User login
Bullous Henoch-Schönlein Purpura in Children
Henoch-Schönlein purpura (HSP) is a systemic, small vessel vasculitis affecting the skin, joints, gastrointestinal tract, and kidneys. It usually is self-limited, but relapses can be seen in one-third of cases.1 The classic cutaneous presentation includes palpable purpura localized to the legs and buttocks. Painful hemorrhagic bullae are uncommonly observed in childhood HSP and often could lead to a diagnostic dilemma. We report the case of a patient who presented with atypical features of painful hemorrhagic bullae and provide a review of the literature.
Case Report
An otherwise healthy 14-year-old adolescent girl presented to the hospital with painful ulcerative lesions covering the arms, legs, lower abdomen, and buttocks of 3 weeks’ duration. The rash first appeared on the ankles and spread in an ascending fashion, starting with bullous formation that was accompanied by joint pain, especially in the ankles and elbows. No abdominal pain was reported. The patient attributed the lesions to prolonged cold exposure followed by a hot bath. She had tried naproxen without any improvement of pain. She was afebrile with normal blood pressure.
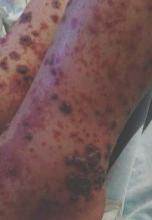
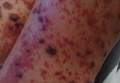
On physical examination, numerous petechiae, palpable purpura, hemorrhagic bullae, and ulcers with surrounding erythematous to violaceous induration as well as central necrosis were noted on the arms, legs (Figure 1), abdomen, and buttocks. The palms, soles, trunk, and face were spared.
Laboratory values on admission revealed leukocytosis (17,500/μL [reference range, 4500–11,000/μL]), elevated erythrocyte sedimentation rate (42 mm/h [reference range, 0–20 mm/h]), elevated C-reactive protein (15.59 mg/L [reference range, 0.08–3.1 mg/L]), elevated C3 (174 mg/dL [reference range, 75–135 mg/dL]), normal C4 (32 mg/dL [reference range, 3–75 mg/dL]), normal blood urea nitrogen (13 mg/dL [reference range, 8–23 mg/dL]), and normal creatinine (0.72 mg/dL [reference range, 0.6–1.2 mg/dL]). Urinalysis showed microscopic hematuria and trace proteinuria. Platelet count was normal.
Diagnostic considerations included HSP, drug-induced leukocytoclastic vasculitis, and bullous pyoderma gangrenosum. The patient was started on oral prednisone 80 mg once daily. Additionally, oral doxycycline 100 mg twice daily was added for prevention of secondary bacterial infections and for anti-inflammatory effects. All nonsteroidal anti-inflammatory drugs were avoided. A commercial ointment containing 8-hydroxyquinoline sulfate 0.3% and triamcinolone acetonide ointment 0.1% were used to minimize skin irritation. Morphine, oxycodone-acetaminophen, and pregabalin followed by gabapentin were used for pain control. Hydrotherapy also was used for the treatment of skin lesions.
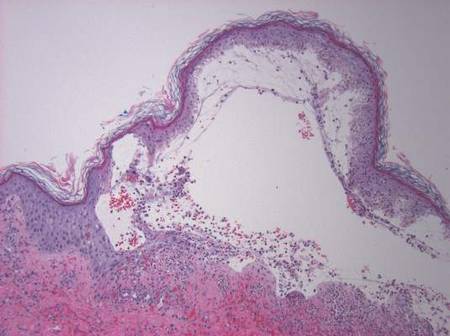
Two skin punch biopsies were performed at different stages. Biopsy of an early palpable purpuric lesion showed small vessel leukocytoclastic vasculitis with perivascular IgA on direct immunofluorescence. A second biopsy from a more hemorrhagic lesion performed 96 hours after admission to the hospital showed subepidermal vesicles with partial epidermal necrosis, confluent neutrophilic infiltrate in the papillary dermis, and small vessel vasculitis (Figures 2 and 3). Gram, periodic acid–Schiff, and acid-fast bacilli staining and cultures were negative. With continued treatment for 7 days, the clinical appearance of the lesions improved. On the tenth day of hospitalization, oral dapsone 25 mg once daily was initiated with the goal of weaning the patient off the prednisone as tolerated. She was discharged on prednisone (60 mg once daily) after 14 days of hospitalization. Dapsone also was continued.
| 
| |
Figure 2. Biopsy of a subepidermal bulla revealed neutrophilic inflammation within bullous space and evidence of dermal hemorrhage (H&E, original magnification ×100). | Figure 3. Leukocytoclastic vasculitis on biopsy (H&E, original magnification ×400). |
At 4-week follow-up, the lesions showed healing with mild residual pigmentation. The patient’s blood pressure and serum urea and creatinine levels were normal but the proteinuria was persistent, so the patient was started on oral lisinopril 5 mg once daily. Tapering of steroids over several months was initiated and the dose of dapsone was increased to 50 mg daily. Follow-up with a nephrologist was arranged to monitor renal function. She continued on lisinopril 5 mg once daily for treatment of nonnephrotic-range proteinuria, which was detected at 6 months following discharge.
Comment
The presence of atypical symptoms such as bullae and painful lesions in patients with suspected HSP can complicate the diagnosis. Initially, one of the top diagnostic considerations in our patient was bullous pyoderma gangrenosum, a neutrophilic dermatosis that typically presents with painful ulcerative lesions and inflammatory bullae. Other causes of bullae in children include erythema multiforme, toxic epidermal necrolysis, epidermolysis bullosa, bullous mastocytosis, pemphigus, bullous pemphigoid, dermatitis herpetiformis, linear IgA dermatosis, bullous impetigo, gangrenous cellulitis, and Vibrio vulnificus infection. However, the clinical symptoms of joint pain and hematuria/proteinuria in our patient as well as the punch biopsy findings pointed toward HSP as the most likely diagnosis.
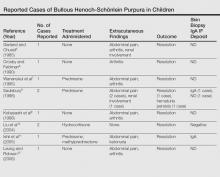
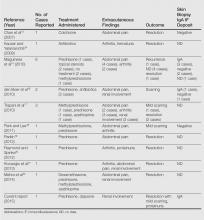
Although bullous lesions are relatively common in adult-onset HSP (16%–60% of patients), they are very rare in pediatric patients (2% of patients).2-4 We performed a PubMed search of articles indexed for MEDLINE for bullous Henoch-Schönlein purpura in childhood using the search term Henoch-Schönlein purpura and bullous. The Table provides a summary of our search results from the English-language literature.5-22
Bullae often develop on several parts of the body but are more commonly observed on the legs.17 Pathergy and edema have been implicated in the pathogenesis, as these findings have been observed in sites such as malleoli and legs, respectively.12 Matrix metalloproteinases secreted in polymorphonuclear neutrophils have been found to be elevated in blister fluid and can cause bullae formation via degrading collagen in the basement membrane.9 Corticosteroids, by virtue of their inhibition of proinflammatory transcription factors (eg, nuclear factor κβ, intranuclear activator protein 1) and decreasing metalloproteinase levels, may be efficacious in bullous HSP. Although there is no consensus, corticosteroid therapy seems to be efficacious in treating the bullae, according to several reports.17-22
The use of glucocorticoids in bullous HSP in childhood remains controversial. Studies report shortening of the duration of abdominal pain, reducing risk of intussusception, decreasing recurrence risk, and reducing the risk of renal involvement with use of steroids in HSP.23-25 The use of systemic steroids has been described in children with bullous HSP to reduce the severity of HSP-related bullae and its associated painful ulcers and necrosis.16,21,25,26 The duration of steroid use ranged from a short burst to a prolonged course of weaning over weeks. Azathioprine also has been used in conjunction with methylprednisolone, prednisone, and dexamethasone.17,22 Because of its anti-IgA antioxidant antineutrophil effects, dapsone has been shown to be effective in the treatment of cutaneous HSP.27 In our patient, we used dapsone to help in weaning the patient off the prednisone. Based on our review of the literature, few cases of bullous HSP in children have reported remission without drug therapy. IgA was not found in all the reported cases in which a skin biopsy was done. As shown by the comparison of the 2 biopsies in our patient, biopsying an early lesion within 48 hours of appearance is essential to make a diagnosis because the biopsy of the older lesion could not rule out bullous pyoderma gangrenosum. Immunoreactants (IgA, C3) are destroyed within 48 hours and might lead to false-negative results on immunofluorescence in old and necrotic lesions.28,29 Most reported cases of bullous HSP showed resolution, but few resulted in scarring and/or pigmentation.10,17,18 Henoch-Schönlein purpura usually is self-limited but relapses can be seen in one-third of cases.1 One of the reported cases of bullous HSP showed recurrence of lesions.15 One of the cases showed persistent hematuria.8 Our patient also was started on lisinopril for persistent proteinuria.
1. Saulsbury FT. Henoch-Schönlein purpura in children. report of 100 patients and the review of literature. Medicine. 1999;78:395-409.
2. Cream JJ, Gumpel JM, Peachey RD. Schönlein-Henoch purpura in the adult. a study of 77 adults with anaphylactoid or Schönlein-Henoch purpura. Q J Med. 1970;39:461-484.
3. Tancrede-Bohin E, Ochonisky S, Vignon-Pennamen MD, et al. Schönlein-Henoch purpura in adult patients. predictive factors for IgA glomerulonephritis in a retrospective study of 57 cases. Arch Dermatol. 1997;133:438-442.
4. Abdel-Al YK, Hejazi Z, Majeed HA. Henoch Schönlein purpura in Arab children. analysis of 52 cases. Trop Geogr Med. 1990;42:52-57.
5. Garland JS, Chusid MJ. Henoch-Schöenlein purpura: association with unusual vesicular lesions. Wis Med J. 1985;84:21-23.
6. Crosby DL, Feldman SD. A pruritic vesicular eruption. Henoch-Schönlein purpura. Arch Dermatol. 1990;126:1497-1498.
7. Wananukul S, Pongprasit P, Korkij W. Henoch-Schönlein purpura presenting as hemorrhagic vesicles and bullae: case report and literature review. Pediatr Dermatol. 1995;12:314-317.
8. Saulsbury FT. Hemorrhagic bullous lesions in Henoch-Schönlein purpura. Pediatr Dermatol. 1998;15:357-359.
9. Kobayashi T, Sakuraoka K, Iwamoto M, et al. A case of anaphylactoid purpura with multiple blister formation: possible pathophysiological role of gelatinase (MMP-9). Dermatology. 1998;197:62-64.
10. Liu PM, Bong CN, Chen HH, et al. Henoch-Schönlein purpura with hemorrhagic bullae in children: report of two cases. J Microbiol Immunol Infect. 2004;37:375-378.
11. Ishii Y, Takizawa T, Arakawa H, et al. Hemorrhagic bullous lesions in Henoch-Schönlein purpura. Pediatr Int. 2005;47:694-697.
12. Leung AK, Robson WL. Hemorrhagic bullous lesions in a child with Henoch-Schönlein purpura. Pediatr Dermatol. 2006;23:139-141.
13. Chan K, Han N, Tang W, et al. Lesions in Henoch-Schönlein purpura. Pediatr Dermatol. 2007;24: 325-326.
14. Kausar S, Yalamanchili A. Management of haemorrhagic bullous lesions in Henoch-Schonlein purpura: is there any consensus? J Dermatolog Treat. 2009;20:88-90.
15. Maguiness S, Balma-Mena A, Pope E, et al. Bullous Henoch-Schönlein purpura in children: a report of 6 cases and review of the literature. Clin Pediatr. 2010;49: 1033-1037.
16. den Boer SL, Pasmans SG, Wulffraat NM, et al. Bullous lesions in Henoch-Schönlein purpura as indication to start systemic prednisone. Acta Paediatr. 2010;99:781-783.
17. Trapani S, Mariotti P, Resti M, et al. Severe hemorrhagic bullous lesions in Henoch Schönlein purpura: three pediatric cases and review of the literature. Rheumatol Int. 2010;30:1355-1359.
18. Park SE, Lee JH. Haemorrhagic bullous lesions in a 3-year-old girl with Henoch-Schönlein purpura. Acta Paediatr. 2011;100:e283-e284.
19. Parikh K. 14-year-old boy with bullous lesions. Pediatr Ann. 2012;41:275-277.
20. Raymond M, Spinks J. Bullous Henoch Schönlein purpura. Arch Dis Child. 2012;97:617.
21. Kocaoglu C, Ozturk R, Unlu Y, et al. Successful treatment of hemorrhagic bullous Henoch-Schönlein purpura with oral corticosteroid: a case report [published online ahead of print April 16, 2013]. Case Rep Pediatr. 2013;2013:680208.
22. Mehra S, Suri D, Dogra S, et al. Hemorrhagic bullous lesions in a girl with Henoch Schönlein purpura. Indian J Pediatr. 2014;81:210-211.
23. Ronkainen J, Koskimies O, Ala-Houhala M, et al. Early prednisone therapy in Henoch-Schönlein purpura: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2006;149:241-247.
24. Weiss PF, Klink AJ, Localio R, et al. Corticosteroids may improve clinical outcomes during hospitalization for Henoch-Schönlein purpura. Pediatrics. 2010;126:674-681.
25. Rosato L, Chehade H, Cachat F. Re: steroids in haemorrhagic bullous Henoch-Schönlein purpura. Acta Paediatr. 2011;100:319-320.
26. Park SJ, Kim JH, Ha TS, et al. The role of corticosteroid in hemorrhagic bullous Henoch Schönlein purpura. Acta Paediatr. 2011;100:e3-e4.
27. Iqbal H, Evans A. Dapsone therapy for Henoch-Schönlein purpura: a case series. Arch Dis Child. 2005;90:985-986.
28. Davin JC, Weening JJ. Diagnosis of Henoch-Schönlein purpura: renal or skin biopsy? Pediatr Nephrol. 2003;18:1201-1203.
29. González LM, Janniger CK, Schwartz RA. Pediatric Henoch-Schönlein purpura. Int J Dermatol. 2009;48: 1157-1165.
Henoch-Schönlein purpura (HSP) is a systemic, small vessel vasculitis affecting the skin, joints, gastrointestinal tract, and kidneys. It usually is self-limited, but relapses can be seen in one-third of cases.1 The classic cutaneous presentation includes palpable purpura localized to the legs and buttocks. Painful hemorrhagic bullae are uncommonly observed in childhood HSP and often could lead to a diagnostic dilemma. We report the case of a patient who presented with atypical features of painful hemorrhagic bullae and provide a review of the literature.
Case Report
An otherwise healthy 14-year-old adolescent girl presented to the hospital with painful ulcerative lesions covering the arms, legs, lower abdomen, and buttocks of 3 weeks’ duration. The rash first appeared on the ankles and spread in an ascending fashion, starting with bullous formation that was accompanied by joint pain, especially in the ankles and elbows. No abdominal pain was reported. The patient attributed the lesions to prolonged cold exposure followed by a hot bath. She had tried naproxen without any improvement of pain. She was afebrile with normal blood pressure.
On physical examination, numerous petechiae, palpable purpura, hemorrhagic bullae, and ulcers with surrounding erythematous to violaceous induration as well as central necrosis were noted on the arms, legs (Figure 1), abdomen, and buttocks. The palms, soles, trunk, and face were spared.
Laboratory values on admission revealed leukocytosis (17,500/μL [reference range, 4500–11,000/μL]), elevated erythrocyte sedimentation rate (42 mm/h [reference range, 0–20 mm/h]), elevated C-reactive protein (15.59 mg/L [reference range, 0.08–3.1 mg/L]), elevated C3 (174 mg/dL [reference range, 75–135 mg/dL]), normal C4 (32 mg/dL [reference range, 3–75 mg/dL]), normal blood urea nitrogen (13 mg/dL [reference range, 8–23 mg/dL]), and normal creatinine (0.72 mg/dL [reference range, 0.6–1.2 mg/dL]). Urinalysis showed microscopic hematuria and trace proteinuria. Platelet count was normal.
Diagnostic considerations included HSP, drug-induced leukocytoclastic vasculitis, and bullous pyoderma gangrenosum. The patient was started on oral prednisone 80 mg once daily. Additionally, oral doxycycline 100 mg twice daily was added for prevention of secondary bacterial infections and for anti-inflammatory effects. All nonsteroidal anti-inflammatory drugs were avoided. A commercial ointment containing 8-hydroxyquinoline sulfate 0.3% and triamcinolone acetonide ointment 0.1% were used to minimize skin irritation. Morphine, oxycodone-acetaminophen, and pregabalin followed by gabapentin were used for pain control. Hydrotherapy also was used for the treatment of skin lesions.
Two skin punch biopsies were performed at different stages. Biopsy of an early palpable purpuric lesion showed small vessel leukocytoclastic vasculitis with perivascular IgA on direct immunofluorescence. A second biopsy from a more hemorrhagic lesion performed 96 hours after admission to the hospital showed subepidermal vesicles with partial epidermal necrosis, confluent neutrophilic infiltrate in the papillary dermis, and small vessel vasculitis (Figures 2 and 3). Gram, periodic acid–Schiff, and acid-fast bacilli staining and cultures were negative. With continued treatment for 7 days, the clinical appearance of the lesions improved. On the tenth day of hospitalization, oral dapsone 25 mg once daily was initiated with the goal of weaning the patient off the prednisone as tolerated. She was discharged on prednisone (60 mg once daily) after 14 days of hospitalization. Dapsone also was continued.

| 
| |
Figure 2. Biopsy of a subepidermal bulla revealed neutrophilic inflammation within bullous space and evidence of dermal hemorrhage (H&E, original magnification ×100). | Figure 3. Leukocytoclastic vasculitis on biopsy (H&E, original magnification ×400). |
At 4-week follow-up, the lesions showed healing with mild residual pigmentation. The patient’s blood pressure and serum urea and creatinine levels were normal but the proteinuria was persistent, so the patient was started on oral lisinopril 5 mg once daily. Tapering of steroids over several months was initiated and the dose of dapsone was increased to 50 mg daily. Follow-up with a nephrologist was arranged to monitor renal function. She continued on lisinopril 5 mg once daily for treatment of nonnephrotic-range proteinuria, which was detected at 6 months following discharge.
Comment
The presence of atypical symptoms such as bullae and painful lesions in patients with suspected HSP can complicate the diagnosis. Initially, one of the top diagnostic considerations in our patient was bullous pyoderma gangrenosum, a neutrophilic dermatosis that typically presents with painful ulcerative lesions and inflammatory bullae. Other causes of bullae in children include erythema multiforme, toxic epidermal necrolysis, epidermolysis bullosa, bullous mastocytosis, pemphigus, bullous pemphigoid, dermatitis herpetiformis, linear IgA dermatosis, bullous impetigo, gangrenous cellulitis, and Vibrio vulnificus infection. However, the clinical symptoms of joint pain and hematuria/proteinuria in our patient as well as the punch biopsy findings pointed toward HSP as the most likely diagnosis.
Although bullous lesions are relatively common in adult-onset HSP (16%–60% of patients), they are very rare in pediatric patients (2% of patients).2-4 We performed a PubMed search of articles indexed for MEDLINE for bullous Henoch-Schönlein purpura in childhood using the search term Henoch-Schönlein purpura and bullous. The Table provides a summary of our search results from the English-language literature.5-22
Bullae often develop on several parts of the body but are more commonly observed on the legs.17 Pathergy and edema have been implicated in the pathogenesis, as these findings have been observed in sites such as malleoli and legs, respectively.12 Matrix metalloproteinases secreted in polymorphonuclear neutrophils have been found to be elevated in blister fluid and can cause bullae formation via degrading collagen in the basement membrane.9 Corticosteroids, by virtue of their inhibition of proinflammatory transcription factors (eg, nuclear factor κβ, intranuclear activator protein 1) and decreasing metalloproteinase levels, may be efficacious in bullous HSP. Although there is no consensus, corticosteroid therapy seems to be efficacious in treating the bullae, according to several reports.17-22
The use of glucocorticoids in bullous HSP in childhood remains controversial. Studies report shortening of the duration of abdominal pain, reducing risk of intussusception, decreasing recurrence risk, and reducing the risk of renal involvement with use of steroids in HSP.23-25 The use of systemic steroids has been described in children with bullous HSP to reduce the severity of HSP-related bullae and its associated painful ulcers and necrosis.16,21,25,26 The duration of steroid use ranged from a short burst to a prolonged course of weaning over weeks. Azathioprine also has been used in conjunction with methylprednisolone, prednisone, and dexamethasone.17,22 Because of its anti-IgA antioxidant antineutrophil effects, dapsone has been shown to be effective in the treatment of cutaneous HSP.27 In our patient, we used dapsone to help in weaning the patient off the prednisone. Based on our review of the literature, few cases of bullous HSP in children have reported remission without drug therapy. IgA was not found in all the reported cases in which a skin biopsy was done. As shown by the comparison of the 2 biopsies in our patient, biopsying an early lesion within 48 hours of appearance is essential to make a diagnosis because the biopsy of the older lesion could not rule out bullous pyoderma gangrenosum. Immunoreactants (IgA, C3) are destroyed within 48 hours and might lead to false-negative results on immunofluorescence in old and necrotic lesions.28,29 Most reported cases of bullous HSP showed resolution, but few resulted in scarring and/or pigmentation.10,17,18 Henoch-Schönlein purpura usually is self-limited but relapses can be seen in one-third of cases.1 One of the reported cases of bullous HSP showed recurrence of lesions.15 One of the cases showed persistent hematuria.8 Our patient also was started on lisinopril for persistent proteinuria.
Henoch-Schönlein purpura (HSP) is a systemic, small vessel vasculitis affecting the skin, joints, gastrointestinal tract, and kidneys. It usually is self-limited, but relapses can be seen in one-third of cases.1 The classic cutaneous presentation includes palpable purpura localized to the legs and buttocks. Painful hemorrhagic bullae are uncommonly observed in childhood HSP and often could lead to a diagnostic dilemma. We report the case of a patient who presented with atypical features of painful hemorrhagic bullae and provide a review of the literature.
Case Report
An otherwise healthy 14-year-old adolescent girl presented to the hospital with painful ulcerative lesions covering the arms, legs, lower abdomen, and buttocks of 3 weeks’ duration. The rash first appeared on the ankles and spread in an ascending fashion, starting with bullous formation that was accompanied by joint pain, especially in the ankles and elbows. No abdominal pain was reported. The patient attributed the lesions to prolonged cold exposure followed by a hot bath. She had tried naproxen without any improvement of pain. She was afebrile with normal blood pressure.
On physical examination, numerous petechiae, palpable purpura, hemorrhagic bullae, and ulcers with surrounding erythematous to violaceous induration as well as central necrosis were noted on the arms, legs (Figure 1), abdomen, and buttocks. The palms, soles, trunk, and face were spared.
Laboratory values on admission revealed leukocytosis (17,500/μL [reference range, 4500–11,000/μL]), elevated erythrocyte sedimentation rate (42 mm/h [reference range, 0–20 mm/h]), elevated C-reactive protein (15.59 mg/L [reference range, 0.08–3.1 mg/L]), elevated C3 (174 mg/dL [reference range, 75–135 mg/dL]), normal C4 (32 mg/dL [reference range, 3–75 mg/dL]), normal blood urea nitrogen (13 mg/dL [reference range, 8–23 mg/dL]), and normal creatinine (0.72 mg/dL [reference range, 0.6–1.2 mg/dL]). Urinalysis showed microscopic hematuria and trace proteinuria. Platelet count was normal.
Diagnostic considerations included HSP, drug-induced leukocytoclastic vasculitis, and bullous pyoderma gangrenosum. The patient was started on oral prednisone 80 mg once daily. Additionally, oral doxycycline 100 mg twice daily was added for prevention of secondary bacterial infections and for anti-inflammatory effects. All nonsteroidal anti-inflammatory drugs were avoided. A commercial ointment containing 8-hydroxyquinoline sulfate 0.3% and triamcinolone acetonide ointment 0.1% were used to minimize skin irritation. Morphine, oxycodone-acetaminophen, and pregabalin followed by gabapentin were used for pain control. Hydrotherapy also was used for the treatment of skin lesions.
Two skin punch biopsies were performed at different stages. Biopsy of an early palpable purpuric lesion showed small vessel leukocytoclastic vasculitis with perivascular IgA on direct immunofluorescence. A second biopsy from a more hemorrhagic lesion performed 96 hours after admission to the hospital showed subepidermal vesicles with partial epidermal necrosis, confluent neutrophilic infiltrate in the papillary dermis, and small vessel vasculitis (Figures 2 and 3). Gram, periodic acid–Schiff, and acid-fast bacilli staining and cultures were negative. With continued treatment for 7 days, the clinical appearance of the lesions improved. On the tenth day of hospitalization, oral dapsone 25 mg once daily was initiated with the goal of weaning the patient off the prednisone as tolerated. She was discharged on prednisone (60 mg once daily) after 14 days of hospitalization. Dapsone also was continued.

| 
| |
Figure 2. Biopsy of a subepidermal bulla revealed neutrophilic inflammation within bullous space and evidence of dermal hemorrhage (H&E, original magnification ×100). | Figure 3. Leukocytoclastic vasculitis on biopsy (H&E, original magnification ×400). |
At 4-week follow-up, the lesions showed healing with mild residual pigmentation. The patient’s blood pressure and serum urea and creatinine levels were normal but the proteinuria was persistent, so the patient was started on oral lisinopril 5 mg once daily. Tapering of steroids over several months was initiated and the dose of dapsone was increased to 50 mg daily. Follow-up with a nephrologist was arranged to monitor renal function. She continued on lisinopril 5 mg once daily for treatment of nonnephrotic-range proteinuria, which was detected at 6 months following discharge.
Comment
The presence of atypical symptoms such as bullae and painful lesions in patients with suspected HSP can complicate the diagnosis. Initially, one of the top diagnostic considerations in our patient was bullous pyoderma gangrenosum, a neutrophilic dermatosis that typically presents with painful ulcerative lesions and inflammatory bullae. Other causes of bullae in children include erythema multiforme, toxic epidermal necrolysis, epidermolysis bullosa, bullous mastocytosis, pemphigus, bullous pemphigoid, dermatitis herpetiformis, linear IgA dermatosis, bullous impetigo, gangrenous cellulitis, and Vibrio vulnificus infection. However, the clinical symptoms of joint pain and hematuria/proteinuria in our patient as well as the punch biopsy findings pointed toward HSP as the most likely diagnosis.
Although bullous lesions are relatively common in adult-onset HSP (16%–60% of patients), they are very rare in pediatric patients (2% of patients).2-4 We performed a PubMed search of articles indexed for MEDLINE for bullous Henoch-Schönlein purpura in childhood using the search term Henoch-Schönlein purpura and bullous. The Table provides a summary of our search results from the English-language literature.5-22
Bullae often develop on several parts of the body but are more commonly observed on the legs.17 Pathergy and edema have been implicated in the pathogenesis, as these findings have been observed in sites such as malleoli and legs, respectively.12 Matrix metalloproteinases secreted in polymorphonuclear neutrophils have been found to be elevated in blister fluid and can cause bullae formation via degrading collagen in the basement membrane.9 Corticosteroids, by virtue of their inhibition of proinflammatory transcription factors (eg, nuclear factor κβ, intranuclear activator protein 1) and decreasing metalloproteinase levels, may be efficacious in bullous HSP. Although there is no consensus, corticosteroid therapy seems to be efficacious in treating the bullae, according to several reports.17-22
The use of glucocorticoids in bullous HSP in childhood remains controversial. Studies report shortening of the duration of abdominal pain, reducing risk of intussusception, decreasing recurrence risk, and reducing the risk of renal involvement with use of steroids in HSP.23-25 The use of systemic steroids has been described in children with bullous HSP to reduce the severity of HSP-related bullae and its associated painful ulcers and necrosis.16,21,25,26 The duration of steroid use ranged from a short burst to a prolonged course of weaning over weeks. Azathioprine also has been used in conjunction with methylprednisolone, prednisone, and dexamethasone.17,22 Because of its anti-IgA antioxidant antineutrophil effects, dapsone has been shown to be effective in the treatment of cutaneous HSP.27 In our patient, we used dapsone to help in weaning the patient off the prednisone. Based on our review of the literature, few cases of bullous HSP in children have reported remission without drug therapy. IgA was not found in all the reported cases in which a skin biopsy was done. As shown by the comparison of the 2 biopsies in our patient, biopsying an early lesion within 48 hours of appearance is essential to make a diagnosis because the biopsy of the older lesion could not rule out bullous pyoderma gangrenosum. Immunoreactants (IgA, C3) are destroyed within 48 hours and might lead to false-negative results on immunofluorescence in old and necrotic lesions.28,29 Most reported cases of bullous HSP showed resolution, but few resulted in scarring and/or pigmentation.10,17,18 Henoch-Schönlein purpura usually is self-limited but relapses can be seen in one-third of cases.1 One of the reported cases of bullous HSP showed recurrence of lesions.15 One of the cases showed persistent hematuria.8 Our patient also was started on lisinopril for persistent proteinuria.
1. Saulsbury FT. Henoch-Schönlein purpura in children. report of 100 patients and the review of literature. Medicine. 1999;78:395-409.
2. Cream JJ, Gumpel JM, Peachey RD. Schönlein-Henoch purpura in the adult. a study of 77 adults with anaphylactoid or Schönlein-Henoch purpura. Q J Med. 1970;39:461-484.
3. Tancrede-Bohin E, Ochonisky S, Vignon-Pennamen MD, et al. Schönlein-Henoch purpura in adult patients. predictive factors for IgA glomerulonephritis in a retrospective study of 57 cases. Arch Dermatol. 1997;133:438-442.
4. Abdel-Al YK, Hejazi Z, Majeed HA. Henoch Schönlein purpura in Arab children. analysis of 52 cases. Trop Geogr Med. 1990;42:52-57.
5. Garland JS, Chusid MJ. Henoch-Schöenlein purpura: association with unusual vesicular lesions. Wis Med J. 1985;84:21-23.
6. Crosby DL, Feldman SD. A pruritic vesicular eruption. Henoch-Schönlein purpura. Arch Dermatol. 1990;126:1497-1498.
7. Wananukul S, Pongprasit P, Korkij W. Henoch-Schönlein purpura presenting as hemorrhagic vesicles and bullae: case report and literature review. Pediatr Dermatol. 1995;12:314-317.
8. Saulsbury FT. Hemorrhagic bullous lesions in Henoch-Schönlein purpura. Pediatr Dermatol. 1998;15:357-359.
9. Kobayashi T, Sakuraoka K, Iwamoto M, et al. A case of anaphylactoid purpura with multiple blister formation: possible pathophysiological role of gelatinase (MMP-9). Dermatology. 1998;197:62-64.
10. Liu PM, Bong CN, Chen HH, et al. Henoch-Schönlein purpura with hemorrhagic bullae in children: report of two cases. J Microbiol Immunol Infect. 2004;37:375-378.
11. Ishii Y, Takizawa T, Arakawa H, et al. Hemorrhagic bullous lesions in Henoch-Schönlein purpura. Pediatr Int. 2005;47:694-697.
12. Leung AK, Robson WL. Hemorrhagic bullous lesions in a child with Henoch-Schönlein purpura. Pediatr Dermatol. 2006;23:139-141.
13. Chan K, Han N, Tang W, et al. Lesions in Henoch-Schönlein purpura. Pediatr Dermatol. 2007;24: 325-326.
14. Kausar S, Yalamanchili A. Management of haemorrhagic bullous lesions in Henoch-Schonlein purpura: is there any consensus? J Dermatolog Treat. 2009;20:88-90.
15. Maguiness S, Balma-Mena A, Pope E, et al. Bullous Henoch-Schönlein purpura in children: a report of 6 cases and review of the literature. Clin Pediatr. 2010;49: 1033-1037.
16. den Boer SL, Pasmans SG, Wulffraat NM, et al. Bullous lesions in Henoch-Schönlein purpura as indication to start systemic prednisone. Acta Paediatr. 2010;99:781-783.
17. Trapani S, Mariotti P, Resti M, et al. Severe hemorrhagic bullous lesions in Henoch Schönlein purpura: three pediatric cases and review of the literature. Rheumatol Int. 2010;30:1355-1359.
18. Park SE, Lee JH. Haemorrhagic bullous lesions in a 3-year-old girl with Henoch-Schönlein purpura. Acta Paediatr. 2011;100:e283-e284.
19. Parikh K. 14-year-old boy with bullous lesions. Pediatr Ann. 2012;41:275-277.
20. Raymond M, Spinks J. Bullous Henoch Schönlein purpura. Arch Dis Child. 2012;97:617.
21. Kocaoglu C, Ozturk R, Unlu Y, et al. Successful treatment of hemorrhagic bullous Henoch-Schönlein purpura with oral corticosteroid: a case report [published online ahead of print April 16, 2013]. Case Rep Pediatr. 2013;2013:680208.
22. Mehra S, Suri D, Dogra S, et al. Hemorrhagic bullous lesions in a girl with Henoch Schönlein purpura. Indian J Pediatr. 2014;81:210-211.
23. Ronkainen J, Koskimies O, Ala-Houhala M, et al. Early prednisone therapy in Henoch-Schönlein purpura: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2006;149:241-247.
24. Weiss PF, Klink AJ, Localio R, et al. Corticosteroids may improve clinical outcomes during hospitalization for Henoch-Schönlein purpura. Pediatrics. 2010;126:674-681.
25. Rosato L, Chehade H, Cachat F. Re: steroids in haemorrhagic bullous Henoch-Schönlein purpura. Acta Paediatr. 2011;100:319-320.
26. Park SJ, Kim JH, Ha TS, et al. The role of corticosteroid in hemorrhagic bullous Henoch Schönlein purpura. Acta Paediatr. 2011;100:e3-e4.
27. Iqbal H, Evans A. Dapsone therapy for Henoch-Schönlein purpura: a case series. Arch Dis Child. 2005;90:985-986.
28. Davin JC, Weening JJ. Diagnosis of Henoch-Schönlein purpura: renal or skin biopsy? Pediatr Nephrol. 2003;18:1201-1203.
29. González LM, Janniger CK, Schwartz RA. Pediatric Henoch-Schönlein purpura. Int J Dermatol. 2009;48: 1157-1165.
1. Saulsbury FT. Henoch-Schönlein purpura in children. report of 100 patients and the review of literature. Medicine. 1999;78:395-409.
2. Cream JJ, Gumpel JM, Peachey RD. Schönlein-Henoch purpura in the adult. a study of 77 adults with anaphylactoid or Schönlein-Henoch purpura. Q J Med. 1970;39:461-484.
3. Tancrede-Bohin E, Ochonisky S, Vignon-Pennamen MD, et al. Schönlein-Henoch purpura in adult patients. predictive factors for IgA glomerulonephritis in a retrospective study of 57 cases. Arch Dermatol. 1997;133:438-442.
4. Abdel-Al YK, Hejazi Z, Majeed HA. Henoch Schönlein purpura in Arab children. analysis of 52 cases. Trop Geogr Med. 1990;42:52-57.
5. Garland JS, Chusid MJ. Henoch-Schöenlein purpura: association with unusual vesicular lesions. Wis Med J. 1985;84:21-23.
6. Crosby DL, Feldman SD. A pruritic vesicular eruption. Henoch-Schönlein purpura. Arch Dermatol. 1990;126:1497-1498.
7. Wananukul S, Pongprasit P, Korkij W. Henoch-Schönlein purpura presenting as hemorrhagic vesicles and bullae: case report and literature review. Pediatr Dermatol. 1995;12:314-317.
8. Saulsbury FT. Hemorrhagic bullous lesions in Henoch-Schönlein purpura. Pediatr Dermatol. 1998;15:357-359.
9. Kobayashi T, Sakuraoka K, Iwamoto M, et al. A case of anaphylactoid purpura with multiple blister formation: possible pathophysiological role of gelatinase (MMP-9). Dermatology. 1998;197:62-64.
10. Liu PM, Bong CN, Chen HH, et al. Henoch-Schönlein purpura with hemorrhagic bullae in children: report of two cases. J Microbiol Immunol Infect. 2004;37:375-378.
11. Ishii Y, Takizawa T, Arakawa H, et al. Hemorrhagic bullous lesions in Henoch-Schönlein purpura. Pediatr Int. 2005;47:694-697.
12. Leung AK, Robson WL. Hemorrhagic bullous lesions in a child with Henoch-Schönlein purpura. Pediatr Dermatol. 2006;23:139-141.
13. Chan K, Han N, Tang W, et al. Lesions in Henoch-Schönlein purpura. Pediatr Dermatol. 2007;24: 325-326.
14. Kausar S, Yalamanchili A. Management of haemorrhagic bullous lesions in Henoch-Schonlein purpura: is there any consensus? J Dermatolog Treat. 2009;20:88-90.
15. Maguiness S, Balma-Mena A, Pope E, et al. Bullous Henoch-Schönlein purpura in children: a report of 6 cases and review of the literature. Clin Pediatr. 2010;49: 1033-1037.
16. den Boer SL, Pasmans SG, Wulffraat NM, et al. Bullous lesions in Henoch-Schönlein purpura as indication to start systemic prednisone. Acta Paediatr. 2010;99:781-783.
17. Trapani S, Mariotti P, Resti M, et al. Severe hemorrhagic bullous lesions in Henoch Schönlein purpura: three pediatric cases and review of the literature. Rheumatol Int. 2010;30:1355-1359.
18. Park SE, Lee JH. Haemorrhagic bullous lesions in a 3-year-old girl with Henoch-Schönlein purpura. Acta Paediatr. 2011;100:e283-e284.
19. Parikh K. 14-year-old boy with bullous lesions. Pediatr Ann. 2012;41:275-277.
20. Raymond M, Spinks J. Bullous Henoch Schönlein purpura. Arch Dis Child. 2012;97:617.
21. Kocaoglu C, Ozturk R, Unlu Y, et al. Successful treatment of hemorrhagic bullous Henoch-Schönlein purpura with oral corticosteroid: a case report [published online ahead of print April 16, 2013]. Case Rep Pediatr. 2013;2013:680208.
22. Mehra S, Suri D, Dogra S, et al. Hemorrhagic bullous lesions in a girl with Henoch Schönlein purpura. Indian J Pediatr. 2014;81:210-211.
23. Ronkainen J, Koskimies O, Ala-Houhala M, et al. Early prednisone therapy in Henoch-Schönlein purpura: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2006;149:241-247.
24. Weiss PF, Klink AJ, Localio R, et al. Corticosteroids may improve clinical outcomes during hospitalization for Henoch-Schönlein purpura. Pediatrics. 2010;126:674-681.
25. Rosato L, Chehade H, Cachat F. Re: steroids in haemorrhagic bullous Henoch-Schönlein purpura. Acta Paediatr. 2011;100:319-320.
26. Park SJ, Kim JH, Ha TS, et al. The role of corticosteroid in hemorrhagic bullous Henoch Schönlein purpura. Acta Paediatr. 2011;100:e3-e4.
27. Iqbal H, Evans A. Dapsone therapy for Henoch-Schönlein purpura: a case series. Arch Dis Child. 2005;90:985-986.
28. Davin JC, Weening JJ. Diagnosis of Henoch-Schönlein purpura: renal or skin biopsy? Pediatr Nephrol. 2003;18:1201-1203.
29. González LM, Janniger CK, Schwartz RA. Pediatric Henoch-Schönlein purpura. Int J Dermatol. 2009;48: 1157-1165.
Practice Points
- The presence of painful hemorrhagic bullae is an uncommon presentation in pediatric patients with Henoch-Schönlein purpura (HSP) and can be a diagnostic challenge.
- Presence of joint pain, abdominal pain, or nephritis could corroborate the diagnosis.
- Early biopsy of the lesion within 48 hours of appearance is important for diagnosis. Presence of IgA deposits on immunofluorescence may aid in diagnosis.
- This finding of bullae in HSP does not seem to have any prognostic significance. Because of the rarity of incidence, there is no consensus on management. Supportive therapy and/or corticosteroids might be effective.