User login
Prenatal acetaminophen exposure may affect ADHD in childhood
Prenatal exposure to acetaminophen was associated with a significantly increased risk of attention-deficit/hyperactivity–like behavioral problems, a hospital diagnosis of hyperkinetic disorder (HKD), or being on ADHD medications in children at age 7 years, based data from more than 60,000 children obtained from the national birth registry and other health registries in Denmark.
The study was published online on Feb. 24, in JAMA Pediatrics (doi:10.1001/jamapediatrics.2013.4914). The risk of ADHD-like behaviors in children at age 7 years increased by 13% among those whose mothers had used acetaminophen overall during pregnancy. When acetaminophen was used in the second and third trimesters, the risk increased by 44%, and when used during all three trimesters, the risk increased by 24%.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
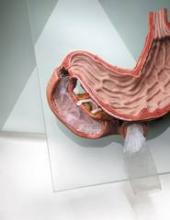
Courtesy: Cleveland Clinic
The associations with these three outcomes were greater when acetaminophen (paracetamol) was used in more than one trimester and with increasing frequency of use (more than 20 weeks during pregnancy). Because exposure to acetaminophen is common, "these associations might explain some of the increasing incidence in HKD/ADHD, but further studies are needed," Zeyan Liew, MPH, and his coauthors concluded. Mr. Liew is with the department of epidemiology, Fielding School of Public Health, University of California, Los Angeles.
The prospective study included 64,322 live-born children and their mothers who were enrolled in the Danish National Birth Cohort during 1996-2002, and data from the national hospital, psychiatric and prescription registries. The mothers had participated in three interviews about acetaminophen use during pregnancy: at the 12th and 30th weeks of gestation, and 6 months after giving birth (28,254 women who had missed at least one of these interviews and their children were excluded).
A subgroup of approximately 41,000 women who had responded to a self-administered questionnaire (the Strengths and Difficulties Questionnaire), when their child was 7 years of age were used to evaluate ADHD-like behaviors; 55% of the women in this group had used acetaminophen at some point in their pregnancy. In the entire group of 64,322 mothers, 56% had used acetaminophen during pregnancy.
Data obtained by the investigators included parental reports of ADHD-like behaviors at age 7 years; a hospital diagnoses of HKD at or after 5 years; and use of ADHD medications, mostly Ritalin. The researchers adjusted for possible confounders, including birthweight; sex; maternal age at child’s birth; socioeconomic status; drug and alcohol use during pregnancy; mother’s self-reports of psychiatric illnesses and childhood behavioral problems; and diseases or conditions that may have prompted the mothers to have used acetaminophen.
The associations between the use of prenatal acetaminophen and the increased risks of HKD or using an ADHD medication also increased when acetaminophen was used during two or more trimesters, and a significant trend appeared with the increasing weeks of use: When used for 20 or more weeks, the risk for an diagnosis of HKD increased by 84% and the risk of having received an ADHD medication increased by 53%, the researchers noted. These results were similar "when restricting to mothers who did not report psychiatric illnesses or episodes of fever, inflammation, and infections during pregnancy," they added.
Referring to evidence from animal and human studies suggesting that acetaminophen may be an endocrine disruptor, the authors wrote: "Maternal hormones, such as sex hormones and thyroid hormones, play critical roles in regulating fetal brain development, and it is possible that acetaminophen may interrupt brain development by interfering with maternal hormones or via neurotoxicity, such as the induction of oxidative stress that can cause neuronal death."
The study strengths included the availability of different endpoints to evaluate different levels of ADHD and the use of prospective data (interviews with the mothers). But the findings were limited by the inability to evaluate the effect of dosage or number of pills, because the mothers were not able to provide this information. Despite the adjustment for confounding variables, "the possibility of unmeasured residual confounding by indication for drug use, ADHD-related genetic factors, or coexposures to other medications cannot be dismissed," the researchers said.
In an accompanying editorial, Miriam Cooper, MRCPsych, of the Institute of Psychological Medicine, Cardiff, Wales, and her associates wrote that while the study’s results are potentially important, "caution should be exercised in ascribing causation to statistical associations between prenatal risk factors and adverse outcomes" and the results "should not change practice." However, the findings should be used as a basis for future research and "underline the importance of not taking a drug’s safety during pregnancy for granted" (doi:10.1001/jamapediatrics.2013.5292).
The study was supported by the Danish Medical Council. Neither the researchers nor the editorialists had relevant financial conflicts to disclose.
There are quite a few problems with the interpretation of this cohort. Most importantly, there is potential bias by indication. For example, women who needed more acetaminophen might have had conditions that could cause the outcome, rather than the acetaminophen. In addition, the doses were not known and more than 20,000 cases were excluded for missing interviews. Therefore, it is possible that they had many more parents with ADHD, which is often a genetic condition. The acetaminophen users had more muscle and joint diseases, more infections, and more psychiatric disease-which can affect the results, independent of acetaminophen.
Dr. Gideon Koren is director of the Motherisk Program at the Hospital for Sick Children, Toronto, and professor of pediatrics, pharmacology, pharmacy, and medical genetics, at the University of Toronto. He has no relevant disclosures.
There are quite a few problems with the interpretation of this cohort. Most importantly, there is potential bias by indication. For example, women who needed more acetaminophen might have had conditions that could cause the outcome, rather than the acetaminophen. In addition, the doses were not known and more than 20,000 cases were excluded for missing interviews. Therefore, it is possible that they had many more parents with ADHD, which is often a genetic condition. The acetaminophen users had more muscle and joint diseases, more infections, and more psychiatric disease-which can affect the results, independent of acetaminophen.
Dr. Gideon Koren is director of the Motherisk Program at the Hospital for Sick Children, Toronto, and professor of pediatrics, pharmacology, pharmacy, and medical genetics, at the University of Toronto. He has no relevant disclosures.
There are quite a few problems with the interpretation of this cohort. Most importantly, there is potential bias by indication. For example, women who needed more acetaminophen might have had conditions that could cause the outcome, rather than the acetaminophen. In addition, the doses were not known and more than 20,000 cases were excluded for missing interviews. Therefore, it is possible that they had many more parents with ADHD, which is often a genetic condition. The acetaminophen users had more muscle and joint diseases, more infections, and more psychiatric disease-which can affect the results, independent of acetaminophen.
Dr. Gideon Koren is director of the Motherisk Program at the Hospital for Sick Children, Toronto, and professor of pediatrics, pharmacology, pharmacy, and medical genetics, at the University of Toronto. He has no relevant disclosures.
Prenatal exposure to acetaminophen was associated with a significantly increased risk of attention-deficit/hyperactivity–like behavioral problems, a hospital diagnosis of hyperkinetic disorder (HKD), or being on ADHD medications in children at age 7 years, based data from more than 60,000 children obtained from the national birth registry and other health registries in Denmark.
The study was published online on Feb. 24, in JAMA Pediatrics (doi:10.1001/jamapediatrics.2013.4914). The risk of ADHD-like behaviors in children at age 7 years increased by 13% among those whose mothers had used acetaminophen overall during pregnancy. When acetaminophen was used in the second and third trimesters, the risk increased by 44%, and when used during all three trimesters, the risk increased by 24%.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Courtesy: Cleveland Clinic
The associations with these three outcomes were greater when acetaminophen (paracetamol) was used in more than one trimester and with increasing frequency of use (more than 20 weeks during pregnancy). Because exposure to acetaminophen is common, "these associations might explain some of the increasing incidence in HKD/ADHD, but further studies are needed," Zeyan Liew, MPH, and his coauthors concluded. Mr. Liew is with the department of epidemiology, Fielding School of Public Health, University of California, Los Angeles.
The prospective study included 64,322 live-born children and their mothers who were enrolled in the Danish National Birth Cohort during 1996-2002, and data from the national hospital, psychiatric and prescription registries. The mothers had participated in three interviews about acetaminophen use during pregnancy: at the 12th and 30th weeks of gestation, and 6 months after giving birth (28,254 women who had missed at least one of these interviews and their children were excluded).
A subgroup of approximately 41,000 women who had responded to a self-administered questionnaire (the Strengths and Difficulties Questionnaire), when their child was 7 years of age were used to evaluate ADHD-like behaviors; 55% of the women in this group had used acetaminophen at some point in their pregnancy. In the entire group of 64,322 mothers, 56% had used acetaminophen during pregnancy.
Data obtained by the investigators included parental reports of ADHD-like behaviors at age 7 years; a hospital diagnoses of HKD at or after 5 years; and use of ADHD medications, mostly Ritalin. The researchers adjusted for possible confounders, including birthweight; sex; maternal age at child’s birth; socioeconomic status; drug and alcohol use during pregnancy; mother’s self-reports of psychiatric illnesses and childhood behavioral problems; and diseases or conditions that may have prompted the mothers to have used acetaminophen.
The associations between the use of prenatal acetaminophen and the increased risks of HKD or using an ADHD medication also increased when acetaminophen was used during two or more trimesters, and a significant trend appeared with the increasing weeks of use: When used for 20 or more weeks, the risk for an diagnosis of HKD increased by 84% and the risk of having received an ADHD medication increased by 53%, the researchers noted. These results were similar "when restricting to mothers who did not report psychiatric illnesses or episodes of fever, inflammation, and infections during pregnancy," they added.
Referring to evidence from animal and human studies suggesting that acetaminophen may be an endocrine disruptor, the authors wrote: "Maternal hormones, such as sex hormones and thyroid hormones, play critical roles in regulating fetal brain development, and it is possible that acetaminophen may interrupt brain development by interfering with maternal hormones or via neurotoxicity, such as the induction of oxidative stress that can cause neuronal death."
The study strengths included the availability of different endpoints to evaluate different levels of ADHD and the use of prospective data (interviews with the mothers). But the findings were limited by the inability to evaluate the effect of dosage or number of pills, because the mothers were not able to provide this information. Despite the adjustment for confounding variables, "the possibility of unmeasured residual confounding by indication for drug use, ADHD-related genetic factors, or coexposures to other medications cannot be dismissed," the researchers said.
In an accompanying editorial, Miriam Cooper, MRCPsych, of the Institute of Psychological Medicine, Cardiff, Wales, and her associates wrote that while the study’s results are potentially important, "caution should be exercised in ascribing causation to statistical associations between prenatal risk factors and adverse outcomes" and the results "should not change practice." However, the findings should be used as a basis for future research and "underline the importance of not taking a drug’s safety during pregnancy for granted" (doi:10.1001/jamapediatrics.2013.5292).
The study was supported by the Danish Medical Council. Neither the researchers nor the editorialists had relevant financial conflicts to disclose.
Prenatal exposure to acetaminophen was associated with a significantly increased risk of attention-deficit/hyperactivity–like behavioral problems, a hospital diagnosis of hyperkinetic disorder (HKD), or being on ADHD medications in children at age 7 years, based data from more than 60,000 children obtained from the national birth registry and other health registries in Denmark.
The study was published online on Feb. 24, in JAMA Pediatrics (doi:10.1001/jamapediatrics.2013.4914). The risk of ADHD-like behaviors in children at age 7 years increased by 13% among those whose mothers had used acetaminophen overall during pregnancy. When acetaminophen was used in the second and third trimesters, the risk increased by 44%, and when used during all three trimesters, the risk increased by 24%.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Courtesy: Cleveland Clinic
The associations with these three outcomes were greater when acetaminophen (paracetamol) was used in more than one trimester and with increasing frequency of use (more than 20 weeks during pregnancy). Because exposure to acetaminophen is common, "these associations might explain some of the increasing incidence in HKD/ADHD, but further studies are needed," Zeyan Liew, MPH, and his coauthors concluded. Mr. Liew is with the department of epidemiology, Fielding School of Public Health, University of California, Los Angeles.
The prospective study included 64,322 live-born children and their mothers who were enrolled in the Danish National Birth Cohort during 1996-2002, and data from the national hospital, psychiatric and prescription registries. The mothers had participated in three interviews about acetaminophen use during pregnancy: at the 12th and 30th weeks of gestation, and 6 months after giving birth (28,254 women who had missed at least one of these interviews and their children were excluded).
A subgroup of approximately 41,000 women who had responded to a self-administered questionnaire (the Strengths and Difficulties Questionnaire), when their child was 7 years of age were used to evaluate ADHD-like behaviors; 55% of the women in this group had used acetaminophen at some point in their pregnancy. In the entire group of 64,322 mothers, 56% had used acetaminophen during pregnancy.
Data obtained by the investigators included parental reports of ADHD-like behaviors at age 7 years; a hospital diagnoses of HKD at or after 5 years; and use of ADHD medications, mostly Ritalin. The researchers adjusted for possible confounders, including birthweight; sex; maternal age at child’s birth; socioeconomic status; drug and alcohol use during pregnancy; mother’s self-reports of psychiatric illnesses and childhood behavioral problems; and diseases or conditions that may have prompted the mothers to have used acetaminophen.
The associations between the use of prenatal acetaminophen and the increased risks of HKD or using an ADHD medication also increased when acetaminophen was used during two or more trimesters, and a significant trend appeared with the increasing weeks of use: When used for 20 or more weeks, the risk for an diagnosis of HKD increased by 84% and the risk of having received an ADHD medication increased by 53%, the researchers noted. These results were similar "when restricting to mothers who did not report psychiatric illnesses or episodes of fever, inflammation, and infections during pregnancy," they added.
Referring to evidence from animal and human studies suggesting that acetaminophen may be an endocrine disruptor, the authors wrote: "Maternal hormones, such as sex hormones and thyroid hormones, play critical roles in regulating fetal brain development, and it is possible that acetaminophen may interrupt brain development by interfering with maternal hormones or via neurotoxicity, such as the induction of oxidative stress that can cause neuronal death."
The study strengths included the availability of different endpoints to evaluate different levels of ADHD and the use of prospective data (interviews with the mothers). But the findings were limited by the inability to evaluate the effect of dosage or number of pills, because the mothers were not able to provide this information. Despite the adjustment for confounding variables, "the possibility of unmeasured residual confounding by indication for drug use, ADHD-related genetic factors, or coexposures to other medications cannot be dismissed," the researchers said.
In an accompanying editorial, Miriam Cooper, MRCPsych, of the Institute of Psychological Medicine, Cardiff, Wales, and her associates wrote that while the study’s results are potentially important, "caution should be exercised in ascribing causation to statistical associations between prenatal risk factors and adverse outcomes" and the results "should not change practice." However, the findings should be used as a basis for future research and "underline the importance of not taking a drug’s safety during pregnancy for granted" (doi:10.1001/jamapediatrics.2013.5292).
The study was supported by the Danish Medical Council. Neither the researchers nor the editorialists had relevant financial conflicts to disclose.
FROM JAMA
Major finding: Children exposed prenatally to acetaminophen were at a greater risk of being diagnosed with hyperkinetic disorder, having ADHD-like behaviors, or being on an ADHD medication, an association that was increased with exposure during more than one trimester and with more weeks of exposure.
Data source: A large prospective study using the Danish National Birth Cohort data and other national health registries evaluated the association between the three outcomes, and maternal use of acetaminophen, based on information obtained from the mothers of more than 64,000 children.
Disclosures: The study was supported by the Danish Medical Research Council. One of the authors was at the University of Arizona, Tucson, when she was involved in the study, but now works at Novartis Farmaceutica SA, in Barcelona.
The future of obesity treatments could include endoscopically placed devices
WASHINGTON – Endoscopically placed devices that include intragastric balloons and gastrointestinal liners are being evaluated for the treatment of obesity and could eventually fill some of the current void in treatment options that fall between lifestyle changes and surgical treatments.
These types of devices are the new paradigm for obesity treatments, and many are already available in Europe and elsewhere, Dr. Richard Rothstein said at a public workshop cosponsored by the Food and Drug Administration and the American Gastroenterological Association. Some have been evaluated in studies that use diabetes control measures as the main outcome endpoint, rather than weight loss, he noted.
Dr. Rothstein, the Joseph M. Huber Professor of Medicine and chair of the department of medicine, Geisel School of Medicine at Dartmouth, Hanover, N.H., provided an overview of some of the devices, which include sleeves, techniques, and devices used to reduce gastric volume such as balloons, and surgical tools used to sew or staple the stomach together.
Endoscopic treatments for obesity are less invasive, less expensive, reversible, and are performed as outpatient procedures, he said, noting that patients may be willing to pay for these procedures out of pocket.
In early studies, the endoscopically delivered EndoBarrier resulted in weight loss and was well tolerated, said Dr. Rothstein, who is an adviser to the manufacturer, GI Dynamics. This is a flexible, tube-shaped liner that forms a physical barrier between food and the intestinal wall, according to the manufacturer. The device, which mimics malabsorptive surgical procedures, has been available for 3 years in Europe, Australia, and South America and is being evaluated in a pivotal U.S. study, the ENDO trial, for treating patients who are obese and have uncontrolled type 2 diabetes.
Andy Levine, who was chief technology officer at GI Dynamics and is now an adviser to the company, said that to date, the EndoBarrier, which completely covers the duodenum and the proximal jejunum and is anchored in the duodenal bulb distal to the pylorus, has been placed in more than 1,300 people worldwide. The device creates a "a complete bypass," so the food from the stomach goes through the flexible soft liner, driven by peristalsis, and the bile and pancreatic enzymes pass on the outside of the liner, with all mixing in the bowel, said Mr. Levine, who is now the leader of business development for medical devices and robotics at the Wyss Institute for Biologically Inspired Engineering at Harvard University, Boston.
In studies, improvements in type 2 diabetes measures are being used as endpoints, since showing improvement in obesity comorbidities was determined to be important for reimbursement, "and the technology had a unique impact on diabetes."
Based on experience with the device to date, Mr. Levine said that for the first 9-10 months, patients lose about 10%-20% of their body weight and many have been able to discontinue insulin and stay on oral therapy. Following removal of the device at 1 year, there is a slow rebound in weight and hemoglobin A1c values. While this might appear to be a temporary solution for a chronic problem, "we’re certainly treating it, and in some ways may be reversing the disease at least for awhile," he said, pointing out that the device addresses the need to break the cycle of continuing weight gain and helps patients discontinue diabetes medications, especially insulin.
A variety of newer, improved versions of endoscopically placed balloons are also being studied, including the ReShape, a double balloon device, Dr. Rothstein said.
During the same session, Richard Thompson, president and CEO of ReShape Medical, a small venture capital–funded company, said that the ReShape Duo intragastric device is made up of two balloons that contain a total of about 900 mL of fluid, which occupy space in the stomach without the discomfort and distension that can occur with one large balloon. This device is designed to reduce the risk of migration, which can occurs when a balloon deflates and migrates to the intestine where it can cause a blockage. If one balloon deflates the other remains inflated, and because there is blue dye in the balloons, when one deflates, the dye is visible in urine to alert the patient, he noted. ReShape Duo has been available in Europe for more than 2 years.
The U.S. trial evaluating the device in 325 patients is almost completed, and the company recently announced that the primary endpoints that assessed weight loss and responder rates had been met. The company plans to file for FDA approval in the second quarter of 2014.
Dr. Rothstein said that other nonsurgical devices being developed include those that do not require endoscopic placement, such as bezoars or balloons that are swallowed by the patient and function as temporary "space-occupying devices," he said. One example is a capsule that contains beads, which swell up in the stomach and take up space after the capsule is swallowed – which may affect satiety and result in weight loss if ingested before a meal, he said.
Techniques that bring aspects of the proximal stomach together represent another approach, using devices to sew and stitch up the stomach, compartmentalizing the stomach, creating "if you will, an endoscopic gastric sleeve," he said, noting there are not much data available on this approach.
Data from studies that are sham-controlled and have adequate sample sizes are needed to determine the role of these therapies, as they are developed, and they need to be shown to have "reasonable durability," and a safety profile with minimal complications, with cost-effectiveness data, Dr. Rothstein said. And as they become available, it will be necessary to "tease out" which patient groups respond best to which therapy, such as balloons or bezoars, or gastric bypass surgery, he added.
WASHINGTON – Endoscopically placed devices that include intragastric balloons and gastrointestinal liners are being evaluated for the treatment of obesity and could eventually fill some of the current void in treatment options that fall between lifestyle changes and surgical treatments.
These types of devices are the new paradigm for obesity treatments, and many are already available in Europe and elsewhere, Dr. Richard Rothstein said at a public workshop cosponsored by the Food and Drug Administration and the American Gastroenterological Association. Some have been evaluated in studies that use diabetes control measures as the main outcome endpoint, rather than weight loss, he noted.
Dr. Rothstein, the Joseph M. Huber Professor of Medicine and chair of the department of medicine, Geisel School of Medicine at Dartmouth, Hanover, N.H., provided an overview of some of the devices, which include sleeves, techniques, and devices used to reduce gastric volume such as balloons, and surgical tools used to sew or staple the stomach together.
Endoscopic treatments for obesity are less invasive, less expensive, reversible, and are performed as outpatient procedures, he said, noting that patients may be willing to pay for these procedures out of pocket.
In early studies, the endoscopically delivered EndoBarrier resulted in weight loss and was well tolerated, said Dr. Rothstein, who is an adviser to the manufacturer, GI Dynamics. This is a flexible, tube-shaped liner that forms a physical barrier between food and the intestinal wall, according to the manufacturer. The device, which mimics malabsorptive surgical procedures, has been available for 3 years in Europe, Australia, and South America and is being evaluated in a pivotal U.S. study, the ENDO trial, for treating patients who are obese and have uncontrolled type 2 diabetes.
Andy Levine, who was chief technology officer at GI Dynamics and is now an adviser to the company, said that to date, the EndoBarrier, which completely covers the duodenum and the proximal jejunum and is anchored in the duodenal bulb distal to the pylorus, has been placed in more than 1,300 people worldwide. The device creates a "a complete bypass," so the food from the stomach goes through the flexible soft liner, driven by peristalsis, and the bile and pancreatic enzymes pass on the outside of the liner, with all mixing in the bowel, said Mr. Levine, who is now the leader of business development for medical devices and robotics at the Wyss Institute for Biologically Inspired Engineering at Harvard University, Boston.
In studies, improvements in type 2 diabetes measures are being used as endpoints, since showing improvement in obesity comorbidities was determined to be important for reimbursement, "and the technology had a unique impact on diabetes."
Based on experience with the device to date, Mr. Levine said that for the first 9-10 months, patients lose about 10%-20% of their body weight and many have been able to discontinue insulin and stay on oral therapy. Following removal of the device at 1 year, there is a slow rebound in weight and hemoglobin A1c values. While this might appear to be a temporary solution for a chronic problem, "we’re certainly treating it, and in some ways may be reversing the disease at least for awhile," he said, pointing out that the device addresses the need to break the cycle of continuing weight gain and helps patients discontinue diabetes medications, especially insulin.
A variety of newer, improved versions of endoscopically placed balloons are also being studied, including the ReShape, a double balloon device, Dr. Rothstein said.
During the same session, Richard Thompson, president and CEO of ReShape Medical, a small venture capital–funded company, said that the ReShape Duo intragastric device is made up of two balloons that contain a total of about 900 mL of fluid, which occupy space in the stomach without the discomfort and distension that can occur with one large balloon. This device is designed to reduce the risk of migration, which can occurs when a balloon deflates and migrates to the intestine where it can cause a blockage. If one balloon deflates the other remains inflated, and because there is blue dye in the balloons, when one deflates, the dye is visible in urine to alert the patient, he noted. ReShape Duo has been available in Europe for more than 2 years.
The U.S. trial evaluating the device in 325 patients is almost completed, and the company recently announced that the primary endpoints that assessed weight loss and responder rates had been met. The company plans to file for FDA approval in the second quarter of 2014.
Dr. Rothstein said that other nonsurgical devices being developed include those that do not require endoscopic placement, such as bezoars or balloons that are swallowed by the patient and function as temporary "space-occupying devices," he said. One example is a capsule that contains beads, which swell up in the stomach and take up space after the capsule is swallowed – which may affect satiety and result in weight loss if ingested before a meal, he said.
Techniques that bring aspects of the proximal stomach together represent another approach, using devices to sew and stitch up the stomach, compartmentalizing the stomach, creating "if you will, an endoscopic gastric sleeve," he said, noting there are not much data available on this approach.
Data from studies that are sham-controlled and have adequate sample sizes are needed to determine the role of these therapies, as they are developed, and they need to be shown to have "reasonable durability," and a safety profile with minimal complications, with cost-effectiveness data, Dr. Rothstein said. And as they become available, it will be necessary to "tease out" which patient groups respond best to which therapy, such as balloons or bezoars, or gastric bypass surgery, he added.
WASHINGTON – Endoscopically placed devices that include intragastric balloons and gastrointestinal liners are being evaluated for the treatment of obesity and could eventually fill some of the current void in treatment options that fall between lifestyle changes and surgical treatments.
These types of devices are the new paradigm for obesity treatments, and many are already available in Europe and elsewhere, Dr. Richard Rothstein said at a public workshop cosponsored by the Food and Drug Administration and the American Gastroenterological Association. Some have been evaluated in studies that use diabetes control measures as the main outcome endpoint, rather than weight loss, he noted.
Dr. Rothstein, the Joseph M. Huber Professor of Medicine and chair of the department of medicine, Geisel School of Medicine at Dartmouth, Hanover, N.H., provided an overview of some of the devices, which include sleeves, techniques, and devices used to reduce gastric volume such as balloons, and surgical tools used to sew or staple the stomach together.
Endoscopic treatments for obesity are less invasive, less expensive, reversible, and are performed as outpatient procedures, he said, noting that patients may be willing to pay for these procedures out of pocket.
In early studies, the endoscopically delivered EndoBarrier resulted in weight loss and was well tolerated, said Dr. Rothstein, who is an adviser to the manufacturer, GI Dynamics. This is a flexible, tube-shaped liner that forms a physical barrier between food and the intestinal wall, according to the manufacturer. The device, which mimics malabsorptive surgical procedures, has been available for 3 years in Europe, Australia, and South America and is being evaluated in a pivotal U.S. study, the ENDO trial, for treating patients who are obese and have uncontrolled type 2 diabetes.
Andy Levine, who was chief technology officer at GI Dynamics and is now an adviser to the company, said that to date, the EndoBarrier, which completely covers the duodenum and the proximal jejunum and is anchored in the duodenal bulb distal to the pylorus, has been placed in more than 1,300 people worldwide. The device creates a "a complete bypass," so the food from the stomach goes through the flexible soft liner, driven by peristalsis, and the bile and pancreatic enzymes pass on the outside of the liner, with all mixing in the bowel, said Mr. Levine, who is now the leader of business development for medical devices and robotics at the Wyss Institute for Biologically Inspired Engineering at Harvard University, Boston.
In studies, improvements in type 2 diabetes measures are being used as endpoints, since showing improvement in obesity comorbidities was determined to be important for reimbursement, "and the technology had a unique impact on diabetes."
Based on experience with the device to date, Mr. Levine said that for the first 9-10 months, patients lose about 10%-20% of their body weight and many have been able to discontinue insulin and stay on oral therapy. Following removal of the device at 1 year, there is a slow rebound in weight and hemoglobin A1c values. While this might appear to be a temporary solution for a chronic problem, "we’re certainly treating it, and in some ways may be reversing the disease at least for awhile," he said, pointing out that the device addresses the need to break the cycle of continuing weight gain and helps patients discontinue diabetes medications, especially insulin.
A variety of newer, improved versions of endoscopically placed balloons are also being studied, including the ReShape, a double balloon device, Dr. Rothstein said.
During the same session, Richard Thompson, president and CEO of ReShape Medical, a small venture capital–funded company, said that the ReShape Duo intragastric device is made up of two balloons that contain a total of about 900 mL of fluid, which occupy space in the stomach without the discomfort and distension that can occur with one large balloon. This device is designed to reduce the risk of migration, which can occurs when a balloon deflates and migrates to the intestine where it can cause a blockage. If one balloon deflates the other remains inflated, and because there is blue dye in the balloons, when one deflates, the dye is visible in urine to alert the patient, he noted. ReShape Duo has been available in Europe for more than 2 years.
The U.S. trial evaluating the device in 325 patients is almost completed, and the company recently announced that the primary endpoints that assessed weight loss and responder rates had been met. The company plans to file for FDA approval in the second quarter of 2014.
Dr. Rothstein said that other nonsurgical devices being developed include those that do not require endoscopic placement, such as bezoars or balloons that are swallowed by the patient and function as temporary "space-occupying devices," he said. One example is a capsule that contains beads, which swell up in the stomach and take up space after the capsule is swallowed – which may affect satiety and result in weight loss if ingested before a meal, he said.
Techniques that bring aspects of the proximal stomach together represent another approach, using devices to sew and stitch up the stomach, compartmentalizing the stomach, creating "if you will, an endoscopic gastric sleeve," he said, noting there are not much data available on this approach.
Data from studies that are sham-controlled and have adequate sample sizes are needed to determine the role of these therapies, as they are developed, and they need to be shown to have "reasonable durability," and a safety profile with minimal complications, with cost-effectiveness data, Dr. Rothstein said. And as they become available, it will be necessary to "tease out" which patient groups respond best to which therapy, such as balloons or bezoars, or gastric bypass surgery, he added.
AT AN FDA/AGA WORKSHOP
Implantable stimulator for OSA wins FDA panel support
SILVER SPRING, MD. – A surgically implanted upper airway stimulation device is a step closer to earning approval as a treatment for adults with refractory moderate to severe obstructive sleep apnea who have failed or cannot tolerate continuous positive airway pressure.
At a meeting on Feb. 20, the Food and Drug Administration’s Anesthesiology and Respiratory Therapy Devices Panel supported approval of the Inspire Upper Airway Stimulation System, voting 12 to 0, with one abstention, that the benefits of the device outweighed its risks as a treatment for adults with moderate to severe obstructive sleep apnea (OSA) who have failed or cannot tolerate continuous positive airway pressure (CPAP) "and who have absence of complete concentric collapse at the level of the soft palate," the indication under FDA review.
The components of the device are a stimulation lead placed around the distal hypoglossal nerve, a pulse generator implanted subcutaneously below the clavicle in the upper chest, and a respiration sensing lead inserted between the fourth and fifth ribs – implanted under general anesthesia, with three surgical incisions. External components include patient and physician programmers. Stimulation of the hypoglossal nerve during the inspiration phase of respiration "results in a forward movement of the base of the tongue, which in turn prevents airway collapse," according to the manufacturer, Inspire Medical Systems.
Panelists had concerns about some safety issues and how the device would be used in practice. But overall, they were enthusiastic about the potential for the device and agreed that the pivotal study, the STAR (Stimulation Therapy for Apnea Reduction) trial, was well done, and had "compelling" results.
The data provided "fairly strong evidence that this is a reasonably safe and effective treatment," said panelist Dr. Andrew Ries, director of the pulmonary rehabilitation program and professor of medicine and family preventive medicine at the University of California, San Diego. There are unanswered questions about how it will be used in the real world, "but it appears to be an important innovative technique," he added.
Panelist Dr. Henry Klar Yaggi of the department of pulmonary, critical care, and sleep medicine, Yale University, New Haven, Conn., said that the patients in the study represent "an underserved population of patients who have an opportunity to benefit" from this therapy.
STAR, a prospective single-arm study, evaluated the device in 126 mostly male, white patients, whose mean age was about 55 years, and who had moderate to severe OSA and had either failed or were intolerant of CPAP. Patients with a BMI greater than 32 kg/m2 were excluded. Only two patients did not complete the study and were considered nonresponders. The apnea-hypopnea index (AHI), the number of apnea or hypopnea events per hour, and the oxygen desaturation index (ODI), the number of times per hour of sleep that blood oxygen levels dropped by at least 4 percentage points from baseline, were the two main outcome measures.
The median AHI score dropped from 29.3 events per hour at baseline to 9 events per hour at 12 months, a 68% reduction that was highly statistically significant (15 or higher indicates moderate to severe apnea). Median ODI scores dropped from 25.4 events per hour at baseline to 7.4 events per hour at 12 months, a 70% reduction that was also highly statistically significant. All five secondary efficacy endpoints – including improvements in a daytime sleepiness scale and the percentage of sleep time with an oxygen saturation level below 90% – were also statistically significant (N. Engl. J. Med. 2014;370:139-49).
The rate of serious adverse events related to the procedure was under 2% (two cases where the neurostimulator had to be repositioned). Nonserious device-related adverse events included 35 cases of tongue weakness in 23 patients; most (27) resolved with no interventions. The three deaths were not considered to be related to the device. Other adverse events included stimulation-related discomfort (99 events in 59 patients) and tongue abrasions or irritation (38 events in 30 patients).
Issues raised by FDA reviewers included the multiple titrations many patients needed to achieve a therapeutic effect of stimulation or to address stimulation-related discomfort, and the possibility that patients might skip CPAP in favor of this device. But the panel agreed that when the multiple amount of titrations needed was considered, the data showed a durable effect for a period of time that they thought was clinically meaningful, and that it would be an advantage to be able to titrate the stimulation.
Although 97% of the patients in the study were white and no black patients were enrolled, none of the panelists thought that there was any reason to expect the device would not be effective in other racial and ethnic groups. The company should include more diverse populations in postmarketing studies, the panel said.
The company has proposed a postmarketing study and is developing a training program for surgeons, sleep physicians, and support staff, including nurses.
The FDA usually follows the recommendations of its advisory panels. Advisory panel members have been cleared of potential conflicts related to the meeting topic. Occasionally, a panelist is given a waiver, but no waivers were given at this meeting.
SILVER SPRING, MD. – A surgically implanted upper airway stimulation device is a step closer to earning approval as a treatment for adults with refractory moderate to severe obstructive sleep apnea who have failed or cannot tolerate continuous positive airway pressure.
At a meeting on Feb. 20, the Food and Drug Administration’s Anesthesiology and Respiratory Therapy Devices Panel supported approval of the Inspire Upper Airway Stimulation System, voting 12 to 0, with one abstention, that the benefits of the device outweighed its risks as a treatment for adults with moderate to severe obstructive sleep apnea (OSA) who have failed or cannot tolerate continuous positive airway pressure (CPAP) "and who have absence of complete concentric collapse at the level of the soft palate," the indication under FDA review.
The components of the device are a stimulation lead placed around the distal hypoglossal nerve, a pulse generator implanted subcutaneously below the clavicle in the upper chest, and a respiration sensing lead inserted between the fourth and fifth ribs – implanted under general anesthesia, with three surgical incisions. External components include patient and physician programmers. Stimulation of the hypoglossal nerve during the inspiration phase of respiration "results in a forward movement of the base of the tongue, which in turn prevents airway collapse," according to the manufacturer, Inspire Medical Systems.
Panelists had concerns about some safety issues and how the device would be used in practice. But overall, they were enthusiastic about the potential for the device and agreed that the pivotal study, the STAR (Stimulation Therapy for Apnea Reduction) trial, was well done, and had "compelling" results.
The data provided "fairly strong evidence that this is a reasonably safe and effective treatment," said panelist Dr. Andrew Ries, director of the pulmonary rehabilitation program and professor of medicine and family preventive medicine at the University of California, San Diego. There are unanswered questions about how it will be used in the real world, "but it appears to be an important innovative technique," he added.
Panelist Dr. Henry Klar Yaggi of the department of pulmonary, critical care, and sleep medicine, Yale University, New Haven, Conn., said that the patients in the study represent "an underserved population of patients who have an opportunity to benefit" from this therapy.
STAR, a prospective single-arm study, evaluated the device in 126 mostly male, white patients, whose mean age was about 55 years, and who had moderate to severe OSA and had either failed or were intolerant of CPAP. Patients with a BMI greater than 32 kg/m2 were excluded. Only two patients did not complete the study and were considered nonresponders. The apnea-hypopnea index (AHI), the number of apnea or hypopnea events per hour, and the oxygen desaturation index (ODI), the number of times per hour of sleep that blood oxygen levels dropped by at least 4 percentage points from baseline, were the two main outcome measures.
The median AHI score dropped from 29.3 events per hour at baseline to 9 events per hour at 12 months, a 68% reduction that was highly statistically significant (15 or higher indicates moderate to severe apnea). Median ODI scores dropped from 25.4 events per hour at baseline to 7.4 events per hour at 12 months, a 70% reduction that was also highly statistically significant. All five secondary efficacy endpoints – including improvements in a daytime sleepiness scale and the percentage of sleep time with an oxygen saturation level below 90% – were also statistically significant (N. Engl. J. Med. 2014;370:139-49).
The rate of serious adverse events related to the procedure was under 2% (two cases where the neurostimulator had to be repositioned). Nonserious device-related adverse events included 35 cases of tongue weakness in 23 patients; most (27) resolved with no interventions. The three deaths were not considered to be related to the device. Other adverse events included stimulation-related discomfort (99 events in 59 patients) and tongue abrasions or irritation (38 events in 30 patients).
Issues raised by FDA reviewers included the multiple titrations many patients needed to achieve a therapeutic effect of stimulation or to address stimulation-related discomfort, and the possibility that patients might skip CPAP in favor of this device. But the panel agreed that when the multiple amount of titrations needed was considered, the data showed a durable effect for a period of time that they thought was clinically meaningful, and that it would be an advantage to be able to titrate the stimulation.
Although 97% of the patients in the study were white and no black patients were enrolled, none of the panelists thought that there was any reason to expect the device would not be effective in other racial and ethnic groups. The company should include more diverse populations in postmarketing studies, the panel said.
The company has proposed a postmarketing study and is developing a training program for surgeons, sleep physicians, and support staff, including nurses.
The FDA usually follows the recommendations of its advisory panels. Advisory panel members have been cleared of potential conflicts related to the meeting topic. Occasionally, a panelist is given a waiver, but no waivers were given at this meeting.
SILVER SPRING, MD. – A surgically implanted upper airway stimulation device is a step closer to earning approval as a treatment for adults with refractory moderate to severe obstructive sleep apnea who have failed or cannot tolerate continuous positive airway pressure.
At a meeting on Feb. 20, the Food and Drug Administration’s Anesthesiology and Respiratory Therapy Devices Panel supported approval of the Inspire Upper Airway Stimulation System, voting 12 to 0, with one abstention, that the benefits of the device outweighed its risks as a treatment for adults with moderate to severe obstructive sleep apnea (OSA) who have failed or cannot tolerate continuous positive airway pressure (CPAP) "and who have absence of complete concentric collapse at the level of the soft palate," the indication under FDA review.
The components of the device are a stimulation lead placed around the distal hypoglossal nerve, a pulse generator implanted subcutaneously below the clavicle in the upper chest, and a respiration sensing lead inserted between the fourth and fifth ribs – implanted under general anesthesia, with three surgical incisions. External components include patient and physician programmers. Stimulation of the hypoglossal nerve during the inspiration phase of respiration "results in a forward movement of the base of the tongue, which in turn prevents airway collapse," according to the manufacturer, Inspire Medical Systems.
Panelists had concerns about some safety issues and how the device would be used in practice. But overall, they were enthusiastic about the potential for the device and agreed that the pivotal study, the STAR (Stimulation Therapy for Apnea Reduction) trial, was well done, and had "compelling" results.
The data provided "fairly strong evidence that this is a reasonably safe and effective treatment," said panelist Dr. Andrew Ries, director of the pulmonary rehabilitation program and professor of medicine and family preventive medicine at the University of California, San Diego. There are unanswered questions about how it will be used in the real world, "but it appears to be an important innovative technique," he added.
Panelist Dr. Henry Klar Yaggi of the department of pulmonary, critical care, and sleep medicine, Yale University, New Haven, Conn., said that the patients in the study represent "an underserved population of patients who have an opportunity to benefit" from this therapy.
STAR, a prospective single-arm study, evaluated the device in 126 mostly male, white patients, whose mean age was about 55 years, and who had moderate to severe OSA and had either failed or were intolerant of CPAP. Patients with a BMI greater than 32 kg/m2 were excluded. Only two patients did not complete the study and were considered nonresponders. The apnea-hypopnea index (AHI), the number of apnea or hypopnea events per hour, and the oxygen desaturation index (ODI), the number of times per hour of sleep that blood oxygen levels dropped by at least 4 percentage points from baseline, were the two main outcome measures.
The median AHI score dropped from 29.3 events per hour at baseline to 9 events per hour at 12 months, a 68% reduction that was highly statistically significant (15 or higher indicates moderate to severe apnea). Median ODI scores dropped from 25.4 events per hour at baseline to 7.4 events per hour at 12 months, a 70% reduction that was also highly statistically significant. All five secondary efficacy endpoints – including improvements in a daytime sleepiness scale and the percentage of sleep time with an oxygen saturation level below 90% – were also statistically significant (N. Engl. J. Med. 2014;370:139-49).
The rate of serious adverse events related to the procedure was under 2% (two cases where the neurostimulator had to be repositioned). Nonserious device-related adverse events included 35 cases of tongue weakness in 23 patients; most (27) resolved with no interventions. The three deaths were not considered to be related to the device. Other adverse events included stimulation-related discomfort (99 events in 59 patients) and tongue abrasions or irritation (38 events in 30 patients).
Issues raised by FDA reviewers included the multiple titrations many patients needed to achieve a therapeutic effect of stimulation or to address stimulation-related discomfort, and the possibility that patients might skip CPAP in favor of this device. But the panel agreed that when the multiple amount of titrations needed was considered, the data showed a durable effect for a period of time that they thought was clinically meaningful, and that it would be an advantage to be able to titrate the stimulation.
Although 97% of the patients in the study were white and no black patients were enrolled, none of the panelists thought that there was any reason to expect the device would not be effective in other racial and ethnic groups. The company should include more diverse populations in postmarketing studies, the panel said.
The company has proposed a postmarketing study and is developing a training program for surgeons, sleep physicians, and support staff, including nurses.
The FDA usually follows the recommendations of its advisory panels. Advisory panel members have been cleared of potential conflicts related to the meeting topic. Occasionally, a panelist is given a waiver, but no waivers were given at this meeting.
AT AN FDA ADVISORY COMMITTEE MEETING
FDA approves droxidopa for neurogenic orthostatic hypotension
Droxidopa, a prodrug that is converted into norepinephrine, has been approved for the treatment of patients with neurogenic orthostatic hypotension, a rare, chronic, and often debilitating condition associated with Parkinson’s disease, multiple system atrophy, and pure autonomic failure, the Food and Drug Administration announced on Feb 18.
The approval is based on two clinical trials of people with NOH, who, over 2 weeks of treatment, reported improvements in dizziness, light-headedness, feeling faint, "or feeling as if they might black out," compared with those taking a placebo, the FDA said in a statement announcing the approval. The most common adverse events reported by patients in the studies were headache, dizziness, nausea, hypertension, and fatigue.
The approval is an accelerated approval, which enables the FDA to approve a drug for a serious disease with few treatment options, based on data showing that the treatment has an effect on a clinical measure that is considered "reasonably likely" to predict longer-term benefit. In the case of droxidopa, approval was based on short-term relief of dizziness. The manufacturer, Chelsea Therapeutics, is required to conduct a postmarketing study to confirm the drug’s benefits.
Droxidopa is "a synthetic catecholamine that is directly converted to norepinephrine (NE) via decarboxylation, resulting in increased levels of NE in the nervous system, both centrally and peripherally," according to the company, which will market the drug under the trade name Northera.
"There are limited treatment options for people with NOH, and we are committed to helping make safe and effective treatments available," Dr. Norman Stockbridge, director of the Division of Cardiovascular and Renal Drugs in the FDA’s Center for Drug Evaluation and Research, said in the statement. People with NOH "are often severely limited in their ability to perform routine daily activities that require walking or standing," he added.
The prescribing information states that droxidopa is indicated for "the treatment of orthostatic dizziness, light-headedness, or the ‘feeling that you are about to black out’ in adult patients with symptomatic neurogenic orthostatic hypotension (NOH) caused by primary autonomic failure [Parkinson’s disease (PD), multiple system atrophy, and pure autonomic failure], dopamine beta-hydroxylase deficiency, and nondiabetic autonomic neuropathy."
The indications and usage statement also adds that effectiveness past 2 weeks "has not been established," and that the "continued effectiveness of Northera should be assessed periodically."
The prescribing information includes a boxed warning about the risk of supine hypertension, and recommends that supine blood pressure be monitored before and during treatment, and more often when doses are increased – and that elevating the head of the bed reduces the risk of supine hypertension.
At a meeting on Jan. 14, the FDA’s Cardiovascular and Renal Drugs Advisory Committee voted 16 to 1 to recommend approval. In the FDA’s briefing documents filed prior to the meeting, the agency did not support approval of the drug, for reasons that included a lack of evidence indicating the drug had a durable effect, as well as safety issues.
Until the approval of droxidopa, the only drug approved for symptomatic NOH was midodrine, a vasoconstrictor that was approved in 1996. But midodrine may soon be off the market because postmarketing studies have failed to provide convincing evidence that treatment improves symptoms, according to the FDA’s briefing documents for the droxidopa panel meeting. Midodrine was approved on the basis of a surrogate endpoint – standing systolic blood pressure – which was considered "reasonably likely" to predict symptomatic benefit, but several postmarketing studies have failed to show that the drug is beneficial, despite its effect on increasing blood pressure.
NOH affects almost 300,000 people in the United States and European Union, according to Chelsea.
A statement on the approval issued by Chelsea said the company had a "preliminary" agreement with the FDA to conduct a postmarketing study to evaluate the clinical effects of droxidopa, which would be a multicenter, placebo-controlled, randomized study of about 1,400 patients enrolled over 6 years.
The prescribing information is available here.
Droxidopa, a prodrug that is converted into norepinephrine, has been approved for the treatment of patients with neurogenic orthostatic hypotension, a rare, chronic, and often debilitating condition associated with Parkinson’s disease, multiple system atrophy, and pure autonomic failure, the Food and Drug Administration announced on Feb 18.
The approval is based on two clinical trials of people with NOH, who, over 2 weeks of treatment, reported improvements in dizziness, light-headedness, feeling faint, "or feeling as if they might black out," compared with those taking a placebo, the FDA said in a statement announcing the approval. The most common adverse events reported by patients in the studies were headache, dizziness, nausea, hypertension, and fatigue.
The approval is an accelerated approval, which enables the FDA to approve a drug for a serious disease with few treatment options, based on data showing that the treatment has an effect on a clinical measure that is considered "reasonably likely" to predict longer-term benefit. In the case of droxidopa, approval was based on short-term relief of dizziness. The manufacturer, Chelsea Therapeutics, is required to conduct a postmarketing study to confirm the drug’s benefits.
Droxidopa is "a synthetic catecholamine that is directly converted to norepinephrine (NE) via decarboxylation, resulting in increased levels of NE in the nervous system, both centrally and peripherally," according to the company, which will market the drug under the trade name Northera.
"There are limited treatment options for people with NOH, and we are committed to helping make safe and effective treatments available," Dr. Norman Stockbridge, director of the Division of Cardiovascular and Renal Drugs in the FDA’s Center for Drug Evaluation and Research, said in the statement. People with NOH "are often severely limited in their ability to perform routine daily activities that require walking or standing," he added.
The prescribing information states that droxidopa is indicated for "the treatment of orthostatic dizziness, light-headedness, or the ‘feeling that you are about to black out’ in adult patients with symptomatic neurogenic orthostatic hypotension (NOH) caused by primary autonomic failure [Parkinson’s disease (PD), multiple system atrophy, and pure autonomic failure], dopamine beta-hydroxylase deficiency, and nondiabetic autonomic neuropathy."
The indications and usage statement also adds that effectiveness past 2 weeks "has not been established," and that the "continued effectiveness of Northera should be assessed periodically."
The prescribing information includes a boxed warning about the risk of supine hypertension, and recommends that supine blood pressure be monitored before and during treatment, and more often when doses are increased – and that elevating the head of the bed reduces the risk of supine hypertension.
At a meeting on Jan. 14, the FDA’s Cardiovascular and Renal Drugs Advisory Committee voted 16 to 1 to recommend approval. In the FDA’s briefing documents filed prior to the meeting, the agency did not support approval of the drug, for reasons that included a lack of evidence indicating the drug had a durable effect, as well as safety issues.
Until the approval of droxidopa, the only drug approved for symptomatic NOH was midodrine, a vasoconstrictor that was approved in 1996. But midodrine may soon be off the market because postmarketing studies have failed to provide convincing evidence that treatment improves symptoms, according to the FDA’s briefing documents for the droxidopa panel meeting. Midodrine was approved on the basis of a surrogate endpoint – standing systolic blood pressure – which was considered "reasonably likely" to predict symptomatic benefit, but several postmarketing studies have failed to show that the drug is beneficial, despite its effect on increasing blood pressure.
NOH affects almost 300,000 people in the United States and European Union, according to Chelsea.
A statement on the approval issued by Chelsea said the company had a "preliminary" agreement with the FDA to conduct a postmarketing study to evaluate the clinical effects of droxidopa, which would be a multicenter, placebo-controlled, randomized study of about 1,400 patients enrolled over 6 years.
The prescribing information is available here.
Droxidopa, a prodrug that is converted into norepinephrine, has been approved for the treatment of patients with neurogenic orthostatic hypotension, a rare, chronic, and often debilitating condition associated with Parkinson’s disease, multiple system atrophy, and pure autonomic failure, the Food and Drug Administration announced on Feb 18.
The approval is based on two clinical trials of people with NOH, who, over 2 weeks of treatment, reported improvements in dizziness, light-headedness, feeling faint, "or feeling as if they might black out," compared with those taking a placebo, the FDA said in a statement announcing the approval. The most common adverse events reported by patients in the studies were headache, dizziness, nausea, hypertension, and fatigue.
The approval is an accelerated approval, which enables the FDA to approve a drug for a serious disease with few treatment options, based on data showing that the treatment has an effect on a clinical measure that is considered "reasonably likely" to predict longer-term benefit. In the case of droxidopa, approval was based on short-term relief of dizziness. The manufacturer, Chelsea Therapeutics, is required to conduct a postmarketing study to confirm the drug’s benefits.
Droxidopa is "a synthetic catecholamine that is directly converted to norepinephrine (NE) via decarboxylation, resulting in increased levels of NE in the nervous system, both centrally and peripherally," according to the company, which will market the drug under the trade name Northera.
"There are limited treatment options for people with NOH, and we are committed to helping make safe and effective treatments available," Dr. Norman Stockbridge, director of the Division of Cardiovascular and Renal Drugs in the FDA’s Center for Drug Evaluation and Research, said in the statement. People with NOH "are often severely limited in their ability to perform routine daily activities that require walking or standing," he added.
The prescribing information states that droxidopa is indicated for "the treatment of orthostatic dizziness, light-headedness, or the ‘feeling that you are about to black out’ in adult patients with symptomatic neurogenic orthostatic hypotension (NOH) caused by primary autonomic failure [Parkinson’s disease (PD), multiple system atrophy, and pure autonomic failure], dopamine beta-hydroxylase deficiency, and nondiabetic autonomic neuropathy."
The indications and usage statement also adds that effectiveness past 2 weeks "has not been established," and that the "continued effectiveness of Northera should be assessed periodically."
The prescribing information includes a boxed warning about the risk of supine hypertension, and recommends that supine blood pressure be monitored before and during treatment, and more often when doses are increased – and that elevating the head of the bed reduces the risk of supine hypertension.
At a meeting on Jan. 14, the FDA’s Cardiovascular and Renal Drugs Advisory Committee voted 16 to 1 to recommend approval. In the FDA’s briefing documents filed prior to the meeting, the agency did not support approval of the drug, for reasons that included a lack of evidence indicating the drug had a durable effect, as well as safety issues.
Until the approval of droxidopa, the only drug approved for symptomatic NOH was midodrine, a vasoconstrictor that was approved in 1996. But midodrine may soon be off the market because postmarketing studies have failed to provide convincing evidence that treatment improves symptoms, according to the FDA’s briefing documents for the droxidopa panel meeting. Midodrine was approved on the basis of a surrogate endpoint – standing systolic blood pressure – which was considered "reasonably likely" to predict symptomatic benefit, but several postmarketing studies have failed to show that the drug is beneficial, despite its effect on increasing blood pressure.
NOH affects almost 300,000 people in the United States and European Union, according to Chelsea.
A statement on the approval issued by Chelsea said the company had a "preliminary" agreement with the FDA to conduct a postmarketing study to evaluate the clinical effects of droxidopa, which would be a multicenter, placebo-controlled, randomized study of about 1,400 patients enrolled over 6 years.
The prescribing information is available here.
No ACS approvals for rivaroxaban, Janssen announces
As expected, the Food and Drug Administration has declined to approve the oral anticoagulant rivaroxaban for treating acute coronary syndromes.
The manufacturer of the factor Xa inhibitor, Janssen Research & Development, announced on Feb. 14 that the FDA has issued a complete response letter regarding rivaroxaban, for reducing the risk of secondary cardiovascular events (MI, stroke, or death) in patients with ACS. This announcement came a month after the FDA’s Cardiovascular and Renal Drugs Advisory Committee voted 10-0 against approval. The company also announced that the FDA issued a complete response letter for the other ACS indication under review, to reduce the risk of stent thrombosis in patients with ACS, in combination with standard antiplatelet therapy.
The FDA issues complete response letters to manufacturers when it decides against approval, with explanations of outstanding issues that need to be resolved before the drug can be reconsidered for approval. The FDA does not publicly announce when these letters are issued, but manufacturers often do so.
Both indications are based on the ATLAS ACS 2 TIMI 51 (Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Aspirin With/Without Thienopyridine Therapy in Subjects With Acute Coronary Syndrome) trial, a phase III study of more than 15,000 patients with recent ACS, which compared two doses of rivaroxaban plus standard antiplatelet therapy (aspirin and a thienopyridine) to placebo plus standard therapy. The primary endpoint – the risk of a composite of cardiovascular death, MI, or stroke – was significantly reduced (P = .039) by 15% among patients on the 2.5-mg twice-daily dose, compared with patients on placebo (N. Engl. J. Med. 2012;366:9-19).
This is the third time the FDA has rejected approval of rivaroxaban for the ACS indication. The company submitted new analyses of the data which included some missing data, but at the FDA meeting in January, the panel said that missing data remained a problem and the P value for the primary endpoint was not low enough to support approval based on a single trial. Members also expressed continuing concerns about bleeding risk and voted against approval.
The company did not explain in the statement why the FDA had decided against approval.
"We are evaluating the contents of the letters and will determine the appropriate next steps," Dr. Paul Burton, vice president, clinical development, Janssen, said in the company’s statement.
Rivaroxaban, marketed as Xarelto, is approved for several indications, including reduction of the risk of stroke and systemic embolism in nonvalvular atrial fibrillation, treatment of deep vein thrombosis, prevention of deep vein thrombosis after hip or knee replacement surgery, and reduction in the risk of deep vein thrombosis and pulmonary embolism.
As expected, the Food and Drug Administration has declined to approve the oral anticoagulant rivaroxaban for treating acute coronary syndromes.
The manufacturer of the factor Xa inhibitor, Janssen Research & Development, announced on Feb. 14 that the FDA has issued a complete response letter regarding rivaroxaban, for reducing the risk of secondary cardiovascular events (MI, stroke, or death) in patients with ACS. This announcement came a month after the FDA’s Cardiovascular and Renal Drugs Advisory Committee voted 10-0 against approval. The company also announced that the FDA issued a complete response letter for the other ACS indication under review, to reduce the risk of stent thrombosis in patients with ACS, in combination with standard antiplatelet therapy.
The FDA issues complete response letters to manufacturers when it decides against approval, with explanations of outstanding issues that need to be resolved before the drug can be reconsidered for approval. The FDA does not publicly announce when these letters are issued, but manufacturers often do so.
Both indications are based on the ATLAS ACS 2 TIMI 51 (Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Aspirin With/Without Thienopyridine Therapy in Subjects With Acute Coronary Syndrome) trial, a phase III study of more than 15,000 patients with recent ACS, which compared two doses of rivaroxaban plus standard antiplatelet therapy (aspirin and a thienopyridine) to placebo plus standard therapy. The primary endpoint – the risk of a composite of cardiovascular death, MI, or stroke – was significantly reduced (P = .039) by 15% among patients on the 2.5-mg twice-daily dose, compared with patients on placebo (N. Engl. J. Med. 2012;366:9-19).
This is the third time the FDA has rejected approval of rivaroxaban for the ACS indication. The company submitted new analyses of the data which included some missing data, but at the FDA meeting in January, the panel said that missing data remained a problem and the P value for the primary endpoint was not low enough to support approval based on a single trial. Members also expressed continuing concerns about bleeding risk and voted against approval.
The company did not explain in the statement why the FDA had decided against approval.
"We are evaluating the contents of the letters and will determine the appropriate next steps," Dr. Paul Burton, vice president, clinical development, Janssen, said in the company’s statement.
Rivaroxaban, marketed as Xarelto, is approved for several indications, including reduction of the risk of stroke and systemic embolism in nonvalvular atrial fibrillation, treatment of deep vein thrombosis, prevention of deep vein thrombosis after hip or knee replacement surgery, and reduction in the risk of deep vein thrombosis and pulmonary embolism.
As expected, the Food and Drug Administration has declined to approve the oral anticoagulant rivaroxaban for treating acute coronary syndromes.
The manufacturer of the factor Xa inhibitor, Janssen Research & Development, announced on Feb. 14 that the FDA has issued a complete response letter regarding rivaroxaban, for reducing the risk of secondary cardiovascular events (MI, stroke, or death) in patients with ACS. This announcement came a month after the FDA’s Cardiovascular and Renal Drugs Advisory Committee voted 10-0 against approval. The company also announced that the FDA issued a complete response letter for the other ACS indication under review, to reduce the risk of stent thrombosis in patients with ACS, in combination with standard antiplatelet therapy.
The FDA issues complete response letters to manufacturers when it decides against approval, with explanations of outstanding issues that need to be resolved before the drug can be reconsidered for approval. The FDA does not publicly announce when these letters are issued, but manufacturers often do so.
Both indications are based on the ATLAS ACS 2 TIMI 51 (Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Aspirin With/Without Thienopyridine Therapy in Subjects With Acute Coronary Syndrome) trial, a phase III study of more than 15,000 patients with recent ACS, which compared two doses of rivaroxaban plus standard antiplatelet therapy (aspirin and a thienopyridine) to placebo plus standard therapy. The primary endpoint – the risk of a composite of cardiovascular death, MI, or stroke – was significantly reduced (P = .039) by 15% among patients on the 2.5-mg twice-daily dose, compared with patients on placebo (N. Engl. J. Med. 2012;366:9-19).
This is the third time the FDA has rejected approval of rivaroxaban for the ACS indication. The company submitted new analyses of the data which included some missing data, but at the FDA meeting in January, the panel said that missing data remained a problem and the P value for the primary endpoint was not low enough to support approval based on a single trial. Members also expressed continuing concerns about bleeding risk and voted against approval.
The company did not explain in the statement why the FDA had decided against approval.
"We are evaluating the contents of the letters and will determine the appropriate next steps," Dr. Paul Burton, vice president, clinical development, Janssen, said in the company’s statement.
Rivaroxaban, marketed as Xarelto, is approved for several indications, including reduction of the risk of stroke and systemic embolism in nonvalvular atrial fibrillation, treatment of deep vein thrombosis, prevention of deep vein thrombosis after hip or knee replacement surgery, and reduction in the risk of deep vein thrombosis and pulmonary embolism.
Pancytopenia warning added to label of hepatitis C drug boceprevir
A warning about cases of pancytopenia linked to the use of the oral hepatitis C antiviral drug boceprevir has been added to the drug’s label, the Food and Drug Administration has announced.
There have been postmarketing reports of "serious cases" of pancytopenia in people treated with boceprevir in combination with peginterferon alfa and ribavirin, according to the FDA. This information has been added to the "Warnings and Precautions" section of the label, with the recommendation that "complete blood counts (with white blood cell differential counts) should be obtained at pretreatment, and at treatment weeks 2, 4, 8, and 12, and should be monitored closely at other time points, as clinically appropriate."
Approved in 2011, boceprevir is an HCV Ns3/4A protease inhibitor approved for the treatment of chronic hepatitis C (CHC) genotype 1 infection, in combination with peginterferon alfa and ribavirin, in adults with compensated liver disease, including cirrhosis, who are previously untreated or who have failed previous interferon and ribavirin therapy, including prior null responders. Boceprevir, in a 200-mg capsule formulation, is marketed as Victrelis by Merck Sharp & Dohme; the recommended dose is 800 mg, three times a day.
The boceprevir Medication Guide will also be updated with this information, according to the FDA.
Serious adverse events associated with boceprevir should be reported to the FDA at 800-332-1088 or at MedWatch.
A warning about cases of pancytopenia linked to the use of the oral hepatitis C antiviral drug boceprevir has been added to the drug’s label, the Food and Drug Administration has announced.
There have been postmarketing reports of "serious cases" of pancytopenia in people treated with boceprevir in combination with peginterferon alfa and ribavirin, according to the FDA. This information has been added to the "Warnings and Precautions" section of the label, with the recommendation that "complete blood counts (with white blood cell differential counts) should be obtained at pretreatment, and at treatment weeks 2, 4, 8, and 12, and should be monitored closely at other time points, as clinically appropriate."
Approved in 2011, boceprevir is an HCV Ns3/4A protease inhibitor approved for the treatment of chronic hepatitis C (CHC) genotype 1 infection, in combination with peginterferon alfa and ribavirin, in adults with compensated liver disease, including cirrhosis, who are previously untreated or who have failed previous interferon and ribavirin therapy, including prior null responders. Boceprevir, in a 200-mg capsule formulation, is marketed as Victrelis by Merck Sharp & Dohme; the recommended dose is 800 mg, three times a day.
The boceprevir Medication Guide will also be updated with this information, according to the FDA.
Serious adverse events associated with boceprevir should be reported to the FDA at 800-332-1088 or at MedWatch.
A warning about cases of pancytopenia linked to the use of the oral hepatitis C antiviral drug boceprevir has been added to the drug’s label, the Food and Drug Administration has announced.
There have been postmarketing reports of "serious cases" of pancytopenia in people treated with boceprevir in combination with peginterferon alfa and ribavirin, according to the FDA. This information has been added to the "Warnings and Precautions" section of the label, with the recommendation that "complete blood counts (with white blood cell differential counts) should be obtained at pretreatment, and at treatment weeks 2, 4, 8, and 12, and should be monitored closely at other time points, as clinically appropriate."
Approved in 2011, boceprevir is an HCV Ns3/4A protease inhibitor approved for the treatment of chronic hepatitis C (CHC) genotype 1 infection, in combination with peginterferon alfa and ribavirin, in adults with compensated liver disease, including cirrhosis, who are previously untreated or who have failed previous interferon and ribavirin therapy, including prior null responders. Boceprevir, in a 200-mg capsule formulation, is marketed as Victrelis by Merck Sharp & Dohme; the recommended dose is 800 mg, three times a day.
The boceprevir Medication Guide will also be updated with this information, according to the FDA.
Serious adverse events associated with boceprevir should be reported to the FDA at 800-332-1088 or at MedWatch.
FDA panel votes against cangrelor approval for PCI, bridge indications
SILVER SPRING, MD. – A Food and Drug Administration advisory panel, citing numerous problems with cangrelor’s clinical trial program, has recommended against approval of the short-acting intravenous antiplatelet drug.
In a 7-2 vote, the meeting of the FDA’s Cardiovascular and Renal Drugs Advisory Committee recommended against approval of cangrelor for the proposed indication, "the reduction of death, MI, stent thrombosis, and ischemic-driven revascularization (IDR) in patients who have not been recently treated with a thienopyridine and who are undergoing PCI." The panel unanimously voted against approval for a second indication for cangrelor, for patients with stents who are at an increased risk for thrombotic events, such as stent thrombosis, when oral P2Y12 therapy is interrupted for surgery.
Cangrelor is a platelet P2Y12 inhibitor administered intravenously; with a half-life of 3-6 minutes, platelet function returns to normal within 1 hour of stopping the infusion of cangrelor, according to the manufacturer, the Medicines Company. The oral P2Y12 inhibitor clopidogrel has a more delayed action, with activity that lasts for days after is stopped.
The PCI indication is based on the results of the multinational CHAMPION PHOENIX study that compared cangrelor to clopidogrel in more than 11,000 patients undergoing PCI. The primary endpoint – a composite of death, MI, IDR, and stent thrombosis (ST) within 48 hours post-PCI – was 4.7% among those in the cangrelor arm, compared with 5.9 % in the clopidogrel arm, a significant difference that represented a 22% reduced risk (N. Engl. J. Med. 2013;368:1303-13). A 300-mg or 600-mg dose of clopidogrel was administered at the start of PCI or after PCI.
There was an increase in bleeding events overall among those on cangrelor (15.6% vs. 11%), but there were no significant differences in GUSTO severe or moderate bleeding events, no significant increase in TIMI major bleeding, and no significant differences in transfusions or fatal bleeding events between the two treatment arms.
The PHOENIX study was conducted after two large studies of patients with stable angina, unstable angina/non–ST-segment elevation MI, CHAMPION PCI (a study comparing cangrelor to clopidogrel during PCI in 8,667 patients) and CHAMPION PLATFORM (a study of 5,301 patients comparing cangrelor to placebo during PCI), were negative and were terminated early. The endpoint for those studies was a composite of death, MI, and IDR at 48 hours. Based on analyses of these studies, the primary endpoint was modified to include ST and an MI component that was based on the Universal Definition of MI. So, the biggest difference between the success of PHOENIX and the failure of the earlier PCI and PLATFORM studies was that the researchers used a different approach to define and track the MIs that patients developed during the studies and that served as primary end points.
Several panelists said they could not ignore the two negative studies and questioned whether PHOENIX could be considered the pivotal trial.
"In the background of two failed trials, and with all of the issues in almost every aspect of this trial, starting from design, oversight, DSMB [Data and Safety Management Board], how they counted efficacy, how they counted safety, it just left me with more uncertainty that I couldn’t defend the risk-benefit" profile, Dr. Stuart Rich, professor of medicine at University of Chicago.
Also voting no, Dr. James DeLemos, professor of medicine at the University of Texas Southwestern Medical Center at Dallas,said that the drug’s features were "intuitively appealing," and acknowledged the challenges in studying a drug that is administered for a short period of time, in a situation with low clinical endpoints. "But when I look at the data in aggregate," including the previous two studies, he added, "I’m just unconvinced that the risk-benefit is clearly favorable for clinically relevant endpoints ... [and] I am bothered that the math does not add up to a clear risk-benefit [profile] for this potent therapy in this situation."
One of the two panelists voting in favor of approval, the panel chair, Dr. Philip Sager, chair of the scientific programs committee at the cardiac safety research consortium, San Francisco, said while he agreed there were issues with the clinical trial, the endpoint was clinically significant, and the different analyses of the data "reassured me that the trial really did show a reduction in the primary endpoint" and that there was a net benefit.
The FDA review produced mixed opinions on whether the drug should be approved for the PCI indication. In briefing documents, filed before the meeting, the two clinical reviewers wrote that they were in favor of approval for the PCI indication. But Dr. Thomas A. Marciniak, clinical team leader in the FDA’s division of cardiovascular and renal products, recommended against approval for the PCI indication, citing multiple flaws in the CHAMPION PHOENIX study design, including the imbalance of clopidogrel dosing and what he considered were the inappropriate delays in clopidogrel administration, in the CHAMPION studies, including PHOENIX.
The reviewers did not support approval for the bridge-to-surgery indication because of the lack of clinical data from the pharmacodynamic trial – the BRIDGE study – which compared cangrelor with placebo in 210 patients with ACS or who were treated with a coronary stent and were at increased risk of thrombotic events after discontinuing an oral platelet inhibitor before coronary artery bypass graft surgery. Levels of platelet inhibition associated with a low risk of thrombotic events such as stent thrombosis were maintained in 99% of those treated with cangrelor, vs. 19% of those on placebo, a statistically significant difference (JAMA 2012;307:265-74).
While panelists said the drug could be effective for this indication, they said that a clinical study was needed to determine the drug’s risk-benefit profile in this setting and that the degree of unmet need for this use was not clear.
In a statement issued by the Medicines Company after the panel meeting, Dr. Clive A. Meanwell, the chairman and chief executive officer, said "we continue to believe in the safety and efficacy of cangrelor and look forward to working with the Agency as it completes its review." The deadline for the FDA decision is April 30.
Cangrelor has not been approved in any country to date.
The FDA usually follows the recommendations of its advisory panels. Advisory panel members have been cleared of potential conflicts related to the meeting topic. Occasionally, a panelist is given a waiver, but no waivers were given at this meeting.
SILVER SPRING, MD. – A Food and Drug Administration advisory panel, citing numerous problems with cangrelor’s clinical trial program, has recommended against approval of the short-acting intravenous antiplatelet drug.
In a 7-2 vote, the meeting of the FDA’s Cardiovascular and Renal Drugs Advisory Committee recommended against approval of cangrelor for the proposed indication, "the reduction of death, MI, stent thrombosis, and ischemic-driven revascularization (IDR) in patients who have not been recently treated with a thienopyridine and who are undergoing PCI." The panel unanimously voted against approval for a second indication for cangrelor, for patients with stents who are at an increased risk for thrombotic events, such as stent thrombosis, when oral P2Y12 therapy is interrupted for surgery.
Cangrelor is a platelet P2Y12 inhibitor administered intravenously; with a half-life of 3-6 minutes, platelet function returns to normal within 1 hour of stopping the infusion of cangrelor, according to the manufacturer, the Medicines Company. The oral P2Y12 inhibitor clopidogrel has a more delayed action, with activity that lasts for days after is stopped.
The PCI indication is based on the results of the multinational CHAMPION PHOENIX study that compared cangrelor to clopidogrel in more than 11,000 patients undergoing PCI. The primary endpoint – a composite of death, MI, IDR, and stent thrombosis (ST) within 48 hours post-PCI – was 4.7% among those in the cangrelor arm, compared with 5.9 % in the clopidogrel arm, a significant difference that represented a 22% reduced risk (N. Engl. J. Med. 2013;368:1303-13). A 300-mg or 600-mg dose of clopidogrel was administered at the start of PCI or after PCI.
There was an increase in bleeding events overall among those on cangrelor (15.6% vs. 11%), but there were no significant differences in GUSTO severe or moderate bleeding events, no significant increase in TIMI major bleeding, and no significant differences in transfusions or fatal bleeding events between the two treatment arms.
The PHOENIX study was conducted after two large studies of patients with stable angina, unstable angina/non–ST-segment elevation MI, CHAMPION PCI (a study comparing cangrelor to clopidogrel during PCI in 8,667 patients) and CHAMPION PLATFORM (a study of 5,301 patients comparing cangrelor to placebo during PCI), were negative and were terminated early. The endpoint for those studies was a composite of death, MI, and IDR at 48 hours. Based on analyses of these studies, the primary endpoint was modified to include ST and an MI component that was based on the Universal Definition of MI. So, the biggest difference between the success of PHOENIX and the failure of the earlier PCI and PLATFORM studies was that the researchers used a different approach to define and track the MIs that patients developed during the studies and that served as primary end points.
Several panelists said they could not ignore the two negative studies and questioned whether PHOENIX could be considered the pivotal trial.
"In the background of two failed trials, and with all of the issues in almost every aspect of this trial, starting from design, oversight, DSMB [Data and Safety Management Board], how they counted efficacy, how they counted safety, it just left me with more uncertainty that I couldn’t defend the risk-benefit" profile, Dr. Stuart Rich, professor of medicine at University of Chicago.
Also voting no, Dr. James DeLemos, professor of medicine at the University of Texas Southwestern Medical Center at Dallas,said that the drug’s features were "intuitively appealing," and acknowledged the challenges in studying a drug that is administered for a short period of time, in a situation with low clinical endpoints. "But when I look at the data in aggregate," including the previous two studies, he added, "I’m just unconvinced that the risk-benefit is clearly favorable for clinically relevant endpoints ... [and] I am bothered that the math does not add up to a clear risk-benefit [profile] for this potent therapy in this situation."
One of the two panelists voting in favor of approval, the panel chair, Dr. Philip Sager, chair of the scientific programs committee at the cardiac safety research consortium, San Francisco, said while he agreed there were issues with the clinical trial, the endpoint was clinically significant, and the different analyses of the data "reassured me that the trial really did show a reduction in the primary endpoint" and that there was a net benefit.
The FDA review produced mixed opinions on whether the drug should be approved for the PCI indication. In briefing documents, filed before the meeting, the two clinical reviewers wrote that they were in favor of approval for the PCI indication. But Dr. Thomas A. Marciniak, clinical team leader in the FDA’s division of cardiovascular and renal products, recommended against approval for the PCI indication, citing multiple flaws in the CHAMPION PHOENIX study design, including the imbalance of clopidogrel dosing and what he considered were the inappropriate delays in clopidogrel administration, in the CHAMPION studies, including PHOENIX.
The reviewers did not support approval for the bridge-to-surgery indication because of the lack of clinical data from the pharmacodynamic trial – the BRIDGE study – which compared cangrelor with placebo in 210 patients with ACS or who were treated with a coronary stent and were at increased risk of thrombotic events after discontinuing an oral platelet inhibitor before coronary artery bypass graft surgery. Levels of platelet inhibition associated with a low risk of thrombotic events such as stent thrombosis were maintained in 99% of those treated with cangrelor, vs. 19% of those on placebo, a statistically significant difference (JAMA 2012;307:265-74).
While panelists said the drug could be effective for this indication, they said that a clinical study was needed to determine the drug’s risk-benefit profile in this setting and that the degree of unmet need for this use was not clear.
In a statement issued by the Medicines Company after the panel meeting, Dr. Clive A. Meanwell, the chairman and chief executive officer, said "we continue to believe in the safety and efficacy of cangrelor and look forward to working with the Agency as it completes its review." The deadline for the FDA decision is April 30.
Cangrelor has not been approved in any country to date.
The FDA usually follows the recommendations of its advisory panels. Advisory panel members have been cleared of potential conflicts related to the meeting topic. Occasionally, a panelist is given a waiver, but no waivers were given at this meeting.
SILVER SPRING, MD. – A Food and Drug Administration advisory panel, citing numerous problems with cangrelor’s clinical trial program, has recommended against approval of the short-acting intravenous antiplatelet drug.
In a 7-2 vote, the meeting of the FDA’s Cardiovascular and Renal Drugs Advisory Committee recommended against approval of cangrelor for the proposed indication, "the reduction of death, MI, stent thrombosis, and ischemic-driven revascularization (IDR) in patients who have not been recently treated with a thienopyridine and who are undergoing PCI." The panel unanimously voted against approval for a second indication for cangrelor, for patients with stents who are at an increased risk for thrombotic events, such as stent thrombosis, when oral P2Y12 therapy is interrupted for surgery.
Cangrelor is a platelet P2Y12 inhibitor administered intravenously; with a half-life of 3-6 minutes, platelet function returns to normal within 1 hour of stopping the infusion of cangrelor, according to the manufacturer, the Medicines Company. The oral P2Y12 inhibitor clopidogrel has a more delayed action, with activity that lasts for days after is stopped.
The PCI indication is based on the results of the multinational CHAMPION PHOENIX study that compared cangrelor to clopidogrel in more than 11,000 patients undergoing PCI. The primary endpoint – a composite of death, MI, IDR, and stent thrombosis (ST) within 48 hours post-PCI – was 4.7% among those in the cangrelor arm, compared with 5.9 % in the clopidogrel arm, a significant difference that represented a 22% reduced risk (N. Engl. J. Med. 2013;368:1303-13). A 300-mg or 600-mg dose of clopidogrel was administered at the start of PCI or after PCI.
There was an increase in bleeding events overall among those on cangrelor (15.6% vs. 11%), but there were no significant differences in GUSTO severe or moderate bleeding events, no significant increase in TIMI major bleeding, and no significant differences in transfusions or fatal bleeding events between the two treatment arms.
The PHOENIX study was conducted after two large studies of patients with stable angina, unstable angina/non–ST-segment elevation MI, CHAMPION PCI (a study comparing cangrelor to clopidogrel during PCI in 8,667 patients) and CHAMPION PLATFORM (a study of 5,301 patients comparing cangrelor to placebo during PCI), were negative and were terminated early. The endpoint for those studies was a composite of death, MI, and IDR at 48 hours. Based on analyses of these studies, the primary endpoint was modified to include ST and an MI component that was based on the Universal Definition of MI. So, the biggest difference between the success of PHOENIX and the failure of the earlier PCI and PLATFORM studies was that the researchers used a different approach to define and track the MIs that patients developed during the studies and that served as primary end points.
Several panelists said they could not ignore the two negative studies and questioned whether PHOENIX could be considered the pivotal trial.
"In the background of two failed trials, and with all of the issues in almost every aspect of this trial, starting from design, oversight, DSMB [Data and Safety Management Board], how they counted efficacy, how they counted safety, it just left me with more uncertainty that I couldn’t defend the risk-benefit" profile, Dr. Stuart Rich, professor of medicine at University of Chicago.
Also voting no, Dr. James DeLemos, professor of medicine at the University of Texas Southwestern Medical Center at Dallas,said that the drug’s features were "intuitively appealing," and acknowledged the challenges in studying a drug that is administered for a short period of time, in a situation with low clinical endpoints. "But when I look at the data in aggregate," including the previous two studies, he added, "I’m just unconvinced that the risk-benefit is clearly favorable for clinically relevant endpoints ... [and] I am bothered that the math does not add up to a clear risk-benefit [profile] for this potent therapy in this situation."
One of the two panelists voting in favor of approval, the panel chair, Dr. Philip Sager, chair of the scientific programs committee at the cardiac safety research consortium, San Francisco, said while he agreed there were issues with the clinical trial, the endpoint was clinically significant, and the different analyses of the data "reassured me that the trial really did show a reduction in the primary endpoint" and that there was a net benefit.
The FDA review produced mixed opinions on whether the drug should be approved for the PCI indication. In briefing documents, filed before the meeting, the two clinical reviewers wrote that they were in favor of approval for the PCI indication. But Dr. Thomas A. Marciniak, clinical team leader in the FDA’s division of cardiovascular and renal products, recommended against approval for the PCI indication, citing multiple flaws in the CHAMPION PHOENIX study design, including the imbalance of clopidogrel dosing and what he considered were the inappropriate delays in clopidogrel administration, in the CHAMPION studies, including PHOENIX.
The reviewers did not support approval for the bridge-to-surgery indication because of the lack of clinical data from the pharmacodynamic trial – the BRIDGE study – which compared cangrelor with placebo in 210 patients with ACS or who were treated with a coronary stent and were at increased risk of thrombotic events after discontinuing an oral platelet inhibitor before coronary artery bypass graft surgery. Levels of platelet inhibition associated with a low risk of thrombotic events such as stent thrombosis were maintained in 99% of those treated with cangrelor, vs. 19% of those on placebo, a statistically significant difference (JAMA 2012;307:265-74).
While panelists said the drug could be effective for this indication, they said that a clinical study was needed to determine the drug’s risk-benefit profile in this setting and that the degree of unmet need for this use was not clear.
In a statement issued by the Medicines Company after the panel meeting, Dr. Clive A. Meanwell, the chairman and chief executive officer, said "we continue to believe in the safety and efficacy of cangrelor and look forward to working with the Agency as it completes its review." The deadline for the FDA decision is April 30.
Cangrelor has not been approved in any country to date.
The FDA usually follows the recommendations of its advisory panels. Advisory panel members have been cleared of potential conflicts related to the meeting topic. Occasionally, a panelist is given a waiver, but no waivers were given at this meeting.
AT AN FDA ADVISORY COMMITTEE MEETING
FDA reviewers support approval of platelet inhibitor cangrelor for PCI indication
The data on cangrelor support approval of the fast-acting antiplatelet drug for reducing thrombotic cardiovascular events in patients with coronary artery disease who are undergoing percutaneous coronary intervention – one of two indications being reviewed by the Food and Drug Administration, according to the agency’s clinical reviewers of the application.
Cangrelor is a platelet P2Y12 inhibitor administered intravenously, and is fast acting and has a short half-life, compared with clopidogrel, the oral P2Y12 inhibitor. The clinical effects of cangrelor persist for about an hour after the infusion is stopped – compared with days after doses when oral antiplatelet drugs are stopped.
In briefing documents filed 2 days before an FDA advisory panel is scheduled to meet to review the data on cangrelor, the reviewers wrote that they recommended approval for the percutaneous coronary intervention (PCI) indication, "the reduction of death, MI, stent thrombosis, and ischemic driven revascularization (IDR) in patients who have not been recently treated with a thienopyridine and who are undergoing PCI." This is based on the results of the CHAMPION PHOENIX study that compared cangrelor to oral clopidogrel in more than 11,000 patients undergoing PCI, which found the primary endpoint – death, MI, IDR, and stent thrombosis at 48 hours post-PCI – was significantly reduced, by 22%, among those treated with cangrelor, compared with those on clopidogrel (N. Engl. J. Med. 2013;368:1303-13).
But they do not recommend approval for the second indication proposed by the manufacturer, the Medicines Company, to "maintain P2Y12 inhibition in patients with acute coronary syndrome or with stents who are at increased risk for thrombotic events (such as stent thrombosis) when oral P2Y12 inhibitor therapy is interrupted due to surgery."
BRIDGE, a pharmacodynamic study, compared cangrelor to placebo in 210 patients with acute coronary syndrome or who were treated with a coronary stent and were at increased risk of thrombotic events after discontinuing an oral platelet inhibitor before coronary artery bypass graft surgery. Levels of platelet inhibition associated with a low risk of thrombotic events such as stent thrombosis were maintained in 99% of those treated with cangrelor, vs. 19% of those on placebo, a statistically significant difference. (JAMA 2012;307:265-74).
Their reasons for not supporting approval include the "lack of clinical data" from this study that support the pharmacodynamic effect at the dose proposed for the bridging indication and that the clinical efficacy results in the PHOENIX study could not be applied to the bridging indication because the cangrelor dose used in the BRIDGE study was fivefold lower than that in the PHOENIX study, the reviewers wrote.
The briefing documents were posted on Feb. 10, 2 days before the agency’s Cardiovascular and Renal Drugs Advisory Committee will meet to review and discuss the data on cangrelor and vote on whether to recommend approval for the indications proposed by the manufacturer.
In the documents, a divergent opinion on approval was expressed by Dr. Thomas A. Marciniak, clinical team leader in the FDA’s division of cardiovascular and renal products. He wrote that he recommended against approval for the PCI indication until another trial successfully corrected what he considered flaws in the cangrelor studies. Among the flaws he cited are the use of the 300-mg loading dose of clopidogrel allowed only in the clopidogrel arm and the "exclusive use" of the 600-mg clopidogrel loading dose in the cangrelor arm, which he commented ‘may explain some of the superiority’ of that arm." (He did not comment on the bridge to surgery indication).
But the clinical reviewers, Dr. Fred Senatore and Nhi Beasley, Pharm.D., of the same division, wrote that while the imbalance in the cangrelor-treated patients who received the higher loading dose "might have played a role in cangrelor achieving its efficacy endpoint," they noted that the 300-mg loading dose is recommended in the clopidogrel label. And the ACCF/AHA 2012 (American College of Cardiology Foundation/American Heart Association) guidelines note that the "optimal clopidogrel loading dose has not been rigorously established and that trials examining the higher loading dose vs. the standard dose ... have only generated a hypothesis suggesting a greater benefit for the higher dose," they added.
The FDA panel will be asked to vote on whether to recommend approval of the two indications, and will be asked to discuss several other questions, including the effect the patient’s diagnosis on entry into the PHOENIX study on the results, and the drug’s overall benefit-risk. In the PHOENIX study, the rates of bleeding events were 15.6% among those on cangrelor vs. 11% of those on clopidogrel.
The data on cangrelor support approval of the fast-acting antiplatelet drug for reducing thrombotic cardiovascular events in patients with coronary artery disease who are undergoing percutaneous coronary intervention – one of two indications being reviewed by the Food and Drug Administration, according to the agency’s clinical reviewers of the application.
Cangrelor is a platelet P2Y12 inhibitor administered intravenously, and is fast acting and has a short half-life, compared with clopidogrel, the oral P2Y12 inhibitor. The clinical effects of cangrelor persist for about an hour after the infusion is stopped – compared with days after doses when oral antiplatelet drugs are stopped.
In briefing documents filed 2 days before an FDA advisory panel is scheduled to meet to review the data on cangrelor, the reviewers wrote that they recommended approval for the percutaneous coronary intervention (PCI) indication, "the reduction of death, MI, stent thrombosis, and ischemic driven revascularization (IDR) in patients who have not been recently treated with a thienopyridine and who are undergoing PCI." This is based on the results of the CHAMPION PHOENIX study that compared cangrelor to oral clopidogrel in more than 11,000 patients undergoing PCI, which found the primary endpoint – death, MI, IDR, and stent thrombosis at 48 hours post-PCI – was significantly reduced, by 22%, among those treated with cangrelor, compared with those on clopidogrel (N. Engl. J. Med. 2013;368:1303-13).
But they do not recommend approval for the second indication proposed by the manufacturer, the Medicines Company, to "maintain P2Y12 inhibition in patients with acute coronary syndrome or with stents who are at increased risk for thrombotic events (such as stent thrombosis) when oral P2Y12 inhibitor therapy is interrupted due to surgery."
BRIDGE, a pharmacodynamic study, compared cangrelor to placebo in 210 patients with acute coronary syndrome or who were treated with a coronary stent and were at increased risk of thrombotic events after discontinuing an oral platelet inhibitor before coronary artery bypass graft surgery. Levels of platelet inhibition associated with a low risk of thrombotic events such as stent thrombosis were maintained in 99% of those treated with cangrelor, vs. 19% of those on placebo, a statistically significant difference. (JAMA 2012;307:265-74).
Their reasons for not supporting approval include the "lack of clinical data" from this study that support the pharmacodynamic effect at the dose proposed for the bridging indication and that the clinical efficacy results in the PHOENIX study could not be applied to the bridging indication because the cangrelor dose used in the BRIDGE study was fivefold lower than that in the PHOENIX study, the reviewers wrote.
The briefing documents were posted on Feb. 10, 2 days before the agency’s Cardiovascular and Renal Drugs Advisory Committee will meet to review and discuss the data on cangrelor and vote on whether to recommend approval for the indications proposed by the manufacturer.
In the documents, a divergent opinion on approval was expressed by Dr. Thomas A. Marciniak, clinical team leader in the FDA’s division of cardiovascular and renal products. He wrote that he recommended against approval for the PCI indication until another trial successfully corrected what he considered flaws in the cangrelor studies. Among the flaws he cited are the use of the 300-mg loading dose of clopidogrel allowed only in the clopidogrel arm and the "exclusive use" of the 600-mg clopidogrel loading dose in the cangrelor arm, which he commented ‘may explain some of the superiority’ of that arm." (He did not comment on the bridge to surgery indication).
But the clinical reviewers, Dr. Fred Senatore and Nhi Beasley, Pharm.D., of the same division, wrote that while the imbalance in the cangrelor-treated patients who received the higher loading dose "might have played a role in cangrelor achieving its efficacy endpoint," they noted that the 300-mg loading dose is recommended in the clopidogrel label. And the ACCF/AHA 2012 (American College of Cardiology Foundation/American Heart Association) guidelines note that the "optimal clopidogrel loading dose has not been rigorously established and that trials examining the higher loading dose vs. the standard dose ... have only generated a hypothesis suggesting a greater benefit for the higher dose," they added.
The FDA panel will be asked to vote on whether to recommend approval of the two indications, and will be asked to discuss several other questions, including the effect the patient’s diagnosis on entry into the PHOENIX study on the results, and the drug’s overall benefit-risk. In the PHOENIX study, the rates of bleeding events were 15.6% among those on cangrelor vs. 11% of those on clopidogrel.
The data on cangrelor support approval of the fast-acting antiplatelet drug for reducing thrombotic cardiovascular events in patients with coronary artery disease who are undergoing percutaneous coronary intervention – one of two indications being reviewed by the Food and Drug Administration, according to the agency’s clinical reviewers of the application.
Cangrelor is a platelet P2Y12 inhibitor administered intravenously, and is fast acting and has a short half-life, compared with clopidogrel, the oral P2Y12 inhibitor. The clinical effects of cangrelor persist for about an hour after the infusion is stopped – compared with days after doses when oral antiplatelet drugs are stopped.
In briefing documents filed 2 days before an FDA advisory panel is scheduled to meet to review the data on cangrelor, the reviewers wrote that they recommended approval for the percutaneous coronary intervention (PCI) indication, "the reduction of death, MI, stent thrombosis, and ischemic driven revascularization (IDR) in patients who have not been recently treated with a thienopyridine and who are undergoing PCI." This is based on the results of the CHAMPION PHOENIX study that compared cangrelor to oral clopidogrel in more than 11,000 patients undergoing PCI, which found the primary endpoint – death, MI, IDR, and stent thrombosis at 48 hours post-PCI – was significantly reduced, by 22%, among those treated with cangrelor, compared with those on clopidogrel (N. Engl. J. Med. 2013;368:1303-13).
But they do not recommend approval for the second indication proposed by the manufacturer, the Medicines Company, to "maintain P2Y12 inhibition in patients with acute coronary syndrome or with stents who are at increased risk for thrombotic events (such as stent thrombosis) when oral P2Y12 inhibitor therapy is interrupted due to surgery."
BRIDGE, a pharmacodynamic study, compared cangrelor to placebo in 210 patients with acute coronary syndrome or who were treated with a coronary stent and were at increased risk of thrombotic events after discontinuing an oral platelet inhibitor before coronary artery bypass graft surgery. Levels of platelet inhibition associated with a low risk of thrombotic events such as stent thrombosis were maintained in 99% of those treated with cangrelor, vs. 19% of those on placebo, a statistically significant difference. (JAMA 2012;307:265-74).
Their reasons for not supporting approval include the "lack of clinical data" from this study that support the pharmacodynamic effect at the dose proposed for the bridging indication and that the clinical efficacy results in the PHOENIX study could not be applied to the bridging indication because the cangrelor dose used in the BRIDGE study was fivefold lower than that in the PHOENIX study, the reviewers wrote.
The briefing documents were posted on Feb. 10, 2 days before the agency’s Cardiovascular and Renal Drugs Advisory Committee will meet to review and discuss the data on cangrelor and vote on whether to recommend approval for the indications proposed by the manufacturer.
In the documents, a divergent opinion on approval was expressed by Dr. Thomas A. Marciniak, clinical team leader in the FDA’s division of cardiovascular and renal products. He wrote that he recommended against approval for the PCI indication until another trial successfully corrected what he considered flaws in the cangrelor studies. Among the flaws he cited are the use of the 300-mg loading dose of clopidogrel allowed only in the clopidogrel arm and the "exclusive use" of the 600-mg clopidogrel loading dose in the cangrelor arm, which he commented ‘may explain some of the superiority’ of that arm." (He did not comment on the bridge to surgery indication).
But the clinical reviewers, Dr. Fred Senatore and Nhi Beasley, Pharm.D., of the same division, wrote that while the imbalance in the cangrelor-treated patients who received the higher loading dose "might have played a role in cangrelor achieving its efficacy endpoint," they noted that the 300-mg loading dose is recommended in the clopidogrel label. And the ACCF/AHA 2012 (American College of Cardiology Foundation/American Heart Association) guidelines note that the "optimal clopidogrel loading dose has not been rigorously established and that trials examining the higher loading dose vs. the standard dose ... have only generated a hypothesis suggesting a greater benefit for the higher dose," they added.
The FDA panel will be asked to vote on whether to recommend approval of the two indications, and will be asked to discuss several other questions, including the effect the patient’s diagnosis on entry into the PHOENIX study on the results, and the drug’s overall benefit-risk. In the PHOENIX study, the rates of bleeding events were 15.6% among those on cangrelor vs. 11% of those on clopidogrel.
Continuous glucose monitor approved for children down to age 2
The Food and Drug Administration has approved a continuous glucose monitor for use in children as young as 2 years, with warnings in the label indicating that the performance of the device in children is less accurate than in adults, the agency announcedon Feb. 3.
Previously approved for use in adults aged 18 years and older, the Dexcom Platinum Continuous Monitoring System is now approved for people with diabetes aged 2-17 years, the agency said. The device has the same sensor and transmitter as the adult system, but the sensor can also be inserted into the upper buttock in addition to the abdomen, the statement said.
"The device can provide valuable glucose trend information to children with diabetes and their families, but it is important that those using this device understand the expected performance of this device compared to blood glucose meters, especially for detecting low glucose, in pediatric patients," Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostics and Radiological Devices in the FDA’s Center for Devices and Radiological Health said in the statement.
The statement summarizes the results of a pivotal study of the device in the clinic and at home in 176 patients aged 2 through 17 years, which compared glucose results provided by the continuous glucose monitoring (CGM) over 7 days of use, with results from a clinical laboratory reference method among those aged 6-17 years, and finger stick samples on a blood glucose meter among those aged 2-17 years. Performance of the device in children was less accurate than in adults, and the performance of the hypoglycemic detection alert feature of the device was "poor relative to that seen in the adult study," particularly at blood glucose levels below 70 mg/dL.
"Despite these limitations, the study did demonstrate that the device is effective for tracking and trending to determine patterns in glucose levels, and for alerting patients when glucose values are approaching potentially dangerously high (hyperglycemic) and/or dangerously low (hypoglycemic) levels," the statement added.
These results are reflected in warnings included in the CGM’s label and displayed on the device’s screen, advising that the blood glucose meter should be used for treatment decisions and that CGM alerts should not be used alone to detect low glucose.
The G4 Platinum (Pediatric) System is manufactured by Dexcom Inc.
This is the first CGM to be approved in children down to age 2 years; another CGM manufactured by Medtronic is approved for use in children after age 6 years, according to an FDA spokesperson.
Along with an insulin pump, CGMs, which measure glucose levels in the interstitial fluid, are a component of the artificial pancreas system, along with an insulin infusion pump, which is being studied in people with type 1 diabetes as a closed loop system of monitoring glucose levels and automatically dispensing the appropriate amount insulin, as determined by a computer algorithm. The CGM is calibrated periodically using the blood glucose values from a blood glucose monitor.
The Food and Drug Administration has approved a continuous glucose monitor for use in children as young as 2 years, with warnings in the label indicating that the performance of the device in children is less accurate than in adults, the agency announcedon Feb. 3.
Previously approved for use in adults aged 18 years and older, the Dexcom Platinum Continuous Monitoring System is now approved for people with diabetes aged 2-17 years, the agency said. The device has the same sensor and transmitter as the adult system, but the sensor can also be inserted into the upper buttock in addition to the abdomen, the statement said.
"The device can provide valuable glucose trend information to children with diabetes and their families, but it is important that those using this device understand the expected performance of this device compared to blood glucose meters, especially for detecting low glucose, in pediatric patients," Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostics and Radiological Devices in the FDA’s Center for Devices and Radiological Health said in the statement.
The statement summarizes the results of a pivotal study of the device in the clinic and at home in 176 patients aged 2 through 17 years, which compared glucose results provided by the continuous glucose monitoring (CGM) over 7 days of use, with results from a clinical laboratory reference method among those aged 6-17 years, and finger stick samples on a blood glucose meter among those aged 2-17 years. Performance of the device in children was less accurate than in adults, and the performance of the hypoglycemic detection alert feature of the device was "poor relative to that seen in the adult study," particularly at blood glucose levels below 70 mg/dL.
"Despite these limitations, the study did demonstrate that the device is effective for tracking and trending to determine patterns in glucose levels, and for alerting patients when glucose values are approaching potentially dangerously high (hyperglycemic) and/or dangerously low (hypoglycemic) levels," the statement added.
These results are reflected in warnings included in the CGM’s label and displayed on the device’s screen, advising that the blood glucose meter should be used for treatment decisions and that CGM alerts should not be used alone to detect low glucose.
The G4 Platinum (Pediatric) System is manufactured by Dexcom Inc.
This is the first CGM to be approved in children down to age 2 years; another CGM manufactured by Medtronic is approved for use in children after age 6 years, according to an FDA spokesperson.
Along with an insulin pump, CGMs, which measure glucose levels in the interstitial fluid, are a component of the artificial pancreas system, along with an insulin infusion pump, which is being studied in people with type 1 diabetes as a closed loop system of monitoring glucose levels and automatically dispensing the appropriate amount insulin, as determined by a computer algorithm. The CGM is calibrated periodically using the blood glucose values from a blood glucose monitor.
The Food and Drug Administration has approved a continuous glucose monitor for use in children as young as 2 years, with warnings in the label indicating that the performance of the device in children is less accurate than in adults, the agency announcedon Feb. 3.
Previously approved for use in adults aged 18 years and older, the Dexcom Platinum Continuous Monitoring System is now approved for people with diabetes aged 2-17 years, the agency said. The device has the same sensor and transmitter as the adult system, but the sensor can also be inserted into the upper buttock in addition to the abdomen, the statement said.
"The device can provide valuable glucose trend information to children with diabetes and their families, but it is important that those using this device understand the expected performance of this device compared to blood glucose meters, especially for detecting low glucose, in pediatric patients," Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostics and Radiological Devices in the FDA’s Center for Devices and Radiological Health said in the statement.
The statement summarizes the results of a pivotal study of the device in the clinic and at home in 176 patients aged 2 through 17 years, which compared glucose results provided by the continuous glucose monitoring (CGM) over 7 days of use, with results from a clinical laboratory reference method among those aged 6-17 years, and finger stick samples on a blood glucose meter among those aged 2-17 years. Performance of the device in children was less accurate than in adults, and the performance of the hypoglycemic detection alert feature of the device was "poor relative to that seen in the adult study," particularly at blood glucose levels below 70 mg/dL.
"Despite these limitations, the study did demonstrate that the device is effective for tracking and trending to determine patterns in glucose levels, and for alerting patients when glucose values are approaching potentially dangerously high (hyperglycemic) and/or dangerously low (hypoglycemic) levels," the statement added.
These results are reflected in warnings included in the CGM’s label and displayed on the device’s screen, advising that the blood glucose meter should be used for treatment decisions and that CGM alerts should not be used alone to detect low glucose.
The G4 Platinum (Pediatric) System is manufactured by Dexcom Inc.
This is the first CGM to be approved in children down to age 2 years; another CGM manufactured by Medtronic is approved for use in children after age 6 years, according to an FDA spokesperson.
Along with an insulin pump, CGMs, which measure glucose levels in the interstitial fluid, are a component of the artificial pancreas system, along with an insulin infusion pump, which is being studied in people with type 1 diabetes as a closed loop system of monitoring glucose levels and automatically dispensing the appropriate amount insulin, as determined by a computer algorithm. The CGM is calibrated periodically using the blood glucose values from a blood glucose monitor.
2014 childhood and adolescent immunization schedule now available
The 2014 childhood and adolescent immunization schedule has been approved, with additions that include the use of one of the meningococcal conjugate vaccines (Menveo) in certain groups of high-risk infants and a list specifying the groups of people at increased risk of hepatitis A.
The schedule will be published in the February 2014 issue of Pediatrics, and is being made available online on Jan. 31 (Pediatrics 2014 [doi: 10.1542/peds.2013-3965]).
Guidance on the use of Menveo (Meningococcal Groups A, C, W-135, and Y Oligosaccharide Diphtheria CRM197 conjugate vaccine) for certain groups of infants at increased risk of disease starting at age 2 months has been added to the meningococcal vaccine footnote. This is based on the Food and Drug Administration licensure of Menveo for use starting at age 2 months in August 2013. Some of the high-risk categories include anatomic or functional asplenia, including sickle cell disease; children with persistent complement component deficiency; and those who travel to or live in an area hyperendemic area for meningococcal disease.
This is the first time that a meningococcal vaccine has been available for use starting at age 2 months, Dr. H. Cody Meissner, professor of pediatrics at Tufts University, Boston, pointed out in an interview.
The hepatitis A vaccine footnote now provides a list of groups at increased risk for hepatitis A. While these groups are well recognized, "it was useful to itemize these groups in the footnote," noted Dr. Meissner, who is a member of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices work group on the harmonized immunization schedule.
The list includes people traveling to or working in countries where there is a high or intermediate endemicity of infection; men having sex with men; people with clotting factor disorders; people with chronic liver disease; users of injection and noninjection illicit drugs; and personal contacts – such as household contacts or regular babysitters – of international adoptees during the first 2 months of arrival in the United States "from a country with high or intermediate endemicity."
The footnote on the tetanus and diphtheria toxoids and acellular pertussis (Tdap) vaccine now states that a dose of the vaccine is recommended for pregnant adolescents every time they get pregnant – preferably during week 27 through week 36 of gestation. Last year, the American Academy of Pediatrics agreed that this vaccine should be given to a pregnant woman, but it withheld the recommendation to vaccinate during every pregnancy until more data became available, Dr. Meissner said. "Additional data now indicate the safety and efficacy of administration of Tdap each time a woman becomes pregnant." This will protect most infants during the first 2 months of life when pertussis can be most severe and until they receive their first DTaP dose at 2 months, he noted.
Other changes include clarification of the intervals between doses in the human papillomavirus (HPV) vaccines footnote, to avoid any misunderstanding of the schedule, said Dr. Meissner, who is also chief of pediatric infectious disease at the Floating Hospital for Children at Tufts Medical Center.
The recommendation for pneumococcal vaccines has not changed from last year, but the footnote provides clarification about the recommendations for PCV13 (Prevnar 13) and PPSV23 in children and adolescents, "which have been stratified according to age and according to degree of risk," he added.
The footnote on Haemophilus influenzae type b (Hib) conjugate vaccine now includes clarification of who should receive the vaccine if immunocompromised.
This is the second year that recommendations and footnotes for ages 0-18 years are included in one schedule, as opposed to previous years, when there were separate schedules and footnotes for 0-7 years and 8-18 years.
The 2014 schedules have been approved by the American Academy of Pediatrics, the Advisory Committee on Immunization Practices, the American Academy of Family Physicians, and the American College of Obstetricians and Gynecologists.
Dr. Meissner said he has no relevant financial disclosures.
You can view the 2014 schedule at our Resources section here.
The 2014 childhood and adolescent immunization schedule has been approved, with additions that include the use of one of the meningococcal conjugate vaccines (Menveo) in certain groups of high-risk infants and a list specifying the groups of people at increased risk of hepatitis A.
The schedule will be published in the February 2014 issue of Pediatrics, and is being made available online on Jan. 31 (Pediatrics 2014 [doi: 10.1542/peds.2013-3965]).
Guidance on the use of Menveo (Meningococcal Groups A, C, W-135, and Y Oligosaccharide Diphtheria CRM197 conjugate vaccine) for certain groups of infants at increased risk of disease starting at age 2 months has been added to the meningococcal vaccine footnote. This is based on the Food and Drug Administration licensure of Menveo for use starting at age 2 months in August 2013. Some of the high-risk categories include anatomic or functional asplenia, including sickle cell disease; children with persistent complement component deficiency; and those who travel to or live in an area hyperendemic area for meningococcal disease.
This is the first time that a meningococcal vaccine has been available for use starting at age 2 months, Dr. H. Cody Meissner, professor of pediatrics at Tufts University, Boston, pointed out in an interview.
The hepatitis A vaccine footnote now provides a list of groups at increased risk for hepatitis A. While these groups are well recognized, "it was useful to itemize these groups in the footnote," noted Dr. Meissner, who is a member of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices work group on the harmonized immunization schedule.
The list includes people traveling to or working in countries where there is a high or intermediate endemicity of infection; men having sex with men; people with clotting factor disorders; people with chronic liver disease; users of injection and noninjection illicit drugs; and personal contacts – such as household contacts or regular babysitters – of international adoptees during the first 2 months of arrival in the United States "from a country with high or intermediate endemicity."
The footnote on the tetanus and diphtheria toxoids and acellular pertussis (Tdap) vaccine now states that a dose of the vaccine is recommended for pregnant adolescents every time they get pregnant – preferably during week 27 through week 36 of gestation. Last year, the American Academy of Pediatrics agreed that this vaccine should be given to a pregnant woman, but it withheld the recommendation to vaccinate during every pregnancy until more data became available, Dr. Meissner said. "Additional data now indicate the safety and efficacy of administration of Tdap each time a woman becomes pregnant." This will protect most infants during the first 2 months of life when pertussis can be most severe and until they receive their first DTaP dose at 2 months, he noted.
Other changes include clarification of the intervals between doses in the human papillomavirus (HPV) vaccines footnote, to avoid any misunderstanding of the schedule, said Dr. Meissner, who is also chief of pediatric infectious disease at the Floating Hospital for Children at Tufts Medical Center.
The recommendation for pneumococcal vaccines has not changed from last year, but the footnote provides clarification about the recommendations for PCV13 (Prevnar 13) and PPSV23 in children and adolescents, "which have been stratified according to age and according to degree of risk," he added.
The footnote on Haemophilus influenzae type b (Hib) conjugate vaccine now includes clarification of who should receive the vaccine if immunocompromised.
This is the second year that recommendations and footnotes for ages 0-18 years are included in one schedule, as opposed to previous years, when there were separate schedules and footnotes for 0-7 years and 8-18 years.
The 2014 schedules have been approved by the American Academy of Pediatrics, the Advisory Committee on Immunization Practices, the American Academy of Family Physicians, and the American College of Obstetricians and Gynecologists.
Dr. Meissner said he has no relevant financial disclosures.
You can view the 2014 schedule at our Resources section here.
The 2014 childhood and adolescent immunization schedule has been approved, with additions that include the use of one of the meningococcal conjugate vaccines (Menveo) in certain groups of high-risk infants and a list specifying the groups of people at increased risk of hepatitis A.
The schedule will be published in the February 2014 issue of Pediatrics, and is being made available online on Jan. 31 (Pediatrics 2014 [doi: 10.1542/peds.2013-3965]).
Guidance on the use of Menveo (Meningococcal Groups A, C, W-135, and Y Oligosaccharide Diphtheria CRM197 conjugate vaccine) for certain groups of infants at increased risk of disease starting at age 2 months has been added to the meningococcal vaccine footnote. This is based on the Food and Drug Administration licensure of Menveo for use starting at age 2 months in August 2013. Some of the high-risk categories include anatomic or functional asplenia, including sickle cell disease; children with persistent complement component deficiency; and those who travel to or live in an area hyperendemic area for meningococcal disease.
This is the first time that a meningococcal vaccine has been available for use starting at age 2 months, Dr. H. Cody Meissner, professor of pediatrics at Tufts University, Boston, pointed out in an interview.
The hepatitis A vaccine footnote now provides a list of groups at increased risk for hepatitis A. While these groups are well recognized, "it was useful to itemize these groups in the footnote," noted Dr. Meissner, who is a member of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices work group on the harmonized immunization schedule.
The list includes people traveling to or working in countries where there is a high or intermediate endemicity of infection; men having sex with men; people with clotting factor disorders; people with chronic liver disease; users of injection and noninjection illicit drugs; and personal contacts – such as household contacts or regular babysitters – of international adoptees during the first 2 months of arrival in the United States "from a country with high or intermediate endemicity."
The footnote on the tetanus and diphtheria toxoids and acellular pertussis (Tdap) vaccine now states that a dose of the vaccine is recommended for pregnant adolescents every time they get pregnant – preferably during week 27 through week 36 of gestation. Last year, the American Academy of Pediatrics agreed that this vaccine should be given to a pregnant woman, but it withheld the recommendation to vaccinate during every pregnancy until more data became available, Dr. Meissner said. "Additional data now indicate the safety and efficacy of administration of Tdap each time a woman becomes pregnant." This will protect most infants during the first 2 months of life when pertussis can be most severe and until they receive their first DTaP dose at 2 months, he noted.
Other changes include clarification of the intervals between doses in the human papillomavirus (HPV) vaccines footnote, to avoid any misunderstanding of the schedule, said Dr. Meissner, who is also chief of pediatric infectious disease at the Floating Hospital for Children at Tufts Medical Center.
The recommendation for pneumococcal vaccines has not changed from last year, but the footnote provides clarification about the recommendations for PCV13 (Prevnar 13) and PPSV23 in children and adolescents, "which have been stratified according to age and according to degree of risk," he added.
The footnote on Haemophilus influenzae type b (Hib) conjugate vaccine now includes clarification of who should receive the vaccine if immunocompromised.
This is the second year that recommendations and footnotes for ages 0-18 years are included in one schedule, as opposed to previous years, when there were separate schedules and footnotes for 0-7 years and 8-18 years.
The 2014 schedules have been approved by the American Academy of Pediatrics, the Advisory Committee on Immunization Practices, the American Academy of Family Physicians, and the American College of Obstetricians and Gynecologists.
Dr. Meissner said he has no relevant financial disclosures.
You can view the 2014 schedule at our Resources section here.
FROM PEDIATRICS