User login
Agent Orange Exposure Increases Lymphoma Risk in Million Veteran Program Cohort
TOPLINE: Agent Orange exposure was associated with a 26% to 71% increased risk for multiple lymphoid cancers in veterans enrolled in the US Department of Veterans Affairs (VA) Million Veterans Program (MVP), while genetic predisposition independently raised risk by 12% to 81% across different lymphoma subtypes. A case-controlled analysis of 255,155 veterans found no significant interaction between genetic risk scores and Agent Orange exposure.
METHODOLOGY:
A case-control study included 255,155 non-Hispanic White veterans (median age 67 years, 92.5% male) enrolled in the VA MVP with genotype and Agent Orange exposure data.
Researchers analyzed five lymphoid malignant neoplasm subtypes: chronic lymphocytic leukemia, diffuse large B-cell lymphoma, follicular lymphoma, marginal zone lymphoma, and multiple myeloma diagnosed from January 1965 through June 2024.
Agent Orange exposure was determined through self-reported survey responses, while polygenic risk scores were derived from genome-wide association studies of lymphoid malignant neoplasms.
Analysis included adjustments for age at enrollment, sex, and the first 10 genetic principal components in logistic regression models evaluating Agent Orange exposure, polygenic risk scores, and their potential interaction.
TAKEAWAY:
Agent Orange exposure significantly increased risk for chronic lymphocytic leukemia (odds ratio [OR], 1.61; 95% CI, 1.40-1.84), diffuse large B-cell lymphoma (OR, 1.26; 95% CI, 1.03-1.53), follicular lymphoma (OR, 1.71; 95% CI, 1.39-2.11), and multiple myeloma (OR, 1.58; 95% CI, 1.35-1.86).
Polygenic risk scores were independently associated with all lymphoma subtypes, with strongest associations for chronic lymphocytic leukemia (OR, 1.81; 95% CI, 1.70-1.93) and multiple myeloma (OR, 1.41; 95% CI, 1.31-1.52).
Analysis in African American participants showed similar associations for multiple myeloma with both Agent Orange exposure (OR, 1.56; 95% CI, 1.18-2.07) and polygenic risk scores (OR, 1.31; 95% CI, 1.15-1.49).
According to the researchers, no significant polygenic risk score and Agent Orange exposure interactions were observed for any lymphoma subtype.
IN PRACTICE: "Our study addressed the public health concerns surrounding Agent Orange exposure and lymphoid malignant neoplasms, finding that both Agent Orange exposure and polygenic risk are independently associated with disease, suggesting potentially distinct and additive pathways that merit further investigation," wrote the authors of the study.
SOURCE: The study was led by researchers at the University of California, Irvine and the Tibor Rubin Veterans Affairs Medical Center, Long Beach, Californiaand was published online on August 13 in JAMA Network Open.
LIMITATIONS: According to the authors, while this represents the largest case-control study of Agent Orange exposure and lymphoid malignant neoplasm risk, the power to detect interaction associations in specific subtypes might be limited. Self-reported Agent Orange exposure data may have introduced survival bias, particularly in aggressive subtypes, as patients with aggressive tumors may have died before joining the MVP. Additionally, about half of the patients were diagnosed with lymphoid malignant neoplasms before self-reporting Agent Orange exposure, potentially introducing recall bias.
DISCLOSURES: The research was supported by a Veterans Affairs Career Development Award Xueyi Teng, PhD, received grants from the George E. Hewitt Foundation for Medical Research Postdoc Fellowship during the study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
TOPLINE: Agent Orange exposure was associated with a 26% to 71% increased risk for multiple lymphoid cancers in veterans enrolled in the US Department of Veterans Affairs (VA) Million Veterans Program (MVP), while genetic predisposition independently raised risk by 12% to 81% across different lymphoma subtypes. A case-controlled analysis of 255,155 veterans found no significant interaction between genetic risk scores and Agent Orange exposure.
METHODOLOGY:
A case-control study included 255,155 non-Hispanic White veterans (median age 67 years, 92.5% male) enrolled in the VA MVP with genotype and Agent Orange exposure data.
Researchers analyzed five lymphoid malignant neoplasm subtypes: chronic lymphocytic leukemia, diffuse large B-cell lymphoma, follicular lymphoma, marginal zone lymphoma, and multiple myeloma diagnosed from January 1965 through June 2024.
Agent Orange exposure was determined through self-reported survey responses, while polygenic risk scores were derived from genome-wide association studies of lymphoid malignant neoplasms.
Analysis included adjustments for age at enrollment, sex, and the first 10 genetic principal components in logistic regression models evaluating Agent Orange exposure, polygenic risk scores, and their potential interaction.
TAKEAWAY:
Agent Orange exposure significantly increased risk for chronic lymphocytic leukemia (odds ratio [OR], 1.61; 95% CI, 1.40-1.84), diffuse large B-cell lymphoma (OR, 1.26; 95% CI, 1.03-1.53), follicular lymphoma (OR, 1.71; 95% CI, 1.39-2.11), and multiple myeloma (OR, 1.58; 95% CI, 1.35-1.86).
Polygenic risk scores were independently associated with all lymphoma subtypes, with strongest associations for chronic lymphocytic leukemia (OR, 1.81; 95% CI, 1.70-1.93) and multiple myeloma (OR, 1.41; 95% CI, 1.31-1.52).
Analysis in African American participants showed similar associations for multiple myeloma with both Agent Orange exposure (OR, 1.56; 95% CI, 1.18-2.07) and polygenic risk scores (OR, 1.31; 95% CI, 1.15-1.49).
According to the researchers, no significant polygenic risk score and Agent Orange exposure interactions were observed for any lymphoma subtype.
IN PRACTICE: "Our study addressed the public health concerns surrounding Agent Orange exposure and lymphoid malignant neoplasms, finding that both Agent Orange exposure and polygenic risk are independently associated with disease, suggesting potentially distinct and additive pathways that merit further investigation," wrote the authors of the study.
SOURCE: The study was led by researchers at the University of California, Irvine and the Tibor Rubin Veterans Affairs Medical Center, Long Beach, Californiaand was published online on August 13 in JAMA Network Open.
LIMITATIONS: According to the authors, while this represents the largest case-control study of Agent Orange exposure and lymphoid malignant neoplasm risk, the power to detect interaction associations in specific subtypes might be limited. Self-reported Agent Orange exposure data may have introduced survival bias, particularly in aggressive subtypes, as patients with aggressive tumors may have died before joining the MVP. Additionally, about half of the patients were diagnosed with lymphoid malignant neoplasms before self-reporting Agent Orange exposure, potentially introducing recall bias.
DISCLOSURES: The research was supported by a Veterans Affairs Career Development Award Xueyi Teng, PhD, received grants from the George E. Hewitt Foundation for Medical Research Postdoc Fellowship during the study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
TOPLINE: Agent Orange exposure was associated with a 26% to 71% increased risk for multiple lymphoid cancers in veterans enrolled in the US Department of Veterans Affairs (VA) Million Veterans Program (MVP), while genetic predisposition independently raised risk by 12% to 81% across different lymphoma subtypes. A case-controlled analysis of 255,155 veterans found no significant interaction between genetic risk scores and Agent Orange exposure.
METHODOLOGY:
A case-control study included 255,155 non-Hispanic White veterans (median age 67 years, 92.5% male) enrolled in the VA MVP with genotype and Agent Orange exposure data.
Researchers analyzed five lymphoid malignant neoplasm subtypes: chronic lymphocytic leukemia, diffuse large B-cell lymphoma, follicular lymphoma, marginal zone lymphoma, and multiple myeloma diagnosed from January 1965 through June 2024.
Agent Orange exposure was determined through self-reported survey responses, while polygenic risk scores were derived from genome-wide association studies of lymphoid malignant neoplasms.
Analysis included adjustments for age at enrollment, sex, and the first 10 genetic principal components in logistic regression models evaluating Agent Orange exposure, polygenic risk scores, and their potential interaction.
TAKEAWAY:
Agent Orange exposure significantly increased risk for chronic lymphocytic leukemia (odds ratio [OR], 1.61; 95% CI, 1.40-1.84), diffuse large B-cell lymphoma (OR, 1.26; 95% CI, 1.03-1.53), follicular lymphoma (OR, 1.71; 95% CI, 1.39-2.11), and multiple myeloma (OR, 1.58; 95% CI, 1.35-1.86).
Polygenic risk scores were independently associated with all lymphoma subtypes, with strongest associations for chronic lymphocytic leukemia (OR, 1.81; 95% CI, 1.70-1.93) and multiple myeloma (OR, 1.41; 95% CI, 1.31-1.52).
Analysis in African American participants showed similar associations for multiple myeloma with both Agent Orange exposure (OR, 1.56; 95% CI, 1.18-2.07) and polygenic risk scores (OR, 1.31; 95% CI, 1.15-1.49).
According to the researchers, no significant polygenic risk score and Agent Orange exposure interactions were observed for any lymphoma subtype.
IN PRACTICE: "Our study addressed the public health concerns surrounding Agent Orange exposure and lymphoid malignant neoplasms, finding that both Agent Orange exposure and polygenic risk are independently associated with disease, suggesting potentially distinct and additive pathways that merit further investigation," wrote the authors of the study.
SOURCE: The study was led by researchers at the University of California, Irvine and the Tibor Rubin Veterans Affairs Medical Center, Long Beach, Californiaand was published online on August 13 in JAMA Network Open.
LIMITATIONS: According to the authors, while this represents the largest case-control study of Agent Orange exposure and lymphoid malignant neoplasm risk, the power to detect interaction associations in specific subtypes might be limited. Self-reported Agent Orange exposure data may have introduced survival bias, particularly in aggressive subtypes, as patients with aggressive tumors may have died before joining the MVP. Additionally, about half of the patients were diagnosed with lymphoid malignant neoplasms before self-reporting Agent Orange exposure, potentially introducing recall bias.
DISCLOSURES: The research was supported by a Veterans Affairs Career Development Award Xueyi Teng, PhD, received grants from the George E. Hewitt Foundation for Medical Research Postdoc Fellowship during the study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Findings from (ImPaCT): Improving Patients With Prostate Cancer’s Access to Germline Testing
Background
With the onset of precision oncology, findings from germline mutational analysis have been helpful in treating patients with cancer and aids in cancer prevention, early detection, and improved overall outcomes. Germline genetic testing is now part of the standard of care for certain types of patients with prostate cancer. There is a very limited body of work that investigated demographic, disease- related and social factors that may be influencing Veterans’ participation in germline genetic testing. This study helps to identify whether certain factors may be influencing decisions on participation in prostate germline testing among Veterans with prostate malignancy.
Methods
The study was conducted using retrospective chart review. Data was collected from the periods of August 1, 2022 to December 31, 2023 among Veterans with prostate cancer who met criteria for germline genetic testing. Demographic and clinical information were collected including age, race, extent of disease (high risk, very high-risk or metastatic disease), significant co-morbidities, educational level, family and personal history of cancer, travel time, germline genetic test findings, impact on treatment approaches, referral for genetic counseling, and whether Veterans agreed or declined germline genetic testing. Data was analyzed using descriptive statistics. A total of 180 charts were reviewed, with 171 meeting the criteria for inclusion. The mean age of the participants is 73, with the youngest being 55 and the oldest being 101 years old. Majority of the participants were African American (77%).
Results
Only about two percent of those who met the inclusion criteria declined to undergo testing with the one living the farthest away from the testing hospital residing 18 miles away. Those who declined testing ranged in age from 67 to 88, majority had high risk prostate cancer and no family history of malignancy, and had 0-1 serious co-morbidity. None of their educational informational was available for review.
Conclusions
Participation in germline genetic testing can be enhanced with adequate patient education and availability of accessible resources, even among patient populations that are not always well-represented in clinical research. The presence of multiple serious co-morbidities and distance from a testing facility do not seem to contribute to hesitancy in germline genetic testing participation.
Background
With the onset of precision oncology, findings from germline mutational analysis have been helpful in treating patients with cancer and aids in cancer prevention, early detection, and improved overall outcomes. Germline genetic testing is now part of the standard of care for certain types of patients with prostate cancer. There is a very limited body of work that investigated demographic, disease- related and social factors that may be influencing Veterans’ participation in germline genetic testing. This study helps to identify whether certain factors may be influencing decisions on participation in prostate germline testing among Veterans with prostate malignancy.
Methods
The study was conducted using retrospective chart review. Data was collected from the periods of August 1, 2022 to December 31, 2023 among Veterans with prostate cancer who met criteria for germline genetic testing. Demographic and clinical information were collected including age, race, extent of disease (high risk, very high-risk or metastatic disease), significant co-morbidities, educational level, family and personal history of cancer, travel time, germline genetic test findings, impact on treatment approaches, referral for genetic counseling, and whether Veterans agreed or declined germline genetic testing. Data was analyzed using descriptive statistics. A total of 180 charts were reviewed, with 171 meeting the criteria for inclusion. The mean age of the participants is 73, with the youngest being 55 and the oldest being 101 years old. Majority of the participants were African American (77%).
Results
Only about two percent of those who met the inclusion criteria declined to undergo testing with the one living the farthest away from the testing hospital residing 18 miles away. Those who declined testing ranged in age from 67 to 88, majority had high risk prostate cancer and no family history of malignancy, and had 0-1 serious co-morbidity. None of their educational informational was available for review.
Conclusions
Participation in germline genetic testing can be enhanced with adequate patient education and availability of accessible resources, even among patient populations that are not always well-represented in clinical research. The presence of multiple serious co-morbidities and distance from a testing facility do not seem to contribute to hesitancy in germline genetic testing participation.
Background
With the onset of precision oncology, findings from germline mutational analysis have been helpful in treating patients with cancer and aids in cancer prevention, early detection, and improved overall outcomes. Germline genetic testing is now part of the standard of care for certain types of patients with prostate cancer. There is a very limited body of work that investigated demographic, disease- related and social factors that may be influencing Veterans’ participation in germline genetic testing. This study helps to identify whether certain factors may be influencing decisions on participation in prostate germline testing among Veterans with prostate malignancy.
Methods
The study was conducted using retrospective chart review. Data was collected from the periods of August 1, 2022 to December 31, 2023 among Veterans with prostate cancer who met criteria for germline genetic testing. Demographic and clinical information were collected including age, race, extent of disease (high risk, very high-risk or metastatic disease), significant co-morbidities, educational level, family and personal history of cancer, travel time, germline genetic test findings, impact on treatment approaches, referral for genetic counseling, and whether Veterans agreed or declined germline genetic testing. Data was analyzed using descriptive statistics. A total of 180 charts were reviewed, with 171 meeting the criteria for inclusion. The mean age of the participants is 73, with the youngest being 55 and the oldest being 101 years old. Majority of the participants were African American (77%).
Results
Only about two percent of those who met the inclusion criteria declined to undergo testing with the one living the farthest away from the testing hospital residing 18 miles away. Those who declined testing ranged in age from 67 to 88, majority had high risk prostate cancer and no family history of malignancy, and had 0-1 serious co-morbidity. None of their educational informational was available for review.
Conclusions
Participation in germline genetic testing can be enhanced with adequate patient education and availability of accessible resources, even among patient populations that are not always well-represented in clinical research. The presence of multiple serious co-morbidities and distance from a testing facility do not seem to contribute to hesitancy in germline genetic testing participation.
Enhancing Molecular Testing Documentation in Prostate Cancer
Background
Prostate cancer is the most common non-cutaneous malignancy at the Veterans Health Administration (VHA) and every year approximately 15,000 Veterans are diagnosed and treated. Many advanced prostate cancer cases harbor genetic mutations that significantly impact prognosis, treatment decisions, and familial screening. In February 2021, the Prostate Cancer Molecular Testing Pathway (PCMTP) flow map was developed to increase appropriate genetic testing.
Methods
VHA initiated the Oncology Clinical Pathways (OCP) program to standardize cancer care for Veterans. The PCMTP was developed by a multidisciplinary team that created interactive templates within the Computerized Patient Record System (CPRS), to facilitate identification of eligible Veterans for germline and comprehensive genomic profiling (CGP). Clinical decision-making for these tests is documented as Health Factors (HF), in CPRS, allowing for assessment of pathway adherence and overall uptake.
Results
The PCMTP has achieved success, as there is over 90% compliance to molecular testing among participating Veterans which exceeds the pathway benchmark of 80%. PCMTP has been utilized at 88 VA sites, by over 700 distinct VA providers, with over 7,000 Veterans participating. This implementation has yielded over 19,200 Health Factors within CPRS.
Conclusions
The PCMTP has markedly improved the documentation and application of germline and CGP testing among Veterans diagnosed with prostate cancer. By facilitating genomic testing in appropriate patients, the PCMTP aims to enhance patient outcomes and optimize the quality of care. Prior to PCMTP establishment, assessing the prevalence of germline and CGP testing in eligible Veterans posed significant challenges. Future work will concentrate on increasing PCMTP utilization, evaluating downstream outcomes from genomic testing, including the identification of pathogenic variants, utilization of genetic counseling services, referrals to clinical trials, and the genomic impact on treatment strategies.
Background
Prostate cancer is the most common non-cutaneous malignancy at the Veterans Health Administration (VHA) and every year approximately 15,000 Veterans are diagnosed and treated. Many advanced prostate cancer cases harbor genetic mutations that significantly impact prognosis, treatment decisions, and familial screening. In February 2021, the Prostate Cancer Molecular Testing Pathway (PCMTP) flow map was developed to increase appropriate genetic testing.
Methods
VHA initiated the Oncology Clinical Pathways (OCP) program to standardize cancer care for Veterans. The PCMTP was developed by a multidisciplinary team that created interactive templates within the Computerized Patient Record System (CPRS), to facilitate identification of eligible Veterans for germline and comprehensive genomic profiling (CGP). Clinical decision-making for these tests is documented as Health Factors (HF), in CPRS, allowing for assessment of pathway adherence and overall uptake.
Results
The PCMTP has achieved success, as there is over 90% compliance to molecular testing among participating Veterans which exceeds the pathway benchmark of 80%. PCMTP has been utilized at 88 VA sites, by over 700 distinct VA providers, with over 7,000 Veterans participating. This implementation has yielded over 19,200 Health Factors within CPRS.
Conclusions
The PCMTP has markedly improved the documentation and application of germline and CGP testing among Veterans diagnosed with prostate cancer. By facilitating genomic testing in appropriate patients, the PCMTP aims to enhance patient outcomes and optimize the quality of care. Prior to PCMTP establishment, assessing the prevalence of germline and CGP testing in eligible Veterans posed significant challenges. Future work will concentrate on increasing PCMTP utilization, evaluating downstream outcomes from genomic testing, including the identification of pathogenic variants, utilization of genetic counseling services, referrals to clinical trials, and the genomic impact on treatment strategies.
Background
Prostate cancer is the most common non-cutaneous malignancy at the Veterans Health Administration (VHA) and every year approximately 15,000 Veterans are diagnosed and treated. Many advanced prostate cancer cases harbor genetic mutations that significantly impact prognosis, treatment decisions, and familial screening. In February 2021, the Prostate Cancer Molecular Testing Pathway (PCMTP) flow map was developed to increase appropriate genetic testing.
Methods
VHA initiated the Oncology Clinical Pathways (OCP) program to standardize cancer care for Veterans. The PCMTP was developed by a multidisciplinary team that created interactive templates within the Computerized Patient Record System (CPRS), to facilitate identification of eligible Veterans for germline and comprehensive genomic profiling (CGP). Clinical decision-making for these tests is documented as Health Factors (HF), in CPRS, allowing for assessment of pathway adherence and overall uptake.
Results
The PCMTP has achieved success, as there is over 90% compliance to molecular testing among participating Veterans which exceeds the pathway benchmark of 80%. PCMTP has been utilized at 88 VA sites, by over 700 distinct VA providers, with over 7,000 Veterans participating. This implementation has yielded over 19,200 Health Factors within CPRS.
Conclusions
The PCMTP has markedly improved the documentation and application of germline and CGP testing among Veterans diagnosed with prostate cancer. By facilitating genomic testing in appropriate patients, the PCMTP aims to enhance patient outcomes and optimize the quality of care. Prior to PCMTP establishment, assessing the prevalence of germline and CGP testing in eligible Veterans posed significant challenges. Future work will concentrate on increasing PCMTP utilization, evaluating downstream outcomes from genomic testing, including the identification of pathogenic variants, utilization of genetic counseling services, referrals to clinical trials, and the genomic impact on treatment strategies.
Analysis of the Frequency of level 1 OncoKB Genomic Alterations in Veterans With Various Solid Organ Malignancies
Purpose
The aim of this study is to quantify the frequency of Memorial Sloan Kettering (MSK) Precision Oncology Knowledge Base (OncoKB) Level 1 genetic alterations in Veterans with various solid organ malignancies and evaluate the clinical benefit and impact of testing on treatment of these patients.
Background
The VA National Precision Oncology Program (NPOP) facilitates comprehensive genomic profiling (CGP) testing of Veterans with advanced cancer. While CGP is increasingly utilized and routinely ordered in patients with advanced solid organ malignancies, the clinical utility and value has not been proven in certain cancers. We present data from 5,979 patients with head and neck (H&N), pancreatic, hepatocellular (HCC), esophageal and kidney cancers who underwent CGP.
Methods
Our cohort consists of Veterans that received CGP testing to identify somatic variants between 1/1/2019 and 4/2/2025. Identified variants and biomarkers were formatted for use with oncoKB-annotator, a publicly available tool to annotate genomic variants with FDA approved drug recommendations stored as Level 1 annotations in OncoKB, and prescribed drugs were extracted from the Veteran Health Administration’s (VHA) Corporate Data Warehouse (CDW). Cancers were grouped by MSK’s OncoTree codes, and summary counts of Veterans tested, Veterans recommended, Veterans prescribed recommended FDA approved drugs were determined. Percentages were calculated using the total number of Veterans tested as the denominator.
Results
Level 1 OncoKB alterations were infrequent in H&N (0.94%), kidney (0.45%), HCC(0.28%), and pancreatic adenocarcinomas (1%). The frequency of Level 1 alterations in esophageal adenocarcinomas (EAC) was 20%. Approximately 98% of the Level 1 alterations in EAC patients were HER2 positivity or MSI-High status, which can be determined by other diagnostic methodologies such as IHC. The remaining 2% of EAC patients with level 1 alterations had BRAF V600E or NTRK rearrangements.
Conclusions
The incidence of level 1 genetic variants in H&N, kidney, HCC and pancreatic adenocarcinoma is very low and would very uncommonly result in clinical benefit. Although there is an expanding number of precision oncology-based therapies available, the proportion of patients with the aforementioned solid organ malignancies who benefitted from CGP was low, suggesting CGP has minimal impact on the treatment of Veterans with these malignancies.
Purpose
The aim of this study is to quantify the frequency of Memorial Sloan Kettering (MSK) Precision Oncology Knowledge Base (OncoKB) Level 1 genetic alterations in Veterans with various solid organ malignancies and evaluate the clinical benefit and impact of testing on treatment of these patients.
Background
The VA National Precision Oncology Program (NPOP) facilitates comprehensive genomic profiling (CGP) testing of Veterans with advanced cancer. While CGP is increasingly utilized and routinely ordered in patients with advanced solid organ malignancies, the clinical utility and value has not been proven in certain cancers. We present data from 5,979 patients with head and neck (H&N), pancreatic, hepatocellular (HCC), esophageal and kidney cancers who underwent CGP.
Methods
Our cohort consists of Veterans that received CGP testing to identify somatic variants between 1/1/2019 and 4/2/2025. Identified variants and biomarkers were formatted for use with oncoKB-annotator, a publicly available tool to annotate genomic variants with FDA approved drug recommendations stored as Level 1 annotations in OncoKB, and prescribed drugs were extracted from the Veteran Health Administration’s (VHA) Corporate Data Warehouse (CDW). Cancers were grouped by MSK’s OncoTree codes, and summary counts of Veterans tested, Veterans recommended, Veterans prescribed recommended FDA approved drugs were determined. Percentages were calculated using the total number of Veterans tested as the denominator.
Results
Level 1 OncoKB alterations were infrequent in H&N (0.94%), kidney (0.45%), HCC(0.28%), and pancreatic adenocarcinomas (1%). The frequency of Level 1 alterations in esophageal adenocarcinomas (EAC) was 20%. Approximately 98% of the Level 1 alterations in EAC patients were HER2 positivity or MSI-High status, which can be determined by other diagnostic methodologies such as IHC. The remaining 2% of EAC patients with level 1 alterations had BRAF V600E or NTRK rearrangements.
Conclusions
The incidence of level 1 genetic variants in H&N, kidney, HCC and pancreatic adenocarcinoma is very low and would very uncommonly result in clinical benefit. Although there is an expanding number of precision oncology-based therapies available, the proportion of patients with the aforementioned solid organ malignancies who benefitted from CGP was low, suggesting CGP has minimal impact on the treatment of Veterans with these malignancies.
Purpose
The aim of this study is to quantify the frequency of Memorial Sloan Kettering (MSK) Precision Oncology Knowledge Base (OncoKB) Level 1 genetic alterations in Veterans with various solid organ malignancies and evaluate the clinical benefit and impact of testing on treatment of these patients.
Background
The VA National Precision Oncology Program (NPOP) facilitates comprehensive genomic profiling (CGP) testing of Veterans with advanced cancer. While CGP is increasingly utilized and routinely ordered in patients with advanced solid organ malignancies, the clinical utility and value has not been proven in certain cancers. We present data from 5,979 patients with head and neck (H&N), pancreatic, hepatocellular (HCC), esophageal and kidney cancers who underwent CGP.
Methods
Our cohort consists of Veterans that received CGP testing to identify somatic variants between 1/1/2019 and 4/2/2025. Identified variants and biomarkers were formatted for use with oncoKB-annotator, a publicly available tool to annotate genomic variants with FDA approved drug recommendations stored as Level 1 annotations in OncoKB, and prescribed drugs were extracted from the Veteran Health Administration’s (VHA) Corporate Data Warehouse (CDW). Cancers were grouped by MSK’s OncoTree codes, and summary counts of Veterans tested, Veterans recommended, Veterans prescribed recommended FDA approved drugs were determined. Percentages were calculated using the total number of Veterans tested as the denominator.
Results
Level 1 OncoKB alterations were infrequent in H&N (0.94%), kidney (0.45%), HCC(0.28%), and pancreatic adenocarcinomas (1%). The frequency of Level 1 alterations in esophageal adenocarcinomas (EAC) was 20%. Approximately 98% of the Level 1 alterations in EAC patients were HER2 positivity or MSI-High status, which can be determined by other diagnostic methodologies such as IHC. The remaining 2% of EAC patients with level 1 alterations had BRAF V600E or NTRK rearrangements.
Conclusions
The incidence of level 1 genetic variants in H&N, kidney, HCC and pancreatic adenocarcinoma is very low and would very uncommonly result in clinical benefit. Although there is an expanding number of precision oncology-based therapies available, the proportion of patients with the aforementioned solid organ malignancies who benefitted from CGP was low, suggesting CGP has minimal impact on the treatment of Veterans with these malignancies.
Pharmacogenomic Testing for Veterans Newly Diagnosed with GI Malignancies
Background
In December of 2023, a workgroup at VA Connecticut Healthcare System (“VACHS”) initiated a quality improvement project to use the weekly GI Tumor Board meeting to identify patients who would benefit from PHASER testing. The PHASER panel includes two genes that are involved in the metabolism of two commonly used chemotherapy drugs in this patient population. Our goal was to identify patients with potentially impaired metabolism of 5FU and/or irinotecan prior to initiating treatment so that the doses of the appropriate drugs could be adjusted, leading to less toxicity for patients while on treatment and fewer lingering side-effects from treatment.
Results
Here we report outcomes based on 12 months of data. We reviewed the charts of all patients who received 5-FU or irinotecan during the period 1/1/24-12/31/24 based on pharmacy records. We separately identified all VACHS patients with newly diagnosed GI cancers in 2024 using data generated by the Tumor Registrar. 39 patients met criteria for PHASER testing. Of those, 37/39 (95%) patients got the testing. The 2 additional patients who were identified during our data analysis will be offered PHASER testing. Of the 37 patients who were tested, 7 patients (19%) had a genetic variant that could potentially impact chemotherapy dosing. 3 of these 7 patients were treated with chemotherapy and did require dose-adjustment. Of note, 100% of patients diagnosed with a new GI malignancy at VA Connecticut in 2024 whose treatment plan included possible chemotherapy with 5FU or Irinotecan got PHASER testing. In one year, this best practice is now our standard procedure.
Conclusions
Despite access to pharmacogenomic testing at VA, there can be variations between VA sites in terms of uptake of this new testing. VA Connecticut’s PHASER testing initiative for patients with GI malignancies is a model that can be replicated throughout VA. This initiative is part of a broader focus at VACHS on “pre-habilitation” and pre-treatment testing that is designed to reduce toxicity of treatment and improve quality of life for cancer survivors.
Background
In December of 2023, a workgroup at VA Connecticut Healthcare System (“VACHS”) initiated a quality improvement project to use the weekly GI Tumor Board meeting to identify patients who would benefit from PHASER testing. The PHASER panel includes two genes that are involved in the metabolism of two commonly used chemotherapy drugs in this patient population. Our goal was to identify patients with potentially impaired metabolism of 5FU and/or irinotecan prior to initiating treatment so that the doses of the appropriate drugs could be adjusted, leading to less toxicity for patients while on treatment and fewer lingering side-effects from treatment.
Results
Here we report outcomes based on 12 months of data. We reviewed the charts of all patients who received 5-FU or irinotecan during the period 1/1/24-12/31/24 based on pharmacy records. We separately identified all VACHS patients with newly diagnosed GI cancers in 2024 using data generated by the Tumor Registrar. 39 patients met criteria for PHASER testing. Of those, 37/39 (95%) patients got the testing. The 2 additional patients who were identified during our data analysis will be offered PHASER testing. Of the 37 patients who were tested, 7 patients (19%) had a genetic variant that could potentially impact chemotherapy dosing. 3 of these 7 patients were treated with chemotherapy and did require dose-adjustment. Of note, 100% of patients diagnosed with a new GI malignancy at VA Connecticut in 2024 whose treatment plan included possible chemotherapy with 5FU or Irinotecan got PHASER testing. In one year, this best practice is now our standard procedure.
Conclusions
Despite access to pharmacogenomic testing at VA, there can be variations between VA sites in terms of uptake of this new testing. VA Connecticut’s PHASER testing initiative for patients with GI malignancies is a model that can be replicated throughout VA. This initiative is part of a broader focus at VACHS on “pre-habilitation” and pre-treatment testing that is designed to reduce toxicity of treatment and improve quality of life for cancer survivors.
Background
In December of 2023, a workgroup at VA Connecticut Healthcare System (“VACHS”) initiated a quality improvement project to use the weekly GI Tumor Board meeting to identify patients who would benefit from PHASER testing. The PHASER panel includes two genes that are involved in the metabolism of two commonly used chemotherapy drugs in this patient population. Our goal was to identify patients with potentially impaired metabolism of 5FU and/or irinotecan prior to initiating treatment so that the doses of the appropriate drugs could be adjusted, leading to less toxicity for patients while on treatment and fewer lingering side-effects from treatment.
Results
Here we report outcomes based on 12 months of data. We reviewed the charts of all patients who received 5-FU or irinotecan during the period 1/1/24-12/31/24 based on pharmacy records. We separately identified all VACHS patients with newly diagnosed GI cancers in 2024 using data generated by the Tumor Registrar. 39 patients met criteria for PHASER testing. Of those, 37/39 (95%) patients got the testing. The 2 additional patients who were identified during our data analysis will be offered PHASER testing. Of the 37 patients who were tested, 7 patients (19%) had a genetic variant that could potentially impact chemotherapy dosing. 3 of these 7 patients were treated with chemotherapy and did require dose-adjustment. Of note, 100% of patients diagnosed with a new GI malignancy at VA Connecticut in 2024 whose treatment plan included possible chemotherapy with 5FU or Irinotecan got PHASER testing. In one year, this best practice is now our standard procedure.
Conclusions
Despite access to pharmacogenomic testing at VA, there can be variations between VA sites in terms of uptake of this new testing. VA Connecticut’s PHASER testing initiative for patients with GI malignancies is a model that can be replicated throughout VA. This initiative is part of a broader focus at VACHS on “pre-habilitation” and pre-treatment testing that is designed to reduce toxicity of treatment and improve quality of life for cancer survivors.
Implementation of an Interdisciplinary Precision Oncology Program at the Madison VA
Background
The William S. Middleton Memorial Veterans Hospital (Madison VA) prioritized the goal of ensuring patients with cancer are receiving guideline-based precision oncology care, including comprehensive genomic profiling (CGP) and germline genomics consultation based on evidence-based medicine and the VA Clinical Pathways. A local Precision Oncology Program was created to assist in review of CGP results including documentation in the electronic medical record (EMR) and recommendations for treatment or additional testing as appropriate. The program, which began in February 2024, focused on patients with prostate cancer initially. This was expanded to all genitourinary cancers in April 2024, non-small cell lung cancers (NSCLC) in August 2024, and all cancers in Dec 2024.
Results
Since the implementation of the Madison VA Precision Oncology Program, CGP was reviewed for 73 unique Veterans leading to 281 recommendations including: 25 FDA approved therapies, 2 off-label standard of care treatment options, 11 patients with potential clinical trial eligibility at the Madison VA. Forty-eight patients had no actionable mutations and 44 were recommended for additional germline genetics counseling. For patients with metastatic prostate cancer, after 1 year of program implementation, an increase was seen in the percentage of patients receiving guideline-based CGP, the percentage of actionable alterations identified, and the percentage of patients identified as potentially eligible for a clinical trial open at the Madison VA based on CGP. The percentage of patients with an interfacility consult to the Clinical Cancer Genetics Service was also increased. For patients with metastatic NSCLC, after 6 months of program implementation, an increase was seen in the percentage of patients appropriately receiving CGP, the percentage of actionable alterations identified, and the percentage of patients on targeted therapy. In all cases where an actionable alteration was not being targeted, the treatment option was not yet appropriate for the stage of disease.
Conclusions
The implementation of preemptive review of all CGP results at the Madison VA through the Precision Oncology Program has increased uptake and awareness of CGP results and potential treatment options, improving the access of targeted treatments and clinical trial opportunities for Veterans with cancer.
Background
The William S. Middleton Memorial Veterans Hospital (Madison VA) prioritized the goal of ensuring patients with cancer are receiving guideline-based precision oncology care, including comprehensive genomic profiling (CGP) and germline genomics consultation based on evidence-based medicine and the VA Clinical Pathways. A local Precision Oncology Program was created to assist in review of CGP results including documentation in the electronic medical record (EMR) and recommendations for treatment or additional testing as appropriate. The program, which began in February 2024, focused on patients with prostate cancer initially. This was expanded to all genitourinary cancers in April 2024, non-small cell lung cancers (NSCLC) in August 2024, and all cancers in Dec 2024.
Results
Since the implementation of the Madison VA Precision Oncology Program, CGP was reviewed for 73 unique Veterans leading to 281 recommendations including: 25 FDA approved therapies, 2 off-label standard of care treatment options, 11 patients with potential clinical trial eligibility at the Madison VA. Forty-eight patients had no actionable mutations and 44 were recommended for additional germline genetics counseling. For patients with metastatic prostate cancer, after 1 year of program implementation, an increase was seen in the percentage of patients receiving guideline-based CGP, the percentage of actionable alterations identified, and the percentage of patients identified as potentially eligible for a clinical trial open at the Madison VA based on CGP. The percentage of patients with an interfacility consult to the Clinical Cancer Genetics Service was also increased. For patients with metastatic NSCLC, after 6 months of program implementation, an increase was seen in the percentage of patients appropriately receiving CGP, the percentage of actionable alterations identified, and the percentage of patients on targeted therapy. In all cases where an actionable alteration was not being targeted, the treatment option was not yet appropriate for the stage of disease.
Conclusions
The implementation of preemptive review of all CGP results at the Madison VA through the Precision Oncology Program has increased uptake and awareness of CGP results and potential treatment options, improving the access of targeted treatments and clinical trial opportunities for Veterans with cancer.
Background
The William S. Middleton Memorial Veterans Hospital (Madison VA) prioritized the goal of ensuring patients with cancer are receiving guideline-based precision oncology care, including comprehensive genomic profiling (CGP) and germline genomics consultation based on evidence-based medicine and the VA Clinical Pathways. A local Precision Oncology Program was created to assist in review of CGP results including documentation in the electronic medical record (EMR) and recommendations for treatment or additional testing as appropriate. The program, which began in February 2024, focused on patients with prostate cancer initially. This was expanded to all genitourinary cancers in April 2024, non-small cell lung cancers (NSCLC) in August 2024, and all cancers in Dec 2024.
Results
Since the implementation of the Madison VA Precision Oncology Program, CGP was reviewed for 73 unique Veterans leading to 281 recommendations including: 25 FDA approved therapies, 2 off-label standard of care treatment options, 11 patients with potential clinical trial eligibility at the Madison VA. Forty-eight patients had no actionable mutations and 44 were recommended for additional germline genetics counseling. For patients with metastatic prostate cancer, after 1 year of program implementation, an increase was seen in the percentage of patients receiving guideline-based CGP, the percentage of actionable alterations identified, and the percentage of patients identified as potentially eligible for a clinical trial open at the Madison VA based on CGP. The percentage of patients with an interfacility consult to the Clinical Cancer Genetics Service was also increased. For patients with metastatic NSCLC, after 6 months of program implementation, an increase was seen in the percentage of patients appropriately receiving CGP, the percentage of actionable alterations identified, and the percentage of patients on targeted therapy. In all cases where an actionable alteration was not being targeted, the treatment option was not yet appropriate for the stage of disease.
Conclusions
The implementation of preemptive review of all CGP results at the Madison VA through the Precision Oncology Program has increased uptake and awareness of CGP results and potential treatment options, improving the access of targeted treatments and clinical trial opportunities for Veterans with cancer.
Trastuzumab Deruxtecan in HER2-Positive Breast Cancer
Study 1 Overview (Cortés et al)
Objective: To compare the efficacy and safety of trastuzumab deruxtecan with those of trastuzumab emtansine in patients with HER2-positive metastatic breast cancer previously treated with trastuzumab and taxane.
Design: Phase 3, multicenter, open-label randomized trial conducted at 169 centers and 15 countries.
Setting and participants: Eligible patients had to have unresectable or metastatic HER2-positive breast cancer that had progressed during or after treatment with trastuzumab and a taxane or had disease that progressed within 6 months after neoadjuvant or adjuvant treatment involving trastuzumab or taxane. Patients with stable or previously treated brain metastases were eligible. Patients were not eligible for the study if they had symptomatic brain metastases, prior exposure to trastuzumab emtansine, or a history of interstitial lung disease.
Intervention: Patients were randomized in a 1-to-1 fashion to receive either trastuzumab deruxtecan 5.4 mg/kg every 3 weeks or trastuzumab emtansine 3.6 mg/kg every 3 weeks. Patients were stratified according to hormone-receptor status, prior treatment with epratuzumab, and the presence or absence of visceral disease.
Main outcome measures: The primary endpoint of the study was progression-free survival as determined by an independent central review. Secondary endpoints included overall survival, overall response, and safety.
Main results: A total of 524 patients were enrolled in the study, with 261 patients randomized to trastuzumab deruxtecan and 263 patients randomized to trastuzumab emtansine. The demographic and baseline characteristics were similar between the 2 cohorts, and 60% of patients in both groups received prior epratuzumab therapy. Stable brain metastases were present in around 20% of patients in each group, and 70% of patients in each group had visceral disease. The median duration of follow-up was 16.2 months with trastuzumab deruxtecan and 15.3 months with trastuzumab emtansine.
The median progression-free survival was not reached in the trastuzumab deruxtecan group and was 6.8 months in the trastuzumab emtansine group (95% CI, 5.6-8.2). At 12 months the percentage of patients alive without disease progression was significantly larger in the trastuzumab deruxtecan group compared with the trastuzumab emtansine group. The hazard ratio for disease progression or death from any cause was 0.28 (95% CI, 0.22-0.37; P < .001). Subgroup analyses showed a benefit in progression-free survival with trastuzumab deruxtecan across all subgroups.
At the time of this analysis, the percentage of patients who were alive at 12 months was 94% with trastuzumab deruxtecan and 85.9% with trastuzumab emtansine. The response rates were significantly higher with trastuzumab deruxtecan compared with trastuzumab emtansine (79.7% vs 34.2%). A complete response was seen in 16% of patients in the trastuzumab deruxtecan arm, compared with 8.7% of patients in the trastuzumab emtansine group. The disease control rate (complete response, partial response, or stable disease) was higher in the trastuzumab deruxtecan group compared with the trastuzumab emtansine group (96.6% vs 76.8%).
Serious adverse events were reported in 19% of patients in the trastuzumab deruxtecan group and 18% of patients in the trastuzumab emtansine group. Discontinuation due to adverse events was higher in the trastuzumab deruxtecan group, with 13.6% of patients discontinuing trastuzumab deruxtecan. Grade 3 or higher adverse events were seen in 52% of patients treated with trastuzumab deruxtecan and 48% of patients treated with trastuzumab emtansine. The most commonly reported adverse event with trastuzumab deruxtecan was nausea/vomiting and fatigue. These adverse events were seen more in the trastuzumab deruxtecan group compared with the trastuzumab emtansine group. No drug-related grade 5 adverse events were reported.
In the trastuzumab deruxtecan group, 10.5% of patients receiving trastuzumab deruxtecan developed interstitial lung disease or pneumonitis. Seven patients had grade 1 events, 18 patients had grade 2 events, and 2 patients had grade 3 events. No grade 4 or 5 events were noted in either treatment group. The median time to onset of interstitial lung disease or pneumonitis in those receiving trastuzumab deruxtecan was 168 days (range, 33-507). Discontinuation of therapy due to interstitial lung disease or pneumonitis occurred in 8% of patients receiving trastuzumab deruxtecan and 1% of patients receiving trastuzumab emtansine.
Conclusion: Trastuzumab deruxtecan significantly decreases the risk of disease progression or death compared to trastuzumab emtansine in patients with HER2-positive metastatic breast cancer who have progressed on prior trastuzumab and taxane-based therapy.
Study 2 Overview (Modi et al)
Objective: To assess the efficacy of trastuzumab deruxtecan in patients with unresectable or metastatic breast cancer with low levels of HER2 expression.
Design: This was a randomized, 2-group, open-label, phase 3 trial.
Setting and participants: The trial was designed with a planned enrollment of 480 patients with hormone receptor–positive disease and 60 patients with hormone receptor–negative disease. Patients were randomized in a 2:1 ratio. Randomization was stratified according to HER2 status (immunohistochemical [IHC] 1+ vs IHC 2+/in situ hybridization [ISH] negative), number of prior lines of therapy, and hormone-receptor status. IHC scores for HER2 expression were determined through central testing. Specimens that had HER2 IHC scores of 2+ were reflexed to ISH. Specimens were considered HER2-low-expressing if they had an IHC score of 1+ or if they had an IHC score of 2+ and were ISH negative.
Eligible patients had to have received chemotherapy for metastatic disease or had disease recurrence during or within 6 months after completing adjuvant chemotherapy. Patients with hormone receptor–positive disease must have had at least 1 line of endocrine therapy. Patients were eligible if they had stable brain metastases. Patients with interstitial lung disease were excluded.
Intervention: Patients were randomized to receive trastuzumab deruxtecan 5.4 mg/kg every 3 weeks or physician’s choice of chemotherapy (capecitabine, eribulin, gemcitabine, paclitaxel, or nab-paclitaxel).
Main outcome measures: The primary endpoint was progression-free survival in patients with hormone receptor–positive disease. Secondary endpoints were progression-free survival among all patients, overall survival in hormone receptor–positive patients, and overall survival in all patients. Additional secondary endpoints included objective response rates, duration of response, and efficacy in hormone receptor–negative patients.
Main results: A total of 373 patients were assigned to the trastuzumab deruxtecan group and 184 patients were assigned to the physician’s choice chemotherapy group; 88% of patients in each cohort were hormone receptor–positive. In the physician’s choice chemotherapy group, 51% received eribulin, 20% received capecitabine, 10% received nab-paclitaxel, 10% received gemcitabine, and 8% received paclitaxel. The demographic and baseline characteristics were similar between both cohorts. The median duration of follow-up was 18.4 months.
The median progression-free survival in the hormone receptor–positive cohort was 10.1 months in the trastuzumab deruxtecan group and 5.4 months in the physician’s choice chemotherapy group (HR, 0.51; 95% CI, 0.4-0.64). Subgroup analyses revealed a benefit across all subgroups. The median progression-free survival among patients with a HER2 IHC score of 1+ and those with a HER2 IHC score of 2+/negative ISH were identical. In patients who received a prior CDK 4/6 inhibitor, the median progression-free survival was also 10 months in the trastuzumab deruxtecan group. In those who were CDK 4/6- naïve, the progression-free survival was 11.7 months. The progression-free survival in all patients was 9.9 months in the trastuzumab deruxtecan group and 5.1 months in the physician’s choice chemotherapy group (HR, 0.46; 95% CI, 0.24-0.89).
The median overall survival in the hormone receptor–positive cohort was 23.9 months in the trastuzumab deruxtecan group compared with 17.5 months in the physician’s choice chemotherapy group (HR, 0.64; 95% CI, 0.48-0.86; P = .003). The median overall survival in the entire population was 23.4 months in the trastuzumab deruxtecan group vs 16.8 months in the physician’s choice chemotherapy group. In the hormone receptor–negative cohort, the median overall survival was 18.2 months in the trastuzumab deruxtecan group and 8.3 months in the physician’s choice chemotherapy group. Complete responses were seen in 3.6% in the trastuzumab deruxtecan group and 0.6% and the physician’s choice chemotherapy group. The median duration of response was 10.7 months in the trastuzumab deruxtecan group and 6.8 months in the physician’s choice chemotherapy group.
Incidence of serious adverse events was 27% in the trastuzumab deruxtecan group and 25% in the physician’s choice chemotherapy group. Grade 3 or higher events occurred in 52% of the trastuzumab deruxtecan group and 67% of the physician’s choice chemotherapy group. Discontinuation due to adverse events occurred in 16% in the trastuzumab deruxtecan group and 18% in the physician’s choice chemotherapy group; 14 patients in the trastuzumab deruxtecan group and 5 patients in the physician’s choice chemotherapy group had an adverse event that was associated with death. Death due to pneumonitis in the trastuzumab deruxtecan group occurred in 2 patients. Drug-related interstitial lung disease or pneumonitis occurred in 45 patients who received trastuzumab deruxtecan. The majority of these events were grade 1 and grade 2. However, 3 patients had grade 5 interstitial lung disease or pneumonitis.
Conclusion: Treatment with trastuzumab deruxtecan led to a significant improvement in progression-free survival compared to physician’s choice chemotherapy in patients with HER2-low metastatic breast cancer.
Commentary
Trastuzumab deruxtecan is an antibody drug conjugate that consists of a humanized anti-HER2 monoclonal antibody linked to a topoisomerase 1 inhibitor. This antibody drug conjugate is unique compared with prior antibody drug conjugates such as trastuzumab emtansine in that it has a high drug-to-antibody ratio (~8). Furthermore, there appears to be a unique bystander effect resulting in off-target cytotoxicity to neighboring tumor cells, enhancing the efficacy of this novel therapy. Prior studies of trastuzumab deruxtecan have shown durable activity in heavily pretreated patients with metastatic HER2-positive breast cancer.1
HER2-positive breast cancer represents approximately 20% of breast cancer cases in women.2 Historically, HER2 positivity has been defined by strong HER2 expression with IHC staining (ie, score 3+) or HER2 amplification through ISH. Conversely, HER2-negative disease has historically been defined as those with IHC scores of 0 or 1+. This group represents approximately 60% of HER2-negative metastatic breast cancer patients.3 These patients have limited targeted treatment options after progressing on primary therapy. Prior data has shown that patients with low HER2 expression represent a heterogeneous population and thus, the historic categorization of HER2 status as positive or negative may in fact not adequately characterize the proportion of patients who may derive clinical benefit from HER2-directed therapies. Nevertheless, there have been no data to date that have shown improved outcomes in low HER2 expressers with anti-HER2 therapies.
The current studies add to the rapidly growing body of literature outlining the efficacy of the novel antibody drug conjugate trastuzumab deruxtecan. The implications of the data presented in these 2 studies are immediately practice changing.
In the DESTINY-Breast03 trial, Cortéz and colleagues show that trastuzumab deruxtecan therapy significantly prolongs progression-free survival compared with trastuzumab emtansine in patients with HER2-positive metastatic breast cancer who have progressed on first-line trastuzumab and taxane-based therapy. With a hazard ratio of 0.28 for disease progression or death, the efficacy of trastuzumab deruxtecan highlighted in this trial clearly makes this the standard of care in the second-line setting for patients with metastatic HER2-positive breast cancer. The overall survival in this trial was immature at the time of this analysis, and thus continued follow-up to validate the results noted here are warranted.
The DESTINY-Breast04 trial by Modi et al expands the cohort of patients who benefit from trastuzumab deruxtecan profoundly. This study defines a population of patients with HER2-low metastatic breast cancer who will now be eligible for HER2-directed therapies. These data show that therapy with trastuzumab deruxtecan leads to a significant and clinically meaningful improvement in both progression-free survival and overall survival compared with chemotherapy in patients with metastatic breast cancer with low expression of HER2. This benefit was seen in both the estrogen receptor–positive cohort as well as the entire population, including pre-treated triple-negative disease. Furthermore, this study does not define a threshold of HER2 expression by IHC that predicts benefit with trastuzumab deruxtecan. Patients with an IHC score of 1+ as well as those with a score of 2+/ISH negative both benefit to a similar extent from trastuzumab deruxtecan. Interestingly, in the DAISY trial, antitumor activity was noted with trastuzumab deruxtecan even in those without any detectable HER2 expression on IHC.4 Given the inconsistency and potential false negatives of IHC along with heterogeneous HER2 expression, further work is needed to better identify patients with low levels of HER2 expression who may benefit from this novel antibody drug conjugate. Thus, a reliable test to quantitatively assess the level of HER2 expression is needed in order to determine more accurately which patients will benefit from trastuzumab deruxtecan.
Last, trastuzumab deruxtecan has been associated with interstitial lung disease and pneumonitis. Interstitial lung disease and pneumonitis occurred in approximately 10% of patients who received trastuzumab deruxtecan in the DESTINY-Breast03 trial and about 12% of patients in the DESTINY-Breast04 trial. Most of these events were grade 1 and grade 2. Nevertheless, clinicians must be aware of this risk and monitor patients frequently for the development of pneumonitis or interstitial lung disease.
Application for Clinical Practice and System Implementation
The results of the current studies show a longer progression-free survival with trastuzumab deruxtecan in both HER2-low expressing metastatic breast cancer and HER2-positive metastatic breast cancer following taxane and trastuzumab-based therapy. These results are clearly practice changing and represent a new standard of care in these patient populations. It is incumbent upon treating oncologists to work with our pathology colleagues to assess HER2 IHC thoroughly in order to identify all potential patients who may benefit from trastuzumab deruxtecan in the metastatic setting. The continued advancement of anti-HER2 therapy will undoubtedly have a significant impact on patient outcomes going forward.
Practice Points
- With a hazard ratio of 0.28 for disease progression or death, the efficacy of trastuzumab deruxtecan highlighted in the DESTINY-Breast03 trial clearly makes this the standard of care in the second-line setting for patients with metastatic HER2-positive breast cancer.
- In the DESTINY-Breast04 trial, a significant and clinically meaningful improvement in both progression-free survival and overall survival compared with chemotherapy was seen in patients with metastatic breast cancer with low expression of HER2, including both the estrogen receptor–positive cohort as well as the entire population, including those with pre-treated triple-negative disease.
—Daniel Isaac, DO, MS
1. Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610-621. doi:10.1056/NEJMoa1914510
2. National Cancer Institute. Cancer stat facts. female breast cancer. Accessed July 25, 2022. https://seer.cancer.gov/statfacts/html/breast.html
3. Schettini F, Chic N, Braso-Maristany F, et al. Clinical, pathological and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. 2021;7(`1):1. doi:10.1038/s41523-020-00208-2
4. Dieras VDE, Deluche E, Lusque A, et al. Trastuzumab deruxtecan for advanced breast cancer patients, regardless of HER2 status: a phase II study with biomarkers analysis. In: Proceedings of Abstracts of the 2021 San Antonio Breast Cancer Symposium, December 7-10, 2021. San Antonio: American Association for Cancer Research, 2021. Abstract.
Study 1 Overview (Cortés et al)
Objective: To compare the efficacy and safety of trastuzumab deruxtecan with those of trastuzumab emtansine in patients with HER2-positive metastatic breast cancer previously treated with trastuzumab and taxane.
Design: Phase 3, multicenter, open-label randomized trial conducted at 169 centers and 15 countries.
Setting and participants: Eligible patients had to have unresectable or metastatic HER2-positive breast cancer that had progressed during or after treatment with trastuzumab and a taxane or had disease that progressed within 6 months after neoadjuvant or adjuvant treatment involving trastuzumab or taxane. Patients with stable or previously treated brain metastases were eligible. Patients were not eligible for the study if they had symptomatic brain metastases, prior exposure to trastuzumab emtansine, or a history of interstitial lung disease.
Intervention: Patients were randomized in a 1-to-1 fashion to receive either trastuzumab deruxtecan 5.4 mg/kg every 3 weeks or trastuzumab emtansine 3.6 mg/kg every 3 weeks. Patients were stratified according to hormone-receptor status, prior treatment with epratuzumab, and the presence or absence of visceral disease.
Main outcome measures: The primary endpoint of the study was progression-free survival as determined by an independent central review. Secondary endpoints included overall survival, overall response, and safety.
Main results: A total of 524 patients were enrolled in the study, with 261 patients randomized to trastuzumab deruxtecan and 263 patients randomized to trastuzumab emtansine. The demographic and baseline characteristics were similar between the 2 cohorts, and 60% of patients in both groups received prior epratuzumab therapy. Stable brain metastases were present in around 20% of patients in each group, and 70% of patients in each group had visceral disease. The median duration of follow-up was 16.2 months with trastuzumab deruxtecan and 15.3 months with trastuzumab emtansine.
The median progression-free survival was not reached in the trastuzumab deruxtecan group and was 6.8 months in the trastuzumab emtansine group (95% CI, 5.6-8.2). At 12 months the percentage of patients alive without disease progression was significantly larger in the trastuzumab deruxtecan group compared with the trastuzumab emtansine group. The hazard ratio for disease progression or death from any cause was 0.28 (95% CI, 0.22-0.37; P < .001). Subgroup analyses showed a benefit in progression-free survival with trastuzumab deruxtecan across all subgroups.
At the time of this analysis, the percentage of patients who were alive at 12 months was 94% with trastuzumab deruxtecan and 85.9% with trastuzumab emtansine. The response rates were significantly higher with trastuzumab deruxtecan compared with trastuzumab emtansine (79.7% vs 34.2%). A complete response was seen in 16% of patients in the trastuzumab deruxtecan arm, compared with 8.7% of patients in the trastuzumab emtansine group. The disease control rate (complete response, partial response, or stable disease) was higher in the trastuzumab deruxtecan group compared with the trastuzumab emtansine group (96.6% vs 76.8%).
Serious adverse events were reported in 19% of patients in the trastuzumab deruxtecan group and 18% of patients in the trastuzumab emtansine group. Discontinuation due to adverse events was higher in the trastuzumab deruxtecan group, with 13.6% of patients discontinuing trastuzumab deruxtecan. Grade 3 or higher adverse events were seen in 52% of patients treated with trastuzumab deruxtecan and 48% of patients treated with trastuzumab emtansine. The most commonly reported adverse event with trastuzumab deruxtecan was nausea/vomiting and fatigue. These adverse events were seen more in the trastuzumab deruxtecan group compared with the trastuzumab emtansine group. No drug-related grade 5 adverse events were reported.
In the trastuzumab deruxtecan group, 10.5% of patients receiving trastuzumab deruxtecan developed interstitial lung disease or pneumonitis. Seven patients had grade 1 events, 18 patients had grade 2 events, and 2 patients had grade 3 events. No grade 4 or 5 events were noted in either treatment group. The median time to onset of interstitial lung disease or pneumonitis in those receiving trastuzumab deruxtecan was 168 days (range, 33-507). Discontinuation of therapy due to interstitial lung disease or pneumonitis occurred in 8% of patients receiving trastuzumab deruxtecan and 1% of patients receiving trastuzumab emtansine.
Conclusion: Trastuzumab deruxtecan significantly decreases the risk of disease progression or death compared to trastuzumab emtansine in patients with HER2-positive metastatic breast cancer who have progressed on prior trastuzumab and taxane-based therapy.
Study 2 Overview (Modi et al)
Objective: To assess the efficacy of trastuzumab deruxtecan in patients with unresectable or metastatic breast cancer with low levels of HER2 expression.
Design: This was a randomized, 2-group, open-label, phase 3 trial.
Setting and participants: The trial was designed with a planned enrollment of 480 patients with hormone receptor–positive disease and 60 patients with hormone receptor–negative disease. Patients were randomized in a 2:1 ratio. Randomization was stratified according to HER2 status (immunohistochemical [IHC] 1+ vs IHC 2+/in situ hybridization [ISH] negative), number of prior lines of therapy, and hormone-receptor status. IHC scores for HER2 expression were determined through central testing. Specimens that had HER2 IHC scores of 2+ were reflexed to ISH. Specimens were considered HER2-low-expressing if they had an IHC score of 1+ or if they had an IHC score of 2+ and were ISH negative.
Eligible patients had to have received chemotherapy for metastatic disease or had disease recurrence during or within 6 months after completing adjuvant chemotherapy. Patients with hormone receptor–positive disease must have had at least 1 line of endocrine therapy. Patients were eligible if they had stable brain metastases. Patients with interstitial lung disease were excluded.
Intervention: Patients were randomized to receive trastuzumab deruxtecan 5.4 mg/kg every 3 weeks or physician’s choice of chemotherapy (capecitabine, eribulin, gemcitabine, paclitaxel, or nab-paclitaxel).
Main outcome measures: The primary endpoint was progression-free survival in patients with hormone receptor–positive disease. Secondary endpoints were progression-free survival among all patients, overall survival in hormone receptor–positive patients, and overall survival in all patients. Additional secondary endpoints included objective response rates, duration of response, and efficacy in hormone receptor–negative patients.
Main results: A total of 373 patients were assigned to the trastuzumab deruxtecan group and 184 patients were assigned to the physician’s choice chemotherapy group; 88% of patients in each cohort were hormone receptor–positive. In the physician’s choice chemotherapy group, 51% received eribulin, 20% received capecitabine, 10% received nab-paclitaxel, 10% received gemcitabine, and 8% received paclitaxel. The demographic and baseline characteristics were similar between both cohorts. The median duration of follow-up was 18.4 months.
The median progression-free survival in the hormone receptor–positive cohort was 10.1 months in the trastuzumab deruxtecan group and 5.4 months in the physician’s choice chemotherapy group (HR, 0.51; 95% CI, 0.4-0.64). Subgroup analyses revealed a benefit across all subgroups. The median progression-free survival among patients with a HER2 IHC score of 1+ and those with a HER2 IHC score of 2+/negative ISH were identical. In patients who received a prior CDK 4/6 inhibitor, the median progression-free survival was also 10 months in the trastuzumab deruxtecan group. In those who were CDK 4/6- naïve, the progression-free survival was 11.7 months. The progression-free survival in all patients was 9.9 months in the trastuzumab deruxtecan group and 5.1 months in the physician’s choice chemotherapy group (HR, 0.46; 95% CI, 0.24-0.89).
The median overall survival in the hormone receptor–positive cohort was 23.9 months in the trastuzumab deruxtecan group compared with 17.5 months in the physician’s choice chemotherapy group (HR, 0.64; 95% CI, 0.48-0.86; P = .003). The median overall survival in the entire population was 23.4 months in the trastuzumab deruxtecan group vs 16.8 months in the physician’s choice chemotherapy group. In the hormone receptor–negative cohort, the median overall survival was 18.2 months in the trastuzumab deruxtecan group and 8.3 months in the physician’s choice chemotherapy group. Complete responses were seen in 3.6% in the trastuzumab deruxtecan group and 0.6% and the physician’s choice chemotherapy group. The median duration of response was 10.7 months in the trastuzumab deruxtecan group and 6.8 months in the physician’s choice chemotherapy group.
Incidence of serious adverse events was 27% in the trastuzumab deruxtecan group and 25% in the physician’s choice chemotherapy group. Grade 3 or higher events occurred in 52% of the trastuzumab deruxtecan group and 67% of the physician’s choice chemotherapy group. Discontinuation due to adverse events occurred in 16% in the trastuzumab deruxtecan group and 18% in the physician’s choice chemotherapy group; 14 patients in the trastuzumab deruxtecan group and 5 patients in the physician’s choice chemotherapy group had an adverse event that was associated with death. Death due to pneumonitis in the trastuzumab deruxtecan group occurred in 2 patients. Drug-related interstitial lung disease or pneumonitis occurred in 45 patients who received trastuzumab deruxtecan. The majority of these events were grade 1 and grade 2. However, 3 patients had grade 5 interstitial lung disease or pneumonitis.
Conclusion: Treatment with trastuzumab deruxtecan led to a significant improvement in progression-free survival compared to physician’s choice chemotherapy in patients with HER2-low metastatic breast cancer.
Commentary
Trastuzumab deruxtecan is an antibody drug conjugate that consists of a humanized anti-HER2 monoclonal antibody linked to a topoisomerase 1 inhibitor. This antibody drug conjugate is unique compared with prior antibody drug conjugates such as trastuzumab emtansine in that it has a high drug-to-antibody ratio (~8). Furthermore, there appears to be a unique bystander effect resulting in off-target cytotoxicity to neighboring tumor cells, enhancing the efficacy of this novel therapy. Prior studies of trastuzumab deruxtecan have shown durable activity in heavily pretreated patients with metastatic HER2-positive breast cancer.1
HER2-positive breast cancer represents approximately 20% of breast cancer cases in women.2 Historically, HER2 positivity has been defined by strong HER2 expression with IHC staining (ie, score 3+) or HER2 amplification through ISH. Conversely, HER2-negative disease has historically been defined as those with IHC scores of 0 or 1+. This group represents approximately 60% of HER2-negative metastatic breast cancer patients.3 These patients have limited targeted treatment options after progressing on primary therapy. Prior data has shown that patients with low HER2 expression represent a heterogeneous population and thus, the historic categorization of HER2 status as positive or negative may in fact not adequately characterize the proportion of patients who may derive clinical benefit from HER2-directed therapies. Nevertheless, there have been no data to date that have shown improved outcomes in low HER2 expressers with anti-HER2 therapies.
The current studies add to the rapidly growing body of literature outlining the efficacy of the novel antibody drug conjugate trastuzumab deruxtecan. The implications of the data presented in these 2 studies are immediately practice changing.
In the DESTINY-Breast03 trial, Cortéz and colleagues show that trastuzumab deruxtecan therapy significantly prolongs progression-free survival compared with trastuzumab emtansine in patients with HER2-positive metastatic breast cancer who have progressed on first-line trastuzumab and taxane-based therapy. With a hazard ratio of 0.28 for disease progression or death, the efficacy of trastuzumab deruxtecan highlighted in this trial clearly makes this the standard of care in the second-line setting for patients with metastatic HER2-positive breast cancer. The overall survival in this trial was immature at the time of this analysis, and thus continued follow-up to validate the results noted here are warranted.
The DESTINY-Breast04 trial by Modi et al expands the cohort of patients who benefit from trastuzumab deruxtecan profoundly. This study defines a population of patients with HER2-low metastatic breast cancer who will now be eligible for HER2-directed therapies. These data show that therapy with trastuzumab deruxtecan leads to a significant and clinically meaningful improvement in both progression-free survival and overall survival compared with chemotherapy in patients with metastatic breast cancer with low expression of HER2. This benefit was seen in both the estrogen receptor–positive cohort as well as the entire population, including pre-treated triple-negative disease. Furthermore, this study does not define a threshold of HER2 expression by IHC that predicts benefit with trastuzumab deruxtecan. Patients with an IHC score of 1+ as well as those with a score of 2+/ISH negative both benefit to a similar extent from trastuzumab deruxtecan. Interestingly, in the DAISY trial, antitumor activity was noted with trastuzumab deruxtecan even in those without any detectable HER2 expression on IHC.4 Given the inconsistency and potential false negatives of IHC along with heterogeneous HER2 expression, further work is needed to better identify patients with low levels of HER2 expression who may benefit from this novel antibody drug conjugate. Thus, a reliable test to quantitatively assess the level of HER2 expression is needed in order to determine more accurately which patients will benefit from trastuzumab deruxtecan.
Last, trastuzumab deruxtecan has been associated with interstitial lung disease and pneumonitis. Interstitial lung disease and pneumonitis occurred in approximately 10% of patients who received trastuzumab deruxtecan in the DESTINY-Breast03 trial and about 12% of patients in the DESTINY-Breast04 trial. Most of these events were grade 1 and grade 2. Nevertheless, clinicians must be aware of this risk and monitor patients frequently for the development of pneumonitis or interstitial lung disease.
Application for Clinical Practice and System Implementation
The results of the current studies show a longer progression-free survival with trastuzumab deruxtecan in both HER2-low expressing metastatic breast cancer and HER2-positive metastatic breast cancer following taxane and trastuzumab-based therapy. These results are clearly practice changing and represent a new standard of care in these patient populations. It is incumbent upon treating oncologists to work with our pathology colleagues to assess HER2 IHC thoroughly in order to identify all potential patients who may benefit from trastuzumab deruxtecan in the metastatic setting. The continued advancement of anti-HER2 therapy will undoubtedly have a significant impact on patient outcomes going forward.
Practice Points
- With a hazard ratio of 0.28 for disease progression or death, the efficacy of trastuzumab deruxtecan highlighted in the DESTINY-Breast03 trial clearly makes this the standard of care in the second-line setting for patients with metastatic HER2-positive breast cancer.
- In the DESTINY-Breast04 trial, a significant and clinically meaningful improvement in both progression-free survival and overall survival compared with chemotherapy was seen in patients with metastatic breast cancer with low expression of HER2, including both the estrogen receptor–positive cohort as well as the entire population, including those with pre-treated triple-negative disease.
—Daniel Isaac, DO, MS
Study 1 Overview (Cortés et al)
Objective: To compare the efficacy and safety of trastuzumab deruxtecan with those of trastuzumab emtansine in patients with HER2-positive metastatic breast cancer previously treated with trastuzumab and taxane.
Design: Phase 3, multicenter, open-label randomized trial conducted at 169 centers and 15 countries.
Setting and participants: Eligible patients had to have unresectable or metastatic HER2-positive breast cancer that had progressed during or after treatment with trastuzumab and a taxane or had disease that progressed within 6 months after neoadjuvant or adjuvant treatment involving trastuzumab or taxane. Patients with stable or previously treated brain metastases were eligible. Patients were not eligible for the study if they had symptomatic brain metastases, prior exposure to trastuzumab emtansine, or a history of interstitial lung disease.
Intervention: Patients were randomized in a 1-to-1 fashion to receive either trastuzumab deruxtecan 5.4 mg/kg every 3 weeks or trastuzumab emtansine 3.6 mg/kg every 3 weeks. Patients were stratified according to hormone-receptor status, prior treatment with epratuzumab, and the presence or absence of visceral disease.
Main outcome measures: The primary endpoint of the study was progression-free survival as determined by an independent central review. Secondary endpoints included overall survival, overall response, and safety.
Main results: A total of 524 patients were enrolled in the study, with 261 patients randomized to trastuzumab deruxtecan and 263 patients randomized to trastuzumab emtansine. The demographic and baseline characteristics were similar between the 2 cohorts, and 60% of patients in both groups received prior epratuzumab therapy. Stable brain metastases were present in around 20% of patients in each group, and 70% of patients in each group had visceral disease. The median duration of follow-up was 16.2 months with trastuzumab deruxtecan and 15.3 months with trastuzumab emtansine.
The median progression-free survival was not reached in the trastuzumab deruxtecan group and was 6.8 months in the trastuzumab emtansine group (95% CI, 5.6-8.2). At 12 months the percentage of patients alive without disease progression was significantly larger in the trastuzumab deruxtecan group compared with the trastuzumab emtansine group. The hazard ratio for disease progression or death from any cause was 0.28 (95% CI, 0.22-0.37; P < .001). Subgroup analyses showed a benefit in progression-free survival with trastuzumab deruxtecan across all subgroups.
At the time of this analysis, the percentage of patients who were alive at 12 months was 94% with trastuzumab deruxtecan and 85.9% with trastuzumab emtansine. The response rates were significantly higher with trastuzumab deruxtecan compared with trastuzumab emtansine (79.7% vs 34.2%). A complete response was seen in 16% of patients in the trastuzumab deruxtecan arm, compared with 8.7% of patients in the trastuzumab emtansine group. The disease control rate (complete response, partial response, or stable disease) was higher in the trastuzumab deruxtecan group compared with the trastuzumab emtansine group (96.6% vs 76.8%).
Serious adverse events were reported in 19% of patients in the trastuzumab deruxtecan group and 18% of patients in the trastuzumab emtansine group. Discontinuation due to adverse events was higher in the trastuzumab deruxtecan group, with 13.6% of patients discontinuing trastuzumab deruxtecan. Grade 3 or higher adverse events were seen in 52% of patients treated with trastuzumab deruxtecan and 48% of patients treated with trastuzumab emtansine. The most commonly reported adverse event with trastuzumab deruxtecan was nausea/vomiting and fatigue. These adverse events were seen more in the trastuzumab deruxtecan group compared with the trastuzumab emtansine group. No drug-related grade 5 adverse events were reported.
In the trastuzumab deruxtecan group, 10.5% of patients receiving trastuzumab deruxtecan developed interstitial lung disease or pneumonitis. Seven patients had grade 1 events, 18 patients had grade 2 events, and 2 patients had grade 3 events. No grade 4 or 5 events were noted in either treatment group. The median time to onset of interstitial lung disease or pneumonitis in those receiving trastuzumab deruxtecan was 168 days (range, 33-507). Discontinuation of therapy due to interstitial lung disease or pneumonitis occurred in 8% of patients receiving trastuzumab deruxtecan and 1% of patients receiving trastuzumab emtansine.
Conclusion: Trastuzumab deruxtecan significantly decreases the risk of disease progression or death compared to trastuzumab emtansine in patients with HER2-positive metastatic breast cancer who have progressed on prior trastuzumab and taxane-based therapy.
Study 2 Overview (Modi et al)
Objective: To assess the efficacy of trastuzumab deruxtecan in patients with unresectable or metastatic breast cancer with low levels of HER2 expression.
Design: This was a randomized, 2-group, open-label, phase 3 trial.
Setting and participants: The trial was designed with a planned enrollment of 480 patients with hormone receptor–positive disease and 60 patients with hormone receptor–negative disease. Patients were randomized in a 2:1 ratio. Randomization was stratified according to HER2 status (immunohistochemical [IHC] 1+ vs IHC 2+/in situ hybridization [ISH] negative), number of prior lines of therapy, and hormone-receptor status. IHC scores for HER2 expression were determined through central testing. Specimens that had HER2 IHC scores of 2+ were reflexed to ISH. Specimens were considered HER2-low-expressing if they had an IHC score of 1+ or if they had an IHC score of 2+ and were ISH negative.
Eligible patients had to have received chemotherapy for metastatic disease or had disease recurrence during or within 6 months after completing adjuvant chemotherapy. Patients with hormone receptor–positive disease must have had at least 1 line of endocrine therapy. Patients were eligible if they had stable brain metastases. Patients with interstitial lung disease were excluded.
Intervention: Patients were randomized to receive trastuzumab deruxtecan 5.4 mg/kg every 3 weeks or physician’s choice of chemotherapy (capecitabine, eribulin, gemcitabine, paclitaxel, or nab-paclitaxel).
Main outcome measures: The primary endpoint was progression-free survival in patients with hormone receptor–positive disease. Secondary endpoints were progression-free survival among all patients, overall survival in hormone receptor–positive patients, and overall survival in all patients. Additional secondary endpoints included objective response rates, duration of response, and efficacy in hormone receptor–negative patients.
Main results: A total of 373 patients were assigned to the trastuzumab deruxtecan group and 184 patients were assigned to the physician’s choice chemotherapy group; 88% of patients in each cohort were hormone receptor–positive. In the physician’s choice chemotherapy group, 51% received eribulin, 20% received capecitabine, 10% received nab-paclitaxel, 10% received gemcitabine, and 8% received paclitaxel. The demographic and baseline characteristics were similar between both cohorts. The median duration of follow-up was 18.4 months.
The median progression-free survival in the hormone receptor–positive cohort was 10.1 months in the trastuzumab deruxtecan group and 5.4 months in the physician’s choice chemotherapy group (HR, 0.51; 95% CI, 0.4-0.64). Subgroup analyses revealed a benefit across all subgroups. The median progression-free survival among patients with a HER2 IHC score of 1+ and those with a HER2 IHC score of 2+/negative ISH were identical. In patients who received a prior CDK 4/6 inhibitor, the median progression-free survival was also 10 months in the trastuzumab deruxtecan group. In those who were CDK 4/6- naïve, the progression-free survival was 11.7 months. The progression-free survival in all patients was 9.9 months in the trastuzumab deruxtecan group and 5.1 months in the physician’s choice chemotherapy group (HR, 0.46; 95% CI, 0.24-0.89).
The median overall survival in the hormone receptor–positive cohort was 23.9 months in the trastuzumab deruxtecan group compared with 17.5 months in the physician’s choice chemotherapy group (HR, 0.64; 95% CI, 0.48-0.86; P = .003). The median overall survival in the entire population was 23.4 months in the trastuzumab deruxtecan group vs 16.8 months in the physician’s choice chemotherapy group. In the hormone receptor–negative cohort, the median overall survival was 18.2 months in the trastuzumab deruxtecan group and 8.3 months in the physician’s choice chemotherapy group. Complete responses were seen in 3.6% in the trastuzumab deruxtecan group and 0.6% and the physician’s choice chemotherapy group. The median duration of response was 10.7 months in the trastuzumab deruxtecan group and 6.8 months in the physician’s choice chemotherapy group.
Incidence of serious adverse events was 27% in the trastuzumab deruxtecan group and 25% in the physician’s choice chemotherapy group. Grade 3 or higher events occurred in 52% of the trastuzumab deruxtecan group and 67% of the physician’s choice chemotherapy group. Discontinuation due to adverse events occurred in 16% in the trastuzumab deruxtecan group and 18% in the physician’s choice chemotherapy group; 14 patients in the trastuzumab deruxtecan group and 5 patients in the physician’s choice chemotherapy group had an adverse event that was associated with death. Death due to pneumonitis in the trastuzumab deruxtecan group occurred in 2 patients. Drug-related interstitial lung disease or pneumonitis occurred in 45 patients who received trastuzumab deruxtecan. The majority of these events were grade 1 and grade 2. However, 3 patients had grade 5 interstitial lung disease or pneumonitis.
Conclusion: Treatment with trastuzumab deruxtecan led to a significant improvement in progression-free survival compared to physician’s choice chemotherapy in patients with HER2-low metastatic breast cancer.
Commentary
Trastuzumab deruxtecan is an antibody drug conjugate that consists of a humanized anti-HER2 monoclonal antibody linked to a topoisomerase 1 inhibitor. This antibody drug conjugate is unique compared with prior antibody drug conjugates such as trastuzumab emtansine in that it has a high drug-to-antibody ratio (~8). Furthermore, there appears to be a unique bystander effect resulting in off-target cytotoxicity to neighboring tumor cells, enhancing the efficacy of this novel therapy. Prior studies of trastuzumab deruxtecan have shown durable activity in heavily pretreated patients with metastatic HER2-positive breast cancer.1
HER2-positive breast cancer represents approximately 20% of breast cancer cases in women.2 Historically, HER2 positivity has been defined by strong HER2 expression with IHC staining (ie, score 3+) or HER2 amplification through ISH. Conversely, HER2-negative disease has historically been defined as those with IHC scores of 0 or 1+. This group represents approximately 60% of HER2-negative metastatic breast cancer patients.3 These patients have limited targeted treatment options after progressing on primary therapy. Prior data has shown that patients with low HER2 expression represent a heterogeneous population and thus, the historic categorization of HER2 status as positive or negative may in fact not adequately characterize the proportion of patients who may derive clinical benefit from HER2-directed therapies. Nevertheless, there have been no data to date that have shown improved outcomes in low HER2 expressers with anti-HER2 therapies.
The current studies add to the rapidly growing body of literature outlining the efficacy of the novel antibody drug conjugate trastuzumab deruxtecan. The implications of the data presented in these 2 studies are immediately practice changing.
In the DESTINY-Breast03 trial, Cortéz and colleagues show that trastuzumab deruxtecan therapy significantly prolongs progression-free survival compared with trastuzumab emtansine in patients with HER2-positive metastatic breast cancer who have progressed on first-line trastuzumab and taxane-based therapy. With a hazard ratio of 0.28 for disease progression or death, the efficacy of trastuzumab deruxtecan highlighted in this trial clearly makes this the standard of care in the second-line setting for patients with metastatic HER2-positive breast cancer. The overall survival in this trial was immature at the time of this analysis, and thus continued follow-up to validate the results noted here are warranted.
The DESTINY-Breast04 trial by Modi et al expands the cohort of patients who benefit from trastuzumab deruxtecan profoundly. This study defines a population of patients with HER2-low metastatic breast cancer who will now be eligible for HER2-directed therapies. These data show that therapy with trastuzumab deruxtecan leads to a significant and clinically meaningful improvement in both progression-free survival and overall survival compared with chemotherapy in patients with metastatic breast cancer with low expression of HER2. This benefit was seen in both the estrogen receptor–positive cohort as well as the entire population, including pre-treated triple-negative disease. Furthermore, this study does not define a threshold of HER2 expression by IHC that predicts benefit with trastuzumab deruxtecan. Patients with an IHC score of 1+ as well as those with a score of 2+/ISH negative both benefit to a similar extent from trastuzumab deruxtecan. Interestingly, in the DAISY trial, antitumor activity was noted with trastuzumab deruxtecan even in those without any detectable HER2 expression on IHC.4 Given the inconsistency and potential false negatives of IHC along with heterogeneous HER2 expression, further work is needed to better identify patients with low levels of HER2 expression who may benefit from this novel antibody drug conjugate. Thus, a reliable test to quantitatively assess the level of HER2 expression is needed in order to determine more accurately which patients will benefit from trastuzumab deruxtecan.
Last, trastuzumab deruxtecan has been associated with interstitial lung disease and pneumonitis. Interstitial lung disease and pneumonitis occurred in approximately 10% of patients who received trastuzumab deruxtecan in the DESTINY-Breast03 trial and about 12% of patients in the DESTINY-Breast04 trial. Most of these events were grade 1 and grade 2. Nevertheless, clinicians must be aware of this risk and monitor patients frequently for the development of pneumonitis or interstitial lung disease.
Application for Clinical Practice and System Implementation
The results of the current studies show a longer progression-free survival with trastuzumab deruxtecan in both HER2-low expressing metastatic breast cancer and HER2-positive metastatic breast cancer following taxane and trastuzumab-based therapy. These results are clearly practice changing and represent a new standard of care in these patient populations. It is incumbent upon treating oncologists to work with our pathology colleagues to assess HER2 IHC thoroughly in order to identify all potential patients who may benefit from trastuzumab deruxtecan in the metastatic setting. The continued advancement of anti-HER2 therapy will undoubtedly have a significant impact on patient outcomes going forward.
Practice Points
- With a hazard ratio of 0.28 for disease progression or death, the efficacy of trastuzumab deruxtecan highlighted in the DESTINY-Breast03 trial clearly makes this the standard of care in the second-line setting for patients with metastatic HER2-positive breast cancer.
- In the DESTINY-Breast04 trial, a significant and clinically meaningful improvement in both progression-free survival and overall survival compared with chemotherapy was seen in patients with metastatic breast cancer with low expression of HER2, including both the estrogen receptor–positive cohort as well as the entire population, including those with pre-treated triple-negative disease.
—Daniel Isaac, DO, MS
1. Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610-621. doi:10.1056/NEJMoa1914510
2. National Cancer Institute. Cancer stat facts. female breast cancer. Accessed July 25, 2022. https://seer.cancer.gov/statfacts/html/breast.html
3. Schettini F, Chic N, Braso-Maristany F, et al. Clinical, pathological and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. 2021;7(`1):1. doi:10.1038/s41523-020-00208-2
4. Dieras VDE, Deluche E, Lusque A, et al. Trastuzumab deruxtecan for advanced breast cancer patients, regardless of HER2 status: a phase II study with biomarkers analysis. In: Proceedings of Abstracts of the 2021 San Antonio Breast Cancer Symposium, December 7-10, 2021. San Antonio: American Association for Cancer Research, 2021. Abstract.
1. Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610-621. doi:10.1056/NEJMoa1914510
2. National Cancer Institute. Cancer stat facts. female breast cancer. Accessed July 25, 2022. https://seer.cancer.gov/statfacts/html/breast.html
3. Schettini F, Chic N, Braso-Maristany F, et al. Clinical, pathological and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. 2021;7(`1):1. doi:10.1038/s41523-020-00208-2
4. Dieras VDE, Deluche E, Lusque A, et al. Trastuzumab deruxtecan for advanced breast cancer patients, regardless of HER2 status: a phase II study with biomarkers analysis. In: Proceedings of Abstracts of the 2021 San Antonio Breast Cancer Symposium, December 7-10, 2021. San Antonio: American Association for Cancer Research, 2021. Abstract.
How to better identify and manage women with elevated breast cancer risk
Breast cancer is the most common invasive cancer in women in the United States; it is estimated that there will be 287,850 new cases of breast cancer in the United States during 2022 with 43,250 deaths.1 Lives are extended and saved every day because of a robust arsenal of treatments and interventions available to those who have been given a diagnosis of breast cancer. And, of course, lives are also extended and saved when we identify women at risk and provide early interventions. But in busy offices where time is short and there are competing demands on our time, proper assessment of a woman’s risk of breast cancer does not always happen. As a result, women with a higher risk of breast cancer may not be getting appropriate management.2,3
Familiarizing yourself with several risk-assessment tools and knowing when genetic testing is needed can make a big difference. Knowing the timing of mammograms and magnetic resonance imaging (MRI) for women deemed to be at high risk is also key. The following review employs a case-based approach (with an accompanying ALGORITHM) to illustrate how best to identify women who are at heightened risk of breast cancer and maximize their care. We also discuss the chemoprophylaxis regimens that may be used for those at increased risk.
CASE
Rachel P, age 37, presents to establish care. She has an Ashkenazi Jewish background and wonders if she should start doing breast cancer screening before age 40. She has 2 children, ages 4 years and 2 years. Her maternal aunt had unilateral breast cancer at age 54, and her maternal grandmother died of ovarian cancer at age 65.
Risk assessment
The risk assessment process (see ALGORITHM) must start with either the clinician or the patient initiating the discussion about breast cancer risk. The clinician may initiate the discussion with a new patient or at an annual physical examination. The patient may start the discussion because they are experiencing new breast symptoms, have anxiety about developing breast cancer, or have a family member with a new cancer diagnosis.
Risk factors. There are single factors that convey enough risk to automatically designate the patient as high risk (see TABLE 14-9). These factors include having a history of chest radiation between the ages of 10 and 30, a history of breast biopsy with either lobular carcinoma in situ (LCIS) or atypical ductal hyperplasia (ADH), past breast and/or ovarian cancer, and either a family or personal history of a high penetrant genetic variant for breast cancer.4-9
In women with previous chest radiation, breast cancer risk correlates with the total dose of radiation.5 For women with a personal history of breast cancer, the younger the age at diagnosis, the higher the risk of contralateral breast cancer.5 Precancerous changes such as ADH, LCIS, and ductal carcinoma in situ (DCIS) also confer moderate increases in risk. Women with these diagnoses will commonly have follow-up with specialists.
Risk assessment tools. There are several models available to assess a woman’s breast cancer risk (see TABLE 210-12). The Gail model (https://bcrisktool.cancer.gov/) is the oldest, quickest, and most widely known. However, the Gail model only accounts for first-degree relatives diagnosed with breast cancer, may underpredict risk in women with a more extensive family history, and has not been studied in women younger than 35. The International Breast Cancer Intervention Study (IBIS) Risk Evaluation Tool (https://ibis-risk-calculator.magview.com/), commonly referred to as the Tyrer-Cuzick model, incorporates second-degree relatives into the prediction model—although women may not know their full family history. Both the IBIS and the Breast Cancer Surveillance Consortium (BCSC) model (https://tools.bcsc-scc.org/BC5yearRisk/intro.htm) include breast density in the prediction algorithm. The choice of tool depends on clinician comfort and individual patient risk factors. There is no evidence that one model is better than another.10-12

Continue to: CASE
CASE
Ms. P’s clinician starts with an assessment using the Gail model. However, when the result comes back with average risk, the clinician decides to follow up with the Tyrer-Cuzick model in order to incorporate Ms. P’s multiple second-degree relatives with breast and ovarian cancer. (The BCSC model was not used because it only includes first-degree relatives.)
Genetic testing
The National Comprehensive Cancer Network (NCCN) guidelines recommend genetic testing if a woman has a first- or second-degree relative with pancreatic cancer, metastatic prostate cancer, male breast cancer, breast cancer at age 45 or younger, 2 or more breast cancers in a single person, 2 or more people on the same side of the family with at least 1 diagnosed at age 50 or younger, or any relative with ovarian cancer (see TABLE 3).7 Before ordering genetic testing, it is useful to refer the patient to a genetic counselor for a thorough discussion of options.
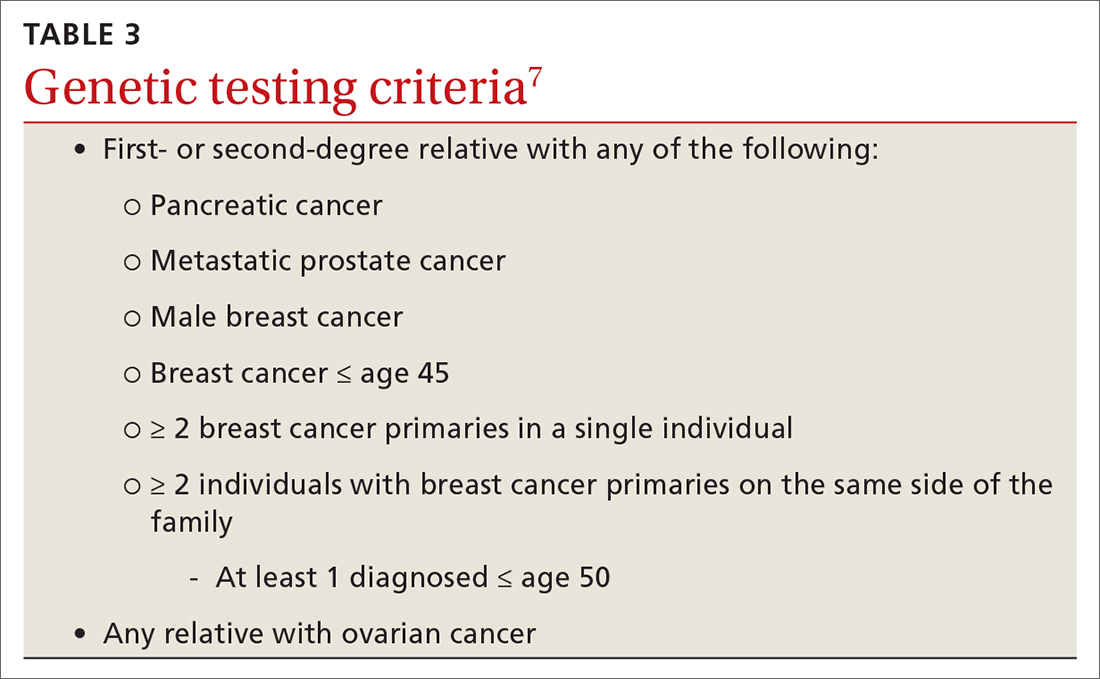
Results of genetic testing may include high-risk variants, moderate-risk variants, and variants of unknown significance (VUS), or be negative for any variants. High-risk variants for breast cancer include BRCA1, BRCA2, PALB2, and cancer syndrome variants such as TP53, PTEN, STK11, and CDH1.5,6,9,13-15 These high-risk variants confer sufficient risk that women with these mutations are automatically categorized in the high-risk group. It is estimated that high-risk variants account for only 25% of the genetic risk for breast cancer.16
BRCA1/2 and PTEN mutations confer greater than 80% lifetime risk, while other high-risk variants such as TP53, CDH1, and STK11 confer risks between 25% and 40%. These variants are also associated with cancers of other organs, depending on the mutation.17
Moderate-risk variants—ATM and CHEK2—do not confer sufficient risk to elevate women into the high-risk group. However, they do qualify these intermediate-risk women to participate in a specialized management strategy.5,9,13,18
VUS are those for which the associated risk is unclear, but more research may be done to categorize the risk.9 The clinical management of women with VUS usually entails close monitoring.
In an effort to better characterize breast cancer risk using a combination of pathogenic variants found in broad multi-gene cancer predisposition panels, researchers have developed a method to combine risks in a “polygenic risk score” (PRS) that can be used to counsel women (see “What is a polygenic risk score for breast cancer?” on page 203).19-21PRS predicts an additional 18% of genetic risk in women of European descent.21
SIDEBAR
What is a polygenic risk score for breast cancer?
- A polygenic risk score (PRS) is a mathematical method to combine results from a variety of different single nucleotide polymorphisms (SNPs; ie, single base pair variants) into a prediction tool that can estimate a woman’s lifetime risk of breast cancer.
- A PRS may be most accurate in determining risk for women with intermediate pathogenic variants, such as ATM and CHEK2. 19,20
- PRS has not been studied in non-White women.21
Continue to: CASE
CASE
Using the assessment results, the clinician talks to Ms. P about her lifetime risk for breast cancer. The Gail model indicates her lifetime risk is 13.3%, just slightly higher than the average (12.5%), and her 5-year risk is 0.5% (average, 0.4%). The IBIS or Tyrer-Cuzick model, which takes into account her second-degree relatives with breast and ovarian cancer and her Ashkenazi ethnicity (which confers increased risk due to elevated risk of BRCA mutations), predicts her lifetime risk of breast cancer to be 20.4%. This categorizes Ms. P as high risk.
Enhanced screening recommendations for women at high risk
TABLE 48,13,22 summarizes screening recommendations for women deemed to be at high risk for breast cancer. The American Cancer Society (ACS), NCCN, and the American College of Radiology (ACR) recommend that women with at least a 20% lifetime risk have yearly magnetic resonance imaging (MRI) and mammography (staggered so that the patient has 1 test every 6 months) starting 10 years before the age of onset for the youngest affected relative but not before age 30.8 For carriers of high-risk (as well as intermediate-risk) genes, NCCN recommends annual MRI screening starting at age 40.13BRCA1/2 screening includes annual MRI starting at age 25 and annual mammography every 6 months starting at age 30.22 Clinicians should counsel women with moderate risk factors (elevated breast density; personal history of ADH, LCIS, or DCIS) about the potential risks and benefits of enhanced screening and chemoprophylaxis.
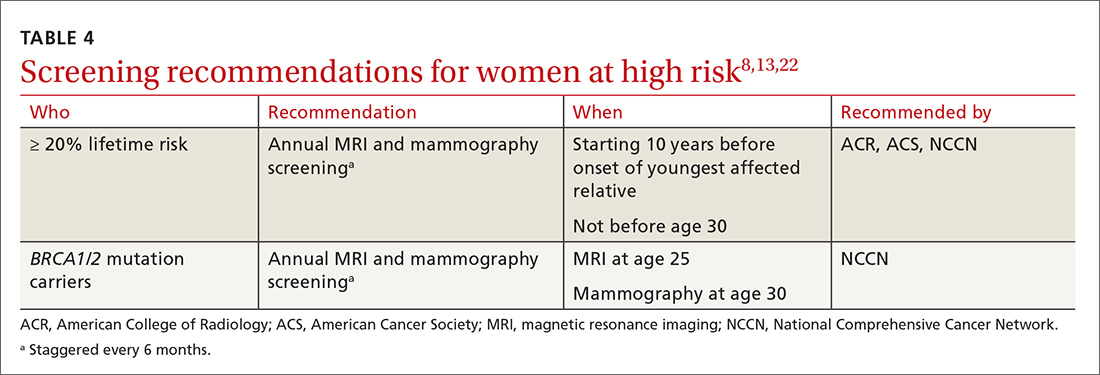
Risk-reduction strategies
Chemoprophylaxis
The US Preventive Services Task Force (USPSTF) recommends that all women at increased risk for breast cancer consider chemoprophylaxis (B recommendation)23 based on convincing evidence that 5 years of treatment with either a synthetic estrogen reuptake modulator (SERM) or an aromatase inhibitor (AI) decreases the incidence of estrogen receptor positive breast cancers. (See TABLE 57,23,24 for absolute risk reduction.) There is no benefit for chemoprophylaxis in women at average risk (D recommendation).23 It is unclear whether chemoprophylaxis is indicated in women with moderate increased risk (ie, who do not meet the 20% lifetime risk criteria). Chemoprophylaxis may not be effective in women with BRCA1 mutations, as they often develop triple-negative breast cancers.
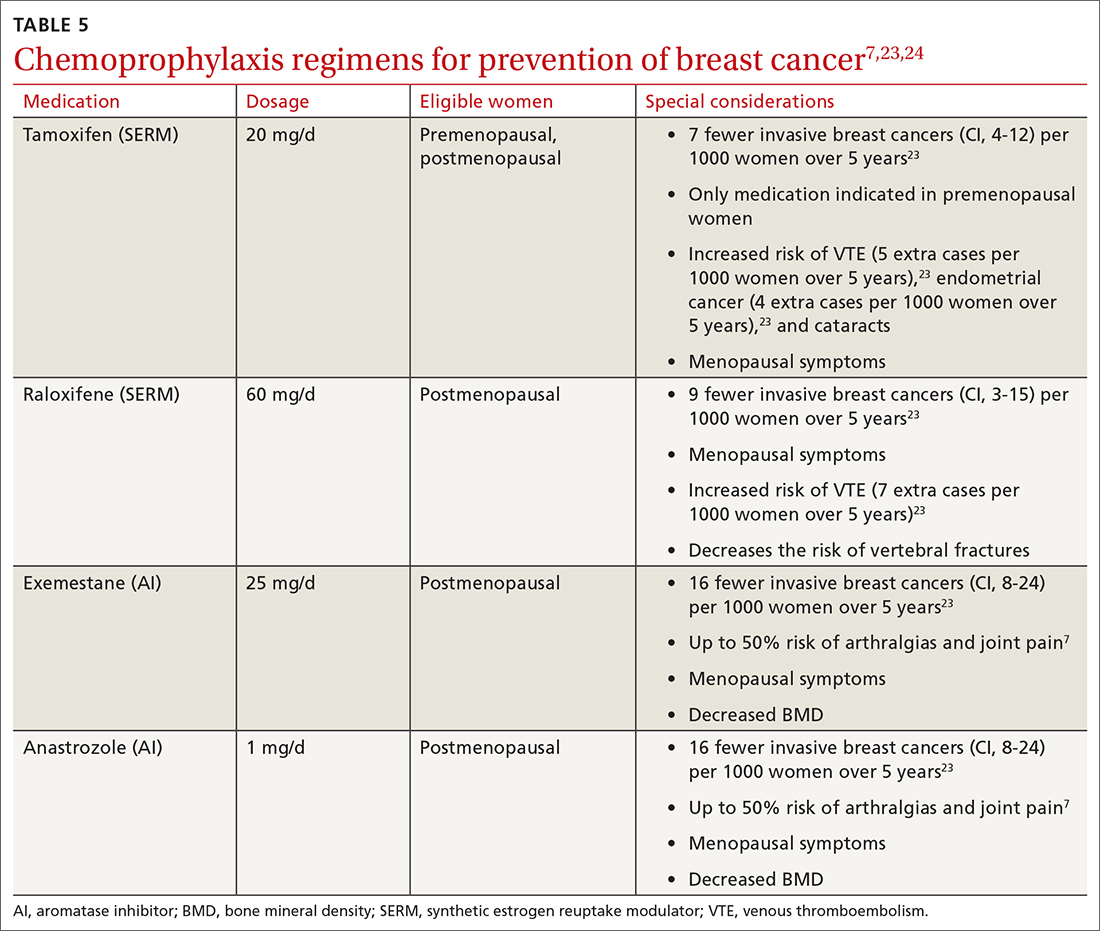
Accurate risk assessment and shared decision-making enable the clinician and patient to discuss the potential risks and benefits of chemoprophylaxis.7,24 The USPSTF did not find that any 1 risk prediction tool was better than another to identify women who should be counseled about chemoprophylaxis. Clinicians should counsel all women taking AIs about optimizing bone health with adequate calcium and vitamin D intake and routine bone density tests.
Surgical risk reduction
The NCCN guidelines state that risk-reducing bilateral mastectomy is reserved for individuals with high-risk gene variants and individuals with prior chest radiation between ages 10 and 30.25 NCCN also recommends discussing risk-reducing mastectomy with all women with BRCA mutations.22
Bilateral mastectomy is the most effective method to reduce breast cancer risk and should be discussed after age 25 in women with BRCA mutations and at least 8 years after chest radiation is completed.26 There is a reduction in breast cancer incidence of 90%.25 Breast imaging for screening (mammography or MRI) is not indicated after risk-reducing mastectomy. However, clinical breast examinations of the surgical site are important, because there is a small risk of developing breast cancer in that area.26
Risk-reducing oophorectomy is the standard of care for women with BRCA mutations to reduce the risk of ovarian cancer. It can also reduce the risk of breast cancer in women with BRCA mutations.27
Continue to: CASE
CASE
Based on her risk assessment results, family history, and genetic heritage, Ms. P qualifies for referral to a genetic counselor for discussion of BRCA testing. The clinician discusses adding annual MRI to Ms. P’s breast cancer screening regimen, based on ACS, NCCN, and ACR recommendations, due to her 20.4% lifetime risk. Discussion of whether and when to start chemoprophylaxis is typically based on breast cancer risk, projected benefit, and the potential impact of medication adverse effects. A high-risk woman is eligible for 5 years of chemoprophylaxis (tamoxifen if premenopausal) based on her lifetime risk. The clinician discusses timing with Ms. P, and even though she is finished with childbearing, she would like to wait until she is age 45, which is before the age at which her aunt was given a diagnosis of breast cancer.
Conclusion
Primary care clinicians are well positioned to identify women with an elevated risk of breast cancer and refer them for enhanced screening and chemoprophylaxis (see ALGORITHM). Shared decision-making with the inclusion of patient decision aids (https://decisionaid.ohri.ca/AZsearch.php?criteria=breast+cancer) about genetic testing, chemoprophylaxis, and prophylactic mastectomy or oophorectomy may help women at intermediate or high risk of breast cancer feel empowered to make decisions about their breast—and overall—health.
CORRESPONDENCE
Sarina Schrager, MD, MS, Professor, Department of Family Medicine and Community Health, University of Wisconsin, 1100 Delaplaine Court, Madison, WI 53715; [email protected]
1. National Cancer Institute. Cancer stat facts: female breast cancer. Accessed May 13, 2022. https://seer.cancer.gov/statfacts/html/breast.html
2. Guerra CE, Sherman M, Armstrong K. Diffusion of breast cancer risk assessment in primary care. J Am Board Fam Med. 2009;22:272-279. doi:10.3122/jabfm.2009.03.080153
3. Hamilton JG, Abdiwahab E, Edwards HM, et al. Primary care providers’ cancer genetic testing-related knowledge, attitudes, and communication behaviors: a systematic review and research agenda. J Gen Intern Med. 2017;32:315-324. doi:10.1007/s11606-016-3943-4
4. Eden KB, Ivlev I, Bensching KL, et al. Use of an online breast cancer risk assessment and patient decision aid in primary care practices. J Womens Health (Larchmt). 2020;29:763-769. doi: 10.1089/jwh.2019.8143
5. Kleibl Z, Kristensen VN. Women at high risk of breast cancer: molecular characteristics, clinical presentation and management. Breast. 2016;28:136-44. doi: 10.1016/j.breast.2016.05.006
6. Sciaraffa T, Guido B, Khan SA, et al. Breast cancer risk assessment and management programs: a practical guide. Breast J. 2020;26:1556-1564. doi: 10.1111/tbj.13967
7. Farkas A, Vanderberg R, Merriam S, et al. Breast cancer chemoprevention: a practical guide for the primary care provider. J Womens Health (Larchmt). 2020;29:46-56. doi: 10.1089/jwh.2018.7643
8. McClintock AH, Golob AL, Laya MB. Breast cancer risk assessment: a step-wise approach for primary care providers on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275. doi: 10.1016/j.mayocp.2020.04.017
9. Catana A, Apostu AP, Antemie RG. Multi gene panel testing for hereditary breast cancer - is it ready to be used? Med Pharm Rep. 2019;92:220-225. doi: 10.15386/mpr-1083
10. Barke LD, Freivogel ME. Breast cancer risk assessment models and high-risk screening. Radiol Clin North Am. 2017;55:457-474. doi: 10.1016/j.rcl.2016.12.013
11. Amir E, Freedman OC, Seruga B, et al. Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst. 2010;102:680-91. doi: 10.1093/jnci/djq088
12. Kim G, Bahl M. Assessing risk of breast cancer: a review of risk prediction models. J Breast Imaging. 2021;3:144-155. doi: 10.1093/jbi/wbab001
13. Narod SA. Which genes for hereditary breast cancer? N Engl J Med. 2021;384:471-473. doi: 10.1056/NEJMe2035083
14. Couch FJ, Shimelis H, Hu C, et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 2017;3:1190-1196. doi: 10.1001/jamaoncol.2017.0424
15. Obeid EI, Hall MJ, Daly MB. Multigene panel testing and breast cancer risk: is it time to scale down? JAMA Oncol. 2017;3:1176-1177. doi: 10.1001/jamaoncol.2017.0342
16. Michailidou K, Lindström S, Dennis J, et al. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551:92-94. doi: 10.1038/nature24284
17. Shiovitz S, Korde LA. Genetics of breast cancer: a topic in evolution. Ann Oncol. 2015;26:1291-1299. doi: 10.1093/annonc/mdv022
18. Hu C, Hart SN, Gnanaolivu R, et al. A population-based study of genes previously implicated in breast cancer. N Engl J Med. 2021;384:440-451. doi: 10.1056/NEJMoa2005936
19. Gao C, Polley EC, Hart SN, et al. Risk of breast cancer among carriers of pathogenic variants in breast cancer predisposition genes varies by polygenic risk score. J Clin Oncol. 2021;39:2564-2573. doi: 10.1200/JCO.20.01992
20. Gallagher S, Hughes E, Wagner S, et al. Association of a polygenic risk score with breast cancer among women carriers of high- and moderate-risk breast cancer genes. JAMA Netw Open. 2020;3:e208501. doi: 10.1001/jamanetworkopen.2020.8501
21. Yanes T, Young MA, Meiser B, et al. Clinical applications of polygenic breast cancer risk: a critical review and perspectives of an emerging field. Breast Cancer Res. 2020;22:21. doi: 10.1186/s13058-020-01260-3
22. Schrager S, Torell E, Ledford K, et al. Managing a woman with BRCA mutations? Shared decision-making is key. J Fam Pract. 2020;69:237-243
23. US Preventive Services Task Force; Owens DK, Davidson KW, Krist AH, et al. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:857-867. doi: 10.1001/jama.2019.11885
24. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol 2015;22:3230-3235. doi: 10.1245/s10434-015-4715-9
25. Britt KL, Cuzick J, Phillips KA. Key steps for effective breast cancer prevention. Nat Rev Cancer. 2020;20:417-436. doi: 10.1038/s41568-020-0266-x
26. Jatoi I, Kemp Z. Risk-reducing mastectomy. JAMA. 2021;325:1781-1782. doi: 10.1001/jama.2020.22414
27. Choi Y, Terry MB, Daly MB, et al. Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2021;7:585-592. doi:10.1001/jamaoncol.2020.7995
Breast cancer is the most common invasive cancer in women in the United States; it is estimated that there will be 287,850 new cases of breast cancer in the United States during 2022 with 43,250 deaths.1 Lives are extended and saved every day because of a robust arsenal of treatments and interventions available to those who have been given a diagnosis of breast cancer. And, of course, lives are also extended and saved when we identify women at risk and provide early interventions. But in busy offices where time is short and there are competing demands on our time, proper assessment of a woman’s risk of breast cancer does not always happen. As a result, women with a higher risk of breast cancer may not be getting appropriate management.2,3
Familiarizing yourself with several risk-assessment tools and knowing when genetic testing is needed can make a big difference. Knowing the timing of mammograms and magnetic resonance imaging (MRI) for women deemed to be at high risk is also key. The following review employs a case-based approach (with an accompanying ALGORITHM) to illustrate how best to identify women who are at heightened risk of breast cancer and maximize their care. We also discuss the chemoprophylaxis regimens that may be used for those at increased risk.

CASE
Rachel P, age 37, presents to establish care. She has an Ashkenazi Jewish background and wonders if she should start doing breast cancer screening before age 40. She has 2 children, ages 4 years and 2 years. Her maternal aunt had unilateral breast cancer at age 54, and her maternal grandmother died of ovarian cancer at age 65.
Risk assessment
The risk assessment process (see ALGORITHM) must start with either the clinician or the patient initiating the discussion about breast cancer risk. The clinician may initiate the discussion with a new patient or at an annual physical examination. The patient may start the discussion because they are experiencing new breast symptoms, have anxiety about developing breast cancer, or have a family member with a new cancer diagnosis.
Risk factors. There are single factors that convey enough risk to automatically designate the patient as high risk (see TABLE 14-9). These factors include having a history of chest radiation between the ages of 10 and 30, a history of breast biopsy with either lobular carcinoma in situ (LCIS) or atypical ductal hyperplasia (ADH), past breast and/or ovarian cancer, and either a family or personal history of a high penetrant genetic variant for breast cancer.4-9

In women with previous chest radiation, breast cancer risk correlates with the total dose of radiation.5 For women with a personal history of breast cancer, the younger the age at diagnosis, the higher the risk of contralateral breast cancer.5 Precancerous changes such as ADH, LCIS, and ductal carcinoma in situ (DCIS) also confer moderate increases in risk. Women with these diagnoses will commonly have follow-up with specialists.
Risk assessment tools. There are several models available to assess a woman’s breast cancer risk (see TABLE 210-12). The Gail model (https://bcrisktool.cancer.gov/) is the oldest, quickest, and most widely known. However, the Gail model only accounts for first-degree relatives diagnosed with breast cancer, may underpredict risk in women with a more extensive family history, and has not been studied in women younger than 35. The International Breast Cancer Intervention Study (IBIS) Risk Evaluation Tool (https://ibis-risk-calculator.magview.com/), commonly referred to as the Tyrer-Cuzick model, incorporates second-degree relatives into the prediction model—although women may not know their full family history. Both the IBIS and the Breast Cancer Surveillance Consortium (BCSC) model (https://tools.bcsc-scc.org/BC5yearRisk/intro.htm) include breast density in the prediction algorithm. The choice of tool depends on clinician comfort and individual patient risk factors. There is no evidence that one model is better than another.10-12

Continue to: CASE
CASE
Ms. P’s clinician starts with an assessment using the Gail model. However, when the result comes back with average risk, the clinician decides to follow up with the Tyrer-Cuzick model in order to incorporate Ms. P’s multiple second-degree relatives with breast and ovarian cancer. (The BCSC model was not used because it only includes first-degree relatives.)
Genetic testing
The National Comprehensive Cancer Network (NCCN) guidelines recommend genetic testing if a woman has a first- or second-degree relative with pancreatic cancer, metastatic prostate cancer, male breast cancer, breast cancer at age 45 or younger, 2 or more breast cancers in a single person, 2 or more people on the same side of the family with at least 1 diagnosed at age 50 or younger, or any relative with ovarian cancer (see TABLE 3).7 Before ordering genetic testing, it is useful to refer the patient to a genetic counselor for a thorough discussion of options.

Results of genetic testing may include high-risk variants, moderate-risk variants, and variants of unknown significance (VUS), or be negative for any variants. High-risk variants for breast cancer include BRCA1, BRCA2, PALB2, and cancer syndrome variants such as TP53, PTEN, STK11, and CDH1.5,6,9,13-15 These high-risk variants confer sufficient risk that women with these mutations are automatically categorized in the high-risk group. It is estimated that high-risk variants account for only 25% of the genetic risk for breast cancer.16
BRCA1/2 and PTEN mutations confer greater than 80% lifetime risk, while other high-risk variants such as TP53, CDH1, and STK11 confer risks between 25% and 40%. These variants are also associated with cancers of other organs, depending on the mutation.17
Moderate-risk variants—ATM and CHEK2—do not confer sufficient risk to elevate women into the high-risk group. However, they do qualify these intermediate-risk women to participate in a specialized management strategy.5,9,13,18
VUS are those for which the associated risk is unclear, but more research may be done to categorize the risk.9 The clinical management of women with VUS usually entails close monitoring.
In an effort to better characterize breast cancer risk using a combination of pathogenic variants found in broad multi-gene cancer predisposition panels, researchers have developed a method to combine risks in a “polygenic risk score” (PRS) that can be used to counsel women (see “What is a polygenic risk score for breast cancer?” on page 203).19-21PRS predicts an additional 18% of genetic risk in women of European descent.21
SIDEBAR
What is a polygenic risk score for breast cancer?
- A polygenic risk score (PRS) is a mathematical method to combine results from a variety of different single nucleotide polymorphisms (SNPs; ie, single base pair variants) into a prediction tool that can estimate a woman’s lifetime risk of breast cancer.
- A PRS may be most accurate in determining risk for women with intermediate pathogenic variants, such as ATM and CHEK2. 19,20
- PRS has not been studied in non-White women.21
Continue to: CASE
CASE
Using the assessment results, the clinician talks to Ms. P about her lifetime risk for breast cancer. The Gail model indicates her lifetime risk is 13.3%, just slightly higher than the average (12.5%), and her 5-year risk is 0.5% (average, 0.4%). The IBIS or Tyrer-Cuzick model, which takes into account her second-degree relatives with breast and ovarian cancer and her Ashkenazi ethnicity (which confers increased risk due to elevated risk of BRCA mutations), predicts her lifetime risk of breast cancer to be 20.4%. This categorizes Ms. P as high risk.
Enhanced screening recommendations for women at high risk
TABLE 48,13,22 summarizes screening recommendations for women deemed to be at high risk for breast cancer. The American Cancer Society (ACS), NCCN, and the American College of Radiology (ACR) recommend that women with at least a 20% lifetime risk have yearly magnetic resonance imaging (MRI) and mammography (staggered so that the patient has 1 test every 6 months) starting 10 years before the age of onset for the youngest affected relative but not before age 30.8 For carriers of high-risk (as well as intermediate-risk) genes, NCCN recommends annual MRI screening starting at age 40.13BRCA1/2 screening includes annual MRI starting at age 25 and annual mammography every 6 months starting at age 30.22 Clinicians should counsel women with moderate risk factors (elevated breast density; personal history of ADH, LCIS, or DCIS) about the potential risks and benefits of enhanced screening and chemoprophylaxis.

Risk-reduction strategies
Chemoprophylaxis
The US Preventive Services Task Force (USPSTF) recommends that all women at increased risk for breast cancer consider chemoprophylaxis (B recommendation)23 based on convincing evidence that 5 years of treatment with either a synthetic estrogen reuptake modulator (SERM) or an aromatase inhibitor (AI) decreases the incidence of estrogen receptor positive breast cancers. (See TABLE 57,23,24 for absolute risk reduction.) There is no benefit for chemoprophylaxis in women at average risk (D recommendation).23 It is unclear whether chemoprophylaxis is indicated in women with moderate increased risk (ie, who do not meet the 20% lifetime risk criteria). Chemoprophylaxis may not be effective in women with BRCA1 mutations, as they often develop triple-negative breast cancers.

Accurate risk assessment and shared decision-making enable the clinician and patient to discuss the potential risks and benefits of chemoprophylaxis.7,24 The USPSTF did not find that any 1 risk prediction tool was better than another to identify women who should be counseled about chemoprophylaxis. Clinicians should counsel all women taking AIs about optimizing bone health with adequate calcium and vitamin D intake and routine bone density tests.
Surgical risk reduction
The NCCN guidelines state that risk-reducing bilateral mastectomy is reserved for individuals with high-risk gene variants and individuals with prior chest radiation between ages 10 and 30.25 NCCN also recommends discussing risk-reducing mastectomy with all women with BRCA mutations.22
Bilateral mastectomy is the most effective method to reduce breast cancer risk and should be discussed after age 25 in women with BRCA mutations and at least 8 years after chest radiation is completed.26 There is a reduction in breast cancer incidence of 90%.25 Breast imaging for screening (mammography or MRI) is not indicated after risk-reducing mastectomy. However, clinical breast examinations of the surgical site are important, because there is a small risk of developing breast cancer in that area.26
Risk-reducing oophorectomy is the standard of care for women with BRCA mutations to reduce the risk of ovarian cancer. It can also reduce the risk of breast cancer in women with BRCA mutations.27
Continue to: CASE
CASE
Based on her risk assessment results, family history, and genetic heritage, Ms. P qualifies for referral to a genetic counselor for discussion of BRCA testing. The clinician discusses adding annual MRI to Ms. P’s breast cancer screening regimen, based on ACS, NCCN, and ACR recommendations, due to her 20.4% lifetime risk. Discussion of whether and when to start chemoprophylaxis is typically based on breast cancer risk, projected benefit, and the potential impact of medication adverse effects. A high-risk woman is eligible for 5 years of chemoprophylaxis (tamoxifen if premenopausal) based on her lifetime risk. The clinician discusses timing with Ms. P, and even though she is finished with childbearing, she would like to wait until she is age 45, which is before the age at which her aunt was given a diagnosis of breast cancer.
Conclusion
Primary care clinicians are well positioned to identify women with an elevated risk of breast cancer and refer them for enhanced screening and chemoprophylaxis (see ALGORITHM). Shared decision-making with the inclusion of patient decision aids (https://decisionaid.ohri.ca/AZsearch.php?criteria=breast+cancer) about genetic testing, chemoprophylaxis, and prophylactic mastectomy or oophorectomy may help women at intermediate or high risk of breast cancer feel empowered to make decisions about their breast—and overall—health.
CORRESPONDENCE
Sarina Schrager, MD, MS, Professor, Department of Family Medicine and Community Health, University of Wisconsin, 1100 Delaplaine Court, Madison, WI 53715; [email protected]
Breast cancer is the most common invasive cancer in women in the United States; it is estimated that there will be 287,850 new cases of breast cancer in the United States during 2022 with 43,250 deaths.1 Lives are extended and saved every day because of a robust arsenal of treatments and interventions available to those who have been given a diagnosis of breast cancer. And, of course, lives are also extended and saved when we identify women at risk and provide early interventions. But in busy offices where time is short and there are competing demands on our time, proper assessment of a woman’s risk of breast cancer does not always happen. As a result, women with a higher risk of breast cancer may not be getting appropriate management.2,3
Familiarizing yourself with several risk-assessment tools and knowing when genetic testing is needed can make a big difference. Knowing the timing of mammograms and magnetic resonance imaging (MRI) for women deemed to be at high risk is also key. The following review employs a case-based approach (with an accompanying ALGORITHM) to illustrate how best to identify women who are at heightened risk of breast cancer and maximize their care. We also discuss the chemoprophylaxis regimens that may be used for those at increased risk.

CASE
Rachel P, age 37, presents to establish care. She has an Ashkenazi Jewish background and wonders if she should start doing breast cancer screening before age 40. She has 2 children, ages 4 years and 2 years. Her maternal aunt had unilateral breast cancer at age 54, and her maternal grandmother died of ovarian cancer at age 65.
Risk assessment
The risk assessment process (see ALGORITHM) must start with either the clinician or the patient initiating the discussion about breast cancer risk. The clinician may initiate the discussion with a new patient or at an annual physical examination. The patient may start the discussion because they are experiencing new breast symptoms, have anxiety about developing breast cancer, or have a family member with a new cancer diagnosis.
Risk factors. There are single factors that convey enough risk to automatically designate the patient as high risk (see TABLE 14-9). These factors include having a history of chest radiation between the ages of 10 and 30, a history of breast biopsy with either lobular carcinoma in situ (LCIS) or atypical ductal hyperplasia (ADH), past breast and/or ovarian cancer, and either a family or personal history of a high penetrant genetic variant for breast cancer.4-9

In women with previous chest radiation, breast cancer risk correlates with the total dose of radiation.5 For women with a personal history of breast cancer, the younger the age at diagnosis, the higher the risk of contralateral breast cancer.5 Precancerous changes such as ADH, LCIS, and ductal carcinoma in situ (DCIS) also confer moderate increases in risk. Women with these diagnoses will commonly have follow-up with specialists.
Risk assessment tools. There are several models available to assess a woman’s breast cancer risk (see TABLE 210-12). The Gail model (https://bcrisktool.cancer.gov/) is the oldest, quickest, and most widely known. However, the Gail model only accounts for first-degree relatives diagnosed with breast cancer, may underpredict risk in women with a more extensive family history, and has not been studied in women younger than 35. The International Breast Cancer Intervention Study (IBIS) Risk Evaluation Tool (https://ibis-risk-calculator.magview.com/), commonly referred to as the Tyrer-Cuzick model, incorporates second-degree relatives into the prediction model—although women may not know their full family history. Both the IBIS and the Breast Cancer Surveillance Consortium (BCSC) model (https://tools.bcsc-scc.org/BC5yearRisk/intro.htm) include breast density in the prediction algorithm. The choice of tool depends on clinician comfort and individual patient risk factors. There is no evidence that one model is better than another.10-12

Continue to: CASE
CASE
Ms. P’s clinician starts with an assessment using the Gail model. However, when the result comes back with average risk, the clinician decides to follow up with the Tyrer-Cuzick model in order to incorporate Ms. P’s multiple second-degree relatives with breast and ovarian cancer. (The BCSC model was not used because it only includes first-degree relatives.)
Genetic testing
The National Comprehensive Cancer Network (NCCN) guidelines recommend genetic testing if a woman has a first- or second-degree relative with pancreatic cancer, metastatic prostate cancer, male breast cancer, breast cancer at age 45 or younger, 2 or more breast cancers in a single person, 2 or more people on the same side of the family with at least 1 diagnosed at age 50 or younger, or any relative with ovarian cancer (see TABLE 3).7 Before ordering genetic testing, it is useful to refer the patient to a genetic counselor for a thorough discussion of options.

Results of genetic testing may include high-risk variants, moderate-risk variants, and variants of unknown significance (VUS), or be negative for any variants. High-risk variants for breast cancer include BRCA1, BRCA2, PALB2, and cancer syndrome variants such as TP53, PTEN, STK11, and CDH1.5,6,9,13-15 These high-risk variants confer sufficient risk that women with these mutations are automatically categorized in the high-risk group. It is estimated that high-risk variants account for only 25% of the genetic risk for breast cancer.16
BRCA1/2 and PTEN mutations confer greater than 80% lifetime risk, while other high-risk variants such as TP53, CDH1, and STK11 confer risks between 25% and 40%. These variants are also associated with cancers of other organs, depending on the mutation.17
Moderate-risk variants—ATM and CHEK2—do not confer sufficient risk to elevate women into the high-risk group. However, they do qualify these intermediate-risk women to participate in a specialized management strategy.5,9,13,18
VUS are those for which the associated risk is unclear, but more research may be done to categorize the risk.9 The clinical management of women with VUS usually entails close monitoring.
In an effort to better characterize breast cancer risk using a combination of pathogenic variants found in broad multi-gene cancer predisposition panels, researchers have developed a method to combine risks in a “polygenic risk score” (PRS) that can be used to counsel women (see “What is a polygenic risk score for breast cancer?” on page 203).19-21PRS predicts an additional 18% of genetic risk in women of European descent.21
SIDEBAR
What is a polygenic risk score for breast cancer?
- A polygenic risk score (PRS) is a mathematical method to combine results from a variety of different single nucleotide polymorphisms (SNPs; ie, single base pair variants) into a prediction tool that can estimate a woman’s lifetime risk of breast cancer.
- A PRS may be most accurate in determining risk for women with intermediate pathogenic variants, such as ATM and CHEK2. 19,20
- PRS has not been studied in non-White women.21
Continue to: CASE
CASE
Using the assessment results, the clinician talks to Ms. P about her lifetime risk for breast cancer. The Gail model indicates her lifetime risk is 13.3%, just slightly higher than the average (12.5%), and her 5-year risk is 0.5% (average, 0.4%). The IBIS or Tyrer-Cuzick model, which takes into account her second-degree relatives with breast and ovarian cancer and her Ashkenazi ethnicity (which confers increased risk due to elevated risk of BRCA mutations), predicts her lifetime risk of breast cancer to be 20.4%. This categorizes Ms. P as high risk.
Enhanced screening recommendations for women at high risk
TABLE 48,13,22 summarizes screening recommendations for women deemed to be at high risk for breast cancer. The American Cancer Society (ACS), NCCN, and the American College of Radiology (ACR) recommend that women with at least a 20% lifetime risk have yearly magnetic resonance imaging (MRI) and mammography (staggered so that the patient has 1 test every 6 months) starting 10 years before the age of onset for the youngest affected relative but not before age 30.8 For carriers of high-risk (as well as intermediate-risk) genes, NCCN recommends annual MRI screening starting at age 40.13BRCA1/2 screening includes annual MRI starting at age 25 and annual mammography every 6 months starting at age 30.22 Clinicians should counsel women with moderate risk factors (elevated breast density; personal history of ADH, LCIS, or DCIS) about the potential risks and benefits of enhanced screening and chemoprophylaxis.

Risk-reduction strategies
Chemoprophylaxis
The US Preventive Services Task Force (USPSTF) recommends that all women at increased risk for breast cancer consider chemoprophylaxis (B recommendation)23 based on convincing evidence that 5 years of treatment with either a synthetic estrogen reuptake modulator (SERM) or an aromatase inhibitor (AI) decreases the incidence of estrogen receptor positive breast cancers. (See TABLE 57,23,24 for absolute risk reduction.) There is no benefit for chemoprophylaxis in women at average risk (D recommendation).23 It is unclear whether chemoprophylaxis is indicated in women with moderate increased risk (ie, who do not meet the 20% lifetime risk criteria). Chemoprophylaxis may not be effective in women with BRCA1 mutations, as they often develop triple-negative breast cancers.

Accurate risk assessment and shared decision-making enable the clinician and patient to discuss the potential risks and benefits of chemoprophylaxis.7,24 The USPSTF did not find that any 1 risk prediction tool was better than another to identify women who should be counseled about chemoprophylaxis. Clinicians should counsel all women taking AIs about optimizing bone health with adequate calcium and vitamin D intake and routine bone density tests.
Surgical risk reduction
The NCCN guidelines state that risk-reducing bilateral mastectomy is reserved for individuals with high-risk gene variants and individuals with prior chest radiation between ages 10 and 30.25 NCCN also recommends discussing risk-reducing mastectomy with all women with BRCA mutations.22
Bilateral mastectomy is the most effective method to reduce breast cancer risk and should be discussed after age 25 in women with BRCA mutations and at least 8 years after chest radiation is completed.26 There is a reduction in breast cancer incidence of 90%.25 Breast imaging for screening (mammography or MRI) is not indicated after risk-reducing mastectomy. However, clinical breast examinations of the surgical site are important, because there is a small risk of developing breast cancer in that area.26
Risk-reducing oophorectomy is the standard of care for women with BRCA mutations to reduce the risk of ovarian cancer. It can also reduce the risk of breast cancer in women with BRCA mutations.27
Continue to: CASE
CASE
Based on her risk assessment results, family history, and genetic heritage, Ms. P qualifies for referral to a genetic counselor for discussion of BRCA testing. The clinician discusses adding annual MRI to Ms. P’s breast cancer screening regimen, based on ACS, NCCN, and ACR recommendations, due to her 20.4% lifetime risk. Discussion of whether and when to start chemoprophylaxis is typically based on breast cancer risk, projected benefit, and the potential impact of medication adverse effects. A high-risk woman is eligible for 5 years of chemoprophylaxis (tamoxifen if premenopausal) based on her lifetime risk. The clinician discusses timing with Ms. P, and even though she is finished with childbearing, she would like to wait until she is age 45, which is before the age at which her aunt was given a diagnosis of breast cancer.
Conclusion
Primary care clinicians are well positioned to identify women with an elevated risk of breast cancer and refer them for enhanced screening and chemoprophylaxis (see ALGORITHM). Shared decision-making with the inclusion of patient decision aids (https://decisionaid.ohri.ca/AZsearch.php?criteria=breast+cancer) about genetic testing, chemoprophylaxis, and prophylactic mastectomy or oophorectomy may help women at intermediate or high risk of breast cancer feel empowered to make decisions about their breast—and overall—health.
CORRESPONDENCE
Sarina Schrager, MD, MS, Professor, Department of Family Medicine and Community Health, University of Wisconsin, 1100 Delaplaine Court, Madison, WI 53715; [email protected]
1. National Cancer Institute. Cancer stat facts: female breast cancer. Accessed May 13, 2022. https://seer.cancer.gov/statfacts/html/breast.html
2. Guerra CE, Sherman M, Armstrong K. Diffusion of breast cancer risk assessment in primary care. J Am Board Fam Med. 2009;22:272-279. doi:10.3122/jabfm.2009.03.080153
3. Hamilton JG, Abdiwahab E, Edwards HM, et al. Primary care providers’ cancer genetic testing-related knowledge, attitudes, and communication behaviors: a systematic review and research agenda. J Gen Intern Med. 2017;32:315-324. doi:10.1007/s11606-016-3943-4
4. Eden KB, Ivlev I, Bensching KL, et al. Use of an online breast cancer risk assessment and patient decision aid in primary care practices. J Womens Health (Larchmt). 2020;29:763-769. doi: 10.1089/jwh.2019.8143
5. Kleibl Z, Kristensen VN. Women at high risk of breast cancer: molecular characteristics, clinical presentation and management. Breast. 2016;28:136-44. doi: 10.1016/j.breast.2016.05.006
6. Sciaraffa T, Guido B, Khan SA, et al. Breast cancer risk assessment and management programs: a practical guide. Breast J. 2020;26:1556-1564. doi: 10.1111/tbj.13967
7. Farkas A, Vanderberg R, Merriam S, et al. Breast cancer chemoprevention: a practical guide for the primary care provider. J Womens Health (Larchmt). 2020;29:46-56. doi: 10.1089/jwh.2018.7643
8. McClintock AH, Golob AL, Laya MB. Breast cancer risk assessment: a step-wise approach for primary care providers on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275. doi: 10.1016/j.mayocp.2020.04.017
9. Catana A, Apostu AP, Antemie RG. Multi gene panel testing for hereditary breast cancer - is it ready to be used? Med Pharm Rep. 2019;92:220-225. doi: 10.15386/mpr-1083
10. Barke LD, Freivogel ME. Breast cancer risk assessment models and high-risk screening. Radiol Clin North Am. 2017;55:457-474. doi: 10.1016/j.rcl.2016.12.013
11. Amir E, Freedman OC, Seruga B, et al. Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst. 2010;102:680-91. doi: 10.1093/jnci/djq088
12. Kim G, Bahl M. Assessing risk of breast cancer: a review of risk prediction models. J Breast Imaging. 2021;3:144-155. doi: 10.1093/jbi/wbab001
13. Narod SA. Which genes for hereditary breast cancer? N Engl J Med. 2021;384:471-473. doi: 10.1056/NEJMe2035083
14. Couch FJ, Shimelis H, Hu C, et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 2017;3:1190-1196. doi: 10.1001/jamaoncol.2017.0424
15. Obeid EI, Hall MJ, Daly MB. Multigene panel testing and breast cancer risk: is it time to scale down? JAMA Oncol. 2017;3:1176-1177. doi: 10.1001/jamaoncol.2017.0342
16. Michailidou K, Lindström S, Dennis J, et al. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551:92-94. doi: 10.1038/nature24284
17. Shiovitz S, Korde LA. Genetics of breast cancer: a topic in evolution. Ann Oncol. 2015;26:1291-1299. doi: 10.1093/annonc/mdv022
18. Hu C, Hart SN, Gnanaolivu R, et al. A population-based study of genes previously implicated in breast cancer. N Engl J Med. 2021;384:440-451. doi: 10.1056/NEJMoa2005936
19. Gao C, Polley EC, Hart SN, et al. Risk of breast cancer among carriers of pathogenic variants in breast cancer predisposition genes varies by polygenic risk score. J Clin Oncol. 2021;39:2564-2573. doi: 10.1200/JCO.20.01992
20. Gallagher S, Hughes E, Wagner S, et al. Association of a polygenic risk score with breast cancer among women carriers of high- and moderate-risk breast cancer genes. JAMA Netw Open. 2020;3:e208501. doi: 10.1001/jamanetworkopen.2020.8501
21. Yanes T, Young MA, Meiser B, et al. Clinical applications of polygenic breast cancer risk: a critical review and perspectives of an emerging field. Breast Cancer Res. 2020;22:21. doi: 10.1186/s13058-020-01260-3
22. Schrager S, Torell E, Ledford K, et al. Managing a woman with BRCA mutations? Shared decision-making is key. J Fam Pract. 2020;69:237-243
23. US Preventive Services Task Force; Owens DK, Davidson KW, Krist AH, et al. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:857-867. doi: 10.1001/jama.2019.11885
24. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol 2015;22:3230-3235. doi: 10.1245/s10434-015-4715-9
25. Britt KL, Cuzick J, Phillips KA. Key steps for effective breast cancer prevention. Nat Rev Cancer. 2020;20:417-436. doi: 10.1038/s41568-020-0266-x
26. Jatoi I, Kemp Z. Risk-reducing mastectomy. JAMA. 2021;325:1781-1782. doi: 10.1001/jama.2020.22414
27. Choi Y, Terry MB, Daly MB, et al. Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2021;7:585-592. doi:10.1001/jamaoncol.2020.7995
1. National Cancer Institute. Cancer stat facts: female breast cancer. Accessed May 13, 2022. https://seer.cancer.gov/statfacts/html/breast.html
2. Guerra CE, Sherman M, Armstrong K. Diffusion of breast cancer risk assessment in primary care. J Am Board Fam Med. 2009;22:272-279. doi:10.3122/jabfm.2009.03.080153
3. Hamilton JG, Abdiwahab E, Edwards HM, et al. Primary care providers’ cancer genetic testing-related knowledge, attitudes, and communication behaviors: a systematic review and research agenda. J Gen Intern Med. 2017;32:315-324. doi:10.1007/s11606-016-3943-4
4. Eden KB, Ivlev I, Bensching KL, et al. Use of an online breast cancer risk assessment and patient decision aid in primary care practices. J Womens Health (Larchmt). 2020;29:763-769. doi: 10.1089/jwh.2019.8143
5. Kleibl Z, Kristensen VN. Women at high risk of breast cancer: molecular characteristics, clinical presentation and management. Breast. 2016;28:136-44. doi: 10.1016/j.breast.2016.05.006
6. Sciaraffa T, Guido B, Khan SA, et al. Breast cancer risk assessment and management programs: a practical guide. Breast J. 2020;26:1556-1564. doi: 10.1111/tbj.13967
7. Farkas A, Vanderberg R, Merriam S, et al. Breast cancer chemoprevention: a practical guide for the primary care provider. J Womens Health (Larchmt). 2020;29:46-56. doi: 10.1089/jwh.2018.7643
8. McClintock AH, Golob AL, Laya MB. Breast cancer risk assessment: a step-wise approach for primary care providers on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275. doi: 10.1016/j.mayocp.2020.04.017
9. Catana A, Apostu AP, Antemie RG. Multi gene panel testing for hereditary breast cancer - is it ready to be used? Med Pharm Rep. 2019;92:220-225. doi: 10.15386/mpr-1083
10. Barke LD, Freivogel ME. Breast cancer risk assessment models and high-risk screening. Radiol Clin North Am. 2017;55:457-474. doi: 10.1016/j.rcl.2016.12.013
11. Amir E, Freedman OC, Seruga B, et al. Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst. 2010;102:680-91. doi: 10.1093/jnci/djq088
12. Kim G, Bahl M. Assessing risk of breast cancer: a review of risk prediction models. J Breast Imaging. 2021;3:144-155. doi: 10.1093/jbi/wbab001
13. Narod SA. Which genes for hereditary breast cancer? N Engl J Med. 2021;384:471-473. doi: 10.1056/NEJMe2035083
14. Couch FJ, Shimelis H, Hu C, et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 2017;3:1190-1196. doi: 10.1001/jamaoncol.2017.0424
15. Obeid EI, Hall MJ, Daly MB. Multigene panel testing and breast cancer risk: is it time to scale down? JAMA Oncol. 2017;3:1176-1177. doi: 10.1001/jamaoncol.2017.0342
16. Michailidou K, Lindström S, Dennis J, et al. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551:92-94. doi: 10.1038/nature24284
17. Shiovitz S, Korde LA. Genetics of breast cancer: a topic in evolution. Ann Oncol. 2015;26:1291-1299. doi: 10.1093/annonc/mdv022
18. Hu C, Hart SN, Gnanaolivu R, et al. A population-based study of genes previously implicated in breast cancer. N Engl J Med. 2021;384:440-451. doi: 10.1056/NEJMoa2005936
19. Gao C, Polley EC, Hart SN, et al. Risk of breast cancer among carriers of pathogenic variants in breast cancer predisposition genes varies by polygenic risk score. J Clin Oncol. 2021;39:2564-2573. doi: 10.1200/JCO.20.01992
20. Gallagher S, Hughes E, Wagner S, et al. Association of a polygenic risk score with breast cancer among women carriers of high- and moderate-risk breast cancer genes. JAMA Netw Open. 2020;3:e208501. doi: 10.1001/jamanetworkopen.2020.8501
21. Yanes T, Young MA, Meiser B, et al. Clinical applications of polygenic breast cancer risk: a critical review and perspectives of an emerging field. Breast Cancer Res. 2020;22:21. doi: 10.1186/s13058-020-01260-3
22. Schrager S, Torell E, Ledford K, et al. Managing a woman with BRCA mutations? Shared decision-making is key. J Fam Pract. 2020;69:237-243
23. US Preventive Services Task Force; Owens DK, Davidson KW, Krist AH, et al. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:857-867. doi: 10.1001/jama.2019.11885
24. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol 2015;22:3230-3235. doi: 10.1245/s10434-015-4715-9
25. Britt KL, Cuzick J, Phillips KA. Key steps for effective breast cancer prevention. Nat Rev Cancer. 2020;20:417-436. doi: 10.1038/s41568-020-0266-x
26. Jatoi I, Kemp Z. Risk-reducing mastectomy. JAMA. 2021;325:1781-1782. doi: 10.1001/jama.2020.22414
27. Choi Y, Terry MB, Daly MB, et al. Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2021;7:585-592. doi:10.1001/jamaoncol.2020.7995
PRACTICE RECOMMENDATIONS
› Assess breast cancer risk in all women starting at age 35. C
› Perform enhanced screening in all women with a lifetime risk of breast cancer > 20%. A
› Discuss chemoprevention for all women at elevated risk for breast cancer. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Patients asking about APOE gene test results? Here’s what to tell them
Advances in Alzheimer disease (AD) genes and biomarkers now allow older adults to undergo testing and learn about their risk for AD.1 Current routes for doing so include testing in cardiology, screening for enrollment in secondary prevention trials (which use these tests to determine trial eligibility),2 and direct-to-consumer (DTC) services that provide these results as part of large panels.3 Patients may also obtain apolipoprotein (APOE) genotype information as part of an assessment of the risks and benefits of treatment with aducanumab (Aduhelm) or other anti-amyloid therapies that have been developed to stop or slow the progression of AD pathologies.
Expanded access to testing, in combination with limited guidance from DTC companies, suggests more older adults may consult their primary care physicians about this testing. In this narrative review, we use a vignette-driven approach to summarize the current scientific knowledge of the topic and to offer guidance on provider-patient discussions and follow-up.
First, a look at APOE genotyping
In cognitively unimpaired older adults, the APOE gene is a known risk factor for mild cognitive impairment (MCI) or AD.3 A person has 2 alleles of the APOE gene, which has 3 variants: ε2, ε3, and ε4. The combination of alleles conveys varying levels of risk for developing clinical symptoms (TABLE 14), with ε4 increasing risk and ε2 decreasing risk compared to the more common ε3; thus the ε4/ε4 genotype conveys the most risk and the ε2/ε2 the least.
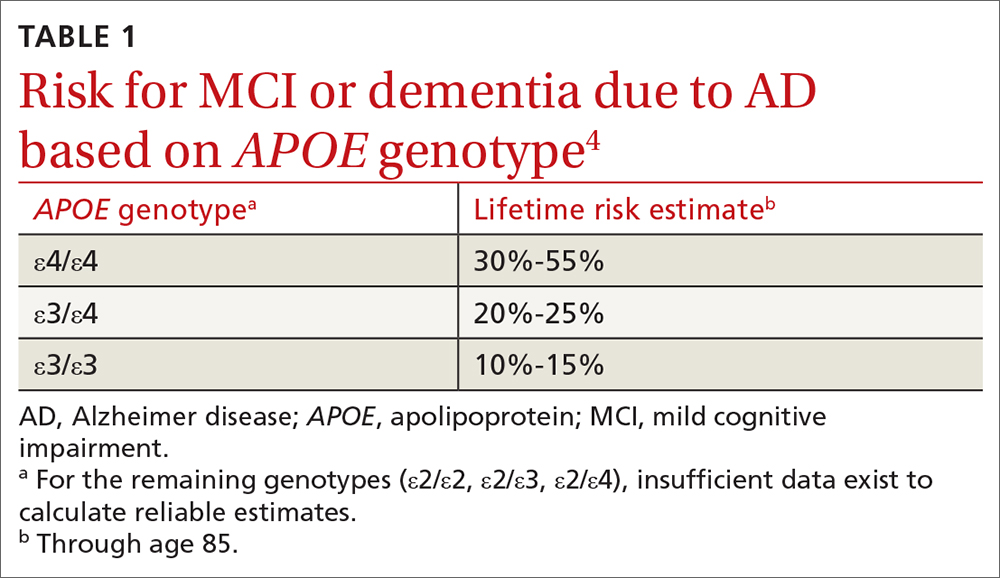
The APOE gene differs from other genes that have been identified in early-onset familial AD. These other genes, which include APP, PSEN1, and PSEN2, are deterministic genes that are fully penetrant. The APOE gene is not deterministic, meaning there is no combination of APOE alleles that are necessary or sufficient to cause late-onset AD dementia.
In clinical trials of amyloid-modifying therapies, the APOE gene has been shown to convey a risk of amyloid-related imaging abnormalities (ARIA).5 That is, in addition to conveying a risk for AD, the gene also conveys a risk for adverse effects of emerging treatments that can result in serious injury or death. This includes the drug aducanumab that was recently approved by the US Food and Drug Administration (FDA).6 In this review, we focus primarily on common clinical scenarios related to APOE. However, in light of the recent controversy over aducanumab and whether the drug should be offered to patients,7-9 we also describe how a patient’s APOE genotype may factor into drug candidacy decisions.
Testing, in clinic and “at home.” To date, practice guidelines have consistently recommended against APOE genetic testing in routine clinical practice. This is primarily due to low clinical prognostic utility and the lack of actionable results. Furthermore, no lifestyle or pharmaceutical interventions based on APOE genotype currently exist (although trials are underway10).
In 2017, the FDA approved marketing of DTC testing for the APOE gene.11 While DTC companies tend to issue standardized test result reports, the content and quality can vary widely. In fact, some provide risk estimates that are too high and too definitive and may not reflect the most recent science.12
Continue to: 7 clinical scenarios and how to approach them
7 clinical scenarios and how to approach them
Six of the following vignettes describe common clinical scenarios in which patients seek medical advice regarding APOE test results. The seventh vignette describes a patient whose APOE genotype may play a role in possible disease-modifying treatments down the road. Each vignette is designed to guide your approach to patient discussions and follow-up. Recommendations and considerations are also summarized in TABLE 213-16.
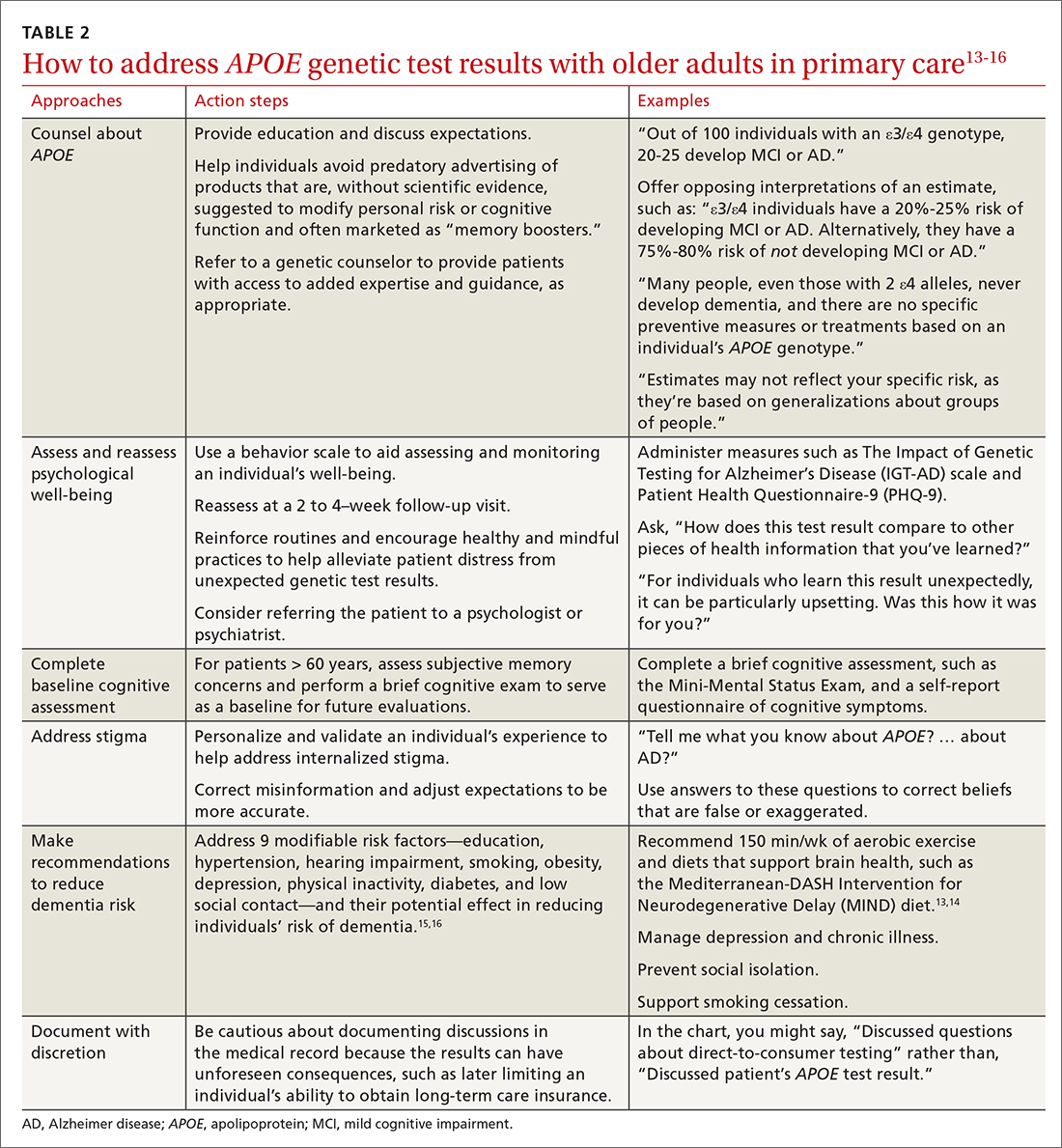
Vignette 1
Janet W, age 65, comes to the clinic for a new patient visit. She has no concerns about her memory but recently purchased DTC genetic testing to learn about her genetic health risks. Her results showed an APOE ε4/ε4 genotype. She is now concerned about developing AD. Her mother was diagnosed with AD in her 70s.
Several important pieces of information can be conveyed by the primary care physician. First, patients such as Ms. W should be told that the APOE gene is not deterministic; many people, even those with 2 ε4 alleles, never develop dementia. Second, no specific preventive measures or treatments exist based on an individual’s APOE genotype (see Vignette 5 for additional discussion).
In this scenario, patients may ask for numeric quantification of their risk for dementia (see TABLE 14 for estimates). When conveying probabilistic risk, consider using simple percentages or pictographs (eg, out of 100 individuals with an ε4/ε4 genotype, 30 to 55 develop MCI or AD). Additionally, because people tend to exhibit confirmatory bias in thinking about probabilistic risk, providing opposing interpretations of an estimate may help them to consider alternative possibilities.17 For example
There are important caveats to the interpretation of APOE risk estimates. Because APOE risk estimates are probabilistic and averaged across a broader spectrum of people in large population cohorts,4 estimates may not accurately reflect a given individual’s risk. The ranges reflect the uncertainty in the estimates. The uncertainty arises from relatively small samples, the rareness of some genotypes (notably ε4/ε4) even in large samples, and variations in methods and sampling that can lead to differences in estimates beyond statistical variation.
Vignette 2
Eric J, age 85, presents for a new patient visit accompanied by his daughter. He lives independently, volunteers at a senior center several times a week, and exercises regularly, and neither he nor his daughter has any concerns about his memory. As a gift, he recently underwent DTC genetic testing and unexpectedly learned his APOE result, which is ε4/ε4. He wants to know about his chances of developing AD.
Risk conveyed by APOE genotype can be modified by a patient’s age. At age 85, Mr. J is healthy, highly functional, and cognitively unimpaired. Given his age, Mr. J has likely “outlived” much of the risk for dementia attributable to the ε4/ε4 genotype. His risk for dementia remains high, but this risk is likely driven more by age than by his APOE genotype. Data for individuals older than age 80 are limited, and thus risk estimates lack precision. Given Mr. J’s good health and functional status, his physician may want to perform a brief cognitive screening test to serve as a baseline for future evaluations.
Continue to: Vignette 3
Vignette 3
Audrey S is a 60-year-old African American woman who comes to the clinic for her annual visit. Because her father had AD, she recently purchased DTC genetic testing to learn about her APOE genotype and risk for AD. Her results are ε3/ε4. She is wondering what this may mean for her future.
Lack of diversity in research cohorts often limits the generalizability of estimates. For example, both the frequency and impact of APOE ε4 differ across racial groups.18 But most of the data on APOE lifetime risk estimates are from largely White patient samples. While APOE ε4 seems to confer increased risk for AD across sociocultural groups, these effects may be attenuated in African American and Hispanic populations.19,20 If Ms. S is interested in numeric risk estimates, the physician can provide the estimate for ε3/ε4 (20%-25% lifetime risk), with the important caveat that this estimate may not be reflective of her individual risk.
It may be prudent to determine whether Ms. S, at age 60, has subjective memory concerns and if she does, to perform a brief cognitive exam to serve as a baseline for future evaluations. Additionally, while the Genetic Information Nondiscrimination Act (GINA, 2008) prohibits health insurers and employers from discriminating based on genetic testing results, no legal provisions exist regarding long-term care, disability, or life insurance. Documented conversations about APOE test results in the medical record may become part of patients’ applications for these insurance products, and physicians should be cautious before documenting such discussions in the medical record.
Vignette 4
Tina L, age 60, comes to the clinic for a routine wellness visit. She recently developed an interest in genealogy and purchased a DNA testing kit to learn more about her family tree. As part of this testing, she unexpectedly learned that she has an APOE ε4/ε4 genotype. She describes feeling distraught and anxious about what the result means for her future.
Ms. L’s reaction to receiving unexpected genetic results highlights a concern of DTC APOE testing. Her experience is quite different from individuals undergoing medically recommended genetic testing or those who are participating in research studies. They receive comprehensive pre-test counseling by licensed genetic counselors. The counseling includes psychological assessment, education, and discussion of expectations.2
In Ms. L’s case, it may be helpful to explain the limits of APOE lifetime risk estimates (see Vignettes 1-3). But it’s also important to address her concerns. There are behavior scales that can aid the assessment and monitoring of an individual’s well-being. The Impact of Genetic Testing for Alzheimer’s Disease (IGT-AD) scale is a tool that assesses psychological impact. It can help physicians to identify, monitor, and address concerns.21 Other useful tools include the Patient Health Questionnaire-9 (PHQ-9) and the Geriatric Depression Scale (GDS) for depression, and a suicide or self-harm assessment.2,22,23 Finally, a follow-up visit at 2 to 4 weeks may be useful to reassess psychological well-being.
Vignette 4 (cont’d)
Ms. L returns to the clinic 2 weeks later, reporting continued anxiety about her APOE test result and feelings of hopelessness and despair.
Continue to: Some patients struggle...
Some patients struggle with knowing their APOE test result. Test result–related distress is often a combination of depression (as with Ms. L), anger, confusion, and grief.24 Cognitions often include worries about uncertainty, stereotyped threat, and internalized stigma.25,26 These issues can spill over to patient concerns about sharing an APOE test result with others.27
Intolerance of uncertainty is a transdiagnostic risk factor that can influence psychological suffering.28 Brief cognitive behavioral interventions that reinforce routines and encourage healthy and mindful practices may help alleviate patient distress from unexpected genetic test results.29 Interventions that personalize and validate an individual’s experience can help address internalized stigma.30 Referral to a psychologist or psychiatrist could be warranted. Additionally, referral to a genetic counselor may help provide patients with access to added expertise and guidance; useful web-based resources for identifying an appropriate referral include https://medlineplus.gov/genetics/understanding/consult/findingprofessional/ and https://findageneticcounselor.nsgc.org/.
Vignette 5
Bob K, age 65, comes to the clinic for his annual exam. He is a current smoker and says he’s hoping to be more physically active now that he is retired. He says that his mother and grandmother both had AD. He recently purchased DTC genetic testing to learn more about his risk for AD. His learned his APOE genotype is ε3/ε4 and is wondering what he can do to decrease his chances of developing AD.
Mr. K likely would have benefited from pre-test counseling regarding the lack of current therapies to modify one’s genetic risk for AD. A pre-test counseling session often includes education about APOE testing and a brief evaluation to assess psychological readiness to undergo testing. Posttest educational information may help Mr. K avoid predatory advertising of products claiming—without scientific evidence—to modify risk for cognitive decline or to improve cognitive function.
There are several important pieces of information that should be communicated to Mr. K. Emerging evidence from randomized controlled trials suggests that healthy lifestyle modifications may benefit cognition in individuals with APOE ε4 alleles.31 It would be prudent to address proper blood pressure control32 and counsel Mr. K on how he may be able to avoid diabetes through exercise and weight maintenance. Lifestyle recommendations for Mr. K could include: smoking cessation, regular aerobic exercise (eg, 150 min/wk), and a brain-healthy diet (eg, the Mediterranean-DASH Intervention for Neurodegenerative Delay [MIND] diet).13,14 Moreover, dementia prevention also includes appropriately managing depression and chronic illnesses and preventing social isolation and hearing loss.15,16 This information should be thoughtfully conveyed, as these interventions can improve overall (especially cardiovascular) health, as well as mitigating one’s personal risk for AD.
Vignette 6
Juan L, age 45, comes in for his annual physical exam. He has a strong family history of heart disease. His cardiologist recently ordered lipid disorder genetic testing for familial hypercholesterolemia. This panel included APOE testing and showed Mr. L’s genotype is ε2/ε4. He read that the APOE gene can be associated with an increased AD risk and asks for information about his genotype.
Mr. L received genetic testing results that were ordered by a physician for another health purpose. Current recommendations for genetic testing in cardiology advise pre-test genetic counseling.33 But this counseling may not include discussion of the relationship of APOE and risk for MCI or AD. This additional information may be unexpected for Mr. L. Moreover, its significance in the context of his present concerns about cardiovascular disease may influence his reaction.
Continue to: The ε2/ε4 genotype...
The ε2/ε4 genotype is rare. One study showed that in healthy adults, the frequency was 7 in 210 (0.02 [0.01-0.04]).34 Given the rarity of the ε2/ε4 genotype, data about it are sparse. However, since the ε4 allele increases risk but the ε2 allele decreases risk, it is likely that any increase in risk is more modest than with ε3/ε4. In addition, it would help Mr. L to know that AD occurs infrequently before age 60.35 Given his relatively young age, he is unlikely to develop AD any time in the near future. In addition, particularly if he starts early, he might be able to mitigate any increased risk through some of the advice provided to Mr. K in Vignette 5.
Vignette 7
Joe J, age 65, comes to the clinic for a new patient visit. He has no concerns about his memory but has a family history of dementia and recently purchased DTC genetic testing to learn about his genetic health risks. His results showed an APOE ε4/ε4 genotype. He is concerned about developing AD. He heard on the news that there is a drug that can treat AD and wants to know if he is a candidate for this treatment.
Mr. J would benefit from the education provided to Ms. W in Vignette 1. Patients such as Mr. J should be advised that while an APOE ε4/ε4 genotype conveys an increased risk for AD, it is not deterministic of the disease. While there are no specific preventive measures or treatments based on APOE genotype, careful medical care and lifestyle factors can offset some of the risk (see Vignette 5 for discussion).
Recently (and controversially), the FDA approved aducanumab, a drug that targets amyloid.6,36 Of note, brain amyloid is more common in individuals with the APOE ε4/ε4 genotype, such as Mr. J. However, there would be no point in testing Mr. J for brain amyloid because at present the drug is only indicated in symptomatic individuals—and, even in this setting, it is controversial. One reason for the controversy is that aducanumab has potentially severe adverse effects. Patients with the ε4/ε4 genotype should know that this genotype carries increased risk for the most serious adverse event, ARIA—which can include brain edema and microhemorrhages.
What lies ahead?
More research is needed to explore the impact that greater AD gene and biomarker testing will have on the health system and workforce development. In addition, graduate schools and training programs will need to prepare clinicians to address probabilistic risk estimates for common diseases, such as AD. Finally, health systems and medical groups that employ clinicians may want to offer simulated training—similar to the vignettes in this article—as a practice requirement or as continuing medical education. This may also allow health systems or medical groups to put in place frameworks that support clinicians in proactively answering questions for patients and families about APOE and other emerging markers of disease risk.
CORRESPONDENCE
Shana Stites, University of Pennsylvania, 3615 Chestnut Street, Philadelphia, PA 19104; [email protected]
1. Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2018;14:535-562. doi: 10.1016/j.jalz.2018.02.018 PMCID:PMC5958625
2. Langlois CM, Bradbury A, Wood EM, et al. Alzheimer’s Prevention Initiative Generation Program: development of an APOE genetic counseling and disclosure process in the context of clinical trials. Alzheimers Dement Transl Res Clin Interv. 2019;5:705-716. doi: 10.1016/j.trci.2019.09.013
3. Frank L, Wesson Ashford J, Bayley PJ, et al. Genetic risk of Alzheimer’s disease: three wishes now that the genie is out of the bottle. J Alzheimers Dis. 2018;66:421-423. doi: 10.3233/JAD-180629
4. Qian J, Wolters FJ, Beiser A, et al. APOE-related risk of mild cognitive impairment and dementia for prevention trials: an analysis of four cohorts. PLOS Med. 2017;14:e1002254. doi: 10.1371/journal.pmed.1002254
5. Sperling RA, Jack CR, Black SE, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7:367-385. doi: 10.1016/j.jalz.2011.05.2351
6. FDA. November 6, 2020: Meeting of the Peripheral and Central Nervous System Drugs Advisory Committee Meeting Announcement. Published November 12, 2020. Accessed January 14, 2021. www.fda.gov/advisory-committees/advisory-committee-calendar/november-6-2020-meeting-peripheral-and-central-nervous-system-drugs-advisory-committee-meeting
7. Cummings J. Why aducanumab is important. Nat Med. 2021;27:1498-1498. doi: 10.1038/s41591-021-01478-4
8. Alexander GC, Karlawish J. The problem of aducanumab for the treatment of Alzheimer disease. Ann Intern Med. 2021;174:1303-1304. doi: 10.7326/M21-2603
9. Mullard A. More Alzheimer’s drugs head for FDA review: what scientists are watching. Nature. 2021;599:544-545. doi: 10.1038/d41586-021-03410-9
10. Rosenberg A, Mangialasche F, Ngandu T, et al. Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: from finger to world-wide fingers. J Prev Alzheimers Dis. 2019:1-8. doi: 10.14283/jpad.2019.41
11. FDA. Commissioner of the FDA allows marketing of first direct-to-consumer tests that provide genetic risk information for certain conditions. Published March 24, 2020. Accessed November 7, 2020. www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-direct-consumer-tests-provide-genetic-risk-information-certain-conditions
12. Blell M, Hunter MA. Direct-to-consumer genetic testing’s red herring: “genetic ancestry” and personalized medicine. Front Med. 2019;6:48. doi: 10.3389/fmed.2019.00048
13. Ekstrand B, Scheers N, Rasmussen MK, et al. Brain foods - the role of diet in brain performance and health. Nutr Rev. 2021;79:693-708. doi: 10.1093/nutrit/nuaa091
14. Cherian L, Wang Y, Fakuda K, et al. Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) diet slows cognitive decline after stroke. J Prev Alzheimers Dis. 2019;6:267-273. doi: 10.14283/jpad.2019.28
15. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396:413-446. doi: 10.1016/S0140-6736(20)30367-6
16. Livingston PG, Sommerlad A, Orgeta V, et al. The Lancet International Commission on Dementia Prevention and Care. 2017. Accessed March 30, 2022. https://discovery.ucl.ac.uk/id/eprint/1567635/1/Livingston_Dementia_prevention_intervention_care.pdf
17. Peters U. What is the function of confirmation bias? Erkenntnis. April 2020. doi: 10.1007/s10670-020-00252-1
18. Barnes LL, Bennett DA. Cognitive resilience in APOE*ε4 carriers—is race important? Nat Rev Neurol. 2015;11:190-191. doi: 10.1038/nrneurol.2015.38
19. Farrer LA. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278:1349. doi: 10.1001/jama.1997.03550160069041
20. Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60:185. doi: 10.1001/archneur.60.2.185
21. Chung WW, Chen CA, Cupples LA, et al. A new scale measuring psychologic impact of genetic susceptibility testing for Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:50-56. doi: 10.1097/WAD.0b013e318188429e
22. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613. doi: 10.1046/j.1525-1497.2001.016009606.x
23. Yesavage JA, Sheikh JI. 9/Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165-173. doi: 10.1300/J018v05n01_09
24. Green RC, Roberts JS, Cupples LA, et al. Disclosure of APOE genotype for risk of Alzheimer’s disease. N Engl J Med. 2009;361:245-254. doi: 10.1056/NEJMoa0809578
25. Lineweaver TT, Bondi MW, Galasko D, et al. Effect of knowledge of APOE genotype on subjective and objective memory performance in healthy older adults. Am J Psychiatry. 2014;171:201-208. doi: 10.1176/appi.ajp.2013.12121590
26. Karlawish J. Understanding the impact of learning an amyloid PET scan result: preliminary findings from the SOKRATES study. Alzheimers Dement J Alzheimers Assoc. 2016;12:P325. doi: 10.1016/j.jalz.2016.06.594
27. Stites SD. Cognitively healthy individuals want to know their risk for Alzheimer’s disease: what should we do? J Alzheimers Dis. 2018;62:499-502. doi: 10.3233/JAD-171089
28. Milne S, Lomax C, Freeston MH. A review of the relationship between intolerance of uncertainty and threat appraisal in anxiety. Cogn Behav Ther. 2019;12:e38. doi: 10.1017/S1754470X19000230
29. Hebert EA, Dugas MJ. Behavioral experiments for intolerance of uncertainty: challenging the unknown in the treatment of generalized anxiety disorder. Cogn Behav Pract. 2019;26:421-436. doi: 10.1016/j.cbpra.2018.07.007
30. Stites SD, Karlawish, J. Stigma of Alzheimer’s disease dementia: considerations for practice. Pract Neurol. Published June 2018. Accessed January 31, 2019. http://practicalneurology.com/2018/06/stigma-of-alzheimers-disease-dementia/
31. Solomon A, Turunen H, Ngandu T, et al. Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol. 2018;75:462. doi: 10.1001/jamaneurol.2017.4365
32. Peters R, Warwick J, Anstey KJ, et al. Blood pressure and dementia: what the SPRINT-MIND trial adds and what we still need to know. Neurology. 2019;92:1017-1018. doi: 10.1212/WNL.0000000000007543
33. Musunuru K, Hershberger RE, Day SM, et al. Genetic testing for inherited cardiovascular diseases: a Scientific Statement from the American Heart Association. Circ Genom Precis Med. 2020;13: e000067. doi: 10.1161/HCG.0000000000000067
34. Margaglione M, Seripa D, Gravina C, et al. Prevalence of apolipoprotein E alleles in healthy subjects and survivors of ischemic stroke. Stroke. 1998;29:399-403. doi: 10.1161/01.STR.29.2.399
35. National Institute on Aging. Alzheimer’s disease genetics fact sheet. Reviewed December 24, 2019. Accessed April 10, 2022. www.nia.nih.gov/health/alzheimers-disease-genetics-fact-sheet
36. Belluck P, Kaplan S, Robbins R. How Aduhelm, an unproven Alzheimer’s drug, got approved. The New York Times. Published July 19, 2021. Updated Oct. 20, 2021. Accessed December 1, 2021. www.nytimes.com/2021/07/19/health/alzheimers-drug-aduhelm-fda.html
Advances in Alzheimer disease (AD) genes and biomarkers now allow older adults to undergo testing and learn about their risk for AD.1 Current routes for doing so include testing in cardiology, screening for enrollment in secondary prevention trials (which use these tests to determine trial eligibility),2 and direct-to-consumer (DTC) services that provide these results as part of large panels.3 Patients may also obtain apolipoprotein (APOE) genotype information as part of an assessment of the risks and benefits of treatment with aducanumab (Aduhelm) or other anti-amyloid therapies that have been developed to stop or slow the progression of AD pathologies.
Expanded access to testing, in combination with limited guidance from DTC companies, suggests more older adults may consult their primary care physicians about this testing. In this narrative review, we use a vignette-driven approach to summarize the current scientific knowledge of the topic and to offer guidance on provider-patient discussions and follow-up.
First, a look at APOE genotyping
In cognitively unimpaired older adults, the APOE gene is a known risk factor for mild cognitive impairment (MCI) or AD.3 A person has 2 alleles of the APOE gene, which has 3 variants: ε2, ε3, and ε4. The combination of alleles conveys varying levels of risk for developing clinical symptoms (TABLE 14), with ε4 increasing risk and ε2 decreasing risk compared to the more common ε3; thus the ε4/ε4 genotype conveys the most risk and the ε2/ε2 the least.

The APOE gene differs from other genes that have been identified in early-onset familial AD. These other genes, which include APP, PSEN1, and PSEN2, are deterministic genes that are fully penetrant. The APOE gene is not deterministic, meaning there is no combination of APOE alleles that are necessary or sufficient to cause late-onset AD dementia.
In clinical trials of amyloid-modifying therapies, the APOE gene has been shown to convey a risk of amyloid-related imaging abnormalities (ARIA).5 That is, in addition to conveying a risk for AD, the gene also conveys a risk for adverse effects of emerging treatments that can result in serious injury or death. This includes the drug aducanumab that was recently approved by the US Food and Drug Administration (FDA).6 In this review, we focus primarily on common clinical scenarios related to APOE. However, in light of the recent controversy over aducanumab and whether the drug should be offered to patients,7-9 we also describe how a patient’s APOE genotype may factor into drug candidacy decisions.
Testing, in clinic and “at home.” To date, practice guidelines have consistently recommended against APOE genetic testing in routine clinical practice. This is primarily due to low clinical prognostic utility and the lack of actionable results. Furthermore, no lifestyle or pharmaceutical interventions based on APOE genotype currently exist (although trials are underway10).
In 2017, the FDA approved marketing of DTC testing for the APOE gene.11 While DTC companies tend to issue standardized test result reports, the content and quality can vary widely. In fact, some provide risk estimates that are too high and too definitive and may not reflect the most recent science.12
Continue to: 7 clinical scenarios and how to approach them
7 clinical scenarios and how to approach them
Six of the following vignettes describe common clinical scenarios in which patients seek medical advice regarding APOE test results. The seventh vignette describes a patient whose APOE genotype may play a role in possible disease-modifying treatments down the road. Each vignette is designed to guide your approach to patient discussions and follow-up. Recommendations and considerations are also summarized in TABLE 213-16.

Vignette 1
Janet W, age 65, comes to the clinic for a new patient visit. She has no concerns about her memory but recently purchased DTC genetic testing to learn about her genetic health risks. Her results showed an APOE ε4/ε4 genotype. She is now concerned about developing AD. Her mother was diagnosed with AD in her 70s.
Several important pieces of information can be conveyed by the primary care physician. First, patients such as Ms. W should be told that the APOE gene is not deterministic; many people, even those with 2 ε4 alleles, never develop dementia. Second, no specific preventive measures or treatments exist based on an individual’s APOE genotype (see Vignette 5 for additional discussion).
In this scenario, patients may ask for numeric quantification of their risk for dementia (see TABLE 14 for estimates). When conveying probabilistic risk, consider using simple percentages or pictographs (eg, out of 100 individuals with an ε4/ε4 genotype, 30 to 55 develop MCI or AD). Additionally, because people tend to exhibit confirmatory bias in thinking about probabilistic risk, providing opposing interpretations of an estimate may help them to consider alternative possibilities.17 For example
There are important caveats to the interpretation of APOE risk estimates. Because APOE risk estimates are probabilistic and averaged across a broader spectrum of people in large population cohorts,4 estimates may not accurately reflect a given individual’s risk. The ranges reflect the uncertainty in the estimates. The uncertainty arises from relatively small samples, the rareness of some genotypes (notably ε4/ε4) even in large samples, and variations in methods and sampling that can lead to differences in estimates beyond statistical variation.
Vignette 2
Eric J, age 85, presents for a new patient visit accompanied by his daughter. He lives independently, volunteers at a senior center several times a week, and exercises regularly, and neither he nor his daughter has any concerns about his memory. As a gift, he recently underwent DTC genetic testing and unexpectedly learned his APOE result, which is ε4/ε4. He wants to know about his chances of developing AD.
Risk conveyed by APOE genotype can be modified by a patient’s age. At age 85, Mr. J is healthy, highly functional, and cognitively unimpaired. Given his age, Mr. J has likely “outlived” much of the risk for dementia attributable to the ε4/ε4 genotype. His risk for dementia remains high, but this risk is likely driven more by age than by his APOE genotype. Data for individuals older than age 80 are limited, and thus risk estimates lack precision. Given Mr. J’s good health and functional status, his physician may want to perform a brief cognitive screening test to serve as a baseline for future evaluations.
Continue to: Vignette 3
Vignette 3
Audrey S is a 60-year-old African American woman who comes to the clinic for her annual visit. Because her father had AD, she recently purchased DTC genetic testing to learn about her APOE genotype and risk for AD. Her results are ε3/ε4. She is wondering what this may mean for her future.
Lack of diversity in research cohorts often limits the generalizability of estimates. For example, both the frequency and impact of APOE ε4 differ across racial groups.18 But most of the data on APOE lifetime risk estimates are from largely White patient samples. While APOE ε4 seems to confer increased risk for AD across sociocultural groups, these effects may be attenuated in African American and Hispanic populations.19,20 If Ms. S is interested in numeric risk estimates, the physician can provide the estimate for ε3/ε4 (20%-25% lifetime risk), with the important caveat that this estimate may not be reflective of her individual risk.
It may be prudent to determine whether Ms. S, at age 60, has subjective memory concerns and if she does, to perform a brief cognitive exam to serve as a baseline for future evaluations. Additionally, while the Genetic Information Nondiscrimination Act (GINA, 2008) prohibits health insurers and employers from discriminating based on genetic testing results, no legal provisions exist regarding long-term care, disability, or life insurance. Documented conversations about APOE test results in the medical record may become part of patients’ applications for these insurance products, and physicians should be cautious before documenting such discussions in the medical record.
Vignette 4
Tina L, age 60, comes to the clinic for a routine wellness visit. She recently developed an interest in genealogy and purchased a DNA testing kit to learn more about her family tree. As part of this testing, she unexpectedly learned that she has an APOE ε4/ε4 genotype. She describes feeling distraught and anxious about what the result means for her future.
Ms. L’s reaction to receiving unexpected genetic results highlights a concern of DTC APOE testing. Her experience is quite different from individuals undergoing medically recommended genetic testing or those who are participating in research studies. They receive comprehensive pre-test counseling by licensed genetic counselors. The counseling includes psychological assessment, education, and discussion of expectations.2
In Ms. L’s case, it may be helpful to explain the limits of APOE lifetime risk estimates (see Vignettes 1-3). But it’s also important to address her concerns. There are behavior scales that can aid the assessment and monitoring of an individual’s well-being. The Impact of Genetic Testing for Alzheimer’s Disease (IGT-AD) scale is a tool that assesses psychological impact. It can help physicians to identify, monitor, and address concerns.21 Other useful tools include the Patient Health Questionnaire-9 (PHQ-9) and the Geriatric Depression Scale (GDS) for depression, and a suicide or self-harm assessment.2,22,23 Finally, a follow-up visit at 2 to 4 weeks may be useful to reassess psychological well-being.
Vignette 4 (cont’d)
Ms. L returns to the clinic 2 weeks later, reporting continued anxiety about her APOE test result and feelings of hopelessness and despair.
Continue to: Some patients struggle...
Some patients struggle with knowing their APOE test result. Test result–related distress is often a combination of depression (as with Ms. L), anger, confusion, and grief.24 Cognitions often include worries about uncertainty, stereotyped threat, and internalized stigma.25,26 These issues can spill over to patient concerns about sharing an APOE test result with others.27
Intolerance of uncertainty is a transdiagnostic risk factor that can influence psychological suffering.28 Brief cognitive behavioral interventions that reinforce routines and encourage healthy and mindful practices may help alleviate patient distress from unexpected genetic test results.29 Interventions that personalize and validate an individual’s experience can help address internalized stigma.30 Referral to a psychologist or psychiatrist could be warranted. Additionally, referral to a genetic counselor may help provide patients with access to added expertise and guidance; useful web-based resources for identifying an appropriate referral include https://medlineplus.gov/genetics/understanding/consult/findingprofessional/ and https://findageneticcounselor.nsgc.org/.
Vignette 5
Bob K, age 65, comes to the clinic for his annual exam. He is a current smoker and says he’s hoping to be more physically active now that he is retired. He says that his mother and grandmother both had AD. He recently purchased DTC genetic testing to learn more about his risk for AD. His learned his APOE genotype is ε3/ε4 and is wondering what he can do to decrease his chances of developing AD.
Mr. K likely would have benefited from pre-test counseling regarding the lack of current therapies to modify one’s genetic risk for AD. A pre-test counseling session often includes education about APOE testing and a brief evaluation to assess psychological readiness to undergo testing. Posttest educational information may help Mr. K avoid predatory advertising of products claiming—without scientific evidence—to modify risk for cognitive decline or to improve cognitive function.
There are several important pieces of information that should be communicated to Mr. K. Emerging evidence from randomized controlled trials suggests that healthy lifestyle modifications may benefit cognition in individuals with APOE ε4 alleles.31 It would be prudent to address proper blood pressure control32 and counsel Mr. K on how he may be able to avoid diabetes through exercise and weight maintenance. Lifestyle recommendations for Mr. K could include: smoking cessation, regular aerobic exercise (eg, 150 min/wk), and a brain-healthy diet (eg, the Mediterranean-DASH Intervention for Neurodegenerative Delay [MIND] diet).13,14 Moreover, dementia prevention also includes appropriately managing depression and chronic illnesses and preventing social isolation and hearing loss.15,16 This information should be thoughtfully conveyed, as these interventions can improve overall (especially cardiovascular) health, as well as mitigating one’s personal risk for AD.
Vignette 6
Juan L, age 45, comes in for his annual physical exam. He has a strong family history of heart disease. His cardiologist recently ordered lipid disorder genetic testing for familial hypercholesterolemia. This panel included APOE testing and showed Mr. L’s genotype is ε2/ε4. He read that the APOE gene can be associated with an increased AD risk and asks for information about his genotype.
Mr. L received genetic testing results that were ordered by a physician for another health purpose. Current recommendations for genetic testing in cardiology advise pre-test genetic counseling.33 But this counseling may not include discussion of the relationship of APOE and risk for MCI or AD. This additional information may be unexpected for Mr. L. Moreover, its significance in the context of his present concerns about cardiovascular disease may influence his reaction.
Continue to: The ε2/ε4 genotype...
The ε2/ε4 genotype is rare. One study showed that in healthy adults, the frequency was 7 in 210 (0.02 [0.01-0.04]).34 Given the rarity of the ε2/ε4 genotype, data about it are sparse. However, since the ε4 allele increases risk but the ε2 allele decreases risk, it is likely that any increase in risk is more modest than with ε3/ε4. In addition, it would help Mr. L to know that AD occurs infrequently before age 60.35 Given his relatively young age, he is unlikely to develop AD any time in the near future. In addition, particularly if he starts early, he might be able to mitigate any increased risk through some of the advice provided to Mr. K in Vignette 5.
Vignette 7
Joe J, age 65, comes to the clinic for a new patient visit. He has no concerns about his memory but has a family history of dementia and recently purchased DTC genetic testing to learn about his genetic health risks. His results showed an APOE ε4/ε4 genotype. He is concerned about developing AD. He heard on the news that there is a drug that can treat AD and wants to know if he is a candidate for this treatment.
Mr. J would benefit from the education provided to Ms. W in Vignette 1. Patients such as Mr. J should be advised that while an APOE ε4/ε4 genotype conveys an increased risk for AD, it is not deterministic of the disease. While there are no specific preventive measures or treatments based on APOE genotype, careful medical care and lifestyle factors can offset some of the risk (see Vignette 5 for discussion).
Recently (and controversially), the FDA approved aducanumab, a drug that targets amyloid.6,36 Of note, brain amyloid is more common in individuals with the APOE ε4/ε4 genotype, such as Mr. J. However, there would be no point in testing Mr. J for brain amyloid because at present the drug is only indicated in symptomatic individuals—and, even in this setting, it is controversial. One reason for the controversy is that aducanumab has potentially severe adverse effects. Patients with the ε4/ε4 genotype should know that this genotype carries increased risk for the most serious adverse event, ARIA—which can include brain edema and microhemorrhages.
What lies ahead?
More research is needed to explore the impact that greater AD gene and biomarker testing will have on the health system and workforce development. In addition, graduate schools and training programs will need to prepare clinicians to address probabilistic risk estimates for common diseases, such as AD. Finally, health systems and medical groups that employ clinicians may want to offer simulated training—similar to the vignettes in this article—as a practice requirement or as continuing medical education. This may also allow health systems or medical groups to put in place frameworks that support clinicians in proactively answering questions for patients and families about APOE and other emerging markers of disease risk.
CORRESPONDENCE
Shana Stites, University of Pennsylvania, 3615 Chestnut Street, Philadelphia, PA 19104; [email protected]
Advances in Alzheimer disease (AD) genes and biomarkers now allow older adults to undergo testing and learn about their risk for AD.1 Current routes for doing so include testing in cardiology, screening for enrollment in secondary prevention trials (which use these tests to determine trial eligibility),2 and direct-to-consumer (DTC) services that provide these results as part of large panels.3 Patients may also obtain apolipoprotein (APOE) genotype information as part of an assessment of the risks and benefits of treatment with aducanumab (Aduhelm) or other anti-amyloid therapies that have been developed to stop or slow the progression of AD pathologies.
Expanded access to testing, in combination with limited guidance from DTC companies, suggests more older adults may consult their primary care physicians about this testing. In this narrative review, we use a vignette-driven approach to summarize the current scientific knowledge of the topic and to offer guidance on provider-patient discussions and follow-up.
First, a look at APOE genotyping
In cognitively unimpaired older adults, the APOE gene is a known risk factor for mild cognitive impairment (MCI) or AD.3 A person has 2 alleles of the APOE gene, which has 3 variants: ε2, ε3, and ε4. The combination of alleles conveys varying levels of risk for developing clinical symptoms (TABLE 14), with ε4 increasing risk and ε2 decreasing risk compared to the more common ε3; thus the ε4/ε4 genotype conveys the most risk and the ε2/ε2 the least.

The APOE gene differs from other genes that have been identified in early-onset familial AD. These other genes, which include APP, PSEN1, and PSEN2, are deterministic genes that are fully penetrant. The APOE gene is not deterministic, meaning there is no combination of APOE alleles that are necessary or sufficient to cause late-onset AD dementia.
In clinical trials of amyloid-modifying therapies, the APOE gene has been shown to convey a risk of amyloid-related imaging abnormalities (ARIA).5 That is, in addition to conveying a risk for AD, the gene also conveys a risk for adverse effects of emerging treatments that can result in serious injury or death. This includes the drug aducanumab that was recently approved by the US Food and Drug Administration (FDA).6 In this review, we focus primarily on common clinical scenarios related to APOE. However, in light of the recent controversy over aducanumab and whether the drug should be offered to patients,7-9 we also describe how a patient’s APOE genotype may factor into drug candidacy decisions.
Testing, in clinic and “at home.” To date, practice guidelines have consistently recommended against APOE genetic testing in routine clinical practice. This is primarily due to low clinical prognostic utility and the lack of actionable results. Furthermore, no lifestyle or pharmaceutical interventions based on APOE genotype currently exist (although trials are underway10).
In 2017, the FDA approved marketing of DTC testing for the APOE gene.11 While DTC companies tend to issue standardized test result reports, the content and quality can vary widely. In fact, some provide risk estimates that are too high and too definitive and may not reflect the most recent science.12
Continue to: 7 clinical scenarios and how to approach them
7 clinical scenarios and how to approach them
Six of the following vignettes describe common clinical scenarios in which patients seek medical advice regarding APOE test results. The seventh vignette describes a patient whose APOE genotype may play a role in possible disease-modifying treatments down the road. Each vignette is designed to guide your approach to patient discussions and follow-up. Recommendations and considerations are also summarized in TABLE 213-16.

Vignette 1
Janet W, age 65, comes to the clinic for a new patient visit. She has no concerns about her memory but recently purchased DTC genetic testing to learn about her genetic health risks. Her results showed an APOE ε4/ε4 genotype. She is now concerned about developing AD. Her mother was diagnosed with AD in her 70s.
Several important pieces of information can be conveyed by the primary care physician. First, patients such as Ms. W should be told that the APOE gene is not deterministic; many people, even those with 2 ε4 alleles, never develop dementia. Second, no specific preventive measures or treatments exist based on an individual’s APOE genotype (see Vignette 5 for additional discussion).
In this scenario, patients may ask for numeric quantification of their risk for dementia (see TABLE 14 for estimates). When conveying probabilistic risk, consider using simple percentages or pictographs (eg, out of 100 individuals with an ε4/ε4 genotype, 30 to 55 develop MCI or AD). Additionally, because people tend to exhibit confirmatory bias in thinking about probabilistic risk, providing opposing interpretations of an estimate may help them to consider alternative possibilities.17 For example
There are important caveats to the interpretation of APOE risk estimates. Because APOE risk estimates are probabilistic and averaged across a broader spectrum of people in large population cohorts,4 estimates may not accurately reflect a given individual’s risk. The ranges reflect the uncertainty in the estimates. The uncertainty arises from relatively small samples, the rareness of some genotypes (notably ε4/ε4) even in large samples, and variations in methods and sampling that can lead to differences in estimates beyond statistical variation.
Vignette 2
Eric J, age 85, presents for a new patient visit accompanied by his daughter. He lives independently, volunteers at a senior center several times a week, and exercises regularly, and neither he nor his daughter has any concerns about his memory. As a gift, he recently underwent DTC genetic testing and unexpectedly learned his APOE result, which is ε4/ε4. He wants to know about his chances of developing AD.
Risk conveyed by APOE genotype can be modified by a patient’s age. At age 85, Mr. J is healthy, highly functional, and cognitively unimpaired. Given his age, Mr. J has likely “outlived” much of the risk for dementia attributable to the ε4/ε4 genotype. His risk for dementia remains high, but this risk is likely driven more by age than by his APOE genotype. Data for individuals older than age 80 are limited, and thus risk estimates lack precision. Given Mr. J’s good health and functional status, his physician may want to perform a brief cognitive screening test to serve as a baseline for future evaluations.
Continue to: Vignette 3
Vignette 3
Audrey S is a 60-year-old African American woman who comes to the clinic for her annual visit. Because her father had AD, she recently purchased DTC genetic testing to learn about her APOE genotype and risk for AD. Her results are ε3/ε4. She is wondering what this may mean for her future.
Lack of diversity in research cohorts often limits the generalizability of estimates. For example, both the frequency and impact of APOE ε4 differ across racial groups.18 But most of the data on APOE lifetime risk estimates are from largely White patient samples. While APOE ε4 seems to confer increased risk for AD across sociocultural groups, these effects may be attenuated in African American and Hispanic populations.19,20 If Ms. S is interested in numeric risk estimates, the physician can provide the estimate for ε3/ε4 (20%-25% lifetime risk), with the important caveat that this estimate may not be reflective of her individual risk.
It may be prudent to determine whether Ms. S, at age 60, has subjective memory concerns and if she does, to perform a brief cognitive exam to serve as a baseline for future evaluations. Additionally, while the Genetic Information Nondiscrimination Act (GINA, 2008) prohibits health insurers and employers from discriminating based on genetic testing results, no legal provisions exist regarding long-term care, disability, or life insurance. Documented conversations about APOE test results in the medical record may become part of patients’ applications for these insurance products, and physicians should be cautious before documenting such discussions in the medical record.
Vignette 4
Tina L, age 60, comes to the clinic for a routine wellness visit. She recently developed an interest in genealogy and purchased a DNA testing kit to learn more about her family tree. As part of this testing, she unexpectedly learned that she has an APOE ε4/ε4 genotype. She describes feeling distraught and anxious about what the result means for her future.
Ms. L’s reaction to receiving unexpected genetic results highlights a concern of DTC APOE testing. Her experience is quite different from individuals undergoing medically recommended genetic testing or those who are participating in research studies. They receive comprehensive pre-test counseling by licensed genetic counselors. The counseling includes psychological assessment, education, and discussion of expectations.2
In Ms. L’s case, it may be helpful to explain the limits of APOE lifetime risk estimates (see Vignettes 1-3). But it’s also important to address her concerns. There are behavior scales that can aid the assessment and monitoring of an individual’s well-being. The Impact of Genetic Testing for Alzheimer’s Disease (IGT-AD) scale is a tool that assesses psychological impact. It can help physicians to identify, monitor, and address concerns.21 Other useful tools include the Patient Health Questionnaire-9 (PHQ-9) and the Geriatric Depression Scale (GDS) for depression, and a suicide or self-harm assessment.2,22,23 Finally, a follow-up visit at 2 to 4 weeks may be useful to reassess psychological well-being.
Vignette 4 (cont’d)
Ms. L returns to the clinic 2 weeks later, reporting continued anxiety about her APOE test result and feelings of hopelessness and despair.
Continue to: Some patients struggle...
Some patients struggle with knowing their APOE test result. Test result–related distress is often a combination of depression (as with Ms. L), anger, confusion, and grief.24 Cognitions often include worries about uncertainty, stereotyped threat, and internalized stigma.25,26 These issues can spill over to patient concerns about sharing an APOE test result with others.27
Intolerance of uncertainty is a transdiagnostic risk factor that can influence psychological suffering.28 Brief cognitive behavioral interventions that reinforce routines and encourage healthy and mindful practices may help alleviate patient distress from unexpected genetic test results.29 Interventions that personalize and validate an individual’s experience can help address internalized stigma.30 Referral to a psychologist or psychiatrist could be warranted. Additionally, referral to a genetic counselor may help provide patients with access to added expertise and guidance; useful web-based resources for identifying an appropriate referral include https://medlineplus.gov/genetics/understanding/consult/findingprofessional/ and https://findageneticcounselor.nsgc.org/.
Vignette 5
Bob K, age 65, comes to the clinic for his annual exam. He is a current smoker and says he’s hoping to be more physically active now that he is retired. He says that his mother and grandmother both had AD. He recently purchased DTC genetic testing to learn more about his risk for AD. His learned his APOE genotype is ε3/ε4 and is wondering what he can do to decrease his chances of developing AD.
Mr. K likely would have benefited from pre-test counseling regarding the lack of current therapies to modify one’s genetic risk for AD. A pre-test counseling session often includes education about APOE testing and a brief evaluation to assess psychological readiness to undergo testing. Posttest educational information may help Mr. K avoid predatory advertising of products claiming—without scientific evidence—to modify risk for cognitive decline or to improve cognitive function.
There are several important pieces of information that should be communicated to Mr. K. Emerging evidence from randomized controlled trials suggests that healthy lifestyle modifications may benefit cognition in individuals with APOE ε4 alleles.31 It would be prudent to address proper blood pressure control32 and counsel Mr. K on how he may be able to avoid diabetes through exercise and weight maintenance. Lifestyle recommendations for Mr. K could include: smoking cessation, regular aerobic exercise (eg, 150 min/wk), and a brain-healthy diet (eg, the Mediterranean-DASH Intervention for Neurodegenerative Delay [MIND] diet).13,14 Moreover, dementia prevention also includes appropriately managing depression and chronic illnesses and preventing social isolation and hearing loss.15,16 This information should be thoughtfully conveyed, as these interventions can improve overall (especially cardiovascular) health, as well as mitigating one’s personal risk for AD.
Vignette 6
Juan L, age 45, comes in for his annual physical exam. He has a strong family history of heart disease. His cardiologist recently ordered lipid disorder genetic testing for familial hypercholesterolemia. This panel included APOE testing and showed Mr. L’s genotype is ε2/ε4. He read that the APOE gene can be associated with an increased AD risk and asks for information about his genotype.
Mr. L received genetic testing results that were ordered by a physician for another health purpose. Current recommendations for genetic testing in cardiology advise pre-test genetic counseling.33 But this counseling may not include discussion of the relationship of APOE and risk for MCI or AD. This additional information may be unexpected for Mr. L. Moreover, its significance in the context of his present concerns about cardiovascular disease may influence his reaction.
Continue to: The ε2/ε4 genotype...
The ε2/ε4 genotype is rare. One study showed that in healthy adults, the frequency was 7 in 210 (0.02 [0.01-0.04]).34 Given the rarity of the ε2/ε4 genotype, data about it are sparse. However, since the ε4 allele increases risk but the ε2 allele decreases risk, it is likely that any increase in risk is more modest than with ε3/ε4. In addition, it would help Mr. L to know that AD occurs infrequently before age 60.35 Given his relatively young age, he is unlikely to develop AD any time in the near future. In addition, particularly if he starts early, he might be able to mitigate any increased risk through some of the advice provided to Mr. K in Vignette 5.
Vignette 7
Joe J, age 65, comes to the clinic for a new patient visit. He has no concerns about his memory but has a family history of dementia and recently purchased DTC genetic testing to learn about his genetic health risks. His results showed an APOE ε4/ε4 genotype. He is concerned about developing AD. He heard on the news that there is a drug that can treat AD and wants to know if he is a candidate for this treatment.
Mr. J would benefit from the education provided to Ms. W in Vignette 1. Patients such as Mr. J should be advised that while an APOE ε4/ε4 genotype conveys an increased risk for AD, it is not deterministic of the disease. While there are no specific preventive measures or treatments based on APOE genotype, careful medical care and lifestyle factors can offset some of the risk (see Vignette 5 for discussion).
Recently (and controversially), the FDA approved aducanumab, a drug that targets amyloid.6,36 Of note, brain amyloid is more common in individuals with the APOE ε4/ε4 genotype, such as Mr. J. However, there would be no point in testing Mr. J for brain amyloid because at present the drug is only indicated in symptomatic individuals—and, even in this setting, it is controversial. One reason for the controversy is that aducanumab has potentially severe adverse effects. Patients with the ε4/ε4 genotype should know that this genotype carries increased risk for the most serious adverse event, ARIA—which can include brain edema and microhemorrhages.
What lies ahead?
More research is needed to explore the impact that greater AD gene and biomarker testing will have on the health system and workforce development. In addition, graduate schools and training programs will need to prepare clinicians to address probabilistic risk estimates for common diseases, such as AD. Finally, health systems and medical groups that employ clinicians may want to offer simulated training—similar to the vignettes in this article—as a practice requirement or as continuing medical education. This may also allow health systems or medical groups to put in place frameworks that support clinicians in proactively answering questions for patients and families about APOE and other emerging markers of disease risk.
CORRESPONDENCE
Shana Stites, University of Pennsylvania, 3615 Chestnut Street, Philadelphia, PA 19104; [email protected]
1. Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2018;14:535-562. doi: 10.1016/j.jalz.2018.02.018 PMCID:PMC5958625
2. Langlois CM, Bradbury A, Wood EM, et al. Alzheimer’s Prevention Initiative Generation Program: development of an APOE genetic counseling and disclosure process in the context of clinical trials. Alzheimers Dement Transl Res Clin Interv. 2019;5:705-716. doi: 10.1016/j.trci.2019.09.013
3. Frank L, Wesson Ashford J, Bayley PJ, et al. Genetic risk of Alzheimer’s disease: three wishes now that the genie is out of the bottle. J Alzheimers Dis. 2018;66:421-423. doi: 10.3233/JAD-180629
4. Qian J, Wolters FJ, Beiser A, et al. APOE-related risk of mild cognitive impairment and dementia for prevention trials: an analysis of four cohorts. PLOS Med. 2017;14:e1002254. doi: 10.1371/journal.pmed.1002254
5. Sperling RA, Jack CR, Black SE, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7:367-385. doi: 10.1016/j.jalz.2011.05.2351
6. FDA. November 6, 2020: Meeting of the Peripheral and Central Nervous System Drugs Advisory Committee Meeting Announcement. Published November 12, 2020. Accessed January 14, 2021. www.fda.gov/advisory-committees/advisory-committee-calendar/november-6-2020-meeting-peripheral-and-central-nervous-system-drugs-advisory-committee-meeting
7. Cummings J. Why aducanumab is important. Nat Med. 2021;27:1498-1498. doi: 10.1038/s41591-021-01478-4
8. Alexander GC, Karlawish J. The problem of aducanumab for the treatment of Alzheimer disease. Ann Intern Med. 2021;174:1303-1304. doi: 10.7326/M21-2603
9. Mullard A. More Alzheimer’s drugs head for FDA review: what scientists are watching. Nature. 2021;599:544-545. doi: 10.1038/d41586-021-03410-9
10. Rosenberg A, Mangialasche F, Ngandu T, et al. Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: from finger to world-wide fingers. J Prev Alzheimers Dis. 2019:1-8. doi: 10.14283/jpad.2019.41
11. FDA. Commissioner of the FDA allows marketing of first direct-to-consumer tests that provide genetic risk information for certain conditions. Published March 24, 2020. Accessed November 7, 2020. www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-direct-consumer-tests-provide-genetic-risk-information-certain-conditions
12. Blell M, Hunter MA. Direct-to-consumer genetic testing’s red herring: “genetic ancestry” and personalized medicine. Front Med. 2019;6:48. doi: 10.3389/fmed.2019.00048
13. Ekstrand B, Scheers N, Rasmussen MK, et al. Brain foods - the role of diet in brain performance and health. Nutr Rev. 2021;79:693-708. doi: 10.1093/nutrit/nuaa091
14. Cherian L, Wang Y, Fakuda K, et al. Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) diet slows cognitive decline after stroke. J Prev Alzheimers Dis. 2019;6:267-273. doi: 10.14283/jpad.2019.28
15. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396:413-446. doi: 10.1016/S0140-6736(20)30367-6
16. Livingston PG, Sommerlad A, Orgeta V, et al. The Lancet International Commission on Dementia Prevention and Care. 2017. Accessed March 30, 2022. https://discovery.ucl.ac.uk/id/eprint/1567635/1/Livingston_Dementia_prevention_intervention_care.pdf
17. Peters U. What is the function of confirmation bias? Erkenntnis. April 2020. doi: 10.1007/s10670-020-00252-1
18. Barnes LL, Bennett DA. Cognitive resilience in APOE*ε4 carriers—is race important? Nat Rev Neurol. 2015;11:190-191. doi: 10.1038/nrneurol.2015.38
19. Farrer LA. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278:1349. doi: 10.1001/jama.1997.03550160069041
20. Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60:185. doi: 10.1001/archneur.60.2.185
21. Chung WW, Chen CA, Cupples LA, et al. A new scale measuring psychologic impact of genetic susceptibility testing for Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:50-56. doi: 10.1097/WAD.0b013e318188429e
22. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613. doi: 10.1046/j.1525-1497.2001.016009606.x
23. Yesavage JA, Sheikh JI. 9/Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165-173. doi: 10.1300/J018v05n01_09
24. Green RC, Roberts JS, Cupples LA, et al. Disclosure of APOE genotype for risk of Alzheimer’s disease. N Engl J Med. 2009;361:245-254. doi: 10.1056/NEJMoa0809578
25. Lineweaver TT, Bondi MW, Galasko D, et al. Effect of knowledge of APOE genotype on subjective and objective memory performance in healthy older adults. Am J Psychiatry. 2014;171:201-208. doi: 10.1176/appi.ajp.2013.12121590
26. Karlawish J. Understanding the impact of learning an amyloid PET scan result: preliminary findings from the SOKRATES study. Alzheimers Dement J Alzheimers Assoc. 2016;12:P325. doi: 10.1016/j.jalz.2016.06.594
27. Stites SD. Cognitively healthy individuals want to know their risk for Alzheimer’s disease: what should we do? J Alzheimers Dis. 2018;62:499-502. doi: 10.3233/JAD-171089
28. Milne S, Lomax C, Freeston MH. A review of the relationship between intolerance of uncertainty and threat appraisal in anxiety. Cogn Behav Ther. 2019;12:e38. doi: 10.1017/S1754470X19000230
29. Hebert EA, Dugas MJ. Behavioral experiments for intolerance of uncertainty: challenging the unknown in the treatment of generalized anxiety disorder. Cogn Behav Pract. 2019;26:421-436. doi: 10.1016/j.cbpra.2018.07.007
30. Stites SD, Karlawish, J. Stigma of Alzheimer’s disease dementia: considerations for practice. Pract Neurol. Published June 2018. Accessed January 31, 2019. http://practicalneurology.com/2018/06/stigma-of-alzheimers-disease-dementia/
31. Solomon A, Turunen H, Ngandu T, et al. Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol. 2018;75:462. doi: 10.1001/jamaneurol.2017.4365
32. Peters R, Warwick J, Anstey KJ, et al. Blood pressure and dementia: what the SPRINT-MIND trial adds and what we still need to know. Neurology. 2019;92:1017-1018. doi: 10.1212/WNL.0000000000007543
33. Musunuru K, Hershberger RE, Day SM, et al. Genetic testing for inherited cardiovascular diseases: a Scientific Statement from the American Heart Association. Circ Genom Precis Med. 2020;13: e000067. doi: 10.1161/HCG.0000000000000067
34. Margaglione M, Seripa D, Gravina C, et al. Prevalence of apolipoprotein E alleles in healthy subjects and survivors of ischemic stroke. Stroke. 1998;29:399-403. doi: 10.1161/01.STR.29.2.399
35. National Institute on Aging. Alzheimer’s disease genetics fact sheet. Reviewed December 24, 2019. Accessed April 10, 2022. www.nia.nih.gov/health/alzheimers-disease-genetics-fact-sheet
36. Belluck P, Kaplan S, Robbins R. How Aduhelm, an unproven Alzheimer’s drug, got approved. The New York Times. Published July 19, 2021. Updated Oct. 20, 2021. Accessed December 1, 2021. www.nytimes.com/2021/07/19/health/alzheimers-drug-aduhelm-fda.html
1. Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2018;14:535-562. doi: 10.1016/j.jalz.2018.02.018 PMCID:PMC5958625
2. Langlois CM, Bradbury A, Wood EM, et al. Alzheimer’s Prevention Initiative Generation Program: development of an APOE genetic counseling and disclosure process in the context of clinical trials. Alzheimers Dement Transl Res Clin Interv. 2019;5:705-716. doi: 10.1016/j.trci.2019.09.013
3. Frank L, Wesson Ashford J, Bayley PJ, et al. Genetic risk of Alzheimer’s disease: three wishes now that the genie is out of the bottle. J Alzheimers Dis. 2018;66:421-423. doi: 10.3233/JAD-180629
4. Qian J, Wolters FJ, Beiser A, et al. APOE-related risk of mild cognitive impairment and dementia for prevention trials: an analysis of four cohorts. PLOS Med. 2017;14:e1002254. doi: 10.1371/journal.pmed.1002254
5. Sperling RA, Jack CR, Black SE, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7:367-385. doi: 10.1016/j.jalz.2011.05.2351
6. FDA. November 6, 2020: Meeting of the Peripheral and Central Nervous System Drugs Advisory Committee Meeting Announcement. Published November 12, 2020. Accessed January 14, 2021. www.fda.gov/advisory-committees/advisory-committee-calendar/november-6-2020-meeting-peripheral-and-central-nervous-system-drugs-advisory-committee-meeting
7. Cummings J. Why aducanumab is important. Nat Med. 2021;27:1498-1498. doi: 10.1038/s41591-021-01478-4
8. Alexander GC, Karlawish J. The problem of aducanumab for the treatment of Alzheimer disease. Ann Intern Med. 2021;174:1303-1304. doi: 10.7326/M21-2603
9. Mullard A. More Alzheimer’s drugs head for FDA review: what scientists are watching. Nature. 2021;599:544-545. doi: 10.1038/d41586-021-03410-9
10. Rosenberg A, Mangialasche F, Ngandu T, et al. Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: from finger to world-wide fingers. J Prev Alzheimers Dis. 2019:1-8. doi: 10.14283/jpad.2019.41
11. FDA. Commissioner of the FDA allows marketing of first direct-to-consumer tests that provide genetic risk information for certain conditions. Published March 24, 2020. Accessed November 7, 2020. www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-direct-consumer-tests-provide-genetic-risk-information-certain-conditions
12. Blell M, Hunter MA. Direct-to-consumer genetic testing’s red herring: “genetic ancestry” and personalized medicine. Front Med. 2019;6:48. doi: 10.3389/fmed.2019.00048
13. Ekstrand B, Scheers N, Rasmussen MK, et al. Brain foods - the role of diet in brain performance and health. Nutr Rev. 2021;79:693-708. doi: 10.1093/nutrit/nuaa091
14. Cherian L, Wang Y, Fakuda K, et al. Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) diet slows cognitive decline after stroke. J Prev Alzheimers Dis. 2019;6:267-273. doi: 10.14283/jpad.2019.28
15. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396:413-446. doi: 10.1016/S0140-6736(20)30367-6
16. Livingston PG, Sommerlad A, Orgeta V, et al. The Lancet International Commission on Dementia Prevention and Care. 2017. Accessed March 30, 2022. https://discovery.ucl.ac.uk/id/eprint/1567635/1/Livingston_Dementia_prevention_intervention_care.pdf
17. Peters U. What is the function of confirmation bias? Erkenntnis. April 2020. doi: 10.1007/s10670-020-00252-1
18. Barnes LL, Bennett DA. Cognitive resilience in APOE*ε4 carriers—is race important? Nat Rev Neurol. 2015;11:190-191. doi: 10.1038/nrneurol.2015.38
19. Farrer LA. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278:1349. doi: 10.1001/jama.1997.03550160069041
20. Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60:185. doi: 10.1001/archneur.60.2.185
21. Chung WW, Chen CA, Cupples LA, et al. A new scale measuring psychologic impact of genetic susceptibility testing for Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:50-56. doi: 10.1097/WAD.0b013e318188429e
22. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613. doi: 10.1046/j.1525-1497.2001.016009606.x
23. Yesavage JA, Sheikh JI. 9/Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165-173. doi: 10.1300/J018v05n01_09
24. Green RC, Roberts JS, Cupples LA, et al. Disclosure of APOE genotype for risk of Alzheimer’s disease. N Engl J Med. 2009;361:245-254. doi: 10.1056/NEJMoa0809578
25. Lineweaver TT, Bondi MW, Galasko D, et al. Effect of knowledge of APOE genotype on subjective and objective memory performance in healthy older adults. Am J Psychiatry. 2014;171:201-208. doi: 10.1176/appi.ajp.2013.12121590
26. Karlawish J. Understanding the impact of learning an amyloid PET scan result: preliminary findings from the SOKRATES study. Alzheimers Dement J Alzheimers Assoc. 2016;12:P325. doi: 10.1016/j.jalz.2016.06.594
27. Stites SD. Cognitively healthy individuals want to know their risk for Alzheimer’s disease: what should we do? J Alzheimers Dis. 2018;62:499-502. doi: 10.3233/JAD-171089
28. Milne S, Lomax C, Freeston MH. A review of the relationship between intolerance of uncertainty and threat appraisal in anxiety. Cogn Behav Ther. 2019;12:e38. doi: 10.1017/S1754470X19000230
29. Hebert EA, Dugas MJ. Behavioral experiments for intolerance of uncertainty: challenging the unknown in the treatment of generalized anxiety disorder. Cogn Behav Pract. 2019;26:421-436. doi: 10.1016/j.cbpra.2018.07.007
30. Stites SD, Karlawish, J. Stigma of Alzheimer’s disease dementia: considerations for practice. Pract Neurol. Published June 2018. Accessed January 31, 2019. http://practicalneurology.com/2018/06/stigma-of-alzheimers-disease-dementia/
31. Solomon A, Turunen H, Ngandu T, et al. Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol. 2018;75:462. doi: 10.1001/jamaneurol.2017.4365
32. Peters R, Warwick J, Anstey KJ, et al. Blood pressure and dementia: what the SPRINT-MIND trial adds and what we still need to know. Neurology. 2019;92:1017-1018. doi: 10.1212/WNL.0000000000007543
33. Musunuru K, Hershberger RE, Day SM, et al. Genetic testing for inherited cardiovascular diseases: a Scientific Statement from the American Heart Association. Circ Genom Precis Med. 2020;13: e000067. doi: 10.1161/HCG.0000000000000067
34. Margaglione M, Seripa D, Gravina C, et al. Prevalence of apolipoprotein E alleles in healthy subjects and survivors of ischemic stroke. Stroke. 1998;29:399-403. doi: 10.1161/01.STR.29.2.399
35. National Institute on Aging. Alzheimer’s disease genetics fact sheet. Reviewed December 24, 2019. Accessed April 10, 2022. www.nia.nih.gov/health/alzheimers-disease-genetics-fact-sheet
36. Belluck P, Kaplan S, Robbins R. How Aduhelm, an unproven Alzheimer’s drug, got approved. The New York Times. Published July 19, 2021. Updated Oct. 20, 2021. Accessed December 1, 2021. www.nytimes.com/2021/07/19/health/alzheimers-drug-aduhelm-fda.html
Transitioning patients with developmental disabilities to adult care
Some adults who have an intellectual or other developmental disability (IDD) require extensive subspecialty care; many, however, depend primarily on their family physician for the bulk of their health care. With that reliance in mind, this article provides (1) an overview of important services that family physicians can provide for their adult patients with IDD and (2) pragmatic clinical suggestions for tailoring that care. Note: We highlight only some high-impact areas of clinical focus; refer to the 2018 Canadian consensus guidelines for a comprehensive approach to optimizing primary care for this population.1
CASE
Laura S, a 24-year-old woman with Down syndrome, is visiting your clinic with her mother to establish care. Ms. S has several medical comorbidities, including type 2 diabetes, hyperlipidemia, repaired congenital heart disease, schizoaffective disorder, and hypothyroidism. She is under the care of multiple specialists, including a cardiologist and an endocrinologist. Her medications include the atypical antipsychotic risperidone, which was prescribed for her through the services of a community mental health center.

Ms. S is due for multiple preventive health screenings. She indicates that she feels nervous today talking about these screenings with a new physician.
First step in care: Proficiency in the lexicon of IDD
Three core concepts of IDD are impairment, disability, and handicap. According to the World Health Organization2:
- impairment “is any loss or abnormality of psychological, physiological, or anatomical structure or function.”
- disability “is any restriction or lack (resulting from an impairment) of ability to perform an activity in the manner or within the range considered normal for a human being.”
- handicap therefore “represents socialization of an impairment or disability, and as such it reflects the consequences for the individual—cultural, social, economic, and environmental—that stem from the presence of impairment and disability.”
Essential transition: Pediatric to adult health care
Health care transition (HCT) is the planned process of transferring care from a pediatric to an adult-based health care setting,3 comprising 3 phases:
- preparation
- transfer from pediatric to adult care
- integration into adult-based care.
Two critical components of a smooth HCT include initiating the transition early in adolescence and providing transition-support resources, which are often lacking, even in large, integrated health systems.4 Got Transition, created by the National Alliance to Advance Adolescent Health, outlines core elements of an organized HCT process (www.gottransition.org) specific to young adults with IDD, including young adults with autism spectrum disorder.5,6
Even young people who are served by a family physician and who intend to remain in that family practice as they age into adulthood require HCT services that include6:
- assessment of readiness to transition to adult care
- update of the medical history
- assessment and promotion of self-care skills
- consent discussions and optimized participation in decision-making
- transition of specialty care from pediatric to adult specialists.
Continue to: For an ideal HCT...
For an ideal HCT, full engagement of the patient, the medical home (physicians, nursing staff, and care coordinators), and the patient’s family (including the primary caregiver or guardian) is critical. In addition to preventive care visits and management of chronic disease, additional domains that require explicit attention in transitioning young people with IDD include health insurance, transportation, employment, and postsecondary education.
Young people who have special health care needs and receive high-quality HCT demonstrate improvements in adherence to care, disease-specific measures, quality of life, self-care skills, satisfaction with care, and health care utilization.7TABLE 13 lists resources identified by Berens and colleagues that are helpful in facilitating the transition.
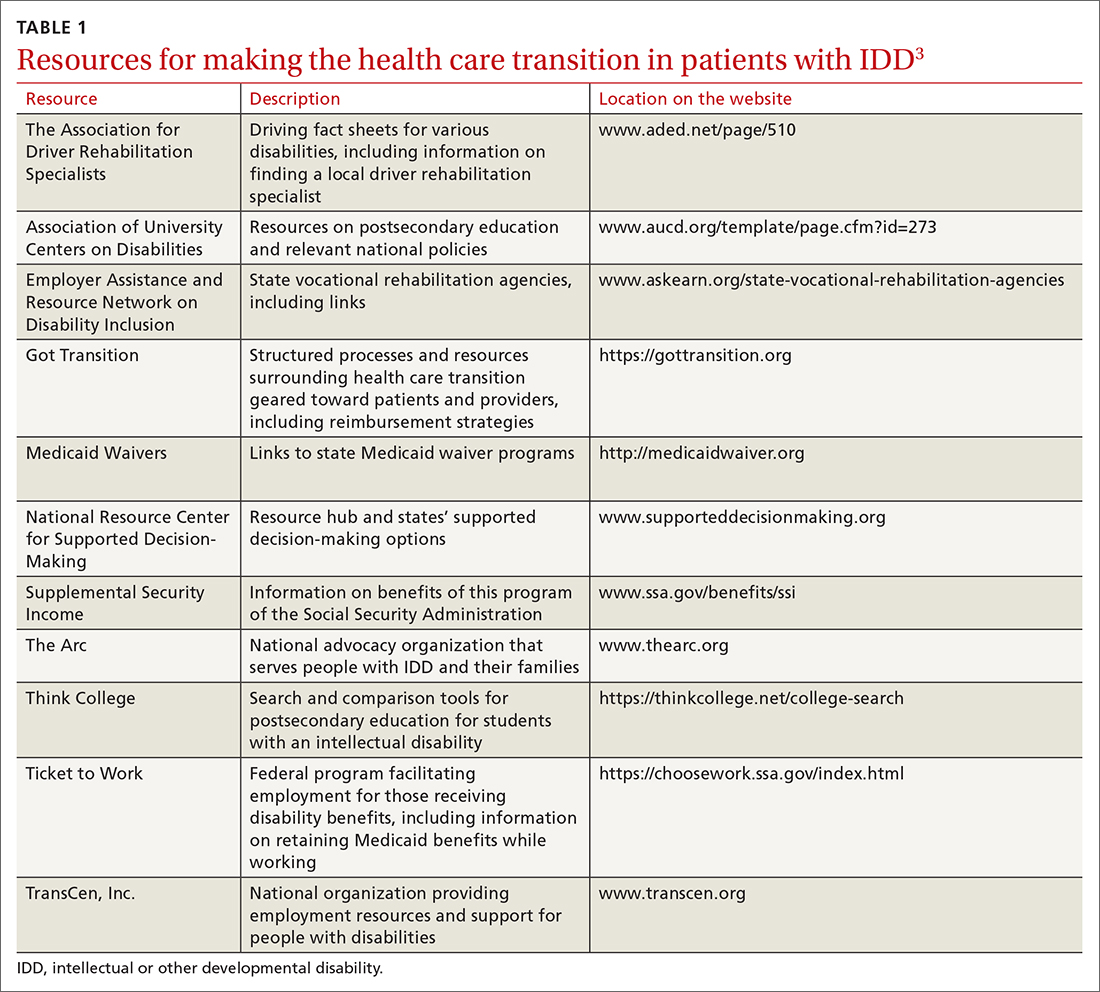
Teach and practice disability etiquette
Societal prejudice harms people with IDD—leading to self-deprecation, alienation from the larger community, and isolation from others with IDD.8 To promote acceptance and inclusivity in residential communities, the workplace, recreational venues, and clinical settings, disability etiquette should be utilized—a set of guidelines on how to interact with patients with IDD. These include speaking to the patient directly, using clear language in an adult voice, and avoiding stereotypes about people with disabilities.9 The entire health care team, including all front-facing staff (receptionists and care and financial coordinators) and clinical staff (physicians, nurses, medical assistants), need to be educated in, and practice, disability etiquette.
Preparing for in-person visits. Pre-visit preparation, ideally by means of dialogue between health care staff and the patient or caregiver (or both), typically by telephone and in advance of the scheduled visit, is often critical for a successful first face-to-face encounter. (See “Pre-visit telephone questionnaire and script for a new adult patient with IDD,” page 287, which we developed for use in our office practice.) Outcomes of the pre-visit preparation should include identifying:
- words or actions that can trigger anxiety or panic
- de-escalation techniques, such as specific calming words and actions
- strategies for optimal communication, physical access, and physical examination.
SIDEBAR
Pre-visit telephone questionnaire and script for a new adult patient with IDD
Introduction
Hello! My name is ______________. I’m a nurse [or medical assistant] from [name of practice]. I understand that [name of patient] is coming to our office for an appointment on [date and time]. I am calling to prepare our health care team to make this first appointment successful for [name of patient] and you.
- How would [name of patient] prefer to be called?
- Who will be accompanying [name of patient] to the appointment? What parts of the appointment will that person remain for?
Describe what to expect, what the patient or caregiver should bring to the appointment, and how long the appointment will last.
- What makes [name of patient] anxious or fearful so that we might avoid doing that? Should we avoid bringing up certain topics? Should we avoid performing any procedures that are customary during a first appointment?
- Does [name of patient] have sensitivities—to light, sound, touch, etc—that we should be aware of?
Offer to have a room ready upon the patient’s arrival if remaining in the waiting area would cause too much anxiety.
- What helps calm [name of patient]? Are there some topics that put [name of patient] at ease?
- How does [name of patient] best communicate?
- Is there anything else the health care team might do to prepare for the appointment?
- Does [name of patient] need personal protective equipment, a wheelchair, oxygen, or other medical equipment upon arrival?
- What would make for a successful first appointment?
- What strategies or techniques have [name of patient’s] providers used in the past that have helped make health care visits successful?
- Is there anything else you want me to know that we haven’t talked about?
- Would it be helpful if I talked with [name of patient] now about their upcoming appointment?
Initial appointments should focus on building trust and rapport with the health care team and desensitizing the patient to the clinical environment.10 Examination techniques used with pediatric patients can be applied to this population: for example, demonstrating an examination maneuver first on the parent or caregiver; beginning the examination with the least invasive or anxiety-provoking components; and stating what you plan to do next—before you do it.
Continue to: Systematic health checks provide great value
Systematic health checks provide great value
A health check is a systematic and comprehensive health assessment that is provided annually to adults with IDD, and includes:
- specific review of signs and symptoms of health conditions that often co-occur in adults with IDD (TABLE 2Calibri11)
- screening for changes in adaptive functioning and secondary disability
- lifestyle counseling
- medication review and counseling
- immunization update
- discussion of caregiver concerns.
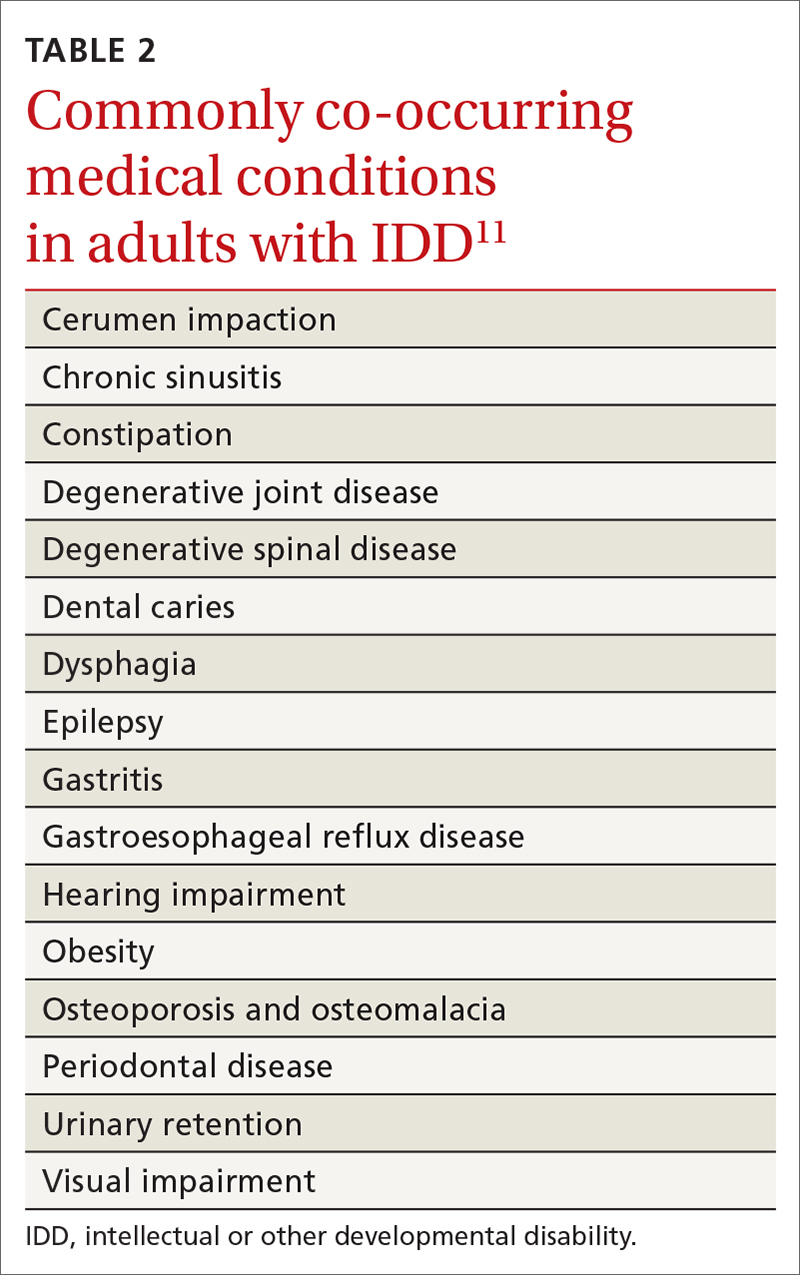
Regarding the last point: Many caregivers are the aging parents of the adult patient with IDD—people who have their own emerging health and support needs. You should initiate conversations about advanced planning for the needs of patients, which often involves engaging siblings and other family members to assume a greater role in caregiving.12
Benefits of the health check. A systematic review of 38 studies, comprising more than 5000 patients with IDD, found that health checks increased the detection of serious conditions, improved screening for sensory impairments, and increased the immunization rate.13 Although many patients with IDD generally understand the need for a periodic health examination, you can enhance their experience by better explaining the rationale for the health check; scheduling sufficient time for the appointment, based on the individual clinical situation; and discussing the value of laboratory testing and referrals to specialists.14
Tailoring preventive care
Many of the preventive services recommendations typically utilized by family physicians, such as guidelines from the US Preventive Services Task Force, have been developed for the general population at average risk of conditions of interest.15 Adults with IDD, depending on the cause of their developmental disability and their behavioral risk profile, might be at significantly higher (or lower) risk of cancer, heart disease, or other conditions than the general population. To address these differences, preventive care guidelines tailored to patients with certain developmental disabilities have been created, including guidelines specific to adults with Down syndrome, fragile X syndrome, Prader-Willi syndrome, Smith-Magenis syndrome, and 22q11.2 deletion (DiGeorge) syndrome.16
Clarifying the molecular genetic etiology of many developmental disabilities has led to more precise understandings about physical and behavioral health issues associated with specific developmental disabilities. For that reason, patients without a known cause for their IDD might benefit from referral to a geneticist—even in early or middle adulthood. Variables generally associated with a higher likelihood of an abnormal genetic test result include17:
- a family history of developmental disability
- a congenital malformation or dysmorphic features
- a dual diagnosis of developmental disability and co-occurring mental illness
- hypotonia
- severe or profound IDD.
Continue to: Successful implementation of preventive health screening tests...
Successful implementation of preventive health screening tests often requires ingenuity and the collective creativity of the patient, family members, staff, and family physician to allay fears and anxieties. Examples: Women who have been advised to undergo screening mammography might feel less anxious by undergoing tandem screening with their sister or mother, and colorectal cancer screening might be more easily accomplished using a fecal DNA test rather than by colonoscopy. Procedural desensitization strategies and preventive care instructional materials targeting people with IDD are posted on YouTube (for example, the “DD CARES Best Practices” series [see www.youtube.com/watch?v=EPJy4zvg4io]) and other websites.
Management of chronic disease
Evidence of health disparities in patients with IDD includes suboptimal management of chronic diseases, such as diabetes18 and hypertension,19 despite contact with a primary care physician. Nonadherence to a medication regimen might be more common in patients who live with their family or in a residential setting where there is a lower degree of supervision—that is, compared to a residence that maintains 24-hour staffing with daily nursing care and supervision. For a patient who is not so closely supervised, reviewing the medication refill history with the pharmacy, or using the so-called brown-bag technique of counting pill bottles brought to appointments, can ensure medication adherence.
CASE
As you interview Ms. S, you note that she is shy, avoids eye contact, and appears generally anxious. You calm her by noticing and complimenting her jewelry and fingernail polish. Ms. S smiles and talks about her favorite polish colors.
Her mother reports that, when Ms. S is stressed, she talks to herself alone in her bedroom. However, you do not observe evidence of schizoaffective disorder, and begin to wonder whether she needs to be taking risperidone.
Essentials of mental health care
It is estimated that one-third of adults with IDD have significant mental and behavioral health care needs.20 Patients with IDD suffer the same psychiatric disorders as the general population; some also engage in problematic behaviors, such as self-injurious actions, physical or verbal aggression (or both), property destruction, and resistance to caregiving assistance.
Continue to: Mental and behavioral health problems...
Mental and behavioral health problems can have a profound impact on the quality of life of patients with IDD, their peers, and their family and other caregivers. If untreated, these problems can lead to premature institutionalization, loss of employment or desired program participation, fractured social relationships, and caregiver withdrawal and burnout.
Initial evaluation of suspected mental and behavioral health problems begins with careful assessment for medical conditions that might be causing pain and distress, stereotypies, and other problematic behaviors. Common sources of pain and discomfort include dental and other oral disease, dysphagia, gastroesophageal reflux disease, gastritis, constipation, allergic disease, headache, musculoskeletal pathology, lower urinary tract disease, and gynecologic disorders.11 Identification and optimal treatment of medical conditions might not eliminate problematic behaviors but often decrease their frequency and intensity.
Psychoactive medications are prescribed for many patients with IDD. Many have behavioral adverse effects, such as akathisia, aggression, and disinhibition—leading to a prescribing cascade of psychoactive medication polypharmacy and escalating dosages.21 Antipsychotic medications are often initiated without a careful diagnosis, explicit outcome targets, or adequate clinical monitoring for effectiveness; in addition, they often lead to insulin resistance, metabolic syndrome, and massive weight gain.21 Even a family physician who is not the prescriber can perform an important advocacy role by critically reviewing psychoactive medications, documenting adverse effects, insisting on a clear therapeutic target, and calling for discontinuation of medications that appear to be ineffective.
Evaluation of mental and behavioral health problems requires a developmental perspective to interpret specific, observable behaviors with a proper clinical lens. For example, many patients with IDD engage in self-talk (soliloquizing) as a means of processing the world around them. This practice might escalate during a time of physical or psychological stress, and the unwary clinician might misinterpret this behavior as psychotic, leading to inappropriate prescribing of antipsychotic medication. Other psychotoform behaviors that, superficially, mimic but are typically not truly psychotic, include talk with or about imaginary friends and repetitive retelling of sometimes elaborate or grandiose tales or assertions. The failure of clinicians to recognize developmentally determined expressions of distress often leads to a misdiagnosis of schizophrenia or other psychotic illness and, consequently, inappropriate psychopharmacotherapy.
Family physicians, familiar with the use of psychiatric scales for diagnosis and treatment monitoring, should use similar scales that have been developed specifically for patients with IDD (TABLE 311). In addition, a psychiatric diagnosis manual, the Diagnostic Manual—Intellectual Disability 2, specific to people with IDD (and analogous to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition) provides modification of diagnostic criteria to account for patients who have difficulty articulating their internal emotional state and inner thoughts.22
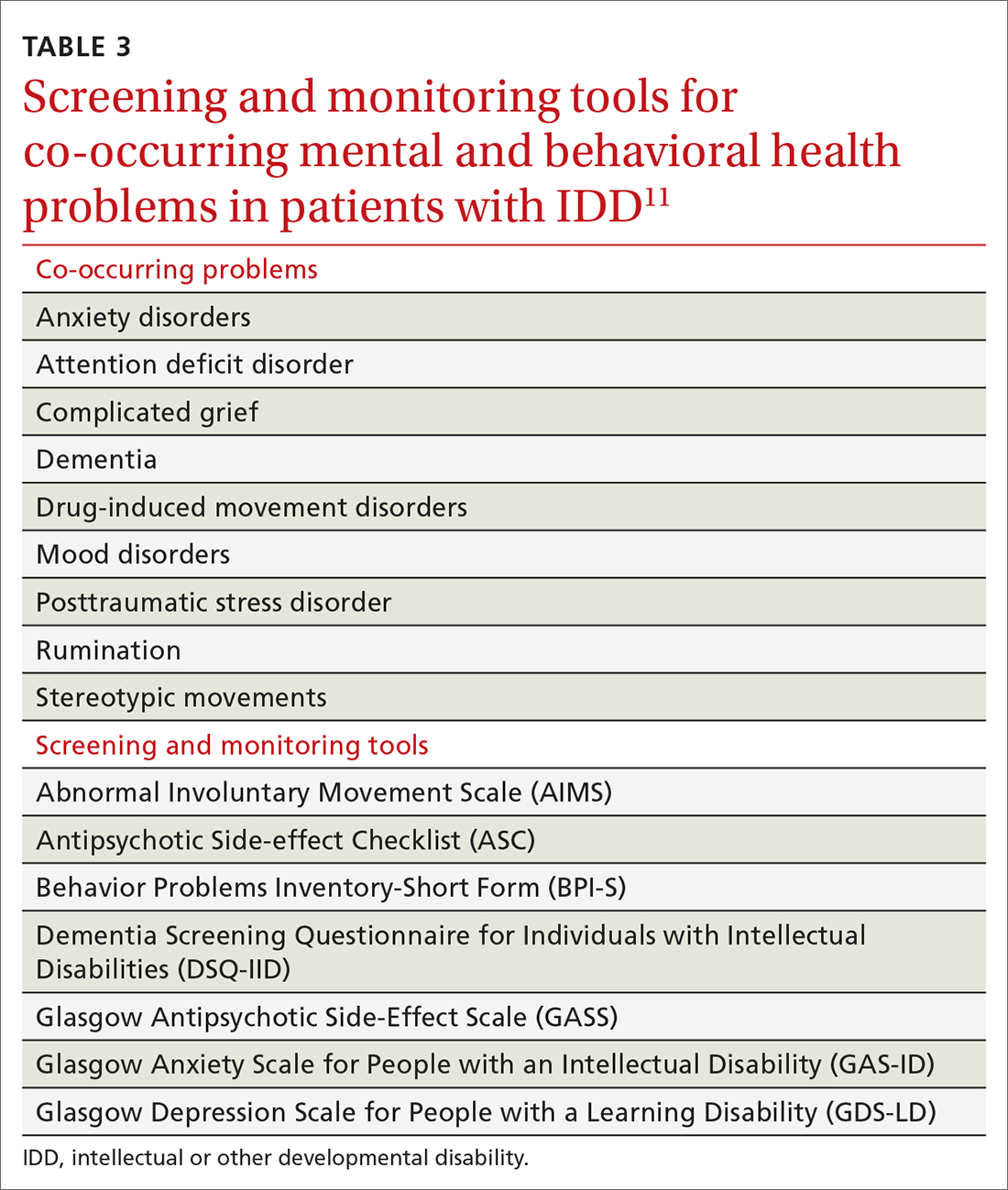
Continue to: Problematic behaviors
Problematic behaviors that are not features of a bona fide psychiatric disorder are often best understood through functional behavioral analysis, which examines antecedents and consequences of problematic behaviors and identifies their predictable outcomes, such as gaining attention, avoiding a task, or securing a desired item. Rather than being given a prescription for psychoactive medication, many adult patients with IDD and problematic behaviors might be best served by having you order consultation with a certified behavior analyst. The analyst will conduct an evaluation and, along with family or residential staff and the patient, craft a behavioral support plan to address core drivers of the undesired behavior. Behavioral support plans might be enriched by multidisciplinary input from a speech and language pathologist, habilitation professionals, occupational and physical therapists, a neuropsychologist, and others.23
Resources to help you address the physical, mental, and behavioral health problems of these patients are available online through Vanderbilt Kennedy Center’s “Toolkit for primary care providers” (https://iddtoolkit.vkcsites.org).
CASE
During your examination, you review Ms. S’s vital signs, including body mass index (BMI). You calculate that she is morbidly obese—BMI, 37—in the setting of a known comorbidity, diabetes.
Ms. S tells you that she is interested in having a healthy lifestyle, but feels frustrated because she does not know how to make the necessary changes. You discuss with her how some medications, including risperidone, can promote weight gain, and that it is important for her mental health provider to carefully reassess whether she needs to continue the drug.
Weight management in a patient population that tends to be sedentary
Patients with IDD are more likely to live a sedentary lifestyle. Compared to adults who do not have IDD, adults with IDD—especially women and patients with Down syndrome—are reported to have a higher prevalence of obesity.24
Continue to: As in the general population...
As in the general population, the greatest success in weight management involves multidisciplinary treatment, including nutritional support, physical activity, behavioral changes, and close follow-up. The importance of such an approach was borne out by the findings of a randomized controlled trial in which a multicomponent intervention—an energy-reduced diet, physical activity, and behavioral sessions—delivered to participants or their caregivers during monthly visits produced clinically meaningful 6-month weight loss.25 Health-promoting behavioral interventions that rely on a dyadic strategy, such as peer health coaches (ie, people with IDD who have been trained as a health coach) or mentors (IDD staff trained as a health coach), might be more successful at changing health behaviors among patients with IDD than traditional office-based, individual patient education and counseling.26
Similarly, undesired weight loss demands careful evaluation and management because such loss can reflect a medically significant condition, such as gastroesophageal reflux, constipation, dysphagia, neglect, and cancer.27
Boosting the amount and effectiveness of physical activity
Young people with IDD participate in physical activity less often than their neurotypical peers; as a result, they tend to be less fit and have a higher prevalence of obesity.28 Based on a meta-analysis, interventions that focus on sport and movement skills training, such as soccer, basketball, and ball-throwing programs, might be more effective than general physical activity programs.28 In addition to year-round sports training and athletic competitions, Special Olympics conducts vital health screenings of athletes and supports community-based initiatives that address bias against patients with IDD, promote inclusion, and foster social relationships (www.specialolympics.org/our-work/inclusive-health?locale=en).
Emphasize regular activity. In adulthood, fewer than 10% of patients with IDD exercise regularly.21 According to the second edition of Physical Activity Guidelines for Americans,29 “all adults, with or without a disability, should get at least 150 minutes of aerobic physical activity a week. Activities can be broken down into smaller amounts, such as about 25 minutes a day every day.”30 Supplementation with muscle-strengthening activities (eg, yoga, weight training, and resistance-band training) provides further health benefit, such as improvement in posture and prevention of future injury.31 An ideal exercise program proposed by Tyler and Baker is based on a daily, “3-2-1” schedule (ie, of every hour of activity, 30 minutes should be of aerobic exercise; 20 minutes, of strength building; and 10 minutes, of flexibility).11 By participating in any type of physical activity, there is potential for considerable health benefit in reducing psychosocial stressors, improving mental health, counteracting metabolic syndromes, and, ultimately, reducing morbidity and mortality related to physical inactivity.
CASE
With permission from Ms. S, you send your progress notes by fax to her mental health provider at the community mental health center and request a call to discuss her case—in particular, to examine potential alternatives to risperidone. With Ms. S’s input, you also co-create an exercise prescription that includes a daily 20-minute walking program with her mother.
At the follow-up visit that is scheduled in 3 months, you anticipate adding a resistance component and balance activity to the exercise prescription to enrich Ms. S’s physical activity regimen.
CORRESPONDENCE
Carl V. Tyler Jr., MD, 14601 Detroit Avenue, Lakewood, OH, 44107; [email protected]
1. Sullivan WF, Diepstra H, Heng J, et al. Primary care of adults with intellectual and developmental disabilities: 2018 Canadian consensus guidelines. Can Fam Physician. 2018;64:254-279.
2. World Health Organization. International Classification of Impairments, Disabilities, and Handicaps: A Manual of Classification Relating to the Consequences of Disease. May 1980. Accessed May 27, 2021. https://apps.who.int/iris/bitstream/handle/10665/41003/9241541261_eng.pdf?sequence=1&isAllowed=y
3. Berens J, Wozow C, Peacock C. Transition to adult care. Phys Med Rehabil Clin N Am. 2020;31:159-170. doi:10.1016/j.pmr.2019.09.004
4. American Academy of Pediatrics; American Academy of Family Physicians; American College of Physicians; Transitions Clinical Report Authoring Group; Cooley WC, Sagerman PJ. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128:182-200. doi:10.1542/peds.2011-0969
5. Dressler PB, Nguyen TK, Moody EJ, et al. Use of transition resources by primary care providers for youth with intellectual and developmental disabilities. Intellect Dev Disabil. 2018;56:56-68. doi:10.1352/1934-9556-56.1.56
6. The National Alliance to Advance Adolescent Health. Six Core Elements of Health Care Transition.™ Got Transition website. Accessed May 27, 2021. www.gottransition.org
7. Schmidt A, Ilango SM, McManus MA, et al. Outcomes of pediatric to adult health care transition interventions: an updated systematic review. J Pediatr Nurs. 2020; 51:92-107. doi: 10.1016/j.pedn.2020.01.002
8. Keith JM, Bennetto L, Rogge RD. The relationship between contact and attitudes: reducing prejudice toward individuals with intellectual and developmental disabilities. Res Dev Disabil. 2015;47:14-26. doi:10.1016/j.ridd.2015.07.032
9. United Spinal Association. Disability Etiquette: Tips on Interacting With People With Disabilities. 2015. Accessed June 9, 2021. www.unitedspinal.org/pdf/DisabilityEtiquette.pdf
10. Nathawad R, Hanks C. Optimizing the office visit for adolescents with special health care needs. Curr Probl Pediatr Adolesc Health Care. 2017;47:182-189. doi:10.1016/j.cppeds.2017.07.002
11. Tyler CV, Baker S. Intellectual Disabilities at Your Fingertips: A Health Care Resource. High Tide Press; 2009.
12. Williamson HJ, Perkins EA. Family caregivers of adults with intellectual and developmental disabilities: outcomes associated with U.S. services and supports. Intellect Dev Disabil. 2014;52:147-159. doi: 10.1352/1934-9556-52.2.147
13. Robertson J, Hatton C, Emerson E, et al. The impact of health checks for people with intellectual disabilities: an updated systematic review of evidence. Res Dev Disabil. 2014;35:2450-2462. doi:10.1016/j.ridd.2014.06.007
14. Perry J, Felce D, Kerr M, et al. Contact with primary care: the experience of people with intellectual disabilities. J Appl Res Intellect Disabil. 2014;27:200-211. doi: 10.1111/jar.12072
15. Recommendation topics. United States Preventive Services Task Force website. 2020. Accessed May 27, 2021. www.uspreventiveservicestaskforce.org
16. Developmental Disabilities Primary Care Initiative. Tools for the Primary Care of People with Developmental Disabilities. 1st ed. MUMS Guideline Clearinghouse; 2011.
17. Jang W, Kim Y, Han E, et al. Chromosomal microarray analysis as a first-tier clinical diagnostic test in patients with developmental delay/intellectual disability, autism spectrum disorders, and multiple congenital anomalies: a prospective multicenter study in Korea. Ann Lab Med. 2019;39:299-310. doi:10.3343/alm.2019.39.3.299
18. Shireman TI, Reichard A, Nazir N, et al. Quality of diabetes care for adults with developmental disabilities. Disabil Health J. 2010;3:179-185. doi:10.1016/j.dhjo.2009.10.004
19. Cyrus AC, Royer J, Carroll DD, et al. Anti-hypertensive medication use and actors related to adherence among adults with intellectual and developmental disabilities. Am J Intellect Dev Disabil. 2019;124:248-262. doi:10.1352/1944-7558-124.3.248
20. IDD/MI diagnosis. National Association for the Dually Diagnosed (NADD) website. 2019. Accessed May 27, 2021. https://thenadd.org/idd-mi-diagnosis
21. Matson JL, Mayville EA, Bielecki J, et al. Reliability of the Matson Evaluation of Drug Side Effects Scale (MEDS). Res Dev Disabil. 1998;19:501-506. doi:10.1016/s0891-4222(98)00021-3
22. Fletcher R, Barnhill J, Cooper SA. (2017). Diagnostic Manual-Intellectual Disability: A Textbook of Diagnosis of Mental Disorders in Persons with Intellectual Disability. 2nd ed. National Association for the Dually Diagnosed (NADD); 2017.
23. Marrus N, Hall L. Intellectual disability and language disorder. Child Adolesc Psychiatr Clin N Am. 2017;26:539-554. doi:10.1016/j.chc.2017.03.001
24. Rimmer JH, Yamaki K. Obesity and intellectual disability. Ment Retard Dev Disabil Res Rev. 2006;12;22-7. doi: 10.1002/mrdd.20091
25. Ptomey LT, Saunders RR, Saunders M, et al. Weight management in adults with intellectual and developmental disabilities: a randomized controlled trial of two dietary approaches. J Appl Res Intellect Disabil. 2018;31(suppl 1):82-96. doi:10.1111/jar.12348
26. Marks B, Sisirak J, Magallanes R, et al. Effectiveness of a HealthMessages peer-to-peer program for people with intellectual and developmental disabilities. Intellect Dev Disabil. 2019;57:242-258. doi:10.1352/1934-9556-57.3.242
27. Escudé C. Clinical Pearls in IDD Health care. HRS, Inc; 2020.
28. Kapsal NJ, Dicke T, Morin AJS, et al. Effects of physical activity on the physical and psychosocial health of youth with intellectual disabilities: a systematic review and meta-analysis. J Phys Act Health. 2019;16:1187-1195. doi:10.1123/jpah.2018-0675
29. Physical Activity Guidelines for Americans. 2nd ed. US Department of Health and Human Services; 2018. Accessed May 29, 2021. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
30. National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention. Physical activity for people with disability. September 2020. Accessed May 27, 2021. www.cdc.gov/ncbddd/disabilityandhealth/features/physical-activity-for-all.html
31. Introduction to strengthening exercises. National Center on Health, Physical Activity and Disability (NCHPAD). 2020. Accessed May 27, 2021. www.nchpad.org/374/2096/Strengthening~Exercises
Some adults who have an intellectual or other developmental disability (IDD) require extensive subspecialty care; many, however, depend primarily on their family physician for the bulk of their health care. With that reliance in mind, this article provides (1) an overview of important services that family physicians can provide for their adult patients with IDD and (2) pragmatic clinical suggestions for tailoring that care. Note: We highlight only some high-impact areas of clinical focus; refer to the 2018 Canadian consensus guidelines for a comprehensive approach to optimizing primary care for this population.1
CASE
Laura S, a 24-year-old woman with Down syndrome, is visiting your clinic with her mother to establish care. Ms. S has several medical comorbidities, including type 2 diabetes, hyperlipidemia, repaired congenital heart disease, schizoaffective disorder, and hypothyroidism. She is under the care of multiple specialists, including a cardiologist and an endocrinologist. Her medications include the atypical antipsychotic risperidone, which was prescribed for her through the services of a community mental health center.

Ms. S is due for multiple preventive health screenings. She indicates that she feels nervous today talking about these screenings with a new physician.
First step in care: Proficiency in the lexicon of IDD
Three core concepts of IDD are impairment, disability, and handicap. According to the World Health Organization2:
- impairment “is any loss or abnormality of psychological, physiological, or anatomical structure or function.”
- disability “is any restriction or lack (resulting from an impairment) of ability to perform an activity in the manner or within the range considered normal for a human being.”
- handicap therefore “represents socialization of an impairment or disability, and as such it reflects the consequences for the individual—cultural, social, economic, and environmental—that stem from the presence of impairment and disability.”
Essential transition: Pediatric to adult health care
Health care transition (HCT) is the planned process of transferring care from a pediatric to an adult-based health care setting,3 comprising 3 phases:
- preparation
- transfer from pediatric to adult care
- integration into adult-based care.
Two critical components of a smooth HCT include initiating the transition early in adolescence and providing transition-support resources, which are often lacking, even in large, integrated health systems.4 Got Transition, created by the National Alliance to Advance Adolescent Health, outlines core elements of an organized HCT process (www.gottransition.org) specific to young adults with IDD, including young adults with autism spectrum disorder.5,6
Even young people who are served by a family physician and who intend to remain in that family practice as they age into adulthood require HCT services that include6:
- assessment of readiness to transition to adult care
- update of the medical history
- assessment and promotion of self-care skills
- consent discussions and optimized participation in decision-making
- transition of specialty care from pediatric to adult specialists.
Continue to: For an ideal HCT...
For an ideal HCT, full engagement of the patient, the medical home (physicians, nursing staff, and care coordinators), and the patient’s family (including the primary caregiver or guardian) is critical. In addition to preventive care visits and management of chronic disease, additional domains that require explicit attention in transitioning young people with IDD include health insurance, transportation, employment, and postsecondary education.
Young people who have special health care needs and receive high-quality HCT demonstrate improvements in adherence to care, disease-specific measures, quality of life, self-care skills, satisfaction with care, and health care utilization.7TABLE 13 lists resources identified by Berens and colleagues that are helpful in facilitating the transition.

Teach and practice disability etiquette
Societal prejudice harms people with IDD—leading to self-deprecation, alienation from the larger community, and isolation from others with IDD.8 To promote acceptance and inclusivity in residential communities, the workplace, recreational venues, and clinical settings, disability etiquette should be utilized—a set of guidelines on how to interact with patients with IDD. These include speaking to the patient directly, using clear language in an adult voice, and avoiding stereotypes about people with disabilities.9 The entire health care team, including all front-facing staff (receptionists and care and financial coordinators) and clinical staff (physicians, nurses, medical assistants), need to be educated in, and practice, disability etiquette.
Preparing for in-person visits. Pre-visit preparation, ideally by means of dialogue between health care staff and the patient or caregiver (or both), typically by telephone and in advance of the scheduled visit, is often critical for a successful first face-to-face encounter. (See “Pre-visit telephone questionnaire and script for a new adult patient with IDD,” page 287, which we developed for use in our office practice.) Outcomes of the pre-visit preparation should include identifying:
- words or actions that can trigger anxiety or panic
- de-escalation techniques, such as specific calming words and actions
- strategies for optimal communication, physical access, and physical examination.
SIDEBAR
Pre-visit telephone questionnaire and script for a new adult patient with IDD
Introduction
Hello! My name is ______________. I’m a nurse [or medical assistant] from [name of practice]. I understand that [name of patient] is coming to our office for an appointment on [date and time]. I am calling to prepare our health care team to make this first appointment successful for [name of patient] and you.
- How would [name of patient] prefer to be called?
- Who will be accompanying [name of patient] to the appointment? What parts of the appointment will that person remain for?
Describe what to expect, what the patient or caregiver should bring to the appointment, and how long the appointment will last.
- What makes [name of patient] anxious or fearful so that we might avoid doing that? Should we avoid bringing up certain topics? Should we avoid performing any procedures that are customary during a first appointment?
- Does [name of patient] have sensitivities—to light, sound, touch, etc—that we should be aware of?
Offer to have a room ready upon the patient’s arrival if remaining in the waiting area would cause too much anxiety.
- What helps calm [name of patient]? Are there some topics that put [name of patient] at ease?
- How does [name of patient] best communicate?
- Is there anything else the health care team might do to prepare for the appointment?
- Does [name of patient] need personal protective equipment, a wheelchair, oxygen, or other medical equipment upon arrival?
- What would make for a successful first appointment?
- What strategies or techniques have [name of patient’s] providers used in the past that have helped make health care visits successful?
- Is there anything else you want me to know that we haven’t talked about?
- Would it be helpful if I talked with [name of patient] now about their upcoming appointment?
Initial appointments should focus on building trust and rapport with the health care team and desensitizing the patient to the clinical environment.10 Examination techniques used with pediatric patients can be applied to this population: for example, demonstrating an examination maneuver first on the parent or caregiver; beginning the examination with the least invasive or anxiety-provoking components; and stating what you plan to do next—before you do it.
Continue to: Systematic health checks provide great value
Systematic health checks provide great value
A health check is a systematic and comprehensive health assessment that is provided annually to adults with IDD, and includes:
- specific review of signs and symptoms of health conditions that often co-occur in adults with IDD (TABLE 2Calibri11)
- screening for changes in adaptive functioning and secondary disability
- lifestyle counseling
- medication review and counseling
- immunization update
- discussion of caregiver concerns.

Regarding the last point: Many caregivers are the aging parents of the adult patient with IDD—people who have their own emerging health and support needs. You should initiate conversations about advanced planning for the needs of patients, which often involves engaging siblings and other family members to assume a greater role in caregiving.12
Benefits of the health check. A systematic review of 38 studies, comprising more than 5000 patients with IDD, found that health checks increased the detection of serious conditions, improved screening for sensory impairments, and increased the immunization rate.13 Although many patients with IDD generally understand the need for a periodic health examination, you can enhance their experience by better explaining the rationale for the health check; scheduling sufficient time for the appointment, based on the individual clinical situation; and discussing the value of laboratory testing and referrals to specialists.14
Tailoring preventive care
Many of the preventive services recommendations typically utilized by family physicians, such as guidelines from the US Preventive Services Task Force, have been developed for the general population at average risk of conditions of interest.15 Adults with IDD, depending on the cause of their developmental disability and their behavioral risk profile, might be at significantly higher (or lower) risk of cancer, heart disease, or other conditions than the general population. To address these differences, preventive care guidelines tailored to patients with certain developmental disabilities have been created, including guidelines specific to adults with Down syndrome, fragile X syndrome, Prader-Willi syndrome, Smith-Magenis syndrome, and 22q11.2 deletion (DiGeorge) syndrome.16
Clarifying the molecular genetic etiology of many developmental disabilities has led to more precise understandings about physical and behavioral health issues associated with specific developmental disabilities. For that reason, patients without a known cause for their IDD might benefit from referral to a geneticist—even in early or middle adulthood. Variables generally associated with a higher likelihood of an abnormal genetic test result include17:
- a family history of developmental disability
- a congenital malformation or dysmorphic features
- a dual diagnosis of developmental disability and co-occurring mental illness
- hypotonia
- severe or profound IDD.
Continue to: Successful implementation of preventive health screening tests...
Successful implementation of preventive health screening tests often requires ingenuity and the collective creativity of the patient, family members, staff, and family physician to allay fears and anxieties. Examples: Women who have been advised to undergo screening mammography might feel less anxious by undergoing tandem screening with their sister or mother, and colorectal cancer screening might be more easily accomplished using a fecal DNA test rather than by colonoscopy. Procedural desensitization strategies and preventive care instructional materials targeting people with IDD are posted on YouTube (for example, the “DD CARES Best Practices” series [see www.youtube.com/watch?v=EPJy4zvg4io]) and other websites.
Management of chronic disease
Evidence of health disparities in patients with IDD includes suboptimal management of chronic diseases, such as diabetes18 and hypertension,19 despite contact with a primary care physician. Nonadherence to a medication regimen might be more common in patients who live with their family or in a residential setting where there is a lower degree of supervision—that is, compared to a residence that maintains 24-hour staffing with daily nursing care and supervision. For a patient who is not so closely supervised, reviewing the medication refill history with the pharmacy, or using the so-called brown-bag technique of counting pill bottles brought to appointments, can ensure medication adherence.
CASE
As you interview Ms. S, you note that she is shy, avoids eye contact, and appears generally anxious. You calm her by noticing and complimenting her jewelry and fingernail polish. Ms. S smiles and talks about her favorite polish colors.
Her mother reports that, when Ms. S is stressed, she talks to herself alone in her bedroom. However, you do not observe evidence of schizoaffective disorder, and begin to wonder whether she needs to be taking risperidone.
Essentials of mental health care
It is estimated that one-third of adults with IDD have significant mental and behavioral health care needs.20 Patients with IDD suffer the same psychiatric disorders as the general population; some also engage in problematic behaviors, such as self-injurious actions, physical or verbal aggression (or both), property destruction, and resistance to caregiving assistance.
Continue to: Mental and behavioral health problems...
Mental and behavioral health problems can have a profound impact on the quality of life of patients with IDD, their peers, and their family and other caregivers. If untreated, these problems can lead to premature institutionalization, loss of employment or desired program participation, fractured social relationships, and caregiver withdrawal and burnout.
Initial evaluation of suspected mental and behavioral health problems begins with careful assessment for medical conditions that might be causing pain and distress, stereotypies, and other problematic behaviors. Common sources of pain and discomfort include dental and other oral disease, dysphagia, gastroesophageal reflux disease, gastritis, constipation, allergic disease, headache, musculoskeletal pathology, lower urinary tract disease, and gynecologic disorders.11 Identification and optimal treatment of medical conditions might not eliminate problematic behaviors but often decrease their frequency and intensity.
Psychoactive medications are prescribed for many patients with IDD. Many have behavioral adverse effects, such as akathisia, aggression, and disinhibition—leading to a prescribing cascade of psychoactive medication polypharmacy and escalating dosages.21 Antipsychotic medications are often initiated without a careful diagnosis, explicit outcome targets, or adequate clinical monitoring for effectiveness; in addition, they often lead to insulin resistance, metabolic syndrome, and massive weight gain.21 Even a family physician who is not the prescriber can perform an important advocacy role by critically reviewing psychoactive medications, documenting adverse effects, insisting on a clear therapeutic target, and calling for discontinuation of medications that appear to be ineffective.
Evaluation of mental and behavioral health problems requires a developmental perspective to interpret specific, observable behaviors with a proper clinical lens. For example, many patients with IDD engage in self-talk (soliloquizing) as a means of processing the world around them. This practice might escalate during a time of physical or psychological stress, and the unwary clinician might misinterpret this behavior as psychotic, leading to inappropriate prescribing of antipsychotic medication. Other psychotoform behaviors that, superficially, mimic but are typically not truly psychotic, include talk with or about imaginary friends and repetitive retelling of sometimes elaborate or grandiose tales or assertions. The failure of clinicians to recognize developmentally determined expressions of distress often leads to a misdiagnosis of schizophrenia or other psychotic illness and, consequently, inappropriate psychopharmacotherapy.
Family physicians, familiar with the use of psychiatric scales for diagnosis and treatment monitoring, should use similar scales that have been developed specifically for patients with IDD (TABLE 311). In addition, a psychiatric diagnosis manual, the Diagnostic Manual—Intellectual Disability 2, specific to people with IDD (and analogous to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition) provides modification of diagnostic criteria to account for patients who have difficulty articulating their internal emotional state and inner thoughts.22

Continue to: Problematic behaviors
Problematic behaviors that are not features of a bona fide psychiatric disorder are often best understood through functional behavioral analysis, which examines antecedents and consequences of problematic behaviors and identifies their predictable outcomes, such as gaining attention, avoiding a task, or securing a desired item. Rather than being given a prescription for psychoactive medication, many adult patients with IDD and problematic behaviors might be best served by having you order consultation with a certified behavior analyst. The analyst will conduct an evaluation and, along with family or residential staff and the patient, craft a behavioral support plan to address core drivers of the undesired behavior. Behavioral support plans might be enriched by multidisciplinary input from a speech and language pathologist, habilitation professionals, occupational and physical therapists, a neuropsychologist, and others.23
Resources to help you address the physical, mental, and behavioral health problems of these patients are available online through Vanderbilt Kennedy Center’s “Toolkit for primary care providers” (https://iddtoolkit.vkcsites.org).
CASE
During your examination, you review Ms. S’s vital signs, including body mass index (BMI). You calculate that she is morbidly obese—BMI, 37—in the setting of a known comorbidity, diabetes.
Ms. S tells you that she is interested in having a healthy lifestyle, but feels frustrated because she does not know how to make the necessary changes. You discuss with her how some medications, including risperidone, can promote weight gain, and that it is important for her mental health provider to carefully reassess whether she needs to continue the drug.
Weight management in a patient population that tends to be sedentary
Patients with IDD are more likely to live a sedentary lifestyle. Compared to adults who do not have IDD, adults with IDD—especially women and patients with Down syndrome—are reported to have a higher prevalence of obesity.24
Continue to: As in the general population...
As in the general population, the greatest success in weight management involves multidisciplinary treatment, including nutritional support, physical activity, behavioral changes, and close follow-up. The importance of such an approach was borne out by the findings of a randomized controlled trial in which a multicomponent intervention—an energy-reduced diet, physical activity, and behavioral sessions—delivered to participants or their caregivers during monthly visits produced clinically meaningful 6-month weight loss.25 Health-promoting behavioral interventions that rely on a dyadic strategy, such as peer health coaches (ie, people with IDD who have been trained as a health coach) or mentors (IDD staff trained as a health coach), might be more successful at changing health behaviors among patients with IDD than traditional office-based, individual patient education and counseling.26
Similarly, undesired weight loss demands careful evaluation and management because such loss can reflect a medically significant condition, such as gastroesophageal reflux, constipation, dysphagia, neglect, and cancer.27
Boosting the amount and effectiveness of physical activity
Young people with IDD participate in physical activity less often than their neurotypical peers; as a result, they tend to be less fit and have a higher prevalence of obesity.28 Based on a meta-analysis, interventions that focus on sport and movement skills training, such as soccer, basketball, and ball-throwing programs, might be more effective than general physical activity programs.28 In addition to year-round sports training and athletic competitions, Special Olympics conducts vital health screenings of athletes and supports community-based initiatives that address bias against patients with IDD, promote inclusion, and foster social relationships (www.specialolympics.org/our-work/inclusive-health?locale=en).
Emphasize regular activity. In adulthood, fewer than 10% of patients with IDD exercise regularly.21 According to the second edition of Physical Activity Guidelines for Americans,29 “all adults, with or without a disability, should get at least 150 minutes of aerobic physical activity a week. Activities can be broken down into smaller amounts, such as about 25 minutes a day every day.”30 Supplementation with muscle-strengthening activities (eg, yoga, weight training, and resistance-band training) provides further health benefit, such as improvement in posture and prevention of future injury.31 An ideal exercise program proposed by Tyler and Baker is based on a daily, “3-2-1” schedule (ie, of every hour of activity, 30 minutes should be of aerobic exercise; 20 minutes, of strength building; and 10 minutes, of flexibility).11 By participating in any type of physical activity, there is potential for considerable health benefit in reducing psychosocial stressors, improving mental health, counteracting metabolic syndromes, and, ultimately, reducing morbidity and mortality related to physical inactivity.
CASE
With permission from Ms. S, you send your progress notes by fax to her mental health provider at the community mental health center and request a call to discuss her case—in particular, to examine potential alternatives to risperidone. With Ms. S’s input, you also co-create an exercise prescription that includes a daily 20-minute walking program with her mother.
At the follow-up visit that is scheduled in 3 months, you anticipate adding a resistance component and balance activity to the exercise prescription to enrich Ms. S’s physical activity regimen.
CORRESPONDENCE
Carl V. Tyler Jr., MD, 14601 Detroit Avenue, Lakewood, OH, 44107; [email protected]
Some adults who have an intellectual or other developmental disability (IDD) require extensive subspecialty care; many, however, depend primarily on their family physician for the bulk of their health care. With that reliance in mind, this article provides (1) an overview of important services that family physicians can provide for their adult patients with IDD and (2) pragmatic clinical suggestions for tailoring that care. Note: We highlight only some high-impact areas of clinical focus; refer to the 2018 Canadian consensus guidelines for a comprehensive approach to optimizing primary care for this population.1
CASE
Laura S, a 24-year-old woman with Down syndrome, is visiting your clinic with her mother to establish care. Ms. S has several medical comorbidities, including type 2 diabetes, hyperlipidemia, repaired congenital heart disease, schizoaffective disorder, and hypothyroidism. She is under the care of multiple specialists, including a cardiologist and an endocrinologist. Her medications include the atypical antipsychotic risperidone, which was prescribed for her through the services of a community mental health center.

Ms. S is due for multiple preventive health screenings. She indicates that she feels nervous today talking about these screenings with a new physician.
First step in care: Proficiency in the lexicon of IDD
Three core concepts of IDD are impairment, disability, and handicap. According to the World Health Organization2:
- impairment “is any loss or abnormality of psychological, physiological, or anatomical structure or function.”
- disability “is any restriction or lack (resulting from an impairment) of ability to perform an activity in the manner or within the range considered normal for a human being.”
- handicap therefore “represents socialization of an impairment or disability, and as such it reflects the consequences for the individual—cultural, social, economic, and environmental—that stem from the presence of impairment and disability.”
Essential transition: Pediatric to adult health care
Health care transition (HCT) is the planned process of transferring care from a pediatric to an adult-based health care setting,3 comprising 3 phases:
- preparation
- transfer from pediatric to adult care
- integration into adult-based care.
Two critical components of a smooth HCT include initiating the transition early in adolescence and providing transition-support resources, which are often lacking, even in large, integrated health systems.4 Got Transition, created by the National Alliance to Advance Adolescent Health, outlines core elements of an organized HCT process (www.gottransition.org) specific to young adults with IDD, including young adults with autism spectrum disorder.5,6
Even young people who are served by a family physician and who intend to remain in that family practice as they age into adulthood require HCT services that include6:
- assessment of readiness to transition to adult care
- update of the medical history
- assessment and promotion of self-care skills
- consent discussions and optimized participation in decision-making
- transition of specialty care from pediatric to adult specialists.
Continue to: For an ideal HCT...
For an ideal HCT, full engagement of the patient, the medical home (physicians, nursing staff, and care coordinators), and the patient’s family (including the primary caregiver or guardian) is critical. In addition to preventive care visits and management of chronic disease, additional domains that require explicit attention in transitioning young people with IDD include health insurance, transportation, employment, and postsecondary education.
Young people who have special health care needs and receive high-quality HCT demonstrate improvements in adherence to care, disease-specific measures, quality of life, self-care skills, satisfaction with care, and health care utilization.7TABLE 13 lists resources identified by Berens and colleagues that are helpful in facilitating the transition.

Teach and practice disability etiquette
Societal prejudice harms people with IDD—leading to self-deprecation, alienation from the larger community, and isolation from others with IDD.8 To promote acceptance and inclusivity in residential communities, the workplace, recreational venues, and clinical settings, disability etiquette should be utilized—a set of guidelines on how to interact with patients with IDD. These include speaking to the patient directly, using clear language in an adult voice, and avoiding stereotypes about people with disabilities.9 The entire health care team, including all front-facing staff (receptionists and care and financial coordinators) and clinical staff (physicians, nurses, medical assistants), need to be educated in, and practice, disability etiquette.
Preparing for in-person visits. Pre-visit preparation, ideally by means of dialogue between health care staff and the patient or caregiver (or both), typically by telephone and in advance of the scheduled visit, is often critical for a successful first face-to-face encounter. (See “Pre-visit telephone questionnaire and script for a new adult patient with IDD,” page 287, which we developed for use in our office practice.) Outcomes of the pre-visit preparation should include identifying:
- words or actions that can trigger anxiety or panic
- de-escalation techniques, such as specific calming words and actions
- strategies for optimal communication, physical access, and physical examination.
SIDEBAR
Pre-visit telephone questionnaire and script for a new adult patient with IDD
Introduction
Hello! My name is ______________. I’m a nurse [or medical assistant] from [name of practice]. I understand that [name of patient] is coming to our office for an appointment on [date and time]. I am calling to prepare our health care team to make this first appointment successful for [name of patient] and you.
- How would [name of patient] prefer to be called?
- Who will be accompanying [name of patient] to the appointment? What parts of the appointment will that person remain for?
Describe what to expect, what the patient or caregiver should bring to the appointment, and how long the appointment will last.
- What makes [name of patient] anxious or fearful so that we might avoid doing that? Should we avoid bringing up certain topics? Should we avoid performing any procedures that are customary during a first appointment?
- Does [name of patient] have sensitivities—to light, sound, touch, etc—that we should be aware of?
Offer to have a room ready upon the patient’s arrival if remaining in the waiting area would cause too much anxiety.
- What helps calm [name of patient]? Are there some topics that put [name of patient] at ease?
- How does [name of patient] best communicate?
- Is there anything else the health care team might do to prepare for the appointment?
- Does [name of patient] need personal protective equipment, a wheelchair, oxygen, or other medical equipment upon arrival?
- What would make for a successful first appointment?
- What strategies or techniques have [name of patient’s] providers used in the past that have helped make health care visits successful?
- Is there anything else you want me to know that we haven’t talked about?
- Would it be helpful if I talked with [name of patient] now about their upcoming appointment?
Initial appointments should focus on building trust and rapport with the health care team and desensitizing the patient to the clinical environment.10 Examination techniques used with pediatric patients can be applied to this population: for example, demonstrating an examination maneuver first on the parent or caregiver; beginning the examination with the least invasive or anxiety-provoking components; and stating what you plan to do next—before you do it.
Continue to: Systematic health checks provide great value
Systematic health checks provide great value
A health check is a systematic and comprehensive health assessment that is provided annually to adults with IDD, and includes:
- specific review of signs and symptoms of health conditions that often co-occur in adults with IDD (TABLE 2Calibri11)
- screening for changes in adaptive functioning and secondary disability
- lifestyle counseling
- medication review and counseling
- immunization update
- discussion of caregiver concerns.

Regarding the last point: Many caregivers are the aging parents of the adult patient with IDD—people who have their own emerging health and support needs. You should initiate conversations about advanced planning for the needs of patients, which often involves engaging siblings and other family members to assume a greater role in caregiving.12
Benefits of the health check. A systematic review of 38 studies, comprising more than 5000 patients with IDD, found that health checks increased the detection of serious conditions, improved screening for sensory impairments, and increased the immunization rate.13 Although many patients with IDD generally understand the need for a periodic health examination, you can enhance their experience by better explaining the rationale for the health check; scheduling sufficient time for the appointment, based on the individual clinical situation; and discussing the value of laboratory testing and referrals to specialists.14
Tailoring preventive care
Many of the preventive services recommendations typically utilized by family physicians, such as guidelines from the US Preventive Services Task Force, have been developed for the general population at average risk of conditions of interest.15 Adults with IDD, depending on the cause of their developmental disability and their behavioral risk profile, might be at significantly higher (or lower) risk of cancer, heart disease, or other conditions than the general population. To address these differences, preventive care guidelines tailored to patients with certain developmental disabilities have been created, including guidelines specific to adults with Down syndrome, fragile X syndrome, Prader-Willi syndrome, Smith-Magenis syndrome, and 22q11.2 deletion (DiGeorge) syndrome.16
Clarifying the molecular genetic etiology of many developmental disabilities has led to more precise understandings about physical and behavioral health issues associated with specific developmental disabilities. For that reason, patients without a known cause for their IDD might benefit from referral to a geneticist—even in early or middle adulthood. Variables generally associated with a higher likelihood of an abnormal genetic test result include17:
- a family history of developmental disability
- a congenital malformation or dysmorphic features
- a dual diagnosis of developmental disability and co-occurring mental illness
- hypotonia
- severe or profound IDD.
Continue to: Successful implementation of preventive health screening tests...
Successful implementation of preventive health screening tests often requires ingenuity and the collective creativity of the patient, family members, staff, and family physician to allay fears and anxieties. Examples: Women who have been advised to undergo screening mammography might feel less anxious by undergoing tandem screening with their sister or mother, and colorectal cancer screening might be more easily accomplished using a fecal DNA test rather than by colonoscopy. Procedural desensitization strategies and preventive care instructional materials targeting people with IDD are posted on YouTube (for example, the “DD CARES Best Practices” series [see www.youtube.com/watch?v=EPJy4zvg4io]) and other websites.
Management of chronic disease
Evidence of health disparities in patients with IDD includes suboptimal management of chronic diseases, such as diabetes18 and hypertension,19 despite contact with a primary care physician. Nonadherence to a medication regimen might be more common in patients who live with their family or in a residential setting where there is a lower degree of supervision—that is, compared to a residence that maintains 24-hour staffing with daily nursing care and supervision. For a patient who is not so closely supervised, reviewing the medication refill history with the pharmacy, or using the so-called brown-bag technique of counting pill bottles brought to appointments, can ensure medication adherence.
CASE
As you interview Ms. S, you note that she is shy, avoids eye contact, and appears generally anxious. You calm her by noticing and complimenting her jewelry and fingernail polish. Ms. S smiles and talks about her favorite polish colors.
Her mother reports that, when Ms. S is stressed, she talks to herself alone in her bedroom. However, you do not observe evidence of schizoaffective disorder, and begin to wonder whether she needs to be taking risperidone.
Essentials of mental health care
It is estimated that one-third of adults with IDD have significant mental and behavioral health care needs.20 Patients with IDD suffer the same psychiatric disorders as the general population; some also engage in problematic behaviors, such as self-injurious actions, physical or verbal aggression (or both), property destruction, and resistance to caregiving assistance.
Continue to: Mental and behavioral health problems...
Mental and behavioral health problems can have a profound impact on the quality of life of patients with IDD, their peers, and their family and other caregivers. If untreated, these problems can lead to premature institutionalization, loss of employment or desired program participation, fractured social relationships, and caregiver withdrawal and burnout.
Initial evaluation of suspected mental and behavioral health problems begins with careful assessment for medical conditions that might be causing pain and distress, stereotypies, and other problematic behaviors. Common sources of pain and discomfort include dental and other oral disease, dysphagia, gastroesophageal reflux disease, gastritis, constipation, allergic disease, headache, musculoskeletal pathology, lower urinary tract disease, and gynecologic disorders.11 Identification and optimal treatment of medical conditions might not eliminate problematic behaviors but often decrease their frequency and intensity.
Psychoactive medications are prescribed for many patients with IDD. Many have behavioral adverse effects, such as akathisia, aggression, and disinhibition—leading to a prescribing cascade of psychoactive medication polypharmacy and escalating dosages.21 Antipsychotic medications are often initiated without a careful diagnosis, explicit outcome targets, or adequate clinical monitoring for effectiveness; in addition, they often lead to insulin resistance, metabolic syndrome, and massive weight gain.21 Even a family physician who is not the prescriber can perform an important advocacy role by critically reviewing psychoactive medications, documenting adverse effects, insisting on a clear therapeutic target, and calling for discontinuation of medications that appear to be ineffective.
Evaluation of mental and behavioral health problems requires a developmental perspective to interpret specific, observable behaviors with a proper clinical lens. For example, many patients with IDD engage in self-talk (soliloquizing) as a means of processing the world around them. This practice might escalate during a time of physical or psychological stress, and the unwary clinician might misinterpret this behavior as psychotic, leading to inappropriate prescribing of antipsychotic medication. Other psychotoform behaviors that, superficially, mimic but are typically not truly psychotic, include talk with or about imaginary friends and repetitive retelling of sometimes elaborate or grandiose tales or assertions. The failure of clinicians to recognize developmentally determined expressions of distress often leads to a misdiagnosis of schizophrenia or other psychotic illness and, consequently, inappropriate psychopharmacotherapy.
Family physicians, familiar with the use of psychiatric scales for diagnosis and treatment monitoring, should use similar scales that have been developed specifically for patients with IDD (TABLE 311). In addition, a psychiatric diagnosis manual, the Diagnostic Manual—Intellectual Disability 2, specific to people with IDD (and analogous to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition) provides modification of diagnostic criteria to account for patients who have difficulty articulating their internal emotional state and inner thoughts.22

Continue to: Problematic behaviors
Problematic behaviors that are not features of a bona fide psychiatric disorder are often best understood through functional behavioral analysis, which examines antecedents and consequences of problematic behaviors and identifies their predictable outcomes, such as gaining attention, avoiding a task, or securing a desired item. Rather than being given a prescription for psychoactive medication, many adult patients with IDD and problematic behaviors might be best served by having you order consultation with a certified behavior analyst. The analyst will conduct an evaluation and, along with family or residential staff and the patient, craft a behavioral support plan to address core drivers of the undesired behavior. Behavioral support plans might be enriched by multidisciplinary input from a speech and language pathologist, habilitation professionals, occupational and physical therapists, a neuropsychologist, and others.23
Resources to help you address the physical, mental, and behavioral health problems of these patients are available online through Vanderbilt Kennedy Center’s “Toolkit for primary care providers” (https://iddtoolkit.vkcsites.org).
CASE
During your examination, you review Ms. S’s vital signs, including body mass index (BMI). You calculate that she is morbidly obese—BMI, 37—in the setting of a known comorbidity, diabetes.
Ms. S tells you that she is interested in having a healthy lifestyle, but feels frustrated because she does not know how to make the necessary changes. You discuss with her how some medications, including risperidone, can promote weight gain, and that it is important for her mental health provider to carefully reassess whether she needs to continue the drug.
Weight management in a patient population that tends to be sedentary
Patients with IDD are more likely to live a sedentary lifestyle. Compared to adults who do not have IDD, adults with IDD—especially women and patients with Down syndrome—are reported to have a higher prevalence of obesity.24
Continue to: As in the general population...
As in the general population, the greatest success in weight management involves multidisciplinary treatment, including nutritional support, physical activity, behavioral changes, and close follow-up. The importance of such an approach was borne out by the findings of a randomized controlled trial in which a multicomponent intervention—an energy-reduced diet, physical activity, and behavioral sessions—delivered to participants or their caregivers during monthly visits produced clinically meaningful 6-month weight loss.25 Health-promoting behavioral interventions that rely on a dyadic strategy, such as peer health coaches (ie, people with IDD who have been trained as a health coach) or mentors (IDD staff trained as a health coach), might be more successful at changing health behaviors among patients with IDD than traditional office-based, individual patient education and counseling.26
Similarly, undesired weight loss demands careful evaluation and management because such loss can reflect a medically significant condition, such as gastroesophageal reflux, constipation, dysphagia, neglect, and cancer.27
Boosting the amount and effectiveness of physical activity
Young people with IDD participate in physical activity less often than their neurotypical peers; as a result, they tend to be less fit and have a higher prevalence of obesity.28 Based on a meta-analysis, interventions that focus on sport and movement skills training, such as soccer, basketball, and ball-throwing programs, might be more effective than general physical activity programs.28 In addition to year-round sports training and athletic competitions, Special Olympics conducts vital health screenings of athletes and supports community-based initiatives that address bias against patients with IDD, promote inclusion, and foster social relationships (www.specialolympics.org/our-work/inclusive-health?locale=en).
Emphasize regular activity. In adulthood, fewer than 10% of patients with IDD exercise regularly.21 According to the second edition of Physical Activity Guidelines for Americans,29 “all adults, with or without a disability, should get at least 150 minutes of aerobic physical activity a week. Activities can be broken down into smaller amounts, such as about 25 minutes a day every day.”30 Supplementation with muscle-strengthening activities (eg, yoga, weight training, and resistance-band training) provides further health benefit, such as improvement in posture and prevention of future injury.31 An ideal exercise program proposed by Tyler and Baker is based on a daily, “3-2-1” schedule (ie, of every hour of activity, 30 minutes should be of aerobic exercise; 20 minutes, of strength building; and 10 minutes, of flexibility).11 By participating in any type of physical activity, there is potential for considerable health benefit in reducing psychosocial stressors, improving mental health, counteracting metabolic syndromes, and, ultimately, reducing morbidity and mortality related to physical inactivity.
CASE
With permission from Ms. S, you send your progress notes by fax to her mental health provider at the community mental health center and request a call to discuss her case—in particular, to examine potential alternatives to risperidone. With Ms. S’s input, you also co-create an exercise prescription that includes a daily 20-minute walking program with her mother.
At the follow-up visit that is scheduled in 3 months, you anticipate adding a resistance component and balance activity to the exercise prescription to enrich Ms. S’s physical activity regimen.
CORRESPONDENCE
Carl V. Tyler Jr., MD, 14601 Detroit Avenue, Lakewood, OH, 44107; [email protected]
1. Sullivan WF, Diepstra H, Heng J, et al. Primary care of adults with intellectual and developmental disabilities: 2018 Canadian consensus guidelines. Can Fam Physician. 2018;64:254-279.
2. World Health Organization. International Classification of Impairments, Disabilities, and Handicaps: A Manual of Classification Relating to the Consequences of Disease. May 1980. Accessed May 27, 2021. https://apps.who.int/iris/bitstream/handle/10665/41003/9241541261_eng.pdf?sequence=1&isAllowed=y
3. Berens J, Wozow C, Peacock C. Transition to adult care. Phys Med Rehabil Clin N Am. 2020;31:159-170. doi:10.1016/j.pmr.2019.09.004
4. American Academy of Pediatrics; American Academy of Family Physicians; American College of Physicians; Transitions Clinical Report Authoring Group; Cooley WC, Sagerman PJ. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128:182-200. doi:10.1542/peds.2011-0969
5. Dressler PB, Nguyen TK, Moody EJ, et al. Use of transition resources by primary care providers for youth with intellectual and developmental disabilities. Intellect Dev Disabil. 2018;56:56-68. doi:10.1352/1934-9556-56.1.56
6. The National Alliance to Advance Adolescent Health. Six Core Elements of Health Care Transition.™ Got Transition website. Accessed May 27, 2021. www.gottransition.org
7. Schmidt A, Ilango SM, McManus MA, et al. Outcomes of pediatric to adult health care transition interventions: an updated systematic review. J Pediatr Nurs. 2020; 51:92-107. doi: 10.1016/j.pedn.2020.01.002
8. Keith JM, Bennetto L, Rogge RD. The relationship between contact and attitudes: reducing prejudice toward individuals with intellectual and developmental disabilities. Res Dev Disabil. 2015;47:14-26. doi:10.1016/j.ridd.2015.07.032
9. United Spinal Association. Disability Etiquette: Tips on Interacting With People With Disabilities. 2015. Accessed June 9, 2021. www.unitedspinal.org/pdf/DisabilityEtiquette.pdf
10. Nathawad R, Hanks C. Optimizing the office visit for adolescents with special health care needs. Curr Probl Pediatr Adolesc Health Care. 2017;47:182-189. doi:10.1016/j.cppeds.2017.07.002
11. Tyler CV, Baker S. Intellectual Disabilities at Your Fingertips: A Health Care Resource. High Tide Press; 2009.
12. Williamson HJ, Perkins EA. Family caregivers of adults with intellectual and developmental disabilities: outcomes associated with U.S. services and supports. Intellect Dev Disabil. 2014;52:147-159. doi: 10.1352/1934-9556-52.2.147
13. Robertson J, Hatton C, Emerson E, et al. The impact of health checks for people with intellectual disabilities: an updated systematic review of evidence. Res Dev Disabil. 2014;35:2450-2462. doi:10.1016/j.ridd.2014.06.007
14. Perry J, Felce D, Kerr M, et al. Contact with primary care: the experience of people with intellectual disabilities. J Appl Res Intellect Disabil. 2014;27:200-211. doi: 10.1111/jar.12072
15. Recommendation topics. United States Preventive Services Task Force website. 2020. Accessed May 27, 2021. www.uspreventiveservicestaskforce.org
16. Developmental Disabilities Primary Care Initiative. Tools for the Primary Care of People with Developmental Disabilities. 1st ed. MUMS Guideline Clearinghouse; 2011.
17. Jang W, Kim Y, Han E, et al. Chromosomal microarray analysis as a first-tier clinical diagnostic test in patients with developmental delay/intellectual disability, autism spectrum disorders, and multiple congenital anomalies: a prospective multicenter study in Korea. Ann Lab Med. 2019;39:299-310. doi:10.3343/alm.2019.39.3.299
18. Shireman TI, Reichard A, Nazir N, et al. Quality of diabetes care for adults with developmental disabilities. Disabil Health J. 2010;3:179-185. doi:10.1016/j.dhjo.2009.10.004
19. Cyrus AC, Royer J, Carroll DD, et al. Anti-hypertensive medication use and actors related to adherence among adults with intellectual and developmental disabilities. Am J Intellect Dev Disabil. 2019;124:248-262. doi:10.1352/1944-7558-124.3.248
20. IDD/MI diagnosis. National Association for the Dually Diagnosed (NADD) website. 2019. Accessed May 27, 2021. https://thenadd.org/idd-mi-diagnosis
21. Matson JL, Mayville EA, Bielecki J, et al. Reliability of the Matson Evaluation of Drug Side Effects Scale (MEDS). Res Dev Disabil. 1998;19:501-506. doi:10.1016/s0891-4222(98)00021-3
22. Fletcher R, Barnhill J, Cooper SA. (2017). Diagnostic Manual-Intellectual Disability: A Textbook of Diagnosis of Mental Disorders in Persons with Intellectual Disability. 2nd ed. National Association for the Dually Diagnosed (NADD); 2017.
23. Marrus N, Hall L. Intellectual disability and language disorder. Child Adolesc Psychiatr Clin N Am. 2017;26:539-554. doi:10.1016/j.chc.2017.03.001
24. Rimmer JH, Yamaki K. Obesity and intellectual disability. Ment Retard Dev Disabil Res Rev. 2006;12;22-7. doi: 10.1002/mrdd.20091
25. Ptomey LT, Saunders RR, Saunders M, et al. Weight management in adults with intellectual and developmental disabilities: a randomized controlled trial of two dietary approaches. J Appl Res Intellect Disabil. 2018;31(suppl 1):82-96. doi:10.1111/jar.12348
26. Marks B, Sisirak J, Magallanes R, et al. Effectiveness of a HealthMessages peer-to-peer program for people with intellectual and developmental disabilities. Intellect Dev Disabil. 2019;57:242-258. doi:10.1352/1934-9556-57.3.242
27. Escudé C. Clinical Pearls in IDD Health care. HRS, Inc; 2020.
28. Kapsal NJ, Dicke T, Morin AJS, et al. Effects of physical activity on the physical and psychosocial health of youth with intellectual disabilities: a systematic review and meta-analysis. J Phys Act Health. 2019;16:1187-1195. doi:10.1123/jpah.2018-0675
29. Physical Activity Guidelines for Americans. 2nd ed. US Department of Health and Human Services; 2018. Accessed May 29, 2021. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
30. National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention. Physical activity for people with disability. September 2020. Accessed May 27, 2021. www.cdc.gov/ncbddd/disabilityandhealth/features/physical-activity-for-all.html
31. Introduction to strengthening exercises. National Center on Health, Physical Activity and Disability (NCHPAD). 2020. Accessed May 27, 2021. www.nchpad.org/374/2096/Strengthening~Exercises
1. Sullivan WF, Diepstra H, Heng J, et al. Primary care of adults with intellectual and developmental disabilities: 2018 Canadian consensus guidelines. Can Fam Physician. 2018;64:254-279.
2. World Health Organization. International Classification of Impairments, Disabilities, and Handicaps: A Manual of Classification Relating to the Consequences of Disease. May 1980. Accessed May 27, 2021. https://apps.who.int/iris/bitstream/handle/10665/41003/9241541261_eng.pdf?sequence=1&isAllowed=y
3. Berens J, Wozow C, Peacock C. Transition to adult care. Phys Med Rehabil Clin N Am. 2020;31:159-170. doi:10.1016/j.pmr.2019.09.004
4. American Academy of Pediatrics; American Academy of Family Physicians; American College of Physicians; Transitions Clinical Report Authoring Group; Cooley WC, Sagerman PJ. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128:182-200. doi:10.1542/peds.2011-0969
5. Dressler PB, Nguyen TK, Moody EJ, et al. Use of transition resources by primary care providers for youth with intellectual and developmental disabilities. Intellect Dev Disabil. 2018;56:56-68. doi:10.1352/1934-9556-56.1.56
6. The National Alliance to Advance Adolescent Health. Six Core Elements of Health Care Transition.™ Got Transition website. Accessed May 27, 2021. www.gottransition.org
7. Schmidt A, Ilango SM, McManus MA, et al. Outcomes of pediatric to adult health care transition interventions: an updated systematic review. J Pediatr Nurs. 2020; 51:92-107. doi: 10.1016/j.pedn.2020.01.002
8. Keith JM, Bennetto L, Rogge RD. The relationship between contact and attitudes: reducing prejudice toward individuals with intellectual and developmental disabilities. Res Dev Disabil. 2015;47:14-26. doi:10.1016/j.ridd.2015.07.032
9. United Spinal Association. Disability Etiquette: Tips on Interacting With People With Disabilities. 2015. Accessed June 9, 2021. www.unitedspinal.org/pdf/DisabilityEtiquette.pdf
10. Nathawad R, Hanks C. Optimizing the office visit for adolescents with special health care needs. Curr Probl Pediatr Adolesc Health Care. 2017;47:182-189. doi:10.1016/j.cppeds.2017.07.002
11. Tyler CV, Baker S. Intellectual Disabilities at Your Fingertips: A Health Care Resource. High Tide Press; 2009.
12. Williamson HJ, Perkins EA. Family caregivers of adults with intellectual and developmental disabilities: outcomes associated with U.S. services and supports. Intellect Dev Disabil. 2014;52:147-159. doi: 10.1352/1934-9556-52.2.147
13. Robertson J, Hatton C, Emerson E, et al. The impact of health checks for people with intellectual disabilities: an updated systematic review of evidence. Res Dev Disabil. 2014;35:2450-2462. doi:10.1016/j.ridd.2014.06.007
14. Perry J, Felce D, Kerr M, et al. Contact with primary care: the experience of people with intellectual disabilities. J Appl Res Intellect Disabil. 2014;27:200-211. doi: 10.1111/jar.12072
15. Recommendation topics. United States Preventive Services Task Force website. 2020. Accessed May 27, 2021. www.uspreventiveservicestaskforce.org
16. Developmental Disabilities Primary Care Initiative. Tools for the Primary Care of People with Developmental Disabilities. 1st ed. MUMS Guideline Clearinghouse; 2011.
17. Jang W, Kim Y, Han E, et al. Chromosomal microarray analysis as a first-tier clinical diagnostic test in patients with developmental delay/intellectual disability, autism spectrum disorders, and multiple congenital anomalies: a prospective multicenter study in Korea. Ann Lab Med. 2019;39:299-310. doi:10.3343/alm.2019.39.3.299
18. Shireman TI, Reichard A, Nazir N, et al. Quality of diabetes care for adults with developmental disabilities. Disabil Health J. 2010;3:179-185. doi:10.1016/j.dhjo.2009.10.004
19. Cyrus AC, Royer J, Carroll DD, et al. Anti-hypertensive medication use and actors related to adherence among adults with intellectual and developmental disabilities. Am J Intellect Dev Disabil. 2019;124:248-262. doi:10.1352/1944-7558-124.3.248
20. IDD/MI diagnosis. National Association for the Dually Diagnosed (NADD) website. 2019. Accessed May 27, 2021. https://thenadd.org/idd-mi-diagnosis
21. Matson JL, Mayville EA, Bielecki J, et al. Reliability of the Matson Evaluation of Drug Side Effects Scale (MEDS). Res Dev Disabil. 1998;19:501-506. doi:10.1016/s0891-4222(98)00021-3
22. Fletcher R, Barnhill J, Cooper SA. (2017). Diagnostic Manual-Intellectual Disability: A Textbook of Diagnosis of Mental Disorders in Persons with Intellectual Disability. 2nd ed. National Association for the Dually Diagnosed (NADD); 2017.
23. Marrus N, Hall L. Intellectual disability and language disorder. Child Adolesc Psychiatr Clin N Am. 2017;26:539-554. doi:10.1016/j.chc.2017.03.001
24. Rimmer JH, Yamaki K. Obesity and intellectual disability. Ment Retard Dev Disabil Res Rev. 2006;12;22-7. doi: 10.1002/mrdd.20091
25. Ptomey LT, Saunders RR, Saunders M, et al. Weight management in adults with intellectual and developmental disabilities: a randomized controlled trial of two dietary approaches. J Appl Res Intellect Disabil. 2018;31(suppl 1):82-96. doi:10.1111/jar.12348
26. Marks B, Sisirak J, Magallanes R, et al. Effectiveness of a HealthMessages peer-to-peer program for people with intellectual and developmental disabilities. Intellect Dev Disabil. 2019;57:242-258. doi:10.1352/1934-9556-57.3.242
27. Escudé C. Clinical Pearls in IDD Health care. HRS, Inc; 2020.
28. Kapsal NJ, Dicke T, Morin AJS, et al. Effects of physical activity on the physical and psychosocial health of youth with intellectual disabilities: a systematic review and meta-analysis. J Phys Act Health. 2019;16:1187-1195. doi:10.1123/jpah.2018-0675
29. Physical Activity Guidelines for Americans. 2nd ed. US Department of Health and Human Services; 2018. Accessed May 29, 2021. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
30. National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention. Physical activity for people with disability. September 2020. Accessed May 27, 2021. www.cdc.gov/ncbddd/disabilityandhealth/features/physical-activity-for-all.html
31. Introduction to strengthening exercises. National Center on Health, Physical Activity and Disability (NCHPAD). 2020. Accessed May 27, 2021. www.nchpad.org/374/2096/Strengthening~Exercises
PRACTICE RECOMMENDATIONS
› Provide young people who have an intellectual or other developmental disability (IDD) with a defined, explicit process for making the transition into the adult health care system. A
› Conduct an annual comprehensive, systematic health assessment for patients who have IDD to improve detection of serious conditions and sensory impairments. A
› Encourage young people and adults with IDD to participate in regular physical activity to reduce psychosocial stressors and counteract metabolic syndromes. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series