User login
FDA issues revised warning for adverse effects associated with canagliflozin
The Food and Drug Administration has issued a new warning and precaution for the type 2 diabetes drug canagliflozin, saying that risks of decreased bone density associated with the drug are more serious than previously stated.
Canagliflozin, a sodium-glucose cotransporter-2 (SGLT2) inhibitor, will now have a revised adverse reactions section on its drug label. Taking canagliflozin can significantly increase an individual’s chances of incurring bone fractures, due to the decreased bone mineral density caused by the drug. According to the FDA, these fractures can start to appear 12 weeks after starting a canagliflozin regimen, and can lead to bone mineral density loss in the hip and lower spine.
“FDA is continuing to evaluate the risk of bone fractures with other drugs in the SGLT2 inhibitor class, including dapagliflozin (Farxiga, Xigduo XR) and empagliflozin (Jardiance, Glyxambi, Synjardy), to determine if additional label changes or studies are needed,” the FDA stated, adding that all health care providers and patients are urged to contact the FDA if they experience adverse effects while taking any of these drugs.
Canagliflozin is marketed as Invokana and Invokamet by Janssen Pharmaceuticals, and was approved by the FDA in March 2013. The FDA is advising all health care professionals to carefully assess patients’ risk for developing bone fractures before prescribing the drug. Individuals who experience side effects while taking canagliflozin should submit a report through the FDA’s MedWatch program, or contact 1-800-332-1088 for more information.
The Food and Drug Administration has issued a new warning and precaution for the type 2 diabetes drug canagliflozin, saying that risks of decreased bone density associated with the drug are more serious than previously stated.
Canagliflozin, a sodium-glucose cotransporter-2 (SGLT2) inhibitor, will now have a revised adverse reactions section on its drug label. Taking canagliflozin can significantly increase an individual’s chances of incurring bone fractures, due to the decreased bone mineral density caused by the drug. According to the FDA, these fractures can start to appear 12 weeks after starting a canagliflozin regimen, and can lead to bone mineral density loss in the hip and lower spine.
“FDA is continuing to evaluate the risk of bone fractures with other drugs in the SGLT2 inhibitor class, including dapagliflozin (Farxiga, Xigduo XR) and empagliflozin (Jardiance, Glyxambi, Synjardy), to determine if additional label changes or studies are needed,” the FDA stated, adding that all health care providers and patients are urged to contact the FDA if they experience adverse effects while taking any of these drugs.
Canagliflozin is marketed as Invokana and Invokamet by Janssen Pharmaceuticals, and was approved by the FDA in March 2013. The FDA is advising all health care professionals to carefully assess patients’ risk for developing bone fractures before prescribing the drug. Individuals who experience side effects while taking canagliflozin should submit a report through the FDA’s MedWatch program, or contact 1-800-332-1088 for more information.
The Food and Drug Administration has issued a new warning and precaution for the type 2 diabetes drug canagliflozin, saying that risks of decreased bone density associated with the drug are more serious than previously stated.
Canagliflozin, a sodium-glucose cotransporter-2 (SGLT2) inhibitor, will now have a revised adverse reactions section on its drug label. Taking canagliflozin can significantly increase an individual’s chances of incurring bone fractures, due to the decreased bone mineral density caused by the drug. According to the FDA, these fractures can start to appear 12 weeks after starting a canagliflozin regimen, and can lead to bone mineral density loss in the hip and lower spine.
“FDA is continuing to evaluate the risk of bone fractures with other drugs in the SGLT2 inhibitor class, including dapagliflozin (Farxiga, Xigduo XR) and empagliflozin (Jardiance, Glyxambi, Synjardy), to determine if additional label changes or studies are needed,” the FDA stated, adding that all health care providers and patients are urged to contact the FDA if they experience adverse effects while taking any of these drugs.
Canagliflozin is marketed as Invokana and Invokamet by Janssen Pharmaceuticals, and was approved by the FDA in March 2013. The FDA is advising all health care professionals to carefully assess patients’ risk for developing bone fractures before prescribing the drug. Individuals who experience side effects while taking canagliflozin should submit a report through the FDA’s MedWatch program, or contact 1-800-332-1088 for more information.
FDA approves antiemesis drug rolapitant
The Food and Drug Administration has approved rolapitant for use in adults undergoing initial and repeat courses of chemotherapy to prevent nausea and vomiting, and it can be used in combination with other antiemetic agents that patients are also taking.
The approval comes after three randomized, double-blind, controlled clinical trials in which 2,800 patients were given either rolapitant or a placebo in combination with granisetron and dexamethasone. All patients were already receiving a chemotherapy regimen of highly or moderately emetogenic chemotherapy drugs, such as cisplatin, anthracycline, and cyclophosphamide. Those taking rolapitant had significantly reduced rates of emesis than those in the control cohort.
The drug, marketed as Varubi by Waltham, Massachusetts–based Tesaro Inc., is taken in tablet form and acts as a substance P/neurokinin 1–receptor antagonist to block emesis caused by chemotherapy.
“Chemotherapy-induced nausea and vomiting remains a major issue that can disrupt patients’ lives and sometimes their therapy,” said Dr. Amy Egan, deputy director of the Office of Drug Evaluation III in the FDA’s Center for Drug Evaluation and Research, in a statement. “Today’s approval provides cancer patients with another treatment option for the prevention of the delayed phase of nausea and vomiting caused by chemotherapy.”
Rolapitant is contraindicated for use with thioridazine, a drug which uses the CYP2D6 enzyme to metabolize – an enzyme which rolapitant inhibits. Taking both drugs together could cause arrhythmia. The most common side effects reported in patients taking rolapitant were neutropenia, decreased appetite, dizziness, and hiccups.
Serious adverse events associated with Varubi should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/Safety/MedWatch/default.htm.
The Food and Drug Administration has approved rolapitant for use in adults undergoing initial and repeat courses of chemotherapy to prevent nausea and vomiting, and it can be used in combination with other antiemetic agents that patients are also taking.
The approval comes after three randomized, double-blind, controlled clinical trials in which 2,800 patients were given either rolapitant or a placebo in combination with granisetron and dexamethasone. All patients were already receiving a chemotherapy regimen of highly or moderately emetogenic chemotherapy drugs, such as cisplatin, anthracycline, and cyclophosphamide. Those taking rolapitant had significantly reduced rates of emesis than those in the control cohort.
The drug, marketed as Varubi by Waltham, Massachusetts–based Tesaro Inc., is taken in tablet form and acts as a substance P/neurokinin 1–receptor antagonist to block emesis caused by chemotherapy.
“Chemotherapy-induced nausea and vomiting remains a major issue that can disrupt patients’ lives and sometimes their therapy,” said Dr. Amy Egan, deputy director of the Office of Drug Evaluation III in the FDA’s Center for Drug Evaluation and Research, in a statement. “Today’s approval provides cancer patients with another treatment option for the prevention of the delayed phase of nausea and vomiting caused by chemotherapy.”
Rolapitant is contraindicated for use with thioridazine, a drug which uses the CYP2D6 enzyme to metabolize – an enzyme which rolapitant inhibits. Taking both drugs together could cause arrhythmia. The most common side effects reported in patients taking rolapitant were neutropenia, decreased appetite, dizziness, and hiccups.
Serious adverse events associated with Varubi should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/Safety/MedWatch/default.htm.
The Food and Drug Administration has approved rolapitant for use in adults undergoing initial and repeat courses of chemotherapy to prevent nausea and vomiting, and it can be used in combination with other antiemetic agents that patients are also taking.
The approval comes after three randomized, double-blind, controlled clinical trials in which 2,800 patients were given either rolapitant or a placebo in combination with granisetron and dexamethasone. All patients were already receiving a chemotherapy regimen of highly or moderately emetogenic chemotherapy drugs, such as cisplatin, anthracycline, and cyclophosphamide. Those taking rolapitant had significantly reduced rates of emesis than those in the control cohort.
The drug, marketed as Varubi by Waltham, Massachusetts–based Tesaro Inc., is taken in tablet form and acts as a substance P/neurokinin 1–receptor antagonist to block emesis caused by chemotherapy.
“Chemotherapy-induced nausea and vomiting remains a major issue that can disrupt patients’ lives and sometimes their therapy,” said Dr. Amy Egan, deputy director of the Office of Drug Evaluation III in the FDA’s Center for Drug Evaluation and Research, in a statement. “Today’s approval provides cancer patients with another treatment option for the prevention of the delayed phase of nausea and vomiting caused by chemotherapy.”
Rolapitant is contraindicated for use with thioridazine, a drug which uses the CYP2D6 enzyme to metabolize – an enzyme which rolapitant inhibits. Taking both drugs together could cause arrhythmia. The most common side effects reported in patients taking rolapitant were neutropenia, decreased appetite, dizziness, and hiccups.
Serious adverse events associated with Varubi should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/Safety/MedWatch/default.htm.
FDA approves aprepitant for 12- to 17-year-olds
The Food and Drug Administration has issued an expanded indication for aprepitant (Emend) for the prevention of acute and delayed phases of chemotherapy-induced nausea and vomiting in patients 12-17 years old and in patients less than 12 years who weigh at least 66 lbs.
The approval is based on the results of a randomized, double-blind, active-comparator–controlled clinical study of 63 patients between 12 and 17 years of age, and 69 patients under the age of 12 years who weighed at least 66 pounds (30 kg). All patients were undergoing either highly or moderately emetogenic cancer chemotherapy and were put on a regimen of either ondansetron alone or ondensatron plus aprepitant, with intravenous dexamethasone administered at the physician’s discretion.
The primary outcome of no vomiting or retching and no use of rescue medication in the 25-120 hours following chemotherapy initiation was observed in 31 of 63 patients taking aprepitant plus ondensatron and in 13 of 69 patients taking ondensatron alone.
In the first 24 hours following chemotherapy initiation, 56% taking aprepitant plus ondensatron and 38% taking ondensatron alone achieved complete response. In the first 120 hours following chemotherapy initiation, 35% taking the combination and 13% taking ondensatron only had complete responses, according to a statement from Merck Research Laboratories.
Aprepitant is contraindicated for patients taking pimozide, as the CYP3A4 inhibitor in aprepitant can cause life-threatening, elevated plasma concentrations if taken with pimozide.
Serious adverse events associated with Emend should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/Safety/MedWatch/default.htm.
The Food and Drug Administration has issued an expanded indication for aprepitant (Emend) for the prevention of acute and delayed phases of chemotherapy-induced nausea and vomiting in patients 12-17 years old and in patients less than 12 years who weigh at least 66 lbs.
The approval is based on the results of a randomized, double-blind, active-comparator–controlled clinical study of 63 patients between 12 and 17 years of age, and 69 patients under the age of 12 years who weighed at least 66 pounds (30 kg). All patients were undergoing either highly or moderately emetogenic cancer chemotherapy and were put on a regimen of either ondansetron alone or ondensatron plus aprepitant, with intravenous dexamethasone administered at the physician’s discretion.
The primary outcome of no vomiting or retching and no use of rescue medication in the 25-120 hours following chemotherapy initiation was observed in 31 of 63 patients taking aprepitant plus ondensatron and in 13 of 69 patients taking ondensatron alone.
In the first 24 hours following chemotherapy initiation, 56% taking aprepitant plus ondensatron and 38% taking ondensatron alone achieved complete response. In the first 120 hours following chemotherapy initiation, 35% taking the combination and 13% taking ondensatron only had complete responses, according to a statement from Merck Research Laboratories.
Aprepitant is contraindicated for patients taking pimozide, as the CYP3A4 inhibitor in aprepitant can cause life-threatening, elevated plasma concentrations if taken with pimozide.
Serious adverse events associated with Emend should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/Safety/MedWatch/default.htm.
The Food and Drug Administration has issued an expanded indication for aprepitant (Emend) for the prevention of acute and delayed phases of chemotherapy-induced nausea and vomiting in patients 12-17 years old and in patients less than 12 years who weigh at least 66 lbs.
The approval is based on the results of a randomized, double-blind, active-comparator–controlled clinical study of 63 patients between 12 and 17 years of age, and 69 patients under the age of 12 years who weighed at least 66 pounds (30 kg). All patients were undergoing either highly or moderately emetogenic cancer chemotherapy and were put on a regimen of either ondansetron alone or ondensatron plus aprepitant, with intravenous dexamethasone administered at the physician’s discretion.
The primary outcome of no vomiting or retching and no use of rescue medication in the 25-120 hours following chemotherapy initiation was observed in 31 of 63 patients taking aprepitant plus ondensatron and in 13 of 69 patients taking ondensatron alone.
In the first 24 hours following chemotherapy initiation, 56% taking aprepitant plus ondensatron and 38% taking ondensatron alone achieved complete response. In the first 120 hours following chemotherapy initiation, 35% taking the combination and 13% taking ondensatron only had complete responses, according to a statement from Merck Research Laboratories.
Aprepitant is contraindicated for patients taking pimozide, as the CYP3A4 inhibitor in aprepitant can cause life-threatening, elevated plasma concentrations if taken with pimozide.
Serious adverse events associated with Emend should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/Safety/MedWatch/default.htm.
ICEID: Flu shots significantly decrease disease severity, duration
ATLANTA – Individuals who neglect to get their annual influenza vaccinations will likely experience more-severe symptoms and a longer duration of the illness if they contract the disease, specifically the A/H3N2 strain.
In a study of 155 influenza patients between 2009 and 2014, 138 (89%) were positive for influenza A virus, 111 (72%) of whom were vaccinated against influenza.
“We know that flu vaccines are about 60% effective, but of that remaining 40%, do they still get severe flu? The data from our study say no,” explained Dr. Eugene V. Millar of the Uniformed Services University of the Health Sciences in Bethesda, Md.
Sixty-six (48%) individuals contracted the A/H3N2 strain of the influenza virus; of these patients, those who did not get vaccinated reported higher average severity scores for upper respiratory symptoms (7 vs. 3), lower respiratory symptoms (7 vs. 3), systemic symptoms (9.5 vs. 6), and total symptoms (22 vs. 12) than did subjects who did get vaccinated (P less than .01).
“People ask me all the time why I bother getting a flu vaccine if it never works,” Dr. Millar said. “I tell them that if you’re walking around and talking to people, then it did work, even if you feel a little lousy; if you didn’t get that vaccination, you’d be on your back.”
Such disparity in the severity and duration of symptoms was not noted in 69 (50%) of the 155 influenza patients who contracted the A/H1N1 strain of the virus, nor in the 3 (2%) subjects who had an “untyped” form of influenza A. However, Dr. Millar cautioned that results regarding H1N1 may have been confounded by a couple of factors.
“As we’ve seen with the [H1N1] pandemic, it was just a pandemic of the sniffles, so it’s very hard to assess symptom severity when the differences are moderate to none,” Dr. Millar explained, adding that the variant strain of H3N2 which became prevalent during the 2014-2015 respiratory season proved to be the far more severe disease. Furthermore, patients found with A/H1N1 were more likely to be put on antivirals, making it impossible to look at vaccine effect.
In total, 884 patients with influenza-like illness were screened for inclusion in the study, from which the sample of 155 subjects was eventually derived. Median age of the 155 subjects was 30.6 (P = .61), mean body mass index was 27.6 kg/m2 (P = .07), males outnumbered females 88 to 67, and 106 subjects were active-duty military at the time they had influenza.
“These are healthy people presenting to outpatient [clinics], it’s very interesting to see if the same thing would hold true for the elderly or people with underlying medical conditions, since those are the people we’re really trying to protect not only from influenza, but its complications, as well, such as secondary bacterial pneumonia.”
Nine subjects (6%) had influenza during the 2009-2010 season, 56 (36%) during the 2010-2011 season, 16 (10%) during the 2011-2012 season, 38 (25%) during the 2012-2013 season, and 36 (23%) during the 2013-2014 season.
This study was supported by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense program carried out via the Uniformed Services University of the Health Sciences, and the National Institute of Allergy and Infectious Diseases, a division of the National Institutes of Health. Dr. Millar did not report any relevant financial disclosures.
ATLANTA – Individuals who neglect to get their annual influenza vaccinations will likely experience more-severe symptoms and a longer duration of the illness if they contract the disease, specifically the A/H3N2 strain.
In a study of 155 influenza patients between 2009 and 2014, 138 (89%) were positive for influenza A virus, 111 (72%) of whom were vaccinated against influenza.
“We know that flu vaccines are about 60% effective, but of that remaining 40%, do they still get severe flu? The data from our study say no,” explained Dr. Eugene V. Millar of the Uniformed Services University of the Health Sciences in Bethesda, Md.
Sixty-six (48%) individuals contracted the A/H3N2 strain of the influenza virus; of these patients, those who did not get vaccinated reported higher average severity scores for upper respiratory symptoms (7 vs. 3), lower respiratory symptoms (7 vs. 3), systemic symptoms (9.5 vs. 6), and total symptoms (22 vs. 12) than did subjects who did get vaccinated (P less than .01).
“People ask me all the time why I bother getting a flu vaccine if it never works,” Dr. Millar said. “I tell them that if you’re walking around and talking to people, then it did work, even if you feel a little lousy; if you didn’t get that vaccination, you’d be on your back.”
Such disparity in the severity and duration of symptoms was not noted in 69 (50%) of the 155 influenza patients who contracted the A/H1N1 strain of the virus, nor in the 3 (2%) subjects who had an “untyped” form of influenza A. However, Dr. Millar cautioned that results regarding H1N1 may have been confounded by a couple of factors.
“As we’ve seen with the [H1N1] pandemic, it was just a pandemic of the sniffles, so it’s very hard to assess symptom severity when the differences are moderate to none,” Dr. Millar explained, adding that the variant strain of H3N2 which became prevalent during the 2014-2015 respiratory season proved to be the far more severe disease. Furthermore, patients found with A/H1N1 were more likely to be put on antivirals, making it impossible to look at vaccine effect.
In total, 884 patients with influenza-like illness were screened for inclusion in the study, from which the sample of 155 subjects was eventually derived. Median age of the 155 subjects was 30.6 (P = .61), mean body mass index was 27.6 kg/m2 (P = .07), males outnumbered females 88 to 67, and 106 subjects were active-duty military at the time they had influenza.
“These are healthy people presenting to outpatient [clinics], it’s very interesting to see if the same thing would hold true for the elderly or people with underlying medical conditions, since those are the people we’re really trying to protect not only from influenza, but its complications, as well, such as secondary bacterial pneumonia.”
Nine subjects (6%) had influenza during the 2009-2010 season, 56 (36%) during the 2010-2011 season, 16 (10%) during the 2011-2012 season, 38 (25%) during the 2012-2013 season, and 36 (23%) during the 2013-2014 season.
This study was supported by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense program carried out via the Uniformed Services University of the Health Sciences, and the National Institute of Allergy and Infectious Diseases, a division of the National Institutes of Health. Dr. Millar did not report any relevant financial disclosures.
ATLANTA – Individuals who neglect to get their annual influenza vaccinations will likely experience more-severe symptoms and a longer duration of the illness if they contract the disease, specifically the A/H3N2 strain.
In a study of 155 influenza patients between 2009 and 2014, 138 (89%) were positive for influenza A virus, 111 (72%) of whom were vaccinated against influenza.
“We know that flu vaccines are about 60% effective, but of that remaining 40%, do they still get severe flu? The data from our study say no,” explained Dr. Eugene V. Millar of the Uniformed Services University of the Health Sciences in Bethesda, Md.
Sixty-six (48%) individuals contracted the A/H3N2 strain of the influenza virus; of these patients, those who did not get vaccinated reported higher average severity scores for upper respiratory symptoms (7 vs. 3), lower respiratory symptoms (7 vs. 3), systemic symptoms (9.5 vs. 6), and total symptoms (22 vs. 12) than did subjects who did get vaccinated (P less than .01).
“People ask me all the time why I bother getting a flu vaccine if it never works,” Dr. Millar said. “I tell them that if you’re walking around and talking to people, then it did work, even if you feel a little lousy; if you didn’t get that vaccination, you’d be on your back.”
Such disparity in the severity and duration of symptoms was not noted in 69 (50%) of the 155 influenza patients who contracted the A/H1N1 strain of the virus, nor in the 3 (2%) subjects who had an “untyped” form of influenza A. However, Dr. Millar cautioned that results regarding H1N1 may have been confounded by a couple of factors.
“As we’ve seen with the [H1N1] pandemic, it was just a pandemic of the sniffles, so it’s very hard to assess symptom severity when the differences are moderate to none,” Dr. Millar explained, adding that the variant strain of H3N2 which became prevalent during the 2014-2015 respiratory season proved to be the far more severe disease. Furthermore, patients found with A/H1N1 were more likely to be put on antivirals, making it impossible to look at vaccine effect.
In total, 884 patients with influenza-like illness were screened for inclusion in the study, from which the sample of 155 subjects was eventually derived. Median age of the 155 subjects was 30.6 (P = .61), mean body mass index was 27.6 kg/m2 (P = .07), males outnumbered females 88 to 67, and 106 subjects were active-duty military at the time they had influenza.
“These are healthy people presenting to outpatient [clinics], it’s very interesting to see if the same thing would hold true for the elderly or people with underlying medical conditions, since those are the people we’re really trying to protect not only from influenza, but its complications, as well, such as secondary bacterial pneumonia.”
Nine subjects (6%) had influenza during the 2009-2010 season, 56 (36%) during the 2010-2011 season, 16 (10%) during the 2011-2012 season, 38 (25%) during the 2012-2013 season, and 36 (23%) during the 2013-2014 season.
This study was supported by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense program carried out via the Uniformed Services University of the Health Sciences, and the National Institute of Allergy and Infectious Diseases, a division of the National Institutes of Health. Dr. Millar did not report any relevant financial disclosures.
AT ICEID 2015
Key clinical point: Although not entirely effective at outright preventing A/H3N2 disease, influenza vaccination can significantly decrease the length and severity of disease.
Major finding: Unvaccinated individuals reported significantly higher severity scores for upper respiratory, lower respiratory, systemic, and total symptoms than did subjects who received influenza vaccinations.
Data source: Retrospective cohort study of 155 individuals between 2009 and 2014.
Disclosures: The Infectious Disease Clinical Research Program and the National Institute of Allergy and Infectious Diseases supported the study. Dr. Millar did not report any relevant financial disclosures.
ICEID: Surveillance program identifies enterovirus strain early
ATLANTA – Implementing surveillance programs at area hospitals is an effective tool for health care providers and public health officials to identify severe acute respiratory illness (SARI) and enterovirus specifically early.
“We do surveillance for respiratory illness [at] three sentinel sites that participate in the Minneapolis-St. Paul metro area,” explained Hannah Friedlander, an epidemiologist with the Minnesota Department of Health in St. Paul, who presented the study. “[But] our surveillance didn’t actually actively look for enterovirus, it looked for rhinovirus, which is known to cross-react with enterovirus on PCRs [polymerase chain reactions],” she said at the International Conference on Emerging Infectious Diseases
To remedy that, the surveillance program – which involves the participation of one pediatric hospital, one hospital serving both children and adults, and one primarily serving adults – added testing for enterovirus to PCRs of all SARI specimens collected from Sept. 1 through Oct. 31, 2014. In total, 363 SARI specimens were collected over that time frame, of which 100 (28%) were found to be pan-EV positive and underwent further evaluation for EV-D68. Ultimately, 64 of the EV-positive specimens were found to be EV-D68 strains.
The vast majority of cases identified as being caused by the EV-D68 strain (73%) were collected between Sept. 6 and Sept. 20.
This indicates that starting surveillance of SARI cases when enteroviruses traditionally become more frequent could allow for faster determination of which strain is most prevalent and what the optimal treatment should be.
“It’s hard to say if this was surprising because we hadn’t previously been looking for enterovirus, so we don’t have another year to compare [these] data to,” Ms. Friedlander explained. “But I think it’s surprising that we saw as much of [enterovirus] as we did.”
Most cases of EV-D68 (64, or 36%) were in patients between the ages of 5 and 11 years, with a median age of 6 years. A total of 52 (81%) EV-D68 cases presented with shortness of breath, and 31 cases (48%) presented with wheezing or cough. Hospital stays of 4 days or fewer occurred in 73% of cases, with a median stay length of 3 days; 33% of EV-D68 patients required admittance to the ICU, and 13% of EV-D68 patients were placed on a mechanical ventilator at some point during treatment.
“This fall is our third year doing this type of surveillance, so at the time the data for this [were] collected, we only had 1 year of surveillance under our belt,” explained Ms. Friedlander. “We now look prospectively for enterovirus, not EV-D68 specifically, so it’ll be interesting to see as the years go on if this was an outlier year.”
Ms. Friedlander and her coinvestigators say they hope that further SARI surveillance will shed more light on the trends of viral pathogens and the impact that these have on hospitalization rates. They implore hospital systems to not only have surveillance programs in place, but also for them to have the flexibility to include additional testing should the need for it arise. That flexibility, Ms. Friedlander said, is what proved crucial in the early identification of EV-D68 in her own study population.
This study was funded by the Council of State and Territorial Epidemiologists, and the Centers for the Disease Control and Prevention. Ms. Friedlander did not report any relevant financial disclosures.
ATLANTA – Implementing surveillance programs at area hospitals is an effective tool for health care providers and public health officials to identify severe acute respiratory illness (SARI) and enterovirus specifically early.
“We do surveillance for respiratory illness [at] three sentinel sites that participate in the Minneapolis-St. Paul metro area,” explained Hannah Friedlander, an epidemiologist with the Minnesota Department of Health in St. Paul, who presented the study. “[But] our surveillance didn’t actually actively look for enterovirus, it looked for rhinovirus, which is known to cross-react with enterovirus on PCRs [polymerase chain reactions],” she said at the International Conference on Emerging Infectious Diseases
To remedy that, the surveillance program – which involves the participation of one pediatric hospital, one hospital serving both children and adults, and one primarily serving adults – added testing for enterovirus to PCRs of all SARI specimens collected from Sept. 1 through Oct. 31, 2014. In total, 363 SARI specimens were collected over that time frame, of which 100 (28%) were found to be pan-EV positive and underwent further evaluation for EV-D68. Ultimately, 64 of the EV-positive specimens were found to be EV-D68 strains.
The vast majority of cases identified as being caused by the EV-D68 strain (73%) were collected between Sept. 6 and Sept. 20.
This indicates that starting surveillance of SARI cases when enteroviruses traditionally become more frequent could allow for faster determination of which strain is most prevalent and what the optimal treatment should be.
“It’s hard to say if this was surprising because we hadn’t previously been looking for enterovirus, so we don’t have another year to compare [these] data to,” Ms. Friedlander explained. “But I think it’s surprising that we saw as much of [enterovirus] as we did.”
Most cases of EV-D68 (64, or 36%) were in patients between the ages of 5 and 11 years, with a median age of 6 years. A total of 52 (81%) EV-D68 cases presented with shortness of breath, and 31 cases (48%) presented with wheezing or cough. Hospital stays of 4 days or fewer occurred in 73% of cases, with a median stay length of 3 days; 33% of EV-D68 patients required admittance to the ICU, and 13% of EV-D68 patients were placed on a mechanical ventilator at some point during treatment.
“This fall is our third year doing this type of surveillance, so at the time the data for this [were] collected, we only had 1 year of surveillance under our belt,” explained Ms. Friedlander. “We now look prospectively for enterovirus, not EV-D68 specifically, so it’ll be interesting to see as the years go on if this was an outlier year.”
Ms. Friedlander and her coinvestigators say they hope that further SARI surveillance will shed more light on the trends of viral pathogens and the impact that these have on hospitalization rates. They implore hospital systems to not only have surveillance programs in place, but also for them to have the flexibility to include additional testing should the need for it arise. That flexibility, Ms. Friedlander said, is what proved crucial in the early identification of EV-D68 in her own study population.
This study was funded by the Council of State and Territorial Epidemiologists, and the Centers for the Disease Control and Prevention. Ms. Friedlander did not report any relevant financial disclosures.
ATLANTA – Implementing surveillance programs at area hospitals is an effective tool for health care providers and public health officials to identify severe acute respiratory illness (SARI) and enterovirus specifically early.
“We do surveillance for respiratory illness [at] three sentinel sites that participate in the Minneapolis-St. Paul metro area,” explained Hannah Friedlander, an epidemiologist with the Minnesota Department of Health in St. Paul, who presented the study. “[But] our surveillance didn’t actually actively look for enterovirus, it looked for rhinovirus, which is known to cross-react with enterovirus on PCRs [polymerase chain reactions],” she said at the International Conference on Emerging Infectious Diseases
To remedy that, the surveillance program – which involves the participation of one pediatric hospital, one hospital serving both children and adults, and one primarily serving adults – added testing for enterovirus to PCRs of all SARI specimens collected from Sept. 1 through Oct. 31, 2014. In total, 363 SARI specimens were collected over that time frame, of which 100 (28%) were found to be pan-EV positive and underwent further evaluation for EV-D68. Ultimately, 64 of the EV-positive specimens were found to be EV-D68 strains.
The vast majority of cases identified as being caused by the EV-D68 strain (73%) were collected between Sept. 6 and Sept. 20.
This indicates that starting surveillance of SARI cases when enteroviruses traditionally become more frequent could allow for faster determination of which strain is most prevalent and what the optimal treatment should be.
“It’s hard to say if this was surprising because we hadn’t previously been looking for enterovirus, so we don’t have another year to compare [these] data to,” Ms. Friedlander explained. “But I think it’s surprising that we saw as much of [enterovirus] as we did.”
Most cases of EV-D68 (64, or 36%) were in patients between the ages of 5 and 11 years, with a median age of 6 years. A total of 52 (81%) EV-D68 cases presented with shortness of breath, and 31 cases (48%) presented with wheezing or cough. Hospital stays of 4 days or fewer occurred in 73% of cases, with a median stay length of 3 days; 33% of EV-D68 patients required admittance to the ICU, and 13% of EV-D68 patients were placed on a mechanical ventilator at some point during treatment.
“This fall is our third year doing this type of surveillance, so at the time the data for this [were] collected, we only had 1 year of surveillance under our belt,” explained Ms. Friedlander. “We now look prospectively for enterovirus, not EV-D68 specifically, so it’ll be interesting to see as the years go on if this was an outlier year.”
Ms. Friedlander and her coinvestigators say they hope that further SARI surveillance will shed more light on the trends of viral pathogens and the impact that these have on hospitalization rates. They implore hospital systems to not only have surveillance programs in place, but also for them to have the flexibility to include additional testing should the need for it arise. That flexibility, Ms. Friedlander said, is what proved crucial in the early identification of EV-D68 in her own study population.
This study was funded by the Council of State and Territorial Epidemiologists, and the Centers for the Disease Control and Prevention. Ms. Friedlander did not report any relevant financial disclosures.
AT ICEID 2015
Key clinical point: Early detection of a potential enterovirus outbreak – specifically, the EV-D68 strain – can be facilitated by adopting a severe acute respiratory infection (SARI) surveillance program.
Major finding: Of 363 SARI samples collected from surveillance programs hospitals, 100 (28%) were pan-EV positive, and 64% of those were identified as EV-D68.
Data source: Retrospective analysis of data collected from 363 SARI cases between Sept. 1, 2014, and Oct. 31, 2014, in the Minneapolis-St. Paul metropolitan area.
Disclosures: Study funded by the Council of State and Territorial Epidemiologists and the Centers for Disease Control and Prevention.
ICEID: Fish-based outbreaks may be on the rise despite drop-off in recent years
ATLANTA – Despite the fact that foodborne outbreaks caused by consumption of fish have decreased over the last 15-20 years, prevalence of such outbreaks still remains relatively high, and the risk of future outbreaks therefore is not one to take lightly.
Kelly A. Walsh of the Center for Disease Control and Prevention’s National Center for Emerging and Zoonotic Infectious Diseases, said her study findings show, “while there was an overall decline in the number of fish outbreaks, they appear to be increasing in recent years,” meaning that public health officials and health care providers should be vigilant, as several popularly consumed fish are the most likely to cause disease.
The two most common etiologic agents behind outbreaks over this time span were scombroid toxin (380 outbreaks, 55%) and ciguatoxin (224 outbreaks, 33%). Onset of symptoms caused by scombroid toxin – including facial and lingual swelling, pruritic rash, diarrhea, and vomiting – can begin in a matter of minutes, and it is typically caused by consumption of tuna and mahi mahi, which were the first (36%) and second most-common (11%) cause of outbreaks between 1998 and 2013, respectively. Ciguatoxin is found in fish such as barracuda and grouper, which caused 6% and 9% of all outbreaks during 1998-2013, respectively. Onset of symptoms can occur in 3-30 hours after consumption of fish, and can cause gastrointestinal, cardiac, and neurologic symptoms, such as fatigue or “aberrant temperature perception,” Ms. Walsh said at the International Conference on Emerging Infectious Diseases.
“Seeing tuna come up most commonly with scombroid toxin is no surprise, but what was surprising is that raw or undercooked fish only accounted for 11% of outbreaks,” Ms. Walsh explained. In instances of undercooked fish causing outbreaks, tuna and salmon were the most commonly reported.
Ms. Walsh and her coinvestigators examined data from the CDC’s Foodborne Disease Outbreak Surveillance System collected between 1998 and 2013, looking specifically at the number of outbreaks, illnesses, hospitalizations, and deaths attributed to fish, as well as which etiologic agents were responsible, the associated fish types these agents were identified in, how the fish were prepared and served for consumption, and the states in which these outbreaks occurred.
In total, 764 outbreaks occurred and led to 4,401 illnesses, with a median of 3 illnesses per outbreak and a range between 2 and 425 illnesses. These outbreaks resulted in 322 hospitalizations and three deaths. However, despite finding that fish-based outbreaks dropped from an average of 62 per year during 1998-2004 to 32 per year during 2005-2012, the number of outbreaks in 2013 alone spiked to 50. The largest outbreak that occurred during 1998-2013 happened in 2012, when 425 illnesses were reported in a multistate outbreak caused by consumption of tuna that was found to have traces of Salmonella Nchanga, and Salmonella Bareilly.
Ms. Walsh and her coinvestigators hope their findings will bring to light the need for better control measures on the fish implicated in recent outbreaks, such as proper fish storage and appropriate preparation of fish. “We’re working with our partners at [the Food and Drug Administration] to inform them of the data that we’ve seen [so] we can inform regulations such as HACCP [Hazard Analysis & Critical Control Points].”
Ms. Walsh did not report any relevant financial disclosures.
ATLANTA – Despite the fact that foodborne outbreaks caused by consumption of fish have decreased over the last 15-20 years, prevalence of such outbreaks still remains relatively high, and the risk of future outbreaks therefore is not one to take lightly.
Kelly A. Walsh of the Center for Disease Control and Prevention’s National Center for Emerging and Zoonotic Infectious Diseases, said her study findings show, “while there was an overall decline in the number of fish outbreaks, they appear to be increasing in recent years,” meaning that public health officials and health care providers should be vigilant, as several popularly consumed fish are the most likely to cause disease.
The two most common etiologic agents behind outbreaks over this time span were scombroid toxin (380 outbreaks, 55%) and ciguatoxin (224 outbreaks, 33%). Onset of symptoms caused by scombroid toxin – including facial and lingual swelling, pruritic rash, diarrhea, and vomiting – can begin in a matter of minutes, and it is typically caused by consumption of tuna and mahi mahi, which were the first (36%) and second most-common (11%) cause of outbreaks between 1998 and 2013, respectively. Ciguatoxin is found in fish such as barracuda and grouper, which caused 6% and 9% of all outbreaks during 1998-2013, respectively. Onset of symptoms can occur in 3-30 hours after consumption of fish, and can cause gastrointestinal, cardiac, and neurologic symptoms, such as fatigue or “aberrant temperature perception,” Ms. Walsh said at the International Conference on Emerging Infectious Diseases.
“Seeing tuna come up most commonly with scombroid toxin is no surprise, but what was surprising is that raw or undercooked fish only accounted for 11% of outbreaks,” Ms. Walsh explained. In instances of undercooked fish causing outbreaks, tuna and salmon were the most commonly reported.
Ms. Walsh and her coinvestigators examined data from the CDC’s Foodborne Disease Outbreak Surveillance System collected between 1998 and 2013, looking specifically at the number of outbreaks, illnesses, hospitalizations, and deaths attributed to fish, as well as which etiologic agents were responsible, the associated fish types these agents were identified in, how the fish were prepared and served for consumption, and the states in which these outbreaks occurred.
In total, 764 outbreaks occurred and led to 4,401 illnesses, with a median of 3 illnesses per outbreak and a range between 2 and 425 illnesses. These outbreaks resulted in 322 hospitalizations and three deaths. However, despite finding that fish-based outbreaks dropped from an average of 62 per year during 1998-2004 to 32 per year during 2005-2012, the number of outbreaks in 2013 alone spiked to 50. The largest outbreak that occurred during 1998-2013 happened in 2012, when 425 illnesses were reported in a multistate outbreak caused by consumption of tuna that was found to have traces of Salmonella Nchanga, and Salmonella Bareilly.
Ms. Walsh and her coinvestigators hope their findings will bring to light the need for better control measures on the fish implicated in recent outbreaks, such as proper fish storage and appropriate preparation of fish. “We’re working with our partners at [the Food and Drug Administration] to inform them of the data that we’ve seen [so] we can inform regulations such as HACCP [Hazard Analysis & Critical Control Points].”
Ms. Walsh did not report any relevant financial disclosures.
ATLANTA – Despite the fact that foodborne outbreaks caused by consumption of fish have decreased over the last 15-20 years, prevalence of such outbreaks still remains relatively high, and the risk of future outbreaks therefore is not one to take lightly.
Kelly A. Walsh of the Center for Disease Control and Prevention’s National Center for Emerging and Zoonotic Infectious Diseases, said her study findings show, “while there was an overall decline in the number of fish outbreaks, they appear to be increasing in recent years,” meaning that public health officials and health care providers should be vigilant, as several popularly consumed fish are the most likely to cause disease.
The two most common etiologic agents behind outbreaks over this time span were scombroid toxin (380 outbreaks, 55%) and ciguatoxin (224 outbreaks, 33%). Onset of symptoms caused by scombroid toxin – including facial and lingual swelling, pruritic rash, diarrhea, and vomiting – can begin in a matter of minutes, and it is typically caused by consumption of tuna and mahi mahi, which were the first (36%) and second most-common (11%) cause of outbreaks between 1998 and 2013, respectively. Ciguatoxin is found in fish such as barracuda and grouper, which caused 6% and 9% of all outbreaks during 1998-2013, respectively. Onset of symptoms can occur in 3-30 hours after consumption of fish, and can cause gastrointestinal, cardiac, and neurologic symptoms, such as fatigue or “aberrant temperature perception,” Ms. Walsh said at the International Conference on Emerging Infectious Diseases.
“Seeing tuna come up most commonly with scombroid toxin is no surprise, but what was surprising is that raw or undercooked fish only accounted for 11% of outbreaks,” Ms. Walsh explained. In instances of undercooked fish causing outbreaks, tuna and salmon were the most commonly reported.
Ms. Walsh and her coinvestigators examined data from the CDC’s Foodborne Disease Outbreak Surveillance System collected between 1998 and 2013, looking specifically at the number of outbreaks, illnesses, hospitalizations, and deaths attributed to fish, as well as which etiologic agents were responsible, the associated fish types these agents were identified in, how the fish were prepared and served for consumption, and the states in which these outbreaks occurred.
In total, 764 outbreaks occurred and led to 4,401 illnesses, with a median of 3 illnesses per outbreak and a range between 2 and 425 illnesses. These outbreaks resulted in 322 hospitalizations and three deaths. However, despite finding that fish-based outbreaks dropped from an average of 62 per year during 1998-2004 to 32 per year during 2005-2012, the number of outbreaks in 2013 alone spiked to 50. The largest outbreak that occurred during 1998-2013 happened in 2012, when 425 illnesses were reported in a multistate outbreak caused by consumption of tuna that was found to have traces of Salmonella Nchanga, and Salmonella Bareilly.
Ms. Walsh and her coinvestigators hope their findings will bring to light the need for better control measures on the fish implicated in recent outbreaks, such as proper fish storage and appropriate preparation of fish. “We’re working with our partners at [the Food and Drug Administration] to inform them of the data that we’ve seen [so] we can inform regulations such as HACCP [Hazard Analysis & Critical Control Points].”
Ms. Walsh did not report any relevant financial disclosures.
AT ICEID 2015
Key clinical point: Although there has been a decline in the number of foodborne outbreaks caused by consumption of fish, recent data point to a slight increase in the last year reported, with specific types of fish still posing a serious threat of widespread illness.
Major finding: A total of 55% of outbreaks between 1998 and 2013 were attributed to scombroid toxin – commonly found in tuna and mahi mahi – and 33% to ciguatoxin – which is prevalent in grouper, among other types of popular fish.
Data source: Retrospective review of data from the CDC’s Foodborne Disease Outbreak Surveillance System for outbreaks attributed to fish from 1998 to 2013.
Disclosures: Ms. Walsh did not report any relevant financial disclosures.
Guidelines ineffective for reprocessing of endoscopes
Reprocessing guidelines currently in place for clinical gastrointestinal endoscopes need to be reevaluated and updated as soon as possible, according to a study published in the American Journal of Infection Control (doi: 10.1016/j.ajic.2015.03.003).
Procedures that follow current guidelines do not fully decontaminate the colonoscopes and gastroscopes after they’re used for procedures, leaving behind microbe levels that are well above the accepted benchmark standards.
“Previous studies show that cleaning endoscope channels is laborious and time consuming, and technicians often skip steps,” wrote the study’s authors – led by Cori L. Ofstead, MSPH, of Ofstead & Associates, St. Paul, Minn. “This is concerning, given that the procedure-related risk of endoscopy may be higher than previously thought [and] without objective verification, clinicians can unknowingly use contaminated endoscopes for procedures.”
The study, conducted at the Mayo Clinic in Rochester, Minn., examined the use of 15 endoscopes involved in 60 separate procedures over the period of Nov. 4-8, 2013, to determine how each endoscope was treated after an operation and whether these cleaning procedures followed guidelines and effectively decontaminated the devices.
“Endoscope testing was performed in a dedicated room adjacent to the procedure room, which allowed for rapid sampling and testing [and] barrier separation [minimized] potential for environmental cross-contamination.” the authors explained.
They maintained aseptic environmental conditions while gathering data, using disinfectant wipes on surfaces, using disposable absorbent pads, and restricting room access.
The researchers wore gloves, impervious gowns, face masks with splash protection, hair nets, and shoe covers; the gloves were changed between each sample collection, and gowns were changed between endoscope collections.
Microbial cultures and rapid indicator tests were conducted for ATP, protein, hemoglobin, and carbohydrate residue. Viable microbe samples were collected from 92% of bedside-cleaned endoscopes, 46% of manually cleaned endoscopes, 64% of “high-level disinfected” endoscopes, and 9% of stored endoscopes. Results from rapid indicator tests showed that some form of contamination was present on 100% of bedside-cleaned endoscopes, 92% of manually cleaned endoscopes, 73% of high-level disinfected endoscopes, and 82% of stored endoscopes which had viable microbes.
“Current guidelines rely on visual inspection to verify cleaning [but] visible contamination was never present, potentially because blood and feces may be difficult to discern from endoscopes’ black exteriors, and microscopic organisms cannot be seen by the naked eye,” the researchers wrote.
Consequently, Ms. Ofstead and colleagues called for a reexamination of current guidelines to move away from the eyeball test, saying that the results found here should pave the way for future studies to devise methods of more effectively cleaning and disinfecting endoscopes in a shorter amount of time.
“In the meantime, methods to ensure effectiveness of reprocessing practices are needed, including the potential use of routine monitoring with rapid indicators and microbiologic cultures,” they suggested.
Ms. Ofstead is president and CEO of Ofstead & Associates, which has received research funding and speaking honoraria related to endoscopic procedures from 3M, Advanced Sterilization Products, Medivators, Invendo Medical, Boston Scientific, and Steris. Three coauthors are employed by Ofstead & Associates, but have no other disclosures to report. The remaining coauthors had no disclosures to report. Funding was provided by 3M, Mayo Clinic, and Ofstead & Associates.
Reprocessing guidelines currently in place for clinical gastrointestinal endoscopes need to be reevaluated and updated as soon as possible, according to a study published in the American Journal of Infection Control (doi: 10.1016/j.ajic.2015.03.003).
Procedures that follow current guidelines do not fully decontaminate the colonoscopes and gastroscopes after they’re used for procedures, leaving behind microbe levels that are well above the accepted benchmark standards.
“Previous studies show that cleaning endoscope channels is laborious and time consuming, and technicians often skip steps,” wrote the study’s authors – led by Cori L. Ofstead, MSPH, of Ofstead & Associates, St. Paul, Minn. “This is concerning, given that the procedure-related risk of endoscopy may be higher than previously thought [and] without objective verification, clinicians can unknowingly use contaminated endoscopes for procedures.”
The study, conducted at the Mayo Clinic in Rochester, Minn., examined the use of 15 endoscopes involved in 60 separate procedures over the period of Nov. 4-8, 2013, to determine how each endoscope was treated after an operation and whether these cleaning procedures followed guidelines and effectively decontaminated the devices.
“Endoscope testing was performed in a dedicated room adjacent to the procedure room, which allowed for rapid sampling and testing [and] barrier separation [minimized] potential for environmental cross-contamination.” the authors explained.
They maintained aseptic environmental conditions while gathering data, using disinfectant wipes on surfaces, using disposable absorbent pads, and restricting room access.
The researchers wore gloves, impervious gowns, face masks with splash protection, hair nets, and shoe covers; the gloves were changed between each sample collection, and gowns were changed between endoscope collections.
Microbial cultures and rapid indicator tests were conducted for ATP, protein, hemoglobin, and carbohydrate residue. Viable microbe samples were collected from 92% of bedside-cleaned endoscopes, 46% of manually cleaned endoscopes, 64% of “high-level disinfected” endoscopes, and 9% of stored endoscopes. Results from rapid indicator tests showed that some form of contamination was present on 100% of bedside-cleaned endoscopes, 92% of manually cleaned endoscopes, 73% of high-level disinfected endoscopes, and 82% of stored endoscopes which had viable microbes.
“Current guidelines rely on visual inspection to verify cleaning [but] visible contamination was never present, potentially because blood and feces may be difficult to discern from endoscopes’ black exteriors, and microscopic organisms cannot be seen by the naked eye,” the researchers wrote.
Consequently, Ms. Ofstead and colleagues called for a reexamination of current guidelines to move away from the eyeball test, saying that the results found here should pave the way for future studies to devise methods of more effectively cleaning and disinfecting endoscopes in a shorter amount of time.
“In the meantime, methods to ensure effectiveness of reprocessing practices are needed, including the potential use of routine monitoring with rapid indicators and microbiologic cultures,” they suggested.
Ms. Ofstead is president and CEO of Ofstead & Associates, which has received research funding and speaking honoraria related to endoscopic procedures from 3M, Advanced Sterilization Products, Medivators, Invendo Medical, Boston Scientific, and Steris. Three coauthors are employed by Ofstead & Associates, but have no other disclosures to report. The remaining coauthors had no disclosures to report. Funding was provided by 3M, Mayo Clinic, and Ofstead & Associates.
Reprocessing guidelines currently in place for clinical gastrointestinal endoscopes need to be reevaluated and updated as soon as possible, according to a study published in the American Journal of Infection Control (doi: 10.1016/j.ajic.2015.03.003).
Procedures that follow current guidelines do not fully decontaminate the colonoscopes and gastroscopes after they’re used for procedures, leaving behind microbe levels that are well above the accepted benchmark standards.
“Previous studies show that cleaning endoscope channels is laborious and time consuming, and technicians often skip steps,” wrote the study’s authors – led by Cori L. Ofstead, MSPH, of Ofstead & Associates, St. Paul, Minn. “This is concerning, given that the procedure-related risk of endoscopy may be higher than previously thought [and] without objective verification, clinicians can unknowingly use contaminated endoscopes for procedures.”
The study, conducted at the Mayo Clinic in Rochester, Minn., examined the use of 15 endoscopes involved in 60 separate procedures over the period of Nov. 4-8, 2013, to determine how each endoscope was treated after an operation and whether these cleaning procedures followed guidelines and effectively decontaminated the devices.
“Endoscope testing was performed in a dedicated room adjacent to the procedure room, which allowed for rapid sampling and testing [and] barrier separation [minimized] potential for environmental cross-contamination.” the authors explained.
They maintained aseptic environmental conditions while gathering data, using disinfectant wipes on surfaces, using disposable absorbent pads, and restricting room access.
The researchers wore gloves, impervious gowns, face masks with splash protection, hair nets, and shoe covers; the gloves were changed between each sample collection, and gowns were changed between endoscope collections.
Microbial cultures and rapid indicator tests were conducted for ATP, protein, hemoglobin, and carbohydrate residue. Viable microbe samples were collected from 92% of bedside-cleaned endoscopes, 46% of manually cleaned endoscopes, 64% of “high-level disinfected” endoscopes, and 9% of stored endoscopes. Results from rapid indicator tests showed that some form of contamination was present on 100% of bedside-cleaned endoscopes, 92% of manually cleaned endoscopes, 73% of high-level disinfected endoscopes, and 82% of stored endoscopes which had viable microbes.
“Current guidelines rely on visual inspection to verify cleaning [but] visible contamination was never present, potentially because blood and feces may be difficult to discern from endoscopes’ black exteriors, and microscopic organisms cannot be seen by the naked eye,” the researchers wrote.
Consequently, Ms. Ofstead and colleagues called for a reexamination of current guidelines to move away from the eyeball test, saying that the results found here should pave the way for future studies to devise methods of more effectively cleaning and disinfecting endoscopes in a shorter amount of time.
“In the meantime, methods to ensure effectiveness of reprocessing practices are needed, including the potential use of routine monitoring with rapid indicators and microbiologic cultures,” they suggested.
Ms. Ofstead is president and CEO of Ofstead & Associates, which has received research funding and speaking honoraria related to endoscopic procedures from 3M, Advanced Sterilization Products, Medivators, Invendo Medical, Boston Scientific, and Steris. Three coauthors are employed by Ofstead & Associates, but have no other disclosures to report. The remaining coauthors had no disclosures to report. Funding was provided by 3M, Mayo Clinic, and Ofstead & Associates.
Weighted CMDS Score Predicts 15-year Diabetes Risk
A modified version of the cardiometabolic disease staging (CMDS) scoring system can be effectively used in a clinical setting to identify patients who are at an elevated risk for developing diabetes, according to a study published in the Journal of Clinical Endocrinology & Metabolism.
“To enhance the application of the CMDS system in clinical settings, we sought to develop a weighted scoring system based on risk factor components in the CMDS system for the prediction of future diabetes by separate identification and weighting of those risk components,” said Dr. Fangjian Guo of the University of Texas Medical Branch, Galveston, and Dr. W. Timothy Garvey of the University of Alabama, Birmingham.
The CMDS scoring method involves these measures:
• Fasting blood glucose of at least 100 mg/dL.
• Two-hour glucose from oral glucose tolerance test (OGTT) of at least 140 mg/dL.
• Waist circumference of 102 cm for men and 88 cm for women.
• Blood pressure (systolic of at least 130 mm Hg and/or diastolic of at least 85 mm Hg) or use of antihypertensive medication.
• HDL cholesterol under 40 mg/dL in men and under 50 mg/dL in women.
• Fasting triglycerides of at least 150 mg/dL or use of antihyperlipidemia medication.
Dr. Guo and Dr. Garvey used data from the CARDIA study and the ARIC study for their investigation.
CARDIA is a large, ongoing population study of 5,115 black and white adults, age 18-30 years, recruited from 1985 to 1986, with follow-up examinations conducted in seven visits over the next 25 years.
ARIC is an ongoing prospective cohort study of 15,792 men and women recruited at age 46-64 years in 1987 from either Mississippi, North Carolina, Minnesota, or Maryland, with follow-ups every 3 years.
The CMDS score was derived based on “verified incident diabetes cases from the CARDIA study” and was validated “in participants from the ARIC study,” after which the researchers “fitted Cox models and derived CMDS scores for diabetes accordingly,” the researchers wrote (J Clin Endocrinol Metab. 2015 Aug 4 doi: 10.1210/jc.2015-2691).
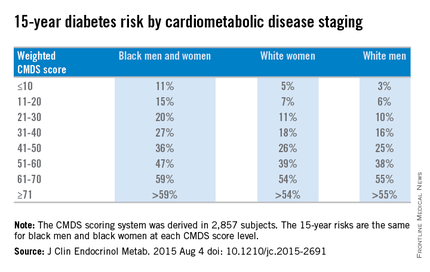
Overall, weighted diabetes CMDS scores were derived for 2,857 subjects and were validated in 6,425 subjects from the ARIC study. Scores in each of the six subcategories carried integer values that, when totaled, equaled a maximum of 100 per subject.
Weighted CMDS scores of 10 or lower were found to indicate a low 15-year risk of diabetes rate: 11% for both black men and women, 5% for white women, and 3% for white men. Weighted CMDS scores of 11-20 indicate a 15% risk for black men and women, 7% for white women, and 6% for white men. Scores of 21-30 indicated a 20% risk for black men and women, 11% for white women, and 10% for white men. Scores of 31-40 indicated a 27% risk for black men and women, 18% for white women, and 16% for white men. Scores of 41-50 indicated a 36% risk for black men and women, 26% for white women, and 25% for white men.
Fifteen-year diabetes-risk rates increased in the higher CMDS score brackets. In the 51-60 range, black men and women have a 47% risk, compared with 39% for white women and 38% for white men. Scores of 61-70 indicated a 59% risk for black men and women, 54% for white women, and 55% for white men. In the final score bracket of 71 and higher, the risk is greater than 59% for black men and women, greater than 54% for white women, and greater than 55% for white men.
“The main strength of this study is the development and validation of a weighted diabetes algorithm using data from two large prospective national cohorts,” Dr. Guo and Dr. Garvey noted, adding that “incident diabetes in the CARDIA study was based on rigorous measures of fasting glucose and 2-hour OGTT glucose, such that the ascertained diabetes cases provide a solid basis for the development of our weighted CMDS scoring system.”
Limitations of the study include the fact that blood glucose measures were not available for ARIC participants after the fourth visit and that diabetes cases were based on hospital records and self-reports. Furthermore, both CARDIA and ARIC looked exclusively at black and white subjects, meaning that other minority groups – particularly Asians – were not included.
This study was supported by the merit review program of the Department of Veterans Affairs, National Institutes of Health, and the UAB Diabetes Research Center. Dr. Guo is supported by an institutional training grant from the Eunice Kennedy Shriver National Institute of Child Health & Human Development at the NIH.
A modified version of the cardiometabolic disease staging (CMDS) scoring system can be effectively used in a clinical setting to identify patients who are at an elevated risk for developing diabetes, according to a study published in the Journal of Clinical Endocrinology & Metabolism.
“To enhance the application of the CMDS system in clinical settings, we sought to develop a weighted scoring system based on risk factor components in the CMDS system for the prediction of future diabetes by separate identification and weighting of those risk components,” said Dr. Fangjian Guo of the University of Texas Medical Branch, Galveston, and Dr. W. Timothy Garvey of the University of Alabama, Birmingham.
The CMDS scoring method involves these measures:
• Fasting blood glucose of at least 100 mg/dL.
• Two-hour glucose from oral glucose tolerance test (OGTT) of at least 140 mg/dL.
• Waist circumference of 102 cm for men and 88 cm for women.
• Blood pressure (systolic of at least 130 mm Hg and/or diastolic of at least 85 mm Hg) or use of antihypertensive medication.
• HDL cholesterol under 40 mg/dL in men and under 50 mg/dL in women.
• Fasting triglycerides of at least 150 mg/dL or use of antihyperlipidemia medication.
Dr. Guo and Dr. Garvey used data from the CARDIA study and the ARIC study for their investigation.
CARDIA is a large, ongoing population study of 5,115 black and white adults, age 18-30 years, recruited from 1985 to 1986, with follow-up examinations conducted in seven visits over the next 25 years.
ARIC is an ongoing prospective cohort study of 15,792 men and women recruited at age 46-64 years in 1987 from either Mississippi, North Carolina, Minnesota, or Maryland, with follow-ups every 3 years.
The CMDS score was derived based on “verified incident diabetes cases from the CARDIA study” and was validated “in participants from the ARIC study,” after which the researchers “fitted Cox models and derived CMDS scores for diabetes accordingly,” the researchers wrote (J Clin Endocrinol Metab. 2015 Aug 4 doi: 10.1210/jc.2015-2691).
Overall, weighted diabetes CMDS scores were derived for 2,857 subjects and were validated in 6,425 subjects from the ARIC study. Scores in each of the six subcategories carried integer values that, when totaled, equaled a maximum of 100 per subject.
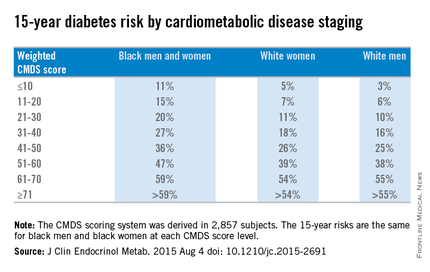
Weighted CMDS scores of 10 or lower were found to indicate a low 15-year risk of diabetes rate: 11% for both black men and women, 5% for white women, and 3% for white men. Weighted CMDS scores of 11-20 indicate a 15% risk for black men and women, 7% for white women, and 6% for white men. Scores of 21-30 indicated a 20% risk for black men and women, 11% for white women, and 10% for white men. Scores of 31-40 indicated a 27% risk for black men and women, 18% for white women, and 16% for white men. Scores of 41-50 indicated a 36% risk for black men and women, 26% for white women, and 25% for white men.
Fifteen-year diabetes-risk rates increased in the higher CMDS score brackets. In the 51-60 range, black men and women have a 47% risk, compared with 39% for white women and 38% for white men. Scores of 61-70 indicated a 59% risk for black men and women, 54% for white women, and 55% for white men. In the final score bracket of 71 and higher, the risk is greater than 59% for black men and women, greater than 54% for white women, and greater than 55% for white men.

“The main strength of this study is the development and validation of a weighted diabetes algorithm using data from two large prospective national cohorts,” Dr. Guo and Dr. Garvey noted, adding that “incident diabetes in the CARDIA study was based on rigorous measures of fasting glucose and 2-hour OGTT glucose, such that the ascertained diabetes cases provide a solid basis for the development of our weighted CMDS scoring system.”
Limitations of the study include the fact that blood glucose measures were not available for ARIC participants after the fourth visit and that diabetes cases were based on hospital records and self-reports. Furthermore, both CARDIA and ARIC looked exclusively at black and white subjects, meaning that other minority groups – particularly Asians – were not included.
This study was supported by the merit review program of the Department of Veterans Affairs, National Institutes of Health, and the UAB Diabetes Research Center. Dr. Guo is supported by an institutional training grant from the Eunice Kennedy Shriver National Institute of Child Health & Human Development at the NIH.
A modified version of the cardiometabolic disease staging (CMDS) scoring system can be effectively used in a clinical setting to identify patients who are at an elevated risk for developing diabetes, according to a study published in the Journal of Clinical Endocrinology & Metabolism.
“To enhance the application of the CMDS system in clinical settings, we sought to develop a weighted scoring system based on risk factor components in the CMDS system for the prediction of future diabetes by separate identification and weighting of those risk components,” said Dr. Fangjian Guo of the University of Texas Medical Branch, Galveston, and Dr. W. Timothy Garvey of the University of Alabama, Birmingham.
The CMDS scoring method involves these measures:
• Fasting blood glucose of at least 100 mg/dL.
• Two-hour glucose from oral glucose tolerance test (OGTT) of at least 140 mg/dL.
• Waist circumference of 102 cm for men and 88 cm for women.
• Blood pressure (systolic of at least 130 mm Hg and/or diastolic of at least 85 mm Hg) or use of antihypertensive medication.
• HDL cholesterol under 40 mg/dL in men and under 50 mg/dL in women.
• Fasting triglycerides of at least 150 mg/dL or use of antihyperlipidemia medication.
Dr. Guo and Dr. Garvey used data from the CARDIA study and the ARIC study for their investigation.
CARDIA is a large, ongoing population study of 5,115 black and white adults, age 18-30 years, recruited from 1985 to 1986, with follow-up examinations conducted in seven visits over the next 25 years.
ARIC is an ongoing prospective cohort study of 15,792 men and women recruited at age 46-64 years in 1987 from either Mississippi, North Carolina, Minnesota, or Maryland, with follow-ups every 3 years.
The CMDS score was derived based on “verified incident diabetes cases from the CARDIA study” and was validated “in participants from the ARIC study,” after which the researchers “fitted Cox models and derived CMDS scores for diabetes accordingly,” the researchers wrote (J Clin Endocrinol Metab. 2015 Aug 4 doi: 10.1210/jc.2015-2691).
Overall, weighted diabetes CMDS scores were derived for 2,857 subjects and were validated in 6,425 subjects from the ARIC study. Scores in each of the six subcategories carried integer values that, when totaled, equaled a maximum of 100 per subject.
Weighted CMDS scores of 10 or lower were found to indicate a low 15-year risk of diabetes rate: 11% for both black men and women, 5% for white women, and 3% for white men. Weighted CMDS scores of 11-20 indicate a 15% risk for black men and women, 7% for white women, and 6% for white men. Scores of 21-30 indicated a 20% risk for black men and women, 11% for white women, and 10% for white men. Scores of 31-40 indicated a 27% risk for black men and women, 18% for white women, and 16% for white men. Scores of 41-50 indicated a 36% risk for black men and women, 26% for white women, and 25% for white men.
Fifteen-year diabetes-risk rates increased in the higher CMDS score brackets. In the 51-60 range, black men and women have a 47% risk, compared with 39% for white women and 38% for white men. Scores of 61-70 indicated a 59% risk for black men and women, 54% for white women, and 55% for white men. In the final score bracket of 71 and higher, the risk is greater than 59% for black men and women, greater than 54% for white women, and greater than 55% for white men.

“The main strength of this study is the development and validation of a weighted diabetes algorithm using data from two large prospective national cohorts,” Dr. Guo and Dr. Garvey noted, adding that “incident diabetes in the CARDIA study was based on rigorous measures of fasting glucose and 2-hour OGTT glucose, such that the ascertained diabetes cases provide a solid basis for the development of our weighted CMDS scoring system.”
Limitations of the study include the fact that blood glucose measures were not available for ARIC participants after the fourth visit and that diabetes cases were based on hospital records and self-reports. Furthermore, both CARDIA and ARIC looked exclusively at black and white subjects, meaning that other minority groups – particularly Asians – were not included.
This study was supported by the merit review program of the Department of Veterans Affairs, National Institutes of Health, and the UAB Diabetes Research Center. Dr. Guo is supported by an institutional training grant from the Eunice Kennedy Shriver National Institute of Child Health & Human Development at the NIH.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Weighted CMDS score predicts 15-year diabetes risk
A modified version of the cardiometabolic disease staging (CMDS) scoring system can be effectively used in a clinical setting to identify patients who are at an elevated risk for developing diabetes, according to a study published in the Journal of Clinical Endocrinology & Metabolism.
“To enhance the application of the CMDS system in clinical settings, we sought to develop a weighted scoring system based on risk factor components in the CMDS system for the prediction of future diabetes by separate identification and weighting of those risk components,” said Dr. Fangjian Guo of the University of Texas Medical Branch, Galveston, and Dr. W. Timothy Garvey of the University of Alabama, Birmingham.
The CMDS scoring method involves these measures:
• Fasting blood glucose of at least 100 mg/dL.
• Two-hour glucose from oral glucose tolerance test (OGTT) of at least 140 mg/dL.
• Waist circumference of 102 cm for men and 88 cm for women.
• Blood pressure (systolic of at least 130 mm Hg and/or diastolic of at least 85 mm Hg) or use of antihypertensive medication.
• HDL cholesterol under 40 mg/dL in men and under 50 mg/dL in women.
• Fasting triglycerides of at least 150 mg/dL or use of antihyperlipidemia medication.
Dr. Guo and Dr. Garvey used data from the CARDIA study and the ARIC study for their investigation.
CARDIA is a large, ongoing population study of 5,115 black and white adults, age 18-30 years, recruited from 1985 to 1986, with follow-up examinations conducted in seven visits over the next 25 years.
ARIC is an ongoing prospective cohort study of 15,792 men and women recruited at age 46-64 years in 1987 from either Mississippi, North Carolina, Minnesota, or Maryland, with follow-ups every 3 years.
The CMDS score was derived based on “verified incident diabetes cases from the CARDIA study” and was validated “in participants from the ARIC study,” after which the researchers “fitted Cox models and derived CMDS scores for diabetes accordingly,” the researchers wrote (J Clin Endocrinol Metab. 2015 Aug 4 doi: 10.1210/jc.2015-2691).
Overall, weighted diabetes CMDS scores were derived for 2,857 subjects and were validated in 6,425 subjects from the ARIC study. Scores in each of the six subcategories carried integer values that, when totaled, equaled a maximum of 100 per subject.
Weighted CMDS scores of 10 or lower were found to indicate a low 15-year risk of diabetes rate: 11% for both black men and women, 5% for white women, and 3% for white men. Weighted CMDS scores of 11-20 indicate a 15% risk for black men and women, 7% for white women, and 6% for white men. Scores of 21-30 indicated a 20% risk for black men and women, 11% for white women, and 10% for white men. Scores of 31-40 indicated a 27% risk for black men and women, 18% for white women, and 16% for white men. Scores of 41-50 indicated a 36% risk for black men and women, 26% for white women, and 25% for white men.
Fifteen-year diabetes-risk rates increased in the higher CMDS score brackets. In the 51-60 range, black men and women have a 47% risk, compared with 39% for white women and 38% for white men. Scores of 61-70 indicated a 59% risk for black men and women, 54% for white women, and 55% for white men. In the final score bracket of 71 and higher, the risk is greater than 59% for black men and women, greater than 54% for white women, and greater than 55% for white men.
“The main strength of this study is the development and validation of a weighted diabetes algorithm using data from two large prospective national cohorts,” Dr. Guo and Dr. Garvey noted, adding that “incident diabetes in the CARDIA study was based on rigorous measures of fasting glucose and 2-hour OGTT glucose, such that the ascertained diabetes cases provide a solid basis for the development of our weighted CMDS scoring system.”
Limitations of the study include the fact that blood glucose measures were not available for ARIC participants after the fourth visit and that diabetes cases were based on hospital records and self-reports. Furthermore, both CARDIA and ARIC looked exclusively at black and white subjects, meaning that other minority groups – particularly Asians – were not included.
This study was supported by the merit review program of the Department of Veterans Affairs, National Institutes of Health, and the UAB Diabetes Research Center. Dr. Guo is supported by an institutional training grant from the Eunice Kennedy Shriver National Institute of Child Health & Human Development at the NIH.
A modified version of the cardiometabolic disease staging (CMDS) scoring system can be effectively used in a clinical setting to identify patients who are at an elevated risk for developing diabetes, according to a study published in the Journal of Clinical Endocrinology & Metabolism.
“To enhance the application of the CMDS system in clinical settings, we sought to develop a weighted scoring system based on risk factor components in the CMDS system for the prediction of future diabetes by separate identification and weighting of those risk components,” said Dr. Fangjian Guo of the University of Texas Medical Branch, Galveston, and Dr. W. Timothy Garvey of the University of Alabama, Birmingham.
The CMDS scoring method involves these measures:
• Fasting blood glucose of at least 100 mg/dL.
• Two-hour glucose from oral glucose tolerance test (OGTT) of at least 140 mg/dL.
• Waist circumference of 102 cm for men and 88 cm for women.
• Blood pressure (systolic of at least 130 mm Hg and/or diastolic of at least 85 mm Hg) or use of antihypertensive medication.
• HDL cholesterol under 40 mg/dL in men and under 50 mg/dL in women.
• Fasting triglycerides of at least 150 mg/dL or use of antihyperlipidemia medication.
Dr. Guo and Dr. Garvey used data from the CARDIA study and the ARIC study for their investigation.
CARDIA is a large, ongoing population study of 5,115 black and white adults, age 18-30 years, recruited from 1985 to 1986, with follow-up examinations conducted in seven visits over the next 25 years.
ARIC is an ongoing prospective cohort study of 15,792 men and women recruited at age 46-64 years in 1987 from either Mississippi, North Carolina, Minnesota, or Maryland, with follow-ups every 3 years.
The CMDS score was derived based on “verified incident diabetes cases from the CARDIA study” and was validated “in participants from the ARIC study,” after which the researchers “fitted Cox models and derived CMDS scores for diabetes accordingly,” the researchers wrote (J Clin Endocrinol Metab. 2015 Aug 4 doi: 10.1210/jc.2015-2691).
Overall, weighted diabetes CMDS scores were derived for 2,857 subjects and were validated in 6,425 subjects from the ARIC study. Scores in each of the six subcategories carried integer values that, when totaled, equaled a maximum of 100 per subject.
Weighted CMDS scores of 10 or lower were found to indicate a low 15-year risk of diabetes rate: 11% for both black men and women, 5% for white women, and 3% for white men. Weighted CMDS scores of 11-20 indicate a 15% risk for black men and women, 7% for white women, and 6% for white men. Scores of 21-30 indicated a 20% risk for black men and women, 11% for white women, and 10% for white men. Scores of 31-40 indicated a 27% risk for black men and women, 18% for white women, and 16% for white men. Scores of 41-50 indicated a 36% risk for black men and women, 26% for white women, and 25% for white men.
Fifteen-year diabetes-risk rates increased in the higher CMDS score brackets. In the 51-60 range, black men and women have a 47% risk, compared with 39% for white women and 38% for white men. Scores of 61-70 indicated a 59% risk for black men and women, 54% for white women, and 55% for white men. In the final score bracket of 71 and higher, the risk is greater than 59% for black men and women, greater than 54% for white women, and greater than 55% for white men.

“The main strength of this study is the development and validation of a weighted diabetes algorithm using data from two large prospective national cohorts,” Dr. Guo and Dr. Garvey noted, adding that “incident diabetes in the CARDIA study was based on rigorous measures of fasting glucose and 2-hour OGTT glucose, such that the ascertained diabetes cases provide a solid basis for the development of our weighted CMDS scoring system.”
Limitations of the study include the fact that blood glucose measures were not available for ARIC participants after the fourth visit and that diabetes cases were based on hospital records and self-reports. Furthermore, both CARDIA and ARIC looked exclusively at black and white subjects, meaning that other minority groups – particularly Asians – were not included.
This study was supported by the merit review program of the Department of Veterans Affairs, National Institutes of Health, and the UAB Diabetes Research Center. Dr. Guo is supported by an institutional training grant from the Eunice Kennedy Shriver National Institute of Child Health & Human Development at the NIH.
A modified version of the cardiometabolic disease staging (CMDS) scoring system can be effectively used in a clinical setting to identify patients who are at an elevated risk for developing diabetes, according to a study published in the Journal of Clinical Endocrinology & Metabolism.
“To enhance the application of the CMDS system in clinical settings, we sought to develop a weighted scoring system based on risk factor components in the CMDS system for the prediction of future diabetes by separate identification and weighting of those risk components,” said Dr. Fangjian Guo of the University of Texas Medical Branch, Galveston, and Dr. W. Timothy Garvey of the University of Alabama, Birmingham.
The CMDS scoring method involves these measures:
• Fasting blood glucose of at least 100 mg/dL.
• Two-hour glucose from oral glucose tolerance test (OGTT) of at least 140 mg/dL.
• Waist circumference of 102 cm for men and 88 cm for women.
• Blood pressure (systolic of at least 130 mm Hg and/or diastolic of at least 85 mm Hg) or use of antihypertensive medication.
• HDL cholesterol under 40 mg/dL in men and under 50 mg/dL in women.
• Fasting triglycerides of at least 150 mg/dL or use of antihyperlipidemia medication.
Dr. Guo and Dr. Garvey used data from the CARDIA study and the ARIC study for their investigation.
CARDIA is a large, ongoing population study of 5,115 black and white adults, age 18-30 years, recruited from 1985 to 1986, with follow-up examinations conducted in seven visits over the next 25 years.
ARIC is an ongoing prospective cohort study of 15,792 men and women recruited at age 46-64 years in 1987 from either Mississippi, North Carolina, Minnesota, or Maryland, with follow-ups every 3 years.
The CMDS score was derived based on “verified incident diabetes cases from the CARDIA study” and was validated “in participants from the ARIC study,” after which the researchers “fitted Cox models and derived CMDS scores for diabetes accordingly,” the researchers wrote (J Clin Endocrinol Metab. 2015 Aug 4 doi: 10.1210/jc.2015-2691).
Overall, weighted diabetes CMDS scores were derived for 2,857 subjects and were validated in 6,425 subjects from the ARIC study. Scores in each of the six subcategories carried integer values that, when totaled, equaled a maximum of 100 per subject.
Weighted CMDS scores of 10 or lower were found to indicate a low 15-year risk of diabetes rate: 11% for both black men and women, 5% for white women, and 3% for white men. Weighted CMDS scores of 11-20 indicate a 15% risk for black men and women, 7% for white women, and 6% for white men. Scores of 21-30 indicated a 20% risk for black men and women, 11% for white women, and 10% for white men. Scores of 31-40 indicated a 27% risk for black men and women, 18% for white women, and 16% for white men. Scores of 41-50 indicated a 36% risk for black men and women, 26% for white women, and 25% for white men.
Fifteen-year diabetes-risk rates increased in the higher CMDS score brackets. In the 51-60 range, black men and women have a 47% risk, compared with 39% for white women and 38% for white men. Scores of 61-70 indicated a 59% risk for black men and women, 54% for white women, and 55% for white men. In the final score bracket of 71 and higher, the risk is greater than 59% for black men and women, greater than 54% for white women, and greater than 55% for white men.

“The main strength of this study is the development and validation of a weighted diabetes algorithm using data from two large prospective national cohorts,” Dr. Guo and Dr. Garvey noted, adding that “incident diabetes in the CARDIA study was based on rigorous measures of fasting glucose and 2-hour OGTT glucose, such that the ascertained diabetes cases provide a solid basis for the development of our weighted CMDS scoring system.”
Limitations of the study include the fact that blood glucose measures were not available for ARIC participants after the fourth visit and that diabetes cases were based on hospital records and self-reports. Furthermore, both CARDIA and ARIC looked exclusively at black and white subjects, meaning that other minority groups – particularly Asians – were not included.
This study was supported by the merit review program of the Department of Veterans Affairs, National Institutes of Health, and the UAB Diabetes Research Center. Dr. Guo is supported by an institutional training grant from the Eunice Kennedy Shriver National Institute of Child Health & Human Development at the NIH.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Key clinical point: The cardiometabolic disease staging (CMDS) scoring system can be used as an effective quantitative measure for predicting an individual’s chances of developing diabetes.
Major finding: CMDS scores that were adjusted according to age and race of each subject were more accurate in predicting risk of developing diabetes within 15 years; black men and women aged 61 years and older were most susceptible.
Data source: CARDIA, an ongoing population-based study of 5,115 black and white adults recruited during 1985-1986, and ARIC, an ongoing prospective cohort study of 15,792 men and women aged 45-64 years recruited in 1987.
Disclosures: This study was supported by the merit review program of the Department of Veterans Affairs, National Institutes of Health, and the UAB Diabetes Research Center. Dr. Guo is supported by an institutional training grant from the Eunice Kennedy Shriver National Institute of Child Health & Human Development at the NIH.
Guidelines ineffective for reprocessing of endoscopes, researchers say
Reprocessing guidelines currently in place for clinical gastrointestinal endoscopes need to be reevaluated and updated as soon as possible, according to a new study published in the American Journal of Infection Control (doi: 10.1016/j.ajic.2015.03.003).
Procedures that follow current guidelines do not include fully decontaminating the colonoscopes and gastroscopes after they’re used for procedures, leaving behind microbe levels that are well above the accepted benchmark standards.
“Previous studies show that cleaning endoscope channels is laborious and time consuming, and technicians often skip steps,” wrote the study’s authors – led by Cori L. Ofstead, MSPH, of Ofstead & Associates, St. Paul, Minn. “This is concerning, given that the procedure-related risk of endoscopy may be higher than previously thought [and] without objective verification, clinicians can unknowingly use contaminated endoscopes for procedures.”
The study, conducted at the Mayo Clinic in Rochester, Minn., examined the use of 15 endoscopes involved in 60 separate procedures over the period of Nov. 4-8, 2013, to determine how each endoscope was treated after an operation and whether these cleaning procedures followed guidelines and effectively decontaminated the devices.
“Endoscope testing was performed in a dedicated room adjacent to the procedure room, which allowed for rapid sampling and testing [and] barrier separation [minimized] potential for environmental cross-contamination.” the authors explained.
They maintained aseptic environmental conditions while gathering data, using disinfectant wipes on surfaces, using disposable absorbent pads, and restricting room access. The researchers wore gloves, impervious gowns, face masks with splash protection, hair nets, and shoe covers; the gloves were changed between each sample collection, and gowns were changed between endoscope collections.
Microbial cultures and rapid indicator tests were conducted for ATP, protein, hemoglobin, and carbohydrate residue. Viable microbe samples were collected from 92% of bedside-cleaned endoscopes, 46% of manually cleaned endoscopes, 64% of “high-level disinfected” endoscopes, and 9% of stored endoscopes. Results from rapid indicator tests showed that some form of contamination was present on 100% of bedside-cleaned endoscopes, 92% of manually cleaned endoscopes, 73% of high-level disinfected endoscopes, and 82% of stored endoscopes which had viable microbes.
“Current guidelines rely on visual inspection to verify cleaning [but] visible contamination was never present, potentially because blood and feces may be difficult to discern from endoscopes’ black exteriors, and microscopic organisms cannot be seen by the naked eye,” the researchers wrote.
Consequently, Ms. Ofstead and colleagues called for a reexamination of current guidelines to move away from the eyeball test, saying that the results found here should pave the way for future studies to devise methods of more effectively cleaning and disinfecting endoscopes in a shorter amount of time.
“In the meantime, methods to ensure effectiveness of reprocessing practices are needed, including the potential use of routine monitoring with rapid indicators and microbiologic cultures,” they suggested.
Ms. Ofstead is president and CEO of Ofstead & Associates, which has received research funding and speaking honoraria related to endoscopic procedures from 3M, Advanced Sterilization Products, Medivators, Invendo Medical, Boston Scientific, and Steris. Three coauthors are employed by Ofstead & Associates, but have no other disclosures to report. The remaining coauthors had no disclosures to report. Funding was provided by 3M, Mayo Clinic, and Ofstead & Associates.
Reprocessing guidelines currently in place for clinical gastrointestinal endoscopes need to be reevaluated and updated as soon as possible, according to a new study published in the American Journal of Infection Control (doi: 10.1016/j.ajic.2015.03.003).
Procedures that follow current guidelines do not include fully decontaminating the colonoscopes and gastroscopes after they’re used for procedures, leaving behind microbe levels that are well above the accepted benchmark standards.
“Previous studies show that cleaning endoscope channels is laborious and time consuming, and technicians often skip steps,” wrote the study’s authors – led by Cori L. Ofstead, MSPH, of Ofstead & Associates, St. Paul, Minn. “This is concerning, given that the procedure-related risk of endoscopy may be higher than previously thought [and] without objective verification, clinicians can unknowingly use contaminated endoscopes for procedures.”
The study, conducted at the Mayo Clinic in Rochester, Minn., examined the use of 15 endoscopes involved in 60 separate procedures over the period of Nov. 4-8, 2013, to determine how each endoscope was treated after an operation and whether these cleaning procedures followed guidelines and effectively decontaminated the devices.
“Endoscope testing was performed in a dedicated room adjacent to the procedure room, which allowed for rapid sampling and testing [and] barrier separation [minimized] potential for environmental cross-contamination.” the authors explained.
They maintained aseptic environmental conditions while gathering data, using disinfectant wipes on surfaces, using disposable absorbent pads, and restricting room access. The researchers wore gloves, impervious gowns, face masks with splash protection, hair nets, and shoe covers; the gloves were changed between each sample collection, and gowns were changed between endoscope collections.
Microbial cultures and rapid indicator tests were conducted for ATP, protein, hemoglobin, and carbohydrate residue. Viable microbe samples were collected from 92% of bedside-cleaned endoscopes, 46% of manually cleaned endoscopes, 64% of “high-level disinfected” endoscopes, and 9% of stored endoscopes. Results from rapid indicator tests showed that some form of contamination was present on 100% of bedside-cleaned endoscopes, 92% of manually cleaned endoscopes, 73% of high-level disinfected endoscopes, and 82% of stored endoscopes which had viable microbes.
“Current guidelines rely on visual inspection to verify cleaning [but] visible contamination was never present, potentially because blood and feces may be difficult to discern from endoscopes’ black exteriors, and microscopic organisms cannot be seen by the naked eye,” the researchers wrote.
Consequently, Ms. Ofstead and colleagues called for a reexamination of current guidelines to move away from the eyeball test, saying that the results found here should pave the way for future studies to devise methods of more effectively cleaning and disinfecting endoscopes in a shorter amount of time.
“In the meantime, methods to ensure effectiveness of reprocessing practices are needed, including the potential use of routine monitoring with rapid indicators and microbiologic cultures,” they suggested.
Ms. Ofstead is president and CEO of Ofstead & Associates, which has received research funding and speaking honoraria related to endoscopic procedures from 3M, Advanced Sterilization Products, Medivators, Invendo Medical, Boston Scientific, and Steris. Three coauthors are employed by Ofstead & Associates, but have no other disclosures to report. The remaining coauthors had no disclosures to report. Funding was provided by 3M, Mayo Clinic, and Ofstead & Associates.
Reprocessing guidelines currently in place for clinical gastrointestinal endoscopes need to be reevaluated and updated as soon as possible, according to a new study published in the American Journal of Infection Control (doi: 10.1016/j.ajic.2015.03.003).
Procedures that follow current guidelines do not include fully decontaminating the colonoscopes and gastroscopes after they’re used for procedures, leaving behind microbe levels that are well above the accepted benchmark standards.
“Previous studies show that cleaning endoscope channels is laborious and time consuming, and technicians often skip steps,” wrote the study’s authors – led by Cori L. Ofstead, MSPH, of Ofstead & Associates, St. Paul, Minn. “This is concerning, given that the procedure-related risk of endoscopy may be higher than previously thought [and] without objective verification, clinicians can unknowingly use contaminated endoscopes for procedures.”
The study, conducted at the Mayo Clinic in Rochester, Minn., examined the use of 15 endoscopes involved in 60 separate procedures over the period of Nov. 4-8, 2013, to determine how each endoscope was treated after an operation and whether these cleaning procedures followed guidelines and effectively decontaminated the devices.
“Endoscope testing was performed in a dedicated room adjacent to the procedure room, which allowed for rapid sampling and testing [and] barrier separation [minimized] potential for environmental cross-contamination.” the authors explained.
They maintained aseptic environmental conditions while gathering data, using disinfectant wipes on surfaces, using disposable absorbent pads, and restricting room access. The researchers wore gloves, impervious gowns, face masks with splash protection, hair nets, and shoe covers; the gloves were changed between each sample collection, and gowns were changed between endoscope collections.
Microbial cultures and rapid indicator tests were conducted for ATP, protein, hemoglobin, and carbohydrate residue. Viable microbe samples were collected from 92% of bedside-cleaned endoscopes, 46% of manually cleaned endoscopes, 64% of “high-level disinfected” endoscopes, and 9% of stored endoscopes. Results from rapid indicator tests showed that some form of contamination was present on 100% of bedside-cleaned endoscopes, 92% of manually cleaned endoscopes, 73% of high-level disinfected endoscopes, and 82% of stored endoscopes which had viable microbes.
“Current guidelines rely on visual inspection to verify cleaning [but] visible contamination was never present, potentially because blood and feces may be difficult to discern from endoscopes’ black exteriors, and microscopic organisms cannot be seen by the naked eye,” the researchers wrote.
Consequently, Ms. Ofstead and colleagues called for a reexamination of current guidelines to move away from the eyeball test, saying that the results found here should pave the way for future studies to devise methods of more effectively cleaning and disinfecting endoscopes in a shorter amount of time.
“In the meantime, methods to ensure effectiveness of reprocessing practices are needed, including the potential use of routine monitoring with rapid indicators and microbiologic cultures,” they suggested.
Ms. Ofstead is president and CEO of Ofstead & Associates, which has received research funding and speaking honoraria related to endoscopic procedures from 3M, Advanced Sterilization Products, Medivators, Invendo Medical, Boston Scientific, and Steris. Three coauthors are employed by Ofstead & Associates, but have no other disclosures to report. The remaining coauthors had no disclosures to report. Funding was provided by 3M, Mayo Clinic, and Ofstead & Associates.
FROM AMERICAN JOURNAL OF INFECTION CONTROL
Key clinical point: Current guidelines for the reprocessing of colonoscopes and gastroscopes need to be reevaluated, as they do not guarantee completely successful decontamination.
Major finding: Contamination levels above indicated benchmark levels were found on 100% of bedside-cleaned endoscopes, 92% of manually cleaned endoscopes, 73% of high-level disinfected endoscopes, and 83% of stored endoscopes.
Data source: Study of 15 endoscopes used during 60 encounters/procedures at the Mayo Clinic from Nov. 4 to Nov. 8, 2013.
Disclosures: Funding was provided by 3M, Mayo Clinic, and Ofstead & Associates, which employs Ms. Ofstead and three coauthors.









