User login
Reflectance Confocal Microscopy Findings in a Small-Diameter Invasive Melanoma
Melanomas have been designated as small melanomas or micromelanomas according to their long-axis diameter (<6 mm and ≤3 mm, respectively).1-3 Because small-diameter melanomas also have the potential to metastasize, particularly if nodular, early diagnosis can be highly rewarding. Deep melanomas with small diameters may have the same potential for metastasis as large-diameter melanomas. In this context, dermoscopy, digital dermoscopic monitoring, and total-body photography are useful in clinical practice. However, these techniques are of limited utility for small, dermoscopic feature–poor melanomas. Conversely, less than 10% of changing lesions, which are spotted via digital dermoscopic surveillance, turn out to be melanomas; therefore, simply removing all changing lesions may result in many unnecessary excisions of benign lesions.4
In vivo reflectance confocal microscopy (RCM) is an advanced technique that allows recognition of the architectural and cellular details of pigmented lesions. Reflectance confocal microscopy has the potential to reduce the rate of unnecessary excisions and to diminish the risk for missing a melanoma.5-7 In meta-analyses, RCM sensitivity was reported as 90% to 93% and specificity was reported as 78% to 82% in detecting melanoma.8,9
We describe a case that highlights the potential role of RCM in the diagnosis of small-diameter melanomas.
Case Report
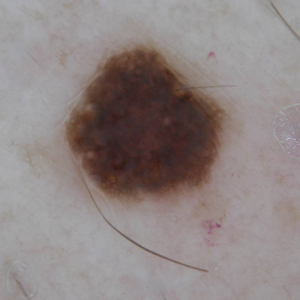
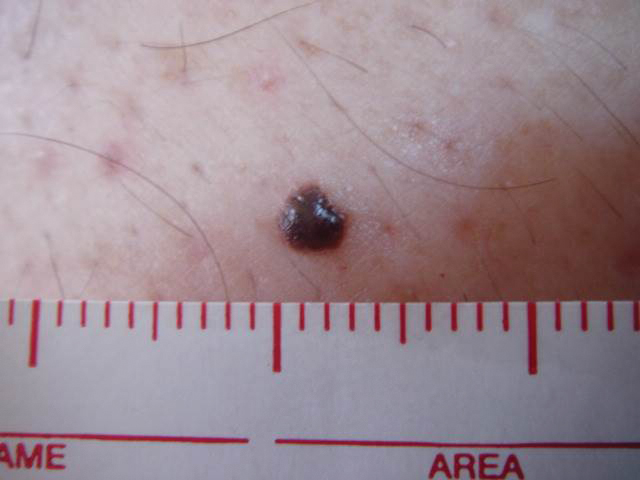
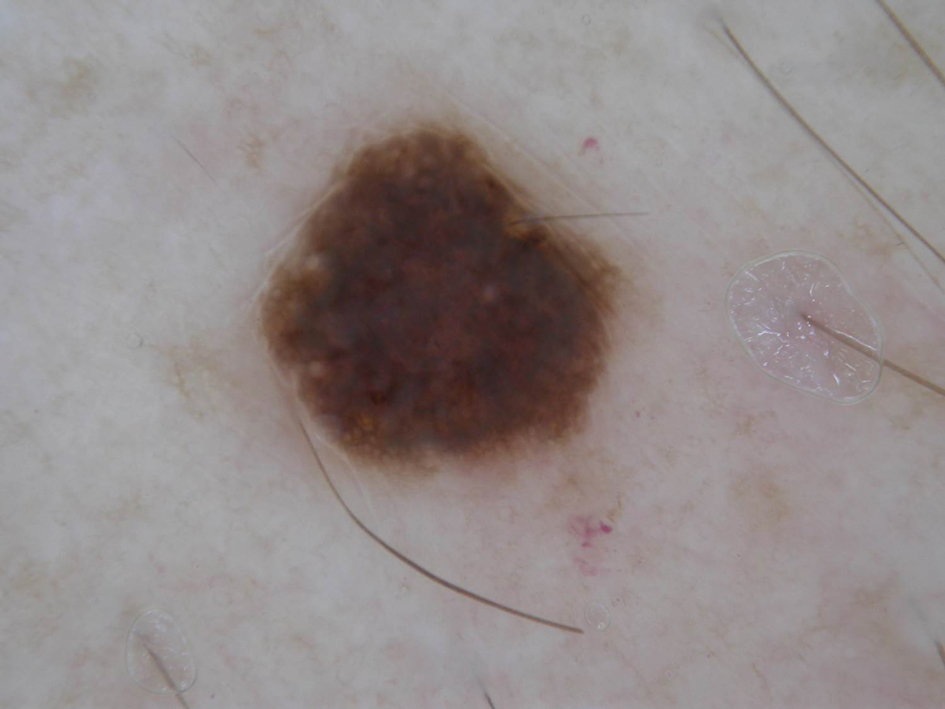
A 57-year-old man with Fitzpatrick skin type III presented to the dermato-oncology unit for evaluation of multiple nevi. He was otherwise healthy and denied a history of skin cancer. Total-body skin examination with dermoscopy was performed, and several mildly atypical lesions were identified. We decided to perform digital dermoscopic monitoring. The patient’s 6-month monitoring appointment had been scheduled, but he did not arrive for the follow-up visit until 10 months after the initial examination. A lesion on the left arm, which initially was 1.5 mm in diameter, had enlarged. It was now a dark brown–gray papule with a 2.5-mm diameter (Figure 1). Dermoscopy revealed grayish globules/dots at the center of the lesion, reticular gray-blue areas, and few milialike cysts; at the periphery, a narrow rim of brownish delicate pigment network also was seen (Figure 2). The clinical and dermoscopic differential diagnosis was either an atypical nevus or an early melanoma. For a more precise diagnosis before excision, the lesion was evaluated with RCM, which takes 10 to 15 minutes to perform.
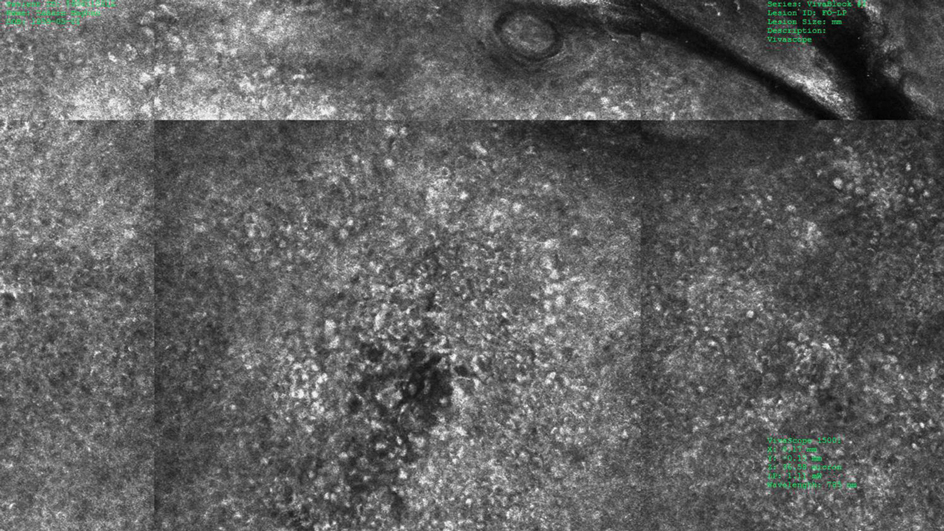
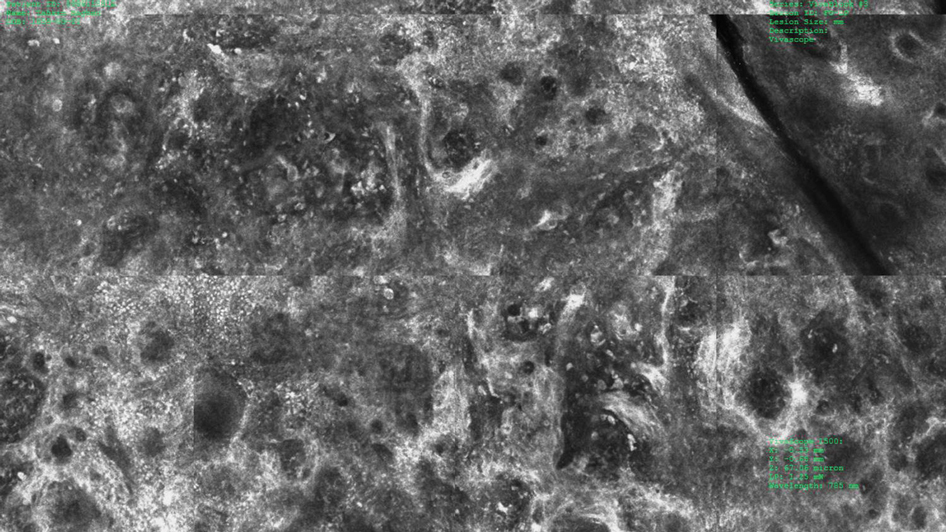
Under RCM at the epidermis level, there was a cobblestone pattern that showed a focus with mild disarrangement and few small, roundish, nucleated cells (Figure 3). A mosaic image, akin to low-magnification microscopy that enables overview of the entire lesion, at the level of the dermoepidermal junction (DEJ) showed an overall irregular meshwork pattern. Higher-magnification optical sections showed marked and diffuse (extending >10% of lesion area) architectural disorder with confluent junctional nests that were irregular to bizarre in shape and uneven in size and spacing as well as edged and nonedged papillae. At the superficial dermal level, atypical bright nucleated cells (>5 cells/mm2) were observed (Figure 4). Bright dots and/or plump bright cells within papillae also were observed. These RCM findings were highly suggestive for melanoma.
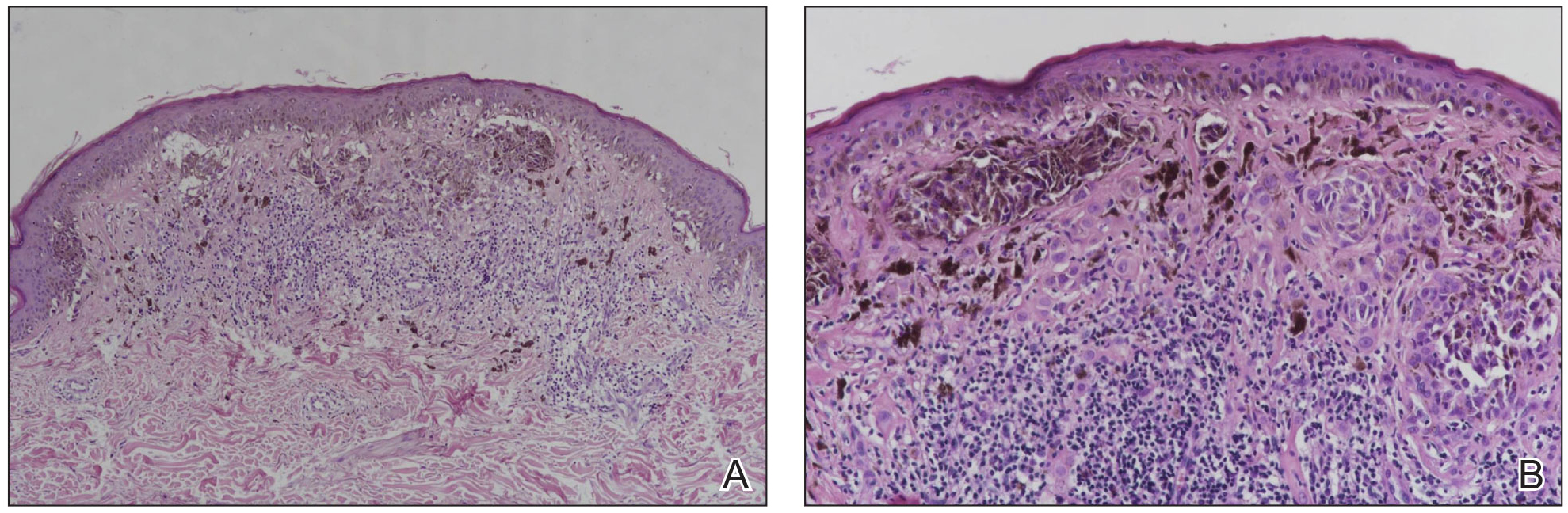
Histopathology showed an asymmetric, junctional, lentiginous, and nested proliferation of atypical epithelioid melanocytes, with few melanocytes in a pagetoid spread. There were small nests of atypical epithelioid melanocytes at the superficial dermis extending to a depth of 0.3 mm. The atypical epithelioid melanocytes displayed angulated hyperchromatic nuclei with conspicuous nucleoli and dusty brown cytoplasm. There was notable inflammation and pigment incontinence at the dermis. There was no evidence of ulceration or mitosis at the dermal component. The diagnosis of a pT1a malignant melanoma was reported (Figure 5).
Comment
A small but enlarging dark gray papule with reticular gray-blue areas under dermoscopy in a 57-year-old man is obviously suspicious for melanoma. In daily practice, this type of small-diameter melanoma is difficult to diagnose with high confidence. We balance our aim to diagnose melanomas early with the need to reduce unnecessary excisions. Reflectance confocal microscopy may allow the clinician to arrive at the correct diagnosis and management decision with confidence before excision of the lesion.
The distinction of a small-diameter melanoma from a nevus via RCM relies on evaluation of the architectural and cellular features. Findings on RCM in small-diameter melanomas have been scarcely reported in the literature; Pupelli et al10 evaluated small melanomas with a diameter of 2 to 5 mm. Among these small-diameter melanomas, the RCM features suggestive for melanomas were the presence of cytologic atypia with cellular pleomorphism, architectural disorder with irregular nests, at least 5 pagetoid cells/mm2, dendrites or tangled lines (ie, short fine lines with no visible nucleus interlacing with the adjacent keratinocytes) within the epidermis, and atypical roundish cells at the DEJ.10
The distinction between an atypical nevus and a small-diameter melanoma using RCM occasionally may be challenging.11 Pellacani et al12 reported an algorithm to distinguish melanoma from atypical nevi. According to this algorithm, when at least 1 of the architectural atypia features (irregular junctional nests, short interconnections between junctional nests, and nonhomogeneous cellularity within junctional nests) and at least 1 of the cytologic atypia features (round pagetoid cells or atypical cells at the DEJ) are observed simultaneously, the lesion is diagnosed as a dysplastic nevus or a melanoma in the first step. In the second step, the RCM diagnosis of melanoma requires at least 1 of 3 parameters: roundish pagetoid cells encompassing at least 50% of the lesional area at the spinous layer, atypical cells involving at least 50% of the lesional area at the DEJ level, and nonedged papillae involving at least 10% of the lesional area.12 Accordingly, our case corresponded with these RCM criteria for a melanoma, given that there were irregular junctional nests, atypical cells at the DEJ, and nonedged papillae involving at least 10% of the lesion.
The current limitations of RCM are the high cost of the device (approximately $58,125–$139,400 for different models), the amount of time needed to train staff in RCM units (seminars, congresses, and special courses organized by the International Confocal Working Group), and the amount of time needed for evaluation of individual lesions (15–20 minutes). However, RCM can be valuable in the clinical diagnosis of difficult lesions, as seen in our case.
Conclusion
Our case highlights the benefit of RCM in allowing the confident diagnosis and correct management of a small-diameter melanoma that turned out to be a melanoma with 0.3-mm Breslow thickness. Even so, histopathologic evaluation remains the gold standard for the diagnosis of melanoma.
- Bergman R, Katz I, Lichtig C, et al. Malignant melanomas with histologic diameters less than 6 mm. J Am Acad Dermatol. 1992;26:462-466.
- Bono A, Tolomio E, Trincone S, et al. Micro-melanoma detection: a clinical study on 206 consecutive cases of pigmented skin lesions with a diameter < or = 3 mm. Br J Dermatol. 2006;155:570-573.
- Bono A, Bartoli C, Baldi M, et al. Micro-melanoma detection. a clinical study on 22 cases of melanoma with a diameter equal to or less than 3 mm. Tumori. 2004;90:128-131.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014;171:1044-1051.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Ferrari B, Pupelli G, Farnetani F, et al. Dermoscopic difficult lesions: an objective evaluation of reflectance confocal microscopy impact for accurate diagnosis. J Eur Acad Dermatol Venereol. 2015;29:1135-1140.
- Dinnes J, Deeks JJ, Saleh D, et al. Reflectance confocal microscopy for diagnosing cutaneous melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD013190.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Pupelli G, Longo C, Veneziano L, et al. Small-diameter melanocytic lesions: morphological analysis by means of in vivo confocal microscopy. Br J Dermatol. 2013;168:1027-1033.
- Carrera C, Marghoob AA. Discriminating nevi from melanomas: clues and pitfalls. Dermatol Clin. 2016;34:395-409.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
Melanomas have been designated as small melanomas or micromelanomas according to their long-axis diameter (<6 mm and ≤3 mm, respectively).1-3 Because small-diameter melanomas also have the potential to metastasize, particularly if nodular, early diagnosis can be highly rewarding. Deep melanomas with small diameters may have the same potential for metastasis as large-diameter melanomas. In this context, dermoscopy, digital dermoscopic monitoring, and total-body photography are useful in clinical practice. However, these techniques are of limited utility for small, dermoscopic feature–poor melanomas. Conversely, less than 10% of changing lesions, which are spotted via digital dermoscopic surveillance, turn out to be melanomas; therefore, simply removing all changing lesions may result in many unnecessary excisions of benign lesions.4
In vivo reflectance confocal microscopy (RCM) is an advanced technique that allows recognition of the architectural and cellular details of pigmented lesions. Reflectance confocal microscopy has the potential to reduce the rate of unnecessary excisions and to diminish the risk for missing a melanoma.5-7 In meta-analyses, RCM sensitivity was reported as 90% to 93% and specificity was reported as 78% to 82% in detecting melanoma.8,9
We describe a case that highlights the potential role of RCM in the diagnosis of small-diameter melanomas.

Case Report
A 57-year-old man with Fitzpatrick skin type III presented to the dermato-oncology unit for evaluation of multiple nevi. He was otherwise healthy and denied a history of skin cancer. Total-body skin examination with dermoscopy was performed, and several mildly atypical lesions were identified. We decided to perform digital dermoscopic monitoring. The patient’s 6-month monitoring appointment had been scheduled, but he did not arrive for the follow-up visit until 10 months after the initial examination. A lesion on the left arm, which initially was 1.5 mm in diameter, had enlarged. It was now a dark brown–gray papule with a 2.5-mm diameter (Figure 1). Dermoscopy revealed grayish globules/dots at the center of the lesion, reticular gray-blue areas, and few milialike cysts; at the periphery, a narrow rim of brownish delicate pigment network also was seen (Figure 2). The clinical and dermoscopic differential diagnosis was either an atypical nevus or an early melanoma. For a more precise diagnosis before excision, the lesion was evaluated with RCM, which takes 10 to 15 minutes to perform.

Under RCM at the epidermis level, there was a cobblestone pattern that showed a focus with mild disarrangement and few small, roundish, nucleated cells (Figure 3). A mosaic image, akin to low-magnification microscopy that enables overview of the entire lesion, at the level of the dermoepidermal junction (DEJ) showed an overall irregular meshwork pattern. Higher-magnification optical sections showed marked and diffuse (extending >10% of lesion area) architectural disorder with confluent junctional nests that were irregular to bizarre in shape and uneven in size and spacing as well as edged and nonedged papillae. At the superficial dermal level, atypical bright nucleated cells (>5 cells/mm2) were observed (Figure 4). Bright dots and/or plump bright cells within papillae also were observed. These RCM findings were highly suggestive for melanoma.

Histopathology showed an asymmetric, junctional, lentiginous, and nested proliferation of atypical epithelioid melanocytes, with few melanocytes in a pagetoid spread. There were small nests of atypical epithelioid melanocytes at the superficial dermis extending to a depth of 0.3 mm. The atypical epithelioid melanocytes displayed angulated hyperchromatic nuclei with conspicuous nucleoli and dusty brown cytoplasm. There was notable inflammation and pigment incontinence at the dermis. There was no evidence of ulceration or mitosis at the dermal component. The diagnosis of a pT1a malignant melanoma was reported (Figure 5).

Comment
A small but enlarging dark gray papule with reticular gray-blue areas under dermoscopy in a 57-year-old man is obviously suspicious for melanoma. In daily practice, this type of small-diameter melanoma is difficult to diagnose with high confidence. We balance our aim to diagnose melanomas early with the need to reduce unnecessary excisions. Reflectance confocal microscopy may allow the clinician to arrive at the correct diagnosis and management decision with confidence before excision of the lesion.

The distinction of a small-diameter melanoma from a nevus via RCM relies on evaluation of the architectural and cellular features. Findings on RCM in small-diameter melanomas have been scarcely reported in the literature; Pupelli et al10 evaluated small melanomas with a diameter of 2 to 5 mm. Among these small-diameter melanomas, the RCM features suggestive for melanomas were the presence of cytologic atypia with cellular pleomorphism, architectural disorder with irregular nests, at least 5 pagetoid cells/mm2, dendrites or tangled lines (ie, short fine lines with no visible nucleus interlacing with the adjacent keratinocytes) within the epidermis, and atypical roundish cells at the DEJ.10
The distinction between an atypical nevus and a small-diameter melanoma using RCM occasionally may be challenging.11 Pellacani et al12 reported an algorithm to distinguish melanoma from atypical nevi. According to this algorithm, when at least 1 of the architectural atypia features (irregular junctional nests, short interconnections between junctional nests, and nonhomogeneous cellularity within junctional nests) and at least 1 of the cytologic atypia features (round pagetoid cells or atypical cells at the DEJ) are observed simultaneously, the lesion is diagnosed as a dysplastic nevus or a melanoma in the first step. In the second step, the RCM diagnosis of melanoma requires at least 1 of 3 parameters: roundish pagetoid cells encompassing at least 50% of the lesional area at the spinous layer, atypical cells involving at least 50% of the lesional area at the DEJ level, and nonedged papillae involving at least 10% of the lesional area.12 Accordingly, our case corresponded with these RCM criteria for a melanoma, given that there were irregular junctional nests, atypical cells at the DEJ, and nonedged papillae involving at least 10% of the lesion.
The current limitations of RCM are the high cost of the device (approximately $58,125–$139,400 for different models), the amount of time needed to train staff in RCM units (seminars, congresses, and special courses organized by the International Confocal Working Group), and the amount of time needed for evaluation of individual lesions (15–20 minutes). However, RCM can be valuable in the clinical diagnosis of difficult lesions, as seen in our case.
Conclusion
Our case highlights the benefit of RCM in allowing the confident diagnosis and correct management of a small-diameter melanoma that turned out to be a melanoma with 0.3-mm Breslow thickness. Even so, histopathologic evaluation remains the gold standard for the diagnosis of melanoma.
Melanomas have been designated as small melanomas or micromelanomas according to their long-axis diameter (<6 mm and ≤3 mm, respectively).1-3 Because small-diameter melanomas also have the potential to metastasize, particularly if nodular, early diagnosis can be highly rewarding. Deep melanomas with small diameters may have the same potential for metastasis as large-diameter melanomas. In this context, dermoscopy, digital dermoscopic monitoring, and total-body photography are useful in clinical practice. However, these techniques are of limited utility for small, dermoscopic feature–poor melanomas. Conversely, less than 10% of changing lesions, which are spotted via digital dermoscopic surveillance, turn out to be melanomas; therefore, simply removing all changing lesions may result in many unnecessary excisions of benign lesions.4
In vivo reflectance confocal microscopy (RCM) is an advanced technique that allows recognition of the architectural and cellular details of pigmented lesions. Reflectance confocal microscopy has the potential to reduce the rate of unnecessary excisions and to diminish the risk for missing a melanoma.5-7 In meta-analyses, RCM sensitivity was reported as 90% to 93% and specificity was reported as 78% to 82% in detecting melanoma.8,9
We describe a case that highlights the potential role of RCM in the diagnosis of small-diameter melanomas.

Case Report
A 57-year-old man with Fitzpatrick skin type III presented to the dermato-oncology unit for evaluation of multiple nevi. He was otherwise healthy and denied a history of skin cancer. Total-body skin examination with dermoscopy was performed, and several mildly atypical lesions were identified. We decided to perform digital dermoscopic monitoring. The patient’s 6-month monitoring appointment had been scheduled, but he did not arrive for the follow-up visit until 10 months after the initial examination. A lesion on the left arm, which initially was 1.5 mm in diameter, had enlarged. It was now a dark brown–gray papule with a 2.5-mm diameter (Figure 1). Dermoscopy revealed grayish globules/dots at the center of the lesion, reticular gray-blue areas, and few milialike cysts; at the periphery, a narrow rim of brownish delicate pigment network also was seen (Figure 2). The clinical and dermoscopic differential diagnosis was either an atypical nevus or an early melanoma. For a more precise diagnosis before excision, the lesion was evaluated with RCM, which takes 10 to 15 minutes to perform.

Under RCM at the epidermis level, there was a cobblestone pattern that showed a focus with mild disarrangement and few small, roundish, nucleated cells (Figure 3). A mosaic image, akin to low-magnification microscopy that enables overview of the entire lesion, at the level of the dermoepidermal junction (DEJ) showed an overall irregular meshwork pattern. Higher-magnification optical sections showed marked and diffuse (extending >10% of lesion area) architectural disorder with confluent junctional nests that were irregular to bizarre in shape and uneven in size and spacing as well as edged and nonedged papillae. At the superficial dermal level, atypical bright nucleated cells (>5 cells/mm2) were observed (Figure 4). Bright dots and/or plump bright cells within papillae also were observed. These RCM findings were highly suggestive for melanoma.

Histopathology showed an asymmetric, junctional, lentiginous, and nested proliferation of atypical epithelioid melanocytes, with few melanocytes in a pagetoid spread. There were small nests of atypical epithelioid melanocytes at the superficial dermis extending to a depth of 0.3 mm. The atypical epithelioid melanocytes displayed angulated hyperchromatic nuclei with conspicuous nucleoli and dusty brown cytoplasm. There was notable inflammation and pigment incontinence at the dermis. There was no evidence of ulceration or mitosis at the dermal component. The diagnosis of a pT1a malignant melanoma was reported (Figure 5).

Comment
A small but enlarging dark gray papule with reticular gray-blue areas under dermoscopy in a 57-year-old man is obviously suspicious for melanoma. In daily practice, this type of small-diameter melanoma is difficult to diagnose with high confidence. We balance our aim to diagnose melanomas early with the need to reduce unnecessary excisions. Reflectance confocal microscopy may allow the clinician to arrive at the correct diagnosis and management decision with confidence before excision of the lesion.

The distinction of a small-diameter melanoma from a nevus via RCM relies on evaluation of the architectural and cellular features. Findings on RCM in small-diameter melanomas have been scarcely reported in the literature; Pupelli et al10 evaluated small melanomas with a diameter of 2 to 5 mm. Among these small-diameter melanomas, the RCM features suggestive for melanomas were the presence of cytologic atypia with cellular pleomorphism, architectural disorder with irregular nests, at least 5 pagetoid cells/mm2, dendrites or tangled lines (ie, short fine lines with no visible nucleus interlacing with the adjacent keratinocytes) within the epidermis, and atypical roundish cells at the DEJ.10
The distinction between an atypical nevus and a small-diameter melanoma using RCM occasionally may be challenging.11 Pellacani et al12 reported an algorithm to distinguish melanoma from atypical nevi. According to this algorithm, when at least 1 of the architectural atypia features (irregular junctional nests, short interconnections between junctional nests, and nonhomogeneous cellularity within junctional nests) and at least 1 of the cytologic atypia features (round pagetoid cells or atypical cells at the DEJ) are observed simultaneously, the lesion is diagnosed as a dysplastic nevus or a melanoma in the first step. In the second step, the RCM diagnosis of melanoma requires at least 1 of 3 parameters: roundish pagetoid cells encompassing at least 50% of the lesional area at the spinous layer, atypical cells involving at least 50% of the lesional area at the DEJ level, and nonedged papillae involving at least 10% of the lesional area.12 Accordingly, our case corresponded with these RCM criteria for a melanoma, given that there were irregular junctional nests, atypical cells at the DEJ, and nonedged papillae involving at least 10% of the lesion.
The current limitations of RCM are the high cost of the device (approximately $58,125–$139,400 for different models), the amount of time needed to train staff in RCM units (seminars, congresses, and special courses organized by the International Confocal Working Group), and the amount of time needed for evaluation of individual lesions (15–20 minutes). However, RCM can be valuable in the clinical diagnosis of difficult lesions, as seen in our case.
Conclusion
Our case highlights the benefit of RCM in allowing the confident diagnosis and correct management of a small-diameter melanoma that turned out to be a melanoma with 0.3-mm Breslow thickness. Even so, histopathologic evaluation remains the gold standard for the diagnosis of melanoma.
- Bergman R, Katz I, Lichtig C, et al. Malignant melanomas with histologic diameters less than 6 mm. J Am Acad Dermatol. 1992;26:462-466.
- Bono A, Tolomio E, Trincone S, et al. Micro-melanoma detection: a clinical study on 206 consecutive cases of pigmented skin lesions with a diameter < or = 3 mm. Br J Dermatol. 2006;155:570-573.
- Bono A, Bartoli C, Baldi M, et al. Micro-melanoma detection. a clinical study on 22 cases of melanoma with a diameter equal to or less than 3 mm. Tumori. 2004;90:128-131.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014;171:1044-1051.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Ferrari B, Pupelli G, Farnetani F, et al. Dermoscopic difficult lesions: an objective evaluation of reflectance confocal microscopy impact for accurate diagnosis. J Eur Acad Dermatol Venereol. 2015;29:1135-1140.
- Dinnes J, Deeks JJ, Saleh D, et al. Reflectance confocal microscopy for diagnosing cutaneous melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD013190.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Pupelli G, Longo C, Veneziano L, et al. Small-diameter melanocytic lesions: morphological analysis by means of in vivo confocal microscopy. Br J Dermatol. 2013;168:1027-1033.
- Carrera C, Marghoob AA. Discriminating nevi from melanomas: clues and pitfalls. Dermatol Clin. 2016;34:395-409.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
- Bergman R, Katz I, Lichtig C, et al. Malignant melanomas with histologic diameters less than 6 mm. J Am Acad Dermatol. 1992;26:462-466.
- Bono A, Tolomio E, Trincone S, et al. Micro-melanoma detection: a clinical study on 206 consecutive cases of pigmented skin lesions with a diameter < or = 3 mm. Br J Dermatol. 2006;155:570-573.
- Bono A, Bartoli C, Baldi M, et al. Micro-melanoma detection. a clinical study on 22 cases of melanoma with a diameter equal to or less than 3 mm. Tumori. 2004;90:128-131.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014;171:1044-1051.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Ferrari B, Pupelli G, Farnetani F, et al. Dermoscopic difficult lesions: an objective evaluation of reflectance confocal microscopy impact for accurate diagnosis. J Eur Acad Dermatol Venereol. 2015;29:1135-1140.
- Dinnes J, Deeks JJ, Saleh D, et al. Reflectance confocal microscopy for diagnosing cutaneous melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD013190.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Pupelli G, Longo C, Veneziano L, et al. Small-diameter melanocytic lesions: morphological analysis by means of in vivo confocal microscopy. Br J Dermatol. 2013;168:1027-1033.
- Carrera C, Marghoob AA. Discriminating nevi from melanomas: clues and pitfalls. Dermatol Clin. 2016;34:395-409.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
Practice Points
- Melanomas with a long-axis diameter smaller than 6 mm are considered small melanomas, and those with diameters of 3 mm and smaller are considered micromelanomas; both are difficult to detect.
- Digital dermoscopic monitoring and reflectance confocal microscopy are important tools in detecting small melanomas.