User login
Malpractice Counsel
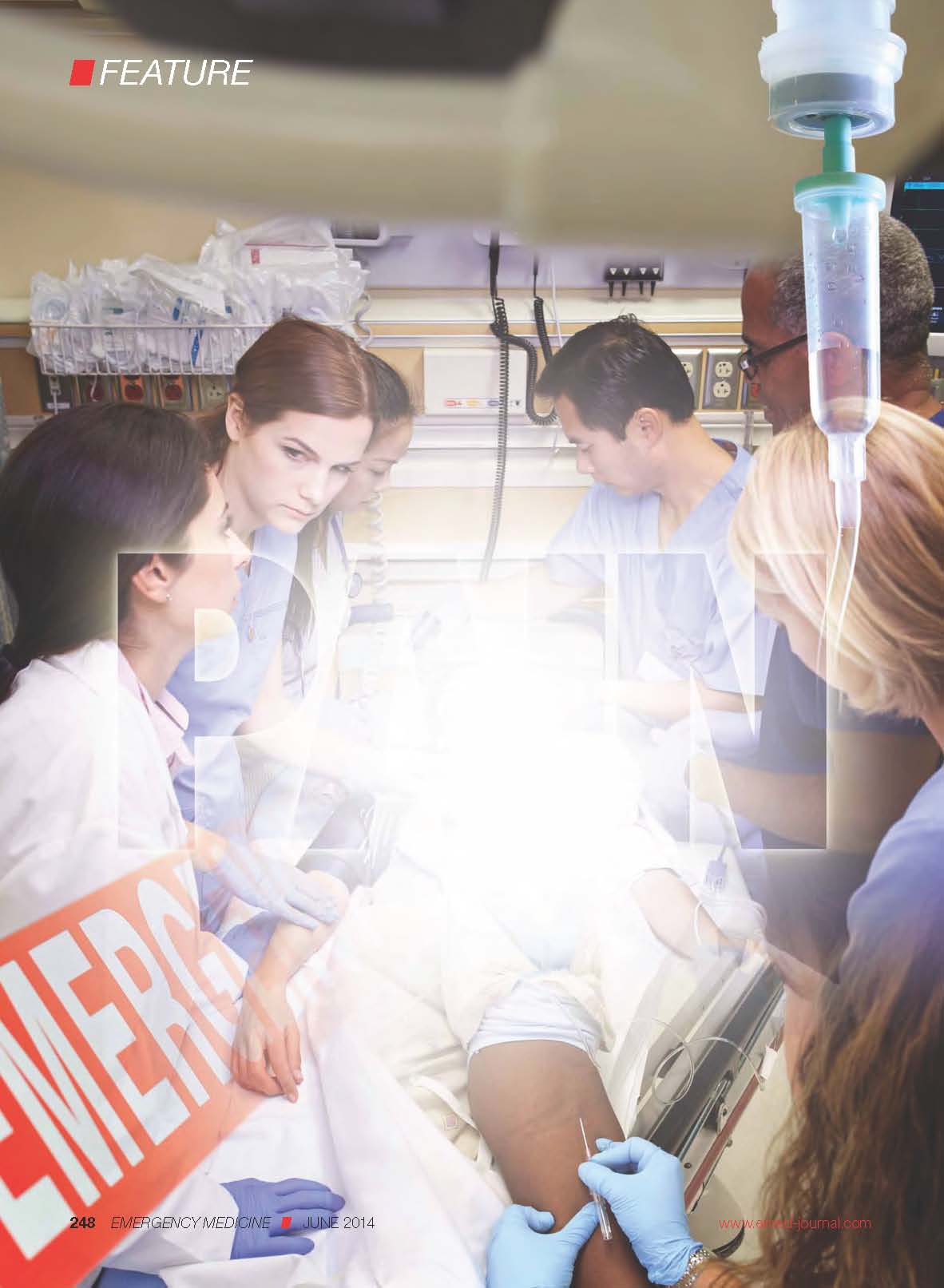
Aortic Rupture
A 59-year-old woman involved in a motor vehicle crash presented to the ED via emergency medical services. The patient had been the front-seat passenger and was wearing a seat belt. She complained of chest wall pain, but denied head injury, loss of consciousness, neck pain, abdominal pain, or shortness of breath. Her past medical history was unremarkable.
A chest X-ray was performed and interpreted as normal by the attending radiologist. Laboratory studies were normal except for mild anemia. The patient was discharged from the hospital with a diagnosis of chest wall contusion. She died 36 hours later from a ruptured thoracic aorta. The family of the patient brought a malpractice suit against the emergency physician (EP) for failing to diagnose and treat acute aortic rupture. At trial, a defense verdict was returned.
Discussion
Aortic rupture from blunt trauma is a devastating injury. More than 90% of patients who have sustained this type of injury in a motor vehicle crash die at the scene.1 For the remaining 10%, 50% die within the following 24 hours.1 The injury occurs in the proximal descending aorta, secondary to the fixation of the vessels between the left subclavian artery and the ligamentum arteriosum; the cause in approximately 80% to 90% of cases is due to blunt trauma. Involvement of the ascending aorta is much less common. Many patients, such as the one in this case, exhibit no external physical findings of injury. Chest pain is the most frequent complaint, followed by dyspnea—both fairly nonspecific symptoms. Physical findings that should raise a suspicion for a thoracic aortic injury include hypotension, hypertension in the upper extremity and hypotension in the lower extremity, unequal BPs in the extremities, external evidence of chest wall trauma, and palpable fractures of the sternum and ribs.2 While it is unclear if this patient had unequal extremity BPs, she did not have any of the other classic findings of aortic rupture. Associated neurological, abdominal, or orthopedic injuries are frequently present as well, and can mask the subtle signs of aortic rupture.
A chest radiograph is often the initial screening test used to evaluate for possible thoracic aortic injury. Suspicious findings include a widened mediastinum (greater than 8 cm), right-sided deviation of the esophagus, depression of the left mainstem bronchus, loss of the aortic knob, and an apical pleural cap. Unfortunately, chest X-ray can be normal, and a normal mediastinum on the radiograph does not exclude the diagnosis.
For patients with suspected thoracic aortic injury, helical computed tomography with angiography is the study of choice. It can accurately identify operative and nonoperative lesions, as well as associated injuries (eg, small pneumothorax, rib fractures). Magnetic resonance angiography provides similar sensitivity and specificity, but is not practical for the majority of trauma patients. Occasionally, aortography can be considered when the CT scan results are indeterminate and when thought to be needed to plan operative intervention. Finally, transesophageal echocardiography can be considered in hemodynamically unstable patients unable to be transferred to the radiology suite.
For most patients, immediate operative intervention is the definitive treatment. For patients with suspected thoracic aortic injury and hypertension, shear forces need to be decreased just as they are for patients with aortic dissection. A short-acting β-blocker like intravenous (IV) esmolol can be used initially to slow HR. Then, an IV arterial vasodilator can be given to decrease BP. To prevent rebound tachycardia and increased shear forces, the β-blocker should always be initiated before the vasodilator is given. Vital-sign targets include an HR of 60 beats/minute and a systolic BP in the range of 100 to 120 mm Hg.
This was a very atypical presentation of a devastating injury. Given the benign presentation, lack of associated injuries, and the normal chest X-ray, a defense verdict appears to be the correct one in this very unfortunate case.
Foot Drop
A 20-year-old woman presented to the ED complaining of severe numbness, tingling, and pain in her left calf. According to the patient, she had attended a New Year’s Eve party, where she spent much of the time dancing. She was awakened by calf pain on the following morning and sought treatment at the ED.
On physical examination, the patient’s vital signs were normal. Examination of the left calf revealed tenderness to palpation; no swelling was noted. The patient was unable to lift her left foot or bear weight on the left leg. She had normal dorsalis pedis and posterior tibial pulses in the affected leg. The remainder of her examination was normal and no testing was performed. The patient was diagnosed with “floppy foot syndrome” and discharged home with a prescription for a nonsteroidal anti-inflammatory drug.
The next day, the patient presented to a different ED because of worsening pain and swelling of the calf. She was admitted to the hospital and the orthopedic service was consulted. The patient was diagnosed with compartment syndrome; however, by that time her condition was complicated by rhabdomyolysis, resulting in acute renal failure.
The patient underwent a fasciotomy. After surgery, she required hemodialysis until her kidney function returned. She had damage to the nerves in her left calf and leg resulting in a permanent foot drop that required prolonged physical therapy following her hospitalization.
The patient sued the initial EP for failure to diagnose compartment syndrome, which resulted in permanent nerve damage and foot drop. A $750,000 settlement was reached.
Discussion
The EP did not appear to have taken this case seriously, as “floppy foot syndrome” is not a recognized diagnosis. No significance was attached to the presence of the foot drop, which is an objective and concerning physical finding.
The differential diagnoses of foot drop are relatively small: a nerve injury, which is the most common cause; a central nervous system event, such as a stroke; or a muscular disorder.3 An injury or problem with the peroneal nerve is the most common cause of foot drop.
While the patient’s history was not typical for the development of compartment syndrome, she potentially participated in strenuous physical activity, which can result in muscle swelling and subsequent compartment syndrome.4 The pain from compartment syndrome is typically described as out of proportion to physical findings; this seems to have been the case for this patient.
The symptoms and findings of compartment syndrome are classically taught as the five “Ps”: pain, paresthesias, paralysis, pallor, and pulselessness, with the symptoms typically presenting in this order. The patient had the first three symptoms, but they were not appreciated in the initial evaluation. Pulselessness is usually the last finding to develop, and tissue damage is frequently present at that point.
Interestingly, approximately 40% of compartment syndromes occur at the level of the tibia and fibula. The lower leg has four compartments: the anterior, which contains the anterior tibial artery and deep peroneal nerve; the lateral, which contains the superficial peroneal nerve; the superficial posterior, which contains the sural nerve; and the deep posterior, which contains the posterior tibial artery and nerve.
As pressure within the enclosed space increases due to swelling, hemorrhage, fracture, etc, the blood supply as well as nerve and muscle functions become compromised. Left untreated, the increased pressure can result in permanent tissue and nerve damage.
Compartment syndrome is a time sensitive diagnosis because of the need for surgical intervention to open the compartment. The EP can measure compartment pressures if he or she has the right equipment and training. A normal compartment pressure is less than 10 mm Hg. When the compartment pressure begins to exceed 30 mm Hg, tissue damage can occur. If unable to measure compartment pressure, an emergent orthopedic consult is indicated.
- Chiesa R, de Moura MR, Lucci C, et al. Traumatic rupture of the thoracic aorta. Acta Chir Belq. 2003;103(4):364-374.
- Ross C, Schwab TM. Cardiac trauma. In: Tintinalli JE, Stapczynski JS, Cline DM, Ma OJ, Cydulka RK, Meckler GD. Ross C. In: Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York, NY: McGraw Hill Medical; 2011:1758-1765.
- Ricarte IF, Figueiredo MM, Fukuda TG, Pedroso JL, Silva GS. Acute foot drop syndrome mimicking peroneal nerve injury: an atypical presentation of ischemic stroke. J Stroke Cerebrovasc Dis. 2014:23(5):1229-1231.
- Aliano K, Gulati S, Stavrides S, Davenport T, Hines G. Low-impact trauma causing acute compartment syndrome of the lower extremities. Am J Emerg Med. 2013;31(5):890.e3-e4.
Aortic Rupture
A 59-year-old woman involved in a motor vehicle crash presented to the ED via emergency medical services. The patient had been the front-seat passenger and was wearing a seat belt. She complained of chest wall pain, but denied head injury, loss of consciousness, neck pain, abdominal pain, or shortness of breath. Her past medical history was unremarkable.
A chest X-ray was performed and interpreted as normal by the attending radiologist. Laboratory studies were normal except for mild anemia. The patient was discharged from the hospital with a diagnosis of chest wall contusion. She died 36 hours later from a ruptured thoracic aorta. The family of the patient brought a malpractice suit against the emergency physician (EP) for failing to diagnose and treat acute aortic rupture. At trial, a defense verdict was returned.
Discussion
Aortic rupture from blunt trauma is a devastating injury. More than 90% of patients who have sustained this type of injury in a motor vehicle crash die at the scene.1 For the remaining 10%, 50% die within the following 24 hours.1 The injury occurs in the proximal descending aorta, secondary to the fixation of the vessels between the left subclavian artery and the ligamentum arteriosum; the cause in approximately 80% to 90% of cases is due to blunt trauma. Involvement of the ascending aorta is much less common. Many patients, such as the one in this case, exhibit no external physical findings of injury. Chest pain is the most frequent complaint, followed by dyspnea—both fairly nonspecific symptoms. Physical findings that should raise a suspicion for a thoracic aortic injury include hypotension, hypertension in the upper extremity and hypotension in the lower extremity, unequal BPs in the extremities, external evidence of chest wall trauma, and palpable fractures of the sternum and ribs.2 While it is unclear if this patient had unequal extremity BPs, she did not have any of the other classic findings of aortic rupture. Associated neurological, abdominal, or orthopedic injuries are frequently present as well, and can mask the subtle signs of aortic rupture.
A chest radiograph is often the initial screening test used to evaluate for possible thoracic aortic injury. Suspicious findings include a widened mediastinum (greater than 8 cm), right-sided deviation of the esophagus, depression of the left mainstem bronchus, loss of the aortic knob, and an apical pleural cap. Unfortunately, chest X-ray can be normal, and a normal mediastinum on the radiograph does not exclude the diagnosis.
For patients with suspected thoracic aortic injury, helical computed tomography with angiography is the study of choice. It can accurately identify operative and nonoperative lesions, as well as associated injuries (eg, small pneumothorax, rib fractures). Magnetic resonance angiography provides similar sensitivity and specificity, but is not practical for the majority of trauma patients. Occasionally, aortography can be considered when the CT scan results are indeterminate and when thought to be needed to plan operative intervention. Finally, transesophageal echocardiography can be considered in hemodynamically unstable patients unable to be transferred to the radiology suite.
For most patients, immediate operative intervention is the definitive treatment. For patients with suspected thoracic aortic injury and hypertension, shear forces need to be decreased just as they are for patients with aortic dissection. A short-acting β-blocker like intravenous (IV) esmolol can be used initially to slow HR. Then, an IV arterial vasodilator can be given to decrease BP. To prevent rebound tachycardia and increased shear forces, the β-blocker should always be initiated before the vasodilator is given. Vital-sign targets include an HR of 60 beats/minute and a systolic BP in the range of 100 to 120 mm Hg.
This was a very atypical presentation of a devastating injury. Given the benign presentation, lack of associated injuries, and the normal chest X-ray, a defense verdict appears to be the correct one in this very unfortunate case.
Foot Drop
A 20-year-old woman presented to the ED complaining of severe numbness, tingling, and pain in her left calf. According to the patient, she had attended a New Year’s Eve party, where she spent much of the time dancing. She was awakened by calf pain on the following morning and sought treatment at the ED.
On physical examination, the patient’s vital signs were normal. Examination of the left calf revealed tenderness to palpation; no swelling was noted. The patient was unable to lift her left foot or bear weight on the left leg. She had normal dorsalis pedis and posterior tibial pulses in the affected leg. The remainder of her examination was normal and no testing was performed. The patient was diagnosed with “floppy foot syndrome” and discharged home with a prescription for a nonsteroidal anti-inflammatory drug.
The next day, the patient presented to a different ED because of worsening pain and swelling of the calf. She was admitted to the hospital and the orthopedic service was consulted. The patient was diagnosed with compartment syndrome; however, by that time her condition was complicated by rhabdomyolysis, resulting in acute renal failure.
The patient underwent a fasciotomy. After surgery, she required hemodialysis until her kidney function returned. She had damage to the nerves in her left calf and leg resulting in a permanent foot drop that required prolonged physical therapy following her hospitalization.
The patient sued the initial EP for failure to diagnose compartment syndrome, which resulted in permanent nerve damage and foot drop. A $750,000 settlement was reached.
Discussion
The EP did not appear to have taken this case seriously, as “floppy foot syndrome” is not a recognized diagnosis. No significance was attached to the presence of the foot drop, which is an objective and concerning physical finding.
The differential diagnoses of foot drop are relatively small: a nerve injury, which is the most common cause; a central nervous system event, such as a stroke; or a muscular disorder.3 An injury or problem with the peroneal nerve is the most common cause of foot drop.
While the patient’s history was not typical for the development of compartment syndrome, she potentially participated in strenuous physical activity, which can result in muscle swelling and subsequent compartment syndrome.4 The pain from compartment syndrome is typically described as out of proportion to physical findings; this seems to have been the case for this patient.
The symptoms and findings of compartment syndrome are classically taught as the five “Ps”: pain, paresthesias, paralysis, pallor, and pulselessness, with the symptoms typically presenting in this order. The patient had the first three symptoms, but they were not appreciated in the initial evaluation. Pulselessness is usually the last finding to develop, and tissue damage is frequently present at that point.
Interestingly, approximately 40% of compartment syndromes occur at the level of the tibia and fibula. The lower leg has four compartments: the anterior, which contains the anterior tibial artery and deep peroneal nerve; the lateral, which contains the superficial peroneal nerve; the superficial posterior, which contains the sural nerve; and the deep posterior, which contains the posterior tibial artery and nerve.
As pressure within the enclosed space increases due to swelling, hemorrhage, fracture, etc, the blood supply as well as nerve and muscle functions become compromised. Left untreated, the increased pressure can result in permanent tissue and nerve damage.
Compartment syndrome is a time sensitive diagnosis because of the need for surgical intervention to open the compartment. The EP can measure compartment pressures if he or she has the right equipment and training. A normal compartment pressure is less than 10 mm Hg. When the compartment pressure begins to exceed 30 mm Hg, tissue damage can occur. If unable to measure compartment pressure, an emergent orthopedic consult is indicated.
Aortic Rupture
A 59-year-old woman involved in a motor vehicle crash presented to the ED via emergency medical services. The patient had been the front-seat passenger and was wearing a seat belt. She complained of chest wall pain, but denied head injury, loss of consciousness, neck pain, abdominal pain, or shortness of breath. Her past medical history was unremarkable.
A chest X-ray was performed and interpreted as normal by the attending radiologist. Laboratory studies were normal except for mild anemia. The patient was discharged from the hospital with a diagnosis of chest wall contusion. She died 36 hours later from a ruptured thoracic aorta. The family of the patient brought a malpractice suit against the emergency physician (EP) for failing to diagnose and treat acute aortic rupture. At trial, a defense verdict was returned.
Discussion
Aortic rupture from blunt trauma is a devastating injury. More than 90% of patients who have sustained this type of injury in a motor vehicle crash die at the scene.1 For the remaining 10%, 50% die within the following 24 hours.1 The injury occurs in the proximal descending aorta, secondary to the fixation of the vessels between the left subclavian artery and the ligamentum arteriosum; the cause in approximately 80% to 90% of cases is due to blunt trauma. Involvement of the ascending aorta is much less common. Many patients, such as the one in this case, exhibit no external physical findings of injury. Chest pain is the most frequent complaint, followed by dyspnea—both fairly nonspecific symptoms. Physical findings that should raise a suspicion for a thoracic aortic injury include hypotension, hypertension in the upper extremity and hypotension in the lower extremity, unequal BPs in the extremities, external evidence of chest wall trauma, and palpable fractures of the sternum and ribs.2 While it is unclear if this patient had unequal extremity BPs, she did not have any of the other classic findings of aortic rupture. Associated neurological, abdominal, or orthopedic injuries are frequently present as well, and can mask the subtle signs of aortic rupture.
A chest radiograph is often the initial screening test used to evaluate for possible thoracic aortic injury. Suspicious findings include a widened mediastinum (greater than 8 cm), right-sided deviation of the esophagus, depression of the left mainstem bronchus, loss of the aortic knob, and an apical pleural cap. Unfortunately, chest X-ray can be normal, and a normal mediastinum on the radiograph does not exclude the diagnosis.
For patients with suspected thoracic aortic injury, helical computed tomography with angiography is the study of choice. It can accurately identify operative and nonoperative lesions, as well as associated injuries (eg, small pneumothorax, rib fractures). Magnetic resonance angiography provides similar sensitivity and specificity, but is not practical for the majority of trauma patients. Occasionally, aortography can be considered when the CT scan results are indeterminate and when thought to be needed to plan operative intervention. Finally, transesophageal echocardiography can be considered in hemodynamically unstable patients unable to be transferred to the radiology suite.
For most patients, immediate operative intervention is the definitive treatment. For patients with suspected thoracic aortic injury and hypertension, shear forces need to be decreased just as they are for patients with aortic dissection. A short-acting β-blocker like intravenous (IV) esmolol can be used initially to slow HR. Then, an IV arterial vasodilator can be given to decrease BP. To prevent rebound tachycardia and increased shear forces, the β-blocker should always be initiated before the vasodilator is given. Vital-sign targets include an HR of 60 beats/minute and a systolic BP in the range of 100 to 120 mm Hg.
This was a very atypical presentation of a devastating injury. Given the benign presentation, lack of associated injuries, and the normal chest X-ray, a defense verdict appears to be the correct one in this very unfortunate case.
Foot Drop
A 20-year-old woman presented to the ED complaining of severe numbness, tingling, and pain in her left calf. According to the patient, she had attended a New Year’s Eve party, where she spent much of the time dancing. She was awakened by calf pain on the following morning and sought treatment at the ED.
On physical examination, the patient’s vital signs were normal. Examination of the left calf revealed tenderness to palpation; no swelling was noted. The patient was unable to lift her left foot or bear weight on the left leg. She had normal dorsalis pedis and posterior tibial pulses in the affected leg. The remainder of her examination was normal and no testing was performed. The patient was diagnosed with “floppy foot syndrome” and discharged home with a prescription for a nonsteroidal anti-inflammatory drug.
The next day, the patient presented to a different ED because of worsening pain and swelling of the calf. She was admitted to the hospital and the orthopedic service was consulted. The patient was diagnosed with compartment syndrome; however, by that time her condition was complicated by rhabdomyolysis, resulting in acute renal failure.
The patient underwent a fasciotomy. After surgery, she required hemodialysis until her kidney function returned. She had damage to the nerves in her left calf and leg resulting in a permanent foot drop that required prolonged physical therapy following her hospitalization.
The patient sued the initial EP for failure to diagnose compartment syndrome, which resulted in permanent nerve damage and foot drop. A $750,000 settlement was reached.
Discussion
The EP did not appear to have taken this case seriously, as “floppy foot syndrome” is not a recognized diagnosis. No significance was attached to the presence of the foot drop, which is an objective and concerning physical finding.
The differential diagnoses of foot drop are relatively small: a nerve injury, which is the most common cause; a central nervous system event, such as a stroke; or a muscular disorder.3 An injury or problem with the peroneal nerve is the most common cause of foot drop.
While the patient’s history was not typical for the development of compartment syndrome, she potentially participated in strenuous physical activity, which can result in muscle swelling and subsequent compartment syndrome.4 The pain from compartment syndrome is typically described as out of proportion to physical findings; this seems to have been the case for this patient.
The symptoms and findings of compartment syndrome are classically taught as the five “Ps”: pain, paresthesias, paralysis, pallor, and pulselessness, with the symptoms typically presenting in this order. The patient had the first three symptoms, but they were not appreciated in the initial evaluation. Pulselessness is usually the last finding to develop, and tissue damage is frequently present at that point.
Interestingly, approximately 40% of compartment syndromes occur at the level of the tibia and fibula. The lower leg has four compartments: the anterior, which contains the anterior tibial artery and deep peroneal nerve; the lateral, which contains the superficial peroneal nerve; the superficial posterior, which contains the sural nerve; and the deep posterior, which contains the posterior tibial artery and nerve.
As pressure within the enclosed space increases due to swelling, hemorrhage, fracture, etc, the blood supply as well as nerve and muscle functions become compromised. Left untreated, the increased pressure can result in permanent tissue and nerve damage.
Compartment syndrome is a time sensitive diagnosis because of the need for surgical intervention to open the compartment. The EP can measure compartment pressures if he or she has the right equipment and training. A normal compartment pressure is less than 10 mm Hg. When the compartment pressure begins to exceed 30 mm Hg, tissue damage can occur. If unable to measure compartment pressure, an emergent orthopedic consult is indicated.
- Chiesa R, de Moura MR, Lucci C, et al. Traumatic rupture of the thoracic aorta. Acta Chir Belq. 2003;103(4):364-374.
- Ross C, Schwab TM. Cardiac trauma. In: Tintinalli JE, Stapczynski JS, Cline DM, Ma OJ, Cydulka RK, Meckler GD. Ross C. In: Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York, NY: McGraw Hill Medical; 2011:1758-1765.
- Ricarte IF, Figueiredo MM, Fukuda TG, Pedroso JL, Silva GS. Acute foot drop syndrome mimicking peroneal nerve injury: an atypical presentation of ischemic stroke. J Stroke Cerebrovasc Dis. 2014:23(5):1229-1231.
- Aliano K, Gulati S, Stavrides S, Davenport T, Hines G. Low-impact trauma causing acute compartment syndrome of the lower extremities. Am J Emerg Med. 2013;31(5):890.e3-e4.
- Chiesa R, de Moura MR, Lucci C, et al. Traumatic rupture of the thoracic aorta. Acta Chir Belq. 2003;103(4):364-374.
- Ross C, Schwab TM. Cardiac trauma. In: Tintinalli JE, Stapczynski JS, Cline DM, Ma OJ, Cydulka RK, Meckler GD. Ross C. In: Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York, NY: McGraw Hill Medical; 2011:1758-1765.
- Ricarte IF, Figueiredo MM, Fukuda TG, Pedroso JL, Silva GS. Acute foot drop syndrome mimicking peroneal nerve injury: an atypical presentation of ischemic stroke. J Stroke Cerebrovasc Dis. 2014:23(5):1229-1231.
- Aliano K, Gulati S, Stavrides S, Davenport T, Hines G. Low-impact trauma causing acute compartment syndrome of the lower extremities. Am J Emerg Med. 2013;31(5):890.e3-e4.
Appropriate Analgesic Use in the Emergency Department
Pain, one of the most common reasons patients present to the ED, may be a primary complaint or a warning sign encouraging further evaluation. The decision to treat pain is one of the most frequent therapeutic decisions made by emergency physicians (EPs) and involves a variety of options and considerations. Moreover, the decision of how to treat pain similarly encompasses a wide selection of variables, including etiology and severity of the pain; intravenous (IV) access; medication allergies; renal function; alcohol use; rapidity of onset; patients’ vital signs; patient preference; and mode of transport upon discharge. Given all of these considerations, there is no perfect analgesic to suit every circumstance. Rather, EPs must tailor their analgesic selection to the individual clinical situation and patient.
The literature over the past 20 years is replete with studies demonstrating the undertreatment or inadequate treatment of pain in the ED.1-5 Often referred to as oligoanalgesia,6 contributing factors include physician concerns regarding adverse side effects, secondary gain, and drug addiction. In addition, the increasing pressure placed on EPs to diagnose and dispose patients quickly likely relegates pain control to a secondary concern.
Further complicating the issue, physicians’ own prejudices and perceptions appear to influence their analgesic prescription practice. For example, several studies have demonstrated that black patients do not receive prescriptions for analgesics similar to those written for white patients in general, and particularly not for opioid analgesics. In a meta-analysis of pain treatment disparity studies, blacks were 22% less likely than whites to receive any analgesics, and 29% less likely than whites to receive opioid treatment for the same type of painful conditions.7 Likewise, Hispanic/Latino patients were also 22% less likely than their white counterparts to receive opioid treatment for similar pain.7 Physicians must keep these common biases in mind when treating patients for pain.
The administration of analgesics and the prescription habits of physicians has never been under greater scrutiny. The Centers for Medicare and Medicaid Services has benchmarked “median time to pain management for long bone fractures” as a core measure, possibly affecting hospital reimbursement rates. Similarly, every patient satisfaction survey specifically inquires about the timeliness and adequacy of pain control. At the same time, though, the increasing problem of prescription opioid abuse has become the nation’s fastest growing drug problem. In 2013, prescription drug abuse was second only to marijuana as the most abused drug category.8 Contributing to this problem are the frequency and ease with which many physicians prescribe opioids. From 1997 to 2007, the milligram-per-person use of prescription opioids in the United States increased from 74 mg per year to 369 mg per year—an increase of 402%.9 As a result, some legislators are now calling for mandatory educational sessions for any physician prescribing medications containing opioids.
Though there are many classes of medications used to treat pain, and numerous individual drugs within each class, this article focuses on several of the more commonly prescribed medications in the ED, including their mechanisms of action, advantages, and disadvantages. The management of pediatric pain and procedural sedation and analgesia are not discussed in this review, as each of these topics deserves a separate detailed discussion.
Recognizing and Quantifying Pain
The first step in treating pain appropriately is recognition. Physicians must specifically inquire about pain and not rely solely on a patient’s unprompted complaint. Several pain scales exist, including the Faces Pain Scale (ie, pictorial representation of a smiling face on one end indicating “no pain” to a frowning face on the opposite end); the verbal quantitative scale or numerical rating scale (ie, “how would you rate your pain on a scale of 0 to 10, with 10 the worst pain ever?”); and the visual analog scale (ie, a 10-cm linear scale marked at one end with “no pain” and “worst pain imaginable” at the opposite end).10,11 Probably the most commonly used scale in the ED is some variation of the numerical rating scale (NRS).1
Each of these scales has its own advantages and disadvantages, but the important point is that patients are given the opportunity to express the type and degree of pain to the healthcare provider. In addition, a pain scale provides a starting point against which the practitioner (or later practitioners) can determine the success (or failure) of a pain treatment strategy.
Three-Step Ladder
In 1996, the World Health Organization developed a three-step analgesic ladder to guide the management of cancer pain.12 Its use has been expanded over time to include treating pain of noncancer etiology. Mild pain (NRS of 1 to 3) is considered Step 1; moderate pain (NRS 4 to 6) is considered Step 2; and severe pain (NRS 7 to 10) is Step 3. For Step 1 (mild pain), acetaminophen or a nonsteroidal anti-inflammatory drug (NSAID) is recommended. For Step 2 (moderate pain), a weak opioid (ie, codeine or hydrocodone) with or without acetaminophen or an NSAID is recommended. Finally, for Step 3 (severe pain), a strong opioid such as morphine or hydromorphone is recommended.
Again, the purpose of the ladder is not to provide a strict protocol for adherence, but rather to provide a reasonable starting point as a guide to the clinician. The key to its successful use is reassessment of the patient to determine if adequate pain relief is achieved.
Routes of Administration
Acetaminophen
Acetaminophen, the active ingredient in Tylenol, was first marketed in the United States in 1955 as an antipyretic and pain reliever.13 It is a synthetic centrally acting analgesic that is metabolized in the liver. Acetaminophen has been used alone or in combination in hundreds of formulations to treat a wide variety of pain and fever-related conditions. In the ED setting, acetaminophen is frequently used as an antipyretic and—either alone or in combination with opioids—for oral pain control.
Acetaminophen is very well tolerated by most patients, with minimal gastrointestinal (GI) distress. It is inexpensive, and the wide variety of formulations (eg, liquid, tablet, suppository) make it useful in a number of clinical scenarios. Acetaminophen is generally considered to be the only nonopioid analgesic that is safe in pregnancy,14 and it has no sedative or addictive effects.
There are, however, some disadvantages to using acetaminophen. Concerns about its safety and accidental overdose have recently led to the introduction of a 4,000-mg maximum daily dose recommendation15 and heightened concern over using multiple medications containing acetaminophen. In January 2014, the US Food and Drug Administration recommended that no combination medication contain more than 325 mg of acetaminophen because of the risk of toxicity when multiple drugs containing acetaminophen are consumed.
In addition to concerns of inadvertent overdose, another disadvantage of acetaminophen is that while it may be an effective antipyretic and analgesic, it has little or no anti-inflammatory properties. Therefore, in cases requiring an anti-inflammatory agent, an NSAID, when appropriate, would be the more effective option.
NSAIDs
Nonsteroidal anti-inflammatory drugs function by inhibiting prostaglandin production in the cyclooxygenase 1 and 2 (COX-1 and COX-2) pathways. Ibuprofen, indomethacin, ketorolac, naproxen, and other NSAIDs are chiefly utilized to control pain, inflammation, and fever via the oral route. As production of various prostaglandins via the COX-2 pathway is thought to contribute to fever, inflammation, and pain, inhibition of this pathway by NSAIDs can help alleviate these symptoms. The COX-1 pathway contributes many factors important to the protection and health of the GI tract, and inhibition of this pathway can lead to GI distress and damage. Unfortunately, the most commonly used and available NSAIDs inhibit both COX pathways simultaneously, and in doing so, prompt the GI symptoms which are the most common adverse side effects of therapy. In addition to GI effects of the COX-1 and COX-2 inhibitors, there are also concerns over the associated antiplatelet effects in patients undergoing surgery or potentially suffering from occult or intracranial bleeding.13
Ibuprofen
This is the most commonly used NSAID in the United States and is available without a prescription. Ibuprofen is typically used to treat mild-to-moderate pain from a musculoskeletal or inflammatory source. As an oral nonprescription medication, it can be used advantageously to treat acute pain in the ED and continued in the outpatient setting. Ibuprofen is neither sedating nor addicting, with a rapid onset of action and a plasma half-life of approximately 2 hours.13 However, there is a dose-dependent feature which allows large doses (eg, 800 mg) to be spaced-out every 8 hours while maintaining effective analgesia. Typical doses range from 200 mg to 800 mg orally every 6 to 8 hours, with a maximum dose of 3.2 g/d. Patients should be instructed to take each dose with a meal or snack to help alleviate GI side effects.
The greatest advantage of ibuprofen and other NSAIDs is their effect on inflammation and the ability to treat the inflammatory cause of pain—not just the symptom. Nonsteroidal anti-inflammatory drugs are well tolerated by most patients and can be obtained without prescription at low cost. Additionally, doses of ibuprofen and acetaminophen can be alternated.
Ketorolac
Often marketed as Toradol, ketorolac is a powerful NSAID available in IV, IM, and oral formulations. Typical doses are 30 mg IV, 60 mg IM, or 10 mg orally every 4 to 6 hours (maximum of 40 mg/d). The basic pharmacology and mechanism of action of ketorolac are similar to ibuprofen. Though ketorolac is useful to treat more severe pain, it should only be used for short-term management of pain (ie, 5 days or less). Ketorolac is often used for postoperative pain, but also is helpful for pain control in patients using opioids. It has been shown to be effective for acute renal colic and can also provide relief for migraine headaches.16,17 In a direct comparison between ketorolac and meperidine (Demerol) for patients suffering from renal stones, ketorolac was found to be more effective and provide longer lasting pain relief.18
The major concern regarding ketorolac relates to potential renal toxicity, and thus caution should be undertaken in prescribing it to patients with known or suspected renal disease. Although this risk is associated with even a single dose, multiple doses increase the danger and therefore should be avoided.
Ketorolac should also be used with caution in patients with asthma. As a subset of asthma patients will experience severe bronchospasm after NSAID administration, clinicians should always determine whether a patient can tolerate NSAIDs.
Renal Toxicity and GI Effects
Concerns over renal toxicity and potential GI distress are the chief disadvantages of NSAID use. While renal toxicity has been reported in patients without pre-existing kidney dysfunction, it is of much greater concern in patients with pre-existing renal disease or decreased glomerular filtration rate. For this reason, care must be exercised when prescribing NSAIDS to elderly patients, patients with diabetes mellitus, or patients with hypertension (or even worse, a combination of these). Prolonged use of NSAIDs can also cause upper GI bleeding. Nonsteroidal anti-inflammatory drugs are contraindicated in pregnant patients.
Opioid analgesics
Morphine
Morphine is the prototypical compound in the opioid class, and has been utilized for more than two centuries since its isolation in 1804.19 Other opioid compounds include codeine, hydrocodone, oxycodone, morphine, and hydromorphone, which represent different functional groups substituted onto the base morphine molecule. All share similar pharmacology, differing primarily only in potency. The onset of action for codeine, hydrocodone, and oxycodone is 30 to 60 minutes when taken orally, with a peak time of 60 to 90 minutes. Codeine and hydrocodone are at the weak-end of the spectrum (Step 2 medications), while morphine and hydromorphone are more potent (Step 3 medications). The majority of opioids and their metabolites are primarily excreted renally (90%-95%).20 Therefore, care must be exercised regarding dosing and frequency when used in patients with renal insufficiency or disease.
Morphine has several features that make it an attractive analgesic for use in the ED. The ability to administer morphine via IV, IM, subcutaneous, or oral route; its quick onset of action; and safety profile are all advantageous. The onset of action is 5 to 10 minutes when given IV. For oral administration, it is 30 to 60 minutes (similar to the other opioids discussed) and can be given as a tablet or syrup.
Morphine decreases the severity of pain, with an apparent increase in tolerance for any remaining discomfort. The potency and central action of morphine make it ideal for managing moderate and severe pain.
As with other analgesics, morphine and its related compounds have associated drawbacks, of which respiratory depression is the most feared, though this is uncommon at therapeutic doses. The respiratory depressant effect is more pronounced in patients with underlying lung disease, depressed mental status, or concurrent use of sedating medication (eg, benzodiazepines).
Another disadvantage to morphine is that it can cause hypotension, limiting its use in certain clinical situations. Repeated or prolonged use may cause slowing or complete arrest of peristaltic waves in the GI tract, which can lead to significant constipation. Pruritus and nausea are two other frequently reported side effects and may necessitate, respectively, coadministration of diphenhydramine (eg, Benadryl) or an antiemetic (eg, Phenergan or Zofran). As an opioid, morphine is potentially addictive, though it is generally thought to require significant intake over several weeks before such problems arise.
The sedation caused by morphine is also of concern, as patients who are under its influence immediately following discharge cannot safely drive, and may actually not be safe to walk or even utilize public transportation. This concern can be lessened by verifying the patient’s mode of transport prior to administration of the medication, ensuring that patients are not overly sedated at the time of discharge and, as much as possible, accompanied home by an adult relative or friend. In comparison to other frequently used opioids, several features can be considered. The duration of action for codeine, hydromorphone, and morphine is similar at 3 to 5 hours.
Hydromorphone
Hydromorphone (Dilaudid) is approximately seven times more potent than morphine, but otherwise very similar. It is frequently the IV opioid of choice for severe pain and also appears to cause pruritus less frequently than morphine. Hydromorphone has a rapid onset of action (1-5 minutes IV; 15-30 minutes orally) and can be titrated to effect when given via the IV route, making it an ideal agent for the pain associated with long bone or pelvic fractures, vasoocclusive crises in patients with sickle cell disease, and renal colic. The oral formulation of hydromorphone can be utilized for severe pain in the appropriate patient population, usually at a dose of 2 to 4 mg every 4 hours.
Codeine
Codeine is frequently prescribed in combination with acetaminophen in a product marketed as Tylenol #3 for mild-to-moderate pain. Because codeine is metabolized in the liver to morphine, it is contraindicated in patients with morphine allergy. Also, the clinician must be aware that codeine is ineffective in 7% to 10% of the population due to an enzyme deficiency.
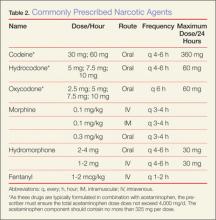
Hydrocodone and oxycodone, which are often combined with acetaminophen and marketed respectively as Vicodin and Percocet, are two additional commonly used compounds in the oral treatment of moderate-to-severe pain. There are numerous preparations containing various strengths of both the opioid and acetaminophen components. Utilizing these medications as initial pain control in the ED can be beneficial, as therapy can be continued as an outpatient. Table 2 provides dosing and frequency information, but the clinician must also be aware of the total amount of acetaminophen administered, especially when being used in conjunction with other medications containing acetaminophen.
Tramadol
Tramadol (Ultram), another oral analgesic with opioid properties, affects several neurotransmitters and is less reliant on the opioid receptors compared to the opioid compounds described above. It is used to treat moderate-to-severe pain and can be considered another option along with codeine, hydrocodone, and oxycodone for oral pain control. Although the complete mechanism of action for tramadol is poorly understood, it appears to be effective in patients who do not respond well to pure opioids, and in patients with neuropathic pain or persistent pain of unclear etiology (eg, fibromyalgia).19 Because of its limited interaction with opioid receptors, tramadol has less potential for abuse and addiction than the opioids. It is also available in several combinations with acetaminophen or ibuprofen.
Conclusion
The ideal medication for treating pain in the ED is one that is effective, easy to administer, and has minimal adverse or residual effects. Generally, the least potent medication that will control pain should be chosen initially. When treating minor traumatic or musculoskeletal pain, initiating pain control with oral medication in the ED may provide patients with relief during imaging or splinting, and help control pain until they can fill outpatient prescriptions. All of the medications discussed in this article can be given in the oral form. However, IV usage may be appropriate when there is need for rapid onset of action, to titrate the medication, or for ease of administration in patients who are vomiting or unable to take anything orally.
When prescribing analgesics for pain control after discharge, the clinician must consider potential relative and absolute contraindications. For acetaminophen, liver toxicity is the major concern, and care must be taken not to exceed recommended daily dosing limits with the multitude of available products containing acetaminophen. Physicians should avoid recommending or prescribing acetaminophen to patients with liver disease (ie, cirrhosis) or habitual alcohol abuse.
Nonsteroidal anti-inflammatory drugs such as ibuprofen and naproxen carry the risk of renal damage, as well as concerns about antiplatelet activity. Because of the tendency for renal function to decline with age, caution must be exercised in using these compounds in older patients and those with preexisting renal dysfunction.
Opioid-containing medications are known for their potency in pain control, but also for their side effects. The most common side effect involves the GI tract, with nausea, vomiting, and constipation. The most dangerous side effect of opioids is respiratory depression; fortunately, this is rarely seen with therapeutic doses. However, somnolence and decreased coordination are significant concerns when prescribing opioids to elderly patients or those already at risk for falls.
There is no ideal single medication to treat all types of pain or situations. However, the choice of medication begins with a decision by the EP to treat pain. Utilizing a stepwise approach, oral medication will usually be effective for mild pain, but IV narcotics will probably be required for severe pain. Frequent reassessment of the patient is critically important for successful pain management in the ED.
Dr Byers is an emergency physician at the Presbyterian Medical Group, department of emergency medicine, Presbyterian Healthcare Services, Albuquerque, New Mexico. Dr Counselman is a distinguished professor of emergency medicine and chairman, department of emergency medicine, Eastern Virginia Medical School and Emergency Physicians of Tidewater, Norfolk, Virginia. He is also associate editor-in-chief of the Emergency Medicine editorial board.
- Todd KH. Pain assessment instruments for use in the emergency department. Emerg Med Clin North Am. 2005;23(2):285-295.
- Cordell WH, Keene KK, Giles BK, Jones JB, Jones JH, Brizendine EJ. The high prevalence of pain in emergency medical care. Am J Emerg Med. 2002;20(3):165-169.
- Tcherny-Lessenot S, Karwowski-Soulié F, Lamarche-Vadel A, Ginsburg C, Brunet F, Vidal-Trecan G. Management and relief of pain in an emergency department from the adult patients’ perspective. J Pain Symptom Manage. 2003;25(6):539-546.
- Karwowski-Soulié F, Lessenot-Tcherny S, Lamarche-Vadel A, et al. Pain in an emergency department: an audit. Eur J Emerg Med. 2006;13(4):218-224.
- Fosnocht DE, Swanson ER, Barton ED. Changing attitudes about pain and pain control in emergency medicine. Emerg Med Clin North Am. 2005;23(2):297-306.
- Wilson JE, Pendelton JM. Oligoanalgesia in the emergency department. Am J Emerg Med. 1989;7(6):620-623.
- Meghani SH, Byun E, Gallagher RM. Time to take stock: a meta-analysis and systemic review of analgesic treatment disparities for pain in the United States. Pain Med. 2012;13(2):150-174.
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schutenberg JE; The University of Michigan Institute for Social Research. Monitoring the future: 2013 overview key findings of on adolescent drug use. http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2013.pdf. Accessed June 6, 2014.
- Manchikanti L, Fellow B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: a ten year perspective. Pain Physician. 2010;13(5):401-435.
- Silka PA, Roth MM, Moreno G, Merrill L, Geiderman JM. Pain scores improve analgesic administration patterns for trauma patients in the emergency department. Acad Emerg Med. 2004;11(3):264-270.
- Nelson BP, Cohen D, Lander O, Crawford N, Viccellio AW, Singer AJ. Mandated pain scales improve frequency of ED analgesic administration. Am J Emerg Med. 2004;22(7):582-585.
- World Health Organization. Cancer pain relief with a guide to opioid availability 2nd ed. 1996. http://whqlibdoc.who.int/publications/9241544821.pdf. Accessed June 6, 2014.
- Grosser T, Smyth EM, FitzGerald GA. Anti-inflammatory, antipyretic, and analgesic agents: pharmacotherapy of gout. In: Brunton LL, Chabner BA, Knollman BC, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. China: The McGraw Hill Companies; 2011:959-1004.
- Scialli AR, Ang R, Breitmeyer J, Royal MA. A review of the literature on the effects of acetaminophen on pregnancy outcome. Reprod Toxicol. 2010;30(4):495-507.
- Schilling A, Corey R, Leonard M, Eghtesad B. Acetaminophen: old drug, new warnings. Cleveland Clin J Med. 2010;77(1):19-27.
- Shrestha M, Singh R, Moreden J, Hayes JE. Ketorolac vs chlorpromazine in the treatment of acute migraine without aura. A prospective, randomized, double-blind trial. Arch Intern Med.1996;156(15):1725-1728.
- Turkewitz LJ, Casaly JS, Dawson GA, Wirth O, Hurst RJ, Gillette PL. Self-administration of parenteral ketorolac tromethamine for head pain. Headache. 1992;32(9):452-454.
- Larkin GL, Peacock WF 4th, Pearl SM, Blair GA, D’Amico F. Efficacy of ketorolac tromethamine versus meperidine in the ED treatment of acute renal colic. Am J Emerg Med. 1999;17(1):6-10.
- Yaksh TL, Wallace MS. Opiods, analgesia and pain management. In: Brunton LL, Chabner BA, Knollman BC, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. China: The McGraw Hill Companies; 2011:481-526.
- DeSandre PL, Quest TE. Management of cancer-related pain. Hematol Oncol Clin N Am. 2010;24(3):643-658.
Pain, one of the most common reasons patients present to the ED, may be a primary complaint or a warning sign encouraging further evaluation. The decision to treat pain is one of the most frequent therapeutic decisions made by emergency physicians (EPs) and involves a variety of options and considerations. Moreover, the decision of how to treat pain similarly encompasses a wide selection of variables, including etiology and severity of the pain; intravenous (IV) access; medication allergies; renal function; alcohol use; rapidity of onset; patients’ vital signs; patient preference; and mode of transport upon discharge. Given all of these considerations, there is no perfect analgesic to suit every circumstance. Rather, EPs must tailor their analgesic selection to the individual clinical situation and patient.
The literature over the past 20 years is replete with studies demonstrating the undertreatment or inadequate treatment of pain in the ED.1-5 Often referred to as oligoanalgesia,6 contributing factors include physician concerns regarding adverse side effects, secondary gain, and drug addiction. In addition, the increasing pressure placed on EPs to diagnose and dispose patients quickly likely relegates pain control to a secondary concern.
Further complicating the issue, physicians’ own prejudices and perceptions appear to influence their analgesic prescription practice. For example, several studies have demonstrated that black patients do not receive prescriptions for analgesics similar to those written for white patients in general, and particularly not for opioid analgesics. In a meta-analysis of pain treatment disparity studies, blacks were 22% less likely than whites to receive any analgesics, and 29% less likely than whites to receive opioid treatment for the same type of painful conditions.7 Likewise, Hispanic/Latino patients were also 22% less likely than their white counterparts to receive opioid treatment for similar pain.7 Physicians must keep these common biases in mind when treating patients for pain.
The administration of analgesics and the prescription habits of physicians has never been under greater scrutiny. The Centers for Medicare and Medicaid Services has benchmarked “median time to pain management for long bone fractures” as a core measure, possibly affecting hospital reimbursement rates. Similarly, every patient satisfaction survey specifically inquires about the timeliness and adequacy of pain control. At the same time, though, the increasing problem of prescription opioid abuse has become the nation’s fastest growing drug problem. In 2013, prescription drug abuse was second only to marijuana as the most abused drug category.8 Contributing to this problem are the frequency and ease with which many physicians prescribe opioids. From 1997 to 2007, the milligram-per-person use of prescription opioids in the United States increased from 74 mg per year to 369 mg per year—an increase of 402%.9 As a result, some legislators are now calling for mandatory educational sessions for any physician prescribing medications containing opioids.
Though there are many classes of medications used to treat pain, and numerous individual drugs within each class, this article focuses on several of the more commonly prescribed medications in the ED, including their mechanisms of action, advantages, and disadvantages. The management of pediatric pain and procedural sedation and analgesia are not discussed in this review, as each of these topics deserves a separate detailed discussion.
Recognizing and Quantifying Pain
The first step in treating pain appropriately is recognition. Physicians must specifically inquire about pain and not rely solely on a patient’s unprompted complaint. Several pain scales exist, including the Faces Pain Scale (ie, pictorial representation of a smiling face on one end indicating “no pain” to a frowning face on the opposite end); the verbal quantitative scale or numerical rating scale (ie, “how would you rate your pain on a scale of 0 to 10, with 10 the worst pain ever?”); and the visual analog scale (ie, a 10-cm linear scale marked at one end with “no pain” and “worst pain imaginable” at the opposite end).10,11 Probably the most commonly used scale in the ED is some variation of the numerical rating scale (NRS).1
Each of these scales has its own advantages and disadvantages, but the important point is that patients are given the opportunity to express the type and degree of pain to the healthcare provider. In addition, a pain scale provides a starting point against which the practitioner (or later practitioners) can determine the success (or failure) of a pain treatment strategy.
Three-Step Ladder
In 1996, the World Health Organization developed a three-step analgesic ladder to guide the management of cancer pain.12 Its use has been expanded over time to include treating pain of noncancer etiology. Mild pain (NRS of 1 to 3) is considered Step 1; moderate pain (NRS 4 to 6) is considered Step 2; and severe pain (NRS 7 to 10) is Step 3. For Step 1 (mild pain), acetaminophen or a nonsteroidal anti-inflammatory drug (NSAID) is recommended. For Step 2 (moderate pain), a weak opioid (ie, codeine or hydrocodone) with or without acetaminophen or an NSAID is recommended. Finally, for Step 3 (severe pain), a strong opioid such as morphine or hydromorphone is recommended.
Again, the purpose of the ladder is not to provide a strict protocol for adherence, but rather to provide a reasonable starting point as a guide to the clinician. The key to its successful use is reassessment of the patient to determine if adequate pain relief is achieved.
Routes of Administration
Acetaminophen
Acetaminophen, the active ingredient in Tylenol, was first marketed in the United States in 1955 as an antipyretic and pain reliever.13 It is a synthetic centrally acting analgesic that is metabolized in the liver. Acetaminophen has been used alone or in combination in hundreds of formulations to treat a wide variety of pain and fever-related conditions. In the ED setting, acetaminophen is frequently used as an antipyretic and—either alone or in combination with opioids—for oral pain control.
Acetaminophen is very well tolerated by most patients, with minimal gastrointestinal (GI) distress. It is inexpensive, and the wide variety of formulations (eg, liquid, tablet, suppository) make it useful in a number of clinical scenarios. Acetaminophen is generally considered to be the only nonopioid analgesic that is safe in pregnancy,14 and it has no sedative or addictive effects.
There are, however, some disadvantages to using acetaminophen. Concerns about its safety and accidental overdose have recently led to the introduction of a 4,000-mg maximum daily dose recommendation15 and heightened concern over using multiple medications containing acetaminophen. In January 2014, the US Food and Drug Administration recommended that no combination medication contain more than 325 mg of acetaminophen because of the risk of toxicity when multiple drugs containing acetaminophen are consumed.
In addition to concerns of inadvertent overdose, another disadvantage of acetaminophen is that while it may be an effective antipyretic and analgesic, it has little or no anti-inflammatory properties. Therefore, in cases requiring an anti-inflammatory agent, an NSAID, when appropriate, would be the more effective option.
NSAIDs
Nonsteroidal anti-inflammatory drugs function by inhibiting prostaglandin production in the cyclooxygenase 1 and 2 (COX-1 and COX-2) pathways. Ibuprofen, indomethacin, ketorolac, naproxen, and other NSAIDs are chiefly utilized to control pain, inflammation, and fever via the oral route. As production of various prostaglandins via the COX-2 pathway is thought to contribute to fever, inflammation, and pain, inhibition of this pathway by NSAIDs can help alleviate these symptoms. The COX-1 pathway contributes many factors important to the protection and health of the GI tract, and inhibition of this pathway can lead to GI distress and damage. Unfortunately, the most commonly used and available NSAIDs inhibit both COX pathways simultaneously, and in doing so, prompt the GI symptoms which are the most common adverse side effects of therapy. In addition to GI effects of the COX-1 and COX-2 inhibitors, there are also concerns over the associated antiplatelet effects in patients undergoing surgery or potentially suffering from occult or intracranial bleeding.13
Ibuprofen
This is the most commonly used NSAID in the United States and is available without a prescription. Ibuprofen is typically used to treat mild-to-moderate pain from a musculoskeletal or inflammatory source. As an oral nonprescription medication, it can be used advantageously to treat acute pain in the ED and continued in the outpatient setting. Ibuprofen is neither sedating nor addicting, with a rapid onset of action and a plasma half-life of approximately 2 hours.13 However, there is a dose-dependent feature which allows large doses (eg, 800 mg) to be spaced-out every 8 hours while maintaining effective analgesia. Typical doses range from 200 mg to 800 mg orally every 6 to 8 hours, with a maximum dose of 3.2 g/d. Patients should be instructed to take each dose with a meal or snack to help alleviate GI side effects.
The greatest advantage of ibuprofen and other NSAIDs is their effect on inflammation and the ability to treat the inflammatory cause of pain—not just the symptom. Nonsteroidal anti-inflammatory drugs are well tolerated by most patients and can be obtained without prescription at low cost. Additionally, doses of ibuprofen and acetaminophen can be alternated.
Ketorolac
Often marketed as Toradol, ketorolac is a powerful NSAID available in IV, IM, and oral formulations. Typical doses are 30 mg IV, 60 mg IM, or 10 mg orally every 4 to 6 hours (maximum of 40 mg/d). The basic pharmacology and mechanism of action of ketorolac are similar to ibuprofen. Though ketorolac is useful to treat more severe pain, it should only be used for short-term management of pain (ie, 5 days or less). Ketorolac is often used for postoperative pain, but also is helpful for pain control in patients using opioids. It has been shown to be effective for acute renal colic and can also provide relief for migraine headaches.16,17 In a direct comparison between ketorolac and meperidine (Demerol) for patients suffering from renal stones, ketorolac was found to be more effective and provide longer lasting pain relief.18
The major concern regarding ketorolac relates to potential renal toxicity, and thus caution should be undertaken in prescribing it to patients with known or suspected renal disease. Although this risk is associated with even a single dose, multiple doses increase the danger and therefore should be avoided.
Ketorolac should also be used with caution in patients with asthma. As a subset of asthma patients will experience severe bronchospasm after NSAID administration, clinicians should always determine whether a patient can tolerate NSAIDs.
Renal Toxicity and GI Effects
Concerns over renal toxicity and potential GI distress are the chief disadvantages of NSAID use. While renal toxicity has been reported in patients without pre-existing kidney dysfunction, it is of much greater concern in patients with pre-existing renal disease or decreased glomerular filtration rate. For this reason, care must be exercised when prescribing NSAIDS to elderly patients, patients with diabetes mellitus, or patients with hypertension (or even worse, a combination of these). Prolonged use of NSAIDs can also cause upper GI bleeding. Nonsteroidal anti-inflammatory drugs are contraindicated in pregnant patients.
Opioid analgesics
Morphine
Morphine is the prototypical compound in the opioid class, and has been utilized for more than two centuries since its isolation in 1804.19 Other opioid compounds include codeine, hydrocodone, oxycodone, morphine, and hydromorphone, which represent different functional groups substituted onto the base morphine molecule. All share similar pharmacology, differing primarily only in potency. The onset of action for codeine, hydrocodone, and oxycodone is 30 to 60 minutes when taken orally, with a peak time of 60 to 90 minutes. Codeine and hydrocodone are at the weak-end of the spectrum (Step 2 medications), while morphine and hydromorphone are more potent (Step 3 medications). The majority of opioids and their metabolites are primarily excreted renally (90%-95%).20 Therefore, care must be exercised regarding dosing and frequency when used in patients with renal insufficiency or disease.
Morphine has several features that make it an attractive analgesic for use in the ED. The ability to administer morphine via IV, IM, subcutaneous, or oral route; its quick onset of action; and safety profile are all advantageous. The onset of action is 5 to 10 minutes when given IV. For oral administration, it is 30 to 60 minutes (similar to the other opioids discussed) and can be given as a tablet or syrup.
Morphine decreases the severity of pain, with an apparent increase in tolerance for any remaining discomfort. The potency and central action of morphine make it ideal for managing moderate and severe pain.
As with other analgesics, morphine and its related compounds have associated drawbacks, of which respiratory depression is the most feared, though this is uncommon at therapeutic doses. The respiratory depressant effect is more pronounced in patients with underlying lung disease, depressed mental status, or concurrent use of sedating medication (eg, benzodiazepines).
Another disadvantage to morphine is that it can cause hypotension, limiting its use in certain clinical situations. Repeated or prolonged use may cause slowing or complete arrest of peristaltic waves in the GI tract, which can lead to significant constipation. Pruritus and nausea are two other frequently reported side effects and may necessitate, respectively, coadministration of diphenhydramine (eg, Benadryl) or an antiemetic (eg, Phenergan or Zofran). As an opioid, morphine is potentially addictive, though it is generally thought to require significant intake over several weeks before such problems arise.
The sedation caused by morphine is also of concern, as patients who are under its influence immediately following discharge cannot safely drive, and may actually not be safe to walk or even utilize public transportation. This concern can be lessened by verifying the patient’s mode of transport prior to administration of the medication, ensuring that patients are not overly sedated at the time of discharge and, as much as possible, accompanied home by an adult relative or friend. In comparison to other frequently used opioids, several features can be considered. The duration of action for codeine, hydromorphone, and morphine is similar at 3 to 5 hours.
Hydromorphone
Hydromorphone (Dilaudid) is approximately seven times more potent than morphine, but otherwise very similar. It is frequently the IV opioid of choice for severe pain and also appears to cause pruritus less frequently than morphine. Hydromorphone has a rapid onset of action (1-5 minutes IV; 15-30 minutes orally) and can be titrated to effect when given via the IV route, making it an ideal agent for the pain associated with long bone or pelvic fractures, vasoocclusive crises in patients with sickle cell disease, and renal colic. The oral formulation of hydromorphone can be utilized for severe pain in the appropriate patient population, usually at a dose of 2 to 4 mg every 4 hours.
Codeine
Codeine is frequently prescribed in combination with acetaminophen in a product marketed as Tylenol #3 for mild-to-moderate pain. Because codeine is metabolized in the liver to morphine, it is contraindicated in patients with morphine allergy. Also, the clinician must be aware that codeine is ineffective in 7% to 10% of the population due to an enzyme deficiency.
Hydrocodone and oxycodone, which are often combined with acetaminophen and marketed respectively as Vicodin and Percocet, are two additional commonly used compounds in the oral treatment of moderate-to-severe pain. There are numerous preparations containing various strengths of both the opioid and acetaminophen components. Utilizing these medications as initial pain control in the ED can be beneficial, as therapy can be continued as an outpatient. Table 2 provides dosing and frequency information, but the clinician must also be aware of the total amount of acetaminophen administered, especially when being used in conjunction with other medications containing acetaminophen.
Tramadol
Tramadol (Ultram), another oral analgesic with opioid properties, affects several neurotransmitters and is less reliant on the opioid receptors compared to the opioid compounds described above. It is used to treat moderate-to-severe pain and can be considered another option along with codeine, hydrocodone, and oxycodone for oral pain control. Although the complete mechanism of action for tramadol is poorly understood, it appears to be effective in patients who do not respond well to pure opioids, and in patients with neuropathic pain or persistent pain of unclear etiology (eg, fibromyalgia).19 Because of its limited interaction with opioid receptors, tramadol has less potential for abuse and addiction than the opioids. It is also available in several combinations with acetaminophen or ibuprofen.
Conclusion
The ideal medication for treating pain in the ED is one that is effective, easy to administer, and has minimal adverse or residual effects. Generally, the least potent medication that will control pain should be chosen initially. When treating minor traumatic or musculoskeletal pain, initiating pain control with oral medication in the ED may provide patients with relief during imaging or splinting, and help control pain until they can fill outpatient prescriptions. All of the medications discussed in this article can be given in the oral form. However, IV usage may be appropriate when there is need for rapid onset of action, to titrate the medication, or for ease of administration in patients who are vomiting or unable to take anything orally.
When prescribing analgesics for pain control after discharge, the clinician must consider potential relative and absolute contraindications. For acetaminophen, liver toxicity is the major concern, and care must be taken not to exceed recommended daily dosing limits with the multitude of available products containing acetaminophen. Physicians should avoid recommending or prescribing acetaminophen to patients with liver disease (ie, cirrhosis) or habitual alcohol abuse.
Nonsteroidal anti-inflammatory drugs such as ibuprofen and naproxen carry the risk of renal damage, as well as concerns about antiplatelet activity. Because of the tendency for renal function to decline with age, caution must be exercised in using these compounds in older patients and those with preexisting renal dysfunction.
Opioid-containing medications are known for their potency in pain control, but also for their side effects. The most common side effect involves the GI tract, with nausea, vomiting, and constipation. The most dangerous side effect of opioids is respiratory depression; fortunately, this is rarely seen with therapeutic doses. However, somnolence and decreased coordination are significant concerns when prescribing opioids to elderly patients or those already at risk for falls.
There is no ideal single medication to treat all types of pain or situations. However, the choice of medication begins with a decision by the EP to treat pain. Utilizing a stepwise approach, oral medication will usually be effective for mild pain, but IV narcotics will probably be required for severe pain. Frequent reassessment of the patient is critically important for successful pain management in the ED.
Dr Byers is an emergency physician at the Presbyterian Medical Group, department of emergency medicine, Presbyterian Healthcare Services, Albuquerque, New Mexico. Dr Counselman is a distinguished professor of emergency medicine and chairman, department of emergency medicine, Eastern Virginia Medical School and Emergency Physicians of Tidewater, Norfolk, Virginia. He is also associate editor-in-chief of the Emergency Medicine editorial board.
Pain, one of the most common reasons patients present to the ED, may be a primary complaint or a warning sign encouraging further evaluation. The decision to treat pain is one of the most frequent therapeutic decisions made by emergency physicians (EPs) and involves a variety of options and considerations. Moreover, the decision of how to treat pain similarly encompasses a wide selection of variables, including etiology and severity of the pain; intravenous (IV) access; medication allergies; renal function; alcohol use; rapidity of onset; patients’ vital signs; patient preference; and mode of transport upon discharge. Given all of these considerations, there is no perfect analgesic to suit every circumstance. Rather, EPs must tailor their analgesic selection to the individual clinical situation and patient.
The literature over the past 20 years is replete with studies demonstrating the undertreatment or inadequate treatment of pain in the ED.1-5 Often referred to as oligoanalgesia,6 contributing factors include physician concerns regarding adverse side effects, secondary gain, and drug addiction. In addition, the increasing pressure placed on EPs to diagnose and dispose patients quickly likely relegates pain control to a secondary concern.
Further complicating the issue, physicians’ own prejudices and perceptions appear to influence their analgesic prescription practice. For example, several studies have demonstrated that black patients do not receive prescriptions for analgesics similar to those written for white patients in general, and particularly not for opioid analgesics. In a meta-analysis of pain treatment disparity studies, blacks were 22% less likely than whites to receive any analgesics, and 29% less likely than whites to receive opioid treatment for the same type of painful conditions.7 Likewise, Hispanic/Latino patients were also 22% less likely than their white counterparts to receive opioid treatment for similar pain.7 Physicians must keep these common biases in mind when treating patients for pain.
The administration of analgesics and the prescription habits of physicians has never been under greater scrutiny. The Centers for Medicare and Medicaid Services has benchmarked “median time to pain management for long bone fractures” as a core measure, possibly affecting hospital reimbursement rates. Similarly, every patient satisfaction survey specifically inquires about the timeliness and adequacy of pain control. At the same time, though, the increasing problem of prescription opioid abuse has become the nation’s fastest growing drug problem. In 2013, prescription drug abuse was second only to marijuana as the most abused drug category.8 Contributing to this problem are the frequency and ease with which many physicians prescribe opioids. From 1997 to 2007, the milligram-per-person use of prescription opioids in the United States increased from 74 mg per year to 369 mg per year—an increase of 402%.9 As a result, some legislators are now calling for mandatory educational sessions for any physician prescribing medications containing opioids.
Though there are many classes of medications used to treat pain, and numerous individual drugs within each class, this article focuses on several of the more commonly prescribed medications in the ED, including their mechanisms of action, advantages, and disadvantages. The management of pediatric pain and procedural sedation and analgesia are not discussed in this review, as each of these topics deserves a separate detailed discussion.
Recognizing and Quantifying Pain
The first step in treating pain appropriately is recognition. Physicians must specifically inquire about pain and not rely solely on a patient’s unprompted complaint. Several pain scales exist, including the Faces Pain Scale (ie, pictorial representation of a smiling face on one end indicating “no pain” to a frowning face on the opposite end); the verbal quantitative scale or numerical rating scale (ie, “how would you rate your pain on a scale of 0 to 10, with 10 the worst pain ever?”); and the visual analog scale (ie, a 10-cm linear scale marked at one end with “no pain” and “worst pain imaginable” at the opposite end).10,11 Probably the most commonly used scale in the ED is some variation of the numerical rating scale (NRS).1
Each of these scales has its own advantages and disadvantages, but the important point is that patients are given the opportunity to express the type and degree of pain to the healthcare provider. In addition, a pain scale provides a starting point against which the practitioner (or later practitioners) can determine the success (or failure) of a pain treatment strategy.
Three-Step Ladder
In 1996, the World Health Organization developed a three-step analgesic ladder to guide the management of cancer pain.12 Its use has been expanded over time to include treating pain of noncancer etiology. Mild pain (NRS of 1 to 3) is considered Step 1; moderate pain (NRS 4 to 6) is considered Step 2; and severe pain (NRS 7 to 10) is Step 3. For Step 1 (mild pain), acetaminophen or a nonsteroidal anti-inflammatory drug (NSAID) is recommended. For Step 2 (moderate pain), a weak opioid (ie, codeine or hydrocodone) with or without acetaminophen or an NSAID is recommended. Finally, for Step 3 (severe pain), a strong opioid such as morphine or hydromorphone is recommended.
Again, the purpose of the ladder is not to provide a strict protocol for adherence, but rather to provide a reasonable starting point as a guide to the clinician. The key to its successful use is reassessment of the patient to determine if adequate pain relief is achieved.
Routes of Administration
Acetaminophen
Acetaminophen, the active ingredient in Tylenol, was first marketed in the United States in 1955 as an antipyretic and pain reliever.13 It is a synthetic centrally acting analgesic that is metabolized in the liver. Acetaminophen has been used alone or in combination in hundreds of formulations to treat a wide variety of pain and fever-related conditions. In the ED setting, acetaminophen is frequently used as an antipyretic and—either alone or in combination with opioids—for oral pain control.
Acetaminophen is very well tolerated by most patients, with minimal gastrointestinal (GI) distress. It is inexpensive, and the wide variety of formulations (eg, liquid, tablet, suppository) make it useful in a number of clinical scenarios. Acetaminophen is generally considered to be the only nonopioid analgesic that is safe in pregnancy,14 and it has no sedative or addictive effects.
There are, however, some disadvantages to using acetaminophen. Concerns about its safety and accidental overdose have recently led to the introduction of a 4,000-mg maximum daily dose recommendation15 and heightened concern over using multiple medications containing acetaminophen. In January 2014, the US Food and Drug Administration recommended that no combination medication contain more than 325 mg of acetaminophen because of the risk of toxicity when multiple drugs containing acetaminophen are consumed.
In addition to concerns of inadvertent overdose, another disadvantage of acetaminophen is that while it may be an effective antipyretic and analgesic, it has little or no anti-inflammatory properties. Therefore, in cases requiring an anti-inflammatory agent, an NSAID, when appropriate, would be the more effective option.
NSAIDs
Nonsteroidal anti-inflammatory drugs function by inhibiting prostaglandin production in the cyclooxygenase 1 and 2 (COX-1 and COX-2) pathways. Ibuprofen, indomethacin, ketorolac, naproxen, and other NSAIDs are chiefly utilized to control pain, inflammation, and fever via the oral route. As production of various prostaglandins via the COX-2 pathway is thought to contribute to fever, inflammation, and pain, inhibition of this pathway by NSAIDs can help alleviate these symptoms. The COX-1 pathway contributes many factors important to the protection and health of the GI tract, and inhibition of this pathway can lead to GI distress and damage. Unfortunately, the most commonly used and available NSAIDs inhibit both COX pathways simultaneously, and in doing so, prompt the GI symptoms which are the most common adverse side effects of therapy. In addition to GI effects of the COX-1 and COX-2 inhibitors, there are also concerns over the associated antiplatelet effects in patients undergoing surgery or potentially suffering from occult or intracranial bleeding.13
Ibuprofen
This is the most commonly used NSAID in the United States and is available without a prescription. Ibuprofen is typically used to treat mild-to-moderate pain from a musculoskeletal or inflammatory source. As an oral nonprescription medication, it can be used advantageously to treat acute pain in the ED and continued in the outpatient setting. Ibuprofen is neither sedating nor addicting, with a rapid onset of action and a plasma half-life of approximately 2 hours.13 However, there is a dose-dependent feature which allows large doses (eg, 800 mg) to be spaced-out every 8 hours while maintaining effective analgesia. Typical doses range from 200 mg to 800 mg orally every 6 to 8 hours, with a maximum dose of 3.2 g/d. Patients should be instructed to take each dose with a meal or snack to help alleviate GI side effects.
The greatest advantage of ibuprofen and other NSAIDs is their effect on inflammation and the ability to treat the inflammatory cause of pain—not just the symptom. Nonsteroidal anti-inflammatory drugs are well tolerated by most patients and can be obtained without prescription at low cost. Additionally, doses of ibuprofen and acetaminophen can be alternated.
Ketorolac
Often marketed as Toradol, ketorolac is a powerful NSAID available in IV, IM, and oral formulations. Typical doses are 30 mg IV, 60 mg IM, or 10 mg orally every 4 to 6 hours (maximum of 40 mg/d). The basic pharmacology and mechanism of action of ketorolac are similar to ibuprofen. Though ketorolac is useful to treat more severe pain, it should only be used for short-term management of pain (ie, 5 days or less). Ketorolac is often used for postoperative pain, but also is helpful for pain control in patients using opioids. It has been shown to be effective for acute renal colic and can also provide relief for migraine headaches.16,17 In a direct comparison between ketorolac and meperidine (Demerol) for patients suffering from renal stones, ketorolac was found to be more effective and provide longer lasting pain relief.18
The major concern regarding ketorolac relates to potential renal toxicity, and thus caution should be undertaken in prescribing it to patients with known or suspected renal disease. Although this risk is associated with even a single dose, multiple doses increase the danger and therefore should be avoided.
Ketorolac should also be used with caution in patients with asthma. As a subset of asthma patients will experience severe bronchospasm after NSAID administration, clinicians should always determine whether a patient can tolerate NSAIDs.
Renal Toxicity and GI Effects
Concerns over renal toxicity and potential GI distress are the chief disadvantages of NSAID use. While renal toxicity has been reported in patients without pre-existing kidney dysfunction, it is of much greater concern in patients with pre-existing renal disease or decreased glomerular filtration rate. For this reason, care must be exercised when prescribing NSAIDS to elderly patients, patients with diabetes mellitus, or patients with hypertension (or even worse, a combination of these). Prolonged use of NSAIDs can also cause upper GI bleeding. Nonsteroidal anti-inflammatory drugs are contraindicated in pregnant patients.
Opioid analgesics
Morphine
Morphine is the prototypical compound in the opioid class, and has been utilized for more than two centuries since its isolation in 1804.19 Other opioid compounds include codeine, hydrocodone, oxycodone, morphine, and hydromorphone, which represent different functional groups substituted onto the base morphine molecule. All share similar pharmacology, differing primarily only in potency. The onset of action for codeine, hydrocodone, and oxycodone is 30 to 60 minutes when taken orally, with a peak time of 60 to 90 minutes. Codeine and hydrocodone are at the weak-end of the spectrum (Step 2 medications), while morphine and hydromorphone are more potent (Step 3 medications). The majority of opioids and their metabolites are primarily excreted renally (90%-95%).20 Therefore, care must be exercised regarding dosing and frequency when used in patients with renal insufficiency or disease.
Morphine has several features that make it an attractive analgesic for use in the ED. The ability to administer morphine via IV, IM, subcutaneous, or oral route; its quick onset of action; and safety profile are all advantageous. The onset of action is 5 to 10 minutes when given IV. For oral administration, it is 30 to 60 minutes (similar to the other opioids discussed) and can be given as a tablet or syrup.
Morphine decreases the severity of pain, with an apparent increase in tolerance for any remaining discomfort. The potency and central action of morphine make it ideal for managing moderate and severe pain.
As with other analgesics, morphine and its related compounds have associated drawbacks, of which respiratory depression is the most feared, though this is uncommon at therapeutic doses. The respiratory depressant effect is more pronounced in patients with underlying lung disease, depressed mental status, or concurrent use of sedating medication (eg, benzodiazepines).
Another disadvantage to morphine is that it can cause hypotension, limiting its use in certain clinical situations. Repeated or prolonged use may cause slowing or complete arrest of peristaltic waves in the GI tract, which can lead to significant constipation. Pruritus and nausea are two other frequently reported side effects and may necessitate, respectively, coadministration of diphenhydramine (eg, Benadryl) or an antiemetic (eg, Phenergan or Zofran). As an opioid, morphine is potentially addictive, though it is generally thought to require significant intake over several weeks before such problems arise.
The sedation caused by morphine is also of concern, as patients who are under its influence immediately following discharge cannot safely drive, and may actually not be safe to walk or even utilize public transportation. This concern can be lessened by verifying the patient’s mode of transport prior to administration of the medication, ensuring that patients are not overly sedated at the time of discharge and, as much as possible, accompanied home by an adult relative or friend. In comparison to other frequently used opioids, several features can be considered. The duration of action for codeine, hydromorphone, and morphine is similar at 3 to 5 hours.
Hydromorphone
Hydromorphone (Dilaudid) is approximately seven times more potent than morphine, but otherwise very similar. It is frequently the IV opioid of choice for severe pain and also appears to cause pruritus less frequently than morphine. Hydromorphone has a rapid onset of action (1-5 minutes IV; 15-30 minutes orally) and can be titrated to effect when given via the IV route, making it an ideal agent for the pain associated with long bone or pelvic fractures, vasoocclusive crises in patients with sickle cell disease, and renal colic. The oral formulation of hydromorphone can be utilized for severe pain in the appropriate patient population, usually at a dose of 2 to 4 mg every 4 hours.
Codeine
Codeine is frequently prescribed in combination with acetaminophen in a product marketed as Tylenol #3 for mild-to-moderate pain. Because codeine is metabolized in the liver to morphine, it is contraindicated in patients with morphine allergy. Also, the clinician must be aware that codeine is ineffective in 7% to 10% of the population due to an enzyme deficiency.
Hydrocodone and oxycodone, which are often combined with acetaminophen and marketed respectively as Vicodin and Percocet, are two additional commonly used compounds in the oral treatment of moderate-to-severe pain. There are numerous preparations containing various strengths of both the opioid and acetaminophen components. Utilizing these medications as initial pain control in the ED can be beneficial, as therapy can be continued as an outpatient. Table 2 provides dosing and frequency information, but the clinician must also be aware of the total amount of acetaminophen administered, especially when being used in conjunction with other medications containing acetaminophen.
Tramadol
Tramadol (Ultram), another oral analgesic with opioid properties, affects several neurotransmitters and is less reliant on the opioid receptors compared to the opioid compounds described above. It is used to treat moderate-to-severe pain and can be considered another option along with codeine, hydrocodone, and oxycodone for oral pain control. Although the complete mechanism of action for tramadol is poorly understood, it appears to be effective in patients who do not respond well to pure opioids, and in patients with neuropathic pain or persistent pain of unclear etiology (eg, fibromyalgia).19 Because of its limited interaction with opioid receptors, tramadol has less potential for abuse and addiction than the opioids. It is also available in several combinations with acetaminophen or ibuprofen.
Conclusion
The ideal medication for treating pain in the ED is one that is effective, easy to administer, and has minimal adverse or residual effects. Generally, the least potent medication that will control pain should be chosen initially. When treating minor traumatic or musculoskeletal pain, initiating pain control with oral medication in the ED may provide patients with relief during imaging or splinting, and help control pain until they can fill outpatient prescriptions. All of the medications discussed in this article can be given in the oral form. However, IV usage may be appropriate when there is need for rapid onset of action, to titrate the medication, or for ease of administration in patients who are vomiting or unable to take anything orally.
When prescribing analgesics for pain control after discharge, the clinician must consider potential relative and absolute contraindications. For acetaminophen, liver toxicity is the major concern, and care must be taken not to exceed recommended daily dosing limits with the multitude of available products containing acetaminophen. Physicians should avoid recommending or prescribing acetaminophen to patients with liver disease (ie, cirrhosis) or habitual alcohol abuse.
Nonsteroidal anti-inflammatory drugs such as ibuprofen and naproxen carry the risk of renal damage, as well as concerns about antiplatelet activity. Because of the tendency for renal function to decline with age, caution must be exercised in using these compounds in older patients and those with preexisting renal dysfunction.
Opioid-containing medications are known for their potency in pain control, but also for their side effects. The most common side effect involves the GI tract, with nausea, vomiting, and constipation. The most dangerous side effect of opioids is respiratory depression; fortunately, this is rarely seen with therapeutic doses. However, somnolence and decreased coordination are significant concerns when prescribing opioids to elderly patients or those already at risk for falls.
There is no ideal single medication to treat all types of pain or situations. However, the choice of medication begins with a decision by the EP to treat pain. Utilizing a stepwise approach, oral medication will usually be effective for mild pain, but IV narcotics will probably be required for severe pain. Frequent reassessment of the patient is critically important for successful pain management in the ED.
Dr Byers is an emergency physician at the Presbyterian Medical Group, department of emergency medicine, Presbyterian Healthcare Services, Albuquerque, New Mexico. Dr Counselman is a distinguished professor of emergency medicine and chairman, department of emergency medicine, Eastern Virginia Medical School and Emergency Physicians of Tidewater, Norfolk, Virginia. He is also associate editor-in-chief of the Emergency Medicine editorial board.
- Todd KH. Pain assessment instruments for use in the emergency department. Emerg Med Clin North Am. 2005;23(2):285-295.
- Cordell WH, Keene KK, Giles BK, Jones JB, Jones JH, Brizendine EJ. The high prevalence of pain in emergency medical care. Am J Emerg Med. 2002;20(3):165-169.
- Tcherny-Lessenot S, Karwowski-Soulié F, Lamarche-Vadel A, Ginsburg C, Brunet F, Vidal-Trecan G. Management and relief of pain in an emergency department from the adult patients’ perspective. J Pain Symptom Manage. 2003;25(6):539-546.
- Karwowski-Soulié F, Lessenot-Tcherny S, Lamarche-Vadel A, et al. Pain in an emergency department: an audit. Eur J Emerg Med. 2006;13(4):218-224.
- Fosnocht DE, Swanson ER, Barton ED. Changing attitudes about pain and pain control in emergency medicine. Emerg Med Clin North Am. 2005;23(2):297-306.
- Wilson JE, Pendelton JM. Oligoanalgesia in the emergency department. Am J Emerg Med. 1989;7(6):620-623.
- Meghani SH, Byun E, Gallagher RM. Time to take stock: a meta-analysis and systemic review of analgesic treatment disparities for pain in the United States. Pain Med. 2012;13(2):150-174.
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schutenberg JE; The University of Michigan Institute for Social Research. Monitoring the future: 2013 overview key findings of on adolescent drug use. http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2013.pdf. Accessed June 6, 2014.
- Manchikanti L, Fellow B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: a ten year perspective. Pain Physician. 2010;13(5):401-435.
- Silka PA, Roth MM, Moreno G, Merrill L, Geiderman JM. Pain scores improve analgesic administration patterns for trauma patients in the emergency department. Acad Emerg Med. 2004;11(3):264-270.
- Nelson BP, Cohen D, Lander O, Crawford N, Viccellio AW, Singer AJ. Mandated pain scales improve frequency of ED analgesic administration. Am J Emerg Med. 2004;22(7):582-585.
- World Health Organization. Cancer pain relief with a guide to opioid availability 2nd ed. 1996. http://whqlibdoc.who.int/publications/9241544821.pdf. Accessed June 6, 2014.
- Grosser T, Smyth EM, FitzGerald GA. Anti-inflammatory, antipyretic, and analgesic agents: pharmacotherapy of gout. In: Brunton LL, Chabner BA, Knollman BC, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. China: The McGraw Hill Companies; 2011:959-1004.
- Scialli AR, Ang R, Breitmeyer J, Royal MA. A review of the literature on the effects of acetaminophen on pregnancy outcome. Reprod Toxicol. 2010;30(4):495-507.
- Schilling A, Corey R, Leonard M, Eghtesad B. Acetaminophen: old drug, new warnings. Cleveland Clin J Med. 2010;77(1):19-27.
- Shrestha M, Singh R, Moreden J, Hayes JE. Ketorolac vs chlorpromazine in the treatment of acute migraine without aura. A prospective, randomized, double-blind trial. Arch Intern Med.1996;156(15):1725-1728.
- Turkewitz LJ, Casaly JS, Dawson GA, Wirth O, Hurst RJ, Gillette PL. Self-administration of parenteral ketorolac tromethamine for head pain. Headache. 1992;32(9):452-454.
- Larkin GL, Peacock WF 4th, Pearl SM, Blair GA, D’Amico F. Efficacy of ketorolac tromethamine versus meperidine in the ED treatment of acute renal colic. Am J Emerg Med. 1999;17(1):6-10.
- Yaksh TL, Wallace MS. Opiods, analgesia and pain management. In: Brunton LL, Chabner BA, Knollman BC, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. China: The McGraw Hill Companies; 2011:481-526.
- DeSandre PL, Quest TE. Management of cancer-related pain. Hematol Oncol Clin N Am. 2010;24(3):643-658.
- Todd KH. Pain assessment instruments for use in the emergency department. Emerg Med Clin North Am. 2005;23(2):285-295.
- Cordell WH, Keene KK, Giles BK, Jones JB, Jones JH, Brizendine EJ. The high prevalence of pain in emergency medical care. Am J Emerg Med. 2002;20(3):165-169.
- Tcherny-Lessenot S, Karwowski-Soulié F, Lamarche-Vadel A, Ginsburg C, Brunet F, Vidal-Trecan G. Management and relief of pain in an emergency department from the adult patients’ perspective. J Pain Symptom Manage. 2003;25(6):539-546.
- Karwowski-Soulié F, Lessenot-Tcherny S, Lamarche-Vadel A, et al. Pain in an emergency department: an audit. Eur J Emerg Med. 2006;13(4):218-224.
- Fosnocht DE, Swanson ER, Barton ED. Changing attitudes about pain and pain control in emergency medicine. Emerg Med Clin North Am. 2005;23(2):297-306.
- Wilson JE, Pendelton JM. Oligoanalgesia in the emergency department. Am J Emerg Med. 1989;7(6):620-623.
- Meghani SH, Byun E, Gallagher RM. Time to take stock: a meta-analysis and systemic review of analgesic treatment disparities for pain in the United States. Pain Med. 2012;13(2):150-174.
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schutenberg JE; The University of Michigan Institute for Social Research. Monitoring the future: 2013 overview key findings of on adolescent drug use. http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2013.pdf. Accessed June 6, 2014.
- Manchikanti L, Fellow B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: a ten year perspective. Pain Physician. 2010;13(5):401-435.
- Silka PA, Roth MM, Moreno G, Merrill L, Geiderman JM. Pain scores improve analgesic administration patterns for trauma patients in the emergency department. Acad Emerg Med. 2004;11(3):264-270.
- Nelson BP, Cohen D, Lander O, Crawford N, Viccellio AW, Singer AJ. Mandated pain scales improve frequency of ED analgesic administration. Am J Emerg Med. 2004;22(7):582-585.
- World Health Organization. Cancer pain relief with a guide to opioid availability 2nd ed. 1996. http://whqlibdoc.who.int/publications/9241544821.pdf. Accessed June 6, 2014.
- Grosser T, Smyth EM, FitzGerald GA. Anti-inflammatory, antipyretic, and analgesic agents: pharmacotherapy of gout. In: Brunton LL, Chabner BA, Knollman BC, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. China: The McGraw Hill Companies; 2011:959-1004.
- Scialli AR, Ang R, Breitmeyer J, Royal MA. A review of the literature on the effects of acetaminophen on pregnancy outcome. Reprod Toxicol. 2010;30(4):495-507.
- Schilling A, Corey R, Leonard M, Eghtesad B. Acetaminophen: old drug, new warnings. Cleveland Clin J Med. 2010;77(1):19-27.
- Shrestha M, Singh R, Moreden J, Hayes JE. Ketorolac vs chlorpromazine in the treatment of acute migraine without aura. A prospective, randomized, double-blind trial. Arch Intern Med.1996;156(15):1725-1728.
- Turkewitz LJ, Casaly JS, Dawson GA, Wirth O, Hurst RJ, Gillette PL. Self-administration of parenteral ketorolac tromethamine for head pain. Headache. 1992;32(9):452-454.
- Larkin GL, Peacock WF 4th, Pearl SM, Blair GA, D’Amico F. Efficacy of ketorolac tromethamine versus meperidine in the ED treatment of acute renal colic. Am J Emerg Med. 1999;17(1):6-10.
- Yaksh TL, Wallace MS. Opiods, analgesia and pain management. In: Brunton LL, Chabner BA, Knollman BC, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. China: The McGraw Hill Companies; 2011:481-526.
- DeSandre PL, Quest TE. Management of cancer-related pain. Hematol Oncol Clin N Am. 2010;24(3):643-658.
Malpractice Counsel
Postpartum Shortness of Breath
A 35-year-old woman presented to the ED after referral by her obstetrician. Six days prior, the patient had given birth to twins without incident. On postpartum hospital day 2, however, she developed mild shortness of breath, and a chest X-ray was ordered. Since there was concern for possible pneumonia, the patient was prescribed oral antibiotics and discharged home on hospital day 4. She continued to complain of shortness of breath following discharge; at that time, the obstetrician referred the patient to the ED for further evaluation.
The patient was evaluated by the EP, who ordered a chest X-ray. He was also concerned that patient had pneumonia and prescribed a different class of antibiotic before discharging the patient home.
One week later, the patient presented back to the same ED with continued shortness of breath. On this visit, she was seen by a physician assistant (PA). Following the history taking and physical examination, a chest X-ray and rapid flu and rapid strep tests were ordered. Both the PA and supervising EP reviewed the chest X-ray and were concerned for pneumonia; however, both the flu and strep tests were negative. A third class of antibiotic was prescribed, and the patient was discharged home.
The chest X-ray on the second ED visit was not interpreted by a radiologist until 3 days later (The patient was seen on a Friday evening and the films were not read until the following Monday morning). The radiologist’s interpretation was “worsening congestive heart failure” (CHF). Two days later (5 days following the second ED visit), the EP was notified of the interpretation discrepancy and made multiple attempts to contact the patient, which included leaving a voice-mail message on her home phone.
The patient returned the call the following day and spoke with one of the ED nurses, who encouraged her to return immediately to the ED. The patient returned to the ED the next day (1 week after her second ED visit) and was admitted to the hospital for CHF secondary to postpartum cardiomyopathy. Unfortunately, she developed an embolus to her kidney, followed by an ischemic cerebrovascular accident, and died 3 weeks after admission.
The patient’s family filed a malpractice lawsuit against the hospital, the EPs, and the PA for negligent delay in making the correct diagnosis, stating that patient’s subsequent stroke and death were a direct result of this delay. Following deliberations, all of the EPs involved in the case were found free of negligence; the PA and the hospital ED, however, were found guilty.
Discussion
Interestingly, the majority of successful malpractice suits against physicians involve cognitive errors and system issues; this case is no exception. Making the correct diagnosis in a patient is a complex process, involving data gathering and synthesis, intuition, clinical experience, and logical thinking. Unfortunately, biases can occur during this process and result in misdiagnosis or delayed diagnosis. These biases include anchoring bias, confirmation bias, premature closure, and diagnosis momentum.1
Anchoring Bias. This occurs when a physician relies too heavily on the first piece of information or one’s initial impression.1 Despite evidence to the contrary, the physician keeps returning to the initial diagnosis (ie, he or she is “anchored” to it).
Confirmation Bias. Related to anchoring bias, in confirmation bias, the physician ignores or discounts evidence that contradicts one’s initial impression and focuses solely on the evidence supporting it.1
Both anchoring bias and confirmation bias appear to have played a role in this case. The differential diagnosis was never broadened beyond pneumonia, despite the fact that one must also consider pulmonary embolism (ie, a hypercoagulable state) and CHF (ie, postpartum cardiomyopathy) in a patient complaining of dyspnea in the postpartum period.
Premature Closure. This occurs when the physician finds a cause that fits the clinical picture and ceases to search for other diagnostic possibilities.1,2
Diagnosis Momentum. A bias that occurs when the diagnosis considered a possibility by one physician becomes a definitive diagnosis as it is passed from one physician to the next; it then becomes accepted without question by physicians down the line.1 This type of bias also seems to have played a role in this case.
There are a few strategies to help prevent or minimize these types of errors. First, as new data are gathered, one should reconsider and reprioritize the differential diagnosis. When certain data points do not fit neatly with an earlier diagnosis, careful attention must be paid to them. This is especially true for the patient receiving appropriate treatment but not showing clinical improvement. While it is usually helpful to know previous working diagnoses, the clinician must try to keep an open mind and consider alternative diagnoses. At the end of the day, the lesson is the need to develop a broad differential diagnosis.2
The system error in this case involves the lack of timely overread of radiology studies. Emergency medicine is a 24-hour, 7-day-a-week specialty; therefore, support services to the ED need to be similarly available. With today’s teleradiology and dedicated night readers, it is difficult to justify not providing such a service. This situation explains, in part, the negligent verdict against the hospital.
Chest Pain in a Man With Type 2 Diabetes
A 54-year-old man presented to the ED with a several-hour history of chest pain and shortness of breath. His medical history was remarkable for type 2 diabetes mellitus. He denied any associated nausea, vomiting, or diaphoresis. He smoked one pack of cigarettes per day and drank alcohol on occasion. The patient further denied having experienced similar symptoms in the past. All vital signs were normal, and he appeared comfortable and in no acute distress. His physical examination was completely unremarkable.
An electrocardiogram (ECG) revealed Q-waves in the anterior leads, but was otherwise nondiagnostic. The results of a portable chest X-ray were interpreted as normal. Cardiac enzyme testing revealed a positive troponin I, but a normal creatine phosphokinase-MB (CPK-MB). The patient’s complete blood count and coagulation studies were normal, and a basic metabolic profile was remarkable only for a blood sugar of 540 mg/dL; his serum bicarbonate value was normal.
The patient was placed on oxygen via nasal cannula, administered an aspirin (325 mg), and given subcutaneous regular insulin for hyperglycemia. The EP contacted a hospitalist to admit the patient. The hospitalist accepted the patient and admitted him to the telemetry floor.
Upon arrival at telemetry, the patient was examined by a nurse who noted rhonchi bilaterally on lung auscultation. Shortly afterward, he became anxious, and the nurse consulted the hospitalist, who ordered administration of lorazepam. Approximately 20 minutes later, the patient went into cardiac arrest and died.
This patient was never examined by the hospitalist prior to coding. An autopsy revealed evidence of severe coronary artery disease, a previous infarction that was at least a few months old with scarring of the left ventricle, and a recent infarction that had begun at least 12 hours prior to death.
The family sued all of the providers involved in the care of the patient. At trial, there was a factual dispute regarding whether the EP informed the hospitalist of the elevated troponin I level. The defendants argued that the patient had sustained irreversible heart damage prior to his arrival at the hospital, and that nothing any of the defendants could have done would have saved his life. The jury deliberated for approximately 2 hours before delivering a verdict in favor of the defense.
Discussion
Clearly, this verdict could have gone the other way. This patient was experiencing a non-ST segment elevation myocardial infarction (MI). The nondiagnostic ECG, coupled with the elevated troponin I, indicated damage of heart muscle from an acute interruption of coronary blood flow.
There are several problems with the management of this case. First, this patient required a cardiology consult for risk stratification. Several scoring systems could have been used to determine whether this patient was a candidate for early (ie, within 4-48 hours) invasive treatments such as percutaneous intervention or a more conservative approach.
Second, having sustained an MI, this patient was at high risk for complications such as ventricular arrhythmias, heart failure, cardiogenic shock, and other serious adverse events. Patients with acute MI should be admitted to the critical care or intensive care unit. In addition to aspirin, this patient should have received nitroglycerin and anticoagulation therapy. Either heparin or a low molecular weight heparin, such as enoxaparin, would have been appropriate if no contraindications existed. Finally, additional therapy including glycoprotein IIB/IIIA inhibitors, clopidogrel, etc, may have been indicated depending upon the timing of percutaneous intervention.1
It appears that both the EP and the hospitalist either failed to appreciate the significance of the elevated troponin I or overlooked it. This patient had normal renal function, so the only explanation—especially in the setting of a middle-aged man complaining of chest pain—was that myocardial damage had occurred.
Alcohol Detoxification in a Young Man
A young man was brought to the ED by a friend. The patient’s sole complaint was the need for help with his alcohol dependency. In addition to alcohol abuse, his medical history was remarkable only for an admission 1 month prior for suicidal ideation. The patient denied suicidal or homicidal ideations on this presentation. The physical examination revealed stable vital signs, but conjunctival injection, slurred speech, and a strong odor of alcohol. A blood alcohol test showed a concentration level of 0.36 g/dL. The patient, however, was alert and able to ambulate without assistance. He was medically cleared by the EP and arrangements were made to admit him to a local detoxification center.
Approximately 4 hours after his arrival, while waiting for transport to the detoxification center, the patient became impatient with the delay, removed his intravenous line, and told the nurse that he was going home via taxi. The nurse encouraged him to call a friend to take him home, to which to the patient agreed. The nurse left the patient to inform the EP of his desire to go home; when she returned, she discovered that the patient had already left the hospital. The physician notified hospital security but not the police. Approximately 2 hours later, the patient was struck by a car and seriously injured.
The patient sued the EP and the hospital for negligence and medical malpractice. The suit alleged that the physician and the hospital should have prevented the patient from leaving the ED. The physician and the hospital requested a dismissal, arguing that the patient did not exhibit any suicidal or homicidal ideation, presented on his own volition, and, though intoxicated, could still make decisions for himself. An appellate court granted the motion, holding that the defendants “lacked authority to confine the plaintiff upon his departure” from the ED.
Discussion
When a patient attempts to leave the ED against medical advice, the treating physician should make an attempt to convince him or her to remain for treatment. Often, something as simple as offering a cup of coffee will change a patient’s mind. In other instances, the use of chemical or physical restraint may be required. The handling of the case ultimately becomes a question of whether the patient was competent to make decisions and whether he presented a danger to himself or others. The extent of intoxication varies by the degree and does not of itself constitute incapability to make decisions. All practicing EPs have cared for patients with blood alcohol levels above the legal limit for driving, but who were functionally sober and able to make decisions. If a patient is competent and does not present a danger to self or others, he or she can decide to leave the ED without further management. However, it is best to release the patient in the company of friends or family, as was urged by the nurse in this case. Obviously, such a patient should not be allowed to drive himself home.
When a patient does leave against medical advice, the physician and nurse should document in the ED record their conversations urging him or her to stay. Alternatively, when a patient is found incompetent of making decisions or is a danger to self or others, he or she must be prevented from leaving the ED. This includes use of the minimal amount of physical or chemical restraint needed to keep the patient from leaving. When there is doubt that the patient is able to make a competent decision, it is better to err on the side of caution and keep him or her in the ED for his own safety and the safety of others.
Postpartum Shortness of Breath
- Penney FT, Datal AK: Understanding diagnostic error. Hospital Medicine Clinics. 2013;2(2):e292-e303.
- Ely JW, Kaldjian LC, D’Alessandro DM. Diagnostic errors in primary care: lessons learned. J Am Board Fam Med. 2012;25(1):87-97.
Chest Pain in a Man With Type 2 Diabetes
- Anantharam V, Lim SH. Treatment of NSTEMI (Non-ST elevation myocardial infarction). Curr Emerg Hosp Med Rep. 2013;1(1):18-28.
Postpartum Shortness of Breath
A 35-year-old woman presented to the ED after referral by her obstetrician. Six days prior, the patient had given birth to twins without incident. On postpartum hospital day 2, however, she developed mild shortness of breath, and a chest X-ray was ordered. Since there was concern for possible pneumonia, the patient was prescribed oral antibiotics and discharged home on hospital day 4. She continued to complain of shortness of breath following discharge; at that time, the obstetrician referred the patient to the ED for further evaluation.
The patient was evaluated by the EP, who ordered a chest X-ray. He was also concerned that patient had pneumonia and prescribed a different class of antibiotic before discharging the patient home.
One week later, the patient presented back to the same ED with continued shortness of breath. On this visit, she was seen by a physician assistant (PA). Following the history taking and physical examination, a chest X-ray and rapid flu and rapid strep tests were ordered. Both the PA and supervising EP reviewed the chest X-ray and were concerned for pneumonia; however, both the flu and strep tests were negative. A third class of antibiotic was prescribed, and the patient was discharged home.
The chest X-ray on the second ED visit was not interpreted by a radiologist until 3 days later (The patient was seen on a Friday evening and the films were not read until the following Monday morning). The radiologist’s interpretation was “worsening congestive heart failure” (CHF). Two days later (5 days following the second ED visit), the EP was notified of the interpretation discrepancy and made multiple attempts to contact the patient, which included leaving a voice-mail message on her home phone.
The patient returned the call the following day and spoke with one of the ED nurses, who encouraged her to return immediately to the ED. The patient returned to the ED the next day (1 week after her second ED visit) and was admitted to the hospital for CHF secondary to postpartum cardiomyopathy. Unfortunately, she developed an embolus to her kidney, followed by an ischemic cerebrovascular accident, and died 3 weeks after admission.
The patient’s family filed a malpractice lawsuit against the hospital, the EPs, and the PA for negligent delay in making the correct diagnosis, stating that patient’s subsequent stroke and death were a direct result of this delay. Following deliberations, all of the EPs involved in the case were found free of negligence; the PA and the hospital ED, however, were found guilty.
Discussion
Interestingly, the majority of successful malpractice suits against physicians involve cognitive errors and system issues; this case is no exception. Making the correct diagnosis in a patient is a complex process, involving data gathering and synthesis, intuition, clinical experience, and logical thinking. Unfortunately, biases can occur during this process and result in misdiagnosis or delayed diagnosis. These biases include anchoring bias, confirmation bias, premature closure, and diagnosis momentum.1
Anchoring Bias. This occurs when a physician relies too heavily on the first piece of information or one’s initial impression.1 Despite evidence to the contrary, the physician keeps returning to the initial diagnosis (ie, he or she is “anchored” to it).
Confirmation Bias. Related to anchoring bias, in confirmation bias, the physician ignores or discounts evidence that contradicts one’s initial impression and focuses solely on the evidence supporting it.1
Both anchoring bias and confirmation bias appear to have played a role in this case. The differential diagnosis was never broadened beyond pneumonia, despite the fact that one must also consider pulmonary embolism (ie, a hypercoagulable state) and CHF (ie, postpartum cardiomyopathy) in a patient complaining of dyspnea in the postpartum period.
Premature Closure. This occurs when the physician finds a cause that fits the clinical picture and ceases to search for other diagnostic possibilities.1,2
Diagnosis Momentum. A bias that occurs when the diagnosis considered a possibility by one physician becomes a definitive diagnosis as it is passed from one physician to the next; it then becomes accepted without question by physicians down the line.1 This type of bias also seems to have played a role in this case.
There are a few strategies to help prevent or minimize these types of errors. First, as new data are gathered, one should reconsider and reprioritize the differential diagnosis. When certain data points do not fit neatly with an earlier diagnosis, careful attention must be paid to them. This is especially true for the patient receiving appropriate treatment but not showing clinical improvement. While it is usually helpful to know previous working diagnoses, the clinician must try to keep an open mind and consider alternative diagnoses. At the end of the day, the lesson is the need to develop a broad differential diagnosis.2
The system error in this case involves the lack of timely overread of radiology studies. Emergency medicine is a 24-hour, 7-day-a-week specialty; therefore, support services to the ED need to be similarly available. With today’s teleradiology and dedicated night readers, it is difficult to justify not providing such a service. This situation explains, in part, the negligent verdict against the hospital.
Chest Pain in a Man With Type 2 Diabetes
A 54-year-old man presented to the ED with a several-hour history of chest pain and shortness of breath. His medical history was remarkable for type 2 diabetes mellitus. He denied any associated nausea, vomiting, or diaphoresis. He smoked one pack of cigarettes per day and drank alcohol on occasion. The patient further denied having experienced similar symptoms in the past. All vital signs were normal, and he appeared comfortable and in no acute distress. His physical examination was completely unremarkable.
An electrocardiogram (ECG) revealed Q-waves in the anterior leads, but was otherwise nondiagnostic. The results of a portable chest X-ray were interpreted as normal. Cardiac enzyme testing revealed a positive troponin I, but a normal creatine phosphokinase-MB (CPK-MB). The patient’s complete blood count and coagulation studies were normal, and a basic metabolic profile was remarkable only for a blood sugar of 540 mg/dL; his serum bicarbonate value was normal.
The patient was placed on oxygen via nasal cannula, administered an aspirin (325 mg), and given subcutaneous regular insulin for hyperglycemia. The EP contacted a hospitalist to admit the patient. The hospitalist accepted the patient and admitted him to the telemetry floor.
Upon arrival at telemetry, the patient was examined by a nurse who noted rhonchi bilaterally on lung auscultation. Shortly afterward, he became anxious, and the nurse consulted the hospitalist, who ordered administration of lorazepam. Approximately 20 minutes later, the patient went into cardiac arrest and died.
This patient was never examined by the hospitalist prior to coding. An autopsy revealed evidence of severe coronary artery disease, a previous infarction that was at least a few months old with scarring of the left ventricle, and a recent infarction that had begun at least 12 hours prior to death.
The family sued all of the providers involved in the care of the patient. At trial, there was a factual dispute regarding whether the EP informed the hospitalist of the elevated troponin I level. The defendants argued that the patient had sustained irreversible heart damage prior to his arrival at the hospital, and that nothing any of the defendants could have done would have saved his life. The jury deliberated for approximately 2 hours before delivering a verdict in favor of the defense.
Discussion
Clearly, this verdict could have gone the other way. This patient was experiencing a non-ST segment elevation myocardial infarction (MI). The nondiagnostic ECG, coupled with the elevated troponin I, indicated damage of heart muscle from an acute interruption of coronary blood flow.
There are several problems with the management of this case. First, this patient required a cardiology consult for risk stratification. Several scoring systems could have been used to determine whether this patient was a candidate for early (ie, within 4-48 hours) invasive treatments such as percutaneous intervention or a more conservative approach.
Second, having sustained an MI, this patient was at high risk for complications such as ventricular arrhythmias, heart failure, cardiogenic shock, and other serious adverse events. Patients with acute MI should be admitted to the critical care or intensive care unit. In addition to aspirin, this patient should have received nitroglycerin and anticoagulation therapy. Either heparin or a low molecular weight heparin, such as enoxaparin, would have been appropriate if no contraindications existed. Finally, additional therapy including glycoprotein IIB/IIIA inhibitors, clopidogrel, etc, may have been indicated depending upon the timing of percutaneous intervention.1
It appears that both the EP and the hospitalist either failed to appreciate the significance of the elevated troponin I or overlooked it. This patient had normal renal function, so the only explanation—especially in the setting of a middle-aged man complaining of chest pain—was that myocardial damage had occurred.
Alcohol Detoxification in a Young Man
A young man was brought to the ED by a friend. The patient’s sole complaint was the need for help with his alcohol dependency. In addition to alcohol abuse, his medical history was remarkable only for an admission 1 month prior for suicidal ideation. The patient denied suicidal or homicidal ideations on this presentation. The physical examination revealed stable vital signs, but conjunctival injection, slurred speech, and a strong odor of alcohol. A blood alcohol test showed a concentration level of 0.36 g/dL. The patient, however, was alert and able to ambulate without assistance. He was medically cleared by the EP and arrangements were made to admit him to a local detoxification center.
Approximately 4 hours after his arrival, while waiting for transport to the detoxification center, the patient became impatient with the delay, removed his intravenous line, and told the nurse that he was going home via taxi. The nurse encouraged him to call a friend to take him home, to which to the patient agreed. The nurse left the patient to inform the EP of his desire to go home; when she returned, she discovered that the patient had already left the hospital. The physician notified hospital security but not the police. Approximately 2 hours later, the patient was struck by a car and seriously injured.
The patient sued the EP and the hospital for negligence and medical malpractice. The suit alleged that the physician and the hospital should have prevented the patient from leaving the ED. The physician and the hospital requested a dismissal, arguing that the patient did not exhibit any suicidal or homicidal ideation, presented on his own volition, and, though intoxicated, could still make decisions for himself. An appellate court granted the motion, holding that the defendants “lacked authority to confine the plaintiff upon his departure” from the ED.
Discussion
When a patient attempts to leave the ED against medical advice, the treating physician should make an attempt to convince him or her to remain for treatment. Often, something as simple as offering a cup of coffee will change a patient’s mind. In other instances, the use of chemical or physical restraint may be required. The handling of the case ultimately becomes a question of whether the patient was competent to make decisions and whether he presented a danger to himself or others. The extent of intoxication varies by the degree and does not of itself constitute incapability to make decisions. All practicing EPs have cared for patients with blood alcohol levels above the legal limit for driving, but who were functionally sober and able to make decisions. If a patient is competent and does not present a danger to self or others, he or she can decide to leave the ED without further management. However, it is best to release the patient in the company of friends or family, as was urged by the nurse in this case. Obviously, such a patient should not be allowed to drive himself home.
When a patient does leave against medical advice, the physician and nurse should document in the ED record their conversations urging him or her to stay. Alternatively, when a patient is found incompetent of making decisions or is a danger to self or others, he or she must be prevented from leaving the ED. This includes use of the minimal amount of physical or chemical restraint needed to keep the patient from leaving. When there is doubt that the patient is able to make a competent decision, it is better to err on the side of caution and keep him or her in the ED for his own safety and the safety of others.
Postpartum Shortness of Breath
A 35-year-old woman presented to the ED after referral by her obstetrician. Six days prior, the patient had given birth to twins without incident. On postpartum hospital day 2, however, she developed mild shortness of breath, and a chest X-ray was ordered. Since there was concern for possible pneumonia, the patient was prescribed oral antibiotics and discharged home on hospital day 4. She continued to complain of shortness of breath following discharge; at that time, the obstetrician referred the patient to the ED for further evaluation.
The patient was evaluated by the EP, who ordered a chest X-ray. He was also concerned that patient had pneumonia and prescribed a different class of antibiotic before discharging the patient home.
One week later, the patient presented back to the same ED with continued shortness of breath. On this visit, she was seen by a physician assistant (PA). Following the history taking and physical examination, a chest X-ray and rapid flu and rapid strep tests were ordered. Both the PA and supervising EP reviewed the chest X-ray and were concerned for pneumonia; however, both the flu and strep tests were negative. A third class of antibiotic was prescribed, and the patient was discharged home.
The chest X-ray on the second ED visit was not interpreted by a radiologist until 3 days later (The patient was seen on a Friday evening and the films were not read until the following Monday morning). The radiologist’s interpretation was “worsening congestive heart failure” (CHF). Two days later (5 days following the second ED visit), the EP was notified of the interpretation discrepancy and made multiple attempts to contact the patient, which included leaving a voice-mail message on her home phone.
The patient returned the call the following day and spoke with one of the ED nurses, who encouraged her to return immediately to the ED. The patient returned to the ED the next day (1 week after her second ED visit) and was admitted to the hospital for CHF secondary to postpartum cardiomyopathy. Unfortunately, she developed an embolus to her kidney, followed by an ischemic cerebrovascular accident, and died 3 weeks after admission.
The patient’s family filed a malpractice lawsuit against the hospital, the EPs, and the PA for negligent delay in making the correct diagnosis, stating that patient’s subsequent stroke and death were a direct result of this delay. Following deliberations, all of the EPs involved in the case were found free of negligence; the PA and the hospital ED, however, were found guilty.
Discussion
Interestingly, the majority of successful malpractice suits against physicians involve cognitive errors and system issues; this case is no exception. Making the correct diagnosis in a patient is a complex process, involving data gathering and synthesis, intuition, clinical experience, and logical thinking. Unfortunately, biases can occur during this process and result in misdiagnosis or delayed diagnosis. These biases include anchoring bias, confirmation bias, premature closure, and diagnosis momentum.1
Anchoring Bias. This occurs when a physician relies too heavily on the first piece of information or one’s initial impression.1 Despite evidence to the contrary, the physician keeps returning to the initial diagnosis (ie, he or she is “anchored” to it).
Confirmation Bias. Related to anchoring bias, in confirmation bias, the physician ignores or discounts evidence that contradicts one’s initial impression and focuses solely on the evidence supporting it.1
Both anchoring bias and confirmation bias appear to have played a role in this case. The differential diagnosis was never broadened beyond pneumonia, despite the fact that one must also consider pulmonary embolism (ie, a hypercoagulable state) and CHF (ie, postpartum cardiomyopathy) in a patient complaining of dyspnea in the postpartum period.
Premature Closure. This occurs when the physician finds a cause that fits the clinical picture and ceases to search for other diagnostic possibilities.1,2
Diagnosis Momentum. A bias that occurs when the diagnosis considered a possibility by one physician becomes a definitive diagnosis as it is passed from one physician to the next; it then becomes accepted without question by physicians down the line.1 This type of bias also seems to have played a role in this case.
There are a few strategies to help prevent or minimize these types of errors. First, as new data are gathered, one should reconsider and reprioritize the differential diagnosis. When certain data points do not fit neatly with an earlier diagnosis, careful attention must be paid to them. This is especially true for the patient receiving appropriate treatment but not showing clinical improvement. While it is usually helpful to know previous working diagnoses, the clinician must try to keep an open mind and consider alternative diagnoses. At the end of the day, the lesson is the need to develop a broad differential diagnosis.2
The system error in this case involves the lack of timely overread of radiology studies. Emergency medicine is a 24-hour, 7-day-a-week specialty; therefore, support services to the ED need to be similarly available. With today’s teleradiology and dedicated night readers, it is difficult to justify not providing such a service. This situation explains, in part, the negligent verdict against the hospital.
Chest Pain in a Man With Type 2 Diabetes
A 54-year-old man presented to the ED with a several-hour history of chest pain and shortness of breath. His medical history was remarkable for type 2 diabetes mellitus. He denied any associated nausea, vomiting, or diaphoresis. He smoked one pack of cigarettes per day and drank alcohol on occasion. The patient further denied having experienced similar symptoms in the past. All vital signs were normal, and he appeared comfortable and in no acute distress. His physical examination was completely unremarkable.
An electrocardiogram (ECG) revealed Q-waves in the anterior leads, but was otherwise nondiagnostic. The results of a portable chest X-ray were interpreted as normal. Cardiac enzyme testing revealed a positive troponin I, but a normal creatine phosphokinase-MB (CPK-MB). The patient’s complete blood count and coagulation studies were normal, and a basic metabolic profile was remarkable only for a blood sugar of 540 mg/dL; his serum bicarbonate value was normal.
The patient was placed on oxygen via nasal cannula, administered an aspirin (325 mg), and given subcutaneous regular insulin for hyperglycemia. The EP contacted a hospitalist to admit the patient. The hospitalist accepted the patient and admitted him to the telemetry floor.
Upon arrival at telemetry, the patient was examined by a nurse who noted rhonchi bilaterally on lung auscultation. Shortly afterward, he became anxious, and the nurse consulted the hospitalist, who ordered administration of lorazepam. Approximately 20 minutes later, the patient went into cardiac arrest and died.
This patient was never examined by the hospitalist prior to coding. An autopsy revealed evidence of severe coronary artery disease, a previous infarction that was at least a few months old with scarring of the left ventricle, and a recent infarction that had begun at least 12 hours prior to death.
The family sued all of the providers involved in the care of the patient. At trial, there was a factual dispute regarding whether the EP informed the hospitalist of the elevated troponin I level. The defendants argued that the patient had sustained irreversible heart damage prior to his arrival at the hospital, and that nothing any of the defendants could have done would have saved his life. The jury deliberated for approximately 2 hours before delivering a verdict in favor of the defense.
Discussion
Clearly, this verdict could have gone the other way. This patient was experiencing a non-ST segment elevation myocardial infarction (MI). The nondiagnostic ECG, coupled with the elevated troponin I, indicated damage of heart muscle from an acute interruption of coronary blood flow.
There are several problems with the management of this case. First, this patient required a cardiology consult for risk stratification. Several scoring systems could have been used to determine whether this patient was a candidate for early (ie, within 4-48 hours) invasive treatments such as percutaneous intervention or a more conservative approach.
Second, having sustained an MI, this patient was at high risk for complications such as ventricular arrhythmias, heart failure, cardiogenic shock, and other serious adverse events. Patients with acute MI should be admitted to the critical care or intensive care unit. In addition to aspirin, this patient should have received nitroglycerin and anticoagulation therapy. Either heparin or a low molecular weight heparin, such as enoxaparin, would have been appropriate if no contraindications existed. Finally, additional therapy including glycoprotein IIB/IIIA inhibitors, clopidogrel, etc, may have been indicated depending upon the timing of percutaneous intervention.1
It appears that both the EP and the hospitalist either failed to appreciate the significance of the elevated troponin I or overlooked it. This patient had normal renal function, so the only explanation—especially in the setting of a middle-aged man complaining of chest pain—was that myocardial damage had occurred.
Alcohol Detoxification in a Young Man
A young man was brought to the ED by a friend. The patient’s sole complaint was the need for help with his alcohol dependency. In addition to alcohol abuse, his medical history was remarkable only for an admission 1 month prior for suicidal ideation. The patient denied suicidal or homicidal ideations on this presentation. The physical examination revealed stable vital signs, but conjunctival injection, slurred speech, and a strong odor of alcohol. A blood alcohol test showed a concentration level of 0.36 g/dL. The patient, however, was alert and able to ambulate without assistance. He was medically cleared by the EP and arrangements were made to admit him to a local detoxification center.
Approximately 4 hours after his arrival, while waiting for transport to the detoxification center, the patient became impatient with the delay, removed his intravenous line, and told the nurse that he was going home via taxi. The nurse encouraged him to call a friend to take him home, to which to the patient agreed. The nurse left the patient to inform the EP of his desire to go home; when she returned, she discovered that the patient had already left the hospital. The physician notified hospital security but not the police. Approximately 2 hours later, the patient was struck by a car and seriously injured.
The patient sued the EP and the hospital for negligence and medical malpractice. The suit alleged that the physician and the hospital should have prevented the patient from leaving the ED. The physician and the hospital requested a dismissal, arguing that the patient did not exhibit any suicidal or homicidal ideation, presented on his own volition, and, though intoxicated, could still make decisions for himself. An appellate court granted the motion, holding that the defendants “lacked authority to confine the plaintiff upon his departure” from the ED.
Discussion
When a patient attempts to leave the ED against medical advice, the treating physician should make an attempt to convince him or her to remain for treatment. Often, something as simple as offering a cup of coffee will change a patient’s mind. In other instances, the use of chemical or physical restraint may be required. The handling of the case ultimately becomes a question of whether the patient was competent to make decisions and whether he presented a danger to himself or others. The extent of intoxication varies by the degree and does not of itself constitute incapability to make decisions. All practicing EPs have cared for patients with blood alcohol levels above the legal limit for driving, but who were functionally sober and able to make decisions. If a patient is competent and does not present a danger to self or others, he or she can decide to leave the ED without further management. However, it is best to release the patient in the company of friends or family, as was urged by the nurse in this case. Obviously, such a patient should not be allowed to drive himself home.
When a patient does leave against medical advice, the physician and nurse should document in the ED record their conversations urging him or her to stay. Alternatively, when a patient is found incompetent of making decisions or is a danger to self or others, he or she must be prevented from leaving the ED. This includes use of the minimal amount of physical or chemical restraint needed to keep the patient from leaving. When there is doubt that the patient is able to make a competent decision, it is better to err on the side of caution and keep him or her in the ED for his own safety and the safety of others.
Postpartum Shortness of Breath
- Penney FT, Datal AK: Understanding diagnostic error. Hospital Medicine Clinics. 2013;2(2):e292-e303.
- Ely JW, Kaldjian LC, D’Alessandro DM. Diagnostic errors in primary care: lessons learned. J Am Board Fam Med. 2012;25(1):87-97.
Chest Pain in a Man With Type 2 Diabetes
- Anantharam V, Lim SH. Treatment of NSTEMI (Non-ST elevation myocardial infarction). Curr Emerg Hosp Med Rep. 2013;1(1):18-28.
Postpartum Shortness of Breath
- Penney FT, Datal AK: Understanding diagnostic error. Hospital Medicine Clinics. 2013;2(2):e292-e303.
- Ely JW, Kaldjian LC, D’Alessandro DM. Diagnostic errors in primary care: lessons learned. J Am Board Fam Med. 2012;25(1):87-97.
Chest Pain in a Man With Type 2 Diabetes
- Anantharam V, Lim SH. Treatment of NSTEMI (Non-ST elevation myocardial infarction). Curr Emerg Hosp Med Rep. 2013;1(1):18-28.
Malpractice Counsel
Case 1: Follow-up in Community-Acquired Methicillin- Resistant Staphylococcus Aureus Pharyngitis
A 73-year-old woman presented to the ED complaining of injuries she sustained following a minor fall at home. The patient’s past medical history was remarkable for diabetes mellitus (DM), hypertension, cerebrovascular accident, and a history of chronic pain. During the course of evaluation, the patient mentioned that she had experienced a sore throat. In addition to X-rays, the emergency physician (EP) ordered a rapid strep test (RST). All studies were normal and the patient was discharged home.
Over the next 2 months, the patient received medical treatment from other medical professionals, but not at the previous hospital. Reportedly, she did not complain of a sore throat during this time period. The patient did return to the same ED approximately 2 months later, complaining of cough and difficulty breathing; she died 5 days thereafter. No autopsy was performed.
A lawsuit was filed on behalf of the patient, stating the hospital breached the standard of care by not reporting the finding of MRSA directly to the treating physician, and that this led directly to the patient’s death by MRSA pneumonia. The jury returned a verdict in favor of the plaintiff for $32 million.
Discussion
This case illustrates two simple but important points: managing community-acquired MRSA (CA-MRSA) infections is a growing challenge for physicians; and hospital follow-up systems for positive findings (ie, fractures, blood cultures, etc) need to be consistently reliable.
It is estimated that 30% of healthy people carry S aureus in their anterior nares; colonization rates for the throat are much less studied. In one recent study, 265 throat swabs were collected from patients aged 14 to 65 years old, who complained of pharyngitis in an outpatient setting.1 A total of 165 S aureus isolates (62.3%) were recovered from the 265 swabs. For the S aureus isolates, 38.2% (63) grew CA-MRSA; the remaining 68.1% (102) were methicillin-sensitive S aureus (MSSA). Interestingly, of the 63 MRSA-positive swabs, over half also grew Group A Streptococcus. The natural disease progression of CA-MRSA pharyngitis is still unknown, as is what to do with a positive throat swab for CA-MRSA. While there are a few case reports of bacteremia and Lemmiere syndrome possibly related to CA-MRSA pharyngitis,2,3 more information is clearly needed. For this case, it is not possible to definitively determine the role of the positive throat swab for CAMRSA in the patient’s subsequent death.
The other teaching point in this case is much simpler and well defined. Simply put, patients expect to be informed of positive findings, whether the result is known at the time of their ED visit or sometime afterward. There needs to be a system in place that consistently and reliably provides important information to either the patient’s treating physician or to the patient. The manners in which this information is communicated are myriad and should take into account hospital resources, the role of the EP and nurse, and what works best for your locality. The “who” and “how” of the contact is not important: reliability, timeliness, and consistency need to be the key drivers of the system.
Case 2: Arterial Occlusion
A 61-year-old woman called emergency medical services (EMS) after noticing her feet felt cold to the touch and having difficulty ambulating. The paramedics noted the patient had normal vital signs and normal circulation in her legs, and she was transported to the ED without incident. Upon arrival to the ED, she was triaged as nonurgent and placed in the minor-care area. On nursing assessment, the patient’s feet were found to be cold, but with palpable pulses bilaterally. Her past medical history was significant for hypertension and DM.
The patient was seen by a physician assistant (PA), who found both feet cool to the touch, but with bilateral pulses present. She was administered intravenous morphine for pain and laboratory studies were ordered. At the time, the PA was concerned about arterial occlusion versus deep vein thrombosis (DVT) versus cellulitis. A venous ultrasound examination was ordered and shown to be negative for DVT. A complete blood count was remarkable only for mild leukocytosis. The PA discussed the case with the supervising EP; they agreed on a diagnosis of cellulitis and discharged the patient home with antibiotics and analgesics.
Approximately 12 hours after discharge, the patient presented back to the ED via ambulance. At that time, she was hypotensive and tachypneic, with a thready palpable pulse. On repeat examination, she no longer had pulses present in her feet. An arteriogram found complete occlusion of her arterial circulation at the level of the knees bilaterally, requiring bilateral belowthe- knee amputation.
The patient sued both the emergency medicine physician and the PA for failure to provide her with the necessary care during her initial ED visit, resulting in loss of limbs. The defendants claimed the patient could not prove gross negligence by clear and convincing evidence, as required by state law. Following the ensuing trial, the jury returned a $5 million verdict in favor of the plaintiff.
Discussion
First, it is important to remember that just because a patient has been triaged to a low-acuity area does not mean she or he must have a minor problem. The provider still must maintain a high level of vigilance— regardless of the location of the patient in the ED.
Second, was this patient in atrial fibrillation, which is responsible in approximately 65% of all peripheral emboli? The abrupt onset of this patient’s symptoms is much more compatible with an embolic origin of her symptoms rather than a thrombus (ie, symptoms of claudication).
Lastly, a diagnosis of cellulitis is inconsistent with the physical findings of the PA, as well as those of the triage nurse and paramedics. This patient’s feet were cool to the touch whereas cellulitis presents with erythema and increased warmth. While the presence of pulses was somewhat reassuring, the cool temperature of the feet and complaint of pain were indicators of the need to evaluate for a possible arterial origin of these findings. However, if this were an embolic phenomenon, peripheral arterial ultrasound would have probably been normal, and the outcome unchanged.
Case 3: Failure to Communicate
A 59-year-old man presented to his primary care physician (PCP) for intermittent right-hand weakness and numbness and tingling in his right arm during the previous 24 hours. As the PCP was concerned that the patient might be experiencing a transient ischemic attack (TIA), the patient and his wife were instructed to go directly to the ED of the local hospital. The PCP wrote a note stating that the patient needed “a stroke work up,” gave the note to the patient, and told him to give it to the ED staff.
The patient went directly to the ED and was immediately seen by a “rapid triage nurse.” He gave the note to the nurse and told her of the PCP’s concerns. The nurse documented on the hospital assessment form that the patient was high priority and needed to be seen immediately. She attached the PCP’s note to the front of the form.
The patient was then seen by the traditional triage nurse. After evaluation, she changed the priority from high to low acuity. The triage nurse later stated later that she never saw the PCP’s note, nor did she obtain any history regarding the concerns of the PCP.
The patient was then evaluated by an EP in the low-acuity (or minor-care) area of the ED. The EP later stated that he never saw the note from the PCP, and had not received any information regarding a suspected TIA or stroke. The EP ordered a right wrist X-ray, diagnosed carpel tunnel syndrome, and prescribed an anti-inflammatory medication as well as follow-up with a hand surgeon.
The initial (rapid triage) nurse saw the patient leaving the ED at the time of discharge and thought he had not been in the ED long enough to have undergone a stroke work up. She reviewed his paperwork and saw the patient had not received the indicated work up. The nurse called the patient’s house and left a message on the answering machine notifying him of the need to return to the ED. The patient arrived back to the ED approximately 2 hours later.
On the second ED presentation that day, the patient was evaluated by a different EP. The patient had blood drawn, an electrocardiogram, and a noncontrast computed tomography scan of the brain, the results of which were all normal. The EP concluded the patient required admission to the hospital for additional work up (eg, carotid Doppler ultrasound). The hospitalist was paged, and told the EP he would be there “as soon as possible.” However, after several hours delay, and no hospitalist, the patient became impatient and expressed the desire to go home. The EP urged the patient to follow up with his PCP in the morning to complete the evaluation.
The next day, before seeing his PCP, the patient suffered an ischemic stroke with right-sided hemiparesis. The case went to trial, and the jury found in favor of the plaintiff.
Discussion
Unfortunately, multiple opportunities were lost in obtaining the correct care at the right time for this patient. Lack of communication and poor communication are frequently cited as causes in medical malpractice cases, and this case perfectly illustrates this problem.
First, the PCP should have called the ED and spoken to the EP directly. This would have provided the PCP the opportunity to express his concerns directly to the treating physician. This kind of one-on-one communication between physicians will always be superior to a hand-written note.
Second, it is unclear why the triage nurse changed the initial nurse’s correct assessment. It is also unclear what happened to the PCP’s note—it was never seen again. Clearly there was miscommunication at this point between the triage nurse and the patient. This case further illustrates the importance of good triage. Once a patient is directed down the wrong pathway (ie, to minor care rather than the main treatment area), the situation becomes much more difficult to correct.
Next, the EP in the low-acuity area was probably falsely assured this patient had only a “minor” problem problem, and not something serious. Emergency physicians must be vigilant to the possibility that the patient can have something seriously wrong even if he or she has been triaged to a low-acuity area. A minor sore throat can turn out to be epiglottitis and a viral stomachache can turn out to be appendicitis. These patients deserve the same quality of history, physical examination, and differential diagnosis as any other patient in the ED.
Finally, while we are not responsible for hospitalists or consultants, we do have a responsibility to our patients. We need to ensure that the care they receive is the appropriate care. Possible alternatives to discharging this patient would have been to call another hospitalist for admission or to seek the input of the chief of the medical staff or the on-call hospital administrator. As EPs, we are frequently required to serve as the primary advocate for our patients.
There is the possibility that even if the patient had been admitted to the hospital, the outcome would have been the same. However, since he might have been a candidate for tissue plasminogen activator or interventional radiology if he had suffered the cerebrovascular accident as an inpatient, he lost his best chance for a good outcome.
- Gowrishankar S, Thenmozhi R, Balaji K, Pandian SK. Emergence of methicillin-resistant, vancomycin-intermediate Staphylococcus aureus among patients associated with group A Streptococcal pharyngitis infection in southern India. Infect Genet Evol. 2013;14:383-389.
- Wang LJ, Du XQ, Nyirimigabo E, Shou ST. A case report: concurrent infectious mononucleosis and community-associated methicillinresistant Staphylococcus aureus bacteremia. Am J Emerg Med. 2013. doi:10.1016/j.ajem.2013.10.033
- Kizhner VZ, Samara GJ, Panesar R, Krespi YP. MRSA bacteremia associated with Lemierre syndrome. Otolaryngol Head Neck Surg. 2011;145(suppl 2):P152,P153.
Case 1: Follow-up in Community-Acquired Methicillin- Resistant Staphylococcus Aureus Pharyngitis
A 73-year-old woman presented to the ED complaining of injuries she sustained following a minor fall at home. The patient’s past medical history was remarkable for diabetes mellitus (DM), hypertension, cerebrovascular accident, and a history of chronic pain. During the course of evaluation, the patient mentioned that she had experienced a sore throat. In addition to X-rays, the emergency physician (EP) ordered a rapid strep test (RST). All studies were normal and the patient was discharged home.
Over the next 2 months, the patient received medical treatment from other medical professionals, but not at the previous hospital. Reportedly, she did not complain of a sore throat during this time period. The patient did return to the same ED approximately 2 months later, complaining of cough and difficulty breathing; she died 5 days thereafter. No autopsy was performed.
A lawsuit was filed on behalf of the patient, stating the hospital breached the standard of care by not reporting the finding of MRSA directly to the treating physician, and that this led directly to the patient’s death by MRSA pneumonia. The jury returned a verdict in favor of the plaintiff for $32 million.
Discussion
This case illustrates two simple but important points: managing community-acquired MRSA (CA-MRSA) infections is a growing challenge for physicians; and hospital follow-up systems for positive findings (ie, fractures, blood cultures, etc) need to be consistently reliable.
It is estimated that 30% of healthy people carry S aureus in their anterior nares; colonization rates for the throat are much less studied. In one recent study, 265 throat swabs were collected from patients aged 14 to 65 years old, who complained of pharyngitis in an outpatient setting.1 A total of 165 S aureus isolates (62.3%) were recovered from the 265 swabs. For the S aureus isolates, 38.2% (63) grew CA-MRSA; the remaining 68.1% (102) were methicillin-sensitive S aureus (MSSA). Interestingly, of the 63 MRSA-positive swabs, over half also grew Group A Streptococcus. The natural disease progression of CA-MRSA pharyngitis is still unknown, as is what to do with a positive throat swab for CA-MRSA. While there are a few case reports of bacteremia and Lemmiere syndrome possibly related to CA-MRSA pharyngitis,2,3 more information is clearly needed. For this case, it is not possible to definitively determine the role of the positive throat swab for CAMRSA in the patient’s subsequent death.
The other teaching point in this case is much simpler and well defined. Simply put, patients expect to be informed of positive findings, whether the result is known at the time of their ED visit or sometime afterward. There needs to be a system in place that consistently and reliably provides important information to either the patient’s treating physician or to the patient. The manners in which this information is communicated are myriad and should take into account hospital resources, the role of the EP and nurse, and what works best for your locality. The “who” and “how” of the contact is not important: reliability, timeliness, and consistency need to be the key drivers of the system.
Case 2: Arterial Occlusion
A 61-year-old woman called emergency medical services (EMS) after noticing her feet felt cold to the touch and having difficulty ambulating. The paramedics noted the patient had normal vital signs and normal circulation in her legs, and she was transported to the ED without incident. Upon arrival to the ED, she was triaged as nonurgent and placed in the minor-care area. On nursing assessment, the patient’s feet were found to be cold, but with palpable pulses bilaterally. Her past medical history was significant for hypertension and DM.
The patient was seen by a physician assistant (PA), who found both feet cool to the touch, but with bilateral pulses present. She was administered intravenous morphine for pain and laboratory studies were ordered. At the time, the PA was concerned about arterial occlusion versus deep vein thrombosis (DVT) versus cellulitis. A venous ultrasound examination was ordered and shown to be negative for DVT. A complete blood count was remarkable only for mild leukocytosis. The PA discussed the case with the supervising EP; they agreed on a diagnosis of cellulitis and discharged the patient home with antibiotics and analgesics.
Approximately 12 hours after discharge, the patient presented back to the ED via ambulance. At that time, she was hypotensive and tachypneic, with a thready palpable pulse. On repeat examination, she no longer had pulses present in her feet. An arteriogram found complete occlusion of her arterial circulation at the level of the knees bilaterally, requiring bilateral belowthe- knee amputation.
The patient sued both the emergency medicine physician and the PA for failure to provide her with the necessary care during her initial ED visit, resulting in loss of limbs. The defendants claimed the patient could not prove gross negligence by clear and convincing evidence, as required by state law. Following the ensuing trial, the jury returned a $5 million verdict in favor of the plaintiff.
Discussion
First, it is important to remember that just because a patient has been triaged to a low-acuity area does not mean she or he must have a minor problem. The provider still must maintain a high level of vigilance— regardless of the location of the patient in the ED.
Second, was this patient in atrial fibrillation, which is responsible in approximately 65% of all peripheral emboli? The abrupt onset of this patient’s symptoms is much more compatible with an embolic origin of her symptoms rather than a thrombus (ie, symptoms of claudication).
Lastly, a diagnosis of cellulitis is inconsistent with the physical findings of the PA, as well as those of the triage nurse and paramedics. This patient’s feet were cool to the touch whereas cellulitis presents with erythema and increased warmth. While the presence of pulses was somewhat reassuring, the cool temperature of the feet and complaint of pain were indicators of the need to evaluate for a possible arterial origin of these findings. However, if this were an embolic phenomenon, peripheral arterial ultrasound would have probably been normal, and the outcome unchanged.
Case 3: Failure to Communicate
A 59-year-old man presented to his primary care physician (PCP) for intermittent right-hand weakness and numbness and tingling in his right arm during the previous 24 hours. As the PCP was concerned that the patient might be experiencing a transient ischemic attack (TIA), the patient and his wife were instructed to go directly to the ED of the local hospital. The PCP wrote a note stating that the patient needed “a stroke work up,” gave the note to the patient, and told him to give it to the ED staff.
The patient went directly to the ED and was immediately seen by a “rapid triage nurse.” He gave the note to the nurse and told her of the PCP’s concerns. The nurse documented on the hospital assessment form that the patient was high priority and needed to be seen immediately. She attached the PCP’s note to the front of the form.
The patient was then seen by the traditional triage nurse. After evaluation, she changed the priority from high to low acuity. The triage nurse later stated later that she never saw the PCP’s note, nor did she obtain any history regarding the concerns of the PCP.
The patient was then evaluated by an EP in the low-acuity (or minor-care) area of the ED. The EP later stated that he never saw the note from the PCP, and had not received any information regarding a suspected TIA or stroke. The EP ordered a right wrist X-ray, diagnosed carpel tunnel syndrome, and prescribed an anti-inflammatory medication as well as follow-up with a hand surgeon.
The initial (rapid triage) nurse saw the patient leaving the ED at the time of discharge and thought he had not been in the ED long enough to have undergone a stroke work up. She reviewed his paperwork and saw the patient had not received the indicated work up. The nurse called the patient’s house and left a message on the answering machine notifying him of the need to return to the ED. The patient arrived back to the ED approximately 2 hours later.
On the second ED presentation that day, the patient was evaluated by a different EP. The patient had blood drawn, an electrocardiogram, and a noncontrast computed tomography scan of the brain, the results of which were all normal. The EP concluded the patient required admission to the hospital for additional work up (eg, carotid Doppler ultrasound). The hospitalist was paged, and told the EP he would be there “as soon as possible.” However, after several hours delay, and no hospitalist, the patient became impatient and expressed the desire to go home. The EP urged the patient to follow up with his PCP in the morning to complete the evaluation.
The next day, before seeing his PCP, the patient suffered an ischemic stroke with right-sided hemiparesis. The case went to trial, and the jury found in favor of the plaintiff.
Discussion
Unfortunately, multiple opportunities were lost in obtaining the correct care at the right time for this patient. Lack of communication and poor communication are frequently cited as causes in medical malpractice cases, and this case perfectly illustrates this problem.
First, the PCP should have called the ED and spoken to the EP directly. This would have provided the PCP the opportunity to express his concerns directly to the treating physician. This kind of one-on-one communication between physicians will always be superior to a hand-written note.
Second, it is unclear why the triage nurse changed the initial nurse’s correct assessment. It is also unclear what happened to the PCP’s note—it was never seen again. Clearly there was miscommunication at this point between the triage nurse and the patient. This case further illustrates the importance of good triage. Once a patient is directed down the wrong pathway (ie, to minor care rather than the main treatment area), the situation becomes much more difficult to correct.
Next, the EP in the low-acuity area was probably falsely assured this patient had only a “minor” problem problem, and not something serious. Emergency physicians must be vigilant to the possibility that the patient can have something seriously wrong even if he or she has been triaged to a low-acuity area. A minor sore throat can turn out to be epiglottitis and a viral stomachache can turn out to be appendicitis. These patients deserve the same quality of history, physical examination, and differential diagnosis as any other patient in the ED.
Finally, while we are not responsible for hospitalists or consultants, we do have a responsibility to our patients. We need to ensure that the care they receive is the appropriate care. Possible alternatives to discharging this patient would have been to call another hospitalist for admission or to seek the input of the chief of the medical staff or the on-call hospital administrator. As EPs, we are frequently required to serve as the primary advocate for our patients.
There is the possibility that even if the patient had been admitted to the hospital, the outcome would have been the same. However, since he might have been a candidate for tissue plasminogen activator or interventional radiology if he had suffered the cerebrovascular accident as an inpatient, he lost his best chance for a good outcome.
Case 1: Follow-up in Community-Acquired Methicillin- Resistant Staphylococcus Aureus Pharyngitis
A 73-year-old woman presented to the ED complaining of injuries she sustained following a minor fall at home. The patient’s past medical history was remarkable for diabetes mellitus (DM), hypertension, cerebrovascular accident, and a history of chronic pain. During the course of evaluation, the patient mentioned that she had experienced a sore throat. In addition to X-rays, the emergency physician (EP) ordered a rapid strep test (RST). All studies were normal and the patient was discharged home.
Over the next 2 months, the patient received medical treatment from other medical professionals, but not at the previous hospital. Reportedly, she did not complain of a sore throat during this time period. The patient did return to the same ED approximately 2 months later, complaining of cough and difficulty breathing; she died 5 days thereafter. No autopsy was performed.
A lawsuit was filed on behalf of the patient, stating the hospital breached the standard of care by not reporting the finding of MRSA directly to the treating physician, and that this led directly to the patient’s death by MRSA pneumonia. The jury returned a verdict in favor of the plaintiff for $32 million.
Discussion
This case illustrates two simple but important points: managing community-acquired MRSA (CA-MRSA) infections is a growing challenge for physicians; and hospital follow-up systems for positive findings (ie, fractures, blood cultures, etc) need to be consistently reliable.
It is estimated that 30% of healthy people carry S aureus in their anterior nares; colonization rates for the throat are much less studied. In one recent study, 265 throat swabs were collected from patients aged 14 to 65 years old, who complained of pharyngitis in an outpatient setting.1 A total of 165 S aureus isolates (62.3%) were recovered from the 265 swabs. For the S aureus isolates, 38.2% (63) grew CA-MRSA; the remaining 68.1% (102) were methicillin-sensitive S aureus (MSSA). Interestingly, of the 63 MRSA-positive swabs, over half also grew Group A Streptococcus. The natural disease progression of CA-MRSA pharyngitis is still unknown, as is what to do with a positive throat swab for CA-MRSA. While there are a few case reports of bacteremia and Lemmiere syndrome possibly related to CA-MRSA pharyngitis,2,3 more information is clearly needed. For this case, it is not possible to definitively determine the role of the positive throat swab for CAMRSA in the patient’s subsequent death.
The other teaching point in this case is much simpler and well defined. Simply put, patients expect to be informed of positive findings, whether the result is known at the time of their ED visit or sometime afterward. There needs to be a system in place that consistently and reliably provides important information to either the patient’s treating physician or to the patient. The manners in which this information is communicated are myriad and should take into account hospital resources, the role of the EP and nurse, and what works best for your locality. The “who” and “how” of the contact is not important: reliability, timeliness, and consistency need to be the key drivers of the system.
Case 2: Arterial Occlusion
A 61-year-old woman called emergency medical services (EMS) after noticing her feet felt cold to the touch and having difficulty ambulating. The paramedics noted the patient had normal vital signs and normal circulation in her legs, and she was transported to the ED without incident. Upon arrival to the ED, she was triaged as nonurgent and placed in the minor-care area. On nursing assessment, the patient’s feet were found to be cold, but with palpable pulses bilaterally. Her past medical history was significant for hypertension and DM.
The patient was seen by a physician assistant (PA), who found both feet cool to the touch, but with bilateral pulses present. She was administered intravenous morphine for pain and laboratory studies were ordered. At the time, the PA was concerned about arterial occlusion versus deep vein thrombosis (DVT) versus cellulitis. A venous ultrasound examination was ordered and shown to be negative for DVT. A complete blood count was remarkable only for mild leukocytosis. The PA discussed the case with the supervising EP; they agreed on a diagnosis of cellulitis and discharged the patient home with antibiotics and analgesics.
Approximately 12 hours after discharge, the patient presented back to the ED via ambulance. At that time, she was hypotensive and tachypneic, with a thready palpable pulse. On repeat examination, she no longer had pulses present in her feet. An arteriogram found complete occlusion of her arterial circulation at the level of the knees bilaterally, requiring bilateral belowthe- knee amputation.
The patient sued both the emergency medicine physician and the PA for failure to provide her with the necessary care during her initial ED visit, resulting in loss of limbs. The defendants claimed the patient could not prove gross negligence by clear and convincing evidence, as required by state law. Following the ensuing trial, the jury returned a $5 million verdict in favor of the plaintiff.
Discussion
First, it is important to remember that just because a patient has been triaged to a low-acuity area does not mean she or he must have a minor problem. The provider still must maintain a high level of vigilance— regardless of the location of the patient in the ED.
Second, was this patient in atrial fibrillation, which is responsible in approximately 65% of all peripheral emboli? The abrupt onset of this patient’s symptoms is much more compatible with an embolic origin of her symptoms rather than a thrombus (ie, symptoms of claudication).
Lastly, a diagnosis of cellulitis is inconsistent with the physical findings of the PA, as well as those of the triage nurse and paramedics. This patient’s feet were cool to the touch whereas cellulitis presents with erythema and increased warmth. While the presence of pulses was somewhat reassuring, the cool temperature of the feet and complaint of pain were indicators of the need to evaluate for a possible arterial origin of these findings. However, if this were an embolic phenomenon, peripheral arterial ultrasound would have probably been normal, and the outcome unchanged.
Case 3: Failure to Communicate
A 59-year-old man presented to his primary care physician (PCP) for intermittent right-hand weakness and numbness and tingling in his right arm during the previous 24 hours. As the PCP was concerned that the patient might be experiencing a transient ischemic attack (TIA), the patient and his wife were instructed to go directly to the ED of the local hospital. The PCP wrote a note stating that the patient needed “a stroke work up,” gave the note to the patient, and told him to give it to the ED staff.
The patient went directly to the ED and was immediately seen by a “rapid triage nurse.” He gave the note to the nurse and told her of the PCP’s concerns. The nurse documented on the hospital assessment form that the patient was high priority and needed to be seen immediately. She attached the PCP’s note to the front of the form.
The patient was then seen by the traditional triage nurse. After evaluation, she changed the priority from high to low acuity. The triage nurse later stated later that she never saw the PCP’s note, nor did she obtain any history regarding the concerns of the PCP.
The patient was then evaluated by an EP in the low-acuity (or minor-care) area of the ED. The EP later stated that he never saw the note from the PCP, and had not received any information regarding a suspected TIA or stroke. The EP ordered a right wrist X-ray, diagnosed carpel tunnel syndrome, and prescribed an anti-inflammatory medication as well as follow-up with a hand surgeon.
The initial (rapid triage) nurse saw the patient leaving the ED at the time of discharge and thought he had not been in the ED long enough to have undergone a stroke work up. She reviewed his paperwork and saw the patient had not received the indicated work up. The nurse called the patient’s house and left a message on the answering machine notifying him of the need to return to the ED. The patient arrived back to the ED approximately 2 hours later.
On the second ED presentation that day, the patient was evaluated by a different EP. The patient had blood drawn, an electrocardiogram, and a noncontrast computed tomography scan of the brain, the results of which were all normal. The EP concluded the patient required admission to the hospital for additional work up (eg, carotid Doppler ultrasound). The hospitalist was paged, and told the EP he would be there “as soon as possible.” However, after several hours delay, and no hospitalist, the patient became impatient and expressed the desire to go home. The EP urged the patient to follow up with his PCP in the morning to complete the evaluation.
The next day, before seeing his PCP, the patient suffered an ischemic stroke with right-sided hemiparesis. The case went to trial, and the jury found in favor of the plaintiff.
Discussion
Unfortunately, multiple opportunities were lost in obtaining the correct care at the right time for this patient. Lack of communication and poor communication are frequently cited as causes in medical malpractice cases, and this case perfectly illustrates this problem.
First, the PCP should have called the ED and spoken to the EP directly. This would have provided the PCP the opportunity to express his concerns directly to the treating physician. This kind of one-on-one communication between physicians will always be superior to a hand-written note.
Second, it is unclear why the triage nurse changed the initial nurse’s correct assessment. It is also unclear what happened to the PCP’s note—it was never seen again. Clearly there was miscommunication at this point between the triage nurse and the patient. This case further illustrates the importance of good triage. Once a patient is directed down the wrong pathway (ie, to minor care rather than the main treatment area), the situation becomes much more difficult to correct.
Next, the EP in the low-acuity area was probably falsely assured this patient had only a “minor” problem problem, and not something serious. Emergency physicians must be vigilant to the possibility that the patient can have something seriously wrong even if he or she has been triaged to a low-acuity area. A minor sore throat can turn out to be epiglottitis and a viral stomachache can turn out to be appendicitis. These patients deserve the same quality of history, physical examination, and differential diagnosis as any other patient in the ED.
Finally, while we are not responsible for hospitalists or consultants, we do have a responsibility to our patients. We need to ensure that the care they receive is the appropriate care. Possible alternatives to discharging this patient would have been to call another hospitalist for admission or to seek the input of the chief of the medical staff or the on-call hospital administrator. As EPs, we are frequently required to serve as the primary advocate for our patients.
There is the possibility that even if the patient had been admitted to the hospital, the outcome would have been the same. However, since he might have been a candidate for tissue plasminogen activator or interventional radiology if he had suffered the cerebrovascular accident as an inpatient, he lost his best chance for a good outcome.
- Gowrishankar S, Thenmozhi R, Balaji K, Pandian SK. Emergence of methicillin-resistant, vancomycin-intermediate Staphylococcus aureus among patients associated with group A Streptococcal pharyngitis infection in southern India. Infect Genet Evol. 2013;14:383-389.
- Wang LJ, Du XQ, Nyirimigabo E, Shou ST. A case report: concurrent infectious mononucleosis and community-associated methicillinresistant Staphylococcus aureus bacteremia. Am J Emerg Med. 2013. doi:10.1016/j.ajem.2013.10.033
- Kizhner VZ, Samara GJ, Panesar R, Krespi YP. MRSA bacteremia associated with Lemierre syndrome. Otolaryngol Head Neck Surg. 2011;145(suppl 2):P152,P153.
- Gowrishankar S, Thenmozhi R, Balaji K, Pandian SK. Emergence of methicillin-resistant, vancomycin-intermediate Staphylococcus aureus among patients associated with group A Streptococcal pharyngitis infection in southern India. Infect Genet Evol. 2013;14:383-389.
- Wang LJ, Du XQ, Nyirimigabo E, Shou ST. A case report: concurrent infectious mononucleosis and community-associated methicillinresistant Staphylococcus aureus bacteremia. Am J Emerg Med. 2013. doi:10.1016/j.ajem.2013.10.033
- Kizhner VZ, Samara GJ, Panesar R, Krespi YP. MRSA bacteremia associated with Lemierre syndrome. Otolaryngol Head Neck Surg. 2011;145(suppl 2):P152,P153.