User login
From smiling to smizing: Assessing the affect of a patient wearing a mask
Although the guidelines for masking in hospitals and other health care settings have been revised and face masks are no longer mandatory, it is important to note that some patients and clinicians will choose to continue wearing masks for various personal or clinical reasons. While effective in reducing transmission of the coronavirus, masks have created challenges in assessing patients’ affective states, which impacts the accuracy of diagnosis and treatment. This article discusses strategies for assessing affect in patients wearing face masks.
How masks complicate assessing affect
One obvious challenge masks present is they prevent clinicians from seeing their patients’ facial expressions. Face masks cover the mouth, nose, and cheeks, all of which are involved in communicating emotions. As a result, clinicians may miss important cues that could inform their assessment of a patient’s affect. For example, when a masked patient is smiling, it is difficult to determine whether their smile is genuine or forced. A study that evaluated the interpretation of 6 emotions (angry, disgusted, fearful, happy, neutral, and sad) in masked patients found that emotion recognition was significantly reduced for all emotions except for fearful and neutral faces.1
Another challenge is the potential for misinterpretation. Health care professionals may rely more heavily on nonverbal cues, such as body language, to interpret a patient’s affect. However, these cues can be influenced by other factors, such as cultural differences and individual variations in communication style. Culture is a key component in assessing nonverbal emotion reading cues.2
Strategies to overcome these challenges
There are several strategies clinicians can use to overcome the difficulties of assessing affect while a patient is wearing a mask:
Focus on other nonverbal cues, such as a patient’s posture and hand gestures. Verbal cues—such as tone of voice, choice of words, and voice inflection—can also provide valuable insights. For example, a patient who speaks in a hesitant or monotone voice may be experiencing anxiety or depression. Clinicians can ask open-ended questions, encouraging patients to expand on their emotions and provide further information about their affect.
Maintain eye contact. Eye contact is an essential component of nonverbal communication. The eyes are “the window of the soul” and can convey various emotions including happiness, sadness, fear, anger, surprise, trust, interest, and empathy. Maintaining eye contact is crucial for building positive relationships with patients, and learning to smile with your eyes (smize) can help build rapport.
Take advantage of technology. Clinicians can leverage telemedicine to assess affect. Telemedicine platforms, which have become increasingly popular during the COVID-19 pandemic, allow clinicians to monitor patients remotely and observe nonverbal cues. Virtual reality technology can also help by documenting physiological responses such as heart rate and skin conductance.
Use standardized assessment tools, as these instruments can aid in assessing affect. For example, the Patient Health Questionnaire-9 and Generalized Anxiety Disorder 7-item scale are standardized questionnaires assessing depression and anxiety, respectively. Administering these tools to patients wearing a face mask can provide information about their affective state.
1. Carbon CC. Wearing face masks strongly confuses counterparts in reading emotions. Front Psychol. 2020;11:566886. doi:10.3389/fpsyg.2020.566886
2. Yuki M, Maddux WW, Masuda T. Are the windows to the soul the same in the East and West? Cultural differences in using the eyes and mouth as cues to recognize emotions in Japan and the United States. J Exp Soc Psychol. 2007;43(2):303-311.
Although the guidelines for masking in hospitals and other health care settings have been revised and face masks are no longer mandatory, it is important to note that some patients and clinicians will choose to continue wearing masks for various personal or clinical reasons. While effective in reducing transmission of the coronavirus, masks have created challenges in assessing patients’ affective states, which impacts the accuracy of diagnosis and treatment. This article discusses strategies for assessing affect in patients wearing face masks.
How masks complicate assessing affect
One obvious challenge masks present is they prevent clinicians from seeing their patients’ facial expressions. Face masks cover the mouth, nose, and cheeks, all of which are involved in communicating emotions. As a result, clinicians may miss important cues that could inform their assessment of a patient’s affect. For example, when a masked patient is smiling, it is difficult to determine whether their smile is genuine or forced. A study that evaluated the interpretation of 6 emotions (angry, disgusted, fearful, happy, neutral, and sad) in masked patients found that emotion recognition was significantly reduced for all emotions except for fearful and neutral faces.1
Another challenge is the potential for misinterpretation. Health care professionals may rely more heavily on nonverbal cues, such as body language, to interpret a patient’s affect. However, these cues can be influenced by other factors, such as cultural differences and individual variations in communication style. Culture is a key component in assessing nonverbal emotion reading cues.2
Strategies to overcome these challenges
There are several strategies clinicians can use to overcome the difficulties of assessing affect while a patient is wearing a mask:
Focus on other nonverbal cues, such as a patient’s posture and hand gestures. Verbal cues—such as tone of voice, choice of words, and voice inflection—can also provide valuable insights. For example, a patient who speaks in a hesitant or monotone voice may be experiencing anxiety or depression. Clinicians can ask open-ended questions, encouraging patients to expand on their emotions and provide further information about their affect.
Maintain eye contact. Eye contact is an essential component of nonverbal communication. The eyes are “the window of the soul” and can convey various emotions including happiness, sadness, fear, anger, surprise, trust, interest, and empathy. Maintaining eye contact is crucial for building positive relationships with patients, and learning to smile with your eyes (smize) can help build rapport.
Take advantage of technology. Clinicians can leverage telemedicine to assess affect. Telemedicine platforms, which have become increasingly popular during the COVID-19 pandemic, allow clinicians to monitor patients remotely and observe nonverbal cues. Virtual reality technology can also help by documenting physiological responses such as heart rate and skin conductance.
Use standardized assessment tools, as these instruments can aid in assessing affect. For example, the Patient Health Questionnaire-9 and Generalized Anxiety Disorder 7-item scale are standardized questionnaires assessing depression and anxiety, respectively. Administering these tools to patients wearing a face mask can provide information about their affective state.
Although the guidelines for masking in hospitals and other health care settings have been revised and face masks are no longer mandatory, it is important to note that some patients and clinicians will choose to continue wearing masks for various personal or clinical reasons. While effective in reducing transmission of the coronavirus, masks have created challenges in assessing patients’ affective states, which impacts the accuracy of diagnosis and treatment. This article discusses strategies for assessing affect in patients wearing face masks.
How masks complicate assessing affect
One obvious challenge masks present is they prevent clinicians from seeing their patients’ facial expressions. Face masks cover the mouth, nose, and cheeks, all of which are involved in communicating emotions. As a result, clinicians may miss important cues that could inform their assessment of a patient’s affect. For example, when a masked patient is smiling, it is difficult to determine whether their smile is genuine or forced. A study that evaluated the interpretation of 6 emotions (angry, disgusted, fearful, happy, neutral, and sad) in masked patients found that emotion recognition was significantly reduced for all emotions except for fearful and neutral faces.1
Another challenge is the potential for misinterpretation. Health care professionals may rely more heavily on nonverbal cues, such as body language, to interpret a patient’s affect. However, these cues can be influenced by other factors, such as cultural differences and individual variations in communication style. Culture is a key component in assessing nonverbal emotion reading cues.2
Strategies to overcome these challenges
There are several strategies clinicians can use to overcome the difficulties of assessing affect while a patient is wearing a mask:
Focus on other nonverbal cues, such as a patient’s posture and hand gestures. Verbal cues—such as tone of voice, choice of words, and voice inflection—can also provide valuable insights. For example, a patient who speaks in a hesitant or monotone voice may be experiencing anxiety or depression. Clinicians can ask open-ended questions, encouraging patients to expand on their emotions and provide further information about their affect.
Maintain eye contact. Eye contact is an essential component of nonverbal communication. The eyes are “the window of the soul” and can convey various emotions including happiness, sadness, fear, anger, surprise, trust, interest, and empathy. Maintaining eye contact is crucial for building positive relationships with patients, and learning to smile with your eyes (smize) can help build rapport.
Take advantage of technology. Clinicians can leverage telemedicine to assess affect. Telemedicine platforms, which have become increasingly popular during the COVID-19 pandemic, allow clinicians to monitor patients remotely and observe nonverbal cues. Virtual reality technology can also help by documenting physiological responses such as heart rate and skin conductance.
Use standardized assessment tools, as these instruments can aid in assessing affect. For example, the Patient Health Questionnaire-9 and Generalized Anxiety Disorder 7-item scale are standardized questionnaires assessing depression and anxiety, respectively. Administering these tools to patients wearing a face mask can provide information about their affective state.
1. Carbon CC. Wearing face masks strongly confuses counterparts in reading emotions. Front Psychol. 2020;11:566886. doi:10.3389/fpsyg.2020.566886
2. Yuki M, Maddux WW, Masuda T. Are the windows to the soul the same in the East and West? Cultural differences in using the eyes and mouth as cues to recognize emotions in Japan and the United States. J Exp Soc Psychol. 2007;43(2):303-311.
1. Carbon CC. Wearing face masks strongly confuses counterparts in reading emotions. Front Psychol. 2020;11:566886. doi:10.3389/fpsyg.2020.566886
2. Yuki M, Maddux WW, Masuda T. Are the windows to the soul the same in the East and West? Cultural differences in using the eyes and mouth as cues to recognize emotions in Japan and the United States. J Exp Soc Psychol. 2007;43(2):303-311.
Food for thought: Dangerous weight loss in an older adult
CASE Fixated on health and nutrition
At the insistence of her daughter, Ms. L, age 75, presents to the emergency department (ED) for self-neglect and severe weight loss, with a body mass index (BMI) of 13.5 kg/m2 (normal: 18.5 to 24.9 kg/m2). When asked why she is in the ED, Ms. L says she doesn’t know. She attributes her significant weight loss (approximately 20 pounds in the last few months) to gastroesophageal reflux disease (GERD). She constantly worries about her esophagus. She had been diagnosed with esophageal dysphagia 7 years ago after undergoing radiofrequency ablation for esophageal cancer. Ms. L fixates on the negative effects certain foods and ingredients might have on her stomach and esophagus.
Following transfer from the ED, Ms. L is involuntarily admitted to our inpatient unit. Although she acknowledges weight loss, she minimizes the severity of her illness and indicates she would like to gain weight, but only by eating healthy foods she is comfortable with, including kale, quinoa, and vegetables. Ms. L says that she has always been interested in “healthful foods” and that she “loves sugar,” but “it’s bad for you,” mentioning that “sugar fuels cancer.” She has daily thoughts about sugar causing cancer. Ms. L also mentions that she stopped eating flour, sugar, fried food, and oils because those foods affect her “stomach acid” and cause “pimples on my face and weight loss.” While in the inpatient unit, Ms. L requests a special diet and demands to know the origin and ingredients of the foods she is offered. She emphasizes that her esophageal cancer diagnosis and dysphagia exacerbate worries that certain foods cause cancer, and wants to continue her diet restrictions. Nonetheless, she says she wants to get healthy, and denies an intense fear of gaining weight or feeling fat.
HISTORY Multiple psychiatric diagnoses
Ms. L lives alone and enjoys spending time with her grandchildren, visiting museums, and listening to classical music. However, her family, social workers, and records from a previous psychiatric hospitalization reveal that Ms. L has a history of psychiatric illness and fears regarding certain types of foods for much of her adult life. Ms. L’s family also described a range of compulsive behaviors, including shoplifting, hoarding art, multiple plastic surgeries, and phases where Ms. L ate only frozen yogurt without sugar.
Ms. L’s daughter reported that Ms. L had seen a psychologist in the late 1990s for depression and had been diagnosed with obsessive-compulsive disorder (OCD) and attention deficit/hyperactivity disorder in the early 2000s. In 2006, during a depressive episode after her divorce, Ms. L had a suicide attempt with pills and alcohol, and was hospitalized. Records from that stay described a history of mood dysregulation with fears regarding food and nutrition. Ms. L was treated with aripiprazole 5 mg/d. A trial of trazodone 25 mg/d did not have any effect. When discharged, she was receiving lamotrigine 100 mg/d. However, her daughter believes she stopped taking all psychiatric medications shortly after discharge.
Her daughter says that in the past 2 years, Ms. L has seen multiple doctors for treatment of somatic gastrointestinal (GI) complaints. A 2018 note from a social worker indicated that Ms. L endorsed taking >80 supplements per day and constantly researched nutrition online. In the months leading to her current hospitalization, Ms. L suffered from severe self-neglect and fear regarding foods she felt were not healthy for her. She had stopped leaving her apartment.
Continue to: EVALUATION Poor insight, normal lab results...
EVALUATION Poor insight, normal lab results
During her evaluation, Ms. L appears cachectic and frail. She has a heavily constricted affect and is guarded, dismissive, and vague. Although her thought processes are linear and goal-directed, her insight into her condition is extremely poor and she appears surprised when clinicians inform her that her self-neglect would lead to death. Instead, Ms. L insists she is eating healthily and demonstrates severe anxiety in relation to her GI symptoms.
Ms. L is oriented to person, place, and time. She scores 27/30 on the Montreal Cognitive Assessment, indicating normal cognition. She denies any depressive symptoms or suicidal intent. She does not appear to be internally preoccupied and denies having auditory or visual hallucinations or manic symptoms.
A neurologic examination reveals that her cranial nerves are normal, and cerebellar function, strength, and sensory testing are intact. Her gait is steady and she walks without a walker. Despite her severely low BMI and recent history of self-neglect, Ms. L’s laboratory results are remarkably normal and show no liver, metabolic, or electrolyte abnormalities, no signs of infection, and normal vitamin B12 levels. She has slightly elevated creatinine and blood urea nitrogen levels, but a normal glomerular filtration rate.
Her medical history is significant for squamous cell esophageal cancer, treated with radiofrequency ablation. Although Ms. L is constantly worried about the recurrence of cancer, pathology reports demonstrate no esophageal dysplasia. However, she does show evidence of an approximately 1 cm × 1 cm mild, noncircumferential esophageal stenosis, likely resulting from radiofrequency ablation.
[polldaddy:11079394]
The authors’ observations
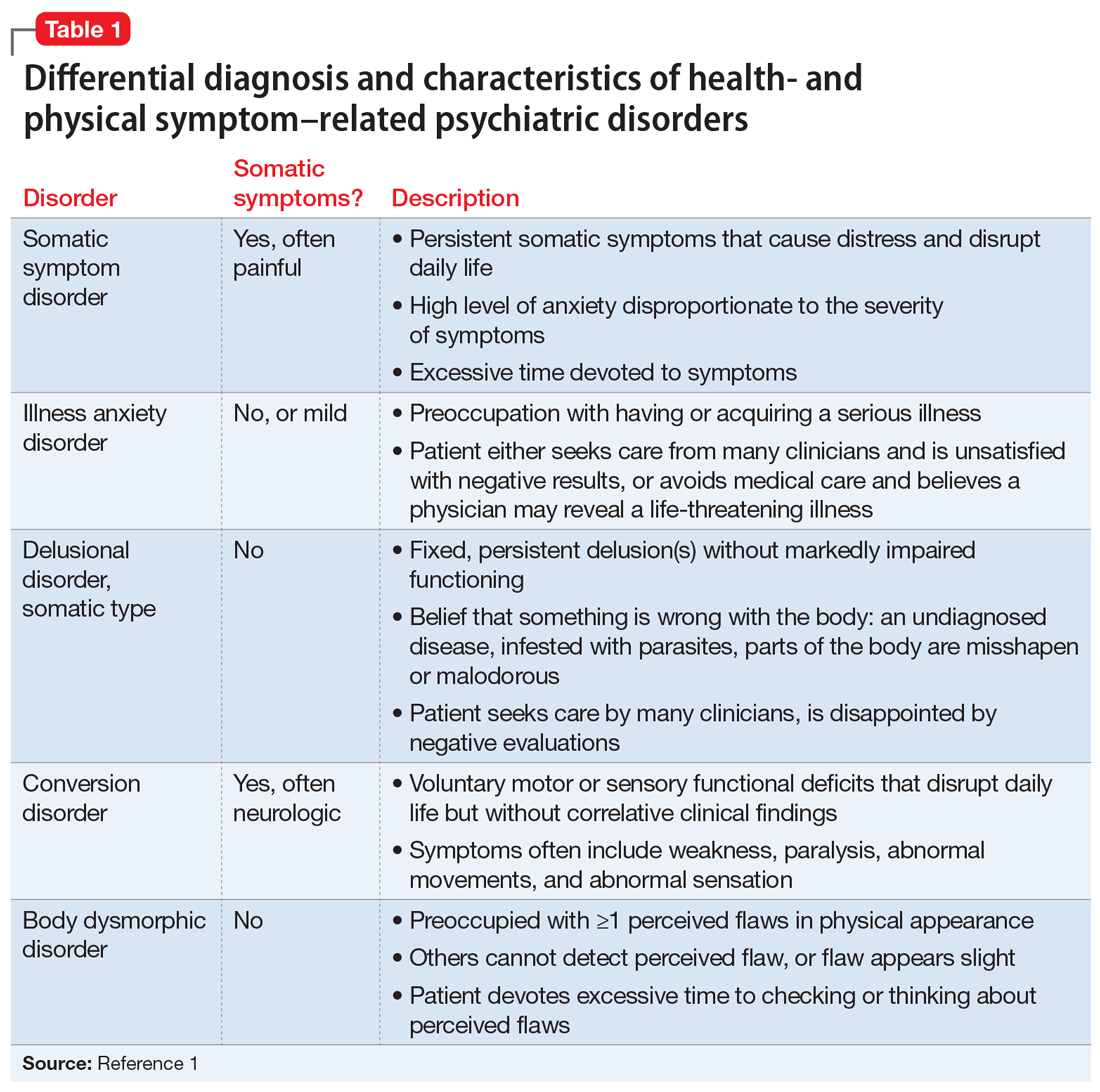
Several health- and physical symptom-related psychiatric disorders have overlapping features, which can complicate the differential diagnosis (Table 11). Ms. L presented to the ED with a severely low BMI of 13.5 kg/m2, obsessions regarding specific types of food, and preoccupations regarding her esophagus. Despite her extensive psychiatric history (including intense fears regarding food), we ruled out a primary psychotic disorder because she did not describe auditory or visual hallucinations and never appeared internally preoccupied. While her BMI and persistent minimization of the extent of her disease meet criteria for anorexia nervosa, she denied body dysmorphia and did not have any fear of gaining weight.
A central element of Ms. L’s presentation was her anxiety regarding how certain types of foods impact her health as well as her anxieties regarding her esophagus. While Ms. L was in remission from esophageal cancer and had a diagnosis of esophageal dysphagia, these preoccupations and obsessions regarding how certain types of foods affect her esophagus drove her to self-neglect and thus represent pathologic thought processes out of proportion to her symptoms. Illness anxiety disorder was considered because Ms. L met many of its criteria: preoccupation with having a serious illness, disproportionate preoccupation with somatic symptoms if they are present, extreme anxiety over health, and performance of health-related behaviors.1 However, illness anxiety disorder is a diagnosis of exclusion, and 1 criterion is that these symptoms cannot be explained by another mental disorder. We felt other diagnoses better fit Ms. L’s condition and ruled out illness anxiety disorder.
Ms. L’s long history of food and non-food–related obsessions and compulsions that interrupted her ability to perform daily activities were strongly suggestive for OCD. Additionally, her intense preoccupation, high level of anxiety, amount of time and energy spent seeking care for her esophagus and GERD symptoms, and the resulting significant disruption of daily life, met criteria for somatic symptom disorder (SSD). However, we did not believe that a diagnosis of OCD and SSD alone explained the entirety of Ms. L’s clinical picture. Despite ruling out anorexia nervosa, Ms. L nonetheless demonstrated disordered eating.
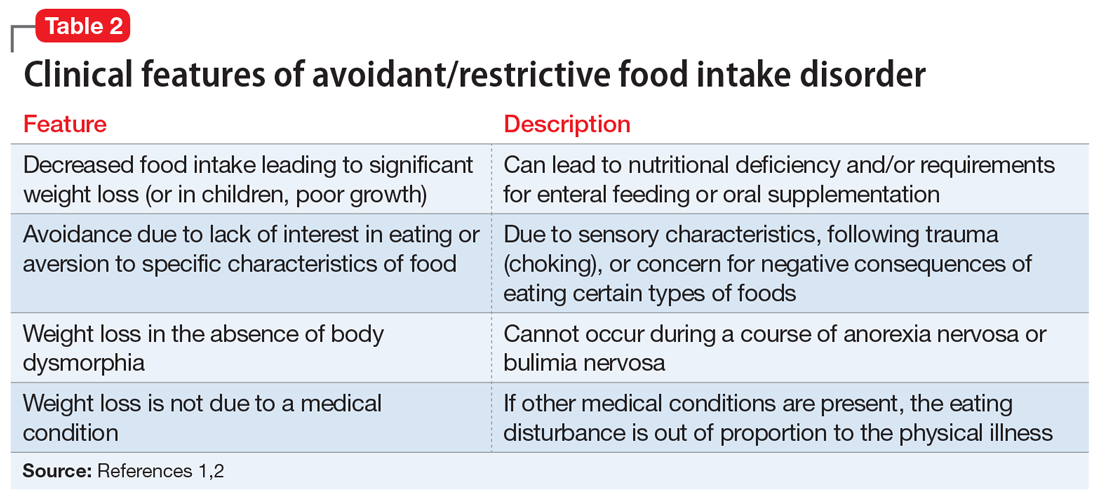
Avoidant/restrictive food intake disorder (ARFID) is an eating disorder in which patients restrict their diet and do not meet nutritional needs for any number of reasons, do not experience body dysmorphia, and do not fear weight gain.1 A common feature of ARFID is a fear of negative consequences from eating specific types of food.2 Table 21,2 summarizes additional clinical features of ARFID. Although ARFID is typically diagnosed in children and adolescents, particularly in individuals with autism with heightened sensory sensitivities, ARFID is also common among adult patients with GI disorders.3 In a retrospective chart review of 410 adults ages 18 to 90 (73% women) referred to a neurogastroenterology care center, 6.3% met the full criteria for ARFID and 17.3% had clinically significant avoidant or restrictive eating behaviors. Among patients with ARFID symptoms, 93% stated that a fear of GI symptoms was the driver of their avoidant or restrictive eating behaviors.2 Patients with GI diseases often develop dietary control and avoidance coping mechanisms to alleviate their symptoms.4 These strategies can exacerbate health anxieties and have a detrimental effect on mental health.5 Patients with GI disorders have a high degree of comorbidity with affective disorders, including anxiety disorders.6 These trends may arise from hypervigilance and the need to gain control over physical symptoms.7 Feeling a need for control, actions driven by anxiety and fear, and the need for compensatory behaviors are cardinal features of OCD and eating disorders.8 Multiple studies have demonstrated comorbidities between irritable bowel syndrome and eating disorders,9 SSD,10 and OCD.11 Taken together with observations that ARFID is also found in patients with GI disorders,2 these findings demonstrate that patients with a history of GI disease are at high risk of developing extreme health anxieties and behavioral coping strategies that can lead to disordered eating.
The rise in “healthy” eating materials online—particularly on social media—has created an atmosphere in which misinformation regarding diet and health is common and widespread. For patients with OCD and a predisposition to health anxiety, such as Ms. L, searching online for nutrition information and healthy living habits can exacerbate food-related anxieties and can lead to a pathological drive for purity and health.12Although not included in DSM-5, orthorexia nervosa was identified in 1997 as a proposed eating disorder best characterized as an obsession with healthy eating with associated restrictive behaviors.13 Patients with this disorder are rarely focused on losing weight, and orthorexic eating behaviors have been associated with both SSD and OCD.12,14 As in Ms. L’s case, patients with orthorexia nervosa demonstrate intrusive obsessions with nutrition, spend excessive amount of time researching nutrition, and fixate on food quality.12 Throughout Ms. L’s hospitalization, even as her food-related magical thinking symptoms improved, she constantly informed her care team that she had been “eating healthily” even though she was severely cachectic. Patients with SSD and OCD prone to health anxieties are at risk of developing pathologic food beliefs and dangerous eating behaviors. These patients may benefit from psychoeducation regarding nutrition and media literacy, which are components of effective eating disorder programs.15
[polldaddy:11079399]
Continue to: The authors' observations...
The authors’ observations
How do we approach the pharmacologic treatment of patients with co-occurring eating, somatic symptom, and anxiety disorders? Olanzapine facilitates recovery in children and adolescents with ARFID by promoting eating and weight gain, and decreasing symptoms of depression and anxiety.16 Patients with orthorexia nervosa also may benefit from treatment with olanzapine, which has decreased food-related fixations, magical thinking, and delusions regarding food.17 Further, orthorexic patients with ARFID have also been shown to respond to SSRIs due to those agents’ efficacy for treating intrusive thoughts, obsessions, and preoccupations from OCD and SSD.18,19 Thus, treating Ms. L’s symptoms with olanzapine and fluoxetine targeted the intersection of several diagnoses on our differential. Olanzapine’s propensity to cause weight gain is favorable in this population, particularly patients such as Ms. L, who do not exhibit body dysmorphia or fear of gaining weight.
OUTCOME Weight gain and fewer fears
Ms. L is prescribed olanzapine 5 mg/d and fluoxetine 20 mg/d. She gains 20.6 pounds in 4 weeks. Importantly, she endorses fewer fears related to foods and expands her palate to include foods she previously considered to be unhealthy, including white bread and farm-raised salmon. Further, she spends less time thinking about food and says she has less anxiety regarding the recurrence of GI symptoms.
1. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; 2013.
2. Murray HB, Bailey AP, Keshishian AC. Prevalence and characteristics of avoidant/restrictive food intake disorder in adult neurogastroenterology patients. Clin Gastroenterol Hepatol. 2020;18(9):1995-2002.e1.
3. Görmez A, Kılıç A, Kırpınar İ. Avoidant/restrictive food intake disorder: an adult case responding to cognitive behavioral therapy. Clinical Case Studies. 2018;17(6):443-452.
4. Reed-Knight B, Squires M, Chitkara DK, et al. Adolescents with irritable bowel syndrome report increased eating-associated symptoms, changes in dietary composition, and altered eating behaviors: a pilot comparison study to healthy adolescents. Neurogastroenterol Motil. 2016;28(12):1915-1920.
5. Melchior C, Desprez C, Riachi G, et al. Anxiety and depression profile is associated with eating disorders in patients with irritable bowel syndrome. Front Psychiatry. 2020;10:928.
6. Mayer EA, Craske M, Naliboff BD. Depression, anxiety, and the gastrointestinal system. J Clin Psychiatry. 2001;62 Suppl 8:28-37.
7. Abraham S, Kellow J. Exploring eating disorder quality of life and functional gastrointestinal disorders among eating disorder patients. J Psychosom Res. 2011;70(4):372-377.
8. Swinbourne JM, Touyz SW. The co-morbidity of eating disorders and anxiety disorders: a review. Eur Eat Disord Rev. 2007;15(4):253-274.
9. Perkins SJ, Keville S, Schmidt U, et al. Eating disorders and irritable bowel syndrome: is there a link? J Psychosom Res. 2005;59(2):57-64.
10. Hausteiner-Wiehle C, Henningsen P. Irritable bowel syndrome: relations with functional, mental, and somatoform disorders. World J Gastroenterol. 2014;20(2):6024-6030.
11. Masand PS, Keuthen NJ, Gupta S, et al. Prevalence of irritable bowel syndrome in obsessive-compulsive disorder. CNS Spectr. 2006;11(1):21-25.
12. Koven NS, Abry AW. The clinical basis of orthorexia nervosa: emerging perspectives. Neuropsychiatr Dis Treat. 2015;11:385-394.
13. Bratman S. Health food junkie. Yoga Journal. 1997;136:42-50.
14. Barthels F, Müller R, Schüth T, et al. Orthorexic eating behavior in patients with somatoform disorders. Eat Weight Disord. 2021;26(1):135-143.
15. Ciao AC, Loth K, Neumark-Sztainer D. Preventing eating disorder pathology: common and unique features of successful eating disorders prevention programs. Curr Psychiatry Rep. 2014;16(7):453.
16. Brewerton TD, D’Agostino M. Adjunctive use of olanzapine in the treatment of avoidant restrictive food intake disorder in children and adolescents in an eating disorders program. J Child Adolesc Psychopharmacol. 2017;27(10):920-922.
17. Moroze RM, Dunn TM, Craig Holland J, et al. Microthinking about micronutrients: a case of transition from obsessions about healthy eating to near-fatal “orthorexia nervosa” and proposed diagnostic criteria. Psychosomatics. 2015;56(4):397-403.
18. Spettigue W, Norris ML, Santos A, et al. Treatment of children and adolescents with avoidant/restrictive food intake disorder: a case series examining the feasibility of family therapy and adjunctive treatments. J Eat Disord. 2018;6:20.
19. Niedzielski A, Kaźmierczak-Wojtaś N. Prevalence of Orthorexia Nervosa and Its Diagnostic Tools-A Literature Review. Int J Environ Res Public Health. 2021;18(10):5488. Published 2021 May 20. doi:10.3390/ijerph18105488 Prevalence of orthorexia nervosa and its diagnostic tools-a literature review. Int J Environ Res Public Health. 2021;18(10):5488.
CASE Fixated on health and nutrition
At the insistence of her daughter, Ms. L, age 75, presents to the emergency department (ED) for self-neglect and severe weight loss, with a body mass index (BMI) of 13.5 kg/m2 (normal: 18.5 to 24.9 kg/m2). When asked why she is in the ED, Ms. L says she doesn’t know. She attributes her significant weight loss (approximately 20 pounds in the last few months) to gastroesophageal reflux disease (GERD). She constantly worries about her esophagus. She had been diagnosed with esophageal dysphagia 7 years ago after undergoing radiofrequency ablation for esophageal cancer. Ms. L fixates on the negative effects certain foods and ingredients might have on her stomach and esophagus.
Following transfer from the ED, Ms. L is involuntarily admitted to our inpatient unit. Although she acknowledges weight loss, she minimizes the severity of her illness and indicates she would like to gain weight, but only by eating healthy foods she is comfortable with, including kale, quinoa, and vegetables. Ms. L says that she has always been interested in “healthful foods” and that she “loves sugar,” but “it’s bad for you,” mentioning that “sugar fuels cancer.” She has daily thoughts about sugar causing cancer. Ms. L also mentions that she stopped eating flour, sugar, fried food, and oils because those foods affect her “stomach acid” and cause “pimples on my face and weight loss.” While in the inpatient unit, Ms. L requests a special diet and demands to know the origin and ingredients of the foods she is offered. She emphasizes that her esophageal cancer diagnosis and dysphagia exacerbate worries that certain foods cause cancer, and wants to continue her diet restrictions. Nonetheless, she says she wants to get healthy, and denies an intense fear of gaining weight or feeling fat.
HISTORY Multiple psychiatric diagnoses
Ms. L lives alone and enjoys spending time with her grandchildren, visiting museums, and listening to classical music. However, her family, social workers, and records from a previous psychiatric hospitalization reveal that Ms. L has a history of psychiatric illness and fears regarding certain types of foods for much of her adult life. Ms. L’s family also described a range of compulsive behaviors, including shoplifting, hoarding art, multiple plastic surgeries, and phases where Ms. L ate only frozen yogurt without sugar.
Ms. L’s daughter reported that Ms. L had seen a psychologist in the late 1990s for depression and had been diagnosed with obsessive-compulsive disorder (OCD) and attention deficit/hyperactivity disorder in the early 2000s. In 2006, during a depressive episode after her divorce, Ms. L had a suicide attempt with pills and alcohol, and was hospitalized. Records from that stay described a history of mood dysregulation with fears regarding food and nutrition. Ms. L was treated with aripiprazole 5 mg/d. A trial of trazodone 25 mg/d did not have any effect. When discharged, she was receiving lamotrigine 100 mg/d. However, her daughter believes she stopped taking all psychiatric medications shortly after discharge.
Her daughter says that in the past 2 years, Ms. L has seen multiple doctors for treatment of somatic gastrointestinal (GI) complaints. A 2018 note from a social worker indicated that Ms. L endorsed taking >80 supplements per day and constantly researched nutrition online. In the months leading to her current hospitalization, Ms. L suffered from severe self-neglect and fear regarding foods she felt were not healthy for her. She had stopped leaving her apartment.
Continue to: EVALUATION Poor insight, normal lab results...
EVALUATION Poor insight, normal lab results
During her evaluation, Ms. L appears cachectic and frail. She has a heavily constricted affect and is guarded, dismissive, and vague. Although her thought processes are linear and goal-directed, her insight into her condition is extremely poor and she appears surprised when clinicians inform her that her self-neglect would lead to death. Instead, Ms. L insists she is eating healthily and demonstrates severe anxiety in relation to her GI symptoms.
Ms. L is oriented to person, place, and time. She scores 27/30 on the Montreal Cognitive Assessment, indicating normal cognition. She denies any depressive symptoms or suicidal intent. She does not appear to be internally preoccupied and denies having auditory or visual hallucinations or manic symptoms.
A neurologic examination reveals that her cranial nerves are normal, and cerebellar function, strength, and sensory testing are intact. Her gait is steady and she walks without a walker. Despite her severely low BMI and recent history of self-neglect, Ms. L’s laboratory results are remarkably normal and show no liver, metabolic, or electrolyte abnormalities, no signs of infection, and normal vitamin B12 levels. She has slightly elevated creatinine and blood urea nitrogen levels, but a normal glomerular filtration rate.
Her medical history is significant for squamous cell esophageal cancer, treated with radiofrequency ablation. Although Ms. L is constantly worried about the recurrence of cancer, pathology reports demonstrate no esophageal dysplasia. However, she does show evidence of an approximately 1 cm × 1 cm mild, noncircumferential esophageal stenosis, likely resulting from radiofrequency ablation.
[polldaddy:11079394]
The authors’ observations
Several health- and physical symptom-related psychiatric disorders have overlapping features, which can complicate the differential diagnosis (Table 11). Ms. L presented to the ED with a severely low BMI of 13.5 kg/m2, obsessions regarding specific types of food, and preoccupations regarding her esophagus. Despite her extensive psychiatric history (including intense fears regarding food), we ruled out a primary psychotic disorder because she did not describe auditory or visual hallucinations and never appeared internally preoccupied. While her BMI and persistent minimization of the extent of her disease meet criteria for anorexia nervosa, she denied body dysmorphia and did not have any fear of gaining weight.

A central element of Ms. L’s presentation was her anxiety regarding how certain types of foods impact her health as well as her anxieties regarding her esophagus. While Ms. L was in remission from esophageal cancer and had a diagnosis of esophageal dysphagia, these preoccupations and obsessions regarding how certain types of foods affect her esophagus drove her to self-neglect and thus represent pathologic thought processes out of proportion to her symptoms. Illness anxiety disorder was considered because Ms. L met many of its criteria: preoccupation with having a serious illness, disproportionate preoccupation with somatic symptoms if they are present, extreme anxiety over health, and performance of health-related behaviors.1 However, illness anxiety disorder is a diagnosis of exclusion, and 1 criterion is that these symptoms cannot be explained by another mental disorder. We felt other diagnoses better fit Ms. L’s condition and ruled out illness anxiety disorder.
Ms. L’s long history of food and non-food–related obsessions and compulsions that interrupted her ability to perform daily activities were strongly suggestive for OCD. Additionally, her intense preoccupation, high level of anxiety, amount of time and energy spent seeking care for her esophagus and GERD symptoms, and the resulting significant disruption of daily life, met criteria for somatic symptom disorder (SSD). However, we did not believe that a diagnosis of OCD and SSD alone explained the entirety of Ms. L’s clinical picture. Despite ruling out anorexia nervosa, Ms. L nonetheless demonstrated disordered eating.
Avoidant/restrictive food intake disorder (ARFID) is an eating disorder in which patients restrict their diet and do not meet nutritional needs for any number of reasons, do not experience body dysmorphia, and do not fear weight gain.1 A common feature of ARFID is a fear of negative consequences from eating specific types of food.2 Table 21,2 summarizes additional clinical features of ARFID. Although ARFID is typically diagnosed in children and adolescents, particularly in individuals with autism with heightened sensory sensitivities, ARFID is also common among adult patients with GI disorders.3 In a retrospective chart review of 410 adults ages 18 to 90 (73% women) referred to a neurogastroenterology care center, 6.3% met the full criteria for ARFID and 17.3% had clinically significant avoidant or restrictive eating behaviors. Among patients with ARFID symptoms, 93% stated that a fear of GI symptoms was the driver of their avoidant or restrictive eating behaviors.2 Patients with GI diseases often develop dietary control and avoidance coping mechanisms to alleviate their symptoms.4 These strategies can exacerbate health anxieties and have a detrimental effect on mental health.5 Patients with GI disorders have a high degree of comorbidity with affective disorders, including anxiety disorders.6 These trends may arise from hypervigilance and the need to gain control over physical symptoms.7 Feeling a need for control, actions driven by anxiety and fear, and the need for compensatory behaviors are cardinal features of OCD and eating disorders.8 Multiple studies have demonstrated comorbidities between irritable bowel syndrome and eating disorders,9 SSD,10 and OCD.11 Taken together with observations that ARFID is also found in patients with GI disorders,2 these findings demonstrate that patients with a history of GI disease are at high risk of developing extreme health anxieties and behavioral coping strategies that can lead to disordered eating.

The rise in “healthy” eating materials online—particularly on social media—has created an atmosphere in which misinformation regarding diet and health is common and widespread. For patients with OCD and a predisposition to health anxiety, such as Ms. L, searching online for nutrition information and healthy living habits can exacerbate food-related anxieties and can lead to a pathological drive for purity and health.12Although not included in DSM-5, orthorexia nervosa was identified in 1997 as a proposed eating disorder best characterized as an obsession with healthy eating with associated restrictive behaviors.13 Patients with this disorder are rarely focused on losing weight, and orthorexic eating behaviors have been associated with both SSD and OCD.12,14 As in Ms. L’s case, patients with orthorexia nervosa demonstrate intrusive obsessions with nutrition, spend excessive amount of time researching nutrition, and fixate on food quality.12 Throughout Ms. L’s hospitalization, even as her food-related magical thinking symptoms improved, she constantly informed her care team that she had been “eating healthily” even though she was severely cachectic. Patients with SSD and OCD prone to health anxieties are at risk of developing pathologic food beliefs and dangerous eating behaviors. These patients may benefit from psychoeducation regarding nutrition and media literacy, which are components of effective eating disorder programs.15
[polldaddy:11079399]
Continue to: The authors' observations...
The authors’ observations
How do we approach the pharmacologic treatment of patients with co-occurring eating, somatic symptom, and anxiety disorders? Olanzapine facilitates recovery in children and adolescents with ARFID by promoting eating and weight gain, and decreasing symptoms of depression and anxiety.16 Patients with orthorexia nervosa also may benefit from treatment with olanzapine, which has decreased food-related fixations, magical thinking, and delusions regarding food.17 Further, orthorexic patients with ARFID have also been shown to respond to SSRIs due to those agents’ efficacy for treating intrusive thoughts, obsessions, and preoccupations from OCD and SSD.18,19 Thus, treating Ms. L’s symptoms with olanzapine and fluoxetine targeted the intersection of several diagnoses on our differential. Olanzapine’s propensity to cause weight gain is favorable in this population, particularly patients such as Ms. L, who do not exhibit body dysmorphia or fear of gaining weight.
OUTCOME Weight gain and fewer fears
Ms. L is prescribed olanzapine 5 mg/d and fluoxetine 20 mg/d. She gains 20.6 pounds in 4 weeks. Importantly, she endorses fewer fears related to foods and expands her palate to include foods she previously considered to be unhealthy, including white bread and farm-raised salmon. Further, she spends less time thinking about food and says she has less anxiety regarding the recurrence of GI symptoms.
CASE Fixated on health and nutrition
At the insistence of her daughter, Ms. L, age 75, presents to the emergency department (ED) for self-neglect and severe weight loss, with a body mass index (BMI) of 13.5 kg/m2 (normal: 18.5 to 24.9 kg/m2). When asked why she is in the ED, Ms. L says she doesn’t know. She attributes her significant weight loss (approximately 20 pounds in the last few months) to gastroesophageal reflux disease (GERD). She constantly worries about her esophagus. She had been diagnosed with esophageal dysphagia 7 years ago after undergoing radiofrequency ablation for esophageal cancer. Ms. L fixates on the negative effects certain foods and ingredients might have on her stomach and esophagus.
Following transfer from the ED, Ms. L is involuntarily admitted to our inpatient unit. Although she acknowledges weight loss, she minimizes the severity of her illness and indicates she would like to gain weight, but only by eating healthy foods she is comfortable with, including kale, quinoa, and vegetables. Ms. L says that she has always been interested in “healthful foods” and that she “loves sugar,” but “it’s bad for you,” mentioning that “sugar fuels cancer.” She has daily thoughts about sugar causing cancer. Ms. L also mentions that she stopped eating flour, sugar, fried food, and oils because those foods affect her “stomach acid” and cause “pimples on my face and weight loss.” While in the inpatient unit, Ms. L requests a special diet and demands to know the origin and ingredients of the foods she is offered. She emphasizes that her esophageal cancer diagnosis and dysphagia exacerbate worries that certain foods cause cancer, and wants to continue her diet restrictions. Nonetheless, she says she wants to get healthy, and denies an intense fear of gaining weight or feeling fat.
HISTORY Multiple psychiatric diagnoses
Ms. L lives alone and enjoys spending time with her grandchildren, visiting museums, and listening to classical music. However, her family, social workers, and records from a previous psychiatric hospitalization reveal that Ms. L has a history of psychiatric illness and fears regarding certain types of foods for much of her adult life. Ms. L’s family also described a range of compulsive behaviors, including shoplifting, hoarding art, multiple plastic surgeries, and phases where Ms. L ate only frozen yogurt without sugar.
Ms. L’s daughter reported that Ms. L had seen a psychologist in the late 1990s for depression and had been diagnosed with obsessive-compulsive disorder (OCD) and attention deficit/hyperactivity disorder in the early 2000s. In 2006, during a depressive episode after her divorce, Ms. L had a suicide attempt with pills and alcohol, and was hospitalized. Records from that stay described a history of mood dysregulation with fears regarding food and nutrition. Ms. L was treated with aripiprazole 5 mg/d. A trial of trazodone 25 mg/d did not have any effect. When discharged, she was receiving lamotrigine 100 mg/d. However, her daughter believes she stopped taking all psychiatric medications shortly after discharge.
Her daughter says that in the past 2 years, Ms. L has seen multiple doctors for treatment of somatic gastrointestinal (GI) complaints. A 2018 note from a social worker indicated that Ms. L endorsed taking >80 supplements per day and constantly researched nutrition online. In the months leading to her current hospitalization, Ms. L suffered from severe self-neglect and fear regarding foods she felt were not healthy for her. She had stopped leaving her apartment.
Continue to: EVALUATION Poor insight, normal lab results...
EVALUATION Poor insight, normal lab results
During her evaluation, Ms. L appears cachectic and frail. She has a heavily constricted affect and is guarded, dismissive, and vague. Although her thought processes are linear and goal-directed, her insight into her condition is extremely poor and she appears surprised when clinicians inform her that her self-neglect would lead to death. Instead, Ms. L insists she is eating healthily and demonstrates severe anxiety in relation to her GI symptoms.
Ms. L is oriented to person, place, and time. She scores 27/30 on the Montreal Cognitive Assessment, indicating normal cognition. She denies any depressive symptoms or suicidal intent. She does not appear to be internally preoccupied and denies having auditory or visual hallucinations or manic symptoms.
A neurologic examination reveals that her cranial nerves are normal, and cerebellar function, strength, and sensory testing are intact. Her gait is steady and she walks without a walker. Despite her severely low BMI and recent history of self-neglect, Ms. L’s laboratory results are remarkably normal and show no liver, metabolic, or electrolyte abnormalities, no signs of infection, and normal vitamin B12 levels. She has slightly elevated creatinine and blood urea nitrogen levels, but a normal glomerular filtration rate.
Her medical history is significant for squamous cell esophageal cancer, treated with radiofrequency ablation. Although Ms. L is constantly worried about the recurrence of cancer, pathology reports demonstrate no esophageal dysplasia. However, she does show evidence of an approximately 1 cm × 1 cm mild, noncircumferential esophageal stenosis, likely resulting from radiofrequency ablation.
[polldaddy:11079394]
The authors’ observations
Several health- and physical symptom-related psychiatric disorders have overlapping features, which can complicate the differential diagnosis (Table 11). Ms. L presented to the ED with a severely low BMI of 13.5 kg/m2, obsessions regarding specific types of food, and preoccupations regarding her esophagus. Despite her extensive psychiatric history (including intense fears regarding food), we ruled out a primary psychotic disorder because she did not describe auditory or visual hallucinations and never appeared internally preoccupied. While her BMI and persistent minimization of the extent of her disease meet criteria for anorexia nervosa, she denied body dysmorphia and did not have any fear of gaining weight.

A central element of Ms. L’s presentation was her anxiety regarding how certain types of foods impact her health as well as her anxieties regarding her esophagus. While Ms. L was in remission from esophageal cancer and had a diagnosis of esophageal dysphagia, these preoccupations and obsessions regarding how certain types of foods affect her esophagus drove her to self-neglect and thus represent pathologic thought processes out of proportion to her symptoms. Illness anxiety disorder was considered because Ms. L met many of its criteria: preoccupation with having a serious illness, disproportionate preoccupation with somatic symptoms if they are present, extreme anxiety over health, and performance of health-related behaviors.1 However, illness anxiety disorder is a diagnosis of exclusion, and 1 criterion is that these symptoms cannot be explained by another mental disorder. We felt other diagnoses better fit Ms. L’s condition and ruled out illness anxiety disorder.
Ms. L’s long history of food and non-food–related obsessions and compulsions that interrupted her ability to perform daily activities were strongly suggestive for OCD. Additionally, her intense preoccupation, high level of anxiety, amount of time and energy spent seeking care for her esophagus and GERD symptoms, and the resulting significant disruption of daily life, met criteria for somatic symptom disorder (SSD). However, we did not believe that a diagnosis of OCD and SSD alone explained the entirety of Ms. L’s clinical picture. Despite ruling out anorexia nervosa, Ms. L nonetheless demonstrated disordered eating.
Avoidant/restrictive food intake disorder (ARFID) is an eating disorder in which patients restrict their diet and do not meet nutritional needs for any number of reasons, do not experience body dysmorphia, and do not fear weight gain.1 A common feature of ARFID is a fear of negative consequences from eating specific types of food.2 Table 21,2 summarizes additional clinical features of ARFID. Although ARFID is typically diagnosed in children and adolescents, particularly in individuals with autism with heightened sensory sensitivities, ARFID is also common among adult patients with GI disorders.3 In a retrospective chart review of 410 adults ages 18 to 90 (73% women) referred to a neurogastroenterology care center, 6.3% met the full criteria for ARFID and 17.3% had clinically significant avoidant or restrictive eating behaviors. Among patients with ARFID symptoms, 93% stated that a fear of GI symptoms was the driver of their avoidant or restrictive eating behaviors.2 Patients with GI diseases often develop dietary control and avoidance coping mechanisms to alleviate their symptoms.4 These strategies can exacerbate health anxieties and have a detrimental effect on mental health.5 Patients with GI disorders have a high degree of comorbidity with affective disorders, including anxiety disorders.6 These trends may arise from hypervigilance and the need to gain control over physical symptoms.7 Feeling a need for control, actions driven by anxiety and fear, and the need for compensatory behaviors are cardinal features of OCD and eating disorders.8 Multiple studies have demonstrated comorbidities between irritable bowel syndrome and eating disorders,9 SSD,10 and OCD.11 Taken together with observations that ARFID is also found in patients with GI disorders,2 these findings demonstrate that patients with a history of GI disease are at high risk of developing extreme health anxieties and behavioral coping strategies that can lead to disordered eating.

The rise in “healthy” eating materials online—particularly on social media—has created an atmosphere in which misinformation regarding diet and health is common and widespread. For patients with OCD and a predisposition to health anxiety, such as Ms. L, searching online for nutrition information and healthy living habits can exacerbate food-related anxieties and can lead to a pathological drive for purity and health.12Although not included in DSM-5, orthorexia nervosa was identified in 1997 as a proposed eating disorder best characterized as an obsession with healthy eating with associated restrictive behaviors.13 Patients with this disorder are rarely focused on losing weight, and orthorexic eating behaviors have been associated with both SSD and OCD.12,14 As in Ms. L’s case, patients with orthorexia nervosa demonstrate intrusive obsessions with nutrition, spend excessive amount of time researching nutrition, and fixate on food quality.12 Throughout Ms. L’s hospitalization, even as her food-related magical thinking symptoms improved, she constantly informed her care team that she had been “eating healthily” even though she was severely cachectic. Patients with SSD and OCD prone to health anxieties are at risk of developing pathologic food beliefs and dangerous eating behaviors. These patients may benefit from psychoeducation regarding nutrition and media literacy, which are components of effective eating disorder programs.15
[polldaddy:11079399]
Continue to: The authors' observations...
The authors’ observations
How do we approach the pharmacologic treatment of patients with co-occurring eating, somatic symptom, and anxiety disorders? Olanzapine facilitates recovery in children and adolescents with ARFID by promoting eating and weight gain, and decreasing symptoms of depression and anxiety.16 Patients with orthorexia nervosa also may benefit from treatment with olanzapine, which has decreased food-related fixations, magical thinking, and delusions regarding food.17 Further, orthorexic patients with ARFID have also been shown to respond to SSRIs due to those agents’ efficacy for treating intrusive thoughts, obsessions, and preoccupations from OCD and SSD.18,19 Thus, treating Ms. L’s symptoms with olanzapine and fluoxetine targeted the intersection of several diagnoses on our differential. Olanzapine’s propensity to cause weight gain is favorable in this population, particularly patients such as Ms. L, who do not exhibit body dysmorphia or fear of gaining weight.
OUTCOME Weight gain and fewer fears
Ms. L is prescribed olanzapine 5 mg/d and fluoxetine 20 mg/d. She gains 20.6 pounds in 4 weeks. Importantly, she endorses fewer fears related to foods and expands her palate to include foods she previously considered to be unhealthy, including white bread and farm-raised salmon. Further, she spends less time thinking about food and says she has less anxiety regarding the recurrence of GI symptoms.
1. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; 2013.
2. Murray HB, Bailey AP, Keshishian AC. Prevalence and characteristics of avoidant/restrictive food intake disorder in adult neurogastroenterology patients. Clin Gastroenterol Hepatol. 2020;18(9):1995-2002.e1.
3. Görmez A, Kılıç A, Kırpınar İ. Avoidant/restrictive food intake disorder: an adult case responding to cognitive behavioral therapy. Clinical Case Studies. 2018;17(6):443-452.
4. Reed-Knight B, Squires M, Chitkara DK, et al. Adolescents with irritable bowel syndrome report increased eating-associated symptoms, changes in dietary composition, and altered eating behaviors: a pilot comparison study to healthy adolescents. Neurogastroenterol Motil. 2016;28(12):1915-1920.
5. Melchior C, Desprez C, Riachi G, et al. Anxiety and depression profile is associated with eating disorders in patients with irritable bowel syndrome. Front Psychiatry. 2020;10:928.
6. Mayer EA, Craske M, Naliboff BD. Depression, anxiety, and the gastrointestinal system. J Clin Psychiatry. 2001;62 Suppl 8:28-37.
7. Abraham S, Kellow J. Exploring eating disorder quality of life and functional gastrointestinal disorders among eating disorder patients. J Psychosom Res. 2011;70(4):372-377.
8. Swinbourne JM, Touyz SW. The co-morbidity of eating disorders and anxiety disorders: a review. Eur Eat Disord Rev. 2007;15(4):253-274.
9. Perkins SJ, Keville S, Schmidt U, et al. Eating disorders and irritable bowel syndrome: is there a link? J Psychosom Res. 2005;59(2):57-64.
10. Hausteiner-Wiehle C, Henningsen P. Irritable bowel syndrome: relations with functional, mental, and somatoform disorders. World J Gastroenterol. 2014;20(2):6024-6030.
11. Masand PS, Keuthen NJ, Gupta S, et al. Prevalence of irritable bowel syndrome in obsessive-compulsive disorder. CNS Spectr. 2006;11(1):21-25.
12. Koven NS, Abry AW. The clinical basis of orthorexia nervosa: emerging perspectives. Neuropsychiatr Dis Treat. 2015;11:385-394.
13. Bratman S. Health food junkie. Yoga Journal. 1997;136:42-50.
14. Barthels F, Müller R, Schüth T, et al. Orthorexic eating behavior in patients with somatoform disorders. Eat Weight Disord. 2021;26(1):135-143.
15. Ciao AC, Loth K, Neumark-Sztainer D. Preventing eating disorder pathology: common and unique features of successful eating disorders prevention programs. Curr Psychiatry Rep. 2014;16(7):453.
16. Brewerton TD, D’Agostino M. Adjunctive use of olanzapine in the treatment of avoidant restrictive food intake disorder in children and adolescents in an eating disorders program. J Child Adolesc Psychopharmacol. 2017;27(10):920-922.
17. Moroze RM, Dunn TM, Craig Holland J, et al. Microthinking about micronutrients: a case of transition from obsessions about healthy eating to near-fatal “orthorexia nervosa” and proposed diagnostic criteria. Psychosomatics. 2015;56(4):397-403.
18. Spettigue W, Norris ML, Santos A, et al. Treatment of children and adolescents with avoidant/restrictive food intake disorder: a case series examining the feasibility of family therapy and adjunctive treatments. J Eat Disord. 2018;6:20.
19. Niedzielski A, Kaźmierczak-Wojtaś N. Prevalence of Orthorexia Nervosa and Its Diagnostic Tools-A Literature Review. Int J Environ Res Public Health. 2021;18(10):5488. Published 2021 May 20. doi:10.3390/ijerph18105488 Prevalence of orthorexia nervosa and its diagnostic tools-a literature review. Int J Environ Res Public Health. 2021;18(10):5488.
1. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; 2013.
2. Murray HB, Bailey AP, Keshishian AC. Prevalence and characteristics of avoidant/restrictive food intake disorder in adult neurogastroenterology patients. Clin Gastroenterol Hepatol. 2020;18(9):1995-2002.e1.
3. Görmez A, Kılıç A, Kırpınar İ. Avoidant/restrictive food intake disorder: an adult case responding to cognitive behavioral therapy. Clinical Case Studies. 2018;17(6):443-452.
4. Reed-Knight B, Squires M, Chitkara DK, et al. Adolescents with irritable bowel syndrome report increased eating-associated symptoms, changes in dietary composition, and altered eating behaviors: a pilot comparison study to healthy adolescents. Neurogastroenterol Motil. 2016;28(12):1915-1920.
5. Melchior C, Desprez C, Riachi G, et al. Anxiety and depression profile is associated with eating disorders in patients with irritable bowel syndrome. Front Psychiatry. 2020;10:928.
6. Mayer EA, Craske M, Naliboff BD. Depression, anxiety, and the gastrointestinal system. J Clin Psychiatry. 2001;62 Suppl 8:28-37.
7. Abraham S, Kellow J. Exploring eating disorder quality of life and functional gastrointestinal disorders among eating disorder patients. J Psychosom Res. 2011;70(4):372-377.
8. Swinbourne JM, Touyz SW. The co-morbidity of eating disorders and anxiety disorders: a review. Eur Eat Disord Rev. 2007;15(4):253-274.
9. Perkins SJ, Keville S, Schmidt U, et al. Eating disorders and irritable bowel syndrome: is there a link? J Psychosom Res. 2005;59(2):57-64.
10. Hausteiner-Wiehle C, Henningsen P. Irritable bowel syndrome: relations with functional, mental, and somatoform disorders. World J Gastroenterol. 2014;20(2):6024-6030.
11. Masand PS, Keuthen NJ, Gupta S, et al. Prevalence of irritable bowel syndrome in obsessive-compulsive disorder. CNS Spectr. 2006;11(1):21-25.
12. Koven NS, Abry AW. The clinical basis of orthorexia nervosa: emerging perspectives. Neuropsychiatr Dis Treat. 2015;11:385-394.
13. Bratman S. Health food junkie. Yoga Journal. 1997;136:42-50.
14. Barthels F, Müller R, Schüth T, et al. Orthorexic eating behavior in patients with somatoform disorders. Eat Weight Disord. 2021;26(1):135-143.
15. Ciao AC, Loth K, Neumark-Sztainer D. Preventing eating disorder pathology: common and unique features of successful eating disorders prevention programs. Curr Psychiatry Rep. 2014;16(7):453.
16. Brewerton TD, D’Agostino M. Adjunctive use of olanzapine in the treatment of avoidant restrictive food intake disorder in children and adolescents in an eating disorders program. J Child Adolesc Psychopharmacol. 2017;27(10):920-922.
17. Moroze RM, Dunn TM, Craig Holland J, et al. Microthinking about micronutrients: a case of transition from obsessions about healthy eating to near-fatal “orthorexia nervosa” and proposed diagnostic criteria. Psychosomatics. 2015;56(4):397-403.
18. Spettigue W, Norris ML, Santos A, et al. Treatment of children and adolescents with avoidant/restrictive food intake disorder: a case series examining the feasibility of family therapy and adjunctive treatments. J Eat Disord. 2018;6:20.
19. Niedzielski A, Kaźmierczak-Wojtaś N. Prevalence of Orthorexia Nervosa and Its Diagnostic Tools-A Literature Review. Int J Environ Res Public Health. 2021;18(10):5488. Published 2021 May 20. doi:10.3390/ijerph18105488 Prevalence of orthorexia nervosa and its diagnostic tools-a literature review. Int J Environ Res Public Health. 2021;18(10):5488.
Elaborate hallucinations, but is it a psychotic disorder?
CASE Visual, auditory, and tactile hallucinations
Mr. B, age 93, is brought to the emergency department by his son after experiencing hallucinations where he reportedly saw and heard individuals in his home. In frustration, Mr. B wielded a knife because he “wanted them to go away.”
Mr. B and his son report that the hallucinations had begun 2 years ago, without prior trauma, medication changes, changes in social situation, or other apparent precipitating events. The hallucinations “come and go,” without preceding symptoms, but have recurring content involving a friendly man named “Harry,” people coming out of the television, 2 children playing, and water covering the floor. Mr. B acknowledges these are hallucinations and had not felt threatened by them until recently, when he wielded the knife. He often tries to talk to them, but they do not reply.
Mr. B also reports intermittent auditory hallucinations including voices at home (non-command) and papers rustling. He also describes tactile hallucinations, where he says he can feel Harry and others prodding him, knocking things out of his hands, or splashing him with water.
Mr. B is admitted to the hospital because he is a danger to himself and others. While on the inpatient unit, Mr. B is pleasant with staff, and eats and sleeps normally; however, he continues to have hallucinations of Harry. Mr. B reports seeing Harry in the hall, and says that Harry pulls out Mr. B’s earpiece and steals his fork. Mr. B also reports hearing a sound “like a bee buzzing.” Mr. B is started on risperidone, 1 mg nightly, for a presumed psychotic disorder.
HISTORY Independent and in good health
Mr. B lives alone and is independent in his activities of daily living. He spends his days at home, often visited by his children, who bring him groceries and other necessities.
Mr. B takes no medications, and has no history of psychiatric treatment; psychotic, manic, or depressive episodes; posttraumatic stress disorder; obsessive-compulsive disorder; or recent emotional stress. His medical history includes chronic progressive hearing loss, which is managed with hearing aids; macular degeneration; and prior bilateral cataract surgeries.
EVALUATION Mental status exam and objective findings
During his evaluation, Mr. B appears well-nourished, and wears glasses and hearing aids. During the interview, he is euthymic with appropriately reactive affect. He is talkative but redirectable, with a goal-directed thought process. Mr. B does not appear to be internally preoccupied. His hearing is impaired, and he often requires questions to be repeated loudly. He is oriented to person, place, and time. There are no signs of delusions, paranoia, thought blocking, thought broadcasting/insertion, or referential thinking. He denies depressed mood, anhedonia, fatigue, sleep changes, or manic symptoms. He denies the occurrence of auditory or visual hallucinations during the evaluation.
Continue to: A neurologic exam shows...
A neurologic exam shows impaired hearing bilaterally and impaired visual acuity. Even with glasses, both eyes have acuity only to finger counting. All other cranial nerves are normal, and Mr. B’s strength, sensation, and cerebellar function are all intact, without rigidity, numbness, or tingling. His gait is steady without a walker, with symmetric arm swing and slight dragging of his feet. His vitals are stable, with normal orthostatic pressures.
Other objective data include a score of 24/30 on the Mini-Mental State Examination, notable for deficits in visuospatial orientation, attention, and calculation, with language and copying limited by poor vision. Mr. B scores 16/22 on the Montreal Cognitive Assessment (MoCA)-Blind (adapted version of MoCA), which is equivalent to a 22/30 on the MoCA, indicating some mild cognitive impairment; however, this modified test is still limited by his poor hearing. His serum and urine laboratory workup show no liver, kidney, metabolic, or electrolyte abnormalities, no sign of infection, negative urine drug screen, and normal B12 and thyroid-stimulating hormone levels. He undergoes a brain MRI, which shows chronic microvascular ischemic change, without mass lesions, infarction, or other pathology.
[polldaddy:10729178]
The authors’ observations
Given Mr. B’s presentation, we ruled out a primary psychotic disorder. He had no psychiatric history, with organized thought, a reactive affect, and no delusions, paranoia, or other psychotic symptoms, all pointing against psychosis. His brain MRI showed no malignancy or other lesions. He had no substance use history to suggest intoxication/withdrawal. His intact attention and orientation did not suggest delirium, and his serum and urine studies were all negative. Although his blaming Harry for knocking things out of his hands could suggest confabulation, Mr. B had no other signs of Korsakoff syndrome, such as ataxia, general confusion, or malnourishment.
We also considered early dementia. There was suspicion for Lewy body dementia given Mr. B’s prominent fluctuating visual hallucinations; however, he displayed no other signs of the disorder, such as parkinsonism, dysautonomia, or sensitivity to the antipsychotic (risperidone 1 mg nightly) started on admission. The presence of 1 core feature of Lewy body dementia—visual hallucinations—indicated a possible, but not probable, diagnosis. Additionally, Mr. B did not have the characteristic features of other types of dementia, such as the stepwise progression of vascular dementia, the behavioral disinhibition of frontotemporal dementia, or the insidious forgetfulness, confusion, language problems, or paranoia that may appear in Alzheimer’s disease. Remarkably, he had a relatively normal brain MRI for his age, given chronic microvascular ischemic changes, and cognitive testing that indicated only mild impairment further pointed against a dementia process.
Charles Bonnet syndrome
Based on Mr. B’s severe vision loss and history of ocular surgeries, we diagnosed him with CBS, described as visual hallucinations in the presence of impaired vision. Charles Bonnet syndrome has been observed in several disorders that affect vision, most commonly macular degeneration, diabetic retinopathy, and glaucoma, with an estimated prevalence of 11% to 39% in older patients with ocular disease.1,2 Visual hallucinations in CBS occur due to ocular disease, likely resulting from changes in afferent sensory input to visual cortical regions of the brain. Table 13 outlines the features of visual hallucinations in patients with CBS. The subsequent disinhibition and spontaneous firing of the visual association cortices leads to the “release hallucinations” of the syndrome.4 The disorder is thought to be significantly underdiagnosed—in a survey of patients with CBS, only 15% had reported their visual hallucinations to a physician.5

Continue to: Mr. B's symptoms...
Mr. B’s symptoms are atypical for CBS, but they fit the diagnosis when considering the entire clinical picture. While hallucinations in CBS are more often simple shapes, complex hallucinations including people and scenes have been noted in several instances.6
Similar to Mr. B’s case, patients with CBS can have recurring figures in their hallucinations, and the images may even move across the visual field.1 Patients with CBS also frequently recognize that their hallucinations are not real, and may or may not be distressed by them.4 Patients with CBS often have hallucinations multiple times daily, lasting from a few seconds to many minutes,7 consistent with Mr. B’s temporary symptoms.
Although auditory and tactile hallucinations are typically not included in CBS, they can also be explained by Mr. B’s significant sensory impairment. Severe hearing impairment in geriatric adults has been associated with auditory hallucinations8; in 1 survey, half of these hallucinations consisted of voices.9 In contrast, tactile hallucinations are not described in sensory deprivation literature. However, in the context of Mr. B’s severe comorbid hearing and vision loss, we propose that these hallucinations reflect his interpretation of sensory events around him, and their integration into his extensive hallucination framework. In other words, Harry poking him and causing him to drop things may be Mr. B’s way of rationalizing events that he has trouble perceiving entirely, or his mild forgetfulness. Mr. B’s social isolation is another factor that may worsen his sensory deprivation and contribute to his extensive hallucinations.10 Additionally, his mild cognitive deficits on testing with chronic microvascular changes on the MRI may suggest a mild vascular-related dementia process, which could also exacerbate his hallucinations. While classic CBS occurs without cognitive impairment, dementia can often co-occur with CBS.11
TREATMENT No significant improvement with medications
During his inpatient stay, Mr. B is treated with risperidone, 1 mg nightly, and is also started on donepezil, 5 mg/d, to treat a possible comorbid dementia. However, he continues to hallucinate without significant improvement.
[polldaddy:10729181]
The authors’ observations
There is no definitive treatment for CBS, and while the hallucinations may spontaneously resolve, per case reports, this typically occurs only as visual loss progresses to total blindness.12 However, many patients can have the hallucinations remit after the underlying ocular etiology is corrected, such as through ocular surgery.13 Other optical interventions, such as special glasses or contact lenses, may help maximize remaining vision.8 In patients without this option, such as Mr. B, there are limited data on beneficial medications for CBS.
Continue to: Evidence for treatment of CBS...
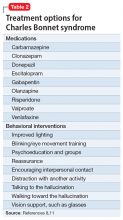
Evidence for treatment of CBS with antipsychotic medications is mixed. Some case studies have found them to be ineffective, while others have found agents such as olanzapine or risperidone to be partially helpful in reducing symptoms.14 There are also data from case reports that may support the use of cholinesterase inhibitors such as donepezil, antiepileptics (carbamazepine, valproate, gabapentin, and clonazepam), and certain antidepressants (escitalopram, venlafaxine) (Table 28,11).3
Addressing loneliness and social isolation
With minimal definitive evidence for pharmacologic management, the most important intervention for treating CBS may be changing the patient’s sensory environment. Specifically, loneliness and social isolation are major exacerbating factors of CBS, and many clinicians advocate for the consistent presence of a sympathetic professional. Reassurance that hallucinations are from ocular disease rather than a primary mental disorder may be extremely relieving for patients.11 A psychoeducation or support group may also be beneficial, not only for giving patients more social contact, but also for teaching them coping skills or strategies to reduce hallucinations, such as distraction, turning on more lights, or even certain eye/blinking movements.11 Table 28,11 (page 49) outlines behavioral interventions for CBS.
Regardless of etiology, Mr. B’s hallucinations significantly affected his quality of life. During his inpatient stay, he was treated with
OUTCOME Home care and family involvement
After discussion with Mr. B and his family about the risks and benefits of medication, the risperidone and donepezil are discontinued. Ultimately, it is determined that Mr. B requires a higher level of home care, both for his safety and to improve his social contact. Mr. B returns home with a combination of a professional home health aide and increased family involvement.
Bottom Line
When evaluating visual hallucinations in older adults, Charles Bonnet syndrome (CBS) should be considered. Sensory deprivation and social isolation are significant risk factors for CBS. While evidence is inconclusive for medical treatment, reassurance and behavioral interventions can often improve symptoms.
Continue to: Related Resources
Related Resources
- Charles Bonnet Syndrome Foundation. http://www.charlesbonnetsyndrome.org
- Schultz G, Melzack R. The Charles Bonnet syndrome: ‘phantom visual images’. Perception. 1991;20:809-825.
- Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet syndrome. Surv Ophthalmol. 2003;48(1):58-72.
Drug Brand Names
Carbamazepine • Tegretol
Clonazepam • Klonopin
Donepezil • Aricept
Escitalopram • Lexapro
Gabapentin • Neurontin
Olanzapine • Zyprexa
Risperidone • Risperdal
Valproate • Depakote
Venlafaxine • Effexor
1. Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet syndrome. Surv Ophthalmol. 2003;48(1):58-72.
2. Cox TM, Ffytche DH. Negative outcome Charles Bonnet syndrome. Br J Ophthalmol. 2014;98(9):1236-1239.
3. Pelak VS. Visual release hallucinations (Charles Bonnet syndrome). UpToDate. Updated February 5, 2019. Accessed September 17, 2020. https://www.uptodate.com/contents/visual-release-hallucinations-charles-bonnet-syndrome
4. Burke W. The neural basis of Charles Bonnet hallucinations: a hypothesis. J Neurol Neurosurg Psychiatry. 2002;73(5):535-541.
5. Scott IU, Schein OD, Feuer WJ, et al. Visual hallucinations in patients with retinal disease. Am J Ophthalmol. 2001;131(5):590-598.
6. Lepore FE. Spontaneous visual phenomena with visual loss: 104 patients with lesions of retinal and neural afferent pathways. Neurology. 1990;40(3 Pt 1):444-447.
7. Nesher R, Nesher G, Epstein E, et al. Charles Bonnet syndrome in glaucoma patients with low vision. J Glaucoma. 2001;10(5):396-400.
8. Pang L. Hallucinations experienced by visually impaired: Charles Bonnet syndrome. Optom Vis Sci. 2016;93(12):1466-1478.
9. Linszen M, Van Zanten G, Teunisse R, et al. Auditory hallucinations in adults with hearing impairment: a large prevalence study. Psychological Medicine. 2019;49(1):132-139.
10. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Social and psychological characteristics of elderly visually handicapped patients with the Charles Bonnet syndrome. Compr Psychiatry. 1999;40(4):315-319.
11. Eperjesi F, Akbarali A. Rehabilitation in Charles Bonnet syndrome: a review of treatment options. Clin Exp Optom. 2004;87(3):149-152.
12. Fernandez A, Lichtshein G, Vieweg WVR. The Charles Bonnet syndrome: a review. J Nen Ment Dis. 1997;185(3):195-200.
13. Rosenbaum F, Harati Y, Rolak L, et al. Visual hallucinations in sane people: Charles Bonnet syndrome. J Am Geriatr Soc. 1987;35(1):66-68.
14. Coletti Moja M, Milano E, Gasverde S, et al. Olanzapine therapy in hallucinatory visions related to Bonnet syndrome. Neurol Sci. 2005;26(3):168-170.
CASE Visual, auditory, and tactile hallucinations
Mr. B, age 93, is brought to the emergency department by his son after experiencing hallucinations where he reportedly saw and heard individuals in his home. In frustration, Mr. B wielded a knife because he “wanted them to go away.”
Mr. B and his son report that the hallucinations had begun 2 years ago, without prior trauma, medication changes, changes in social situation, or other apparent precipitating events. The hallucinations “come and go,” without preceding symptoms, but have recurring content involving a friendly man named “Harry,” people coming out of the television, 2 children playing, and water covering the floor. Mr. B acknowledges these are hallucinations and had not felt threatened by them until recently, when he wielded the knife. He often tries to talk to them, but they do not reply.
Mr. B also reports intermittent auditory hallucinations including voices at home (non-command) and papers rustling. He also describes tactile hallucinations, where he says he can feel Harry and others prodding him, knocking things out of his hands, or splashing him with water.
Mr. B is admitted to the hospital because he is a danger to himself and others. While on the inpatient unit, Mr. B is pleasant with staff, and eats and sleeps normally; however, he continues to have hallucinations of Harry. Mr. B reports seeing Harry in the hall, and says that Harry pulls out Mr. B’s earpiece and steals his fork. Mr. B also reports hearing a sound “like a bee buzzing.” Mr. B is started on risperidone, 1 mg nightly, for a presumed psychotic disorder.
HISTORY Independent and in good health
Mr. B lives alone and is independent in his activities of daily living. He spends his days at home, often visited by his children, who bring him groceries and other necessities.
Mr. B takes no medications, and has no history of psychiatric treatment; psychotic, manic, or depressive episodes; posttraumatic stress disorder; obsessive-compulsive disorder; or recent emotional stress. His medical history includes chronic progressive hearing loss, which is managed with hearing aids; macular degeneration; and prior bilateral cataract surgeries.
EVALUATION Mental status exam and objective findings
During his evaluation, Mr. B appears well-nourished, and wears glasses and hearing aids. During the interview, he is euthymic with appropriately reactive affect. He is talkative but redirectable, with a goal-directed thought process. Mr. B does not appear to be internally preoccupied. His hearing is impaired, and he often requires questions to be repeated loudly. He is oriented to person, place, and time. There are no signs of delusions, paranoia, thought blocking, thought broadcasting/insertion, or referential thinking. He denies depressed mood, anhedonia, fatigue, sleep changes, or manic symptoms. He denies the occurrence of auditory or visual hallucinations during the evaluation.
Continue to: A neurologic exam shows...
A neurologic exam shows impaired hearing bilaterally and impaired visual acuity. Even with glasses, both eyes have acuity only to finger counting. All other cranial nerves are normal, and Mr. B’s strength, sensation, and cerebellar function are all intact, without rigidity, numbness, or tingling. His gait is steady without a walker, with symmetric arm swing and slight dragging of his feet. His vitals are stable, with normal orthostatic pressures.
Other objective data include a score of 24/30 on the Mini-Mental State Examination, notable for deficits in visuospatial orientation, attention, and calculation, with language and copying limited by poor vision. Mr. B scores 16/22 on the Montreal Cognitive Assessment (MoCA)-Blind (adapted version of MoCA), which is equivalent to a 22/30 on the MoCA, indicating some mild cognitive impairment; however, this modified test is still limited by his poor hearing. His serum and urine laboratory workup show no liver, kidney, metabolic, or electrolyte abnormalities, no sign of infection, negative urine drug screen, and normal B12 and thyroid-stimulating hormone levels. He undergoes a brain MRI, which shows chronic microvascular ischemic change, without mass lesions, infarction, or other pathology.
[polldaddy:10729178]
The authors’ observations
Given Mr. B’s presentation, we ruled out a primary psychotic disorder. He had no psychiatric history, with organized thought, a reactive affect, and no delusions, paranoia, or other psychotic symptoms, all pointing against psychosis. His brain MRI showed no malignancy or other lesions. He had no substance use history to suggest intoxication/withdrawal. His intact attention and orientation did not suggest delirium, and his serum and urine studies were all negative. Although his blaming Harry for knocking things out of his hands could suggest confabulation, Mr. B had no other signs of Korsakoff syndrome, such as ataxia, general confusion, or malnourishment.
We also considered early dementia. There was suspicion for Lewy body dementia given Mr. B’s prominent fluctuating visual hallucinations; however, he displayed no other signs of the disorder, such as parkinsonism, dysautonomia, or sensitivity to the antipsychotic (risperidone 1 mg nightly) started on admission. The presence of 1 core feature of Lewy body dementia—visual hallucinations—indicated a possible, but not probable, diagnosis. Additionally, Mr. B did not have the characteristic features of other types of dementia, such as the stepwise progression of vascular dementia, the behavioral disinhibition of frontotemporal dementia, or the insidious forgetfulness, confusion, language problems, or paranoia that may appear in Alzheimer’s disease. Remarkably, he had a relatively normal brain MRI for his age, given chronic microvascular ischemic changes, and cognitive testing that indicated only mild impairment further pointed against a dementia process.
Charles Bonnet syndrome
Based on Mr. B’s severe vision loss and history of ocular surgeries, we diagnosed him with CBS, described as visual hallucinations in the presence of impaired vision. Charles Bonnet syndrome has been observed in several disorders that affect vision, most commonly macular degeneration, diabetic retinopathy, and glaucoma, with an estimated prevalence of 11% to 39% in older patients with ocular disease.1,2 Visual hallucinations in CBS occur due to ocular disease, likely resulting from changes in afferent sensory input to visual cortical regions of the brain. Table 13 outlines the features of visual hallucinations in patients with CBS. The subsequent disinhibition and spontaneous firing of the visual association cortices leads to the “release hallucinations” of the syndrome.4 The disorder is thought to be significantly underdiagnosed—in a survey of patients with CBS, only 15% had reported their visual hallucinations to a physician.5

Continue to: Mr. B's symptoms...
Mr. B’s symptoms are atypical for CBS, but they fit the diagnosis when considering the entire clinical picture. While hallucinations in CBS are more often simple shapes, complex hallucinations including people and scenes have been noted in several instances.6
Similar to Mr. B’s case, patients with CBS can have recurring figures in their hallucinations, and the images may even move across the visual field.1 Patients with CBS also frequently recognize that their hallucinations are not real, and may or may not be distressed by them.4 Patients with CBS often have hallucinations multiple times daily, lasting from a few seconds to many minutes,7 consistent with Mr. B’s temporary symptoms.
Although auditory and tactile hallucinations are typically not included in CBS, they can also be explained by Mr. B’s significant sensory impairment. Severe hearing impairment in geriatric adults has been associated with auditory hallucinations8; in 1 survey, half of these hallucinations consisted of voices.9 In contrast, tactile hallucinations are not described in sensory deprivation literature. However, in the context of Mr. B’s severe comorbid hearing and vision loss, we propose that these hallucinations reflect his interpretation of sensory events around him, and their integration into his extensive hallucination framework. In other words, Harry poking him and causing him to drop things may be Mr. B’s way of rationalizing events that he has trouble perceiving entirely, or his mild forgetfulness. Mr. B’s social isolation is another factor that may worsen his sensory deprivation and contribute to his extensive hallucinations.10 Additionally, his mild cognitive deficits on testing with chronic microvascular changes on the MRI may suggest a mild vascular-related dementia process, which could also exacerbate his hallucinations. While classic CBS occurs without cognitive impairment, dementia can often co-occur with CBS.11
TREATMENT No significant improvement with medications
During his inpatient stay, Mr. B is treated with risperidone, 1 mg nightly, and is also started on donepezil, 5 mg/d, to treat a possible comorbid dementia. However, he continues to hallucinate without significant improvement.
[polldaddy:10729181]
The authors’ observations
There is no definitive treatment for CBS, and while the hallucinations may spontaneously resolve, per case reports, this typically occurs only as visual loss progresses to total blindness.12 However, many patients can have the hallucinations remit after the underlying ocular etiology is corrected, such as through ocular surgery.13 Other optical interventions, such as special glasses or contact lenses, may help maximize remaining vision.8 In patients without this option, such as Mr. B, there are limited data on beneficial medications for CBS.
Continue to: Evidence for treatment of CBS...
Evidence for treatment of CBS with antipsychotic medications is mixed. Some case studies have found them to be ineffective, while others have found agents such as olanzapine or risperidone to be partially helpful in reducing symptoms.14 There are also data from case reports that may support the use of cholinesterase inhibitors such as donepezil, antiepileptics (carbamazepine, valproate, gabapentin, and clonazepam), and certain antidepressants (escitalopram, venlafaxine) (Table 28,11).3
Addressing loneliness and social isolation
With minimal definitive evidence for pharmacologic management, the most important intervention for treating CBS may be changing the patient’s sensory environment. Specifically, loneliness and social isolation are major exacerbating factors of CBS, and many clinicians advocate for the consistent presence of a sympathetic professional. Reassurance that hallucinations are from ocular disease rather than a primary mental disorder may be extremely relieving for patients.11 A psychoeducation or support group may also be beneficial, not only for giving patients more social contact, but also for teaching them coping skills or strategies to reduce hallucinations, such as distraction, turning on more lights, or even certain eye/blinking movements.11 Table 28,11 (page 49) outlines behavioral interventions for CBS.
Regardless of etiology, Mr. B’s hallucinations significantly affected his quality of life. During his inpatient stay, he was treated with
OUTCOME Home care and family involvement
After discussion with Mr. B and his family about the risks and benefits of medication, the risperidone and donepezil are discontinued. Ultimately, it is determined that Mr. B requires a higher level of home care, both for his safety and to improve his social contact. Mr. B returns home with a combination of a professional home health aide and increased family involvement.
Bottom Line
When evaluating visual hallucinations in older adults, Charles Bonnet syndrome (CBS) should be considered. Sensory deprivation and social isolation are significant risk factors for CBS. While evidence is inconclusive for medical treatment, reassurance and behavioral interventions can often improve symptoms.
Continue to: Related Resources
Related Resources
- Charles Bonnet Syndrome Foundation. http://www.charlesbonnetsyndrome.org
- Schultz G, Melzack R. The Charles Bonnet syndrome: ‘phantom visual images’. Perception. 1991;20:809-825.
- Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet syndrome. Surv Ophthalmol. 2003;48(1):58-72.
Drug Brand Names
Carbamazepine • Tegretol
Clonazepam • Klonopin
Donepezil • Aricept
Escitalopram • Lexapro
Gabapentin • Neurontin
Olanzapine • Zyprexa
Risperidone • Risperdal
Valproate • Depakote
Venlafaxine • Effexor
CASE Visual, auditory, and tactile hallucinations
Mr. B, age 93, is brought to the emergency department by his son after experiencing hallucinations where he reportedly saw and heard individuals in his home. In frustration, Mr. B wielded a knife because he “wanted them to go away.”
Mr. B and his son report that the hallucinations had begun 2 years ago, without prior trauma, medication changes, changes in social situation, or other apparent precipitating events. The hallucinations “come and go,” without preceding symptoms, but have recurring content involving a friendly man named “Harry,” people coming out of the television, 2 children playing, and water covering the floor. Mr. B acknowledges these are hallucinations and had not felt threatened by them until recently, when he wielded the knife. He often tries to talk to them, but they do not reply.
Mr. B also reports intermittent auditory hallucinations including voices at home (non-command) and papers rustling. He also describes tactile hallucinations, where he says he can feel Harry and others prodding him, knocking things out of his hands, or splashing him with water.
Mr. B is admitted to the hospital because he is a danger to himself and others. While on the inpatient unit, Mr. B is pleasant with staff, and eats and sleeps normally; however, he continues to have hallucinations of Harry. Mr. B reports seeing Harry in the hall, and says that Harry pulls out Mr. B’s earpiece and steals his fork. Mr. B also reports hearing a sound “like a bee buzzing.” Mr. B is started on risperidone, 1 mg nightly, for a presumed psychotic disorder.
HISTORY Independent and in good health
Mr. B lives alone and is independent in his activities of daily living. He spends his days at home, often visited by his children, who bring him groceries and other necessities.
Mr. B takes no medications, and has no history of psychiatric treatment; psychotic, manic, or depressive episodes; posttraumatic stress disorder; obsessive-compulsive disorder; or recent emotional stress. His medical history includes chronic progressive hearing loss, which is managed with hearing aids; macular degeneration; and prior bilateral cataract surgeries.
EVALUATION Mental status exam and objective findings
During his evaluation, Mr. B appears well-nourished, and wears glasses and hearing aids. During the interview, he is euthymic with appropriately reactive affect. He is talkative but redirectable, with a goal-directed thought process. Mr. B does not appear to be internally preoccupied. His hearing is impaired, and he often requires questions to be repeated loudly. He is oriented to person, place, and time. There are no signs of delusions, paranoia, thought blocking, thought broadcasting/insertion, or referential thinking. He denies depressed mood, anhedonia, fatigue, sleep changes, or manic symptoms. He denies the occurrence of auditory or visual hallucinations during the evaluation.
Continue to: A neurologic exam shows...
A neurologic exam shows impaired hearing bilaterally and impaired visual acuity. Even with glasses, both eyes have acuity only to finger counting. All other cranial nerves are normal, and Mr. B’s strength, sensation, and cerebellar function are all intact, without rigidity, numbness, or tingling. His gait is steady without a walker, with symmetric arm swing and slight dragging of his feet. His vitals are stable, with normal orthostatic pressures.
Other objective data include a score of 24/30 on the Mini-Mental State Examination, notable for deficits in visuospatial orientation, attention, and calculation, with language and copying limited by poor vision. Mr. B scores 16/22 on the Montreal Cognitive Assessment (MoCA)-Blind (adapted version of MoCA), which is equivalent to a 22/30 on the MoCA, indicating some mild cognitive impairment; however, this modified test is still limited by his poor hearing. His serum and urine laboratory workup show no liver, kidney, metabolic, or electrolyte abnormalities, no sign of infection, negative urine drug screen, and normal B12 and thyroid-stimulating hormone levels. He undergoes a brain MRI, which shows chronic microvascular ischemic change, without mass lesions, infarction, or other pathology.
[polldaddy:10729178]
The authors’ observations
Given Mr. B’s presentation, we ruled out a primary psychotic disorder. He had no psychiatric history, with organized thought, a reactive affect, and no delusions, paranoia, or other psychotic symptoms, all pointing against psychosis. His brain MRI showed no malignancy or other lesions. He had no substance use history to suggest intoxication/withdrawal. His intact attention and orientation did not suggest delirium, and his serum and urine studies were all negative. Although his blaming Harry for knocking things out of his hands could suggest confabulation, Mr. B had no other signs of Korsakoff syndrome, such as ataxia, general confusion, or malnourishment.
We also considered early dementia. There was suspicion for Lewy body dementia given Mr. B’s prominent fluctuating visual hallucinations; however, he displayed no other signs of the disorder, such as parkinsonism, dysautonomia, or sensitivity to the antipsychotic (risperidone 1 mg nightly) started on admission. The presence of 1 core feature of Lewy body dementia—visual hallucinations—indicated a possible, but not probable, diagnosis. Additionally, Mr. B did not have the characteristic features of other types of dementia, such as the stepwise progression of vascular dementia, the behavioral disinhibition of frontotemporal dementia, or the insidious forgetfulness, confusion, language problems, or paranoia that may appear in Alzheimer’s disease. Remarkably, he had a relatively normal brain MRI for his age, given chronic microvascular ischemic changes, and cognitive testing that indicated only mild impairment further pointed against a dementia process.
Charles Bonnet syndrome
Based on Mr. B’s severe vision loss and history of ocular surgeries, we diagnosed him with CBS, described as visual hallucinations in the presence of impaired vision. Charles Bonnet syndrome has been observed in several disorders that affect vision, most commonly macular degeneration, diabetic retinopathy, and glaucoma, with an estimated prevalence of 11% to 39% in older patients with ocular disease.1,2 Visual hallucinations in CBS occur due to ocular disease, likely resulting from changes in afferent sensory input to visual cortical regions of the brain. Table 13 outlines the features of visual hallucinations in patients with CBS. The subsequent disinhibition and spontaneous firing of the visual association cortices leads to the “release hallucinations” of the syndrome.4 The disorder is thought to be significantly underdiagnosed—in a survey of patients with CBS, only 15% had reported their visual hallucinations to a physician.5

Continue to: Mr. B's symptoms...
Mr. B’s symptoms are atypical for CBS, but they fit the diagnosis when considering the entire clinical picture. While hallucinations in CBS are more often simple shapes, complex hallucinations including people and scenes have been noted in several instances.6
Similar to Mr. B’s case, patients with CBS can have recurring figures in their hallucinations, and the images may even move across the visual field.1 Patients with CBS also frequently recognize that their hallucinations are not real, and may or may not be distressed by them.4 Patients with CBS often have hallucinations multiple times daily, lasting from a few seconds to many minutes,7 consistent with Mr. B’s temporary symptoms.
Although auditory and tactile hallucinations are typically not included in CBS, they can also be explained by Mr. B’s significant sensory impairment. Severe hearing impairment in geriatric adults has been associated with auditory hallucinations8; in 1 survey, half of these hallucinations consisted of voices.9 In contrast, tactile hallucinations are not described in sensory deprivation literature. However, in the context of Mr. B’s severe comorbid hearing and vision loss, we propose that these hallucinations reflect his interpretation of sensory events around him, and their integration into his extensive hallucination framework. In other words, Harry poking him and causing him to drop things may be Mr. B’s way of rationalizing events that he has trouble perceiving entirely, or his mild forgetfulness. Mr. B’s social isolation is another factor that may worsen his sensory deprivation and contribute to his extensive hallucinations.10 Additionally, his mild cognitive deficits on testing with chronic microvascular changes on the MRI may suggest a mild vascular-related dementia process, which could also exacerbate his hallucinations. While classic CBS occurs without cognitive impairment, dementia can often co-occur with CBS.11
TREATMENT No significant improvement with medications
During his inpatient stay, Mr. B is treated with risperidone, 1 mg nightly, and is also started on donepezil, 5 mg/d, to treat a possible comorbid dementia. However, he continues to hallucinate without significant improvement.
[polldaddy:10729181]
The authors’ observations
There is no definitive treatment for CBS, and while the hallucinations may spontaneously resolve, per case reports, this typically occurs only as visual loss progresses to total blindness.12 However, many patients can have the hallucinations remit after the underlying ocular etiology is corrected, such as through ocular surgery.13 Other optical interventions, such as special glasses or contact lenses, may help maximize remaining vision.8 In patients without this option, such as Mr. B, there are limited data on beneficial medications for CBS.
Continue to: Evidence for treatment of CBS...
Evidence for treatment of CBS with antipsychotic medications is mixed. Some case studies have found them to be ineffective, while others have found agents such as olanzapine or risperidone to be partially helpful in reducing symptoms.14 There are also data from case reports that may support the use of cholinesterase inhibitors such as donepezil, antiepileptics (carbamazepine, valproate, gabapentin, and clonazepam), and certain antidepressants (escitalopram, venlafaxine) (Table 28,11).3
Addressing loneliness and social isolation
With minimal definitive evidence for pharmacologic management, the most important intervention for treating CBS may be changing the patient’s sensory environment. Specifically, loneliness and social isolation are major exacerbating factors of CBS, and many clinicians advocate for the consistent presence of a sympathetic professional. Reassurance that hallucinations are from ocular disease rather than a primary mental disorder may be extremely relieving for patients.11 A psychoeducation or support group may also be beneficial, not only for giving patients more social contact, but also for teaching them coping skills or strategies to reduce hallucinations, such as distraction, turning on more lights, or even certain eye/blinking movements.11 Table 28,11 (page 49) outlines behavioral interventions for CBS.
Regardless of etiology, Mr. B’s hallucinations significantly affected his quality of life. During his inpatient stay, he was treated with
OUTCOME Home care and family involvement
After discussion with Mr. B and his family about the risks and benefits of medication, the risperidone and donepezil are discontinued. Ultimately, it is determined that Mr. B requires a higher level of home care, both for his safety and to improve his social contact. Mr. B returns home with a combination of a professional home health aide and increased family involvement.
Bottom Line
When evaluating visual hallucinations in older adults, Charles Bonnet syndrome (CBS) should be considered. Sensory deprivation and social isolation are significant risk factors for CBS. While evidence is inconclusive for medical treatment, reassurance and behavioral interventions can often improve symptoms.
Continue to: Related Resources
Related Resources
- Charles Bonnet Syndrome Foundation. http://www.charlesbonnetsyndrome.org
- Schultz G, Melzack R. The Charles Bonnet syndrome: ‘phantom visual images’. Perception. 1991;20:809-825.
- Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet syndrome. Surv Ophthalmol. 2003;48(1):58-72.
Drug Brand Names
Carbamazepine • Tegretol
Clonazepam • Klonopin
Donepezil • Aricept
Escitalopram • Lexapro
Gabapentin • Neurontin
Olanzapine • Zyprexa
Risperidone • Risperdal
Valproate • Depakote
Venlafaxine • Effexor
1. Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet syndrome. Surv Ophthalmol. 2003;48(1):58-72.
2. Cox TM, Ffytche DH. Negative outcome Charles Bonnet syndrome. Br J Ophthalmol. 2014;98(9):1236-1239.
3. Pelak VS. Visual release hallucinations (Charles Bonnet syndrome). UpToDate. Updated February 5, 2019. Accessed September 17, 2020. https://www.uptodate.com/contents/visual-release-hallucinations-charles-bonnet-syndrome
4. Burke W. The neural basis of Charles Bonnet hallucinations: a hypothesis. J Neurol Neurosurg Psychiatry. 2002;73(5):535-541.
5. Scott IU, Schein OD, Feuer WJ, et al. Visual hallucinations in patients with retinal disease. Am J Ophthalmol. 2001;131(5):590-598.
6. Lepore FE. Spontaneous visual phenomena with visual loss: 104 patients with lesions of retinal and neural afferent pathways. Neurology. 1990;40(3 Pt 1):444-447.
7. Nesher R, Nesher G, Epstein E, et al. Charles Bonnet syndrome in glaucoma patients with low vision. J Glaucoma. 2001;10(5):396-400.
8. Pang L. Hallucinations experienced by visually impaired: Charles Bonnet syndrome. Optom Vis Sci. 2016;93(12):1466-1478.
9. Linszen M, Van Zanten G, Teunisse R, et al. Auditory hallucinations in adults with hearing impairment: a large prevalence study. Psychological Medicine. 2019;49(1):132-139.
10. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Social and psychological characteristics of elderly visually handicapped patients with the Charles Bonnet syndrome. Compr Psychiatry. 1999;40(4):315-319.
11. Eperjesi F, Akbarali A. Rehabilitation in Charles Bonnet syndrome: a review of treatment options. Clin Exp Optom. 2004;87(3):149-152.
12. Fernandez A, Lichtshein G, Vieweg WVR. The Charles Bonnet syndrome: a review. J Nen Ment Dis. 1997;185(3):195-200.
13. Rosenbaum F, Harati Y, Rolak L, et al. Visual hallucinations in sane people: Charles Bonnet syndrome. J Am Geriatr Soc. 1987;35(1):66-68.
14. Coletti Moja M, Milano E, Gasverde S, et al. Olanzapine therapy in hallucinatory visions related to Bonnet syndrome. Neurol Sci. 2005;26(3):168-170.
1. Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet syndrome. Surv Ophthalmol. 2003;48(1):58-72.
2. Cox TM, Ffytche DH. Negative outcome Charles Bonnet syndrome. Br J Ophthalmol. 2014;98(9):1236-1239.
3. Pelak VS. Visual release hallucinations (Charles Bonnet syndrome). UpToDate. Updated February 5, 2019. Accessed September 17, 2020. https://www.uptodate.com/contents/visual-release-hallucinations-charles-bonnet-syndrome
4. Burke W. The neural basis of Charles Bonnet hallucinations: a hypothesis. J Neurol Neurosurg Psychiatry. 2002;73(5):535-541.
5. Scott IU, Schein OD, Feuer WJ, et al. Visual hallucinations in patients with retinal disease. Am J Ophthalmol. 2001;131(5):590-598.
6. Lepore FE. Spontaneous visual phenomena with visual loss: 104 patients with lesions of retinal and neural afferent pathways. Neurology. 1990;40(3 Pt 1):444-447.
7. Nesher R, Nesher G, Epstein E, et al. Charles Bonnet syndrome in glaucoma patients with low vision. J Glaucoma. 2001;10(5):396-400.
8. Pang L. Hallucinations experienced by visually impaired: Charles Bonnet syndrome. Optom Vis Sci. 2016;93(12):1466-1478.
9. Linszen M, Van Zanten G, Teunisse R, et al. Auditory hallucinations in adults with hearing impairment: a large prevalence study. Psychological Medicine. 2019;49(1):132-139.
10. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Social and psychological characteristics of elderly visually handicapped patients with the Charles Bonnet syndrome. Compr Psychiatry. 1999;40(4):315-319.
11. Eperjesi F, Akbarali A. Rehabilitation in Charles Bonnet syndrome: a review of treatment options. Clin Exp Optom. 2004;87(3):149-152.
12. Fernandez A, Lichtshein G, Vieweg WVR. The Charles Bonnet syndrome: a review. J Nen Ment Dis. 1997;185(3):195-200.
13. Rosenbaum F, Harati Y, Rolak L, et al. Visual hallucinations in sane people: Charles Bonnet syndrome. J Am Geriatr Soc. 1987;35(1):66-68.
14. Coletti Moja M, Milano E, Gasverde S, et al. Olanzapine therapy in hallucinatory visions related to Bonnet syndrome. Neurol Sci. 2005;26(3):168-170.
How to document SUICIDE risk
Despite the high prevalence of suicide and its impact on society,1 psychiatric practitioners achieve only modest success at predicting and preventing suicide. With this in mind, evaluating suicide risk when designing a safe treatment plan for a patient admitted with acute suicidal ideation or after a suicide attempt can be daunting. The mnemonic SUICIDE can remind you of the key elements to include in these patients’ charts.
Suicide assessment. Evaluate the patient for suicide risk factors and protective factors.2 Key risk factors include previous suicide attempts, family history of suicide, access to lethal means, history of a psychiatric disorder, history of alcohol and substance abuse, recent loss of a loved one, and severe hopelessness. Protective factors can be family and community support, problem-solving skills, and religious beliefs that discourage suicide.
Unpredictable and unpreventable. You are responsible for performing a comprehensive risk assessment, responding appropriately to those risks, and instituting a safe discharge plan. Despite your best effort, you might not be able to avert a suicide, and you must provide the patient and family members with this information and document it.
Interventions. Proceed with biological, psychological, and social treatment options. Review the patient’s medications and, when appropriate, consider suicide protective drugs, such as clozapine or lithium.3 Advocate for substance abuse rehabilitation. Encourage psychotherapy and work with your patient to improve social stressors.
Clear and comprehensive documentation. Detailed, accurate, and thorough daily notes are key. Go beyond reporting “no SI/ HI” (suicidal ideation/homicidal ideation). Report what the patient said and how she (he) said it. If the patient denies current suicidal ideation, ask when her (his) last suicidal thought occurred. How did the patient respond to that thought? Why? Avoid using a suicide contract—it is not a substitute for a thorough suicide assessment; it isn’t a legal document; it does not protect against legal liability; and it is ineffective.4
Intent. Evaluate the lethality of the suicidal attempt or plan. Ask about the means of suicide that were used or considered, whether the patient made provisions to ensure or avoid discovery, and how long she (he) has been planning the attempt.
Discuss the treatment plan with a collateral informant (family, friend, community support, outpatient providers). Facilitate safe discharge by including social support in planning. Request that all methods of self-harm, including guns and old prescriptions, be removed from the patient’s home. Help family members and friends identify signs of suicidal ideation.
Educate, engage, and empathize with the patent. Use the Collaborative Assessment and Management of Suicidality (CAMS), which is a structured, evidence-based method of risk assessment and treatment planning. CAMS provides a framework to involve the patient in the assessment of her suicidality and to design a suicide-specific treatment plan.5
A mnemonic can’t prevent all suicides
But using SUICIDE can ensure that the key elements of the evaluation, treatment, and discharge of a patient admitted for acute suicidal ideation or after a suicide attempt are addressed to the best of your ability during an inpatient hospitalization. In doing so, you’ll better identify modifiable risk and protective factors that will inform this plan.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. National Vital Statistics Reports, vol 61 no 4. Hyattsville, MD: National Center for Health Statistics. http://www.cdc.gov/nchs/data/nvsr/nvsr61/ nvsr61_04.pdf. Published May 8, 2013. Accessed August 18, 2014.
2. Fowler JC. Suicide risk assessment in clinical practice: pragmatic guidelines for imperfect assessments. Psychotherapy (Chic). 2012;49(1):81-90.
3. Wasserman D, Rihmer Z, Rujescu D, et al; European Psychiatric Association. The European Psychiatric Association (EPA) guidance on suicide treatment and prevention. Eur Psychiatry. 2012;27(2):129-141.
4. Garvey KA, Penn JV, Campbell AL, et al. Contracting for safety with patients: clinical practice and forensic implications. J Am Acad Psychiatry Law. 2009;37(3): 363-370.
5. Jobes DA. The Collaborative Assessment and Management of Suicidality (CAMS): an evolving evidence‐based clinical approach to suicidal risk. Suicide Life Threat Behav. 2012;42(6):640-653.
Despite the high prevalence of suicide and its impact on society,1 psychiatric practitioners achieve only modest success at predicting and preventing suicide. With this in mind, evaluating suicide risk when designing a safe treatment plan for a patient admitted with acute suicidal ideation or after a suicide attempt can be daunting. The mnemonic SUICIDE can remind you of the key elements to include in these patients’ charts.
Suicide assessment. Evaluate the patient for suicide risk factors and protective factors.2 Key risk factors include previous suicide attempts, family history of suicide, access to lethal means, history of a psychiatric disorder, history of alcohol and substance abuse, recent loss of a loved one, and severe hopelessness. Protective factors can be family and community support, problem-solving skills, and religious beliefs that discourage suicide.
Unpredictable and unpreventable. You are responsible for performing a comprehensive risk assessment, responding appropriately to those risks, and instituting a safe discharge plan. Despite your best effort, you might not be able to avert a suicide, and you must provide the patient and family members with this information and document it.
Interventions. Proceed with biological, psychological, and social treatment options. Review the patient’s medications and, when appropriate, consider suicide protective drugs, such as clozapine or lithium.3 Advocate for substance abuse rehabilitation. Encourage psychotherapy and work with your patient to improve social stressors.
Clear and comprehensive documentation. Detailed, accurate, and thorough daily notes are key. Go beyond reporting “no SI/ HI” (suicidal ideation/homicidal ideation). Report what the patient said and how she (he) said it. If the patient denies current suicidal ideation, ask when her (his) last suicidal thought occurred. How did the patient respond to that thought? Why? Avoid using a suicide contract—it is not a substitute for a thorough suicide assessment; it isn’t a legal document; it does not protect against legal liability; and it is ineffective.4
Intent. Evaluate the lethality of the suicidal attempt or plan. Ask about the means of suicide that were used or considered, whether the patient made provisions to ensure or avoid discovery, and how long she (he) has been planning the attempt.
Discuss the treatment plan with a collateral informant (family, friend, community support, outpatient providers). Facilitate safe discharge by including social support in planning. Request that all methods of self-harm, including guns and old prescriptions, be removed from the patient’s home. Help family members and friends identify signs of suicidal ideation.
Educate, engage, and empathize with the patent. Use the Collaborative Assessment and Management of Suicidality (CAMS), which is a structured, evidence-based method of risk assessment and treatment planning. CAMS provides a framework to involve the patient in the assessment of her suicidality and to design a suicide-specific treatment plan.5
A mnemonic can’t prevent all suicides
But using SUICIDE can ensure that the key elements of the evaluation, treatment, and discharge of a patient admitted for acute suicidal ideation or after a suicide attempt are addressed to the best of your ability during an inpatient hospitalization. In doing so, you’ll better identify modifiable risk and protective factors that will inform this plan.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Despite the high prevalence of suicide and its impact on society,1 psychiatric practitioners achieve only modest success at predicting and preventing suicide. With this in mind, evaluating suicide risk when designing a safe treatment plan for a patient admitted with acute suicidal ideation or after a suicide attempt can be daunting. The mnemonic SUICIDE can remind you of the key elements to include in these patients’ charts.
Suicide assessment. Evaluate the patient for suicide risk factors and protective factors.2 Key risk factors include previous suicide attempts, family history of suicide, access to lethal means, history of a psychiatric disorder, history of alcohol and substance abuse, recent loss of a loved one, and severe hopelessness. Protective factors can be family and community support, problem-solving skills, and religious beliefs that discourage suicide.
Unpredictable and unpreventable. You are responsible for performing a comprehensive risk assessment, responding appropriately to those risks, and instituting a safe discharge plan. Despite your best effort, you might not be able to avert a suicide, and you must provide the patient and family members with this information and document it.
Interventions. Proceed with biological, psychological, and social treatment options. Review the patient’s medications and, when appropriate, consider suicide protective drugs, such as clozapine or lithium.3 Advocate for substance abuse rehabilitation. Encourage psychotherapy and work with your patient to improve social stressors.
Clear and comprehensive documentation. Detailed, accurate, and thorough daily notes are key. Go beyond reporting “no SI/ HI” (suicidal ideation/homicidal ideation). Report what the patient said and how she (he) said it. If the patient denies current suicidal ideation, ask when her (his) last suicidal thought occurred. How did the patient respond to that thought? Why? Avoid using a suicide contract—it is not a substitute for a thorough suicide assessment; it isn’t a legal document; it does not protect against legal liability; and it is ineffective.4
Intent. Evaluate the lethality of the suicidal attempt or plan. Ask about the means of suicide that were used or considered, whether the patient made provisions to ensure or avoid discovery, and how long she (he) has been planning the attempt.
Discuss the treatment plan with a collateral informant (family, friend, community support, outpatient providers). Facilitate safe discharge by including social support in planning. Request that all methods of self-harm, including guns and old prescriptions, be removed from the patient’s home. Help family members and friends identify signs of suicidal ideation.
Educate, engage, and empathize with the patent. Use the Collaborative Assessment and Management of Suicidality (CAMS), which is a structured, evidence-based method of risk assessment and treatment planning. CAMS provides a framework to involve the patient in the assessment of her suicidality and to design a suicide-specific treatment plan.5
A mnemonic can’t prevent all suicides
But using SUICIDE can ensure that the key elements of the evaluation, treatment, and discharge of a patient admitted for acute suicidal ideation or after a suicide attempt are addressed to the best of your ability during an inpatient hospitalization. In doing so, you’ll better identify modifiable risk and protective factors that will inform this plan.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. National Vital Statistics Reports, vol 61 no 4. Hyattsville, MD: National Center for Health Statistics. http://www.cdc.gov/nchs/data/nvsr/nvsr61/ nvsr61_04.pdf. Published May 8, 2013. Accessed August 18, 2014.
2. Fowler JC. Suicide risk assessment in clinical practice: pragmatic guidelines for imperfect assessments. Psychotherapy (Chic). 2012;49(1):81-90.
3. Wasserman D, Rihmer Z, Rujescu D, et al; European Psychiatric Association. The European Psychiatric Association (EPA) guidance on suicide treatment and prevention. Eur Psychiatry. 2012;27(2):129-141.
4. Garvey KA, Penn JV, Campbell AL, et al. Contracting for safety with patients: clinical practice and forensic implications. J Am Acad Psychiatry Law. 2009;37(3): 363-370.
5. Jobes DA. The Collaborative Assessment and Management of Suicidality (CAMS): an evolving evidence‐based clinical approach to suicidal risk. Suicide Life Threat Behav. 2012;42(6):640-653.
1. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. National Vital Statistics Reports, vol 61 no 4. Hyattsville, MD: National Center for Health Statistics. http://www.cdc.gov/nchs/data/nvsr/nvsr61/ nvsr61_04.pdf. Published May 8, 2013. Accessed August 18, 2014.
2. Fowler JC. Suicide risk assessment in clinical practice: pragmatic guidelines for imperfect assessments. Psychotherapy (Chic). 2012;49(1):81-90.
3. Wasserman D, Rihmer Z, Rujescu D, et al; European Psychiatric Association. The European Psychiatric Association (EPA) guidance on suicide treatment and prevention. Eur Psychiatry. 2012;27(2):129-141.
4. Garvey KA, Penn JV, Campbell AL, et al. Contracting for safety with patients: clinical practice and forensic implications. J Am Acad Psychiatry Law. 2009;37(3): 363-370.
5. Jobes DA. The Collaborative Assessment and Management of Suicidality (CAMS): an evolving evidence‐based clinical approach to suicidal risk. Suicide Life Threat Behav. 2012;42(6):640-653.
Epilepsy or something else?
CASE: Seizure-like symptoms
Ms. T, age 20, is brought to the emergency room (ER) by her father because she refuses to eat and drink, is unable to function at home, lies in bed all day, and does not attend to her activities of daily living (ADLs). Ms. T lives with her family, is not enrolled in school, and is unemployed. In the ER she initially is uncooperative and mute and then suddenly becomes agitated and has a seizure-like episode characterized by jerking of her trunk followed by random, asymmetrical movements of her legs and arms, closing both eyes, weeping, foaming at the mouth, moaning, and marked unresponsiveness. The episode lasts for >5 minutes.
The authors’ observations
Based on Ms. T’s presentation, the medical team considered acute epileptic seizures. Asymmetrical jerking of the body may be seen in frontal lobe epilepsy or seizures of the supplementary sensorimotor area. Frontal lobe epilepsy can present with bilateral asynchronous motor activity with consciousness during the event and a lack of postictal confusion.1 Seizures of the supplementary sensorimotor area—also known as the secondary motor area—are particularly problematic because typically they present with bilateral asymmetric tonic posturing followed by a few clonic movements, intact consciousness, and rarely postictal confusion. Adding to the diagnostic uncertainty, some “soft signs” thought to indicate PNES (eg, pelvic thrusting, crying) are common with frontal lobe epilepsy.1,2
PNES are episodes of altered movement, sensation, or experience that may be mistaken for epileptic seizures but are not a consequence of abnormal cortical discharges. Instead they are caused by physiological or psychological factors.3 Behaviors or signs that strongly suggest PNES include:
- gradual onset or termination
- pseudosleep, when the patient appears to be asleep but electroencephalography (EEG) findings indicate he or she is awake
- discontinuous (stop-and-go), irregular, or asynchronous (out-of-phase) activity—including side-to-side head movement, pelvic thrusting, and opisthotonic posturing—stuttering, and weeping4
- eye closure.5
Ms. T’s father said his daughter had been hospitalized several times for episodes characterized by pelvic thrusting, stuttering, and pseudosleep, which raised the possibility of PNES. Definitive diagnosis of PNES comes from video EEG when a patient is observed having typical seizures without accompanying EEG abnormalities.6
EVALUATION: Inconclusive data
Ms. T is admitted to the medical unit to rule out a seizure disorder. Physical examination is unremarkable and laboratory tests are within normal limits. The neurology service requests a head MRI, which is inconclusive. Inpatient video EEG with 24-hour monitoring does not indicate acute epileptic seizures. Ms. T’s father says that she has experienced many paroxysmal motor episodes and all neurologic tests, exams, and labs have failed to find a cause for these episodes. She did not receive any antiepileptic medications. A psychiatric consult is requested to clarify the diagnosis. Ms. T is transferred to an inpatient psychiatric unit for further evaluation and management.
The authors’ observations
Fleisher et al7 suggested that traumatic events may lead to presentations similar to PNES. Because Ms. T was molested by a family friend as a child, we considered posttraumatic stress disorder (PTSD) in the differential diagnosis, although she has not reported symptoms of intrusive recollections, avoidance, numbing, or hyperarousal.
We also considered conversion disorder and dissociative disorder. Patients with conversion disorder have ≥1 symptoms or signs that affect voluntary motor or sensory function that cannot be explained by a neurologic or general medical condition.8 Dissociative disorder is a disruption in usually integrated functions of consciousness, memory, identity, or perception of the environment.8 The presentation of patients with PNES may resemble that of patients with dissociative disorder.8 In a study of 45 adult PNES patients, Bowman et al8 found that PNES often are comorbid with other psychiatric disorders, including somatoform disorders (89%), dissociative disorders (91%), affective disorders (64%), personality disorders (62%), PTSD (49%), and other anxiety disorders (47%).
TREATMENT: Managing aggression
In the psychiatric unit, Ms. T initially is irritable and disorganized with poor oral intake and regressed behavior; she often is found in the fetal position, crying and talking in a childish manner. Throughout her admission, she receives several anxiolytics and antipsychotics—including lorazepam, up to 6 mg/d, clonazepam, up to 3 mg/d, haloperidol, up to 10 mg/d, and quetiapine, up to 200 mg/d—to help manage her aggressive behaviors after her seizure-like episodes. Further evaluation reveals that Ms. T has no psychotic symptoms, overt delusions, or perceptual disturbances and her thought process is coherent and clear. She has no history of substance abuse. Her ability to perform ADLs improves within a few days. She complains of depressed mood and engages in head banging, which requires close observation.
Ms. T has a history of mood and behavioral problems since early childhood characterized by episodic dysphoric mood, anxiety, and agitation. She has had trials of several antidepressants, including sertraline, fluoxetine, venlafaxine, and escitalopram, and anxiolytics, including lorazepam, clonazepam, and alprazolam. Her outpatient psychiatrist describes a history of physical and sexual abuse starting at age 7. At age 9, after her mother died from breast cancer, Ms. T and her siblings were moved to foster care, where she was physically abused by the staff. She remained in foster care until age 18.
The authors’ observations
PNES pose a diagnostic and therapeutic challenge. Many PNES patients seek medical attention for their seizures. PNES patients misdiagnosed as having epilepsy have a worse prognosis because they do not receive appropriate treatment9 and may experience side effects if antiepileptics are prescribed.10 Finally, the financial burden of medical care can be significant. Ms. T had several hospitalizations, including extensive neurologic workup, intensive care unit admissions for intubation, and use of antiepileptics with almost no benefit.
Psychosocial assessments of PNES patients have revealed that sexual abuse, family conflicts, and death of a family member often play an important role.11 It is possible that as a result of childhood trauma, Ms. T exhibited a regressed and primitive defense mechanism to deal with the trauma. PNES usually are considered when a patient presents with:
- absence of therapeutic response to antiepileptics
- loss of response (therapeutic failure) to antiepileptics
- paradoxical response to antiepileptics (worsening or unexpected responses)
- atypical, multiple, or inconsistent seizures
- seizures that occur soon after emotional stress.12
We concluded Ms. T had PNES because of the unusual presentations of her seizures, negative video EEG findings, failure to respond to antiepileptics, lack of risk factors for epilepsy, and aggressive behaviors before or after the seizures ( Table ).4,10,11,13 Diagnosing PNES early allows clinicians to focus on appropriate treatment modalities (eg, psychotherapy, antidepressants), prevents costly neurologic workups and treatments (eg, routine EEGs, trials of several antiepileptics), and provides patients with diagnostic assurance.10
Table
Characteristics of psychogenic nonepileptic seizures
| Characteristic | Comment |
|---|---|
| Duration | May be prolonged |
| Timing | Usually occur only during the day |
| Physical harm | Rare |
| Tongue biting | Rare |
| Urinary incontinence | Rare |
| Motor activity | Prolonged |
| Cyanosis | No |
| Postictal confusion | Rare |
| Related to medication changes | No |
| Interictal EEG | Normal |
| Ictal EEG | Normal |
| Presence of secondary gain | Common |
| EEG: electroencephalography Source: References 4,10,11,13 | |
3 components of treatment
Presenting the PNES diagnosis to the patient. The neurologist and the psychiatrist should convey to the patient that they see the symptoms as “real” and not “all in your head.”14
Withdrawing antiepileptic medications. Antiepileptic medication withdrawal is recommended when a thorough diagnostic workup shows no evidence of epileptic seizures.15 Oto et al16 reported 49% of PNES patients became seizure-free 12 months after discontinuing antiepileptics.
Psychotherapy and pharmacotherapy. Open-label studies of psychological treatments for PNES have demonstrated that a cognitive-behavioral therapy-based approach and brief augmented psychodynamic interpersonal therapy could reduce seizures.17 In a pilot, randomized, placebo-controlled trial, PNES patients who received flexibly dosed sertraline reported a 45% reduction in seizures compared with an 8% increase in the placebo group.18 Similar improvements in seizure frequency have been reported in PNES patients with anxiety or depression treated with venlafaxine.19
OUTCOME: Support, improvement
During the next several days, Ms. T has random episodes of seizures with foaming of the mouth and unresponsiveness. These episodes last from 5 to 30 minutes and require transfer to the ER. After each episode, Ms. T is medically cleared and sent back to the psychiatric unit. The neurologist recommends avoiding antiepileptics. Ms. T responds well to the structured inpatient setting and supportive psychotherapy. Her episodes decrease and her mood becomes more stable. She refrains from self-injurious behaviors and is discharged home with outpatient follow-up.
Related Resource
- Marsh P, Benbadis S, Fernandez F. Psychogenic nonepileptic seizures: ways to win over skeptical patients. Current Psychiatry. 2008;7(1):21-35.
Drug Brand Names
- Alprazolam • Xanax
- Clonazepam • Klonopin
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Lorazepam • Ativan
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kellinghaus C, Lüders HO. Frontal lobe epilepsy. Epileptic Disord. 2004;6(4):223-239.
2. Kanner AM, Morris HH, Lüders H, et al. Supplementary motor seizures mimicking pseudoseizures: some clinical differences. Neurology. 1990;40(9):1404-1407.
3. Hall-Patch L, Brown R, House A, et al. Acceptability and effectiveness of a strategy for the communication of the diagnosis of psychogenic nonepileptic seizures. Epilepsia. 2010;51(1):70-78.
4. Reuber M, Elger CE. Psychogenic nonepileptic seizures: review and update. Epilepsy Behav. 2003;4(3):205-216.
5. Chung SS, Gerber P, Kirlin KA. Ictal eye closure is a reliable indicator for psychogenic nonepileptic seizures. Neurology. 2006;66(11):1730-1731.
6. Mostacci B, Bisulli F, Alvisi L, et al. Ictal characteristics of psychogenic nonepileptic seizures: what we have learned from video/EEG recordings—a literature review. Epilepsy Behav. 2011;22(2):144-153.
7. Fleisher W, Staley D, Krawetz P, et al. Comparative study of trauma-related phenomena in subjects with pseudoseizures and subjects with epilepsy. Am J Psychiatry. 2002;159(4):660-663.
8. Bowman ES, Markand ON. Psychodynamics and psychiatric diagnoses of pseudoseizure subjects. Am J Psychiatry. 1996;153(1):57-63.
9. Benbadis SR. The EEG in nonepileptic seizures. J Clin Neurophysiol. 2006;23(4):340-352.
10. Brown RJ, Syed TU, Benbadis S, et al. Psychogenic nonepileptic seizures. Epilepsy Behav. 2011;22(1):85-93.
11. Bodde NM, Brooks JL, Baker GA, et al. Psychogenic non-epileptic seizures—definition, etiology, treatment and prognostic issues: a critical review. Seizure. 2009;18(8):543-553.
12. Alsaadi TM, Marquez AV. Psychogenic nonepileptic seizures. Am Fam Physician. 2005;72(5):849-856.
13. Bradley WG, Daroff RB, Fenichel GM, et al. eds. Neurology in clinical practice: principles of diagnosis and management. 4th ed. Philadelphia, PA: Butterworth Heinemann; 2004:19-20, 1971–1972.
14. Harden CL, Ferrando SJ. Delivering the diagnosis of psychogenic pseudoseizures: should the neurologist or the psychiatrist be responsible? Epilepsy Behav. 2001;2(6):519-523.
15. Oto M, Espie CA, Duncan R. An exploratory randomized controlled trial of immediate versus delayed withdrawal of antiepileptic drugs in patients with psychogenic nonepileptic attacks (PNEAs). Epilepsia. 2010;51(10):1994-1999.
16. Oto M, Espie C, Pelosi A, et al. The safety of antiepileptic drug withdrawal in patients with non-epileptic seizures. J Neurol Neurosurg Psychiatry. 2005;76(12):1682-1685.
17. Goldstein LH, Mellers JD. Recent developments in our understanding of the semiology and treatment of psychogenic nonepileptic seizures. Curr Neurol Neurosci Rep. 2012;12(4):436-444.
18. LaFrance WC, Jr, Keitner GI, Papandonatos GD, et al. Pilot pharmacologic randomized controlled trial for psychogenic nonepileptic seizures. Neurology. 2010;75(13):1166-1173.
19. Pintor L, Baillés E, Matrai S, et al. Efficiency of venlafaxine in patients with psychogenic nonepileptic seizures and anxiety and/or depressive disorders. J Neuropsychiatry Clin Neurosci. 2010;22(4):401-408.
CASE: Seizure-like symptoms
Ms. T, age 20, is brought to the emergency room (ER) by her father because she refuses to eat and drink, is unable to function at home, lies in bed all day, and does not attend to her activities of daily living (ADLs). Ms. T lives with her family, is not enrolled in school, and is unemployed. In the ER she initially is uncooperative and mute and then suddenly becomes agitated and has a seizure-like episode characterized by jerking of her trunk followed by random, asymmetrical movements of her legs and arms, closing both eyes, weeping, foaming at the mouth, moaning, and marked unresponsiveness. The episode lasts for >5 minutes.
The authors’ observations
Based on Ms. T’s presentation, the medical team considered acute epileptic seizures. Asymmetrical jerking of the body may be seen in frontal lobe epilepsy or seizures of the supplementary sensorimotor area. Frontal lobe epilepsy can present with bilateral asynchronous motor activity with consciousness during the event and a lack of postictal confusion.1 Seizures of the supplementary sensorimotor area—also known as the secondary motor area—are particularly problematic because typically they present with bilateral asymmetric tonic posturing followed by a few clonic movements, intact consciousness, and rarely postictal confusion. Adding to the diagnostic uncertainty, some “soft signs” thought to indicate PNES (eg, pelvic thrusting, crying) are common with frontal lobe epilepsy.1,2
PNES are episodes of altered movement, sensation, or experience that may be mistaken for epileptic seizures but are not a consequence of abnormal cortical discharges. Instead they are caused by physiological or psychological factors.3 Behaviors or signs that strongly suggest PNES include:
- gradual onset or termination
- pseudosleep, when the patient appears to be asleep but electroencephalography (EEG) findings indicate he or she is awake
- discontinuous (stop-and-go), irregular, or asynchronous (out-of-phase) activity—including side-to-side head movement, pelvic thrusting, and opisthotonic posturing—stuttering, and weeping4
- eye closure.5
Ms. T’s father said his daughter had been hospitalized several times for episodes characterized by pelvic thrusting, stuttering, and pseudosleep, which raised the possibility of PNES. Definitive diagnosis of PNES comes from video EEG when a patient is observed having typical seizures without accompanying EEG abnormalities.6
EVALUATION: Inconclusive data
Ms. T is admitted to the medical unit to rule out a seizure disorder. Physical examination is unremarkable and laboratory tests are within normal limits. The neurology service requests a head MRI, which is inconclusive. Inpatient video EEG with 24-hour monitoring does not indicate acute epileptic seizures. Ms. T’s father says that she has experienced many paroxysmal motor episodes and all neurologic tests, exams, and labs have failed to find a cause for these episodes. She did not receive any antiepileptic medications. A psychiatric consult is requested to clarify the diagnosis. Ms. T is transferred to an inpatient psychiatric unit for further evaluation and management.
The authors’ observations
Fleisher et al7 suggested that traumatic events may lead to presentations similar to PNES. Because Ms. T was molested by a family friend as a child, we considered posttraumatic stress disorder (PTSD) in the differential diagnosis, although she has not reported symptoms of intrusive recollections, avoidance, numbing, or hyperarousal.
We also considered conversion disorder and dissociative disorder. Patients with conversion disorder have ≥1 symptoms or signs that affect voluntary motor or sensory function that cannot be explained by a neurologic or general medical condition.8 Dissociative disorder is a disruption in usually integrated functions of consciousness, memory, identity, or perception of the environment.8 The presentation of patients with PNES may resemble that of patients with dissociative disorder.8 In a study of 45 adult PNES patients, Bowman et al8 found that PNES often are comorbid with other psychiatric disorders, including somatoform disorders (89%), dissociative disorders (91%), affective disorders (64%), personality disorders (62%), PTSD (49%), and other anxiety disorders (47%).
TREATMENT: Managing aggression
In the psychiatric unit, Ms. T initially is irritable and disorganized with poor oral intake and regressed behavior; she often is found in the fetal position, crying and talking in a childish manner. Throughout her admission, she receives several anxiolytics and antipsychotics—including lorazepam, up to 6 mg/d, clonazepam, up to 3 mg/d, haloperidol, up to 10 mg/d, and quetiapine, up to 200 mg/d—to help manage her aggressive behaviors after her seizure-like episodes. Further evaluation reveals that Ms. T has no psychotic symptoms, overt delusions, or perceptual disturbances and her thought process is coherent and clear. She has no history of substance abuse. Her ability to perform ADLs improves within a few days. She complains of depressed mood and engages in head banging, which requires close observation.
Ms. T has a history of mood and behavioral problems since early childhood characterized by episodic dysphoric mood, anxiety, and agitation. She has had trials of several antidepressants, including sertraline, fluoxetine, venlafaxine, and escitalopram, and anxiolytics, including lorazepam, clonazepam, and alprazolam. Her outpatient psychiatrist describes a history of physical and sexual abuse starting at age 7. At age 9, after her mother died from breast cancer, Ms. T and her siblings were moved to foster care, where she was physically abused by the staff. She remained in foster care until age 18.
The authors’ observations
PNES pose a diagnostic and therapeutic challenge. Many PNES patients seek medical attention for their seizures. PNES patients misdiagnosed as having epilepsy have a worse prognosis because they do not receive appropriate treatment9 and may experience side effects if antiepileptics are prescribed.10 Finally, the financial burden of medical care can be significant. Ms. T had several hospitalizations, including extensive neurologic workup, intensive care unit admissions for intubation, and use of antiepileptics with almost no benefit.
Psychosocial assessments of PNES patients have revealed that sexual abuse, family conflicts, and death of a family member often play an important role.11 It is possible that as a result of childhood trauma, Ms. T exhibited a regressed and primitive defense mechanism to deal with the trauma. PNES usually are considered when a patient presents with:
- absence of therapeutic response to antiepileptics
- loss of response (therapeutic failure) to antiepileptics
- paradoxical response to antiepileptics (worsening or unexpected responses)
- atypical, multiple, or inconsistent seizures
- seizures that occur soon after emotional stress.12
We concluded Ms. T had PNES because of the unusual presentations of her seizures, negative video EEG findings, failure to respond to antiepileptics, lack of risk factors for epilepsy, and aggressive behaviors before or after the seizures ( Table ).4,10,11,13 Diagnosing PNES early allows clinicians to focus on appropriate treatment modalities (eg, psychotherapy, antidepressants), prevents costly neurologic workups and treatments (eg, routine EEGs, trials of several antiepileptics), and provides patients with diagnostic assurance.10
Table
Characteristics of psychogenic nonepileptic seizures
| Characteristic | Comment |
|---|---|
| Duration | May be prolonged |
| Timing | Usually occur only during the day |
| Physical harm | Rare |
| Tongue biting | Rare |
| Urinary incontinence | Rare |
| Motor activity | Prolonged |
| Cyanosis | No |
| Postictal confusion | Rare |
| Related to medication changes | No |
| Interictal EEG | Normal |
| Ictal EEG | Normal |
| Presence of secondary gain | Common |
| EEG: electroencephalography Source: References 4,10,11,13 | |
3 components of treatment
Presenting the PNES diagnosis to the patient. The neurologist and the psychiatrist should convey to the patient that they see the symptoms as “real” and not “all in your head.”14
Withdrawing antiepileptic medications. Antiepileptic medication withdrawal is recommended when a thorough diagnostic workup shows no evidence of epileptic seizures.15 Oto et al16 reported 49% of PNES patients became seizure-free 12 months after discontinuing antiepileptics.
Psychotherapy and pharmacotherapy. Open-label studies of psychological treatments for PNES have demonstrated that a cognitive-behavioral therapy-based approach and brief augmented psychodynamic interpersonal therapy could reduce seizures.17 In a pilot, randomized, placebo-controlled trial, PNES patients who received flexibly dosed sertraline reported a 45% reduction in seizures compared with an 8% increase in the placebo group.18 Similar improvements in seizure frequency have been reported in PNES patients with anxiety or depression treated with venlafaxine.19
OUTCOME: Support, improvement
During the next several days, Ms. T has random episodes of seizures with foaming of the mouth and unresponsiveness. These episodes last from 5 to 30 minutes and require transfer to the ER. After each episode, Ms. T is medically cleared and sent back to the psychiatric unit. The neurologist recommends avoiding antiepileptics. Ms. T responds well to the structured inpatient setting and supportive psychotherapy. Her episodes decrease and her mood becomes more stable. She refrains from self-injurious behaviors and is discharged home with outpatient follow-up.
Related Resource
- Marsh P, Benbadis S, Fernandez F. Psychogenic nonepileptic seizures: ways to win over skeptical patients. Current Psychiatry. 2008;7(1):21-35.
Drug Brand Names
- Alprazolam • Xanax
- Clonazepam • Klonopin
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Lorazepam • Ativan
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Seizure-like symptoms
Ms. T, age 20, is brought to the emergency room (ER) by her father because she refuses to eat and drink, is unable to function at home, lies in bed all day, and does not attend to her activities of daily living (ADLs). Ms. T lives with her family, is not enrolled in school, and is unemployed. In the ER she initially is uncooperative and mute and then suddenly becomes agitated and has a seizure-like episode characterized by jerking of her trunk followed by random, asymmetrical movements of her legs and arms, closing both eyes, weeping, foaming at the mouth, moaning, and marked unresponsiveness. The episode lasts for >5 minutes.
The authors’ observations
Based on Ms. T’s presentation, the medical team considered acute epileptic seizures. Asymmetrical jerking of the body may be seen in frontal lobe epilepsy or seizures of the supplementary sensorimotor area. Frontal lobe epilepsy can present with bilateral asynchronous motor activity with consciousness during the event and a lack of postictal confusion.1 Seizures of the supplementary sensorimotor area—also known as the secondary motor area—are particularly problematic because typically they present with bilateral asymmetric tonic posturing followed by a few clonic movements, intact consciousness, and rarely postictal confusion. Adding to the diagnostic uncertainty, some “soft signs” thought to indicate PNES (eg, pelvic thrusting, crying) are common with frontal lobe epilepsy.1,2
PNES are episodes of altered movement, sensation, or experience that may be mistaken for epileptic seizures but are not a consequence of abnormal cortical discharges. Instead they are caused by physiological or psychological factors.3 Behaviors or signs that strongly suggest PNES include:
- gradual onset or termination
- pseudosleep, when the patient appears to be asleep but electroencephalography (EEG) findings indicate he or she is awake
- discontinuous (stop-and-go), irregular, or asynchronous (out-of-phase) activity—including side-to-side head movement, pelvic thrusting, and opisthotonic posturing—stuttering, and weeping4
- eye closure.5
Ms. T’s father said his daughter had been hospitalized several times for episodes characterized by pelvic thrusting, stuttering, and pseudosleep, which raised the possibility of PNES. Definitive diagnosis of PNES comes from video EEG when a patient is observed having typical seizures without accompanying EEG abnormalities.6
EVALUATION: Inconclusive data
Ms. T is admitted to the medical unit to rule out a seizure disorder. Physical examination is unremarkable and laboratory tests are within normal limits. The neurology service requests a head MRI, which is inconclusive. Inpatient video EEG with 24-hour monitoring does not indicate acute epileptic seizures. Ms. T’s father says that she has experienced many paroxysmal motor episodes and all neurologic tests, exams, and labs have failed to find a cause for these episodes. She did not receive any antiepileptic medications. A psychiatric consult is requested to clarify the diagnosis. Ms. T is transferred to an inpatient psychiatric unit for further evaluation and management.
The authors’ observations
Fleisher et al7 suggested that traumatic events may lead to presentations similar to PNES. Because Ms. T was molested by a family friend as a child, we considered posttraumatic stress disorder (PTSD) in the differential diagnosis, although she has not reported symptoms of intrusive recollections, avoidance, numbing, or hyperarousal.
We also considered conversion disorder and dissociative disorder. Patients with conversion disorder have ≥1 symptoms or signs that affect voluntary motor or sensory function that cannot be explained by a neurologic or general medical condition.8 Dissociative disorder is a disruption in usually integrated functions of consciousness, memory, identity, or perception of the environment.8 The presentation of patients with PNES may resemble that of patients with dissociative disorder.8 In a study of 45 adult PNES patients, Bowman et al8 found that PNES often are comorbid with other psychiatric disorders, including somatoform disorders (89%), dissociative disorders (91%), affective disorders (64%), personality disorders (62%), PTSD (49%), and other anxiety disorders (47%).
TREATMENT: Managing aggression
In the psychiatric unit, Ms. T initially is irritable and disorganized with poor oral intake and regressed behavior; she often is found in the fetal position, crying and talking in a childish manner. Throughout her admission, she receives several anxiolytics and antipsychotics—including lorazepam, up to 6 mg/d, clonazepam, up to 3 mg/d, haloperidol, up to 10 mg/d, and quetiapine, up to 200 mg/d—to help manage her aggressive behaviors after her seizure-like episodes. Further evaluation reveals that Ms. T has no psychotic symptoms, overt delusions, or perceptual disturbances and her thought process is coherent and clear. She has no history of substance abuse. Her ability to perform ADLs improves within a few days. She complains of depressed mood and engages in head banging, which requires close observation.
Ms. T has a history of mood and behavioral problems since early childhood characterized by episodic dysphoric mood, anxiety, and agitation. She has had trials of several antidepressants, including sertraline, fluoxetine, venlafaxine, and escitalopram, and anxiolytics, including lorazepam, clonazepam, and alprazolam. Her outpatient psychiatrist describes a history of physical and sexual abuse starting at age 7. At age 9, after her mother died from breast cancer, Ms. T and her siblings were moved to foster care, where she was physically abused by the staff. She remained in foster care until age 18.
The authors’ observations
PNES pose a diagnostic and therapeutic challenge. Many PNES patients seek medical attention for their seizures. PNES patients misdiagnosed as having epilepsy have a worse prognosis because they do not receive appropriate treatment9 and may experience side effects if antiepileptics are prescribed.10 Finally, the financial burden of medical care can be significant. Ms. T had several hospitalizations, including extensive neurologic workup, intensive care unit admissions for intubation, and use of antiepileptics with almost no benefit.
Psychosocial assessments of PNES patients have revealed that sexual abuse, family conflicts, and death of a family member often play an important role.11 It is possible that as a result of childhood trauma, Ms. T exhibited a regressed and primitive defense mechanism to deal with the trauma. PNES usually are considered when a patient presents with:
- absence of therapeutic response to antiepileptics
- loss of response (therapeutic failure) to antiepileptics
- paradoxical response to antiepileptics (worsening or unexpected responses)
- atypical, multiple, or inconsistent seizures
- seizures that occur soon after emotional stress.12
We concluded Ms. T had PNES because of the unusual presentations of her seizures, negative video EEG findings, failure to respond to antiepileptics, lack of risk factors for epilepsy, and aggressive behaviors before or after the seizures ( Table ).4,10,11,13 Diagnosing PNES early allows clinicians to focus on appropriate treatment modalities (eg, psychotherapy, antidepressants), prevents costly neurologic workups and treatments (eg, routine EEGs, trials of several antiepileptics), and provides patients with diagnostic assurance.10
Table
Characteristics of psychogenic nonepileptic seizures
| Characteristic | Comment |
|---|---|
| Duration | May be prolonged |
| Timing | Usually occur only during the day |
| Physical harm | Rare |
| Tongue biting | Rare |
| Urinary incontinence | Rare |
| Motor activity | Prolonged |
| Cyanosis | No |
| Postictal confusion | Rare |
| Related to medication changes | No |
| Interictal EEG | Normal |
| Ictal EEG | Normal |
| Presence of secondary gain | Common |
| EEG: electroencephalography Source: References 4,10,11,13 | |
3 components of treatment
Presenting the PNES diagnosis to the patient. The neurologist and the psychiatrist should convey to the patient that they see the symptoms as “real” and not “all in your head.”14
Withdrawing antiepileptic medications. Antiepileptic medication withdrawal is recommended when a thorough diagnostic workup shows no evidence of epileptic seizures.15 Oto et al16 reported 49% of PNES patients became seizure-free 12 months after discontinuing antiepileptics.
Psychotherapy and pharmacotherapy. Open-label studies of psychological treatments for PNES have demonstrated that a cognitive-behavioral therapy-based approach and brief augmented psychodynamic interpersonal therapy could reduce seizures.17 In a pilot, randomized, placebo-controlled trial, PNES patients who received flexibly dosed sertraline reported a 45% reduction in seizures compared with an 8% increase in the placebo group.18 Similar improvements in seizure frequency have been reported in PNES patients with anxiety or depression treated with venlafaxine.19
OUTCOME: Support, improvement
During the next several days, Ms. T has random episodes of seizures with foaming of the mouth and unresponsiveness. These episodes last from 5 to 30 minutes and require transfer to the ER. After each episode, Ms. T is medically cleared and sent back to the psychiatric unit. The neurologist recommends avoiding antiepileptics. Ms. T responds well to the structured inpatient setting and supportive psychotherapy. Her episodes decrease and her mood becomes more stable. She refrains from self-injurious behaviors and is discharged home with outpatient follow-up.
Related Resource
- Marsh P, Benbadis S, Fernandez F. Psychogenic nonepileptic seizures: ways to win over skeptical patients. Current Psychiatry. 2008;7(1):21-35.
Drug Brand Names
- Alprazolam • Xanax
- Clonazepam • Klonopin
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Lorazepam • Ativan
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kellinghaus C, Lüders HO. Frontal lobe epilepsy. Epileptic Disord. 2004;6(4):223-239.
2. Kanner AM, Morris HH, Lüders H, et al. Supplementary motor seizures mimicking pseudoseizures: some clinical differences. Neurology. 1990;40(9):1404-1407.
3. Hall-Patch L, Brown R, House A, et al. Acceptability and effectiveness of a strategy for the communication of the diagnosis of psychogenic nonepileptic seizures. Epilepsia. 2010;51(1):70-78.
4. Reuber M, Elger CE. Psychogenic nonepileptic seizures: review and update. Epilepsy Behav. 2003;4(3):205-216.
5. Chung SS, Gerber P, Kirlin KA. Ictal eye closure is a reliable indicator for psychogenic nonepileptic seizures. Neurology. 2006;66(11):1730-1731.
6. Mostacci B, Bisulli F, Alvisi L, et al. Ictal characteristics of psychogenic nonepileptic seizures: what we have learned from video/EEG recordings—a literature review. Epilepsy Behav. 2011;22(2):144-153.
7. Fleisher W, Staley D, Krawetz P, et al. Comparative study of trauma-related phenomena in subjects with pseudoseizures and subjects with epilepsy. Am J Psychiatry. 2002;159(4):660-663.
8. Bowman ES, Markand ON. Psychodynamics and psychiatric diagnoses of pseudoseizure subjects. Am J Psychiatry. 1996;153(1):57-63.
9. Benbadis SR. The EEG in nonepileptic seizures. J Clin Neurophysiol. 2006;23(4):340-352.
10. Brown RJ, Syed TU, Benbadis S, et al. Psychogenic nonepileptic seizures. Epilepsy Behav. 2011;22(1):85-93.
11. Bodde NM, Brooks JL, Baker GA, et al. Psychogenic non-epileptic seizures—definition, etiology, treatment and prognostic issues: a critical review. Seizure. 2009;18(8):543-553.
12. Alsaadi TM, Marquez AV. Psychogenic nonepileptic seizures. Am Fam Physician. 2005;72(5):849-856.
13. Bradley WG, Daroff RB, Fenichel GM, et al. eds. Neurology in clinical practice: principles of diagnosis and management. 4th ed. Philadelphia, PA: Butterworth Heinemann; 2004:19-20, 1971–1972.
14. Harden CL, Ferrando SJ. Delivering the diagnosis of psychogenic pseudoseizures: should the neurologist or the psychiatrist be responsible? Epilepsy Behav. 2001;2(6):519-523.
15. Oto M, Espie CA, Duncan R. An exploratory randomized controlled trial of immediate versus delayed withdrawal of antiepileptic drugs in patients with psychogenic nonepileptic attacks (PNEAs). Epilepsia. 2010;51(10):1994-1999.
16. Oto M, Espie C, Pelosi A, et al. The safety of antiepileptic drug withdrawal in patients with non-epileptic seizures. J Neurol Neurosurg Psychiatry. 2005;76(12):1682-1685.
17. Goldstein LH, Mellers JD. Recent developments in our understanding of the semiology and treatment of psychogenic nonepileptic seizures. Curr Neurol Neurosci Rep. 2012;12(4):436-444.
18. LaFrance WC, Jr, Keitner GI, Papandonatos GD, et al. Pilot pharmacologic randomized controlled trial for psychogenic nonepileptic seizures. Neurology. 2010;75(13):1166-1173.
19. Pintor L, Baillés E, Matrai S, et al. Efficiency of venlafaxine in patients with psychogenic nonepileptic seizures and anxiety and/or depressive disorders. J Neuropsychiatry Clin Neurosci. 2010;22(4):401-408.
1. Kellinghaus C, Lüders HO. Frontal lobe epilepsy. Epileptic Disord. 2004;6(4):223-239.
2. Kanner AM, Morris HH, Lüders H, et al. Supplementary motor seizures mimicking pseudoseizures: some clinical differences. Neurology. 1990;40(9):1404-1407.
3. Hall-Patch L, Brown R, House A, et al. Acceptability and effectiveness of a strategy for the communication of the diagnosis of psychogenic nonepileptic seizures. Epilepsia. 2010;51(1):70-78.
4. Reuber M, Elger CE. Psychogenic nonepileptic seizures: review and update. Epilepsy Behav. 2003;4(3):205-216.
5. Chung SS, Gerber P, Kirlin KA. Ictal eye closure is a reliable indicator for psychogenic nonepileptic seizures. Neurology. 2006;66(11):1730-1731.
6. Mostacci B, Bisulli F, Alvisi L, et al. Ictal characteristics of psychogenic nonepileptic seizures: what we have learned from video/EEG recordings—a literature review. Epilepsy Behav. 2011;22(2):144-153.
7. Fleisher W, Staley D, Krawetz P, et al. Comparative study of trauma-related phenomena in subjects with pseudoseizures and subjects with epilepsy. Am J Psychiatry. 2002;159(4):660-663.
8. Bowman ES, Markand ON. Psychodynamics and psychiatric diagnoses of pseudoseizure subjects. Am J Psychiatry. 1996;153(1):57-63.
9. Benbadis SR. The EEG in nonepileptic seizures. J Clin Neurophysiol. 2006;23(4):340-352.
10. Brown RJ, Syed TU, Benbadis S, et al. Psychogenic nonepileptic seizures. Epilepsy Behav. 2011;22(1):85-93.
11. Bodde NM, Brooks JL, Baker GA, et al. Psychogenic non-epileptic seizures—definition, etiology, treatment and prognostic issues: a critical review. Seizure. 2009;18(8):543-553.
12. Alsaadi TM, Marquez AV. Psychogenic nonepileptic seizures. Am Fam Physician. 2005;72(5):849-856.
13. Bradley WG, Daroff RB, Fenichel GM, et al. eds. Neurology in clinical practice: principles of diagnosis and management. 4th ed. Philadelphia, PA: Butterworth Heinemann; 2004:19-20, 1971–1972.
14. Harden CL, Ferrando SJ. Delivering the diagnosis of psychogenic pseudoseizures: should the neurologist or the psychiatrist be responsible? Epilepsy Behav. 2001;2(6):519-523.
15. Oto M, Espie CA, Duncan R. An exploratory randomized controlled trial of immediate versus delayed withdrawal of antiepileptic drugs in patients with psychogenic nonepileptic attacks (PNEAs). Epilepsia. 2010;51(10):1994-1999.
16. Oto M, Espie C, Pelosi A, et al. The safety of antiepileptic drug withdrawal in patients with non-epileptic seizures. J Neurol Neurosurg Psychiatry. 2005;76(12):1682-1685.
17. Goldstein LH, Mellers JD. Recent developments in our understanding of the semiology and treatment of psychogenic nonepileptic seizures. Curr Neurol Neurosci Rep. 2012;12(4):436-444.
18. LaFrance WC, Jr, Keitner GI, Papandonatos GD, et al. Pilot pharmacologic randomized controlled trial for psychogenic nonepileptic seizures. Neurology. 2010;75(13):1166-1173.
19. Pintor L, Baillés E, Matrai S, et al. Efficiency of venlafaxine in patients with psychogenic nonepileptic seizures and anxiety and/or depressive disorders. J Neuropsychiatry Clin Neurosci. 2010;22(4):401-408.
The hidden danger of hand sanitizer
Discuss this article at www.facebook.com/CurrentPsychiatry
The Centers for Disease Control and Prevention recommends that all health care professionals use an ethanol-based hand sanitizer to decontaminate their hands before and after direct contact with patients to prevent infection.1,2 As a result, many psychiatric hospitals use alcohol-based hand sanitizers as a primary infection control measure.
Patient misuse of these products as intoxicants has been reported in prisons, emergency rooms, and medical units.3-7 We report 2 cases of psychiatric inpatients who intentionally ingested alcohol-based hand sanitizers to become intoxicated; there were no permanent toxic effects in either case.
Case 1
Mr. F, age 52, is diagnosed with polysubstance dependence and bipolar disorder and hospitalized for acute exacerbation of mania characterized by unrestrained buying sprees, racing thoughts, grandiosity, and a persistently irritable mood. On day 3 of admission, he presents as stuporous and disorganized, with a strong odor of alcohol on his breath. He admits drinking an alcohol-based hand sanitizer foaming solution, an empty bottle of which is found in his room. His serum alcohol level is 176 mg/dL; the threshold concentration above which a person is considered legally drunk when operating a motor vehicle is 100 mg/dL. Other laboratory values, including urine toxicology, were negative.
Case 2
Mr. V, age 47, has schizophrenia, cocaine dependence, and antisocial personality disorder. He is admitted for command auditory hallucinations and a suicide attempt by overdose. On day 6 of hospitalization, staff members find him delirious and confused. Mr. V confesses to drinking an alcohol-based hand sanitizer solution for the past 3 days. His vital signs are stable, and his serum alcohol level is 142 mg/dL.
Limiting access
Hand sanitizer has a much higher alcohol concentration than several common alcoholic drinks Table8,9 Ethyl alcohol, the active ingredient in hand sanitizers, is responsible for the adverse effects seen in our patients; the inactive ingredients—glycerin, propylene glycol, tocopherol acetate, isopropyl myristate, and aminomethyl propanol—generally are recognized as safe by the FDA and the Cosmetic Ingredient Review Expert Panel.10,11 Although hospitals routinely restrict patients’ access to traditional forms of alcohol, hand sanitizer is easily accessible in many facilities. In our cases, having the alcohol-based sanitizer placed throughout the unit and readily available to patients made it easy for at-risk patients to become intoxicated. As suggested by Weiner,7 replacing bottles of hand sanitizer with self-contained, wall-mounted dispensers that are difficult for patients to remove might decrease the likelihood of ingestion.
Table
Alcohol content of hand sanitizers and beverages
| Product | Percentage of alcohol by volume |
|---|---|
| Beer | 5% alcohol8 |
| Wine | 12% alcohol8 |
| Distilled spirits | 40% alcohol8 |
| Purell Foaming Hand Sanitizer | 62% ethyl alcohol9 |
| Purell Instant Hand Sanitizer | 62% ethyl alcohol, 5% isopropanol by volume9 |
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. The Joint Commission National patient safety goals. http://www.jointcommission.org/standards_information/npsgs.aspx. Accessed December 8 2011.
2. Emadi A, Coberly L. Intoxication of a hospitalized patient with an isopropanol-based hand sanitizer. N Engl J Med. 2007;356(5):530-531.
3. Boyce JM, Pittet D. Guideline for hand hygiene in health-care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Infect Control Hosp Epidemiol. 2002;23(12 suppl):S3-S40.
4. Bookstaver PB, Norris LB, Michels JE. Ingestion of hand sanitizer by a hospitalized patient with a history of alcohol abuse. Am J Health Syst Pharm. 2008;65(23):2203-2204.
5. Doyon S, Welsh C. Intoxication of a prison inmate with an ethyl alcohol-based hand sanitizer. N Engl J Med. 2007;356(5):529-530.
6. Thanarajasingam G, Diedrich DA, Mueller PS. Intentional ingestion of ethanol-based hand sanitizer by a hospitalized patient with alcoholism. Mayo Clin Proc. 2007;82(10):1288-1289.
7. Weiner SG. Changing dispensers may prevent intoxication from isopropanol and ethyl alcohol-based hand sanitizers. Ann Emerg Med. 2007;50(4):486.-
8. National Institute on Alcohol Abuse and Alcoholism What’s a “standard” drink? http://rethinkingdrinking.niaaa.nih.gov/WhatCountsDrink/WhatsAStandardDrink.asp. Accessed February 17, 2012.
9. GOJO Industries. Purell instant hand sanitizier. http://www.gojo.com/United-States/Brands/PURELL/MSDS.aspx. Accessed December 8, 2011.
10. U.S. Food and Drug Administration. Generally Recognized as Safe (GRAS) notice inventory. http://www.fda.gov/Food/FoodIngredientsPackaging/GenerallyRecognizedasSafeGRAS/
GRASListings/default.htm. Accessed February 21, 2012.
11. The Cosmetic Ingredient Review Find ingredient reviews and documents. http://www.cir-safety.org/ingredients/glossary/all. Accessed February 21, 2012.
Discuss this article at www.facebook.com/CurrentPsychiatry
The Centers for Disease Control and Prevention recommends that all health care professionals use an ethanol-based hand sanitizer to decontaminate their hands before and after direct contact with patients to prevent infection.1,2 As a result, many psychiatric hospitals use alcohol-based hand sanitizers as a primary infection control measure.
Patient misuse of these products as intoxicants has been reported in prisons, emergency rooms, and medical units.3-7 We report 2 cases of psychiatric inpatients who intentionally ingested alcohol-based hand sanitizers to become intoxicated; there were no permanent toxic effects in either case.
Case 1
Mr. F, age 52, is diagnosed with polysubstance dependence and bipolar disorder and hospitalized for acute exacerbation of mania characterized by unrestrained buying sprees, racing thoughts, grandiosity, and a persistently irritable mood. On day 3 of admission, he presents as stuporous and disorganized, with a strong odor of alcohol on his breath. He admits drinking an alcohol-based hand sanitizer foaming solution, an empty bottle of which is found in his room. His serum alcohol level is 176 mg/dL; the threshold concentration above which a person is considered legally drunk when operating a motor vehicle is 100 mg/dL. Other laboratory values, including urine toxicology, were negative.
Case 2
Mr. V, age 47, has schizophrenia, cocaine dependence, and antisocial personality disorder. He is admitted for command auditory hallucinations and a suicide attempt by overdose. On day 6 of hospitalization, staff members find him delirious and confused. Mr. V confesses to drinking an alcohol-based hand sanitizer solution for the past 3 days. His vital signs are stable, and his serum alcohol level is 142 mg/dL.
Limiting access
Hand sanitizer has a much higher alcohol concentration than several common alcoholic drinks Table8,9 Ethyl alcohol, the active ingredient in hand sanitizers, is responsible for the adverse effects seen in our patients; the inactive ingredients—glycerin, propylene glycol, tocopherol acetate, isopropyl myristate, and aminomethyl propanol—generally are recognized as safe by the FDA and the Cosmetic Ingredient Review Expert Panel.10,11 Although hospitals routinely restrict patients’ access to traditional forms of alcohol, hand sanitizer is easily accessible in many facilities. In our cases, having the alcohol-based sanitizer placed throughout the unit and readily available to patients made it easy for at-risk patients to become intoxicated. As suggested by Weiner,7 replacing bottles of hand sanitizer with self-contained, wall-mounted dispensers that are difficult for patients to remove might decrease the likelihood of ingestion.
Table
Alcohol content of hand sanitizers and beverages
| Product | Percentage of alcohol by volume |
|---|---|
| Beer | 5% alcohol8 |
| Wine | 12% alcohol8 |
| Distilled spirits | 40% alcohol8 |
| Purell Foaming Hand Sanitizer | 62% ethyl alcohol9 |
| Purell Instant Hand Sanitizer | 62% ethyl alcohol, 5% isopropanol by volume9 |
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
The Centers for Disease Control and Prevention recommends that all health care professionals use an ethanol-based hand sanitizer to decontaminate their hands before and after direct contact with patients to prevent infection.1,2 As a result, many psychiatric hospitals use alcohol-based hand sanitizers as a primary infection control measure.
Patient misuse of these products as intoxicants has been reported in prisons, emergency rooms, and medical units.3-7 We report 2 cases of psychiatric inpatients who intentionally ingested alcohol-based hand sanitizers to become intoxicated; there were no permanent toxic effects in either case.
Case 1
Mr. F, age 52, is diagnosed with polysubstance dependence and bipolar disorder and hospitalized for acute exacerbation of mania characterized by unrestrained buying sprees, racing thoughts, grandiosity, and a persistently irritable mood. On day 3 of admission, he presents as stuporous and disorganized, with a strong odor of alcohol on his breath. He admits drinking an alcohol-based hand sanitizer foaming solution, an empty bottle of which is found in his room. His serum alcohol level is 176 mg/dL; the threshold concentration above which a person is considered legally drunk when operating a motor vehicle is 100 mg/dL. Other laboratory values, including urine toxicology, were negative.
Case 2
Mr. V, age 47, has schizophrenia, cocaine dependence, and antisocial personality disorder. He is admitted for command auditory hallucinations and a suicide attempt by overdose. On day 6 of hospitalization, staff members find him delirious and confused. Mr. V confesses to drinking an alcohol-based hand sanitizer solution for the past 3 days. His vital signs are stable, and his serum alcohol level is 142 mg/dL.
Limiting access
Hand sanitizer has a much higher alcohol concentration than several common alcoholic drinks Table8,9 Ethyl alcohol, the active ingredient in hand sanitizers, is responsible for the adverse effects seen in our patients; the inactive ingredients—glycerin, propylene glycol, tocopherol acetate, isopropyl myristate, and aminomethyl propanol—generally are recognized as safe by the FDA and the Cosmetic Ingredient Review Expert Panel.10,11 Although hospitals routinely restrict patients’ access to traditional forms of alcohol, hand sanitizer is easily accessible in many facilities. In our cases, having the alcohol-based sanitizer placed throughout the unit and readily available to patients made it easy for at-risk patients to become intoxicated. As suggested by Weiner,7 replacing bottles of hand sanitizer with self-contained, wall-mounted dispensers that are difficult for patients to remove might decrease the likelihood of ingestion.
Table
Alcohol content of hand sanitizers and beverages
| Product | Percentage of alcohol by volume |
|---|---|
| Beer | 5% alcohol8 |
| Wine | 12% alcohol8 |
| Distilled spirits | 40% alcohol8 |
| Purell Foaming Hand Sanitizer | 62% ethyl alcohol9 |
| Purell Instant Hand Sanitizer | 62% ethyl alcohol, 5% isopropanol by volume9 |
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. The Joint Commission National patient safety goals. http://www.jointcommission.org/standards_information/npsgs.aspx. Accessed December 8 2011.
2. Emadi A, Coberly L. Intoxication of a hospitalized patient with an isopropanol-based hand sanitizer. N Engl J Med. 2007;356(5):530-531.
3. Boyce JM, Pittet D. Guideline for hand hygiene in health-care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Infect Control Hosp Epidemiol. 2002;23(12 suppl):S3-S40.
4. Bookstaver PB, Norris LB, Michels JE. Ingestion of hand sanitizer by a hospitalized patient with a history of alcohol abuse. Am J Health Syst Pharm. 2008;65(23):2203-2204.
5. Doyon S, Welsh C. Intoxication of a prison inmate with an ethyl alcohol-based hand sanitizer. N Engl J Med. 2007;356(5):529-530.
6. Thanarajasingam G, Diedrich DA, Mueller PS. Intentional ingestion of ethanol-based hand sanitizer by a hospitalized patient with alcoholism. Mayo Clin Proc. 2007;82(10):1288-1289.
7. Weiner SG. Changing dispensers may prevent intoxication from isopropanol and ethyl alcohol-based hand sanitizers. Ann Emerg Med. 2007;50(4):486.-
8. National Institute on Alcohol Abuse and Alcoholism What’s a “standard” drink? http://rethinkingdrinking.niaaa.nih.gov/WhatCountsDrink/WhatsAStandardDrink.asp. Accessed February 17, 2012.
9. GOJO Industries. Purell instant hand sanitizier. http://www.gojo.com/United-States/Brands/PURELL/MSDS.aspx. Accessed December 8, 2011.
10. U.S. Food and Drug Administration. Generally Recognized as Safe (GRAS) notice inventory. http://www.fda.gov/Food/FoodIngredientsPackaging/GenerallyRecognizedasSafeGRAS/
GRASListings/default.htm. Accessed February 21, 2012.
11. The Cosmetic Ingredient Review Find ingredient reviews and documents. http://www.cir-safety.org/ingredients/glossary/all. Accessed February 21, 2012.
1. The Joint Commission National patient safety goals. http://www.jointcommission.org/standards_information/npsgs.aspx. Accessed December 8 2011.
2. Emadi A, Coberly L. Intoxication of a hospitalized patient with an isopropanol-based hand sanitizer. N Engl J Med. 2007;356(5):530-531.
3. Boyce JM, Pittet D. Guideline for hand hygiene in health-care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Infect Control Hosp Epidemiol. 2002;23(12 suppl):S3-S40.
4. Bookstaver PB, Norris LB, Michels JE. Ingestion of hand sanitizer by a hospitalized patient with a history of alcohol abuse. Am J Health Syst Pharm. 2008;65(23):2203-2204.
5. Doyon S, Welsh C. Intoxication of a prison inmate with an ethyl alcohol-based hand sanitizer. N Engl J Med. 2007;356(5):529-530.
6. Thanarajasingam G, Diedrich DA, Mueller PS. Intentional ingestion of ethanol-based hand sanitizer by a hospitalized patient with alcoholism. Mayo Clin Proc. 2007;82(10):1288-1289.
7. Weiner SG. Changing dispensers may prevent intoxication from isopropanol and ethyl alcohol-based hand sanitizers. Ann Emerg Med. 2007;50(4):486.-
8. National Institute on Alcohol Abuse and Alcoholism What’s a “standard” drink? http://rethinkingdrinking.niaaa.nih.gov/WhatCountsDrink/WhatsAStandardDrink.asp. Accessed February 17, 2012.
9. GOJO Industries. Purell instant hand sanitizier. http://www.gojo.com/United-States/Brands/PURELL/MSDS.aspx. Accessed December 8, 2011.
10. U.S. Food and Drug Administration. Generally Recognized as Safe (GRAS) notice inventory. http://www.fda.gov/Food/FoodIngredientsPackaging/GenerallyRecognizedasSafeGRAS/
GRASListings/default.htm. Accessed February 21, 2012.
11. The Cosmetic Ingredient Review Find ingredient reviews and documents. http://www.cir-safety.org/ingredients/glossary/all. Accessed February 21, 2012.