User login
Clinical Guidelines: ADA 2017 Standards of Medical Care in Diabetes
In 2012, 29.1 million Americans, or 9.3% of the population, had diabetes. Of this number, 21 million were diagnosed, and 8.1 million were undiagnosed. Each year almost 1.5 million Americans receive a new diagnosis of diabetes. The management of diabetes relies upon excellent primary care. Each year the American Diabetes Association reviews new evidence and publishes an updated Standards of Care in the January issue of Diabetes Care. Here we give a short overview of the guidelines with emphasis on fundamentals and changes in the standards over the past year.
Self-management education and support, nutrition therapy, and physical activity
All patients should participate in ongoing diabetes self-management education (DSME) to facilitate the knowledge, skills, and abilities necessary to obtain optimal self-care and incorporate the needs, goals, and life experiences of the person with diabetes as they face new challenges throughout a lifetime of diabetes.
In addition, each patient should receive individualized medical nutrition therapy (MNT) provided by a registered dietitian with knowledge regarding diabetes-specific MNT. Most patients should increase aerobic physical activity to 150 min/week. Providers should encourage patients to reduce the amount of time spent sedentary by briefly standing, walking, or performing other light physical activities every 30 minutes.
Glycemic targets
A reasonable hemoglobin A1c goal for many diabetic nonpregnant adults is less than 7%. A less stringent goal under 8% may be appropriate for patients with a history of severe hypoglycemia, limited life expectancy, advanced microvascular and macrovascular complications, and extensive comorbid conditions. HbA1c measurements should be done at diagnosis and routinely to monitor glycemic control. To aid in achieving glycemic targets, self-monitoring blood glucose (SMBG) allows patients to evaluate their individual response to therapy. Integrating SMBG data into diabetes management can help guide MNT, adjust medications, determine physical activity requirements, and prevent hypoglycemia. Individuals at risk for hypoglycemia should be asked about symptomatic and asymptomatic hypoglycemia at each encounter and counseled regarding treatment of hypoglycemic events.
Obesity management
There is strong and consistent evidence that obesity management may be beneficial in the treatment of type 2 diabetes. For overweight and obese patients with type 2 diabetes, interventions should be high intensity (more than 16 sessions in 6 months) and focus on diet, physical activity, and behavioral therapy designed to achieve a greater than 5% weight loss (energy deficit of 500-750 kcal/day).
For select patients, weight loss medications may be effective as adjuncts to lifestyle changes. When choosing additional pharmacologic interventions to improve glycemic control in overweight or obese patients, providers should use medications that promote weight loss or are weight neutral including metformin, sodium-glucose cotransporter-2 (SGLT-2) inhibitors, glucagon-like peptide-1 (GLP-1) agonists, and dipeptidyl peptidase-4 inhibitors (DPP-4) versus those that cause weight gain such as insulin secretagogues, thiazolidinediones, and insulin.
Metabolic surgery should be recommended to patients with type 2 diabetes and body mass index above 40 kg/m2 (BMI above 37.5 kg/m2 in Asian Americans), regardless of adequate glycemic control and for patients with BMI above 35 kg/m2 (more than 32.5 kg/m2 in Asian Americans) without adequate glycemic control despite lifestyle modifications and optimal medical therapy. Metabolic surgery should be considered for appropriate candidates with BMIs as low as 30 if hyperglycemia is inadequately controlled despite optical medical control by either oral or injectable medications.
CV disease and risk management: BP, lipids, antiplatelet therapy, and glycemic medication management
Atherosclerotic cardiovascular disease is the leading cause of morbidity and mortality for individuals with diabetes. Screening for atherosclerotic cardiovascular disease is not recommended; rather, the emphasis is on careful risk factor management.
If systolic blood pressure (SBP) is confirmed to be above 140 mm Hg and/or the diastolic blood pressure (DBP) is confirmed to be above 90 mm Hg, pharmacologic therapy should be initiated. A meta-analysis of randomized trials of adults with type 2 diabetes comparing intensive blood pressure targets (upper limit of 130 mm Hg SBP and 80 mm Hg DBP) with standard targets (upper limit of 140-160 mm Hg SBP and 85-100 mm Hg DBP) found no significant reduction in mortality or nonfatal MI. There was a statistically significant, 35% relative risk (RR) reduction in stroke with intensive targets, but intensive targets were associated with an increased risk for adverse events such as hypotension and syncope. Recommendations suggest that antihypertension treatment in adults with diabetes without albuminuria should include any of the classes of medication demonstrated to reduce cardiovascular events in patients with diabetes, such as ACE inhibitors, angiotensin receptor blockers (ARBs), thiazide-like diuretics, or dihydropyridine calcium-channel blockers. ACE inhibitors and ARBs continue to be recommended as first-line medications for the treatment of hypertension in patients with diabetes and elevated urine albumin/creatinine ratios (above 30 mg/g creatinine). The standards also suggest consideration of administering one or more antihypertensive medications at bedtime, which may improve cardiovascular outcomes.
For patients aged 40-75 years who have diabetes without additional atherosclerotic CV disease risk factors, a moderate-intensity statin should be considered. If there are additional cardiovascular risk factors, then a high-intensity statin should be considered. For patients who are younger than 40 years of age and have diabetes with additional atherosclerotic CV disease risk factors, a less strong recommendation is to consider using moderate-intensity or high-intensity statins. For patients older than 75 years with diabetes without additional atherosclerotic CV disease risk factors, consider using moderate-intensity statin therapy; high-intensity statin therapy may be considered in older adults with risk factors for atherosclerotic cardiovascular disease.
Both women and men who are at least 50 years old and have diabetes with at least one additional cardiovascular risk factor should consider taking daily aspirin therapy (75-162 mg/day) if they do not have any risk for excessive bleeding.
In patients with long-standing suboptimally controlled type 2 diabetes and established atherosclerotic CV disease, empagliflozin or liraglutide should be considered as they have been shown to reduce cardiovascular and all-cause mortality when added to standard care.
Microvascular disease and foot care
Large prospective studies have demonstrated that optimized glucose control can reduce the onset and progression of diabetic microvascular complications. Diabetic kidney disease occurs in about 20%-40% of persons with diabetes. Annual screening includes estimated glomerular filtration rate and spot urine albumin-to-creatinine ratio. Treatment includes ACE inhibitors or ARBs in addition to a target blood pressure of under 140/90 mm Hg.
Diabetic retinopathy screening includes a dilated eye exam by an eye care professional. Treatment includes laser photocoagulation therapy for high risk nonproliferative retinopathy or proliferative retinopathy, or intravitreal injections of antivascular endothelial growth factor agents for central-involved diabetic macular edema.
Diabetic peripheral neuropathy screening includes annual 10-g monofilament and 128-HZ tuning fork vibration sensation. Medications for painful diabetic neuropathy may include gabapentin, pregabalin, duloxetine, and other agents.
Neuropathy and vascular disease are contributors to diabetic foot ulcers and amputation. A comprehensive foot examination along with appropriate risk factor oriented history to include neuropathic and vascular components (pulses, claudication) should be performed annually, while all patients with diabetes should have their feet checked at every visit.
Older adults
Prioritizing treatment goals in older adults is important in this heterogeneous population. Cardiovascular risk factor treatment is likely to be beneficial.
In setting HbA1c goals, functional status, and comorbid conditions should be considered. Metformin can still be a first-line agent for many older adults with type 2 diabetes, with consideration to renal status (creatinine clearance above 30 mL/min per 1.73 m2) and heart failure. DPP-4s have few side effects and low risk of hypoglycemia. GLP-1 receptor agonists have a low risk of hypoglycemia but may be associated with GI side effects and weight loss. SGLT-2 inhibitors have a low risk of hypoglycemia, and attention should be paid to renal thresholds for use. Thiazolidinediones should be used cautiously in those with heart failure or at elevated fracture risk. Sulfonylureas should be used cautiously because of their elevated risk of hypoglycemia. When used, a short-acting sulfonylurea – such as glipizide – is preferred, as long-acting sulfonylureas are contraindicated because of even greater hypoglycemic risk. Single-injection basal insulin may be appropriate for many with ease of use and efficacy.
The bottom line
Diabetes is a rapidly changing field and each year the American Diabetes Association updates the Standards of Medical Care document to be consistent with the latest evidence. Highlights of the standards include emphasis on diabetes self-management education, individualized glycemic goal setting, obesity management, setting blood pressure targets to less than 140/90 mm Hg, as well as statins and daily aspirin for most people with diabetes. In addition, ADA now recommends the use of specific antihyperglycemic medications to reduce cardiovascular and all-cause mortality in patients with diabetes and established cardiovascular disease.
Reference
American Diabetes Association Standards of Medical Care in Diabetes – 2017. Diabetes Care 2017; 40 (sup 1):S1-S138
Dr. Skolnik is professor of family and community medicine, Temple University School of Medicine, Philadelphia, and associate director, Family Medicine Residency Program, Abington-Jefferson Health, Abington, Pa. Dr. Johnson is associate professor at the University of North Dakota School of Medicine and Health Sciences, and practices at the Altru Diabetes Center, Grand Forks. Ms. Neuman practices at St. Mark’s Hospital, Salt Lake City.
In 2012, 29.1 million Americans, or 9.3% of the population, had diabetes. Of this number, 21 million were diagnosed, and 8.1 million were undiagnosed. Each year almost 1.5 million Americans receive a new diagnosis of diabetes. The management of diabetes relies upon excellent primary care. Each year the American Diabetes Association reviews new evidence and publishes an updated Standards of Care in the January issue of Diabetes Care. Here we give a short overview of the guidelines with emphasis on fundamentals and changes in the standards over the past year.
Self-management education and support, nutrition therapy, and physical activity
All patients should participate in ongoing diabetes self-management education (DSME) to facilitate the knowledge, skills, and abilities necessary to obtain optimal self-care and incorporate the needs, goals, and life experiences of the person with diabetes as they face new challenges throughout a lifetime of diabetes.
In addition, each patient should receive individualized medical nutrition therapy (MNT) provided by a registered dietitian with knowledge regarding diabetes-specific MNT. Most patients should increase aerobic physical activity to 150 min/week. Providers should encourage patients to reduce the amount of time spent sedentary by briefly standing, walking, or performing other light physical activities every 30 minutes.
Glycemic targets
A reasonable hemoglobin A1c goal for many diabetic nonpregnant adults is less than 7%. A less stringent goal under 8% may be appropriate for patients with a history of severe hypoglycemia, limited life expectancy, advanced microvascular and macrovascular complications, and extensive comorbid conditions. HbA1c measurements should be done at diagnosis and routinely to monitor glycemic control. To aid in achieving glycemic targets, self-monitoring blood glucose (SMBG) allows patients to evaluate their individual response to therapy. Integrating SMBG data into diabetes management can help guide MNT, adjust medications, determine physical activity requirements, and prevent hypoglycemia. Individuals at risk for hypoglycemia should be asked about symptomatic and asymptomatic hypoglycemia at each encounter and counseled regarding treatment of hypoglycemic events.
Obesity management
There is strong and consistent evidence that obesity management may be beneficial in the treatment of type 2 diabetes. For overweight and obese patients with type 2 diabetes, interventions should be high intensity (more than 16 sessions in 6 months) and focus on diet, physical activity, and behavioral therapy designed to achieve a greater than 5% weight loss (energy deficit of 500-750 kcal/day).
For select patients, weight loss medications may be effective as adjuncts to lifestyle changes. When choosing additional pharmacologic interventions to improve glycemic control in overweight or obese patients, providers should use medications that promote weight loss or are weight neutral including metformin, sodium-glucose cotransporter-2 (SGLT-2) inhibitors, glucagon-like peptide-1 (GLP-1) agonists, and dipeptidyl peptidase-4 inhibitors (DPP-4) versus those that cause weight gain such as insulin secretagogues, thiazolidinediones, and insulin.
Metabolic surgery should be recommended to patients with type 2 diabetes and body mass index above 40 kg/m2 (BMI above 37.5 kg/m2 in Asian Americans), regardless of adequate glycemic control and for patients with BMI above 35 kg/m2 (more than 32.5 kg/m2 in Asian Americans) without adequate glycemic control despite lifestyle modifications and optimal medical therapy. Metabolic surgery should be considered for appropriate candidates with BMIs as low as 30 if hyperglycemia is inadequately controlled despite optical medical control by either oral or injectable medications.
CV disease and risk management: BP, lipids, antiplatelet therapy, and glycemic medication management
Atherosclerotic cardiovascular disease is the leading cause of morbidity and mortality for individuals with diabetes. Screening for atherosclerotic cardiovascular disease is not recommended; rather, the emphasis is on careful risk factor management.
If systolic blood pressure (SBP) is confirmed to be above 140 mm Hg and/or the diastolic blood pressure (DBP) is confirmed to be above 90 mm Hg, pharmacologic therapy should be initiated. A meta-analysis of randomized trials of adults with type 2 diabetes comparing intensive blood pressure targets (upper limit of 130 mm Hg SBP and 80 mm Hg DBP) with standard targets (upper limit of 140-160 mm Hg SBP and 85-100 mm Hg DBP) found no significant reduction in mortality or nonfatal MI. There was a statistically significant, 35% relative risk (RR) reduction in stroke with intensive targets, but intensive targets were associated with an increased risk for adverse events such as hypotension and syncope. Recommendations suggest that antihypertension treatment in adults with diabetes without albuminuria should include any of the classes of medication demonstrated to reduce cardiovascular events in patients with diabetes, such as ACE inhibitors, angiotensin receptor blockers (ARBs), thiazide-like diuretics, or dihydropyridine calcium-channel blockers. ACE inhibitors and ARBs continue to be recommended as first-line medications for the treatment of hypertension in patients with diabetes and elevated urine albumin/creatinine ratios (above 30 mg/g creatinine). The standards also suggest consideration of administering one or more antihypertensive medications at bedtime, which may improve cardiovascular outcomes.
For patients aged 40-75 years who have diabetes without additional atherosclerotic CV disease risk factors, a moderate-intensity statin should be considered. If there are additional cardiovascular risk factors, then a high-intensity statin should be considered. For patients who are younger than 40 years of age and have diabetes with additional atherosclerotic CV disease risk factors, a less strong recommendation is to consider using moderate-intensity or high-intensity statins. For patients older than 75 years with diabetes without additional atherosclerotic CV disease risk factors, consider using moderate-intensity statin therapy; high-intensity statin therapy may be considered in older adults with risk factors for atherosclerotic cardiovascular disease.
Both women and men who are at least 50 years old and have diabetes with at least one additional cardiovascular risk factor should consider taking daily aspirin therapy (75-162 mg/day) if they do not have any risk for excessive bleeding.
In patients with long-standing suboptimally controlled type 2 diabetes and established atherosclerotic CV disease, empagliflozin or liraglutide should be considered as they have been shown to reduce cardiovascular and all-cause mortality when added to standard care.
Microvascular disease and foot care
Large prospective studies have demonstrated that optimized glucose control can reduce the onset and progression of diabetic microvascular complications. Diabetic kidney disease occurs in about 20%-40% of persons with diabetes. Annual screening includes estimated glomerular filtration rate and spot urine albumin-to-creatinine ratio. Treatment includes ACE inhibitors or ARBs in addition to a target blood pressure of under 140/90 mm Hg.
Diabetic retinopathy screening includes a dilated eye exam by an eye care professional. Treatment includes laser photocoagulation therapy for high risk nonproliferative retinopathy or proliferative retinopathy, or intravitreal injections of antivascular endothelial growth factor agents for central-involved diabetic macular edema.
Diabetic peripheral neuropathy screening includes annual 10-g monofilament and 128-HZ tuning fork vibration sensation. Medications for painful diabetic neuropathy may include gabapentin, pregabalin, duloxetine, and other agents.
Neuropathy and vascular disease are contributors to diabetic foot ulcers and amputation. A comprehensive foot examination along with appropriate risk factor oriented history to include neuropathic and vascular components (pulses, claudication) should be performed annually, while all patients with diabetes should have their feet checked at every visit.
Older adults
Prioritizing treatment goals in older adults is important in this heterogeneous population. Cardiovascular risk factor treatment is likely to be beneficial.
In setting HbA1c goals, functional status, and comorbid conditions should be considered. Metformin can still be a first-line agent for many older adults with type 2 diabetes, with consideration to renal status (creatinine clearance above 30 mL/min per 1.73 m2) and heart failure. DPP-4s have few side effects and low risk of hypoglycemia. GLP-1 receptor agonists have a low risk of hypoglycemia but may be associated with GI side effects and weight loss. SGLT-2 inhibitors have a low risk of hypoglycemia, and attention should be paid to renal thresholds for use. Thiazolidinediones should be used cautiously in those with heart failure or at elevated fracture risk. Sulfonylureas should be used cautiously because of their elevated risk of hypoglycemia. When used, a short-acting sulfonylurea – such as glipizide – is preferred, as long-acting sulfonylureas are contraindicated because of even greater hypoglycemic risk. Single-injection basal insulin may be appropriate for many with ease of use and efficacy.
The bottom line
Diabetes is a rapidly changing field and each year the American Diabetes Association updates the Standards of Medical Care document to be consistent with the latest evidence. Highlights of the standards include emphasis on diabetes self-management education, individualized glycemic goal setting, obesity management, setting blood pressure targets to less than 140/90 mm Hg, as well as statins and daily aspirin for most people with diabetes. In addition, ADA now recommends the use of specific antihyperglycemic medications to reduce cardiovascular and all-cause mortality in patients with diabetes and established cardiovascular disease.
Reference
American Diabetes Association Standards of Medical Care in Diabetes – 2017. Diabetes Care 2017; 40 (sup 1):S1-S138
Dr. Skolnik is professor of family and community medicine, Temple University School of Medicine, Philadelphia, and associate director, Family Medicine Residency Program, Abington-Jefferson Health, Abington, Pa. Dr. Johnson is associate professor at the University of North Dakota School of Medicine and Health Sciences, and practices at the Altru Diabetes Center, Grand Forks. Ms. Neuman practices at St. Mark’s Hospital, Salt Lake City.
In 2012, 29.1 million Americans, or 9.3% of the population, had diabetes. Of this number, 21 million were diagnosed, and 8.1 million were undiagnosed. Each year almost 1.5 million Americans receive a new diagnosis of diabetes. The management of diabetes relies upon excellent primary care. Each year the American Diabetes Association reviews new evidence and publishes an updated Standards of Care in the January issue of Diabetes Care. Here we give a short overview of the guidelines with emphasis on fundamentals and changes in the standards over the past year.
Self-management education and support, nutrition therapy, and physical activity
All patients should participate in ongoing diabetes self-management education (DSME) to facilitate the knowledge, skills, and abilities necessary to obtain optimal self-care and incorporate the needs, goals, and life experiences of the person with diabetes as they face new challenges throughout a lifetime of diabetes.
In addition, each patient should receive individualized medical nutrition therapy (MNT) provided by a registered dietitian with knowledge regarding diabetes-specific MNT. Most patients should increase aerobic physical activity to 150 min/week. Providers should encourage patients to reduce the amount of time spent sedentary by briefly standing, walking, or performing other light physical activities every 30 minutes.
Glycemic targets
A reasonable hemoglobin A1c goal for many diabetic nonpregnant adults is less than 7%. A less stringent goal under 8% may be appropriate for patients with a history of severe hypoglycemia, limited life expectancy, advanced microvascular and macrovascular complications, and extensive comorbid conditions. HbA1c measurements should be done at diagnosis and routinely to monitor glycemic control. To aid in achieving glycemic targets, self-monitoring blood glucose (SMBG) allows patients to evaluate their individual response to therapy. Integrating SMBG data into diabetes management can help guide MNT, adjust medications, determine physical activity requirements, and prevent hypoglycemia. Individuals at risk for hypoglycemia should be asked about symptomatic and asymptomatic hypoglycemia at each encounter and counseled regarding treatment of hypoglycemic events.
Obesity management
There is strong and consistent evidence that obesity management may be beneficial in the treatment of type 2 diabetes. For overweight and obese patients with type 2 diabetes, interventions should be high intensity (more than 16 sessions in 6 months) and focus on diet, physical activity, and behavioral therapy designed to achieve a greater than 5% weight loss (energy deficit of 500-750 kcal/day).
For select patients, weight loss medications may be effective as adjuncts to lifestyle changes. When choosing additional pharmacologic interventions to improve glycemic control in overweight or obese patients, providers should use medications that promote weight loss or are weight neutral including metformin, sodium-glucose cotransporter-2 (SGLT-2) inhibitors, glucagon-like peptide-1 (GLP-1) agonists, and dipeptidyl peptidase-4 inhibitors (DPP-4) versus those that cause weight gain such as insulin secretagogues, thiazolidinediones, and insulin.
Metabolic surgery should be recommended to patients with type 2 diabetes and body mass index above 40 kg/m2 (BMI above 37.5 kg/m2 in Asian Americans), regardless of adequate glycemic control and for patients with BMI above 35 kg/m2 (more than 32.5 kg/m2 in Asian Americans) without adequate glycemic control despite lifestyle modifications and optimal medical therapy. Metabolic surgery should be considered for appropriate candidates with BMIs as low as 30 if hyperglycemia is inadequately controlled despite optical medical control by either oral or injectable medications.
CV disease and risk management: BP, lipids, antiplatelet therapy, and glycemic medication management
Atherosclerotic cardiovascular disease is the leading cause of morbidity and mortality for individuals with diabetes. Screening for atherosclerotic cardiovascular disease is not recommended; rather, the emphasis is on careful risk factor management.
If systolic blood pressure (SBP) is confirmed to be above 140 mm Hg and/or the diastolic blood pressure (DBP) is confirmed to be above 90 mm Hg, pharmacologic therapy should be initiated. A meta-analysis of randomized trials of adults with type 2 diabetes comparing intensive blood pressure targets (upper limit of 130 mm Hg SBP and 80 mm Hg DBP) with standard targets (upper limit of 140-160 mm Hg SBP and 85-100 mm Hg DBP) found no significant reduction in mortality or nonfatal MI. There was a statistically significant, 35% relative risk (RR) reduction in stroke with intensive targets, but intensive targets were associated with an increased risk for adverse events such as hypotension and syncope. Recommendations suggest that antihypertension treatment in adults with diabetes without albuminuria should include any of the classes of medication demonstrated to reduce cardiovascular events in patients with diabetes, such as ACE inhibitors, angiotensin receptor blockers (ARBs), thiazide-like diuretics, or dihydropyridine calcium-channel blockers. ACE inhibitors and ARBs continue to be recommended as first-line medications for the treatment of hypertension in patients with diabetes and elevated urine albumin/creatinine ratios (above 30 mg/g creatinine). The standards also suggest consideration of administering one or more antihypertensive medications at bedtime, which may improve cardiovascular outcomes.
For patients aged 40-75 years who have diabetes without additional atherosclerotic CV disease risk factors, a moderate-intensity statin should be considered. If there are additional cardiovascular risk factors, then a high-intensity statin should be considered. For patients who are younger than 40 years of age and have diabetes with additional atherosclerotic CV disease risk factors, a less strong recommendation is to consider using moderate-intensity or high-intensity statins. For patients older than 75 years with diabetes without additional atherosclerotic CV disease risk factors, consider using moderate-intensity statin therapy; high-intensity statin therapy may be considered in older adults with risk factors for atherosclerotic cardiovascular disease.
Both women and men who are at least 50 years old and have diabetes with at least one additional cardiovascular risk factor should consider taking daily aspirin therapy (75-162 mg/day) if they do not have any risk for excessive bleeding.
In patients with long-standing suboptimally controlled type 2 diabetes and established atherosclerotic CV disease, empagliflozin or liraglutide should be considered as they have been shown to reduce cardiovascular and all-cause mortality when added to standard care.
Microvascular disease and foot care
Large prospective studies have demonstrated that optimized glucose control can reduce the onset and progression of diabetic microvascular complications. Diabetic kidney disease occurs in about 20%-40% of persons with diabetes. Annual screening includes estimated glomerular filtration rate and spot urine albumin-to-creatinine ratio. Treatment includes ACE inhibitors or ARBs in addition to a target blood pressure of under 140/90 mm Hg.
Diabetic retinopathy screening includes a dilated eye exam by an eye care professional. Treatment includes laser photocoagulation therapy for high risk nonproliferative retinopathy or proliferative retinopathy, or intravitreal injections of antivascular endothelial growth factor agents for central-involved diabetic macular edema.
Diabetic peripheral neuropathy screening includes annual 10-g monofilament and 128-HZ tuning fork vibration sensation. Medications for painful diabetic neuropathy may include gabapentin, pregabalin, duloxetine, and other agents.
Neuropathy and vascular disease are contributors to diabetic foot ulcers and amputation. A comprehensive foot examination along with appropriate risk factor oriented history to include neuropathic and vascular components (pulses, claudication) should be performed annually, while all patients with diabetes should have their feet checked at every visit.
Older adults
Prioritizing treatment goals in older adults is important in this heterogeneous population. Cardiovascular risk factor treatment is likely to be beneficial.
In setting HbA1c goals, functional status, and comorbid conditions should be considered. Metformin can still be a first-line agent for many older adults with type 2 diabetes, with consideration to renal status (creatinine clearance above 30 mL/min per 1.73 m2) and heart failure. DPP-4s have few side effects and low risk of hypoglycemia. GLP-1 receptor agonists have a low risk of hypoglycemia but may be associated with GI side effects and weight loss. SGLT-2 inhibitors have a low risk of hypoglycemia, and attention should be paid to renal thresholds for use. Thiazolidinediones should be used cautiously in those with heart failure or at elevated fracture risk. Sulfonylureas should be used cautiously because of their elevated risk of hypoglycemia. When used, a short-acting sulfonylurea – such as glipizide – is preferred, as long-acting sulfonylureas are contraindicated because of even greater hypoglycemic risk. Single-injection basal insulin may be appropriate for many with ease of use and efficacy.
The bottom line
Diabetes is a rapidly changing field and each year the American Diabetes Association updates the Standards of Medical Care document to be consistent with the latest evidence. Highlights of the standards include emphasis on diabetes self-management education, individualized glycemic goal setting, obesity management, setting blood pressure targets to less than 140/90 mm Hg, as well as statins and daily aspirin for most people with diabetes. In addition, ADA now recommends the use of specific antihyperglycemic medications to reduce cardiovascular and all-cause mortality in patients with diabetes and established cardiovascular disease.
Reference
American Diabetes Association Standards of Medical Care in Diabetes – 2017. Diabetes Care 2017; 40 (sup 1):S1-S138
Dr. Skolnik is professor of family and community medicine, Temple University School of Medicine, Philadelphia, and associate director, Family Medicine Residency Program, Abington-Jefferson Health, Abington, Pa. Dr. Johnson is associate professor at the University of North Dakota School of Medicine and Health Sciences, and practices at the Altru Diabetes Center, Grand Forks. Ms. Neuman practices at St. Mark’s Hospital, Salt Lake City.
Fatty liver disease in type 2 diabetes: Common and often unmanaged
Purpose The objective of this pilot study was to evaluate the prevalence and management of nonalcoholic fatty liver disease in a rural type 2 diabetes population.
Methods We randomly selected 100 patients with type 2 diabetes from a large rural clinic/hospital system in the upper Midwest and conducted a chart review to determine the prevalence of abnormal results of serum liver function tests and liver imaging (eg, computed tomography, ultrasound, magnetic resonance imaging). We also determined the number of patients who were given a diagnosis of fatty liver disease and who among those were subsequently managed for the condition.
Results Of the 100 subjects, 40 had abnormal serum liver function testing, and half of those individuals underwent imaging. This resulted in a diagnosis of fatty liver disease in 11 (27.5% of the 40 with abnormal liver function). Only 4 patients received specific interventions for fatty liver disease.
Conclusion In this rural population, fatty liver disease was common and untreated, suggesting a possible need for a change in screening and management protocols.
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in the United States.1 In particular, NAFLD and the related inflammatory nonalcoholic steatohepatitis (NASH) often develop in individuals who are obese or who have prediabetes or type 2 diabetes, affecting up to 75% of patients with these conditions.2
In addition to NAFLD and NASH, other liver diseases associated with type 2 diabetes include cirrhosis, hepatocellular carcinoma, liver failure, and hepatitis.1,3,4 Of patients with type 2 diabetes, more than 600,000 have cirrhosis; 4.4% of diabetes-related deaths have been attributed to cirrhosis.4,5
NAFLD and NASH share a common pathophysiology in type 2 diabetes with respect to insulin resistance, which results in hyperlipidemias that enhance fatty deposits in the liver.1,2 Hepatic fat accumulation is also associated with increasing measures of inflammation, including C-reactive protein.6
Resultant liver function test abnormalities and characteristic appearance on imaging studies (ultrasound, computed tomography [CT], or magnetic resonance imaging [MRI]) may be similar in NAFLD and NASH.1-3 Liver biopsy therefore is necessary to distinguish NAFLD from NASH, with NASH showing characteristic inflammatory and fibrotic changes.1,2 Evaluations of patients with minor liver test abnormalities reveal that up to 98% may have liver disease, most often fatty liver disease.7
Weight loss is a strategy for managing NAFLD and NASH, although large randomized controlled trials are lacking.8-10 Several agents used for diabetes and dyslipidemias, including glucagon-like peptide-1 (GLP-1) mimetics, metformin, thiazolidinediones, and statins, have been studied as possible treatments for NAFLD and NASH.8-13 Currently, these medications carry cautions or warnings about using them in patients with liver disease and are not indicated as treatments for NAFLD or NASH.
SUBJECTS AND METHODS
One hundred patients were randomly selected from a type 2 diabetes patient database at the Altru Health System (Grand Forks, ND) for cross-sectional analysis. Manual data extraction from “paper charts” was necessary in some cases, limiting the size of the study.
All subjects had a diagnosis of type 2 diabetes confirmed by American Diabetes Association criteria, were between the ages of 18 and 64 years, and had no known liver disease other than that associated with their diabetes. Other criteria included visiting a health care provider regarding diabetes management within the last year and having undergone laboratory blood testing of liver function within the last 5 years. The study population comprised an equal number of men and women.
We collected data about abnormal liver function from blood test results, including levels of aspartate transaminase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP); results of radiologic imaging of the liver (ultrasound, MRI, or CT) and liver biopsy; any interventions (medication changes, lifestyle management, or surgery); and referral to a specialist (gastroenterologist or surgeon).
DATA ANALYSIS
Of the 100 subjects, 40 had at least one abnormal serum liver function test (AST, ALT, or ALP), although we could find no record of tests for one of the 100 subjects. Of the 40 with abnormal test results, 17 (42.5%) were women and 23 (57.5%) were men. None of these patients had highly elevated levels of AST, ALT, or ALP.
Of the 40 patients with abnormal serum liver function tests, only 10 (25%) were specifically referred for imaging studies related to a possible diagnosis of fatty liver disease. Four of these 10 patients (1 woman and 3 men) had both ultrasound and CT imaging. Another 10 subjects (25%) had incidental findings of fatty liver disease on imaging performed for another presumed diagnosis or symptom, eg, abdominal pain. Overall, 11 (6 men, 5 women) of the 40 subjects (27.5%) with at least one abnormal liver function test received a diagnosis of fatty liver disease based on imaging findings. None of the subjects had a diagnosis of cirrhosis or other end-stage liver disease.
A medical intervention was offered to 4 of the 11 patients (2 women and 2 men) who received a diagnosis of fatty liver disease. Practitioners specifically referred one woman for medical weight loss management and another for weight loss surgery. One man was advised to stop taking metformin, and another was referred to a dietician for lifestyle weight loss management. No patient was referred to a gastroenterologist or any other specialist for further evaluation or biopsy.
DISCUSSION
Although a small sample size limits the strength of this pilot study, the finding that fatty liver disease is common in patients with type 2 diabetes in a rural community hospital population supports other published data. As only half of the subjects with abnormal liver function tests had imaging studies, it’s likely that some patients in the study group who did not undergo imaging also had NAFLD, NASH, or other liver disease, but it was not diagnosed.
While no specific screening guidelines for fatty liver disease in patients with type 2 diabetes have been issued, clinical interest in this area has been growing, and this study suggests some avenues for further exploration.3 In the institution where this study was conducted, it appears that liver function tests were most likely to be performed in conjunction with routine monitoring of the use of statins, metformin, or other medications or because of a symptom such as abdominal pain. Yet given the widespread availability and relatively low cost of such tests, periodic monitoring of serum liver function in patients with type 2 diabetes may be warranted.14
Patients found to have persistent or recurrent abnormal liver function tests could then be referred for further evaluation with ultrasound, CT, or MRI.14 Ultrasound has the benefit of lower cost and avoidance of intravenous contrast, which may be important for patients with renal dysfunction.14 Based on the results of these tests, appropriate medical interventions could then follow.
·Acknowledgements·
The author thanks Bonnie Lee and Diane Vold for their assistance in the design of this study and its preparation for publication. The author also thanks William Zaks, MD, PhD, and James Brosseau, MD, MPH, for their guidance and feedback.
1. Caldwell SH, Oelsner DH, Iezzoni JC, et al. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664-669.
2. Pagano G, Pacini G, Musso G, et al. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology. 2002;35:367-372.
3. Tolman KG, Fonseca V, Dalpiaz A, et al. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care. 2007;30:734-743.
4. de Marco R, Locatelli F, Zoppini G, et al. Cause-specific mortality in type 2 diabetes: the Verona Diabetes Study. Diabetes Care. 1999;22:756-761.
5. Koehler E, Watt K, Charlton M. Fatty liver and liver transplantation. Clin Liver Dis. 2009;13:621-630.
6. Saremi A, Allison M, Ditomasso D, et al. Preliminary report: hepatic fat and inflammation in type 2 diabetes mellitus. Metabolism. 2010;59:430-432.
7. Hultcrantz R, Glaumann H, Lindberg G, et al. Liver investigation in 149 asymptomatic patients with moderately elevated activities of serum aminotransferases. Scand J Gastroenterol. 1986;21:109-113.
8. Schreuder TC, Verwer BJ, van Nieuwkerk CM, et al. Nonalcoholic fatty liver disease: an overview of current insights in pathogenesis, diagnosis and treatment. World J Gastroenterol. 2008;14:2474-2486.
9. Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, et al. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010;(1):CD007340.-
10. Medina J, Fernández-Salazar LI, García-Buey L, et al. Approach to the pathogenesis and treatment of nonalcoholic steatohepatitis. Diabetes Care. 2004;27:2057-2066.
11. Khashab M, Chalasani N. Use of insulin sensitizers in NASH. Endocrinol Metab Clin North Am. 2007;36:1067-1087.
12. Ding X, Saxena NK, Lin S, et al. Exendin-4, a glucagon-like protein (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43:173-181.
13. Matafome P, Nunes E, Louro T, et al. A role for atorvastatin and insulin combination in protecting from liver injury in a model of type 2 diabetes with hyperlipidemia. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:241-251.
14. Ratziu V, Bellentani S, Cortez-Pinto H, et al. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372-384.
CORRESPONDENCE Eric L. Johnson, MD, 501 N. Columbia Road, Stop 9037, Grand Forks, ND 58202; [email protected]
Purpose The objective of this pilot study was to evaluate the prevalence and management of nonalcoholic fatty liver disease in a rural type 2 diabetes population.
Methods We randomly selected 100 patients with type 2 diabetes from a large rural clinic/hospital system in the upper Midwest and conducted a chart review to determine the prevalence of abnormal results of serum liver function tests and liver imaging (eg, computed tomography, ultrasound, magnetic resonance imaging). We also determined the number of patients who were given a diagnosis of fatty liver disease and who among those were subsequently managed for the condition.
Results Of the 100 subjects, 40 had abnormal serum liver function testing, and half of those individuals underwent imaging. This resulted in a diagnosis of fatty liver disease in 11 (27.5% of the 40 with abnormal liver function). Only 4 patients received specific interventions for fatty liver disease.
Conclusion In this rural population, fatty liver disease was common and untreated, suggesting a possible need for a change in screening and management protocols.
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in the United States.1 In particular, NAFLD and the related inflammatory nonalcoholic steatohepatitis (NASH) often develop in individuals who are obese or who have prediabetes or type 2 diabetes, affecting up to 75% of patients with these conditions.2
In addition to NAFLD and NASH, other liver diseases associated with type 2 diabetes include cirrhosis, hepatocellular carcinoma, liver failure, and hepatitis.1,3,4 Of patients with type 2 diabetes, more than 600,000 have cirrhosis; 4.4% of diabetes-related deaths have been attributed to cirrhosis.4,5
NAFLD and NASH share a common pathophysiology in type 2 diabetes with respect to insulin resistance, which results in hyperlipidemias that enhance fatty deposits in the liver.1,2 Hepatic fat accumulation is also associated with increasing measures of inflammation, including C-reactive protein.6
Resultant liver function test abnormalities and characteristic appearance on imaging studies (ultrasound, computed tomography [CT], or magnetic resonance imaging [MRI]) may be similar in NAFLD and NASH.1-3 Liver biopsy therefore is necessary to distinguish NAFLD from NASH, with NASH showing characteristic inflammatory and fibrotic changes.1,2 Evaluations of patients with minor liver test abnormalities reveal that up to 98% may have liver disease, most often fatty liver disease.7
Weight loss is a strategy for managing NAFLD and NASH, although large randomized controlled trials are lacking.8-10 Several agents used for diabetes and dyslipidemias, including glucagon-like peptide-1 (GLP-1) mimetics, metformin, thiazolidinediones, and statins, have been studied as possible treatments for NAFLD and NASH.8-13 Currently, these medications carry cautions or warnings about using them in patients with liver disease and are not indicated as treatments for NAFLD or NASH.
SUBJECTS AND METHODS
One hundred patients were randomly selected from a type 2 diabetes patient database at the Altru Health System (Grand Forks, ND) for cross-sectional analysis. Manual data extraction from “paper charts” was necessary in some cases, limiting the size of the study.
All subjects had a diagnosis of type 2 diabetes confirmed by American Diabetes Association criteria, were between the ages of 18 and 64 years, and had no known liver disease other than that associated with their diabetes. Other criteria included visiting a health care provider regarding diabetes management within the last year and having undergone laboratory blood testing of liver function within the last 5 years. The study population comprised an equal number of men and women.
We collected data about abnormal liver function from blood test results, including levels of aspartate transaminase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP); results of radiologic imaging of the liver (ultrasound, MRI, or CT) and liver biopsy; any interventions (medication changes, lifestyle management, or surgery); and referral to a specialist (gastroenterologist or surgeon).
DATA ANALYSIS
Of the 100 subjects, 40 had at least one abnormal serum liver function test (AST, ALT, or ALP), although we could find no record of tests for one of the 100 subjects. Of the 40 with abnormal test results, 17 (42.5%) were women and 23 (57.5%) were men. None of these patients had highly elevated levels of AST, ALT, or ALP.
Of the 40 patients with abnormal serum liver function tests, only 10 (25%) were specifically referred for imaging studies related to a possible diagnosis of fatty liver disease. Four of these 10 patients (1 woman and 3 men) had both ultrasound and CT imaging. Another 10 subjects (25%) had incidental findings of fatty liver disease on imaging performed for another presumed diagnosis or symptom, eg, abdominal pain. Overall, 11 (6 men, 5 women) of the 40 subjects (27.5%) with at least one abnormal liver function test received a diagnosis of fatty liver disease based on imaging findings. None of the subjects had a diagnosis of cirrhosis or other end-stage liver disease.
A medical intervention was offered to 4 of the 11 patients (2 women and 2 men) who received a diagnosis of fatty liver disease. Practitioners specifically referred one woman for medical weight loss management and another for weight loss surgery. One man was advised to stop taking metformin, and another was referred to a dietician for lifestyle weight loss management. No patient was referred to a gastroenterologist or any other specialist for further evaluation or biopsy.
DISCUSSION
Although a small sample size limits the strength of this pilot study, the finding that fatty liver disease is common in patients with type 2 diabetes in a rural community hospital population supports other published data. As only half of the subjects with abnormal liver function tests had imaging studies, it’s likely that some patients in the study group who did not undergo imaging also had NAFLD, NASH, or other liver disease, but it was not diagnosed.
While no specific screening guidelines for fatty liver disease in patients with type 2 diabetes have been issued, clinical interest in this area has been growing, and this study suggests some avenues for further exploration.3 In the institution where this study was conducted, it appears that liver function tests were most likely to be performed in conjunction with routine monitoring of the use of statins, metformin, or other medications or because of a symptom such as abdominal pain. Yet given the widespread availability and relatively low cost of such tests, periodic monitoring of serum liver function in patients with type 2 diabetes may be warranted.14
Patients found to have persistent or recurrent abnormal liver function tests could then be referred for further evaluation with ultrasound, CT, or MRI.14 Ultrasound has the benefit of lower cost and avoidance of intravenous contrast, which may be important for patients with renal dysfunction.14 Based on the results of these tests, appropriate medical interventions could then follow.
·Acknowledgements·
The author thanks Bonnie Lee and Diane Vold for their assistance in the design of this study and its preparation for publication. The author also thanks William Zaks, MD, PhD, and James Brosseau, MD, MPH, for their guidance and feedback.
Purpose The objective of this pilot study was to evaluate the prevalence and management of nonalcoholic fatty liver disease in a rural type 2 diabetes population.
Methods We randomly selected 100 patients with type 2 diabetes from a large rural clinic/hospital system in the upper Midwest and conducted a chart review to determine the prevalence of abnormal results of serum liver function tests and liver imaging (eg, computed tomography, ultrasound, magnetic resonance imaging). We also determined the number of patients who were given a diagnosis of fatty liver disease and who among those were subsequently managed for the condition.
Results Of the 100 subjects, 40 had abnormal serum liver function testing, and half of those individuals underwent imaging. This resulted in a diagnosis of fatty liver disease in 11 (27.5% of the 40 with abnormal liver function). Only 4 patients received specific interventions for fatty liver disease.
Conclusion In this rural population, fatty liver disease was common and untreated, suggesting a possible need for a change in screening and management protocols.
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in the United States.1 In particular, NAFLD and the related inflammatory nonalcoholic steatohepatitis (NASH) often develop in individuals who are obese or who have prediabetes or type 2 diabetes, affecting up to 75% of patients with these conditions.2
In addition to NAFLD and NASH, other liver diseases associated with type 2 diabetes include cirrhosis, hepatocellular carcinoma, liver failure, and hepatitis.1,3,4 Of patients with type 2 diabetes, more than 600,000 have cirrhosis; 4.4% of diabetes-related deaths have been attributed to cirrhosis.4,5
NAFLD and NASH share a common pathophysiology in type 2 diabetes with respect to insulin resistance, which results in hyperlipidemias that enhance fatty deposits in the liver.1,2 Hepatic fat accumulation is also associated with increasing measures of inflammation, including C-reactive protein.6
Resultant liver function test abnormalities and characteristic appearance on imaging studies (ultrasound, computed tomography [CT], or magnetic resonance imaging [MRI]) may be similar in NAFLD and NASH.1-3 Liver biopsy therefore is necessary to distinguish NAFLD from NASH, with NASH showing characteristic inflammatory and fibrotic changes.1,2 Evaluations of patients with minor liver test abnormalities reveal that up to 98% may have liver disease, most often fatty liver disease.7
Weight loss is a strategy for managing NAFLD and NASH, although large randomized controlled trials are lacking.8-10 Several agents used for diabetes and dyslipidemias, including glucagon-like peptide-1 (GLP-1) mimetics, metformin, thiazolidinediones, and statins, have been studied as possible treatments for NAFLD and NASH.8-13 Currently, these medications carry cautions or warnings about using them in patients with liver disease and are not indicated as treatments for NAFLD or NASH.
SUBJECTS AND METHODS
One hundred patients were randomly selected from a type 2 diabetes patient database at the Altru Health System (Grand Forks, ND) for cross-sectional analysis. Manual data extraction from “paper charts” was necessary in some cases, limiting the size of the study.
All subjects had a diagnosis of type 2 diabetes confirmed by American Diabetes Association criteria, were between the ages of 18 and 64 years, and had no known liver disease other than that associated with their diabetes. Other criteria included visiting a health care provider regarding diabetes management within the last year and having undergone laboratory blood testing of liver function within the last 5 years. The study population comprised an equal number of men and women.
We collected data about abnormal liver function from blood test results, including levels of aspartate transaminase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP); results of radiologic imaging of the liver (ultrasound, MRI, or CT) and liver biopsy; any interventions (medication changes, lifestyle management, or surgery); and referral to a specialist (gastroenterologist or surgeon).
DATA ANALYSIS
Of the 100 subjects, 40 had at least one abnormal serum liver function test (AST, ALT, or ALP), although we could find no record of tests for one of the 100 subjects. Of the 40 with abnormal test results, 17 (42.5%) were women and 23 (57.5%) were men. None of these patients had highly elevated levels of AST, ALT, or ALP.
Of the 40 patients with abnormal serum liver function tests, only 10 (25%) were specifically referred for imaging studies related to a possible diagnosis of fatty liver disease. Four of these 10 patients (1 woman and 3 men) had both ultrasound and CT imaging. Another 10 subjects (25%) had incidental findings of fatty liver disease on imaging performed for another presumed diagnosis or symptom, eg, abdominal pain. Overall, 11 (6 men, 5 women) of the 40 subjects (27.5%) with at least one abnormal liver function test received a diagnosis of fatty liver disease based on imaging findings. None of the subjects had a diagnosis of cirrhosis or other end-stage liver disease.
A medical intervention was offered to 4 of the 11 patients (2 women and 2 men) who received a diagnosis of fatty liver disease. Practitioners specifically referred one woman for medical weight loss management and another for weight loss surgery. One man was advised to stop taking metformin, and another was referred to a dietician for lifestyle weight loss management. No patient was referred to a gastroenterologist or any other specialist for further evaluation or biopsy.
DISCUSSION
Although a small sample size limits the strength of this pilot study, the finding that fatty liver disease is common in patients with type 2 diabetes in a rural community hospital population supports other published data. As only half of the subjects with abnormal liver function tests had imaging studies, it’s likely that some patients in the study group who did not undergo imaging also had NAFLD, NASH, or other liver disease, but it was not diagnosed.
While no specific screening guidelines for fatty liver disease in patients with type 2 diabetes have been issued, clinical interest in this area has been growing, and this study suggests some avenues for further exploration.3 In the institution where this study was conducted, it appears that liver function tests were most likely to be performed in conjunction with routine monitoring of the use of statins, metformin, or other medications or because of a symptom such as abdominal pain. Yet given the widespread availability and relatively low cost of such tests, periodic monitoring of serum liver function in patients with type 2 diabetes may be warranted.14
Patients found to have persistent or recurrent abnormal liver function tests could then be referred for further evaluation with ultrasound, CT, or MRI.14 Ultrasound has the benefit of lower cost and avoidance of intravenous contrast, which may be important for patients with renal dysfunction.14 Based on the results of these tests, appropriate medical interventions could then follow.
·Acknowledgements·
The author thanks Bonnie Lee and Diane Vold for their assistance in the design of this study and its preparation for publication. The author also thanks William Zaks, MD, PhD, and James Brosseau, MD, MPH, for their guidance and feedback.
1. Caldwell SH, Oelsner DH, Iezzoni JC, et al. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664-669.
2. Pagano G, Pacini G, Musso G, et al. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology. 2002;35:367-372.
3. Tolman KG, Fonseca V, Dalpiaz A, et al. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care. 2007;30:734-743.
4. de Marco R, Locatelli F, Zoppini G, et al. Cause-specific mortality in type 2 diabetes: the Verona Diabetes Study. Diabetes Care. 1999;22:756-761.
5. Koehler E, Watt K, Charlton M. Fatty liver and liver transplantation. Clin Liver Dis. 2009;13:621-630.
6. Saremi A, Allison M, Ditomasso D, et al. Preliminary report: hepatic fat and inflammation in type 2 diabetes mellitus. Metabolism. 2010;59:430-432.
7. Hultcrantz R, Glaumann H, Lindberg G, et al. Liver investigation in 149 asymptomatic patients with moderately elevated activities of serum aminotransferases. Scand J Gastroenterol. 1986;21:109-113.
8. Schreuder TC, Verwer BJ, van Nieuwkerk CM, et al. Nonalcoholic fatty liver disease: an overview of current insights in pathogenesis, diagnosis and treatment. World J Gastroenterol. 2008;14:2474-2486.
9. Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, et al. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010;(1):CD007340.-
10. Medina J, Fernández-Salazar LI, García-Buey L, et al. Approach to the pathogenesis and treatment of nonalcoholic steatohepatitis. Diabetes Care. 2004;27:2057-2066.
11. Khashab M, Chalasani N. Use of insulin sensitizers in NASH. Endocrinol Metab Clin North Am. 2007;36:1067-1087.
12. Ding X, Saxena NK, Lin S, et al. Exendin-4, a glucagon-like protein (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43:173-181.
13. Matafome P, Nunes E, Louro T, et al. A role for atorvastatin and insulin combination in protecting from liver injury in a model of type 2 diabetes with hyperlipidemia. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:241-251.
14. Ratziu V, Bellentani S, Cortez-Pinto H, et al. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372-384.
CORRESPONDENCE Eric L. Johnson, MD, 501 N. Columbia Road, Stop 9037, Grand Forks, ND 58202; [email protected]
1. Caldwell SH, Oelsner DH, Iezzoni JC, et al. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664-669.
2. Pagano G, Pacini G, Musso G, et al. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology. 2002;35:367-372.
3. Tolman KG, Fonseca V, Dalpiaz A, et al. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care. 2007;30:734-743.
4. de Marco R, Locatelli F, Zoppini G, et al. Cause-specific mortality in type 2 diabetes: the Verona Diabetes Study. Diabetes Care. 1999;22:756-761.
5. Koehler E, Watt K, Charlton M. Fatty liver and liver transplantation. Clin Liver Dis. 2009;13:621-630.
6. Saremi A, Allison M, Ditomasso D, et al. Preliminary report: hepatic fat and inflammation in type 2 diabetes mellitus. Metabolism. 2010;59:430-432.
7. Hultcrantz R, Glaumann H, Lindberg G, et al. Liver investigation in 149 asymptomatic patients with moderately elevated activities of serum aminotransferases. Scand J Gastroenterol. 1986;21:109-113.
8. Schreuder TC, Verwer BJ, van Nieuwkerk CM, et al. Nonalcoholic fatty liver disease: an overview of current insights in pathogenesis, diagnosis and treatment. World J Gastroenterol. 2008;14:2474-2486.
9. Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, et al. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010;(1):CD007340.-
10. Medina J, Fernández-Salazar LI, García-Buey L, et al. Approach to the pathogenesis and treatment of nonalcoholic steatohepatitis. Diabetes Care. 2004;27:2057-2066.
11. Khashab M, Chalasani N. Use of insulin sensitizers in NASH. Endocrinol Metab Clin North Am. 2007;36:1067-1087.
12. Ding X, Saxena NK, Lin S, et al. Exendin-4, a glucagon-like protein (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43:173-181.
13. Matafome P, Nunes E, Louro T, et al. A role for atorvastatin and insulin combination in protecting from liver injury in a model of type 2 diabetes with hyperlipidemia. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:241-251.
14. Ratziu V, Bellentani S, Cortez-Pinto H, et al. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372-384.
CORRESPONDENCE Eric L. Johnson, MD, 501 N. Columbia Road, Stop 9037, Grand Forks, ND 58202; [email protected]
Glycemic variability: Too often overlooked in type 2 diabetes?
• Consider evaluating 24-hour variability in glucose levels with patients’ self-monitoring glucose meters (in addition to monitoring glycosylated hemoglobin [HbA1c] levels at regular intervals). C
• If glycemic goals are unmet 2 to 3 months after initiating treatment with exercise and diet or with oral agent monotherapy, consider starting insulin therapy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Though we have the knowledge and the means to reduce complications of type 2 diabetes mellitus (T2DM), most patients may not be reaching all the glycemic goals necessary to achieve optimal risk reduction.1 Maintaining an acceptable level of glycosylated hemoglobin (HbA1c) is one of the important glycemic goals. But that measurement is an average of glucose levels occurring over the prior 3 months. Regardless of a given HbA1c measurement, an emerging body of evidence supports the presumption that glycemic variability over each 24-hour cycle is an independent risk factor for vascular complications.2-15
In this article, I review the literature pertaining to the risk associated with glycemic variability and to the benefit in correcting it. I also review the comparative outcomes achievable with normal human insulin and insulin analogs, as well as the advisability of starting insulin earlier in the management process.
Glycemic variability increases vascular risk independently
HbA1c, considered the gold standard for monitoring glycemic control in patients with T2DM, is an average of the full range of glucose values in the preceding 3 months, including fasting plasma glucose (FPG) and 2-hour postprandial glucose (PPG) levels. Studies have linked lowering HbA1c to reducing the risk and progression of micro- and macrovascular complications associated with diabetes.16,17 But evidence shows that other glycemic values are also important.
The Diabetes Control and Complications Trial (DCCT) was a landmark study in which patients with type 1 diabetes mellitus who received targeted intensive insulin therapy experienced delayed onset and slowed progression of micro-vascular complications compared with those who received conventional insulin treatment.16 Interestingly, this study also reported that patients randomized to receive conventional insulin treatment did not exhibit a reduction in the risk of progression of microvascular disease despite having HbA1c values comparable to those in the intensive-treatment group. One hypothesis is that glucose excursions occurred more frequently in the conventionally treated group, which received fewer daily insulin injections.5
Acute glucose fluctuations during the postprandial period trigger oxidative stress and are more predictive of atherosclerosis development than are FPG or HbA1c6,7 (see “Implications of glycemic variability” below). This suggests that therapy for patients with T2DM should not only target HbA1c as a long-term goal, but also aim to avoid acute glucose fluctuations as an immediate goal. Several studies have shown that postprandial hyperglycemia is an independent risk factor for vascular complications in patients with T2DM.2,7-9,12,14,15
Evidence of increased vascular risk with glycemic variability. The Diabetes Epidemiology: COllaborative analysis of Diagnostic criteria in Europe study (DECODE) followed more than 25,000 patients for more than 7 years and found that increased mortality was more closely associated with increased 2-hour PPG levels than with FPG.14 In the Framingham Offspring Study, Meigs et al9 reported that, in nondiabetic subjects, an elevated glucose level 2 hours after an oral challenge increased the relative risk for cardiovascular disease by up to 40%, independent of fasting hyperglycemia.
Mixed outcomes with Hba1c reduction only. Macrovascular risk reduction with intensive HbA1c management was not apparent in 3 recent studies—Action to Control Cardiovascular Risk in Diabetes (ACCORD),18 Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE),19 and Veterans Affairs Diabetes Trial (VADT).20 The ACCORD study, in fact, showed an increase in cardiovascular events in the intensively managed group (HbA1c target <6.0%). Indeed, previous studies had suggested an association between fasting hypoglycemia and poor cardiovascular outcomes.3,4 Retrospective subanalysis of the ACCORD study suggested that patients with poorer glycemic control had a greater risk of hypoglycemia independent of HbA1c values, and that patients who had difficulty reaching lower HbA1c levels may have had poorer cardiovascular outcomes.21
The apparent absence of a reduction in macrovascular events in the ACCORD, ADVANCE, and VADT studies also suggests an additive effect of nonglycemic risk factors that frequently accompany diabetes—ie, hypertension, hyperlipidemia, and hypercoagulability/pro-inflammatory states.
Long-term follow-up in the United Kingdom Prospective Diabetes Study (UKPDS) showed ongoing risk reduction for both microvascular and macrovascular complications.22 A separate meta-analysis showed a significant 10% reduction in cardiovascular events with intensive glycemic control when data were combined from the ACCORD trial, ADVANCE trial, VADT, and the UKPDS.23
An improvement in long-term outcomes for patients with T2DM might be expected when initiating a targeted, intensified, multi-factorial interventional regimen to reduce not only HbA1c, but also glucose variability. The STENO-2 trial showed that a targeted multifactorial treatment regimen in patients with T2DM could decrease long-term vascular complications.24
Consider assessing true variability in your patients. Because postprandial glucose levels alone may not equate to overall glycemic variability, you may want to ask select patients to take readings with their glucose meters at various times of the day across several days to get a more accurate picture.5
Normal physiologic insulin secretion prevents glucose fluctuations in healthy adults. in patients with diabetes, abnormalities in insulin secretion are part of the pathophysiologic process, resulting in chronic sustained hyperglycemia and acute daily fluctuations in glucose levels. These glycemic disorders are associated with a state of increased oxidative stress and possible subsequent development of vascular complications.
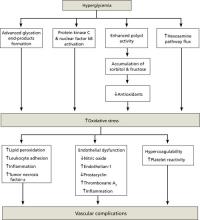
Cellular response to hyperglycemia. oxidative stress, the imbalance between production of reactive oxygen species and the ability to eliminate them, is central to the pathogenesis of cardiovascular complications of diabetes, including accelerated atherosclerotic macrovascular disease (FIGURE 1). Both insulin resistance and hyperglycemia are implicated in the pathogenesis of these complications.65,66 hyperglycemia is hypothesized to induce vascular injury via at least 4 biochemical pathways: enhanced polyol activity leading to sorbitol and fructose accumulation; increased formation of advanced glycation end products; activation of protein kinase c and nuclear factor kB; and increased hexosamine pathway flux.67 endothelium activation is a pro-inflammatory, proliferative, and pro-coagulatory setting, ultimately leading to arterial narrowing and susceptibility to atheroma deposition. hyperglycemia can also induce alterations in the coagulation system, resulting in increased thrombosis.68
Association of glycemic variability with oxidative stress. macrovascular complications, particularly cardiovascular disease, contribute significantly to the increased morbidity and mortality with diabetes.24 oxidative stress has been implicated as a major factor in the development of these complications.66-68 other cell-culture evidence suggests that normal protective mechanisms of oxidative stress are impaired by chronic hyperglycemia. When exposed to intermittent glycemic variability, cells have exhibited more pronounced toxicity.69,70 risso et al71 further established that variability in glycemic control resulted in more endothelial cell damage than did chronic sustained hyperglycemia.
Despite the experimental evidence that suggests glycemic variability is associated with increased risk of vascular complications, there are limited clinical data establishing glycemic variability as an independent predictor of these complications. monnier et al72 provided data in patients with type 2 diabetes mellitus (T2Dm) to support the concept of acute glucose fluctuations as a more important trigger of oxidative stress than chronic hyperglycemia. if these data are confirmed in larger clinical trials, a monitoring paradigm for patients with T2Dm could include increased focus on preventing glucose excursions in addition to reducing HbA1c.
FIGURE 1
How oxidative stress secondary to hyperglycemia leads to vascular complications in diabetes66-68
Following through with targeted, intensified management
Consider the following treatment goals for patients with T2DM: (1) lowering HbA1c levels; (2) lowering fasting blood glucose levels; (3) minimizing glycemic variability, including postprandial glucose excursions. TABLE 1 lists the values that the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists (AACE) have assigned to these glycemic-control goals.
In addition to managing glycemic levels, reducing risk of cardiovascular disease in T2DM involves aggressive interventions, as needed, to correct blood pressure and lipid levels.24,25
TABLE 1
Aim to reach 3 glycemic goals in treating type 2 diabetes mellitus
| ADA | AACE | |
|---|---|---|
| Fasting blood glucose (mg/dL) | 90-130 | <110 |
| Postprandial plasma glucose (mg/dL) | <180 | <140 |
| HbA1c (%) | <7* | ≤6.5 |
| *Recommended “in general”; however, the guideline indicates that for “the individual patient,” HbA1c should be as close to normal (<6%) as possible without causing hypoglycemia. | ||
| AACE, American Association of clinical endocrinologists; ADA, American Diabetes Association; HbA1c, glycosylated hemoglobin. | ||
| Sources: ADA, http://care.diabetesjournals.org/content/33/Supplement_1/S11/T11.expansion.htm; AACE, http://www.metcare.com/files/physician-resources/clinical-guidelines/dm-guidelines.pdf | ||
Challenges to achieving glycemic control
Despite current recommendations for more aggressive management of patients with T2DM,25 estimates are that as many as 60% of patients with T2DM do not achieve glycemic targets, and, as the disease progresses, many of the available treatment options fail to sustain levels previously reached.1,26,27
A shortcoming of older treatment strategies still in use is the slow transition to more effective therapy, resulting in long periods of inadequate glycemic control.1 Brown et al27 found that patients receiving monotherapy with either a sulfonylurea or metformin had HbA1c levels >8% for a mean of 20 months and 14 months, respectively, before treatment was changed. Current recommendations call for treatment changes within 2 to 3 months of initiation of therapy if the HbA1c goal is not reached.27-29
Turning to insulin earlier. Insulin is most effective for lowering HbA1c and delaying subsequent complications related to diabetes; however, there is often reluctance to using it early in diabetes management. Consequently, by the time insulin therapy is started, many patients will have had unacceptable glycemic levels for 10 years or more and may already be developing complications.27 And, as noted, the HbA1c level is an average measurement that does not detect glycemic variability. Continuous glucose monitoring will likely lead to more responsive adjustments in treatment regimens and to improved quality of care for patients with T2DM.
Insulin has many beneficial effects
Insulin exerts an anti-inflammatory effect by reducing the increase in C-reactive protein and serum amyloid A.30 It also partially restores insulin-stimulated endothelial function,31 facilitates vasodilation by increasing nitric oxide production,32 and improves fibrinolytic profiles.33 Early initiation of insulin therapy can increase peripheral insulin sensitivity and preserve beta cell function.34-36
When oral agents have failed, insulin can significantly improve patients’ beta cell function,34,35,37 and short periods of insulin therapy in patients newly diagnosed with T2DM may even set the foundation for better long-term control.38,39
But not all insulin is alike
Ideally, insulin therapy should mimic physiologic insulin secretion. However, conventional human insulin products fail to do so because of their suboptimal pharmacodynamic profiles. With recombinant DNA technology, molecular modifications of the human insulin molecule have overcome some of the limitations of conventional human insulin products.
Unfortunately, many practitioners still hold insulin in reserve until combination therapy with oral agents has failed, possibly resulting in years of suboptimal glycemic control. Newer strategies recommend earlier initiation of insulin—ie, once diet and exercise fail, or when treatment with 1 oral agent fails. The development of insulin analogs is a significant milestone on the road to achieving improved outcomes for patients with T2DM.
Rapid-acting agents
Compared with regular human insulin, newer rapid-acting insulin analogs may improve glycemic control when used at mealtimes. However, due to their shorter half-lives, these insulin analogs require augmentation with basal insulin to control hyperglycemia between meals and during the night.
Insulin lispro was the first commercially available rapid-acting insulin analog, introduced in 1996. This agent differs from human insulin by an inversion of amino acid residues in positions 28 and 29 of the insulin B-chain. Inversion prevents the formation of hexamers and dimers that tend to diffuse more slowly, thereby facilitating a rapid uptake of the insulin analog into blood and tissues.40,41 The second such agent, marketed in 2000, was insulin aspart, in which aspartic acid replaces proline at position 28 of the B-chain of human insulin.41,42 The most recent rapid-acting analog is insulin glulisine, in which lysine replaces asparagine near the N-terminus of the B-chain, and glutamic acid replaces lysine near the C-terminus of human insulin.
The molecular changes made in creating these analogs allows them to dissociate quickly into monomers that are absorbed rapidly and achieve faster peak levels compared with regular human insulin.41,42 These changes do not, however, interfere with the analogs’ ability to bind to the insulin receptor.43,44
Dosing considerations. Absorption of regular human insulin is not sufficiently rapid at mealtimes to control prandial glucose levels.45 Therefore, it is essential to give regular insulin 30 to 60 minutes before meals. For patients who have erratic daily schedules, adhering to this sort of routine can be difficult. But even if scheduling is not a problem, the prolonged duration of action of human insulin can predispose patients to hypoglycemia. Moreover, absorption of regular insulin can vary dramatically from day to day.46,47
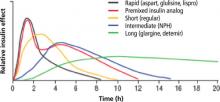
The insulin analogs correct the pharmacokinetic and pharmacodynamic deficiencies of regular insulin, producing plasma profiles that more closely simulate normal, physiologic meal-stimulated insulin release.48-50 The 3 rapid-acting agents (aspart, glulisine, lispro) have very similar onset and duration of action, with peak effect occurring close to injection time (TABLE 2 and FIGURE 2).48-50
Advantages of rapid-acting agents. These agents can be administered closer to meals, giving patients more flexibility and likely tighter postprandial glucose control, with reductions in glycemic excursions. Another advantage is the ability to better match insulin dose to anticipated carbohydrate in-take, affording better postprandial control.51-53 Rapid-acting analogs also result in fewer episodes of hypoglycemia. In a meta-analysis of 2576 patients, hypoglycemic events occurred 25% less often with insulin lispro compared with regular human insulin in patients with type 1 diabetes mellitus (T1DM).52 In clinical trials, insulin aspart and insulin glulisine have also caused fewer hypoglycemic events compared with regular human insulin.51-53
Long-acting agents
Basal, or long-acting, insulins are important for maintaining normoglycemia over 24 hours. Neutral protamine Hagedorn (NPH) insulin reaches its peak effect 4 to 10 hours after injection, and its total effect lasts only 12 to 18 hours. NPH is therefore often dosed twice daily. Absorption of NPH can vary significantly, causing day-to-day blood glucose fluctuations.46,47 Therefore, this agent’s activity does not closely resemble normal physiologic basal insulin secretion.
The newer long-acting insulin analogs—insulin detemir and insulin glargine—were designed to more closely replicate normal physiologic basal insulin secretion. Insulin glargine was first to reach the market, in 2001. It contains glycine instead of asparagine in the alpha-chain and 2 arginine residues at the C-terminus, and the addition of zinc enhances the aggregation and slow release at a neutral pH. Insulin glargine precipitates in the subcutaneous tissue, which slows its absorption and results in a relatively flat insulin plasma profile and extended action.54,55 Insulin detemir is a combination of the original insulin molecule and a saturated fatty acid (myristic acid). Insulin detemir is designed to bind albumin (98% albumin-bound in circulation) through this fatty acid chain in the plasma after injection, resulting in an extended plasma profile.54,56 Insulin glargine and NPH form crystalline depots, but detemir is soluble and the subcutaneous depot remains in a liquid state; this may account for differences in absorption variability.56
Advantages of the long-acting insulin analogs. Compared with conventional basal insulin such as NPH, the analogs have a prolonged duration of action (up to 24 hours) without pronounced peaks, permitting once-daily dosing in many patients (TABLE 2 and FIGURE 2).46,47,50,55 The pharmacodynamic and pharmacokinetic properties of the long- acting agents make them less likely than NPH to cause nocturnal and overall hypoglycemia, a benefit that has been observed in several clinical trials.57-61
Comparative clinical trials evaluating glycemic variability. Both of the long-acting analogs have shown lower within-subject variability in blood and plasma glucose measurements when compared with NPH.62-64 In head-to-head comparisons of the analogs in glucose clamp studies, insulin detemir has demonstrated less within-subject variability of blood glucose levels than insulin glargine, in patients with T1DM or T2DM.56,64 In clinical practice, different patients may have better results with one of these basal insulins as opposed to the other, and treatment choices will need to be tailored to the individual patient.
TABLE 2
Pharmacokinetic properties giving insulin analogs an advantage over regular insulin46-50
| Insulin preparation | Onset of action | Peak action | Duration of action |
|---|---|---|---|
| Short-acting | |||
| Regular | 30-60 minutes | 2-3 hours | 8-10 hours |
| Lispro | 5-15 minutes | 30-90 minutes | 4-6 hours |
| Aspart | 5-15 minutes | 30-90 minutes | 4-6 hours |
| Glulisine | 20 minutes | 90 minutes | 5.3 hours |
| Long-acting | |||
| NPH | 2-4 hours | 4-10 hours | 12-18 hours |
| Glargine | 2-4 hours | Relatively flat | Up to 24 hours |
| Detemir | 1-2 hours | Relatively flat | Up to 24 hours |
| NPH, neutral protamine Hagedorn. | |||
FIGURE 2
Pharmacokinetic profiles of human insulin and insulin analogs
Adapted from: Burton S. J Fam Pract. 2006;55(12 suppl):S10-S17.
CORRESPONDENCE Eric L. Johnson, MD, University of North Dakota School of Medicine and Health Sciences, Department of Family & Community Medicine, 501 North Columbia Road, Grand Forks, ND 58202-9037; [email protected]
1. Koro CE, Bowlin SJ, Bourgeois N, et al. Glycemic control from 1988 to 2000 among U.S. adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care. 2004;27:17-20.
2. Kadowaki S, Okamura T, Hozawa A, et al. Relationship of elevated casual blood glucose level with coronary heart disease, cardiovascular disease and all-cause mortality in a representative sample of the Japanese population. NIPPON DATA80. Diabetologia. 2008;51:575-582.
3. Wändell PE, Theobald H. The association between low fasting glucose value and mortality. Curr Diabetes Rev. 2007;3:274-279.
4. Wei M, Gibbons LW, Mitchell TL, et al. Low fasting plasma glucose level as a predictor of cardiovascular disease and all-cause mortality. Circulation. 2000;101:2047-2052.
5. Hirsch IB, Brownlee M. Should minimal blood glucose variability become the gold standard of glycemic control? J Diabetes Complications. 2005;19:178-181.
6. Temelkova-Kurktschiev TS, Koehler C, Henkel E, et al. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care. 2000;23:1830-1834.
7. Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in Type II diabetes: the epidemiological evidence. Diabetologia. 2001;44:2107-2114.
8. Chiasson JL, Josse RG, Gomis R, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486-494.
9. Meigs JB, Nathan DM, D’Agostino RB, Sr, et al. Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care. 2002;25:1845-1850.
10. Balkau B, Shipley M, Jarrett RJ, et al. High blood glucose concentration is a risk factor for mortality in middle-aged nondiabetic men. 20-year follow-up in the Whitehall Study, the Paris Prospective Study, and the Helsinki Policemen Study. Diabetes Care. 1998;21:360-367.
11. Hanefeld M, Fischer S, Julius U, et al. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia. 1996;39:1577-1583.
12. Donahue RP, Abbott RD, Reed DM, et al. Postchallenge glucose concentration and coronary heart disease in men of Japanese ancestry. Honolulu Heart Program. Diabetes. 1987;36:689-692.
13. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet. 1999;354:617-621.
14. Balkau B, Hu G, Qiao Q, et al. Prediction of the risk of cardiovascular mortality using a score that includes glucose as a risk factor. The DECODE Study. Diabetologia. 2004;47:2118-2128.
15. Hanefeld M, Fischer S, Julius U, et al. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia. 1996;39:1577-1583.
16. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977-986.
17. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405-412.
18. The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559.
19. The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560-2572.
20. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129-139.
21. Miller ME, Bonds DE, Gerstein HC, et al. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444.-
22. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
23. Turnbull FM, Abraira C, Anderson RJ, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;11:2288-2298.
24. Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383-393.
25. Standards of medical care in diabetes—2010 Diabetes Care. 2010;33(suppl 1):S11-S61.
26. Barnett AH. Treating to goal: challenges of current management. Eur J Endocrinol. 2004;151(suppl 2):T3-T7.
27. Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27:1535-1540.
28. Nathan DM. Clinical practice. Initial management of glycemia in type 2 diabetes mellitus. N Engl J Med. 2002;347:1342-1349.
29. Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association; European Association for the Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetes Care. 2009;32:193-203.
30. Chaudhuri A, Janicke D, Wilson MF, et al. Anti-inflammatory and profibrinolytic effect of insulin in acute ST-segment-elevation myocardial infarction. Circulation. 2004;109:849-854.
31. Rask-Madsen C, Ihlemann N, Krarup T, et al. Insulin therapy improves insulin-stimulated endothelial function in patients with type 2 diabetes and ischemic heart disease. Diabetes. 2001;50:2611-2618.
32. Chaudhuri A, Kanjwal Y, Mohanty P, et al. Insulin-induced vasodilatation of internal carotid artery. Metabolism. 1999;48:1470-1473.
33. Melidonis A, Stefanidis A, Tournis S, et al. The role of strict metabolic control by insulin infusion on fibrinolytic profile during an acute coronary event in diabetic patients. Clin Cardiol. 2000;23:160-164.
34. Garvey WT, Olefsky JM, Griffin J, et al. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985;34:222-234.
35. Rolla A. The pathophysiological basis for intensive insulin replacement. Int J Obes Relat Metab Disord. 2004;28(suppl 2):S3-S7.
36. Alvarsson M, Sundkvist G, Lager I, et al. Beneficial effects of insulin versus sulphonylurea on insulin secretion and metabolic control in recently diagnosed type 2 diabetic patients. Diabetes Care. 2003;26:2231-2237.
37. Glaser B, Leibovich G, Nesher R, et al. Improved beta-cell function after intensive insulin treatment in severe non-insulin-dependent diabetes. Acta Endocrinol (Copenh). 1988;118:365-373.
38. Andrews WJ, Vasquez B, Nagulesparan M, et al. Insulin therapy in obese, non-insulin-dependent diabetes induces improvements in insulin action and secretion that are maintained for two weeks after insulin withdrawal. Diabetes. 1984;33:634-642.
39. Ryan EA, Imes S, Wallace C. Short-term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care. 2004;27:1028-1032.
40. Heise T, Heinemann L. Rapid and long-acting analogues as an approach to improve insulin therapy: an evidence-based medicine assessment. Curr Pharm Des. 2001;7:1303-1325.
41. Brems DN, Alter LA, Beckage MJ, et al. Altering the association properties of insulin by amino acid replacement. Protein Eng. 1992;5:527-533.
42. Garber AJ. Pharmacologic modifications of hormones to improve their therapeutic potential for diabetes management. Diabetes Obes Metab. 2005;7:666-674.
43. Hansen BF, Danielsen GM, Drejer K, et al. Sustained signalling from the insulin receptor after stimulation with insulin analogues exhibiting increased mitogenic potency. Biochem J. 1996;315(pt 1):271-279.
44. Slieker LJ, Brooke GS, DiMarchi RD, et al. Modifications in the B10 and B26-30 regions of the B chain of human insulin alter affinity for the human IGF-I receptor more than for the insulin receptor. Diabetologia. 1997;40(suppl 2):S54-S61.
45. Dimitriadis GD, Gerich JE. Importance of timing of preprandial subcutaneous insulin administration in the management of diabetes mellitus. Diabetes Care. 1983;6:374-377.
46. Roy B, Chou MC, Field JB. Time-action characteristics of regular and NPH insulin in insulin-treated diabetics. J Clin Endocrinol Metab. 1980;50:475-479.
47. Binder C, Lauritzen T, Faber O, et al. Insulin pharmacokinetics. Diabetes Care. 1984;7:188-199.
48. Kang S, Creagh FM, Peters JR, et al. Comparison of subcutaneous soluble human insulin and insulin analogues (AspB9, GluB27; AspB10; AspB28) on meal-related plasma glucose excursions in type 1 diabetic subjects. Diabetes Care. 1991;14:571-577.
49. Becker RH, Frick AD, Burger F, et al. A comparison of the steady-state pharmacokinetics and pharmacodynamics of a novel rapid-acting insulin analog, insulin glulisine, and regular human insulin in healthy volunteers using the euglycemic clamp technique. Exp Clin Endocrinol Diabetes. 2005;113:292-297.
50. Hirsch IB. Insulin analogues. N Engl J Med. 2005;352:174-183.
51. Lindholm A, McEwen J, Riis AP. Improved postprandial glycemic control with insulin aspart. A randomized double-blind cross-over trial in type 1 diabetes. Diabetes Care. 1999;22:801-805.
52. Brunelle BL, Llewelyn J, Anderson JH, Jr, et al. Meta-analysis of the effect of insulin lispro on severe hypoglycemia in patients with type 1 diabetes. Diabetes Care. 1998;21:1726-1731.
53. Home PD, Lindholm A, Hylleberg B, et al. Improved glycemic control with insulin aspart: a multicenter randomized double-blind crossover trial in type 1 diabetic patients. UK Insulin Aspart Study Group. Diabetes Care. 1998;21:1904-1909.
54. Heise T, Pieber TR. Towards peakless, reproducible and long-acting insulins. An assessment of the basal analogues based on isoglycaemic clamp studies. Diabetes Obes Metab. 2007;5:648-659.
55. Bolli GB, Di Marchi RD, Park GD, et al. Insulin analogues and their potential in the management of diabetes mellitus. Diabetologia. 1999;42:1151-1167.
56. Klein O, Lynge J, Endahl L, et al. Albumin-bound basal insulin analogues (insulin detemir and NN344): comparable time-action profiles but less variability than insulin glargine in type 2 diabetes. Diabetes Obes Metab. 2007;9:290-299.
57. Riddle MC, Rosenstock J, Gerich J. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080-3086.
58. Yki-Jarvinen H, Dressler A, Ziemen M. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care. 2000;23:1130-1136.
59. Rosenstock J, Schwartz SL, Clark CM, Jr, et al. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care. 2001;24:631-636.
60. Hermansen K, Davies M, Derezinski T, et al. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care. 2006;29:1269-1274.
61. Philis-Tsimikas A, Charpentier G, Clauson P, et al. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther. 2006;28:1569-1581.
62. Haak T, Tiengo A, Draeger E, et al. Lower within-subject variability of fasting blood glucose and reduced weight gain with insulin detemir compared to NPH insulin in patients with type 2 diabetes. Diabetes Obes Metab. 2005;7:56-64.
63. Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49:2142-2148.
64. Heise T, Nosek L, Ronn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53:1614-1620.
65. Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106:453-458.
66. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615-1625.
67. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813-820.
68. Ceriello A. Coagulation activation in diabetes mellitus: the role of hyperglycaemia and therapeutic prospects. Diabetologia. 1993;36:1119-1125.
69. Quagliaro L, Piconi L, Assaloni R, et al. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52:2795-2804.
70. Jones SC, Saunders HJ, Qi W, et al. Intermittent high glucose enhances cell growth and collagen synthesis in cultured human tubulointerstitial cells. Diabetologia. 1999;42:1113-1119.
71. Risso A, Mercuri F, Quagliaro L, et al. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am J Physiol Endocrinol Metab. 2001;281:E924-E930.
72. Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681-1687.
• Consider evaluating 24-hour variability in glucose levels with patients’ self-monitoring glucose meters (in addition to monitoring glycosylated hemoglobin [HbA1c] levels at regular intervals). C
• If glycemic goals are unmet 2 to 3 months after initiating treatment with exercise and diet or with oral agent monotherapy, consider starting insulin therapy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Though we have the knowledge and the means to reduce complications of type 2 diabetes mellitus (T2DM), most patients may not be reaching all the glycemic goals necessary to achieve optimal risk reduction.1 Maintaining an acceptable level of glycosylated hemoglobin (HbA1c) is one of the important glycemic goals. But that measurement is an average of glucose levels occurring over the prior 3 months. Regardless of a given HbA1c measurement, an emerging body of evidence supports the presumption that glycemic variability over each 24-hour cycle is an independent risk factor for vascular complications.2-15
In this article, I review the literature pertaining to the risk associated with glycemic variability and to the benefit in correcting it. I also review the comparative outcomes achievable with normal human insulin and insulin analogs, as well as the advisability of starting insulin earlier in the management process.
Glycemic variability increases vascular risk independently
HbA1c, considered the gold standard for monitoring glycemic control in patients with T2DM, is an average of the full range of glucose values in the preceding 3 months, including fasting plasma glucose (FPG) and 2-hour postprandial glucose (PPG) levels. Studies have linked lowering HbA1c to reducing the risk and progression of micro- and macrovascular complications associated with diabetes.16,17 But evidence shows that other glycemic values are also important.
The Diabetes Control and Complications Trial (DCCT) was a landmark study in which patients with type 1 diabetes mellitus who received targeted intensive insulin therapy experienced delayed onset and slowed progression of micro-vascular complications compared with those who received conventional insulin treatment.16 Interestingly, this study also reported that patients randomized to receive conventional insulin treatment did not exhibit a reduction in the risk of progression of microvascular disease despite having HbA1c values comparable to those in the intensive-treatment group. One hypothesis is that glucose excursions occurred more frequently in the conventionally treated group, which received fewer daily insulin injections.5
Acute glucose fluctuations during the postprandial period trigger oxidative stress and are more predictive of atherosclerosis development than are FPG or HbA1c6,7 (see “Implications of glycemic variability” below). This suggests that therapy for patients with T2DM should not only target HbA1c as a long-term goal, but also aim to avoid acute glucose fluctuations as an immediate goal. Several studies have shown that postprandial hyperglycemia is an independent risk factor for vascular complications in patients with T2DM.2,7-9,12,14,15
Evidence of increased vascular risk with glycemic variability. The Diabetes Epidemiology: COllaborative analysis of Diagnostic criteria in Europe study (DECODE) followed more than 25,000 patients for more than 7 years and found that increased mortality was more closely associated with increased 2-hour PPG levels than with FPG.14 In the Framingham Offspring Study, Meigs et al9 reported that, in nondiabetic subjects, an elevated glucose level 2 hours after an oral challenge increased the relative risk for cardiovascular disease by up to 40%, independent of fasting hyperglycemia.
Mixed outcomes with Hba1c reduction only. Macrovascular risk reduction with intensive HbA1c management was not apparent in 3 recent studies—Action to Control Cardiovascular Risk in Diabetes (ACCORD),18 Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE),19 and Veterans Affairs Diabetes Trial (VADT).20 The ACCORD study, in fact, showed an increase in cardiovascular events in the intensively managed group (HbA1c target <6.0%). Indeed, previous studies had suggested an association between fasting hypoglycemia and poor cardiovascular outcomes.3,4 Retrospective subanalysis of the ACCORD study suggested that patients with poorer glycemic control had a greater risk of hypoglycemia independent of HbA1c values, and that patients who had difficulty reaching lower HbA1c levels may have had poorer cardiovascular outcomes.21
The apparent absence of a reduction in macrovascular events in the ACCORD, ADVANCE, and VADT studies also suggests an additive effect of nonglycemic risk factors that frequently accompany diabetes—ie, hypertension, hyperlipidemia, and hypercoagulability/pro-inflammatory states.
Long-term follow-up in the United Kingdom Prospective Diabetes Study (UKPDS) showed ongoing risk reduction for both microvascular and macrovascular complications.22 A separate meta-analysis showed a significant 10% reduction in cardiovascular events with intensive glycemic control when data were combined from the ACCORD trial, ADVANCE trial, VADT, and the UKPDS.23
An improvement in long-term outcomes for patients with T2DM might be expected when initiating a targeted, intensified, multi-factorial interventional regimen to reduce not only HbA1c, but also glucose variability. The STENO-2 trial showed that a targeted multifactorial treatment regimen in patients with T2DM could decrease long-term vascular complications.24
Consider assessing true variability in your patients. Because postprandial glucose levels alone may not equate to overall glycemic variability, you may want to ask select patients to take readings with their glucose meters at various times of the day across several days to get a more accurate picture.5
Normal physiologic insulin secretion prevents glucose fluctuations in healthy adults. in patients with diabetes, abnormalities in insulin secretion are part of the pathophysiologic process, resulting in chronic sustained hyperglycemia and acute daily fluctuations in glucose levels. These glycemic disorders are associated with a state of increased oxidative stress and possible subsequent development of vascular complications.
Cellular response to hyperglycemia. oxidative stress, the imbalance between production of reactive oxygen species and the ability to eliminate them, is central to the pathogenesis of cardiovascular complications of diabetes, including accelerated atherosclerotic macrovascular disease (FIGURE 1). Both insulin resistance and hyperglycemia are implicated in the pathogenesis of these complications.65,66 hyperglycemia is hypothesized to induce vascular injury via at least 4 biochemical pathways: enhanced polyol activity leading to sorbitol and fructose accumulation; increased formation of advanced glycation end products; activation of protein kinase c and nuclear factor kB; and increased hexosamine pathway flux.67 endothelium activation is a pro-inflammatory, proliferative, and pro-coagulatory setting, ultimately leading to arterial narrowing and susceptibility to atheroma deposition. hyperglycemia can also induce alterations in the coagulation system, resulting in increased thrombosis.68
Association of glycemic variability with oxidative stress. macrovascular complications, particularly cardiovascular disease, contribute significantly to the increased morbidity and mortality with diabetes.24 oxidative stress has been implicated as a major factor in the development of these complications.66-68 other cell-culture evidence suggests that normal protective mechanisms of oxidative stress are impaired by chronic hyperglycemia. When exposed to intermittent glycemic variability, cells have exhibited more pronounced toxicity.69,70 risso et al71 further established that variability in glycemic control resulted in more endothelial cell damage than did chronic sustained hyperglycemia.
Despite the experimental evidence that suggests glycemic variability is associated with increased risk of vascular complications, there are limited clinical data establishing glycemic variability as an independent predictor of these complications. monnier et al72 provided data in patients with type 2 diabetes mellitus (T2Dm) to support the concept of acute glucose fluctuations as a more important trigger of oxidative stress than chronic hyperglycemia. if these data are confirmed in larger clinical trials, a monitoring paradigm for patients with T2Dm could include increased focus on preventing glucose excursions in addition to reducing HbA1c.
FIGURE 1
How oxidative stress secondary to hyperglycemia leads to vascular complications in diabetes66-68
Following through with targeted, intensified management
Consider the following treatment goals for patients with T2DM: (1) lowering HbA1c levels; (2) lowering fasting blood glucose levels; (3) minimizing glycemic variability, including postprandial glucose excursions. TABLE 1 lists the values that the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists (AACE) have assigned to these glycemic-control goals.
In addition to managing glycemic levels, reducing risk of cardiovascular disease in T2DM involves aggressive interventions, as needed, to correct blood pressure and lipid levels.24,25
TABLE 1
Aim to reach 3 glycemic goals in treating type 2 diabetes mellitus
| ADA | AACE | |
|---|---|---|
| Fasting blood glucose (mg/dL) | 90-130 | <110 |
| Postprandial plasma glucose (mg/dL) | <180 | <140 |
| HbA1c (%) | <7* | ≤6.5 |
| *Recommended “in general”; however, the guideline indicates that for “the individual patient,” HbA1c should be as close to normal (<6%) as possible without causing hypoglycemia. | ||
| AACE, American Association of clinical endocrinologists; ADA, American Diabetes Association; HbA1c, glycosylated hemoglobin. | ||
| Sources: ADA, http://care.diabetesjournals.org/content/33/Supplement_1/S11/T11.expansion.htm; AACE, http://www.metcare.com/files/physician-resources/clinical-guidelines/dm-guidelines.pdf | ||
Challenges to achieving glycemic control
Despite current recommendations for more aggressive management of patients with T2DM,25 estimates are that as many as 60% of patients with T2DM do not achieve glycemic targets, and, as the disease progresses, many of the available treatment options fail to sustain levels previously reached.1,26,27
A shortcoming of older treatment strategies still in use is the slow transition to more effective therapy, resulting in long periods of inadequate glycemic control.1 Brown et al27 found that patients receiving monotherapy with either a sulfonylurea or metformin had HbA1c levels >8% for a mean of 20 months and 14 months, respectively, before treatment was changed. Current recommendations call for treatment changes within 2 to 3 months of initiation of therapy if the HbA1c goal is not reached.27-29
Turning to insulin earlier. Insulin is most effective for lowering HbA1c and delaying subsequent complications related to diabetes; however, there is often reluctance to using it early in diabetes management. Consequently, by the time insulin therapy is started, many patients will have had unacceptable glycemic levels for 10 years or more and may already be developing complications.27 And, as noted, the HbA1c level is an average measurement that does not detect glycemic variability. Continuous glucose monitoring will likely lead to more responsive adjustments in treatment regimens and to improved quality of care for patients with T2DM.
Insulin has many beneficial effects
Insulin exerts an anti-inflammatory effect by reducing the increase in C-reactive protein and serum amyloid A.30 It also partially restores insulin-stimulated endothelial function,31 facilitates vasodilation by increasing nitric oxide production,32 and improves fibrinolytic profiles.33 Early initiation of insulin therapy can increase peripheral insulin sensitivity and preserve beta cell function.34-36
When oral agents have failed, insulin can significantly improve patients’ beta cell function,34,35,37 and short periods of insulin therapy in patients newly diagnosed with T2DM may even set the foundation for better long-term control.38,39
But not all insulin is alike
Ideally, insulin therapy should mimic physiologic insulin secretion. However, conventional human insulin products fail to do so because of their suboptimal pharmacodynamic profiles. With recombinant DNA technology, molecular modifications of the human insulin molecule have overcome some of the limitations of conventional human insulin products.
Unfortunately, many practitioners still hold insulin in reserve until combination therapy with oral agents has failed, possibly resulting in years of suboptimal glycemic control. Newer strategies recommend earlier initiation of insulin—ie, once diet and exercise fail, or when treatment with 1 oral agent fails. The development of insulin analogs is a significant milestone on the road to achieving improved outcomes for patients with T2DM.
Rapid-acting agents
Compared with regular human insulin, newer rapid-acting insulin analogs may improve glycemic control when used at mealtimes. However, due to their shorter half-lives, these insulin analogs require augmentation with basal insulin to control hyperglycemia between meals and during the night.
Insulin lispro was the first commercially available rapid-acting insulin analog, introduced in 1996. This agent differs from human insulin by an inversion of amino acid residues in positions 28 and 29 of the insulin B-chain. Inversion prevents the formation of hexamers and dimers that tend to diffuse more slowly, thereby facilitating a rapid uptake of the insulin analog into blood and tissues.40,41 The second such agent, marketed in 2000, was insulin aspart, in which aspartic acid replaces proline at position 28 of the B-chain of human insulin.41,42 The most recent rapid-acting analog is insulin glulisine, in which lysine replaces asparagine near the N-terminus of the B-chain, and glutamic acid replaces lysine near the C-terminus of human insulin.
The molecular changes made in creating these analogs allows them to dissociate quickly into monomers that are absorbed rapidly and achieve faster peak levels compared with regular human insulin.41,42 These changes do not, however, interfere with the analogs’ ability to bind to the insulin receptor.43,44
Dosing considerations. Absorption of regular human insulin is not sufficiently rapid at mealtimes to control prandial glucose levels.45 Therefore, it is essential to give regular insulin 30 to 60 minutes before meals. For patients who have erratic daily schedules, adhering to this sort of routine can be difficult. But even if scheduling is not a problem, the prolonged duration of action of human insulin can predispose patients to hypoglycemia. Moreover, absorption of regular insulin can vary dramatically from day to day.46,47
The insulin analogs correct the pharmacokinetic and pharmacodynamic deficiencies of regular insulin, producing plasma profiles that more closely simulate normal, physiologic meal-stimulated insulin release.48-50 The 3 rapid-acting agents (aspart, glulisine, lispro) have very similar onset and duration of action, with peak effect occurring close to injection time (TABLE 2 and FIGURE 2).48-50
Advantages of rapid-acting agents. These agents can be administered closer to meals, giving patients more flexibility and likely tighter postprandial glucose control, with reductions in glycemic excursions. Another advantage is the ability to better match insulin dose to anticipated carbohydrate in-take, affording better postprandial control.51-53 Rapid-acting analogs also result in fewer episodes of hypoglycemia. In a meta-analysis of 2576 patients, hypoglycemic events occurred 25% less often with insulin lispro compared with regular human insulin in patients with type 1 diabetes mellitus (T1DM).52 In clinical trials, insulin aspart and insulin glulisine have also caused fewer hypoglycemic events compared with regular human insulin.51-53
Long-acting agents
Basal, or long-acting, insulins are important for maintaining normoglycemia over 24 hours. Neutral protamine Hagedorn (NPH) insulin reaches its peak effect 4 to 10 hours after injection, and its total effect lasts only 12 to 18 hours. NPH is therefore often dosed twice daily. Absorption of NPH can vary significantly, causing day-to-day blood glucose fluctuations.46,47 Therefore, this agent’s activity does not closely resemble normal physiologic basal insulin secretion.
The newer long-acting insulin analogs—insulin detemir and insulin glargine—were designed to more closely replicate normal physiologic basal insulin secretion. Insulin glargine was first to reach the market, in 2001. It contains glycine instead of asparagine in the alpha-chain and 2 arginine residues at the C-terminus, and the addition of zinc enhances the aggregation and slow release at a neutral pH. Insulin glargine precipitates in the subcutaneous tissue, which slows its absorption and results in a relatively flat insulin plasma profile and extended action.54,55 Insulin detemir is a combination of the original insulin molecule and a saturated fatty acid (myristic acid). Insulin detemir is designed to bind albumin (98% albumin-bound in circulation) through this fatty acid chain in the plasma after injection, resulting in an extended plasma profile.54,56 Insulin glargine and NPH form crystalline depots, but detemir is soluble and the subcutaneous depot remains in a liquid state; this may account for differences in absorption variability.56
Advantages of the long-acting insulin analogs. Compared with conventional basal insulin such as NPH, the analogs have a prolonged duration of action (up to 24 hours) without pronounced peaks, permitting once-daily dosing in many patients (TABLE 2 and FIGURE 2).46,47,50,55 The pharmacodynamic and pharmacokinetic properties of the long- acting agents make them less likely than NPH to cause nocturnal and overall hypoglycemia, a benefit that has been observed in several clinical trials.57-61
Comparative clinical trials evaluating glycemic variability. Both of the long-acting analogs have shown lower within-subject variability in blood and plasma glucose measurements when compared with NPH.62-64 In head-to-head comparisons of the analogs in glucose clamp studies, insulin detemir has demonstrated less within-subject variability of blood glucose levels than insulin glargine, in patients with T1DM or T2DM.56,64 In clinical practice, different patients may have better results with one of these basal insulins as opposed to the other, and treatment choices will need to be tailored to the individual patient.
TABLE 2
Pharmacokinetic properties giving insulin analogs an advantage over regular insulin46-50
| Insulin preparation | Onset of action | Peak action | Duration of action |
|---|---|---|---|
| Short-acting | |||
| Regular | 30-60 minutes | 2-3 hours | 8-10 hours |
| Lispro | 5-15 minutes | 30-90 minutes | 4-6 hours |
| Aspart | 5-15 minutes | 30-90 minutes | 4-6 hours |
| Glulisine | 20 minutes | 90 minutes | 5.3 hours |
| Long-acting | |||
| NPH | 2-4 hours | 4-10 hours | 12-18 hours |
| Glargine | 2-4 hours | Relatively flat | Up to 24 hours |
| Detemir | 1-2 hours | Relatively flat | Up to 24 hours |
| NPH, neutral protamine Hagedorn. | |||
FIGURE 2
Pharmacokinetic profiles of human insulin and insulin analogs
Adapted from: Burton S. J Fam Pract. 2006;55(12 suppl):S10-S17.
CORRESPONDENCE Eric L. Johnson, MD, University of North Dakota School of Medicine and Health Sciences, Department of Family & Community Medicine, 501 North Columbia Road, Grand Forks, ND 58202-9037; [email protected]
• Consider evaluating 24-hour variability in glucose levels with patients’ self-monitoring glucose meters (in addition to monitoring glycosylated hemoglobin [HbA1c] levels at regular intervals). C
• If glycemic goals are unmet 2 to 3 months after initiating treatment with exercise and diet or with oral agent monotherapy, consider starting insulin therapy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Though we have the knowledge and the means to reduce complications of type 2 diabetes mellitus (T2DM), most patients may not be reaching all the glycemic goals necessary to achieve optimal risk reduction.1 Maintaining an acceptable level of glycosylated hemoglobin (HbA1c) is one of the important glycemic goals. But that measurement is an average of glucose levels occurring over the prior 3 months. Regardless of a given HbA1c measurement, an emerging body of evidence supports the presumption that glycemic variability over each 24-hour cycle is an independent risk factor for vascular complications.2-15
In this article, I review the literature pertaining to the risk associated with glycemic variability and to the benefit in correcting it. I also review the comparative outcomes achievable with normal human insulin and insulin analogs, as well as the advisability of starting insulin earlier in the management process.
Glycemic variability increases vascular risk independently
HbA1c, considered the gold standard for monitoring glycemic control in patients with T2DM, is an average of the full range of glucose values in the preceding 3 months, including fasting plasma glucose (FPG) and 2-hour postprandial glucose (PPG) levels. Studies have linked lowering HbA1c to reducing the risk and progression of micro- and macrovascular complications associated with diabetes.16,17 But evidence shows that other glycemic values are also important.
The Diabetes Control and Complications Trial (DCCT) was a landmark study in which patients with type 1 diabetes mellitus who received targeted intensive insulin therapy experienced delayed onset and slowed progression of micro-vascular complications compared with those who received conventional insulin treatment.16 Interestingly, this study also reported that patients randomized to receive conventional insulin treatment did not exhibit a reduction in the risk of progression of microvascular disease despite having HbA1c values comparable to those in the intensive-treatment group. One hypothesis is that glucose excursions occurred more frequently in the conventionally treated group, which received fewer daily insulin injections.5
Acute glucose fluctuations during the postprandial period trigger oxidative stress and are more predictive of atherosclerosis development than are FPG or HbA1c6,7 (see “Implications of glycemic variability” below). This suggests that therapy for patients with T2DM should not only target HbA1c as a long-term goal, but also aim to avoid acute glucose fluctuations as an immediate goal. Several studies have shown that postprandial hyperglycemia is an independent risk factor for vascular complications in patients with T2DM.2,7-9,12,14,15
Evidence of increased vascular risk with glycemic variability. The Diabetes Epidemiology: COllaborative analysis of Diagnostic criteria in Europe study (DECODE) followed more than 25,000 patients for more than 7 years and found that increased mortality was more closely associated with increased 2-hour PPG levels than with FPG.14 In the Framingham Offspring Study, Meigs et al9 reported that, in nondiabetic subjects, an elevated glucose level 2 hours after an oral challenge increased the relative risk for cardiovascular disease by up to 40%, independent of fasting hyperglycemia.
Mixed outcomes with Hba1c reduction only. Macrovascular risk reduction with intensive HbA1c management was not apparent in 3 recent studies—Action to Control Cardiovascular Risk in Diabetes (ACCORD),18 Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE),19 and Veterans Affairs Diabetes Trial (VADT).20 The ACCORD study, in fact, showed an increase in cardiovascular events in the intensively managed group (HbA1c target <6.0%). Indeed, previous studies had suggested an association between fasting hypoglycemia and poor cardiovascular outcomes.3,4 Retrospective subanalysis of the ACCORD study suggested that patients with poorer glycemic control had a greater risk of hypoglycemia independent of HbA1c values, and that patients who had difficulty reaching lower HbA1c levels may have had poorer cardiovascular outcomes.21
The apparent absence of a reduction in macrovascular events in the ACCORD, ADVANCE, and VADT studies also suggests an additive effect of nonglycemic risk factors that frequently accompany diabetes—ie, hypertension, hyperlipidemia, and hypercoagulability/pro-inflammatory states.
Long-term follow-up in the United Kingdom Prospective Diabetes Study (UKPDS) showed ongoing risk reduction for both microvascular and macrovascular complications.22 A separate meta-analysis showed a significant 10% reduction in cardiovascular events with intensive glycemic control when data were combined from the ACCORD trial, ADVANCE trial, VADT, and the UKPDS.23
An improvement in long-term outcomes for patients with T2DM might be expected when initiating a targeted, intensified, multi-factorial interventional regimen to reduce not only HbA1c, but also glucose variability. The STENO-2 trial showed that a targeted multifactorial treatment regimen in patients with T2DM could decrease long-term vascular complications.24
Consider assessing true variability in your patients. Because postprandial glucose levels alone may not equate to overall glycemic variability, you may want to ask select patients to take readings with their glucose meters at various times of the day across several days to get a more accurate picture.5
Normal physiologic insulin secretion prevents glucose fluctuations in healthy adults. in patients with diabetes, abnormalities in insulin secretion are part of the pathophysiologic process, resulting in chronic sustained hyperglycemia and acute daily fluctuations in glucose levels. These glycemic disorders are associated with a state of increased oxidative stress and possible subsequent development of vascular complications.
Cellular response to hyperglycemia. oxidative stress, the imbalance between production of reactive oxygen species and the ability to eliminate them, is central to the pathogenesis of cardiovascular complications of diabetes, including accelerated atherosclerotic macrovascular disease (FIGURE 1). Both insulin resistance and hyperglycemia are implicated in the pathogenesis of these complications.65,66 hyperglycemia is hypothesized to induce vascular injury via at least 4 biochemical pathways: enhanced polyol activity leading to sorbitol and fructose accumulation; increased formation of advanced glycation end products; activation of protein kinase c and nuclear factor kB; and increased hexosamine pathway flux.67 endothelium activation is a pro-inflammatory, proliferative, and pro-coagulatory setting, ultimately leading to arterial narrowing and susceptibility to atheroma deposition. hyperglycemia can also induce alterations in the coagulation system, resulting in increased thrombosis.68
Association of glycemic variability with oxidative stress. macrovascular complications, particularly cardiovascular disease, contribute significantly to the increased morbidity and mortality with diabetes.24 oxidative stress has been implicated as a major factor in the development of these complications.66-68 other cell-culture evidence suggests that normal protective mechanisms of oxidative stress are impaired by chronic hyperglycemia. When exposed to intermittent glycemic variability, cells have exhibited more pronounced toxicity.69,70 risso et al71 further established that variability in glycemic control resulted in more endothelial cell damage than did chronic sustained hyperglycemia.
Despite the experimental evidence that suggests glycemic variability is associated with increased risk of vascular complications, there are limited clinical data establishing glycemic variability as an independent predictor of these complications. monnier et al72 provided data in patients with type 2 diabetes mellitus (T2Dm) to support the concept of acute glucose fluctuations as a more important trigger of oxidative stress than chronic hyperglycemia. if these data are confirmed in larger clinical trials, a monitoring paradigm for patients with T2Dm could include increased focus on preventing glucose excursions in addition to reducing HbA1c.
FIGURE 1
How oxidative stress secondary to hyperglycemia leads to vascular complications in diabetes66-68
Following through with targeted, intensified management
Consider the following treatment goals for patients with T2DM: (1) lowering HbA1c levels; (2) lowering fasting blood glucose levels; (3) minimizing glycemic variability, including postprandial glucose excursions. TABLE 1 lists the values that the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists (AACE) have assigned to these glycemic-control goals.
In addition to managing glycemic levels, reducing risk of cardiovascular disease in T2DM involves aggressive interventions, as needed, to correct blood pressure and lipid levels.24,25
TABLE 1
Aim to reach 3 glycemic goals in treating type 2 diabetes mellitus
| ADA | AACE | |
|---|---|---|
| Fasting blood glucose (mg/dL) | 90-130 | <110 |
| Postprandial plasma glucose (mg/dL) | <180 | <140 |
| HbA1c (%) | <7* | ≤6.5 |
| *Recommended “in general”; however, the guideline indicates that for “the individual patient,” HbA1c should be as close to normal (<6%) as possible without causing hypoglycemia. | ||
| AACE, American Association of clinical endocrinologists; ADA, American Diabetes Association; HbA1c, glycosylated hemoglobin. | ||
| Sources: ADA, http://care.diabetesjournals.org/content/33/Supplement_1/S11/T11.expansion.htm; AACE, http://www.metcare.com/files/physician-resources/clinical-guidelines/dm-guidelines.pdf | ||
Challenges to achieving glycemic control
Despite current recommendations for more aggressive management of patients with T2DM,25 estimates are that as many as 60% of patients with T2DM do not achieve glycemic targets, and, as the disease progresses, many of the available treatment options fail to sustain levels previously reached.1,26,27
A shortcoming of older treatment strategies still in use is the slow transition to more effective therapy, resulting in long periods of inadequate glycemic control.1 Brown et al27 found that patients receiving monotherapy with either a sulfonylurea or metformin had HbA1c levels >8% for a mean of 20 months and 14 months, respectively, before treatment was changed. Current recommendations call for treatment changes within 2 to 3 months of initiation of therapy if the HbA1c goal is not reached.27-29
Turning to insulin earlier. Insulin is most effective for lowering HbA1c and delaying subsequent complications related to diabetes; however, there is often reluctance to using it early in diabetes management. Consequently, by the time insulin therapy is started, many patients will have had unacceptable glycemic levels for 10 years or more and may already be developing complications.27 And, as noted, the HbA1c level is an average measurement that does not detect glycemic variability. Continuous glucose monitoring will likely lead to more responsive adjustments in treatment regimens and to improved quality of care for patients with T2DM.
Insulin has many beneficial effects
Insulin exerts an anti-inflammatory effect by reducing the increase in C-reactive protein and serum amyloid A.30 It also partially restores insulin-stimulated endothelial function,31 facilitates vasodilation by increasing nitric oxide production,32 and improves fibrinolytic profiles.33 Early initiation of insulin therapy can increase peripheral insulin sensitivity and preserve beta cell function.34-36
When oral agents have failed, insulin can significantly improve patients’ beta cell function,34,35,37 and short periods of insulin therapy in patients newly diagnosed with T2DM may even set the foundation for better long-term control.38,39
But not all insulin is alike
Ideally, insulin therapy should mimic physiologic insulin secretion. However, conventional human insulin products fail to do so because of their suboptimal pharmacodynamic profiles. With recombinant DNA technology, molecular modifications of the human insulin molecule have overcome some of the limitations of conventional human insulin products.
Unfortunately, many practitioners still hold insulin in reserve until combination therapy with oral agents has failed, possibly resulting in years of suboptimal glycemic control. Newer strategies recommend earlier initiation of insulin—ie, once diet and exercise fail, or when treatment with 1 oral agent fails. The development of insulin analogs is a significant milestone on the road to achieving improved outcomes for patients with T2DM.
Rapid-acting agents
Compared with regular human insulin, newer rapid-acting insulin analogs may improve glycemic control when used at mealtimes. However, due to their shorter half-lives, these insulin analogs require augmentation with basal insulin to control hyperglycemia between meals and during the night.
Insulin lispro was the first commercially available rapid-acting insulin analog, introduced in 1996. This agent differs from human insulin by an inversion of amino acid residues in positions 28 and 29 of the insulin B-chain. Inversion prevents the formation of hexamers and dimers that tend to diffuse more slowly, thereby facilitating a rapid uptake of the insulin analog into blood and tissues.40,41 The second such agent, marketed in 2000, was insulin aspart, in which aspartic acid replaces proline at position 28 of the B-chain of human insulin.41,42 The most recent rapid-acting analog is insulin glulisine, in which lysine replaces asparagine near the N-terminus of the B-chain, and glutamic acid replaces lysine near the C-terminus of human insulin.
The molecular changes made in creating these analogs allows them to dissociate quickly into monomers that are absorbed rapidly and achieve faster peak levels compared with regular human insulin.41,42 These changes do not, however, interfere with the analogs’ ability to bind to the insulin receptor.43,44
Dosing considerations. Absorption of regular human insulin is not sufficiently rapid at mealtimes to control prandial glucose levels.45 Therefore, it is essential to give regular insulin 30 to 60 minutes before meals. For patients who have erratic daily schedules, adhering to this sort of routine can be difficult. But even if scheduling is not a problem, the prolonged duration of action of human insulin can predispose patients to hypoglycemia. Moreover, absorption of regular insulin can vary dramatically from day to day.46,47
The insulin analogs correct the pharmacokinetic and pharmacodynamic deficiencies of regular insulin, producing plasma profiles that more closely simulate normal, physiologic meal-stimulated insulin release.48-50 The 3 rapid-acting agents (aspart, glulisine, lispro) have very similar onset and duration of action, with peak effect occurring close to injection time (TABLE 2 and FIGURE 2).48-50
Advantages of rapid-acting agents. These agents can be administered closer to meals, giving patients more flexibility and likely tighter postprandial glucose control, with reductions in glycemic excursions. Another advantage is the ability to better match insulin dose to anticipated carbohydrate in-take, affording better postprandial control.51-53 Rapid-acting analogs also result in fewer episodes of hypoglycemia. In a meta-analysis of 2576 patients, hypoglycemic events occurred 25% less often with insulin lispro compared with regular human insulin in patients with type 1 diabetes mellitus (T1DM).52 In clinical trials, insulin aspart and insulin glulisine have also caused fewer hypoglycemic events compared with regular human insulin.51-53
Long-acting agents
Basal, or long-acting, insulins are important for maintaining normoglycemia over 24 hours. Neutral protamine Hagedorn (NPH) insulin reaches its peak effect 4 to 10 hours after injection, and its total effect lasts only 12 to 18 hours. NPH is therefore often dosed twice daily. Absorption of NPH can vary significantly, causing day-to-day blood glucose fluctuations.46,47 Therefore, this agent’s activity does not closely resemble normal physiologic basal insulin secretion.
The newer long-acting insulin analogs—insulin detemir and insulin glargine—were designed to more closely replicate normal physiologic basal insulin secretion. Insulin glargine was first to reach the market, in 2001. It contains glycine instead of asparagine in the alpha-chain and 2 arginine residues at the C-terminus, and the addition of zinc enhances the aggregation and slow release at a neutral pH. Insulin glargine precipitates in the subcutaneous tissue, which slows its absorption and results in a relatively flat insulin plasma profile and extended action.54,55 Insulin detemir is a combination of the original insulin molecule and a saturated fatty acid (myristic acid). Insulin detemir is designed to bind albumin (98% albumin-bound in circulation) through this fatty acid chain in the plasma after injection, resulting in an extended plasma profile.54,56 Insulin glargine and NPH form crystalline depots, but detemir is soluble and the subcutaneous depot remains in a liquid state; this may account for differences in absorption variability.56
Advantages of the long-acting insulin analogs. Compared with conventional basal insulin such as NPH, the analogs have a prolonged duration of action (up to 24 hours) without pronounced peaks, permitting once-daily dosing in many patients (TABLE 2 and FIGURE 2).46,47,50,55 The pharmacodynamic and pharmacokinetic properties of the long- acting agents make them less likely than NPH to cause nocturnal and overall hypoglycemia, a benefit that has been observed in several clinical trials.57-61
Comparative clinical trials evaluating glycemic variability. Both of the long-acting analogs have shown lower within-subject variability in blood and plasma glucose measurements when compared with NPH.62-64 In head-to-head comparisons of the analogs in glucose clamp studies, insulin detemir has demonstrated less within-subject variability of blood glucose levels than insulin glargine, in patients with T1DM or T2DM.56,64 In clinical practice, different patients may have better results with one of these basal insulins as opposed to the other, and treatment choices will need to be tailored to the individual patient.
TABLE 2
Pharmacokinetic properties giving insulin analogs an advantage over regular insulin46-50
| Insulin preparation | Onset of action | Peak action | Duration of action |
|---|---|---|---|
| Short-acting | |||
| Regular | 30-60 minutes | 2-3 hours | 8-10 hours |
| Lispro | 5-15 minutes | 30-90 minutes | 4-6 hours |
| Aspart | 5-15 minutes | 30-90 minutes | 4-6 hours |
| Glulisine | 20 minutes | 90 minutes | 5.3 hours |
| Long-acting | |||
| NPH | 2-4 hours | 4-10 hours | 12-18 hours |
| Glargine | 2-4 hours | Relatively flat | Up to 24 hours |
| Detemir | 1-2 hours | Relatively flat | Up to 24 hours |
| NPH, neutral protamine Hagedorn. | |||
FIGURE 2
Pharmacokinetic profiles of human insulin and insulin analogs
Adapted from: Burton S. J Fam Pract. 2006;55(12 suppl):S10-S17.
CORRESPONDENCE Eric L. Johnson, MD, University of North Dakota School of Medicine and Health Sciences, Department of Family & Community Medicine, 501 North Columbia Road, Grand Forks, ND 58202-9037; [email protected]
1. Koro CE, Bowlin SJ, Bourgeois N, et al. Glycemic control from 1988 to 2000 among U.S. adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care. 2004;27:17-20.
2. Kadowaki S, Okamura T, Hozawa A, et al. Relationship of elevated casual blood glucose level with coronary heart disease, cardiovascular disease and all-cause mortality in a representative sample of the Japanese population. NIPPON DATA80. Diabetologia. 2008;51:575-582.
3. Wändell PE, Theobald H. The association between low fasting glucose value and mortality. Curr Diabetes Rev. 2007;3:274-279.
4. Wei M, Gibbons LW, Mitchell TL, et al. Low fasting plasma glucose level as a predictor of cardiovascular disease and all-cause mortality. Circulation. 2000;101:2047-2052.
5. Hirsch IB, Brownlee M. Should minimal blood glucose variability become the gold standard of glycemic control? J Diabetes Complications. 2005;19:178-181.
6. Temelkova-Kurktschiev TS, Koehler C, Henkel E, et al. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care. 2000;23:1830-1834.
7. Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in Type II diabetes: the epidemiological evidence. Diabetologia. 2001;44:2107-2114.
8. Chiasson JL, Josse RG, Gomis R, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486-494.
9. Meigs JB, Nathan DM, D’Agostino RB, Sr, et al. Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care. 2002;25:1845-1850.
10. Balkau B, Shipley M, Jarrett RJ, et al. High blood glucose concentration is a risk factor for mortality in middle-aged nondiabetic men. 20-year follow-up in the Whitehall Study, the Paris Prospective Study, and the Helsinki Policemen Study. Diabetes Care. 1998;21:360-367.
11. Hanefeld M, Fischer S, Julius U, et al. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia. 1996;39:1577-1583.
12. Donahue RP, Abbott RD, Reed DM, et al. Postchallenge glucose concentration and coronary heart disease in men of Japanese ancestry. Honolulu Heart Program. Diabetes. 1987;36:689-692.
13. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet. 1999;354:617-621.
14. Balkau B, Hu G, Qiao Q, et al. Prediction of the risk of cardiovascular mortality using a score that includes glucose as a risk factor. The DECODE Study. Diabetologia. 2004;47:2118-2128.
15. Hanefeld M, Fischer S, Julius U, et al. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia. 1996;39:1577-1583.
16. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977-986.
17. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405-412.
18. The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559.
19. The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560-2572.
20. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129-139.
21. Miller ME, Bonds DE, Gerstein HC, et al. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444.-
22. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
23. Turnbull FM, Abraira C, Anderson RJ, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;11:2288-2298.
24. Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383-393.
25. Standards of medical care in diabetes—2010 Diabetes Care. 2010;33(suppl 1):S11-S61.
26. Barnett AH. Treating to goal: challenges of current management. Eur J Endocrinol. 2004;151(suppl 2):T3-T7.
27. Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27:1535-1540.
28. Nathan DM. Clinical practice. Initial management of glycemia in type 2 diabetes mellitus. N Engl J Med. 2002;347:1342-1349.
29. Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association; European Association for the Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetes Care. 2009;32:193-203.
30. Chaudhuri A, Janicke D, Wilson MF, et al. Anti-inflammatory and profibrinolytic effect of insulin in acute ST-segment-elevation myocardial infarction. Circulation. 2004;109:849-854.
31. Rask-Madsen C, Ihlemann N, Krarup T, et al. Insulin therapy improves insulin-stimulated endothelial function in patients with type 2 diabetes and ischemic heart disease. Diabetes. 2001;50:2611-2618.
32. Chaudhuri A, Kanjwal Y, Mohanty P, et al. Insulin-induced vasodilatation of internal carotid artery. Metabolism. 1999;48:1470-1473.
33. Melidonis A, Stefanidis A, Tournis S, et al. The role of strict metabolic control by insulin infusion on fibrinolytic profile during an acute coronary event in diabetic patients. Clin Cardiol. 2000;23:160-164.
34. Garvey WT, Olefsky JM, Griffin J, et al. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985;34:222-234.
35. Rolla A. The pathophysiological basis for intensive insulin replacement. Int J Obes Relat Metab Disord. 2004;28(suppl 2):S3-S7.
36. Alvarsson M, Sundkvist G, Lager I, et al. Beneficial effects of insulin versus sulphonylurea on insulin secretion and metabolic control in recently diagnosed type 2 diabetic patients. Diabetes Care. 2003;26:2231-2237.
37. Glaser B, Leibovich G, Nesher R, et al. Improved beta-cell function after intensive insulin treatment in severe non-insulin-dependent diabetes. Acta Endocrinol (Copenh). 1988;118:365-373.
38. Andrews WJ, Vasquez B, Nagulesparan M, et al. Insulin therapy in obese, non-insulin-dependent diabetes induces improvements in insulin action and secretion that are maintained for two weeks after insulin withdrawal. Diabetes. 1984;33:634-642.
39. Ryan EA, Imes S, Wallace C. Short-term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care. 2004;27:1028-1032.
40. Heise T, Heinemann L. Rapid and long-acting analogues as an approach to improve insulin therapy: an evidence-based medicine assessment. Curr Pharm Des. 2001;7:1303-1325.
41. Brems DN, Alter LA, Beckage MJ, et al. Altering the association properties of insulin by amino acid replacement. Protein Eng. 1992;5:527-533.
42. Garber AJ. Pharmacologic modifications of hormones to improve their therapeutic potential for diabetes management. Diabetes Obes Metab. 2005;7:666-674.
43. Hansen BF, Danielsen GM, Drejer K, et al. Sustained signalling from the insulin receptor after stimulation with insulin analogues exhibiting increased mitogenic potency. Biochem J. 1996;315(pt 1):271-279.
44. Slieker LJ, Brooke GS, DiMarchi RD, et al. Modifications in the B10 and B26-30 regions of the B chain of human insulin alter affinity for the human IGF-I receptor more than for the insulin receptor. Diabetologia. 1997;40(suppl 2):S54-S61.
45. Dimitriadis GD, Gerich JE. Importance of timing of preprandial subcutaneous insulin administration in the management of diabetes mellitus. Diabetes Care. 1983;6:374-377.
46. Roy B, Chou MC, Field JB. Time-action characteristics of regular and NPH insulin in insulin-treated diabetics. J Clin Endocrinol Metab. 1980;50:475-479.
47. Binder C, Lauritzen T, Faber O, et al. Insulin pharmacokinetics. Diabetes Care. 1984;7:188-199.
48. Kang S, Creagh FM, Peters JR, et al. Comparison of subcutaneous soluble human insulin and insulin analogues (AspB9, GluB27; AspB10; AspB28) on meal-related plasma glucose excursions in type 1 diabetic subjects. Diabetes Care. 1991;14:571-577.
49. Becker RH, Frick AD, Burger F, et al. A comparison of the steady-state pharmacokinetics and pharmacodynamics of a novel rapid-acting insulin analog, insulin glulisine, and regular human insulin in healthy volunteers using the euglycemic clamp technique. Exp Clin Endocrinol Diabetes. 2005;113:292-297.
50. Hirsch IB. Insulin analogues. N Engl J Med. 2005;352:174-183.
51. Lindholm A, McEwen J, Riis AP. Improved postprandial glycemic control with insulin aspart. A randomized double-blind cross-over trial in type 1 diabetes. Diabetes Care. 1999;22:801-805.
52. Brunelle BL, Llewelyn J, Anderson JH, Jr, et al. Meta-analysis of the effect of insulin lispro on severe hypoglycemia in patients with type 1 diabetes. Diabetes Care. 1998;21:1726-1731.
53. Home PD, Lindholm A, Hylleberg B, et al. Improved glycemic control with insulin aspart: a multicenter randomized double-blind crossover trial in type 1 diabetic patients. UK Insulin Aspart Study Group. Diabetes Care. 1998;21:1904-1909.
54. Heise T, Pieber TR. Towards peakless, reproducible and long-acting insulins. An assessment of the basal analogues based on isoglycaemic clamp studies. Diabetes Obes Metab. 2007;5:648-659.
55. Bolli GB, Di Marchi RD, Park GD, et al. Insulin analogues and their potential in the management of diabetes mellitus. Diabetologia. 1999;42:1151-1167.
56. Klein O, Lynge J, Endahl L, et al. Albumin-bound basal insulin analogues (insulin detemir and NN344): comparable time-action profiles but less variability than insulin glargine in type 2 diabetes. Diabetes Obes Metab. 2007;9:290-299.
57. Riddle MC, Rosenstock J, Gerich J. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080-3086.
58. Yki-Jarvinen H, Dressler A, Ziemen M. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care. 2000;23:1130-1136.
59. Rosenstock J, Schwartz SL, Clark CM, Jr, et al. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care. 2001;24:631-636.
60. Hermansen K, Davies M, Derezinski T, et al. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care. 2006;29:1269-1274.
61. Philis-Tsimikas A, Charpentier G, Clauson P, et al. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther. 2006;28:1569-1581.
62. Haak T, Tiengo A, Draeger E, et al. Lower within-subject variability of fasting blood glucose and reduced weight gain with insulin detemir compared to NPH insulin in patients with type 2 diabetes. Diabetes Obes Metab. 2005;7:56-64.
63. Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49:2142-2148.
64. Heise T, Nosek L, Ronn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53:1614-1620.
65. Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106:453-458.
66. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615-1625.
67. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813-820.
68. Ceriello A. Coagulation activation in diabetes mellitus: the role of hyperglycaemia and therapeutic prospects. Diabetologia. 1993;36:1119-1125.
69. Quagliaro L, Piconi L, Assaloni R, et al. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52:2795-2804.
70. Jones SC, Saunders HJ, Qi W, et al. Intermittent high glucose enhances cell growth and collagen synthesis in cultured human tubulointerstitial cells. Diabetologia. 1999;42:1113-1119.
71. Risso A, Mercuri F, Quagliaro L, et al. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am J Physiol Endocrinol Metab. 2001;281:E924-E930.
72. Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681-1687.
1. Koro CE, Bowlin SJ, Bourgeois N, et al. Glycemic control from 1988 to 2000 among U.S. adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care. 2004;27:17-20.
2. Kadowaki S, Okamura T, Hozawa A, et al. Relationship of elevated casual blood glucose level with coronary heart disease, cardiovascular disease and all-cause mortality in a representative sample of the Japanese population. NIPPON DATA80. Diabetologia. 2008;51:575-582.
3. Wändell PE, Theobald H. The association between low fasting glucose value and mortality. Curr Diabetes Rev. 2007;3:274-279.
4. Wei M, Gibbons LW, Mitchell TL, et al. Low fasting plasma glucose level as a predictor of cardiovascular disease and all-cause mortality. Circulation. 2000;101:2047-2052.
5. Hirsch IB, Brownlee M. Should minimal blood glucose variability become the gold standard of glycemic control? J Diabetes Complications. 2005;19:178-181.
6. Temelkova-Kurktschiev TS, Koehler C, Henkel E, et al. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care. 2000;23:1830-1834.
7. Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in Type II diabetes: the epidemiological evidence. Diabetologia. 2001;44:2107-2114.
8. Chiasson JL, Josse RG, Gomis R, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486-494.
9. Meigs JB, Nathan DM, D’Agostino RB, Sr, et al. Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care. 2002;25:1845-1850.
10. Balkau B, Shipley M, Jarrett RJ, et al. High blood glucose concentration is a risk factor for mortality in middle-aged nondiabetic men. 20-year follow-up in the Whitehall Study, the Paris Prospective Study, and the Helsinki Policemen Study. Diabetes Care. 1998;21:360-367.
11. Hanefeld M, Fischer S, Julius U, et al. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia. 1996;39:1577-1583.
12. Donahue RP, Abbott RD, Reed DM, et al. Postchallenge glucose concentration and coronary heart disease in men of Japanese ancestry. Honolulu Heart Program. Diabetes. 1987;36:689-692.
13. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet. 1999;354:617-621.
14. Balkau B, Hu G, Qiao Q, et al. Prediction of the risk of cardiovascular mortality using a score that includes glucose as a risk factor. The DECODE Study. Diabetologia. 2004;47:2118-2128.
15. Hanefeld M, Fischer S, Julius U, et al. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia. 1996;39:1577-1583.
16. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977-986.
17. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405-412.
18. The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559.
19. The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560-2572.
20. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129-139.
21. Miller ME, Bonds DE, Gerstein HC, et al. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444.-
22. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
23. Turnbull FM, Abraira C, Anderson RJ, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;11:2288-2298.
24. Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383-393.
25. Standards of medical care in diabetes—2010 Diabetes Care. 2010;33(suppl 1):S11-S61.
26. Barnett AH. Treating to goal: challenges of current management. Eur J Endocrinol. 2004;151(suppl 2):T3-T7.
27. Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27:1535-1540.
28. Nathan DM. Clinical practice. Initial management of glycemia in type 2 diabetes mellitus. N Engl J Med. 2002;347:1342-1349.
29. Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association; European Association for the Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetes Care. 2009;32:193-203.
30. Chaudhuri A, Janicke D, Wilson MF, et al. Anti-inflammatory and profibrinolytic effect of insulin in acute ST-segment-elevation myocardial infarction. Circulation. 2004;109:849-854.
31. Rask-Madsen C, Ihlemann N, Krarup T, et al. Insulin therapy improves insulin-stimulated endothelial function in patients with type 2 diabetes and ischemic heart disease. Diabetes. 2001;50:2611-2618.
32. Chaudhuri A, Kanjwal Y, Mohanty P, et al. Insulin-induced vasodilatation of internal carotid artery. Metabolism. 1999;48:1470-1473.
33. Melidonis A, Stefanidis A, Tournis S, et al. The role of strict metabolic control by insulin infusion on fibrinolytic profile during an acute coronary event in diabetic patients. Clin Cardiol. 2000;23:160-164.
34. Garvey WT, Olefsky JM, Griffin J, et al. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985;34:222-234.
35. Rolla A. The pathophysiological basis for intensive insulin replacement. Int J Obes Relat Metab Disord. 2004;28(suppl 2):S3-S7.
36. Alvarsson M, Sundkvist G, Lager I, et al. Beneficial effects of insulin versus sulphonylurea on insulin secretion and metabolic control in recently diagnosed type 2 diabetic patients. Diabetes Care. 2003;26:2231-2237.
37. Glaser B, Leibovich G, Nesher R, et al. Improved beta-cell function after intensive insulin treatment in severe non-insulin-dependent diabetes. Acta Endocrinol (Copenh). 1988;118:365-373.
38. Andrews WJ, Vasquez B, Nagulesparan M, et al. Insulin therapy in obese, non-insulin-dependent diabetes induces improvements in insulin action and secretion that are maintained for two weeks after insulin withdrawal. Diabetes. 1984;33:634-642.
39. Ryan EA, Imes S, Wallace C. Short-term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care. 2004;27:1028-1032.
40. Heise T, Heinemann L. Rapid and long-acting analogues as an approach to improve insulin therapy: an evidence-based medicine assessment. Curr Pharm Des. 2001;7:1303-1325.
41. Brems DN, Alter LA, Beckage MJ, et al. Altering the association properties of insulin by amino acid replacement. Protein Eng. 1992;5:527-533.
42. Garber AJ. Pharmacologic modifications of hormones to improve their therapeutic potential for diabetes management. Diabetes Obes Metab. 2005;7:666-674.
43. Hansen BF, Danielsen GM, Drejer K, et al. Sustained signalling from the insulin receptor after stimulation with insulin analogues exhibiting increased mitogenic potency. Biochem J. 1996;315(pt 1):271-279.
44. Slieker LJ, Brooke GS, DiMarchi RD, et al. Modifications in the B10 and B26-30 regions of the B chain of human insulin alter affinity for the human IGF-I receptor more than for the insulin receptor. Diabetologia. 1997;40(suppl 2):S54-S61.
45. Dimitriadis GD, Gerich JE. Importance of timing of preprandial subcutaneous insulin administration in the management of diabetes mellitus. Diabetes Care. 1983;6:374-377.
46. Roy B, Chou MC, Field JB. Time-action characteristics of regular and NPH insulin in insulin-treated diabetics. J Clin Endocrinol Metab. 1980;50:475-479.
47. Binder C, Lauritzen T, Faber O, et al. Insulin pharmacokinetics. Diabetes Care. 1984;7:188-199.
48. Kang S, Creagh FM, Peters JR, et al. Comparison of subcutaneous soluble human insulin and insulin analogues (AspB9, GluB27; AspB10; AspB28) on meal-related plasma glucose excursions in type 1 diabetic subjects. Diabetes Care. 1991;14:571-577.
49. Becker RH, Frick AD, Burger F, et al. A comparison of the steady-state pharmacokinetics and pharmacodynamics of a novel rapid-acting insulin analog, insulin glulisine, and regular human insulin in healthy volunteers using the euglycemic clamp technique. Exp Clin Endocrinol Diabetes. 2005;113:292-297.
50. Hirsch IB. Insulin analogues. N Engl J Med. 2005;352:174-183.
51. Lindholm A, McEwen J, Riis AP. Improved postprandial glycemic control with insulin aspart. A randomized double-blind cross-over trial in type 1 diabetes. Diabetes Care. 1999;22:801-805.
52. Brunelle BL, Llewelyn J, Anderson JH, Jr, et al. Meta-analysis of the effect of insulin lispro on severe hypoglycemia in patients with type 1 diabetes. Diabetes Care. 1998;21:1726-1731.
53. Home PD, Lindholm A, Hylleberg B, et al. Improved glycemic control with insulin aspart: a multicenter randomized double-blind crossover trial in type 1 diabetic patients. UK Insulin Aspart Study Group. Diabetes Care. 1998;21:1904-1909.
54. Heise T, Pieber TR. Towards peakless, reproducible and long-acting insulins. An assessment of the basal analogues based on isoglycaemic clamp studies. Diabetes Obes Metab. 2007;5:648-659.
55. Bolli GB, Di Marchi RD, Park GD, et al. Insulin analogues and their potential in the management of diabetes mellitus. Diabetologia. 1999;42:1151-1167.
56. Klein O, Lynge J, Endahl L, et al. Albumin-bound basal insulin analogues (insulin detemir and NN344): comparable time-action profiles but less variability than insulin glargine in type 2 diabetes. Diabetes Obes Metab. 2007;9:290-299.
57. Riddle MC, Rosenstock J, Gerich J. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080-3086.
58. Yki-Jarvinen H, Dressler A, Ziemen M. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care. 2000;23:1130-1136.
59. Rosenstock J, Schwartz SL, Clark CM, Jr, et al. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care. 2001;24:631-636.
60. Hermansen K, Davies M, Derezinski T, et al. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care. 2006;29:1269-1274.
61. Philis-Tsimikas A, Charpentier G, Clauson P, et al. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther. 2006;28:1569-1581.
62. Haak T, Tiengo A, Draeger E, et al. Lower within-subject variability of fasting blood glucose and reduced weight gain with insulin detemir compared to NPH insulin in patients with type 2 diabetes. Diabetes Obes Metab. 2005;7:56-64.
63. Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49:2142-2148.
64. Heise T, Nosek L, Ronn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53:1614-1620.
65. Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106:453-458.
66. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615-1625.
67. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813-820.
68. Ceriello A. Coagulation activation in diabetes mellitus: the role of hyperglycaemia and therapeutic prospects. Diabetologia. 1993;36:1119-1125.
69. Quagliaro L, Piconi L, Assaloni R, et al. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52:2795-2804.
70. Jones SC, Saunders HJ, Qi W, et al. Intermittent high glucose enhances cell growth and collagen synthesis in cultured human tubulointerstitial cells. Diabetologia. 1999;42:1113-1119.
71. Risso A, Mercuri F, Quagliaro L, et al. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am J Physiol Endocrinol Metab. 2001;281:E924-E930.
72. Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681-1687.