User login
How to identify balance disorders and reduce fall risk
CASE Mr. J, a 75-year-old man, presents to your family practice reporting that he feels increasingly unsteady and slow while walking. He fell twice last year, without resulting injury. He now worries about tripping while walking around the house and relies on his spouse to run errands.
Clearly, Mr. J is experiencing a problem with balance. What management approach should you undertake to prevent him from falling?

Balance disorders are common in older people and drastically hinder quality of life.1-4 Patients often describe imbalance as vague symptoms: dizziness, unsteadiness, faintness, spinning sensations.5,6 Importantly, balance disorders disrupt normal gait and contribute to falls that are a major cause of disability and morbidity in older people. Almost 30% of people older than 65 years report 1 or more falls annually.7 Factors that increase the risk of falls include impaired mobility, previously reported falls, reduced psychological functioning, chronic medical conditions, and polypharmacy.7,8
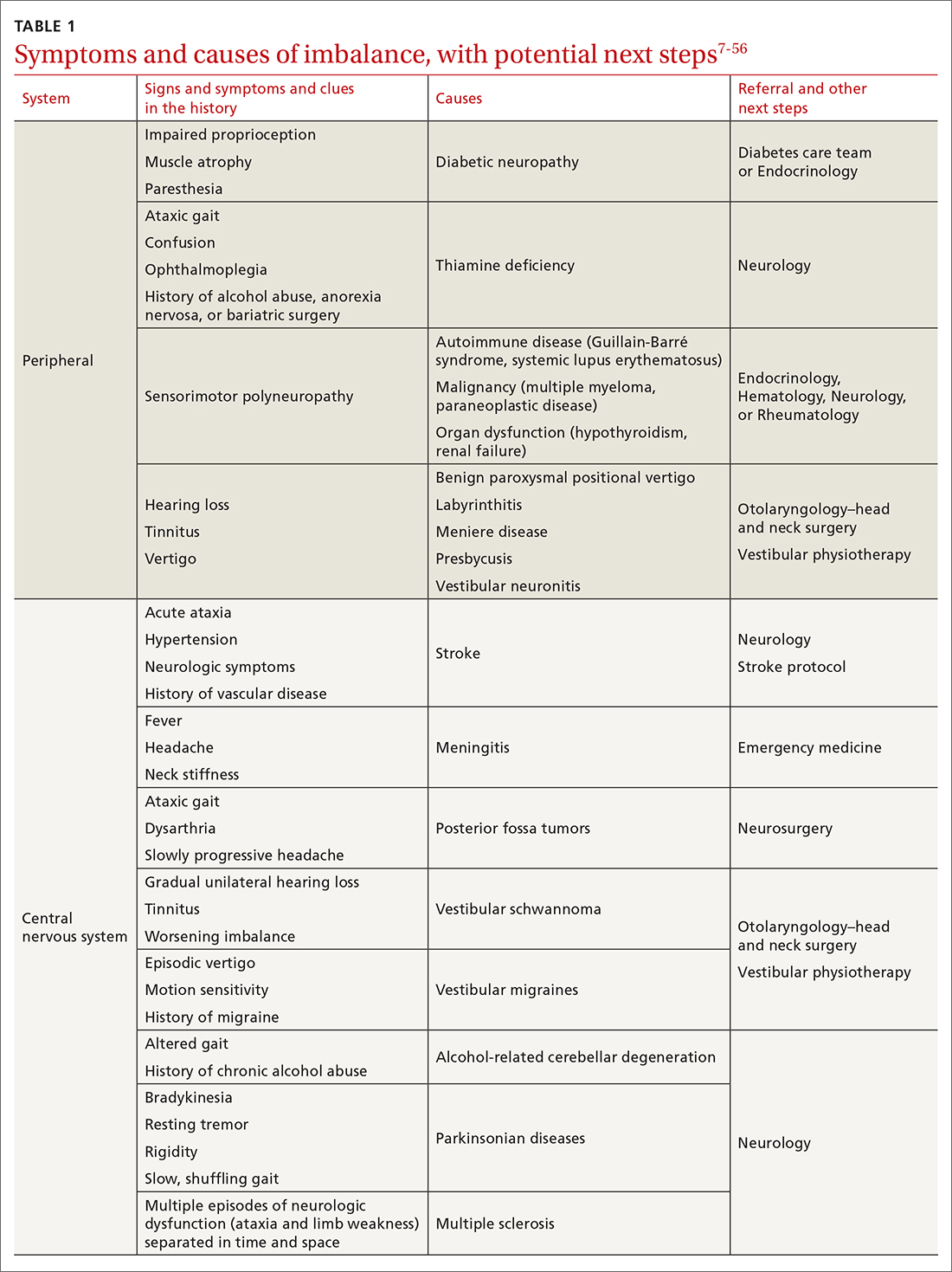
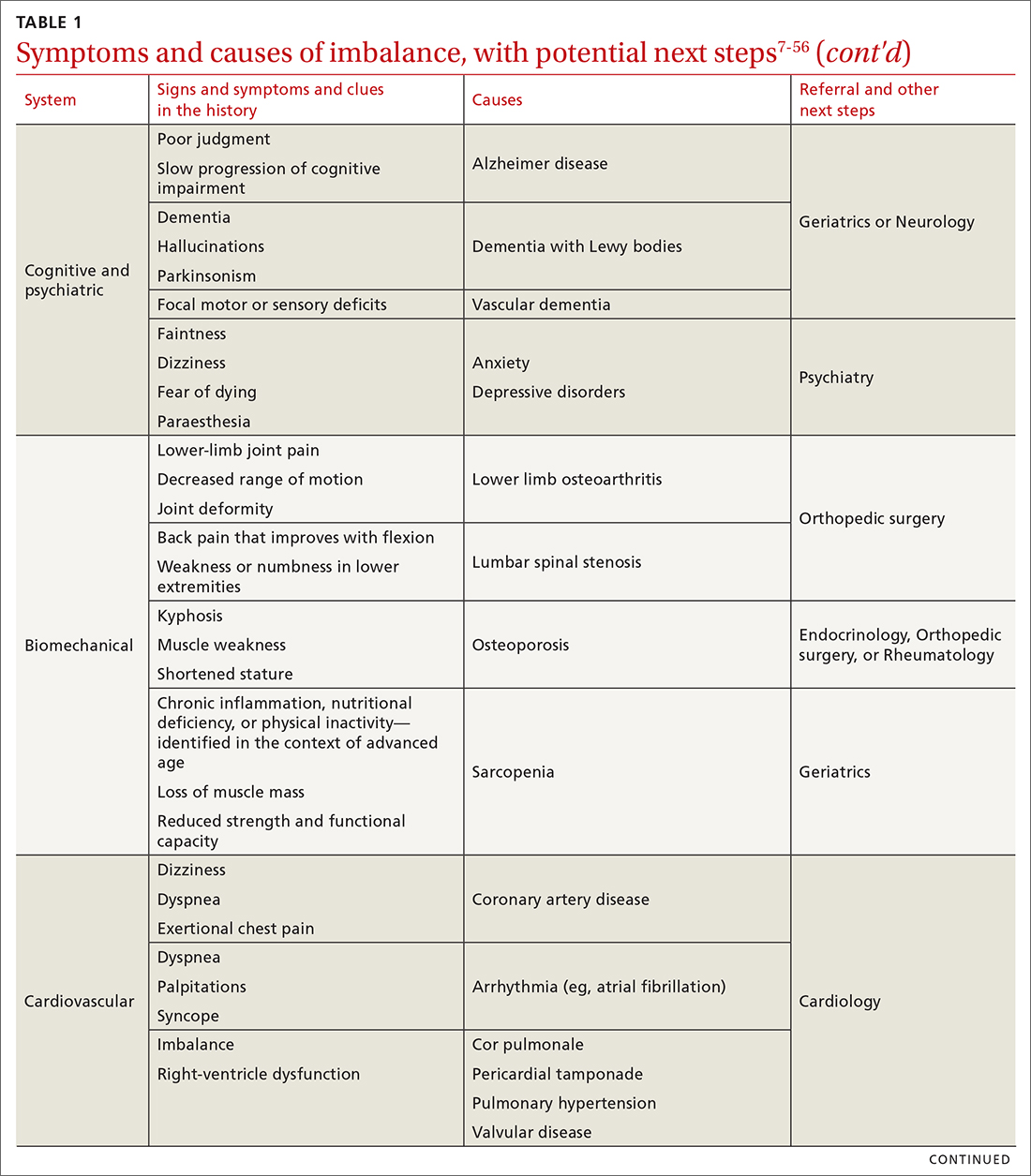
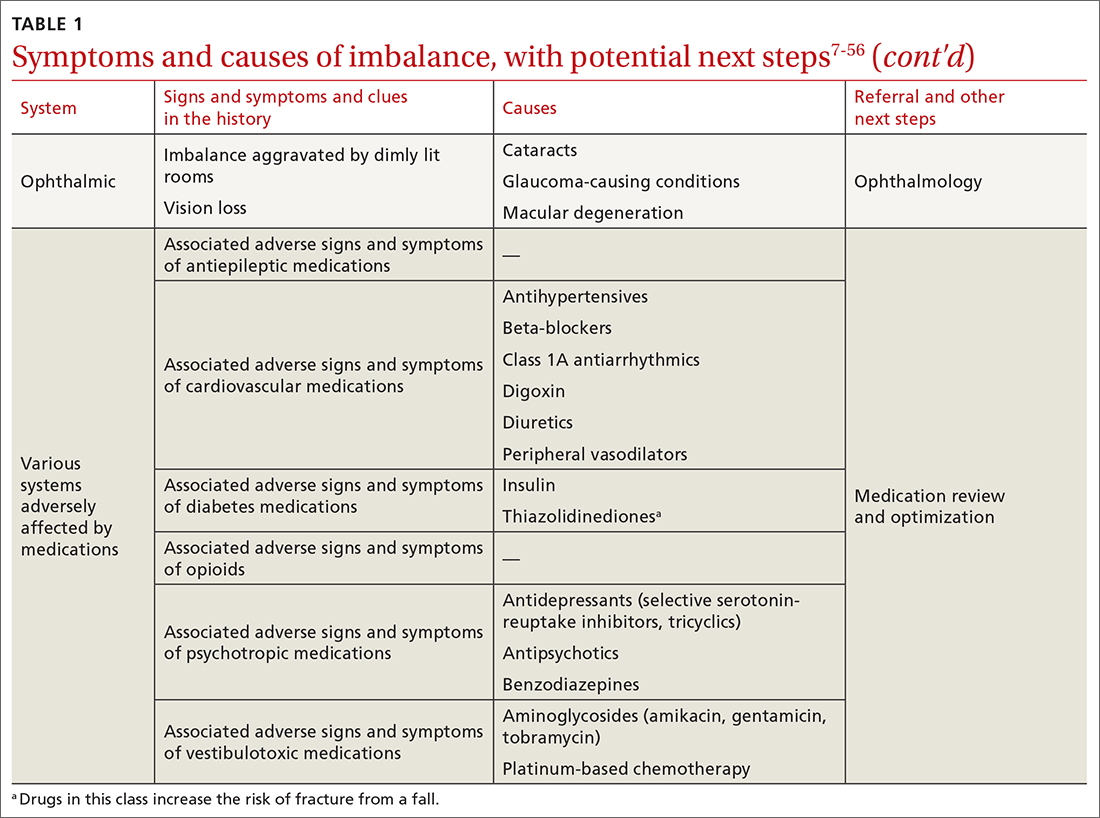
The cause of any single case of imbalance is often multifactorial, resulting from dysfunction of multiple body systems (TABLE 17-56); in our clinical experience, most patients with imbalance and who are at risk of falls do not have a detectable deficit of the vestibular system. These alterations in function arise in 3 key systems—vision, proprioception, and vestibular function—which signal to, and are incorporated by, the cerebellum to mediate balance. Cognitive and neurologic decline are also factors in imbalance.
Considering that 20% of falls result in serious injury in older populations, it is important to identify balance disorders and implement preventive strategies to mitigate harmful consequences of falls on patients’ health and independence.7,57 In this article, we answer the question that the case presentation raises about the proper management approach to imbalance in family practice, including assessment of risk and rehabilitation strategies to reduce the risk of falls. Our insights and recommendations are based on our clinical experience and a review of the medical literature from the past 40 years.
CASE Mr. J has a history of hypertension, age-related hearing loss, and osteoarthritis of the knees; he has not had surgery for the arthritis. His medications are antihypertensives and extra-strength acetaminophen for knee pain.
Making the diagnosis of a balance disorder
History
A thorough clinical history, often including a collateral history from caregivers, narrows the differential diagnosis. Information regarding onset, duration, timing, character, and previous episodes of imbalance is essential. Symptoms of imbalance are often challenging for the patient to describe: They might use terms such as vertigo or dizziness, when, in fact, on further questioning, they are describing balance difficulties. Inquiry into (1) their use of assistive walking devices and (2) development or exacerbation of neurologic, musculoskeletal, auditory, visual, and mood symptoms is necessary. Note the current level of their mobility, episodes of pain or fatigue, previous falls and associated injuries, fear of falling, balance confidence, and sensations that precede falls.58
Continue to: The medical and surgical histories
The medical and surgical histories are key pieces of information. The history of smoking, alcohol habits, and substance use is relevant.
A robust medication history is essential to evaluate a patient’s risk of falling. Polypharmacy—typically, defined as taking 4 or more medications—has been repeatedly associated with a heightened risk of falls.53,59-61 Moreover, a dose-dependent association between polypharmacy and hospitalization following falls has been identified, and demonstrates that taking 10 or more medications greatly increases the risk of hospitalization.59 Studies of polypharmacy cement the importance of inquiring about medication use when assessing imbalance, particularly in older patients.
Physical examination
A focused and detailed physical examination provides insight into systems that should be investigated:
- Obtain vital signs, including orthostatic vitals to test for orthostatic hypotension62; keep in mind that symptoms of orthostatic dizziness can occur without orthostatic hypotension.
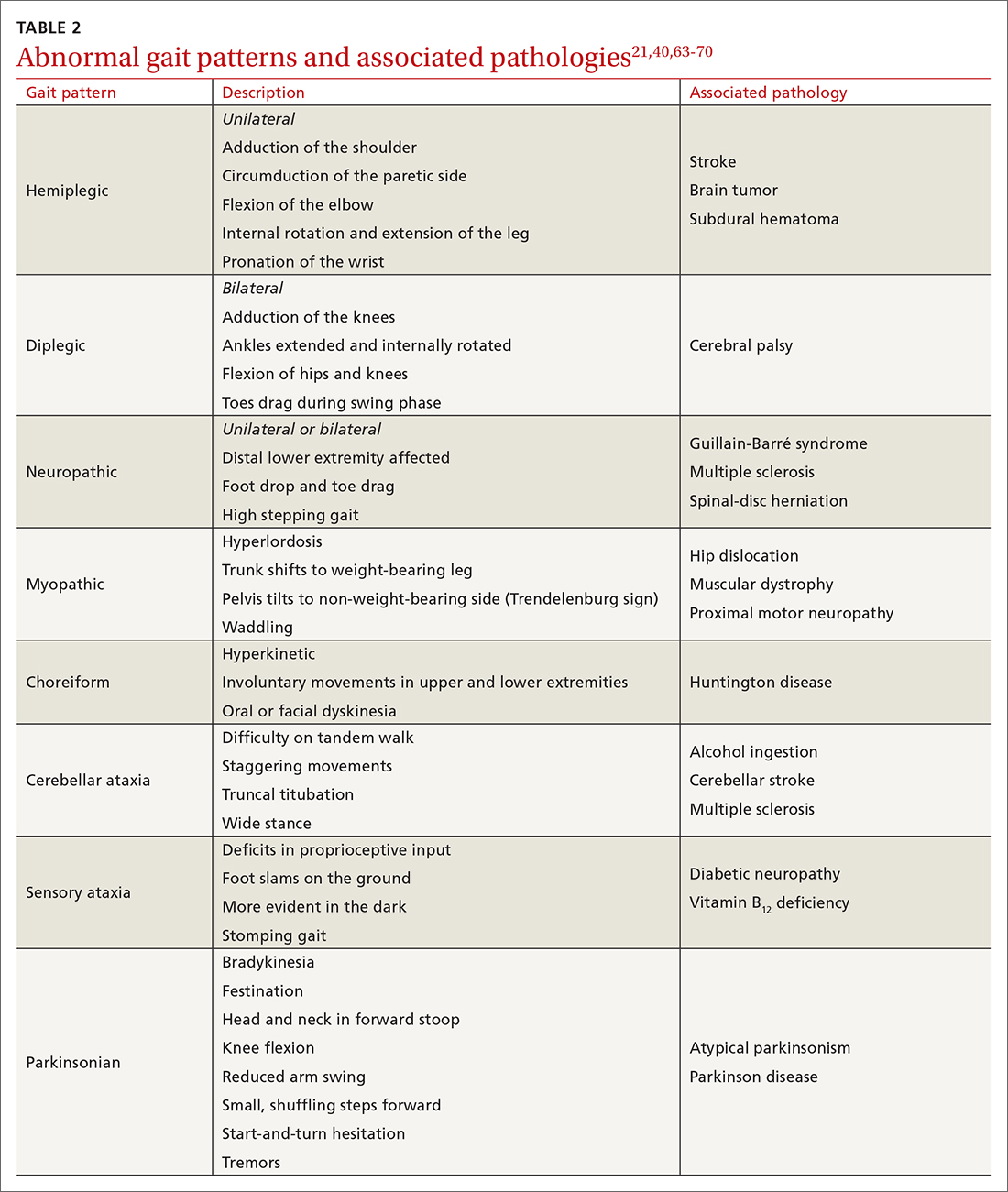
- Examine gait, which can distinguish between causes of imbalance (TABLE 2).21,40,63-70
- Perform a cardiac examination.
- Assess visual acuity and visual fields; test for nystagmus and identify any optic-nerve and retinal abnormalities.
- Evaluate lower-limb sensation, proprioception, and motor function.
- Evaluate suspected vestibular dysfunction, including dysfunction with positional testing (the Dix-Hallpike maneuver71). The patient is taken from sitting to supine while the head is rotated 45° to the tested side by the examiner. As the patient moves into a supine position, the neck is extended 30° off the table and held for at least 30 seconds. The maneuver is positive if torsional nystagmus is noted while the head is held rotated during neck extension. The maneuver is negative if the patient reports dizziness, vertigo, unsteadiness, or “pressure in the head.” Torsional nystagmus must be present to confirm a diagnosis of benign paroxysmal positional vertigo.
- If you suspect a central nervous system cause of imbalance, assess the cranial nerves, coordination, strength, and, of course, balance.
CASE
Mr. J’s physical examination showed normal vital signs without significant postural changes in blood pressure. Gait analysis revealed a slowed gait, with reduced range of motion in both knees over the entire gait cycle. Audiometry revealed symmetric moderate sensorineural hearing loss characteristic of presbycusis.
Diagnostic investigations
Consider focused investigations into imbalance based on the history and physical examination. We discourage overly broad testing and imaging; in primary care, cost and limited access to technology can bar robust investigations into causes of imbalance. However, identification of acute pathologies should prompt immediate referral to the emergency department. Furthermore, specific symptoms (TABLE 17-56) should prompt referral to specialists for assessment.
Continue to: In the emergency department...
In the emergency department and academic hospitals, key investigations can identify causes of imbalance:
- Electrocardiography and Holter monitoring test for cardiac arrhythmias.
- Echocardiography identifies structural abnormalities.
- Radiography and computed tomography are useful for detecting musculoskeletal abnormalities.
- Bone densitometry can identify osteoporosis.
- Head and spinal cord magnetic resonance imaging can be used to identify lesions of the central nervous system.
- Computed tomographic angiography of the head and neck is useful for identifying stroke, cerebral atrophy, and stenotic lesions of the carotid and vertebral arteries.
- Nerve conduction studies and levels of serum vitamin B12, hemoglobin A1C, thyroid-stimulating hormone, and random cortisol can uncover causes of peripheral neuropathy.
- Bedside cognitive screening tests can be used to measure cognitive decline.72
- Suspicion of vestibular disease requires audiometry and vestibular testing, including videonystagmography, head impulse testing, and vestibular evoked myogenic potentials.
In many cases of imbalance, no specific underlying correctable cause is discovered.
Management of imbalance
Pharmacotherapy
Targeted pharmacotherapy can be utilized in select clinical scenarios:
- Medical treatment of peripheral neuropathy should target the underlying condition.
- Cognitive behavioral therapy and antidepressants are useful for treating anxiety and depressive disorders.73
- Musculoskeletal pain can be managed with acetaminophen and topical nonsteroidal anti-inflammatory drugs (NSAIDs), using a short course of an oral NSAID when needed.74
- Cardiovascular disease management might include any of several classes of pharmacotherapy, including antiplatelet and lipid-lowering medications, antiarrhythmic drugs, and antihypertensive agents.
- Acute episodes of vertigo due to vestibular neuritis or labyrinthitis can be managed with an antiemetic.46
Surgical treatment
Surgery is infrequently considered for patients with imbalance. Examples of indications include microsurgical resection of vestibular schwannoma, resection of central nervous system tumors, lens replacement surgery for cataract, surgical management of severe spinal fracture, and hip or knee arthroplasty in select patients.
Tools for assessing the risk of falls
Scoring systems called falls risk assessment tools, or FRAT, have been developed to gauge a patient’s risk of falling. The various FRATs differ in specificity and sensitivity for predicting the risk of falls, and are typically designed for specific clinical environments, such as hospital inpatient care or long-term care facilities. Specifically, FRATs attempt to classify risk using sets of risk factors known to be associated with falls.
Continue to: Research abounds into...
Research abounds into the validity of commonly used FRATs across institutions, patient populations, and clinical environments:
The Johns Hopkins FRATa determines risk using metrics such as age, fall history, incontinence, cognition, mobility, and medications75; it is predominantly used for assessment in hospital inpatient units. This tool has been validated repeatedly.76,77
Peninsula Health FRATb stratifies patients in subacute and residential aged-care settings, based on risk factors that include recent falls, medications, psychological status, and cognition.78
FRAT-upc is a web-based tool that generates falls risk using risk factors that users input. This tool has been studied in the context of patients older than 65 years living in the community.79
Although FRATs are reasonably useful for predicting falls, their utility varies by patient population and clinical context. Moreover, it has been suggested that FRATs neglect environmental and personal factors when assessing risk by focusing primarily on bodily factors.80 Implementing a FRAT requires extensive consideration of the target population and should be accompanied by clinical judgment that is grounded in an individual patient’s circumstances.81
Continue to: Preventing falls in primary care
Preventing falls in primary care
An approach to preventing falls includes the development of individualized programs that account for frailty, a syndrome of physiologic decline associated with aging. Because frailty leads to diminished balance and mobility, a patient’s frailty index—determined using the 5 frailty phenotype criteria (exhaustion, weight loss, low physical activity, weakness, slowness)82 or the Canadian Study of Health and Aging Clinical Frailty Scale83—is a useful tool for predicting falls risk and readmission for falls following trauma-related injury. Prevention of falls in communities is critical for reducing mortality and allowing older people to maintain their independence and quality of life.
Exercise. In some areas, exercise and falls prevention programs are accessible to seniors.84 Community exercise programs that focus on balance retraining and muscle strengthening can reduce the risk of falls.73,85 The Choosing Wisely initiative of the ABIM [American Board of Internal Medicine] Foundation recommends that exercise programs be designed around an accurate functional baseline of the patient to avoid underdosed strength training.54
Multifactorial risk assessment in high-risk patients can reduce the rate of falls. Such an assessment includes examination of orthostatic blood pressure, vision and hearing, bone health, gait, activities of daily living, cognition, and environmental hazards, and enables provision of necessary interventions.73,86 Hearing amplification, specifically, correlates with enhanced postural control, slowed cognitive decline, and a reduced likelihood of falls.87-93 The mechanism behind improved balance performance might be reduced cognitive load through supporting a patient’s listening needs.88-90
Pharmacotherapy. Optimizing medications and performing a complete medication review before prescribing new medications is highly recommended to avoid unnecessary polypharmacy7,8,18,53-56 (TABLE 17-56).
Management of comorbidities associated with a higher risk of falls, including arthritis, cancer, stroke, diabetes, depression, kidney disease, chronic obstructive pulmonary disease, cognitive impairment, hypertension, and atrial fibrillation, is essential.94-96
Continue to: Home safety interventions
Home safety interventions, through occupational therapy, are important. These include removing unsafe mats and step-overs and installing nonslip strips on stairs, double-sided tape under mats, and handrails.73-97
Screening for risk of falls. The Centers for Disease Control and Prevention recommends that (1) all patients older than 65 years and (2) any patient presenting with an acute fall undergo screening for their risk of falls.98 When a patient is identified as at risk of falling, you can, when appropriate, assess modifiable risk factors and facilitate interventions.98 This strategy is supported by a 2018 statement from the US Preventive Services Task Force99 that recommends identifying high-risk patients who have:
- a history of falling
- a balance disturbance that causes a deficit of mobility or function
- poor performance on clinical tests, such as the 3-meter Timed Up and Go (TUG) assessment (www.cdc.gov/steadi/pdf/TUG_test-print.pdf).
An increased risk of falls should prompt you to refer the patient to community programs and physiotherapy in accordance with the individual’s personal goals99; a balance and vestibular physiotherapist is ideally positioned to accurately assess and manage patients at risk of falls. Specifically, the Task Force identified exercise programs and multifactorial interventions as being beneficial in preventing falls in high-risk older people.99
Balance assessment and rehabilitation in specialty centers
An individualized rehabilitation program aims to restore safe mobility by testing and addressing specific balance deficits, improving functional balance, and increasing balance confidence. Collaboration with colleagues from physiotherapy and occupational therapy aids in tailoring individualized programs.
Many tests are available to assess balance, determine the risk of falls, and guide rehabilitation:
- The timed 10-meter walk testd and the TUG test are simple assessments that measure functional mobility; both have normalized values for the risk of falls. A TUG time of ≥ 12 seconds suggests a high risk of falls.
- The 30-second chair stande evaluates functional lower-extremity strength in older patients. The test can indicate if lower-extremity strength is contributing to a patient’s imbalance.
- The modified clinical test of sensory interaction in balancef is a static balance test that measures the integrity of sensory inputs. The test can suggest if 1 or more sensory systems are compromised.
- The mini balance evaluation systems testg is similar: It can differentiate balance deficits by underlying system and allows individualization of a rehabilitation program.
- The functional gait assessmenth is a modification of the dynamic gait index that assesses postural stability during everyday dynamic activities, including tasks such as walking with head turns and pivots.
- The Berg Balance Scalei continues to be used extensively to assess balance.
Continue to: The mini balance evaluation systems test...
The mini balance evaluation systems test, functional gait index, and Berg Balance Scale all have normative age-graded values to predict fall risk.
CASE
Mr. J was referred for balance assessment and to a rehabilitation program. He underwent balance physiotherapy, including multifactorial balance assessment, joined a community exercise program, was fitted with hearing aids, and had his home environment optimized by an occupational therapist. (See examples of “home safety interventions” under “Preventing falls in primary care.”)
3 months later. Mr. J says he feels stronger on his feet. His knee pain has eased, and he is more confident walking around his home. He continues to engage in exercise programs and is comfortable running errands with his spouse.
CORRESPONDENCE
Jason A. Beyea, MD, PhD, FRCSC, Division of OtolaryngologyHead and Neck Surgery, Queen’s University, 144 Brock Street, Kingston, Ontario, Canada, K7L 5G2; [email protected]
a www.hopkinsmedicine.org/institute_nursing/models_tools/jhfrat_acute%20care%20original_6_22_17.pdf
c www.ncbi.nlm.nih.gov/pmc/articles/PMC4376110/figure/figure14/?report=objectonly
e www.cdc.gov/steadi/pdf/STEADI-Assessment-30Sec-508.pdf
f www.mdapp.co/mctsib-modified-clinical-test-of-sensory-interaction-in-balance-calculator-404/
g www.sralab.org/sites/default/files/2017-07/MiniBEST_revised_final_3_8_13.pdf
1. Larocca NG. Impact of walking impairment in multiple sclerosis: perspectives of patients and care partners. Patient. 2011;4:189-201. doi: 10.2165/11591150-000000000-00000
2. TB, ZF, ES, et al. The relationship of balance disorders with falling, the effect of health problems, and social life on postural balance in the elderly living in a district in Turkey. Geriatrics (Basel). 2019;4:37. doi: 10.3390/geriatrics4020037
3. R, Sixt E, Landahl S, et al. Prevalence of dizziness and vertigo in an urban elderly population. J Vestib Res. 2004;14:47-52.
4. Sturnieks DL, St George R, Lord SR. Balance disorders in the elderly. Neurophysiol Clin. 2008;38:467-478. doi: 10.1016/j.neucli.2008.09.001
5. Boult C, Murphy J, Sloane P, et al. The relation of dizziness to functional decline. J Am Geriatr Soc. 1991;39:858-861. doi: 10.1111/j.1532-5415.1991.tb04451.x
6. Lin HW, Bhattacharyya N. Balance disorders in the elderly: epidemiology and functional impact. Laryngoscope. 2012;122:1858-1861. doi: 10.1002/lary.23376
7. Jia H, Lubetkin EI, DeMichele K, et al. Prevalence, risk factors, and burden of disease for falls and balance or walking problems among older adults in the U.S. Prev Med. 2019;126:105737. doi: 10.1016/j.ypmed.2019.05.025
8. Al-Momani M, Al-Momani F, Alghadir AH, et al. Factors related to gait and balance deficits in older adults. Clin Interv Aging. 2016;11:1043-1049. doi: 10.2147/CIA.S112282
9. Agrawal Y, Ward BK, Minor LB. Vestibular dysfunction: prevalence, impact and need for targeted treatment. J Vestib Res. 2013;23:113-117. doi: 10.3233/VES-130498
10. Altinsoy B, Erboy F, Tanriverdi H, et al. Syncope as a presentation of acute pulmonary embolism. Ther Clin Risk Manag. 2016;12:1023-1028. doi: 10.2147/TCRM.S105722
11. Belvederi Murri M, Triolo F, Coni A, et al. Instrumental assessment of balance and gait in depression: a systematic review. Psychiatry Res. 2020;284:112687. doi: 10.1016/j.psychres.2019.112687
12. Bhattacharyya N, Gubbels SP, Schwartz SR, et al. Clinical practice guideline: benign paroxysmal positional vertigo (update). Otolaryngol Head Neck Surg. 2017;156(suppl 3):S1-S47. doi: 10.1177/0194599816689667
13. S, Schwarm S, Grevenrath P, et al. Prevalence, aetiologies and prognosis of the symptom dizziness in primary care - a systematic review. BMC Fam Pract. 2018;19:33. doi: 10.1186/s12875-017-0695-0
14. Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev. 2010;23:467-492. doi: 10.1128/CMR.00070-09
15. Chad DA. Lumbar spinal stenosis. Neurol Clin. 2007;25:407-418. doi: 10.1016/j.ncl.2007.01.003
16. Conrad BP, Shokat MS, Abbasi AZ, et al. Associations of self-report measures with gait, range of motion and proprioception in patients with lumbar spinal stenosis. Gait Posture. 2013;38:987-992. doi: 10.1016/j.gaitpost.2013.05.010
17. de Luna RA, Mihailovic A, Nguyen AM, et al. The association of glaucomatous visual field loss and balance. Transl Vis Sci Technol. 2017;6:8. doi: 10.1167/tvst.6.3.8
18. DiSogra RM. Common aminoglycosides and platinum-based ototoxic drugs: cochlear/vestibular side effects and incidence. Semin Hear. 2019;40:104-107. doi: 10.1055/s-0039-1684040
19. Ebersbach G, Moreau C, Gandor F, et al. Clinical syndromes: parkinsonian gait. Mov Disord. 2013;28:1552-1559. doi: 10.1002/mds.25675
20. Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S-1127S. doi: 10.3945/ajcn.2010.28608A
21. Filli L, Sutter T, Easthope CS, et al. Profiling walking dysfunction in multiple sclerosis: characterisation, classification and progression over time. Sci Rep. 2018;8:4984. doi: 10.1038/s41598-018-22676-0
22. Fritz NE, Kegelmeyer DA, Kloos AD, et al. Motor performance differentiates individuals with Lewy body dementia, Parkinson’s and Alzheimer’s disease. Gait Posture. 2016;50:1-7. doi: 10.1016/j.gaitpost.2016.08.009
23. Furman JM, Jacob RG. A clinical taxonomy of dizziness and anxiety in the otoneurological setting. J Anxiety Disord. 2001;15:9-26. doi: 10.1016/s0887-6185(00)00040-2
24. Furman JM, Marcus DA, Balaban CD. Vestibular migraine: clinical aspects and pathophysiology. Lancet Neurol. 2013;12:706-715. doi: 10.1016/S1474-4422(13)70107-8
25. Gerson LW, Jarjoura D, McCord G. Risk of imbalance in elderly people with impaired hearing or vision. Age Ageing. 1989;18:31-34. doi: 10.1093/ageing/18.1.31
26. Goudakos JK, Markou KD, Franco-Vidal V, et al. Corticosteroids in the treatment of vestibular neuritis: a systematic review and meta-analysis. Otol Neurotol. 2010;31:183-189. doi: 10.1097/MAO.0b013e3181ca843d
27. Green AD, CS, Bastian L, et al. Does this woman have osteoporosis? JAMA. 2004;292:2890-2900. doi: 10.1001/jama.292.23.2890
28. Hallemans A, Ortibus E, Meire F, et al. Low vision affects dynamic stability of gait. Gait Posture. 2010;32:547-551. doi: 10.1016/j.gaitpost.2010.07.018
29. Handelsman JA. Vestibulotoxicity: strategies for clinical diagnosis and rehabilitation. Int J Audiol. 2018;57(suppl 4):S99-S107. doi: 10.1080/14992027.2018.1468092
30. Head VA, Wakerley BR. Guillain-Barré syndrome in general practice: clinical features suggestive of early diagnosis. Br J Gen Pract. 2016;66:218-219. doi: 10.3399/bjgp16X684733
31. Helbostad JL, Vereijken B, Hesseberg K, et al. Altered vision destabilizes gait in older persons. Gait Posture. 2009;30:233-238. doi: 10.1016/j.gaitpost.2009.05.004
32. Hsu W-L, Chen C-Y, Tsauo J-Y, et al. Balance control in elderly people with osteoporosis. J Formos Med Assoc. 2014;113:334-339. doi: 10.1016/j.jfma.2014.02.006
33. Kim H-S, Yun DH, Yoo SD, et al. Balance control and knee osteoarthritis severity. Ann Rehabil Med. 2011;35:701-709. doi: 10.5535/arm.2011.35.5.701
34. Li L, Simonsick EM, Ferrucci L, et al. Hearing loss and gait speed among older adults in the United States. Gait Posture. 2013;38:25-29.
35. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89:88-100. doi: 10.1212/WNL.0000000000004058
36. Milner KA, Funk M, Richards S, et al. Gender differences in symptom presentation associated with coronary heart disease. Am J Cardiol. 1999;84:396-399. doi: 10.1016/s0002-9149(99)00322-7
37. Paillard T, F, Bru N, et al. The impact of time of day on the gait and balance control of Alzheimer’s patients. Chronobiol Int. 2016;33:161-168. doi: 10.3109/07420528.2015.1124885
38. Paldor I, Chen AS, Kaye AH. Growth rate of vestibular schwannoma. J Clin Neurosci. 2016;32:1-8. doi: 10.1016/j.jocn.2016.05.003
39. Picorelli AMA, Hatton AL, Gane EM, et al. Balance performance in older adults with hip osteoarthritis: a systematic review. Gait Posture. 2018;65:89-99. doi: 10.1016/j.gaitpost.2018.07.001
40. Raccagni C, Nonnekes J, Bloem BR, et al. Gait and postural disorders in parkinsonism: a clinical approach. J Neurol. 2020;267:3169-3176. doi: 10.1007/s00415-019-09382-1
41. Shanmugarajah PD, Hoggard N, Currie S, et al. Alcohol-related cerebellar degeneration: not all down to toxicity? Cerebellum Ataxias. 2016;3:17. doi: 10.1186/s40673-016-0055-1
42. Shih RY, Smirniotopoulos JG. Posterior fossa tumors in adult patients. Neuroimaging Clin N Am. 2016;26:493-510. doi: 10.1016/j.nic.2016.06.003
43. Smith EE. Clinical presentations and epidemiology of vascular dementia. Clin Sci (Lond). 2017;131:1059-1068. doi: 10.1042/CS20160607
44. Streur M, Ratcliffe SJ, Ball J, et al. Symptom clusters in adults with chronic atrial fibrillation. J Cardiovasc Nurs. 2017;32:296-303. doi: 10.1097/JCN.0000000000000344
45. Strupp M, M, JA. Peripheral vestibular disorders: an update. Curr Opin Neurol. 2019;32:165-173. doi: 10.1097/WCO.0000000000000649
46. Thompson TL, Amedee R. Vertigo: a review of common peripheral and central vestibular disorders. Ochsner J. 2009;9:20-26.
47. Timar B, Timar R, L, et al. The impact of diabetic neuropathy on balance and on the risk of falls in patients with type 2 diabetes mellitus: a cross-sectional study. PLoS One. 2016;11:e0154654. doi: 10.1371/journal.pone.0154654
48. Walls R, Hockberger R, Gausche-Hill M. Peripheral nerve disorders. In: Rosen’s Emergency Medicine: Concepts and Clinical Practice. 9th ed. Elsevier, Inc; 2018:1307-1320.
49. Watson JC, Dyck PJB. Peripheral neuropathy: a practical approach to diagnosis and symptom management. Mayo Clin Proc. 2015;90:940-951. doi: 10.1016/j.mayocp.2015.05.004
50. Whitfield KC, Bourassa MW, Adamolekun B, et al. Thiamine deficiency disorders: diagnosis, prevalence, and a roadmap for global control programs. Ann N Y Acad Sci. 2018;1430:3-43. doi: 10.1111/nyas.13919
51. Wu V, Sykes EA, Beyea MM, et al. Approach to Meniere disease management. Can Fam Physician. 2019;65:463-467.
52. Yew KS, Cheng EM. Diagnosis of acute stroke. Am Fam Physician. 2015;91:528-536.
53. Seppala LJ, van de Glind EMM, Daams JG, et al; . Fall-risk-increasing drugs: a systematic review and meta-analysis: III. Others. J Am Med Dir Assoc. 2018;19:372.e1-372.e8. doi: 10.1016/j.jamda.2017.12.099
54. ABIM Foundation. Choosing wisely. Choosing Wisely website. 2021. Accessed November 11. 2021. www.choosingwisely.org/
55. Berlie HD, Garwood CL. Diabetes medications related to an increased risk of falls and fall-related morbidity in the elderly. Ann Pharmacother. 2010;44:712-717. doi: 10.1345/aph.1M551
56. Hartikainen S, E, Louhivuori K. Medication as a risk factor for falls: critical systematic review. J Gerontol A Biol Sci Med Sci. 2007;62:1172-1181. doi: 10.1093/gerona/62.10.1172
57. Khanuja K, Joki J, Bachmann G, et al. Gait and balance in the aging population: Fall prevention using innovation and technology. Maturitas. 2018;110:51-56. doi: 10.1016/j.maturitas.2018.01.021
58. Salzman B. Gait and balance disorders in older adults. Am Fam Physician. 2010;82:61-68.
59. Zaninotto P, Huang YT, Di Gessa G, et al. Polypharmacy is a risk factor for hospital admission due to a fall: evidence from the English Longitudinal Study of Ageing. BMC Public Health. 2020;20:1804. doi: 10.1186/s12889-020-09920-x
60. Morin L, Calderon A, Welmer AK, et al. Polypharmacy and injurious falls in older adults: a nationwide nested case-control study. Clin Epidemiol. 2019;11:483-493. doi: 10.2147/CLEP.S201614
61. Dhalwani NN, Fahami R, Sathanapally H, et al. Association between polypharmacy and falls in older adults: a longitudinal study from England. BMJ Open. 2017;7:e016358. doi: 10.1136/bmjopen-2017-016358
62. Arnold AC, Raj SR. Orthostatic hypotension: a practical approach to investigation and management. Can J Cardiol. 2017;33:1725-1728. doi: 10.1016/j.cjca.2017.05.007
63. Alexander NB. Differential diagnosis of gait disorders in older adults. Clin Geriatr Med. 1996;12:689-703.
64. Baker JM. Gait disorders. Am J Med. 2018;131:602-607. doi: 10.1016/j.amjmed.2017.11.051
65. Cameron MH, Wagner JM. Gait abnormalities in multiple sclerosis: pathogenesis, evaluation, and advances in treatment. Curr Neurol Neurosci Rep. 2011;11:507-515. doi: 10.1007/s11910-011-0214-y
66. Chen C-L, Chen H-C, Tang SF-T, et al. Gait performance with compensatory adaptations in stroke patients with different degrees of motor recovery. Am J Phys Med Rehabil. 2003;82:925-935. doi: 10.1097/01.PHM.0000098040.13355.B5
67. Marsden J, Harris C. Cerebellar ataxia: pathophysiology and rehabilitation. Clin Rehabil. 2011;25:195-216. doi: 10.1177/0269215510382495
68. Mirek E, Filip M, W, et al. Three-dimensional trunk and lower limbs characteristics during gait in patients with Huntington’s disease. Front Neurosci. 2017;11:566. doi: 10.3389/fnins.2017.00566
69. Paramanandam V, Lizarraga KJ, Soh D, et al. Unusual gait disorders: a phenomenological approach and classification. Expert Rev Neurother. 2019;19:119-132. doi: 10.1080/14737175.2019.1562337
70. Sahyouni R, Goshtasbi K, Mahmoodi A, et al. Chronic subdural hematoma: a historical and clinical perspective. World Neurosurg. 2017;108:948-953. doi: 10.1016/j.wneu.2017.09.064
71. Talmud JD, Coffey R, Edemekong PF. Dix Hallpike maneuver. StatPearls [Internet]. StatPearls Publishing Updated September 5, 2021. Accessed December 6, 2021. www.ncbi.nlm.nih.gov/books/NBK459307/
72. Molnar FJ, Benjamin S, Hawkins SA, et al. One size does not fit all: choosing practical cognitive screening tools for your practice. J Am Geriatr Soc. 2020;68:2207-2213. doi: 10.1111/jgs.16713
73. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012:CD007146. doi: 10.1002/14651858.CD007146.pub3
74. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150. doi: 10.14336/AD.2017.0306
75. Poe SS, Cvach M, Dawson PB, Straus H, Hill EE. The Johns Hopkins Fall Risk Assessment Tool: postimplementation evaluation. J Nurs Care Qual. 2007;22:293-298. doi: 10.1097/01.NCQ.0000290408.74027.39
76. Poe SS, Dawson PB, Cvach M, et al. The Johns Hopkins Fall Risk Assessment Tool: a study of reliability and validity. J Nurs Care Qual. 2018;33:10-19. doi: 10.1097/NCQ.0000000000000301
77. Klinkenberg WD, Potter P. Validity of the Johns Hopkins Fall Risk Assessment Tool for predicting falls on inpatient medicine services. J Nurs Care Qual. 2017;32:108-113. doi: 10.1097/NCQ.0000000000000210
78. Stapleton C, Hough P, Oldmeadow L, et al. Four-item fall risk screening tool for subacute and residential aged care: the first step in fall prevention. Australas J Ageing. 2009;28:139-143. doi: 10.1111/j.1741-6612.2009.00375.x
79. Cattelani L, Palumbo P, Palmerini L, et al. FRAT-up, a Web-based fall-risk assessment tool for elderly people living in the community. J Med Internet Res. 2015;17:e41. doi: 10.2196/jmir.4064
80. De Clercq H, Naudé A, Bornman J. Factors included in adult fall risk assessment tools (FRATs): a systematic review. Ageing Soc. 2020;41:2558-2582. doi: 10.1017/S0144686X2000046X
81. Yap G, Melder A. Accuracy of validated falls risk assessment tools and clinical judgement. Centre for Clinical Effectiveness, Monash Innovation and Quality. Monash Health. February 5, 2020. Accessed November 11, 2021. https://monashhealth.org/wp-content/uploads/2019/01/Rapid-Review_Falls-risk-tools-FINAL.pdf
82. Chittrakul J, Siviroj P, Sungkarat S, et al. Physical frailty and fall risk in community-dwelling older adults: a cross-sectional study. J Aging Res. 2020;2020:3964973. doi: 10.1155/2020/3964973
83. Hatcher VH, Galet C, Lilienthal M, et al. Association of clinical frailty scores with hospital readmission for falls after index admission for trauma-related injury. JAMA Netw Open. 2019;2:e1912409. doi: 10.1001/jamanetworkopen.2019.12409
84. Exercise and fall prevention programs. Government of Ontario Ministry of Health. Updated April 9, 2019. Accessed November 11. 2021. www.ontario.ca/page/exercise-and-falls-prevention-programs
85. Sherrington C, Fairhall NJ, Wallbank GK, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1:CD012424. doi: 10.1002/14651858.CD012424.pub2
86. Hopewell S, Copsey B, Nicolson P, et al. Multifactorial interventions for preventing falls in older people living in the community: a systematic review and meta-analysis of 41 trials and almost 20 000 participants. Br J Sports Med. 2020;54:1340-1350. doi: 10.1136/bjsports-2019-100732
87. Jafari Z, Kolb BE, Mohajerani MH. Age-related hearing loss and tinnitus, dementia risk, and auditory amplification outcomes. Ageing Res Rev. 2019;56:100963. doi: 10.1016/j.arr.2019.100963
88. Griffiths TD, Lad M, Kumar S, et al. How can hearing loss cause dementia? Neuron. 2020;108:401-412. doi: 10.1016/j.neuron.2020.08.003
89. Martini A, Castiglione A, Bovo R, et al. Aging, cognitive load, dementia and hearing loss. Audiol Neurootol. 2014;19(suppl 1):2-5. doi: 10.1159/000371593
90. Vitkovic J, Le C, Lee S-L, et al. The contribution of hearing and hearing loss to balance control. Audiol Neurootol. 2016;21:195-202. doi: 10.1159/000445100
91. Maheu M, Behtani L, Nooristani M, et al. Vestibular function modulates the benefit of hearing aids in people with hearing loss during static postural control. Ear Hear. 2019;40:1418-1424. doi: 10.1097/AUD.0000000000000720
92. Negahban H, Bavarsad Cheshmeh Ali M, Nassadj G. Effect of hearing aids on static balance function in elderly with hearing loss. Gait Posture. 2017;58:126-129. doi: 10.1016/j.gaitpost.2017.07.112
93. Mahmoudi E, Basu T, Langa K, et al. Can hearing aids delay time to diagnosis of dementia, depression, or falls in older adults? J Am Geriatr Soc. 2019;67:2362-2369. doi: 10.1111/jgs.16109
94. Paliwal Y, Slattum PW, Ratliff SM. Chronic health conditions as a risk factor for falls among the community-dwelling US older adults: a zero-inflated regression modeling approach. Biomed Res Int. 2017;2017:5146378. doi: 10.1155/2017/5146378
95. Deandrea S, Lucenteforte E, Bravi F, et al. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology. 2010;21:658-668. doi: 10.1097/EDE.0b013e3181e89905
96. Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51-61. doi: 10.1016/j.maturitas.2013.02.009
97. Stevens M, Holman CD, Bennett N. Preventing falls in older people: impact of an intervention to reduce environmental hazards in the home. J Am Geriatr Soc. 2001;49:1442-1447. doi: 10.1046/j.1532-5415.2001.4911235.x
98. Clinical resources. Centers for Disease Control and Prevention STEADI-Older Adult Fall Prevention website. 2020. Accessed November 12, 2021. www.cdc.gov/steadi/materials.html
99. ; Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1696-1704. doi: 10.1001/jama.2018.3097
CASE Mr. J, a 75-year-old man, presents to your family practice reporting that he feels increasingly unsteady and slow while walking. He fell twice last year, without resulting injury. He now worries about tripping while walking around the house and relies on his spouse to run errands.
Clearly, Mr. J is experiencing a problem with balance. What management approach should you undertake to prevent him from falling?

Balance disorders are common in older people and drastically hinder quality of life.1-4 Patients often describe imbalance as vague symptoms: dizziness, unsteadiness, faintness, spinning sensations.5,6 Importantly, balance disorders disrupt normal gait and contribute to falls that are a major cause of disability and morbidity in older people. Almost 30% of people older than 65 years report 1 or more falls annually.7 Factors that increase the risk of falls include impaired mobility, previously reported falls, reduced psychological functioning, chronic medical conditions, and polypharmacy.7,8
The cause of any single case of imbalance is often multifactorial, resulting from dysfunction of multiple body systems (TABLE 17-56); in our clinical experience, most patients with imbalance and who are at risk of falls do not have a detectable deficit of the vestibular system. These alterations in function arise in 3 key systems—vision, proprioception, and vestibular function—which signal to, and are incorporated by, the cerebellum to mediate balance. Cognitive and neurologic decline are also factors in imbalance.

Considering that 20% of falls result in serious injury in older populations, it is important to identify balance disorders and implement preventive strategies to mitigate harmful consequences of falls on patients’ health and independence.7,57 In this article, we answer the question that the case presentation raises about the proper management approach to imbalance in family practice, including assessment of risk and rehabilitation strategies to reduce the risk of falls. Our insights and recommendations are based on our clinical experience and a review of the medical literature from the past 40 years.

CASE Mr. J has a history of hypertension, age-related hearing loss, and osteoarthritis of the knees; he has not had surgery for the arthritis. His medications are antihypertensives and extra-strength acetaminophen for knee pain.

Making the diagnosis of a balance disorder
History
A thorough clinical history, often including a collateral history from caregivers, narrows the differential diagnosis. Information regarding onset, duration, timing, character, and previous episodes of imbalance is essential. Symptoms of imbalance are often challenging for the patient to describe: They might use terms such as vertigo or dizziness, when, in fact, on further questioning, they are describing balance difficulties. Inquiry into (1) their use of assistive walking devices and (2) development or exacerbation of neurologic, musculoskeletal, auditory, visual, and mood symptoms is necessary. Note the current level of their mobility, episodes of pain or fatigue, previous falls and associated injuries, fear of falling, balance confidence, and sensations that precede falls.58
Continue to: The medical and surgical histories
The medical and surgical histories are key pieces of information. The history of smoking, alcohol habits, and substance use is relevant.
A robust medication history is essential to evaluate a patient’s risk of falling. Polypharmacy—typically, defined as taking 4 or more medications—has been repeatedly associated with a heightened risk of falls.53,59-61 Moreover, a dose-dependent association between polypharmacy and hospitalization following falls has been identified, and demonstrates that taking 10 or more medications greatly increases the risk of hospitalization.59 Studies of polypharmacy cement the importance of inquiring about medication use when assessing imbalance, particularly in older patients.
Physical examination
A focused and detailed physical examination provides insight into systems that should be investigated:
- Obtain vital signs, including orthostatic vitals to test for orthostatic hypotension62; keep in mind that symptoms of orthostatic dizziness can occur without orthostatic hypotension.
- Examine gait, which can distinguish between causes of imbalance (TABLE 2).21,40,63-70
- Perform a cardiac examination.
- Assess visual acuity and visual fields; test for nystagmus and identify any optic-nerve and retinal abnormalities.
- Evaluate lower-limb sensation, proprioception, and motor function.
- Evaluate suspected vestibular dysfunction, including dysfunction with positional testing (the Dix-Hallpike maneuver71). The patient is taken from sitting to supine while the head is rotated 45° to the tested side by the examiner. As the patient moves into a supine position, the neck is extended 30° off the table and held for at least 30 seconds. The maneuver is positive if torsional nystagmus is noted while the head is held rotated during neck extension. The maneuver is negative if the patient reports dizziness, vertigo, unsteadiness, or “pressure in the head.” Torsional nystagmus must be present to confirm a diagnosis of benign paroxysmal positional vertigo.
- If you suspect a central nervous system cause of imbalance, assess the cranial nerves, coordination, strength, and, of course, balance.

CASE
Mr. J’s physical examination showed normal vital signs without significant postural changes in blood pressure. Gait analysis revealed a slowed gait, with reduced range of motion in both knees over the entire gait cycle. Audiometry revealed symmetric moderate sensorineural hearing loss characteristic of presbycusis.
Diagnostic investigations
Consider focused investigations into imbalance based on the history and physical examination. We discourage overly broad testing and imaging; in primary care, cost and limited access to technology can bar robust investigations into causes of imbalance. However, identification of acute pathologies should prompt immediate referral to the emergency department. Furthermore, specific symptoms (TABLE 17-56) should prompt referral to specialists for assessment.
Continue to: In the emergency department...
In the emergency department and academic hospitals, key investigations can identify causes of imbalance:
- Electrocardiography and Holter monitoring test for cardiac arrhythmias.
- Echocardiography identifies structural abnormalities.
- Radiography and computed tomography are useful for detecting musculoskeletal abnormalities.
- Bone densitometry can identify osteoporosis.
- Head and spinal cord magnetic resonance imaging can be used to identify lesions of the central nervous system.
- Computed tomographic angiography of the head and neck is useful for identifying stroke, cerebral atrophy, and stenotic lesions of the carotid and vertebral arteries.
- Nerve conduction studies and levels of serum vitamin B12, hemoglobin A1C, thyroid-stimulating hormone, and random cortisol can uncover causes of peripheral neuropathy.
- Bedside cognitive screening tests can be used to measure cognitive decline.72
- Suspicion of vestibular disease requires audiometry and vestibular testing, including videonystagmography, head impulse testing, and vestibular evoked myogenic potentials.
In many cases of imbalance, no specific underlying correctable cause is discovered.
Management of imbalance
Pharmacotherapy
Targeted pharmacotherapy can be utilized in select clinical scenarios:
- Medical treatment of peripheral neuropathy should target the underlying condition.
- Cognitive behavioral therapy and antidepressants are useful for treating anxiety and depressive disorders.73
- Musculoskeletal pain can be managed with acetaminophen and topical nonsteroidal anti-inflammatory drugs (NSAIDs), using a short course of an oral NSAID when needed.74
- Cardiovascular disease management might include any of several classes of pharmacotherapy, including antiplatelet and lipid-lowering medications, antiarrhythmic drugs, and antihypertensive agents.
- Acute episodes of vertigo due to vestibular neuritis or labyrinthitis can be managed with an antiemetic.46
Surgical treatment
Surgery is infrequently considered for patients with imbalance. Examples of indications include microsurgical resection of vestibular schwannoma, resection of central nervous system tumors, lens replacement surgery for cataract, surgical management of severe spinal fracture, and hip or knee arthroplasty in select patients.
Tools for assessing the risk of falls
Scoring systems called falls risk assessment tools, or FRAT, have been developed to gauge a patient’s risk of falling. The various FRATs differ in specificity and sensitivity for predicting the risk of falls, and are typically designed for specific clinical environments, such as hospital inpatient care or long-term care facilities. Specifically, FRATs attempt to classify risk using sets of risk factors known to be associated with falls.
Continue to: Research abounds into...
Research abounds into the validity of commonly used FRATs across institutions, patient populations, and clinical environments:
The Johns Hopkins FRATa determines risk using metrics such as age, fall history, incontinence, cognition, mobility, and medications75; it is predominantly used for assessment in hospital inpatient units. This tool has been validated repeatedly.76,77
Peninsula Health FRATb stratifies patients in subacute and residential aged-care settings, based on risk factors that include recent falls, medications, psychological status, and cognition.78
FRAT-upc is a web-based tool that generates falls risk using risk factors that users input. This tool has been studied in the context of patients older than 65 years living in the community.79
Although FRATs are reasonably useful for predicting falls, their utility varies by patient population and clinical context. Moreover, it has been suggested that FRATs neglect environmental and personal factors when assessing risk by focusing primarily on bodily factors.80 Implementing a FRAT requires extensive consideration of the target population and should be accompanied by clinical judgment that is grounded in an individual patient’s circumstances.81
Continue to: Preventing falls in primary care
Preventing falls in primary care
An approach to preventing falls includes the development of individualized programs that account for frailty, a syndrome of physiologic decline associated with aging. Because frailty leads to diminished balance and mobility, a patient’s frailty index—determined using the 5 frailty phenotype criteria (exhaustion, weight loss, low physical activity, weakness, slowness)82 or the Canadian Study of Health and Aging Clinical Frailty Scale83—is a useful tool for predicting falls risk and readmission for falls following trauma-related injury. Prevention of falls in communities is critical for reducing mortality and allowing older people to maintain their independence and quality of life.
Exercise. In some areas, exercise and falls prevention programs are accessible to seniors.84 Community exercise programs that focus on balance retraining and muscle strengthening can reduce the risk of falls.73,85 The Choosing Wisely initiative of the ABIM [American Board of Internal Medicine] Foundation recommends that exercise programs be designed around an accurate functional baseline of the patient to avoid underdosed strength training.54
Multifactorial risk assessment in high-risk patients can reduce the rate of falls. Such an assessment includes examination of orthostatic blood pressure, vision and hearing, bone health, gait, activities of daily living, cognition, and environmental hazards, and enables provision of necessary interventions.73,86 Hearing amplification, specifically, correlates with enhanced postural control, slowed cognitive decline, and a reduced likelihood of falls.87-93 The mechanism behind improved balance performance might be reduced cognitive load through supporting a patient’s listening needs.88-90
Pharmacotherapy. Optimizing medications and performing a complete medication review before prescribing new medications is highly recommended to avoid unnecessary polypharmacy7,8,18,53-56 (TABLE 17-56).
Management of comorbidities associated with a higher risk of falls, including arthritis, cancer, stroke, diabetes, depression, kidney disease, chronic obstructive pulmonary disease, cognitive impairment, hypertension, and atrial fibrillation, is essential.94-96
Continue to: Home safety interventions
Home safety interventions, through occupational therapy, are important. These include removing unsafe mats and step-overs and installing nonslip strips on stairs, double-sided tape under mats, and handrails.73-97
Screening for risk of falls. The Centers for Disease Control and Prevention recommends that (1) all patients older than 65 years and (2) any patient presenting with an acute fall undergo screening for their risk of falls.98 When a patient is identified as at risk of falling, you can, when appropriate, assess modifiable risk factors and facilitate interventions.98 This strategy is supported by a 2018 statement from the US Preventive Services Task Force99 that recommends identifying high-risk patients who have:
- a history of falling
- a balance disturbance that causes a deficit of mobility or function
- poor performance on clinical tests, such as the 3-meter Timed Up and Go (TUG) assessment (www.cdc.gov/steadi/pdf/TUG_test-print.pdf).
An increased risk of falls should prompt you to refer the patient to community programs and physiotherapy in accordance with the individual’s personal goals99; a balance and vestibular physiotherapist is ideally positioned to accurately assess and manage patients at risk of falls. Specifically, the Task Force identified exercise programs and multifactorial interventions as being beneficial in preventing falls in high-risk older people.99
Balance assessment and rehabilitation in specialty centers
An individualized rehabilitation program aims to restore safe mobility by testing and addressing specific balance deficits, improving functional balance, and increasing balance confidence. Collaboration with colleagues from physiotherapy and occupational therapy aids in tailoring individualized programs.
Many tests are available to assess balance, determine the risk of falls, and guide rehabilitation:
- The timed 10-meter walk testd and the TUG test are simple assessments that measure functional mobility; both have normalized values for the risk of falls. A TUG time of ≥ 12 seconds suggests a high risk of falls.
- The 30-second chair stande evaluates functional lower-extremity strength in older patients. The test can indicate if lower-extremity strength is contributing to a patient’s imbalance.
- The modified clinical test of sensory interaction in balancef is a static balance test that measures the integrity of sensory inputs. The test can suggest if 1 or more sensory systems are compromised.
- The mini balance evaluation systems testg is similar: It can differentiate balance deficits by underlying system and allows individualization of a rehabilitation program.
- The functional gait assessmenth is a modification of the dynamic gait index that assesses postural stability during everyday dynamic activities, including tasks such as walking with head turns and pivots.
- The Berg Balance Scalei continues to be used extensively to assess balance.
Continue to: The mini balance evaluation systems test...
The mini balance evaluation systems test, functional gait index, and Berg Balance Scale all have normative age-graded values to predict fall risk.
CASE
Mr. J was referred for balance assessment and to a rehabilitation program. He underwent balance physiotherapy, including multifactorial balance assessment, joined a community exercise program, was fitted with hearing aids, and had his home environment optimized by an occupational therapist. (See examples of “home safety interventions” under “Preventing falls in primary care.”)
3 months later. Mr. J says he feels stronger on his feet. His knee pain has eased, and he is more confident walking around his home. He continues to engage in exercise programs and is comfortable running errands with his spouse.
CORRESPONDENCE
Jason A. Beyea, MD, PhD, FRCSC, Division of OtolaryngologyHead and Neck Surgery, Queen’s University, 144 Brock Street, Kingston, Ontario, Canada, K7L 5G2; [email protected]
a www.hopkinsmedicine.org/institute_nursing/models_tools/jhfrat_acute%20care%20original_6_22_17.pdf
c www.ncbi.nlm.nih.gov/pmc/articles/PMC4376110/figure/figure14/?report=objectonly
e www.cdc.gov/steadi/pdf/STEADI-Assessment-30Sec-508.pdf
f www.mdapp.co/mctsib-modified-clinical-test-of-sensory-interaction-in-balance-calculator-404/
g www.sralab.org/sites/default/files/2017-07/MiniBEST_revised_final_3_8_13.pdf
CASE Mr. J, a 75-year-old man, presents to your family practice reporting that he feels increasingly unsteady and slow while walking. He fell twice last year, without resulting injury. He now worries about tripping while walking around the house and relies on his spouse to run errands.
Clearly, Mr. J is experiencing a problem with balance. What management approach should you undertake to prevent him from falling?

Balance disorders are common in older people and drastically hinder quality of life.1-4 Patients often describe imbalance as vague symptoms: dizziness, unsteadiness, faintness, spinning sensations.5,6 Importantly, balance disorders disrupt normal gait and contribute to falls that are a major cause of disability and morbidity in older people. Almost 30% of people older than 65 years report 1 or more falls annually.7 Factors that increase the risk of falls include impaired mobility, previously reported falls, reduced psychological functioning, chronic medical conditions, and polypharmacy.7,8
The cause of any single case of imbalance is often multifactorial, resulting from dysfunction of multiple body systems (TABLE 17-56); in our clinical experience, most patients with imbalance and who are at risk of falls do not have a detectable deficit of the vestibular system. These alterations in function arise in 3 key systems—vision, proprioception, and vestibular function—which signal to, and are incorporated by, the cerebellum to mediate balance. Cognitive and neurologic decline are also factors in imbalance.

Considering that 20% of falls result in serious injury in older populations, it is important to identify balance disorders and implement preventive strategies to mitigate harmful consequences of falls on patients’ health and independence.7,57 In this article, we answer the question that the case presentation raises about the proper management approach to imbalance in family practice, including assessment of risk and rehabilitation strategies to reduce the risk of falls. Our insights and recommendations are based on our clinical experience and a review of the medical literature from the past 40 years.

CASE Mr. J has a history of hypertension, age-related hearing loss, and osteoarthritis of the knees; he has not had surgery for the arthritis. His medications are antihypertensives and extra-strength acetaminophen for knee pain.

Making the diagnosis of a balance disorder
History
A thorough clinical history, often including a collateral history from caregivers, narrows the differential diagnosis. Information regarding onset, duration, timing, character, and previous episodes of imbalance is essential. Symptoms of imbalance are often challenging for the patient to describe: They might use terms such as vertigo or dizziness, when, in fact, on further questioning, they are describing balance difficulties. Inquiry into (1) their use of assistive walking devices and (2) development or exacerbation of neurologic, musculoskeletal, auditory, visual, and mood symptoms is necessary. Note the current level of their mobility, episodes of pain or fatigue, previous falls and associated injuries, fear of falling, balance confidence, and sensations that precede falls.58
Continue to: The medical and surgical histories
The medical and surgical histories are key pieces of information. The history of smoking, alcohol habits, and substance use is relevant.
A robust medication history is essential to evaluate a patient’s risk of falling. Polypharmacy—typically, defined as taking 4 or more medications—has been repeatedly associated with a heightened risk of falls.53,59-61 Moreover, a dose-dependent association between polypharmacy and hospitalization following falls has been identified, and demonstrates that taking 10 or more medications greatly increases the risk of hospitalization.59 Studies of polypharmacy cement the importance of inquiring about medication use when assessing imbalance, particularly in older patients.
Physical examination
A focused and detailed physical examination provides insight into systems that should be investigated:
- Obtain vital signs, including orthostatic vitals to test for orthostatic hypotension62; keep in mind that symptoms of orthostatic dizziness can occur without orthostatic hypotension.
- Examine gait, which can distinguish between causes of imbalance (TABLE 2).21,40,63-70
- Perform a cardiac examination.
- Assess visual acuity and visual fields; test for nystagmus and identify any optic-nerve and retinal abnormalities.
- Evaluate lower-limb sensation, proprioception, and motor function.
- Evaluate suspected vestibular dysfunction, including dysfunction with positional testing (the Dix-Hallpike maneuver71). The patient is taken from sitting to supine while the head is rotated 45° to the tested side by the examiner. As the patient moves into a supine position, the neck is extended 30° off the table and held for at least 30 seconds. The maneuver is positive if torsional nystagmus is noted while the head is held rotated during neck extension. The maneuver is negative if the patient reports dizziness, vertigo, unsteadiness, or “pressure in the head.” Torsional nystagmus must be present to confirm a diagnosis of benign paroxysmal positional vertigo.
- If you suspect a central nervous system cause of imbalance, assess the cranial nerves, coordination, strength, and, of course, balance.

CASE
Mr. J’s physical examination showed normal vital signs without significant postural changes in blood pressure. Gait analysis revealed a slowed gait, with reduced range of motion in both knees over the entire gait cycle. Audiometry revealed symmetric moderate sensorineural hearing loss characteristic of presbycusis.
Diagnostic investigations
Consider focused investigations into imbalance based on the history and physical examination. We discourage overly broad testing and imaging; in primary care, cost and limited access to technology can bar robust investigations into causes of imbalance. However, identification of acute pathologies should prompt immediate referral to the emergency department. Furthermore, specific symptoms (TABLE 17-56) should prompt referral to specialists for assessment.
Continue to: In the emergency department...
In the emergency department and academic hospitals, key investigations can identify causes of imbalance:
- Electrocardiography and Holter monitoring test for cardiac arrhythmias.
- Echocardiography identifies structural abnormalities.
- Radiography and computed tomography are useful for detecting musculoskeletal abnormalities.
- Bone densitometry can identify osteoporosis.
- Head and spinal cord magnetic resonance imaging can be used to identify lesions of the central nervous system.
- Computed tomographic angiography of the head and neck is useful for identifying stroke, cerebral atrophy, and stenotic lesions of the carotid and vertebral arteries.
- Nerve conduction studies and levels of serum vitamin B12, hemoglobin A1C, thyroid-stimulating hormone, and random cortisol can uncover causes of peripheral neuropathy.
- Bedside cognitive screening tests can be used to measure cognitive decline.72
- Suspicion of vestibular disease requires audiometry and vestibular testing, including videonystagmography, head impulse testing, and vestibular evoked myogenic potentials.
In many cases of imbalance, no specific underlying correctable cause is discovered.
Management of imbalance
Pharmacotherapy
Targeted pharmacotherapy can be utilized in select clinical scenarios:
- Medical treatment of peripheral neuropathy should target the underlying condition.
- Cognitive behavioral therapy and antidepressants are useful for treating anxiety and depressive disorders.73
- Musculoskeletal pain can be managed with acetaminophen and topical nonsteroidal anti-inflammatory drugs (NSAIDs), using a short course of an oral NSAID when needed.74
- Cardiovascular disease management might include any of several classes of pharmacotherapy, including antiplatelet and lipid-lowering medications, antiarrhythmic drugs, and antihypertensive agents.
- Acute episodes of vertigo due to vestibular neuritis or labyrinthitis can be managed with an antiemetic.46
Surgical treatment
Surgery is infrequently considered for patients with imbalance. Examples of indications include microsurgical resection of vestibular schwannoma, resection of central nervous system tumors, lens replacement surgery for cataract, surgical management of severe spinal fracture, and hip or knee arthroplasty in select patients.
Tools for assessing the risk of falls
Scoring systems called falls risk assessment tools, or FRAT, have been developed to gauge a patient’s risk of falling. The various FRATs differ in specificity and sensitivity for predicting the risk of falls, and are typically designed for specific clinical environments, such as hospital inpatient care or long-term care facilities. Specifically, FRATs attempt to classify risk using sets of risk factors known to be associated with falls.
Continue to: Research abounds into...
Research abounds into the validity of commonly used FRATs across institutions, patient populations, and clinical environments:
The Johns Hopkins FRATa determines risk using metrics such as age, fall history, incontinence, cognition, mobility, and medications75; it is predominantly used for assessment in hospital inpatient units. This tool has been validated repeatedly.76,77
Peninsula Health FRATb stratifies patients in subacute and residential aged-care settings, based on risk factors that include recent falls, medications, psychological status, and cognition.78
FRAT-upc is a web-based tool that generates falls risk using risk factors that users input. This tool has been studied in the context of patients older than 65 years living in the community.79
Although FRATs are reasonably useful for predicting falls, their utility varies by patient population and clinical context. Moreover, it has been suggested that FRATs neglect environmental and personal factors when assessing risk by focusing primarily on bodily factors.80 Implementing a FRAT requires extensive consideration of the target population and should be accompanied by clinical judgment that is grounded in an individual patient’s circumstances.81
Continue to: Preventing falls in primary care
Preventing falls in primary care
An approach to preventing falls includes the development of individualized programs that account for frailty, a syndrome of physiologic decline associated with aging. Because frailty leads to diminished balance and mobility, a patient’s frailty index—determined using the 5 frailty phenotype criteria (exhaustion, weight loss, low physical activity, weakness, slowness)82 or the Canadian Study of Health and Aging Clinical Frailty Scale83—is a useful tool for predicting falls risk and readmission for falls following trauma-related injury. Prevention of falls in communities is critical for reducing mortality and allowing older people to maintain their independence and quality of life.
Exercise. In some areas, exercise and falls prevention programs are accessible to seniors.84 Community exercise programs that focus on balance retraining and muscle strengthening can reduce the risk of falls.73,85 The Choosing Wisely initiative of the ABIM [American Board of Internal Medicine] Foundation recommends that exercise programs be designed around an accurate functional baseline of the patient to avoid underdosed strength training.54
Multifactorial risk assessment in high-risk patients can reduce the rate of falls. Such an assessment includes examination of orthostatic blood pressure, vision and hearing, bone health, gait, activities of daily living, cognition, and environmental hazards, and enables provision of necessary interventions.73,86 Hearing amplification, specifically, correlates with enhanced postural control, slowed cognitive decline, and a reduced likelihood of falls.87-93 The mechanism behind improved balance performance might be reduced cognitive load through supporting a patient’s listening needs.88-90
Pharmacotherapy. Optimizing medications and performing a complete medication review before prescribing new medications is highly recommended to avoid unnecessary polypharmacy7,8,18,53-56 (TABLE 17-56).
Management of comorbidities associated with a higher risk of falls, including arthritis, cancer, stroke, diabetes, depression, kidney disease, chronic obstructive pulmonary disease, cognitive impairment, hypertension, and atrial fibrillation, is essential.94-96
Continue to: Home safety interventions
Home safety interventions, through occupational therapy, are important. These include removing unsafe mats and step-overs and installing nonslip strips on stairs, double-sided tape under mats, and handrails.73-97
Screening for risk of falls. The Centers for Disease Control and Prevention recommends that (1) all patients older than 65 years and (2) any patient presenting with an acute fall undergo screening for their risk of falls.98 When a patient is identified as at risk of falling, you can, when appropriate, assess modifiable risk factors and facilitate interventions.98 This strategy is supported by a 2018 statement from the US Preventive Services Task Force99 that recommends identifying high-risk patients who have:
- a history of falling
- a balance disturbance that causes a deficit of mobility or function
- poor performance on clinical tests, such as the 3-meter Timed Up and Go (TUG) assessment (www.cdc.gov/steadi/pdf/TUG_test-print.pdf).
An increased risk of falls should prompt you to refer the patient to community programs and physiotherapy in accordance with the individual’s personal goals99; a balance and vestibular physiotherapist is ideally positioned to accurately assess and manage patients at risk of falls. Specifically, the Task Force identified exercise programs and multifactorial interventions as being beneficial in preventing falls in high-risk older people.99
Balance assessment and rehabilitation in specialty centers
An individualized rehabilitation program aims to restore safe mobility by testing and addressing specific balance deficits, improving functional balance, and increasing balance confidence. Collaboration with colleagues from physiotherapy and occupational therapy aids in tailoring individualized programs.
Many tests are available to assess balance, determine the risk of falls, and guide rehabilitation:
- The timed 10-meter walk testd and the TUG test are simple assessments that measure functional mobility; both have normalized values for the risk of falls. A TUG time of ≥ 12 seconds suggests a high risk of falls.
- The 30-second chair stande evaluates functional lower-extremity strength in older patients. The test can indicate if lower-extremity strength is contributing to a patient’s imbalance.
- The modified clinical test of sensory interaction in balancef is a static balance test that measures the integrity of sensory inputs. The test can suggest if 1 or more sensory systems are compromised.
- The mini balance evaluation systems testg is similar: It can differentiate balance deficits by underlying system and allows individualization of a rehabilitation program.
- The functional gait assessmenth is a modification of the dynamic gait index that assesses postural stability during everyday dynamic activities, including tasks such as walking with head turns and pivots.
- The Berg Balance Scalei continues to be used extensively to assess balance.
Continue to: The mini balance evaluation systems test...
The mini balance evaluation systems test, functional gait index, and Berg Balance Scale all have normative age-graded values to predict fall risk.
CASE
Mr. J was referred for balance assessment and to a rehabilitation program. He underwent balance physiotherapy, including multifactorial balance assessment, joined a community exercise program, was fitted with hearing aids, and had his home environment optimized by an occupational therapist. (See examples of “home safety interventions” under “Preventing falls in primary care.”)
3 months later. Mr. J says he feels stronger on his feet. His knee pain has eased, and he is more confident walking around his home. He continues to engage in exercise programs and is comfortable running errands with his spouse.
CORRESPONDENCE
Jason A. Beyea, MD, PhD, FRCSC, Division of OtolaryngologyHead and Neck Surgery, Queen’s University, 144 Brock Street, Kingston, Ontario, Canada, K7L 5G2; [email protected]
a www.hopkinsmedicine.org/institute_nursing/models_tools/jhfrat_acute%20care%20original_6_22_17.pdf
c www.ncbi.nlm.nih.gov/pmc/articles/PMC4376110/figure/figure14/?report=objectonly
e www.cdc.gov/steadi/pdf/STEADI-Assessment-30Sec-508.pdf
f www.mdapp.co/mctsib-modified-clinical-test-of-sensory-interaction-in-balance-calculator-404/
g www.sralab.org/sites/default/files/2017-07/MiniBEST_revised_final_3_8_13.pdf
1. Larocca NG. Impact of walking impairment in multiple sclerosis: perspectives of patients and care partners. Patient. 2011;4:189-201. doi: 10.2165/11591150-000000000-00000
2. TB, ZF, ES, et al. The relationship of balance disorders with falling, the effect of health problems, and social life on postural balance in the elderly living in a district in Turkey. Geriatrics (Basel). 2019;4:37. doi: 10.3390/geriatrics4020037
3. R, Sixt E, Landahl S, et al. Prevalence of dizziness and vertigo in an urban elderly population. J Vestib Res. 2004;14:47-52.
4. Sturnieks DL, St George R, Lord SR. Balance disorders in the elderly. Neurophysiol Clin. 2008;38:467-478. doi: 10.1016/j.neucli.2008.09.001
5. Boult C, Murphy J, Sloane P, et al. The relation of dizziness to functional decline. J Am Geriatr Soc. 1991;39:858-861. doi: 10.1111/j.1532-5415.1991.tb04451.x
6. Lin HW, Bhattacharyya N. Balance disorders in the elderly: epidemiology and functional impact. Laryngoscope. 2012;122:1858-1861. doi: 10.1002/lary.23376
7. Jia H, Lubetkin EI, DeMichele K, et al. Prevalence, risk factors, and burden of disease for falls and balance or walking problems among older adults in the U.S. Prev Med. 2019;126:105737. doi: 10.1016/j.ypmed.2019.05.025
8. Al-Momani M, Al-Momani F, Alghadir AH, et al. Factors related to gait and balance deficits in older adults. Clin Interv Aging. 2016;11:1043-1049. doi: 10.2147/CIA.S112282
9. Agrawal Y, Ward BK, Minor LB. Vestibular dysfunction: prevalence, impact and need for targeted treatment. J Vestib Res. 2013;23:113-117. doi: 10.3233/VES-130498
10. Altinsoy B, Erboy F, Tanriverdi H, et al. Syncope as a presentation of acute pulmonary embolism. Ther Clin Risk Manag. 2016;12:1023-1028. doi: 10.2147/TCRM.S105722
11. Belvederi Murri M, Triolo F, Coni A, et al. Instrumental assessment of balance and gait in depression: a systematic review. Psychiatry Res. 2020;284:112687. doi: 10.1016/j.psychres.2019.112687
12. Bhattacharyya N, Gubbels SP, Schwartz SR, et al. Clinical practice guideline: benign paroxysmal positional vertigo (update). Otolaryngol Head Neck Surg. 2017;156(suppl 3):S1-S47. doi: 10.1177/0194599816689667
13. S, Schwarm S, Grevenrath P, et al. Prevalence, aetiologies and prognosis of the symptom dizziness in primary care - a systematic review. BMC Fam Pract. 2018;19:33. doi: 10.1186/s12875-017-0695-0
14. Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev. 2010;23:467-492. doi: 10.1128/CMR.00070-09
15. Chad DA. Lumbar spinal stenosis. Neurol Clin. 2007;25:407-418. doi: 10.1016/j.ncl.2007.01.003
16. Conrad BP, Shokat MS, Abbasi AZ, et al. Associations of self-report measures with gait, range of motion and proprioception in patients with lumbar spinal stenosis. Gait Posture. 2013;38:987-992. doi: 10.1016/j.gaitpost.2013.05.010
17. de Luna RA, Mihailovic A, Nguyen AM, et al. The association of glaucomatous visual field loss and balance. Transl Vis Sci Technol. 2017;6:8. doi: 10.1167/tvst.6.3.8
18. DiSogra RM. Common aminoglycosides and platinum-based ototoxic drugs: cochlear/vestibular side effects and incidence. Semin Hear. 2019;40:104-107. doi: 10.1055/s-0039-1684040
19. Ebersbach G, Moreau C, Gandor F, et al. Clinical syndromes: parkinsonian gait. Mov Disord. 2013;28:1552-1559. doi: 10.1002/mds.25675
20. Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S-1127S. doi: 10.3945/ajcn.2010.28608A
21. Filli L, Sutter T, Easthope CS, et al. Profiling walking dysfunction in multiple sclerosis: characterisation, classification and progression over time. Sci Rep. 2018;8:4984. doi: 10.1038/s41598-018-22676-0
22. Fritz NE, Kegelmeyer DA, Kloos AD, et al. Motor performance differentiates individuals with Lewy body dementia, Parkinson’s and Alzheimer’s disease. Gait Posture. 2016;50:1-7. doi: 10.1016/j.gaitpost.2016.08.009
23. Furman JM, Jacob RG. A clinical taxonomy of dizziness and anxiety in the otoneurological setting. J Anxiety Disord. 2001;15:9-26. doi: 10.1016/s0887-6185(00)00040-2
24. Furman JM, Marcus DA, Balaban CD. Vestibular migraine: clinical aspects and pathophysiology. Lancet Neurol. 2013;12:706-715. doi: 10.1016/S1474-4422(13)70107-8
25. Gerson LW, Jarjoura D, McCord G. Risk of imbalance in elderly people with impaired hearing or vision. Age Ageing. 1989;18:31-34. doi: 10.1093/ageing/18.1.31
26. Goudakos JK, Markou KD, Franco-Vidal V, et al. Corticosteroids in the treatment of vestibular neuritis: a systematic review and meta-analysis. Otol Neurotol. 2010;31:183-189. doi: 10.1097/MAO.0b013e3181ca843d
27. Green AD, CS, Bastian L, et al. Does this woman have osteoporosis? JAMA. 2004;292:2890-2900. doi: 10.1001/jama.292.23.2890
28. Hallemans A, Ortibus E, Meire F, et al. Low vision affects dynamic stability of gait. Gait Posture. 2010;32:547-551. doi: 10.1016/j.gaitpost.2010.07.018
29. Handelsman JA. Vestibulotoxicity: strategies for clinical diagnosis and rehabilitation. Int J Audiol. 2018;57(suppl 4):S99-S107. doi: 10.1080/14992027.2018.1468092
30. Head VA, Wakerley BR. Guillain-Barré syndrome in general practice: clinical features suggestive of early diagnosis. Br J Gen Pract. 2016;66:218-219. doi: 10.3399/bjgp16X684733
31. Helbostad JL, Vereijken B, Hesseberg K, et al. Altered vision destabilizes gait in older persons. Gait Posture. 2009;30:233-238. doi: 10.1016/j.gaitpost.2009.05.004
32. Hsu W-L, Chen C-Y, Tsauo J-Y, et al. Balance control in elderly people with osteoporosis. J Formos Med Assoc. 2014;113:334-339. doi: 10.1016/j.jfma.2014.02.006
33. Kim H-S, Yun DH, Yoo SD, et al. Balance control and knee osteoarthritis severity. Ann Rehabil Med. 2011;35:701-709. doi: 10.5535/arm.2011.35.5.701
34. Li L, Simonsick EM, Ferrucci L, et al. Hearing loss and gait speed among older adults in the United States. Gait Posture. 2013;38:25-29.
35. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89:88-100. doi: 10.1212/WNL.0000000000004058
36. Milner KA, Funk M, Richards S, et al. Gender differences in symptom presentation associated with coronary heart disease. Am J Cardiol. 1999;84:396-399. doi: 10.1016/s0002-9149(99)00322-7
37. Paillard T, F, Bru N, et al. The impact of time of day on the gait and balance control of Alzheimer’s patients. Chronobiol Int. 2016;33:161-168. doi: 10.3109/07420528.2015.1124885
38. Paldor I, Chen AS, Kaye AH. Growth rate of vestibular schwannoma. J Clin Neurosci. 2016;32:1-8. doi: 10.1016/j.jocn.2016.05.003
39. Picorelli AMA, Hatton AL, Gane EM, et al. Balance performance in older adults with hip osteoarthritis: a systematic review. Gait Posture. 2018;65:89-99. doi: 10.1016/j.gaitpost.2018.07.001
40. Raccagni C, Nonnekes J, Bloem BR, et al. Gait and postural disorders in parkinsonism: a clinical approach. J Neurol. 2020;267:3169-3176. doi: 10.1007/s00415-019-09382-1
41. Shanmugarajah PD, Hoggard N, Currie S, et al. Alcohol-related cerebellar degeneration: not all down to toxicity? Cerebellum Ataxias. 2016;3:17. doi: 10.1186/s40673-016-0055-1
42. Shih RY, Smirniotopoulos JG. Posterior fossa tumors in adult patients. Neuroimaging Clin N Am. 2016;26:493-510. doi: 10.1016/j.nic.2016.06.003
43. Smith EE. Clinical presentations and epidemiology of vascular dementia. Clin Sci (Lond). 2017;131:1059-1068. doi: 10.1042/CS20160607
44. Streur M, Ratcliffe SJ, Ball J, et al. Symptom clusters in adults with chronic atrial fibrillation. J Cardiovasc Nurs. 2017;32:296-303. doi: 10.1097/JCN.0000000000000344
45. Strupp M, M, JA. Peripheral vestibular disorders: an update. Curr Opin Neurol. 2019;32:165-173. doi: 10.1097/WCO.0000000000000649
46. Thompson TL, Amedee R. Vertigo: a review of common peripheral and central vestibular disorders. Ochsner J. 2009;9:20-26.
47. Timar B, Timar R, L, et al. The impact of diabetic neuropathy on balance and on the risk of falls in patients with type 2 diabetes mellitus: a cross-sectional study. PLoS One. 2016;11:e0154654. doi: 10.1371/journal.pone.0154654
48. Walls R, Hockberger R, Gausche-Hill M. Peripheral nerve disorders. In: Rosen’s Emergency Medicine: Concepts and Clinical Practice. 9th ed. Elsevier, Inc; 2018:1307-1320.
49. Watson JC, Dyck PJB. Peripheral neuropathy: a practical approach to diagnosis and symptom management. Mayo Clin Proc. 2015;90:940-951. doi: 10.1016/j.mayocp.2015.05.004
50. Whitfield KC, Bourassa MW, Adamolekun B, et al. Thiamine deficiency disorders: diagnosis, prevalence, and a roadmap for global control programs. Ann N Y Acad Sci. 2018;1430:3-43. doi: 10.1111/nyas.13919
51. Wu V, Sykes EA, Beyea MM, et al. Approach to Meniere disease management. Can Fam Physician. 2019;65:463-467.
52. Yew KS, Cheng EM. Diagnosis of acute stroke. Am Fam Physician. 2015;91:528-536.
53. Seppala LJ, van de Glind EMM, Daams JG, et al; . Fall-risk-increasing drugs: a systematic review and meta-analysis: III. Others. J Am Med Dir Assoc. 2018;19:372.e1-372.e8. doi: 10.1016/j.jamda.2017.12.099
54. ABIM Foundation. Choosing wisely. Choosing Wisely website. 2021. Accessed November 11. 2021. www.choosingwisely.org/
55. Berlie HD, Garwood CL. Diabetes medications related to an increased risk of falls and fall-related morbidity in the elderly. Ann Pharmacother. 2010;44:712-717. doi: 10.1345/aph.1M551
56. Hartikainen S, E, Louhivuori K. Medication as a risk factor for falls: critical systematic review. J Gerontol A Biol Sci Med Sci. 2007;62:1172-1181. doi: 10.1093/gerona/62.10.1172
57. Khanuja K, Joki J, Bachmann G, et al. Gait and balance in the aging population: Fall prevention using innovation and technology. Maturitas. 2018;110:51-56. doi: 10.1016/j.maturitas.2018.01.021
58. Salzman B. Gait and balance disorders in older adults. Am Fam Physician. 2010;82:61-68.
59. Zaninotto P, Huang YT, Di Gessa G, et al. Polypharmacy is a risk factor for hospital admission due to a fall: evidence from the English Longitudinal Study of Ageing. BMC Public Health. 2020;20:1804. doi: 10.1186/s12889-020-09920-x
60. Morin L, Calderon A, Welmer AK, et al. Polypharmacy and injurious falls in older adults: a nationwide nested case-control study. Clin Epidemiol. 2019;11:483-493. doi: 10.2147/CLEP.S201614
61. Dhalwani NN, Fahami R, Sathanapally H, et al. Association between polypharmacy and falls in older adults: a longitudinal study from England. BMJ Open. 2017;7:e016358. doi: 10.1136/bmjopen-2017-016358
62. Arnold AC, Raj SR. Orthostatic hypotension: a practical approach to investigation and management. Can J Cardiol. 2017;33:1725-1728. doi: 10.1016/j.cjca.2017.05.007
63. Alexander NB. Differential diagnosis of gait disorders in older adults. Clin Geriatr Med. 1996;12:689-703.
64. Baker JM. Gait disorders. Am J Med. 2018;131:602-607. doi: 10.1016/j.amjmed.2017.11.051
65. Cameron MH, Wagner JM. Gait abnormalities in multiple sclerosis: pathogenesis, evaluation, and advances in treatment. Curr Neurol Neurosci Rep. 2011;11:507-515. doi: 10.1007/s11910-011-0214-y
66. Chen C-L, Chen H-C, Tang SF-T, et al. Gait performance with compensatory adaptations in stroke patients with different degrees of motor recovery. Am J Phys Med Rehabil. 2003;82:925-935. doi: 10.1097/01.PHM.0000098040.13355.B5
67. Marsden J, Harris C. Cerebellar ataxia: pathophysiology and rehabilitation. Clin Rehabil. 2011;25:195-216. doi: 10.1177/0269215510382495
68. Mirek E, Filip M, W, et al. Three-dimensional trunk and lower limbs characteristics during gait in patients with Huntington’s disease. Front Neurosci. 2017;11:566. doi: 10.3389/fnins.2017.00566
69. Paramanandam V, Lizarraga KJ, Soh D, et al. Unusual gait disorders: a phenomenological approach and classification. Expert Rev Neurother. 2019;19:119-132. doi: 10.1080/14737175.2019.1562337
70. Sahyouni R, Goshtasbi K, Mahmoodi A, et al. Chronic subdural hematoma: a historical and clinical perspective. World Neurosurg. 2017;108:948-953. doi: 10.1016/j.wneu.2017.09.064
71. Talmud JD, Coffey R, Edemekong PF. Dix Hallpike maneuver. StatPearls [Internet]. StatPearls Publishing Updated September 5, 2021. Accessed December 6, 2021. www.ncbi.nlm.nih.gov/books/NBK459307/
72. Molnar FJ, Benjamin S, Hawkins SA, et al. One size does not fit all: choosing practical cognitive screening tools for your practice. J Am Geriatr Soc. 2020;68:2207-2213. doi: 10.1111/jgs.16713
73. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012:CD007146. doi: 10.1002/14651858.CD007146.pub3
74. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150. doi: 10.14336/AD.2017.0306
75. Poe SS, Cvach M, Dawson PB, Straus H, Hill EE. The Johns Hopkins Fall Risk Assessment Tool: postimplementation evaluation. J Nurs Care Qual. 2007;22:293-298. doi: 10.1097/01.NCQ.0000290408.74027.39
76. Poe SS, Dawson PB, Cvach M, et al. The Johns Hopkins Fall Risk Assessment Tool: a study of reliability and validity. J Nurs Care Qual. 2018;33:10-19. doi: 10.1097/NCQ.0000000000000301
77. Klinkenberg WD, Potter P. Validity of the Johns Hopkins Fall Risk Assessment Tool for predicting falls on inpatient medicine services. J Nurs Care Qual. 2017;32:108-113. doi: 10.1097/NCQ.0000000000000210
78. Stapleton C, Hough P, Oldmeadow L, et al. Four-item fall risk screening tool for subacute and residential aged care: the first step in fall prevention. Australas J Ageing. 2009;28:139-143. doi: 10.1111/j.1741-6612.2009.00375.x
79. Cattelani L, Palumbo P, Palmerini L, et al. FRAT-up, a Web-based fall-risk assessment tool for elderly people living in the community. J Med Internet Res. 2015;17:e41. doi: 10.2196/jmir.4064
80. De Clercq H, Naudé A, Bornman J. Factors included in adult fall risk assessment tools (FRATs): a systematic review. Ageing Soc. 2020;41:2558-2582. doi: 10.1017/S0144686X2000046X
81. Yap G, Melder A. Accuracy of validated falls risk assessment tools and clinical judgement. Centre for Clinical Effectiveness, Monash Innovation and Quality. Monash Health. February 5, 2020. Accessed November 11, 2021. https://monashhealth.org/wp-content/uploads/2019/01/Rapid-Review_Falls-risk-tools-FINAL.pdf
82. Chittrakul J, Siviroj P, Sungkarat S, et al. Physical frailty and fall risk in community-dwelling older adults: a cross-sectional study. J Aging Res. 2020;2020:3964973. doi: 10.1155/2020/3964973
83. Hatcher VH, Galet C, Lilienthal M, et al. Association of clinical frailty scores with hospital readmission for falls after index admission for trauma-related injury. JAMA Netw Open. 2019;2:e1912409. doi: 10.1001/jamanetworkopen.2019.12409
84. Exercise and fall prevention programs. Government of Ontario Ministry of Health. Updated April 9, 2019. Accessed November 11. 2021. www.ontario.ca/page/exercise-and-falls-prevention-programs
85. Sherrington C, Fairhall NJ, Wallbank GK, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1:CD012424. doi: 10.1002/14651858.CD012424.pub2
86. Hopewell S, Copsey B, Nicolson P, et al. Multifactorial interventions for preventing falls in older people living in the community: a systematic review and meta-analysis of 41 trials and almost 20 000 participants. Br J Sports Med. 2020;54:1340-1350. doi: 10.1136/bjsports-2019-100732
87. Jafari Z, Kolb BE, Mohajerani MH. Age-related hearing loss and tinnitus, dementia risk, and auditory amplification outcomes. Ageing Res Rev. 2019;56:100963. doi: 10.1016/j.arr.2019.100963
88. Griffiths TD, Lad M, Kumar S, et al. How can hearing loss cause dementia? Neuron. 2020;108:401-412. doi: 10.1016/j.neuron.2020.08.003
89. Martini A, Castiglione A, Bovo R, et al. Aging, cognitive load, dementia and hearing loss. Audiol Neurootol. 2014;19(suppl 1):2-5. doi: 10.1159/000371593
90. Vitkovic J, Le C, Lee S-L, et al. The contribution of hearing and hearing loss to balance control. Audiol Neurootol. 2016;21:195-202. doi: 10.1159/000445100
91. Maheu M, Behtani L, Nooristani M, et al. Vestibular function modulates the benefit of hearing aids in people with hearing loss during static postural control. Ear Hear. 2019;40:1418-1424. doi: 10.1097/AUD.0000000000000720
92. Negahban H, Bavarsad Cheshmeh Ali M, Nassadj G. Effect of hearing aids on static balance function in elderly with hearing loss. Gait Posture. 2017;58:126-129. doi: 10.1016/j.gaitpost.2017.07.112
93. Mahmoudi E, Basu T, Langa K, et al. Can hearing aids delay time to diagnosis of dementia, depression, or falls in older adults? J Am Geriatr Soc. 2019;67:2362-2369. doi: 10.1111/jgs.16109
94. Paliwal Y, Slattum PW, Ratliff SM. Chronic health conditions as a risk factor for falls among the community-dwelling US older adults: a zero-inflated regression modeling approach. Biomed Res Int. 2017;2017:5146378. doi: 10.1155/2017/5146378
95. Deandrea S, Lucenteforte E, Bravi F, et al. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology. 2010;21:658-668. doi: 10.1097/EDE.0b013e3181e89905
96. Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51-61. doi: 10.1016/j.maturitas.2013.02.009
97. Stevens M, Holman CD, Bennett N. Preventing falls in older people: impact of an intervention to reduce environmental hazards in the home. J Am Geriatr Soc. 2001;49:1442-1447. doi: 10.1046/j.1532-5415.2001.4911235.x
98. Clinical resources. Centers for Disease Control and Prevention STEADI-Older Adult Fall Prevention website. 2020. Accessed November 12, 2021. www.cdc.gov/steadi/materials.html
99. ; Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1696-1704. doi: 10.1001/jama.2018.3097
1. Larocca NG. Impact of walking impairment in multiple sclerosis: perspectives of patients and care partners. Patient. 2011;4:189-201. doi: 10.2165/11591150-000000000-00000
2. TB, ZF, ES, et al. The relationship of balance disorders with falling, the effect of health problems, and social life on postural balance in the elderly living in a district in Turkey. Geriatrics (Basel). 2019;4:37. doi: 10.3390/geriatrics4020037
3. R, Sixt E, Landahl S, et al. Prevalence of dizziness and vertigo in an urban elderly population. J Vestib Res. 2004;14:47-52.
4. Sturnieks DL, St George R, Lord SR. Balance disorders in the elderly. Neurophysiol Clin. 2008;38:467-478. doi: 10.1016/j.neucli.2008.09.001
5. Boult C, Murphy J, Sloane P, et al. The relation of dizziness to functional decline. J Am Geriatr Soc. 1991;39:858-861. doi: 10.1111/j.1532-5415.1991.tb04451.x
6. Lin HW, Bhattacharyya N. Balance disorders in the elderly: epidemiology and functional impact. Laryngoscope. 2012;122:1858-1861. doi: 10.1002/lary.23376
7. Jia H, Lubetkin EI, DeMichele K, et al. Prevalence, risk factors, and burden of disease for falls and balance or walking problems among older adults in the U.S. Prev Med. 2019;126:105737. doi: 10.1016/j.ypmed.2019.05.025
8. Al-Momani M, Al-Momani F, Alghadir AH, et al. Factors related to gait and balance deficits in older adults. Clin Interv Aging. 2016;11:1043-1049. doi: 10.2147/CIA.S112282
9. Agrawal Y, Ward BK, Minor LB. Vestibular dysfunction: prevalence, impact and need for targeted treatment. J Vestib Res. 2013;23:113-117. doi: 10.3233/VES-130498
10. Altinsoy B, Erboy F, Tanriverdi H, et al. Syncope as a presentation of acute pulmonary embolism. Ther Clin Risk Manag. 2016;12:1023-1028. doi: 10.2147/TCRM.S105722
11. Belvederi Murri M, Triolo F, Coni A, et al. Instrumental assessment of balance and gait in depression: a systematic review. Psychiatry Res. 2020;284:112687. doi: 10.1016/j.psychres.2019.112687
12. Bhattacharyya N, Gubbels SP, Schwartz SR, et al. Clinical practice guideline: benign paroxysmal positional vertigo (update). Otolaryngol Head Neck Surg. 2017;156(suppl 3):S1-S47. doi: 10.1177/0194599816689667
13. S, Schwarm S, Grevenrath P, et al. Prevalence, aetiologies and prognosis of the symptom dizziness in primary care - a systematic review. BMC Fam Pract. 2018;19:33. doi: 10.1186/s12875-017-0695-0
14. Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev. 2010;23:467-492. doi: 10.1128/CMR.00070-09
15. Chad DA. Lumbar spinal stenosis. Neurol Clin. 2007;25:407-418. doi: 10.1016/j.ncl.2007.01.003
16. Conrad BP, Shokat MS, Abbasi AZ, et al. Associations of self-report measures with gait, range of motion and proprioception in patients with lumbar spinal stenosis. Gait Posture. 2013;38:987-992. doi: 10.1016/j.gaitpost.2013.05.010
17. de Luna RA, Mihailovic A, Nguyen AM, et al. The association of glaucomatous visual field loss and balance. Transl Vis Sci Technol. 2017;6:8. doi: 10.1167/tvst.6.3.8
18. DiSogra RM. Common aminoglycosides and platinum-based ototoxic drugs: cochlear/vestibular side effects and incidence. Semin Hear. 2019;40:104-107. doi: 10.1055/s-0039-1684040
19. Ebersbach G, Moreau C, Gandor F, et al. Clinical syndromes: parkinsonian gait. Mov Disord. 2013;28:1552-1559. doi: 10.1002/mds.25675
20. Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S-1127S. doi: 10.3945/ajcn.2010.28608A
21. Filli L, Sutter T, Easthope CS, et al. Profiling walking dysfunction in multiple sclerosis: characterisation, classification and progression over time. Sci Rep. 2018;8:4984. doi: 10.1038/s41598-018-22676-0
22. Fritz NE, Kegelmeyer DA, Kloos AD, et al. Motor performance differentiates individuals with Lewy body dementia, Parkinson’s and Alzheimer’s disease. Gait Posture. 2016;50:1-7. doi: 10.1016/j.gaitpost.2016.08.009
23. Furman JM, Jacob RG. A clinical taxonomy of dizziness and anxiety in the otoneurological setting. J Anxiety Disord. 2001;15:9-26. doi: 10.1016/s0887-6185(00)00040-2
24. Furman JM, Marcus DA, Balaban CD. Vestibular migraine: clinical aspects and pathophysiology. Lancet Neurol. 2013;12:706-715. doi: 10.1016/S1474-4422(13)70107-8
25. Gerson LW, Jarjoura D, McCord G. Risk of imbalance in elderly people with impaired hearing or vision. Age Ageing. 1989;18:31-34. doi: 10.1093/ageing/18.1.31
26. Goudakos JK, Markou KD, Franco-Vidal V, et al. Corticosteroids in the treatment of vestibular neuritis: a systematic review and meta-analysis. Otol Neurotol. 2010;31:183-189. doi: 10.1097/MAO.0b013e3181ca843d
27. Green AD, CS, Bastian L, et al. Does this woman have osteoporosis? JAMA. 2004;292:2890-2900. doi: 10.1001/jama.292.23.2890
28. Hallemans A, Ortibus E, Meire F, et al. Low vision affects dynamic stability of gait. Gait Posture. 2010;32:547-551. doi: 10.1016/j.gaitpost.2010.07.018
29. Handelsman JA. Vestibulotoxicity: strategies for clinical diagnosis and rehabilitation. Int J Audiol. 2018;57(suppl 4):S99-S107. doi: 10.1080/14992027.2018.1468092
30. Head VA, Wakerley BR. Guillain-Barré syndrome in general practice: clinical features suggestive of early diagnosis. Br J Gen Pract. 2016;66:218-219. doi: 10.3399/bjgp16X684733
31. Helbostad JL, Vereijken B, Hesseberg K, et al. Altered vision destabilizes gait in older persons. Gait Posture. 2009;30:233-238. doi: 10.1016/j.gaitpost.2009.05.004
32. Hsu W-L, Chen C-Y, Tsauo J-Y, et al. Balance control in elderly people with osteoporosis. J Formos Med Assoc. 2014;113:334-339. doi: 10.1016/j.jfma.2014.02.006
33. Kim H-S, Yun DH, Yoo SD, et al. Balance control and knee osteoarthritis severity. Ann Rehabil Med. 2011;35:701-709. doi: 10.5535/arm.2011.35.5.701
34. Li L, Simonsick EM, Ferrucci L, et al. Hearing loss and gait speed among older adults in the United States. Gait Posture. 2013;38:25-29.
35. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89:88-100. doi: 10.1212/WNL.0000000000004058
36. Milner KA, Funk M, Richards S, et al. Gender differences in symptom presentation associated with coronary heart disease. Am J Cardiol. 1999;84:396-399. doi: 10.1016/s0002-9149(99)00322-7
37. Paillard T, F, Bru N, et al. The impact of time of day on the gait and balance control of Alzheimer’s patients. Chronobiol Int. 2016;33:161-168. doi: 10.3109/07420528.2015.1124885
38. Paldor I, Chen AS, Kaye AH. Growth rate of vestibular schwannoma. J Clin Neurosci. 2016;32:1-8. doi: 10.1016/j.jocn.2016.05.003
39. Picorelli AMA, Hatton AL, Gane EM, et al. Balance performance in older adults with hip osteoarthritis: a systematic review. Gait Posture. 2018;65:89-99. doi: 10.1016/j.gaitpost.2018.07.001
40. Raccagni C, Nonnekes J, Bloem BR, et al. Gait and postural disorders in parkinsonism: a clinical approach. J Neurol. 2020;267:3169-3176. doi: 10.1007/s00415-019-09382-1
41. Shanmugarajah PD, Hoggard N, Currie S, et al. Alcohol-related cerebellar degeneration: not all down to toxicity? Cerebellum Ataxias. 2016;3:17. doi: 10.1186/s40673-016-0055-1
42. Shih RY, Smirniotopoulos JG. Posterior fossa tumors in adult patients. Neuroimaging Clin N Am. 2016;26:493-510. doi: 10.1016/j.nic.2016.06.003
43. Smith EE. Clinical presentations and epidemiology of vascular dementia. Clin Sci (Lond). 2017;131:1059-1068. doi: 10.1042/CS20160607
44. Streur M, Ratcliffe SJ, Ball J, et al. Symptom clusters in adults with chronic atrial fibrillation. J Cardiovasc Nurs. 2017;32:296-303. doi: 10.1097/JCN.0000000000000344
45. Strupp M, M, JA. Peripheral vestibular disorders: an update. Curr Opin Neurol. 2019;32:165-173. doi: 10.1097/WCO.0000000000000649
46. Thompson TL, Amedee R. Vertigo: a review of common peripheral and central vestibular disorders. Ochsner J. 2009;9:20-26.
47. Timar B, Timar R, L, et al. The impact of diabetic neuropathy on balance and on the risk of falls in patients with type 2 diabetes mellitus: a cross-sectional study. PLoS One. 2016;11:e0154654. doi: 10.1371/journal.pone.0154654
48. Walls R, Hockberger R, Gausche-Hill M. Peripheral nerve disorders. In: Rosen’s Emergency Medicine: Concepts and Clinical Practice. 9th ed. Elsevier, Inc; 2018:1307-1320.
49. Watson JC, Dyck PJB. Peripheral neuropathy: a practical approach to diagnosis and symptom management. Mayo Clin Proc. 2015;90:940-951. doi: 10.1016/j.mayocp.2015.05.004
50. Whitfield KC, Bourassa MW, Adamolekun B, et al. Thiamine deficiency disorders: diagnosis, prevalence, and a roadmap for global control programs. Ann N Y Acad Sci. 2018;1430:3-43. doi: 10.1111/nyas.13919
51. Wu V, Sykes EA, Beyea MM, et al. Approach to Meniere disease management. Can Fam Physician. 2019;65:463-467.
52. Yew KS, Cheng EM. Diagnosis of acute stroke. Am Fam Physician. 2015;91:528-536.
53. Seppala LJ, van de Glind EMM, Daams JG, et al; . Fall-risk-increasing drugs: a systematic review and meta-analysis: III. Others. J Am Med Dir Assoc. 2018;19:372.e1-372.e8. doi: 10.1016/j.jamda.2017.12.099
54. ABIM Foundation. Choosing wisely. Choosing Wisely website. 2021. Accessed November 11. 2021. www.choosingwisely.org/
55. Berlie HD, Garwood CL. Diabetes medications related to an increased risk of falls and fall-related morbidity in the elderly. Ann Pharmacother. 2010;44:712-717. doi: 10.1345/aph.1M551
56. Hartikainen S, E, Louhivuori K. Medication as a risk factor for falls: critical systematic review. J Gerontol A Biol Sci Med Sci. 2007;62:1172-1181. doi: 10.1093/gerona/62.10.1172
57. Khanuja K, Joki J, Bachmann G, et al. Gait and balance in the aging population: Fall prevention using innovation and technology. Maturitas. 2018;110:51-56. doi: 10.1016/j.maturitas.2018.01.021
58. Salzman B. Gait and balance disorders in older adults. Am Fam Physician. 2010;82:61-68.
59. Zaninotto P, Huang YT, Di Gessa G, et al. Polypharmacy is a risk factor for hospital admission due to a fall: evidence from the English Longitudinal Study of Ageing. BMC Public Health. 2020;20:1804. doi: 10.1186/s12889-020-09920-x
60. Morin L, Calderon A, Welmer AK, et al. Polypharmacy and injurious falls in older adults: a nationwide nested case-control study. Clin Epidemiol. 2019;11:483-493. doi: 10.2147/CLEP.S201614
61. Dhalwani NN, Fahami R, Sathanapally H, et al. Association between polypharmacy and falls in older adults: a longitudinal study from England. BMJ Open. 2017;7:e016358. doi: 10.1136/bmjopen-2017-016358
62. Arnold AC, Raj SR. Orthostatic hypotension: a practical approach to investigation and management. Can J Cardiol. 2017;33:1725-1728. doi: 10.1016/j.cjca.2017.05.007
63. Alexander NB. Differential diagnosis of gait disorders in older adults. Clin Geriatr Med. 1996;12:689-703.
64. Baker JM. Gait disorders. Am J Med. 2018;131:602-607. doi: 10.1016/j.amjmed.2017.11.051
65. Cameron MH, Wagner JM. Gait abnormalities in multiple sclerosis: pathogenesis, evaluation, and advances in treatment. Curr Neurol Neurosci Rep. 2011;11:507-515. doi: 10.1007/s11910-011-0214-y
66. Chen C-L, Chen H-C, Tang SF-T, et al. Gait performance with compensatory adaptations in stroke patients with different degrees of motor recovery. Am J Phys Med Rehabil. 2003;82:925-935. doi: 10.1097/01.PHM.0000098040.13355.B5
67. Marsden J, Harris C. Cerebellar ataxia: pathophysiology and rehabilitation. Clin Rehabil. 2011;25:195-216. doi: 10.1177/0269215510382495
68. Mirek E, Filip M, W, et al. Three-dimensional trunk and lower limbs characteristics during gait in patients with Huntington’s disease. Front Neurosci. 2017;11:566. doi: 10.3389/fnins.2017.00566
69. Paramanandam V, Lizarraga KJ, Soh D, et al. Unusual gait disorders: a phenomenological approach and classification. Expert Rev Neurother. 2019;19:119-132. doi: 10.1080/14737175.2019.1562337
70. Sahyouni R, Goshtasbi K, Mahmoodi A, et al. Chronic subdural hematoma: a historical and clinical perspective. World Neurosurg. 2017;108:948-953. doi: 10.1016/j.wneu.2017.09.064
71. Talmud JD, Coffey R, Edemekong PF. Dix Hallpike maneuver. StatPearls [Internet]. StatPearls Publishing Updated September 5, 2021. Accessed December 6, 2021. www.ncbi.nlm.nih.gov/books/NBK459307/
72. Molnar FJ, Benjamin S, Hawkins SA, et al. One size does not fit all: choosing practical cognitive screening tools for your practice. J Am Geriatr Soc. 2020;68:2207-2213. doi: 10.1111/jgs.16713
73. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012:CD007146. doi: 10.1002/14651858.CD007146.pub3
74. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150. doi: 10.14336/AD.2017.0306
75. Poe SS, Cvach M, Dawson PB, Straus H, Hill EE. The Johns Hopkins Fall Risk Assessment Tool: postimplementation evaluation. J Nurs Care Qual. 2007;22:293-298. doi: 10.1097/01.NCQ.0000290408.74027.39
76. Poe SS, Dawson PB, Cvach M, et al. The Johns Hopkins Fall Risk Assessment Tool: a study of reliability and validity. J Nurs Care Qual. 2018;33:10-19. doi: 10.1097/NCQ.0000000000000301
77. Klinkenberg WD, Potter P. Validity of the Johns Hopkins Fall Risk Assessment Tool for predicting falls on inpatient medicine services. J Nurs Care Qual. 2017;32:108-113. doi: 10.1097/NCQ.0000000000000210
78. Stapleton C, Hough P, Oldmeadow L, et al. Four-item fall risk screening tool for subacute and residential aged care: the first step in fall prevention. Australas J Ageing. 2009;28:139-143. doi: 10.1111/j.1741-6612.2009.00375.x
79. Cattelani L, Palumbo P, Palmerini L, et al. FRAT-up, a Web-based fall-risk assessment tool for elderly people living in the community. J Med Internet Res. 2015;17:e41. doi: 10.2196/jmir.4064
80. De Clercq H, Naudé A, Bornman J. Factors included in adult fall risk assessment tools (FRATs): a systematic review. Ageing Soc. 2020;41:2558-2582. doi: 10.1017/S0144686X2000046X
81. Yap G, Melder A. Accuracy of validated falls risk assessment tools and clinical judgement. Centre for Clinical Effectiveness, Monash Innovation and Quality. Monash Health. February 5, 2020. Accessed November 11, 2021. https://monashhealth.org/wp-content/uploads/2019/01/Rapid-Review_Falls-risk-tools-FINAL.pdf
82. Chittrakul J, Siviroj P, Sungkarat S, et al. Physical frailty and fall risk in community-dwelling older adults: a cross-sectional study. J Aging Res. 2020;2020:3964973. doi: 10.1155/2020/3964973
83. Hatcher VH, Galet C, Lilienthal M, et al. Association of clinical frailty scores with hospital readmission for falls after index admission for trauma-related injury. JAMA Netw Open. 2019;2:e1912409. doi: 10.1001/jamanetworkopen.2019.12409
84. Exercise and fall prevention programs. Government of Ontario Ministry of Health. Updated April 9, 2019. Accessed November 11. 2021. www.ontario.ca/page/exercise-and-falls-prevention-programs
85. Sherrington C, Fairhall NJ, Wallbank GK, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1:CD012424. doi: 10.1002/14651858.CD012424.pub2
86. Hopewell S, Copsey B, Nicolson P, et al. Multifactorial interventions for preventing falls in older people living in the community: a systematic review and meta-analysis of 41 trials and almost 20 000 participants. Br J Sports Med. 2020;54:1340-1350. doi: 10.1136/bjsports-2019-100732
87. Jafari Z, Kolb BE, Mohajerani MH. Age-related hearing loss and tinnitus, dementia risk, and auditory amplification outcomes. Ageing Res Rev. 2019;56:100963. doi: 10.1016/j.arr.2019.100963
88. Griffiths TD, Lad M, Kumar S, et al. How can hearing loss cause dementia? Neuron. 2020;108:401-412. doi: 10.1016/j.neuron.2020.08.003
89. Martini A, Castiglione A, Bovo R, et al. Aging, cognitive load, dementia and hearing loss. Audiol Neurootol. 2014;19(suppl 1):2-5. doi: 10.1159/000371593
90. Vitkovic J, Le C, Lee S-L, et al. The contribution of hearing and hearing loss to balance control. Audiol Neurootol. 2016;21:195-202. doi: 10.1159/000445100
91. Maheu M, Behtani L, Nooristani M, et al. Vestibular function modulates the benefit of hearing aids in people with hearing loss during static postural control. Ear Hear. 2019;40:1418-1424. doi: 10.1097/AUD.0000000000000720
92. Negahban H, Bavarsad Cheshmeh Ali M, Nassadj G. Effect of hearing aids on static balance function in elderly with hearing loss. Gait Posture. 2017;58:126-129. doi: 10.1016/j.gaitpost.2017.07.112
93. Mahmoudi E, Basu T, Langa K, et al. Can hearing aids delay time to diagnosis of dementia, depression, or falls in older adults? J Am Geriatr Soc. 2019;67:2362-2369. doi: 10.1111/jgs.16109
94. Paliwal Y, Slattum PW, Ratliff SM. Chronic health conditions as a risk factor for falls among the community-dwelling US older adults: a zero-inflated regression modeling approach. Biomed Res Int. 2017;2017:5146378. doi: 10.1155/2017/5146378
95. Deandrea S, Lucenteforte E, Bravi F, et al. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology. 2010;21:658-668. doi: 10.1097/EDE.0b013e3181e89905
96. Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51-61. doi: 10.1016/j.maturitas.2013.02.009
97. Stevens M, Holman CD, Bennett N. Preventing falls in older people: impact of an intervention to reduce environmental hazards in the home. J Am Geriatr Soc. 2001;49:1442-1447. doi: 10.1046/j.1532-5415.2001.4911235.x
98. Clinical resources. Centers for Disease Control and Prevention STEADI-Older Adult Fall Prevention website. 2020. Accessed November 12, 2021. www.cdc.gov/steadi/materials.html
99. ; Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1696-1704. doi: 10.1001/jama.2018.3097
PRACTICE RECOMMENDATIONS
› Utilize a falls-prevention program for older patients that focuses on balance and functional exercises. A
› Perform a multifactorial assessment of the risk of falls in older patients that includes optimizing medications, managing comorbidities, and addressing environmental hazards. B
› Use a systems-based approach to presentations of imbalance to direct your clinical judgment and highlight the need for referral to specialists for management and rehabilitation. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Standardizing your approach to dizziness and vertigo
Dizziness. Vertigo. These 2 terms are often used interchangeably by patients, with the sensations described as imbalance, lightheadedness, disorientation, presyncope, confusion—among others. While dizziness is a broad term that is often used to describe all the aforementioned sensations, including vertigo, true vertigo (a specific type of dizziness) is defined as the perception of movement within one’s visual field while stationary.1 Because patients are not usually aware of the distinction, their reports of signs and symptoms can cause much confusion for health care providers, thereby delaying a diagnosis.
International studies have reported the prevalence of both dizziness and vertigo to be between 15% and 36%.2,3 Over half of all patients with dizziness and vertigo are cared for by the family physician (FP), and the sensations combined account for approximately 5% of all family medicine visits.4,5 Additionally, between 2.5% and 4% of all emergency department (ED) visits stem from complaints of dizziness and vertigo, with an incidence of up to 25% in those >65 years of age.6,7
Causes of dizziness and vertigo are broad, ranging from the benign to the life-threatening. It has been reported that upwards of 50% of patients presenting to the FP’s office for dizziness leave without a diagnosis.8 Given the confusion surrounding the terms and their broad differential, this review aims to provide FPs with the tools to accurately discern benign from ominous causes.
Nonvestibular benign causes vastly outnumber life-threatening ones
Causes of dizziness are classified as either vestibular (these cause true vertigo) or nonvestibular in origin, with nonvestibular causes being more common.7
Nonvestibular etiologies: Numerous and varied
Nonvestibular causes are broad, spanning many different body systems. Cardiovascular causes of dizziness may include orthostatic hypotension, cardiac arrhythmia, myocardial infarction, and carotid artery stenosis.4,9 Metabolic causes include complications of diabetes such as hypoglycemia and peripheral neuropathy.4,9 Psychiatric conditions such as anxiety, depression, and bipolar disorder can manifest as dizziness, disorientation, or psychogenic vertigo.4,10 Medications including nonsteroidal anti-inflammatory drugs, anticonvulsants, antipsychotics, and sedatives can all contribute to dizziness.11 Other causes of dizziness include Parkinson’s disease, musculoskeletal disorders, and gait disorders.4,9 Especially in the elderly, sensory deficit (peripheral neuropathy), poor vision, and polypharmacy (≥5 medications) are common causes of dizziness.12
Vestibular etiologies of dizziness = true vertigo
Vestibular causes of a patient’s feelings of dizziness manifest as true vertigo and can be categorized as either central (a dysfunction of one or more parts of the central nervous system that help process balance and spatial information or along the pathway where these sensations are interpreted) or peripheral (a dysfunction of the balance organs of the inner ear) in origin.
Central vestibular causes include vertebrobasilar ischemic stroke, vertebrobasilar insufficiency (transient ischemic attack), vestibular migraines, and meningioma of the cerebellopontine angle and posterior fossa.13
Continue to: Peripheral vestibular causes
Peripheral vestibular causes. Benign paroxysmal positional vertigo (BPPV) represents the most common peripheral diagnosis. It is caused by dislodged otoliths in the posterior semicircular canal. While the majority of BPPV cases are idiopathic in nature, up to 15% may result from previous head injury.14 Other peripheral vestibular causes include vestibular neuronitis, viral labyrinthitis, Meniere’s disease, vestibular schwannoma, perilymphatic fistula, superior semicircular canal dehiscence (SSCD), and head trauma (basilar skull fracture).13
Start with a history: Is it dizziness or true vertigo?
The clinical history typically guides the differential diagnosis (FIGURE). Identifying true vertigo from among other sensations helps to limit the differential because true vertigo is caused by vestibular etiologies only. True vertigo is often reported by patients as “seeing the room spin;” this stems from the perception of motion.1 A notable exception is that patients with orthostatic hypotension will often describe spinning sensations lasting seconds to minutes when they rise from a seated or supine position.
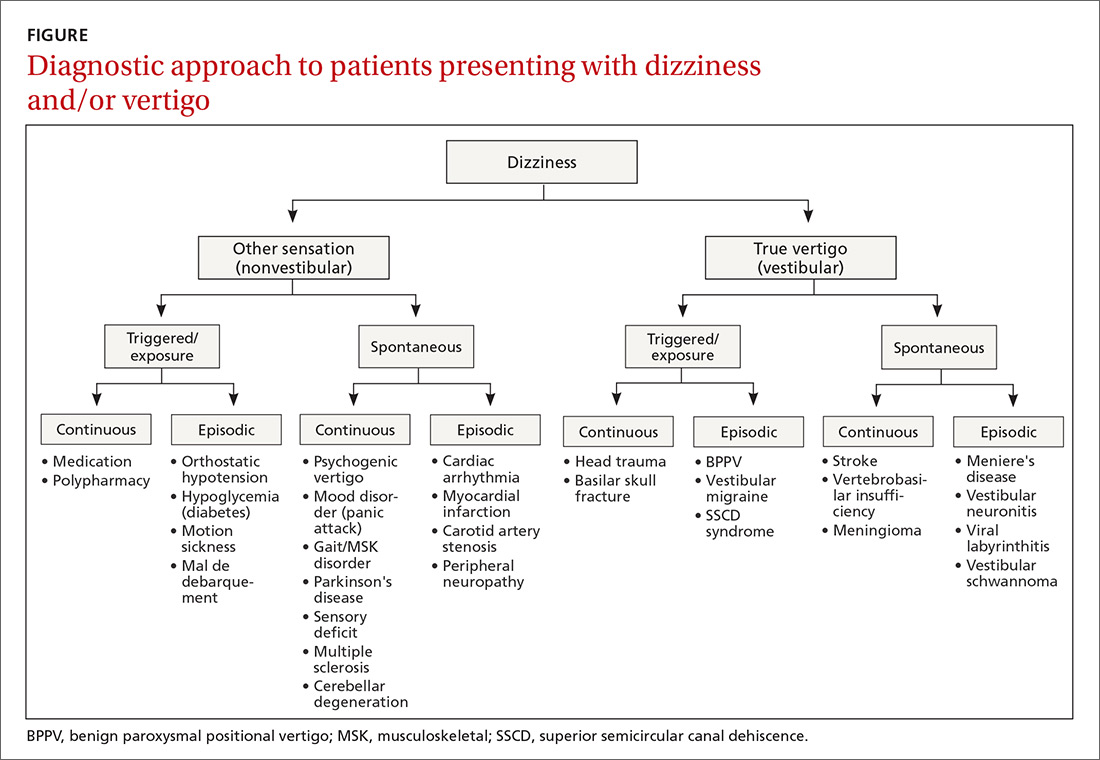
Never depend solely, however, on patient-reported sensations, as not all patients with true vertigo report spinning, and some patients with nonvestibular causes interpret their dizziness as a spinning sensation.15 Therefore, it is important to tease out specifics about the timing, triggers, and associated symptoms in order to further delineate possible causes (TABLE).16
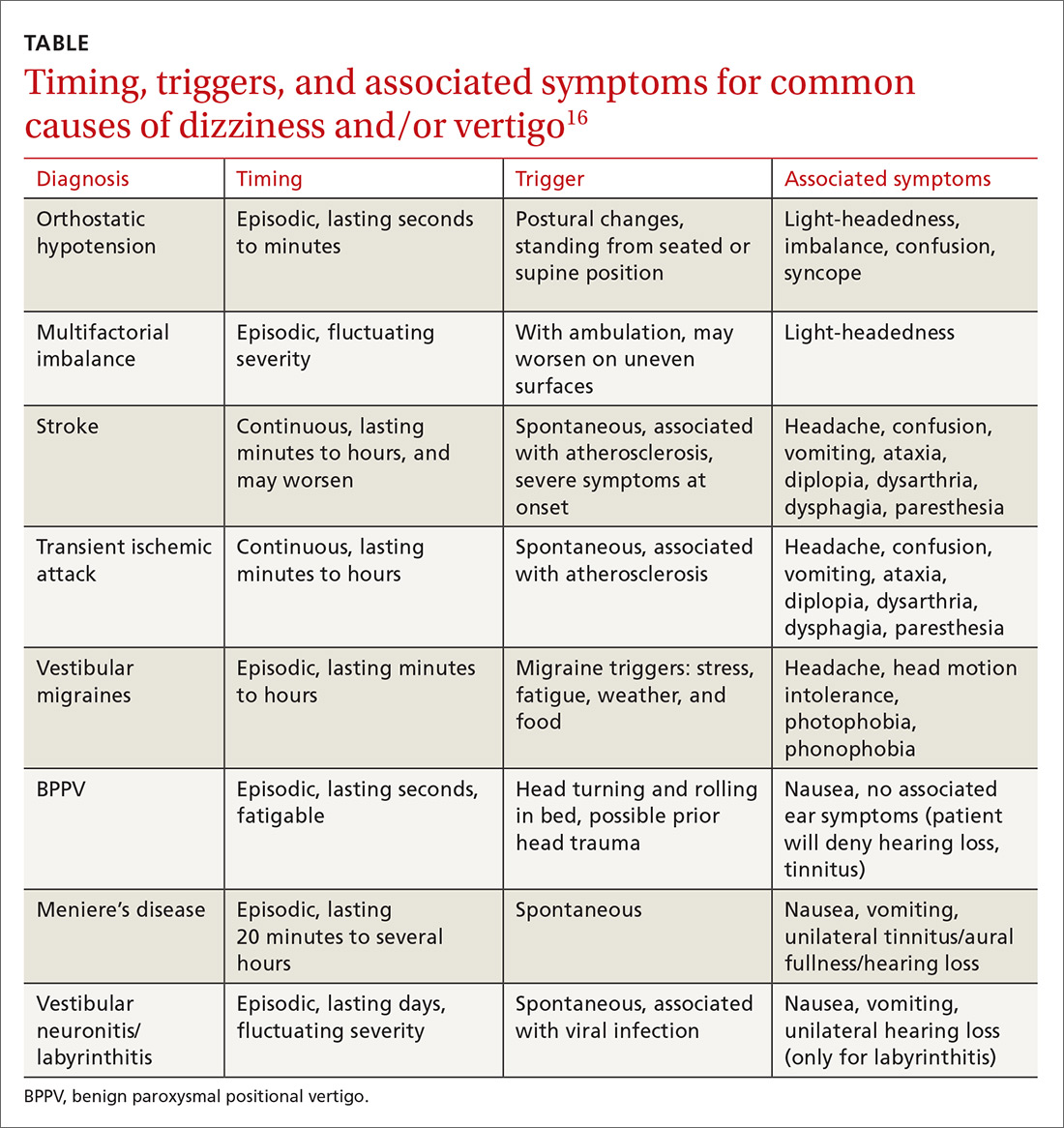
Make a list of current medications. Gather a comprehensive list of current medications, especially from elderly patients, because polypharmacy is a major contributor to dizziness in this population.12 Keep in mind that elderly patients presenting with dizziness/vertigo may have multifactorial balance difficulties, which can be revealed by a detailed history.
Physical exam: May be broad or focused
Given the broad range of causes for dizziness, cardiovascular, head/neck, and neurologic examinations may be performed as part of the work-up, as the clinical history warrants. More typically, time is spent ruling out the following common causes.
Continue to: Orthostatic hypotension
Orthostatic hypotension. Orthostatic vitals are recommended initially in all patients with dizziness, although these may be normal in patients with orthostatic hypotension.17 A diagnosis of orthostatic hypotension can be made with systolic blood pressure decreases of 20 mm Hg or diastolic pressure decreases of ≥10 mm Hg within 3 minutes of standing.18 An increase in heart rate >30 beats per minute after rising from a supine position may indicate autonomic disturbances such as postural orthostatic tachycardia syndrome.19 However, physical examination findings alone are insufficient to make the diagnosis of orthostatic hypotension, and determining the underlying cause of the orthostatic hypotension (dehydration, cardiac dysfunction, pure-autonomic failure, medication adverse effect) is vital.18
BPPV. Perform the Dix-Hallpike maneuver (see https://collections.lib.utah.edu/details?id=177177 for a demonstration of the maneuver) on patients presenting with dizziness with features suggestive of BPPV (eg, attacks of dizziness triggered by head movements).20,21
As BPPV is the most common cause of vestibular dizziness, a negative Dix-Hallpike can be helpful in refining the differential diagnosis.20,21 The maneuver begins with the patient seated, looking directly ahead. To test the left side, ask the patient to turn his/her head 45 degrees to the left. Then direct the patient to lie back, so that the patient’s head is off the edge of the examination table and hyperextended, while maintaining the same head orientation. To test the right side, repeat the procedure with the patient turning his/her head to the right.
Torsional nystagmus is necessary for a positive Dix-Hallpike, which is diagnostic for BPPV. The laterality of BPPV can be determined by paying attention to the fast phase of the torsional nystagmus; the superior pole of the eye beats toward the affected side.14 The patient may report severe dizziness or vertigo during the Dix-Hallpike, but without torsional nystagmus, the test is negative, and the patient does not have BPPV.14
Neurologic causes. Perform a complete neurologic examination in patients who clearly do not have a history of orthostatic hypotension and who have a Dix-Hallpike test that is negative or not indicated.4 Also perform cerebellar testing including rapid-alternating movements, a finger-to-nose test, and a heel-to-shin test. Round out the neurologic exam with an assessment of gait and a Romberg’s test (see https://www.youtube.com/watch?v=U5a4lbmwmOw for a demonstration of Romberg’s test). Romberg’s test is performed by having the patient place his/her feet together with hands at sides and eyes closed. The patient is observed for up to a minute, with a positive test denoted by a loss of balance.
Continue to: Abnormal gait may indicate...
Abnormal gait may indicate peripheral neuropathy, while a positive Romberg’s test suggests involvement of the proprioceptive receptors and/or their pathway.
Central/peripheral vestibular causes. The head impulse, nystagmus, test of skew (HINTS) examination can differentiate between central and peripheral vestibular causes of dizziness and rule out stroke (a central vestibular cause).22 (See https://collections.lib.utah.edu/details?id=177180 for a video demonstrating the steps involved in performing the HINTS examination.) The head impulse (HI) portion of the exam is performed by moving the patient’s head side to side, while having the patient focus on the examiner’s nose. Rapid movements of both eyes (“abnormal” HI) suggest a peripheral etiology, while no eye movement with gaze fixated on the examiner’s nose (“normal” HI) is concerning for stroke or another central cause of vertigo.22
Nystagmus is assessed by having the patient follow the examiner’s finger as it moves in a horizontal direction. Spontaneous horizontal unidirectional nystagmus suggests a peripheral cause, while vertical or torsional bidirectional (direction-changing) nystagmus points to a central cause.22
The test of skew is executed by covering and uncovering each of the patient’s eyes, while asking the patient to look ahead. Vertical deviation of the eye after uncovering suggests a central etiology, more specifically one involving the brainstem.22
Diagnostic testing/imaging has a limited, but pivotal role
There is a limited role for routine laboratory testing in patients with dizziness. However, for those patients with underlying medical conditions (eg, diabetes), which may contribute to the symptoms, routine blood work can be ordered (ie, finger-stick blood glucose test).22
Continue to: More worrisome suspicions
More worrisome suspicions. Patients suspected of cardiac causes should have a full cardiac work-up performed.22 For suspected stroke, brain tumor, or head trauma, specific computed tomography or magnetic resonance imaging can be arranged.22 Carotid doppler can be used if dizziness is suspected to be caused by orthostatic hypotension or a vascular cause.23
Audiologic and vestibular testing. Audiologic testing is not routinely recommended and is only warranted in instances when patients report hearing loss or changes. Referral to an otolaryngologist for vestibular testing is warranted once life-threatening and alternate etiologies have been ruled out, and a vestibular disorder remains at the top of the differential.24
Treatment hinges on cause and may be multifaceted
Treatment hinges on the specific cause of the patient’s dizziness and may involve useful maneuvers, medication, physiotherapy, or perhaps even surgery.
Employ a particle repositioning maneuver for BPPV
A positive Dix-Hallpike test should prompt the use of a particle repositioning maneuver (PRM) to treat BPPV.21 The goal of PRMs, such as the Epley maneuver (see https://www.youtube.com/watch?v=9SLm76jQg3g for a demonstration of this maneuver), is to move the head in such a way as to return displaced otoliths in the semicircular canal back to the utricle. The Epley maneuver is specific for treating posterior semicircular canal BPPV, which is the most common variant.
Performing the Epley maneuver. To perform the Epley PRM for correction of an otolith in the left posterior semicircular canal, ask the patient to sit and look straight ahead. Lay the patient back, while asking the patient to turn his/her head 45 degrees to the left side. Then ask the patient to turn his/her head 45 degrees to the right side. Instruct the patient to maintain the same 45-degree head orientation, while rolling over to his/her right shoulder, ending in the right decubitus position. Conclude the maneuver by having the patient sit up.
Continue to: Performing the barbecue roll maneuver
Performing the barbecue roll maneuver. Different PRMs exist to treat less common variants of BPPV, including the “barbecue roll” maneuver for horizontal BPPV (see https://www.youtube.com/watch?v=mwTmM6uF5yA for a demonstration of this maneuver).25 The barbecue roll maneuver is initiated with the patient looking ahead and lying back. For a left-sided horizontal canal otolith, the patient first turns to the left decubitus position, then moves clockwise to the right decubitus position, stopping at each position for approximately 20 seconds, all while maintaining a straight head position. The patient then turns clockwise into a prone position, pausing, and finally turning into the left decubitus position again. The maneuver is completed with the patient sitting up.
Medications are used to treat symptoms and/or underlying causes
Adjustments in antihypertensives can be made in cases of orthostatic hypotension.17 Antiemetics (ondansetron, promethazine, metoclopramide), antihistamines (meclizine, dimenhydrinate, diphenhydramine), and benzodiazepines (lorazepam, diazepam) may be used during acute and brief vertiginous episodes to decrease symptom severity after central causes have been ruled out.26,27 However, patients with BPPV should avoid these medications as they may blunt central compensation and increase the risk of falls.27 Research has shown betahistine to improve vertigo control only in patients with Meniere’s disease and only when taken regularly and prophylactically.28 Therefore, do not prescribe betahistine for all other causes of dizziness/vertigo.28
Consider physiotherapy
All patients with dizziness/vertigo, and particularly those presenting with primary balance concerns, may benefit from vestibular rehabilitation therapy (VRT). This is an exercise-based program focusing on habituation of dizziness and improvement of postural stability.29 VRT can improve dizziness associated with central and peripheral vestibular lesions, vertigo of uncertain etiology, and psychogenic vertigo.30 Typically, the vestibular physiotherapist will provide home exercises for the patient, reducing the cost and inconvenience of attending multiple sessions.
Surgery and referrals
Referrals for surgery are rare and are typically reserved for refractory causes of vestibular disease, such as Meniere’s disease, BPPV, SSCD syndrome.31
Referral to the ED is warranted for symptom control if an acute vertiginous episode is refractory to initial management. Emergent or urgent neurology consultation is indicated for suspected or confirmed central disorders. Urgent cardiology referral is recommended for patients with symptoms of presyncope/syncope, arrhythmia, or persistent orthostatic hypotension after conservative management. Outpatient referral to an otolaryngologist is warranted if the patient has failed a course of balance physiotherapy, has a persistently positive Dix-Hallpike test after a PRM and vestibular/balance physiotherapy, or has asymmetric hearing loss.
Continue to: Management starts with primary and secondary prevention
Management starts with primary and secondary prevention
Patient education is essential for avoiding potential triggers of dizziness. Patients with orthostatic hypotension should be educated about the need to correct the underlying mechanism, including the need for adequate hydration and recognition of offending medications and contributory conditions/situations (caffeine, heat, standing quickly).17 Encouraging balance maintenance through exercise and physiotherapy can help with gait and musculoskeletal disorders, and reducing harmful habits (smoking, poor diet, no exercise) can lead to overall improved cardiovascular health.32 Advise those with Meniere’s disease to avoid potential triggers such as caffeine, high sodium foods, and alcohol.33
CORRESPONDENCE
Jason A. Beyea, MD, PhD, FRCSC, Otology/Neurotology, Assistant Professor, Department of Otolaryngology, Queen's University, 144 Brock Street, Kingston, Ontario, Canada, K7L 5G2; [email protected].
1. Bisdorff A, Von Brevern M, Lempert T, et al. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res. 2009;19:1-13.
2. Mendel B, Bergenius J, Langius-Eklöf A. Dizziness: a common, troublesome symptom but often treatable. J Vestib Res. 2010;20:391-398.
3. Gopinath B, McMahon CM, Rochtchina E, et al. Dizziness and vertigo in an older population: the Blue Mountains prospective cross‐sectional study. Clin Otolaryngol. 2009;34:552-556.
4. Post RE, Dickerson LM. Dizziness: a diagnostic approach. Am Fam Physician. 2010;82:361-368.
5. Sloan PD. Dizziness in primary care. Results from the National Ambulatory Care Survey. Fam Pract. 1989;29:33-38.
6. Kerber KA, Meurer WJ, West BT, et al. Dizziness presentations in US emergency departments, 1995–2004. Acad Emerg Med. 2008;15:744-750.
7. Newman-Toker DE, Hsieh YH, Camargo CA Jr, et al. Spectrum of dizziness visits to US emergency departments: cross-sectional analysis from a nationally representative sample. Mayo Clin Proc. 2008;83:765-775.
8. Ponka D, Kirlew M. Top 10 differential diagnoses in family medicine: vertigo and dizziness. Can Fam Physician. 2007;53:1959.
9. Chan Y. Differential diagnosis of dizziness. Curr Opin in Otolaryngol Head Neck Surg. 2009;17:200-203.
10. Staab JP, Ruckenstein MJ. Expanding the differential diagnosis of chronic dizziness. Arch Otolaryngol Head Neck Surg. 2007;133:170-176.
11. Kutz JW Jr. The dizzy patient. Med Clin North Am. 2010;94:989-1002.
12. Jahn K, Kressig RW, Bridenbaugh SA, et al. Dizziness and unstable gait in old age: etiology, diagnosis and treatment. Dtsch Ärztebl Int. 2015;112:387-393.
13. Thompson TL, Amedee R. Vertigo: a review of common peripheral and central vestibular disorders. Ochsner J. 2009;9:20-26.
14. Parnes LS, Agrawal SK, Atlas J. Diagnosis and management of benign paroxysmal positional vertigo (BPPV). CMAJ. 2003;169:681-693.
15. Newman-Toker DE, Dy FJ, Stanton VA, et al. How often is dizziness from primary cardiovascular disease true vertigo? A systematic review. J Gen Intern Med. 2008;23:2087-2094.
16. Newman-Toker DE, Edlow JA. TiTrATE: a novel, evidence-based approach to diagnosing acute dizziness and vertigo. Neurol Clin. 2015;33:577-599.
17. Shibao C, Lipsitz LA, Biaggioni I. ASH position paper: evaluation and treatment of orthostatic hypotension. J Clin Hypertens (Greenwich). 2013;15:147-153.
18. Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res. 1996;6:125-126.
19. Agarwal AK, Garg R, Ritch A, et al. Postural orthostatic tachycardia syndrome. Postgrad Med J. 2007;83:478-480.
20. Halker RB, Barrs DM, Wellik KE, et al. Establishing a diagnosis of benign paroxysmal positional vertigo through the dix-hallpike and side-lying maneuvers: a critically appraised topic. Neurologist. 2008;14:201-204.
21. Hilton MP, Pinder DK. The Epley (canalith repositioning) manoeuvre for benign paroxysmal positional vertigo. Cochrane Database Syst Rev. 2014;(12):CD003162.
22. Kattah JC, Talkad AV, Wang DZ, et al. HINTS to diagnose stroke in the acute vestibular syndrome. Stroke. 2009;40:3504-3510.
23. Hamaguchi T, Iwasa K, Okino S, et al. Carotid duplex ultrasonography during head-up tilt in patients with orthostatic hypotension. Eur Neurol. 2007;57:219-222.
24. Canadian Society of Otolaryngology - Head & Neck Surgery. Five Things Physicians and Patients Should Question [Internet]. Choosing Wisely Canada. 2016 [cited 2017 August 17]. Available at: https://choosingwiselycanada.org/wp-content/uploads/2017/02/Hospital-medicine.pdf. Accessed August 30, 2017.
25. Lee SH, Kim JS. Benign paroxysmal positional vertigo. J Clin Neurol. 2010;6:51-63.
26. Zatonski T, Temporale H, Holanowska J, et al. Current views of treatment of vertigo and dizziness. J Med Diagn Meth. 2014;2:150.
27. Wipperman J. Dizziness and vertigo. Prim Care Clin Office Pract. 2014;41:115-131
28. Murdin L, Hussain K, Schilder AG. Betahistine for symptoms of vertigo. Cochrane Database Syst Rev. 2016;(6):CD010696.
29. Han BI, Song HS, Kim JS. Vestibular rehabilitation therapy: review of indications, mechanisms, and key exercises. J Clin Neurol. 2011;7:184-196.
30. Jung JY, Kim JS, Chung PS, et al. Effect of vestibular rehabilitation on dizziness in the elderly. Am J Otolaryngol. 2009;30:295-299.
31. Semaan MT, Megerian CA. Meniere’s disease: a challenging and relentless disorder. Otolaryngol Clin North Am. 2011;44:383-403.
32. Pirker W, Katzenschlager R. Gait disorders in adults and the elderly. Wien Klin Wochenschr. 2017;129:81-95.
33. Kirby SE, Yardley L. Physical and psychological triggers for attacks in Ménière’s disease: the patient perspective. Psychother Psychosom. 2012;81:396-398.
Dizziness. Vertigo. These 2 terms are often used interchangeably by patients, with the sensations described as imbalance, lightheadedness, disorientation, presyncope, confusion—among others. While dizziness is a broad term that is often used to describe all the aforementioned sensations, including vertigo, true vertigo (a specific type of dizziness) is defined as the perception of movement within one’s visual field while stationary.1 Because patients are not usually aware of the distinction, their reports of signs and symptoms can cause much confusion for health care providers, thereby delaying a diagnosis.
International studies have reported the prevalence of both dizziness and vertigo to be between 15% and 36%.2,3 Over half of all patients with dizziness and vertigo are cared for by the family physician (FP), and the sensations combined account for approximately 5% of all family medicine visits.4,5 Additionally, between 2.5% and 4% of all emergency department (ED) visits stem from complaints of dizziness and vertigo, with an incidence of up to 25% in those >65 years of age.6,7
Causes of dizziness and vertigo are broad, ranging from the benign to the life-threatening. It has been reported that upwards of 50% of patients presenting to the FP’s office for dizziness leave without a diagnosis.8 Given the confusion surrounding the terms and their broad differential, this review aims to provide FPs with the tools to accurately discern benign from ominous causes.
Nonvestibular benign causes vastly outnumber life-threatening ones
Causes of dizziness are classified as either vestibular (these cause true vertigo) or nonvestibular in origin, with nonvestibular causes being more common.7
Nonvestibular etiologies: Numerous and varied
Nonvestibular causes are broad, spanning many different body systems. Cardiovascular causes of dizziness may include orthostatic hypotension, cardiac arrhythmia, myocardial infarction, and carotid artery stenosis.4,9 Metabolic causes include complications of diabetes such as hypoglycemia and peripheral neuropathy.4,9 Psychiatric conditions such as anxiety, depression, and bipolar disorder can manifest as dizziness, disorientation, or psychogenic vertigo.4,10 Medications including nonsteroidal anti-inflammatory drugs, anticonvulsants, antipsychotics, and sedatives can all contribute to dizziness.11 Other causes of dizziness include Parkinson’s disease, musculoskeletal disorders, and gait disorders.4,9 Especially in the elderly, sensory deficit (peripheral neuropathy), poor vision, and polypharmacy (≥5 medications) are common causes of dizziness.12
Vestibular etiologies of dizziness = true vertigo
Vestibular causes of a patient’s feelings of dizziness manifest as true vertigo and can be categorized as either central (a dysfunction of one or more parts of the central nervous system that help process balance and spatial information or along the pathway where these sensations are interpreted) or peripheral (a dysfunction of the balance organs of the inner ear) in origin.
Central vestibular causes include vertebrobasilar ischemic stroke, vertebrobasilar insufficiency (transient ischemic attack), vestibular migraines, and meningioma of the cerebellopontine angle and posterior fossa.13
Continue to: Peripheral vestibular causes
Peripheral vestibular causes. Benign paroxysmal positional vertigo (BPPV) represents the most common peripheral diagnosis. It is caused by dislodged otoliths in the posterior semicircular canal. While the majority of BPPV cases are idiopathic in nature, up to 15% may result from previous head injury.14 Other peripheral vestibular causes include vestibular neuronitis, viral labyrinthitis, Meniere’s disease, vestibular schwannoma, perilymphatic fistula, superior semicircular canal dehiscence (SSCD), and head trauma (basilar skull fracture).13
Start with a history: Is it dizziness or true vertigo?
The clinical history typically guides the differential diagnosis (FIGURE). Identifying true vertigo from among other sensations helps to limit the differential because true vertigo is caused by vestibular etiologies only. True vertigo is often reported by patients as “seeing the room spin;” this stems from the perception of motion.1 A notable exception is that patients with orthostatic hypotension will often describe spinning sensations lasting seconds to minutes when they rise from a seated or supine position.

Never depend solely, however, on patient-reported sensations, as not all patients with true vertigo report spinning, and some patients with nonvestibular causes interpret their dizziness as a spinning sensation.15 Therefore, it is important to tease out specifics about the timing, triggers, and associated symptoms in order to further delineate possible causes (TABLE).16

Make a list of current medications. Gather a comprehensive list of current medications, especially from elderly patients, because polypharmacy is a major contributor to dizziness in this population.12 Keep in mind that elderly patients presenting with dizziness/vertigo may have multifactorial balance difficulties, which can be revealed by a detailed history.
Physical exam: May be broad or focused
Given the broad range of causes for dizziness, cardiovascular, head/neck, and neurologic examinations may be performed as part of the work-up, as the clinical history warrants. More typically, time is spent ruling out the following common causes.
Continue to: Orthostatic hypotension
Orthostatic hypotension. Orthostatic vitals are recommended initially in all patients with dizziness, although these may be normal in patients with orthostatic hypotension.17 A diagnosis of orthostatic hypotension can be made with systolic blood pressure decreases of 20 mm Hg or diastolic pressure decreases of ≥10 mm Hg within 3 minutes of standing.18 An increase in heart rate >30 beats per minute after rising from a supine position may indicate autonomic disturbances such as postural orthostatic tachycardia syndrome.19 However, physical examination findings alone are insufficient to make the diagnosis of orthostatic hypotension, and determining the underlying cause of the orthostatic hypotension (dehydration, cardiac dysfunction, pure-autonomic failure, medication adverse effect) is vital.18
BPPV. Perform the Dix-Hallpike maneuver (see https://collections.lib.utah.edu/details?id=177177 for a demonstration of the maneuver) on patients presenting with dizziness with features suggestive of BPPV (eg, attacks of dizziness triggered by head movements).20,21
As BPPV is the most common cause of vestibular dizziness, a negative Dix-Hallpike can be helpful in refining the differential diagnosis.20,21 The maneuver begins with the patient seated, looking directly ahead. To test the left side, ask the patient to turn his/her head 45 degrees to the left. Then direct the patient to lie back, so that the patient’s head is off the edge of the examination table and hyperextended, while maintaining the same head orientation. To test the right side, repeat the procedure with the patient turning his/her head to the right.
Torsional nystagmus is necessary for a positive Dix-Hallpike, which is diagnostic for BPPV. The laterality of BPPV can be determined by paying attention to the fast phase of the torsional nystagmus; the superior pole of the eye beats toward the affected side.14 The patient may report severe dizziness or vertigo during the Dix-Hallpike, but without torsional nystagmus, the test is negative, and the patient does not have BPPV.14
Neurologic causes. Perform a complete neurologic examination in patients who clearly do not have a history of orthostatic hypotension and who have a Dix-Hallpike test that is negative or not indicated.4 Also perform cerebellar testing including rapid-alternating movements, a finger-to-nose test, and a heel-to-shin test. Round out the neurologic exam with an assessment of gait and a Romberg’s test (see https://www.youtube.com/watch?v=U5a4lbmwmOw for a demonstration of Romberg’s test). Romberg’s test is performed by having the patient place his/her feet together with hands at sides and eyes closed. The patient is observed for up to a minute, with a positive test denoted by a loss of balance.
Continue to: Abnormal gait may indicate...
Abnormal gait may indicate peripheral neuropathy, while a positive Romberg’s test suggests involvement of the proprioceptive receptors and/or their pathway.
Central/peripheral vestibular causes. The head impulse, nystagmus, test of skew (HINTS) examination can differentiate between central and peripheral vestibular causes of dizziness and rule out stroke (a central vestibular cause).22 (See https://collections.lib.utah.edu/details?id=177180 for a video demonstrating the steps involved in performing the HINTS examination.) The head impulse (HI) portion of the exam is performed by moving the patient’s head side to side, while having the patient focus on the examiner’s nose. Rapid movements of both eyes (“abnormal” HI) suggest a peripheral etiology, while no eye movement with gaze fixated on the examiner’s nose (“normal” HI) is concerning for stroke or another central cause of vertigo.22
Nystagmus is assessed by having the patient follow the examiner’s finger as it moves in a horizontal direction. Spontaneous horizontal unidirectional nystagmus suggests a peripheral cause, while vertical or torsional bidirectional (direction-changing) nystagmus points to a central cause.22
The test of skew is executed by covering and uncovering each of the patient’s eyes, while asking the patient to look ahead. Vertical deviation of the eye after uncovering suggests a central etiology, more specifically one involving the brainstem.22
Diagnostic testing/imaging has a limited, but pivotal role
There is a limited role for routine laboratory testing in patients with dizziness. However, for those patients with underlying medical conditions (eg, diabetes), which may contribute to the symptoms, routine blood work can be ordered (ie, finger-stick blood glucose test).22
Continue to: More worrisome suspicions
More worrisome suspicions. Patients suspected of cardiac causes should have a full cardiac work-up performed.22 For suspected stroke, brain tumor, or head trauma, specific computed tomography or magnetic resonance imaging can be arranged.22 Carotid doppler can be used if dizziness is suspected to be caused by orthostatic hypotension or a vascular cause.23
Audiologic and vestibular testing. Audiologic testing is not routinely recommended and is only warranted in instances when patients report hearing loss or changes. Referral to an otolaryngologist for vestibular testing is warranted once life-threatening and alternate etiologies have been ruled out, and a vestibular disorder remains at the top of the differential.24
Treatment hinges on cause and may be multifaceted
Treatment hinges on the specific cause of the patient’s dizziness and may involve useful maneuvers, medication, physiotherapy, or perhaps even surgery.
Employ a particle repositioning maneuver for BPPV
A positive Dix-Hallpike test should prompt the use of a particle repositioning maneuver (PRM) to treat BPPV.21 The goal of PRMs, such as the Epley maneuver (see https://www.youtube.com/watch?v=9SLm76jQg3g for a demonstration of this maneuver), is to move the head in such a way as to return displaced otoliths in the semicircular canal back to the utricle. The Epley maneuver is specific for treating posterior semicircular canal BPPV, which is the most common variant.
Performing the Epley maneuver. To perform the Epley PRM for correction of an otolith in the left posterior semicircular canal, ask the patient to sit and look straight ahead. Lay the patient back, while asking the patient to turn his/her head 45 degrees to the left side. Then ask the patient to turn his/her head 45 degrees to the right side. Instruct the patient to maintain the same 45-degree head orientation, while rolling over to his/her right shoulder, ending in the right decubitus position. Conclude the maneuver by having the patient sit up.
Continue to: Performing the barbecue roll maneuver
Performing the barbecue roll maneuver. Different PRMs exist to treat less common variants of BPPV, including the “barbecue roll” maneuver for horizontal BPPV (see https://www.youtube.com/watch?v=mwTmM6uF5yA for a demonstration of this maneuver).25 The barbecue roll maneuver is initiated with the patient looking ahead and lying back. For a left-sided horizontal canal otolith, the patient first turns to the left decubitus position, then moves clockwise to the right decubitus position, stopping at each position for approximately 20 seconds, all while maintaining a straight head position. The patient then turns clockwise into a prone position, pausing, and finally turning into the left decubitus position again. The maneuver is completed with the patient sitting up.
Medications are used to treat symptoms and/or underlying causes
Adjustments in antihypertensives can be made in cases of orthostatic hypotension.17 Antiemetics (ondansetron, promethazine, metoclopramide), antihistamines (meclizine, dimenhydrinate, diphenhydramine), and benzodiazepines (lorazepam, diazepam) may be used during acute and brief vertiginous episodes to decrease symptom severity after central causes have been ruled out.26,27 However, patients with BPPV should avoid these medications as they may blunt central compensation and increase the risk of falls.27 Research has shown betahistine to improve vertigo control only in patients with Meniere’s disease and only when taken regularly and prophylactically.28 Therefore, do not prescribe betahistine for all other causes of dizziness/vertigo.28
Consider physiotherapy
All patients with dizziness/vertigo, and particularly those presenting with primary balance concerns, may benefit from vestibular rehabilitation therapy (VRT). This is an exercise-based program focusing on habituation of dizziness and improvement of postural stability.29 VRT can improve dizziness associated with central and peripheral vestibular lesions, vertigo of uncertain etiology, and psychogenic vertigo.30 Typically, the vestibular physiotherapist will provide home exercises for the patient, reducing the cost and inconvenience of attending multiple sessions.
Surgery and referrals
Referrals for surgery are rare and are typically reserved for refractory causes of vestibular disease, such as Meniere’s disease, BPPV, SSCD syndrome.31
Referral to the ED is warranted for symptom control if an acute vertiginous episode is refractory to initial management. Emergent or urgent neurology consultation is indicated for suspected or confirmed central disorders. Urgent cardiology referral is recommended for patients with symptoms of presyncope/syncope, arrhythmia, or persistent orthostatic hypotension after conservative management. Outpatient referral to an otolaryngologist is warranted if the patient has failed a course of balance physiotherapy, has a persistently positive Dix-Hallpike test after a PRM and vestibular/balance physiotherapy, or has asymmetric hearing loss.
Continue to: Management starts with primary and secondary prevention
Management starts with primary and secondary prevention
Patient education is essential for avoiding potential triggers of dizziness. Patients with orthostatic hypotension should be educated about the need to correct the underlying mechanism, including the need for adequate hydration and recognition of offending medications and contributory conditions/situations (caffeine, heat, standing quickly).17 Encouraging balance maintenance through exercise and physiotherapy can help with gait and musculoskeletal disorders, and reducing harmful habits (smoking, poor diet, no exercise) can lead to overall improved cardiovascular health.32 Advise those with Meniere’s disease to avoid potential triggers such as caffeine, high sodium foods, and alcohol.33
CORRESPONDENCE
Jason A. Beyea, MD, PhD, FRCSC, Otology/Neurotology, Assistant Professor, Department of Otolaryngology, Queen's University, 144 Brock Street, Kingston, Ontario, Canada, K7L 5G2; [email protected].
Dizziness. Vertigo. These 2 terms are often used interchangeably by patients, with the sensations described as imbalance, lightheadedness, disorientation, presyncope, confusion—among others. While dizziness is a broad term that is often used to describe all the aforementioned sensations, including vertigo, true vertigo (a specific type of dizziness) is defined as the perception of movement within one’s visual field while stationary.1 Because patients are not usually aware of the distinction, their reports of signs and symptoms can cause much confusion for health care providers, thereby delaying a diagnosis.
International studies have reported the prevalence of both dizziness and vertigo to be between 15% and 36%.2,3 Over half of all patients with dizziness and vertigo are cared for by the family physician (FP), and the sensations combined account for approximately 5% of all family medicine visits.4,5 Additionally, between 2.5% and 4% of all emergency department (ED) visits stem from complaints of dizziness and vertigo, with an incidence of up to 25% in those >65 years of age.6,7
Causes of dizziness and vertigo are broad, ranging from the benign to the life-threatening. It has been reported that upwards of 50% of patients presenting to the FP’s office for dizziness leave without a diagnosis.8 Given the confusion surrounding the terms and their broad differential, this review aims to provide FPs with the tools to accurately discern benign from ominous causes.
Nonvestibular benign causes vastly outnumber life-threatening ones
Causes of dizziness are classified as either vestibular (these cause true vertigo) or nonvestibular in origin, with nonvestibular causes being more common.7
Nonvestibular etiologies: Numerous and varied
Nonvestibular causes are broad, spanning many different body systems. Cardiovascular causes of dizziness may include orthostatic hypotension, cardiac arrhythmia, myocardial infarction, and carotid artery stenosis.4,9 Metabolic causes include complications of diabetes such as hypoglycemia and peripheral neuropathy.4,9 Psychiatric conditions such as anxiety, depression, and bipolar disorder can manifest as dizziness, disorientation, or psychogenic vertigo.4,10 Medications including nonsteroidal anti-inflammatory drugs, anticonvulsants, antipsychotics, and sedatives can all contribute to dizziness.11 Other causes of dizziness include Parkinson’s disease, musculoskeletal disorders, and gait disorders.4,9 Especially in the elderly, sensory deficit (peripheral neuropathy), poor vision, and polypharmacy (≥5 medications) are common causes of dizziness.12
Vestibular etiologies of dizziness = true vertigo
Vestibular causes of a patient’s feelings of dizziness manifest as true vertigo and can be categorized as either central (a dysfunction of one or more parts of the central nervous system that help process balance and spatial information or along the pathway where these sensations are interpreted) or peripheral (a dysfunction of the balance organs of the inner ear) in origin.
Central vestibular causes include vertebrobasilar ischemic stroke, vertebrobasilar insufficiency (transient ischemic attack), vestibular migraines, and meningioma of the cerebellopontine angle and posterior fossa.13
Continue to: Peripheral vestibular causes
Peripheral vestibular causes. Benign paroxysmal positional vertigo (BPPV) represents the most common peripheral diagnosis. It is caused by dislodged otoliths in the posterior semicircular canal. While the majority of BPPV cases are idiopathic in nature, up to 15% may result from previous head injury.14 Other peripheral vestibular causes include vestibular neuronitis, viral labyrinthitis, Meniere’s disease, vestibular schwannoma, perilymphatic fistula, superior semicircular canal dehiscence (SSCD), and head trauma (basilar skull fracture).13
Start with a history: Is it dizziness or true vertigo?
The clinical history typically guides the differential diagnosis (FIGURE). Identifying true vertigo from among other sensations helps to limit the differential because true vertigo is caused by vestibular etiologies only. True vertigo is often reported by patients as “seeing the room spin;” this stems from the perception of motion.1 A notable exception is that patients with orthostatic hypotension will often describe spinning sensations lasting seconds to minutes when they rise from a seated or supine position.

Never depend solely, however, on patient-reported sensations, as not all patients with true vertigo report spinning, and some patients with nonvestibular causes interpret their dizziness as a spinning sensation.15 Therefore, it is important to tease out specifics about the timing, triggers, and associated symptoms in order to further delineate possible causes (TABLE).16

Make a list of current medications. Gather a comprehensive list of current medications, especially from elderly patients, because polypharmacy is a major contributor to dizziness in this population.12 Keep in mind that elderly patients presenting with dizziness/vertigo may have multifactorial balance difficulties, which can be revealed by a detailed history.
Physical exam: May be broad or focused
Given the broad range of causes for dizziness, cardiovascular, head/neck, and neurologic examinations may be performed as part of the work-up, as the clinical history warrants. More typically, time is spent ruling out the following common causes.
Continue to: Orthostatic hypotension
Orthostatic hypotension. Orthostatic vitals are recommended initially in all patients with dizziness, although these may be normal in patients with orthostatic hypotension.17 A diagnosis of orthostatic hypotension can be made with systolic blood pressure decreases of 20 mm Hg or diastolic pressure decreases of ≥10 mm Hg within 3 minutes of standing.18 An increase in heart rate >30 beats per minute after rising from a supine position may indicate autonomic disturbances such as postural orthostatic tachycardia syndrome.19 However, physical examination findings alone are insufficient to make the diagnosis of orthostatic hypotension, and determining the underlying cause of the orthostatic hypotension (dehydration, cardiac dysfunction, pure-autonomic failure, medication adverse effect) is vital.18
BPPV. Perform the Dix-Hallpike maneuver (see https://collections.lib.utah.edu/details?id=177177 for a demonstration of the maneuver) on patients presenting with dizziness with features suggestive of BPPV (eg, attacks of dizziness triggered by head movements).20,21
As BPPV is the most common cause of vestibular dizziness, a negative Dix-Hallpike can be helpful in refining the differential diagnosis.20,21 The maneuver begins with the patient seated, looking directly ahead. To test the left side, ask the patient to turn his/her head 45 degrees to the left. Then direct the patient to lie back, so that the patient’s head is off the edge of the examination table and hyperextended, while maintaining the same head orientation. To test the right side, repeat the procedure with the patient turning his/her head to the right.
Torsional nystagmus is necessary for a positive Dix-Hallpike, which is diagnostic for BPPV. The laterality of BPPV can be determined by paying attention to the fast phase of the torsional nystagmus; the superior pole of the eye beats toward the affected side.14 The patient may report severe dizziness or vertigo during the Dix-Hallpike, but without torsional nystagmus, the test is negative, and the patient does not have BPPV.14
Neurologic causes. Perform a complete neurologic examination in patients who clearly do not have a history of orthostatic hypotension and who have a Dix-Hallpike test that is negative or not indicated.4 Also perform cerebellar testing including rapid-alternating movements, a finger-to-nose test, and a heel-to-shin test. Round out the neurologic exam with an assessment of gait and a Romberg’s test (see https://www.youtube.com/watch?v=U5a4lbmwmOw for a demonstration of Romberg’s test). Romberg’s test is performed by having the patient place his/her feet together with hands at sides and eyes closed. The patient is observed for up to a minute, with a positive test denoted by a loss of balance.
Continue to: Abnormal gait may indicate...
Abnormal gait may indicate peripheral neuropathy, while a positive Romberg’s test suggests involvement of the proprioceptive receptors and/or their pathway.
Central/peripheral vestibular causes. The head impulse, nystagmus, test of skew (HINTS) examination can differentiate between central and peripheral vestibular causes of dizziness and rule out stroke (a central vestibular cause).22 (See https://collections.lib.utah.edu/details?id=177180 for a video demonstrating the steps involved in performing the HINTS examination.) The head impulse (HI) portion of the exam is performed by moving the patient’s head side to side, while having the patient focus on the examiner’s nose. Rapid movements of both eyes (“abnormal” HI) suggest a peripheral etiology, while no eye movement with gaze fixated on the examiner’s nose (“normal” HI) is concerning for stroke or another central cause of vertigo.22
Nystagmus is assessed by having the patient follow the examiner’s finger as it moves in a horizontal direction. Spontaneous horizontal unidirectional nystagmus suggests a peripheral cause, while vertical or torsional bidirectional (direction-changing) nystagmus points to a central cause.22
The test of skew is executed by covering and uncovering each of the patient’s eyes, while asking the patient to look ahead. Vertical deviation of the eye after uncovering suggests a central etiology, more specifically one involving the brainstem.22
Diagnostic testing/imaging has a limited, but pivotal role
There is a limited role for routine laboratory testing in patients with dizziness. However, for those patients with underlying medical conditions (eg, diabetes), which may contribute to the symptoms, routine blood work can be ordered (ie, finger-stick blood glucose test).22
Continue to: More worrisome suspicions
More worrisome suspicions. Patients suspected of cardiac causes should have a full cardiac work-up performed.22 For suspected stroke, brain tumor, or head trauma, specific computed tomography or magnetic resonance imaging can be arranged.22 Carotid doppler can be used if dizziness is suspected to be caused by orthostatic hypotension or a vascular cause.23
Audiologic and vestibular testing. Audiologic testing is not routinely recommended and is only warranted in instances when patients report hearing loss or changes. Referral to an otolaryngologist for vestibular testing is warranted once life-threatening and alternate etiologies have been ruled out, and a vestibular disorder remains at the top of the differential.24
Treatment hinges on cause and may be multifaceted
Treatment hinges on the specific cause of the patient’s dizziness and may involve useful maneuvers, medication, physiotherapy, or perhaps even surgery.
Employ a particle repositioning maneuver for BPPV
A positive Dix-Hallpike test should prompt the use of a particle repositioning maneuver (PRM) to treat BPPV.21 The goal of PRMs, such as the Epley maneuver (see https://www.youtube.com/watch?v=9SLm76jQg3g for a demonstration of this maneuver), is to move the head in such a way as to return displaced otoliths in the semicircular canal back to the utricle. The Epley maneuver is specific for treating posterior semicircular canal BPPV, which is the most common variant.
Performing the Epley maneuver. To perform the Epley PRM for correction of an otolith in the left posterior semicircular canal, ask the patient to sit and look straight ahead. Lay the patient back, while asking the patient to turn his/her head 45 degrees to the left side. Then ask the patient to turn his/her head 45 degrees to the right side. Instruct the patient to maintain the same 45-degree head orientation, while rolling over to his/her right shoulder, ending in the right decubitus position. Conclude the maneuver by having the patient sit up.
Continue to: Performing the barbecue roll maneuver
Performing the barbecue roll maneuver. Different PRMs exist to treat less common variants of BPPV, including the “barbecue roll” maneuver for horizontal BPPV (see https://www.youtube.com/watch?v=mwTmM6uF5yA for a demonstration of this maneuver).25 The barbecue roll maneuver is initiated with the patient looking ahead and lying back. For a left-sided horizontal canal otolith, the patient first turns to the left decubitus position, then moves clockwise to the right decubitus position, stopping at each position for approximately 20 seconds, all while maintaining a straight head position. The patient then turns clockwise into a prone position, pausing, and finally turning into the left decubitus position again. The maneuver is completed with the patient sitting up.
Medications are used to treat symptoms and/or underlying causes
Adjustments in antihypertensives can be made in cases of orthostatic hypotension.17 Antiemetics (ondansetron, promethazine, metoclopramide), antihistamines (meclizine, dimenhydrinate, diphenhydramine), and benzodiazepines (lorazepam, diazepam) may be used during acute and brief vertiginous episodes to decrease symptom severity after central causes have been ruled out.26,27 However, patients with BPPV should avoid these medications as they may blunt central compensation and increase the risk of falls.27 Research has shown betahistine to improve vertigo control only in patients with Meniere’s disease and only when taken regularly and prophylactically.28 Therefore, do not prescribe betahistine for all other causes of dizziness/vertigo.28
Consider physiotherapy
All patients with dizziness/vertigo, and particularly those presenting with primary balance concerns, may benefit from vestibular rehabilitation therapy (VRT). This is an exercise-based program focusing on habituation of dizziness and improvement of postural stability.29 VRT can improve dizziness associated with central and peripheral vestibular lesions, vertigo of uncertain etiology, and psychogenic vertigo.30 Typically, the vestibular physiotherapist will provide home exercises for the patient, reducing the cost and inconvenience of attending multiple sessions.
Surgery and referrals
Referrals for surgery are rare and are typically reserved for refractory causes of vestibular disease, such as Meniere’s disease, BPPV, SSCD syndrome.31
Referral to the ED is warranted for symptom control if an acute vertiginous episode is refractory to initial management. Emergent or urgent neurology consultation is indicated for suspected or confirmed central disorders. Urgent cardiology referral is recommended for patients with symptoms of presyncope/syncope, arrhythmia, or persistent orthostatic hypotension after conservative management. Outpatient referral to an otolaryngologist is warranted if the patient has failed a course of balance physiotherapy, has a persistently positive Dix-Hallpike test after a PRM and vestibular/balance physiotherapy, or has asymmetric hearing loss.
Continue to: Management starts with primary and secondary prevention
Management starts with primary and secondary prevention
Patient education is essential for avoiding potential triggers of dizziness. Patients with orthostatic hypotension should be educated about the need to correct the underlying mechanism, including the need for adequate hydration and recognition of offending medications and contributory conditions/situations (caffeine, heat, standing quickly).17 Encouraging balance maintenance through exercise and physiotherapy can help with gait and musculoskeletal disorders, and reducing harmful habits (smoking, poor diet, no exercise) can lead to overall improved cardiovascular health.32 Advise those with Meniere’s disease to avoid potential triggers such as caffeine, high sodium foods, and alcohol.33
CORRESPONDENCE
Jason A. Beyea, MD, PhD, FRCSC, Otology/Neurotology, Assistant Professor, Department of Otolaryngology, Queen's University, 144 Brock Street, Kingston, Ontario, Canada, K7L 5G2; [email protected].
1. Bisdorff A, Von Brevern M, Lempert T, et al. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res. 2009;19:1-13.
2. Mendel B, Bergenius J, Langius-Eklöf A. Dizziness: a common, troublesome symptom but often treatable. J Vestib Res. 2010;20:391-398.
3. Gopinath B, McMahon CM, Rochtchina E, et al. Dizziness and vertigo in an older population: the Blue Mountains prospective cross‐sectional study. Clin Otolaryngol. 2009;34:552-556.
4. Post RE, Dickerson LM. Dizziness: a diagnostic approach. Am Fam Physician. 2010;82:361-368.
5. Sloan PD. Dizziness in primary care. Results from the National Ambulatory Care Survey. Fam Pract. 1989;29:33-38.
6. Kerber KA, Meurer WJ, West BT, et al. Dizziness presentations in US emergency departments, 1995–2004. Acad Emerg Med. 2008;15:744-750.
7. Newman-Toker DE, Hsieh YH, Camargo CA Jr, et al. Spectrum of dizziness visits to US emergency departments: cross-sectional analysis from a nationally representative sample. Mayo Clin Proc. 2008;83:765-775.
8. Ponka D, Kirlew M. Top 10 differential diagnoses in family medicine: vertigo and dizziness. Can Fam Physician. 2007;53:1959.
9. Chan Y. Differential diagnosis of dizziness. Curr Opin in Otolaryngol Head Neck Surg. 2009;17:200-203.
10. Staab JP, Ruckenstein MJ. Expanding the differential diagnosis of chronic dizziness. Arch Otolaryngol Head Neck Surg. 2007;133:170-176.
11. Kutz JW Jr. The dizzy patient. Med Clin North Am. 2010;94:989-1002.
12. Jahn K, Kressig RW, Bridenbaugh SA, et al. Dizziness and unstable gait in old age: etiology, diagnosis and treatment. Dtsch Ärztebl Int. 2015;112:387-393.
13. Thompson TL, Amedee R. Vertigo: a review of common peripheral and central vestibular disorders. Ochsner J. 2009;9:20-26.
14. Parnes LS, Agrawal SK, Atlas J. Diagnosis and management of benign paroxysmal positional vertigo (BPPV). CMAJ. 2003;169:681-693.
15. Newman-Toker DE, Dy FJ, Stanton VA, et al. How often is dizziness from primary cardiovascular disease true vertigo? A systematic review. J Gen Intern Med. 2008;23:2087-2094.
16. Newman-Toker DE, Edlow JA. TiTrATE: a novel, evidence-based approach to diagnosing acute dizziness and vertigo. Neurol Clin. 2015;33:577-599.
17. Shibao C, Lipsitz LA, Biaggioni I. ASH position paper: evaluation and treatment of orthostatic hypotension. J Clin Hypertens (Greenwich). 2013;15:147-153.
18. Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res. 1996;6:125-126.
19. Agarwal AK, Garg R, Ritch A, et al. Postural orthostatic tachycardia syndrome. Postgrad Med J. 2007;83:478-480.
20. Halker RB, Barrs DM, Wellik KE, et al. Establishing a diagnosis of benign paroxysmal positional vertigo through the dix-hallpike and side-lying maneuvers: a critically appraised topic. Neurologist. 2008;14:201-204.
21. Hilton MP, Pinder DK. The Epley (canalith repositioning) manoeuvre for benign paroxysmal positional vertigo. Cochrane Database Syst Rev. 2014;(12):CD003162.
22. Kattah JC, Talkad AV, Wang DZ, et al. HINTS to diagnose stroke in the acute vestibular syndrome. Stroke. 2009;40:3504-3510.
23. Hamaguchi T, Iwasa K, Okino S, et al. Carotid duplex ultrasonography during head-up tilt in patients with orthostatic hypotension. Eur Neurol. 2007;57:219-222.
24. Canadian Society of Otolaryngology - Head & Neck Surgery. Five Things Physicians and Patients Should Question [Internet]. Choosing Wisely Canada. 2016 [cited 2017 August 17]. Available at: https://choosingwiselycanada.org/wp-content/uploads/2017/02/Hospital-medicine.pdf. Accessed August 30, 2017.
25. Lee SH, Kim JS. Benign paroxysmal positional vertigo. J Clin Neurol. 2010;6:51-63.
26. Zatonski T, Temporale H, Holanowska J, et al. Current views of treatment of vertigo and dizziness. J Med Diagn Meth. 2014;2:150.
27. Wipperman J. Dizziness and vertigo. Prim Care Clin Office Pract. 2014;41:115-131
28. Murdin L, Hussain K, Schilder AG. Betahistine for symptoms of vertigo. Cochrane Database Syst Rev. 2016;(6):CD010696.
29. Han BI, Song HS, Kim JS. Vestibular rehabilitation therapy: review of indications, mechanisms, and key exercises. J Clin Neurol. 2011;7:184-196.
30. Jung JY, Kim JS, Chung PS, et al. Effect of vestibular rehabilitation on dizziness in the elderly. Am J Otolaryngol. 2009;30:295-299.
31. Semaan MT, Megerian CA. Meniere’s disease: a challenging and relentless disorder. Otolaryngol Clin North Am. 2011;44:383-403.
32. Pirker W, Katzenschlager R. Gait disorders in adults and the elderly. Wien Klin Wochenschr. 2017;129:81-95.
33. Kirby SE, Yardley L. Physical and psychological triggers for attacks in Ménière’s disease: the patient perspective. Psychother Psychosom. 2012;81:396-398.
1. Bisdorff A, Von Brevern M, Lempert T, et al. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res. 2009;19:1-13.
2. Mendel B, Bergenius J, Langius-Eklöf A. Dizziness: a common, troublesome symptom but often treatable. J Vestib Res. 2010;20:391-398.
3. Gopinath B, McMahon CM, Rochtchina E, et al. Dizziness and vertigo in an older population: the Blue Mountains prospective cross‐sectional study. Clin Otolaryngol. 2009;34:552-556.
4. Post RE, Dickerson LM. Dizziness: a diagnostic approach. Am Fam Physician. 2010;82:361-368.
5. Sloan PD. Dizziness in primary care. Results from the National Ambulatory Care Survey. Fam Pract. 1989;29:33-38.
6. Kerber KA, Meurer WJ, West BT, et al. Dizziness presentations in US emergency departments, 1995–2004. Acad Emerg Med. 2008;15:744-750.
7. Newman-Toker DE, Hsieh YH, Camargo CA Jr, et al. Spectrum of dizziness visits to US emergency departments: cross-sectional analysis from a nationally representative sample. Mayo Clin Proc. 2008;83:765-775.
8. Ponka D, Kirlew M. Top 10 differential diagnoses in family medicine: vertigo and dizziness. Can Fam Physician. 2007;53:1959.
9. Chan Y. Differential diagnosis of dizziness. Curr Opin in Otolaryngol Head Neck Surg. 2009;17:200-203.
10. Staab JP, Ruckenstein MJ. Expanding the differential diagnosis of chronic dizziness. Arch Otolaryngol Head Neck Surg. 2007;133:170-176.
11. Kutz JW Jr. The dizzy patient. Med Clin North Am. 2010;94:989-1002.
12. Jahn K, Kressig RW, Bridenbaugh SA, et al. Dizziness and unstable gait in old age: etiology, diagnosis and treatment. Dtsch Ärztebl Int. 2015;112:387-393.
13. Thompson TL, Amedee R. Vertigo: a review of common peripheral and central vestibular disorders. Ochsner J. 2009;9:20-26.
14. Parnes LS, Agrawal SK, Atlas J. Diagnosis and management of benign paroxysmal positional vertigo (BPPV). CMAJ. 2003;169:681-693.
15. Newman-Toker DE, Dy FJ, Stanton VA, et al. How often is dizziness from primary cardiovascular disease true vertigo? A systematic review. J Gen Intern Med. 2008;23:2087-2094.
16. Newman-Toker DE, Edlow JA. TiTrATE: a novel, evidence-based approach to diagnosing acute dizziness and vertigo. Neurol Clin. 2015;33:577-599.
17. Shibao C, Lipsitz LA, Biaggioni I. ASH position paper: evaluation and treatment of orthostatic hypotension. J Clin Hypertens (Greenwich). 2013;15:147-153.
18. Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res. 1996;6:125-126.
19. Agarwal AK, Garg R, Ritch A, et al. Postural orthostatic tachycardia syndrome. Postgrad Med J. 2007;83:478-480.
20. Halker RB, Barrs DM, Wellik KE, et al. Establishing a diagnosis of benign paroxysmal positional vertigo through the dix-hallpike and side-lying maneuvers: a critically appraised topic. Neurologist. 2008;14:201-204.
21. Hilton MP, Pinder DK. The Epley (canalith repositioning) manoeuvre for benign paroxysmal positional vertigo. Cochrane Database Syst Rev. 2014;(12):CD003162.
22. Kattah JC, Talkad AV, Wang DZ, et al. HINTS to diagnose stroke in the acute vestibular syndrome. Stroke. 2009;40:3504-3510.
23. Hamaguchi T, Iwasa K, Okino S, et al. Carotid duplex ultrasonography during head-up tilt in patients with orthostatic hypotension. Eur Neurol. 2007;57:219-222.
24. Canadian Society of Otolaryngology - Head & Neck Surgery. Five Things Physicians and Patients Should Question [Internet]. Choosing Wisely Canada. 2016 [cited 2017 August 17]. Available at: https://choosingwiselycanada.org/wp-content/uploads/2017/02/Hospital-medicine.pdf. Accessed August 30, 2017.
25. Lee SH, Kim JS. Benign paroxysmal positional vertigo. J Clin Neurol. 2010;6:51-63.
26. Zatonski T, Temporale H, Holanowska J, et al. Current views of treatment of vertigo and dizziness. J Med Diagn Meth. 2014;2:150.
27. Wipperman J. Dizziness and vertigo. Prim Care Clin Office Pract. 2014;41:115-131
28. Murdin L, Hussain K, Schilder AG. Betahistine for symptoms of vertigo. Cochrane Database Syst Rev. 2016;(6):CD010696.
29. Han BI, Song HS, Kim JS. Vestibular rehabilitation therapy: review of indications, mechanisms, and key exercises. J Clin Neurol. 2011;7:184-196.
30. Jung JY, Kim JS, Chung PS, et al. Effect of vestibular rehabilitation on dizziness in the elderly. Am J Otolaryngol. 2009;30:295-299.
31. Semaan MT, Megerian CA. Meniere’s disease: a challenging and relentless disorder. Otolaryngol Clin North Am. 2011;44:383-403.
32. Pirker W, Katzenschlager R. Gait disorders in adults and the elderly. Wien Klin Wochenschr. 2017;129:81-95.
33. Kirby SE, Yardley L. Physical and psychological triggers for attacks in Ménière’s disease: the patient perspective. Psychother Psychosom. 2012;81:396-398.
From The Journal of Family Practice | 2018;67(8):490-492,495-498.
PRACTICE RECOMMENDATIONS
› Employ the Dix-Hallpike maneuver to diagnose patients presenting with dizziness with features suggestive of benign paroxysmal positional vertigo (BPPV). A
› Use the head impulse, nystagmus, test of skew (HINTS) examination to differentiate between central and peripheral vestibular causes of dizziness and rule out stroke. B
› Prescribe betahistine only for patients with Meniere’s disease and not for patients with other causes of dizziness and/or vertigo. B
› Rely on antiemetics, antihistamines, and benzodiazepines to manage acute and brief episodes of vertigo, but not to treat BPPV because they blunt central compensation. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series

