User login
Bedside Assessment of the Necessity of Daily Lab Testing for Patients Nearing Discharge
As part of the Choosing Wisely® campaign, the Society of Hospital Medicine recommends against performing “repetitive complete blood count [CBC] and chemistry testing in the face of clinical and lab stability.”1 This recommendation stems from a body of research that shows that frequent or excessive phlebotomy can have negative consequences, including iatrogenic anemia (termed hospital-acquired anemia), which may necessitate blood transfusion.2 The downstream effects of potentially unnecessary testing, including the evaluation of false-positive results, must also be considered. Additional important effects include patient discomfort and disruption of sleep and unproductive work by hospital staff, including nurses, phlebotomists, and laboratory technicians.
Though interventions to reduce unnecessary daily labs have been previously evaluated, there are no studies that focus on decreasing lab testing on patients deemed clinically stable and close to discharge. This is in part due to the absence of clear criteria or guidelines to define clinical stability in the context of lab utilization.
We therefore aimed to implement a multifaceted, patient-centered initiative—the Necessity of Labs Assessed Bedside (NO LABS)—that focused on reducing lab testing in patients at 24 to 48 hours before discharge. We targeted the 24 to 48-hour period before the anticipated date of discharge, as this may be a period of greater stability and provide an opportunity to identify and decrease unnecessary testing.
METHODS
The study took place at Mount Sinai Hospital, which is an 1174-bed tertiary care teaching hospital in New York City. We targeted 2 inpatient medicine units where virtually all patients are assigned to a hospitalist rotating for a 2- to 4-week period, for the period of July 1, 2015, to July 31, 2016. These units employed bedside interdisciplinary rounds (IDR) attended by the hospitalist, social worker, case manager, nurse, nurse manager, and medical director. Bedside IDR focuses on the daily plan and patient safety by
As described by Dunn et al.,3 the IDR script included the following: a review of the plan of care by the hospitalist, identifying a patient’s personal goals for the day, a brief update of discharge planning (as appropriate), and a safety assessment performed by the nurse (identifying Foley catheters, falls risk, etc). We incorporated an inquiry into the daily IDR script identifying clinically stable patients for discharge in the next 24 to 48 hours (based on physician judgment), followed by a prompt to the hospitalist to discontinue labs when appropriate. The unit medical director and nurse manager were both tasked with prompting the hospitalist at the bedside. Our hospital utilizes computerized physician order entry. Lab orders were then discontinued by the clinician during rounds using a computer on wheels (or after rounds when one was not available). The hospitalist, unit medical director, and nurse manager were reminded about the project through weekly e-mails and in-person communication.
To assess whether the prompt was being incorporated consistently, an observer was added to rounds beginning in the second month of the project. The observer was present at least 3 times a week for the subsequent 3 months of the project. Our intervention also included education geared towards hospitalists, including a brief presentation on reducing unnecessary lab testing during a monthly hospitalist faculty meeting (the first and sixth month of the intervention). The group’s data on laboratory testing within the 24 to 48 hours prior to discharge were also presented at these monthly meetings (beginning 2 months into the intervention and monthly thereafter). Lastly, we provided the unit staff with unit-level metrics, biweekly for the first 3 months and every 2 to 3 months thereafter.
We extracted electronic medical record (EMR) data on lab utilization for patients on the 2 hospitalist units for the intervention period. Baseline data were obtained from July 1, 2014, to June 30, 2015. Patients with a length of stay (LOS) ≤7 days (75th percentile) were included; on these units, longer stays were considered more likely to have complex social issues delaying discharge and thus less likely to require laboratory testing. We tracked ordering for 4 common lab tests: basic metabolic panel, CBC, CBC with differential, and the comprehensive metabolic panel. The primary outcome was the monthly percentage of patients for whom testing was ordered in the 24 and 48 hours preceding discharge. A secondary outcome was testing at 72 hours preceding discharge to identify any potential compensatory (increased) testing the evening prior. We applied a quasi-experimental interrupted time series design with a segmented regression analysis to estimate changes before and after our intervention, expressed in acute changes (change in intercept) and over time (changes in trend) while adjusting for preintervention trends. All analyses were performed with SAS v9.4 statistical software (SAS Institute, Cary, NC). Our project was deemed a quality improvement project and thus an IRB submission was not required.
RESULTS
There were 1579 discharges in the preintervention period and 1308 discharges in the postintervention period. The average age of the patient population was similar in the baseline and postintervention groups (61.5 vs 59.3 years; P = 0.400), and there was no difference in the mean LOS before and after implementation (3.67 vs 3.68 days; P = 0.817).
DISCUSSION
Our structured, multifaceted approach effectively reduced daily lab testing in the 24 to 48 hours prior to discharge. Bedside IDR provided a unique opportunity to effectively communicate to the patient about necessary (or unnecessary) testing. Moreover, given the complexity of identifying clinical stability, our strategy focused on the onset of discharge planning, a more easily discernible and less obtrusive focal point to promote the discontinuation of lab testing.
Though the nature of bundled interventions can make it difficult to identify which intervention is most effective, we believe that all interventions were effective in different capacities during various phases in the intervention period. We believe that the decrease in lab testing in the 24 to 48 hours preceding discharge was primarily driven by the new rounding structure. This is evident in the significant decrease seen in the first few months of the intervention period. Six months into the intervention, we begin to see a decrease at 72 hours prior to discharge. Additionally, we see a decrease in the mean number of labs per patient day over the entire hospitalization period. We attribute these results to a gradual shift in the culture in our division as a direct consequence of educational sessions and individual feedback provided during this time.
To our knowledge, this is the first study to use anticipated discharge as a correlate for clinical stability and therefore as an opportunity to prompt discontinuation of laboratory testing. Other studies evaluated interventions targeting the EMR and the ease with which providers can order recurring labs. These include restricting recurring orders in the EMR,4 a robust education and awareness campaign targeting house staff,5 and other multifaceted approaches to decreasing lab utilization,6 all of which have shown promising results. While these approaches show varying degrees of success, ours is unique in its focus on the period prior to discharge. In addition, the intervention can be readily implemented in settings that utilize scripted IDR. It also brings high-value decision-making to the bedside by informing the patient that in the setting of presumed clinical stability, no additional tests are warranted.
Our study has several limitations. First, while interdisciplinary discharge rounds are widely implemented,7,8 our rounds occur at the bedside and employ a script, potentially limiting generalizability. The structured prompting may be feasible during structured IDR in a standard conference room setting, though we did not assess this model. Second, bedside rounds only included patients who were able to participate. Rounding on patients unable to participate, such as patients with delirium with agitation, was done outside the patient room rather than at the bedside. A modified script was used in these instances (absent questions addressed to the patient), allowing for the prompt to be incorporated. These patients were included in the analysis. Lastly, as previously stated, we cannot clearly identify which intervention (the prompt, education, or feedback) most effectively led to a sustained decrease in lab ordering.
Our structured, multifaceted intervention reduced laboratory testing during the last 48 hours of admission. Hospitals that aim to decrease potentially unnecessary lab testing should consider implementing a bundle, including a prompt at a uniform and structured point during the hospitalization of patients who are expected to be discharged within 24 to 48 hours, clinician education, an audit, and feedback.
Disclosure
All authors report no conflicts of interest to disclose.
1. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: Five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. PubMed
2. Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20(6):520-524. PubMed
3. Dunn AS, Reyna, M, Radbill B, et al. The impact of bedside interdisciplinary rounds on length of stay and complications. J Hosp Med. 2017;3:137-142. PubMed
4. Iturrate E, Jubelt L, Volpicelli F, Hochman K. Optimize Your Electronic Medical Record to Increase Value: Reducing Laboratory Overutilization. Am J Med. 2016;129(2):215-220. PubMed
5. Wheeler D, Marcus P, Nguyen J, et al. Evaluation of a Resident-Led Project to Decrease Phlebotomy Rates in the Hospital: Think Twice, Stick Once. JAMA Intern Med. 2016;176(5):708-710. PubMed
6. Corson AH, Fan VS, White T, et al. A Multifaceted Hospitalist Quality Improvement Intervention: Decreased Frequency of Common Labs. J Hosp Med. 2015;10(6):390-395. PubMed
7. Bhamidipati VS, Elliott DJ, Justice EM, Belleh E, Sonnad SS, Robinson EJ. Structure and outcomes of interdisciplinary rounds in hospitalized medicine patients: A systematic review and suggested taxonomy. J Hosp Med. 2016;11(7):513-523. PubMed
8. O’Leary, KJ, Sehgal NL, Terrell G, Williams MV, High Performance Teams and the Hospital of the Future Project Team. Interdisciplinary teamwork in hospitals: a review and practical recommendations for improvement. J Hosp Med. 2012;7(1):48-54. PubMed
As part of the Choosing Wisely® campaign, the Society of Hospital Medicine recommends against performing “repetitive complete blood count [CBC] and chemistry testing in the face of clinical and lab stability.”1 This recommendation stems from a body of research that shows that frequent or excessive phlebotomy can have negative consequences, including iatrogenic anemia (termed hospital-acquired anemia), which may necessitate blood transfusion.2 The downstream effects of potentially unnecessary testing, including the evaluation of false-positive results, must also be considered. Additional important effects include patient discomfort and disruption of sleep and unproductive work by hospital staff, including nurses, phlebotomists, and laboratory technicians.
Though interventions to reduce unnecessary daily labs have been previously evaluated, there are no studies that focus on decreasing lab testing on patients deemed clinically stable and close to discharge. This is in part due to the absence of clear criteria or guidelines to define clinical stability in the context of lab utilization.
We therefore aimed to implement a multifaceted, patient-centered initiative—the Necessity of Labs Assessed Bedside (NO LABS)—that focused on reducing lab testing in patients at 24 to 48 hours before discharge. We targeted the 24 to 48-hour period before the anticipated date of discharge, as this may be a period of greater stability and provide an opportunity to identify and decrease unnecessary testing.
METHODS
The study took place at Mount Sinai Hospital, which is an 1174-bed tertiary care teaching hospital in New York City. We targeted 2 inpatient medicine units where virtually all patients are assigned to a hospitalist rotating for a 2- to 4-week period, for the period of July 1, 2015, to July 31, 2016. These units employed bedside interdisciplinary rounds (IDR) attended by the hospitalist, social worker, case manager, nurse, nurse manager, and medical director. Bedside IDR focuses on the daily plan and patient safety by
As described by Dunn et al.,3 the IDR script included the following: a review of the plan of care by the hospitalist, identifying a patient’s personal goals for the day, a brief update of discharge planning (as appropriate), and a safety assessment performed by the nurse (identifying Foley catheters, falls risk, etc). We incorporated an inquiry into the daily IDR script identifying clinically stable patients for discharge in the next 24 to 48 hours (based on physician judgment), followed by a prompt to the hospitalist to discontinue labs when appropriate. The unit medical director and nurse manager were both tasked with prompting the hospitalist at the bedside. Our hospital utilizes computerized physician order entry. Lab orders were then discontinued by the clinician during rounds using a computer on wheels (or after rounds when one was not available). The hospitalist, unit medical director, and nurse manager were reminded about the project through weekly e-mails and in-person communication.
To assess whether the prompt was being incorporated consistently, an observer was added to rounds beginning in the second month of the project. The observer was present at least 3 times a week for the subsequent 3 months of the project. Our intervention also included education geared towards hospitalists, including a brief presentation on reducing unnecessary lab testing during a monthly hospitalist faculty meeting (the first and sixth month of the intervention). The group’s data on laboratory testing within the 24 to 48 hours prior to discharge were also presented at these monthly meetings (beginning 2 months into the intervention and monthly thereafter). Lastly, we provided the unit staff with unit-level metrics, biweekly for the first 3 months and every 2 to 3 months thereafter.
We extracted electronic medical record (EMR) data on lab utilization for patients on the 2 hospitalist units for the intervention period. Baseline data were obtained from July 1, 2014, to June 30, 2015. Patients with a length of stay (LOS) ≤7 days (75th percentile) were included; on these units, longer stays were considered more likely to have complex social issues delaying discharge and thus less likely to require laboratory testing. We tracked ordering for 4 common lab tests: basic metabolic panel, CBC, CBC with differential, and the comprehensive metabolic panel. The primary outcome was the monthly percentage of patients for whom testing was ordered in the 24 and 48 hours preceding discharge. A secondary outcome was testing at 72 hours preceding discharge to identify any potential compensatory (increased) testing the evening prior. We applied a quasi-experimental interrupted time series design with a segmented regression analysis to estimate changes before and after our intervention, expressed in acute changes (change in intercept) and over time (changes in trend) while adjusting for preintervention trends. All analyses were performed with SAS v9.4 statistical software (SAS Institute, Cary, NC). Our project was deemed a quality improvement project and thus an IRB submission was not required.
RESULTS
There were 1579 discharges in the preintervention period and 1308 discharges in the postintervention period. The average age of the patient population was similar in the baseline and postintervention groups (61.5 vs 59.3 years; P = 0.400), and there was no difference in the mean LOS before and after implementation (3.67 vs 3.68 days; P = 0.817).
DISCUSSION
Our structured, multifaceted approach effectively reduced daily lab testing in the 24 to 48 hours prior to discharge. Bedside IDR provided a unique opportunity to effectively communicate to the patient about necessary (or unnecessary) testing. Moreover, given the complexity of identifying clinical stability, our strategy focused on the onset of discharge planning, a more easily discernible and less obtrusive focal point to promote the discontinuation of lab testing.
Though the nature of bundled interventions can make it difficult to identify which intervention is most effective, we believe that all interventions were effective in different capacities during various phases in the intervention period. We believe that the decrease in lab testing in the 24 to 48 hours preceding discharge was primarily driven by the new rounding structure. This is evident in the significant decrease seen in the first few months of the intervention period. Six months into the intervention, we begin to see a decrease at 72 hours prior to discharge. Additionally, we see a decrease in the mean number of labs per patient day over the entire hospitalization period. We attribute these results to a gradual shift in the culture in our division as a direct consequence of educational sessions and individual feedback provided during this time.
To our knowledge, this is the first study to use anticipated discharge as a correlate for clinical stability and therefore as an opportunity to prompt discontinuation of laboratory testing. Other studies evaluated interventions targeting the EMR and the ease with which providers can order recurring labs. These include restricting recurring orders in the EMR,4 a robust education and awareness campaign targeting house staff,5 and other multifaceted approaches to decreasing lab utilization,6 all of which have shown promising results. While these approaches show varying degrees of success, ours is unique in its focus on the period prior to discharge. In addition, the intervention can be readily implemented in settings that utilize scripted IDR. It also brings high-value decision-making to the bedside by informing the patient that in the setting of presumed clinical stability, no additional tests are warranted.
Our study has several limitations. First, while interdisciplinary discharge rounds are widely implemented,7,8 our rounds occur at the bedside and employ a script, potentially limiting generalizability. The structured prompting may be feasible during structured IDR in a standard conference room setting, though we did not assess this model. Second, bedside rounds only included patients who were able to participate. Rounding on patients unable to participate, such as patients with delirium with agitation, was done outside the patient room rather than at the bedside. A modified script was used in these instances (absent questions addressed to the patient), allowing for the prompt to be incorporated. These patients were included in the analysis. Lastly, as previously stated, we cannot clearly identify which intervention (the prompt, education, or feedback) most effectively led to a sustained decrease in lab ordering.
Our structured, multifaceted intervention reduced laboratory testing during the last 48 hours of admission. Hospitals that aim to decrease potentially unnecessary lab testing should consider implementing a bundle, including a prompt at a uniform and structured point during the hospitalization of patients who are expected to be discharged within 24 to 48 hours, clinician education, an audit, and feedback.
Disclosure
All authors report no conflicts of interest to disclose.
As part of the Choosing Wisely® campaign, the Society of Hospital Medicine recommends against performing “repetitive complete blood count [CBC] and chemistry testing in the face of clinical and lab stability.”1 This recommendation stems from a body of research that shows that frequent or excessive phlebotomy can have negative consequences, including iatrogenic anemia (termed hospital-acquired anemia), which may necessitate blood transfusion.2 The downstream effects of potentially unnecessary testing, including the evaluation of false-positive results, must also be considered. Additional important effects include patient discomfort and disruption of sleep and unproductive work by hospital staff, including nurses, phlebotomists, and laboratory technicians.
Though interventions to reduce unnecessary daily labs have been previously evaluated, there are no studies that focus on decreasing lab testing on patients deemed clinically stable and close to discharge. This is in part due to the absence of clear criteria or guidelines to define clinical stability in the context of lab utilization.
We therefore aimed to implement a multifaceted, patient-centered initiative—the Necessity of Labs Assessed Bedside (NO LABS)—that focused on reducing lab testing in patients at 24 to 48 hours before discharge. We targeted the 24 to 48-hour period before the anticipated date of discharge, as this may be a period of greater stability and provide an opportunity to identify and decrease unnecessary testing.
METHODS
The study took place at Mount Sinai Hospital, which is an 1174-bed tertiary care teaching hospital in New York City. We targeted 2 inpatient medicine units where virtually all patients are assigned to a hospitalist rotating for a 2- to 4-week period, for the period of July 1, 2015, to July 31, 2016. These units employed bedside interdisciplinary rounds (IDR) attended by the hospitalist, social worker, case manager, nurse, nurse manager, and medical director. Bedside IDR focuses on the daily plan and patient safety by
As described by Dunn et al.,3 the IDR script included the following: a review of the plan of care by the hospitalist, identifying a patient’s personal goals for the day, a brief update of discharge planning (as appropriate), and a safety assessment performed by the nurse (identifying Foley catheters, falls risk, etc). We incorporated an inquiry into the daily IDR script identifying clinically stable patients for discharge in the next 24 to 48 hours (based on physician judgment), followed by a prompt to the hospitalist to discontinue labs when appropriate. The unit medical director and nurse manager were both tasked with prompting the hospitalist at the bedside. Our hospital utilizes computerized physician order entry. Lab orders were then discontinued by the clinician during rounds using a computer on wheels (or after rounds when one was not available). The hospitalist, unit medical director, and nurse manager were reminded about the project through weekly e-mails and in-person communication.
To assess whether the prompt was being incorporated consistently, an observer was added to rounds beginning in the second month of the project. The observer was present at least 3 times a week for the subsequent 3 months of the project. Our intervention also included education geared towards hospitalists, including a brief presentation on reducing unnecessary lab testing during a monthly hospitalist faculty meeting (the first and sixth month of the intervention). The group’s data on laboratory testing within the 24 to 48 hours prior to discharge were also presented at these monthly meetings (beginning 2 months into the intervention and monthly thereafter). Lastly, we provided the unit staff with unit-level metrics, biweekly for the first 3 months and every 2 to 3 months thereafter.
We extracted electronic medical record (EMR) data on lab utilization for patients on the 2 hospitalist units for the intervention period. Baseline data were obtained from July 1, 2014, to June 30, 2015. Patients with a length of stay (LOS) ≤7 days (75th percentile) were included; on these units, longer stays were considered more likely to have complex social issues delaying discharge and thus less likely to require laboratory testing. We tracked ordering for 4 common lab tests: basic metabolic panel, CBC, CBC with differential, and the comprehensive metabolic panel. The primary outcome was the monthly percentage of patients for whom testing was ordered in the 24 and 48 hours preceding discharge. A secondary outcome was testing at 72 hours preceding discharge to identify any potential compensatory (increased) testing the evening prior. We applied a quasi-experimental interrupted time series design with a segmented regression analysis to estimate changes before and after our intervention, expressed in acute changes (change in intercept) and over time (changes in trend) while adjusting for preintervention trends. All analyses were performed with SAS v9.4 statistical software (SAS Institute, Cary, NC). Our project was deemed a quality improvement project and thus an IRB submission was not required.
RESULTS
There were 1579 discharges in the preintervention period and 1308 discharges in the postintervention period. The average age of the patient population was similar in the baseline and postintervention groups (61.5 vs 59.3 years; P = 0.400), and there was no difference in the mean LOS before and after implementation (3.67 vs 3.68 days; P = 0.817).
DISCUSSION
Our structured, multifaceted approach effectively reduced daily lab testing in the 24 to 48 hours prior to discharge. Bedside IDR provided a unique opportunity to effectively communicate to the patient about necessary (or unnecessary) testing. Moreover, given the complexity of identifying clinical stability, our strategy focused on the onset of discharge planning, a more easily discernible and less obtrusive focal point to promote the discontinuation of lab testing.
Though the nature of bundled interventions can make it difficult to identify which intervention is most effective, we believe that all interventions were effective in different capacities during various phases in the intervention period. We believe that the decrease in lab testing in the 24 to 48 hours preceding discharge was primarily driven by the new rounding structure. This is evident in the significant decrease seen in the first few months of the intervention period. Six months into the intervention, we begin to see a decrease at 72 hours prior to discharge. Additionally, we see a decrease in the mean number of labs per patient day over the entire hospitalization period. We attribute these results to a gradual shift in the culture in our division as a direct consequence of educational sessions and individual feedback provided during this time.
To our knowledge, this is the first study to use anticipated discharge as a correlate for clinical stability and therefore as an opportunity to prompt discontinuation of laboratory testing. Other studies evaluated interventions targeting the EMR and the ease with which providers can order recurring labs. These include restricting recurring orders in the EMR,4 a robust education and awareness campaign targeting house staff,5 and other multifaceted approaches to decreasing lab utilization,6 all of which have shown promising results. While these approaches show varying degrees of success, ours is unique in its focus on the period prior to discharge. In addition, the intervention can be readily implemented in settings that utilize scripted IDR. It also brings high-value decision-making to the bedside by informing the patient that in the setting of presumed clinical stability, no additional tests are warranted.
Our study has several limitations. First, while interdisciplinary discharge rounds are widely implemented,7,8 our rounds occur at the bedside and employ a script, potentially limiting generalizability. The structured prompting may be feasible during structured IDR in a standard conference room setting, though we did not assess this model. Second, bedside rounds only included patients who were able to participate. Rounding on patients unable to participate, such as patients with delirium with agitation, was done outside the patient room rather than at the bedside. A modified script was used in these instances (absent questions addressed to the patient), allowing for the prompt to be incorporated. These patients were included in the analysis. Lastly, as previously stated, we cannot clearly identify which intervention (the prompt, education, or feedback) most effectively led to a sustained decrease in lab ordering.
Our structured, multifaceted intervention reduced laboratory testing during the last 48 hours of admission. Hospitals that aim to decrease potentially unnecessary lab testing should consider implementing a bundle, including a prompt at a uniform and structured point during the hospitalization of patients who are expected to be discharged within 24 to 48 hours, clinician education, an audit, and feedback.
Disclosure
All authors report no conflicts of interest to disclose.
1. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: Five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. PubMed
2. Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20(6):520-524. PubMed
3. Dunn AS, Reyna, M, Radbill B, et al. The impact of bedside interdisciplinary rounds on length of stay and complications. J Hosp Med. 2017;3:137-142. PubMed
4. Iturrate E, Jubelt L, Volpicelli F, Hochman K. Optimize Your Electronic Medical Record to Increase Value: Reducing Laboratory Overutilization. Am J Med. 2016;129(2):215-220. PubMed
5. Wheeler D, Marcus P, Nguyen J, et al. Evaluation of a Resident-Led Project to Decrease Phlebotomy Rates in the Hospital: Think Twice, Stick Once. JAMA Intern Med. 2016;176(5):708-710. PubMed
6. Corson AH, Fan VS, White T, et al. A Multifaceted Hospitalist Quality Improvement Intervention: Decreased Frequency of Common Labs. J Hosp Med. 2015;10(6):390-395. PubMed
7. Bhamidipati VS, Elliott DJ, Justice EM, Belleh E, Sonnad SS, Robinson EJ. Structure and outcomes of interdisciplinary rounds in hospitalized medicine patients: A systematic review and suggested taxonomy. J Hosp Med. 2016;11(7):513-523. PubMed
8. O’Leary, KJ, Sehgal NL, Terrell G, Williams MV, High Performance Teams and the Hospital of the Future Project Team. Interdisciplinary teamwork in hospitals: a review and practical recommendations for improvement. J Hosp Med. 2012;7(1):48-54. PubMed
1. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: Five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. PubMed
2. Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20(6):520-524. PubMed
3. Dunn AS, Reyna, M, Radbill B, et al. The impact of bedside interdisciplinary rounds on length of stay and complications. J Hosp Med. 2017;3:137-142. PubMed
4. Iturrate E, Jubelt L, Volpicelli F, Hochman K. Optimize Your Electronic Medical Record to Increase Value: Reducing Laboratory Overutilization. Am J Med. 2016;129(2):215-220. PubMed
5. Wheeler D, Marcus P, Nguyen J, et al. Evaluation of a Resident-Led Project to Decrease Phlebotomy Rates in the Hospital: Think Twice, Stick Once. JAMA Intern Med. 2016;176(5):708-710. PubMed
6. Corson AH, Fan VS, White T, et al. A Multifaceted Hospitalist Quality Improvement Intervention: Decreased Frequency of Common Labs. J Hosp Med. 2015;10(6):390-395. PubMed
7. Bhamidipati VS, Elliott DJ, Justice EM, Belleh E, Sonnad SS, Robinson EJ. Structure and outcomes of interdisciplinary rounds in hospitalized medicine patients: A systematic review and suggested taxonomy. J Hosp Med. 2016;11(7):513-523. PubMed
8. O’Leary, KJ, Sehgal NL, Terrell G, Williams MV, High Performance Teams and the Hospital of the Future Project Team. Interdisciplinary teamwork in hospitals: a review and practical recommendations for improvement. J Hosp Med. 2012;7(1):48-54. PubMed
© 2018 Society of Hospital Medicine
The value of using ultrasound to rule out deep vein thrombosis in cases of cellulitis
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Because of overlapping clinical manifestations, clinicians often order ultrasound to rule out deep vein thrombosis (DVT) in cases of cellulitis. Ultrasound testing is performed for 16% to 73% of patients diagnosed with cellulitis. Although testing is common, the pooled incidence of DVT is low (3.1%). Few data elucidate which patients with cellulitis are more likely to have concurrent DVT and require further testing. The Wells clinical prediction rule with
CASE REPORT
A 50-year-old man presented to the emergency department with a 3-day-old cut on his anterior right shin. Associated redness, warmth, pain, and swelling had progressed. The patient had no history of prior DVT or pulmonary embolism (PE). His temperature was 38.5°C, and his white blood cell count of 18,000. On review of systems, he denied shortness of breath and chest pain. He was diagnosed with cellulitis and administered intravenous fluids and cefazolin. The clinician wondered whether to perform lower extremity ultrasound to rule out concurrent DVT.
WHY YOU MIGHT THINK ULTRASOUND IS HELPFUL IN RULING OUT DVT IN CELLULITIS
Lower extremity cellulitis, a common infection of the skin and subcutaneous tissues, is characterized by unilateral erythema, pain, warmth, and swelling. The infection usually follows a skin breach that allows bacteria to enter. DVT may present similarly, and symptoms can include mild leukocytosis and elevated temperature. Because of the clinical similarities, clinicians often order compression ultrasound of the extremity to rule out concurrent DVT in cellulitis. Further impetus for testing stems from fear of the potential complications of untreated DVT, including post-thrombotic syndrome, chronic venous insufficiency, and venous ulceration. A subsequent PE can be fatal, or can cause significant morbidity, including chronic VTE with associated pulmonary hypertension. An estimated quarter of all PEs present as sudden death.1
WHY ULTRASOUND IS NOT HELPFUL IN THIS SETTING
Studies have shown that ultrasound is ordered for 16% to 73% of patients with a cellulitis diagnosis.2,3 Although testing is commonly performed, a meta-analysis of 9 studies of cellulitis patients who underwent ultrasound testing for concurrent DVT revealed a low pooled incidence of total DVT (3.1%) and proximal DVT (2.1%).4 Maze et al.2 retrospectively reviewed 1515 cellulitis cases (identified by International Classification of Diseases, Ninth Revision codes) at a single center in New Zealand over 3 years. Of the 1515 patients, 240 (16%) had ultrasound performed, and only 3 (1.3%) were found to have DVT. Two of the 3 had active malignancy, and the third had injected battery acid into the area. In a 5-year retrospective cohort study at a Veterans Administration hospital in Connecticut, Gunderson and Chang3 reviewed the cases of 183 patients with cellulitis and found ultrasound testing commonly performed (73% of cases) to assess for DVT. Only 1 patient (<1%) was diagnosed with new DVT in the ipsilateral leg, and acute DVT was diagnosed in the contralateral leg of 2 other patients. Overall, these studies indicate the incidence of concurrent DVT in cellulitis is low, regardless of the frequency of ultrasound testing.
Although the cost of a single ultrasound test is not prohibitive, annual total costs hospital-wide and nationally are large. In the United States, the charge for a unilateral duplex ultrasound of the extremity ranges from $260 to $1300, and there is an additional charge for interpretation by a radiologist.5 In a retrospective study spanning 3.5 years and involving 2 community hospitals in Michigan, an estimated $290,000 was spent on ultrasound tests defined as unnecessary for patients with cellulitis.6 A limitation of the study was defining a test as unnecessary based on its result being negative.
DOES WELLS SCORE WITH D-DIMER HELP DEFINE A LOW-RISK POPULATION?
The Wells clinical prediction rule is commonly used to assess the pretest probability of DVT in patients presenting with unilateral leg symptoms. The Wells score is often combined with
WHEN MIGHT ULTRASOUND
Investigators have described possible DVT risk factors in patients with cellulitis, but definitive associations are lacking because of the insufficient number of patients studied.8,9 The most consistently identified DVT risk factor is history of previous thromboembolism. In a retrospective analysis of patients with cellulitis, Afzal et al.6 found that, of the 66.8% who underwent ultrasound testing, 5.5% were identified as having concurrent DVT. The authors performed univariate analyses of 15 potential risk factors, including active malignancy, oral contraceptive pill use, recent hospitalization, and surgery. A higher incidence of DVT was found for patients with history of VTE (odds ratio [OR], 5.7; 95% confidence interval [CI], 2.3-13.7), calf swelling (OR, 4.5; 95% CI, 1.3-15.8), CVA (OR, 3.5; 95% CI, 1.2-10.1), or hypertension (OR, 3.5; 95% CI, 0.98-12.2). Given the wide confidence intervals, paucity of studies, and lack of definitive data in the setting of cellulitis, clinicians may want to consider the risk factors established in larger trials in other settings, including known immobility (OR, <2); thrombophilia, CHF, and CVA with hemiparesis (OR, 2-9); and trauma and recent surgery (OR, >10).10
WHAT YOU SHOULD DO INSTEAD
As the incidence of concurrent VTE in patients with cellulitis is low, the essential step is to make a clear diagnosis of cellulitis based on its established signs and symptoms. A 2-center trial of 145 patients found that cellulitis was diagnosed accurately by general medicine and emergency medicine physicians 72% of the time, with evaluation by dermatologists and infectious disease specialists used as the gold standard. Only 5% of the misdiagnosed patients were diagnosed with DVT; stasis dermatitis was the most common alternative diagnosis. Taking a thorough history may elicit risk factors consistent with cellulitis, such as a recent injury with a break in the skin. On examination, cellulitis should be suspected for patients with fever and localized pain, redness, swelling, and warmth—the cardinal signs of dolor, rubor, tumor, and calor. An injury or entry site and leukocytosis also support the diagnosis of cellulitis. Distinct margins of erythema on the skin are highly suspicious for erysipelas.11 Other physical findings (eg, laceration, purulent drainage, lymphangitic spread, fluctuating mass) also are consistent with a diagnosis of cellulitis.
The patient’s history is also essential in determining whether any DVT risk factors are present. Past medical history of VTE or CVA, or recent history of surgery, immobility, or trauma, should alert the clinician to the possibility of DVT. Family history of VTE increases the likelihood of DVT. Acute shortness of breath or chest pain in the setting of concerning lower extremity findings for DVT should raise concern for DVT and concurrent PE.
If the classic features of cellulitis are present, empiric antibiotics should be initiated. Routine ultrasound testing for all patients with cellulitis is of low value. However, as the incidence of DVT in this population is not negligible, those with VTE risk factors should be targeted for testing. Studies in the setting of cellulitis provide little guidance regarding specific risk factors that can be used to determine who should undergo further testing. Given this limitation, we suggest that clinicians incorporate into their decision making the well-established VTE risk factors identified for large populations studied in other settings, such as the postoperative period. Specifically, clinicians should consider ultrasound testing for patients with cellulitis and prior history of VTE; immobility; thrombophilia, CHF, and CVA with hemiparesis; or trauma and recent surgery.10-12 Ultrasound should also be considered for patients with cellulitis that does not improve and for patients whose localized symptoms worsen despite use of antibiotics.
RECOMMENDATIONS
Do not routinely perform ultrasound to rule out concurrent DVT in cases of cellulitis.
Consider compression ultrasound if there is a history of VTE; immobility; thrombophilia, CHF, and CVA with hemiparesis; or trauma and recent surgery. Also consider it for patients who do not respond to antibiotics.
- In cases of cellulitis, avoid use of the Wells score alone or with
D -dimer testing, as it likely overestimates the DVT risk.
CONCLUSION
The current evidence shows that, for most patients with cellulitis, routine ultrasound testing for DVT is unnecessary. Ultrasound should be considered for patients with potent VTE risk factors. If symptoms do not improve, or if they worsen despite use of antibiotics, clinicians should be alert to potential anchoring bias and consider DVT. The Wells clinical prediction rule overestimates the incidence of DVT in cellulitis and has little value in this setting.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
1. Heit JA. The epidemiology of venous thromboembolism in the community: implications for prevention and management. J Thromb Thrombolysis. 2006;21(1):23-29. PubMed
2. Maze MJ, Pithie A, Dawes T, Chambers ST. An audit of venous duplex ultrasonography in patients with lower limb cellulitis. N Z Med J. 2011;124(1329):53-56. PubMed
3. Gunderson CG, Chang JJ. Overuse of compression ultrasound for patients with lower extremity cellulitis. Thromb Res. 2014;134(4):846-850. PubMed
4. Gunderson CG, Chang JJ. Risk of deep vein thrombosis in patients with cellulitis and erysipelas: a systematic review and meta-analysis. Thromb Res. 2013;132(3):336-340. PubMed
5. Extremity ultrasound (nonvascular) cost and procedure information. http://www.newchoicehealth.com/procedures/extremity-ultrasound-nonvascular. Accessed February 15, 2016.
6. Afzal MZ, Saleh MM, Razvi S, Hashmi H, Lampen R. Utility of lower extremity Doppler in patients with lower extremity cellulitis: a need to change the practice? South Med J. 2015;108(7):439-444. PubMed
7. Goodacre S, Sutton AJ, Sampson FC. Meta-analysis: the value of clinical assessment in the diagnosis of deep venous thrombosis. Ann Intern Med. 2005;143(2):129-139. PubMed
8. Maze MJ, Skea S, Pithie A, Metcalf S, Pearson JF, Chambers ST. Prevalence of concurrent deep vein thrombosis in patients with lower limb cellulitis: a prospective cohort study. BMC Infect Dis. 2013;13:141. PubMed
9. Bersier D, Bounameaux H. Cellulitis and deep vein thrombosis: a controversial association. J Thromb Haemost. 2003;1(4):867-868. PubMed
10. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107(23 suppl 1):I9-I16. PubMed
11. Rabuka CE, Azoulay LY, Kahn SR. Predictors of a positive duplex scan in patients with a clinical presentation compatible with deep vein thrombosis or cellulitis. Can J Infect Dis. 2003;14(4):210-214. PubMed
12. Samama MM. An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the Sirius Study. Arch Intern Med. 2000;160(22):3415-3420. PubMed
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Because of overlapping clinical manifestations, clinicians often order ultrasound to rule out deep vein thrombosis (DVT) in cases of cellulitis. Ultrasound testing is performed for 16% to 73% of patients diagnosed with cellulitis. Although testing is common, the pooled incidence of DVT is low (3.1%). Few data elucidate which patients with cellulitis are more likely to have concurrent DVT and require further testing. The Wells clinical prediction rule with
CASE REPORT
A 50-year-old man presented to the emergency department with a 3-day-old cut on his anterior right shin. Associated redness, warmth, pain, and swelling had progressed. The patient had no history of prior DVT or pulmonary embolism (PE). His temperature was 38.5°C, and his white blood cell count of 18,000. On review of systems, he denied shortness of breath and chest pain. He was diagnosed with cellulitis and administered intravenous fluids and cefazolin. The clinician wondered whether to perform lower extremity ultrasound to rule out concurrent DVT.
WHY YOU MIGHT THINK ULTRASOUND IS HELPFUL IN RULING OUT DVT IN CELLULITIS
Lower extremity cellulitis, a common infection of the skin and subcutaneous tissues, is characterized by unilateral erythema, pain, warmth, and swelling. The infection usually follows a skin breach that allows bacteria to enter. DVT may present similarly, and symptoms can include mild leukocytosis and elevated temperature. Because of the clinical similarities, clinicians often order compression ultrasound of the extremity to rule out concurrent DVT in cellulitis. Further impetus for testing stems from fear of the potential complications of untreated DVT, including post-thrombotic syndrome, chronic venous insufficiency, and venous ulceration. A subsequent PE can be fatal, or can cause significant morbidity, including chronic VTE with associated pulmonary hypertension. An estimated quarter of all PEs present as sudden death.1
WHY ULTRASOUND IS NOT HELPFUL IN THIS SETTING
Studies have shown that ultrasound is ordered for 16% to 73% of patients with a cellulitis diagnosis.2,3 Although testing is commonly performed, a meta-analysis of 9 studies of cellulitis patients who underwent ultrasound testing for concurrent DVT revealed a low pooled incidence of total DVT (3.1%) and proximal DVT (2.1%).4 Maze et al.2 retrospectively reviewed 1515 cellulitis cases (identified by International Classification of Diseases, Ninth Revision codes) at a single center in New Zealand over 3 years. Of the 1515 patients, 240 (16%) had ultrasound performed, and only 3 (1.3%) were found to have DVT. Two of the 3 had active malignancy, and the third had injected battery acid into the area. In a 5-year retrospective cohort study at a Veterans Administration hospital in Connecticut, Gunderson and Chang3 reviewed the cases of 183 patients with cellulitis and found ultrasound testing commonly performed (73% of cases) to assess for DVT. Only 1 patient (<1%) was diagnosed with new DVT in the ipsilateral leg, and acute DVT was diagnosed in the contralateral leg of 2 other patients. Overall, these studies indicate the incidence of concurrent DVT in cellulitis is low, regardless of the frequency of ultrasound testing.
Although the cost of a single ultrasound test is not prohibitive, annual total costs hospital-wide and nationally are large. In the United States, the charge for a unilateral duplex ultrasound of the extremity ranges from $260 to $1300, and there is an additional charge for interpretation by a radiologist.5 In a retrospective study spanning 3.5 years and involving 2 community hospitals in Michigan, an estimated $290,000 was spent on ultrasound tests defined as unnecessary for patients with cellulitis.6 A limitation of the study was defining a test as unnecessary based on its result being negative.
DOES WELLS SCORE WITH D-DIMER HELP DEFINE A LOW-RISK POPULATION?
The Wells clinical prediction rule is commonly used to assess the pretest probability of DVT in patients presenting with unilateral leg symptoms. The Wells score is often combined with
WHEN MIGHT ULTRASOUND
Investigators have described possible DVT risk factors in patients with cellulitis, but definitive associations are lacking because of the insufficient number of patients studied.8,9 The most consistently identified DVT risk factor is history of previous thromboembolism. In a retrospective analysis of patients with cellulitis, Afzal et al.6 found that, of the 66.8% who underwent ultrasound testing, 5.5% were identified as having concurrent DVT. The authors performed univariate analyses of 15 potential risk factors, including active malignancy, oral contraceptive pill use, recent hospitalization, and surgery. A higher incidence of DVT was found for patients with history of VTE (odds ratio [OR], 5.7; 95% confidence interval [CI], 2.3-13.7), calf swelling (OR, 4.5; 95% CI, 1.3-15.8), CVA (OR, 3.5; 95% CI, 1.2-10.1), or hypertension (OR, 3.5; 95% CI, 0.98-12.2). Given the wide confidence intervals, paucity of studies, and lack of definitive data in the setting of cellulitis, clinicians may want to consider the risk factors established in larger trials in other settings, including known immobility (OR, <2); thrombophilia, CHF, and CVA with hemiparesis (OR, 2-9); and trauma and recent surgery (OR, >10).10
WHAT YOU SHOULD DO INSTEAD
As the incidence of concurrent VTE in patients with cellulitis is low, the essential step is to make a clear diagnosis of cellulitis based on its established signs and symptoms. A 2-center trial of 145 patients found that cellulitis was diagnosed accurately by general medicine and emergency medicine physicians 72% of the time, with evaluation by dermatologists and infectious disease specialists used as the gold standard. Only 5% of the misdiagnosed patients were diagnosed with DVT; stasis dermatitis was the most common alternative diagnosis. Taking a thorough history may elicit risk factors consistent with cellulitis, such as a recent injury with a break in the skin. On examination, cellulitis should be suspected for patients with fever and localized pain, redness, swelling, and warmth—the cardinal signs of dolor, rubor, tumor, and calor. An injury or entry site and leukocytosis also support the diagnosis of cellulitis. Distinct margins of erythema on the skin are highly suspicious for erysipelas.11 Other physical findings (eg, laceration, purulent drainage, lymphangitic spread, fluctuating mass) also are consistent with a diagnosis of cellulitis.
The patient’s history is also essential in determining whether any DVT risk factors are present. Past medical history of VTE or CVA, or recent history of surgery, immobility, or trauma, should alert the clinician to the possibility of DVT. Family history of VTE increases the likelihood of DVT. Acute shortness of breath or chest pain in the setting of concerning lower extremity findings for DVT should raise concern for DVT and concurrent PE.
If the classic features of cellulitis are present, empiric antibiotics should be initiated. Routine ultrasound testing for all patients with cellulitis is of low value. However, as the incidence of DVT in this population is not negligible, those with VTE risk factors should be targeted for testing. Studies in the setting of cellulitis provide little guidance regarding specific risk factors that can be used to determine who should undergo further testing. Given this limitation, we suggest that clinicians incorporate into their decision making the well-established VTE risk factors identified for large populations studied in other settings, such as the postoperative period. Specifically, clinicians should consider ultrasound testing for patients with cellulitis and prior history of VTE; immobility; thrombophilia, CHF, and CVA with hemiparesis; or trauma and recent surgery.10-12 Ultrasound should also be considered for patients with cellulitis that does not improve and for patients whose localized symptoms worsen despite use of antibiotics.
RECOMMENDATIONS
Do not routinely perform ultrasound to rule out concurrent DVT in cases of cellulitis.
Consider compression ultrasound if there is a history of VTE; immobility; thrombophilia, CHF, and CVA with hemiparesis; or trauma and recent surgery. Also consider it for patients who do not respond to antibiotics.
- In cases of cellulitis, avoid use of the Wells score alone or with
D -dimer testing, as it likely overestimates the DVT risk.
CONCLUSION
The current evidence shows that, for most patients with cellulitis, routine ultrasound testing for DVT is unnecessary. Ultrasound should be considered for patients with potent VTE risk factors. If symptoms do not improve, or if they worsen despite use of antibiotics, clinicians should be alert to potential anchoring bias and consider DVT. The Wells clinical prediction rule overestimates the incidence of DVT in cellulitis and has little value in this setting.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Because of overlapping clinical manifestations, clinicians often order ultrasound to rule out deep vein thrombosis (DVT) in cases of cellulitis. Ultrasound testing is performed for 16% to 73% of patients diagnosed with cellulitis. Although testing is common, the pooled incidence of DVT is low (3.1%). Few data elucidate which patients with cellulitis are more likely to have concurrent DVT and require further testing. The Wells clinical prediction rule with
CASE REPORT
A 50-year-old man presented to the emergency department with a 3-day-old cut on his anterior right shin. Associated redness, warmth, pain, and swelling had progressed. The patient had no history of prior DVT or pulmonary embolism (PE). His temperature was 38.5°C, and his white blood cell count of 18,000. On review of systems, he denied shortness of breath and chest pain. He was diagnosed with cellulitis and administered intravenous fluids and cefazolin. The clinician wondered whether to perform lower extremity ultrasound to rule out concurrent DVT.
WHY YOU MIGHT THINK ULTRASOUND IS HELPFUL IN RULING OUT DVT IN CELLULITIS
Lower extremity cellulitis, a common infection of the skin and subcutaneous tissues, is characterized by unilateral erythema, pain, warmth, and swelling. The infection usually follows a skin breach that allows bacteria to enter. DVT may present similarly, and symptoms can include mild leukocytosis and elevated temperature. Because of the clinical similarities, clinicians often order compression ultrasound of the extremity to rule out concurrent DVT in cellulitis. Further impetus for testing stems from fear of the potential complications of untreated DVT, including post-thrombotic syndrome, chronic venous insufficiency, and venous ulceration. A subsequent PE can be fatal, or can cause significant morbidity, including chronic VTE with associated pulmonary hypertension. An estimated quarter of all PEs present as sudden death.1
WHY ULTRASOUND IS NOT HELPFUL IN THIS SETTING
Studies have shown that ultrasound is ordered for 16% to 73% of patients with a cellulitis diagnosis.2,3 Although testing is commonly performed, a meta-analysis of 9 studies of cellulitis patients who underwent ultrasound testing for concurrent DVT revealed a low pooled incidence of total DVT (3.1%) and proximal DVT (2.1%).4 Maze et al.2 retrospectively reviewed 1515 cellulitis cases (identified by International Classification of Diseases, Ninth Revision codes) at a single center in New Zealand over 3 years. Of the 1515 patients, 240 (16%) had ultrasound performed, and only 3 (1.3%) were found to have DVT. Two of the 3 had active malignancy, and the third had injected battery acid into the area. In a 5-year retrospective cohort study at a Veterans Administration hospital in Connecticut, Gunderson and Chang3 reviewed the cases of 183 patients with cellulitis and found ultrasound testing commonly performed (73% of cases) to assess for DVT. Only 1 patient (<1%) was diagnosed with new DVT in the ipsilateral leg, and acute DVT was diagnosed in the contralateral leg of 2 other patients. Overall, these studies indicate the incidence of concurrent DVT in cellulitis is low, regardless of the frequency of ultrasound testing.
Although the cost of a single ultrasound test is not prohibitive, annual total costs hospital-wide and nationally are large. In the United States, the charge for a unilateral duplex ultrasound of the extremity ranges from $260 to $1300, and there is an additional charge for interpretation by a radiologist.5 In a retrospective study spanning 3.5 years and involving 2 community hospitals in Michigan, an estimated $290,000 was spent on ultrasound tests defined as unnecessary for patients with cellulitis.6 A limitation of the study was defining a test as unnecessary based on its result being negative.
DOES WELLS SCORE WITH D-DIMER HELP DEFINE A LOW-RISK POPULATION?
The Wells clinical prediction rule is commonly used to assess the pretest probability of DVT in patients presenting with unilateral leg symptoms. The Wells score is often combined with
WHEN MIGHT ULTRASOUND
Investigators have described possible DVT risk factors in patients with cellulitis, but definitive associations are lacking because of the insufficient number of patients studied.8,9 The most consistently identified DVT risk factor is history of previous thromboembolism. In a retrospective analysis of patients with cellulitis, Afzal et al.6 found that, of the 66.8% who underwent ultrasound testing, 5.5% were identified as having concurrent DVT. The authors performed univariate analyses of 15 potential risk factors, including active malignancy, oral contraceptive pill use, recent hospitalization, and surgery. A higher incidence of DVT was found for patients with history of VTE (odds ratio [OR], 5.7; 95% confidence interval [CI], 2.3-13.7), calf swelling (OR, 4.5; 95% CI, 1.3-15.8), CVA (OR, 3.5; 95% CI, 1.2-10.1), or hypertension (OR, 3.5; 95% CI, 0.98-12.2). Given the wide confidence intervals, paucity of studies, and lack of definitive data in the setting of cellulitis, clinicians may want to consider the risk factors established in larger trials in other settings, including known immobility (OR, <2); thrombophilia, CHF, and CVA with hemiparesis (OR, 2-9); and trauma and recent surgery (OR, >10).10
WHAT YOU SHOULD DO INSTEAD
As the incidence of concurrent VTE in patients with cellulitis is low, the essential step is to make a clear diagnosis of cellulitis based on its established signs and symptoms. A 2-center trial of 145 patients found that cellulitis was diagnosed accurately by general medicine and emergency medicine physicians 72% of the time, with evaluation by dermatologists and infectious disease specialists used as the gold standard. Only 5% of the misdiagnosed patients were diagnosed with DVT; stasis dermatitis was the most common alternative diagnosis. Taking a thorough history may elicit risk factors consistent with cellulitis, such as a recent injury with a break in the skin. On examination, cellulitis should be suspected for patients with fever and localized pain, redness, swelling, and warmth—the cardinal signs of dolor, rubor, tumor, and calor. An injury or entry site and leukocytosis also support the diagnosis of cellulitis. Distinct margins of erythema on the skin are highly suspicious for erysipelas.11 Other physical findings (eg, laceration, purulent drainage, lymphangitic spread, fluctuating mass) also are consistent with a diagnosis of cellulitis.
The patient’s history is also essential in determining whether any DVT risk factors are present. Past medical history of VTE or CVA, or recent history of surgery, immobility, or trauma, should alert the clinician to the possibility of DVT. Family history of VTE increases the likelihood of DVT. Acute shortness of breath or chest pain in the setting of concerning lower extremity findings for DVT should raise concern for DVT and concurrent PE.
If the classic features of cellulitis are present, empiric antibiotics should be initiated. Routine ultrasound testing for all patients with cellulitis is of low value. However, as the incidence of DVT in this population is not negligible, those with VTE risk factors should be targeted for testing. Studies in the setting of cellulitis provide little guidance regarding specific risk factors that can be used to determine who should undergo further testing. Given this limitation, we suggest that clinicians incorporate into their decision making the well-established VTE risk factors identified for large populations studied in other settings, such as the postoperative period. Specifically, clinicians should consider ultrasound testing for patients with cellulitis and prior history of VTE; immobility; thrombophilia, CHF, and CVA with hemiparesis; or trauma and recent surgery.10-12 Ultrasound should also be considered for patients with cellulitis that does not improve and for patients whose localized symptoms worsen despite use of antibiotics.
RECOMMENDATIONS
Do not routinely perform ultrasound to rule out concurrent DVT in cases of cellulitis.
Consider compression ultrasound if there is a history of VTE; immobility; thrombophilia, CHF, and CVA with hemiparesis; or trauma and recent surgery. Also consider it for patients who do not respond to antibiotics.
- In cases of cellulitis, avoid use of the Wells score alone or with
D -dimer testing, as it likely overestimates the DVT risk.
CONCLUSION
The current evidence shows that, for most patients with cellulitis, routine ultrasound testing for DVT is unnecessary. Ultrasound should be considered for patients with potent VTE risk factors. If symptoms do not improve, or if they worsen despite use of antibiotics, clinicians should be alert to potential anchoring bias and consider DVT. The Wells clinical prediction rule overestimates the incidence of DVT in cellulitis and has little value in this setting.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
1. Heit JA. The epidemiology of venous thromboembolism in the community: implications for prevention and management. J Thromb Thrombolysis. 2006;21(1):23-29. PubMed
2. Maze MJ, Pithie A, Dawes T, Chambers ST. An audit of venous duplex ultrasonography in patients with lower limb cellulitis. N Z Med J. 2011;124(1329):53-56. PubMed
3. Gunderson CG, Chang JJ. Overuse of compression ultrasound for patients with lower extremity cellulitis. Thromb Res. 2014;134(4):846-850. PubMed
4. Gunderson CG, Chang JJ. Risk of deep vein thrombosis in patients with cellulitis and erysipelas: a systematic review and meta-analysis. Thromb Res. 2013;132(3):336-340. PubMed
5. Extremity ultrasound (nonvascular) cost and procedure information. http://www.newchoicehealth.com/procedures/extremity-ultrasound-nonvascular. Accessed February 15, 2016.
6. Afzal MZ, Saleh MM, Razvi S, Hashmi H, Lampen R. Utility of lower extremity Doppler in patients with lower extremity cellulitis: a need to change the practice? South Med J. 2015;108(7):439-444. PubMed
7. Goodacre S, Sutton AJ, Sampson FC. Meta-analysis: the value of clinical assessment in the diagnosis of deep venous thrombosis. Ann Intern Med. 2005;143(2):129-139. PubMed
8. Maze MJ, Skea S, Pithie A, Metcalf S, Pearson JF, Chambers ST. Prevalence of concurrent deep vein thrombosis in patients with lower limb cellulitis: a prospective cohort study. BMC Infect Dis. 2013;13:141. PubMed
9. Bersier D, Bounameaux H. Cellulitis and deep vein thrombosis: a controversial association. J Thromb Haemost. 2003;1(4):867-868. PubMed
10. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107(23 suppl 1):I9-I16. PubMed
11. Rabuka CE, Azoulay LY, Kahn SR. Predictors of a positive duplex scan in patients with a clinical presentation compatible with deep vein thrombosis or cellulitis. Can J Infect Dis. 2003;14(4):210-214. PubMed
12. Samama MM. An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the Sirius Study. Arch Intern Med. 2000;160(22):3415-3420. PubMed
1. Heit JA. The epidemiology of venous thromboembolism in the community: implications for prevention and management. J Thromb Thrombolysis. 2006;21(1):23-29. PubMed
2. Maze MJ, Pithie A, Dawes T, Chambers ST. An audit of venous duplex ultrasonography in patients with lower limb cellulitis. N Z Med J. 2011;124(1329):53-56. PubMed
3. Gunderson CG, Chang JJ. Overuse of compression ultrasound for patients with lower extremity cellulitis. Thromb Res. 2014;134(4):846-850. PubMed
4. Gunderson CG, Chang JJ. Risk of deep vein thrombosis in patients with cellulitis and erysipelas: a systematic review and meta-analysis. Thromb Res. 2013;132(3):336-340. PubMed
5. Extremity ultrasound (nonvascular) cost and procedure information. http://www.newchoicehealth.com/procedures/extremity-ultrasound-nonvascular. Accessed February 15, 2016.
6. Afzal MZ, Saleh MM, Razvi S, Hashmi H, Lampen R. Utility of lower extremity Doppler in patients with lower extremity cellulitis: a need to change the practice? South Med J. 2015;108(7):439-444. PubMed
7. Goodacre S, Sutton AJ, Sampson FC. Meta-analysis: the value of clinical assessment in the diagnosis of deep venous thrombosis. Ann Intern Med. 2005;143(2):129-139. PubMed
8. Maze MJ, Skea S, Pithie A, Metcalf S, Pearson JF, Chambers ST. Prevalence of concurrent deep vein thrombosis in patients with lower limb cellulitis: a prospective cohort study. BMC Infect Dis. 2013;13:141. PubMed
9. Bersier D, Bounameaux H. Cellulitis and deep vein thrombosis: a controversial association. J Thromb Haemost. 2003;1(4):867-868. PubMed
10. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107(23 suppl 1):I9-I16. PubMed
11. Rabuka CE, Azoulay LY, Kahn SR. Predictors of a positive duplex scan in patients with a clinical presentation compatible with deep vein thrombosis or cellulitis. Can J Infect Dis. 2003;14(4):210-214. PubMed
12. Samama MM. An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the Sirius Study. Arch Intern Med. 2000;160(22):3415-3420. PubMed
© 2017 Society of Hospital Medicine
Are we causing anemia by ordering unnecessary blood tests?
A 68-year-old woman is admitted for community-acquired pneumonia. She receives antibiotics, and her condition begins to improve after 2 days. She has her blood drawn daily throughout her admission.
On hospital day 3, she complains of fatigue, and on day 4, laboratory results show that her hemoglobin and hematocrit values have fallen. To make sure this result is not spurious, her blood is drawn again to repeat the test. On day 5, her hemoglobin level has dropped to 7.0 g/dL, which is 2 g/dL lower than at admission, and she receives a transfusion.
On day 7, her hemoglobin level is stable at 8.5 g/dL, and her physicians decide to discharge her. The morning of her discharge, as a nurse is about to draw her blood, the patient asks, “Are all these blood tests really necessary?”
DO WE DRAW TOO MUCH BLOOD?
This case portrays a common occurrence. Significant amounts of blood are drawn from patients, especially in critical care. Clinical uncertainty drives most laboratory testing ordered by physicians. Too often, however, these tests lead to more testing and interventions, without a clear benefit to the patient.1
When blood testing leads to more testing, a patient’s hemoglobin and hematocrit can fall. Symptomatic iatrogenic anemia is associated with significant morbidity for patients with preexisting cardiopulmonary disease.
We draw much larger volumes of blood than most testing guidelines say are necessary. One author2 has noted that 50 to 60 mL of blood is removed for each set of tests, owing to the size of collection tubes, multiple reagents needed for each test, and the possibility that tests may need to be rerun. Yet about 3 mL of blood is sufficient to perform most laboratory tests even if the test needs to be rerun.2
CAN BLOOD DRAWS CAUSE ANEMIA?
A relationship between the volume of blood drawn and iatrogenic anemia was first described in 2005, when Thavendiranathan et al3 found that in adult patients on general medicine floors, the volume of blood drawn strongly predicted decreased hemoglobin and hematocrit levels. For every 100 mL of blood drawn, hemoglobin levels fell by an average of 0.7 g/dL, and 13.9% of the patients in the study had iron studies and fecal occult blood tests performed to investigate anemia.
Kurniali et al4 reported that during an average admission, 65% of patients experienced a drop in hemoglobin of 1.0 g/dL or more, and 49% developed anemia.
Salisbury et al,5 in 2011, studied 17,676 patients with acute myocardial infarction across 57 centers and found a correlation between the volume of blood taken and the development of anemia. On average, for every 50 mL of blood drawn, the risk of moderate to severe iatrogenic anemia increased by 18%. They also found significant variation in blood loss from testing in patients who developed moderate or severe anemia. The authors believed this indicated that moderate to severe anemia was more frequent at centers with higher than average diagnostic blood loss.5
This relationship has also been described in patients in intensive care, where it contributes to anemia of chronic disease. While anemia of critical illness is multifactorial, phlebotomy contributes to anemia in both short- and long-term stays in the intensive care unit.6
CHOOSING WISELY GUIDELINES
The Choosing Wisely initiative of the American Board of Internal Medicine Foundation collects recommendations by a number of medical specialty societies to reduce overuse of healthcare resources.7 The Critical Care Societies Collaborative recommends ordering diagnostic tests only when they answer specific clinical questions rather than routinely. The Society of Hospital Medicine also recommends against repeat complete blood cell count and blood chemistry testing because it may contribute to anemia, which is of particular concern in patients with cardiorespiratory disease.
POSSIBLE HARM
The Critical Care Societies Collaborative, in its Choosing Wisely Guidelines, specifically cites anemia as a potential harm of unnecessary phlebotomy, noting it may result in transfusion, with its associated risks and costs. In addition, aggressive investigation of incidental and nonpathologic results of routine studies is wasteful and exposes the patient to additional risks.
REDUCING PHLEBOTOMY DECREASES IATROGENIC ANEMIA
Since the relationship between excessive phlebotomy and iatrogenic anemia was described, hospitals have attempted to address the problem.
In 2011, Stuebing and Miner8 described an intervention in which the house staff and attending physicians on non-intensive care surgical services were given weekly reports of the cost of the laboratory services for the previous week. They found that simply making providers aware of the cost of their tests reduced the number of tests ordered and resulted in significant hospital savings.
Another strategy is to use pediatric collection tubes in adult patients. A 2008 study in which all blood samples were drawn using pediatric tubes reduced the blood volume removed per patient by almost 75% in inpatient and critical care patients, without the need for repeat blood draws.9 However, Kurniali et al found that the use of pediatric collection tubes did not significantly change hemoglobin fluctuations throughout patient hospital stays.4
Corson et al10 in 2015 described an intervention involving detailing, auditing, and giving feedback regarding the frequency of laboratory tests commonly ordered by a group of hospitalists. The intervention resulted in a modest reduction in the number of common laboratory tests ordered per patient day and in hospital costs, without any changes in the length of hospital stay, mortality rate, or readmission rate.10
THE CLINICAL BOTTOM LINE
As a general principle, diagnostic testing should be done to answer specific diagnostic questions and to guide management. Ordering of diagnostic tests should be decided on a day-to-day basis rather than scheduled automatically or done reflexively. In the case of blood draws, the volume of blood drawn is significantly increased by unnecessary testing, resulting in higher rates of hospital-acquired anemia.
- Ezzie ME, Aberegg SK, O’Brien JM Jr. Laboratory testing in the intensive care unit. Crit Care Clin 2007; 23:435–465.
- Stefanini M. Iatrogenic anemia (can it be prevented?). J Thromb Haemost 2014; 12:1591.
- Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med 2005; 20:520–524.
- Kurniali PC, Curry S, Brennan KW, et al. A retrospective study investigating the incidence and predisposing factors of hospital-acquired anemia. Anemia 2014; 2014:634582.
- Salisbury AC, Reid KJ, Alexander KP, et al. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch Intern Med 2011; 171:1646–1653.
- Walsh TS, Lee RJ, Maciver CR, et al. Anemia during and at discharge from intensive care: the impact of restrictive blood transfusion practice. Intensive Care Med 2006; 32:100–109.
- American Board of Internal Medicine Foundation. Choosing Wisely. www.abimfoundation.org/Initiatives/Choosing-Wisely.aspx. Accessed April 19, 2016.
- Stuebing EA, Miner TJ. Surgical vampires and rising health care expenditure: reducing the cost of daily phlebotomy. Arch Surg 2011; 146:524–527.
- Sanchez-Giron F, Alvarez-Mora F. Reduction of blood loss from laboratory testing in hospitalized adult patients using small-volume (pediatric) tubes. Arch Pathol Lab Med 2008; 132:1916–1919.
- Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: decreased frequency of common labs. J Hosp Med 2015; 10:390–395.
A 68-year-old woman is admitted for community-acquired pneumonia. She receives antibiotics, and her condition begins to improve after 2 days. She has her blood drawn daily throughout her admission.
On hospital day 3, she complains of fatigue, and on day 4, laboratory results show that her hemoglobin and hematocrit values have fallen. To make sure this result is not spurious, her blood is drawn again to repeat the test. On day 5, her hemoglobin level has dropped to 7.0 g/dL, which is 2 g/dL lower than at admission, and she receives a transfusion.
On day 7, her hemoglobin level is stable at 8.5 g/dL, and her physicians decide to discharge her. The morning of her discharge, as a nurse is about to draw her blood, the patient asks, “Are all these blood tests really necessary?”
DO WE DRAW TOO MUCH BLOOD?
This case portrays a common occurrence. Significant amounts of blood are drawn from patients, especially in critical care. Clinical uncertainty drives most laboratory testing ordered by physicians. Too often, however, these tests lead to more testing and interventions, without a clear benefit to the patient.1
When blood testing leads to more testing, a patient’s hemoglobin and hematocrit can fall. Symptomatic iatrogenic anemia is associated with significant morbidity for patients with preexisting cardiopulmonary disease.
We draw much larger volumes of blood than most testing guidelines say are necessary. One author2 has noted that 50 to 60 mL of blood is removed for each set of tests, owing to the size of collection tubes, multiple reagents needed for each test, and the possibility that tests may need to be rerun. Yet about 3 mL of blood is sufficient to perform most laboratory tests even if the test needs to be rerun.2
CAN BLOOD DRAWS CAUSE ANEMIA?
A relationship between the volume of blood drawn and iatrogenic anemia was first described in 2005, when Thavendiranathan et al3 found that in adult patients on general medicine floors, the volume of blood drawn strongly predicted decreased hemoglobin and hematocrit levels. For every 100 mL of blood drawn, hemoglobin levels fell by an average of 0.7 g/dL, and 13.9% of the patients in the study had iron studies and fecal occult blood tests performed to investigate anemia.
Kurniali et al4 reported that during an average admission, 65% of patients experienced a drop in hemoglobin of 1.0 g/dL or more, and 49% developed anemia.
Salisbury et al,5 in 2011, studied 17,676 patients with acute myocardial infarction across 57 centers and found a correlation between the volume of blood taken and the development of anemia. On average, for every 50 mL of blood drawn, the risk of moderate to severe iatrogenic anemia increased by 18%. They also found significant variation in blood loss from testing in patients who developed moderate or severe anemia. The authors believed this indicated that moderate to severe anemia was more frequent at centers with higher than average diagnostic blood loss.5
This relationship has also been described in patients in intensive care, where it contributes to anemia of chronic disease. While anemia of critical illness is multifactorial, phlebotomy contributes to anemia in both short- and long-term stays in the intensive care unit.6
CHOOSING WISELY GUIDELINES
The Choosing Wisely initiative of the American Board of Internal Medicine Foundation collects recommendations by a number of medical specialty societies to reduce overuse of healthcare resources.7 The Critical Care Societies Collaborative recommends ordering diagnostic tests only when they answer specific clinical questions rather than routinely. The Society of Hospital Medicine also recommends against repeat complete blood cell count and blood chemistry testing because it may contribute to anemia, which is of particular concern in patients with cardiorespiratory disease.
POSSIBLE HARM
The Critical Care Societies Collaborative, in its Choosing Wisely Guidelines, specifically cites anemia as a potential harm of unnecessary phlebotomy, noting it may result in transfusion, with its associated risks and costs. In addition, aggressive investigation of incidental and nonpathologic results of routine studies is wasteful and exposes the patient to additional risks.
REDUCING PHLEBOTOMY DECREASES IATROGENIC ANEMIA
Since the relationship between excessive phlebotomy and iatrogenic anemia was described, hospitals have attempted to address the problem.
In 2011, Stuebing and Miner8 described an intervention in which the house staff and attending physicians on non-intensive care surgical services were given weekly reports of the cost of the laboratory services for the previous week. They found that simply making providers aware of the cost of their tests reduced the number of tests ordered and resulted in significant hospital savings.
Another strategy is to use pediatric collection tubes in adult patients. A 2008 study in which all blood samples were drawn using pediatric tubes reduced the blood volume removed per patient by almost 75% in inpatient and critical care patients, without the need for repeat blood draws.9 However, Kurniali et al found that the use of pediatric collection tubes did not significantly change hemoglobin fluctuations throughout patient hospital stays.4
Corson et al10 in 2015 described an intervention involving detailing, auditing, and giving feedback regarding the frequency of laboratory tests commonly ordered by a group of hospitalists. The intervention resulted in a modest reduction in the number of common laboratory tests ordered per patient day and in hospital costs, without any changes in the length of hospital stay, mortality rate, or readmission rate.10
THE CLINICAL BOTTOM LINE
As a general principle, diagnostic testing should be done to answer specific diagnostic questions and to guide management. Ordering of diagnostic tests should be decided on a day-to-day basis rather than scheduled automatically or done reflexively. In the case of blood draws, the volume of blood drawn is significantly increased by unnecessary testing, resulting in higher rates of hospital-acquired anemia.
A 68-year-old woman is admitted for community-acquired pneumonia. She receives antibiotics, and her condition begins to improve after 2 days. She has her blood drawn daily throughout her admission.
On hospital day 3, she complains of fatigue, and on day 4, laboratory results show that her hemoglobin and hematocrit values have fallen. To make sure this result is not spurious, her blood is drawn again to repeat the test. On day 5, her hemoglobin level has dropped to 7.0 g/dL, which is 2 g/dL lower than at admission, and she receives a transfusion.
On day 7, her hemoglobin level is stable at 8.5 g/dL, and her physicians decide to discharge her. The morning of her discharge, as a nurse is about to draw her blood, the patient asks, “Are all these blood tests really necessary?”
DO WE DRAW TOO MUCH BLOOD?
This case portrays a common occurrence. Significant amounts of blood are drawn from patients, especially in critical care. Clinical uncertainty drives most laboratory testing ordered by physicians. Too often, however, these tests lead to more testing and interventions, without a clear benefit to the patient.1
When blood testing leads to more testing, a patient’s hemoglobin and hematocrit can fall. Symptomatic iatrogenic anemia is associated with significant morbidity for patients with preexisting cardiopulmonary disease.
We draw much larger volumes of blood than most testing guidelines say are necessary. One author2 has noted that 50 to 60 mL of blood is removed for each set of tests, owing to the size of collection tubes, multiple reagents needed for each test, and the possibility that tests may need to be rerun. Yet about 3 mL of blood is sufficient to perform most laboratory tests even if the test needs to be rerun.2
CAN BLOOD DRAWS CAUSE ANEMIA?
A relationship between the volume of blood drawn and iatrogenic anemia was first described in 2005, when Thavendiranathan et al3 found that in adult patients on general medicine floors, the volume of blood drawn strongly predicted decreased hemoglobin and hematocrit levels. For every 100 mL of blood drawn, hemoglobin levels fell by an average of 0.7 g/dL, and 13.9% of the patients in the study had iron studies and fecal occult blood tests performed to investigate anemia.
Kurniali et al4 reported that during an average admission, 65% of patients experienced a drop in hemoglobin of 1.0 g/dL or more, and 49% developed anemia.
Salisbury et al,5 in 2011, studied 17,676 patients with acute myocardial infarction across 57 centers and found a correlation between the volume of blood taken and the development of anemia. On average, for every 50 mL of blood drawn, the risk of moderate to severe iatrogenic anemia increased by 18%. They also found significant variation in blood loss from testing in patients who developed moderate or severe anemia. The authors believed this indicated that moderate to severe anemia was more frequent at centers with higher than average diagnostic blood loss.5
This relationship has also been described in patients in intensive care, where it contributes to anemia of chronic disease. While anemia of critical illness is multifactorial, phlebotomy contributes to anemia in both short- and long-term stays in the intensive care unit.6
CHOOSING WISELY GUIDELINES
The Choosing Wisely initiative of the American Board of Internal Medicine Foundation collects recommendations by a number of medical specialty societies to reduce overuse of healthcare resources.7 The Critical Care Societies Collaborative recommends ordering diagnostic tests only when they answer specific clinical questions rather than routinely. The Society of Hospital Medicine also recommends against repeat complete blood cell count and blood chemistry testing because it may contribute to anemia, which is of particular concern in patients with cardiorespiratory disease.
POSSIBLE HARM
The Critical Care Societies Collaborative, in its Choosing Wisely Guidelines, specifically cites anemia as a potential harm of unnecessary phlebotomy, noting it may result in transfusion, with its associated risks and costs. In addition, aggressive investigation of incidental and nonpathologic results of routine studies is wasteful and exposes the patient to additional risks.
REDUCING PHLEBOTOMY DECREASES IATROGENIC ANEMIA
Since the relationship between excessive phlebotomy and iatrogenic anemia was described, hospitals have attempted to address the problem.
In 2011, Stuebing and Miner8 described an intervention in which the house staff and attending physicians on non-intensive care surgical services were given weekly reports of the cost of the laboratory services for the previous week. They found that simply making providers aware of the cost of their tests reduced the number of tests ordered and resulted in significant hospital savings.
Another strategy is to use pediatric collection tubes in adult patients. A 2008 study in which all blood samples were drawn using pediatric tubes reduced the blood volume removed per patient by almost 75% in inpatient and critical care patients, without the need for repeat blood draws.9 However, Kurniali et al found that the use of pediatric collection tubes did not significantly change hemoglobin fluctuations throughout patient hospital stays.4
Corson et al10 in 2015 described an intervention involving detailing, auditing, and giving feedback regarding the frequency of laboratory tests commonly ordered by a group of hospitalists. The intervention resulted in a modest reduction in the number of common laboratory tests ordered per patient day and in hospital costs, without any changes in the length of hospital stay, mortality rate, or readmission rate.10
THE CLINICAL BOTTOM LINE
As a general principle, diagnostic testing should be done to answer specific diagnostic questions and to guide management. Ordering of diagnostic tests should be decided on a day-to-day basis rather than scheduled automatically or done reflexively. In the case of blood draws, the volume of blood drawn is significantly increased by unnecessary testing, resulting in higher rates of hospital-acquired anemia.
- Ezzie ME, Aberegg SK, O’Brien JM Jr. Laboratory testing in the intensive care unit. Crit Care Clin 2007; 23:435–465.
- Stefanini M. Iatrogenic anemia (can it be prevented?). J Thromb Haemost 2014; 12:1591.
- Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med 2005; 20:520–524.
- Kurniali PC, Curry S, Brennan KW, et al. A retrospective study investigating the incidence and predisposing factors of hospital-acquired anemia. Anemia 2014; 2014:634582.
- Salisbury AC, Reid KJ, Alexander KP, et al. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch Intern Med 2011; 171:1646–1653.
- Walsh TS, Lee RJ, Maciver CR, et al. Anemia during and at discharge from intensive care: the impact of restrictive blood transfusion practice. Intensive Care Med 2006; 32:100–109.
- American Board of Internal Medicine Foundation. Choosing Wisely. www.abimfoundation.org/Initiatives/Choosing-Wisely.aspx. Accessed April 19, 2016.
- Stuebing EA, Miner TJ. Surgical vampires and rising health care expenditure: reducing the cost of daily phlebotomy. Arch Surg 2011; 146:524–527.
- Sanchez-Giron F, Alvarez-Mora F. Reduction of blood loss from laboratory testing in hospitalized adult patients using small-volume (pediatric) tubes. Arch Pathol Lab Med 2008; 132:1916–1919.
- Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: decreased frequency of common labs. J Hosp Med 2015; 10:390–395.
- Ezzie ME, Aberegg SK, O’Brien JM Jr. Laboratory testing in the intensive care unit. Crit Care Clin 2007; 23:435–465.
- Stefanini M. Iatrogenic anemia (can it be prevented?). J Thromb Haemost 2014; 12:1591.
- Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med 2005; 20:520–524.
- Kurniali PC, Curry S, Brennan KW, et al. A retrospective study investigating the incidence and predisposing factors of hospital-acquired anemia. Anemia 2014; 2014:634582.
- Salisbury AC, Reid KJ, Alexander KP, et al. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch Intern Med 2011; 171:1646–1653.
- Walsh TS, Lee RJ, Maciver CR, et al. Anemia during and at discharge from intensive care: the impact of restrictive blood transfusion practice. Intensive Care Med 2006; 32:100–109.
- American Board of Internal Medicine Foundation. Choosing Wisely. www.abimfoundation.org/Initiatives/Choosing-Wisely.aspx. Accessed April 19, 2016.
- Stuebing EA, Miner TJ. Surgical vampires and rising health care expenditure: reducing the cost of daily phlebotomy. Arch Surg 2011; 146:524–527.
- Sanchez-Giron F, Alvarez-Mora F. Reduction of blood loss from laboratory testing in hospitalized adult patients using small-volume (pediatric) tubes. Arch Pathol Lab Med 2008; 132:1916–1919.
- Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: decreased frequency of common labs. J Hosp Med 2015; 10:390–395.
Improving Anticoagulation Transitions
Anticoagulants are among the prescriptions with the highest risk of an adverse drug event (ADE) after discharge, and many of these events are preventable.[1, 2, 3] In recognition of the high risk for adverse events, the Institute for Healthcare Improvement Map details several key features of a safe anticoagulation management program, including the recommendation during the transition period that clinicians ensure proper lab monitoring and establish capacity for follow‐up testing.[4]
Despite the potential for harm, most hospitals do not have a structured process for the transmission of vital information related to warfarin management from the inpatient to the ambulatory setting. Our aim was to develop a concise report containing the essential information regarding the patient's anticoagulation regimen, the Safe Transitions Anticoagulation Report (STAR), and a process to ensure the report can be readily accessed and utilized by ambulatory clinicians.
METHODS
We assembled an interdisciplinary team to develop a new report and workflow to ensure that information on inpatient warfarin management was transmitted to outpatient providers in a reliable and structured manner. Explicit goals were to maximize use of the electronic medical record (EMR) to autopopulate aspects of the new report and create a process that worked seamlessly into the workflow. The final items were selected based on the risk of harm if not conveyed and feasibility of incorporation through the EMR:
- Warfarin doses: the 7 warfarin doses immediately prior to discharge
- International normalized ratio (INR) values: the 7 INR values immediately prior to discharge
- Bridging anticoagulation: the low‐molecular‐weight heparin (LMWH) prescribed as a bridging anticoagulant, if any
The STAR resides in both the discharge summary and the after visit summary (AVS) for patients discharged on warfarin. At our institution, the AVS contains a medication list, discharge instructions, and appointments, and is automatically produced through our EMR. Our institution utilizes the Epic EMR (Epic Systems Corp., Verona, WI) in the hospital, ambulatory clinics, and faculty practices.
A process was developed where a structured table is automatically created (Figure 1). A field was added to our EMR's discharge summary template asking whether the patient is being discharged on warfarin. Answering yes produces a second question asking whether the patient is also being discharged on bridging anticoagulation with LMWH. The STAR is not inserted into the discharge summary if the clinician completing the discharge summary deletes the anticoagulation question. The STAR is automatically created for patients discharged on warfarin and inserted into the AVS by the EMR regardless of whether the discharge summary has been completed. Patients are instructed by their nurse to bring their AVS to their follow‐up appointments.
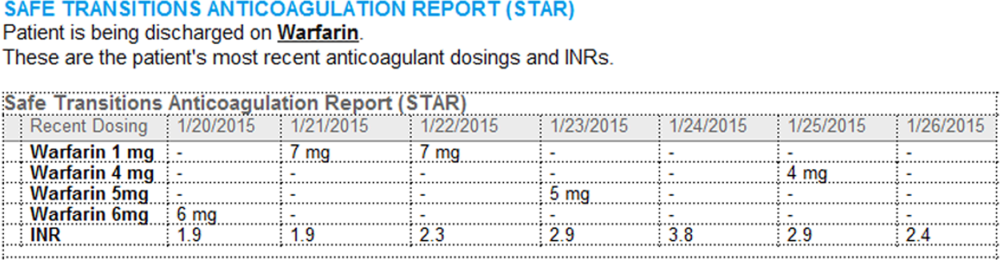
The STAR project team utilized plan‐do‐study‐act cycles to test small changes and make revisions. The workflow was piloted on 2 medical/surgical units from January 2014 through March 2014, and revised based on feedback from clinicians and nursing staff. The STAR initiative was fully implemented across our institution in April 2014.
The study was evaluated by the institutional review board of the Icahn School of Medicine at Mount Sinai, and full review was waived.
Outcomes
Our primary outcomes were the timeliness of laboratory monitoring and quality of anticoagulation management for patients with an established relationship at 1 of the main outpatient practices in our system. Our institution has an anticoagulation clinic for patients followed at the general medicine clinic. We defined an established relationship as having been seen in the same practice on at least 2 occasions in the 12 months prior to admission. The primary outcomes were the percentage of patients who had an INR measurement done and the percentage who had a therapeutic INR value within 10 days after discharge. As the 10‐day period is arbitrary, we also assessed these outcomes for the 3‐, 7‐, and 30‐day periods after discharge. The therapeutic range was defined for all patients as an INR of 2.0 to 3.0, as this is the target range for the large majority of patients on warfarin in our system. Outcomes during the intervention period were compared to baseline values during the preintervention period. For patients with multiple admissions, the first admission during each period was included.
Ambulatory Physician Survey
We surveyed ambulatory care physicians at the main practices for our health system. The survey assessed how often the STAR was viewed and incorporated into decision making, whether the report improved workflow, and whether ambulatory providers perceived that the report increased patient safety. The survey was distributed at the 6‐month interval during the intervention phase. The survey was disseminated electronically on 3 occasions, and a paper version was distributed on 1 occasion to housestaff and general medicine faculty.
Statistical Analysis
Comparisons for categorical data were performed using the 2 test. P values were based on 2‐tailed tests of significance, and a value 0.05 was considered significant.
RESULTS
The STAR was embedded in the discharge summary for 1370 (78.6%) discharges during the intervention period. A total of 505 patients in the preintervention period and 292 patients in the intervention period were established patients at an affiliated practice and comprised the study population. Demographics and indications for warfarin for the preintervention and intervention groups are listed in the Table 1.
| Preintervention Group, N=505 | Intervention Group, N=292 | P Value | |
|---|---|---|---|
| |||
| Age, y | 66.7 | 68.0 | 0.29 |
| Male gender, n (%) | 236 (46.7) | 153 (52.4) | 0.12 |
| Discharged on bridging agent, n (%) | 90 (17.8) | 36 (12.3) | 0.04 |
| Average length of stay, d | 7.1 | 7.6 | 0.46 |
| Newly prescribed warfarin, n (%) | 147 (29.1) | 62 (21.2) | 0.01 |
| INR 2.03.0 range at discharge, n (%) | 187 (37.0) | 137 (46.9) | 0.02 |
| Warfarin indication, n (%)* | |||
| Venous thromboembolism | 93 (18.4) | 39 (13.4) | |
| Atrial fibrillation | 204 (40.3) | 127 (43.5) | |
| Mechanical heart valve | 19 (3.8) | 17 (6.5) | |
| Prevention of thromboembolism | 142 (28.1) | 94 (32.2) | |
| Intracardiac thrombosis | 3 (0.5) | 6 (2.1) | |
| Thrombophilia | 4 (0.8) | 5 (1.7) | |
| Other | 19 (3.8) | 12 (4.1) | |
| No indication | 32 (6.3) | 1 (3.8) | |
The frequency of INR testing within 10 days of discharge was similar for the preintervention and intervention periods (41.4% and 47.6%, respectively, P=0.09). Similarly, the likelihood of having at least 1 therapeutic INR value within 10 days of discharge was not statistically different for the groups (17.0% and 21.2%, P=0.14). The pattern was similar for the 3‐, 7‐, and 30‐day periods; a higher percentage of the intervention group had INR testing and attained a therapeutic INR value, though for no time period did this reach statistical significance. This pattern was also found when limiting the analysis to patients discharged home rather than to a facility, patients on warfarin, prior to admission, and patients started on warfarin during the hospitalization.
A total of 87of 207 (42.0%) clinicians responded to the survey. Of respondents, 75% reported that they had seen 1 patient who had been discharged on warfarin since the STAR initiative had begun, 58% of whom reported having viewed the STAR. Most respondents who viewed the STAR found it to be helpful or very helpful in guiding warfarin management (67%), improving their workflow and efficiency (58%), and improving patient safety (77%). Approximately one‐third of respondents who had viewed the STAR (34%) reported that they selected a different dose than they would have chosen had the STAR not been available.
DISCUSSION
We developed a concise report that is seamlessly created and inserted into the discharge summary. Though the STAR was perceived as improving patient safety by ambulatory care providers, there was no impact on attaining a therapeutic INR after discharge. There are several possible explanations for a lack of benefit. Most notably, our intervention was comprised of a stand‐alone EMR‐based tool and focused on 1 component of the transitions process. Given the complexity of healthcare delivery and anticoagulant management, it is likely that broader interventions are required to improve clinical outcomes over the transition period. Potential targets of multifaceted approaches may include improving access to care, providing greater access to anticoagulation clinics, enhancing patient education, and promoting direct physician‐physician communication. Bundled interventions will likely need to include involvement of an interdisciplinary team, such as pharmacist involvement in the medication reconciliation process.
The transition period from the hospital to the outpatient setting has the potential to jeopardize patient safety if vital information is not reliably transmitted across venues.[5, 6, 7, 8] Forster and colleagues noted an 11% incidence of ADEs in the posthospitalization period, of which 60% were either preventable or ameliorable.[3] To decrease the risk to patient safety during the transitions period, the Transitions of Care Consensus policy statement by the Society of Hospital Medicine and other medical organizations called for incorporation of standard data transfer forms (templates and transmission protocols).[9] Despite the high risk and preventable nature of many of the events, few specific tools have been developed. As part of broader initiatives to improve the transitions process, the STAR may have the potential to be a means for health systems to improve the quality of the transition of care for patients on anticoagulants.
Our study has several limitations. First, it was performed at a single health system. It is unknown whether the EMR‐based report could be similarly employed at other systems. Second, our study was unable to assess clinical endpoints. Given the lack of effect on attaining a therapeutic INR, it is unlikely that downstream outcomes, such as thromboembolism, were impacted. Lastly, we were unable to examine whether our intervention improved the care of patients whose outpatient provider was external to our system.
The STAR is a concise tool developed to provide essential anticoagulant‐related information to ambulatory providers. Though the report was perceived as improving patient safety, our finding of a lack of impact on attaining a therapeutic INR after discharge suggests that the tool would need to be a component of a broader multifaceted intervention to impact clinical outcomes.
Disclosures
This project was funded by a grant from the Cardinal Health E3 Foundation. The authors report no conflicts of interest.
- , , , Medication errors: experience of the United States Pharmacopeia (USP) MEDMARX reporting system. J Clin Pharm. 2003;43:760–767.
- , , , , The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138:161–167.
- , , , , Adverse drug events occurring following hospital discharge. J Gen Int Med. 2005;204:317–323.
- IHI Improvement Map. Available at: http://app.ihi.org/imap/tool/#Process=54aa289b‐16fd‐4a64‐8329‐3941dfc565d1. Accessed February 20 2015.
- , , , Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2:314–323.
- , , , , , Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297:831–841.
- Transitions of care in patients receiving oral anticoagulants: general principles, procedures, and impact of new oral anticoagulants. J Cardiovasc Nurs. 2013;28:54–65.
- Care transitions in anticoagulation management for patients with atrial fibrillation: an emphasis on safety. Oschner J. 2013;13:419–427.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4:364–370.
Anticoagulants are among the prescriptions with the highest risk of an adverse drug event (ADE) after discharge, and many of these events are preventable.[1, 2, 3] In recognition of the high risk for adverse events, the Institute for Healthcare Improvement Map details several key features of a safe anticoagulation management program, including the recommendation during the transition period that clinicians ensure proper lab monitoring and establish capacity for follow‐up testing.[4]
Despite the potential for harm, most hospitals do not have a structured process for the transmission of vital information related to warfarin management from the inpatient to the ambulatory setting. Our aim was to develop a concise report containing the essential information regarding the patient's anticoagulation regimen, the Safe Transitions Anticoagulation Report (STAR), and a process to ensure the report can be readily accessed and utilized by ambulatory clinicians.
METHODS
We assembled an interdisciplinary team to develop a new report and workflow to ensure that information on inpatient warfarin management was transmitted to outpatient providers in a reliable and structured manner. Explicit goals were to maximize use of the electronic medical record (EMR) to autopopulate aspects of the new report and create a process that worked seamlessly into the workflow. The final items were selected based on the risk of harm if not conveyed and feasibility of incorporation through the EMR:
- Warfarin doses: the 7 warfarin doses immediately prior to discharge
- International normalized ratio (INR) values: the 7 INR values immediately prior to discharge
- Bridging anticoagulation: the low‐molecular‐weight heparin (LMWH) prescribed as a bridging anticoagulant, if any
The STAR resides in both the discharge summary and the after visit summary (AVS) for patients discharged on warfarin. At our institution, the AVS contains a medication list, discharge instructions, and appointments, and is automatically produced through our EMR. Our institution utilizes the Epic EMR (Epic Systems Corp., Verona, WI) in the hospital, ambulatory clinics, and faculty practices.
A process was developed where a structured table is automatically created (Figure 1). A field was added to our EMR's discharge summary template asking whether the patient is being discharged on warfarin. Answering yes produces a second question asking whether the patient is also being discharged on bridging anticoagulation with LMWH. The STAR is not inserted into the discharge summary if the clinician completing the discharge summary deletes the anticoagulation question. The STAR is automatically created for patients discharged on warfarin and inserted into the AVS by the EMR regardless of whether the discharge summary has been completed. Patients are instructed by their nurse to bring their AVS to their follow‐up appointments.

The STAR project team utilized plan‐do‐study‐act cycles to test small changes and make revisions. The workflow was piloted on 2 medical/surgical units from January 2014 through March 2014, and revised based on feedback from clinicians and nursing staff. The STAR initiative was fully implemented across our institution in April 2014.
The study was evaluated by the institutional review board of the Icahn School of Medicine at Mount Sinai, and full review was waived.
Outcomes
Our primary outcomes were the timeliness of laboratory monitoring and quality of anticoagulation management for patients with an established relationship at 1 of the main outpatient practices in our system. Our institution has an anticoagulation clinic for patients followed at the general medicine clinic. We defined an established relationship as having been seen in the same practice on at least 2 occasions in the 12 months prior to admission. The primary outcomes were the percentage of patients who had an INR measurement done and the percentage who had a therapeutic INR value within 10 days after discharge. As the 10‐day period is arbitrary, we also assessed these outcomes for the 3‐, 7‐, and 30‐day periods after discharge. The therapeutic range was defined for all patients as an INR of 2.0 to 3.0, as this is the target range for the large majority of patients on warfarin in our system. Outcomes during the intervention period were compared to baseline values during the preintervention period. For patients with multiple admissions, the first admission during each period was included.
Ambulatory Physician Survey
We surveyed ambulatory care physicians at the main practices for our health system. The survey assessed how often the STAR was viewed and incorporated into decision making, whether the report improved workflow, and whether ambulatory providers perceived that the report increased patient safety. The survey was distributed at the 6‐month interval during the intervention phase. The survey was disseminated electronically on 3 occasions, and a paper version was distributed on 1 occasion to housestaff and general medicine faculty.
Statistical Analysis
Comparisons for categorical data were performed using the 2 test. P values were based on 2‐tailed tests of significance, and a value 0.05 was considered significant.
RESULTS
The STAR was embedded in the discharge summary for 1370 (78.6%) discharges during the intervention period. A total of 505 patients in the preintervention period and 292 patients in the intervention period were established patients at an affiliated practice and comprised the study population. Demographics and indications for warfarin for the preintervention and intervention groups are listed in the Table 1.
| Preintervention Group, N=505 | Intervention Group, N=292 | P Value | |
|---|---|---|---|
| |||
| Age, y | 66.7 | 68.0 | 0.29 |
| Male gender, n (%) | 236 (46.7) | 153 (52.4) | 0.12 |
| Discharged on bridging agent, n (%) | 90 (17.8) | 36 (12.3) | 0.04 |
| Average length of stay, d | 7.1 | 7.6 | 0.46 |
| Newly prescribed warfarin, n (%) | 147 (29.1) | 62 (21.2) | 0.01 |
| INR 2.03.0 range at discharge, n (%) | 187 (37.0) | 137 (46.9) | 0.02 |
| Warfarin indication, n (%)* | |||
| Venous thromboembolism | 93 (18.4) | 39 (13.4) | |
| Atrial fibrillation | 204 (40.3) | 127 (43.5) | |
| Mechanical heart valve | 19 (3.8) | 17 (6.5) | |
| Prevention of thromboembolism | 142 (28.1) | 94 (32.2) | |
| Intracardiac thrombosis | 3 (0.5) | 6 (2.1) | |
| Thrombophilia | 4 (0.8) | 5 (1.7) | |
| Other | 19 (3.8) | 12 (4.1) | |
| No indication | 32 (6.3) | 1 (3.8) | |
The frequency of INR testing within 10 days of discharge was similar for the preintervention and intervention periods (41.4% and 47.6%, respectively, P=0.09). Similarly, the likelihood of having at least 1 therapeutic INR value within 10 days of discharge was not statistically different for the groups (17.0% and 21.2%, P=0.14). The pattern was similar for the 3‐, 7‐, and 30‐day periods; a higher percentage of the intervention group had INR testing and attained a therapeutic INR value, though for no time period did this reach statistical significance. This pattern was also found when limiting the analysis to patients discharged home rather than to a facility, patients on warfarin, prior to admission, and patients started on warfarin during the hospitalization.
A total of 87of 207 (42.0%) clinicians responded to the survey. Of respondents, 75% reported that they had seen 1 patient who had been discharged on warfarin since the STAR initiative had begun, 58% of whom reported having viewed the STAR. Most respondents who viewed the STAR found it to be helpful or very helpful in guiding warfarin management (67%), improving their workflow and efficiency (58%), and improving patient safety (77%). Approximately one‐third of respondents who had viewed the STAR (34%) reported that they selected a different dose than they would have chosen had the STAR not been available.
DISCUSSION
We developed a concise report that is seamlessly created and inserted into the discharge summary. Though the STAR was perceived as improving patient safety by ambulatory care providers, there was no impact on attaining a therapeutic INR after discharge. There are several possible explanations for a lack of benefit. Most notably, our intervention was comprised of a stand‐alone EMR‐based tool and focused on 1 component of the transitions process. Given the complexity of healthcare delivery and anticoagulant management, it is likely that broader interventions are required to improve clinical outcomes over the transition period. Potential targets of multifaceted approaches may include improving access to care, providing greater access to anticoagulation clinics, enhancing patient education, and promoting direct physician‐physician communication. Bundled interventions will likely need to include involvement of an interdisciplinary team, such as pharmacist involvement in the medication reconciliation process.
The transition period from the hospital to the outpatient setting has the potential to jeopardize patient safety if vital information is not reliably transmitted across venues.[5, 6, 7, 8] Forster and colleagues noted an 11% incidence of ADEs in the posthospitalization period, of which 60% were either preventable or ameliorable.[3] To decrease the risk to patient safety during the transitions period, the Transitions of Care Consensus policy statement by the Society of Hospital Medicine and other medical organizations called for incorporation of standard data transfer forms (templates and transmission protocols).[9] Despite the high risk and preventable nature of many of the events, few specific tools have been developed. As part of broader initiatives to improve the transitions process, the STAR may have the potential to be a means for health systems to improve the quality of the transition of care for patients on anticoagulants.
Our study has several limitations. First, it was performed at a single health system. It is unknown whether the EMR‐based report could be similarly employed at other systems. Second, our study was unable to assess clinical endpoints. Given the lack of effect on attaining a therapeutic INR, it is unlikely that downstream outcomes, such as thromboembolism, were impacted. Lastly, we were unable to examine whether our intervention improved the care of patients whose outpatient provider was external to our system.
The STAR is a concise tool developed to provide essential anticoagulant‐related information to ambulatory providers. Though the report was perceived as improving patient safety, our finding of a lack of impact on attaining a therapeutic INR after discharge suggests that the tool would need to be a component of a broader multifaceted intervention to impact clinical outcomes.
Disclosures
This project was funded by a grant from the Cardinal Health E3 Foundation. The authors report no conflicts of interest.
Anticoagulants are among the prescriptions with the highest risk of an adverse drug event (ADE) after discharge, and many of these events are preventable.[1, 2, 3] In recognition of the high risk for adverse events, the Institute for Healthcare Improvement Map details several key features of a safe anticoagulation management program, including the recommendation during the transition period that clinicians ensure proper lab monitoring and establish capacity for follow‐up testing.[4]
Despite the potential for harm, most hospitals do not have a structured process for the transmission of vital information related to warfarin management from the inpatient to the ambulatory setting. Our aim was to develop a concise report containing the essential information regarding the patient's anticoagulation regimen, the Safe Transitions Anticoagulation Report (STAR), and a process to ensure the report can be readily accessed and utilized by ambulatory clinicians.
METHODS
We assembled an interdisciplinary team to develop a new report and workflow to ensure that information on inpatient warfarin management was transmitted to outpatient providers in a reliable and structured manner. Explicit goals were to maximize use of the electronic medical record (EMR) to autopopulate aspects of the new report and create a process that worked seamlessly into the workflow. The final items were selected based on the risk of harm if not conveyed and feasibility of incorporation through the EMR:
- Warfarin doses: the 7 warfarin doses immediately prior to discharge
- International normalized ratio (INR) values: the 7 INR values immediately prior to discharge
- Bridging anticoagulation: the low‐molecular‐weight heparin (LMWH) prescribed as a bridging anticoagulant, if any
The STAR resides in both the discharge summary and the after visit summary (AVS) for patients discharged on warfarin. At our institution, the AVS contains a medication list, discharge instructions, and appointments, and is automatically produced through our EMR. Our institution utilizes the Epic EMR (Epic Systems Corp., Verona, WI) in the hospital, ambulatory clinics, and faculty practices.
A process was developed where a structured table is automatically created (Figure 1). A field was added to our EMR's discharge summary template asking whether the patient is being discharged on warfarin. Answering yes produces a second question asking whether the patient is also being discharged on bridging anticoagulation with LMWH. The STAR is not inserted into the discharge summary if the clinician completing the discharge summary deletes the anticoagulation question. The STAR is automatically created for patients discharged on warfarin and inserted into the AVS by the EMR regardless of whether the discharge summary has been completed. Patients are instructed by their nurse to bring their AVS to their follow‐up appointments.

The STAR project team utilized plan‐do‐study‐act cycles to test small changes and make revisions. The workflow was piloted on 2 medical/surgical units from January 2014 through March 2014, and revised based on feedback from clinicians and nursing staff. The STAR initiative was fully implemented across our institution in April 2014.
The study was evaluated by the institutional review board of the Icahn School of Medicine at Mount Sinai, and full review was waived.
Outcomes
Our primary outcomes were the timeliness of laboratory monitoring and quality of anticoagulation management for patients with an established relationship at 1 of the main outpatient practices in our system. Our institution has an anticoagulation clinic for patients followed at the general medicine clinic. We defined an established relationship as having been seen in the same practice on at least 2 occasions in the 12 months prior to admission. The primary outcomes were the percentage of patients who had an INR measurement done and the percentage who had a therapeutic INR value within 10 days after discharge. As the 10‐day period is arbitrary, we also assessed these outcomes for the 3‐, 7‐, and 30‐day periods after discharge. The therapeutic range was defined for all patients as an INR of 2.0 to 3.0, as this is the target range for the large majority of patients on warfarin in our system. Outcomes during the intervention period were compared to baseline values during the preintervention period. For patients with multiple admissions, the first admission during each period was included.
Ambulatory Physician Survey
We surveyed ambulatory care physicians at the main practices for our health system. The survey assessed how often the STAR was viewed and incorporated into decision making, whether the report improved workflow, and whether ambulatory providers perceived that the report increased patient safety. The survey was distributed at the 6‐month interval during the intervention phase. The survey was disseminated electronically on 3 occasions, and a paper version was distributed on 1 occasion to housestaff and general medicine faculty.
Statistical Analysis
Comparisons for categorical data were performed using the 2 test. P values were based on 2‐tailed tests of significance, and a value 0.05 was considered significant.
RESULTS
The STAR was embedded in the discharge summary for 1370 (78.6%) discharges during the intervention period. A total of 505 patients in the preintervention period and 292 patients in the intervention period were established patients at an affiliated practice and comprised the study population. Demographics and indications for warfarin for the preintervention and intervention groups are listed in the Table 1.
| Preintervention Group, N=505 | Intervention Group, N=292 | P Value | |
|---|---|---|---|
| |||
| Age, y | 66.7 | 68.0 | 0.29 |
| Male gender, n (%) | 236 (46.7) | 153 (52.4) | 0.12 |
| Discharged on bridging agent, n (%) | 90 (17.8) | 36 (12.3) | 0.04 |
| Average length of stay, d | 7.1 | 7.6 | 0.46 |
| Newly prescribed warfarin, n (%) | 147 (29.1) | 62 (21.2) | 0.01 |
| INR 2.03.0 range at discharge, n (%) | 187 (37.0) | 137 (46.9) | 0.02 |
| Warfarin indication, n (%)* | |||
| Venous thromboembolism | 93 (18.4) | 39 (13.4) | |
| Atrial fibrillation | 204 (40.3) | 127 (43.5) | |
| Mechanical heart valve | 19 (3.8) | 17 (6.5) | |
| Prevention of thromboembolism | 142 (28.1) | 94 (32.2) | |
| Intracardiac thrombosis | 3 (0.5) | 6 (2.1) | |
| Thrombophilia | 4 (0.8) | 5 (1.7) | |
| Other | 19 (3.8) | 12 (4.1) | |
| No indication | 32 (6.3) | 1 (3.8) | |
The frequency of INR testing within 10 days of discharge was similar for the preintervention and intervention periods (41.4% and 47.6%, respectively, P=0.09). Similarly, the likelihood of having at least 1 therapeutic INR value within 10 days of discharge was not statistically different for the groups (17.0% and 21.2%, P=0.14). The pattern was similar for the 3‐, 7‐, and 30‐day periods; a higher percentage of the intervention group had INR testing and attained a therapeutic INR value, though for no time period did this reach statistical significance. This pattern was also found when limiting the analysis to patients discharged home rather than to a facility, patients on warfarin, prior to admission, and patients started on warfarin during the hospitalization.
A total of 87of 207 (42.0%) clinicians responded to the survey. Of respondents, 75% reported that they had seen 1 patient who had been discharged on warfarin since the STAR initiative had begun, 58% of whom reported having viewed the STAR. Most respondents who viewed the STAR found it to be helpful or very helpful in guiding warfarin management (67%), improving their workflow and efficiency (58%), and improving patient safety (77%). Approximately one‐third of respondents who had viewed the STAR (34%) reported that they selected a different dose than they would have chosen had the STAR not been available.
DISCUSSION
We developed a concise report that is seamlessly created and inserted into the discharge summary. Though the STAR was perceived as improving patient safety by ambulatory care providers, there was no impact on attaining a therapeutic INR after discharge. There are several possible explanations for a lack of benefit. Most notably, our intervention was comprised of a stand‐alone EMR‐based tool and focused on 1 component of the transitions process. Given the complexity of healthcare delivery and anticoagulant management, it is likely that broader interventions are required to improve clinical outcomes over the transition period. Potential targets of multifaceted approaches may include improving access to care, providing greater access to anticoagulation clinics, enhancing patient education, and promoting direct physician‐physician communication. Bundled interventions will likely need to include involvement of an interdisciplinary team, such as pharmacist involvement in the medication reconciliation process.
The transition period from the hospital to the outpatient setting has the potential to jeopardize patient safety if vital information is not reliably transmitted across venues.[5, 6, 7, 8] Forster and colleagues noted an 11% incidence of ADEs in the posthospitalization period, of which 60% were either preventable or ameliorable.[3] To decrease the risk to patient safety during the transitions period, the Transitions of Care Consensus policy statement by the Society of Hospital Medicine and other medical organizations called for incorporation of standard data transfer forms (templates and transmission protocols).[9] Despite the high risk and preventable nature of many of the events, few specific tools have been developed. As part of broader initiatives to improve the transitions process, the STAR may have the potential to be a means for health systems to improve the quality of the transition of care for patients on anticoagulants.
Our study has several limitations. First, it was performed at a single health system. It is unknown whether the EMR‐based report could be similarly employed at other systems. Second, our study was unable to assess clinical endpoints. Given the lack of effect on attaining a therapeutic INR, it is unlikely that downstream outcomes, such as thromboembolism, were impacted. Lastly, we were unable to examine whether our intervention improved the care of patients whose outpatient provider was external to our system.
The STAR is a concise tool developed to provide essential anticoagulant‐related information to ambulatory providers. Though the report was perceived as improving patient safety, our finding of a lack of impact on attaining a therapeutic INR after discharge suggests that the tool would need to be a component of a broader multifaceted intervention to impact clinical outcomes.
Disclosures
This project was funded by a grant from the Cardinal Health E3 Foundation. The authors report no conflicts of interest.
- , , , Medication errors: experience of the United States Pharmacopeia (USP) MEDMARX reporting system. J Clin Pharm. 2003;43:760–767.
- , , , , The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138:161–167.
- , , , , Adverse drug events occurring following hospital discharge. J Gen Int Med. 2005;204:317–323.
- IHI Improvement Map. Available at: http://app.ihi.org/imap/tool/#Process=54aa289b‐16fd‐4a64‐8329‐3941dfc565d1. Accessed February 20 2015.
- , , , Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2:314–323.
- , , , , , Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297:831–841.
- Transitions of care in patients receiving oral anticoagulants: general principles, procedures, and impact of new oral anticoagulants. J Cardiovasc Nurs. 2013;28:54–65.
- Care transitions in anticoagulation management for patients with atrial fibrillation: an emphasis on safety. Oschner J. 2013;13:419–427.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4:364–370.
- , , , Medication errors: experience of the United States Pharmacopeia (USP) MEDMARX reporting system. J Clin Pharm. 2003;43:760–767.
- , , , , The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138:161–167.
- , , , , Adverse drug events occurring following hospital discharge. J Gen Int Med. 2005;204:317–323.
- IHI Improvement Map. Available at: http://app.ihi.org/imap/tool/#Process=54aa289b‐16fd‐4a64‐8329‐3941dfc565d1. Accessed February 20 2015.
- , , , Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2:314–323.
- , , , , , Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297:831–841.
- Transitions of care in patients receiving oral anticoagulants: general principles, procedures, and impact of new oral anticoagulants. J Cardiovasc Nurs. 2013;28:54–65.
- Care transitions in anticoagulation management for patients with atrial fibrillation: an emphasis on safety. Oschner J. 2013;13:419–427.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4:364–370.
Risk of Perioperative Morbidity, Post-Op Mortality Higher for Current Smokers
Clinical question: Is there an association between current and past smoking on outcomes among patients having major surgery?
Background: Smoking is associated with adverse postoperative outcomes, but it is not known whether the associations are dose-dependent or limited to patients with smoking-related diseases. Smoking-related effects on postoperative events among patients having major surgery are also not well established.
Study design: Retrospective cohort study.
Setting: Four hundred forty-eight non-VA hospitals across the U.S., Canada, Lebanon, and the United Arab Emirates.
Synopsis: Data from 607,558 adult patients undergoing major surgery were obtained from the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database. After adjusting for confounders (cardiopulmonary diseases and cancer), the effects of current and past smoking (quit >1 year prior) on 30-day postoperative outcomes were measured.
There were 125,192 (21%) current smokers and 78,763 (13%) past smokers. Increased odds of post-op mortality were noted in current smokers only (odds ratio [OR] 1.17; 95% CI, 1.10–1.24). The adjusted odds ratios were higher for arterial and respiratory events among current smokers compared with past smokers (OR 1.65; 95% CI, 1.51–1.81 vs. OR 1.20; CI, 1.09–1.31 for arterial events, respectively) and (OR, 1.45; CI, 1.40–1.51 vs. OR, 1.13; CI, 1.08–1.18, for respiratory events, respectively). No significant effects on venous events were observed.
There was an increased adjusted odds of mortality for current smokers with <10 pack-years, while the effects on arterial and respiratory events increased incrementally with increased pack-years. Smoking was associated with adverse post-op outcomes regardless of smoking-related diseases. Variability in hospital quality or surgical strategies may have confounded the results.
Bottom line: Among patients undergoing major surgery, current but not past smoking was associated with higher mortality; smoking cessation for at least a year prior to surgery may decrease postoperative adverse events.
Citation: Musallam KM, Rosendaal FR, Zaatari G, et al. Smoking and the risk of mortality and vascular and respiratory events in patients undergoing major surgery. JAMA Surg. 2013;148:755-762.
Clinical question: Is there an association between current and past smoking on outcomes among patients having major surgery?
Background: Smoking is associated with adverse postoperative outcomes, but it is not known whether the associations are dose-dependent or limited to patients with smoking-related diseases. Smoking-related effects on postoperative events among patients having major surgery are also not well established.
Study design: Retrospective cohort study.
Setting: Four hundred forty-eight non-VA hospitals across the U.S., Canada, Lebanon, and the United Arab Emirates.
Synopsis: Data from 607,558 adult patients undergoing major surgery were obtained from the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database. After adjusting for confounders (cardiopulmonary diseases and cancer), the effects of current and past smoking (quit >1 year prior) on 30-day postoperative outcomes were measured.
There were 125,192 (21%) current smokers and 78,763 (13%) past smokers. Increased odds of post-op mortality were noted in current smokers only (odds ratio [OR] 1.17; 95% CI, 1.10–1.24). The adjusted odds ratios were higher for arterial and respiratory events among current smokers compared with past smokers (OR 1.65; 95% CI, 1.51–1.81 vs. OR 1.20; CI, 1.09–1.31 for arterial events, respectively) and (OR, 1.45; CI, 1.40–1.51 vs. OR, 1.13; CI, 1.08–1.18, for respiratory events, respectively). No significant effects on venous events were observed.
There was an increased adjusted odds of mortality for current smokers with <10 pack-years, while the effects on arterial and respiratory events increased incrementally with increased pack-years. Smoking was associated with adverse post-op outcomes regardless of smoking-related diseases. Variability in hospital quality or surgical strategies may have confounded the results.
Bottom line: Among patients undergoing major surgery, current but not past smoking was associated with higher mortality; smoking cessation for at least a year prior to surgery may decrease postoperative adverse events.
Citation: Musallam KM, Rosendaal FR, Zaatari G, et al. Smoking and the risk of mortality and vascular and respiratory events in patients undergoing major surgery. JAMA Surg. 2013;148:755-762.
Clinical question: Is there an association between current and past smoking on outcomes among patients having major surgery?
Background: Smoking is associated with adverse postoperative outcomes, but it is not known whether the associations are dose-dependent or limited to patients with smoking-related diseases. Smoking-related effects on postoperative events among patients having major surgery are also not well established.
Study design: Retrospective cohort study.
Setting: Four hundred forty-eight non-VA hospitals across the U.S., Canada, Lebanon, and the United Arab Emirates.
Synopsis: Data from 607,558 adult patients undergoing major surgery were obtained from the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database. After adjusting for confounders (cardiopulmonary diseases and cancer), the effects of current and past smoking (quit >1 year prior) on 30-day postoperative outcomes were measured.
There were 125,192 (21%) current smokers and 78,763 (13%) past smokers. Increased odds of post-op mortality were noted in current smokers only (odds ratio [OR] 1.17; 95% CI, 1.10–1.24). The adjusted odds ratios were higher for arterial and respiratory events among current smokers compared with past smokers (OR 1.65; 95% CI, 1.51–1.81 vs. OR 1.20; CI, 1.09–1.31 for arterial events, respectively) and (OR, 1.45; CI, 1.40–1.51 vs. OR, 1.13; CI, 1.08–1.18, for respiratory events, respectively). No significant effects on venous events were observed.
There was an increased adjusted odds of mortality for current smokers with <10 pack-years, while the effects on arterial and respiratory events increased incrementally with increased pack-years. Smoking was associated with adverse post-op outcomes regardless of smoking-related diseases. Variability in hospital quality or surgical strategies may have confounded the results.
Bottom line: Among patients undergoing major surgery, current but not past smoking was associated with higher mortality; smoking cessation for at least a year prior to surgery may decrease postoperative adverse events.
Citation: Musallam KM, Rosendaal FR, Zaatari G, et al. Smoking and the risk of mortality and vascular and respiratory events in patients undergoing major surgery. JAMA Surg. 2013;148:755-762.
Reviews of Reseach on Perioperative Morbidity, Capnography with Diabetic Ketoacidosis in the ED, Mortality Rate for Elective Surgeries
In This Edition
Literature At A Glance
A guide to this month’s studies
- Early treatment with intravenous tPA for acute stroke
- Perioperative morbidity, mortality for current smokers
- Statins associated with musculoskeletal conditions
- Antithrombotic medications in patients with history of stroke
- Extended prophylaxis with aspirin for patients after total hip arthroplasty
- Prognosis for symptomatic subsegmental pulmonary embolism
- Video-based educational workshops for academic hospitalists
- Increased mortality for elective surgeries on Fridays, weekends
- Basal plus correction insulin regimen and Type 2 diabetes
- Capnography to diagnose diabetic ketoacidosis in the ED
- How publicly reported mortality rates correlate with hospitals’ overall mortality
- Cost savings and preventable acute-care visits for Medicare patients
Early tPA in Acute Stroke Is Associated with Better Short-Term Outcomes in Routine Clinical Practice
Clinical question: Does early treatment with intravenous (IV) tissue plasminogen activator (tPA) result in better outcomes among patients with acute ischemic stroke in routine clinical practice?
Background: IV tPA for acute ischemic stroke is beneficial if given in the first 4.5 hours after symptom onset. However, pooled data from clinical trials have been limited in characterizing the extent to which onset-to-treatment (OTT) with IV tPA influences outcomes and how effective tPA is in routine clinical practice.
Study design: Data analysis from a stroke registry.
Setting: One thousand three hundred ninety-five U.S. hospitals participating in the Get with the Guidelines—Stroke Program.
Synopsis: Data were analyzed from 58,353 tPA-treated patients within 4.5 hours of symptom onset. Clinical outcomes were compared among patients treated in the 0-90-, 91-180-, and 181-270-minute OTT windows. Patient factors strongly associated with shorter OTT were greater stroke severity (odds ratio [OR] 2.8; 95% confidence interval [CI], 2.5-3.1 per five-point increase), arrival by ambulance (OR 5.9; 95% CI, 4.5-7.3), and arrival during regular hours (OR 4.6; 95% CI, 3.8-5.4). Faster OTT, in 15-minute increments, was associated with reduced in-hospital mortality (OR 0.96; 95% CI, 0.95-0.98; P<.001), reduced symptomatic intracranial hemorrhage (OR 0.96; 95% CI, 0.95-0.98; P<.001), increased achievement of independent ambulation at discharge (OR 1.04; 95% CI, 1.03-1.05; P<.001), and increased discharge to home (OR 1.03; 95% CI, 1.02-1.04; P<.001).
Data collected were dependent on the accuracy and completeness of the chart abstraction, and only short-term outcomes were reported. Although no post-discharge outcomes were reported, previous studies have shown that functional status at discharge strongly correlates with three-month disability outcomes.
Bottom line: In routine clinical practice, earlier tPA for acute ischemic strokes results in better short-term clinical outcomes.
Citation: Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309:2480-2488.
Current Smokers Have Higher Perioperative Morbidity and Mortality Compared to Past Smokers
Clinical question: Is there an association between current and past smoking on outcomes among patients having major surgery?
Background: Smoking is associated with adverse postoperative outcomes, but it is not known whether the associations are dose-dependent or limited to patients with smoking-related diseases. Smoking-related effects on postoperative events among patients having major surgery are also not well established.
Study design: Retrospective cohort study.
Setting: Four hundred forty-eight non-VA hospitals across the U.S., Canada, Lebanon, and the United Arab Emirates.
Synopsis: Data from 607,558 adult patients undergoing major surgery were obtained from the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database. After adjusting for confounders (cardiopulmonary diseases and cancer), the effects of current and past smoking (quit >1 year prior) on 30-day post-operative outcomes were measured.
There were 125,192 (21%) current smokers and 78,763 (13%) past smokers. Increased odds of post-op mortality were noted in current smokers only (odds ratio [OR] 1.17; 95% CI, 1.10-1.24). The adjusted odds ratios were higher for arterial and respiratory events among current smokers compared with past smokers (OR 1.65; 95% CI, 1.51-1.81 vs. OR 1.20; CI, 1.09-1.31 for arterial events, respectively) and (OR, 1.45; CI, 1.40-1.51 vs. OR, 1.13; CI, 1.08-1.18, for respiratory events, respectively). No significant effects on venous events were observed.
There was an increased adjusted odds of mortality for current smokers with <10 pack-years, while the effects on arterial and respiratory events increased incrementally with increased pack-years. Smoking was associated with adverse post-op outcomes regardless of smoking-related diseases. Variability in hospital quality or surgical strategies may have confounded the results.
Bottom line: Among patients undergoing major surgery, current but not past smoking was associated with higher mortality; smoking cessation for at least a year prior to surgery may decrease post-operative adverse events.
Citation: Musallam KM, Rosendaal FR, Zaatari G, et al. Smoking and the risk of mortality and vascular and respiratory events in patients undergoing major surgery. JAMA Surg. 2013 Jun 19:1-8. doi: 10.1001/jamasurg.2013.2360 [Epub ahead of print].
Statins Associated with Several Musculoskeletal Conditions
Clinical question: Is statin use associated with musculoskeletal adverse events, including arthropathy and injury, in physically active individuals?
Background: Statin-induced musculoskeletal adverse events (AEs) include myalgias, muscle weakness, cramps, rhabdomyolysis, and tendinous disease. The full spectrum of AEs is unknown because randomized clinical trials have not been powered to detect uncommon AEs.
Study design: Retrospective cohort study with propensity score matching.
Setting: San Antonio military area.
Synopsis: A total of 46,249 patients aged 30 to 85 years who met study criteria were propensity-matched into 6,967 statin users and 6,967 nonusers. The occurrence of musculoskeletal conditions were categorized using ICD-9 codes: Msk1, all musculoskeletal diseases; Msk1a, arthropathies and related diseases; Msk1b, injury-related diseases; and Msk2, drug-associated musculoskeletal pain. Of these, statin users had a higher odds ratio (OR) for Msk1 (OR 1.19; 95% CI, 1.08-1.30), Msk1b (1.13; 1.05-1.21), and Msk2 (1.09; 1.02-1.18). Msk1b (arthropathies) had an OR of 1.07 (0.9-1.16, P=0.07). Simvastatin was used by 73.5% of patients, and years of simvastatin use was not a significant predictor of any of the outcome measures. Secondary and sensitivity analyses showed higher adjusted ORs for statin users in all groups. This study was limited by the use of ICD-9-CM codes for identification of baseline characteristics, and the musculoskeletal diagnosis groups used were not validated.
Bottom line: Statin use is associated with an increased likelihood of musculoskeletal conditions, arthropathies, injuries, and pain.
Citation: Mansi I, Frei CR, Pugh M, Makris U, Mortensen EM. Statins and musculoskeletal conditions, arthropathies, and injuries. JAMA Intern Med. 2013;173:1318-1326.
Evidence-Based Guidelines on Periprocedural Management of Antithrombotic Medications in Patients with History of Stroke
Clinical question: What is the evidence for the periprocedural management of antithrombotics in patients with ischemic cerebrovascular accidents (CVAs)?
Background: Evidence-based guidelines are needed to help clinicians determine the thromboembolic risk of temporary discontinuation of antithrombotic medications, the perioperative bleeding risks of continuing antithrombotic agents, whether bridging therapy should be used, and the appropriate timing of antithrombotic agent discontinuation.
Study design: Systematic literature review with practice recommendations.
Setting: American Academy of Neurology Guideline Development Subcommittee convened an expert panel to review and provide recommendations.
Synopsis: Researchers analyzed 133 literature reviews via MEDLINE and EMBASE. Aspirin in stroke patients:
- Should routinely be continued for dental procedures (Level A);
- Should probably be continued for invasive ocular anesthesia, cataract surgery, dermatologic procedures, transrectal ultrasound-guided prostate biopsy, spinal/epidural procedures, and carpal tunnel surgery (Level B); and
- Should possibly be continued for vitreoretinal surgery, electromyogram (EMG), transbronchial lung biopsy, colonoscopic polypectomy, upper endoscopy and biopsy/sphincterotomy, and abdominal ultrasound-guided biopsies (Level C).
Warfarin in stroke patients:
- Should routinely be continued for dental procedures (Level A); and
- Should possibly continued for dermatologic procedures (Level B) and EMG, prostate procedures, inguinal hemiorrhaphy, and endothermal ablation of great saphenous vein (Level C).
- There is a lack of evidence on warfarin for ophthalmologic procedures, with the exception of ocular anesthesia, where it probably does not increase clinically significant bleeding (Level B).
There was not enough evidence to support or refute a recommendation regarding heparin bridge therapy in reducing thromboembolism in chronically anticoagulated patients (Level B).
Bottom line: These are the most up-to-date guidelines for anticoagulant and antiplatelet agents in patients with transient ischemic attacks and strokes undergoing procedures, but further research is needed in many areas.
Citation: Armstrong MJ, Gronseth G Anderson DC, et al. Summary of evidence-based guideline: periprocedural management of antithrombotic medications in patients with ischemic cerebrovascular disease: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2065-2069.
Extended Prophylaxis with Aspirin Was Noninferior to Extended Prophylaxis with Low-Molecular-Weight Heparin
Clinical question: Is aspirin as effective as low-molecular-weight heparin (LMWH) for the extended prophylaxis of venous thromboembolism (VTE) after total hip arthroplasty (THA)?
Background: Deep vein thrombosis (DVT) and pulmonary embolism (PE) are common complications after THA. After initial prophylaxis, LMWH given for up to 30 days has been shown to reduce VTE compared with placebo. However, LMWH is costly and may increase the risk of minor bleeding. Aspirin is a potentially simple, low-cost alternative.
Study design: Randomized, placebo-controlled trial.
Setting: Twelve university-affiliated orthopedic hospitals in Canada.
Synopsis: Patients undergoing elective THA without hip fracture, metastatic cancer, or bleeding precluding anticoagulants were eligible. All patients received dalteparin for 10 days and were then randomized to aspirin 81 mg daily or to continue dalteparin. The primary outcome was symptomatic proximal DVT or PE during 90 days’ follow-up. The study was terminated early due to slow enrollment. At that time, 2,364 patients had been enrolled, and an analysis by an independent data safety and monitoring board determined that continuing the study was unlikely to alter the main findings. Extended prophylaxis with aspirin was noninferior to LMWH for the primary outcome, which occurred in 0.3% vs. 1.3%, respectively (95% CI, -0.5% to 2.5%, P<.001 for noninferiority). There were no significant differences in major or minor bleeding.
Though the early termination is a concern, the sample size was large and the results do not suggest inadequate power as a reason for lack of superiority for LMWH. Also, all patients received 10 days of LMWH, which indicates a period of LMWH after discharge will still be needed for most patients prior to initiating aspirin.
Bottom line: After initial LMWH prophylaxis for 10 days, extended prophylaxis with aspirin can be considered, particularly for patients for whom LMWH may not be feasible.
Citation: Anderson DR, Dunbar MJ, Bohm ER, et al. Aspirin versus low-molecular-weight heparin for extended venous thromboembolism prophylaxis after total hip arthroplasty: a randomized trial. Ann Intern Med. 2013;158:800-806.
Symptomatic Subsegmental Pulmonary Embolism (PE) Has a Prognosis Similar to Proximal PE
Clinical question: Is the prognosis of a symptomatic subsegmental pulmonary embolism (PE) similar to that of a more proximal PE?
Background: The use of multidetector computed tomography angiography (CTA) has allowed for better assessment of the pulmonary vasculature and increased detection of distal emboli. Prior studies have raised questions on the clinical importance of subsegmental PE but have been limited by small size or retrospective design.
Study design: Combined data from two prospective trials of management of suspected PE.
Setting: Twelve hospitals in the Netherlands and four tertiary-care emergency departments in Canada.
Synopsis: The study cohort consisted of 3,769 patients with suspected PE, of which 2,688 underwent CTA. Of patients diagnosed with PE, 15.5% had isolated subsegmental emboli. All patients were treated with anticoagulation. During three months of follow-up, the incidence of symptomatic recurrence for subsegmental PE was similar to patients with proximal PE (3.6% vs. 2.5%, respectively). The mortality rates for patients with subsegmental and proximal PE were also similar (10.3% vs. 6.3%, respectively).
The study may have been underpowered to detect small differences in event rates; however, there was no trend suggesting that subsegmental PE had better outcomes than more proximal PE. Also, the study did not specifically investigate whether any management strategy is preferred based on thrombus location on CTA.
Bottom line: Clinicians should continue to anticoagulate patients with subsegmental PE as the prognosis is similar to those with proximal PE.
Citation: Den Exter PL, van Es J, Klok FA, et al. Risk profile and clinical outcome of symptomatic subsegmental pulmonary embolism. Blood. 2013;122:1144-1149.
Video-Based Educational Workshop for Academic Hospitalists and House Staff May Improve Professionalism
Clinical question: Can video-based education promote professionalism among academic hospitalists and house staff?
Background: Unprofessional behavior by academic hospitalists and residents can negatively impact the learning environment and patient safety. This behavior increases throughout training, and faculty behavior can be influential. There is a paucity of educational materials to train hospitalists and house staff to recognize and ameliorate unprofessional behaviors.
Study design: Educational survey study.
Setting: University of Chicago, Northwestern University, and NorthShore University Health System teaching hospitals.
Synopsis: Three videos were developed displaying three types of unprofessional behavior: disparaging other physicians, “blocking” admissions, and misrepresenting tests to expedite their completion. There were 44 hospitalists and 244 house staff who received a 60-minute workshop in which they watched the videos using a viewing tool and discussed the videos in small groups.
For all three videos, more than three-quarters of both hospitalists and house staff felt the behavior was unprofessional or somewhat unprofessional. Hospitalists and house staff found the workshop useful and effective (65.9% and 77.1%, respectively) and would change their behavior as a result of the workshop (65.9% and 67.2%, respectively). Those who perceived the videos as “very realistic” were more likely to report intent to change behavior (93% vs. 53%, P=0.01).
This study is limited by its small sample size and possible selection bias. Those interested or concerned about unprofessional behavior may have been more likely to attend the workshop.
Bottom line: Video-based professionalism education is a feasible and well-received way to educate hospitalists and residents about unprofessional behavior and may even affect their future behavior.
Citation: Farnan JM, O’Leary KJ, Didwania A, et al. Promoting professionalism via a video-based educational workshop for academic hospitalists and housestaff. J Hosp Med. 2013;8:386-389.
Friday and Weekend Elective Surgeries Have Increased Mortality
Clinical question: How can the association between mortality and the day of elective surgical procedures be assessed?
Background: Several studies have described the “weekend effect” for both surgical and medical patients, with higher mortality and length of stay in patients admitted on the weekend compared to weekdays. Two potential explanations are poorer quality of care being delivered on the weekend or more severely ill patients being operated on or admitted on the weekend.
Study design: Retrospective analysis of national hospital administrative data.
Setting: All acute-care and specialist hospitals in England from 2008 to 2011.
Synopsis: There were 4,133,346 elective, inpatient surgical procedures studied. Friday surgeries had an adjusted odds ratio of death within 30 days and within two days of 1.44 [95% CI, 1.39-1.50] and 1.42 [95% CI, 1.26-1.60], respectively, when compared with Monday. Weekend surgeries had an adjusted odds ratio of death within 30 days and within two days of 1.82 [95% CI, 1.71-1.94] and 2.67 [95% CI, 2.30-3.09], respectively, when compared with Monday. There were significant trends toward higher mortality at the end of the workweek and weekends for four high-risk procedures: esophagus and/or stomach excision, colon and/or rectum excisions, coronary artery bypass graft, and lung excision. For lower-risk procedures, there was a significant increase in mortality for Friday surgeries but not weekend surgeries. As with all studies using administrative data, inherent selection biases could not be adjusted for Friday or weekend procedures.
Bottom line: Elective surgeries that occur on the weekend and later in the week have an increased risk of mortality, implying that the weekend effect is due to poorer quality of care during weekends, rather than higher-acuity patients presenting on weekends.
Citation: Aylin P, Alexandrescu R, Jen MH, Mayer EK, Bottle A. Day of week of procedure and 30 day mortality for elective surgery: retrospective analysis of hospital episode statistics. BMJ. 2013;346:f2424.
Basal Plus Correction Insulin Regimen Is Effective in Hospitalized Patients with Type 2 Diabetes
Clinical question: Does a basal plus correction insulin regimen (as needed with meals) result in similar glycemic control and lower rates of hypoglycemia compared to a basal-bolus regimen?
Background: Basal bolus is the preferred insulin regimen for non-critically-ill hospitalized patients as per clinical guidelines. But use is limited due to the complexity of the regimen and the fear of inducing hypoglycemia. A less complex, easier-to-implement basal plus correction insulin regimen may be an effective alternative.
Study design: Multicenter, prospective, open-label, randomized study.
Setting: Six hospitals in the U.S.
Synopsis: A group of 375 medical and surgical patients with Type 2 diabetes treated with diet, oral anti-diabetic agents, or low-dose insulin (≤ 0.4 units/kg/day) were randomized to:
- Basal-bolus insulin regimen with glargine once daily and fixed doses of glusiline before meals;
- Basal plus correction insulin (“basal plus”) regimen with glargine once daily and glusiline sliding scale insulin (SSI) before meals; or
- Regular SSI alone.
After the first day of therapy, treatment with basal-bolus and basal-plus regimens resulted in similar improvements in daily blood glucose (BG) (P=0.16), and both were superior to SSI alone (P=0.04). Both regimens also resulted in less treatment failure (defined as mean daily BG of >240 mg/dl or >2 consecutive BG >240 mg/dl) than did treatment with SSI. Hypoglycemia (BG <70 mg/dl) occurred in 16%, 13%, and 3% of patients in the basal-bolus, basal-plus, and SSI groups, respectively (P=0.02). There were no between-group differences in the frequency of severe hypoglycemia (<40 mg/dl; P=0.76).
The study was not powered to evaluate hospital complications (infection, mortality, hospital stay, and readmissions) across groups.
Bottom line: The basal-plus regimen resulted in glycemic control similar to standard basal-bolus regimen and is an effective alternative for the initial management of hyperglycemia in general medical and surgical patients with Type 2 diabetes.
Citation: Umpierrez GE, Smiley D, Hermayer K, et al. Randomized study comparing a basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes: Basal plus trial. Diabetes Care. 2013;36:2169-2174.
Capnography Can Help Diagnose Diabetic Ketoacidosis in the ED
Clinical question: Can capnography be used as a screening tool to identify patients with diabetic ketoacidosis (DKA)?
Background: Metabolic acidosis is a major criterion for diagnosing DKA. Previous studies have shown that end-tidal carbon dioxide (ETCO2) measurement by capnography can provide an accurate estimation of arterial carbon dioxide tension (PaCO2) and may be a noninvasive, fast, inexpensive measurement of acidosis in DKA. However, those studies were in pediatric patients and had small sample sizes.
Study design: Cross-sectional, prospective descriptive-analytic study.
Setting: The ED of Imam Reza Medical Research and Training Hospital, Tabriz, East Azarbaijan, Iran.
Synopsis: A total of 181 adult patients older than 18 with suspected DKA and blood sugar >250 mg/dl were included in the study. Simultaneous capnography and arterial blood gas (ABG) were obtained on all patients. Urine ketones, complete blood count, serum levels of potassium, urea, and creatinine were collected. Sixty-two patients were found to have DKA, while 119 had other conditions associated with metabolic acidosis. There was a significant linear relationship between pH and ETCO2 (P>0.0001, relative risk (R)=0.253), PaCO2 and ETCO2 (P>0.0001, R=0.572), and bicarbonate (HCO3) and ETCO2 (P>0.0001, R=0.730). ETCO2 values >24.5 mmHg had a sensitivity and specificity of 0.90 for ruling out DKA. No cutoff point could be determined for ruling in DKA.
The study was open to selection bias as patient collection was only done during the day, so eligible subjects may have been missed. Moreover, though the study suggests that capnography has a role in ruling out DKA, the exact cutoff value is unclear. Other studies found that higher values were needed to exclude diagnosis.
Bottom line: Using ETCO2 values >24.5 mmHg, capnography can help exclude the diagnosis of DKA in adult patients with elevated BG.
Citation: Soleimanour H, Taghizadieh A, Niafar M, Rahmani F, Golzari S, Esfanjani RM. Predictive value of capnography for diagnosis in patients with suspected diabetic ketoacidosis in the emergency department. West J Emerg Med. 2013. doi: 10.5811/westjem.2013.4.14296.
Publicly Reported Mortality Correlates with Overall Mortality
Clinical question: Are publicly reported mortality rates associated with a hospital’s overall medical and surgical mortality rate?
Background: Public reporting of mortality has become an important strategy in Medicare’s quality-improvement initiative. However, the mortality rate for only three conditions, acute myocardial infarction, congestive heart failure, and pneumonia are reported. It is unclear if these rates correlate to a hospital’s overall mortality rate.
Study design: Retrospective cohort.
Setting: National Medicare fee-for-service population.
Synopsis: Using 2008-2009 data from 2,322 acute-care hospitals with 6.7 million admissions, an aggregate mortality rate for the three publicly reported conditions, a standardized 30-day mortality rate for selected medical and surgical conditions, and an overall average composite mortality score was calculated for each hospital. Based on their mortality for the three publicly reported conditions, hospitals were grouped into quartiles from highest (top-performing hospitals) to lowest mortality (poor-performing hospitals).
Top-performing hospitals had a 3.6% (9.4%vs 13.0%; P<.001) lower mortality rate than poor-performing hospitals and an odds ratio >5 of being a top performer in overall mortality (OR 5.3; 95% CI, 4.3-6.5). They also had an 81% lower chance of being in the worst-performing quartile in overall mortality (OR 0.19; 95% CI, 0.14-0.27). Conversely, poor-performing hospitals had a 4.5 times higher risk of being in the lowest quartile in overall mortality. The study is limited by the use of administrative data, which limits the ability to adjust for severity of illness, overall health, and socioeconomic status of each hospital’s population.
Bottom line: A hospital’s mortality performance on the three publicly reported conditions may predict mortality rates across a wide range of medical and surgical conditions.
Citation: McCrum ML, Joynt KE, Orav EJ, Gawande AA, Jha AK. Mortality for publicly reported conditions and overall hospital mortality rates. JAMA Intern Med. 2013;173:1351-1357.
Cost Savings in Decreasing Preventable Acute-Care Visits Are Limited among High-Cost Medicare Utilizers
Clinical question: What role do preventable acute-care visits play in the overall costs of care for the highest Medicare utilizers?
Background: Some 10% of Medicare patients account for more than half the costs. Interventions targeted at decreasing acute-care costs (ED visits and inpatient hospitalizations) for this high-cost population are widespread, but it is unknown what impact they can have.
Study design: Retrospective cohort.
Setting: National Medicare fee-for-service population.
Synopsis: Standardized total costs were created for fee-for-service Medicare patients for 2009 and 2010 in order to identify high-cost and persistently high-cost patients. Algorithms were used to identify preventable ED visits and hospitalizations in both the high-cost and non-high-cost cohorts.
Of the more than 1 million patients in the sample Medicare population, as many as 113,341 were high-cost. As much as 73% of acute-care spending was attributable to this cohort. Overall, 10% of acute-care costs were felt to be preventable in the high-cost group, 13.5% in the persistently high-cost group, and 19% in the non-high-cost group for 2010. The most common reasons for preventable acute care in the high-cost cohort were heart failure, bacterial pneumonia, and chronic obstructive pulmonary disease. Catastrophic events (myocardial infarction, stroke, sepsis), cancer, and orthopedic procedures drove overall inpatient costs in the high-cost group.
Preventable costs were higher per capita in areas with higher numbers of primary-care and specialist physicians, but it’s unclear if this was a supply or demand issue. The study also used algorithms that possibly overestimate the amount of preventable acute care.
Bottom line: In the highest Medicare utilizers, cost savings aimed at preventable acute care may be limited and might be better targeted at efficiency during acute-care episodes.
Citation: Joynt KE, Gawande AA, Orav EJ, Jha AK. Contribution of preventable acute care spending to total spending for high-cost Medicare patients. JAMA. 2013;309:2572-2578.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Early treatment with intravenous tPA for acute stroke
- Perioperative morbidity, mortality for current smokers
- Statins associated with musculoskeletal conditions
- Antithrombotic medications in patients with history of stroke
- Extended prophylaxis with aspirin for patients after total hip arthroplasty
- Prognosis for symptomatic subsegmental pulmonary embolism
- Video-based educational workshops for academic hospitalists
- Increased mortality for elective surgeries on Fridays, weekends
- Basal plus correction insulin regimen and Type 2 diabetes
- Capnography to diagnose diabetic ketoacidosis in the ED
- How publicly reported mortality rates correlate with hospitals’ overall mortality
- Cost savings and preventable acute-care visits for Medicare patients
Early tPA in Acute Stroke Is Associated with Better Short-Term Outcomes in Routine Clinical Practice
Clinical question: Does early treatment with intravenous (IV) tissue plasminogen activator (tPA) result in better outcomes among patients with acute ischemic stroke in routine clinical practice?
Background: IV tPA for acute ischemic stroke is beneficial if given in the first 4.5 hours after symptom onset. However, pooled data from clinical trials have been limited in characterizing the extent to which onset-to-treatment (OTT) with IV tPA influences outcomes and how effective tPA is in routine clinical practice.
Study design: Data analysis from a stroke registry.
Setting: One thousand three hundred ninety-five U.S. hospitals participating in the Get with the Guidelines—Stroke Program.
Synopsis: Data were analyzed from 58,353 tPA-treated patients within 4.5 hours of symptom onset. Clinical outcomes were compared among patients treated in the 0-90-, 91-180-, and 181-270-minute OTT windows. Patient factors strongly associated with shorter OTT were greater stroke severity (odds ratio [OR] 2.8; 95% confidence interval [CI], 2.5-3.1 per five-point increase), arrival by ambulance (OR 5.9; 95% CI, 4.5-7.3), and arrival during regular hours (OR 4.6; 95% CI, 3.8-5.4). Faster OTT, in 15-minute increments, was associated with reduced in-hospital mortality (OR 0.96; 95% CI, 0.95-0.98; P<.001), reduced symptomatic intracranial hemorrhage (OR 0.96; 95% CI, 0.95-0.98; P<.001), increased achievement of independent ambulation at discharge (OR 1.04; 95% CI, 1.03-1.05; P<.001), and increased discharge to home (OR 1.03; 95% CI, 1.02-1.04; P<.001).
Data collected were dependent on the accuracy and completeness of the chart abstraction, and only short-term outcomes were reported. Although no post-discharge outcomes were reported, previous studies have shown that functional status at discharge strongly correlates with three-month disability outcomes.
Bottom line: In routine clinical practice, earlier tPA for acute ischemic strokes results in better short-term clinical outcomes.
Citation: Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309:2480-2488.
Current Smokers Have Higher Perioperative Morbidity and Mortality Compared to Past Smokers
Clinical question: Is there an association between current and past smoking on outcomes among patients having major surgery?
Background: Smoking is associated with adverse postoperative outcomes, but it is not known whether the associations are dose-dependent or limited to patients with smoking-related diseases. Smoking-related effects on postoperative events among patients having major surgery are also not well established.
Study design: Retrospective cohort study.
Setting: Four hundred forty-eight non-VA hospitals across the U.S., Canada, Lebanon, and the United Arab Emirates.
Synopsis: Data from 607,558 adult patients undergoing major surgery were obtained from the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database. After adjusting for confounders (cardiopulmonary diseases and cancer), the effects of current and past smoking (quit >1 year prior) on 30-day post-operative outcomes were measured.
There were 125,192 (21%) current smokers and 78,763 (13%) past smokers. Increased odds of post-op mortality were noted in current smokers only (odds ratio [OR] 1.17; 95% CI, 1.10-1.24). The adjusted odds ratios were higher for arterial and respiratory events among current smokers compared with past smokers (OR 1.65; 95% CI, 1.51-1.81 vs. OR 1.20; CI, 1.09-1.31 for arterial events, respectively) and (OR, 1.45; CI, 1.40-1.51 vs. OR, 1.13; CI, 1.08-1.18, for respiratory events, respectively). No significant effects on venous events were observed.
There was an increased adjusted odds of mortality for current smokers with <10 pack-years, while the effects on arterial and respiratory events increased incrementally with increased pack-years. Smoking was associated with adverse post-op outcomes regardless of smoking-related diseases. Variability in hospital quality or surgical strategies may have confounded the results.
Bottom line: Among patients undergoing major surgery, current but not past smoking was associated with higher mortality; smoking cessation for at least a year prior to surgery may decrease post-operative adverse events.
Citation: Musallam KM, Rosendaal FR, Zaatari G, et al. Smoking and the risk of mortality and vascular and respiratory events in patients undergoing major surgery. JAMA Surg. 2013 Jun 19:1-8. doi: 10.1001/jamasurg.2013.2360 [Epub ahead of print].
Statins Associated with Several Musculoskeletal Conditions
Clinical question: Is statin use associated with musculoskeletal adverse events, including arthropathy and injury, in physically active individuals?
Background: Statin-induced musculoskeletal adverse events (AEs) include myalgias, muscle weakness, cramps, rhabdomyolysis, and tendinous disease. The full spectrum of AEs is unknown because randomized clinical trials have not been powered to detect uncommon AEs.
Study design: Retrospective cohort study with propensity score matching.
Setting: San Antonio military area.
Synopsis: A total of 46,249 patients aged 30 to 85 years who met study criteria were propensity-matched into 6,967 statin users and 6,967 nonusers. The occurrence of musculoskeletal conditions were categorized using ICD-9 codes: Msk1, all musculoskeletal diseases; Msk1a, arthropathies and related diseases; Msk1b, injury-related diseases; and Msk2, drug-associated musculoskeletal pain. Of these, statin users had a higher odds ratio (OR) for Msk1 (OR 1.19; 95% CI, 1.08-1.30), Msk1b (1.13; 1.05-1.21), and Msk2 (1.09; 1.02-1.18). Msk1b (arthropathies) had an OR of 1.07 (0.9-1.16, P=0.07). Simvastatin was used by 73.5% of patients, and years of simvastatin use was not a significant predictor of any of the outcome measures. Secondary and sensitivity analyses showed higher adjusted ORs for statin users in all groups. This study was limited by the use of ICD-9-CM codes for identification of baseline characteristics, and the musculoskeletal diagnosis groups used were not validated.
Bottom line: Statin use is associated with an increased likelihood of musculoskeletal conditions, arthropathies, injuries, and pain.
Citation: Mansi I, Frei CR, Pugh M, Makris U, Mortensen EM. Statins and musculoskeletal conditions, arthropathies, and injuries. JAMA Intern Med. 2013;173:1318-1326.
Evidence-Based Guidelines on Periprocedural Management of Antithrombotic Medications in Patients with History of Stroke
Clinical question: What is the evidence for the periprocedural management of antithrombotics in patients with ischemic cerebrovascular accidents (CVAs)?
Background: Evidence-based guidelines are needed to help clinicians determine the thromboembolic risk of temporary discontinuation of antithrombotic medications, the perioperative bleeding risks of continuing antithrombotic agents, whether bridging therapy should be used, and the appropriate timing of antithrombotic agent discontinuation.
Study design: Systematic literature review with practice recommendations.
Setting: American Academy of Neurology Guideline Development Subcommittee convened an expert panel to review and provide recommendations.
Synopsis: Researchers analyzed 133 literature reviews via MEDLINE and EMBASE. Aspirin in stroke patients:
- Should routinely be continued for dental procedures (Level A);
- Should probably be continued for invasive ocular anesthesia, cataract surgery, dermatologic procedures, transrectal ultrasound-guided prostate biopsy, spinal/epidural procedures, and carpal tunnel surgery (Level B); and
- Should possibly be continued for vitreoretinal surgery, electromyogram (EMG), transbronchial lung biopsy, colonoscopic polypectomy, upper endoscopy and biopsy/sphincterotomy, and abdominal ultrasound-guided biopsies (Level C).
Warfarin in stroke patients:
- Should routinely be continued for dental procedures (Level A); and
- Should possibly continued for dermatologic procedures (Level B) and EMG, prostate procedures, inguinal hemiorrhaphy, and endothermal ablation of great saphenous vein (Level C).
- There is a lack of evidence on warfarin for ophthalmologic procedures, with the exception of ocular anesthesia, where it probably does not increase clinically significant bleeding (Level B).
There was not enough evidence to support or refute a recommendation regarding heparin bridge therapy in reducing thromboembolism in chronically anticoagulated patients (Level B).
Bottom line: These are the most up-to-date guidelines for anticoagulant and antiplatelet agents in patients with transient ischemic attacks and strokes undergoing procedures, but further research is needed in many areas.
Citation: Armstrong MJ, Gronseth G Anderson DC, et al. Summary of evidence-based guideline: periprocedural management of antithrombotic medications in patients with ischemic cerebrovascular disease: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2065-2069.
Extended Prophylaxis with Aspirin Was Noninferior to Extended Prophylaxis with Low-Molecular-Weight Heparin
Clinical question: Is aspirin as effective as low-molecular-weight heparin (LMWH) for the extended prophylaxis of venous thromboembolism (VTE) after total hip arthroplasty (THA)?
Background: Deep vein thrombosis (DVT) and pulmonary embolism (PE) are common complications after THA. After initial prophylaxis, LMWH given for up to 30 days has been shown to reduce VTE compared with placebo. However, LMWH is costly and may increase the risk of minor bleeding. Aspirin is a potentially simple, low-cost alternative.
Study design: Randomized, placebo-controlled trial.
Setting: Twelve university-affiliated orthopedic hospitals in Canada.
Synopsis: Patients undergoing elective THA without hip fracture, metastatic cancer, or bleeding precluding anticoagulants were eligible. All patients received dalteparin for 10 days and were then randomized to aspirin 81 mg daily or to continue dalteparin. The primary outcome was symptomatic proximal DVT or PE during 90 days’ follow-up. The study was terminated early due to slow enrollment. At that time, 2,364 patients had been enrolled, and an analysis by an independent data safety and monitoring board determined that continuing the study was unlikely to alter the main findings. Extended prophylaxis with aspirin was noninferior to LMWH for the primary outcome, which occurred in 0.3% vs. 1.3%, respectively (95% CI, -0.5% to 2.5%, P<.001 for noninferiority). There were no significant differences in major or minor bleeding.
Though the early termination is a concern, the sample size was large and the results do not suggest inadequate power as a reason for lack of superiority for LMWH. Also, all patients received 10 days of LMWH, which indicates a period of LMWH after discharge will still be needed for most patients prior to initiating aspirin.
Bottom line: After initial LMWH prophylaxis for 10 days, extended prophylaxis with aspirin can be considered, particularly for patients for whom LMWH may not be feasible.
Citation: Anderson DR, Dunbar MJ, Bohm ER, et al. Aspirin versus low-molecular-weight heparin for extended venous thromboembolism prophylaxis after total hip arthroplasty: a randomized trial. Ann Intern Med. 2013;158:800-806.
Symptomatic Subsegmental Pulmonary Embolism (PE) Has a Prognosis Similar to Proximal PE
Clinical question: Is the prognosis of a symptomatic subsegmental pulmonary embolism (PE) similar to that of a more proximal PE?
Background: The use of multidetector computed tomography angiography (CTA) has allowed for better assessment of the pulmonary vasculature and increased detection of distal emboli. Prior studies have raised questions on the clinical importance of subsegmental PE but have been limited by small size or retrospective design.
Study design: Combined data from two prospective trials of management of suspected PE.
Setting: Twelve hospitals in the Netherlands and four tertiary-care emergency departments in Canada.
Synopsis: The study cohort consisted of 3,769 patients with suspected PE, of which 2,688 underwent CTA. Of patients diagnosed with PE, 15.5% had isolated subsegmental emboli. All patients were treated with anticoagulation. During three months of follow-up, the incidence of symptomatic recurrence for subsegmental PE was similar to patients with proximal PE (3.6% vs. 2.5%, respectively). The mortality rates for patients with subsegmental and proximal PE were also similar (10.3% vs. 6.3%, respectively).
The study may have been underpowered to detect small differences in event rates; however, there was no trend suggesting that subsegmental PE had better outcomes than more proximal PE. Also, the study did not specifically investigate whether any management strategy is preferred based on thrombus location on CTA.
Bottom line: Clinicians should continue to anticoagulate patients with subsegmental PE as the prognosis is similar to those with proximal PE.
Citation: Den Exter PL, van Es J, Klok FA, et al. Risk profile and clinical outcome of symptomatic subsegmental pulmonary embolism. Blood. 2013;122:1144-1149.
Video-Based Educational Workshop for Academic Hospitalists and House Staff May Improve Professionalism
Clinical question: Can video-based education promote professionalism among academic hospitalists and house staff?
Background: Unprofessional behavior by academic hospitalists and residents can negatively impact the learning environment and patient safety. This behavior increases throughout training, and faculty behavior can be influential. There is a paucity of educational materials to train hospitalists and house staff to recognize and ameliorate unprofessional behaviors.
Study design: Educational survey study.
Setting: University of Chicago, Northwestern University, and NorthShore University Health System teaching hospitals.
Synopsis: Three videos were developed displaying three types of unprofessional behavior: disparaging other physicians, “blocking” admissions, and misrepresenting tests to expedite their completion. There were 44 hospitalists and 244 house staff who received a 60-minute workshop in which they watched the videos using a viewing tool and discussed the videos in small groups.
For all three videos, more than three-quarters of both hospitalists and house staff felt the behavior was unprofessional or somewhat unprofessional. Hospitalists and house staff found the workshop useful and effective (65.9% and 77.1%, respectively) and would change their behavior as a result of the workshop (65.9% and 67.2%, respectively). Those who perceived the videos as “very realistic” were more likely to report intent to change behavior (93% vs. 53%, P=0.01).
This study is limited by its small sample size and possible selection bias. Those interested or concerned about unprofessional behavior may have been more likely to attend the workshop.
Bottom line: Video-based professionalism education is a feasible and well-received way to educate hospitalists and residents about unprofessional behavior and may even affect their future behavior.
Citation: Farnan JM, O’Leary KJ, Didwania A, et al. Promoting professionalism via a video-based educational workshop for academic hospitalists and housestaff. J Hosp Med. 2013;8:386-389.
Friday and Weekend Elective Surgeries Have Increased Mortality
Clinical question: How can the association between mortality and the day of elective surgical procedures be assessed?
Background: Several studies have described the “weekend effect” for both surgical and medical patients, with higher mortality and length of stay in patients admitted on the weekend compared to weekdays. Two potential explanations are poorer quality of care being delivered on the weekend or more severely ill patients being operated on or admitted on the weekend.
Study design: Retrospective analysis of national hospital administrative data.
Setting: All acute-care and specialist hospitals in England from 2008 to 2011.
Synopsis: There were 4,133,346 elective, inpatient surgical procedures studied. Friday surgeries had an adjusted odds ratio of death within 30 days and within two days of 1.44 [95% CI, 1.39-1.50] and 1.42 [95% CI, 1.26-1.60], respectively, when compared with Monday. Weekend surgeries had an adjusted odds ratio of death within 30 days and within two days of 1.82 [95% CI, 1.71-1.94] and 2.67 [95% CI, 2.30-3.09], respectively, when compared with Monday. There were significant trends toward higher mortality at the end of the workweek and weekends for four high-risk procedures: esophagus and/or stomach excision, colon and/or rectum excisions, coronary artery bypass graft, and lung excision. For lower-risk procedures, there was a significant increase in mortality for Friday surgeries but not weekend surgeries. As with all studies using administrative data, inherent selection biases could not be adjusted for Friday or weekend procedures.
Bottom line: Elective surgeries that occur on the weekend and later in the week have an increased risk of mortality, implying that the weekend effect is due to poorer quality of care during weekends, rather than higher-acuity patients presenting on weekends.
Citation: Aylin P, Alexandrescu R, Jen MH, Mayer EK, Bottle A. Day of week of procedure and 30 day mortality for elective surgery: retrospective analysis of hospital episode statistics. BMJ. 2013;346:f2424.
Basal Plus Correction Insulin Regimen Is Effective in Hospitalized Patients with Type 2 Diabetes
Clinical question: Does a basal plus correction insulin regimen (as needed with meals) result in similar glycemic control and lower rates of hypoglycemia compared to a basal-bolus regimen?
Background: Basal bolus is the preferred insulin regimen for non-critically-ill hospitalized patients as per clinical guidelines. But use is limited due to the complexity of the regimen and the fear of inducing hypoglycemia. A less complex, easier-to-implement basal plus correction insulin regimen may be an effective alternative.
Study design: Multicenter, prospective, open-label, randomized study.
Setting: Six hospitals in the U.S.
Synopsis: A group of 375 medical and surgical patients with Type 2 diabetes treated with diet, oral anti-diabetic agents, or low-dose insulin (≤ 0.4 units/kg/day) were randomized to:
- Basal-bolus insulin regimen with glargine once daily and fixed doses of glusiline before meals;
- Basal plus correction insulin (“basal plus”) regimen with glargine once daily and glusiline sliding scale insulin (SSI) before meals; or
- Regular SSI alone.
After the first day of therapy, treatment with basal-bolus and basal-plus regimens resulted in similar improvements in daily blood glucose (BG) (P=0.16), and both were superior to SSI alone (P=0.04). Both regimens also resulted in less treatment failure (defined as mean daily BG of >240 mg/dl or >2 consecutive BG >240 mg/dl) than did treatment with SSI. Hypoglycemia (BG <70 mg/dl) occurred in 16%, 13%, and 3% of patients in the basal-bolus, basal-plus, and SSI groups, respectively (P=0.02). There were no between-group differences in the frequency of severe hypoglycemia (<40 mg/dl; P=0.76).
The study was not powered to evaluate hospital complications (infection, mortality, hospital stay, and readmissions) across groups.
Bottom line: The basal-plus regimen resulted in glycemic control similar to standard basal-bolus regimen and is an effective alternative for the initial management of hyperglycemia in general medical and surgical patients with Type 2 diabetes.
Citation: Umpierrez GE, Smiley D, Hermayer K, et al. Randomized study comparing a basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes: Basal plus trial. Diabetes Care. 2013;36:2169-2174.
Capnography Can Help Diagnose Diabetic Ketoacidosis in the ED
Clinical question: Can capnography be used as a screening tool to identify patients with diabetic ketoacidosis (DKA)?
Background: Metabolic acidosis is a major criterion for diagnosing DKA. Previous studies have shown that end-tidal carbon dioxide (ETCO2) measurement by capnography can provide an accurate estimation of arterial carbon dioxide tension (PaCO2) and may be a noninvasive, fast, inexpensive measurement of acidosis in DKA. However, those studies were in pediatric patients and had small sample sizes.
Study design: Cross-sectional, prospective descriptive-analytic study.
Setting: The ED of Imam Reza Medical Research and Training Hospital, Tabriz, East Azarbaijan, Iran.
Synopsis: A total of 181 adult patients older than 18 with suspected DKA and blood sugar >250 mg/dl were included in the study. Simultaneous capnography and arterial blood gas (ABG) were obtained on all patients. Urine ketones, complete blood count, serum levels of potassium, urea, and creatinine were collected. Sixty-two patients were found to have DKA, while 119 had other conditions associated with metabolic acidosis. There was a significant linear relationship between pH and ETCO2 (P>0.0001, relative risk (R)=0.253), PaCO2 and ETCO2 (P>0.0001, R=0.572), and bicarbonate (HCO3) and ETCO2 (P>0.0001, R=0.730). ETCO2 values >24.5 mmHg had a sensitivity and specificity of 0.90 for ruling out DKA. No cutoff point could be determined for ruling in DKA.
The study was open to selection bias as patient collection was only done during the day, so eligible subjects may have been missed. Moreover, though the study suggests that capnography has a role in ruling out DKA, the exact cutoff value is unclear. Other studies found that higher values were needed to exclude diagnosis.
Bottom line: Using ETCO2 values >24.5 mmHg, capnography can help exclude the diagnosis of DKA in adult patients with elevated BG.
Citation: Soleimanour H, Taghizadieh A, Niafar M, Rahmani F, Golzari S, Esfanjani RM. Predictive value of capnography for diagnosis in patients with suspected diabetic ketoacidosis in the emergency department. West J Emerg Med. 2013. doi: 10.5811/westjem.2013.4.14296.
Publicly Reported Mortality Correlates with Overall Mortality
Clinical question: Are publicly reported mortality rates associated with a hospital’s overall medical and surgical mortality rate?
Background: Public reporting of mortality has become an important strategy in Medicare’s quality-improvement initiative. However, the mortality rate for only three conditions, acute myocardial infarction, congestive heart failure, and pneumonia are reported. It is unclear if these rates correlate to a hospital’s overall mortality rate.
Study design: Retrospective cohort.
Setting: National Medicare fee-for-service population.
Synopsis: Using 2008-2009 data from 2,322 acute-care hospitals with 6.7 million admissions, an aggregate mortality rate for the three publicly reported conditions, a standardized 30-day mortality rate for selected medical and surgical conditions, and an overall average composite mortality score was calculated for each hospital. Based on their mortality for the three publicly reported conditions, hospitals were grouped into quartiles from highest (top-performing hospitals) to lowest mortality (poor-performing hospitals).
Top-performing hospitals had a 3.6% (9.4%vs 13.0%; P<.001) lower mortality rate than poor-performing hospitals and an odds ratio >5 of being a top performer in overall mortality (OR 5.3; 95% CI, 4.3-6.5). They also had an 81% lower chance of being in the worst-performing quartile in overall mortality (OR 0.19; 95% CI, 0.14-0.27). Conversely, poor-performing hospitals had a 4.5 times higher risk of being in the lowest quartile in overall mortality. The study is limited by the use of administrative data, which limits the ability to adjust for severity of illness, overall health, and socioeconomic status of each hospital’s population.
Bottom line: A hospital’s mortality performance on the three publicly reported conditions may predict mortality rates across a wide range of medical and surgical conditions.
Citation: McCrum ML, Joynt KE, Orav EJ, Gawande AA, Jha AK. Mortality for publicly reported conditions and overall hospital mortality rates. JAMA Intern Med. 2013;173:1351-1357.
Cost Savings in Decreasing Preventable Acute-Care Visits Are Limited among High-Cost Medicare Utilizers
Clinical question: What role do preventable acute-care visits play in the overall costs of care for the highest Medicare utilizers?
Background: Some 10% of Medicare patients account for more than half the costs. Interventions targeted at decreasing acute-care costs (ED visits and inpatient hospitalizations) for this high-cost population are widespread, but it is unknown what impact they can have.
Study design: Retrospective cohort.
Setting: National Medicare fee-for-service population.
Synopsis: Standardized total costs were created for fee-for-service Medicare patients for 2009 and 2010 in order to identify high-cost and persistently high-cost patients. Algorithms were used to identify preventable ED visits and hospitalizations in both the high-cost and non-high-cost cohorts.
Of the more than 1 million patients in the sample Medicare population, as many as 113,341 were high-cost. As much as 73% of acute-care spending was attributable to this cohort. Overall, 10% of acute-care costs were felt to be preventable in the high-cost group, 13.5% in the persistently high-cost group, and 19% in the non-high-cost group for 2010. The most common reasons for preventable acute care in the high-cost cohort were heart failure, bacterial pneumonia, and chronic obstructive pulmonary disease. Catastrophic events (myocardial infarction, stroke, sepsis), cancer, and orthopedic procedures drove overall inpatient costs in the high-cost group.
Preventable costs were higher per capita in areas with higher numbers of primary-care and specialist physicians, but it’s unclear if this was a supply or demand issue. The study also used algorithms that possibly overestimate the amount of preventable acute care.
Bottom line: In the highest Medicare utilizers, cost savings aimed at preventable acute care may be limited and might be better targeted at efficiency during acute-care episodes.
Citation: Joynt KE, Gawande AA, Orav EJ, Jha AK. Contribution of preventable acute care spending to total spending for high-cost Medicare patients. JAMA. 2013;309:2572-2578.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Early treatment with intravenous tPA for acute stroke
- Perioperative morbidity, mortality for current smokers
- Statins associated with musculoskeletal conditions
- Antithrombotic medications in patients with history of stroke
- Extended prophylaxis with aspirin for patients after total hip arthroplasty
- Prognosis for symptomatic subsegmental pulmonary embolism
- Video-based educational workshops for academic hospitalists
- Increased mortality for elective surgeries on Fridays, weekends
- Basal plus correction insulin regimen and Type 2 diabetes
- Capnography to diagnose diabetic ketoacidosis in the ED
- How publicly reported mortality rates correlate with hospitals’ overall mortality
- Cost savings and preventable acute-care visits for Medicare patients
Early tPA in Acute Stroke Is Associated with Better Short-Term Outcomes in Routine Clinical Practice
Clinical question: Does early treatment with intravenous (IV) tissue plasminogen activator (tPA) result in better outcomes among patients with acute ischemic stroke in routine clinical practice?
Background: IV tPA for acute ischemic stroke is beneficial if given in the first 4.5 hours after symptom onset. However, pooled data from clinical trials have been limited in characterizing the extent to which onset-to-treatment (OTT) with IV tPA influences outcomes and how effective tPA is in routine clinical practice.
Study design: Data analysis from a stroke registry.
Setting: One thousand three hundred ninety-five U.S. hospitals participating in the Get with the Guidelines—Stroke Program.
Synopsis: Data were analyzed from 58,353 tPA-treated patients within 4.5 hours of symptom onset. Clinical outcomes were compared among patients treated in the 0-90-, 91-180-, and 181-270-minute OTT windows. Patient factors strongly associated with shorter OTT were greater stroke severity (odds ratio [OR] 2.8; 95% confidence interval [CI], 2.5-3.1 per five-point increase), arrival by ambulance (OR 5.9; 95% CI, 4.5-7.3), and arrival during regular hours (OR 4.6; 95% CI, 3.8-5.4). Faster OTT, in 15-minute increments, was associated with reduced in-hospital mortality (OR 0.96; 95% CI, 0.95-0.98; P<.001), reduced symptomatic intracranial hemorrhage (OR 0.96; 95% CI, 0.95-0.98; P<.001), increased achievement of independent ambulation at discharge (OR 1.04; 95% CI, 1.03-1.05; P<.001), and increased discharge to home (OR 1.03; 95% CI, 1.02-1.04; P<.001).
Data collected were dependent on the accuracy and completeness of the chart abstraction, and only short-term outcomes were reported. Although no post-discharge outcomes were reported, previous studies have shown that functional status at discharge strongly correlates with three-month disability outcomes.
Bottom line: In routine clinical practice, earlier tPA for acute ischemic strokes results in better short-term clinical outcomes.
Citation: Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309:2480-2488.
Current Smokers Have Higher Perioperative Morbidity and Mortality Compared to Past Smokers
Clinical question: Is there an association between current and past smoking on outcomes among patients having major surgery?
Background: Smoking is associated with adverse postoperative outcomes, but it is not known whether the associations are dose-dependent or limited to patients with smoking-related diseases. Smoking-related effects on postoperative events among patients having major surgery are also not well established.
Study design: Retrospective cohort study.
Setting: Four hundred forty-eight non-VA hospitals across the U.S., Canada, Lebanon, and the United Arab Emirates.
Synopsis: Data from 607,558 adult patients undergoing major surgery were obtained from the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database. After adjusting for confounders (cardiopulmonary diseases and cancer), the effects of current and past smoking (quit >1 year prior) on 30-day post-operative outcomes were measured.
There were 125,192 (21%) current smokers and 78,763 (13%) past smokers. Increased odds of post-op mortality were noted in current smokers only (odds ratio [OR] 1.17; 95% CI, 1.10-1.24). The adjusted odds ratios were higher for arterial and respiratory events among current smokers compared with past smokers (OR 1.65; 95% CI, 1.51-1.81 vs. OR 1.20; CI, 1.09-1.31 for arterial events, respectively) and (OR, 1.45; CI, 1.40-1.51 vs. OR, 1.13; CI, 1.08-1.18, for respiratory events, respectively). No significant effects on venous events were observed.
There was an increased adjusted odds of mortality for current smokers with <10 pack-years, while the effects on arterial and respiratory events increased incrementally with increased pack-years. Smoking was associated with adverse post-op outcomes regardless of smoking-related diseases. Variability in hospital quality or surgical strategies may have confounded the results.
Bottom line: Among patients undergoing major surgery, current but not past smoking was associated with higher mortality; smoking cessation for at least a year prior to surgery may decrease post-operative adverse events.
Citation: Musallam KM, Rosendaal FR, Zaatari G, et al. Smoking and the risk of mortality and vascular and respiratory events in patients undergoing major surgery. JAMA Surg. 2013 Jun 19:1-8. doi: 10.1001/jamasurg.2013.2360 [Epub ahead of print].
Statins Associated with Several Musculoskeletal Conditions
Clinical question: Is statin use associated with musculoskeletal adverse events, including arthropathy and injury, in physically active individuals?
Background: Statin-induced musculoskeletal adverse events (AEs) include myalgias, muscle weakness, cramps, rhabdomyolysis, and tendinous disease. The full spectrum of AEs is unknown because randomized clinical trials have not been powered to detect uncommon AEs.
Study design: Retrospective cohort study with propensity score matching.
Setting: San Antonio military area.
Synopsis: A total of 46,249 patients aged 30 to 85 years who met study criteria were propensity-matched into 6,967 statin users and 6,967 nonusers. The occurrence of musculoskeletal conditions were categorized using ICD-9 codes: Msk1, all musculoskeletal diseases; Msk1a, arthropathies and related diseases; Msk1b, injury-related diseases; and Msk2, drug-associated musculoskeletal pain. Of these, statin users had a higher odds ratio (OR) for Msk1 (OR 1.19; 95% CI, 1.08-1.30), Msk1b (1.13; 1.05-1.21), and Msk2 (1.09; 1.02-1.18). Msk1b (arthropathies) had an OR of 1.07 (0.9-1.16, P=0.07). Simvastatin was used by 73.5% of patients, and years of simvastatin use was not a significant predictor of any of the outcome measures. Secondary and sensitivity analyses showed higher adjusted ORs for statin users in all groups. This study was limited by the use of ICD-9-CM codes for identification of baseline characteristics, and the musculoskeletal diagnosis groups used were not validated.
Bottom line: Statin use is associated with an increased likelihood of musculoskeletal conditions, arthropathies, injuries, and pain.
Citation: Mansi I, Frei CR, Pugh M, Makris U, Mortensen EM. Statins and musculoskeletal conditions, arthropathies, and injuries. JAMA Intern Med. 2013;173:1318-1326.
Evidence-Based Guidelines on Periprocedural Management of Antithrombotic Medications in Patients with History of Stroke
Clinical question: What is the evidence for the periprocedural management of antithrombotics in patients with ischemic cerebrovascular accidents (CVAs)?
Background: Evidence-based guidelines are needed to help clinicians determine the thromboembolic risk of temporary discontinuation of antithrombotic medications, the perioperative bleeding risks of continuing antithrombotic agents, whether bridging therapy should be used, and the appropriate timing of antithrombotic agent discontinuation.
Study design: Systematic literature review with practice recommendations.
Setting: American Academy of Neurology Guideline Development Subcommittee convened an expert panel to review and provide recommendations.
Synopsis: Researchers analyzed 133 literature reviews via MEDLINE and EMBASE. Aspirin in stroke patients:
- Should routinely be continued for dental procedures (Level A);
- Should probably be continued for invasive ocular anesthesia, cataract surgery, dermatologic procedures, transrectal ultrasound-guided prostate biopsy, spinal/epidural procedures, and carpal tunnel surgery (Level B); and
- Should possibly be continued for vitreoretinal surgery, electromyogram (EMG), transbronchial lung biopsy, colonoscopic polypectomy, upper endoscopy and biopsy/sphincterotomy, and abdominal ultrasound-guided biopsies (Level C).
Warfarin in stroke patients:
- Should routinely be continued for dental procedures (Level A); and
- Should possibly continued for dermatologic procedures (Level B) and EMG, prostate procedures, inguinal hemiorrhaphy, and endothermal ablation of great saphenous vein (Level C).
- There is a lack of evidence on warfarin for ophthalmologic procedures, with the exception of ocular anesthesia, where it probably does not increase clinically significant bleeding (Level B).
There was not enough evidence to support or refute a recommendation regarding heparin bridge therapy in reducing thromboembolism in chronically anticoagulated patients (Level B).
Bottom line: These are the most up-to-date guidelines for anticoagulant and antiplatelet agents in patients with transient ischemic attacks and strokes undergoing procedures, but further research is needed in many areas.
Citation: Armstrong MJ, Gronseth G Anderson DC, et al. Summary of evidence-based guideline: periprocedural management of antithrombotic medications in patients with ischemic cerebrovascular disease: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2065-2069.
Extended Prophylaxis with Aspirin Was Noninferior to Extended Prophylaxis with Low-Molecular-Weight Heparin
Clinical question: Is aspirin as effective as low-molecular-weight heparin (LMWH) for the extended prophylaxis of venous thromboembolism (VTE) after total hip arthroplasty (THA)?
Background: Deep vein thrombosis (DVT) and pulmonary embolism (PE) are common complications after THA. After initial prophylaxis, LMWH given for up to 30 days has been shown to reduce VTE compared with placebo. However, LMWH is costly and may increase the risk of minor bleeding. Aspirin is a potentially simple, low-cost alternative.
Study design: Randomized, placebo-controlled trial.
Setting: Twelve university-affiliated orthopedic hospitals in Canada.
Synopsis: Patients undergoing elective THA without hip fracture, metastatic cancer, or bleeding precluding anticoagulants were eligible. All patients received dalteparin for 10 days and were then randomized to aspirin 81 mg daily or to continue dalteparin. The primary outcome was symptomatic proximal DVT or PE during 90 days’ follow-up. The study was terminated early due to slow enrollment. At that time, 2,364 patients had been enrolled, and an analysis by an independent data safety and monitoring board determined that continuing the study was unlikely to alter the main findings. Extended prophylaxis with aspirin was noninferior to LMWH for the primary outcome, which occurred in 0.3% vs. 1.3%, respectively (95% CI, -0.5% to 2.5%, P<.001 for noninferiority). There were no significant differences in major or minor bleeding.
Though the early termination is a concern, the sample size was large and the results do not suggest inadequate power as a reason for lack of superiority for LMWH. Also, all patients received 10 days of LMWH, which indicates a period of LMWH after discharge will still be needed for most patients prior to initiating aspirin.
Bottom line: After initial LMWH prophylaxis for 10 days, extended prophylaxis with aspirin can be considered, particularly for patients for whom LMWH may not be feasible.
Citation: Anderson DR, Dunbar MJ, Bohm ER, et al. Aspirin versus low-molecular-weight heparin for extended venous thromboembolism prophylaxis after total hip arthroplasty: a randomized trial. Ann Intern Med. 2013;158:800-806.
Symptomatic Subsegmental Pulmonary Embolism (PE) Has a Prognosis Similar to Proximal PE
Clinical question: Is the prognosis of a symptomatic subsegmental pulmonary embolism (PE) similar to that of a more proximal PE?
Background: The use of multidetector computed tomography angiography (CTA) has allowed for better assessment of the pulmonary vasculature and increased detection of distal emboli. Prior studies have raised questions on the clinical importance of subsegmental PE but have been limited by small size or retrospective design.
Study design: Combined data from two prospective trials of management of suspected PE.
Setting: Twelve hospitals in the Netherlands and four tertiary-care emergency departments in Canada.
Synopsis: The study cohort consisted of 3,769 patients with suspected PE, of which 2,688 underwent CTA. Of patients diagnosed with PE, 15.5% had isolated subsegmental emboli. All patients were treated with anticoagulation. During three months of follow-up, the incidence of symptomatic recurrence for subsegmental PE was similar to patients with proximal PE (3.6% vs. 2.5%, respectively). The mortality rates for patients with subsegmental and proximal PE were also similar (10.3% vs. 6.3%, respectively).
The study may have been underpowered to detect small differences in event rates; however, there was no trend suggesting that subsegmental PE had better outcomes than more proximal PE. Also, the study did not specifically investigate whether any management strategy is preferred based on thrombus location on CTA.
Bottom line: Clinicians should continue to anticoagulate patients with subsegmental PE as the prognosis is similar to those with proximal PE.
Citation: Den Exter PL, van Es J, Klok FA, et al. Risk profile and clinical outcome of symptomatic subsegmental pulmonary embolism. Blood. 2013;122:1144-1149.
Video-Based Educational Workshop for Academic Hospitalists and House Staff May Improve Professionalism
Clinical question: Can video-based education promote professionalism among academic hospitalists and house staff?
Background: Unprofessional behavior by academic hospitalists and residents can negatively impact the learning environment and patient safety. This behavior increases throughout training, and faculty behavior can be influential. There is a paucity of educational materials to train hospitalists and house staff to recognize and ameliorate unprofessional behaviors.
Study design: Educational survey study.
Setting: University of Chicago, Northwestern University, and NorthShore University Health System teaching hospitals.
Synopsis: Three videos were developed displaying three types of unprofessional behavior: disparaging other physicians, “blocking” admissions, and misrepresenting tests to expedite their completion. There were 44 hospitalists and 244 house staff who received a 60-minute workshop in which they watched the videos using a viewing tool and discussed the videos in small groups.
For all three videos, more than three-quarters of both hospitalists and house staff felt the behavior was unprofessional or somewhat unprofessional. Hospitalists and house staff found the workshop useful and effective (65.9% and 77.1%, respectively) and would change their behavior as a result of the workshop (65.9% and 67.2%, respectively). Those who perceived the videos as “very realistic” were more likely to report intent to change behavior (93% vs. 53%, P=0.01).
This study is limited by its small sample size and possible selection bias. Those interested or concerned about unprofessional behavior may have been more likely to attend the workshop.
Bottom line: Video-based professionalism education is a feasible and well-received way to educate hospitalists and residents about unprofessional behavior and may even affect their future behavior.
Citation: Farnan JM, O’Leary KJ, Didwania A, et al. Promoting professionalism via a video-based educational workshop for academic hospitalists and housestaff. J Hosp Med. 2013;8:386-389.
Friday and Weekend Elective Surgeries Have Increased Mortality
Clinical question: How can the association between mortality and the day of elective surgical procedures be assessed?
Background: Several studies have described the “weekend effect” for both surgical and medical patients, with higher mortality and length of stay in patients admitted on the weekend compared to weekdays. Two potential explanations are poorer quality of care being delivered on the weekend or more severely ill patients being operated on or admitted on the weekend.
Study design: Retrospective analysis of national hospital administrative data.
Setting: All acute-care and specialist hospitals in England from 2008 to 2011.
Synopsis: There were 4,133,346 elective, inpatient surgical procedures studied. Friday surgeries had an adjusted odds ratio of death within 30 days and within two days of 1.44 [95% CI, 1.39-1.50] and 1.42 [95% CI, 1.26-1.60], respectively, when compared with Monday. Weekend surgeries had an adjusted odds ratio of death within 30 days and within two days of 1.82 [95% CI, 1.71-1.94] and 2.67 [95% CI, 2.30-3.09], respectively, when compared with Monday. There were significant trends toward higher mortality at the end of the workweek and weekends for four high-risk procedures: esophagus and/or stomach excision, colon and/or rectum excisions, coronary artery bypass graft, and lung excision. For lower-risk procedures, there was a significant increase in mortality for Friday surgeries but not weekend surgeries. As with all studies using administrative data, inherent selection biases could not be adjusted for Friday or weekend procedures.
Bottom line: Elective surgeries that occur on the weekend and later in the week have an increased risk of mortality, implying that the weekend effect is due to poorer quality of care during weekends, rather than higher-acuity patients presenting on weekends.
Citation: Aylin P, Alexandrescu R, Jen MH, Mayer EK, Bottle A. Day of week of procedure and 30 day mortality for elective surgery: retrospective analysis of hospital episode statistics. BMJ. 2013;346:f2424.
Basal Plus Correction Insulin Regimen Is Effective in Hospitalized Patients with Type 2 Diabetes
Clinical question: Does a basal plus correction insulin regimen (as needed with meals) result in similar glycemic control and lower rates of hypoglycemia compared to a basal-bolus regimen?
Background: Basal bolus is the preferred insulin regimen for non-critically-ill hospitalized patients as per clinical guidelines. But use is limited due to the complexity of the regimen and the fear of inducing hypoglycemia. A less complex, easier-to-implement basal plus correction insulin regimen may be an effective alternative.
Study design: Multicenter, prospective, open-label, randomized study.
Setting: Six hospitals in the U.S.
Synopsis: A group of 375 medical and surgical patients with Type 2 diabetes treated with diet, oral anti-diabetic agents, or low-dose insulin (≤ 0.4 units/kg/day) were randomized to:
- Basal-bolus insulin regimen with glargine once daily and fixed doses of glusiline before meals;
- Basal plus correction insulin (“basal plus”) regimen with glargine once daily and glusiline sliding scale insulin (SSI) before meals; or
- Regular SSI alone.
After the first day of therapy, treatment with basal-bolus and basal-plus regimens resulted in similar improvements in daily blood glucose (BG) (P=0.16), and both were superior to SSI alone (P=0.04). Both regimens also resulted in less treatment failure (defined as mean daily BG of >240 mg/dl or >2 consecutive BG >240 mg/dl) than did treatment with SSI. Hypoglycemia (BG <70 mg/dl) occurred in 16%, 13%, and 3% of patients in the basal-bolus, basal-plus, and SSI groups, respectively (P=0.02). There were no between-group differences in the frequency of severe hypoglycemia (<40 mg/dl; P=0.76).
The study was not powered to evaluate hospital complications (infection, mortality, hospital stay, and readmissions) across groups.
Bottom line: The basal-plus regimen resulted in glycemic control similar to standard basal-bolus regimen and is an effective alternative for the initial management of hyperglycemia in general medical and surgical patients with Type 2 diabetes.
Citation: Umpierrez GE, Smiley D, Hermayer K, et al. Randomized study comparing a basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes: Basal plus trial. Diabetes Care. 2013;36:2169-2174.
Capnography Can Help Diagnose Diabetic Ketoacidosis in the ED
Clinical question: Can capnography be used as a screening tool to identify patients with diabetic ketoacidosis (DKA)?
Background: Metabolic acidosis is a major criterion for diagnosing DKA. Previous studies have shown that end-tidal carbon dioxide (ETCO2) measurement by capnography can provide an accurate estimation of arterial carbon dioxide tension (PaCO2) and may be a noninvasive, fast, inexpensive measurement of acidosis in DKA. However, those studies were in pediatric patients and had small sample sizes.
Study design: Cross-sectional, prospective descriptive-analytic study.
Setting: The ED of Imam Reza Medical Research and Training Hospital, Tabriz, East Azarbaijan, Iran.
Synopsis: A total of 181 adult patients older than 18 with suspected DKA and blood sugar >250 mg/dl were included in the study. Simultaneous capnography and arterial blood gas (ABG) were obtained on all patients. Urine ketones, complete blood count, serum levels of potassium, urea, and creatinine were collected. Sixty-two patients were found to have DKA, while 119 had other conditions associated with metabolic acidosis. There was a significant linear relationship between pH and ETCO2 (P>0.0001, relative risk (R)=0.253), PaCO2 and ETCO2 (P>0.0001, R=0.572), and bicarbonate (HCO3) and ETCO2 (P>0.0001, R=0.730). ETCO2 values >24.5 mmHg had a sensitivity and specificity of 0.90 for ruling out DKA. No cutoff point could be determined for ruling in DKA.
The study was open to selection bias as patient collection was only done during the day, so eligible subjects may have been missed. Moreover, though the study suggests that capnography has a role in ruling out DKA, the exact cutoff value is unclear. Other studies found that higher values were needed to exclude diagnosis.
Bottom line: Using ETCO2 values >24.5 mmHg, capnography can help exclude the diagnosis of DKA in adult patients with elevated BG.
Citation: Soleimanour H, Taghizadieh A, Niafar M, Rahmani F, Golzari S, Esfanjani RM. Predictive value of capnography for diagnosis in patients with suspected diabetic ketoacidosis in the emergency department. West J Emerg Med. 2013. doi: 10.5811/westjem.2013.4.14296.
Publicly Reported Mortality Correlates with Overall Mortality
Clinical question: Are publicly reported mortality rates associated with a hospital’s overall medical and surgical mortality rate?
Background: Public reporting of mortality has become an important strategy in Medicare’s quality-improvement initiative. However, the mortality rate for only three conditions, acute myocardial infarction, congestive heart failure, and pneumonia are reported. It is unclear if these rates correlate to a hospital’s overall mortality rate.
Study design: Retrospective cohort.
Setting: National Medicare fee-for-service population.
Synopsis: Using 2008-2009 data from 2,322 acute-care hospitals with 6.7 million admissions, an aggregate mortality rate for the three publicly reported conditions, a standardized 30-day mortality rate for selected medical and surgical conditions, and an overall average composite mortality score was calculated for each hospital. Based on their mortality for the three publicly reported conditions, hospitals were grouped into quartiles from highest (top-performing hospitals) to lowest mortality (poor-performing hospitals).
Top-performing hospitals had a 3.6% (9.4%vs 13.0%; P<.001) lower mortality rate than poor-performing hospitals and an odds ratio >5 of being a top performer in overall mortality (OR 5.3; 95% CI, 4.3-6.5). They also had an 81% lower chance of being in the worst-performing quartile in overall mortality (OR 0.19; 95% CI, 0.14-0.27). Conversely, poor-performing hospitals had a 4.5 times higher risk of being in the lowest quartile in overall mortality. The study is limited by the use of administrative data, which limits the ability to adjust for severity of illness, overall health, and socioeconomic status of each hospital’s population.
Bottom line: A hospital’s mortality performance on the three publicly reported conditions may predict mortality rates across a wide range of medical and surgical conditions.
Citation: McCrum ML, Joynt KE, Orav EJ, Gawande AA, Jha AK. Mortality for publicly reported conditions and overall hospital mortality rates. JAMA Intern Med. 2013;173:1351-1357.
Cost Savings in Decreasing Preventable Acute-Care Visits Are Limited among High-Cost Medicare Utilizers
Clinical question: What role do preventable acute-care visits play in the overall costs of care for the highest Medicare utilizers?
Background: Some 10% of Medicare patients account for more than half the costs. Interventions targeted at decreasing acute-care costs (ED visits and inpatient hospitalizations) for this high-cost population are widespread, but it is unknown what impact they can have.
Study design: Retrospective cohort.
Setting: National Medicare fee-for-service population.
Synopsis: Standardized total costs were created for fee-for-service Medicare patients for 2009 and 2010 in order to identify high-cost and persistently high-cost patients. Algorithms were used to identify preventable ED visits and hospitalizations in both the high-cost and non-high-cost cohorts.
Of the more than 1 million patients in the sample Medicare population, as many as 113,341 were high-cost. As much as 73% of acute-care spending was attributable to this cohort. Overall, 10% of acute-care costs were felt to be preventable in the high-cost group, 13.5% in the persistently high-cost group, and 19% in the non-high-cost group for 2010. The most common reasons for preventable acute care in the high-cost cohort were heart failure, bacterial pneumonia, and chronic obstructive pulmonary disease. Catastrophic events (myocardial infarction, stroke, sepsis), cancer, and orthopedic procedures drove overall inpatient costs in the high-cost group.
Preventable costs were higher per capita in areas with higher numbers of primary-care and specialist physicians, but it’s unclear if this was a supply or demand issue. The study also used algorithms that possibly overestimate the amount of preventable acute care.
Bottom line: In the highest Medicare utilizers, cost savings aimed at preventable acute care may be limited and might be better targeted at efficiency during acute-care episodes.
Citation: Joynt KE, Gawande AA, Orav EJ, Jha AK. Contribution of preventable acute care spending to total spending for high-cost Medicare patients. JAMA. 2013;309:2572-2578.