User login
Optimizing diagnostic testing for venous thromboembolism
When a patient presents with suspected venous thromboembolism, ie, deep vein thrombosis or pulmonary embolism, what diagnostic tests are needed to confirm the diagnosis? The clinical signs and symptoms of venous thromboembolism are nonspecific and often difficult to interpret. Therefore, it is essential for clinicians to use a standardized, structured approach to diagnosis that incorporates clinical findings and laboratory testing, as well as judicious use of diagnostic imaging. But while information is important, clinicians must also strive to avoid unnecessary testing, not only to decrease costs, but also to avoid potential harm.
If the diagnosis is confirmed, does the patient need testing for an underlying thrombophilic disorder? Such screening is often considered after a thromboembolic event occurs. However, a growing body of evidence indicates that the results of thrombophilia testing can be misinterpreted and potentially harmful.1 We need to understand the utility of this testing as well as when and how it should be used. Patients and thrombosis specialists should be involved in deciding whether to perform these tests.
In this article, we provide practical information about how to diagnose venous thromboembolism, including strategies to optimize testing in suspected cases. We also offer guidance on how to decide whether further thrombophilia testing is warranted.
COMMON AND SERIOUS
Venous thromboembolism is a major cause of morbidity and death. Approximately 900,000 cases of pulmonary embolism and deep vein thrombosis occur in the United States each year, causing 60,000 to 300,000 deaths,2 with the number of cases projected to double over the next 40 years.3
INITIAL APPROACH: PRETEST PROBABILITY
Given the morbidity and mortality associated with venous thromboembolism, prompt recognition and diagnosis are imperative. Clinical diagnosis alone is insufficient, with confirmed disease found in only 15% to 25% of patients suspected of having venous thromboembolism.4–8 Therefore, the pretest probability should be coupled with objective testing.
The Wells score shows good discrimination in the outpatient and emergency department settings, but it has been invalidated in the inpatient setting, and thus it should not be used in inpatients.10
LABORATORY TESTS FOR SUSPECTED VENOUS THROMBOEMBOLISM
Employing an understanding of diagnostic testing is fundamental to identifying patients with venous thromboembolism.
D-dimer is a byproduct of fibrinolysis.
D-dimer testing has very high sensitivity for venous thromboembolism (> 90%) but low specificity (about 50%), and levels can be elevated in a variety of situations such as advanced age, acute inflammation, and cancer.15 The standard threshold is 500 μg/L, but because the D-dimer level increases with age, some clinicians advocate using an age-adjusted threshold for patients age 50 or older (age in years × 10 μg/L) to increase the diagnostic yield.16
Of the laboratory tests for D-dimer, the enzyme-linked immunosorbent assay has the highest sensitivity and highest negative predictive value (100%) and may be preferred over the other test methodologies.17
With its high sensitivity, D-dimer testing is clinically useful for ruling out venous thromboembolism, particularly when the pretest probability is low, but it lacks the specificity required for diagnosing and treating the disease if positive. Thus, it is not useful for ruling in venous thromboembolism. If the patient has a high pretest probability, we can omit D-dimer testing in favor of imaging studies.
Other laboratory tests such as arterial blood gas and brain natriuretic peptide levels have been proposed as markers of pulmonary embolism, but studies suggest they have limited utility in predicting the presence of disease.18,19
DIAGNOSTIC TESTS FOR DEEP VEIN THROMBOSIS
Ultrasonography
If the pretest probability of deep vein thrombosis is high or a D-dimer test is found to be positive, the next step in evaluation is compression ultrasonography.
While some guidelines recommend scanning only the proximal leg, many facilities in the United States scan the whole leg, which may reveal distal deep vein thrombosis.20 The clinical significance of isolated distal deep vein thrombosis is unknown, and a selective anticoagulation approach may be used if this condition is discovered. The 2012 and 2016 American College of Chest Physicians (ACCP) guidelines on diagnosis and management of venous thromboembolism address this topic.20,21
Deep vein thrombosis in the arm should be evaluated in the same manner as in the lower extremities.
Venography
Invasive and therefore no longer often used, venography is considered the gold standard for diagnosing deep vein thrombosis. Computed tomographic (CT) or magnetic resonance (MR) venography is most useful if the patient has aberrant anatomy such as a deformity of the leg, or in situations where the use of ultrasonography is difficult or unreliable, such as in the setting of severe obesity. CT or MR venography may be considered when looking for thrombosis in noncompressible veins of the thorax and abdomen (eg, the subclavian vein, iliac vein, and inferior vena cava) if ultrasonography is negative but clinical suspicion is high. Venous-phase CT angiography is particularly useful in diagnosing deep vein thrombosis in the inferior vena cava and iliac vein when deep vein thrombosis is clinically suspected but cannot be visualized on duplex ultrasonography.
DIAGNOSTIC TESTS FOR PULMONARY EMBOLISM
Computed tomography
Imaging is warranted in patients who have a high pretest probability of pulmonary embolism, or in whom the D-dimer assay was positive but the pretest probability was low or moderate.
Once the gold standard, pulmonary angiography is no longer recommended for the initial diagnosis of pulmonary embolism because it is invasive, often unavailable, less sophisticated, and more expensive than noninvasive imaging techniques such as CT angiography. It is still used, however, in catheter-directed thrombolysis.
Thus, multiphasic CT angiography, as guided by pretest probability and the D-dimer level, is the imaging test of choice in the evaluation of pulmonary embolism. It can also offer insight into thrombotic burden and can reveal concurrent or alternative diagnoses (eg, pneumonia).
Ventilation-perfusion scanning
When CT angiography is unavailable or the patient should not be exposed to contrast medium (eg, due to concern for contrast-induced nephropathy or contrast allergy), ventilation-perfusion (V/Q) scanning remains an option for ruling out pulmonary embolism.22
Anderson et al23 compared CT angiography and V/Q scanning in a study in 1,417 patients considered likely to have acute pulmonary embolism. Rates of symptomatic pulmonary embolism during 3-month follow-up were similar in patients who initially had negative results on V/Q scanning compared with those who initially had negative results on CT angiography. However, this study used single-detector CT scanners for one-third of the patients. Therefore, the results may have been different if current technology had been used.
Limitations of V/Q scanning include length of time to perform (30–45 minutes), cost, inability to identify other causes of symptoms, and difficulty with interpretation when other pulmonary pathology is present (eg, lung infiltrate). V/Q scanning is helpful when negative but is often reported based on probability (low, intermediate, or high) and may not provide adequate guidance. Therefore, CT angiography should be used whenever possible for diagnosing pulmonary embolism.
Other tests for pulmonary embolism
Electrocardiography, transthoracic echocardiography, and chest radiography may aid in the search for alternative diagnoses and assess the degree of right heart strain as a sequela of pulmonary embolism, but they do not confirm the diagnosis.
ORDER IMAGING ONLY IF NEEDED
Diagnostic imaging can be optimized by avoiding unnecessary tests that carry both costs and clinical risks.
Most patients in whom acute pulmonary embolism is discovered will not need testing for deep vein thrombosis, as they will receive anticoagulation regardless. Similarly, many patients with acute symptomatic deep vein thrombosis do not need testing for pulmonary embolism with chest CT imaging, as they too will receive anticoagulation regardless.
Therefore, clinicians are encouraged to use diagnostic reasoning while practicing high-value care (including estimating pretest probability and measuring D-dimer when appropriate), ordering additional tests judiciously and only if indicated.
THROMBOEMBOLISM IS CONFIRMED—IS FURTHER TESTING WARRANTED?
Once acute venous thromboembolism is confirmed, key considerations include whether the event was provoked or unprovoked (ie, idiopathic) and whether the patient needs indefinite anticoagulation (eg, after 2 or more unprovoked events).
Was the event provoked or unprovoked?
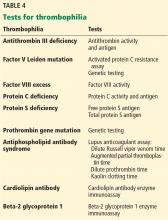
Even in cases of unprovoked venous thromboembolism, no clear consensus exists as to which patients should be tested for thrombophilia. Experts do advocate, however, that it be done only in highly selected patients and that it be coordinated with the patient, family members, and an expert in this testing. Patients for whom further testing may be considered include those with venous thromboembolism in unusual sites (eg, the cavernous sinus), with warfarin-induced skin necrosis, or with recurrent pregnancy loss.
While screening for malignancy may seem prudent in the case of unexplained venous thromboembolism, the use of CT imaging for this purpose has been found to be of low yield. In one study,24 it was not found to detect additional neoplasms, and it can lead to additional cost and no added benefit for patients.
The American Board of Internal Medicine’s Choosing Wisely campaign strongly recommends consultation with an expert in thrombophilia (eg, a hematologist) before testing.25 Ordering multiple tests in bundles (hypercoagulability panels) is unlikely to alter management, could have a negative clinical impact on patients, and is generally not recommended.
The ‘4 Ps’ approach to testing
- Patient selection
- Pretest counseling
- Proper laboratory interpretation
- Provision of education and advice.
Importantly, testing should be reserved for patients in whom the pretest probability of the thrombophilic disease is moderate to high, such as testing for antiphospholipid antibody syndrome in patients with systemic lupus erythematosus or recurrent miscarriage.
Venous thromboembolism in a patient who is known to have a malignant disease does not typically warrant further thrombophilia testing, as the event was likely a sequela of the malignancy. The evaluation and management of venous thromboembolism with concurrent neoplasm is covered elsewhere.21
WHAT IF VENOUS THROMBOEMBOLISM IS DISCOVERED INCIDENTALLY?
Thrombophilia testing should be approached the same regardless of whether the venous thromboembolism was diagnosed intentionally or incidentally. First, determine whether the thrombosis was provoked or unprovoked, then order additional tests only if indicated, as recommended. Alternative approaches such as forgoing anticoagulation (but performing serial imaging, if indicated) may be reasonable if the thrombus is deemed clinically irrelevant (eg, nonocclusive, asymptomatic, subsegmental pulmonary embolism in the absence of proximal deep vein thrombosis; isolated distal deep vein thrombosis).25,27
It is still debatable whether the increasing incidence of asymptomatic pulmonary embolism due to enhanced sensitivity of noninvasive diagnostic imaging warrants a change in diagnostic approach.28
FACTORS TO CONSIDER BEFORE THROMBOPHILIA TESTING
Important factors to consider before testing for thrombophilia are29:
- How will the results affect the anticoagulation plan?
- How may the patient’s clinical status and medications influence the results?
- Has the patient expressed a desire to understand why venous thromboembolism occurred?
- Will the results have a potential impact on the patient’s family members?
How will the results of thrombophilia testing affect anticoagulation management?
Because the goal of any diagnostic test is to find out what type of care the patient needs, clinicians must determine whether knowledge of an underlying thrombophilia will alter the short-term or long-term anticoagulation therapy the patient is receiving for an acute venous thromboembolic event.
As most acute episodes of venous thromboembolism require an initial 3 months of anticoagulation (with the exception of some nonclinically relevant events such as isolated distal deep vein thrombosis without extension on reimaging), testing in the acute setting does not change the short-term management of anticoagulation. Many hospitals have advocated for outpatient-only thrombophilia testing (if testing does occur), as testing in the acute setting may render test results uninterpretable (see What factors can influence thrombophilia testing? below) and can inappropriately affect the long-term management of anticoagulation. We recommend against testing in the inpatient setting.
To determine the duration of anticoagulation, clinicians must balance the risk of recurrent venous thromboembolism and the risk of bleeding. If a patient is at significant risk of bleeding or does not tolerate anticoagulation, clinicians may consider stopping therapy instead of evaluating for thrombophilia. For patients with provoked venous thromboembolism, anticoagulation should generally be limited to 3 months, as the risk of recurrence does not outweigh the risk of bleeding with continued anticoagulation therapy.
Patients with unprovoked venous thromboembolism have a risk of recurrence twice as high as those with provoked venous thromboembolism and generally need a longer duration of anticoagulation.30,31 Once a patient with an unprovoked venous thromboembolic event has completed the initial 3 months of anticoagulation, a formal risk-benefit evaluation should be performed to determine whether to continue it.
Up to 42% of patients with unprovoked venous thromboembolism may have 1 or more thrombotic disorders, and some clinicians believe that detecting an underlying thrombophilia will aid in decisions regarding duration of therapy.32 However, the risk of recurrent venous thromboembolism in these patients does not differ significantly from that in patients without an underlying thrombophilia.33–35 As such, it has been suggested that the unprovoked character of the thrombotic event, rather than an underlying thrombophilia, determines the risk of future recurrence and should be used instead of testing to guide the duration of anticoagulation therapy.32
For more information, see the 2016 ACCP guideline update on antithrombotic therapy for venous thromboembolism.27
What factors can influence the results of thrombophilia testing?
For example, antithrombin is consumed during thrombus formation; therefore, antithrombin levels may be transiently suppressed in acute venous thromboembolism. Moreover, since antithrombin binds to unfractionated heparin, low-molecular-weight heparin, and fondaparinux and mediates their activity as anticoagulants, antithrombin levels may be decreased by heparin therapy.
Similarly, vitamin K antagonists (eg, warfarin) suppress protein C and S activity levels by inhibiting vitamin K epoxide reductase and may falsely indicate a protein C or S deficiency.
Direct oral anticoagulants can cause false-positive results on lupus anticoagulant assays (dilute Russell viper venom time, augmented partial thromboplastin time), raise protein C, protein S, and antithrombin activity levels, and normalize activated protein C resistance assays, leading to missed diagnoses.41
Since estrogen therapy and pregnancy lead to increases in C4b binding protein, resulting in decreased free protein S, these situations can result in clinicians falsely labeling patients as having congenital protein S deficiency when in fact the patient had a transient reduction in protein S levels.33
Therefore, to optimize accuracy and interpretation of results, thrombophilia testing should ideally be performed when the patient:
- Is past the acute event and out of the hospital
- Is not pregnant
- Has received the required 3 months of anticoagulation and is off this therapy.
For warfarin, most recommendations say that testing should be performed after the patient has been off therapy for 2 to 6 weeks.42 Low-molecular-weight heparins and direct oral anticoagulants should be discontinued for at least 48 to 72 hours, or longer if the patient has kidney impairment, as these medications are renally eliminated.
Genetic tests such as factor V Leiden and prothrombin gene mutation are not affected by these factors and do not require repeat or confirmatory testing.
What if the patient or family wants to understand why an event occurred?
Some experts advocate thrombophilia testing of asymptomatic family members to identify carriers who may need prophylaxis against venous thromboembolism in high-risk situations such as pregnancy, oral contraceptive use, hospitalization, and surgery.29 Asymptomatic family members of a first-degree relative with a history of venous thromboembolism have a 2 times higher risk of an index event.43 Thus, it may be argued that these asymptomatic individuals should receive prophylactic measures in any high-risk situation, based on the family history itself rather than results of thrombophilia testing.
Occasionally, patients and family members want to know the cause of the thrombotic event and want to be tested. In these instances, pretest counseling for the patient and family about the potential implications of testing and shared decision-making between the provider and patient are of utmost importance.29
What is the impact on family members if thrombophilia is diagnosed?
While positive test results can give patients some satisfaction, this knowledge may also cause unnecessary worry, as the patient knows he or she has a hematologic disorder and could possible die of venous thromboembolism.
Thrombophilia testing can have other adverse consequences. For example, while the Genetic Information Nondiscrimination Act of 2008 protects against denial of health insurance benefits based on genetic information, known carriers of thrombophilia may have trouble obtaining life or disability insurance.44
Unfortunately, it is not uncommon for thrombophilia testing to be inappropriately performed, interpreted, or followed up. These suboptimal approaches can lead to unnecessary exposure to high-risk therapeutic anticoagulation, excessive durations of therapy, and labeling with an unconfirmed or incorrect diagnosis. Additionally, there are significant costs associated with thrombophilia testing, including the cost of the tests and anticoagulant medications and management of adverse events such as bleeding.
WHAT ARE THE ALTERNATIVES TO THROMBOPHILIA TESTING?
Because discovered thrombophilias (eg, factor V Leiden mutation, prothrombin gene mutation) have not consistently shown a strong correlation with increased recurrence of venous thromboembolism, alternative approaches are emerging to determine the duration of therapy for unprovoked events.
Clinical prediction tools based on patient characteristics and laboratory markers that are more consistently associated with recurrent venous thromboembolism (eg, male sex, persistently elevated D-dimer) have been developed to aid clinicians dealing with this challenging question. Several prediction tools are available:
The “Men Continue and HERDOO2” rule (HERDOO2 = hyperpigmentation, edema, or redness in either leg; D-dimer level ≥ 250 μg/L; obesity with body mass index ≥ 30 kg/m2; or older age, ≥ 65)45
The DASH score (D-dimer, age, sex, and hormonal therapy)46
The Vienna score,47,48 at http://cemsiis.meduniwien.ac.at/en/kb/science-research/software/clinical-software/recurrent-vte/.
SUMMARY OF THROMBOPHILIA TESTING RECOMMENDATIONS
Test for thrombophilia only when…
- Discussing with a specialist (eg, hematologist) who has an understanding of thrombophilia
- Using the 4 Ps approach
- A patient requests testing to understand why a thrombotic event occurred, and the patient understands the implications of testing (ie, received counseling) for self and for family
- An expert deems identification of asymptomatic family members important for those who may be carriers of a detected thrombophilia
- The patient with a venous thromboembolic event has completed 3 months of anticoagulation and has been off anticoagulation for the appropriate length of time
- The results will change management.
Forgo thrombophilia testing when…
- A patient has a provoked venous thromboembolic event
- You do not intend to discontinue anticoagulation (ie, anticoagulation is indefinite)
- The patient is in the acute (eg, inpatient) setting
- The patient is on anticoagulants that may render test results uninterpretable
- The patient is pregnant or on oral contraceptives
- Use of alternative patient characteristics and laboratory markers to predict venous thromboembolism recurrence may be an option.
OPTIMIZING THE DIAGNOSIS
With the incidence of venous thromboembolism rapidly increasing, optimizing its diagnosis from both a financial and clinical perspective is becoming increasingly important. Clinicians should be familiar with the use of pretest probability scoring for venous thromboembolism, as well as which diagnostic tests are preferred if further workup is indicated. They should strive to minimize or avoid indiscriminate thrombophilia testing, which may lead to increased healthcare costs and patient exposure to potentially harmful anticoagulation.
Testing for thrombophilia should be based on whether a venous thromboembolic event was provoked or unprovoked. Patients with provoked venous thromboembolism or those receiving indefinite anticoagulation therapy should not be tested for thrombophilia. If testing is being considered in a patient with unprovoked venous thromboembolism, a specialist who is able to implement the 4 Ps approach should be consulted to ensure well-informed, shared decision-making with patients and family members.
- National Institute for Health and Care Excellence (NICE). Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. https://www.nice.org.uk/guidance/cg144. Accessed June 13, 2017.
- Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 2008; 28:370–372.
- Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol 2011; 86:217–220.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e419S–e494S.
- Pengo V, Lensing AW, Prins MH, et al; Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350:2257–2264.
- Kahn SR, Hirsch A, Shrier I. Effect of postthrombotic syndrome on health-related quality of life after deep venous thrombosis. Arch Intern Med 2002; 162:1144–1148.
- Wells PS, Owen C, Doucette S, Fergusson D, Tran H. Does this patient have deep vein thrombosis? JAMA 2006; 295:199–207.
- Ljungqvist M, Söderberg M, Moritz P, Ahlgren A, Lärfars G. Evaluation of Wells score and repeated D-dimer in diagnosing venous thromboembolism. Eur J Intern Med 2008; 19:285–288.
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med 2001; 135:98–107.
- Silveira PC, Ip IK, Goldhaber SZ, Piazza G, Benson CB, Khorasani R. Performance of Wells score for deep vein thrombosis in the inpatient setting. JAMA Intern Med 2015; 175:1112–1117.
- Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997; 350:1795–1798.
- Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med 2003; 349:1227–1235.
- van Belle A, Büller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 2006; 295:172–179.
- Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemos 2000; 83:416–420.
- Schrecengost JE, LeGallo RD, Boyd JC, et al. Comparison of diagnostic accuracies in outpatients and hospitalized patients of D-dimer testing for the evaluation of suspected pulmonary embolism. Clin Chem 2003; 49:1483–1490.
- Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA 2014; 311:1117–1124.
- Pulivarthi S, Gurram MK. Effectiveness of D-dimer as a screening test for venous thromboembolism: an update. N Am J Med Sci 2014; 6:491–499.
- Söhne M, Ten Wolde M, Boomsma F, Reitsma JB, Douketis JD, Büller HR. Brain natriuretic peptide in hemodynamically stable acute pulmonary embolism. J Thromb Haemost 2006; 4:552–556.
- Stein PD, Goldhaber SZ, Henry JW, Miller AC. Arterial blood gas analysis in the assessment of suspected acute pulmonary embolism. Chest 1996; 109:78–81.
- Bates SM, Jaeschke R, Stevens SM, et al; American College of Chest Physicians. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e351S–e418S.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest 2016; 149:315–352.
- PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA 1990; 263:2753–2759.
- Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA 2007; 298:2743–2753.
- Carrier M. Cancer screening in unprovoked venous thromboembolism. N Engl J Med 2015; 373:2475.
- American Society of Hematology. Don’t test for thrombophilia in adult patients with venous thromboembolism (VTE) occurring in the setting of major transient risk factors (surgery, trauma or prolonged immobility). www.choosingwisely.org/clinician-lists/american-society-hematology-testing-for-thrombophilia-in-adults/. Accessed June 13, 2017.
- Cushman M. Thrombophilia testing in women with venous thrombosis: the 4 Ps approach. Clin Chem 2014; 60:134–137.
- Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO; American College of Chest Physicians. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl): e691S–e736S.
- Ritchie G, McGurk S, McCreath C, Graham C, Murchison JT. Prospective evaluation of unsuspected pulmonary embolism on contrast enhanced multidetector CT (MDCT) scanning. Thorax 2007; 62:536–540.
- Moll S. Thrombophilia: clinical-practical aspects. J Thromb Thrombolysis 2015; 39:367–378.
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica 2007; 92:199–205.
- Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036.
- Kearon C, Julian JA, Kovacs MJ, et al; ELATE Investigators. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008; 112:4432–4436.
- Lijfering WM, Middeldorp S, Veeger NJ, et al. Risk of recurrent venous thrombosis in homozygous carriers and double heterozygous carriers of factor V Leiden and prothrombin G20210A. Circulation 2010; 121:1706–1712.
- Hron G, Eichinger S, Weltermann A, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med 2006; 119:50–53.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Lijfering WM. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113:5314–5322.
- Segal JB. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation. JAMA 2009; 301:2472–2485.
- Juul K. Factor V Leiden and the risk for venous thromboembolism in the adult Danish population. Ann Intern Med 2004; 140: 330–337.
- Emmerich J. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism: pooled analysis of 8 case-control studies including 2310 cases and 3204 controls. Thromb Haemost 2001; 86: 809–816.
- Garcia D. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood 2013; 122:817–824.
- Gosselin R, Adcock DM. The laboratory’s 2015 perspective on direct oral anticoagulant testing. J Thromb Haemost 2016; 14:886–893.
- Marlar RA, Gausman JN. Protein S abnormalities: a diagnostic nightmare. Am J Hematol 2011; 86:418–421.
- Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med 2009; 169:610–615.
- Middeldorp S. Evidence-based approach to thrombophilia testing. J Thromb Thrombolysis 2011; 31:275–281.
- Rodger MA, Le Gal G, Anderson DR, et al, for the REVERSE II Study Investigators. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ 2017; 356:j1065.
- Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012; 10:1019–1025.
- Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation 2010; 121:1630–1636.
- Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008; 179:417–426.
When a patient presents with suspected venous thromboembolism, ie, deep vein thrombosis or pulmonary embolism, what diagnostic tests are needed to confirm the diagnosis? The clinical signs and symptoms of venous thromboembolism are nonspecific and often difficult to interpret. Therefore, it is essential for clinicians to use a standardized, structured approach to diagnosis that incorporates clinical findings and laboratory testing, as well as judicious use of diagnostic imaging. But while information is important, clinicians must also strive to avoid unnecessary testing, not only to decrease costs, but also to avoid potential harm.
If the diagnosis is confirmed, does the patient need testing for an underlying thrombophilic disorder? Such screening is often considered after a thromboembolic event occurs. However, a growing body of evidence indicates that the results of thrombophilia testing can be misinterpreted and potentially harmful.1 We need to understand the utility of this testing as well as when and how it should be used. Patients and thrombosis specialists should be involved in deciding whether to perform these tests.
In this article, we provide practical information about how to diagnose venous thromboembolism, including strategies to optimize testing in suspected cases. We also offer guidance on how to decide whether further thrombophilia testing is warranted.
COMMON AND SERIOUS
Venous thromboembolism is a major cause of morbidity and death. Approximately 900,000 cases of pulmonary embolism and deep vein thrombosis occur in the United States each year, causing 60,000 to 300,000 deaths,2 with the number of cases projected to double over the next 40 years.3
INITIAL APPROACH: PRETEST PROBABILITY
Given the morbidity and mortality associated with venous thromboembolism, prompt recognition and diagnosis are imperative. Clinical diagnosis alone is insufficient, with confirmed disease found in only 15% to 25% of patients suspected of having venous thromboembolism.4–8 Therefore, the pretest probability should be coupled with objective testing.
The Wells score shows good discrimination in the outpatient and emergency department settings, but it has been invalidated in the inpatient setting, and thus it should not be used in inpatients.10
LABORATORY TESTS FOR SUSPECTED VENOUS THROMBOEMBOLISM
Employing an understanding of diagnostic testing is fundamental to identifying patients with venous thromboembolism.
D-dimer is a byproduct of fibrinolysis.
D-dimer testing has very high sensitivity for venous thromboembolism (> 90%) but low specificity (about 50%), and levels can be elevated in a variety of situations such as advanced age, acute inflammation, and cancer.15 The standard threshold is 500 μg/L, but because the D-dimer level increases with age, some clinicians advocate using an age-adjusted threshold for patients age 50 or older (age in years × 10 μg/L) to increase the diagnostic yield.16
Of the laboratory tests for D-dimer, the enzyme-linked immunosorbent assay has the highest sensitivity and highest negative predictive value (100%) and may be preferred over the other test methodologies.17
With its high sensitivity, D-dimer testing is clinically useful for ruling out venous thromboembolism, particularly when the pretest probability is low, but it lacks the specificity required for diagnosing and treating the disease if positive. Thus, it is not useful for ruling in venous thromboembolism. If the patient has a high pretest probability, we can omit D-dimer testing in favor of imaging studies.
Other laboratory tests such as arterial blood gas and brain natriuretic peptide levels have been proposed as markers of pulmonary embolism, but studies suggest they have limited utility in predicting the presence of disease.18,19
DIAGNOSTIC TESTS FOR DEEP VEIN THROMBOSIS
Ultrasonography
If the pretest probability of deep vein thrombosis is high or a D-dimer test is found to be positive, the next step in evaluation is compression ultrasonography.
While some guidelines recommend scanning only the proximal leg, many facilities in the United States scan the whole leg, which may reveal distal deep vein thrombosis.20 The clinical significance of isolated distal deep vein thrombosis is unknown, and a selective anticoagulation approach may be used if this condition is discovered. The 2012 and 2016 American College of Chest Physicians (ACCP) guidelines on diagnosis and management of venous thromboembolism address this topic.20,21
Deep vein thrombosis in the arm should be evaluated in the same manner as in the lower extremities.
Venography
Invasive and therefore no longer often used, venography is considered the gold standard for diagnosing deep vein thrombosis. Computed tomographic (CT) or magnetic resonance (MR) venography is most useful if the patient has aberrant anatomy such as a deformity of the leg, or in situations where the use of ultrasonography is difficult or unreliable, such as in the setting of severe obesity. CT or MR venography may be considered when looking for thrombosis in noncompressible veins of the thorax and abdomen (eg, the subclavian vein, iliac vein, and inferior vena cava) if ultrasonography is negative but clinical suspicion is high. Venous-phase CT angiography is particularly useful in diagnosing deep vein thrombosis in the inferior vena cava and iliac vein when deep vein thrombosis is clinically suspected but cannot be visualized on duplex ultrasonography.
DIAGNOSTIC TESTS FOR PULMONARY EMBOLISM
Computed tomography
Imaging is warranted in patients who have a high pretest probability of pulmonary embolism, or in whom the D-dimer assay was positive but the pretest probability was low or moderate.
Once the gold standard, pulmonary angiography is no longer recommended for the initial diagnosis of pulmonary embolism because it is invasive, often unavailable, less sophisticated, and more expensive than noninvasive imaging techniques such as CT angiography. It is still used, however, in catheter-directed thrombolysis.
Thus, multiphasic CT angiography, as guided by pretest probability and the D-dimer level, is the imaging test of choice in the evaluation of pulmonary embolism. It can also offer insight into thrombotic burden and can reveal concurrent or alternative diagnoses (eg, pneumonia).
Ventilation-perfusion scanning
When CT angiography is unavailable or the patient should not be exposed to contrast medium (eg, due to concern for contrast-induced nephropathy or contrast allergy), ventilation-perfusion (V/Q) scanning remains an option for ruling out pulmonary embolism.22
Anderson et al23 compared CT angiography and V/Q scanning in a study in 1,417 patients considered likely to have acute pulmonary embolism. Rates of symptomatic pulmonary embolism during 3-month follow-up were similar in patients who initially had negative results on V/Q scanning compared with those who initially had negative results on CT angiography. However, this study used single-detector CT scanners for one-third of the patients. Therefore, the results may have been different if current technology had been used.
Limitations of V/Q scanning include length of time to perform (30–45 minutes), cost, inability to identify other causes of symptoms, and difficulty with interpretation when other pulmonary pathology is present (eg, lung infiltrate). V/Q scanning is helpful when negative but is often reported based on probability (low, intermediate, or high) and may not provide adequate guidance. Therefore, CT angiography should be used whenever possible for diagnosing pulmonary embolism.
Other tests for pulmonary embolism
Electrocardiography, transthoracic echocardiography, and chest radiography may aid in the search for alternative diagnoses and assess the degree of right heart strain as a sequela of pulmonary embolism, but they do not confirm the diagnosis.
ORDER IMAGING ONLY IF NEEDED
Diagnostic imaging can be optimized by avoiding unnecessary tests that carry both costs and clinical risks.
Most patients in whom acute pulmonary embolism is discovered will not need testing for deep vein thrombosis, as they will receive anticoagulation regardless. Similarly, many patients with acute symptomatic deep vein thrombosis do not need testing for pulmonary embolism with chest CT imaging, as they too will receive anticoagulation regardless.
Therefore, clinicians are encouraged to use diagnostic reasoning while practicing high-value care (including estimating pretest probability and measuring D-dimer when appropriate), ordering additional tests judiciously and only if indicated.
THROMBOEMBOLISM IS CONFIRMED—IS FURTHER TESTING WARRANTED?
Once acute venous thromboembolism is confirmed, key considerations include whether the event was provoked or unprovoked (ie, idiopathic) and whether the patient needs indefinite anticoagulation (eg, after 2 or more unprovoked events).
Was the event provoked or unprovoked?
Even in cases of unprovoked venous thromboembolism, no clear consensus exists as to which patients should be tested for thrombophilia. Experts do advocate, however, that it be done only in highly selected patients and that it be coordinated with the patient, family members, and an expert in this testing. Patients for whom further testing may be considered include those with venous thromboembolism in unusual sites (eg, the cavernous sinus), with warfarin-induced skin necrosis, or with recurrent pregnancy loss.
While screening for malignancy may seem prudent in the case of unexplained venous thromboembolism, the use of CT imaging for this purpose has been found to be of low yield. In one study,24 it was not found to detect additional neoplasms, and it can lead to additional cost and no added benefit for patients.
The American Board of Internal Medicine’s Choosing Wisely campaign strongly recommends consultation with an expert in thrombophilia (eg, a hematologist) before testing.25 Ordering multiple tests in bundles (hypercoagulability panels) is unlikely to alter management, could have a negative clinical impact on patients, and is generally not recommended.
The ‘4 Ps’ approach to testing
- Patient selection
- Pretest counseling
- Proper laboratory interpretation
- Provision of education and advice.
Importantly, testing should be reserved for patients in whom the pretest probability of the thrombophilic disease is moderate to high, such as testing for antiphospholipid antibody syndrome in patients with systemic lupus erythematosus or recurrent miscarriage.
Venous thromboembolism in a patient who is known to have a malignant disease does not typically warrant further thrombophilia testing, as the event was likely a sequela of the malignancy. The evaluation and management of venous thromboembolism with concurrent neoplasm is covered elsewhere.21
WHAT IF VENOUS THROMBOEMBOLISM IS DISCOVERED INCIDENTALLY?
Thrombophilia testing should be approached the same regardless of whether the venous thromboembolism was diagnosed intentionally or incidentally. First, determine whether the thrombosis was provoked or unprovoked, then order additional tests only if indicated, as recommended. Alternative approaches such as forgoing anticoagulation (but performing serial imaging, if indicated) may be reasonable if the thrombus is deemed clinically irrelevant (eg, nonocclusive, asymptomatic, subsegmental pulmonary embolism in the absence of proximal deep vein thrombosis; isolated distal deep vein thrombosis).25,27
It is still debatable whether the increasing incidence of asymptomatic pulmonary embolism due to enhanced sensitivity of noninvasive diagnostic imaging warrants a change in diagnostic approach.28
FACTORS TO CONSIDER BEFORE THROMBOPHILIA TESTING
Important factors to consider before testing for thrombophilia are29:
- How will the results affect the anticoagulation plan?
- How may the patient’s clinical status and medications influence the results?
- Has the patient expressed a desire to understand why venous thromboembolism occurred?
- Will the results have a potential impact on the patient’s family members?
How will the results of thrombophilia testing affect anticoagulation management?
Because the goal of any diagnostic test is to find out what type of care the patient needs, clinicians must determine whether knowledge of an underlying thrombophilia will alter the short-term or long-term anticoagulation therapy the patient is receiving for an acute venous thromboembolic event.
As most acute episodes of venous thromboembolism require an initial 3 months of anticoagulation (with the exception of some nonclinically relevant events such as isolated distal deep vein thrombosis without extension on reimaging), testing in the acute setting does not change the short-term management of anticoagulation. Many hospitals have advocated for outpatient-only thrombophilia testing (if testing does occur), as testing in the acute setting may render test results uninterpretable (see What factors can influence thrombophilia testing? below) and can inappropriately affect the long-term management of anticoagulation. We recommend against testing in the inpatient setting.
To determine the duration of anticoagulation, clinicians must balance the risk of recurrent venous thromboembolism and the risk of bleeding. If a patient is at significant risk of bleeding or does not tolerate anticoagulation, clinicians may consider stopping therapy instead of evaluating for thrombophilia. For patients with provoked venous thromboembolism, anticoagulation should generally be limited to 3 months, as the risk of recurrence does not outweigh the risk of bleeding with continued anticoagulation therapy.
Patients with unprovoked venous thromboembolism have a risk of recurrence twice as high as those with provoked venous thromboembolism and generally need a longer duration of anticoagulation.30,31 Once a patient with an unprovoked venous thromboembolic event has completed the initial 3 months of anticoagulation, a formal risk-benefit evaluation should be performed to determine whether to continue it.
Up to 42% of patients with unprovoked venous thromboembolism may have 1 or more thrombotic disorders, and some clinicians believe that detecting an underlying thrombophilia will aid in decisions regarding duration of therapy.32 However, the risk of recurrent venous thromboembolism in these patients does not differ significantly from that in patients without an underlying thrombophilia.33–35 As such, it has been suggested that the unprovoked character of the thrombotic event, rather than an underlying thrombophilia, determines the risk of future recurrence and should be used instead of testing to guide the duration of anticoagulation therapy.32
For more information, see the 2016 ACCP guideline update on antithrombotic therapy for venous thromboembolism.27
What factors can influence the results of thrombophilia testing?
For example, antithrombin is consumed during thrombus formation; therefore, antithrombin levels may be transiently suppressed in acute venous thromboembolism. Moreover, since antithrombin binds to unfractionated heparin, low-molecular-weight heparin, and fondaparinux and mediates their activity as anticoagulants, antithrombin levels may be decreased by heparin therapy.
Similarly, vitamin K antagonists (eg, warfarin) suppress protein C and S activity levels by inhibiting vitamin K epoxide reductase and may falsely indicate a protein C or S deficiency.
Direct oral anticoagulants can cause false-positive results on lupus anticoagulant assays (dilute Russell viper venom time, augmented partial thromboplastin time), raise protein C, protein S, and antithrombin activity levels, and normalize activated protein C resistance assays, leading to missed diagnoses.41
Since estrogen therapy and pregnancy lead to increases in C4b binding protein, resulting in decreased free protein S, these situations can result in clinicians falsely labeling patients as having congenital protein S deficiency when in fact the patient had a transient reduction in protein S levels.33
Therefore, to optimize accuracy and interpretation of results, thrombophilia testing should ideally be performed when the patient:
- Is past the acute event and out of the hospital
- Is not pregnant
- Has received the required 3 months of anticoagulation and is off this therapy.
For warfarin, most recommendations say that testing should be performed after the patient has been off therapy for 2 to 6 weeks.42 Low-molecular-weight heparins and direct oral anticoagulants should be discontinued for at least 48 to 72 hours, or longer if the patient has kidney impairment, as these medications are renally eliminated.
Genetic tests such as factor V Leiden and prothrombin gene mutation are not affected by these factors and do not require repeat or confirmatory testing.
What if the patient or family wants to understand why an event occurred?
Some experts advocate thrombophilia testing of asymptomatic family members to identify carriers who may need prophylaxis against venous thromboembolism in high-risk situations such as pregnancy, oral contraceptive use, hospitalization, and surgery.29 Asymptomatic family members of a first-degree relative with a history of venous thromboembolism have a 2 times higher risk of an index event.43 Thus, it may be argued that these asymptomatic individuals should receive prophylactic measures in any high-risk situation, based on the family history itself rather than results of thrombophilia testing.
Occasionally, patients and family members want to know the cause of the thrombotic event and want to be tested. In these instances, pretest counseling for the patient and family about the potential implications of testing and shared decision-making between the provider and patient are of utmost importance.29
What is the impact on family members if thrombophilia is diagnosed?
While positive test results can give patients some satisfaction, this knowledge may also cause unnecessary worry, as the patient knows he or she has a hematologic disorder and could possible die of venous thromboembolism.
Thrombophilia testing can have other adverse consequences. For example, while the Genetic Information Nondiscrimination Act of 2008 protects against denial of health insurance benefits based on genetic information, known carriers of thrombophilia may have trouble obtaining life or disability insurance.44
Unfortunately, it is not uncommon for thrombophilia testing to be inappropriately performed, interpreted, or followed up. These suboptimal approaches can lead to unnecessary exposure to high-risk therapeutic anticoagulation, excessive durations of therapy, and labeling with an unconfirmed or incorrect diagnosis. Additionally, there are significant costs associated with thrombophilia testing, including the cost of the tests and anticoagulant medications and management of adverse events such as bleeding.
WHAT ARE THE ALTERNATIVES TO THROMBOPHILIA TESTING?
Because discovered thrombophilias (eg, factor V Leiden mutation, prothrombin gene mutation) have not consistently shown a strong correlation with increased recurrence of venous thromboembolism, alternative approaches are emerging to determine the duration of therapy for unprovoked events.
Clinical prediction tools based on patient characteristics and laboratory markers that are more consistently associated with recurrent venous thromboembolism (eg, male sex, persistently elevated D-dimer) have been developed to aid clinicians dealing with this challenging question. Several prediction tools are available:
The “Men Continue and HERDOO2” rule (HERDOO2 = hyperpigmentation, edema, or redness in either leg; D-dimer level ≥ 250 μg/L; obesity with body mass index ≥ 30 kg/m2; or older age, ≥ 65)45
The DASH score (D-dimer, age, sex, and hormonal therapy)46
The Vienna score,47,48 at http://cemsiis.meduniwien.ac.at/en/kb/science-research/software/clinical-software/recurrent-vte/.
SUMMARY OF THROMBOPHILIA TESTING RECOMMENDATIONS
Test for thrombophilia only when…
- Discussing with a specialist (eg, hematologist) who has an understanding of thrombophilia
- Using the 4 Ps approach
- A patient requests testing to understand why a thrombotic event occurred, and the patient understands the implications of testing (ie, received counseling) for self and for family
- An expert deems identification of asymptomatic family members important for those who may be carriers of a detected thrombophilia
- The patient with a venous thromboembolic event has completed 3 months of anticoagulation and has been off anticoagulation for the appropriate length of time
- The results will change management.
Forgo thrombophilia testing when…
- A patient has a provoked venous thromboembolic event
- You do not intend to discontinue anticoagulation (ie, anticoagulation is indefinite)
- The patient is in the acute (eg, inpatient) setting
- The patient is on anticoagulants that may render test results uninterpretable
- The patient is pregnant or on oral contraceptives
- Use of alternative patient characteristics and laboratory markers to predict venous thromboembolism recurrence may be an option.
OPTIMIZING THE DIAGNOSIS
With the incidence of venous thromboembolism rapidly increasing, optimizing its diagnosis from both a financial and clinical perspective is becoming increasingly important. Clinicians should be familiar with the use of pretest probability scoring for venous thromboembolism, as well as which diagnostic tests are preferred if further workup is indicated. They should strive to minimize or avoid indiscriminate thrombophilia testing, which may lead to increased healthcare costs and patient exposure to potentially harmful anticoagulation.
Testing for thrombophilia should be based on whether a venous thromboembolic event was provoked or unprovoked. Patients with provoked venous thromboembolism or those receiving indefinite anticoagulation therapy should not be tested for thrombophilia. If testing is being considered in a patient with unprovoked venous thromboembolism, a specialist who is able to implement the 4 Ps approach should be consulted to ensure well-informed, shared decision-making with patients and family members.
When a patient presents with suspected venous thromboembolism, ie, deep vein thrombosis or pulmonary embolism, what diagnostic tests are needed to confirm the diagnosis? The clinical signs and symptoms of venous thromboembolism are nonspecific and often difficult to interpret. Therefore, it is essential for clinicians to use a standardized, structured approach to diagnosis that incorporates clinical findings and laboratory testing, as well as judicious use of diagnostic imaging. But while information is important, clinicians must also strive to avoid unnecessary testing, not only to decrease costs, but also to avoid potential harm.
If the diagnosis is confirmed, does the patient need testing for an underlying thrombophilic disorder? Such screening is often considered after a thromboembolic event occurs. However, a growing body of evidence indicates that the results of thrombophilia testing can be misinterpreted and potentially harmful.1 We need to understand the utility of this testing as well as when and how it should be used. Patients and thrombosis specialists should be involved in deciding whether to perform these tests.
In this article, we provide practical information about how to diagnose venous thromboembolism, including strategies to optimize testing in suspected cases. We also offer guidance on how to decide whether further thrombophilia testing is warranted.
COMMON AND SERIOUS
Venous thromboembolism is a major cause of morbidity and death. Approximately 900,000 cases of pulmonary embolism and deep vein thrombosis occur in the United States each year, causing 60,000 to 300,000 deaths,2 with the number of cases projected to double over the next 40 years.3
INITIAL APPROACH: PRETEST PROBABILITY
Given the morbidity and mortality associated with venous thromboembolism, prompt recognition and diagnosis are imperative. Clinical diagnosis alone is insufficient, with confirmed disease found in only 15% to 25% of patients suspected of having venous thromboembolism.4–8 Therefore, the pretest probability should be coupled with objective testing.
The Wells score shows good discrimination in the outpatient and emergency department settings, but it has been invalidated in the inpatient setting, and thus it should not be used in inpatients.10
LABORATORY TESTS FOR SUSPECTED VENOUS THROMBOEMBOLISM
Employing an understanding of diagnostic testing is fundamental to identifying patients with venous thromboembolism.
D-dimer is a byproduct of fibrinolysis.
D-dimer testing has very high sensitivity for venous thromboembolism (> 90%) but low specificity (about 50%), and levels can be elevated in a variety of situations such as advanced age, acute inflammation, and cancer.15 The standard threshold is 500 μg/L, but because the D-dimer level increases with age, some clinicians advocate using an age-adjusted threshold for patients age 50 or older (age in years × 10 μg/L) to increase the diagnostic yield.16
Of the laboratory tests for D-dimer, the enzyme-linked immunosorbent assay has the highest sensitivity and highest negative predictive value (100%) and may be preferred over the other test methodologies.17
With its high sensitivity, D-dimer testing is clinically useful for ruling out venous thromboembolism, particularly when the pretest probability is low, but it lacks the specificity required for diagnosing and treating the disease if positive. Thus, it is not useful for ruling in venous thromboembolism. If the patient has a high pretest probability, we can omit D-dimer testing in favor of imaging studies.
Other laboratory tests such as arterial blood gas and brain natriuretic peptide levels have been proposed as markers of pulmonary embolism, but studies suggest they have limited utility in predicting the presence of disease.18,19
DIAGNOSTIC TESTS FOR DEEP VEIN THROMBOSIS
Ultrasonography
If the pretest probability of deep vein thrombosis is high or a D-dimer test is found to be positive, the next step in evaluation is compression ultrasonography.
While some guidelines recommend scanning only the proximal leg, many facilities in the United States scan the whole leg, which may reveal distal deep vein thrombosis.20 The clinical significance of isolated distal deep vein thrombosis is unknown, and a selective anticoagulation approach may be used if this condition is discovered. The 2012 and 2016 American College of Chest Physicians (ACCP) guidelines on diagnosis and management of venous thromboembolism address this topic.20,21
Deep vein thrombosis in the arm should be evaluated in the same manner as in the lower extremities.
Venography
Invasive and therefore no longer often used, venography is considered the gold standard for diagnosing deep vein thrombosis. Computed tomographic (CT) or magnetic resonance (MR) venography is most useful if the patient has aberrant anatomy such as a deformity of the leg, or in situations where the use of ultrasonography is difficult or unreliable, such as in the setting of severe obesity. CT or MR venography may be considered when looking for thrombosis in noncompressible veins of the thorax and abdomen (eg, the subclavian vein, iliac vein, and inferior vena cava) if ultrasonography is negative but clinical suspicion is high. Venous-phase CT angiography is particularly useful in diagnosing deep vein thrombosis in the inferior vena cava and iliac vein when deep vein thrombosis is clinically suspected but cannot be visualized on duplex ultrasonography.
DIAGNOSTIC TESTS FOR PULMONARY EMBOLISM
Computed tomography
Imaging is warranted in patients who have a high pretest probability of pulmonary embolism, or in whom the D-dimer assay was positive but the pretest probability was low or moderate.
Once the gold standard, pulmonary angiography is no longer recommended for the initial diagnosis of pulmonary embolism because it is invasive, often unavailable, less sophisticated, and more expensive than noninvasive imaging techniques such as CT angiography. It is still used, however, in catheter-directed thrombolysis.
Thus, multiphasic CT angiography, as guided by pretest probability and the D-dimer level, is the imaging test of choice in the evaluation of pulmonary embolism. It can also offer insight into thrombotic burden and can reveal concurrent or alternative diagnoses (eg, pneumonia).
Ventilation-perfusion scanning
When CT angiography is unavailable or the patient should not be exposed to contrast medium (eg, due to concern for contrast-induced nephropathy or contrast allergy), ventilation-perfusion (V/Q) scanning remains an option for ruling out pulmonary embolism.22
Anderson et al23 compared CT angiography and V/Q scanning in a study in 1,417 patients considered likely to have acute pulmonary embolism. Rates of symptomatic pulmonary embolism during 3-month follow-up were similar in patients who initially had negative results on V/Q scanning compared with those who initially had negative results on CT angiography. However, this study used single-detector CT scanners for one-third of the patients. Therefore, the results may have been different if current technology had been used.
Limitations of V/Q scanning include length of time to perform (30–45 minutes), cost, inability to identify other causes of symptoms, and difficulty with interpretation when other pulmonary pathology is present (eg, lung infiltrate). V/Q scanning is helpful when negative but is often reported based on probability (low, intermediate, or high) and may not provide adequate guidance. Therefore, CT angiography should be used whenever possible for diagnosing pulmonary embolism.
Other tests for pulmonary embolism
Electrocardiography, transthoracic echocardiography, and chest radiography may aid in the search for alternative diagnoses and assess the degree of right heart strain as a sequela of pulmonary embolism, but they do not confirm the diagnosis.
ORDER IMAGING ONLY IF NEEDED
Diagnostic imaging can be optimized by avoiding unnecessary tests that carry both costs and clinical risks.
Most patients in whom acute pulmonary embolism is discovered will not need testing for deep vein thrombosis, as they will receive anticoagulation regardless. Similarly, many patients with acute symptomatic deep vein thrombosis do not need testing for pulmonary embolism with chest CT imaging, as they too will receive anticoagulation regardless.
Therefore, clinicians are encouraged to use diagnostic reasoning while practicing high-value care (including estimating pretest probability and measuring D-dimer when appropriate), ordering additional tests judiciously and only if indicated.
THROMBOEMBOLISM IS CONFIRMED—IS FURTHER TESTING WARRANTED?
Once acute venous thromboembolism is confirmed, key considerations include whether the event was provoked or unprovoked (ie, idiopathic) and whether the patient needs indefinite anticoagulation (eg, after 2 or more unprovoked events).
Was the event provoked or unprovoked?
Even in cases of unprovoked venous thromboembolism, no clear consensus exists as to which patients should be tested for thrombophilia. Experts do advocate, however, that it be done only in highly selected patients and that it be coordinated with the patient, family members, and an expert in this testing. Patients for whom further testing may be considered include those with venous thromboembolism in unusual sites (eg, the cavernous sinus), with warfarin-induced skin necrosis, or with recurrent pregnancy loss.
While screening for malignancy may seem prudent in the case of unexplained venous thromboembolism, the use of CT imaging for this purpose has been found to be of low yield. In one study,24 it was not found to detect additional neoplasms, and it can lead to additional cost and no added benefit for patients.
The American Board of Internal Medicine’s Choosing Wisely campaign strongly recommends consultation with an expert in thrombophilia (eg, a hematologist) before testing.25 Ordering multiple tests in bundles (hypercoagulability panels) is unlikely to alter management, could have a negative clinical impact on patients, and is generally not recommended.
The ‘4 Ps’ approach to testing
- Patient selection
- Pretest counseling
- Proper laboratory interpretation
- Provision of education and advice.
Importantly, testing should be reserved for patients in whom the pretest probability of the thrombophilic disease is moderate to high, such as testing for antiphospholipid antibody syndrome in patients with systemic lupus erythematosus or recurrent miscarriage.
Venous thromboembolism in a patient who is known to have a malignant disease does not typically warrant further thrombophilia testing, as the event was likely a sequela of the malignancy. The evaluation and management of venous thromboembolism with concurrent neoplasm is covered elsewhere.21
WHAT IF VENOUS THROMBOEMBOLISM IS DISCOVERED INCIDENTALLY?
Thrombophilia testing should be approached the same regardless of whether the venous thromboembolism was diagnosed intentionally or incidentally. First, determine whether the thrombosis was provoked or unprovoked, then order additional tests only if indicated, as recommended. Alternative approaches such as forgoing anticoagulation (but performing serial imaging, if indicated) may be reasonable if the thrombus is deemed clinically irrelevant (eg, nonocclusive, asymptomatic, subsegmental pulmonary embolism in the absence of proximal deep vein thrombosis; isolated distal deep vein thrombosis).25,27
It is still debatable whether the increasing incidence of asymptomatic pulmonary embolism due to enhanced sensitivity of noninvasive diagnostic imaging warrants a change in diagnostic approach.28
FACTORS TO CONSIDER BEFORE THROMBOPHILIA TESTING
Important factors to consider before testing for thrombophilia are29:
- How will the results affect the anticoagulation plan?
- How may the patient’s clinical status and medications influence the results?
- Has the patient expressed a desire to understand why venous thromboembolism occurred?
- Will the results have a potential impact on the patient’s family members?
How will the results of thrombophilia testing affect anticoagulation management?
Because the goal of any diagnostic test is to find out what type of care the patient needs, clinicians must determine whether knowledge of an underlying thrombophilia will alter the short-term or long-term anticoagulation therapy the patient is receiving for an acute venous thromboembolic event.
As most acute episodes of venous thromboembolism require an initial 3 months of anticoagulation (with the exception of some nonclinically relevant events such as isolated distal deep vein thrombosis without extension on reimaging), testing in the acute setting does not change the short-term management of anticoagulation. Many hospitals have advocated for outpatient-only thrombophilia testing (if testing does occur), as testing in the acute setting may render test results uninterpretable (see What factors can influence thrombophilia testing? below) and can inappropriately affect the long-term management of anticoagulation. We recommend against testing in the inpatient setting.
To determine the duration of anticoagulation, clinicians must balance the risk of recurrent venous thromboembolism and the risk of bleeding. If a patient is at significant risk of bleeding or does not tolerate anticoagulation, clinicians may consider stopping therapy instead of evaluating for thrombophilia. For patients with provoked venous thromboembolism, anticoagulation should generally be limited to 3 months, as the risk of recurrence does not outweigh the risk of bleeding with continued anticoagulation therapy.
Patients with unprovoked venous thromboembolism have a risk of recurrence twice as high as those with provoked venous thromboembolism and generally need a longer duration of anticoagulation.30,31 Once a patient with an unprovoked venous thromboembolic event has completed the initial 3 months of anticoagulation, a formal risk-benefit evaluation should be performed to determine whether to continue it.
Up to 42% of patients with unprovoked venous thromboembolism may have 1 or more thrombotic disorders, and some clinicians believe that detecting an underlying thrombophilia will aid in decisions regarding duration of therapy.32 However, the risk of recurrent venous thromboembolism in these patients does not differ significantly from that in patients without an underlying thrombophilia.33–35 As such, it has been suggested that the unprovoked character of the thrombotic event, rather than an underlying thrombophilia, determines the risk of future recurrence and should be used instead of testing to guide the duration of anticoagulation therapy.32
For more information, see the 2016 ACCP guideline update on antithrombotic therapy for venous thromboembolism.27
What factors can influence the results of thrombophilia testing?
For example, antithrombin is consumed during thrombus formation; therefore, antithrombin levels may be transiently suppressed in acute venous thromboembolism. Moreover, since antithrombin binds to unfractionated heparin, low-molecular-weight heparin, and fondaparinux and mediates their activity as anticoagulants, antithrombin levels may be decreased by heparin therapy.
Similarly, vitamin K antagonists (eg, warfarin) suppress protein C and S activity levels by inhibiting vitamin K epoxide reductase and may falsely indicate a protein C or S deficiency.
Direct oral anticoagulants can cause false-positive results on lupus anticoagulant assays (dilute Russell viper venom time, augmented partial thromboplastin time), raise protein C, protein S, and antithrombin activity levels, and normalize activated protein C resistance assays, leading to missed diagnoses.41
Since estrogen therapy and pregnancy lead to increases in C4b binding protein, resulting in decreased free protein S, these situations can result in clinicians falsely labeling patients as having congenital protein S deficiency when in fact the patient had a transient reduction in protein S levels.33
Therefore, to optimize accuracy and interpretation of results, thrombophilia testing should ideally be performed when the patient:
- Is past the acute event and out of the hospital
- Is not pregnant
- Has received the required 3 months of anticoagulation and is off this therapy.
For warfarin, most recommendations say that testing should be performed after the patient has been off therapy for 2 to 6 weeks.42 Low-molecular-weight heparins and direct oral anticoagulants should be discontinued for at least 48 to 72 hours, or longer if the patient has kidney impairment, as these medications are renally eliminated.
Genetic tests such as factor V Leiden and prothrombin gene mutation are not affected by these factors and do not require repeat or confirmatory testing.
What if the patient or family wants to understand why an event occurred?
Some experts advocate thrombophilia testing of asymptomatic family members to identify carriers who may need prophylaxis against venous thromboembolism in high-risk situations such as pregnancy, oral contraceptive use, hospitalization, and surgery.29 Asymptomatic family members of a first-degree relative with a history of venous thromboembolism have a 2 times higher risk of an index event.43 Thus, it may be argued that these asymptomatic individuals should receive prophylactic measures in any high-risk situation, based on the family history itself rather than results of thrombophilia testing.
Occasionally, patients and family members want to know the cause of the thrombotic event and want to be tested. In these instances, pretest counseling for the patient and family about the potential implications of testing and shared decision-making between the provider and patient are of utmost importance.29
What is the impact on family members if thrombophilia is diagnosed?
While positive test results can give patients some satisfaction, this knowledge may also cause unnecessary worry, as the patient knows he or she has a hematologic disorder and could possible die of venous thromboembolism.
Thrombophilia testing can have other adverse consequences. For example, while the Genetic Information Nondiscrimination Act of 2008 protects against denial of health insurance benefits based on genetic information, known carriers of thrombophilia may have trouble obtaining life or disability insurance.44
Unfortunately, it is not uncommon for thrombophilia testing to be inappropriately performed, interpreted, or followed up. These suboptimal approaches can lead to unnecessary exposure to high-risk therapeutic anticoagulation, excessive durations of therapy, and labeling with an unconfirmed or incorrect diagnosis. Additionally, there are significant costs associated with thrombophilia testing, including the cost of the tests and anticoagulant medications and management of adverse events such as bleeding.
WHAT ARE THE ALTERNATIVES TO THROMBOPHILIA TESTING?
Because discovered thrombophilias (eg, factor V Leiden mutation, prothrombin gene mutation) have not consistently shown a strong correlation with increased recurrence of venous thromboembolism, alternative approaches are emerging to determine the duration of therapy for unprovoked events.
Clinical prediction tools based on patient characteristics and laboratory markers that are more consistently associated with recurrent venous thromboembolism (eg, male sex, persistently elevated D-dimer) have been developed to aid clinicians dealing with this challenging question. Several prediction tools are available:
The “Men Continue and HERDOO2” rule (HERDOO2 = hyperpigmentation, edema, or redness in either leg; D-dimer level ≥ 250 μg/L; obesity with body mass index ≥ 30 kg/m2; or older age, ≥ 65)45
The DASH score (D-dimer, age, sex, and hormonal therapy)46
The Vienna score,47,48 at http://cemsiis.meduniwien.ac.at/en/kb/science-research/software/clinical-software/recurrent-vte/.
SUMMARY OF THROMBOPHILIA TESTING RECOMMENDATIONS
Test for thrombophilia only when…
- Discussing with a specialist (eg, hematologist) who has an understanding of thrombophilia
- Using the 4 Ps approach
- A patient requests testing to understand why a thrombotic event occurred, and the patient understands the implications of testing (ie, received counseling) for self and for family
- An expert deems identification of asymptomatic family members important for those who may be carriers of a detected thrombophilia
- The patient with a venous thromboembolic event has completed 3 months of anticoagulation and has been off anticoagulation for the appropriate length of time
- The results will change management.
Forgo thrombophilia testing when…
- A patient has a provoked venous thromboembolic event
- You do not intend to discontinue anticoagulation (ie, anticoagulation is indefinite)
- The patient is in the acute (eg, inpatient) setting
- The patient is on anticoagulants that may render test results uninterpretable
- The patient is pregnant or on oral contraceptives
- Use of alternative patient characteristics and laboratory markers to predict venous thromboembolism recurrence may be an option.
OPTIMIZING THE DIAGNOSIS
With the incidence of venous thromboembolism rapidly increasing, optimizing its diagnosis from both a financial and clinical perspective is becoming increasingly important. Clinicians should be familiar with the use of pretest probability scoring for venous thromboembolism, as well as which diagnostic tests are preferred if further workup is indicated. They should strive to minimize or avoid indiscriminate thrombophilia testing, which may lead to increased healthcare costs and patient exposure to potentially harmful anticoagulation.
Testing for thrombophilia should be based on whether a venous thromboembolic event was provoked or unprovoked. Patients with provoked venous thromboembolism or those receiving indefinite anticoagulation therapy should not be tested for thrombophilia. If testing is being considered in a patient with unprovoked venous thromboembolism, a specialist who is able to implement the 4 Ps approach should be consulted to ensure well-informed, shared decision-making with patients and family members.
- National Institute for Health and Care Excellence (NICE). Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. https://www.nice.org.uk/guidance/cg144. Accessed June 13, 2017.
- Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 2008; 28:370–372.
- Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol 2011; 86:217–220.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e419S–e494S.
- Pengo V, Lensing AW, Prins MH, et al; Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350:2257–2264.
- Kahn SR, Hirsch A, Shrier I. Effect of postthrombotic syndrome on health-related quality of life after deep venous thrombosis. Arch Intern Med 2002; 162:1144–1148.
- Wells PS, Owen C, Doucette S, Fergusson D, Tran H. Does this patient have deep vein thrombosis? JAMA 2006; 295:199–207.
- Ljungqvist M, Söderberg M, Moritz P, Ahlgren A, Lärfars G. Evaluation of Wells score and repeated D-dimer in diagnosing venous thromboembolism. Eur J Intern Med 2008; 19:285–288.
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med 2001; 135:98–107.
- Silveira PC, Ip IK, Goldhaber SZ, Piazza G, Benson CB, Khorasani R. Performance of Wells score for deep vein thrombosis in the inpatient setting. JAMA Intern Med 2015; 175:1112–1117.
- Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997; 350:1795–1798.
- Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med 2003; 349:1227–1235.
- van Belle A, Büller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 2006; 295:172–179.
- Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemos 2000; 83:416–420.
- Schrecengost JE, LeGallo RD, Boyd JC, et al. Comparison of diagnostic accuracies in outpatients and hospitalized patients of D-dimer testing for the evaluation of suspected pulmonary embolism. Clin Chem 2003; 49:1483–1490.
- Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA 2014; 311:1117–1124.
- Pulivarthi S, Gurram MK. Effectiveness of D-dimer as a screening test for venous thromboembolism: an update. N Am J Med Sci 2014; 6:491–499.
- Söhne M, Ten Wolde M, Boomsma F, Reitsma JB, Douketis JD, Büller HR. Brain natriuretic peptide in hemodynamically stable acute pulmonary embolism. J Thromb Haemost 2006; 4:552–556.
- Stein PD, Goldhaber SZ, Henry JW, Miller AC. Arterial blood gas analysis in the assessment of suspected acute pulmonary embolism. Chest 1996; 109:78–81.
- Bates SM, Jaeschke R, Stevens SM, et al; American College of Chest Physicians. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e351S–e418S.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest 2016; 149:315–352.
- PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA 1990; 263:2753–2759.
- Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA 2007; 298:2743–2753.
- Carrier M. Cancer screening in unprovoked venous thromboembolism. N Engl J Med 2015; 373:2475.
- American Society of Hematology. Don’t test for thrombophilia in adult patients with venous thromboembolism (VTE) occurring in the setting of major transient risk factors (surgery, trauma or prolonged immobility). www.choosingwisely.org/clinician-lists/american-society-hematology-testing-for-thrombophilia-in-adults/. Accessed June 13, 2017.
- Cushman M. Thrombophilia testing in women with venous thrombosis: the 4 Ps approach. Clin Chem 2014; 60:134–137.
- Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO; American College of Chest Physicians. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl): e691S–e736S.
- Ritchie G, McGurk S, McCreath C, Graham C, Murchison JT. Prospective evaluation of unsuspected pulmonary embolism on contrast enhanced multidetector CT (MDCT) scanning. Thorax 2007; 62:536–540.
- Moll S. Thrombophilia: clinical-practical aspects. J Thromb Thrombolysis 2015; 39:367–378.
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica 2007; 92:199–205.
- Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036.
- Kearon C, Julian JA, Kovacs MJ, et al; ELATE Investigators. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008; 112:4432–4436.
- Lijfering WM, Middeldorp S, Veeger NJ, et al. Risk of recurrent venous thrombosis in homozygous carriers and double heterozygous carriers of factor V Leiden and prothrombin G20210A. Circulation 2010; 121:1706–1712.
- Hron G, Eichinger S, Weltermann A, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med 2006; 119:50–53.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Lijfering WM. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113:5314–5322.
- Segal JB. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation. JAMA 2009; 301:2472–2485.
- Juul K. Factor V Leiden and the risk for venous thromboembolism in the adult Danish population. Ann Intern Med 2004; 140: 330–337.
- Emmerich J. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism: pooled analysis of 8 case-control studies including 2310 cases and 3204 controls. Thromb Haemost 2001; 86: 809–816.
- Garcia D. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood 2013; 122:817–824.
- Gosselin R, Adcock DM. The laboratory’s 2015 perspective on direct oral anticoagulant testing. J Thromb Haemost 2016; 14:886–893.
- Marlar RA, Gausman JN. Protein S abnormalities: a diagnostic nightmare. Am J Hematol 2011; 86:418–421.
- Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med 2009; 169:610–615.
- Middeldorp S. Evidence-based approach to thrombophilia testing. J Thromb Thrombolysis 2011; 31:275–281.
- Rodger MA, Le Gal G, Anderson DR, et al, for the REVERSE II Study Investigators. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ 2017; 356:j1065.
- Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012; 10:1019–1025.
- Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation 2010; 121:1630–1636.
- Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008; 179:417–426.
- National Institute for Health and Care Excellence (NICE). Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. https://www.nice.org.uk/guidance/cg144. Accessed June 13, 2017.
- Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 2008; 28:370–372.
- Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol 2011; 86:217–220.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e419S–e494S.
- Pengo V, Lensing AW, Prins MH, et al; Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350:2257–2264.
- Kahn SR, Hirsch A, Shrier I. Effect of postthrombotic syndrome on health-related quality of life after deep venous thrombosis. Arch Intern Med 2002; 162:1144–1148.
- Wells PS, Owen C, Doucette S, Fergusson D, Tran H. Does this patient have deep vein thrombosis? JAMA 2006; 295:199–207.
- Ljungqvist M, Söderberg M, Moritz P, Ahlgren A, Lärfars G. Evaluation of Wells score and repeated D-dimer in diagnosing venous thromboembolism. Eur J Intern Med 2008; 19:285–288.
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med 2001; 135:98–107.
- Silveira PC, Ip IK, Goldhaber SZ, Piazza G, Benson CB, Khorasani R. Performance of Wells score for deep vein thrombosis in the inpatient setting. JAMA Intern Med 2015; 175:1112–1117.
- Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997; 350:1795–1798.
- Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med 2003; 349:1227–1235.
- van Belle A, Büller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 2006; 295:172–179.
- Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemos 2000; 83:416–420.
- Schrecengost JE, LeGallo RD, Boyd JC, et al. Comparison of diagnostic accuracies in outpatients and hospitalized patients of D-dimer testing for the evaluation of suspected pulmonary embolism. Clin Chem 2003; 49:1483–1490.
- Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA 2014; 311:1117–1124.
- Pulivarthi S, Gurram MK. Effectiveness of D-dimer as a screening test for venous thromboembolism: an update. N Am J Med Sci 2014; 6:491–499.
- Söhne M, Ten Wolde M, Boomsma F, Reitsma JB, Douketis JD, Büller HR. Brain natriuretic peptide in hemodynamically stable acute pulmonary embolism. J Thromb Haemost 2006; 4:552–556.
- Stein PD, Goldhaber SZ, Henry JW, Miller AC. Arterial blood gas analysis in the assessment of suspected acute pulmonary embolism. Chest 1996; 109:78–81.
- Bates SM, Jaeschke R, Stevens SM, et al; American College of Chest Physicians. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e351S–e418S.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest 2016; 149:315–352.
- PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA 1990; 263:2753–2759.
- Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA 2007; 298:2743–2753.
- Carrier M. Cancer screening in unprovoked venous thromboembolism. N Engl J Med 2015; 373:2475.
- American Society of Hematology. Don’t test for thrombophilia in adult patients with venous thromboembolism (VTE) occurring in the setting of major transient risk factors (surgery, trauma or prolonged immobility). www.choosingwisely.org/clinician-lists/american-society-hematology-testing-for-thrombophilia-in-adults/. Accessed June 13, 2017.
- Cushman M. Thrombophilia testing in women with venous thrombosis: the 4 Ps approach. Clin Chem 2014; 60:134–137.
- Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO; American College of Chest Physicians. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl): e691S–e736S.
- Ritchie G, McGurk S, McCreath C, Graham C, Murchison JT. Prospective evaluation of unsuspected pulmonary embolism on contrast enhanced multidetector CT (MDCT) scanning. Thorax 2007; 62:536–540.
- Moll S. Thrombophilia: clinical-practical aspects. J Thromb Thrombolysis 2015; 39:367–378.
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica 2007; 92:199–205.
- Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036.
- Kearon C, Julian JA, Kovacs MJ, et al; ELATE Investigators. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008; 112:4432–4436.
- Lijfering WM, Middeldorp S, Veeger NJ, et al. Risk of recurrent venous thrombosis in homozygous carriers and double heterozygous carriers of factor V Leiden and prothrombin G20210A. Circulation 2010; 121:1706–1712.
- Hron G, Eichinger S, Weltermann A, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med 2006; 119:50–53.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Lijfering WM. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113:5314–5322.
- Segal JB. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation. JAMA 2009; 301:2472–2485.
- Juul K. Factor V Leiden and the risk for venous thromboembolism in the adult Danish population. Ann Intern Med 2004; 140: 330–337.
- Emmerich J. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism: pooled analysis of 8 case-control studies including 2310 cases and 3204 controls. Thromb Haemost 2001; 86: 809–816.
- Garcia D. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood 2013; 122:817–824.
- Gosselin R, Adcock DM. The laboratory’s 2015 perspective on direct oral anticoagulant testing. J Thromb Haemost 2016; 14:886–893.
- Marlar RA, Gausman JN. Protein S abnormalities: a diagnostic nightmare. Am J Hematol 2011; 86:418–421.
- Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med 2009; 169:610–615.
- Middeldorp S. Evidence-based approach to thrombophilia testing. J Thromb Thrombolysis 2011; 31:275–281.
- Rodger MA, Le Gal G, Anderson DR, et al, for the REVERSE II Study Investigators. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ 2017; 356:j1065.
- Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012; 10:1019–1025.
- Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation 2010; 121:1630–1636.
- Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008; 179:417–426.
KEY POINTS
- A pretest clinical prediction tool such as the Wells score can help in deciding whether a patient with suspected venous thromboembolism warrants further workup.
- A clinical prediction tool should be used in concert with additional laboratory testing (eg, D-dimer) and imaging in patients at risk.
- In many cases, screening for thrombophilia to determine the cause of a venous thromboembolic event may be unwarranted.
- Testing for thrombophilia should be based on whether a venous thromboembolic event was provoked or unprovoked.
Dashboards and P4P in VTE Prophylaxis
The Affordable Care Act explicitly outlines improving the value of healthcare by increasing quality and decreasing costs. It emphasizes value‐based purchasing, the transparency of performance metrics, and the use of payment incentives to reward quality.[1, 2] Venous thromboembolism (VTE) prophylaxis is one of these publicly reported performance measures. The National Quality Forum recommends that each patient be evaluated on hospital admission and during their hospitalization for VTE risk level and for appropriate thromboprophylaxis to be used, if required.[3] Similarly, the Joint Commission includes appropriate VTE prophylaxis in its Core Measures.[4] Patient experience and performance metrics, including VTE prophylaxis, constitute the hospital value‐based purchasing (VBP) component of healthcare reform.[5] For a hypothetical 327‐bed hospital, an estimated $1.7 million of a hospital's inpatient payments from Medicare will be at risk from VBP alone.[2]
VTE prophylaxis is a common target of quality improvement projects. Effective, safe, and cost‐effective measures to prevent VTE exist, including pharmacologic and mechanical prophylaxis.[6, 7] Despite these measures, compliance rates are often below 50%.[8] Different interventions have been pursued to ensure appropriate VTE prophylaxis, including computerized provider order entry (CPOE), electronic alerts, mandatory VTE risk assessment and prophylaxis, and provider education campaigns.[9] Recent studies show that CPOE systems with mandatory fields can increase VTE prophylaxis rates to above 80%, yet the goal of a high reliability health system is for 100% of patients to receive recommended therapy.[10, 11, 12, 13, 14, 15] Interventions to improve prophylaxis rates that have included multiple strategies, such as computerized order sets, feedback, and education, have been the most effective, increasing compliance to above 90%.[9, 11, 16] These systems can be enhanced with additional interventions such as providing individualized provider education and feedback, understanding of work flow, and ensuring patients receive the prescribed therapies.[12] For example, a physician dashboard could be employed to provide a snapshot and historical trend of key performance indicators using graphical displays and indicators.[17]
Dashboards and pay‐for‐performance programs have been increasingly used to increase the visibility of these metrics, provide feedback, visually display benchmarks and goals, and proactively monitor for achievements and setbacks.[18] Although these strategies are often addressed at departmental (or greater) levels, applying them at the level of the individual provider may assist hospitals in reducing preventable harm and achieving safety and quality goals, especially at higher benchmarks. With their expanding role, hospitalists provide a key opportunity to lead improvement efforts and to study the impact of dashboards and pay‐for performance at the provider level to achieve VTE prophylaxis performance targets. Hospitalists are often the front‐line provider for inpatients and deliver up to 70% of inpatient general medical services.[19] The objective of our study was to evaluate the impact of providing individual provider feedback and employing a pay‐for‐performance program on baseline performance of VTE prophylaxis among hospitalists. We hypothesized that performance feedback through the use of a dashboard would increase appropriate VTE prophylaxis, and this effect would be further augmented by incorporation of a pay‐for‐performance program.
METHODS
Hospitalist Dashboard
In 2010, hospitalist program leaders met with hospital administrators to create a hospitalist dashboard that would provide regularly updated summaries of performance measures for individual hospitalists. The final set of metrics identified included appropriate VTE prophylaxis, length of stay, patients discharged per day, discharges before 3 pm, depth of coding, patient satisfaction, readmissions, communication with the primary care provider, and time to signature for discharge summaries (Figure 1A). The dashboard was introduced at a general hospitalist meeting during which its purpose, methodology, and accessibility were described; it was subsequently implemented in January 2011.
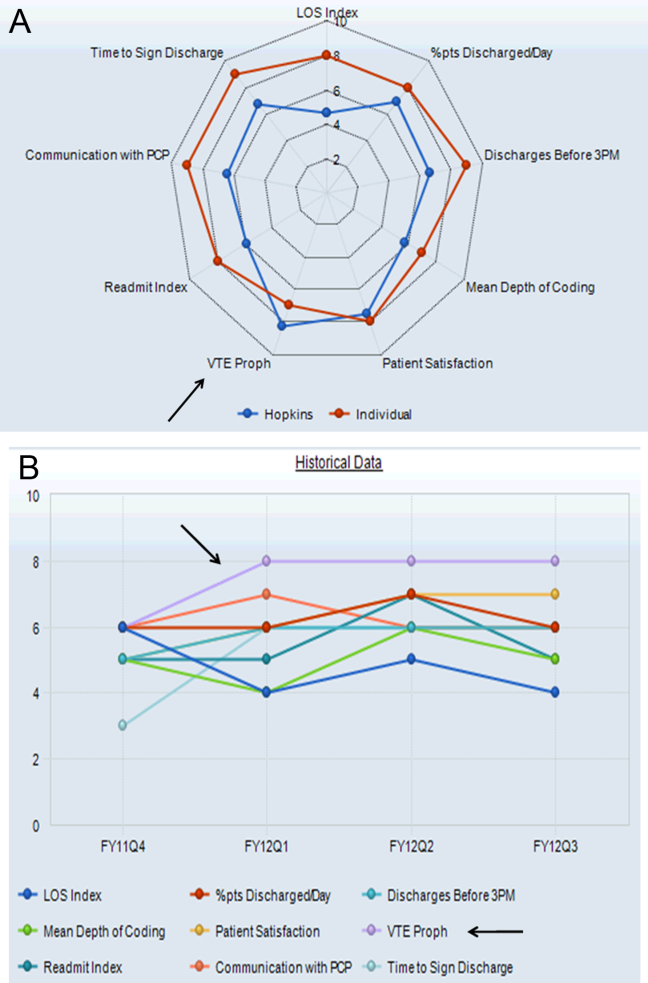
Benchmarks were established for each metric, standardized to establish a scale ranging from 1 through 9, and incorporated into the dashboard (Figure 1A). Higher scores (creating a larger geometric shape) were desirable. For the VTE prophylaxis measure, scores of 1 through 9 corresponded to <60%, 60% to 64.9%, 65% to 69.9%, 70% to 74.9%, 75% to 79.9%, 80% to 84.9%, 85% to 89.9%, 90% to 94.9%, and 95% American College of Chest Physicians (ACCP)‐compliant VTE prophylaxis, respectively.[12, 20] Each provider was able to access the aggregated dashboard (showing the group mean) and his/her individualized dashboard using an individualized login and password for the institutional portal. This portal is used during the provider's workflow, including medical record review and order entry. Both a polygonal summary graphic (Figure 1A) and trend (Figure 1B) view of the dashboard were available to the provider. A comparison of the individual provider to the hospitalist group average was displayed (Figure 1A). At monthly program meetings, the dashboard, group results, and trends were discussed.
Venous Thromboembolism Prophylaxis Compliance
Our study was performed in a tertiary academic medical center with an approximately 20‐member hospitalist group (the precise membership varied over time), whose responsibilities include, among other clinical duties, staffing a 17‐bed general medicine unit with telemetry. The scope of diagnoses and acuity of patients admitted to the hospitalist service is similar to the housestaff services. Some hospitalist faculty serve both as hospitalist and nonhospitalist general medicine service team attendings, but the comparison groups were staffed by hospitalists for <20% of the time. For admissions, all hospitalists use a standardized general medicine admission order set that is integrated into the CPOE system (Sunrise Clinical Manager; Allscripts, Chicago, IL) and completed for all admitted patients. A mandatory VTE risk screen, which includes an assessment of VTE risk factors and pharmacological prophylaxis contraindications, must be completed by the ordering physician as part of this order set (Figure 2A). The system then prompts the provider with a risk‐appropriate VTE prophylaxis recommendation that the provider may subsequently order, including mechanical prophylaxis (Figure 2B). Based on ACCP VTE prevention guidelines, risk‐appropriate prophylaxis was determined using an electronic algorithm that categorized patients into risk categories based on the presence of major VTE risk factors (Figure 2A).[12, 15, 20] If none of these were present, the provider selected No major risk factors known. Both an assessment of current use of anticoagulation and a clinically high risk of bleeding were also included (Figure 2A). If none of these were present, the provider selected No contraindications known. This algorithm is published in detail elsewhere and has been shown to not increase major bleeding episodes.[12, 15] The VTE risk assessment, but not the VTE order itself, was a mandatory field. This allowed the physician discretion to choose among various pharmacological agents and mechanical mechanisms based on patient and physician preferences.
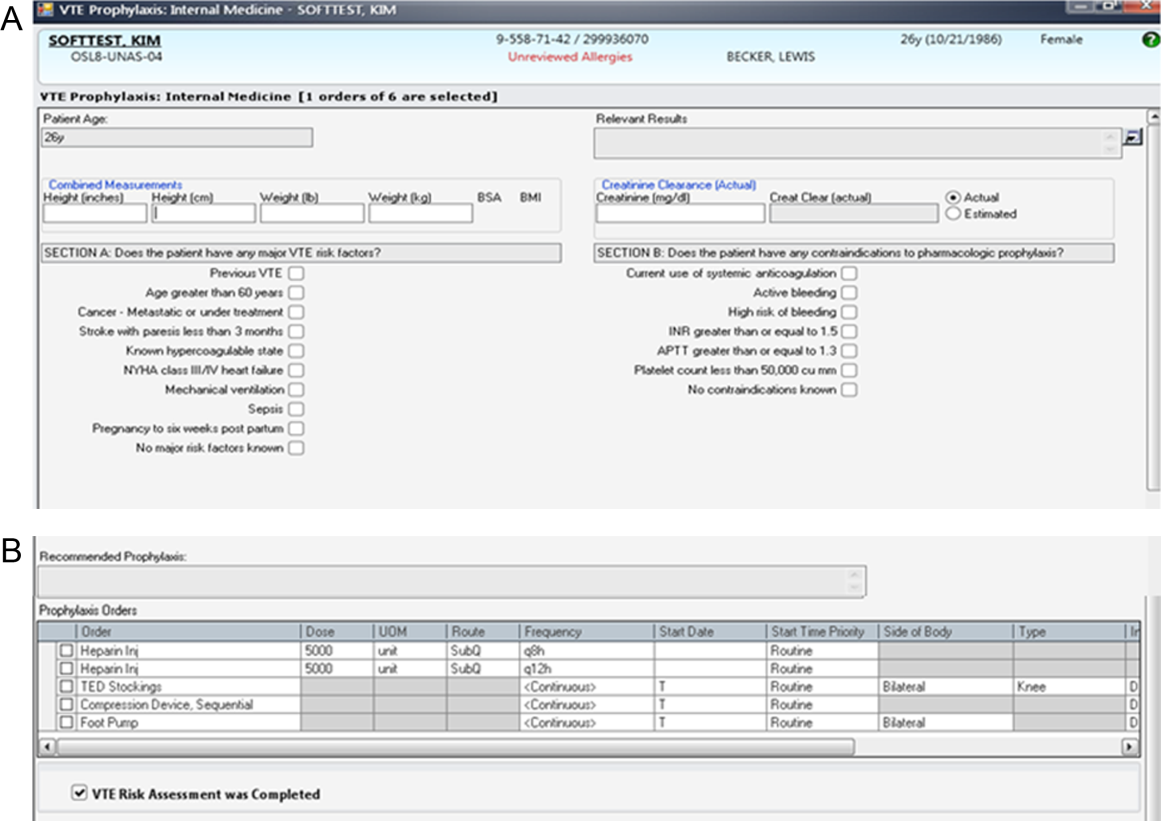
Compliance of risk‐appropriate VTE prophylaxis was determined 24 hours after the admission order set was completed using an automated electronic query of the CPOE system. Low molecular‐weight heparin prescription was included in the compliance algorithm as acceptable prophylaxis. Prescription of pharmacological VTE prophylaxis when a contraindication was present was considered noncompliant. The metric was assigned to the attending physician who billed for the first inpatient encounter.
Pay‐for‐Performance Program
In July 2011, a pay‐for‐performance program was added to the dashboard. All full‐time and part‐time hospitalists were eligible. The financial incentive was determined according to hospital priority and funds available. The VTE prophylaxis metric was prorated by clinical effort, with a maximum of $0.50 per work relative value unit (RVU). To optimize performance, a threshold of 80% compliance had to be surpassed before any payment was made. Progressively increasing percentages of the incentive were earned as compliance increased from 80% to 100%, corresponding to dashboard scores of 6, 7, 8, and 9: <80% (scores 1 to 5)=no payment; 80% to 84.9% (score 6)=$0.125 per RVU; 85% to 89.9% (score 7)=$0.25 per RVU; 90% to 94.9% (score 8)=$0.375 per RVU; and 95% (score 9)=$0.50 per RVU (maximum incentive). Payments were accrued quarterly and paid at the end of the fiscal year as a cumulative, separate performance supplement.
Individualized physician feedback through the dashboard was continued during the pay‐for‐performance period. Average hospitalist group compliance continued to be displayed on the electronic dashboard and was explicitly reviewed at monthly hospitalist meetings.
The VTE prophylaxis order set and data collection and analyses were approved by the Johns Hopkins Medicine Institutional Review Board. The dashboard and pay‐for‐performance program were initiated by the institution as part of a proof of concept quality improvement project.
Analysis
We examined all inpatient admissions to the hospitalist unit from 2008 to 2012. We included patients admitted to and discharged from the hospitalist unit and excluded patients transferred into/out of the unit and encounters with a length of stay <24 hours. VTE prophylaxis orders were queried from the CPOE system 24 hours after the patient was admitted to determine compliance.
After allowing for a run‐in period (2008), we analyzed the change in percent compliance for 3 periods: (1) CPOE‐based VTE order set alone (baseline [BASE], January 2009 to December 2010); (2) group and individual physician feedback using the dashboard (dashboard only [DASH], January to June 2011); and (3) dashboard tied to the pay‐for‐performance program (dashboard with pay‐for‐performance [P4P], July 2011 to December 2012). The CPOE‐based VTE order set was used during all 3 periods. We used the other medical services as a control to ensure that there were no temporal trends toward improved prophylaxis on a service without the intervention. VTE prophylaxis compliance was examined by calculating percent compliance using the same algorithm for the 4 resident‐staffed general medicine service teams at our institution, which utilized the same CPOE system but did not receive the dashboard or pay‐for‐performance interventions. We used locally weighted scatterplot smoothing, a locally weighted regression of percent compliance over time, to graphically display changes in group compliance over time.[21, 22]
We also performed linear regression to assess the rate of change in group compliance and included spline terms that allowed slope to vary for each of the 3 time periods.[23, 24] Clustered analysis accounted for potentially correlated serial measurements of compliance for an individual provider. A separate analysis examined the effect of provider turnover and individual provider improvement during each of the 3 periods. Tests of significance were 2‐sided, with an level of 0.05. Statistical analysis was performed using Stata 12.1 (StataCorp LP, College Station, TX).
RESULTS
Venous Thromboembolism Prophylaxis Compliance
We analyzed 3144 inpatient admissions by 38 hospitalists from 2009 to 2012. The 5 most frequent coded diagnoses were heart failure, acute kidney failure, syncope, pneumonia, and chest pain. Patients had a median length of stay of 3 days [interquartile range: 26]. During the dashboard‐only period, on average, providers improved in compliance by 4% (95% confidence interval [CI]: 35; P<0.001). With the addition of the pay‐for‐performance program, providers improved by an additional 4% (95% CI: 35; P<0.001). Group compliance significantly improved from 86% (95% CI: 8588) during the BASE period of the CPOE‐based VTE order set to 90% (95% CI: 8893) during the DASH period (P=0.01) and 94% (95% CI: 9396) during the subsequent P4P program (P=0.01) (Figure 3). Both inappropriate prophylaxis and lack of prophylaxis, when indicated, resulted in a non‐compliance rating. During the 3 periods, inappropriate prophylaxis decreased from 7.9% to 6.2% to 2.6% during the BASE, DASH, and subsequent P4P periods, respectively. Similarly, lack of prophylaxis when indicated decreased from 6.1% to 3.2% to 3.1% during the BASE, DASH, and subsequent P4P periods, respectively.
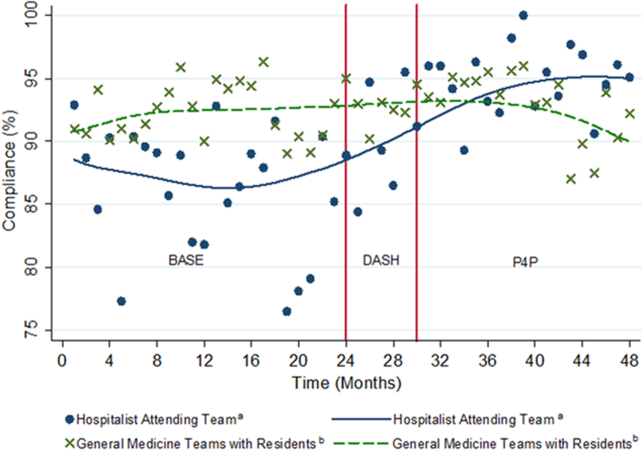
The average compliance of the 4 non‐hospitalist general medicine service teams was initially higher than that of the hospitalist service during the CPOE‐based VTE order set (90%) and DASH (92%) periods, but subsequently plateaued and was exceeded by the hospitalist service during the combined P4P (92%) period (Figure 3). However, there was no statistically significant difference between the general medicine service teams and hospitalist service during the DASH (P=0.15) and subsequent P4P (P=0.76) periods.
We also analyzed the rate of VTE prophylaxis compliance improvement (slope) with cut points at each time period transition (Figure 3). Risk‐appropriate VTE prophylaxis during the BASE period did not exhibit significant improvement as indicated by the slope (P=0.23) (Figure 3). In contrast, during the DASH period, VTE prophylaxis compliance significantly increased by 1.58% per month (95% CI: 0.41‐2.76; P=0.01). The addition of the P4P program, however, did not further significantly increase the rate of compliance (P=0.78).
A subgroup analysis restricted to the 19 providers present during all 3 periods was performed to assess for potential confounding from physician turnover. The percent compliance increased in a similar fashion: BASE period of CPOE‐based VTE order set, 85% (95% CI: 8386); DASH, 90% (95% CI: 8893); and P4P, 94% (95% CI: 9296).
Pay‐for‐Performance Program
Nineteen providers met the threshold for pay‐for‐performance (80% appropriate VTE prophylaxis), with 9 providers in the intermediate categories (80%94.9%) and 10 in the full incentive category (95%). The mean individual payout for the incentive was $633 (standard deviation 350), with a total disbursement of $12,029. The majority of payments (17 of 19) were under $1000.
DISCUSSION
A key component of healthcare reform has been value‐based purchasing, which emphasizes extrinsic motivation through the transparency of performance metrics and use of payment incentives to reward quality. Our study evaluates the impact of both extrinsic (payments) and intrinsic (professionalism and peer norms) motivation. It specifically attributed an individual performance metric, VTE prophylaxis, to an attending physician, provided both individualized and group feedback using an electronic dashboard, and incorporated a pay‐for‐performance program. Prescription of risk‐appropriate VTE prophylaxis significantly increased with the implementation of the dashboard and subsequent pay‐for performance program. The fastest rate of improvement occurred after the addition of the dashboard. Sensitivity analyses for provider turnover and comparisons to the general medicine services showed our results to be independent of a general trend of improvement, both at the provider and institutional levels.
Our prior studies demonstrated that order sets significantly improve performance, from a baseline compliance of risk‐appropriate VTE prophylaxis of 66% to 84%.[13, 15, 25] In the current study, compliance was relatively flat during the BASE period, which included these order sets. The greatest rate of continued improvement in compliance occurred during the DASH period, emphasizing both the importance of provider feedback and receptivity and adaptability in the prescribing behavior of hospitalists. Because the goal of a high‐reliability health system is for 100% of patients to receive recommended therapy, multiple approaches are necessary for success.
Nationally, benchmarks for performance measures continue to be raised, with the highest performers achieving above 95%.[26] Additional interventions, such as dashboards and pay‐for‐performance programs, supplement CPOE systems to achieve high reliability. In our study, the compliance rate during the baseline period, which included a CPOE‐based, clinical support‐enabled VTE order set, was 86%. Initially the compliance of the general medicine teams with residents exceeded that of the hospitalist attending teams, which may reflect a greater willingness of resident teams to comply with order sets and automated recommendations. This emphasizes the importance of continuous individual feedback and provider education at the attending physician level to enhance both guideline compliance and decrease provider care variation. Ultimately, with the addition of the dashboard and subsequent pay‐for‐performance program, compliance was increased to 90% and 94%, respectively. Although the major mechanism used by policymakers to improve quality of care is extrinsic motivation, this study demonstrates that intrinsic motivation through peer norms can enhance extrinsic efforts and may be more influential. Both of these programs, dashboards and pay‐for‐performance, may ultimately assist institutions in changing provider behavior and achieving these harder‐to‐achieve higher benchmarks.
We recognize that there are several limitations to our study. First, this is a single‐site program limited to an attending‐physician‐only service. There was strong data support and a defined CPOE algorithm for this initiative. Multi‐site studies will need to overcome the additional challenges of varying service structures and electronic medical record and provider order entry systems. Second, it is difficult to show actual changes in VTE events over time with appropriate prophylaxis. Although VTE prophylaxis is recommended for patients with VTE risk factors, there are conflicting findings about whether prophylaxis prevents VTE events in lower‐risk patients, and current studies suggest that most patients with VTE events are severely ill and develop VTE despite receiving prophylaxis.[27, 28, 29] Our study was underpowered to detect these potential differences in VTE rates, and although the algorithm has been shown to not increase bleeding rates, we did not measure bleeding rates during this study.[12, 15] Our institutional experience suggests that the majority of VTE events occur despite appropriate prophylaxis.[30] Also, VTE prophylaxis may be ordered, but intervening events, such as procedures and changes in risk status or patient refusal, may prevent patients from receiving appropriate prophylaxis.[31, 32] Similarly, hospitals with higher quality scores have higher VTE prophylaxis rates but worse risk‐adjusted VTE rates, which may result from increased surveillance for VTE, suggesting surveillance bias limits the usefulness of the VTE quality measure.[33, 34] Nevertheless, VTE prophylaxis remains a publicly reported Core Measure tied to financial incentives.[4, 5] Third, there may be an unmeasured factor specific to the hospitalist program, which could potentially account for an overall improvement in quality of care. Although the rate of increase in appropriate prophylaxis was not statistically significant during the baseline period, there did appear to be some improvement in prophylaxis toward the end of the period. However, there were no other VTE‐related provider feedback programs being simultaneously pursued during this study. VTE prophylaxis for the non‐hospitalist services showed a relatively stable, non‐increasing compliance rate for the general medical services. Although it was possible for successful residents to age into the hospitalist service, thereby improving rates of prophylaxis based on changes in group makeup, our subgroup analysis of the providers present throughout all phases of the study showed our results to be robust. Similarly, there may have been a cross‐contamination effect of hospitalist faculty who attended on both hospitalist and non‐hospitalist general medicine service teams. This, however, would attenuate any impact of the programs, and thus the effects may in fact be greater than reported. Fourth, establishment of both the dashboard and pay‐for‐performance program required significant institutional and program leadership and resources. To be successful, the dashboard must be in the provider's workflow, transparent, minimize reporter burden, use existing systems, and be actively fed back to providers, ideally those directly entering orders. Our greatest rate of improvement occurred during the feedback‐only phase of this study, emphasizing the importance of physician feedback, provider‐level accountability, and engagement. We suspect that the relatively modest pay‐for‐performance incentive served mainly as a means of engaging providers in self‐monitoring, rather than as a means to change behavior through true incentivization. Although we did not track individual physician views of the dashboard, we reinforced trends, deviations, and expectations at regularly scheduled meetings and provided feedback and patient‐level data to individual providers. Fifth, the design of the pay‐for‐performance program may have also influenced its effectiveness. These types of programs may be more effective when they provide frequent visible, small payments rather than one large payment, and when the payment is framed as a loss rather than a gain.[35] Finally, physician champions and consistent feedback through departmental meetings or visual displays may be required for program success. The initial resources to create the dashboard, continued maintenance and monitoring of performance, and payment of financial incentives all require institutional commitment. A partnership of physicians, program leaders, and institutional administrators is necessary for both initial and continued success.
To achieve performance goals and benchmarks, multiple strategies that combine extrinsic and intrinsic motivation are necessary. As shown by our study, the use of a dashboard and pay‐for‐performance can be tailored to an institution's goals, in line with national standards. The specific goal (risk‐appropriate VTE prophylaxis) and benchmarks (80%, 85%, 90%, 95%) can be individualized to a particular institution. For example, if readmission rates are above target, readmissions could be added as a dashboard metric. The specific benchmark would be determined by historical trends and administrative targets. Similarly, the overall financial incentives could be adjusted based on the financial resources available. Other process measures, such as influenza vaccination screening and administration, could also be targeted. For all of these objectives, continued provider feedback and engagement are critical for progressive success, especially to decrease variability in care at the attending physician level. Incorporating the value‐based purchasing philosophy from the Affordable Care Act, our study suggests that the combination of standardized order sets, real‐time dashboards, and physician‐level incentives may assist hospitals in achieving quality and safety benchmarks, especially at higher targets.
Acknowledgements
The authors thank Meir Gottlieb, BS, from Salar Inc. for data support; Murali Padmanaban, BS, from Johns Hopkins University for his assistance in linking the administrative billing data with real‐time physician orders; and Hsin‐Chieh Yeh, PhD, from the Bloomberg School of Public Health for her statistical advice and additional review. We also thank Mr. Ronald R. Peterson, President, Johns Hopkins Health System and Johns Hopkins Hospital, for providing funding support for the physician incentive payments.
Disclosures: Drs. Michtalik and Brotman had full access to all of the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Study concept and design: Drs. Michtalik, Streiff, Finkelstein, Pronovost, and Brotman. Acquisition of data: Drs. Michtalik, Streiff, Brotman and Mr. Carolan, Mr. Lau, Mrs. Durkin. Analysis and interpretation of data: Drs. Michtalik, Haut, Streiff, Brotman and Mr. Carolan, Mr. Lau. Drafting of the manuscript: Drs. Michtalik and Brotman. Critical revision of the manuscript for important intellectual content: Drs. Michtalik, Haut, Streiff, Finkelstein, Pronovost, Brotman and Mr. Carolan, Mr. Lau, Mrs. Durkin. Statistical analysis and supervision: Drs. Michtalik and Brotman. Obtaining funding: Drs. Streiff and Brotman. Technical support: Dr. Streiff and Mr. Carolan, Mr. Lau, Mrs. Durkin
This study was supported by a National Institutes of Health grant T32 HP10025‐17‐00 (Dr. Michtalik), the National Institutes of Health/Johns Hopkins Institute for Clinical and Translational Research KL2 Award 5KL2RR025006 (Dr. Michtalik), the Agency for Healthcare Research and Quality Mentored Clinical Scientist Development K08 Awards 1K08HS017952‐01 (Dr. Haut) and 1K08HS022331‐01A1 (Dr. Michtalik), and the Johns Hopkins Hospitalist Scholars Fund. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Dr. Haut receives royalties from Lippincott, Williams & Wilkins. Dr. Streiff has received research funding from Portola and Bristol Myers Squibb, honoraria for CME lectures from Sanofi‐Aventis and Ortho‐McNeil, consulted for Eisai, Daiichi‐Sankyo, Boerhinger‐Ingelheim, Janssen Healthcare, and Pfizer. Mr. Lau, Drs. Haut, Streiff, and Pronovost are supported by a contract from the Patient‐Centered Outcomes Research Institute (PCORI) titled Preventing Venous Thromboembolism: Empowering Patients and Enabling Patient‐Centered Care via Health Information Technology (CE‐12‐11‐4489). Dr. Brotman has received research support from Siemens Healthcare Diagnostics, Bristol‐Myers Squibb, the Agency for Healthcare Research and Quality, Centers for Medicare & Medicaid Services, the Amerigroup Corporation, and the Guerrieri Family Foundation. He has received honoraria from the Gerson Lehrman Group, the Dunn Group, and from Quantia Communications, and received royalties from McGraw‐Hill.
- Medicare Program, Centers for Medicare 76(88):26490–26547.
- . Quality meets finance: payments at risk with value‐based purchasing, readmission, and hospital‐acquired conditions force hospitalists to focus. Hospitalist. 2013;17(1):31.
- National Quality Forum. March 2009. Safe practices for better healthcare—2009 update. Available at: http://www.qualityforum.org/Publications/2009/03/Safe_Practices_for_Better_Healthcare%E2%80%932009_Update.aspx. Accessed November 1, 2014.
- Joint Commission on Accreditation of Healthcare Organizations. Approved: more options for hospital core measures. Jt Comm Perspect. 2009;29(4):1–6.
- Centers for Medicare 208(2):227–240.
- , . Thromboprophylaxis in nonsurgical patients. Hematology Am Soc Hematol Educ Program. 2012;2012:631–637.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2008;371(9610):387–394.
- , . Practices to prevent venous thromboembolism: a brief review. BMJ Qual Saf. 2014;23(3):187–195.
- , , , et al. Improving hospital venous thromboembolism prophylaxis with electronic decision support. J Hosp Med. 2013;8(3):115–120.
- , , . Innovative approaches to increase deep vein thrombosis prophylaxis rate resulting in a decrease in hospital‐acquired deep vein thrombosis at a tertiary‐care teaching hospital. J Hosp Med. 2008;3(2):148–155.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
- , , , et al. Improved prophylaxis and decreased rates of preventable harm with the use of a mandatory computerized clinical decision support tool for prophylaxis for venous thromboembolism in trauma. Arch Surg. 2012;147(10):901–907.
- , . Designing and implementing effective venous thromboembolism prevention protocols: lessons from collaborative efforts. J Thromb Thrombolysis. 2010;29(2):159–166.
- , , , et al. Impact of a venous thromboembolism prophylaxis "smart order set": improved compliance, fewer events. Am J Hematol. 2013;88(7):545–549.
- , . Improving adherence to venous thromoembolism prophylaxis using multiple interventions. BMJ. 2012;344:e3935.
- Health Resources and Services Administration of the U.S. Department of Health and Human Services. Managing data for performance improvement. Available at: http://www.hrsa.gov/quality/toolbox/methodology/performanceimprovement/part2.html. Accessed December 18, 2014.
- , . Improving patient safety by taking systems seriously. JAMA. 2008;299(4):445–447.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , , , et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines (8th edition). Chest. 2008;133(6 suppl):381S–453S.
- . Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74(368):829–836.
- , . Locally weighted regression: An approach to regression analysis by local fitting. J Am Stat Assoc. 1988;83(403):596–610.
- , , , . Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. 2nd ed. New York, NY: Springer; 2012.
- . Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer‐Verlag; 2001.
- , , , et al. Eliminating healthcare disparities via mandatory clinical decision support: the venous thromboembolism (VTE) example [published online ahead of print November 4, 2014]. Med Care. doi: 10.1097/MLR.0000000000000251.
- Joint Commission. Improving America's hospitals: the Joint Commission's annual report on quality and safety. 2012. Available at: http://www.jointcommission.org/assets/1/18/TJC_Annual_Report_2012.pdf. Accessed September 8, 2013.
- , , , et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577–1584.
- , , , et al. Incidence of hospital‐acquired venous thromboembolic codes in medical patients hospitalized in academic medical centers. J Hosp Med. 2014;9(4):221–225.
- , , , , . No association between hospital‐reported perioperative venous thromboembolism prophylaxis and outcome rates in publicly reported data. JAMA Surg. 2014;149(4):400–401.
- , , , , . Linking processes and outcomes: a key strategy to prevent and report harm from venous thromboembolism in surgical patients. JAMA Surg. 2013;148(3):299–300.
- , , , et al. Patterns of non‐administration of ordered doses of venous thromboembolism prophylaxis: implications for novel intervention strategies. PLoS One. 2013;8(6):e66311.
- , , , et al. Nonadministration of thromboprophylaxis in hospitalized patients with HIV: a missed opportunity for prevention? J Hosp Med. 2014;9(4):215–220.
- , , , et al. Evaluation of surveillance bias and the validity of the venous thromboembolism quality measure. JAMA. 2013;310(14):1482–1489.
- , . Surveillance bias in outcomes reporting. JAMA. 2011;305(23):2462–2463.
- . Pay for performance in health care: an international overview of initiatives. Med Care Res Rev. 2012;69(3):251–276.
The Affordable Care Act explicitly outlines improving the value of healthcare by increasing quality and decreasing costs. It emphasizes value‐based purchasing, the transparency of performance metrics, and the use of payment incentives to reward quality.[1, 2] Venous thromboembolism (VTE) prophylaxis is one of these publicly reported performance measures. The National Quality Forum recommends that each patient be evaluated on hospital admission and during their hospitalization for VTE risk level and for appropriate thromboprophylaxis to be used, if required.[3] Similarly, the Joint Commission includes appropriate VTE prophylaxis in its Core Measures.[4] Patient experience and performance metrics, including VTE prophylaxis, constitute the hospital value‐based purchasing (VBP) component of healthcare reform.[5] For a hypothetical 327‐bed hospital, an estimated $1.7 million of a hospital's inpatient payments from Medicare will be at risk from VBP alone.[2]
VTE prophylaxis is a common target of quality improvement projects. Effective, safe, and cost‐effective measures to prevent VTE exist, including pharmacologic and mechanical prophylaxis.[6, 7] Despite these measures, compliance rates are often below 50%.[8] Different interventions have been pursued to ensure appropriate VTE prophylaxis, including computerized provider order entry (CPOE), electronic alerts, mandatory VTE risk assessment and prophylaxis, and provider education campaigns.[9] Recent studies show that CPOE systems with mandatory fields can increase VTE prophylaxis rates to above 80%, yet the goal of a high reliability health system is for 100% of patients to receive recommended therapy.[10, 11, 12, 13, 14, 15] Interventions to improve prophylaxis rates that have included multiple strategies, such as computerized order sets, feedback, and education, have been the most effective, increasing compliance to above 90%.[9, 11, 16] These systems can be enhanced with additional interventions such as providing individualized provider education and feedback, understanding of work flow, and ensuring patients receive the prescribed therapies.[12] For example, a physician dashboard could be employed to provide a snapshot and historical trend of key performance indicators using graphical displays and indicators.[17]
Dashboards and pay‐for‐performance programs have been increasingly used to increase the visibility of these metrics, provide feedback, visually display benchmarks and goals, and proactively monitor for achievements and setbacks.[18] Although these strategies are often addressed at departmental (or greater) levels, applying them at the level of the individual provider may assist hospitals in reducing preventable harm and achieving safety and quality goals, especially at higher benchmarks. With their expanding role, hospitalists provide a key opportunity to lead improvement efforts and to study the impact of dashboards and pay‐for performance at the provider level to achieve VTE prophylaxis performance targets. Hospitalists are often the front‐line provider for inpatients and deliver up to 70% of inpatient general medical services.[19] The objective of our study was to evaluate the impact of providing individual provider feedback and employing a pay‐for‐performance program on baseline performance of VTE prophylaxis among hospitalists. We hypothesized that performance feedback through the use of a dashboard would increase appropriate VTE prophylaxis, and this effect would be further augmented by incorporation of a pay‐for‐performance program.
METHODS
Hospitalist Dashboard
In 2010, hospitalist program leaders met with hospital administrators to create a hospitalist dashboard that would provide regularly updated summaries of performance measures for individual hospitalists. The final set of metrics identified included appropriate VTE prophylaxis, length of stay, patients discharged per day, discharges before 3 pm, depth of coding, patient satisfaction, readmissions, communication with the primary care provider, and time to signature for discharge summaries (Figure 1A). The dashboard was introduced at a general hospitalist meeting during which its purpose, methodology, and accessibility were described; it was subsequently implemented in January 2011.

Benchmarks were established for each metric, standardized to establish a scale ranging from 1 through 9, and incorporated into the dashboard (Figure 1A). Higher scores (creating a larger geometric shape) were desirable. For the VTE prophylaxis measure, scores of 1 through 9 corresponded to <60%, 60% to 64.9%, 65% to 69.9%, 70% to 74.9%, 75% to 79.9%, 80% to 84.9%, 85% to 89.9%, 90% to 94.9%, and 95% American College of Chest Physicians (ACCP)‐compliant VTE prophylaxis, respectively.[12, 20] Each provider was able to access the aggregated dashboard (showing the group mean) and his/her individualized dashboard using an individualized login and password for the institutional portal. This portal is used during the provider's workflow, including medical record review and order entry. Both a polygonal summary graphic (Figure 1A) and trend (Figure 1B) view of the dashboard were available to the provider. A comparison of the individual provider to the hospitalist group average was displayed (Figure 1A). At monthly program meetings, the dashboard, group results, and trends were discussed.
Venous Thromboembolism Prophylaxis Compliance
Our study was performed in a tertiary academic medical center with an approximately 20‐member hospitalist group (the precise membership varied over time), whose responsibilities include, among other clinical duties, staffing a 17‐bed general medicine unit with telemetry. The scope of diagnoses and acuity of patients admitted to the hospitalist service is similar to the housestaff services. Some hospitalist faculty serve both as hospitalist and nonhospitalist general medicine service team attendings, but the comparison groups were staffed by hospitalists for <20% of the time. For admissions, all hospitalists use a standardized general medicine admission order set that is integrated into the CPOE system (Sunrise Clinical Manager; Allscripts, Chicago, IL) and completed for all admitted patients. A mandatory VTE risk screen, which includes an assessment of VTE risk factors and pharmacological prophylaxis contraindications, must be completed by the ordering physician as part of this order set (Figure 2A). The system then prompts the provider with a risk‐appropriate VTE prophylaxis recommendation that the provider may subsequently order, including mechanical prophylaxis (Figure 2B). Based on ACCP VTE prevention guidelines, risk‐appropriate prophylaxis was determined using an electronic algorithm that categorized patients into risk categories based on the presence of major VTE risk factors (Figure 2A).[12, 15, 20] If none of these were present, the provider selected No major risk factors known. Both an assessment of current use of anticoagulation and a clinically high risk of bleeding were also included (Figure 2A). If none of these were present, the provider selected No contraindications known. This algorithm is published in detail elsewhere and has been shown to not increase major bleeding episodes.[12, 15] The VTE risk assessment, but not the VTE order itself, was a mandatory field. This allowed the physician discretion to choose among various pharmacological agents and mechanical mechanisms based on patient and physician preferences.

Compliance of risk‐appropriate VTE prophylaxis was determined 24 hours after the admission order set was completed using an automated electronic query of the CPOE system. Low molecular‐weight heparin prescription was included in the compliance algorithm as acceptable prophylaxis. Prescription of pharmacological VTE prophylaxis when a contraindication was present was considered noncompliant. The metric was assigned to the attending physician who billed for the first inpatient encounter.
Pay‐for‐Performance Program
In July 2011, a pay‐for‐performance program was added to the dashboard. All full‐time and part‐time hospitalists were eligible. The financial incentive was determined according to hospital priority and funds available. The VTE prophylaxis metric was prorated by clinical effort, with a maximum of $0.50 per work relative value unit (RVU). To optimize performance, a threshold of 80% compliance had to be surpassed before any payment was made. Progressively increasing percentages of the incentive were earned as compliance increased from 80% to 100%, corresponding to dashboard scores of 6, 7, 8, and 9: <80% (scores 1 to 5)=no payment; 80% to 84.9% (score 6)=$0.125 per RVU; 85% to 89.9% (score 7)=$0.25 per RVU; 90% to 94.9% (score 8)=$0.375 per RVU; and 95% (score 9)=$0.50 per RVU (maximum incentive). Payments were accrued quarterly and paid at the end of the fiscal year as a cumulative, separate performance supplement.
Individualized physician feedback through the dashboard was continued during the pay‐for‐performance period. Average hospitalist group compliance continued to be displayed on the electronic dashboard and was explicitly reviewed at monthly hospitalist meetings.
The VTE prophylaxis order set and data collection and analyses were approved by the Johns Hopkins Medicine Institutional Review Board. The dashboard and pay‐for‐performance program were initiated by the institution as part of a proof of concept quality improvement project.
Analysis
We examined all inpatient admissions to the hospitalist unit from 2008 to 2012. We included patients admitted to and discharged from the hospitalist unit and excluded patients transferred into/out of the unit and encounters with a length of stay <24 hours. VTE prophylaxis orders were queried from the CPOE system 24 hours after the patient was admitted to determine compliance.
After allowing for a run‐in period (2008), we analyzed the change in percent compliance for 3 periods: (1) CPOE‐based VTE order set alone (baseline [BASE], January 2009 to December 2010); (2) group and individual physician feedback using the dashboard (dashboard only [DASH], January to June 2011); and (3) dashboard tied to the pay‐for‐performance program (dashboard with pay‐for‐performance [P4P], July 2011 to December 2012). The CPOE‐based VTE order set was used during all 3 periods. We used the other medical services as a control to ensure that there were no temporal trends toward improved prophylaxis on a service without the intervention. VTE prophylaxis compliance was examined by calculating percent compliance using the same algorithm for the 4 resident‐staffed general medicine service teams at our institution, which utilized the same CPOE system but did not receive the dashboard or pay‐for‐performance interventions. We used locally weighted scatterplot smoothing, a locally weighted regression of percent compliance over time, to graphically display changes in group compliance over time.[21, 22]
We also performed linear regression to assess the rate of change in group compliance and included spline terms that allowed slope to vary for each of the 3 time periods.[23, 24] Clustered analysis accounted for potentially correlated serial measurements of compliance for an individual provider. A separate analysis examined the effect of provider turnover and individual provider improvement during each of the 3 periods. Tests of significance were 2‐sided, with an level of 0.05. Statistical analysis was performed using Stata 12.1 (StataCorp LP, College Station, TX).
RESULTS
Venous Thromboembolism Prophylaxis Compliance
We analyzed 3144 inpatient admissions by 38 hospitalists from 2009 to 2012. The 5 most frequent coded diagnoses were heart failure, acute kidney failure, syncope, pneumonia, and chest pain. Patients had a median length of stay of 3 days [interquartile range: 26]. During the dashboard‐only period, on average, providers improved in compliance by 4% (95% confidence interval [CI]: 35; P<0.001). With the addition of the pay‐for‐performance program, providers improved by an additional 4% (95% CI: 35; P<0.001). Group compliance significantly improved from 86% (95% CI: 8588) during the BASE period of the CPOE‐based VTE order set to 90% (95% CI: 8893) during the DASH period (P=0.01) and 94% (95% CI: 9396) during the subsequent P4P program (P=0.01) (Figure 3). Both inappropriate prophylaxis and lack of prophylaxis, when indicated, resulted in a non‐compliance rating. During the 3 periods, inappropriate prophylaxis decreased from 7.9% to 6.2% to 2.6% during the BASE, DASH, and subsequent P4P periods, respectively. Similarly, lack of prophylaxis when indicated decreased from 6.1% to 3.2% to 3.1% during the BASE, DASH, and subsequent P4P periods, respectively.

The average compliance of the 4 non‐hospitalist general medicine service teams was initially higher than that of the hospitalist service during the CPOE‐based VTE order set (90%) and DASH (92%) periods, but subsequently plateaued and was exceeded by the hospitalist service during the combined P4P (92%) period (Figure 3). However, there was no statistically significant difference between the general medicine service teams and hospitalist service during the DASH (P=0.15) and subsequent P4P (P=0.76) periods.
We also analyzed the rate of VTE prophylaxis compliance improvement (slope) with cut points at each time period transition (Figure 3). Risk‐appropriate VTE prophylaxis during the BASE period did not exhibit significant improvement as indicated by the slope (P=0.23) (Figure 3). In contrast, during the DASH period, VTE prophylaxis compliance significantly increased by 1.58% per month (95% CI: 0.41‐2.76; P=0.01). The addition of the P4P program, however, did not further significantly increase the rate of compliance (P=0.78).
A subgroup analysis restricted to the 19 providers present during all 3 periods was performed to assess for potential confounding from physician turnover. The percent compliance increased in a similar fashion: BASE period of CPOE‐based VTE order set, 85% (95% CI: 8386); DASH, 90% (95% CI: 8893); and P4P, 94% (95% CI: 9296).
Pay‐for‐Performance Program
Nineteen providers met the threshold for pay‐for‐performance (80% appropriate VTE prophylaxis), with 9 providers in the intermediate categories (80%94.9%) and 10 in the full incentive category (95%). The mean individual payout for the incentive was $633 (standard deviation 350), with a total disbursement of $12,029. The majority of payments (17 of 19) were under $1000.
DISCUSSION
A key component of healthcare reform has been value‐based purchasing, which emphasizes extrinsic motivation through the transparency of performance metrics and use of payment incentives to reward quality. Our study evaluates the impact of both extrinsic (payments) and intrinsic (professionalism and peer norms) motivation. It specifically attributed an individual performance metric, VTE prophylaxis, to an attending physician, provided both individualized and group feedback using an electronic dashboard, and incorporated a pay‐for‐performance program. Prescription of risk‐appropriate VTE prophylaxis significantly increased with the implementation of the dashboard and subsequent pay‐for performance program. The fastest rate of improvement occurred after the addition of the dashboard. Sensitivity analyses for provider turnover and comparisons to the general medicine services showed our results to be independent of a general trend of improvement, both at the provider and institutional levels.
Our prior studies demonstrated that order sets significantly improve performance, from a baseline compliance of risk‐appropriate VTE prophylaxis of 66% to 84%.[13, 15, 25] In the current study, compliance was relatively flat during the BASE period, which included these order sets. The greatest rate of continued improvement in compliance occurred during the DASH period, emphasizing both the importance of provider feedback and receptivity and adaptability in the prescribing behavior of hospitalists. Because the goal of a high‐reliability health system is for 100% of patients to receive recommended therapy, multiple approaches are necessary for success.
Nationally, benchmarks for performance measures continue to be raised, with the highest performers achieving above 95%.[26] Additional interventions, such as dashboards and pay‐for‐performance programs, supplement CPOE systems to achieve high reliability. In our study, the compliance rate during the baseline period, which included a CPOE‐based, clinical support‐enabled VTE order set, was 86%. Initially the compliance of the general medicine teams with residents exceeded that of the hospitalist attending teams, which may reflect a greater willingness of resident teams to comply with order sets and automated recommendations. This emphasizes the importance of continuous individual feedback and provider education at the attending physician level to enhance both guideline compliance and decrease provider care variation. Ultimately, with the addition of the dashboard and subsequent pay‐for‐performance program, compliance was increased to 90% and 94%, respectively. Although the major mechanism used by policymakers to improve quality of care is extrinsic motivation, this study demonstrates that intrinsic motivation through peer norms can enhance extrinsic efforts and may be more influential. Both of these programs, dashboards and pay‐for‐performance, may ultimately assist institutions in changing provider behavior and achieving these harder‐to‐achieve higher benchmarks.
We recognize that there are several limitations to our study. First, this is a single‐site program limited to an attending‐physician‐only service. There was strong data support and a defined CPOE algorithm for this initiative. Multi‐site studies will need to overcome the additional challenges of varying service structures and electronic medical record and provider order entry systems. Second, it is difficult to show actual changes in VTE events over time with appropriate prophylaxis. Although VTE prophylaxis is recommended for patients with VTE risk factors, there are conflicting findings about whether prophylaxis prevents VTE events in lower‐risk patients, and current studies suggest that most patients with VTE events are severely ill and develop VTE despite receiving prophylaxis.[27, 28, 29] Our study was underpowered to detect these potential differences in VTE rates, and although the algorithm has been shown to not increase bleeding rates, we did not measure bleeding rates during this study.[12, 15] Our institutional experience suggests that the majority of VTE events occur despite appropriate prophylaxis.[30] Also, VTE prophylaxis may be ordered, but intervening events, such as procedures and changes in risk status or patient refusal, may prevent patients from receiving appropriate prophylaxis.[31, 32] Similarly, hospitals with higher quality scores have higher VTE prophylaxis rates but worse risk‐adjusted VTE rates, which may result from increased surveillance for VTE, suggesting surveillance bias limits the usefulness of the VTE quality measure.[33, 34] Nevertheless, VTE prophylaxis remains a publicly reported Core Measure tied to financial incentives.[4, 5] Third, there may be an unmeasured factor specific to the hospitalist program, which could potentially account for an overall improvement in quality of care. Although the rate of increase in appropriate prophylaxis was not statistically significant during the baseline period, there did appear to be some improvement in prophylaxis toward the end of the period. However, there were no other VTE‐related provider feedback programs being simultaneously pursued during this study. VTE prophylaxis for the non‐hospitalist services showed a relatively stable, non‐increasing compliance rate for the general medical services. Although it was possible for successful residents to age into the hospitalist service, thereby improving rates of prophylaxis based on changes in group makeup, our subgroup analysis of the providers present throughout all phases of the study showed our results to be robust. Similarly, there may have been a cross‐contamination effect of hospitalist faculty who attended on both hospitalist and non‐hospitalist general medicine service teams. This, however, would attenuate any impact of the programs, and thus the effects may in fact be greater than reported. Fourth, establishment of both the dashboard and pay‐for‐performance program required significant institutional and program leadership and resources. To be successful, the dashboard must be in the provider's workflow, transparent, minimize reporter burden, use existing systems, and be actively fed back to providers, ideally those directly entering orders. Our greatest rate of improvement occurred during the feedback‐only phase of this study, emphasizing the importance of physician feedback, provider‐level accountability, and engagement. We suspect that the relatively modest pay‐for‐performance incentive served mainly as a means of engaging providers in self‐monitoring, rather than as a means to change behavior through true incentivization. Although we did not track individual physician views of the dashboard, we reinforced trends, deviations, and expectations at regularly scheduled meetings and provided feedback and patient‐level data to individual providers. Fifth, the design of the pay‐for‐performance program may have also influenced its effectiveness. These types of programs may be more effective when they provide frequent visible, small payments rather than one large payment, and when the payment is framed as a loss rather than a gain.[35] Finally, physician champions and consistent feedback through departmental meetings or visual displays may be required for program success. The initial resources to create the dashboard, continued maintenance and monitoring of performance, and payment of financial incentives all require institutional commitment. A partnership of physicians, program leaders, and institutional administrators is necessary for both initial and continued success.
To achieve performance goals and benchmarks, multiple strategies that combine extrinsic and intrinsic motivation are necessary. As shown by our study, the use of a dashboard and pay‐for‐performance can be tailored to an institution's goals, in line with national standards. The specific goal (risk‐appropriate VTE prophylaxis) and benchmarks (80%, 85%, 90%, 95%) can be individualized to a particular institution. For example, if readmission rates are above target, readmissions could be added as a dashboard metric. The specific benchmark would be determined by historical trends and administrative targets. Similarly, the overall financial incentives could be adjusted based on the financial resources available. Other process measures, such as influenza vaccination screening and administration, could also be targeted. For all of these objectives, continued provider feedback and engagement are critical for progressive success, especially to decrease variability in care at the attending physician level. Incorporating the value‐based purchasing philosophy from the Affordable Care Act, our study suggests that the combination of standardized order sets, real‐time dashboards, and physician‐level incentives may assist hospitals in achieving quality and safety benchmarks, especially at higher targets.
Acknowledgements
The authors thank Meir Gottlieb, BS, from Salar Inc. for data support; Murali Padmanaban, BS, from Johns Hopkins University for his assistance in linking the administrative billing data with real‐time physician orders; and Hsin‐Chieh Yeh, PhD, from the Bloomberg School of Public Health for her statistical advice and additional review. We also thank Mr. Ronald R. Peterson, President, Johns Hopkins Health System and Johns Hopkins Hospital, for providing funding support for the physician incentive payments.
Disclosures: Drs. Michtalik and Brotman had full access to all of the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Study concept and design: Drs. Michtalik, Streiff, Finkelstein, Pronovost, and Brotman. Acquisition of data: Drs. Michtalik, Streiff, Brotman and Mr. Carolan, Mr. Lau, Mrs. Durkin. Analysis and interpretation of data: Drs. Michtalik, Haut, Streiff, Brotman and Mr. Carolan, Mr. Lau. Drafting of the manuscript: Drs. Michtalik and Brotman. Critical revision of the manuscript for important intellectual content: Drs. Michtalik, Haut, Streiff, Finkelstein, Pronovost, Brotman and Mr. Carolan, Mr. Lau, Mrs. Durkin. Statistical analysis and supervision: Drs. Michtalik and Brotman. Obtaining funding: Drs. Streiff and Brotman. Technical support: Dr. Streiff and Mr. Carolan, Mr. Lau, Mrs. Durkin
This study was supported by a National Institutes of Health grant T32 HP10025‐17‐00 (Dr. Michtalik), the National Institutes of Health/Johns Hopkins Institute for Clinical and Translational Research KL2 Award 5KL2RR025006 (Dr. Michtalik), the Agency for Healthcare Research and Quality Mentored Clinical Scientist Development K08 Awards 1K08HS017952‐01 (Dr. Haut) and 1K08HS022331‐01A1 (Dr. Michtalik), and the Johns Hopkins Hospitalist Scholars Fund. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Dr. Haut receives royalties from Lippincott, Williams & Wilkins. Dr. Streiff has received research funding from Portola and Bristol Myers Squibb, honoraria for CME lectures from Sanofi‐Aventis and Ortho‐McNeil, consulted for Eisai, Daiichi‐Sankyo, Boerhinger‐Ingelheim, Janssen Healthcare, and Pfizer. Mr. Lau, Drs. Haut, Streiff, and Pronovost are supported by a contract from the Patient‐Centered Outcomes Research Institute (PCORI) titled Preventing Venous Thromboembolism: Empowering Patients and Enabling Patient‐Centered Care via Health Information Technology (CE‐12‐11‐4489). Dr. Brotman has received research support from Siemens Healthcare Diagnostics, Bristol‐Myers Squibb, the Agency for Healthcare Research and Quality, Centers for Medicare & Medicaid Services, the Amerigroup Corporation, and the Guerrieri Family Foundation. He has received honoraria from the Gerson Lehrman Group, the Dunn Group, and from Quantia Communications, and received royalties from McGraw‐Hill.
The Affordable Care Act explicitly outlines improving the value of healthcare by increasing quality and decreasing costs. It emphasizes value‐based purchasing, the transparency of performance metrics, and the use of payment incentives to reward quality.[1, 2] Venous thromboembolism (VTE) prophylaxis is one of these publicly reported performance measures. The National Quality Forum recommends that each patient be evaluated on hospital admission and during their hospitalization for VTE risk level and for appropriate thromboprophylaxis to be used, if required.[3] Similarly, the Joint Commission includes appropriate VTE prophylaxis in its Core Measures.[4] Patient experience and performance metrics, including VTE prophylaxis, constitute the hospital value‐based purchasing (VBP) component of healthcare reform.[5] For a hypothetical 327‐bed hospital, an estimated $1.7 million of a hospital's inpatient payments from Medicare will be at risk from VBP alone.[2]
VTE prophylaxis is a common target of quality improvement projects. Effective, safe, and cost‐effective measures to prevent VTE exist, including pharmacologic and mechanical prophylaxis.[6, 7] Despite these measures, compliance rates are often below 50%.[8] Different interventions have been pursued to ensure appropriate VTE prophylaxis, including computerized provider order entry (CPOE), electronic alerts, mandatory VTE risk assessment and prophylaxis, and provider education campaigns.[9] Recent studies show that CPOE systems with mandatory fields can increase VTE prophylaxis rates to above 80%, yet the goal of a high reliability health system is for 100% of patients to receive recommended therapy.[10, 11, 12, 13, 14, 15] Interventions to improve prophylaxis rates that have included multiple strategies, such as computerized order sets, feedback, and education, have been the most effective, increasing compliance to above 90%.[9, 11, 16] These systems can be enhanced with additional interventions such as providing individualized provider education and feedback, understanding of work flow, and ensuring patients receive the prescribed therapies.[12] For example, a physician dashboard could be employed to provide a snapshot and historical trend of key performance indicators using graphical displays and indicators.[17]
Dashboards and pay‐for‐performance programs have been increasingly used to increase the visibility of these metrics, provide feedback, visually display benchmarks and goals, and proactively monitor for achievements and setbacks.[18] Although these strategies are often addressed at departmental (or greater) levels, applying them at the level of the individual provider may assist hospitals in reducing preventable harm and achieving safety and quality goals, especially at higher benchmarks. With their expanding role, hospitalists provide a key opportunity to lead improvement efforts and to study the impact of dashboards and pay‐for performance at the provider level to achieve VTE prophylaxis performance targets. Hospitalists are often the front‐line provider for inpatients and deliver up to 70% of inpatient general medical services.[19] The objective of our study was to evaluate the impact of providing individual provider feedback and employing a pay‐for‐performance program on baseline performance of VTE prophylaxis among hospitalists. We hypothesized that performance feedback through the use of a dashboard would increase appropriate VTE prophylaxis, and this effect would be further augmented by incorporation of a pay‐for‐performance program.
METHODS
Hospitalist Dashboard
In 2010, hospitalist program leaders met with hospital administrators to create a hospitalist dashboard that would provide regularly updated summaries of performance measures for individual hospitalists. The final set of metrics identified included appropriate VTE prophylaxis, length of stay, patients discharged per day, discharges before 3 pm, depth of coding, patient satisfaction, readmissions, communication with the primary care provider, and time to signature for discharge summaries (Figure 1A). The dashboard was introduced at a general hospitalist meeting during which its purpose, methodology, and accessibility were described; it was subsequently implemented in January 2011.

Benchmarks were established for each metric, standardized to establish a scale ranging from 1 through 9, and incorporated into the dashboard (Figure 1A). Higher scores (creating a larger geometric shape) were desirable. For the VTE prophylaxis measure, scores of 1 through 9 corresponded to <60%, 60% to 64.9%, 65% to 69.9%, 70% to 74.9%, 75% to 79.9%, 80% to 84.9%, 85% to 89.9%, 90% to 94.9%, and 95% American College of Chest Physicians (ACCP)‐compliant VTE prophylaxis, respectively.[12, 20] Each provider was able to access the aggregated dashboard (showing the group mean) and his/her individualized dashboard using an individualized login and password for the institutional portal. This portal is used during the provider's workflow, including medical record review and order entry. Both a polygonal summary graphic (Figure 1A) and trend (Figure 1B) view of the dashboard were available to the provider. A comparison of the individual provider to the hospitalist group average was displayed (Figure 1A). At monthly program meetings, the dashboard, group results, and trends were discussed.
Venous Thromboembolism Prophylaxis Compliance
Our study was performed in a tertiary academic medical center with an approximately 20‐member hospitalist group (the precise membership varied over time), whose responsibilities include, among other clinical duties, staffing a 17‐bed general medicine unit with telemetry. The scope of diagnoses and acuity of patients admitted to the hospitalist service is similar to the housestaff services. Some hospitalist faculty serve both as hospitalist and nonhospitalist general medicine service team attendings, but the comparison groups were staffed by hospitalists for <20% of the time. For admissions, all hospitalists use a standardized general medicine admission order set that is integrated into the CPOE system (Sunrise Clinical Manager; Allscripts, Chicago, IL) and completed for all admitted patients. A mandatory VTE risk screen, which includes an assessment of VTE risk factors and pharmacological prophylaxis contraindications, must be completed by the ordering physician as part of this order set (Figure 2A). The system then prompts the provider with a risk‐appropriate VTE prophylaxis recommendation that the provider may subsequently order, including mechanical prophylaxis (Figure 2B). Based on ACCP VTE prevention guidelines, risk‐appropriate prophylaxis was determined using an electronic algorithm that categorized patients into risk categories based on the presence of major VTE risk factors (Figure 2A).[12, 15, 20] If none of these were present, the provider selected No major risk factors known. Both an assessment of current use of anticoagulation and a clinically high risk of bleeding were also included (Figure 2A). If none of these were present, the provider selected No contraindications known. This algorithm is published in detail elsewhere and has been shown to not increase major bleeding episodes.[12, 15] The VTE risk assessment, but not the VTE order itself, was a mandatory field. This allowed the physician discretion to choose among various pharmacological agents and mechanical mechanisms based on patient and physician preferences.

Compliance of risk‐appropriate VTE prophylaxis was determined 24 hours after the admission order set was completed using an automated electronic query of the CPOE system. Low molecular‐weight heparin prescription was included in the compliance algorithm as acceptable prophylaxis. Prescription of pharmacological VTE prophylaxis when a contraindication was present was considered noncompliant. The metric was assigned to the attending physician who billed for the first inpatient encounter.
Pay‐for‐Performance Program
In July 2011, a pay‐for‐performance program was added to the dashboard. All full‐time and part‐time hospitalists were eligible. The financial incentive was determined according to hospital priority and funds available. The VTE prophylaxis metric was prorated by clinical effort, with a maximum of $0.50 per work relative value unit (RVU). To optimize performance, a threshold of 80% compliance had to be surpassed before any payment was made. Progressively increasing percentages of the incentive were earned as compliance increased from 80% to 100%, corresponding to dashboard scores of 6, 7, 8, and 9: <80% (scores 1 to 5)=no payment; 80% to 84.9% (score 6)=$0.125 per RVU; 85% to 89.9% (score 7)=$0.25 per RVU; 90% to 94.9% (score 8)=$0.375 per RVU; and 95% (score 9)=$0.50 per RVU (maximum incentive). Payments were accrued quarterly and paid at the end of the fiscal year as a cumulative, separate performance supplement.
Individualized physician feedback through the dashboard was continued during the pay‐for‐performance period. Average hospitalist group compliance continued to be displayed on the electronic dashboard and was explicitly reviewed at monthly hospitalist meetings.
The VTE prophylaxis order set and data collection and analyses were approved by the Johns Hopkins Medicine Institutional Review Board. The dashboard and pay‐for‐performance program were initiated by the institution as part of a proof of concept quality improvement project.
Analysis
We examined all inpatient admissions to the hospitalist unit from 2008 to 2012. We included patients admitted to and discharged from the hospitalist unit and excluded patients transferred into/out of the unit and encounters with a length of stay <24 hours. VTE prophylaxis orders were queried from the CPOE system 24 hours after the patient was admitted to determine compliance.
After allowing for a run‐in period (2008), we analyzed the change in percent compliance for 3 periods: (1) CPOE‐based VTE order set alone (baseline [BASE], January 2009 to December 2010); (2) group and individual physician feedback using the dashboard (dashboard only [DASH], January to June 2011); and (3) dashboard tied to the pay‐for‐performance program (dashboard with pay‐for‐performance [P4P], July 2011 to December 2012). The CPOE‐based VTE order set was used during all 3 periods. We used the other medical services as a control to ensure that there were no temporal trends toward improved prophylaxis on a service without the intervention. VTE prophylaxis compliance was examined by calculating percent compliance using the same algorithm for the 4 resident‐staffed general medicine service teams at our institution, which utilized the same CPOE system but did not receive the dashboard or pay‐for‐performance interventions. We used locally weighted scatterplot smoothing, a locally weighted regression of percent compliance over time, to graphically display changes in group compliance over time.[21, 22]
We also performed linear regression to assess the rate of change in group compliance and included spline terms that allowed slope to vary for each of the 3 time periods.[23, 24] Clustered analysis accounted for potentially correlated serial measurements of compliance for an individual provider. A separate analysis examined the effect of provider turnover and individual provider improvement during each of the 3 periods. Tests of significance were 2‐sided, with an level of 0.05. Statistical analysis was performed using Stata 12.1 (StataCorp LP, College Station, TX).
RESULTS
Venous Thromboembolism Prophylaxis Compliance
We analyzed 3144 inpatient admissions by 38 hospitalists from 2009 to 2012. The 5 most frequent coded diagnoses were heart failure, acute kidney failure, syncope, pneumonia, and chest pain. Patients had a median length of stay of 3 days [interquartile range: 26]. During the dashboard‐only period, on average, providers improved in compliance by 4% (95% confidence interval [CI]: 35; P<0.001). With the addition of the pay‐for‐performance program, providers improved by an additional 4% (95% CI: 35; P<0.001). Group compliance significantly improved from 86% (95% CI: 8588) during the BASE period of the CPOE‐based VTE order set to 90% (95% CI: 8893) during the DASH period (P=0.01) and 94% (95% CI: 9396) during the subsequent P4P program (P=0.01) (Figure 3). Both inappropriate prophylaxis and lack of prophylaxis, when indicated, resulted in a non‐compliance rating. During the 3 periods, inappropriate prophylaxis decreased from 7.9% to 6.2% to 2.6% during the BASE, DASH, and subsequent P4P periods, respectively. Similarly, lack of prophylaxis when indicated decreased from 6.1% to 3.2% to 3.1% during the BASE, DASH, and subsequent P4P periods, respectively.

The average compliance of the 4 non‐hospitalist general medicine service teams was initially higher than that of the hospitalist service during the CPOE‐based VTE order set (90%) and DASH (92%) periods, but subsequently plateaued and was exceeded by the hospitalist service during the combined P4P (92%) period (Figure 3). However, there was no statistically significant difference between the general medicine service teams and hospitalist service during the DASH (P=0.15) and subsequent P4P (P=0.76) periods.
We also analyzed the rate of VTE prophylaxis compliance improvement (slope) with cut points at each time period transition (Figure 3). Risk‐appropriate VTE prophylaxis during the BASE period did not exhibit significant improvement as indicated by the slope (P=0.23) (Figure 3). In contrast, during the DASH period, VTE prophylaxis compliance significantly increased by 1.58% per month (95% CI: 0.41‐2.76; P=0.01). The addition of the P4P program, however, did not further significantly increase the rate of compliance (P=0.78).
A subgroup analysis restricted to the 19 providers present during all 3 periods was performed to assess for potential confounding from physician turnover. The percent compliance increased in a similar fashion: BASE period of CPOE‐based VTE order set, 85% (95% CI: 8386); DASH, 90% (95% CI: 8893); and P4P, 94% (95% CI: 9296).
Pay‐for‐Performance Program
Nineteen providers met the threshold for pay‐for‐performance (80% appropriate VTE prophylaxis), with 9 providers in the intermediate categories (80%94.9%) and 10 in the full incentive category (95%). The mean individual payout for the incentive was $633 (standard deviation 350), with a total disbursement of $12,029. The majority of payments (17 of 19) were under $1000.
DISCUSSION
A key component of healthcare reform has been value‐based purchasing, which emphasizes extrinsic motivation through the transparency of performance metrics and use of payment incentives to reward quality. Our study evaluates the impact of both extrinsic (payments) and intrinsic (professionalism and peer norms) motivation. It specifically attributed an individual performance metric, VTE prophylaxis, to an attending physician, provided both individualized and group feedback using an electronic dashboard, and incorporated a pay‐for‐performance program. Prescription of risk‐appropriate VTE prophylaxis significantly increased with the implementation of the dashboard and subsequent pay‐for performance program. The fastest rate of improvement occurred after the addition of the dashboard. Sensitivity analyses for provider turnover and comparisons to the general medicine services showed our results to be independent of a general trend of improvement, both at the provider and institutional levels.
Our prior studies demonstrated that order sets significantly improve performance, from a baseline compliance of risk‐appropriate VTE prophylaxis of 66% to 84%.[13, 15, 25] In the current study, compliance was relatively flat during the BASE period, which included these order sets. The greatest rate of continued improvement in compliance occurred during the DASH period, emphasizing both the importance of provider feedback and receptivity and adaptability in the prescribing behavior of hospitalists. Because the goal of a high‐reliability health system is for 100% of patients to receive recommended therapy, multiple approaches are necessary for success.
Nationally, benchmarks for performance measures continue to be raised, with the highest performers achieving above 95%.[26] Additional interventions, such as dashboards and pay‐for‐performance programs, supplement CPOE systems to achieve high reliability. In our study, the compliance rate during the baseline period, which included a CPOE‐based, clinical support‐enabled VTE order set, was 86%. Initially the compliance of the general medicine teams with residents exceeded that of the hospitalist attending teams, which may reflect a greater willingness of resident teams to comply with order sets and automated recommendations. This emphasizes the importance of continuous individual feedback and provider education at the attending physician level to enhance both guideline compliance and decrease provider care variation. Ultimately, with the addition of the dashboard and subsequent pay‐for‐performance program, compliance was increased to 90% and 94%, respectively. Although the major mechanism used by policymakers to improve quality of care is extrinsic motivation, this study demonstrates that intrinsic motivation through peer norms can enhance extrinsic efforts and may be more influential. Both of these programs, dashboards and pay‐for‐performance, may ultimately assist institutions in changing provider behavior and achieving these harder‐to‐achieve higher benchmarks.
We recognize that there are several limitations to our study. First, this is a single‐site program limited to an attending‐physician‐only service. There was strong data support and a defined CPOE algorithm for this initiative. Multi‐site studies will need to overcome the additional challenges of varying service structures and electronic medical record and provider order entry systems. Second, it is difficult to show actual changes in VTE events over time with appropriate prophylaxis. Although VTE prophylaxis is recommended for patients with VTE risk factors, there are conflicting findings about whether prophylaxis prevents VTE events in lower‐risk patients, and current studies suggest that most patients with VTE events are severely ill and develop VTE despite receiving prophylaxis.[27, 28, 29] Our study was underpowered to detect these potential differences in VTE rates, and although the algorithm has been shown to not increase bleeding rates, we did not measure bleeding rates during this study.[12, 15] Our institutional experience suggests that the majority of VTE events occur despite appropriate prophylaxis.[30] Also, VTE prophylaxis may be ordered, but intervening events, such as procedures and changes in risk status or patient refusal, may prevent patients from receiving appropriate prophylaxis.[31, 32] Similarly, hospitals with higher quality scores have higher VTE prophylaxis rates but worse risk‐adjusted VTE rates, which may result from increased surveillance for VTE, suggesting surveillance bias limits the usefulness of the VTE quality measure.[33, 34] Nevertheless, VTE prophylaxis remains a publicly reported Core Measure tied to financial incentives.[4, 5] Third, there may be an unmeasured factor specific to the hospitalist program, which could potentially account for an overall improvement in quality of care. Although the rate of increase in appropriate prophylaxis was not statistically significant during the baseline period, there did appear to be some improvement in prophylaxis toward the end of the period. However, there were no other VTE‐related provider feedback programs being simultaneously pursued during this study. VTE prophylaxis for the non‐hospitalist services showed a relatively stable, non‐increasing compliance rate for the general medical services. Although it was possible for successful residents to age into the hospitalist service, thereby improving rates of prophylaxis based on changes in group makeup, our subgroup analysis of the providers present throughout all phases of the study showed our results to be robust. Similarly, there may have been a cross‐contamination effect of hospitalist faculty who attended on both hospitalist and non‐hospitalist general medicine service teams. This, however, would attenuate any impact of the programs, and thus the effects may in fact be greater than reported. Fourth, establishment of both the dashboard and pay‐for‐performance program required significant institutional and program leadership and resources. To be successful, the dashboard must be in the provider's workflow, transparent, minimize reporter burden, use existing systems, and be actively fed back to providers, ideally those directly entering orders. Our greatest rate of improvement occurred during the feedback‐only phase of this study, emphasizing the importance of physician feedback, provider‐level accountability, and engagement. We suspect that the relatively modest pay‐for‐performance incentive served mainly as a means of engaging providers in self‐monitoring, rather than as a means to change behavior through true incentivization. Although we did not track individual physician views of the dashboard, we reinforced trends, deviations, and expectations at regularly scheduled meetings and provided feedback and patient‐level data to individual providers. Fifth, the design of the pay‐for‐performance program may have also influenced its effectiveness. These types of programs may be more effective when they provide frequent visible, small payments rather than one large payment, and when the payment is framed as a loss rather than a gain.[35] Finally, physician champions and consistent feedback through departmental meetings or visual displays may be required for program success. The initial resources to create the dashboard, continued maintenance and monitoring of performance, and payment of financial incentives all require institutional commitment. A partnership of physicians, program leaders, and institutional administrators is necessary for both initial and continued success.
To achieve performance goals and benchmarks, multiple strategies that combine extrinsic and intrinsic motivation are necessary. As shown by our study, the use of a dashboard and pay‐for‐performance can be tailored to an institution's goals, in line with national standards. The specific goal (risk‐appropriate VTE prophylaxis) and benchmarks (80%, 85%, 90%, 95%) can be individualized to a particular institution. For example, if readmission rates are above target, readmissions could be added as a dashboard metric. The specific benchmark would be determined by historical trends and administrative targets. Similarly, the overall financial incentives could be adjusted based on the financial resources available. Other process measures, such as influenza vaccination screening and administration, could also be targeted. For all of these objectives, continued provider feedback and engagement are critical for progressive success, especially to decrease variability in care at the attending physician level. Incorporating the value‐based purchasing philosophy from the Affordable Care Act, our study suggests that the combination of standardized order sets, real‐time dashboards, and physician‐level incentives may assist hospitals in achieving quality and safety benchmarks, especially at higher targets.
Acknowledgements
The authors thank Meir Gottlieb, BS, from Salar Inc. for data support; Murali Padmanaban, BS, from Johns Hopkins University for his assistance in linking the administrative billing data with real‐time physician orders; and Hsin‐Chieh Yeh, PhD, from the Bloomberg School of Public Health for her statistical advice and additional review. We also thank Mr. Ronald R. Peterson, President, Johns Hopkins Health System and Johns Hopkins Hospital, for providing funding support for the physician incentive payments.
Disclosures: Drs. Michtalik and Brotman had full access to all of the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Study concept and design: Drs. Michtalik, Streiff, Finkelstein, Pronovost, and Brotman. Acquisition of data: Drs. Michtalik, Streiff, Brotman and Mr. Carolan, Mr. Lau, Mrs. Durkin. Analysis and interpretation of data: Drs. Michtalik, Haut, Streiff, Brotman and Mr. Carolan, Mr. Lau. Drafting of the manuscript: Drs. Michtalik and Brotman. Critical revision of the manuscript for important intellectual content: Drs. Michtalik, Haut, Streiff, Finkelstein, Pronovost, Brotman and Mr. Carolan, Mr. Lau, Mrs. Durkin. Statistical analysis and supervision: Drs. Michtalik and Brotman. Obtaining funding: Drs. Streiff and Brotman. Technical support: Dr. Streiff and Mr. Carolan, Mr. Lau, Mrs. Durkin
This study was supported by a National Institutes of Health grant T32 HP10025‐17‐00 (Dr. Michtalik), the National Institutes of Health/Johns Hopkins Institute for Clinical and Translational Research KL2 Award 5KL2RR025006 (Dr. Michtalik), the Agency for Healthcare Research and Quality Mentored Clinical Scientist Development K08 Awards 1K08HS017952‐01 (Dr. Haut) and 1K08HS022331‐01A1 (Dr. Michtalik), and the Johns Hopkins Hospitalist Scholars Fund. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Dr. Haut receives royalties from Lippincott, Williams & Wilkins. Dr. Streiff has received research funding from Portola and Bristol Myers Squibb, honoraria for CME lectures from Sanofi‐Aventis and Ortho‐McNeil, consulted for Eisai, Daiichi‐Sankyo, Boerhinger‐Ingelheim, Janssen Healthcare, and Pfizer. Mr. Lau, Drs. Haut, Streiff, and Pronovost are supported by a contract from the Patient‐Centered Outcomes Research Institute (PCORI) titled Preventing Venous Thromboembolism: Empowering Patients and Enabling Patient‐Centered Care via Health Information Technology (CE‐12‐11‐4489). Dr. Brotman has received research support from Siemens Healthcare Diagnostics, Bristol‐Myers Squibb, the Agency for Healthcare Research and Quality, Centers for Medicare & Medicaid Services, the Amerigroup Corporation, and the Guerrieri Family Foundation. He has received honoraria from the Gerson Lehrman Group, the Dunn Group, and from Quantia Communications, and received royalties from McGraw‐Hill.
- Medicare Program, Centers for Medicare 76(88):26490–26547.
- . Quality meets finance: payments at risk with value‐based purchasing, readmission, and hospital‐acquired conditions force hospitalists to focus. Hospitalist. 2013;17(1):31.
- National Quality Forum. March 2009. Safe practices for better healthcare—2009 update. Available at: http://www.qualityforum.org/Publications/2009/03/Safe_Practices_for_Better_Healthcare%E2%80%932009_Update.aspx. Accessed November 1, 2014.
- Joint Commission on Accreditation of Healthcare Organizations. Approved: more options for hospital core measures. Jt Comm Perspect. 2009;29(4):1–6.
- Centers for Medicare 208(2):227–240.
- , . Thromboprophylaxis in nonsurgical patients. Hematology Am Soc Hematol Educ Program. 2012;2012:631–637.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2008;371(9610):387–394.
- , . Practices to prevent venous thromboembolism: a brief review. BMJ Qual Saf. 2014;23(3):187–195.
- , , , et al. Improving hospital venous thromboembolism prophylaxis with electronic decision support. J Hosp Med. 2013;8(3):115–120.
- , , . Innovative approaches to increase deep vein thrombosis prophylaxis rate resulting in a decrease in hospital‐acquired deep vein thrombosis at a tertiary‐care teaching hospital. J Hosp Med. 2008;3(2):148–155.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
- , , , et al. Improved prophylaxis and decreased rates of preventable harm with the use of a mandatory computerized clinical decision support tool for prophylaxis for venous thromboembolism in trauma. Arch Surg. 2012;147(10):901–907.
- , . Designing and implementing effective venous thromboembolism prevention protocols: lessons from collaborative efforts. J Thromb Thrombolysis. 2010;29(2):159–166.
- , , , et al. Impact of a venous thromboembolism prophylaxis "smart order set": improved compliance, fewer events. Am J Hematol. 2013;88(7):545–549.
- , . Improving adherence to venous thromoembolism prophylaxis using multiple interventions. BMJ. 2012;344:e3935.
- Health Resources and Services Administration of the U.S. Department of Health and Human Services. Managing data for performance improvement. Available at: http://www.hrsa.gov/quality/toolbox/methodology/performanceimprovement/part2.html. Accessed December 18, 2014.
- , . Improving patient safety by taking systems seriously. JAMA. 2008;299(4):445–447.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , , , et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines (8th edition). Chest. 2008;133(6 suppl):381S–453S.
- . Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74(368):829–836.
- , . Locally weighted regression: An approach to regression analysis by local fitting. J Am Stat Assoc. 1988;83(403):596–610.
- , , , . Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. 2nd ed. New York, NY: Springer; 2012.
- . Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer‐Verlag; 2001.
- , , , et al. Eliminating healthcare disparities via mandatory clinical decision support: the venous thromboembolism (VTE) example [published online ahead of print November 4, 2014]. Med Care. doi: 10.1097/MLR.0000000000000251.
- Joint Commission. Improving America's hospitals: the Joint Commission's annual report on quality and safety. 2012. Available at: http://www.jointcommission.org/assets/1/18/TJC_Annual_Report_2012.pdf. Accessed September 8, 2013.
- , , , et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577–1584.
- , , , et al. Incidence of hospital‐acquired venous thromboembolic codes in medical patients hospitalized in academic medical centers. J Hosp Med. 2014;9(4):221–225.
- , , , , . No association between hospital‐reported perioperative venous thromboembolism prophylaxis and outcome rates in publicly reported data. JAMA Surg. 2014;149(4):400–401.
- , , , , . Linking processes and outcomes: a key strategy to prevent and report harm from venous thromboembolism in surgical patients. JAMA Surg. 2013;148(3):299–300.
- , , , et al. Patterns of non‐administration of ordered doses of venous thromboembolism prophylaxis: implications for novel intervention strategies. PLoS One. 2013;8(6):e66311.
- , , , et al. Nonadministration of thromboprophylaxis in hospitalized patients with HIV: a missed opportunity for prevention? J Hosp Med. 2014;9(4):215–220.
- , , , et al. Evaluation of surveillance bias and the validity of the venous thromboembolism quality measure. JAMA. 2013;310(14):1482–1489.
- , . Surveillance bias in outcomes reporting. JAMA. 2011;305(23):2462–2463.
- . Pay for performance in health care: an international overview of initiatives. Med Care Res Rev. 2012;69(3):251–276.
- Medicare Program, Centers for Medicare 76(88):26490–26547.
- . Quality meets finance: payments at risk with value‐based purchasing, readmission, and hospital‐acquired conditions force hospitalists to focus. Hospitalist. 2013;17(1):31.
- National Quality Forum. March 2009. Safe practices for better healthcare—2009 update. Available at: http://www.qualityforum.org/Publications/2009/03/Safe_Practices_for_Better_Healthcare%E2%80%932009_Update.aspx. Accessed November 1, 2014.
- Joint Commission on Accreditation of Healthcare Organizations. Approved: more options for hospital core measures. Jt Comm Perspect. 2009;29(4):1–6.
- Centers for Medicare 208(2):227–240.
- , . Thromboprophylaxis in nonsurgical patients. Hematology Am Soc Hematol Educ Program. 2012;2012:631–637.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2008;371(9610):387–394.
- , . Practices to prevent venous thromboembolism: a brief review. BMJ Qual Saf. 2014;23(3):187–195.
- , , , et al. Improving hospital venous thromboembolism prophylaxis with electronic decision support. J Hosp Med. 2013;8(3):115–120.
- , , . Innovative approaches to increase deep vein thrombosis prophylaxis rate resulting in a decrease in hospital‐acquired deep vein thrombosis at a tertiary‐care teaching hospital. J Hosp Med. 2008;3(2):148–155.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
- , , , et al. Improved prophylaxis and decreased rates of preventable harm with the use of a mandatory computerized clinical decision support tool for prophylaxis for venous thromboembolism in trauma. Arch Surg. 2012;147(10):901–907.
- , . Designing and implementing effective venous thromboembolism prevention protocols: lessons from collaborative efforts. J Thromb Thrombolysis. 2010;29(2):159–166.
- , , , et al. Impact of a venous thromboembolism prophylaxis "smart order set": improved compliance, fewer events. Am J Hematol. 2013;88(7):545–549.
- , . Improving adherence to venous thromoembolism prophylaxis using multiple interventions. BMJ. 2012;344:e3935.
- Health Resources and Services Administration of the U.S. Department of Health and Human Services. Managing data for performance improvement. Available at: http://www.hrsa.gov/quality/toolbox/methodology/performanceimprovement/part2.html. Accessed December 18, 2014.
- , . Improving patient safety by taking systems seriously. JAMA. 2008;299(4):445–447.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , , , et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines (8th edition). Chest. 2008;133(6 suppl):381S–453S.
- . Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74(368):829–836.
- , . Locally weighted regression: An approach to regression analysis by local fitting. J Am Stat Assoc. 1988;83(403):596–610.
- , , , . Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. 2nd ed. New York, NY: Springer; 2012.
- . Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer‐Verlag; 2001.
- , , , et al. Eliminating healthcare disparities via mandatory clinical decision support: the venous thromboembolism (VTE) example [published online ahead of print November 4, 2014]. Med Care. doi: 10.1097/MLR.0000000000000251.
- Joint Commission. Improving America's hospitals: the Joint Commission's annual report on quality and safety. 2012. Available at: http://www.jointcommission.org/assets/1/18/TJC_Annual_Report_2012.pdf. Accessed September 8, 2013.
- , , , et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577–1584.
- , , , et al. Incidence of hospital‐acquired venous thromboembolic codes in medical patients hospitalized in academic medical centers. J Hosp Med. 2014;9(4):221–225.
- , , , , . No association between hospital‐reported perioperative venous thromboembolism prophylaxis and outcome rates in publicly reported data. JAMA Surg. 2014;149(4):400–401.
- , , , , . Linking processes and outcomes: a key strategy to prevent and report harm from venous thromboembolism in surgical patients. JAMA Surg. 2013;148(3):299–300.
- , , , et al. Patterns of non‐administration of ordered doses of venous thromboembolism prophylaxis: implications for novel intervention strategies. PLoS One. 2013;8(6):e66311.
- , , , et al. Nonadministration of thromboprophylaxis in hospitalized patients with HIV: a missed opportunity for prevention? J Hosp Med. 2014;9(4):215–220.
- , , , et al. Evaluation of surveillance bias and the validity of the venous thromboembolism quality measure. JAMA. 2013;310(14):1482–1489.
- , . Surveillance bias in outcomes reporting. JAMA. 2011;305(23):2462–2463.
- . Pay for performance in health care: an international overview of initiatives. Med Care Res Rev. 2012;69(3):251–276.
© 2014 Society of Hospital Medicine
Thromboembolism Prophylaxis Preferences
The 2012 American College of Chest Physicians (ACCP) guidelines on antithrombotic and thrombolytic therapy conducted a systematic review focusing on patient values and preferences regarding antithrombotic therapy, including thromboprophylaxis.[1] They found that patient values and preferences are highly variable and should be considered when developing future clinical practice guidelines. Notably, there were no studies evaluating patient preferences for venous thromboembolism (VTE) prophylaxis, which is prescribed for the vast majority of hospitalized patients.
Historically, interventions to prevent VTE have focused on increasing prescriptions of prophylaxis. At the Johns Hopkins Hospital, we implemented a mandatory clinical decision support tool in our computerized provider order entry system.[2] Following implementation of this tool, prescription of risk‐appropriate VTE prophylaxis dramatically increased for both medical and surgical patients.[3, 4, 5] These efforts were made with the implicit and incorrect assumption that prescribed medication doses will always be administered to patients, when in fact patient refusal is a leading cause of nonadministration. Studies of VTE prophylaxis administration have reported that 10% to 12% of doses are not administered to patients.[6] Alarmingly, it has been reported that among medically ill patients, between 10% and 30% of doses are not administered, with patient refusal as the most frequently documented reason.
The purpose of this study was to assess patient preferences regarding pharmacological VTE prophylaxis.
METHODS
Study Design
A sample of consecutive hospitalized patients on select medicine and surgical floors previously identified as low‐ and high‐performing units at our institution in regard to administration rates of pharmacologic VTE prophylaxis was assembled from a daily electronic report of patients prescribed pharmacological VTE prophylaxis (Allscripts Sunrise, Chicago, IL) from December 2012 to March 2013. These units were identified in a study conducted at our institution as the lowest‐ and highest‐performing units in regard to incidence of administration of ordered pharmacologic VTE prophylaxis. From this data analysis, we chose the 2 lowest‐performing and 2 highest‐performing units on the medical and surgical service. To be eligible for this study, patients had to have an active order for 1 of the following VTE prophylaxis regimens: unfractionated heparin 5000 units or 7500 units administered subcutaneously every 8 or 12 hours, enoxaparin 30 mg administered subcutaneously every 12 hours or 40 mg administered subcutaneously every 24 hours. Participants had to be at least 18 years of age and hospitalized for at least 2 days on their respective units. Patients who were nonEnglish speaking, those previously enrolled in this study, or those unable to provide consent were excluded from the study.
Data Collection
Demographic information was collected, including patient‐reported education level. To determine their preference for VTE prophylaxis, patients were provided a survey, which included being asked, Would you prefer a pill or a shot to prevent blood clots, if they both worked equally well. The survey was created by the study team to collect information from patients regarding their baseline knowledge of VTE and preference regarding pharmacologic prophylaxis. Additional data included the patient's education level to determine potential association with preference. The survey was verbally administered by 1 investigator (A.W.) to all patients. Patients were asked to explain their rationale for their stated preference in regard to VTE prophylaxis. Patient rationale was subsequently coded to allow for uniformity among patient responses based on patterns in responses. Our electronic medication record allows us to identify patients who refused their medication through nursing documentation. Patients with documented refusal of ordered pharmacologic VTE prophylaxis were asked about the rationale for their refusal. This study was approved by the Johns Hopkins Medicine Institutional Review Board.
Statistical Analysis
Quantitative data from the surveys were analyzed using Minitab (Minitab Inc., State College, PA). A [2] test analysis was performed for categorical data, as appropriate. A P value 0.05 was considered to be statistically significant.
RESULTS
Quantitative Results
We interviewed patients regarding their preferred route of administration of VTE prophylaxis. Overall, 339 patients were screened for this study. Sixty patients were not eligible to participate. Forty‐seven were unable to provide consent, and 13 were nonEnglish speaking. Of the 269 remaining eligible patients, 227 (84.4%) consented to participate.
Baseline demographics of the participants are presented in Table 1, categorized on the basis of their preferred route of administration for VTE prophylaxis. A majority of patients indicated a preference for an oral formulation of pharmacologic VTE prophylaxis. There was no association between education level or service type on preference. Preference for an oral formulation was largely influenced by patient‐reported pain and bruising associated with subcutaneous administration (Table 2). A substantial majority of patients reporting a preference for a subcutaneous formulation and emphasized a belief that this route was associated with a faster onset of action. Among patients who preferred an oral formulation (n=137), 71 patients (51.8%) were documented as having refused at least 1 dose of ordered VTE prophylaxis. Patients who preferred a subcutaneous route of VTE prophylaxis were less likely to refuse prophylaxis, with only 22 patients (35.5%) having a documented refusal of at least 1 dose (P0.0001).
| Enteral, n=137 | Parenteral, n=62 | No Preference, n=28 | |
|---|---|---|---|
| |||
| Age, y, mean ( SD) | 49.5 (14.7) | 51.7 (16.1) | 48.9 (14.6) |
| Male, n (%) | 74 (54.0) | 38 (61.3) | 15 (53.6) |
| Race n (%) | |||
| Caucasian | 81 (59.1) | 31 (50.0) | 14 (50.0) |
| African American | 50 (36.5) | 28 (45.2) | 14 (50.0) |
| Education level, n (%) | |||
| High school or less | 46 (33.6) | 27 (43.5) | 14 (50.0) |
| College | 68 (49.6) | 21 (33.9) | 9 (32.1) |
| Advanced degree | 10 (7.3) | 8 (12.9) | 2 (7.1) |
| Unable to obtain | 13 (9.5) | 6 (9.7) | 3 (10.8) |
| Past history of VTE, n (%) | 12 (8.8) | 9 (14.5) | 2 (7.1) |
| Type of unit, n (%) | |||
| Medical | 59 (43.1) | 24 (38.7) | 17 (60.7) |
| Surgical | 78 (56.9) | 38 (61.3) | 11 (39.3) |
| Documented refusal of ordered prophylaxis, n (%) | 71 (51.8) | 20 (32.3) | 9 (32.1) |
| Length of hospital stay prior to inclusion in study, d, median (IQR) | 4.0 (3.07.0) | 3.0 (3.05.0) | 4.0 (2.05.0) |
| Patients preferring enteral route, n (%) | 137 (60.4) |
| Dislike of needles | 41 (30.0) |
| Pain from injection | 38 (27.7) |
| Ease of use | 18 (13.1) |
| Bruising from injection | 9 (6.6) |
| Other/no rationale | 31 (22.6) |
| Patients preferring injection route, n (%) | 62 (27.5) |
| Faster onset of action | 25 (40.3) |
| Pill burden | 11 (17.7) |
| Ease of use | 9 (14.5) |
| Other/no rationale | 17 (27.5) |
| Patients with no preference, n (%) | 28 (12.4) |
DISCUSSION
Using a mixed‐methods approach, we report the first survey evaluating patient preferences regarding pharmacologic VTE prophylaxis. We found that a majority of patients preferred an oral route of administration. Nevertheless, a substantial number of patients favored a subcutaneous route of administration believing it to be associated with a faster onset of action. Of interest, patients favoring subcutaneous injections were significantly less likely to refuse doses of ordered VTE prophylaxis. Given that all patients were prescribed a subcutaneous form of VTE prophylaxis, matching patient preference to VTE prophylaxis prescription could potentially increase adherence and reduce patient refusal of ordered prophylaxis. Considering the large number of patients who preferred an oral route of administration, the availability of an oral formulation may potentially result in improved adherence to inpatient VTE prophylaxis.
Our findings have significant implications for healthcare providers, and for patient safety and quality‐improvement researchers. VTE prophylaxis is an important patient‐safety practice, particularly for medically ill patients, which is believed to be underprescribed.[7] Recent studies have demonstrated that a significant number of doses of VTE prophylaxis are not administered, primarily due to patient refusal.[6] Our data indicate that tailoring the route of prophylaxis administration to patient preference may represent a feasible strategy to improve VTE prophylaxis administration rates. Recently, several target‐specific oral anticoagulants (TSOACs) have been approved for a variety of clinical indications, and all have been investigated for VTE prophylaxis.[7, 8, 9, 10, 11, 12, 13, 14, 15] However, no agent is currently US Food & Drug Administration (FDA) approved for primary prevention of VTE, although apixaban and rivaroxaban are FDA approved for VTE prevention in joint replacement.[13, 14] Although in some instances these TSOACs were noted to demonstrate only equivalent efficacy to standard subcutaneous forms of VTE prophylaxis, our data suggest that perhaps in some patients, use of these agents may result in better outcomes due to improved adherence to therapy due to a preferred oral route of administration. We think this hypothesis warrants further investigation.
Our study also underscores the importance of considering patient preferences when caring for patients as emphasized by the 2012 ACCP guidelines.[1] Our results indicate that consideration of patient preferences may lead to better patient care and better outcomes. Interestingly, there were no differences in preference based on education level or the type of service to which the patient was admitted. Clarification of uninformed opinions regarding the rationale for preference may also lead to more informed decisions by patients.
This study has a number of limitations. We only included patients on the internal medicine and general surgical services. It is possible that patients on other specialty services may have different opinions regarding prophylaxis that were not captured in our sample. Similarly, our sample size was limited, and approximately 15% of potential subjects did not participate. We do believe that our population is reflective of our institution based upon our previously published evaluation of multiple hospital units and the inclusion of low‐ and high‐performing units on both the medical and surgical services. Nevertheless, we believe that much more investigation of patient perspectives on VTE prophylaxis needs to be done to inform decision making, including the impact of patient preferences on VTE‐related outcomes. Additionally, we did not evaluate potential predictors of preference including admission diagnosis and duration of hospital length of stay.
In conclusion, we conducted a mixed‐methods analysis of patient preferences regarding pharmacologic VTE prophylaxis. Matching patient preference to ordered VTE prophylaxis may increase adherence to ordered prophylaxis. In this era of increasingly patient‐centered healthcare and expanding options for VTE prophylaxis, we believe information on patient preferences will be helpful to tailoring options for prevention and treatment.
ACKNOWLEDGMENTS
Disclosures: Dr. Haut is the primary investigator of the Mentored Clinician Scientist Development Award K08 1K08HS017952‐01 from the Agency for Healthcare Research and Quality entitled Does Screening Variability Make DVT an Unreliable Quality Measure of Trauma Care? Dr. Haut receives royalties from Lippincott, Williams, & Wilkins for a book he coauthored (Avoiding Common ICU Errors). He has received honoraria for various speaking engagements regarding clinical, quality, and safety topics and has given expert witness testimony in various medical malpractice cases. Dr. Streiff has received research funding from Sanofi‐Aventis and Bristol‐Myers Squibb; honoraria for Continuing Medial Education lectures from Sanofi‐Aventis and Ortho‐McNeil; consulted for Sanofi‐Aventis, Eisai, Daiichi‐Sankyo, and Janssen HealthCare; and has given expert witness testimony in various medical malpractice cases. Mr. Lau, Drs. Haut, Streiff, and Shermock are supported by a contract from the Patient‐Centered Outcomes Research Institute titled Preventing Venous Thromboembolism: Empowering Patients and Enabling Patient‐Centered Care via Health Information Technology (CE‐12‐11‐4489). Ms. Hobson has given expert witness testimony in various medical malpractice cases. All others have no relevant funding or conflicts of interest to report.
- , , , et al. Patient values and preferences in decision making for antithrombotic therapy: a systematic review. Chest. 2012;141(2):e1S–e23S.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
- , , , et al. Impact of a venous thromboembolism (VTE) prophylaxis “smart order set”: improved compliance, fewer events. Am J Hematol. 2013;88(7):545–549.
- , , , et al. Improved prophylaxis and decreased preventable harm with a mandatory computerized clinical decision support tool for venous thromboembolism (VTE) prophylaxis in trauma patients. Arch Surg. 2012;147(10):901–907.
- , , , , . Linking processes and outcomes: a key strategy to prevent and report harm from venous thromboembolism in surgical patients. JAMA Surg. 2013;148(3):299–300.
- , , , et al. Patterns of non‐administration of ordered doses of venous thromboembolism prophylaxis: implications for intervention strategies. PLoS One. 2013;8(6):e66311.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2008;371:387–394.
- , , , et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765–2775.
- , , , et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthoplasty. N Engl J Med. 2008;358:2776–2786.
- , , , , , . Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361:594–604.
- , , , , , . Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE‐2): a randomized double‐blind trial. Lancet. 2010;275:807–815.
- , , , et al. Rivaroxaban for the prevention of venous thromboembolism after hip or knee arthroplasty. Pooled analysis of four studies. Thromb Haemost. 2011;105:444–453.
- , , , et al. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med. 2011;365:2167–2177.
- , , , et al. Efficacy and safety of thromboprophylaxis with low‐molecular‐weight heparin or rivaroxaban in hip and knee replacement surgery: findings from the ORTHO‐TEP registry. Thromb Haemost. 2013;109:154–163.
- , , , et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:513–523.
The 2012 American College of Chest Physicians (ACCP) guidelines on antithrombotic and thrombolytic therapy conducted a systematic review focusing on patient values and preferences regarding antithrombotic therapy, including thromboprophylaxis.[1] They found that patient values and preferences are highly variable and should be considered when developing future clinical practice guidelines. Notably, there were no studies evaluating patient preferences for venous thromboembolism (VTE) prophylaxis, which is prescribed for the vast majority of hospitalized patients.
Historically, interventions to prevent VTE have focused on increasing prescriptions of prophylaxis. At the Johns Hopkins Hospital, we implemented a mandatory clinical decision support tool in our computerized provider order entry system.[2] Following implementation of this tool, prescription of risk‐appropriate VTE prophylaxis dramatically increased for both medical and surgical patients.[3, 4, 5] These efforts were made with the implicit and incorrect assumption that prescribed medication doses will always be administered to patients, when in fact patient refusal is a leading cause of nonadministration. Studies of VTE prophylaxis administration have reported that 10% to 12% of doses are not administered to patients.[6] Alarmingly, it has been reported that among medically ill patients, between 10% and 30% of doses are not administered, with patient refusal as the most frequently documented reason.
The purpose of this study was to assess patient preferences regarding pharmacological VTE prophylaxis.
METHODS
Study Design
A sample of consecutive hospitalized patients on select medicine and surgical floors previously identified as low‐ and high‐performing units at our institution in regard to administration rates of pharmacologic VTE prophylaxis was assembled from a daily electronic report of patients prescribed pharmacological VTE prophylaxis (Allscripts Sunrise, Chicago, IL) from December 2012 to March 2013. These units were identified in a study conducted at our institution as the lowest‐ and highest‐performing units in regard to incidence of administration of ordered pharmacologic VTE prophylaxis. From this data analysis, we chose the 2 lowest‐performing and 2 highest‐performing units on the medical and surgical service. To be eligible for this study, patients had to have an active order for 1 of the following VTE prophylaxis regimens: unfractionated heparin 5000 units or 7500 units administered subcutaneously every 8 or 12 hours, enoxaparin 30 mg administered subcutaneously every 12 hours or 40 mg administered subcutaneously every 24 hours. Participants had to be at least 18 years of age and hospitalized for at least 2 days on their respective units. Patients who were nonEnglish speaking, those previously enrolled in this study, or those unable to provide consent were excluded from the study.
Data Collection
Demographic information was collected, including patient‐reported education level. To determine their preference for VTE prophylaxis, patients were provided a survey, which included being asked, Would you prefer a pill or a shot to prevent blood clots, if they both worked equally well. The survey was created by the study team to collect information from patients regarding their baseline knowledge of VTE and preference regarding pharmacologic prophylaxis. Additional data included the patient's education level to determine potential association with preference. The survey was verbally administered by 1 investigator (A.W.) to all patients. Patients were asked to explain their rationale for their stated preference in regard to VTE prophylaxis. Patient rationale was subsequently coded to allow for uniformity among patient responses based on patterns in responses. Our electronic medication record allows us to identify patients who refused their medication through nursing documentation. Patients with documented refusal of ordered pharmacologic VTE prophylaxis were asked about the rationale for their refusal. This study was approved by the Johns Hopkins Medicine Institutional Review Board.
Statistical Analysis
Quantitative data from the surveys were analyzed using Minitab (Minitab Inc., State College, PA). A [2] test analysis was performed for categorical data, as appropriate. A P value 0.05 was considered to be statistically significant.
RESULTS
Quantitative Results
We interviewed patients regarding their preferred route of administration of VTE prophylaxis. Overall, 339 patients were screened for this study. Sixty patients were not eligible to participate. Forty‐seven were unable to provide consent, and 13 were nonEnglish speaking. Of the 269 remaining eligible patients, 227 (84.4%) consented to participate.
Baseline demographics of the participants are presented in Table 1, categorized on the basis of their preferred route of administration for VTE prophylaxis. A majority of patients indicated a preference for an oral formulation of pharmacologic VTE prophylaxis. There was no association between education level or service type on preference. Preference for an oral formulation was largely influenced by patient‐reported pain and bruising associated with subcutaneous administration (Table 2). A substantial majority of patients reporting a preference for a subcutaneous formulation and emphasized a belief that this route was associated with a faster onset of action. Among patients who preferred an oral formulation (n=137), 71 patients (51.8%) were documented as having refused at least 1 dose of ordered VTE prophylaxis. Patients who preferred a subcutaneous route of VTE prophylaxis were less likely to refuse prophylaxis, with only 22 patients (35.5%) having a documented refusal of at least 1 dose (P0.0001).
| Enteral, n=137 | Parenteral, n=62 | No Preference, n=28 | |
|---|---|---|---|
| |||
| Age, y, mean ( SD) | 49.5 (14.7) | 51.7 (16.1) | 48.9 (14.6) |
| Male, n (%) | 74 (54.0) | 38 (61.3) | 15 (53.6) |
| Race n (%) | |||
| Caucasian | 81 (59.1) | 31 (50.0) | 14 (50.0) |
| African American | 50 (36.5) | 28 (45.2) | 14 (50.0) |
| Education level, n (%) | |||
| High school or less | 46 (33.6) | 27 (43.5) | 14 (50.0) |
| College | 68 (49.6) | 21 (33.9) | 9 (32.1) |
| Advanced degree | 10 (7.3) | 8 (12.9) | 2 (7.1) |
| Unable to obtain | 13 (9.5) | 6 (9.7) | 3 (10.8) |
| Past history of VTE, n (%) | 12 (8.8) | 9 (14.5) | 2 (7.1) |
| Type of unit, n (%) | |||
| Medical | 59 (43.1) | 24 (38.7) | 17 (60.7) |
| Surgical | 78 (56.9) | 38 (61.3) | 11 (39.3) |
| Documented refusal of ordered prophylaxis, n (%) | 71 (51.8) | 20 (32.3) | 9 (32.1) |
| Length of hospital stay prior to inclusion in study, d, median (IQR) | 4.0 (3.07.0) | 3.0 (3.05.0) | 4.0 (2.05.0) |
| Patients preferring enteral route, n (%) | 137 (60.4) |
| Dislike of needles | 41 (30.0) |
| Pain from injection | 38 (27.7) |
| Ease of use | 18 (13.1) |
| Bruising from injection | 9 (6.6) |
| Other/no rationale | 31 (22.6) |
| Patients preferring injection route, n (%) | 62 (27.5) |
| Faster onset of action | 25 (40.3) |
| Pill burden | 11 (17.7) |
| Ease of use | 9 (14.5) |
| Other/no rationale | 17 (27.5) |
| Patients with no preference, n (%) | 28 (12.4) |
DISCUSSION
Using a mixed‐methods approach, we report the first survey evaluating patient preferences regarding pharmacologic VTE prophylaxis. We found that a majority of patients preferred an oral route of administration. Nevertheless, a substantial number of patients favored a subcutaneous route of administration believing it to be associated with a faster onset of action. Of interest, patients favoring subcutaneous injections were significantly less likely to refuse doses of ordered VTE prophylaxis. Given that all patients were prescribed a subcutaneous form of VTE prophylaxis, matching patient preference to VTE prophylaxis prescription could potentially increase adherence and reduce patient refusal of ordered prophylaxis. Considering the large number of patients who preferred an oral route of administration, the availability of an oral formulation may potentially result in improved adherence to inpatient VTE prophylaxis.
Our findings have significant implications for healthcare providers, and for patient safety and quality‐improvement researchers. VTE prophylaxis is an important patient‐safety practice, particularly for medically ill patients, which is believed to be underprescribed.[7] Recent studies have demonstrated that a significant number of doses of VTE prophylaxis are not administered, primarily due to patient refusal.[6] Our data indicate that tailoring the route of prophylaxis administration to patient preference may represent a feasible strategy to improve VTE prophylaxis administration rates. Recently, several target‐specific oral anticoagulants (TSOACs) have been approved for a variety of clinical indications, and all have been investigated for VTE prophylaxis.[7, 8, 9, 10, 11, 12, 13, 14, 15] However, no agent is currently US Food & Drug Administration (FDA) approved for primary prevention of VTE, although apixaban and rivaroxaban are FDA approved for VTE prevention in joint replacement.[13, 14] Although in some instances these TSOACs were noted to demonstrate only equivalent efficacy to standard subcutaneous forms of VTE prophylaxis, our data suggest that perhaps in some patients, use of these agents may result in better outcomes due to improved adherence to therapy due to a preferred oral route of administration. We think this hypothesis warrants further investigation.
Our study also underscores the importance of considering patient preferences when caring for patients as emphasized by the 2012 ACCP guidelines.[1] Our results indicate that consideration of patient preferences may lead to better patient care and better outcomes. Interestingly, there were no differences in preference based on education level or the type of service to which the patient was admitted. Clarification of uninformed opinions regarding the rationale for preference may also lead to more informed decisions by patients.
This study has a number of limitations. We only included patients on the internal medicine and general surgical services. It is possible that patients on other specialty services may have different opinions regarding prophylaxis that were not captured in our sample. Similarly, our sample size was limited, and approximately 15% of potential subjects did not participate. We do believe that our population is reflective of our institution based upon our previously published evaluation of multiple hospital units and the inclusion of low‐ and high‐performing units on both the medical and surgical services. Nevertheless, we believe that much more investigation of patient perspectives on VTE prophylaxis needs to be done to inform decision making, including the impact of patient preferences on VTE‐related outcomes. Additionally, we did not evaluate potential predictors of preference including admission diagnosis and duration of hospital length of stay.
In conclusion, we conducted a mixed‐methods analysis of patient preferences regarding pharmacologic VTE prophylaxis. Matching patient preference to ordered VTE prophylaxis may increase adherence to ordered prophylaxis. In this era of increasingly patient‐centered healthcare and expanding options for VTE prophylaxis, we believe information on patient preferences will be helpful to tailoring options for prevention and treatment.
ACKNOWLEDGMENTS
Disclosures: Dr. Haut is the primary investigator of the Mentored Clinician Scientist Development Award K08 1K08HS017952‐01 from the Agency for Healthcare Research and Quality entitled Does Screening Variability Make DVT an Unreliable Quality Measure of Trauma Care? Dr. Haut receives royalties from Lippincott, Williams, & Wilkins for a book he coauthored (Avoiding Common ICU Errors). He has received honoraria for various speaking engagements regarding clinical, quality, and safety topics and has given expert witness testimony in various medical malpractice cases. Dr. Streiff has received research funding from Sanofi‐Aventis and Bristol‐Myers Squibb; honoraria for Continuing Medial Education lectures from Sanofi‐Aventis and Ortho‐McNeil; consulted for Sanofi‐Aventis, Eisai, Daiichi‐Sankyo, and Janssen HealthCare; and has given expert witness testimony in various medical malpractice cases. Mr. Lau, Drs. Haut, Streiff, and Shermock are supported by a contract from the Patient‐Centered Outcomes Research Institute titled Preventing Venous Thromboembolism: Empowering Patients and Enabling Patient‐Centered Care via Health Information Technology (CE‐12‐11‐4489). Ms. Hobson has given expert witness testimony in various medical malpractice cases. All others have no relevant funding or conflicts of interest to report.
The 2012 American College of Chest Physicians (ACCP) guidelines on antithrombotic and thrombolytic therapy conducted a systematic review focusing on patient values and preferences regarding antithrombotic therapy, including thromboprophylaxis.[1] They found that patient values and preferences are highly variable and should be considered when developing future clinical practice guidelines. Notably, there were no studies evaluating patient preferences for venous thromboembolism (VTE) prophylaxis, which is prescribed for the vast majority of hospitalized patients.
Historically, interventions to prevent VTE have focused on increasing prescriptions of prophylaxis. At the Johns Hopkins Hospital, we implemented a mandatory clinical decision support tool in our computerized provider order entry system.[2] Following implementation of this tool, prescription of risk‐appropriate VTE prophylaxis dramatically increased for both medical and surgical patients.[3, 4, 5] These efforts were made with the implicit and incorrect assumption that prescribed medication doses will always be administered to patients, when in fact patient refusal is a leading cause of nonadministration. Studies of VTE prophylaxis administration have reported that 10% to 12% of doses are not administered to patients.[6] Alarmingly, it has been reported that among medically ill patients, between 10% and 30% of doses are not administered, with patient refusal as the most frequently documented reason.
The purpose of this study was to assess patient preferences regarding pharmacological VTE prophylaxis.
METHODS
Study Design
A sample of consecutive hospitalized patients on select medicine and surgical floors previously identified as low‐ and high‐performing units at our institution in regard to administration rates of pharmacologic VTE prophylaxis was assembled from a daily electronic report of patients prescribed pharmacological VTE prophylaxis (Allscripts Sunrise, Chicago, IL) from December 2012 to March 2013. These units were identified in a study conducted at our institution as the lowest‐ and highest‐performing units in regard to incidence of administration of ordered pharmacologic VTE prophylaxis. From this data analysis, we chose the 2 lowest‐performing and 2 highest‐performing units on the medical and surgical service. To be eligible for this study, patients had to have an active order for 1 of the following VTE prophylaxis regimens: unfractionated heparin 5000 units or 7500 units administered subcutaneously every 8 or 12 hours, enoxaparin 30 mg administered subcutaneously every 12 hours or 40 mg administered subcutaneously every 24 hours. Participants had to be at least 18 years of age and hospitalized for at least 2 days on their respective units. Patients who were nonEnglish speaking, those previously enrolled in this study, or those unable to provide consent were excluded from the study.
Data Collection
Demographic information was collected, including patient‐reported education level. To determine their preference for VTE prophylaxis, patients were provided a survey, which included being asked, Would you prefer a pill or a shot to prevent blood clots, if they both worked equally well. The survey was created by the study team to collect information from patients regarding their baseline knowledge of VTE and preference regarding pharmacologic prophylaxis. Additional data included the patient's education level to determine potential association with preference. The survey was verbally administered by 1 investigator (A.W.) to all patients. Patients were asked to explain their rationale for their stated preference in regard to VTE prophylaxis. Patient rationale was subsequently coded to allow for uniformity among patient responses based on patterns in responses. Our electronic medication record allows us to identify patients who refused their medication through nursing documentation. Patients with documented refusal of ordered pharmacologic VTE prophylaxis were asked about the rationale for their refusal. This study was approved by the Johns Hopkins Medicine Institutional Review Board.
Statistical Analysis
Quantitative data from the surveys were analyzed using Minitab (Minitab Inc., State College, PA). A [2] test analysis was performed for categorical data, as appropriate. A P value 0.05 was considered to be statistically significant.
RESULTS
Quantitative Results
We interviewed patients regarding their preferred route of administration of VTE prophylaxis. Overall, 339 patients were screened for this study. Sixty patients were not eligible to participate. Forty‐seven were unable to provide consent, and 13 were nonEnglish speaking. Of the 269 remaining eligible patients, 227 (84.4%) consented to participate.
Baseline demographics of the participants are presented in Table 1, categorized on the basis of their preferred route of administration for VTE prophylaxis. A majority of patients indicated a preference for an oral formulation of pharmacologic VTE prophylaxis. There was no association between education level or service type on preference. Preference for an oral formulation was largely influenced by patient‐reported pain and bruising associated with subcutaneous administration (Table 2). A substantial majority of patients reporting a preference for a subcutaneous formulation and emphasized a belief that this route was associated with a faster onset of action. Among patients who preferred an oral formulation (n=137), 71 patients (51.8%) were documented as having refused at least 1 dose of ordered VTE prophylaxis. Patients who preferred a subcutaneous route of VTE prophylaxis were less likely to refuse prophylaxis, with only 22 patients (35.5%) having a documented refusal of at least 1 dose (P0.0001).
| Enteral, n=137 | Parenteral, n=62 | No Preference, n=28 | |
|---|---|---|---|
| |||
| Age, y, mean ( SD) | 49.5 (14.7) | 51.7 (16.1) | 48.9 (14.6) |
| Male, n (%) | 74 (54.0) | 38 (61.3) | 15 (53.6) |
| Race n (%) | |||
| Caucasian | 81 (59.1) | 31 (50.0) | 14 (50.0) |
| African American | 50 (36.5) | 28 (45.2) | 14 (50.0) |
| Education level, n (%) | |||
| High school or less | 46 (33.6) | 27 (43.5) | 14 (50.0) |
| College | 68 (49.6) | 21 (33.9) | 9 (32.1) |
| Advanced degree | 10 (7.3) | 8 (12.9) | 2 (7.1) |
| Unable to obtain | 13 (9.5) | 6 (9.7) | 3 (10.8) |
| Past history of VTE, n (%) | 12 (8.8) | 9 (14.5) | 2 (7.1) |
| Type of unit, n (%) | |||
| Medical | 59 (43.1) | 24 (38.7) | 17 (60.7) |
| Surgical | 78 (56.9) | 38 (61.3) | 11 (39.3) |
| Documented refusal of ordered prophylaxis, n (%) | 71 (51.8) | 20 (32.3) | 9 (32.1) |
| Length of hospital stay prior to inclusion in study, d, median (IQR) | 4.0 (3.07.0) | 3.0 (3.05.0) | 4.0 (2.05.0) |
| Patients preferring enteral route, n (%) | 137 (60.4) |
| Dislike of needles | 41 (30.0) |
| Pain from injection | 38 (27.7) |
| Ease of use | 18 (13.1) |
| Bruising from injection | 9 (6.6) |
| Other/no rationale | 31 (22.6) |
| Patients preferring injection route, n (%) | 62 (27.5) |
| Faster onset of action | 25 (40.3) |
| Pill burden | 11 (17.7) |
| Ease of use | 9 (14.5) |
| Other/no rationale | 17 (27.5) |
| Patients with no preference, n (%) | 28 (12.4) |
DISCUSSION
Using a mixed‐methods approach, we report the first survey evaluating patient preferences regarding pharmacologic VTE prophylaxis. We found that a majority of patients preferred an oral route of administration. Nevertheless, a substantial number of patients favored a subcutaneous route of administration believing it to be associated with a faster onset of action. Of interest, patients favoring subcutaneous injections were significantly less likely to refuse doses of ordered VTE prophylaxis. Given that all patients were prescribed a subcutaneous form of VTE prophylaxis, matching patient preference to VTE prophylaxis prescription could potentially increase adherence and reduce patient refusal of ordered prophylaxis. Considering the large number of patients who preferred an oral route of administration, the availability of an oral formulation may potentially result in improved adherence to inpatient VTE prophylaxis.
Our findings have significant implications for healthcare providers, and for patient safety and quality‐improvement researchers. VTE prophylaxis is an important patient‐safety practice, particularly for medically ill patients, which is believed to be underprescribed.[7] Recent studies have demonstrated that a significant number of doses of VTE prophylaxis are not administered, primarily due to patient refusal.[6] Our data indicate that tailoring the route of prophylaxis administration to patient preference may represent a feasible strategy to improve VTE prophylaxis administration rates. Recently, several target‐specific oral anticoagulants (TSOACs) have been approved for a variety of clinical indications, and all have been investigated for VTE prophylaxis.[7, 8, 9, 10, 11, 12, 13, 14, 15] However, no agent is currently US Food & Drug Administration (FDA) approved for primary prevention of VTE, although apixaban and rivaroxaban are FDA approved for VTE prevention in joint replacement.[13, 14] Although in some instances these TSOACs were noted to demonstrate only equivalent efficacy to standard subcutaneous forms of VTE prophylaxis, our data suggest that perhaps in some patients, use of these agents may result in better outcomes due to improved adherence to therapy due to a preferred oral route of administration. We think this hypothesis warrants further investigation.
Our study also underscores the importance of considering patient preferences when caring for patients as emphasized by the 2012 ACCP guidelines.[1] Our results indicate that consideration of patient preferences may lead to better patient care and better outcomes. Interestingly, there were no differences in preference based on education level or the type of service to which the patient was admitted. Clarification of uninformed opinions regarding the rationale for preference may also lead to more informed decisions by patients.
This study has a number of limitations. We only included patients on the internal medicine and general surgical services. It is possible that patients on other specialty services may have different opinions regarding prophylaxis that were not captured in our sample. Similarly, our sample size was limited, and approximately 15% of potential subjects did not participate. We do believe that our population is reflective of our institution based upon our previously published evaluation of multiple hospital units and the inclusion of low‐ and high‐performing units on both the medical and surgical services. Nevertheless, we believe that much more investigation of patient perspectives on VTE prophylaxis needs to be done to inform decision making, including the impact of patient preferences on VTE‐related outcomes. Additionally, we did not evaluate potential predictors of preference including admission diagnosis and duration of hospital length of stay.
In conclusion, we conducted a mixed‐methods analysis of patient preferences regarding pharmacologic VTE prophylaxis. Matching patient preference to ordered VTE prophylaxis may increase adherence to ordered prophylaxis. In this era of increasingly patient‐centered healthcare and expanding options for VTE prophylaxis, we believe information on patient preferences will be helpful to tailoring options for prevention and treatment.
ACKNOWLEDGMENTS
Disclosures: Dr. Haut is the primary investigator of the Mentored Clinician Scientist Development Award K08 1K08HS017952‐01 from the Agency for Healthcare Research and Quality entitled Does Screening Variability Make DVT an Unreliable Quality Measure of Trauma Care? Dr. Haut receives royalties from Lippincott, Williams, & Wilkins for a book he coauthored (Avoiding Common ICU Errors). He has received honoraria for various speaking engagements regarding clinical, quality, and safety topics and has given expert witness testimony in various medical malpractice cases. Dr. Streiff has received research funding from Sanofi‐Aventis and Bristol‐Myers Squibb; honoraria for Continuing Medial Education lectures from Sanofi‐Aventis and Ortho‐McNeil; consulted for Sanofi‐Aventis, Eisai, Daiichi‐Sankyo, and Janssen HealthCare; and has given expert witness testimony in various medical malpractice cases. Mr. Lau, Drs. Haut, Streiff, and Shermock are supported by a contract from the Patient‐Centered Outcomes Research Institute titled Preventing Venous Thromboembolism: Empowering Patients and Enabling Patient‐Centered Care via Health Information Technology (CE‐12‐11‐4489). Ms. Hobson has given expert witness testimony in various medical malpractice cases. All others have no relevant funding or conflicts of interest to report.
- , , , et al. Patient values and preferences in decision making for antithrombotic therapy: a systematic review. Chest. 2012;141(2):e1S–e23S.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
- , , , et al. Impact of a venous thromboembolism (VTE) prophylaxis “smart order set”: improved compliance, fewer events. Am J Hematol. 2013;88(7):545–549.
- , , , et al. Improved prophylaxis and decreased preventable harm with a mandatory computerized clinical decision support tool for venous thromboembolism (VTE) prophylaxis in trauma patients. Arch Surg. 2012;147(10):901–907.
- , , , , . Linking processes and outcomes: a key strategy to prevent and report harm from venous thromboembolism in surgical patients. JAMA Surg. 2013;148(3):299–300.
- , , , et al. Patterns of non‐administration of ordered doses of venous thromboembolism prophylaxis: implications for intervention strategies. PLoS One. 2013;8(6):e66311.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2008;371:387–394.
- , , , et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765–2775.
- , , , et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthoplasty. N Engl J Med. 2008;358:2776–2786.
- , , , , , . Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361:594–604.
- , , , , , . Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE‐2): a randomized double‐blind trial. Lancet. 2010;275:807–815.
- , , , et al. Rivaroxaban for the prevention of venous thromboembolism after hip or knee arthroplasty. Pooled analysis of four studies. Thromb Haemost. 2011;105:444–453.
- , , , et al. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med. 2011;365:2167–2177.
- , , , et al. Efficacy and safety of thromboprophylaxis with low‐molecular‐weight heparin or rivaroxaban in hip and knee replacement surgery: findings from the ORTHO‐TEP registry. Thromb Haemost. 2013;109:154–163.
- , , , et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:513–523.
- , , , et al. Patient values and preferences in decision making for antithrombotic therapy: a systematic review. Chest. 2012;141(2):e1S–e23S.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
- , , , et al. Impact of a venous thromboembolism (VTE) prophylaxis “smart order set”: improved compliance, fewer events. Am J Hematol. 2013;88(7):545–549.
- , , , et al. Improved prophylaxis and decreased preventable harm with a mandatory computerized clinical decision support tool for venous thromboembolism (VTE) prophylaxis in trauma patients. Arch Surg. 2012;147(10):901–907.
- , , , , . Linking processes and outcomes: a key strategy to prevent and report harm from venous thromboembolism in surgical patients. JAMA Surg. 2013;148(3):299–300.
- , , , et al. Patterns of non‐administration of ordered doses of venous thromboembolism prophylaxis: implications for intervention strategies. PLoS One. 2013;8(6):e66311.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2008;371:387–394.
- , , , et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765–2775.
- , , , et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthoplasty. N Engl J Med. 2008;358:2776–2786.
- , , , , , . Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361:594–604.
- , , , , , . Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE‐2): a randomized double‐blind trial. Lancet. 2010;275:807–815.
- , , , et al. Rivaroxaban for the prevention of venous thromboembolism after hip or knee arthroplasty. Pooled analysis of four studies. Thromb Haemost. 2011;105:444–453.
- , , , et al. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med. 2011;365:2167–2177.
- , , , et al. Efficacy and safety of thromboprophylaxis with low‐molecular‐weight heparin or rivaroxaban in hip and knee replacement surgery: findings from the ORTHO‐TEP registry. Thromb Haemost. 2013;109:154–163.
- , , , et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:513–523.
Thromboprophylaxis in Patients with HIV
Patients with human immunodeficiency virus (HIV) are at a 2‐ to 10‐fold greater risk for venous thromboembolism (VTE) compared with the general population.[1] Although antiphospholipid antibodies and protein S deficiency have often been cited as reasons for the thrombophilia associated with HIV, previous studies have also documented an increased risk of VTE with declining CD4+ cell count.[2, 3, 4, 5, 6, 7, 8] Worsening immune function places HIV patients at increased risk for opportunistic and nonopportunistic infections and malignancies, all independently associated with an increased risk of VTE.[5, 9, 10, 11, 12] Although increasing use of antiretroviral therapy has greatly decreased these sequelae, these complications of HIV infection are associated with an increased frequency of hospitalization.[13, 14, 15, 16] HIV infection and associated inflammation has been implicated in cardiovascular conditions such as cardiomyopathy, pulmonary hypertension, and myocardial infarction.[17, 18] Additionally, progression of HIV infection appears to influence T‐cell activation and differentiation in a manner that leads to early immunosenescence in infected individuals.[19, 20]
VTE prophylaxis is effective.[21] Virtually all efforts to decrease VTE have been focused on improving the prescription of prophylaxis with varying degrees of success.[22] These interventions have been employed with the tacit assumption that medication prescribed for inpatients will always be administered. However, at our institution, recent research has demonstrated that a significant proportion of prescribed thromboprophylaxis doses are not administered to hospitalized patients.[23] Refusal by the patient or a family member was the most commonly documented reason for dose nonadministration. In addition, the rate of thromboprophylaxis nonadministration varied greatly between nursing units with distinct patient populations. We hypothesized that nonadministration of VTE prophylaxis may be more common in patients with HIV, and this phenomenon may contribute to their increased risk for VTE.
The purpose of this study was to determine if the proportion of nonadministered thromboprophylaxis is greater among hospitalized patients with HIV and to characterize documented reasons for dose nonadministration.
METHODS
This study was conducted at The Johns Hopkins Hospital (JHH), a large, urban, academic medical center in Baltimore, Maryland. This single‐center retrospective cohort study utilized an existing dataset containing dose administration data extracted from an electronic medication administration record (eMAR). This dataset included information for all prescribed doses of thromboprophylaxis (heparin 5000 U subcutaneously every 8 or 12 hours, heparin 7500 U subcutaneously every 12 hours, enoxaparin 30 mg subcutaneously every 12 hours, or enoxaparin 40 mg subcutaneously daily) for patients hospitalized on medicine units at JHH from November 2007 to December 2008. This time period follows the implementation of an electronic order set for VTE prophylaxis.[24, 25] Data available for each dose included drug name, dose, frequency, patient demographics, and whether or not the dose was administered. Each dose not administered included a reason for nonadministration, which was chosen from a dropdown menu of responses on the eMAR by the nurse at the time the dose was due. A separate electronic report was obtained from an internal administrative database, which identified all patients within the dose administration dataset who had the International Classification of Diseases, 9th Revision code 042 (HIV diagnosis). A report identifying patient history numbers with matching diagnostic code for HIV was appended to the dose administration dataset using a relational database (Microsoft Access; Microsoft Corp., Redmond, WA) prior to analysis. The dose administration data were obtained previously for a separate analysis.[23] Approval for this study was granted from the institutional review board of Johns Hopkins Medicine.
Our analytic plan included comparisons between patients with and without HIV on a dose, patient, and unit level. As JHH operates a nursing unit dedicated to the inpatient care of patients with HIV, we included analyses of dose characteristics between this unit and other medicine units. It should be noted that patients without a diagnosis of HIV are sometimes cared for on this unit. Therefore, the electronic medical record for each patient without the diagnosis code for HIV hospitalized on this unit was reviewed to determine HIV status. An analysis was performed comparing visit identification numbers with diagnosis codes to identify potential seroconversions during the study period. Although we planned to compare nonadministration and documented refusal of doses on the unit level, a lack of patients with HIV on a number of units limited our ability to perform these analyses.
Statistical Analysis
The percent of doses not administered was calculated as the number of doses not administered divided by the number of doses prescribed. Likewise, the percent of prescribed doses documented as refused was calculated as the number of prescribed doses documented as refused divided by the number of doses prescribed. For each comparison, an odds ratio (OR) with 95% confidence interval (CI) was reported. Univariate and multivariate regression analyses were performed to assess the relationship between patient factors and dose nonadministration and documented refusal, respectively. Generalized estimating equations (GEE) using a logit link and an exchangeable correlation structure were used in these analyses. The GEE technique was used to account for within‐individual correlation of administration and documented refusal status.
Categorical data were compared using the two‐sided [2] test. Parametric and nonparametric continuous data were compared using the Student t test and Mann‐Whitney U test, respectively. A P value of <0.05 was considered statistically significant for all analyses. Analyses were performed using Minitab 15 (Minitab Inc., State College, PA) and Stata (StataCorp, College Station, TX).
RESULTS
During the 8‐month study period, 42,870 doses of thromboprophylaxis were prescribed during 4947 patient admissions to 13 individual medicine units. Overall, the diagnosis code for HIV was present in 12% of patient visits. The proportion of nonadministered doses per unit ranged from 6% to 27%, whereas the number of doses prescribed per unit ranged from 34 to 7301.
Patient characteristics were described on the visit level (Table 1). Patients with HIV were significantly younger, more often male and black, and had a longer length of stay compared with patients without HIV. Patients hospitalized on the HIV care unit had similar characteristics to the overall population of patients with HIV. It should be noted that not all patients cared for on this unit had a diagnosis of HIV, as patients from other medicine services are sometimes cared for in this location.
| Patients Without HIV | Patients With HIV | P | |
|---|---|---|---|
| |||
| Visits, n | 4,364 | 583 | N/A |
| Male, n (%) | 2,039 (47) | 370 (64) | <0.001 |
| Mean ageSD, y | 5618 | 469 | <0.001 |
| Race, n (%) | |||
| African American | 2,603 (60) | 522 (90) | <0.001 |
| Caucasian | 1,610 (37) | 53 (9) | <0.001 |
| Asian, Pacific Islander, other | 151 (4) | 8 (1) | 0.006 |
| Median length of stay (IQR), d | 3 (15) | 4 (27) | 0.002 |
| Marital status, n (%) | |||
| Single | 2,051 (47) | 471 (81) | <0.001 |
| Married | 1,405 (32) | 71 (12) | <0.001 |
| Widowed | 486 (11) | 10 (1) | <0.001 |
| Divorced | 402 (9) | 28 (5) | <0.001 |
| Separated | 33 (1) | 3 (1) | 0.607 |
| Unknown | 5 (0) | 0 (0) | 0.465 |
| Payor, n (%) | |||
| Medicare | 1,771 (41) | 133 (23) | <0.001 |
| Medicaid | 1,343 (31) | 392 (67) | <0.001 |
| Commercial | 1,181 (27) | 43 (7) | <0.001 |
| Other including self‐pay | 69 (1) | 15 (3) | 0.087 |
Overall, 17% of prescribed prophylaxis doses were not administered. A greater proportion of prescribed doses were not administered to patients with HIV compared with patients without HIV (23.5% vs 16.1%, OR: 1.59, 95% CI: 1.49‐1.70, P<0.001) (Table 2). Using a GEE and univariate regression, HIV diagnosis was associated with nonadministration of doses (OR: 1.37, 95% CI: 1.17‐1.60, P<0.001) (Table 3). Race, age, length of stay, and drug (heparin vs enoxaparin) were each associated with nonadministration. There was no significant association between nonadministration and sex, marital status, or payor. When stratified by nursing unit, there was substantial variation in the proportion of nonadministered doses between units. Within each unit, the proportion of doses not administered varied when stratified by HIV status. For example, on unit A, the proportion of doses not administered was greater for patients with HIV compared with patients without HIV (33.3% vs 12.9%, OR: 3.38, 95% CI: 2.61 to 4.37, P<0.001) (Figure 1). However, on unit K, the proportion of doses not administered to patients with HIV was 2‐fold less than in patients without HIV (7.2% vs 14.3%, OR: 0.47, 95% CI: 0.30‐0.74, P<0.001). Unit‐level analysis was not possible in regression models due to drastic imbalance in the prevalence of HIV across units. When comparing doses prescribed in the HIV care unit to all other medicine units, the proportion not administered (23.9% vs 16.3%, OR: 1.61, 95% CI: 1.49‐1.73, P<0.001) closely resembled the values seen when comparing patients with and without HIV hospital wide (23.5% vs 16.1%). However, when doses on the HIV care unit were stratified by HIV status, the doses not administered were 2‐fold greater, as a proportion, for patients with HIV compared with those without HIV (26.4% vs 13.1%, OR: 2.39, 95% CI: 1.93‐2.96, P<0.001).
| Doses Prescribed | Doses Not Administered (% of Doses Prescribed) | Doses Documented as Refused (% of All Doses Prescribed) | |
|---|---|---|---|
| |||
| All patients with HIV | 5,681 | 1,334 (23.5%)a | 935 (16.5%)a |
| All patients without HIV | 37,189 | 6,005 (16.1%) | 3,935 (10.6%) |
| HIV care unit | 4,452 | 1,063 (23.9%)a | 709 (15.9%)a |
| All other units | 38,418 | 6,276 (16.3%) | 4,161 (10.8%) |
| HIV care unit: patients with HIV | 3,602 | 952 (26.4%)a | 651 (18.1%)a |
| HIV care unit: patients without HIV | 850 | 111 (13.1%) | 58 (6.8%) |
| All other units: patients with HIV | 2,079 | 382 (18.4%)b | 284 (13.7%)a |
| All other units: patients without HIV | 36,339 | 5,894 (16.2%) | 3,877 (10.7%) |
| Nonadministered, n (%) | P | Documented as Refused, n (%) | P | |
|---|---|---|---|---|
| ||||
| Race | 0.001 | 0.072 | ||
| African American | 2,601 (17.8) | 1,708 (11.7) | ||
| Caucasian | 4,379 (16.4) | 2,922 (10.9) | ||
| Asian, Pacific Islander, other | 359 (23.4) | 240 (15.6) | ||
| HIV status | <0.001 | 0.002 | ||
| Negative | 6,005 (16.2) | 3,935 (10.6) | ||
| Positive | 1,344 (23.5) | 935 (16.5) | ||
| Age, y | <0.001 | <0.001 | ||
| 19 | 59 (20.6) | 44 (15.3) | ||
| 2029 | 1,260 (33.8) | 1,000 (26.8) | ||
| 3039 | 1,088 (28.1) | 845 (21.8) | ||
| 4049 | 1,628 (21.0) | 1,104 (14.2) | ||
| 5059 | 1,493 (16.1) | 953 (10.3) | ||
| 6069 | 900 (12.6) | 515 (7.2) | ||
| 7079 | 571 (9.6) | 250 (4.2) | ||
| 8089 | 252 (6.2) | 95 (2.3) | ||
| 90 | 88 (11.5) | 84 (8.4) | ||
| Sex | 0.372 | 0.919 | ||
| Male | 3,689 (17.3) | 2,392 (11.2) | ||
| Female | 3,650 (17.0) | 2,478 (11.5) | ||
| Drug | <0.001 | <0.001 | ||
| Heparin | 6,833 (18.4) | 4,515 (12.2) | ||
| Enoxaparin | 506 (8.9) | 355 (6.2) | ||
| Length of stay, d | <0.001 | <0.001 | ||
| 01 | 446 (24.3) | 282 (15.4) | ||
| 23 | 1,463 (19.4) | 971 (12.9) | ||
| 47 | 2,332 (18.9) | 1,620 (13.1) | ||
| 8 | 3,098 (14.6) | 1,997 (9.4) | ||
The results of the multivariate regression analyses with GEE are displayed in Table 4. HIV diagnosis, non‐African American race, and heparin (as compared with enoxaparin) were associated with increased likelihood of nonadministration. Increasing age and increasing length of stay were associated with decreased likelihood of nonadministration by a small but significant amount.
| OR of Nonadministration | 95% CI, P | OR of Documented Refusal | 95% CI, P | |
|---|---|---|---|---|
| ||||
| Race | ||||
| African American | 1.00 | Reference | 1.00 | Reference |
| Caucasian | 1.62 | 1.44‐1.81, <0.001 | 1.53 | 1.32‐1.77, <0.001 |
| Asian, Pacific Islander, Other | 1.54 | 1.19‐2.00, 0.001 | 1.48 | 1.07‐2.01, 0.019 |
| HIV status | ||||
| Negative | 1.00 | Reference | 1.00 | Reference |
| Positive | 1.21 | 1.001.45, 0.039 | 1.29 | 1.06‐1.56, 0.012 |
| Age, per year | 0.97 | 0.97‐0.98, <0.001 | 0.97 | 0.96‐0.97, <0.001 |
| Drug | ||||
| Heparin | 1.00 | Reference | 1.00 | Reference |
| Enoxaparin | 0.45 | 0.40‐0.51, <0.001 | 0.53 | 0.47‐0.61, <0.001 |
| Length of stay, per day | 0.991 | 0.987‐0.995, <0.001 | 0.989 | 0.983‐0.993, <0.001 |
The most commonly documented reason for nonadministration was refusal by the patient or family member (66% of all doses not administered). The second most common reason, patient condition not appropriate, accounted for an additional 10% of doses. Across all nursing units, the proportion of prescribed doses that were documented as refused was significantly greater for patients with HIV compared with patients without HIV (16.5% vs 10.6%, OR: 1.66, 95% CI: 1.54‐1.80, P<0.0001) (Table 2). Using the GEE and multivariate regression, HIV diagnosis, non‐African American race, and heparin were associated with increased risk of documented dose refusal. Age and length of stay were inversely related to the likelihood of documented dose refusal. When all administered doses were excluded from the analysis, the association between these variables and documented dose refusal were not as strong. Age and length of stay remained significantly inversely related; however, the other factors were no longer significantly positively associated with documented dose refusal.
Within the HIV care unit, the proportion of prescribed doses documented as refused was greater for patients with HIV compared with patients without HIV (18.1% vs 6.8%, OR: 3.01, 95% CI: 2.28‐3.99, P<0.0001). For all other medicine units, the proportion of nonadministered doses documented as refused was also greater for patients with HIV compared with patients without HIV (13.7% vs 10.7%, OR: 1.32, 95% CI: 1.16‐1.51, P<0.0001).
DISCUSSION
We have identified that nonadministration of thromboprophylaxis was more common among patients with HIV at our institution. Substantial variation in the proportion of doses not administered existed on the nursing unit level, as well as within each unit when stratified by HIV status. This disparity in dose administration was observed on the HIV care unit as well, as the proportion not administered was about 2‐fold greater for patients with HIV compared with those without HIV. Documented dose refusal appeared to account for the majority of nonadministered doses in our cohort. Our analysis also demonstrated that HIV diagnosis is significantly associated with both dose nonadministration and documented dose refusal at our institution.
Medication refusal is a well‐recognized phenomenon among hospitalized patients. A recent study of medication administration in hospitalized patients in the United Kingdom noted that refusal accounted for about 45% of omitted doses.[26] Fanikos et al. also found that documented refusal of doses contributed significantly to the overall number of VTE prophylaxis doses not administered to patients.[27] In our study, the proportion of nonadministered doses documented as refused by the patient or family member was significantly greater in patients with HIV than in patients without HIV across all units. Interestingly, the difference was greater on the HIV care unit when doses were stratified by HIV status. This observation leads us to hypothesize that specific hospital care environments may influence dose nonadministration and refusal rates among our patient population.
Based on regression analyses, increasing age and length of stay were associated with a decreased likelihood of any particular dose not being administered and with any particular dose being documented as refused. It is important to note that our GEE did not take into account date or time of each dose, and therefore we cannot make conclusions as to the likelihood of dose nonadministration or refusal of doses in relation to each other on a time scale. One cannot assume that a dose due later in a hospital course was more or less likely to be given than a dose due on the first hospital day. Although we did not expect these findings, one can hypothesize that patients who are older or have longer stays may be perceived to have more severe illness, and therefore greater need for prophylaxis, from nursing staff and others involved in their care. The associations were small but significant and warrant future investigation.
To our knowledge, this is the first investigation comparing the proportion of nonadministered doses of thromboprophylaxis between patients with and without HIV. Our data show that nonadministered doses and refused doses of thromboprophylaxis are more frequent among patients with HIV. In addition, we noted that nonadministration was more common on the dedicated HIV care unit compared with other units. We cannot currently offer a clear explanation for the disparity observed between units, and more specifically, within the HIV care unit. However, it is possible that a unique culture of care and provider‐specific factors may contribute.
Our study was limited by a number of factors. Seroconversion among patients during the study period was possible; however, our analysis revealed only 2 instances among nearly 4000 unique patients. A more significant limitation was the level of analysis allowed by the dataset. We examined dose characteristics on a dose and unit level, but the ability to analyze doses based on the prescriber and nurse level may have provided valuable insight into the specific reasons behind the observations presented here. Additionally, the specific unit assigned to a given dose in our dataset represented the discharge location for the corresponding patient, making it possible that some amount of nonadministered doses may be attributed to the incorrect unit. However, we do not believe that unit‐to‐unit transfers would be frequent enough to influence the overall results. In addition, we did not link nonadministration of thromboprophylaxis with VTE events, as these data were not present in the current dataset. Although this is a limitation of the current study, we believe that the notion that missed doses of thromboprophylaxis place patients at higher risk for VTE is plausible, as the efficacy of thromboprophylaxis is well established.[28, 29, 30] It is important to note that the reason for nonadministration selected by the nurse on the eMAR may not always represent the only reason or even the true reason for dose nonadministration. It is possible that dose refusal may be over‐represented in our sample, in part due to inaccurate documentation. Recent investigations at JHH have identified varying attitudes on the part of the patient and the nurse regarding thromboprophylaxis. A questionnaire and interview of patients showed a large knowledge gap regarding thromboprophylaxis, with many individuals unable to explain its role or significance in their medical care.[31] A common theme was also observed in a survey of nurses regarding VTE prophylaxis: doses were sometimes considered optional for reasons such as ambulation status, perceived severity of illness, or reason for hospitalization. Some nurses also reported that after an initial refused dose, they may continue to document subsequent doses as refused, sometimes without offering the dose to the patient.[32] As variation in practice was observed between individual nurses, it is also likely that the culture of care may vary between units, influencing thromboprophylaxis nonadministration rates as well as documentation of doses as refused. The dose‐level data used for the GEE analyses did not include date and time of administration, which limited the ability of the GEE to more completely account for autocorrelation.
To further investigate the findings of this and related studies, we intend to more closely analyze data at multiple levels with the goal of identifying an appropriate and feasible target for intervention. Additionally, further investigation should be performed with the goal of determining the relationship between decreased exposure to thromboprophylaxis and VTE. However, as patients with HIV appear to be at increased risk of VTE, ensuring that thromboprophylaxis is delivered appropriately and consistently should be an important goal for all who provide care to this population.
- , , , , . Venous thromboembolism in patients with HIV/AIDS: a case‐control study. J Acquir Immune Defic Syndr. 2008;48(3):310–314.
- , , . AIDS and thrombosis: retrospective study of 131 HIV‐infected patients. AIDS Patient Care STDS. 2001;15(6):311–320.
- , , , et al. HIV and risk of venous thromboembolism: a Danish nationwide population‐based cohort study. HIV Med. 2011;12(4):202–210.
- , , , . Epidemiology of thrombosis in HIV‐infected individuals. The adult/adolescent spectrum of HIV disease project. AIDS. 2000;14(3):321–324.
- , , . Thrombotic complications in patients infected with HIV in the era of highly active antiretroviral therapy: a case series. Clin Infect Dis. 2004;39(8):1214–1222.
- , , . Circulating coagulation inhibitors in the acquired immunodeficiency syndrome. Ann Intern Med. 1986;104(2):175–180.
- , . The pathogenesis of the antiphospholipid syndrome. N Engl J Med. 2013;368(11):1033–1044.
- , , , . Acquired protein C and protein S deficiency in HIV‐infected patients. Clin Appl Thromb Hemost. 2003;9(4):325–331.
- , , , et al. Antiphospholipid syndrome associated with cytomegalovirus infection: case report and review. Clin Infect Dis. 1997;24(2):197–200.
- , . Viral infections and antiphospholipid antibodies. Semin Arthritis Rheum. 2002;31(4):256–263.
- , . AIDS‐defining and non‐AIDS‐defining malignancies: cancer occurrence in the antiretroviral therapy era. Curr Opin Oncol. 2007;19(5):446–451.
- , , , , . Pathogenesis, clinical and laboratory aspects of thrombosis in cancer. J Thromb Thrombolysis. 2007;24(1):29–38.
- , , , et al. Patterns of diagnoses in hospital admissions in a multistate cohort of HIV‐positive adults in 2001. Med Care. 2005;43(9 suppl):III3–III14.
- , , , et al. Opportunistic infections as causes of death in HIV‐infected patients in the HAART era in France. Scand J Infect Dis. 2005;37(6‐7):482–487.
- , , , et al. Rates of hospitalizations and associated diagnoses in a large multisite cohort of HIV patients in the United States, 1994–2005. AIDS. 2008;22(11):1345–1354.
- , , . Hospitalizations for metabolic conditions, opportunistic infections, and injection drug use among HIV patients: trends between 1996 and 2000 in 12 states. J Acquir Immune Defic Syndr. 2005;40(5):609–616.
- , , , , , . Cardiovascular disease in HIV infection. Am Heart J. 2006;151:1147–1155.
- , , , et al. Epidemiological evidence for cardiovascular disease in HIV‐infected patients and relationship to highly active antiretroviral therapy. Circulation. 2008;118(2):e29–e35.
- , , , et al. Immune activation and CD8+ T‐cell differentiation towards senescence in HIV‐1 infection. PLoS Biol. 2004;2(2):E20.
- , , , , . CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV‐1 and HIV‐2 but only indirectly to the viral load. J Immunol. 2002;169(6):3400–3406.
- , . Thromboprophylaxis in nonsurgical patients. Hematology Am Soc Hematol Educ Program. 2012;2012:631–637.
- , . Practices to prevent venous thromboembolism [published online ahead of print May 24, 2013]. BMJ Qual Saf. doi:10.1136/bmjqs‐2012‐001782.
- , , , et al. Patterns of non‐administration of ordered doses of venous thromboembolism prophylaxis: implications for novel intervention strategies. PLoS One. 2013;8(6):e66311.
- , , , et al. Lessons from The Johns Hopkins multi‐disciplinary venous thromboembolism (VTE) prevention collaborative. BMJ. 2012;344:e3935.
- , , , et al. Impact of a venous thromboembolism prophylaxis “smart order set”: improved compliance, fewer events [published online ahead of print April 4, 2013]. Am J Hematol. doi: 10.1002/ajh.23450.
- , , . Dose omissions in hospitalized patients in a UK hospital: an analysis of the relative contribution of adverse drug reactions. Drug Saf. 2012;35(8):677–683.
- , , , et al. Adherence to pharmacological thromboprophylaxis orders in hospitalized patients. Am J Med. 2010;123(6):536–541.
- , , , et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in medical patients with enoxaparin study group. N Engl J Med. 1999;341(11):793–800.
- , , , et al. Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7):874–879.
- , , , et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332(7537):325–329.
- , , , et al. Patient perspectives on pharmacological venous thromboembolism prophylaxis at The Johns Hopkins Hospital. J Thromb Thrombolysis. 2013;35(3):416.
- , , , et al. Culture of care and documented patient refusal of pharmacologic venous thromboembolism prophylaxis. J Thromb Thrombolysis. 2011;31(3):367–400.
Patients with human immunodeficiency virus (HIV) are at a 2‐ to 10‐fold greater risk for venous thromboembolism (VTE) compared with the general population.[1] Although antiphospholipid antibodies and protein S deficiency have often been cited as reasons for the thrombophilia associated with HIV, previous studies have also documented an increased risk of VTE with declining CD4+ cell count.[2, 3, 4, 5, 6, 7, 8] Worsening immune function places HIV patients at increased risk for opportunistic and nonopportunistic infections and malignancies, all independently associated with an increased risk of VTE.[5, 9, 10, 11, 12] Although increasing use of antiretroviral therapy has greatly decreased these sequelae, these complications of HIV infection are associated with an increased frequency of hospitalization.[13, 14, 15, 16] HIV infection and associated inflammation has been implicated in cardiovascular conditions such as cardiomyopathy, pulmonary hypertension, and myocardial infarction.[17, 18] Additionally, progression of HIV infection appears to influence T‐cell activation and differentiation in a manner that leads to early immunosenescence in infected individuals.[19, 20]
VTE prophylaxis is effective.[21] Virtually all efforts to decrease VTE have been focused on improving the prescription of prophylaxis with varying degrees of success.[22] These interventions have been employed with the tacit assumption that medication prescribed for inpatients will always be administered. However, at our institution, recent research has demonstrated that a significant proportion of prescribed thromboprophylaxis doses are not administered to hospitalized patients.[23] Refusal by the patient or a family member was the most commonly documented reason for dose nonadministration. In addition, the rate of thromboprophylaxis nonadministration varied greatly between nursing units with distinct patient populations. We hypothesized that nonadministration of VTE prophylaxis may be more common in patients with HIV, and this phenomenon may contribute to their increased risk for VTE.
The purpose of this study was to determine if the proportion of nonadministered thromboprophylaxis is greater among hospitalized patients with HIV and to characterize documented reasons for dose nonadministration.
METHODS
This study was conducted at The Johns Hopkins Hospital (JHH), a large, urban, academic medical center in Baltimore, Maryland. This single‐center retrospective cohort study utilized an existing dataset containing dose administration data extracted from an electronic medication administration record (eMAR). This dataset included information for all prescribed doses of thromboprophylaxis (heparin 5000 U subcutaneously every 8 or 12 hours, heparin 7500 U subcutaneously every 12 hours, enoxaparin 30 mg subcutaneously every 12 hours, or enoxaparin 40 mg subcutaneously daily) for patients hospitalized on medicine units at JHH from November 2007 to December 2008. This time period follows the implementation of an electronic order set for VTE prophylaxis.[24, 25] Data available for each dose included drug name, dose, frequency, patient demographics, and whether or not the dose was administered. Each dose not administered included a reason for nonadministration, which was chosen from a dropdown menu of responses on the eMAR by the nurse at the time the dose was due. A separate electronic report was obtained from an internal administrative database, which identified all patients within the dose administration dataset who had the International Classification of Diseases, 9th Revision code 042 (HIV diagnosis). A report identifying patient history numbers with matching diagnostic code for HIV was appended to the dose administration dataset using a relational database (Microsoft Access; Microsoft Corp., Redmond, WA) prior to analysis. The dose administration data were obtained previously for a separate analysis.[23] Approval for this study was granted from the institutional review board of Johns Hopkins Medicine.
Our analytic plan included comparisons between patients with and without HIV on a dose, patient, and unit level. As JHH operates a nursing unit dedicated to the inpatient care of patients with HIV, we included analyses of dose characteristics between this unit and other medicine units. It should be noted that patients without a diagnosis of HIV are sometimes cared for on this unit. Therefore, the electronic medical record for each patient without the diagnosis code for HIV hospitalized on this unit was reviewed to determine HIV status. An analysis was performed comparing visit identification numbers with diagnosis codes to identify potential seroconversions during the study period. Although we planned to compare nonadministration and documented refusal of doses on the unit level, a lack of patients with HIV on a number of units limited our ability to perform these analyses.
Statistical Analysis
The percent of doses not administered was calculated as the number of doses not administered divided by the number of doses prescribed. Likewise, the percent of prescribed doses documented as refused was calculated as the number of prescribed doses documented as refused divided by the number of doses prescribed. For each comparison, an odds ratio (OR) with 95% confidence interval (CI) was reported. Univariate and multivariate regression analyses were performed to assess the relationship between patient factors and dose nonadministration and documented refusal, respectively. Generalized estimating equations (GEE) using a logit link and an exchangeable correlation structure were used in these analyses. The GEE technique was used to account for within‐individual correlation of administration and documented refusal status.
Categorical data were compared using the two‐sided [2] test. Parametric and nonparametric continuous data were compared using the Student t test and Mann‐Whitney U test, respectively. A P value of <0.05 was considered statistically significant for all analyses. Analyses were performed using Minitab 15 (Minitab Inc., State College, PA) and Stata (StataCorp, College Station, TX).
RESULTS
During the 8‐month study period, 42,870 doses of thromboprophylaxis were prescribed during 4947 patient admissions to 13 individual medicine units. Overall, the diagnosis code for HIV was present in 12% of patient visits. The proportion of nonadministered doses per unit ranged from 6% to 27%, whereas the number of doses prescribed per unit ranged from 34 to 7301.
Patient characteristics were described on the visit level (Table 1). Patients with HIV were significantly younger, more often male and black, and had a longer length of stay compared with patients without HIV. Patients hospitalized on the HIV care unit had similar characteristics to the overall population of patients with HIV. It should be noted that not all patients cared for on this unit had a diagnosis of HIV, as patients from other medicine services are sometimes cared for in this location.
| Patients Without HIV | Patients With HIV | P | |
|---|---|---|---|
| |||
| Visits, n | 4,364 | 583 | N/A |
| Male, n (%) | 2,039 (47) | 370 (64) | <0.001 |
| Mean ageSD, y | 5618 | 469 | <0.001 |
| Race, n (%) | |||
| African American | 2,603 (60) | 522 (90) | <0.001 |
| Caucasian | 1,610 (37) | 53 (9) | <0.001 |
| Asian, Pacific Islander, other | 151 (4) | 8 (1) | 0.006 |
| Median length of stay (IQR), d | 3 (15) | 4 (27) | 0.002 |
| Marital status, n (%) | |||
| Single | 2,051 (47) | 471 (81) | <0.001 |
| Married | 1,405 (32) | 71 (12) | <0.001 |
| Widowed | 486 (11) | 10 (1) | <0.001 |
| Divorced | 402 (9) | 28 (5) | <0.001 |
| Separated | 33 (1) | 3 (1) | 0.607 |
| Unknown | 5 (0) | 0 (0) | 0.465 |
| Payor, n (%) | |||
| Medicare | 1,771 (41) | 133 (23) | <0.001 |
| Medicaid | 1,343 (31) | 392 (67) | <0.001 |
| Commercial | 1,181 (27) | 43 (7) | <0.001 |
| Other including self‐pay | 69 (1) | 15 (3) | 0.087 |
Overall, 17% of prescribed prophylaxis doses were not administered. A greater proportion of prescribed doses were not administered to patients with HIV compared with patients without HIV (23.5% vs 16.1%, OR: 1.59, 95% CI: 1.49‐1.70, P<0.001) (Table 2). Using a GEE and univariate regression, HIV diagnosis was associated with nonadministration of doses (OR: 1.37, 95% CI: 1.17‐1.60, P<0.001) (Table 3). Race, age, length of stay, and drug (heparin vs enoxaparin) were each associated with nonadministration. There was no significant association between nonadministration and sex, marital status, or payor. When stratified by nursing unit, there was substantial variation in the proportion of nonadministered doses between units. Within each unit, the proportion of doses not administered varied when stratified by HIV status. For example, on unit A, the proportion of doses not administered was greater for patients with HIV compared with patients without HIV (33.3% vs 12.9%, OR: 3.38, 95% CI: 2.61 to 4.37, P<0.001) (Figure 1). However, on unit K, the proportion of doses not administered to patients with HIV was 2‐fold less than in patients without HIV (7.2% vs 14.3%, OR: 0.47, 95% CI: 0.30‐0.74, P<0.001). Unit‐level analysis was not possible in regression models due to drastic imbalance in the prevalence of HIV across units. When comparing doses prescribed in the HIV care unit to all other medicine units, the proportion not administered (23.9% vs 16.3%, OR: 1.61, 95% CI: 1.49‐1.73, P<0.001) closely resembled the values seen when comparing patients with and without HIV hospital wide (23.5% vs 16.1%). However, when doses on the HIV care unit were stratified by HIV status, the doses not administered were 2‐fold greater, as a proportion, for patients with HIV compared with those without HIV (26.4% vs 13.1%, OR: 2.39, 95% CI: 1.93‐2.96, P<0.001).
| Doses Prescribed | Doses Not Administered (% of Doses Prescribed) | Doses Documented as Refused (% of All Doses Prescribed) | |
|---|---|---|---|
| |||
| All patients with HIV | 5,681 | 1,334 (23.5%)a | 935 (16.5%)a |
| All patients without HIV | 37,189 | 6,005 (16.1%) | 3,935 (10.6%) |
| HIV care unit | 4,452 | 1,063 (23.9%)a | 709 (15.9%)a |
| All other units | 38,418 | 6,276 (16.3%) | 4,161 (10.8%) |
| HIV care unit: patients with HIV | 3,602 | 952 (26.4%)a | 651 (18.1%)a |
| HIV care unit: patients without HIV | 850 | 111 (13.1%) | 58 (6.8%) |
| All other units: patients with HIV | 2,079 | 382 (18.4%)b | 284 (13.7%)a |
| All other units: patients without HIV | 36,339 | 5,894 (16.2%) | 3,877 (10.7%) |
| Nonadministered, n (%) | P | Documented as Refused, n (%) | P | |
|---|---|---|---|---|
| ||||
| Race | 0.001 | 0.072 | ||
| African American | 2,601 (17.8) | 1,708 (11.7) | ||
| Caucasian | 4,379 (16.4) | 2,922 (10.9) | ||
| Asian, Pacific Islander, other | 359 (23.4) | 240 (15.6) | ||
| HIV status | <0.001 | 0.002 | ||
| Negative | 6,005 (16.2) | 3,935 (10.6) | ||
| Positive | 1,344 (23.5) | 935 (16.5) | ||
| Age, y | <0.001 | <0.001 | ||
| 19 | 59 (20.6) | 44 (15.3) | ||
| 2029 | 1,260 (33.8) | 1,000 (26.8) | ||
| 3039 | 1,088 (28.1) | 845 (21.8) | ||
| 4049 | 1,628 (21.0) | 1,104 (14.2) | ||
| 5059 | 1,493 (16.1) | 953 (10.3) | ||
| 6069 | 900 (12.6) | 515 (7.2) | ||
| 7079 | 571 (9.6) | 250 (4.2) | ||
| 8089 | 252 (6.2) | 95 (2.3) | ||
| 90 | 88 (11.5) | 84 (8.4) | ||
| Sex | 0.372 | 0.919 | ||
| Male | 3,689 (17.3) | 2,392 (11.2) | ||
| Female | 3,650 (17.0) | 2,478 (11.5) | ||
| Drug | <0.001 | <0.001 | ||
| Heparin | 6,833 (18.4) | 4,515 (12.2) | ||
| Enoxaparin | 506 (8.9) | 355 (6.2) | ||
| Length of stay, d | <0.001 | <0.001 | ||
| 01 | 446 (24.3) | 282 (15.4) | ||
| 23 | 1,463 (19.4) | 971 (12.9) | ||
| 47 | 2,332 (18.9) | 1,620 (13.1) | ||
| 8 | 3,098 (14.6) | 1,997 (9.4) | ||

The results of the multivariate regression analyses with GEE are displayed in Table 4. HIV diagnosis, non‐African American race, and heparin (as compared with enoxaparin) were associated with increased likelihood of nonadministration. Increasing age and increasing length of stay were associated with decreased likelihood of nonadministration by a small but significant amount.
| OR of Nonadministration | 95% CI, P | OR of Documented Refusal | 95% CI, P | |
|---|---|---|---|---|
| ||||
| Race | ||||
| African American | 1.00 | Reference | 1.00 | Reference |
| Caucasian | 1.62 | 1.44‐1.81, <0.001 | 1.53 | 1.32‐1.77, <0.001 |
| Asian, Pacific Islander, Other | 1.54 | 1.19‐2.00, 0.001 | 1.48 | 1.07‐2.01, 0.019 |
| HIV status | ||||
| Negative | 1.00 | Reference | 1.00 | Reference |
| Positive | 1.21 | 1.001.45, 0.039 | 1.29 | 1.06‐1.56, 0.012 |
| Age, per year | 0.97 | 0.97‐0.98, <0.001 | 0.97 | 0.96‐0.97, <0.001 |
| Drug | ||||
| Heparin | 1.00 | Reference | 1.00 | Reference |
| Enoxaparin | 0.45 | 0.40‐0.51, <0.001 | 0.53 | 0.47‐0.61, <0.001 |
| Length of stay, per day | 0.991 | 0.987‐0.995, <0.001 | 0.989 | 0.983‐0.993, <0.001 |
The most commonly documented reason for nonadministration was refusal by the patient or family member (66% of all doses not administered). The second most common reason, patient condition not appropriate, accounted for an additional 10% of doses. Across all nursing units, the proportion of prescribed doses that were documented as refused was significantly greater for patients with HIV compared with patients without HIV (16.5% vs 10.6%, OR: 1.66, 95% CI: 1.54‐1.80, P<0.0001) (Table 2). Using the GEE and multivariate regression, HIV diagnosis, non‐African American race, and heparin were associated with increased risk of documented dose refusal. Age and length of stay were inversely related to the likelihood of documented dose refusal. When all administered doses were excluded from the analysis, the association between these variables and documented dose refusal were not as strong. Age and length of stay remained significantly inversely related; however, the other factors were no longer significantly positively associated with documented dose refusal.
Within the HIV care unit, the proportion of prescribed doses documented as refused was greater for patients with HIV compared with patients without HIV (18.1% vs 6.8%, OR: 3.01, 95% CI: 2.28‐3.99, P<0.0001). For all other medicine units, the proportion of nonadministered doses documented as refused was also greater for patients with HIV compared with patients without HIV (13.7% vs 10.7%, OR: 1.32, 95% CI: 1.16‐1.51, P<0.0001).
DISCUSSION
We have identified that nonadministration of thromboprophylaxis was more common among patients with HIV at our institution. Substantial variation in the proportion of doses not administered existed on the nursing unit level, as well as within each unit when stratified by HIV status. This disparity in dose administration was observed on the HIV care unit as well, as the proportion not administered was about 2‐fold greater for patients with HIV compared with those without HIV. Documented dose refusal appeared to account for the majority of nonadministered doses in our cohort. Our analysis also demonstrated that HIV diagnosis is significantly associated with both dose nonadministration and documented dose refusal at our institution.
Medication refusal is a well‐recognized phenomenon among hospitalized patients. A recent study of medication administration in hospitalized patients in the United Kingdom noted that refusal accounted for about 45% of omitted doses.[26] Fanikos et al. also found that documented refusal of doses contributed significantly to the overall number of VTE prophylaxis doses not administered to patients.[27] In our study, the proportion of nonadministered doses documented as refused by the patient or family member was significantly greater in patients with HIV than in patients without HIV across all units. Interestingly, the difference was greater on the HIV care unit when doses were stratified by HIV status. This observation leads us to hypothesize that specific hospital care environments may influence dose nonadministration and refusal rates among our patient population.
Based on regression analyses, increasing age and length of stay were associated with a decreased likelihood of any particular dose not being administered and with any particular dose being documented as refused. It is important to note that our GEE did not take into account date or time of each dose, and therefore we cannot make conclusions as to the likelihood of dose nonadministration or refusal of doses in relation to each other on a time scale. One cannot assume that a dose due later in a hospital course was more or less likely to be given than a dose due on the first hospital day. Although we did not expect these findings, one can hypothesize that patients who are older or have longer stays may be perceived to have more severe illness, and therefore greater need for prophylaxis, from nursing staff and others involved in their care. The associations were small but significant and warrant future investigation.
To our knowledge, this is the first investigation comparing the proportion of nonadministered doses of thromboprophylaxis between patients with and without HIV. Our data show that nonadministered doses and refused doses of thromboprophylaxis are more frequent among patients with HIV. In addition, we noted that nonadministration was more common on the dedicated HIV care unit compared with other units. We cannot currently offer a clear explanation for the disparity observed between units, and more specifically, within the HIV care unit. However, it is possible that a unique culture of care and provider‐specific factors may contribute.
Our study was limited by a number of factors. Seroconversion among patients during the study period was possible; however, our analysis revealed only 2 instances among nearly 4000 unique patients. A more significant limitation was the level of analysis allowed by the dataset. We examined dose characteristics on a dose and unit level, but the ability to analyze doses based on the prescriber and nurse level may have provided valuable insight into the specific reasons behind the observations presented here. Additionally, the specific unit assigned to a given dose in our dataset represented the discharge location for the corresponding patient, making it possible that some amount of nonadministered doses may be attributed to the incorrect unit. However, we do not believe that unit‐to‐unit transfers would be frequent enough to influence the overall results. In addition, we did not link nonadministration of thromboprophylaxis with VTE events, as these data were not present in the current dataset. Although this is a limitation of the current study, we believe that the notion that missed doses of thromboprophylaxis place patients at higher risk for VTE is plausible, as the efficacy of thromboprophylaxis is well established.[28, 29, 30] It is important to note that the reason for nonadministration selected by the nurse on the eMAR may not always represent the only reason or even the true reason for dose nonadministration. It is possible that dose refusal may be over‐represented in our sample, in part due to inaccurate documentation. Recent investigations at JHH have identified varying attitudes on the part of the patient and the nurse regarding thromboprophylaxis. A questionnaire and interview of patients showed a large knowledge gap regarding thromboprophylaxis, with many individuals unable to explain its role or significance in their medical care.[31] A common theme was also observed in a survey of nurses regarding VTE prophylaxis: doses were sometimes considered optional for reasons such as ambulation status, perceived severity of illness, or reason for hospitalization. Some nurses also reported that after an initial refused dose, they may continue to document subsequent doses as refused, sometimes without offering the dose to the patient.[32] As variation in practice was observed between individual nurses, it is also likely that the culture of care may vary between units, influencing thromboprophylaxis nonadministration rates as well as documentation of doses as refused. The dose‐level data used for the GEE analyses did not include date and time of administration, which limited the ability of the GEE to more completely account for autocorrelation.
To further investigate the findings of this and related studies, we intend to more closely analyze data at multiple levels with the goal of identifying an appropriate and feasible target for intervention. Additionally, further investigation should be performed with the goal of determining the relationship between decreased exposure to thromboprophylaxis and VTE. However, as patients with HIV appear to be at increased risk of VTE, ensuring that thromboprophylaxis is delivered appropriately and consistently should be an important goal for all who provide care to this population.
Patients with human immunodeficiency virus (HIV) are at a 2‐ to 10‐fold greater risk for venous thromboembolism (VTE) compared with the general population.[1] Although antiphospholipid antibodies and protein S deficiency have often been cited as reasons for the thrombophilia associated with HIV, previous studies have also documented an increased risk of VTE with declining CD4+ cell count.[2, 3, 4, 5, 6, 7, 8] Worsening immune function places HIV patients at increased risk for opportunistic and nonopportunistic infections and malignancies, all independently associated with an increased risk of VTE.[5, 9, 10, 11, 12] Although increasing use of antiretroviral therapy has greatly decreased these sequelae, these complications of HIV infection are associated with an increased frequency of hospitalization.[13, 14, 15, 16] HIV infection and associated inflammation has been implicated in cardiovascular conditions such as cardiomyopathy, pulmonary hypertension, and myocardial infarction.[17, 18] Additionally, progression of HIV infection appears to influence T‐cell activation and differentiation in a manner that leads to early immunosenescence in infected individuals.[19, 20]
VTE prophylaxis is effective.[21] Virtually all efforts to decrease VTE have been focused on improving the prescription of prophylaxis with varying degrees of success.[22] These interventions have been employed with the tacit assumption that medication prescribed for inpatients will always be administered. However, at our institution, recent research has demonstrated that a significant proportion of prescribed thromboprophylaxis doses are not administered to hospitalized patients.[23] Refusal by the patient or a family member was the most commonly documented reason for dose nonadministration. In addition, the rate of thromboprophylaxis nonadministration varied greatly between nursing units with distinct patient populations. We hypothesized that nonadministration of VTE prophylaxis may be more common in patients with HIV, and this phenomenon may contribute to their increased risk for VTE.
The purpose of this study was to determine if the proportion of nonadministered thromboprophylaxis is greater among hospitalized patients with HIV and to characterize documented reasons for dose nonadministration.
METHODS
This study was conducted at The Johns Hopkins Hospital (JHH), a large, urban, academic medical center in Baltimore, Maryland. This single‐center retrospective cohort study utilized an existing dataset containing dose administration data extracted from an electronic medication administration record (eMAR). This dataset included information for all prescribed doses of thromboprophylaxis (heparin 5000 U subcutaneously every 8 or 12 hours, heparin 7500 U subcutaneously every 12 hours, enoxaparin 30 mg subcutaneously every 12 hours, or enoxaparin 40 mg subcutaneously daily) for patients hospitalized on medicine units at JHH from November 2007 to December 2008. This time period follows the implementation of an electronic order set for VTE prophylaxis.[24, 25] Data available for each dose included drug name, dose, frequency, patient demographics, and whether or not the dose was administered. Each dose not administered included a reason for nonadministration, which was chosen from a dropdown menu of responses on the eMAR by the nurse at the time the dose was due. A separate electronic report was obtained from an internal administrative database, which identified all patients within the dose administration dataset who had the International Classification of Diseases, 9th Revision code 042 (HIV diagnosis). A report identifying patient history numbers with matching diagnostic code for HIV was appended to the dose administration dataset using a relational database (Microsoft Access; Microsoft Corp., Redmond, WA) prior to analysis. The dose administration data were obtained previously for a separate analysis.[23] Approval for this study was granted from the institutional review board of Johns Hopkins Medicine.
Our analytic plan included comparisons between patients with and without HIV on a dose, patient, and unit level. As JHH operates a nursing unit dedicated to the inpatient care of patients with HIV, we included analyses of dose characteristics between this unit and other medicine units. It should be noted that patients without a diagnosis of HIV are sometimes cared for on this unit. Therefore, the electronic medical record for each patient without the diagnosis code for HIV hospitalized on this unit was reviewed to determine HIV status. An analysis was performed comparing visit identification numbers with diagnosis codes to identify potential seroconversions during the study period. Although we planned to compare nonadministration and documented refusal of doses on the unit level, a lack of patients with HIV on a number of units limited our ability to perform these analyses.
Statistical Analysis
The percent of doses not administered was calculated as the number of doses not administered divided by the number of doses prescribed. Likewise, the percent of prescribed doses documented as refused was calculated as the number of prescribed doses documented as refused divided by the number of doses prescribed. For each comparison, an odds ratio (OR) with 95% confidence interval (CI) was reported. Univariate and multivariate regression analyses were performed to assess the relationship between patient factors and dose nonadministration and documented refusal, respectively. Generalized estimating equations (GEE) using a logit link and an exchangeable correlation structure were used in these analyses. The GEE technique was used to account for within‐individual correlation of administration and documented refusal status.
Categorical data were compared using the two‐sided [2] test. Parametric and nonparametric continuous data were compared using the Student t test and Mann‐Whitney U test, respectively. A P value of <0.05 was considered statistically significant for all analyses. Analyses were performed using Minitab 15 (Minitab Inc., State College, PA) and Stata (StataCorp, College Station, TX).
RESULTS
During the 8‐month study period, 42,870 doses of thromboprophylaxis were prescribed during 4947 patient admissions to 13 individual medicine units. Overall, the diagnosis code for HIV was present in 12% of patient visits. The proportion of nonadministered doses per unit ranged from 6% to 27%, whereas the number of doses prescribed per unit ranged from 34 to 7301.
Patient characteristics were described on the visit level (Table 1). Patients with HIV were significantly younger, more often male and black, and had a longer length of stay compared with patients without HIV. Patients hospitalized on the HIV care unit had similar characteristics to the overall population of patients with HIV. It should be noted that not all patients cared for on this unit had a diagnosis of HIV, as patients from other medicine services are sometimes cared for in this location.
| Patients Without HIV | Patients With HIV | P | |
|---|---|---|---|
| |||
| Visits, n | 4,364 | 583 | N/A |
| Male, n (%) | 2,039 (47) | 370 (64) | <0.001 |
| Mean ageSD, y | 5618 | 469 | <0.001 |
| Race, n (%) | |||
| African American | 2,603 (60) | 522 (90) | <0.001 |
| Caucasian | 1,610 (37) | 53 (9) | <0.001 |
| Asian, Pacific Islander, other | 151 (4) | 8 (1) | 0.006 |
| Median length of stay (IQR), d | 3 (15) | 4 (27) | 0.002 |
| Marital status, n (%) | |||
| Single | 2,051 (47) | 471 (81) | <0.001 |
| Married | 1,405 (32) | 71 (12) | <0.001 |
| Widowed | 486 (11) | 10 (1) | <0.001 |
| Divorced | 402 (9) | 28 (5) | <0.001 |
| Separated | 33 (1) | 3 (1) | 0.607 |
| Unknown | 5 (0) | 0 (0) | 0.465 |
| Payor, n (%) | |||
| Medicare | 1,771 (41) | 133 (23) | <0.001 |
| Medicaid | 1,343 (31) | 392 (67) | <0.001 |
| Commercial | 1,181 (27) | 43 (7) | <0.001 |
| Other including self‐pay | 69 (1) | 15 (3) | 0.087 |
Overall, 17% of prescribed prophylaxis doses were not administered. A greater proportion of prescribed doses were not administered to patients with HIV compared with patients without HIV (23.5% vs 16.1%, OR: 1.59, 95% CI: 1.49‐1.70, P<0.001) (Table 2). Using a GEE and univariate regression, HIV diagnosis was associated with nonadministration of doses (OR: 1.37, 95% CI: 1.17‐1.60, P<0.001) (Table 3). Race, age, length of stay, and drug (heparin vs enoxaparin) were each associated with nonadministration. There was no significant association between nonadministration and sex, marital status, or payor. When stratified by nursing unit, there was substantial variation in the proportion of nonadministered doses between units. Within each unit, the proportion of doses not administered varied when stratified by HIV status. For example, on unit A, the proportion of doses not administered was greater for patients with HIV compared with patients without HIV (33.3% vs 12.9%, OR: 3.38, 95% CI: 2.61 to 4.37, P<0.001) (Figure 1). However, on unit K, the proportion of doses not administered to patients with HIV was 2‐fold less than in patients without HIV (7.2% vs 14.3%, OR: 0.47, 95% CI: 0.30‐0.74, P<0.001). Unit‐level analysis was not possible in regression models due to drastic imbalance in the prevalence of HIV across units. When comparing doses prescribed in the HIV care unit to all other medicine units, the proportion not administered (23.9% vs 16.3%, OR: 1.61, 95% CI: 1.49‐1.73, P<0.001) closely resembled the values seen when comparing patients with and without HIV hospital wide (23.5% vs 16.1%). However, when doses on the HIV care unit were stratified by HIV status, the doses not administered were 2‐fold greater, as a proportion, for patients with HIV compared with those without HIV (26.4% vs 13.1%, OR: 2.39, 95% CI: 1.93‐2.96, P<0.001).
| Doses Prescribed | Doses Not Administered (% of Doses Prescribed) | Doses Documented as Refused (% of All Doses Prescribed) | |
|---|---|---|---|
| |||
| All patients with HIV | 5,681 | 1,334 (23.5%)a | 935 (16.5%)a |
| All patients without HIV | 37,189 | 6,005 (16.1%) | 3,935 (10.6%) |
| HIV care unit | 4,452 | 1,063 (23.9%)a | 709 (15.9%)a |
| All other units | 38,418 | 6,276 (16.3%) | 4,161 (10.8%) |
| HIV care unit: patients with HIV | 3,602 | 952 (26.4%)a | 651 (18.1%)a |
| HIV care unit: patients without HIV | 850 | 111 (13.1%) | 58 (6.8%) |
| All other units: patients with HIV | 2,079 | 382 (18.4%)b | 284 (13.7%)a |
| All other units: patients without HIV | 36,339 | 5,894 (16.2%) | 3,877 (10.7%) |
| Nonadministered, n (%) | P | Documented as Refused, n (%) | P | |
|---|---|---|---|---|
| ||||
| Race | 0.001 | 0.072 | ||
| African American | 2,601 (17.8) | 1,708 (11.7) | ||
| Caucasian | 4,379 (16.4) | 2,922 (10.9) | ||
| Asian, Pacific Islander, other | 359 (23.4) | 240 (15.6) | ||
| HIV status | <0.001 | 0.002 | ||
| Negative | 6,005 (16.2) | 3,935 (10.6) | ||
| Positive | 1,344 (23.5) | 935 (16.5) | ||
| Age, y | <0.001 | <0.001 | ||
| 19 | 59 (20.6) | 44 (15.3) | ||
| 2029 | 1,260 (33.8) | 1,000 (26.8) | ||
| 3039 | 1,088 (28.1) | 845 (21.8) | ||
| 4049 | 1,628 (21.0) | 1,104 (14.2) | ||
| 5059 | 1,493 (16.1) | 953 (10.3) | ||
| 6069 | 900 (12.6) | 515 (7.2) | ||
| 7079 | 571 (9.6) | 250 (4.2) | ||
| 8089 | 252 (6.2) | 95 (2.3) | ||
| 90 | 88 (11.5) | 84 (8.4) | ||
| Sex | 0.372 | 0.919 | ||
| Male | 3,689 (17.3) | 2,392 (11.2) | ||
| Female | 3,650 (17.0) | 2,478 (11.5) | ||
| Drug | <0.001 | <0.001 | ||
| Heparin | 6,833 (18.4) | 4,515 (12.2) | ||
| Enoxaparin | 506 (8.9) | 355 (6.2) | ||
| Length of stay, d | <0.001 | <0.001 | ||
| 01 | 446 (24.3) | 282 (15.4) | ||
| 23 | 1,463 (19.4) | 971 (12.9) | ||
| 47 | 2,332 (18.9) | 1,620 (13.1) | ||
| 8 | 3,098 (14.6) | 1,997 (9.4) | ||

The results of the multivariate regression analyses with GEE are displayed in Table 4. HIV diagnosis, non‐African American race, and heparin (as compared with enoxaparin) were associated with increased likelihood of nonadministration. Increasing age and increasing length of stay were associated with decreased likelihood of nonadministration by a small but significant amount.
| OR of Nonadministration | 95% CI, P | OR of Documented Refusal | 95% CI, P | |
|---|---|---|---|---|
| ||||
| Race | ||||
| African American | 1.00 | Reference | 1.00 | Reference |
| Caucasian | 1.62 | 1.44‐1.81, <0.001 | 1.53 | 1.32‐1.77, <0.001 |
| Asian, Pacific Islander, Other | 1.54 | 1.19‐2.00, 0.001 | 1.48 | 1.07‐2.01, 0.019 |
| HIV status | ||||
| Negative | 1.00 | Reference | 1.00 | Reference |
| Positive | 1.21 | 1.001.45, 0.039 | 1.29 | 1.06‐1.56, 0.012 |
| Age, per year | 0.97 | 0.97‐0.98, <0.001 | 0.97 | 0.96‐0.97, <0.001 |
| Drug | ||||
| Heparin | 1.00 | Reference | 1.00 | Reference |
| Enoxaparin | 0.45 | 0.40‐0.51, <0.001 | 0.53 | 0.47‐0.61, <0.001 |
| Length of stay, per day | 0.991 | 0.987‐0.995, <0.001 | 0.989 | 0.983‐0.993, <0.001 |
The most commonly documented reason for nonadministration was refusal by the patient or family member (66% of all doses not administered). The second most common reason, patient condition not appropriate, accounted for an additional 10% of doses. Across all nursing units, the proportion of prescribed doses that were documented as refused was significantly greater for patients with HIV compared with patients without HIV (16.5% vs 10.6%, OR: 1.66, 95% CI: 1.54‐1.80, P<0.0001) (Table 2). Using the GEE and multivariate regression, HIV diagnosis, non‐African American race, and heparin were associated with increased risk of documented dose refusal. Age and length of stay were inversely related to the likelihood of documented dose refusal. When all administered doses were excluded from the analysis, the association between these variables and documented dose refusal were not as strong. Age and length of stay remained significantly inversely related; however, the other factors were no longer significantly positively associated with documented dose refusal.
Within the HIV care unit, the proportion of prescribed doses documented as refused was greater for patients with HIV compared with patients without HIV (18.1% vs 6.8%, OR: 3.01, 95% CI: 2.28‐3.99, P<0.0001). For all other medicine units, the proportion of nonadministered doses documented as refused was also greater for patients with HIV compared with patients without HIV (13.7% vs 10.7%, OR: 1.32, 95% CI: 1.16‐1.51, P<0.0001).
DISCUSSION
We have identified that nonadministration of thromboprophylaxis was more common among patients with HIV at our institution. Substantial variation in the proportion of doses not administered existed on the nursing unit level, as well as within each unit when stratified by HIV status. This disparity in dose administration was observed on the HIV care unit as well, as the proportion not administered was about 2‐fold greater for patients with HIV compared with those without HIV. Documented dose refusal appeared to account for the majority of nonadministered doses in our cohort. Our analysis also demonstrated that HIV diagnosis is significantly associated with both dose nonadministration and documented dose refusal at our institution.
Medication refusal is a well‐recognized phenomenon among hospitalized patients. A recent study of medication administration in hospitalized patients in the United Kingdom noted that refusal accounted for about 45% of omitted doses.[26] Fanikos et al. also found that documented refusal of doses contributed significantly to the overall number of VTE prophylaxis doses not administered to patients.[27] In our study, the proportion of nonadministered doses documented as refused by the patient or family member was significantly greater in patients with HIV than in patients without HIV across all units. Interestingly, the difference was greater on the HIV care unit when doses were stratified by HIV status. This observation leads us to hypothesize that specific hospital care environments may influence dose nonadministration and refusal rates among our patient population.
Based on regression analyses, increasing age and length of stay were associated with a decreased likelihood of any particular dose not being administered and with any particular dose being documented as refused. It is important to note that our GEE did not take into account date or time of each dose, and therefore we cannot make conclusions as to the likelihood of dose nonadministration or refusal of doses in relation to each other on a time scale. One cannot assume that a dose due later in a hospital course was more or less likely to be given than a dose due on the first hospital day. Although we did not expect these findings, one can hypothesize that patients who are older or have longer stays may be perceived to have more severe illness, and therefore greater need for prophylaxis, from nursing staff and others involved in their care. The associations were small but significant and warrant future investigation.
To our knowledge, this is the first investigation comparing the proportion of nonadministered doses of thromboprophylaxis between patients with and without HIV. Our data show that nonadministered doses and refused doses of thromboprophylaxis are more frequent among patients with HIV. In addition, we noted that nonadministration was more common on the dedicated HIV care unit compared with other units. We cannot currently offer a clear explanation for the disparity observed between units, and more specifically, within the HIV care unit. However, it is possible that a unique culture of care and provider‐specific factors may contribute.
Our study was limited by a number of factors. Seroconversion among patients during the study period was possible; however, our analysis revealed only 2 instances among nearly 4000 unique patients. A more significant limitation was the level of analysis allowed by the dataset. We examined dose characteristics on a dose and unit level, but the ability to analyze doses based on the prescriber and nurse level may have provided valuable insight into the specific reasons behind the observations presented here. Additionally, the specific unit assigned to a given dose in our dataset represented the discharge location for the corresponding patient, making it possible that some amount of nonadministered doses may be attributed to the incorrect unit. However, we do not believe that unit‐to‐unit transfers would be frequent enough to influence the overall results. In addition, we did not link nonadministration of thromboprophylaxis with VTE events, as these data were not present in the current dataset. Although this is a limitation of the current study, we believe that the notion that missed doses of thromboprophylaxis place patients at higher risk for VTE is plausible, as the efficacy of thromboprophylaxis is well established.[28, 29, 30] It is important to note that the reason for nonadministration selected by the nurse on the eMAR may not always represent the only reason or even the true reason for dose nonadministration. It is possible that dose refusal may be over‐represented in our sample, in part due to inaccurate documentation. Recent investigations at JHH have identified varying attitudes on the part of the patient and the nurse regarding thromboprophylaxis. A questionnaire and interview of patients showed a large knowledge gap regarding thromboprophylaxis, with many individuals unable to explain its role or significance in their medical care.[31] A common theme was also observed in a survey of nurses regarding VTE prophylaxis: doses were sometimes considered optional for reasons such as ambulation status, perceived severity of illness, or reason for hospitalization. Some nurses also reported that after an initial refused dose, they may continue to document subsequent doses as refused, sometimes without offering the dose to the patient.[32] As variation in practice was observed between individual nurses, it is also likely that the culture of care may vary between units, influencing thromboprophylaxis nonadministration rates as well as documentation of doses as refused. The dose‐level data used for the GEE analyses did not include date and time of administration, which limited the ability of the GEE to more completely account for autocorrelation.
To further investigate the findings of this and related studies, we intend to more closely analyze data at multiple levels with the goal of identifying an appropriate and feasible target for intervention. Additionally, further investigation should be performed with the goal of determining the relationship between decreased exposure to thromboprophylaxis and VTE. However, as patients with HIV appear to be at increased risk of VTE, ensuring that thromboprophylaxis is delivered appropriately and consistently should be an important goal for all who provide care to this population.
- , , , , . Venous thromboembolism in patients with HIV/AIDS: a case‐control study. J Acquir Immune Defic Syndr. 2008;48(3):310–314.
- , , . AIDS and thrombosis: retrospective study of 131 HIV‐infected patients. AIDS Patient Care STDS. 2001;15(6):311–320.
- , , , et al. HIV and risk of venous thromboembolism: a Danish nationwide population‐based cohort study. HIV Med. 2011;12(4):202–210.
- , , , . Epidemiology of thrombosis in HIV‐infected individuals. The adult/adolescent spectrum of HIV disease project. AIDS. 2000;14(3):321–324.
- , , . Thrombotic complications in patients infected with HIV in the era of highly active antiretroviral therapy: a case series. Clin Infect Dis. 2004;39(8):1214–1222.
- , , . Circulating coagulation inhibitors in the acquired immunodeficiency syndrome. Ann Intern Med. 1986;104(2):175–180.
- , . The pathogenesis of the antiphospholipid syndrome. N Engl J Med. 2013;368(11):1033–1044.
- , , , . Acquired protein C and protein S deficiency in HIV‐infected patients. Clin Appl Thromb Hemost. 2003;9(4):325–331.
- , , , et al. Antiphospholipid syndrome associated with cytomegalovirus infection: case report and review. Clin Infect Dis. 1997;24(2):197–200.
- , . Viral infections and antiphospholipid antibodies. Semin Arthritis Rheum. 2002;31(4):256–263.
- , . AIDS‐defining and non‐AIDS‐defining malignancies: cancer occurrence in the antiretroviral therapy era. Curr Opin Oncol. 2007;19(5):446–451.
- , , , , . Pathogenesis, clinical and laboratory aspects of thrombosis in cancer. J Thromb Thrombolysis. 2007;24(1):29–38.
- , , , et al. Patterns of diagnoses in hospital admissions in a multistate cohort of HIV‐positive adults in 2001. Med Care. 2005;43(9 suppl):III3–III14.
- , , , et al. Opportunistic infections as causes of death in HIV‐infected patients in the HAART era in France. Scand J Infect Dis. 2005;37(6‐7):482–487.
- , , , et al. Rates of hospitalizations and associated diagnoses in a large multisite cohort of HIV patients in the United States, 1994–2005. AIDS. 2008;22(11):1345–1354.
- , , . Hospitalizations for metabolic conditions, opportunistic infections, and injection drug use among HIV patients: trends between 1996 and 2000 in 12 states. J Acquir Immune Defic Syndr. 2005;40(5):609–616.
- , , , , , . Cardiovascular disease in HIV infection. Am Heart J. 2006;151:1147–1155.
- , , , et al. Epidemiological evidence for cardiovascular disease in HIV‐infected patients and relationship to highly active antiretroviral therapy. Circulation. 2008;118(2):e29–e35.
- , , , et al. Immune activation and CD8+ T‐cell differentiation towards senescence in HIV‐1 infection. PLoS Biol. 2004;2(2):E20.
- , , , , . CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV‐1 and HIV‐2 but only indirectly to the viral load. J Immunol. 2002;169(6):3400–3406.
- , . Thromboprophylaxis in nonsurgical patients. Hematology Am Soc Hematol Educ Program. 2012;2012:631–637.
- , . Practices to prevent venous thromboembolism [published online ahead of print May 24, 2013]. BMJ Qual Saf. doi:10.1136/bmjqs‐2012‐001782.
- , , , et al. Patterns of non‐administration of ordered doses of venous thromboembolism prophylaxis: implications for novel intervention strategies. PLoS One. 2013;8(6):e66311.
- , , , et al. Lessons from The Johns Hopkins multi‐disciplinary venous thromboembolism (VTE) prevention collaborative. BMJ. 2012;344:e3935.
- , , , et al. Impact of a venous thromboembolism prophylaxis “smart order set”: improved compliance, fewer events [published online ahead of print April 4, 2013]. Am J Hematol. doi: 10.1002/ajh.23450.
- , , . Dose omissions in hospitalized patients in a UK hospital: an analysis of the relative contribution of adverse drug reactions. Drug Saf. 2012;35(8):677–683.
- , , , et al. Adherence to pharmacological thromboprophylaxis orders in hospitalized patients. Am J Med. 2010;123(6):536–541.
- , , , et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in medical patients with enoxaparin study group. N Engl J Med. 1999;341(11):793–800.
- , , , et al. Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7):874–879.
- , , , et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332(7537):325–329.
- , , , et al. Patient perspectives on pharmacological venous thromboembolism prophylaxis at The Johns Hopkins Hospital. J Thromb Thrombolysis. 2013;35(3):416.
- , , , et al. Culture of care and documented patient refusal of pharmacologic venous thromboembolism prophylaxis. J Thromb Thrombolysis. 2011;31(3):367–400.
- , , , , . Venous thromboembolism in patients with HIV/AIDS: a case‐control study. J Acquir Immune Defic Syndr. 2008;48(3):310–314.
- , , . AIDS and thrombosis: retrospective study of 131 HIV‐infected patients. AIDS Patient Care STDS. 2001;15(6):311–320.
- , , , et al. HIV and risk of venous thromboembolism: a Danish nationwide population‐based cohort study. HIV Med. 2011;12(4):202–210.
- , , , . Epidemiology of thrombosis in HIV‐infected individuals. The adult/adolescent spectrum of HIV disease project. AIDS. 2000;14(3):321–324.
- , , . Thrombotic complications in patients infected with HIV in the era of highly active antiretroviral therapy: a case series. Clin Infect Dis. 2004;39(8):1214–1222.
- , , . Circulating coagulation inhibitors in the acquired immunodeficiency syndrome. Ann Intern Med. 1986;104(2):175–180.
- , . The pathogenesis of the antiphospholipid syndrome. N Engl J Med. 2013;368(11):1033–1044.
- , , , . Acquired protein C and protein S deficiency in HIV‐infected patients. Clin Appl Thromb Hemost. 2003;9(4):325–331.
- , , , et al. Antiphospholipid syndrome associated with cytomegalovirus infection: case report and review. Clin Infect Dis. 1997;24(2):197–200.
- , . Viral infections and antiphospholipid antibodies. Semin Arthritis Rheum. 2002;31(4):256–263.
- , . AIDS‐defining and non‐AIDS‐defining malignancies: cancer occurrence in the antiretroviral therapy era. Curr Opin Oncol. 2007;19(5):446–451.
- , , , , . Pathogenesis, clinical and laboratory aspects of thrombosis in cancer. J Thromb Thrombolysis. 2007;24(1):29–38.
- , , , et al. Patterns of diagnoses in hospital admissions in a multistate cohort of HIV‐positive adults in 2001. Med Care. 2005;43(9 suppl):III3–III14.
- , , , et al. Opportunistic infections as causes of death in HIV‐infected patients in the HAART era in France. Scand J Infect Dis. 2005;37(6‐7):482–487.
- , , , et al. Rates of hospitalizations and associated diagnoses in a large multisite cohort of HIV patients in the United States, 1994–2005. AIDS. 2008;22(11):1345–1354.
- , , . Hospitalizations for metabolic conditions, opportunistic infections, and injection drug use among HIV patients: trends between 1996 and 2000 in 12 states. J Acquir Immune Defic Syndr. 2005;40(5):609–616.
- , , , , , . Cardiovascular disease in HIV infection. Am Heart J. 2006;151:1147–1155.
- , , , et al. Epidemiological evidence for cardiovascular disease in HIV‐infected patients and relationship to highly active antiretroviral therapy. Circulation. 2008;118(2):e29–e35.
- , , , et al. Immune activation and CD8+ T‐cell differentiation towards senescence in HIV‐1 infection. PLoS Biol. 2004;2(2):E20.
- , , , , . CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV‐1 and HIV‐2 but only indirectly to the viral load. J Immunol. 2002;169(6):3400–3406.
- , . Thromboprophylaxis in nonsurgical patients. Hematology Am Soc Hematol Educ Program. 2012;2012:631–637.
- , . Practices to prevent venous thromboembolism [published online ahead of print May 24, 2013]. BMJ Qual Saf. doi:10.1136/bmjqs‐2012‐001782.
- , , , et al. Patterns of non‐administration of ordered doses of venous thromboembolism prophylaxis: implications for novel intervention strategies. PLoS One. 2013;8(6):e66311.
- , , , et al. Lessons from The Johns Hopkins multi‐disciplinary venous thromboembolism (VTE) prevention collaborative. BMJ. 2012;344:e3935.
- , , , et al. Impact of a venous thromboembolism prophylaxis “smart order set”: improved compliance, fewer events [published online ahead of print April 4, 2013]. Am J Hematol. doi: 10.1002/ajh.23450.
- , , . Dose omissions in hospitalized patients in a UK hospital: an analysis of the relative contribution of adverse drug reactions. Drug Saf. 2012;35(8):677–683.
- , , , et al. Adherence to pharmacological thromboprophylaxis orders in hospitalized patients. Am J Med. 2010;123(6):536–541.
- , , , et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in medical patients with enoxaparin study group. N Engl J Med. 1999;341(11):793–800.
- , , , et al. Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7):874–879.
- , , , et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332(7537):325–329.
- , , , et al. Patient perspectives on pharmacological venous thromboembolism prophylaxis at The Johns Hopkins Hospital. J Thromb Thrombolysis. 2013;35(3):416.
- , , , et al. Culture of care and documented patient refusal of pharmacologic venous thromboembolism prophylaxis. J Thromb Thrombolysis. 2011;31(3):367–400.
© 2014 Society of Hospital Medicine
Retrievable Vena Cava Filters
Vena cava filters were introduced in the 1960s as a mechanical means to prevent pulmonary embolism (PE).1 Since that time, the number of filters placed has grown steadily, to over 49,000 annually in the United States alone.2 However, patients with vena cava filters can develop complications from the filter itself, which can lead to significant morbidity and, rarely, mortality. In particular, the interruption of venous flow caused by the filter can precipitate lower extremity deep vein thrombosis (DVT),3 as well as vena caval thrombosis involving the filter itself. This has led some experts to recommend indefinite anticoagulation in patients with vena caval filters,4, 5 potentially exposing many patients to the risks of anticoagulation. Given these long‐term safety concerns, there has been recent enthusiasm for the development of optional filters. Optional vena cava filters can be classified into 2 types: temporary and retrievable. Temporary filters, which are not currently available in the United States, are held in place by a tether or catheter5 and cannot be used as permanent devices. Retrievable filters, on the other hand, maintain their position by hooks, radial pressure, or barbs and can either be removed within a prescribed time period after placement or remain in place permanently. In this way, optional filters offer the possibility of avoiding long‐term filter complications in patients with temporary contraindications to anticoagulation. Not surprisingly, the use of retrievable filters has increased dramatically, with many filters being placed for prophylactic indications in patients without known venous thromboembolism (VTE).6 In this work we review the different types of retrievable vena cava filters, current indications for placement, complications, and areas for future research.
Filter Design and Efficacy
Currently, there are 5 U.S. Food and Drug Administration (FDA)‐approved filters in the United States that can be used as retrievable filters: ALN (ALN Implants Chirurgicaux, Ghisonaccia, France); Celect (Cook Medical Incorporated, Bloomington, IN); Gunther‐Tulip (Cook Medical Incorporated, Bloomington, IN); G2 (Bard Peripheral Vascular, Tempe, AZ); and OptEase (Cordis Corporation, Miami Lakes, FL) (Table 1). Three more devices are in U.S. clinical trials: SafeFlo (Rafael Medical Technologies, Hasselt, Belgium); Crux (Crux Biomedical, Portola Valley, CA); and Option (Rex Medical, Conshohocken, PA). Filters are constructed from magnetic resonance imaging (MRI)‐compatible, nonferromagnetic alloys and are produced in either a hexagonal or conical shape. There are potential advantages and disadvantages to both designs. A hexagonal design is thought to be better for trapping small thrombi, but conical filters may have a decreased propensity toward thrombosis.7 When a hexagonal filter becomes partially occluded in vitro, flow disturbances can lead to turbulence, stasis, and progressive clot formation.7 Some clinical studies have demonstrated an increased incidence of thrombosis with hexagonal filters,8 but further investigation is needed to determine if a true correlation exists. Comparisons of the 2 types of filter design are limited but have shown no difference in their efficacy in the prevention of PE.9 Therefore, filter choice is usually dependent upon the physician performing the procedure, although other factors, such as caval size, clot extent, available venous access, and route of retrieval also may affect this decision. Furthermore, retrospective reviews have shown no difference in efficacy between retrievable and permanent filters.10
| Filter | Image | Insertion Site | Retrieval Site | Maximum Successful Documented Dwell Time |
|---|---|---|---|---|
| Gunther‐Tulip (photo courtesy of Cook Medical Incorporated, Bloomington, IN) | Femoral or jugular | Jugular | 204 days42 | |
| Optease (photo courtesy of Cordis Corporation, Miami Lakes, FL) | Femoral or jugular | Femoral | 48 days43 | |
| ALN (photo courtesy of ALN Implants Chirurgicaux, Ghisonaccia, France) | Femoral, jugular, or brachial | Jugular | 352 days44 | |
| Celect (photo courtesy of Cook Medical Incorporated, Bloomington, IN) | Femoral or jugular | Jugular | 357 days45 | |
| G2 (photo courtesy of Bard Peripheral Vascular, Tempe, AZ) | Femoral or jugular | Jugular | 300 days46 |
Insertion of filters is typically performed under fluoroscopy in the operating room or interventional radiology suite. Placement can also occur at the bedside using intravascular ultrasound. This option is particularly useful for critically ill patients who are not stable enough to leave the intensive care unit (ICU) for insertion. The safety of this approach has been documented for both retrievable and permanent filters.11, 12 Duplex ultrasonography has been used to allow bedside placement of permanent filters, but published experience with this modality in placement of retrievable filters is lacking.13, 14
There are no set time limits for retrieving filters, although the retrieval success rate decreases as the time postplacement increases. Rather, the decision to remove them is based on the clinical situation. Table 1 shows data on some of the longest documented successful dwell times for the various retrievable filters. Prior to filter retrieval, a venogram is performed to ensure that there is no clot in the inferior vena cava (IVC) or common iliac veins (Figure 1). Removal of a retrievable filter involves snaring one end of the filter with a hook and then slipping a sheath over the filter, which retracts the filter from the vessel wall as it is being pulled into the sheath (Figure 2). Retrieval rates from various studies are listed in Table 2. Common reasons for nonretrieval include loss to follow up,15 ongoing contraindications to anticoagulation,11, 1618 presence of large thrombi in the filter,16, 1820 poor patient prognosis,16, 18 unrelated death,1618 and filter tilting or embedment.19, 21
| Study | Total Number of Patients | Study Type | Filter Type | Follow‐Up Duration (months) | PE [number (%)] | IVC Thrombosis [number (%)] | DVT [number (%)] | Retrieval Attempted/ Successful Retrieval [number (%)] | Mean Duration Between Filter Placement and Retrieval (days) |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Millward et al., 200116 | 90 | RO/PO | G | 3.4 | 0 | 1/39 (2.6) | 1/39 (2.6) | 53 (59)/52 (98) | 9 |
| de Gregorio et al., 200319 | 87 | RO | G | N/R | 0 | 0 | 0 | 69 (79)/68 (99) | 13 |
| Wicky et al., 200317 | 71 | RO | G | 30 | 0 | 0 | 0 | 47 (66)/33 (70) | 8.2 |
| Rosenthal et al., 200411 | 94 | PO | O | N/R | 0 | 0 | 1 (1.1) | 34 (36)/31 (91) | 19 |
| Grande et al., 200515 | 106 | RO | R | N/R | 3 (2.8) | 0 | 0 | 15 (14)/14 (93) | 150 |
| Oliva et al., 200547 | 27 | PO | O | N/R | 0 | 0 | 1/27 (3.7) | 21 (78)/21 (100) | 11.1 |
| Hoppe et al., 200618 | 41 | PO | G | 3 | 1 (2.4) | 1 (2.4) | 1 (2.4) | 23 (57)/23 (100) | 11.1 |
| Kalva et al., 200648 | 96 | RO | R | 5.3 | 1 (1.0) | 0 | 10/53 (18) | 11 (12)/9 (82) | 117 |
| Meier et al., 200635 | 37 | PO | O | 5 | 0 | 1/5 (20) | 1/5 (20) | 32 (86)/32 (100) | 16 |
| Ray et al., 200649 | 197 | RO | G, R | N/R | 1 (0.5)‐G | 2 (1.0)‐G | 0 | 94 (48)/80 (85) | 11 (G)/28 (R) |
| Rosenthal et al., 200650 | 127 | RO | G, R, O | N/R | 0 | 0 | 0 | 70 (52)/66 (94) | 71 |
| Looby et al., 200721 | 147 | RO | G | N/R | 1 (0.7) | 0 | 0 | 45 (31)/36 (80) | 33.6 |
| Yamagami et al., 200751 | 86 | RO | G | N/R | 0 | N/R | N/R | 80 (93)/77 (96) | 13.4 |
| Kim et al, 200852 | 427 | RO | G, P, R, G2 | 10.4 | 20 (4.7) | 2 (0.5) | 54 (12.6) | 60 (15.5)/46 (69.7) | 20.4 |
Indications for Filter Placement
Patients with Known VTE
Suggested indications for the use of vena cava filters in patients with proven VTE are listed in Table 3. For patients at risk for either recurrent or severe bleeding (eg, multiple falls, recurrent gastrointestinal or intracranial hemorrhage) or most patients who have failed treatment with therapeutic anticoagulation, a permanent filter is usually the preferred mechanical option. However, for certain conditions (such as Trousseau's syndrome, heparin‐induced thrombocytopenia, antiphospholipid syndrome, or anatomic abnormalities such as thoracic outlet syndrome‐Paget‐von Schroetter syndrome, or May‐Thurner syndrome‐iliac vein compression syndrome), vena cava filters have been shown either to be ineffective or to worsen thrombosis. In these cases, alternative therapies must be used, based on the underlying disorder and the clinical situation.
| Anticipated Transient Need for Anticoagulation | Anticipated Long‐Term Need for Anticoagulation* | |
|---|---|---|
| ||
| Transient bleeding risk in a patient at high risk for recurrent thromboembolism | Retrievable filter appropriate | Retrievable filter appropriate |
| Permanent, or likely recurrent, bleeding risk | Retrievable filter with extended dwell time | Permanent filter appropriate |
| No unusual bleeding risk | No filter indicated | No filter indicated |
A retrievable filter should only be considered in patients who have a transient contraindication to anticoagulation (Table 5). Such contraindications include isolated but treatable episodes of hemorrhage, urgent surgeries, or procedures associated with a high risk of bleeding, and trauma. The risk of recurrent VTE in the absence of anticoagulation has been estimated at 40% in the first month after VTE and then 10% during the second and third months.22 Therefore, it is reasonable to place a retrievable filter in perioperative patients who cannot be treated with therapeutic anticoagulation during the first 30 days after an acute VTE. If more than 30 days have passed since the thrombotic event, a filter is probably not necessary for patients who will have temporary interruptions in anticoagulation therapy. Instead, bridging anticoagulation (eg, unfractionated heparin [UFH] or low molecular weight heparin [LMWH]) can be given while warfarin is being held prior to surgery. Then, the patient can be transitioned back to warfarin therapy with prophylactic and then therapeutic LMWH or UFH in the postoperative period.
|
| Recurrent VTE |
| Idiopathic VTE |
| Near‐fatal thrombosis |
| Thrombosis at an unusual site (eg, mesenteric vein) |
| VTE in high‐risk thrombophilic disorders: |
| Antiphospholipid antibody syndrome |
| Protein C or S deficiency |
| Antithrombin III deficiency |
| Heterozygous mutations for both the Factor V Leiden and the Prothrombin gene mutation (compound heterozygosity) |
| Homozygous Factor V Leiden mutation |
| Cancer‐associated VTE |
| Major trauma |
| Peripartum |
| Isolated and treatable causes of hemorrhage (eg, peptic ulcer) |
| Bleeding complications after procedures or surgeries53 |
| Liver or kidney biopsy |
| Urgent surgery associated with a high bleeding risk54 |
| Cardiac (coronary artery bypass or valve replacement) |
| Vascular (aortic aneurysm repair, peripheral artery bypass) |
| Neurosurgical (intracranial or spinal) |
| Urologic (prostate and bladder) |
| Major cancer surgery |
Controversy remains regarding the use of retrievable filters in patients with calf vein DVT. It also exists for patients with massive or submassive PE who are receiving anticoagulation therapy but are at high risk for poor outcomes should another PEeven if smalloccur while they are on anticoagulation therapy. Vena cava filters are generally not recommended for patients with distal VTE unless they have a persistent contraindication to anticoagulation therapy and have shown clot propagation on serial duplex studies. At least 1 institution, however, has noted an increased use of filter placement in this population since the advent of retrievable filters.23 Randomized controlled trials and practice guidelines are still lacking in this area. Therefore, there is currently insufficient evidence to recommend retrievable filters for distal VTE.
There is also insufficient evidence to recommend filters for patients with massive or submassive PE who can tolerate anticoagulation therapy. Only 1 registry study has compared patients with massive PE (defined by a systolic blood pressure 90 mmHg at presentation) who were treated with vena cava filters to those who were not.24 Though there was a reduction in recurrent PE and mortality at 90 days in patients who received filters, this result requires further confirmation due to the small number of patients who received filters (11 patients) and a possible selection bias (patients who received filters were, on average, 16 years younger than those who did not). More evidence will be needed to weigh not only the cost but the risks of filter insertion (such as insertion site hematoma, increased incidence of DVT, or contrast nephropathy) against any benefit. Until then, routine filter use in patients with massive or submassive PE cannot be routinely recommended, but may be considered in those with massive PE and impending hemodynamic collapse.
Prophylaxis in High‐Risk Patients
Controversy also exists in the use of retrievable filters in patients without VTE who are at high risk for thromboembolic events. Currently, there are no randomized controlled trials that have established the efficacy of retrievable filters as prophylaxis in these patients. However, there are a number of prospective and retrospective studies that examine this topic, particularly in trauma patients.
Trauma
The Eastern Association for the Surgery of Trauma currently recommends that prophylactic filters be considered in trauma patients who are at increased risk for bleeding and prolonged immobilization (level III).25 These patients include those with severe closed head injury, incomplete spinal cord injury with paraplegia or quadriplegia, multiple long bone fractures, and complex pelvic fractures with multiple long bone fractures. The largest study to date on retrievable filters in trauma patients was done by the American Association for the Surgery of Trauma.26 The incidence of new PE after filter placement was 0.5%, which compares favorably with permanent filter recipients (PE 0.7%) and historical controls (2.1%).27 OptEase filters were more commonly associated with caval thrombosis. The majority of filters (78%) were not retrieved, primarily because patients were lost to follow up. Failure to retrieve filters has become a major issue as these devices grow in popularity.28, 29 In this situation, the benefit of using retrievable filters could be mitigated by the same long‐term complications associated with permanent filters. Therefore, well‐coordinated patient follow‐up is essential to ensure optimal use of retrievable filters. Furthermore, randomized studies of retrievable filters are urgently needed to confirm that vena cava filters are associated with net benefit compared with conventional approaches to VTE prophylaxis (enoxaparin, sequential compression devices) in trauma patients.
Other High‐Risk Situations
The use of permanent filters has been studied in neurosurgical, bariatric, orthopedic, and pregnant patients. However, there are very few studies that look at the use of retrievable filters specifically in these populations. One such study was done in obese (body mass index [BMI] > 55 kg/m2) patients undergoing gastric bypass surgery.30 Filter retrieval rates were high (87%), and there were no DVTs or PEs prior to or after removal. The authors attributed their high removal rates to a dedicated follow‐up program and close collaboration with the interventional radiologists. More research needs to be done comparing outcomes with filters to conventional pharmacologic VTE prophylaxis before these devices can be recommended in these patients.
Filter Complications
During Filter Placement
Complications related to both retrievable and nonretrievable filter placement are rare but have been documented in several studies. Failure of the filter to deploy properly has been reported.21 The same study also noted pneumothorax as a complication in some patients whose filters were inserted via the jugular vein.21 Therefore, location of access and retrieval should be an important consideration for patients with significant underlying pulmonary disease. Insertion site thrombosis and arteriovenous fistula formation have been reported primarily with permanent filters31, 32; that risk could be extrapolated to retrievable filters given that the method of placement is the same. Iodine contrast‐induced nephropathy is of concern for high‐risk patients, although the procedure can be performed using gadolinium‐based contrast, carbon dioxide contrast, or without contrast (under ultrasound guidance).
During Filter Retrieval
Filter tilting and clot trapping under the filter that occurs during the filter removal process are infrequent causes of non‐retrieval. Tilting of the filter sometimes can pose problems, but if this occurs, the filter can be repositioned so that the degree of tilt no longer precludes removal. Severe cases of tilting that lead to nonretrieval are very rare. When thrombus is trapped in the filter (Figure 3), retrieval often depends on the amount of thrombus. A visual scale to assist in judgment of thrombus volume has been developed to assist in retrieval decision‐making.33 In some cases, catheter‐directed thrombolysis has been used to facilitate thrombus dissolution.34
VTE After Placement
Table 2 lists the incidence of VTE after retrievable filter placement. The overall incidence of PE is low, but that of DVT varies widely. These data raise the possibility that some filters may not be removed due to the occurrence of a new DVT, thereby becoming permanent filters with the associated risks of recurrent DVT, caval thrombosis, and PE. Only a few studies have investigated the differences in the rate of PE between permanent and retrievable filters and have shown no differences.29 The long‐term complication rates of retrievable filters and how they may differ from permanent filters warrants further investigation.
Some studies have also noted the development of PE after filter retrieval.35, 36 It is possible that a subclinical DVT was present at the time of removal or that the filter was retrieved before the risk of thrombosis had resolved. Therefore, consideration should be given to the use of duplex ultrasound evaluation for DVT prior to filter removal to ensure that patients with active thrombosis receive therapeutic anticoagulation for an appropriate duration.
Because of the concern for DVT and PE associated with retrievable filters, anticoagulation should ideally occur before and after retrieval, once the bleeding risk has become acceptable. Consensus guidelines support this practice,5, 37 though one systematic review has found insufficient evidence regarding the use of anticoagulation in patients with vena cava filters.4 Retrospective reviews have shown that filters can be both placed and removed without bleeding complications, even in patients who are therapeutically anticoagulated with warfarin and/or LMWH.38, 39 Further investigation would be useful to confirm whether this is an effective approach to VTE prevention at the time of retrieval.
Other Adverse Events
Other complications that have been associated with retrievable filters include migration, fracture, infection, and perforation. It may be difficult to estimate the true incidence of these complications, as most of the literature on this topic comes from case reports. Vena cava perforation with hooks may be not uncommon but in most cases is not clinically significant.40 Filter fracture is more common but rarely reported. Filter migration toward the heart is a very rare but potentially life‐threatening complication. The Recovery filter was taken off the market due in part to concerns about migration.26 As the use of retrievable filters increases, complications related to filters will need to be monitored.
Ongoing and Future Research
Other types of removable filters are currently in development. Convertible filters that can be converted into a stent once they are no longer needed are under investigation. Other devices, such as absorbable or drug‐eluting filters, are also being studied.5 In addition, there is ongoing research to better characterize the safety and efficacy of available filters. The Prevention du Risque d'Embolie Pulmonaire par Interruption Cave (PREPIC) 2 will assess their use in the first prospective, randomized, controlled trial of retrievable filters in patients with acute VTE receiving anticoagulation (
Conclusions
There is growing concern over the increased use of vena caval filters for the prevention of PE.41 Retrievable filters offer the possibility of protection without the risk of long‐term complications attributable to permanent filters. The advent of these devices has lead to an increase in overall filter use but also could result in filter placement without adequate consideration of the potential complications or consequences of nonretrieval. More evidence is needed in order to establish best practice guidelines for retrievable filter use. Until these data are available, these devices should be used only in patients with acute VTE who are at risk for recurrent thromboembolism and have a transient risk for bleeding.
- ,,, et al.A vena caval filter for the prevention of pulmonary embolus.Surg Forum.1967;18.
- ,,.Twenty‐one‐year trends in the use of inferior vena cava filters.Arch Intern Med.2004;164:1541–1545.
- ,,, et al.A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep‐vein thrombosis. Prevention du Risque d'Embolie Pulmonaire par Interruption Cave Study Group.N Engl J Med.1998;338:409–415.
- ,.The need for anticoagulation following inferior vena cava filter placement: systematic review.Cardiovasc Intervent Radiol.2008;31:316–324.
- ,,, et al.Guidelines for the use of retrievable and convertible vena cava filters: report from the Society of Interventional Radiology multidisciplinary consensus conference.J Vasc Interv Radiol.2006;17:449–459.
- ,,, et al.Changing patterns in the use of inferior vena cava filters: review of a single center experience.J Am Coll Surg.2007;205:564–569.
- ,,.Hemodynamic effects of clot entrapment in the TrapEase inferior vena cava filter.J Vasc Interv Radiol.2004;15:485–490.
- ,,, et al.Vena cava filters and inferior vena cava thrombosis.J Vasc Surg.2007;45:789–794.
- ,,, et al.Clinical comparison of two optional vena cava filters.J Vasc Interv Radiol.2007;18:505–511.
- ,,, et al.Use of retrievable compared to permanent inferior vena cava filters: a single‐institution experience.Cardiovasc Intervent Radiol.2008;31:308–315.
- ,,, et al.Role of prophylactic temporary inferior vena cava filters placed at the ICU bedside under intravascular ultrasound guidance in patients with multiple trauma.J Vasc Surg.2004;40:958–964.
- ,,.Bedside vena cava filter placement with intravascular ultrasound: a simple, accurate, single venous access method.J Vasc Surg.2007;46:1284–1286.
- ,,.The bedside insertion of inferior vena cava filters using ultrasound guidance.Perspect Vasc Surg Endovasc Ther.2007;19:78–84.
- ,,, et al.Comparison of bedside transabdominal duplex ultrasound versus contrast venography for inferior vena cava filter placement: what is the best imaging modality?Ann Vasc Surg.2005;19:229–234.
- ,,, et al.Experience with the recovery filter as a retrievable inferior vena cava filter.J Vasc Interv Radiol.2005;16:1189–1193.
- ,,, et al.Gunther Tulip retrievable vena cava filter: results from the Registry of the Canadian Interventional Radiology Association.J Vasc Interv Radiol.2001;12:1053–1058.
- ,,, et al.Clinical experience with retrievable Gunther Tulip vena cava filters.J Endovasc Ther.2003;10:994–1000.
- ,,, et al.Gunther Tulip filter retrievability multicenter study including CT follow‐up: final report.J Vasc Interv Radiol.2006;17:1017–1023.
- ,,, et al.The Gunther Tulip retrievable filter: prolonged temporary filtration by repositioning within the inferior vena cava.J Vasc Interv Radiol.2003;14:1259–1265.
- ,,, et al.Retrievable inferior vena cava filters: early clinical experience.J Cardiovasc Surg (Torino).2005;46:163–169.
- ,,, et al.Gunther Tulip retrievable inferior vena caval filters: indications, efficacy, retrieval, and complications.Cardiovasc Intervent Radiol.2007;30:59–65.
- ,.Management of anticoagulation before and after elective surgery.N Engl J Med.1997;336:1506–1511.
- ,,, et al.Changes in inferior vena cava filter placement over the past decade at a large community‐based academic health center.J Vasc Surg.2008;47:157–165.
- ,,, et al.Massive pulmonary embolism.Circulation.2006;113:577–582.
- ,,, et al.Practice management guidelines for the prevention of venous thromboembolism in trauma patients: the EAST practice management guidelines work group.J Trauma.2002;53:142–164.
- ,,, et al.Practice patterns and outcomes of retrievable vena cava filters in trauma patients: an AAST multicenter study.J Trauma.2007;62:17–24; discussion 24‐25.
- ,.Inferior vena cava interruption. In: Crowther M, et al., eds.Evidence‐Based Hematology.West Sussex, UK:Wiley‐Blackwell Publishing;2008:99–109.
- ,,, et al.Are temporary inferior vena cava filters really temporary?Am J Surg.2005;190:858–863.
- ,,, et al.Retrievable vena cava filters for preventing pulmonary embolism in trauma patients: a cautionary tale.J Trauma.2006;60:35–40.
- ,,, et al.Safety, feasibility, and outcome of retrievable vena cava filters in high‐risk surgical patients.J Vasc Surg.2007;45:784–788; discussion 788.
- ,,, et al.Femoral arteriovenous fistula after placement of a Kimray‐Greenfield filter.AJR Am J Roentgenol.1988;151:681–682.
- ,,, et al.Prophylactic Greenfield filters: acute complications and long‐term follow‐up.J Trauma.1996;41:231–236; discussion 236‐237.
- ,,.Estimation of trapped thrombus volumes in retrievable inferior vena cava filters: a visual scale.J Vasc Interv Radiol.2007;18:273–276.
- ,,, et al.Endovascular recanalization of the thrombosed filter‐bearing inferior vena cava.J Vasc Interv Radiol.2003;14:893–903.
- ,,, et al.Early experience with the retrievable OptEase vena cava filter in high‐risk trauma patients.Eur J Vasc Endovasc Surg.2006;32:589–595.
- ,,, et al.Current trends in vena caval filtration with the introduction of a retrievable filter at a level I trauma center.J Trauma.2004;57:32–36.
- ,,, et al.Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:401S–428S.
- ,,, et al.Safety of inferior vena cava filter retrieval in anticoagulated patients.Chest.2007;132:31–36.
- ,,, et al.Should anticoagulant therapy be stopped or reversed before venous intervention?Can Assoc Radiol J.1999;50:306–309.
- ,,.Retrievable vena cava filters: clinical experience.Curr Opin Pulm Med.2006;12:304–309.
- .Use of emboli‐blocking filters increases, but rigorous data are lacking.JAMA.2006;295:989–990.
- ,,, et al.Extended interval for retrieval of vena cava filters is safe and may maximize protection against pulmonary embolism.Am J Surg.2006;192:789–794.
- ,,, et al.Long‐term retrievability of IVC filters: should we abandon permanent devices?Cardiovasc Intervent Radiol.2007;30:820–827.
- ,,, et al.A prospective long‐term study of 220 patients with a retrievable vena cava filter for secondary prevention of venous thromboembolism.Chest.2007;131:223–229.
- ,,, et al.The Cook Celect filter: the UK and global experience so far. In:European Congress of Radiology.2008;European Society of Radiology:Vienna, Austria.
- ,,, et al.Multicenter retrievability trial of the recovery G2 filter.J Vasc Interv Radiol.2008;19:S28.
- ,,, et al.The Jonas study: evaluation of the retrievability of the Cordis OptEase inferior vena cava filter.J Vasc Interv Radiol.2005;16:1439–1445.
- ,,, et al.“Recovery” vena cava filter: experience in 96 patients.Cardiovasc Intervent Radiol.2006;29:559–564.
- ,,, et al.Outcomes with retrievable inferior vena cava filters: a multicenter study.J Vasc Interv Radiol.2006;17:1595–1604.
- ,,, et al.Retrievable inferior vena cava filters: initial clinical results.Ann Vasc Surg.2006;20:157–165.
- ,,, et al.Evaluation of retrievability of the Günther Tulip vena cava filter.Cardiovasc Intervent Radiol.2007;30:226–231.
- ,,, et al.A comparison of clinical outcomes with retrievable and permanent inferior vena cava filters.J Vasc Interv Radiol.2008;19:393–399.
- .The management of anticoagulation before and after procedures.Med Clin North Am.2001;85:1109–1116.
- ,,, et al.The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines, 8th ed.Chest.2008;133:299S–339S.
Vena cava filters were introduced in the 1960s as a mechanical means to prevent pulmonary embolism (PE).1 Since that time, the number of filters placed has grown steadily, to over 49,000 annually in the United States alone.2 However, patients with vena cava filters can develop complications from the filter itself, which can lead to significant morbidity and, rarely, mortality. In particular, the interruption of venous flow caused by the filter can precipitate lower extremity deep vein thrombosis (DVT),3 as well as vena caval thrombosis involving the filter itself. This has led some experts to recommend indefinite anticoagulation in patients with vena caval filters,4, 5 potentially exposing many patients to the risks of anticoagulation. Given these long‐term safety concerns, there has been recent enthusiasm for the development of optional filters. Optional vena cava filters can be classified into 2 types: temporary and retrievable. Temporary filters, which are not currently available in the United States, are held in place by a tether or catheter5 and cannot be used as permanent devices. Retrievable filters, on the other hand, maintain their position by hooks, radial pressure, or barbs and can either be removed within a prescribed time period after placement or remain in place permanently. In this way, optional filters offer the possibility of avoiding long‐term filter complications in patients with temporary contraindications to anticoagulation. Not surprisingly, the use of retrievable filters has increased dramatically, with many filters being placed for prophylactic indications in patients without known venous thromboembolism (VTE).6 In this work we review the different types of retrievable vena cava filters, current indications for placement, complications, and areas for future research.
Filter Design and Efficacy
Currently, there are 5 U.S. Food and Drug Administration (FDA)‐approved filters in the United States that can be used as retrievable filters: ALN (ALN Implants Chirurgicaux, Ghisonaccia, France); Celect (Cook Medical Incorporated, Bloomington, IN); Gunther‐Tulip (Cook Medical Incorporated, Bloomington, IN); G2 (Bard Peripheral Vascular, Tempe, AZ); and OptEase (Cordis Corporation, Miami Lakes, FL) (Table 1). Three more devices are in U.S. clinical trials: SafeFlo (Rafael Medical Technologies, Hasselt, Belgium); Crux (Crux Biomedical, Portola Valley, CA); and Option (Rex Medical, Conshohocken, PA). Filters are constructed from magnetic resonance imaging (MRI)‐compatible, nonferromagnetic alloys and are produced in either a hexagonal or conical shape. There are potential advantages and disadvantages to both designs. A hexagonal design is thought to be better for trapping small thrombi, but conical filters may have a decreased propensity toward thrombosis.7 When a hexagonal filter becomes partially occluded in vitro, flow disturbances can lead to turbulence, stasis, and progressive clot formation.7 Some clinical studies have demonstrated an increased incidence of thrombosis with hexagonal filters,8 but further investigation is needed to determine if a true correlation exists. Comparisons of the 2 types of filter design are limited but have shown no difference in their efficacy in the prevention of PE.9 Therefore, filter choice is usually dependent upon the physician performing the procedure, although other factors, such as caval size, clot extent, available venous access, and route of retrieval also may affect this decision. Furthermore, retrospective reviews have shown no difference in efficacy between retrievable and permanent filters.10
| Filter | Image | Insertion Site | Retrieval Site | Maximum Successful Documented Dwell Time |
|---|---|---|---|---|
| Gunther‐Tulip (photo courtesy of Cook Medical Incorporated, Bloomington, IN) | Femoral or jugular | Jugular | 204 days42 | |
| Optease (photo courtesy of Cordis Corporation, Miami Lakes, FL) | Femoral or jugular | Femoral | 48 days43 | |
| ALN (photo courtesy of ALN Implants Chirurgicaux, Ghisonaccia, France) | Femoral, jugular, or brachial | Jugular | 352 days44 | |
| Celect (photo courtesy of Cook Medical Incorporated, Bloomington, IN) | Femoral or jugular | Jugular | 357 days45 | |
| G2 (photo courtesy of Bard Peripheral Vascular, Tempe, AZ) | Femoral or jugular | Jugular | 300 days46 |
Insertion of filters is typically performed under fluoroscopy in the operating room or interventional radiology suite. Placement can also occur at the bedside using intravascular ultrasound. This option is particularly useful for critically ill patients who are not stable enough to leave the intensive care unit (ICU) for insertion. The safety of this approach has been documented for both retrievable and permanent filters.11, 12 Duplex ultrasonography has been used to allow bedside placement of permanent filters, but published experience with this modality in placement of retrievable filters is lacking.13, 14
There are no set time limits for retrieving filters, although the retrieval success rate decreases as the time postplacement increases. Rather, the decision to remove them is based on the clinical situation. Table 1 shows data on some of the longest documented successful dwell times for the various retrievable filters. Prior to filter retrieval, a venogram is performed to ensure that there is no clot in the inferior vena cava (IVC) or common iliac veins (Figure 1). Removal of a retrievable filter involves snaring one end of the filter with a hook and then slipping a sheath over the filter, which retracts the filter from the vessel wall as it is being pulled into the sheath (Figure 2). Retrieval rates from various studies are listed in Table 2. Common reasons for nonretrieval include loss to follow up,15 ongoing contraindications to anticoagulation,11, 1618 presence of large thrombi in the filter,16, 1820 poor patient prognosis,16, 18 unrelated death,1618 and filter tilting or embedment.19, 21


| Study | Total Number of Patients | Study Type | Filter Type | Follow‐Up Duration (months) | PE [number (%)] | IVC Thrombosis [number (%)] | DVT [number (%)] | Retrieval Attempted/ Successful Retrieval [number (%)] | Mean Duration Between Filter Placement and Retrieval (days) |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Millward et al., 200116 | 90 | RO/PO | G | 3.4 | 0 | 1/39 (2.6) | 1/39 (2.6) | 53 (59)/52 (98) | 9 |
| de Gregorio et al., 200319 | 87 | RO | G | N/R | 0 | 0 | 0 | 69 (79)/68 (99) | 13 |
| Wicky et al., 200317 | 71 | RO | G | 30 | 0 | 0 | 0 | 47 (66)/33 (70) | 8.2 |
| Rosenthal et al., 200411 | 94 | PO | O | N/R | 0 | 0 | 1 (1.1) | 34 (36)/31 (91) | 19 |
| Grande et al., 200515 | 106 | RO | R | N/R | 3 (2.8) | 0 | 0 | 15 (14)/14 (93) | 150 |
| Oliva et al., 200547 | 27 | PO | O | N/R | 0 | 0 | 1/27 (3.7) | 21 (78)/21 (100) | 11.1 |
| Hoppe et al., 200618 | 41 | PO | G | 3 | 1 (2.4) | 1 (2.4) | 1 (2.4) | 23 (57)/23 (100) | 11.1 |
| Kalva et al., 200648 | 96 | RO | R | 5.3 | 1 (1.0) | 0 | 10/53 (18) | 11 (12)/9 (82) | 117 |
| Meier et al., 200635 | 37 | PO | O | 5 | 0 | 1/5 (20) | 1/5 (20) | 32 (86)/32 (100) | 16 |
| Ray et al., 200649 | 197 | RO | G, R | N/R | 1 (0.5)‐G | 2 (1.0)‐G | 0 | 94 (48)/80 (85) | 11 (G)/28 (R) |
| Rosenthal et al., 200650 | 127 | RO | G, R, O | N/R | 0 | 0 | 0 | 70 (52)/66 (94) | 71 |
| Looby et al., 200721 | 147 | RO | G | N/R | 1 (0.7) | 0 | 0 | 45 (31)/36 (80) | 33.6 |
| Yamagami et al., 200751 | 86 | RO | G | N/R | 0 | N/R | N/R | 80 (93)/77 (96) | 13.4 |
| Kim et al, 200852 | 427 | RO | G, P, R, G2 | 10.4 | 20 (4.7) | 2 (0.5) | 54 (12.6) | 60 (15.5)/46 (69.7) | 20.4 |
Indications for Filter Placement
Patients with Known VTE
Suggested indications for the use of vena cava filters in patients with proven VTE are listed in Table 3. For patients at risk for either recurrent or severe bleeding (eg, multiple falls, recurrent gastrointestinal or intracranial hemorrhage) or most patients who have failed treatment with therapeutic anticoagulation, a permanent filter is usually the preferred mechanical option. However, for certain conditions (such as Trousseau's syndrome, heparin‐induced thrombocytopenia, antiphospholipid syndrome, or anatomic abnormalities such as thoracic outlet syndrome‐Paget‐von Schroetter syndrome, or May‐Thurner syndrome‐iliac vein compression syndrome), vena cava filters have been shown either to be ineffective or to worsen thrombosis. In these cases, alternative therapies must be used, based on the underlying disorder and the clinical situation.
| Anticipated Transient Need for Anticoagulation | Anticipated Long‐Term Need for Anticoagulation* | |
|---|---|---|
| ||
| Transient bleeding risk in a patient at high risk for recurrent thromboembolism | Retrievable filter appropriate | Retrievable filter appropriate |
| Permanent, or likely recurrent, bleeding risk | Retrievable filter with extended dwell time | Permanent filter appropriate |
| No unusual bleeding risk | No filter indicated | No filter indicated |
A retrievable filter should only be considered in patients who have a transient contraindication to anticoagulation (Table 5). Such contraindications include isolated but treatable episodes of hemorrhage, urgent surgeries, or procedures associated with a high risk of bleeding, and trauma. The risk of recurrent VTE in the absence of anticoagulation has been estimated at 40% in the first month after VTE and then 10% during the second and third months.22 Therefore, it is reasonable to place a retrievable filter in perioperative patients who cannot be treated with therapeutic anticoagulation during the first 30 days after an acute VTE. If more than 30 days have passed since the thrombotic event, a filter is probably not necessary for patients who will have temporary interruptions in anticoagulation therapy. Instead, bridging anticoagulation (eg, unfractionated heparin [UFH] or low molecular weight heparin [LMWH]) can be given while warfarin is being held prior to surgery. Then, the patient can be transitioned back to warfarin therapy with prophylactic and then therapeutic LMWH or UFH in the postoperative period.
|
| Recurrent VTE |
| Idiopathic VTE |
| Near‐fatal thrombosis |
| Thrombosis at an unusual site (eg, mesenteric vein) |
| VTE in high‐risk thrombophilic disorders: |
| Antiphospholipid antibody syndrome |
| Protein C or S deficiency |
| Antithrombin III deficiency |
| Heterozygous mutations for both the Factor V Leiden and the Prothrombin gene mutation (compound heterozygosity) |
| Homozygous Factor V Leiden mutation |
| Cancer‐associated VTE |
| Major trauma |
| Peripartum |
| Isolated and treatable causes of hemorrhage (eg, peptic ulcer) |
| Bleeding complications after procedures or surgeries53 |
| Liver or kidney biopsy |
| Urgent surgery associated with a high bleeding risk54 |
| Cardiac (coronary artery bypass or valve replacement) |
| Vascular (aortic aneurysm repair, peripheral artery bypass) |
| Neurosurgical (intracranial or spinal) |
| Urologic (prostate and bladder) |
| Major cancer surgery |
Controversy remains regarding the use of retrievable filters in patients with calf vein DVT. It also exists for patients with massive or submassive PE who are receiving anticoagulation therapy but are at high risk for poor outcomes should another PEeven if smalloccur while they are on anticoagulation therapy. Vena cava filters are generally not recommended for patients with distal VTE unless they have a persistent contraindication to anticoagulation therapy and have shown clot propagation on serial duplex studies. At least 1 institution, however, has noted an increased use of filter placement in this population since the advent of retrievable filters.23 Randomized controlled trials and practice guidelines are still lacking in this area. Therefore, there is currently insufficient evidence to recommend retrievable filters for distal VTE.
There is also insufficient evidence to recommend filters for patients with massive or submassive PE who can tolerate anticoagulation therapy. Only 1 registry study has compared patients with massive PE (defined by a systolic blood pressure 90 mmHg at presentation) who were treated with vena cava filters to those who were not.24 Though there was a reduction in recurrent PE and mortality at 90 days in patients who received filters, this result requires further confirmation due to the small number of patients who received filters (11 patients) and a possible selection bias (patients who received filters were, on average, 16 years younger than those who did not). More evidence will be needed to weigh not only the cost but the risks of filter insertion (such as insertion site hematoma, increased incidence of DVT, or contrast nephropathy) against any benefit. Until then, routine filter use in patients with massive or submassive PE cannot be routinely recommended, but may be considered in those with massive PE and impending hemodynamic collapse.
Prophylaxis in High‐Risk Patients
Controversy also exists in the use of retrievable filters in patients without VTE who are at high risk for thromboembolic events. Currently, there are no randomized controlled trials that have established the efficacy of retrievable filters as prophylaxis in these patients. However, there are a number of prospective and retrospective studies that examine this topic, particularly in trauma patients.
Trauma
The Eastern Association for the Surgery of Trauma currently recommends that prophylactic filters be considered in trauma patients who are at increased risk for bleeding and prolonged immobilization (level III).25 These patients include those with severe closed head injury, incomplete spinal cord injury with paraplegia or quadriplegia, multiple long bone fractures, and complex pelvic fractures with multiple long bone fractures. The largest study to date on retrievable filters in trauma patients was done by the American Association for the Surgery of Trauma.26 The incidence of new PE after filter placement was 0.5%, which compares favorably with permanent filter recipients (PE 0.7%) and historical controls (2.1%).27 OptEase filters were more commonly associated with caval thrombosis. The majority of filters (78%) were not retrieved, primarily because patients were lost to follow up. Failure to retrieve filters has become a major issue as these devices grow in popularity.28, 29 In this situation, the benefit of using retrievable filters could be mitigated by the same long‐term complications associated with permanent filters. Therefore, well‐coordinated patient follow‐up is essential to ensure optimal use of retrievable filters. Furthermore, randomized studies of retrievable filters are urgently needed to confirm that vena cava filters are associated with net benefit compared with conventional approaches to VTE prophylaxis (enoxaparin, sequential compression devices) in trauma patients.
Other High‐Risk Situations
The use of permanent filters has been studied in neurosurgical, bariatric, orthopedic, and pregnant patients. However, there are very few studies that look at the use of retrievable filters specifically in these populations. One such study was done in obese (body mass index [BMI] > 55 kg/m2) patients undergoing gastric bypass surgery.30 Filter retrieval rates were high (87%), and there were no DVTs or PEs prior to or after removal. The authors attributed their high removal rates to a dedicated follow‐up program and close collaboration with the interventional radiologists. More research needs to be done comparing outcomes with filters to conventional pharmacologic VTE prophylaxis before these devices can be recommended in these patients.
Filter Complications
During Filter Placement
Complications related to both retrievable and nonretrievable filter placement are rare but have been documented in several studies. Failure of the filter to deploy properly has been reported.21 The same study also noted pneumothorax as a complication in some patients whose filters were inserted via the jugular vein.21 Therefore, location of access and retrieval should be an important consideration for patients with significant underlying pulmonary disease. Insertion site thrombosis and arteriovenous fistula formation have been reported primarily with permanent filters31, 32; that risk could be extrapolated to retrievable filters given that the method of placement is the same. Iodine contrast‐induced nephropathy is of concern for high‐risk patients, although the procedure can be performed using gadolinium‐based contrast, carbon dioxide contrast, or without contrast (under ultrasound guidance).
During Filter Retrieval
Filter tilting and clot trapping under the filter that occurs during the filter removal process are infrequent causes of non‐retrieval. Tilting of the filter sometimes can pose problems, but if this occurs, the filter can be repositioned so that the degree of tilt no longer precludes removal. Severe cases of tilting that lead to nonretrieval are very rare. When thrombus is trapped in the filter (Figure 3), retrieval often depends on the amount of thrombus. A visual scale to assist in judgment of thrombus volume has been developed to assist in retrieval decision‐making.33 In some cases, catheter‐directed thrombolysis has been used to facilitate thrombus dissolution.34

VTE After Placement
Table 2 lists the incidence of VTE after retrievable filter placement. The overall incidence of PE is low, but that of DVT varies widely. These data raise the possibility that some filters may not be removed due to the occurrence of a new DVT, thereby becoming permanent filters with the associated risks of recurrent DVT, caval thrombosis, and PE. Only a few studies have investigated the differences in the rate of PE between permanent and retrievable filters and have shown no differences.29 The long‐term complication rates of retrievable filters and how they may differ from permanent filters warrants further investigation.
Some studies have also noted the development of PE after filter retrieval.35, 36 It is possible that a subclinical DVT was present at the time of removal or that the filter was retrieved before the risk of thrombosis had resolved. Therefore, consideration should be given to the use of duplex ultrasound evaluation for DVT prior to filter removal to ensure that patients with active thrombosis receive therapeutic anticoagulation for an appropriate duration.
Because of the concern for DVT and PE associated with retrievable filters, anticoagulation should ideally occur before and after retrieval, once the bleeding risk has become acceptable. Consensus guidelines support this practice,5, 37 though one systematic review has found insufficient evidence regarding the use of anticoagulation in patients with vena cava filters.4 Retrospective reviews have shown that filters can be both placed and removed without bleeding complications, even in patients who are therapeutically anticoagulated with warfarin and/or LMWH.38, 39 Further investigation would be useful to confirm whether this is an effective approach to VTE prevention at the time of retrieval.
Other Adverse Events
Other complications that have been associated with retrievable filters include migration, fracture, infection, and perforation. It may be difficult to estimate the true incidence of these complications, as most of the literature on this topic comes from case reports. Vena cava perforation with hooks may be not uncommon but in most cases is not clinically significant.40 Filter fracture is more common but rarely reported. Filter migration toward the heart is a very rare but potentially life‐threatening complication. The Recovery filter was taken off the market due in part to concerns about migration.26 As the use of retrievable filters increases, complications related to filters will need to be monitored.
Ongoing and Future Research
Other types of removable filters are currently in development. Convertible filters that can be converted into a stent once they are no longer needed are under investigation. Other devices, such as absorbable or drug‐eluting filters, are also being studied.5 In addition, there is ongoing research to better characterize the safety and efficacy of available filters. The Prevention du Risque d'Embolie Pulmonaire par Interruption Cave (PREPIC) 2 will assess their use in the first prospective, randomized, controlled trial of retrievable filters in patients with acute VTE receiving anticoagulation (
Conclusions
There is growing concern over the increased use of vena caval filters for the prevention of PE.41 Retrievable filters offer the possibility of protection without the risk of long‐term complications attributable to permanent filters. The advent of these devices has lead to an increase in overall filter use but also could result in filter placement without adequate consideration of the potential complications or consequences of nonretrieval. More evidence is needed in order to establish best practice guidelines for retrievable filter use. Until these data are available, these devices should be used only in patients with acute VTE who are at risk for recurrent thromboembolism and have a transient risk for bleeding.
Vena cava filters were introduced in the 1960s as a mechanical means to prevent pulmonary embolism (PE).1 Since that time, the number of filters placed has grown steadily, to over 49,000 annually in the United States alone.2 However, patients with vena cava filters can develop complications from the filter itself, which can lead to significant morbidity and, rarely, mortality. In particular, the interruption of venous flow caused by the filter can precipitate lower extremity deep vein thrombosis (DVT),3 as well as vena caval thrombosis involving the filter itself. This has led some experts to recommend indefinite anticoagulation in patients with vena caval filters,4, 5 potentially exposing many patients to the risks of anticoagulation. Given these long‐term safety concerns, there has been recent enthusiasm for the development of optional filters. Optional vena cava filters can be classified into 2 types: temporary and retrievable. Temporary filters, which are not currently available in the United States, are held in place by a tether or catheter5 and cannot be used as permanent devices. Retrievable filters, on the other hand, maintain their position by hooks, radial pressure, or barbs and can either be removed within a prescribed time period after placement or remain in place permanently. In this way, optional filters offer the possibility of avoiding long‐term filter complications in patients with temporary contraindications to anticoagulation. Not surprisingly, the use of retrievable filters has increased dramatically, with many filters being placed for prophylactic indications in patients without known venous thromboembolism (VTE).6 In this work we review the different types of retrievable vena cava filters, current indications for placement, complications, and areas for future research.
Filter Design and Efficacy
Currently, there are 5 U.S. Food and Drug Administration (FDA)‐approved filters in the United States that can be used as retrievable filters: ALN (ALN Implants Chirurgicaux, Ghisonaccia, France); Celect (Cook Medical Incorporated, Bloomington, IN); Gunther‐Tulip (Cook Medical Incorporated, Bloomington, IN); G2 (Bard Peripheral Vascular, Tempe, AZ); and OptEase (Cordis Corporation, Miami Lakes, FL) (Table 1). Three more devices are in U.S. clinical trials: SafeFlo (Rafael Medical Technologies, Hasselt, Belgium); Crux (Crux Biomedical, Portola Valley, CA); and Option (Rex Medical, Conshohocken, PA). Filters are constructed from magnetic resonance imaging (MRI)‐compatible, nonferromagnetic alloys and are produced in either a hexagonal or conical shape. There are potential advantages and disadvantages to both designs. A hexagonal design is thought to be better for trapping small thrombi, but conical filters may have a decreased propensity toward thrombosis.7 When a hexagonal filter becomes partially occluded in vitro, flow disturbances can lead to turbulence, stasis, and progressive clot formation.7 Some clinical studies have demonstrated an increased incidence of thrombosis with hexagonal filters,8 but further investigation is needed to determine if a true correlation exists. Comparisons of the 2 types of filter design are limited but have shown no difference in their efficacy in the prevention of PE.9 Therefore, filter choice is usually dependent upon the physician performing the procedure, although other factors, such as caval size, clot extent, available venous access, and route of retrieval also may affect this decision. Furthermore, retrospective reviews have shown no difference in efficacy between retrievable and permanent filters.10
| Filter | Image | Insertion Site | Retrieval Site | Maximum Successful Documented Dwell Time |
|---|---|---|---|---|
| Gunther‐Tulip (photo courtesy of Cook Medical Incorporated, Bloomington, IN) | Femoral or jugular | Jugular | 204 days42 | |
| Optease (photo courtesy of Cordis Corporation, Miami Lakes, FL) | Femoral or jugular | Femoral | 48 days43 | |
| ALN (photo courtesy of ALN Implants Chirurgicaux, Ghisonaccia, France) | Femoral, jugular, or brachial | Jugular | 352 days44 | |
| Celect (photo courtesy of Cook Medical Incorporated, Bloomington, IN) | Femoral or jugular | Jugular | 357 days45 | |
| G2 (photo courtesy of Bard Peripheral Vascular, Tempe, AZ) | Femoral or jugular | Jugular | 300 days46 |
Insertion of filters is typically performed under fluoroscopy in the operating room or interventional radiology suite. Placement can also occur at the bedside using intravascular ultrasound. This option is particularly useful for critically ill patients who are not stable enough to leave the intensive care unit (ICU) for insertion. The safety of this approach has been documented for both retrievable and permanent filters.11, 12 Duplex ultrasonography has been used to allow bedside placement of permanent filters, but published experience with this modality in placement of retrievable filters is lacking.13, 14
There are no set time limits for retrieving filters, although the retrieval success rate decreases as the time postplacement increases. Rather, the decision to remove them is based on the clinical situation. Table 1 shows data on some of the longest documented successful dwell times for the various retrievable filters. Prior to filter retrieval, a venogram is performed to ensure that there is no clot in the inferior vena cava (IVC) or common iliac veins (Figure 1). Removal of a retrievable filter involves snaring one end of the filter with a hook and then slipping a sheath over the filter, which retracts the filter from the vessel wall as it is being pulled into the sheath (Figure 2). Retrieval rates from various studies are listed in Table 2. Common reasons for nonretrieval include loss to follow up,15 ongoing contraindications to anticoagulation,11, 1618 presence of large thrombi in the filter,16, 1820 poor patient prognosis,16, 18 unrelated death,1618 and filter tilting or embedment.19, 21


| Study | Total Number of Patients | Study Type | Filter Type | Follow‐Up Duration (months) | PE [number (%)] | IVC Thrombosis [number (%)] | DVT [number (%)] | Retrieval Attempted/ Successful Retrieval [number (%)] | Mean Duration Between Filter Placement and Retrieval (days) |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Millward et al., 200116 | 90 | RO/PO | G | 3.4 | 0 | 1/39 (2.6) | 1/39 (2.6) | 53 (59)/52 (98) | 9 |
| de Gregorio et al., 200319 | 87 | RO | G | N/R | 0 | 0 | 0 | 69 (79)/68 (99) | 13 |
| Wicky et al., 200317 | 71 | RO | G | 30 | 0 | 0 | 0 | 47 (66)/33 (70) | 8.2 |
| Rosenthal et al., 200411 | 94 | PO | O | N/R | 0 | 0 | 1 (1.1) | 34 (36)/31 (91) | 19 |
| Grande et al., 200515 | 106 | RO | R | N/R | 3 (2.8) | 0 | 0 | 15 (14)/14 (93) | 150 |
| Oliva et al., 200547 | 27 | PO | O | N/R | 0 | 0 | 1/27 (3.7) | 21 (78)/21 (100) | 11.1 |
| Hoppe et al., 200618 | 41 | PO | G | 3 | 1 (2.4) | 1 (2.4) | 1 (2.4) | 23 (57)/23 (100) | 11.1 |
| Kalva et al., 200648 | 96 | RO | R | 5.3 | 1 (1.0) | 0 | 10/53 (18) | 11 (12)/9 (82) | 117 |
| Meier et al., 200635 | 37 | PO | O | 5 | 0 | 1/5 (20) | 1/5 (20) | 32 (86)/32 (100) | 16 |
| Ray et al., 200649 | 197 | RO | G, R | N/R | 1 (0.5)‐G | 2 (1.0)‐G | 0 | 94 (48)/80 (85) | 11 (G)/28 (R) |
| Rosenthal et al., 200650 | 127 | RO | G, R, O | N/R | 0 | 0 | 0 | 70 (52)/66 (94) | 71 |
| Looby et al., 200721 | 147 | RO | G | N/R | 1 (0.7) | 0 | 0 | 45 (31)/36 (80) | 33.6 |
| Yamagami et al., 200751 | 86 | RO | G | N/R | 0 | N/R | N/R | 80 (93)/77 (96) | 13.4 |
| Kim et al, 200852 | 427 | RO | G, P, R, G2 | 10.4 | 20 (4.7) | 2 (0.5) | 54 (12.6) | 60 (15.5)/46 (69.7) | 20.4 |
Indications for Filter Placement
Patients with Known VTE
Suggested indications for the use of vena cava filters in patients with proven VTE are listed in Table 3. For patients at risk for either recurrent or severe bleeding (eg, multiple falls, recurrent gastrointestinal or intracranial hemorrhage) or most patients who have failed treatment with therapeutic anticoagulation, a permanent filter is usually the preferred mechanical option. However, for certain conditions (such as Trousseau's syndrome, heparin‐induced thrombocytopenia, antiphospholipid syndrome, or anatomic abnormalities such as thoracic outlet syndrome‐Paget‐von Schroetter syndrome, or May‐Thurner syndrome‐iliac vein compression syndrome), vena cava filters have been shown either to be ineffective or to worsen thrombosis. In these cases, alternative therapies must be used, based on the underlying disorder and the clinical situation.
| Anticipated Transient Need for Anticoagulation | Anticipated Long‐Term Need for Anticoagulation* | |
|---|---|---|
| ||
| Transient bleeding risk in a patient at high risk for recurrent thromboembolism | Retrievable filter appropriate | Retrievable filter appropriate |
| Permanent, or likely recurrent, bleeding risk | Retrievable filter with extended dwell time | Permanent filter appropriate |
| No unusual bleeding risk | No filter indicated | No filter indicated |
A retrievable filter should only be considered in patients who have a transient contraindication to anticoagulation (Table 5). Such contraindications include isolated but treatable episodes of hemorrhage, urgent surgeries, or procedures associated with a high risk of bleeding, and trauma. The risk of recurrent VTE in the absence of anticoagulation has been estimated at 40% in the first month after VTE and then 10% during the second and third months.22 Therefore, it is reasonable to place a retrievable filter in perioperative patients who cannot be treated with therapeutic anticoagulation during the first 30 days after an acute VTE. If more than 30 days have passed since the thrombotic event, a filter is probably not necessary for patients who will have temporary interruptions in anticoagulation therapy. Instead, bridging anticoagulation (eg, unfractionated heparin [UFH] or low molecular weight heparin [LMWH]) can be given while warfarin is being held prior to surgery. Then, the patient can be transitioned back to warfarin therapy with prophylactic and then therapeutic LMWH or UFH in the postoperative period.
|
| Recurrent VTE |
| Idiopathic VTE |
| Near‐fatal thrombosis |
| Thrombosis at an unusual site (eg, mesenteric vein) |
| VTE in high‐risk thrombophilic disorders: |
| Antiphospholipid antibody syndrome |
| Protein C or S deficiency |
| Antithrombin III deficiency |
| Heterozygous mutations for both the Factor V Leiden and the Prothrombin gene mutation (compound heterozygosity) |
| Homozygous Factor V Leiden mutation |
| Cancer‐associated VTE |
| Major trauma |
| Peripartum |
| Isolated and treatable causes of hemorrhage (eg, peptic ulcer) |
| Bleeding complications after procedures or surgeries53 |
| Liver or kidney biopsy |
| Urgent surgery associated with a high bleeding risk54 |
| Cardiac (coronary artery bypass or valve replacement) |
| Vascular (aortic aneurysm repair, peripheral artery bypass) |
| Neurosurgical (intracranial or spinal) |
| Urologic (prostate and bladder) |
| Major cancer surgery |
Controversy remains regarding the use of retrievable filters in patients with calf vein DVT. It also exists for patients with massive or submassive PE who are receiving anticoagulation therapy but are at high risk for poor outcomes should another PEeven if smalloccur while they are on anticoagulation therapy. Vena cava filters are generally not recommended for patients with distal VTE unless they have a persistent contraindication to anticoagulation therapy and have shown clot propagation on serial duplex studies. At least 1 institution, however, has noted an increased use of filter placement in this population since the advent of retrievable filters.23 Randomized controlled trials and practice guidelines are still lacking in this area. Therefore, there is currently insufficient evidence to recommend retrievable filters for distal VTE.
There is also insufficient evidence to recommend filters for patients with massive or submassive PE who can tolerate anticoagulation therapy. Only 1 registry study has compared patients with massive PE (defined by a systolic blood pressure 90 mmHg at presentation) who were treated with vena cava filters to those who were not.24 Though there was a reduction in recurrent PE and mortality at 90 days in patients who received filters, this result requires further confirmation due to the small number of patients who received filters (11 patients) and a possible selection bias (patients who received filters were, on average, 16 years younger than those who did not). More evidence will be needed to weigh not only the cost but the risks of filter insertion (such as insertion site hematoma, increased incidence of DVT, or contrast nephropathy) against any benefit. Until then, routine filter use in patients with massive or submassive PE cannot be routinely recommended, but may be considered in those with massive PE and impending hemodynamic collapse.
Prophylaxis in High‐Risk Patients
Controversy also exists in the use of retrievable filters in patients without VTE who are at high risk for thromboembolic events. Currently, there are no randomized controlled trials that have established the efficacy of retrievable filters as prophylaxis in these patients. However, there are a number of prospective and retrospective studies that examine this topic, particularly in trauma patients.
Trauma
The Eastern Association for the Surgery of Trauma currently recommends that prophylactic filters be considered in trauma patients who are at increased risk for bleeding and prolonged immobilization (level III).25 These patients include those with severe closed head injury, incomplete spinal cord injury with paraplegia or quadriplegia, multiple long bone fractures, and complex pelvic fractures with multiple long bone fractures. The largest study to date on retrievable filters in trauma patients was done by the American Association for the Surgery of Trauma.26 The incidence of new PE after filter placement was 0.5%, which compares favorably with permanent filter recipients (PE 0.7%) and historical controls (2.1%).27 OptEase filters were more commonly associated with caval thrombosis. The majority of filters (78%) were not retrieved, primarily because patients were lost to follow up. Failure to retrieve filters has become a major issue as these devices grow in popularity.28, 29 In this situation, the benefit of using retrievable filters could be mitigated by the same long‐term complications associated with permanent filters. Therefore, well‐coordinated patient follow‐up is essential to ensure optimal use of retrievable filters. Furthermore, randomized studies of retrievable filters are urgently needed to confirm that vena cava filters are associated with net benefit compared with conventional approaches to VTE prophylaxis (enoxaparin, sequential compression devices) in trauma patients.
Other High‐Risk Situations
The use of permanent filters has been studied in neurosurgical, bariatric, orthopedic, and pregnant patients. However, there are very few studies that look at the use of retrievable filters specifically in these populations. One such study was done in obese (body mass index [BMI] > 55 kg/m2) patients undergoing gastric bypass surgery.30 Filter retrieval rates were high (87%), and there were no DVTs or PEs prior to or after removal. The authors attributed their high removal rates to a dedicated follow‐up program and close collaboration with the interventional radiologists. More research needs to be done comparing outcomes with filters to conventional pharmacologic VTE prophylaxis before these devices can be recommended in these patients.
Filter Complications
During Filter Placement
Complications related to both retrievable and nonretrievable filter placement are rare but have been documented in several studies. Failure of the filter to deploy properly has been reported.21 The same study also noted pneumothorax as a complication in some patients whose filters were inserted via the jugular vein.21 Therefore, location of access and retrieval should be an important consideration for patients with significant underlying pulmonary disease. Insertion site thrombosis and arteriovenous fistula formation have been reported primarily with permanent filters31, 32; that risk could be extrapolated to retrievable filters given that the method of placement is the same. Iodine contrast‐induced nephropathy is of concern for high‐risk patients, although the procedure can be performed using gadolinium‐based contrast, carbon dioxide contrast, or without contrast (under ultrasound guidance).
During Filter Retrieval
Filter tilting and clot trapping under the filter that occurs during the filter removal process are infrequent causes of non‐retrieval. Tilting of the filter sometimes can pose problems, but if this occurs, the filter can be repositioned so that the degree of tilt no longer precludes removal. Severe cases of tilting that lead to nonretrieval are very rare. When thrombus is trapped in the filter (Figure 3), retrieval often depends on the amount of thrombus. A visual scale to assist in judgment of thrombus volume has been developed to assist in retrieval decision‐making.33 In some cases, catheter‐directed thrombolysis has been used to facilitate thrombus dissolution.34

VTE After Placement
Table 2 lists the incidence of VTE after retrievable filter placement. The overall incidence of PE is low, but that of DVT varies widely. These data raise the possibility that some filters may not be removed due to the occurrence of a new DVT, thereby becoming permanent filters with the associated risks of recurrent DVT, caval thrombosis, and PE. Only a few studies have investigated the differences in the rate of PE between permanent and retrievable filters and have shown no differences.29 The long‐term complication rates of retrievable filters and how they may differ from permanent filters warrants further investigation.
Some studies have also noted the development of PE after filter retrieval.35, 36 It is possible that a subclinical DVT was present at the time of removal or that the filter was retrieved before the risk of thrombosis had resolved. Therefore, consideration should be given to the use of duplex ultrasound evaluation for DVT prior to filter removal to ensure that patients with active thrombosis receive therapeutic anticoagulation for an appropriate duration.
Because of the concern for DVT and PE associated with retrievable filters, anticoagulation should ideally occur before and after retrieval, once the bleeding risk has become acceptable. Consensus guidelines support this practice,5, 37 though one systematic review has found insufficient evidence regarding the use of anticoagulation in patients with vena cava filters.4 Retrospective reviews have shown that filters can be both placed and removed without bleeding complications, even in patients who are therapeutically anticoagulated with warfarin and/or LMWH.38, 39 Further investigation would be useful to confirm whether this is an effective approach to VTE prevention at the time of retrieval.
Other Adverse Events
Other complications that have been associated with retrievable filters include migration, fracture, infection, and perforation. It may be difficult to estimate the true incidence of these complications, as most of the literature on this topic comes from case reports. Vena cava perforation with hooks may be not uncommon but in most cases is not clinically significant.40 Filter fracture is more common but rarely reported. Filter migration toward the heart is a very rare but potentially life‐threatening complication. The Recovery filter was taken off the market due in part to concerns about migration.26 As the use of retrievable filters increases, complications related to filters will need to be monitored.
Ongoing and Future Research
Other types of removable filters are currently in development. Convertible filters that can be converted into a stent once they are no longer needed are under investigation. Other devices, such as absorbable or drug‐eluting filters, are also being studied.5 In addition, there is ongoing research to better characterize the safety and efficacy of available filters. The Prevention du Risque d'Embolie Pulmonaire par Interruption Cave (PREPIC) 2 will assess their use in the first prospective, randomized, controlled trial of retrievable filters in patients with acute VTE receiving anticoagulation (
Conclusions
There is growing concern over the increased use of vena caval filters for the prevention of PE.41 Retrievable filters offer the possibility of protection without the risk of long‐term complications attributable to permanent filters. The advent of these devices has lead to an increase in overall filter use but also could result in filter placement without adequate consideration of the potential complications or consequences of nonretrieval. More evidence is needed in order to establish best practice guidelines for retrievable filter use. Until these data are available, these devices should be used only in patients with acute VTE who are at risk for recurrent thromboembolism and have a transient risk for bleeding.
- ,,, et al.A vena caval filter for the prevention of pulmonary embolus.Surg Forum.1967;18.
- ,,.Twenty‐one‐year trends in the use of inferior vena cava filters.Arch Intern Med.2004;164:1541–1545.
- ,,, et al.A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep‐vein thrombosis. Prevention du Risque d'Embolie Pulmonaire par Interruption Cave Study Group.N Engl J Med.1998;338:409–415.
- ,.The need for anticoagulation following inferior vena cava filter placement: systematic review.Cardiovasc Intervent Radiol.2008;31:316–324.
- ,,, et al.Guidelines for the use of retrievable and convertible vena cava filters: report from the Society of Interventional Radiology multidisciplinary consensus conference.J Vasc Interv Radiol.2006;17:449–459.
- ,,, et al.Changing patterns in the use of inferior vena cava filters: review of a single center experience.J Am Coll Surg.2007;205:564–569.
- ,,.Hemodynamic effects of clot entrapment in the TrapEase inferior vena cava filter.J Vasc Interv Radiol.2004;15:485–490.
- ,,, et al.Vena cava filters and inferior vena cava thrombosis.J Vasc Surg.2007;45:789–794.
- ,,, et al.Clinical comparison of two optional vena cava filters.J Vasc Interv Radiol.2007;18:505–511.
- ,,, et al.Use of retrievable compared to permanent inferior vena cava filters: a single‐institution experience.Cardiovasc Intervent Radiol.2008;31:308–315.
- ,,, et al.Role of prophylactic temporary inferior vena cava filters placed at the ICU bedside under intravascular ultrasound guidance in patients with multiple trauma.J Vasc Surg.2004;40:958–964.
- ,,.Bedside vena cava filter placement with intravascular ultrasound: a simple, accurate, single venous access method.J Vasc Surg.2007;46:1284–1286.
- ,,.The bedside insertion of inferior vena cava filters using ultrasound guidance.Perspect Vasc Surg Endovasc Ther.2007;19:78–84.
- ,,, et al.Comparison of bedside transabdominal duplex ultrasound versus contrast venography for inferior vena cava filter placement: what is the best imaging modality?Ann Vasc Surg.2005;19:229–234.
- ,,, et al.Experience with the recovery filter as a retrievable inferior vena cava filter.J Vasc Interv Radiol.2005;16:1189–1193.
- ,,, et al.Gunther Tulip retrievable vena cava filter: results from the Registry of the Canadian Interventional Radiology Association.J Vasc Interv Radiol.2001;12:1053–1058.
- ,,, et al.Clinical experience with retrievable Gunther Tulip vena cava filters.J Endovasc Ther.2003;10:994–1000.
- ,,, et al.Gunther Tulip filter retrievability multicenter study including CT follow‐up: final report.J Vasc Interv Radiol.2006;17:1017–1023.
- ,,, et al.The Gunther Tulip retrievable filter: prolonged temporary filtration by repositioning within the inferior vena cava.J Vasc Interv Radiol.2003;14:1259–1265.
- ,,, et al.Retrievable inferior vena cava filters: early clinical experience.J Cardiovasc Surg (Torino).2005;46:163–169.
- ,,, et al.Gunther Tulip retrievable inferior vena caval filters: indications, efficacy, retrieval, and complications.Cardiovasc Intervent Radiol.2007;30:59–65.
- ,.Management of anticoagulation before and after elective surgery.N Engl J Med.1997;336:1506–1511.
- ,,, et al.Changes in inferior vena cava filter placement over the past decade at a large community‐based academic health center.J Vasc Surg.2008;47:157–165.
- ,,, et al.Massive pulmonary embolism.Circulation.2006;113:577–582.
- ,,, et al.Practice management guidelines for the prevention of venous thromboembolism in trauma patients: the EAST practice management guidelines work group.J Trauma.2002;53:142–164.
- ,,, et al.Practice patterns and outcomes of retrievable vena cava filters in trauma patients: an AAST multicenter study.J Trauma.2007;62:17–24; discussion 24‐25.
- ,.Inferior vena cava interruption. In: Crowther M, et al., eds.Evidence‐Based Hematology.West Sussex, UK:Wiley‐Blackwell Publishing;2008:99–109.
- ,,, et al.Are temporary inferior vena cava filters really temporary?Am J Surg.2005;190:858–863.
- ,,, et al.Retrievable vena cava filters for preventing pulmonary embolism in trauma patients: a cautionary tale.J Trauma.2006;60:35–40.
- ,,, et al.Safety, feasibility, and outcome of retrievable vena cava filters in high‐risk surgical patients.J Vasc Surg.2007;45:784–788; discussion 788.
- ,,, et al.Femoral arteriovenous fistula after placement of a Kimray‐Greenfield filter.AJR Am J Roentgenol.1988;151:681–682.
- ,,, et al.Prophylactic Greenfield filters: acute complications and long‐term follow‐up.J Trauma.1996;41:231–236; discussion 236‐237.
- ,,.Estimation of trapped thrombus volumes in retrievable inferior vena cava filters: a visual scale.J Vasc Interv Radiol.2007;18:273–276.
- ,,, et al.Endovascular recanalization of the thrombosed filter‐bearing inferior vena cava.J Vasc Interv Radiol.2003;14:893–903.
- ,,, et al.Early experience with the retrievable OptEase vena cava filter in high‐risk trauma patients.Eur J Vasc Endovasc Surg.2006;32:589–595.
- ,,, et al.Current trends in vena caval filtration with the introduction of a retrievable filter at a level I trauma center.J Trauma.2004;57:32–36.
- ,,, et al.Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:401S–428S.
- ,,, et al.Safety of inferior vena cava filter retrieval in anticoagulated patients.Chest.2007;132:31–36.
- ,,, et al.Should anticoagulant therapy be stopped or reversed before venous intervention?Can Assoc Radiol J.1999;50:306–309.
- ,,.Retrievable vena cava filters: clinical experience.Curr Opin Pulm Med.2006;12:304–309.
- .Use of emboli‐blocking filters increases, but rigorous data are lacking.JAMA.2006;295:989–990.
- ,,, et al.Extended interval for retrieval of vena cava filters is safe and may maximize protection against pulmonary embolism.Am J Surg.2006;192:789–794.
- ,,, et al.Long‐term retrievability of IVC filters: should we abandon permanent devices?Cardiovasc Intervent Radiol.2007;30:820–827.
- ,,, et al.A prospective long‐term study of 220 patients with a retrievable vena cava filter for secondary prevention of venous thromboembolism.Chest.2007;131:223–229.
- ,,, et al.The Cook Celect filter: the UK and global experience so far. In:European Congress of Radiology.2008;European Society of Radiology:Vienna, Austria.
- ,,, et al.Multicenter retrievability trial of the recovery G2 filter.J Vasc Interv Radiol.2008;19:S28.
- ,,, et al.The Jonas study: evaluation of the retrievability of the Cordis OptEase inferior vena cava filter.J Vasc Interv Radiol.2005;16:1439–1445.
- ,,, et al.“Recovery” vena cava filter: experience in 96 patients.Cardiovasc Intervent Radiol.2006;29:559–564.
- ,,, et al.Outcomes with retrievable inferior vena cava filters: a multicenter study.J Vasc Interv Radiol.2006;17:1595–1604.
- ,,, et al.Retrievable inferior vena cava filters: initial clinical results.Ann Vasc Surg.2006;20:157–165.
- ,,, et al.Evaluation of retrievability of the Günther Tulip vena cava filter.Cardiovasc Intervent Radiol.2007;30:226–231.
- ,,, et al.A comparison of clinical outcomes with retrievable and permanent inferior vena cava filters.J Vasc Interv Radiol.2008;19:393–399.
- .The management of anticoagulation before and after procedures.Med Clin North Am.2001;85:1109–1116.
- ,,, et al.The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines, 8th ed.Chest.2008;133:299S–339S.
- ,,, et al.A vena caval filter for the prevention of pulmonary embolus.Surg Forum.1967;18.
- ,,.Twenty‐one‐year trends in the use of inferior vena cava filters.Arch Intern Med.2004;164:1541–1545.
- ,,, et al.A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep‐vein thrombosis. Prevention du Risque d'Embolie Pulmonaire par Interruption Cave Study Group.N Engl J Med.1998;338:409–415.
- ,.The need for anticoagulation following inferior vena cava filter placement: systematic review.Cardiovasc Intervent Radiol.2008;31:316–324.
- ,,, et al.Guidelines for the use of retrievable and convertible vena cava filters: report from the Society of Interventional Radiology multidisciplinary consensus conference.J Vasc Interv Radiol.2006;17:449–459.
- ,,, et al.Changing patterns in the use of inferior vena cava filters: review of a single center experience.J Am Coll Surg.2007;205:564–569.
- ,,.Hemodynamic effects of clot entrapment in the TrapEase inferior vena cava filter.J Vasc Interv Radiol.2004;15:485–490.
- ,,, et al.Vena cava filters and inferior vena cava thrombosis.J Vasc Surg.2007;45:789–794.
- ,,, et al.Clinical comparison of two optional vena cava filters.J Vasc Interv Radiol.2007;18:505–511.
- ,,, et al.Use of retrievable compared to permanent inferior vena cava filters: a single‐institution experience.Cardiovasc Intervent Radiol.2008;31:308–315.
- ,,, et al.Role of prophylactic temporary inferior vena cava filters placed at the ICU bedside under intravascular ultrasound guidance in patients with multiple trauma.J Vasc Surg.2004;40:958–964.
- ,,.Bedside vena cava filter placement with intravascular ultrasound: a simple, accurate, single venous access method.J Vasc Surg.2007;46:1284–1286.
- ,,.The bedside insertion of inferior vena cava filters using ultrasound guidance.Perspect Vasc Surg Endovasc Ther.2007;19:78–84.
- ,,, et al.Comparison of bedside transabdominal duplex ultrasound versus contrast venography for inferior vena cava filter placement: what is the best imaging modality?Ann Vasc Surg.2005;19:229–234.
- ,,, et al.Experience with the recovery filter as a retrievable inferior vena cava filter.J Vasc Interv Radiol.2005;16:1189–1193.
- ,,, et al.Gunther Tulip retrievable vena cava filter: results from the Registry of the Canadian Interventional Radiology Association.J Vasc Interv Radiol.2001;12:1053–1058.
- ,,, et al.Clinical experience with retrievable Gunther Tulip vena cava filters.J Endovasc Ther.2003;10:994–1000.
- ,,, et al.Gunther Tulip filter retrievability multicenter study including CT follow‐up: final report.J Vasc Interv Radiol.2006;17:1017–1023.
- ,,, et al.The Gunther Tulip retrievable filter: prolonged temporary filtration by repositioning within the inferior vena cava.J Vasc Interv Radiol.2003;14:1259–1265.
- ,,, et al.Retrievable inferior vena cava filters: early clinical experience.J Cardiovasc Surg (Torino).2005;46:163–169.
- ,,, et al.Gunther Tulip retrievable inferior vena caval filters: indications, efficacy, retrieval, and complications.Cardiovasc Intervent Radiol.2007;30:59–65.
- ,.Management of anticoagulation before and after elective surgery.N Engl J Med.1997;336:1506–1511.
- ,,, et al.Changes in inferior vena cava filter placement over the past decade at a large community‐based academic health center.J Vasc Surg.2008;47:157–165.
- ,,, et al.Massive pulmonary embolism.Circulation.2006;113:577–582.
- ,,, et al.Practice management guidelines for the prevention of venous thromboembolism in trauma patients: the EAST practice management guidelines work group.J Trauma.2002;53:142–164.
- ,,, et al.Practice patterns and outcomes of retrievable vena cava filters in trauma patients: an AAST multicenter study.J Trauma.2007;62:17–24; discussion 24‐25.
- ,.Inferior vena cava interruption. In: Crowther M, et al., eds.Evidence‐Based Hematology.West Sussex, UK:Wiley‐Blackwell Publishing;2008:99–109.
- ,,, et al.Are temporary inferior vena cava filters really temporary?Am J Surg.2005;190:858–863.
- ,,, et al.Retrievable vena cava filters for preventing pulmonary embolism in trauma patients: a cautionary tale.J Trauma.2006;60:35–40.
- ,,, et al.Safety, feasibility, and outcome of retrievable vena cava filters in high‐risk surgical patients.J Vasc Surg.2007;45:784–788; discussion 788.
- ,,, et al.Femoral arteriovenous fistula after placement of a Kimray‐Greenfield filter.AJR Am J Roentgenol.1988;151:681–682.
- ,,, et al.Prophylactic Greenfield filters: acute complications and long‐term follow‐up.J Trauma.1996;41:231–236; discussion 236‐237.
- ,,.Estimation of trapped thrombus volumes in retrievable inferior vena cava filters: a visual scale.J Vasc Interv Radiol.2007;18:273–276.
- ,,, et al.Endovascular recanalization of the thrombosed filter‐bearing inferior vena cava.J Vasc Interv Radiol.2003;14:893–903.
- ,,, et al.Early experience with the retrievable OptEase vena cava filter in high‐risk trauma patients.Eur J Vasc Endovasc Surg.2006;32:589–595.
- ,,, et al.Current trends in vena caval filtration with the introduction of a retrievable filter at a level I trauma center.J Trauma.2004;57:32–36.
- ,,, et al.Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:401S–428S.
- ,,, et al.Safety of inferior vena cava filter retrieval in anticoagulated patients.Chest.2007;132:31–36.
- ,,, et al.Should anticoagulant therapy be stopped or reversed before venous intervention?Can Assoc Radiol J.1999;50:306–309.
- ,,.Retrievable vena cava filters: clinical experience.Curr Opin Pulm Med.2006;12:304–309.
- .Use of emboli‐blocking filters increases, but rigorous data are lacking.JAMA.2006;295:989–990.
- ,,, et al.Extended interval for retrieval of vena cava filters is safe and may maximize protection against pulmonary embolism.Am J Surg.2006;192:789–794.
- ,,, et al.Long‐term retrievability of IVC filters: should we abandon permanent devices?Cardiovasc Intervent Radiol.2007;30:820–827.
- ,,, et al.A prospective long‐term study of 220 patients with a retrievable vena cava filter for secondary prevention of venous thromboembolism.Chest.2007;131:223–229.
- ,,, et al.The Cook Celect filter: the UK and global experience so far. In:European Congress of Radiology.2008;European Society of Radiology:Vienna, Austria.
- ,,, et al.Multicenter retrievability trial of the recovery G2 filter.J Vasc Interv Radiol.2008;19:S28.
- ,,, et al.The Jonas study: evaluation of the retrievability of the Cordis OptEase inferior vena cava filter.J Vasc Interv Radiol.2005;16:1439–1445.
- ,,, et al.“Recovery” vena cava filter: experience in 96 patients.Cardiovasc Intervent Radiol.2006;29:559–564.
- ,,, et al.Outcomes with retrievable inferior vena cava filters: a multicenter study.J Vasc Interv Radiol.2006;17:1595–1604.
- ,,, et al.Retrievable inferior vena cava filters: initial clinical results.Ann Vasc Surg.2006;20:157–165.
- ,,, et al.Evaluation of retrievability of the Günther Tulip vena cava filter.Cardiovasc Intervent Radiol.2007;30:226–231.
- ,,, et al.A comparison of clinical outcomes with retrievable and permanent inferior vena cava filters.J Vasc Interv Radiol.2008;19:393–399.
- .The management of anticoagulation before and after procedures.Med Clin North Am.2001;85:1109–1116.
- ,,, et al.The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines, 8th ed.Chest.2008;133:299S–339S.