User login
In reply: Menopause, vitamin D, and oral health
In Reply: Dr. Mascitelli and colleagues bring up an excellent point regarding the role of vitamin D. Vitamin D deficiency (and insufficiency) is such a widespead problem that it deserves attention in both dental and medical circles, and to be fair, it deserves an article of its own. Low vitamin D has been associated with bone loss and an increased risk for certain cancers and other chronic diseases.1 The literature also suggests that low levels of vitamin D are associated with periodontal disease,2 and that supplementation with vitamin D (and calcium) leads to better periodontal health.3,4 However, since vitamin D supplementation is not a recognized way to treat periodontitis, mentioning it with therapies adjudicated as treatment modalities (such as removal of biofilm, which we stressed in our paper) risks misinterpretation by clinicians less versed in periodontal and dental conditions in general.
Nevertheless, the comment brings to light that medical, dental, and nutritional colleagues are very interested in learning more about the pathophysiologic commonalities in the diseases we treat and in a common postmenopausal patient cohort. Our paper focused more closely on what periodontitis is, and on the more primary etiologic pathophysiology—what common resorptive pathways it shares with osteoporosis in the postmenopausal cohort, and biofilm, the primary etiology of periodontitis. But there is need for more discussion and research into bone development (during childhood and adolescence as well) and the role of nutrition during all stages of life.
- Holick M. Vitamin D deficiency. N Eng J Med 2007; 357:266–281.
- Dietrich T, Joshipura KJ, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am J Clin Nutr 2004; 80:108–113.
- Miley DD, Garcia MN, Hildebolt CF, et al. Cross-sectional study of vitamin d and calcium supplementation effects on chronic periodontitis. J Periodontol 2009; 80:1433–1439.
- Amano Y, Komiyama K, Makishima M. Vitamin D and periodontal disease. J Oral Sci 2009; 51:11–20.
In Reply: Dr. Mascitelli and colleagues bring up an excellent point regarding the role of vitamin D. Vitamin D deficiency (and insufficiency) is such a widespead problem that it deserves attention in both dental and medical circles, and to be fair, it deserves an article of its own. Low vitamin D has been associated with bone loss and an increased risk for certain cancers and other chronic diseases.1 The literature also suggests that low levels of vitamin D are associated with periodontal disease,2 and that supplementation with vitamin D (and calcium) leads to better periodontal health.3,4 However, since vitamin D supplementation is not a recognized way to treat periodontitis, mentioning it with therapies adjudicated as treatment modalities (such as removal of biofilm, which we stressed in our paper) risks misinterpretation by clinicians less versed in periodontal and dental conditions in general.
Nevertheless, the comment brings to light that medical, dental, and nutritional colleagues are very interested in learning more about the pathophysiologic commonalities in the diseases we treat and in a common postmenopausal patient cohort. Our paper focused more closely on what periodontitis is, and on the more primary etiologic pathophysiology—what common resorptive pathways it shares with osteoporosis in the postmenopausal cohort, and biofilm, the primary etiology of periodontitis. But there is need for more discussion and research into bone development (during childhood and adolescence as well) and the role of nutrition during all stages of life.
In Reply: Dr. Mascitelli and colleagues bring up an excellent point regarding the role of vitamin D. Vitamin D deficiency (and insufficiency) is such a widespead problem that it deserves attention in both dental and medical circles, and to be fair, it deserves an article of its own. Low vitamin D has been associated with bone loss and an increased risk for certain cancers and other chronic diseases.1 The literature also suggests that low levels of vitamin D are associated with periodontal disease,2 and that supplementation with vitamin D (and calcium) leads to better periodontal health.3,4 However, since vitamin D supplementation is not a recognized way to treat periodontitis, mentioning it with therapies adjudicated as treatment modalities (such as removal of biofilm, which we stressed in our paper) risks misinterpretation by clinicians less versed in periodontal and dental conditions in general.
Nevertheless, the comment brings to light that medical, dental, and nutritional colleagues are very interested in learning more about the pathophysiologic commonalities in the diseases we treat and in a common postmenopausal patient cohort. Our paper focused more closely on what periodontitis is, and on the more primary etiologic pathophysiology—what common resorptive pathways it shares with osteoporosis in the postmenopausal cohort, and biofilm, the primary etiology of periodontitis. But there is need for more discussion and research into bone development (during childhood and adolescence as well) and the role of nutrition during all stages of life.
- Holick M. Vitamin D deficiency. N Eng J Med 2007; 357:266–281.
- Dietrich T, Joshipura KJ, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am J Clin Nutr 2004; 80:108–113.
- Miley DD, Garcia MN, Hildebolt CF, et al. Cross-sectional study of vitamin d and calcium supplementation effects on chronic periodontitis. J Periodontol 2009; 80:1433–1439.
- Amano Y, Komiyama K, Makishima M. Vitamin D and periodontal disease. J Oral Sci 2009; 51:11–20.
- Holick M. Vitamin D deficiency. N Eng J Med 2007; 357:266–281.
- Dietrich T, Joshipura KJ, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am J Clin Nutr 2004; 80:108–113.
- Miley DD, Garcia MN, Hildebolt CF, et al. Cross-sectional study of vitamin d and calcium supplementation effects on chronic periodontitis. J Periodontol 2009; 80:1433–1439.
- Amano Y, Komiyama K, Makishima M. Vitamin D and periodontal disease. J Oral Sci 2009; 51:11–20.
How menopause affects oral health, and what we can do about it
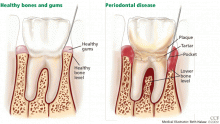
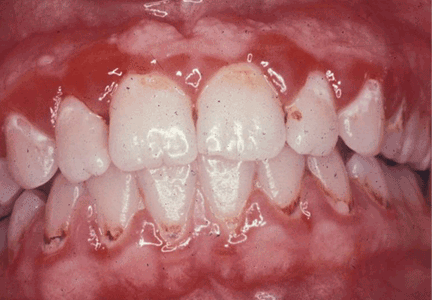
Menopause can bring oral health problems that physicians ought to keep in mind. The same processes that lead to loss of bone in the spine and hips can also lead to loss of the alveolar bone of the jaws, resulting in periodontal disease, loose teeth, and tooth loss. Although the mouth is traditionally the dentist’s responsibility, patients may need encouragement from their physicians to practice good oral hygiene and to see their dentists, and should be referred to a periodontist at the first sign of periodontal disease.
Moreover, bisphosphonates, the class of drugs most often prescribed for osteoporosis, have been linked by case reports (unfairly, we believe) to osteonecrosis of the jaw. This low-evidence-level information, its far-reaching interpretation, and misinformation in the lay media about hormonal changes associated with menopause have led to confusion among women; for clarification and reliable information, they are driven to ask their physicians challenging questions related to oral health.
This article reviews the published studies of the association between menopause and periodontal disease, specifically, the effects of hormonal changes, osteoporosis, and bisphosphonate use on the periodontal status of postmenopausal women. We will highlight the interrelationship of dental health and postmenopausal health and underscore the need for cross-communication and patient referral between physicians and dentists.
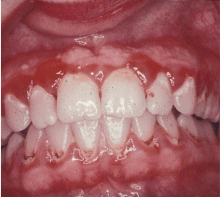
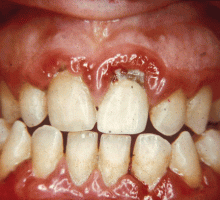
GINGIVITIS CAN PROGRESS TO PERIODONTITIS
AS ESTROGEN DECLINES, SO DO THE BONES AND, MAYBE, THE TEETH
The rate of bone loss in postmenopausal women predicts tooth loss—for every 1%-per-year decrease in whole-body bone mineral density, the risk of tooth loss increases more than four times.2 In fact, Kribbs3 found that women with severe osteoporosis were three times more likely than healthy, age-matched controls to be edentulous (ie, to have fewer teeth).
Although a number of studies have found that the density of the alveolar bone in the mandible correlated with the density of the bone in the rest of the skeleton and that generalized bone loss may render the jaw susceptible to accelerated alveolar bone resorption,3–11 these findings are not universal. In a longitudinal study, Famili et al12 found no association between systemic bone loss, periodontal disease, and edentulism. This shows that the relationship between alveolar bone loss and systemic bone loss is multifactorial and not yet fully understood.13
Nevertheless, the American Academy of Periodontology considers osteoporosis to be a risk factor for periodontal disease.10 In fact, alveolar bone loss has been related not only to osteoporosis but also to osteopenia.14
Bone mineral density has also been studied in relation to the loss of periodontal ligament—the collagenous attachment of tooth to bone. Klemetti et al15 found that healthy postmenopausal women with high bone mineral density seemed to retain teeth more readily than those with low bone density or those with osteoporosis, even if they had deep periodontal pockets (a sign of periodontal disease). These findings were reiterated when osteoporotic women were found to have significantly greater loss of attachment compared with nonosteoporotic women.7
However, Hildebolt16 reported that loss of tooth attachment correlated with tooth loss but not with the density of the vertebrae or the proximal femur. This study called into question the findings of the previous studies and provoked debate.
Tezal et al17 found that low bone mineral density was related to the loss of interproximal alveolar bone (the alveolar bone between adjacent teeth) and, to a lesser extent, ligamentous attachment loss. These data implicated osteoporosis as a possible risk indicator for periodontal disease in white women. (This study was limited to white women because of different demographics in the incidence of osteoporosis.)
Another study showed only a weak correlation between changes in alveolar bone height (in periodontal disease, bone height decreases) and attachment levels. Although a correlation might be present, the relationship was complex and required further examination. The authors found no clear association between clinical attachment levels and bone mineral density in the lumbar spine, but they recognized that attachment loss often precedes the loss of alveolar bone by a significant time period.13
Several studies have found a possible relationship between the bone density in the jaw and the density in the rest of the skeleton. It appears that loss of bone mineral density in the hip, wrist, and lumbar areas is correlated with low density in the mandible. Taguchi et al18 reported that the density in the lumbar spine correlated with the density of the mandibular cortex in early menopause, and with the density of both the cortex and cancellous bone in later menopause.
But whatever the statistical measurement, the susceptibility to progressive periodontitis increases after menopause, and the primary cause is bacterial plaque. The best hedge against this increased susceptibility is regular dental care to remove bacterial plaque biofilm under the gum-line.
HORMONE THERAPY PRESERVES BONE IN THE JAW
Hormones have long been recognized as having some role in periodontal disease.
Payne et al19 reported that postmenopausal women who were estrogen-deficient had a higher frequency of sites with a net loss of alveolar bone density at follow-up. Furthermore, estrogen-deficient women undergoing supportive periodontal therapy following treatment of moderate to severe periodontitis had three times as many sites losing more than 0.4 mm of interproximal alveolar bone height. Patients who had sufficient estrogen levels did not lose bone during 1 year of follow-up.20
Estrogen replacement improves bone density in postmenopausal women. In a 3-year randomized trial in postmenopausal women with moderate or advanced periodontal disease, estrogen therapy significantly increased alveolar bone mass compared with placebo (P = .04), and it increased bone density in the femur but not the lumbar spine.21 Furthermore, women receiving hormonal therapy had significantly less gingival inflammation, lower plaque scores, and less loss of attachment.
On the other hand, a report by Albandar and Kingman22 suggested that women who comply with hormonal therapy also comply with oral hygiene instructions. This compliance could explain the lower gingival inflammation scores, lower plaque scores, and lesser loss of attachment.
Norderyd et al,23 in a cross-sectional study, found less periodontal disease in postmenopausal women who were on estrogen therapy than in those who were not, although the difference was not statistically significant.
In a 5-year longitudinal study of 69 postmenopausal women receiving estrogen, a moderate but significant relationship was found between bone mineral density of the lumbar spine and the mandible, and estrogen replacement therapy had a positive effect on the mandibular bone mass.24
In a longitudinal study of 24 postmenopausal women, estrogen-deficient women had a mean net loss of alveolar bone density over time, while estrogen-sufficient women had a mean net gain, suggesting that estrogen deficiency may be a risk factor for alveolar bone loss.20 More-recent studies had similar findings. A cross-sectional study by Meisel et al25 found that hormone therapy significantly reduced the extent of clinical attachment loss and, hence, periodontal disease.
The findings of these studies are generally consistent, suggesting that estrogen builds up mandibular bone mass and attenuates the severity of periodontal disease in postmenopausal women.26
DOES ESTROGEN THERAPY PROTECT THE TEETH?
Studies of the Leisure World,27 Framingham,28 and Nurses Health Study29 cohorts suggest that hormone therapy protects against tooth loss in postmenopausal women.
On the other hand, Taguchi et al30 evaluated more than 300 postmenopausal Japanese women and found no significant difference in the total number of teeth between estrogen users and nonusers. The population in this study was younger than in the other studies mentioned above,27–29 which may explain the negative finding. However, the duration of estrogen use was significantly associated with the total number of teeth remaining, independent of age.30 Meisel et al25 reported that women receiving hormonal therapy had more teeth, though the difference was not significant.
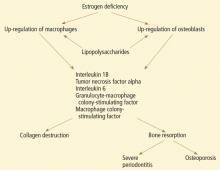
CYTOKINES, PERIODONTITIS, AND SKELETAL BONE LOSS
Studies suggest that low estrogen production after menopause is associated with increased production of interleukin 1 (IL-1), IL-6, IL-8, IL-10, tumor necrosis factor alpha, granulocyte colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor, which stimulate mature osteoclasts, modulate bone cell proliferation, and induce resorption of both skeletal and alveolar bone.31–34
In this regard, osteoporosis and periodontitis appear to be mediated by common cytokines. Managing osteoporosis, removing bacterial plaque biofilm with good oral hygiene, and regular dental visits are important in avoiding periodontitis in susceptible women.
BISPHOSPHONATES PROTECT BONE
In the skeleton
Bisphosphonates, the most commonly prescribed therapy for osteoporosis, inhibit systemic bone resorption and reduce the incidence of vertebral and nonvertebral fractures. Among the bisphosphonates, alendronate (Fosamax), risedronate (Actonel), and intravenous zoledronic acid (Reclast) have been shown to reduce the risk of both hip and vertebral fractures, whereas ibandronate (Boniva) has only been shown to decrease the risk of vertebral fracture.36 Specific findings:
- In the Fracture Intervention Trial,37 alendronate reduced the risk of vertebral fracture by 47% and hip fracture by 51% in women with low bone mineral density and previous vertebral fractures.
- In the Hip Intervention Program,38 risedronate decreased the risk of hip fracture by 40% in postmenopausal women 70 to 79 years old with osteoporosis, but not in those 80 years and older, who are at high risk of falls. Risedronate also reduced vertebral fracture risk by 49% after 3 years of treatment.39
- In the Health Outcomes and Reduced Incidence With Zoledronic Acid Once Yearly Recurrent Fracture Trial,40 annual infusion of zoledronic acid after a hip fracture reduced the rates of new clinical vertebral and nonvertebral fractures and death from all causes.
In the jaw
Not surprisingly, recent studies suggest that bisphosphonates slow the resorption of alveolar bone of the maxilla and mandible as well. Alendronate and risedronate, in particular, have been noted to improve periodontal status.41–43 Findings:
- In a cross-sectional study by Palomo et al,41 postmenopausal women with low bone density using risedronate for at least 3 months showed significantly less plaque accumulation, less gingival inflammation, lower probing-depth measurments, less periodontal attachment loss, and greater alveolar bone levels.
- In a double-blind, controlled, prospective study by Rocha et al,42 6 months of alendronate therapy significantly improved periodontal disease as assessed radiographically and clinically in 40 postmenopausal women with established periodontal disease.
- Jeffcoat et al43 reported that 2 years of alendronate treatment significantly reduced alveolar bone loss relative to placebo in patients with low mandibular bone mineral density at baseline but not in those with normal baseline mandibular bone mineral density.
DO BISPHOSPHONATES CAUSE OSTEONECROSIS OF THE JAW?
The intravenous bisphosphonates most commonly used to treat hypercalcemia of malignancy, multiple myeloma, or metastatic bone disease are47:
- Pamidronate (Aredia) 90 mg infused over 2 to 24 hours every 3 to 4 weeks
- Zoledronic acid (Zometa) 4 mg infused over 15 minutes monthly.
The doses of bisphosphonates indicated for the treatment of osteoporosis are much lower,1 eg:
- Alendronate 70 mg by mouth once a week
- Risedronate 35 mg by mouth once a week or 150 mg once a month
- Ibandronate 150 mg by mouth once a month
- Ibandronate 3 mg intravenously every 3 months
- Zoledronic acid 5 mg intravenously once a year.
Moreover, less than 1% of an oral dose is absorbed by the gastrointestinal tract,49 whereas more than 50% of the dose of bisphosphonates given intravenously is bioavailable,50 which may account for the lower incidence of jaw ostenonecrosis with oral agents.
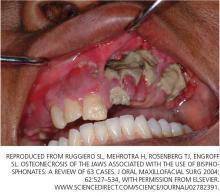
Osteonecrosis of the jaw can occur spontaneously but is more often associated with dental procedures that traumatize bone, such as tooth extraction.51 In a systematic review,45 patients with multiple myeloma and metastatic cancer to the bone who were receiving intravenous bisphosphonates accounted for 94% of published cases. Sixty percent of cases were preceded by dental surgical procedures, and in 39% of cases that occurred spontaneously the lesions were located on bony exostoses, a possible source of trauma. Of 63 cases reported by Ruggiero et al,47 56 patients were receiving intravenous bisphosphonates and 7 were receiving oral bisphosphonates. Older age (> 65 years), chronic systemic steroid use, periodontitis, and prolonged use of bisphosphonates have also been associated with a higher risk of osteonecrosis of the jaw.51
The risk of developing osteonecrosis of the jaw in people taking bisphosphonates in doses recommended by the US Food and Drug Administration for treating osteoporosis is very low (the incidence is calculated at 0.7 per 100,000 person-years of exposure to alendronate).51,52 In a 3-year prospective study in more than 7,000 women with post-menopausal osteoporosis, the incidence of osteonecrosis of the jaw was no different in those treated with zoledronic acid 5 mg intravenously than in those receiving placebo.53 In a randomized, placebo-controlled study of the effect of 2 years of alendronate treatment on alveolar bone loss involving 335 patients with periodontal disease, no cases of osteonecrosis of the jaw were reported.43
The American Dental Association (ADA) released a statement noting that osteonecrosis of the jaw can occur with or without bisphosphonate use.51 To date, a true cause-and-effect relationship between osteonecrosis of the jaw and bisphosphonate use has not been established. Further studies are needed to fully explore this relationship. Our group is currently exploring novel periodontal assessments comparing the oral health of postmenopausal women with osteoporosis who are on no bone therapy vs postmenopausal women with osteoporosis treated with bisphosphonates for 2 or more years.
Discussion of treatment for bisphosphonate-associated osteonecrosis of the jaw is beyond the scope of this article.
REGULAR DENTAL CARE IS ESSENTIAL
Regardless of whether the patient is receiving a bisphosphonate drug, physicians caring for postmenopausal women should be vigilant and encourage their patients to seek regular dental evaluation for prevention and early management of oral disorders. Conversely, dentists should be aware of the potential effects of menopause and its treatments on bone and dental health.
Questions from postmenopausal women can be managed, in part, by returning to the basics suggested by the ADA:
- Regular dental examinations; regular professional cleaning to remove bacterial plaque biofilm under the gum-line where a toothbrush will not reach
- Daily oral hygiene practices to remove biofilm at and above the gum-line including brushing twice daily with an ADA-accepted toothpaste
- Replacing the toothbrush every 3 to 4 months (or sooner if the bristles begin to look frayed)
- Cleaning interproximally (between teeth) with floss or interdental cleaner
- Maintaining a balanced diet
- No smoking.
- North American Menopause Society. Menopause Practice: A Clinician’s Guide. 3rd ed; 2007.
- Krall EA, Garcia RI, Dawson-Hughes B. Increased risk of tooth loss is related to bone loss at the whole body, hip and spine. Calcif Tissue Int 1996; 59:433–437.
- Kribbs PJ. Comparison of mandibular bone in normal and osteoporotic women. J Prosthet Dent 1990; 63:218–222.
- Kribbs PJ, Chesnut CH, Ott SM, Kilcoyne RF. Relationship between mandibular and skeletal bone in an osteoporotic population. J Prosthet Dent 1989; 62:703–707.
- Kribbs PJ, Chestnut CH, Ott SM, Kilcyne RE. Relationship between mandibular and skeletal bone in a population of normal women. J Prosthet Dent 1990; 63:86–89.
- Kribbs PJ, Smith DE, Chestnut CH. Oral findings in osteoporosis. Part II: relationship between residual ridge and alveolar bone resorption and generalized skeletal osteopenia. J Prosthet Dent 1983; 50:719–724.
- von Wowern N, Klausen B, Kollerup G. Osteoporosis: a risk factor in periodontal disease. J Periodontol 1994; 65:1134–1138.
- Wactawski-Wende J, Grossi SG, Trevisan M, et al. The role of osteopenia in oral bone loss and periodontal disease. J Peridontol 1996; 67(suppl 10):1076–1084.
- Ronderos M, Jacobs DR, Himes JH, Pihlstrom BL. Associations of periodontal disease with femoral bone mineral density and estrogen replacement therapy: cross-sectional evaluation of US adults from the NHANES III. J Clin Periodontol 2000; 27:778–86.
- American Dental Association Council on Access, Prevention and Interprofessional Relations. Women’s Oral Health Issues. November 2006.
- Jeffcoat MK, Lewis CE, Reddy MS, Wang CY, Redford M. Post-menopausal bone loss and its relationship to oral bone loss. Periodontol 2000 2000; 23:94–102.
- Famili P, Cauley J, Suzuki JB, Weyant R. Longitudinal study of periodontal disease and edentulism with rates of bone loss in older women. J Periodontol 2005; 76:11–15.
- Pilgram TK, Hildebolt CF, Yokoyama N, et al. Relationships between longitudinal changes in radiographic alveolar bone height and probing depth measurements: data from postmenopausal women. J Periodontol 1999; 70:829–833.
- Jeffcoat MK, Lewis CE, Reddy MS, et al. Oral bone loss and systemic osteopenia, osteoporosis. InMarcus R, Feldman D, Kelsey J, editors. Osteoporosis. New York Academic Press 1996:969–990.
- Klemetti E, Collin HL, Forss H, Markkanen H, Lassila V. Mineral status of skeletal and advanced periodontal disease. J Clin Periodontol 1994; 21:184–188.
- Hildebolt CF. Osteoporosis and oral bone loss. Dentomaxillofac Radiol 1997; 26:3–15.
- Tezal M, Wactawski-Wende J, Grossi SG, Ho AW, Dunford R, Genco RJ. The relationship between bone mineral density and periodontitis in postmenopausal women. J Periodontol 2000; 71:1492–1498.
- Taguchi A, Tanimoto K, Suei Y, Ohama K, Wada T. Relationship between the mandibular and lumbar vertebral bone mineral density at different postmenopausal stages. Dentomaxillofac Radiol 1996; 25:130–135.
- Payne JB, Reinhardt RA, Nummikoski PV, Patil KD. Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporos Int 1999; 10:34–40.
- Payne JB, Zachs NR, Reinhardt RA, Nummikoski PV, Patil K. The association between estrogen status and alveolar bone density changes in postmenopausal women with a history of periodontitis. J Periodontol 1997; 68:24–31.
- Civitelli R, Pilgram TK, Dotson M, et al. Alveolar and postcranial bone density in postmenopausal women receiving hormone/estrogen replacement: a randomized, double blind, placebo-controlled trial. Arch Intern Med 2002; 162:1409–1415.
- Albandar JM, Kingman A. Gingival recession, gingival bleeding, and dental calculus in adults 30 years of age and older in the United States, 1988–1994. J Periodontol 1999; 70:30–43.
- Norderyd OM, Grossi SG, Machtel EE, et al. Periodontal status of women taking postmenopausal estrogen supplementation. J Periodontol 1993; 64:957–962.
- Jacobs R, Ghyselen J, Koninckx P, van Steenberghe D. Long-term bone mass evaluation of mandible and lumbar spine in a group of women receiving hormone replacement therapy. Eur J Oral Sci 1996; 104:10–16.
- Meisel P, Reifenberger J, Haase R, Nauck M, Bandt C, Kocher T. Women are periodontally healthier than men, but why don’t they have more teeth than men? Menopause 2008; 15:270–275.
- Genco RJ, Grossi SG. Is estrogen deficiency a risk factor for periodontal disease? Compend Contin Educ Dent Suppl 1998; 22:S23–S29.
- Paganini-Hill A. The benefits of estrogen replacement therapy on oral health. The Leisure World cohort. Arch Intern Med 1995; 155:2325–2329.
- Krall EA, Dawson-Hughes B, Hannan MT, Wilson PW, Kiel DP. Post-menopausal estrogen replacement and tooth retention. Am J Med 1997; 102:536–542.
- Grodstein F, Colditz GA, Stampfer MJ. Postmenopausal hormone use and tooth loss: a prospective study. J Am Dent Assoc 1996; 127:370–377.
- Taguchi A, Sanada M, Suei Y, et al. Effect of estrogen use on tooth retention, oral bone height, and oral bone porosity in Japanese postmenopausal women. Menopause 2004; 11:556–562.
- Pacifici R. Estrogen, cytokines and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res 1996; 11:1043–1051.
- Pacifici R. Is there a causal role for IL-1 in postmenopausal bone loss? Calcif Tissue Int 1992; 50:295–299.
- Girasole G, Jilka RL, Passeri G, et al. 17 beta-estradiol inhibits interleukin-6 production by bone marrow-derived stromal cells and osteoblasts in vitro: a potential mechanism for the antiosteoporotic effect of estrogens. J Clin Invest 1992; 89:883–891.
- Pacifici R, Brown C, Pusheck E, et al. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci USA 1991; 88:5134–5138.
- Brennan RM, Genco RJ, Wilding GE, Hovey KM, Trevisan M, Wactawski-Wende J. Bacterial species in subgingival plaque and oral bone loss in postmenopausal women. J Periodontol 2007; 78:1051–1061.
- Chestnut CH, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in post-menopausal osteoporosis. J Bone Miner Res 2004; 19:1241–1249.
- Black DM, Cummings SR, Karpf DB, et al. Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures: Fracture Intervention Trial Research Group. Lancet 1996; 348:1535–1541.
- McClung MR, Geusen P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med 2001; 344:333–340.
- Reginster JY, Minne HW, Sorensen OH, et al. Randomized trial of effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 2000; 11:83–91.
- Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007; 357:1799–1809.
- Palomo L, Bissada N, Liu J. Periodontal assessment of postmenopausal women receiving risedronate. Menopause 2005; 12:685–690.
- Rocha ML, Malacara JM, Sánchez-Marin FJ, Vazquez de la Torre CJ, Fajardo ME. Effect of alendronate on periodontal disease in postmenopausal women: a randomized placebo-controlled trial. J Periodontol 2004; 75:1579–1585.
- Jeffcoat MK, Cizza G, Shih WJ, Genco R, Lombardi A. Efficacy of bisphosphonates for the control of alveolar bone loss in periodontitis. J Int Acad Periodontol 2007; 9:70–76.
- Carey JJ, Palomo L. Bisphosphonates and osteonecrosis of the jaw: innocent association or significant risk? Cleve Clin J Med 2008; 75:871–879.
- Woo SB, Hellstein JW, Kalamare JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med 2006; 144:753–761.
- Dodson TB, Raje NS, Caruso PA, Rosenberg AE. Case records of the Massachusetts General Hospital. Case 9–2008. A 65-year-old woman with a nonhealing ulcer of the jaw. N Engl J Med 2008; 358:1283–1291.
- Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofacial Surg 2004; 62:527–534.
- Palomo L, Liu J, Bissada NF. Skeletal bone diseases impact the periodontium: a review of bisphosphonate therapy. Expert Opin Pharmacother 2007; 8:309–315.
- Ezra A, Golomb G. Administration routes and delivery systems of bisphosphonates for the treatment of bone resorption. Adv Drug Deliv Rev 2000; 42:175–195.
- Berenson JR, Rosen L, Vescio R, et al. Pharmacokinetics of pamidronate disodium in patients with cancer with normal or impaired renal function. J Clin Pharmacol 1997; 37:285–290.
- American Dental Association Council on Scientific Affairs. Dental management of patients receiving oral bisphosphonate therapy: expert panel recommendations. J Am Dent Assoc 2006; 137:1144–1150.
- Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofacial Surg 2007; 65:369–376.
- Grbic JT, Landesberg R, Lin SQ, et al; Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly Pivotal Fracture Trial Research Group. Incidence of osteonecrosis of the jaw in women with postmenopausal osteoporosis in the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly Pivotal Fracture Trial. J Am Dent Assoc 2008; 139:32–40.
Menopause can bring oral health problems that physicians ought to keep in mind. The same processes that lead to loss of bone in the spine and hips can also lead to loss of the alveolar bone of the jaws, resulting in periodontal disease, loose teeth, and tooth loss. Although the mouth is traditionally the dentist’s responsibility, patients may need encouragement from their physicians to practice good oral hygiene and to see their dentists, and should be referred to a periodontist at the first sign of periodontal disease.
Moreover, bisphosphonates, the class of drugs most often prescribed for osteoporosis, have been linked by case reports (unfairly, we believe) to osteonecrosis of the jaw. This low-evidence-level information, its far-reaching interpretation, and misinformation in the lay media about hormonal changes associated with menopause have led to confusion among women; for clarification and reliable information, they are driven to ask their physicians challenging questions related to oral health.
This article reviews the published studies of the association between menopause and periodontal disease, specifically, the effects of hormonal changes, osteoporosis, and bisphosphonate use on the periodontal status of postmenopausal women. We will highlight the interrelationship of dental health and postmenopausal health and underscore the need for cross-communication and patient referral between physicians and dentists.
GINGIVITIS CAN PROGRESS TO PERIODONTITIS
AS ESTROGEN DECLINES, SO DO THE BONES AND, MAYBE, THE TEETH
The rate of bone loss in postmenopausal women predicts tooth loss—for every 1%-per-year decrease in whole-body bone mineral density, the risk of tooth loss increases more than four times.2 In fact, Kribbs3 found that women with severe osteoporosis were three times more likely than healthy, age-matched controls to be edentulous (ie, to have fewer teeth).
Although a number of studies have found that the density of the alveolar bone in the mandible correlated with the density of the bone in the rest of the skeleton and that generalized bone loss may render the jaw susceptible to accelerated alveolar bone resorption,3–11 these findings are not universal. In a longitudinal study, Famili et al12 found no association between systemic bone loss, periodontal disease, and edentulism. This shows that the relationship between alveolar bone loss and systemic bone loss is multifactorial and not yet fully understood.13
Nevertheless, the American Academy of Periodontology considers osteoporosis to be a risk factor for periodontal disease.10 In fact, alveolar bone loss has been related not only to osteoporosis but also to osteopenia.14
Bone mineral density has also been studied in relation to the loss of periodontal ligament—the collagenous attachment of tooth to bone. Klemetti et al15 found that healthy postmenopausal women with high bone mineral density seemed to retain teeth more readily than those with low bone density or those with osteoporosis, even if they had deep periodontal pockets (a sign of periodontal disease). These findings were reiterated when osteoporotic women were found to have significantly greater loss of attachment compared with nonosteoporotic women.7
However, Hildebolt16 reported that loss of tooth attachment correlated with tooth loss but not with the density of the vertebrae or the proximal femur. This study called into question the findings of the previous studies and provoked debate.
Tezal et al17 found that low bone mineral density was related to the loss of interproximal alveolar bone (the alveolar bone between adjacent teeth) and, to a lesser extent, ligamentous attachment loss. These data implicated osteoporosis as a possible risk indicator for periodontal disease in white women. (This study was limited to white women because of different demographics in the incidence of osteoporosis.)
Another study showed only a weak correlation between changes in alveolar bone height (in periodontal disease, bone height decreases) and attachment levels. Although a correlation might be present, the relationship was complex and required further examination. The authors found no clear association between clinical attachment levels and bone mineral density in the lumbar spine, but they recognized that attachment loss often precedes the loss of alveolar bone by a significant time period.13
Several studies have found a possible relationship between the bone density in the jaw and the density in the rest of the skeleton. It appears that loss of bone mineral density in the hip, wrist, and lumbar areas is correlated with low density in the mandible. Taguchi et al18 reported that the density in the lumbar spine correlated with the density of the mandibular cortex in early menopause, and with the density of both the cortex and cancellous bone in later menopause.
But whatever the statistical measurement, the susceptibility to progressive periodontitis increases after menopause, and the primary cause is bacterial plaque. The best hedge against this increased susceptibility is regular dental care to remove bacterial plaque biofilm under the gum-line.
HORMONE THERAPY PRESERVES BONE IN THE JAW
Hormones have long been recognized as having some role in periodontal disease.
Payne et al19 reported that postmenopausal women who were estrogen-deficient had a higher frequency of sites with a net loss of alveolar bone density at follow-up. Furthermore, estrogen-deficient women undergoing supportive periodontal therapy following treatment of moderate to severe periodontitis had three times as many sites losing more than 0.4 mm of interproximal alveolar bone height. Patients who had sufficient estrogen levels did not lose bone during 1 year of follow-up.20
Estrogen replacement improves bone density in postmenopausal women. In a 3-year randomized trial in postmenopausal women with moderate or advanced periodontal disease, estrogen therapy significantly increased alveolar bone mass compared with placebo (P = .04), and it increased bone density in the femur but not the lumbar spine.21 Furthermore, women receiving hormonal therapy had significantly less gingival inflammation, lower plaque scores, and less loss of attachment.
On the other hand, a report by Albandar and Kingman22 suggested that women who comply with hormonal therapy also comply with oral hygiene instructions. This compliance could explain the lower gingival inflammation scores, lower plaque scores, and lesser loss of attachment.
Norderyd et al,23 in a cross-sectional study, found less periodontal disease in postmenopausal women who were on estrogen therapy than in those who were not, although the difference was not statistically significant.
In a 5-year longitudinal study of 69 postmenopausal women receiving estrogen, a moderate but significant relationship was found between bone mineral density of the lumbar spine and the mandible, and estrogen replacement therapy had a positive effect on the mandibular bone mass.24
In a longitudinal study of 24 postmenopausal women, estrogen-deficient women had a mean net loss of alveolar bone density over time, while estrogen-sufficient women had a mean net gain, suggesting that estrogen deficiency may be a risk factor for alveolar bone loss.20 More-recent studies had similar findings. A cross-sectional study by Meisel et al25 found that hormone therapy significantly reduced the extent of clinical attachment loss and, hence, periodontal disease.
The findings of these studies are generally consistent, suggesting that estrogen builds up mandibular bone mass and attenuates the severity of periodontal disease in postmenopausal women.26
DOES ESTROGEN THERAPY PROTECT THE TEETH?
Studies of the Leisure World,27 Framingham,28 and Nurses Health Study29 cohorts suggest that hormone therapy protects against tooth loss in postmenopausal women.
On the other hand, Taguchi et al30 evaluated more than 300 postmenopausal Japanese women and found no significant difference in the total number of teeth between estrogen users and nonusers. The population in this study was younger than in the other studies mentioned above,27–29 which may explain the negative finding. However, the duration of estrogen use was significantly associated with the total number of teeth remaining, independent of age.30 Meisel et al25 reported that women receiving hormonal therapy had more teeth, though the difference was not significant.
CYTOKINES, PERIODONTITIS, AND SKELETAL BONE LOSS
Studies suggest that low estrogen production after menopause is associated with increased production of interleukin 1 (IL-1), IL-6, IL-8, IL-10, tumor necrosis factor alpha, granulocyte colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor, which stimulate mature osteoclasts, modulate bone cell proliferation, and induce resorption of both skeletal and alveolar bone.31–34
In this regard, osteoporosis and periodontitis appear to be mediated by common cytokines. Managing osteoporosis, removing bacterial plaque biofilm with good oral hygiene, and regular dental visits are important in avoiding periodontitis in susceptible women.
BISPHOSPHONATES PROTECT BONE
In the skeleton
Bisphosphonates, the most commonly prescribed therapy for osteoporosis, inhibit systemic bone resorption and reduce the incidence of vertebral and nonvertebral fractures. Among the bisphosphonates, alendronate (Fosamax), risedronate (Actonel), and intravenous zoledronic acid (Reclast) have been shown to reduce the risk of both hip and vertebral fractures, whereas ibandronate (Boniva) has only been shown to decrease the risk of vertebral fracture.36 Specific findings:
- In the Fracture Intervention Trial,37 alendronate reduced the risk of vertebral fracture by 47% and hip fracture by 51% in women with low bone mineral density and previous vertebral fractures.
- In the Hip Intervention Program,38 risedronate decreased the risk of hip fracture by 40% in postmenopausal women 70 to 79 years old with osteoporosis, but not in those 80 years and older, who are at high risk of falls. Risedronate also reduced vertebral fracture risk by 49% after 3 years of treatment.39
- In the Health Outcomes and Reduced Incidence With Zoledronic Acid Once Yearly Recurrent Fracture Trial,40 annual infusion of zoledronic acid after a hip fracture reduced the rates of new clinical vertebral and nonvertebral fractures and death from all causes.
In the jaw
Not surprisingly, recent studies suggest that bisphosphonates slow the resorption of alveolar bone of the maxilla and mandible as well. Alendronate and risedronate, in particular, have been noted to improve periodontal status.41–43 Findings:
- In a cross-sectional study by Palomo et al,41 postmenopausal women with low bone density using risedronate for at least 3 months showed significantly less plaque accumulation, less gingival inflammation, lower probing-depth measurments, less periodontal attachment loss, and greater alveolar bone levels.
- In a double-blind, controlled, prospective study by Rocha et al,42 6 months of alendronate therapy significantly improved periodontal disease as assessed radiographically and clinically in 40 postmenopausal women with established periodontal disease.
- Jeffcoat et al43 reported that 2 years of alendronate treatment significantly reduced alveolar bone loss relative to placebo in patients with low mandibular bone mineral density at baseline but not in those with normal baseline mandibular bone mineral density.
DO BISPHOSPHONATES CAUSE OSTEONECROSIS OF THE JAW?
The intravenous bisphosphonates most commonly used to treat hypercalcemia of malignancy, multiple myeloma, or metastatic bone disease are47:
- Pamidronate (Aredia) 90 mg infused over 2 to 24 hours every 3 to 4 weeks
- Zoledronic acid (Zometa) 4 mg infused over 15 minutes monthly.
The doses of bisphosphonates indicated for the treatment of osteoporosis are much lower,1 eg:
- Alendronate 70 mg by mouth once a week
- Risedronate 35 mg by mouth once a week or 150 mg once a month
- Ibandronate 150 mg by mouth once a month
- Ibandronate 3 mg intravenously every 3 months
- Zoledronic acid 5 mg intravenously once a year.
Moreover, less than 1% of an oral dose is absorbed by the gastrointestinal tract,49 whereas more than 50% of the dose of bisphosphonates given intravenously is bioavailable,50 which may account for the lower incidence of jaw ostenonecrosis with oral agents.
Osteonecrosis of the jaw can occur spontaneously but is more often associated with dental procedures that traumatize bone, such as tooth extraction.51 In a systematic review,45 patients with multiple myeloma and metastatic cancer to the bone who were receiving intravenous bisphosphonates accounted for 94% of published cases. Sixty percent of cases were preceded by dental surgical procedures, and in 39% of cases that occurred spontaneously the lesions were located on bony exostoses, a possible source of trauma. Of 63 cases reported by Ruggiero et al,47 56 patients were receiving intravenous bisphosphonates and 7 were receiving oral bisphosphonates. Older age (> 65 years), chronic systemic steroid use, periodontitis, and prolonged use of bisphosphonates have also been associated with a higher risk of osteonecrosis of the jaw.51
The risk of developing osteonecrosis of the jaw in people taking bisphosphonates in doses recommended by the US Food and Drug Administration for treating osteoporosis is very low (the incidence is calculated at 0.7 per 100,000 person-years of exposure to alendronate).51,52 In a 3-year prospective study in more than 7,000 women with post-menopausal osteoporosis, the incidence of osteonecrosis of the jaw was no different in those treated with zoledronic acid 5 mg intravenously than in those receiving placebo.53 In a randomized, placebo-controlled study of the effect of 2 years of alendronate treatment on alveolar bone loss involving 335 patients with periodontal disease, no cases of osteonecrosis of the jaw were reported.43
The American Dental Association (ADA) released a statement noting that osteonecrosis of the jaw can occur with or without bisphosphonate use.51 To date, a true cause-and-effect relationship between osteonecrosis of the jaw and bisphosphonate use has not been established. Further studies are needed to fully explore this relationship. Our group is currently exploring novel periodontal assessments comparing the oral health of postmenopausal women with osteoporosis who are on no bone therapy vs postmenopausal women with osteoporosis treated with bisphosphonates for 2 or more years.
Discussion of treatment for bisphosphonate-associated osteonecrosis of the jaw is beyond the scope of this article.
REGULAR DENTAL CARE IS ESSENTIAL
Regardless of whether the patient is receiving a bisphosphonate drug, physicians caring for postmenopausal women should be vigilant and encourage their patients to seek regular dental evaluation for prevention and early management of oral disorders. Conversely, dentists should be aware of the potential effects of menopause and its treatments on bone and dental health.
Questions from postmenopausal women can be managed, in part, by returning to the basics suggested by the ADA:
- Regular dental examinations; regular professional cleaning to remove bacterial plaque biofilm under the gum-line where a toothbrush will not reach
- Daily oral hygiene practices to remove biofilm at and above the gum-line including brushing twice daily with an ADA-accepted toothpaste
- Replacing the toothbrush every 3 to 4 months (or sooner if the bristles begin to look frayed)
- Cleaning interproximally (between teeth) with floss or interdental cleaner
- Maintaining a balanced diet
- No smoking.
Menopause can bring oral health problems that physicians ought to keep in mind. The same processes that lead to loss of bone in the spine and hips can also lead to loss of the alveolar bone of the jaws, resulting in periodontal disease, loose teeth, and tooth loss. Although the mouth is traditionally the dentist’s responsibility, patients may need encouragement from their physicians to practice good oral hygiene and to see their dentists, and should be referred to a periodontist at the first sign of periodontal disease.
Moreover, bisphosphonates, the class of drugs most often prescribed for osteoporosis, have been linked by case reports (unfairly, we believe) to osteonecrosis of the jaw. This low-evidence-level information, its far-reaching interpretation, and misinformation in the lay media about hormonal changes associated with menopause have led to confusion among women; for clarification and reliable information, they are driven to ask their physicians challenging questions related to oral health.
This article reviews the published studies of the association between menopause and periodontal disease, specifically, the effects of hormonal changes, osteoporosis, and bisphosphonate use on the periodontal status of postmenopausal women. We will highlight the interrelationship of dental health and postmenopausal health and underscore the need for cross-communication and patient referral between physicians and dentists.
GINGIVITIS CAN PROGRESS TO PERIODONTITIS
AS ESTROGEN DECLINES, SO DO THE BONES AND, MAYBE, THE TEETH
The rate of bone loss in postmenopausal women predicts tooth loss—for every 1%-per-year decrease in whole-body bone mineral density, the risk of tooth loss increases more than four times.2 In fact, Kribbs3 found that women with severe osteoporosis were three times more likely than healthy, age-matched controls to be edentulous (ie, to have fewer teeth).
Although a number of studies have found that the density of the alveolar bone in the mandible correlated with the density of the bone in the rest of the skeleton and that generalized bone loss may render the jaw susceptible to accelerated alveolar bone resorption,3–11 these findings are not universal. In a longitudinal study, Famili et al12 found no association between systemic bone loss, periodontal disease, and edentulism. This shows that the relationship between alveolar bone loss and systemic bone loss is multifactorial and not yet fully understood.13
Nevertheless, the American Academy of Periodontology considers osteoporosis to be a risk factor for periodontal disease.10 In fact, alveolar bone loss has been related not only to osteoporosis but also to osteopenia.14
Bone mineral density has also been studied in relation to the loss of periodontal ligament—the collagenous attachment of tooth to bone. Klemetti et al15 found that healthy postmenopausal women with high bone mineral density seemed to retain teeth more readily than those with low bone density or those with osteoporosis, even if they had deep periodontal pockets (a sign of periodontal disease). These findings were reiterated when osteoporotic women were found to have significantly greater loss of attachment compared with nonosteoporotic women.7
However, Hildebolt16 reported that loss of tooth attachment correlated with tooth loss but not with the density of the vertebrae or the proximal femur. This study called into question the findings of the previous studies and provoked debate.
Tezal et al17 found that low bone mineral density was related to the loss of interproximal alveolar bone (the alveolar bone between adjacent teeth) and, to a lesser extent, ligamentous attachment loss. These data implicated osteoporosis as a possible risk indicator for periodontal disease in white women. (This study was limited to white women because of different demographics in the incidence of osteoporosis.)
Another study showed only a weak correlation between changes in alveolar bone height (in periodontal disease, bone height decreases) and attachment levels. Although a correlation might be present, the relationship was complex and required further examination. The authors found no clear association between clinical attachment levels and bone mineral density in the lumbar spine, but they recognized that attachment loss often precedes the loss of alveolar bone by a significant time period.13
Several studies have found a possible relationship between the bone density in the jaw and the density in the rest of the skeleton. It appears that loss of bone mineral density in the hip, wrist, and lumbar areas is correlated with low density in the mandible. Taguchi et al18 reported that the density in the lumbar spine correlated with the density of the mandibular cortex in early menopause, and with the density of both the cortex and cancellous bone in later menopause.
But whatever the statistical measurement, the susceptibility to progressive periodontitis increases after menopause, and the primary cause is bacterial plaque. The best hedge against this increased susceptibility is regular dental care to remove bacterial plaque biofilm under the gum-line.
HORMONE THERAPY PRESERVES BONE IN THE JAW
Hormones have long been recognized as having some role in periodontal disease.
Payne et al19 reported that postmenopausal women who were estrogen-deficient had a higher frequency of sites with a net loss of alveolar bone density at follow-up. Furthermore, estrogen-deficient women undergoing supportive periodontal therapy following treatment of moderate to severe periodontitis had three times as many sites losing more than 0.4 mm of interproximal alveolar bone height. Patients who had sufficient estrogen levels did not lose bone during 1 year of follow-up.20
Estrogen replacement improves bone density in postmenopausal women. In a 3-year randomized trial in postmenopausal women with moderate or advanced periodontal disease, estrogen therapy significantly increased alveolar bone mass compared with placebo (P = .04), and it increased bone density in the femur but not the lumbar spine.21 Furthermore, women receiving hormonal therapy had significantly less gingival inflammation, lower plaque scores, and less loss of attachment.
On the other hand, a report by Albandar and Kingman22 suggested that women who comply with hormonal therapy also comply with oral hygiene instructions. This compliance could explain the lower gingival inflammation scores, lower plaque scores, and lesser loss of attachment.
Norderyd et al,23 in a cross-sectional study, found less periodontal disease in postmenopausal women who were on estrogen therapy than in those who were not, although the difference was not statistically significant.
In a 5-year longitudinal study of 69 postmenopausal women receiving estrogen, a moderate but significant relationship was found between bone mineral density of the lumbar spine and the mandible, and estrogen replacement therapy had a positive effect on the mandibular bone mass.24
In a longitudinal study of 24 postmenopausal women, estrogen-deficient women had a mean net loss of alveolar bone density over time, while estrogen-sufficient women had a mean net gain, suggesting that estrogen deficiency may be a risk factor for alveolar bone loss.20 More-recent studies had similar findings. A cross-sectional study by Meisel et al25 found that hormone therapy significantly reduced the extent of clinical attachment loss and, hence, periodontal disease.
The findings of these studies are generally consistent, suggesting that estrogen builds up mandibular bone mass and attenuates the severity of periodontal disease in postmenopausal women.26
DOES ESTROGEN THERAPY PROTECT THE TEETH?
Studies of the Leisure World,27 Framingham,28 and Nurses Health Study29 cohorts suggest that hormone therapy protects against tooth loss in postmenopausal women.
On the other hand, Taguchi et al30 evaluated more than 300 postmenopausal Japanese women and found no significant difference in the total number of teeth between estrogen users and nonusers. The population in this study was younger than in the other studies mentioned above,27–29 which may explain the negative finding. However, the duration of estrogen use was significantly associated with the total number of teeth remaining, independent of age.30 Meisel et al25 reported that women receiving hormonal therapy had more teeth, though the difference was not significant.
CYTOKINES, PERIODONTITIS, AND SKELETAL BONE LOSS
Studies suggest that low estrogen production after menopause is associated with increased production of interleukin 1 (IL-1), IL-6, IL-8, IL-10, tumor necrosis factor alpha, granulocyte colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor, which stimulate mature osteoclasts, modulate bone cell proliferation, and induce resorption of both skeletal and alveolar bone.31–34
In this regard, osteoporosis and periodontitis appear to be mediated by common cytokines. Managing osteoporosis, removing bacterial plaque biofilm with good oral hygiene, and regular dental visits are important in avoiding periodontitis in susceptible women.
BISPHOSPHONATES PROTECT BONE
In the skeleton
Bisphosphonates, the most commonly prescribed therapy for osteoporosis, inhibit systemic bone resorption and reduce the incidence of vertebral and nonvertebral fractures. Among the bisphosphonates, alendronate (Fosamax), risedronate (Actonel), and intravenous zoledronic acid (Reclast) have been shown to reduce the risk of both hip and vertebral fractures, whereas ibandronate (Boniva) has only been shown to decrease the risk of vertebral fracture.36 Specific findings:
- In the Fracture Intervention Trial,37 alendronate reduced the risk of vertebral fracture by 47% and hip fracture by 51% in women with low bone mineral density and previous vertebral fractures.
- In the Hip Intervention Program,38 risedronate decreased the risk of hip fracture by 40% in postmenopausal women 70 to 79 years old with osteoporosis, but not in those 80 years and older, who are at high risk of falls. Risedronate also reduced vertebral fracture risk by 49% after 3 years of treatment.39
- In the Health Outcomes and Reduced Incidence With Zoledronic Acid Once Yearly Recurrent Fracture Trial,40 annual infusion of zoledronic acid after a hip fracture reduced the rates of new clinical vertebral and nonvertebral fractures and death from all causes.
In the jaw
Not surprisingly, recent studies suggest that bisphosphonates slow the resorption of alveolar bone of the maxilla and mandible as well. Alendronate and risedronate, in particular, have been noted to improve periodontal status.41–43 Findings:
- In a cross-sectional study by Palomo et al,41 postmenopausal women with low bone density using risedronate for at least 3 months showed significantly less plaque accumulation, less gingival inflammation, lower probing-depth measurments, less periodontal attachment loss, and greater alveolar bone levels.
- In a double-blind, controlled, prospective study by Rocha et al,42 6 months of alendronate therapy significantly improved periodontal disease as assessed radiographically and clinically in 40 postmenopausal women with established periodontal disease.
- Jeffcoat et al43 reported that 2 years of alendronate treatment significantly reduced alveolar bone loss relative to placebo in patients with low mandibular bone mineral density at baseline but not in those with normal baseline mandibular bone mineral density.
DO BISPHOSPHONATES CAUSE OSTEONECROSIS OF THE JAW?
The intravenous bisphosphonates most commonly used to treat hypercalcemia of malignancy, multiple myeloma, or metastatic bone disease are47:
- Pamidronate (Aredia) 90 mg infused over 2 to 24 hours every 3 to 4 weeks
- Zoledronic acid (Zometa) 4 mg infused over 15 minutes monthly.
The doses of bisphosphonates indicated for the treatment of osteoporosis are much lower,1 eg:
- Alendronate 70 mg by mouth once a week
- Risedronate 35 mg by mouth once a week or 150 mg once a month
- Ibandronate 150 mg by mouth once a month
- Ibandronate 3 mg intravenously every 3 months
- Zoledronic acid 5 mg intravenously once a year.
Moreover, less than 1% of an oral dose is absorbed by the gastrointestinal tract,49 whereas more than 50% of the dose of bisphosphonates given intravenously is bioavailable,50 which may account for the lower incidence of jaw ostenonecrosis with oral agents.
Osteonecrosis of the jaw can occur spontaneously but is more often associated with dental procedures that traumatize bone, such as tooth extraction.51 In a systematic review,45 patients with multiple myeloma and metastatic cancer to the bone who were receiving intravenous bisphosphonates accounted for 94% of published cases. Sixty percent of cases were preceded by dental surgical procedures, and in 39% of cases that occurred spontaneously the lesions were located on bony exostoses, a possible source of trauma. Of 63 cases reported by Ruggiero et al,47 56 patients were receiving intravenous bisphosphonates and 7 were receiving oral bisphosphonates. Older age (> 65 years), chronic systemic steroid use, periodontitis, and prolonged use of bisphosphonates have also been associated with a higher risk of osteonecrosis of the jaw.51
The risk of developing osteonecrosis of the jaw in people taking bisphosphonates in doses recommended by the US Food and Drug Administration for treating osteoporosis is very low (the incidence is calculated at 0.7 per 100,000 person-years of exposure to alendronate).51,52 In a 3-year prospective study in more than 7,000 women with post-menopausal osteoporosis, the incidence of osteonecrosis of the jaw was no different in those treated with zoledronic acid 5 mg intravenously than in those receiving placebo.53 In a randomized, placebo-controlled study of the effect of 2 years of alendronate treatment on alveolar bone loss involving 335 patients with periodontal disease, no cases of osteonecrosis of the jaw were reported.43
The American Dental Association (ADA) released a statement noting that osteonecrosis of the jaw can occur with or without bisphosphonate use.51 To date, a true cause-and-effect relationship between osteonecrosis of the jaw and bisphosphonate use has not been established. Further studies are needed to fully explore this relationship. Our group is currently exploring novel periodontal assessments comparing the oral health of postmenopausal women with osteoporosis who are on no bone therapy vs postmenopausal women with osteoporosis treated with bisphosphonates for 2 or more years.
Discussion of treatment for bisphosphonate-associated osteonecrosis of the jaw is beyond the scope of this article.
REGULAR DENTAL CARE IS ESSENTIAL
Regardless of whether the patient is receiving a bisphosphonate drug, physicians caring for postmenopausal women should be vigilant and encourage their patients to seek regular dental evaluation for prevention and early management of oral disorders. Conversely, dentists should be aware of the potential effects of menopause and its treatments on bone and dental health.
Questions from postmenopausal women can be managed, in part, by returning to the basics suggested by the ADA:
- Regular dental examinations; regular professional cleaning to remove bacterial plaque biofilm under the gum-line where a toothbrush will not reach
- Daily oral hygiene practices to remove biofilm at and above the gum-line including brushing twice daily with an ADA-accepted toothpaste
- Replacing the toothbrush every 3 to 4 months (or sooner if the bristles begin to look frayed)
- Cleaning interproximally (between teeth) with floss or interdental cleaner
- Maintaining a balanced diet
- No smoking.
- North American Menopause Society. Menopause Practice: A Clinician’s Guide. 3rd ed; 2007.
- Krall EA, Garcia RI, Dawson-Hughes B. Increased risk of tooth loss is related to bone loss at the whole body, hip and spine. Calcif Tissue Int 1996; 59:433–437.
- Kribbs PJ. Comparison of mandibular bone in normal and osteoporotic women. J Prosthet Dent 1990; 63:218–222.
- Kribbs PJ, Chesnut CH, Ott SM, Kilcoyne RF. Relationship between mandibular and skeletal bone in an osteoporotic population. J Prosthet Dent 1989; 62:703–707.
- Kribbs PJ, Chestnut CH, Ott SM, Kilcyne RE. Relationship between mandibular and skeletal bone in a population of normal women. J Prosthet Dent 1990; 63:86–89.
- Kribbs PJ, Smith DE, Chestnut CH. Oral findings in osteoporosis. Part II: relationship between residual ridge and alveolar bone resorption and generalized skeletal osteopenia. J Prosthet Dent 1983; 50:719–724.
- von Wowern N, Klausen B, Kollerup G. Osteoporosis: a risk factor in periodontal disease. J Periodontol 1994; 65:1134–1138.
- Wactawski-Wende J, Grossi SG, Trevisan M, et al. The role of osteopenia in oral bone loss and periodontal disease. J Peridontol 1996; 67(suppl 10):1076–1084.
- Ronderos M, Jacobs DR, Himes JH, Pihlstrom BL. Associations of periodontal disease with femoral bone mineral density and estrogen replacement therapy: cross-sectional evaluation of US adults from the NHANES III. J Clin Periodontol 2000; 27:778–86.
- American Dental Association Council on Access, Prevention and Interprofessional Relations. Women’s Oral Health Issues. November 2006.
- Jeffcoat MK, Lewis CE, Reddy MS, Wang CY, Redford M. Post-menopausal bone loss and its relationship to oral bone loss. Periodontol 2000 2000; 23:94–102.
- Famili P, Cauley J, Suzuki JB, Weyant R. Longitudinal study of periodontal disease and edentulism with rates of bone loss in older women. J Periodontol 2005; 76:11–15.
- Pilgram TK, Hildebolt CF, Yokoyama N, et al. Relationships between longitudinal changes in radiographic alveolar bone height and probing depth measurements: data from postmenopausal women. J Periodontol 1999; 70:829–833.
- Jeffcoat MK, Lewis CE, Reddy MS, et al. Oral bone loss and systemic osteopenia, osteoporosis. InMarcus R, Feldman D, Kelsey J, editors. Osteoporosis. New York Academic Press 1996:969–990.
- Klemetti E, Collin HL, Forss H, Markkanen H, Lassila V. Mineral status of skeletal and advanced periodontal disease. J Clin Periodontol 1994; 21:184–188.
- Hildebolt CF. Osteoporosis and oral bone loss. Dentomaxillofac Radiol 1997; 26:3–15.
- Tezal M, Wactawski-Wende J, Grossi SG, Ho AW, Dunford R, Genco RJ. The relationship between bone mineral density and periodontitis in postmenopausal women. J Periodontol 2000; 71:1492–1498.
- Taguchi A, Tanimoto K, Suei Y, Ohama K, Wada T. Relationship between the mandibular and lumbar vertebral bone mineral density at different postmenopausal stages. Dentomaxillofac Radiol 1996; 25:130–135.
- Payne JB, Reinhardt RA, Nummikoski PV, Patil KD. Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporos Int 1999; 10:34–40.
- Payne JB, Zachs NR, Reinhardt RA, Nummikoski PV, Patil K. The association between estrogen status and alveolar bone density changes in postmenopausal women with a history of periodontitis. J Periodontol 1997; 68:24–31.
- Civitelli R, Pilgram TK, Dotson M, et al. Alveolar and postcranial bone density in postmenopausal women receiving hormone/estrogen replacement: a randomized, double blind, placebo-controlled trial. Arch Intern Med 2002; 162:1409–1415.
- Albandar JM, Kingman A. Gingival recession, gingival bleeding, and dental calculus in adults 30 years of age and older in the United States, 1988–1994. J Periodontol 1999; 70:30–43.
- Norderyd OM, Grossi SG, Machtel EE, et al. Periodontal status of women taking postmenopausal estrogen supplementation. J Periodontol 1993; 64:957–962.
- Jacobs R, Ghyselen J, Koninckx P, van Steenberghe D. Long-term bone mass evaluation of mandible and lumbar spine in a group of women receiving hormone replacement therapy. Eur J Oral Sci 1996; 104:10–16.
- Meisel P, Reifenberger J, Haase R, Nauck M, Bandt C, Kocher T. Women are periodontally healthier than men, but why don’t they have more teeth than men? Menopause 2008; 15:270–275.
- Genco RJ, Grossi SG. Is estrogen deficiency a risk factor for periodontal disease? Compend Contin Educ Dent Suppl 1998; 22:S23–S29.
- Paganini-Hill A. The benefits of estrogen replacement therapy on oral health. The Leisure World cohort. Arch Intern Med 1995; 155:2325–2329.
- Krall EA, Dawson-Hughes B, Hannan MT, Wilson PW, Kiel DP. Post-menopausal estrogen replacement and tooth retention. Am J Med 1997; 102:536–542.
- Grodstein F, Colditz GA, Stampfer MJ. Postmenopausal hormone use and tooth loss: a prospective study. J Am Dent Assoc 1996; 127:370–377.
- Taguchi A, Sanada M, Suei Y, et al. Effect of estrogen use on tooth retention, oral bone height, and oral bone porosity in Japanese postmenopausal women. Menopause 2004; 11:556–562.
- Pacifici R. Estrogen, cytokines and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res 1996; 11:1043–1051.
- Pacifici R. Is there a causal role for IL-1 in postmenopausal bone loss? Calcif Tissue Int 1992; 50:295–299.
- Girasole G, Jilka RL, Passeri G, et al. 17 beta-estradiol inhibits interleukin-6 production by bone marrow-derived stromal cells and osteoblasts in vitro: a potential mechanism for the antiosteoporotic effect of estrogens. J Clin Invest 1992; 89:883–891.
- Pacifici R, Brown C, Pusheck E, et al. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci USA 1991; 88:5134–5138.
- Brennan RM, Genco RJ, Wilding GE, Hovey KM, Trevisan M, Wactawski-Wende J. Bacterial species in subgingival plaque and oral bone loss in postmenopausal women. J Periodontol 2007; 78:1051–1061.
- Chestnut CH, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in post-menopausal osteoporosis. J Bone Miner Res 2004; 19:1241–1249.
- Black DM, Cummings SR, Karpf DB, et al. Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures: Fracture Intervention Trial Research Group. Lancet 1996; 348:1535–1541.
- McClung MR, Geusen P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med 2001; 344:333–340.
- Reginster JY, Minne HW, Sorensen OH, et al. Randomized trial of effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 2000; 11:83–91.
- Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007; 357:1799–1809.
- Palomo L, Bissada N, Liu J. Periodontal assessment of postmenopausal women receiving risedronate. Menopause 2005; 12:685–690.
- Rocha ML, Malacara JM, Sánchez-Marin FJ, Vazquez de la Torre CJ, Fajardo ME. Effect of alendronate on periodontal disease in postmenopausal women: a randomized placebo-controlled trial. J Periodontol 2004; 75:1579–1585.
- Jeffcoat MK, Cizza G, Shih WJ, Genco R, Lombardi A. Efficacy of bisphosphonates for the control of alveolar bone loss in periodontitis. J Int Acad Periodontol 2007; 9:70–76.
- Carey JJ, Palomo L. Bisphosphonates and osteonecrosis of the jaw: innocent association or significant risk? Cleve Clin J Med 2008; 75:871–879.
- Woo SB, Hellstein JW, Kalamare JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med 2006; 144:753–761.
- Dodson TB, Raje NS, Caruso PA, Rosenberg AE. Case records of the Massachusetts General Hospital. Case 9–2008. A 65-year-old woman with a nonhealing ulcer of the jaw. N Engl J Med 2008; 358:1283–1291.
- Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofacial Surg 2004; 62:527–534.
- Palomo L, Liu J, Bissada NF. Skeletal bone diseases impact the periodontium: a review of bisphosphonate therapy. Expert Opin Pharmacother 2007; 8:309–315.
- Ezra A, Golomb G. Administration routes and delivery systems of bisphosphonates for the treatment of bone resorption. Adv Drug Deliv Rev 2000; 42:175–195.
- Berenson JR, Rosen L, Vescio R, et al. Pharmacokinetics of pamidronate disodium in patients with cancer with normal or impaired renal function. J Clin Pharmacol 1997; 37:285–290.
- American Dental Association Council on Scientific Affairs. Dental management of patients receiving oral bisphosphonate therapy: expert panel recommendations. J Am Dent Assoc 2006; 137:1144–1150.
- Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofacial Surg 2007; 65:369–376.
- Grbic JT, Landesberg R, Lin SQ, et al; Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly Pivotal Fracture Trial Research Group. Incidence of osteonecrosis of the jaw in women with postmenopausal osteoporosis in the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly Pivotal Fracture Trial. J Am Dent Assoc 2008; 139:32–40.
- North American Menopause Society. Menopause Practice: A Clinician’s Guide. 3rd ed; 2007.
- Krall EA, Garcia RI, Dawson-Hughes B. Increased risk of tooth loss is related to bone loss at the whole body, hip and spine. Calcif Tissue Int 1996; 59:433–437.
- Kribbs PJ. Comparison of mandibular bone in normal and osteoporotic women. J Prosthet Dent 1990; 63:218–222.
- Kribbs PJ, Chesnut CH, Ott SM, Kilcoyne RF. Relationship between mandibular and skeletal bone in an osteoporotic population. J Prosthet Dent 1989; 62:703–707.
- Kribbs PJ, Chestnut CH, Ott SM, Kilcyne RE. Relationship between mandibular and skeletal bone in a population of normal women. J Prosthet Dent 1990; 63:86–89.
- Kribbs PJ, Smith DE, Chestnut CH. Oral findings in osteoporosis. Part II: relationship between residual ridge and alveolar bone resorption and generalized skeletal osteopenia. J Prosthet Dent 1983; 50:719–724.
- von Wowern N, Klausen B, Kollerup G. Osteoporosis: a risk factor in periodontal disease. J Periodontol 1994; 65:1134–1138.
- Wactawski-Wende J, Grossi SG, Trevisan M, et al. The role of osteopenia in oral bone loss and periodontal disease. J Peridontol 1996; 67(suppl 10):1076–1084.
- Ronderos M, Jacobs DR, Himes JH, Pihlstrom BL. Associations of periodontal disease with femoral bone mineral density and estrogen replacement therapy: cross-sectional evaluation of US adults from the NHANES III. J Clin Periodontol 2000; 27:778–86.
- American Dental Association Council on Access, Prevention and Interprofessional Relations. Women’s Oral Health Issues. November 2006.
- Jeffcoat MK, Lewis CE, Reddy MS, Wang CY, Redford M. Post-menopausal bone loss and its relationship to oral bone loss. Periodontol 2000 2000; 23:94–102.
- Famili P, Cauley J, Suzuki JB, Weyant R. Longitudinal study of periodontal disease and edentulism with rates of bone loss in older women. J Periodontol 2005; 76:11–15.
- Pilgram TK, Hildebolt CF, Yokoyama N, et al. Relationships between longitudinal changes in radiographic alveolar bone height and probing depth measurements: data from postmenopausal women. J Periodontol 1999; 70:829–833.
- Jeffcoat MK, Lewis CE, Reddy MS, et al. Oral bone loss and systemic osteopenia, osteoporosis. InMarcus R, Feldman D, Kelsey J, editors. Osteoporosis. New York Academic Press 1996:969–990.
- Klemetti E, Collin HL, Forss H, Markkanen H, Lassila V. Mineral status of skeletal and advanced periodontal disease. J Clin Periodontol 1994; 21:184–188.
- Hildebolt CF. Osteoporosis and oral bone loss. Dentomaxillofac Radiol 1997; 26:3–15.
- Tezal M, Wactawski-Wende J, Grossi SG, Ho AW, Dunford R, Genco RJ. The relationship between bone mineral density and periodontitis in postmenopausal women. J Periodontol 2000; 71:1492–1498.
- Taguchi A, Tanimoto K, Suei Y, Ohama K, Wada T. Relationship between the mandibular and lumbar vertebral bone mineral density at different postmenopausal stages. Dentomaxillofac Radiol 1996; 25:130–135.
- Payne JB, Reinhardt RA, Nummikoski PV, Patil KD. Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporos Int 1999; 10:34–40.
- Payne JB, Zachs NR, Reinhardt RA, Nummikoski PV, Patil K. The association between estrogen status and alveolar bone density changes in postmenopausal women with a history of periodontitis. J Periodontol 1997; 68:24–31.
- Civitelli R, Pilgram TK, Dotson M, et al. Alveolar and postcranial bone density in postmenopausal women receiving hormone/estrogen replacement: a randomized, double blind, placebo-controlled trial. Arch Intern Med 2002; 162:1409–1415.
- Albandar JM, Kingman A. Gingival recession, gingival bleeding, and dental calculus in adults 30 years of age and older in the United States, 1988–1994. J Periodontol 1999; 70:30–43.
- Norderyd OM, Grossi SG, Machtel EE, et al. Periodontal status of women taking postmenopausal estrogen supplementation. J Periodontol 1993; 64:957–962.
- Jacobs R, Ghyselen J, Koninckx P, van Steenberghe D. Long-term bone mass evaluation of mandible and lumbar spine in a group of women receiving hormone replacement therapy. Eur J Oral Sci 1996; 104:10–16.
- Meisel P, Reifenberger J, Haase R, Nauck M, Bandt C, Kocher T. Women are periodontally healthier than men, but why don’t they have more teeth than men? Menopause 2008; 15:270–275.
- Genco RJ, Grossi SG. Is estrogen deficiency a risk factor for periodontal disease? Compend Contin Educ Dent Suppl 1998; 22:S23–S29.
- Paganini-Hill A. The benefits of estrogen replacement therapy on oral health. The Leisure World cohort. Arch Intern Med 1995; 155:2325–2329.
- Krall EA, Dawson-Hughes B, Hannan MT, Wilson PW, Kiel DP. Post-menopausal estrogen replacement and tooth retention. Am J Med 1997; 102:536–542.
- Grodstein F, Colditz GA, Stampfer MJ. Postmenopausal hormone use and tooth loss: a prospective study. J Am Dent Assoc 1996; 127:370–377.
- Taguchi A, Sanada M, Suei Y, et al. Effect of estrogen use on tooth retention, oral bone height, and oral bone porosity in Japanese postmenopausal women. Menopause 2004; 11:556–562.
- Pacifici R. Estrogen, cytokines and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res 1996; 11:1043–1051.
- Pacifici R. Is there a causal role for IL-1 in postmenopausal bone loss? Calcif Tissue Int 1992; 50:295–299.
- Girasole G, Jilka RL, Passeri G, et al. 17 beta-estradiol inhibits interleukin-6 production by bone marrow-derived stromal cells and osteoblasts in vitro: a potential mechanism for the antiosteoporotic effect of estrogens. J Clin Invest 1992; 89:883–891.
- Pacifici R, Brown C, Pusheck E, et al. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci USA 1991; 88:5134–5138.
- Brennan RM, Genco RJ, Wilding GE, Hovey KM, Trevisan M, Wactawski-Wende J. Bacterial species in subgingival plaque and oral bone loss in postmenopausal women. J Periodontol 2007; 78:1051–1061.
- Chestnut CH, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in post-menopausal osteoporosis. J Bone Miner Res 2004; 19:1241–1249.
- Black DM, Cummings SR, Karpf DB, et al. Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures: Fracture Intervention Trial Research Group. Lancet 1996; 348:1535–1541.
- McClung MR, Geusen P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med 2001; 344:333–340.
- Reginster JY, Minne HW, Sorensen OH, et al. Randomized trial of effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 2000; 11:83–91.
- Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007; 357:1799–1809.
- Palomo L, Bissada N, Liu J. Periodontal assessment of postmenopausal women receiving risedronate. Menopause 2005; 12:685–690.
- Rocha ML, Malacara JM, Sánchez-Marin FJ, Vazquez de la Torre CJ, Fajardo ME. Effect of alendronate on periodontal disease in postmenopausal women: a randomized placebo-controlled trial. J Periodontol 2004; 75:1579–1585.
- Jeffcoat MK, Cizza G, Shih WJ, Genco R, Lombardi A. Efficacy of bisphosphonates for the control of alveolar bone loss in periodontitis. J Int Acad Periodontol 2007; 9:70–76.
- Carey JJ, Palomo L. Bisphosphonates and osteonecrosis of the jaw: innocent association or significant risk? Cleve Clin J Med 2008; 75:871–879.
- Woo SB, Hellstein JW, Kalamare JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med 2006; 144:753–761.
- Dodson TB, Raje NS, Caruso PA, Rosenberg AE. Case records of the Massachusetts General Hospital. Case 9–2008. A 65-year-old woman with a nonhealing ulcer of the jaw. N Engl J Med 2008; 358:1283–1291.
- Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofacial Surg 2004; 62:527–534.
- Palomo L, Liu J, Bissada NF. Skeletal bone diseases impact the periodontium: a review of bisphosphonate therapy. Expert Opin Pharmacother 2007; 8:309–315.
- Ezra A, Golomb G. Administration routes and delivery systems of bisphosphonates for the treatment of bone resorption. Adv Drug Deliv Rev 2000; 42:175–195.
- Berenson JR, Rosen L, Vescio R, et al. Pharmacokinetics of pamidronate disodium in patients with cancer with normal or impaired renal function. J Clin Pharmacol 1997; 37:285–290.
- American Dental Association Council on Scientific Affairs. Dental management of patients receiving oral bisphosphonate therapy: expert panel recommendations. J Am Dent Assoc 2006; 137:1144–1150.
- Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofacial Surg 2007; 65:369–376.
- Grbic JT, Landesberg R, Lin SQ, et al; Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly Pivotal Fracture Trial Research Group. Incidence of osteonecrosis of the jaw in women with postmenopausal osteoporosis in the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly Pivotal Fracture Trial. J Am Dent Assoc 2008; 139:32–40.
KEY POINTS
- Physicians should be vigilant for dental problems and should encourage their patients to practice good oral hygiene and to seek regular dental care.
- Available information suggests that hormone therapy and bisphosphonate drugs may be developed to protect against alveolar bone loss and perhaps slow the progression of periodontal disease.
- Bisphosphonate-associated osteonecrosis of the jaw is rare, and most of the reported cases have been in cancer patients who received high doses of bisphosphonates intravenously and who had other risk factors for it.