User login
Stroke: Secondary prevention of ischemic events
Patients who suffer a stroke rarely have just one vascular risk factor. Therefore, the approach to secondary stroke prevention must be multifactorial. In fact, it has been estimated that 80% of recurrent strokes could be prevented through the application of a comprehensive, multifactorial approach that includes lifestyle modification and optimal medical management.1 Such an achievement would save millions of people from disability and functional decline, as well as millions of dollars in related medical costs.
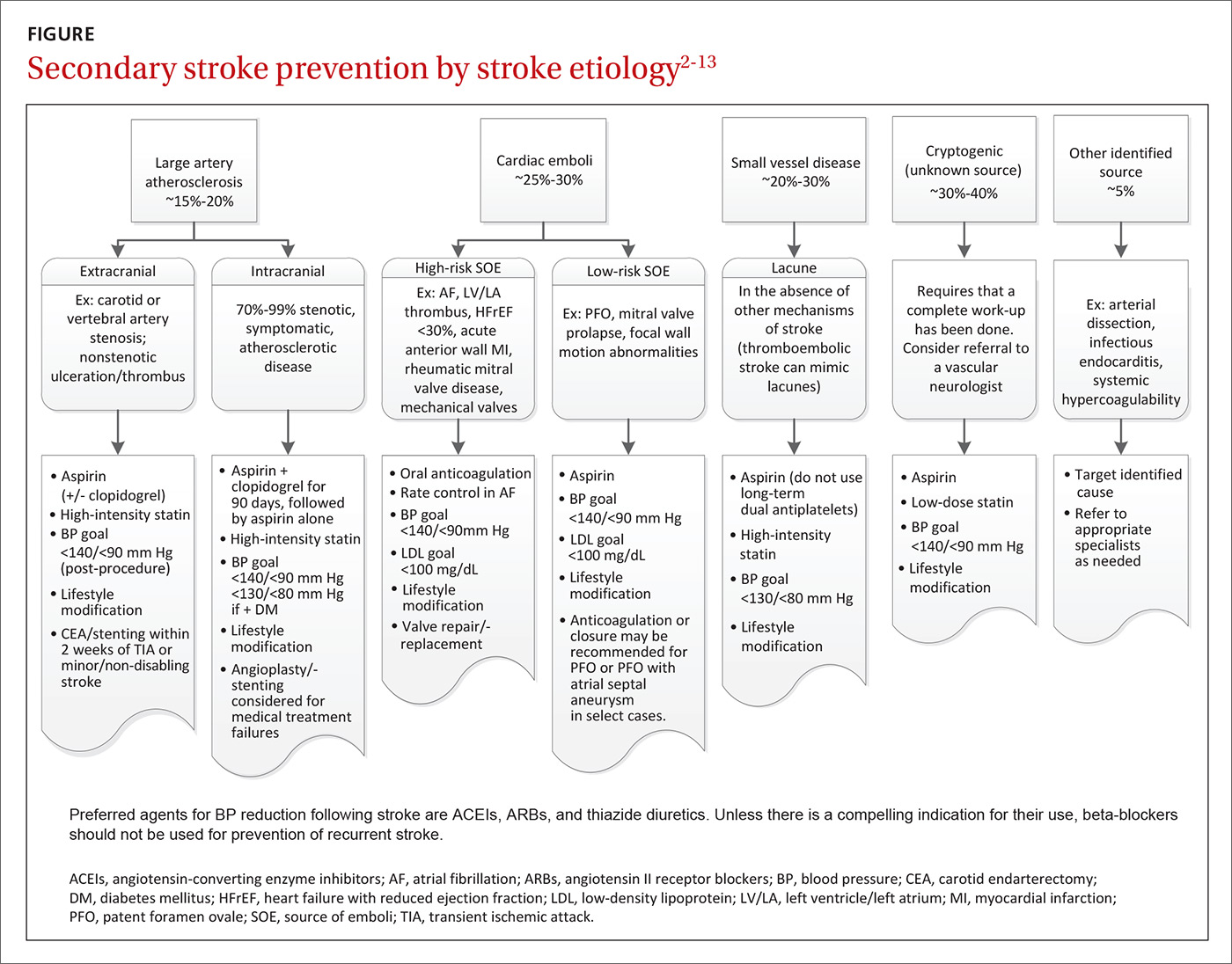
The initial approach to patients with stroke is focused on stabilization and a rapid work-up to identify the most likely etiology. Common causes of stroke include large artery atherosclerosis, cardiac emboli, and small vessel disease; less common causes include dissection, aortic emboli, and non-atherosclerotic vascular disease. If a complete diagnostic work-up is unrevealing, the stroke is said to be cryptogenic. Determining the correct etiology of a stroke is paramount to preventing secondary stroke (FIGURE2-13).
Effective secondary prevention strategies designed to prevent a stroke or transient ischemic attack (TIA) in a patient with a known history of either event include lifestyle modifications, medications, and when appropriate, mechanical interventions. As a primary care physician (PCP), you are uniquely positioned to spearhead the prevention of secondary strokes: Not only are you at the forefront of prevention and the use of techniques such as motivational interviewing, but you also have longstanding relationships with many of your patients. In fact, the success of many interventions is improved by the informed, enduring, and trusting nature of relationships between patients and their PCPs.
In the first part of this 2-part series, we focused on subacute stroke management and outlined the recommended work-up for subacute stroke/TIA (see “Stroke: A road map to subacute management,” 2017;66:366-374). In this part, we focus on secondary prevention. The more common modifiable conditions encountered in primary care are discussed here, while many of the more rare etiologies (hypercoagulable states, sickle cell disease, and vasculitis) are outside the scope of this article.
Lifestyle interventions: Target tobacco use, obesity, alcohol intake
Lifestyle modifications can have a positive impact on many of America’s most prevalent diseases, and stroke is no exception.14 Many of the disease states identified as risk factors for stroke (type 2 diabetes, hypertension, dyslipidemia) are exacerbated by tobacco use, obesity, and excessive alcohol intake.
Does your patient smoke? Up to 25% of all strokes are directly attributable to cigarette smoking.15 Smoking raises an individual’s risk for stroke in a dose-dependent fashion.15,16 One study demonstrated that, compared to never-smokers, women ages 15 to 49 years who smoked a half-pack per day had an odds ratio for ischemic stroke of 2.2; those who smoked 2 packs per day had an odds ratio of 9.1.17 After cessation, stroke risk generally returns to baseline within 5 years.16 Thus, smoking cessation is among the most significant steps a patient can take to reduce the risk of both primary and secondary stroke.
Is your patient overweight? While obesity in and of itself is a risk factor for stroke, a focus on nutrition and physical activity as mechanisms for weight loss is far superior to focusing on either element alone. Physical activity—consisting of at least 40 minutes of moderate intensity aerobic exercise 3 to 4 times per week—and a diet that emphasizes fruits and vegetables, whole grains, and healthy fats, have both independently demonstrated benefits in secondary stroke prevention and are important parts of American College of Cardiology (ACC)/American Heart Association (AHA) guidelines.2,3
The Mediterranean Diet, which emphasizes consumption of fruits and vegetables, legumes, tree nuts, olive oil, and lean protein, has long been associated with cardiovascular benefit.18 One prospective, randomized, single-blinded trial involving approximately 600 patients that looked at secondary prevention of coronary heart disease found that following the diet significantly reduced mortality compared with a usual prudent post-infarct diet (number needed to treat [NNT]=30 over 4 years).19
Is alcohol consumption an issue? Chronic heavy alcohol intake contributes to the development of hemorrhagic and ischemic stroke through multiple mechanisms, including alcohol-induced hypertension, alcoholic cardiomyopathy, and atrial fibrillation (AF). Light or moderate alcohol consumption has a paradoxical mild protective effect on ischemic stroke, thought to possibly be mediated by an increase in high-density lipoprotein (HDL) level and mild antiplatelet effect.3
AHA/American Stroke Association (ASA) guidelines indicate that no more than one standard drink per day for women and 2 drinks per day for men is reasonable.3 Counsel patients who drink in excess of this about the benefits of decreasing alcohol intake or abstaining altogether.
Choosing medications to manage BP, cholesterol, and clotting
Optimize blood pressure control. Blood pressure (BP) plays a critical role in both the management and prevention of stroke and is considered to be the most important modifiable risk factor in both primary and secondary stroke prevention.20 In the first 24 to 48 hours following a cerebral ischemic event that is not eligible for thrombolysis, permissive hypertension (treating BP only if it exceeds 220/120 mm Hg unless there is a concurrent medical illness that requires you do so) is appropriate, as hypotension or rapid fluctuations in BP can be harmful.21
This flexibility does not continue into the subacute phase of management (at a minimum, after the initial 48 hours) or into secondary prevention. Initiation and titration of oral agents to gradually achieve a BP <140/90 mm Hg or a reduction of 10/5 mm Hg for patients already within optimal range are the most widely recognized goals.3,20 Patients with stroke secondary to small vessel disease may benefit from an even lower goal of <130/<80 mm Hg.11 Encourage patients to monitor their BP at home for added accuracy and consistency.22
Pharmacologic BP management is appropriate for patients who are consistently above optimal range despite attempting recommended lifestyle modifications. The data are relatively consistent with respect to the effects of different drug classes after a stroke: beta-blockers have no effect on any outcome; thiazide diuretics significantly reduce stroke and total vascular events; angiotensin-converting enzyme (ACE) inhibitors significantly reduce myocardial infarction (MI); and the combination of an ACE inhibitor and thiazide diuretic reduces stroke, MI, and combined vascular events.4
This has led many stroke specialists to recommend the combination of an ACE inhibitor or angiotensin II receptor blocker (ARB) and a thiazide diuretic as a first-line approach to secondary stroke prevention rather than a beta-blocker (assuming there is no additional indication for a beta-blocker). Similarly, there is ample evidence to show that the magnitude of BP reduction is proportional to the reduction in recurrent vascular events.3
Make use of statin therapy—regardless of LDL. The SPARCL (Stroke Prevention by Aggressive Reduction in Cholesterol Levels) trial5 explored the potential role of statin medication for secondary stroke prevention. Researchers randomly assigned almost 5000 participants who’d had a stroke or TIA one to 6 months before study entry (but had no known history of coronary artery disease) to placebo or a high-intensity statin (80 mg/d atorvastatin). The statin group demonstrated a 4.9-year absolute risk reduction in fatal or nonfatal recurrent stroke of 1.9% (NNT=53).
Given these findings and those from other studies, the AHA and ASA recommend treating patients with stroke or TIA presumed to be of atherosclerotic origin with high-intensity statin therapy, regardless of low-density lipoprotein (LDL) level.3 Of note, statins are not indicated for the secondary prevention of hemorrhagic stroke.
Select antiplatelet therapy based on ischemic stroke subtype. Investigators are still trying to determine the optimal antiplatelet for secondary stroke prevention; it is likely that the ideal choice depends largely on the etiology of the stroke. Trials that did not select patients based on subtype of ischemic stroke have not shown a long-term benefit from dual antiplatelet therapy (clopidogrel and aspirin),23,24 and one double-blind, multicenter trial involving more than 3000 patients with recent stroke secondary to small vessel disease demonstrated harm from such therapy in terms of a significantly increased risk of bleeding and death.6
However, a 2011 study compared aggressive medical management (aspirin 325 mg/d plus clopidogrel 75 mg/d for 90 days) alone to aggressive medical management plus percutaneous transluminal angioplasty and stenting (PTAS). The study involved almost 500 patients who'd had a recent TIA or stroke attributed to intracranial atherosclerotic stenosis. The authors found that the 30-day rate of stroke or death was 14.7% in the PTAS group vs 5.8% in the medical management group.25
Similarly, a randomized double-blind, placebo-controlled trial published in 2013 involving over 5000 patients in China found that short-term use of dual antiplatelets (clopidogrel and aspirin for the first 21 days after an ischemic event, followed by aspirin monotherapy for 90 days) had an absolute risk reduction of 3.5% without increasing the risk of major bleeding in patients with high-risk TIA or minor stroke.26
All stroke patients who do not have an indication for oral anticoagulation should be placed on long-term daily aspirin (75-325 mg); research has shown that lower doses are as effective as higher doses but with a lower risk of adverse gastrointestinal effects, including bleeding.3,20 Aspirin 81 mg/d is a common effective dose.
For patients who cannot tolerate aspirin due to allergy, clopidogrel 75 mg/d is a reasonable alternative. Long-term studies of aspirin vs clopidogrel7 and clopidogrel vs extended-release dipyridamole8 showed no difference in secondary stroke prevention. The International Stroke Trial27 and Chinese Acute Stroke Trial28 both indicate that aspirin should be started as soon as possible after the onset of an acute stroke.
This special population should probably get antiplatelets, too. One recent study explored the use of an antiplatelet vs anticoagulation therapy for stroke patients with carotid artery dissection. The CADISS (Cervical Artery Dissection in Stroke Study) trial29 randomized 250 patients with extracranial carotid and vertebral artery dissection with onset of symptoms within the previous 7 days to either antiplatelet or anticoagulation therapy and found no difference in the primary outcomes of recurrent stroke or death. The study also demonstrated a low risk of recurrent stroke in this population, which was 2% at 3-month follow-up.
Most patients with cervical artery dissection, therefore, are now treated with antiplatelet therapy. That said, situations may still arise in which anticoagulation can be considered, and consultation with a neurologist for guidance on choice of therapy is recommended.
Is an anticoagulant in order? Which agent, when
The most common cause of cardioembolic stroke is AF, which accounts for at least 15% of ischemic strokes, a number that rises in those over the age of 80.20,30,31 A meta-analysis of more than 28,000 patients with non-valvular AF demonstrated that warfarin reduced the risk of stroke by 64%.32
The rate of intracerebral hemorrhages during oral anticoagulation ranges from 0.3% to 0.6% per year.33 The risk of bleeding complications can be mitigated by keeping international normalized ratios ≤3.0, maintaining good BP control, and avoiding concurrent use of antiplatelets in the absence of a clear indication for them.33
Several risk assessment scores, such as the HAS-BLED,34 can help with estimating the risk of hemorrhagic complications, although these scores have their limitations.35,36 Even in an older population (mean age 83 years) with a high risk for falls, warfarin provided a net benefit in a composite endpoint of out-of-hospital death or hospitalization for stroke, MI, or hemorrhage in a retrospective study of over 1200 Medicare beneficiaries.37
AF is not the only cause of cardioembolic stroke to consider. Additional high-risk factors warranting anticoagulation include rheumatic mitral valve disease, the presence of mechanical aortic or mitral valves, known mural thrombus, and acute anterior ST segment elevation myocardial infarctions (STEMIs) with resulting anterior apical dyskinesis/akinesis and concurrent ischemic stroke/TIA.3 (The specific management of each of these situations is beyond the scope of this paper.)
The choice of anticoagulation agent is based on multiple factors, including cost, risk of non-reversible bleeding, drug interactions, renal function, and patient preference. Approved options currently include warfarin/vitamin K antagonist therapy, apixaban, rivaroxaban, dabigatran and edoxaban.3 Choice of therapy will continue to evolve as reversal agents, such as idarucizumab, are developed. Idarucizumab, a reversal agent for dabigatran, received approval from the US Food and Drug Administration in October 2015.38
When to start anticoagulation. There are limited data regarding the optimal timing of initiation of anticoagulation following a stroke; however, a recent multicenter prospective study supported the common practice of initiating anticoagulation therapy within 4 to 14 days of the event.39 Individual patient factors must be taken into consideration, including the size of the stroke (the larger the stroke, the higher the risk for hemorrhagic transformation), BP control, any additional risk factors for bleeding, and the estimated risk of early recurrent stroke.
Bridging patients onto anticoagulation with unfractionated or low-molecular-weight heparin in the setting of acute stroke is not recommended.40 Results from randomized controlled trials involving unfractionated heparin, heparinoids, and low-molecular-weight heparin have not reported any benefit to these agents over aspirin at preventing early stroke recurrence.27,41,42
For immobile or hospitalized patients. Subcutaneous heparin for the prevention of deep vein thrombosis (DVT) during immobility and hospitalization is recommended.43 Patients who cannot tolerate anticoagulation should be maintained on low-dose antiplatelet therapy. Experts do not recommend dual treatment with aspirin and anticoagulation in most cases. However, recent coronary artery stent placement does require temporary dual treatment, with duration dependent on the type of stent placed.
A role for glycemic control? Still to be determined
The specific role of diabetic management in secondary stroke prevention remains unclear. The 2008 ACCORD trial,44 a randomized study involving over 10,000 patients with a median glycated hemoglobin level of 8.1%, investigated intensive hyperglycemic control (targeting a glycated hemoglobin level <6.0% vs <7.9%) as a means of decreasing cardiovascular risk. However, the trial ended 17 months early because of an increase in all-cause mortality in the intensive treatment arm compared with the standard management group. The same trial was also unable to demonstrate a decrease in stroke risk with a decrease in A1c.44
More recently, the IRIS (Insulin Resistance Intervention after Stroke) trial45 (2016) found a 2.8% absolute risk reduction in stroke or MI among participants who had a stroke or TIA in the previous 6 months who were treated with pioglitazone vs placebo over 4.8 years (NNT=36). Participants were required to have insulin resistance, but were excluded if they had diabetes. The authors did, however, report a notable increase in the risk of bone fractures requiring surgery or hospitalization in the pioglitazone arm (5.1% vs 3.2%; number needed to harm [NNH]=53).
The impact this single study should have on standard secondary prevention is not yet clear. The authors concluded, “It seems reasonable to consider individual treatment preference and risk of drug-related adverse events in addition to potential benefits when making patient-specific decisions regarding therapy.”45
Determining whether mechanical interventions are needed
Almost all conditions leading to stroke warrant active medical management, but a few benefit from procedural intervention, as well.
Extracranial carotid atherosclerosis. Carotid endarterectomy or carotid artery stenting is recommended as secondary prevention for patients with a history of stroke or TIA who have ipsilateral high-grade extracranial carotid stenosis of 70% to 99% and, in some cases, 50% to 69%.3,9,20 In patients with mild non-disabling stroke, the optimal timing for these procedures is within 2 weeks of the ischemic event. A delay of 6 weeks is generally preferred for moderate or larger strokes to allow for some healing of the injured brain.
The choice of procedure is based on risk profile, with the most important factor being age. For patients >70 years, endarterectomy is preferred because stenting is associated with an increased risk of stroke.3,9,10 Experts do not recommend either procedure for patients who have had a severe disabling stroke. Generally speaking, these procedures have higher rates of success when they are performed in centers that perform a higher number of these procedures.10
Vertebrobasilar atherosclerosis. Due to generally good compensatory blood flow of the contralateral vertebral artery in the setting of vertebral artery stenosis, and an unacceptably high complication rate of angioplasty and stenting in the basilar artery, medical management is typically the first-line approach. If a patient has recurrent symptoms in the setting of optimal medical management and a focal lesion that is amenable to an endovascular intervention (most commonly a vertebral artery origin high-grade stenosis), angioplasty and stenting may be considered.10
Intracranial atherosclerosis. Similarly, medical management is the preferred strategy for intracranial atherosclerosis. Angioplasty and/or stenting are reserved for complex cases or recurrence despite adherence to secondary stroke prevention measures. Ideally, these patients should be managed with long-term aspirin 81 mg/d, adjunctive clopidogrel 75 mg/d for 90 days post stroke, a high-intensity statin, BP optimization, and any relevant lifestyle interventions.13
Patent foramen ovale. Research to date has not shown that closure of a patent foramen ovale (PFO) is superior to medical therapy for secondary stroke prevention in patients <60 years with cryptogenic stroke.12,46,47 The decision to anticoagulate these patients should be based on the presence or absence of a DVT and not on a PFO alone. In patients with an identified DVT and a contraindication to oral anticoagulation, inferior vena cava filter placement should be considered. For patients with ongoing prothrombotic risk thought to increase the chances of future paradoxical embolism, closure of the PFO may be considered.
CORRESPONDENCE
Stephen A. Martin, MD, EdM, Barre Family Health Center, 151 Worcester Road, Barre, MA 01005; [email protected].
1. Hackam DG, Spence JD. Combining multiple approaches for the secondary prevention of vascular events after stroke: a quantitative modeling study. Stroke. 2007;38:1881-1885.
2. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S76-S99.
3. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236.
4. Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke. 2003;34:2741-2748.
5. Amarenco P, Bogousslavsky J, Callahan A, et al, for the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559.
6. Benavente OR, Hart RG, McClure LA, et al, for the SPS3 Investigators. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
7. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329-1339.
8. Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238-1251.
9. Diethrich EB, N’diaye M, Reid DB. The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST): implications for clinical practice. In: Henry M, Diethrich EB, Polydorou A, eds. The Carotid and Supra-Aortic Trunks: Diagnosis, Angioplasty and Stenting. 2nd ed. Oxford, UK: Wiley-Blackwell; 2011.
10. Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. Circulation. 2011;124:489-532.
11. SPS3 Study Group. Blood pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507-515.
12. Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092-1100.
13. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003.
14. Romero JR, Morris J, Pikula A. Stroke prevention: modifying risk factors. Ther Adv Cardiovasc Dis. 2008;2:287-303.
15. Hankey GJ. Smoking and risk of stroke. J Cardiovasc Risk. 1999;6:207-211.
16. Shah RS, Cole JW. Smoking and stroke: the more you smoke the more you stroke. Expert Rev Cardiovasc Ther. 2010;8:917-932.
17. Bhat VM, Cole JW, Sorkin JD, et al. Dose-response relationship between cigarette smoking and risk of ischemic stroke in young women. Stroke. 2008;39:2439-2443.
18. Lakkur S, Judd SE. Diet and stroke: recent evidence supporting a Mediterranean-style diet and food in the primary prevention of stroke. Stroke. 2015;46:2007-2011.
19. de Lorgeril M, Salen P, Martin JL, et al. Mediterranean dietary pattern in a randomized trial: prolonged survival and possible reduced cancer rate. Arch Intern Med. 1998;158:1181-1187.
20. Davis SM, Donnan GA. Clinical practice. Secondary prevention after ischemic stroke or transient ischemic attack. N Engl J Med. 2012;366:1914-1922.
21. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
22. Magid DJ, Green BB. Home blood pressure monitoring: take it to the bank. JAMA. 2013;310:40-41.
23. Diener H-C, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2004;364:331-337.
24. Bhatt DL, Fox KAA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706-1717.
25. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003.
26. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
27. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet. 1997;349:1569-1581.
28. CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet. 1997;349:1641-1649.
29. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14:361-367.
30. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) Study Group. Lancet. 1993;342:1255-1262.
31. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983-988.
32. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Int Med. 2007;146:857-867.
33. Hart RG, Tonarelli SB, Pearce LA. Avoiding central nervous system bleeding during antithrombotic therapy. Recent data and ideas. Stroke. 2005;36:1588-1593.
34. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093-1100.
35. Quinn GR, Singer DE, Chang Y, et al. How well do stroke risk scores predict hemorrhage in patients with atrial fibrillation? Am J Cardiol. 2016;118:697-699.
36. Gorman EW, Perkel D, Dennis D, et al. Validation of the HAS-BLED tool in atrial fibrillation patients receiving rivaroxaban. J Atr Fibrillation. 2016;9:1461.
37. Gage BF, Birman-Deych E, Kerzner R, et al. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med. 2005;118:612-617.
38. US Food and Drug Administration. FDA approves Praxbind, the first reversal agent for the anticoagulant Pradaxa. October 16, 2015. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm467300.htm. Accessed May 26, 2017.
39. Paciaroni M, Agnelli G, Falocci N, et al. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: effect of anticoagulation and its timing: the RAF Study. Stroke. 2015;46:2175-2182.
40. Sandercock PA, Counsell C, Kane EJ. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev. 2015;3:CD000024.
41. Bath PM, Lindenstrom E, Boysen G, et al. Tinzaparin in acute ischaemic stroke (TAIST): a randomised aspirin-controlled trial. Lancet. 2001;358:702-710.
42. Berge E, Abdelnoor M, Nakstad PH, et al. Low molecular-weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: a double-blind randomised study. HAEST Study Group. Heparin in Acute Embolic Stroke Trial. Lancet. 2000;355:1205-1210.
43. Sherman DG, Albers GW, Bladin C, et al. The efficacy and safety of enoxaparin versus unfractionated heparin for the prevention of venous thromboembolism after acute ischaemic stroke (PREVAIL Study): an open-label randomised comparison. Lancet. 2007;369:1347-1355.
44. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559.
45. Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321-1331.
46. Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083-1091.
47. Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991-999.
Patients who suffer a stroke rarely have just one vascular risk factor. Therefore, the approach to secondary stroke prevention must be multifactorial. In fact, it has been estimated that 80% of recurrent strokes could be prevented through the application of a comprehensive, multifactorial approach that includes lifestyle modification and optimal medical management.1 Such an achievement would save millions of people from disability and functional decline, as well as millions of dollars in related medical costs.
The initial approach to patients with stroke is focused on stabilization and a rapid work-up to identify the most likely etiology. Common causes of stroke include large artery atherosclerosis, cardiac emboli, and small vessel disease; less common causes include dissection, aortic emboli, and non-atherosclerotic vascular disease. If a complete diagnostic work-up is unrevealing, the stroke is said to be cryptogenic. Determining the correct etiology of a stroke is paramount to preventing secondary stroke (FIGURE2-13).

Effective secondary prevention strategies designed to prevent a stroke or transient ischemic attack (TIA) in a patient with a known history of either event include lifestyle modifications, medications, and when appropriate, mechanical interventions. As a primary care physician (PCP), you are uniquely positioned to spearhead the prevention of secondary strokes: Not only are you at the forefront of prevention and the use of techniques such as motivational interviewing, but you also have longstanding relationships with many of your patients. In fact, the success of many interventions is improved by the informed, enduring, and trusting nature of relationships between patients and their PCPs.
In the first part of this 2-part series, we focused on subacute stroke management and outlined the recommended work-up for subacute stroke/TIA (see “Stroke: A road map to subacute management,” 2017;66:366-374). In this part, we focus on secondary prevention. The more common modifiable conditions encountered in primary care are discussed here, while many of the more rare etiologies (hypercoagulable states, sickle cell disease, and vasculitis) are outside the scope of this article.
Lifestyle interventions: Target tobacco use, obesity, alcohol intake
Lifestyle modifications can have a positive impact on many of America’s most prevalent diseases, and stroke is no exception.14 Many of the disease states identified as risk factors for stroke (type 2 diabetes, hypertension, dyslipidemia) are exacerbated by tobacco use, obesity, and excessive alcohol intake.
Does your patient smoke? Up to 25% of all strokes are directly attributable to cigarette smoking.15 Smoking raises an individual’s risk for stroke in a dose-dependent fashion.15,16 One study demonstrated that, compared to never-smokers, women ages 15 to 49 years who smoked a half-pack per day had an odds ratio for ischemic stroke of 2.2; those who smoked 2 packs per day had an odds ratio of 9.1.17 After cessation, stroke risk generally returns to baseline within 5 years.16 Thus, smoking cessation is among the most significant steps a patient can take to reduce the risk of both primary and secondary stroke.
Is your patient overweight? While obesity in and of itself is a risk factor for stroke, a focus on nutrition and physical activity as mechanisms for weight loss is far superior to focusing on either element alone. Physical activity—consisting of at least 40 minutes of moderate intensity aerobic exercise 3 to 4 times per week—and a diet that emphasizes fruits and vegetables, whole grains, and healthy fats, have both independently demonstrated benefits in secondary stroke prevention and are important parts of American College of Cardiology (ACC)/American Heart Association (AHA) guidelines.2,3
The Mediterranean Diet, which emphasizes consumption of fruits and vegetables, legumes, tree nuts, olive oil, and lean protein, has long been associated with cardiovascular benefit.18 One prospective, randomized, single-blinded trial involving approximately 600 patients that looked at secondary prevention of coronary heart disease found that following the diet significantly reduced mortality compared with a usual prudent post-infarct diet (number needed to treat [NNT]=30 over 4 years).19
Is alcohol consumption an issue? Chronic heavy alcohol intake contributes to the development of hemorrhagic and ischemic stroke through multiple mechanisms, including alcohol-induced hypertension, alcoholic cardiomyopathy, and atrial fibrillation (AF). Light or moderate alcohol consumption has a paradoxical mild protective effect on ischemic stroke, thought to possibly be mediated by an increase in high-density lipoprotein (HDL) level and mild antiplatelet effect.3
AHA/American Stroke Association (ASA) guidelines indicate that no more than one standard drink per day for women and 2 drinks per day for men is reasonable.3 Counsel patients who drink in excess of this about the benefits of decreasing alcohol intake or abstaining altogether.
Choosing medications to manage BP, cholesterol, and clotting
Optimize blood pressure control. Blood pressure (BP) plays a critical role in both the management and prevention of stroke and is considered to be the most important modifiable risk factor in both primary and secondary stroke prevention.20 In the first 24 to 48 hours following a cerebral ischemic event that is not eligible for thrombolysis, permissive hypertension (treating BP only if it exceeds 220/120 mm Hg unless there is a concurrent medical illness that requires you do so) is appropriate, as hypotension or rapid fluctuations in BP can be harmful.21
This flexibility does not continue into the subacute phase of management (at a minimum, after the initial 48 hours) or into secondary prevention. Initiation and titration of oral agents to gradually achieve a BP <140/90 mm Hg or a reduction of 10/5 mm Hg for patients already within optimal range are the most widely recognized goals.3,20 Patients with stroke secondary to small vessel disease may benefit from an even lower goal of <130/<80 mm Hg.11 Encourage patients to monitor their BP at home for added accuracy and consistency.22
Pharmacologic BP management is appropriate for patients who are consistently above optimal range despite attempting recommended lifestyle modifications. The data are relatively consistent with respect to the effects of different drug classes after a stroke: beta-blockers have no effect on any outcome; thiazide diuretics significantly reduce stroke and total vascular events; angiotensin-converting enzyme (ACE) inhibitors significantly reduce myocardial infarction (MI); and the combination of an ACE inhibitor and thiazide diuretic reduces stroke, MI, and combined vascular events.4
This has led many stroke specialists to recommend the combination of an ACE inhibitor or angiotensin II receptor blocker (ARB) and a thiazide diuretic as a first-line approach to secondary stroke prevention rather than a beta-blocker (assuming there is no additional indication for a beta-blocker). Similarly, there is ample evidence to show that the magnitude of BP reduction is proportional to the reduction in recurrent vascular events.3
Make use of statin therapy—regardless of LDL. The SPARCL (Stroke Prevention by Aggressive Reduction in Cholesterol Levels) trial5 explored the potential role of statin medication for secondary stroke prevention. Researchers randomly assigned almost 5000 participants who’d had a stroke or TIA one to 6 months before study entry (but had no known history of coronary artery disease) to placebo or a high-intensity statin (80 mg/d atorvastatin). The statin group demonstrated a 4.9-year absolute risk reduction in fatal or nonfatal recurrent stroke of 1.9% (NNT=53).
Given these findings and those from other studies, the AHA and ASA recommend treating patients with stroke or TIA presumed to be of atherosclerotic origin with high-intensity statin therapy, regardless of low-density lipoprotein (LDL) level.3 Of note, statins are not indicated for the secondary prevention of hemorrhagic stroke.
Select antiplatelet therapy based on ischemic stroke subtype. Investigators are still trying to determine the optimal antiplatelet for secondary stroke prevention; it is likely that the ideal choice depends largely on the etiology of the stroke. Trials that did not select patients based on subtype of ischemic stroke have not shown a long-term benefit from dual antiplatelet therapy (clopidogrel and aspirin),23,24 and one double-blind, multicenter trial involving more than 3000 patients with recent stroke secondary to small vessel disease demonstrated harm from such therapy in terms of a significantly increased risk of bleeding and death.6
However, a 2011 study compared aggressive medical management (aspirin 325 mg/d plus clopidogrel 75 mg/d for 90 days) alone to aggressive medical management plus percutaneous transluminal angioplasty and stenting (PTAS). The study involved almost 500 patients who'd had a recent TIA or stroke attributed to intracranial atherosclerotic stenosis. The authors found that the 30-day rate of stroke or death was 14.7% in the PTAS group vs 5.8% in the medical management group.25
Similarly, a randomized double-blind, placebo-controlled trial published in 2013 involving over 5000 patients in China found that short-term use of dual antiplatelets (clopidogrel and aspirin for the first 21 days after an ischemic event, followed by aspirin monotherapy for 90 days) had an absolute risk reduction of 3.5% without increasing the risk of major bleeding in patients with high-risk TIA or minor stroke.26
All stroke patients who do not have an indication for oral anticoagulation should be placed on long-term daily aspirin (75-325 mg); research has shown that lower doses are as effective as higher doses but with a lower risk of adverse gastrointestinal effects, including bleeding.3,20 Aspirin 81 mg/d is a common effective dose.
For patients who cannot tolerate aspirin due to allergy, clopidogrel 75 mg/d is a reasonable alternative. Long-term studies of aspirin vs clopidogrel7 and clopidogrel vs extended-release dipyridamole8 showed no difference in secondary stroke prevention. The International Stroke Trial27 and Chinese Acute Stroke Trial28 both indicate that aspirin should be started as soon as possible after the onset of an acute stroke.
This special population should probably get antiplatelets, too. One recent study explored the use of an antiplatelet vs anticoagulation therapy for stroke patients with carotid artery dissection. The CADISS (Cervical Artery Dissection in Stroke Study) trial29 randomized 250 patients with extracranial carotid and vertebral artery dissection with onset of symptoms within the previous 7 days to either antiplatelet or anticoagulation therapy and found no difference in the primary outcomes of recurrent stroke or death. The study also demonstrated a low risk of recurrent stroke in this population, which was 2% at 3-month follow-up.
Most patients with cervical artery dissection, therefore, are now treated with antiplatelet therapy. That said, situations may still arise in which anticoagulation can be considered, and consultation with a neurologist for guidance on choice of therapy is recommended.
Is an anticoagulant in order? Which agent, when
The most common cause of cardioembolic stroke is AF, which accounts for at least 15% of ischemic strokes, a number that rises in those over the age of 80.20,30,31 A meta-analysis of more than 28,000 patients with non-valvular AF demonstrated that warfarin reduced the risk of stroke by 64%.32
The rate of intracerebral hemorrhages during oral anticoagulation ranges from 0.3% to 0.6% per year.33 The risk of bleeding complications can be mitigated by keeping international normalized ratios ≤3.0, maintaining good BP control, and avoiding concurrent use of antiplatelets in the absence of a clear indication for them.33
Several risk assessment scores, such as the HAS-BLED,34 can help with estimating the risk of hemorrhagic complications, although these scores have their limitations.35,36 Even in an older population (mean age 83 years) with a high risk for falls, warfarin provided a net benefit in a composite endpoint of out-of-hospital death or hospitalization for stroke, MI, or hemorrhage in a retrospective study of over 1200 Medicare beneficiaries.37
AF is not the only cause of cardioembolic stroke to consider. Additional high-risk factors warranting anticoagulation include rheumatic mitral valve disease, the presence of mechanical aortic or mitral valves, known mural thrombus, and acute anterior ST segment elevation myocardial infarctions (STEMIs) with resulting anterior apical dyskinesis/akinesis and concurrent ischemic stroke/TIA.3 (The specific management of each of these situations is beyond the scope of this paper.)
The choice of anticoagulation agent is based on multiple factors, including cost, risk of non-reversible bleeding, drug interactions, renal function, and patient preference. Approved options currently include warfarin/vitamin K antagonist therapy, apixaban, rivaroxaban, dabigatran and edoxaban.3 Choice of therapy will continue to evolve as reversal agents, such as idarucizumab, are developed. Idarucizumab, a reversal agent for dabigatran, received approval from the US Food and Drug Administration in October 2015.38
When to start anticoagulation. There are limited data regarding the optimal timing of initiation of anticoagulation following a stroke; however, a recent multicenter prospective study supported the common practice of initiating anticoagulation therapy within 4 to 14 days of the event.39 Individual patient factors must be taken into consideration, including the size of the stroke (the larger the stroke, the higher the risk for hemorrhagic transformation), BP control, any additional risk factors for bleeding, and the estimated risk of early recurrent stroke.
Bridging patients onto anticoagulation with unfractionated or low-molecular-weight heparin in the setting of acute stroke is not recommended.40 Results from randomized controlled trials involving unfractionated heparin, heparinoids, and low-molecular-weight heparin have not reported any benefit to these agents over aspirin at preventing early stroke recurrence.27,41,42
For immobile or hospitalized patients. Subcutaneous heparin for the prevention of deep vein thrombosis (DVT) during immobility and hospitalization is recommended.43 Patients who cannot tolerate anticoagulation should be maintained on low-dose antiplatelet therapy. Experts do not recommend dual treatment with aspirin and anticoagulation in most cases. However, recent coronary artery stent placement does require temporary dual treatment, with duration dependent on the type of stent placed.
A role for glycemic control? Still to be determined
The specific role of diabetic management in secondary stroke prevention remains unclear. The 2008 ACCORD trial,44 a randomized study involving over 10,000 patients with a median glycated hemoglobin level of 8.1%, investigated intensive hyperglycemic control (targeting a glycated hemoglobin level <6.0% vs <7.9%) as a means of decreasing cardiovascular risk. However, the trial ended 17 months early because of an increase in all-cause mortality in the intensive treatment arm compared with the standard management group. The same trial was also unable to demonstrate a decrease in stroke risk with a decrease in A1c.44
More recently, the IRIS (Insulin Resistance Intervention after Stroke) trial45 (2016) found a 2.8% absolute risk reduction in stroke or MI among participants who had a stroke or TIA in the previous 6 months who were treated with pioglitazone vs placebo over 4.8 years (NNT=36). Participants were required to have insulin resistance, but were excluded if they had diabetes. The authors did, however, report a notable increase in the risk of bone fractures requiring surgery or hospitalization in the pioglitazone arm (5.1% vs 3.2%; number needed to harm [NNH]=53).
The impact this single study should have on standard secondary prevention is not yet clear. The authors concluded, “It seems reasonable to consider individual treatment preference and risk of drug-related adverse events in addition to potential benefits when making patient-specific decisions regarding therapy.”45
Determining whether mechanical interventions are needed
Almost all conditions leading to stroke warrant active medical management, but a few benefit from procedural intervention, as well.
Extracranial carotid atherosclerosis. Carotid endarterectomy or carotid artery stenting is recommended as secondary prevention for patients with a history of stroke or TIA who have ipsilateral high-grade extracranial carotid stenosis of 70% to 99% and, in some cases, 50% to 69%.3,9,20 In patients with mild non-disabling stroke, the optimal timing for these procedures is within 2 weeks of the ischemic event. A delay of 6 weeks is generally preferred for moderate or larger strokes to allow for some healing of the injured brain.
The choice of procedure is based on risk profile, with the most important factor being age. For patients >70 years, endarterectomy is preferred because stenting is associated with an increased risk of stroke.3,9,10 Experts do not recommend either procedure for patients who have had a severe disabling stroke. Generally speaking, these procedures have higher rates of success when they are performed in centers that perform a higher number of these procedures.10
Vertebrobasilar atherosclerosis. Due to generally good compensatory blood flow of the contralateral vertebral artery in the setting of vertebral artery stenosis, and an unacceptably high complication rate of angioplasty and stenting in the basilar artery, medical management is typically the first-line approach. If a patient has recurrent symptoms in the setting of optimal medical management and a focal lesion that is amenable to an endovascular intervention (most commonly a vertebral artery origin high-grade stenosis), angioplasty and stenting may be considered.10
Intracranial atherosclerosis. Similarly, medical management is the preferred strategy for intracranial atherosclerosis. Angioplasty and/or stenting are reserved for complex cases or recurrence despite adherence to secondary stroke prevention measures. Ideally, these patients should be managed with long-term aspirin 81 mg/d, adjunctive clopidogrel 75 mg/d for 90 days post stroke, a high-intensity statin, BP optimization, and any relevant lifestyle interventions.13
Patent foramen ovale. Research to date has not shown that closure of a patent foramen ovale (PFO) is superior to medical therapy for secondary stroke prevention in patients <60 years with cryptogenic stroke.12,46,47 The decision to anticoagulate these patients should be based on the presence or absence of a DVT and not on a PFO alone. In patients with an identified DVT and a contraindication to oral anticoagulation, inferior vena cava filter placement should be considered. For patients with ongoing prothrombotic risk thought to increase the chances of future paradoxical embolism, closure of the PFO may be considered.
CORRESPONDENCE
Stephen A. Martin, MD, EdM, Barre Family Health Center, 151 Worcester Road, Barre, MA 01005; [email protected].
Patients who suffer a stroke rarely have just one vascular risk factor. Therefore, the approach to secondary stroke prevention must be multifactorial. In fact, it has been estimated that 80% of recurrent strokes could be prevented through the application of a comprehensive, multifactorial approach that includes lifestyle modification and optimal medical management.1 Such an achievement would save millions of people from disability and functional decline, as well as millions of dollars in related medical costs.
The initial approach to patients with stroke is focused on stabilization and a rapid work-up to identify the most likely etiology. Common causes of stroke include large artery atherosclerosis, cardiac emboli, and small vessel disease; less common causes include dissection, aortic emboli, and non-atherosclerotic vascular disease. If a complete diagnostic work-up is unrevealing, the stroke is said to be cryptogenic. Determining the correct etiology of a stroke is paramount to preventing secondary stroke (FIGURE2-13).

Effective secondary prevention strategies designed to prevent a stroke or transient ischemic attack (TIA) in a patient with a known history of either event include lifestyle modifications, medications, and when appropriate, mechanical interventions. As a primary care physician (PCP), you are uniquely positioned to spearhead the prevention of secondary strokes: Not only are you at the forefront of prevention and the use of techniques such as motivational interviewing, but you also have longstanding relationships with many of your patients. In fact, the success of many interventions is improved by the informed, enduring, and trusting nature of relationships between patients and their PCPs.
In the first part of this 2-part series, we focused on subacute stroke management and outlined the recommended work-up for subacute stroke/TIA (see “Stroke: A road map to subacute management,” 2017;66:366-374). In this part, we focus on secondary prevention. The more common modifiable conditions encountered in primary care are discussed here, while many of the more rare etiologies (hypercoagulable states, sickle cell disease, and vasculitis) are outside the scope of this article.
Lifestyle interventions: Target tobacco use, obesity, alcohol intake
Lifestyle modifications can have a positive impact on many of America’s most prevalent diseases, and stroke is no exception.14 Many of the disease states identified as risk factors for stroke (type 2 diabetes, hypertension, dyslipidemia) are exacerbated by tobacco use, obesity, and excessive alcohol intake.
Does your patient smoke? Up to 25% of all strokes are directly attributable to cigarette smoking.15 Smoking raises an individual’s risk for stroke in a dose-dependent fashion.15,16 One study demonstrated that, compared to never-smokers, women ages 15 to 49 years who smoked a half-pack per day had an odds ratio for ischemic stroke of 2.2; those who smoked 2 packs per day had an odds ratio of 9.1.17 After cessation, stroke risk generally returns to baseline within 5 years.16 Thus, smoking cessation is among the most significant steps a patient can take to reduce the risk of both primary and secondary stroke.
Is your patient overweight? While obesity in and of itself is a risk factor for stroke, a focus on nutrition and physical activity as mechanisms for weight loss is far superior to focusing on either element alone. Physical activity—consisting of at least 40 minutes of moderate intensity aerobic exercise 3 to 4 times per week—and a diet that emphasizes fruits and vegetables, whole grains, and healthy fats, have both independently demonstrated benefits in secondary stroke prevention and are important parts of American College of Cardiology (ACC)/American Heart Association (AHA) guidelines.2,3
The Mediterranean Diet, which emphasizes consumption of fruits and vegetables, legumes, tree nuts, olive oil, and lean protein, has long been associated with cardiovascular benefit.18 One prospective, randomized, single-blinded trial involving approximately 600 patients that looked at secondary prevention of coronary heart disease found that following the diet significantly reduced mortality compared with a usual prudent post-infarct diet (number needed to treat [NNT]=30 over 4 years).19
Is alcohol consumption an issue? Chronic heavy alcohol intake contributes to the development of hemorrhagic and ischemic stroke through multiple mechanisms, including alcohol-induced hypertension, alcoholic cardiomyopathy, and atrial fibrillation (AF). Light or moderate alcohol consumption has a paradoxical mild protective effect on ischemic stroke, thought to possibly be mediated by an increase in high-density lipoprotein (HDL) level and mild antiplatelet effect.3
AHA/American Stroke Association (ASA) guidelines indicate that no more than one standard drink per day for women and 2 drinks per day for men is reasonable.3 Counsel patients who drink in excess of this about the benefits of decreasing alcohol intake or abstaining altogether.
Choosing medications to manage BP, cholesterol, and clotting
Optimize blood pressure control. Blood pressure (BP) plays a critical role in both the management and prevention of stroke and is considered to be the most important modifiable risk factor in both primary and secondary stroke prevention.20 In the first 24 to 48 hours following a cerebral ischemic event that is not eligible for thrombolysis, permissive hypertension (treating BP only if it exceeds 220/120 mm Hg unless there is a concurrent medical illness that requires you do so) is appropriate, as hypotension or rapid fluctuations in BP can be harmful.21
This flexibility does not continue into the subacute phase of management (at a minimum, after the initial 48 hours) or into secondary prevention. Initiation and titration of oral agents to gradually achieve a BP <140/90 mm Hg or a reduction of 10/5 mm Hg for patients already within optimal range are the most widely recognized goals.3,20 Patients with stroke secondary to small vessel disease may benefit from an even lower goal of <130/<80 mm Hg.11 Encourage patients to monitor their BP at home for added accuracy and consistency.22
Pharmacologic BP management is appropriate for patients who are consistently above optimal range despite attempting recommended lifestyle modifications. The data are relatively consistent with respect to the effects of different drug classes after a stroke: beta-blockers have no effect on any outcome; thiazide diuretics significantly reduce stroke and total vascular events; angiotensin-converting enzyme (ACE) inhibitors significantly reduce myocardial infarction (MI); and the combination of an ACE inhibitor and thiazide diuretic reduces stroke, MI, and combined vascular events.4
This has led many stroke specialists to recommend the combination of an ACE inhibitor or angiotensin II receptor blocker (ARB) and a thiazide diuretic as a first-line approach to secondary stroke prevention rather than a beta-blocker (assuming there is no additional indication for a beta-blocker). Similarly, there is ample evidence to show that the magnitude of BP reduction is proportional to the reduction in recurrent vascular events.3
Make use of statin therapy—regardless of LDL. The SPARCL (Stroke Prevention by Aggressive Reduction in Cholesterol Levels) trial5 explored the potential role of statin medication for secondary stroke prevention. Researchers randomly assigned almost 5000 participants who’d had a stroke or TIA one to 6 months before study entry (but had no known history of coronary artery disease) to placebo or a high-intensity statin (80 mg/d atorvastatin). The statin group demonstrated a 4.9-year absolute risk reduction in fatal or nonfatal recurrent stroke of 1.9% (NNT=53).
Given these findings and those from other studies, the AHA and ASA recommend treating patients with stroke or TIA presumed to be of atherosclerotic origin with high-intensity statin therapy, regardless of low-density lipoprotein (LDL) level.3 Of note, statins are not indicated for the secondary prevention of hemorrhagic stroke.
Select antiplatelet therapy based on ischemic stroke subtype. Investigators are still trying to determine the optimal antiplatelet for secondary stroke prevention; it is likely that the ideal choice depends largely on the etiology of the stroke. Trials that did not select patients based on subtype of ischemic stroke have not shown a long-term benefit from dual antiplatelet therapy (clopidogrel and aspirin),23,24 and one double-blind, multicenter trial involving more than 3000 patients with recent stroke secondary to small vessel disease demonstrated harm from such therapy in terms of a significantly increased risk of bleeding and death.6
However, a 2011 study compared aggressive medical management (aspirin 325 mg/d plus clopidogrel 75 mg/d for 90 days) alone to aggressive medical management plus percutaneous transluminal angioplasty and stenting (PTAS). The study involved almost 500 patients who'd had a recent TIA or stroke attributed to intracranial atherosclerotic stenosis. The authors found that the 30-day rate of stroke or death was 14.7% in the PTAS group vs 5.8% in the medical management group.25
Similarly, a randomized double-blind, placebo-controlled trial published in 2013 involving over 5000 patients in China found that short-term use of dual antiplatelets (clopidogrel and aspirin for the first 21 days after an ischemic event, followed by aspirin monotherapy for 90 days) had an absolute risk reduction of 3.5% without increasing the risk of major bleeding in patients with high-risk TIA or minor stroke.26
All stroke patients who do not have an indication for oral anticoagulation should be placed on long-term daily aspirin (75-325 mg); research has shown that lower doses are as effective as higher doses but with a lower risk of adverse gastrointestinal effects, including bleeding.3,20 Aspirin 81 mg/d is a common effective dose.
For patients who cannot tolerate aspirin due to allergy, clopidogrel 75 mg/d is a reasonable alternative. Long-term studies of aspirin vs clopidogrel7 and clopidogrel vs extended-release dipyridamole8 showed no difference in secondary stroke prevention. The International Stroke Trial27 and Chinese Acute Stroke Trial28 both indicate that aspirin should be started as soon as possible after the onset of an acute stroke.
This special population should probably get antiplatelets, too. One recent study explored the use of an antiplatelet vs anticoagulation therapy for stroke patients with carotid artery dissection. The CADISS (Cervical Artery Dissection in Stroke Study) trial29 randomized 250 patients with extracranial carotid and vertebral artery dissection with onset of symptoms within the previous 7 days to either antiplatelet or anticoagulation therapy and found no difference in the primary outcomes of recurrent stroke or death. The study also demonstrated a low risk of recurrent stroke in this population, which was 2% at 3-month follow-up.
Most patients with cervical artery dissection, therefore, are now treated with antiplatelet therapy. That said, situations may still arise in which anticoagulation can be considered, and consultation with a neurologist for guidance on choice of therapy is recommended.
Is an anticoagulant in order? Which agent, when
The most common cause of cardioembolic stroke is AF, which accounts for at least 15% of ischemic strokes, a number that rises in those over the age of 80.20,30,31 A meta-analysis of more than 28,000 patients with non-valvular AF demonstrated that warfarin reduced the risk of stroke by 64%.32
The rate of intracerebral hemorrhages during oral anticoagulation ranges from 0.3% to 0.6% per year.33 The risk of bleeding complications can be mitigated by keeping international normalized ratios ≤3.0, maintaining good BP control, and avoiding concurrent use of antiplatelets in the absence of a clear indication for them.33
Several risk assessment scores, such as the HAS-BLED,34 can help with estimating the risk of hemorrhagic complications, although these scores have their limitations.35,36 Even in an older population (mean age 83 years) with a high risk for falls, warfarin provided a net benefit in a composite endpoint of out-of-hospital death or hospitalization for stroke, MI, or hemorrhage in a retrospective study of over 1200 Medicare beneficiaries.37
AF is not the only cause of cardioembolic stroke to consider. Additional high-risk factors warranting anticoagulation include rheumatic mitral valve disease, the presence of mechanical aortic or mitral valves, known mural thrombus, and acute anterior ST segment elevation myocardial infarctions (STEMIs) with resulting anterior apical dyskinesis/akinesis and concurrent ischemic stroke/TIA.3 (The specific management of each of these situations is beyond the scope of this paper.)
The choice of anticoagulation agent is based on multiple factors, including cost, risk of non-reversible bleeding, drug interactions, renal function, and patient preference. Approved options currently include warfarin/vitamin K antagonist therapy, apixaban, rivaroxaban, dabigatran and edoxaban.3 Choice of therapy will continue to evolve as reversal agents, such as idarucizumab, are developed. Idarucizumab, a reversal agent for dabigatran, received approval from the US Food and Drug Administration in October 2015.38
When to start anticoagulation. There are limited data regarding the optimal timing of initiation of anticoagulation following a stroke; however, a recent multicenter prospective study supported the common practice of initiating anticoagulation therapy within 4 to 14 days of the event.39 Individual patient factors must be taken into consideration, including the size of the stroke (the larger the stroke, the higher the risk for hemorrhagic transformation), BP control, any additional risk factors for bleeding, and the estimated risk of early recurrent stroke.
Bridging patients onto anticoagulation with unfractionated or low-molecular-weight heparin in the setting of acute stroke is not recommended.40 Results from randomized controlled trials involving unfractionated heparin, heparinoids, and low-molecular-weight heparin have not reported any benefit to these agents over aspirin at preventing early stroke recurrence.27,41,42
For immobile or hospitalized patients. Subcutaneous heparin for the prevention of deep vein thrombosis (DVT) during immobility and hospitalization is recommended.43 Patients who cannot tolerate anticoagulation should be maintained on low-dose antiplatelet therapy. Experts do not recommend dual treatment with aspirin and anticoagulation in most cases. However, recent coronary artery stent placement does require temporary dual treatment, with duration dependent on the type of stent placed.
A role for glycemic control? Still to be determined
The specific role of diabetic management in secondary stroke prevention remains unclear. The 2008 ACCORD trial,44 a randomized study involving over 10,000 patients with a median glycated hemoglobin level of 8.1%, investigated intensive hyperglycemic control (targeting a glycated hemoglobin level <6.0% vs <7.9%) as a means of decreasing cardiovascular risk. However, the trial ended 17 months early because of an increase in all-cause mortality in the intensive treatment arm compared with the standard management group. The same trial was also unable to demonstrate a decrease in stroke risk with a decrease in A1c.44
More recently, the IRIS (Insulin Resistance Intervention after Stroke) trial45 (2016) found a 2.8% absolute risk reduction in stroke or MI among participants who had a stroke or TIA in the previous 6 months who were treated with pioglitazone vs placebo over 4.8 years (NNT=36). Participants were required to have insulin resistance, but were excluded if they had diabetes. The authors did, however, report a notable increase in the risk of bone fractures requiring surgery or hospitalization in the pioglitazone arm (5.1% vs 3.2%; number needed to harm [NNH]=53).
The impact this single study should have on standard secondary prevention is not yet clear. The authors concluded, “It seems reasonable to consider individual treatment preference and risk of drug-related adverse events in addition to potential benefits when making patient-specific decisions regarding therapy.”45
Determining whether mechanical interventions are needed
Almost all conditions leading to stroke warrant active medical management, but a few benefit from procedural intervention, as well.
Extracranial carotid atherosclerosis. Carotid endarterectomy or carotid artery stenting is recommended as secondary prevention for patients with a history of stroke or TIA who have ipsilateral high-grade extracranial carotid stenosis of 70% to 99% and, in some cases, 50% to 69%.3,9,20 In patients with mild non-disabling stroke, the optimal timing for these procedures is within 2 weeks of the ischemic event. A delay of 6 weeks is generally preferred for moderate or larger strokes to allow for some healing of the injured brain.
The choice of procedure is based on risk profile, with the most important factor being age. For patients >70 years, endarterectomy is preferred because stenting is associated with an increased risk of stroke.3,9,10 Experts do not recommend either procedure for patients who have had a severe disabling stroke. Generally speaking, these procedures have higher rates of success when they are performed in centers that perform a higher number of these procedures.10
Vertebrobasilar atherosclerosis. Due to generally good compensatory blood flow of the contralateral vertebral artery in the setting of vertebral artery stenosis, and an unacceptably high complication rate of angioplasty and stenting in the basilar artery, medical management is typically the first-line approach. If a patient has recurrent symptoms in the setting of optimal medical management and a focal lesion that is amenable to an endovascular intervention (most commonly a vertebral artery origin high-grade stenosis), angioplasty and stenting may be considered.10
Intracranial atherosclerosis. Similarly, medical management is the preferred strategy for intracranial atherosclerosis. Angioplasty and/or stenting are reserved for complex cases or recurrence despite adherence to secondary stroke prevention measures. Ideally, these patients should be managed with long-term aspirin 81 mg/d, adjunctive clopidogrel 75 mg/d for 90 days post stroke, a high-intensity statin, BP optimization, and any relevant lifestyle interventions.13
Patent foramen ovale. Research to date has not shown that closure of a patent foramen ovale (PFO) is superior to medical therapy for secondary stroke prevention in patients <60 years with cryptogenic stroke.12,46,47 The decision to anticoagulate these patients should be based on the presence or absence of a DVT and not on a PFO alone. In patients with an identified DVT and a contraindication to oral anticoagulation, inferior vena cava filter placement should be considered. For patients with ongoing prothrombotic risk thought to increase the chances of future paradoxical embolism, closure of the PFO may be considered.
CORRESPONDENCE
Stephen A. Martin, MD, EdM, Barre Family Health Center, 151 Worcester Road, Barre, MA 01005; [email protected].
1. Hackam DG, Spence JD. Combining multiple approaches for the secondary prevention of vascular events after stroke: a quantitative modeling study. Stroke. 2007;38:1881-1885.
2. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S76-S99.
3. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236.
4. Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke. 2003;34:2741-2748.
5. Amarenco P, Bogousslavsky J, Callahan A, et al, for the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559.
6. Benavente OR, Hart RG, McClure LA, et al, for the SPS3 Investigators. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
7. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329-1339.
8. Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238-1251.
9. Diethrich EB, N’diaye M, Reid DB. The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST): implications for clinical practice. In: Henry M, Diethrich EB, Polydorou A, eds. The Carotid and Supra-Aortic Trunks: Diagnosis, Angioplasty and Stenting. 2nd ed. Oxford, UK: Wiley-Blackwell; 2011.
10. Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. Circulation. 2011;124:489-532.
11. SPS3 Study Group. Blood pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507-515.
12. Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092-1100.
13. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003.
14. Romero JR, Morris J, Pikula A. Stroke prevention: modifying risk factors. Ther Adv Cardiovasc Dis. 2008;2:287-303.
15. Hankey GJ. Smoking and risk of stroke. J Cardiovasc Risk. 1999;6:207-211.
16. Shah RS, Cole JW. Smoking and stroke: the more you smoke the more you stroke. Expert Rev Cardiovasc Ther. 2010;8:917-932.
17. Bhat VM, Cole JW, Sorkin JD, et al. Dose-response relationship between cigarette smoking and risk of ischemic stroke in young women. Stroke. 2008;39:2439-2443.
18. Lakkur S, Judd SE. Diet and stroke: recent evidence supporting a Mediterranean-style diet and food in the primary prevention of stroke. Stroke. 2015;46:2007-2011.
19. de Lorgeril M, Salen P, Martin JL, et al. Mediterranean dietary pattern in a randomized trial: prolonged survival and possible reduced cancer rate. Arch Intern Med. 1998;158:1181-1187.
20. Davis SM, Donnan GA. Clinical practice. Secondary prevention after ischemic stroke or transient ischemic attack. N Engl J Med. 2012;366:1914-1922.
21. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
22. Magid DJ, Green BB. Home blood pressure monitoring: take it to the bank. JAMA. 2013;310:40-41.
23. Diener H-C, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2004;364:331-337.
24. Bhatt DL, Fox KAA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706-1717.
25. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003.
26. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
27. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet. 1997;349:1569-1581.
28. CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet. 1997;349:1641-1649.
29. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14:361-367.
30. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) Study Group. Lancet. 1993;342:1255-1262.
31. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983-988.
32. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Int Med. 2007;146:857-867.
33. Hart RG, Tonarelli SB, Pearce LA. Avoiding central nervous system bleeding during antithrombotic therapy. Recent data and ideas. Stroke. 2005;36:1588-1593.
34. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093-1100.
35. Quinn GR, Singer DE, Chang Y, et al. How well do stroke risk scores predict hemorrhage in patients with atrial fibrillation? Am J Cardiol. 2016;118:697-699.
36. Gorman EW, Perkel D, Dennis D, et al. Validation of the HAS-BLED tool in atrial fibrillation patients receiving rivaroxaban. J Atr Fibrillation. 2016;9:1461.
37. Gage BF, Birman-Deych E, Kerzner R, et al. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med. 2005;118:612-617.
38. US Food and Drug Administration. FDA approves Praxbind, the first reversal agent for the anticoagulant Pradaxa. October 16, 2015. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm467300.htm. Accessed May 26, 2017.
39. Paciaroni M, Agnelli G, Falocci N, et al. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: effect of anticoagulation and its timing: the RAF Study. Stroke. 2015;46:2175-2182.
40. Sandercock PA, Counsell C, Kane EJ. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev. 2015;3:CD000024.
41. Bath PM, Lindenstrom E, Boysen G, et al. Tinzaparin in acute ischaemic stroke (TAIST): a randomised aspirin-controlled trial. Lancet. 2001;358:702-710.
42. Berge E, Abdelnoor M, Nakstad PH, et al. Low molecular-weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: a double-blind randomised study. HAEST Study Group. Heparin in Acute Embolic Stroke Trial. Lancet. 2000;355:1205-1210.
43. Sherman DG, Albers GW, Bladin C, et al. The efficacy and safety of enoxaparin versus unfractionated heparin for the prevention of venous thromboembolism after acute ischaemic stroke (PREVAIL Study): an open-label randomised comparison. Lancet. 2007;369:1347-1355.
44. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559.
45. Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321-1331.
46. Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083-1091.
47. Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991-999.
1. Hackam DG, Spence JD. Combining multiple approaches for the secondary prevention of vascular events after stroke: a quantitative modeling study. Stroke. 2007;38:1881-1885.
2. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S76-S99.
3. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236.
4. Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke. 2003;34:2741-2748.
5. Amarenco P, Bogousslavsky J, Callahan A, et al, for the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559.
6. Benavente OR, Hart RG, McClure LA, et al, for the SPS3 Investigators. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
7. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329-1339.
8. Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238-1251.
9. Diethrich EB, N’diaye M, Reid DB. The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST): implications for clinical practice. In: Henry M, Diethrich EB, Polydorou A, eds. The Carotid and Supra-Aortic Trunks: Diagnosis, Angioplasty and Stenting. 2nd ed. Oxford, UK: Wiley-Blackwell; 2011.
10. Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. Circulation. 2011;124:489-532.
11. SPS3 Study Group. Blood pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507-515.
12. Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092-1100.
13. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003.
14. Romero JR, Morris J, Pikula A. Stroke prevention: modifying risk factors. Ther Adv Cardiovasc Dis. 2008;2:287-303.
15. Hankey GJ. Smoking and risk of stroke. J Cardiovasc Risk. 1999;6:207-211.
16. Shah RS, Cole JW. Smoking and stroke: the more you smoke the more you stroke. Expert Rev Cardiovasc Ther. 2010;8:917-932.
17. Bhat VM, Cole JW, Sorkin JD, et al. Dose-response relationship between cigarette smoking and risk of ischemic stroke in young women. Stroke. 2008;39:2439-2443.
18. Lakkur S, Judd SE. Diet and stroke: recent evidence supporting a Mediterranean-style diet and food in the primary prevention of stroke. Stroke. 2015;46:2007-2011.
19. de Lorgeril M, Salen P, Martin JL, et al. Mediterranean dietary pattern in a randomized trial: prolonged survival and possible reduced cancer rate. Arch Intern Med. 1998;158:1181-1187.
20. Davis SM, Donnan GA. Clinical practice. Secondary prevention after ischemic stroke or transient ischemic attack. N Engl J Med. 2012;366:1914-1922.
21. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
22. Magid DJ, Green BB. Home blood pressure monitoring: take it to the bank. JAMA. 2013;310:40-41.
23. Diener H-C, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2004;364:331-337.
24. Bhatt DL, Fox KAA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706-1717.
25. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993-1003.
26. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
27. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet. 1997;349:1569-1581.
28. CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet. 1997;349:1641-1649.
29. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14:361-367.
30. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) Study Group. Lancet. 1993;342:1255-1262.
31. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983-988.
32. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Int Med. 2007;146:857-867.
33. Hart RG, Tonarelli SB, Pearce LA. Avoiding central nervous system bleeding during antithrombotic therapy. Recent data and ideas. Stroke. 2005;36:1588-1593.
34. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093-1100.
35. Quinn GR, Singer DE, Chang Y, et al. How well do stroke risk scores predict hemorrhage in patients with atrial fibrillation? Am J Cardiol. 2016;118:697-699.
36. Gorman EW, Perkel D, Dennis D, et al. Validation of the HAS-BLED tool in atrial fibrillation patients receiving rivaroxaban. J Atr Fibrillation. 2016;9:1461.
37. Gage BF, Birman-Deych E, Kerzner R, et al. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med. 2005;118:612-617.
38. US Food and Drug Administration. FDA approves Praxbind, the first reversal agent for the anticoagulant Pradaxa. October 16, 2015. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm467300.htm. Accessed May 26, 2017.
39. Paciaroni M, Agnelli G, Falocci N, et al. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: effect of anticoagulation and its timing: the RAF Study. Stroke. 2015;46:2175-2182.
40. Sandercock PA, Counsell C, Kane EJ. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev. 2015;3:CD000024.
41. Bath PM, Lindenstrom E, Boysen G, et al. Tinzaparin in acute ischaemic stroke (TAIST): a randomised aspirin-controlled trial. Lancet. 2001;358:702-710.
42. Berge E, Abdelnoor M, Nakstad PH, et al. Low molecular-weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: a double-blind randomised study. HAEST Study Group. Heparin in Acute Embolic Stroke Trial. Lancet. 2000;355:1205-1210.
43. Sherman DG, Albers GW, Bladin C, et al. The efficacy and safety of enoxaparin versus unfractionated heparin for the prevention of venous thromboembolism after acute ischaemic stroke (PREVAIL Study): an open-label randomised comparison. Lancet. 2007;369:1347-1355.
44. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559.
45. Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321-1331.
46. Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083-1091.
47. Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991-999.
From The Journal of Family Practice | 2017;66(7):420-422,424-427.
PRACTICE RECOMMENDATIONS
› Encourage lifestyle modifications, including smoking cessation, alcohol moderation, appropriate diet, and exercise to reduce the risk of recurrent stroke. A
› Optimize blood pressure control using an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker and a thiazide diuretic. A
› Only use beta-blockers if there is another indication for them. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Stroke: A road map for subacute management
CASE › A 68-year-old woman with hypertension and hyperlipidemia comes into your office for evaluation of a 30-minute episode of sudden-onset right-hand weakness and difficulty speaking that occurred 4 days earlier. The patient, who is also a smoker, has come in at the insistence of her daughter. On examination, her blood pressure (BP) is 145/88 mm Hg and her heart rate is 76 beats/minute and regular. She appears well and her language function is normal. The rest of her examination is normal. How would you proceed?
Stroke—the death of nerve cells due to a lack of blood supply from either infarction or hemorrhage—strikes nearly 800,000 people in the United States every year.1,2 Of these events, 130,000 are fatal, making stroke the fifth leading cause of death.3 Effective, early evaluation and cause-specific treatment are crucial parts of stroke care.
Research has helped to clarify the critical role primary care physicians play in recognizing, triaging, and managing stroke and transient ischemic attacks (TIA). This article reviews what we know about the different ways that a stroke and a TIA can present, the appropriate diagnostic work-up for patients presenting with symptoms of either event, and management strategies for subacute care (24 hours to up to 14 days after a stroke has occurred).4,5 Unless otherwise specified, this review will focus on ischemic stroke because 87% of strokes are attributable to ischemia.1
A follow-up to this article on secondary stroke prevention will appear in the journal next month.
Look to onset more than type of symptoms for clues
Stroke presents as a sudden onset of neurologic deficits (language, motor, sensory, cerebellar, or brainstem functions) (TABLE 14). Because presenting symptoms can vary widely, sudden onset, rather than particular symptoms, should raise a red flag for potential stroke.
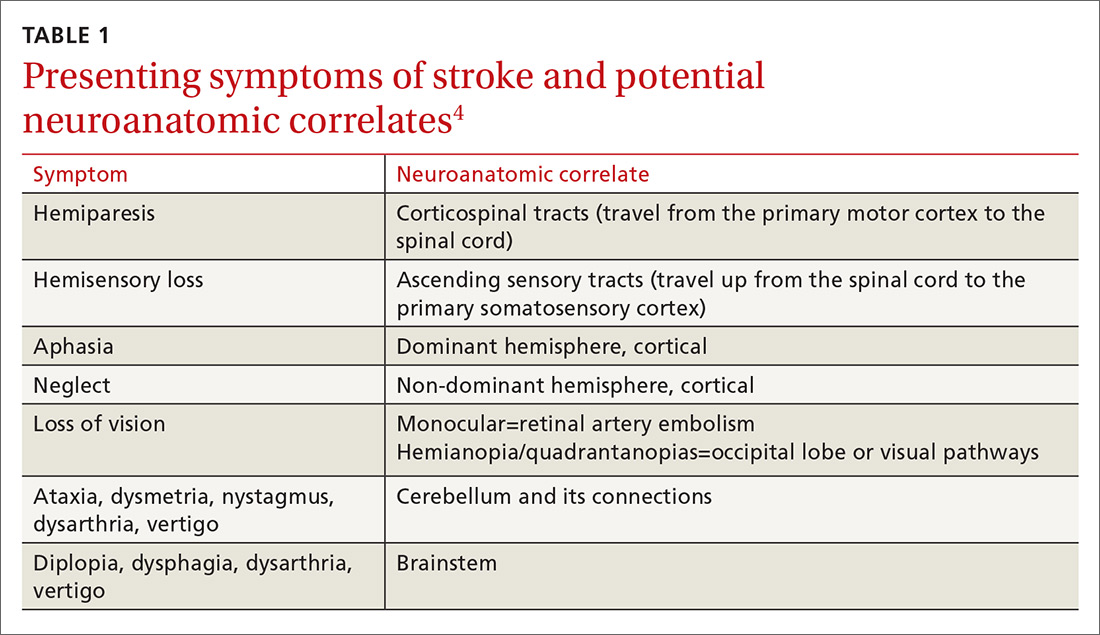
The differential diagnosis includes: seizure, complex migraine, medication effect (eg, slurred speech or confusion after taking a central nervous system [CNS] depressant), toxin exposure, electrolyte abnormalities (especially hypoglycemia), concussion/trauma, infection of the CNS, peripheral vertigo, demyelination, intracranial mass, Bell’s palsy, and psychogenic disorders. The history and physical, along with laboratory findings and brain imaging (detailed later in this article), will guide the FP toward (or away from) these various etiologies.
Optimal triage is a subject of ongoing interest and research
If stroke or TIA remains a possibility after an initial assessment, it’s time to stratify patients by risk.
One of the most widely accepted tools is the ABCD2 score (see TABLE 26). Clinicians can employ the ABCD2 risk stratification tool when trying to determine whether it is reasonable to pursue an expedited work-up (ie, <1 day) in the outpatient setting or recommend that the patient be evaluated in an emergency department (ED). The 90-day stroke rate following a TIA ranges from 3% with an ABCD2 score of 0 to 3 to 18% with a score of 6 or 7. A score of 0 to 3 is considered relatively low risk; in the absence of other compelling factors, rapid outpatient evaluation is appropriate. For patients with an ABCD2 score ≥4, referral to the ED or direct admission to the hospital is advised.
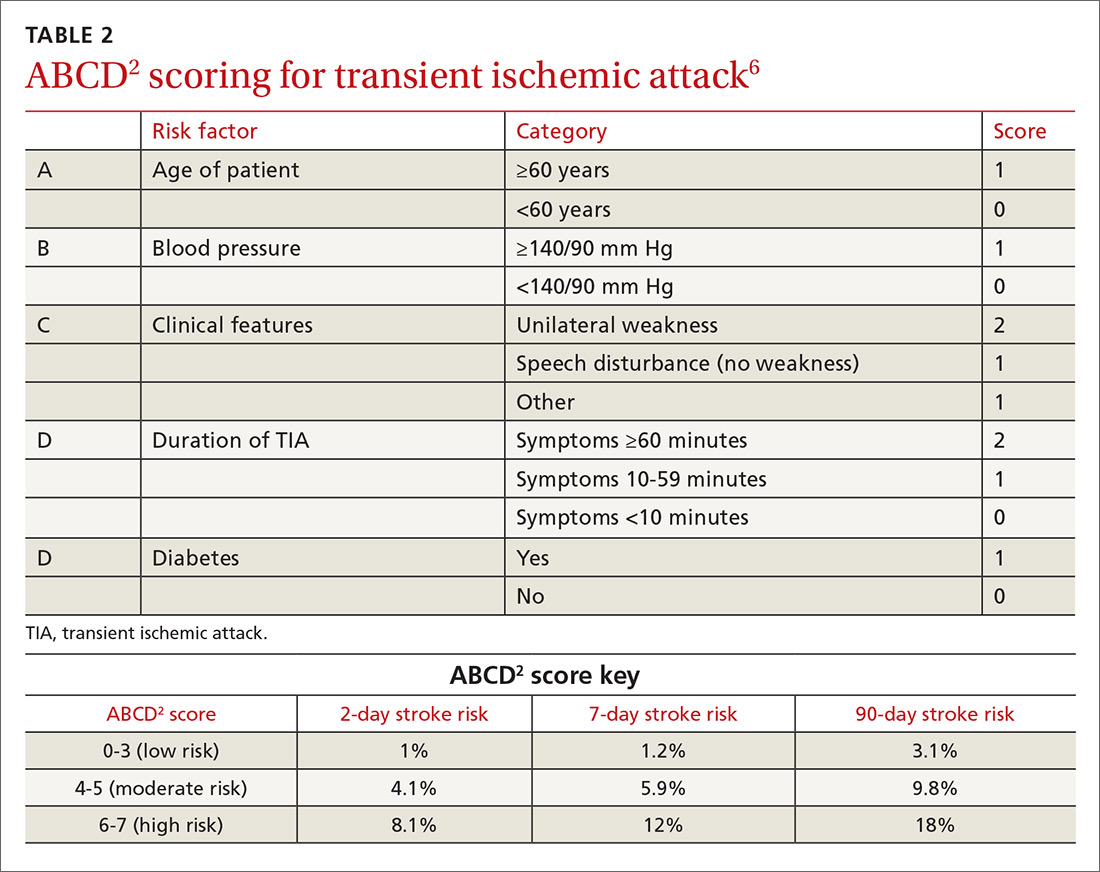
The validity of the ABCD2 score for risk stratification has been studied extensively with conflicting results.7-10 As with any assessment tool, it should be used as a guide, and should not supplant a full assessment of the patient or the judgment of the examining physician. In making the decision regarding inpatient or outpatient evaluation, it’s also important to consider available resources, access to specialists, and patient preference.
In a 2016 population-based study, the 30-day recurrent stroke/TIA rate for patients hospitalized for TIA was 3% compared with 10.7% for those discharged from the ED with referral to a stroke clinic and 10.6% for those discharged from the ED without a referral to a stroke clinic.11 These data suggest that only patients for whom you have a low clinical suspicion of stroke/TIA should be worked up as outpatients, and that hospital admission is advised in moderate- and high-risk cases. The findings also highlight the critical role that primary care physicians can play in triaging and managing these patients for secondary prevention.
CASE › This patient’s recent history of sudden-onset right-sided weakness and expressive language dysfunction is suspicious for left hemispheric ischemia. She has several risk factors for stroke, and her ABCD2 score is 5 (hypertension, age ≥60 years, unilateral weakness, and duration 10-59 min), which places her at moderate risk. Thus, the recommendation would be to have her go directly to an ED for rapid evaluation.
The diagnostic work-up
Even when a patient is sent to the ED, the FP plays a critical role in his or her continuing care. FPs will often coordinate with inpatient care and manage transition of care to the outpatient setting. (And in many communities, the ED or hospital physicians may themselves be family practitioners.)
In terms of care, not even an aspirin should be administered in a case like this because the patient has not yet had any neuroimaging, and differentiation of ischemic from hemorrhagic stroke cannot be made on clinical grounds alone. Once an ischemic stroke is confirmed, determining the etiology is critical given the significant management differences between the different types of stroke (atherosclerotic, cardioembolic, lacunar, or other).
Which imaging method, and when?
While a computerized tomography (CT) scan is the preferred initial imaging strategy for acute stroke to discern the ischemic type from the hemorrhagic, MRI is preferred for the evaluation of acute ischemic stroke because the method has a higher sensitivity for infarction and a greater ability to identify findings (such as demyelination) that would suggest an alternative diagnosis.
In addition to evaluating the brain parenchyma, physicians must also assess the cerebral vasculature. CT angiography (CTA) or MR angiography (MRA) of the head and neck are preferred over carotid ultrasound because they are capable of evaluating the entire cerebrovascular system12,13 and can be instrumental in identifying potential causes of stroke, as well as guiding therapeutic decisions. Carotid ultrasound is a reasonable alternative for patients presenting with symptoms indicative of anterior circulation involvement when CTA and MRA are unavailable or contraindicated, but it will not identify intracranial vascular disease, proximal common carotid disease, or vertebrobasilar disease.
Getting to the cause of suspected stroke: Labs and other diagnostic tests
A routine work-up includes BP checks, routine labs (complete blood count, complete metabolic panel, coagulation profile, and troponin), an electrocardiogram (EKG), a transthoracic echocardiogram (TTE) with bubble study if possible, and a minimum of 24 to 48 hours of cardiac rhythm monitoring. Cardiac rhythm monitoring should be extended in the setting of clinical concern for unidentified paroxysmal atrial fibrillation, such as an embolism without a proximal vascular source, multiple embolic infarcts in different vascular territories, a dilated left atrium, or other risk factors for atrial fibrillation that include smoking, systolic hypertension, diabetes, and heart failure (see TABLE 312,13,17,18).14-16 This standard diagnostic work-up will identify the cause of stroke in 70% to 80% of patients.19
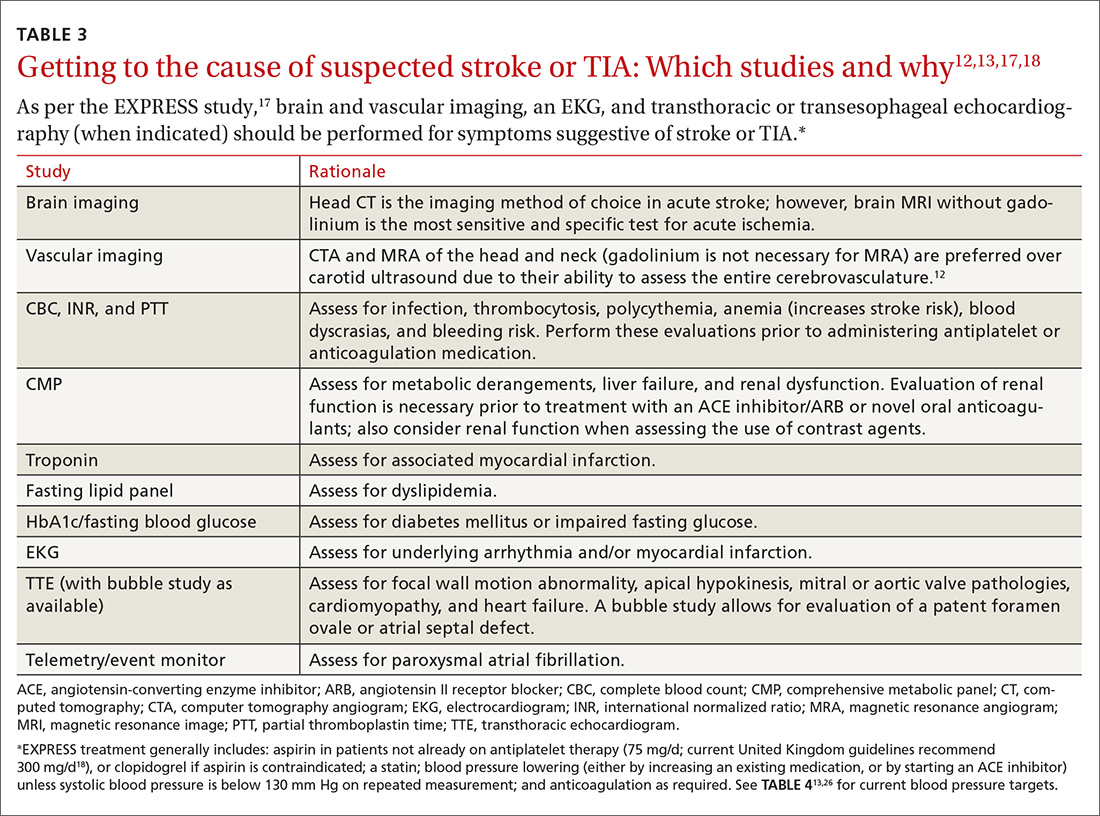
Additional investigations to consider if the etiology is not yet elucidated include a transesophageal echocardiogram (TEE), cerebral angiography, a coagulopathy evaluation, a lumbar puncture, and a vasculitis work-up. If available, consultation with a neurologist is appropriate for any patient who has had a stroke or TIA. Patients with unclear etiologies or for whom there are questions concerning strategies for preventing secondary stroke should be referred to Neurology and preferably a stroke specialist.
Timing matters, even when symptoms have resolved (ie, TIA).11,20 The EXPRESS trial17 (the Early use of eXisting PREventive Strategies for Stroke) looked at the effect of urgent assessment and treatment (≤1 day) of patients presenting with a TIA or minor stroke on the risk of recurrent stroke within 90 days. The diagnostic work-up included brain and vascular imaging together with an EKG. This intensive approach led to an absolute risk reduction of 8.2% (from 10.3% to 2.1%) in the risk of recurrent stroke at 90 days (number needed to treat [NNT]=12).17
Expedited work-up and treatment was also recently evaluated in a non-trial, real-world setting and was associated with reducing recurrent stroke by more than half the rate reported in older studies.20 Overall, the data suggest that evaluation within 24 hours confers substantial benefit, and that this evaluation can happen in an outpatient setting.21-23
Acute management: Use of tPA
Once imaging rules out intracranial hemorrhage, patients should be treated with tissue plasminogen activator (tPA) or an endovascular intervention as per guidelines.24 For patients with ischemic stroke ineligible for tPA or endovascular treatments, the initial focus is to determine the etiology of the symptoms so that the best strategies for prevention of secondary stroke may be employed.
Aspirin should be provided within 24 to 48 hours to all patients after intracranial hemorrhage is ruled out. Aspirin should be delayed for 24 hours in those given thrombolytics. The initial recommended dose of aspirin is 325 mg with continued low-dose (81 mg) aspirin daily.13 The addition of clopidogrel to aspirin within 24 hours of an event and continued for 21 days, followed by aspirin alone, was shown to be beneficial in a Chinese population with high-risk TIA (ABCD2 score ≥4) or minor stroke (National Institutes of Health Stroke Scale [NIHSS] ≤3).25 Anticoagulation with heparin, warfarin, or a novel oral anticoagulant is generally not indicated in the acute setting due to the risk of hemorrhagic transformation.
Acute BP management depends upon the type of stroke (ischemic or hemorrhagic), eligibility for thrombolytics, timing of presentation, and possible comorbidities such as myocardial infarction or aortic dissection (see TABLE 413,26). In the absence of contraindications, high-intensity statins should be initiated in all patients able to take oral medications.
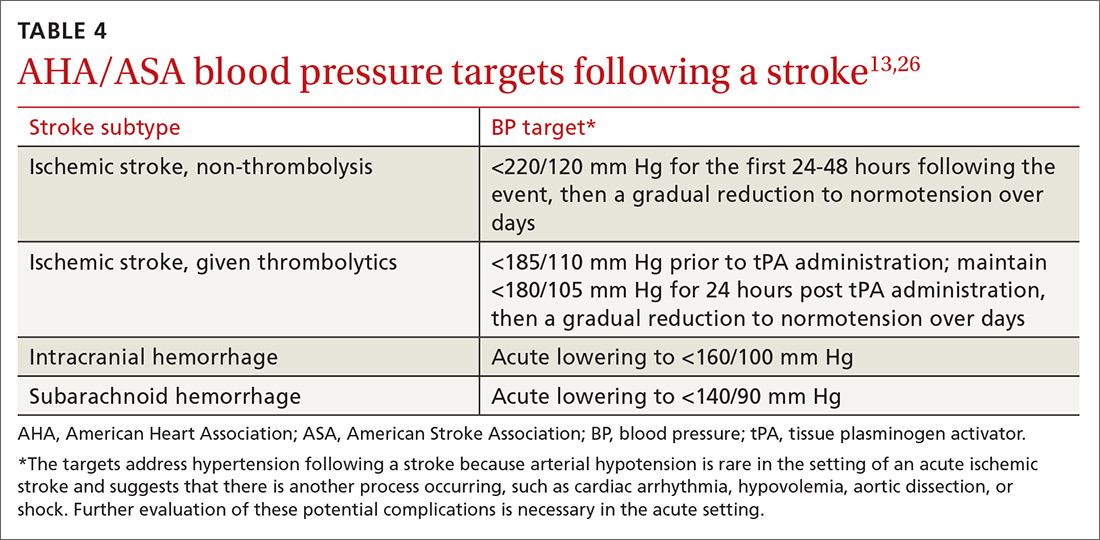
CASE › You appropriately referred your patient to the local ED. A head CT with head and neck CTA was performed. While the head CT did not show any abnormalities, the CTA demonstrated high-grade left internal carotid artery stenosis. The patient was given an initial dose of aspirin 325 mg and a high-intensity statin and admitted for further management. An MRI revealed a small shower of emboli in the left hemisphere, confirming the diagnosis of stroke over TIA. Labs were marginally remarkable with a low-density lipoprotein level of 115 mg/dL and an HbA1c of 6.2. Telemetry monitoring did not reveal any arrhythmias, and TTE was normal. BP remained in the high-normal to low-hypertensive range.
A Vascular Surgery consultation was obtained and the patient underwent a left carotid endarterectomy the following day. She did well without surgical complications. Her BP medications were adjusted; a combination of an angiotensin-converting enzyme inhibitor and a thiazide diuretic achieved a goal BP <140/90 mm Hg.
Permissive hypertension was not indicated due to her presentation >48 hours beyond the acute event. Low-dose aspirin and a high-intensity statin were continued, for secondary stroke prevention in the setting of atherosclerotic disease. She received smoking cessation counseling, which will continue.
CORRESPONDENCE
Stephen A. Martin, MD, EdM, Barre Family Health Center, 151 Worcester Road, Barre, MA 01005; [email protected].
1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146-e603.
2. Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064-2089.
3. Kochanek KD, Murphy SL, Xu J, et al. Mortality in the United States, 2013. NCHS Data Brief. 2014:1-8. Available at: https://www.cdc.gov/nchs/data/databriefs/db178.pdf. Accessed June 5, 2016.
4. Flossmann E, Redgrave JN, Briley D, et al. Reliability of clinical diagnosis of the symptomatic vascular territory in patients with recent transient ischemic attack or minor stroke. Stroke. 2008;39:2457-2460.
5. Josephson SA, Sidney S, Pham TN, et al. Higher ABCD2 score predicts patients most likely to have true transient ischemic attack. Stroke. 2008;39:3096-3098. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18688003. Accessed June 5, 2016.
6. Hankey GJ. The ABCD, California, and unified ABCD2 risk scores predicted stroke within 2, 7, and 90 days after TIA. Evid Based Med. 2007;12:88.
7. Sheehan OC, Kyne L, Kelly LA, et al. Population-based study of ABCD2 score, carotid stenosis, and atrial fibrillation for early stroke prediction after transient ischemic attack: the North Dublin TIA study. Stroke. 2010;41:844-850.
8. Rothwell PM, Giles MF, Flossmann E, et al. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet. 2005; 366:29-36.
9. Tsivgoulis G, Spengos K, Manta P, et al. Validation of the ABCD score in identifying individuals at high early risk of stroke after a transient ischemic attack: a hospital-based case series study. Stroke. 2006;37:2892-2897.
10. Kiyohara T, Kamouchi M, Kumai Y, et al. ABCD3 and ABCD3-I scores are superior to ABCD2 score in the prediction of short- and long-term risks of stroke after transient ischemic attack. Stroke. 2014;45:418-425.
11. Sacco RL, Rundek T. The value of urgent specialized care for TIA and minor stroke. N Engl J Med. 2016;374:1577-1579.
12. Demchuk AM, Menon BK, Goyal M. Comparing vessel imaging: noncontrast computed tomography/computed tomographic angiography should be the new minimum standard in acute disabling stroke. Stroke. 2016;47:273-281.
13. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
14. Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467-2477.
15. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478-2486.
16. Christophersen IE, Yin X, Larson MG, et al. A comparison of the CHARGE-AF and the CHA2DS2-VASc risk scores for prediction of atrial fibrillation ni the Framingham Heart Study. Am Heart J. 2016;178:45-54.
17. Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370:1432-1442.
18. National Institute for Health and Care Excellence. Stroke and transient ischaemic attack in over 16s: diagnosis and initial management. Available at: https://www.nice.org.uk/guidance/cg68. Published 2008. Accessed February 5, 2017.
19. Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429-438.
20. Amarenco P, Lavallée PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542.
21. Joshi JK, Ouyang B, Prabhakaran S. Should TIA patients be hospitalized or referred to a same-day clinic? A decision analysis. Neurology. 2011;77:2082-2088.
22. Mijalski C, Silver B. TIA management: should TIA patients be admitted? should TIA patients get combination antiplatelet therapy? The Neurohospitalist. 2015;5:151-160.
23. Silver B, Adeoye O. Management of patients with transient ischemic attack in the emergency department. Neurology. 2016;86:1568-1569.
24. Demaerschalk BM, Kleindorfer DO, Adeoye OM, et al. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47:581-641.
25. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
26. Hemphill JC, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015;46:2032-2060.
CASE › A 68-year-old woman with hypertension and hyperlipidemia comes into your office for evaluation of a 30-minute episode of sudden-onset right-hand weakness and difficulty speaking that occurred 4 days earlier. The patient, who is also a smoker, has come in at the insistence of her daughter. On examination, her blood pressure (BP) is 145/88 mm Hg and her heart rate is 76 beats/minute and regular. She appears well and her language function is normal. The rest of her examination is normal. How would you proceed?
Stroke—the death of nerve cells due to a lack of blood supply from either infarction or hemorrhage—strikes nearly 800,000 people in the United States every year.1,2 Of these events, 130,000 are fatal, making stroke the fifth leading cause of death.3 Effective, early evaluation and cause-specific treatment are crucial parts of stroke care.
Research has helped to clarify the critical role primary care physicians play in recognizing, triaging, and managing stroke and transient ischemic attacks (TIA). This article reviews what we know about the different ways that a stroke and a TIA can present, the appropriate diagnostic work-up for patients presenting with symptoms of either event, and management strategies for subacute care (24 hours to up to 14 days after a stroke has occurred).4,5 Unless otherwise specified, this review will focus on ischemic stroke because 87% of strokes are attributable to ischemia.1
A follow-up to this article on secondary stroke prevention will appear in the journal next month.

Look to onset more than type of symptoms for clues
Stroke presents as a sudden onset of neurologic deficits (language, motor, sensory, cerebellar, or brainstem functions) (TABLE 14). Because presenting symptoms can vary widely, sudden onset, rather than particular symptoms, should raise a red flag for potential stroke.

The differential diagnosis includes: seizure, complex migraine, medication effect (eg, slurred speech or confusion after taking a central nervous system [CNS] depressant), toxin exposure, electrolyte abnormalities (especially hypoglycemia), concussion/trauma, infection of the CNS, peripheral vertigo, demyelination, intracranial mass, Bell’s palsy, and psychogenic disorders. The history and physical, along with laboratory findings and brain imaging (detailed later in this article), will guide the FP toward (or away from) these various etiologies.
Optimal triage is a subject of ongoing interest and research
If stroke or TIA remains a possibility after an initial assessment, it’s time to stratify patients by risk.
One of the most widely accepted tools is the ABCD2 score (see TABLE 26). Clinicians can employ the ABCD2 risk stratification tool when trying to determine whether it is reasonable to pursue an expedited work-up (ie, <1 day) in the outpatient setting or recommend that the patient be evaluated in an emergency department (ED). The 90-day stroke rate following a TIA ranges from 3% with an ABCD2 score of 0 to 3 to 18% with a score of 6 or 7. A score of 0 to 3 is considered relatively low risk; in the absence of other compelling factors, rapid outpatient evaluation is appropriate. For patients with an ABCD2 score ≥4, referral to the ED or direct admission to the hospital is advised.

The validity of the ABCD2 score for risk stratification has been studied extensively with conflicting results.7-10 As with any assessment tool, it should be used as a guide, and should not supplant a full assessment of the patient or the judgment of the examining physician. In making the decision regarding inpatient or outpatient evaluation, it’s also important to consider available resources, access to specialists, and patient preference.
In a 2016 population-based study, the 30-day recurrent stroke/TIA rate for patients hospitalized for TIA was 3% compared with 10.7% for those discharged from the ED with referral to a stroke clinic and 10.6% for those discharged from the ED without a referral to a stroke clinic.11 These data suggest that only patients for whom you have a low clinical suspicion of stroke/TIA should be worked up as outpatients, and that hospital admission is advised in moderate- and high-risk cases. The findings also highlight the critical role that primary care physicians can play in triaging and managing these patients for secondary prevention.
CASE › This patient’s recent history of sudden-onset right-sided weakness and expressive language dysfunction is suspicious for left hemispheric ischemia. She has several risk factors for stroke, and her ABCD2 score is 5 (hypertension, age ≥60 years, unilateral weakness, and duration 10-59 min), which places her at moderate risk. Thus, the recommendation would be to have her go directly to an ED for rapid evaluation.
The diagnostic work-up
Even when a patient is sent to the ED, the FP plays a critical role in his or her continuing care. FPs will often coordinate with inpatient care and manage transition of care to the outpatient setting. (And in many communities, the ED or hospital physicians may themselves be family practitioners.)
In terms of care, not even an aspirin should be administered in a case like this because the patient has not yet had any neuroimaging, and differentiation of ischemic from hemorrhagic stroke cannot be made on clinical grounds alone. Once an ischemic stroke is confirmed, determining the etiology is critical given the significant management differences between the different types of stroke (atherosclerotic, cardioembolic, lacunar, or other).
Which imaging method, and when?
While a computerized tomography (CT) scan is the preferred initial imaging strategy for acute stroke to discern the ischemic type from the hemorrhagic, MRI is preferred for the evaluation of acute ischemic stroke because the method has a higher sensitivity for infarction and a greater ability to identify findings (such as demyelination) that would suggest an alternative diagnosis.
In addition to evaluating the brain parenchyma, physicians must also assess the cerebral vasculature. CT angiography (CTA) or MR angiography (MRA) of the head and neck are preferred over carotid ultrasound because they are capable of evaluating the entire cerebrovascular system12,13 and can be instrumental in identifying potential causes of stroke, as well as guiding therapeutic decisions. Carotid ultrasound is a reasonable alternative for patients presenting with symptoms indicative of anterior circulation involvement when CTA and MRA are unavailable or contraindicated, but it will not identify intracranial vascular disease, proximal common carotid disease, or vertebrobasilar disease.
Getting to the cause of suspected stroke: Labs and other diagnostic tests
A routine work-up includes BP checks, routine labs (complete blood count, complete metabolic panel, coagulation profile, and troponin), an electrocardiogram (EKG), a transthoracic echocardiogram (TTE) with bubble study if possible, and a minimum of 24 to 48 hours of cardiac rhythm monitoring. Cardiac rhythm monitoring should be extended in the setting of clinical concern for unidentified paroxysmal atrial fibrillation, such as an embolism without a proximal vascular source, multiple embolic infarcts in different vascular territories, a dilated left atrium, or other risk factors for atrial fibrillation that include smoking, systolic hypertension, diabetes, and heart failure (see TABLE 312,13,17,18).14-16 This standard diagnostic work-up will identify the cause of stroke in 70% to 80% of patients.19

Additional investigations to consider if the etiology is not yet elucidated include a transesophageal echocardiogram (TEE), cerebral angiography, a coagulopathy evaluation, a lumbar puncture, and a vasculitis work-up. If available, consultation with a neurologist is appropriate for any patient who has had a stroke or TIA. Patients with unclear etiologies or for whom there are questions concerning strategies for preventing secondary stroke should be referred to Neurology and preferably a stroke specialist.
Timing matters, even when symptoms have resolved (ie, TIA).11,20 The EXPRESS trial17 (the Early use of eXisting PREventive Strategies for Stroke) looked at the effect of urgent assessment and treatment (≤1 day) of patients presenting with a TIA or minor stroke on the risk of recurrent stroke within 90 days. The diagnostic work-up included brain and vascular imaging together with an EKG. This intensive approach led to an absolute risk reduction of 8.2% (from 10.3% to 2.1%) in the risk of recurrent stroke at 90 days (number needed to treat [NNT]=12).17
Expedited work-up and treatment was also recently evaluated in a non-trial, real-world setting and was associated with reducing recurrent stroke by more than half the rate reported in older studies.20 Overall, the data suggest that evaluation within 24 hours confers substantial benefit, and that this evaluation can happen in an outpatient setting.21-23
Acute management: Use of tPA
Once imaging rules out intracranial hemorrhage, patients should be treated with tissue plasminogen activator (tPA) or an endovascular intervention as per guidelines.24 For patients with ischemic stroke ineligible for tPA or endovascular treatments, the initial focus is to determine the etiology of the symptoms so that the best strategies for prevention of secondary stroke may be employed.
Aspirin should be provided within 24 to 48 hours to all patients after intracranial hemorrhage is ruled out. Aspirin should be delayed for 24 hours in those given thrombolytics. The initial recommended dose of aspirin is 325 mg with continued low-dose (81 mg) aspirin daily.13 The addition of clopidogrel to aspirin within 24 hours of an event and continued for 21 days, followed by aspirin alone, was shown to be beneficial in a Chinese population with high-risk TIA (ABCD2 score ≥4) or minor stroke (National Institutes of Health Stroke Scale [NIHSS] ≤3).25 Anticoagulation with heparin, warfarin, or a novel oral anticoagulant is generally not indicated in the acute setting due to the risk of hemorrhagic transformation.
Acute BP management depends upon the type of stroke (ischemic or hemorrhagic), eligibility for thrombolytics, timing of presentation, and possible comorbidities such as myocardial infarction or aortic dissection (see TABLE 413,26). In the absence of contraindications, high-intensity statins should be initiated in all patients able to take oral medications.

CASE › You appropriately referred your patient to the local ED. A head CT with head and neck CTA was performed. While the head CT did not show any abnormalities, the CTA demonstrated high-grade left internal carotid artery stenosis. The patient was given an initial dose of aspirin 325 mg and a high-intensity statin and admitted for further management. An MRI revealed a small shower of emboli in the left hemisphere, confirming the diagnosis of stroke over TIA. Labs were marginally remarkable with a low-density lipoprotein level of 115 mg/dL and an HbA1c of 6.2. Telemetry monitoring did not reveal any arrhythmias, and TTE was normal. BP remained in the high-normal to low-hypertensive range.
A Vascular Surgery consultation was obtained and the patient underwent a left carotid endarterectomy the following day. She did well without surgical complications. Her BP medications were adjusted; a combination of an angiotensin-converting enzyme inhibitor and a thiazide diuretic achieved a goal BP <140/90 mm Hg.
Permissive hypertension was not indicated due to her presentation >48 hours beyond the acute event. Low-dose aspirin and a high-intensity statin were continued, for secondary stroke prevention in the setting of atherosclerotic disease. She received smoking cessation counseling, which will continue.
CORRESPONDENCE
Stephen A. Martin, MD, EdM, Barre Family Health Center, 151 Worcester Road, Barre, MA 01005; [email protected].
CASE › A 68-year-old woman with hypertension and hyperlipidemia comes into your office for evaluation of a 30-minute episode of sudden-onset right-hand weakness and difficulty speaking that occurred 4 days earlier. The patient, who is also a smoker, has come in at the insistence of her daughter. On examination, her blood pressure (BP) is 145/88 mm Hg and her heart rate is 76 beats/minute and regular. She appears well and her language function is normal. The rest of her examination is normal. How would you proceed?
Stroke—the death of nerve cells due to a lack of blood supply from either infarction or hemorrhage—strikes nearly 800,000 people in the United States every year.1,2 Of these events, 130,000 are fatal, making stroke the fifth leading cause of death.3 Effective, early evaluation and cause-specific treatment are crucial parts of stroke care.
Research has helped to clarify the critical role primary care physicians play in recognizing, triaging, and managing stroke and transient ischemic attacks (TIA). This article reviews what we know about the different ways that a stroke and a TIA can present, the appropriate diagnostic work-up for patients presenting with symptoms of either event, and management strategies for subacute care (24 hours to up to 14 days after a stroke has occurred).4,5 Unless otherwise specified, this review will focus on ischemic stroke because 87% of strokes are attributable to ischemia.1
A follow-up to this article on secondary stroke prevention will appear in the journal next month.

Look to onset more than type of symptoms for clues
Stroke presents as a sudden onset of neurologic deficits (language, motor, sensory, cerebellar, or brainstem functions) (TABLE 14). Because presenting symptoms can vary widely, sudden onset, rather than particular symptoms, should raise a red flag for potential stroke.

The differential diagnosis includes: seizure, complex migraine, medication effect (eg, slurred speech or confusion after taking a central nervous system [CNS] depressant), toxin exposure, electrolyte abnormalities (especially hypoglycemia), concussion/trauma, infection of the CNS, peripheral vertigo, demyelination, intracranial mass, Bell’s palsy, and psychogenic disorders. The history and physical, along with laboratory findings and brain imaging (detailed later in this article), will guide the FP toward (or away from) these various etiologies.
Optimal triage is a subject of ongoing interest and research
If stroke or TIA remains a possibility after an initial assessment, it’s time to stratify patients by risk.
One of the most widely accepted tools is the ABCD2 score (see TABLE 26). Clinicians can employ the ABCD2 risk stratification tool when trying to determine whether it is reasonable to pursue an expedited work-up (ie, <1 day) in the outpatient setting or recommend that the patient be evaluated in an emergency department (ED). The 90-day stroke rate following a TIA ranges from 3% with an ABCD2 score of 0 to 3 to 18% with a score of 6 or 7. A score of 0 to 3 is considered relatively low risk; in the absence of other compelling factors, rapid outpatient evaluation is appropriate. For patients with an ABCD2 score ≥4, referral to the ED or direct admission to the hospital is advised.

The validity of the ABCD2 score for risk stratification has been studied extensively with conflicting results.7-10 As with any assessment tool, it should be used as a guide, and should not supplant a full assessment of the patient or the judgment of the examining physician. In making the decision regarding inpatient or outpatient evaluation, it’s also important to consider available resources, access to specialists, and patient preference.
In a 2016 population-based study, the 30-day recurrent stroke/TIA rate for patients hospitalized for TIA was 3% compared with 10.7% for those discharged from the ED with referral to a stroke clinic and 10.6% for those discharged from the ED without a referral to a stroke clinic.11 These data suggest that only patients for whom you have a low clinical suspicion of stroke/TIA should be worked up as outpatients, and that hospital admission is advised in moderate- and high-risk cases. The findings also highlight the critical role that primary care physicians can play in triaging and managing these patients for secondary prevention.
CASE › This patient’s recent history of sudden-onset right-sided weakness and expressive language dysfunction is suspicious for left hemispheric ischemia. She has several risk factors for stroke, and her ABCD2 score is 5 (hypertension, age ≥60 years, unilateral weakness, and duration 10-59 min), which places her at moderate risk. Thus, the recommendation would be to have her go directly to an ED for rapid evaluation.
The diagnostic work-up
Even when a patient is sent to the ED, the FP plays a critical role in his or her continuing care. FPs will often coordinate with inpatient care and manage transition of care to the outpatient setting. (And in many communities, the ED or hospital physicians may themselves be family practitioners.)
In terms of care, not even an aspirin should be administered in a case like this because the patient has not yet had any neuroimaging, and differentiation of ischemic from hemorrhagic stroke cannot be made on clinical grounds alone. Once an ischemic stroke is confirmed, determining the etiology is critical given the significant management differences between the different types of stroke (atherosclerotic, cardioembolic, lacunar, or other).
Which imaging method, and when?
While a computerized tomography (CT) scan is the preferred initial imaging strategy for acute stroke to discern the ischemic type from the hemorrhagic, MRI is preferred for the evaluation of acute ischemic stroke because the method has a higher sensitivity for infarction and a greater ability to identify findings (such as demyelination) that would suggest an alternative diagnosis.
In addition to evaluating the brain parenchyma, physicians must also assess the cerebral vasculature. CT angiography (CTA) or MR angiography (MRA) of the head and neck are preferred over carotid ultrasound because they are capable of evaluating the entire cerebrovascular system12,13 and can be instrumental in identifying potential causes of stroke, as well as guiding therapeutic decisions. Carotid ultrasound is a reasonable alternative for patients presenting with symptoms indicative of anterior circulation involvement when CTA and MRA are unavailable or contraindicated, but it will not identify intracranial vascular disease, proximal common carotid disease, or vertebrobasilar disease.
Getting to the cause of suspected stroke: Labs and other diagnostic tests
A routine work-up includes BP checks, routine labs (complete blood count, complete metabolic panel, coagulation profile, and troponin), an electrocardiogram (EKG), a transthoracic echocardiogram (TTE) with bubble study if possible, and a minimum of 24 to 48 hours of cardiac rhythm monitoring. Cardiac rhythm monitoring should be extended in the setting of clinical concern for unidentified paroxysmal atrial fibrillation, such as an embolism without a proximal vascular source, multiple embolic infarcts in different vascular territories, a dilated left atrium, or other risk factors for atrial fibrillation that include smoking, systolic hypertension, diabetes, and heart failure (see TABLE 312,13,17,18).14-16 This standard diagnostic work-up will identify the cause of stroke in 70% to 80% of patients.19

Additional investigations to consider if the etiology is not yet elucidated include a transesophageal echocardiogram (TEE), cerebral angiography, a coagulopathy evaluation, a lumbar puncture, and a vasculitis work-up. If available, consultation with a neurologist is appropriate for any patient who has had a stroke or TIA. Patients with unclear etiologies or for whom there are questions concerning strategies for preventing secondary stroke should be referred to Neurology and preferably a stroke specialist.
Timing matters, even when symptoms have resolved (ie, TIA).11,20 The EXPRESS trial17 (the Early use of eXisting PREventive Strategies for Stroke) looked at the effect of urgent assessment and treatment (≤1 day) of patients presenting with a TIA or minor stroke on the risk of recurrent stroke within 90 days. The diagnostic work-up included brain and vascular imaging together with an EKG. This intensive approach led to an absolute risk reduction of 8.2% (from 10.3% to 2.1%) in the risk of recurrent stroke at 90 days (number needed to treat [NNT]=12).17
Expedited work-up and treatment was also recently evaluated in a non-trial, real-world setting and was associated with reducing recurrent stroke by more than half the rate reported in older studies.20 Overall, the data suggest that evaluation within 24 hours confers substantial benefit, and that this evaluation can happen in an outpatient setting.21-23
Acute management: Use of tPA
Once imaging rules out intracranial hemorrhage, patients should be treated with tissue plasminogen activator (tPA) or an endovascular intervention as per guidelines.24 For patients with ischemic stroke ineligible for tPA or endovascular treatments, the initial focus is to determine the etiology of the symptoms so that the best strategies for prevention of secondary stroke may be employed.
Aspirin should be provided within 24 to 48 hours to all patients after intracranial hemorrhage is ruled out. Aspirin should be delayed for 24 hours in those given thrombolytics. The initial recommended dose of aspirin is 325 mg with continued low-dose (81 mg) aspirin daily.13 The addition of clopidogrel to aspirin within 24 hours of an event and continued for 21 days, followed by aspirin alone, was shown to be beneficial in a Chinese population with high-risk TIA (ABCD2 score ≥4) or minor stroke (National Institutes of Health Stroke Scale [NIHSS] ≤3).25 Anticoagulation with heparin, warfarin, or a novel oral anticoagulant is generally not indicated in the acute setting due to the risk of hemorrhagic transformation.
Acute BP management depends upon the type of stroke (ischemic or hemorrhagic), eligibility for thrombolytics, timing of presentation, and possible comorbidities such as myocardial infarction or aortic dissection (see TABLE 413,26). In the absence of contraindications, high-intensity statins should be initiated in all patients able to take oral medications.

CASE › You appropriately referred your patient to the local ED. A head CT with head and neck CTA was performed. While the head CT did not show any abnormalities, the CTA demonstrated high-grade left internal carotid artery stenosis. The patient was given an initial dose of aspirin 325 mg and a high-intensity statin and admitted for further management. An MRI revealed a small shower of emboli in the left hemisphere, confirming the diagnosis of stroke over TIA. Labs were marginally remarkable with a low-density lipoprotein level of 115 mg/dL and an HbA1c of 6.2. Telemetry monitoring did not reveal any arrhythmias, and TTE was normal. BP remained in the high-normal to low-hypertensive range.
A Vascular Surgery consultation was obtained and the patient underwent a left carotid endarterectomy the following day. She did well without surgical complications. Her BP medications were adjusted; a combination of an angiotensin-converting enzyme inhibitor and a thiazide diuretic achieved a goal BP <140/90 mm Hg.
Permissive hypertension was not indicated due to her presentation >48 hours beyond the acute event. Low-dose aspirin and a high-intensity statin were continued, for secondary stroke prevention in the setting of atherosclerotic disease. She received smoking cessation counseling, which will continue.
CORRESPONDENCE
Stephen A. Martin, MD, EdM, Barre Family Health Center, 151 Worcester Road, Barre, MA 01005; [email protected].
1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146-e603.
2. Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064-2089.
3. Kochanek KD, Murphy SL, Xu J, et al. Mortality in the United States, 2013. NCHS Data Brief. 2014:1-8. Available at: https://www.cdc.gov/nchs/data/databriefs/db178.pdf. Accessed June 5, 2016.
4. Flossmann E, Redgrave JN, Briley D, et al. Reliability of clinical diagnosis of the symptomatic vascular territory in patients with recent transient ischemic attack or minor stroke. Stroke. 2008;39:2457-2460.
5. Josephson SA, Sidney S, Pham TN, et al. Higher ABCD2 score predicts patients most likely to have true transient ischemic attack. Stroke. 2008;39:3096-3098. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18688003. Accessed June 5, 2016.
6. Hankey GJ. The ABCD, California, and unified ABCD2 risk scores predicted stroke within 2, 7, and 90 days after TIA. Evid Based Med. 2007;12:88.
7. Sheehan OC, Kyne L, Kelly LA, et al. Population-based study of ABCD2 score, carotid stenosis, and atrial fibrillation for early stroke prediction after transient ischemic attack: the North Dublin TIA study. Stroke. 2010;41:844-850.
8. Rothwell PM, Giles MF, Flossmann E, et al. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet. 2005; 366:29-36.
9. Tsivgoulis G, Spengos K, Manta P, et al. Validation of the ABCD score in identifying individuals at high early risk of stroke after a transient ischemic attack: a hospital-based case series study. Stroke. 2006;37:2892-2897.
10. Kiyohara T, Kamouchi M, Kumai Y, et al. ABCD3 and ABCD3-I scores are superior to ABCD2 score in the prediction of short- and long-term risks of stroke after transient ischemic attack. Stroke. 2014;45:418-425.
11. Sacco RL, Rundek T. The value of urgent specialized care for TIA and minor stroke. N Engl J Med. 2016;374:1577-1579.
12. Demchuk AM, Menon BK, Goyal M. Comparing vessel imaging: noncontrast computed tomography/computed tomographic angiography should be the new minimum standard in acute disabling stroke. Stroke. 2016;47:273-281.
13. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
14. Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467-2477.
15. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478-2486.
16. Christophersen IE, Yin X, Larson MG, et al. A comparison of the CHARGE-AF and the CHA2DS2-VASc risk scores for prediction of atrial fibrillation ni the Framingham Heart Study. Am Heart J. 2016;178:45-54.
17. Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370:1432-1442.
18. National Institute for Health and Care Excellence. Stroke and transient ischaemic attack in over 16s: diagnosis and initial management. Available at: https://www.nice.org.uk/guidance/cg68. Published 2008. Accessed February 5, 2017.
19. Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429-438.
20. Amarenco P, Lavallée PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542.
21. Joshi JK, Ouyang B, Prabhakaran S. Should TIA patients be hospitalized or referred to a same-day clinic? A decision analysis. Neurology. 2011;77:2082-2088.
22. Mijalski C, Silver B. TIA management: should TIA patients be admitted? should TIA patients get combination antiplatelet therapy? The Neurohospitalist. 2015;5:151-160.
23. Silver B, Adeoye O. Management of patients with transient ischemic attack in the emergency department. Neurology. 2016;86:1568-1569.
24. Demaerschalk BM, Kleindorfer DO, Adeoye OM, et al. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47:581-641.
25. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
26. Hemphill JC, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015;46:2032-2060.
1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146-e603.
2. Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064-2089.
3. Kochanek KD, Murphy SL, Xu J, et al. Mortality in the United States, 2013. NCHS Data Brief. 2014:1-8. Available at: https://www.cdc.gov/nchs/data/databriefs/db178.pdf. Accessed June 5, 2016.
4. Flossmann E, Redgrave JN, Briley D, et al. Reliability of clinical diagnosis of the symptomatic vascular territory in patients with recent transient ischemic attack or minor stroke. Stroke. 2008;39:2457-2460.
5. Josephson SA, Sidney S, Pham TN, et al. Higher ABCD2 score predicts patients most likely to have true transient ischemic attack. Stroke. 2008;39:3096-3098. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18688003. Accessed June 5, 2016.
6. Hankey GJ. The ABCD, California, and unified ABCD2 risk scores predicted stroke within 2, 7, and 90 days after TIA. Evid Based Med. 2007;12:88.
7. Sheehan OC, Kyne L, Kelly LA, et al. Population-based study of ABCD2 score, carotid stenosis, and atrial fibrillation for early stroke prediction after transient ischemic attack: the North Dublin TIA study. Stroke. 2010;41:844-850.
8. Rothwell PM, Giles MF, Flossmann E, et al. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet. 2005; 366:29-36.
9. Tsivgoulis G, Spengos K, Manta P, et al. Validation of the ABCD score in identifying individuals at high early risk of stroke after a transient ischemic attack: a hospital-based case series study. Stroke. 2006;37:2892-2897.
10. Kiyohara T, Kamouchi M, Kumai Y, et al. ABCD3 and ABCD3-I scores are superior to ABCD2 score in the prediction of short- and long-term risks of stroke after transient ischemic attack. Stroke. 2014;45:418-425.
11. Sacco RL, Rundek T. The value of urgent specialized care for TIA and minor stroke. N Engl J Med. 2016;374:1577-1579.
12. Demchuk AM, Menon BK, Goyal M. Comparing vessel imaging: noncontrast computed tomography/computed tomographic angiography should be the new minimum standard in acute disabling stroke. Stroke. 2016;47:273-281.
13. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870-947.
14. Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467-2477.
15. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478-2486.
16. Christophersen IE, Yin X, Larson MG, et al. A comparison of the CHARGE-AF and the CHA2DS2-VASc risk scores for prediction of atrial fibrillation ni the Framingham Heart Study. Am Heart J. 2016;178:45-54.
17. Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370:1432-1442.
18. National Institute for Health and Care Excellence. Stroke and transient ischaemic attack in over 16s: diagnosis and initial management. Available at: https://www.nice.org.uk/guidance/cg68. Published 2008. Accessed February 5, 2017.
19. Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429-438.
20. Amarenco P, Lavallée PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542.
21. Joshi JK, Ouyang B, Prabhakaran S. Should TIA patients be hospitalized or referred to a same-day clinic? A decision analysis. Neurology. 2011;77:2082-2088.
22. Mijalski C, Silver B. TIA management: should TIA patients be admitted? should TIA patients get combination antiplatelet therapy? The Neurohospitalist. 2015;5:151-160.
23. Silver B, Adeoye O. Management of patients with transient ischemic attack in the emergency department. Neurology. 2016;86:1568-1569.
24. Demaerschalk BM, Kleindorfer DO, Adeoye OM, et al. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47:581-641.
25. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
26. Hemphill JC, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015;46:2032-2060.
PRACTICE RECOMMENDATIONS
› Perform an urgent work-up on patients presenting with symptoms of a transient ischemic attack or stroke. A
› Employ the ABCD2 risk stratification tool when determining whether it is reasonable to pursue an expedited work-up in the outpatient setting or recommend that a patient be evaluated in an emergency department. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series