User login
Dehydration in terminal illness: Which path forward?
CASE 1
A 94-year-old white woman, who had been in excellent health (other than pernicious anemia, treated with monthly cyanocobalamin injections), suddenly developed gastrointestinal distress 2 weeks earlier. A work-up performed by her physician revealed advanced pancreatic cancer.
Over the next 2 weeks, she experienced pain and nausea. A left-sided fistula developed externally at her flank that drained feces and induced considerable discomfort. An indwelling drain was placed, which provided some relief, but the patient’s dyspepsia, pain, and nausea escalated.
One month into her disease course, an oncologist reported on her potential treatment options and prognosis. Her life expectancy was about 3 months without treatment. This could be extended by 1 to 2 months with extensive surgical and chemotherapeutic interventions, but would further diminish her quality of life. The patient declined further treatment.
Her clinical status declined, and her quality of life significantly deteriorated. At 3 months, she felt life had lost meaning and was not worth living. She began asking for a morphine overdose, stating a desire to end her life.
After several discussions with the oncologist, one of the patient’s adult children suggested that her mother stop eating and drinking in order to diminish discomfort and hasten her demise. This plan was adopted, and the patient declined food and drank only enough to swish for oral comfort.
CASE 2
An 83-year-old woman with advanced Parkinson’s disease had become increasingly disabled. Her gait and motor skills were dramatically and progressively compromised. Pharmacotherapy yielded only transient improvement and considerable adverse effects of choreiform hyperkinesia and hallucinations, which were troublesome and embarrassing. Her social, physical, and personal well-being declined to the point that she was placed in a nursing home.
Despite this help, worsening parkinsonism progressively diminished her physical capacity. She became largely bedridden and developed decubitus ulcerations, especially at the coccyx, which produced severe pain and distress.
Continue to: The confluence of pain...
The confluence of pain, bedfastness, constipation, and social isolation yielded a loss of interest and joy in life. The patient required assistance with almost every aspect of daily life, including eating. As the illness progressed, she prayed at night that God would “take her.” Each morning, she spoke of disappointment upon reawakening. She overtly expressed her lack of desire to live to her family. Medical interventions were increasingly ineffective.
After repeated family and physician discussions had focused on her death wishes, one adult daughter recommended her mother stop eating and drinking; her food intake was already minimal. Although she did not endorse this plan verbally, the patient’s oral intake significantly diminished. Within 2 weeks, her physical state had declined, and she died one night during sleep.
Adequate hydration is stressed in physician education and practice. A conventional expectation to normalize fluid balance is important to restore health and improve well-being. In addition to being good medical practice, it can also show patients (and their families) that we care about their well-being.1-3
Treating dehydration in individuals with terminal illness is controversial from both medical and ethical standpoints. While the natural tendency of physicians is to restore full hydration to their patients, in select cases of imminent death, being fully hydrated may prolong discomfort.1,2 Emphasis in this population should be consistently placed on improving comfort care and quality of life, rather than prolonging life or delaying death.3-5
Continue to: A multifactorial, patient-based decision
A multifactorial, patient-based decision
Years ago, before the advent of hospitalizing people with terminal illnesses, dying at home amongst loved ones was believed to be peaceful. Nevertheless, questions arise about the practical vs ethical approach to caring for patients with terminal illness.2 Sometimes it is difficult to find a balance between potential health care benefits and the burdens induced by medical, legal, moral, and/or social pressures. Our medical communities and the general population uphold preserving dignity at the end of life, which is supported by organizations such as Compassion & Choices (a nonprofit group that seeks to improve and expand options for end of life care; https://www.compassionandchoices.org).
Allowing for voluntary, patient-determined dehydration in those with terminal illness can offer greater comfort than maintaining the physiologic degrees of fluid balance. There are 3 key considerations to bear in mind:
- Hydration is usually a standard part of quality medical care.1
- Selectively allowing dehydration in patients who are dying can facilitate comfort.1-5
- Dehydration may be a deliberate strategy to hasten death.6
When is dehydration appropriate?
Hydration is not favored whenever doing so may increase discomfort and prolong pain without meaningful life.3 In people with terminal illness, hydration may reduce quality of life.7
The data support dehydration in certain patients. A randomized controlled trial involving 129 patients receiving hospice care compared parenteral hydration with placebo, documenting that rehydration did not improve symptoms or quality of life; there was no significant difference between patients who were hydrated and those who were dehydrated.7 In fact, dehydration may even yield greater patient comfort.8
Case reports, retrospective chart reviews, and testimonials from health care professionals have reported that being less hydrated can diminish nausea, vomiting, diarrhea, ascites, edema, and urinary or bowel incontinence, with less skin breakdown.8 Hydration, on the other hand, may exacerbate dyspnea, coughing, and choking, increasing the need for suctioning.
Continue to: A component of palliative care
A component of palliative care. When death is imminent, palliation becomes key. Pain may be more manageable with less fluids, an important goal for this population.6,8 Dehydration is associated with an accumulation of opioids throughout body fluid volumes, which may decrease pain, consciousness, and/or agony.2 Pharmacotherapies might also have greater efficacy in a dehydrated patient.9 In addition, tissue shrinkage might mitigate pain from tumors, especially those in confined spaces.8
Hospice care and palliative medicine confirm that routine hydration is not always advisable; allowing for dehydration is a conventional practice, especially in older adults with terminal illness.7 However, do not deny access to liquids if a patient wants them, and never force unwanted fluids by any route.8 Facilitate oral care in the form of swishing fluids, elective drinking, or providing mouth lubrication for any patients selectively allowed to become dehydrated.3,8
The role of the physician in decision-making
Patients with terminal illness sometimes do not want fluids and may actively decline food and drink.10 This can be emotionally distressing for family members and/or caregivers to witness. Physicians can address this concern by compassionately explaining: “I know you are concerned that your relative is not eating or drinking, but there is no indication that hydration or parenteral feeding will improve function or quality of life.”10 This can generate a discussion between physicians and families by acknowledging concerns, relieving distress, and leading to what is ultimately best for the patient.
Implications for practice: Individualized autonomy
Physicians must identify patients who wish to die by purposely becoming dehydrated and uphold the important physician obligation to hydrate those with a recoverable illness. Allowing for a moderate degree of dehydration might provide greater comfort in select people with terminal illness. Some individuals for whom life has lost meaning may choose dehydration as a means to hasten their departure.4-6 Allowing individualized autonomy over life and death choices is part of a physician’s obligation to their patients. It can be difficult for caregivers, but it is medically indicated to comply with a patient’s desire for comfort when death is imminent.
Providing palliation as a priority over treatment is sometimes challenging, but comfort care takes preference and is always coordinated with the person’s own wishes. Facilitating dehydration removes assisted-suicide issues or requests and thus affords everyone involved more emotional comfort. An advantage of this method is that a decisional patient maintains full control over the direction of their choices and helps preserve dignity during the end of life.
CORRESPONDENCE
Steven Lippmann, MD, Department of Psychiatry, University of Louisville School of Medicine, 401 East Chestnut Street, Suite 610, Louisville, KY 40202; [email protected]
1. Burge FI. Dehydration and provision of fluids in palliative care. What is the evidence? Can Fam Physician. 1996;42:2383-2388.
2. Printz LA. Is withholding hydration a valid comfort measure in the terminally ill? Geriatrics. 1988;43:84-88.
3. Lippmann S. Palliative dehydration. Prim Care Companion CNS Disord. 2015;17: doi: 10.4088/PCC.15101797.
4. Bernat JL, Gert B, Mogielnicki RP. Patient refusal of hydration and nutrition: an alternative to physician-assisted suicide or voluntary active euthanasia. Arch Intern Med. 1993;153:2723-2728.
5. Sullivan RJ. Accepting death without artificial nutrition or hydration. J Gen Intern Med.1993;8:220-224.
6. Miller FG, Meier DE. Voluntary death: a comparison of terminal dehydration and physician-assisted suicide. Ann Intern Med. 1998;128:559-562.
7. Bruera E, Hui D, Dalal S, et al. Parenteral hydration in patients with advanced cancer: a multicenter, double-blind, placebo-controlled randomized trial. J Clin Oncol. 2013;31:111-118.
8. Forrow L, Smith HS. Pain management in end of life: palliative care. In: Warfield CA, Bajwa ZH, ed. Principles and Practice of Pain Management. 2nd ed. New York, NY: McGraw-Hill; 2004.
9. Zerwekh JV. The dehydration question. Nursing. 1983;13:47-51.
10. Bailey F, Harman S. Palliative care: The last hours and days of life. www.uptodate.com. September, 2016. Accessed on September 11, 2018.
CASE 1
A 94-year-old white woman, who had been in excellent health (other than pernicious anemia, treated with monthly cyanocobalamin injections), suddenly developed gastrointestinal distress 2 weeks earlier. A work-up performed by her physician revealed advanced pancreatic cancer.
Over the next 2 weeks, she experienced pain and nausea. A left-sided fistula developed externally at her flank that drained feces and induced considerable discomfort. An indwelling drain was placed, which provided some relief, but the patient’s dyspepsia, pain, and nausea escalated.
One month into her disease course, an oncologist reported on her potential treatment options and prognosis. Her life expectancy was about 3 months without treatment. This could be extended by 1 to 2 months with extensive surgical and chemotherapeutic interventions, but would further diminish her quality of life. The patient declined further treatment.
Her clinical status declined, and her quality of life significantly deteriorated. At 3 months, she felt life had lost meaning and was not worth living. She began asking for a morphine overdose, stating a desire to end her life.
After several discussions with the oncologist, one of the patient’s adult children suggested that her mother stop eating and drinking in order to diminish discomfort and hasten her demise. This plan was adopted, and the patient declined food and drank only enough to swish for oral comfort.
CASE 2
An 83-year-old woman with advanced Parkinson’s disease had become increasingly disabled. Her gait and motor skills were dramatically and progressively compromised. Pharmacotherapy yielded only transient improvement and considerable adverse effects of choreiform hyperkinesia and hallucinations, which were troublesome and embarrassing. Her social, physical, and personal well-being declined to the point that she was placed in a nursing home.
Despite this help, worsening parkinsonism progressively diminished her physical capacity. She became largely bedridden and developed decubitus ulcerations, especially at the coccyx, which produced severe pain and distress.
Continue to: The confluence of pain...
The confluence of pain, bedfastness, constipation, and social isolation yielded a loss of interest and joy in life. The patient required assistance with almost every aspect of daily life, including eating. As the illness progressed, she prayed at night that God would “take her.” Each morning, she spoke of disappointment upon reawakening. She overtly expressed her lack of desire to live to her family. Medical interventions were increasingly ineffective.
After repeated family and physician discussions had focused on her death wishes, one adult daughter recommended her mother stop eating and drinking; her food intake was already minimal. Although she did not endorse this plan verbally, the patient’s oral intake significantly diminished. Within 2 weeks, her physical state had declined, and she died one night during sleep.
Adequate hydration is stressed in physician education and practice. A conventional expectation to normalize fluid balance is important to restore health and improve well-being. In addition to being good medical practice, it can also show patients (and their families) that we care about their well-being.1-3
Treating dehydration in individuals with terminal illness is controversial from both medical and ethical standpoints. While the natural tendency of physicians is to restore full hydration to their patients, in select cases of imminent death, being fully hydrated may prolong discomfort.1,2 Emphasis in this population should be consistently placed on improving comfort care and quality of life, rather than prolonging life or delaying death.3-5
Continue to: A multifactorial, patient-based decision
A multifactorial, patient-based decision
Years ago, before the advent of hospitalizing people with terminal illnesses, dying at home amongst loved ones was believed to be peaceful. Nevertheless, questions arise about the practical vs ethical approach to caring for patients with terminal illness.2 Sometimes it is difficult to find a balance between potential health care benefits and the burdens induced by medical, legal, moral, and/or social pressures. Our medical communities and the general population uphold preserving dignity at the end of life, which is supported by organizations such as Compassion & Choices (a nonprofit group that seeks to improve and expand options for end of life care; https://www.compassionandchoices.org).
Allowing for voluntary, patient-determined dehydration in those with terminal illness can offer greater comfort than maintaining the physiologic degrees of fluid balance. There are 3 key considerations to bear in mind:
- Hydration is usually a standard part of quality medical care.1
- Selectively allowing dehydration in patients who are dying can facilitate comfort.1-5
- Dehydration may be a deliberate strategy to hasten death.6
When is dehydration appropriate?
Hydration is not favored whenever doing so may increase discomfort and prolong pain without meaningful life.3 In people with terminal illness, hydration may reduce quality of life.7
The data support dehydration in certain patients. A randomized controlled trial involving 129 patients receiving hospice care compared parenteral hydration with placebo, documenting that rehydration did not improve symptoms or quality of life; there was no significant difference between patients who were hydrated and those who were dehydrated.7 In fact, dehydration may even yield greater patient comfort.8
Case reports, retrospective chart reviews, and testimonials from health care professionals have reported that being less hydrated can diminish nausea, vomiting, diarrhea, ascites, edema, and urinary or bowel incontinence, with less skin breakdown.8 Hydration, on the other hand, may exacerbate dyspnea, coughing, and choking, increasing the need for suctioning.
Continue to: A component of palliative care
A component of palliative care. When death is imminent, palliation becomes key. Pain may be more manageable with less fluids, an important goal for this population.6,8 Dehydration is associated with an accumulation of opioids throughout body fluid volumes, which may decrease pain, consciousness, and/or agony.2 Pharmacotherapies might also have greater efficacy in a dehydrated patient.9 In addition, tissue shrinkage might mitigate pain from tumors, especially those in confined spaces.8
Hospice care and palliative medicine confirm that routine hydration is not always advisable; allowing for dehydration is a conventional practice, especially in older adults with terminal illness.7 However, do not deny access to liquids if a patient wants them, and never force unwanted fluids by any route.8 Facilitate oral care in the form of swishing fluids, elective drinking, or providing mouth lubrication for any patients selectively allowed to become dehydrated.3,8
The role of the physician in decision-making
Patients with terminal illness sometimes do not want fluids and may actively decline food and drink.10 This can be emotionally distressing for family members and/or caregivers to witness. Physicians can address this concern by compassionately explaining: “I know you are concerned that your relative is not eating or drinking, but there is no indication that hydration or parenteral feeding will improve function or quality of life.”10 This can generate a discussion between physicians and families by acknowledging concerns, relieving distress, and leading to what is ultimately best for the patient.
Implications for practice: Individualized autonomy
Physicians must identify patients who wish to die by purposely becoming dehydrated and uphold the important physician obligation to hydrate those with a recoverable illness. Allowing for a moderate degree of dehydration might provide greater comfort in select people with terminal illness. Some individuals for whom life has lost meaning may choose dehydration as a means to hasten their departure.4-6 Allowing individualized autonomy over life and death choices is part of a physician’s obligation to their patients. It can be difficult for caregivers, but it is medically indicated to comply with a patient’s desire for comfort when death is imminent.
Providing palliation as a priority over treatment is sometimes challenging, but comfort care takes preference and is always coordinated with the person’s own wishes. Facilitating dehydration removes assisted-suicide issues or requests and thus affords everyone involved more emotional comfort. An advantage of this method is that a decisional patient maintains full control over the direction of their choices and helps preserve dignity during the end of life.
CORRESPONDENCE
Steven Lippmann, MD, Department of Psychiatry, University of Louisville School of Medicine, 401 East Chestnut Street, Suite 610, Louisville, KY 40202; [email protected]
CASE 1
A 94-year-old white woman, who had been in excellent health (other than pernicious anemia, treated with monthly cyanocobalamin injections), suddenly developed gastrointestinal distress 2 weeks earlier. A work-up performed by her physician revealed advanced pancreatic cancer.
Over the next 2 weeks, she experienced pain and nausea. A left-sided fistula developed externally at her flank that drained feces and induced considerable discomfort. An indwelling drain was placed, which provided some relief, but the patient’s dyspepsia, pain, and nausea escalated.
One month into her disease course, an oncologist reported on her potential treatment options and prognosis. Her life expectancy was about 3 months without treatment. This could be extended by 1 to 2 months with extensive surgical and chemotherapeutic interventions, but would further diminish her quality of life. The patient declined further treatment.
Her clinical status declined, and her quality of life significantly deteriorated. At 3 months, she felt life had lost meaning and was not worth living. She began asking for a morphine overdose, stating a desire to end her life.
After several discussions with the oncologist, one of the patient’s adult children suggested that her mother stop eating and drinking in order to diminish discomfort and hasten her demise. This plan was adopted, and the patient declined food and drank only enough to swish for oral comfort.
CASE 2
An 83-year-old woman with advanced Parkinson’s disease had become increasingly disabled. Her gait and motor skills were dramatically and progressively compromised. Pharmacotherapy yielded only transient improvement and considerable adverse effects of choreiform hyperkinesia and hallucinations, which were troublesome and embarrassing. Her social, physical, and personal well-being declined to the point that she was placed in a nursing home.
Despite this help, worsening parkinsonism progressively diminished her physical capacity. She became largely bedridden and developed decubitus ulcerations, especially at the coccyx, which produced severe pain and distress.
Continue to: The confluence of pain...
The confluence of pain, bedfastness, constipation, and social isolation yielded a loss of interest and joy in life. The patient required assistance with almost every aspect of daily life, including eating. As the illness progressed, she prayed at night that God would “take her.” Each morning, she spoke of disappointment upon reawakening. She overtly expressed her lack of desire to live to her family. Medical interventions were increasingly ineffective.
After repeated family and physician discussions had focused on her death wishes, one adult daughter recommended her mother stop eating and drinking; her food intake was already minimal. Although she did not endorse this plan verbally, the patient’s oral intake significantly diminished. Within 2 weeks, her physical state had declined, and she died one night during sleep.
Adequate hydration is stressed in physician education and practice. A conventional expectation to normalize fluid balance is important to restore health and improve well-being. In addition to being good medical practice, it can also show patients (and their families) that we care about their well-being.1-3
Treating dehydration in individuals with terminal illness is controversial from both medical and ethical standpoints. While the natural tendency of physicians is to restore full hydration to their patients, in select cases of imminent death, being fully hydrated may prolong discomfort.1,2 Emphasis in this population should be consistently placed on improving comfort care and quality of life, rather than prolonging life or delaying death.3-5
Continue to: A multifactorial, patient-based decision
A multifactorial, patient-based decision
Years ago, before the advent of hospitalizing people with terminal illnesses, dying at home amongst loved ones was believed to be peaceful. Nevertheless, questions arise about the practical vs ethical approach to caring for patients with terminal illness.2 Sometimes it is difficult to find a balance between potential health care benefits and the burdens induced by medical, legal, moral, and/or social pressures. Our medical communities and the general population uphold preserving dignity at the end of life, which is supported by organizations such as Compassion & Choices (a nonprofit group that seeks to improve and expand options for end of life care; https://www.compassionandchoices.org).
Allowing for voluntary, patient-determined dehydration in those with terminal illness can offer greater comfort than maintaining the physiologic degrees of fluid balance. There are 3 key considerations to bear in mind:
- Hydration is usually a standard part of quality medical care.1
- Selectively allowing dehydration in patients who are dying can facilitate comfort.1-5
- Dehydration may be a deliberate strategy to hasten death.6
When is dehydration appropriate?
Hydration is not favored whenever doing so may increase discomfort and prolong pain without meaningful life.3 In people with terminal illness, hydration may reduce quality of life.7
The data support dehydration in certain patients. A randomized controlled trial involving 129 patients receiving hospice care compared parenteral hydration with placebo, documenting that rehydration did not improve symptoms or quality of life; there was no significant difference between patients who were hydrated and those who were dehydrated.7 In fact, dehydration may even yield greater patient comfort.8
Case reports, retrospective chart reviews, and testimonials from health care professionals have reported that being less hydrated can diminish nausea, vomiting, diarrhea, ascites, edema, and urinary or bowel incontinence, with less skin breakdown.8 Hydration, on the other hand, may exacerbate dyspnea, coughing, and choking, increasing the need for suctioning.
Continue to: A component of palliative care
A component of palliative care. When death is imminent, palliation becomes key. Pain may be more manageable with less fluids, an important goal for this population.6,8 Dehydration is associated with an accumulation of opioids throughout body fluid volumes, which may decrease pain, consciousness, and/or agony.2 Pharmacotherapies might also have greater efficacy in a dehydrated patient.9 In addition, tissue shrinkage might mitigate pain from tumors, especially those in confined spaces.8
Hospice care and palliative medicine confirm that routine hydration is not always advisable; allowing for dehydration is a conventional practice, especially in older adults with terminal illness.7 However, do not deny access to liquids if a patient wants them, and never force unwanted fluids by any route.8 Facilitate oral care in the form of swishing fluids, elective drinking, or providing mouth lubrication for any patients selectively allowed to become dehydrated.3,8
The role of the physician in decision-making
Patients with terminal illness sometimes do not want fluids and may actively decline food and drink.10 This can be emotionally distressing for family members and/or caregivers to witness. Physicians can address this concern by compassionately explaining: “I know you are concerned that your relative is not eating or drinking, but there is no indication that hydration or parenteral feeding will improve function or quality of life.”10 This can generate a discussion between physicians and families by acknowledging concerns, relieving distress, and leading to what is ultimately best for the patient.
Implications for practice: Individualized autonomy
Physicians must identify patients who wish to die by purposely becoming dehydrated and uphold the important physician obligation to hydrate those with a recoverable illness. Allowing for a moderate degree of dehydration might provide greater comfort in select people with terminal illness. Some individuals for whom life has lost meaning may choose dehydration as a means to hasten their departure.4-6 Allowing individualized autonomy over life and death choices is part of a physician’s obligation to their patients. It can be difficult for caregivers, but it is medically indicated to comply with a patient’s desire for comfort when death is imminent.
Providing palliation as a priority over treatment is sometimes challenging, but comfort care takes preference and is always coordinated with the person’s own wishes. Facilitating dehydration removes assisted-suicide issues or requests and thus affords everyone involved more emotional comfort. An advantage of this method is that a decisional patient maintains full control over the direction of their choices and helps preserve dignity during the end of life.
CORRESPONDENCE
Steven Lippmann, MD, Department of Psychiatry, University of Louisville School of Medicine, 401 East Chestnut Street, Suite 610, Louisville, KY 40202; [email protected]
1. Burge FI. Dehydration and provision of fluids in palliative care. What is the evidence? Can Fam Physician. 1996;42:2383-2388.
2. Printz LA. Is withholding hydration a valid comfort measure in the terminally ill? Geriatrics. 1988;43:84-88.
3. Lippmann S. Palliative dehydration. Prim Care Companion CNS Disord. 2015;17: doi: 10.4088/PCC.15101797.
4. Bernat JL, Gert B, Mogielnicki RP. Patient refusal of hydration and nutrition: an alternative to physician-assisted suicide or voluntary active euthanasia. Arch Intern Med. 1993;153:2723-2728.
5. Sullivan RJ. Accepting death without artificial nutrition or hydration. J Gen Intern Med.1993;8:220-224.
6. Miller FG, Meier DE. Voluntary death: a comparison of terminal dehydration and physician-assisted suicide. Ann Intern Med. 1998;128:559-562.
7. Bruera E, Hui D, Dalal S, et al. Parenteral hydration in patients with advanced cancer: a multicenter, double-blind, placebo-controlled randomized trial. J Clin Oncol. 2013;31:111-118.
8. Forrow L, Smith HS. Pain management in end of life: palliative care. In: Warfield CA, Bajwa ZH, ed. Principles and Practice of Pain Management. 2nd ed. New York, NY: McGraw-Hill; 2004.
9. Zerwekh JV. The dehydration question. Nursing. 1983;13:47-51.
10. Bailey F, Harman S. Palliative care: The last hours and days of life. www.uptodate.com. September, 2016. Accessed on September 11, 2018.
1. Burge FI. Dehydration and provision of fluids in palliative care. What is the evidence? Can Fam Physician. 1996;42:2383-2388.
2. Printz LA. Is withholding hydration a valid comfort measure in the terminally ill? Geriatrics. 1988;43:84-88.
3. Lippmann S. Palliative dehydration. Prim Care Companion CNS Disord. 2015;17: doi: 10.4088/PCC.15101797.
4. Bernat JL, Gert B, Mogielnicki RP. Patient refusal of hydration and nutrition: an alternative to physician-assisted suicide or voluntary active euthanasia. Arch Intern Med. 1993;153:2723-2728.
5. Sullivan RJ. Accepting death without artificial nutrition or hydration. J Gen Intern Med.1993;8:220-224.
6. Miller FG, Meier DE. Voluntary death: a comparison of terminal dehydration and physician-assisted suicide. Ann Intern Med. 1998;128:559-562.
7. Bruera E, Hui D, Dalal S, et al. Parenteral hydration in patients with advanced cancer: a multicenter, double-blind, placebo-controlled randomized trial. J Clin Oncol. 2013;31:111-118.
8. Forrow L, Smith HS. Pain management in end of life: palliative care. In: Warfield CA, Bajwa ZH, ed. Principles and Practice of Pain Management. 2nd ed. New York, NY: McGraw-Hill; 2004.
9. Zerwekh JV. The dehydration question. Nursing. 1983;13:47-51.
10. Bailey F, Harman S. Palliative care: The last hours and days of life. www.uptodate.com. September, 2016. Accessed on September 11, 2018.
Are Antipsychotic Medications Safe During Pregnancy?
Psychiatric illnesses are prevalent in about 25% of the US adult population.1 Approximately 21% to 33% of women are prescribed antipsychotic drugs during pregnancies,and about 50% experience a relapse of symptoms related to mental illness.2,3 In 2015, the Pregnancy and Lactation Labeling Rule removed the A, B, C, D, and X categories for medications prescribed during gestation. Labels now include more information and detail about these pharmaceuticals regarding potential risks to a mother and fetus.4 As with all other pharmacotherapies during pregnancy, teratogenicity and medicinal adverse effects (AEs) must be balanced against the risk of nonpharmacotherapy.
Medications
Antipsychotic medications often are prescribed to treat people with a wide range of psychiatric conditions, including schizophrenia, bipolar disorder, depression, anxiety, and personality disorders.5 Commonly, second-generation antipsychotic medications are selected for pregnant women. Olanzapine, haloperidol, risperidone, and quetiapine freely pass through the placenta.5 During gestation, when an antipsychotic agent is strongly indicated, it is prudent to select one of the second-generation versions or haloperidol.
Risks vs Benefits
Physicians should always consider the risk-to-benefit ratio of these medicines for both the pregnant woman and the fetus.6 The National Pregnancy Registry for Atypical Antipsychotics was established to evaluate the safety and efficacy of these drugs during pregnancy and the postpartum period.4,6
Even during the first trimester of pregnancy most antipsychotic medications prescribed to women, are documented to cause few major fetal malformations.7 Research during 487 pregnancies revealed that the risk of a malformed infant previously exposed in utero to antipsychotic drugs was 1.4%, compared with 1.1% for those not exposed.6 Risperidone, however is an exception, because evidence has shown that it results in more cardiac malformations and congenital anomalies than do other medications.7
Complications
Maternal complications, such as increased weight gain, gestational diabetes mellitus, hypertension, and venous thromboembolism, are reported in pregnant women prescribed antipsychotic medications.3 Sudden discontinuation of these drugs might interfere with activities of daily living, allow more psychotic symptoms in the mother, impair prenatal self-care, and increase the risk for suicide or infanticide.8 Fetal complications might include prematurity, intrauterine growth retardation, distress, suboptimal birth weights, low Apgar scores, neonatal hypoglycemia, and congenital defects. Stillbirths can occur as well.9
Neonates exposed to antipsychotic medications in utero can experience withdrawal symptoms after delivery. They might exhibit agitation, feeding disorders, hypotonia, hypertonia, respiratory distress, somnolence, and tremor.10 Extrapyramidal symptoms, such as abnormal movements, restlessness, stiffness, and tremors, may occur more often when prescribing first-generation rather than with second-generation antipsychotic drugs.11 These clinical manifestations occur from a few hours after birth to 1 month later. The management of withdrawal symptoms is not clear, though symptomatic intervention is recommended.11
However, studies have shown that documented AEs are not significantly increased in the patients or infants exposed to antipsychotic medications compared with those of a control group.7 Furthermore, pregnant women with mental illness who remain untreated or who discontinue these drugs during a gestation evidence increased maternal morbidity12;they also exhibit more complications, such as placental abnormalities, antepartum hemorrhage, or preeclampsia.6 Hence, when medications are indicated, physicians should encourage patients to continue taking these medications after being educated about the risks and benefits of pharmacotherapy.6
Conclusions
The advantages of prescribing antipsychotic drugs during pregnancy include better psychiatric, obstetric, and neonatal health. Although antipsychotic medications continue to be safe during pregnancy, only necessary prescribing of indicated antipsychotic medicine and maintaining the safest possible therapeutic profile is an optimal approach to treat pregnant women requiring these medications.12 The efficacy of these medications also depends on an individual assessment of the patient’s health and lifestyle. When obtaining a patient history, physicians should include a review of smoking, alcohol consumption, substance abuse, and prior and/or concomitant use of other medications. Demographics, medical comorbidities, and psychiatric illnesses have a role in the clinical outcome.13 Physicians also should consider dosage, timing, and duration of medication exposure.
A baby born with birth defects can be devastating to the mother and is always balanced against the risk of less intervention. Apart from guiding patients regarding antipsychotic medication intake, pregnant women should be educated about regular prenatal checkups, taking vitamins and other supplements, monitoring for gestational diabetes mellitus, a proper diet, and exercise. Physicians and their patients should always minimize exposure to smoking or drugs and medications, especially polypharmacy.13 A higher level of prenatal care is advised whenever a physician suspects complications, including a referral to a maternal-fetal specialist.
1. Centers for Disease Control and Prevention. CDC report: mental illness surveillance among adults in the United States. https://www.cdc.gov/mentalhealthsurveillance/fact_sheet.html. Archived document. Updated December 2, 2011. Accessed April 10, 2018.
2. L evenson JL, ed . The American Psychiatric Publishing Textbook of Psychosomatic Medicine: Psychiatric Care of the Medically Ill. 2nd ed. Arlington, VA: American Psychiatric Publishing; 2011 .
3. Kulkarni J, Worsley R, Gilbert H, et al. A prospective cohort study of antipsychotic medications in pregnancy: the first 147 pregnancies and 100 one year old babies. PLoS One. 2014;9(5):e94788.
4. US Food and Drug Administration. Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. https://www.fda.gov/downloads/aboutfda/reportsmanualsforms/reports/economicanalyses/ucm427798.pdf. Accessed April 10, 2018.
5. Ennis ZN, Damkier P. Pregnancy exposure to olanzapine, quetiapine, risperidone, aripiprazole and risk of congenital malformations. A systematic review. Basic Clin Pharmacol Toxicol. 2015;116(4):315-320.
6. Cohen LS, Viguera AC, McInerney KA, et al. Reproductive safety of second-generation antipsychotics: current data from the Massachusetts General Hospital National Pregnancy Registry for Atypical Antipsychotics. Am J Psychiatry. 2016:173(3):263-270.
7. Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry. 2016;73(9):938-946.
8. Galbally M, Snellen M, Power J. Antipsychotic drugs in pregnancy: a review of their maternal and fetal effects. Ther Adv Drug Saf. 2014;5(2):100-109.
9 . Crawford MB, DeLisi LE. Issues related to sex differences in antipsychotic treatment. Curr Opin Psychiatry. 2016;29(3):211-217.
10. Chisolm MS, Payne JL. Management of psychotropic drugs during pregnancy. BMJ. 2016;352:h5918.
11. US Food and Drug Administration. FDA drug safety communication: antipsychotic drug labels updated on use during pregnancy and risk of abnormal muscle movements and withdrawal symptoms in newborns. https://www.fda.gov/Drugs/DrugSafety/ucm243903.htm. Updated August 4, 2017. Accessed April 10, 2018.
12 . Tosato S, Albert U, Tomassi S, et al. A systematized review of atypical antipsychotics in pregnant women: balancing between risks of untreated illness and risks of drug-related adverse effects. J Clin Psychiatry. 2017;78(5):e477-e489.
13. Petersen I, Sammon CJ, McCrea RL, et al. Risks associated with antipsychotic treatment in pregnancy: comparative cohort studies based on electronic health records. Schizophr Res. 2016; 76(2-3):349-356.
Psychiatric illnesses are prevalent in about 25% of the US adult population.1 Approximately 21% to 33% of women are prescribed antipsychotic drugs during pregnancies,and about 50% experience a relapse of symptoms related to mental illness.2,3 In 2015, the Pregnancy and Lactation Labeling Rule removed the A, B, C, D, and X categories for medications prescribed during gestation. Labels now include more information and detail about these pharmaceuticals regarding potential risks to a mother and fetus.4 As with all other pharmacotherapies during pregnancy, teratogenicity and medicinal adverse effects (AEs) must be balanced against the risk of nonpharmacotherapy.
Medications
Antipsychotic medications often are prescribed to treat people with a wide range of psychiatric conditions, including schizophrenia, bipolar disorder, depression, anxiety, and personality disorders.5 Commonly, second-generation antipsychotic medications are selected for pregnant women. Olanzapine, haloperidol, risperidone, and quetiapine freely pass through the placenta.5 During gestation, when an antipsychotic agent is strongly indicated, it is prudent to select one of the second-generation versions or haloperidol.
Risks vs Benefits
Physicians should always consider the risk-to-benefit ratio of these medicines for both the pregnant woman and the fetus.6 The National Pregnancy Registry for Atypical Antipsychotics was established to evaluate the safety and efficacy of these drugs during pregnancy and the postpartum period.4,6
Even during the first trimester of pregnancy most antipsychotic medications prescribed to women, are documented to cause few major fetal malformations.7 Research during 487 pregnancies revealed that the risk of a malformed infant previously exposed in utero to antipsychotic drugs was 1.4%, compared with 1.1% for those not exposed.6 Risperidone, however is an exception, because evidence has shown that it results in more cardiac malformations and congenital anomalies than do other medications.7
Complications
Maternal complications, such as increased weight gain, gestational diabetes mellitus, hypertension, and venous thromboembolism, are reported in pregnant women prescribed antipsychotic medications.3 Sudden discontinuation of these drugs might interfere with activities of daily living, allow more psychotic symptoms in the mother, impair prenatal self-care, and increase the risk for suicide or infanticide.8 Fetal complications might include prematurity, intrauterine growth retardation, distress, suboptimal birth weights, low Apgar scores, neonatal hypoglycemia, and congenital defects. Stillbirths can occur as well.9
Neonates exposed to antipsychotic medications in utero can experience withdrawal symptoms after delivery. They might exhibit agitation, feeding disorders, hypotonia, hypertonia, respiratory distress, somnolence, and tremor.10 Extrapyramidal symptoms, such as abnormal movements, restlessness, stiffness, and tremors, may occur more often when prescribing first-generation rather than with second-generation antipsychotic drugs.11 These clinical manifestations occur from a few hours after birth to 1 month later. The management of withdrawal symptoms is not clear, though symptomatic intervention is recommended.11
However, studies have shown that documented AEs are not significantly increased in the patients or infants exposed to antipsychotic medications compared with those of a control group.7 Furthermore, pregnant women with mental illness who remain untreated or who discontinue these drugs during a gestation evidence increased maternal morbidity12;they also exhibit more complications, such as placental abnormalities, antepartum hemorrhage, or preeclampsia.6 Hence, when medications are indicated, physicians should encourage patients to continue taking these medications after being educated about the risks and benefits of pharmacotherapy.6
Conclusions
The advantages of prescribing antipsychotic drugs during pregnancy include better psychiatric, obstetric, and neonatal health. Although antipsychotic medications continue to be safe during pregnancy, only necessary prescribing of indicated antipsychotic medicine and maintaining the safest possible therapeutic profile is an optimal approach to treat pregnant women requiring these medications.12 The efficacy of these medications also depends on an individual assessment of the patient’s health and lifestyle. When obtaining a patient history, physicians should include a review of smoking, alcohol consumption, substance abuse, and prior and/or concomitant use of other medications. Demographics, medical comorbidities, and psychiatric illnesses have a role in the clinical outcome.13 Physicians also should consider dosage, timing, and duration of medication exposure.
A baby born with birth defects can be devastating to the mother and is always balanced against the risk of less intervention. Apart from guiding patients regarding antipsychotic medication intake, pregnant women should be educated about regular prenatal checkups, taking vitamins and other supplements, monitoring for gestational diabetes mellitus, a proper diet, and exercise. Physicians and their patients should always minimize exposure to smoking or drugs and medications, especially polypharmacy.13 A higher level of prenatal care is advised whenever a physician suspects complications, including a referral to a maternal-fetal specialist.
Psychiatric illnesses are prevalent in about 25% of the US adult population.1 Approximately 21% to 33% of women are prescribed antipsychotic drugs during pregnancies,and about 50% experience a relapse of symptoms related to mental illness.2,3 In 2015, the Pregnancy and Lactation Labeling Rule removed the A, B, C, D, and X categories for medications prescribed during gestation. Labels now include more information and detail about these pharmaceuticals regarding potential risks to a mother and fetus.4 As with all other pharmacotherapies during pregnancy, teratogenicity and medicinal adverse effects (AEs) must be balanced against the risk of nonpharmacotherapy.
Medications
Antipsychotic medications often are prescribed to treat people with a wide range of psychiatric conditions, including schizophrenia, bipolar disorder, depression, anxiety, and personality disorders.5 Commonly, second-generation antipsychotic medications are selected for pregnant women. Olanzapine, haloperidol, risperidone, and quetiapine freely pass through the placenta.5 During gestation, when an antipsychotic agent is strongly indicated, it is prudent to select one of the second-generation versions or haloperidol.
Risks vs Benefits
Physicians should always consider the risk-to-benefit ratio of these medicines for both the pregnant woman and the fetus.6 The National Pregnancy Registry for Atypical Antipsychotics was established to evaluate the safety and efficacy of these drugs during pregnancy and the postpartum period.4,6
Even during the first trimester of pregnancy most antipsychotic medications prescribed to women, are documented to cause few major fetal malformations.7 Research during 487 pregnancies revealed that the risk of a malformed infant previously exposed in utero to antipsychotic drugs was 1.4%, compared with 1.1% for those not exposed.6 Risperidone, however is an exception, because evidence has shown that it results in more cardiac malformations and congenital anomalies than do other medications.7
Complications
Maternal complications, such as increased weight gain, gestational diabetes mellitus, hypertension, and venous thromboembolism, are reported in pregnant women prescribed antipsychotic medications.3 Sudden discontinuation of these drugs might interfere with activities of daily living, allow more psychotic symptoms in the mother, impair prenatal self-care, and increase the risk for suicide or infanticide.8 Fetal complications might include prematurity, intrauterine growth retardation, distress, suboptimal birth weights, low Apgar scores, neonatal hypoglycemia, and congenital defects. Stillbirths can occur as well.9
Neonates exposed to antipsychotic medications in utero can experience withdrawal symptoms after delivery. They might exhibit agitation, feeding disorders, hypotonia, hypertonia, respiratory distress, somnolence, and tremor.10 Extrapyramidal symptoms, such as abnormal movements, restlessness, stiffness, and tremors, may occur more often when prescribing first-generation rather than with second-generation antipsychotic drugs.11 These clinical manifestations occur from a few hours after birth to 1 month later. The management of withdrawal symptoms is not clear, though symptomatic intervention is recommended.11
However, studies have shown that documented AEs are not significantly increased in the patients or infants exposed to antipsychotic medications compared with those of a control group.7 Furthermore, pregnant women with mental illness who remain untreated or who discontinue these drugs during a gestation evidence increased maternal morbidity12;they also exhibit more complications, such as placental abnormalities, antepartum hemorrhage, or preeclampsia.6 Hence, when medications are indicated, physicians should encourage patients to continue taking these medications after being educated about the risks and benefits of pharmacotherapy.6
Conclusions
The advantages of prescribing antipsychotic drugs during pregnancy include better psychiatric, obstetric, and neonatal health. Although antipsychotic medications continue to be safe during pregnancy, only necessary prescribing of indicated antipsychotic medicine and maintaining the safest possible therapeutic profile is an optimal approach to treat pregnant women requiring these medications.12 The efficacy of these medications also depends on an individual assessment of the patient’s health and lifestyle. When obtaining a patient history, physicians should include a review of smoking, alcohol consumption, substance abuse, and prior and/or concomitant use of other medications. Demographics, medical comorbidities, and psychiatric illnesses have a role in the clinical outcome.13 Physicians also should consider dosage, timing, and duration of medication exposure.
A baby born with birth defects can be devastating to the mother and is always balanced against the risk of less intervention. Apart from guiding patients regarding antipsychotic medication intake, pregnant women should be educated about regular prenatal checkups, taking vitamins and other supplements, monitoring for gestational diabetes mellitus, a proper diet, and exercise. Physicians and their patients should always minimize exposure to smoking or drugs and medications, especially polypharmacy.13 A higher level of prenatal care is advised whenever a physician suspects complications, including a referral to a maternal-fetal specialist.
1. Centers for Disease Control and Prevention. CDC report: mental illness surveillance among adults in the United States. https://www.cdc.gov/mentalhealthsurveillance/fact_sheet.html. Archived document. Updated December 2, 2011. Accessed April 10, 2018.
2. L evenson JL, ed . The American Psychiatric Publishing Textbook of Psychosomatic Medicine: Psychiatric Care of the Medically Ill. 2nd ed. Arlington, VA: American Psychiatric Publishing; 2011 .
3. Kulkarni J, Worsley R, Gilbert H, et al. A prospective cohort study of antipsychotic medications in pregnancy: the first 147 pregnancies and 100 one year old babies. PLoS One. 2014;9(5):e94788.
4. US Food and Drug Administration. Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. https://www.fda.gov/downloads/aboutfda/reportsmanualsforms/reports/economicanalyses/ucm427798.pdf. Accessed April 10, 2018.
5. Ennis ZN, Damkier P. Pregnancy exposure to olanzapine, quetiapine, risperidone, aripiprazole and risk of congenital malformations. A systematic review. Basic Clin Pharmacol Toxicol. 2015;116(4):315-320.
6. Cohen LS, Viguera AC, McInerney KA, et al. Reproductive safety of second-generation antipsychotics: current data from the Massachusetts General Hospital National Pregnancy Registry for Atypical Antipsychotics. Am J Psychiatry. 2016:173(3):263-270.
7. Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry. 2016;73(9):938-946.
8. Galbally M, Snellen M, Power J. Antipsychotic drugs in pregnancy: a review of their maternal and fetal effects. Ther Adv Drug Saf. 2014;5(2):100-109.
9 . Crawford MB, DeLisi LE. Issues related to sex differences in antipsychotic treatment. Curr Opin Psychiatry. 2016;29(3):211-217.
10. Chisolm MS, Payne JL. Management of psychotropic drugs during pregnancy. BMJ. 2016;352:h5918.
11. US Food and Drug Administration. FDA drug safety communication: antipsychotic drug labels updated on use during pregnancy and risk of abnormal muscle movements and withdrawal symptoms in newborns. https://www.fda.gov/Drugs/DrugSafety/ucm243903.htm. Updated August 4, 2017. Accessed April 10, 2018.
12 . Tosato S, Albert U, Tomassi S, et al. A systematized review of atypical antipsychotics in pregnant women: balancing between risks of untreated illness and risks of drug-related adverse effects. J Clin Psychiatry. 2017;78(5):e477-e489.
13. Petersen I, Sammon CJ, McCrea RL, et al. Risks associated with antipsychotic treatment in pregnancy: comparative cohort studies based on electronic health records. Schizophr Res. 2016; 76(2-3):349-356.
1. Centers for Disease Control and Prevention. CDC report: mental illness surveillance among adults in the United States. https://www.cdc.gov/mentalhealthsurveillance/fact_sheet.html. Archived document. Updated December 2, 2011. Accessed April 10, 2018.
2. L evenson JL, ed . The American Psychiatric Publishing Textbook of Psychosomatic Medicine: Psychiatric Care of the Medically Ill. 2nd ed. Arlington, VA: American Psychiatric Publishing; 2011 .
3. Kulkarni J, Worsley R, Gilbert H, et al. A prospective cohort study of antipsychotic medications in pregnancy: the first 147 pregnancies and 100 one year old babies. PLoS One. 2014;9(5):e94788.
4. US Food and Drug Administration. Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. https://www.fda.gov/downloads/aboutfda/reportsmanualsforms/reports/economicanalyses/ucm427798.pdf. Accessed April 10, 2018.
5. Ennis ZN, Damkier P. Pregnancy exposure to olanzapine, quetiapine, risperidone, aripiprazole and risk of congenital malformations. A systematic review. Basic Clin Pharmacol Toxicol. 2015;116(4):315-320.
6. Cohen LS, Viguera AC, McInerney KA, et al. Reproductive safety of second-generation antipsychotics: current data from the Massachusetts General Hospital National Pregnancy Registry for Atypical Antipsychotics. Am J Psychiatry. 2016:173(3):263-270.
7. Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry. 2016;73(9):938-946.
8. Galbally M, Snellen M, Power J. Antipsychotic drugs in pregnancy: a review of their maternal and fetal effects. Ther Adv Drug Saf. 2014;5(2):100-109.
9 . Crawford MB, DeLisi LE. Issues related to sex differences in antipsychotic treatment. Curr Opin Psychiatry. 2016;29(3):211-217.
10. Chisolm MS, Payne JL. Management of psychotropic drugs during pregnancy. BMJ. 2016;352:h5918.
11. US Food and Drug Administration. FDA drug safety communication: antipsychotic drug labels updated on use during pregnancy and risk of abnormal muscle movements and withdrawal symptoms in newborns. https://www.fda.gov/Drugs/DrugSafety/ucm243903.htm. Updated August 4, 2017. Accessed April 10, 2018.
12 . Tosato S, Albert U, Tomassi S, et al. A systematized review of atypical antipsychotics in pregnant women: balancing between risks of untreated illness and risks of drug-related adverse effects. J Clin Psychiatry. 2017;78(5):e477-e489.
13. Petersen I, Sammon CJ, McCrea RL, et al. Risks associated with antipsychotic treatment in pregnancy: comparative cohort studies based on electronic health records. Schizophr Res. 2016; 76(2-3):349-356.
A 95-year-old man with treatment-resistant depression
CASE Depressed, avoidant
Mr. R, age 95, has a history of recurrent major depressive disorder. He presents to the emergency department with depressive symptoms that began 6 weeks ago. His symptoms include depressed mood, hopelessness, anhedonia, anxiety, and insomnia. Co-occurring anorexia nervosa has resulted in a 20-lb weight loss. He denies suicidal ideation.
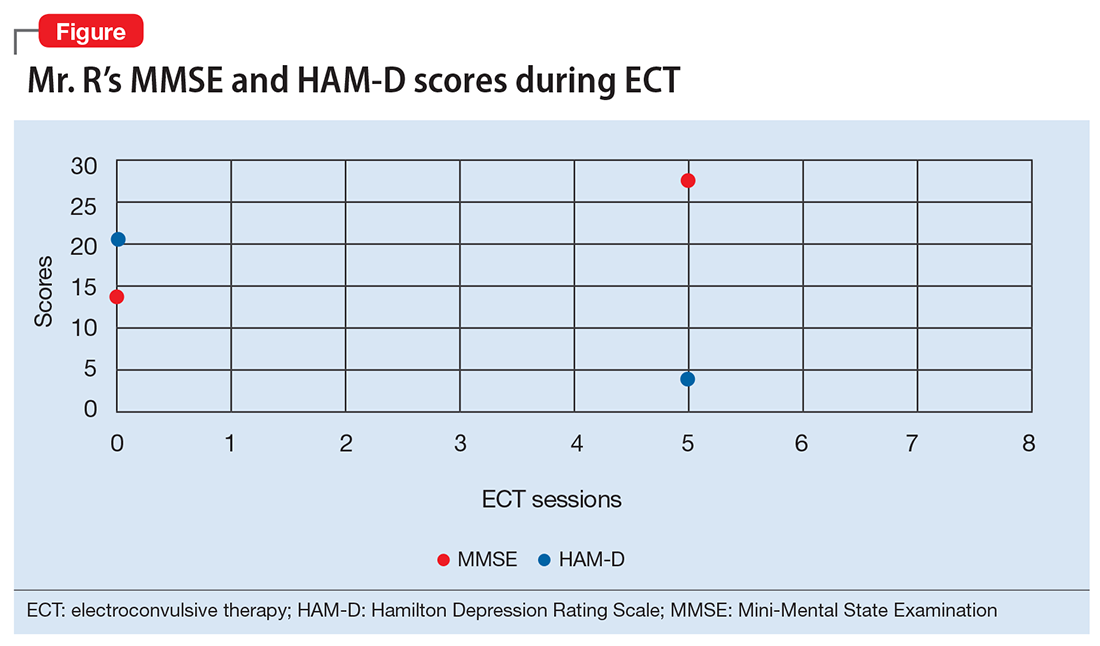
A mental status examination reveals profound psychomotor agitation, dysphoric mood, tearfulness, and mood-congruent delusions. Mr. R’s Mini-Mental State Examination (MMSE) score is 14/30; his Hamilton Depression Rating Scale (HAM-D) score is 21, indicating severe depression (19 to 22). However, the examiner feels that these scores may not reflect an accurate assessment because Mr. R gave flippant responses and did not cooperate during the interview. Physical examination is unremarkable. Previous medication trials included buspirone, escitalopram, and risperidone; none of these medications successfully alleviated his depressive symptoms.
On admission, Mr. R is given oral mirtazapine, 15 mg/d, and quetiapine, 25 mg/d, to target depressive mood, insomnia, and weight loss. Urgent intervention is indicated because his depressive symptoms are profoundly causing failure to thrive and are compromising his physical health. Mr. R’s deterioration concerns the physician team. Because of a history of failed pharmacotherapy trials, the team reassesses Mr. R’s treatment options.
[polldaddy:9903171]
The authors’ observations
The physician team recommends that Mr. R undergo ECT to obtain rapid relief from his depressive symptoms. After discussion of the potential risks and benefits, Mr. R agrees to this treatment. Quetiapine is discontinued prior to initiating ECT to avoid unnecessary medications; mirtazapine is continued.
Mr. R’s lack of response to previous antidepressants and significant deterioration were concerning. The physicians wanted to avoid higher-dose medications because of the risk of falls or somnolence. Their clinical experience and the literature supporting ECT for patients of Mr. R’s age lead them to select ECT as the most appropriate therapeutic option.
ECT has no absolute contraindications.1 The rate of ECT use in the United States has fluctuated over time because of factors unrelated to the efficacy and availability of ECT or alternative treatments.2 This form of intervention is also somewhat stigmatized.
Some psychiatrists are reluctant to prescribe ECT for geriatric patients because of concerns of potential neurocognitive or medical complications and risks during anesthesia. However, in the United States, older patients with depression are more likely to be treated with ECT than their younger counterparts.3 ECT usually induces greater immediate efficacy than antidepressants.4
Evidence supports using ECT in older patients
Multiple studies have found that ECT is a rapid, safe, and efficacious intervention for treating older persons with depression. Patients age >60 who receive ECT plus pharmacotherapy have lower HAM-D scores than those receiving pharmacotherapy alone.5 Overall, the rates of remission for depression range from 50% to 70%; yet geriatric patients who receive only ECT have response rates around 90%.6 Older age, presence of psychotic symptoms, and shorter duration of illness can predict a rapidly positive ECT response.7
When treated with ECT, older patients, including those age >85, have fewer subsequent episodes of depression compared with those who receive pharmacotherapy alone.1 Older individuals with physical illness or cognitive impairment respond to and tolerate ECT much like younger patients.6 Older patients receiving ECT may experience less cognitive decline than younger ones.7 Those in their ninth decade of life with treatment-resistant depression, psychotic features, and post-stroke depression often respond robustly with improvement following ECT.8
Remission rates also depend on the technique of administration. Interactions between electrode placement and stimulus parameter dosage affect efficacy and adverse effects.9 Right-sided, unilateral ECT induces less cognitive dysfunction compared with bilateral electrode placement,9 but bilateral ECT is more clinically effective.10 However, the efficacy of right-sided ECT is more dose-sensitive, and some data suggest that suboptimal response is due to insufficient stimulus dosages.11 One double-blind randomized controlled trial documented that when using a high-dose stimulus parameter, unilateral ECT is as effective as bilateral ECT.12 When there is a suboptimal response to unilateral ECT, bilateral ECT might be beneficial.12,13 For preventing relapse in older patients, increasing the interval between ECT treatments is more effective than stopping ECT abruptly.13
[polldaddy:9903172]
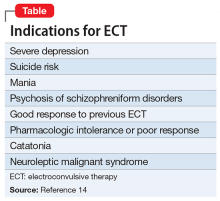
Indications of ECT
ECT is indicated for patients with severe depression, mania, and other conditions (Table).14 The most common indication for ECT in older persons is a history of treatment-resistant depression, with melancholia, psychosis, or suicidal ideations.1-6,12 There are also age-related and clinical factors to consider with ECT. This treatment provides a safe, rapid remission for patients age >65, even after adjusting for somatic conditions, duration of illness, medication resistance, or case severity.15 Compared with younger patients, older adults may not tolerate antidepressants as well because of age-related pharmacokinetic alterations, including increased sensitivity to anticholinergic and/or hypotensive effects.1
Factors that favor ECT include a previous good response to it; patient preference; and an indication for rapid intervention, such as suicidality, catatonia, dehydration, malnutrition, or a suboptimal result from pharmacotherapy.3 Mortality among individuals age >85 who receive ECT reportedly is lower than that among their counterparts who receive alternative treatments.16 ECT has been administered safely and effectively in patients with comorbid medical illnesses such as stroke, cerebral aneurysm, cardiovascular disease with ischemia or arrhythmia, dementia, and osteoporosis.17
Neurocognitive effects
Reports on the effects of ECT on neurocognitive functioning have varied. In some studies, performance improved or did not change in severely depressed older patients who received ECT.18,19 In older people who receive ECT, MMSE scores often return to baseline by the end of treatment.20 There often is only mild transient cognitive impairment in patients with late-life depression who receive ECT. Areas of concern include attention span, orientation, and speed of mental processing.20 Physicians should conduct cognitive tests before, during, and after ECT sessions to monitor their patient’s mental status.20
Cognitive stability can be maintained by administering ECT twice a week; applying right-sided, unilateral electrode placement; and using short, ultra-brief stimulus pulse width parameters.21 Cognitive impairment induced by ECT is not associated with age in geriatric patients with depression.22 Older adults who experienced longer postictal reorientation time periods have better outcomes than others who reach orientation faster; their intellectual impairment returned to baseline.20 Falling is another complication associated with ECT. A longitudinal cohort study found the incident of falls among patients receiving ECT was 13%.22 Risk factors for falls during a course of ECT include the number of treatments and the presence of coexisting Parkinson’s disease.23
OUTCOME Improvement
Mr. R receives 8 sessions of right-sided, unilateral ECT with an individualized dosage titration method. Treatments are completed with a stimulus intensity at 6 times seizure threshold, with an ultra-brief pulse width at 0.3 milliseconds. Mr. R’s mood and affect begin to improve after 3 ECT sessions. His MMSE score increases to 28/30 (Figure). His clinical improvement is progressively sustained; he develops an increasingly jovial attitude and experiences less anxiety. Mr. R’s confidence, appetite, and sleep also improve. There are no complications with treatment, and Mr. R has no complaints. After 8 ECT sessions, Mr. R has no affective symptoms and does not experience any cognitive impairment.
The authors’ observations
Depression among older people is a growing public health concern. It is a leading cause of disability, and often leads to nursing home placement.24 ECT is a safe, effective treatment for late-life depression, but is underutilized in patients age >75 because of concerns for cognitive impairment.6 However, there is evidence that response rates to ECT are higher in patients ages 45 to 85, compared with young individuals ages 18 to 45.25 ECT is a viable intervention for older depressed patients, particularly for those who do not tolerate or fail to respond to pharmacotherapy. Many of these patients are at risk for drug-induced toxicities or interactions or suicide.1
1. Kerner N, Prudic J. Current electroconvulsive therapy practice and research in the geriatric population. Neuropsychiatry (London). 2014;4(1):33-54.
2. Dombrovski AY, Mulsant BH. The evidence for electroconvulsive therapy (ECT) in the treatment of severe late-life depression. ECT: the preferred treatment for severe depression in late life. Int Psychogeriatr. 2007;19(1):10-14,27-35; discussion 24-26.
3. Olfson M, Marcus S, Sackeim HA, et al. Use of ECT for the inpatient treatment of recurrent major depression. Am J Psychiatry. 1998;155(1):22-29.
4. Salzman C, Wong E, Wright BC. Drug and ECT treatment of depression in the elderly, 1996-2001: a literature review. Biol Psychiatry. 2002;52(3):265-284.
5. Kellner CH, Husain MM, Knapp RG, et al; CORE/PRIDE Work Group. A novel strategy for continuation ect in geriatric depression: phase 2 of the PRIDE study. A
6. Tew JD Jr, Mulsant BH, Haskett RF, et al. Acute efficacy of ECT in the treatment of major depression in the old-old. Am J Psychiatry. 1999;156(12):1865-1870.
7. Dombrovski AY, Mulsant BH, Haskett RF, et al. Predictors of remission after electroconvulsive therapy in unipolar major depression. J Clin Psychiatry. 2005;66(8):1043-1049.
8. Charles K. UpToDate. Unipolar major depression in adults: indications for efficacy of electroconvulsive therapy (ECT). https://www.uptodate.com/contents/unipolar-major-depression-in-adults-indications-for-and-efficacy-of-electroconvulsive-therapy-ect. Updated May 16, 2017. Accessed November 26, 2017.
9. Sackeim HA, Prudic J, Devanand DP, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57(5):425-434.
10. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
11. Lisanby SH. Electroconvulsive therapy for depression. N Engl J Med. 2007;357(19):1939-1945.
12. Stoppe A, Louzã M, Rosa M, et al. Fixed high dose electroconvulsive therapy in elderly with depression: a double-blind, randomized comparison of efficacy and tolerability between unilateral and bilateral electrode placement. J ECT. 2006;22(2):92-99.
13. Geduldig ET, Kellner CH. Electroconvulsive therapy in the elderly: new findings in geriatric depression. Curr Psychiatry Rep. 2016;18(4):40.
14. Practice guideline for the treatment of patients with major depressive disorder (revision). American Psychiatric Association. Am J Psychiatry. 2000;157(suppl 4):1-45.
15. Rhebergen D, Huisman A, Bouckaert F, et al. Older age is associated with rapid remission of depression after electroconvulsive therapy: a latent class growth analysis. Am J Geriatr Psychiatry. 2015;23(3):274-282.
16. Philibert RA, Richards L, Lynch CF, et al. Effect of ECT on mortality and clinical outcome in geriatric unipolar depression. J Clin Psychiatry. 1995;56(9):390-394.
17. Tomac TA, Rummans TA, Pileggi TS, et al. Safety and efficacy of electroconvulsive therapy in patients over age 85. Am J Geriatr Psychiatry. 1997;5(2):126-130.
18. Verwijk E, Comijs HC, Kok RM, et al. Short and long-term neurocognitive functioning after electroconvulsive therapy in depressed elderly: a prospective naturalistic study. Int Psychogeriatr. 2014;26(2):315-324.
19. Flint AJ, Gagnon N. Effective use of electroconvulsive therapy in late life depression. Can J Psychiatry. 2002;47(8):734-741.
20. Bjolseth TM, Engedal K, Benth JS, et al. Speed of recovery from disorientation may predict the treatment outcome of electroconvulsive therapy (ECT) in elderly patients with major depression. J Affect Disord. 2016;190:178-186.
21. Sackeim HA, Prudic J, Nobler MS, et al. Ultra-brief pulse ECT and the affective and cognitive consequences of ECT. J ECT. 2001;17(1):77.
22. Bjolseth TM, Engedal K, Benth JS, et al. Baseline cognitive function does not predict the treatment outcome of electroconvulsive therapy (ECT) in late-life depression. J Affect Disord. 2015;185:67-75.
23. de Carle AJ, Kohn R. Electroconvulsive therapy and falls in the elderly. J ECT. 2000;16(3):252-257.
24. Hoover DR, Siegel M, Lucas J, et al. Depression in the first year of stay for elderly long-term nursing home residents in the USA. Int Psychogeriatr. 2010;22:1161.
25. O’Connor MK, Knapp R, Husain M, et al. The influence of age on the response of major depression to electroconvulsive therapy: a C.O.R.E. Report. Am J Geriatr Psychiatry. 2001; 9:382.
CASE Depressed, avoidant
Mr. R, age 95, has a history of recurrent major depressive disorder. He presents to the emergency department with depressive symptoms that began 6 weeks ago. His symptoms include depressed mood, hopelessness, anhedonia, anxiety, and insomnia. Co-occurring anorexia nervosa has resulted in a 20-lb weight loss. He denies suicidal ideation.
A mental status examination reveals profound psychomotor agitation, dysphoric mood, tearfulness, and mood-congruent delusions. Mr. R’s Mini-Mental State Examination (MMSE) score is 14/30; his Hamilton Depression Rating Scale (HAM-D) score is 21, indicating severe depression (19 to 22). However, the examiner feels that these scores may not reflect an accurate assessment because Mr. R gave flippant responses and did not cooperate during the interview. Physical examination is unremarkable. Previous medication trials included buspirone, escitalopram, and risperidone; none of these medications successfully alleviated his depressive symptoms.
On admission, Mr. R is given oral mirtazapine, 15 mg/d, and quetiapine, 25 mg/d, to target depressive mood, insomnia, and weight loss. Urgent intervention is indicated because his depressive symptoms are profoundly causing failure to thrive and are compromising his physical health. Mr. R’s deterioration concerns the physician team. Because of a history of failed pharmacotherapy trials, the team reassesses Mr. R’s treatment options.
[polldaddy:9903171]
The authors’ observations
The physician team recommends that Mr. R undergo ECT to obtain rapid relief from his depressive symptoms. After discussion of the potential risks and benefits, Mr. R agrees to this treatment. Quetiapine is discontinued prior to initiating ECT to avoid unnecessary medications; mirtazapine is continued.
Mr. R’s lack of response to previous antidepressants and significant deterioration were concerning. The physicians wanted to avoid higher-dose medications because of the risk of falls or somnolence. Their clinical experience and the literature supporting ECT for patients of Mr. R’s age lead them to select ECT as the most appropriate therapeutic option.
ECT has no absolute contraindications.1 The rate of ECT use in the United States has fluctuated over time because of factors unrelated to the efficacy and availability of ECT or alternative treatments.2 This form of intervention is also somewhat stigmatized.
Some psychiatrists are reluctant to prescribe ECT for geriatric patients because of concerns of potential neurocognitive or medical complications and risks during anesthesia. However, in the United States, older patients with depression are more likely to be treated with ECT than their younger counterparts.3 ECT usually induces greater immediate efficacy than antidepressants.4
Evidence supports using ECT in older patients
Multiple studies have found that ECT is a rapid, safe, and efficacious intervention for treating older persons with depression. Patients age >60 who receive ECT plus pharmacotherapy have lower HAM-D scores than those receiving pharmacotherapy alone.5 Overall, the rates of remission for depression range from 50% to 70%; yet geriatric patients who receive only ECT have response rates around 90%.6 Older age, presence of psychotic symptoms, and shorter duration of illness can predict a rapidly positive ECT response.7
When treated with ECT, older patients, including those age >85, have fewer subsequent episodes of depression compared with those who receive pharmacotherapy alone.1 Older individuals with physical illness or cognitive impairment respond to and tolerate ECT much like younger patients.6 Older patients receiving ECT may experience less cognitive decline than younger ones.7 Those in their ninth decade of life with treatment-resistant depression, psychotic features, and post-stroke depression often respond robustly with improvement following ECT.8
Remission rates also depend on the technique of administration. Interactions between electrode placement and stimulus parameter dosage affect efficacy and adverse effects.9 Right-sided, unilateral ECT induces less cognitive dysfunction compared with bilateral electrode placement,9 but bilateral ECT is more clinically effective.10 However, the efficacy of right-sided ECT is more dose-sensitive, and some data suggest that suboptimal response is due to insufficient stimulus dosages.11 One double-blind randomized controlled trial documented that when using a high-dose stimulus parameter, unilateral ECT is as effective as bilateral ECT.12 When there is a suboptimal response to unilateral ECT, bilateral ECT might be beneficial.12,13 For preventing relapse in older patients, increasing the interval between ECT treatments is more effective than stopping ECT abruptly.13
[polldaddy:9903172]
Indications of ECT
ECT is indicated for patients with severe depression, mania, and other conditions (Table).14 The most common indication for ECT in older persons is a history of treatment-resistant depression, with melancholia, psychosis, or suicidal ideations.1-6,12 There are also age-related and clinical factors to consider with ECT. This treatment provides a safe, rapid remission for patients age >65, even after adjusting for somatic conditions, duration of illness, medication resistance, or case severity.15 Compared with younger patients, older adults may not tolerate antidepressants as well because of age-related pharmacokinetic alterations, including increased sensitivity to anticholinergic and/or hypotensive effects.1
Factors that favor ECT include a previous good response to it; patient preference; and an indication for rapid intervention, such as suicidality, catatonia, dehydration, malnutrition, or a suboptimal result from pharmacotherapy.3 Mortality among individuals age >85 who receive ECT reportedly is lower than that among their counterparts who receive alternative treatments.16 ECT has been administered safely and effectively in patients with comorbid medical illnesses such as stroke, cerebral aneurysm, cardiovascular disease with ischemia or arrhythmia, dementia, and osteoporosis.17
Neurocognitive effects
Reports on the effects of ECT on neurocognitive functioning have varied. In some studies, performance improved or did not change in severely depressed older patients who received ECT.18,19 In older people who receive ECT, MMSE scores often return to baseline by the end of treatment.20 There often is only mild transient cognitive impairment in patients with late-life depression who receive ECT. Areas of concern include attention span, orientation, and speed of mental processing.20 Physicians should conduct cognitive tests before, during, and after ECT sessions to monitor their patient’s mental status.20
Cognitive stability can be maintained by administering ECT twice a week; applying right-sided, unilateral electrode placement; and using short, ultra-brief stimulus pulse width parameters.21 Cognitive impairment induced by ECT is not associated with age in geriatric patients with depression.22 Older adults who experienced longer postictal reorientation time periods have better outcomes than others who reach orientation faster; their intellectual impairment returned to baseline.20 Falling is another complication associated with ECT. A longitudinal cohort study found the incident of falls among patients receiving ECT was 13%.22 Risk factors for falls during a course of ECT include the number of treatments and the presence of coexisting Parkinson’s disease.23
OUTCOME Improvement
Mr. R receives 8 sessions of right-sided, unilateral ECT with an individualized dosage titration method. Treatments are completed with a stimulus intensity at 6 times seizure threshold, with an ultra-brief pulse width at 0.3 milliseconds. Mr. R’s mood and affect begin to improve after 3 ECT sessions. His MMSE score increases to 28/30 (Figure). His clinical improvement is progressively sustained; he develops an increasingly jovial attitude and experiences less anxiety. Mr. R’s confidence, appetite, and sleep also improve. There are no complications with treatment, and Mr. R has no complaints. After 8 ECT sessions, Mr. R has no affective symptoms and does not experience any cognitive impairment.

The authors’ observations
Depression among older people is a growing public health concern. It is a leading cause of disability, and often leads to nursing home placement.24 ECT is a safe, effective treatment for late-life depression, but is underutilized in patients age >75 because of concerns for cognitive impairment.6 However, there is evidence that response rates to ECT are higher in patients ages 45 to 85, compared with young individuals ages 18 to 45.25 ECT is a viable intervention for older depressed patients, particularly for those who do not tolerate or fail to respond to pharmacotherapy. Many of these patients are at risk for drug-induced toxicities or interactions or suicide.1
CASE Depressed, avoidant
Mr. R, age 95, has a history of recurrent major depressive disorder. He presents to the emergency department with depressive symptoms that began 6 weeks ago. His symptoms include depressed mood, hopelessness, anhedonia, anxiety, and insomnia. Co-occurring anorexia nervosa has resulted in a 20-lb weight loss. He denies suicidal ideation.
A mental status examination reveals profound psychomotor agitation, dysphoric mood, tearfulness, and mood-congruent delusions. Mr. R’s Mini-Mental State Examination (MMSE) score is 14/30; his Hamilton Depression Rating Scale (HAM-D) score is 21, indicating severe depression (19 to 22). However, the examiner feels that these scores may not reflect an accurate assessment because Mr. R gave flippant responses and did not cooperate during the interview. Physical examination is unremarkable. Previous medication trials included buspirone, escitalopram, and risperidone; none of these medications successfully alleviated his depressive symptoms.
On admission, Mr. R is given oral mirtazapine, 15 mg/d, and quetiapine, 25 mg/d, to target depressive mood, insomnia, and weight loss. Urgent intervention is indicated because his depressive symptoms are profoundly causing failure to thrive and are compromising his physical health. Mr. R’s deterioration concerns the physician team. Because of a history of failed pharmacotherapy trials, the team reassesses Mr. R’s treatment options.
[polldaddy:9903171]
The authors’ observations
The physician team recommends that Mr. R undergo ECT to obtain rapid relief from his depressive symptoms. After discussion of the potential risks and benefits, Mr. R agrees to this treatment. Quetiapine is discontinued prior to initiating ECT to avoid unnecessary medications; mirtazapine is continued.
Mr. R’s lack of response to previous antidepressants and significant deterioration were concerning. The physicians wanted to avoid higher-dose medications because of the risk of falls or somnolence. Their clinical experience and the literature supporting ECT for patients of Mr. R’s age lead them to select ECT as the most appropriate therapeutic option.
ECT has no absolute contraindications.1 The rate of ECT use in the United States has fluctuated over time because of factors unrelated to the efficacy and availability of ECT or alternative treatments.2 This form of intervention is also somewhat stigmatized.
Some psychiatrists are reluctant to prescribe ECT for geriatric patients because of concerns of potential neurocognitive or medical complications and risks during anesthesia. However, in the United States, older patients with depression are more likely to be treated with ECT than their younger counterparts.3 ECT usually induces greater immediate efficacy than antidepressants.4
Evidence supports using ECT in older patients
Multiple studies have found that ECT is a rapid, safe, and efficacious intervention for treating older persons with depression. Patients age >60 who receive ECT plus pharmacotherapy have lower HAM-D scores than those receiving pharmacotherapy alone.5 Overall, the rates of remission for depression range from 50% to 70%; yet geriatric patients who receive only ECT have response rates around 90%.6 Older age, presence of psychotic symptoms, and shorter duration of illness can predict a rapidly positive ECT response.7
When treated with ECT, older patients, including those age >85, have fewer subsequent episodes of depression compared with those who receive pharmacotherapy alone.1 Older individuals with physical illness or cognitive impairment respond to and tolerate ECT much like younger patients.6 Older patients receiving ECT may experience less cognitive decline than younger ones.7 Those in their ninth decade of life with treatment-resistant depression, psychotic features, and post-stroke depression often respond robustly with improvement following ECT.8
Remission rates also depend on the technique of administration. Interactions between electrode placement and stimulus parameter dosage affect efficacy and adverse effects.9 Right-sided, unilateral ECT induces less cognitive dysfunction compared with bilateral electrode placement,9 but bilateral ECT is more clinically effective.10 However, the efficacy of right-sided ECT is more dose-sensitive, and some data suggest that suboptimal response is due to insufficient stimulus dosages.11 One double-blind randomized controlled trial documented that when using a high-dose stimulus parameter, unilateral ECT is as effective as bilateral ECT.12 When there is a suboptimal response to unilateral ECT, bilateral ECT might be beneficial.12,13 For preventing relapse in older patients, increasing the interval between ECT treatments is more effective than stopping ECT abruptly.13
[polldaddy:9903172]
Indications of ECT
ECT is indicated for patients with severe depression, mania, and other conditions (Table).14 The most common indication for ECT in older persons is a history of treatment-resistant depression, with melancholia, psychosis, or suicidal ideations.1-6,12 There are also age-related and clinical factors to consider with ECT. This treatment provides a safe, rapid remission for patients age >65, even after adjusting for somatic conditions, duration of illness, medication resistance, or case severity.15 Compared with younger patients, older adults may not tolerate antidepressants as well because of age-related pharmacokinetic alterations, including increased sensitivity to anticholinergic and/or hypotensive effects.1
Factors that favor ECT include a previous good response to it; patient preference; and an indication for rapid intervention, such as suicidality, catatonia, dehydration, malnutrition, or a suboptimal result from pharmacotherapy.3 Mortality among individuals age >85 who receive ECT reportedly is lower than that among their counterparts who receive alternative treatments.16 ECT has been administered safely and effectively in patients with comorbid medical illnesses such as stroke, cerebral aneurysm, cardiovascular disease with ischemia or arrhythmia, dementia, and osteoporosis.17
Neurocognitive effects
Reports on the effects of ECT on neurocognitive functioning have varied. In some studies, performance improved or did not change in severely depressed older patients who received ECT.18,19 In older people who receive ECT, MMSE scores often return to baseline by the end of treatment.20 There often is only mild transient cognitive impairment in patients with late-life depression who receive ECT. Areas of concern include attention span, orientation, and speed of mental processing.20 Physicians should conduct cognitive tests before, during, and after ECT sessions to monitor their patient’s mental status.20
Cognitive stability can be maintained by administering ECT twice a week; applying right-sided, unilateral electrode placement; and using short, ultra-brief stimulus pulse width parameters.21 Cognitive impairment induced by ECT is not associated with age in geriatric patients with depression.22 Older adults who experienced longer postictal reorientation time periods have better outcomes than others who reach orientation faster; their intellectual impairment returned to baseline.20 Falling is another complication associated with ECT. A longitudinal cohort study found the incident of falls among patients receiving ECT was 13%.22 Risk factors for falls during a course of ECT include the number of treatments and the presence of coexisting Parkinson’s disease.23
OUTCOME Improvement
Mr. R receives 8 sessions of right-sided, unilateral ECT with an individualized dosage titration method. Treatments are completed with a stimulus intensity at 6 times seizure threshold, with an ultra-brief pulse width at 0.3 milliseconds. Mr. R’s mood and affect begin to improve after 3 ECT sessions. His MMSE score increases to 28/30 (Figure). His clinical improvement is progressively sustained; he develops an increasingly jovial attitude and experiences less anxiety. Mr. R’s confidence, appetite, and sleep also improve. There are no complications with treatment, and Mr. R has no complaints. After 8 ECT sessions, Mr. R has no affective symptoms and does not experience any cognitive impairment.

The authors’ observations
Depression among older people is a growing public health concern. It is a leading cause of disability, and often leads to nursing home placement.24 ECT is a safe, effective treatment for late-life depression, but is underutilized in patients age >75 because of concerns for cognitive impairment.6 However, there is evidence that response rates to ECT are higher in patients ages 45 to 85, compared with young individuals ages 18 to 45.25 ECT is a viable intervention for older depressed patients, particularly for those who do not tolerate or fail to respond to pharmacotherapy. Many of these patients are at risk for drug-induced toxicities or interactions or suicide.1
1. Kerner N, Prudic J. Current electroconvulsive therapy practice and research in the geriatric population. Neuropsychiatry (London). 2014;4(1):33-54.
2. Dombrovski AY, Mulsant BH. The evidence for electroconvulsive therapy (ECT) in the treatment of severe late-life depression. ECT: the preferred treatment for severe depression in late life. Int Psychogeriatr. 2007;19(1):10-14,27-35; discussion 24-26.
3. Olfson M, Marcus S, Sackeim HA, et al. Use of ECT for the inpatient treatment of recurrent major depression. Am J Psychiatry. 1998;155(1):22-29.
4. Salzman C, Wong E, Wright BC. Drug and ECT treatment of depression in the elderly, 1996-2001: a literature review. Biol Psychiatry. 2002;52(3):265-284.
5. Kellner CH, Husain MM, Knapp RG, et al; CORE/PRIDE Work Group. A novel strategy for continuation ect in geriatric depression: phase 2 of the PRIDE study. A
6. Tew JD Jr, Mulsant BH, Haskett RF, et al. Acute efficacy of ECT in the treatment of major depression in the old-old. Am J Psychiatry. 1999;156(12):1865-1870.
7. Dombrovski AY, Mulsant BH, Haskett RF, et al. Predictors of remission after electroconvulsive therapy in unipolar major depression. J Clin Psychiatry. 2005;66(8):1043-1049.
8. Charles K. UpToDate. Unipolar major depression in adults: indications for efficacy of electroconvulsive therapy (ECT). https://www.uptodate.com/contents/unipolar-major-depression-in-adults-indications-for-and-efficacy-of-electroconvulsive-therapy-ect. Updated May 16, 2017. Accessed November 26, 2017.
9. Sackeim HA, Prudic J, Devanand DP, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57(5):425-434.
10. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
11. Lisanby SH. Electroconvulsive therapy for depression. N Engl J Med. 2007;357(19):1939-1945.
12. Stoppe A, Louzã M, Rosa M, et al. Fixed high dose electroconvulsive therapy in elderly with depression: a double-blind, randomized comparison of efficacy and tolerability between unilateral and bilateral electrode placement. J ECT. 2006;22(2):92-99.
13. Geduldig ET, Kellner CH. Electroconvulsive therapy in the elderly: new findings in geriatric depression. Curr Psychiatry Rep. 2016;18(4):40.
14. Practice guideline for the treatment of patients with major depressive disorder (revision). American Psychiatric Association. Am J Psychiatry. 2000;157(suppl 4):1-45.
15. Rhebergen D, Huisman A, Bouckaert F, et al. Older age is associated with rapid remission of depression after electroconvulsive therapy: a latent class growth analysis. Am J Geriatr Psychiatry. 2015;23(3):274-282.
16. Philibert RA, Richards L, Lynch CF, et al. Effect of ECT on mortality and clinical outcome in geriatric unipolar depression. J Clin Psychiatry. 1995;56(9):390-394.
17. Tomac TA, Rummans TA, Pileggi TS, et al. Safety and efficacy of electroconvulsive therapy in patients over age 85. Am J Geriatr Psychiatry. 1997;5(2):126-130.
18. Verwijk E, Comijs HC, Kok RM, et al. Short and long-term neurocognitive functioning after electroconvulsive therapy in depressed elderly: a prospective naturalistic study. Int Psychogeriatr. 2014;26(2):315-324.
19. Flint AJ, Gagnon N. Effective use of electroconvulsive therapy in late life depression. Can J Psychiatry. 2002;47(8):734-741.
20. Bjolseth TM, Engedal K, Benth JS, et al. Speed of recovery from disorientation may predict the treatment outcome of electroconvulsive therapy (ECT) in elderly patients with major depression. J Affect Disord. 2016;190:178-186.
21. Sackeim HA, Prudic J, Nobler MS, et al. Ultra-brief pulse ECT and the affective and cognitive consequences of ECT. J ECT. 2001;17(1):77.
22. Bjolseth TM, Engedal K, Benth JS, et al. Baseline cognitive function does not predict the treatment outcome of electroconvulsive therapy (ECT) in late-life depression. J Affect Disord. 2015;185:67-75.
23. de Carle AJ, Kohn R. Electroconvulsive therapy and falls in the elderly. J ECT. 2000;16(3):252-257.
24. Hoover DR, Siegel M, Lucas J, et al. Depression in the first year of stay for elderly long-term nursing home residents in the USA. Int Psychogeriatr. 2010;22:1161.
25. O’Connor MK, Knapp R, Husain M, et al. The influence of age on the response of major depression to electroconvulsive therapy: a C.O.R.E. Report. Am J Geriatr Psychiatry. 2001; 9:382.
1. Kerner N, Prudic J. Current electroconvulsive therapy practice and research in the geriatric population. Neuropsychiatry (London). 2014;4(1):33-54.
2. Dombrovski AY, Mulsant BH. The evidence for electroconvulsive therapy (ECT) in the treatment of severe late-life depression. ECT: the preferred treatment for severe depression in late life. Int Psychogeriatr. 2007;19(1):10-14,27-35; discussion 24-26.
3. Olfson M, Marcus S, Sackeim HA, et al. Use of ECT for the inpatient treatment of recurrent major depression. Am J Psychiatry. 1998;155(1):22-29.
4. Salzman C, Wong E, Wright BC. Drug and ECT treatment of depression in the elderly, 1996-2001: a literature review. Biol Psychiatry. 2002;52(3):265-284.
5. Kellner CH, Husain MM, Knapp RG, et al; CORE/PRIDE Work Group. A novel strategy for continuation ect in geriatric depression: phase 2 of the PRIDE study. A
6. Tew JD Jr, Mulsant BH, Haskett RF, et al. Acute efficacy of ECT in the treatment of major depression in the old-old. Am J Psychiatry. 1999;156(12):1865-1870.
7. Dombrovski AY, Mulsant BH, Haskett RF, et al. Predictors of remission after electroconvulsive therapy in unipolar major depression. J Clin Psychiatry. 2005;66(8):1043-1049.
8. Charles K. UpToDate. Unipolar major depression in adults: indications for efficacy of electroconvulsive therapy (ECT). https://www.uptodate.com/contents/unipolar-major-depression-in-adults-indications-for-and-efficacy-of-electroconvulsive-therapy-ect. Updated May 16, 2017. Accessed November 26, 2017.
9. Sackeim HA, Prudic J, Devanand DP, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57(5):425-434.
10. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
11. Lisanby SH. Electroconvulsive therapy for depression. N Engl J Med. 2007;357(19):1939-1945.
12. Stoppe A, Louzã M, Rosa M, et al. Fixed high dose electroconvulsive therapy in elderly with depression: a double-blind, randomized comparison of efficacy and tolerability between unilateral and bilateral electrode placement. J ECT. 2006;22(2):92-99.
13. Geduldig ET, Kellner CH. Electroconvulsive therapy in the elderly: new findings in geriatric depression. Curr Psychiatry Rep. 2016;18(4):40.
14. Practice guideline for the treatment of patients with major depressive disorder (revision). American Psychiatric Association. Am J Psychiatry. 2000;157(suppl 4):1-45.
15. Rhebergen D, Huisman A, Bouckaert F, et al. Older age is associated with rapid remission of depression after electroconvulsive therapy: a latent class growth analysis. Am J Geriatr Psychiatry. 2015;23(3):274-282.
16. Philibert RA, Richards L, Lynch CF, et al. Effect of ECT on mortality and clinical outcome in geriatric unipolar depression. J Clin Psychiatry. 1995;56(9):390-394.
17. Tomac TA, Rummans TA, Pileggi TS, et al. Safety and efficacy of electroconvulsive therapy in patients over age 85. Am J Geriatr Psychiatry. 1997;5(2):126-130.
18. Verwijk E, Comijs HC, Kok RM, et al. Short and long-term neurocognitive functioning after electroconvulsive therapy in depressed elderly: a prospective naturalistic study. Int Psychogeriatr. 2014;26(2):315-324.
19. Flint AJ, Gagnon N. Effective use of electroconvulsive therapy in late life depression. Can J Psychiatry. 2002;47(8):734-741.
20. Bjolseth TM, Engedal K, Benth JS, et al. Speed of recovery from disorientation may predict the treatment outcome of electroconvulsive therapy (ECT) in elderly patients with major depression. J Affect Disord. 2016;190:178-186.
21. Sackeim HA, Prudic J, Nobler MS, et al. Ultra-brief pulse ECT and the affective and cognitive consequences of ECT. J ECT. 2001;17(1):77.
22. Bjolseth TM, Engedal K, Benth JS, et al. Baseline cognitive function does not predict the treatment outcome of electroconvulsive therapy (ECT) in late-life depression. J Affect Disord. 2015;185:67-75.
23. de Carle AJ, Kohn R. Electroconvulsive therapy and falls in the elderly. J ECT. 2000;16(3):252-257.
24. Hoover DR, Siegel M, Lucas J, et al. Depression in the first year of stay for elderly long-term nursing home residents in the USA. Int Psychogeriatr. 2010;22:1161.
25. O’Connor MK, Knapp R, Husain M, et al. The influence of age on the response of major depression to electroconvulsive therapy: a C.O.R.E. Report. Am J Geriatr Psychiatry. 2001; 9:382.
What can we do about the Zika virus in the United States?
Since Florida has seen several new cases of local mosquito-borne infection, controlling and preventing Zika infection has great urgency. Zika virus involves an arthropod-borne infection transmitted by Aedes aegypti and Aedes albopictus mosquitoes. Other modes of transmission include the maternal-fetal route, any sexual contact, blood transfusions, organ or tissue transplantation, and laboratory exposure.1
The first case of Zika infection in the United States and its territories occurred through international travel. According to the Centers for Disease Control and Prevention, as of October 12, 2016, there were 3807 travel-associated cases of Zika infection in the United States and 84 instances in its territories.2 As for local transmission, there were 128 people evidencing a Zika infection in the United States and 25,871 in US territories.2 Regions between Texas and Florida are at high risk because Aedes mosquitoes primarily inhabit the gulf coast.3 Many cases have occurred despite repellent use and eradication efforts, possibly due to resistance acquired by these mosquitoes.1
Control measures include using insect repellents, aerial spraying of insecticides, eliminating mosquito breeding sites, covering water tanks, and using mosquito nets or door and window screens. Infection during pregnancy is the greatest concern because of congenital anomalies (including microcephaly) that negatively affect brain development.4
Before a possible conception or any sexual contact, women exposed to Zika—with or without symptoms—must wait at least 8 weeks; men with or without symptoms should abstain for 6 months.4 Individuals should avoid traveling to areas with Zika infestation, wear long-sleeved clothing treated with permethrin, and minimize outside exposure, especially in evening hours.4
The World Health Organization is utilizing genetically modified mosquitoes to diminish Aedes populations; trials conducted in affected areas of Brazil revealed that the number of Aedes mosquitoes was reduced by 90%.5 This method of mosquito control is currently being studied in the United States.6 Vaccinations to prevent Zika infection are also under investigation.
Physicians should educate patients regarding the clinical manifestations and complications of Zika virus infection; people need to know that the Zika virus can be sexually transmitted. Doctors should also counsel patients to curtail travel to areas that have Zika infestations, or to at least wear protective clothing while in such areas to minimize mosquito bite risk. Educating travelers about appropriate postponement of sexual contact after any exposure to the Zika virus is also essential.4
Hema Madhuri Mekala, MD
Priyanga Jayakumar, MD
Rajashekar Reddy Yeruva, MD
Steven Lippmann, MD
Louisville, KY
1. Centers for Disease Control and Prevention. Zika virus: Transmission & risks. Available at: http://www.cdc.gov/zika/transmission/index.html. Accessed October 14, 2016.
2. Centers for Disease Control and Prevention. Zika virus: Case counts in the US. Available at: http://www.cdc.gov/zika/geo/united-states.html. Accessed October 14, 2016.
3. Castro L, Chen X, Dimitrov NB, et al. The University of Texas at Austin. Texas Arbovirus Risk. 2015. Available at: http://hdl.handle.net/2152/31934. Accessed October 14, 2016.
4. Centers for Disease Control and Prevention. Zika virus: Zika is in your area: What to do. Available at: http://www.cdc.gov/zika/intheus/what-to-do.html. Accessed October 14, 2016.
5. FL KEYS NEWS. Available at: http://www.flkeysnews.com/opinion/opn-columns-blogs/article83328707.html. Accessed October 14, 2016.
6. Ernst KC, Haenchen S, Dickinson K, et al. Awareness and support of release of genetically modified “sterile” mosquitoes, Key West, Florida, USA. Emerg Infect Dis. 2015;21:320-324.
Since Florida has seen several new cases of local mosquito-borne infection, controlling and preventing Zika infection has great urgency. Zika virus involves an arthropod-borne infection transmitted by Aedes aegypti and Aedes albopictus mosquitoes. Other modes of transmission include the maternal-fetal route, any sexual contact, blood transfusions, organ or tissue transplantation, and laboratory exposure.1
The first case of Zika infection in the United States and its territories occurred through international travel. According to the Centers for Disease Control and Prevention, as of October 12, 2016, there were 3807 travel-associated cases of Zika infection in the United States and 84 instances in its territories.2 As for local transmission, there were 128 people evidencing a Zika infection in the United States and 25,871 in US territories.2 Regions between Texas and Florida are at high risk because Aedes mosquitoes primarily inhabit the gulf coast.3 Many cases have occurred despite repellent use and eradication efforts, possibly due to resistance acquired by these mosquitoes.1
Control measures include using insect repellents, aerial spraying of insecticides, eliminating mosquito breeding sites, covering water tanks, and using mosquito nets or door and window screens. Infection during pregnancy is the greatest concern because of congenital anomalies (including microcephaly) that negatively affect brain development.4
Before a possible conception or any sexual contact, women exposed to Zika—with or without symptoms—must wait at least 8 weeks; men with or without symptoms should abstain for 6 months.4 Individuals should avoid traveling to areas with Zika infestation, wear long-sleeved clothing treated with permethrin, and minimize outside exposure, especially in evening hours.4
The World Health Organization is utilizing genetically modified mosquitoes to diminish Aedes populations; trials conducted in affected areas of Brazil revealed that the number of Aedes mosquitoes was reduced by 90%.5 This method of mosquito control is currently being studied in the United States.6 Vaccinations to prevent Zika infection are also under investigation.
Physicians should educate patients regarding the clinical manifestations and complications of Zika virus infection; people need to know that the Zika virus can be sexually transmitted. Doctors should also counsel patients to curtail travel to areas that have Zika infestations, or to at least wear protective clothing while in such areas to minimize mosquito bite risk. Educating travelers about appropriate postponement of sexual contact after any exposure to the Zika virus is also essential.4
Hema Madhuri Mekala, MD
Priyanga Jayakumar, MD
Rajashekar Reddy Yeruva, MD
Steven Lippmann, MD
Louisville, KY
Since Florida has seen several new cases of local mosquito-borne infection, controlling and preventing Zika infection has great urgency. Zika virus involves an arthropod-borne infection transmitted by Aedes aegypti and Aedes albopictus mosquitoes. Other modes of transmission include the maternal-fetal route, any sexual contact, blood transfusions, organ or tissue transplantation, and laboratory exposure.1
The first case of Zika infection in the United States and its territories occurred through international travel. According to the Centers for Disease Control and Prevention, as of October 12, 2016, there were 3807 travel-associated cases of Zika infection in the United States and 84 instances in its territories.2 As for local transmission, there were 128 people evidencing a Zika infection in the United States and 25,871 in US territories.2 Regions between Texas and Florida are at high risk because Aedes mosquitoes primarily inhabit the gulf coast.3 Many cases have occurred despite repellent use and eradication efforts, possibly due to resistance acquired by these mosquitoes.1
Control measures include using insect repellents, aerial spraying of insecticides, eliminating mosquito breeding sites, covering water tanks, and using mosquito nets or door and window screens. Infection during pregnancy is the greatest concern because of congenital anomalies (including microcephaly) that negatively affect brain development.4
Before a possible conception or any sexual contact, women exposed to Zika—with or without symptoms—must wait at least 8 weeks; men with or without symptoms should abstain for 6 months.4 Individuals should avoid traveling to areas with Zika infestation, wear long-sleeved clothing treated with permethrin, and minimize outside exposure, especially in evening hours.4
The World Health Organization is utilizing genetically modified mosquitoes to diminish Aedes populations; trials conducted in affected areas of Brazil revealed that the number of Aedes mosquitoes was reduced by 90%.5 This method of mosquito control is currently being studied in the United States.6 Vaccinations to prevent Zika infection are also under investigation.
Physicians should educate patients regarding the clinical manifestations and complications of Zika virus infection; people need to know that the Zika virus can be sexually transmitted. Doctors should also counsel patients to curtail travel to areas that have Zika infestations, or to at least wear protective clothing while in such areas to minimize mosquito bite risk. Educating travelers about appropriate postponement of sexual contact after any exposure to the Zika virus is also essential.4
Hema Madhuri Mekala, MD
Priyanga Jayakumar, MD
Rajashekar Reddy Yeruva, MD
Steven Lippmann, MD
Louisville, KY
1. Centers for Disease Control and Prevention. Zika virus: Transmission & risks. Available at: http://www.cdc.gov/zika/transmission/index.html. Accessed October 14, 2016.
2. Centers for Disease Control and Prevention. Zika virus: Case counts in the US. Available at: http://www.cdc.gov/zika/geo/united-states.html. Accessed October 14, 2016.
3. Castro L, Chen X, Dimitrov NB, et al. The University of Texas at Austin. Texas Arbovirus Risk. 2015. Available at: http://hdl.handle.net/2152/31934. Accessed October 14, 2016.
4. Centers for Disease Control and Prevention. Zika virus: Zika is in your area: What to do. Available at: http://www.cdc.gov/zika/intheus/what-to-do.html. Accessed October 14, 2016.
5. FL KEYS NEWS. Available at: http://www.flkeysnews.com/opinion/opn-columns-blogs/article83328707.html. Accessed October 14, 2016.
6. Ernst KC, Haenchen S, Dickinson K, et al. Awareness and support of release of genetically modified “sterile” mosquitoes, Key West, Florida, USA. Emerg Infect Dis. 2015;21:320-324.
1. Centers for Disease Control and Prevention. Zika virus: Transmission & risks. Available at: http://www.cdc.gov/zika/transmission/index.html. Accessed October 14, 2016.
2. Centers for Disease Control and Prevention. Zika virus: Case counts in the US. Available at: http://www.cdc.gov/zika/geo/united-states.html. Accessed October 14, 2016.
3. Castro L, Chen X, Dimitrov NB, et al. The University of Texas at Austin. Texas Arbovirus Risk. 2015. Available at: http://hdl.handle.net/2152/31934. Accessed October 14, 2016.
4. Centers for Disease Control and Prevention. Zika virus: Zika is in your area: What to do. Available at: http://www.cdc.gov/zika/intheus/what-to-do.html. Accessed October 14, 2016.
5. FL KEYS NEWS. Available at: http://www.flkeysnews.com/opinion/opn-columns-blogs/article83328707.html. Accessed October 14, 2016.
6. Ernst KC, Haenchen S, Dickinson K, et al. Awareness and support of release of genetically modified “sterile” mosquitoes, Key West, Florida, USA. Emerg Infect Dis. 2015;21:320-324.