degree of pyuria when making a presumptive diagnosis of UTI [but] lack of pyuria on an initial urinalysis may result in delayed diagnosis and delayed antimicrobial therapy,” explained Dr. Nader Shaikh of the University of Pittsburgh and his associates. “We hypothesized, based on some preliminary data in adults, that gram-positive organisms would cause less inflammation of the urinary tract and consequently cause less pyuria on urinalysis than infections caused by gram-negative organisms, in which pyuria is observed in the vast majority of cases.”
Dr. Shaikh and his coinvestigators looked at patients under the age of 18 years admitted to the Children’s Hospital of Pittsburgh at the University of Pittsburgh Medical Center between 2007 and 2013 with a UTI. Of 46,158 relevant hospital visits over the course of the study period, 1,181 children were ultimately selected for inclusion. Urine samples were tested for the presence of uropathogens, followed by analysis for the presence of pyuria, defined as having 5 or more white blood cells per high-powered field or at least 10 white blood cells per mm3 of urine (Pediatrics. 2016. doi: 10.1542/peds.2016-0087).
Only 150 subjects (13%) did not have pyuria; the remaining 1,031 (87%) of subjects did. Escherichia coli was found in 999 of the 1,181 (85%) subjects included, of which 892 (89%) had pyuria. Additionally, of the 27 children found to have Staphylococcus saprophyticus, all had pyuria. High rates of pyuria also were found in children whose urine had Proteus species and Enterobacter species uropathogens: 25 of 31 (81%) and 13 of 15 (87%), respectively.
However, the presence of three other pathogens suggested a lower association between pyuria and UTI. Pseudomonas aeruginosa was found in 13 children, of which 8 (62%) had pyuria; similarly, those with Enterococcus species uropathogens had a 54% chance of having pyuria (19 of 35) and those with Klebsiella species had a 74% chance (34 of 46). The takeaway, therefore, is that pyuria may not always be present in cases of UTI, especially if these pathogens are the cause of it; in fact, “the odds of pyuria were three to five times lower” with these pathogens, compared with E. coli.
“The most recent American Academy of Pediatrics guideline suggests that pyuria should be present when diagnosing a UTI [but] only 90% of children with UTI exhibit pyuria even when the urine specimen is collected by bladder catheterization or suprapubic aspiration,” Dr. Shaikh and his associates found, adding that the “findings of our study offer an additional explanation for the absence of pyuria – some uropathogens may not elicit a strong host inflammatory response – [and] suggest that bedside biomarkers that are more sensitive and specific than pyuria are needed to improve the accuracy of early diagnosis.”
There was no outside funding involved with this study. Dr. Shaikh and his coauthors did not report any relevant financial disclosures.
Shaikh et al. demonstrate that certain urinary pathogens fail to reliably elicit pyuria. In 1,181 children where both a urine culture and concomitant urinalysis were performed, only 87% of the time was pyuria found in the setting of a positive culture. The authors further found that Enterococcus, Klebsiella, and Pseudomonas species were less likely to elicit pyuria or a positive leukocyte esterase test despite causing a urinary tract infection.
So how does the urinalysis help? More pointedly, why do a urinalysis? The report by Shaikh et al. agrees with a previously published meta-analysis that reveals pyuria to be absent in at least 10% of urines that culture positive. For a condition as common as UTI, this is too high a false-negative rate. Shaikh et al. conclude that a urine culture should be obtained in all children suspected of UTI. Indeed, the AAP guidelines are consistent with this statement. The AAP guidelines recognize that a clinician may have a low level of suspicion for UTI and may choose not to treat. However, given recent analyses of the utility of urinalysis and the report by Shaikh et al., it is difficult to see how a negative urinalysis might reassure a clinician if there are signs of a UTI. Hence, if one is considering treating for presumptive UTI, a culture is needed. If one is considering waiting and not treating, but suspects a UTI, a culture is still needed. Shaikh et al. conclude that new biomarkers are needed if we really want help with the “point of care” testing. Although we wait for new biomarkers, we should recognize the limitations of a negative urinalysis and still get that urine culture.
Dr. Aaron Friedman is the former dean of the University of Minnesota Medical School, Minneapolis. These comments are excerpted from a commentary accompanying Dr. Shaikh and his associates’ report (Pediatrics. 2016. doi: 10.1542/peds.2016-1247). Dr. Friedman did not report any relevant financial disclosures or sources of external funding.
Shaikh et al. demonstrate that certain urinary pathogens fail to reliably elicit pyuria. In 1,181 children where both a urine culture and concomitant urinalysis were performed, only 87% of the time was pyuria found in the setting of a positive culture. The authors further found that Enterococcus, Klebsiella, and Pseudomonas species were less likely to elicit pyuria or a positive leukocyte esterase test despite causing a urinary tract infection.
So how does the urinalysis help? More pointedly, why do a urinalysis? The report by Shaikh et al. agrees with a previously published meta-analysis that reveals pyuria to be absent in at least 10% of urines that culture positive. For a condition as common as UTI, this is too high a false-negative rate. Shaikh et al. conclude that a urine culture should be obtained in all children suspected of UTI. Indeed, the AAP guidelines are consistent with this statement. The AAP guidelines recognize that a clinician may have a low level of suspicion for UTI and may choose not to treat. However, given recent analyses of the utility of urinalysis and the report by Shaikh et al., it is difficult to see how a negative urinalysis might reassure a clinician if there are signs of a UTI. Hence, if one is considering treating for presumptive UTI, a culture is needed. If one is considering waiting and not treating, but suspects a UTI, a culture is still needed. Shaikh et al. conclude that new biomarkers are needed if we really want help with the “point of care” testing. Although we wait for new biomarkers, we should recognize the limitations of a negative urinalysis and still get that urine culture.
Dr. Aaron Friedman is the former dean of the University of Minnesota Medical School, Minneapolis. These comments are excerpted from a commentary accompanying Dr. Shaikh and his associates’ report (Pediatrics. 2016. doi: 10.1542/peds.2016-1247). Dr. Friedman did not report any relevant financial disclosures or sources of external funding.
Shaikh et al. demonstrate that certain urinary pathogens fail to reliably elicit pyuria. In 1,181 children where both a urine culture and concomitant urinalysis were performed, only 87% of the time was pyuria found in the setting of a positive culture. The authors further found that Enterococcus, Klebsiella, and Pseudomonas species were less likely to elicit pyuria or a positive leukocyte esterase test despite causing a urinary tract infection.
So how does the urinalysis help? More pointedly, why do a urinalysis? The report by Shaikh et al. agrees with a previously published meta-analysis that reveals pyuria to be absent in at least 10% of urines that culture positive. For a condition as common as UTI, this is too high a false-negative rate. Shaikh et al. conclude that a urine culture should be obtained in all children suspected of UTI. Indeed, the AAP guidelines are consistent with this statement. The AAP guidelines recognize that a clinician may have a low level of suspicion for UTI and may choose not to treat. However, given recent analyses of the utility of urinalysis and the report by Shaikh et al., it is difficult to see how a negative urinalysis might reassure a clinician if there are signs of a UTI. Hence, if one is considering treating for presumptive UTI, a culture is needed. If one is considering waiting and not treating, but suspects a UTI, a culture is still needed. Shaikh et al. conclude that new biomarkers are needed if we really want help with the “point of care” testing. Although we wait for new biomarkers, we should recognize the limitations of a negative urinalysis and still get that urine culture.
Dr. Aaron Friedman is the former dean of the University of Minnesota Medical School, Minneapolis. These comments are excerpted from a commentary accompanying Dr. Shaikh and his associates’ report (Pediatrics. 2016. doi: 10.1542/peds.2016-1247). Dr. Friedman did not report any relevant financial disclosures or sources of external funding.
Urine cultures should be performed in all children clinically suspected of having a urinary tract infection (UTI), results of a study in Pediatrics suggest.
That’s because pyuria may be absent in some children with certain uropathogens.
“Clinicians rely heavily on the degree of pyuria when making a presumptive diagnosis of UTI [but] lack of pyuria on an initial urinalysis may result in delayed diagnosis and delayed antimicrobial therapy,” explained Dr. Nader Shaikh of the University of Pittsburgh and his associates. “We hypothesized, based on some preliminary data in adults, that gram-positive organisms would cause less inflammation of the urinary tract and consequently cause less pyuria on urinalysis than infections caused by gram-negative organisms, in which pyuria is observed in the vast majority of cases.”
Dr. Shaikh and his coinvestigators looked at patients under the age of 18 years admitted to the Children’s Hospital of Pittsburgh at the University of Pittsburgh Medical Center between 2007 and 2013 with a UTI. Of 46,158 relevant hospital visits over the course of the study period, 1,181 children were ultimately selected for inclusion. Urine samples were tested for the presence of uropathogens, followed by analysis for the presence of pyuria, defined as having 5 or more white blood cells per high-powered field or at least 10 white blood cells per mm3 of urine (Pediatrics. 2016. doi: 10.1542/peds.2016-0087).
Only 150 subjects (13%) did not have pyuria; the remaining 1,031 (87%) of subjects did. Escherichia coli was found in 999 of the 1,181 (85%) subjects included, of which 892 (89%) had pyuria. Additionally, of the 27 children found to have Staphylococcus saprophyticus, all had pyuria. High rates of pyuria also were found in children whose urine had Proteus species and Enterobacter species uropathogens: 25 of 31 (81%) and 13 of 15 (87%), respectively.
However, the presence of three other pathogens suggested a lower association between pyuria and UTI. Pseudomonas aeruginosa was found in 13 children, of which 8 (62%) had pyuria; similarly, those with Enterococcus species uropathogens had a 54% chance of having pyuria (19 of 35) and those with Klebsiella species had a 74% chance (34 of 46). The takeaway, therefore, is that pyuria may not always be present in cases of UTI, especially if these pathogens are the cause of it; in fact, “the odds of pyuria were three to five times lower” with these pathogens, compared with E. coli.
“The most recent American Academy of Pediatrics guideline suggests that pyuria should be present when diagnosing a UTI [but] only 90% of children with UTI exhibit pyuria even when the urine specimen is collected by bladder catheterization or suprapubic aspiration,” Dr. Shaikh and his associates found, adding that the “findings of our study offer an additional explanation for the absence of pyuria – some uropathogens may not elicit a strong host inflammatory response – [and] suggest that bedside biomarkers that are more sensitive and specific than pyuria are needed to improve the accuracy of early diagnosis.”
There was no outside funding involved with this study. Dr. Shaikh and his coauthors did not report any relevant financial disclosures.
Urine cultures should be performed in all children clinically suspected of having a urinary tract infection (UTI), results of a study in Pediatrics suggest.
That’s because pyuria may be absent in some children with certain uropathogens.
“Clinicians rely heavily on the degree of pyuria when making a presumptive diagnosis of UTI [but] lack of pyuria on an initial urinalysis may result in delayed diagnosis and delayed antimicrobial therapy,” explained Dr. Nader Shaikh of the University of Pittsburgh and his associates. “We hypothesized, based on some preliminary data in adults, that gram-positive organisms would cause less inflammation of the urinary tract and consequently cause less pyuria on urinalysis than infections caused by gram-negative organisms, in which pyuria is observed in the vast majority of cases.”
Dr. Shaikh and his coinvestigators looked at patients under the age of 18 years admitted to the Children’s Hospital of Pittsburgh at the University of Pittsburgh Medical Center between 2007 and 2013 with a UTI. Of 46,158 relevant hospital visits over the course of the study period, 1,181 children were ultimately selected for inclusion. Urine samples were tested for the presence of uropathogens, followed by analysis for the presence of pyuria, defined as having 5 or more white blood cells per high-powered field or at least 10 white blood cells per mm3 of urine (Pediatrics. 2016. doi: 10.1542/peds.2016-0087).
Only 150 subjects (13%) did not have pyuria; the remaining 1,031 (87%) of subjects did. Escherichia coli was found in 999 of the 1,181 (85%) subjects included, of which 892 (89%) had pyuria. Additionally, of the 27 children found to have Staphylococcus saprophyticus, all had pyuria. High rates of pyuria also were found in children whose urine had Proteus species and Enterobacter species uropathogens: 25 of 31 (81%) and 13 of 15 (87%), respectively.
However, the presence of three other pathogens suggested a lower association between pyuria and UTI. Pseudomonas aeruginosa was found in 13 children, of which 8 (62%) had pyuria; similarly, those with Enterococcus species uropathogens had a 54% chance of having pyuria (19 of 35) and those with Klebsiella species had a 74% chance (34 of 46). The takeaway, therefore, is that pyuria may not always be present in cases of UTI, especially if these pathogens are the cause of it; in fact, “the odds of pyuria were three to five times lower” with these pathogens, compared with E. coli.
“The most recent American Academy of Pediatrics guideline suggests that pyuria should be present when diagnosing a UTI [but] only 90% of children with UTI exhibit pyuria even when the urine specimen is collected by bladder catheterization or suprapubic aspiration,” Dr. Shaikh and his associates found, adding that the “findings of our study offer an additional explanation for the absence of pyuria – some uropathogens may not elicit a strong host inflammatory response – [and] suggest that bedside biomarkers that are more sensitive and specific than pyuria are needed to improve the accuracy of early diagnosis.”
There was no outside funding involved with this study. Dr. Shaikh and his coauthors did not report any relevant financial disclosures.
FROM PEDIATRICS
Key clinical point: Certain pathogens found in urine, such as E. coli, are more reliable than others at indicating a child’s risk for pyuria.
Major finding: 89% of subjects with E. coli in their urinary tract also had pyuria, compared with 62% of those with P. aeruginosa, 54.3% of those with a member of the Enterococcus species, and 74% of those with a member of the Klebsiella species found in their urinary tract.
Data source: Retrospective review of 1,181 children diagnosed with UTIs between 2007 and 2013 at the Children’s Hospital of Pittsburgh at UPMC.
Disclosures: The study had no external funding. Dr. Shaikh and his coauthors did not report any relevant financial disclosures.
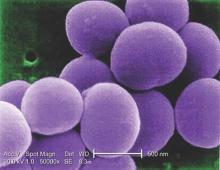
Glucocorticoids increase risk of S. aureus bacteremia
Use of systemic glucocorticoids significantly increased risk for community-acquired Staphylococcus aureus bacteremia (CA-SAB) in a dose-dependent fashion, based on data from a large Danish registry.
On average, current users of systemic glucocorticoids had an adjusted 2.5-fold increased risk of CA-SAB, compared with nonusers. The risk was most pronounced in long-term users of glucocorticoids, including patients with connective tissue disease and patients with chronic pulmonary disease. Among new users of glucocorticoids, the risk of CA-SAB was highest for patients with cancer, in the retrospective, case-control study published by Mayo Clinic Proceedings.
Dr. Jesper Smit of Aalborg (Denmark) University and his colleagues, looked at all 2,638 patients admitted with first-time CA-SAB and 26,379 matched population controls in Northern Denmark medical databases between January 1, 2000, and December 31, 2011.
New glucocorticoid users had an odds ratio for CA-SAB of 2.7, slightly higher than the OR of 2.3 for long-term users. Former glucocorticoid users had a considerably lower OR for CA-SAB of 1.3.
Risk of CA-SAB rose in a dose-dependent fashion as 90-day cumulative doses increased. For subjects taking a cumulative dose of 150 mg or less, the adjusted OR for CA-SAB was 1.3. At a cumulative dose of 500-1000 mg, OR rose to 2.4. At a cumulative dose greater than 1000 mg, OR was 6.2.
Risk did not differ based on individuals’ sex, age group, or the severity of any comorbidity.
“This is the first study to specifically investigate whether the use of glucocorticoids is associated with increased risk of CA-SAB,” the authors concluded, adding that “these results extend the current knowledge of risk factors for CA-SAB and may serve as a reminder for clinicians to carefully weigh the elevated risk against the potential beneficial effect of glucocorticoid therapy, particularly in patients with concomitant CA-SAB risk factors.”
This study was supported by grants from Heinrich Kopp, Hertha Christensen, and North Denmark Health Sciences Research foundation. The authors did not report any relevant financial disclosures.
Use of systemic glucocorticoids significantly increased risk for community-acquired Staphylococcus aureus bacteremia (CA-SAB) in a dose-dependent fashion, based on data from a large Danish registry.
On average, current users of systemic glucocorticoids had an adjusted 2.5-fold increased risk of CA-SAB, compared with nonusers. The risk was most pronounced in long-term users of glucocorticoids, including patients with connective tissue disease and patients with chronic pulmonary disease. Among new users of glucocorticoids, the risk of CA-SAB was highest for patients with cancer, in the retrospective, case-control study published by Mayo Clinic Proceedings.
Dr. Jesper Smit of Aalborg (Denmark) University and his colleagues, looked at all 2,638 patients admitted with first-time CA-SAB and 26,379 matched population controls in Northern Denmark medical databases between January 1, 2000, and December 31, 2011.
New glucocorticoid users had an odds ratio for CA-SAB of 2.7, slightly higher than the OR of 2.3 for long-term users. Former glucocorticoid users had a considerably lower OR for CA-SAB of 1.3.
Risk of CA-SAB rose in a dose-dependent fashion as 90-day cumulative doses increased. For subjects taking a cumulative dose of 150 mg or less, the adjusted OR for CA-SAB was 1.3. At a cumulative dose of 500-1000 mg, OR rose to 2.4. At a cumulative dose greater than 1000 mg, OR was 6.2.
Risk did not differ based on individuals’ sex, age group, or the severity of any comorbidity.
“This is the first study to specifically investigate whether the use of glucocorticoids is associated with increased risk of CA-SAB,” the authors concluded, adding that “these results extend the current knowledge of risk factors for CA-SAB and may serve as a reminder for clinicians to carefully weigh the elevated risk against the potential beneficial effect of glucocorticoid therapy, particularly in patients with concomitant CA-SAB risk factors.”
This study was supported by grants from Heinrich Kopp, Hertha Christensen, and North Denmark Health Sciences Research foundation. The authors did not report any relevant financial disclosures.
Use of systemic glucocorticoids significantly increased risk for community-acquired Staphylococcus aureus bacteremia (CA-SAB) in a dose-dependent fashion, based on data from a large Danish registry.
On average, current users of systemic glucocorticoids had an adjusted 2.5-fold increased risk of CA-SAB, compared with nonusers. The risk was most pronounced in long-term users of glucocorticoids, including patients with connective tissue disease and patients with chronic pulmonary disease. Among new users of glucocorticoids, the risk of CA-SAB was highest for patients with cancer, in the retrospective, case-control study published by Mayo Clinic Proceedings.
Dr. Jesper Smit of Aalborg (Denmark) University and his colleagues, looked at all 2,638 patients admitted with first-time CA-SAB and 26,379 matched population controls in Northern Denmark medical databases between January 1, 2000, and December 31, 2011.
New glucocorticoid users had an odds ratio for CA-SAB of 2.7, slightly higher than the OR of 2.3 for long-term users. Former glucocorticoid users had a considerably lower OR for CA-SAB of 1.3.
Risk of CA-SAB rose in a dose-dependent fashion as 90-day cumulative doses increased. For subjects taking a cumulative dose of 150 mg or less, the adjusted OR for CA-SAB was 1.3. At a cumulative dose of 500-1000 mg, OR rose to 2.4. At a cumulative dose greater than 1000 mg, OR was 6.2.
Risk did not differ based on individuals’ sex, age group, or the severity of any comorbidity.
“This is the first study to specifically investigate whether the use of glucocorticoids is associated with increased risk of CA-SAB,” the authors concluded, adding that “these results extend the current knowledge of risk factors for CA-SAB and may serve as a reminder for clinicians to carefully weigh the elevated risk against the potential beneficial effect of glucocorticoid therapy, particularly in patients with concomitant CA-SAB risk factors.”
This study was supported by grants from Heinrich Kopp, Hertha Christensen, and North Denmark Health Sciences Research foundation. The authors did not report any relevant financial disclosures.
FROM MAYO CLINIC PROCEEDINGS
Key clinical point: Taking glucocorticoids can significantly increase the risk of contracting community-acquired Staphylococcus aureus bacteremia (CA-SAB).
Major finding: New glucocorticoid users had an odds ratio for CA-SAB of 2.7, slightly higher than the OR of 2.3 for long-term users. Former glucocorticoid users had a considerably lower OR for CA-SAB of 1.3.
Data source: Retrospective, case-control study of all adults with first-time CA-SAB in Northern Denmark medical registries between 2000 and 2011.
Disclosures: Study supported by grants from Heinrich Kopp, Hertha Christensen, and North Denmark Health Sciences Research foundation. The authors did not report any relevant financial disclosures.
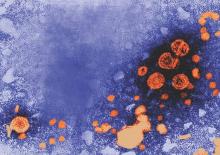
Tenofovir may prevent mother-to-child transmission of hepatitis B
The antiviral drug tenofovir disoproxil fumarate (TDF) can be used by expectant mothers infected with the hepatitis B virus to decrease HBV DNA levels and, therefore, significantly decrease the risk of passing the virus onto their children, according to a new study.
“A small but growing body of evidence has suggested that antiviral treatment may reduce the risk of mother-to-child transmission among mothers with an HBV DNA level of more than 6 log10 copies per milliliter, although quality studies are lacking and the existing studies have shown conflicting results,” wrote Dr. Calvin Q. Pan of New York University and coauthors in a study published in the New England Journal of Medicine (2016;374[24]:2324-34. doi: 10.1056/NEJMoa1508660).
An international research team recruited pregnant women in China – all of whom were positive for hepatitis B e antigen and had a viral load of at least 200,000 IU/mL – from March 2012 through June 2013. A total of 200 women were selected for inclusion and randomized into either a control cohort, or a cohort taking TDF; 100 mothers with 88 infants ultimately completed the study in the control group, while 97 mothers with 92 children completed the study in the TDF cohort.
Women on TDF were given a 300-mg dose orally every day, beginning 30-32 weeks into their pregnancy and continuing through 4 weeks after giving birth. Follow-up examinations were conducted at 4, 12, 24, and 28 weeks post partum, and the primary outcome was determining transmission of HBV from mother to child, with transmission defined as an infant having an HBV DNA level higher than 20 IU/mL. Patients in the control group were given “usual care without antiviral therapy.”
Mothers on TDF had lower HBV DNA levels at delivery, with 68% (66/97) dropping to under 200,000 IU/mL levels versus just 2% (2/100) in the control group having levels under that threshold (P less than .001). At 28-week follow-up examinations, intent-to-treat analysis showed that 5% (5/97) of infants born to mothers on TDF were found to have HBV DNA levels higher than 20 IU/mL, compared with 18% of infants born to mothers in the control group (P = .007). In per-protocol analysis, none of the infants whose mothers were taking TDF had contracted the disease; on the other hand, 7% (6/88) of those in the control group had (P = .01). Rates of birth defects and HBV serologic outcomes were not significantly different between the two cohorts.
“TDF [may] be useful for preventing mother-to-child transmission, which is a critical step toward the global eradication of HBV and a reduction in the incidence of liver cancer,” Dr. Pan and his colleagues said, adding that “the postpartum cessation of TDF requires close monitoring” in order to further understand the drug’s longer-term effects.
The study was funded by Gilead Sciences. Dr. Pan reported grant support from Gilead Sciences during the conduct of the study; grant support from Merck, grant support and personal fees from Gilead Sciences and Bristol-Myers Squibb; and personal fees from Johnson and Johnson and AbbVie outside the submitted work.
The antiviral drug tenofovir disoproxil fumarate (TDF) can be used by expectant mothers infected with the hepatitis B virus to decrease HBV DNA levels and, therefore, significantly decrease the risk of passing the virus onto their children, according to a new study.
“A small but growing body of evidence has suggested that antiviral treatment may reduce the risk of mother-to-child transmission among mothers with an HBV DNA level of more than 6 log10 copies per milliliter, although quality studies are lacking and the existing studies have shown conflicting results,” wrote Dr. Calvin Q. Pan of New York University and coauthors in a study published in the New England Journal of Medicine (2016;374[24]:2324-34. doi: 10.1056/NEJMoa1508660).
An international research team recruited pregnant women in China – all of whom were positive for hepatitis B e antigen and had a viral load of at least 200,000 IU/mL – from March 2012 through June 2013. A total of 200 women were selected for inclusion and randomized into either a control cohort, or a cohort taking TDF; 100 mothers with 88 infants ultimately completed the study in the control group, while 97 mothers with 92 children completed the study in the TDF cohort.
Women on TDF were given a 300-mg dose orally every day, beginning 30-32 weeks into their pregnancy and continuing through 4 weeks after giving birth. Follow-up examinations were conducted at 4, 12, 24, and 28 weeks post partum, and the primary outcome was determining transmission of HBV from mother to child, with transmission defined as an infant having an HBV DNA level higher than 20 IU/mL. Patients in the control group were given “usual care without antiviral therapy.”
Mothers on TDF had lower HBV DNA levels at delivery, with 68% (66/97) dropping to under 200,000 IU/mL levels versus just 2% (2/100) in the control group having levels under that threshold (P less than .001). At 28-week follow-up examinations, intent-to-treat analysis showed that 5% (5/97) of infants born to mothers on TDF were found to have HBV DNA levels higher than 20 IU/mL, compared with 18% of infants born to mothers in the control group (P = .007). In per-protocol analysis, none of the infants whose mothers were taking TDF had contracted the disease; on the other hand, 7% (6/88) of those in the control group had (P = .01). Rates of birth defects and HBV serologic outcomes were not significantly different between the two cohorts.
“TDF [may] be useful for preventing mother-to-child transmission, which is a critical step toward the global eradication of HBV and a reduction in the incidence of liver cancer,” Dr. Pan and his colleagues said, adding that “the postpartum cessation of TDF requires close monitoring” in order to further understand the drug’s longer-term effects.
The study was funded by Gilead Sciences. Dr. Pan reported grant support from Gilead Sciences during the conduct of the study; grant support from Merck, grant support and personal fees from Gilead Sciences and Bristol-Myers Squibb; and personal fees from Johnson and Johnson and AbbVie outside the submitted work.
The antiviral drug tenofovir disoproxil fumarate (TDF) can be used by expectant mothers infected with the hepatitis B virus to decrease HBV DNA levels and, therefore, significantly decrease the risk of passing the virus onto their children, according to a new study.
“A small but growing body of evidence has suggested that antiviral treatment may reduce the risk of mother-to-child transmission among mothers with an HBV DNA level of more than 6 log10 copies per milliliter, although quality studies are lacking and the existing studies have shown conflicting results,” wrote Dr. Calvin Q. Pan of New York University and coauthors in a study published in the New England Journal of Medicine (2016;374[24]:2324-34. doi: 10.1056/NEJMoa1508660).
An international research team recruited pregnant women in China – all of whom were positive for hepatitis B e antigen and had a viral load of at least 200,000 IU/mL – from March 2012 through June 2013. A total of 200 women were selected for inclusion and randomized into either a control cohort, or a cohort taking TDF; 100 mothers with 88 infants ultimately completed the study in the control group, while 97 mothers with 92 children completed the study in the TDF cohort.
Women on TDF were given a 300-mg dose orally every day, beginning 30-32 weeks into their pregnancy and continuing through 4 weeks after giving birth. Follow-up examinations were conducted at 4, 12, 24, and 28 weeks post partum, and the primary outcome was determining transmission of HBV from mother to child, with transmission defined as an infant having an HBV DNA level higher than 20 IU/mL. Patients in the control group were given “usual care without antiviral therapy.”
Mothers on TDF had lower HBV DNA levels at delivery, with 68% (66/97) dropping to under 200,000 IU/mL levels versus just 2% (2/100) in the control group having levels under that threshold (P less than .001). At 28-week follow-up examinations, intent-to-treat analysis showed that 5% (5/97) of infants born to mothers on TDF were found to have HBV DNA levels higher than 20 IU/mL, compared with 18% of infants born to mothers in the control group (P = .007). In per-protocol analysis, none of the infants whose mothers were taking TDF had contracted the disease; on the other hand, 7% (6/88) of those in the control group had (P = .01). Rates of birth defects and HBV serologic outcomes were not significantly different between the two cohorts.
“TDF [may] be useful for preventing mother-to-child transmission, which is a critical step toward the global eradication of HBV and a reduction in the incidence of liver cancer,” Dr. Pan and his colleagues said, adding that “the postpartum cessation of TDF requires close monitoring” in order to further understand the drug’s longer-term effects.
The study was funded by Gilead Sciences. Dr. Pan reported grant support from Gilead Sciences during the conduct of the study; grant support from Merck, grant support and personal fees from Gilead Sciences and Bristol-Myers Squibb; and personal fees from Johnson and Johnson and AbbVie outside the submitted work.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: A 300-mg daily dose of tenofovir disoproxil fumarate significantly reduced the risk of pregnant mothers with HBV infection transmitting the disease to their children.
Major finding: In an intent-to-treat analysis, only 5% of children born to mothers taking TDF during pregnancy were found to have contracted the disease, versus 18% in the control group (P = .007); similarly, in the per-protocol analysis, 0% of children born to mothers taking TDF had HBV vs. 7% in the control group (P = .01).
Data source: A multicenter, open label, randomized, parallel-group study of 197 HBV-positive pregnant women from March 2012 through June 2013.
Disclosures: The study was funded by Gilead Sciences. Dr. Pan reported grant support from Gilead Sciences during the conduct of the study; grant support from Merck, grant support and personal fees from Gilead Sciences and Bristol-Myers Squibb; and personal fees from Johnson and Johnson and AbbVie outside the submitted work.
Dually active antiretrovirals protect HIV patients against HBV infection
Dually active antiretroviral therapy (DAART) for HIV-infected patients may be the best treatment option to also protect these individuals against hepatitis B virus (HBV) infection, according to results of a study in the Journal of Infectious Diseases.
“Vaccination against HBV remains the mainstay of preventing HBV acquisition both in HIV-infected and uninfected individuals,” states the study, led by Roger Kouyos, Ph.D., of University Hospital Zürich. “However, owing to HIV’s effect on the immune system, mounting and maintaining a protective immune response against HBV is sometimes unattainable, with a success rate between 18% and 71%.”
Dr. Kouyos and his colleagues collected CD4, CD8, and HIV-1 viral load data for 1,716 patients in the Swiss HIV Cohort Study – an ongoing, prospective cohort study that began in 1988 – and updated the data continuously during follow-up visits every 3 months, on average. Subjects were enrolled if they had negative baseline HBV serology and at least one additional HBV test after the initial one, and were either a man who had sex with men, a heterosexual, or an intravenous drug user – the three major groups examined by the study (J Infect Dis. 2016 May 18. doi:10.1093/infdis/jiw195).
DAART treatment consisted of tenofovir, lamivudine, and emtricitabine. Antiretroviral treatment history was also collected, and at follow-up examinations every 6 months, subjects were asked about occasional sex partners and frequency of condom use during the preceding 6 months. All were tested for at least one HBV marker: surface antigen (HBsAg), anti–hepatitis B core antibodies (AntiHBc), or hepatitis B virus DNA.
Of the 1,716 subjects enrolled, 177 had HBV. Of these, 86 (49%) were men who had sex with men. Most subjects underwent at least two HBV tests, with the median time between tests being 29 months (range of 12-58 months). In the transmission group, the overall incidence rate of HBV was 16 (95% confidence interval, 14-19), while it was 25 (21-31) for men who had sex with men, 9 (6-11) for heterosexuals, and 28 (21-38) for intravenous drug users.
DAART, however, was shown to significantly reduce HBV-infection risk. Univariate analysis yielded a hazard ratio of 0.4 (95% CI, 0.2-0.6), and multivariate analysis showed even more significant effects for intravenous drug users (3.8, 2.4-6.1), men who had sex with men (2.7, 1.7-4.2), and those who had unprotected sex (1.9, 1.4-2.6).
“Our study confirms the importance of viral suppression (and the implicit adherence) in reaching the protective effect of DAART,” the authors concluded, noting that “Our study adds to the growing body of evidence that early antiretroviral therapy initiation, regardless of CD4 counts, has a strong beneficial public health impact, including preexposure prophylaxis of HBV coinfections.”
The study was supported by the Swiss HIV Cohort Study, the Swiss National Science Foundation, the European Community’s Seventh Framework Program, and the Union Bank of Switzerland. Dr. Kouyos reported travel grants from Gilead. Other coauthors reported research, educational, and travel grants from Roche, Abbott, Bristol-Myers Squibb, Gilead, Astra-Zeneca, GlaxoSmithKline, Pfizer, and Astellas.
Dually active antiretroviral therapy (DAART) for HIV-infected patients may be the best treatment option to also protect these individuals against hepatitis B virus (HBV) infection, according to results of a study in the Journal of Infectious Diseases.
“Vaccination against HBV remains the mainstay of preventing HBV acquisition both in HIV-infected and uninfected individuals,” states the study, led by Roger Kouyos, Ph.D., of University Hospital Zürich. “However, owing to HIV’s effect on the immune system, mounting and maintaining a protective immune response against HBV is sometimes unattainable, with a success rate between 18% and 71%.”
Dr. Kouyos and his colleagues collected CD4, CD8, and HIV-1 viral load data for 1,716 patients in the Swiss HIV Cohort Study – an ongoing, prospective cohort study that began in 1988 – and updated the data continuously during follow-up visits every 3 months, on average. Subjects were enrolled if they had negative baseline HBV serology and at least one additional HBV test after the initial one, and were either a man who had sex with men, a heterosexual, or an intravenous drug user – the three major groups examined by the study (J Infect Dis. 2016 May 18. doi:10.1093/infdis/jiw195).
DAART treatment consisted of tenofovir, lamivudine, and emtricitabine. Antiretroviral treatment history was also collected, and at follow-up examinations every 6 months, subjects were asked about occasional sex partners and frequency of condom use during the preceding 6 months. All were tested for at least one HBV marker: surface antigen (HBsAg), anti–hepatitis B core antibodies (AntiHBc), or hepatitis B virus DNA.
Of the 1,716 subjects enrolled, 177 had HBV. Of these, 86 (49%) were men who had sex with men. Most subjects underwent at least two HBV tests, with the median time between tests being 29 months (range of 12-58 months). In the transmission group, the overall incidence rate of HBV was 16 (95% confidence interval, 14-19), while it was 25 (21-31) for men who had sex with men, 9 (6-11) for heterosexuals, and 28 (21-38) for intravenous drug users.
DAART, however, was shown to significantly reduce HBV-infection risk. Univariate analysis yielded a hazard ratio of 0.4 (95% CI, 0.2-0.6), and multivariate analysis showed even more significant effects for intravenous drug users (3.8, 2.4-6.1), men who had sex with men (2.7, 1.7-4.2), and those who had unprotected sex (1.9, 1.4-2.6).
“Our study confirms the importance of viral suppression (and the implicit adherence) in reaching the protective effect of DAART,” the authors concluded, noting that “Our study adds to the growing body of evidence that early antiretroviral therapy initiation, regardless of CD4 counts, has a strong beneficial public health impact, including preexposure prophylaxis of HBV coinfections.”
The study was supported by the Swiss HIV Cohort Study, the Swiss National Science Foundation, the European Community’s Seventh Framework Program, and the Union Bank of Switzerland. Dr. Kouyos reported travel grants from Gilead. Other coauthors reported research, educational, and travel grants from Roche, Abbott, Bristol-Myers Squibb, Gilead, Astra-Zeneca, GlaxoSmithKline, Pfizer, and Astellas.
Dually active antiretroviral therapy (DAART) for HIV-infected patients may be the best treatment option to also protect these individuals against hepatitis B virus (HBV) infection, according to results of a study in the Journal of Infectious Diseases.
“Vaccination against HBV remains the mainstay of preventing HBV acquisition both in HIV-infected and uninfected individuals,” states the study, led by Roger Kouyos, Ph.D., of University Hospital Zürich. “However, owing to HIV’s effect on the immune system, mounting and maintaining a protective immune response against HBV is sometimes unattainable, with a success rate between 18% and 71%.”
Dr. Kouyos and his colleagues collected CD4, CD8, and HIV-1 viral load data for 1,716 patients in the Swiss HIV Cohort Study – an ongoing, prospective cohort study that began in 1988 – and updated the data continuously during follow-up visits every 3 months, on average. Subjects were enrolled if they had negative baseline HBV serology and at least one additional HBV test after the initial one, and were either a man who had sex with men, a heterosexual, or an intravenous drug user – the three major groups examined by the study (J Infect Dis. 2016 May 18. doi:10.1093/infdis/jiw195).
DAART treatment consisted of tenofovir, lamivudine, and emtricitabine. Antiretroviral treatment history was also collected, and at follow-up examinations every 6 months, subjects were asked about occasional sex partners and frequency of condom use during the preceding 6 months. All were tested for at least one HBV marker: surface antigen (HBsAg), anti–hepatitis B core antibodies (AntiHBc), or hepatitis B virus DNA.
Of the 1,716 subjects enrolled, 177 had HBV. Of these, 86 (49%) were men who had sex with men. Most subjects underwent at least two HBV tests, with the median time between tests being 29 months (range of 12-58 months). In the transmission group, the overall incidence rate of HBV was 16 (95% confidence interval, 14-19), while it was 25 (21-31) for men who had sex with men, 9 (6-11) for heterosexuals, and 28 (21-38) for intravenous drug users.
DAART, however, was shown to significantly reduce HBV-infection risk. Univariate analysis yielded a hazard ratio of 0.4 (95% CI, 0.2-0.6), and multivariate analysis showed even more significant effects for intravenous drug users (3.8, 2.4-6.1), men who had sex with men (2.7, 1.7-4.2), and those who had unprotected sex (1.9, 1.4-2.6).
“Our study confirms the importance of viral suppression (and the implicit adherence) in reaching the protective effect of DAART,” the authors concluded, noting that “Our study adds to the growing body of evidence that early antiretroviral therapy initiation, regardless of CD4 counts, has a strong beneficial public health impact, including preexposure prophylaxis of HBV coinfections.”
The study was supported by the Swiss HIV Cohort Study, the Swiss National Science Foundation, the European Community’s Seventh Framework Program, and the Union Bank of Switzerland. Dr. Kouyos reported travel grants from Gilead. Other coauthors reported research, educational, and travel grants from Roche, Abbott, Bristol-Myers Squibb, Gilead, Astra-Zeneca, GlaxoSmithKline, Pfizer, and Astellas.
FROM THE JOURNAL OF INFECTIOUS DISEASES
Key clinical point: DAART for HIV and hepatitis B virus consisting of tenofovir, lamivudine, and emtricitabine can be an effective preexposure prophylaxis against HBV coinfection.
Major finding: HBV infection was negatively associated with DAART therapy (0.4, 95% CI, 0.2-0.6), with even stronger indications of protection after adjusting for condomless sex, drug use, and men having sex with men.
Data source: Prospective cohort study of 1,716 patients, with 177 incident HBV cases, from the Swiss HIV Cohort Study.
Disclosures: The study was supported by the Swiss HIV Cohort Study, the Swiss National Science Foundation, the European Community’s Seventh Framework Program, and the Union Bank of Switzerland. Dr. Kouyos reported travel grants from Gilead. Other coauthors reported research, educational, and travel grants from Roche, Abbott, Bristol-Myers Squibb, Gilead, Astra-Zeneca, GlaxoSmithKline, Pfizer, and Astellas.
High school students’ health risk behaviors mixed, CDC says
Data from the latest National Youth Risk Behavior Survey show that although many harmful behaviors are declining among high school students across the United States, work still needs to be done to eliminate risks that linger and quell those starting to crop up.
“We are encouraged to see that high school students are making some better choices; unfortunately, [we] are also facing some big challenges,” Dr. Stephanie Zaza, director of the division of adolescent and school health at the Centers for Disease Control and Prevention, said June 9 during a teleconference.
More than 15,000 high school students – located in 37 states and 19 large, urban school districts – participated in the survey, which analyzed risk behaviors in key health and wellness categories. Smoking has decreased significantly since 1991, the inaugural year of the survey, dropping from 70% to 32% for students who had ever tried a cigarette. Hispanic males (37%) and students in 11th grade (38%) had the highest rates of cigarette use. However, e-cigarette use continues to be a growing issue, with 45% of high school students reporting having used an electronic vapor product at some point. This category includes not just e-cigarettes, but vape pipes and e-hookahs.
Opiate use also continues to be a challenging issue for public health, with 17% of high school students reporting that they have used a prescription drug despite not having a doctor’s prescription to use it. Those drugs include OxyContin (oxycodone), Percocet (acetaminophen/oxycodone), Vicodin (acetaminophen/hydrocodone), and codeine. Other problematic drugs among these students are the stimulants Adderall (dextroamphetamine plus amphetamine) and Ritalin (methylphenidate hydrochloride), and Xanax (alprazolam), a benzodiazepine. Males were more likely to use drugs without a prescription than female students were: 18% vs.16%, respectively.
Results related to interpersonal violence – such as schoolyard fighting and physical bullying – were “mixed,” according to Dr. Zaza. Students who had engaged in a physical fight in the last year registered 23%, a sharp decrease from the 42% logged in 1991; however, bullying rates continue to remain high, with 20% of students reporting being involved with physical bullying and 16% reporting involvement with cyberbullying in some capacity.
On a more positive note, students seem to be eating better, with dietary statistics looking increasingly optimistic. In particular, consumption of sugar-heavy drinks and sodas dropped significantly from 27% in 2013 to 20% last year. But Dr. Zaza warned that dietary improvements are offset by continued sedentary lifestyles. Although TV watching has declined, it has been effectively replaced by increased time in front of computers and video games; use of a computer for tasks unrelated to school for more than 3 hours daily rose, from 22% in 2003 to 42% in 2015.
The percentage of sexually active high school students – those who have had sexual intercourse in the 3 months before participating in the survey – has dropped to 30%, from 38% in 1991. However, despite this, condom use among sexually active high school students also declined, going from 63% in 2003 to 57% in 2015.
Also alarming is that 21% of sexually active students reported consuming drugs or alcohol before their last sexual encounter. Testing for HIV among sexually active high school student also has decreased, from 13% in 2013 to 10% in 2015.
A total of 42% of students reported texting or emailing while driving in the 30 days prior to taking the survey, which is steady with the data from 2013.
“There’s no single solution to improving health risk behaviors among high school students, and we can all collaborate to help address these health risks using interventions that are based on the best science available,” Dr. Zaza said. “We hope that public health professionals, educators, youth service providers, policy makers, and parents can use these data to guide their planning decisions, and help schools and communities reduce youth health risk behaviors.”
The CDC conducted and released the survey.
Data from the latest National Youth Risk Behavior Survey show that although many harmful behaviors are declining among high school students across the United States, work still needs to be done to eliminate risks that linger and quell those starting to crop up.
“We are encouraged to see that high school students are making some better choices; unfortunately, [we] are also facing some big challenges,” Dr. Stephanie Zaza, director of the division of adolescent and school health at the Centers for Disease Control and Prevention, said June 9 during a teleconference.
More than 15,000 high school students – located in 37 states and 19 large, urban school districts – participated in the survey, which analyzed risk behaviors in key health and wellness categories. Smoking has decreased significantly since 1991, the inaugural year of the survey, dropping from 70% to 32% for students who had ever tried a cigarette. Hispanic males (37%) and students in 11th grade (38%) had the highest rates of cigarette use. However, e-cigarette use continues to be a growing issue, with 45% of high school students reporting having used an electronic vapor product at some point. This category includes not just e-cigarettes, but vape pipes and e-hookahs.
Opiate use also continues to be a challenging issue for public health, with 17% of high school students reporting that they have used a prescription drug despite not having a doctor’s prescription to use it. Those drugs include OxyContin (oxycodone), Percocet (acetaminophen/oxycodone), Vicodin (acetaminophen/hydrocodone), and codeine. Other problematic drugs among these students are the stimulants Adderall (dextroamphetamine plus amphetamine) and Ritalin (methylphenidate hydrochloride), and Xanax (alprazolam), a benzodiazepine. Males were more likely to use drugs without a prescription than female students were: 18% vs.16%, respectively.
Results related to interpersonal violence – such as schoolyard fighting and physical bullying – were “mixed,” according to Dr. Zaza. Students who had engaged in a physical fight in the last year registered 23%, a sharp decrease from the 42% logged in 1991; however, bullying rates continue to remain high, with 20% of students reporting being involved with physical bullying and 16% reporting involvement with cyberbullying in some capacity.
On a more positive note, students seem to be eating better, with dietary statistics looking increasingly optimistic. In particular, consumption of sugar-heavy drinks and sodas dropped significantly from 27% in 2013 to 20% last year. But Dr. Zaza warned that dietary improvements are offset by continued sedentary lifestyles. Although TV watching has declined, it has been effectively replaced by increased time in front of computers and video games; use of a computer for tasks unrelated to school for more than 3 hours daily rose, from 22% in 2003 to 42% in 2015.
The percentage of sexually active high school students – those who have had sexual intercourse in the 3 months before participating in the survey – has dropped to 30%, from 38% in 1991. However, despite this, condom use among sexually active high school students also declined, going from 63% in 2003 to 57% in 2015.
Also alarming is that 21% of sexually active students reported consuming drugs or alcohol before their last sexual encounter. Testing for HIV among sexually active high school student also has decreased, from 13% in 2013 to 10% in 2015.
A total of 42% of students reported texting or emailing while driving in the 30 days prior to taking the survey, which is steady with the data from 2013.
“There’s no single solution to improving health risk behaviors among high school students, and we can all collaborate to help address these health risks using interventions that are based on the best science available,” Dr. Zaza said. “We hope that public health professionals, educators, youth service providers, policy makers, and parents can use these data to guide their planning decisions, and help schools and communities reduce youth health risk behaviors.”
The CDC conducted and released the survey.
Data from the latest National Youth Risk Behavior Survey show that although many harmful behaviors are declining among high school students across the United States, work still needs to be done to eliminate risks that linger and quell those starting to crop up.
“We are encouraged to see that high school students are making some better choices; unfortunately, [we] are also facing some big challenges,” Dr. Stephanie Zaza, director of the division of adolescent and school health at the Centers for Disease Control and Prevention, said June 9 during a teleconference.
More than 15,000 high school students – located in 37 states and 19 large, urban school districts – participated in the survey, which analyzed risk behaviors in key health and wellness categories. Smoking has decreased significantly since 1991, the inaugural year of the survey, dropping from 70% to 32% for students who had ever tried a cigarette. Hispanic males (37%) and students in 11th grade (38%) had the highest rates of cigarette use. However, e-cigarette use continues to be a growing issue, with 45% of high school students reporting having used an electronic vapor product at some point. This category includes not just e-cigarettes, but vape pipes and e-hookahs.
Opiate use also continues to be a challenging issue for public health, with 17% of high school students reporting that they have used a prescription drug despite not having a doctor’s prescription to use it. Those drugs include OxyContin (oxycodone), Percocet (acetaminophen/oxycodone), Vicodin (acetaminophen/hydrocodone), and codeine. Other problematic drugs among these students are the stimulants Adderall (dextroamphetamine plus amphetamine) and Ritalin (methylphenidate hydrochloride), and Xanax (alprazolam), a benzodiazepine. Males were more likely to use drugs without a prescription than female students were: 18% vs.16%, respectively.
Results related to interpersonal violence – such as schoolyard fighting and physical bullying – were “mixed,” according to Dr. Zaza. Students who had engaged in a physical fight in the last year registered 23%, a sharp decrease from the 42% logged in 1991; however, bullying rates continue to remain high, with 20% of students reporting being involved with physical bullying and 16% reporting involvement with cyberbullying in some capacity.
On a more positive note, students seem to be eating better, with dietary statistics looking increasingly optimistic. In particular, consumption of sugar-heavy drinks and sodas dropped significantly from 27% in 2013 to 20% last year. But Dr. Zaza warned that dietary improvements are offset by continued sedentary lifestyles. Although TV watching has declined, it has been effectively replaced by increased time in front of computers and video games; use of a computer for tasks unrelated to school for more than 3 hours daily rose, from 22% in 2003 to 42% in 2015.
The percentage of sexually active high school students – those who have had sexual intercourse in the 3 months before participating in the survey – has dropped to 30%, from 38% in 1991. However, despite this, condom use among sexually active high school students also declined, going from 63% in 2003 to 57% in 2015.
Also alarming is that 21% of sexually active students reported consuming drugs or alcohol before their last sexual encounter. Testing for HIV among sexually active high school student also has decreased, from 13% in 2013 to 10% in 2015.
A total of 42% of students reported texting or emailing while driving in the 30 days prior to taking the survey, which is steady with the data from 2013.
“There’s no single solution to improving health risk behaviors among high school students, and we can all collaborate to help address these health risks using interventions that are based on the best science available,” Dr. Zaza said. “We hope that public health professionals, educators, youth service providers, policy makers, and parents can use these data to guide their planning decisions, and help schools and communities reduce youth health risk behaviors.”
The CDC conducted and released the survey.
Bevacizumab offers best value of anti-VEGF drugs to treat DME
Despite recent data showing that aflibercept is the most effective anti–vascular endothelial growth factor (anti-VEGF) treatment available for patients with diabetic macular edema, bevacizumab is still by far the best drug option available from a cost-effectiveness perspective, according to a post hoc analysis.
Dr. Joshua D. Stein of the University of Michigan, Ann Arbor, and his coauthors analyzed the anti-VEGF drugs prescribed to 624 diabetic macular edema (DME) patients – 209 taking aflibercept, 207 taking bevacizumab, and 208 taking ranibizumab – enrolled in the Diabetic Retinopathy Clinical Research Network Comparative Effectiveness Trial for incremental cost-effectiveness ratios (ICERs).
“On the basis of 2015 wholesale acquisition costs, aflibercept (2 mg) costs $1,850, ranibizumab (0.3mg) costs $1,170, and bevacizumab repackaged at compounding pharmacies into syringes for ophthalmologic use containing 1.25 mg of bevacizumab costs approximately $60 per dose,” Dr. Stein and his coauthors wrote (JAMA Ophthalmol. 2016 June 9. doi: 10.1001/jamaophthalmol.2016.1669). “Considering that these medicines may be given 9 to 11 times in the first year of treatment and, on average, 17 times during 5 years, total costs can be substantial.”
Data from the randomized clinical trial were used to calculate projected benefit, costs, and cost-effectiveness of aflibercept and ranibizumab compared with bevacizumab as baseline. In addition, the investigators also determined both ICERs and Quality Life-Adjusted Years (QALY) for each drug over periods of 1 and 10 years. Results indicated that for 1 year of treatment, the ICER of aflibercept was $1.11 million per QALY and for ranibizumab, $1.73 million per QALY. Over the course of 10 years, aflibercept would come out to $349,000 per QALY, while ranibizumab would be $603,000 per QALY. In an analysis of a subgroup with highly reduced eyesight due to DME, the 10-year ICER of aflibercept would be $287,000 per QALY and for ranibizumab, $817,000, according to Dr. Stein and his associates.
Over a 1-year period, bevacizumab would cost $4,100, compared to $26,100 for aflibercept and $18,600 for ranibizumab. Over a 10-year period, those costs would jump up to $102,500 for aflibercept and $79,400 for ranibizumab, while bevacizumab would cost $39,800. Overall, the costs of aflibercept and ranibizumab would have to decrease by 69% and 80%, respectively, for the costs to become competitive with bevacizumab.
“Aflibercept (2.0 mg) and ranibizumab (0.3 mg) are not cost-effective relative to bevacizumab for treatment of DME unless their prices decrease substantially [and] in contexts where bevacizumab is unavailable for DME treatment, aflibercept is not cost-effective relative to ranibizumab,” the authors concluded, adding that bevacizumab makes the most sense as a primary anti-VEGF treatment because it allows for the greatest overall value.
The National Eye Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, and the U.S. Department of Health and Human Services funded the study. Dr. Stein did not report any relevant financial disclosures; however, other coauthors reported potentially relevant disclosures.
Despite recent data showing that aflibercept is the most effective anti–vascular endothelial growth factor (anti-VEGF) treatment available for patients with diabetic macular edema, bevacizumab is still by far the best drug option available from a cost-effectiveness perspective, according to a post hoc analysis.
Dr. Joshua D. Stein of the University of Michigan, Ann Arbor, and his coauthors analyzed the anti-VEGF drugs prescribed to 624 diabetic macular edema (DME) patients – 209 taking aflibercept, 207 taking bevacizumab, and 208 taking ranibizumab – enrolled in the Diabetic Retinopathy Clinical Research Network Comparative Effectiveness Trial for incremental cost-effectiveness ratios (ICERs).
“On the basis of 2015 wholesale acquisition costs, aflibercept (2 mg) costs $1,850, ranibizumab (0.3mg) costs $1,170, and bevacizumab repackaged at compounding pharmacies into syringes for ophthalmologic use containing 1.25 mg of bevacizumab costs approximately $60 per dose,” Dr. Stein and his coauthors wrote (JAMA Ophthalmol. 2016 June 9. doi: 10.1001/jamaophthalmol.2016.1669). “Considering that these medicines may be given 9 to 11 times in the first year of treatment and, on average, 17 times during 5 years, total costs can be substantial.”
Data from the randomized clinical trial were used to calculate projected benefit, costs, and cost-effectiveness of aflibercept and ranibizumab compared with bevacizumab as baseline. In addition, the investigators also determined both ICERs and Quality Life-Adjusted Years (QALY) for each drug over periods of 1 and 10 years. Results indicated that for 1 year of treatment, the ICER of aflibercept was $1.11 million per QALY and for ranibizumab, $1.73 million per QALY. Over the course of 10 years, aflibercept would come out to $349,000 per QALY, while ranibizumab would be $603,000 per QALY. In an analysis of a subgroup with highly reduced eyesight due to DME, the 10-year ICER of aflibercept would be $287,000 per QALY and for ranibizumab, $817,000, according to Dr. Stein and his associates.
Over a 1-year period, bevacizumab would cost $4,100, compared to $26,100 for aflibercept and $18,600 for ranibizumab. Over a 10-year period, those costs would jump up to $102,500 for aflibercept and $79,400 for ranibizumab, while bevacizumab would cost $39,800. Overall, the costs of aflibercept and ranibizumab would have to decrease by 69% and 80%, respectively, for the costs to become competitive with bevacizumab.
“Aflibercept (2.0 mg) and ranibizumab (0.3 mg) are not cost-effective relative to bevacizumab for treatment of DME unless their prices decrease substantially [and] in contexts where bevacizumab is unavailable for DME treatment, aflibercept is not cost-effective relative to ranibizumab,” the authors concluded, adding that bevacizumab makes the most sense as a primary anti-VEGF treatment because it allows for the greatest overall value.
The National Eye Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, and the U.S. Department of Health and Human Services funded the study. Dr. Stein did not report any relevant financial disclosures; however, other coauthors reported potentially relevant disclosures.
Despite recent data showing that aflibercept is the most effective anti–vascular endothelial growth factor (anti-VEGF) treatment available for patients with diabetic macular edema, bevacizumab is still by far the best drug option available from a cost-effectiveness perspective, according to a post hoc analysis.
Dr. Joshua D. Stein of the University of Michigan, Ann Arbor, and his coauthors analyzed the anti-VEGF drugs prescribed to 624 diabetic macular edema (DME) patients – 209 taking aflibercept, 207 taking bevacizumab, and 208 taking ranibizumab – enrolled in the Diabetic Retinopathy Clinical Research Network Comparative Effectiveness Trial for incremental cost-effectiveness ratios (ICERs).
“On the basis of 2015 wholesale acquisition costs, aflibercept (2 mg) costs $1,850, ranibizumab (0.3mg) costs $1,170, and bevacizumab repackaged at compounding pharmacies into syringes for ophthalmologic use containing 1.25 mg of bevacizumab costs approximately $60 per dose,” Dr. Stein and his coauthors wrote (JAMA Ophthalmol. 2016 June 9. doi: 10.1001/jamaophthalmol.2016.1669). “Considering that these medicines may be given 9 to 11 times in the first year of treatment and, on average, 17 times during 5 years, total costs can be substantial.”
Data from the randomized clinical trial were used to calculate projected benefit, costs, and cost-effectiveness of aflibercept and ranibizumab compared with bevacizumab as baseline. In addition, the investigators also determined both ICERs and Quality Life-Adjusted Years (QALY) for each drug over periods of 1 and 10 years. Results indicated that for 1 year of treatment, the ICER of aflibercept was $1.11 million per QALY and for ranibizumab, $1.73 million per QALY. Over the course of 10 years, aflibercept would come out to $349,000 per QALY, while ranibizumab would be $603,000 per QALY. In an analysis of a subgroup with highly reduced eyesight due to DME, the 10-year ICER of aflibercept would be $287,000 per QALY and for ranibizumab, $817,000, according to Dr. Stein and his associates.
Over a 1-year period, bevacizumab would cost $4,100, compared to $26,100 for aflibercept and $18,600 for ranibizumab. Over a 10-year period, those costs would jump up to $102,500 for aflibercept and $79,400 for ranibizumab, while bevacizumab would cost $39,800. Overall, the costs of aflibercept and ranibizumab would have to decrease by 69% and 80%, respectively, for the costs to become competitive with bevacizumab.
“Aflibercept (2.0 mg) and ranibizumab (0.3 mg) are not cost-effective relative to bevacizumab for treatment of DME unless their prices decrease substantially [and] in contexts where bevacizumab is unavailable for DME treatment, aflibercept is not cost-effective relative to ranibizumab,” the authors concluded, adding that bevacizumab makes the most sense as a primary anti-VEGF treatment because it allows for the greatest overall value.
The National Eye Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, and the U.S. Department of Health and Human Services funded the study. Dr. Stein did not report any relevant financial disclosures; however, other coauthors reported potentially relevant disclosures.
FROM JAMA OPHTHALMOLOGY
Key clinical point: Bevacizumab remains the best option, in terms of price and value, over aflibercept and ranibizumab for treatment of diabetic macular edema.
Major finding: Based on incremental cost-effectiveness ratios (ICERs) over 1- and 10-year periods, the costs of aflibercept and ranibizumab would have to decrease by 69% and 80%, respectively, to match the cost of bevacizumab over the same time periods.
Data source: Post hoc analysis of patients enrolled in the Diabetic Retinopathy Clinical Research Network Comparative Effectiveness Trial at 1-year follow-up.
Disclosures: The National Eye Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, and the U.S. Department of Health and Human Services funded the study. Dr. Stein did not report any relevant financial disclosures.
Study Finds Most Antidepressants Ineffective or Harmful in Children, Adolescents
Most antidepressants prescribed to children and adolescents with acute major depression might not be nearly as effective as they are believed to be – and one might be harmful, according to a retrospective review published June 8 and including more than 5,000 participants.
“Our analysis found robust evidence to suggest a significantly increased risk for suicidality for young people given venlafaxine,” wrote Dr. Andrea Cipriani, Xinyu Zhou, Ph.D., and their colleagues. “Unfortunately, due to the absence of reliable data on suicidality for many antidepressants, it was not possible to comprehensively assess the risk of suicidality for all drugs.”
The review looked at 34 double-blind, randomized, controlled trials investigating at least one of 14 major drugs typically prescribed as antidepressants for pediatric patients. In addition to venlafaxine, the researchers looked at amitriptyline, citalopram, clomipramine, desipramine, duloxetine, escitalopram, fluoxetine, imipramine, mirtazapine, nefazodone, nortriptyline, paroxetine, and sertraline (Lancet. 2016 Jun 8. doi: 10.1016/S0140-6736[16]30385-3).
All trials were published before May 31, 2015, and were found in the PubMed, Cochrane Library, Web of Science, Embase, CINAHL, PsycINFO, and LiLACS databases, as well as regulatory agencies’ websites, and international registers for published and unpublished trials. Trials either compared one or more drugs against one another, or one or more drugs against a placebo.
Fluoxetine was the only drug found to be significantly more effective than placebo, and it also was found to be significantly more effective than nortriptyline. In addition, fluoxetine proved better tolerated than imipramine and duloxetine. “However, the clinical interpretation of these findings is limited not only by the uncertainty around these estimates, but also by the potential bias due to selective reporting and the small number of trials,” said Dr. Cipriani of the University of Oxford (England) and Dr. Zhou of the First Affiliated Hospital of Chongqing Medical University in China.
Regardless of which treatment clinicians choose, children and adolescents prescribed antidepressants should be monitored closely. Clinical guidelines for young people with major depression recommend psychotherapy, particularly cognitive-behavioral therapy or interpersonal therapy as first-line interventions, and “fluoxetine should be considered only for those patients with moderate to severe depression who do not have access to psychotherapy or have not responded to nonpharmacological interventions,” the researchers said.
The study was funded by the National Basic Research Program of China. The authors did not report any relevant financial disclosures.
The study by Dr. Andrea Cipriani, Xinyu Zhou, Ph.D., and their colleagues has “disturbing implications for clinical practice” in that it concludes that the risk-benefit profile of antidepressants in the acute treatment of major depression in children and adolescents “does not seem to offer a clear advantage” for these young patients, Dr. Jon Jureidini wrote in an accompanying editorial (Lancet. 2016 Jun 8. doi: 10.1016/S0140-6736[16]30385-2).
For clinicians, the implications are that every decision about whether and what to prescribe requires a calculation on the part of the clinician that is both complex and intuitive, he wrote.
“With research evidence as an important part of that calculation, we now know that we need to make a conscious correction for favorable misrepresentation of outcomes in published and unpublished study reports,” Dr. Jureidini wrote. “Only if the discounted benefit outweighs the boosted harm should the treatment be prescribed. For antidepressants in adolescents, this equation will rarely favor prescribing; in younger children, almost never.”
Dr. Jureidini is a research leader for the Robinson Research Institute at the University of Adelaide in North Adelaide, Australia.
The study by Dr. Andrea Cipriani, Xinyu Zhou, Ph.D., and their colleagues has “disturbing implications for clinical practice” in that it concludes that the risk-benefit profile of antidepressants in the acute treatment of major depression in children and adolescents “does not seem to offer a clear advantage” for these young patients, Dr. Jon Jureidini wrote in an accompanying editorial (Lancet. 2016 Jun 8. doi: 10.1016/S0140-6736[16]30385-2).
For clinicians, the implications are that every decision about whether and what to prescribe requires a calculation on the part of the clinician that is both complex and intuitive, he wrote.
“With research evidence as an important part of that calculation, we now know that we need to make a conscious correction for favorable misrepresentation of outcomes in published and unpublished study reports,” Dr. Jureidini wrote. “Only if the discounted benefit outweighs the boosted harm should the treatment be prescribed. For antidepressants in adolescents, this equation will rarely favor prescribing; in younger children, almost never.”
Dr. Jureidini is a research leader for the Robinson Research Institute at the University of Adelaide in North Adelaide, Australia.
The study by Dr. Andrea Cipriani, Xinyu Zhou, Ph.D., and their colleagues has “disturbing implications for clinical practice” in that it concludes that the risk-benefit profile of antidepressants in the acute treatment of major depression in children and adolescents “does not seem to offer a clear advantage” for these young patients, Dr. Jon Jureidini wrote in an accompanying editorial (Lancet. 2016 Jun 8. doi: 10.1016/S0140-6736[16]30385-2).
For clinicians, the implications are that every decision about whether and what to prescribe requires a calculation on the part of the clinician that is both complex and intuitive, he wrote.
“With research evidence as an important part of that calculation, we now know that we need to make a conscious correction for favorable misrepresentation of outcomes in published and unpublished study reports,” Dr. Jureidini wrote. “Only if the discounted benefit outweighs the boosted harm should the treatment be prescribed. For antidepressants in adolescents, this equation will rarely favor prescribing; in younger children, almost never.”
Dr. Jureidini is a research leader for the Robinson Research Institute at the University of Adelaide in North Adelaide, Australia.
Most antidepressants prescribed to children and adolescents with acute major depression might not be nearly as effective as they are believed to be – and one might be harmful, according to a retrospective review published June 8 and including more than 5,000 participants.
“Our analysis found robust evidence to suggest a significantly increased risk for suicidality for young people given venlafaxine,” wrote Dr. Andrea Cipriani, Xinyu Zhou, Ph.D., and their colleagues. “Unfortunately, due to the absence of reliable data on suicidality for many antidepressants, it was not possible to comprehensively assess the risk of suicidality for all drugs.”
The review looked at 34 double-blind, randomized, controlled trials investigating at least one of 14 major drugs typically prescribed as antidepressants for pediatric patients. In addition to venlafaxine, the researchers looked at amitriptyline, citalopram, clomipramine, desipramine, duloxetine, escitalopram, fluoxetine, imipramine, mirtazapine, nefazodone, nortriptyline, paroxetine, and sertraline (Lancet. 2016 Jun 8. doi: 10.1016/S0140-6736[16]30385-3).
All trials were published before May 31, 2015, and were found in the PubMed, Cochrane Library, Web of Science, Embase, CINAHL, PsycINFO, and LiLACS databases, as well as regulatory agencies’ websites, and international registers for published and unpublished trials. Trials either compared one or more drugs against one another, or one or more drugs against a placebo.
Fluoxetine was the only drug found to be significantly more effective than placebo, and it also was found to be significantly more effective than nortriptyline. In addition, fluoxetine proved better tolerated than imipramine and duloxetine. “However, the clinical interpretation of these findings is limited not only by the uncertainty around these estimates, but also by the potential bias due to selective reporting and the small number of trials,” said Dr. Cipriani of the University of Oxford (England) and Dr. Zhou of the First Affiliated Hospital of Chongqing Medical University in China.
Regardless of which treatment clinicians choose, children and adolescents prescribed antidepressants should be monitored closely. Clinical guidelines for young people with major depression recommend psychotherapy, particularly cognitive-behavioral therapy or interpersonal therapy as first-line interventions, and “fluoxetine should be considered only for those patients with moderate to severe depression who do not have access to psychotherapy or have not responded to nonpharmacological interventions,” the researchers said.
The study was funded by the National Basic Research Program of China. The authors did not report any relevant financial disclosures.
Most antidepressants prescribed to children and adolescents with acute major depression might not be nearly as effective as they are believed to be – and one might be harmful, according to a retrospective review published June 8 and including more than 5,000 participants.
“Our analysis found robust evidence to suggest a significantly increased risk for suicidality for young people given venlafaxine,” wrote Dr. Andrea Cipriani, Xinyu Zhou, Ph.D., and their colleagues. “Unfortunately, due to the absence of reliable data on suicidality for many antidepressants, it was not possible to comprehensively assess the risk of suicidality for all drugs.”
The review looked at 34 double-blind, randomized, controlled trials investigating at least one of 14 major drugs typically prescribed as antidepressants for pediatric patients. In addition to venlafaxine, the researchers looked at amitriptyline, citalopram, clomipramine, desipramine, duloxetine, escitalopram, fluoxetine, imipramine, mirtazapine, nefazodone, nortriptyline, paroxetine, and sertraline (Lancet. 2016 Jun 8. doi: 10.1016/S0140-6736[16]30385-3).
All trials were published before May 31, 2015, and were found in the PubMed, Cochrane Library, Web of Science, Embase, CINAHL, PsycINFO, and LiLACS databases, as well as regulatory agencies’ websites, and international registers for published and unpublished trials. Trials either compared one or more drugs against one another, or one or more drugs against a placebo.
Fluoxetine was the only drug found to be significantly more effective than placebo, and it also was found to be significantly more effective than nortriptyline. In addition, fluoxetine proved better tolerated than imipramine and duloxetine. “However, the clinical interpretation of these findings is limited not only by the uncertainty around these estimates, but also by the potential bias due to selective reporting and the small number of trials,” said Dr. Cipriani of the University of Oxford (England) and Dr. Zhou of the First Affiliated Hospital of Chongqing Medical University in China.
Regardless of which treatment clinicians choose, children and adolescents prescribed antidepressants should be monitored closely. Clinical guidelines for young people with major depression recommend psychotherapy, particularly cognitive-behavioral therapy or interpersonal therapy as first-line interventions, and “fluoxetine should be considered only for those patients with moderate to severe depression who do not have access to psychotherapy or have not responded to nonpharmacological interventions,” the researchers said.
The study was funded by the National Basic Research Program of China. The authors did not report any relevant financial disclosures.
FROM THE LANCET
Study finds most antidepressants ineffective or harmful in children, adolescents
Most antidepressants prescribed to children and adolescents with acute major depression might not be nearly as effective as they are believed to be – and one might be harmful, according to a retrospective review published June 8 and including more than 5,000 participants.
“Our analysis found robust evidence to suggest a significantly increased risk for suicidality for young people given venlafaxine,” wrote Dr. Andrea Cipriani, Xinyu Zhou, Ph.D., and their colleagues. “Unfortunately, due to the absence of reliable data on suicidality for many antidepressants, it was not possible to comprehensively assess the risk of suicidality for all drugs.”
The review looked at 34 double-blind, randomized, controlled trials investigating at least one of 14 major drugs typically prescribed as antidepressants for pediatric patients. In addition to venlafaxine, the researchers looked at amitriptyline, citalopram, clomipramine, desipramine, duloxetine, escitalopram, fluoxetine, imipramine, mirtazapine, nefazodone, nortriptyline, paroxetine, and sertraline (Lancet. 2016 Jun 8. doi: 10.1016/S0140-6736[16]30385-3).
All trials were published before May 31, 2015, and were found in the PubMed, Cochrane Library, Web of Science, Embase, CINAHL, PsycINFO, and LiLACS databases, as well as regulatory agencies’ websites, and international registers for published and unpublished trials. Trials either compared one or more drugs against one another, or one or more drugs against a placebo.
Fluoxetine was the only drug found to be significantly more effective than placebo, and it also was found to be significantly more effective than nortriptyline. In addition, fluoxetine proved better tolerated than imipramine and duloxetine. “However, the clinical interpretation of these findings is limited not only by the uncertainty around these estimates, but also by the potential bias due to selective reporting and the small number of trials,” said Dr. Cipriani of the University of Oxford (England) and Dr. Zhou of the First Affiliated Hospital of Chongqing Medical University in China.
Regardless of which treatment clinicians choose, children and adolescents prescribed antidepressants should be monitored closely. Clinical guidelines for young people with major depression recommend psychotherapy, particularly cognitive-behavioral therapy or interpersonal therapy as first-line interventions, and “fluoxetine should be considered only for those patients with moderate to severe depression who do not have access to psychotherapy or have not responded to nonpharmacological interventions,” the researchers said.
The study was funded by the National Basic Research Program of China. The authors did not report any relevant financial disclosures.
The study by Dr. Andrea Cipriani, Xinyu Zhou, Ph.D., and their colleagues has “disturbing implications for clinical practice” in that it concludes that the risk-benefit profile of antidepressants in the acute treatment of major depression in children and adolescents “does not seem to offer a clear advantage” for these young patients, Dr. Jon Jureidini wrote in an accompanying editorial (Lancet. 2016 Jun 8. doi: 10.1016/S0140-6736[16]30385-2).
For clinicians, the implications are that every decision about whether and what to prescribe requires a calculation on the part of the clinician that is both complex and intuitive, he wrote.
“With research evidence as an important part of that calculation, we now know that we need to make a conscious correction for favorable misrepresentation of outcomes in published and unpublished study reports,” Dr. Jureidini wrote. “Only if the discounted benefit outweighs the boosted harm should the treatment be prescribed. For antidepressants in adolescents, this equation will rarely favor prescribing; in younger children, almost never.”
Dr. Jureidini is a research leader for the Robinson Research Institute at the University of Adelaide in North Adelaide, Australia.
The study by Dr. Andrea Cipriani, Xinyu Zhou, Ph.D., and their colleagues has “disturbing implications for clinical practice” in that it concludes that the risk-benefit profile of antidepressants in the acute treatment of major depression in children and adolescents “does not seem to offer a clear advantage” for these young patients, Dr. Jon Jureidini wrote in an accompanying editorial (Lancet. 2016 Jun 8. doi: 10.1016/S0140-6736[16]30385-2).
For clinicians, the implications are that every decision about whether and what to prescribe requires a calculation on the part of the clinician that is both complex and intuitive, he wrote.
“With research evidence as an important part of that calculation, we now know that we need to make a conscious correction for favorable misrepresentation of outcomes in published and unpublished study reports,” Dr. Jureidini wrote. “Only if the discounted benefit outweighs the boosted harm should the treatment be prescribed. For antidepressants in adolescents, this equation will rarely favor prescribing; in younger children, almost never.”
Dr. Jureidini is a research leader for the Robinson Research Institute at the University of Adelaide in North Adelaide, Australia.
The study by Dr. Andrea Cipriani, Xinyu Zhou, Ph.D., and their colleagues has “disturbing implications for clinical practice” in that it concludes that the risk-benefit profile of antidepressants in the acute treatment of major depression in children and adolescents “does not seem to offer a clear advantage” for these young patients, Dr. Jon Jureidini wrote in an accompanying editorial (Lancet. 2016 Jun 8. doi: 10.1016/S0140-6736[16]30385-2).
For clinicians, the implications are that every decision about whether and what to prescribe requires a calculation on the part of the clinician that is both complex and intuitive, he wrote.
“With research evidence as an important part of that calculation, we now know that we need to make a conscious correction for favorable misrepresentation of outcomes in published and unpublished study reports,” Dr. Jureidini wrote. “Only if the discounted benefit outweighs the boosted harm should the treatment be prescribed. For antidepressants in adolescents, this equation will rarely favor prescribing; in younger children, almost never.”
Dr. Jureidini is a research leader for the Robinson Research Institute at the University of Adelaide in North Adelaide, Australia.
Most antidepressants prescribed to children and adolescents with acute major depression might not be nearly as effective as they are believed to be – and one might be harmful, according to a retrospective review published June 8 and including more than 5,000 participants.
“Our analysis found robust evidence to suggest a significantly increased risk for suicidality for young people given venlafaxine,” wrote Dr. Andrea Cipriani, Xinyu Zhou, Ph.D., and their colleagues. “Unfortunately, due to the absence of reliable data on suicidality for many antidepressants, it was not possible to comprehensively assess the risk of suicidality for all drugs.”
The review looked at 34 double-blind, randomized, controlled trials investigating at least one of 14 major drugs typically prescribed as antidepressants for pediatric patients. In addition to venlafaxine, the researchers looked at amitriptyline, citalopram, clomipramine, desipramine, duloxetine, escitalopram, fluoxetine, imipramine, mirtazapine, nefazodone, nortriptyline, paroxetine, and sertraline (Lancet. 2016 Jun 8. doi: 10.1016/S0140-6736[16]30385-3).
All trials were published before May 31, 2015, and were found in the PubMed, Cochrane Library, Web of Science, Embase, CINAHL, PsycINFO, and LiLACS databases, as well as regulatory agencies’ websites, and international registers for published and unpublished trials. Trials either compared one or more drugs against one another, or one or more drugs against a placebo.
Fluoxetine was the only drug found to be significantly more effective than placebo, and it also was found to be significantly more effective than nortriptyline. In addition, fluoxetine proved better tolerated than imipramine and duloxetine. “However, the clinical interpretation of these findings is limited not only by the uncertainty around these estimates, but also by the potential bias due to selective reporting and the small number of trials,” said Dr. Cipriani of the University of Oxford (England) and Dr. Zhou of the First Affiliated Hospital of Chongqing Medical University in China.
Regardless of which treatment clinicians choose, children and adolescents prescribed antidepressants should be monitored closely. Clinical guidelines for young people with major depression recommend psychotherapy, particularly cognitive-behavioral therapy or interpersonal therapy as first-line interventions, and “fluoxetine should be considered only for those patients with moderate to severe depression who do not have access to psychotherapy or have not responded to nonpharmacological interventions,” the researchers said.
The study was funded by the National Basic Research Program of China. The authors did not report any relevant financial disclosures.
Most antidepressants prescribed to children and adolescents with acute major depression might not be nearly as effective as they are believed to be – and one might be harmful, according to a retrospective review published June 8 and including more than 5,000 participants.
“Our analysis found robust evidence to suggest a significantly increased risk for suicidality for young people given venlafaxine,” wrote Dr. Andrea Cipriani, Xinyu Zhou, Ph.D., and their colleagues. “Unfortunately, due to the absence of reliable data on suicidality for many antidepressants, it was not possible to comprehensively assess the risk of suicidality for all drugs.”
The review looked at 34 double-blind, randomized, controlled trials investigating at least one of 14 major drugs typically prescribed as antidepressants for pediatric patients. In addition to venlafaxine, the researchers looked at amitriptyline, citalopram, clomipramine, desipramine, duloxetine, escitalopram, fluoxetine, imipramine, mirtazapine, nefazodone, nortriptyline, paroxetine, and sertraline (Lancet. 2016 Jun 8. doi: 10.1016/S0140-6736[16]30385-3).
All trials were published before May 31, 2015, and were found in the PubMed, Cochrane Library, Web of Science, Embase, CINAHL, PsycINFO, and LiLACS databases, as well as regulatory agencies’ websites, and international registers for published and unpublished trials. Trials either compared one or more drugs against one another, or one or more drugs against a placebo.
Fluoxetine was the only drug found to be significantly more effective than placebo, and it also was found to be significantly more effective than nortriptyline. In addition, fluoxetine proved better tolerated than imipramine and duloxetine. “However, the clinical interpretation of these findings is limited not only by the uncertainty around these estimates, but also by the potential bias due to selective reporting and the small number of trials,” said Dr. Cipriani of the University of Oxford (England) and Dr. Zhou of the First Affiliated Hospital of Chongqing Medical University in China.
Regardless of which treatment clinicians choose, children and adolescents prescribed antidepressants should be monitored closely. Clinical guidelines for young people with major depression recommend psychotherapy, particularly cognitive-behavioral therapy or interpersonal therapy as first-line interventions, and “fluoxetine should be considered only for those patients with moderate to severe depression who do not have access to psychotherapy or have not responded to nonpharmacological interventions,” the researchers said.
The study was funded by the National Basic Research Program of China. The authors did not report any relevant financial disclosures.
FROM THE LANCET
Key clinical point: The majority of antidepressant drugs prescribed to children and adolescents are ineffective, with only fluoxetine showing significant improvement.
Major finding: Fluoxetine showed the most promising results among all 14 antidepressants studied.
Data source: Retrospective review of 34 studies with 5,260 participants investigating 14 distinct drugs used to treat depression in children and adolescents.
Disclosures: The study was funded by the National Basic Research Program of China. Authors did not report any relevant financial disclosures.
Building owners, managers must do more to prevent Legionnaires’ disease
Building owners, managers, and administrators of hospitals and other health care facilities around the country are being urged to shore up their water system management facilities to prevent further outbreaks of Legionnaires’ disease, which is the focus of the Center for Disease Control and Prevention’s latest Vital Signs report.
“Almost all Legionnaires’ disease outbreaks are preventable with improvements in water system management,” explained CDC Director Tom Frieden, adding that “At the end of the day, building owners and managers need to take steps to reduce the risk of Legionnaires’ disease [and] work together to reduce this risk and limit the number of people exposed, infected, and hospitalized or, potentially, fatally infected.”
For the report, the CDC investigated 27 outbreaks of Legionnaires’ disease in the United States from 2000 through 2014, which involved a total of 415 cases and 65 fatalities. In each outbreak analysis, the location, source of exposure, and problems with environmental controls of Legionella – the bacterium that causes the disease – were evaluated.
Hotels and resorts accounted for 44% of all outbreaks over the 15-year period, followed by long-term care facilities (19%) and hospitals (15%). However, outbreaks at the latter two location types accounted for 85% of all deaths, while outbreaks at hotels and resorts accounted for only 6%. Potable water was the most common direct cause of Legionella infections, followed by water from cooling towers, hot tubs, industrial equipment, and decorative fountains.
Additionally, 23 of the investigations yielded enough information to determine the exact cause of the outbreak, all of which were caused by at least one of 4 issues. The first was process failures, such as not having a proper water system management program in place to handle Legionella; this was found in two-thirds of the outbreaks. The second major cause was human error, such as not replacing filters or tubing as recommended by manufacturers, which was a cause in half of the outbreaks. The third was equipment breakdown, which was found in one-third of the outbreaks. Finally, reasons external to the buildings themselves – such as water main breaks or disruptions caused by nearby construction – factored into one-third of the outbreaks.
“Large, recent outbreaks of Legionnaires’ disease in New York City and Flint, Michigan, have brought attention to the disease and highlight the need for us to understand why these outbreaks happen and how best to prevent them, [which is] why this Vital Signs is targeted to a specific audience that we in public health don’t talk [to] often enough: building owners and managers,” Dr. Frieden said. “It’s not a traditional public health audience, [but] they are the key to environmental controls in buildings that we live in, get our health care in, and work in everyday.”
To that end, Dr. Frieden announced the release of a new CDC toolkit entitled “Developing a Water Management Program to Reduce Legionella Growth & Spread in Buildings: A Practical Guide to Implementing Industry Standards,” which building owners, managers, and administrators can turn to for guidance on how to implement effective water system management protocols in their buildings.
Legionnaires’ disease is a serious lung infection caused by inhalation of the bacteria Legionella, which can be found in water and inhaled as airborne mist. Elderly individuals, as well as those with suppressed immune systems because of underlying illnesses, are at a heightened risk for Legionnaires’ disease, which would explain the higher death rates observed at hospitals and long-term care facilities. Dr. Frieden stated that outbreaks and cases of Legionnaires’ disease are on the rise nationally, with about 5,000 infections and 20 outbreaks occurring annually; roughly 10% of infections result in death.
The uptick in recent cases is likely because of “the aging of the population, the increase in chronic illness, [an] increase in immunosuppression through use of medication to treat a variety of conditions [and] an aging plumbing infrastructure and that makes maintenance all the more challenging,” according to Dr. Frieden. “It is also possible that increased use of diagnostic tests and more reliable reporting are contributing to some of the rising rates.”
Building owners, managers, and administrators of hospitals and other health care facilities around the country are being urged to shore up their water system management facilities to prevent further outbreaks of Legionnaires’ disease, which is the focus of the Center for Disease Control and Prevention’s latest Vital Signs report.
“Almost all Legionnaires’ disease outbreaks are preventable with improvements in water system management,” explained CDC Director Tom Frieden, adding that “At the end of the day, building owners and managers need to take steps to reduce the risk of Legionnaires’ disease [and] work together to reduce this risk and limit the number of people exposed, infected, and hospitalized or, potentially, fatally infected.”
For the report, the CDC investigated 27 outbreaks of Legionnaires’ disease in the United States from 2000 through 2014, which involved a total of 415 cases and 65 fatalities. In each outbreak analysis, the location, source of exposure, and problems with environmental controls of Legionella – the bacterium that causes the disease – were evaluated.
Hotels and resorts accounted for 44% of all outbreaks over the 15-year period, followed by long-term care facilities (19%) and hospitals (15%). However, outbreaks at the latter two location types accounted for 85% of all deaths, while outbreaks at hotels and resorts accounted for only 6%. Potable water was the most common direct cause of Legionella infections, followed by water from cooling towers, hot tubs, industrial equipment, and decorative fountains.
Additionally, 23 of the investigations yielded enough information to determine the exact cause of the outbreak, all of which were caused by at least one of 4 issues. The first was process failures, such as not having a proper water system management program in place to handle Legionella; this was found in two-thirds of the outbreaks. The second major cause was human error, such as not replacing filters or tubing as recommended by manufacturers, which was a cause in half of the outbreaks. The third was equipment breakdown, which was found in one-third of the outbreaks. Finally, reasons external to the buildings themselves – such as water main breaks or disruptions caused by nearby construction – factored into one-third of the outbreaks.
“Large, recent outbreaks of Legionnaires’ disease in New York City and Flint, Michigan, have brought attention to the disease and highlight the need for us to understand why these outbreaks happen and how best to prevent them, [which is] why this Vital Signs is targeted to a specific audience that we in public health don’t talk [to] often enough: building owners and managers,” Dr. Frieden said. “It’s not a traditional public health audience, [but] they are the key to environmental controls in buildings that we live in, get our health care in, and work in everyday.”
To that end, Dr. Frieden announced the release of a new CDC toolkit entitled “Developing a Water Management Program to Reduce Legionella Growth & Spread in Buildings: A Practical Guide to Implementing Industry Standards,” which building owners, managers, and administrators can turn to for guidance on how to implement effective water system management protocols in their buildings.
Legionnaires’ disease is a serious lung infection caused by inhalation of the bacteria Legionella, which can be found in water and inhaled as airborne mist. Elderly individuals, as well as those with suppressed immune systems because of underlying illnesses, are at a heightened risk for Legionnaires’ disease, which would explain the higher death rates observed at hospitals and long-term care facilities. Dr. Frieden stated that outbreaks and cases of Legionnaires’ disease are on the rise nationally, with about 5,000 infections and 20 outbreaks occurring annually; roughly 10% of infections result in death.
The uptick in recent cases is likely because of “the aging of the population, the increase in chronic illness, [an] increase in immunosuppression through use of medication to treat a variety of conditions [and] an aging plumbing infrastructure and that makes maintenance all the more challenging,” according to Dr. Frieden. “It is also possible that increased use of diagnostic tests and more reliable reporting are contributing to some of the rising rates.”
Building owners, managers, and administrators of hospitals and other health care facilities around the country are being urged to shore up their water system management facilities to prevent further outbreaks of Legionnaires’ disease, which is the focus of the Center for Disease Control and Prevention’s latest Vital Signs report.
“Almost all Legionnaires’ disease outbreaks are preventable with improvements in water system management,” explained CDC Director Tom Frieden, adding that “At the end of the day, building owners and managers need to take steps to reduce the risk of Legionnaires’ disease [and] work together to reduce this risk and limit the number of people exposed, infected, and hospitalized or, potentially, fatally infected.”
For the report, the CDC investigated 27 outbreaks of Legionnaires’ disease in the United States from 2000 through 2014, which involved a total of 415 cases and 65 fatalities. In each outbreak analysis, the location, source of exposure, and problems with environmental controls of Legionella – the bacterium that causes the disease – were evaluated.
Hotels and resorts accounted for 44% of all outbreaks over the 15-year period, followed by long-term care facilities (19%) and hospitals (15%). However, outbreaks at the latter two location types accounted for 85% of all deaths, while outbreaks at hotels and resorts accounted for only 6%. Potable water was the most common direct cause of Legionella infections, followed by water from cooling towers, hot tubs, industrial equipment, and decorative fountains.
Additionally, 23 of the investigations yielded enough information to determine the exact cause of the outbreak, all of which were caused by at least one of 4 issues. The first was process failures, such as not having a proper water system management program in place to handle Legionella; this was found in two-thirds of the outbreaks. The second major cause was human error, such as not replacing filters or tubing as recommended by manufacturers, which was a cause in half of the outbreaks. The third was equipment breakdown, which was found in one-third of the outbreaks. Finally, reasons external to the buildings themselves – such as water main breaks or disruptions caused by nearby construction – factored into one-third of the outbreaks.
“Large, recent outbreaks of Legionnaires’ disease in New York City and Flint, Michigan, have brought attention to the disease and highlight the need for us to understand why these outbreaks happen and how best to prevent them, [which is] why this Vital Signs is targeted to a specific audience that we in public health don’t talk [to] often enough: building owners and managers,” Dr. Frieden said. “It’s not a traditional public health audience, [but] they are the key to environmental controls in buildings that we live in, get our health care in, and work in everyday.”
To that end, Dr. Frieden announced the release of a new CDC toolkit entitled “Developing a Water Management Program to Reduce Legionella Growth & Spread in Buildings: A Practical Guide to Implementing Industry Standards,” which building owners, managers, and administrators can turn to for guidance on how to implement effective water system management protocols in their buildings.
Legionnaires’ disease is a serious lung infection caused by inhalation of the bacteria Legionella, which can be found in water and inhaled as airborne mist. Elderly individuals, as well as those with suppressed immune systems because of underlying illnesses, are at a heightened risk for Legionnaires’ disease, which would explain the higher death rates observed at hospitals and long-term care facilities. Dr. Frieden stated that outbreaks and cases of Legionnaires’ disease are on the rise nationally, with about 5,000 infections and 20 outbreaks occurring annually; roughly 10% of infections result in death.
The uptick in recent cases is likely because of “the aging of the population, the increase in chronic illness, [an] increase in immunosuppression through use of medication to treat a variety of conditions [and] an aging plumbing infrastructure and that makes maintenance all the more challenging,” according to Dr. Frieden. “It is also possible that increased use of diagnostic tests and more reliable reporting are contributing to some of the rising rates.”
FROM CDC VITAL SIGNS
USPSTF Updates Recommendations for Syphilis Screening
Because syphilis infection rates have increased over the last decade, health care providers should screen patients for the disease more frequently to detect and treat it earlier and more effectively, particular in high-risk subgroups.
The United States Preventive Services Task Force made this recommendation in a JAMA article published online, the first update to the syphilis screening recommendation since 2004 (JAMA. 2016;315[21]:2321-2327. doi: 10.1001/jama.2016.5824).
“Individuals with previous syphilis infection, an infected sexual partner, current HIV infection, or more than 4 sex partners in the preceding year are at increased risk of acquiring syphilis,” the report states. “Higher prevalence rates are associated with sociodemographic groups including men who have sex with men (MSM), young adult men, sex workers, adults in correctional facilities, and individuals who are black or live in metropolitan areas in the southern and western United States. Men who have sex with men accounted for 61% of all primary and secondary syphilis cases reported in 2014.”
A systematic review of trials and studies investigating syphilis screening effectiveness, testing accuracy, and screening risks in nonpregnant adults and adolescents – all of which were included in the Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews through October 2015, along with the Ovid MEDLINE database from January 2004 through October 2015 – was undertaken to update the 2004 recommendations.
Studies with clinical application were emphasized more heavily, along with those that focused on target populations of asymptomatic, sexually active, adolescent and older men and women. In total, 2,000 English-language titles and abstracts were reviewed, from which 448 full-text studies underwent further review for inclusion. From these, only nine were ultimately selected for inclusion, four of which were observational studies, three of which were observational studies of test accuracy, and two of which were observational studies of testing sequences.
The first four studies – Bissessor et al. 2010, Cohen et al. 2005, Zou et al. 2013, and Bissessor et al. 2011 – were included to answer the investigation’s key question 1 (KQ1) – “What is the effectiveness of screening for syphilis in reducing complications of the disease and transmission or acquisition of other sexually transmitted infections in asymptomatic, nonpregnant, sexually active adults and adolescents? What is the effectiveness of specific screening intervals and screening among population subgroups?”
The remaining five studies – Wong et al. 2011, Tsang et al. 2007, Juárez-Figueroa et al. 2007, Mishra et al. 2011, and Binnicker et al. 2012 – were aimed at KQ3, asking “What is the accuracy of currently used screening tests and strategies (e.g., sequence of tests) for detecting syphilis infection?”
Regarding KQ1, all four studies found that MSM and HIV-infected men who were screened routinely every 3 months had a significantly higher rate of syphilis detection compared with those who were screened annually. The studies, all of which came from Europe and Australia, detected syphilis early in those who were screened every 3 months in 8.1% of HIV-infected MSM versus 3.1% of those who screened annually (P = .001), detected early-latent syphilis in 1.7% of routine screeners versus 0.4% of annual screeners (P = .008), and detected early syphilis in higher-risk MSM in 53% of regular screeners versus just 16% of annual screeners (P = .001). The cohort comprised 6,789 asymptomatic MSM.
In addition, newly acquired syphilis in HIV-positive MSM was found in 7.3 cases per 1,000 patient-years (95% CI, 5.2-9.9) for those who were regularly screened, versus 2.8 cases per 1,000 patient-years (95% CI, 1.8-4.0) for those who were screened annually.
Of the five studies investigating KQ3, three (Wong, Tsang, Juárez-Figueroa) were observational studies of treponemal and nontreponemal tests to detect syphilis. Results indicated that both tests had a sensitivity of 85.3%-98%, and specificity of 91%-100%. However, the remaining two studies (Mishra, Binnicker) used reverse screening algorithms to confirm positive test results from treponemal and nontreponemal, finding higher rates of false positives in the rapid plasma reagin than in the traditional enzyme analyses: 0.6% vs. 0.0% in one study (P = .03) and 0.26% vs. 0.13% in the other.
“Additional research on syphilis screening is needed to directly compare the effectiveness of different screening strategies for identifying individuals at increased risk of infection, co-testing for concurrent STIs, and different screening intervals among various patient populations,” wrote the USPSTF investigators, adding that research also is needed on evaluating the instruments used in risk assessment, as well as diagnostic accuracy.
Furthermore, while the data from these studies have shed light on syphilis testing and screening, several other important components of disease treatment and prevention – namely, “the effectiveness of screening in reducing syphilis complications and transmission, effectiveness of risk assessment methods, and harms related to screening” – were not adequately addressed.
This study was funded by the Agency for Healthcare Research and Quality, “under a contract to support the USPSTF.” The authors did not report any relevant financial disclosures.
As the 1990s ended, syphilis was on the decline. At least in part due to safer sexual behaviors prompted by the AIDS epidemic, the rate of incident syphilis declined to fewer than 4 cases per 100,000 by the year 2000, a historic nadir. Now, in 2016, hopes for eradication have long since faded, as have many of the gains realized by the effort. Rates of syphilis have trended steadily upward since 2000, and the CDC’s syphilis elimination efforts officially ended as of December 2013.
The good news is that fixing what has gone wrong does not require huge capital investment, breakthrough technological advances, or massive restructuring of our health care system. Improvements are at hand and require mostly focus and commitment on the part of the health care community. First, awareness of the problem needs to be increased, particularly in clinical settings where patients at higher risk for syphilis are being followed up. These high-risk populations include MSM, HIV-infected persons, and younger sexually active persons, particularly persons of color and those from socioeconomically disadvantaged populations. The syphilis demographic overlaps considerably with the HIV demographic. For example, in 2014, half of all MSM diagnosed with syphilis were also coinfected with HIV. Younger men (aged 20-29 years) have a prevalence rate nearly 3 times that of the national average for men, and persons of color are particularly at risk, with black individuals disproportionately affected in the United States. Rates of primary and secondary syphilis were 18.9 cases per 100000 in blacks compared with 3.5 per 100000 in whites. Rates in other ethnic groups (aside from Asians, whose rates were lowest of all) were intermediate between blacks and whites.
Although imperfect, serologic syphilis screening is highly sensitive and specific in high-prevalence populations, is inexpensive and technically simple, and has minimal potential for harm. These factors argue for much more widespread and comprehensive screening of groups at high risk for syphilis. Because treatment of early syphilis is also highly effective, identifying untreated infected persons by means of the recommended screening strategy has great potential for both eliminating the consequences of later-stage infection and substantially reducing transmission from those with early infection.
Dr. Meredith E. Clement is with the division of infectious diseases at Duke University, Durham, N.C. Dr. Charles B. Hicks is with the department of medicine at the University of California, San Diego. Dr Hicks reported ties with Bristol-Myers Squibb, Gilead Sciences, Merck, Janssen Virology, ViiV Healthcare, AstraZeneca, and UpToDate. He served as associate editor and author for NEJM Journal Watch. Dr. Clement has received royalties from UpToDate. These comments were excerpted from the editorial accompanying the study (JAMA. 2016;315[21]:2281-3).
As the 1990s ended, syphilis was on the decline. At least in part due to safer sexual behaviors prompted by the AIDS epidemic, the rate of incident syphilis declined to fewer than 4 cases per 100,000 by the year 2000, a historic nadir. Now, in 2016, hopes for eradication have long since faded, as have many of the gains realized by the effort. Rates of syphilis have trended steadily upward since 2000, and the CDC’s syphilis elimination efforts officially ended as of December 2013.
The good news is that fixing what has gone wrong does not require huge capital investment, breakthrough technological advances, or massive restructuring of our health care system. Improvements are at hand and require mostly focus and commitment on the part of the health care community. First, awareness of the problem needs to be increased, particularly in clinical settings where patients at higher risk for syphilis are being followed up. These high-risk populations include MSM, HIV-infected persons, and younger sexually active persons, particularly persons of color and those from socioeconomically disadvantaged populations. The syphilis demographic overlaps considerably with the HIV demographic. For example, in 2014, half of all MSM diagnosed with syphilis were also coinfected with HIV. Younger men (aged 20-29 years) have a prevalence rate nearly 3 times that of the national average for men, and persons of color are particularly at risk, with black individuals disproportionately affected in the United States. Rates of primary and secondary syphilis were 18.9 cases per 100000 in blacks compared with 3.5 per 100000 in whites. Rates in other ethnic groups (aside from Asians, whose rates were lowest of all) were intermediate between blacks and whites.
Although imperfect, serologic syphilis screening is highly sensitive and specific in high-prevalence populations, is inexpensive and technically simple, and has minimal potential for harm. These factors argue for much more widespread and comprehensive screening of groups at high risk for syphilis. Because treatment of early syphilis is also highly effective, identifying untreated infected persons by means of the recommended screening strategy has great potential for both eliminating the consequences of later-stage infection and substantially reducing transmission from those with early infection.
Dr. Meredith E. Clement is with the division of infectious diseases at Duke University, Durham, N.C. Dr. Charles B. Hicks is with the department of medicine at the University of California, San Diego. Dr Hicks reported ties with Bristol-Myers Squibb, Gilead Sciences, Merck, Janssen Virology, ViiV Healthcare, AstraZeneca, and UpToDate. He served as associate editor and author for NEJM Journal Watch. Dr. Clement has received royalties from UpToDate. These comments were excerpted from the editorial accompanying the study (JAMA. 2016;315[21]:2281-3).
As the 1990s ended, syphilis was on the decline. At least in part due to safer sexual behaviors prompted by the AIDS epidemic, the rate of incident syphilis declined to fewer than 4 cases per 100,000 by the year 2000, a historic nadir. Now, in 2016, hopes for eradication have long since faded, as have many of the gains realized by the effort. Rates of syphilis have trended steadily upward since 2000, and the CDC’s syphilis elimination efforts officially ended as of December 2013.
The good news is that fixing what has gone wrong does not require huge capital investment, breakthrough technological advances, or massive restructuring of our health care system. Improvements are at hand and require mostly focus and commitment on the part of the health care community. First, awareness of the problem needs to be increased, particularly in clinical settings where patients at higher risk for syphilis are being followed up. These high-risk populations include MSM, HIV-infected persons, and younger sexually active persons, particularly persons of color and those from socioeconomically disadvantaged populations. The syphilis demographic overlaps considerably with the HIV demographic. For example, in 2014, half of all MSM diagnosed with syphilis were also coinfected with HIV. Younger men (aged 20-29 years) have a prevalence rate nearly 3 times that of the national average for men, and persons of color are particularly at risk, with black individuals disproportionately affected in the United States. Rates of primary and secondary syphilis were 18.9 cases per 100000 in blacks compared with 3.5 per 100000 in whites. Rates in other ethnic groups (aside from Asians, whose rates were lowest of all) were intermediate between blacks and whites.
Although imperfect, serologic syphilis screening is highly sensitive and specific in high-prevalence populations, is inexpensive and technically simple, and has minimal potential for harm. These factors argue for much more widespread and comprehensive screening of groups at high risk for syphilis. Because treatment of early syphilis is also highly effective, identifying untreated infected persons by means of the recommended screening strategy has great potential for both eliminating the consequences of later-stage infection and substantially reducing transmission from those with early infection.
Dr. Meredith E. Clement is with the division of infectious diseases at Duke University, Durham, N.C. Dr. Charles B. Hicks is with the department of medicine at the University of California, San Diego. Dr Hicks reported ties with Bristol-Myers Squibb, Gilead Sciences, Merck, Janssen Virology, ViiV Healthcare, AstraZeneca, and UpToDate. He served as associate editor and author for NEJM Journal Watch. Dr. Clement has received royalties from UpToDate. These comments were excerpted from the editorial accompanying the study (JAMA. 2016;315[21]:2281-3).
Because syphilis infection rates have increased over the last decade, health care providers should screen patients for the disease more frequently to detect and treat it earlier and more effectively, particular in high-risk subgroups.
The United States Preventive Services Task Force made this recommendation in a JAMA article published online, the first update to the syphilis screening recommendation since 2004 (JAMA. 2016;315[21]:2321-2327. doi: 10.1001/jama.2016.5824).
“Individuals with previous syphilis infection, an infected sexual partner, current HIV infection, or more than 4 sex partners in the preceding year are at increased risk of acquiring syphilis,” the report states. “Higher prevalence rates are associated with sociodemographic groups including men who have sex with men (MSM), young adult men, sex workers, adults in correctional facilities, and individuals who are black or live in metropolitan areas in the southern and western United States. Men who have sex with men accounted for 61% of all primary and secondary syphilis cases reported in 2014.”
A systematic review of trials and studies investigating syphilis screening effectiveness, testing accuracy, and screening risks in nonpregnant adults and adolescents – all of which were included in the Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews through October 2015, along with the Ovid MEDLINE database from January 2004 through October 2015 – was undertaken to update the 2004 recommendations.
Studies with clinical application were emphasized more heavily, along with those that focused on target populations of asymptomatic, sexually active, adolescent and older men and women. In total, 2,000 English-language titles and abstracts were reviewed, from which 448 full-text studies underwent further review for inclusion. From these, only nine were ultimately selected for inclusion, four of which were observational studies, three of which were observational studies of test accuracy, and two of which were observational studies of testing sequences.
The first four studies – Bissessor et al. 2010, Cohen et al. 2005, Zou et al. 2013, and Bissessor et al. 2011 – were included to answer the investigation’s key question 1 (KQ1) – “What is the effectiveness of screening for syphilis in reducing complications of the disease and transmission or acquisition of other sexually transmitted infections in asymptomatic, nonpregnant, sexually active adults and adolescents? What is the effectiveness of specific screening intervals and screening among population subgroups?”
The remaining five studies – Wong et al. 2011, Tsang et al. 2007, Juárez-Figueroa et al. 2007, Mishra et al. 2011, and Binnicker et al. 2012 – were aimed at KQ3, asking “What is the accuracy of currently used screening tests and strategies (e.g., sequence of tests) for detecting syphilis infection?”
Regarding KQ1, all four studies found that MSM and HIV-infected men who were screened routinely every 3 months had a significantly higher rate of syphilis detection compared with those who were screened annually. The studies, all of which came from Europe and Australia, detected syphilis early in those who were screened every 3 months in 8.1% of HIV-infected MSM versus 3.1% of those who screened annually (P = .001), detected early-latent syphilis in 1.7% of routine screeners versus 0.4% of annual screeners (P = .008), and detected early syphilis in higher-risk MSM in 53% of regular screeners versus just 16% of annual screeners (P = .001). The cohort comprised 6,789 asymptomatic MSM.
In addition, newly acquired syphilis in HIV-positive MSM was found in 7.3 cases per 1,000 patient-years (95% CI, 5.2-9.9) for those who were regularly screened, versus 2.8 cases per 1,000 patient-years (95% CI, 1.8-4.0) for those who were screened annually.
Of the five studies investigating KQ3, three (Wong, Tsang, Juárez-Figueroa) were observational studies of treponemal and nontreponemal tests to detect syphilis. Results indicated that both tests had a sensitivity of 85.3%-98%, and specificity of 91%-100%. However, the remaining two studies (Mishra, Binnicker) used reverse screening algorithms to confirm positive test results from treponemal and nontreponemal, finding higher rates of false positives in the rapid plasma reagin than in the traditional enzyme analyses: 0.6% vs. 0.0% in one study (P = .03) and 0.26% vs. 0.13% in the other.
“Additional research on syphilis screening is needed to directly compare the effectiveness of different screening strategies for identifying individuals at increased risk of infection, co-testing for concurrent STIs, and different screening intervals among various patient populations,” wrote the USPSTF investigators, adding that research also is needed on evaluating the instruments used in risk assessment, as well as diagnostic accuracy.
Furthermore, while the data from these studies have shed light on syphilis testing and screening, several other important components of disease treatment and prevention – namely, “the effectiveness of screening in reducing syphilis complications and transmission, effectiveness of risk assessment methods, and harms related to screening” – were not adequately addressed.
This study was funded by the Agency for Healthcare Research and Quality, “under a contract to support the USPSTF.” The authors did not report any relevant financial disclosures.
Because syphilis infection rates have increased over the last decade, health care providers should screen patients for the disease more frequently to detect and treat it earlier and more effectively, particular in high-risk subgroups.
The United States Preventive Services Task Force made this recommendation in a JAMA article published online, the first update to the syphilis screening recommendation since 2004 (JAMA. 2016;315[21]:2321-2327. doi: 10.1001/jama.2016.5824).
“Individuals with previous syphilis infection, an infected sexual partner, current HIV infection, or more than 4 sex partners in the preceding year are at increased risk of acquiring syphilis,” the report states. “Higher prevalence rates are associated with sociodemographic groups including men who have sex with men (MSM), young adult men, sex workers, adults in correctional facilities, and individuals who are black or live in metropolitan areas in the southern and western United States. Men who have sex with men accounted for 61% of all primary and secondary syphilis cases reported in 2014.”
A systematic review of trials and studies investigating syphilis screening effectiveness, testing accuracy, and screening risks in nonpregnant adults and adolescents – all of which were included in the Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews through October 2015, along with the Ovid MEDLINE database from January 2004 through October 2015 – was undertaken to update the 2004 recommendations.
Studies with clinical application were emphasized more heavily, along with those that focused on target populations of asymptomatic, sexually active, adolescent and older men and women. In total, 2,000 English-language titles and abstracts were reviewed, from which 448 full-text studies underwent further review for inclusion. From these, only nine were ultimately selected for inclusion, four of which were observational studies, three of which were observational studies of test accuracy, and two of which were observational studies of testing sequences.
The first four studies – Bissessor et al. 2010, Cohen et al. 2005, Zou et al. 2013, and Bissessor et al. 2011 – were included to answer the investigation’s key question 1 (KQ1) – “What is the effectiveness of screening for syphilis in reducing complications of the disease and transmission or acquisition of other sexually transmitted infections in asymptomatic, nonpregnant, sexually active adults and adolescents? What is the effectiveness of specific screening intervals and screening among population subgroups?”
The remaining five studies – Wong et al. 2011, Tsang et al. 2007, Juárez-Figueroa et al. 2007, Mishra et al. 2011, and Binnicker et al. 2012 – were aimed at KQ3, asking “What is the accuracy of currently used screening tests and strategies (e.g., sequence of tests) for detecting syphilis infection?”
Regarding KQ1, all four studies found that MSM and HIV-infected men who were screened routinely every 3 months had a significantly higher rate of syphilis detection compared with those who were screened annually. The studies, all of which came from Europe and Australia, detected syphilis early in those who were screened every 3 months in 8.1% of HIV-infected MSM versus 3.1% of those who screened annually (P = .001), detected early-latent syphilis in 1.7% of routine screeners versus 0.4% of annual screeners (P = .008), and detected early syphilis in higher-risk MSM in 53% of regular screeners versus just 16% of annual screeners (P = .001). The cohort comprised 6,789 asymptomatic MSM.
In addition, newly acquired syphilis in HIV-positive MSM was found in 7.3 cases per 1,000 patient-years (95% CI, 5.2-9.9) for those who were regularly screened, versus 2.8 cases per 1,000 patient-years (95% CI, 1.8-4.0) for those who were screened annually.
Of the five studies investigating KQ3, three (Wong, Tsang, Juárez-Figueroa) were observational studies of treponemal and nontreponemal tests to detect syphilis. Results indicated that both tests had a sensitivity of 85.3%-98%, and specificity of 91%-100%. However, the remaining two studies (Mishra, Binnicker) used reverse screening algorithms to confirm positive test results from treponemal and nontreponemal, finding higher rates of false positives in the rapid plasma reagin than in the traditional enzyme analyses: 0.6% vs. 0.0% in one study (P = .03) and 0.26% vs. 0.13% in the other.
“Additional research on syphilis screening is needed to directly compare the effectiveness of different screening strategies for identifying individuals at increased risk of infection, co-testing for concurrent STIs, and different screening intervals among various patient populations,” wrote the USPSTF investigators, adding that research also is needed on evaluating the instruments used in risk assessment, as well as diagnostic accuracy.
Furthermore, while the data from these studies have shed light on syphilis testing and screening, several other important components of disease treatment and prevention – namely, “the effectiveness of screening in reducing syphilis complications and transmission, effectiveness of risk assessment methods, and harms related to screening” – were not adequately addressed.
This study was funded by the Agency for Healthcare Research and Quality, “under a contract to support the USPSTF.” The authors did not report any relevant financial disclosures.
FROM JAMA